Oncosec - Immunotherapie pd-1 + pIL12 - 500 Beiträge pro Seite
eröffnet am 01.10.14 00:25:00 von
neuester Beitrag 04.11.15 14:04:39 von
neuester Beitrag 04.11.15 14:04:39 von
Beiträge: 16
ID: 1.200.011
ID: 1.200.011
Aufrufe heute: 0
Gesamt: 7.212
Gesamt: 7.212
Aktive User: 0
ISIN: US68234L4059 · WKN: A3D1RU · Symbol: ONCSQ
0,0002
USD
0,00 %
0,0000 USD
Letzter Kurs 24.04.24 Nasdaq OTC
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 52,25 | +93,52 | |
| 1,9000 | +59,66 | |
| 0,6000 | +57,48 | |
| 2,2999 | +25,68 | |
| 1,9200 | +23,87 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,8500 | -12,97 | |
| 2,2900 | -17,63 | |
| 2,6700 | -28,03 | |
| 9,6900 | -33,06 | |
| 5,1700 | -33,72 |
Oncosec ist so etwas wie ein Spin-Off der Nasdaq-notierten Immunotherapie-Firma Inovio und hat deren Elektroporation-Technologie lizenziert, verwendet sie aber in anderer Weise als Inovio selbst für eine Plattformtherapie gegen Krebserkrankungen allgemein. In der Pipeline sind wegen der einfacheren Zugänglichkeit aber erst einmal drei Hautkrebstypen.
Bei Melanom können sie in der noch laufenden PII respektable Studienzwischenergebnisse vorweisen, welche wegen der geringen Nebenwirkungen schon für sich genommen eine hohe Zulassungswahrscheinlichkeit rechtfertigen würde:
Background: Interleukin-12 (IL-12) promotes anti-tumor activity through multiple mechanisms, including augmentation of adaptive and innate immune responses. Intratumoral (IT) delivery of IL-12 via electroporation (EP) avoids systemic toxicity while promoting systemic antitumor immunity. This phase 2 study explores the systemic efficacy, clinical response and safety of IT plasmid IL-12 injection (pIL-12) followed by EP in patients (pts) with advanced melanoma. Methods: This single-arm, open-label phase 2 study plans to enroll 30 pts with in-transit or M1a melanoma. One treatment cycle consists of IT pIL-12-EP on days 1, 5, 8 in up to four lesions per cycle. A maximum of four cycles at 12-week intervals are allowed. ORR was assessed by a modification of RECIST for cutaneous lesions with restaging performed every 12 weeks. The primary endpoint is best ORR within 24 weeks of first treatment. Pre- and post-treatment tumor biopsies were obtained in all patients. Ongoing analyses to assess safety and emerging efficacy data are being utilized to inform future studies.
Results: 29 pts have been enrolled and have received at least one treatment cycle. The ORR is 33% (9/27), with 11% CR (3/27). Regression of non-injected lesions was seen in 62% (13/21) of pts with evaluable lesions. Transient pain (56.5%) and inflammation (17.4%) at the treatment site were the most common grade 1/2 drug-related adverse events (AEs), with no grade 3/4 drug-related AEs. Exploratory analyses indicate a doubling of intratumoral NK cells from pre-treatment through day 11 and at day 39, and increased frequency in activated circulating NK cells.
Conclusions: Local treatment with pIL-12-EP is well tolerated without severe systemic side effects. Regression of treated and non-treated tumors suggests successful induction of systemic anti-tumor response. Local and systemic increases in NK cells are consistent with the expected pharmacodynamic effect of IL-12. Based on these data, an expansion protocol to evaluate increased treatment frequency is planned for melanoma patients.
Clinical trial information: NCT01502293
Was bedeutet das?
Für die Studie hat man Patienten mit metastasierendem Melanom rekrutiert, einer Krankheitsphase für die es so gut wie keine effektive Behandlung mehr gibt.
Bei der Immunopulse Behandlung hat man in einem der Tumore die Poren der Zellen mittels Elektroporation , über einen elektrischen Impuls, geöffnet und dabei Plasmide in die Zelle transportiert, also Genmaterial, welches die (Krebs)Zelle dazu bringt Interleukin-12 zu produzieren. Interleukin-12 ist bereits dafür bekannt den Körper zu einer Immunreaktion zu veranlassen, konnte aber bis jetzt wegen starker Toxizität nicht therapeutisch verwendet werden.
In 11% der Fälle ist der behandelte Tumor komplett verschwunden.
In der Studie konnte bei 62% der Patienten in Tumoren die NICHT behandelt wurden eine Schrumpfen bzw. Verschwinden festgestellt werden. Man behandelt also lokal, hat aber systemisch im ganzen Körper eine therapeutische Wirkung.
Dieses Wochenende wurde eine weitere Studie mit Mäusen präsentiert, welche Indikatoren untersucht, die nahelegen, dass Immunopulse im pd-1 Bereich ein Wirkung zeigt.
Abstract:
http://www.reddit.com/r/ONCS/comments/2gng7w/esmo_abstract/
komplette Version:
http://oncosec.com/QR-Downloads/ESMO-2014-Poster_FINAL-09181…
In dieser Studie 20 complete regressions in den 20 Mäusen, also komplettes Verschwinden des direkt behandelten Tumors bei allen Mäusen der Studie, und 40% der Metastasen) sammelten sie Indizien zur Immunwirkung, einschließlich pd-1.
pd-1:
Eine Kombinationsstudie im Menschen mit anti-pd-1 Antikörpern ist das erklärte Ziel von Oncosec. Dr.Pierce, der Chief Scientific Officer geht davon aus dass Immunopulse pd-1 non-responders in responders umwandeln kann, also die Wirksamkeit von pd-1/pdl-1 Therapien drastisch erhöht.
Die folgende Tabelle der wertvollsten Entwicklungsprojekte zeigt wie wertvoll auch eine pd-1 verstärkende Technologie wäre: 4 der 10 wertvollsten Projekte sind pd-1/pdl-1. Bild einfügen klappt anscheinend nicht, deswegen ein Link.

http://www.flickr.com/photos/40608934@N04/15206773619/
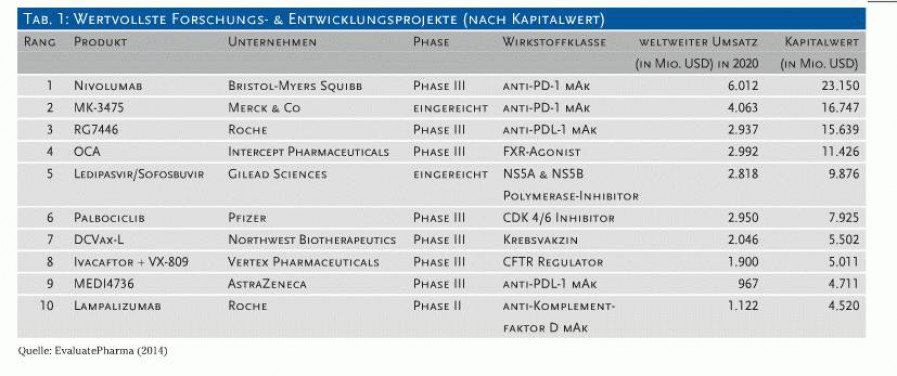
Anfang September wurde die erste pd-1 Therapie von der FDA approved, pembrolizumab Handelsname Keytruda von Merck (in obiger Tabelle noch als eingereicht eingetragen).
Dr.Pierce war maßgeblich an der Entwicklung dieses Antikörpers im Team von Merck beteiligt, bevor er im Dezember 2013 zu Oncosec wechselte mit der Überzeugung hier die pd-1-/pdl-1Therapien verbessern zu können.
Fazit:
Die pIL12 Ergebnisse sind schon ganz gut, mit pd-1 Wirkung würde Oncosec in eine andere Liga befördert.
Soweit ein erster Überblick.
Hinweis:
Oncosec ist gegenwärtig noch an der OTC gelistet. Mit Consors kann ich dort handeln, aber mit vielen deutschen Brokern nicht (mehr).
Bei Melanom können sie in der noch laufenden PII respektable Studienzwischenergebnisse vorweisen, welche wegen der geringen Nebenwirkungen schon für sich genommen eine hohe Zulassungswahrscheinlichkeit rechtfertigen würde:
Background: Interleukin-12 (IL-12) promotes anti-tumor activity through multiple mechanisms, including augmentation of adaptive and innate immune responses. Intratumoral (IT) delivery of IL-12 via electroporation (EP) avoids systemic toxicity while promoting systemic antitumor immunity. This phase 2 study explores the systemic efficacy, clinical response and safety of IT plasmid IL-12 injection (pIL-12) followed by EP in patients (pts) with advanced melanoma. Methods: This single-arm, open-label phase 2 study plans to enroll 30 pts with in-transit or M1a melanoma. One treatment cycle consists of IT pIL-12-EP on days 1, 5, 8 in up to four lesions per cycle. A maximum of four cycles at 12-week intervals are allowed. ORR was assessed by a modification of RECIST for cutaneous lesions with restaging performed every 12 weeks. The primary endpoint is best ORR within 24 weeks of first treatment. Pre- and post-treatment tumor biopsies were obtained in all patients. Ongoing analyses to assess safety and emerging efficacy data are being utilized to inform future studies.
Results: 29 pts have been enrolled and have received at least one treatment cycle. The ORR is 33% (9/27), with 11% CR (3/27). Regression of non-injected lesions was seen in 62% (13/21) of pts with evaluable lesions. Transient pain (56.5%) and inflammation (17.4%) at the treatment site were the most common grade 1/2 drug-related adverse events (AEs), with no grade 3/4 drug-related AEs. Exploratory analyses indicate a doubling of intratumoral NK cells from pre-treatment through day 11 and at day 39, and increased frequency in activated circulating NK cells.
Conclusions: Local treatment with pIL-12-EP is well tolerated without severe systemic side effects. Regression of treated and non-treated tumors suggests successful induction of systemic anti-tumor response. Local and systemic increases in NK cells are consistent with the expected pharmacodynamic effect of IL-12. Based on these data, an expansion protocol to evaluate increased treatment frequency is planned for melanoma patients.
Clinical trial information: NCT01502293
Was bedeutet das?
Für die Studie hat man Patienten mit metastasierendem Melanom rekrutiert, einer Krankheitsphase für die es so gut wie keine effektive Behandlung mehr gibt.
Bei der Immunopulse Behandlung hat man in einem der Tumore die Poren der Zellen mittels Elektroporation , über einen elektrischen Impuls, geöffnet und dabei Plasmide in die Zelle transportiert, also Genmaterial, welches die (Krebs)Zelle dazu bringt Interleukin-12 zu produzieren. Interleukin-12 ist bereits dafür bekannt den Körper zu einer Immunreaktion zu veranlassen, konnte aber bis jetzt wegen starker Toxizität nicht therapeutisch verwendet werden.
In 11% der Fälle ist der behandelte Tumor komplett verschwunden.
In der Studie konnte bei 62% der Patienten in Tumoren die NICHT behandelt wurden eine Schrumpfen bzw. Verschwinden festgestellt werden. Man behandelt also lokal, hat aber systemisch im ganzen Körper eine therapeutische Wirkung.
Dieses Wochenende wurde eine weitere Studie mit Mäusen präsentiert, welche Indikatoren untersucht, die nahelegen, dass Immunopulse im pd-1 Bereich ein Wirkung zeigt.
Abstract:
http://www.reddit.com/r/ONCS/comments/2gng7w/esmo_abstract/
komplette Version:
http://oncosec.com/QR-Downloads/ESMO-2014-Poster_FINAL-09181…
In dieser Studie 20 complete regressions in den 20 Mäusen, also komplettes Verschwinden des direkt behandelten Tumors bei allen Mäusen der Studie, und 40% der Metastasen) sammelten sie Indizien zur Immunwirkung, einschließlich pd-1.
pd-1:
Eine Kombinationsstudie im Menschen mit anti-pd-1 Antikörpern ist das erklärte Ziel von Oncosec. Dr.Pierce, der Chief Scientific Officer geht davon aus dass Immunopulse pd-1 non-responders in responders umwandeln kann, also die Wirksamkeit von pd-1/pdl-1 Therapien drastisch erhöht.
Die folgende Tabelle der wertvollsten Entwicklungsprojekte zeigt wie wertvoll auch eine pd-1 verstärkende Technologie wäre: 4 der 10 wertvollsten Projekte sind pd-1/pdl-1. Bild einfügen klappt anscheinend nicht, deswegen ein Link.

http://www.flickr.com/photos/40608934@N04/15206773619/

Anfang September wurde die erste pd-1 Therapie von der FDA approved, pembrolizumab Handelsname Keytruda von Merck (in obiger Tabelle noch als eingereicht eingetragen).
Dr.Pierce war maßgeblich an der Entwicklung dieses Antikörpers im Team von Merck beteiligt, bevor er im Dezember 2013 zu Oncosec wechselte mit der Überzeugung hier die pd-1-/pdl-1Therapien verbessern zu können.
Fazit:
Die pIL12 Ergebnisse sind schon ganz gut, mit pd-1 Wirkung würde Oncosec in eine andere Liga befördert.
Soweit ein erster Überblick.
Hinweis:
Oncosec ist gegenwärtig noch an der OTC gelistet. Mit Consors kann ich dort handeln, aber mit vielen deutschen Brokern nicht (mehr).
http://ir.oncosec.com/company-news/detail/1634/uc-san-franci…
Zitat:
This Phase II clinical trial will be conducted as a multicenter Investigator Sponsored Trial (IST), with UCSF and Dr. Alain Algazi as the sponsor. Merck will supply pembrolizumab, and OncoSec will provide electroporation devices and plasmid IL-12.
lässt hoffen dass Oncosec nur geringe Kosten hat.
Grossartig, bin erleichtert dass die Kooperation mit Merck und in dieser Form zustandekam.
Zitat:
UC San Francisco and OncoSec Medical Collaborate to Evaluate Investigational Combination of ImmunoPulse and Anti-PD-1 Treatment
Download PDF
Investigator Sponsored Trial Led by Dr. Alain Algazi and Supported by OncoSec and Merck Will Evaluate Combination of KEYTRUDA® and ImmunoPulse in Metastatic Melanoma
SAN DIEGO-- OncoSec Medical Inc. (OTCQB: ONCS), a company developing DNA-based intratumoral cancer immunotherapies, has entered a clinical collaboration with the University of California, San Francisco (UCSF), to evaluate the safety, tolerability and efficacy of the combination of KEYTRUDA® (pembrolizumab), Merck’s anti-PD-1 therapy, and OncoSec’s ImmunoPulse (intratumoral IL-12) in metastatic melanoma.
Recent data suggest that patients who are PD-L1 positive and have increased tumor-infiltrating lymphocytes (TILs) are more likely to respond to anti-PD-1/PD-L1 mAbs compared to patients who are PD-L1 negative. Therefore, therapies that promote TIL generation and PD-L1 positivity may play an important role in augmenting the clinical efficacy of these agents.
Interleukin-12 (IL-12) is an inflammatory cytokine believed to be a master regulator of the immune system, promoting up-regulation of both the innate and adaptive immune responses. More specifically, IL-12 stimulates the production of another cytokine, interferon gamma (IFN-γ), which results in the stimulation of antigen processing and presentation machinery, leading to increased TILs and anti-tumor cytotoxic T-cell (CTL) activity.
ImmunoPulse, an investigational intratumoral immunotherapy, uses plasmid DNA that encodes for IL-12 and delivers it directly into the tumor using a proprietary electroporation device. Preclinical and clinical data suggest that local delivery and expression of IL-12 with ImmunoPulse promotes tumor immunogenicity and increases TILs without the toxicities associated with systemic IL-12 administration. Recent interim data from OncoSec’s ongoing Phase II study have demonstrated that plasmid IL-12 electroporation treatment increases IFN-γ production and increased expression of genes related to antigen processing and presentation, including the expression of PD-L1.
Punit Dhillon, President and CEO of OncoSec, said, “This collaboration with Dr. Algazi and UCSF with support from Merck marks the first clinical trial to evaluate the combination of an anti-PD-1 antibody with an intratumoral therapy using electroporation. Over the course of the last year, OncoSec has continually stated the need to evaluate intratumoral therapies that have the ability to convert the anti-PD-1 non-responder population to responders. The design of this clinical trial will assess this hypothesis, and we believe the combination of OncoSec’s intratumoral immunotherapies and checkpoint inhibitors holds significant promise for the treatment of melanoma and other cancers. We look forward to sharing the results from this clinical trial in the future."
Dr. Robert Pierce, Chief Scientific Officer of OncoSec, said, “There is a strong rationale for combining a treatment like ImmunoPulse, which increases TILs, with a T cell checkpoint therapy like pembrolizumab, which then acts on those TILs. This study is designed to test whether this combination increases patients’ TILs and improves anti-tumor efficacy in low-TIL melanoma patients.”
Dr. Alain Algazi, principal investigator at UCSF, said, “The PD-1 antibody pembrolizumab takes the brakes off of the anti-melanoma immune responses. ImmunoPulse with IL-12 has the potential to bring immune cells and signals into the tumor so that, when pembrolizumab takes the brakes off the immune response, the results could be devastating for the tumor and great for our patients.”
This Phase II clinical trial will be conducted as a multicenter Investigator Sponsored Trial (IST), with UCSF and Dr. Alain Algazi as the sponsor. Merck will supply pembrolizumab, and OncoSec will provide electroporation devices and plasmid IL-12. Enrollment is expected to begin in Q1 2015.
About OncoSec Medical
OncoSec Medical Inc. is a biopharmaceutical company developing its investigational ImmunoPulse intratumoral cancer immunotherapy. OncoSec Medical's core technology is designed to enhance the local delivery and uptake of DNA IL-12 and other DNA-based immune-targeting agents. Clinical studies of ImmunoPulse have demonstrated an acceptable safety profile and preliminary evidence of anti-tumor activity in the treatment of various skin cancers, as well as the potential to initiate a systemic immune response without the systemic toxicities associated with other treatments. OncoSec's lead program evaluating ImmunoPulse for the treatment of metastatic melanoma is currently in Phase 2 development, and is being conducted in collaboration with several prominent academic medical centers. As the company continues to evaluate ImmunoPulse in its current indications, it is also focused on identifying and developing new immune-targeting agents, investigating additional tumor indications, and evaluating combination-based immunotherapy approaches. For more information, please visit www.oncosec.com.
This press release contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Any statements in this release that are not historical facts may be considered such “forward-looking statements.” Forward-looking statements are based on management’s current preliminary expectations and are subject to risks and uncertainties, which may cause our results to differ materially and adversely from the statements contained herein. Some of the potential risks and uncertainties that could cause actual results to differ from those predicted include our ability to raise additional funding, our ability to acquire, develop or commercialize new products, uncertainties inherent in pre-clinical studies and clinical trials, unexpected new data, safety and technical issues, competition, and market conditions. These and additional risks and uncertainties are more fully described in OncoSec Medical’s filings with the Securities and Exchange Commission. Undue reliance should not be placed on forward-looking statements, which speak only as of the date they are made. OncoSec Medical disclaims any obligation to update any forward-looking statements to reflect new information, events or circumstances after the date they are made, or to reflect the occurrence of unanticipated events.
UC Disclaimer
The information stated above was prepared by OncoSec Medical Inc. and reflects solely the opinion of the corporation. Nothing in this statement shall be construed to imply any support or endorsement of OncoSec, or any of its products, by The Regents of the University of California, its officers, agents and employees.
Investor Relations:
OncoSec Medical Inc.
Jordyn Kopin, 855-662-6732
investors@oncosec.com
or
Public Relations:
Dian Griesel Int’l.
Laura Radocaj, 212-825-3210
lradocaj@dgicomm.com
Zitat:
This Phase II clinical trial will be conducted as a multicenter Investigator Sponsored Trial (IST), with UCSF and Dr. Alain Algazi as the sponsor. Merck will supply pembrolizumab, and OncoSec will provide electroporation devices and plasmid IL-12.
lässt hoffen dass Oncosec nur geringe Kosten hat.
Grossartig, bin erleichtert dass die Kooperation mit Merck und in dieser Form zustandekam.
Zitat:
UC San Francisco and OncoSec Medical Collaborate to Evaluate Investigational Combination of ImmunoPulse and Anti-PD-1 Treatment
Download PDF
Investigator Sponsored Trial Led by Dr. Alain Algazi and Supported by OncoSec and Merck Will Evaluate Combination of KEYTRUDA® and ImmunoPulse in Metastatic Melanoma
SAN DIEGO-- OncoSec Medical Inc. (OTCQB: ONCS), a company developing DNA-based intratumoral cancer immunotherapies, has entered a clinical collaboration with the University of California, San Francisco (UCSF), to evaluate the safety, tolerability and efficacy of the combination of KEYTRUDA® (pembrolizumab), Merck’s anti-PD-1 therapy, and OncoSec’s ImmunoPulse (intratumoral IL-12) in metastatic melanoma.
Recent data suggest that patients who are PD-L1 positive and have increased tumor-infiltrating lymphocytes (TILs) are more likely to respond to anti-PD-1/PD-L1 mAbs compared to patients who are PD-L1 negative. Therefore, therapies that promote TIL generation and PD-L1 positivity may play an important role in augmenting the clinical efficacy of these agents.
Interleukin-12 (IL-12) is an inflammatory cytokine believed to be a master regulator of the immune system, promoting up-regulation of both the innate and adaptive immune responses. More specifically, IL-12 stimulates the production of another cytokine, interferon gamma (IFN-γ), which results in the stimulation of antigen processing and presentation machinery, leading to increased TILs and anti-tumor cytotoxic T-cell (CTL) activity.
ImmunoPulse, an investigational intratumoral immunotherapy, uses plasmid DNA that encodes for IL-12 and delivers it directly into the tumor using a proprietary electroporation device. Preclinical and clinical data suggest that local delivery and expression of IL-12 with ImmunoPulse promotes tumor immunogenicity and increases TILs without the toxicities associated with systemic IL-12 administration. Recent interim data from OncoSec’s ongoing Phase II study have demonstrated that plasmid IL-12 electroporation treatment increases IFN-γ production and increased expression of genes related to antigen processing and presentation, including the expression of PD-L1.
Punit Dhillon, President and CEO of OncoSec, said, “This collaboration with Dr. Algazi and UCSF with support from Merck marks the first clinical trial to evaluate the combination of an anti-PD-1 antibody with an intratumoral therapy using electroporation. Over the course of the last year, OncoSec has continually stated the need to evaluate intratumoral therapies that have the ability to convert the anti-PD-1 non-responder population to responders. The design of this clinical trial will assess this hypothesis, and we believe the combination of OncoSec’s intratumoral immunotherapies and checkpoint inhibitors holds significant promise for the treatment of melanoma and other cancers. We look forward to sharing the results from this clinical trial in the future."
Dr. Robert Pierce, Chief Scientific Officer of OncoSec, said, “There is a strong rationale for combining a treatment like ImmunoPulse, which increases TILs, with a T cell checkpoint therapy like pembrolizumab, which then acts on those TILs. This study is designed to test whether this combination increases patients’ TILs and improves anti-tumor efficacy in low-TIL melanoma patients.”
Dr. Alain Algazi, principal investigator at UCSF, said, “The PD-1 antibody pembrolizumab takes the brakes off of the anti-melanoma immune responses. ImmunoPulse with IL-12 has the potential to bring immune cells and signals into the tumor so that, when pembrolizumab takes the brakes off the immune response, the results could be devastating for the tumor and great for our patients.”
This Phase II clinical trial will be conducted as a multicenter Investigator Sponsored Trial (IST), with UCSF and Dr. Alain Algazi as the sponsor. Merck will supply pembrolizumab, and OncoSec will provide electroporation devices and plasmid IL-12. Enrollment is expected to begin in Q1 2015.
About OncoSec Medical
OncoSec Medical Inc. is a biopharmaceutical company developing its investigational ImmunoPulse intratumoral cancer immunotherapy. OncoSec Medical's core technology is designed to enhance the local delivery and uptake of DNA IL-12 and other DNA-based immune-targeting agents. Clinical studies of ImmunoPulse have demonstrated an acceptable safety profile and preliminary evidence of anti-tumor activity in the treatment of various skin cancers, as well as the potential to initiate a systemic immune response without the systemic toxicities associated with other treatments. OncoSec's lead program evaluating ImmunoPulse for the treatment of metastatic melanoma is currently in Phase 2 development, and is being conducted in collaboration with several prominent academic medical centers. As the company continues to evaluate ImmunoPulse in its current indications, it is also focused on identifying and developing new immune-targeting agents, investigating additional tumor indications, and evaluating combination-based immunotherapy approaches. For more information, please visit www.oncosec.com.
This press release contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Any statements in this release that are not historical facts may be considered such “forward-looking statements.” Forward-looking statements are based on management’s current preliminary expectations and are subject to risks and uncertainties, which may cause our results to differ materially and adversely from the statements contained herein. Some of the potential risks and uncertainties that could cause actual results to differ from those predicted include our ability to raise additional funding, our ability to acquire, develop or commercialize new products, uncertainties inherent in pre-clinical studies and clinical trials, unexpected new data, safety and technical issues, competition, and market conditions. These and additional risks and uncertainties are more fully described in OncoSec Medical’s filings with the Securities and Exchange Commission. Undue reliance should not be placed on forward-looking statements, which speak only as of the date they are made. OncoSec Medical disclaims any obligation to update any forward-looking statements to reflect new information, events or circumstances after the date they are made, or to reflect the occurrence of unanticipated events.
UC Disclaimer
The information stated above was prepared by OncoSec Medical Inc. and reflects solely the opinion of the corporation. Nothing in this statement shall be construed to imply any support or endorsement of OncoSec, or any of its products, by The Regents of the University of California, its officers, agents and employees.
Investor Relations:
OncoSec Medical Inc.
Jordyn Kopin, 855-662-6732
investors@oncosec.com
or
Public Relations:
Dian Griesel Int’l.
Laura Radocaj, 212-825-3210
lradocaj@dgicomm.com
Antwort auf Beitrag Nr.: 48.407.621 von logel am 25.11.14 13:24:37CEO Dhillon kommentiert die Meldung:
http://punitdhillon.com/2014/11/25/oncosec-ucsf-and-merck-in…
OncoSec, UCSF, and Merck Initiate a Landmark Phase IIb Combination Trial of Breakthrough Drug KEYTRUDA® And ImmunoPulse to Treat Metastatic Melanoma
OncoSec Medical is changing the cancer experience. Today changes the course of OncoSec as we announced a collaboration with the University of California San Francisco (UCSF), to evaluate the safety, tolerability and efficacy of the combination of Merck’s anti-PD-1 drug, KEYTRUDA® (pembrolizumab), and OncoSec’s ImmunoPulse therapy (intratumoral IL- 12), in a phase II metastatic melanoma study with patients non-responsive to KEYTRUDA®. This is an Investigator Sponsored Trial led by renowned Oncologist Dr. Alain Algazi at the prestigious University of California San Francisco and supported by OncoSec Medical and Merck Research Labs. This powerful combination is expected to treat the larger population of approximately 70% of the patients for which it is not currently effective by one of the most exciting breakthrough drugs in treatment of cancer.
For the last year, OncoSec has been focused on the the tremendous unmet medical need of metastatic melanoma patients, who don’t respond to PD-1/PDL1 therapeutics. Unfortunately, these patients represent approximately 70% of metastatic melanoma patients. OncoSec data – both preclinical and clinical – demonstrates that intratumoral Immunopulse therapy with IL–12 drives an increase in TILs. Based on these data, we have proposed that Immunpulse IL-12 will “convert” anti-PD-1 non-responders into responders, leading to potentially enhanced clinical efficacy of PD-1. The field of immunotherapy is beginning to focus on where we’ve been for the last year – an awareness that PD-1 non-responders constitute a tremendous unmet medical need and business opportunity.
This collaboration is significant because of the speed of being able to get into a clinical trial to test our hypothesis and as a critical and positive step in the development of IL-12 immunotherapy. I believe this is yet another sign giving validation of the value of intratumoral immunotherapy and combinatorial approaches as part of an overall therapeutic regimen to treat cancer.
Key Highlights:
Only minority of patients respond to KEYTRUDA®
Patients who do not respond to KEYTRUDA® do not have immune cells (i.e. the correct TILs) already present in the tumor
ImmunoPulse has the potential to bring the correct immune cells into the tumor
Thus, ImmunoPulse has the potential to address the approximately 70% of patients who do not respond to KEYTRUDA®
ImmunoPulse, uses plasmid DNA that encodes for IL-12 and delivers it directly into the tumor using a proprietary delivery device. Preclinical and clinical data suggest that local delivery and expression of IL-12 with ImmunoPulse promotes tumor immunogenicity and increases tumor-infiltrating lymphocytes (TILs). Over 70 patients have been treated with ImmunoPulse, and no serious drug related toxicities have been reported to-date, which demonstrates the strong safety profile of this therapy.
ImmunoPulse has the potential to be the ultimate combination therapy with a new class of immunotherapies called, checkpoint inhibitors. Merck’s recently approved KEYTRUDA® (pembrolizumab), is the first anti-PD-1 (programmed death receptor-1) checkpoint therapy approved in the United States for metastatic melanoma, based on strong Phase 2 data, that showed a 24 percent overall response rate. Despite the deserved excitement, there still remains an unmet medical need for the approximately 70 percent of patients who do not respond to this drug. New data suggest that 24% of the Melanoma patients who best respond to KEYTRUDA® are those who already have immune cells (i.e. TILs) in the tumor, and the approximately 70 percent of the patients that have little or no immune cells in the tumor do not respond to KEYTRUDA®. Since IL- 12 promotes tumor immunogenicity and increases the number of TILs in the tumor, the combination of our therapy to increase TILs in the tumor, and KEYTRUDA® which activates these cells to kill the tumor, present an exciting landmark study to address the vast majority of the metastatic melanoma patients, as well as, have profound implications on many other tumor indications.
Dr. Alain Algazi, Principal Investigator at UCSF said, “Merck’s PD-1 antibody, KEYTRUDA®, takes the brakes off of the anti-melanoma immune responses. ImmunoPulse with IL-12 has the potential to bring immune cells and signals into the tumor so that, when KEYTRUDA® takes the brakes off the immune response, the results could be devastating for the tumor and great for our patients, allowing us to potentially use this combination approach to address a much larger population of patients with aggressive cancer then with KEYTRUDA® alone.”
Dr. Robert Pierce, Chief Scientific Officer, stated, “There is a strong rationale for combining a treatment like ImmunoPulse, which increases TILs, with a T cell checkpoint therapy like KEYTRUDA, to improve anti-tumor efficacy in low-TIL melanoma patients, which make up approximately 70% of the metastatic melanoma patients”
“We are excited and proud to announce this collaboration with Dr. Algazi and UCSF with support from Merck. The achievement of this milestone marks the first clinical trial to evaluate the combination of an anti-PD-1 antibody with an intratumoral therapy using electroporation,” commented Punit Dhillon, President and CEO of OncoSec. “Over the course of the last year, OncoSec has continually stated the need to evaluate intratumoral therapies that have the ability to convert the anti-PD-1 non-responder population to responders. We believe the combination of OncoSec’s ImmunoPulse and checkpoint inhibitors holds significant promise for the treatment of melanoma and other cancers. The caner immunotherapy market is expected to expand up to $35 billion in the next 10 years. Intralesional therapies have the potential to gain a significant portion of this market share.”
This Phase II clinical trial will be conducted as a multicenter Investigator Sponsored Trial (IST), with UCSF and Dr. Alain Algazi as the sponsor. Merck will supply pembrolizumab, and OncoSec will provide electroporation devices and plasmid IL-12. Enrollment is expected to begin in Q1 2015.
About Checkpoint Inhibitors and Combination Approaches
Checkpoint inhibitors have shown promise in a hard-to-treat cancer such as metastatic melanoma. The objective response rate (ORR) with checkpoint inhibitors, like Yervoy (anti-CTLA-4) or Keytruda (anti-PD-1), is in the 10-40% range. There is an obvious need to improve responses and address the needs of the remaining melanoma patients who do not respond to these therapies, thus interventions that complement checkpoint inhibitors are needed.
Mechanistically, checkpoint inhibitors allow the immune cells to recognize and kill the tumors, however, if there are no immune cells already present in the tumor then these checkpoint inhibitors may not be effective. Therefore checkpoint inhibitors may be helped by combining with agents that enhance the infiltration of immune cells into the tumor. It is widely believed and demonstrated in pre-clinical studies (and maybe in early clinical studies) that combining checkpoint inhibitors with complementary immune therapies that can engage a broader immune response could lead to increased efficacy. Of equal importance, is to find potential combination therapies that not only have the potential to improve efficacy, but also pose minimal risk in increasing potential immune- related toxicities.
About KEYTRUDA®
KEYTRUDA is a human programmed death receptor-1 (PD-1)-blocking antibody indicated for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor. This indication is approved under accelerated approval based on tumor response rate and durability of response. An improvement in survival or disease-related symptoms has not yet been established.
About metastatic melanoma
Skin cancer is one of the most common types of cancers diagnosed in the United States, with an estimated 2 million new cases annually. Melanoma is the most deadly type of skin cancer, leading to 75% of all skin cancer related deaths due to systemic metastatic spread. Of the 76,000 new Melanoma diagnoses estimated to occur in 2014, approximately 12% will have progressed to stage III or IV melanoma already with a 5- year survival rate of only 15%. Even today, treatment options for melanoma remain limited.
Alain Algazi, M.D. Melanoma Specialist
Dr. Alain Algazi, an oncologist, is a skin cancer specialist in the Melanoma Center at the UCSF Helen Diller Family Comprehensive Cancer Center. He treats patients with high- risk and advanced melanoma as well as those with extensive squamous cell and merkel cell carcinoma of the skin. These trials give patients access to new medications, including drugs called BRAF inhibitors that cause melanoma tumors to shrink in a majority of patients treated. He is looking for ways to improve the effectiveness of these medications.
Algazi earned a medical degree at the UCLA School of Medicine and completed an internal medicine residency at UCLA Medical Center. He completed a fellowship in hematology and oncology at UCSF. He is a member of the American Society of Clinical Oncology and American Association for Cancer Research. He is a clinical instructor at UCSF.
About Merck
Merck & Co., Inc. provides various health solutions through its prescription medicines, vaccines, biologic therapies, animal health, and consumer care products worldwide. The companys Pharmaceutical segment offers human health pharmaceutical products, such as therapeutic and preventive agents for the treatment of human disorders in the areas of cardiovascular, diabetes and obesity, respiratory, women’s health and endocrine, inflammatory and infectious diseases, oncology, ophthalmology, immunology, infectious diseases, and others. This segment also provides vaccines, including preventive pediatric, adolescent, and adult vaccines. Its Animal Health segment discovers, develops, manufactures, and markets animal health products comprising vaccines, antibiotics, and anti-inflammatory drugs for respiratory diseases, as well as products for the treatment of fertility disorders. The company was founded in 1891 and is headquartered in Whitehouse Station, New Jersey.
http://punitdhillon.com/2014/11/25/oncosec-ucsf-and-merck-in…
OncoSec, UCSF, and Merck Initiate a Landmark Phase IIb Combination Trial of Breakthrough Drug KEYTRUDA® And ImmunoPulse to Treat Metastatic Melanoma
OncoSec Medical is changing the cancer experience. Today changes the course of OncoSec as we announced a collaboration with the University of California San Francisco (UCSF), to evaluate the safety, tolerability and efficacy of the combination of Merck’s anti-PD-1 drug, KEYTRUDA® (pembrolizumab), and OncoSec’s ImmunoPulse therapy (intratumoral IL- 12), in a phase II metastatic melanoma study with patients non-responsive to KEYTRUDA®. This is an Investigator Sponsored Trial led by renowned Oncologist Dr. Alain Algazi at the prestigious University of California San Francisco and supported by OncoSec Medical and Merck Research Labs. This powerful combination is expected to treat the larger population of approximately 70% of the patients for which it is not currently effective by one of the most exciting breakthrough drugs in treatment of cancer.
For the last year, OncoSec has been focused on the the tremendous unmet medical need of metastatic melanoma patients, who don’t respond to PD-1/PDL1 therapeutics. Unfortunately, these patients represent approximately 70% of metastatic melanoma patients. OncoSec data – both preclinical and clinical – demonstrates that intratumoral Immunopulse therapy with IL–12 drives an increase in TILs. Based on these data, we have proposed that Immunpulse IL-12 will “convert” anti-PD-1 non-responders into responders, leading to potentially enhanced clinical efficacy of PD-1. The field of immunotherapy is beginning to focus on where we’ve been for the last year – an awareness that PD-1 non-responders constitute a tremendous unmet medical need and business opportunity.
This collaboration is significant because of the speed of being able to get into a clinical trial to test our hypothesis and as a critical and positive step in the development of IL-12 immunotherapy. I believe this is yet another sign giving validation of the value of intratumoral immunotherapy and combinatorial approaches as part of an overall therapeutic regimen to treat cancer.
Key Highlights:
Only minority of patients respond to KEYTRUDA®
Patients who do not respond to KEYTRUDA® do not have immune cells (i.e. the correct TILs) already present in the tumor
ImmunoPulse has the potential to bring the correct immune cells into the tumor
Thus, ImmunoPulse has the potential to address the approximately 70% of patients who do not respond to KEYTRUDA®
ImmunoPulse, uses plasmid DNA that encodes for IL-12 and delivers it directly into the tumor using a proprietary delivery device. Preclinical and clinical data suggest that local delivery and expression of IL-12 with ImmunoPulse promotes tumor immunogenicity and increases tumor-infiltrating lymphocytes (TILs). Over 70 patients have been treated with ImmunoPulse, and no serious drug related toxicities have been reported to-date, which demonstrates the strong safety profile of this therapy.
ImmunoPulse has the potential to be the ultimate combination therapy with a new class of immunotherapies called, checkpoint inhibitors. Merck’s recently approved KEYTRUDA® (pembrolizumab), is the first anti-PD-1 (programmed death receptor-1) checkpoint therapy approved in the United States for metastatic melanoma, based on strong Phase 2 data, that showed a 24 percent overall response rate. Despite the deserved excitement, there still remains an unmet medical need for the approximately 70 percent of patients who do not respond to this drug. New data suggest that 24% of the Melanoma patients who best respond to KEYTRUDA® are those who already have immune cells (i.e. TILs) in the tumor, and the approximately 70 percent of the patients that have little or no immune cells in the tumor do not respond to KEYTRUDA®. Since IL- 12 promotes tumor immunogenicity and increases the number of TILs in the tumor, the combination of our therapy to increase TILs in the tumor, and KEYTRUDA® which activates these cells to kill the tumor, present an exciting landmark study to address the vast majority of the metastatic melanoma patients, as well as, have profound implications on many other tumor indications.
Dr. Alain Algazi, Principal Investigator at UCSF said, “Merck’s PD-1 antibody, KEYTRUDA®, takes the brakes off of the anti-melanoma immune responses. ImmunoPulse with IL-12 has the potential to bring immune cells and signals into the tumor so that, when KEYTRUDA® takes the brakes off the immune response, the results could be devastating for the tumor and great for our patients, allowing us to potentially use this combination approach to address a much larger population of patients with aggressive cancer then with KEYTRUDA® alone.”
Dr. Robert Pierce, Chief Scientific Officer, stated, “There is a strong rationale for combining a treatment like ImmunoPulse, which increases TILs, with a T cell checkpoint therapy like KEYTRUDA, to improve anti-tumor efficacy in low-TIL melanoma patients, which make up approximately 70% of the metastatic melanoma patients”
“We are excited and proud to announce this collaboration with Dr. Algazi and UCSF with support from Merck. The achievement of this milestone marks the first clinical trial to evaluate the combination of an anti-PD-1 antibody with an intratumoral therapy using electroporation,” commented Punit Dhillon, President and CEO of OncoSec. “Over the course of the last year, OncoSec has continually stated the need to evaluate intratumoral therapies that have the ability to convert the anti-PD-1 non-responder population to responders. We believe the combination of OncoSec’s ImmunoPulse and checkpoint inhibitors holds significant promise for the treatment of melanoma and other cancers. The caner immunotherapy market is expected to expand up to $35 billion in the next 10 years. Intralesional therapies have the potential to gain a significant portion of this market share.”
This Phase II clinical trial will be conducted as a multicenter Investigator Sponsored Trial (IST), with UCSF and Dr. Alain Algazi as the sponsor. Merck will supply pembrolizumab, and OncoSec will provide electroporation devices and plasmid IL-12. Enrollment is expected to begin in Q1 2015.
About Checkpoint Inhibitors and Combination Approaches
Checkpoint inhibitors have shown promise in a hard-to-treat cancer such as metastatic melanoma. The objective response rate (ORR) with checkpoint inhibitors, like Yervoy (anti-CTLA-4) or Keytruda (anti-PD-1), is in the 10-40% range. There is an obvious need to improve responses and address the needs of the remaining melanoma patients who do not respond to these therapies, thus interventions that complement checkpoint inhibitors are needed.
Mechanistically, checkpoint inhibitors allow the immune cells to recognize and kill the tumors, however, if there are no immune cells already present in the tumor then these checkpoint inhibitors may not be effective. Therefore checkpoint inhibitors may be helped by combining with agents that enhance the infiltration of immune cells into the tumor. It is widely believed and demonstrated in pre-clinical studies (and maybe in early clinical studies) that combining checkpoint inhibitors with complementary immune therapies that can engage a broader immune response could lead to increased efficacy. Of equal importance, is to find potential combination therapies that not only have the potential to improve efficacy, but also pose minimal risk in increasing potential immune- related toxicities.
About KEYTRUDA®
KEYTRUDA is a human programmed death receptor-1 (PD-1)-blocking antibody indicated for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor. This indication is approved under accelerated approval based on tumor response rate and durability of response. An improvement in survival or disease-related symptoms has not yet been established.
About metastatic melanoma
Skin cancer is one of the most common types of cancers diagnosed in the United States, with an estimated 2 million new cases annually. Melanoma is the most deadly type of skin cancer, leading to 75% of all skin cancer related deaths due to systemic metastatic spread. Of the 76,000 new Melanoma diagnoses estimated to occur in 2014, approximately 12% will have progressed to stage III or IV melanoma already with a 5- year survival rate of only 15%. Even today, treatment options for melanoma remain limited.
Alain Algazi, M.D. Melanoma Specialist
Dr. Alain Algazi, an oncologist, is a skin cancer specialist in the Melanoma Center at the UCSF Helen Diller Family Comprehensive Cancer Center. He treats patients with high- risk and advanced melanoma as well as those with extensive squamous cell and merkel cell carcinoma of the skin. These trials give patients access to new medications, including drugs called BRAF inhibitors that cause melanoma tumors to shrink in a majority of patients treated. He is looking for ways to improve the effectiveness of these medications.
Algazi earned a medical degree at the UCLA School of Medicine and completed an internal medicine residency at UCLA Medical Center. He completed a fellowship in hematology and oncology at UCSF. He is a member of the American Society of Clinical Oncology and American Association for Cancer Research. He is a clinical instructor at UCSF.
About Merck
Merck & Co., Inc. provides various health solutions through its prescription medicines, vaccines, biologic therapies, animal health, and consumer care products worldwide. The companys Pharmaceutical segment offers human health pharmaceutical products, such as therapeutic and preventive agents for the treatment of human disorders in the areas of cardiovascular, diabetes and obesity, respiratory, women’s health and endocrine, inflammatory and infectious diseases, oncology, ophthalmology, immunology, infectious diseases, and others. This segment also provides vaccines, including preventive pediatric, adolescent, and adult vaccines. Its Animal Health segment discovers, develops, manufactures, and markets animal health products comprising vaccines, antibiotics, and anti-inflammatory drugs for respiratory diseases, as well as products for the treatment of fertility disorders. The company was founded in 1891 and is headquartered in Whitehouse Station, New Jersey.
Endpoint PII Studiendaten
http://content.stockpr.com/_news/oncosec/2014-12-05_OncoSec_…
bestätigt Daten aus Zwischenergebnissen.
Es gibt einen berechtigten Einwand gegen diese Studie, und auch gegen die geplante PIIb Studie mit Merck und der University of California.
Beide sind Investigator Sponsored, also durchgeführt von den Firmen selbst und nicht Sponsored, also ausgelagert an eine separate Firma welche die Studien stellvertretend durchführt.
Die Daten sind dadurch einer grösseren Manipulationsgefahr ausgesetzt und werden von der FDA mit Recht als weniger aussagekräftig eingestuft.
Eine vorzeitige Zulassung nach guten Daten in der Kombistudie mit Merck, und (was manche hoffen) wie bei Mercks Pembrolizumab ohne dass PIII verlangt wird halte ich deswegen für unwahrscheinlich.
[spoiler]OncoSec Reports Positive Top-Line Six-Month Primary Endpoint Data from Phase II Melanoma Trial of ImmunoPulse Monotherapy
Download PDF
Best Overall Response Rate of 31 Percent
Complete Response Rate of 14 Percent
Disease Control Rate of 48 Percent
SAN DIEGO-- OncoSec Medical Inc. (OTCQB: ONCS), a company developing DNA-based intratumoral cancer immunotherapies, released top-line six-month data from the first Phase II trial of its investigational intratumoral plasmid IL-12 electroporation (pIL-12 EP) monotherapy (ImmunoPulse IL-12) in patients with Stage III and IV metastatic melanoma. Dr. Robert H. Pierce, Chief Scientific Officer at OncoSec, a co-author of the study, presented these data today in an abstract at the Melanoma Bridge 2014 conference in Naples, Italy.
In this Phase II study, 30 patients with stage III-IV melanoma received up to four cycles of pIL-12 EP into superficial cutaneous, subcutaneous and nodal lesions on Days 1, 5 and 8 of each 12-week cycle. Tumor responses were evaluated using modified RECIST criteria for cutaneous lesions. The primary endpoint of the study was best overall response rate (bORR) by modified RECIST. In the 29 response-evaluable patients, bORR was 31 percent (9/29), with 14 percent (4/29) of patients achieving a complete response (CR). Regression of at least one non-injected, non-electroporated lesion was observed in 50 percent (13/26) of patients.
Dr. Mai H. Le, Chief Medical Officer, stated, “Along with the Phase I long-term survival analysis presented yesterday, these data continue to support the use of pIL-12 EP as a treatment for metastatic melanoma. Importantly, our observation that non-treated lesions regress in approximately half of the patients suggests that local, intratumoral pIL-12 EP successfully induces a more global anti-tumor immune-mediated response.”
The most common treatment-related adverse event (AE) reported was transient Grade 1/2 pain at the treatment site, reported in 87 percent (26/30) of patients. The reports of pain were directly associated with the procedure and the median duration of pain was one minute. Only one Grade 3 adverse event of pain was reported. No other Grade 3 or worse adverse events were observed and there were no reports of any treatment-related serious adverse events (SAEs).
Analysis of tissue samples from patients treated with pIL-12 EP showed a gene expression pattern consistent with generation of an inflammatory response with increased CD8+ TILs (tumor-infiltrating lymphocytes) and the induction of key immune co-stimulatory molecules. These findings were corroborated by the results of preliminary preclinical experiments testing pIL-12 EP in the B16.F10 mouse melanoma model, which also indicated the presence of CD8+ TILs (tumor-infiltrating lymphocytes) and the induction of adaptive resistance mechanisms in distant tumors.
Dr. Pierce said, “We are pleased to see such good concordance between our findings from patient biopsy samples and the B16.F10 mouse model. This gives us confidence that we can use this experimental model to deepen our understanding of how ImmunoPulse re-programs the immune system to drive a systemic anti-cancer immune response. Taken together, these clinical and preclinical data provide further evidence for combining this approach with checkpoint inhibitors such as anti-PD-1.”
Punit Dhillon, President and Chief Executive Officer, added, “Induction of systemic anti-tumor immune responses in metastatic melanoma with a local IL-12 therapy, like ImmunoPulse, may provide an important and convenient therapeutic option for treating this devastating disease, particularly with other therapies that block immune checkpoints. In light of this data, we are excited about our recently announced combination trial of ImmunoPulse IL-12 with Merck's recently approved PD-1 inhibitor, Keytruda®, in a Phase IIb study.” [/spoiler]
http://content.stockpr.com/_news/oncosec/2014-12-05_OncoSec_…
bestätigt Daten aus Zwischenergebnissen.
Es gibt einen berechtigten Einwand gegen diese Studie, und auch gegen die geplante PIIb Studie mit Merck und der University of California.
Beide sind Investigator Sponsored, also durchgeführt von den Firmen selbst und nicht Sponsored, also ausgelagert an eine separate Firma welche die Studien stellvertretend durchführt.
Die Daten sind dadurch einer grösseren Manipulationsgefahr ausgesetzt und werden von der FDA mit Recht als weniger aussagekräftig eingestuft.
Eine vorzeitige Zulassung nach guten Daten in der Kombistudie mit Merck, und (was manche hoffen) wie bei Mercks Pembrolizumab ohne dass PIII verlangt wird halte ich deswegen für unwahrscheinlich.
[spoiler]OncoSec Reports Positive Top-Line Six-Month Primary Endpoint Data from Phase II Melanoma Trial of ImmunoPulse Monotherapy
Download PDF
Best Overall Response Rate of 31 Percent
Complete Response Rate of 14 Percent
Disease Control Rate of 48 Percent
SAN DIEGO-- OncoSec Medical Inc. (OTCQB: ONCS), a company developing DNA-based intratumoral cancer immunotherapies, released top-line six-month data from the first Phase II trial of its investigational intratumoral plasmid IL-12 electroporation (pIL-12 EP) monotherapy (ImmunoPulse IL-12) in patients with Stage III and IV metastatic melanoma. Dr. Robert H. Pierce, Chief Scientific Officer at OncoSec, a co-author of the study, presented these data today in an abstract at the Melanoma Bridge 2014 conference in Naples, Italy.
In this Phase II study, 30 patients with stage III-IV melanoma received up to four cycles of pIL-12 EP into superficial cutaneous, subcutaneous and nodal lesions on Days 1, 5 and 8 of each 12-week cycle. Tumor responses were evaluated using modified RECIST criteria for cutaneous lesions. The primary endpoint of the study was best overall response rate (bORR) by modified RECIST. In the 29 response-evaluable patients, bORR was 31 percent (9/29), with 14 percent (4/29) of patients achieving a complete response (CR). Regression of at least one non-injected, non-electroporated lesion was observed in 50 percent (13/26) of patients.
Dr. Mai H. Le, Chief Medical Officer, stated, “Along with the Phase I long-term survival analysis presented yesterday, these data continue to support the use of pIL-12 EP as a treatment for metastatic melanoma. Importantly, our observation that non-treated lesions regress in approximately half of the patients suggests that local, intratumoral pIL-12 EP successfully induces a more global anti-tumor immune-mediated response.”
The most common treatment-related adverse event (AE) reported was transient Grade 1/2 pain at the treatment site, reported in 87 percent (26/30) of patients. The reports of pain were directly associated with the procedure and the median duration of pain was one minute. Only one Grade 3 adverse event of pain was reported. No other Grade 3 or worse adverse events were observed and there were no reports of any treatment-related serious adverse events (SAEs).
Analysis of tissue samples from patients treated with pIL-12 EP showed a gene expression pattern consistent with generation of an inflammatory response with increased CD8+ TILs (tumor-infiltrating lymphocytes) and the induction of key immune co-stimulatory molecules. These findings were corroborated by the results of preliminary preclinical experiments testing pIL-12 EP in the B16.F10 mouse melanoma model, which also indicated the presence of CD8+ TILs (tumor-infiltrating lymphocytes) and the induction of adaptive resistance mechanisms in distant tumors.
Dr. Pierce said, “We are pleased to see such good concordance between our findings from patient biopsy samples and the B16.F10 mouse model. This gives us confidence that we can use this experimental model to deepen our understanding of how ImmunoPulse re-programs the immune system to drive a systemic anti-cancer immune response. Taken together, these clinical and preclinical data provide further evidence for combining this approach with checkpoint inhibitors such as anti-PD-1.”
Punit Dhillon, President and Chief Executive Officer, added, “Induction of systemic anti-tumor immune responses in metastatic melanoma with a local IL-12 therapy, like ImmunoPulse, may provide an important and convenient therapeutic option for treating this devastating disease, particularly with other therapies that block immune checkpoints. In light of this data, we are excited about our recently announced combination trial of ImmunoPulse IL-12 with Merck's recently approved PD-1 inhibitor, Keytruda®, in a Phase IIb study.” [/spoiler]
Antwort auf Beitrag Nr.: 48.506.096 von logel am 06.12.14 14:21:43
was bedeutet das für Oncosec med.? Machen die Betrug ?
Wie siehst Du deren Zukunft ?
Vielen Dank im voraus.
Hallo Logel - Frage !
Hallo Logel,was bedeutet das für Oncosec med.? Machen die Betrug ?
Wie siehst Du deren Zukunft ?
Vielen Dank im voraus.
Antwort auf Beitrag Nr.: 48.512.270 von bayernland1 am 08.12.14 09:33:22Oh je nein, Betrug wollte ich nicht andeuten.
Es heisst nur dass es eine kleine Firma ist die entweder sparen oder mit Kapitalerhöhungen verwässern muss. Sponsored Studien sind um ein Vielfaches teurer. Mir ist es durchaus recht dass man sich für die Sparvariante entschieden habt.
Die kommende PIIb wird durch die UCSF, die University of California San Francisco durchgeführt und mitfinanziert. Da ist genügend Abstand zu Oncosec dass ich den Resultaten vertrauen werde. Aber die FDA wird aufgrund der Daten vermutlich keine vorgezogene Zulassung gewähren, auch wenn sie gut ausfallen.
Es heisst nur dass es eine kleine Firma ist die entweder sparen oder mit Kapitalerhöhungen verwässern muss. Sponsored Studien sind um ein Vielfaches teurer. Mir ist es durchaus recht dass man sich für die Sparvariante entschieden habt.
Die kommende PIIb wird durch die UCSF, die University of California San Francisco durchgeführt und mitfinanziert. Da ist genügend Abstand zu Oncosec dass ich den Resultaten vertrauen werde. Aber die FDA wird aufgrund der Daten vermutlich keine vorgezogene Zulassung gewähren, auch wenn sie gut ausfallen.
Antwort auf Beitrag Nr.: 48.513.074 von logel am 08.12.14 10:48:40
Vielen Dank für die schnelle Antwort !
Gruß.Bayernland1.
Danke
Vielen Dank für die schnelle Antwort !
Gruß.Bayernland1.
Tvec vs. Oncosec
Vergleich von Oncosec zum Konkurrenten Tvec:http://docs.google.com/document/d/1aaIQRFno3T5W7PDDsPffAt6v7…
Für einzelne der Tabellen muss man im Google Message Board für Oncosec angemeldet sein, glaube ich.
Demnach steht Oncosec recht gut da.
Antwort auf Beitrag Nr.: 48.578.864 von logel am 15.12.14 21:44:52
danke für Deine interessanten Beiträge zu dieser Firma.
Ich habe vor kurzem im letzten Q-Bericht dieser Firma gelesen, das Kapital ist noch für 12 Monate ausreichend. Kommt evtl. in absehbarer Zeit eine
Ausgabe neuer Aktien um an frisches/neues Kapital zu kommen ?
Gruß.Bayernland1
Oncosec med.
Hallo Logel,danke für Deine interessanten Beiträge zu dieser Firma.
Ich habe vor kurzem im letzten Q-Bericht dieser Firma gelesen, das Kapital ist noch für 12 Monate ausreichend. Kommt evtl. in absehbarer Zeit eine
Ausgabe neuer Aktien um an frisches/neues Kapital zu kommen ?
Gruß.Bayernland1
Antwort auf Beitrag Nr.: 48.581.267 von bayernland1 am 16.12.14 09:23:18Der Zeitpunkt der nächsten KE ist immer eine spannende Frage bei kleinen Biotechs.
Leider weiss ich da auch nicht mehr.
Leider weiss ich da auch nicht mehr.
Ich hoffe, das in der nächsten Woche richtungsweisende Ergebnisse bekannt werden! 



Antwort auf Beitrag Nr.: 48.748.181 von hebbelman am 11.01.15 19:55:00
Keine KE in absehbarer Zeit geplant.
From Shallum Furbush who actually went to the symposium:
Key points I got from the presentation
IL-12 works great with just 1 dose, strong indications from Dr. Le and Dr. Pierce that P2B expanded (yes first time I heard them call it a P2B for the expansion study) is showing significant improvement over the P2 final we saw.
People that respond, really respond well, about 80% are stable or show ORR, about 50% have shrinkage of 20% or more (30% needed to qualify as ORR) and apparently some of those who did not qualify during the study have sense hit a point where they would have qualified, so a longer trial would have shown higher ORR values.
--------------------------------
Quote:I showed up about an hour early and checked in with Jordan, (we all got name tags, there was about 80-90 tags laid out on the desk I estimate) I was quickly joined by a HF manager and he and I talked for a bit, he has held a position for 2 years roughly and has been considering adding more to his HF. He stated that the company was performing quite well and he was very satisfied with the growth they have accomplished this last year. About this time Punit arrived, and we shook hands and began discussing the company. After I made the comment to him that I was quite happy with everything but the stock price he said "don't worry about the day to day movements" which I don't. He went on to say that he too was dissatisfied with how the stock was performing, which was one of the reasons that he was doing the presentation, he believed that most people do not fully understand what ONCS was/is all about. He stated that one of the big problems is the high percentage of retail holders (see if we all sell the stock will go up), retail tends to have less patience with stock price and the constant selling effects price. He was hoping to gain additional institutional investors, which led me to ask about institutional investors. He mentioned 4 names (I don't care who they are, so don't remember the names), and said they all were still holders (so I assume we have heard who they were in the past). I brought up doing a R./S, and he stated that they were discussing it, a R/S would be oriented around an event with positive movement. Dr. Pierce showed up, and we all introduced ourselves, shortly there after Punit had to take off. Dr. Pierce began talking about immunotherapy and how it is so new and poorly understood by scientists and investors, he talked about Keytruda, how Merck actually acquired it from some other company they bought, then sat on it for several years till BMY released their P1 Opdivo study. Then the race was on. He talked about Yervoy, how it would land you in the ICU, etc. Dr. Le joined us, and the conversation continued, no significant details that I recall, just a lot of confirming information about immunotherapy, how IL-12 works, IL-15 was discussed.
Conference started, (I'll leave this to you to listen to when posted), key things, first immunotherapy was in 1890 with a 50% CR injecting bacteria into patients with cancer. There have been studies injecting melanoma with various agents giving a 80% CR but does nothing for OS, (sell your PVCT now), The key to stimulating immune response is a small release of the tumor cells that triggers the immune response, if you kill the tumor to quick you get no systemic response. Some of the CR's were at day 600 for immunotherapy. All the CR's for IL-12 were durrable and long lasting with excellent outcomes, even for the ones who did not get a CR, along with this was just 1 dose. 1 patient had a relapse after the study was done was retreated in the last 2 weeks. The Relapse seen was minor, (even the one reported in P2 study) where a person with multiple lesions was treated had a CR, the relapse was 1 lesion only. IL-12 allows for use of less PD1 which will be cost effective. CART does not work in solid tumors, however according to Dr. Khort, you can use CART to convert the solid tumor environment to respond to CART (I assume this means 2 CART treatments which doubles the cost and risks. He went on to say that he believes that IL-12 can be used in B-Cell cancers, perhaps in conjunction with CART and probably eliminating CART entirely (several years from now, CART is probably a good investment for the time being, but staying in it longer would be dangerous IMO)
After the conference I spoke at great length with Dr. Pierce and the lead Dr. from our H&N study. Breast Cancer trial is 10 patients with a biopsy pre and post therapy, 28 days long. This is in a population that has already received surgery and chemo, the breast cancer returns with surface lesions. Both Genetech (if I spelled that right) and Merck released data at DAllas Breast cancer symposium showing that TIL was a key to this working. So if 10 patients show an increase in TIL..... Oh, by the way for every 10% increase in TIL there is a corresponding increase in either ORR or OS (can't remember which), in melanoma the TIL was increased by hunderds of % (as in more than 10).
Alright, so still talking with Dr. Pierce and Dr. Algazi ( I believe is who it was), and topic is still the breast cancer trial. 10 patients, 28 days for data, so depending on how quickly this gets filled. Dr. Telli, sounded very excited about using IL-12 on her patients, and talked about the need and the potential during the symposium. I don't know what % of patients that are triple negative experience a relapse, but going off of her enthusiasm level I'd guess a couple months to get 10 patients into this. So 3-4 months for data available to Genentech and Merck, Probably Aug/Sept before we hear. P2B trial in Melanoma will take longer, just because TIL is not created overnight, Dr. Pierce said it will take a few months to create these T-Cells and for the immune system to train them to fight the cancer, so I guess it will be next year before we see data on this, though Merck will likely see it earlier. Remember this will be a 10 patient study with a subgroup that demonstrate a known biomarker that does not respond well to PD1, only 10% have an ORR, so with the small population size I would think 0-2 responses should be expected. How quickly will the trial fill up? What happens if the first 3 patients all have a response? What about 5? At what point in time will Merck say, scratch that we want a full trial? What if only 1 responds but PD1 dosage was cut in half thus decreasing cost of PD1 therapy? All these questions Dr. Pierce and I discussed, as being points of interest. Dr. Pierce then went of to talk with someone else leaving me with Dr. Algazi, we talked at great length about H&N cancer, how it is currently being treated, the horror of having your jaw amputated and the potential of using the immune system to save a persons jaw. He seemed very excited, and made the comment that he was the only one not going to make any money off of the trial. I'm begining to see why he was so excited about it. I left Dr. Algazi and went to the back of the room to get a glass of wine, and started talking with Punit again. He asked if I had gotten all my questions answere, so I pulled out my notebook and flipped through it, landing on questions about the larger warehouse, he said right now they have a corporate office and 2 research facilities, they are closing all 3 and moving to the larger building, this should be a cost saving event not an added cost. He brought up last year they had 12 employees and now have 55, then mentioned having 65 in the future, not sure if this was an example or a current planned expansion. I asked about NeoPulse, and again, not sure if it was an example, or this was the offered deal, but he said if they partnered NeoPulse for marketing they would have to provide the people to train how to use NeoPulse as well as equipment and it would become a break even, more importantly if NeoPulse was marketed for $10m what does that say about the value of their tech? It would be hard to get a $1B deal for ImmunoPulse if NeoPulse went for $10m (his dollar figure, not mine). Finally I asked him about the costs of treating a patient for 1 year with ImmunoPulse, he said $4-5k, about 20 to produce the IL-12 and that cost will drop as they mass produce. Add another $10k, per person for lab tests needed for the studies. So a study is running $15k per person to do, which aint to shabby if you ask me. He did not say it outright but I got a strong feeling that he does not believe another round of dilution will happen, he expects lucrative partnerships to happen as soon as data is in from these 2 studies. In addition, ImmunoPulse will have the ability to reach all (yes they said all, I have doubts about brain cancer myself) areas of the body, so I could see a rapid expansion of indications happening end of year. That pretty well concludes my interactions to the best I recall, I got a great deal of information and feel 1000x more comfortable with this investment. If I missed something ask, its hard to recall everything without a prompt.
http://investorshub.advfn.com/boards/read_msg.aspx?message_i…
JP Morgan Healthcare Conference
Für bestehende Aktionäre ein Bericht mit vielen interessanten Details. Furbush kennt man auch von Seeking-Alpha.Keine KE in absehbarer Zeit geplant.
From Shallum Furbush who actually went to the symposium:
Key points I got from the presentation
IL-12 works great with just 1 dose, strong indications from Dr. Le and Dr. Pierce that P2B expanded (yes first time I heard them call it a P2B for the expansion study) is showing significant improvement over the P2 final we saw.
People that respond, really respond well, about 80% are stable or show ORR, about 50% have shrinkage of 20% or more (30% needed to qualify as ORR) and apparently some of those who did not qualify during the study have sense hit a point where they would have qualified, so a longer trial would have shown higher ORR values.
--------------------------------
Quote:I showed up about an hour early and checked in with Jordan, (we all got name tags, there was about 80-90 tags laid out on the desk I estimate) I was quickly joined by a HF manager and he and I talked for a bit, he has held a position for 2 years roughly and has been considering adding more to his HF. He stated that the company was performing quite well and he was very satisfied with the growth they have accomplished this last year. About this time Punit arrived, and we shook hands and began discussing the company. After I made the comment to him that I was quite happy with everything but the stock price he said "don't worry about the day to day movements" which I don't. He went on to say that he too was dissatisfied with how the stock was performing, which was one of the reasons that he was doing the presentation, he believed that most people do not fully understand what ONCS was/is all about. He stated that one of the big problems is the high percentage of retail holders (see if we all sell the stock will go up), retail tends to have less patience with stock price and the constant selling effects price. He was hoping to gain additional institutional investors, which led me to ask about institutional investors. He mentioned 4 names (I don't care who they are, so don't remember the names), and said they all were still holders (so I assume we have heard who they were in the past). I brought up doing a R./S, and he stated that they were discussing it, a R/S would be oriented around an event with positive movement. Dr. Pierce showed up, and we all introduced ourselves, shortly there after Punit had to take off. Dr. Pierce began talking about immunotherapy and how it is so new and poorly understood by scientists and investors, he talked about Keytruda, how Merck actually acquired it from some other company they bought, then sat on it for several years till BMY released their P1 Opdivo study. Then the race was on. He talked about Yervoy, how it would land you in the ICU, etc. Dr. Le joined us, and the conversation continued, no significant details that I recall, just a lot of confirming information about immunotherapy, how IL-12 works, IL-15 was discussed.
Conference started, (I'll leave this to you to listen to when posted), key things, first immunotherapy was in 1890 with a 50% CR injecting bacteria into patients with cancer. There have been studies injecting melanoma with various agents giving a 80% CR but does nothing for OS, (sell your PVCT now), The key to stimulating immune response is a small release of the tumor cells that triggers the immune response, if you kill the tumor to quick you get no systemic response. Some of the CR's were at day 600 for immunotherapy. All the CR's for IL-12 were durrable and long lasting with excellent outcomes, even for the ones who did not get a CR, along with this was just 1 dose. 1 patient had a relapse after the study was done was retreated in the last 2 weeks. The Relapse seen was minor, (even the one reported in P2 study) where a person with multiple lesions was treated had a CR, the relapse was 1 lesion only. IL-12 allows for use of less PD1 which will be cost effective. CART does not work in solid tumors, however according to Dr. Khort, you can use CART to convert the solid tumor environment to respond to CART (I assume this means 2 CART treatments which doubles the cost and risks. He went on to say that he believes that IL-12 can be used in B-Cell cancers, perhaps in conjunction with CART and probably eliminating CART entirely (several years from now, CART is probably a good investment for the time being, but staying in it longer would be dangerous IMO)
After the conference I spoke at great length with Dr. Pierce and the lead Dr. from our H&N study. Breast Cancer trial is 10 patients with a biopsy pre and post therapy, 28 days long. This is in a population that has already received surgery and chemo, the breast cancer returns with surface lesions. Both Genetech (if I spelled that right) and Merck released data at DAllas Breast cancer symposium showing that TIL was a key to this working. So if 10 patients show an increase in TIL..... Oh, by the way for every 10% increase in TIL there is a corresponding increase in either ORR or OS (can't remember which), in melanoma the TIL was increased by hunderds of % (as in more than 10).
Alright, so still talking with Dr. Pierce and Dr. Algazi ( I believe is who it was), and topic is still the breast cancer trial. 10 patients, 28 days for data, so depending on how quickly this gets filled. Dr. Telli, sounded very excited about using IL-12 on her patients, and talked about the need and the potential during the symposium. I don't know what % of patients that are triple negative experience a relapse, but going off of her enthusiasm level I'd guess a couple months to get 10 patients into this. So 3-4 months for data available to Genentech and Merck, Probably Aug/Sept before we hear. P2B trial in Melanoma will take longer, just because TIL is not created overnight, Dr. Pierce said it will take a few months to create these T-Cells and for the immune system to train them to fight the cancer, so I guess it will be next year before we see data on this, though Merck will likely see it earlier. Remember this will be a 10 patient study with a subgroup that demonstrate a known biomarker that does not respond well to PD1, only 10% have an ORR, so with the small population size I would think 0-2 responses should be expected. How quickly will the trial fill up? What happens if the first 3 patients all have a response? What about 5? At what point in time will Merck say, scratch that we want a full trial? What if only 1 responds but PD1 dosage was cut in half thus decreasing cost of PD1 therapy? All these questions Dr. Pierce and I discussed, as being points of interest. Dr. Pierce then went of to talk with someone else leaving me with Dr. Algazi, we talked at great length about H&N cancer, how it is currently being treated, the horror of having your jaw amputated and the potential of using the immune system to save a persons jaw. He seemed very excited, and made the comment that he was the only one not going to make any money off of the trial. I'm begining to see why he was so excited about it. I left Dr. Algazi and went to the back of the room to get a glass of wine, and started talking with Punit again. He asked if I had gotten all my questions answere, so I pulled out my notebook and flipped through it, landing on questions about the larger warehouse, he said right now they have a corporate office and 2 research facilities, they are closing all 3 and moving to the larger building, this should be a cost saving event not an added cost. He brought up last year they had 12 employees and now have 55, then mentioned having 65 in the future, not sure if this was an example or a current planned expansion. I asked about NeoPulse, and again, not sure if it was an example, or this was the offered deal, but he said if they partnered NeoPulse for marketing they would have to provide the people to train how to use NeoPulse as well as equipment and it would become a break even, more importantly if NeoPulse was marketed for $10m what does that say about the value of their tech? It would be hard to get a $1B deal for ImmunoPulse if NeoPulse went for $10m (his dollar figure, not mine). Finally I asked him about the costs of treating a patient for 1 year with ImmunoPulse, he said $4-5k, about 20 to produce the IL-12 and that cost will drop as they mass produce. Add another $10k, per person for lab tests needed for the studies. So a study is running $15k per person to do, which aint to shabby if you ask me. He did not say it outright but I got a strong feeling that he does not believe another round of dilution will happen, he expects lucrative partnerships to happen as soon as data is in from these 2 studies. In addition, ImmunoPulse will have the ability to reach all (yes they said all, I have doubts about brain cancer myself) areas of the body, so I could see a rapid expansion of indications happening end of year. That pretty well concludes my interactions to the best I recall, I got a great deal of information and feel 1000x more comfortable with this investment. If I missed something ask, its hard to recall everything without a prompt.
http://investorshub.advfn.com/boards/read_msg.aspx?message_i…
Zusammenfassung zum aktuellen Stand der Dinge
ein Artikel auf Seeking-Alpha :http://seekingalpha.com/article/3037956-oncosec-medical-has-…
muss gestehen ich habe die Hälfte meiner Oncosec letzte Woche verkauft und dafür Medigene gekauft. Die Technologien sollten sich gut ergänzen, denke ich.
p2b-Studie gelistet bei clinicaltrials.gov
Die p2b-Studie zur Verstärkung der Wirkung von Pembrolizumab ist jetzt bei Clinicaltrials.gov eingetragen und soll wohl im Juli beginnen. Wurde auch Zeit.Angeblich gibt es schon eine Warteliste, so das das Enrollment nicht allzulange dauern sollte.
https://clinicaltrials.gov/ct2/show/NCT02493361?term=pil-12+…
Der erste Patient für die Kombistudie ist gewonnen. Man kann nur hoffen dass die lange Zeitdauer bis zur Rekrutierung für Vorbereitungen verwendet wurde - siehe die umfangreichen Ausschlusskriterien im Studienprotokoll. Und dass die weiteren Patienten jetzt schneller rekrutiert werden können. Für meine laienhaften Begriffe zieht sich der Beginn sowieso schon unverständlich lange hin.
Nach Studienbeginn sollen die ersten Zwischenergebnisse nach drei Monaten veröffentlicht werden. Wie viele Patienten dafür mindestens benötigt werden ist mir aber bisher nicht bekannt.
http://ir.oncosec.com/press-releases/detail/1835/oncosec-ann…
Nach Studienbeginn sollen die ersten Zwischenergebnisse nach drei Monaten veröffentlicht werden. Wie viele Patienten dafür mindestens benötigt werden ist mir aber bisher nicht bekannt.
http://ir.oncosec.com/press-releases/detail/1835/oncosec-ann…
Kapitalerhöhung
Beim Wettrennen zwischen Verwässerung und Fortschritt bei Technologie und klinischen Studien muss ich bei ONCS bedrückt feststellen, dass die Verwässerung weit vorne liegt. Unerwartete KE an ausgesuchte Investoren mit grossem Abschlag zum aktuellen Kurs UND zusätzlich Warrants, ohne dass die Firma das Geld nach bisheriger Kommunikation überhaupt gebraucht hat.
Die Kapitalerhöhung ist gar nicht mal so gross, aber man hat den Eindruck da bedient sich jemand auf dem Rücken der Normalaktionäre.
Das gibt ein Blutbad heute, und die Frage stellt sich ob angenommener wissenschaftlicher Fortschritt ein hoch bezahltes lausiges Management kompensieren kann.
OncoSec Announces $7.5 Million Registered Direct Offering
http://ir.oncosec.com/press-releases/detail/1846/oncosec-ann…
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| -2,08 | |
| -29,23 | |
| 0,00 | |
| -0,11 | |
| 0,00 | |
| -8,43 | |
| +8,11 | |
| -2,97 | |
| -3,87 | |
| +1,20 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 205 | ||
| 186 | ||
| 166 | ||
| 69 | ||
| 32 | ||
| 29 | ||
| 28 | ||
| 26 | ||
| 25 | ||
| 25 |





















