Mit Delcath Systems "seperat" die Chemo einsetzen! - 500 Beiträge pro Seite
eröffnet am 17.02.11 16:12:42 von
neuester Beitrag 12.03.14 23:10:28 von
neuester Beitrag 12.03.14 23:10:28 von
Beiträge: 68
ID: 1.163.842
ID: 1.163.842
Aufrufe heute: 0
Gesamt: 8.861
Gesamt: 8.861
Aktive User: 0
ISIN: US24661P8077 · WKN: A2PT5P · Symbol: DCTH
4,6950
USD
+4,92 %
+0,2200 USD
Letzter Kurs 18.04.24 Nasdaq
Neuigkeiten
Werte aus der Branche Gesundheitswesen
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,8000 | +99.999,00 | |
| 0,8486 | +34,00 | |
| 3,2500 | +22,18 | |
| 0,6050 | +21,00 | |
| 1,4101 | +20,52 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 3,0300 | -14,65 | |
| 4,5200 | -16,30 | |
| 1,5000 | -17,58 | |
| 0,5383 | -22,06 | |
| 1,0700 | -27,21 |
Delcath System's Two Chances of Legitimacy in 2011
2 comments | by: Chemistfrog February 17, 2011 | about: DCTH Font Size: PrintEmail Recommend 1 Share this page
Share0 In the world of biotechnology and big pharmaceuticals there has always been a delicate balance of dosage amount to obtain the desired efficacy versus the side effects associated with the higher dosages.
One company with a very innovative means by which to have the best of both worlds is Delcath Systems (NASDAQ: DCTH). Delcath’s innovative device involves the use of melphalan hydrochloride to treat metastatic melanoma of the liver (melanoma that has spread to the liver). Melphalan is typically administered orally or intravenously for multiple myeloma, ovarian cancer and occasionally malignant melanoma. However, administered levels required to treat melanoma were not effective, and earlier investigations found other applications for the drug.
Their novel device is in the form of their device/drug combination termed a “chemosaturation system”. Their proprietary system, as best described in this video, basically involves isolating the organ’s (liver for this application) return blood flow to the body by inflatable balloons placed in the vena cava.
The mephalan hydrochloride solution is directly injected into the artery supplying blood to the liver (the hepatic artery) to deliver a dose up to 100 times more concentrated than traditional chemotherapy would have. Then the return blood is rerouted after treatment through a filter device to remove the mephalan hydrochloride, and then it is returned to the patient’s body via a catheter in the jugular.
After treatment, the catheters are simply removed, balloons deflated and the patient is prevented from having his entire biologic system have to deal with the side effects often associated with chemotherapy. The targeted organ containing the malignant melanoma meanwhile received a substantial dose thereby increasing efficacy while maintaining a safety profile more palatable to the patient (and the FDA). For more technical information, please see the Delcath website.
Delcath Systems applied for an NDA for their device on December 22, 2010 with a request for priority drug review. The data in it exceeded their primary endpoints as noted here: "The FDA told us what result they would want to see" to approve PHP – an average duration of hepatic progression-free survival of 7.7 months in PHP patients and an average 4.0-month survival in control patients, Dr. Nutting said in an interview. “The results exceeded that," he noted, with an average hepatic progression-free survival of 245 days (8.2 months) in the patients randomized to initial PHP and an average of 49 days (1.6 months) in control patients who received best alternative care. The hazard ratio for the PHP patients compared with the controls for this primary end point was 0.301 (P =.0001).”
The typical FDA NDA acceptance deadline for this type of application is 60 days, though it may take a little longer with their deadlines being somewhat later this previous year. This puts the NDA acceptance with priority review at sometime from February 22nd to the end of the month. If given priority review, we should hear back from the FDA for its decision on whether to approve the novel device sometime in June.
Not to be forgotten in all the attention Delcath is getting for its submission to the FDA here in the US, on December 6 they submitted their CE Mark Technical File for marketing in the European Union. Per their CEO, Eamonn Hobbs, they expect a decision for CE Mark approval sometime in mid 2011 also. June and July could be an exciting time for Delcath Systems!
2010 marked a year of substantial volatility of the DCTH stock with the stock trading from 5.1 to well over 16 dollars per share. Current market cap of the company per Google Finance is about $469 million, which could be considered low for a company with a device that if approved for its current application request for metastatic melanoma of the liver could be generating good revenue over the next few years.
However, many other trials are likely being planned for other application such as other liver cancers as well as cancers of other organs that may be isolated from the rest of the human body and treated with the Delcath system. This sort of current application as well as potential applications could very well be drawing attention from big Pharma as a merger or acquisition target. Actually, there were rumors back in October 2010 pertaining to Delcath being a takeover target for Bristol-Meyers (BMY).
While I don’t give much credit to these types of rumors, it is interesting to note that the stock rose over 12% on these rumors. Additionally, Jim Cramer mentioned on his February 3rd program’s “Lightning Round” pertaining to Delcath that “This is in the sweet spot. Companies like these are getting bought every day. I'm going to say that I like your stock.”
2011 looks to be a promising and exciting year for a company on the verge of greatness or on the verge of defeat. I need to review the phase III data a little more closely to give an opinion for FDA approval in June of this year. However, I have viewed it enough to give an opinion on the NDA acceptance with priority review. I believe Delcath Systems will have their NDA accepted sometime the last week of February of 2011 with the request for priority review. This will cast DCTH into the spotlight as opinions and rumors will circulate at a feverish pace pertaining to buyout, FDA approval and company worth.
Regardless of the FDA’s NDA acceptance, there is still the Mark CE decision also coming at about the same time as the FDA decision so there are two chances for this company to succeed and start marketing its novel device. Essentially, DCTH should see catalyst after catalyst after catalyst this year and most of it in the first half of the year. Investors in for the “long haul” have the potential for real gains meanwhile traders riding the dips and spikes will have an exciting time to say the least.
This brings me to one big question, why with all the potential here is the short interest in the stock so high?! With a short interest of almost 21% and over 13 days to cover, per this article, somebody’s going to be getting concerned fairly soon now. Will it be the “longs” or the “shorts” that will come out on top with DCTH? Hopefully, the ones coming out on top will be the patients and healthcare providers that need another weapon in their arsenal against an unforgivable enemy, cancer.
Good luck, place DCTH on your watch list and feel free to post your opinions on the company, its stock and its novel cancer treatment device.
Disclosure: I am long DCTH.
2 comments | by: Chemistfrog February 17, 2011 | about: DCTH Font Size: PrintEmail Recommend 1 Share this page
Share0 In the world of biotechnology and big pharmaceuticals there has always been a delicate balance of dosage amount to obtain the desired efficacy versus the side effects associated with the higher dosages.
One company with a very innovative means by which to have the best of both worlds is Delcath Systems (NASDAQ: DCTH). Delcath’s innovative device involves the use of melphalan hydrochloride to treat metastatic melanoma of the liver (melanoma that has spread to the liver). Melphalan is typically administered orally or intravenously for multiple myeloma, ovarian cancer and occasionally malignant melanoma. However, administered levels required to treat melanoma were not effective, and earlier investigations found other applications for the drug.
Their novel device is in the form of their device/drug combination termed a “chemosaturation system”. Their proprietary system, as best described in this video, basically involves isolating the organ’s (liver for this application) return blood flow to the body by inflatable balloons placed in the vena cava.
The mephalan hydrochloride solution is directly injected into the artery supplying blood to the liver (the hepatic artery) to deliver a dose up to 100 times more concentrated than traditional chemotherapy would have. Then the return blood is rerouted after treatment through a filter device to remove the mephalan hydrochloride, and then it is returned to the patient’s body via a catheter in the jugular.
After treatment, the catheters are simply removed, balloons deflated and the patient is prevented from having his entire biologic system have to deal with the side effects often associated with chemotherapy. The targeted organ containing the malignant melanoma meanwhile received a substantial dose thereby increasing efficacy while maintaining a safety profile more palatable to the patient (and the FDA). For more technical information, please see the Delcath website.
Delcath Systems applied for an NDA for their device on December 22, 2010 with a request for priority drug review. The data in it exceeded their primary endpoints as noted here: "The FDA told us what result they would want to see" to approve PHP – an average duration of hepatic progression-free survival of 7.7 months in PHP patients and an average 4.0-month survival in control patients, Dr. Nutting said in an interview. “The results exceeded that," he noted, with an average hepatic progression-free survival of 245 days (8.2 months) in the patients randomized to initial PHP and an average of 49 days (1.6 months) in control patients who received best alternative care. The hazard ratio for the PHP patients compared with the controls for this primary end point was 0.301 (P =.0001).”
The typical FDA NDA acceptance deadline for this type of application is 60 days, though it may take a little longer with their deadlines being somewhat later this previous year. This puts the NDA acceptance with priority review at sometime from February 22nd to the end of the month. If given priority review, we should hear back from the FDA for its decision on whether to approve the novel device sometime in June.
Not to be forgotten in all the attention Delcath is getting for its submission to the FDA here in the US, on December 6 they submitted their CE Mark Technical File for marketing in the European Union. Per their CEO, Eamonn Hobbs, they expect a decision for CE Mark approval sometime in mid 2011 also. June and July could be an exciting time for Delcath Systems!
2010 marked a year of substantial volatility of the DCTH stock with the stock trading from 5.1 to well over 16 dollars per share. Current market cap of the company per Google Finance is about $469 million, which could be considered low for a company with a device that if approved for its current application request for metastatic melanoma of the liver could be generating good revenue over the next few years.
However, many other trials are likely being planned for other application such as other liver cancers as well as cancers of other organs that may be isolated from the rest of the human body and treated with the Delcath system. This sort of current application as well as potential applications could very well be drawing attention from big Pharma as a merger or acquisition target. Actually, there were rumors back in October 2010 pertaining to Delcath being a takeover target for Bristol-Meyers (BMY).
While I don’t give much credit to these types of rumors, it is interesting to note that the stock rose over 12% on these rumors. Additionally, Jim Cramer mentioned on his February 3rd program’s “Lightning Round” pertaining to Delcath that “This is in the sweet spot. Companies like these are getting bought every day. I'm going to say that I like your stock.”
2011 looks to be a promising and exciting year for a company on the verge of greatness or on the verge of defeat. I need to review the phase III data a little more closely to give an opinion for FDA approval in June of this year. However, I have viewed it enough to give an opinion on the NDA acceptance with priority review. I believe Delcath Systems will have their NDA accepted sometime the last week of February of 2011 with the request for priority review. This will cast DCTH into the spotlight as opinions and rumors will circulate at a feverish pace pertaining to buyout, FDA approval and company worth.
Regardless of the FDA’s NDA acceptance, there is still the Mark CE decision also coming at about the same time as the FDA decision so there are two chances for this company to succeed and start marketing its novel device. Essentially, DCTH should see catalyst after catalyst after catalyst this year and most of it in the first half of the year. Investors in for the “long haul” have the potential for real gains meanwhile traders riding the dips and spikes will have an exciting time to say the least.
This brings me to one big question, why with all the potential here is the short interest in the stock so high?! With a short interest of almost 21% and over 13 days to cover, per this article, somebody’s going to be getting concerned fairly soon now. Will it be the “longs” or the “shorts” that will come out on top with DCTH? Hopefully, the ones coming out on top will be the patients and healthcare providers that need another weapon in their arsenal against an unforgivable enemy, cancer.
Good luck, place DCTH on your watch list and feel free to post your opinions on the company, its stock and its novel cancer treatment device.
Disclosure: I am long DCTH.
Antwort auf Beitrag Nr.: 41.059.316 von Magnetfeldfredy am 17.02.11 16:12:42Super News:
Delcath Achieves ISO 13485 Certification
ShareretweetEmailPrintCompanies elcath Systems, Inc. Related Quotes
elcath Systems, Inc. Related Quotes
Symbol Price Change
DCTH 11.05 +0.10
{"s" : "dcth","k" : "a00,a50,b00,b60,c10,g00,h00,l10,p20,t10,v00","o" : "","j" : ""} Press Release Source: Delcath Systems, Inc. On Thursday February 17, 2011, 9:55 am
NEW YORK, Feb. 17, 2011 /PRNewswire/ -- Delcath Systems, Inc. (Nasdaq CTH - News) today announced that the Company has achieved ISO 13485:2003 Certification—an internationally recognized quality standard designed to ensure that medical device manufacturers have the necessary comprehensive management systems in place to safely design, develop, manufacture and distribute medical devices in the European Union (EU). ISO 13485 Certification is a regulatory requirement of the EU's Medical Device Directive, and represents an important step toward attaining European CE Mark approval for the Company's proprietary Hepatic ChemoSAT™ Delivery System.
CTH - News) today announced that the Company has achieved ISO 13485:2003 Certification—an internationally recognized quality standard designed to ensure that medical device manufacturers have the necessary comprehensive management systems in place to safely design, develop, manufacture and distribute medical devices in the European Union (EU). ISO 13485 Certification is a regulatory requirement of the EU's Medical Device Directive, and represents an important step toward attaining European CE Mark approval for the Company's proprietary Hepatic ChemoSAT™ Delivery System.
Commenting on the announcement, Eamonn P. Hobbs, CEO & President of Delcath Systems, said, "ISO 13485 Certification confirms that our manufacturing and quality systems meet the high standards required of medical device companies selling into Europe, and we are pleased to have achieved this important milestone toward the receipt of CE Mark approval. Our technical file for CE Mark is pending, and we continue to expect CE Mark approval for our product in mid-2011."
About Delcath Systems
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Delcath's proprietary system for chemosaturation is designed to administer high dose chemotherapy and other therapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath concluded a Phase III metastatic melanoma study, and the Company recently completed a multi–arm Phase II trial to treat other liver cancers. The Company has not yet received FDA approval for commercial sale of its system. For more information, please visit the Company's website at www.delcath.com.
The Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward–looking statements made by the Company or on its behalf. This news release contains forward–looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to the acceptability of the Phase III clinical trial data by the FDA, acceptance of the Company's NDA by the FDA, acceptance of the Company's CE Mark Technical File by its Notified Body, receipt of CE Mark approval, adoption, use and resulting sales in the EU, if any, approval of the Company's NDA by the FDA or other regulatory authorities of the current or future drug delivery system for the treatment of metastatic melanoma, the potential of the chemosaturation system as a treatment for patients with terminal metastatic disease in the liver, actions by the FDA or other regulatory agencies, our ability to successfully enter into distribution and strategic partnership agreements in foreign markets and the corresponding revenue associated with such foreign markets, and uncertainties regarding our ability to obtain financial and other resources for any research, development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward–looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward–looking statements to reflect events or circumstances after the date they are made.
Follow Yahoo! Finance on Twitter; become a fan on Facebook.
Delcath Achieves ISO 13485 Certification
ShareretweetEmailPrintCompanies
 elcath Systems, Inc. Related Quotes
elcath Systems, Inc. Related QuotesSymbol Price Change
DCTH 11.05 +0.10
{"s" : "dcth","k" : "a00,a50,b00,b60,c10,g00,h00,l10,p20,t10,v00","o" : "","j" : ""} Press Release Source: Delcath Systems, Inc. On Thursday February 17, 2011, 9:55 am
NEW YORK, Feb. 17, 2011 /PRNewswire/ -- Delcath Systems, Inc. (Nasdaq
 CTH - News) today announced that the Company has achieved ISO 13485:2003 Certification—an internationally recognized quality standard designed to ensure that medical device manufacturers have the necessary comprehensive management systems in place to safely design, develop, manufacture and distribute medical devices in the European Union (EU). ISO 13485 Certification is a regulatory requirement of the EU's Medical Device Directive, and represents an important step toward attaining European CE Mark approval for the Company's proprietary Hepatic ChemoSAT™ Delivery System.
CTH - News) today announced that the Company has achieved ISO 13485:2003 Certification—an internationally recognized quality standard designed to ensure that medical device manufacturers have the necessary comprehensive management systems in place to safely design, develop, manufacture and distribute medical devices in the European Union (EU). ISO 13485 Certification is a regulatory requirement of the EU's Medical Device Directive, and represents an important step toward attaining European CE Mark approval for the Company's proprietary Hepatic ChemoSAT™ Delivery System.Commenting on the announcement, Eamonn P. Hobbs, CEO & President of Delcath Systems, said, "ISO 13485 Certification confirms that our manufacturing and quality systems meet the high standards required of medical device companies selling into Europe, and we are pleased to have achieved this important milestone toward the receipt of CE Mark approval. Our technical file for CE Mark is pending, and we continue to expect CE Mark approval for our product in mid-2011."

About Delcath Systems
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Delcath's proprietary system for chemosaturation is designed to administer high dose chemotherapy and other therapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath concluded a Phase III metastatic melanoma study, and the Company recently completed a multi–arm Phase II trial to treat other liver cancers. The Company has not yet received FDA approval for commercial sale of its system. For more information, please visit the Company's website at www.delcath.com.
The Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward–looking statements made by the Company or on its behalf. This news release contains forward–looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to the acceptability of the Phase III clinical trial data by the FDA, acceptance of the Company's NDA by the FDA, acceptance of the Company's CE Mark Technical File by its Notified Body, receipt of CE Mark approval, adoption, use and resulting sales in the EU, if any, approval of the Company's NDA by the FDA or other regulatory authorities of the current or future drug delivery system for the treatment of metastatic melanoma, the potential of the chemosaturation system as a treatment for patients with terminal metastatic disease in the liver, actions by the FDA or other regulatory agencies, our ability to successfully enter into distribution and strategic partnership agreements in foreign markets and the corresponding revenue associated with such foreign markets, and uncertainties regarding our ability to obtain financial and other resources for any research, development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward–looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward–looking statements to reflect events or circumstances after the date they are made.
Follow Yahoo! Finance on Twitter; become a fan on Facebook.
wie geht es hier jetzt weiter?
Langsamer Anstieg auf die $10,00?
Langsamer Anstieg auf die $10,00?
Antwort auf Beitrag Nr.: 41.276.242 von Poppholz am 28.03.11 13:46:09Erstmals müssen die den FDA Approval Antrag hinbekommen, der erste Versuch scheiterte ja jämmerlich!
Antwort auf Beitrag Nr.: 41.276.402 von Magnetfeldfredy am 28.03.11 14:17:30Wahnsinn, die FDA nimmt nicht mal den Zulassungsantrag an, die Europäer erteilen die Zulassung, Vervielfachungspotential!

Delcath Receives Notice of European Regulatory Approval for Hepatic CHEMOSAT Delivery System
Email
Print
..
Companies:
Delcath Systems, Inc.
.
Related Quotes
Symbol
Price
Change
DCTH
8.75
+1.49
Press Release Source: Delcath Systems, Inc. On Wednesday April 13, 2011, 12:26 pm
NEW YORK, April 13, 2011 /PRNewswire/ -- Delcath Systems, Inc. (NASDAQ CTH - News) today announced that the Company has been notified of CE Mark approval for its proprietary Hepatic CHEMOSAT™ Delivery System. The product has been approved with an indication for the percutaneous intra-arterial administration of a chemotherapeutic agent (melphalan hydrochloride) to the liver.
CTH - News) today announced that the Company has been notified of CE Mark approval for its proprietary Hepatic CHEMOSAT™ Delivery System. The product has been approved with an indication for the percutaneous intra-arterial administration of a chemotherapeutic agent (melphalan hydrochloride) to the liver.
CE Marking confirms that a medical device complies with the Essential Requirements of the Medical Device Directive, and that the device has been subjected to conformity assessment procedures. Receipt of the CE Mark allows Delcath to market and sell the product in countries in the European Economic Area.
"Receipt of our CE Mark represents the first regulatory approval for this product, and marks the beginning of the Company's transition into a fully commercial enterprise," said Eamonn P. Hobbs, CEO & President of Delcath Systems. "With its rising liver cancer rates, Europe represents a large opportunity for this product. We believe the Hepatic CHEMOSAT Delivery System may ultimately fulfill an annual unmet clinical need for as many as 100,000 liver cancer patients in this region. With the CE Mark in hand, we will now begin to build inventory and establish the commercialization infrastructure in Europe, including assembling a direct sales organization to cover countries in Northern Europe and establishing a network of third party distributors in Southern Europe. We will also begin establishing and training initial sites in select European countries as Centers of Clinical Excellence for the chemosaturation procedure."
About Delcath Systems
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Delcath's proprietary system for chemosaturation is designed to administer high dose chemotherapy and other therapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath concluded a Phase III metastatic melanoma study, and the Company recently completed a multi–arm Phase II trial to treat other liver cancers. The Company has not yet received FDA approval for commercial sale of its system. For more information, please visit the Company's website at www.delcath.com.
The Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward–looking statements made by the Company or on its behalf. This news release contains forward–looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to the time required to build inventory and establish commercial operations in Europe, adoption, use and resulting sales for the Hepatic CHEMOSAT delivery system in the EEA, if any, our ability to successfully commercialize the chemosaturation system and the potential of the chemosaturation system as a treatment for patients with terminal metastatic disease in the liver, acceptability of the Phase III clinical trial data by the FDA, our ability to address the issues raised in the Refusal to File letter received from the FDA and the timing of our re-submission of our NDA, re-submission and acceptance of the Company's NDA by the FDA, approval of the Company's NDA for the treatment of metastatic melanoma to the liver, adoption, use and resulting sales in the United States, if any, approval of the current or future chemosaturation system for other indications, actions by the FDA or other foreign regulatory agencies, our ability to successfully enter into distribution and strategic partnership agreements in foreign markets and the corresponding revenue associated with such foreign markets, and uncertainties regarding our ability to obtain financial and other resources for any research, development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward–looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward–looking statements to reflect events or circumstances after the date they are made


Delcath Receives Notice of European Regulatory Approval for Hepatic CHEMOSAT Delivery System
..
Companies:
Delcath Systems, Inc.
.
Related Quotes
Symbol
Price
Change
DCTH
8.75
+1.49
Press Release Source: Delcath Systems, Inc. On Wednesday April 13, 2011, 12:26 pm
NEW YORK, April 13, 2011 /PRNewswire/ -- Delcath Systems, Inc. (NASDAQ
 CTH - News) today announced that the Company has been notified of CE Mark approval for its proprietary Hepatic CHEMOSAT™ Delivery System. The product has been approved with an indication for the percutaneous intra-arterial administration of a chemotherapeutic agent (melphalan hydrochloride) to the liver.
CTH - News) today announced that the Company has been notified of CE Mark approval for its proprietary Hepatic CHEMOSAT™ Delivery System. The product has been approved with an indication for the percutaneous intra-arterial administration of a chemotherapeutic agent (melphalan hydrochloride) to the liver.CE Marking confirms that a medical device complies with the Essential Requirements of the Medical Device Directive, and that the device has been subjected to conformity assessment procedures. Receipt of the CE Mark allows Delcath to market and sell the product in countries in the European Economic Area.
"Receipt of our CE Mark represents the first regulatory approval for this product, and marks the beginning of the Company's transition into a fully commercial enterprise," said Eamonn P. Hobbs, CEO & President of Delcath Systems. "With its rising liver cancer rates, Europe represents a large opportunity for this product. We believe the Hepatic CHEMOSAT Delivery System may ultimately fulfill an annual unmet clinical need for as many as 100,000 liver cancer patients in this region. With the CE Mark in hand, we will now begin to build inventory and establish the commercialization infrastructure in Europe, including assembling a direct sales organization to cover countries in Northern Europe and establishing a network of third party distributors in Southern Europe. We will also begin establishing and training initial sites in select European countries as Centers of Clinical Excellence for the chemosaturation procedure."
About Delcath Systems
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Delcath's proprietary system for chemosaturation is designed to administer high dose chemotherapy and other therapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath concluded a Phase III metastatic melanoma study, and the Company recently completed a multi–arm Phase II trial to treat other liver cancers. The Company has not yet received FDA approval for commercial sale of its system. For more information, please visit the Company's website at www.delcath.com.
The Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward–looking statements made by the Company or on its behalf. This news release contains forward–looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to the time required to build inventory and establish commercial operations in Europe, adoption, use and resulting sales for the Hepatic CHEMOSAT delivery system in the EEA, if any, our ability to successfully commercialize the chemosaturation system and the potential of the chemosaturation system as a treatment for patients with terminal metastatic disease in the liver, acceptability of the Phase III clinical trial data by the FDA, our ability to address the issues raised in the Refusal to File letter received from the FDA and the timing of our re-submission of our NDA, re-submission and acceptance of the Company's NDA by the FDA, approval of the Company's NDA for the treatment of metastatic melanoma to the liver, adoption, use and resulting sales in the United States, if any, approval of the current or future chemosaturation system for other indications, actions by the FDA or other foreign regulatory agencies, our ability to successfully enter into distribution and strategic partnership agreements in foreign markets and the corresponding revenue associated with such foreign markets, and uncertainties regarding our ability to obtain financial and other resources for any research, development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward–looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward–looking statements to reflect events or circumstances after the date they are made
Antwort auf Beitrag Nr.: 41.276.402 von Magnetfeldfredy am 28.03.11 14:17:30werden die das hinbekommen?
nach der eu zulassung hört es sich erstmal nach einer formsache an.
nach der eu zulassung hört es sich erstmal nach einer formsache an.

Antwort auf Beitrag Nr.: 41.495.303 von GuHu1 am 12.05.11 22:50:04Eigentlich schon, aber wir Dendronen wissen ja bestens was man mit der FDA alles erleben kann!

Delcath Announces Fiscal 2011 Third Quarter Results and Recent Developments
Quarterly Investor Call to be Held Monday November 7, 2011 at 4:30pm ET--
NEW YORK, Nov. 7, 2011 /PRNewswire via COMTEX/ --
Delcath Systems (NASDAQ: DCTH) today reported financial results for the fiscal 2011 third quarter ended September 30, 2011. Highlights for the quarter and recent developments include:
Research & Development: Accelerated development of Generation Two of the Delcath Hepatic CHEMOSAT® Delivery system, which has demonstrated significantly higher filtration efficiency of melphalan in pre-clinical testing compared to Generation One of the system.
European Commercialization: Submission of CE Mark application for high-efficiency filter of Generation Two CHEMOSAT system, and earlier than anticipated availability of Generation Two for initial launch in European markets in early Q1 2012, subject to CE Mark approval.
Establishment of European Business Entity & Headquarters: Formation of Delcath Systems Ltd. (Delcath Limited), an Irish company headquartered in Galway, Ireland, under which Delcath will conduct its European operations. Delcath Limited received a development grant from IDA Ireland to support the hiring of staff over the next three years.
U.S. Regulatory: Pre-New Drug Application (NDA) meeting scheduled with U.S. Food and Drug Administration (FDA) for mid-January 2012.
International Regulatory: Completion of product notification process for CHEMOSAT with the Medicines and Medical Device Safety Authority in New Zealand, and submission of applications to obtain regulatory approval for CHEMOSAT for several other markets including Australia, Singapore and Hong Kong. The Company also submitted an application to obtain European CE Mark approval for the Generation Two high efficiency filter.
Clinical Trial Data Update: Updated efficacy results from the Company's Phase 3 trial showed the potential for CHEMOSAT as a promising treatment option for patients with metastatic melanoma in the liver, and were presented at the European Multidisciplinary Cancer Congress; Positive Phase 2 trial results from the neuroendocrine tumor cohort showing a 70% overall response rate were presented at the Cardiovascular and Interventional Radiological Society of Europe conference; announced encouraging top-line results for the hepatobiliary cohort and top-line results for metastatic colorectal cohort of the Phase 2 trial.
Leadership Team Expansion: Addition of Graham G. Miao, M.S, MBA, Ph.D., as Executive Vice President, Chief Financial Officer; appointment of David McDonald, to newly created role of Executive Vice President, Business Development
Common Stock Offering: Successfully completed the sale of 5,000,000 shares of common stock in July 2011 for $23.6 million in net proceeds.
"Our company had a productive third quarter, with progress made in several areas toward commercialization of our CHEMOSAT system," said Eamonn P. Hobbs, President and CEO of Delcath. "While some of our goals have yet to be achieved, we are pleased that we will be able to meet emerging interest in CHEMOSAT with faster than expected development of our Generation Two version of the system, which we believe will not only improve filtration efficiency, but potentially lead to new therapeutic possibilities as well. Along with the positive clinical data released during the quarter, these and other developments have positioned us well to begin realizing the potential of the CHEMOSAT system in 2012."
For the three months ended September 30, 2011, the Company's operating loss was $12.2 million, which included approximately $900,000 in non-cash stock-based compensation expense. This compares to an operating loss for the same period in the prior year of $7.4 million, which included approximately $1.4 million in non-cash stock-based compensation expense. General and administrative (G&A) expenses were $5.7 million for the third quarter of 2011, compared to $3.2 million for the same period in the prior year. The increase in G&A was primarily due to an expansion in staff as the Company continued its progress in transitioning from a development stage company to a commercial enterprise and preparations for commercialization in Europe. Research and development (R&D) expenses were $6.4 million for the third quarter of 2011, compared to $4.3 million for the same period in the prior year. The increase in R&D expenses was primarily due to our expanded research and development activities and regulatory expenses related to the preparation of our NDA submission for the FDA.
For the nine months ended September 30, 2011, the Company's operating loss was $30.5 million, which included approximately $3.4 million in non-cash stock-based compensation expense. This compares to an operating loss for the nine months ended September 30, 2010 of $21.2 million, which included approximately $3.9 million in non-cash stock-based compensation expense. G&A expenses were $15.1 million for the nine months ended September 30, 2011, compared to $9.4 million for the nine months ended September 30, 2010. The increase in G&A was primarily due to an expansion in staff as the Company continued its progress in transitioning from a development stage company to a commercial enterprise and preparations for commercialization in Europe. R&D expenses were $15.3 million for the nine months ended September 30, 2011, compared to $11.8 million during the first nine months of 2010. During 2010, the Company was incurring expenses related to wrapping up its Phase III clinical trial. The reduction in trial related expenses during 2011 was more than offset by an increase in expenses related to our expanded research and development activities and regulatory expenses related to our submission to the FDA.
At September 30, 2011, cash, cash equivalents and certificates of deposit were $44.7 million, as compared to $54.3 million at September 30, 2010.
Conference Call and Webcast
The Company will host a conference call today, November 7, 2011 at 4:30 p.m. ET, to discuss its recent corporate developments and update its progress. Eamonn Hobbs, President and Chief Executive Officer, will host the call. To participate in the live call by telephone, please dial 800-322-5044 for domestic participants and 617-614-4927 for international participants, both using passcode 65515511. Participants are asked to call the above numbers 5-10 minutes prior to the starting time. To access the live webcast of the call, go to Delcath's website at www.delcath.com.
In addition, a taped replay of the conference call will also be available beginning approximately two hours after the call's conclusion and will be available for seven days. This replay can be accessed by dialing 888-286-8010 for domestic callers and 617-801-6888 for international callers, both using passcode 65413528. An archived webcast will also be available at www.delcath.com.
About Delcath Systems
Delcath Systems, Inc. is a development stage specialty pharmaceutical and medical device company focused on oncology. Delcath's proprietary system for chemosaturation is designed to administer high dose chemotherapy and other therapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath concluded a Phase 3 metastatic melanoma study, and the Company recently completed a multi-arm Phase 2 trial to treat other liver cancers. The Company obtained authorization to affix a CE Mark for the Delcath Hepatic CHEMOSAT® delivery system in April 2011. The Company has not yet received FDA approval for commercial sale of its system in the United States. For more information, please visit the Company's website at http://www.delcath.com/.
Quarterly Investor Call to be Held Monday November 7, 2011 at 4:30pm ET--
NEW YORK, Nov. 7, 2011 /PRNewswire via COMTEX/ --
Delcath Systems (NASDAQ: DCTH) today reported financial results for the fiscal 2011 third quarter ended September 30, 2011. Highlights for the quarter and recent developments include:
Research & Development: Accelerated development of Generation Two of the Delcath Hepatic CHEMOSAT® Delivery system, which has demonstrated significantly higher filtration efficiency of melphalan in pre-clinical testing compared to Generation One of the system.
European Commercialization: Submission of CE Mark application for high-efficiency filter of Generation Two CHEMOSAT system, and earlier than anticipated availability of Generation Two for initial launch in European markets in early Q1 2012, subject to CE Mark approval.
Establishment of European Business Entity & Headquarters: Formation of Delcath Systems Ltd. (Delcath Limited), an Irish company headquartered in Galway, Ireland, under which Delcath will conduct its European operations. Delcath Limited received a development grant from IDA Ireland to support the hiring of staff over the next three years.
U.S. Regulatory: Pre-New Drug Application (NDA) meeting scheduled with U.S. Food and Drug Administration (FDA) for mid-January 2012.
International Regulatory: Completion of product notification process for CHEMOSAT with the Medicines and Medical Device Safety Authority in New Zealand, and submission of applications to obtain regulatory approval for CHEMOSAT for several other markets including Australia, Singapore and Hong Kong. The Company also submitted an application to obtain European CE Mark approval for the Generation Two high efficiency filter.
Clinical Trial Data Update: Updated efficacy results from the Company's Phase 3 trial showed the potential for CHEMOSAT as a promising treatment option for patients with metastatic melanoma in the liver, and were presented at the European Multidisciplinary Cancer Congress; Positive Phase 2 trial results from the neuroendocrine tumor cohort showing a 70% overall response rate were presented at the Cardiovascular and Interventional Radiological Society of Europe conference; announced encouraging top-line results for the hepatobiliary cohort and top-line results for metastatic colorectal cohort of the Phase 2 trial.
Leadership Team Expansion: Addition of Graham G. Miao, M.S, MBA, Ph.D., as Executive Vice President, Chief Financial Officer; appointment of David McDonald, to newly created role of Executive Vice President, Business Development
Common Stock Offering: Successfully completed the sale of 5,000,000 shares of common stock in July 2011 for $23.6 million in net proceeds.
"Our company had a productive third quarter, with progress made in several areas toward commercialization of our CHEMOSAT system," said Eamonn P. Hobbs, President and CEO of Delcath. "While some of our goals have yet to be achieved, we are pleased that we will be able to meet emerging interest in CHEMOSAT with faster than expected development of our Generation Two version of the system, which we believe will not only improve filtration efficiency, but potentially lead to new therapeutic possibilities as well. Along with the positive clinical data released during the quarter, these and other developments have positioned us well to begin realizing the potential of the CHEMOSAT system in 2012."
For the three months ended September 30, 2011, the Company's operating loss was $12.2 million, which included approximately $900,000 in non-cash stock-based compensation expense. This compares to an operating loss for the same period in the prior year of $7.4 million, which included approximately $1.4 million in non-cash stock-based compensation expense. General and administrative (G&A) expenses were $5.7 million for the third quarter of 2011, compared to $3.2 million for the same period in the prior year. The increase in G&A was primarily due to an expansion in staff as the Company continued its progress in transitioning from a development stage company to a commercial enterprise and preparations for commercialization in Europe. Research and development (R&D) expenses were $6.4 million for the third quarter of 2011, compared to $4.3 million for the same period in the prior year. The increase in R&D expenses was primarily due to our expanded research and development activities and regulatory expenses related to the preparation of our NDA submission for the FDA.
For the nine months ended September 30, 2011, the Company's operating loss was $30.5 million, which included approximately $3.4 million in non-cash stock-based compensation expense. This compares to an operating loss for the nine months ended September 30, 2010 of $21.2 million, which included approximately $3.9 million in non-cash stock-based compensation expense. G&A expenses were $15.1 million for the nine months ended September 30, 2011, compared to $9.4 million for the nine months ended September 30, 2010. The increase in G&A was primarily due to an expansion in staff as the Company continued its progress in transitioning from a development stage company to a commercial enterprise and preparations for commercialization in Europe. R&D expenses were $15.3 million for the nine months ended September 30, 2011, compared to $11.8 million during the first nine months of 2010. During 2010, the Company was incurring expenses related to wrapping up its Phase III clinical trial. The reduction in trial related expenses during 2011 was more than offset by an increase in expenses related to our expanded research and development activities and regulatory expenses related to our submission to the FDA.
At September 30, 2011, cash, cash equivalents and certificates of deposit were $44.7 million, as compared to $54.3 million at September 30, 2010.
Conference Call and Webcast
The Company will host a conference call today, November 7, 2011 at 4:30 p.m. ET, to discuss its recent corporate developments and update its progress. Eamonn Hobbs, President and Chief Executive Officer, will host the call. To participate in the live call by telephone, please dial 800-322-5044 for domestic participants and 617-614-4927 for international participants, both using passcode 65515511. Participants are asked to call the above numbers 5-10 minutes prior to the starting time. To access the live webcast of the call, go to Delcath's website at www.delcath.com.
In addition, a taped replay of the conference call will also be available beginning approximately two hours after the call's conclusion and will be available for seven days. This replay can be accessed by dialing 888-286-8010 for domestic callers and 617-801-6888 for international callers, both using passcode 65413528. An archived webcast will also be available at www.delcath.com.
About Delcath Systems
Delcath Systems, Inc. is a development stage specialty pharmaceutical and medical device company focused on oncology. Delcath's proprietary system for chemosaturation is designed to administer high dose chemotherapy and other therapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath concluded a Phase 3 metastatic melanoma study, and the Company recently completed a multi-arm Phase 2 trial to treat other liver cancers. The Company obtained authorization to affix a CE Mark for the Delcath Hepatic CHEMOSAT® delivery system in April 2011. The Company has not yet received FDA approval for commercial sale of its system in the United States. For more information, please visit the Company's website at http://www.delcath.com/.
NEW YORK, Dec. 21, 2011 /PRNewswire/ -- Delcath Systems, Inc. (NASDAQ: DCTH) appointed Gregory Gores, M.D. to the Company's Medical Advisory Board.
"As a widely respected hepatologist and 2011 President of the International Liver Cancer Association (ILCA), Dr. Gores will contribute a wealth of clinical knowledge on hepatotoxicity and drug induced liver pathology to our Medical Advisory Board," said Eamonn P. Hobbs, CEO & President of Delcath Systems. "His insight will help provide valuable support to the commercialization of the Delcath Hepatic CHEMOSAT® Delivery System in Europe and the rest of the world, as well as to our regulatory process in the United States."
Dr. Gores is the Reuben R. Eisenberg Endowed Professor in Gastroenterology and Hepatology, professor of Medicine, and chair of the Division of Gastroenterology and Hepatology at the Mayo Clinic in Rochester, Minnesota. His research is focused on the fundamental mechanisms underpinning cell death in the liver, employing models relevant to human disease. He has published more than 400 original articles, chapters, reviews, and editorials. Dr. Gores serves on the editorial boards for the American Journal of Physiology, American Journal of Gastroenterology, and Nature Reviews in Clinical Gastroenterology and Hepatology and is a past Associate Editor for Hepatology.
Dr. Gores has served as a standing member for two NIH Study Sections and recently chaired the Hepatobiliary Pathobiology Study Section. He has served on the Grants Review Committee for the American Liver Foundation. Dr. Gores is a past president of the American Association for the Study of Liver Diseases and has participated in many activities and committees of this organization. He has been elected into the honorific societies of the American Society for Clinical Investigation and the American Association of Physicians. He is a Mayo Distinguished Investigator.
"The efficacy results of Delcath's Phase 3 trial are impressive," said Dr. Gores. "I am excited to be joining Delcath's Medical Advisory Board. The Hepatic CHEMOSAT Delivery System provides a minimally invasive, repeatable means for delivering high-dose chemotherapy to the liver and has the potential to complement existing systemic therapies that often fail to adequately treat primary or metastatic liver tumors. This is an innovative technology, and I look forward to contributing to its clinical and commercial development."
About Delcath Systems
Delcath Systems, Inc. is a development stage specialty pharmaceutical and medical device company focused on oncology. Delcath's proprietary system for chemosaturation is designed to administer high dose chemotherapy and other chemotherapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath concluded a Phase III metastatic melanoma study, and the Company recently completed a multi-arm Phase II trial to treat other liver cancers. The Company obtained authorization to affix a CE Mark for the Hepatic CHEMOSAT Delivery System in April 2011. The Company has not yet received FDA approval for commercial sale of its system in the United States. For more information, please visit the Company's website at http://www.delcath.com/.
"As a widely respected hepatologist and 2011 President of the International Liver Cancer Association (ILCA), Dr. Gores will contribute a wealth of clinical knowledge on hepatotoxicity and drug induced liver pathology to our Medical Advisory Board," said Eamonn P. Hobbs, CEO & President of Delcath Systems. "His insight will help provide valuable support to the commercialization of the Delcath Hepatic CHEMOSAT® Delivery System in Europe and the rest of the world, as well as to our regulatory process in the United States."
Dr. Gores is the Reuben R. Eisenberg Endowed Professor in Gastroenterology and Hepatology, professor of Medicine, and chair of the Division of Gastroenterology and Hepatology at the Mayo Clinic in Rochester, Minnesota. His research is focused on the fundamental mechanisms underpinning cell death in the liver, employing models relevant to human disease. He has published more than 400 original articles, chapters, reviews, and editorials. Dr. Gores serves on the editorial boards for the American Journal of Physiology, American Journal of Gastroenterology, and Nature Reviews in Clinical Gastroenterology and Hepatology and is a past Associate Editor for Hepatology.
Dr. Gores has served as a standing member for two NIH Study Sections and recently chaired the Hepatobiliary Pathobiology Study Section. He has served on the Grants Review Committee for the American Liver Foundation. Dr. Gores is a past president of the American Association for the Study of Liver Diseases and has participated in many activities and committees of this organization. He has been elected into the honorific societies of the American Society for Clinical Investigation and the American Association of Physicians. He is a Mayo Distinguished Investigator.
"The efficacy results of Delcath's Phase 3 trial are impressive," said Dr. Gores. "I am excited to be joining Delcath's Medical Advisory Board. The Hepatic CHEMOSAT Delivery System provides a minimally invasive, repeatable means for delivering high-dose chemotherapy to the liver and has the potential to complement existing systemic therapies that often fail to adequately treat primary or metastatic liver tumors. This is an innovative technology, and I look forward to contributing to its clinical and commercial development."
About Delcath Systems
Delcath Systems, Inc. is a development stage specialty pharmaceutical and medical device company focused on oncology. Delcath's proprietary system for chemosaturation is designed to administer high dose chemotherapy and other chemotherapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath concluded a Phase III metastatic melanoma study, and the Company recently completed a multi-arm Phase II trial to treat other liver cancers. The Company obtained authorization to affix a CE Mark for the Hepatic CHEMOSAT Delivery System in April 2011. The Company has not yet received FDA approval for commercial sale of its system in the United States. For more information, please visit the Company's website at http://www.delcath.com/.
Agreement with Premier Cancer Center Marks Commercial Launch in Germany
NEW YORK, Dec. 27, 2011 /PRNewswire/ -- Delcath Systems, Inc. (NASDAQ: DCTH) announced today that the Company has entered into an initial launch and training agreement for the Delcath Hepatic CHEMOSAT® Delivery system with Johann Wolfgang Goethe University Hospital (J.W. Goethe), a premier European cancer treatment and research center located in Frankfurt, Germany. Under the terms of the agreement, the Company will provide J.W. Goethe with logistics and clinical training support in the performance of chemosaturation therapy using the CHEMOSAT system. The Company expects to conduct the training using the Generation Two version of the CHEMOSAT system, pending CE Mark approval, and for training to begin at the J.W. Goethe University Hospital in February 2012.
University Professor Dr. Thomas J. Vogl, Director of the Institute for Diagnostic and Interventional Radiology at J.W. Goethe, said, "Our team is excited to bring use of the CHEMOSAT system to Germany. Clinical research suggests that chemosaturation therapy using the CHEMOSAT system will offer us a clinically significant tool in treating liver metastases with melphalan. We're eager to begin providing therapy to our patients and exploring the additional potential benefits that the newest generation product from Delcath provides."
"This agreement marks another key milestone in the execution of our commercialization strategy for the CHEMOSAT system in the European Union," said Eamonn P. Hobbs, President and CEO of Delcath Systems. "Germany is the largest market in the EU and we are excited to be entering it with a partner as prestigious as J.W. Goethe. CHEMOSAT will be available to patients in Germany soon, and this latest agreement further positions us to begin realizing the system's potential throughout Europe in 2012."
About the Johann Wolfgang Goethe University Hospital
Founded in 1914, J.W. Goethe University Hospital is considered to be one of the leading university hospitals in Germany. Twenty-five research institutes working in close cooperation with the Medical Department bear witness to the hospital's strong academic approach. This makes sure that patients coming to the University Hospital for treatment enjoy the benefits resulting from timely implementation of research findings. Every year, around 47,200 and 220,000 patients respectively receive in- and outpatient treatment. The University Hospital possesses special interdisciplinary competence in the fields of neurological science, oncology and cardiovascular medicine.
NEW YORK, Dec. 27, 2011 /PRNewswire/ -- Delcath Systems, Inc. (NASDAQ: DCTH) announced today that the Company has entered into an initial launch and training agreement for the Delcath Hepatic CHEMOSAT® Delivery system with Johann Wolfgang Goethe University Hospital (J.W. Goethe), a premier European cancer treatment and research center located in Frankfurt, Germany. Under the terms of the agreement, the Company will provide J.W. Goethe with logistics and clinical training support in the performance of chemosaturation therapy using the CHEMOSAT system. The Company expects to conduct the training using the Generation Two version of the CHEMOSAT system, pending CE Mark approval, and for training to begin at the J.W. Goethe University Hospital in February 2012.
University Professor Dr. Thomas J. Vogl, Director of the Institute for Diagnostic and Interventional Radiology at J.W. Goethe, said, "Our team is excited to bring use of the CHEMOSAT system to Germany. Clinical research suggests that chemosaturation therapy using the CHEMOSAT system will offer us a clinically significant tool in treating liver metastases with melphalan. We're eager to begin providing therapy to our patients and exploring the additional potential benefits that the newest generation product from Delcath provides."
"This agreement marks another key milestone in the execution of our commercialization strategy for the CHEMOSAT system in the European Union," said Eamonn P. Hobbs, President and CEO of Delcath Systems. "Germany is the largest market in the EU and we are excited to be entering it with a partner as prestigious as J.W. Goethe. CHEMOSAT will be available to patients in Germany soon, and this latest agreement further positions us to begin realizing the system's potential throughout Europe in 2012."
About the Johann Wolfgang Goethe University Hospital
Founded in 1914, J.W. Goethe University Hospital is considered to be one of the leading university hospitals in Germany. Twenty-five research institutes working in close cooperation with the Medical Department bear witness to the hospital's strong academic approach. This makes sure that patients coming to the University Hospital for treatment enjoy the benefits resulting from timely implementation of research findings. Every year, around 47,200 and 220,000 patients respectively receive in- and outpatient treatment. The University Hospital possesses special interdisciplinary competence in the fields of neurological science, oncology and cardiovascular medicine.
NEW YORK, Dec. 28, 2011 /PRNewswire/ --Delcath Systems, Inc. (NASDAQ: DCTH) today announced that the Company's Galway, Ireland location has achieved ISO 13485:2003 Certification—an internationally recognized quality standard designed to ensure that medical device manufacturers have the necessary comprehensive quality management systems in place to safely design, develop, manufacture and distribute medical devices in the European Union (EU). ISO 13485 Certification is a regulatory requirement of the EU's Medical Device Directive, and represents an important step toward commercialization of the Delcath Hepatic CHEMOSAT® Delivery System following its European CE Mark approval in April 2011.
"ISO 13485 Certification of our Galway facility confirms that our manufacturing and quality systems meet the high standards required of medical device companies selling into Europe," said Eamonn P. Hobbs, CEO & President of Delcath Systems. "This achievement represents one more important milestone toward commercialization of CHEMOSAT in the EU, and we are looking forward to a successful initial launch of the product early next year."
About Delcath Systems
Delcath Systems, Inc. is a development stage specialty pharmaceutical and medical device company focused on oncology. Delcath's proprietary system for chemosaturation is designed to administer high dose chemotherapy and other chemotherapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath concluded a Phase III metastatic melanoma study, and the Company recently completed a multi-arm Phase II trial to treat other liver cancers. The Company obtained authorization to affix a CE Mark for the Hepatic CHEMOSAT Delivery System in April 2011. The Company has not yet received FDA approval for commercial sale of its system in the United States. For more information, please visit the Company's website at http://www.delcath.com/.
"ISO 13485 Certification of our Galway facility confirms that our manufacturing and quality systems meet the high standards required of medical device companies selling into Europe," said Eamonn P. Hobbs, CEO & President of Delcath Systems. "This achievement represents one more important milestone toward commercialization of CHEMOSAT in the EU, and we are looking forward to a successful initial launch of the product early next year."
About Delcath Systems
Delcath Systems, Inc. is a development stage specialty pharmaceutical and medical device company focused on oncology. Delcath's proprietary system for chemosaturation is designed to administer high dose chemotherapy and other chemotherapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath concluded a Phase III metastatic melanoma study, and the Company recently completed a multi-arm Phase II trial to treat other liver cancers. The Company obtained authorization to affix a CE Mark for the Hepatic CHEMOSAT Delivery System in April 2011. The Company has not yet received FDA approval for commercial sale of its system in the United States. For more information, please visit the Company's website at http://www.delcath.com/.
So mal 3 nette Neuigkeiten von Delcath... der Kurs hat schon entsprechend reagiert 

Delcath (DCTH) to Continue with NDA Filing Following FDA Meeting
January 13, 2012 8:15 AM EST
On January 12, 2012, Delcath Systems, Inc. (Nasdaq: DCTH) met with the United States Food & Drug Administration (FDA) to conduct the scheduled pre-New Drug Application (NDA) meeting.
Based upon the meeting and FDA correspondence received in response to our meeting request and the briefing packet we submitted, we are satisfied with the responses that we received from the FDA to certain questions we had regarding the NDA submission.
Accordingly, we will continue with the preparation of our NDA submission as planned and expect to make the submission in the second quarter of 2012.

January 13, 2012 8:15 AM EST
On January 12, 2012, Delcath Systems, Inc. (Nasdaq: DCTH) met with the United States Food & Drug Administration (FDA) to conduct the scheduled pre-New Drug Application (NDA) meeting.
Based upon the meeting and FDA correspondence received in response to our meeting request and the briefing packet we submitted, we are satisfied with the responses that we received from the FDA to certain questions we had regarding the NDA submission.
Accordingly, we will continue with the preparation of our NDA submission as planned and expect to make the submission in the second quarter of 2012.

NEW YORK, Feb. 16, 2012 /PRNewswire/ -- Delcath Systems, Inc. (NASDAQ: DCTH) announced today that the Company has entered into an initial launch and training agreement for the Delcath Hepatic CHEMOSAT® Delivery system with University Medical Center Schleswig-Holstein (Kiel Campus), one of the largest European centers for medical care and the second cancer center in Germany to commercially utilize the CHEMOSAT system to treat patients. Under the terms of the agreement, the Company will provide the University Medical Center Schleswig-Holstein with logistics and clinical training support in the performance of chemosaturation therapy using the CHEMOSAT system. Training at the University Medical Center Schleswig-Holstein is expected to begin in April 2012.
Prof. Dr. med. Thomas Becker, Director of the Department of General and Thoracic Surgery, said, "I am excited that our medical center will begin using Delcath's CHEMOSAT system to treat cancers in the liver. Gaining experience with this device and procedure is in line with our international reputation for leadership in medical care as well as excellent research results."
Axel Hauschild, MD, Professor of Dermatology in the Department of Dermatology, said, "Our team is committed to translating novel scientific developments and findings into new options for therapies. Clinical research suggests that CHEMOSAT will offer us a clinically significant tool in treating melanoma liver metastases with melphalan, and we're eager to begin providing therapy to our patients."
"We are proud of the addition of University Medical Center Schleswig-Holstein to the growing number of European sites providing therapy with the CHEMOSAT system," said Eamonn P. Hobbs, President and CEO of Delcath Systems. "This second clinical site in Germany not only expands the access in the largest market in Europe, but provides further validation that our years of research and development have produced a product that's attracting the interest of Europe's best cancer centers."
About the University Medical Center Schleswig-Holstein
The University Medical Center Schleswig-Holstein is one of the largest European centers for medical care. As the only maximum care provider in Schleswig-Holstein it covers the entire spectrum of modern medical and health care. At the University Medical Center Schleswig-Holstein, 2,000 physicians, scientists and researchers, and 3,600 health staff treat over 360,000 inpatients and outpatients in 80 clinics and institutes.
SOURCE Delcath Systems, Inc.
Prof. Dr. med. Thomas Becker, Director of the Department of General and Thoracic Surgery, said, "I am excited that our medical center will begin using Delcath's CHEMOSAT system to treat cancers in the liver. Gaining experience with this device and procedure is in line with our international reputation for leadership in medical care as well as excellent research results."
Axel Hauschild, MD, Professor of Dermatology in the Department of Dermatology, said, "Our team is committed to translating novel scientific developments and findings into new options for therapies. Clinical research suggests that CHEMOSAT will offer us a clinically significant tool in treating melanoma liver metastases with melphalan, and we're eager to begin providing therapy to our patients."
"We are proud of the addition of University Medical Center Schleswig-Holstein to the growing number of European sites providing therapy with the CHEMOSAT system," said Eamonn P. Hobbs, President and CEO of Delcath Systems. "This second clinical site in Germany not only expands the access in the largest market in Europe, but provides further validation that our years of research and development have produced a product that's attracting the interest of Europe's best cancer centers."
About the University Medical Center Schleswig-Holstein
The University Medical Center Schleswig-Holstein is one of the largest European centers for medical care. As the only maximum care provider in Schleswig-Holstein it covers the entire spectrum of modern medical and health care. At the University Medical Center Schleswig-Holstein, 2,000 physicians, scientists and researchers, and 3,600 health staff treat over 360,000 inpatients and outpatients in 80 clinics and institutes.
SOURCE Delcath Systems, Inc.
Johann Wolfgang Goethe University Hospital Becomes Second Training Center in Europe to Treat Patients with Delcath Hepatic CHEMOSAT® Delivery System
NEW YORK, Feb. 27, 2012 /PRNewswire/ -- Delcath Systems, Inc., (NASDAQ: DCTH) announced today that the first patients in Germany have been treated with the Delcath Hepatic CHEMOSAT® Delivery System at the Johann Wolfgang Goethe University Hospital, a premier European cancer treatment and research center located in Frankfurt. The cases were treated as part of the initial launch and training agreement the Company announced with the hospital in December 2011.
Two patients were treated for inoperable, liver-dominant metastases, one from cutaneous melanoma and one from breast cancer. The treating physicians reported that both patients were treated successfully without procedure-related complications.
Dr. Thomas J. Vogl, Director of the Institute for Diagnostic and Interventional Radiology at J.W. Goethe, said, "We believe this technology has significant potential to help control cancers in the liver. We're pleased to be the first cancer center to begin offering this important treatment option to patients in Germany, and are eager to further explore its role in the treatment of multiple tumor types including breast cancer."
"Delcath's partnership with J.W. Goethe reinforces the potential of CHEMOSAT," said Eamonn P. Hobbs, president and CEO of Delcath. "We recently treated our first patients in Milan and are eager to continue our expansion across Europe. Opening another CHEMOSAT treatment center and treating patients in the continent's largest market is another step forward in the commercialization of this technology."
About the Johann Wolfgang Goethe University Hospital
Founded in 1914, J.W. Goethe University Hospital is considered to be one of the leading university hospitals in Germany. Twenty-five research institutes working in close cooperation with the Medical Department bear witness to the hospital's strong academic approach. This makes sure that patients coming to the University Hospital for treatment enjoy the benefits resulting from timely implementation of research findings. Every year, around 47,200 and 220,000 patients respectively receive in- and outpatient treatment. The University Hospital possesses special interdisciplinary competence in the fields of neurological science, oncology and cardiovascular medicine.
SOURCE Delcath Systems, Inc.
NEW YORK, Feb. 27, 2012 /PRNewswire/ -- Delcath Systems, Inc., (NASDAQ: DCTH) announced today that the first patients in Germany have been treated with the Delcath Hepatic CHEMOSAT® Delivery System at the Johann Wolfgang Goethe University Hospital, a premier European cancer treatment and research center located in Frankfurt. The cases were treated as part of the initial launch and training agreement the Company announced with the hospital in December 2011.
Two patients were treated for inoperable, liver-dominant metastases, one from cutaneous melanoma and one from breast cancer. The treating physicians reported that both patients were treated successfully without procedure-related complications.
Dr. Thomas J. Vogl, Director of the Institute for Diagnostic and Interventional Radiology at J.W. Goethe, said, "We believe this technology has significant potential to help control cancers in the liver. We're pleased to be the first cancer center to begin offering this important treatment option to patients in Germany, and are eager to further explore its role in the treatment of multiple tumor types including breast cancer."
"Delcath's partnership with J.W. Goethe reinforces the potential of CHEMOSAT," said Eamonn P. Hobbs, president and CEO of Delcath. "We recently treated our first patients in Milan and are eager to continue our expansion across Europe. Opening another CHEMOSAT treatment center and treating patients in the continent's largest market is another step forward in the commercialization of this technology."
About the Johann Wolfgang Goethe University Hospital
Founded in 1914, J.W. Goethe University Hospital is considered to be one of the leading university hospitals in Germany. Twenty-five research institutes working in close cooperation with the Medical Department bear witness to the hospital's strong academic approach. This makes sure that patients coming to the University Hospital for treatment enjoy the benefits resulting from timely implementation of research findings. Every year, around 47,200 and 220,000 patients respectively receive in- and outpatient treatment. The University Hospital possesses special interdisciplinary competence in the fields of neurological science, oncology and cardiovascular medicine.
SOURCE Delcath Systems, Inc.
Delcath Selects Quintiles to Support EU Launch of CHEMOSAT
Agreement to Accelerate Market Access and Generate Advocacy among Oncology and Hepatology Specialists
NEW YORK, March 9, 2012 /PRNewswire/ -- Delcath Systems, Inc. (NASDAQ: DCTH) announced today that Quintiles will provide a specialized team of Medical Science Liaisons (MSLs) to support the launch of the Hepatic CHEMOSAT® Delivery system for the treatment of cancers in the liver in France, Germany, Italy, Netherlands, Spain, Ireland and the United Kingdom. In addition, it will provide medical communication to support patient advocacy, key opinion leader development, and field force materials.
Comprised of medical doctors, nurses, pharmacologists, and Ph.D. cancer specialists, the MSL team will draw on their understanding of the local healthcare environment and solid key opinion leader relationships to educate medical oncologists and hepatology specialists on the clinical benefits of CHEMOSAT.
"This agreement gives us a highly professional medical science team in Europe with which we can begin communicating to and educating medical oncologists and liver specialists on the benefits of chemosaturation therapy," said Eamonn P. Hobbs, President and CEO of Delcath Systems. "Quintiles brings a compelling combination of scientific and medical knowledge, as well as broad experience in the European oncology marketplace that will help to support our own sales and marketing efforts. This agreement also represents yet another important milestone in our preparations for the commercial launch of CHEMOSAT in Europe, and we look forward to working with such a reputable partner to begin realizing the potential of CHEMOSAT."
Chris Pepler, Quintiles' Head of Commercial Services in Europe, Middle East and Africa, said, "The complexities in the European health care market can pose significant operational and market-place risk for companies based internationally and looking to launch here. Successfully navigating local health care systems requires deep local knowledge and expertise. We're drawing on this local experience, alongside our consistent global standards of quality and delivery to provide comprehensive commercial solutions as Delcath launches their product in multiple markets across Europe."
About Quintiles
Quintiles is the only fully integrated biopharmaceutical services company offering clinical, commercial, consulting and capital solutions worldwide. The Quintiles network of more than 20,000 engaged professionals in 60 countries works with an unwavering commitment to patients, safety and ethics. Quintiles helps biopharmaceutical companies navigate risk and seize opportunities in an environment where change is constant. For more information, please visit www.quintiles.com.
Agreement to Accelerate Market Access and Generate Advocacy among Oncology and Hepatology Specialists
NEW YORK, March 9, 2012 /PRNewswire/ -- Delcath Systems, Inc. (NASDAQ: DCTH) announced today that Quintiles will provide a specialized team of Medical Science Liaisons (MSLs) to support the launch of the Hepatic CHEMOSAT® Delivery system for the treatment of cancers in the liver in France, Germany, Italy, Netherlands, Spain, Ireland and the United Kingdom. In addition, it will provide medical communication to support patient advocacy, key opinion leader development, and field force materials.
Comprised of medical doctors, nurses, pharmacologists, and Ph.D. cancer specialists, the MSL team will draw on their understanding of the local healthcare environment and solid key opinion leader relationships to educate medical oncologists and hepatology specialists on the clinical benefits of CHEMOSAT.
"This agreement gives us a highly professional medical science team in Europe with which we can begin communicating to and educating medical oncologists and liver specialists on the benefits of chemosaturation therapy," said Eamonn P. Hobbs, President and CEO of Delcath Systems. "Quintiles brings a compelling combination of scientific and medical knowledge, as well as broad experience in the European oncology marketplace that will help to support our own sales and marketing efforts. This agreement also represents yet another important milestone in our preparations for the commercial launch of CHEMOSAT in Europe, and we look forward to working with such a reputable partner to begin realizing the potential of CHEMOSAT."
Chris Pepler, Quintiles' Head of Commercial Services in Europe, Middle East and Africa, said, "The complexities in the European health care market can pose significant operational and market-place risk for companies based internationally and looking to launch here. Successfully navigating local health care systems requires deep local knowledge and expertise. We're drawing on this local experience, alongside our consistent global standards of quality and delivery to provide comprehensive commercial solutions as Delcath launches their product in multiple markets across Europe."
About Quintiles
Quintiles is the only fully integrated biopharmaceutical services company offering clinical, commercial, consulting and capital solutions worldwide. The Quintiles network of more than 20,000 engaged professionals in 60 countries works with an unwavering commitment to patients, safety and ethics. Quintiles helps biopharmaceutical companies navigate risk and seize opportunities in an environment where change is constant. For more information, please visit www.quintiles.com.
Antwort auf Beitrag Nr.: 42.877.108 von McNay am 09.03.12 12:50:51Hier liegt eine Tenbagger Chance :
:
..
Delcath Reports Second Quarter 2012 Financial Results And Highlights Recent Accomplishments
--Conference Call and Webcast Today at 8:30 a.m. ET--
Press Release: Delcath Systems, Inc. – 1 hour 2 minutes ago.. .
.
Email
Print
... .
.
Companies:.
.
.Delcath Systems, Inc.
.
.
.
RELATED QUOTES.
.
Symbol
Price
Change
DCTH
1.74
.
.
..
.
.
..
.
.
NEW YORK, Aug. 7, 2012 /PRNewswire/ -- Delcath Systems (DCTH) today announced financial results and highlights for its second quarter 2012 ended June 30, 2012 and recent corporate accomplishments.
Highlights for the second quarter 2012 and recent weeks include:
•Recognition of first product revenue in Delcath history
•FDA acceptance of the IND Amendments for use of the Generation 2 filter in the U.S. Expanded Access Program, compassionate use, and future clinical trials
•New drug application (NDA) filing with the FDA for the Company's proprietary chemosaturation system on target for mid-August 2012
•Addition of five leading cancer centers in the EU to the CHEMOSAT® initial launch and training program; a total 13 CHEMOSAT centers have been signed with presence in all seven key target EU markets
•Deployment of Quintiles trained medical science liaisons field force in the key target markets in Europe
•To date 16 CHEMOSAT procedures performed at five EU centers; 13 patients with liver dominant metastases from multiple tumor types have been treated, including cutaneous melanoma, ocular melanoma, gastric cancer, breast cancer and cholangiocarcinoma
•Raised $21.1 million in net proceeds through a follow on offering to fund operational progress and CHEMOSAT European launch
•Board of Directors strengthened with the addition of pharmaceutical industry veterans Laura A. Brege and Tasos G. Konidaris.
"During the second quarter, we made important progress with the European launch of CHEMOSAT, and generated our first product revenue in the Company's history," said Eamonn P. Hobbs, President and CEO of Delcath. "We have now signed agreements with 13 leading European Union cancer centers, exceeding our original expectations, and intend to sign additional centers in the coming months. Our medical science liaisons are in the field in our seven target markets educating oncologists on the potential of the CHEMOSAT treatment for their patients with liver dominant disease. To date, patients afflicted with liver metastases from five different types of cancer have been treated with CHEMOSAT. During the remainder of the year, our commercial focus in Europe will be to continue to bring clinical centers on line while we work with referring physicians to expand clinical adoption of the CHEMOSAT system as an important therapeutic option for these patients.
"In the U.S., our New Drug Application (NDA) is on schedule and we expect to submit the file to the FDA by mid-August," continued Mr. Hobbs. "Our amendments to our Investigational New Drug (IND) application to include Generation 2 in our Expanded Access Program and all future clinical trials and compassionate use cases were accepted by the FDA. Additionally, after consultation with the FDA, we have agreed to include the addition of Generation 2 in our NDA submission as a technical change to the Chemistry, Manufacturing, and Control module."
For the three months ended June 30, 2012, Delcath's operating loss was $15.4 million, which included approximately $1.0 million in non-cash stock-based compensation expense. Operating loss for the three months ended June 30, 2011 was $10.5 million, which included approximately $1.2 million in non-cash stock-based compensation expense. Selling, general and administrative (SG&A) expenses were $7.2 million for the second quarter of 2012, compared to $5.2 million for the same period in 2011. The increase was primarily due to an increase in sales, marketing and operational support staff in the EU. Research and development (R&D) expenses were $8.2 million for the second quarter of 2012, compared to $5.2 million for the same period in 2011. The increase was primarily due to global regulatory efforts including continued preparation of the NDA submission to the FDA and the training and deployment of third party medical science liaisons.
At June 30, 2012, cash, cash equivalents and certificates of deposit were $29.3 million, compared to $30.8 million at December 31, 2011. Gross cash spend in the second quarter 2012 was $14.2 million, as compared to $8.2 million in the same period in the prior year. The increase was primarily driven by NDA submission related costs and staff increases in various functions to support EU commercialization. Average monthly operating gross spend was $4.7 million in the second quarter, a decrease from $4.9 million in the first quarter of 2012. Following the anticipated NDA submission, Delcath expects average monthly cash spend to decrease to between $3 million to $4 million for the fourth quarter of 2012.
Conference Call and Webcast
The Company will host a conference call today, August 7, 2012 at 8:30 a.m. ET. To participate in the live call by telephone, please dial 800-762-8779 for domestic participants and 480-629-9645 for international participants. To access the live webcast, go to the Events & Presentations page on Delcath's website at http://www.delcath.com/investors/events/.
A taped replay of the conference call will also be available beginning approximately two hours after the call's conclusion and will be available for seven days. This replay can be accessed by dialing 800-406-7325 for domestic callers and 303-590-3030 for international callers, both using passcode 4548817. An archived webcast will also be available at http://www.delcath.com/investors/events/.
About Delcath Systems
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Delcath's proprietary system for chemosaturation is designed to administer high dose chemotherapy and other therapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath announced that its randomized Phase III clinical trial for patients with metastatic melanoma in the liver had successfully achieved the study's primary endpoint of extended hepatic progression-free survival. The Company also completed a multi-arm Phase II trial to treat other liver cancers. The Company obtained authorization to affix a CE Mark for the Delcath Hepatic CHEMOSAT® delivery system in April 2011 and for the second generation hemofiltration cartridge for CHEMOSAT in April 2012. The right to affix the CE mark allows the Company to market and sell the CHEMOSAT system in Europe. The Company has not yet received FDA approval for commercial sale of its system in the United States. The Company continues with the preparation of its NDA submission and intends to seek FDA approval for commercial sale of its chemosaturation system with melphalan. For more information, please visit the Company's website at www.delcath.com.
Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by the Company or on its behalf. This news release contains forward-looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to: the benefits of the Generation 2 CHEMOSAT system and market acceptance of the same, patient outcomes using the Generation 2 system, the timing of the supply and distribution of the CHEMOSAT system to early launch centers in Europe, the time required to build inventory and establish commercial operations in Europe, adoption, use and resulting sales, if any, for the Hepatic CHEMOSAT delivery system in the EEA, our ability to successfully commercialize the chemosaturation system and the potential of the chemosaturation system as a treatment for patients with terminal metastatic disease in the liver, acceptability of the Phase III clinical trial data by the FDA, our ability to address the issues raised in the Refusal to File letter received from the FDA and the timing of our re-submission of our NDA, re-submission and acceptance of the Company's NDA by the FDA, approval of the Company's NDA for the treatment of metastatic melanoma to the liver, adoption, use and resulting sales, if any, for the chemosaturation system in the United States, approval of the current or future chemosaturation system for other indications, actions by the FDA or other foreign regulatory agencies, our ability to obtain reimbursement for the CHEMOSAT system, our ability to successfully enter into distribution and strategic partnership agreements in foreign markets and the corresponding revenue associated with such foreign markets, uncertainties relating to the results of research and development projects and future clinical trials, the timing and use, if any, of the line of credit from SVB, and our ability to access this facility, and uncertainties regarding our ability to obtain financial and other resources for any research, development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward-looking statements to reflect events or circumstances after the date they are made.
 :
:..
Delcath Reports Second Quarter 2012 Financial Results And Highlights Recent Accomplishments
--Conference Call and Webcast Today at 8:30 a.m. ET--
Press Release: Delcath Systems, Inc. – 1 hour 2 minutes ago.. .
.
... .
.
Companies:.
.
.Delcath Systems, Inc.
.
.
.
RELATED QUOTES.
.
Symbol
Price
Change
DCTH
1.74
.
.
..
.
.
..
.
.
NEW YORK, Aug. 7, 2012 /PRNewswire/ -- Delcath Systems (DCTH) today announced financial results and highlights for its second quarter 2012 ended June 30, 2012 and recent corporate accomplishments.
Highlights for the second quarter 2012 and recent weeks include:
•Recognition of first product revenue in Delcath history
•FDA acceptance of the IND Amendments for use of the Generation 2 filter in the U.S. Expanded Access Program, compassionate use, and future clinical trials
•New drug application (NDA) filing with the FDA for the Company's proprietary chemosaturation system on target for mid-August 2012
•Addition of five leading cancer centers in the EU to the CHEMOSAT® initial launch and training program; a total 13 CHEMOSAT centers have been signed with presence in all seven key target EU markets
•Deployment of Quintiles trained medical science liaisons field force in the key target markets in Europe
•To date 16 CHEMOSAT procedures performed at five EU centers; 13 patients with liver dominant metastases from multiple tumor types have been treated, including cutaneous melanoma, ocular melanoma, gastric cancer, breast cancer and cholangiocarcinoma
•Raised $21.1 million in net proceeds through a follow on offering to fund operational progress and CHEMOSAT European launch
•Board of Directors strengthened with the addition of pharmaceutical industry veterans Laura A. Brege and Tasos G. Konidaris.
"During the second quarter, we made important progress with the European launch of CHEMOSAT, and generated our first product revenue in the Company's history," said Eamonn P. Hobbs, President and CEO of Delcath. "We have now signed agreements with 13 leading European Union cancer centers, exceeding our original expectations, and intend to sign additional centers in the coming months. Our medical science liaisons are in the field in our seven target markets educating oncologists on the potential of the CHEMOSAT treatment for their patients with liver dominant disease. To date, patients afflicted with liver metastases from five different types of cancer have been treated with CHEMOSAT. During the remainder of the year, our commercial focus in Europe will be to continue to bring clinical centers on line while we work with referring physicians to expand clinical adoption of the CHEMOSAT system as an important therapeutic option for these patients.
"In the U.S., our New Drug Application (NDA) is on schedule and we expect to submit the file to the FDA by mid-August," continued Mr. Hobbs. "Our amendments to our Investigational New Drug (IND) application to include Generation 2 in our Expanded Access Program and all future clinical trials and compassionate use cases were accepted by the FDA. Additionally, after consultation with the FDA, we have agreed to include the addition of Generation 2 in our NDA submission as a technical change to the Chemistry, Manufacturing, and Control module."
For the three months ended June 30, 2012, Delcath's operating loss was $15.4 million, which included approximately $1.0 million in non-cash stock-based compensation expense. Operating loss for the three months ended June 30, 2011 was $10.5 million, which included approximately $1.2 million in non-cash stock-based compensation expense. Selling, general and administrative (SG&A) expenses were $7.2 million for the second quarter of 2012, compared to $5.2 million for the same period in 2011. The increase was primarily due to an increase in sales, marketing and operational support staff in the EU. Research and development (R&D) expenses were $8.2 million for the second quarter of 2012, compared to $5.2 million for the same period in 2011. The increase was primarily due to global regulatory efforts including continued preparation of the NDA submission to the FDA and the training and deployment of third party medical science liaisons.
At June 30, 2012, cash, cash equivalents and certificates of deposit were $29.3 million, compared to $30.8 million at December 31, 2011. Gross cash spend in the second quarter 2012 was $14.2 million, as compared to $8.2 million in the same period in the prior year. The increase was primarily driven by NDA submission related costs and staff increases in various functions to support EU commercialization. Average monthly operating gross spend was $4.7 million in the second quarter, a decrease from $4.9 million in the first quarter of 2012. Following the anticipated NDA submission, Delcath expects average monthly cash spend to decrease to between $3 million to $4 million for the fourth quarter of 2012.
Conference Call and Webcast
The Company will host a conference call today, August 7, 2012 at 8:30 a.m. ET. To participate in the live call by telephone, please dial 800-762-8779 for domestic participants and 480-629-9645 for international participants. To access the live webcast, go to the Events & Presentations page on Delcath's website at http://www.delcath.com/investors/events/.
A taped replay of the conference call will also be available beginning approximately two hours after the call's conclusion and will be available for seven days. This replay can be accessed by dialing 800-406-7325 for domestic callers and 303-590-3030 for international callers, both using passcode 4548817. An archived webcast will also be available at http://www.delcath.com/investors/events/.
About Delcath Systems
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Delcath's proprietary system for chemosaturation is designed to administer high dose chemotherapy and other therapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath announced that its randomized Phase III clinical trial for patients with metastatic melanoma in the liver had successfully achieved the study's primary endpoint of extended hepatic progression-free survival. The Company also completed a multi-arm Phase II trial to treat other liver cancers. The Company obtained authorization to affix a CE Mark for the Delcath Hepatic CHEMOSAT® delivery system in April 2011 and for the second generation hemofiltration cartridge for CHEMOSAT in April 2012. The right to affix the CE mark allows the Company to market and sell the CHEMOSAT system in Europe. The Company has not yet received FDA approval for commercial sale of its system in the United States. The Company continues with the preparation of its NDA submission and intends to seek FDA approval for commercial sale of its chemosaturation system with melphalan. For more information, please visit the Company's website at www.delcath.com.
Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by the Company or on its behalf. This news release contains forward-looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to: the benefits of the Generation 2 CHEMOSAT system and market acceptance of the same, patient outcomes using the Generation 2 system, the timing of the supply and distribution of the CHEMOSAT system to early launch centers in Europe, the time required to build inventory and establish commercial operations in Europe, adoption, use and resulting sales, if any, for the Hepatic CHEMOSAT delivery system in the EEA, our ability to successfully commercialize the chemosaturation system and the potential of the chemosaturation system as a treatment for patients with terminal metastatic disease in the liver, acceptability of the Phase III clinical trial data by the FDA, our ability to address the issues raised in the Refusal to File letter received from the FDA and the timing of our re-submission of our NDA, re-submission and acceptance of the Company's NDA by the FDA, approval of the Company's NDA for the treatment of metastatic melanoma to the liver, adoption, use and resulting sales, if any, for the chemosaturation system in the United States, approval of the current or future chemosaturation system for other indications, actions by the FDA or other foreign regulatory agencies, our ability to obtain reimbursement for the CHEMOSAT system, our ability to successfully enter into distribution and strategic partnership agreements in foreign markets and the corresponding revenue associated with such foreign markets, uncertainties relating to the results of research and development projects and future clinical trials, the timing and use, if any, of the line of credit from SVB, and our ability to access this facility, and uncertainties regarding our ability to obtain financial and other resources for any research, development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward-looking statements to reflect events or circumstances after the date they are made.
Antwort auf Beitrag Nr.: 43.465.311 von Magnetfeldfredy am 07.08.12 13:39:00anscheinend sind die Einschätzungen aber nicht von allen Marktteilnehmern so gut eingeschätzt.
Der Kurs dümpelt weiterhin bei (aktuell) $ 2,- rum.
Habe den Wert weiter auf meiner Watchlist.

Der Kurs dümpelt weiterhin bei (aktuell) $ 2,- rum.
Habe den Wert weiter auf meiner Watchlist.

Der Umsatz liegt bei 106 000$, der operative Verlust bei 15Mio$. Delcath hat in seiner Geschichte noch keinen Cent Gewinn gemacht. Darüber hinaus neigt man dazu, die Erwartungen der Analysten zu verfehlen. Im letzten Quartal konnte man diese Erwartungen aber um einen Cent schlagen.
Darin begründet sich wahrscheinlich auch die leichte Aufwärtstendenz der Aktie seit Juni. Jetzt ist Delcath an der 100er (ca. 2,14$). Möglicherweise wird dieser Widerstand noch erreicht oder sogar leicht überwunden. Aber der Stochastik ist 1. stark überkauft 2. gedreht und 3. seine Signallinie geschnitten, so dass eine stärkere Korrektur bevorstehen sollte. Diese sollte oberhalb von 1,64$ enden, da ansonsten die Aufwärtstendenz zumindest angeknackst wäre.
Darin begründet sich wahrscheinlich auch die leichte Aufwärtstendenz der Aktie seit Juni. Jetzt ist Delcath an der 100er (ca. 2,14$). Möglicherweise wird dieser Widerstand noch erreicht oder sogar leicht überwunden. Aber der Stochastik ist 1. stark überkauft 2. gedreht und 3. seine Signallinie geschnitten, so dass eine stärkere Korrektur bevorstehen sollte. Diese sollte oberhalb von 1,64$ enden, da ansonsten die Aufwärtstendenz zumindest angeknackst wäre.
Antwort auf Beitrag Nr.: 43.497.008 von sdaktien am 15.08.12 17:18:59..Delcath Submits New Drug Application For Proprietary Chemosaturation System To The U.S. Food And Drug Administration
Press Release: Delcath Systems, Inc. – 52 minutes ago....Email
Share0Print.....Companies:...Delcath Systems, Inc. . ..RELATED QUOTES.
.Symbol Price Change
DCTH 2.07 +0.06
..........
NEW YORK, Aug. 15, 2012 /PRNewswire/ -- Delcath Systems, Inc. (DCTH) announced today that it has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration seeking approval for the Company's proprietary chemosaturation system for use with melphalan hydrochloride in the treatment of patients with unresectable metastatic melanoma in the liver. The Company included its Generation 2 filter in its NDA submission as a technical change to the Chemistry, Manufacturing, and Control (CMC) module.
"Our team has achieved a significant milestone with the filing of our NDA," said Eamonn P. Hobbs, President and CEO of Delcath Systems. "We believe that our chemosaturation system provides the opportunity to satisfy a high unmet medical need to treat patients with unresectable metastatic melanoma in the liver. We also believe including our Generation 2 filter in the CMC module represents the fastest regulatory review path for the Generation 2 system, and that it is in the best interest of U.S. patients that we accelerate the potential availability of Generation 2."
"We have requested priority review of our NDA by the FDA. Assuming the NDA is accepted and that priority review is granted, our expected Prescription Drug User Fee Act (PDUFA) date would be in February of next year. Based upon the strength of our Phase 1, 2 and 3 data, along with the limited treatment options available for patients with unresectable melanoma metastases in the liver, we believe that our application meets the FDA's criteria for priority review."
In Delcath's Phase 3 clinical trial (April 2010 data cutoff), comparing treatment with the Company's proprietary chemosaturation system to best alternative care (BAC) revealed that patients treated with chemosaturation therapy experienced a statistically significant extension in median hepatic progression free survival (hPFS) of 5.4 months (p=0.0001, hazard ratio 0.39) longer than patients treated with BAC according to independent review committee (IRC) blinded intent-to-treat (ITT) analysis. Previously reported investigator ITT analysis of these data showed an extension in median hPFS of 6.4 months (p<0.0001, hazard ratio 0.28) longer than patients treated with BAC. Priority review is granted by the FDA to those products that address significant unmet medical needs or have the potential to provide significant improvement compared to marketed products. The FDA has previously granted Delcath two orphan drug designations for melphalan in ocular and cutaneous melanoma, which will provide the Company with exclusivity in these indications for seven years if the NDA is accepted, reviewed and approved.
Press Release: Delcath Systems, Inc. – 52 minutes ago....Email
Share0Print.....Companies:...Delcath Systems, Inc. . ..RELATED QUOTES.
.Symbol Price Change
DCTH 2.07 +0.06
..........
NEW YORK, Aug. 15, 2012 /PRNewswire/ -- Delcath Systems, Inc. (DCTH) announced today that it has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration seeking approval for the Company's proprietary chemosaturation system for use with melphalan hydrochloride in the treatment of patients with unresectable metastatic melanoma in the liver. The Company included its Generation 2 filter in its NDA submission as a technical change to the Chemistry, Manufacturing, and Control (CMC) module.
"Our team has achieved a significant milestone with the filing of our NDA," said Eamonn P. Hobbs, President and CEO of Delcath Systems. "We believe that our chemosaturation system provides the opportunity to satisfy a high unmet medical need to treat patients with unresectable metastatic melanoma in the liver. We also believe including our Generation 2 filter in the CMC module represents the fastest regulatory review path for the Generation 2 system, and that it is in the best interest of U.S. patients that we accelerate the potential availability of Generation 2."
"We have requested priority review of our NDA by the FDA. Assuming the NDA is accepted and that priority review is granted, our expected Prescription Drug User Fee Act (PDUFA) date would be in February of next year. Based upon the strength of our Phase 1, 2 and 3 data, along with the limited treatment options available for patients with unresectable melanoma metastases in the liver, we believe that our application meets the FDA's criteria for priority review."
In Delcath's Phase 3 clinical trial (April 2010 data cutoff), comparing treatment with the Company's proprietary chemosaturation system to best alternative care (BAC) revealed that patients treated with chemosaturation therapy experienced a statistically significant extension in median hepatic progression free survival (hPFS) of 5.4 months (p=0.0001, hazard ratio 0.39) longer than patients treated with BAC according to independent review committee (IRC) blinded intent-to-treat (ITT) analysis. Previously reported investigator ITT analysis of these data showed an extension in median hPFS of 6.4 months (p<0.0001, hazard ratio 0.28) longer than patients treated with BAC. Priority review is granted by the FDA to those products that address significant unmet medical needs or have the potential to provide significant improvement compared to marketed products. The FDA has previously granted Delcath two orphan drug designations for melphalan in ocular and cutaneous melanoma, which will provide the Company with exclusivity in these indications for seven years if the NDA is accepted, reviewed and approved.
Antwort auf Beitrag Nr.: 43.497.008 von sdaktien am 15.08.12 17:18:59knapp über der Linie von $1,64 hat sich die Aktie ja eine Weile gehalten und ist anschließend weiter eingebrochen.
Somit genau wie von Dir angesprochen.
Nun haben wir Charttechnisch aber ein anderes Bild, da wir uns nun aus der Seitwärtsbewegung in den steigenden Bereich bewegen.
Wie ist Deine Einschätzung, ist dies nur eien Korrektur oder bereits die Gegenbewegung?
An den Basiszahlen hat sich nicht wirklich etwas geändert, somit würde (wenn überhaupt) nur die Charttechnik greifen.
Somit genau wie von Dir angesprochen.
Nun haben wir Charttechnisch aber ein anderes Bild, da wir uns nun aus der Seitwärtsbewegung in den steigenden Bereich bewegen.
Wie ist Deine Einschätzung, ist dies nur eien Korrektur oder bereits die Gegenbewegung?
An den Basiszahlen hat sich nicht wirklich etwas geändert, somit würde (wenn überhaupt) nur die Charttechnik greifen.
Also grundsätzlich hat sich an der technischen Beurteilung nicht viel verändert. Immer noch ist die 100er für den Kursverlauf prägend und immer noch nicht hat Delcath diese Marke überwunden (dürfte momentan bei ca. 1,65$ liegen). Richtig ist, dass die Aktie ihre 38er überwunden hat, daraus aber eine Aufwärtsbewegung abzuleiten halte ich für verfrüht, zumal diese in der Seitwärtsbewegung seit dem Sommer auch immer wieder mal geschnitten wurde, ohne das es sich ausgewirkt hat.
Markttechnisch ist auch nicht alles in Butter. Für einen richtigen Kursaufschwung zieht das Handelsvolumen nicht mit. Der Stochastik ist bereits überkauft, steigt aber noch an.
Wahrscheinlich wird es eine ähnliche Konstellation geben wie im August. Ein Erreichen oder Überschreiten der 100er und dann einen Rücksetzer. Möglicherweise hält die Aktie dann bei ca. 1,25$. Da könnte sich eine Unterstüzung ausbilden. Für sehr stark halte ich die aber nicht. Ich denke, wichtig ist vor allem, dass die 1,11$ nicht mehr unterschritten wird, da sich der Abwärtstrend ansonsten weiter fortsetzen sollte.
Ich hab noch einen kurzen Blick auf die Fundamentaldaten geworfen. Demnach war das Ergebnis Q3 mit -0,18$ etwas besser als von den Analysten erwartet. an der Gesamteinschätzung hat das aber nicht viel geändert.
Der Anstieg heute hängt wohl an einer Pressemitteilung, genannt Investor Presentation.
Markttechnisch ist auch nicht alles in Butter. Für einen richtigen Kursaufschwung zieht das Handelsvolumen nicht mit. Der Stochastik ist bereits überkauft, steigt aber noch an.
Wahrscheinlich wird es eine ähnliche Konstellation geben wie im August. Ein Erreichen oder Überschreiten der 100er und dann einen Rücksetzer. Möglicherweise hält die Aktie dann bei ca. 1,25$. Da könnte sich eine Unterstüzung ausbilden. Für sehr stark halte ich die aber nicht. Ich denke, wichtig ist vor allem, dass die 1,11$ nicht mehr unterschritten wird, da sich der Abwärtstrend ansonsten weiter fortsetzen sollte.
Ich hab noch einen kurzen Blick auf die Fundamentaldaten geworfen. Demnach war das Ergebnis Q3 mit -0,18$ etwas besser als von den Analysten erwartet. an der Gesamteinschätzung hat das aber nicht viel geändert.
Der Anstieg heute hängt wohl an einer Pressemitteilung, genannt Investor Presentation.
Antwort auf Beitrag Nr.: 44.000.914 von sdaktien am 09.01.13 17:31:12Meiner Meinung nach wird es ein pre-run auf die FDA Entscheidung im Juni geben!
Wann weiß keiner, auf alle Fälle ist das jetzige Kursniveau sehr interessant für diese Annahme!
Wann weiß keiner, auf alle Fälle ist das jetzige Kursniveau sehr interessant für diese Annahme!
Antwort auf Beitrag Nr.: 44.009.780 von Magnetfeldfredy am 11.01.13 12:02:53PDUFA Termin ist am 15 Juni 
http://www.google.com/finance?q=NASDAQ%3ADCTH&ei=Xd4OUZiAJ-f…
Aktuell auf meiner Beobachtungsliste

http://www.google.com/finance?q=NASDAQ%3ADCTH&ei=Xd4OUZiAJ-f…
Aktuell auf meiner Beobachtungsliste

Antwort auf Beitrag Nr.: 44.098.460 von multimediaperle am 03.02.13 23:14:09Bei mir schon lange im Depot, besonders jetzt nachdem beim Konkurrenten Celsion die Phase III gefloppt ist!
Warum sollte das Präparat von Delcath besser sein als das von Celsion?
Kurzfristig ist Delcath markttechnisch interessant. Die Aktie ist an der 100er, der Stochastik scheint aber schon im neutralen Bereich nach oben zu drehen, seine Signallinie hat er bereits überschritten. Wenn sich das bestätigt, solle Delcath zumindest seine 100er nachhaltig überwinden.
Kurzfristig ist Delcath markttechnisch interessant. Die Aktie ist an der 100er, der Stochastik scheint aber schon im neutralen Bereich nach oben zu drehen, seine Signallinie hat er bereits überschritten. Wenn sich das bestätigt, solle Delcath zumindest seine 100er nachhaltig überwinden.
Antwort auf Beitrag Nr.: 44.099.174 von sdaktien am 04.02.13 10:43:45Celsion hat die primären Endpunkte der Phase III nicht erreicht, also eine Konkurrenz weniger!
Super Nachricht heute Delcath erhält die Ersattung
 in Deutschland:
in Deutschland:
DELCATH ANNOUNCES REIMBURSEMENT FOR CHEMOSAT IN GERMANY
Press Release: Delcath Systems, Inc – 1 hour 49 minutes ago
Email
ShareTweet
Print
RELATED QUOTES
Symbol Price Change
DCTH 1.51
NEW YORK, February 4, 2013 - Delcath Systems, Inc. (DCTH) today announced that the Institut für das Entgeltsystem im Krankenhaus (InEk), the German federal reimbursement agency, has established a reimbursement pathway for the treatment of patients with liver metastases with the Delcath Hepatic CHEMOSAT® Delivery System for melphalan hydrochloride. The decision by the InEK followed an endorsement by the German Radiology Association, which prompted 47 cancer centers throughout Germany to submit applications under the Neue Untersuchungs- und Behandlungsmethoden (NUB) scheme for new technology reimbursement at specific hospitals. The Value 4 status given to the CHEMOSAT procedure, while not mandating reimbursement, allows participating cancer centers to negotiate reimbursement coverage for the CHEMOSAT procedure with all insurers serving their region. Under the NUB scheme, reimbursement pathways will potentially be available for treatment with CHEMOSAT regardless of primary cancer origin.
Eamonn P. Hobbs, President & CEO of Delcath said, "This is excellent news for both patients in Germany and Delcath, as it represents a significant positive step in our efforts to fully commercialize CHEMOSAT in Europe. This is the first reimbursement mechanism for our procedure in Germany, the biggest market for CHEMOSAT in the European Union. It is important to note that the application for coverage was supported by 47 cancer centers across the country, which we believe speaks to the medical need physicians in Germany see for CHEMOSAT. We will continue to work closely with the participating hospitals to achieve reimbursement with the insurers. With a direct sales force in place and training of the additional centers in Germany on the way, we are now in a good position to begin growing this market for CHEMOSAT."
About Delcath Systems
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Delcath`s proprietary system for chemosaturation is designed to administer high dose chemotherapy and other therapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company`s initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath announced that its randomized Phase 3 clinical trial for patients with metastatic melanoma in the liver had successfully achieved the study`s primary endpoint of extended hepatic progression-free survival. The Company also completed a multi-arm Phase 2 trial to treat other liver cancers. The Company obtained authorization to affix a CE Mark for the Generation Two CHEMOSAT® delivery system for melphalan hydrochloride in April 2012. The right to affix the CE mark allows the Company to market and sell the CHEMOSAT system for melphalan hydrochloride in Europe. In October 2012, the Company satisfied all of the requirements to affix the CE Mark to the Hepatic CHEMOSAT Delivery System device for intra-hepatic arterial delivery and extracorporeal filtration of doxorubicin hydrochloride injection, providing a regulatory pathway for the CHEMOSAT Delivery System to deliver and filter doxorubicin for countries in Asia that accept the CE Marking as part of their national regulatory requirements. The Company has not yet received FDA approval for commercial sale of its system in the United States. The Company`s NDA has been accepted for filing and substantive review by the FDA. For more information, please visit the Company`s website at www.delcath.com.
Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by the Company or on its behalf. This news release contains forward-looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to: whether each of the 47 cancer centers in Germany will successfully negotiate and receive reimbursement for the CHEMOSAT procedure in their region and the amount of reimbursement to be provided, patient outcomes using the CHEMOSAT system in the EU, patient outcomes using the chemosaturation system under the EAP, timing of completion of the FDA`s review of our NDA, the extent to which the FDA may request additional information or data and our ability to provide the same in a timely manner, acceptability of the Phase 1, 2 and 3 clinical trial data by the FDA, FDA approval of the Company`s NDA for the treatment of ocular metastatic melanoma to the liver, adoption, use and resulting sales, if any, in the United States, adoption, use and resulting sales, if any, for the CHEMOSAT system to deliver and filter melphalan in the EEA, our ability to successfully commercialize the chemosaturation system and the potential of the chemosaturation system as a treatment for patients with primary and metastatic disease in the liver, market acceptance of the Gen Two CHEMOSAT system and patient outcomes using the same, approval of the current or future chemosaturation system for delivery and filtration of melphalan, doxorubicin or other chemotherapeutic agents for various indications in the US and/or in foreign markets, actions by the FDA or other foreign regulatory agencies, our ability to successfully enter into strategic partnership and distribution arrangements in foreign markets including Australia and key Asian markets and timing an revenue, if any, of the same, the approval of the Hepatic CHEMOSAT Delivery System device to deliver and filter doxorubicin in key Asian markets and patient outcomes using the same, our ability to obtain reimbursement for the CHEMOSAT system, uncertainties relating to the timing and results of research and development projects, uncertainties relating to the timing and results of future clinical trials, and uncertainties regarding our ability to obtain financial and other resources for any research, development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward-looking statements to reflect events or circumstances after the date they are made.
Contact Information:
Investors: Financial Media EU Consumer/Medical Media
Gregory Gin/Patty Eisenhaur Janine McCargo Julie Johnson
EVC Group EVC Group Bliss Integrated
646-445-4801/951-316-0577 646-688-0425 503-883-9103
This announcement is distributed by Thomson Reuters on behalf of Thomson Reuters clients.
The owner of this announcement warrants that:
(i) the releases contained herein are protected by copyright and other applicable laws; and
(ii) they are solely responsible for the content, accuracy and originality of the
information contained therein.
Source: Delcath Systems, Inc via Thomson Reuters ONE
HUG#1675162
@yahoofinance on Twitter, become a fan on Facebook
RELATED CONTENT
ArQule enrolls first patient in Phase 3 trial …
FDA to Review Sanofi's Lemtrada
Threshold/Merck KGaA Drug in Study
FDA gives new drug application approval to Is …
FDA Clears Teleflex Needles
CTI announces DSMB recommendation to continue …
ImmunoGen slides as analyst cuts rating
Germany warns euro crisis not over
Associated Press
Germany: Sexism debate prompts surge in complaints
Associated Press
Recent Quotes
Portfolios
Recent Quotes
Symbol
Price Change % Chg Chart
DCTH
1.51
DNDN
5.95
KERX
7.11
AMRN
8.45
ARNA
8.58
PZG
2.25
TOPO.CO
2.18 -0.08 -3.54%
EXEL
4.765
GIG
1.485
VUZI
0.099
Search for share prices
Save List to Portfolio »
Recent Quotes News
DNDN: Sharp Increase in FDA Drug Approvals a Major Factor in Biotech Industry's Success - Marketwire
KERX: Today's Market News To Trade On: 5 Stocks Moving On News - at Seeking Alpha
AMRN: Weekly Stock Watch, Week Of 4 February 2013 - at Seeking Alpha
AMRN: Keryx slide attributable to Zerenex market exclusivity worries, The Street says - theflyonthewall.com
AMRN: Keryx Falls On Questions About Zerenex Market Exclusivity - at TheStreet
More Recent Quotes News »
There are no comments yet
Leave a comment... Comment Guidelines
Post As
TOP STORIES »
European Political Worries Halt Risk Asset Rally
Reuters
Stronger U.S. and Chinese economic data supported world equity markets on Monday, while the euro dipped and Spanish …
Which Super Bowl Ads Stole the Show?MarketWatch
Low Rates Force Companies To Pour Cash Into PensionsThe Wall Street Journal
Harvard President: Zuckerberg Dropped Out, but His Staff Didn’tOff the Cuff
Humana Profit Slips; 2013 Outlook AffirmedMarketWatch
Sign up for The Daily Download, the new Yahoo! Finance morning newsletter that gives you the day's top news and stocks to watch, all in one convenient place.
ANZEIGE
TRADING CENTER
Compare Brokers »
TODAY ON YAHOO!1 - 6 of 48
Bowl ads that sparked the biggest backlash
Ravens resist comeback attempt to win
High-paying careers without grad school
Cameras capture moment not meant for TV
Kaepernick misses chance to join legends
Whatever happened to this '80s icon?
YAHOO! FINANCE ON FACEBOOK
@YAHOOFINANCE ON TWITTER
POLL
With gas prices on the rise, how is it impacting your spending?
I'm cutting back on discretionary items
Spending about the same
I'm feeling good about the economy and spending more
Vote
See Results
Super Nachricht heute Delcath erhält die Ersattung

 in Deutschland:
in Deutschland:DELCATH ANNOUNCES REIMBURSEMENT FOR CHEMOSAT IN GERMANY
Press Release: Delcath Systems, Inc – 1 hour 49 minutes ago
ShareTweet
RELATED QUOTES
Symbol Price Change
DCTH 1.51
NEW YORK, February 4, 2013 - Delcath Systems, Inc. (DCTH) today announced that the Institut für das Entgeltsystem im Krankenhaus (InEk), the German federal reimbursement agency, has established a reimbursement pathway for the treatment of patients with liver metastases with the Delcath Hepatic CHEMOSAT® Delivery System for melphalan hydrochloride. The decision by the InEK followed an endorsement by the German Radiology Association, which prompted 47 cancer centers throughout Germany to submit applications under the Neue Untersuchungs- und Behandlungsmethoden (NUB) scheme for new technology reimbursement at specific hospitals. The Value 4 status given to the CHEMOSAT procedure, while not mandating reimbursement, allows participating cancer centers to negotiate reimbursement coverage for the CHEMOSAT procedure with all insurers serving their region. Under the NUB scheme, reimbursement pathways will potentially be available for treatment with CHEMOSAT regardless of primary cancer origin.
Eamonn P. Hobbs, President & CEO of Delcath said, "This is excellent news for both patients in Germany and Delcath, as it represents a significant positive step in our efforts to fully commercialize CHEMOSAT in Europe. This is the first reimbursement mechanism for our procedure in Germany, the biggest market for CHEMOSAT in the European Union. It is important to note that the application for coverage was supported by 47 cancer centers across the country, which we believe speaks to the medical need physicians in Germany see for CHEMOSAT. We will continue to work closely with the participating hospitals to achieve reimbursement with the insurers. With a direct sales force in place and training of the additional centers in Germany on the way, we are now in a good position to begin growing this market for CHEMOSAT."
About Delcath Systems
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Delcath`s proprietary system for chemosaturation is designed to administer high dose chemotherapy and other therapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company`s initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath announced that its randomized Phase 3 clinical trial for patients with metastatic melanoma in the liver had successfully achieved the study`s primary endpoint of extended hepatic progression-free survival. The Company also completed a multi-arm Phase 2 trial to treat other liver cancers. The Company obtained authorization to affix a CE Mark for the Generation Two CHEMOSAT® delivery system for melphalan hydrochloride in April 2012. The right to affix the CE mark allows the Company to market and sell the CHEMOSAT system for melphalan hydrochloride in Europe. In October 2012, the Company satisfied all of the requirements to affix the CE Mark to the Hepatic CHEMOSAT Delivery System device for intra-hepatic arterial delivery and extracorporeal filtration of doxorubicin hydrochloride injection, providing a regulatory pathway for the CHEMOSAT Delivery System to deliver and filter doxorubicin for countries in Asia that accept the CE Marking as part of their national regulatory requirements. The Company has not yet received FDA approval for commercial sale of its system in the United States. The Company`s NDA has been accepted for filing and substantive review by the FDA. For more information, please visit the Company`s website at www.delcath.com.
Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by the Company or on its behalf. This news release contains forward-looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to: whether each of the 47 cancer centers in Germany will successfully negotiate and receive reimbursement for the CHEMOSAT procedure in their region and the amount of reimbursement to be provided, patient outcomes using the CHEMOSAT system in the EU, patient outcomes using the chemosaturation system under the EAP, timing of completion of the FDA`s review of our NDA, the extent to which the FDA may request additional information or data and our ability to provide the same in a timely manner, acceptability of the Phase 1, 2 and 3 clinical trial data by the FDA, FDA approval of the Company`s NDA for the treatment of ocular metastatic melanoma to the liver, adoption, use and resulting sales, if any, in the United States, adoption, use and resulting sales, if any, for the CHEMOSAT system to deliver and filter melphalan in the EEA, our ability to successfully commercialize the chemosaturation system and the potential of the chemosaturation system as a treatment for patients with primary and metastatic disease in the liver, market acceptance of the Gen Two CHEMOSAT system and patient outcomes using the same, approval of the current or future chemosaturation system for delivery and filtration of melphalan, doxorubicin or other chemotherapeutic agents for various indications in the US and/or in foreign markets, actions by the FDA or other foreign regulatory agencies, our ability to successfully enter into strategic partnership and distribution arrangements in foreign markets including Australia and key Asian markets and timing an revenue, if any, of the same, the approval of the Hepatic CHEMOSAT Delivery System device to deliver and filter doxorubicin in key Asian markets and patient outcomes using the same, our ability to obtain reimbursement for the CHEMOSAT system, uncertainties relating to the timing and results of research and development projects, uncertainties relating to the timing and results of future clinical trials, and uncertainties regarding our ability to obtain financial and other resources for any research, development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward-looking statements to reflect events or circumstances after the date they are made.
Contact Information:
Investors: Financial Media EU Consumer/Medical Media
Gregory Gin/Patty Eisenhaur Janine McCargo Julie Johnson
EVC Group EVC Group Bliss Integrated
646-445-4801/951-316-0577 646-688-0425 503-883-9103
This announcement is distributed by Thomson Reuters on behalf of Thomson Reuters clients.
The owner of this announcement warrants that:
(i) the releases contained herein are protected by copyright and other applicable laws; and
(ii) they are solely responsible for the content, accuracy and originality of the
information contained therein.
Source: Delcath Systems, Inc via Thomson Reuters ONE
HUG#1675162
@yahoofinance on Twitter, become a fan on Facebook
RELATED CONTENT
ArQule enrolls first patient in Phase 3 trial …
FDA to Review Sanofi's Lemtrada
Threshold/Merck KGaA Drug in Study
FDA gives new drug application approval to Is …
FDA Clears Teleflex Needles
CTI announces DSMB recommendation to continue …
ImmunoGen slides as analyst cuts rating
Germany warns euro crisis not over
Associated Press
Germany: Sexism debate prompts surge in complaints
Associated Press
Recent Quotes
Portfolios
Recent Quotes
Symbol
Price Change % Chg Chart
DCTH
1.51
DNDN
5.95
KERX
7.11
AMRN
8.45
ARNA
8.58
PZG
2.25
TOPO.CO
2.18 -0.08 -3.54%
EXEL
4.765
GIG
1.485
VUZI
0.099
Search for share prices
Save List to Portfolio »
Recent Quotes News
DNDN: Sharp Increase in FDA Drug Approvals a Major Factor in Biotech Industry's Success - Marketwire
KERX: Today's Market News To Trade On: 5 Stocks Moving On News - at Seeking Alpha
AMRN: Weekly Stock Watch, Week Of 4 February 2013 - at Seeking Alpha
AMRN: Keryx slide attributable to Zerenex market exclusivity worries, The Street says - theflyonthewall.com
AMRN: Keryx Falls On Questions About Zerenex Market Exclusivity - at TheStreet
More Recent Quotes News »
There are no comments yet
Leave a comment... Comment Guidelines
Post As
TOP STORIES »
European Political Worries Halt Risk Asset Rally
Reuters
Stronger U.S. and Chinese economic data supported world equity markets on Monday, while the euro dipped and Spanish …
Which Super Bowl Ads Stole the Show?MarketWatch
Low Rates Force Companies To Pour Cash Into PensionsThe Wall Street Journal
Harvard President: Zuckerberg Dropped Out, but His Staff Didn’tOff the Cuff
Humana Profit Slips; 2013 Outlook AffirmedMarketWatch
Sign up for The Daily Download, the new Yahoo! Finance morning newsletter that gives you the day's top news and stocks to watch, all in one convenient place.
ANZEIGE
TRADING CENTER
Compare Brokers »
TODAY ON YAHOO!1 - 6 of 48
Bowl ads that sparked the biggest backlash
Ravens resist comeback attempt to win
High-paying careers without grad school
Cameras capture moment not meant for TV
Kaepernick misses chance to join legends
Whatever happened to this '80s icon?
YAHOO! FINANCE ON FACEBOOK
@YAHOOFINANCE ON TWITTER
POLL
With gas prices on the rise, how is it impacting your spending?
I'm cutting back on discretionary items
Spending about the same
I'm feeling good about the economy and spending more
Vote
See Results
Wenn sich Delcath auf dem Niveau hält, wäre man durch die 100er durch. Die liegt knapp über 1,5$
Antwort auf Beitrag Nr.: 44.101.010 von sdaktien am 04.02.13 16:33:59http://us.rd.yahoo.com/finance/external/pssa/SIG=12q943jls/*…
Antwort auf Beitrag Nr.: 44.101.825 von Magnetfeldfredy am 04.02.13 18:44:01bin gestern auch mal rein.gutes volumen und nähe tageshoch geschlossen.nachbörslich 1,65 hoch.sollte jetzt bis 2 dollar erst mal laufen.

Antwort auf Beitrag Nr.: 44.102.994 von ultrawuffsn am 05.02.13 04:23:00Everyone saw the collapse of Celsion Corporation (CLSN) last week, which is something I already covered. As you may know, their drug Thermodox failed Phase III trials for the treatment of hepatocellular carcinoma (HCC) - a form a liver cancer.
Liver cancer is one of the most common cancers in the world, and seems to be have rapidly increasing incidence in the United States especially (as we "catch up" to the rest of the world). About 30,000 new patients last year get diagnosed with liver cancer, with the vast majority of these cases being HCC.
The reason that there was so much hope for Thermodox leading up to the HEAT trial data release was because it offered a giant upgrade to our current chemotherapy regimen for liver cancer (and even others as well). Liver cancer is notoriously tricky, which is why there is a lot of emphasis on improvements on basic chemotherapy delivery. After all, finding new liver cancer drugs with new mechanisms of action is easier said than done.
But, had Thermodox succeeded, it would not have had trouble with healthcare reimbursement or general acceptance amongst oncologists. It used heat-activated doxorubicin, which is one of the most common chemotherapeutic agents out there.
Now that Thermodox seems to be out of the question, a lot of the attention turns to Delcath Systems (DCTH). Delcath is developing a system known as CHEMOSAT, which is a potential improvement to chemotherapy delivery to the liver - much like Thermodox. The difference is that CHEMOSAT isolates the liver's blood flow through a system of catheters, which allows a chemotherapeutic compound to be pumped directly into the liver while limiting damage to the rest of the patient's body through circulatory isolation.
The company had some trouble in recent years with the approval process, although these were largely manufacturing related and represented no real threat to the survival of CHEMOSAT's program.
Drawing more comparisons between Thermodox and CHEMOSAT, you can see that Thermodox failed the Phase III HEAT trial because it wasn't able to demonstrated statistically significant improvement in progression free survival of its treatment arm versus placebo arm. CHEMOSAT, in its pivotal Phase III trials, did exactly that. Also worth noting, both Phase III trials were conducted under special protocol assessment.
Anyway, I also believe that Delcath's market capitalization, which is approximately $113 million right now, is not representative of CHEMOSAT's real market potential after a potential FDA approval (which is estimated to be well over $2 billion). This makes me think that either DCTH would immediately have a jump following FDA approval, or have a gradual run-up as we head towards the PDUFA date of June 15th 2013.
Another point the bulls make is that CHEMOSAT is already approved and ready to go in Europe, and seems to be quite popular. Just today (February 4th) we got news that their healthcare reimbursement situation in Germany is looking terrific as well. Germany is key, of course, because it is the biggest liver cancer market in Europe.
While DCTH is inexpensive in terms of valuation, I think that the company is not all that cash rich ($28 million, as mentioned in the Q3 2012 earnings press release). The company does have $35 million in equity financing that won't be dilutive to shareholders, although they may want to raise cash for the marketing budget of CHEMOSAT going into 2014. I think there's a fair chance that we'll see an equity financing in the second half of 2013, following a hypothetical FDA approval of CHEMOSAT.
Celsion has a market capitalization of about $46 million at this point, which is clearly much lower than that of Delcath but justified. This low valuation does reduce Celsion's risk to the downside, but the results of the HEAT trial bring question as to whether or not Thermodox is worth anything.
Even if the company wanted to pursue strategic alternatives for the drug, I think they'd have a very hard time finding a buyer. If the company wanted to finance another Phase III trial for Thermodox, they'd probably have to raise the capital through share dilution. I really can't imagine anything that would put CLSN investors in a more sour mood than an equity financing following a Phase III trial failure.
I'm not sure if remaining CLSN shareholders are willing to accept their losses, but I do think that anyone willing to buy and hold DCTH as we get close to the PDUFA for CHEMOSAT could make very substantial gains just on the anticipation of the event. Given that DCTH has a catalyst ahead while CLSN doesn't, I think Delcath is the no-brainer option when it comes to current liver cancer investments.



 5 dollar wir kommen.
5 dollar wir kommen.
Liver cancer is one of the most common cancers in the world, and seems to be have rapidly increasing incidence in the United States especially (as we "catch up" to the rest of the world). About 30,000 new patients last year get diagnosed with liver cancer, with the vast majority of these cases being HCC.
The reason that there was so much hope for Thermodox leading up to the HEAT trial data release was because it offered a giant upgrade to our current chemotherapy regimen for liver cancer (and even others as well). Liver cancer is notoriously tricky, which is why there is a lot of emphasis on improvements on basic chemotherapy delivery. After all, finding new liver cancer drugs with new mechanisms of action is easier said than done.
But, had Thermodox succeeded, it would not have had trouble with healthcare reimbursement or general acceptance amongst oncologists. It used heat-activated doxorubicin, which is one of the most common chemotherapeutic agents out there.
Now that Thermodox seems to be out of the question, a lot of the attention turns to Delcath Systems (DCTH). Delcath is developing a system known as CHEMOSAT, which is a potential improvement to chemotherapy delivery to the liver - much like Thermodox. The difference is that CHEMOSAT isolates the liver's blood flow through a system of catheters, which allows a chemotherapeutic compound to be pumped directly into the liver while limiting damage to the rest of the patient's body through circulatory isolation.
The company had some trouble in recent years with the approval process, although these were largely manufacturing related and represented no real threat to the survival of CHEMOSAT's program.
Drawing more comparisons between Thermodox and CHEMOSAT, you can see that Thermodox failed the Phase III HEAT trial because it wasn't able to demonstrated statistically significant improvement in progression free survival of its treatment arm versus placebo arm. CHEMOSAT, in its pivotal Phase III trials, did exactly that. Also worth noting, both Phase III trials were conducted under special protocol assessment.
Anyway, I also believe that Delcath's market capitalization, which is approximately $113 million right now, is not representative of CHEMOSAT's real market potential after a potential FDA approval (which is estimated to be well over $2 billion). This makes me think that either DCTH would immediately have a jump following FDA approval, or have a gradual run-up as we head towards the PDUFA date of June 15th 2013.
Another point the bulls make is that CHEMOSAT is already approved and ready to go in Europe, and seems to be quite popular. Just today (February 4th) we got news that their healthcare reimbursement situation in Germany is looking terrific as well. Germany is key, of course, because it is the biggest liver cancer market in Europe.
While DCTH is inexpensive in terms of valuation, I think that the company is not all that cash rich ($28 million, as mentioned in the Q3 2012 earnings press release). The company does have $35 million in equity financing that won't be dilutive to shareholders, although they may want to raise cash for the marketing budget of CHEMOSAT going into 2014. I think there's a fair chance that we'll see an equity financing in the second half of 2013, following a hypothetical FDA approval of CHEMOSAT.
Celsion has a market capitalization of about $46 million at this point, which is clearly much lower than that of Delcath but justified. This low valuation does reduce Celsion's risk to the downside, but the results of the HEAT trial bring question as to whether or not Thermodox is worth anything.
Even if the company wanted to pursue strategic alternatives for the drug, I think they'd have a very hard time finding a buyer. If the company wanted to finance another Phase III trial for Thermodox, they'd probably have to raise the capital through share dilution. I really can't imagine anything that would put CLSN investors in a more sour mood than an equity financing following a Phase III trial failure.
I'm not sure if remaining CLSN shareholders are willing to accept their losses, but I do think that anyone willing to buy and hold DCTH as we get close to the PDUFA for CHEMOSAT could make very substantial gains just on the anticipation of the event. Given that DCTH has a catalyst ahead while CLSN doesn't, I think Delcath is the no-brainer option when it comes to current liver cancer investments.



 5 dollar wir kommen.
5 dollar wir kommen.
Antwort auf Beitrag Nr.: 44.102.995 von ultrawuffsn am 05.02.13 04:34:37Schon ein paar Tage alt, aber immer noch aktuell:
Delcath Systems
Delcath Systems (DCTH) is a development stage, specialty pharmaceutical and medical device company focused on oncology, initially cancers in the liver. This stock is trading up 8.4% to $1.54 in recent trading.
Today’s Range: $1.40-$1.55
52-Week Range: $1.01-$4.74
Volume: 3.25 million
Three-Month Average Volume: 1.02 million
From a technical perspective, DCTH is ripping higher here right above its 50-day moving average of $1.35 with monster upside volume. This stock has been uptrending for the last month, with shares moving higher from its recent low of $1.16 to its intraday high of $1.55. During that move, shares of DCTH have been consistently making higher lows and higher highs, which is bullish technical price action. That move has now pushed DCTH within range of triggering a near-term breakout trade. That trade will hit if DCTH manages to clear some near-term overhead resistance at $1.67 with high volume.
Traders should now look for long-biased trades in DCTH as long as it’s trending above its 50-day at $1.35, and then once it sustains a move or close $1.67 with volume that hits near or above 1.02 million shares. If that breakout triggers soon, then DCTH will set up to re-test or possibly take out its next major overhead resistance levels at $1.84 to $2.24.
To see more stocks that are making notable moves higher today, check out the Stocks Under $10 Moving Higher portfolio on Stockpickr.
-- Written by Roberto Pedone in Winderemere, Fla.
Delcath Systems
Delcath Systems (DCTH) is a development stage, specialty pharmaceutical and medical device company focused on oncology, initially cancers in the liver. This stock is trading up 8.4% to $1.54 in recent trading.
Today’s Range: $1.40-$1.55
52-Week Range: $1.01-$4.74
Volume: 3.25 million
Three-Month Average Volume: 1.02 million
From a technical perspective, DCTH is ripping higher here right above its 50-day moving average of $1.35 with monster upside volume. This stock has been uptrending for the last month, with shares moving higher from its recent low of $1.16 to its intraday high of $1.55. During that move, shares of DCTH have been consistently making higher lows and higher highs, which is bullish technical price action. That move has now pushed DCTH within range of triggering a near-term breakout trade. That trade will hit if DCTH manages to clear some near-term overhead resistance at $1.67 with high volume.
Traders should now look for long-biased trades in DCTH as long as it’s trending above its 50-day at $1.35, and then once it sustains a move or close $1.67 with volume that hits near or above 1.02 million shares. If that breakout triggers soon, then DCTH will set up to re-test or possibly take out its next major overhead resistance levels at $1.84 to $2.24.
To see more stocks that are making notable moves higher today, check out the Stocks Under $10 Moving Higher portfolio on Stockpickr.
-- Written by Roberto Pedone in Winderemere, Fla.
Antwort auf Beitrag Nr.: 44.104.333 von Magnetfeldfredy am 05.02.13 12:10:11Delcath gets boost from German government
Enlarge Image
Donna Abbott Vlahos | The Business Review
Thomas Cosey Jr. examines the bonding of a double-balloon catheter at Delcath’s Queensbury facility.
Barbara Pinckney
Reporter-
The Business Review
Email
Delcath Systems Inc. is poised to grow its market in Germany after getting the green light from the country’s federal reimbursement agency.
The New York City-based company, which has its manufacturing opertions in Queensbury, Warren County, makes a liver chemotherapy system called Chemostat, and has been setting up delivery channels in Europe for the past year. The decision from the German agency means that while insurers in that country are not mandated to cover Chemostat, hospitals are now permitted to negotiate for coverage.
Eamonn Hobbs, president & CEO of Delcath (Nasdaq: DCTH) said this presents a “significant positive step” in the company’s efforts to fully commercialize Chemostat in Europe.
“This is the first reimbursement mechanism for our procedure in Germany, the biggest market for CHEMOSAT in the European Union,” he said. “With a direct sales force in place and training of the additional centers in Germany on the way, we are now in a good position to begin growing this market for Chemostat.”
Delcath, which last week trimmed its local workforce, earned $146,000 from European sales in the first nine months of 2011—the first product sales in its 24-year history. It is awaiting FDA approval to sell Chemostat in the United States.
Pinckney covers banking, health care, media and advertising.
Enlarge Image
Donna Abbott Vlahos | The Business Review
Thomas Cosey Jr. examines the bonding of a double-balloon catheter at Delcath’s Queensbury facility.
Barbara Pinckney
Reporter-
The Business Review
Delcath Systems Inc. is poised to grow its market in Germany after getting the green light from the country’s federal reimbursement agency.
The New York City-based company, which has its manufacturing opertions in Queensbury, Warren County, makes a liver chemotherapy system called Chemostat, and has been setting up delivery channels in Europe for the past year. The decision from the German agency means that while insurers in that country are not mandated to cover Chemostat, hospitals are now permitted to negotiate for coverage.
Eamonn Hobbs, president & CEO of Delcath (Nasdaq: DCTH) said this presents a “significant positive step” in the company’s efforts to fully commercialize Chemostat in Europe.
“This is the first reimbursement mechanism for our procedure in Germany, the biggest market for CHEMOSAT in the European Union,” he said. “With a direct sales force in place and training of the additional centers in Germany on the way, we are now in a good position to begin growing this market for Chemostat.”
Delcath, which last week trimmed its local workforce, earned $146,000 from European sales in the first nine months of 2011—the first product sales in its 24-year history. It is awaiting FDA approval to sell Chemostat in the United States.
Pinckney covers banking, health care, media and advertising.
Antwort auf Beitrag Nr.: 41.276.242 von Poppholz am 28.03.11 13:46:09hammerstark das teil.

Antwort auf Beitrag Nr.: 44.106.821 von ultrawuffsn am 05.02.13 20:25:44immer noch auf meiner Watchlist.
Charttechnisch scheint der Boden gefunden zu sein.
Mal sehen was passiert.
Gegebenenfalls habe ich demnächst mal wieder ein wenig Spielgeld frei.

Charttechnisch scheint der Boden gefunden zu sein.
Mal sehen was passiert.
Gegebenenfalls habe ich demnächst mal wieder ein wenig Spielgeld frei.

bin eingestiegen.
Charttechnisch sieht es gut aus.
Bin gespannt.

Charttechnisch sieht es gut aus.
Bin gespannt.

Antwort auf Beitrag Nr.: 44.161.479 von Poppholz am 20.02.13 07:37:43Auch fundamental sieht es langfristig gut aus, EU-Zulassung ist schon lange da, Erstattung in Deutschland schon möglich, viele Behandlungszentren in Europa und USA FDA PDUFA Date im Juni......
Und er Hauptkonkurrent Celsion hat die Phase III endpoints nicht erreicht....
Frage mich schon lange warum Delcath so weit unten steht?
Und er Hauptkonkurrent Celsion hat die Phase III endpoints nicht erreicht....
Frage mich schon lange warum Delcath so weit unten steht?

Antwort auf Beitrag Nr.: 44.161.479 von Poppholz am 20.02.13 07:37:43erstmal seitwärts mit leichter Tendenz nach unten.
Aktuell $1,47
Aktuell $1,47
Eigentlich sieht Delcath nicht schlecht aus, der Kurs ging im Februar zurück, ebenso das Handelsvolumen. 38er und 100er generieren ein Kaufsignal. Das ist zwar nicht so ausgeprägt, aber immerhin.
Nur irgendwie werd ich den Verdacht nicht los, dass die Aktie nicht aus dem Quark kommt. Haber aber nichts dagegen Contra-Indikator zu sein.
Nur irgendwie werd ich den Verdacht nicht los, dass die Aktie nicht aus dem Quark kommt. Haber aber nichts dagegen Contra-Indikator zu sein.
Nachtrag: Genau bei 1.65$ befindet sich eine ehemalige Unterstützung, die durch den Sturz im November zu einem Widerstand geworden ist und ihren Ursprung im August hat. Genau da ist Delcath erst einmal gescheitert. In diesem Bereich befindet sich auch die 200er, was diesen Widerstand noch massiver macht.
Andererseits:Bricht Delcath da nach oben durch, könnte es massiv nach oben gehen.
Andererseits:Bricht Delcath da nach oben durch, könnte es massiv nach oben gehen.
Antwort auf Beitrag Nr.: 44.183.814 von sdaktien am 25.02.13 17:52:44sehe ich auch so.
Mal sehen was die nächsten Wochen bringen.
Mal sehen was die nächsten Wochen bringen.
Delcath Systems, Inc. : DELCATH ANNOUNCES ODAC MEETING REVIEW DATE FOR ITS PROPRIETARY DRUG/DEVICE COMBINATION PRODUCT DELCATH HEPATIC DELIVERY SYSTEM
02/27/2013| 07:41am US/Eastern
DELCATH ANNOUNCES ODAC MEETING REVIEW DATE FOR ITS PROPRIETARY DRUG/DEVICE COMBINATION PRODUCT DELCATH HEPATIC DELIVERY SYSTEM
Meeting to be Held May 2, 2013
NEW YORK, Feb. 27, 2013 -- Delcath Systems, Inc. (NASDAQ: DCTH) today announced that the U.S. Food and Drug Administration's (FDA) Oncologic Drugs Advisory Committee (ODAC) will review the Company's pending New Drug Application (NDA) for a drug/device combination product with the proposed trade name Melblez KitTM (Melblez (melphalan) for Injection for use with the Delcath Hepatic Delivery System. The ODAC meeting will be convened Thursday, May 2, 2013, to review the NDA with a proposed indication for the treatment of patients with unresectable ocular melanoma that is metastatic to the liver.
ODAC panels advise the U.S. Food and Drug Administration on the safety and efficacy of proposed new cancer therapies. The FDA is not legally bound to follow the advice of its advisory committees regarding new drug applications. Delcath's NDA was accepted by the FDA for substantive review on October 15, 2012, and was assigned a Prescription Drug User Fee Act (PDUFA) goal date of June 15, 2013.
Eamonn P. Hobbs, President & CEO of Delcath Systems, said, "Our team is actively preparing for the ODAC meeting and we look forward to presenting our data for the safety and efficacy of Delcath's system for the treatment of patients with unresectable ocular melanoma metastatic to the liver to the ODAC panel."
The FDA will publish materials, including webcast information, pertaining to the meeting at http://www.fda.gov/AdvisoryCommittees/WhatsNew/default.htm.
Changes to the Advisory Committee meetings calendars may also be found on the FDA website at http://www.fda.gov/AdvisoryCommittees/Calendar/default.htm
About Delcath Systems
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Our proprietary drug/device combination product Delcath Hepatic Delivery System is designed to administer high dose chemotherapy and other therapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath announced that its randomized Phase 3 clinical trial for patients with metastatic melanoma in the liver had successfully achieved the study's primary endpoint of extended hepatic progression-free survival. The Company also completed a multi-arm Phase 2 trial to treat other liver cancers. Outside of the United States, our proprietary product to deliver and filter melphalan hydrochloride is marketed under the trade name Delcath Hepatic CHEMOSAT® Delivery System (CHEMOSAT Delivery System for Melphalan.) The Company obtained authorization to affix a CE Mark for the Generation Two CHEMOSAT Delivery System for Melphalan in April 2012. The right to affix the CE mark allows the Company to market and sell the CHEMOSAT Delivery System for Melphalan in Europe. In October 2012, the Company satisfied all of the requirements to affix the CE Mark to the Hepatic CHEMOSAT Delivery System device for intra-hepatic arterial delivery and extracorporeal filtration of doxorubicin hydrochloride injection (CHEMOSAT Delivery System for Doxorubicin), providing a regulatory pathway for the CHEMOSAT Delivery System for Doxorubicin for countries in Asia that accept the CE Marking as part of their national regulatory requirements. The Company has not yet received FDA approval for commercial sale of its system in the United States. The Company's NDA has been accepted for filing and substantive review by the FDA. For more information, please visit the Company's website at www.delcath.com.
Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by the Company or on its behalf. This news release contains forward-looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to: the outcome of the ODAC meeting, and the impact, if any, of the advisory panel's recommendation on the FDA's decision regarding the Company's new drug application (NDA), timing of completion of the FDA's review of our NDA, the extent to which the FDA may request additional information or data and our ability to provide the same in a timely manner, acceptability of the Phase 1, 2 and 3 clinical trial data by the FDA, FDA approval of the Company's NDA for the treatment of ocular metastatic melanoma to the liver, adoption, use and resulting sales, if any, in the United States, adoption, use and resulting sales, if any, for the CHEMOSAT system to deliver and filter melphalan in the EEA, our ability to successfully commercialize the chemosaturation system and the potential of the chemosaturation system as a treatment for patients with primary and metastatic disease in the liver, market acceptance of the Gen Two CHEMOSAT system and patient outcomes using the same, approval of the current or future chemosaturation system for delivery and filtration of melphalan, doxorubicin or other chemotherapeutic agents for various indications in the US and/or in foreign markets, actions by the FDA or other foreign regulatory agencies, our ability to successfully enter into strategic partnership and distribution arrangements in foreign markets including Australia and key Asian markets and timing an revenue, if any, of the same, the approval of the Hepatic CHEMOSAT Delivery System device to deliver and filter doxorubicin in key Asian markets and patient outcomes using the same, our ability to obtain reimbursement for the CHEMOSAT system, uncertainties relating to the timing and results of research and development projects, uncertainties relating to the timing and results of future clinical trials, and uncertainties regarding our ability to obtain financial and other resources for any research, development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward-looking statements to reflect events or circumstances after the date they are made.
Contact Information:
Investor Contact: Media Contact:
Doug Sherk/Gregory Gin Chris Gale
EVC Group EVC Group
415-568-4887/646-445-4801 646-201-5431
cgale@evcgroup.com
http://www.4-traders.com/DELCATH-SYSTEMS-INC-9017/news/Delca…
02/27/2013| 07:41am US/Eastern
DELCATH ANNOUNCES ODAC MEETING REVIEW DATE FOR ITS PROPRIETARY DRUG/DEVICE COMBINATION PRODUCT DELCATH HEPATIC DELIVERY SYSTEM
Meeting to be Held May 2, 2013
NEW YORK, Feb. 27, 2013 -- Delcath Systems, Inc. (NASDAQ: DCTH) today announced that the U.S. Food and Drug Administration's (FDA) Oncologic Drugs Advisory Committee (ODAC) will review the Company's pending New Drug Application (NDA) for a drug/device combination product with the proposed trade name Melblez KitTM (Melblez (melphalan) for Injection for use with the Delcath Hepatic Delivery System. The ODAC meeting will be convened Thursday, May 2, 2013, to review the NDA with a proposed indication for the treatment of patients with unresectable ocular melanoma that is metastatic to the liver.
ODAC panels advise the U.S. Food and Drug Administration on the safety and efficacy of proposed new cancer therapies. The FDA is not legally bound to follow the advice of its advisory committees regarding new drug applications. Delcath's NDA was accepted by the FDA for substantive review on October 15, 2012, and was assigned a Prescription Drug User Fee Act (PDUFA) goal date of June 15, 2013.
Eamonn P. Hobbs, President & CEO of Delcath Systems, said, "Our team is actively preparing for the ODAC meeting and we look forward to presenting our data for the safety and efficacy of Delcath's system for the treatment of patients with unresectable ocular melanoma metastatic to the liver to the ODAC panel."
The FDA will publish materials, including webcast information, pertaining to the meeting at http://www.fda.gov/AdvisoryCommittees/WhatsNew/default.htm.
Changes to the Advisory Committee meetings calendars may also be found on the FDA website at http://www.fda.gov/AdvisoryCommittees/Calendar/default.htm
About Delcath Systems
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Our proprietary drug/device combination product Delcath Hepatic Delivery System is designed to administer high dose chemotherapy and other therapeutic agents to diseased organs or regions of the body, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. In 2010, Delcath announced that its randomized Phase 3 clinical trial for patients with metastatic melanoma in the liver had successfully achieved the study's primary endpoint of extended hepatic progression-free survival. The Company also completed a multi-arm Phase 2 trial to treat other liver cancers. Outside of the United States, our proprietary product to deliver and filter melphalan hydrochloride is marketed under the trade name Delcath Hepatic CHEMOSAT® Delivery System (CHEMOSAT Delivery System for Melphalan.) The Company obtained authorization to affix a CE Mark for the Generation Two CHEMOSAT Delivery System for Melphalan in April 2012. The right to affix the CE mark allows the Company to market and sell the CHEMOSAT Delivery System for Melphalan in Europe. In October 2012, the Company satisfied all of the requirements to affix the CE Mark to the Hepatic CHEMOSAT Delivery System device for intra-hepatic arterial delivery and extracorporeal filtration of doxorubicin hydrochloride injection (CHEMOSAT Delivery System for Doxorubicin), providing a regulatory pathway for the CHEMOSAT Delivery System for Doxorubicin for countries in Asia that accept the CE Marking as part of their national regulatory requirements. The Company has not yet received FDA approval for commercial sale of its system in the United States. The Company's NDA has been accepted for filing and substantive review by the FDA. For more information, please visit the Company's website at www.delcath.com.
Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by the Company or on its behalf. This news release contains forward-looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to: the outcome of the ODAC meeting, and the impact, if any, of the advisory panel's recommendation on the FDA's decision regarding the Company's new drug application (NDA), timing of completion of the FDA's review of our NDA, the extent to which the FDA may request additional information or data and our ability to provide the same in a timely manner, acceptability of the Phase 1, 2 and 3 clinical trial data by the FDA, FDA approval of the Company's NDA for the treatment of ocular metastatic melanoma to the liver, adoption, use and resulting sales, if any, in the United States, adoption, use and resulting sales, if any, for the CHEMOSAT system to deliver and filter melphalan in the EEA, our ability to successfully commercialize the chemosaturation system and the potential of the chemosaturation system as a treatment for patients with primary and metastatic disease in the liver, market acceptance of the Gen Two CHEMOSAT system and patient outcomes using the same, approval of the current or future chemosaturation system for delivery and filtration of melphalan, doxorubicin or other chemotherapeutic agents for various indications in the US and/or in foreign markets, actions by the FDA or other foreign regulatory agencies, our ability to successfully enter into strategic partnership and distribution arrangements in foreign markets including Australia and key Asian markets and timing an revenue, if any, of the same, the approval of the Hepatic CHEMOSAT Delivery System device to deliver and filter doxorubicin in key Asian markets and patient outcomes using the same, our ability to obtain reimbursement for the CHEMOSAT system, uncertainties relating to the timing and results of research and development projects, uncertainties relating to the timing and results of future clinical trials, and uncertainties regarding our ability to obtain financial and other resources for any research, development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward-looking statements to reflect events or circumstances after the date they are made.
Contact Information:
Investor Contact: Media Contact:
Doug Sherk/Gregory Gin Chris Gale
EVC Group EVC Group
415-568-4887/646-445-4801 646-201-5431
cgale@evcgroup.com
http://www.4-traders.com/DELCATH-SYSTEMS-INC-9017/news/Delca…
aktuell läuft es doch ganz gut:
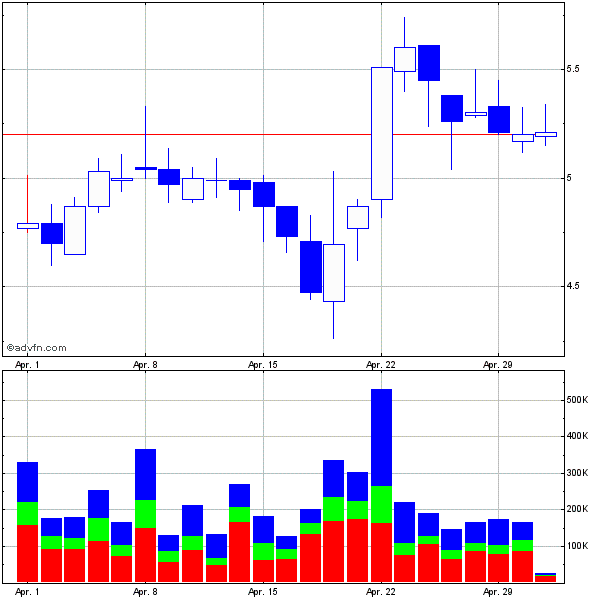
Potential nach oben ist ja genug vorhanden.

Potential nach oben ist ja genug vorhanden.

Antwort auf Beitrag Nr.: 44.183.814 von sdaktien am 25.02.13 17:52:44die Unterstützung ist sauber genommen worden.
Aktuell: $ 1,74
Aktuell: $ 1,74
Antwort auf Beitrag Nr.: 44.211.732 von Poppholz am 04.03.13 16:37:23Delcath scheint nicht nur technisch auszubrechen, heute Präsentation, mehr auf:
www.delcath.com
inclusive neuer März Präsentation!
www.delcath.com
inclusive neuer März Präsentation!
Antwort auf Beitrag Nr.: 44.212.728 von Magnetfeldfredy am 04.03.13 19:56:38die Vorfreude ist immer das schönste am Fest.


aktuell vorbörslich
$1,83 zu $2,03

$1,83 zu $2,03

Antwort auf Beitrag Nr.: 44.221.136 von Poppholz am 06.03.13 16:03:07Delcath kann in 1-2 Jahren zum Tenbagger werden!

Antwort auf Beitrag Nr.: 44.225.595 von Magnetfeldfredy am 07.03.13 14:35:00http://us.rd.yahoo.com/finance/external/pssa/SIG=13jdeccuk/*…
Antwort auf Beitrag Nr.: 44.211.732 von Poppholz am 04.03.13 16:37:23Das Handelsvolumen in New York ist ordentlich, der Stochastik ist aber überhitzt. Ich kann mir vorstellen, dass Delcath trotzdem noch weitersteigt Bis wohin ist schwer zu sagen. Ein nächster Widerstand wäre die Zone zwischen 2,3$ und 2,4$. Muss aber nicht sein.
Die 38er und die 200er sind jetzt dran mit dem Kaufsignal. Möglich, dass dies zu erneuten starken Kurssteigerungen führt. Wenn nicht, wäre es wichtig, dass Delcath in die Nähe des Schnittpunkts konsolidiert
Am 13. kommen die Zahlen für 2012. Hoffentlich gibt es keine böse Überraschung. In der Vergangenheit hat man die Analysten gerne mal enttäuscht.
Ich setz mal den Originalartikel von seeking alpha hier ein:
http://seekingalpha.com/article/1259171-delcath-systems-bull…
Die 38er und die 200er sind jetzt dran mit dem Kaufsignal. Möglich, dass dies zu erneuten starken Kurssteigerungen führt. Wenn nicht, wäre es wichtig, dass Delcath in die Nähe des Schnittpunkts konsolidiert
Am 13. kommen die Zahlen für 2012. Hoffentlich gibt es keine böse Überraschung. In der Vergangenheit hat man die Analysten gerne mal enttäuscht.
Ich setz mal den Originalartikel von seeking alpha hier ein:
http://seekingalpha.com/article/1259171-delcath-systems-bull…
Antwort auf Beitrag Nr.: 44.233.806 von sdaktien am 09.03.13 18:13:22gestern ist der Kurs schön bei $2,10 beblieben.
Mal sehen was heute und morgen passiert.
Mal sehen was heute und morgen passiert.
zur Kenntnis: heutiges Statement vom CEO Eamonn P. Hobbs
+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
copy by reuters.com
+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
"Delcath für das vierte Quartal und das gesamte Jahr 2012
ERGEBNISSE
* Reuters ist nicht verantwortlich für den Inhalt dieser Pressemitteilung."
+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
Mi 13. März 2013 04.02 Uhr EDT
Die besten Ergebnisse beim Drucken dieser Ankündigung, klicken Sie bitte auf den folgenden Link:
- Telefonkonferenz und Webcast heute um 4:30 Uhr ET -
NEW YORK, 13. März 2013 - Delcath Systems, Inc. (NASDAQ: DCTH) meldete heute die Finanzergebnisse
und operativen Highlights des vierten steuerlichen Quartal und das Gesamtjahr zum 31. Dezember 2012.
Highlights aus dem Geschäftsjahr 2012 und den letzten Wochen sind:
* TMAcceptance von der US Food and Drug Administration (FDA) für die inhaltliche Überprüfung der
Firma New Drug Application (NDA) für seine firmeneigene Medikamentenentwicklung / Gerätekombination Produkt mit dem
vorgeschlagen Markennamen Melblez Kit (Melblez (Melphalan) zur Injektion zur Verwendung mit dem Delcath
Hepatischen Delivery System), mit einer beabsichtigten Indikation zur Behandlung von Patienten mit
inoperablem okuläre Melanom mit Metastasen in der Leber - die FDA zugeordnet einen PDUFA Ziel Termin Juni
15, 2013
* Ankündigung eines Oncologic Drugs Advisory Committee (ODAC) Sitzung des Gremiums am 2. Mai 2013
Beurteilung des Unternehmens NDA
* Einleitung der US Expanded Access Program (EAP) und Therapie der ersten EAP Patienten in den USA
* Q4 Verwendung der Barmittel um 33% reduziert, um Q3 im Vergleich
* Erste kommerzielle Umsatz in der Unternehmensgeschichte
* (R) Therapie der ersten Patienten in Europa mit dem CHEMOSAT Leber Delivery System für Melphalan
Hydrochlorid
* Wert 4 interim Erstattung Versorgung in Deutschland erteilt und identifiziert eine bestehende DRG-Code für
teilweise Rückerstattung in Italien-Kliniken Vorlage Zwischenbericht Erstattung Anwendungen sowohl in
Ländern
* Erhalt der CE-Zulassung für CHEMOSAT Leber Delivery System zu liefern und zu filtern
Doxorubicin-Hydrochlorid Injektion
"Letztes Jahr war ein bedeutender Leistungen für Delcath, von der FDA die Akzeptanz unserer führten
NDA für die Melblez Kit System für inhaltliche Überprüfung ", sagte Eamonn P. Hobbs, Präsident und CEO von
Delcath Systems. "Submission unserer Anwendung war der Höhepunkt einer intensiven Anstrengungen unserer
Team und FDA Akzeptanz unserer NDA und Benennung eines 15. Juni 2013 PDUFA Ziel aktuell sind die
wichtigsten Entwicklungen in unserer Geschichte. Im Laufe des Jahres haben wir auch festgestellt unseren ersten
kommerzielle Präsenz in Europa, unsere erste kommerzielle Vertrieb und in den letzten Wochen begonnen
Verhandlungen mit der FDA auf unserem klinischen Entwicklungsprogramm mit dem Ziel der Erweiterung des Etiketts
für konzentrierte, Therapie Leber-directed ".
"Wir stellten CHEMOSAT in Europa im vergangenen Jahr, eine Präsenz für die Behandlung in
alle sieben unseren Zielländern ", fuhr Mr. Hobbs." Patienten mit Leber-dominante Erkrankung
aus verschiedenen Tumorarten behandelt worden sind, und Arzt Interesse an der therapeutischen Ansatz
mit CHEMOSAT stark bleibt. Wir glauben Veröffentlichung unserer Phase-3-Melanom-und Phase 2
Multi-Histologie Daten aus klinischen Studien weiter fahren dieses Interesse. Entwicklung von attraktiven
Erstattung ist ein wichtiger Treiber der Verwertung und Umsatzwachstum. Wir arbeiten eng mit
Experten und unseren Partner Krankenhäuser zu bringen verschiedenen Zwischenbericht Erstattungsmechanismen online, und in
parallel in Anwendungen Prozesses engagiert permanent, überzeugende Erstattung zu etablieren.
Obwohl diese Mechanismen Online langsamer als wir erwartet gekommen sind, hat die Einnahmen ausgewirkt
Rampe, Arzt Interesse an unserer Therapie ist stark und wir bleiben optimistisch über die langfristigen
Aussichten für CHEMOSAT in Europa. "
"In den Vereinigten Staaten, wir weiterhin eng mit der FDA in ihrer Bewertung des Zulassungsantrags und
sind für unsere Präsentation auf der ODAC Panel Vorbereitung am 2. Mai. Unsere Zusammenarbeit mit der FDA hat
konstruktiv, und wir sind zuversichtlich, dass die Agentur die Überprüfung auf dem PDUFA Ziel Schluss
Datum. Wenn unsere NDA zugelassen ist, ist unser Plan, in den USA im vierten Quartal starten,
sich zunächst auf die Krankenhäuser, die in unserem kürzlich gestarteten EAP teilnehmen und haben
nahmen in unseren klinischen Studien. Mit diesem Ziel vor Augen sind wir derzeit, einen
sehr seltenen Preise und Vermarktungsstrategie in den USA für inoperablen Metastasen okulären
Melanom ", schloss Mr. Hobbs.
Vierte Quartal und das Gesamtjahr Financial Results
Für die drei Monate zum 31. Dezember 2012, erfasst Delcath Umsatz von $ 0,2 Millionen. Betriebs-
Verlust betrug $ 11,8 Mio., die ungefähr $ 0,9 Mio. nicht zahlungswirksame aktienbasierte enthalten
Aufwendungen, wie mit einem operativen Verlust von $ 16,0 Millionen, verglichen, einschließlich $ 0.900.000
in unbaren Vergütungsaufwand im gleichen Vorjahreszeitraum. Vertriebs-und allgemeine
Verwaltungskosten (SG & A) betrugen $ 6,4 Mio. im vierten Quartal 2012, verglichen mit $ 6,1
Millionen für den gleichen Zeitraum in 2011. Die höhere SG & A-Kosten ist vor allem auf erhöhte EU
Kommerzialisierung Aufwendungen. Forschung und Entwicklung (R & D) beliefen sich auf $ 5.600.000 für das
vierten Quartal 2012 auf $ 9.800.000 für den gleichen Zeitraum im Jahr 2011 verglichen. Der untere R & D
Aufwand spiegelt niedrigere Beratungskosten im Anschluss an die Vorlage des NDA am 15. August 2012.
Für das Jahr zum 31. Dezember 2012, erfasst Delcath Umsatz von $ 0.300.000 und eine zusätzliche
$ 30.000 von unrealisierten Erträgen im Zusammenhang mit Bestellungen von Vertriebspartnern. Die operativen
Verlust betrug $ 53,9 Mio., die ungefähr $ 3,8 Mio. nicht zahlungswirksame aktienbasierte enthalten
Aufwendungen. Der Anstieg der Kosten ist in erster Linie die EU-Vermarktung im Zusammenhang
Bemühungen. Der operative Verlust für das Geschäftsjahr zum 31. Dezember 2011 46.500.000 $, die im Lieferumfang enthalten
ca. US $ 4,3 Mio. im unbaren Vergütungsaufwand. SG & A-Aufwendungen betrugen $ 28,0
Millionen für das Geschäftsjahr zum 31. Dezember 2012, um $ 21.300.000 für das Geschäftsjahr im Vergleich
31. Dezember 2011. R & D Aufwendungen betrugen $ 26.200.000 für das Geschäftsjahr zum 31. Dezember 2012 gegenüber
um $ 25.200.000 im Geschäftsjahr zum 31. Dezember 2011.
Am 31. Dezember 2012 waren Zahlungsmittel, Zahlungsmitteläquivalente und Certificates of Deposit $ 23.700.000,
verglichen mit $ 30.800.000 am 31. Dezember 2011. Für das vierte Quartal Cash Auslastung lag
$ 9.700.000, eine 33% ige Reduktion auf $ 14.600.000 im dritten Quartal verglichen. Dies stimmte
mit der Gesellschaft angekündigten Erwartungen für durchschnittlichen monatlichen Cash-Auslastung zwischen
$ 3.000.000 und $ 4 Millionen für das vierte Quartal. Der Rückgang in der Verwendung der Barmittel war in erster Linie
angetrieben durch eine Verringerung Beratung nach Einreichung des NDA sowie insgesamt
effektives Kostenmanagement.
Das Unternehmen erfolgreich seine "auf dem Markt" (ATM) Aktienemission Programm, und zwischen
1. Januar 2013 und 28. Februar 2013 hob ca. 20.900.000 $, bevor Aufwendungen.
Am 28. Februar 2013 waren der Gesellschaft Zahlungsmittel und Zahlungsmitteläquivalente rund $ 38.000.000
(Ungeprüft). Das Unternehmen hat in eine neue ATM Aktienemission Programm eingegeben, unter denen die
Unternehmen verkaufen können bis zu $ 50 Millionen seiner Stammaktien von Zeit zu Zeit einmal die zugehörige Regal
Registration Statement wirksam wird.
"Wir bleiben verpflichtet, zur Erhaltung unserer reduzierte vierteljährliche Nutzung von $ 9.000.000 auf $ 12
Millionen für das Jahr 2013 ", sagte Graham Miao, Ph.D., Delcath Chief Financial Officer." Wir glauben, dass
mit unseren aktuellen Barbestand und den Zugang zu Kapital haben wir die finanziellen Mittel und
Flexibilität, um unsere operativen Plan für die nächsten 12 Monate und darüber hinaus führen. "
Telefonkonferenz und Webcast
Das Unternehmen wird eine Telefonkonferenz heute, 13. März 2013 16.30 Uhr ET. Die Einwahlnummern
für die Telefonkonferenz sind 877-299-4454 (US-Teilnehmer) und 617-597-5447 (International
Teilnehmer), beide Zahlen erfordern Passcode 99502530. Um die Live-Übertragung im Internet, gehen Sie zum
Events & Presentations Seite im Investor Relations-Bereich der Website des Unternehmens unter
http://www.delcath.com/investors/events/ http://www.delcath.com/investors/events/.
Eine Aufzeichnung der Telefonkonferenz wird ab Anfang etwa zwei Stunden nach dem Anruf des
Abschluss und wird für sieben Tage zur Verfügung. Einwahlnummern für die Wiedergabe sind 888-286-8010
und 617-801-6888 für die USA und internationale Anrufer bzw.. Die Wiederholung Passcode für beide
US-und internationale Anrufer lautet 41198605. Eine archivierte Webcast wird auch verfügbar sein
http://www.delcath.com/investors/events/ http://www.delcath.com/investors/events/.
Über Delcath Systems
Delcath Systems, Inc. ist ein spezialisiertes Pharma-und Medizintechnik-Unternehmen fokussiert auf
Onkologie. Unsere firmeneigene Medikamentenentwicklung / Gerätekombination Produkt, das Delcath Leber Delivery System ist
entwickelt, um Hochdosis-Chemotherapie und andere therapeutische Mittel in erkrankte Organe oder verwalten
Regionen des Körpers, während die Steuerung die systemische Exposition von diesen Agenten. Die Gesellschaft
Fokus liegt zunächst auf die Behandlung von primären und metastatischen Leberkrebs. Im Jahr 2010 Delcath
bekannt, dass seine randomisierten klinischen Phase-3-Studie für Patienten mit metastasierendem Melanom in der
Leber hatte erfolgreich den primären Endpunkt der Studie von erweiterten Leber progressionsfreie erreicht
Überleben. Das Unternehmen schloss zudem eine Multi-Arm-Phase-2-Studie zu anderen Leberkrebs zu behandeln.
Außerhalb der Vereinigten Staaten, an unsere eigene Produkt zu liefern und zu filtern Melphalan
Hydrochlorid wird unter dem Handelsnamen Delcath Leber CHEMOSAT (R) Delivery System vermarktet
(CHEMOSAT Delivery System für Melphalan.) Die Gesellschaft Genehmigung zum Anbringen einer CE-Kennzeichnung erhalten
für die Generation Zwei CHEMOSAT Delivery System für Melphalan im April 2012. Das Recht auf Anbringung
die CE-Kennzeichnung ermöglicht es dem Unternehmen zu vermarkten und zu verkaufen CHEMOSAT Delivery System für Melphalan in
Europa. Im Oktober 2012 wird das Unternehmen alle Anforderungen zur Anbringung der CE-Kennzeichnung die zufrieden
Leber CHEMOSAT Delivery System für Intra-arteriellen Lieferung und extrakorporale
Filtration von Doxorubicin-Hydrochlorid Injektion (CHEMOSAT Delivery System für Doxorubicin),
Bereitstellung eines regulatorischen Weg für die CHEMOSAT Delivery System für Doxorubicin für die Länder
Asien, die die CE-Kennzeichnung als Teil ihrer nationalen regulatorischen Anforderungen zu akzeptieren. Das Unternehmen
noch nicht FDA-Zulassung für den kommerziellen Verkauf seines Systems in den Vereinigten Staaten erhalten. Die
Gesellschaft NDA für Ablage und inhaltliche Überprüfung durch die FDA akzeptiert. Weitere
Informationen, besuchen Sie bitte die Website des Unternehmens unter www.delcath.com http://www.delcath.com/.
Private Securities Litigation Reform Act von 1995 bietet einen sicheren Hafen für zukunftsweisende
Aussagen des Unternehmens oder in seinem Namen gemacht. Diese Presseinformation enthält bestimmte in die Zukunft gerichtete
Aussagen, die gewissen Risiken und Unsicherheiten, welche die tatsächlichen Ergebnisse können, sind
erheblich von jenen beschrieben. Faktoren, die solche Abweichungen verursachen können, gehören, sind aber
nicht beschränkt auf, Unsicherheiten in Bezug auf: das Ergebnis der ODAC-Sitzung, und die Auswirkungen, wenn
überhaupt, der Beirat der Empfehlung auf die Entscheidung der FDA bezüglich der Gesellschaft neues Medikament
Application (NDA), Zeitpunkt der Fertigstellung der FDA Überprüfung unserer NDA, das Ausmaß, in dem die
FDA kann zusätzliche Informationen oder Daten und unsere Fähigkeit, die gleiche rechtzeitig liefern
Weise Akzeptanz der Phase 1, 2 und 3 Daten aus klinischen Studien von der FDA, FDA-Zulassung des
Gesellschaft NDA für die Behandlung von Augenkrankheiten metastasierendem Melanom in der Leber, die Annahme, Verwendung und
daraus resultierenden Umsätze, wenn überhaupt, in den Vereinigten Staaten, die Annahme, Verwendung und daraus resultierenden Umsätzen, falls vorhanden, für die
CHEMOSAT System zu liefern und zu filtern Melphalan im EWR, unsere Fähigkeit, erfolgreich
Vermarktung der Chemosättigungs System und das Potential des Systems als Chemosättigungs
Behandlung für Patienten mit primären und metastatischen Erkrankung in der Leber, die Marktakzeptanz der
Gen Zwei CHEMOSAT und Behandlungsergebnisse mit dem gleichen, mit Zustimmung des gegenwärtigen oder zukünftigen
Chemosättigungs-Systems für die Lieferung und Filtration von Melphalan, Doxorubicin oder anderen
Chemotherapeutika für verschiedene Indikationen in den USA und / oder in ausländischen Märkten, Aktionen
die FDA oder anderen ausländischen Aufsichtsbehörden, unsere Fähigkeit, erfolgreich in strategischen geben
Partnerschaft und Vertriebsvereinbarungen in ausländischen Märkten, darunter Australien und asiatischen Schlüsselmärkten
Märkte und Timing ein Umsatz, wenn überhaupt, von der gleichen, die Zustimmung der Leber CHEMOSAT Lieferung
System-Gerät zu liefern und zu filtern Doxorubicin in wichtigen asiatischen Märkten und Behandlungsergebnisse mit
das gleiche, über unsere Fähigkeit, die Erstattung für die CHEMOSAT System zu erhalten, Unsicherheiten
das Timing und die Ergebnisse von Forschungs-und Entwicklungsprojekten, Unsicherheiten in Bezug auf den Zeitpunkt
und die Ergebnisse zukünftiger klinischer Studien sowie Unsicherheiten in Bezug auf unsere Fähigkeit, finanzielle erhalten
und andere Ressourcen für alle Forschungs-, Entwicklungs-und Vermarktungsaktivitäten. Diese Faktoren,
und andere, werden von Zeit zu Zeit in unseren Einreichungen bei der Securities and Exchange diskutiert
Kommission. Sie sollten kein unangemessenes Vertrauen in diese zukunftsgerichteten Aussagen setzen sollten, sprechen die
nur zum Datum ihrer Veröffentlichung. Wir übernehmen keine Verpflichtung zur öffentlichen Aktualisierung oder Überarbeitung dieser
zukunftsgerichtete Aussagen, um Ereignisse oder Umstände nach dem Datum ihrer Veröffentlichung widerspiegeln.
Kontakt-Informationen:
Investoren: Financial Media
Gregory Gin / Patty Eisenhaur Chris Gale
EVC Gruppe EVC-Gruppe
646-445-4801/951-316-0577 646-201-5431
-------------------------------------------------- -------------------------------------------------
Diese Ankündigung wird von Thomson Reuters im Auftrag der Thomson Reuters Clients verteilt.
Der Besitzer dieser Ankündigung gewährleistet, dass:
(I) die Veröffentlichungen enthaltenen werden durch Urheberrechte und andere anwendbare Gesetze geschützt, und
(Ii) sie sind allein verantwortlich für den Inhalt, die Richtigkeit und die Originalität der
darin enthaltenen Informationen.
Quelle: Delcath Systems, Inc via Thomson Reuters ONE
+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
copy by reuters.com
+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
"Delcath für das vierte Quartal und das gesamte Jahr 2012
ERGEBNISSE
* Reuters ist nicht verantwortlich für den Inhalt dieser Pressemitteilung."
+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
Mi 13. März 2013 04.02 Uhr EDT
Die besten Ergebnisse beim Drucken dieser Ankündigung, klicken Sie bitte auf den folgenden Link:
- Telefonkonferenz und Webcast heute um 4:30 Uhr ET -
NEW YORK, 13. März 2013 - Delcath Systems, Inc. (NASDAQ: DCTH) meldete heute die Finanzergebnisse
und operativen Highlights des vierten steuerlichen Quartal und das Gesamtjahr zum 31. Dezember 2012.
Highlights aus dem Geschäftsjahr 2012 und den letzten Wochen sind:
* TMAcceptance von der US Food and Drug Administration (FDA) für die inhaltliche Überprüfung der
Firma New Drug Application (NDA) für seine firmeneigene Medikamentenentwicklung / Gerätekombination Produkt mit dem
vorgeschlagen Markennamen Melblez Kit (Melblez (Melphalan) zur Injektion zur Verwendung mit dem Delcath
Hepatischen Delivery System), mit einer beabsichtigten Indikation zur Behandlung von Patienten mit
inoperablem okuläre Melanom mit Metastasen in der Leber - die FDA zugeordnet einen PDUFA Ziel Termin Juni
15, 2013
* Ankündigung eines Oncologic Drugs Advisory Committee (ODAC) Sitzung des Gremiums am 2. Mai 2013
Beurteilung des Unternehmens NDA
* Einleitung der US Expanded Access Program (EAP) und Therapie der ersten EAP Patienten in den USA
* Q4 Verwendung der Barmittel um 33% reduziert, um Q3 im Vergleich
* Erste kommerzielle Umsatz in der Unternehmensgeschichte
* (R) Therapie der ersten Patienten in Europa mit dem CHEMOSAT Leber Delivery System für Melphalan
Hydrochlorid
* Wert 4 interim Erstattung Versorgung in Deutschland erteilt und identifiziert eine bestehende DRG-Code für
teilweise Rückerstattung in Italien-Kliniken Vorlage Zwischenbericht Erstattung Anwendungen sowohl in
Ländern
* Erhalt der CE-Zulassung für CHEMOSAT Leber Delivery System zu liefern und zu filtern
Doxorubicin-Hydrochlorid Injektion
"Letztes Jahr war ein bedeutender Leistungen für Delcath, von der FDA die Akzeptanz unserer führten
NDA für die Melblez Kit System für inhaltliche Überprüfung ", sagte Eamonn P. Hobbs, Präsident und CEO von
Delcath Systems. "Submission unserer Anwendung war der Höhepunkt einer intensiven Anstrengungen unserer
Team und FDA Akzeptanz unserer NDA und Benennung eines 15. Juni 2013 PDUFA Ziel aktuell sind die
wichtigsten Entwicklungen in unserer Geschichte. Im Laufe des Jahres haben wir auch festgestellt unseren ersten
kommerzielle Präsenz in Europa, unsere erste kommerzielle Vertrieb und in den letzten Wochen begonnen
Verhandlungen mit der FDA auf unserem klinischen Entwicklungsprogramm mit dem Ziel der Erweiterung des Etiketts
für konzentrierte, Therapie Leber-directed ".
"Wir stellten CHEMOSAT in Europa im vergangenen Jahr, eine Präsenz für die Behandlung in
alle sieben unseren Zielländern ", fuhr Mr. Hobbs." Patienten mit Leber-dominante Erkrankung
aus verschiedenen Tumorarten behandelt worden sind, und Arzt Interesse an der therapeutischen Ansatz
mit CHEMOSAT stark bleibt. Wir glauben Veröffentlichung unserer Phase-3-Melanom-und Phase 2
Multi-Histologie Daten aus klinischen Studien weiter fahren dieses Interesse. Entwicklung von attraktiven
Erstattung ist ein wichtiger Treiber der Verwertung und Umsatzwachstum. Wir arbeiten eng mit
Experten und unseren Partner Krankenhäuser zu bringen verschiedenen Zwischenbericht Erstattungsmechanismen online, und in
parallel in Anwendungen Prozesses engagiert permanent, überzeugende Erstattung zu etablieren.
Obwohl diese Mechanismen Online langsamer als wir erwartet gekommen sind, hat die Einnahmen ausgewirkt
Rampe, Arzt Interesse an unserer Therapie ist stark und wir bleiben optimistisch über die langfristigen
Aussichten für CHEMOSAT in Europa. "
"In den Vereinigten Staaten, wir weiterhin eng mit der FDA in ihrer Bewertung des Zulassungsantrags und
sind für unsere Präsentation auf der ODAC Panel Vorbereitung am 2. Mai. Unsere Zusammenarbeit mit der FDA hat
konstruktiv, und wir sind zuversichtlich, dass die Agentur die Überprüfung auf dem PDUFA Ziel Schluss
Datum. Wenn unsere NDA zugelassen ist, ist unser Plan, in den USA im vierten Quartal starten,
sich zunächst auf die Krankenhäuser, die in unserem kürzlich gestarteten EAP teilnehmen und haben
nahmen in unseren klinischen Studien. Mit diesem Ziel vor Augen sind wir derzeit, einen
sehr seltenen Preise und Vermarktungsstrategie in den USA für inoperablen Metastasen okulären
Melanom ", schloss Mr. Hobbs.
Vierte Quartal und das Gesamtjahr Financial Results
Für die drei Monate zum 31. Dezember 2012, erfasst Delcath Umsatz von $ 0,2 Millionen. Betriebs-
Verlust betrug $ 11,8 Mio., die ungefähr $ 0,9 Mio. nicht zahlungswirksame aktienbasierte enthalten
Aufwendungen, wie mit einem operativen Verlust von $ 16,0 Millionen, verglichen, einschließlich $ 0.900.000
in unbaren Vergütungsaufwand im gleichen Vorjahreszeitraum. Vertriebs-und allgemeine
Verwaltungskosten (SG & A) betrugen $ 6,4 Mio. im vierten Quartal 2012, verglichen mit $ 6,1
Millionen für den gleichen Zeitraum in 2011. Die höhere SG & A-Kosten ist vor allem auf erhöhte EU
Kommerzialisierung Aufwendungen. Forschung und Entwicklung (R & D) beliefen sich auf $ 5.600.000 für das
vierten Quartal 2012 auf $ 9.800.000 für den gleichen Zeitraum im Jahr 2011 verglichen. Der untere R & D
Aufwand spiegelt niedrigere Beratungskosten im Anschluss an die Vorlage des NDA am 15. August 2012.
Für das Jahr zum 31. Dezember 2012, erfasst Delcath Umsatz von $ 0.300.000 und eine zusätzliche
$ 30.000 von unrealisierten Erträgen im Zusammenhang mit Bestellungen von Vertriebspartnern. Die operativen
Verlust betrug $ 53,9 Mio., die ungefähr $ 3,8 Mio. nicht zahlungswirksame aktienbasierte enthalten
Aufwendungen. Der Anstieg der Kosten ist in erster Linie die EU-Vermarktung im Zusammenhang
Bemühungen. Der operative Verlust für das Geschäftsjahr zum 31. Dezember 2011 46.500.000 $, die im Lieferumfang enthalten
ca. US $ 4,3 Mio. im unbaren Vergütungsaufwand. SG & A-Aufwendungen betrugen $ 28,0
Millionen für das Geschäftsjahr zum 31. Dezember 2012, um $ 21.300.000 für das Geschäftsjahr im Vergleich
31. Dezember 2011. R & D Aufwendungen betrugen $ 26.200.000 für das Geschäftsjahr zum 31. Dezember 2012 gegenüber
um $ 25.200.000 im Geschäftsjahr zum 31. Dezember 2011.
Am 31. Dezember 2012 waren Zahlungsmittel, Zahlungsmitteläquivalente und Certificates of Deposit $ 23.700.000,
verglichen mit $ 30.800.000 am 31. Dezember 2011. Für das vierte Quartal Cash Auslastung lag
$ 9.700.000, eine 33% ige Reduktion auf $ 14.600.000 im dritten Quartal verglichen. Dies stimmte
mit der Gesellschaft angekündigten Erwartungen für durchschnittlichen monatlichen Cash-Auslastung zwischen
$ 3.000.000 und $ 4 Millionen für das vierte Quartal. Der Rückgang in der Verwendung der Barmittel war in erster Linie
angetrieben durch eine Verringerung Beratung nach Einreichung des NDA sowie insgesamt
effektives Kostenmanagement.
Das Unternehmen erfolgreich seine "auf dem Markt" (ATM) Aktienemission Programm, und zwischen
1. Januar 2013 und 28. Februar 2013 hob ca. 20.900.000 $, bevor Aufwendungen.
Am 28. Februar 2013 waren der Gesellschaft Zahlungsmittel und Zahlungsmitteläquivalente rund $ 38.000.000
(Ungeprüft). Das Unternehmen hat in eine neue ATM Aktienemission Programm eingegeben, unter denen die
Unternehmen verkaufen können bis zu $ 50 Millionen seiner Stammaktien von Zeit zu Zeit einmal die zugehörige Regal
Registration Statement wirksam wird.
"Wir bleiben verpflichtet, zur Erhaltung unserer reduzierte vierteljährliche Nutzung von $ 9.000.000 auf $ 12
Millionen für das Jahr 2013 ", sagte Graham Miao, Ph.D., Delcath Chief Financial Officer." Wir glauben, dass
mit unseren aktuellen Barbestand und den Zugang zu Kapital haben wir die finanziellen Mittel und
Flexibilität, um unsere operativen Plan für die nächsten 12 Monate und darüber hinaus führen. "
Telefonkonferenz und Webcast
Das Unternehmen wird eine Telefonkonferenz heute, 13. März 2013 16.30 Uhr ET. Die Einwahlnummern
für die Telefonkonferenz sind 877-299-4454 (US-Teilnehmer) und 617-597-5447 (International
Teilnehmer), beide Zahlen erfordern Passcode 99502530. Um die Live-Übertragung im Internet, gehen Sie zum
Events & Presentations Seite im Investor Relations-Bereich der Website des Unternehmens unter
http://www.delcath.com/investors/events/ http://www.delcath.com/investors/events/.
Eine Aufzeichnung der Telefonkonferenz wird ab Anfang etwa zwei Stunden nach dem Anruf des
Abschluss und wird für sieben Tage zur Verfügung. Einwahlnummern für die Wiedergabe sind 888-286-8010
und 617-801-6888 für die USA und internationale Anrufer bzw.. Die Wiederholung Passcode für beide
US-und internationale Anrufer lautet 41198605. Eine archivierte Webcast wird auch verfügbar sein
http://www.delcath.com/investors/events/ http://www.delcath.com/investors/events/.
Über Delcath Systems
Delcath Systems, Inc. ist ein spezialisiertes Pharma-und Medizintechnik-Unternehmen fokussiert auf
Onkologie. Unsere firmeneigene Medikamentenentwicklung / Gerätekombination Produkt, das Delcath Leber Delivery System ist
entwickelt, um Hochdosis-Chemotherapie und andere therapeutische Mittel in erkrankte Organe oder verwalten
Regionen des Körpers, während die Steuerung die systemische Exposition von diesen Agenten. Die Gesellschaft
Fokus liegt zunächst auf die Behandlung von primären und metastatischen Leberkrebs. Im Jahr 2010 Delcath
bekannt, dass seine randomisierten klinischen Phase-3-Studie für Patienten mit metastasierendem Melanom in der
Leber hatte erfolgreich den primären Endpunkt der Studie von erweiterten Leber progressionsfreie erreicht
Überleben. Das Unternehmen schloss zudem eine Multi-Arm-Phase-2-Studie zu anderen Leberkrebs zu behandeln.
Außerhalb der Vereinigten Staaten, an unsere eigene Produkt zu liefern und zu filtern Melphalan
Hydrochlorid wird unter dem Handelsnamen Delcath Leber CHEMOSAT (R) Delivery System vermarktet
(CHEMOSAT Delivery System für Melphalan.) Die Gesellschaft Genehmigung zum Anbringen einer CE-Kennzeichnung erhalten
für die Generation Zwei CHEMOSAT Delivery System für Melphalan im April 2012. Das Recht auf Anbringung
die CE-Kennzeichnung ermöglicht es dem Unternehmen zu vermarkten und zu verkaufen CHEMOSAT Delivery System für Melphalan in
Europa. Im Oktober 2012 wird das Unternehmen alle Anforderungen zur Anbringung der CE-Kennzeichnung die zufrieden
Leber CHEMOSAT Delivery System für Intra-arteriellen Lieferung und extrakorporale
Filtration von Doxorubicin-Hydrochlorid Injektion (CHEMOSAT Delivery System für Doxorubicin),
Bereitstellung eines regulatorischen Weg für die CHEMOSAT Delivery System für Doxorubicin für die Länder
Asien, die die CE-Kennzeichnung als Teil ihrer nationalen regulatorischen Anforderungen zu akzeptieren. Das Unternehmen
noch nicht FDA-Zulassung für den kommerziellen Verkauf seines Systems in den Vereinigten Staaten erhalten. Die
Gesellschaft NDA für Ablage und inhaltliche Überprüfung durch die FDA akzeptiert. Weitere
Informationen, besuchen Sie bitte die Website des Unternehmens unter www.delcath.com http://www.delcath.com/.
Private Securities Litigation Reform Act von 1995 bietet einen sicheren Hafen für zukunftsweisende
Aussagen des Unternehmens oder in seinem Namen gemacht. Diese Presseinformation enthält bestimmte in die Zukunft gerichtete
Aussagen, die gewissen Risiken und Unsicherheiten, welche die tatsächlichen Ergebnisse können, sind
erheblich von jenen beschrieben. Faktoren, die solche Abweichungen verursachen können, gehören, sind aber
nicht beschränkt auf, Unsicherheiten in Bezug auf: das Ergebnis der ODAC-Sitzung, und die Auswirkungen, wenn
überhaupt, der Beirat der Empfehlung auf die Entscheidung der FDA bezüglich der Gesellschaft neues Medikament
Application (NDA), Zeitpunkt der Fertigstellung der FDA Überprüfung unserer NDA, das Ausmaß, in dem die
FDA kann zusätzliche Informationen oder Daten und unsere Fähigkeit, die gleiche rechtzeitig liefern
Weise Akzeptanz der Phase 1, 2 und 3 Daten aus klinischen Studien von der FDA, FDA-Zulassung des
Gesellschaft NDA für die Behandlung von Augenkrankheiten metastasierendem Melanom in der Leber, die Annahme, Verwendung und
daraus resultierenden Umsätze, wenn überhaupt, in den Vereinigten Staaten, die Annahme, Verwendung und daraus resultierenden Umsätzen, falls vorhanden, für die
CHEMOSAT System zu liefern und zu filtern Melphalan im EWR, unsere Fähigkeit, erfolgreich
Vermarktung der Chemosättigungs System und das Potential des Systems als Chemosättigungs
Behandlung für Patienten mit primären und metastatischen Erkrankung in der Leber, die Marktakzeptanz der
Gen Zwei CHEMOSAT und Behandlungsergebnisse mit dem gleichen, mit Zustimmung des gegenwärtigen oder zukünftigen
Chemosättigungs-Systems für die Lieferung und Filtration von Melphalan, Doxorubicin oder anderen
Chemotherapeutika für verschiedene Indikationen in den USA und / oder in ausländischen Märkten, Aktionen
die FDA oder anderen ausländischen Aufsichtsbehörden, unsere Fähigkeit, erfolgreich in strategischen geben
Partnerschaft und Vertriebsvereinbarungen in ausländischen Märkten, darunter Australien und asiatischen Schlüsselmärkten
Märkte und Timing ein Umsatz, wenn überhaupt, von der gleichen, die Zustimmung der Leber CHEMOSAT Lieferung
System-Gerät zu liefern und zu filtern Doxorubicin in wichtigen asiatischen Märkten und Behandlungsergebnisse mit
das gleiche, über unsere Fähigkeit, die Erstattung für die CHEMOSAT System zu erhalten, Unsicherheiten
das Timing und die Ergebnisse von Forschungs-und Entwicklungsprojekten, Unsicherheiten in Bezug auf den Zeitpunkt
und die Ergebnisse zukünftiger klinischer Studien sowie Unsicherheiten in Bezug auf unsere Fähigkeit, finanzielle erhalten
und andere Ressourcen für alle Forschungs-, Entwicklungs-und Vermarktungsaktivitäten. Diese Faktoren,
und andere, werden von Zeit zu Zeit in unseren Einreichungen bei der Securities and Exchange diskutiert
Kommission. Sie sollten kein unangemessenes Vertrauen in diese zukunftsgerichteten Aussagen setzen sollten, sprechen die
nur zum Datum ihrer Veröffentlichung. Wir übernehmen keine Verpflichtung zur öffentlichen Aktualisierung oder Überarbeitung dieser
zukunftsgerichtete Aussagen, um Ereignisse oder Umstände nach dem Datum ihrer Veröffentlichung widerspiegeln.
Kontakt-Informationen:
Investoren: Financial Media
Gregory Gin / Patty Eisenhaur Chris Gale
EVC Gruppe EVC-Gruppe
646-445-4801/951-316-0577 646-201-5431
-------------------------------------------------- -------------------------------------------------
Diese Ankündigung wird von Thomson Reuters im Auftrag der Thomson Reuters Clients verteilt.
Der Besitzer dieser Ankündigung gewährleistet, dass:
(I) die Veröffentlichungen enthaltenen werden durch Urheberrechte und andere anwendbare Gesetze geschützt, und
(Ii) sie sind allein verantwortlich für den Inhalt, die Richtigkeit und die Originalität der
darin enthaltenen Informationen.
Quelle: Delcath Systems, Inc via Thomson Reuters ONE
Vielen Dank für die Mitteilung
http://www.delcath.com/investors/news/1795896/
Die gleiche Meldung gibt es hier, allerdings mit mehr Zahlenmaterial und auf Englisch.
Demnach lag der Verlust 2012 bei 0,85$. Erwartet worden waren aber 0,94$. Es ist aber immer noch eine Verlustausweitung von 0,68$ in 2011.
Anleger in den USA sind davon nicht begeistert. Laut CNBC ist die Aktie im nachbörslichen Handel um ca. 10% gefallen. Mal sehen was da morgen kommt.
http://www.delcath.com/investors/news/1795896/
Die gleiche Meldung gibt es hier, allerdings mit mehr Zahlenmaterial und auf Englisch.
Demnach lag der Verlust 2012 bei 0,85$. Erwartet worden waren aber 0,94$. Es ist aber immer noch eine Verlustausweitung von 0,68$ in 2011.
Anleger in den USA sind davon nicht begeistert. Laut CNBC ist die Aktie im nachbörslichen Handel um ca. 10% gefallen. Mal sehen was da morgen kommt.
Chemostat works:
http://www.ourcoloradonews.com/lonetree/news/procedure-attac…
Cool, die Chemo nur in der Leber einsetzen, hochdosiert, und dann über Filter wieder raus aus dem Körper!
http://www.ourcoloradonews.com/lonetree/news/procedure-attac…
Cool, die Chemo nur in der Leber einsetzen, hochdosiert, und dann über Filter wieder raus aus dem Körper!
Antwort auf Beitrag Nr.: 44.349.845 von Magnetfeldfredy am 02.04.13 22:27:50http://ih.advfn.com/p.php?pid=nmona&article=57418399
Sieht übel aus... 16-0 gewählt ohne Enthaltungen
After Hours: 0.360 -0.433 (-54.58%)
Sieht übel aus... 16-0 gewählt ohne Enthaltungen
After Hours: 0.360 -0.433 (-54.58%)
Antwort auf Beitrag Nr.: 44.558.063 von multimediaperle am 03.05.13 01:05:41Stimmt, aber Delcath ist nicht tot, Delcath hat die EU-Zulassung und der neue Gen2 Filter, der in der EU eingesetzt wird, filtert 98% der Chemo wieder aus dem Blut gegenüber 72 % beim alten Filter!
Das brutale Ergebnis in den USA basiert auf Gen1 Filter, d.h. EU + ROW kann weiter behandeln, USA braucht neue Trials für Gen2 Filter!
Ohne Europäische Zulassung wäre Delcath tot, so ist es ein Schnäppchen wenn man ein paar Jahre Zeit hat, zudem hat Delcath 38 Millionen US Dollar cash und keine Schulden!
Trotzdem megascheiße!
Das brutale Ergebnis in den USA basiert auf Gen1 Filter, d.h. EU + ROW kann weiter behandeln, USA braucht neue Trials für Gen2 Filter!
Ohne Europäische Zulassung wäre Delcath tot, so ist es ein Schnäppchen wenn man ein paar Jahre Zeit hat, zudem hat Delcath 38 Millionen US Dollar cash und keine Schulden!
Trotzdem megascheiße!
Wie sagtest du Anfang des Jahres so treffend: Ein Konkurrent weniger.
Nun hat es Delcath selbst erwischt. Ich hab nochmal in den Annual Report Daten bei EDGAR geschaut. Demnach verbrennt Delcath jedes Jahr ca. 42Mio$ (Forschung ist teuer).
Wenn die also 38Mio$ Cash haben ist absehbar, dass das Unternehmen trocken läuft so lange man keine neuen Aktien ausgibt (denn Umsätze sind fast nicht vorhanden).
Um im Bild zu bleiben: Delcath ist nicht tot, liegt aber inzwischen auf der Intensivstation.
Nun hat es Delcath selbst erwischt. Ich hab nochmal in den Annual Report Daten bei EDGAR geschaut. Demnach verbrennt Delcath jedes Jahr ca. 42Mio$ (Forschung ist teuer).
Wenn die also 38Mio$ Cash haben ist absehbar, dass das Unternehmen trocken läuft so lange man keine neuen Aktien ausgibt (denn Umsätze sind fast nicht vorhanden).
Um im Bild zu bleiben: Delcath ist nicht tot, liegt aber inzwischen auf der Intensivstation.
Antwort auf Beitrag Nr.: 44.559.485 von sdaktien am 03.05.13 10:24:24Leider ist es so wie Du es ausführst, gut nur das Delcath in der EU und in Asien zugelassen ist, auch der neue Filter Gen2 ist zugelassen, nur im Herstellerland der USA, wird es verweigert, Korruption, Machtspiele.....
Antwort auf Beitrag Nr.: 44.567.717 von Magnetfeldfredy am 04.05.13 14:08:52wie viel Geld kommt durch die Vermarktung in EU und Asien rein?
Der Kurs fällt immer weiter, anscheinend reichen die Einnahmen nicht zur Kostendeckung aus.
Besteht die Möglichkeit, dass auch in den USA noch eine Markteinführung kommen wird?
Der Kurs fällt immer weiter, anscheinend reichen die Einnahmen nicht zur Kostendeckung aus.
Besteht die Möglichkeit, dass auch in den USA noch eine Markteinführung kommen wird?
Antwort auf Beitrag Nr.: 44.797.813 von Poppholz am 06.06.13 11:52:34Delcath die Entäuschung pur, EU-Zulassung aber kaum revenues, Phase III angeblich super erreicht und dann 16:0 im AdCom gegen Delcath, reiner Betrug für mich und viel Geld verloren mit dem Chemodreck!

Antwort auf Beitrag Nr.: 44.809.297 von Magnetfeldfredy am 07.06.13 18:50:39gab es denn eine Begründung dafür?
Haben wir hier ein Ähnliches Problem wie damals bei Dendreon, wo sich die Zulassung nur "verzögert" hat?
Aktuell scheint jegliche Phantasie aus der Aktie raus zu sein. Bei dem Kurs scheint nicht einmal ein Konkurent die Bude schlucken zu wollen, um das Wissen für sich zu nutzen.

Haben wir hier ein Ähnliches Problem wie damals bei Dendreon, wo sich die Zulassung nur "verzögert" hat?
Aktuell scheint jegliche Phantasie aus der Aktie raus zu sein. Bei dem Kurs scheint nicht einmal ein Konkurent die Bude schlucken zu wollen, um das Wissen für sich zu nutzen.

Antwort auf Beitrag Nr.: 44.809.297 von Magnetfeldfredy am 07.06.13 18:50:39@Magnetfeldfredy
Bist noch Investiert
Die Chancen am PDUFA 13 September sind mehr als gering aber bei der FDA weiss man nie ev gibt es eine Überraschung
ev gibt es eine Überraschung 
Bist noch Investiert

Die Chancen am PDUFA 13 September sind mehr als gering aber bei der FDA weiss man nie
 ev gibt es eine Überraschung
ev gibt es eine Überraschung 
Antwort auf Beitrag Nr.: 45.273.345 von multimediaperle am 18.08.13 15:55:39



U.S. FDA GRANTS ORPHAN DRUG DESIGNATION TO DELCATH SYSTEMS FOR USE OF MELPHALAN HYDROCHLORIDE IN HCC
Print
table.hugin { border-color:black;} td.hugin { padding: 3px; border-color:black;}
New York, NY - October 1, 2013 - Delcath Systems, Inc. (NASDAQ: DCTH), a specialty pharmaceutical and medical device company focused on oncology, announced today that the U.S. Food & Drug Administration (FDA) has granted the Company orphan drug designation for melphalan in the treatment of patients with hepatocellular carcinoma (HCC, or primary liver cancer).
Orphan-drug designation is granted by the FDA Office of Orphan Products Development to novel drugs or biologics that treat a rare disease or condition affecting fewer than 200,000 patients in the U.S. The designation provides the drug developer with a seven-year period of U.S. marketing exclusivity if the drug is the first of its type approved for the specified indication or if it demonstrates superior safety, efficacy, or a major contribution to patient care versus another drug of its type previously granted the designation for the same indication. It also provides tax credits for clinical research costs, the ability to apply for annual grant funding, clinical research trial design assistance and waiver of Prescription Drug User Fee Act (PDUFA) filing fees.
Melphalan for use with the Delcath Hepatic Delivery System is not currently approved in the United States for the treatment of patients with HCC.
About Delcath Systems
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Our proprietary drug/device combination product, the Delcath Hepatic Delivery System, is designed to administer high dose chemotherapy and other therapeutic agents to the liver, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. Outside of the United States, our proprietary product to deliver and filter melphalan hydrochloride is marketed under the trade name Delcath Hepatic CHEMOSAT® Delivery System for melphalan hydrochloride. The Company obtained authorization to affix a CE Mark for the Generation Two CHEMOSAT Delivery System for Melphalan in April 2012. The right to affix the CE mark allows the Company to market and sell the CHEMOSAT Delivery System for Melphalan in Europe. In addition, the Company has initiated plans to investigate the Melphalan Hydrochloride for Injection for use with the Delcath Hepatic Delivery System for primary liver cancer. For more information, please visit the Company's website at www.delcath.com.
Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by the Company or on its behalf. This news release contains forward-looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to: the timing and results of the Company's future clinical trials including without limitation the HCC trials, FDA approval of the melphalan Delcath Hepatic Delivery System for the treatment of HCC,, the Company's ability to benefit from the orphan drug designation for melphalan for the treatment of HCC, the Company's ability to satisfy the requirements of the FDA's Complete Response Letter and provide the same in a timely manner, clinical adoption, use and resulting sales, if any, for the CHEMOSAT system to deliver and filter melphalan in Europe, our ability to successfully commercialize the chemosaturation system and the potential of the chemosaturation system as a treatment for patients with primary and metastatic disease in the liver, our ability to obtain reimbursement for the CHEMOSAT system in various markets, approval of the current or future chemosaturation system for delivery and filtration of melphalan, doxorubicin or other chemotherapeutic agents for various indications in the US and/or in foreign markets, actions by the FDA or other foreign regulatory agencies, our ability to successfully enter into strategic partnership and distribution arrangements in foreign markets including Australia and key Asian markets and timing and revenue, if any, of the same, uncertainties relating to the timing and results of research and development projects,, and uncertainties regarding our ability to obtain financial and other resources for any research, development, clinical trials and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward-looking statements to reflect events or circumstances after the date they are made.
Contact Information:
Investor Contact: Media Contact:
Michael Polyviou/Patty Eisenhaur John Carter
EVC Group EVC Group
212-850-6020/951-316-0577 212-850-6021




U.S. FDA GRANTS ORPHAN DRUG DESIGNATION TO DELCATH SYSTEMS FOR USE OF MELPHALAN HYDROCHLORIDE IN HCC
table.hugin { border-color:black;} td.hugin { padding: 3px; border-color:black;}
New York, NY - October 1, 2013 - Delcath Systems, Inc. (NASDAQ: DCTH), a specialty pharmaceutical and medical device company focused on oncology, announced today that the U.S. Food & Drug Administration (FDA) has granted the Company orphan drug designation for melphalan in the treatment of patients with hepatocellular carcinoma (HCC, or primary liver cancer).
Orphan-drug designation is granted by the FDA Office of Orphan Products Development to novel drugs or biologics that treat a rare disease or condition affecting fewer than 200,000 patients in the U.S. The designation provides the drug developer with a seven-year period of U.S. marketing exclusivity if the drug is the first of its type approved for the specified indication or if it demonstrates superior safety, efficacy, or a major contribution to patient care versus another drug of its type previously granted the designation for the same indication. It also provides tax credits for clinical research costs, the ability to apply for annual grant funding, clinical research trial design assistance and waiver of Prescription Drug User Fee Act (PDUFA) filing fees.
Melphalan for use with the Delcath Hepatic Delivery System is not currently approved in the United States for the treatment of patients with HCC.
About Delcath Systems
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Our proprietary drug/device combination product, the Delcath Hepatic Delivery System, is designed to administer high dose chemotherapy and other therapeutic agents to the liver, while controlling the systemic exposure of those agents. The Company's initial focus is on the treatment of primary and metastatic liver cancers. Outside of the United States, our proprietary product to deliver and filter melphalan hydrochloride is marketed under the trade name Delcath Hepatic CHEMOSAT® Delivery System for melphalan hydrochloride. The Company obtained authorization to affix a CE Mark for the Generation Two CHEMOSAT Delivery System for Melphalan in April 2012. The right to affix the CE mark allows the Company to market and sell the CHEMOSAT Delivery System for Melphalan in Europe. In addition, the Company has initiated plans to investigate the Melphalan Hydrochloride for Injection for use with the Delcath Hepatic Delivery System for primary liver cancer. For more information, please visit the Company's website at www.delcath.com.
Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by the Company or on its behalf. This news release contains forward-looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to: the timing and results of the Company's future clinical trials including without limitation the HCC trials, FDA approval of the melphalan Delcath Hepatic Delivery System for the treatment of HCC,, the Company's ability to benefit from the orphan drug designation for melphalan for the treatment of HCC, the Company's ability to satisfy the requirements of the FDA's Complete Response Letter and provide the same in a timely manner, clinical adoption, use and resulting sales, if any, for the CHEMOSAT system to deliver and filter melphalan in Europe, our ability to successfully commercialize the chemosaturation system and the potential of the chemosaturation system as a treatment for patients with primary and metastatic disease in the liver, our ability to obtain reimbursement for the CHEMOSAT system in various markets, approval of the current or future chemosaturation system for delivery and filtration of melphalan, doxorubicin or other chemotherapeutic agents for various indications in the US and/or in foreign markets, actions by the FDA or other foreign regulatory agencies, our ability to successfully enter into strategic partnership and distribution arrangements in foreign markets including Australia and key Asian markets and timing and revenue, if any, of the same, uncertainties relating to the timing and results of research and development projects,, and uncertainties regarding our ability to obtain financial and other resources for any research, development, clinical trials and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward-looking statements to reflect events or circumstances after the date they are made.
Contact Information:
Investor Contact: Media Contact:
Michael Polyviou/Patty Eisenhaur John Carter
EVC Group EVC Group
212-850-6020/951-316-0577 212-850-6021
Antwort auf Beitrag Nr.: 45.555.011 von ultrawuffsn am 02.10.13 14:37:15Diff. abs / Diff. rel: 0,124 EUR / 58,22%
Datum / Uhrzeit: 02.10.13 / 14:33:47
Geld / Brief: Geld: 02.10.13 - 14:47:27, Brief: 02.10.13 - 14:47:27 0,285 EUR / 0,347 EUR
Datum / Uhrzeit: 02.10.13 / 14:33:47
Geld / Brief: Geld: 02.10.13 - 14:47:27, Brief: 02.10.13 - 14:47:27 0,285 EUR / 0,347 EUR
Antwort auf Beitrag Nr.: 45.555.101 von ultrawuffsn am 02.10.13 14:48:02geht ab 0,45.

Antwort auf Beitrag Nr.: 45.555.495 von ultrawuffsn am 02.10.13 15:32:580,47 geht weiter
geht weiter
 geht weiter
geht weiter
Antwort auf Beitrag Nr.: 45.555.823 von ultrawuffsn am 02.10.13 16:09:38gleich über 0,50.

NEW YORK, March 12, 2014 /PRNewswire/ -- Delcath Systems, Inc. (NASDAQ CTH) today reported financial results and operational developments for the fiscal fourth quarter and full year ended December 31, 2013. Developments for the quarter and recent weeks subsequent to quarter end are as follows:
CTH) today reported financial results and operational developments for the fiscal fourth quarter and full year ended December 31, 2013. Developments for the quarter and recent weeks subsequent to quarter end are as follows:
The Company's cash and cash equivalents increased in the fourth quarter 2013 and the first quarter of 2014; cash position as of February 28, 2014 was $32.7 million; fourth quarter 2013 cash utilization was $6.2 million
2014 average quarterly cash utilization expected to be between $5 and $6 million
In December 2013Delcath received comments from FDA on its proposed HCC trial protocol to investigate Melphalan HDS for first-line treatment of patients with unresectable advanced hepatocellular carcinoma (HCC) or primary liver cancer; a supplemental Investigational New Drug (IND) application incorporating FDA comments was submitted to FDA in February 2014; FDA review of the IND is pending
The Company received the approval from shareholders authorizing its Board of Directors, at its discretion, to effect a reverse stock split of the common stock at a specific ratio within a range from 1-for-8 to 1-for-16, inclusive, on or prior to December 31, 2014
"During our fourth quarter we continued to make steady progress on our main priorities of CHEMOSAT clinical adoption in Europe and our clinical development program for HCC," commented Jennifer K. Simpson, Interim Co-President and Co-CEO. "We are optimistic that clinical adoption is progressing in Germany and the U.K., key target markets that we believe offer the best near-term revenue opportunities. Experience with CHEMOSAT therapy continues to build, with 89 procedures performed on 61 patients since we first introduced CHEMOSAT in Europe. Concurrently, we had dialogue with the FDA on our supplemental IND submission and with the clinical institutions that will be conducting our HCC studies. As a result of our February IND submission, and assuming scientific review and institutional review board approvals, we now anticipate clinical institutions to become activated in the second quarter of 2014."
Financial Results
For the fourth quarter ended December 31, 2013, total revenue was $0.3 million compared with total revenue of $0.2 million in the fourth quarter 2012. Operating expenses decreased by approximately 52% to $5.8 million from $12.0 million for the same period in 2012. The decrease is primarily due to a significant reduction in expenses related to the Company's NDA submission to the FDA, as well as the Company's overall cost management efforts. Operating loss was $5.5 million, which included non-cash stock-based compensation income of $0.3 million, as compared with an operating loss of $11.8 million, including $0.9 million in non-cash stock-based compensation expense, in the year ago period.
For the full year ended December 31, 2013, total revenue was approximately $0.8 million of which $0.3 million was related to the recognition of previously deferred revenue. Total operating expenses decreased by approximately 38% to $33.3 million from $54.2 million for the same period in 2012. Operating loss for the year was $33.0 million, which included $0.3 million in non-cash stock-based compensation expense, as compared with an operating loss of $53.9 million, including $3.8 million in non-cash stock-based compensation expense, in the year ago period.
In 2013, Delcath raised approximately $43.2 million before related expenses, including approximately $26.7 million through the Company's At-the-Market equity offering program, $9.0 million through its Committed Equity Financing Facility program, and approximately $7.5 million through a registered direct offering.
Cash and cash equivalents as of December 31, 2013 were $31.2 million, compared with $23.7 million at December 31, 2012. During the year, cash used in operating activities was $34.1 million, a 32% reduction compared to $50.0 million in the comparable period in 2012. The decrease in cash utilization was in part due to a reduction in NDA submission-related costs, and improved organizational and operational efficiencies.
During the first quarter through February 28, 2014, the Company raised approximately $4.5 million before related expenses through our At-the-Market offering program. As of February 28, 2014, the Company's cash and cash equivalents were $32.7 million, including $0.4 million in accounts receivables collection.
"During the second half of 2013, we made significant progress in streamlining our business operations and improving our operational efficiencies, while focusing on our key priorities. As a result, we successfully reduced our operating costs and cash burn to more sustainable levels, and we believe we have sufficient resources to execute our plan into the first half of 2015," said Graham G. Miao, Interim Co-President and Co-CEO.
On February 24, 2014, shareholders approved an amendment to the Company's Amended and Restated Certificate of Incorporation authorizing the Board of Directors to affect a reverse stock split of the Company's common stock at a specific ratio within a range from 1-for-8 to 1-for-16, inclusive, on or prior to December 31, 2014. If deemed necessary by the Board, a reverse stock split may enable the Company to regain compliance with NASDAQ's $1.00 minimum bid price requirement by June 9, 2014, and maintain its listing on the NASDAQ Capital Market.
Conference Call and Webcast
The Company will host a conference call today, March 12, 2014 at 4:30 p.m. ET to discuss its financial results for the fourth quarter and full year of 2013 ended December 31, 2013, and provide an update on recent corporate progress. The dial-in numbers for the conference call are 800-706-7749 (U.S. participants) and 617-614-3474 (international participants); both numbers require passcode 27241849. To access the live webcast, go to the Events & Presentations page on the Investor Relations section of the Company's website at http://www.delcath.com/investors/events/.
A taped replay of the call will be available beginning approximately two hours after the call's conclusion and will be available for seven days. Dial-in numbers for the replay are 888-286-8010 and 617-801-6888 for U.S. and International callers, respectively. The replay passcode for both U.S. and International callers is 49674508. An archived webcast will also be available at http://www.delcath.com/investors/events/.
 CTH) today reported financial results and operational developments for the fiscal fourth quarter and full year ended December 31, 2013. Developments for the quarter and recent weeks subsequent to quarter end are as follows:
CTH) today reported financial results and operational developments for the fiscal fourth quarter and full year ended December 31, 2013. Developments for the quarter and recent weeks subsequent to quarter end are as follows:The Company's cash and cash equivalents increased in the fourth quarter 2013 and the first quarter of 2014; cash position as of February 28, 2014 was $32.7 million; fourth quarter 2013 cash utilization was $6.2 million
2014 average quarterly cash utilization expected to be between $5 and $6 million
In December 2013Delcath received comments from FDA on its proposed HCC trial protocol to investigate Melphalan HDS for first-line treatment of patients with unresectable advanced hepatocellular carcinoma (HCC) or primary liver cancer; a supplemental Investigational New Drug (IND) application incorporating FDA comments was submitted to FDA in February 2014; FDA review of the IND is pending
The Company received the approval from shareholders authorizing its Board of Directors, at its discretion, to effect a reverse stock split of the common stock at a specific ratio within a range from 1-for-8 to 1-for-16, inclusive, on or prior to December 31, 2014
"During our fourth quarter we continued to make steady progress on our main priorities of CHEMOSAT clinical adoption in Europe and our clinical development program for HCC," commented Jennifer K. Simpson, Interim Co-President and Co-CEO. "We are optimistic that clinical adoption is progressing in Germany and the U.K., key target markets that we believe offer the best near-term revenue opportunities. Experience with CHEMOSAT therapy continues to build, with 89 procedures performed on 61 patients since we first introduced CHEMOSAT in Europe. Concurrently, we had dialogue with the FDA on our supplemental IND submission and with the clinical institutions that will be conducting our HCC studies. As a result of our February IND submission, and assuming scientific review and institutional review board approvals, we now anticipate clinical institutions to become activated in the second quarter of 2014."
Financial Results
For the fourth quarter ended December 31, 2013, total revenue was $0.3 million compared with total revenue of $0.2 million in the fourth quarter 2012. Operating expenses decreased by approximately 52% to $5.8 million from $12.0 million for the same period in 2012. The decrease is primarily due to a significant reduction in expenses related to the Company's NDA submission to the FDA, as well as the Company's overall cost management efforts. Operating loss was $5.5 million, which included non-cash stock-based compensation income of $0.3 million, as compared with an operating loss of $11.8 million, including $0.9 million in non-cash stock-based compensation expense, in the year ago period.
For the full year ended December 31, 2013, total revenue was approximately $0.8 million of which $0.3 million was related to the recognition of previously deferred revenue. Total operating expenses decreased by approximately 38% to $33.3 million from $54.2 million for the same period in 2012. Operating loss for the year was $33.0 million, which included $0.3 million in non-cash stock-based compensation expense, as compared with an operating loss of $53.9 million, including $3.8 million in non-cash stock-based compensation expense, in the year ago period.
In 2013, Delcath raised approximately $43.2 million before related expenses, including approximately $26.7 million through the Company's At-the-Market equity offering program, $9.0 million through its Committed Equity Financing Facility program, and approximately $7.5 million through a registered direct offering.
Cash and cash equivalents as of December 31, 2013 were $31.2 million, compared with $23.7 million at December 31, 2012. During the year, cash used in operating activities was $34.1 million, a 32% reduction compared to $50.0 million in the comparable period in 2012. The decrease in cash utilization was in part due to a reduction in NDA submission-related costs, and improved organizational and operational efficiencies.
During the first quarter through February 28, 2014, the Company raised approximately $4.5 million before related expenses through our At-the-Market offering program. As of February 28, 2014, the Company's cash and cash equivalents were $32.7 million, including $0.4 million in accounts receivables collection.
"During the second half of 2013, we made significant progress in streamlining our business operations and improving our operational efficiencies, while focusing on our key priorities. As a result, we successfully reduced our operating costs and cash burn to more sustainable levels, and we believe we have sufficient resources to execute our plan into the first half of 2015," said Graham G. Miao, Interim Co-President and Co-CEO.
On February 24, 2014, shareholders approved an amendment to the Company's Amended and Restated Certificate of Incorporation authorizing the Board of Directors to affect a reverse stock split of the Company's common stock at a specific ratio within a range from 1-for-8 to 1-for-16, inclusive, on or prior to December 31, 2014. If deemed necessary by the Board, a reverse stock split may enable the Company to regain compliance with NASDAQ's $1.00 minimum bid price requirement by June 9, 2014, and maintain its listing on the NASDAQ Capital Market.
Conference Call and Webcast
The Company will host a conference call today, March 12, 2014 at 4:30 p.m. ET to discuss its financial results for the fourth quarter and full year of 2013 ended December 31, 2013, and provide an update on recent corporate progress. The dial-in numbers for the conference call are 800-706-7749 (U.S. participants) and 617-614-3474 (international participants); both numbers require passcode 27241849. To access the live webcast, go to the Events & Presentations page on the Investor Relations section of the Company's website at http://www.delcath.com/investors/events/.
A taped replay of the call will be available beginning approximately two hours after the call's conclusion and will be available for seven days. Dial-in numbers for the replay are 888-286-8010 and 617-801-6888 for U.S. and International callers, respectively. The replay passcode for both U.S. and International callers is 49674508. An archived webcast will also be available at http://www.delcath.com/investors/events/.
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +1,56 | |
| +0,25 | |
| -1,36 | |
| -0,71 | |
| +0,10 | |
| -1,08 | |
| -0,18 | |
| -4,35 | |
| +140,00 | |
| -1,52 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 172 | ||
| 120 | ||
| 78 | ||
| 57 | ||
| 56 | ||
| 54 | ||
| 54 | ||
| 53 | ||
| 44 | ||
| 40 |
Mit Delcath Systems "seperat" die Chemo einsetzen!





















