Oncolytics Biotech, Reolysin gegen Krebs - 500 Beiträge pro Seite
eröffnet am 06.10.10 01:08:50 von
neuester Beitrag 30.05.18 07:24:32 von
neuester Beitrag 30.05.18 07:24:32 von
Beiträge: 228
ID: 1.160.315
ID: 1.160.315
Aufrufe heute: 0
Gesamt: 15.653
Gesamt: 15.653
Aktive User: 0
ISIN: CA6823108759 · WKN: A2JMW5 · Symbol: ONC
1,4400
CAD
+0,70 %
+0,0100 CAD
Letzter Kurs 22:10:05 Toronto
Neuigkeiten
14.02.24 · PR Newswire (engl.) |
03.11.23 · PR Newswire (engl.) |
23.10.23 · PR Newswire (engl.) |
23.10.23 · PR Newswire (engl.) |
16.10.23 · PR Newswire (engl.) |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 3.000,00 | +74.900,00 | |
| 2,4700 | +33,51 | |
| 1,5400 | +25,71 | |
| 12,400 | +23,26 | |
| 0,6889 | +16,78 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,8501 | -17,47 | |
| 0,8963 | -17,77 | |
| 5,0550 | -22,71 | |
| 4,6600 | -28,75 | |
| 0,6021 | -35,26 |
Oncolytics Biotech (oncolyticsbiotech.com) ist eine Biotech AG aus Kanada und betreibt derzeit 12 Studien gegen unterschiedlichste Krebsformen
Jahrelang wurde versucht, Krebs mit seinem Produkt Reolysin (Info http://www.oncolyticsbiotech.com/tech.html) als Monotherapie zu bekämpfen. Die Erfolge hielten sich in Grenzen. Seit ca 3 Jahren laufen Studien mit einer Kombitherapie, Reolysin in Verbindung mit Chemo oder Bestrahlung (Studien http://www.oncolyticsbiotech.com/tech.html). Anscheinend mit großem Erfolg. Der Kurs der Aktie, der noch Ende 2008 bei 1 US$ lag, liegt heute bei 5 $ und hat sich allein in den letzten zwei Monaten verdoppelt.
Die Umsätze an der NASDAG dümpelten lange Zeit um 35 K am Tag, stieg vor wenigen Wochen rasant an, heute wurden über 500 k gehandelt
Infos gibt es hier auf Yahoo http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
hier gab es vor mehr als 5 Jahren Beiträge zu dieser AG
reguau
Jahrelang wurde versucht, Krebs mit seinem Produkt Reolysin (Info http://www.oncolyticsbiotech.com/tech.html) als Monotherapie zu bekämpfen. Die Erfolge hielten sich in Grenzen. Seit ca 3 Jahren laufen Studien mit einer Kombitherapie, Reolysin in Verbindung mit Chemo oder Bestrahlung (Studien http://www.oncolyticsbiotech.com/tech.html). Anscheinend mit großem Erfolg. Der Kurs der Aktie, der noch Ende 2008 bei 1 US$ lag, liegt heute bei 5 $ und hat sich allein in den letzten zwei Monaten verdoppelt.
Die Umsätze an der NASDAG dümpelten lange Zeit um 35 K am Tag, stieg vor wenigen Wochen rasant an, heute wurden über 500 k gehandelt
Infos gibt es hier auf Yahoo http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
hier gab es vor mehr als 5 Jahren Beiträge zu dieser AG
reguau
kaufe niemals auf ath

Oncolytics Biotech® Inc. Collaborators Present Data from U.K. Translational Colorectal Cancer Study at the International Symposium on CTL and Immunostimulation
CALGARY, Oct. 26 /PRNewswire-FirstCall/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ: ONCY) announced today that interim data from a U.K. translational clinical trial (REO 013) investigating intravenous administration of REOLYSIN® in patients with metastatic colorectal cancer prior to surgical resection of liver metastases was presented at the International Symposium on Cytotoxic T-lymphocytes (CTL) and Immunostimulation being held in Pamplona, Spain. The presentation was given by principal investigator Professor Alan Melcher of Leeds Institute of Molecular Medicine, University of Leeds, UK.
The trial is an open-label, non-randomized, single centre study of REOLYSIN given intravenously to patients for five consecutive days in advance of their scheduled operations to remove colorectal cancer deposits metastatic to the liver. Patients were treated with intravenous REOLYSIN at 1x1010 TCID50, one to three weeks prior to planned surgery. After surgery, the tumour and surrounding liver tissue was assessed for viral status and anti-tumour effects.
On histological analysis of six patients to date, there was evidence of replication and tumour cell death in the tumours of four of six patients, two of which had confirmed Kras mutations in codon 12 (the most common ras mutation). The other two of these four patients' samples were still being analyzed for Kras status. There was no evidence of replication in samples analyzed from two of the six patients. The researchers concluded that reovirus can be successfully delivered specifically to colorectal liver metastases following intravenous administration as a monotherapy and that pre-operative treatment was safe, suggesting that application of oncolytic viral therapy can be widened to the neoadjuvant setting. Further translational studies with biological endpoints, particularly co-administering reovirus with chemotherapy, would further inform how to maximize the efficacy of this novel biotherapy in cancer patients.
"This compelling data shows that following even a single course of treatment with REOLYSIN that reovirus can target and replicate specifically in metastatic colorectal cancer tumours and that there is a correlation with Kras status," said Dr. Brad Thompson, President and CEO of Oncolytics. "The interim findings from this study are further supportive of our decision to conduct a Phase I study of REOLYSIN in combination with FOLFIRI in patients with oxaliplatin refractory/intolerant Kras mutant colorectal cancer."
reguau
CALGARY, Oct. 26 /PRNewswire-FirstCall/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ: ONCY) announced today that interim data from a U.K. translational clinical trial (REO 013) investigating intravenous administration of REOLYSIN® in patients with metastatic colorectal cancer prior to surgical resection of liver metastases was presented at the International Symposium on Cytotoxic T-lymphocytes (CTL) and Immunostimulation being held in Pamplona, Spain. The presentation was given by principal investigator Professor Alan Melcher of Leeds Institute of Molecular Medicine, University of Leeds, UK.
The trial is an open-label, non-randomized, single centre study of REOLYSIN given intravenously to patients for five consecutive days in advance of their scheduled operations to remove colorectal cancer deposits metastatic to the liver. Patients were treated with intravenous REOLYSIN at 1x1010 TCID50, one to three weeks prior to planned surgery. After surgery, the tumour and surrounding liver tissue was assessed for viral status and anti-tumour effects.
On histological analysis of six patients to date, there was evidence of replication and tumour cell death in the tumours of four of six patients, two of which had confirmed Kras mutations in codon 12 (the most common ras mutation). The other two of these four patients' samples were still being analyzed for Kras status. There was no evidence of replication in samples analyzed from two of the six patients. The researchers concluded that reovirus can be successfully delivered specifically to colorectal liver metastases following intravenous administration as a monotherapy and that pre-operative treatment was safe, suggesting that application of oncolytic viral therapy can be widened to the neoadjuvant setting. Further translational studies with biological endpoints, particularly co-administering reovirus with chemotherapy, would further inform how to maximize the efficacy of this novel biotherapy in cancer patients.
"This compelling data shows that following even a single course of treatment with REOLYSIN that reovirus can target and replicate specifically in metastatic colorectal cancer tumours and that there is a correlation with Kras status," said Dr. Brad Thompson, President and CEO of Oncolytics. "The interim findings from this study are further supportive of our decision to conduct a Phase I study of REOLYSIN in combination with FOLFIRI in patients with oxaliplatin refractory/intolerant Kras mutant colorectal cancer."
reguau
Übersetzung von Englisch nach Deutsch
Oncolytics Biotech Inc. ® Teams präsentieren Daten aus Großbritannien Translational Darmkrebs Studium an der International Symposium on CTL und Immunstimulation
CALGARY, 26. Oktober / PRNewswire / - Oncolytics Biotech Inc. ("Oncolytics") (TSX: ONC, NASDAQ: ONCY) meldete heute, dass vorläufige Daten aus einer britischen translationale klinische Studie (REO 013) untersucht intravenösen Verabreichung von REOLYSIN ® bei Patienten mit metastasiertem kolorektalem Karzinom vor der chirurgischen Resektion von Lebermetastasen wurde auf dem Internationalen Symposium auf zytotoxischen T-Lymphozyten (CTL) und Immunstimulation in Pamplona, Spanien präsentiert. Die Präsentation wurde vom Principal Investigator Prof. Alan Melcher von Leeds Institute of Molecular Medicine, University of Leeds, UK gegeben.
Die Studie ist eine Open-Label, nicht-randomisierte, monozentrische Studie von REOLYSIN intravenös an Patienten an fünf aufeinanderfolgenden Tagen gegeben im Voraus über ihre geplante Operationen zu Darmkrebs Einlagen mit Metastasen in der Leber zu entfernen. Die Patienten wurden mit intravenösen REOLYSIN bei 1x1010 TCID50, 02.59 Wochen vor der geplanten Operation behandelt. Nach der Operation wurde der Tumor und die umliegenden Lebergewebes für virale Status und Anti-Tumor-Effekte bewertet.
Bei der histologischen Analyse der sechs Patienten auf dem neuesten Stand, gab es Hinweise auf die Replikation und Tod von Tumorzellen in den Tumoren von vier von sechs Patienten, von denen zwei KRAS-Mutationen im Codon 12 bestätigt hatte (die häufigste ras Mutation). Die beiden anderen der vier Patienten-Proben wurden noch für KRAS-Status untersucht. Es gab keine Hinweise auf die Replikation in Proben von zwei der sechs Patienten analysiert. Die Forscher folgerten, dass Reovirus erfolgreich geliefert werden, um speziell kolorektalen Lebermetastasen nach intravenöser Verabreichung als Monotherapie als auch die präoperative Behandlung war sicher, was darauf hindeutet, dass die Anwendung von onkolytischen virale Therapie kann die neoadjuvante Einstellung erweitert werden. Weitere translationale Studien mit biologischen Endpunkte, insbesondere gleichzeitiger Verabreichung von Reovirus mit Chemotherapie würden weitere informieren, wie die Wirksamkeit dieses neuartigen Biotherapie bei Krebspatienten zu maximieren.
"Das überzeugende Daten zeigen, dass folgende auch nur einen einzigen Behandlungszyklus mit REOLYSIN dass Reovirus Ziel und replizieren kann speziell bei metastasierendem Dickdarmkrebs Tumoren und dass es eine Korrelation mit KRAS-Status", sagte Dr. Brad Thompson, Präsident und CEO von Oncolytics. "Die vorläufigen Ergebnisse dieser Studie werden weiter unterstützt unsere Entscheidung zu einer Phase I Studie von REOLYSIN in Kombination mit FOLFIRI bei Patienten mit refraktärer Oxaliplatin / intolerant KRAS-Mutationen Darmkrebs führen."
reguau
Oncolytics Biotech Inc. ® Teams präsentieren Daten aus Großbritannien Translational Darmkrebs Studium an der International Symposium on CTL und Immunstimulation
CALGARY, 26. Oktober / PRNewswire / - Oncolytics Biotech Inc. ("Oncolytics") (TSX: ONC, NASDAQ: ONCY) meldete heute, dass vorläufige Daten aus einer britischen translationale klinische Studie (REO 013) untersucht intravenösen Verabreichung von REOLYSIN ® bei Patienten mit metastasiertem kolorektalem Karzinom vor der chirurgischen Resektion von Lebermetastasen wurde auf dem Internationalen Symposium auf zytotoxischen T-Lymphozyten (CTL) und Immunstimulation in Pamplona, Spanien präsentiert. Die Präsentation wurde vom Principal Investigator Prof. Alan Melcher von Leeds Institute of Molecular Medicine, University of Leeds, UK gegeben.
Die Studie ist eine Open-Label, nicht-randomisierte, monozentrische Studie von REOLYSIN intravenös an Patienten an fünf aufeinanderfolgenden Tagen gegeben im Voraus über ihre geplante Operationen zu Darmkrebs Einlagen mit Metastasen in der Leber zu entfernen. Die Patienten wurden mit intravenösen REOLYSIN bei 1x1010 TCID50, 02.59 Wochen vor der geplanten Operation behandelt. Nach der Operation wurde der Tumor und die umliegenden Lebergewebes für virale Status und Anti-Tumor-Effekte bewertet.
Bei der histologischen Analyse der sechs Patienten auf dem neuesten Stand, gab es Hinweise auf die Replikation und Tod von Tumorzellen in den Tumoren von vier von sechs Patienten, von denen zwei KRAS-Mutationen im Codon 12 bestätigt hatte (die häufigste ras Mutation). Die beiden anderen der vier Patienten-Proben wurden noch für KRAS-Status untersucht. Es gab keine Hinweise auf die Replikation in Proben von zwei der sechs Patienten analysiert. Die Forscher folgerten, dass Reovirus erfolgreich geliefert werden, um speziell kolorektalen Lebermetastasen nach intravenöser Verabreichung als Monotherapie als auch die präoperative Behandlung war sicher, was darauf hindeutet, dass die Anwendung von onkolytischen virale Therapie kann die neoadjuvante Einstellung erweitert werden. Weitere translationale Studien mit biologischen Endpunkte, insbesondere gleichzeitiger Verabreichung von Reovirus mit Chemotherapie würden weitere informieren, wie die Wirksamkeit dieses neuartigen Biotherapie bei Krebspatienten zu maximieren.
"Das überzeugende Daten zeigen, dass folgende auch nur einen einzigen Behandlungszyklus mit REOLYSIN dass Reovirus Ziel und replizieren kann speziell bei metastasierendem Dickdarmkrebs Tumoren und dass es eine Korrelation mit KRAS-Status", sagte Dr. Brad Thompson, Präsident und CEO von Oncolytics. "Die vorläufigen Ergebnisse dieser Studie werden weiter unterstützt unsere Entscheidung zu einer Phase I Studie von REOLYSIN in Kombination mit FOLFIRI bei Patienten mit refraktärer Oxaliplatin / intolerant KRAS-Mutationen Darmkrebs führen."
reguau
10/28/2010 11:14:40 AM ET News Release Index
Oncolytics Biotech® Inc. Announces Cdn$25 Million Bought Deal Financing
CALGARY, AB - October 28, 2010 - Oncolytics Biotech Inc. ("Oncolytics" or the "Company") (TSX:ONC, NASDAQ:ONCY) today announced that it has entered into an agreement with a syndicate of underwriters (the "Underwriters") pursuant to which they have agreed to purchase, on a bought deal basis, 5,440,000 units (the "Units") of the Company at a price of Cdn$4.60 per Unit for gross proceeds to the Company of approximately Cdn$25 million (the "Offering"). In addition, the Corporation has agreed to grant to the Underwriters an option (the "Over-Allotment Option") to purchase up to an additional 15% of the number of Units sold under the Offering at a price of Cdn$4.60 per Unit, on the same terms and conditions as the Offering, exercisable at any time, in whole or in part, until the date that is 30 days following the closing of the Offering. In the event that the Over-Allotment Option is exercised in its entirety, the aggregate gross proceeds of the Offering to Oncolytics will be approximately Cdn$28.75 million. Each Unit will consist of one common share of Oncolytics and one-half of one common share purchase warrant (each whole common share purchase warrant, a "Warrant"). Each Warrant will entitle the holder to acquire one common share of Oncolytics at a price of Cdn$6.15 at any time prior to the date which is two years following completion of the Offering.
The Units will be offered in Canada by way of a shelf prospectus supplement to a short-form base shelf prospectus dated June 10, 2010, that has been filed in the provinces of British Columbia, Alberta, Manitoba and Ontario pursuant to National Instrument 44-101 ("NI 44-101") and National Instrument 44-102 ("NI 44-102").
Oncolytics intends to use the net proceeds from the Offering to fund its ongoing Phase III combination REOLYSIN® and paclitaxel/carboplatin trial for patients with platinum-failed head and neck cancers, its other clinical development and research and development activities, and for general corporate and working capital purposes.
The transaction is subject to the receipt of all necessary regulatory and stock exchange approvals. The transaction is expected to close on or about November 16, 2010.
Oncolytics Biotech® Inc. Announces Cdn$25 Million Bought Deal Financing
CALGARY, AB - October 28, 2010 - Oncolytics Biotech Inc. ("Oncolytics" or the "Company") (TSX:ONC, NASDAQ:ONCY) today announced that it has entered into an agreement with a syndicate of underwriters (the "Underwriters") pursuant to which they have agreed to purchase, on a bought deal basis, 5,440,000 units (the "Units") of the Company at a price of Cdn$4.60 per Unit for gross proceeds to the Company of approximately Cdn$25 million (the "Offering"). In addition, the Corporation has agreed to grant to the Underwriters an option (the "Over-Allotment Option") to purchase up to an additional 15% of the number of Units sold under the Offering at a price of Cdn$4.60 per Unit, on the same terms and conditions as the Offering, exercisable at any time, in whole or in part, until the date that is 30 days following the closing of the Offering. In the event that the Over-Allotment Option is exercised in its entirety, the aggregate gross proceeds of the Offering to Oncolytics will be approximately Cdn$28.75 million. Each Unit will consist of one common share of Oncolytics and one-half of one common share purchase warrant (each whole common share purchase warrant, a "Warrant"). Each Warrant will entitle the holder to acquire one common share of Oncolytics at a price of Cdn$6.15 at any time prior to the date which is two years following completion of the Offering.
The Units will be offered in Canada by way of a shelf prospectus supplement to a short-form base shelf prospectus dated June 10, 2010, that has been filed in the provinces of British Columbia, Alberta, Manitoba and Ontario pursuant to National Instrument 44-101 ("NI 44-101") and National Instrument 44-102 ("NI 44-102").
Oncolytics intends to use the net proceeds from the Offering to fund its ongoing Phase III combination REOLYSIN® and paclitaxel/carboplatin trial for patients with platinum-failed head and neck cancers, its other clinical development and research and development activities, and for general corporate and working capital purposes.
The transaction is subject to the receipt of all necessary regulatory and stock exchange approvals. The transaction is expected to close on or about November 16, 2010.
ONCOLYTICS BIOTECH INC. CLOSES CDN$28.77 MILLION BOUGHT DEAL FINANCING
Oncolytics Biotech Inc. has closed its previously announced $25-million financing in which it entered into an agreement with a syndicate of underwriters pursuant to which they purchased, on a bought deal basis, 5.44 million units of the company at a price of $4.60 per unit for gross proceeds to the company of approximately $25-million. The Offering was conducted through a syndicate of underwriters led by Paradigm Capital Inc., and including RBC Dominion Securities Inc., Canaccord Genuity Corp., and Bloom Burton & Co. Inc. (collectively the "Underwriters").
In connection with the Offering, the Underwriters have exercised in full an option (the "Over-Allotment Option") to purchase an additional 816,000 Units sold under the Offering at a price of Cdn$4.60 per Unit, on the same terms and conditions as the Offering. With the Over-Allotment Option exercised in full, the aggregate gross proceeds of the Offering to Oncolytics is approximately Cdn$28.77 million.
reguau
Oncolytics Biotech Inc. has closed its previously announced $25-million financing in which it entered into an agreement with a syndicate of underwriters pursuant to which they purchased, on a bought deal basis, 5.44 million units of the company at a price of $4.60 per unit for gross proceeds to the company of approximately $25-million. The Offering was conducted through a syndicate of underwriters led by Paradigm Capital Inc., and including RBC Dominion Securities Inc., Canaccord Genuity Corp., and Bloom Burton & Co. Inc. (collectively the "Underwriters").
In connection with the Offering, the Underwriters have exercised in full an option (the "Over-Allotment Option") to purchase an additional 816,000 Units sold under the Offering at a price of Cdn$4.60 per Unit, on the same terms and conditions as the Offering. With the Over-Allotment Option exercised in full, the aggregate gross proceeds of the Offering to Oncolytics is approximately Cdn$28.77 million.
reguau
They have completed pre-clinical research on pediatric sarcomas and Reolysin. "These results suggest that Reolysin alone or in combination with other cytotoxic agents may be effective therapy in pediatric sarcomas". This could mean that very soon there could be a pediatric sarcoma trial starting up. One of the members of the Onc Medical Board is Dr. Richard Gorlick who lost a leg to childhood sarcoma http://sarcomahelp.org/profiles/gorlick....
Systemic administration of reovirus (Reolysin) inhibits growth of human sarcoma xenografts
Pooja Hingorani MD1,*,†, Wendong Zhang BS2, Juan Lin PhD3, Laibin Liu BS4, Chandan Guha MD4, E. Anders Kolb MD5Article first published online: 8 NOV 2010
DOI: 10.1002/cncr.25741
Copyright © 2010 American Cancer Society
Systemic administration of reovirus (Reolysin) inhibits growth of human sarcoma xenografts
Pooja Hingorani MD1,*,†, Wendong Zhang BS2, Juan Lin PhD3, Laibin Liu BS4, Chandan Guha MD4, E. Anders Kolb MD5Article first published online: 8 NOV 2010
DOI: 10.1002/cncr.25741
Copyright © 2010 American Cancer Society
Q3 RESULTS 10-Nov-10 07:34 am Press Release Source: Oncolytics Biotech Inc. On Wednesday November 10, 2010, 7:30 am
CALGARY, Nov. 10 /PRNewswire-FirstCall/ - Oncolytics Biotech Inc. (TSX:ONC.to - News) ("Oncolytics" or the "Company") today announced its financial results and operational highlights for the quarter ended September 30, 2010.
"We are excited to be conducting our first Phase 3 study," said Dr. Brad Thompson, President and CEO of Oncolytics. "Moving into the fourth quarter the Company's fundamentals remain strong and we are well positioned to execute on our clinical strategy in 2011 and beyond."
Selected Highlights
Since July 1, 2010 the Company has announced:
Clinical Program
•Receipt of a No Objection Letter from Health Canada to conduct the Company's Phase 3 trial examining REOLYSIN® in combination with paclitaxel and carboplatin in patients with platinum-refractory head and neck cancers at centres in Canada;
•A randomized Phase 2 trial of weekly paclitaxel versus weekly paclitaxel with REOLYSIN in patients with persistent or recurrent ovarian, fallopian tube or primary peritoneal cancer to be conducted by the Gynecologic Oncology Group (GOG). The Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, U.S. National Cancer Institute (NCI), which is part of the National Institutes of Health, will sponsor the trial under its Clinical Trials Agreement with Oncolytics;
•A presentation of interim data from a U.K. translational clinical trial investigating intravenous administration of REOLYSIN in patients with metastatic colorectal cancer prior to surgical resection of liver metastases. The researchers concluded that reovirus can be successfully delivered specifically to colorectal liver metastases following intravenous administration as a monotherapy and that pre-operative treatment was safe, suggesting that application of oncolytic viral therapy can be widened to the neoadjuvant setting;
Preclinical Program
•An abstract, entitled "REOLYSIN induces endoplasmic reticular stress in multiple myeloma and enhances the activity of bortezomib", indicating that the combination of REOLYSIN and bortezomib significantly reduced tumor burden in both xenograft and syngeneic multiple myeloma mouse models. The authors concluded that REOLYSIN is a promising anticancer agent that displays activity against multiple myeloma alone and in combination with bortezomib and warrants further investigation for the treatment of multiple myeloma and other malignancies;
Intellectual Property
•Grant of U.S. Patent, # 7,803,385 entitled "Reoviruses Having Modified Sequences." This is a composition of matter patent that covers the reovirus variant the Company is using in its clinical trial program and expires in 2028; and
Financial
•Completion of a bought deal financing issuing 6,256,000 units of the Company at a price of $4.60 per Unit for gross proceeds to the Company of approximately $28.77 million.
CALGARY, Nov. 10 /PRNewswire-FirstCall/ - Oncolytics Biotech Inc. (TSX:ONC.to - News) ("Oncolytics" or the "Company") today announced its financial results and operational highlights for the quarter ended September 30, 2010.
"We are excited to be conducting our first Phase 3 study," said Dr. Brad Thompson, President and CEO of Oncolytics. "Moving into the fourth quarter the Company's fundamentals remain strong and we are well positioned to execute on our clinical strategy in 2011 and beyond."
Selected Highlights
Since July 1, 2010 the Company has announced:
Clinical Program
•Receipt of a No Objection Letter from Health Canada to conduct the Company's Phase 3 trial examining REOLYSIN® in combination with paclitaxel and carboplatin in patients with platinum-refractory head and neck cancers at centres in Canada;
•A randomized Phase 2 trial of weekly paclitaxel versus weekly paclitaxel with REOLYSIN in patients with persistent or recurrent ovarian, fallopian tube or primary peritoneal cancer to be conducted by the Gynecologic Oncology Group (GOG). The Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, U.S. National Cancer Institute (NCI), which is part of the National Institutes of Health, will sponsor the trial under its Clinical Trials Agreement with Oncolytics;
•A presentation of interim data from a U.K. translational clinical trial investigating intravenous administration of REOLYSIN in patients with metastatic colorectal cancer prior to surgical resection of liver metastases. The researchers concluded that reovirus can be successfully delivered specifically to colorectal liver metastases following intravenous administration as a monotherapy and that pre-operative treatment was safe, suggesting that application of oncolytic viral therapy can be widened to the neoadjuvant setting;
Preclinical Program
•An abstract, entitled "REOLYSIN induces endoplasmic reticular stress in multiple myeloma and enhances the activity of bortezomib", indicating that the combination of REOLYSIN and bortezomib significantly reduced tumor burden in both xenograft and syngeneic multiple myeloma mouse models. The authors concluded that REOLYSIN is a promising anticancer agent that displays activity against multiple myeloma alone and in combination with bortezomib and warrants further investigation for the treatment of multiple myeloma and other malignancies;
Intellectual Property
•Grant of U.S. Patent, # 7,803,385 entitled "Reoviruses Having Modified Sequences." This is a composition of matter patent that covers the reovirus variant the Company is using in its clinical trial program and expires in 2028; and
Financial
•Completion of a bought deal financing issuing 6,256,000 units of the Company at a price of $4.60 per Unit for gross proceeds to the Company of approximately $28.77 million.
Oncolytics Biotech® Inc. Announces Phase I Study in Pediatric Patients with Relapsed or Refractory Solid Tumors to be Conducted by the Children's Oncology Group and Sponsored by the National Cancer Institute
CALGARY, Nov. 18 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) announced today that the Children's Oncology Group (COG) intends to conduct a Phase I trial of REOLYSIN® in combination with cyclophosphamide in pediatric patients with relapsed or refractory solid tumors. The study will be conducted in collaboration with the Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, U.S. National Cancer Institute (NCI), which is part of the National Institutes of Health, under its Clinical Trials Agreement with Oncolytics. Oncolytics will provide clinical supplies of REOLYSIN for this study. The study chair will be Dr. E. Anders Kolb of the Nemours/Alfred I. duPont Hospital for Children.
The study is an open label, multicentre, dose escalation Phase I study of REOLYSIN in patients aged three to 21 years with relapsed or refractory solid tumors. Patients will receive intravenous REOLYSIN on days one through five of each 28-day treatment cycle. Some patients will also receive oral cyclophosphamide on days one through 21. Treatments repeat every 28 days for up to 12 cycles in the absence of disease progression or unacceptable toxicity.
The primary objectives of the trial include estimating maximum tolerated dose, and defining and describing the toxicities of REOLYSIN and REOLYSIN plus oral cyclophosphamide in this patient population. Secondary objectives include defining antitumor activity of REOLYSIN within the confines of a Phase I study, evaluating the development of neutralizing antibodies to REOLYSIN following intravenous administration of REOLYSIN alone and in combination with cyclophosphamide, and assessing the biologic activity of REOLYSIN. After completion of study treatment, patients are to be followed up periodically for up to one year.
"This trial builds on a range of completed preclinical work and clinical studies in adult patients, including a U.K. Phase I clinical study examining the use of REOLYSIN in conjunction with cyclophosphamide and a U.S. Phase II clinical study in sarcoma using REOLYSIN as a monotherapy," said Dr. Brad Thompson, President and CEO of Oncolytics. "It is very important to investigate the use of an agent in adults prior to proceeding to use in pediatric patients. With the work in adults well advanced, we can now move ahead in younger patients."
CALGARY, Nov. 18 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) announced today that the Children's Oncology Group (COG) intends to conduct a Phase I trial of REOLYSIN® in combination with cyclophosphamide in pediatric patients with relapsed or refractory solid tumors. The study will be conducted in collaboration with the Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, U.S. National Cancer Institute (NCI), which is part of the National Institutes of Health, under its Clinical Trials Agreement with Oncolytics. Oncolytics will provide clinical supplies of REOLYSIN for this study. The study chair will be Dr. E. Anders Kolb of the Nemours/Alfred I. duPont Hospital for Children.
The study is an open label, multicentre, dose escalation Phase I study of REOLYSIN in patients aged three to 21 years with relapsed or refractory solid tumors. Patients will receive intravenous REOLYSIN on days one through five of each 28-day treatment cycle. Some patients will also receive oral cyclophosphamide on days one through 21. Treatments repeat every 28 days for up to 12 cycles in the absence of disease progression or unacceptable toxicity.
The primary objectives of the trial include estimating maximum tolerated dose, and defining and describing the toxicities of REOLYSIN and REOLYSIN plus oral cyclophosphamide in this patient population. Secondary objectives include defining antitumor activity of REOLYSIN within the confines of a Phase I study, evaluating the development of neutralizing antibodies to REOLYSIN following intravenous administration of REOLYSIN alone and in combination with cyclophosphamide, and assessing the biologic activity of REOLYSIN. After completion of study treatment, patients are to be followed up periodically for up to one year.
"This trial builds on a range of completed preclinical work and clinical studies in adult patients, including a U.K. Phase I clinical study examining the use of REOLYSIN in conjunction with cyclophosphamide and a U.S. Phase II clinical study in sarcoma using REOLYSIN as a monotherapy," said Dr. Brad Thompson, President and CEO of Oncolytics. "It is very important to investigate the use of an agent in adults prior to proceeding to use in pediatric patients. With the work in adults well advanced, we can now move ahead in younger patients."
Übersetzung von Englisch nach Deutsch
Oncolytics Biotech Inc. kündigt ® Phase I Studie bei pädiatrischen Patienten mit rezidiviertem oder refraktären soliden Tumoren durch die Children's Oncology Group und gefördert durch das National Cancer Institute durchgeführt werden
CALGARY, Nov. 18 / PRNewswire / - Oncolytics Biotech Inc. ("Oncolytics") (TSX: ONC, NASDAQ: ONCY) gab heute bekannt, dass die Children's Oncology Group (COG) zur Durchführung einer Phase I-Studie von REOLYSIN ® in Kombination mit Absicht Cyclophosphamid bei pädiatrischen Patienten mit rezidivierten oder refraktären soliden Tumoren. Die Studie wird in Zusammenarbeit mit der Krebstherapie Evaluation Program, Division of Cancer Treatment und Diagnose, US National Cancer Institute (NCI), das Teil der National Institutes of Health ist nach seinen Clinical Trials Abkommen mit Oncolytics durchgeführt werden. Oncolytics wird die klinische Versorgung von REOLYSIN für diese Studie. Die Studie Stuhl Dr. E. Anders Kolb der Nemours / Alfred I. DuPont Klinik für Kinder werden.
Die Studie ist eine offene, multizentrische Dosisfindungsstudie der Phase I-Studie bei Patienten mit REOLYSIN im Alter von drei bis 21 Jahren mit rezidivierender oder refraktärer soliden Tumoren. Die Patienten werden intravenös REOLYSIN an den Tagen eins bis fünf jeweils 28-tägigen Behandlungszyklus erhalten. Einige Patienten erhalten auch mündliche Cyclophosphamid an den Tagen eins bis 21. Behandlungen wiederholen alle 28 Tage für bis zu 12 Zyklen in der Abwesenheit von Krankheitsprogression oder inakzeptabler Toxizität.
Die primären Ziele der Studie sind die Schätzung maximal verträgliche Dosis und der Beschreibung und Definition der Toxizitäten von REOLYSIN und REOLYSIN plus Cyclophosphamid oral in dieser Patientenpopulation. Sekundäre Ziele sind die Definition Antitumor-Aktivität von REOLYSIN innerhalb der Grenzen einer Phase I Studie, die Beurteilung der Entwicklung von neutralisierenden Antikörpern gegen nach intravenöser Verabreichung von REOLYSIN allein und in Kombination mit Cyclophosphamid REOLYSIN und die Beurteilung der biologischen Aktivität von REOLYSIN. Nach Abschluss der Studie Behandlung werden die Patienten in regelmäßigen Abständen werden bis zu einem Jahr.
"Diese Studie baut auf einer Reihe von präklinischen Arbeiten abgeschlossen und klinischen Studien bei erwachsenen Patienten, einschließlich einer britischen Phase I der klinischen Studie zur Untersuchung der Verwendung von REOLYSIN in Kombination mit Cyclophosphamid und einer US-Phase II der klinischen Studie in Sarkom mit REOLYSIN als Monotherapie" sagte Dr. Brad Thompson, Präsident und CEO von Oncolytics. "Es ist sehr wichtig, die Verwendung eines Mittels an Erwachsenen vor fortfahren bei pädiatrischen Patienten nutzen zu untersuchen. Mit der Arbeit bei Erwachsenen weit fortgeschritten, können wir jetzt vorankommen bei jüngeren
Oncolytics Biotech Inc. kündigt ® Phase I Studie bei pädiatrischen Patienten mit rezidiviertem oder refraktären soliden Tumoren durch die Children's Oncology Group und gefördert durch das National Cancer Institute durchgeführt werden
CALGARY, Nov. 18 / PRNewswire / - Oncolytics Biotech Inc. ("Oncolytics") (TSX: ONC, NASDAQ: ONCY) gab heute bekannt, dass die Children's Oncology Group (COG) zur Durchführung einer Phase I-Studie von REOLYSIN ® in Kombination mit Absicht Cyclophosphamid bei pädiatrischen Patienten mit rezidivierten oder refraktären soliden Tumoren. Die Studie wird in Zusammenarbeit mit der Krebstherapie Evaluation Program, Division of Cancer Treatment und Diagnose, US National Cancer Institute (NCI), das Teil der National Institutes of Health ist nach seinen Clinical Trials Abkommen mit Oncolytics durchgeführt werden. Oncolytics wird die klinische Versorgung von REOLYSIN für diese Studie. Die Studie Stuhl Dr. E. Anders Kolb der Nemours / Alfred I. DuPont Klinik für Kinder werden.
Die Studie ist eine offene, multizentrische Dosisfindungsstudie der Phase I-Studie bei Patienten mit REOLYSIN im Alter von drei bis 21 Jahren mit rezidivierender oder refraktärer soliden Tumoren. Die Patienten werden intravenös REOLYSIN an den Tagen eins bis fünf jeweils 28-tägigen Behandlungszyklus erhalten. Einige Patienten erhalten auch mündliche Cyclophosphamid an den Tagen eins bis 21. Behandlungen wiederholen alle 28 Tage für bis zu 12 Zyklen in der Abwesenheit von Krankheitsprogression oder inakzeptabler Toxizität.
Die primären Ziele der Studie sind die Schätzung maximal verträgliche Dosis und der Beschreibung und Definition der Toxizitäten von REOLYSIN und REOLYSIN plus Cyclophosphamid oral in dieser Patientenpopulation. Sekundäre Ziele sind die Definition Antitumor-Aktivität von REOLYSIN innerhalb der Grenzen einer Phase I Studie, die Beurteilung der Entwicklung von neutralisierenden Antikörpern gegen nach intravenöser Verabreichung von REOLYSIN allein und in Kombination mit Cyclophosphamid REOLYSIN und die Beurteilung der biologischen Aktivität von REOLYSIN. Nach Abschluss der Studie Behandlung werden die Patienten in regelmäßigen Abständen werden bis zu einem Jahr.
"Diese Studie baut auf einer Reihe von präklinischen Arbeiten abgeschlossen und klinischen Studien bei erwachsenen Patienten, einschließlich einer britischen Phase I der klinischen Studie zur Untersuchung der Verwendung von REOLYSIN in Kombination mit Cyclophosphamid und einer US-Phase II der klinischen Studie in Sarkom mit REOLYSIN als Monotherapie" sagte Dr. Brad Thompson, Präsident und CEO von Oncolytics. "Es ist sehr wichtig, die Verwendung eines Mittels an Erwachsenen vor fortfahren bei pädiatrischen Patienten nutzen zu untersuchen. Mit der Arbeit bei Erwachsenen weit fortgeschritten, können wir jetzt vorankommen bei jüngeren
Oncolytic Viruses: Are They The Future of Cancer Therapy?
http://www.bioportfolio.com/news/article..." target="_blank" rel="nofollow ugc noopener">http://www.bioportfolio.com/news/article...
"Reoviruses: The Most Promising Option?"
"These combinations are showing extremely good results in human trials, particularly in refractory head and neck cancer patients. Many head and neck cancer patients treated with a combination of Reolysin and chemotherapy to date have experienced dramatic and prolonged tumor shrinkage, without increasing adverse side effects. A pivotal 180-to-480-patient Phase III head and neck cancer study commenced this year and could generate survival data in a few years. Non-small cell lung cancer (NSCLC) is another potential target for this treatment combination. The Cancer Therapy & Research Center at the University of Texas Health Science Centerâ??a big proponent of oncolytic virusesâ??has committed to funding up to five Phase II clinical trials using Reolysin in combination with chemotherapy against a variety of advanced cancers. And based on solid evidence that Reolysin could be effective as an anticancer agent targeting tumor types with activated Ras pathway, Oncolytics Biotech and clinical collaborators are actively testing Reolysin in ovarian cancer, colorectal cancer (though only in patients harboring mutated Kras gene), melanoma, sarcoma, and pancreatic cancer, for which tumor response data could be available in a year or two."
Onkolytische Viren: sie sind die Zukunft der Krebstherapie?
http://www.bioportfolio.com/news/article ...
"Reoviren: die vielversprechendste Option?"
"Diese Kombinationen sind extrem gute Ergebnisse in Studien am Menschen, vor allem im Kopf-Hals-refraktären Krebs-Patienten. Viele Kopf-Hals-Krebs-Patienten mit einer Kombination von REOLYSIN und Chemotherapie bisher behandelt haben erlebt dramatische und anhaltende Schrumpfung des Tumors, ohne Erhöhung Nebenwirkungen . Eine zentrale 180-zu-480-Patienten Phase-III-Kopf-und Halstumoren Studie in diesem Jahr begonnenen und konnte Überleben Daten in ein paar Jahren zu erzeugen. Nicht-kleinzelliges Lungenkarzinom (NSCLC) ist ein weiteres potenzielles Ziel für diese Behandlung Kombination. Der Krebs Therapy & Research Center an der University of Texas Health Science Center "? ein großer Befürworter von onkolytischen virusesâ? hat sich verpflichtet, bis zu fünf Phase-II-Studien mit REOLYSIN in Kombination mit Chemotherapie gegen eine Vielzahl von fortgeschrittenen Krebserkrankungen Finanzierung. Und auf der Grundlage solider Beweise, dass REOLYSIN wirksam sein könnte als Antikrebsmittel Targeting Tumorarten mit aktivierter Ras-Signalweg, Oncolytics Biotech und klinischen Partnern aktiv Prüfung Reolysin bei Eierstockkrebs, Dickdarmkrebs, Melanom, Sarkom (allerdings nur bei Patienten beherbergen KRAS-Gen mutiert), und der Bauchspeicheldrüse Krebs, bei denen Tumor-Antwort-Daten könnte in ein oder zwei Jahre zur Verfügung. "
http://www.bioportfolio.com/news/article..." target="_blank" rel="nofollow ugc noopener">http://www.bioportfolio.com/news/article...
"Reoviruses: The Most Promising Option?"
"These combinations are showing extremely good results in human trials, particularly in refractory head and neck cancer patients. Many head and neck cancer patients treated with a combination of Reolysin and chemotherapy to date have experienced dramatic and prolonged tumor shrinkage, without increasing adverse side effects. A pivotal 180-to-480-patient Phase III head and neck cancer study commenced this year and could generate survival data in a few years. Non-small cell lung cancer (NSCLC) is another potential target for this treatment combination. The Cancer Therapy & Research Center at the University of Texas Health Science Centerâ??a big proponent of oncolytic virusesâ??has committed to funding up to five Phase II clinical trials using Reolysin in combination with chemotherapy against a variety of advanced cancers. And based on solid evidence that Reolysin could be effective as an anticancer agent targeting tumor types with activated Ras pathway, Oncolytics Biotech and clinical collaborators are actively testing Reolysin in ovarian cancer, colorectal cancer (though only in patients harboring mutated Kras gene), melanoma, sarcoma, and pancreatic cancer, for which tumor response data could be available in a year or two."
Onkolytische Viren: sie sind die Zukunft der Krebstherapie?
http://www.bioportfolio.com/news/article ...
"Reoviren: die vielversprechendste Option?"
"Diese Kombinationen sind extrem gute Ergebnisse in Studien am Menschen, vor allem im Kopf-Hals-refraktären Krebs-Patienten. Viele Kopf-Hals-Krebs-Patienten mit einer Kombination von REOLYSIN und Chemotherapie bisher behandelt haben erlebt dramatische und anhaltende Schrumpfung des Tumors, ohne Erhöhung Nebenwirkungen . Eine zentrale 180-zu-480-Patienten Phase-III-Kopf-und Halstumoren Studie in diesem Jahr begonnenen und konnte Überleben Daten in ein paar Jahren zu erzeugen. Nicht-kleinzelliges Lungenkarzinom (NSCLC) ist ein weiteres potenzielles Ziel für diese Behandlung Kombination. Der Krebs Therapy & Research Center an der University of Texas Health Science Center "? ein großer Befürworter von onkolytischen virusesâ? hat sich verpflichtet, bis zu fünf Phase-II-Studien mit REOLYSIN in Kombination mit Chemotherapie gegen eine Vielzahl von fortgeschrittenen Krebserkrankungen Finanzierung. Und auf der Grundlage solider Beweise, dass REOLYSIN wirksam sein könnte als Antikrebsmittel Targeting Tumorarten mit aktivierter Ras-Signalweg, Oncolytics Biotech und klinischen Partnern aktiv Prüfung Reolysin bei Eierstockkrebs, Dickdarmkrebs, Melanom, Sarkom (allerdings nur bei Patienten beherbergen KRAS-Gen mutiert), und der Bauchspeicheldrüse Krebs, bei denen Tumor-Antwort-Daten könnte in ein oder zwei Jahre zur Verfügung. "
11/29/2010 11:22:33 AM ET News Release Index
Oncolytics Biotech® Inc. Announces Publication of Phase I Clinical Trial Results Examining Combination of REOLYSIN® and Docetaxel in Clinical Cancer Research
CALGARY, AB, --- November 29, 2010 - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) announced today that a paper entitled "REO-10: A Phase I Study of Intravenous Reovirus and Docetaxel in Patients with Advanced Cancer," was recently published by Comins et al in the journal Clinical Cancer Research (Clin Cancer Res 16(22):5564-5572).
The paper reports final results from a combination REOLYSIN and docetaxel trial, (REO 010) designed to evaluate the anti-tumour effects of systemic administration of REOLYSIN in combination with docetaxel (Taxotere®) in patients with advanced cancers. Patients received docetaxel on day one (75mg/m2) and escalating doses of reovirus up to 3 x 1010 TCID50 on days one through five, every three weeks. The principal investigator was Professor Hardev Pandha of the Royal Surrey County Hospital, U.K.
Twenty-five patients were enrolled, with 24 being exposed to treatment and 23 completing at least one cycle of therapy. Sixteen patients were suitable for response assessment. The combination was deemed to be safe and well tolerated and a maximum tolerated dose was not reached. Antitumour activity was seen with one complete response (in the liver of a breast cancer patient with no evidence of disease recurrence at the end of the study, following eight cycles of treatment) and three partial responses. A disease control rate (combined complete response, partial response and stable disease) of 88% was observed. The authors concluded that the combination of reovirus and docetaxel is safe, with evidence of objective disease response, and warrants further evaluation in a Phase II study at a recommended schedule of docetaxel (75mg/m2, three times weekly) and reovirus (3 x 1010 TCID50, days one to five, every three weeks).
Eligible patients included those who had been diagnosed with advanced or metastatic solid tumours including bladder, lung, prostate or upper gastro-intestinal cancers that were refractory (had not responded) to standard therapy or for which no curative standard therapy existed. The primary objective of the trial was to determine the MTD, Dose-Limiting Toxicity, recommended dose and dosing schedule and safety profile of REOLYSIN when administered in combination with docetaxel. Secondary objectives included the evaluation of immune response to the drug combination, the body's response to the drug combination compared to chemotherapy alone and any evidence of anti-tumour activity.
"These findings demonstrate a clear benefit for patients, even at lower doses, and provide us with additional patient data on another REOLYSIN/chemotherapy combination that we may elect to advance into later stage testing in the future," said Dr. Brad Thompson, President and CEO of Oncolytics.
Oncolytics Biotech® Inc. Announces Publication of Phase I Clinical Trial Results Examining Combination of REOLYSIN® and Docetaxel in Clinical Cancer Research
CALGARY, AB, --- November 29, 2010 - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) announced today that a paper entitled "REO-10: A Phase I Study of Intravenous Reovirus and Docetaxel in Patients with Advanced Cancer," was recently published by Comins et al in the journal Clinical Cancer Research (Clin Cancer Res 16(22):5564-5572).
The paper reports final results from a combination REOLYSIN and docetaxel trial, (REO 010) designed to evaluate the anti-tumour effects of systemic administration of REOLYSIN in combination with docetaxel (Taxotere®) in patients with advanced cancers. Patients received docetaxel on day one (75mg/m2) and escalating doses of reovirus up to 3 x 1010 TCID50 on days one through five, every three weeks. The principal investigator was Professor Hardev Pandha of the Royal Surrey County Hospital, U.K.
Twenty-five patients were enrolled, with 24 being exposed to treatment and 23 completing at least one cycle of therapy. Sixteen patients were suitable for response assessment. The combination was deemed to be safe and well tolerated and a maximum tolerated dose was not reached. Antitumour activity was seen with one complete response (in the liver of a breast cancer patient with no evidence of disease recurrence at the end of the study, following eight cycles of treatment) and three partial responses. A disease control rate (combined complete response, partial response and stable disease) of 88% was observed. The authors concluded that the combination of reovirus and docetaxel is safe, with evidence of objective disease response, and warrants further evaluation in a Phase II study at a recommended schedule of docetaxel (75mg/m2, three times weekly) and reovirus (3 x 1010 TCID50, days one to five, every three weeks).
Eligible patients included those who had been diagnosed with advanced or metastatic solid tumours including bladder, lung, prostate or upper gastro-intestinal cancers that were refractory (had not responded) to standard therapy or for which no curative standard therapy existed. The primary objective of the trial was to determine the MTD, Dose-Limiting Toxicity, recommended dose and dosing schedule and safety profile of REOLYSIN when administered in combination with docetaxel. Secondary objectives included the evaluation of immune response to the drug combination, the body's response to the drug combination compared to chemotherapy alone and any evidence of anti-tumour activity.
"These findings demonstrate a clear benefit for patients, even at lower doses, and provide us with additional patient data on another REOLYSIN/chemotherapy combination that we may elect to advance into later stage testing in the future," said Dr. Brad Thompson, President and CEO of Oncolytics.
CALGARY, Dec. 1 /PRNewswire/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ: ONCY) announced today the start of enrollment in a randomized Phase II ovarian cancer study. The Gynecologic Oncology Group (GOG) is conducting the randomized Phase II trial of weekly paclitaxel versus weekly paclitaxel with REOLYSIN® in patients with persistent or recurrent ovarian, fallopian tube or primary peritoneal cancer (GOG186H). The study is being sponsored by the Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, U.S. National Cancer Institute (NCI), which is part of the National Institutes of Health, under its Clinical Trials Agreement with Oncolytics. Oncolytics will provide clinical supplies of REOLYSIN for this study. The Study Chair is Dr. David E. Cohn of The Ohio State University Comprehensive Cancer Center - Arthur G. James Cancer Hospital and Richard J. Solove Research Institute.
"Treating recurrent ovarian cancer is challenging since the response rates to many chemotherapy regimens is relatively poor," said Dr. David E. Cohn. "Novel and targeted approaches to treating this disease hold promise in improving the outcome for women with recurrent ovarian cancer, while potentially limiting the side effects associated with standard chemotherapy."
The study is a randomized Phase II trial of weekly paclitaxel versus weekly paclitaxel with REOLYSIN in patients with persistent or recurrent ovarian, fallopian tube or primary peritoneal cancer. Patients will be randomized to receive either paclitaxel alone or paclitaxel plus REOLYSIN. Patients in both arms will receive treatment with paclitaxel, with the second arm also receiving intravenous REOLYSIN. Patients will receive standard doses of paclitaxel on days one, eight, and 15 every 28 days. In the second arm, patients will also receive, on days one through five of each 28-day cycle, intravenous REOLYSIN at a dose of 3x1010 TCID50.
The primary objectives of the trial are to estimate the progression-free survival hazard ratio of the combination of weekly paclitaxel with REOLYSIN to weekly paclitaxel alone in patients with persistent or recurrent ovarian, fallopian tube, or primary peritoneal cancer and to determine the frequency and severity of adverse events associated with treatment with weekly paclitaxel alone and weekly paclitaxel with REOLYSIN as assessed by Common Terminology Criteria for Adverse Events (CTCAE). The secondary objectives are to estimate the progression-free survival and overall survival of patients treated with weekly paclitaxel alone and weekly paclitaxel with REOLYSIN; to estimate (and compare) the proportion of patients who respond to the regimen on each arm of the study (according to RECIST 1.1 with measurable patients and by CA-125 for those patients with detectible disease only); and to characterize and compare progression-free survival and overall survival in patients with measurable disease (RECIST 1.1 criteria) and patients with detectable (nonmeasurable) disease. The study is expected to enroll up to 150 patients.
Information on the study will be available at href="http://www.clinicaltrials.gov/">www.clinicaltrials.gov.
"Treating recurrent ovarian cancer is challenging since the response rates to many chemotherapy regimens is relatively poor," said Dr. David E. Cohn. "Novel and targeted approaches to treating this disease hold promise in improving the outcome for women with recurrent ovarian cancer, while potentially limiting the side effects associated with standard chemotherapy."
The study is a randomized Phase II trial of weekly paclitaxel versus weekly paclitaxel with REOLYSIN in patients with persistent or recurrent ovarian, fallopian tube or primary peritoneal cancer. Patients will be randomized to receive either paclitaxel alone or paclitaxel plus REOLYSIN. Patients in both arms will receive treatment with paclitaxel, with the second arm also receiving intravenous REOLYSIN. Patients will receive standard doses of paclitaxel on days one, eight, and 15 every 28 days. In the second arm, patients will also receive, on days one through five of each 28-day cycle, intravenous REOLYSIN at a dose of 3x1010 TCID50.
The primary objectives of the trial are to estimate the progression-free survival hazard ratio of the combination of weekly paclitaxel with REOLYSIN to weekly paclitaxel alone in patients with persistent or recurrent ovarian, fallopian tube, or primary peritoneal cancer and to determine the frequency and severity of adverse events associated with treatment with weekly paclitaxel alone and weekly paclitaxel with REOLYSIN as assessed by Common Terminology Criteria for Adverse Events (CTCAE). The secondary objectives are to estimate the progression-free survival and overall survival of patients treated with weekly paclitaxel alone and weekly paclitaxel with REOLYSIN; to estimate (and compare) the proportion of patients who respond to the regimen on each arm of the study (according to RECIST 1.1 with measurable patients and by CA-125 for those patients with detectible disease only); and to characterize and compare progression-free survival and overall survival in patients with measurable disease (RECIST 1.1 criteria) and patients with detectable (nonmeasurable) disease. The study is expected to enroll up to 150 patients.
Information on the study will be available at href="http://www.clinicaltrials.gov/">www.clinicaltrials.gov.
Antwort auf Beitrag Nr.: 40.623.687 von reguau am 01.12.10 14:39:00Written by Douglas W. Loe, PhD, MBA Monday, 22 November 2010 01:42
Chemotherapy—as any cancer patient will tell you—is not for the faint of heart, but it can kill many forms of cancer. Some form of chemotherapy, originally discovered as a cancer treatment almost 70 years ago, is still routinely prescribed for most types of the disease. The treatment works by targeting fast-growing cells, like those typically found in rapidly growing tumors. But while chemotherapy can shrink tumors, they often grow back and become resistant, or refractory to the treatment.
To combat this resistance, chemotherapy is now often used in combination with other treatments that have different mechanisms for attacking and killing cancer cells. Doctors must be cautious when combining treatments to ensure that the regimen does not become too toxic for patients to tolerate. The goal is to introduce drugs that can be used synergistically with chemotherapy to not only extend life, but to improve quality of life while undergoing treatment.
The Potential of Oncolytic Viruses
One approach that has proven quite promising is known as oncolytic virotherapeutics. Here, viruses are harnessed to infect, multiply within and subsequently lyse cancer cells; the virus targets tumors without affecting normal tissue.
Several types of oncolytic viruses have been developed to date. These include the adenovirus, which is a non-enveloped virus with a double-stranded, linear DNA genome that forms particles that are 70 to 90 nm in size. There are multiple engineered versions of this virus in clinical trials, including Onyx-015 and H101. The latter has been approved in China and is sold by Shanghai Sunway Biotech.
A second form of oncolytic virus is Newcastle-disease virus (NDV). This is an enveloped virus with a single-stranded, negative-sense RNA genome that forms pleiomorphic particles ranging from 150 to 300 nm. Naturally attenuated versions, such as PV701, are in clinical development. Although still in Phase I testing, slow virus infusion rather than injection seems to mitigate side effects. The Maryland-based private firm Wellstate Biologics has two Phase I open-label PV701 cancer trials ongoing.
Poxviruses are a family of enveloped viruses that contain a double-stranded, linear DNA genome and form particles that are 200 nm in diameter and 300 nm in length. Myxoma and vaccinia are family members that are under therapeutic development. Among several candidates, the most advanced is probably Jennerex’s JX-594, which is entering a Phase II liver cancer trial in partnership with Transgene (see below) and which could enter a Phase II colorectal cancer study imminently, testing JX-594 in patients refractory to Eli Lilly’s (NYSE: LLY ) leading EGFr-targeted antibody drug Erbitux. Interim data are already available from nine patients in a small Phase I/II liver cancer study showing disease stabilization in at least five patients when JX-594 is administered with Bayer’s approved liver cancer drug sorafenib (branded as Nexavar). JX-594 performed just as well in a separate Phase I liver cancer study where it was tested as monotherapy and not in combination with Nexavar. Interim data from 24 patients in that trial showed disease stabilization at two months in most JX-594-treated patients, providing solid evidence of JX-594 oncolytic virus potency at treating this cancer form.
Oncolytic Viruses: An Approved Product on the Horizon?
Oncolytic Viruses in Cancer Therapy
ONCOLYTICS BIOTECH
4.81 +0.13 (+2.85%)
Intraday | 3 Month | 6 Month | 1 Year
Quotes delayed at least 20 mins.
It may come as a surprise to some that the herpes simplex virus is also under consideration as an oncolytic virus. This is an enveloped virus with a double-stranded, linear DNA genome that forms particles that are 150 to 200 nm. Many engineered versions are in clinical trials for the treatment of patients with cancer, such as OncoVEXGM-CSF, G207, HSV-1716 and NV1020. The most advanced of these is OncoVEXGM-CSF, a combination of the oncolytic virus OncoVEX plus granulocyte macrophage-colony stimulating factor (GM-CSF) developed by Massachusetts-based private firm BioVex; the combination is already well-advanced in a 360-patient Phase III advanced melanoma trial and a 528-patient Phase III head & neck cancer trial design has been endorsed by the U.S. FDA under a Special Protocol Assessment and could begin later this year, or perhaps early 2011. G207 was developed by the German firm Medigene, which recently completed a Phase II brain cancer trial, and was also developing NV1020, which recently completed testing in a 27-patient Phase II liver metastasis from colon cancer trial. However, neither oncolytic virus formulation is identified in Medigene’s clinical pipeline at present. UK-based Crusade Laboratories tested HSV-1716 in a Phase I oral cancer trial; a Phase III GBM trial is being planned as are Phase I/II ovarian cancer and liver cancer trials. And lastly, BioSante Pharmaceuticals’ (Nasdaq:BPAX) adenovirus CG0070 was recently sold to private biotech firm Cold Genesys; the virus performed well in a 35-patient Phase I bladder cancer trial conducted by innovator Cell Genesys, and based on those data, the virus could advance into Phase I/II bladder cancer testing at company discretion.
Picornaviruses are a family of non-enveloped viruses with single-stranded, positive-sense RNA genomes that form particles that range from 18 to 30 nm. Members of this family that are being tested as oncolytic therapeutics include coxsackieviruses and engineered versions of poliovirus. The latter is in development at a few locations, including research institutes at Duke University and Stony Brook University, and has shown some preclinical efficacy against GBM and neuroblastoma. The firm Viralytics is developing the coxsackievirus A21 in a Phase I advanced melanoma study.
Vesicular stomatitis virus (VSV) is an enveloped virus with a single-stranded, negative-sense RNA that forms 65 to 185 nm bullet-shaped particles. This virus is still in the research stage; two constructs have recently been tested at the Mt. Sinai School of Medicine in New York.
Reoviruses: The Most Promising Option?
Finally, we come to what some consider the most promising form of oncolytic virus: the reovirus. This is a non-enveloped virus with a double-stranded, segmented RNA genome that forms particles that are 60 to 90 nm. The reovirus preferentially replicates in cancer cells that feature a common mutation known as an “activated Ras pathway,” while sparing normal cells. This makes it intrinsically tumor selective without the need for any genetic manipulation.
Reovirus is a virus with no known associated disease. It replicates in the cytoplasm and therefore does not integrate into the cell’s DNA. Reovirus is found everywhere in nature and has been isolated from untreated sewage, river and stagnant waters. Exposure to reovirus is common in humans, with half of all children by the age of 12 having been exposed and up to 100% testing positive by adulthood.
Tumors bearing an activated Ras pathway cannot activate the anti-viral response mediated by the host cellular protein, PKR. Studies have shown that reovirus actively replicates in transformed cell lines with an active Ras signaling pathway, eventually killing the host cell and freeing the viral progeny that go on to infect and kill more Ras-activated tumor cells. When normal cells are infected with reovirus, the immune system can neutralize the virus. Approximately one-third of human cancers have activating mutations in the Ras gene itself, and it is possible that more than two-thirds of cancer cells have an activated Ras signaling pathway because of activating mutations in genes upstream or downstream of Ras.
How Reoviruses Might Help
While it has been demonstrated in animal studies that reovirus is capable of treating metastatic cancer in immunocompetent mice, it has also been shown that reovirus used in conjunction with immunosuppressive drugs can effectively prolong animal survival. Combining IV reovirus therapy with Cyclosporine A, an immune suppressant, significantly inhibited tumor regrowth. In a model of disseminated LLC metastatic lung cancer in C57BL mice, treatment with reovirus and either Cyclosporine A or T cell depleting antibodies (anti-CD4 and anti-CD8 Ab) led to an increase in survival compared to treatment with reovirus alone.
The above results supported the development of clinical protocols in which immune suppressive drugs could be combined with a systemically administered reovirus in the treatment of cancer. The combination of reovirus with various chemotherapies in human colorectal cancer cell lines demonstrated synergistic cytotoxic activity. In addition to modulating the immune response, the use of chemotherapies along with reovirus treatment may enhance intratumoral spread of the virus.
Calgary-based Oncolytics Biotech, Inc.(Nasdaq:ONCY) has developed a biologic agent, Reolysin, from naturallyoccurring reovirus. The virus has demonstrated impressive results in clinical trials on its own, but particularly in combination with certain chemotherapeutics. In preclinical studies in a wide variety of cancer cell lines, investigators found that when used together, reovirus and chemotherapy resulted in more efficient and synergistic anti-cancer activity than when each agent was used on its own.
These combinations are showing extremely good results in human trials, particularly in refractory head and neck cancer patients. Many head and neck cancer patients treated with a combination of Reolysin and chemotherapy to date have experienced dramatic and prolonged tumor shrinkage, without increasing adverse side effects. A pivotal 180-to-480-patient Phase III head and neck cancer study commenced this year and could generate survival data in a few years. Non-small cell lung cancer (NSCLC) is another potential target for this treatment combination. The Cancer Therapy & Research Center at the University of Texas Health Science Center—a big proponent of oncolytic viruses—has committed to funding up to five Phase II clinical trials using Reolysin in combination with chemotherapy against a variety of advanced cancers. And based on solid evidence that Reolysin could be effective as an anticancer agent targeting tumor types with activated Ras pathway, Oncolytics Biotech and clinical collaborators are actively testing Reolysin in ovarian cancer, colorectal cancer (though only in patients harboring mutated Kras gene), melanoma, sarcoma, and pancreatic cancer, for which tumor response data could be available in a year or two.
It is difficult to provide a crystal-clear economic forecast for oncolytic viruses as a whole, but an indicator of their potential future sales earnings can be derived from examining four recently-launched anticancer therapies already on the market. One of these is erlotinib, branded as Tarceva, developed by OSI Pharmaceuticals and launched in 2004 by Roche/Genentech and OSI. An oral small molecule tyrosine kinase inhibitor drug that is prescribed for patients with advanced-stage non-small cell lung cancer, it earned $20 million in 2004, $387 million in 2005, and $813 million in 2006. Sales reached $1.2 billion in 2009. Another is the immunomodulatory and anti-angiogenic drug thalidomide (branded as Thalomid), developed by Celgene and launched in 2003 for treating multiple myeloma, which enjoyed sales of $224 million that first year and reached $505 million by 2008. A third is lenalidomide (branded as Revlimid and also developed by Celgene), a drug structurally related to Thalomid that generated even more robust sales growth after its launch in 2006, achieving blockbuster sales in 2008 of $1.3 billion that grew to $1.7 billion last year. And a fourth is the oral alkylating agent temozolamide, branded as Temodar by Schering-Plough (now part of Merck) and approved as a glioblastoma (brain cancer) drug; Temodar generated $180 million in sales in 2001 that grew to $703 million in 2006 (its first full-year of sales in the U.S.) and exceeded US$1 billion in 2009. Though survival benefit of 11 weeks is modest, the drug (along with Roche’s Avastin) is still standard-of-care for patients with advanced brain cancer.
Partners and capital markets are now recognizing value in oncolytic viruses
The year-over-year, steadily increasing demand for the four drugs described above provides supporting evidence that demand for new and effective agents in oncology remains strong, giving us confidence that Reolysin could be similarly embraced if it performs well in Phase III testing. We are optimistic that global oncology-focused pharmaceutical firms will be keen to partner with Oncolytics once Reolysin Phase III head and neck cancer data are available, if not before, and capital markets are exhibiting optimism in Reolysin’s medical prospects through the company’s share price strength this year and through well-subscribed equity offerings in 2009/10.
And in other commercial advances in oncolytic virus development, Jennerex’s JX-594 is now partnered with the French biotechnology firm Transgene in a $116 million alliance that could pay Jennerex a double-digit royalty on future JX-594 sales if the drug performs well in pivotal liver cancer or colorectal cancer trials, or in other cancer indications contemplated in the alliance. The Transgene alliance seems to be focused on European and Middle East marketing rights and thus provides an opportunity for U.S. or Japan-based firms to partner with Jennerex in those regions.
As we have seen above, there are a number of oncolytic viruses that have shown potential use in cancer treatment and demand for more effective agents is strong. Future research studies will give us an even clearer perspective on which, if any, of these viruses offer the most effective route toward a reliable and commercially viable complement to chemotherapy for oncologists and their patients.
About the Author
Douglas W. Loe, Ph.D, MBA, is a consistently topped ranked healthcare and biotechnology analyst. He has covered Canadian biotech since 2000, initially as part of the research team at Yorkton Securities (now Macquarie Capital Markets), and has been with Versant since Fall 2002 where he covers a broad spectrum of drug development, medical technology, and healthcare services firms. Doug holds a MBA from Queen’s University and a Ph.D in biochemistry from the University of Guelph, working in the area of cancer chemotherapy and multidrug resistance, followed by post-doctoral training at the Queen’s University Cancer Research Institute. During his scientific career, he published multiple abstracts, peer-reviewed manuscripts and reviews related to P-glycoprotein and MRP-mediated multidrug resistance. He can be reached at DLoe@versantpartners.com This e-mail address is being protected from spambots. You need JavaScript enabled to view it . Versant Partners is a member of the Canadian Investor Protection Fund (CIPF).
Douglas W. Loe, PhD, MBA authored and submitted this article solely for the purpose of providing key expert analysis and opinion. No compensation was exchanged by either party in consideration for the publication of this material.
If you are a science or medical expert willing to share your market intelligence with our readers, please contact us.
Read the full report: http://biomedreports.com/2010112260394/oncolytic-viruses-are…
Chemotherapy—as any cancer patient will tell you—is not for the faint of heart, but it can kill many forms of cancer. Some form of chemotherapy, originally discovered as a cancer treatment almost 70 years ago, is still routinely prescribed for most types of the disease. The treatment works by targeting fast-growing cells, like those typically found in rapidly growing tumors. But while chemotherapy can shrink tumors, they often grow back and become resistant, or refractory to the treatment.
To combat this resistance, chemotherapy is now often used in combination with other treatments that have different mechanisms for attacking and killing cancer cells. Doctors must be cautious when combining treatments to ensure that the regimen does not become too toxic for patients to tolerate. The goal is to introduce drugs that can be used synergistically with chemotherapy to not only extend life, but to improve quality of life while undergoing treatment.
The Potential of Oncolytic Viruses
One approach that has proven quite promising is known as oncolytic virotherapeutics. Here, viruses are harnessed to infect, multiply within and subsequently lyse cancer cells; the virus targets tumors without affecting normal tissue.
Several types of oncolytic viruses have been developed to date. These include the adenovirus, which is a non-enveloped virus with a double-stranded, linear DNA genome that forms particles that are 70 to 90 nm in size. There are multiple engineered versions of this virus in clinical trials, including Onyx-015 and H101. The latter has been approved in China and is sold by Shanghai Sunway Biotech.
A second form of oncolytic virus is Newcastle-disease virus (NDV). This is an enveloped virus with a single-stranded, negative-sense RNA genome that forms pleiomorphic particles ranging from 150 to 300 nm. Naturally attenuated versions, such as PV701, are in clinical development. Although still in Phase I testing, slow virus infusion rather than injection seems to mitigate side effects. The Maryland-based private firm Wellstate Biologics has two Phase I open-label PV701 cancer trials ongoing.
Poxviruses are a family of enveloped viruses that contain a double-stranded, linear DNA genome and form particles that are 200 nm in diameter and 300 nm in length. Myxoma and vaccinia are family members that are under therapeutic development. Among several candidates, the most advanced is probably Jennerex’s JX-594, which is entering a Phase II liver cancer trial in partnership with Transgene (see below) and which could enter a Phase II colorectal cancer study imminently, testing JX-594 in patients refractory to Eli Lilly’s (NYSE: LLY ) leading EGFr-targeted antibody drug Erbitux. Interim data are already available from nine patients in a small Phase I/II liver cancer study showing disease stabilization in at least five patients when JX-594 is administered with Bayer’s approved liver cancer drug sorafenib (branded as Nexavar). JX-594 performed just as well in a separate Phase I liver cancer study where it was tested as monotherapy and not in combination with Nexavar. Interim data from 24 patients in that trial showed disease stabilization at two months in most JX-594-treated patients, providing solid evidence of JX-594 oncolytic virus potency at treating this cancer form.
Oncolytic Viruses: An Approved Product on the Horizon?
Oncolytic Viruses in Cancer Therapy
ONCOLYTICS BIOTECH
4.81 +0.13 (+2.85%)
Intraday | 3 Month | 6 Month | 1 Year
Quotes delayed at least 20 mins.
It may come as a surprise to some that the herpes simplex virus is also under consideration as an oncolytic virus. This is an enveloped virus with a double-stranded, linear DNA genome that forms particles that are 150 to 200 nm. Many engineered versions are in clinical trials for the treatment of patients with cancer, such as OncoVEXGM-CSF, G207, HSV-1716 and NV1020. The most advanced of these is OncoVEXGM-CSF, a combination of the oncolytic virus OncoVEX plus granulocyte macrophage-colony stimulating factor (GM-CSF) developed by Massachusetts-based private firm BioVex; the combination is already well-advanced in a 360-patient Phase III advanced melanoma trial and a 528-patient Phase III head & neck cancer trial design has been endorsed by the U.S. FDA under a Special Protocol Assessment and could begin later this year, or perhaps early 2011. G207 was developed by the German firm Medigene, which recently completed a Phase II brain cancer trial, and was also developing NV1020, which recently completed testing in a 27-patient Phase II liver metastasis from colon cancer trial. However, neither oncolytic virus formulation is identified in Medigene’s clinical pipeline at present. UK-based Crusade Laboratories tested HSV-1716 in a Phase I oral cancer trial; a Phase III GBM trial is being planned as are Phase I/II ovarian cancer and liver cancer trials. And lastly, BioSante Pharmaceuticals’ (Nasdaq:BPAX) adenovirus CG0070 was recently sold to private biotech firm Cold Genesys; the virus performed well in a 35-patient Phase I bladder cancer trial conducted by innovator Cell Genesys, and based on those data, the virus could advance into Phase I/II bladder cancer testing at company discretion.
Picornaviruses are a family of non-enveloped viruses with single-stranded, positive-sense RNA genomes that form particles that range from 18 to 30 nm. Members of this family that are being tested as oncolytic therapeutics include coxsackieviruses and engineered versions of poliovirus. The latter is in development at a few locations, including research institutes at Duke University and Stony Brook University, and has shown some preclinical efficacy against GBM and neuroblastoma. The firm Viralytics is developing the coxsackievirus A21 in a Phase I advanced melanoma study.
Vesicular stomatitis virus (VSV) is an enveloped virus with a single-stranded, negative-sense RNA that forms 65 to 185 nm bullet-shaped particles. This virus is still in the research stage; two constructs have recently been tested at the Mt. Sinai School of Medicine in New York.
Reoviruses: The Most Promising Option?
Finally, we come to what some consider the most promising form of oncolytic virus: the reovirus. This is a non-enveloped virus with a double-stranded, segmented RNA genome that forms particles that are 60 to 90 nm. The reovirus preferentially replicates in cancer cells that feature a common mutation known as an “activated Ras pathway,” while sparing normal cells. This makes it intrinsically tumor selective without the need for any genetic manipulation.
Reovirus is a virus with no known associated disease. It replicates in the cytoplasm and therefore does not integrate into the cell’s DNA. Reovirus is found everywhere in nature and has been isolated from untreated sewage, river and stagnant waters. Exposure to reovirus is common in humans, with half of all children by the age of 12 having been exposed and up to 100% testing positive by adulthood.
Tumors bearing an activated Ras pathway cannot activate the anti-viral response mediated by the host cellular protein, PKR. Studies have shown that reovirus actively replicates in transformed cell lines with an active Ras signaling pathway, eventually killing the host cell and freeing the viral progeny that go on to infect and kill more Ras-activated tumor cells. When normal cells are infected with reovirus, the immune system can neutralize the virus. Approximately one-third of human cancers have activating mutations in the Ras gene itself, and it is possible that more than two-thirds of cancer cells have an activated Ras signaling pathway because of activating mutations in genes upstream or downstream of Ras.
How Reoviruses Might Help
While it has been demonstrated in animal studies that reovirus is capable of treating metastatic cancer in immunocompetent mice, it has also been shown that reovirus used in conjunction with immunosuppressive drugs can effectively prolong animal survival. Combining IV reovirus therapy with Cyclosporine A, an immune suppressant, significantly inhibited tumor regrowth. In a model of disseminated LLC metastatic lung cancer in C57BL mice, treatment with reovirus and either Cyclosporine A or T cell depleting antibodies (anti-CD4 and anti-CD8 Ab) led to an increase in survival compared to treatment with reovirus alone.
The above results supported the development of clinical protocols in which immune suppressive drugs could be combined with a systemically administered reovirus in the treatment of cancer. The combination of reovirus with various chemotherapies in human colorectal cancer cell lines demonstrated synergistic cytotoxic activity. In addition to modulating the immune response, the use of chemotherapies along with reovirus treatment may enhance intratumoral spread of the virus.
Calgary-based Oncolytics Biotech, Inc.(Nasdaq:ONCY) has developed a biologic agent, Reolysin, from naturallyoccurring reovirus. The virus has demonstrated impressive results in clinical trials on its own, but particularly in combination with certain chemotherapeutics. In preclinical studies in a wide variety of cancer cell lines, investigators found that when used together, reovirus and chemotherapy resulted in more efficient and synergistic anti-cancer activity than when each agent was used on its own.
These combinations are showing extremely good results in human trials, particularly in refractory head and neck cancer patients. Many head and neck cancer patients treated with a combination of Reolysin and chemotherapy to date have experienced dramatic and prolonged tumor shrinkage, without increasing adverse side effects. A pivotal 180-to-480-patient Phase III head and neck cancer study commenced this year and could generate survival data in a few years. Non-small cell lung cancer (NSCLC) is another potential target for this treatment combination. The Cancer Therapy & Research Center at the University of Texas Health Science Center—a big proponent of oncolytic viruses—has committed to funding up to five Phase II clinical trials using Reolysin in combination with chemotherapy against a variety of advanced cancers. And based on solid evidence that Reolysin could be effective as an anticancer agent targeting tumor types with activated Ras pathway, Oncolytics Biotech and clinical collaborators are actively testing Reolysin in ovarian cancer, colorectal cancer (though only in patients harboring mutated Kras gene), melanoma, sarcoma, and pancreatic cancer, for which tumor response data could be available in a year or two.
It is difficult to provide a crystal-clear economic forecast for oncolytic viruses as a whole, but an indicator of their potential future sales earnings can be derived from examining four recently-launched anticancer therapies already on the market. One of these is erlotinib, branded as Tarceva, developed by OSI Pharmaceuticals and launched in 2004 by Roche/Genentech and OSI. An oral small molecule tyrosine kinase inhibitor drug that is prescribed for patients with advanced-stage non-small cell lung cancer, it earned $20 million in 2004, $387 million in 2005, and $813 million in 2006. Sales reached $1.2 billion in 2009. Another is the immunomodulatory and anti-angiogenic drug thalidomide (branded as Thalomid), developed by Celgene and launched in 2003 for treating multiple myeloma, which enjoyed sales of $224 million that first year and reached $505 million by 2008. A third is lenalidomide (branded as Revlimid and also developed by Celgene), a drug structurally related to Thalomid that generated even more robust sales growth after its launch in 2006, achieving blockbuster sales in 2008 of $1.3 billion that grew to $1.7 billion last year. And a fourth is the oral alkylating agent temozolamide, branded as Temodar by Schering-Plough (now part of Merck) and approved as a glioblastoma (brain cancer) drug; Temodar generated $180 million in sales in 2001 that grew to $703 million in 2006 (its first full-year of sales in the U.S.) and exceeded US$1 billion in 2009. Though survival benefit of 11 weeks is modest, the drug (along with Roche’s Avastin) is still standard-of-care for patients with advanced brain cancer.
Partners and capital markets are now recognizing value in oncolytic viruses
The year-over-year, steadily increasing demand for the four drugs described above provides supporting evidence that demand for new and effective agents in oncology remains strong, giving us confidence that Reolysin could be similarly embraced if it performs well in Phase III testing. We are optimistic that global oncology-focused pharmaceutical firms will be keen to partner with Oncolytics once Reolysin Phase III head and neck cancer data are available, if not before, and capital markets are exhibiting optimism in Reolysin’s medical prospects through the company’s share price strength this year and through well-subscribed equity offerings in 2009/10.
And in other commercial advances in oncolytic virus development, Jennerex’s JX-594 is now partnered with the French biotechnology firm Transgene in a $116 million alliance that could pay Jennerex a double-digit royalty on future JX-594 sales if the drug performs well in pivotal liver cancer or colorectal cancer trials, or in other cancer indications contemplated in the alliance. The Transgene alliance seems to be focused on European and Middle East marketing rights and thus provides an opportunity for U.S. or Japan-based firms to partner with Jennerex in those regions.
As we have seen above, there are a number of oncolytic viruses that have shown potential use in cancer treatment and demand for more effective agents is strong. Future research studies will give us an even clearer perspective on which, if any, of these viruses offer the most effective route toward a reliable and commercially viable complement to chemotherapy for oncologists and their patients.
About the Author
Douglas W. Loe, Ph.D, MBA, is a consistently topped ranked healthcare and biotechnology analyst. He has covered Canadian biotech since 2000, initially as part of the research team at Yorkton Securities (now Macquarie Capital Markets), and has been with Versant since Fall 2002 where he covers a broad spectrum of drug development, medical technology, and healthcare services firms. Doug holds a MBA from Queen’s University and a Ph.D in biochemistry from the University of Guelph, working in the area of cancer chemotherapy and multidrug resistance, followed by post-doctoral training at the Queen’s University Cancer Research Institute. During his scientific career, he published multiple abstracts, peer-reviewed manuscripts and reviews related to P-glycoprotein and MRP-mediated multidrug resistance. He can be reached at DLoe@versantpartners.com This e-mail address is being protected from spambots. You need JavaScript enabled to view it . Versant Partners is a member of the Canadian Investor Protection Fund (CIPF).
Douglas W. Loe, PhD, MBA authored and submitted this article solely for the purpose of providing key expert analysis and opinion. No compensation was exchanged by either party in consideration for the publication of this material.
If you are a science or medical expert willing to share your market intelligence with our readers, please contact us.
Read the full report: http://biomedreports.com/2010112260394/oncolytic-viruses-are…
Oncolytics continues to push their pipeline further PDF | Print | E-mail
Written by Patrick Crutcher
Thursday, 02 December 2010 04:15
Oncolytics Biotech Inc.(NASDAQ: ONCY) is another company pushing the limits of immunotherapeutics. Yesterday, they announced that enrollment had in a randomized Phase 2 ovarian cancer study using Reolysin. They currently have 11 ongoing studies.
This study will be a randomized phase 2 trial of weekly paclitaxel versus weekly paclitaxel with Reolysin in patients with persistent or recurrent ovarian, fallopian tube or primary peritoneal cancer (GOG186H). They recently also began a Phase 1 trial in pediatric patients with relapsed or refractory solid tumors and announced some promising publications regarding their lead candidate, Reolysin.
Oncoloytics' Reolysin is an immunotherapy that works by using the reovirus to replicate in cancer cells that with a mutation known as an “activated Ras pathway,” but it spares normal cells; the reovirus then replicates in those cells with an active Ras signaling pathway, eventually killing the host cell and continuing this cycle. It has made some impressive results as a monotherapy, but has a particularly synergistic effect in combination with certain chemotherapeutics.
With ONCY conducting eleven(11) clinical trials in the U.K. and U.S., it makes you wonder how they've done it without heavily diluting. This has only been possible thru collaborative agreements with the National Cancer Institute(NCI) in the U.S. to conduct multiple clinical trials, the Cancer Therapy and Research Center at the University of Texas Health Science Center has committed to funding up to five(5) Phase II clinical trials using Reolysin in combination with chemotherapy against a variety of advanced cancers, and sponsored studies in the UK. Oncolytics Biotech and clinical collaborators are actively testing Reolysin in ovarian cancer, colorectal cancer ( only patients with mutated Kras gene), melanoma, sarcoma, and pancreatic cancer.
ONCOLYTICS BIOTECH
4.82 +0.14 (+2.99%)
Intraday |
3 Month |
6 Month |
1 Year
Quotes delayed at least 20 mins.
Financially, these agreements have kept them afloat except for the occasional capital raise. More importantly, they are running a Phase 3 trial examining REOLYSIN in combination with paclitaxel and carboplatin in patients with platinum-refractory head and neck cancers that's being conducted under a Special Protocol Assessment(SPA). As we get close to top-line results later on down the road, you can be sure that volatility will increase. The Street and big pharma will be watching.
With a pipeline of 11 ongoing trials, we can expect ONCY to begin announcing results from these trials over the next 1-2 years. Note that they raised roughly $28 million back in early November and should have enough cash to see them through until 2012. By then, we should have more complete knowledge on the potential for Reolysin.
Disclosure: No position.
ONCY begins Phase 2 in ovarian cancer - http://bit.ly/hsAHPA
Recent report on ONCY - http://biomedreports.com/2010112260394/oncolytic-viruses-are…
Recent ONCY news - http://www.integratir.com/newsrelease.asp?ticker=t.onc
ONCY pipeline - http://www.oncolyticsbiotech.com/clinical.html
Read the full report: http://biomedreports.com/2010120260837/oncolytics-continues-…
Written by Patrick Crutcher
Thursday, 02 December 2010 04:15
Oncolytics Biotech Inc.(NASDAQ: ONCY) is another company pushing the limits of immunotherapeutics. Yesterday, they announced that enrollment had in a randomized Phase 2 ovarian cancer study using Reolysin. They currently have 11 ongoing studies.
This study will be a randomized phase 2 trial of weekly paclitaxel versus weekly paclitaxel with Reolysin in patients with persistent or recurrent ovarian, fallopian tube or primary peritoneal cancer (GOG186H). They recently also began a Phase 1 trial in pediatric patients with relapsed or refractory solid tumors and announced some promising publications regarding their lead candidate, Reolysin.
Oncoloytics' Reolysin is an immunotherapy that works by using the reovirus to replicate in cancer cells that with a mutation known as an “activated Ras pathway,” but it spares normal cells; the reovirus then replicates in those cells with an active Ras signaling pathway, eventually killing the host cell and continuing this cycle. It has made some impressive results as a monotherapy, but has a particularly synergistic effect in combination with certain chemotherapeutics.
With ONCY conducting eleven(11) clinical trials in the U.K. and U.S., it makes you wonder how they've done it without heavily diluting. This has only been possible thru collaborative agreements with the National Cancer Institute(NCI) in the U.S. to conduct multiple clinical trials, the Cancer Therapy and Research Center at the University of Texas Health Science Center has committed to funding up to five(5) Phase II clinical trials using Reolysin in combination with chemotherapy against a variety of advanced cancers, and sponsored studies in the UK. Oncolytics Biotech and clinical collaborators are actively testing Reolysin in ovarian cancer, colorectal cancer ( only patients with mutated Kras gene), melanoma, sarcoma, and pancreatic cancer.
ONCOLYTICS BIOTECH
4.82 +0.14 (+2.99%)
Intraday |
3 Month |
6 Month |
1 Year
Quotes delayed at least 20 mins.
Financially, these agreements have kept them afloat except for the occasional capital raise. More importantly, they are running a Phase 3 trial examining REOLYSIN in combination with paclitaxel and carboplatin in patients with platinum-refractory head and neck cancers that's being conducted under a Special Protocol Assessment(SPA). As we get close to top-line results later on down the road, you can be sure that volatility will increase. The Street and big pharma will be watching.
With a pipeline of 11 ongoing trials, we can expect ONCY to begin announcing results from these trials over the next 1-2 years. Note that they raised roughly $28 million back in early November and should have enough cash to see them through until 2012. By then, we should have more complete knowledge on the potential for Reolysin.
Disclosure: No position.
ONCY begins Phase 2 in ovarian cancer - http://bit.ly/hsAHPA
Recent report on ONCY - http://biomedreports.com/2010112260394/oncolytic-viruses-are…
Recent ONCY news - http://www.integratir.com/newsrelease.asp?ticker=t.onc
ONCY pipeline - http://www.oncolyticsbiotech.com/clinical.html
Read the full report: http://biomedreports.com/2010120260837/oncolytics-continues-…
KursdatenBörse Nasdaq
Aktuell 5,74 USD
Zeit 06.12.1021:05
Diff. Vortag +13,22%
Tages-Vol. --
Gehandelte Stück 453.701
Aktuell 5,74 USD
Zeit 06.12.1021:05
Diff. Vortag +13,22%
Tages-Vol. --
Gehandelte Stück 453.701
Antwort auf Beitrag Nr.: 40.657.653 von reguau am 06.12.10 21:22:08müßt Ihr mal lesen..........
http://www.thestreet.com/_yahoo/story/10941504/1/biotechs-br…
http://www.thestreet.com/_yahoo/story/10941504/1/biotechs-br…
KursdatenBörse Nasdaq
Aktuell 6,10 USD
Zeit 09.12.1022:00
Diff. Vortag +5,72%
Tages-Vol. --
Gehandelte Stück 140.900
Aktuell 6,10 USD
Zeit 09.12.1022:00
Diff. Vortag +5,72%
Tages-Vol. --
Gehandelte Stück 140.900
Antwort auf Beitrag Nr.: 40.685.725 von reguau am 10.12.10 00:41:07By Mark Bern at Seeking Alpha
Thread: Borrell: Entscheidung über Nord Stream 2 ist keine EU-Sache
As to how many of us 'gades are likely to be hit by cancer of some sort, I believe the ratio is about 1 in 4 here in America will get cancer. That is not encouraging. But, then again, if we can profit from it while we wait to see if genes are among the more susceptible pool, why not?
The english version of how it works:
Resolyn is a man-made version of a naturally occurring virus (reovirus) that most people contract multiple times throughout their lifetime. This type of virus is not harmful. They actually help keep us healthy by cleaning up things like cancer without our even knowing it. I suppose you could say that (at least some) people who don't get cancer probably were protected by getting a reovirus on occasion.
How it works: cells in the human body have receptors for nourishment that stay closed over 95% of the time once we stop growing. Think of the receptor as an on/off switch. When the cell needs nourishment the receptor click to the on position. Only when it is in the on position can a reovirus enter. So our normal cells are protected most of the time. When a reovirus enters a cell, it devours it. The reovirus will continue to feed on cells until there are no more cells in the on position and then it dies naturally. A very small number of normal cells are destroyed by reovirus, but the number is insignificant enough that our body can replace them. (I have a few extra cells of cellulose I could spare.) Cancer cells also have receptors, similar to normal cells. But since cancer cells grow more rapidly, the receptors are in the "on" position most of the time leaving them susceptible to the reovirus.
Tests have shown that the reovirus will actually travel via the blood stream (it is administered intravenously) and search out cancers that have metastasized to other organs throughout the body. Apparently the virus doesn't always move beyond the first tumor, thus the need to test the product on multiple cancer types and in conjunction with other agents to test the potential increase in specific efficacies. The company estimates that about 70% of all cancers can be treated using the Resolyn treatment. That's a huge market. I suspect that treatments won't be cheap. I also suspect that patients who would otherwise die and their insurance companies will pay whatever it takes. I can't help but think that life insurance companies would want to support any legislation to get the treatments covered by Medicare/Medicaid. I also can't help but think that if the treatment works they could find a price point at which they are very profitable but, at the same time, brings down the overall cost of cancer treatment. Multiple radiation/chemo treatments (potentially several times) for a prolonged life expectancy of 1-5 years is high cost compared to a treatment that cures the stuff. It does require several applications, but hey, I think a cure that lets me live is better than a treatment that just keeps me alive while I degenerate over 1-5 years is a no brainer!
The company hold several patents primarily on the production process used to manufacture their agent, Resolyn. If another company wants to compete, they'll have to discover another process to make the stuff.
Thread: Borrell: Entscheidung über Nord Stream 2 ist keine EU-Sache
As to how many of us 'gades are likely to be hit by cancer of some sort, I believe the ratio is about 1 in 4 here in America will get cancer. That is not encouraging. But, then again, if we can profit from it while we wait to see if genes are among the more susceptible pool, why not?
The english version of how it works:
Resolyn is a man-made version of a naturally occurring virus (reovirus) that most people contract multiple times throughout their lifetime. This type of virus is not harmful. They actually help keep us healthy by cleaning up things like cancer without our even knowing it. I suppose you could say that (at least some) people who don't get cancer probably were protected by getting a reovirus on occasion.
How it works: cells in the human body have receptors for nourishment that stay closed over 95% of the time once we stop growing. Think of the receptor as an on/off switch. When the cell needs nourishment the receptor click to the on position. Only when it is in the on position can a reovirus enter. So our normal cells are protected most of the time. When a reovirus enters a cell, it devours it. The reovirus will continue to feed on cells until there are no more cells in the on position and then it dies naturally. A very small number of normal cells are destroyed by reovirus, but the number is insignificant enough that our body can replace them. (I have a few extra cells of cellulose I could spare.) Cancer cells also have receptors, similar to normal cells. But since cancer cells grow more rapidly, the receptors are in the "on" position most of the time leaving them susceptible to the reovirus.
Tests have shown that the reovirus will actually travel via the blood stream (it is administered intravenously) and search out cancers that have metastasized to other organs throughout the body. Apparently the virus doesn't always move beyond the first tumor, thus the need to test the product on multiple cancer types and in conjunction with other agents to test the potential increase in specific efficacies. The company estimates that about 70% of all cancers can be treated using the Resolyn treatment. That's a huge market. I suspect that treatments won't be cheap. I also suspect that patients who would otherwise die and their insurance companies will pay whatever it takes. I can't help but think that life insurance companies would want to support any legislation to get the treatments covered by Medicare/Medicaid. I also can't help but think that if the treatment works they could find a price point at which they are very profitable but, at the same time, brings down the overall cost of cancer treatment. Multiple radiation/chemo treatments (potentially several times) for a prolonged life expectancy of 1-5 years is high cost compared to a treatment that cures the stuff. It does require several applications, but hey, I think a cure that lets me live is better than a treatment that just keeps me alive while I degenerate over 1-5 years is a no brainer!
The company hold several patents primarily on the production process used to manufacture their agent, Resolyn. If another company wants to compete, they'll have to discover another process to make the stuff.
Paclitaxel With or Without Viral Therapy in Treating Patients With Recurrent or Persistent Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cancer
This study is currently recruiting participants.
Verified by National Cancer Institute (NCI), December 2010
First Received: September 9, 2010 Last Updated: December 15, 2010 History of Changes
Sponsor: Gynecologic Oncology Group
Collaborator: National Cancer Institute (NCI)
RATIONALE: Drugs used in chemotherapy, such as paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. Viral therapy may be able to kill tumor cells without damaging normal cells. Giving chemotherapy together with viral therapy may kill more tumor cells.
PURPOSE: This randomized phase II trial is studying the side effects and how well giving paclitaxel with or without viral therapy works in treating patients with ovarian epithelial, fallopian tube, or primary peritoneal cancer.
Further study details as provided by National Cancer Institute (NCI):
Primary Outcome Measures:
•Progression-free survival (PFS) [ Designated as safety issue: No ]
•Frequency and severity of adverse events as assessed by CTCAE v4.0 [ Designated as safety issue: Yes ]
Secondary Outcome Measures:
•Median PFS and overall survival (OS) [ Designated as safety issue: No ]
•Tumor response by RECIST and CA-125 criteria [ Designated as safety issue: No ]
•Comparison of PFS and OS between patients with measurable and non-measurable disease [ Designated as safety issue: No ]
Estimated Enrollment: 110
Study Start Date: December 2010
Estimated Primary Completion Date: December 2012 (Final data collection date for primary outcome measure)
This study is currently recruiting participants.
Verified by National Cancer Institute (NCI), December 2010
First Received: September 9, 2010 Last Updated: December 15, 2010 History of Changes
Sponsor: Gynecologic Oncology Group
Collaborator: National Cancer Institute (NCI)
RATIONALE: Drugs used in chemotherapy, such as paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. Viral therapy may be able to kill tumor cells without damaging normal cells. Giving chemotherapy together with viral therapy may kill more tumor cells.
PURPOSE: This randomized phase II trial is studying the side effects and how well giving paclitaxel with or without viral therapy works in treating patients with ovarian epithelial, fallopian tube, or primary peritoneal cancer.
Further study details as provided by National Cancer Institute (NCI):
Primary Outcome Measures:
•Progression-free survival (PFS) [ Designated as safety issue: No ]
•Frequency and severity of adverse events as assessed by CTCAE v4.0 [ Designated as safety issue: Yes ]
Secondary Outcome Measures:
•Median PFS and overall survival (OS) [ Designated as safety issue: No ]
•Tumor response by RECIST and CA-125 criteria [ Designated as safety issue: No ]
•Comparison of PFS and OS between patients with measurable and non-measurable disease [ Designated as safety issue: No ]
Estimated Enrollment: 110
Study Start Date: December 2010
Estimated Primary Completion Date: December 2012 (Final data collection date for primary outcome measure)
Antwort auf Beitrag Nr.: 40.723.109 von reguau am 16.12.10 17:20:08Auszug aus dem Yahoo-Board vom 24.12.2010
Re: I believe this was orchestrated down
24-Dec-10 06:34 am
Medimune stock went up $18. last Friday on good news that their ca drug worked.Up over 100% in 1 day.
DNDN went from $5 to $20/ share a year before FDA approval on test results only marginally better than the standard.
The stock market is forward looking .
DNDN went from $20 to $40 before FDA eventual approval.
Good news doubles share price immediately.
After approval is too late to invest.
You have to be in it to win it.
ONCY MGMT giving themselves options at $6.66/share & they are accumulating & not selling.
I think $6.66 is the bottom. Buy low & sell.........
$1 Billion in sales = $100 in share price.
REO is like 10 different drugs for 10 different kinds of CA.
10 drugs x $100/ share = $1,000/ share potential.
What other stock has the potential to double from here 143 times in the next few years?
Dont laugh , I think of Cisco up 86,000% in 10 years.
Dont let them steal your stock at this cheap price. [/b]
Sentiment : Strong Buy
IMHO Jacci
Re: I believe this was orchestrated down
24-Dec-10 06:34 am
Medimune stock went up $18. last Friday on good news that their ca drug worked.Up over 100% in 1 day.
DNDN went from $5 to $20/ share a year before FDA approval on test results only marginally better than the standard.
The stock market is forward looking .
DNDN went from $20 to $40 before FDA eventual approval.
Good news doubles share price immediately.
After approval is too late to invest.
You have to be in it to win it.
ONCY MGMT giving themselves options at $6.66/share & they are accumulating & not selling.
I think $6.66 is the bottom. Buy low & sell.........
$1 Billion in sales = $100 in share price.
REO is like 10 different drugs for 10 different kinds of CA.
10 drugs x $100/ share = $1,000/ share potential.
What other stock has the potential to double from here 143 times in the next few years?
Dont laugh , I think of Cisco up 86,000% in 10 years.
Dont let them steal your stock at this cheap price. [/b]
Sentiment : Strong Buy
IMHO Jacci
Antwort auf Beitrag Nr.: 40.760.471 von jacci am 24.12.10 14:06:20New article link
http://biotuesday.ca/2010/05/18/oncolytics-ready-for-final-s…
Partial cut 'n paste:
Oncolytics ready for final step with Reolysin
May 18, 2010 by leonardzehr · Leave a Comment
After a 10-year journey, Oncolytics Biotech (TSX:ONC (2.87 ↑2.14%); NASDAQ:ONCY ) is ready to take the final clinical step with its sole Reolysin drug as a treatment for a broad range of cancers.
“Our product has really shown a breadth of activity in a wide variety of cancers that we had hoped it would way back when we started out,” CEO Dr. Brad Thompson said in an exclusive interview with BioTuesday.ca. “It doesn’t matter what kind of drug combination we use or the amount of radiation, we typically get a clinical benefit no matter what the indication is, which is honestly pretty remarkable.”
Since 2000, when Reolysin first entered the clinic, Oncolytics has enrolled some 330 patients in multiple Phase 1 and 2 studies. “When we lump all indications together, you see this pattern, where people have a three-in-four chance of stabilizing or having a tumour regress fairly significantly. And an even higher percentage in metastatic lesions,” he adds.
The company is now gearing up to start its first Phase 3 pivotal study with Reolysin in patients with advanced head and neck cancer at 25 centres in Britain and the U.S., under a special protocol assessment from the FDA, with plans to expand to Belgium, for multijurisdictional approval in Europe and Canada.
Reolysin will be tested in combination with two chemotherapy drugs, paclitaxel and carboplatin, versus chemotherapy alone in a two-stage clinical trial. The first stage is designed to enrol 80 patients. The second stage is adaptive, and designed to enrol between 100 and 400 patients, with the most probable statistical enrolment now being 200 patients, Dr. Thompson explains.
The company expects to complete enrolment of 80 patients by this Christmas and release some initial data next spring or summer, he adds. The primary endpoint is overall survival, with key secondary endpoints including progression free survival, response rate and duration of response, as well as safety.
The U.S. National Cancer Institute estimates that 48,000 people in the U.S. alone contracted head and neck cancer in 2009 and that 11,260 died. European incidence and mortality are substantially higher than in the U.S., standing at 140,000 new cases each year and 69,000 deaths.
The decision to pursue a Phase 3 trial in advanced head and neck cancer followed positive results in mid-stage testing in Britain, combining Reolysin with paclitaxel and carboplatin, where the company demonstrated an overall response rate of 42% and a total clinical benefit rate of 74%. The company is currently conducting a confirmatory Phase 2 trial in the U.S. with patients having the same disease.
So what gives Reolysin such high marks?
Reolysin is a formulation of human reovirus, an acronym for respiratory enteric orphan virus. While the reovirus occurs naturally in nature, it is not known to cause any disease in humans. Its cancer-killing link comes from its ability to reproduce in various cancer cells that have an activated Ras pathway, one of the most important genetic defects leading to cancer.
Click on Link for MORE>>>>>>
http://biotuesday.ca/2010/05/18/oncolytics-ready-for-final-s…
Partial cut 'n paste:
Oncolytics ready for final step with Reolysin
May 18, 2010 by leonardzehr · Leave a Comment
After a 10-year journey, Oncolytics Biotech (TSX:ONC (2.87 ↑2.14%); NASDAQ:ONCY ) is ready to take the final clinical step with its sole Reolysin drug as a treatment for a broad range of cancers.
“Our product has really shown a breadth of activity in a wide variety of cancers that we had hoped it would way back when we started out,” CEO Dr. Brad Thompson said in an exclusive interview with BioTuesday.ca. “It doesn’t matter what kind of drug combination we use or the amount of radiation, we typically get a clinical benefit no matter what the indication is, which is honestly pretty remarkable.”
Since 2000, when Reolysin first entered the clinic, Oncolytics has enrolled some 330 patients in multiple Phase 1 and 2 studies. “When we lump all indications together, you see this pattern, where people have a three-in-four chance of stabilizing or having a tumour regress fairly significantly. And an even higher percentage in metastatic lesions,” he adds.
The company is now gearing up to start its first Phase 3 pivotal study with Reolysin in patients with advanced head and neck cancer at 25 centres in Britain and the U.S., under a special protocol assessment from the FDA, with plans to expand to Belgium, for multijurisdictional approval in Europe and Canada.
Reolysin will be tested in combination with two chemotherapy drugs, paclitaxel and carboplatin, versus chemotherapy alone in a two-stage clinical trial. The first stage is designed to enrol 80 patients. The second stage is adaptive, and designed to enrol between 100 and 400 patients, with the most probable statistical enrolment now being 200 patients, Dr. Thompson explains.
The company expects to complete enrolment of 80 patients by this Christmas and release some initial data next spring or summer, he adds. The primary endpoint is overall survival, with key secondary endpoints including progression free survival, response rate and duration of response, as well as safety.
The U.S. National Cancer Institute estimates that 48,000 people in the U.S. alone contracted head and neck cancer in 2009 and that 11,260 died. European incidence and mortality are substantially higher than in the U.S., standing at 140,000 new cases each year and 69,000 deaths.
The decision to pursue a Phase 3 trial in advanced head and neck cancer followed positive results in mid-stage testing in Britain, combining Reolysin with paclitaxel and carboplatin, where the company demonstrated an overall response rate of 42% and a total clinical benefit rate of 74%. The company is currently conducting a confirmatory Phase 2 trial in the U.S. with patients having the same disease.
So what gives Reolysin such high marks?
Reolysin is a formulation of human reovirus, an acronym for respiratory enteric orphan virus. While the reovirus occurs naturally in nature, it is not known to cause any disease in humans. Its cancer-killing link comes from its ability to reproduce in various cancer cells that have an activated Ras pathway, one of the most important genetic defects leading to cancer.
Click on Link for MORE>>>>>>
Antwort auf Beitrag Nr.: 40.760.857 von jacci am 24.12.10 20:02:4010.01.2011 13:34
Oncolytics Biotech(R) Inc. Announces Opening of Enrollment in U.S. Phase I Colorectal Cancer Study
CALGARY, Jan. 10 /PRNewswire-FirstCall/ -- Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) announced today that a U.S. Phase I study of REOLYSIN(R) in combination with FOLFIRI (Folinic Acid (leucovorin) + Fluorouracil (5-FU) + Irinotecan) in patients with oxaliplatin refractory/intolerant Kras mutant colorectal cancer (REO 022) is now open to enrollment. The principal investigator is Dr. Sanjay Goel of the Montefiore Medical Center at The Albert Einstein College of Medicine in New York.
"We continue to advance REOLYSIN into clinical testing in new indications where we believe the product may have sound clinical and commercial potential," said Dr. Brad Thompson, President and CEO of Oncolytics. "We are focusing our clinical program increasingly to look at patients with Kras mutant cancers by either pre-screening patients for Kras status, as in the case of this study and the Phase II trial we are running in non-small cell lung cancer (REO 016), or by selecting indications with widespread Kras involvement, such as our ongoing Phase II study in advanced pancreatic cancer (REO 017)."
The trial is a Phase I dose escalation study with three dose levels, comprising cohorts of three to six patients, to determine a maximum tolerated dose and dose-limiting toxicities with the combination of REOLYSIN and FOLFIRI. FOLFIRI will be administered on the first day of a two week (14-day) cycle, while REOLYSIN will be administered on days one through five of a four week (28-day) cycle.
Eligible patients include those with histologically confirmed cancer of the colon or rectum with Kras mutation and measurable disease. They must have progressed on or within 190 days after last dose of oxaliplatin regimen as front-line therapy in the metastatic setting or be intolerant to oxaliplatin. The study is expected to enroll 12 to 20 patients.
The rationale for conducting the study is based on signals of efficacy seen in a range of preclinical and clinical work with REOLYSIN. This includes a National Cancer Institute screen of seven colorectal cancer cell lines (four with ras mutations), all of which were susceptible to REOLYSIN; preclinical research into the efficacy of REOLYSIN in combination with various chemotherapeutic agents in colorectal cancer cell lines; observation of CEA responses and stable disease in colorectal patients in a phase I study of REOLYSIN as a monotherapy; and interim results from a translational study with REOLYSIN as a monotherapy that is currently ongoing, which showed evidence of viral replication and tumour cell death in four of six patients with metastatic colorectal cancer analyzed to date, two of which had confirmed Kras mutations in codon 12.
About Colorectal Cancer The American Cancer Society estimates that nearly 143,000 Americans were diagnosed with colorectal cancer and an estimated 51,370 died from the disease in 2010. The prognosis for patients diagnosed with colorectal cancer at the localized stage is good with a five-year survival rate of 91%, however only about 39% of cases are diagnosed at this stage; five-year survival rates drop to 70% with the spread to adjacent organs or lymph nodes and 11% for distant metastases. Colorectal cancer is the third leading cause of cancer death among both men and women in the United States.
About Oncolytics Biotech Inc. Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. REOLYSIN preferentially replicates in cancer cells that have an activated RAS pathway. Approximately two thirds of all cancers have an activated RAS pathway, including most metastatic disease. A large number of mutations, including mutations in EGFR, Her2 or Kras along the RAS pathway lead to RAS pathway activation. For further information about Oncolytics, please visit: http://www.oncolyticsbiotech.com/.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements, including the Company's expectations related to the Phase I dose escalation study of REOLYSIN in combination with FOLFIRI (Folinic Acid (leucovorin) + Fluorouracil (5-FU) + Irinotecan) in patients with oxaliplatin refractory/intolerant Kras mutant colorectal cancer, and the Company's belief as to the potential of REOLYSIN as a cancer therapeutic, involve known and unknown risks and uncertainties, which could cause the Company's actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of REOLYSIN as a cancer treatment, the tolerability of REOLYSIN outside a controlled test, the success and timely completion of clinical studies and trials, the Company's ability to successfully commercialize REOLYSIN, uncertainties related to the research and development of pharmaceuticals and uncertainties related to the regulatory process. Investors should consult the Company's quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake to update these forward-looking statements, except as required by applicable laws.
Oncolytics Biotech Inc.
CONTACT:
The Equicom Group The Investor Relations Group
Nick Hurst Erika Moran
300 5th Ave. SW, 10th Floor 11 Stone St, 3rd Floor
Calgary, Alberta, T2P 3C4 New York, NY 10004
Tel: 403.218.2835 Tel: 212.825.3210
Fax: 403.218.2830 Fax: 212.825.3229
nhurst@equicomgroup.com emoran@investorrelationsgroup.com
© 2011 PR Newswire
Oncolytics Biotech(R) Inc. Announces Opening of Enrollment in U.S. Phase I Colorectal Cancer Study
CALGARY, Jan. 10 /PRNewswire-FirstCall/ -- Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) announced today that a U.S. Phase I study of REOLYSIN(R) in combination with FOLFIRI (Folinic Acid (leucovorin) + Fluorouracil (5-FU) + Irinotecan) in patients with oxaliplatin refractory/intolerant Kras mutant colorectal cancer (REO 022) is now open to enrollment. The principal investigator is Dr. Sanjay Goel of the Montefiore Medical Center at The Albert Einstein College of Medicine in New York.
"We continue to advance REOLYSIN into clinical testing in new indications where we believe the product may have sound clinical and commercial potential," said Dr. Brad Thompson, President and CEO of Oncolytics. "We are focusing our clinical program increasingly to look at patients with Kras mutant cancers by either pre-screening patients for Kras status, as in the case of this study and the Phase II trial we are running in non-small cell lung cancer (REO 016), or by selecting indications with widespread Kras involvement, such as our ongoing Phase II study in advanced pancreatic cancer (REO 017)."
The trial is a Phase I dose escalation study with three dose levels, comprising cohorts of three to six patients, to determine a maximum tolerated dose and dose-limiting toxicities with the combination of REOLYSIN and FOLFIRI. FOLFIRI will be administered on the first day of a two week (14-day) cycle, while REOLYSIN will be administered on days one through five of a four week (28-day) cycle.
Eligible patients include those with histologically confirmed cancer of the colon or rectum with Kras mutation and measurable disease. They must have progressed on or within 190 days after last dose of oxaliplatin regimen as front-line therapy in the metastatic setting or be intolerant to oxaliplatin. The study is expected to enroll 12 to 20 patients.
The rationale for conducting the study is based on signals of efficacy seen in a range of preclinical and clinical work with REOLYSIN. This includes a National Cancer Institute screen of seven colorectal cancer cell lines (four with ras mutations), all of which were susceptible to REOLYSIN; preclinical research into the efficacy of REOLYSIN in combination with various chemotherapeutic agents in colorectal cancer cell lines; observation of CEA responses and stable disease in colorectal patients in a phase I study of REOLYSIN as a monotherapy; and interim results from a translational study with REOLYSIN as a monotherapy that is currently ongoing, which showed evidence of viral replication and tumour cell death in four of six patients with metastatic colorectal cancer analyzed to date, two of which had confirmed Kras mutations in codon 12.
About Colorectal Cancer The American Cancer Society estimates that nearly 143,000 Americans were diagnosed with colorectal cancer and an estimated 51,370 died from the disease in 2010. The prognosis for patients diagnosed with colorectal cancer at the localized stage is good with a five-year survival rate of 91%, however only about 39% of cases are diagnosed at this stage; five-year survival rates drop to 70% with the spread to adjacent organs or lymph nodes and 11% for distant metastases. Colorectal cancer is the third leading cause of cancer death among both men and women in the United States.
About Oncolytics Biotech Inc. Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. REOLYSIN preferentially replicates in cancer cells that have an activated RAS pathway. Approximately two thirds of all cancers have an activated RAS pathway, including most metastatic disease. A large number of mutations, including mutations in EGFR, Her2 or Kras along the RAS pathway lead to RAS pathway activation. For further information about Oncolytics, please visit: http://www.oncolyticsbiotech.com/.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements, including the Company's expectations related to the Phase I dose escalation study of REOLYSIN in combination with FOLFIRI (Folinic Acid (leucovorin) + Fluorouracil (5-FU) + Irinotecan) in patients with oxaliplatin refractory/intolerant Kras mutant colorectal cancer, and the Company's belief as to the potential of REOLYSIN as a cancer therapeutic, involve known and unknown risks and uncertainties, which could cause the Company's actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of REOLYSIN as a cancer treatment, the tolerability of REOLYSIN outside a controlled test, the success and timely completion of clinical studies and trials, the Company's ability to successfully commercialize REOLYSIN, uncertainties related to the research and development of pharmaceuticals and uncertainties related to the regulatory process. Investors should consult the Company's quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake to update these forward-looking statements, except as required by applicable laws.
Oncolytics Biotech Inc.
CONTACT:
The Equicom Group The Investor Relations Group
Nick Hurst Erika Moran
300 5th Ave. SW, 10th Floor 11 Stone St, 3rd Floor
Calgary, Alberta, T2P 3C4 New York, NY 10004
Tel: 403.218.2835 Tel: 212.825.3210
Fax: 403.218.2830 Fax: 212.825.3229
nhurst@equicomgroup.com emoran@investorrelationsgroup.com
© 2011 PR Newswire
Colorectal , KRas+, Reolysin, and our Trials 2 minutes ago From the 850+ member, free, moderated, oncyV2 board at: http://finance.groups.yahoo.com/group/on...
Colorectal , KRas+, Reolysin, and our Trials
Oncolytics announced opening of enrollment in a Phase I colorectal cancer Trial today, in their PR at http://www.prnewswire.com/news-releases/...
At ASCO 2008, one of the big stories was that if colorectal cancer or non-small cell lung cancer were found to have a "mutant" or "active" KRas gene (KRas+), then the standard therapy (that uses an EGFR blocker) doesn't work. EGFR blockers include Erbitux (cetuximab), Tarceva (erlotinib), Vectibix (panitumumab), and Iressa (gefitinib). Their combined 2008 sales were $2.31 Billion. In 2009, their combined sales were $3.43 Billion.
Here's a very informative 6 minute video by Dr John Marshall, from Sep 26, 2008, in a discussion of colorectal cancer where he spells out the KRas+ story from ASCO 2008 … http://www.youtube.com/watch?v=0iFTcPX_r...
He concludes with "Colon Cancer became two separate diseases at ASCO this year … all around the KRas mutation versus wild type … and it's going to change everything." He did not present any therapy to fill the gap … to look after the KRas+ colorectal cancer patients.
Oncolytics’ colorectal Trial will be of interest to us of course, but it will be of at least as much interest to the FDA, the NCI, and to our potential partners. This Trial has the potential of being the beginning of a very big deal. Because Reolysin has not previously been administered with FOLFIRI, this short Phase I Trial is required. A randomized Phase II Trial will follow directly, on successful results from the Phase I Trial.
As transcribed by PullDaTrigger, we have Brad saying this at the Canaccord Genuity conference (Aug 8, 2010)”
<??? 14:33> “with colorectal cancer, we had clinical data on carbo-tax combinations so we could go straight into phase II, but we needed to go back into the lab because of the Irinotecan component of FOLFIRI, and we're just about to start enrolment in a phase I study with Reo combined with FOLFIRI - again, same thing, while patients fail first line in this case are screened for EGFR status and KRas, if they have the KRas mutation they'll go onto our study. In this case it’s a much bigger percentage however, it's almost half of the patients in 2nd line colorectal are now being excluded from EGFR status because they have KRAS mutations so this is a significant patient group for us.”
Also, from PullDaTrigger’s tran of Brad’s talk at the Bio Investor Conference (Oct 6, 2010), we have:
Colorectal - "half not eligible for the standard of care...should be starting any day now, a quick phase I study looking at FOLFIRI and Reolysin, and then we'll proceed into a randomized phase II study immediately thereafter. We've had very good response rates in colorectal with Reolysin as a mono-therapy and metastatic colorectal cancer in a number of our studies and so anticipation is that the addition of the FOLFIRI will simply enhance that, especially with the [KRas+] pre-screened patient population"
Although a Phase I Trial is carried out to determine safety; on the completion of this “quick phase I study” , I wouldn’t be surprised to see some efficacy data presented “immediately thereafter” when Oncolytics proceeds into a randomized Phase II study. Colorectal cancer is the third leading cause of cancer death among both men and women in the United States.
reguau
Colorectal , KRas+, Reolysin, and our Trials
Oncolytics announced opening of enrollment in a Phase I colorectal cancer Trial today, in their PR at http://www.prnewswire.com/news-releases/...
At ASCO 2008, one of the big stories was that if colorectal cancer or non-small cell lung cancer were found to have a "mutant" or "active" KRas gene (KRas+), then the standard therapy (that uses an EGFR blocker) doesn't work. EGFR blockers include Erbitux (cetuximab), Tarceva (erlotinib), Vectibix (panitumumab), and Iressa (gefitinib). Their combined 2008 sales were $2.31 Billion. In 2009, their combined sales were $3.43 Billion.
Here's a very informative 6 minute video by Dr John Marshall, from Sep 26, 2008, in a discussion of colorectal cancer where he spells out the KRas+ story from ASCO 2008 … http://www.youtube.com/watch?v=0iFTcPX_r...
He concludes with "Colon Cancer became two separate diseases at ASCO this year … all around the KRas mutation versus wild type … and it's going to change everything." He did not present any therapy to fill the gap … to look after the KRas+ colorectal cancer patients.
Oncolytics’ colorectal Trial will be of interest to us of course, but it will be of at least as much interest to the FDA, the NCI, and to our potential partners. This Trial has the potential of being the beginning of a very big deal. Because Reolysin has not previously been administered with FOLFIRI, this short Phase I Trial is required. A randomized Phase II Trial will follow directly, on successful results from the Phase I Trial.
As transcribed by PullDaTrigger, we have Brad saying this at the Canaccord Genuity conference (Aug 8, 2010)”
<??? 14:33> “with colorectal cancer, we had clinical data on carbo-tax combinations so we could go straight into phase II, but we needed to go back into the lab because of the Irinotecan component of FOLFIRI, and we're just about to start enrolment in a phase I study with Reo combined with FOLFIRI - again, same thing, while patients fail first line in this case are screened for EGFR status and KRas, if they have the KRas mutation they'll go onto our study. In this case it’s a much bigger percentage however, it's almost half of the patients in 2nd line colorectal are now being excluded from EGFR status because they have KRAS mutations so this is a significant patient group for us.”
Also, from PullDaTrigger’s tran of Brad’s talk at the Bio Investor Conference (Oct 6, 2010), we have:
Colorectal - "half not eligible for the standard of care...should be starting any day now, a quick phase I study looking at FOLFIRI and Reolysin, and then we'll proceed into a randomized phase II study immediately thereafter. We've had very good response rates in colorectal with Reolysin as a mono-therapy and metastatic colorectal cancer in a number of our studies and so anticipation is that the addition of the FOLFIRI will simply enhance that, especially with the [KRas+] pre-screened patient population"
Although a Phase I Trial is carried out to determine safety; on the completion of this “quick phase I study” , I wouldn’t be surprised to see some efficacy data presented “immediately thereafter” when Oncolytics proceeds into a randomized Phase II study. Colorectal cancer is the third leading cause of cancer death among both men and women in the United States.
reguau
CALGARY, Jan. 18 /PRNewswire-FirstCall/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC.to - News) announced today that the Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, U.S. National Cancer Institute (NCI), which is part of the National Institutes of Health, has agreed to sponsor a 2-Arm randomized Phase II study of carboplatin, paclitaxel plus REOLYSIN® versus carboplatin and paclitaxel alone in the first line treatment of patients with recurrent or metastatic pancreatic cancer. The NCI is sponsoring the trial under its Clinical Trials Agreement with Oncolytics, while Oncolytics will provide clinical supplies of REOLYSIN. The Principal Investigator is Dr. Tanios Bekaii-Saab of The Ohio State University Comprehensive Cancer Center - Arthur G. James Cancer Hospital and Richard J. Solove Research Institute.
"Pancreatic cancer is typically diagnosed at a later stage, when there is a poor prognosis for long-term survival," said Dr. Tanios Bekaii-Saab, principal investigator. "Although the standard of care has evolved over the last few years, it has only managed to generate very modest improvements in response rate and median overall survival. Given these poor outcomes, we are eager to evaluate new and promising treatments in this indication."
The study is an open-label, multi-institution, 2-arm Phase II randomized study of patients with metastatic pancreatic cancer. Patients will be randomized to receive either carboplatin, paclitaxel plus REOLYSIN (Arm A) or carboplatin and paclitaxel alone (Arm B). Patients in both arms will receive treatment every three weeks (21-day cycles). Patients in both arms will be receiving standard intravenous doses of paclitaxel and carboplatin on day one only. In Arm A, patients will also receive intravenous REOLYSIN at a dose of 3x1010 TCID50 on days one through five. Tumor response assessment will be done by CT scan and conducted every eight weeks. Patients that progress on carboplatin and paclitaxel (Arm B) will have REOLYSIN added. If patients experience significant toxicity related to carboplatin and/or paclitaxel they may continue with single agent REOLYSIN.
The primary objective of the trial is to assess improvement in progression-free survival with REOLYSIN, carboplatin and paclitaxel relative to carboplatin and paclitaxel alone in patients with metastatic pancreatic cancer. The primary endpoint is progression free survival in both arms. Secondary endpoints include overall response rate and overall survival. The study is expected to enroll approximately 70 patients.
"We continue to expand our clinical relationship with the NCI, as they push forward innovative research initiatives," said Dr. Brad Thompson, President and CEO of Oncolytics. "Partnering with the NCI allows us to cost-effectively expand the evaluation of REOLYSIN into new indications, with a focus on identifying additional targets for future late-stage clinical testing."
This is the fourth clinical trial using REOLYSIN to be sponsored by the NCI. The NCI is currently conducting a Phase II metastatic melanoma trial, a Phase I/II ovarian, peritoneal and fallopian tube cancer trial, and has announced its intention to sponsor a randomized Phase II trial in patients with persistent or recurrent ovarian, fallopian tube or primary peritoneal cancer.
reguau
"Pancreatic cancer is typically diagnosed at a later stage, when there is a poor prognosis for long-term survival," said Dr. Tanios Bekaii-Saab, principal investigator. "Although the standard of care has evolved over the last few years, it has only managed to generate very modest improvements in response rate and median overall survival. Given these poor outcomes, we are eager to evaluate new and promising treatments in this indication."
The study is an open-label, multi-institution, 2-arm Phase II randomized study of patients with metastatic pancreatic cancer. Patients will be randomized to receive either carboplatin, paclitaxel plus REOLYSIN (Arm A) or carboplatin and paclitaxel alone (Arm B). Patients in both arms will receive treatment every three weeks (21-day cycles). Patients in both arms will be receiving standard intravenous doses of paclitaxel and carboplatin on day one only. In Arm A, patients will also receive intravenous REOLYSIN at a dose of 3x1010 TCID50 on days one through five. Tumor response assessment will be done by CT scan and conducted every eight weeks. Patients that progress on carboplatin and paclitaxel (Arm B) will have REOLYSIN added. If patients experience significant toxicity related to carboplatin and/or paclitaxel they may continue with single agent REOLYSIN.
The primary objective of the trial is to assess improvement in progression-free survival with REOLYSIN, carboplatin and paclitaxel relative to carboplatin and paclitaxel alone in patients with metastatic pancreatic cancer. The primary endpoint is progression free survival in both arms. Secondary endpoints include overall response rate and overall survival. The study is expected to enroll approximately 70 patients.
"We continue to expand our clinical relationship with the NCI, as they push forward innovative research initiatives," said Dr. Brad Thompson, President and CEO of Oncolytics. "Partnering with the NCI allows us to cost-effectively expand the evaluation of REOLYSIN into new indications, with a focus on identifying additional targets for future late-stage clinical testing."
This is the fourth clinical trial using REOLYSIN to be sponsored by the NCI. The NCI is currently conducting a Phase II metastatic melanoma trial, a Phase I/II ovarian, peritoneal and fallopian tube cancer trial, and has announced its intention to sponsor a randomized Phase II trial in patients with persistent or recurrent ovarian, fallopian tube or primary peritoneal cancer.
reguau
Antwort auf Beitrag Nr.: 40.833.075 von reguau am 10.01.11 15:43:30das ist bereits die vierte studie, die das nationale krebs institut (nci) der usa sponsort
absolut neu ist, dass patienten, die im arm B nur mit carboplatin und paclitaxel behandelten patienten die möglichkeit erhalten, anschließend mit reolysin weiter behandelt zu werden.
reguau
absolut neu ist, dass patienten, die im arm B nur mit carboplatin und paclitaxel behandelten patienten die möglichkeit erhalten, anschließend mit reolysin weiter behandelt zu werden.
reguau
Die Leerverkäufer könnten Probleme bekommen.
KursdatenBörse Nasdaq
Aktuell 6,89 USD
Zeit 18.01.1120:25
Diff. Vortag +9,37%
Tages-Vol. --
Gehandelte Stück 380.134
wohl wahr
reguau
Aktuell 6,89 USD
Zeit 18.01.1120:25
Diff. Vortag +9,37%
Tages-Vol. --
Gehandelte Stück 380.134
wohl wahr
reguau
Oncolytics Biotech Target Raised To C$8 From C$5.25 By Canaccord Genuity >ONCY
7:10a ET January 19, 2011 (Dow Jones)
Oncolytics Biotech Target Raised To C$8 From C$5.25 By Canaccord Genuity >ONCY
(END) Dow Jones Newswires (212-416-2400)
01-19-11 0710ET
Copyright (c) 2011 Dow Jones & Company, Inc.BT201101190025562011-01-19 12:10:00.0006F64HVKTJ94S62MRA6L4LOAJC5DJNF
jacci
7:10a ET January 19, 2011 (Dow Jones)
Oncolytics Biotech Target Raised To C$8 From C$5.25 By Canaccord Genuity >ONCY
(END) Dow Jones Newswires (212-416-2400)
01-19-11 0710ET
Copyright (c) 2011 Dow Jones & Company, Inc.BT201101190025562011-01-19 12:10:00.0006F64HVKTJ94S62MRA6L4LOAJC5DJNF
jacci
Ein Aufbäumen der Short-Fraktion- heute??
phase 1 trial for children recruiting 10-Feb-11 01:50 pm Recruiting started for phase 1 "Viral Therapy in Treating Young Patients With Relapsed or Refractory Solid Tumors" at
Children's Hospital of Orange County
Orange, California, United States, 92868
Contact: Violet Shen 714-532-8636
http://clinicaltrials.gov/ct2/show/NCT01...
Age of patient population for Reolysin now range from 3 yr olds to seniors!
Children's Hospital of Orange County
Orange, California, United States, 92868
Contact: Violet Shen 714-532-8636
http://clinicaltrials.gov/ct2/show/NCT01...
Age of patient population for Reolysin now range from 3 yr olds to seniors!
Oncolytics Biotech® Inc. Meets Primary Endpoint for First Part of U.S. Phase 2 Pancreatic Cancer Clinical Trial
CALGARY, Feb. 14 /PRNewswire-FirstCall/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC.to - News) today announced preliminary results from a U.S. Phase 2 clinical trial (REO 017) using intravenous administration of REOLYSIN® in combination with gemcitabine (Gemzar®) in patients with advanced pancreatic cancer. The trial is being conducted at the Cancer Therapy & Research Center at The University of Texas Health Science Center at San Antonio (CTRC). The Principal Investigator is Dr. Monica Mita of the CTRC.
"When we opened the study we anticipated positive results based on preclinical models and on the high frequency of Ras pathway abnormalities in pancreatic cancer," said Dr. Monica Mita. "We are, however, impressed by the fact that the study met the endpoint so early. We are eager to complete the study and to proceed to the next step of development for this combination."
The trial is a single arm, open-label, Phase 2 study of REOLYSIN given intravenously with gemcitabine every three weeks. The study's primary objective is to determine the clinical benefit rate (complete response (CR) + partial response (PR) + stable disease (SD)) of REOLYSIN in combination with gemcitabine in patients with advanced or metastatic pancreatic adenocarcinoma with measurable disease who have not received any prior chemotherapy or biotherapy. The secondary objectives are to determine progression-free survival, and the safety and tolerability of REOLYSIN when administered in combination with gemcitabine. Seventeen evaluable patients with pancreatic cancer were expected to be treated in the first stage and if three or more patients received clinical benefit, the study would then proceed to the next stage. This endpoint was met after six evaluable patients were enrolled. All patients treated reported symptomatic improvement. Three of six patients showed SD for 12 weeks or greater. In addition, one patient had stable disease at nine weeks of treatment, but was taken off of the study for alternative treatment, and one patient had a PR of less than 12 weeks duration, and then died from a medical issue unrelated to treatment. The Company now intends to complete enrollment in the first stage of the study and then continue with further studies.
"This study is supportive of the decision to conduct the randomized Phase II study being sponsored by the NCI examining REOLYSIN in combination with carboplatin/paclitaxel," said Dr. Brad Thompson, President and CEO of Oncolytics.
CALGARY, Feb. 14 /PRNewswire-FirstCall/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC.to - News) today announced preliminary results from a U.S. Phase 2 clinical trial (REO 017) using intravenous administration of REOLYSIN® in combination with gemcitabine (Gemzar®) in patients with advanced pancreatic cancer. The trial is being conducted at the Cancer Therapy & Research Center at The University of Texas Health Science Center at San Antonio (CTRC). The Principal Investigator is Dr. Monica Mita of the CTRC.
"When we opened the study we anticipated positive results based on preclinical models and on the high frequency of Ras pathway abnormalities in pancreatic cancer," said Dr. Monica Mita. "We are, however, impressed by the fact that the study met the endpoint so early. We are eager to complete the study and to proceed to the next step of development for this combination."
The trial is a single arm, open-label, Phase 2 study of REOLYSIN given intravenously with gemcitabine every three weeks. The study's primary objective is to determine the clinical benefit rate (complete response (CR) + partial response (PR) + stable disease (SD)) of REOLYSIN in combination with gemcitabine in patients with advanced or metastatic pancreatic adenocarcinoma with measurable disease who have not received any prior chemotherapy or biotherapy. The secondary objectives are to determine progression-free survival, and the safety and tolerability of REOLYSIN when administered in combination with gemcitabine. Seventeen evaluable patients with pancreatic cancer were expected to be treated in the first stage and if three or more patients received clinical benefit, the study would then proceed to the next stage. This endpoint was met after six evaluable patients were enrolled. All patients treated reported symptomatic improvement. Three of six patients showed SD for 12 weeks or greater. In addition, one patient had stable disease at nine weeks of treatment, but was taken off of the study for alternative treatment, and one patient had a PR of less than 12 weeks duration, and then died from a medical issue unrelated to treatment. The Company now intends to complete enrollment in the first stage of the study and then continue with further studies.
"This study is supportive of the decision to conduct the randomized Phase II study being sponsored by the NCI examining REOLYSIN in combination with carboplatin/paclitaxel," said Dr. Brad Thompson, President and CEO of Oncolytics.
Oncolytics Biotech(R) Inc. Announces Start of Enrollment in Randomized Phase II Pancreatic Cancer StudyLast update: 2/16/2011 7:30:00 AMCALGARY, Feb 16, 2011 /PRNewswire via COMTEX/ -- Oncolytics Biotech Inc. ("Oncolytics") (CA:ONC)(ONCY) announced today that enrollment has begun in a 2-Arm randomized Phase II study of carboplatin, paclitaxel plus REOLYSIN(R) versus carboplatin and paclitaxel alone in the first line treatment of patients with recurrent or metastatic pancreatic cancer. The Principal Investigator is Dr. Tanios Bekaii-Saab, Medical Director of Gastrointestinal Oncology at The Ohio State University Comprehensive Cancer Center - Arthur G. James Cancer Hospital and Richard J. Solove Research Institute. The Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, U.S. National Cancer Institute (NCI), which is part of the National Institutes of Health, is sponsoring the trial under its Clinical Trials Agreement with Oncolytics, while Oncolytics will provide clinical supplies of REOLYSIN. "Pancreatic cancer has seen very little progress over the last few decades," said Dr. Tanios Bekaii-Saab, principal investigator. "This clinical trial will allow us to evaluate a promising new treatment for pancreatic cancer with the goal of improving long-term survival for patients who typically have a poor prognosis." The study is an open-label, multi-institution, 2-Arm Phase II randomized study of patients with metastatic pancreatic cancer. Patients will be randomized to receive either carboplatin, paclitaxel plus REOLYSIN (Arm A) or carboplatin and paclitaxel alone (Arm B). Patients in both arms will receive treatment every three weeks (21-day cycles). Patients in both arms will be receiving standard intravenous doses of paclitaxel and carboplatin on day one only. In Arm A, patients will also receive intravenous REOLYSIN at a dose of 3x1010 TCID50 on days one through five. Tumor response assessment will be done by CT scan and conducted every eight weeks. Patients that progress on carboplatin and paclitaxel (Arm B) will have REOLYSIN added. If patients experience significant toxicity related to carboplatin and/or paclitaxel they may continue with single agent REOLYSIN. The primary objective of the trial is to assess improvement in progression-free survival with REOLYSIN, carboplatin and paclitaxel relative to carboplatin and paclitaxel alone in patients with metastatic pancreatic cancer. The primary endpoint is progression free survival in both arms. Secondary endpoints include overall response rate and overall survival. The study is expected to enroll approximately 70 patients.
hier mal etwas grundsätzliches zur wirkweise des reolysin:
How Absent Reoviruses Kill Cancer
ScienceDaily (Feb. 20, 2011) — Reoviruses are successfully being used in clinical trials to treat patients with cancer. Not only does the virus cause cancer cells to die, it also forces them to release pro-inflammatory chemokines and cytokines, which in turn causes the patient's immune system to attack the disease. New research published by BioMed Central's open access journal Molecular Cancer shows that reovirus infected cancer cells secrete proteins which, even when isolated, result in the death of cancer cells.
Normal human cells are protected from reovirus infection by a protein called PKR. However a cellular signalling protein (Ras), which can block PKR activity, is abnormally activated in many types of cancer and provides a window of opportunity for reovirus infection. A multi-centre study, involving labs in the UK and America, collected growth media from reovirus infected melanoma cells. The researchers showed that this media contained a range of small pro-inflammatory proteins, including an interleukin (IL-8) and Type 1 Interferon (INF-β), which recruited and activated white blood cells, specifically Natural Killer (NK) cells, dendritic cells (DC) and anti melanoma cytotoxic T cells (CTL).
Whilst the exact details behind this mode of action of cell signalling in response to viral infection are unclear, the release of cytokines was dependent on both 'inactive' PKR and a specific nuclear factor (NF-κβ). According to Prof Alan Melcher, from Leeds Institute of Molecular Medicine, "Bystander immune-mediated therapy may well be an important component in the treatment of cancer by reoviruses, and may have potential in treating cancer even in the absence of live virus."
reguau
How Absent Reoviruses Kill Cancer
ScienceDaily (Feb. 20, 2011) — Reoviruses are successfully being used in clinical trials to treat patients with cancer. Not only does the virus cause cancer cells to die, it also forces them to release pro-inflammatory chemokines and cytokines, which in turn causes the patient's immune system to attack the disease. New research published by BioMed Central's open access journal Molecular Cancer shows that reovirus infected cancer cells secrete proteins which, even when isolated, result in the death of cancer cells.
Normal human cells are protected from reovirus infection by a protein called PKR. However a cellular signalling protein (Ras), which can block PKR activity, is abnormally activated in many types of cancer and provides a window of opportunity for reovirus infection. A multi-centre study, involving labs in the UK and America, collected growth media from reovirus infected melanoma cells. The researchers showed that this media contained a range of small pro-inflammatory proteins, including an interleukin (IL-8) and Type 1 Interferon (INF-β), which recruited and activated white blood cells, specifically Natural Killer (NK) cells, dendritic cells (DC) and anti melanoma cytotoxic T cells (CTL).
Whilst the exact details behind this mode of action of cell signalling in response to viral infection are unclear, the release of cytokines was dependent on both 'inactive' PKR and a specific nuclear factor (NF-κβ). According to Prof Alan Melcher, from Leeds Institute of Molecular Medicine, "Bystander immune-mediated therapy may well be an important component in the treatment of cancer by reoviruses, and may have potential in treating cancer even in the absence of live virus."
reguau
und hier die übersetzung
Wie abwesend Reoviren Kill Krebs
ScienceDaily (20 Februar 2011) - Reoviren sind erfolgreich in klinischen Studien zur Behandlung von Patienten mit Krebs zu behandeln. Nicht nur, dass das Virus Krebs-Zellen zu sterben, sondern zwingt sie zu pro-inflammatorischen Chemokine und Zytokine, die wiederum bewirkt, dass der Patient das Immunsystem, die Krankheit Angriff freizugeben. Neue Forschungsergebnisse von Open-Access-BioMed Central Zeitschrift Molecular Cancer veröffentlicht zeigt, dass Reovirus infizierten Krebszellen Proteine, die, auch wenn isoliert, führen den Tod von Krebszellen absondern.
Normale menschliche Zellen aus Reovirus Infektion durch ein Protein namens PKR geschützt. Doch ein zelluläres Signal-Protein (Ras), die PKR-Aktivität blockieren kann, ist ungewöhnlich in vielen Arten von Krebs aktiviert und bietet eine gute Gelegenheit für Reovirus-Infektion. Eine multizentrische Studie, die Labors in Großbritannien und Amerika, gesammelt Nährmedien von Reovirus infizierten Melanomzellen. Die Forscher zeigten, dass diese Medien eine Reihe von kleinen pro-inflammatorische Proteine enthalten, einschließlich einer Interleukin (IL-8) und Typ-1-Interferon (INF-β), die rekrutiert und aktiviert weißen Blutkörperchen, speziell Natural Killer (NK) Zellen, dendritischen Zellen (DC) und Anti Melanom zytotoxischen T-Zellen (CTL).
Während die genauen Details hinter dieser Wirkungsweise der zellulären Signalübertragung in Reaktion auf virale Infektion unklar sind, wurde die Freisetzung von Zytokinen abhängig sowohl "inaktiv" PKR und einer bestimmten nuclear factor (NF-κβ). Laut Prof. Alan Melcher, aus Leeds Institute of Molecular Medicine "Bystander immunvermittelte Therapie kann auch ein wichtiger Bestandteil in der Behandlung von Krebs durch Reoviren werden und kann potenzielle Behandlung von Krebs auch in Abwesenheit von lebenden Virus."
Wie abwesend Reoviren Kill Krebs
ScienceDaily (20 Februar 2011) - Reoviren sind erfolgreich in klinischen Studien zur Behandlung von Patienten mit Krebs zu behandeln. Nicht nur, dass das Virus Krebs-Zellen zu sterben, sondern zwingt sie zu pro-inflammatorischen Chemokine und Zytokine, die wiederum bewirkt, dass der Patient das Immunsystem, die Krankheit Angriff freizugeben. Neue Forschungsergebnisse von Open-Access-BioMed Central Zeitschrift Molecular Cancer veröffentlicht zeigt, dass Reovirus infizierten Krebszellen Proteine, die, auch wenn isoliert, führen den Tod von Krebszellen absondern.
Normale menschliche Zellen aus Reovirus Infektion durch ein Protein namens PKR geschützt. Doch ein zelluläres Signal-Protein (Ras), die PKR-Aktivität blockieren kann, ist ungewöhnlich in vielen Arten von Krebs aktiviert und bietet eine gute Gelegenheit für Reovirus-Infektion. Eine multizentrische Studie, die Labors in Großbritannien und Amerika, gesammelt Nährmedien von Reovirus infizierten Melanomzellen. Die Forscher zeigten, dass diese Medien eine Reihe von kleinen pro-inflammatorische Proteine enthalten, einschließlich einer Interleukin (IL-8) und Typ-1-Interferon (INF-β), die rekrutiert und aktiviert weißen Blutkörperchen, speziell Natural Killer (NK) Zellen, dendritischen Zellen (DC) und Anti Melanom zytotoxischen T-Zellen (CTL).
Während die genauen Details hinter dieser Wirkungsweise der zellulären Signalübertragung in Reaktion auf virale Infektion unklar sind, wurde die Freisetzung von Zytokinen abhängig sowohl "inaktiv" PKR und einer bestimmten nuclear factor (NF-κβ). Laut Prof. Alan Melcher, aus Leeds Institute of Molecular Medicine "Bystander immunvermittelte Therapie kann auch ein wichtiger Bestandteil in der Behandlung von Krebs durch Reoviren werden und kann potenzielle Behandlung von Krebs auch in Abwesenheit von lebenden Virus."
Re: ONCY Enrollment in Metastatic Colorectal Cancer Completed 23-Feb-11 09:21 am Oncolytics Biotech® Inc. Announces Completion of Enrollment in Translational Clinical Trial Investigating REOLYSIN® in Patients with Metastatic Colorectal Cancer
Press Release Source: Oncolytics Biotech Inc. On Wednesday February 23, 2011, 7:30 am EST
CALGARY, Feb. 23 /PRNewswire-FirstCall/ -
Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC.to - News) (NASDAQ:ONCY - News) announced today that enrollment has been completed in a U.K. translational clinical trial investigating intravenous administration of REOLYSIN in patients with metastatic colorectal cancer prior to surgical resection of liver metastases (REO 013). The principal investigator is Professor Alan Melcher of St. James's University Hospital and the trial is sponsored by the University of Leeds, UK.
"The early results from this study reported in 2010 concluded that reovirus can be successfully delivered specifically to colorectal liver metastases following intravenous administration as a monotherapy," said Dr. Brad Thompson, President and CEO of Oncolytics. "This is supportive of observations that REOLYSIN was active against metastatic liver lesions in a number of different combination therapy studies."
The trial was an open-label, non-randomized, single centre study of REOLYSIN given intravenously to patients for five consecutive days in advance of their scheduled operations to remove colorectal cancer deposits metastatic to the liver. After surgery, the tumour and surrounding liver tissue were assessed for viral status and anti-tumour effects.
The primary objectives of the trial are to assess the presence, replication and anti-cancer effects of reovirus within liver metastases after intravenous administration of REOLYSIN by examination of the resected tumour. Secondary objectives include assessing the anti-tumour activity and safety profile of REOLYSIN, and monitoring the humoral and cellular immune response to REOLYSIN.
Eligible patients included those with histologically proven colorectal cancer, planned for potentially curative surgical resection of liver metastases. A total of 10 patients were treated in the study. The results are expected to be fully reported in 2011.
Press Release Source: Oncolytics Biotech Inc. On Wednesday February 23, 2011, 7:30 am EST
CALGARY, Feb. 23 /PRNewswire-FirstCall/ -
Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC.to - News) (NASDAQ:ONCY - News) announced today that enrollment has been completed in a U.K. translational clinical trial investigating intravenous administration of REOLYSIN in patients with metastatic colorectal cancer prior to surgical resection of liver metastases (REO 013). The principal investigator is Professor Alan Melcher of St. James's University Hospital and the trial is sponsored by the University of Leeds, UK.
"The early results from this study reported in 2010 concluded that reovirus can be successfully delivered specifically to colorectal liver metastases following intravenous administration as a monotherapy," said Dr. Brad Thompson, President and CEO of Oncolytics. "This is supportive of observations that REOLYSIN was active against metastatic liver lesions in a number of different combination therapy studies."
The trial was an open-label, non-randomized, single centre study of REOLYSIN given intravenously to patients for five consecutive days in advance of their scheduled operations to remove colorectal cancer deposits metastatic to the liver. After surgery, the tumour and surrounding liver tissue were assessed for viral status and anti-tumour effects.
The primary objectives of the trial are to assess the presence, replication and anti-cancer effects of reovirus within liver metastases after intravenous administration of REOLYSIN by examination of the resected tumour. Secondary objectives include assessing the anti-tumour activity and safety profile of REOLYSIN, and monitoring the humoral and cellular immune response to REOLYSIN.
Eligible patients included those with histologically proven colorectal cancer, planned for potentially curative surgical resection of liver metastases. A total of 10 patients were treated in the study. The results are expected to be fully reported in 2011.
Antwort auf Beitrag Nr.: 41.093.772 von reguau am 23.02.11 17:43:00Canaccord on the REO-013 translational Trial
February 23, 2011
Oncolytics Biotech Inc. (ONC : TSX : C$6.22) - Buy - Target: C$9.00
Lining up the catalysts: CRC liver mets data expected this year
The completion of enrolment in the translational clinical study of monotherapy
Reolysin in patients with metastatic colorectal cancer (CRC) suggests that
additional clinical data should be expected in 2011. Interim data from this
trial (reported in October 2010) provided evidence of Reolysin activity in
KRAS-positive liver metastases when administered alone, suggesting that the
viral therapy was working as expected. We now anticipate final data from this
study this year, as well as Phase I glial cancer data and the results from two
Phase II lung cancer studies (SCC and NSCLC). The key data expected in 2011 will
be an interim analysis of the Phase III study in head and neck cancer, expected
after mid-year.
We continue to recommend investors BUY Oncolytics in anticipation of this data.
Investment highlights An interim analysis of the Phase III is scheduled
following enrolment of the first 80 patients into the study; this analysis is
expected around the middle of the year. This data will be the major catalyst for
the stock in 2011, in our view. Data from two studies in lung cancer (NSCLC and
SCC of the lung) are expected in the first half of 2011. We believe that
positive results from either study could attract partnering interest. Reolysin
could be an attractive asset for prospective big pharma partners. We expect that
Oncolytics will partner this drug in 2011, providing validation for the
technology. We are also encouraged by the recent purchase of a private
competitor for $1 billion.
Valuation We value Oncolytics using a probability-weighted NPV model of lead
drug Reolysin. Based on this analysis, we arrive at a target price of C$9.00,
which supports our BUY recommendation.
February 23, 2011
Oncolytics Biotech Inc. (ONC : TSX : C$6.22) - Buy - Target: C$9.00
Lining up the catalysts: CRC liver mets data expected this year
The completion of enrolment in the translational clinical study of monotherapy
Reolysin in patients with metastatic colorectal cancer (CRC) suggests that
additional clinical data should be expected in 2011. Interim data from this
trial (reported in October 2010) provided evidence of Reolysin activity in
KRAS-positive liver metastases when administered alone, suggesting that the
viral therapy was working as expected. We now anticipate final data from this
study this year, as well as Phase I glial cancer data and the results from two
Phase II lung cancer studies (SCC and NSCLC). The key data expected in 2011 will
be an interim analysis of the Phase III study in head and neck cancer, expected
after mid-year.
We continue to recommend investors BUY Oncolytics in anticipation of this data.
Investment highlights An interim analysis of the Phase III is scheduled
following enrolment of the first 80 patients into the study; this analysis is
expected around the middle of the year. This data will be the major catalyst for
the stock in 2011, in our view. Data from two studies in lung cancer (NSCLC and
SCC of the lung) are expected in the first half of 2011. We believe that
positive results from either study could attract partnering interest. Reolysin
could be an attractive asset for prospective big pharma partners. We expect that
Oncolytics will partner this drug in 2011, providing validation for the
technology. We are also encouraged by the recent purchase of a private
competitor for $1 billion.
Valuation We value Oncolytics using a probability-weighted NPV model of lead
drug Reolysin. Based on this analysis, we arrive at a target price of C$9.00,
which supports our BUY recommendation.
Antwort auf Beitrag Nr.: 41.103.230 von jacci am 24.02.11 21:58:19es sind in 2011 noch einige daten zu erwarten, oncolytics ist zunehmend in den focus der presse gerückt, was nicht unwesentlich zu dem kursanstieg in 2010 beigetragen haben dürfte.
die technologie scheint im kampf gegen krebs erfolgversprechend zu sein
allerdings liegt der focus von oncolytics jetzt nicht mehr (wie noch bei der reo 13 studie) auf alleintherapie, sondern auf co therapie mit zugelassenen krebs medikamenten
beeindruckend ist dabei die vielzahl der indikationen
reo 13 und reo 14 sowie die vom nci gesponserten trials waren mono anwendungen, reo 15 bis reo 22 sind co anwendungen (http://www.oncolyticsbiotech.com/clinical.html)
cannacord hat sein kursziel auf 9 can$ angehoben und nicht unewrähnt gelassen, daß ein mitbewerber für 1 mrd can$ erworben wurde, das würde bei oncolytics einem kurs von ca 15 can$ entsprechen
nach den vorleigenden presseberichten ist oncolytics mit einer phase III und einer vielzahl von Phase II in vielen indikationen von allen firmen, die mit oncolytischen viren forschen, am weitesten
dendreon, mit einer einzigen indikation hat eine marktkapitalisierung von ca 3,5 mrd us$
reguau
die technologie scheint im kampf gegen krebs erfolgversprechend zu sein
allerdings liegt der focus von oncolytics jetzt nicht mehr (wie noch bei der reo 13 studie) auf alleintherapie, sondern auf co therapie mit zugelassenen krebs medikamenten
beeindruckend ist dabei die vielzahl der indikationen
reo 13 und reo 14 sowie die vom nci gesponserten trials waren mono anwendungen, reo 15 bis reo 22 sind co anwendungen (http://www.oncolyticsbiotech.com/clinical.html)
cannacord hat sein kursziel auf 9 can$ angehoben und nicht unewrähnt gelassen, daß ein mitbewerber für 1 mrd can$ erworben wurde, das würde bei oncolytics einem kurs von ca 15 can$ entsprechen
nach den vorleigenden presseberichten ist oncolytics mit einer phase III und einer vielzahl von Phase II in vielen indikationen von allen firmen, die mit oncolytischen viren forschen, am weitesten
dendreon, mit einer einzigen indikation hat eine marktkapitalisierung von ca 3,5 mrd us$
reguau
Antwort auf Beitrag Nr.: 41.103.755 von reguau am 25.02.11 00:35:31Fool.com erwähnt Oncolytics wieder......
http://www.fool.com/investing/general/2011/02/25/12-small-ca…
http://www.fool.com/investing/general/2011/02/25/12-small-ca…
Antwort auf Beitrag Nr.: 41.111.976 von jacci am 26.02.11 18:41:06CALGARY, March 1 /PRNewswire/ - Oncolytics Biotech Inc. ("Oncolytics" or the "Corporation") (TSX: ONC) (NASDAQ: ONCY) today announced that the Corporation's biggest institutional shareholder, has exercised 1,248,800 warrants, issued in connection with the financing that closed on November 8, 2010, providing the Corporation with proceeds of approximately $7.7 million. Oncolytics now has approximately 71.1 million shares issued and outstanding, and cash on hand and available for operations of approximately $55 million. The Corporation plans to use the proceeds to fund an additional randomized clinical trial.
Link: http://www.prnewswire.com/news-releases/...
that is fine.........
Link: http://www.prnewswire.com/news-releases/...
that is fine.........
Antwort auf Beitrag Nr.: 41.130.133 von jacci am 02.03.11 06:30:32das ist mehr als fine
zum einen heisst das, dass der grösste investor in die gesellschaft auch grösstes vertrauen in die wirksamkeit des reolysin und in die geschäftsführung hat
zum anderen, dass oncolytics genügend cash auf der hand hat um auch eine grosse randomisierte studie durch zu führen
2011 könnte das jahr für oncolytics werden, eine gesellschaft, die hier in deutschland bisher noch keine beachtung gefunden hat
reguau
zum einen heisst das, dass der grösste investor in die gesellschaft auch grösstes vertrauen in die wirksamkeit des reolysin und in die geschäftsführung hat
zum anderen, dass oncolytics genügend cash auf der hand hat um auch eine grosse randomisierte studie durch zu führen
2011 könnte das jahr für oncolytics werden, eine gesellschaft, die hier in deutschland bisher noch keine beachtung gefunden hat
reguau
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
Secondary metastatic disease in the UK H&N Trial 2-Mar-11 09:29 pm Secondary metastatic disease in the UK H&N Trial
I’ve started doing some transcribing of today’s RBC presentation and have found something interesting in the Biomarkers section. Watch for the part below that mentions the 80% Stable Disease or better in metastatic lesions … I expand on that below the tran.
=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-
Biomarkers
{29:40} The use of biomarkers has evolved in our program over time. When we first started out there really wasn’t any adequate proof markers. With the Ras pathway, there’s … our virus requires an activated Ras pathway and fortunately a lot of things activate the Ras pathway: mutations, overexpressions, many of the elements on the pathway actually lead to that metabolic state; but the vast majority in most indications are caused by EGFR mutation overexpression, or KRas mutation overexpression. So if you lump those two together, you actually have a pretty good subset. You can do that in two different ways. You can screen for that now, and we have approved kits in Europe for both, and an approved kit for EGFR in the States, and soon to be approved KRas kits in the US. We have a first line non-small cell lung Study running in Phase II where we’re screening for KRas and EGFR status, and a second line colorectal Study where we’re screening for KRas and EGFR status, as pre-screening agents.
Now the other way you can screen is by looking at the high incidences of Ras that are just naturally occurring with respect to different indications. Pancreatic for example is over 90% KRas mutant specifically. We have a randomized, and a single arm, pancreatic Study running in that. The kind of different one is metastatic disease. Metastatic disease is usually over 90% Ras activated. If you look at metastatic disease specifically, and look for clinical responses there, you get what you’d expect to get, which is very high rates of Clinical Response ... our best is out of our Head and Neck data, out of our Phase II. If you segregate and grade the clinical Responses in lung, or liver, or lymph nodes separately by RECIST, you get 80% Stable Disease or better, and 40 odd percent actual durable Partial or Complete Responses in metastatic lesions, which given the importance of metastatic disease and lifespan, it’s kind of interesting. So biomarkers are really important for us, and that’s the two ways we handle them … we either screen by metastatic disease or indication, or by using a kit.
=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-
The metastatic lesions being discussed above also show up now on page 5 of the Current Investor Presentation on the Oncolytics website here: http://www.oncolyticsbiotech.com/pdf/ONC...
These are not the H&N cancers per se, but are the distant cancers that (I’m assuming) were created by metastases from the primary H&N tumor(s). From the Current Investor Presentation we find out that 15 of the 19 evaluable H&N patients had remote metastatic disease (in addition to their primary H&N cancer) … either in their lungs (5 patients), lymph nodes (5 patients), or liver (5 patients).
In these 15 patients with metastatic lesions, 6 patients had Stable Disease, 4 had Partial Responses, and 2 had Complete Responses. Three of the patients had Progressive Disease. So 12 out of 15 (80%) had Stable Disease or better (“Clinical Benefit Rate”), and 6 out of 15 (40%) had either a PR or a CR (combined as “Response Rate”). Not bad for cancers that we weren’t “officially” trying to treat. The presence of these extra cancers gives us a feel for just how severe the condition is, of the patient population (carboplatin failed) that we’re working with here (and also in our Phase III H&N Trial). I expect that a large part of the success can be attributed to Oncolytics’ early switch over from direct tumor injection to intravenous therapy.
rjc
man beachte die erfolgsraten bei einer krebserkrankung, die eigentlich als nicht behandelbar gilt
reguau
Secondary metastatic disease in the UK H&N Trial 2-Mar-11 09:29 pm Secondary metastatic disease in the UK H&N Trial
I’ve started doing some transcribing of today’s RBC presentation and have found something interesting in the Biomarkers section. Watch for the part below that mentions the 80% Stable Disease or better in metastatic lesions … I expand on that below the tran.
=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-
Biomarkers
{29:40} The use of biomarkers has evolved in our program over time. When we first started out there really wasn’t any adequate proof markers. With the Ras pathway, there’s … our virus requires an activated Ras pathway and fortunately a lot of things activate the Ras pathway: mutations, overexpressions, many of the elements on the pathway actually lead to that metabolic state; but the vast majority in most indications are caused by EGFR mutation overexpression, or KRas mutation overexpression. So if you lump those two together, you actually have a pretty good subset. You can do that in two different ways. You can screen for that now, and we have approved kits in Europe for both, and an approved kit for EGFR in the States, and soon to be approved KRas kits in the US. We have a first line non-small cell lung Study running in Phase II where we’re screening for KRas and EGFR status, and a second line colorectal Study where we’re screening for KRas and EGFR status, as pre-screening agents.
Now the other way you can screen is by looking at the high incidences of Ras that are just naturally occurring with respect to different indications. Pancreatic for example is over 90% KRas mutant specifically. We have a randomized, and a single arm, pancreatic Study running in that. The kind of different one is metastatic disease. Metastatic disease is usually over 90% Ras activated. If you look at metastatic disease specifically, and look for clinical responses there, you get what you’d expect to get, which is very high rates of Clinical Response ... our best is out of our Head and Neck data, out of our Phase II. If you segregate and grade the clinical Responses in lung, or liver, or lymph nodes separately by RECIST, you get 80% Stable Disease or better, and 40 odd percent actual durable Partial or Complete Responses in metastatic lesions, which given the importance of metastatic disease and lifespan, it’s kind of interesting. So biomarkers are really important for us, and that’s the two ways we handle them … we either screen by metastatic disease or indication, or by using a kit.
=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-
The metastatic lesions being discussed above also show up now on page 5 of the Current Investor Presentation on the Oncolytics website here: http://www.oncolyticsbiotech.com/pdf/ONC...
These are not the H&N cancers per se, but are the distant cancers that (I’m assuming) were created by metastases from the primary H&N tumor(s). From the Current Investor Presentation we find out that 15 of the 19 evaluable H&N patients had remote metastatic disease (in addition to their primary H&N cancer) … either in their lungs (5 patients), lymph nodes (5 patients), or liver (5 patients).
In these 15 patients with metastatic lesions, 6 patients had Stable Disease, 4 had Partial Responses, and 2 had Complete Responses. Three of the patients had Progressive Disease. So 12 out of 15 (80%) had Stable Disease or better (“Clinical Benefit Rate”), and 6 out of 15 (40%) had either a PR or a CR (combined as “Response Rate”). Not bad for cancers that we weren’t “officially” trying to treat. The presence of these extra cancers gives us a feel for just how severe the condition is, of the patient population (carboplatin failed) that we’re working with here (and also in our Phase III H&N Trial). I expect that a large part of the success can be attributed to Oncolytics’ early switch over from direct tumor injection to intravenous therapy.
rjc
man beachte die erfolgsraten bei einer krebserkrankung, die eigentlich als nicht behandelbar gilt
reguau
Antwort auf Beitrag Nr.: 41.137.725 von reguau am 03.03.11 07:57:16http://www.oncolyticsbiotech.com/pdf/ONCYInvestorPresentatio…
Antwort auf Beitrag Nr.: 41.139.910 von jacci am 03.03.11 12:52:02http://www.smartbrief.com/news/aanp/industryPR-detail.jsp?id…
Oncolytics Biotech® Inc. Completes Patient Enrollment in U.S. Phase 2 Clinical Trial Investigating REOLYSIN® in Combination with Paclitaxel and Carboplatin in Head and Neck Cancers
.
Companies:Oncolytics Biotech, Inc.Oncolytics Biotech, Inc..Related Quotes
Symbol Price Change
ONC.TO 5.70 0.00
CALGARY, March 24 /PRNewswire/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC.to - News) today announced that it has completed patient enrollment in its U.S. Phase 2 clinical trial (REO 015) using intravenous administration of REOLYSIN in combination with paclitaxel and carboplatin in patients with advanced head and neck cancers.
"This study was performed in part to confirm the results of our UK Phase II study, which enrolled a slightly different patient population, and to support our ongoing Phase III study in platinum resistant head and neck cancers," said Dr. Brad Thompson, President and CEO of Oncolytics. "This U.S. study examined a greater proportion of patients with prior taxane exposure than either of the other two studies we have conducted in this indication."
This trial was a 14-patient, single arm, open-label, dose-targeted, non-randomized trial of REOLYSIN given intravenously in combination with a standard dosage of paclitaxel and carboplatin.
Eligible patients included those with advanced or metastatic head and neck cancers that were refractory to standard therapy or for which no curative standard therapy existed. The primary objective of the Phase 2 trial was to measure tumor responses and duration of response, and to describe any evidence of antitumor activity. The secondary objective is to determine the safety and tolerability of REOLYSIN when administered in combination with paclitaxel and carboplatin to patients with advanced or metastatic head and neck cancers. The results are expected to be fully reported in 2011.
reguau
.
Companies:Oncolytics Biotech, Inc.Oncolytics Biotech, Inc..Related Quotes
Symbol Price Change
ONC.TO 5.70 0.00
CALGARY, March 24 /PRNewswire/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC.to - News) today announced that it has completed patient enrollment in its U.S. Phase 2 clinical trial (REO 015) using intravenous administration of REOLYSIN in combination with paclitaxel and carboplatin in patients with advanced head and neck cancers.
"This study was performed in part to confirm the results of our UK Phase II study, which enrolled a slightly different patient population, and to support our ongoing Phase III study in platinum resistant head and neck cancers," said Dr. Brad Thompson, President and CEO of Oncolytics. "This U.S. study examined a greater proportion of patients with prior taxane exposure than either of the other two studies we have conducted in this indication."
This trial was a 14-patient, single arm, open-label, dose-targeted, non-randomized trial of REOLYSIN given intravenously in combination with a standard dosage of paclitaxel and carboplatin.
Eligible patients included those with advanced or metastatic head and neck cancers that were refractory to standard therapy or for which no curative standard therapy existed. The primary objective of the Phase 2 trial was to measure tumor responses and duration of response, and to describe any evidence of antitumor activity. The secondary objective is to determine the safety and tolerability of REOLYSIN when administered in combination with paclitaxel and carboplatin to patients with advanced or metastatic head and neck cancers. The results are expected to be fully reported in 2011.
reguau
Antwort auf Beitrag Nr.: 41.260.018 von reguau am 24.03.11 14:07:25http://media.integratir.com/T.ONC/financials/2010_Financials…
schönen Sonntag
jacci
schönen Sonntag
jacci
Antwort auf Beitrag Nr.: 41.271.500 von jacci am 27.03.11 07:53:19http://seekingalpha.com/article/260439-4-biotech-companies-w…
interessant.............
interessant.............
Antwort auf Beitrag Nr.: 41.284.303 von jacci am 29.03.11 18:10:22http://seekingalpha.com/article/260439-4-biotech-companies-w…
nochmals Link
nochmals Link
Antwort auf Beitrag Nr.: 41.284.320 von jacci am 29.03.11 18:13:00http://www.newswise.com/articles/the-future-of-cancer-clinic…
Oncolytics Biotech® Inc. Announces Positive Data from Translational Clinical Trial Investigating REOLYSIN® in Patients with Metastatic Colorectal Cancer
CALGARY, April 20 /PRNewswire-FirstCall/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC.to - News) (NASDAQ:ONCY - News) today announced interim data from a U.K. translational clinical trial (REO 013) investigating intravenous administration of REOLYSIN in patients with metastatic colorectal cancer prior to surgical resection of liver metastases. The principal investigator of the study was Professor Alan Melcher of Leeds Institute of Molecular Medicine, University of Leeds, UK.
The trial was an open-label, non-randomized, single centre study of REOLYSIN given intravenously to patients for five consecutive days in advance of their scheduled operations to remove colorectal cancer deposits metastatic to the liver. Patients were treated with intravenous REOLYSIN at 1x1010 TCID50, one to three weeks prior to planned surgery. After surgery, the tumour and surrounding liver tissue were assessed for viral status and anti-tumour effects.
On initial histological analysis of the 10 treated patients to date, there was evidence of selective delivery of virus to tumour versus normal liver and viral replication in the majority (seven) of patients. In two patients, only necrotic tumour was found; in one of these cases virus was detected in immune cells in the tumour. In six of 10 patients there was no evidence of virus in the normal liver surrounding the tumour, with virus found only rarely in liver cells in the other four patients. Final and complete results from the study are expected to be presented in 2011.
"These data suggest reovirus can be intravenously administered as a monotherapy and successfully delivered specifically and selectively to colorectal liver metastases without affecting surrounding normal liver tissue," said Dr. Brad Thompson, President and CEO of Oncolytics. "Based on these results, we are now planning a further translational study co-administering reovirus with FOLFIRI (Folinic Acid (leucovorin) + Fluorouracil (5-FU) + Irinotecan), which would further build our knowledge of how to maximize the efficacy of this novel therapy in cancer patients. This study would be complementary to our ongoing Phase I study of REOLYSIN in combination with FOLFIRI in patients with oxaliplatin refractory/intolerant Kras mutant colorectal cancer."
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements, including the Company's expectations related to the results reported from the U.K. translational study for patients with metastatic colorectal cancer with liver metastases, and the Company's belief as to the potential of REOLYSIN as a cancer therapeutic, involve known and unknown risks and uncertainties, which could cause the Company's actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of REOLYSIN as a cancer treatment, the tolerability of REOLYSIN outside a controlled test, the success and timely completion of clinical studies and trials, the Company's ability to successfully commercialize REOLYSIN, uncertainties related to the research and development of pharmaceuticals and uncertainties related to the regulatory process. Investors should consult the Company's quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake to update these forward-looking statements, except as required by applicable laws.
CALGARY, April 20 /PRNewswire-FirstCall/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC.to - News) (NASDAQ:ONCY - News) today announced interim data from a U.K. translational clinical trial (REO 013) investigating intravenous administration of REOLYSIN in patients with metastatic colorectal cancer prior to surgical resection of liver metastases. The principal investigator of the study was Professor Alan Melcher of Leeds Institute of Molecular Medicine, University of Leeds, UK.
The trial was an open-label, non-randomized, single centre study of REOLYSIN given intravenously to patients for five consecutive days in advance of their scheduled operations to remove colorectal cancer deposits metastatic to the liver. Patients were treated with intravenous REOLYSIN at 1x1010 TCID50, one to three weeks prior to planned surgery. After surgery, the tumour and surrounding liver tissue were assessed for viral status and anti-tumour effects.
On initial histological analysis of the 10 treated patients to date, there was evidence of selective delivery of virus to tumour versus normal liver and viral replication in the majority (seven) of patients. In two patients, only necrotic tumour was found; in one of these cases virus was detected in immune cells in the tumour. In six of 10 patients there was no evidence of virus in the normal liver surrounding the tumour, with virus found only rarely in liver cells in the other four patients. Final and complete results from the study are expected to be presented in 2011.
"These data suggest reovirus can be intravenously administered as a monotherapy and successfully delivered specifically and selectively to colorectal liver metastases without affecting surrounding normal liver tissue," said Dr. Brad Thompson, President and CEO of Oncolytics. "Based on these results, we are now planning a further translational study co-administering reovirus with FOLFIRI (Folinic Acid (leucovorin) + Fluorouracil (5-FU) + Irinotecan), which would further build our knowledge of how to maximize the efficacy of this novel therapy in cancer patients. This study would be complementary to our ongoing Phase I study of REOLYSIN in combination with FOLFIRI in patients with oxaliplatin refractory/intolerant Kras mutant colorectal cancer."
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements, including the Company's expectations related to the results reported from the U.K. translational study for patients with metastatic colorectal cancer with liver metastases, and the Company's belief as to the potential of REOLYSIN as a cancer therapeutic, involve known and unknown risks and uncertainties, which could cause the Company's actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of REOLYSIN as a cancer treatment, the tolerability of REOLYSIN outside a controlled test, the success and timely completion of clinical studies and trials, the Company's ability to successfully commercialize REOLYSIN, uncertainties related to the research and development of pharmaceuticals and uncertainties related to the regulatory process. Investors should consult the Company's quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake to update these forward-looking statements, except as required by applicable laws.
ONCOLYTICS BIOTECH INC.
ONCOLYTICS BIOTECH INC.ONCY 5.68 0.02
Today 5d 1m 3m 1y 5y 10y
Detailed Chart...
ONCOLYTICS BIOTECH INC.ONC:CA 5.46 -0.03
Today 5d 1m 3m 1y 5y 10y
Detailed Chart... Oncolytics Biotech® Inc. Announces Phase I Multiple Myeloma Cancer Study
CALGARY, May 10 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) announced today that the Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, U.S. National Cancer Institute (NCI), which is part of the National Institutes of Health, has agreed to sponsor a Phase I study of REOLYSIN® alone in patients with relapsed multiple myeloma. The NCI is sponsoring the trial under its Clinical Trials Agreement with Oncolytics, while Oncolytics will provide clinical supplies of REOLYSIN. The Principal Investigator is Dr. Craig Hofmeister of The Ohio State University Comprehensive Cancer Center - Arthur G. James Cancer Hospital and Richard J. Solove Research Institute.
"While progression free survivals have improved with novel therapies over the last decade, the cure fraction remains low and additional options are needed," said Dr. Craig Hofmeister, principal investigator. "Multiple myeloma cells are frequently RAS activated, especially in relapse, so we are very interested in looking at REOLYSIN for these patients."
The study will initially be a proof of concept, open-label Phase I study of REOLYSIN in patients with relapsed multiple myeloma. Approximately 12 patients will receive REOLYSIN, in a dose escalation up to 3 x 1010 TCID50 per day administered intravenously on days one through five every 28 days.
The primary endpoint for the dose escalation portion of this study will be adverse events using CTCAE criteria. Correlative studies will focus on the efficiency with which reovirus replicates in patient myeloma cells. Investigators will use standard cohorts-of-three phase I dose escalation design with three to six patients being treated at each dose level. Secondary endpoints will include clinical benefit, duration of response, and time to progression.
This is the sixth clinical trial using REOLYSIN to be sponsored by the NCI. The NCI is currently conducting a Phase II metastatic melanoma trial, a Phase I/II and a randomized Phase II ovarian, peritoneal and fallopian tube cancer trials, a randomized Phase II trial in pancreatic cancer and a Phase I trial in pediatric patients with relapsed or refractory solid tumors.
About Myeloma
The American Cancer Society estimates that 20,180 Americans were diagnosed with myeloma and an estimated 10,650 Americans were expected to die from the disease in 2010. The prognosis for patients diagnosed with myeloma varies but the five-year survival rate for the period between 1999 and 2005 was approximately 37%.
About the NCI
The U.S. National Cancer Institute (NCI) is part of the National Institutes of Health and the U.S. Department of Health and Human Services. NCI's main responsibilities include coordinating the National Cancer Program; conducting and supporting cancer-related research; training physicians and scientists; and disseminating state-of-the-art information about cancer detection, diagnosis, treatment, prevention, control, palliative care, and survivorship.
About The Ohio State University Comprehensive Cancer Center
The Ohio State University Comprehensive Cancer Center - Arthur G. James Cancer Hospital and Richard J. Solove Research Institute (cancer.osu.edu) is one of only 40 Comprehensive Cancer Centers in the United States designated by the National Cancer Institute. Ranked by U.S. News & World Report among the top cancer hospitals in the nation, The James is the 205-bed adult patient-care component of the cancer program at The Ohio State University. The OSUCCC-James is one of only seven funded programs in the country approved by the NCI to conduct both Phase I and Phase II clinical trials.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
das ist mittlerweile die sechste studie, die vom nci gesponsert wird
reguau
ONCOLYTICS BIOTECH INC.ONCY 5.68 0.02
Today 5d 1m 3m 1y 5y 10y
Detailed Chart...
ONCOLYTICS BIOTECH INC.ONC:CA 5.46 -0.03
Today 5d 1m 3m 1y 5y 10y
Detailed Chart... Oncolytics Biotech® Inc. Announces Phase I Multiple Myeloma Cancer Study
CALGARY, May 10 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) announced today that the Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, U.S. National Cancer Institute (NCI), which is part of the National Institutes of Health, has agreed to sponsor a Phase I study of REOLYSIN® alone in patients with relapsed multiple myeloma. The NCI is sponsoring the trial under its Clinical Trials Agreement with Oncolytics, while Oncolytics will provide clinical supplies of REOLYSIN. The Principal Investigator is Dr. Craig Hofmeister of The Ohio State University Comprehensive Cancer Center - Arthur G. James Cancer Hospital and Richard J. Solove Research Institute.
"While progression free survivals have improved with novel therapies over the last decade, the cure fraction remains low and additional options are needed," said Dr. Craig Hofmeister, principal investigator. "Multiple myeloma cells are frequently RAS activated, especially in relapse, so we are very interested in looking at REOLYSIN for these patients."
The study will initially be a proof of concept, open-label Phase I study of REOLYSIN in patients with relapsed multiple myeloma. Approximately 12 patients will receive REOLYSIN, in a dose escalation up to 3 x 1010 TCID50 per day administered intravenously on days one through five every 28 days.
The primary endpoint for the dose escalation portion of this study will be adverse events using CTCAE criteria. Correlative studies will focus on the efficiency with which reovirus replicates in patient myeloma cells. Investigators will use standard cohorts-of-three phase I dose escalation design with three to six patients being treated at each dose level. Secondary endpoints will include clinical benefit, duration of response, and time to progression.
This is the sixth clinical trial using REOLYSIN to be sponsored by the NCI. The NCI is currently conducting a Phase II metastatic melanoma trial, a Phase I/II and a randomized Phase II ovarian, peritoneal and fallopian tube cancer trials, a randomized Phase II trial in pancreatic cancer and a Phase I trial in pediatric patients with relapsed or refractory solid tumors.
About Myeloma
The American Cancer Society estimates that 20,180 Americans were diagnosed with myeloma and an estimated 10,650 Americans were expected to die from the disease in 2010. The prognosis for patients diagnosed with myeloma varies but the five-year survival rate for the period between 1999 and 2005 was approximately 37%.
About the NCI
The U.S. National Cancer Institute (NCI) is part of the National Institutes of Health and the U.S. Department of Health and Human Services. NCI's main responsibilities include coordinating the National Cancer Program; conducting and supporting cancer-related research; training physicians and scientists; and disseminating state-of-the-art information about cancer detection, diagnosis, treatment, prevention, control, palliative care, and survivorship.
About The Ohio State University Comprehensive Cancer Center
The Ohio State University Comprehensive Cancer Center - Arthur G. James Cancer Hospital and Richard J. Solove Research Institute (cancer.osu.edu) is one of only 40 Comprehensive Cancer Centers in the United States designated by the National Cancer Institute. Ranked by U.S. News & World Report among the top cancer hospitals in the nation, The James is the 205-bed adult patient-care component of the cancer program at The Ohio State University. The OSUCCC-James is one of only seven funded programs in the country approved by the NCI to conduct both Phase I and Phase II clinical trials.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
das ist mittlerweile die sechste studie, die vom nci gesponsert wird
reguau
Re: Liefervertrag mit Sigma-Aldrich für die kommerzielle Herstellung - CALGARY, May 11 / PRNewswire / - Oncolytics Biotech Inc. (TSX: ONC.to - News) (NASDAQ: ONCY - News) ("Oncolytics" oder das "Unternehmen") gab heute bekannt, dass es zu einem kommerziellen Liefervertrag mit SAFC, eine Division von Sigma-Aldrich Corporation, hat für die kommerzielle Herstellung von REOLYSIN ®, Oncolytics proprietären Reovirus getreten. Gemäß den Bedingungen der Vereinbarung, SAFC Prozessvalidierung des Produktes durchführen wird, wird weiterhin klinischen Anforderungen liefern und Gewerbe auf Zulassung des Produktes liefern.
"Als ein weltweit führender Anbieter von qualitativ hochwertigen und zuverlässigen Biologics Manufacturing Services sind wir bestrebt, mit den Unternehmen auf die Entwicklung neuartiger Produkte auf den Markt konzentriert arbeiten", sagte Gilles Cottier, Präsident von SAFC. "SAFC hat eng mit Oncolytics gearbeitet zu entwickeln, Scale-up und Optimierung der Herstellung von REOLYSIN, die Förderung an die kommerzielle Ebene."
"Diese Vereinbarung stellt einen bedeutenden Schritt vorwärts im Bemühen um die Vermarktung von REOLYSIN wie wir weitere klinische Versorgung produzieren und bauen Inventar für potenzielle gewerbliche Verkäufe vorzubereiten", sagte Dr. Matt Coffey, Chief Operating Officer von Oncolytics. "Nachdem wir mit ein weltweit anerkannter Marktführer auf die effiziente Herstellung von REOLYSIN seit einigen Jahren, wir fühlen uns gut mit diesem Element unseres Gesamtplans zur REOLYSIN vermarkten positioniert gearbeitet."
"Als ein weltweit führender Anbieter von qualitativ hochwertigen und zuverlässigen Biologics Manufacturing Services sind wir bestrebt, mit den Unternehmen auf die Entwicklung neuartiger Produkte auf den Markt konzentriert arbeiten", sagte Gilles Cottier, Präsident von SAFC. "SAFC hat eng mit Oncolytics gearbeitet zu entwickeln, Scale-up und Optimierung der Herstellung von REOLYSIN, die Förderung an die kommerzielle Ebene."
"Diese Vereinbarung stellt einen bedeutenden Schritt vorwärts im Bemühen um die Vermarktung von REOLYSIN wie wir weitere klinische Versorgung produzieren und bauen Inventar für potenzielle gewerbliche Verkäufe vorzubereiten", sagte Dr. Matt Coffey, Chief Operating Officer von Oncolytics. "Nachdem wir mit ein weltweit anerkannter Marktführer auf die effiziente Herstellung von REOLYSIN seit einigen Jahren, wir fühlen uns gut mit diesem Element unseres Gesamtplans zur REOLYSIN vermarkten positioniert gearbeitet."
INTERVIEW-Oncolytics says in talks on partnership deals
15 hours ago by Thomson Reuters
* Companies across the globe show partnering interest
* Could launch reolysin in late 2013 if regulators approve
By S. John Tilak
TORONTO, May 13 (Reuters) - Oncolytics Biotech Inc <ONC.TO> <ONCY.O> says it has received interest from potential marketing partners across the world for its cancer-combating therapy, reolysin, and is in discussions with several of them as it looks to launch the much-heralded treatment as early as 2013.
"We're looking to cover all regions with partnerships," Chief Executive Brad Thompson told Reuters in an interview this week.
Oncolytics, a Calgary-based biotechnology company, develops specialized viruses to treat cancer. Reolysin is its lead product and is a formulation of a human reovirus that is associated with respiratory and gastrointestinal diseases.
The company says reolysin -- a short form of the term respiratory enteric orphan virus -- is designed to destroy cancer cells alone or in combination with chemotherapy treatments. It is currently being tested on humans.
Partnerships are key for smaller biotech companies, which need the scale of global or regional pharmaceutical companies to distribute and market their products.
"Basically you engage in (partnering) activities and you run like you don't have a partner. You do those two things in parallel," Thompson said.
Thompson declined to comment on when a deal could be signed other than to say it is likely to be before the product is launched. Analysts expect an agreement much earlier, possibly this year.
"We'll do a partnership when it gives us a better financial outcome than not having a partner does," Thompson said.
The timing of regulatory approval depends on factors such as how quickly people are enrolled for studies that are underway in several countries.
Late-stage clinical studies for head and neck cancer have been approved in six countries and that should expand to 10 or 11 countries by the end of the summer, Thompson said.
"It's possible that if everything goes correctly to have product approval in late 2013," he said.
Earlier this week, Oncolytics signed a commercial supply agreement with SAFC, a unit of Sigma-Aldrich Corp <SIAL.O>, for the commercial manufacture of reolysin.
"The deal is absolutely critical for us to be ready to file for product approval," Thompson said.
The company owns a patent until 2028 that covers methods for making and using modified reoviruses and also covers pharmaceutical compositions that include them. (Reporting by S. John Tilak; editing by Peter Galloway)
reguau
.
15 hours ago by Thomson Reuters
* Companies across the globe show partnering interest
* Could launch reolysin in late 2013 if regulators approve
By S. John Tilak
TORONTO, May 13 (Reuters) - Oncolytics Biotech Inc <ONC.TO> <ONCY.O> says it has received interest from potential marketing partners across the world for its cancer-combating therapy, reolysin, and is in discussions with several of them as it looks to launch the much-heralded treatment as early as 2013.
"We're looking to cover all regions with partnerships," Chief Executive Brad Thompson told Reuters in an interview this week.
Oncolytics, a Calgary-based biotechnology company, develops specialized viruses to treat cancer. Reolysin is its lead product and is a formulation of a human reovirus that is associated with respiratory and gastrointestinal diseases.
The company says reolysin -- a short form of the term respiratory enteric orphan virus -- is designed to destroy cancer cells alone or in combination with chemotherapy treatments. It is currently being tested on humans.
Partnerships are key for smaller biotech companies, which need the scale of global or regional pharmaceutical companies to distribute and market their products.
"Basically you engage in (partnering) activities and you run like you don't have a partner. You do those two things in parallel," Thompson said.
Thompson declined to comment on when a deal could be signed other than to say it is likely to be before the product is launched. Analysts expect an agreement much earlier, possibly this year.
"We'll do a partnership when it gives us a better financial outcome than not having a partner does," Thompson said.
The timing of regulatory approval depends on factors such as how quickly people are enrolled for studies that are underway in several countries.
Late-stage clinical studies for head and neck cancer have been approved in six countries and that should expand to 10 or 11 countries by the end of the summer, Thompson said.
"It's possible that if everything goes correctly to have product approval in late 2013," he said.
Earlier this week, Oncolytics signed a commercial supply agreement with SAFC, a unit of Sigma-Aldrich Corp <SIAL.O>, for the commercial manufacture of reolysin.
"The deal is absolutely critical for us to be ready to file for product approval," Thompson said.
The company owns a patent until 2028 that covers methods for making and using modified reoviruses and also covers pharmaceutical compositions that include them. (Reporting by S. John Tilak; editing by Peter Galloway)
reguau
.
Oncolytics may launch cancer treatment in 2013
Firm has received interest from potential partners
By S. John Tilak, Reuters May 14, 2011 Oncolytics Biotech Inc says it has received interest from potential marketing partners across the world for its cancer-combating therapy, reolysin, and is in discussions with several of them as it looks to launch the much-heralded treatment as early as 2013.
"We're looking to cover all regions with partnerships," chief executive Brad Thompson said this week.
Oncolytics, a Calgary-based biotechnology company, develops specialized viruses to treat cancer. Reolysin is its lead product and is a formulation of a human reovirus that is associated with respiratory and gastrointestinal diseases.
The company says reolysin -a short form of the term respiratory enteric orphan virus -is designed to destroy cancer cells alone or in combination with chemotherapy treatments. It is currently being tested on humans.
Partnerships are key for smaller biotech companies, which need the scale of global or regional pharmaceutical companies to distribute and market their products.
"Basically you engage in (partnering) activities and you run like you don't have a partner. You do those two things in parallel," Thompson said.
Thompson declined to comment on when a deal could be signed, other than to say it is likely to be before the product is launched. Analysts expect an agreement much earlier, possibly this year.
"We'll do a partnership when it gives us a better financial outcome than not having a partner does," Thompson said.
The timing of regulatory approval depends on factors such as how quickly people are enrolled for studies that are underway in several countries.
Late-stage clinical studies for head and neck cancer have been approved in six countries and that should expand to 10 or 11 countries by the end of the summer, Thompson said.
"It's possible that if everything goes correctly to have product approval in late 2013," he said.
Earlier this week, Oncolytics signed a supply agreement with SAFC, a unit of Sigma-Aldrich Corp, for the commercial manufacture of reolysin.
"The deal is absolutely critical or us to be ready to file for prodct approval," Thompson says the ompany owns a patent until 2028 hat covers methods for making and sing modified reoviruses and also overs pharmaceutical compositions hat include them.
© Copyright (c) The Calgary Herald
Read more: http://www.calgaryherald.com/health/Oncolytics+launch+cancer…
reguau
Firm has received interest from potential partners
By S. John Tilak, Reuters May 14, 2011 Oncolytics Biotech Inc says it has received interest from potential marketing partners across the world for its cancer-combating therapy, reolysin, and is in discussions with several of them as it looks to launch the much-heralded treatment as early as 2013.
"We're looking to cover all regions with partnerships," chief executive Brad Thompson said this week.
Oncolytics, a Calgary-based biotechnology company, develops specialized viruses to treat cancer. Reolysin is its lead product and is a formulation of a human reovirus that is associated with respiratory and gastrointestinal diseases.
The company says reolysin -a short form of the term respiratory enteric orphan virus -is designed to destroy cancer cells alone or in combination with chemotherapy treatments. It is currently being tested on humans.
Partnerships are key for smaller biotech companies, which need the scale of global or regional pharmaceutical companies to distribute and market their products.
"Basically you engage in (partnering) activities and you run like you don't have a partner. You do those two things in parallel," Thompson said.
Thompson declined to comment on when a deal could be signed, other than to say it is likely to be before the product is launched. Analysts expect an agreement much earlier, possibly this year.
"We'll do a partnership when it gives us a better financial outcome than not having a partner does," Thompson said.
The timing of regulatory approval depends on factors such as how quickly people are enrolled for studies that are underway in several countries.
Late-stage clinical studies for head and neck cancer have been approved in six countries and that should expand to 10 or 11 countries by the end of the summer, Thompson said.
"It's possible that if everything goes correctly to have product approval in late 2013," he said.
Earlier this week, Oncolytics signed a supply agreement with SAFC, a unit of Sigma-Aldrich Corp, for the commercial manufacture of reolysin.
"The deal is absolutely critical or us to be ready to file for prodct approval," Thompson says the ompany owns a patent until 2028 hat covers methods for making and sing modified reoviruses and also overs pharmaceutical compositions hat include them.
© Copyright (c) The Calgary Herald
Read more: http://www.calgaryherald.com/health/Oncolytics+launch+cancer…
reguau
Antwort auf Beitrag Nr.: 41.505.029 von reguau am 15.05.11 16:21:10interessantes Infoboard......
http://investorshub.advfn.com/boards/board.aspx?board_id=814
http://investorshub.advfn.com/boards/board.aspx?board_id=814
Oncolytics Biotech Phase III-Studie zu Reolysin könnte ric.
31.05.11 09:20
Meldung Endingen (aktiencheck.de AG) - Die Experten von "Global Biotech Investing" halten die Aktie von Oncolytics Biotch (ISIN CA6823101077 / WKN 936874 ) für ein interessantes Investment.
Die gerade gestartete Phase III-Studie zu Reolysin könnte richtungsweisend für Oncolytics Biotech sein. Reolysin dürfte nach Einschätzung der Experten zu den vielversprechendsten "Targeted agents" zählen, an denen momentan in der Onkologie geforscht werde. Oncolytics Biotech habe sich darauf spezialisiert, onkolytische Viren zu entwickeln, die potenziell zur Krebstherapie eingesetzt werden könnten. In einer Ersteinschätzung würden die Analysten von Oppenheimer & Co. den Titel mit "perform" einstufen.
Die Experten von "Global Biotech Investing" halten die Aktie von Oncolytics Biotech für ein spannendes Investment. (Ausgabe 11 vom 30.05.2011) (31.05.2011/ac/a/a)
immerhin, soweit ich weiß, die erste empfehlung in deutscher sprache
reguau
31.05.11 09:20
Meldung Endingen (aktiencheck.de AG) - Die Experten von "Global Biotech Investing" halten die Aktie von Oncolytics Biotch (ISIN CA6823101077 / WKN 936874 ) für ein interessantes Investment.
Die gerade gestartete Phase III-Studie zu Reolysin könnte richtungsweisend für Oncolytics Biotech sein. Reolysin dürfte nach Einschätzung der Experten zu den vielversprechendsten "Targeted agents" zählen, an denen momentan in der Onkologie geforscht werde. Oncolytics Biotech habe sich darauf spezialisiert, onkolytische Viren zu entwickeln, die potenziell zur Krebstherapie eingesetzt werden könnten. In einer Ersteinschätzung würden die Analysten von Oppenheimer & Co. den Titel mit "perform" einstufen.
Die Experten von "Global Biotech Investing" halten die Aktie von Oncolytics Biotech für ein spannendes Investment. (Ausgabe 11 vom 30.05.2011) (31.05.2011/ac/a/a)
immerhin, soweit ich weiß, die erste empfehlung in deutscher sprache
reguau
Interim results - ONCY reovirus combo response rate = 42% 5-Nov-11 01:27 pm
Slide #5
Head and Neck Cancer Patients
Interim Results (REO 011) Compared to Historical Controls
1. Docetaxel = 6.7%
2. Carboplatin/paclitaxel = 10.0%
3. REOLYSIN + carboplatin/paclitaxel = 42% response rate
http://www.oncolyticsbiotech.com/pdf/Onc...
Focused Clinical Program
• Lead product is REOLYSIN®
• Phase III REOLYSIN and paclitaxel/carboplatin in platinum-refractory
head and neck cancer patients (international study)
• Randomized Phase 2 REOLYSIN and paclitaxel in ovarian and
reproductive organ cancer patients (US)
• Randomized Phase 2 REOLYSIN and paclitaxel/carboplatin in recurrent
or metastatic pancreatic cancer (US)
Slide #5
Head and Neck Cancer Patients
Interim Results (REO 011) Compared to Historical Controls
1. Docetaxel = 6.7%
2. Carboplatin/paclitaxel = 10.0%
3. REOLYSIN + carboplatin/paclitaxel = 42% response rate
http://www.oncolyticsbiotech.com/pdf/Onc...
Focused Clinical Program
• Lead product is REOLYSIN®
• Phase III REOLYSIN and paclitaxel/carboplatin in platinum-refractory
head and neck cancer patients (international study)
• Randomized Phase 2 REOLYSIN and paclitaxel in ovarian and
reproductive organ cancer patients (US)
• Randomized Phase 2 REOLYSIN and paclitaxel/carboplatin in recurrent
or metastatic pancreatic cancer (US)
ONCY reovirus in Osteosarcoma 5-Nov-11 02:06 pm
Seher A. Khan, PhD
School of Pharmacy
Lake Erie College of Osteopathic Medicine
Erie, Pennsylvania
>>Ras Pathways: Reovirus is a naturally occurring virus that is relatively harmless in humans but can grow in cells where Ras pathways are activated. Activated Ras pathways inhibit the antiviral effects of a cellular kinase (PKR; double-stranded RNA-activated protein kinase), rendering tumor cells to viral replication and lysis. Reolysin is an IV formulation of reovirus serotype 3 dearing strain used to treat different cancers. In a phase II open-label, single-agent study, Reolysin was administered every 28 days in patients with bone or soft-tissue sarcoma with lung metastasis.
Preliminary data suggest that reovirus was well-tolerated, with 5 out of 33 patients attaining stable disease for >6 months.<<
http://www.uspharmacist.com/content/c/30...
Seher A. Khan, PhD
School of Pharmacy
Lake Erie College of Osteopathic Medicine
Erie, Pennsylvania
>>Ras Pathways: Reovirus is a naturally occurring virus that is relatively harmless in humans but can grow in cells where Ras pathways are activated. Activated Ras pathways inhibit the antiviral effects of a cellular kinase (PKR; double-stranded RNA-activated protein kinase), rendering tumor cells to viral replication and lysis. Reolysin is an IV formulation of reovirus serotype 3 dearing strain used to treat different cancers. In a phase II open-label, single-agent study, Reolysin was administered every 28 days in patients with bone or soft-tissue sarcoma with lung metastasis.
Preliminary data suggest that reovirus was well-tolerated, with 5 out of 33 patients attaining stable disease for >6 months.<<
http://www.uspharmacist.com/content/c/30...
Oncolytics Biotech® Inc. Announces Positive Results of U.S. Phase 2 Clinical Trial Investigating REOLYSIN® in Combination with Paclitaxel and Carboplatin in Head and Neck Cancers
* Reuters is not responsible for the content in this press release.
Mon Nov 14, 2011 7:01am EST
Oncolytics Biotech® Inc. Announces Positive Results of U.S. Phase 2 Clinical Trial Investigating REOLYSIN® in Combination with Paclitaxel and Carboplatin in Head and Neck Cancers
PR Newswire
CALGARY, Nov. 14, 2011
CALGARY, Nov. 14, 2011 /PRNewswire/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) today announced that positive results from a Phase 2 clinical trial (REO 015) using intravenous administration of REOLYSIN® in combination with paclitaxel and carboplatin in patients with advanced head and neck cancers will be presented at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics. The conference is being held in San Francisco, CA from November 12th to 16th 2011. The Principal Investigator of the U.S. study was Dr. Monica Mita of the Cancer Therapy & Research Center at The University of Texas Health Science Center at San Antonio (CTRC).
The U.S. trial was a single arm open-label, phase-2 study of REOLYSIN given intravenously with paclitaxel (175 mg/m2) and carboplatin (AUC 5) every three weeks in patients with platinum-refractory recurrent and/or metastatic squamous cell cancers of the oral cavity, larynx, or pharynx. The primary end point was to determine the objective response rate (complete response (CR) + partial response (PR)) of the treatment regimen in the study population. Secondary objectives included the determination of disease control rate (CR + PR + stable disease (SD)) and the safety and tolerability of the treatment regimen.
Of the 14 enrolled patients, all had received prior chemotherapy, radiotherapy, or combinations thereof for their metastatic or recurrent disease. Ten of the 14 patients received prior chemotherapy treatment with taxanes. Of the 13 patients evaluable for response, four had PRs, for an objective response rate of 31%. Six patients had SD or better for 12 weeks or longer for a disease control rate (SD or better) of 46%. Two of the four patients with PRs and both patients with SD had received prior treatment with taxanes.
"This study was undertaken as a confirmatory trial of an earlier study performed in the UK with a similar patient population (REO 011). It confirmed the high rate of responses observed in the U.K. study, and was noteworthy as this U.S. patient population included a higher proportion of patients that had failed prior taxane-based therapy," said Dr. Matt Coffey, COO of Oncolytics. "Historically, head and neck cancer has been characterized by a very low rate of success in treatment of patients who have already failed chemotherapy. Standard second-line therapies for platinum refractory head and neck cancer using currently approved cytotoxics have yielded 3 to 7% response rates1."
"These data further confirm the utility of REOLYSIN in this indication, even in a heavily pre-treated patient population," said Dr. Brad Thompson, President and CEO of Oncolytics. "These results are an important step towards making this potential therapy available to head and neck cancer patients, and represent a significant milestone for Oncolytics."
1León X et al. (2005) A retrospective analysis of the outcome of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck refractory to a platinum-based chemotherapy. ClinOncol 17: 418-424; Specenier P et al. (2011) Weekly Docetaxel in Patients With Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck. Am J ClinOncol 34: 472-7.
Conference Call Information
Dr. Brad Thompson, President and CEO of Oncolytics, will host a conference call and webcast on Monday, November 14th, 2011 at 6:30 a.m. MT (8:30 a.m. ET) to discuss in more depth the presentation covering the U.S. Phase II head and neck cancer data. To access the conference call by telephone, dial 1-647-427-7450 or 1-888-231-8191. A live audio webcast will also be available at the following link: http://www.newswire.ca/en/webcast/viewEvent.cgi?eventID=3743… or through the Company's website at www.oncolyticsbiotech.com. Please connect at least 10 minutes prior to the webcast to ensure adequate time for any software download that may be needed. A replay of the webcast will be available at www.oncolyticsbiotech.com and will also be available by telephone through November 21st, 2011. To access the telephone replay, dial 1-416-849-0833 or 1-855-859-2056 and enter reservation number 27729561 followed by the number sign.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolyticviruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit:
* Reuters is not responsible for the content in this press release.
Mon Nov 14, 2011 7:01am EST
Oncolytics Biotech® Inc. Announces Positive Results of U.S. Phase 2 Clinical Trial Investigating REOLYSIN® in Combination with Paclitaxel and Carboplatin in Head and Neck Cancers
PR Newswire
CALGARY, Nov. 14, 2011
CALGARY, Nov. 14, 2011 /PRNewswire/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) today announced that positive results from a Phase 2 clinical trial (REO 015) using intravenous administration of REOLYSIN® in combination with paclitaxel and carboplatin in patients with advanced head and neck cancers will be presented at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics. The conference is being held in San Francisco, CA from November 12th to 16th 2011. The Principal Investigator of the U.S. study was Dr. Monica Mita of the Cancer Therapy & Research Center at The University of Texas Health Science Center at San Antonio (CTRC).
The U.S. trial was a single arm open-label, phase-2 study of REOLYSIN given intravenously with paclitaxel (175 mg/m2) and carboplatin (AUC 5) every three weeks in patients with platinum-refractory recurrent and/or metastatic squamous cell cancers of the oral cavity, larynx, or pharynx. The primary end point was to determine the objective response rate (complete response (CR) + partial response (PR)) of the treatment regimen in the study population. Secondary objectives included the determination of disease control rate (CR + PR + stable disease (SD)) and the safety and tolerability of the treatment regimen.
Of the 14 enrolled patients, all had received prior chemotherapy, radiotherapy, or combinations thereof for their metastatic or recurrent disease. Ten of the 14 patients received prior chemotherapy treatment with taxanes. Of the 13 patients evaluable for response, four had PRs, for an objective response rate of 31%. Six patients had SD or better for 12 weeks or longer for a disease control rate (SD or better) of 46%. Two of the four patients with PRs and both patients with SD had received prior treatment with taxanes.
"This study was undertaken as a confirmatory trial of an earlier study performed in the UK with a similar patient population (REO 011). It confirmed the high rate of responses observed in the U.K. study, and was noteworthy as this U.S. patient population included a higher proportion of patients that had failed prior taxane-based therapy," said Dr. Matt Coffey, COO of Oncolytics. "Historically, head and neck cancer has been characterized by a very low rate of success in treatment of patients who have already failed chemotherapy. Standard second-line therapies for platinum refractory head and neck cancer using currently approved cytotoxics have yielded 3 to 7% response rates1."
"These data further confirm the utility of REOLYSIN in this indication, even in a heavily pre-treated patient population," said Dr. Brad Thompson, President and CEO of Oncolytics. "These results are an important step towards making this potential therapy available to head and neck cancer patients, and represent a significant milestone for Oncolytics."
1León X et al. (2005) A retrospective analysis of the outcome of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck refractory to a platinum-based chemotherapy. ClinOncol 17: 418-424; Specenier P et al. (2011) Weekly Docetaxel in Patients With Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck. Am J ClinOncol 34: 472-7.
Conference Call Information
Dr. Brad Thompson, President and CEO of Oncolytics, will host a conference call and webcast on Monday, November 14th, 2011 at 6:30 a.m. MT (8:30 a.m. ET) to discuss in more depth the presentation covering the U.S. Phase II head and neck cancer data. To access the conference call by telephone, dial 1-647-427-7450 or 1-888-231-8191. A live audio webcast will also be available at the following link: http://www.newswire.ca/en/webcast/viewEvent.cgi?eventID=3743… or through the Company's website at www.oncolyticsbiotech.com. Please connect at least 10 minutes prior to the webcast to ensure adequate time for any software download that may be needed. A replay of the webcast will be available at www.oncolyticsbiotech.com and will also be available by telephone through November 21st, 2011. To access the telephone replay, dial 1-416-849-0833 or 1-855-859-2056 and enter reservation number 27729561 followed by the number sign.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolyticviruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit:
Oncolytics Biotech® Inc. Collaborators to Present Positive Phase 2 REOLYSIN Clinical Data in Pancreatic Cancer at the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics Conference
* Reuters is not responsible for the content in this press release.
Mon Nov 14, 2011 7:01am EST
Oncolytics Biotech® Inc. Collaborators to Present Positive Phase 2 REOLYSIN Clinical Data in Pancreatic Cancer at the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics Conference
PR Newswire
CALGARY, Nov. 14, 2011
CALGARY, Nov. 14, 2011 /PRNewswire/ - Oncolytics Biotech Inc. (TSX:ONC, NASDAQ:ONCY) ("Oncolytics") today announced that interim results from a Phase 2 clinical trial using intravenous administration of REOLYSIN® in combination with gemcitabine (Gemzar®) in patients with advanced pancreatic cancer will be presented at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics. The conference is being held in San Francisco, CA from November 12th to 16th, 2011.
The poster, entitled "A Phase II Study of REOLYSIN® in Combination with Gemcitabine in Patients with Advanced Pancreatic Adenocarcinoma", authored by Mita et al, indicated that as of the date of submission of the poster that 12 patients were evaluable for response. All patients except one reported symptomatic improvement. Seven patients had stable disease (SD) for 12 weeks or longer, for a clinical benefit rate (complete response (CR) + partial response (PR) + SD) of 58%.This included two patients who had SD for 36 weeks or longer. An additional patient had an unconfirmed PR of less than six weeks. The treatment was well tolerated with manageable adverse events.
This study is using a one sample, two-stage design. In the first stage, 17 patients were to be enrolled, and best response noted. If less than three responses (defined as CR or PR or SD for 12 weeks or more) were observed, the study would have concluded that the combination was inactive and been terminated. If three or more responses were observed among the 17 patents, the study would enroll an additional 16 patients for a total of 33 evaluable patients. As previously disclosed, this endpoint was met after six evaluable patients were enrolled. If at least eight responses are observed out of 33 patients, the study will conclude that the drug combination is active. This study will be updated if and when this endpoint is reached.
"Pancreatic cancer typically offers a dismal prognosis, so it is highly encouraging to see the clinical benefit rate reported in this study. The median progression free survival for pancreatic patients on gemcitabine is nine weeks1, whilst the majority of the patients on this study had not progressed as of 12 weeks," said Dr. Brad Thompson, President and CEO of Oncolytics. "We intend to continue testing REOLYSIN with various chemotherapeutic agents including gemcitabine and carboplatin/paclitaxel across multiple trials to further understand its potential in the treatment of pancreatic cancer."
1Burris,H.A. et al. (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J ClinOncol 15(6):2403-2413.
Conference Call Information
Dr. Brad Thompson, President and CEO of Oncolytics, will host a conference call and webcast on Monday, November 14th, 2011 at 6:30 a.m. MT (8:30 a.m. ET) to discuss in more depth the presentation covering the U.S. Phase II pancreatic cancer data. To access the conference call by telephone, dial 1-647-427-7450 or 1-888-231-8191. A live audio webcast will also be available at the following link: http://www.newswire.ca/en/webcast/viewEvent.cgi?eventID=3743… or through the Company's website at www.oncolyticsbiotech.com. Please connect at least 10 minutes prior to the webcast to ensure adequate time for any software download that may be needed. A replay of the webcast will be available at www.oncolyticsbiotech.com and will also be available by telephone through November 21st, 2011. To access the telephone replay, dial 1-416-849-0833 or 1-855-859-2056 and enter reservation number 27729561 followed by the number sign.
About Pancreatic Cancer
The American Cancer Society estimates that 44,030 Americans will be diagnosed with pancreatic cancer and 37,660 Americans will die from the disease in 2011, making this type of cancer the fourth leading cause of cancer death for both men and women in the United States. For more information about pancreatic cancer, please go to www.cancer.org.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
* Reuters is not responsible for the content in this press release.
Mon Nov 14, 2011 7:01am EST
Oncolytics Biotech® Inc. Collaborators to Present Positive Phase 2 REOLYSIN Clinical Data in Pancreatic Cancer at the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics Conference
PR Newswire
CALGARY, Nov. 14, 2011
CALGARY, Nov. 14, 2011 /PRNewswire/ - Oncolytics Biotech Inc. (TSX:ONC, NASDAQ:ONCY) ("Oncolytics") today announced that interim results from a Phase 2 clinical trial using intravenous administration of REOLYSIN® in combination with gemcitabine (Gemzar®) in patients with advanced pancreatic cancer will be presented at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics. The conference is being held in San Francisco, CA from November 12th to 16th, 2011.
The poster, entitled "A Phase II Study of REOLYSIN® in Combination with Gemcitabine in Patients with Advanced Pancreatic Adenocarcinoma", authored by Mita et al, indicated that as of the date of submission of the poster that 12 patients were evaluable for response. All patients except one reported symptomatic improvement. Seven patients had stable disease (SD) for 12 weeks or longer, for a clinical benefit rate (complete response (CR) + partial response (PR) + SD) of 58%.This included two patients who had SD for 36 weeks or longer. An additional patient had an unconfirmed PR of less than six weeks. The treatment was well tolerated with manageable adverse events.
This study is using a one sample, two-stage design. In the first stage, 17 patients were to be enrolled, and best response noted. If less than three responses (defined as CR or PR or SD for 12 weeks or more) were observed, the study would have concluded that the combination was inactive and been terminated. If three or more responses were observed among the 17 patents, the study would enroll an additional 16 patients for a total of 33 evaluable patients. As previously disclosed, this endpoint was met after six evaluable patients were enrolled. If at least eight responses are observed out of 33 patients, the study will conclude that the drug combination is active. This study will be updated if and when this endpoint is reached.
"Pancreatic cancer typically offers a dismal prognosis, so it is highly encouraging to see the clinical benefit rate reported in this study. The median progression free survival for pancreatic patients on gemcitabine is nine weeks1, whilst the majority of the patients on this study had not progressed as of 12 weeks," said Dr. Brad Thompson, President and CEO of Oncolytics. "We intend to continue testing REOLYSIN with various chemotherapeutic agents including gemcitabine and carboplatin/paclitaxel across multiple trials to further understand its potential in the treatment of pancreatic cancer."
1Burris,H.A. et al. (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J ClinOncol 15(6):2403-2413.
Conference Call Information
Dr. Brad Thompson, President and CEO of Oncolytics, will host a conference call and webcast on Monday, November 14th, 2011 at 6:30 a.m. MT (8:30 a.m. ET) to discuss in more depth the presentation covering the U.S. Phase II pancreatic cancer data. To access the conference call by telephone, dial 1-647-427-7450 or 1-888-231-8191. A live audio webcast will also be available at the following link: http://www.newswire.ca/en/webcast/viewEvent.cgi?eventID=3743… or through the Company's website at www.oncolyticsbiotech.com. Please connect at least 10 minutes prior to the webcast to ensure adequate time for any software download that may be needed. A replay of the webcast will be available at www.oncolyticsbiotech.com and will also be available by telephone through November 21st, 2011. To access the telephone replay, dial 1-416-849-0833 or 1-855-859-2056 and enter reservation number 27729561 followed by the number sign.
About Pancreatic Cancer
The American Cancer Society estimates that 44,030 Americans will be diagnosed with pancreatic cancer and 37,660 Americans will die from the disease in 2011, making this type of cancer the fourth leading cause of cancer death for both men and women in the United States. For more information about pancreatic cancer, please go to www.cancer.org.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
Oncolytics Biotech® Inc. Announces Phase 2 Clinical Trial of REOLYSIN® in Combination with Gemcitabine in Pancreatic Cancer Reaches Primary Endpoint
CALGARY, AB, December 2, 2011 --- Oncolytics Biotech Inc. (TSX:ONC, NASDAQ:ONCY) (“Oncolytics”) today announced that interim data from a Phase 2 clinical trial using intravenous administration of REOLYSIN® in combination with gemcitabine (Gemzar®) in patients with advanced pancreatic cancer (REO 017) indicated that the clinical study had successfully reached its primary endpoint, and that the drug combination is active.
To date, eight patients of 13 evaluable patients in the study had stable disease (SD) for 12 weeks or longer, for a clinical benefit rate (complete response (CR) + partial response (PR) + SD) of 62%. An additional patient had an unconfirmed PR of less than six weeks.
The study is using a one sample, two-stage design. In the first stage, 17 patients were to be enrolled, and best response noted. If less than three responses (defined as CR or PR or SD for 12 weeks or more) were observed, the study would have concluded that the combination was inactive and been terminated. If three or more responses were observed among the 17 patents, the study would enroll an additional 16 patients for a total of 33 evaluable patients. As previously disclosed, this initial endpoint was met after six evaluable patients were enrolled. If at least eight responses were observed out of 33 patients, the study would have reached its primary endpoint and conclude that the drug combination is active.
“These results far exceeded our initial expectations, having met the primary endpoint in a study designed to enroll 33 patients with just over a third of enrolment completed,” said Dr. Matt Coffey, Chief Operating Officer of Oncolytics. “We will continue to enroll in this study to evaluate the progression free survival of the patient group, and will continue testing REOLYSIN with various chemotherapeutic agents including gemcitabine and carboplatin/paclitaxel across multiple trials to further understand its potential in the treatment of pancreatic cancer.”
About Pancreatic Cancer
The American Cancer Society estimates that 44,030 Americans will be diagnosed with pancreatic cancer and 37,660 Americans will die from the disease in 2011, making this type of cancer the fourth leading cause of cancer death for both men and women in the United States. For more information about pancreatic cancer, please go to www.cancer.org.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics’ clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements, including the implication of the results generated to date with respect to REOLYSIN in the treatment of pancreatic cancer; the Company’s belief as to the potential of REOLYSIN as a cancer therapeutic; the Company’s expectations as to the success of its research and development programs in 2011 and beyond, the Company’s planned operations, the value of the additional patents and intellectual property; the Company’s expectations related to the applications of the patented technology; the Company’s expectations as to adequacy of its existing capital resources; the design, timing, success of planned clinical trial programs; and other statements related to anticipated developments in the Company’s business and technologies involve known and unknown risks and uncertainties, which could cause the Company’s actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of REOLYSIN as a cancer treatment, the success and timely completion of clinical studies and trials, the Company’s ability to successfully commercialize REOLYSIN, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. Investors should consult the Company’s quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake to update these forward-looking statements, except as required by applicable laws.
FOR FURTHER INFORMATION PLEASE CONTACT:
The Equicom Group
Nick Hurst
300 5th Ave. SW, 10th Floor
Calgary, Alberta T2P 3C4
Tel: 403.218.2835
Fax: 403.218.2830
nhurst@equicomgroup.com
reguau
CALGARY, AB, December 2, 2011 --- Oncolytics Biotech Inc. (TSX:ONC, NASDAQ:ONCY) (“Oncolytics”) today announced that interim data from a Phase 2 clinical trial using intravenous administration of REOLYSIN® in combination with gemcitabine (Gemzar®) in patients with advanced pancreatic cancer (REO 017) indicated that the clinical study had successfully reached its primary endpoint, and that the drug combination is active.
To date, eight patients of 13 evaluable patients in the study had stable disease (SD) for 12 weeks or longer, for a clinical benefit rate (complete response (CR) + partial response (PR) + SD) of 62%. An additional patient had an unconfirmed PR of less than six weeks.
The study is using a one sample, two-stage design. In the first stage, 17 patients were to be enrolled, and best response noted. If less than three responses (defined as CR or PR or SD for 12 weeks or more) were observed, the study would have concluded that the combination was inactive and been terminated. If three or more responses were observed among the 17 patents, the study would enroll an additional 16 patients for a total of 33 evaluable patients. As previously disclosed, this initial endpoint was met after six evaluable patients were enrolled. If at least eight responses were observed out of 33 patients, the study would have reached its primary endpoint and conclude that the drug combination is active.
“These results far exceeded our initial expectations, having met the primary endpoint in a study designed to enroll 33 patients with just over a third of enrolment completed,” said Dr. Matt Coffey, Chief Operating Officer of Oncolytics. “We will continue to enroll in this study to evaluate the progression free survival of the patient group, and will continue testing REOLYSIN with various chemotherapeutic agents including gemcitabine and carboplatin/paclitaxel across multiple trials to further understand its potential in the treatment of pancreatic cancer.”
About Pancreatic Cancer
The American Cancer Society estimates that 44,030 Americans will be diagnosed with pancreatic cancer and 37,660 Americans will die from the disease in 2011, making this type of cancer the fourth leading cause of cancer death for both men and women in the United States. For more information about pancreatic cancer, please go to www.cancer.org.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics’ clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements, including the implication of the results generated to date with respect to REOLYSIN in the treatment of pancreatic cancer; the Company’s belief as to the potential of REOLYSIN as a cancer therapeutic; the Company’s expectations as to the success of its research and development programs in 2011 and beyond, the Company’s planned operations, the value of the additional patents and intellectual property; the Company’s expectations related to the applications of the patented technology; the Company’s expectations as to adequacy of its existing capital resources; the design, timing, success of planned clinical trial programs; and other statements related to anticipated developments in the Company’s business and technologies involve known and unknown risks and uncertainties, which could cause the Company’s actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of REOLYSIN as a cancer treatment, the success and timely completion of clinical studies and trials, the Company’s ability to successfully commercialize REOLYSIN, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. Investors should consult the Company’s quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake to update these forward-looking statements, except as required by applicable laws.
FOR FURTHER INFORMATION PLEASE CONTACT:
The Equicom Group
Nick Hurst
300 5th Ave. SW, 10th Floor
Calgary, Alberta T2P 3C4
Tel: 403.218.2835
Fax: 403.218.2830
nhurst@equicomgroup.com
reguau
Re: Monika Mita posters on ONCY website. 28 minutes ago
Very positive results in the treatment of ADVANCED pancreatic cancer from:
"A Phase II Study of REOLYSIN® in Combination with Gemcitabine in Patients With Advanced Pancreatic Adenocarcinoma"
"Conclusions
• The first stage endpoint of the study has been
reached and patient accrual is continuing
• REOLYSIN® combined with gemcitabine has
demonstrated clinical benefit in patients with
unresectable pancreatic cancer"
http://media.integratir.com/T.ONC/ppt/Po...
Very positive results in the treatment of ADVANCED pancreatic cancer from:
"A Phase II Study of REOLYSIN® in Combination with Gemcitabine in Patients With Advanced Pancreatic Adenocarcinoma"
"Conclusions
• The first stage endpoint of the study has been
reached and patient accrual is continuing
• REOLYSIN® combined with gemcitabine has
demonstrated clinical benefit in patients with
unresectable pancreatic cancer"
http://media.integratir.com/T.ONC/ppt/Po...
sie steigt seit tagen, ohne meldung mit steigendem volumen
KursdatenBörse Nasdaq
Aktuell 5,52 USD
Zeit 02.02.1217:00
Diff. Vortag +8,88%
Tages-Vol. --
Gehandelte Stück 125.924
KursdatenBörse Nasdaq
Aktuell 5,52 USD
Zeit 02.02.1217:00
Diff. Vortag +8,88%
Tages-Vol. --
Gehandelte Stück 125.924
KursdatenBörse Nasdaq
Aktuell 5,58 USD
Zeit 02.02.1222:13
Diff. Vortag +10,06%
Tages-Vol. --
Gehandelte Stück 399.420
reguau
Aktuell 5,58 USD
Zeit 02.02.1222:13
Diff. Vortag +10,06%
Tages-Vol. --
Gehandelte Stück 399.420
reguau
See more news releases in: Biotechnology, Contracts, Clinical Trials & Medical Discoveries
Oncolytics Biotech® Inc. Enters into Sponsorship Agreement with NCIC CTG for Randomized Phase II Study in Prostate Cancer
0 0 0
My news for Investors AtLeast one of the check box should be selected You are following news about Follow the latest news about ONCY
CALGARY, Feb. 21, 2012 /PRNewswire/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ: ONCY) announced today that it has entered into an agreement whereby the NCIC Clinical Trials Group (CTG) at Queen's University in Kingston, Ontario, will sponsor and conduct a randomized Phase II study of REOLYSIN® in patients with recurrent or metastatic castration resistant prostate cancer.
"Third party sponsored trials remain an important part of our overall clinical strategy. We are pleased to be conducting another randomized study, especially in Canada, with an experienced and capable group like the NCIC CTG," said Dr. Brad Thompson, President and CEO of Oncolytics. "This study will build on both our early preclinical and clinical prostate cancer work, as well as our growing understanding of REOLYSIN in combination with approved chemotherapeutic agents. As a Company we continue to expand our clinical program to include studies of frequently diagnosed cancers."
The study will be an open-label, randomized, non-blinded, Phase II clinical study of REOLYSIN given in combination with docetaxel versus docetaxel alone. Approximately 40 response evaluable patients will be enrolled in each arm.
About Prostate Cancer
The Canadian Cancer Society estimates that 25,500 Canadians were diagnosed with prostate cancer and nearly 4,100 Canadians were expected to die from the disease in 2011. The prognosis for patients diagnosed with prostate cancer varies but the five-year survival rate for the period between 2004 and 2006 was approximately 95%.
About the NCIC Clinical Trials Group at Queen's University
The NCIC Clinical Trials Group (NCIC CTG) is a cancer clinical trials cooperative group that conducts Phase I-III trials testing anti-cancer and supportive therapies across Canada and internationally. It is a national research program of the Canadian Cancer Society. The NCIC CTG's Central Office is located at Queen's University in Kingston, Ontario, Canada. The Group is committed to assessing all modalities of therapy for a spectrum of cancer types. More than 60 member institutions, from major cancer centres to community hospitals, enroll patients in NCIC Clinical Trials Group studies.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements, including the Company's expectations related to the Phase II prostate cancer trial sponsored by the NCIC CTG, and the Company's belief as to the potential of REOLYSIN as a cancer therapeutic, involve known and unknown risks and uncertainties, which could cause the Company's actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of REOLYSIN as a cancer treatment, the tolerability of REOLYSIN outside a controlled test, the success and timely completion of clinical studies and trials, the Company's ability to successfully commercialize REOLYSIN, uncertainties related to the research and development of pharmaceuticals and uncertainties related to the regulatory process. Investors should consult the Company's quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake to update these forward-looking statements, except as required by applicable laws.
SOURCE Oncolytics Biotech Inc.
Oncolytics Biotech® Inc. Enters into Sponsorship Agreement with NCIC CTG for Randomized Phase II Study in Prostate Cancer
0 0 0
My news for Investors AtLeast one of the check box should be selected You are following news about Follow the latest news about ONCY
CALGARY, Feb. 21, 2012 /PRNewswire/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ: ONCY) announced today that it has entered into an agreement whereby the NCIC Clinical Trials Group (CTG) at Queen's University in Kingston, Ontario, will sponsor and conduct a randomized Phase II study of REOLYSIN® in patients with recurrent or metastatic castration resistant prostate cancer.
"Third party sponsored trials remain an important part of our overall clinical strategy. We are pleased to be conducting another randomized study, especially in Canada, with an experienced and capable group like the NCIC CTG," said Dr. Brad Thompson, President and CEO of Oncolytics. "This study will build on both our early preclinical and clinical prostate cancer work, as well as our growing understanding of REOLYSIN in combination with approved chemotherapeutic agents. As a Company we continue to expand our clinical program to include studies of frequently diagnosed cancers."
The study will be an open-label, randomized, non-blinded, Phase II clinical study of REOLYSIN given in combination with docetaxel versus docetaxel alone. Approximately 40 response evaluable patients will be enrolled in each arm.
About Prostate Cancer
The Canadian Cancer Society estimates that 25,500 Canadians were diagnosed with prostate cancer and nearly 4,100 Canadians were expected to die from the disease in 2011. The prognosis for patients diagnosed with prostate cancer varies but the five-year survival rate for the period between 2004 and 2006 was approximately 95%.
About the NCIC Clinical Trials Group at Queen's University
The NCIC Clinical Trials Group (NCIC CTG) is a cancer clinical trials cooperative group that conducts Phase I-III trials testing anti-cancer and supportive therapies across Canada and internationally. It is a national research program of the Canadian Cancer Society. The NCIC CTG's Central Office is located at Queen's University in Kingston, Ontario, Canada. The Group is committed to assessing all modalities of therapy for a spectrum of cancer types. More than 60 member institutions, from major cancer centres to community hospitals, enroll patients in NCIC Clinical Trials Group studies.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements, including the Company's expectations related to the Phase II prostate cancer trial sponsored by the NCIC CTG, and the Company's belief as to the potential of REOLYSIN as a cancer therapeutic, involve known and unknown risks and uncertainties, which could cause the Company's actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of REOLYSIN as a cancer treatment, the tolerability of REOLYSIN outside a controlled test, the success and timely completion of clinical studies and trials, the Company's ability to successfully commercialize REOLYSIN, uncertainties related to the research and development of pharmaceuticals and uncertainties related to the regulatory process. Investors should consult the Company's quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake to update these forward-looking statements, except as required by applicable laws.
SOURCE Oncolytics Biotech Inc.
04/02/2012 07:00:00
Oncolytics Biotech® Inc. Completes Enrollment in First Stage of Phase III Study in Head and Neck Cancers
CALGARY, April 2, 2012 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) announced today that it has completed enrollment in the first, 80 patient stage of its Phase III clinical trial examining REOLYSIN® in combination with paclitaxel and carboplatin in patients with platinum-refractory head and neck cancers (REO 018).
"This is an important milestone for this study," said Dr. Brad Thompson, President and CEO of Oncolytics. "A data analysis that will involve examining evolving progression free survival will now be performed on this patient group to determine the probability of success in the second stage of the study."
The randomized, two-arm, double-blind, multi-centre, two-stage, adaptive Phase III trial will assess the intravenous administration of REOLYSIN with the chemotherapy combination of paclitaxel and carboplatin versus the chemotherapy alone in patients with metastatic or recurrent squamous cell carcinoma of the head and neck, or squamous cell cancer of the nasopharynx, who have progressed on or after prior platinum-based chemotherapy. All patients will receive treatment every three weeks (21 day cycles) with paclitaxel and carboplatin and will also receive, on a blinded basis, either intravenous placebo or intravenous REOLYSIN. All dosing takes place in the first five days of each cycle, with all patients receiving standard intravenous doses of paclitaxel and carboplatin on day one only, and on days one through five, either intravenous placebo or intravenous REOLYSIN at a dose of 3x1010 TCID50. Patients may continue to receive the trial combination therapy for up to eight, 21-day cycles and, thereafter, blinded placebo or blinded REOLYSIN until the patient has progressive disease or meets other criteria for removal from the trial.
The primary endpoint for the trial is overall survival (OS). Secondary endpoints include progression free survival (PFS), objective response rate (complete response (CR) + partial response (PR)) and duration of response, and safety and tolerability of REOLYSIN when administered in combination with paclitaxel and carboplatin. The first stage of the trial is non-adaptive, and was designed to enroll 80 patients. The second stage is adaptive, and is designed to enroll between 100 and 400 patients with the most probable statistical enrollment being 195 patients in this stage. This adaptive trial design allows data evaluation to determine if the probability of reaching a statistically significant endpoint has been achieved.
The trial is currently being conducted in more than 80 centres in 12 countries in North America and Europe, including the U.S. following an agreement with the U.S. Food and Drug Administration (FDA) under the Special Protocol Assessment (SPA) process with respect to the trial's design.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
Oncolytics Biotech® Inc. Completes Enrollment in First Stage of Phase III Study in Head and Neck Cancers
CALGARY, April 2, 2012 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) announced today that it has completed enrollment in the first, 80 patient stage of its Phase III clinical trial examining REOLYSIN® in combination with paclitaxel and carboplatin in patients with platinum-refractory head and neck cancers (REO 018).
"This is an important milestone for this study," said Dr. Brad Thompson, President and CEO of Oncolytics. "A data analysis that will involve examining evolving progression free survival will now be performed on this patient group to determine the probability of success in the second stage of the study."
The randomized, two-arm, double-blind, multi-centre, two-stage, adaptive Phase III trial will assess the intravenous administration of REOLYSIN with the chemotherapy combination of paclitaxel and carboplatin versus the chemotherapy alone in patients with metastatic or recurrent squamous cell carcinoma of the head and neck, or squamous cell cancer of the nasopharynx, who have progressed on or after prior platinum-based chemotherapy. All patients will receive treatment every three weeks (21 day cycles) with paclitaxel and carboplatin and will also receive, on a blinded basis, either intravenous placebo or intravenous REOLYSIN. All dosing takes place in the first five days of each cycle, with all patients receiving standard intravenous doses of paclitaxel and carboplatin on day one only, and on days one through five, either intravenous placebo or intravenous REOLYSIN at a dose of 3x1010 TCID50. Patients may continue to receive the trial combination therapy for up to eight, 21-day cycles and, thereafter, blinded placebo or blinded REOLYSIN until the patient has progressive disease or meets other criteria for removal from the trial.
The primary endpoint for the trial is overall survival (OS). Secondary endpoints include progression free survival (PFS), objective response rate (complete response (CR) + partial response (PR)) and duration of response, and safety and tolerability of REOLYSIN when administered in combination with paclitaxel and carboplatin. The first stage of the trial is non-adaptive, and was designed to enroll 80 patients. The second stage is adaptive, and is designed to enroll between 100 and 400 patients with the most probable statistical enrollment being 195 patients in this stage. This adaptive trial design allows data evaluation to determine if the probability of reaching a statistically significant endpoint has been achieved.
The trial is currently being conducted in more than 80 centres in 12 countries in North America and Europe, including the U.S. following an agreement with the U.S. Food and Drug Administration (FDA) under the Special Protocol Assessment (SPA) process with respect to the trial's design.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
Re: Another Canadian NCIC trial starting up? 29-Apr-12 12:30 am
I wonder if it will be another randomized trial - my guess would be yes.
It's really great and significant to see that Canada's NCIC may soon be sponsoring multiple randomized Reo trials.
So now:
2 sponsored NCIC trials in Canada.
3 sponsored trials at University of Texas CTRC.
6 sponsored NCI trials in US.
1 UK sponsored trial - University of Leeds translational colorectal trial.
Total of at least 12 sponsored Reolysin trials to date - amazing!
reguau
I wonder if it will be another randomized trial - my guess would be yes.
It's really great and significant to see that Canada's NCIC may soon be sponsoring multiple randomized Reo trials.
So now:
2 sponsored NCIC trials in Canada.
3 sponsored trials at University of Texas CTRC.
6 sponsored NCI trials in US.
1 UK sponsored trial - University of Leeds translational colorectal trial.
Total of at least 12 sponsored Reolysin trials to date - amazing!
reguau
05/03/2012 07:30:00
Oncolytics Biotech® Inc. Enters into Sponsorship Agreement with NCIC CTG for Randomized Phase II Study in Colorectal Cancer
CALGARY, May 3, 2012 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) announced today that it has entered into an agreement whereby the NCIC Clinical Trials Group (CTG) at Queen's University in Kingston, Ontario, will sponsor and conduct a randomized Phase II study of REOLYSIN® in patients with advanced or metastatic colorectal cancer.
"We are pleased to be conducting a second randomized study with the NCIC CTG," said Dr. Brad Thompson, President and CEO of Oncolytics. "This study will build on both our early preclinical and clinical colorectal cancer work, as well as preclinical research combining REOLYSIN with Avastin®. As a Company, we intend to continue expanding our clinical program to include randomized studies of frequently diagnosed cancers."
The study will be an open-label, randomized, non-blinded, Phase II clinical study of REOLYSIN given in combination with FOLFOX-6 plus bevacizumab (Avastin) versus FOLFOX-6 plus bevacizumab alone. Approximately 50 response evaluable patients will be enrolled in each arm, after a six to nine patient safety run in.
About Colorectal Cancer
The Canadian Cancer Society estimates that 22,200 Canadians were diagnosed with colorectal cancer and 8,900 Canadians were expected to die from the disease in 2011. The American Cancer Society estimates that 143,460 Americans will be diagnosed with colorectal cancer and 51,690 Americans are expected to die of the disease in 2012.
About the NCIC Clinical Trials Group at Queen's University
The NCIC Clinical Trials Group (NCIC CTG) is a cancer clinical trials cooperative group that conducts Phase I-III trials testing anti-cancer and supportive therapies across Canada and internationally. It is a national research program of the Canadian Cancer Society. The NCIC CTG's Central Office is located at Queen's University in Kingston, Ontario, Canada. The Group is committed to assessing all modalities of therapy for a spectrum of cancer types. More than 60 member institutions, from major cancer centres to community hospitals, enroll patients in NCIC Clinical Trials Group studies.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
Oncolytics Biotech® Inc. Enters into Sponsorship Agreement with NCIC CTG for Randomized Phase II Study in Colorectal Cancer
CALGARY, May 3, 2012 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC, NASDAQ:ONCY) announced today that it has entered into an agreement whereby the NCIC Clinical Trials Group (CTG) at Queen's University in Kingston, Ontario, will sponsor and conduct a randomized Phase II study of REOLYSIN® in patients with advanced or metastatic colorectal cancer.
"We are pleased to be conducting a second randomized study with the NCIC CTG," said Dr. Brad Thompson, President and CEO of Oncolytics. "This study will build on both our early preclinical and clinical colorectal cancer work, as well as preclinical research combining REOLYSIN with Avastin®. As a Company, we intend to continue expanding our clinical program to include randomized studies of frequently diagnosed cancers."
The study will be an open-label, randomized, non-blinded, Phase II clinical study of REOLYSIN given in combination with FOLFOX-6 plus bevacizumab (Avastin) versus FOLFOX-6 plus bevacizumab alone. Approximately 50 response evaluable patients will be enrolled in each arm, after a six to nine patient safety run in.
About Colorectal Cancer
The Canadian Cancer Society estimates that 22,200 Canadians were diagnosed with colorectal cancer and 8,900 Canadians were expected to die from the disease in 2011. The American Cancer Society estimates that 143,460 Americans will be diagnosed with colorectal cancer and 51,690 Americans are expected to die of the disease in 2012.
About the NCIC Clinical Trials Group at Queen's University
The NCIC Clinical Trials Group (NCIC CTG) is a cancer clinical trials cooperative group that conducts Phase I-III trials testing anti-cancer and supportive therapies across Canada and internationally. It is a national research program of the Canadian Cancer Society. The NCIC CTG's Central Office is located at Queen's University in Kingston, Ontario, Canada. The Group is committed to assessing all modalities of therapy for a spectrum of cancer types. More than 60 member institutions, from major cancer centres to community hospitals, enroll patients in NCIC Clinical Trials Group studies.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
Message Boards Settings Oncolytics Biotech Inc. - Quote Info
Search :
in Oncolytics Biotech Inc. boardStocks O categoryStocks (A to Z) categoryAll Yahoo! Message BoardsThe Web
Yahoo! Message Boards > Business & Finance > Investments > Stocks (A to Z) > Stocks O > Oncolytics Biotech Inc.
View all Topics | View all Messages < Newer Topic | Older Topic >
Another indication for reovirus - Acute Myeloid Leukemia 57 minutes ago
http://online.liebertpub.com/doi/pdf/10....
Search :
in Oncolytics Biotech Inc. boardStocks O categoryStocks (A to Z) categoryAll Yahoo! Message BoardsThe Web
Yahoo! Message Boards > Business & Finance > Investments > Stocks (A to Z) > Stocks O > Oncolytics Biotech Inc.
View all Topics | View all Messages < Newer Topic | Older Topic >
Another indication for reovirus - Acute Myeloid Leukemia 57 minutes ago
http://online.liebertpub.com/doi/pdf/10....
und hier ein kommentar zu weiteren anwendungsmöglichkeiten des reovirus
Re: Another indication for reovirus - Acute Myeloid Leukemia 7 minutes ago
From right near the end of the paper it says this:
"Since a number of other oncolytic viruses, including adenovirus, myxoma virus, and vesicular stomatitis virus, have also been shown to induce death in leukemic cells of myeloid origin, future work should also include testing reovirus in combination with other viruses for the treatment of leukemia."
This is a clear statement of what many of us have suggested in the past. The use of oncolytic viruses is opening up a whole new world of possibilities as regards the treatment of cancer. Combinations of oncolytic viruses may be just the thing to eradicate or hold in abeyance many types of cancer. Maybe oncolytic virus cocktails will do the same thing against cancers as drug cocktails have done against AIDS.
Re: Another indication for reovirus - Acute Myeloid Leukemia 7 minutes ago
From right near the end of the paper it says this:
"Since a number of other oncolytic viruses, including adenovirus, myxoma virus, and vesicular stomatitis virus, have also been shown to induce death in leukemic cells of myeloid origin, future work should also include testing reovirus in combination with other viruses for the treatment of leukemia."
This is a clear statement of what many of us have suggested in the past. The use of oncolytic viruses is opening up a whole new world of possibilities as regards the treatment of cancer. Combinations of oncolytic viruses may be just the thing to eradicate or hold in abeyance many types of cancer. Maybe oncolytic virus cocktails will do the same thing against cancers as drug cocktails have done against AIDS.
View all Topics | View all Messages < Newer Topic | Older Topic >
Re: ONCY Short jumps 20.3% 10-May-12 04:20 pm
500,000 shares traded today on Nas and Toronto. 3,383,406 short to April 30. As an assumption assume 800,000 short shares have been covered since May 1. That still leaves a 2.5 million shares short. I believe it is likely that this rally could continue for a few days as shorts cover some more shares. Any one agree or disagree?
Oncolytics Biotech Inc. (ONCY)
At 3:59PM EDKursdatenBörse Nasdaq
Aktuell 3,96 USD
Zeit 10.05.1222:00
Diff. Vortag +9,09%
Tages-Vol. --
Gehandelte Stück 312.438
T: 3.96 0.33 (9.09%)
KursdatenBörse Toronto Stock Exchange
Aktuell 3,97 CAD
Zeit 10.05.1221:59
Diff. Vortag +9,67%
Tages-Vol. 734.747,00
Gehandelte Stück 188.676
reguau
Re: ONCY Short jumps 20.3% 10-May-12 04:20 pm
500,000 shares traded today on Nas and Toronto. 3,383,406 short to April 30. As an assumption assume 800,000 short shares have been covered since May 1. That still leaves a 2.5 million shares short. I believe it is likely that this rally could continue for a few days as shorts cover some more shares. Any one agree or disagree?
Oncolytics Biotech Inc. (ONCY)
At 3:59PM EDKursdatenBörse Nasdaq
Aktuell 3,96 USD
Zeit 10.05.1222:00
Diff. Vortag +9,09%
Tages-Vol. --
Gehandelte Stück 312.438
T: 3.96 0.33 (9.09%)
KursdatenBörse Toronto Stock Exchange
Aktuell 3,97 CAD
Zeit 10.05.1221:59
Diff. Vortag +9,67%
Tages-Vol. 734.747,00
Gehandelte Stück 188.676
reguau
Oncolytics Biotech® Inc. Enters into Sponsorship Agreement with NCIC CTG for Randomized Phase II Study in Non-Small Cell Lung Cancer
Press Release: Oncolytics Biotech Inc. – Tue, Jun 5, 2012 7:00 AM EDTShare0EmailPrintCompanies:Oncolytics Biotech Inc.Oncolytics Biotech Inc.RELATED QUOTESSymbol Price Change
ONC.TO 3.87 +0.01
--Company to Host Conference Call to Discuss Randomized Phase II Program--
CALGARY , June 5, 2012 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (ONC.TO) (ONCY) announced today that it has entered into an agreement whereby the NCIC Clinical Trials Group (CTG) at Queen's University in Kingston, Ontario, will sponsor and conduct a randomized Phase II study of REOLYSIN® in patients with advanced or metastatic non-small cell lung cancer ("NSCLC").
"This agreement expands our program with the NCIC CTG to a total of three randomized studies, including the colorectal and prostate studies we announced earlier in 2012," said Dr. Brad Thompson , President and CEO of Oncolytics. "This study builds upon both of our existing NSCLC clinical studies, as well as clinical research combining REOLYSIN® with docetaxel."
The study will be an open-label, randomized, non-blinded, Phase II clinical study of REOLYSIN. Patients with squamous cell histology will be treated with REOLYSIN given in combination with docetaxel versus docetaxel alone. Patients with non-squamous cell histology will be treated with REOLYSIN given in combination with pemetrexed versus pemetrexed alone. Approximately 150 total response evaluable patients will be enrolled, after a patient safety run in.
Conference Call Details
Dr. Brad Thompson , President and CEO of Oncolytics, will host a conference call and webcast on Tuesday, June 5th, 2012 at 7:00 a.m. MT (9:00 a.m. ET) to discuss in more depth the Company's randomized Phase II clinical trial program. To access the conference call by telephone, dial 1-647-427-7450 or 1-888-231-8191. A live audio webcast will also be available at the following link: http://www.newswire.ca/en/webcast/detail/986501/1062975 or through the Company's website at www.oncolyticsbiotech.com/presentations. Please connect at least 10 minutes prior to the webcast to ensure adequate time for any software download that may be needed. A replay of the webcast will be available at www.oncolyticsbiotech.com and will also be available by telephone through June 12th, 2012 . To access the telephone replay, dial 1-416-849-0833 or 1-855-859-2056 and enter reservation number 88541070 followed by the number sign.
About Lung Cancer
The Canadian Cancer Society estimates that 25,600 Canadians will be diagnosed with lung cancer and that 20,200 Canadians are expected to die from the disease in 2012. The American Cancer Society estimates that 226,160 new cases of lung cancer will be diagnosed in the United States and that 160,340 Americans are expected to die from the disease in 2012. Approximately 80% of all lung cancer cases are of the non-small cell type.
About the NCIC Clinical Trials Group at Queen's University
The NCIC Clinical Trials Group (NCIC CTG) is a cancer clinical trials cooperative group that conducts Phase I-III trials testing anti-cancer and supportive therapies across Canada and internationally. It is a national research program of the Canadian Cancer Society. The NCIC CTG's Central Office is located at Queen's University in Kingston, Ontario, Canada . The Group is committed to assessing all modalities of therapy for a spectrum of cancer types. More than 60 member institutions, from major cancer centres to community hospitals, enroll patients in NCIC Clinical Trials Group studies.
Press Release: Oncolytics Biotech Inc. – Tue, Jun 5, 2012 7:00 AM EDTShare0EmailPrintCompanies:Oncolytics Biotech Inc.Oncolytics Biotech Inc.RELATED QUOTESSymbol Price Change
ONC.TO 3.87 +0.01
--Company to Host Conference Call to Discuss Randomized Phase II Program--
CALGARY , June 5, 2012 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (ONC.TO) (ONCY) announced today that it has entered into an agreement whereby the NCIC Clinical Trials Group (CTG) at Queen's University in Kingston, Ontario, will sponsor and conduct a randomized Phase II study of REOLYSIN® in patients with advanced or metastatic non-small cell lung cancer ("NSCLC").
"This agreement expands our program with the NCIC CTG to a total of three randomized studies, including the colorectal and prostate studies we announced earlier in 2012," said Dr. Brad Thompson , President and CEO of Oncolytics. "This study builds upon both of our existing NSCLC clinical studies, as well as clinical research combining REOLYSIN® with docetaxel."
The study will be an open-label, randomized, non-blinded, Phase II clinical study of REOLYSIN. Patients with squamous cell histology will be treated with REOLYSIN given in combination with docetaxel versus docetaxel alone. Patients with non-squamous cell histology will be treated with REOLYSIN given in combination with pemetrexed versus pemetrexed alone. Approximately 150 total response evaluable patients will be enrolled, after a patient safety run in.
Conference Call Details
Dr. Brad Thompson , President and CEO of Oncolytics, will host a conference call and webcast on Tuesday, June 5th, 2012 at 7:00 a.m. MT (9:00 a.m. ET) to discuss in more depth the Company's randomized Phase II clinical trial program. To access the conference call by telephone, dial 1-647-427-7450 or 1-888-231-8191. A live audio webcast will also be available at the following link: http://www.newswire.ca/en/webcast/detail/986501/1062975 or through the Company's website at www.oncolyticsbiotech.com/presentations. Please connect at least 10 minutes prior to the webcast to ensure adequate time for any software download that may be needed. A replay of the webcast will be available at www.oncolyticsbiotech.com and will also be available by telephone through June 12th, 2012 . To access the telephone replay, dial 1-416-849-0833 or 1-855-859-2056 and enter reservation number 88541070 followed by the number sign.
About Lung Cancer
The Canadian Cancer Society estimates that 25,600 Canadians will be diagnosed with lung cancer and that 20,200 Canadians are expected to die from the disease in 2012. The American Cancer Society estimates that 226,160 new cases of lung cancer will be diagnosed in the United States and that 160,340 Americans are expected to die from the disease in 2012. Approximately 80% of all lung cancer cases are of the non-small cell type.
About the NCIC Clinical Trials Group at Queen's University
The NCIC Clinical Trials Group (NCIC CTG) is a cancer clinical trials cooperative group that conducts Phase I-III trials testing anti-cancer and supportive therapies across Canada and internationally. It is a national research program of the Canadian Cancer Society. The NCIC CTG's Central Office is located at Queen's University in Kingston, Ontario, Canada . The Group is committed to assessing all modalities of therapy for a spectrum of cancer types. More than 60 member institutions, from major cancer centres to community hospitals, enroll patients in NCIC Clinical Trials Group studies.
vom yahoo message board:
Analysis - pg 1 6-Jun-12 07:23 pm
We know from the two Phase II trials that Reo/Carb/Pax is is effective and safe in the treatment of second line squamous cell carcinoma of the head and neck.
What we don't know is if Reo/Carb/Pac is meaningly more effective than just Carb/Pac in the treatment of second line squamous cell carcinoma of the head and neck.
The trial is not comparing reo/carb/pac against any other treatments i.e. no erbitux etc. If reo/carb/pac is statistically more effective than carb/pac then we should have a marketable product - to paraphrase what Geneman says another treatment option to be presented to these patients.
http://finance.groups.yahoo.com/group/on...
Dr. Harrington
"... between the three of us we'vre demonstrated across a range of different tumor types that when we combine the virus with certain cytotoxic chemotherapy drugs including platins and taxanes the virus is able to kill far better than just on its own and there are a number of mechanisms that underlie that - it can influence the rate at which the virus replicates and the rate at which it kills the cancer cells. It also appears to be able to render the cancer cells more suceptible to the effects of the chemotherapy through a process that's called apoptosis so it makes them more likely to commit suicide in response to the chemotherapy... and the signal that we got from that Phase I study because there was a relatively large number of patients wih head and neck tumors - we saw that there appeared to be significant activity in that group of patients. Now that mirrored data that we had generated in my lab here at the Institute of Cancer Research demonstrating that the combination of virus with both platin and taxanes was potently active in these types of tumors. So in the phase II component of the trial we only recruited patients with tumors in the head and neck region and we saw really very impressive response rates in those patients "
Q What results do you hope to see in the Phase III trial?
"Unfortunately the group of patients we're recruiting into this study are patients who are receiving so called second line therapy with platin refractory disease have an extremely poor prognosis. The average survival for this group of patients would be somewhere between three and four months. In our phase I/II study we showed that that had been prolonged to as much as seven months albeit not in a randomized trial. So we are hoping that in the randomized Phase III study where the primary end point is overall survival that we will see a significant improvemnet in outcomes of this population of patients who are receiving palliative treatments for a disease that really carries an apalling prognosis."
http://www.youtube.com/watch?v=aMnLaE23v...
Analysis - pg 1 6-Jun-12 07:23 pm
We know from the two Phase II trials that Reo/Carb/Pax is is effective and safe in the treatment of second line squamous cell carcinoma of the head and neck.
What we don't know is if Reo/Carb/Pac is meaningly more effective than just Carb/Pac in the treatment of second line squamous cell carcinoma of the head and neck.
The trial is not comparing reo/carb/pac against any other treatments i.e. no erbitux etc. If reo/carb/pac is statistically more effective than carb/pac then we should have a marketable product - to paraphrase what Geneman says another treatment option to be presented to these patients.
http://finance.groups.yahoo.com/group/on...
Dr. Harrington
"... between the three of us we'vre demonstrated across a range of different tumor types that when we combine the virus with certain cytotoxic chemotherapy drugs including platins and taxanes the virus is able to kill far better than just on its own and there are a number of mechanisms that underlie that - it can influence the rate at which the virus replicates and the rate at which it kills the cancer cells. It also appears to be able to render the cancer cells more suceptible to the effects of the chemotherapy through a process that's called apoptosis so it makes them more likely to commit suicide in response to the chemotherapy... and the signal that we got from that Phase I study because there was a relatively large number of patients wih head and neck tumors - we saw that there appeared to be significant activity in that group of patients. Now that mirrored data that we had generated in my lab here at the Institute of Cancer Research demonstrating that the combination of virus with both platin and taxanes was potently active in these types of tumors. So in the phase II component of the trial we only recruited patients with tumors in the head and neck region and we saw really very impressive response rates in those patients "
Q What results do you hope to see in the Phase III trial?
"Unfortunately the group of patients we're recruiting into this study are patients who are receiving so called second line therapy with platin refractory disease have an extremely poor prognosis. The average survival for this group of patients would be somewhere between three and four months. In our phase I/II study we showed that that had been prolonged to as much as seven months albeit not in a randomized trial. So we are hoping that in the randomized Phase III study where the primary end point is overall survival that we will see a significant improvemnet in outcomes of this population of patients who are receiving palliative treatments for a disease that really carries an apalling prognosis."
http://www.youtube.com/watch?v=aMnLaE23v...
und Teil 2:
Re: Analysis - pg 2 6-Jun-12 07:24 pm
So we get the following analysis keeping in mind that average is different from median:
Dr. Harrington says three or four months for average survival for this group of patients Dr. Harrington says that Phase I/II study reo/pax/carb increased this average to as much as seven months
Therefore the average survival improvement is about 75% according to Harrington.
Dr. Vermorken estimates in the investor presentation a 4.5 months median survival for this group of patients on carb/pax alone
Looking at the the overall survival curve in the presentation and counting down to the 12th patient it looks like the median
survival time hits out to somewhere around 240 days or 8 months.
Therefore using Vermorkens median estimate of 4.5 on carb/pax alone and the estimate of 8 months from the survival chart we get a 77% improvement.
Therefore the Harrington average estimate increase equals the Vermorken median estimate within about 2% as best as I can figure.
What is there to be concerned about?
As far as I can see it the main concern is Vermorken's median survival estimate of 4.5 for carb/pax is low and will be greater and come closer to 8 months than he expects.
I'll go with Vermorken's estimate of 4.5 months for carb/pac alone - after all he is the world class expert and has been working with H&N patients for years.
I think the PH III improvement will approach the 75% they saw in the Phase I/II H&N patients and even if they only get 30 to 40% improvement I think that would also be statistically significant.
Re: Analysis - pg 2 6-Jun-12 07:24 pm
So we get the following analysis keeping in mind that average is different from median:
Dr. Harrington says three or four months for average survival for this group of patients Dr. Harrington says that Phase I/II study reo/pax/carb increased this average to as much as seven months
Therefore the average survival improvement is about 75% according to Harrington.
Dr. Vermorken estimates in the investor presentation a 4.5 months median survival for this group of patients on carb/pax alone
Looking at the the overall survival curve in the presentation and counting down to the 12th patient it looks like the median
survival time hits out to somewhere around 240 days or 8 months.
Therefore using Vermorkens median estimate of 4.5 on carb/pax alone and the estimate of 8 months from the survival chart we get a 77% improvement.
Therefore the Harrington average estimate increase equals the Vermorken median estimate within about 2% as best as I can figure.
What is there to be concerned about?
As far as I can see it the main concern is Vermorken's median survival estimate of 4.5 for carb/pax is low and will be greater and come closer to 8 months than he expects.
I'll go with Vermorken's estimate of 4.5 months for carb/pac alone - after all he is the world class expert and has been working with H&N patients for years.
I think the PH III improvement will approach the 75% they saw in the Phase I/II H&N patients and even if they only get 30 to 40% improvement I think that would also be statistically significant.
REO-013 related article 13-Jun-12 02:23 pm
Here’s an article (with Harrington and Melcher contributing) to go with the REO-013 results PR
http://wtaq.com/news/articles/2012/jun/1...
rjc
=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-
Cold virus hitches a ride to kill cancer: study
Wednesday, June 13, 2012 1:01 p.m. CDT
By Kate Kelland
LONDON (Reuters) - In a significant step forward for the development of a potential new cancer treatment, scientists have found how a common cold virus can kill tumors and trigger an immune response, like a vaccine, when injected into the blood stream.
Researchers from Britain's Leeds University and the Institute of Cancer Research (ICR) said by hitching a ride on blood cells, the virus was protected from antibodies in the blood stream that might otherwise neutralize its cancer-fighting abilities.
The findings suggest viral therapies like this, called reovirus, could be injected into the blood stream at routine outpatient appointments - like standard chemotherapy - making them potentially suitable for treating a range of cancers.
The study, part-funded by the charity Cancer Research UK and conducted on 10 patients with advanced bowel cancer, confirmed that reovirus attacks on two fronts - killing cancer cells directly and triggering an immune response that helps eliminate leftover cancer cells.
"Viral treatments like reovirus are showing real promise in patient trials. This study gives us the very good news that it should be possible to deliver these treatments with a simple injection into the blood stream," said Kevin Harrington from ICR, who co-led the study and published it in the journal Science Translational Medicine on Wednesday.
Harrington said if viral treatments had been found only to work when injected directly into tumors, that would have been a significant barrier to their widespread use.
"But the finding that they can hitch a ride on blood cells will potentially make them relevant to a broad range of cancers," he said.
Reovirus is being investigated by several research teams around the world - including scientists at Canada's Oncolytics Biotech Inc - because it has shown an ability to infect and kill cancer cells without affecting normal tissue.
"We also confirmed that reovirus was specifically targeting cancer cells and leaving normal cells alone, which we hope should mean fewer side-effects for patients," Harrington said.
Cancer killed 7.6 million people worldwide in 2008, the most recent year for which the World Health Organisation has full data. The number of cancer cases is expected to surge by more than 75 percent across the world by 2030.
The 10 patients in the study were all due to have surgery on tumors that had spread to their livers. During outpatient appointments in the weeks before their surgery, they were given up to five doses of the experimental reovirus treatment.
When researchers looked at pieces of tissue removed during surgery up to four weeks later, they found what they described as "viral factories" of active virus in the tumor - but not in the normal liver tissue.
This confirmed the reovirus had been delivered specifically to the cancer cells after being injected into the blood stream.
"It seems that reovirus is even cleverer than we had thought," said University of Leeds' researcher Alan Melcher.
"By piggybacking on blood cells, the virus is managing to hide from the body's natural immune response and reach its target intact. This could be hugely significant for the uptake of viral therapies like this in clinical practice."
reguau
Here’s an article (with Harrington and Melcher contributing) to go with the REO-013 results PR
http://wtaq.com/news/articles/2012/jun/1...
rjc
=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-
Cold virus hitches a ride to kill cancer: study
Wednesday, June 13, 2012 1:01 p.m. CDT
By Kate Kelland
LONDON (Reuters) - In a significant step forward for the development of a potential new cancer treatment, scientists have found how a common cold virus can kill tumors and trigger an immune response, like a vaccine, when injected into the blood stream.
Researchers from Britain's Leeds University and the Institute of Cancer Research (ICR) said by hitching a ride on blood cells, the virus was protected from antibodies in the blood stream that might otherwise neutralize its cancer-fighting abilities.
The findings suggest viral therapies like this, called reovirus, could be injected into the blood stream at routine outpatient appointments - like standard chemotherapy - making them potentially suitable for treating a range of cancers.
The study, part-funded by the charity Cancer Research UK and conducted on 10 patients with advanced bowel cancer, confirmed that reovirus attacks on two fronts - killing cancer cells directly and triggering an immune response that helps eliminate leftover cancer cells.
"Viral treatments like reovirus are showing real promise in patient trials. This study gives us the very good news that it should be possible to deliver these treatments with a simple injection into the blood stream," said Kevin Harrington from ICR, who co-led the study and published it in the journal Science Translational Medicine on Wednesday.
Harrington said if viral treatments had been found only to work when injected directly into tumors, that would have been a significant barrier to their widespread use.
"But the finding that they can hitch a ride on blood cells will potentially make them relevant to a broad range of cancers," he said.
Reovirus is being investigated by several research teams around the world - including scientists at Canada's Oncolytics Biotech Inc - because it has shown an ability to infect and kill cancer cells without affecting normal tissue.
"We also confirmed that reovirus was specifically targeting cancer cells and leaving normal cells alone, which we hope should mean fewer side-effects for patients," Harrington said.
Cancer killed 7.6 million people worldwide in 2008, the most recent year for which the World Health Organisation has full data. The number of cancer cases is expected to surge by more than 75 percent across the world by 2030.
The 10 patients in the study were all due to have surgery on tumors that had spread to their livers. During outpatient appointments in the weeks before their surgery, they were given up to five doses of the experimental reovirus treatment.
When researchers looked at pieces of tissue removed during surgery up to four weeks later, they found what they described as "viral factories" of active virus in the tumor - but not in the normal liver tissue.
This confirmed the reovirus had been delivered specifically to the cancer cells after being injected into the blood stream.
"It seems that reovirus is even cleverer than we had thought," said University of Leeds' researcher Alan Melcher.
"By piggybacking on blood cells, the virus is managing to hide from the body's natural immune response and reach its target intact. This could be hugely significant for the uptake of viral therapies like this in clinical practice."
reguau
Key quotes from Matt's interview 13-Jun-12 09:02 pm
1:32 Matt: What was maybe more significant was that we found that the virus was able to hitchhike on the immune cells and get carried to the tumor itself allowing it to avoid being neutralized by the antibodies.
1:46 Interviewer: Does it have potential for treating other diseases as well?
Matt: We're actually running a phase III that's actively enrolling in fourteen countries (in head and neck cancer). We're also running studies in lung and colorectal and pancreatic and prostrate. So we believe this product will be broadly applicable to a host of human diseases.
4:52 Interviewer: How soon do you think this reovirus treatment could be in practical clinical use widely?
Matt: We would like to file to have the agent be sold in the market in 2013.
reguau
1:32 Matt: What was maybe more significant was that we found that the virus was able to hitchhike on the immune cells and get carried to the tumor itself allowing it to avoid being neutralized by the antibodies.
1:46 Interviewer: Does it have potential for treating other diseases as well?
Matt: We're actually running a phase III that's actively enrolling in fourteen countries (in head and neck cancer). We're also running studies in lung and colorectal and pancreatic and prostrate. So we believe this product will be broadly applicable to a host of human diseases.
4:52 Interviewer: How soon do you think this reovirus treatment could be in practical clinical use widely?
Matt: We would like to file to have the agent be sold in the market in 2013.
reguau
06/20/2012 07:00:00
Oncolytics Announces Independent Data Monitoring Committee Recommends Continued Enrollment in Phase III Study of REOLYSIN® in Head and Neck Cancers Following Review of Safety Data
CALGARY, June 20, 2012 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX: ONC) NASDAQ: ONCY) announced today that the Company's independent Data Monitoring Committee (DMC) has reviewed the safety data for the first stage of its Phase III trial of REOLYSIN in combination with carboplatin and paclitaxel for the treatment of head and neck cancers (REO 018). Based on the review of the safety data the DMC has recommended that enrollment continue in the study.
"The safety data from the first stage of the Phase III study met our expectations and confirms the positive safety data from our other clinical studies using REOLYSIN," said Dr. Brad Thompson, President and CEO of Oncolytics. "We will now focus our time and attention on conducting the statistical analysis of the efficacy of the first stage's patient population, which is forthcoming."
The study design stipulates that the study will proceed to full enrollment in Stage 2 (ranging from 100 to 400 additional patients) provided that the DMC concludes that safety data in Stage 1 is acceptable for continuation to Stage 2, and an independent statistical analysis of Progression-Free Survival (PFS, a measure of efficacy) in Stage 1 predicts probability of success in Stage 2. The data collection for this analysis is currently being performed.
The safety analysis was performed on the 80 patients enrolled in Stage 1 of the study, once every patient had sufficient follow up after starting treatment on the study (six weeks). The statistical analysis will be performed once every patient has had sufficient follow up after starting treatment (12 weeks) to determine potential differences in PFS between the control and test arms of the study.
The study is a randomized, two-arm, double-blind, multi-centre, two-stage, adaptive Phase III trial assessing the intravenous administration of REOLYSIN with the chemotherapy combination of paclitaxel and carboplatin versus the chemotherapy alone in patients with metastatic or recurrent squamous cell carcinoma of the head and neck, or squamous cell cancer of the nasopharynx, who have progressed on or after prior platinum-based chemotherapy. On April 2, 2012, the Company announced that enrollment in the non-adaptive, 80-patient first stage of the trial had been completed. Enrollment is currently underway in the adaptive second stage, which is designed to enroll between 100 and 400 patients. This adaptive trial design allows data evolution to determine if the probably of reaching a statically significant endpoint has been achieved. The trial is currently being conducted at more than 80 centres in 14 countries in North America and Europe, including the United States following an agreement with the U.S. Food and Drug Administration (FDA) under the Special Protocol Assessment (SPA) process with respect to the trial's design.
Oncolytics Announces Independent Data Monitoring Committee Recommends Continued Enrollment in Phase III Study of REOLYSIN® in Head and Neck Cancers Following Review of Safety Data
CALGARY, June 20, 2012 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX: ONC) NASDAQ: ONCY) announced today that the Company's independent Data Monitoring Committee (DMC) has reviewed the safety data for the first stage of its Phase III trial of REOLYSIN in combination with carboplatin and paclitaxel for the treatment of head and neck cancers (REO 018). Based on the review of the safety data the DMC has recommended that enrollment continue in the study.
"The safety data from the first stage of the Phase III study met our expectations and confirms the positive safety data from our other clinical studies using REOLYSIN," said Dr. Brad Thompson, President and CEO of Oncolytics. "We will now focus our time and attention on conducting the statistical analysis of the efficacy of the first stage's patient population, which is forthcoming."
The study design stipulates that the study will proceed to full enrollment in Stage 2 (ranging from 100 to 400 additional patients) provided that the DMC concludes that safety data in Stage 1 is acceptable for continuation to Stage 2, and an independent statistical analysis of Progression-Free Survival (PFS, a measure of efficacy) in Stage 1 predicts probability of success in Stage 2. The data collection for this analysis is currently being performed.
The safety analysis was performed on the 80 patients enrolled in Stage 1 of the study, once every patient had sufficient follow up after starting treatment on the study (six weeks). The statistical analysis will be performed once every patient has had sufficient follow up after starting treatment (12 weeks) to determine potential differences in PFS between the control and test arms of the study.
The study is a randomized, two-arm, double-blind, multi-centre, two-stage, adaptive Phase III trial assessing the intravenous administration of REOLYSIN with the chemotherapy combination of paclitaxel and carboplatin versus the chemotherapy alone in patients with metastatic or recurrent squamous cell carcinoma of the head and neck, or squamous cell cancer of the nasopharynx, who have progressed on or after prior platinum-based chemotherapy. On April 2, 2012, the Company announced that enrollment in the non-adaptive, 80-patient first stage of the trial had been completed. Enrollment is currently underway in the adaptive second stage, which is designed to enroll between 100 and 400 patients. This adaptive trial design allows data evolution to determine if the probably of reaching a statically significant endpoint has been achieved. The trial is currently being conducted at more than 80 centres in 14 countries in North America and Europe, including the United States following an agreement with the U.S. Food and Drug Administration (FDA) under the Special Protocol Assessment (SPA) process with respect to the trial's design.
Oncolytics Biotech® Inc. Enters into Sponsorship Agreement with NCIC CTG for Randomized Phase II Study in Advanced and Metastatic Breast Cancers
Source: PR Newswire (http://s.tt/1fl9X)
CALGARY, June 21, 2012 /PRNewswire/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC) (NASDAQ: ONCY) today announced that it has entered into an agreement whereby the NCIC Clinical Trials Group (NCIC CTG) at Queen's University in Kingston, Ontario, will sponsor and conduct a randomized Phase II study of REOLYSIN® in patients with advanced or metastatic breast cancer.
"We are excited to announce our fourth randomized study with the NCIC CTG," said Dr. Brad Thompson, President and CEO of Oncolytics. "This study will build upon pre-clinical research conducted with REOLYSIN in human breast cancer primary tumour samples."
The study will be an open-label, randomized, non-blinded, Phase II clinical study of REOLYSIN given in combination with paclitaxel versus paclitaxel alone. Approximately 50 response-evaluable patients will be enrolled in each arm, after a six- to nine-patient safety run-in.
About Breast Cancer
The Canadian Cancer Society estimates that 22,900 Canadians will be diagnosed with breast cancer and 5,155 Canadians are expected to die from the disease in 2012. The American Cancer Society estimates that 229,060 Americans will be diagnosed with breast cancer and 39,920 will die from the disease in 2012.
About the NCIC Clinical Trials Group at Queen's University
The NCIC Clinical Trials Group (NCIC CTG) is a cancer clinical trials cooperative group that conducts Phase I-III trials testing anti-cancer and supportive therapies across Canada and internationally. It is a national research program of the Canadian Cancer Society. The NCIC CTG's Central Office is located at Queen's University in Kingston, Ontario, Canada.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN®, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements, including the Company's expectations related to the Phase II breast cancer trial sponsored by the NCIC CTG, and the Company's belief as to the potential of REOLYSIN as a cancer therapeutic, involve known and unknown risks and uncertainties, which could cause the Company's actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of REOLYSIN as a cancer treatment, the tolerability of REOLYSIN outside a controlled test, the success and timely completion of clinical studies and trials, the Company's ability to successfully commercialize REOLYSIN, uncertainties related to the research and development of pharmaceuticals and uncertainties related to the regulatory process. Investors should consult the Company's quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake to update these forward-looking statements, except as required by applicable laws.
Source: PR Newswire (http://s.tt/1fl9X)
Source: PR Newswire (http://s.tt/1fl9X)
CALGARY, June 21, 2012 /PRNewswire/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX:ONC) (NASDAQ: ONCY) today announced that it has entered into an agreement whereby the NCIC Clinical Trials Group (NCIC CTG) at Queen's University in Kingston, Ontario, will sponsor and conduct a randomized Phase II study of REOLYSIN® in patients with advanced or metastatic breast cancer.
"We are excited to announce our fourth randomized study with the NCIC CTG," said Dr. Brad Thompson, President and CEO of Oncolytics. "This study will build upon pre-clinical research conducted with REOLYSIN in human breast cancer primary tumour samples."
The study will be an open-label, randomized, non-blinded, Phase II clinical study of REOLYSIN given in combination with paclitaxel versus paclitaxel alone. Approximately 50 response-evaluable patients will be enrolled in each arm, after a six- to nine-patient safety run-in.
About Breast Cancer
The Canadian Cancer Society estimates that 22,900 Canadians will be diagnosed with breast cancer and 5,155 Canadians are expected to die from the disease in 2012. The American Cancer Society estimates that 229,060 Americans will be diagnosed with breast cancer and 39,920 will die from the disease in 2012.
About the NCIC Clinical Trials Group at Queen's University
The NCIC Clinical Trials Group (NCIC CTG) is a cancer clinical trials cooperative group that conducts Phase I-III trials testing anti-cancer and supportive therapies across Canada and internationally. It is a national research program of the Canadian Cancer Society. The NCIC CTG's Central Office is located at Queen's University in Kingston, Ontario, Canada.
About Oncolytics Biotech Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of human trials including a Phase III trial in head and neck cancers using REOLYSIN®, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements, including the Company's expectations related to the Phase II breast cancer trial sponsored by the NCIC CTG, and the Company's belief as to the potential of REOLYSIN as a cancer therapeutic, involve known and unknown risks and uncertainties, which could cause the Company's actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of REOLYSIN as a cancer treatment, the tolerability of REOLYSIN outside a controlled test, the success and timely completion of clinical studies and trials, the Company's ability to successfully commercialize REOLYSIN, uncertainties related to the research and development of pharmaceuticals and uncertainties related to the regulatory process. Investors should consult the Company's quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake to update these forward-looking statements, except as required by applicable laws.
Source: PR Newswire (http://s.tt/1fl9X)
Brad excited about NCIC sponsorship 21-Jun-12 07:28 pm
From 44:15 to 47:28 of the AGM webcast:
QUESTION: Can you share any more information about the breast cancer study that you alluded to, or do we have to wait until tomorrow?
BRAD: I think you have to wait for it tomorrow. I just couldn't help saying it because it's after the markets closed. I'm so excited about it. (My comment: you can hear the glee in Brad's voice). Honestly, the development of our relationship in Canada with the NCIC has been one of those amazing stories that one doesn't get to talk about very often. I mean starting at Christmas time when we had no relationship, we're now coming up on having four randomized phase II studies with, you know, 600 plus patients going to be involved with them. There are going to be very few centers in Canada (and I'm sure we'll batter down the walls of resistance on the west coast for the few centers that aren't going to be enrolling) that won't be running at least one REOLYSIN study in Canada in this year (by the middle or late this year). That's a remarkable transformation and change. It's so nice to actually be running clinical studies in our home country again in any size. I mean we are running patients in our phase III study, but it's a small number of centers. I think reovirus is going to be a very very topical discussion inside the clinical community in Canada in the coming year and the breast is just sort of the last leg on that stool. I can't tell you how thrilled we are. The enrollment rates in these studies should be very good - all of them. And I'm just thrilled to be in those indications - lung, colorectal, prostate, breast - I mean that says it all. Those are MAJOR indications.
From 44:15 to 47:28 of the AGM webcast:
QUESTION: Can you share any more information about the breast cancer study that you alluded to, or do we have to wait until tomorrow?
BRAD: I think you have to wait for it tomorrow. I just couldn't help saying it because it's after the markets closed. I'm so excited about it. (My comment: you can hear the glee in Brad's voice). Honestly, the development of our relationship in Canada with the NCIC has been one of those amazing stories that one doesn't get to talk about very often. I mean starting at Christmas time when we had no relationship, we're now coming up on having four randomized phase II studies with, you know, 600 plus patients going to be involved with them. There are going to be very few centers in Canada (and I'm sure we'll batter down the walls of resistance on the west coast for the few centers that aren't going to be enrolling) that won't be running at least one REOLYSIN study in Canada in this year (by the middle or late this year). That's a remarkable transformation and change. It's so nice to actually be running clinical studies in our home country again in any size. I mean we are running patients in our phase III study, but it's a small number of centers. I think reovirus is going to be a very very topical discussion inside the clinical community in Canada in the coming year and the breast is just sort of the last leg on that stool. I can't tell you how thrilled we are. The enrollment rates in these studies should be very good - all of them. And I'm just thrilled to be in those indications - lung, colorectal, prostate, breast - I mean that says it all. Those are MAJOR indications.
News
Follow Us
Back to News
11/08/2012 06:30:00
Oncolytics Biotech® Inc. Announces Third Quarter 2012 Results
CALGARY, Nov. 8, 2012 /CNW/ - Oncolytics Biotech Inc. (TSX: ONC) (NASDAQ: ONCY) ("Oncolytics" or the "Company") today announced its financial results and operational highlights for the quarter ended September 30, 2012.
"We continue to make progress across the entire breadth of our clinical program and completed enrollment in multiple trials this quarter," said Dr. Brad Thompson, President and CEO of Oncolytics. "Our near term focus remains on completing the initial follow-up on the expanded 167 patient group enrolled in the REO 018 study so that we can receive our first randomized clinical data for REOLYSIN®."
Selected Highlights
During the quarter the Company has made a number of significant announcements:
Management
• Appointment of Dr. Alan Tuchman to the role of Chief Medical Officer and Senior Vice President, Clinical and Medical Development.
Clinical Trial Program
• Expanded enrollment in the first stage of our Phase III head and neck cancer clinical trial (REO 18) to include 167 patients, all of whom have now been enrolled, and introduced an additional patient segregation to differentiate between patients with local recurrent disease, with or without metastases, and patients with distal metastases while maintaining the blind;
• Reaching the primary endpoint in the first stage of a U.S. Phase II clinical trial in patients with squamous cell carcinoma of the lung (SCCLC) using intravenous administration of REOLYSIN in combination with carboplatin and paclitaxel (REO 021);
• Completion of enrollment in a U.K. Phase I clinical trial using intravenously-administered REOLYSIN in combination with cyclophosphamide in patients with advanced malignancies (REO 012); and
• Completion of enrollment in a U.S. Phase I clinical trial using intravenously-administered REOLYSIN in combination with FOLFIRI in patients with colorectal cancer (REO 022).
Since September 30, 2012, the Company also announced completion of patient enrollment in a U.S. Phase II clinical trial using intravenous administration of REOLYSIN in combination with gemcitabine (Gemzar®) in patients with advanced or metastatic pancreatic cancer (REO 017).
http://www.oncolyticsbiotech.com/news_items/details?press_re…
Follow Us
Back to News
11/08/2012 06:30:00
Oncolytics Biotech® Inc. Announces Third Quarter 2012 Results
CALGARY, Nov. 8, 2012 /CNW/ - Oncolytics Biotech Inc. (TSX: ONC) (NASDAQ: ONCY) ("Oncolytics" or the "Company") today announced its financial results and operational highlights for the quarter ended September 30, 2012.
"We continue to make progress across the entire breadth of our clinical program and completed enrollment in multiple trials this quarter," said Dr. Brad Thompson, President and CEO of Oncolytics. "Our near term focus remains on completing the initial follow-up on the expanded 167 patient group enrolled in the REO 018 study so that we can receive our first randomized clinical data for REOLYSIN®."
Selected Highlights
During the quarter the Company has made a number of significant announcements:
Management
• Appointment of Dr. Alan Tuchman to the role of Chief Medical Officer and Senior Vice President, Clinical and Medical Development.
Clinical Trial Program
• Expanded enrollment in the first stage of our Phase III head and neck cancer clinical trial (REO 18) to include 167 patients, all of whom have now been enrolled, and introduced an additional patient segregation to differentiate between patients with local recurrent disease, with or without metastases, and patients with distal metastases while maintaining the blind;
• Reaching the primary endpoint in the first stage of a U.S. Phase II clinical trial in patients with squamous cell carcinoma of the lung (SCCLC) using intravenous administration of REOLYSIN in combination with carboplatin and paclitaxel (REO 021);
• Completion of enrollment in a U.K. Phase I clinical trial using intravenously-administered REOLYSIN in combination with cyclophosphamide in patients with advanced malignancies (REO 012); and
• Completion of enrollment in a U.S. Phase I clinical trial using intravenously-administered REOLYSIN in combination with FOLFIRI in patients with colorectal cancer (REO 022).
Since September 30, 2012, the Company also announced completion of patient enrollment in a U.S. Phase II clinical trial using intravenous administration of REOLYSIN in combination with gemcitabine (Gemzar®) in patients with advanced or metastatic pancreatic cancer (REO 017).
http://www.oncolyticsbiotech.com/news_items/details?press_re…
12/13/2012 06:30:00
Oncolytics Biotech Inc. Announces Positive Top Line REOLYSIN® Data for First Endpoint in Randomized Phase III Study in Head and Neck Cancers
CALGARY, Dec. 13, 2012 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics" or the "Company") (TSX: ONC) (NASDAQ: ONCY) today announced initial positive top line data from the first endpoint in its double-blinded randomized Phase III clinical study examining REOLYSIN in combination with carboplatin and paclitaxel in second-line patients with platinum-refractory, taxane-naïve head and neck cancers (REO 018).
The endpoint examines initial percentage tumour changes between the pre-treatment and first post-treatment scans (typically performed at six weeks post-first treatment) of all patients enrolled in the study. The analysis was designed to assess early differences in response between loco-regional tumours and metastatic tumours, as classified and observed by the investigators. This is the first, and to this point only, endpoint to be un-blinded for this study.
The first analysis compared the relative percentages of patients in the test and control arms with tumours that had either stabilized or exhibited shrinkage. For the purposes of this endpoint, the definition of tumour stabilization was restricted to zero percent growth only. Of the 105 total patients with evaluable metastatic tumours, 86 percent (n=50) of those in the test arm of the study exhibited tumour stabilization or shrinkage, compared with 67 percent of patients (n=55) in the control arm. This was statistically significant, with a p-value of 0.025.
The second analysis examined the magnitude of tumour response on a per patient basis using a comparison of percentage tumour shrinkage at six weeks in each patient with evaluable metastatic tumours. This analysis showed that REOLYSIN in combination with carboplatin and paclitaxel was statistically significantly better than carboplatin and paclitaxel alone at stabilizing or shrinking metastatic tumours, yielding a p-value of 0.03.
At the six week point, there is a numeric trend in favour of the test group towards differing activity between the test and control groups in patients with loco-regional tumours.
In an intragroup analysis of the test arm, an improvement in the percentage of patients' metastatic tumours over loco-regional tumours was noted (p=0.083) and an improvement of magnitude of response in metastatic tumours over loco-regional tumours was also noted (p=0.13). By contrast, in an intragroup analysis of the control arm, no statistical differences were noted between the responses of patients with evaluable metastatic tumours and patients with evaluable loco-regional tumours.
Oncolytics Biotech Inc. Announces Positive Top Line REOLYSIN® Data for First Endpoint in Randomized Phase III Study in Head and Neck Cancers
CALGARY, Dec. 13, 2012 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics" or the "Company") (TSX: ONC) (NASDAQ: ONCY) today announced initial positive top line data from the first endpoint in its double-blinded randomized Phase III clinical study examining REOLYSIN in combination with carboplatin and paclitaxel in second-line patients with platinum-refractory, taxane-naïve head and neck cancers (REO 018).
The endpoint examines initial percentage tumour changes between the pre-treatment and first post-treatment scans (typically performed at six weeks post-first treatment) of all patients enrolled in the study. The analysis was designed to assess early differences in response between loco-regional tumours and metastatic tumours, as classified and observed by the investigators. This is the first, and to this point only, endpoint to be un-blinded for this study.
The first analysis compared the relative percentages of patients in the test and control arms with tumours that had either stabilized or exhibited shrinkage. For the purposes of this endpoint, the definition of tumour stabilization was restricted to zero percent growth only. Of the 105 total patients with evaluable metastatic tumours, 86 percent (n=50) of those in the test arm of the study exhibited tumour stabilization or shrinkage, compared with 67 percent of patients (n=55) in the control arm. This was statistically significant, with a p-value of 0.025.
The second analysis examined the magnitude of tumour response on a per patient basis using a comparison of percentage tumour shrinkage at six weeks in each patient with evaluable metastatic tumours. This analysis showed that REOLYSIN in combination with carboplatin and paclitaxel was statistically significantly better than carboplatin and paclitaxel alone at stabilizing or shrinking metastatic tumours, yielding a p-value of 0.03.
At the six week point, there is a numeric trend in favour of the test group towards differing activity between the test and control groups in patients with loco-regional tumours.
In an intragroup analysis of the test arm, an improvement in the percentage of patients' metastatic tumours over loco-regional tumours was noted (p=0.083) and an improvement of magnitude of response in metastatic tumours over loco-regional tumours was also noted (p=0.13). By contrast, in an intragroup analysis of the control arm, no statistical differences were noted between the responses of patients with evaluable metastatic tumours and patients with evaluable loco-regional tumours.
3.4615 0.3615(11.66%) 10:23AM EST - Nasdaq Real Time Price
Add to Portfolio
Prev Close: 3.10
Open: 3.14
Bid: 3.40 x 1700
Ask: 3.41 x 400
1y Target Est: 8.50
Beta: 2.39
Next Earnings Date: N/A
Day's Range: 3.1310 - 3.54
52wk Range: 1.60 - 5.86
Volume: 427,853
Avg Vol (3m): 202,711
Market Cap: 261.52M
P/E (ttm): N/A
EPS (ttm): -0.54
Div & Yield: N/A (N/A
über 400K in der ersten Stunde an nasdaq gehandelt
Add to Portfolio
Prev Close: 3.10
Open: 3.14
Bid: 3.40 x 1700
Ask: 3.41 x 400
1y Target Est: 8.50
Beta: 2.39
Next Earnings Date: N/A
Day's Range: 3.1310 - 3.54
52wk Range: 1.60 - 5.86
Volume: 427,853
Avg Vol (3m): 202,711
Market Cap: 261.52M
P/E (ttm): N/A
EPS (ttm): -0.54
Div & Yield: N/A (N/A
über 400K in der ersten Stunde an nasdaq gehandelt
Na langsam scheint es vorwärts zu gehen!
nicht ohne Grund
02/08/2013 06:30:00
Oncolytics Biotech® Inc. Announces Additional Positive REOLYSIN® Clinical Trial Data from Phase 2 Study in Squamous Cell Carcinoma of the Lung
CALGARY, Feb. 8, 2013 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX: ONC) (NASDAQ: ONCY) today announced results examining percent overall tumour shrinkage data from its U.S. Phase 2 clinical trial in patients with squamous cell carcinoma of the lung (SCCLC) using intravenous administration of REOLYSIN® in combination with carboplatin and paclitaxel (REO 021).
The analysis examined percent best overall tumour changes between pre-treatment and up to six treatment cycles. Of 20 evaluable patients, 19 (95%) exhibited overall tumour shrinkage, (mean (20 patients): 33.7% shrinkage). A waterfall graph showing individual patient data will be available on the Company's website at http://www.oncolyticsbiotech.com/presentations.
"It's exciting to have 95% of patients in this study exhibit tumour shrinkage and these results further suggest that REOLYSIN may have potential use in neoadjuvant (pre-surgical) settings," said Dr. Brad Thompson , President and CEO of Oncolytics. "Based on these findings we intend to continue to look at REOLYSIN as a treatment for cancers of the lung and cancers that metastasize to the lung."
The study enrolled patients with metastatic or recurrent squamous cell carcinoma of the lung. The primary endpoint of the study is objective tumour response rates, and the secondary objectives include progression free survival and overall survival. To date, the Company has observed nine partial responses (PR), nine stable disease (SD) and three progressive disease (PD) by RECIST criteria for a disease control rate (complete response (CR) + PR + SD)) of 86%. The study continues to enroll patients.
02/08/2013 06:30:00
Oncolytics Biotech® Inc. Announces Additional Positive REOLYSIN® Clinical Trial Data from Phase 2 Study in Squamous Cell Carcinoma of the Lung
CALGARY, Feb. 8, 2013 /CNW/ - Oncolytics Biotech Inc. ("Oncolytics") (TSX: ONC) (NASDAQ: ONCY) today announced results examining percent overall tumour shrinkage data from its U.S. Phase 2 clinical trial in patients with squamous cell carcinoma of the lung (SCCLC) using intravenous administration of REOLYSIN® in combination with carboplatin and paclitaxel (REO 021).
The analysis examined percent best overall tumour changes between pre-treatment and up to six treatment cycles. Of 20 evaluable patients, 19 (95%) exhibited overall tumour shrinkage, (mean (20 patients): 33.7% shrinkage). A waterfall graph showing individual patient data will be available on the Company's website at http://www.oncolyticsbiotech.com/presentations.
"It's exciting to have 95% of patients in this study exhibit tumour shrinkage and these results further suggest that REOLYSIN may have potential use in neoadjuvant (pre-surgical) settings," said Dr. Brad Thompson , President and CEO of Oncolytics. "Based on these findings we intend to continue to look at REOLYSIN as a treatment for cancers of the lung and cancers that metastasize to the lung."
The study enrolled patients with metastatic or recurrent squamous cell carcinoma of the lung. The primary endpoint of the study is objective tumour response rates, and the secondary objectives include progression free survival and overall survival. To date, the Company has observed nine partial responses (PR), nine stable disease (SD) and three progressive disease (PD) by RECIST criteria for a disease control rate (complete response (CR) + PR + SD)) of 86%. The study continues to enroll patients.
am Freitag wurden in NY und Tornto 10,47 Mio Aktien gehandelt, mehr als 10 %
Kursdaten
Börse
Nasdaq
Aktuell
4,35 USD
Zeit
08.02.1322:00
Diff. Vortag
+21,85%
Tages-Vol.
--
Gehandelte Stück
8,89 Mio.
Kursdaten
Börse
Nasdaq
Aktuell
4,35 USD
Zeit
08.02.1322:00
Diff. Vortag
+21,85%
Tages-Vol.
--
Gehandelte Stück
8,89 Mio.
eine Zusammenfassung über Möglichkeiten und Risiken aus em OncyV2 Board:
--- In oncyV2@yahoogroups.com, "john" wrote:
>
> Hey Baba -- as Gilly's name came up you would be interested in what he posted
on yahoo about 10 days ago. One of the best summaries that I have seen. Go
Cardinals.
> John
> "My daughter presenting to an Invst club, here's how I summed it up for her.
Long-winded, sorry.
> This is a high risk, high reward name. Downside to zero in theory is much %
higher outcome than most stocks (somewhat true of research stage biotech in
general). That said, upside potential is enormous as well. The upside is
literally 100s of times the downside. That is a rare reward:risk ratio in the
investing world. Even if you think the odds are 90% towards failure, 10%
success, the Expected Return:Expected Risk profile is 10x+, extraordinarily good
investment profile IMO. My personal belief is that the odds of success are
closer to 50% here given the following pluses & minuses along with the
preliminary neoadjuvant (pre-surgical shrinkage) measures released on Dec 12th,
which were encouraging regarding hoped for metastatic results in the Ph3 trial.
> Positives: 1) Greater likelihood of Ph3 success given "control group" is
well-known Carboplatin & Paclitaxil drugs that have long track records (already
in generic forms), and the late stage nature of the patient population – Control
Group PFS & OS should be fairly predictable, and less susceptible to placebo
effect, thus less likely to provide surprisingly difficult comparison in the Ph3
study (the bane of randomized blinded studies), and greater upside upon success
than other biotechs given: 2) Metastasis market is huge indication and basically
unaddressed right now (no competition, I believe $20BN+ potential for this
indication alone), 3) Success in one indication likely means success in several
indications given the RAS pathway and kRAS angles, 4) Potential for "annuity"
like revenue streams given fact patients may need to stay on reolysin, 5)
Potential for pre-surgical treatments (an incremental revenue market), 6) Value
to the company as the foundation of a whole new platform for big Pharma (virus
biologic field, along with big IP portfolio to back Big Pharma's entry, Amgen
recently purchased OncoVex as its entry into the virus field), 6) Attractive
Pharma-like margin potential (70%+ Gross Margins) given low-cost formulation, 7)
Attractive cumulative safety profile from all studies.
>
> Negatives: 1) The viral field is new and unknown, FDA will tread particularly
slowly & carefully, 2) The company's trial sizes to date have been unusually
small, 3) While trial results have been generally encouraging so far, none of
results have been from a randomized blinded study, 4) The Ph3 is heavily
weighted towards eastern European patient population, where pre-qualifications
and predictability may be more questionable, and 5) Even without these prior
negatives, odds are generally stacked against Ph3 trials (~90% failure rates),
6) It's unclear whether other biotechs/Pharmas can circumvent the IP portfolio
and develop competitive drugs with similar viruses or variants of reolysin
formulation.
>
> My Assumptions:
> 2.5MM new metastatic disease patients diagnosed globally every year. 50% go on
reolysin. $18,000/year treatment = $22.5BN Revenue for primary use. No credit
for other indications, neoadjuvant, recurring maintenance, etc. 70% Gross
margin, 25% SG&A to sell/distribute the product. $10BN+ pretax, $6.5BN+ after
taxes, 100MM shares o/s by time company reaches profitability. $65/share
earnings per share. 15 multiple, discount back by 5 years required to build that
business up post favorable Phase3 results at a 25% higher risk equity return
requirement, $323+/share value in todays $s upon favorable results. Could easily
see a $100-$200/share buyout in a year's time by big Pharma IMO upon favorable
Ph3 results. Should unfavorable results occur, still potential for neoadjuvant
and other primary indications, and the IP portfolio likely worth something to
others pursuing viral field, but would take substantially more dilution to reach
any of these endgames. Stock likely down to ~$1s.
>
> Regarding PFS & OS. We can only speculate but we have clues…They still have
not unblinded the Ph 3 study which has Overall Survival (OS) as it's primary
endpoint goal, and Progression Free Survival (PFS) as a secondary goal, but one
which will give them a statistically significant early read on OS (and could
have palliative benefit in its own right). Rather, the company took a
preliminary check at 6 weeks that did not include peaks at PFS or OS, but
reflect endpoints you'd measure to determine whether treatment made sense prior
to surgeries (tumor size measurements looking for shrinkage or stable size,
different sizing criteria - tighter limits). Recall, they've now split the Ph 3
into two groups, local regional (more primary tumor like) and metastasized. Also
recall the September unblinded results showed PFS medians at over 2x as good in
the metastacized group (120 days) than local regional. This recent test showed
zero tumor growth at 6 weeks in the separate control groups at ~67% of patients,
with a 98% correlation to each other in the 2 control groups (statistical
equals), suggesting the control groups are on the same trajectory local
regionally and metastasized given just the chemo. The tumor-sizing curve looked
better for the Reo + chemo in the local regional group, but not significantly,
suggesting activity but not statistical significance yet (but just 6 weeks into
treatment). The curve looked better in the Reo + chemo for the metastasized
group, with 86% of patients showing no tumor growth at 6 weeks. This was
statistically significant. So, this quick read gives us two important clues to
the puzzle. 1) The company looks like it's on the right track looking at
metastatic cancers (with no competition), the likely focus of the next part of
this Phase 3, and all of their other current randomized studies are metastatic
diseases (pancreatic, lung, ovarian, prostate, I think). 2) The meaningful PFS
improvement we saw in the unblinded metastatic group in September is unlikely to
be due to better control group statistics (the bane of randomized trials),
suggesting it is likely due to reo. In which case, the reo PFS in metastacized
should be 2-3x better than control group, which would be indicative of an
extremely favorable result likely to be at least doubling expected lifespan
(OS). Expect an initial unblinding in the next 30 days or so, and also expect to
see results in a few of the other randomized studies soon (including pancreatic
which I'm particularly optimistic about). So, no true confirmations yet, but
encouraging clues regarding progress.
>
> Sentiment: Strong Buy
--- In oncyV2@yahoogroups.com, "john" wrote:
>
> Hey Baba -- as Gilly's name came up you would be interested in what he posted
on yahoo about 10 days ago. One of the best summaries that I have seen. Go
Cardinals.
> John
> "My daughter presenting to an Invst club, here's how I summed it up for her.
Long-winded, sorry.
> This is a high risk, high reward name. Downside to zero in theory is much %
higher outcome than most stocks (somewhat true of research stage biotech in
general). That said, upside potential is enormous as well. The upside is
literally 100s of times the downside. That is a rare reward:risk ratio in the
investing world. Even if you think the odds are 90% towards failure, 10%
success, the Expected Return:Expected Risk profile is 10x+, extraordinarily good
investment profile IMO. My personal belief is that the odds of success are
closer to 50% here given the following pluses & minuses along with the
preliminary neoadjuvant (pre-surgical shrinkage) measures released on Dec 12th,
which were encouraging regarding hoped for metastatic results in the Ph3 trial.
> Positives: 1) Greater likelihood of Ph3 success given "control group" is
well-known Carboplatin & Paclitaxil drugs that have long track records (already
in generic forms), and the late stage nature of the patient population – Control
Group PFS & OS should be fairly predictable, and less susceptible to placebo
effect, thus less likely to provide surprisingly difficult comparison in the Ph3
study (the bane of randomized blinded studies), and greater upside upon success
than other biotechs given: 2) Metastasis market is huge indication and basically
unaddressed right now (no competition, I believe $20BN+ potential for this
indication alone), 3) Success in one indication likely means success in several
indications given the RAS pathway and kRAS angles, 4) Potential for "annuity"
like revenue streams given fact patients may need to stay on reolysin, 5)
Potential for pre-surgical treatments (an incremental revenue market), 6) Value
to the company as the foundation of a whole new platform for big Pharma (virus
biologic field, along with big IP portfolio to back Big Pharma's entry, Amgen
recently purchased OncoVex as its entry into the virus field), 6) Attractive
Pharma-like margin potential (70%+ Gross Margins) given low-cost formulation, 7)
Attractive cumulative safety profile from all studies.
>
> Negatives: 1) The viral field is new and unknown, FDA will tread particularly
slowly & carefully, 2) The company's trial sizes to date have been unusually
small, 3) While trial results have been generally encouraging so far, none of
results have been from a randomized blinded study, 4) The Ph3 is heavily
weighted towards eastern European patient population, where pre-qualifications
and predictability may be more questionable, and 5) Even without these prior
negatives, odds are generally stacked against Ph3 trials (~90% failure rates),
6) It's unclear whether other biotechs/Pharmas can circumvent the IP portfolio
and develop competitive drugs with similar viruses or variants of reolysin
formulation.
>
> My Assumptions:
> 2.5MM new metastatic disease patients diagnosed globally every year. 50% go on
reolysin. $18,000/year treatment = $22.5BN Revenue for primary use. No credit
for other indications, neoadjuvant, recurring maintenance, etc. 70% Gross
margin, 25% SG&A to sell/distribute the product. $10BN+ pretax, $6.5BN+ after
taxes, 100MM shares o/s by time company reaches profitability. $65/share
earnings per share. 15 multiple, discount back by 5 years required to build that
business up post favorable Phase3 results at a 25% higher risk equity return
requirement, $323+/share value in todays $s upon favorable results. Could easily
see a $100-$200/share buyout in a year's time by big Pharma IMO upon favorable
Ph3 results. Should unfavorable results occur, still potential for neoadjuvant
and other primary indications, and the IP portfolio likely worth something to
others pursuing viral field, but would take substantially more dilution to reach
any of these endgames. Stock likely down to ~$1s.
>
> Regarding PFS & OS. We can only speculate but we have clues…They still have
not unblinded the Ph 3 study which has Overall Survival (OS) as it's primary
endpoint goal, and Progression Free Survival (PFS) as a secondary goal, but one
which will give them a statistically significant early read on OS (and could
have palliative benefit in its own right). Rather, the company took a
preliminary check at 6 weeks that did not include peaks at PFS or OS, but
reflect endpoints you'd measure to determine whether treatment made sense prior
to surgeries (tumor size measurements looking for shrinkage or stable size,
different sizing criteria - tighter limits). Recall, they've now split the Ph 3
into two groups, local regional (more primary tumor like) and metastasized. Also
recall the September unblinded results showed PFS medians at over 2x as good in
the metastacized group (120 days) than local regional. This recent test showed
zero tumor growth at 6 weeks in the separate control groups at ~67% of patients,
with a 98% correlation to each other in the 2 control groups (statistical
equals), suggesting the control groups are on the same trajectory local
regionally and metastasized given just the chemo. The tumor-sizing curve looked
better for the Reo + chemo in the local regional group, but not significantly,
suggesting activity but not statistical significance yet (but just 6 weeks into
treatment). The curve looked better in the Reo + chemo for the metastasized
group, with 86% of patients showing no tumor growth at 6 weeks. This was
statistically significant. So, this quick read gives us two important clues to
the puzzle. 1) The company looks like it's on the right track looking at
metastatic cancers (with no competition), the likely focus of the next part of
this Phase 3, and all of their other current randomized studies are metastatic
diseases (pancreatic, lung, ovarian, prostate, I think). 2) The meaningful PFS
improvement we saw in the unblinded metastatic group in September is unlikely to
be due to better control group statistics (the bane of randomized trials),
suggesting it is likely due to reo. In which case, the reo PFS in metastacized
should be 2-3x better than control group, which would be indicative of an
extremely favorable result likely to be at least doubling expected lifespan
(OS). Expect an initial unblinding in the next 30 days or so, and also expect to
see results in a few of the other randomized studies soon (including pancreatic
which I'm particularly optimistic about). So, no true confirmations yet, but
encouraging clues regarding progress.
>
> Sentiment: Strong Buy
eine aktuelle Zusammenfassung aus dem oncyv2 Board Teil 1
Oncolytics Biotech, Inc. (ONCY)
Product: Reolysin and 360 patents worldwide (including methods for oncolytic viral administration)
Stock Price: $3.87
Net loss for 2012 expected to be $36 million
Oncolytics Biotech, Inc., is a development stage company that is in the process of developing Reolysin, a virus formulation that can be used in the treatment of cancer. It is undergoing 19 clinical trials, including a Phase III clinical trial. This is a high-risk stock, essentially worth nothing if the product does not achieve FDA approval. The company has 76.69 M shares; equity has been its main route of financing.
This stock is market-indifferent with a high risk, but a great reward potential. The potential for this stock could be upwards of $100/share. If it is approved for one strain of cancer, it will be prescribed off-label for other types of cancers as well. Although this market is competitive, Reolysin works in conjunction with a common therapy, chemotherapy. Oncolytics is currently undergoing a Phase II clinical trial with Pancreatic cancer and a Phase III clinical trial involving metastatic cancer. Since there are no effective treatments for either of these diseases, success in either of these trials could fast-track Reolysin for FDA approval, or approval in other countries (Europe is usually quicker to approve new products to market). Oncolytics would also have no competition in these markets, as there are no other effective treatments. If it is approved to go to market in the next year, Oncolytics has already partnered with Sigma-Aldrich Co. to manufacture the product and seems to be ready for commercialization.
In the following report I discuss the Stock Potential, The Product: Reolysin, The Key Clinical Trials, other viruses being developed and their impact on Oncolytics, Failed viruses, and a Conclusion including some key points about the company and why it is worth buying.
Stock Potential
For Reolysin being approved for metastatic cancer alone, the potential is enormous. The following assumptions have been made:
2.5 MM new metastatic disease patients diagnosed in the developed world each year
50% of those patients go on Reolysin= 1.25 MM patients
$18,000/year price for treatment
70% Gross Margin (this is relatively cheap to manufacture)
25% SG&A expense
100MM shares by the time the company reaches profitability
Based on these assumptions, pretax income would be over $10BN and Net Income over $6.5 BN.
With 100 MM shares, the EPS would be $65/share and assuming a 15 P/E multiple, discounted back 5 years required to build the business up after favorable Phase III results at a 25% higher risk equity return requirement, this could have the potential to be a $320+/share current value stock. However, I expect that upon favorable Phase III results, there will more likely be a $100-$200/share buyout by big Pharma.
The Product: Reolysin, a Reovirus
The reovirus is a Baltimore Class III virus. This means it is a double-stranded (DS) RNA virus. Double-stranded RNA viruses do not enter the nucleus and do all of their replicating in the cytoplasm. The Reolysin strand isolated by Oncolytics Biotech, Inc., has not been engineered or modified at all. It is in its original wild-type form and can be found in nature.
Reoviruses are found in nature and it is estimated that 50% of our population has contracted it by the age of 12, and approximately 90% of the world's population has been infected with (and, therefore, built up antibodies for) the reovirus. It also exhibits no symptoms and is not known to cause disease, especially since humans have become so resistant to its effects. It is also widespread in nature and extremely common to be found in water sources and the bowels of humans.
The reovirus is built with its own DS RNA, which is contained in a capsid along with some enzymes that are necessary to its reproduction. The virus binds to cell receptor proteins and enters the body, where it is transported to the lysosome. Instead of the lysosome destroying the virus, it dissolves the outer shell and frees the inner protein shell and its genetic materials into the cytoplasm. This dissolving of the outer shell signals for the virus to start replicating in the cytoplasm. It synthesizes its own mRNA from one of its strands and its own enzymes. When enough protein is made, the genetic material and protein is encapsulated and the cell undergoes lysis. The cell being destroyed releases the virus back into the body, which is how it spreads throughout the body.
The ras gene is controller gene that turns the ras pathways on or off. This pathways are a series of reactions in the cell that result in the cell receiving a signal to grow and divide. It contains a series of reactions, one of the reactions turns off the cell defense for reovirus, so the cell does not recognize the reovirus for destruction when it enters the cell. In cancer, often the ras gene has a mutation and it creates uncontrolled cell division (permanently turns on the pathway). The cells do not have enough time to copy all of the cell materials and this insufficient growth leads to abnormal cells (cancer cells), this is one of the most common causes of cancer.
The reovirus naturally is tumor selective, as it destroys only cancer cells with activated ras pathways. While the immune system's response would normally destroy the reovirus, it is deactivated when the ras pathway is activated, in cancer cells. The reovirus, therefore, replicates in and destroys cancer cell, while in normal cells the body's natural immune response kills the virus quickly leaving no effect on those cells. This is what makes this virus so valuable in cancer treatment. It kills cancer cells naturally and has little or no effect on healthy cells.
This virus is already existent in nature with mild symptoms (at most). Whether or not it is used to treat cancer, it will keep replicating and mutating. It will continue to infect people, as it has for centuries, and people will continue to build up antibodies against different strains. So its use as a cancer treatment does not expose it to any additional risk to the possibility of mutation than it has in nature. It cannot change the DNA of humans because it does not enter the nucleus or use human genetic material to replicate. It has also performed well in regards to safety in all clinical trials. There have been no adverse events during any of the trials.
Clark, Madigan, Martinko, and Stahl. Biology of Microorganisms. 13th ed. San Francisco: Pearson Education, 2012. Print.
Key Clinical Trials
Oncolytics is currently undergoing 19 active clinical trials. The two key ones that I believe will mean the most are the Phase III Head and Neck/Metastatic Cancer trial and the Phase II Pancreatic Cancer trial. The Phase III trial is important because it is the last stage of FDA testing. However, the metastatic and Pancreatic cancer trials are important because there are currently no effective treatments for either of these cancers. If either of these passes their trials, Reolysin could be fast-tracked toward approval. Another fact to note is that on the rare occasion that these clinical trials yielded full recoveries, the patients who stopped taking Reolysin had their cancer come back. This is an important fact to note because it signifies that people who take this virus, would have to take it for their entire lives.
Head and Neck cancer type: Platinum-refractory recurrent and/or metastatic squamous cell cancers
Treatment: Paclitaxel (175 mg/m2) and Carboplatin (AUC 5) every three weeks in combination with the reovirus
Phase II results:
13 patients evaluable for response, four had PRs, for an objective response rate of 31%. Six patients had SD or better for 12 weeks or longer for a disease control rate (SD or better) of 46% compared to 3%-13% historical rates in chemotherapy alone.
Phase III trial:
The Phase III trial is randomizied, two-arm (meaning there is a test and a control group), double blind, multi-center (different places trained to administer virus and perform study), two stage adaptive trial. This trial was designed with an initial phase and an adaptive second phase. The initial stage had 80 patients enrolled and had a requirement to pass an independent safety evaluation after 6 weeks as well as an independent statistical analysis of the probability of success after 12 weeks. The second stage was designed to have a larger number of patients enrolled.
This first stage was analyzed while still blinded and unexpected results were discovered, however, so the second-stage did not continue as planned. A protocol amendment was submitted to the FDA to change the trial after it was noted (while the trial was still blinded) that the patients with metastatic disease had a statistically significant greater progression free survival rates than the patients with local regional head and neck cancers. This signified two separate test groups to be analyzed and the trial was redesigned.
The new study splits the patients into two categories: local recurrent disease and metastasis. Also it increases the number of enrolled patients to 160 in order to achieve statistical significance when separating the groups. While still blinded, these groups will be analyzed both together and separately to determine if further clinical trials will be necessary to determine effectiveness of Reolysin for either groups. This replaced the second stage of trials, though a further support trial may be required to strengthen statistical significance.
In December, an unblinding occurred pertaining only to the secondary endpoint of determining if Reolysin adds tumor-specific differential activity between metastatic and loco-regional cancers. The partial unblinding did not measure the primary endpoints of Progression-Free Survival (PFS) or Overall Survival (OS), all parties remain blinded to this data so as to not invalidate any of the results of these two primary goals when the study is complete. It measured pre-treatment and post-treatment percentage changes from the patients in the study (after 6 weeks of treatment). The data from the unblinding was supportive of the decision to separate the groups in order to highlight the metastatic results. Of all the people in the metastatic test group (with reo), 86% experienced stabilization (no growth at all) or tumor shrinkage, while 71% of those in the loco regional test group experienced this result. The RECIST criteria for tumor stabilization remains blinded and will be measured at the end of the study for both of these groups; however these initial measurements demonstrate promise in the new study particularly with metastatic cancer, which will likely be the focus the company takes in its route to approval.
Also, this unblinding revealed positive results in the effectiveness of Reolysin as a cancer treatment relative to the control groups. It was observed in the test group that 86% of the 50 patients had exhibited tumor stabilization (0% growth) or shrinkage, while only 67% of the control group (chemo only) experienced it. At a p-value of .025, this is a statistically significant result. This shows promising evidence for the success of the Phase III trial that is currently in progress. However, the criteria for RECIST was not unblinded, so it can not be known if this data will translate to positive PFS and OS rates (primary study goals) for Reolysin at the end of the study.
Figure 1: Results of a 3rd party study on Reolysin on different strains of Head and Neck cancers in the lab. It was done on the cells in lab, it is not a clinical study.
This new Phase III trial looks promising for Oncolytics. Changing the trial was a good move for getting FDA approval due to the potential it has for metastatic cancer. More than 2.6 million people die of metastatic cancer every year, this is a large amount of people who would need this treatment. Because there is no cure for metastatic cancer, nor very effective treatment options, this would make Reolysin one of the only effective treatments for this type of cancer.
However promising the virus looks in the lab though, problems may arise in clinical trials with the actual effectiveness of the virus. Because our immune system's response to this virus is already so strong, it is possible that the immune system will seek out and destroy this virus before it can infect the cancer cells. This problem, however, has been addressed by the use of chemotherapy as a way to suppress the body's immune system, which had not been done in previous oncolytic virus trials done by other companies. Reolysin has also been effective in the earlier clinical trials, and the unblinding of the secondary goal...
Single page viewpage 1 / 2| Next »
Oncolytics Biotech, Inc. (ONCY)
Product: Reolysin and 360 patents worldwide (including methods for oncolytic viral administration)
Stock Price: $3.87
Net loss for 2012 expected to be $36 million
Oncolytics Biotech, Inc., is a development stage company that is in the process of developing Reolysin, a virus formulation that can be used in the treatment of cancer. It is undergoing 19 clinical trials, including a Phase III clinical trial. This is a high-risk stock, essentially worth nothing if the product does not achieve FDA approval. The company has 76.69 M shares; equity has been its main route of financing.
This stock is market-indifferent with a high risk, but a great reward potential. The potential for this stock could be upwards of $100/share. If it is approved for one strain of cancer, it will be prescribed off-label for other types of cancers as well. Although this market is competitive, Reolysin works in conjunction with a common therapy, chemotherapy. Oncolytics is currently undergoing a Phase II clinical trial with Pancreatic cancer and a Phase III clinical trial involving metastatic cancer. Since there are no effective treatments for either of these diseases, success in either of these trials could fast-track Reolysin for FDA approval, or approval in other countries (Europe is usually quicker to approve new products to market). Oncolytics would also have no competition in these markets, as there are no other effective treatments. If it is approved to go to market in the next year, Oncolytics has already partnered with Sigma-Aldrich Co. to manufacture the product and seems to be ready for commercialization.
In the following report I discuss the Stock Potential, The Product: Reolysin, The Key Clinical Trials, other viruses being developed and their impact on Oncolytics, Failed viruses, and a Conclusion including some key points about the company and why it is worth buying.
Stock Potential
For Reolysin being approved for metastatic cancer alone, the potential is enormous. The following assumptions have been made:
2.5 MM new metastatic disease patients diagnosed in the developed world each year
50% of those patients go on Reolysin= 1.25 MM patients
$18,000/year price for treatment
70% Gross Margin (this is relatively cheap to manufacture)
25% SG&A expense
100MM shares by the time the company reaches profitability
Based on these assumptions, pretax income would be over $10BN and Net Income over $6.5 BN.
With 100 MM shares, the EPS would be $65/share and assuming a 15 P/E multiple, discounted back 5 years required to build the business up after favorable Phase III results at a 25% higher risk equity return requirement, this could have the potential to be a $320+/share current value stock. However, I expect that upon favorable Phase III results, there will more likely be a $100-$200/share buyout by big Pharma.
The Product: Reolysin, a Reovirus
The reovirus is a Baltimore Class III virus. This means it is a double-stranded (DS) RNA virus. Double-stranded RNA viruses do not enter the nucleus and do all of their replicating in the cytoplasm. The Reolysin strand isolated by Oncolytics Biotech, Inc., has not been engineered or modified at all. It is in its original wild-type form and can be found in nature.
Reoviruses are found in nature and it is estimated that 50% of our population has contracted it by the age of 12, and approximately 90% of the world's population has been infected with (and, therefore, built up antibodies for) the reovirus. It also exhibits no symptoms and is not known to cause disease, especially since humans have become so resistant to its effects. It is also widespread in nature and extremely common to be found in water sources and the bowels of humans.
The reovirus is built with its own DS RNA, which is contained in a capsid along with some enzymes that are necessary to its reproduction. The virus binds to cell receptor proteins and enters the body, where it is transported to the lysosome. Instead of the lysosome destroying the virus, it dissolves the outer shell and frees the inner protein shell and its genetic materials into the cytoplasm. This dissolving of the outer shell signals for the virus to start replicating in the cytoplasm. It synthesizes its own mRNA from one of its strands and its own enzymes. When enough protein is made, the genetic material and protein is encapsulated and the cell undergoes lysis. The cell being destroyed releases the virus back into the body, which is how it spreads throughout the body.
The ras gene is controller gene that turns the ras pathways on or off. This pathways are a series of reactions in the cell that result in the cell receiving a signal to grow and divide. It contains a series of reactions, one of the reactions turns off the cell defense for reovirus, so the cell does not recognize the reovirus for destruction when it enters the cell. In cancer, often the ras gene has a mutation and it creates uncontrolled cell division (permanently turns on the pathway). The cells do not have enough time to copy all of the cell materials and this insufficient growth leads to abnormal cells (cancer cells), this is one of the most common causes of cancer.
The reovirus naturally is tumor selective, as it destroys only cancer cells with activated ras pathways. While the immune system's response would normally destroy the reovirus, it is deactivated when the ras pathway is activated, in cancer cells. The reovirus, therefore, replicates in and destroys cancer cell, while in normal cells the body's natural immune response kills the virus quickly leaving no effect on those cells. This is what makes this virus so valuable in cancer treatment. It kills cancer cells naturally and has little or no effect on healthy cells.
This virus is already existent in nature with mild symptoms (at most). Whether or not it is used to treat cancer, it will keep replicating and mutating. It will continue to infect people, as it has for centuries, and people will continue to build up antibodies against different strains. So its use as a cancer treatment does not expose it to any additional risk to the possibility of mutation than it has in nature. It cannot change the DNA of humans because it does not enter the nucleus or use human genetic material to replicate. It has also performed well in regards to safety in all clinical trials. There have been no adverse events during any of the trials.
Clark, Madigan, Martinko, and Stahl. Biology of Microorganisms. 13th ed. San Francisco: Pearson Education, 2012. Print.
Key Clinical Trials
Oncolytics is currently undergoing 19 active clinical trials. The two key ones that I believe will mean the most are the Phase III Head and Neck/Metastatic Cancer trial and the Phase II Pancreatic Cancer trial. The Phase III trial is important because it is the last stage of FDA testing. However, the metastatic and Pancreatic cancer trials are important because there are currently no effective treatments for either of these cancers. If either of these passes their trials, Reolysin could be fast-tracked toward approval. Another fact to note is that on the rare occasion that these clinical trials yielded full recoveries, the patients who stopped taking Reolysin had their cancer come back. This is an important fact to note because it signifies that people who take this virus, would have to take it for their entire lives.
Head and Neck cancer type: Platinum-refractory recurrent and/or metastatic squamous cell cancers
Treatment: Paclitaxel (175 mg/m2) and Carboplatin (AUC 5) every three weeks in combination with the reovirus
Phase II results:
13 patients evaluable for response, four had PRs, for an objective response rate of 31%. Six patients had SD or better for 12 weeks or longer for a disease control rate (SD or better) of 46% compared to 3%-13% historical rates in chemotherapy alone.
Phase III trial:
The Phase III trial is randomizied, two-arm (meaning there is a test and a control group), double blind, multi-center (different places trained to administer virus and perform study), two stage adaptive trial. This trial was designed with an initial phase and an adaptive second phase. The initial stage had 80 patients enrolled and had a requirement to pass an independent safety evaluation after 6 weeks as well as an independent statistical analysis of the probability of success after 12 weeks. The second stage was designed to have a larger number of patients enrolled.
This first stage was analyzed while still blinded and unexpected results were discovered, however, so the second-stage did not continue as planned. A protocol amendment was submitted to the FDA to change the trial after it was noted (while the trial was still blinded) that the patients with metastatic disease had a statistically significant greater progression free survival rates than the patients with local regional head and neck cancers. This signified two separate test groups to be analyzed and the trial was redesigned.
The new study splits the patients into two categories: local recurrent disease and metastasis. Also it increases the number of enrolled patients to 160 in order to achieve statistical significance when separating the groups. While still blinded, these groups will be analyzed both together and separately to determine if further clinical trials will be necessary to determine effectiveness of Reolysin for either groups. This replaced the second stage of trials, though a further support trial may be required to strengthen statistical significance.
In December, an unblinding occurred pertaining only to the secondary endpoint of determining if Reolysin adds tumor-specific differential activity between metastatic and loco-regional cancers. The partial unblinding did not measure the primary endpoints of Progression-Free Survival (PFS) or Overall Survival (OS), all parties remain blinded to this data so as to not invalidate any of the results of these two primary goals when the study is complete. It measured pre-treatment and post-treatment percentage changes from the patients in the study (after 6 weeks of treatment). The data from the unblinding was supportive of the decision to separate the groups in order to highlight the metastatic results. Of all the people in the metastatic test group (with reo), 86% experienced stabilization (no growth at all) or tumor shrinkage, while 71% of those in the loco regional test group experienced this result. The RECIST criteria for tumor stabilization remains blinded and will be measured at the end of the study for both of these groups; however these initial measurements demonstrate promise in the new study particularly with metastatic cancer, which will likely be the focus the company takes in its route to approval.
Also, this unblinding revealed positive results in the effectiveness of Reolysin as a cancer treatment relative to the control groups. It was observed in the test group that 86% of the 50 patients had exhibited tumor stabilization (0% growth) or shrinkage, while only 67% of the control group (chemo only) experienced it. At a p-value of .025, this is a statistically significant result. This shows promising evidence for the success of the Phase III trial that is currently in progress. However, the criteria for RECIST was not unblinded, so it can not be known if this data will translate to positive PFS and OS rates (primary study goals) for Reolysin at the end of the study.
Figure 1: Results of a 3rd party study on Reolysin on different strains of Head and Neck cancers in the lab. It was done on the cells in lab, it is not a clinical study.
This new Phase III trial looks promising for Oncolytics. Changing the trial was a good move for getting FDA approval due to the potential it has for metastatic cancer. More than 2.6 million people die of metastatic cancer every year, this is a large amount of people who would need this treatment. Because there is no cure for metastatic cancer, nor very effective treatment options, this would make Reolysin one of the only effective treatments for this type of cancer.
However promising the virus looks in the lab though, problems may arise in clinical trials with the actual effectiveness of the virus. Because our immune system's response to this virus is already so strong, it is possible that the immune system will seek out and destroy this virus before it can infect the cancer cells. This problem, however, has been addressed by the use of chemotherapy as a way to suppress the body's immune system, which had not been done in previous oncolytic virus trials done by other companies. Reolysin has also been effective in the earlier clinical trials, and the unblinding of the secondary goal...
Single page viewpage 1 / 2| Next »
Antwort auf Beitrag Nr.: 44.123.019 von twisterfriend am 09.02.13 19:14:29
Zitat von twisterfriend: Na langsam scheint es vorwärts zu gehen!
Antwort auf Beitrag Nr.: 48.192.136 von gerdass am 31.10.14 22:25:15Es gibt bisher noch nichts Neues zu vermelden. Die Aktie ist von unter 0,50USD auf 1 USD zurück gekommen. Die Umsätze haben seit Mittwoch letzter Woche stark zugenommen, Freitag wurden an der Nasdaq und in Toronto mehr als 6 Mio Aktien (von 88 Mio) gehandelt, der Kurs hat dabei mehr als 20 % zugelegt.
Ein Börsenblatt (Sierra World Equity Review) hat gestern gemutmaßt, Merck und Oncolytics würden Mitte Dezember ein Joint Venture verkünden.
M.E. erklärt das den steilen Anstieg nicht.
Vielleicht kommt diese Woche etwas, es stehen genug Phase II Trials aus.
Ein Börsenblatt (Sierra World Equity Review) hat gestern gemutmaßt, Merck und Oncolytics würden Mitte Dezember ein Joint Venture verkünden.
M.E. erklärt das den steilen Anstieg nicht.
Vielleicht kommt diese Woche etwas, es stehen genug Phase II Trials aus.
Antwort auf Beitrag Nr.: 48.202.363 von reguau am 03.11.14 11:02:12Und heute wieder 13 cent runter
und heute?
Antwort auf Beitrag Nr.: 48.202.363 von reguau am 03.11.14 11:02:12
Pipeline
Zitat von reguau: Es gibt bisher noch nichts Neues zu vermelden. Die Aktie ist von unter 0,50USD auf 1 USD zurück gekommen. Die Umsätze haben seit Mittwoch letzter Woche stark zugenommen, Freitag wurden an der Nasdaq und in Toronto mehr als 6 Mio Aktien (von 88 Mio) gehandelt, der Kurs hat dabei mehr als 20 % zugelegt.
Ein Börsenblatt (Sierra World Equity Review) hat gestern gemutmaßt, Merck und Oncolytics würden Mitte Dezember ein Joint Venture verkünden.
M.E. erklärt das den steilen Anstieg nicht.
Vielleicht kommt diese Woche etwas, es stehen genug Phase II Trials aus.
Pipeline

Pipeline





$0.7* 0.0812.9%

mal die nächsten Tage im Auge behalten das Teil
et voilá:
heute News:
Oncolytics Biotech® Inc. Announces Receipt of Orphan Drug Designation from the U.S. FDA for Cancer of the Fallopian Tube
Date : 03/02/2015 @ 6:30AM
Source : PR Newswire (US)
Stock : Oncolytics Biotech, Inc. (MM) (ONCY)
Quote : 0.7285 0.0 (0.00%) @ 6:52AM
CALGARY, March 2, 2015 /PRNewswire/ - Oncolytics Biotech® Inc. ("Oncolytics") (TSX.ONC, NASDAQ.ONCY), a clinical-stage biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation for its lead product candidate, REOLYSIN®, for the treatment of cancer of the fallopian tube. The designation was granted on the basis of the Company's December 2014 application for an Orphan Drug Designation encompassing ovarian, fallopian tube and primary peritoneal cancers which are generally treated as one indication. On February 11, 2015, the Company announced that it had received Orphan Drug Designation for ovarian cancer.
"The FDA's recognition of ovarian and fallopian tube cancers as distinctly separate indications paves the way for a more targeted approach to the treatment of gynecological cancers," said Dr. Brad Thompson, President and CEO of Oncolytics. "We are pleased to have secured our third Orphan Drug Designation in the United States and look forward to continuing our development and commercialization program for REOLYSIN®."
Oncolytics has supported two sponsored clinical studies assessing REOLYSIN® in the treatment of cancers of the fallopian tube. The first was a Phase 1/2 clinical trial (OSU-07022) for patients with metastatic ovarian, peritoneal and fallopian tube cancers using concurrent intravenous and intraperitoneal administration of REOLYSIN® that provided evidence of viral targeting and replication in peritoneal and ovarian cancer cells. The second is an ongoing randomized Phase II trial (GOG186H) of weekly paclitaxel versus weekly paclitaxel with REOLYSIN® in patients with persistent or recurrent ovarian, fallopian tube or primary peritoneal cancer. The second trial completed enrollment in September 2014.
The FDA grants Orphan Drug Designation status to products that treat rare diseases, providing incentives to sponsors developing drugs or biologics. The FDA defines rare diseases as those affecting fewer than 200,000 people in the United States at any given time. Orphan Drug Designation provides the sponsor certain benefits and incentives, including a period of marketing exclusivity if regulatory approval is ultimately received for the designated indication, potential tax credits for certain activities, eligibility for orphan drug grants, and the waiver of certain administrative fees. The receipt of Orphan Drug Designation status does not change the regulatory requirements or process for obtaining marketing approval. For more information, please visit: http://www.fda.gov/forindustry/DevelopingProductsforrareDise…
About Fallopian Tube Cancer
The incidence rate of fallopian tube cancers is estimated to be 0.37 per 100,000 women. Approximately 15,750 patients are affected with fallopian tube cancer at any time in the United States. The median survival of women in the U.S. with fallopian tube cancers is 58 months, or just under five years.
About Oncolytics Biotech® Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of later-stage, randomized human trials in various indications using REOLYSIN®, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
This press release contains forward-looking statements within the meaning of the U.S. Securities Act of 1933, as amended, and U.S. Securities Exchange Act of 1934, as amended, and forward-looking information within the meaning of Canadian securities laws. Statements, other than statements of historical facts, included in this press release that address activities, events or developments that Oncolytics expects or anticipates will or may occur in the future, including such things as, the Company's expectations related to the granting of Orphan Drug Designation for REOLYSIN®, the Company's belief as to the potential of REOLYSIN® as a cancer therapeutic, and other such matters are forward-looking statements and forward-looking information and involve known and unknown risks and uncertainties, which could cause the Company's actual results to differ materially from those in the forward-looking statements and forward-looking information. Such risks and uncertainties include, among others, risks related to the statistical sufficiency of patient enrollment numbers in separate patient groups, the availability of funds and resources to pursue research and development projects, the efficacy of REOLYSIN® as a cancer treatment, the tolerability of REOLYSIN® outside a controlled test, the success and timely completion of clinical studies and trials, the Company's ability to successfully commercialize REOLYSIN®, uncertainties related to the research and development of pharmaceuticals and uncertainties related to the regulatory process. Investors should consult the Company's quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements and forward-looking information. Investors are cautioned against placing undue reliance on forward-looking statements and forward-looking information. The Company does not undertake to update these forward-looking statements and forward-looking information, except as required by applicable laws.
SOURCE Oncolytics Biotech Inc.
Copyright 2015 PR Newswire
http://ih.advfn.com/p.php?pid=nmona&article=65679751
heute News:
Oncolytics Biotech® Inc. Announces Receipt of Orphan Drug Designation from the U.S. FDA for Cancer of the Fallopian Tube
Date : 03/02/2015 @ 6:30AM
Source : PR Newswire (US)
Stock : Oncolytics Biotech, Inc. (MM) (ONCY)
Quote : 0.7285 0.0 (0.00%) @ 6:52AM
CALGARY, March 2, 2015 /PRNewswire/ - Oncolytics Biotech® Inc. ("Oncolytics") (TSX.ONC, NASDAQ.ONCY), a clinical-stage biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation for its lead product candidate, REOLYSIN®, for the treatment of cancer of the fallopian tube. The designation was granted on the basis of the Company's December 2014 application for an Orphan Drug Designation encompassing ovarian, fallopian tube and primary peritoneal cancers which are generally treated as one indication. On February 11, 2015, the Company announced that it had received Orphan Drug Designation for ovarian cancer.
"The FDA's recognition of ovarian and fallopian tube cancers as distinctly separate indications paves the way for a more targeted approach to the treatment of gynecological cancers," said Dr. Brad Thompson, President and CEO of Oncolytics. "We are pleased to have secured our third Orphan Drug Designation in the United States and look forward to continuing our development and commercialization program for REOLYSIN®."
Oncolytics has supported two sponsored clinical studies assessing REOLYSIN® in the treatment of cancers of the fallopian tube. The first was a Phase 1/2 clinical trial (OSU-07022) for patients with metastatic ovarian, peritoneal and fallopian tube cancers using concurrent intravenous and intraperitoneal administration of REOLYSIN® that provided evidence of viral targeting and replication in peritoneal and ovarian cancer cells. The second is an ongoing randomized Phase II trial (GOG186H) of weekly paclitaxel versus weekly paclitaxel with REOLYSIN® in patients with persistent or recurrent ovarian, fallopian tube or primary peritoneal cancer. The second trial completed enrollment in September 2014.
The FDA grants Orphan Drug Designation status to products that treat rare diseases, providing incentives to sponsors developing drugs or biologics. The FDA defines rare diseases as those affecting fewer than 200,000 people in the United States at any given time. Orphan Drug Designation provides the sponsor certain benefits and incentives, including a period of marketing exclusivity if regulatory approval is ultimately received for the designated indication, potential tax credits for certain activities, eligibility for orphan drug grants, and the waiver of certain administrative fees. The receipt of Orphan Drug Designation status does not change the regulatory requirements or process for obtaining marketing approval. For more information, please visit: http://www.fda.gov/forindustry/DevelopingProductsforrareDise…
About Fallopian Tube Cancer
The incidence rate of fallopian tube cancers is estimated to be 0.37 per 100,000 women. Approximately 15,750 patients are affected with fallopian tube cancer at any time in the United States. The median survival of women in the U.S. with fallopian tube cancers is 58 months, or just under five years.
About Oncolytics Biotech® Inc.
Oncolytics is a Calgary-based biotechnology company focused on the development of oncolytic viruses as potential cancer therapeutics. Oncolytics' clinical program includes a variety of later-stage, randomized human trials in various indications using REOLYSIN®, its proprietary formulation of the human reovirus. For further information about Oncolytics, please visit: www.oncolyticsbiotech.com.
This press release contains forward-looking statements within the meaning of the U.S. Securities Act of 1933, as amended, and U.S. Securities Exchange Act of 1934, as amended, and forward-looking information within the meaning of Canadian securities laws. Statements, other than statements of historical facts, included in this press release that address activities, events or developments that Oncolytics expects or anticipates will or may occur in the future, including such things as, the Company's expectations related to the granting of Orphan Drug Designation for REOLYSIN®, the Company's belief as to the potential of REOLYSIN® as a cancer therapeutic, and other such matters are forward-looking statements and forward-looking information and involve known and unknown risks and uncertainties, which could cause the Company's actual results to differ materially from those in the forward-looking statements and forward-looking information. Such risks and uncertainties include, among others, risks related to the statistical sufficiency of patient enrollment numbers in separate patient groups, the availability of funds and resources to pursue research and development projects, the efficacy of REOLYSIN® as a cancer treatment, the tolerability of REOLYSIN® outside a controlled test, the success and timely completion of clinical studies and trials, the Company's ability to successfully commercialize REOLYSIN®, uncertainties related to the research and development of pharmaceuticals and uncertainties related to the regulatory process. Investors should consult the Company's quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements and forward-looking information. Investors are cautioned against placing undue reliance on forward-looking statements and forward-looking information. The Company does not undertake to update these forward-looking statements and forward-looking information, except as required by applicable laws.
SOURCE Oncolytics Biotech Inc.
Copyright 2015 PR Newswire
http://ih.advfn.com/p.php?pid=nmona&article=65679751
Und noch dazu eine schöne W-Formation im Chart:
3 Jahre

1 Jahr

10 Tage
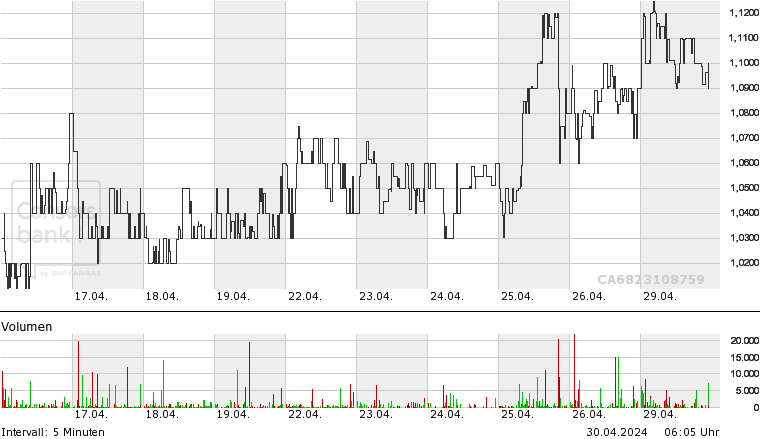

3 Jahre

1 Jahr

10 Tage

Anfang 2013, also vor zwei Jahren, stand Oncolytics noch über 4,50$.
Die Frage ist, wohin und wie schnell sich ONCY in diesen Bereicht zurückfindet.
In der momentanen Biotech-Hausse ist ONCY nach mehreren FDA-Gehnehmigungen in den letzten Wochen im Bereich Krebs auf jeden Fall einer der interessantesten Biotech-Werte, wie ich finde.
Die Marktkapitalisierung ist auch nicht sehr hoch.
Die Frage ist, wohin und wie schnell sich ONCY in diesen Bereicht zurückfindet.
In der momentanen Biotech-Hausse ist ONCY nach mehreren FDA-Gehnehmigungen in den letzten Wochen im Bereich Krebs auf jeden Fall einer der interessantesten Biotech-Werte, wie ich finde.
Die Marktkapitalisierung ist auch nicht sehr hoch.
Vorbörslich geht es jedenfalls schon um fast 20% hoch, bei hohem Volumen:
Oncolytics Biotech, Inc. (ONCY) Vorbörslich Trading - NASDAQ.com
*$.87
*0.1415
*19.42%
*03/02/2015 08:11
Oncolytics Biotech, Inc. (ONCY) Vorbörslich Trading - NASDAQ.com
*$.87
*0.1415
*19.42%
*03/02/2015 08:11
Antwort auf Beitrag Nr.: 49.216.478 von NurFundamentalesIstBares am 02.03.15 14:14:17Oncolytics Biotech Inc. (ONCY)
-NasdaqCM
Watchlist
1.07 Up 0.34(46.88%
Volume: 11,999,775
und damit noch nicht genug
After hours
1,21 fast 500 k
-NasdaqCM
Watchlist
1.07 Up 0.34(46.88%
Volume: 11,999,775
und damit noch nicht genug
After hours
1,21 fast 500 k
Antwort auf Beitrag Nr.: 48.260.041 von gerdass am 07.11.14 20:56:49
Zitat von gerdass: Pipeline
Google Übersetzung des oben nenannten Liinks:
CALGARY, 3. März 2015 / PRNewswire / - Oncolytics Biotech® Inc. ("Oncolytics") (TSX: ONC, NASDAQ: ONCY), konzentrierte sich eine klinische Phase-Biotechnologie-Unternehmen auf die Entwicklung onkolytischer Viren als potentielle Krebstherapeutika, gab heute bekannt, dass die US Food and Drug Administration (FDA) hat die Orphan Drug Designation für seine führende Produktkandidat, REOLYSIN ®, für die Behandlung von primären peritonealen Krebsarten gewährt. Die Bezeichnung wurde auf der Grundlage von Dezember 2014 den Antrag des Unternehmens für eine Orphan Drug Designation umfasst Eierstock, Eileiter und primären peritonealen Krebsarten, die in der Regel als eine Indikation behandelt werden, gewährt. Am 11. Februar 2015 gab das Unternehmen bekannt, dass es die Orphan Drug Designation für Eierstockkrebs und Krebs der Eileiter am 2. März 2015 erhalten hatte.
"Dies ist das vierte Indikation, für die wir die Orphan Drug Designation in den Vereinigten Staaten erhalten haben, und die dritte in einer gynäkologischen Krebsindikation", sagte Dr. Brad Thompson, Präsident und CEO von Oncolytics. "Das Orphan Drug Designation sind ein wichtiger Schritt für die laufende Programm von Oncolytics zur Entwicklung und Vermarktung REOLYSIN ® als Therapeutikum für zielgerichtete Krebspatientengruppen."
Oncolytics hat zwei gesponserten klinischen Studien, in denen REOLYSIN ® in der Behandlung von Bauchfellkrebs unterstützt. Die erste war eine Phase 1/2 klinischen Studie (OSU-07022) für Patienten mit metastatischem Eierstock-, Peritonealdialyse und Eileiterkrebs mit gleichzeitige intravenöse und intraperitoneale Verabreichung von REOLYSIN ®, die Anzeichen von Virus Targeting und Replikation in Bauch und Eierstockkrebszellen zur Verfügung gestellt. Die zweite ist eine laufende randomisierte Phase-II-Studie (GOG186H) der wöchentlichen Paclitaxel im Vergleich zu Paclitaxel wöchentlich mit REOLYSIN ® bei Patienten mit persistierendem oder rezidivierendem Eierstock-, Eileiter oder primären Bauchfellkrebs. Die zweite Studie abgeschlossen Immatrikulation in September 2014.
Die FDA erteilt Orphan-Drug-Status für Produkte, die zur Behandlung seltener Krankheiten, die Schaffung von Anreizen für Sponsoren zur Entwicklung Drogen oder Biologika. Die FDA definiert seltene Krankheiten wie die zu einem bestimmten Zeitpunkt die weniger als 200.000 Menschen in den Vereinigten Staaten. Orphan Drug Designation bietet dem Sponsor bestimmte Vorteile und Anreize, einschließlich einer Frist von Marktexklusivität, wenn die Zulassung letztlich für die vorgesehene Indikation potenziellen Steuergutschriften für bestimmte Tätigkeiten, Eignung für Orphan-Drug-Zuschüsse und der Verzicht auf bestimmte Verwaltungsgebühren erhalten. Der Erhalt von Orphan-Drug-Status auch nicht den gesetzlichen Anforderungen oder Verfahren zur Erlangung der Marktzulassung zu ändern. Für weitere Informationen, besuchen Sie bitte:
http://www.fda.gov/forindustry/DevelopingProductsforrareDise…
CALGARY, 3. März 2015 / PRNewswire / - Oncolytics Biotech® Inc. ("Oncolytics") (TSX: ONC, NASDAQ: ONCY), konzentrierte sich eine klinische Phase-Biotechnologie-Unternehmen auf die Entwicklung onkolytischer Viren als potentielle Krebstherapeutika, gab heute bekannt, dass die US Food and Drug Administration (FDA) hat die Orphan Drug Designation für seine führende Produktkandidat, REOLYSIN ®, für die Behandlung von primären peritonealen Krebsarten gewährt. Die Bezeichnung wurde auf der Grundlage von Dezember 2014 den Antrag des Unternehmens für eine Orphan Drug Designation umfasst Eierstock, Eileiter und primären peritonealen Krebsarten, die in der Regel als eine Indikation behandelt werden, gewährt. Am 11. Februar 2015 gab das Unternehmen bekannt, dass es die Orphan Drug Designation für Eierstockkrebs und Krebs der Eileiter am 2. März 2015 erhalten hatte.
"Dies ist das vierte Indikation, für die wir die Orphan Drug Designation in den Vereinigten Staaten erhalten haben, und die dritte in einer gynäkologischen Krebsindikation", sagte Dr. Brad Thompson, Präsident und CEO von Oncolytics. "Das Orphan Drug Designation sind ein wichtiger Schritt für die laufende Programm von Oncolytics zur Entwicklung und Vermarktung REOLYSIN ® als Therapeutikum für zielgerichtete Krebspatientengruppen."
Oncolytics hat zwei gesponserten klinischen Studien, in denen REOLYSIN ® in der Behandlung von Bauchfellkrebs unterstützt. Die erste war eine Phase 1/2 klinischen Studie (OSU-07022) für Patienten mit metastatischem Eierstock-, Peritonealdialyse und Eileiterkrebs mit gleichzeitige intravenöse und intraperitoneale Verabreichung von REOLYSIN ®, die Anzeichen von Virus Targeting und Replikation in Bauch und Eierstockkrebszellen zur Verfügung gestellt. Die zweite ist eine laufende randomisierte Phase-II-Studie (GOG186H) der wöchentlichen Paclitaxel im Vergleich zu Paclitaxel wöchentlich mit REOLYSIN ® bei Patienten mit persistierendem oder rezidivierendem Eierstock-, Eileiter oder primären Bauchfellkrebs. Die zweite Studie abgeschlossen Immatrikulation in September 2014.
Die FDA erteilt Orphan-Drug-Status für Produkte, die zur Behandlung seltener Krankheiten, die Schaffung von Anreizen für Sponsoren zur Entwicklung Drogen oder Biologika. Die FDA definiert seltene Krankheiten wie die zu einem bestimmten Zeitpunkt die weniger als 200.000 Menschen in den Vereinigten Staaten. Orphan Drug Designation bietet dem Sponsor bestimmte Vorteile und Anreize, einschließlich einer Frist von Marktexklusivität, wenn die Zulassung letztlich für die vorgesehene Indikation potenziellen Steuergutschriften für bestimmte Tätigkeiten, Eignung für Orphan-Drug-Zuschüsse und der Verzicht auf bestimmte Verwaltungsgebühren erhalten. Der Erhalt von Orphan-Drug-Status auch nicht den gesetzlichen Anforderungen oder Verfahren zur Erlangung der Marktzulassung zu ändern. Für weitere Informationen, besuchen Sie bitte:
http://www.fda.gov/forindustry/DevelopingProductsforrareDise…
Antwort auf Beitrag Nr.: 49.225.631 von sirlacoura am 03.03.15 12:54:59Bin drin

Lach....Edi Edi Edi...wärste mal gestern mit mir hier eingestiegen, hättest du jetzt schon 60 % grün in deinem Depot ;-)
Antwort auf Beitrag Nr.: 49.226.207 von sirlacoura am 03.03.15 13:53:48Tja schade aber auch 
Aber mit der heutigen News dürften doch nochmals 60% drin sein
Gratulation läuft gut bei dir Bruder

Aber mit der heutigen News dürften doch nochmals 60% drin sein

Gratulation läuft gut bei dir Bruder

Antwort auf Beitrag Nr.: 49.226.288 von Edison09 am 03.03.15 14:00:58
Bin auch mit an Bord
Zitat von Edison09: Tja schade aber auch
Aber mit der heutigen News dürften doch nochmals 60% drin sein
Gratulation läuft gut bei dir Bruder
Bin auch mit an Bord

Antwort auf Beitrag Nr.: 49.226.318 von Earthfire am 03.03.15 14:03:43Willkommen 

Antwort auf Beitrag Nr.: 49.226.378 von Edison09 am 03.03.15 14:11:15
Danke....
Zitat von Edison09: Willkommen
Danke....

https://www.americanbulls.com/SignalPage.aspx?lang=de&Ticker…
Oncolytics Biotech
Signalaktualisierung Unsere heutige Systemempfehlung lautet LONG BEIBEHALTEN. Die vorherige Empfehlung KAUFEN wurde am 02/13/2015, 17 Tage zuvor ausgegeben, als der Aktienkurs 0.4933 betrug. Seit dem ist ONCY um +116.91% gestiegen.
Oncolytics Biotech
Signalaktualisierung Unsere heutige Systemempfehlung lautet LONG BEIBEHALTEN. Die vorherige Empfehlung KAUFEN wurde am 02/13/2015, 17 Tage zuvor ausgegeben, als der Aktienkurs 0.4933 betrug. Seit dem ist ONCY um +116.91% gestiegen.
2$ wir kommen 



Schönen Dank an Printi für den Tip gestern !!





Schönen Dank an Printi für den Tip gestern !!


Antwort auf Beitrag Nr.: 49.227.401 von Earthfire am 03.03.15 15:40:57ja sieht nicht schlecht aus hier

Antwort auf Beitrag Nr.: 49.227.437 von printguru am 03.03.15 15:43:10
Wenn die Gewinnmitnahmen abeben.....poppt die heute noch richtig hoch
Zitat von printguru: ja sieht nicht schlecht aus hier
Wenn die Gewinnmitnahmen abeben.....poppt die heute noch richtig hoch

Sch....e habe den Einstieg verpasst

Danke an die- oder denjenigen, die/der mir in Stuttgart um 14.45 Uhr welche für 1,066 Euro verkauft hat 

Der Chart erinnert mich an cytx auch an der 1,40$ abgeprallt 
Hoffe die fängt sich wieder und endet nicht cytx

Hoffe die fängt sich wieder und endet nicht cytx
Antwort auf Beitrag Nr.: 49.227.773 von Edison09 am 03.03.15 16:08:34ja wollen wir es hoffen.....
habe inzwischen etwas umgeschichtet in Aeterna AEZS mal schauen ob da auch was geht
wenn die 1,2$ bei ONCY unterschritten werden fliege ich raus...
LG Printi
habe inzwischen etwas umgeschichtet in Aeterna AEZS mal schauen ob da auch was geht

wenn die 1,2$ bei ONCY unterschritten werden fliege ich raus...
LG Printi
Antwort auf Beitrag Nr.: 49.227.503 von gogo123 am 03.03.15 15:48:15Sei froh............eine FDA durch und nun das......Mist
Antwort auf Beitrag Nr.: 49.227.878 von printguru am 03.03.15 16:19:24Wow wen Phase 3 durchgeht könnte das die zweite santhera-pharmaceuticals werden
Es gibt bis heute noch kein einziges Medikament, welches von der FDA (Food and Drug Administration) zur Behandlung von wiederkehrendem Gebärmutterkrebs zugelassen ist. Aeterna könnte so auf einen Markt ohne Konkurrenz vorstoßen, den Experten alleine in den USA und Europa auf 650 Millionen Dollar jährlich schätzen. Im ersten Halbjahr 2015 sollten die Daten der entscheidenden Phase III Studie vorlegen. Weitere Indikationen für Zoptarelin Doxorubicin sind Anwendungen gegen Eierstock- und Prostatakrebs, die sich derzeit in der klinischen Phase II befinden. Wie wir bereits vermuteten hat Aeterna Kooperationen und Lizenzen bezüglich Zoptarelin Doxorubicin abgeschlossen. Zum einen sicherte sich Sinopharm die Lizenz am in Phase III befindlichen Krebswirkstoff. Sinopharm ist nicht irgendein Unternehmen, sondern der größte Pharmakonzern Chinas.
Es gibt bis heute noch kein einziges Medikament, welches von der FDA (Food and Drug Administration) zur Behandlung von wiederkehrendem Gebärmutterkrebs zugelassen ist. Aeterna könnte so auf einen Markt ohne Konkurrenz vorstoßen, den Experten alleine in den USA und Europa auf 650 Millionen Dollar jährlich schätzen. Im ersten Halbjahr 2015 sollten die Daten der entscheidenden Phase III Studie vorlegen. Weitere Indikationen für Zoptarelin Doxorubicin sind Anwendungen gegen Eierstock- und Prostatakrebs, die sich derzeit in der klinischen Phase II befinden. Wie wir bereits vermuteten hat Aeterna Kooperationen und Lizenzen bezüglich Zoptarelin Doxorubicin abgeschlossen. Zum einen sicherte sich Sinopharm die Lizenz am in Phase III befindlichen Krebswirkstoff. Sinopharm ist nicht irgendein Unternehmen, sondern der größte Pharmakonzern Chinas.
Holla die Waldfee....25 Millionen gehandelte Shares bis jetzt. Das sind mehr als 1/4 der sich im Umlauf befindlichen Aktien...
Antwort auf Beitrag Nr.: 49.228.985 von Edison09 am 03.03.15 17:26:46Falsches Forum Edi
Hier darfst du ONYC pushen aber nicht Aeterna

Hier darfst du ONYC pushen aber nicht Aeterna

Wer gewinnt das Rennen heute cytx oder oncy ???
Ich tippe und hoffe auf oncy
Ich tippe und hoffe auf oncy

Cytori hat grad einen mega Satz gemacht Edi. sagte doch, lass laufen...ist zwar noch nicht ende, schätze aber das cyt das rennen heute macht.
hier hoffe ich, dass din Gewinnmitnahmen bald durch sind. Das Volumen ist riesig. Über 28 Millionen bis jetzt.
hier hoffe ich, dass din Gewinnmitnahmen bald durch sind. Das Volumen ist riesig. Über 28 Millionen bis jetzt.
AEZS scheint wieder nur eine Verpuffung zu sein...was haltet Ihr von StemCells?
Antwort auf Beitrag Nr.: 49.230.395 von NurFundamentalesIstBares am 03.03.15 19:35:47Nix....und von Aeterna zzt auch nix.
Gäääähhhhhhnnn
Also ein bisschen mehr hatten wir uns wohl alle erhofft
Kann mir nicht erklären warum der Kurs nicht angesprungen ist????
Vielleicht morgen

Also ein bisschen mehr hatten wir uns wohl alle erhofft

Kann mir nicht erklären warum der Kurs nicht angesprungen ist????
Vielleicht morgen

Antwort auf Beitrag Nr.: 49.230.788 von Edison09 am 03.03.15 20:17:19Nur mit Kurzfristein- und aussteigern steigt der Kurs eben nicht weiter!
Antwort auf Beitrag Nr.: 49.232.678 von twisterfriend am 04.03.15 07:23:52Das Volumen ist mit Kurzfristein- und -aussteigern nicht zu erklären.
An der NASDAQ und in Toronto wurden gestern ca 40 % der im Umlauf befindlichen Aktien gehandelt, nimmt man die beiden vorherigen Tage hinzu, waren es ca 50 %.
Ich habe gestern die Realtime Kurse auf Yahoo an der Nasdaq verfolgt und bin fester Überzeugung, daß hier der Kurs erheblich manipuliert wurde. Immer wenn der Kurs nachgab, teilweise sogar um 10 Cent mit einem Trade, erfolgte im Sekundentakt ein Trade auf altem, höheren Niveau.
Es droht ein Delisting von der NASDAQ, da der Kurs unter einem Dollar lag. Nur wenn innerhalb der nächsten Wochen der Kurs, ich glaube, 10 Tage über einem Dollar liegt, ist das auch bereits angedrohte Delisting vorerst abgewendet.
Mein Bauchgefühl sagt nichts Gutes, da Meldungen bisher ausgeblieben sind, bzw. die New, die die letzten Tage kamen, nicht ausreichen, um ein derartig bhohes Volumen zu erklären.
An der NASDAQ und in Toronto wurden gestern ca 40 % der im Umlauf befindlichen Aktien gehandelt, nimmt man die beiden vorherigen Tage hinzu, waren es ca 50 %.
Ich habe gestern die Realtime Kurse auf Yahoo an der Nasdaq verfolgt und bin fester Überzeugung, daß hier der Kurs erheblich manipuliert wurde. Immer wenn der Kurs nachgab, teilweise sogar um 10 Cent mit einem Trade, erfolgte im Sekundentakt ein Trade auf altem, höheren Niveau.
Es droht ein Delisting von der NASDAQ, da der Kurs unter einem Dollar lag. Nur wenn innerhalb der nächsten Wochen der Kurs, ich glaube, 10 Tage über einem Dollar liegt, ist das auch bereits angedrohte Delisting vorerst abgewendet.
Mein Bauchgefühl sagt nichts Gutes, da Meldungen bisher ausgeblieben sind, bzw. die New, die die letzten Tage kamen, nicht ausreichen, um ein derartig bhohes Volumen zu erklären.
Antwort auf Beitrag Nr.: 49.233.200 von reguau am 04.03.15 08:41:23
Das Meldungen ausblieben, ist nicht richtig.
02.03.2015
http://de.advfn.com/p.php?pid=nmona&article=65679747
03.03.2015
http://de.advfn.com/p.php?pid=nmona&article=65698267" target="_blank" rel="nofollow ugc noopener">http://de.advfn.com/p.php?pid=nmona&article=65698267
Das Meldungen ausblieben, ist nicht richtig.
02.03.2015
http://de.advfn.com/p.php?pid=nmona&article=65679747
03.03.2015
http://de.advfn.com/p.php?pid=nmona&article=65698267" target="_blank" rel="nofollow ugc noopener">http://de.advfn.com/p.php?pid=nmona&article=65698267
Was mich bei dem riesigen Volumen stutzig macht ist, dass der Kurs trotz neuer News vom Tageshoch bei ca. 1,37 $ minimal im Minus bei 1,15 $ geschlossen hat. Kann mir darauf noch keinen Reim machen...grübel...
Antwort auf Beitrag Nr.: 49.235.495 von sirlacoura am 04.03.15 12:04:44Oncolytics hat für Reolysin für insgesamt 4 Krebsindikationen von der FDA den Status Orphan Drug Destination bekommen.
Damit verbunden ist ein vereinfachtes Zulassungsverfahren, aber noch lange nicht die eigentliche Zulassung.
Die letzten beiden Meldungen sind vom 02. und 03. März mit den beiden letzten Statusmeldungen. Die beiden vorherigen sind völlig wirkungslos verpufft und die Genehmigungen für die beiden jüngsten sind ebenfalls nicht der Renner.
Das rechtfertigt nicht einen Umsatz von 50 % der Aktien eines Unternehmens an 3 Tagen. Auch eine Übernahme steht nicht an, dann wäre der Kurs wirklich angesprungen.
Heute im Premarket kommt der Kurs bereits deutlich wieder zurück.
Oncolytics Biotech Inc. (ONCY)
-NasdaqCM
Watchlist
1.16 Mar 3, 4:00PM EST
Pre-Market : 1.06 Down 0.10 (8.62%) 9:19AM EST - Nasdaq Real Time Price
Ich bleibe bei meiner Meinung, der Kurs wurde manipuliert.
Damit verbunden ist ein vereinfachtes Zulassungsverfahren, aber noch lange nicht die eigentliche Zulassung.
Die letzten beiden Meldungen sind vom 02. und 03. März mit den beiden letzten Statusmeldungen. Die beiden vorherigen sind völlig wirkungslos verpufft und die Genehmigungen für die beiden jüngsten sind ebenfalls nicht der Renner.
Das rechtfertigt nicht einen Umsatz von 50 % der Aktien eines Unternehmens an 3 Tagen. Auch eine Übernahme steht nicht an, dann wäre der Kurs wirklich angesprungen.
Heute im Premarket kommt der Kurs bereits deutlich wieder zurück.
Oncolytics Biotech Inc. (ONCY)
-NasdaqCM
Watchlist
1.16 Mar 3, 4:00PM EST
Pre-Market : 1.06 Down 0.10 (8.62%) 9:19AM EST - Nasdaq Real Time Price
Ich bleibe bei meiner Meinung, der Kurs wurde manipuliert.
Antwort auf Beitrag Nr.: 49.247.939 von gerdass am 05.03.15 13:22:54Nichts, was relevant wäre, ein weiteres Mausmodell und premarketkurse, die weder vom Volumen, noch vom Kurs her beeindruckend sind.
Fakt ist, der Kurs lag ei ast 1,40 $ und kam innerhalb von 2 Tagen auf 1,01 zurück. Bei den Umsatzvolumina nach meiner Meinung auch gestern heftig gestützt.
Fakt ist, der Kurs lag ei ast 1,40 $ und kam innerhalb von 2 Tagen auf 1,01 zurück. Bei den Umsatzvolumina nach meiner Meinung auch gestern heftig gestützt.
Antwort auf Beitrag Nr.: 49.248.080 von reguau am 05.03.15 13:32:28
gestützt
von wem denn
Zitat von reguau: Nichts, was relevant wäre, ein weiteres Mausmodell und premarketkurse, die weder vom Volumen, noch vom Kurs her beeindruckend sind.
Fakt ist, der Kurs lag ei ast 1,40 $ und kam innerhalb von 2 Tagen auf 1,01 zurück. Bei den Umsatzvolumina nach meiner Meinung auch gestern heftig gestützt.
gestützt
von wem denn

über 130 Tsd Aktien
ist das nix
http://www.nasdaq.com/symbol/oncy/premarket
mal sachte
das Teil hat ja erst gezündet
ist das nix
http://www.nasdaq.com/symbol/oncy/premarket
mal sachte
das Teil hat ja erst gezündet
schon immer brauchten die US-Jungen bis zu 3 Tage um eine NEWS zu verstehen
das war schon immer so
.
http://www.nasdaq.com/symbol/oncy/after-hours

das war schon immer so
.
http://www.nasdaq.com/symbol/oncy/after-hours
Oncolytics Biotech Price to Book Value:7.068 for March 5, 2015
.
http://ycharts.com/companies/ONCY/price_to_book_value
.
http://ycharts.com/companies/ONCY/price_to_book_value
Antwort auf Beitrag Nr.: 49.254.575 von gerdass am 05.03.15 22:28:16Wieso ging es hier so in den letzten paar Tagen rasant runter?
Gewinnmitnahmen?
Gewinnmitnahmen?
Bin jetzt hier mal mit dabei.sieht mir aus das wir wieder den Dollar ins Visier nehmen.
Jetzt gilt es erst einmal den Zeitraum bis zum 27.04.2015 im Auge zu behalten!
Antwort auf Beitrag Nr.: 49.579.310 von gerdass am 16.04.15 14:13:49
.
http://ih.advfn.com/p.php?pid=nmona&article=66460405&symbol=…
Zitat von gerdass: http://www.nasdaq.com/symbol/oncy/premarket
.
http://ih.advfn.com/p.php?pid=nmona&article=66460405&symbol=…

Kann mir einer sagen, wieviel Cash die noch haben.
Und wie lange die damit auskommen, ohne KE ? (burnrate)
Vielen Dank (überlege hier einzusteigen)
Und wie lange die damit auskommen, ohne KE ? (burnrate)
Vielen Dank (überlege hier einzusteigen)
Antwort auf Beitrag Nr.: 49.595.585 von Moneyplus am 18.04.15 19:23:13
so weit mir bekannt ist haben sie ca. 28 Mio, Dollar
das soll für 18 mMnate reichen
Zitat von Moneyplus: Kann mir einer sagen, wieviel Cash die noch haben.
Und wie lange die damit auskommen, ohne KE ? (burnrate)
Vielen Dank (überlege hier einzusteigen)
so weit mir bekannt ist haben sie ca. 28 Mio, Dollar
das soll für 18 mMnate reichen
beambe investierte erneut 70 Tsd. Dollar bei .65
alles wird gut
alles wird gut

Antwort auf Beitrag Nr.: 49.595.801 von gerdass am 18.04.15 21:08:18

Zitat von gerdass: stark unterbewertet
.
http://ycharts.com/companies/ONCY/price_to_book_value
Zeit zum investieren gab es
unter 3 DOLLAR verkaufe ich nicht eine Aktie
mein Tip an D
nix verschenken
nix verschenken
da ich drüben 10 verschiedene User bin
einer ist beambe
einen Anderen kennt jeder
einer ist beambe
einen Anderen kennt jeder

alles wird vörmlichst aufgesaugt
Short Term Indicators Average: 100% Buy
.
http://www2.barchart.com/opinions/stocks/ONCY
.
Overall Average: 80% Buy
.
http://www2.barchart.com/opinions/stocks/ONCY
.
Overall Average: 80% Buy
Antwort auf Beitrag Nr.: 49.602.065 von gerdass am 20.04.15 14:23:10
na
geht doch
Zitat von gerdass:![]()
.
http://ih.advfn.com/p.php?pid=staticchart&s=N%5EONCY&p=0&t=1…
na
geht doch

Antwort auf Beitrag Nr.: 49.604.201 von gerdass am 20.04.15 18:25:49
Overall Average: 72% Buy
Zitat von gerdass: Short Term Indicators Average: 100% Buy
.
http://www2.barchart.com/opinions/stocks/ONCY
.
Overall Average: 80% Buy
Overall Average: 72% Buy

Antwort auf Beitrag Nr.: 40.270.746 von reguau am 06.10.10 01:08:50
Zitat von reguau: Oncolytics Biotech (oncolyticsbiotech.com) ist eine Biotech AG aus Kanada und betreibt derzeit 12 Studien gegen unterschiedlichste Krebsformen
Jahrelang wurde versucht, Krebs mit seinem Produkt Reolysin (Info http://www.oncolyticsbiotech.com/tech.html) als Monotherapie zu bekämpfen. Die Erfolge hielten sich in Grenzen. Seit ca 3 Jahren laufen Studien mit einer Kombitherapie, Reolysin in Verbindung mit Chemo oder Bestrahlung (Studien http://www.oncolyticsbiotech.com/tech.html). Anscheinend mit großem Erfolg. Der Kurs der Aktie, der noch Ende 2008 bei 1 US$ lag, liegt heute bei 5 $ und hat sich allein in den letzten zwei Monaten verdoppelt.
Die Umsätze an der NASDAG dümpelten lange Zeit um 35 K am Tag, stieg vor wenigen Wochen rasant an, heute wurden über 500 k gehandelt
Infos gibt es hier auf Yahoo http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
hier gab es vor mehr als 5 Jahren Beiträge zu dieser AG
reguau

.
.
Prost

Oncolytics Biotech, Reolysin gegen Krebs
Antwort auf Beitrag Nr.: 49.604.222 von gerdass am 20.04.15 18:27:42
Zitat von gerdass:Zitat von gerdass:![]()
.
http://ih.advfn.com/p.php?pid=staticchart&s=N%5EONCY&p=0&t=1…
na
geht doch

Oncolytics Biotech, Reolysin gegen Krebs

http://www.nasdaq.com/symbol/oncy/time-sales/url]" target="_blank" rel="nofollow ugc noopener">http://www.nasdaq.com/symbol/oncy/time-sales/url]
Oncolytics Biotech, (ONCY)
0.87 ▲ 0.109 (14.32%)
0.87 ▲ 0.109 (14.32%)
Watchlist 



 Was ist denn hier los?
Was ist denn hier los? 
oh may Got--Volume $$$

das ist erst der Anfang

Verlückt
aber gut
aber gut

Oncolytics Biotech, (ONCY)
0.7385 ▲ 0.1595 (27.55%)
Volume: 3,738,826 @ 3:59:59 PM ET
http://investorshub.advfn.com/Oncolytics-Biotech-ONCY-814/
0.7385 ▲ 0.1595 (27.55%)
Volume: 3,738,826 @ 3:59:59 PM ET
http://investorshub.advfn.com/Oncolytics-Biotech-ONCY-814/
Clinical Trials
Oncolytics Biotech is conducting clinical trials in multiple indications with the objective of developing REOLYSIN® as a human cancer therapeutic. The current clinical program comprises human trials using REOLYSIN® both alone and in combination with chemotherapy, delivered via local and/or intravenous administration. In May 2010, the Company commenced enrollment for its first Phase III clinical trial, REO 018, investigating the intravenous administration of REOLYSIN® in combination with paclitaxel and carboplatin in patients with platinum-refractory head and neck cancers.
Trial Number Phase Trial Name Location Status
MAYO-MC1472 Phase I REOLYSIN® in Combination with GM-CSF in Pediatric Patients with Relapsed or Refractory Brain Tumours US Ongoing
NCI-9603 (NCI Trial) Translational Study Intravenous Administration of REOLYSIN® in Combination with Dexamethasone and Carfilzomib for Patients with Relapsed or Refractory Myeloma US Ongoing
IND 213 (NCIC CTG Trial) Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel for Patients with Advanced or Metastatic Breast Cancer Canada Ongoing
IND 211 (NCIC CTG Trial) Phase II Intravenous Administration of REOLYSIN® in Combination with Docetaxel or Pemetrexed for Patients with Previously-Treated Advanced or Metastatic Non-Small Cell Lung Cancer Canada Ongoing
IND 210 (NCIC CTG Trial) Phase II Intravenous Administration of REOLYSIN® in Combination with FOLFOX-6 Plus Bevacizumab (Avastin®) Versus FOLFOX-6 Plus Bevacizumab Alone in Patients with Advanced or Metastatic Colorectal Cancer Canada Ongoing
IND 209 (NCIC CTG Trial) Phase II Intravenous Administration of REOLYSIN® in Combination with Docetaxel for Patients with Recurrent or Metastatic Castration Resistant Prostate Cancer Canada Ongoing
OSU-11148 (NCI Trial) Phase I Intravenous Administration of REOLYSIN® for Patients with Relapsed Multiple Myeloma US Ongoing
NCI-8601/OSU-10045 (NCI Trial) Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Metastatic Pancreatic Cancer US Ongoing
COG-ADVL1014 (NCI / COG Trial) Phase I Intravenous Administration of REOLYSIN® in Combination with Cyclophosphamide for Pediatric Patients with Relapsed or Refractory Solid Tumors US Complete
GOG-0186H (NCI / GOG Trial) Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel for Patients with Persistent or Recurrent Ovarian, Fallopian Tube or Primary Peritoneal Cancer US Ongoing
REO 022 Phase I Intravenous Administration of REOLYSIN® in Combination with FOLFIRI and Bevacizumab in FOLFIRI naive Patients with Mutant Metastatic Colorectal Cancer US Ongoing
REO 021 Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Squamous Cell Carcinoma Lung Cancer US Ongoing
REO 020 Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Metastatic Melanoma US Complete
REO 018 Phase III Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Platinum-Refractory Head and Neck Cancers International Complete
REO 017 Phase II Intravenous Administration of REOLYSIN® in Combination with Gemcitabine for Patients with Advanced Pancreatic Cancer US Ongoing
REO 016 Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Non-Small Cell Lung Cancer US Ongoing
REO 015 Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Advanced Head and Neck Cancers US Complete
REO 014 Phase II Intravenous Administration of REOLYSIN® for Patients with Metastatic Sarcomas US Complete
REO 013 Brain Phase I Intravenous Administration of REOLYSIN® in Patients Prior to Surgical Resection of Recurrent High Grade Primary or Metastatic Brain Tumors UK Ongoing
REO 013 Translational Study Intravenous Administration of REOLYSIN® for Patients with Metastatic Colorectal Cancer UK Complete
MAYO-MC0672 (NCI Trial) Phase II Intravenous Administration of REOLYSIN® for Patients with Metastatic Melanoma US Complete
OSU-07022 (NCI Trial) Phase I/II Systemic and Intraperitoneal Administration of REOLYSIN® for Patients with Metastatic Ovarian, Peritoneal and Fallopian Tube Cancers US Ongoing
REO 012 Phase I Intravenous Administration of REOLYSIN® in Combination with Cyclophosphamide for Patients with Advanced Malignancies UK Complete
REO 011 Phase I/II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Advanced Head and Neck Cancers UK Complete
REO 010 Phase I Intravenous Administration of REOLYSIN® in Combination with Docetaxel for Patients with Advanced Malignancies UK Complete
REO 009 Phase I Intravenous Administration of REOLYSIN® in Combination with Gemcitabine for Patients with Advanced Malignancies UK Complete
REO 008 Phase II Intratumoral Administration of REOLYSIN® in Combination with Low-Dose Radiation for Patients with Advanced Malignancies UK Complete
REO 007 Phase I/II Infusion Monotherapy of REOLYSIN® for Patients with Recurrent Malignant Gliomas US Complete
REO 006 Phase I Local Administration of REOLYSIN® in Combination with Radiation for Patients with Advanced Cancers UK Complete
REO 005 Phase I Systemic Administration of REOLYSIN® for Patients with Various Metastatic Tumors UK Complete
REO 004 Phase I Systemic Administration of REOLYSIN® for Patients with Various Metastatic Tumors US Complete
REO 003 Phase I/II Local Monotherapy of REOLYSIN® for Patients with Recurrent Malignant Gliomas Canada Complete
REO 002 Translational Study Local Monotherapy of REOLYSIN® for Patients with T2 Prostate Cancer Canada Complete
REO 001 Phase I Local Monotherapy of REOLYSIN® for Patients with Subcutaneous Tumors Canada Complete
REOLYSIN® is currently available exclusively in a clinical trial setting. It has not been approved in any jurisdiction and is not available for purchase. Oncolytics and its clinical partners are conducting clinical trials in a number of countries worldwide, including Canada, the United States and the United Kingdom.
The Company and its employees have no influence upon the inclusion of a patient in a clinical trial. If the patient’s physician determines that he or she may be a suitable trial candidate, they can contact a participating center. Contact information, as well as inclusion and exclusion criteria, for US and international participating centers, can be found at www.clinicaltrials.gov for studies with a US component.
http://www.oncolyticsbiotech.com/clinical-trials/default.asp…
Oncolytics Biotech is conducting clinical trials in multiple indications with the objective of developing REOLYSIN® as a human cancer therapeutic. The current clinical program comprises human trials using REOLYSIN® both alone and in combination with chemotherapy, delivered via local and/or intravenous administration. In May 2010, the Company commenced enrollment for its first Phase III clinical trial, REO 018, investigating the intravenous administration of REOLYSIN® in combination with paclitaxel and carboplatin in patients with platinum-refractory head and neck cancers.
Trial Number Phase Trial Name Location Status
MAYO-MC1472 Phase I REOLYSIN® in Combination with GM-CSF in Pediatric Patients with Relapsed or Refractory Brain Tumours US Ongoing
NCI-9603 (NCI Trial) Translational Study Intravenous Administration of REOLYSIN® in Combination with Dexamethasone and Carfilzomib for Patients with Relapsed or Refractory Myeloma US Ongoing
IND 213 (NCIC CTG Trial) Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel for Patients with Advanced or Metastatic Breast Cancer Canada Ongoing
IND 211 (NCIC CTG Trial) Phase II Intravenous Administration of REOLYSIN® in Combination with Docetaxel or Pemetrexed for Patients with Previously-Treated Advanced or Metastatic Non-Small Cell Lung Cancer Canada Ongoing
IND 210 (NCIC CTG Trial) Phase II Intravenous Administration of REOLYSIN® in Combination with FOLFOX-6 Plus Bevacizumab (Avastin®) Versus FOLFOX-6 Plus Bevacizumab Alone in Patients with Advanced or Metastatic Colorectal Cancer Canada Ongoing
IND 209 (NCIC CTG Trial) Phase II Intravenous Administration of REOLYSIN® in Combination with Docetaxel for Patients with Recurrent or Metastatic Castration Resistant Prostate Cancer Canada Ongoing
OSU-11148 (NCI Trial) Phase I Intravenous Administration of REOLYSIN® for Patients with Relapsed Multiple Myeloma US Ongoing
NCI-8601/OSU-10045 (NCI Trial) Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Metastatic Pancreatic Cancer US Ongoing
COG-ADVL1014 (NCI / COG Trial) Phase I Intravenous Administration of REOLYSIN® in Combination with Cyclophosphamide for Pediatric Patients with Relapsed or Refractory Solid Tumors US Complete
GOG-0186H (NCI / GOG Trial) Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel for Patients with Persistent or Recurrent Ovarian, Fallopian Tube or Primary Peritoneal Cancer US Ongoing
REO 022 Phase I Intravenous Administration of REOLYSIN® in Combination with FOLFIRI and Bevacizumab in FOLFIRI naive Patients with Mutant Metastatic Colorectal Cancer US Ongoing
REO 021 Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Squamous Cell Carcinoma Lung Cancer US Ongoing
REO 020 Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Metastatic Melanoma US Complete
REO 018 Phase III Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Platinum-Refractory Head and Neck Cancers International Complete
REO 017 Phase II Intravenous Administration of REOLYSIN® in Combination with Gemcitabine for Patients with Advanced Pancreatic Cancer US Ongoing
REO 016 Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Non-Small Cell Lung Cancer US Ongoing
REO 015 Phase II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Advanced Head and Neck Cancers US Complete
REO 014 Phase II Intravenous Administration of REOLYSIN® for Patients with Metastatic Sarcomas US Complete
REO 013 Brain Phase I Intravenous Administration of REOLYSIN® in Patients Prior to Surgical Resection of Recurrent High Grade Primary or Metastatic Brain Tumors UK Ongoing
REO 013 Translational Study Intravenous Administration of REOLYSIN® for Patients with Metastatic Colorectal Cancer UK Complete
MAYO-MC0672 (NCI Trial) Phase II Intravenous Administration of REOLYSIN® for Patients with Metastatic Melanoma US Complete
OSU-07022 (NCI Trial) Phase I/II Systemic and Intraperitoneal Administration of REOLYSIN® for Patients with Metastatic Ovarian, Peritoneal and Fallopian Tube Cancers US Ongoing
REO 012 Phase I Intravenous Administration of REOLYSIN® in Combination with Cyclophosphamide for Patients with Advanced Malignancies UK Complete
REO 011 Phase I/II Intravenous Administration of REOLYSIN® in Combination with Paclitaxel and Carboplatin for Patients with Advanced Head and Neck Cancers UK Complete
REO 010 Phase I Intravenous Administration of REOLYSIN® in Combination with Docetaxel for Patients with Advanced Malignancies UK Complete
REO 009 Phase I Intravenous Administration of REOLYSIN® in Combination with Gemcitabine for Patients with Advanced Malignancies UK Complete
REO 008 Phase II Intratumoral Administration of REOLYSIN® in Combination with Low-Dose Radiation for Patients with Advanced Malignancies UK Complete
REO 007 Phase I/II Infusion Monotherapy of REOLYSIN® for Patients with Recurrent Malignant Gliomas US Complete
REO 006 Phase I Local Administration of REOLYSIN® in Combination with Radiation for Patients with Advanced Cancers UK Complete
REO 005 Phase I Systemic Administration of REOLYSIN® for Patients with Various Metastatic Tumors UK Complete
REO 004 Phase I Systemic Administration of REOLYSIN® for Patients with Various Metastatic Tumors US Complete
REO 003 Phase I/II Local Monotherapy of REOLYSIN® for Patients with Recurrent Malignant Gliomas Canada Complete
REO 002 Translational Study Local Monotherapy of REOLYSIN® for Patients with T2 Prostate Cancer Canada Complete
REO 001 Phase I Local Monotherapy of REOLYSIN® for Patients with Subcutaneous Tumors Canada Complete
REOLYSIN® is currently available exclusively in a clinical trial setting. It has not been approved in any jurisdiction and is not available for purchase. Oncolytics and its clinical partners are conducting clinical trials in a number of countries worldwide, including Canada, the United States and the United Kingdom.
The Company and its employees have no influence upon the inclusion of a patient in a clinical trial. If the patient’s physician determines that he or she may be a suitable trial candidate, they can contact a participating center. Contact information, as well as inclusion and exclusion criteria, for US and international participating centers, can be found at www.clinicaltrials.gov for studies with a US component.
http://www.oncolyticsbiotech.com/clinical-trials/default.asp…
wurde ja auch mal Zeit 

Antwort auf Beitrag Nr.: 50.180.217 von Ahwas am 14.07.15 07:22:47
son ist es
http://www.nasdaq.com/symbol/oncy/after-hours
Zitat von Ahwas: wurde ja auch mal Zeit
son ist es

http://www.nasdaq.com/symbol/oncy/after-hours
Antwort auf Beitrag Nr.: 50.180.391 von gerdass am 14.07.15 08:05:36Es gibt keine neuen Meldungen
Zur Erinnerung, Anfang März stieg der Kurs ebenfalls ohne neuen Meldungen unter hohen Umsätzen auf 1,00 € um dann wieder auf den heutigen Stand abzustürzen.
Und noch etwas, das Delisting droht, auch ein ähnliches Szenario wie März, damals wurde gemunkelt, jemand würde versuchen, den Kurs auf über 1 Dollar zu pushen (Grenze für Delisting).
Zur Erinnerung, Anfang März stieg der Kurs ebenfalls ohne neuen Meldungen unter hohen Umsätzen auf 1,00 € um dann wieder auf den heutigen Stand abzustürzen.
Und noch etwas, das Delisting droht, auch ein ähnliches Szenario wie März, damals wurde gemunkelt, jemand würde versuchen, den Kurs auf über 1 Dollar zu pushen (Grenze für Delisting).
das Problem ist
dass wir stark unterbewertet sind
http://ycharts.com/companies/ONCY/price_to_book_value
dass wir stark unterbewertet sind
http://ycharts.com/companies/ONCY/price_to_book_value
Antwort auf Beitrag Nr.: 50.180.421 von reguau am 14.07.15 08:12:33
schau dir die ganzen NEWS an
http://ih.advfn.com/stock-market/NASDAQ/ONCY/stock-price
Zitat von reguau: Es gibt keine neuen Meldungen
Zur Erinnerung, Anfang März stieg der Kurs ebenfalls ohne neuen Meldungen unter hohen Umsätzen auf 1,00 € um dann wieder auf den heutigen Stand abzustürzen.
Und noch etwas, das Delisting droht, auch ein ähnliches Szenario wie März, damals wurde gemunkelt, jemand würde versuchen, den Kurs auf über 1 Dollar zu pushen (Grenze für Delisting).
schau dir die ganzen NEWS an
http://ih.advfn.com/stock-market/NASDAQ/ONCY/stock-price
Antwort auf Beitrag Nr.: 50.180.679 von gerdass am 14.07.15 08:41:55
Kannst du das bitte mal kurz übersetzen? My english is not so good :-(
Zitat von gerdass: schau dir die ganzen NEWS an
http://ih.advfn.com/stock-market/NASDAQ/ONCY/stock-price
Kannst du das bitte mal kurz übersetzen? My english is not so good :-(
Antwort auf Beitrag Nr.: 50.180.853 von Ahwas am 14.07.15 09:05:26
https://translate.google.de/
Zitat von Ahwas:Zitat von gerdass: schau dir die ganzen NEWS an
http://ih.advfn.com/stock-market/NASDAQ/ONCY/stock-price
Kannst du das bitte mal kurz übersetzen? My english is not so good :-(
https://translate.google.de/
Antwort auf Beitrag Nr.: 50.181.027 von gerdass am 14.07.15 09:21:00
Danke, aber da komme ich hier (bei der Arbeit) nicht rein.
Zitat von gerdass:Zitat von Ahwas: ...
Kannst du das bitte mal kurz übersetzen? My english is not so good :-(
https://translate.google.de/
Danke, aber da komme ich hier (bei der Arbeit) nicht rein.
Wird sich heute zeigen wo die Reise hin geht :Entweder die 1 $ Marke wird angekratzt oder der Kurs geht wieder unter 60Cent. Denke ich aber nicht da wir drüben Nachbörslich auf Hoch geschlossen haben
Antwort auf Beitrag Nr.: 50.184.342 von gerdass am 14.07.15 15:15:53und schon ist die Party wieder vorbei
Antwort auf Beitrag Nr.: 50.185.371 von reguau am 14.07.15 17:12:23
nö
hat ja noch nicht angefangen
Zitat von reguau: und schon ist die Party wieder vorbei
nö
hat ja noch nicht angefangen

Antwort auf Beitrag Nr.: 50.501.217 von Ahwas am 27.08.15 14:26:24
bin seit gestern auch mit dabei, hatte die Aktie die ganze Zeit auf der Watchlist aber mir war die Bewertung noch zu hoch, aktuell nachdem das Nasdaq delisting verdaut ist, notiert Sie sogar unter Cash Level, daher glasklarer Kauf.
Freue mich auf spannende Diskussionen und viele Grüße
Sonic
Neiinvestment
So Hallo Onco-ianer,bin seit gestern auch mit dabei, hatte die Aktie die ganze Zeit auf der Watchlist aber mir war die Bewertung noch zu hoch, aktuell nachdem das Nasdaq delisting verdaut ist, notiert Sie sogar unter Cash Level, daher glasklarer Kauf.
Freue mich auf spannende Diskussionen und viele Grüße
Sonic
5. November Ergebnisse des dritten Quatrals 2015. Da steht unter anderen : Accumulated deficit (260,192,408) also Bilanzverlust von 260 Millionen. Mutig wer hier investiert ist.
Liquidity
As at September 30, 2015, we had cash and cash equivalents, short-term investments and working capital positions as follows:
September 30,
2015
$
December 31,
2014
$
Cash and cash equivalents 27,962,462 14,152,825
Short-term investments 2,060,977 2,031,685
Shareholders’ equity 27,724,279 13,819,193
We do not have any debt other than trade accounts payable and we have potential contingent obligations relating to the completion
of our research and development of REOLYSIN®
As at September 30, 2015, we had cash and cash equivalents, short-term investments and working capital positions as follows:
September 30,
2015
$
December 31,
2014
$
Cash and cash equivalents 27,962,462 14,152,825
Short-term investments 2,060,977 2,031,685
Shareholders’ equity 27,724,279 13,819,193
We do not have any debt other than trade accounts payable and we have potential contingent obligations relating to the completion
of our research and development of REOLYSIN®
Antwort auf Beitrag Nr.: 50.970.816 von crystalsonic am 30.10.15 15:49:21Hi, ich suche eine Biotech oder Pharmafirma die ein Gegenmittel für Blutvertüner haben. Kennst Du eine Firma vielleicht Phase II oder III
lg
hbc02
lg
hbc02
Wie ist das zu vestehen? Suchst du einen Hersteller der Blutverdünner herstellt? Das Gegenteil wirst du nicht antreffen. Das wäre gesundheitsschädlich.
Ist noch wer hier investiert?
Gab es hier einen reverse split??
Gab es hier einen reverse split??
Antwort auf Beitrag Nr.: 57.852.507 von Ahwas am 29.05.18 08:05:30

Oncolytics Biotech Aktienzusammenlegung (reverse split)
https://globenewswire.com/news-release/2018/05/22/1510438/0/…
Antwort auf Beitrag Nr.: 57.861.645 von MichaBu am 29.05.18 22:45:05
Danke!!!
Zitat von MichaBu: https://globenewswire.com/news-release/2018/05/22/1510438/0/…
Danke!!!
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +2,00 | |
| 0,00 | |
| -0,37 | |
| -0,70 | |
| +2,92 | |
| -5,99 | |
| +4,28 | |
| 0,00 | |
| -0,27 | |
| 0,00 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 203 | ||
| 92 | ||
| 70 | ||
| 56 | ||
| 55 | ||
| 45 | ||
| 45 | ||
| 40 | ||
| 33 | ||
| 27 |





















