4NX Newlink hat den Zuschlag bekommen - 500 Beiträge pro Seite
eröffnet am 20.10.14 14:28:19 von
neuester Beitrag 31.07.15 14:55:16 von
neuester Beitrag 31.07.15 14:55:16 von
Beiträge: 112
ID: 1.200.925
ID: 1.200.925
Aufrufe heute: 0
Gesamt: 6.971
Gesamt: 6.971
Aktive User: 0
ISIN: US55028X1090 · WKN: A2P12E · Symbol: LUMO
2,8100
USD
+4,46 %
+0,1200 USD
Letzter Kurs 17:44:11 Nasdaq
Neuigkeiten
18.04.24 · globenewswire |
20.03.24 · globenewswire |
07.03.24 · globenewswire |
26.02.24 · globenewswire |
05.02.24 · globenewswire |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,4800 | +33,82 | |
| 2,4400 | +27,75 | |
| 0,7560 | +21,94 | |
| 0,5900 | +21,90 | |
| 0,7315 | +17,11 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 5,3300 | -16,33 | |
| 2,9250 | -17,37 | |
| 3,2700 | -18,25 | |
| 27,00 | -49,30 | |
| 3,5200 | -52,17 |
keiner hat es hier mitbekommen. Sie ist schon 29 % im Plus

hier steht alles wichtige drin
NewLink Genetics Announces Exclusive Worldwide Licensing Agreement for Development of NLG919, an IDO Inhibitor in Phase 1, and Research Collaboration for the Discovery of Next Generation IDO/TDO Inhibitors
NewLink Genetics Announces Exclusive Worldwide Licensing Agreement for Development of NLG919, an IDO Inhibitor in Phase 1, and Research Collaboration for the Discovery of Next Generation IDO/TDO Inhibitors
Antwort auf Beitrag Nr.: 48.083.779 von mfierke am 20.10.14 14:29:50so ist besser lesbar
http://www.wallstreet-online.de/nachricht/7093813-newlink-ge…
http://www.wallstreet-online.de/nachricht/7093813-newlink-ge…
Der Kursanstieg dürfte aber eher Folge dessen sein, dass Newlink 800 Dosen Ebola-Impfstoff an die WHO geliefert hat.
Einen Impfstoff kann man millionenfach verkaufen, ein Medikament wahrscheinlich nur für ein paar Tausend Patienten. Entsprechend groß dürfte das Potential von Newlink sein.
Nur meine Meinung, recherchiert mal selbst.
LG, ER
Einen Impfstoff kann man millionenfach verkaufen, ein Medikament wahrscheinlich nur für ein paar Tausend Patienten. Entsprechend groß dürfte das Potential von Newlink sein.
Nur meine Meinung, recherchiert mal selbst.
LG, ER
Meine Meinung: Zu spät, um mit diesem Wert Spaß zu haben.
Andrew
Andrew

On the financial front, the only Ebola-related company that made a significant upturn Monday was NewLink Genetics Corp. NLNK, +7.12% , which was up nearly 19% in recent action to $34.88, but some of its gain may not have been Ebola related.
NewLink probably benefited from Canada’s announcement over the weekend that it was shipping 800 Ebola vaccines to WHO in an effort to stanch the spread of the disease in West Africa. NewLink’s BioProtection Systems Corp. has been licensed to further develop the vaccine.
NewLink, however, also announced a contract with the Roche Group’s RHHBY, +1.26% Genentech to develop a cancer-fighting drug. NewLink received an upfront payment of $150 million and could get more than $1 billion over the life of the pact.
http://www.marketwatch.com/story/small-victories-recorded-in…
NewLink probably benefited from Canada’s announcement over the weekend that it was shipping 800 Ebola vaccines to WHO in an effort to stanch the spread of the disease in West Africa. NewLink’s BioProtection Systems Corp. has been licensed to further develop the vaccine.
NewLink, however, also announced a contract with the Roche Group’s RHHBY, +1.26% Genentech to develop a cancer-fighting drug. NewLink received an upfront payment of $150 million and could get more than $1 billion over the life of the pact.
http://www.marketwatch.com/story/small-victories-recorded-in…
About inhibition of the IDO pathway
IDO pathway inhibitors are another class of immune check point inhibitors akin to the recently developed antibodies targeting CTLA-4, PD-1, and PD-L1 that represent potential breakthrough approaches to cancer therapy. The IDO pathway regulates immune response by suppressing T-cell activation which enables local tumor immune escape. Recent studies have demonstrated that the IDO pathway is active in many cancers, both within tumor cells as a direct defense against T-cell attack, and also within antigen presenting cells in tumor draining lymph nodes whereby this pathway promotes peripheral tolerance to tumor associated antigens (TAAs). When hijacked by developing cancers in this manner, the IDO pathway may facilitate the survival, growth, invasion and metastasis of malignant cells whose expression of TAAs might otherwise be recognized and attacked by the immune system. NewLink has a number of active programs directed at synthesizing inhibitors to the IDO pathway and additionally has discovered novel tryptophan-2,3-dioxygenase (TDO) specific inhibitors that are potential anti-cancer compounds which could function individually or in combination with IDO inhibition.
http://www.marketwatch.com/story/newlink-genetics-announces-…
IDO pathway inhibitors are another class of immune check point inhibitors akin to the recently developed antibodies targeting CTLA-4, PD-1, and PD-L1 that represent potential breakthrough approaches to cancer therapy. The IDO pathway regulates immune response by suppressing T-cell activation which enables local tumor immune escape. Recent studies have demonstrated that the IDO pathway is active in many cancers, both within tumor cells as a direct defense against T-cell attack, and also within antigen presenting cells in tumor draining lymph nodes whereby this pathway promotes peripheral tolerance to tumor associated antigens (TAAs). When hijacked by developing cancers in this manner, the IDO pathway may facilitate the survival, growth, invasion and metastasis of malignant cells whose expression of TAAs might otherwise be recognized and attacked by the immune system. NewLink has a number of active programs directed at synthesizing inhibitors to the IDO pathway and additionally has discovered novel tryptophan-2,3-dioxygenase (TDO) specific inhibitors that are potential anti-cancer compounds which could function individually or in combination with IDO inhibition.
http://www.marketwatch.com/story/newlink-genetics-announces-…
NewLink Ebola vaccine trial begins in U.S. while Glaxo takes its jab to Mali
October 16, 2014 | By Emily Mullin
Ebola virus under an electron microscope--Courtesy of CDC
The race is on to test an experimental Ebola vaccine as West Africa grapples with an out-of-control outbreak and the U.S. scrambles to rectify breaches in protocol after a patient with the virus died at a Dallas hospital and a healthcare worker tested positive for the infection.
A Canadian-made vaccine owned by NewLink Genetics ($NLNK) is moving into Phase I trials at the Walter Reed Army Institute of Research in Silver Spring, MD. Meanwhile, GlaxoSmithKline ($GSK) is now testing its investigational Ebola vaccine in Mali, a West African country that has remained untouched by the disease.
Both vaccines are flying through the regulatory process at lightning speed. Typically, it would take 6 to 11 months to move an experimental vaccine from preclinical testing into clinical trials. But the jabs are being fast-tracked by the World Health Organization in an effort to halt the spread of the disease as fast as possible.
This webinar will introduce a robust, highly sensitive biomarker development program that leverages the revolutionary metabolomics discovery platform and expands the menu of compounds that can be measured routinely and reliably.
Developed by the Public Health Agency of Canada, the latest Ebola vaccine to enter clinical trials--dubbed rVSV-ZEBOV--uses a weakened vesicular stomatitis virus as a vector to carry Ebola proteins intended to spur an immune response against exposure to the real virus. Canada said in a statement that is supplying 20 vials of the experimental for use in the trial.
The trial will test NewLink's vaccine on a small group of people to "assess its safety, determine the appropriate dosage and identify any side effects."
Earlier this month, NewLink said additional trials will soon be underway in Germany, Switzerland and in an unnamed African country that has not experienced any Ebola cases. A statement from the Public Health Agency of Canada indicated that data from the first Phase I trial in Maryland should be available in December 2014.
In Mali, GlaxoSmithKline has teamed up with the Center for Vaccine Development of Mali and the Ministry of Health of Mali to administer its cAd3-ZEBOV vaccine to a total of 40 healthcare workers. Glaxo has been working with the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases on its jab, which uses a chimpanzee cold virus vector containing two ebolavirus gene segments that switch on a protein to activate an immune response in the body. A parallel trial in Gambia is expected to begin soon.
- get the statement from the Public Health Agency of Canada
- read more about GlaxoSmithKline's Mali trial
http://www.fiercevaccines.com/story/newlink-ebola-vaccine-tr…
October 16, 2014 | By Emily Mullin
Ebola virus under an electron microscope--Courtesy of CDC
The race is on to test an experimental Ebola vaccine as West Africa grapples with an out-of-control outbreak and the U.S. scrambles to rectify breaches in protocol after a patient with the virus died at a Dallas hospital and a healthcare worker tested positive for the infection.
A Canadian-made vaccine owned by NewLink Genetics ($NLNK) is moving into Phase I trials at the Walter Reed Army Institute of Research in Silver Spring, MD. Meanwhile, GlaxoSmithKline ($GSK) is now testing its investigational Ebola vaccine in Mali, a West African country that has remained untouched by the disease.
Both vaccines are flying through the regulatory process at lightning speed. Typically, it would take 6 to 11 months to move an experimental vaccine from preclinical testing into clinical trials. But the jabs are being fast-tracked by the World Health Organization in an effort to halt the spread of the disease as fast as possible.
This webinar will introduce a robust, highly sensitive biomarker development program that leverages the revolutionary metabolomics discovery platform and expands the menu of compounds that can be measured routinely and reliably.
Developed by the Public Health Agency of Canada, the latest Ebola vaccine to enter clinical trials--dubbed rVSV-ZEBOV--uses a weakened vesicular stomatitis virus as a vector to carry Ebola proteins intended to spur an immune response against exposure to the real virus. Canada said in a statement that is supplying 20 vials of the experimental for use in the trial.
The trial will test NewLink's vaccine on a small group of people to "assess its safety, determine the appropriate dosage and identify any side effects."
Earlier this month, NewLink said additional trials will soon be underway in Germany, Switzerland and in an unnamed African country that has not experienced any Ebola cases. A statement from the Public Health Agency of Canada indicated that data from the first Phase I trial in Maryland should be available in December 2014.
In Mali, GlaxoSmithKline has teamed up with the Center for Vaccine Development of Mali and the Ministry of Health of Mali to administer its cAd3-ZEBOV vaccine to a total of 40 healthcare workers. Glaxo has been working with the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases on its jab, which uses a chimpanzee cold virus vector containing two ebolavirus gene segments that switch on a protein to activate an immune response in the body. A parallel trial in Gambia is expected to begin soon.
- get the statement from the Public Health Agency of Canada
- read more about GlaxoSmithKline's Mali trial
http://www.fiercevaccines.com/story/newlink-ebola-vaccine-tr…
Die beiden Impfstoffe von GSK und NewLink haben also beide Fast-Track-Status. Die Marktkapitalisierung von NLNK liegt mit 819 Mio USD jedoch noch nicht einmal bei 1% der MK von GSK (105 Mrd USD).
Ebola dürfte für GSK keine bedeutsame Umsatzrelevanz haben. Bei NLNK sieht es diesbezüglich anders aus.
LG, ER
Ebola dürfte für GSK keine bedeutsame Umsatzrelevanz haben. Bei NLNK sieht es diesbezüglich anders aus.
LG, ER
The first phase 1 trial of the rVSV vaccine is slated to begin soon in the United States. Ideally, the immunogenicity outcomes in this trial will be compared with those obtained with the GSK–NIAID vaccine. The government of Canada has donated 800 vials of rVSV to the WHO, and discussions about expanding phase 1 trials to European and sub-Saharan African sites are at an advanced stage.
Participants in the Geneva meeting stressed that phase 1 trials should be expedited and their results shared broadly in order to facilitate rapid progression to phase 2. If the results in phase 1 are favorable, the consensus was that phase 2a studies should be conducted in Africa but outside the current Ebola outbreak zone and should proceed in parallel with phase 2b studies conducted in exposed populations. This approach will provide robust efficacy and safety data as quickly as possible. Results from phase 2a trials in unexposed populations would inform the use of these vaccines in expanded populations, including children and people who are HIV-positive. The phase 2b trials in exposed populations would enroll people who are at the highest risk for Ebola virus disease, including frontline workers at Ebola treatment facilities.
http://www.nejm.org/doi/full/10.1056/NEJMp1412166?query=feat…
Participants in the Geneva meeting stressed that phase 1 trials should be expedited and their results shared broadly in order to facilitate rapid progression to phase 2. If the results in phase 1 are favorable, the consensus was that phase 2a studies should be conducted in Africa but outside the current Ebola outbreak zone and should proceed in parallel with phase 2b studies conducted in exposed populations. This approach will provide robust efficacy and safety data as quickly as possible. Results from phase 2a trials in unexposed populations would inform the use of these vaccines in expanded populations, including children and people who are HIV-positive. The phase 2b trials in exposed populations would enroll people who are at the highest risk for Ebola virus disease, including frontline workers at Ebola treatment facilities.
http://www.nejm.org/doi/full/10.1056/NEJMp1412166?query=feat…
Antwort auf Beitrag Nr.: 48.089.551 von extremrelaxer am 21.10.14 08:27:43Ohoooooh:
Trouble in Newlink License with Canada
https://ca.news.yahoo.com/canada-urged-cancel-ebola-vaccine-…
TORONTO - A prominent law professor is urging the federal government to terminate an American company's licence for a Canadian-made Ebola vaccine.
The company, NewLink Genetics, doesn't have the capacity to develop the much-needed vaccine, argues Amir Attaran, a professor of law and population health at the University of Ottawa.
"The mistake Canada has made has been to keep this bad marriage with NewLink and try to make it better. Canada should either be terminating the licence agreement outright or simply issuing another licence on non-commercial terms to someone else," Attaran told The Canadian Press in an interview.
"Either of those would work. Neither of them have been done. And that's absolutely shameful."
Brian Wiley, NewLink's vice president for business development, said in an email that the company would not comment on Attaran's suggestion.
But he did confirm that a deal announced Monday — with Genentech, a division of Swiss drugmaker Roche — does not involve the Ebola vaccine. The deal, which will pay NewLink US$150 million upfront and potentially more than US$1 billion, is for a cancer therapy in early stage testing.
Attaran said he has written to federal Health Minister Rona Ambrose outlining why he believes Canada should strip NewLink of the vaccine licence. Her department's press office did not immediately respond on Monday to queries about Attaran's proposal.
Canada began shipping 800 vials of the vaccine to Geneva on Monday, having donated the vaccine to the World Health Organization. The donated vaccine will be used in clinical trials aimed at determining whether it is safe to use in people and what an effective dose is.
The vaccine, known as VSV-EBOV, was designed by scientists at the National Microbiology Laboratory in Winnipeg. It was licensed to NewLink, of Ames, Iowa, in 2010. NewLink's chief focus is the development of cancer vaccines.
At the time, Canada's options for commercializing the Ebola vaccines or drugs its scientists were developing were limited. Prior to this outbreak, fewer than 3,000 people had been known to have been infected with Ebola, in sporadic outbreaks in poor African countries over a period spanning nearly 40 years.
The prospects for recouping the considerable costs of developing and licensing a drug or vaccine were nil. The major players in the pharmaceutical world — those with deep pockets and regulatory know-how — weren't interested.
It appears from filings the company made with the U.S. Securities and Exchange Commission that NewLink paid merely $205,000 for the rights to the vaccine, Attaran said. The company is also required to pay Canada royalties — the rate will be in "the low single-digit percentage" of the price — on sales of the vaccine.
Many of those involved in charting the Ebola response believe an effective vaccine will be needed to bring this raging epidemic under control. And although another — made by scientists at the U.S. National Institutes of Health — is further along in the testing process, many scientists point to the Canadian vaccine as the better option.
For one thing, the Winnipeg-designed vaccine is expected to require only a single dose. It is thought the American vaccine will require a priming dose followed by a booster with another vaccine that will serve to stimulate the immune system.
A two-dose regimen — with two different vaccines — would be hugely challenging to deliver, especially in countries where the health-care systems have collapsed under the weight of the Ebola outbreak.
As well, in studies in primates the Canadian vaccine was shown to be effective at both protecting against infection and mitigating the severity of the disease when given after an animal was infected. There are no data to suggest the American vaccine would work in both pre- and post-exposure scenarios.
Because of these advantages, many are eager to see VSV-EBOV tested and to see the scale up of production of the vaccine.
But the fact that the licence is held by a small company with no experience bringing a product through the regulatory process and with no vaccine production capacity of its own is seen to be impeding the ability to make, test and — if it is safe and effective — eventually disseminate the vaccine in Ebola-affected countries.
The first human clinical trial of the vaccine began last week in Bethesda, Md. It had been expected that it and other trials would have begun weeks earlier, but there were delays that people knowledgeable about the process attribute to NewLink's size and inexperience.
In its most recent annual report, filed on March 31 of this year, its Ebola vaccine appears to be almost an afterthought, rating only a single mention. NewLink described itself as a "development stage company" and noted that it has incurred significant losses since its inception. At the end of 2013, the company's accumulated deficit was $136 million.
The company has been receiving significant assistance from the Biomedical Advanced Research and Development Authority, a branch of the U.S. Department of Health and Human Services that fosters development of drugs and vaccines for bio-threats like pandemic influenza and anthrax.
The contract between Canada and NewLink gives the Canadian government some options it could employ if it believes NewLink lacks the capacity to develop the vaccine. And Attaran insisted the government should have exercised one of these options before now.
"West Africa is burning, he said. "It is past time that Canada terminate the contract with them (NewLink)."
Attaran noted that in the four years since it acquired the licence to the vaccine, NewLink had not conducted a single human trial with it until now.
In the licence agreement, Canada retains the ability to make or have made the vaccine for use in Canada during an emergency.
It also has the right to let other manufacturers make the vaccine for use in other countries "for compassionate care purposes" if NewLink has not received regulatory approval for the vaccine in the target country. At the current time NewLink has not received regulatory approval for VSV-EBOV anywhere.
— With files from the Associated Press
Follow @HelenBranswell on Twitter
Trouble in Newlink License with Canada
https://ca.news.yahoo.com/canada-urged-cancel-ebola-vaccine-…
TORONTO - A prominent law professor is urging the federal government to terminate an American company's licence for a Canadian-made Ebola vaccine.
The company, NewLink Genetics, doesn't have the capacity to develop the much-needed vaccine, argues Amir Attaran, a professor of law and population health at the University of Ottawa.
"The mistake Canada has made has been to keep this bad marriage with NewLink and try to make it better. Canada should either be terminating the licence agreement outright or simply issuing another licence on non-commercial terms to someone else," Attaran told The Canadian Press in an interview.
"Either of those would work. Neither of them have been done. And that's absolutely shameful."
Brian Wiley, NewLink's vice president for business development, said in an email that the company would not comment on Attaran's suggestion.
But he did confirm that a deal announced Monday — with Genentech, a division of Swiss drugmaker Roche — does not involve the Ebola vaccine. The deal, which will pay NewLink US$150 million upfront and potentially more than US$1 billion, is for a cancer therapy in early stage testing.
Attaran said he has written to federal Health Minister Rona Ambrose outlining why he believes Canada should strip NewLink of the vaccine licence. Her department's press office did not immediately respond on Monday to queries about Attaran's proposal.
Canada began shipping 800 vials of the vaccine to Geneva on Monday, having donated the vaccine to the World Health Organization. The donated vaccine will be used in clinical trials aimed at determining whether it is safe to use in people and what an effective dose is.
The vaccine, known as VSV-EBOV, was designed by scientists at the National Microbiology Laboratory in Winnipeg. It was licensed to NewLink, of Ames, Iowa, in 2010. NewLink's chief focus is the development of cancer vaccines.
At the time, Canada's options for commercializing the Ebola vaccines or drugs its scientists were developing were limited. Prior to this outbreak, fewer than 3,000 people had been known to have been infected with Ebola, in sporadic outbreaks in poor African countries over a period spanning nearly 40 years.
The prospects for recouping the considerable costs of developing and licensing a drug or vaccine were nil. The major players in the pharmaceutical world — those with deep pockets and regulatory know-how — weren't interested.
It appears from filings the company made with the U.S. Securities and Exchange Commission that NewLink paid merely $205,000 for the rights to the vaccine, Attaran said. The company is also required to pay Canada royalties — the rate will be in "the low single-digit percentage" of the price — on sales of the vaccine.
Many of those involved in charting the Ebola response believe an effective vaccine will be needed to bring this raging epidemic under control. And although another — made by scientists at the U.S. National Institutes of Health — is further along in the testing process, many scientists point to the Canadian vaccine as the better option.
For one thing, the Winnipeg-designed vaccine is expected to require only a single dose. It is thought the American vaccine will require a priming dose followed by a booster with another vaccine that will serve to stimulate the immune system.
A two-dose regimen — with two different vaccines — would be hugely challenging to deliver, especially in countries where the health-care systems have collapsed under the weight of the Ebola outbreak.
As well, in studies in primates the Canadian vaccine was shown to be effective at both protecting against infection and mitigating the severity of the disease when given after an animal was infected. There are no data to suggest the American vaccine would work in both pre- and post-exposure scenarios.
Because of these advantages, many are eager to see VSV-EBOV tested and to see the scale up of production of the vaccine.
But the fact that the licence is held by a small company with no experience bringing a product through the regulatory process and with no vaccine production capacity of its own is seen to be impeding the ability to make, test and — if it is safe and effective — eventually disseminate the vaccine in Ebola-affected countries.
The first human clinical trial of the vaccine began last week in Bethesda, Md. It had been expected that it and other trials would have begun weeks earlier, but there were delays that people knowledgeable about the process attribute to NewLink's size and inexperience.
In its most recent annual report, filed on March 31 of this year, its Ebola vaccine appears to be almost an afterthought, rating only a single mention. NewLink described itself as a "development stage company" and noted that it has incurred significant losses since its inception. At the end of 2013, the company's accumulated deficit was $136 million.
The company has been receiving significant assistance from the Biomedical Advanced Research and Development Authority, a branch of the U.S. Department of Health and Human Services that fosters development of drugs and vaccines for bio-threats like pandemic influenza and anthrax.
The contract between Canada and NewLink gives the Canadian government some options it could employ if it believes NewLink lacks the capacity to develop the vaccine. And Attaran insisted the government should have exercised one of these options before now.
"West Africa is burning, he said. "It is past time that Canada terminate the contract with them (NewLink)."
Attaran noted that in the four years since it acquired the licence to the vaccine, NewLink had not conducted a single human trial with it until now.
In the licence agreement, Canada retains the ability to make or have made the vaccine for use in Canada during an emergency.
It also has the right to let other manufacturers make the vaccine for use in other countries "for compassionate care purposes" if NewLink has not received regulatory approval for the vaccine in the target country. At the current time NewLink has not received regulatory approval for VSV-EBOV anywhere.
— With files from the Associated Press
Follow @HelenBranswell on Twitter
Why the Work of Dr. Nancy J. Sullivan Could Be Key to a Potential Ebola Vaccine
http://online.wsj.com/articles/ebola-vaccine-push-ramps-up-1…
http://online.wsj.com/articles/ebola-vaccine-push-ramps-up-1…
Top Scientist: This Version Of Ebola Looks Like ‘A Very Different Bug’
October 20, 2014 7:54 PM
http://www.endtimeupdates.com/top-scientist-this-version-of-…
October 20, 2014 7:54 PM
http://www.endtimeupdates.com/top-scientist-this-version-of-…
Antwort auf Beitrag Nr.: 48.089.581 von Halde20 am 21.10.14 08:31:14Man ärgert sich, dass ein so kleines Unternehmen den Zuschlag bekommen hat. Das ist natürlich nachvollziehbar.
Für uns Investoren ergeben sich aus dem Umstand der geringen Marktkapitalisierung aber natürlich entsprechende Chancen auf überproportionale Kursgewinne im Erfolgsfall.
Die Alternative ist GSK mit einer über 100-fachen Marktkapitalisierung.
Dass angesichts des unerwartet heftigen Ausbruchs die großen Imfstoffhersteller sich gerne in den Allerwertesten beißen würden, ist klar. NewLink schwimmt entsprechend jetzt in einem Haifischbecken voller Missgunst...
LG, ER
Für uns Investoren ergeben sich aus dem Umstand der geringen Marktkapitalisierung aber natürlich entsprechende Chancen auf überproportionale Kursgewinne im Erfolgsfall.
Die Alternative ist GSK mit einer über 100-fachen Marktkapitalisierung.
Dass angesichts des unerwartet heftigen Ausbruchs die großen Imfstoffhersteller sich gerne in den Allerwertesten beißen würden, ist klar. NewLink schwimmt entsprechend jetzt in einem Haifischbecken voller Missgunst...
LG, ER
Antwort auf Beitrag Nr.: 48.089.869 von extremrelaxer am 21.10.14 08:54:52Aber so richtig in die Pötte kommt Newlink bisher irgendwie auch nicht....

Antwort auf Beitrag Nr.: 48.091.867 von Halde20 am 21.10.14 12:03:12
Ja, gestern GAP-Schluss und Kontaktbildung mit den Bollinger-Bändern, aus charttechnischer Sicht wahrscheinlich sinnvoll, um einen Rebound vorzubeugen.
Aber jetzt könnte es ruhig weiter aufwärts gehen.
LG, ER
Zitat von Halde20: Aber so richtig in die Pötte kommt Newlink bisher irgendwie auch nicht....
Ja, gestern GAP-Schluss und Kontaktbildung mit den Bollinger-Bändern, aus charttechnischer Sicht wahrscheinlich sinnvoll, um einen Rebound vorzubeugen.
Aber jetzt könnte es ruhig weiter aufwärts gehen.
LG, ER
NewLink Vaccine
NewLink’s vaccine is one with a weakened live virus that can still replicate. The Ames, Iowa-based company has started or will soon begin first-stage clinical trials in the U.S., Canada, Switzerland, Germany, and two African nations
http://www.bloomberg.com/news/2014-10-21/ebola-vaccine-trial…
NewLink’s vaccine is one with a weakened live virus that can still replicate. The Ames, Iowa-based company has started or will soon begin first-stage clinical trials in the U.S., Canada, Switzerland, Germany, and two African nations
http://www.bloomberg.com/news/2014-10-21/ebola-vaccine-trial…
October 21, 2014 6:23 AM EDT Send to a Friend
Get Alerts NLNK Hot Sheet
Price: $31.20 -0.76%
Overall Analyst Rating:
BUY (Up Up)
Revenue Growth %: -8.6%
Trade NLNK Now!
Join SI Premium – FREE
(Updated - October 21, 2014 6:34 AM EDT)
WHO says NewLink Genetics' (NASDAQ: NLNK) Ebola vaccine will arrive in Geneva today, according to Bloomberg headlines. The vaccine will be stored at an Geneva hospital. Vaccines may be used in Africa starting in January.
The WHO is in discussions with Gavi on Ebola vaccination funding.
The vaccinations may start with health workers.
The organization also sees Ebola vaccinations costing 'hundreds of millions.'
Commentary comes from the WHO's Dr Marie-Paule Kieny, who is speaking at a Geneva briefing.
Last weekend, Canada said it would start shipping 800 vials of an experimental Ebola vaccine, which was expected to arrive at the WHO on Monday.
“Canada views this experimental Ebola vaccine as a global resource and in the interest of global public health, we are sharing it with our international partners to help address the Ebola outbreak in West Africa,” Canadian Health Minister Rona Ambrose said on Saturday.
Canada licensed NewLink Genetics, through its wholly owned subsidiary BioProtection Systems Corp. to further develop the vaccine for use in humans.
NewLink is indicated higher early Tuesday.
http://www.streetinsider.com/FDA/WHO+says+NewLinks+%28NLNK%2…
Get Alerts NLNK Hot Sheet
Price: $31.20 -0.76%
Overall Analyst Rating:
BUY (Up Up)
Revenue Growth %: -8.6%
Trade NLNK Now!
Join SI Premium – FREE
(Updated - October 21, 2014 6:34 AM EDT)
WHO says NewLink Genetics' (NASDAQ: NLNK) Ebola vaccine will arrive in Geneva today, according to Bloomberg headlines. The vaccine will be stored at an Geneva hospital. Vaccines may be used in Africa starting in January.
The WHO is in discussions with Gavi on Ebola vaccination funding.
The vaccinations may start with health workers.
The organization also sees Ebola vaccinations costing 'hundreds of millions.'
Commentary comes from the WHO's Dr Marie-Paule Kieny, who is speaking at a Geneva briefing.
Last weekend, Canada said it would start shipping 800 vials of an experimental Ebola vaccine, which was expected to arrive at the WHO on Monday.
“Canada views this experimental Ebola vaccine as a global resource and in the interest of global public health, we are sharing it with our international partners to help address the Ebola outbreak in West Africa,” Canadian Health Minister Rona Ambrose said on Saturday.
Canada licensed NewLink Genetics, through its wholly owned subsidiary BioProtection Systems Corp. to further develop the vaccine for use in humans.
NewLink is indicated higher early Tuesday.
http://www.streetinsider.com/FDA/WHO+says+NewLinks+%28NLNK%2…
(Reuters) - Human testing of an experimental Ebola vaccine developed by the Canadian health agency and licensed to NewLink Genetics Corp has begun, Canadian health minister Rona Ambrose said.
The early-stage trial will test the vaccine, VSV-EBOV, on a small group of people to determine whether the vaccine is safe and the appropriate dose necessary to provide immunity, she added.
Results of the study are expected in December.
(Reporting by Natalie Grover in Bangalore)
Read more: http://www.businessinsider.com/r-human-testing-begins-on-new…
http://www.businessinsider.com/r-human-testing-begins-on-new…
The early-stage trial will test the vaccine, VSV-EBOV, on a small group of people to determine whether the vaccine is safe and the appropriate dose necessary to provide immunity, she added.
Results of the study are expected in December.
(Reporting by Natalie Grover in Bangalore)
Read more: http://www.businessinsider.com/r-human-testing-begins-on-new…
http://www.businessinsider.com/r-human-testing-begins-on-new…
Tens of thousands expected to get Ebola vaccines from January - WHO
Tue Oct 21, 2014 4:35pm BST
10/21/2014
* Clinical trials now under way to yield data on dosage
* Health workers should get GSK, NewLink vaccines in Jan
* Johnson & Johnson, Russia also have candidate vaccines
By Stephanie Nebehay
GENEVA, Oct 21 (Reuters) - Tens of thousands of people in West Africa are expected to begin getting experimental Ebola vaccines from January, but population-wide immunisation is still far off, the World Health Organization (WHO) said on Tuesday.
Initial clinical trials of vaccines from GlaxoSmithKline and NewLink Genetics are already under way. Some 500 volunteers are due to take part in countries including the United States, Britain, Germany, Switzerland, Mali, Gabon and Kenya.
The tests will generate safety and immune-response data in December. The vaccines can then be rolled out early next year to groups including frontline healthcare workers, said Marie-Paule Kieny, the WHO assistant director-general for health systems and innovation.
"These data are absolutely crucial to allow decision-making on what dose level should go into efficacy testing in Africa," Kieny told a news briefing.
Determining the dosage will dictate the yield or overall amount of vaccine available for the large clinical trials in Africa, she said.
"There is still a possibility that it will fail, but everybody is putting things in order in order for being able to move to West Africa in January," Kieny said. "When I say deployed, I am not talking about mass vaccination, I am talking about utilisation in the tens of thousands of doses in the first couple of months of the year."
West Africa's Ebola outbreak, which began in March, has killed 4,546 out of 9,191 known cases in Guinea, Liberia and Sierra Leone, according to WHO, which has declared outbreaks in Senegal and Nigeria over.
There have been a handful of Ebola cases in Spain and the United States, which on Monday issued stringent new protocols for health workers treating Ebola victims.
FUNDING THE ROLL-OUT
Vaccine makers and regulatory authorities are moving quickly to speed up trials and approval for the vaccines, Kieny said. Donors stand ready to help finance the roll-out, expected to cost hundreds of millions of dollars, she added.
"The funding scenario has not been worked out. But what we are working on for the time being is the assumption that the funding will come from the countries who are helping with the response, so indeed the U.S., UK, France, Norway, Germany and many others as well as from the GAVI," she said.
The Geneva-based GAVI alliance procures vaccines at affordable prices for use in developing countries.
While the GSK and the NewLink vaccines, the latter developed by Canada's Public Health Agency and licensed to the U.S. firm, are considered "lead candidates", others are being developed.
Johnson & Johnson has a candidate vaccine that is expected to start clinical trials in January, Kieny said.
Inovio Pharmaceuticals is developing a DNA vaccine that would enter clinical tests early next year. Protein Sciences is developing a vaccine that should reach trials in the first quarter of 2015, she said. Both are U.S.-based companies.
Russia has been developing Ebola vaccines, but their status is less clear, Kieny said.
"The best that I know is that one of the vaccines is either currently or has already been in clinical trials, in Phase I clinical trials, to assess safety and immunogenicity in Russia," she said. "We are currently discussing with them to know a bit more about what are the results and what are their plans."
Experimental drugs against Ebola include Fujifilm's flu drug Avigan, or favipiravir, the safety and efficacy of which the French government will evaluate in a clinical trial in Guinea, she said. The trials are expected to start in coming weeks, she said.
Asked whether this would be the first drug used in trials in Ebola-affected West African countries, Kieny said: "If it starts on time, as far as I am aware, yes."
British scientists said on Tuesday that Avigan might also have potential against norovirus, or the winter vomiting bug. {ID:nL6N0SG324]
The U.S.-made drug ZMapp from Mapp Biopharmaceutical has been given to a handful of infected health workers evacuated from the region, but this has been on an ad hoc basis, she said. (Additional reporting by Tom Miles and Ben Hirschler; Editing by Larry King)
http://uk.reuters.com/article/2014/10/21/health-ebola-vaccin…
Tue Oct 21, 2014 4:35pm BST
10/21/2014
* Clinical trials now under way to yield data on dosage
* Health workers should get GSK, NewLink vaccines in Jan
* Johnson & Johnson, Russia also have candidate vaccines
By Stephanie Nebehay
GENEVA, Oct 21 (Reuters) - Tens of thousands of people in West Africa are expected to begin getting experimental Ebola vaccines from January, but population-wide immunisation is still far off, the World Health Organization (WHO) said on Tuesday.
Initial clinical trials of vaccines from GlaxoSmithKline and NewLink Genetics are already under way. Some 500 volunteers are due to take part in countries including the United States, Britain, Germany, Switzerland, Mali, Gabon and Kenya.
The tests will generate safety and immune-response data in December. The vaccines can then be rolled out early next year to groups including frontline healthcare workers, said Marie-Paule Kieny, the WHO assistant director-general for health systems and innovation.
"These data are absolutely crucial to allow decision-making on what dose level should go into efficacy testing in Africa," Kieny told a news briefing.
Determining the dosage will dictate the yield or overall amount of vaccine available for the large clinical trials in Africa, she said.
"There is still a possibility that it will fail, but everybody is putting things in order in order for being able to move to West Africa in January," Kieny said. "When I say deployed, I am not talking about mass vaccination, I am talking about utilisation in the tens of thousands of doses in the first couple of months of the year."
West Africa's Ebola outbreak, which began in March, has killed 4,546 out of 9,191 known cases in Guinea, Liberia and Sierra Leone, according to WHO, which has declared outbreaks in Senegal and Nigeria over.
There have been a handful of Ebola cases in Spain and the United States, which on Monday issued stringent new protocols for health workers treating Ebola victims.
FUNDING THE ROLL-OUT
Vaccine makers and regulatory authorities are moving quickly to speed up trials and approval for the vaccines, Kieny said. Donors stand ready to help finance the roll-out, expected to cost hundreds of millions of dollars, she added.
"The funding scenario has not been worked out. But what we are working on for the time being is the assumption that the funding will come from the countries who are helping with the response, so indeed the U.S., UK, France, Norway, Germany and many others as well as from the GAVI," she said.
The Geneva-based GAVI alliance procures vaccines at affordable prices for use in developing countries.
While the GSK and the NewLink vaccines, the latter developed by Canada's Public Health Agency and licensed to the U.S. firm, are considered "lead candidates", others are being developed.
Johnson & Johnson has a candidate vaccine that is expected to start clinical trials in January, Kieny said.
Inovio Pharmaceuticals is developing a DNA vaccine that would enter clinical tests early next year. Protein Sciences is developing a vaccine that should reach trials in the first quarter of 2015, she said. Both are U.S.-based companies.
Russia has been developing Ebola vaccines, but their status is less clear, Kieny said.
"The best that I know is that one of the vaccines is either currently or has already been in clinical trials, in Phase I clinical trials, to assess safety and immunogenicity in Russia," she said. "We are currently discussing with them to know a bit more about what are the results and what are their plans."
Experimental drugs against Ebola include Fujifilm's flu drug Avigan, or favipiravir, the safety and efficacy of which the French government will evaluate in a clinical trial in Guinea, she said. The trials are expected to start in coming weeks, she said.
Asked whether this would be the first drug used in trials in Ebola-affected West African countries, Kieny said: "If it starts on time, as far as I am aware, yes."
British scientists said on Tuesday that Avigan might also have potential against norovirus, or the winter vomiting bug. {ID:nL6N0SG324]
The U.S.-made drug ZMapp from Mapp Biopharmaceutical has been given to a handful of infected health workers evacuated from the region, but this has been on an ad hoc basis, she said. (Additional reporting by Tom Miles and Ben Hirschler; Editing by Larry King)
http://uk.reuters.com/article/2014/10/21/health-ebola-vaccin…
Quelle: SDA
In der Schweiz wird Ebola-Impfstoff getestet
Ebola-Impfstoff wird laut WHO auch in Schweiz getestet
1 / 1
WHO-Generaldirektorin Marie Paule Kieny am Dienstag vor den Medien
(Bildquelle: Keystone)
Im Kampf gegen Ebola wird nach Angaben der Weltgesundheitsorganisation (WHO) in Kürze auch in der Schweiz ein Impfstoff getestet. Dabei handelt es sich um Teile der Charge von 800 Ampullen, die Kanada zur Verfügung gestellt hat.
Das sagte die stellvertretende WHO-Generaldirektorin Marie Paule Kieny am Dienstag an einer Medienkonferenz in Genf. Der Stoff, der laut Kieny beim Transport auf minus 80 Grad Celsius gekühlt werden muss, wird zunächst nach Genf gebracht und von dort weiter an verschiedene europäische Testlabors - unter anderem in Genf und Lausanne - verteilt.
Bis Dezember hoffe man auf erste gesicherte Erkenntnisse über die Sicherheit der Impfstoffe. Mit dem Einsatz in Afrika könne gegebenenfalls im Januar begonnen werden, sagte Kieny. Zunächst werde es jedoch keine flächendeckenden Impfungen geben, weil die Dosen nicht in entsprechendem Umfang zur Verfügung stünden.
Neben dem kanadischen Produkt gebe es einen weiteren vielversprechenden Impfstoff, der gerade getestet werde. Zudem werden laut Kieny etwa in Russland Impfstoffe entwickelt.
Derzeit gibt es nach ihren Worten zudem vier Medikamente, die getestet werden und in wenigen Wochen in Afrika zum Einsatz kommen könnten. Auch die Behandlung von Patienten mit dem Blut genesener Ebola-Kranker habe sich als vielversprechend erwiesen.
Der Impfstoff rVSV-ZEBOV, der von den kanadischen Gesundheitsbehörden entwickelt wurde, wird in den USA und Europa zunächst an gesunden Freiwilligen getestet werden. Die Lizenz zur Vermarktung des Impfstoffs hat die US-Firma NewLink Genetics. Das Mittel soll über ein geschwächtes Virus für eine Viehkrankheit (Vesicular Stomatitis Virus) wirken, dem ein Ebola-Gen eingefügt wurde.
Der zweite Kandidatimpfstoff, der bereits an Gesunden erprobt wird (cAd3-ZEBOV) kommt vom britischen Pharmakonzern GlaxoSmithKline (GSK) und besteht aus einem genetisch veränderten Schimpansen-Adenovirus, das für den Menschen ungefährlich ist. In dieses wurden Gene von Oberflächenproteinen von zwei Ebola-Stämmen eingefügt. Nach der Impfung sollen sie zu einer Immunreaktion gegen das Virus führen.
Die ersten Tests an Menschen begannen im September in den USA und in Grossbritannien, um die Unbedenklichkeit des Stoffs sicherzustellen. Weitere Tests der Phase I sind in Mali und Gambia vorgesehen. Laut WHO dürfte GSK Anfang 2015 etwa 10'000 Dosen des Impfstoffs zur Verfügung haben.
http://www.1815.ch/ausland/politik/ebola-impfstoff-wird-laut…
In der Schweiz wird Ebola-Impfstoff getestet
Ebola-Impfstoff wird laut WHO auch in Schweiz getestet
1 / 1
WHO-Generaldirektorin Marie Paule Kieny am Dienstag vor den Medien
(Bildquelle: Keystone)
Im Kampf gegen Ebola wird nach Angaben der Weltgesundheitsorganisation (WHO) in Kürze auch in der Schweiz ein Impfstoff getestet. Dabei handelt es sich um Teile der Charge von 800 Ampullen, die Kanada zur Verfügung gestellt hat.
Das sagte die stellvertretende WHO-Generaldirektorin Marie Paule Kieny am Dienstag an einer Medienkonferenz in Genf. Der Stoff, der laut Kieny beim Transport auf minus 80 Grad Celsius gekühlt werden muss, wird zunächst nach Genf gebracht und von dort weiter an verschiedene europäische Testlabors - unter anderem in Genf und Lausanne - verteilt.
Bis Dezember hoffe man auf erste gesicherte Erkenntnisse über die Sicherheit der Impfstoffe. Mit dem Einsatz in Afrika könne gegebenenfalls im Januar begonnen werden, sagte Kieny. Zunächst werde es jedoch keine flächendeckenden Impfungen geben, weil die Dosen nicht in entsprechendem Umfang zur Verfügung stünden.
Neben dem kanadischen Produkt gebe es einen weiteren vielversprechenden Impfstoff, der gerade getestet werde. Zudem werden laut Kieny etwa in Russland Impfstoffe entwickelt.
Derzeit gibt es nach ihren Worten zudem vier Medikamente, die getestet werden und in wenigen Wochen in Afrika zum Einsatz kommen könnten. Auch die Behandlung von Patienten mit dem Blut genesener Ebola-Kranker habe sich als vielversprechend erwiesen.
Der Impfstoff rVSV-ZEBOV, der von den kanadischen Gesundheitsbehörden entwickelt wurde, wird in den USA und Europa zunächst an gesunden Freiwilligen getestet werden. Die Lizenz zur Vermarktung des Impfstoffs hat die US-Firma NewLink Genetics. Das Mittel soll über ein geschwächtes Virus für eine Viehkrankheit (Vesicular Stomatitis Virus) wirken, dem ein Ebola-Gen eingefügt wurde.
Der zweite Kandidatimpfstoff, der bereits an Gesunden erprobt wird (cAd3-ZEBOV) kommt vom britischen Pharmakonzern GlaxoSmithKline (GSK) und besteht aus einem genetisch veränderten Schimpansen-Adenovirus, das für den Menschen ungefährlich ist. In dieses wurden Gene von Oberflächenproteinen von zwei Ebola-Stämmen eingefügt. Nach der Impfung sollen sie zu einer Immunreaktion gegen das Virus führen.
Die ersten Tests an Menschen begannen im September in den USA und in Grossbritannien, um die Unbedenklichkeit des Stoffs sicherzustellen. Weitere Tests der Phase I sind in Mali und Gambia vorgesehen. Laut WHO dürfte GSK Anfang 2015 etwa 10'000 Dosen des Impfstoffs zur Verfügung haben.
http://www.1815.ch/ausland/politik/ebola-impfstoff-wird-laut…
The Strongest Biotech Stock In The Market
October 21, 2014 12:05 PM
http://www.benzinga.com/trading-ideas/long-ideas/14/10/49360…
October 21, 2014 12:05 PM
http://www.benzinga.com/trading-ideas/long-ideas/14/10/49360…
Ebola-Impfstoffe: WHO beginnt in zwei Woche mit Tests
von euronews_international 1:11 Min.
Noch sind Schutzkleidung, Quarantänestationen und Desinfektionsmittel die einzigen wirksamen Vorbeugemaßnahmen gegen Ebola. Das soll sich so schnell wie möglich ändern. Die Weltgesundheitsorganisation (WHO) will in Kürze mit eigenen Tests neuer Impfstoffe beginnen. Zwei Kandidaten sind derzeit im Rennen: ein Präparat von GlaxoSmithKline sowie eines von NewLink Genetics und der kanadischen Gesundheitsbehörde. Ein wirksamer Impfstoff verspricht den Herstellern gute Gewinne und Hochrisikogruppen einen dringend benötigten Schutz. Denn alle bisher aufgetretenen Infektionen außerhalb Afrikas betrafen medizinisches Personal. “Die Tests werden in den kommenden zwei Wochen beginnen”, so Marie Paule Kieny, die Stellvertretende Generaldirektorin bei der WHO. “Die Versuche werden sechs bis zwölf Monate dauern. Erste Ergebnisse zu Sicherheit, Immunreaktion und Dosierung erwarten wir aber bis zum Ende des Jahres. Kanada hatte der WHO jüngst 800 Phiolen des experimentellen Stoffes übergeben. Beide Präparate sollen unter WHO-Aufsicht in Deutschland, Gabun und Kenia getestet werden. Eigene Tests der Herstellerlabore laufen bereits, unter anderem in den USA und Großbritannien.
https://de.screen.yahoo.com/aktuelle-nachrichten-videos/ebol…
von euronews_international 1:11 Min.
Noch sind Schutzkleidung, Quarantänestationen und Desinfektionsmittel die einzigen wirksamen Vorbeugemaßnahmen gegen Ebola. Das soll sich so schnell wie möglich ändern. Die Weltgesundheitsorganisation (WHO) will in Kürze mit eigenen Tests neuer Impfstoffe beginnen. Zwei Kandidaten sind derzeit im Rennen: ein Präparat von GlaxoSmithKline sowie eines von NewLink Genetics und der kanadischen Gesundheitsbehörde. Ein wirksamer Impfstoff verspricht den Herstellern gute Gewinne und Hochrisikogruppen einen dringend benötigten Schutz. Denn alle bisher aufgetretenen Infektionen außerhalb Afrikas betrafen medizinisches Personal. “Die Tests werden in den kommenden zwei Wochen beginnen”, so Marie Paule Kieny, die Stellvertretende Generaldirektorin bei der WHO. “Die Versuche werden sechs bis zwölf Monate dauern. Erste Ergebnisse zu Sicherheit, Immunreaktion und Dosierung erwarten wir aber bis zum Ende des Jahres. Kanada hatte der WHO jüngst 800 Phiolen des experimentellen Stoffes übergeben. Beide Präparate sollen unter WHO-Aufsicht in Deutschland, Gabun und Kenia getestet werden. Eigene Tests der Herstellerlabore laufen bereits, unter anderem in den USA und Großbritannien.
https://de.screen.yahoo.com/aktuelle-nachrichten-videos/ebol…
Erneute Kursexplosion, der Run beginnt / geht weiter
Langfrist-Chart:
Noch viel Platz nach oben, Wiederstand oberhalb von 50 USD
Noch viel Platz nach oben, Wiederstand oberhalb von 50 USD
Ist außer mir hier noch jemand investiert?
Kann doch nicht sein, dass alle auf Tekmira oder IBIO setzen?
Kann doch nicht sein, dass alle auf Tekmira oder IBIO setzen?
Experimental Vaccines
Experimental vaccines that have shown promise in early-stage clinical trials are being fast-tracked, but a cure for Ebola remains illusive. Since a virus rather than bacteria causes Ebola, it's relatively straightforward to vaccinate healthy individuals and stimulate the immune system to develop antibodies, but curing someone that has already contracted the disease is an entirely different story.
Several companies have already been working on vaccines. GlaxoSmithKline plc GSK, +0.28% believes that it could have a million doses available next year. The Public Health Agency of Canada and NewLink Genetics Corp. NLNK, +7.16% are testing a second vaccine, while Johnson and Johnson JNJ, +0.87% plan clinical trials for a third vaccine early next year, with the blessing of the FDA.
These vaccines are based on modified viruses that express an Ebola protein strong enough to stimulate an immune response, but weak enough to avoid making patients sick. By establishing a firewall among healthy people, researchers are hoping to at least contain the disease in West African countries using these vaccines, while ZMapp and other cures are still being investigated.
http://www.marketwatch.com/story/vaccines-medical-oxygen-and…
Experimental vaccines that have shown promise in early-stage clinical trials are being fast-tracked, but a cure for Ebola remains illusive. Since a virus rather than bacteria causes Ebola, it's relatively straightforward to vaccinate healthy individuals and stimulate the immune system to develop antibodies, but curing someone that has already contracted the disease is an entirely different story.
Several companies have already been working on vaccines. GlaxoSmithKline plc GSK, +0.28% believes that it could have a million doses available next year. The Public Health Agency of Canada and NewLink Genetics Corp. NLNK, +7.16% are testing a second vaccine, while Johnson and Johnson JNJ, +0.87% plan clinical trials for a third vaccine early next year, with the blessing of the FDA.
These vaccines are based on modified viruses that express an Ebola protein strong enough to stimulate an immune response, but weak enough to avoid making patients sick. By establishing a firewall among healthy people, researchers are hoping to at least contain the disease in West African countries using these vaccines, while ZMapp and other cures are still being investigated.
http://www.marketwatch.com/story/vaccines-medical-oxygen-and…
Motif Investing’s “Fighting Ebola” stock basket as of Oct. 21
http://www.marketwatch.com/story/fighting-ebola-though-inves…
http://www.marketwatch.com/story/fighting-ebola-though-inves…
Ebola vaccine could be ready in 3 months: CEO
Leanne Miller | @LeanneBMiller
7 Hours AgoCNBC.com
Hundreds of thousands of doses a new Ebola vaccine could be ready to send to West Africa within the next three months, NewLink Genetics CEO Charles Link said Tuesday.
"We think by the end of the year we could have hundreds of thousands of doses, if the dose that's chosen is a moderate-size dose," he said. "It could be hundreds of thousands of doses, maybe more."
Read More 100 doses of Ebola drug available: Sarepta CEO
A CDC safety training course on Ebola in Anniston, Ala.
Tami Chappell | Reuters
A CDC safety training course on Ebola in Anniston, Ala.
On CNBC's "Fast Money," Link said that Phase 1 data of NewLink's vaccine is expected in December 2014.
The drug is currently being tested on 500 human volunteers in the United States, Britain, Germany, Switzerland, Mali, Gabon and Kenya. That Phase 1 data will determine the efficacy of the drug based on the human's antibody response to the vaccine.
Read More Ebola: A closer look at this 'frightening disease'
"That data will be used to make a choice to then launch the larger scale trial (in West Africa) in a phased version in January of next year but it's a little hard to nail that date down today," said Link.
GlaxoSmithKline, Johnson & Johnson, Inovio Pharmaceuticals and Sarepta Therapeutics are also among the companies developing Ebola vaccines.
http://www.cnbc.com/id/102108377
Leanne Miller | @LeanneBMiller
7 Hours AgoCNBC.com
Hundreds of thousands of doses a new Ebola vaccine could be ready to send to West Africa within the next three months, NewLink Genetics CEO Charles Link said Tuesday.
"We think by the end of the year we could have hundreds of thousands of doses, if the dose that's chosen is a moderate-size dose," he said. "It could be hundreds of thousands of doses, maybe more."
Read More 100 doses of Ebola drug available: Sarepta CEO
A CDC safety training course on Ebola in Anniston, Ala.
Tami Chappell | Reuters
A CDC safety training course on Ebola in Anniston, Ala.
On CNBC's "Fast Money," Link said that Phase 1 data of NewLink's vaccine is expected in December 2014.
The drug is currently being tested on 500 human volunteers in the United States, Britain, Germany, Switzerland, Mali, Gabon and Kenya. That Phase 1 data will determine the efficacy of the drug based on the human's antibody response to the vaccine.
Read More Ebola: A closer look at this 'frightening disease'
"That data will be used to make a choice to then launch the larger scale trial (in West Africa) in a phased version in January of next year but it's a little hard to nail that date down today," said Link.
GlaxoSmithKline, Johnson & Johnson, Inovio Pharmaceuticals and Sarepta Therapeutics are also among the companies developing Ebola vaccines.
http://www.cnbc.com/id/102108377
Der Weg nach oben ist frei:
Nächstes Kursziel 53,48 USD (ATH)
NewLink Genetics (NASDAQ: NLNK ) may have fewer short sellers in its shares next week, given news out this week that giant drugmaker Roche (NASDAQOTH: RHHBY ) is teaming up with the company.
Roche's Genentech is handing over $150 million upfront to partner up with NewLink on its immunology drugs for the treatment of cancer. If that partnership works out, NewLink could earn more than $1 billion in milestones.
In September, NewLink also launched a phase 1 trial that's studying its Ebola vaccine in healthy volunteers. The vaccine, which the Public Health Agency of Canada initially developed, has shown promise in primate studies, and the phase 1 trial will be conducted with help from the Department of Defense.
Despite the deal with Roche and the Ebola vaccine trial, NewLink is still a risky bet since it doesn't have any products on the market. But Roche's cash infusion should give NewLink plenty of dry powder to advance these drugs through clinic, as well as others including drug candidates for pancreatic cancer and melanoma.
Regardless, with sellers short 35% of the company's shares available for trading, representing nearly 18 days of average trading volume, there may be an opportunity for upside.
http://www.fool.com/investing/general/2014/10/22/3-biotech-s…
Roche's Genentech is handing over $150 million upfront to partner up with NewLink on its immunology drugs for the treatment of cancer. If that partnership works out, NewLink could earn more than $1 billion in milestones.
In September, NewLink also launched a phase 1 trial that's studying its Ebola vaccine in healthy volunteers. The vaccine, which the Public Health Agency of Canada initially developed, has shown promise in primate studies, and the phase 1 trial will be conducted with help from the Department of Defense.
Despite the deal with Roche and the Ebola vaccine trial, NewLink is still a risky bet since it doesn't have any products on the market. But Roche's cash infusion should give NewLink plenty of dry powder to advance these drugs through clinic, as well as others including drug candidates for pancreatic cancer and melanoma.
Regardless, with sellers short 35% of the company's shares available for trading, representing nearly 18 days of average trading volume, there may be an opportunity for upside.
http://www.fool.com/investing/general/2014/10/22/3-biotech-s…
Na die ist doch heute wieder gut gelaufen
sie ist in aller Munde, dann wollen wir mal sehen
Ich schätz mal, sie läuft noch bis zum ATH von 53 USD hoch und dürfte diese Marke m.E. dann erst im Dezember oder Januar im Rahmen der ersten Ergebnisse der Impfungen überwinden.
Andere Einschätzungen zum Kursverlauf?
LG, ER
Andere Einschätzungen zum Kursverlauf?
LG, ER
Ebola: Massenimpfung ab Januar?
22.10.2014
Weltgesundheitsorganisation (WHO): Anfang Januar 2015 können die ersten Impfstoff-Chargen gegen Ebola in Westafrika verteilt werden. Bis dahin muss der Stoff noch getestet werden (Schnelltest). Auch in Deutschland werden derzeit Mutige gesucht, die sich das Zeug in den Körper spritzen lassen.
Schon im Januar sollen große Impfaktionen in Westafrika gestartet werden. Bis dahin müssen die Vakzine jedoch noch getestet werden. Welche Nebenwirkungen sie haben oder ob sie überhaupt helfen, ist noch nicht sicher.
Wie die stellvertretende WHO-Generalsekretärin Marie-Paule Kieny in einer Pressekonferenz am Mittewoch erklärte, gebe es derzeit zwei Kandidaten für eine Impfung, die als erfolgsversprechend gelten:
Eine Vakzine, entwickelt von der Firma GlaxoSmithKline (GSK), namens cAd3-EBO
Eine Vakzine, entwickelt vom kanadischen Gesundheitsministerium und lizenziert durch die US-Firma NewLink Genetics Corp, namens VSV-EBOV
http://www.mmnews.de/index.php/i-news/24626-ebola-massenimpf…
Die 800 Impfungen in Genf, welche in dem Artikel erwähnt werden dürften meiner Recherche zu Folge allerdings von Newlink sein!
...
22.10.2014
Weltgesundheitsorganisation (WHO): Anfang Januar 2015 können die ersten Impfstoff-Chargen gegen Ebola in Westafrika verteilt werden. Bis dahin muss der Stoff noch getestet werden (Schnelltest). Auch in Deutschland werden derzeit Mutige gesucht, die sich das Zeug in den Körper spritzen lassen.
Schon im Januar sollen große Impfaktionen in Westafrika gestartet werden. Bis dahin müssen die Vakzine jedoch noch getestet werden. Welche Nebenwirkungen sie haben oder ob sie überhaupt helfen, ist noch nicht sicher.
Wie die stellvertretende WHO-Generalsekretärin Marie-Paule Kieny in einer Pressekonferenz am Mittewoch erklärte, gebe es derzeit zwei Kandidaten für eine Impfung, die als erfolgsversprechend gelten:
Eine Vakzine, entwickelt von der Firma GlaxoSmithKline (GSK), namens cAd3-EBO
Eine Vakzine, entwickelt vom kanadischen Gesundheitsministerium und lizenziert durch die US-Firma NewLink Genetics Corp, namens VSV-EBOV
http://www.mmnews.de/index.php/i-news/24626-ebola-massenimpf…
Die 800 Impfungen in Genf, welche in dem Artikel erwähnt werden dürften meiner Recherche zu Folge allerdings von Newlink sein!

...
Mali confirms first Ebola case
Oct 24 2014, 04:48 ET
http://seekingalpha.com/news/2057315-mali-confirms-first-ebo…
Oct 24 2014, 04:48 ET
http://seekingalpha.com/news/2057315-mali-confirms-first-ebo…
NewLink Genetics' vaccine, called VSV-EBOV, recently received clearance from the FDA to enter early-stage studies, with officials in Canada later announcing that trials of the vaccine started in the US.
http://www.firstwordpharma.com/node/1242288#axzz3HH3j3jSb
http://www.firstwordpharma.com/node/1242288#axzz3HH3j3jSb
Reuters More: Reuters Health
Human Testing Begins On NewLink Genetics' Ebola Vaccine
http://www.businessinsider.com/r-human-testing-begins-on-new…
Human Testing Begins On NewLink Genetics' Ebola Vaccine
http://www.businessinsider.com/r-human-testing-begins-on-new…
Schneller Schutz gesucht Ebola-Impfstudien im Eilverfahren
17:20 Uhrvon Jana Schlütter
Der kanadische Impfstoff wird auch in Deutschland getestet.
Der kanadische Impfstoff wird auch in Deutschland getestet. - Foto: Reuters
Noch nie wurde eine Impfung innerhalb von sechs Monaten zugelassen. Doch angesichts der Ebola-Epidemie in Westafrika ist genau das das Ziel der WHO.
Wenn es um den Ebola-Ausbruch in Westafrika geht, ist nichts normal. Weder die Zahl der Erkrankten, die offiziell auf 10 141 gestiegen ist, noch die internationale Ausbreitung oder die Tatsache, dass es eine UN-Mission zur Ebola-Bekämpfung gibt. Die Entwicklung der Impfstoffe ist keine Ausnahme. Bereits im Juni sollen sie millionenfach einsatzbereit sein – obwohl die ersten Sicherheitstests am Menschen gerade begonnen haben.
WERBUNG
„Das gab es noch nie in der Geschichte der Medizin“, sagte die stellvertretende WHO-Generalsekretärin Marie-Paule Kieny nach einem Treffen von etwa 90 Delegierten von Regulierungsbehörden, Pharmaindustrie, Hilfsorganisationen, Unis, Stiftungen und Regierungen in Genf. Die klinische Studien sollen nicht mindestens zwei bis vier Jahre, sondern sechs Monate dauern. „Eine Impfung ist kein Wundermittel. Aber wenn sie sicher ist und schützt, könnte sie helfen, diese Flut zurückzudrängen“, sagt Kieny.
Die Impfstoff-Kandidaten
Es wird kein komplettes Ebola-Virus verwendet, weder abgetötet noch abgeschwächt. Eine Infektion über die Impfung ist deshalb ausgeschlossen. Zwei Impfstoffe werden derzeit am Menschen getestet. Dabei geht es um die Verträglichkeit und die Suche nach einer Dosis, die eine ausreichende Immunantwort hervorruft (Phase eins). Der GSK-Impfstoff, entwickelt von GlaxoSmithKline und den Nationalen Gesundheitsinstituten der USA, nutzt ein nicht vermehrungsfähiges Schimpansen-Erkältungsvirus. Das veränderte Adenovirus wird in einen Muskel gespritzt, es schleust genetisches Material für ein Ebola-Oberflächeneiweiß in menschliche Zellen ein. Sie produzieren das Eiweiß und präsentieren es auf ihrer Oberfläche dem Immunsystem. So können sich Abwehrstoffe (Antikörper) bilden. In den USA, in Großbritannien und in Mali wurden einige gesunde Freiwillige damit geimpft. Die Tests in Lausanne, Schweiz, beginnen in den nächsten Tagen. Vorläufige Daten sollen Ende November vorliegen.
Mit der Konzeption eines weiteren Ebola-Impfstoffes begann der deutsche Virologe Heinz Feldmann vor mehr als fünfzehn Jahren an der Universität Marburg. Er testete ihn später in einem Labor der kanadischen Regierung an Makaken. Das Ergebnis veröffentlichte sein Team im Jahr 2005: Die Tiere waren zu 100 Prozent gegen Ebola geschützt. 2013 lagerte Kanada 1500 Impfstoff-Ampullen ein. 800 davon spendete Kanada der WHO, die Pakete kamen am letzten Dienstag in der Schweiz an. Die kanadische Impfung VSV-ZEBOV, entwickelt von der Public Health Agency Canada und lizenziert an die Firma New Link Genetics in Iowa, ist ein Lebendimpfstoff. Ein Gen im Erbgut von abgeschwächten Vesikulären Stomatitis-Viren wurde durch ein Gen für ein Ebola-Eiweiß ersetzt, damit das Immunsystem Antikörper gegen Ebola bilden kann. In den USA bekommen seit letzter Woche nach und nach 39 Freiwillige den Impfstoff oder ein Placebo gespritzt. Tests in Hamburg und in Genf sollen in den nächsten Tagen starten. Gabun und Kenia werden folgen.
Fünf weitere Impfstoffe - auch aus Russland
Zuvor hatte es Verzögerungen gegeben. Ende September beklagte sich der Marburger Virologe Stephan Becker, dass New Link Genetics Papiere zurückhalte. „Die Firma ist jetzt sehr kooperativ“, sagt Marylyn Addo vom Universitätsklinikum Hamburg-Eppendorf, die den deutschen Teil der Studie leitet. 30 Freiwillige zwischen 18 und 55 Jahren sollen in Hamburg die Spritze bekommen. Ärzte und Krankenschwestern, die in Westafrika helfen wollen, können sich ebenfalls melden. „Wir weisen sie aber nachdrücklich darauf hin, dass keiner weiß, ob die Impfung schützt“, sagt Addo. Die Gesundheit der Probanden wird sechs Monate überwacht. In Marburg suchen Wissenschaftler nach Antikörpern in ihrem Blut.
Besserer Schutz. Marylyn Addo leitet die Ebola-Impfstoffstudie am Universitätsklinikum Hamburg-Eppendorf.Bild vergrößernBesserer Schutz. Marylyn Addo leitet die Ebola-Impfstoffstudie am Universitätsklinikum Hamburg-Eppendorf. - Foto: dpa
Der Impfstoff hat einen großen Vorteil: Eventuell hilft er dem Körper noch bis zu zwei Tage nach der Ansteckung. Deshalb bekam ihn 2009 eine Forscherin des Bernhard-Nocht-Instituts in Hamburg nach einem Laborunfall. Nachteile: Bei Patienten mit schwachem Immunsystem könnte der Lebendimpfstoff Komplikationen verursachen. Außerdem ist unklar, wie hoch die Dosis für einen Schutz vor oder nach der Ansteckung sein muss.
Darüber hinaus gibt es mindestens fünf weitere Impfstoffe, die im Tierversuch vielversprechend waren. Darunter sind drei aus Russland. Die Firma Johnson und Johnson könnte im Januar mit Sicherheitstests beginnen. „Wir wissen nicht, welcher Kandidat erfolgreich sein wird und welcher versagt“, sagt Kieny.
Die Tests in Westafrika
Die WHO wartet nicht auf endgültige Daten aus der Phase eins. Stattdessen beginnen die WHO-Teams ab Dezember mit Impfungen in den betroffenen Staaten Westafrikas (Phase zwei und drei).
Den Anfang macht Liberia. Dort werden etwa 18 000 Menschen, vor allem Helfer an der Ebola-Front, zufällig drei Gruppen zugeteilt: Sie bekommen innerhalb von drei bis vier Monaten entweder den GSK-Impfstoff, den kanadischen Impfstoff oder eine Impfung gegen eine andere Infektionskrankheit (als Placebo). Während dieser Epidemie ein Placebo einzusetzen, war umstritten – kein Helfer wäre gern in dieser Gruppe. Doch es fehlen Basisdaten, gegen die man die Impfung vergleichen kann. Für einen zuverlässigen und schnellen Nachweis der Wirksamkeit brauche man ein Placebo, argumentierte GSK.
Die WHO-Experten stimmten dem zwar zu. Gleichzeitig wählten sie für Sierra Leone ein schrittweises Vorgehen (Step-Wedge-Studie) mit etwa 8000 Krankenpflegern, Totengräbern, Ambulanzfahrern und Reinigungskräften. Weil nicht alle gleichzeitig geimpft werden, kann man die zu unterschiedlichen Zeiten immunisierten Gruppen miteinander vergleichen. Niemand bekommt ein Placebo. Dafür gibt es unzählige Faktoren, die das Ergebnis verfälschen können, schließlich verändert sich die Epidemie ständig. Ob die Impfung schützt, ist ohnehin schwer nachweisbar, weil alle Helfer weiter in Schutzkleidung arbeiten müssen. „Allerdings wissen wir, dass es Infektionen gibt“, sagt Klaus Cichutek, der Präsident des Paul-Ehrlich-Instituts. „Trotzdem muss man viele Menschen in die Studien einschließen, um daraus Schlüsse zu ziehen.“ Die WHO rechnet im April mit den ersten Daten zur Effektivität.
Zuversichtlich. Marie-Paule Kieny, die stellvertretende WHO-Generalsekretärin, erläutert das Vorgehen bei den Impfstoffstudien.Bild vergrößernZuversichtlich. Marie-Paule Kieny, die stellvertretende WHO-Generalsekretärin, erläutert das Vorgehen bei den Impfstoffstudien. - Foto: dpa
Wann und wie in Guinea geimpft wird, steht nicht fest. „Wir arbeiten daran“, sagt Kieny. Um Vertrauen zu schaffen, beginnen die WHO und ihre Partner derzeit in allen drei Ländern mit Aufklärungskampagnen über die Impfungen und bauen die nötige Infrastruktur für die Studien auf. Dazu gehört vor allem eine lückenlose Kühlkette. Denn beide Impfstoffe müssen bei minus 80 Grad Celsius gelagert werden – eine Herausforderung in Westafrika, wo Elektrizität nicht selbstverständlich ist.
Ab Juni 2015 genug Impfstoff für Massenimpfungen
Ab April kann GSK 230 000 Impfungen pro Monat produzieren, ab Ende 2015 etwa eine Million – sofern die Firma die Fläschchen nicht allein abfüllen muss, denn das würde die Verfügbarkeit anderer Impfstoffe beeinträchtigen. In welcher Menge die kanadische Impfung bereit stehen kann, richtet sich nach der Dosis und schwankt im Frühjahr 2015 zwischen 50 000 und fünf Millionen. Ab Juni soll es genügend Impfstoff geben, um über Massenimpfungen nachzudenken, sagt Kieny: „Falls die Epidemie das rechtfertigt und falls die Impfung sicher und effektiv ist.“ Geld werde kein Problem sein. Viele Partner der WHO hätten ihre Unterstützung zugesagt, auch um für die nächste Epidemie gewappnet zu sein. „Die Impfungen geben den Helfern die nötige Hoffnung und Vertrauen, um sich weiter um Patienten zu kümmern und gegen das Virus zu kämpfen“, sagt Jeremy Farrar, der Direktor des britischen Wellcome Trust.
http://www.tagesspiegel.de/wissen/schneller-schutz-gesucht-e…
17:20 Uhrvon Jana Schlütter
Der kanadische Impfstoff wird auch in Deutschland getestet.
Der kanadische Impfstoff wird auch in Deutschland getestet. - Foto: Reuters
Noch nie wurde eine Impfung innerhalb von sechs Monaten zugelassen. Doch angesichts der Ebola-Epidemie in Westafrika ist genau das das Ziel der WHO.
Wenn es um den Ebola-Ausbruch in Westafrika geht, ist nichts normal. Weder die Zahl der Erkrankten, die offiziell auf 10 141 gestiegen ist, noch die internationale Ausbreitung oder die Tatsache, dass es eine UN-Mission zur Ebola-Bekämpfung gibt. Die Entwicklung der Impfstoffe ist keine Ausnahme. Bereits im Juni sollen sie millionenfach einsatzbereit sein – obwohl die ersten Sicherheitstests am Menschen gerade begonnen haben.
WERBUNG
„Das gab es noch nie in der Geschichte der Medizin“, sagte die stellvertretende WHO-Generalsekretärin Marie-Paule Kieny nach einem Treffen von etwa 90 Delegierten von Regulierungsbehörden, Pharmaindustrie, Hilfsorganisationen, Unis, Stiftungen und Regierungen in Genf. Die klinische Studien sollen nicht mindestens zwei bis vier Jahre, sondern sechs Monate dauern. „Eine Impfung ist kein Wundermittel. Aber wenn sie sicher ist und schützt, könnte sie helfen, diese Flut zurückzudrängen“, sagt Kieny.
Die Impfstoff-Kandidaten
Es wird kein komplettes Ebola-Virus verwendet, weder abgetötet noch abgeschwächt. Eine Infektion über die Impfung ist deshalb ausgeschlossen. Zwei Impfstoffe werden derzeit am Menschen getestet. Dabei geht es um die Verträglichkeit und die Suche nach einer Dosis, die eine ausreichende Immunantwort hervorruft (Phase eins). Der GSK-Impfstoff, entwickelt von GlaxoSmithKline und den Nationalen Gesundheitsinstituten der USA, nutzt ein nicht vermehrungsfähiges Schimpansen-Erkältungsvirus. Das veränderte Adenovirus wird in einen Muskel gespritzt, es schleust genetisches Material für ein Ebola-Oberflächeneiweiß in menschliche Zellen ein. Sie produzieren das Eiweiß und präsentieren es auf ihrer Oberfläche dem Immunsystem. So können sich Abwehrstoffe (Antikörper) bilden. In den USA, in Großbritannien und in Mali wurden einige gesunde Freiwillige damit geimpft. Die Tests in Lausanne, Schweiz, beginnen in den nächsten Tagen. Vorläufige Daten sollen Ende November vorliegen.
Mit der Konzeption eines weiteren Ebola-Impfstoffes begann der deutsche Virologe Heinz Feldmann vor mehr als fünfzehn Jahren an der Universität Marburg. Er testete ihn später in einem Labor der kanadischen Regierung an Makaken. Das Ergebnis veröffentlichte sein Team im Jahr 2005: Die Tiere waren zu 100 Prozent gegen Ebola geschützt. 2013 lagerte Kanada 1500 Impfstoff-Ampullen ein. 800 davon spendete Kanada der WHO, die Pakete kamen am letzten Dienstag in der Schweiz an. Die kanadische Impfung VSV-ZEBOV, entwickelt von der Public Health Agency Canada und lizenziert an die Firma New Link Genetics in Iowa, ist ein Lebendimpfstoff. Ein Gen im Erbgut von abgeschwächten Vesikulären Stomatitis-Viren wurde durch ein Gen für ein Ebola-Eiweiß ersetzt, damit das Immunsystem Antikörper gegen Ebola bilden kann. In den USA bekommen seit letzter Woche nach und nach 39 Freiwillige den Impfstoff oder ein Placebo gespritzt. Tests in Hamburg und in Genf sollen in den nächsten Tagen starten. Gabun und Kenia werden folgen.
Fünf weitere Impfstoffe - auch aus Russland
Zuvor hatte es Verzögerungen gegeben. Ende September beklagte sich der Marburger Virologe Stephan Becker, dass New Link Genetics Papiere zurückhalte. „Die Firma ist jetzt sehr kooperativ“, sagt Marylyn Addo vom Universitätsklinikum Hamburg-Eppendorf, die den deutschen Teil der Studie leitet. 30 Freiwillige zwischen 18 und 55 Jahren sollen in Hamburg die Spritze bekommen. Ärzte und Krankenschwestern, die in Westafrika helfen wollen, können sich ebenfalls melden. „Wir weisen sie aber nachdrücklich darauf hin, dass keiner weiß, ob die Impfung schützt“, sagt Addo. Die Gesundheit der Probanden wird sechs Monate überwacht. In Marburg suchen Wissenschaftler nach Antikörpern in ihrem Blut.
Besserer Schutz. Marylyn Addo leitet die Ebola-Impfstoffstudie am Universitätsklinikum Hamburg-Eppendorf.Bild vergrößernBesserer Schutz. Marylyn Addo leitet die Ebola-Impfstoffstudie am Universitätsklinikum Hamburg-Eppendorf. - Foto: dpa
Der Impfstoff hat einen großen Vorteil: Eventuell hilft er dem Körper noch bis zu zwei Tage nach der Ansteckung. Deshalb bekam ihn 2009 eine Forscherin des Bernhard-Nocht-Instituts in Hamburg nach einem Laborunfall. Nachteile: Bei Patienten mit schwachem Immunsystem könnte der Lebendimpfstoff Komplikationen verursachen. Außerdem ist unklar, wie hoch die Dosis für einen Schutz vor oder nach der Ansteckung sein muss.
Darüber hinaus gibt es mindestens fünf weitere Impfstoffe, die im Tierversuch vielversprechend waren. Darunter sind drei aus Russland. Die Firma Johnson und Johnson könnte im Januar mit Sicherheitstests beginnen. „Wir wissen nicht, welcher Kandidat erfolgreich sein wird und welcher versagt“, sagt Kieny.
Die Tests in Westafrika
Die WHO wartet nicht auf endgültige Daten aus der Phase eins. Stattdessen beginnen die WHO-Teams ab Dezember mit Impfungen in den betroffenen Staaten Westafrikas (Phase zwei und drei).
Den Anfang macht Liberia. Dort werden etwa 18 000 Menschen, vor allem Helfer an der Ebola-Front, zufällig drei Gruppen zugeteilt: Sie bekommen innerhalb von drei bis vier Monaten entweder den GSK-Impfstoff, den kanadischen Impfstoff oder eine Impfung gegen eine andere Infektionskrankheit (als Placebo). Während dieser Epidemie ein Placebo einzusetzen, war umstritten – kein Helfer wäre gern in dieser Gruppe. Doch es fehlen Basisdaten, gegen die man die Impfung vergleichen kann. Für einen zuverlässigen und schnellen Nachweis der Wirksamkeit brauche man ein Placebo, argumentierte GSK.
Die WHO-Experten stimmten dem zwar zu. Gleichzeitig wählten sie für Sierra Leone ein schrittweises Vorgehen (Step-Wedge-Studie) mit etwa 8000 Krankenpflegern, Totengräbern, Ambulanzfahrern und Reinigungskräften. Weil nicht alle gleichzeitig geimpft werden, kann man die zu unterschiedlichen Zeiten immunisierten Gruppen miteinander vergleichen. Niemand bekommt ein Placebo. Dafür gibt es unzählige Faktoren, die das Ergebnis verfälschen können, schließlich verändert sich die Epidemie ständig. Ob die Impfung schützt, ist ohnehin schwer nachweisbar, weil alle Helfer weiter in Schutzkleidung arbeiten müssen. „Allerdings wissen wir, dass es Infektionen gibt“, sagt Klaus Cichutek, der Präsident des Paul-Ehrlich-Instituts. „Trotzdem muss man viele Menschen in die Studien einschließen, um daraus Schlüsse zu ziehen.“ Die WHO rechnet im April mit den ersten Daten zur Effektivität.
Zuversichtlich. Marie-Paule Kieny, die stellvertretende WHO-Generalsekretärin, erläutert das Vorgehen bei den Impfstoffstudien.Bild vergrößernZuversichtlich. Marie-Paule Kieny, die stellvertretende WHO-Generalsekretärin, erläutert das Vorgehen bei den Impfstoffstudien. - Foto: dpa
Wann und wie in Guinea geimpft wird, steht nicht fest. „Wir arbeiten daran“, sagt Kieny. Um Vertrauen zu schaffen, beginnen die WHO und ihre Partner derzeit in allen drei Ländern mit Aufklärungskampagnen über die Impfungen und bauen die nötige Infrastruktur für die Studien auf. Dazu gehört vor allem eine lückenlose Kühlkette. Denn beide Impfstoffe müssen bei minus 80 Grad Celsius gelagert werden – eine Herausforderung in Westafrika, wo Elektrizität nicht selbstverständlich ist.
Ab Juni 2015 genug Impfstoff für Massenimpfungen
Ab April kann GSK 230 000 Impfungen pro Monat produzieren, ab Ende 2015 etwa eine Million – sofern die Firma die Fläschchen nicht allein abfüllen muss, denn das würde die Verfügbarkeit anderer Impfstoffe beeinträchtigen. In welcher Menge die kanadische Impfung bereit stehen kann, richtet sich nach der Dosis und schwankt im Frühjahr 2015 zwischen 50 000 und fünf Millionen. Ab Juni soll es genügend Impfstoff geben, um über Massenimpfungen nachzudenken, sagt Kieny: „Falls die Epidemie das rechtfertigt und falls die Impfung sicher und effektiv ist.“ Geld werde kein Problem sein. Viele Partner der WHO hätten ihre Unterstützung zugesagt, auch um für die nächste Epidemie gewappnet zu sein. „Die Impfungen geben den Helfern die nötige Hoffnung und Vertrauen, um sich weiter um Patienten zu kümmern und gegen das Virus zu kämpfen“, sagt Jeremy Farrar, der Direktor des britischen Wellcome Trust.
http://www.tagesspiegel.de/wissen/schneller-schutz-gesucht-e…
Danke für die news, ich habe vermutet das aus NEWLINK GENETICS was richtiges gutes kommt. Die hat seti einer woche tgl.ein neues hoch erzielt. Bin gespannt wie sie morgen eröffnet. Noch wird dieser Thraed ganz wenig angenommen, könnte auch gut sein.
"In welcher Menge die kanadische Impfung bereit stehen kann, richtet sich nach der Dosis und schwankt im Frühjahr 2015 zwischen 50 000 und fünf Millionen."
http://www.tagesspiegel.de/wissen/schneller-schutz-gesucht-e…
http://www.tagesspiegel.de/wissen/schneller-schutz-gesucht-e…
Die WHO wartet nicht auf endgültige Daten aus der Phase eins. Stattdessen beginnen die WHO-Teams ab Dezember mit Impfungen in den betroffenen Staaten Westafrikas (Phase zwei und drei).
http://www.tagesspiegel.de/wissen/schneller-schutz-gesucht-e…
http://www.tagesspiegel.de/wissen/schneller-schutz-gesucht-e…
According to press reports, the European Union has agreed to finance clinical trials of a vaccine, earmarking $31 million for research.
http://www.globalresearch.ca/why-is-there-no-vaccine-for-ebo…
http://www.globalresearch.ca/why-is-there-no-vaccine-for-ebo…
Roche konnte durch zusätzlichen Verkauf von Tamiflu 2009 1,74 Mrd. USD mehr erzielen, obwohl dieses Medikament dann eigentlich kaum gebraucht wurde. Das verdeutlicht das Potential von Medikamenten wie Tekmira oder Impfungen wie die von NewLink:
"In 2009, Roche saw sales of its Tamiflu antiviral medication jump by $1.74 billion as governments around the world snapped up drugs in anticipation of a bird flu outbreak that never materialized. The U.S. has provided billions of dollars of incentives to reinvigorate flu vaccine production by companies like Glaxo and Novartis. Because of complex testing and manufacturing procedures, vaccines are expensive to produce and only a handful of companies compete in the space."
http://abcnews.go.com/Health/wireStory/drugmakers-bet-ebola-…
"In 2009, Roche saw sales of its Tamiflu antiviral medication jump by $1.74 billion as governments around the world snapped up drugs in anticipation of a bird flu outbreak that never materialized. The U.S. has provided billions of dollars of incentives to reinvigorate flu vaccine production by companies like Glaxo and Novartis. Because of complex testing and manufacturing procedures, vaccines are expensive to produce and only a handful of companies compete in the space."
http://abcnews.go.com/Health/wireStory/drugmakers-bet-ebola-…
Antwort auf Beitrag Nr.: 48.135.863 von mfierke am 26.10.14 19:17:54
Ja das ist wirklich erstaunlich! Um genau zu sein, tummeln wir uns hier zu zweit in diesem Threat!
Bei IBIO und ähnlichen Buden, die nur mit Andeutungen, "man sei in die Produktion involviert" versuchen, ihren Kurs in die Höhe zu schrauben finden sich hier bei W hingegen unendlich viele Interessierte...
hingegen unendlich viele Interessierte...
Man kann manchmal nur staunen!
LG, ER
Zitat von mfierke: Danke für die news, ich habe vermutet das aus NEWLINK GENETICS was richtiges gutes kommt. Die hat seti einer woche tgl.ein neues hoch erzielt. Bin gespannt wie sie morgen eröffnet. Noch wird dieser Thraed ganz wenig angenommen, könnte auch gut sein.
Ja das ist wirklich erstaunlich! Um genau zu sein, tummeln wir uns hier zu zweit in diesem Threat!

Bei IBIO und ähnlichen Buden, die nur mit Andeutungen, "man sei in die Produktion involviert" versuchen, ihren Kurs in die Höhe zu schrauben finden sich hier bei W
 hingegen unendlich viele Interessierte...
hingegen unendlich viele Interessierte...Man kann manchmal nur staunen!
LG, ER
Antwort auf Beitrag Nr.: 48.136.073 von extremrelaxer am 26.10.14 19:51:45Das wir hier zu zweit sind, kann auch sehr gut sein. Wir haben ruhe vor den Basher und pushern.
Ich sage jetzt auch gute Nacht und freue mich mal auf morgen früh:keks
Ich sage jetzt auch gute Nacht und freue mich mal auf morgen früh:keks

guten morgen allen hier, seit ihr noch die news am lesen??
gut, gelllle ?
gut, gelllle ?
Guten Morgen,
anscheinend hat hier noch keiner etwas von dem "Phasensprung" mitbekommen!
AUFWACHEN!
Am Wochenende wurde mitgeteilt, dass wir Anfang nächsten Jahres bei unserem Ebola-Impfstoff bereits mit Phase 3 anfangen werden!
anscheinend hat hier noch keiner etwas von dem "Phasensprung" mitbekommen!
AUFWACHEN!
Am Wochenende wurde mitgeteilt, dass wir Anfang nächsten Jahres bei unserem Ebola-Impfstoff bereits mit Phase 3 anfangen werden!

Antwort auf Beitrag Nr.: 48.138.350 von extremrelaxer am 27.10.14 09:29:10getaxt wird schon richtig hoch, mal sehen was der Pre-Market raus macht
jetzt 39,30 zu 41,79 $
jetzt 39,30 zu 41,79 $
Best-case- und Worst-case-Szenarium des Ebola-Verlaufs in West-Afrika:
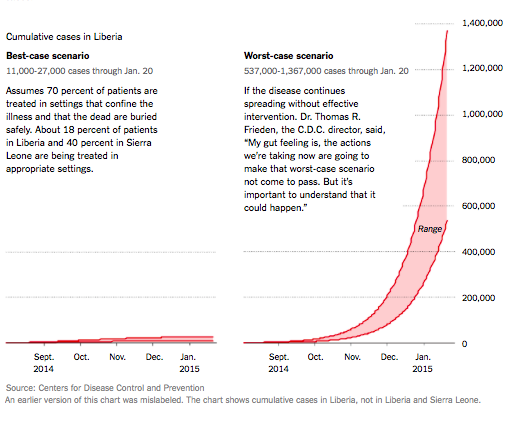
http://seekingalpha.com/article/2599785-ebola-hits-nyc-time-…

http://seekingalpha.com/article/2599785-ebola-hits-nyc-time-…
AMES, Iowa, Oct 27, 2014 (GLOBE NEWSWIRE via COMTEX) --
NewLink Genetics Corporation NLNK, -6.58% will release its third quarter 2014 financial results on Thursday, November 6, 2014. The company has scheduled a conference call for 8:30 AM ET the same day to discuss the results and to give an update on clinical and business development activities.
NewLink's senior management team will host the conference call, which will be open to all listeners. There will also be a question and answer session following the prepared remarks.
Access to the live call is available by dialing (855) 469-0612 (domestic) or (484) 756-4268 (international) five minutes prior to the start of the call. The call can also be accessed through a webcast via a link provided on the Investors and Media homepage of NewLink's website at www.linkp.com. A replay of the call will be available approximately two hours after the completion of the call and can be accessed by dialing (855) 859-2056 (domestic) or (404) 537-3406 (international) and using the passcode 25598868. The replay will be available for two weeks from the date of the call and the webcast will also be archived on the website.
NewLink is a biopharmaceutical company focused on discovering, developing and commercializing novel immuno-oncology products to improve treatment options for patients with cancer. NewLink's portfolio includes biologic and small molecule immunotherapy product candidates intended to treat a wide range of oncology indications. NewLink's product candidates are designed to harness multiple components of the immune system to combat cancer without significant incremental toxicity, either as a monotherapy or in combination with other treatment regimens. For more information please visit http://www.linkp.com.
http://www.marketwatch.com/story/newlink-genetics-to-host-it…
NewLink Genetics Corporation NLNK, -6.58% will release its third quarter 2014 financial results on Thursday, November 6, 2014. The company has scheduled a conference call for 8:30 AM ET the same day to discuss the results and to give an update on clinical and business development activities.
NewLink's senior management team will host the conference call, which will be open to all listeners. There will also be a question and answer session following the prepared remarks.
Access to the live call is available by dialing (855) 469-0612 (domestic) or (484) 756-4268 (international) five minutes prior to the start of the call. The call can also be accessed through a webcast via a link provided on the Investors and Media homepage of NewLink's website at www.linkp.com. A replay of the call will be available approximately two hours after the completion of the call and can be accessed by dialing (855) 859-2056 (domestic) or (404) 537-3406 (international) and using the passcode 25598868. The replay will be available for two weeks from the date of the call and the webcast will also be archived on the website.
NewLink is a biopharmaceutical company focused on discovering, developing and commercializing novel immuno-oncology products to improve treatment options for patients with cancer. NewLink's portfolio includes biologic and small molecule immunotherapy product candidates intended to treat a wide range of oncology indications. NewLink's product candidates are designed to harness multiple components of the immune system to combat cancer without significant incremental toxicity, either as a monotherapy or in combination with other treatment regimens. For more information please visit http://www.linkp.com.
http://www.marketwatch.com/story/newlink-genetics-to-host-it…
Big Pharma Bets on Bucks to Be Made on Ebola Vaccine
Drugmakers are racing to develop vaccines and drugs to address the worst outbreak of Ebola in history. It's unclear who will pay for their products, but companies are betting that governments and aid groups will foot the bill. Governments and corporations now are shifting millions of dollars to fight Ebola. Experts say drugmakers are wagering that international groups and wealthier governments like the U.S. will buy Ebola vaccines and drugs in mass quantities to stockpile them for future use once they're deemed safe. While there are no reliable estimates of the potential market size for an Ebola drug or vaccine, some drugmakers have already seen their stocks rise on the potential of the therapies in their pipelines.
Johnson & Johnson said last week it will begin safety testing in early January of a vaccine combination that could protect against an Ebola strain that is "highly similar" to the virus that triggered the current outbreak.
Human trials of a vaccine co-developed by the U.S. National Institutes of Health and GlaxoSmithKline are being funded by the company, its charitable trust and funds from the U.S. and U.K. governments. It is being tested for safety in the U.S., U.K., and Mali.
A small U.S. drugmaker, NewLink Genetics, holds the license on one front-runner vaccine, which was initially developed by the Public Health Agency of Canada and has been sent to the U.S. Walter Reed Army Institute of Research in Maryland for testing on healthy volunteers, with preliminary safety results expected by December.
IN-DEPTH
New Testing Starts on Experimental Ebola Vaccine
Trial Ebola Vaccine Set for January Rollout in West Africa
Third Ebola Vaccine Gets Cash Infusion from U.S.
-- The Associated Press
First published October 27th 2014, 1:10 pm
http://www.nbcnews.com/storyline/ebola-virus-outbreak/big-ph…
Drugmakers are racing to develop vaccines and drugs to address the worst outbreak of Ebola in history. It's unclear who will pay for their products, but companies are betting that governments and aid groups will foot the bill. Governments and corporations now are shifting millions of dollars to fight Ebola. Experts say drugmakers are wagering that international groups and wealthier governments like the U.S. will buy Ebola vaccines and drugs in mass quantities to stockpile them for future use once they're deemed safe. While there are no reliable estimates of the potential market size for an Ebola drug or vaccine, some drugmakers have already seen their stocks rise on the potential of the therapies in their pipelines.
Johnson & Johnson said last week it will begin safety testing in early January of a vaccine combination that could protect against an Ebola strain that is "highly similar" to the virus that triggered the current outbreak.
Human trials of a vaccine co-developed by the U.S. National Institutes of Health and GlaxoSmithKline are being funded by the company, its charitable trust and funds from the U.S. and U.K. governments. It is being tested for safety in the U.S., U.K., and Mali.
A small U.S. drugmaker, NewLink Genetics, holds the license on one front-runner vaccine, which was initially developed by the Public Health Agency of Canada and has been sent to the U.S. Walter Reed Army Institute of Research in Maryland for testing on healthy volunteers, with preliminary safety results expected by December.
IN-DEPTH
New Testing Starts on Experimental Ebola Vaccine
Trial Ebola Vaccine Set for January Rollout in West Africa
Third Ebola Vaccine Gets Cash Infusion from U.S.
-- The Associated Press
First published October 27th 2014, 1:10 pm
http://www.nbcnews.com/storyline/ebola-virus-outbreak/big-ph…
GEN News Highlights
More »
Oct 27, 2014
EbolaWatch: Costs Could Hit $6 Billion by 2016
VSV-ZEBOV: The Ebola vaccine candidate developed by Canadian and U.S. researchers has begun trials stateside and has been distributed to the WHO for trials in Switzerland starting November 1.
At NIH’s Clinical Center, researchers from the National Institute of Allergy and Infectious Diseases (NIAID) on October 22 began conducting a Phase I trial of VSV-ZEBOV for safety and immunogenicity in healthy adults through a prime-boost strategy. The Walter Reed Army Institute of Research is simultaneously testing the vaccine candidate as a single dose at its Clinical Trials Center.
VSV-EBOV was developed by the Public Health Agency of Canada (PHAC), and has been licensed by PHAC to BioProtection Systems, a subsidiary of NewLink Genetics.
On October 20, the first of 800 vials of VSV-EBOV were shipped to the WHO, which will begin its own clinical trials in Lausanne, Switzerland. Results are expected in December. Should the vaccine succeed, it would be shipped in January to African countries stricken with Ebola.
http://www.genengnews.com/gen-news-highlights/ebolawatch-a-5…
More »
Oct 27, 2014
EbolaWatch: Costs Could Hit $6 Billion by 2016
VSV-ZEBOV: The Ebola vaccine candidate developed by Canadian and U.S. researchers has begun trials stateside and has been distributed to the WHO for trials in Switzerland starting November 1.
At NIH’s Clinical Center, researchers from the National Institute of Allergy and Infectious Diseases (NIAID) on October 22 began conducting a Phase I trial of VSV-ZEBOV for safety and immunogenicity in healthy adults through a prime-boost strategy. The Walter Reed Army Institute of Research is simultaneously testing the vaccine candidate as a single dose at its Clinical Trials Center.
VSV-EBOV was developed by the Public Health Agency of Canada (PHAC), and has been licensed by PHAC to BioProtection Systems, a subsidiary of NewLink Genetics.
On October 20, the first of 800 vials of VSV-EBOV were shipped to the WHO, which will begin its own clinical trials in Lausanne, Switzerland. Results are expected in December. Should the vaccine succeed, it would be shipped in January to African countries stricken with Ebola.
http://www.genengnews.com/gen-news-highlights/ebolawatch-a-5…
Ebola news: Boy hospitalized in NY; doctor 'looks better;' vaccines in the works
http://www.oregonlive.com/health/index.ssf/2014/10/ebola_new…
http://www.oregonlive.com/health/index.ssf/2014/10/ebola_new…
6 Mrd USD nur für 2016 veranschlagt. Da dürften die beiden Vorreiter bei Impfstoffen gegen Ebola GSK und Newlink einiges vom Kuchen abbekommen können:
GEN News Highlights
More »
Oct 27, 2014
EbolaWatch: Costs Could Hit $6 Billion by 2016
Alex Philippidis
The global cost of preventing and containing Ebola will soar to $5.9 billion by 2016, Frost & Sullivan projects in a new video. A key reason why, the consultancy contends, is U.S. regulatory red tape delaying commercialization of new rapid diagnostics.
$129M COMMITTED: Prime Minister David Cameron said October 23 the U.K. will commit £80 million ($128.8 million) to fight Ebola. Most of the money—some £50 million ($80.5 million)—will be spent to create 200 Ebola care units in Sierra Leone, allowing individuals who suspect they have the virus to be examined and, if needed, isolated.
Of the remainder, £20 million ($32.2 million) will be set aside for the UN Ebola Multi-Partner Trust Fund, established to fight the disease by UN Secretary General Ban Ki-Moon, and £10 million ($16.1 million) toward burials of Ebola victims.
Cameron said the U.K. has spent £125 million ($201 million) to date fighting Ebola, and has urged European leaders to set aside a combined £800 million (about $1.3 billion).
$100M MORE: Paul G. Allen, the Microsoft co-founder-turned-philanthropist, said October 23 he has raised his investment in the fight against Ebola to $100 million, toward several efforts:
Developing and manufacturing two medevac containment units, for U.S. State Department use in evacuating medical professionals.
Joining with the World Health Organization (WHO) to enhance its capacity to coordinate logistics involved in transporting aid workers.
Establishing the $2.5 million Ebola Medevac Fund to address the gap between insurance coverage and transport costs. Grants will be matched by the Paul G. Allen Family Foundation.
Helping provide training, medical workers and lab equipment for relief efforts in Liberia, through a donation to University of Massachusetts Medical School.
Launching the online donation platform TackleEbola.com, designed to coordinate and optimize individual global giving toward solutions to treat, contain and prevent the spread of Ebola.
...
http://www.genengnews.com/gen-news-highlights/ebolawatch-a-5…
GEN News Highlights
More »
Oct 27, 2014
EbolaWatch: Costs Could Hit $6 Billion by 2016
Alex Philippidis
The global cost of preventing and containing Ebola will soar to $5.9 billion by 2016, Frost & Sullivan projects in a new video. A key reason why, the consultancy contends, is U.S. regulatory red tape delaying commercialization of new rapid diagnostics.
$129M COMMITTED: Prime Minister David Cameron said October 23 the U.K. will commit £80 million ($128.8 million) to fight Ebola. Most of the money—some £50 million ($80.5 million)—will be spent to create 200 Ebola care units in Sierra Leone, allowing individuals who suspect they have the virus to be examined and, if needed, isolated.
Of the remainder, £20 million ($32.2 million) will be set aside for the UN Ebola Multi-Partner Trust Fund, established to fight the disease by UN Secretary General Ban Ki-Moon, and £10 million ($16.1 million) toward burials of Ebola victims.
Cameron said the U.K. has spent £125 million ($201 million) to date fighting Ebola, and has urged European leaders to set aside a combined £800 million (about $1.3 billion).
$100M MORE: Paul G. Allen, the Microsoft co-founder-turned-philanthropist, said October 23 he has raised his investment in the fight against Ebola to $100 million, toward several efforts:
Developing and manufacturing two medevac containment units, for U.S. State Department use in evacuating medical professionals.
Joining with the World Health Organization (WHO) to enhance its capacity to coordinate logistics involved in transporting aid workers.
Establishing the $2.5 million Ebola Medevac Fund to address the gap between insurance coverage and transport costs. Grants will be matched by the Paul G. Allen Family Foundation.
Helping provide training, medical workers and lab equipment for relief efforts in Liberia, through a donation to University of Massachusetts Medical School.
Launching the online donation platform TackleEbola.com, designed to coordinate and optimize individual global giving toward solutions to treat, contain and prevent the spread of Ebola.
...
http://www.genengnews.com/gen-news-highlights/ebolawatch-a-5…
n race for Ebola vaccines, technical hurdles loom large
By Reuters
Published: 15:50 GMT, 27 October 2014 | Updated: 15:50 GMT, 27 October 2014
By Ben Hirschler
LONDON, Oct 27 (Reuters) - Drugmakers sprinting to develop Ebola vaccines face a series of technical hurdles if they are to get millions of doses ready for use next year -- even assuming clinical trials are successful.
The challenges include finding sufficient sterile capacity for filling and packaging finished vials, getting fast quality approvals from regulators, and building a supply chain in Africa for products that must be stored at minus 80 degrees Celsius.
With the World Health Organization (WHO) now expecting vaccine efficacy trials to start in West Africa in December, a month earlier than anticipated, the development programme is clearly gaining momentum.
But industry executives, health experts and governments are still grappling with a host of issues -- and some differences of opinion -- on topics ranging from clinical trial design to factory biosafety levels, according to people who attended a high-level meeting of officials in Geneva last week.
"There is not a funding problem," said one person at the meeting. "The real issues are all around the practicalities."
Because it has proved so difficult to slow the world's worst Ebola epidemic, which has killed around half of the 10,000 officially reported cases so far, many health experts now see a vaccine as a vital tool in controlling the disease.
Far from coming too late, experts at last week's meeting concluded that "vaccines will have a significant impact on the further evolution of the epidemic in any scenario, from best-case to worst-case".
That has increased the pressure on all parties to expedite development, production and distribution.
Companies have pledged affordable prices as they race to develop the first vaccines for a previously neglected disease, in collaboration with donors and government agencies.
Two leading vaccine candidates from GlaxoSmithKline and NewLink Genetics are already in human safety trials, and another five should begin testing in the first quarter of next year. One from Johnson & Johnson will start trials in January.
The three leading companies hope to make millions of doses over the course of 2015, with GSK ordering five production lines and J&J investing $200 million.
However, the exact number of doses depends on manufacturing yields -- something that can vary widely, depending on how well cell cultures grow -- and how much vaccine has to be injected into the arm of each person to ensure protection.
The Norwegian Institute of Public Health estimates that 10 million to 27 million doses may be needed, or considerably more if the current epidemic spreads to neighbouring countries, according to a document circulated at the WHO meeting.
BIOSAFETY LEVEL 2
Translating bulk vaccine production into vials of product ready for widespread use in Liberia, Sierra Leone and Guinea, where the disease is still spreading rapidly, also needs a massive packaging effort.
"One of the main bottlenecks is filling capacity," said WHO assistant director general Marie-Paule Kieny, echoing the concerns of vaccine developers.
Because Ebola vaccines use genetically modified organisms, the sterile filling of vials must be done in a so-called biosafety level 2 (BSL2) facility, which only a few companies possess.
Those with BSL2 operations include GSK, Sanofi, Merck and Novartis, but in many cases their factories are already working on other important vaccines against diseases such as measles, mumps, rubella and rotavirus.
Options now being actively pursued include sharing capacity between companies and talks with regulators as to whether the BSL2 requirement for Ebola vaccines might be relaxed, although this may cause concern given past biosafety lapses.
Another hurdle that must be overcome is accelerating the quality approval process required by regulators for finished vaccines, which typically involves lengthy checks at various stages in the production process.
The hope is to run some quality checks in parallel, rather than sequentially. That will require the buy-in not only of regulatory authorities in producing countries but also those in Africa to prevent consignments being delayed at customs.
Further consultations are planned for this week to clarify and simplify such regulatory hurdles, and also fine-tune plans for clinical trials, where a U.S.-led plan for a placebo-controlled study in Liberia has sparked some controversy.
While experts agree that testing experimental Ebola vaccines against a control or placebo shot will give the most definitive results, a number of experts question whether this is either practical or ethical in the current outbreak.
Further down the road, the final piece that needs to be put in place is a system for procuring and delivering the millions of vaccine doses that may be ready for deployment later in 2015, through a supply chain that will need specialist refrigeration.
GAVI, the global vaccines alliance, is already working to figure out how to bulk buy a future vaccine at a reasonable price and its board will consider ideas on Dec. 10-11. (Editing by Giles Elgood)
Read more: http://www.dailymail.co.uk/wires/reuters/article-2809775/In-…
Follow us: @MailOnline on Twitter | DailyMail on Facebook
http://www.dailymail.co.uk/wires/reuters/article-2809775/In-…
By Reuters
Published: 15:50 GMT, 27 October 2014 | Updated: 15:50 GMT, 27 October 2014
By Ben Hirschler
LONDON, Oct 27 (Reuters) - Drugmakers sprinting to develop Ebola vaccines face a series of technical hurdles if they are to get millions of doses ready for use next year -- even assuming clinical trials are successful.
The challenges include finding sufficient sterile capacity for filling and packaging finished vials, getting fast quality approvals from regulators, and building a supply chain in Africa for products that must be stored at minus 80 degrees Celsius.
With the World Health Organization (WHO) now expecting vaccine efficacy trials to start in West Africa in December, a month earlier than anticipated, the development programme is clearly gaining momentum.
But industry executives, health experts and governments are still grappling with a host of issues -- and some differences of opinion -- on topics ranging from clinical trial design to factory biosafety levels, according to people who attended a high-level meeting of officials in Geneva last week.
"There is not a funding problem," said one person at the meeting. "The real issues are all around the practicalities."
Because it has proved so difficult to slow the world's worst Ebola epidemic, which has killed around half of the 10,000 officially reported cases so far, many health experts now see a vaccine as a vital tool in controlling the disease.
Far from coming too late, experts at last week's meeting concluded that "vaccines will have a significant impact on the further evolution of the epidemic in any scenario, from best-case to worst-case".
That has increased the pressure on all parties to expedite development, production and distribution.
Companies have pledged affordable prices as they race to develop the first vaccines for a previously neglected disease, in collaboration with donors and government agencies.
Two leading vaccine candidates from GlaxoSmithKline and NewLink Genetics are already in human safety trials, and another five should begin testing in the first quarter of next year. One from Johnson & Johnson will start trials in January.
The three leading companies hope to make millions of doses over the course of 2015, with GSK ordering five production lines and J&J investing $200 million.
However, the exact number of doses depends on manufacturing yields -- something that can vary widely, depending on how well cell cultures grow -- and how much vaccine has to be injected into the arm of each person to ensure protection.
The Norwegian Institute of Public Health estimates that 10 million to 27 million doses may be needed, or considerably more if the current epidemic spreads to neighbouring countries, according to a document circulated at the WHO meeting.
BIOSAFETY LEVEL 2
Translating bulk vaccine production into vials of product ready for widespread use in Liberia, Sierra Leone and Guinea, where the disease is still spreading rapidly, also needs a massive packaging effort.
"One of the main bottlenecks is filling capacity," said WHO assistant director general Marie-Paule Kieny, echoing the concerns of vaccine developers.
Because Ebola vaccines use genetically modified organisms, the sterile filling of vials must be done in a so-called biosafety level 2 (BSL2) facility, which only a few companies possess.
Those with BSL2 operations include GSK, Sanofi, Merck and Novartis, but in many cases their factories are already working on other important vaccines against diseases such as measles, mumps, rubella and rotavirus.
Options now being actively pursued include sharing capacity between companies and talks with regulators as to whether the BSL2 requirement for Ebola vaccines might be relaxed, although this may cause concern given past biosafety lapses.
Another hurdle that must be overcome is accelerating the quality approval process required by regulators for finished vaccines, which typically involves lengthy checks at various stages in the production process.
The hope is to run some quality checks in parallel, rather than sequentially. That will require the buy-in not only of regulatory authorities in producing countries but also those in Africa to prevent consignments being delayed at customs.
Further consultations are planned for this week to clarify and simplify such regulatory hurdles, and also fine-tune plans for clinical trials, where a U.S.-led plan for a placebo-controlled study in Liberia has sparked some controversy.
While experts agree that testing experimental Ebola vaccines against a control or placebo shot will give the most definitive results, a number of experts question whether this is either practical or ethical in the current outbreak.
Further down the road, the final piece that needs to be put in place is a system for procuring and delivering the millions of vaccine doses that may be ready for deployment later in 2015, through a supply chain that will need specialist refrigeration.
GAVI, the global vaccines alliance, is already working to figure out how to bulk buy a future vaccine at a reasonable price and its board will consider ideas on Dec. 10-11. (Editing by Giles Elgood)
Read more: http://www.dailymail.co.uk/wires/reuters/article-2809775/In-…
Follow us: @MailOnline on Twitter | DailyMail on Facebook
http://www.dailymail.co.uk/wires/reuters/article-2809775/In-…
Antwort auf Beitrag Nr.: 48.143.525 von extremrelaxer am 27.10.14 16:54:45Bin heute hier eingestiegen.die Aktie hat meiner Meinung nach beste Zukunftsperspektiven.Fühle mich gut aufgehoben.
Antwort auf Beitrag Nr.: 48.136.121 von mfierke am 26.10.14 20:02:43Hallo,
na da sehe ich ja schon zwei bekannte Namen...
Du hättest mir ja mal einen Tipp geben können o. zählst Du mich auch zu den Bashern/Pushern?
Na ich lese mich mal ein wenig ein ...
...
na da sehe ich ja schon zwei bekannte Namen...
Du hättest mir ja mal einen Tipp geben können o. zählst Du mich auch zu den Bashern/Pushern?
Na ich lese mich mal ein wenig ein
 ...
...
Antwort auf Beitrag Nr.: 48.145.010 von BSA01 am 27.10.14 18:58:19Dein Bild ha<t mir die Sprache verschlagen, daher konnte ich Dir das leider nicht flüstern. kleines Späßle,
habe ich nicht daran gedacht
habe ich nicht daran gedacht
More Than 13,500 People Infected With Ebola
http://online.wsj.com/articles/more-than-13-500-people-infec…
http://online.wsj.com/articles/more-than-13-500-people-infec…
"Ebola-Aktien" gibt es ja inzwischen mehr als genug. Bleibt die Frage, welche Unternehmen durch Prävention oder Bekämpfung mittel- und langfristig von der Krankheit profitieren werden und ob die Kurse der beteiligten Unternehmen nach einem Abebben der Neuerkrankungen auf erhöhtem Niveau verbleiben oder dann kollabieren.
Aktuell zeigen sich bei den "Ebola-Aktien" immer ähnliche Tendenzen, hierbei zeigt sich ein Ansteigen der Aktienkurse bei entsprechendem Newsflow und ein Sinken der Kurse bei Nachlassen des selben.
Die relative Stärke bei den Aktienkursen ist ein brauchbarer Indikator, bei welchen Aktien wirklich langfristig ein Potential besteht und bei welchen Kursanstiegen es sich um "Frick-Zacks" oder "Nebelkerzen" handelt.
Mittelfristig (< 12 Monate) gehe ich von einer Konsolidierung der Neuerkrankungsfälle aus, d.h. keine exponentielle, sondern dann eine lineare bzw. später abfallende Zahl von Neuerkrankungsfällen. Aufgrund der zahllosen verbuddelten Leichen und dem Umstand, dass diese Leichen weiterhin infektiös sind, glaube ich nicht, dass es bei dieser Epidemie zu einem plötzlichen Ende kommen wird, sondern diese Erkrankungsepidemie langfristig in den westafrikanischen Ländern festsetzen wird.
Potential sehe ich daher insbesondere für die Impfstoffhersteller, welche an präventiven Impfstoffen arbeiten. Aufgrund der niedrigen MK sehe ich unter diesen für Newlink das größte Potential.
Aber auch hier muss man wohl Sitzfleisch haben. Ein Überschreiten des All-Time-Highs wird es m.E. wahrscheinlich erst mit Bekanntgabe der ersten Studienergebnisse im Dezember geben, außer wir erleben am 06.11. im Rahmen der Zahlen eine positive Überraschung...
Ich werde hier jedenfalls einen langen Atem haben.
LG, ER
Aktuell zeigen sich bei den "Ebola-Aktien" immer ähnliche Tendenzen, hierbei zeigt sich ein Ansteigen der Aktienkurse bei entsprechendem Newsflow und ein Sinken der Kurse bei Nachlassen des selben.
Die relative Stärke bei den Aktienkursen ist ein brauchbarer Indikator, bei welchen Aktien wirklich langfristig ein Potential besteht und bei welchen Kursanstiegen es sich um "Frick-Zacks" oder "Nebelkerzen" handelt.
Mittelfristig (< 12 Monate) gehe ich von einer Konsolidierung der Neuerkrankungsfälle aus, d.h. keine exponentielle, sondern dann eine lineare bzw. später abfallende Zahl von Neuerkrankungsfällen. Aufgrund der zahllosen verbuddelten Leichen und dem Umstand, dass diese Leichen weiterhin infektiös sind, glaube ich nicht, dass es bei dieser Epidemie zu einem plötzlichen Ende kommen wird, sondern diese Erkrankungsepidemie langfristig in den westafrikanischen Ländern festsetzen wird.
Potential sehe ich daher insbesondere für die Impfstoffhersteller, welche an präventiven Impfstoffen arbeiten. Aufgrund der niedrigen MK sehe ich unter diesen für Newlink das größte Potential.
Aber auch hier muss man wohl Sitzfleisch haben. Ein Überschreiten des All-Time-Highs wird es m.E. wahrscheinlich erst mit Bekanntgabe der ersten Studienergebnisse im Dezember geben, außer wir erleben am 06.11. im Rahmen der Zahlen eine positive Überraschung...
Ich werde hier jedenfalls einen langen Atem haben.
LG, ER
Nach Australien verweigert jetzt auch Kanada Menschen aus den westafrikanischen Ebola-Ländern die Einreise.
http://nachrichten168.eu/nachrichten/kanada-macht-grenzen-fu…
http://nachrichten168.eu/nachrichten/kanada-macht-grenzen-fu…
Posted October 30, 2014 - 11:18pm
NewLink moving toward large Ebola vaccine trial
- See more at: http://amestrib.com/news/newlink-moving-toward-large-ebola-v…
By Melissa Erickson
Associate Editor
merickson@amestrib.com
Ames-based medical vaccine research company NewLink Genetics is on the forefront of research efforts to create a vaccine that has already been shown effective in both pre- and post-exposure vaccination of primates exposed to the deadly Ebola virus.
U.S. Sen. Tom Harkin, D-Iowa, visited NewLink Thursday to hear an update on the company’s progress and to pledge his support for working to raise federal funding for biomedical research in his last months in office.
NewLink CEO Charles Link said the company has been working with the Canadian government, the U.S. government and other research companies to work “as diligently and as rapidly as possible within the bounds of safety” to prepare the Ebola vaccine for a large human trial later this year.
Right now they are working to determine the appropriate dose, Link said, and will start a 30,000 person trial in Western Africa.
If a medium-size dose of the vaccine is used, NewLink could have between 500,000 and 5 million doses available by the end of December and many more by the end of the first quarter of 2015 , and if a higher dose is required, the company would have about 50,000 doses available at the end of the year, Link said.
“We think one of the medium doses is probably likely to be the effective dose, but we need data to be able to prove that, that’s what we’re trying to do right now,” he said.
“I assure you that there are literally hundreds of people moving heaven and earth trying to move these things forward, but it has to be done in a very scientifically responsible, ethically responsible way and with all the appropriate medical safety involved.”
Harkin, who is retiring at the end of this year, told NewLink employees Thursday that he plans to argue for lifting the caps on funding for the National Institutes of Health when Congress starts their budget talks in the next month.
“The last 10 years, the funding for NIH, actually in real terms, has gone down,” Harkin said. “I’m going to make the argument, and I think the biomedical community is 100 percent behind this, to raise the caps so that we can fund NIH at an appropriate level. The idea is to make it a separate type of item that is funded without regard to whether you’re cutting something else.”
After meeting with employees, Harkin told reporters he is “somewhat upset” by the response to the Ebola outbreak.
“We have people who are trying to second guess (the Center for Disease Control and Prevention) and inject political considerations into responding to the Ebola outbreak, but they’re not scientifically based and that’s what bothers me,” he said. “There’s enough to be concerned about with Ebola and all the other viruses without unduly stoking fears in people.”
Harkin said the CDC is the “gold standard” of the world and should be trusted rather than letting politicians “scare everybody to death.”
“Let’s follow the advice of people who know what this is about and calm down a little bit and then put our efforts behind the development of vaccines and procedures that really do answer the problem,” he said.
As Harkin left NewLink Thursday afternoon, U.S. Rep. Steve King, R-Iowa, arrived for a visit of his own.
When asked by reporters if he thought it would be possible to raise the caps on NIH funding, King said it is too early to say.
“I would not say that we have an Ebola problem because there wasn’t enough money spent at the CDC,” King said. “I think there’s some people on the other side of the aisle that have asserted that.”
King said the epidemic needs to be isolated and that bringing people who have been exposed to it into the U.S. is a mistake.
“American citizens that need to come home, and that would include our health care personnel, they need to wait 21 days in a quarantine to come back into the United States,” King said.
“What if we had outbreaks that popped up in our major cities across the country? Within a week or two weeks, that could happen. If that’s the case, where does it stop then? It’s a lot easier to stop it by not letting it in.”
King also said Tom Friedan, director of the CDC, is no longer a reliable voice and needs to step down, and that he believes any military personnel sent to help with the Ebola response should be volunteers.
“I haven’t met anybody that says that 4,000 troops can be sent over there and bunked in a hotel with Liberians and expect that they’ll not come back having contracted Ebola,” he said.
“We’re in the early stages of what could be a massive epidemic and on the other hand we might be in the early stages of eradicating it from our continent and getting it controlled in Africa. We don’t know yet, but it’s better to overreact on the side of safety and then move ourselves back towards practicality as we learn.”
- See more at: http://amestrib.com/news/newlink-moving-toward-large-ebola-v…
http://amestrib.com/news/newlink-moving-toward-large-ebola-v…
NewLink moving toward large Ebola vaccine trial
- See more at: http://amestrib.com/news/newlink-moving-toward-large-ebola-v…
By Melissa Erickson
Associate Editor
merickson@amestrib.com
Ames-based medical vaccine research company NewLink Genetics is on the forefront of research efforts to create a vaccine that has already been shown effective in both pre- and post-exposure vaccination of primates exposed to the deadly Ebola virus.
U.S. Sen. Tom Harkin, D-Iowa, visited NewLink Thursday to hear an update on the company’s progress and to pledge his support for working to raise federal funding for biomedical research in his last months in office.
NewLink CEO Charles Link said the company has been working with the Canadian government, the U.S. government and other research companies to work “as diligently and as rapidly as possible within the bounds of safety” to prepare the Ebola vaccine for a large human trial later this year.
Right now they are working to determine the appropriate dose, Link said, and will start a 30,000 person trial in Western Africa.
If a medium-size dose of the vaccine is used, NewLink could have between 500,000 and 5 million doses available by the end of December and many more by the end of the first quarter of 2015 , and if a higher dose is required, the company would have about 50,000 doses available at the end of the year, Link said.
“We think one of the medium doses is probably likely to be the effective dose, but we need data to be able to prove that, that’s what we’re trying to do right now,” he said.
“I assure you that there are literally hundreds of people moving heaven and earth trying to move these things forward, but it has to be done in a very scientifically responsible, ethically responsible way and with all the appropriate medical safety involved.”
Harkin, who is retiring at the end of this year, told NewLink employees Thursday that he plans to argue for lifting the caps on funding for the National Institutes of Health when Congress starts their budget talks in the next month.
“The last 10 years, the funding for NIH, actually in real terms, has gone down,” Harkin said. “I’m going to make the argument, and I think the biomedical community is 100 percent behind this, to raise the caps so that we can fund NIH at an appropriate level. The idea is to make it a separate type of item that is funded without regard to whether you’re cutting something else.”
After meeting with employees, Harkin told reporters he is “somewhat upset” by the response to the Ebola outbreak.
“We have people who are trying to second guess (the Center for Disease Control and Prevention) and inject political considerations into responding to the Ebola outbreak, but they’re not scientifically based and that’s what bothers me,” he said. “There’s enough to be concerned about with Ebola and all the other viruses without unduly stoking fears in people.”
Harkin said the CDC is the “gold standard” of the world and should be trusted rather than letting politicians “scare everybody to death.”
“Let’s follow the advice of people who know what this is about and calm down a little bit and then put our efforts behind the development of vaccines and procedures that really do answer the problem,” he said.
As Harkin left NewLink Thursday afternoon, U.S. Rep. Steve King, R-Iowa, arrived for a visit of his own.
When asked by reporters if he thought it would be possible to raise the caps on NIH funding, King said it is too early to say.
“I would not say that we have an Ebola problem because there wasn’t enough money spent at the CDC,” King said. “I think there’s some people on the other side of the aisle that have asserted that.”
King said the epidemic needs to be isolated and that bringing people who have been exposed to it into the U.S. is a mistake.
“American citizens that need to come home, and that would include our health care personnel, they need to wait 21 days in a quarantine to come back into the United States,” King said.
“What if we had outbreaks that popped up in our major cities across the country? Within a week or two weeks, that could happen. If that’s the case, where does it stop then? It’s a lot easier to stop it by not letting it in.”
King also said Tom Friedan, director of the CDC, is no longer a reliable voice and needs to step down, and that he believes any military personnel sent to help with the Ebola response should be volunteers.
“I haven’t met anybody that says that 4,000 troops can be sent over there and bunked in a hotel with Liberians and expect that they’ll not come back having contracted Ebola,” he said.
“We’re in the early stages of what could be a massive epidemic and on the other hand we might be in the early stages of eradicating it from our continent and getting it controlled in Africa. We don’t know yet, but it’s better to overreact on the side of safety and then move ourselves back towards practicality as we learn.”
- See more at: http://amestrib.com/news/newlink-moving-toward-large-ebola-v…
http://amestrib.com/news/newlink-moving-toward-large-ebola-v…
NewLink announced yesterday that it has started human trials for its experimental Ebola vaccine VSV-EBOV in Europe, Gabon, and Kenya
Published: October 30, 2014 at 7:12 am ESTBy: Hannah Ishmael
...
The trials are being funded by the UK charity Wellcome Trust under a $5 million grant. The program is being overseen by the World Health Organization (WHO). The trials will allow several global partners to gather essential data for the vaccine. Canada has donated 800 vials of the vaccine to be used in the trials.
...
http://www.bidnessetc.com/28252-newlink-begins-human-trials-…
5 Mio USD für die 1. Studie mit 800 Impfdosen also. Das würde 6.250 USD pro Studienteilnehmer bzw. pro verimpfter Dosis entsprechen.
Published: October 30, 2014 at 7:12 am ESTBy: Hannah Ishmael
...
The trials are being funded by the UK charity Wellcome Trust under a $5 million grant. The program is being overseen by the World Health Organization (WHO). The trials will allow several global partners to gather essential data for the vaccine. Canada has donated 800 vials of the vaccine to be used in the trials.
...
http://www.bidnessetc.com/28252-newlink-begins-human-trials-…
5 Mio USD für die 1. Studie mit 800 Impfdosen also. Das würde 6.250 USD pro Studienteilnehmer bzw. pro verimpfter Dosis entsprechen.
Science 31 October 2014:
Vol. 346 no. 6209 pp. 534-535
DOI: 10.1126/science.346.6209.534
In Depth
Infectious Diseases
The Ebola vaccine underdog
NewLink CEO Charles Link.
"PHOTO: AP PHOTO/CHARLIE NEIBERGALL"
In the race to develop an Ebola vaccine, little NewLink Genetics has been in the shadow of pharmaceutical giant GlaxoSmithKline (GSK).
Both companies have rushed experimental vaccines into small, early-stage trials. Hopes are high that the vaccines can be ready for large efficacy trials in hard-hit West Africa in January—and if they work, for real-world use in the spring. GSK's efforts have received extensive media attention, and, with its substantial manufacturing capacity and experience, the mammoth U.K.-based company is widely assumed to be in the lead. In contrast, NewLink, a cancer drug company based in Ames, Iowa, with just 120 employees, has until recently avoided media coverage and drawn criticism for delaying the launch of its studies.
But a different picture emerged after NewLink broke its media silence following a high-level meeting on Ebola vaccines held by the World Health Organization on 23 October. At the meeting, NewLink executives said that, under a best-case scenario, the company might have 12 million doses of vaccine by April. That number would far outstrip GSK's estimate of 230,000 doses by that date.
There are many caveats. If NewLink's vaccine requires a high dose to be effective, far fewer people could be immunized. And NewLink's vaccine, which combines an Ebola gene with a weakened vesicular stomatitis virus (VSV), a livestock pathogen, poses unique risks.
NewLink CEO Charles Link Jr., an oncologist who previously worked at the National Cancer Institute, spoke with Science about the charges of delay and why he is optimistic about the higher projections. This interview has been edited for brevity and clarity.
Q:You recently completed a $1 billion deal with Genentech to develop a cancer immunotherapy. Did those negotiations delay work on the Ebola vaccine and influence your decision to avoid media?
A:I really don't feel there were any delays. Things are moving so quickly that we're right on the edge of moving too quickly. There's a huge push and pull between wanting to do the right thing for humanity and needing to do things safely, scientifically, and ethically in healthy volunteers who are receiving the vaccine. Our view was we didn't want to hype anything. We just wanted to work on the project. Ebola came first, [the Genentech] negotiation came second, and PR came third. We've been trying to play it low-key, but it's difficult to play it low-key with all this attention.
Q:You licensed the vaccine from the Canadian government for a mere $200,000. Although you have received small contracts from the U.S. government to develop the vaccine, did you have trouble getting substantial funding to support the Ebola program?
A:No doubt. At first, the board didn't see much commercial potential in it. But when the crisis began to evolve, everybody was: “Let's go, let's make this happen.” There was no hesitancy once the crisis began.
Q:What about your projection of 12 million doses available in April?
A:The key question is what is going to be the dose of the vaccine.
Q:Studies under way are looking at doses of 106 virus particles up to 108. The 12 million is based on 106, right?
A:Yes, so if a dose needs to be 106 or 105 virus particles, we're going to have plenty of vaccine for West Africa if it works.
Q:Why do you think the lower doses might suffice?
A:Even though this vaccine is based on an attenuated virus, it is replicating at least some in humans. In talking to experts who have worked with a lot of attenuated vaccines, you may only need 104 [virus particles] to create the immunologic effect—and we may amend our studies to look at those lower doses.
Q o you think the vaccines are going to be safe and effective?
o you think the vaccines are going to be safe and effective?
A:In the primate model, such a wide variety of these vaccines work that I really believe one of them is going to be effective in humans. That is my hope and dream here—ours or someone else's.
Q:What about side effects? The VSV vaccine was used in 2009 to treat a lab worker who had a needlestick injury in Germany. What happened?
A:The woman developed a temperature of 38.5°[C]. I don't think you can have a vaccine that causes high fever in a significant portion of subjects, especially where fever is the first indication of Ebola. But she was given a dose of 5 × 107 [virus particles], based on an extrapolation from monkey studies. We're hoping that at the lower doses people might have low-grade fevers, but there won't be high-grade fevers.
Q:Is there a risk of VSV spreading from vaccinated people and infecting livestock?
A:It's a legitimate concern and we're looking at ways to evaluate that.
Q:Producing the vaccine in bulk will require large-scale manufacturing capacity. Have you considered linking with big pharma companies that know how to mass-produce vaccine, including putting it into vials?
A:We are in fact in active discussions with a big company about just that potential.
http://www.sciencemag.org/content/346/6209/534.full
Vol. 346 no. 6209 pp. 534-535
DOI: 10.1126/science.346.6209.534
In Depth
Infectious Diseases
The Ebola vaccine underdog
NewLink CEO Charles Link.
"PHOTO: AP PHOTO/CHARLIE NEIBERGALL"
In the race to develop an Ebola vaccine, little NewLink Genetics has been in the shadow of pharmaceutical giant GlaxoSmithKline (GSK).
Both companies have rushed experimental vaccines into small, early-stage trials. Hopes are high that the vaccines can be ready for large efficacy trials in hard-hit West Africa in January—and if they work, for real-world use in the spring. GSK's efforts have received extensive media attention, and, with its substantial manufacturing capacity and experience, the mammoth U.K.-based company is widely assumed to be in the lead. In contrast, NewLink, a cancer drug company based in Ames, Iowa, with just 120 employees, has until recently avoided media coverage and drawn criticism for delaying the launch of its studies.
But a different picture emerged after NewLink broke its media silence following a high-level meeting on Ebola vaccines held by the World Health Organization on 23 October. At the meeting, NewLink executives said that, under a best-case scenario, the company might have 12 million doses of vaccine by April. That number would far outstrip GSK's estimate of 230,000 doses by that date.
There are many caveats. If NewLink's vaccine requires a high dose to be effective, far fewer people could be immunized. And NewLink's vaccine, which combines an Ebola gene with a weakened vesicular stomatitis virus (VSV), a livestock pathogen, poses unique risks.
NewLink CEO Charles Link Jr., an oncologist who previously worked at the National Cancer Institute, spoke with Science about the charges of delay and why he is optimistic about the higher projections. This interview has been edited for brevity and clarity.
Q:You recently completed a $1 billion deal with Genentech to develop a cancer immunotherapy. Did those negotiations delay work on the Ebola vaccine and influence your decision to avoid media?
A:I really don't feel there were any delays. Things are moving so quickly that we're right on the edge of moving too quickly. There's a huge push and pull between wanting to do the right thing for humanity and needing to do things safely, scientifically, and ethically in healthy volunteers who are receiving the vaccine. Our view was we didn't want to hype anything. We just wanted to work on the project. Ebola came first, [the Genentech] negotiation came second, and PR came third. We've been trying to play it low-key, but it's difficult to play it low-key with all this attention.
Q:You licensed the vaccine from the Canadian government for a mere $200,000. Although you have received small contracts from the U.S. government to develop the vaccine, did you have trouble getting substantial funding to support the Ebola program?
A:No doubt. At first, the board didn't see much commercial potential in it. But when the crisis began to evolve, everybody was: “Let's go, let's make this happen.” There was no hesitancy once the crisis began.
Q:What about your projection of 12 million doses available in April?
A:The key question is what is going to be the dose of the vaccine.
Q:Studies under way are looking at doses of 106 virus particles up to 108. The 12 million is based on 106, right?
A:Yes, so if a dose needs to be 106 or 105 virus particles, we're going to have plenty of vaccine for West Africa if it works.
Q:Why do you think the lower doses might suffice?
A:Even though this vaccine is based on an attenuated virus, it is replicating at least some in humans. In talking to experts who have worked with a lot of attenuated vaccines, you may only need 104 [virus particles] to create the immunologic effect—and we may amend our studies to look at those lower doses.
Q
 o you think the vaccines are going to be safe and effective?
o you think the vaccines are going to be safe and effective?A:In the primate model, such a wide variety of these vaccines work that I really believe one of them is going to be effective in humans. That is my hope and dream here—ours or someone else's.
Q:What about side effects? The VSV vaccine was used in 2009 to treat a lab worker who had a needlestick injury in Germany. What happened?
A:The woman developed a temperature of 38.5°[C]. I don't think you can have a vaccine that causes high fever in a significant portion of subjects, especially where fever is the first indication of Ebola. But she was given a dose of 5 × 107 [virus particles], based on an extrapolation from monkey studies. We're hoping that at the lower doses people might have low-grade fevers, but there won't be high-grade fevers.
Q:Is there a risk of VSV spreading from vaccinated people and infecting livestock?
A:It's a legitimate concern and we're looking at ways to evaluate that.
Q:Producing the vaccine in bulk will require large-scale manufacturing capacity. Have you considered linking with big pharma companies that know how to mass-produce vaccine, including putting it into vials?
A:We are in fact in active discussions with a big company about just that potential.
http://www.sciencemag.org/content/346/6209/534.full
Ehemaliges Mitglied der Reagan-Regierung: Die Ebola-Story stinkt
http://www.shortnews.de/id/1117901/ehemaliges-mitglied-der-r…
http://www.shortnews.de/id/1117901/ehemaliges-mitglied-der-r…
Fleißige Forscher
Weltweit befassen sich etliche Labors mit dem Thema. Die wichtigsten Ansätze: GlaxoSmithKline (GSK) untersucht zusammen mit dem amerikanischen National Institute of Allergy and Infectious Diseases, welche Potenziale in Adenoviren stecken. Biologen verwenden das chimpanzee-derived replication-defective adenovirus (ChAd), um Antigene des Ebolavirus im menschlichen Körper zu exprimieren und dem Immunsystem zu präsentieren. Momentan laufen zwei große Studien mit dem Konstrukt cAd3-EBO Z: In Großbritannien sollen 60 Probanden verschiedene Dosen des Impfstoffs erhalten. Ähnliche Untersuchungen planen Wissenschaftler in den USA mit 26 Personen. Auch das Vesicular stomatitis Indiana virus (VSIV), ein Verwandter des Tollwutvirus, hat Potenziale. Nach einer VSIV-Infektion erkranken Rinder oder Pferde schwer, während Menschen nur leichte, grippeähnliche Symptome verspüren. Ein wichtiger Vertreter ist VSV-EBOV. Molekularbiologen haben Erbinformation für das virale VSV-Glykoprotein durch die RNA für ein Ebola-Glykoprotein ausgetauscht. Unser Immunsystem reagiert, indem es Antikörper herstellt. Die Public Health Agency of Canada setzt große Erwartungen in VSV-EBOV. Zusammen mit der NewLinks Genetics Corporation hat sie eine Phase-I-Studie in die Wege geleitet.
VSV-EBOV unter der Lupe
Dazu noch einige Studien: Ratten und Meerschweinchen profitierten nachweislich vom Impfstoff. In Studien überlebten 80 bis 100 Prozent aller Tiere. Menschenaffen wurden ebenfalls zuverlässig geschützt – es kam zu einer starken Immunantwort mit hohen Serumtitern. Ein Großteil aller Primaten blieb am Leben, falls unmittelbar nach einer Virusinfektion die Impfung als Postexpositionsprophylaxe verabreicht wurde. Erhielten Tiere die Vakzine aber erst 48 Stunden nach der Exposition, starben zwei Drittel. VSV-EBOV wirkt allerdings hoch spezifisch. Ein Impfstoff mit dem Glykoprotein des Ebola-Zaire-Virus schützt nicht vor verwandten Erregern wie dem Ebola-Sudan-Virus. Forscher haben VSV-EBOV bislang nur in Tierexperimenten untersucht – mit einer Ausnahme: Als sich vor mehreren Jahren eine Mitarbeiterin des Bernhard-Nocht-Instituts für Tropenmedizin mit einer kontaminierten Nadel gestochen hatte, bekam sie VSV-EBOV und zeigte eine nachweisbare Immunreaktion. Ob sie sich tatsächlich infiziert hatte, blieb unklar.
Warten oder handeln?
Sollten VSV-EBOV- oder ChAd-Vakzine zu höheren Antikörpertitern führen, wäre die logische Konsequenz, Phase-2- und später Phase-3-Studien durchzuführen. An diesem Thema scheiden sich die Geister: GSK ist in der Lage, mittelfristig etwa 10.000 Impfdosen für Phase-2-Studien produzieren, um die optimale Menge zu ermitteln. Gesundheitspolitiker würden lieber sofort mit Phase-3-Studien beginnen, um keine Zeit zu verlieren. Auch hier müsste ein Serum gegen Placebo getestet werden, was Experten teilweise als unverantwortlich kritisieren. Ira Longini von der University of Florida schlägt als Alternative vor, mit dem „Stepped-wedge-Design“ ohne Placeboarm zu arbeiten. Wissenschaftler vergleichen lediglich Probanden, die zu unterschiedlichen Zeitpunkten geimpft wurden. Ripley Ballou, Chef der Impfstoffforschung bei GSK, hätte lieber eine randomisierte Studie mit Ärzten und Pflegekräften vor Ort. Angesichts des dramatischen Anstiegs von Ebola-Fällen lehnen es einige Forscher generell ab, weitere Studien durchzuführen. Die Public Health Agency of Canada ging recht pragmatisch vor. Sie lieferte 800 Dosen ihres experimentellen Impfstoffs VSV-EBOV an den WHO-Sitz in Genf. Zusammen mit Vertretern aller betroffenen westafrikanischen Länder überlegen die obersten Gesundheitswächter jetzt, wie sie die Vakzine verteilen könnten.
http://news.doccheck.com/de/newsletter/1289/8862/?utm_source…
Weltweit befassen sich etliche Labors mit dem Thema. Die wichtigsten Ansätze: GlaxoSmithKline (GSK) untersucht zusammen mit dem amerikanischen National Institute of Allergy and Infectious Diseases, welche Potenziale in Adenoviren stecken. Biologen verwenden das chimpanzee-derived replication-defective adenovirus (ChAd), um Antigene des Ebolavirus im menschlichen Körper zu exprimieren und dem Immunsystem zu präsentieren. Momentan laufen zwei große Studien mit dem Konstrukt cAd3-EBO Z: In Großbritannien sollen 60 Probanden verschiedene Dosen des Impfstoffs erhalten. Ähnliche Untersuchungen planen Wissenschaftler in den USA mit 26 Personen. Auch das Vesicular stomatitis Indiana virus (VSIV), ein Verwandter des Tollwutvirus, hat Potenziale. Nach einer VSIV-Infektion erkranken Rinder oder Pferde schwer, während Menschen nur leichte, grippeähnliche Symptome verspüren. Ein wichtiger Vertreter ist VSV-EBOV. Molekularbiologen haben Erbinformation für das virale VSV-Glykoprotein durch die RNA für ein Ebola-Glykoprotein ausgetauscht. Unser Immunsystem reagiert, indem es Antikörper herstellt. Die Public Health Agency of Canada setzt große Erwartungen in VSV-EBOV. Zusammen mit der NewLinks Genetics Corporation hat sie eine Phase-I-Studie in die Wege geleitet.
VSV-EBOV unter der Lupe
Dazu noch einige Studien: Ratten und Meerschweinchen profitierten nachweislich vom Impfstoff. In Studien überlebten 80 bis 100 Prozent aller Tiere. Menschenaffen wurden ebenfalls zuverlässig geschützt – es kam zu einer starken Immunantwort mit hohen Serumtitern. Ein Großteil aller Primaten blieb am Leben, falls unmittelbar nach einer Virusinfektion die Impfung als Postexpositionsprophylaxe verabreicht wurde. Erhielten Tiere die Vakzine aber erst 48 Stunden nach der Exposition, starben zwei Drittel. VSV-EBOV wirkt allerdings hoch spezifisch. Ein Impfstoff mit dem Glykoprotein des Ebola-Zaire-Virus schützt nicht vor verwandten Erregern wie dem Ebola-Sudan-Virus. Forscher haben VSV-EBOV bislang nur in Tierexperimenten untersucht – mit einer Ausnahme: Als sich vor mehreren Jahren eine Mitarbeiterin des Bernhard-Nocht-Instituts für Tropenmedizin mit einer kontaminierten Nadel gestochen hatte, bekam sie VSV-EBOV und zeigte eine nachweisbare Immunreaktion. Ob sie sich tatsächlich infiziert hatte, blieb unklar.
Warten oder handeln?
Sollten VSV-EBOV- oder ChAd-Vakzine zu höheren Antikörpertitern führen, wäre die logische Konsequenz, Phase-2- und später Phase-3-Studien durchzuführen. An diesem Thema scheiden sich die Geister: GSK ist in der Lage, mittelfristig etwa 10.000 Impfdosen für Phase-2-Studien produzieren, um die optimale Menge zu ermitteln. Gesundheitspolitiker würden lieber sofort mit Phase-3-Studien beginnen, um keine Zeit zu verlieren. Auch hier müsste ein Serum gegen Placebo getestet werden, was Experten teilweise als unverantwortlich kritisieren. Ira Longini von der University of Florida schlägt als Alternative vor, mit dem „Stepped-wedge-Design“ ohne Placeboarm zu arbeiten. Wissenschaftler vergleichen lediglich Probanden, die zu unterschiedlichen Zeitpunkten geimpft wurden. Ripley Ballou, Chef der Impfstoffforschung bei GSK, hätte lieber eine randomisierte Studie mit Ärzten und Pflegekräften vor Ort. Angesichts des dramatischen Anstiegs von Ebola-Fällen lehnen es einige Forscher generell ab, weitere Studien durchzuführen. Die Public Health Agency of Canada ging recht pragmatisch vor. Sie lieferte 800 Dosen ihres experimentellen Impfstoffs VSV-EBOV an den WHO-Sitz in Genf. Zusammen mit Vertretern aller betroffenen westafrikanischen Länder überlegen die obersten Gesundheitswächter jetzt, wie sie die Vakzine verteilen könnten.
http://news.doccheck.com/de/newsletter/1289/8862/?utm_source…
NewLink Genetics-Aktie: Ebola-Killer dringend gesucht
http://www.boerse-online.de/nachrichten/aktien/NewLink-Genet…
http://www.boerse-online.de/nachrichten/aktien/NewLink-Genet…
Immune response key to beating Ebola
by Chris Defrancesco
Immune response key to beating Ebola
Antibodies in the blood, made by cells (B lymphocytes), are part of the body’s natural defense against infectious pathogens such as the Ebola virus. This microscopic rendering depicts the Ebola virus (the strands) surrounded by blood cells (the disks). Credit: Shutterstock
Of the nine Ebola virus disease patients known to have been treated in the United States, seven are now free of the disease.
By almost any measure, that's an impressive success rate for a disease that the World Health Organization says has a mortality rate of nearly 70 percent.
Although the number of patients is small, it's likely that part of the improvement in survival in the U.S. is due to the health care infrastructure that is able to provide basics such as fluid replacement and other supportive care. It is also possible that experimental treatments and plasma (blood) transfusions from Ebola survivors have been effective.
Could it mean that science is close to getting the upper hand?
"I, for one, am optimistic we'll have a vaccine for Ebola virus disease sooner rather than later," says Dr. Paul Skolnik, an infectious diseases physician who chairs the Department of Medicine and co-directs the Ebola Task Force at UConn Health. "The fact that plasma from patients who have recovered from certain viral diseases, like hepatitis B, varicella (chickenpox), and rabies, can help to treat these illnesses gives optimism that a vaccine can be made to help prevent Ebola. We're very good at making these types of vaccines."
Several patients, including Nina Pham, one of the nurses who contracted Ebola after caring for Ebola patient Eric Duncan in Dallas, Texas, were treated in this manner and were cured. Doctors at a National Institutes of Health facility in Maryland treated Pham with plasma donated from an Ebola survivor. She was discharged last week.
"The use of an immunoglobulin, or antibody, preparation to treat infectious disease is a time-honored and well-known therapy," Skolnik says. "The plasma, or blood, from a patient who's recovered from Ebola virus disease is useful because it contains antibodies to fight the virus. Simply put, these antibodies bind to the virus so it can be gobbled up by other cells. There may be other factors in plasma that are important. Based on first principles, this is why we believe it may be possible to develop a preventive vaccine for Ebola. The evidence suggests that antibodies may be helpful to treat the infection, as well as prevent it."
It's a similar concept to what makes other vaccines effective. Inactivated or molecularly produced parts of an infectious pathogen are introduced to the immune system, which triggers the body to recognize the parent virus and marshal a response to prevent it by creating antibodies against the infection. This is referred to as "active immunity," because the body must create the antibodies for the defense.
"It's often harder to develop vaccines for viruses that are kept in check mostly by the cellular arm of the immune system (infection fighting cells), Skolnik says. "This is the case, for instance, with HIV infection."
Transfusion of Ebola-survivor plasma is an example of "passive immunotherapy," he says. The donor blood already has the antibodies, because the donor's immune system already had created them.
Put another way, while a vaccine introduces the plans to build an army to fight a specific enemy, the transfusion supplies the army, already trained and ready to fight.
"The advantage of an Ebola vaccine, once we have one, is it can be mass produced and therefore mass distributed to prevent disease from occurring," Skolnik says. "Plasma therapy is used once patients are already sick and requires donor and host to have compatible blood types – there aren't a whole lot of Ebola survivors in the world to serve as donors. A vaccine would be preferable, more cost-effective, and much easier to use across broad populations as a preventative measure, rather than a therapy."
Clinical trials for two experimental Ebola vaccines are underway in the U.S. and overseas, and a third therapy, a two-vaccine regimen, is expected to start testing in January.
http://medicalxpress.com/news/2014-10-immune-response-key-eb…
by Chris Defrancesco
Immune response key to beating Ebola
Antibodies in the blood, made by cells (B lymphocytes), are part of the body’s natural defense against infectious pathogens such as the Ebola virus. This microscopic rendering depicts the Ebola virus (the strands) surrounded by blood cells (the disks). Credit: Shutterstock
Of the nine Ebola virus disease patients known to have been treated in the United States, seven are now free of the disease.
By almost any measure, that's an impressive success rate for a disease that the World Health Organization says has a mortality rate of nearly 70 percent.
Although the number of patients is small, it's likely that part of the improvement in survival in the U.S. is due to the health care infrastructure that is able to provide basics such as fluid replacement and other supportive care. It is also possible that experimental treatments and plasma (blood) transfusions from Ebola survivors have been effective.
Could it mean that science is close to getting the upper hand?
"I, for one, am optimistic we'll have a vaccine for Ebola virus disease sooner rather than later," says Dr. Paul Skolnik, an infectious diseases physician who chairs the Department of Medicine and co-directs the Ebola Task Force at UConn Health. "The fact that plasma from patients who have recovered from certain viral diseases, like hepatitis B, varicella (chickenpox), and rabies, can help to treat these illnesses gives optimism that a vaccine can be made to help prevent Ebola. We're very good at making these types of vaccines."
Several patients, including Nina Pham, one of the nurses who contracted Ebola after caring for Ebola patient Eric Duncan in Dallas, Texas, were treated in this manner and were cured. Doctors at a National Institutes of Health facility in Maryland treated Pham with plasma donated from an Ebola survivor. She was discharged last week.
"The use of an immunoglobulin, or antibody, preparation to treat infectious disease is a time-honored and well-known therapy," Skolnik says. "The plasma, or blood, from a patient who's recovered from Ebola virus disease is useful because it contains antibodies to fight the virus. Simply put, these antibodies bind to the virus so it can be gobbled up by other cells. There may be other factors in plasma that are important. Based on first principles, this is why we believe it may be possible to develop a preventive vaccine for Ebola. The evidence suggests that antibodies may be helpful to treat the infection, as well as prevent it."
It's a similar concept to what makes other vaccines effective. Inactivated or molecularly produced parts of an infectious pathogen are introduced to the immune system, which triggers the body to recognize the parent virus and marshal a response to prevent it by creating antibodies against the infection. This is referred to as "active immunity," because the body must create the antibodies for the defense.
"It's often harder to develop vaccines for viruses that are kept in check mostly by the cellular arm of the immune system (infection fighting cells), Skolnik says. "This is the case, for instance, with HIV infection."
Transfusion of Ebola-survivor plasma is an example of "passive immunotherapy," he says. The donor blood already has the antibodies, because the donor's immune system already had created them.
Put another way, while a vaccine introduces the plans to build an army to fight a specific enemy, the transfusion supplies the army, already trained and ready to fight.
"The advantage of an Ebola vaccine, once we have one, is it can be mass produced and therefore mass distributed to prevent disease from occurring," Skolnik says. "Plasma therapy is used once patients are already sick and requires donor and host to have compatible blood types – there aren't a whole lot of Ebola survivors in the world to serve as donors. A vaccine would be preferable, more cost-effective, and much easier to use across broad populations as a preventative measure, rather than a therapy."
Clinical trials for two experimental Ebola vaccines are underway in the U.S. and overseas, and a third therapy, a two-vaccine regimen, is expected to start testing in January.
http://medicalxpress.com/news/2014-10-immune-response-key-eb…
EBOLA RESPONSE ROADMAP SITUATION REPORT
31 OCTOBER 2014
http://apps.who.int/iris/bitstream/10665/137424/1/roadmapsit…
31 OCTOBER 2014
http://apps.who.int/iris/bitstream/10665/137424/1/roadmapsit…
!
Dieser Beitrag wurde vom System automatisch gesperrt. Bei Fragen wenden Sie sich bitte an feedback@wallstreet-online.de
S.Leone Ebola outbreak 'catastrophic': aid group MSF
Auszug aus dem Artikel:
"The World Health Organization (WHO) published revised figures on Friday showing 4,951 people have died of Ebola and there was a total of 13,567 reported cases.
"The WHO says there is a correction factor of 2.5, so maybe it is 2.5 times higher and maybe that is not far from the truth. It could be 10,000, 15,000 or 20,000," said Zachariah."
http://news.yahoo.com/leone-ebola-outbreak-catastrophic-aid-…
Auszug aus dem Artikel:
"The World Health Organization (WHO) published revised figures on Friday showing 4,951 people have died of Ebola and there was a total of 13,567 reported cases.
"The WHO says there is a correction factor of 2.5, so maybe it is 2.5 times higher and maybe that is not far from the truth. It could be 10,000, 15,000 or 20,000," said Zachariah."
http://news.yahoo.com/leone-ebola-outbreak-catastrophic-aid-…
Scientists try to predict number of US Ebola cases
Posted: Nov 01, 2014 3:34 PM Updated: Nov 02, 2014 4:11 AM
http://www.wtvm.com/story/27182396/scientists-try-to-predict…
Posted: Nov 01, 2014 3:34 PM Updated: Nov 02, 2014 4:11 AM
http://www.wtvm.com/story/27182396/scientists-try-to-predict…
Follow the Government Money When Picking Ebola Plays
http://blogs.barrons.com/stockstowatchtoday/2014/10/27/follo…
http://blogs.barrons.com/stockstowatchtoday/2014/10/27/follo…
Die aktuellen Erkrankungs- und Todeszahlen zeigen weiterhin einen Exponentialverlauf:
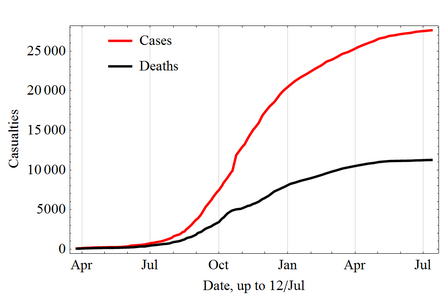
http://en.wikipedia.org/wiki/Ebola_virus_epidemic_in_West_Af…

http://en.wikipedia.org/wiki/Ebola_virus_epidemic_in_West_Af…
Bei den Neuerkrankungszahlen pro Tag zeigt sich eine dramatische Entwicklung:
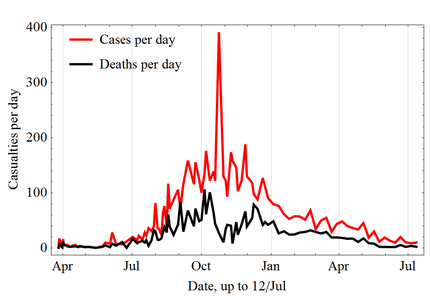
http://en.wikipedia.org/wiki/Ebola_virus_epidemic_in_West_Af…

http://en.wikipedia.org/wiki/Ebola_virus_epidemic_in_West_Af…
Ebola Vaccines May Be Paid For by Group Backed by Bill Gates
http://www.swissinfo.ch/eng/ebola-vaccines-may-be-paid-for-b…
http://www.swissinfo.ch/eng/ebola-vaccines-may-be-paid-for-b…
11/4/2014 7:56:53 AM
Government of Canada announces Ebola-related investment
WINNIPEG, Nov. 3, 2014 /CNW/ - Today, the Honourable Rona Ambrose, Minister of Health, and Dr. Gregory Taylor, Chief Public Health Officer of Canada, announced a $30.5 million investment and further actions to strengthen Canada's domestic preparedness and international response to the Ebola outbreak in West Africa.
Ebola Medical Countermeasures Research and Development
The Government of Canada is committing $23.5 million to support further research and development of Ebola medical countermeasures namely Canada's Ebola vaccine and monoclonal antibody treatments.
http://www.biospace.com/News/canadian-government-commits-30-…
Government of Canada announces Ebola-related investment
WINNIPEG, Nov. 3, 2014 /CNW/ - Today, the Honourable Rona Ambrose, Minister of Health, and Dr. Gregory Taylor, Chief Public Health Officer of Canada, announced a $30.5 million investment and further actions to strengthen Canada's domestic preparedness and international response to the Ebola outbreak in West Africa.
Ebola Medical Countermeasures Research and Development
The Government of Canada is committing $23.5 million to support further research and development of Ebola medical countermeasures namely Canada's Ebola vaccine and monoclonal antibody treatments.
http://www.biospace.com/News/canadian-government-commits-30-…
MEDIZINREPORT
Ebola: Passiert die Impfung die Zielgerade?
Dtsch Arztebl 2014; 111(45): A-1958 / B-1670 / C-1600
http://www.aerzteblatt.de/archiv/163467/Ebola-Passiert-die-I…
Ebola: Passiert die Impfung die Zielgerade?
Dtsch Arztebl 2014; 111(45): A-1958 / B-1670 / C-1600
http://www.aerzteblatt.de/archiv/163467/Ebola-Passiert-die-I…
Press Release
NewLink Genetics Corporation Reports Third Quarter 2014 Financial Results
Published: Nov 6, 2014 7:01 a.m. ET
AMES, Iowa, Nov 06, 2014 (GLOBE NEWSWIRE via COMTEX) --
NewLink Genetics Corporation NLNK, +5.89% today reported consolidated financial results for the third quarter of 2014 and progress in its clinical and business development programs.
"As we announced in August, we continue to develop the science around combining our IDO pathway product candidates with treatment modalities including other checkpoint inhibitors, chemotherapy, and our own HyperAcute immunotherapies," said Dr. Charles Link, Chairman and Chief Executive Officer. "The license and collaboration agreement with Genentech, which we announced a few weeks ago, is the most important recent step in realizing that vision, in addition to providing us with substantial new capital to develop further our HyperAcute vaccine technology."
NewLink reported a net loss of $5.6 million or $.20 per share for the third quarter of 2014 compared to a net loss of $8.1 million or $.32 per share for the comparable period in 2013.
Research and development expense in the third quarter of 2014 was $10.9 million compared to $6.1 million during the comparable period in 2013. The increase was primarily due to increases in compensation including share based compensation, as well as increases in contract research and manufacturing, clinical trial expense, and other expenses.
"We continue to look forward to seeing the second interim look on the IMPRESS trial in the first quarter of 2015," said Dr. Nicholas Vahanian, President and Chief Medical Officer of NewLink.
General and administrative expense in the third quarter of 2014 was $4.9 million compared to $2.3 million during the comparable period in 2013. The increase was primarily due to an increase in share-based compensation expense, as well as increases in consulting and legal fees, travel expense, and medical affairs and marketing. Excluding costs associated with the transition in the chief financial officer function, general and administrative expense for the quarter would have been $2.8 million, as set forth in the attached reconciliation.
NewLink ended the quarter on September 30, 2014, with cash, cash equivalents, and certificates of deposit totaling $67.7 million and, if the Hart-Scott-Rodino waiting period with respect to the Genentech collaboration expires as anticipated, expects to end the year with approximately $180 million in cash, cash equivalents and certificates of deposit.
NewLink has an additional $13.9 million of shares available for sale under its at-the-market offering (ATM) and has sold no shares under the ATM since March 31, 2014. NewLink ended the third quarter of 2014 with 27,929,874 shares outstanding.
Conference call
The company has scheduled a conference call for 8:30 AM ET today to discuss the results and to give an update on clinical and business development activities.
NewLink's senior management team will host the conference call, which will be open to all listeners. There will also be a question and answer session following the prepared remarks.
Access to the live call is available by dialing (855) 469-0612 (domestic) or (484) 756-4268 (international) five minutes prior to the start of the call. The call can also be accessed through a webcast via a link provided on the Investors and Media homepage of NewLink's website at www.linkp.com. A replay of the call will be available approximately two hours after the completion of the call and can be accessed by dialing (855) 859-2056 (domestic) or (404) 537-3406 (international) and using the passcode 25598868. The replay will be available for two weeks from the date of the call and the webcast will also be archived on the website.
About NewLink Genetics Corporation
NewLink is a biopharmaceutical company focused on discovering, developing and commercializing novel immunotherapeutic products to improve treatment options for patients with cancer. NewLink's portfolio includes biologic and small molecule immunotherapy product candidates intended to treat a wide range of oncology indications. NewLink's product candidates are designed to harness multiple components of the immune system to combat cancer without significant incremental toxicity, either as a monotherapy or in combination with other treatment regimens. For more information please visit http://www.linkp.com.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements of NewLink that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this press release are forward-looking statements, within the meaning of The Private Securities Litigation Reform Act of 1995. The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "target," "potential," "will," "could," "should," "seek," or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, statements about: NewLink's financial guidance for 2014; enrollment in its clinical trials for product candidates based on NewLink's HyperAcute® and IDO platform technologies; its timing of release of clinical data from ongoing clinical studies; its plans related to moving additional indications into clinical development; NewLink's future financial performance, results of operations, cash position and sufficiency of capital resources to fund its operating requirements; and any other statements other than statements of historical fact. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that NewLink makes due to a number of important factors, including those risks discussed in "Risk Factors" and elsewhere in NewLink's Annual Report on Form 10-K for the year ended December 31, 2013, Form S-3 Registration Statement filed December 28, 2012 and in its other filings with the Securities and Exchange Commission. The forward-looking statements in this press release represent NewLink's views as of the date of this press release. NewLink anticipates that subsequent events and developments will cause its views to change. However, while it may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. You should, therefore, not rely on these forward-looking statements as representing NewLink's views as of any date subsequent to the date of this press release.
CONTACT: Jack Henneman Chief Financial Officer 515-598-2561 Investor@linkp.com
Copyright (C) 2014 GlobeNewswire, Inc. All rights reserved.
http://www.marketwatch.com/story/newlink-genetics-corporatio…
NewLink Genetics Corporation Reports Third Quarter 2014 Financial Results
Published: Nov 6, 2014 7:01 a.m. ET
AMES, Iowa, Nov 06, 2014 (GLOBE NEWSWIRE via COMTEX) --
NewLink Genetics Corporation NLNK, +5.89% today reported consolidated financial results for the third quarter of 2014 and progress in its clinical and business development programs.
"As we announced in August, we continue to develop the science around combining our IDO pathway product candidates with treatment modalities including other checkpoint inhibitors, chemotherapy, and our own HyperAcute immunotherapies," said Dr. Charles Link, Chairman and Chief Executive Officer. "The license and collaboration agreement with Genentech, which we announced a few weeks ago, is the most important recent step in realizing that vision, in addition to providing us with substantial new capital to develop further our HyperAcute vaccine technology."
NewLink reported a net loss of $5.6 million or $.20 per share for the third quarter of 2014 compared to a net loss of $8.1 million or $.32 per share for the comparable period in 2013.
Research and development expense in the third quarter of 2014 was $10.9 million compared to $6.1 million during the comparable period in 2013. The increase was primarily due to increases in compensation including share based compensation, as well as increases in contract research and manufacturing, clinical trial expense, and other expenses.
"We continue to look forward to seeing the second interim look on the IMPRESS trial in the first quarter of 2015," said Dr. Nicholas Vahanian, President and Chief Medical Officer of NewLink.
General and administrative expense in the third quarter of 2014 was $4.9 million compared to $2.3 million during the comparable period in 2013. The increase was primarily due to an increase in share-based compensation expense, as well as increases in consulting and legal fees, travel expense, and medical affairs and marketing. Excluding costs associated with the transition in the chief financial officer function, general and administrative expense for the quarter would have been $2.8 million, as set forth in the attached reconciliation.
NewLink ended the quarter on September 30, 2014, with cash, cash equivalents, and certificates of deposit totaling $67.7 million and, if the Hart-Scott-Rodino waiting period with respect to the Genentech collaboration expires as anticipated, expects to end the year with approximately $180 million in cash, cash equivalents and certificates of deposit.
NewLink has an additional $13.9 million of shares available for sale under its at-the-market offering (ATM) and has sold no shares under the ATM since March 31, 2014. NewLink ended the third quarter of 2014 with 27,929,874 shares outstanding.
Conference call
The company has scheduled a conference call for 8:30 AM ET today to discuss the results and to give an update on clinical and business development activities.
NewLink's senior management team will host the conference call, which will be open to all listeners. There will also be a question and answer session following the prepared remarks.
Access to the live call is available by dialing (855) 469-0612 (domestic) or (484) 756-4268 (international) five minutes prior to the start of the call. The call can also be accessed through a webcast via a link provided on the Investors and Media homepage of NewLink's website at www.linkp.com. A replay of the call will be available approximately two hours after the completion of the call and can be accessed by dialing (855) 859-2056 (domestic) or (404) 537-3406 (international) and using the passcode 25598868. The replay will be available for two weeks from the date of the call and the webcast will also be archived on the website.
About NewLink Genetics Corporation
NewLink is a biopharmaceutical company focused on discovering, developing and commercializing novel immunotherapeutic products to improve treatment options for patients with cancer. NewLink's portfolio includes biologic and small molecule immunotherapy product candidates intended to treat a wide range of oncology indications. NewLink's product candidates are designed to harness multiple components of the immune system to combat cancer without significant incremental toxicity, either as a monotherapy or in combination with other treatment regimens. For more information please visit http://www.linkp.com.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements of NewLink that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this press release are forward-looking statements, within the meaning of The Private Securities Litigation Reform Act of 1995. The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "target," "potential," "will," "could," "should," "seek," or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, statements about: NewLink's financial guidance for 2014; enrollment in its clinical trials for product candidates based on NewLink's HyperAcute® and IDO platform technologies; its timing of release of clinical data from ongoing clinical studies; its plans related to moving additional indications into clinical development; NewLink's future financial performance, results of operations, cash position and sufficiency of capital resources to fund its operating requirements; and any other statements other than statements of historical fact. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that NewLink makes due to a number of important factors, including those risks discussed in "Risk Factors" and elsewhere in NewLink's Annual Report on Form 10-K for the year ended December 31, 2013, Form S-3 Registration Statement filed December 28, 2012 and in its other filings with the Securities and Exchange Commission. The forward-looking statements in this press release represent NewLink's views as of the date of this press release. NewLink anticipates that subsequent events and developments will cause its views to change. However, while it may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. You should, therefore, not rely on these forward-looking statements as representing NewLink's views as of any date subsequent to the date of this press release.
CONTACT: Jack Henneman Chief Financial Officer 515-598-2561 Investor@linkp.com
Copyright (C) 2014 GlobeNewswire, Inc. All rights reserved.
http://www.marketwatch.com/story/newlink-genetics-corporatio…
Wenn man am Ende des Jahres 180 Mio Cash und Cash-Äquivalent haben möchte, dann wird Newlink entsprechend bereits für 2014 einen Gewinn aufweisen können. Ursächlich hierfür ist die Lizensvereinbarung mit Genentech.
NewLink Genetics (NASDAQ: NLNK) reported Q3 EPS of ($0.20), $0.20 better than the analyst estimate of ($0.40). Revenue for the quarter came in at $2.8 million versus the consensus estimate of $310 thousand.
http://www.streetinsider.com/Earnings/NewLink+Genetics+%28NL…
http://www.streetinsider.com/Earnings/NewLink+Genetics+%28NL…
NewLink Genetics income loss shrinks in third quarter
http://www.desmoinesregister.com/story/money/business/2014/1…
http://www.desmoinesregister.com/story/money/business/2014/1…
NewLink Genetics beats by $0.20, beats on revenue
Nov 6 2014, 07:21 ET | About: NewLink Genetics Corporation (NLNK) | By: Mamta Mayani, SA News Editor [Contact this editor with comments or a news tip]
NewLink Genetics (NASDAQ:NLNK): Q3 EPS of -$0.20 beats by $0.20.
Revenue of $2.8M (+976.9% Y/Y) beats by $2.49M.
http://seekingalpha.com/news/2100915-newlink-genetics-beats-…
Nov 6 2014, 07:21 ET | About: NewLink Genetics Corporation (NLNK) | By: Mamta Mayani, SA News Editor [Contact this editor with comments or a news tip]
NewLink Genetics (NASDAQ:NLNK): Q3 EPS of -$0.20 beats by $0.20.
Revenue of $2.8M (+976.9% Y/Y) beats by $2.49M.
http://seekingalpha.com/news/2100915-newlink-genetics-beats-…
"The Livestock Vaccine (VSV-EBOV)- This vaccine uses a virus found in livestock, modified to include part of the gene from the Ebola virus. It's Canadian, but licensed to NewLink Genetics of Iowa, which will have up to 12 million doses of the vaccine ready by April. Human testing began in October. "Due to the sheer volume of shots that could be available quickly, this is our best hope to beat down this disease the fastest," says Dr. Stephen Shrewsbury, author ofDefy Your DNA: How the New Gene Patch Personalized Medicines Will Help You Overcome Your Greatest Health Challenges(10 Finger Press/2013)."
http://forums.digitalmedianet.com/articles/viewarticle.jsp?i…
http://forums.digitalmedianet.com/articles/viewarticle.jsp?i…
Antwort auf Beitrag Nr.: 48.247.678 von extremrelaxer am 06.11.14 19:40:3512 Millionen Impfdosen bis April, das ist eine Zahl die selbst mich überrascht!
Bei einem "Nischenprodukt" kann man m.E. einen Preis von über 100 USD pro Impfung erwarten. Damit würde Newlink bis April mehr Umsatz generieren, als das Unternehmen derzeit als Marktkapitalisierung hat!
Nur meine Meinung und keine Empfehlung, aber das lässt doch aufhorchen!
LG, ER
Bei einem "Nischenprodukt" kann man m.E. einen Preis von über 100 USD pro Impfung erwarten. Damit würde Newlink bis April mehr Umsatz generieren, als das Unternehmen derzeit als Marktkapitalisierung hat!
Nur meine Meinung und keine Empfehlung, aber das lässt doch aufhorchen!
LG, ER
"London: The Europe Union and drugmakers pledged on Thursday to invest 280 million euros ($350 million) in Ebola research, with the lion’s share going to the testing and manufacture of potential vaccines."
http://www.newsyaps.com/eu-drugmakers-pledge-350-million-for…
http://www.newsyaps.com/eu-drugmakers-pledge-350-million-for…
FIVE PRIORITY PROJECTS
With a sum bill of 3.3 billion euros for a duration 2014 to 2024, Europe’s IMI intrigue is a world’s biggest public-private partnership in life sciences.
It began in 2008 and now has 46 ongoing projects, some of that are focused on specific health issues such as Alzheimer’s disease, cancer and obesity. Others engage work on broader hurdles in drug development.
In a box of Ebola, 5 obligatory projects have been identified involving a 3 stages of vaccine clinical trials; vaccine manufacturing; vaccine ride and storage; regimens for vaccination deployment; and fast evidence tests.
http://healthmedicinet.com/eu-scheme-commits-350-million-for…
With a sum bill of 3.3 billion euros for a duration 2014 to 2024, Europe’s IMI intrigue is a world’s biggest public-private partnership in life sciences.
It began in 2008 and now has 46 ongoing projects, some of that are focused on specific health issues such as Alzheimer’s disease, cancer and obesity. Others engage work on broader hurdles in drug development.
In a box of Ebola, 5 obligatory projects have been identified involving a 3 stages of vaccine clinical trials; vaccine manufacturing; vaccine ride and storage; regimens for vaccination deployment; and fast evidence tests.
http://healthmedicinet.com/eu-scheme-commits-350-million-for…
Antwort auf Beitrag Nr.: 48.247.876 von extremrelaxer am 06.11.14 19:51:15Wenn die 3,3 Mrd. USD für Impfungen ausgegeben werden, dann dürfte Newlink einen beträchtlichen Anteil davon verbuchen.
Angesichts dessen hat Newlink eine lächerliche Marktkapitalisierung!
Angesichts dessen hat Newlink eine lächerliche Marktkapitalisierung!
Antwort auf Beitrag Nr.: 48.247.927 von extremrelaxer am 06.11.14 19:54:50Sorry, natürlich 3,3 Mrd. Euro (nicht USD)
NewLink Genetics' (NLNK) CEO Charles Link on Q3 2014 Results - Earnings Call Transcript
http://seekingalpha.com/article/2651345-newlink-genetics-nln…
http://seekingalpha.com/article/2651345-newlink-genetics-nln…
An Ebola vaccine licensed by Ames-based NewLink Genetics has been "well-tolerated" in early human trials, the company's chief executive said Thursday.
http://www.desmoinesregister.com/story/money/business/2014/1…
Das sieht gut aus, damit dürfte der kombinierten Phase-2- und Phase-3-Studie ab Dezember nichts im Wege stehen!
http://www.desmoinesregister.com/story/money/business/2014/1…
Das sieht gut aus, damit dürfte der kombinierten Phase-2- und Phase-3-Studie ab Dezember nichts im Wege stehen!
"The first dose level in healthy volunteers at Walter Reed Medical Center has been completed, and next dose level has begun. The vaccine was well-tolerated thus far, but the study is ongoing," NewLink CEO Charles Link said in a conference call with analysts Thursday.
http://www.desmoinesregister.com/story/money/business/2014/1…
http://www.desmoinesregister.com/story/money/business/2014/1…
President Obama Requests $6 Billion To Fight Ebola
http://news.silobreaker.com/president-obama-requests-6-billi…
http://news.silobreaker.com/president-obama-requests-6-billi…
NewLink CEO: Clinical trials of Ebola vaccine promising
http://amestrib.com/news/newlink-ceo-clinical-trials-ebola-v…
http://amestrib.com/news/newlink-ceo-clinical-trials-ebola-v…
UPDATE: Stifel Reiterates On NewLink Genetics On Good Risk/Reward
Dwight Einhorn, Benzinga Staff Writer
November 07, 2014 8:40 AM
Related NLNK
Mid-Morning Market Update: Markets Edge Lower; DirecTV Profit Tops Estimates
Ebola Vaccine Contenders
In a report published Friday, Stifel analyst Stephen Willey reiterated a Buy rating on NewLink Genetics Corp (NASDAQ: NLNK [FREE Stock Trend Analysis]), and raised the price target from $51.00 to $55.00.
In the report, Stifel noted, “After recently-signing one of the most economically-significant P1 oncology transactions in recent memory, investor attention has re-focused to the second P3 IMPRESS interim analysis – the results of which management now expects to communicate in 1Q15. While we've acknowledged longer-term visibility into the development/monetization of the Ebola vaccine remains limited, we believe the recent Janssen/Bavarian Nordic transaction suggests investors shouldn't ignore the optionality here – particularly given NewLink appears to be furthest ahead in the race to achieve both P1 safety/immunogenicity data and million-dose manufacturing scale. Volatility should remain high into the IMPRESS second interim analysis – but we continue to like the risk/reward here. Target increases to $55 (previously $51) on inclusion of IDO collaborative revenue.”
NewLink Genetics closed on Thursday at $34.69.
Read more: http://www.benzinga.com/analyst-ratings/analyst-color/14/11/…
http://www.benzinga.com/analyst-ratings/analyst-color/14/11/…
Dwight Einhorn, Benzinga Staff Writer
November 07, 2014 8:40 AM
Related NLNK
Mid-Morning Market Update: Markets Edge Lower; DirecTV Profit Tops Estimates
Ebola Vaccine Contenders
In a report published Friday, Stifel analyst Stephen Willey reiterated a Buy rating on NewLink Genetics Corp (NASDAQ: NLNK [FREE Stock Trend Analysis]), and raised the price target from $51.00 to $55.00.
In the report, Stifel noted, “After recently-signing one of the most economically-significant P1 oncology transactions in recent memory, investor attention has re-focused to the second P3 IMPRESS interim analysis – the results of which management now expects to communicate in 1Q15. While we've acknowledged longer-term visibility into the development/monetization of the Ebola vaccine remains limited, we believe the recent Janssen/Bavarian Nordic transaction suggests investors shouldn't ignore the optionality here – particularly given NewLink appears to be furthest ahead in the race to achieve both P1 safety/immunogenicity data and million-dose manufacturing scale. Volatility should remain high into the IMPRESS second interim analysis – but we continue to like the risk/reward here. Target increases to $55 (previously $51) on inclusion of IDO collaborative revenue.”
NewLink Genetics closed on Thursday at $34.69.
Read more: http://www.benzinga.com/analyst-ratings/analyst-color/14/11/…
http://www.benzinga.com/analyst-ratings/analyst-color/14/11/…
Deutschland
Impfstoff-Tests gegen Ebola starten
In Deutschland soll jetzt die klinische Phase-I-Prüfung des experimentellen Ebola-Impfstoffs rVSV-ZEBOV beginnen.
LANGEN. Unter Leitung von Professor Marylyn Addo vom Deutschen Zentrum für Infektionsforschung (DZIF) wird jetzt eine klinische Studie mit rVSV-ZEBOV am Uniklinikum Hamburg-Eppendorf starten, berichtet das Paul-Ehrlich-Institut (PEI) als Zulassungsbehörde.
Die Studie wird vom Bundesgesundheitsministerium finanziert. Der Impfstoff wird an gesunden Erwachsenen geprüft, darunter möglicherweise auch Personal zur Versorgung von Ebola-Kranken in Deutschland oder Afrika.
Der von der kanadischen Gesundheitsbehörde Health Canada entwickelte Impfstoff wird vom Unternehmen NewLink Genetics in Lizenz hergestellt, so das PEI in einer Mitteilung. Der Vektorimpfstoff basiert auf dem Vesikulären Stomatitis Virus (VSV) als Übertragungsvehikel.
Auf der Oberfläche trägt es das zellrezeptorbindende Ebolavirus-Protein, wogegen das Immunsystem der Geimpften Antikörper bildet. Die Antikörper sollen bei Kontakt mit Ebolaviren die Krankheit verhindern. In präklinischen Studien wurden alle damit geimpften Altweltaffen vor der Ebola-Infektion geschützt.
Ebenfalls ab nächster Woche erproben Tübinger Wissenschaftler um Professor Peter Kremsner in Lambaréné in Gabun den gleichen Impfstoff an Probanden aus Afrika. Diese Tests sind besonders wichtig, weil das Mittel möglichst schnell in den Risikogebieten Westafrikas eingesetzt werden soll. (eis/dpa)
http://www.aerztezeitung.de/news/article/872844/deutschland-…
Impfstoff-Tests gegen Ebola starten
In Deutschland soll jetzt die klinische Phase-I-Prüfung des experimentellen Ebola-Impfstoffs rVSV-ZEBOV beginnen.
LANGEN. Unter Leitung von Professor Marylyn Addo vom Deutschen Zentrum für Infektionsforschung (DZIF) wird jetzt eine klinische Studie mit rVSV-ZEBOV am Uniklinikum Hamburg-Eppendorf starten, berichtet das Paul-Ehrlich-Institut (PEI) als Zulassungsbehörde.
Die Studie wird vom Bundesgesundheitsministerium finanziert. Der Impfstoff wird an gesunden Erwachsenen geprüft, darunter möglicherweise auch Personal zur Versorgung von Ebola-Kranken in Deutschland oder Afrika.
Der von der kanadischen Gesundheitsbehörde Health Canada entwickelte Impfstoff wird vom Unternehmen NewLink Genetics in Lizenz hergestellt, so das PEI in einer Mitteilung. Der Vektorimpfstoff basiert auf dem Vesikulären Stomatitis Virus (VSV) als Übertragungsvehikel.
Auf der Oberfläche trägt es das zellrezeptorbindende Ebolavirus-Protein, wogegen das Immunsystem der Geimpften Antikörper bildet. Die Antikörper sollen bei Kontakt mit Ebolaviren die Krankheit verhindern. In präklinischen Studien wurden alle damit geimpften Altweltaffen vor der Ebola-Infektion geschützt.
Ebenfalls ab nächster Woche erproben Tübinger Wissenschaftler um Professor Peter Kremsner in Lambaréné in Gabun den gleichen Impfstoff an Probanden aus Afrika. Diese Tests sind besonders wichtig, weil das Mittel möglichst schnell in den Risikogebieten Westafrikas eingesetzt werden soll. (eis/dpa)
http://www.aerztezeitung.de/news/article/872844/deutschland-…
NewLink In Talks With Merck
NewLink Genetics Corp (NASDAQ:NLNK) and Merck & Co., Inc. (NYSE:MRK) are said to be in talks to increase production of NewLink’s experimental Ebola vaccine. The vaccine under consideration was developed by the Public Health Agency of Canada and licensed to NewLink. Accelerated human trials for the vaccine, which is still in developmental stage, started in the US last month. The vaccine’s potential role in curbing the ongoing Ebola outbreak, which has killed over 5,000 people so far, has been driving NewLink’s stock price during the past few months. The company is now looking to secure Merck’s help to scale up the vaccine’s production. NewLink’s share price was up 0.48% in after-hours trading yesterday, and is likely to gain more.
http://www.bidnessetc.com/29606-novartis-ag-drug-gets-positi…
NewLink Genetics Corp (NASDAQ:NLNK) and Merck & Co., Inc. (NYSE:MRK) are said to be in talks to increase production of NewLink’s experimental Ebola vaccine. The vaccine under consideration was developed by the Public Health Agency of Canada and licensed to NewLink. Accelerated human trials for the vaccine, which is still in developmental stage, started in the US last month. The vaccine’s potential role in curbing the ongoing Ebola outbreak, which has killed over 5,000 people so far, has been driving NewLink’s stock price during the past few months. The company is now looking to secure Merck’s help to scale up the vaccine’s production. NewLink’s share price was up 0.48% in after-hours trading yesterday, and is likely to gain more.
http://www.bidnessetc.com/29606-novartis-ag-drug-gets-positi…
Bloomberg: NewLink, Merck in Ebola vaccine production talks
November 21, 2014 | By Carly Helfand
To scale up production of its Ebola vaccine, NewLink Genetics ($NLNK) is going to need some manufacturing help. And for that, it's reportedly in talks with vaccine giant Merck ($MRK).
Ebolavirus under an electron microscope--Courtesy of CDC
Merck has production technology, dubbed Vero, that it could use to up the vaccine's manufacturing capacity, a source told Bloomberg. The technique, which the New Jersey pharma giant already uses to make rotavirus vaccine RotaTeq, uses cells from African green monkeys, the news service notes.
NewLink's prospect, known as VSV-EBOV, is currently in Phase I trials that began in October at the Walter Reed Army Institute of Research in Silver Spring, MD. And last week, Canada--which developed the vaccine before licensing it to NewLink in 2010–began a domestic trial of the vaccine at its its national microbiology laboratory. The Iowa-based company has said it could churn out 12 million doses on its own by next April, depending on the amount of vaccine needed to generate an immune response, Bloomberg says.
http://www.fiercevaccines.com/story/bloomberg-newlink-merck-…
Wenn sich NewLink jetzt an Merck wendet, um noch über die eigene Kapazität von 12 Millionen Impfdosen bis April 2015 hinaus Impfstoff zur Verfügung stellen zu können, dann erwartet das Unternehmen ein gigantischer Umsatz.
Bei einem Impfstoffpreis von 50-100 USD pro Impfung würden die 12 Millionen Impfdosen für NewLink einen Umsatz von 600 Mio bis 1,2 Mrd. USD bios April 2015 bedeuten. Das entspricht der kompletten Marktkapitalisierung von NewLink. Hinzu kommt noch der letzten Monat durchgeführte 1 Mrd. USD-Deal.
Diese Aktie ist m.E. vollkommen unterbewertet!
LG, ER
November 21, 2014 | By Carly Helfand
To scale up production of its Ebola vaccine, NewLink Genetics ($NLNK) is going to need some manufacturing help. And for that, it's reportedly in talks with vaccine giant Merck ($MRK).
Ebolavirus under an electron microscope--Courtesy of CDC
Merck has production technology, dubbed Vero, that it could use to up the vaccine's manufacturing capacity, a source told Bloomberg. The technique, which the New Jersey pharma giant already uses to make rotavirus vaccine RotaTeq, uses cells from African green monkeys, the news service notes.
NewLink's prospect, known as VSV-EBOV, is currently in Phase I trials that began in October at the Walter Reed Army Institute of Research in Silver Spring, MD. And last week, Canada--which developed the vaccine before licensing it to NewLink in 2010–began a domestic trial of the vaccine at its its national microbiology laboratory. The Iowa-based company has said it could churn out 12 million doses on its own by next April, depending on the amount of vaccine needed to generate an immune response, Bloomberg says.
http://www.fiercevaccines.com/story/bloomberg-newlink-merck-…
Wenn sich NewLink jetzt an Merck wendet, um noch über die eigene Kapazität von 12 Millionen Impfdosen bis April 2015 hinaus Impfstoff zur Verfügung stellen zu können, dann erwartet das Unternehmen ein gigantischer Umsatz.
Bei einem Impfstoffpreis von 50-100 USD pro Impfung würden die 12 Millionen Impfdosen für NewLink einen Umsatz von 600 Mio bis 1,2 Mrd. USD bios April 2015 bedeuten. Das entspricht der kompletten Marktkapitalisierung von NewLink. Hinzu kommt noch der letzten Monat durchgeführte 1 Mrd. USD-Deal.
Diese Aktie ist m.E. vollkommen unterbewertet!
LG, ER
He pointed out that the vaccine is currently in five separate clinical trials on humans and in about a 10-day period, a team of staffers, working 16 to 18-hour days, were able to get FDA approval within four days. “I didn’t even know such a thing was possible,” Link said in a statement. - See more at: http://www.biospace.com/News/merck-co-newlink-genetics-in-secret-talks-on/355742#sthash.nuaVvBmL.dpuf
http://www.biospace.com/News/merck-co-newlink-genetics-in-se…
http://www.biospace.com/News/merck-co-newlink-genetics-in-se…
Widerstandszone zwischen 35,40 und 35,50 USD durchbrochen - 2. RUN beginnt
ist schon seltsam wie wenig diese Aktie hier beachtung findet. Für mich ein toller Wert
Antwort auf Beitrag Nr.: 48.424.224 von mfierke am 26.11.14 19:50:40Finde ich auch. Ich bin vor ein paar Wochen zu 24,50 Euro eingestiegen. Danach ging es mal hoch und runter und jetzt stehen wir schon ein paar Tage recht stabil. Laut einer Mitteilung soll bis jetzt bei der Studie in Genf über den Impfstoff alles im grünen Bereich sein. Die Chancen stehen also sehr gut das sich dieser Impfstoff durchsetzt. Man muß jetzt jeden Tag mit neuen Ergebnissen rechnen 

Antwort auf Beitrag Nr.: 48.532.949 von Tableman am 10.12.14 10:08:05Hallo,
gestern nahm plötzlich das Volumen zu und der Kurs zog 10% an. Ich glaube da wissen schon manche mehr und wir werden in den kommenden Tagen gute Ergebnisse der Tests bekommen Mich wundert nur das diese Aktie in Deutschland und auch hier im Forum gar keine Beachtung findet
Mich wundert nur das diese Aktie in Deutschland und auch hier im Forum gar keine Beachtung findet
Auf eine erfolgreiche nächste Woche
gestern nahm plötzlich das Volumen zu und der Kurs zog 10% an. Ich glaube da wissen schon manche mehr und wir werden in den kommenden Tagen gute Ergebnisse der Tests bekommen
 Mich wundert nur das diese Aktie in Deutschland und auch hier im Forum gar keine Beachtung findet
Mich wundert nur das diese Aktie in Deutschland und auch hier im Forum gar keine Beachtung findet
Auf eine erfolgreiche nächste Woche

Die Aktie wird steigen - lest selbst
... One of the two vaccines being tested is a vaccine developed and tested by the NIH and pharmaceutical company GlaxoSmithKline (GSK), and the other vaccine is coming from biotech company NewLink Genetics and the pharmaceutical company Merck.
... When the trial starts, the vaccines will initially be given to 600 people to collect additional data on the vaccine’s safety. If all goes well, the second part of the trial will launch with 27,000 people.
______________
HEALTH EBOLA
Two Ebola Vaccines Are Heading to Trials in Liberia
Alexandra Sifferlin @acsifferlin Jan. 23, 2015
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, speaks during a House Energy and Commerce Committee subcommittee hearing on the U.S. public health response to the Ebola outbreak in Washington, D.C., Oct. 2014.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, speaks during a House Energy and Commerce Committee subcommittee hearing on the U.S. public health response to the Ebola outbreak in Washington, D.C., Oct. 2014.
Andrew Harrer—Bloomberg/Getty Images
Two vaccines will start trials in February
The long-awaited vaccine for Ebola is heading to clinical trials in Liberia.
Two vaccines, with the National Institutes of Health’s (NIH) support, will start efficacy testing in Liberia in the beginning of February.
The NIH is launching the trial in collaboration with the Liberian Ministry of Health. The trial will test two vaccines against a placebo. People in Liberia who agree to participate in the trial will be split evenly into three groups. Two groups will test separate vaccines and the third group will be given a placebo. The trial will take place in Montserrado County, which includes the capital Monrovia, one of the country’s hardest-hit regions.
MORE: TIME Person of the Year: Ebola Fighters
The vaccines have already undergone early safety trials at various sites in the U.S., Europe, and in parts of Africa. “There were no significant safety concerns and [the vaccine] induced the type of response that was quite comparable to the animal response of the monkeys,” says Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID). Prior trials in monkeys had shown the vaccine made the animals immune to the virus.
Initially the target date for the vaccine trial in West Africa had been end of January, but some logistics still need to be worked out. Fauci told TIME that he can say with almost certainty that the trials will indeed launch in early February. “There are a couple of minor issues that we are just ironing out with regard to the protocol with the FDA,” says Fauci. “Nothing that’s a show stopper.”
One of the two vaccines being tested is a vaccine developed and tested by the NIH and pharmaceutical company GlaxoSmithKline (GSK), and the other vaccine is coming from biotech company NewLink Genetics and the pharmaceutical company Merck.
When the trial starts, the vaccines will initially be given to 600 people to collect additional data on the vaccine’s safety. If all goes well, the second part of the trial will launch with 27,000 people.
... One of the two vaccines being tested is a vaccine developed and tested by the NIH and pharmaceutical company GlaxoSmithKline (GSK), and the other vaccine is coming from biotech company NewLink Genetics and the pharmaceutical company Merck.
... When the trial starts, the vaccines will initially be given to 600 people to collect additional data on the vaccine’s safety. If all goes well, the second part of the trial will launch with 27,000 people.
______________
HEALTH EBOLA
Two Ebola Vaccines Are Heading to Trials in Liberia
Alexandra Sifferlin @acsifferlin Jan. 23, 2015
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, speaks during a House Energy and Commerce Committee subcommittee hearing on the U.S. public health response to the Ebola outbreak in Washington, D.C., Oct. 2014.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, speaks during a House Energy and Commerce Committee subcommittee hearing on the U.S. public health response to the Ebola outbreak in Washington, D.C., Oct. 2014.
Andrew Harrer—Bloomberg/Getty Images
Two vaccines will start trials in February
The long-awaited vaccine for Ebola is heading to clinical trials in Liberia.
Two vaccines, with the National Institutes of Health’s (NIH) support, will start efficacy testing in Liberia in the beginning of February.
The NIH is launching the trial in collaboration with the Liberian Ministry of Health. The trial will test two vaccines against a placebo. People in Liberia who agree to participate in the trial will be split evenly into three groups. Two groups will test separate vaccines and the third group will be given a placebo. The trial will take place in Montserrado County, which includes the capital Monrovia, one of the country’s hardest-hit regions.
MORE: TIME Person of the Year: Ebola Fighters
The vaccines have already undergone early safety trials at various sites in the U.S., Europe, and in parts of Africa. “There were no significant safety concerns and [the vaccine] induced the type of response that was quite comparable to the animal response of the monkeys,” says Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID). Prior trials in monkeys had shown the vaccine made the animals immune to the virus.
Initially the target date for the vaccine trial in West Africa had been end of January, but some logistics still need to be worked out. Fauci told TIME that he can say with almost certainty that the trials will indeed launch in early February. “There are a couple of minor issues that we are just ironing out with regard to the protocol with the FDA,” says Fauci. “Nothing that’s a show stopper.”
One of the two vaccines being tested is a vaccine developed and tested by the NIH and pharmaceutical company GlaxoSmithKline (GSK), and the other vaccine is coming from biotech company NewLink Genetics and the pharmaceutical company Merck.
When the trial starts, the vaccines will initially be given to 600 people to collect additional data on the vaccine’s safety. If all goes well, the second part of the trial will launch with 27,000 people.
Antwort auf Beitrag Nr.: 48.875.750 von Geheimnisvoller am 24.01.15 21:23:46die Kommentare sind aber vielversprechend :
https://de.search.yahoo.com/search?p=rVSV-ZEBOV-GP+%2B+Newli…
https://de.search.yahoo.com/search?p=rVSV-ZEBOV-GP+%2B+Newli…
Neuer Ebola-Impfstoff bietet bei Versuch 100-prozentigen Schutz
PARIS (AFP)--Es ist womöglich ein entscheidender Durchbruch im Kampf gegen Ebola: Ein experimenteller Impfstoff hat bei klinischen Versuchen in Afrika einen 100-prozentigen Schutz vor der tödlichen Krankheit geboten. Damit stehe die Welt davor, mit dem Mittel VSV-ZEBOV einen "wirksamen Ebola-Impfstoff" zu bekommen, erklärte die Weltgesundheitsorganisation WHO am Freitag. "Das ist eine extrem vielversprechende Entwicklung", erklärte WHO-Chefin Margaret Chan. An dem Versuch nahmen mehr als 4000 Menschen inGuinea teil, wo die monatelange Ebola-Epidemie in Westafrika im Dezember 2013 ihren Ausgang genommen hatte.
Die Ergebnisse zu den Versuchen wurden am Freitag im Fachmagazin The Lancet veröffentlicht. Sven Trelle von der Universität Bern erklärte, der Wirkstoff biete etwa nach einer Woche einen "100-prozentigen Schutz vor Ebola". Hinter dem neuen Impfstoff stehen der US-Pharmakonzern Merck, die WHO sowie die Regierungen Kanadas, Norwegens und Guineas.
az-maja schrieb am 31.07.2015, 14:21 Uhr
news WHO: Durchbruch bei Impfstoff gegen Ebola
Fr. 31.07.2015, 13:21
Ein Impfstoff gegen die tödliche Ebola-Krankheit ist nach Angaben der Weltgesundheitsorganisation in unmittelbarer Reichweite.
Der Wirkstoff VSV-EBOV sei an Menschen in Guinea getestet worden und zeige seine hohe Wirksamkeit, teilte die WHO am Freitag in Genf mit.
„Das ist eine extrem vielversprechende Entwicklung”, erklärte die WHO-Generaldirektorin Margaret Chan. Ein effektiver Wirkstoff könne in Zukunft viele Menschenleben retten. Die WHO empfahl, den Wirkstoff VSV-EBOV der US-Unternehmen Merck 6MK (A0YD8Q) A0YD8Q 53,23-53,52
und NewLink Genetics 4NX (A1JH4X) A1JH4X 50,12-50,73
weiter zu testen.
quelle : www.bild.de
PARIS (AFP)--Es ist womöglich ein entscheidender Durchbruch im Kampf gegen Ebola: Ein experimenteller Impfstoff hat bei klinischen Versuchen in Afrika einen 100-prozentigen Schutz vor der tödlichen Krankheit geboten. Damit stehe die Welt davor, mit dem Mittel VSV-ZEBOV einen "wirksamen Ebola-Impfstoff" zu bekommen, erklärte die Weltgesundheitsorganisation WHO am Freitag. "Das ist eine extrem vielversprechende Entwicklung", erklärte WHO-Chefin Margaret Chan. An dem Versuch nahmen mehr als 4000 Menschen inGuinea teil, wo die monatelange Ebola-Epidemie in Westafrika im Dezember 2013 ihren Ausgang genommen hatte.
Die Ergebnisse zu den Versuchen wurden am Freitag im Fachmagazin The Lancet veröffentlicht. Sven Trelle von der Universität Bern erklärte, der Wirkstoff biete etwa nach einer Woche einen "100-prozentigen Schutz vor Ebola". Hinter dem neuen Impfstoff stehen der US-Pharmakonzern Merck, die WHO sowie die Regierungen Kanadas, Norwegens und Guineas.
az-maja schrieb am 31.07.2015, 14:21 Uhr
news WHO: Durchbruch bei Impfstoff gegen Ebola
Fr. 31.07.2015, 13:21
Ein Impfstoff gegen die tödliche Ebola-Krankheit ist nach Angaben der Weltgesundheitsorganisation in unmittelbarer Reichweite.
Der Wirkstoff VSV-EBOV sei an Menschen in Guinea getestet worden und zeige seine hohe Wirksamkeit, teilte die WHO am Freitag in Genf mit.
„Das ist eine extrem vielversprechende Entwicklung”, erklärte die WHO-Generaldirektorin Margaret Chan. Ein effektiver Wirkstoff könne in Zukunft viele Menschenleben retten. Die WHO empfahl, den Wirkstoff VSV-EBOV der US-Unternehmen Merck 6MK (A0YD8Q) A0YD8Q 53,23-53,52
und NewLink Genetics 4NX (A1JH4X) A1JH4X 50,12-50,73
weiter zu testen.
quelle : www.bild.de
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| 0,00 | |
| +1,04 | |
| +2,38 | |
| +1,81 | |
| -2,09 | |
| +3,65 | |
| +1,00 | |
| +2,63 | |
| +3,81 | |
| +0,74 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 210 | ||
| 94 | ||
| 77 | ||
| 66 | ||
| 55 | ||
| 36 | ||
| 34 | ||
| 30 | ||
| 30 | ||
| 29 |



















