CBMX - Combimatrix - 500 Beiträge pro Seite
eröffnet am 22.11.07 22:55:33 von
neuester Beitrag 17.08.08 11:38:53 von
neuester Beitrag 17.08.08 11:38:53 von
Beiträge: 17
ID: 1.135.572
ID: 1.135.572
Aufrufe heute: 0
Gesamt: 8.283
Gesamt: 8.283
Aktive User: 0
Top-Diskussionen
| Titel | letzter Beitrag | Aufrufe |
|---|---|---|
| 20.04.24, 12:11 | 507 | |
| vor 54 Minuten | 260 | |
| heute 00:58 | 229 | |
| gestern 22:37 | 166 | |
| gestern 22:13 | 153 | |
| heute 00:36 | 150 | |
| heute 01:03 | 137 | |
| gestern 21:35 | 132 |
Meistdiskutierte Wertpapiere
| Platz | vorher | Wertpapier | Kurs | Perf. % | Anzahl | ||
|---|---|---|---|---|---|---|---|
| 1. | 1. | 17.940,00 | +1,09 | 201 | |||
| 2. | 2. | 142,05 | -3,40 | 127 | |||
| 3. | 3. | 2.331,05 | +0,19 | 80 | |||
| 4. | 4. | 6,8920 | +3,61 | 69 | |||
| 5. | 5. | 717,02 | +0,47 | 51 | |||
| 6. | 6. | 3,7225 | -1,33 | 40 | |||
| 7. | 7. | 0,6005 | -11,04 | 37 | |||
| 8. | 8. | 2,4900 | -3,11 | 37 |
An die alten Hasen von CBMX - dann mache ich doch mal hier einen Thread auf...
Amit Kumar der CEO hat in den letzten Wochen knapp 100k $ hier reingesteckt.
Hat es was zu bedeuten?
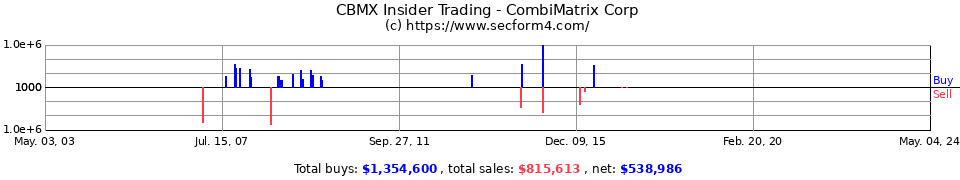
Amit Kumar der CEO hat in den letzten Wochen knapp 100k $ hier reingesteckt.
Hat es was zu bedeuten?

Vielleicht wollte er nur unterstützend eingreifen oder er hat investiert weil er von seinem Laden hoffentlich noch bessere
Zeiten erwartet.
Habe selbst 500 Stück im Depot und erwarte eigentlich auch bessere Zeiten. Seit dem Split Mitte August ging's ja erst noch in der gewohnten Richtung weiter, aber Mitte September war hoffentlich der Turnaround. In den letzten Wochen tendiert das Teil zwar seitwärst,
hat aber seit Mitte September immerhin knapp 30 Prozent zugelegt und ist trotz der Turbulenzen an den Börsen nicht wieder in den Keller abgerutscht. Ich bin momentan zwar tiefrot in der Verlustzone sehe aber langfristig Licht am Ende des Tunnels und vielleicht sehen wir ja irgendwann mal wieder so Kurse wie Ende März 2006 oder noch besser wie Ende Januar 2004.
Zeiten erwartet.
Habe selbst 500 Stück im Depot und erwarte eigentlich auch bessere Zeiten. Seit dem Split Mitte August ging's ja erst noch in der gewohnten Richtung weiter, aber Mitte September war hoffentlich der Turnaround. In den letzten Wochen tendiert das Teil zwar seitwärst,
hat aber seit Mitte September immerhin knapp 30 Prozent zugelegt und ist trotz der Turbulenzen an den Börsen nicht wieder in den Keller abgerutscht. Ich bin momentan zwar tiefrot in der Verlustzone sehe aber langfristig Licht am Ende des Tunnels und vielleicht sehen wir ja irgendwann mal wieder so Kurse wie Ende März 2006 oder noch besser wie Ende Januar 2004.
Mir geht es ähnlich, bin auch tief in den roten Zahlen und warte auf bessere Zeiten. Es schaut ja auch gar nicht ganz so schlecht aus.
Also hoffen wir weiter auf bessere Zeiten, denn jetzt auszusteigen heißt Verluste realisieren, darauf habe ich noch keinen Bock!!
Also hoffen wir weiter auf bessere Zeiten, denn jetzt auszusteigen heißt Verluste realisieren, darauf habe ich noch keinen Bock!!
CombiMatrix Molecular Diagnostics Launches Microarray Test for Detection of Autism Spectrum Disorder
Tuesday December 18, 9:00 am ET
MUKILTEO, Wash., Dec. 18, 2007 (PRIME NEWSWIRE) -- CombiMatrix Corporation (NasdaqGM:CBMX - News) announced today that its subsidiary, CombiMatrix Molecular Diagnostics (CMDX), has completed the clinical validation of the first of its ATScan(tm) suite of BAC (Bacterial Artificial Chromosome) array CGH (Comparative Genomic Hybridization) based tests. ATScan is designed to detect known genomic copy-number variations (CNVs) associated with Autism Spectrum Disorder (ASD) and this test is now available to physicians and consumers.
While there is considerable debate as to all of the possible causes of ASD, numerous studies are reporting that structural changes, and in particular CNVs of specific chromosomal regions, can be involved as predisposition factors. CMDX has incorporated this latest genomic information onto a DNA MicroArray utilizing its BAC technology. This development underscores CMDX's unique partnership with thought leaders in genomics such as Dr. Steve Scherer of The Center for Applied Genomics (TCAG) and Hospital for Sick Children in Toronto, Canada who recently published confirmatory studies for a role of the SHANK3 gene on chromosome 22q as an ASD susceptibility factor (AJHG 2007; 81: 1289-1297).
``If children analyzed by the new CMDX array are found to carry specific CNVs, they would become candidates for careful monitoring and early intervention,'' stated Dr. Mansoor Mohammed, President and CEO of CMDX. ``CMDX is committed to ensuring the latest data available that may assist in clinical categorization and diagnosis in ASD be represented on our microarrays. For example, relevant data presented at the recent American Society of Human Genetics meeting implicating a role for chromosome 16p11.2 is also already now covered by our ATscan array,'' concluded Dr. Mohammed.
ASD is one of the most frequently occurring development-related disorders in children (1 in 150 children born in the U.S. are diagnosed with ADS, as noted by the U.S. Centers for Disease Control). The American Academy of Pediatrics has recently recommended that children should be evaluated for autism at least twice before the age of two, as early intervention can impact the severity of symptoms (see http://www.reuters.com/article/healthNews/idUSN2952020920071… Typically, children are evaluated and diagnosed through somewhat subjective behavioral analyses, and objective methods to determine predisposition do not exist. ``While this test does not yet capture all of the genetic factors contributing to ASD, it does contain what we believe to be the most updated, accurate and clinically relevant information for any microarray product available today for this and other developmental disorders,'' stated Dr. Amit Kumar, President and CEO of CombiMatrix Corporation.
ABOUT COMBIMATRIX CORPORATION
CombiMatrix Corporation is a diversified biotechnology company that develops and sells proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security, as well as other potential markets where our products and services could be utilized. The technologies we have developed include methods to produce DNA arrays for use in identifying and determining the roles of genes, gene mutations and proteins. These technologies have a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology.
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com.
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory and competitive developments and general economic conditions. Our Annual Report on Form S-1, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Investor Relations
Amit Kumar
(425) 493-2000
Fax (425) 493-2010
Tuesday December 18, 9:00 am ET
MUKILTEO, Wash., Dec. 18, 2007 (PRIME NEWSWIRE) -- CombiMatrix Corporation (NasdaqGM:CBMX - News) announced today that its subsidiary, CombiMatrix Molecular Diagnostics (CMDX), has completed the clinical validation of the first of its ATScan(tm) suite of BAC (Bacterial Artificial Chromosome) array CGH (Comparative Genomic Hybridization) based tests. ATScan is designed to detect known genomic copy-number variations (CNVs) associated with Autism Spectrum Disorder (ASD) and this test is now available to physicians and consumers.
While there is considerable debate as to all of the possible causes of ASD, numerous studies are reporting that structural changes, and in particular CNVs of specific chromosomal regions, can be involved as predisposition factors. CMDX has incorporated this latest genomic information onto a DNA MicroArray utilizing its BAC technology. This development underscores CMDX's unique partnership with thought leaders in genomics such as Dr. Steve Scherer of The Center for Applied Genomics (TCAG) and Hospital for Sick Children in Toronto, Canada who recently published confirmatory studies for a role of the SHANK3 gene on chromosome 22q as an ASD susceptibility factor (AJHG 2007; 81: 1289-1297).
``If children analyzed by the new CMDX array are found to carry specific CNVs, they would become candidates for careful monitoring and early intervention,'' stated Dr. Mansoor Mohammed, President and CEO of CMDX. ``CMDX is committed to ensuring the latest data available that may assist in clinical categorization and diagnosis in ASD be represented on our microarrays. For example, relevant data presented at the recent American Society of Human Genetics meeting implicating a role for chromosome 16p11.2 is also already now covered by our ATscan array,'' concluded Dr. Mohammed.
ASD is one of the most frequently occurring development-related disorders in children (1 in 150 children born in the U.S. are diagnosed with ADS, as noted by the U.S. Centers for Disease Control). The American Academy of Pediatrics has recently recommended that children should be evaluated for autism at least twice before the age of two, as early intervention can impact the severity of symptoms (see http://www.reuters.com/article/healthNews/idUSN2952020920071… Typically, children are evaluated and diagnosed through somewhat subjective behavioral analyses, and objective methods to determine predisposition do not exist. ``While this test does not yet capture all of the genetic factors contributing to ASD, it does contain what we believe to be the most updated, accurate and clinically relevant information for any microarray product available today for this and other developmental disorders,'' stated Dr. Amit Kumar, President and CEO of CombiMatrix Corporation.
ABOUT COMBIMATRIX CORPORATION
CombiMatrix Corporation is a diversified biotechnology company that develops and sells proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security, as well as other potential markets where our products and services could be utilized. The technologies we have developed include methods to produce DNA arrays for use in identifying and determining the roles of genes, gene mutations and proteins. These technologies have a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology.
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com.
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory and competitive developments and general economic conditions. Our Annual Report on Form S-1, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Investor Relations
Amit Kumar
(425) 493-2000
Fax (425) 493-2010
Seit 3 Monaten nur noch nach oben....

Hallöchen an alle noch Investierten:
New Discoveries Confirm and Enhance CombiMatrix's Microarray Test for Autism
MUKILTEO, Wash., Jan. 17, 2008 (PRIME NEWSWIRE) -- CombiMatrix Corporation (Nasdaq:CBMX) announced that today's publication in the American Journal of Human Genetics by Dr. Steve Scherer and colleagues further implicate genomic copy number variation (CNV) in the etiology of Autism Spectrum Disorder (ASD) elevating the importance of the CBMX ATScan(tm) 1.0 microarray test. The study reports the discovery of a chromosome 16p11.2 region in one percent of ASD patients identical to that described last week in the New England Journal of Medicine (Daly et al., NEJM), as well as numerous additional previously unreported ASD-risk genes. CBMX is proud to announce that, as a function of its unparalleled ability to rapidly enhance and clinically validate its genomic arrays, these new loci exist or are being added to the ATScan(tm) 1.0 establishing the CBMX platform as the most up-to-date and comprehensive diagnostic test of its kind for ASD. Moreover, as a function of CBMX's commitment to responsible and affordable diagnostics testing, the enhanced ATScan(tm) remains priced as the most affordable comparable test in the industry. We encourage physicians and patients to contact us at 949-753-0624 for more information.
In the American Journal of Human Genetics publication, researchers at the Hospital for Sick Children (SickKids) in Toronto discovered several new genomic variations, increasing the number of identified CNV regions associated to ASD. Dr. Steve Scherer, senior scientist in Genetics & Genomic Biology at SickKids and professor of Molecular Genetics at the University of Toronto noted that, "Our finding of CNVs in seven percent of autistic children not seen in their parents or controls has important clinical implications. Our data indicates that the application of microarrays can have great utility in clinical assessment of undetected syndromes underlying ASD, making it crucial to rapidly translate our basic research discoveries into validated clinical tests available to families," he said.
Dr. Mansoor Mohammed, President and CEO of CombiMatrix Molecular Diagnostics noted that, "We have dedicated considerable effort in mining the literature and leveraging our partnerships to move significant evidence-based research into quality-assured clinical diagnostics. These recent discoveries validate our business plan to establish ourselves as the preeminent provider of genome-based diagnostics. Not only am I proud to see the fruition of our efforts in the rapid development and deployment of our ATScan(tm) test, but also of the exciting pipeline of products we are currently developing and our commitment to their clinical relevance and affordability."
The following are links to the American Journal of Human Genetics article referenced above, as well as the press release from the Hospital for Sick Children (SickKids): http://www.ajhg.org/AJHG/latestarticles; http://www.sickkids.ca/mediaroom/custom/structuralchanges08
New Discoveries Confirm and Enhance CombiMatrix's Microarray Test for Autism
MUKILTEO, Wash., Jan. 17, 2008 (PRIME NEWSWIRE) -- CombiMatrix Corporation (Nasdaq:CBMX) announced that today's publication in the American Journal of Human Genetics by Dr. Steve Scherer and colleagues further implicate genomic copy number variation (CNV) in the etiology of Autism Spectrum Disorder (ASD) elevating the importance of the CBMX ATScan(tm) 1.0 microarray test. The study reports the discovery of a chromosome 16p11.2 region in one percent of ASD patients identical to that described last week in the New England Journal of Medicine (Daly et al., NEJM), as well as numerous additional previously unreported ASD-risk genes. CBMX is proud to announce that, as a function of its unparalleled ability to rapidly enhance and clinically validate its genomic arrays, these new loci exist or are being added to the ATScan(tm) 1.0 establishing the CBMX platform as the most up-to-date and comprehensive diagnostic test of its kind for ASD. Moreover, as a function of CBMX's commitment to responsible and affordable diagnostics testing, the enhanced ATScan(tm) remains priced as the most affordable comparable test in the industry. We encourage physicians and patients to contact us at 949-753-0624 for more information.
In the American Journal of Human Genetics publication, researchers at the Hospital for Sick Children (SickKids) in Toronto discovered several new genomic variations, increasing the number of identified CNV regions associated to ASD. Dr. Steve Scherer, senior scientist in Genetics & Genomic Biology at SickKids and professor of Molecular Genetics at the University of Toronto noted that, "Our finding of CNVs in seven percent of autistic children not seen in their parents or controls has important clinical implications. Our data indicates that the application of microarrays can have great utility in clinical assessment of undetected syndromes underlying ASD, making it crucial to rapidly translate our basic research discoveries into validated clinical tests available to families," he said.
Dr. Mansoor Mohammed, President and CEO of CombiMatrix Molecular Diagnostics noted that, "We have dedicated considerable effort in mining the literature and leveraging our partnerships to move significant evidence-based research into quality-assured clinical diagnostics. These recent discoveries validate our business plan to establish ourselves as the preeminent provider of genome-based diagnostics. Not only am I proud to see the fruition of our efforts in the rapid development and deployment of our ATScan(tm) test, but also of the exciting pipeline of products we are currently developing and our commitment to their clinical relevance and affordability."
The following are links to the American Journal of Human Genetics article referenced above, as well as the press release from the Hospital for Sick Children (SickKids): http://www.ajhg.org/AJHG/latestarticles; http://www.sickkids.ca/mediaroom/custom/structuralchanges08
Daily Cancer News
Advertisement
HemeScan™ Evaluates Prognosis in Chronic Lymphocytic Leukemia
A genetic test developed by CombiMatrix will soon be available to determine prognosis for chronic lymphocytic leukemia (CLL). The test, known as HemeScan™, has proven useful in the management of hematological malignancies such as leukemia and lymphoma.
Chronic lymphocytic leukemia is the most common form of adult leukemia. The American Cancer Society estimated that approximately 8,000 people would be diagnosed with CLL in 2007. Currently, there are approximately 60,000 people in the United States living with CLL.
Chronic lymphocytic leukemia is characterized by the production of atypical lymphocytes. Lymphocytes are specialized immune cells that exist in two forms: B- and T-cells. These cells are produced in the bone marrow and each serves a specific function in helping the body fight infection.
Disease characteristics and outcomes for CLL vary greatly from patient to patient. In order to determine the best course of treatment for each patient, researchers are evaluating ways to more accurately predict individual outcomes. Genetic evaluation is one possible way to better understand certain disease characteristics and to then individualize treatment plans accordingly. A number of genes have already been identified that play a part in the development of certain types of cancer. Genetic testing analyzes the patient’s genetic code to look for any genetic alterations that may indicate an increased risk for developing a specific disease. Genetic testing may also be helpful in evaluating prognosis for certain types of cancer.
In addition to using genetic testing to predict disease prognosis, recent studies have shown that identification of specific abnormal chromosomes allow patients to be categorized by levels of prognostic risk based on an International Prognostic Scoring System (IPSS).The IPSS score stratifies the patient into “good”, “intermediate”, and “poor” risk categories based on the patient’s genetic test results. HemeScan integrates these results to provide important information for treatment planning and patient management.
HemeScan has proven to be a useful and reliable test for evaluating prognosis and determining treatment for CLL and other cancers resulting from chromosomal abnormalities (errors in the genetic code that cause cells to grow and reproduce abnormally). The HemeScan test uses fluid samples removed from the bone marrow to provide an overview of all the genetic abnormalities, as well as identify established genetic markers that may be helpful in predicting the disease’s clinical course. At this point researchers have validated the use of HemeScan for CLL, as well as acute lymphoblastic leukemia and myelodysplastic syndrome. Current testing is underway for use of HemeScan in multiple myeloma.
HemeScan was developed in collaboration with several academic centers including M.D. Anderson Cancer Center in Houston, The University of Texas Health Science Center in San Antonio, and the Netherlands Cancer Institute in Amsterdam.
Reference: CombiMatrix. CombiMatrix Will Collaborate with Clarient to Market, Sell Novel Genomics-Based Test for Chronic Lymphocytic Leukemia. Available at http://investor.combimatrix.com/releasedetail.cfm?ReleaseID=… January 31, 2008.
Copyright Leukemia Information Center on CancerConsultants.com
Advertisement
HemeScan™ Evaluates Prognosis in Chronic Lymphocytic Leukemia
A genetic test developed by CombiMatrix will soon be available to determine prognosis for chronic lymphocytic leukemia (CLL). The test, known as HemeScan™, has proven useful in the management of hematological malignancies such as leukemia and lymphoma.
Chronic lymphocytic leukemia is the most common form of adult leukemia. The American Cancer Society estimated that approximately 8,000 people would be diagnosed with CLL in 2007. Currently, there are approximately 60,000 people in the United States living with CLL.
Chronic lymphocytic leukemia is characterized by the production of atypical lymphocytes. Lymphocytes are specialized immune cells that exist in two forms: B- and T-cells. These cells are produced in the bone marrow and each serves a specific function in helping the body fight infection.
Disease characteristics and outcomes for CLL vary greatly from patient to patient. In order to determine the best course of treatment for each patient, researchers are evaluating ways to more accurately predict individual outcomes. Genetic evaluation is one possible way to better understand certain disease characteristics and to then individualize treatment plans accordingly. A number of genes have already been identified that play a part in the development of certain types of cancer. Genetic testing analyzes the patient’s genetic code to look for any genetic alterations that may indicate an increased risk for developing a specific disease. Genetic testing may also be helpful in evaluating prognosis for certain types of cancer.
In addition to using genetic testing to predict disease prognosis, recent studies have shown that identification of specific abnormal chromosomes allow patients to be categorized by levels of prognostic risk based on an International Prognostic Scoring System (IPSS).The IPSS score stratifies the patient into “good”, “intermediate”, and “poor” risk categories based on the patient’s genetic test results. HemeScan integrates these results to provide important information for treatment planning and patient management.
HemeScan has proven to be a useful and reliable test for evaluating prognosis and determining treatment for CLL and other cancers resulting from chromosomal abnormalities (errors in the genetic code that cause cells to grow and reproduce abnormally). The HemeScan test uses fluid samples removed from the bone marrow to provide an overview of all the genetic abnormalities, as well as identify established genetic markers that may be helpful in predicting the disease’s clinical course. At this point researchers have validated the use of HemeScan for CLL, as well as acute lymphoblastic leukemia and myelodysplastic syndrome. Current testing is underway for use of HemeScan in multiple myeloma.
HemeScan was developed in collaboration with several academic centers including M.D. Anderson Cancer Center in Houston, The University of Texas Health Science Center in San Antonio, and the Netherlands Cancer Institute in Amsterdam.
Reference: CombiMatrix. CombiMatrix Will Collaborate with Clarient to Market, Sell Novel Genomics-Based Test for Chronic Lymphocytic Leukemia. Available at http://investor.combimatrix.com/releasedetail.cfm?ReleaseID=… January 31, 2008.
Copyright Leukemia Information Center on CancerConsultants.com
Federal Court Awards CombiMatrix Corporation $31.4 Million in Lawsuit Against National Union Fire Ins. Co.
Wednesday February 13, 9:00 am ET
MUKILTEO, Wash., Feb. 13, 2008 (PRIME NEWSWIRE) -- The United States District Court for the Central District of California has issued a ruling in favor of CombiMatrix Corporation (NasdaqGM:CBMX - News) and its former parent company. The Court awarded CombiMatrix and its former parent company $31,381,474 in monetary damages, to be paid by their insurance carrier, National Union Fire Ins. Co. of Pittsburgh, PA, which had refused to defend and indemnify CombiMatrix under its director and officer's insurance policy with National Union. In accordance with an agreement between CombiMatrix and its former parent company, all proceeds from the lawsuit will be paid to CombiMatrix.
CombiMatrix and its former parent company were represented in the lawsuit by Richard B. Specter of Corbett, Steelman & Specter in Irvine, California. CombiMatrix intends to file a request for attorneys' fees in the next 30 days and a final judgment is expected to be entered by the U.S. District Court at a later date.
ABOUT COMBIMATRIX CORPORATION
CombiMatrix Corporation is a diversified biotechnology company that develops and sells proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security, as well as other potential markets where our products and services could be utilized. The technologies we have developed include methods to produce DNA arrays for use in identifying and determining the roles of genes, gene mutations and proteins. These technologies have a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology.
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com.
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory and competitive developments and general economic conditions. Our Annual Report, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Investor Relations
Amit Kumar
(425) 493-2000
Fax: (425) 493-2010
Wednesday February 13, 9:00 am ET
MUKILTEO, Wash., Feb. 13, 2008 (PRIME NEWSWIRE) -- The United States District Court for the Central District of California has issued a ruling in favor of CombiMatrix Corporation (NasdaqGM:CBMX - News) and its former parent company. The Court awarded CombiMatrix and its former parent company $31,381,474 in monetary damages, to be paid by their insurance carrier, National Union Fire Ins. Co. of Pittsburgh, PA, which had refused to defend and indemnify CombiMatrix under its director and officer's insurance policy with National Union. In accordance with an agreement between CombiMatrix and its former parent company, all proceeds from the lawsuit will be paid to CombiMatrix.
CombiMatrix and its former parent company were represented in the lawsuit by Richard B. Specter of Corbett, Steelman & Specter in Irvine, California. CombiMatrix intends to file a request for attorneys' fees in the next 30 days and a final judgment is expected to be entered by the U.S. District Court at a later date.
ABOUT COMBIMATRIX CORPORATION
CombiMatrix Corporation is a diversified biotechnology company that develops and sells proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security, as well as other potential markets where our products and services could be utilized. The technologies we have developed include methods to produce DNA arrays for use in identifying and determining the roles of genes, gene mutations and proteins. These technologies have a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology.
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com.
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory and competitive developments and general economic conditions. Our Annual Report, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Investor Relations
Amit Kumar
(425) 493-2000
Fax: (425) 493-2010
Antwort auf Beitrag Nr.: 33.355.138 von DagobertDuck57 am 13.02.08 15:18:59Man sollte noch darauf hinweisen, dass der Laden derzeit ca. 60 Mio $ wert ist!!!
Genetic Diagnostics Expert, Dr. Kavita Reddy, Joins CombiMatrix as Laboratory Director
Friday February 15, 8:00 am ET
MUKILTEO, Wash., Feb. 15, 2008 (PRIME NEWSWIRE) -- CombiMatrix Corporation (NasdaqGM:CBMX - News) announced today that industry veteran, Dr. Kavita Reddy, has joined CombiMatrix Molecular Diagnostics (CMDX) as its Laboratory Director. Dr. Reddy is a Diplomat of the American Board of Medical Genetics in Clinical Molecular Genetics and Clinical Cytogenetics and is also licensed in New York and California in both cytogenetics and molecular genetics.
``I have served in a directorship role for several of the industry's largest clinical diagnostics providers, including most recently, as the Senior Director of Genzyme Genetics Manhattan,'' said Dr. Reddy. ``Throughout the course of my career, I have paid particular attention to cutting-edge methodologies within the sphere of medical genetics, and none have held my fascination as much as genome-based microarray technologies. Those who are well-informed have recognized that the latter is transforming how we view medical diagnostics. I am convinced that the robust and innovative genome microarray platforms and diverse test menu of CMDX is perfectly positioned to lead the industry in this rapidly growing market segment,'' concluded Dr. Reddy.
``I am delighted that Dr. Reddy has joined our company,'' said Dr. Mansoor Mohammed, President and Chief Executive Officer of CMDX. ``Dr. Reddy has published numerous peer reviewed articles across a broad spectrum of genetic diagnostic topics, and she is a primary reference in the genetic diagnosis of hematological malignancies. Our ability to attract such high caliber talent is a potent endorsement of our credibility and business model. I look forward to the additive expertise of Dr. Reddy as we grow our business, particularly as we add to our innovative menu of genomics-based oncology tests,'' concluded Dr. Mohammed.
Dr. Reddy earned her Ph.D. from the Bangalore University, India, and was then awarded a Welcome Trust Postdoctoral fellowship to the prestigious MRC Radiobiology Unit of the United Kingdom. Dr. Reddy joins CMDX from Genzyme Genetics Manhattan where she was Senior Director. Dr. Reddy has also been Associate Director and Scientific Director with Quest Diagnostics Inc. at the Nichols Institute and has been a consultant with the City of Hope Cancer Center and US Labs (now Laboratory Corporation of America). She has been the recipient of the Achievement of Excellence award from Quest Diagnostics as well as the Young Scientist award from the Indian Society of Human Genetics and has published numerous peer reviewed articles in the field of molecular diagnostics.
ABOUT COMBIMATRIX CORPORATION
CombiMatrix Corporation is a diversified biotechnology business, having its focus on the development of proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where our products and services could be utilized. The technologies we have developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs. CombiMatrix Molecular Diagnostics, Inc. (``CMDX''), a wholly owned subsidiary of the Company located in Irvine, California, has developed capabilities of producing arrays that utilize bacterial artificial chromosomes, which also enable genetic analysis.
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com or call toll free: 1-800-710-0624.
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory, and competitive developments, and general economic conditions. Our Annual Report, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations, and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Investor Relations
Amit Kumar
(425) 493-2000
Fax: (425) 493-2010
Friday February 15, 8:00 am ET
MUKILTEO, Wash., Feb. 15, 2008 (PRIME NEWSWIRE) -- CombiMatrix Corporation (NasdaqGM:CBMX - News) announced today that industry veteran, Dr. Kavita Reddy, has joined CombiMatrix Molecular Diagnostics (CMDX) as its Laboratory Director. Dr. Reddy is a Diplomat of the American Board of Medical Genetics in Clinical Molecular Genetics and Clinical Cytogenetics and is also licensed in New York and California in both cytogenetics and molecular genetics.
``I have served in a directorship role for several of the industry's largest clinical diagnostics providers, including most recently, as the Senior Director of Genzyme Genetics Manhattan,'' said Dr. Reddy. ``Throughout the course of my career, I have paid particular attention to cutting-edge methodologies within the sphere of medical genetics, and none have held my fascination as much as genome-based microarray technologies. Those who are well-informed have recognized that the latter is transforming how we view medical diagnostics. I am convinced that the robust and innovative genome microarray platforms and diverse test menu of CMDX is perfectly positioned to lead the industry in this rapidly growing market segment,'' concluded Dr. Reddy.
``I am delighted that Dr. Reddy has joined our company,'' said Dr. Mansoor Mohammed, President and Chief Executive Officer of CMDX. ``Dr. Reddy has published numerous peer reviewed articles across a broad spectrum of genetic diagnostic topics, and she is a primary reference in the genetic diagnosis of hematological malignancies. Our ability to attract such high caliber talent is a potent endorsement of our credibility and business model. I look forward to the additive expertise of Dr. Reddy as we grow our business, particularly as we add to our innovative menu of genomics-based oncology tests,'' concluded Dr. Mohammed.
Dr. Reddy earned her Ph.D. from the Bangalore University, India, and was then awarded a Welcome Trust Postdoctoral fellowship to the prestigious MRC Radiobiology Unit of the United Kingdom. Dr. Reddy joins CMDX from Genzyme Genetics Manhattan where she was Senior Director. Dr. Reddy has also been Associate Director and Scientific Director with Quest Diagnostics Inc. at the Nichols Institute and has been a consultant with the City of Hope Cancer Center and US Labs (now Laboratory Corporation of America). She has been the recipient of the Achievement of Excellence award from Quest Diagnostics as well as the Young Scientist award from the Indian Society of Human Genetics and has published numerous peer reviewed articles in the field of molecular diagnostics.
ABOUT COMBIMATRIX CORPORATION
CombiMatrix Corporation is a diversified biotechnology business, having its focus on the development of proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where our products and services could be utilized. The technologies we have developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs. CombiMatrix Molecular Diagnostics, Inc. (``CMDX''), a wholly owned subsidiary of the Company located in Irvine, California, has developed capabilities of producing arrays that utilize bacterial artificial chromosomes, which also enable genetic analysis.
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com or call toll free: 1-800-710-0624.
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory, and competitive developments, and general economic conditions. Our Annual Report, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations, and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Investor Relations
Amit Kumar
(425) 493-2000
Fax: (425) 493-2010
Antwort auf Beitrag Nr.: 33.381.381 von DagobertDuck57 am 15.02.08 14:37:11Frisches Geld bring gute Leute... Die Wachstumsstory stimmt... 

CombiMatrix Molecular Diagnostics Announces the Validation and Launch of the HerScan Test for Assessment of Newly Diagnosed Breast Cancer
Tuesday February 26, 6:00 am ET
The HerScan Test is the First Array-based Diagnostic to Comprehensively Analyze HER2 Gene Status and Patients' Tumor Genome
MUKILTEO, Wash., Feb. 26, 2008 (PRIME NEWSWIRE) -- CombiMatrix Corporation (NasdaqGM:CBMX - News) announced today that its subsidiary, CombiMatrix Molecular Diagnostics (CMDX), has completed clinical validation and has commercially launched the HerScan(tm) test, the first Bacterial Artificial Chromosome (BAC) Comparative Genomic Hybridization (CGH) array-based test for breast cancer. The HerScan test is designed to detect amplification of the HER2 gene in early breast cancer while simultaneously giving clinicians a complete profile of a patient's tumor genome.
``The HerScan test is the first diagnostic, in our portfolio of array-based diagnostic offerings, for a solid tumor,'' stated Dr. Amit Kumar, President and CEO of CombiMatrix Corporation. ``The flexibility of our BAC Array Platform is underscored by the rapid release of this product, our previous releases, and additional diagnostic products in our pipeline. We look forward to the wide acceptance of the HerScan test for breast cancer testing and the benefit we expect it will have for patients.''
``CMDX's validation and launch of the HerScan test, underscores my decision to join this incredibly innovative company,'' stated Dr. Kavita Reddy, Clinical Laboratory Director of CMDX and former Senior Director of Genzyme Genetics. ``Through CMDX, I am convinced that my desire to play a role in positively impacting the future of medical diagnostics can be best realized.''
The College of American Pathologists (CAP) and American Society of Clinical Oncology (ASCO) have recently recommended that HER2 status be determined in all invasive breast cancers as a prognostic marker for which patients are most likely to benefit from the breast cancer drug trastuzumab (Herceptin(r)). However, it is estimated that approximately 20 per cent of HER2 testing currently being performed by the two available methods, immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH), may be inaccurate. Although new CAP and ASCO guidelines have been issued to address inconsistencies arising from the inherent subjectivity of FISH and IHC techniques, problems with these existing methods remain unresolved (Journal of National Cancer Institute 99:1064-1065; 2007). With the HerScan test, HER2 gene copy number is objectively analyzed for the determination of amplification status (amplification, gain, no change, or loss) at the genomic level. Moreover, since the HerScan test simultaneously determines the copy number status of chromosome 17 in its entirety, differentiation of locus specific amplification versus ploidy gains is possible. In addition, the HerScan test comprehensively monitors the copy number status of loci across the entire genome. For example, also provided in this one test, is information about genomic stability and the copy number status of additional genes of prognostic significance, including TP53, c-MYC and TOP2A.
Breast cancer is the most common malignancy in women in the United States, which has over 200,000 new cases diagnosed every year. Approximately 20 per cent of newly diagnosed breast cancer cases have extra copies of the HER2 gene on chromosome 17 (called HER2-positive), and thus these patients have a concomitant poor prognosis due to the aggressive disease characteristics conferred by the extra dosage of the HER2 gene product. HER2-positive tumors are likely to respond to the Herceptin drug because it is designed to target the product of the HER2 oncogene. Conversely, it is important to accurately identify patients with normal copy number or loss of the HER2 gene (HER2-negative) because, for these patients, the risks of side effects, including cardiotoxicity, are greater than any potential benefit of the drug.
The HerScan test is designed to give pathologists and oncologists an objective measure of HER2 gene copy number with simultaneous analysis of the entire tumor genome. As a function of the enumeration of the HER2 gene through the HerScan test, patients are assigned to one of four categories of HER2 gene status: Amplification, Gain, Normal, or Loss. As part of the validation of the HerScan test, clinical testing was completed on over 100 cases of invasive ductal and invasive lobular carcinoma with known IHC and/or FISH results. The HerScan test accurately and reproducibly determined HER2 status, and in addition it clearly revealed the genomic subtypes previously reported in Cancer Research 64: 8541-8549; 2004. These subtypes include tumors showing gain of chromosome 1q; loss of chromosome 16q; amplification of the c-MYC oncogene on chromosome 8; and loss of the tumor suppressor gene P53 on chromosome 17. These additional markers, which are not simultaneously available by IHC or FISH, provide additional relevant information, enabling the clinician to make better patient management decisions and recommendations.
``The HerScan test is designed to give clinicians and their patients a precise determination of the status of the HER2 gene so that appropriate treatment decisions can be made based on the presence or absence of this important prognostic marker,'' said Dr. Shelly Gunn, Medical Director of CMDX. ``Since this is an array-based test, it also provides clinicians additional information on the genomic instability of the tumor enabling greater prognostic information,'' concluded Dr. Gunn.
``We are pleased with the performance of the HerScan test in our series of validation cases,'' said Dr. Mansoor Mohammed, President and CEO of CMDX. ``While array CGH tests were first introduced to the clinical community for the diagnosis of developmental disorders and are now an indispensable tool in that arena, it is noteworthy that array CGH was historically first developed with cancers such as breast cancer in mind. We are very proud to be the first to deliver this transformatory test to the clinical community, and we look forward to adding additional solid tumor oncology tests in the near future as we continue to leverage the information gleaned from the complete sequencing of the human genome through our partnership with the world renowned Centre for Applied Genomics in Toronto,'' concluded Dr. Mohammed.
ABOUT COMBIMATRIX CORPORATION
CombiMatrix Corporation is a diversified biotechnology business having its focus on the development of proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where our products and services could be utilized. The technologies we have developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs. CombiMatrix Molecular Diagnostics, Inc. (``CMDX''), a wholly owned subsidiary of the Company located in Irvine, California, has developed capabilities of producing arrays that utilize bacterial artificial chromosomes, which also enable genetic analysis.
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com or call toll free: 1-800-710-0624.
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory, and competitive developments, and general economic conditions. Our Annual Report, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations, and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Amit Kumar, Ph.D., President & CEO
(425) 493-2000
Fax: (425) 493-2010
Tuesday February 26, 6:00 am ET
The HerScan Test is the First Array-based Diagnostic to Comprehensively Analyze HER2 Gene Status and Patients' Tumor Genome
MUKILTEO, Wash., Feb. 26, 2008 (PRIME NEWSWIRE) -- CombiMatrix Corporation (NasdaqGM:CBMX - News) announced today that its subsidiary, CombiMatrix Molecular Diagnostics (CMDX), has completed clinical validation and has commercially launched the HerScan(tm) test, the first Bacterial Artificial Chromosome (BAC) Comparative Genomic Hybridization (CGH) array-based test for breast cancer. The HerScan test is designed to detect amplification of the HER2 gene in early breast cancer while simultaneously giving clinicians a complete profile of a patient's tumor genome.
``The HerScan test is the first diagnostic, in our portfolio of array-based diagnostic offerings, for a solid tumor,'' stated Dr. Amit Kumar, President and CEO of CombiMatrix Corporation. ``The flexibility of our BAC Array Platform is underscored by the rapid release of this product, our previous releases, and additional diagnostic products in our pipeline. We look forward to the wide acceptance of the HerScan test for breast cancer testing and the benefit we expect it will have for patients.''
``CMDX's validation and launch of the HerScan test, underscores my decision to join this incredibly innovative company,'' stated Dr. Kavita Reddy, Clinical Laboratory Director of CMDX and former Senior Director of Genzyme Genetics. ``Through CMDX, I am convinced that my desire to play a role in positively impacting the future of medical diagnostics can be best realized.''
The College of American Pathologists (CAP) and American Society of Clinical Oncology (ASCO) have recently recommended that HER2 status be determined in all invasive breast cancers as a prognostic marker for which patients are most likely to benefit from the breast cancer drug trastuzumab (Herceptin(r)). However, it is estimated that approximately 20 per cent of HER2 testing currently being performed by the two available methods, immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH), may be inaccurate. Although new CAP and ASCO guidelines have been issued to address inconsistencies arising from the inherent subjectivity of FISH and IHC techniques, problems with these existing methods remain unresolved (Journal of National Cancer Institute 99:1064-1065; 2007). With the HerScan test, HER2 gene copy number is objectively analyzed for the determination of amplification status (amplification, gain, no change, or loss) at the genomic level. Moreover, since the HerScan test simultaneously determines the copy number status of chromosome 17 in its entirety, differentiation of locus specific amplification versus ploidy gains is possible. In addition, the HerScan test comprehensively monitors the copy number status of loci across the entire genome. For example, also provided in this one test, is information about genomic stability and the copy number status of additional genes of prognostic significance, including TP53, c-MYC and TOP2A.
Breast cancer is the most common malignancy in women in the United States, which has over 200,000 new cases diagnosed every year. Approximately 20 per cent of newly diagnosed breast cancer cases have extra copies of the HER2 gene on chromosome 17 (called HER2-positive), and thus these patients have a concomitant poor prognosis due to the aggressive disease characteristics conferred by the extra dosage of the HER2 gene product. HER2-positive tumors are likely to respond to the Herceptin drug because it is designed to target the product of the HER2 oncogene. Conversely, it is important to accurately identify patients with normal copy number or loss of the HER2 gene (HER2-negative) because, for these patients, the risks of side effects, including cardiotoxicity, are greater than any potential benefit of the drug.
The HerScan test is designed to give pathologists and oncologists an objective measure of HER2 gene copy number with simultaneous analysis of the entire tumor genome. As a function of the enumeration of the HER2 gene through the HerScan test, patients are assigned to one of four categories of HER2 gene status: Amplification, Gain, Normal, or Loss. As part of the validation of the HerScan test, clinical testing was completed on over 100 cases of invasive ductal and invasive lobular carcinoma with known IHC and/or FISH results. The HerScan test accurately and reproducibly determined HER2 status, and in addition it clearly revealed the genomic subtypes previously reported in Cancer Research 64: 8541-8549; 2004. These subtypes include tumors showing gain of chromosome 1q; loss of chromosome 16q; amplification of the c-MYC oncogene on chromosome 8; and loss of the tumor suppressor gene P53 on chromosome 17. These additional markers, which are not simultaneously available by IHC or FISH, provide additional relevant information, enabling the clinician to make better patient management decisions and recommendations.
``The HerScan test is designed to give clinicians and their patients a precise determination of the status of the HER2 gene so that appropriate treatment decisions can be made based on the presence or absence of this important prognostic marker,'' said Dr. Shelly Gunn, Medical Director of CMDX. ``Since this is an array-based test, it also provides clinicians additional information on the genomic instability of the tumor enabling greater prognostic information,'' concluded Dr. Gunn.
``We are pleased with the performance of the HerScan test in our series of validation cases,'' said Dr. Mansoor Mohammed, President and CEO of CMDX. ``While array CGH tests were first introduced to the clinical community for the diagnosis of developmental disorders and are now an indispensable tool in that arena, it is noteworthy that array CGH was historically first developed with cancers such as breast cancer in mind. We are very proud to be the first to deliver this transformatory test to the clinical community, and we look forward to adding additional solid tumor oncology tests in the near future as we continue to leverage the information gleaned from the complete sequencing of the human genome through our partnership with the world renowned Centre for Applied Genomics in Toronto,'' concluded Dr. Mohammed.
ABOUT COMBIMATRIX CORPORATION
CombiMatrix Corporation is a diversified biotechnology business having its focus on the development of proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where our products and services could be utilized. The technologies we have developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs. CombiMatrix Molecular Diagnostics, Inc. (``CMDX''), a wholly owned subsidiary of the Company located in Irvine, California, has developed capabilities of producing arrays that utilize bacterial artificial chromosomes, which also enable genetic analysis.
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com or call toll free: 1-800-710-0624.
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory, and competitive developments, and general economic conditions. Our Annual Report, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations, and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Amit Kumar, Ph.D., President & CEO
(425) 493-2000
Fax: (425) 493-2010
Noch einmal 3,6 Milliönchen zusätzlich 

Federal Court Increases Award in Favor of CombiMatrix to $35.7 Million
Thursday April 24, 6:00 am ET
MUKILTEO, Wash., April 24, 2008 (PRIME NEWSWIRE) -- The United States District Court for the Central District of California increased the award previously issued in favor of CombiMatrix Corporation (NasdaqGM:CBMX - News). The Court originally awarded $32.1 million to CombiMatrix to be paid by National Union Fire Ins. Co. of Pittsburgh, PA, a member company of American International Group (AIG). On April 23, 2008, the Court awarded CombiMatrix an additional $3.6 million in attorneys' fees and litigation costs, thereby increasing the overall award to $35.7 million. This award will be entered as a Final Judgment at a later date, and will continue to earn interest until paid.
``CombiMatrix, which has roughly 6 million shares of stock outstanding, is pleased by the verdict,'' commented Amit Kumar, Ph.D., President and CEO of CombiMatrix Corporation.
CombiMatrix is represented in this litigation by Richard B. Specter of Corbett, Steelman & Specter in Irvine, California.
ABOUT COMBIMATRIX CORPORATION
We are a diversified biotechnology business that develops proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where our products and services could be utilized. The technologies we have developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs. CombiMatrix Molecular Diagnostics, Inc., a wholly owned subsidiary located in Irvine, California, has developed capabilities of producing arrays that utilize bacterial artificial chromosomes, which also enable genetic analysis.
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com or by calling 1-800-985 CBMX (2269). Additional information about our laboratory, CombiMatrix Molecular Diagnostics, is available at http://www.cmdiagnostics.com or by calling 1-800-710-0624.
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory and competitive developments and general economic conditions. Our Annual Report on Form 10-K, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Dr. Amit Kumar, President and Chief Executive Officer
(425) 493-2000
Fax: (425) 493-2010
--------------------------------------------------------------------------------
Source: CombiMatrix Corporation


Federal Court Increases Award in Favor of CombiMatrix to $35.7 Million
Thursday April 24, 6:00 am ET
MUKILTEO, Wash., April 24, 2008 (PRIME NEWSWIRE) -- The United States District Court for the Central District of California increased the award previously issued in favor of CombiMatrix Corporation (NasdaqGM:CBMX - News). The Court originally awarded $32.1 million to CombiMatrix to be paid by National Union Fire Ins. Co. of Pittsburgh, PA, a member company of American International Group (AIG). On April 23, 2008, the Court awarded CombiMatrix an additional $3.6 million in attorneys' fees and litigation costs, thereby increasing the overall award to $35.7 million. This award will be entered as a Final Judgment at a later date, and will continue to earn interest until paid.
``CombiMatrix, which has roughly 6 million shares of stock outstanding, is pleased by the verdict,'' commented Amit Kumar, Ph.D., President and CEO of CombiMatrix Corporation.
CombiMatrix is represented in this litigation by Richard B. Specter of Corbett, Steelman & Specter in Irvine, California.
ABOUT COMBIMATRIX CORPORATION
We are a diversified biotechnology business that develops proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where our products and services could be utilized. The technologies we have developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs. CombiMatrix Molecular Diagnostics, Inc., a wholly owned subsidiary located in Irvine, California, has developed capabilities of producing arrays that utilize bacterial artificial chromosomes, which also enable genetic analysis.
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com or by calling 1-800-985 CBMX (2269). Additional information about our laboratory, CombiMatrix Molecular Diagnostics, is available at http://www.cmdiagnostics.com or by calling 1-800-710-0624.
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory and competitive developments and general economic conditions. Our Annual Report on Form 10-K, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Dr. Amit Kumar, President and Chief Executive Officer
(425) 493-2000
Fax: (425) 493-2010
--------------------------------------------------------------------------------
Source: CombiMatrix Corporation
CombiMatrix Announces the Acceptance of Its Multi-Institute Study On the Use of HemeScan for CLL by Journal, Expert Opinions in Molecular Diagnostics 

Wednesday June 4, 6:00 am ET
Publication Represents First Peer-Reviewed Study of a Commercial Clinically Validated Diagnostic Array CGH-Based Test for Cancer
MUKILTEO, Wash., June 4, 2008 (PRIME NEWSWIRE) -- CombiMatrix Corporation (NasdaqGM:CBMX - News) announced today that its subsidiary, Combimatrix Molecular Diagnostics (CMDX), has results from a multi-institute evaluation of its HemeScan(tm) test for CLL that were accepted for publication by the journal, Expert Opinions in Molecular Diagnostics (2008) 2(6): 1-10. The study, which was led by Dr. Shelly Gunn, Medical Director of CMDX, and which included collaborators from the prestigious M.D. Anderson Cancer Center, Texas, the Netherlands Cancer Institute, Amsterdam, and the University of Texas Health Science Center, San Antonio, evaluated over 170 patients with chronic lymphocytic leukemia (CLL) by the HemeScan test. The results of the study concluded that CMDX's HemeScan test is a powerful and cost-effective tool for genome-wide risk assessment in the clinical evaluation of CLL.
``This article is the first peer-reviewed publication of the clinical evaluation of an array CGH (Comparative Genomic Hybridization) diagnostics test addressing cancer. CombiMatrix is pleased to be the first to have launched a CGH array for cancer and now, is pleased to have completed this clinical study, which enables another first for the company,'' noted Dr. Mansoor Mohammed, President and CEO of CombiMatrix Molecular Diagnostics. ``We are also pleased to have completed this study in collaboration with several leading cancer researchers and their institutes. This study is the first in what we anticipate to be a series of publications, which will clearly establish the clinical utility of CMDX's suite of oncology tests.''
Dr. Shelly Gunn, Medical Director of CMDX, notes, ``The publication of peer-reviewed studies is a hallmark of legitimizing new diagnostic tests and their concomitant platforms. Publications have set the standard for the use of new medical innovations.'' Dr. Gunn further adds, ``I am proud to have contributed, together with Dr. Mohammed, pioneering publications documenting the use of array CGH in solving clinical diagnostic challenges, and I am equally proud to now author the first study documenting the use of this transformatory technology toward solving diagnostic challenges, in a commercial setting, for the oncology arena''.
Mr. Ron Andrews, CEO of Clarient (NasdaqCM:CLRT - News), commented, ``As I have previously noted, we evaluated several leading platforms and tests before deciding upon CMDX's HemeScan test as a suitable addition to our world class portfolio of cancer diagnostics. This recent publication by CMDX validates our assessment of the HemeScan test for CLL and fits with our model of launching the most comprehensive and robust diagnostic test menu in the industry.''
ABOUT COMBIMATRIX CORPORATION
We are a diversified biotechnology business, through the development of proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where our products and services could be utilized. The technologies we have developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. We have also developed the capabilities of producing arrays that utilize bacterial artificial chromosomes on our arrays, also enabling genetic analysis. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs. CombiMatrix Molecular Diagnostics, Inc. (``CMDX''), our wholly owned subsidiary located in Irvine, California, has developed capabilities of producing arrays that utilize bacterial artificial chromosomes, which also enable genetic analysis.
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com or by calling 1-800-985 CBMX (2269). Additional information about our laboratory, CombiMatrix Molecular Diagnostics, is available at http://www.cmdiagnostics.com or by calling 1-800-710-0624.
About Clarient
Clarient combines innovative technologies with world class expertise to assess and characterize cancer. Clarient's mission is to provide the services, resources and critical information to improve the quality and reduce the cost of patient care as well as accelerating the drug development process. The Company's principal customers include pathologists, oncologists, hospitals and biopharmaceutical companies. The rise of individualized medicine as the new direction in oncology has created the need for a centralized resource providing leading diagnostic technologies such as flow cytometry and molecular testing. Clarient is that resource, having created a state-of-the-art commercial cancer laboratory providing the most advanced oncology testing and drug development services available both onsite and over the web. Clarient is a Safeguard Scientifics, Inc. partner company. http://www.clarientinc.com
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory, and competitive developments, and general economic conditions. Our Annual Report on Form 10-K, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations, and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Amit Kumar, Ph.D., President & CEO
(425) 493-2000
Fax: (425) 493-2010
Source: CombiMatrix Corporation


Wednesday June 4, 6:00 am ET
Publication Represents First Peer-Reviewed Study of a Commercial Clinically Validated Diagnostic Array CGH-Based Test for Cancer
MUKILTEO, Wash., June 4, 2008 (PRIME NEWSWIRE) -- CombiMatrix Corporation (NasdaqGM:CBMX - News) announced today that its subsidiary, Combimatrix Molecular Diagnostics (CMDX), has results from a multi-institute evaluation of its HemeScan(tm) test for CLL that were accepted for publication by the journal, Expert Opinions in Molecular Diagnostics (2008) 2(6): 1-10. The study, which was led by Dr. Shelly Gunn, Medical Director of CMDX, and which included collaborators from the prestigious M.D. Anderson Cancer Center, Texas, the Netherlands Cancer Institute, Amsterdam, and the University of Texas Health Science Center, San Antonio, evaluated over 170 patients with chronic lymphocytic leukemia (CLL) by the HemeScan test. The results of the study concluded that CMDX's HemeScan test is a powerful and cost-effective tool for genome-wide risk assessment in the clinical evaluation of CLL.
``This article is the first peer-reviewed publication of the clinical evaluation of an array CGH (Comparative Genomic Hybridization) diagnostics test addressing cancer. CombiMatrix is pleased to be the first to have launched a CGH array for cancer and now, is pleased to have completed this clinical study, which enables another first for the company,'' noted Dr. Mansoor Mohammed, President and CEO of CombiMatrix Molecular Diagnostics. ``We are also pleased to have completed this study in collaboration with several leading cancer researchers and their institutes. This study is the first in what we anticipate to be a series of publications, which will clearly establish the clinical utility of CMDX's suite of oncology tests.''
Dr. Shelly Gunn, Medical Director of CMDX, notes, ``The publication of peer-reviewed studies is a hallmark of legitimizing new diagnostic tests and their concomitant platforms. Publications have set the standard for the use of new medical innovations.'' Dr. Gunn further adds, ``I am proud to have contributed, together with Dr. Mohammed, pioneering publications documenting the use of array CGH in solving clinical diagnostic challenges, and I am equally proud to now author the first study documenting the use of this transformatory technology toward solving diagnostic challenges, in a commercial setting, for the oncology arena''.
Mr. Ron Andrews, CEO of Clarient (NasdaqCM:CLRT - News), commented, ``As I have previously noted, we evaluated several leading platforms and tests before deciding upon CMDX's HemeScan test as a suitable addition to our world class portfolio of cancer diagnostics. This recent publication by CMDX validates our assessment of the HemeScan test for CLL and fits with our model of launching the most comprehensive and robust diagnostic test menu in the industry.''
ABOUT COMBIMATRIX CORPORATION
We are a diversified biotechnology business, through the development of proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where our products and services could be utilized. The technologies we have developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. We have also developed the capabilities of producing arrays that utilize bacterial artificial chromosomes on our arrays, also enabling genetic analysis. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs. CombiMatrix Molecular Diagnostics, Inc. (``CMDX''), our wholly owned subsidiary located in Irvine, California, has developed capabilities of producing arrays that utilize bacterial artificial chromosomes, which also enable genetic analysis.
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com or by calling 1-800-985 CBMX (2269). Additional information about our laboratory, CombiMatrix Molecular Diagnostics, is available at http://www.cmdiagnostics.com or by calling 1-800-710-0624.
About Clarient
Clarient combines innovative technologies with world class expertise to assess and characterize cancer. Clarient's mission is to provide the services, resources and critical information to improve the quality and reduce the cost of patient care as well as accelerating the drug development process. The Company's principal customers include pathologists, oncologists, hospitals and biopharmaceutical companies. The rise of individualized medicine as the new direction in oncology has created the need for a centralized resource providing leading diagnostic technologies such as flow cytometry and molecular testing. Clarient is that resource, having created a state-of-the-art commercial cancer laboratory providing the most advanced oncology testing and drug development services available both onsite and over the web. Clarient is a Safeguard Scientifics, Inc. partner company. http://www.clarientinc.com
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory, and competitive developments, and general economic conditions. Our Annual Report on Form 10-K, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations, and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Amit Kumar, Ph.D., President & CEO
(425) 493-2000
Fax: (425) 493-2010
Source: CombiMatrix Corporation
Renowned Prostate Cancer Expert, Dr. Colin Collins, Joins CombiMatrix's Scientific Advisory Board 

Wednesday August 6, 6:00 am ET
MUKILTEO, Wash., Aug. 6, 2008 (PRIME NEWSWIRE) -- CombiMatrix Corporation (NasdaqGM:CBMX - News) announced today that renowned cancer expert, Colin Collins, Ph.D., has joined the Scientific Advisory Board (SAB) of its subsidiary reference laboratory Combimatrix Molecular Diagnostics (CMDX).
Dr. Collins is an Associate Professor of Urology at the Cancer Research Institute and Department of Laboratory Medicine at the University of California, San Francisco (UCSF). He is one of the world's leading authorities in the emerging field of oncogenomics, which is the study of the causal basis of cancer. More specifically, Dr. Collins is considered a leading authority on prostate cancer, which is one of the leading causes of death among adult males. As one of the most prolific authors and innovators in his genre, Dr. Collins has published widely and holds multiple patents in the cancer field. His research has led to the identification of several genetic markers that have helped to better risk stratify cancer patients, which approach, in turn, has facilitated the administration of better therapeutic options. In addition to his work in prostate cancer, Dr. Collins's laboratory was responsible for the discovery of the ZNF217 gene and for elucidating its critical role in breast cancer. Over expression of this gene functions to make tumor cells hardier and more resistant to certain drugs. Additionally, Dr. Collins invented, and was awarded a patent for, a new method of analyzing the structure of tumor genomes called End Sequence Profiling (ESP). The latter has been credited with fundamentally transforming the way in which tumors are studied.
``The application of genomic discoveries to our approach in diagnosing, and ultimately treating, cancers has transformed the molecular diagnostics industry,'' said Dr. Collins. ``I strongly believe that the company best able to efficiently convert high caliber genomic discoveries to high caliber diagnostic tests will lead the industry in this growing and lucrative sector of the diagnostic market. I am convinced that CombiMatrix is ideally positioned to do just this, and I am excited to have the opportunity to help them expand that market leadership position.''
``I am very pleased to have someone of Dr. Collins's considerable accomplishment and innovativeness in the field of cancer research join our science advisory board,'' said Dr. Mansoor Mohammed, President and Chief Executive Officer of CMDX. ``Our ability to engage world class scientists like Dr. Collins is testament to our caliber as a company. He brings to our team a wealth of knowledge and experience that will help to accelerate our efforts to expand our continually growing portfolio of revolutionary tests to include, amongst others, our anticipated prostate cancer test by the end of the year.''
More information about Dr. Collins can be found at http://urology.ucsf.edu/faculty/facCollins.html.
ABOUT COMBIMATRIX CORPORATION
CombiMatrix Corporation is a diversified biotechnology business, through the development of proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where our products and services could be utilized. The technologies we have developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs. CombiMatrix Molecular Diagnostics, Inc. (``CMDX''), a wholly owned subsidiary of the Company located in Irvine, California, has developed capabilities of producing arrays that utilize bacterial artificial chromosomes, which also enable genetic analysis..
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com or call toll free: 1-800-710-0624.
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory, and competitive developments, and general economic conditions. Our Annual Report, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations, and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Investor Relations
Amit Kumar
(425) 493-2000
Fax: (425) 493-2010
Source: CombiMatrix Corporation


Wednesday August 6, 6:00 am ET
MUKILTEO, Wash., Aug. 6, 2008 (PRIME NEWSWIRE) -- CombiMatrix Corporation (NasdaqGM:CBMX - News) announced today that renowned cancer expert, Colin Collins, Ph.D., has joined the Scientific Advisory Board (SAB) of its subsidiary reference laboratory Combimatrix Molecular Diagnostics (CMDX).
Dr. Collins is an Associate Professor of Urology at the Cancer Research Institute and Department of Laboratory Medicine at the University of California, San Francisco (UCSF). He is one of the world's leading authorities in the emerging field of oncogenomics, which is the study of the causal basis of cancer. More specifically, Dr. Collins is considered a leading authority on prostate cancer, which is one of the leading causes of death among adult males. As one of the most prolific authors and innovators in his genre, Dr. Collins has published widely and holds multiple patents in the cancer field. His research has led to the identification of several genetic markers that have helped to better risk stratify cancer patients, which approach, in turn, has facilitated the administration of better therapeutic options. In addition to his work in prostate cancer, Dr. Collins's laboratory was responsible for the discovery of the ZNF217 gene and for elucidating its critical role in breast cancer. Over expression of this gene functions to make tumor cells hardier and more resistant to certain drugs. Additionally, Dr. Collins invented, and was awarded a patent for, a new method of analyzing the structure of tumor genomes called End Sequence Profiling (ESP). The latter has been credited with fundamentally transforming the way in which tumors are studied.
``The application of genomic discoveries to our approach in diagnosing, and ultimately treating, cancers has transformed the molecular diagnostics industry,'' said Dr. Collins. ``I strongly believe that the company best able to efficiently convert high caliber genomic discoveries to high caliber diagnostic tests will lead the industry in this growing and lucrative sector of the diagnostic market. I am convinced that CombiMatrix is ideally positioned to do just this, and I am excited to have the opportunity to help them expand that market leadership position.''
``I am very pleased to have someone of Dr. Collins's considerable accomplishment and innovativeness in the field of cancer research join our science advisory board,'' said Dr. Mansoor Mohammed, President and Chief Executive Officer of CMDX. ``Our ability to engage world class scientists like Dr. Collins is testament to our caliber as a company. He brings to our team a wealth of knowledge and experience that will help to accelerate our efforts to expand our continually growing portfolio of revolutionary tests to include, amongst others, our anticipated prostate cancer test by the end of the year.''
More information about Dr. Collins can be found at http://urology.ucsf.edu/faculty/facCollins.html.
ABOUT COMBIMATRIX CORPORATION
CombiMatrix Corporation is a diversified biotechnology business, through the development of proprietary technologies, products and services in the areas of drug development, genetic analysis, molecular diagnostics, nanotechnology research, defense and homeland security markets, as well as other potential markets where our products and services could be utilized. The technologies we have developed include a platform technology to rapidly produce customizable, in-situ synthesized, oligonucleotide arrays for use in identifying and determining the roles of genes, gene mutations and proteins. This technology has a wide range of potential applications in the areas of genomics, proteomics, biosensors, drug discovery, drug development, diagnostics, combinatorial chemistry, material sciences and nanotechnology. Other technologies include proprietary molecular synthesis and screening methods for the discovery of potential new drugs. CombiMatrix Molecular Diagnostics, Inc. (``CMDX''), a wholly owned subsidiary of the Company located in Irvine, California, has developed capabilities of producing arrays that utilize bacterial artificial chromosomes, which also enable genetic analysis..
Additional information about CombiMatrix Corporation is available at http://www.combimatrix.com or call toll free: 1-800-710-0624.
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995
This news release contains forward-looking statements within the meaning of the ``safe harbor'' provisions of the Private Securities Litigation Reform Act of 1995. These statements are based upon our current expectations and speak only as of the date hereof. Our actual results may differ materially and adversely from those expressed in any forward-looking statements as a result of various factors and uncertainties, including the recent economic slowdown affecting technology companies, our ability to successfully develop products, rapid technological change in our markets, changes in demand for our future products, legislative, regulatory, and competitive developments, and general economic conditions. Our Annual Report, recent and forthcoming Quarterly Reports on Form 10-Q, recent Current Reports on Forms 8-K and 8-K/A, and other SEC filings discuss some of the important risk factors that may affect our business, results of operations, and financial condition. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Contact:
CombiMatrix Corporation
Investor Relations
Amit Kumar
(425) 493-2000
Fax: (425) 493-2010
Source: CombiMatrix Corporation
das sieht doch mal nicht schlecht aus... 


Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 201 | ||
| 127 | ||
| 80 | ||
| 69 | ||
| 51 | ||
| 40 | ||
| 37 | ||
| 37 | ||
| 35 | ||
| 33 |
| Wertpapier | Beiträge | |
|---|---|---|
| 29 | ||
| 27 | ||
| 27 | ||
| 26 | ||
| 25 | ||
| 25 | ||
| 25 | ||
| 23 | ||
| 20 | ||
| 17 |






















