ZGNX nächster Turnaroundwert mit großem Potential - 500 Beiträge pro Seite
eröffnet am 30.11.11 23:50:09 von
neuester Beitrag 21.04.15 10:53:47 von
neuester Beitrag 21.04.15 10:53:47 von
Beiträge: 172
ID: 1.170.742
ID: 1.170.742
Aufrufe heute: 0
Gesamt: 23.798
Gesamt: 23.798
Aktive User: 0
ISIN: US98978L2043 · WKN: A14V7E
26,67
USD
+1,56 %
+0,41 USD
Letzter Kurs 05.03.22 NYSE
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 3,5800 | +922,86 | |
| 0,8800 | +95,56 | |
| 384,00 | +20,00 | |
| 3,0980 | +19,34 | |
| 3,0650 | +18,34 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,5000 | -24,62 | |
| 14,510 | -32,32 | |
| 71,33 | -33,92 | |
| 3,6400 | -38,62 | |
| 0,7000 | -61,85 |
TICKER: ZGNX
Kurs: $1.80
Börse: Nasdaq
Mein Kursziel: $ 5.00
52-Week High: $ 6.90
Mkt Cap (Mil) 117.33
Shares Out (Mil) 65.18
Float (Mil) 11.24
Insider buys: http://www.insidercow.com/history/company.jsp?company=zgnx&B…

Kurs: $1.80
Börse: Nasdaq
Mein Kursziel: $ 5.00
52-Week High: $ 6.90
Mkt Cap (Mil) 117.33
Shares Out (Mil) 65.18
Float (Mil) 11.24
Insider buys: http://www.insidercow.com/history/company.jsp?company=zgnx&B…
DD on ZGNX 100 Mill $$$ Mkt Cap 71 Mill in Cash & approved Migraine drug doing 40 mill in sales 2011 & projected to do 80 mill in 2012 BUT their big dollar drug has phase 3 trials completed & positive on their ( First to Market ) Extended release Hydrocodone drug & also ( First to Market ) APAP free or Acetaminophen free ( causes Liver Toxicity) & FDA cracking down on it. ZGNX according to last CC has already been approached by Pharmas looking to partner up on drug & will make a decision in early 2012 whether to partner or go alone. Why go alone ??? Huge market for Hydrocodone ( Over 6 Billion Dollars a Year ) & Oppenhiemer has opined that ZGNX drug will do around 500 Million a year in sales. Insiders know the potential & have bought over 56% of the total outstanding shares 36.4 Million shares including the 7.1 Million shares purchased in September. Coupled with the 18 Million shares just purchased in the last 3 months by Institutions which now totals over 25 million shares & growing . BTW 18 million shares bought 11 thousand shares sold ???? FLOAT is around 4 Million shares 4 MILLION ...This is a sure shot double/triple/??? easily could be another INHX $2 to $10 quickly on FDA or Partner news or a straight buyout..INSANE Valuation here
ZGNX webcast von gestern
http://ir.zogenix.com/phoenix.zhtml?c=220862&p=irol-IRHome
http://ir.zogenix.com/phoenix.zhtml?c=220862&p=irol-IRHome
From S.E.C. filing of latest 10Q
As of September 30, 2011, we had cash and cash equivalents of $70.8 million. We believe that our cash and cash equivalents as of September 30, 2011, together with future revenue and borrowings available under our $10.0 million revolving credit facility, will be sufficient to fund our operations into the second quarter of 2013.
As of September 30, 2011, we had cash and cash equivalents of $70.8 million. We believe that our cash and cash equivalents as of September 30, 2011, together with future revenue and borrowings available under our $10.0 million revolving credit facility, will be sufficient to fund our operations into the second quarter of 2013.
Ich habe noch nie einen Wert gesehen bei dem die Insiderkäufe im Vergleich zu den außenstehenden Aktien zu extrem sind.
Hier kommt was auf jeden Fall.
ZGNX ist keine Tradingaktie, sondern ein Investment.
Im ersten Quartal 2012 wird das Migränemedikamet von ZGNX entweder von FDA zugelassen oder nicht.
Aktuell sind ca. 900.000 short.
Hier kommt was auf jeden Fall.
ZGNX ist keine Tradingaktie, sondern ein Investment.
Im ersten Quartal 2012 wird das Migränemedikamet von ZGNX entweder von FDA zugelassen oder nicht.
Aktuell sind ca. 900.000 short.
ZGNX ist aktuell mit dem 1.65 fachen des Cashbestands bewertet.
LÄCHERLICH!
LÄCHERLICH!
Antwort auf Beitrag Nr.: 42.424.648 von Kursziel1000 am 01.12.11 11:59:53Alleinunterhalter??? Naja, nicht viel zu finden, aber seit einigen Tagen gehts langsam hoch....es sieht zumindest mal nicht schlecht aus

!
Dieser Beitrag wurde von MODernist moderiert. Grund: auf eigenen Wunsch des Users
Antwort auf Beitrag Nr.: 42.456.491 von sebirem am 08.12.11 18:18:26wobei ein Reverse nicht schlimm ist, sofern er nur alle paar "Jubeljahre"
geschieht!
Der Wert wird erst wieder um die 1-1,25 USD heiß und dann wäre ich dabei
also abwarten...
geschieht!
Der Wert wird erst wieder um die 1-1,25 USD heiß und dann wäre ich dabei

also abwarten...
Antwort auf Beitrag Nr.: 42.463.336 von sebirem am 10.12.11 09:13:07Dann sag mir bitte mal wie die Insider buys bei $2 erklärst, wenn die Aktie auf $1 fallen wird lol
Ich bin gespannt...
Ich bin gespannt...
Antwort auf Beitrag Nr.: 42.456.491 von sebirem am 08.12.11 18:18:26GENTA ist ne Bruchbude und kurz vor dem Bankkrott im Vergleich zu ZGNX.
Des weiteren ist ZGNX an der Nasdaq gelistet und nicht wie GENTA am Bulletin Board OTC.
Des weiteren ist ZGNX an der Nasdaq gelistet und nicht wie GENTA am Bulletin Board OTC.
Antwort auf Beitrag Nr.: 42.464.097 von Kursziel1000 am 10.12.11 15:56:48(wollte hier kein Vergleich ziehen...
Genta ist natürlich ne Bruchbude und =0 Wert!!)
Ja das mit den Insiderkäufen ist schon interessant!
Kann sicherlich nur noch interessanter werden, momentan bleibe
ich noch an der Seitenlinie.
Tja so ist das,sehr spekulativ, aber denke die Range die ich mir hier gesetzt habe,ist in Ordnung. Ansonsten habe ich leider pech gehabt, wenn
der Wert explodieren sollte
Wir werden sehen!
Genta ist natürlich ne Bruchbude und =0 Wert!!)
Ja das mit den Insiderkäufen ist schon interessant!
Kann sicherlich nur noch interessanter werden, momentan bleibe
ich noch an der Seitenlinie.
Tja so ist das,sehr spekulativ, aber denke die Range die ich mir hier gesetzt habe,ist in Ordnung. Ansonsten habe ich leider pech gehabt, wenn
der Wert explodieren sollte

Wir werden sehen!
Antwort auf Beitrag Nr.: 42.464.184 von sebirem am 10.12.11 16:48:50Die extremen Isiderkäufe machen für mich den Wert konservativ.
Hast jemals so heftige Insiderkäufe bei einem Wert gesehen?
Bei mir ist das schon sehr lange her...
Hast jemals so heftige Insiderkäufe bei einem Wert gesehen?
Bei mir ist das schon sehr lange her...
ZGNX news - Zogenix Completes Zohydro(TM) Pre-NDA Meetings With FDA
Zohydro NDA Submission on Track
SAN DIEGO, Dec. 20, 2011 (GLOBE NEWSWIRE) -- Zogenix, Inc. (Nasdaq:ZGNX - News), a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain, today announced that it has concluded its pre-New Drug Application (NDA) meetings with the U.S. Food & Drug Administration (FDA) related to its lead investigational product candidate, Zohydro(TM) (hydrocodone bitartrate extended-release capsules). The purpose of the meetings was to discuss the non-clinical, clinical and Chemistry, Manufacturing and Controls (CMC) development of Zohydro, and to agree on the submission requirements for the NDA submission under 505(b)(2) of the Federal Food, Drug, and Cosmetic Act. After a detailed review of the submission timeline, Zogenix plans to submit the NDA for Zohydro early in the second quarter of 2012.
Stephen Farr, PhD, president and chief operating officer of Zogenix, said, "The completion of our pre-NDA meetings with the FDA brings us one more important step closer to potentially gaining approval for Zohydro. We appreciate the informative interactions, timeliness, and clarity provided by the FDA, as well as the full support of Alkermes, our CMC partner, in the pre-NDA meeting process."
Zohydro is being evaluated for the management of moderate to severe chronic pain in patients requiring continuous around-the-clock opioid therapy for an extended period of time. If approved, Zohydro could be the first extended-release hydrocodone therapy available without acetaminophen, which is associated with an increased risk of liver toxicity when used in high doses over time.
Hydrocodone pain products represent the largest prescription drug category in the United States, with over 128 million prescriptions filled in 2010. The Company believes Zohydro's ability to provide consistent 12-hour pain relief, without exposure to acetaminophen, will position the product well in this large market.
About Zohydro
Zohydro is a novel, oral, single entity (without acetaminophen) extended-release capsule formulation of hydrocodone bitartrate. When used in high dosages over time, acetaminophen can cause liver toxicity. If approved, Zohydro could be the first single-entity hydrocodone therapy available. Zohydro uses Alkermes' patented Spheriodal Oral Drug Absorption System (SODAS(R)) drug delivery technology which serves to enhance the release profile of hydrocodone to provide consistent 12-hour pain relief relative to existing immediate release combination products. Capsule strengths utilized in the Phase 3 studies included 10, 20, 30, 40 and 50 mg capsules.
About Chronic Pain
The American Pain Society estimated in 1999 that 9% of the U.S. adult population suffers from moderate to severe non-cancer related chronic pain. Chronic pain can be treated with both immediate-release and extended-release opioids. Marketed hydrocodone products are the most commonly prescribed pharmaceuticals in the U.S., generating $3.2 billion in sales during the 12 months ended December 2010 (Wolters Kluwer Pharma Solutions, Source Pharmaceutical Audit Suite Retail, January 2010 -- December 2010). All of these hydrocodone products contain an analgesic combination ingredient, primarily acetaminophen. Acetaminophen may cause liver toxicity when used in high dosages over time.
About Zogenix
Zogenix, Inc. (Nasdaq:ZGNX - News), with offices in San Diego and Emeryville, California, is a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain. Zogenix's first commercial product, SUMAVEL(R) DosePro(R) (sumatriptan injection) Needle-free Delivery System, was launched in January 2010 for the acute treatment of migraine and cluster headache. Zogenix's lead investigational product candidate, Zohydro(TM) (hydrocodone bitartrate), is a novel, oral, single-entity (without acetaminophen) extended-release capsule formulation currently in Phase 3 clinical trials for the management of moderate to severe chronic pain in patients requiring around-the-clock opioid therapy. Zogenix's second investigational DosePro product candidate, Relday(TM), is a proprietary, long-acting injectable formulation of risperidone for the treatment of schizophrenia.
Forward Looking Statements
Zogenix cautions you that statements included in this press release that are not a description of historical facts are forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests," "assuming," "designed" and similar expressions are intended to identify forward-looking statements. These statements are based on the company's current beliefs and expectations. These forward-looking statements include statements regarding: the potential for, and timing of, an NDA submission for Zohydro; the potential for Zohydro to be the first approved oral, single-entity extended-release formulation of hydrocodone; and the size of the opioid pain market and the potential of Zohydro to be well positioned in that market. The inclusion of forward-looking statements should not be regarded as a representation by Zogenix that any of its plans will be achieved. Actual results may differ from those set forth in this release due to the risk and uncertainties inherent in Zogenix's business, including, without limitation: the top-line data Zogenix has reported for Zohydro is based on preliminary analysis of key efficacy and safety data, and such data may change following a more comprehensive review of the data related to the clinical trial, and may also change in connection with the continued review of such data as part of Zogenix's planned submission and the FDA's review of the NDA for Zohydro; the progress, timing and results of the planned bioequivalence study for Zohydro; the potential that earlier clinical trials may not be predictive of future results; the potential for Zohydro to receive regulatory approval on a timely basis or at all; the potential for adverse safety findings relating to Zohydro to delay or prevent regulatory approval or commercialization; the impact of any inability to raise sufficient capital to fund ongoing operations; the ability of Zogenix and its licensors to obtain, maintain and successfully enforce adequate patent and other intellectual property protection of its products and product candidates and the ability to operate its business without infringing the intellectual property rights of others; and other risks described in Zogenix's filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Zogenix undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.
SODAS(R) is a trademark of Alkermes plc.
SUMAVEL(R), DosePro(R), ReldayTM and ZohydroTM are trademarks of Zogenix, Inc.
Contact:
INVESTORS:Zack Kubow | The Ruth Group646.536.7020 | zkubow@theruthgroup.comMEDIA:Victoria Aguiar | The Ruth Group646.536.7013 | vaguiar@theruthgroup.com
Zohydro NDA Submission on Track
SAN DIEGO, Dec. 20, 2011 (GLOBE NEWSWIRE) -- Zogenix, Inc. (Nasdaq:ZGNX - News), a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain, today announced that it has concluded its pre-New Drug Application (NDA) meetings with the U.S. Food & Drug Administration (FDA) related to its lead investigational product candidate, Zohydro(TM) (hydrocodone bitartrate extended-release capsules). The purpose of the meetings was to discuss the non-clinical, clinical and Chemistry, Manufacturing and Controls (CMC) development of Zohydro, and to agree on the submission requirements for the NDA submission under 505(b)(2) of the Federal Food, Drug, and Cosmetic Act. After a detailed review of the submission timeline, Zogenix plans to submit the NDA for Zohydro early in the second quarter of 2012.
Stephen Farr, PhD, president and chief operating officer of Zogenix, said, "The completion of our pre-NDA meetings with the FDA brings us one more important step closer to potentially gaining approval for Zohydro. We appreciate the informative interactions, timeliness, and clarity provided by the FDA, as well as the full support of Alkermes, our CMC partner, in the pre-NDA meeting process."
Zohydro is being evaluated for the management of moderate to severe chronic pain in patients requiring continuous around-the-clock opioid therapy for an extended period of time. If approved, Zohydro could be the first extended-release hydrocodone therapy available without acetaminophen, which is associated with an increased risk of liver toxicity when used in high doses over time.
Hydrocodone pain products represent the largest prescription drug category in the United States, with over 128 million prescriptions filled in 2010. The Company believes Zohydro's ability to provide consistent 12-hour pain relief, without exposure to acetaminophen, will position the product well in this large market.
About Zohydro
Zohydro is a novel, oral, single entity (without acetaminophen) extended-release capsule formulation of hydrocodone bitartrate. When used in high dosages over time, acetaminophen can cause liver toxicity. If approved, Zohydro could be the first single-entity hydrocodone therapy available. Zohydro uses Alkermes' patented Spheriodal Oral Drug Absorption System (SODAS(R)) drug delivery technology which serves to enhance the release profile of hydrocodone to provide consistent 12-hour pain relief relative to existing immediate release combination products. Capsule strengths utilized in the Phase 3 studies included 10, 20, 30, 40 and 50 mg capsules.
About Chronic Pain
The American Pain Society estimated in 1999 that 9% of the U.S. adult population suffers from moderate to severe non-cancer related chronic pain. Chronic pain can be treated with both immediate-release and extended-release opioids. Marketed hydrocodone products are the most commonly prescribed pharmaceuticals in the U.S., generating $3.2 billion in sales during the 12 months ended December 2010 (Wolters Kluwer Pharma Solutions, Source Pharmaceutical Audit Suite Retail, January 2010 -- December 2010). All of these hydrocodone products contain an analgesic combination ingredient, primarily acetaminophen. Acetaminophen may cause liver toxicity when used in high dosages over time.
About Zogenix
Zogenix, Inc. (Nasdaq:ZGNX - News), with offices in San Diego and Emeryville, California, is a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain. Zogenix's first commercial product, SUMAVEL(R) DosePro(R) (sumatriptan injection) Needle-free Delivery System, was launched in January 2010 for the acute treatment of migraine and cluster headache. Zogenix's lead investigational product candidate, Zohydro(TM) (hydrocodone bitartrate), is a novel, oral, single-entity (without acetaminophen) extended-release capsule formulation currently in Phase 3 clinical trials for the management of moderate to severe chronic pain in patients requiring around-the-clock opioid therapy. Zogenix's second investigational DosePro product candidate, Relday(TM), is a proprietary, long-acting injectable formulation of risperidone for the treatment of schizophrenia.
Forward Looking Statements
Zogenix cautions you that statements included in this press release that are not a description of historical facts are forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests," "assuming," "designed" and similar expressions are intended to identify forward-looking statements. These statements are based on the company's current beliefs and expectations. These forward-looking statements include statements regarding: the potential for, and timing of, an NDA submission for Zohydro; the potential for Zohydro to be the first approved oral, single-entity extended-release formulation of hydrocodone; and the size of the opioid pain market and the potential of Zohydro to be well positioned in that market. The inclusion of forward-looking statements should not be regarded as a representation by Zogenix that any of its plans will be achieved. Actual results may differ from those set forth in this release due to the risk and uncertainties inherent in Zogenix's business, including, without limitation: the top-line data Zogenix has reported for Zohydro is based on preliminary analysis of key efficacy and safety data, and such data may change following a more comprehensive review of the data related to the clinical trial, and may also change in connection with the continued review of such data as part of Zogenix's planned submission and the FDA's review of the NDA for Zohydro; the progress, timing and results of the planned bioequivalence study for Zohydro; the potential that earlier clinical trials may not be predictive of future results; the potential for Zohydro to receive regulatory approval on a timely basis or at all; the potential for adverse safety findings relating to Zohydro to delay or prevent regulatory approval or commercialization; the impact of any inability to raise sufficient capital to fund ongoing operations; the ability of Zogenix and its licensors to obtain, maintain and successfully enforce adequate patent and other intellectual property protection of its products and product candidates and the ability to operate its business without infringing the intellectual property rights of others; and other risks described in Zogenix's filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Zogenix undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.
SODAS(R) is a trademark of Alkermes plc.
SUMAVEL(R), DosePro(R), ReldayTM and ZohydroTM are trademarks of Zogenix, Inc.
Contact:
INVESTORS:Zack Kubow | The Ruth Group646.536.7020 | zkubow@theruthgroup.comMEDIA:Victoria Aguiar | The Ruth Group646.536.7013 | vaguiar@theruthgroup.com
Zogenix, Astellas To End Co-Promotion Agreement For Sumavel Drug
Pharmaceutical company Zogenix Inc. (ZGNX) said it agreed to end its co-promotion of the Sumavel DosePro needle-free delivery system with Astellas Pharma U.S. Inc., a U.S. subsidiary of Astellas Pharma Inc. (4503.TO), on March 31 next year.
Sumavel DosePro is Zogenix's first commercial product and was launched with Astellas in January of last year to treat migraines and cluster headaches. Zogenix develops drugs for the treatment of nervous system disorders and pain.
Beginning in the second quarter of 2012, Zogenix will assume full responsibility for the commercialization of Sumavel, focusing on headache specialists, neurologists and primary care physicians who treat a lot of migraine patients.
Zogenix Chief Executive Roger Hawley said ending the co-promotion will lower its expenses, since Zogenix will no longer pay a service fee to Astellas beginning in the second quarter of 2012. He said the company is exploring the potential of modestly expanding its sales force and considering other Sumavel promotion partners.
Neither company will incur a penalty payment for terminating the agreement, which was originally slated to expire in June 2013. The companies plan to agree on a detailed customer transition plan by February next year, with the goal of uninterrupted access and service to physicians receiving the drug from Astellas.
Last month, Zogenix said its third-quarter loss edged lower on higher sales of Sumavel.
Shares closed Tuesday at $1.60 and were inactive premarket. The stock is down 72% year to date.
-By Ben Fox Rubin, Dow Jones Newswires; 212-416-3108; ben.rubin@dowjones.com;
Pharmaceutical company Zogenix Inc. (ZGNX) said it agreed to end its co-promotion of the Sumavel DosePro needle-free delivery system with Astellas Pharma U.S. Inc., a U.S. subsidiary of Astellas Pharma Inc. (4503.TO), on March 31 next year.
Sumavel DosePro is Zogenix's first commercial product and was launched with Astellas in January of last year to treat migraines and cluster headaches. Zogenix develops drugs for the treatment of nervous system disorders and pain.
Beginning in the second quarter of 2012, Zogenix will assume full responsibility for the commercialization of Sumavel, focusing on headache specialists, neurologists and primary care physicians who treat a lot of migraine patients.
Zogenix Chief Executive Roger Hawley said ending the co-promotion will lower its expenses, since Zogenix will no longer pay a service fee to Astellas beginning in the second quarter of 2012. He said the company is exploring the potential of modestly expanding its sales force and considering other Sumavel promotion partners.
Neither company will incur a penalty payment for terminating the agreement, which was originally slated to expire in June 2013. The companies plan to agree on a detailed customer transition plan by February next year, with the goal of uninterrupted access and service to physicians receiving the drug from Astellas.
Last month, Zogenix said its third-quarter loss edged lower on higher sales of Sumavel.
Shares closed Tuesday at $1.60 and were inactive premarket. The stock is down 72% year to date.
-By Ben Fox Rubin, Dow Jones Newswires; 212-416-3108; ben.rubin@dowjones.com;
ZGNX on ABC " 'Zo-Hydro' Contains Pure Hydrocodone"
http://abcnews.go.com/WNT/video/zohydro-pure-hydrocodone-152…
http://abcnews.go.com/WNT/video/zohydro-pure-hydrocodone-152…
Top Biotech Pick For 2012: Zogenix ( ZGNX )
http://seekingalpha.com/article/317105-top-biotech-pick-for-…
http://seekingalpha.com/article/317105-top-biotech-pick-for-…
sehr interessant... mehr sag i net..
mehr sag i net..
 mehr sag i net..
mehr sag i net..
Zogenix (NASDAQ: ZGNX) is now covered by analysts at William Blair. The analysts set an “outperform” rating and a $6.00 price target on the stock.
Sie ist mit Sicherheit auf dem Besten Weg dorthin :
wenn das so wäre.....???





Antwort auf Beitrag Nr.: 42.572.014 von ElGordo am 10.01.12 09:41:01Lies mal Dicker...
Bloomberg
Super Painkiller Needs Extra Scrutiny From FDA, NY Senator Says
January 09, 2012, 5:07 PM EST
By Anna Edney
Jan. 9 (Bloomberg) -- Powerful painkillers being researched by Zogenix Inc., a specialty pharmaceutical company, should be severely restricted if U.S. regulators decide to approve them, a New York senator said today.
Senator Charles Schumer, a Democrat, wrote Food and Drug Administration Commissioner Margaret Hamburg urging her to proceed with caution before allowing what he called “super” drugs that are 10 times more powerful than Vicodin on the market. Zogenix and at least three other companies are researching pure-form hydrocodone pain relievers, according to a statement from Schumer.
The products have never been sold in the U.S. and would be the most powerful prescription painkillers available, Schumer said. The senator urged FDA to closely track any approved hydrocodone products and take precautions to deter abuse as well as closely monitor advertising and sales.
“It’s tremendously concerning that at the same time policy makers and law enforcement professionals are waging a war on the growing prescription drug crisis, new and more powerful super- drugs could well be on their way, flooding the market,” Schumer said.
Zogenix’s product, Zohydro, began final phases of testing in March 2010, according to the company’s website. Zogenix plans to submit an application to the FDA for approval in the second quarter of 2012, Victoria Aguiar, a spokeswoman for Zogenix, said in an e-mail.
The company is attempting to develop a safer pain treatment than Vicodin, which contains acetaminophen and can cause liver toxicity, Aguiar said. Zogenix also plans to implement a risk mitigation strategy that recognizes the abuse potential of its product.
Zogenix rose less than 1 percent to $2.84 at the close in New York. The shares have declined 53 percent in the past 12 months.
--Editor: Andrew Pollack, Adriel Bettelheim

Bloomberg
Super Painkiller Needs Extra Scrutiny From FDA, NY Senator Says
January 09, 2012, 5:07 PM EST
By Anna Edney
Jan. 9 (Bloomberg) -- Powerful painkillers being researched by Zogenix Inc., a specialty pharmaceutical company, should be severely restricted if U.S. regulators decide to approve them, a New York senator said today.
Senator Charles Schumer, a Democrat, wrote Food and Drug Administration Commissioner Margaret Hamburg urging her to proceed with caution before allowing what he called “super” drugs that are 10 times more powerful than Vicodin on the market. Zogenix and at least three other companies are researching pure-form hydrocodone pain relievers, according to a statement from Schumer.
The products have never been sold in the U.S. and would be the most powerful prescription painkillers available, Schumer said. The senator urged FDA to closely track any approved hydrocodone products and take precautions to deter abuse as well as closely monitor advertising and sales.
“It’s tremendously concerning that at the same time policy makers and law enforcement professionals are waging a war on the growing prescription drug crisis, new and more powerful super- drugs could well be on their way, flooding the market,” Schumer said.
Zogenix’s product, Zohydro, began final phases of testing in March 2010, according to the company’s website. Zogenix plans to submit an application to the FDA for approval in the second quarter of 2012, Victoria Aguiar, a spokeswoman for Zogenix, said in an e-mail.
The company is attempting to develop a safer pain treatment than Vicodin, which contains acetaminophen and can cause liver toxicity, Aguiar said. Zogenix also plans to implement a risk mitigation strategy that recognizes the abuse potential of its product.
Zogenix rose less than 1 percent to $2.84 at the close in New York. The shares have declined 53 percent in the past 12 months.
--Editor: Andrew Pollack, Adriel Bettelheim
Zogenix, Inc Traded with a strange Volume - NASDAQ:ZGNX
Jan 10th, 2012
Zogenix, Inc (NASDAQ:ZGNX) is a specialty pharmaceutical company with two product candidates in late-stage development for the treatment of central nervous system (CNS) disorders and pain. Zogenix, Inc witnessed volume of 1.74 million shares during last trade however it holds an average trading capacity of 454,700 shares. ZGNX last trade opened at $2.90 reached intraday low of $2.32 and went +0.71% up to close at $2.84.
ZGNX has intra-day market capitalization $185.12 million and an enterprise value at $168.99 million. Trailing twelve months price to sales ratio of the stock was 4.81 while price to book ratio in most recent quarter was 6.07. In profitability ratios, net profit margin in past twelve months appeared at -162.00% whereas operating profit margin for the same period at -193.42%.
The company made a return on asset of -54.11% in past twelve months and return on equity of -779.22% for similar period. In the period of trailing 12 months it generated revenue amounted to $38.48 million gaining $1.30 revenue per share. Its year over year, quarterly growth of revenue was 47.30%.
According to preceding quarter balance sheet results, the company had $70.85 million cash in hand making cash per share at 1.09. The total of $56.01 million debt was there putting a total debt to equity ratio 185.68. Moreover its current ratio according to same quarter results was 2.70 and book value per share was 0.47.
Looking at the trading information, the stock price history displayed that its S&P500 52 Week Change illustrated 0.49% where the stock current price exhibited up beat from its 50 day moving average price of $1.86 and remained below from its 200 Day Moving Average price of $2.85.
ZGNX holds 65.18 million outstanding shares with 39.09 million floating shares where insider possessed 38.97% and institutions kept 39.10%.
THIS IS NOT A RECOMMENDATION TO BUY OR SELL ANY SECURITY!
Jan 10th, 2012
Zogenix, Inc (NASDAQ:ZGNX) is a specialty pharmaceutical company with two product candidates in late-stage development for the treatment of central nervous system (CNS) disorders and pain. Zogenix, Inc witnessed volume of 1.74 million shares during last trade however it holds an average trading capacity of 454,700 shares. ZGNX last trade opened at $2.90 reached intraday low of $2.32 and went +0.71% up to close at $2.84.
ZGNX has intra-day market capitalization $185.12 million and an enterprise value at $168.99 million. Trailing twelve months price to sales ratio of the stock was 4.81 while price to book ratio in most recent quarter was 6.07. In profitability ratios, net profit margin in past twelve months appeared at -162.00% whereas operating profit margin for the same period at -193.42%.
The company made a return on asset of -54.11% in past twelve months and return on equity of -779.22% for similar period. In the period of trailing 12 months it generated revenue amounted to $38.48 million gaining $1.30 revenue per share. Its year over year, quarterly growth of revenue was 47.30%.
According to preceding quarter balance sheet results, the company had $70.85 million cash in hand making cash per share at 1.09. The total of $56.01 million debt was there putting a total debt to equity ratio 185.68. Moreover its current ratio according to same quarter results was 2.70 and book value per share was 0.47.
Looking at the trading information, the stock price history displayed that its S&P500 52 Week Change illustrated 0.49% where the stock current price exhibited up beat from its 50 day moving average price of $1.86 and remained below from its 200 Day Moving Average price of $2.85.
ZGNX holds 65.18 million outstanding shares with 39.09 million floating shares where insider possessed 38.97% and institutions kept 39.10%.
THIS IS NOT A RECOMMENDATION TO BUY OR SELL ANY SECURITY!
will hier nur n kurzen Comment zum Chart geben...
(glaube Zogenix wird zwischen der 100- und 200 Tage Linie seitwärts pendeln 2,2-3,3USD ca. oder dann wieder nach oben ausbrechen, mal schauen...)
was mich nachdenklich macht, wie kommt so etwas zu stande?
Computer Programm?Technische Gegegnreaktion?Menschliches Handeln?Ist mir schon öfter aufgefallen auch nach sukzessiven Anstieg und dann schon direkt nach 50% folgen Gewinnmitnahmen...echt arm!So ist die Börse!

(glaube Zogenix wird zwischen der 100- und 200 Tage Linie seitwärts pendeln 2,2-3,3USD ca. oder dann wieder nach oben ausbrechen, mal schauen...)
was mich nachdenklich macht, wie kommt so etwas zu stande?
Computer Programm?Technische Gegegnreaktion?Menschliches Handeln?Ist mir schon öfter aufgefallen auch nach sukzessiven Anstieg und dann schon direkt nach 50% folgen Gewinnmitnahmen...echt arm!So ist die Börse!

Gestern nachbörsliche Ankündigung...
http://www.bloomberg.com/news/2012-02-03/aeropostale-micron-…
Nächste Woche sollte turbulent werden, hohes Volumen wird für ordentlichen Umsatz sorgen.
http://www.bloomberg.com/news/2012-02-03/aeropostale-micron-…
Nächste Woche sollte turbulent werden, hohes Volumen wird für ordentlichen Umsatz sorgen.
Wie soll man diese News sehen ?
Negativ ?
Negativ ?
Antwort auf Beitrag Nr.: 42.702.348 von Wohnwunsch am 05.02.12 18:32:50Mal sehen was hier kommt in den nächsten 3 Monaten
Antwort auf Beitrag Nr.: 42.715.481 von Wohnwunsch am 07.02.12 21:34:32Hier wird es bald einen Newsflow geben.
Höre das dies innerhalb der nächsten 30-45Tage geschehen soll.
Also wer noch nicht investiert ist sollte sich die Aktie mal genauer anschauen!
Höre das dies innerhalb der nächsten 30-45Tage geschehen soll.
Also wer noch nicht investiert ist sollte sich die Aktie mal genauer anschauen!
Antwort auf Beitrag Nr.: 42.567.819 von Kursziel1000 am 09.01.12 12:00:43Hiervscheint heute auch in grossem Stil gedeckelt zu werden
Größere Blaue Balken und der Kurs bewegt sich 0,0
Oh man heute könnte einem schlecht werden bei meinen Werten


Größere Blaue Balken und der Kurs bewegt sich 0,0
Oh man heute könnte einem schlecht werden bei meinen Werten


Und ab in den Keller, am besten geh ich mit in den Keller denn lachen kann ich nur noch dort wenn das so weiter geht
Antwort auf Beitrag Nr.: 42.567.819 von Kursziel1000 am 09.01.12 12:00:43Und ab in den Keller, heute grosser Ausverkauf
Oh wie ist das zum kotzen
Wer will nochmal wer hat noch nicht ?
Oh wie ist das zum kotzen
Wer will nochmal wer hat noch nicht ?
Höre auf mit rumheulen, Kleiner!
Wenn du die Schwankungen nicht verträgst, lege dir ein Sparbuch bei der Sparkasse zu!
Wenn du die Schwankungen nicht verträgst, lege dir ein Sparbuch bei der Sparkasse zu!
Antwort auf Beitrag Nr.: 42.752.416 von mr.krabs am 15.02.12 09:55:38Hier läuft auch nicht mehr viel ausser Richtung Süde
Zogenix, Inc. Earnings Conference Call (Q4 2011)
Scheduled to start Thu, Mar 8, 2012, 4:30 pm Eastern
Scheduled to start Thu, Mar 8, 2012, 4:30 pm Eastern
Antwort auf Beitrag Nr.: 42.792.231 von Wohnwunsch am 22.02.12 22:12:33Also heute sieht es sehr sehr gut aus, Volumen stimmt auch
Auf jedenfall bis jetzt

Auf jedenfall bis jetzt

Antwort auf Beitrag Nr.: 42.852.407 von Wohnwunsch am 05.03.12 16:34:59Sieht sehr sehr gut aus
Go Zogenix go go go



Go Zogenix go go go



Antwort auf Beitrag Nr.: 42.567.819 von Kursziel1000 am 09.01.12 12:00:43Sehr gut gestartet und dann wieder ins Minus
SCHADE


SCHADE


GAP bei $1.95 sollte hier morgen geschlossen werden.
Die Umsatzschätzungen von den Analysten wurden leicht verfehlt.
Die Umsatzschätzungen von den Analysten wurden leicht verfehlt.
Antwort auf Beitrag Nr.: 42.567.819 von Kursziel1000 am 09.01.12 12:00:43Was ist denn hier los, aus welchem Grund so stark im Minus ?
Warum dieser Kursrutsch, wascläuft hier jetzt ?
Antwort auf Beitrag Nr.: 42.874.515 von Kursziel1000 am 08.03.12 23:54:07Also ist auch diese Aktie ein Flopp und hier werden wir wohl nie die 5$ sehen
Wohl eher der $ wird hier bald kommen??????




Wohl eher der $ wird hier bald kommen??????




ZGNX 2.03 NDA filing 04/30/2012
Wohin geht jetzt wohl die Reise
Und sehen wir hier demnächst nochmal die 2,80-3,00$ ?
 :
:
Was ist deine Meinung Kursziel1000 ?
Und sehen wir hier demnächst nochmal die 2,80-3,00$ ?
 :
:Was ist deine Meinung Kursziel1000 ?
Antwort auf Beitrag Nr.: 42.881.977 von Wohnwunsch am 10.03.12 13:35:175$ bis spätestens Mitte des Jahres! In der Zwischenzeit höre ich mir Dein Geheule an!
Oh man oh man, was ist mit dieser Ktie jetzt los
Antwort auf Beitrag Nr.: 42.702.348 von Wohnwunsch am 05.02.12 18:32:50Also das nenne ich mal einen massiven Kursverlust innerhalb ein paar Tagen
Mir wird schon ganz schlecht bei dem Kursverlauf



Mir wird schon ganz schlecht bei dem Kursverlauf



Antwort auf Beitrag Nr.: 42.567.819 von Kursziel1000 am 09.01.12 12:00:43Kursverfall scheint auch weiterhin bei Zogenix voll in Takt zu sein
LEIDER


LEIDER


Kleiner Tipp an "Wünsch-dir-was".
Wenn man keine Ahnung hat, einfach mal die Fresse halten.
Danke
Wenn man keine Ahnung hat, einfach mal die Fresse halten.
Danke

ZGNX $1.96 ready for gap fill @$2.48 next week!
NDA filing 04/30/2012
Neues Tageshoch $2.18
Zogenix and Battelle Ink Marketing Agreement for DosePro(R) Drug Delivery Technology
Zogenix, Inc. (Nasdaq:ZGNX), a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain, and Battelle, the world's largest independent research and development organization, today finalized their previously announced collaborative agreement to advance out-licensing opportunities and development of Zogenix's DosePro® drug delivery technology. Battelle and Zogenix will co-market DosePro technology to potential pharmaceutical and government clients with the objective of licensing the system for use with innovative therapeutics that would be enabled or enhanced by DosePro's unique needle-free delivery system.
DosePro offers increased safety and convenience compared to needle-based injection via syringes or auto-injectors by providing a pre-filled, single-use, disposable, needle-free drug delivery system that is easy to use and preferred by patients. Compared to other delivery technologies, DosePro has the potential to solve the significant challenges of delivering viscous drug formulations, such as high concentration biologics, which cannot be delivered with traditional needle-based injection.
The DosePro drug delivery technology is covered by more than 46 internationally issued patents extending through 2026. Zogenix has produced in excess of 1.5 million units of the company's migraine therapy, SUMAVEL® DosePro®, which has received approval in the United States and Europe using the DosePro technology. Patient experience has demonstrated that patients will switch from oral to injectable formulations when provided the option of using SUMAVEL DosePro, despite the availability of a needle-based product for over a decade.
John Turanin, Vice President and General Manager, DosePro Technology, at Zogenix, states, "Battelle has a strong reputation for product development that has earned them a 'who's who' client list in the pharmaceutical industry. Collaborating on DosePro provides additional support of our technology and the backing of a significant technical business partner. We expect the out-licensing effort to accelerate now that we are working with Battelle. We have already trained their business development team and are expanding laboratory capabilities to begin working on DosePro product candidates."
Barbara Kunz, President of Battelle Health and Life Sciences Global Business, said, "This collaboration enables Battelle to expand our platform of innovative drug delivery solutions to our pharmaceutical customers. We believe DosePro will be able to assist our clients with addressing many of the challenges they face today, in particular, the delivery of highly viscous drug formulations."
Battelle's business development professionals responsible for life and health sciences will market DosePro to their customers in strategic product planning meetings, at conferences, in trade publications, and through other marketing communications. The technical teams from both Battelle and Zogenix are working together to create a center of excellence for DosePro technology development and testing within Battelle's laboratories. Battelle has the option to enter into an agreement with Zogenix to jointly develop and commercialize an iteration of the DosePro technology which delivers a larger dose (1.2mL) than the current dose size of 0.5mL.
For more information on licensing opportunities using the DosePro platform contact Michael Chansler from Battelle at (206) 588-9827 or chanslerm@battelle.org.
About DosePro®
The DosePro system is a first-in-class, easy-to-use drug delivery system designed for self-administration of a pre-filled, single dose of liquid drug, subcutaneously, without a needle. The platform is currently used by Zogenix's first commercial product, SUMAVEL DosePro®, and its investigational candidate, Relday. The Company believes that DosePro offers several benefits to patients compared to other subcutaneous delivery methods, and that it has the potential to become a preferred delivery option for patients and physicians. These benefits include less anxiety or fear due to the lack of a needle, easier disposal without the need for a sharps container, no risk of needle stick injury or contamination, an easy-to-use three step process, no need to fill or manipulate the device, reliable performance, discreet use and portability. In several clinical trials and market research studies, DosePro has been shown to be preferred by patients over conventional needle-based systems.
About Zogenix
Zogenix, Inc. (Nasdaq:ZGNX), with offices in San Diego and Emeryville, California, is a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain. Zogenix's first commercial product, SUMAVEL® DosePro® (sumatriptan injection) Needle-free Delivery System, was launched in January 2010 for the acute treatment of migraine and cluster headache. Zogenix's lead investigational product candidate, Zohydro™ (hydrocodone bitartrate), is a novel, oral, 12-hour extended-release formulation of hydrocodone without acetaminophen for the treatment of moderate to severe chronic pain requiring around the clock opioid therapy which has recently completed Phase 3 clinical trials. Zogenix's second DosePro investigational product candidate, Relday™, is a proprietary, long-acting injectable formulation of risperidone for the treatment of schizophrenia.
About Battelle
As the world's largest independent research and development organization, Battelle provides innovative solutions to the world's most pressing needs through its four global businesses: Laboratory Management; National Security; Health and Life Sciences; and Energy, Environment and Material Sciences. It advances scientific discovery and application by conducting $6.5 billion in global R&D annually through contract research, laboratory management and technology commercialization. Headquartered in Columbus, Ohio, Battelle oversees 22,000 employees in more than 130 locations worldwide, including seven national laboratories which Battelle manages or co-manages for the U.S. Department of Energy and the U.S. Department of Homeland Security and a nuclear energy lab in the United Kingdom.
Battelle also is one of the nation's leading charitable trusts focusing on societal and economic impact and actively supporting and promoting science, technology, engineering and mathematics (STEM) education.
Forward Looking Statements
Zogenix cautions you that statements included in this press release that are not a description of historical facts are forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests," "assuming," "designed" and similar expressions are intended to identify forward-looking statements. These statements are based on the company's current beliefs and expectations. These forward-looking statements include statements regarding the ability to successfully out-license the DosePro technology and the timing thereof, the ability of DosePro to solve the significant challenges of delivering viscous drug formulations, Battelle's exercise of the DosePro 1.2 mL option, the expected duration of patent protection for the DosePro technology and the likelihood that patients will switch from oral to injectable formulations when provided the option. The inclusion of forward-looking statements should not be regarded as a representation by Zogenix that any of its plans will be achieved. Actual results may differ from those set forth in this release due to the risk and uncertainties inherent in Zogenix's business, including, without limitation: difficulties in identifying, negotiating, executing and carrying out strategic transactions relating to DosePro and obtaining regulatory approval for other DosePro products; risks associated with the development of a larger volume, second generation version of the DosePro technology to accommodate drug formulation volumes greater than 0.5 mL; and the scope, validity and duration of patent protection and other intellectual property rights for DosePro; and other risks described in the company's prior press releases and filings with the Securities and Exchange Commission.
You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Zogenix undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.
SUMAVEL®, DosePro®, ReldayTM and ZohydroTM are trademarks of Zogenix, Inc.
CONTACT: ZOGENIX MEDIA:
Victoria Aguiar | The Ruth Group
646.536.7013 | vaguiar@theruthgroup.com
BATTELLE:
Katy Delaney
614.424.7208 | delaneyk@battelle.org
T.R. Massey
614.424.5544 | masseytr@battelle.org
Zogenix, Inc. (Nasdaq:ZGNX), a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain, and Battelle, the world's largest independent research and development organization, today finalized their previously announced collaborative agreement to advance out-licensing opportunities and development of Zogenix's DosePro® drug delivery technology. Battelle and Zogenix will co-market DosePro technology to potential pharmaceutical and government clients with the objective of licensing the system for use with innovative therapeutics that would be enabled or enhanced by DosePro's unique needle-free delivery system.
DosePro offers increased safety and convenience compared to needle-based injection via syringes or auto-injectors by providing a pre-filled, single-use, disposable, needle-free drug delivery system that is easy to use and preferred by patients. Compared to other delivery technologies, DosePro has the potential to solve the significant challenges of delivering viscous drug formulations, such as high concentration biologics, which cannot be delivered with traditional needle-based injection.
The DosePro drug delivery technology is covered by more than 46 internationally issued patents extending through 2026. Zogenix has produced in excess of 1.5 million units of the company's migraine therapy, SUMAVEL® DosePro®, which has received approval in the United States and Europe using the DosePro technology. Patient experience has demonstrated that patients will switch from oral to injectable formulations when provided the option of using SUMAVEL DosePro, despite the availability of a needle-based product for over a decade.
John Turanin, Vice President and General Manager, DosePro Technology, at Zogenix, states, "Battelle has a strong reputation for product development that has earned them a 'who's who' client list in the pharmaceutical industry. Collaborating on DosePro provides additional support of our technology and the backing of a significant technical business partner. We expect the out-licensing effort to accelerate now that we are working with Battelle. We have already trained their business development team and are expanding laboratory capabilities to begin working on DosePro product candidates."
Barbara Kunz, President of Battelle Health and Life Sciences Global Business, said, "This collaboration enables Battelle to expand our platform of innovative drug delivery solutions to our pharmaceutical customers. We believe DosePro will be able to assist our clients with addressing many of the challenges they face today, in particular, the delivery of highly viscous drug formulations."
Battelle's business development professionals responsible for life and health sciences will market DosePro to their customers in strategic product planning meetings, at conferences, in trade publications, and through other marketing communications. The technical teams from both Battelle and Zogenix are working together to create a center of excellence for DosePro technology development and testing within Battelle's laboratories. Battelle has the option to enter into an agreement with Zogenix to jointly develop and commercialize an iteration of the DosePro technology which delivers a larger dose (1.2mL) than the current dose size of 0.5mL.
For more information on licensing opportunities using the DosePro platform contact Michael Chansler from Battelle at (206) 588-9827 or chanslerm@battelle.org.
About DosePro®
The DosePro system is a first-in-class, easy-to-use drug delivery system designed for self-administration of a pre-filled, single dose of liquid drug, subcutaneously, without a needle. The platform is currently used by Zogenix's first commercial product, SUMAVEL DosePro®, and its investigational candidate, Relday. The Company believes that DosePro offers several benefits to patients compared to other subcutaneous delivery methods, and that it has the potential to become a preferred delivery option for patients and physicians. These benefits include less anxiety or fear due to the lack of a needle, easier disposal without the need for a sharps container, no risk of needle stick injury or contamination, an easy-to-use three step process, no need to fill or manipulate the device, reliable performance, discreet use and portability. In several clinical trials and market research studies, DosePro has been shown to be preferred by patients over conventional needle-based systems.
About Zogenix
Zogenix, Inc. (Nasdaq:ZGNX), with offices in San Diego and Emeryville, California, is a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain. Zogenix's first commercial product, SUMAVEL® DosePro® (sumatriptan injection) Needle-free Delivery System, was launched in January 2010 for the acute treatment of migraine and cluster headache. Zogenix's lead investigational product candidate, Zohydro™ (hydrocodone bitartrate), is a novel, oral, 12-hour extended-release formulation of hydrocodone without acetaminophen for the treatment of moderate to severe chronic pain requiring around the clock opioid therapy which has recently completed Phase 3 clinical trials. Zogenix's second DosePro investigational product candidate, Relday™, is a proprietary, long-acting injectable formulation of risperidone for the treatment of schizophrenia.
About Battelle
As the world's largest independent research and development organization, Battelle provides innovative solutions to the world's most pressing needs through its four global businesses: Laboratory Management; National Security; Health and Life Sciences; and Energy, Environment and Material Sciences. It advances scientific discovery and application by conducting $6.5 billion in global R&D annually through contract research, laboratory management and technology commercialization. Headquartered in Columbus, Ohio, Battelle oversees 22,000 employees in more than 130 locations worldwide, including seven national laboratories which Battelle manages or co-manages for the U.S. Department of Energy and the U.S. Department of Homeland Security and a nuclear energy lab in the United Kingdom.
Battelle also is one of the nation's leading charitable trusts focusing on societal and economic impact and actively supporting and promoting science, technology, engineering and mathematics (STEM) education.
Forward Looking Statements
Zogenix cautions you that statements included in this press release that are not a description of historical facts are forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests," "assuming," "designed" and similar expressions are intended to identify forward-looking statements. These statements are based on the company's current beliefs and expectations. These forward-looking statements include statements regarding the ability to successfully out-license the DosePro technology and the timing thereof, the ability of DosePro to solve the significant challenges of delivering viscous drug formulations, Battelle's exercise of the DosePro 1.2 mL option, the expected duration of patent protection for the DosePro technology and the likelihood that patients will switch from oral to injectable formulations when provided the option. The inclusion of forward-looking statements should not be regarded as a representation by Zogenix that any of its plans will be achieved. Actual results may differ from those set forth in this release due to the risk and uncertainties inherent in Zogenix's business, including, without limitation: difficulties in identifying, negotiating, executing and carrying out strategic transactions relating to DosePro and obtaining regulatory approval for other DosePro products; risks associated with the development of a larger volume, second generation version of the DosePro technology to accommodate drug formulation volumes greater than 0.5 mL; and the scope, validity and duration of patent protection and other intellectual property rights for DosePro; and other risks described in the company's prior press releases and filings with the Securities and Exchange Commission.
You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Zogenix undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.
SUMAVEL®, DosePro®, ReldayTM and ZohydroTM are trademarks of Zogenix, Inc.
CONTACT: ZOGENIX MEDIA:
Victoria Aguiar | The Ruth Group
646.536.7013 | vaguiar@theruthgroup.com
BATTELLE:
Katy Delaney
614.424.7208 | delaneyk@battelle.org
T.R. Massey
614.424.5544 | masseytr@battelle.org
Antwort auf Beitrag Nr.: 42.977.037 von Kursziel1000 am 30.03.12 01:40:00Und das ist fûr uns jetzt negativ oder positiv ?
Der Kurs ging ja am Freitad wieder kräftig nach Süden
:
Der Kurs ging ja am Freitad wieder kräftig nach Süden
:

Aktuelle Kurse unter $2 sind klare Kaufkurse!
Also es ist schon traurig wenn man den Verlauf ansieht,
ich bleib jetzt erstmal dabei auch wenn ich hier gut im
Minus bin.
Mal sehen was kommt
ich bleib jetzt erstmal dabei auch wenn ich hier gut im
Minus bin.
Mal sehen was kommt
Antwort auf Beitrag Nr.: 43.039.196 von Wohnwunsch am 14.04.12 14:20:42Was ist denn Dein Durschschnittskurs hier?
Antwort auf Beitrag Nr.: 43.040.327 von Wohnwunsch am 15.04.12 09:21:56Na da würde ich am Montag deine BWOW in den ersten 15-30Min verkaufen und dann bei ZGNX verbilligen....
Antwort auf Beitrag Nr.: 43.040.475 von Kursziel1000 am 15.04.12 11:13:18Da bin ich mal gespannt was bei WOW am Montag geht
ZGNX $2.00 - gap to fill @$2.48
Also momentan sieht diese Aktie eher wie eine Geldverbrennungsmaschine aus
Schade, der Verlauf spricht für sich
Schade, der Verlauf spricht für sich
Antwort auf Beitrag Nr.: 43.104.775 von Wohnwunsch am 30.04.12 16:12:29Leider hat Zogenix wieder im Minus geschlossen, mittlerweile
Scheint das leider der Trend bei dieser Aktie zu sein.
Momentan bin ich von diesem Invest nicht mehr überzeugt
Scheint das leider der Trend bei dieser Aktie zu sein.
Momentan bin ich von diesem Invest nicht mehr überzeugt
Antwort auf Beitrag Nr.: 43.106.083 von Wohnwunsch am 30.04.12 22:15:24Na wenn Du anfängst bearisch zu ZGNX eingestellt zu sein, dann werte ich das als bullischen Indikator die Aktie zu kaufen bzw. aufzustocken!
Antwort auf Beitrag Nr.: 43.106.393 von Kursziel1000 am 01.05.12 00:41:06Na wenn es so sein sollte dann bleib ich erstmal bearish vielleicht gewinnen wir dann
beide Bit Zogenix
Ich muss aber ehrlich sagen, das ich damals zeitgleich ein Invest in Zogenix und Biodelivery getätigt
habe und bei Zogenix bin ich fett im Minus, bei Biodelivery gute 90% im Plus.
Aktuell ist die wahre Perle "noch" Biodelivery
beide Bit Zogenix
Ich muss aber ehrlich sagen, das ich damals zeitgleich ein Invest in Zogenix und Biodelivery getätigt
habe und bei Zogenix bin ich fett im Minus, bei Biodelivery gute 90% im Plus.
Aktuell ist die wahre Perle "noch" Biodelivery
Antwort auf Beitrag Nr.: 43.106.723 von Wohnwunsch am 01.05.12 09:48:34Du hast ja auch viel später gekauft als ich das getan habe.
Antwort auf Beitrag Nr.: 43.107.397 von Kursziel1000 am 01.05.12 14:55:07Ja leider und deshalb fett im Minus


Wenn man fett im minus bei einer Aktie ist bei der man fest davon überzeugt ist , dass sie auf jeden Fall kurzfristig bis mittelfristig steigen wird, dann sollte man mit einem Double Down seinen Einstandskurs verbilligen imo.
Aktueller Kurs $1.92
Aktueller Kurs $1.92
Antwort auf Beitrag Nr.: 43.108.011 von Kursziel1000 am 01.05.12 19:23:13Hoffen wir mal das der Anstieg von Zogenix mal wieder nachhaltig ist.
Zeit würde es jetzt werden, denn der Kurs hat in den letzten Monaten stark
nachgegeben, leider.
Ich hoff wir laufen jetzt mal nachhaltig über die 2,xx$
Zeit würde es jetzt werden, denn der Kurs hat in den letzten Monaten stark
nachgegeben, leider.
Ich hoff wir laufen jetzt mal nachhaltig über die 2,xx$
Sieht nach dem Motto aus wie gewonnen so geronnen aus ?
LEIDER WIEDER NICHT NACHHALTIG
LEIDER WIEDER NICHT NACHHALTIG
Nur die Harten kommen in den Garten ... Aktie wird heute schön steigen!
Zogenix Submits New Drug Application (NDA) to U.S. Food and Drug Administration (FDA) for Zohydro™ for Treatment of Chronic Pain
SAN DIEGO, May 2, 2012 /PRNewswire/ -- Zogenix, Inc. (ZGNX), a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain, announced today that the Company has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for Zohydro™ (hydrocodone bitartrate extended-release capsules), Zogenix's lead investigational product candidate for the treatment of chronic pain.
Zohydro is a novel, oral, single-entity (without acetaminophen) extended-release formulation of various strengths of hydrocodone intended for administration every 12 hours for around the clock management of moderate to severe chronic pain. If approved, Zohydro could be the first hydrocodone product to offer the benefit of less frequent dosing and the ability to treat patients with chronic pain without the risk of acetaminophen-related liver injury. Currently, hydrocodone is only available in immediate-release, combination products, most commonly with the analgesic acetaminophen, and requires dosing every 4 to 6 hours. Zohydro, classified as a Drug Enforcement Agency (DEA) Schedule II drug product, would carry more strict prescription and dispensing rules as compared to the currently available hydrocodone combination products. In addition, Zogenix has included in the NDA a comprehensive Risk Evaluation and Mitigation Strategy (REMS) that is consistent with current FDA and industry-wide guidelines for extended-release opioid products. The REMS is intended to control inappropriate prescribing, misuse and abuse of extended-release opioids while maintaining patient access to essential pain medications.
"The NDA submission for Zohydro is a significant milestone, bringing us another step closer to making this important acetaminophen-free hydrocodone treatment option available to patients in need of around the clock therapy for chronic pain," said Stephen Farr, Ph.D., president and chief operating officer of Zogenix. "Hydrocodone is often a physician's first opioid recommendation for treating acute, moderate or moderately severe pain. However, many patients are being treated with hydrocodone combination products that include acetaminophen and, when used in high dosages or over long periods of time, put themselves at risk for developing liver injury. Zohydro could provide a significant new treatment alternative that does not contribute to this health risk."
The NDA submission is based on data from over 1,100 patients with chronic pain participating in the pivotal Phase 3 efficacy study (Study 801), and an open-label Phase 3 safety study (Study 802) of Zohydro. Study 801 successfully met its primary efficacy endpoint, demonstrating that Zohydro resulted in significantly (p=0.008) improved chronic pain relief compared to placebo. The two key secondary endpoints in this study - the proportion of patients with at least 30% improvement in pain intensity and the improvement of overall satisfaction of medication - were also met. Additional study endpoints were supportive of the efficacy of Zohydro compared to placebo. The study demonstrated that Zohydro was generally safe and well tolerated. Overall, the most commonly reported adverse events (greater than or equal to 2%) in the placebo-controlled pivotal Phase 3 efficacy Study 801 in opioid-experienced patients were consistent with those typically seen with chronic opioid therapy and were constipation, nausea, somnolence, fatigue, headache, dizziness, dry mouth, vomiting and pruritus. Study 802, in which patients received Zohydro for up to 12 months, further demonstrated that Zohydro was generally safe and well tolerated, and the incidence of adverse events was consistent with that seen in the pivotal Phase 3 efficacy study.
In conjunction with Zohydro's NDA submission, Zogenix is required to make a milestone payment of $1.0 million to Alkermes Pharma Ireland Limited (APIL), a subsidiary of Alkermes, plc, under the Company's exclusive license agreement with APIL in the U.S. for Zohydro.
About Zohydro
Zohydro is a novel, oral, single-entity extended-release formulation of hydrocodone without acetaminophen for the management of moderate to severe chronic pain in patients requiring around the clock opioid therapy. If approved, Zohydro could be the first single-entity hydrocodone therapy, avoiding the potential for liver injury associated with the use of acetaminophen in high doses or over long periods of time.
Zohydro uses APIL's patented Spheroidal Oral Drug Absorption System (SODAS®) drug delivery technology which serves to enhance the release profile of hydrocodone to provide extended-release pain relief relative to existing immediate-release combination products.
About Chronic Pain
Chronic pain is defined as ongoing or recurrent pain that adversely affects an individual's well-being. An estimated 116 million people in the United States are burdened with chronic pain, at an estimated national economic cost of $560 to $635 billion annually.
Chronic pain can be treated with both immediate-release and extended-release opioids. Currently marketed hydrocodone products are only immediate-release and contain an analgesic combination ingredient, primarily acetaminophen. Acetaminophen may cause liver injury when used in high dosages, over long periods of time or in accidental overdoses due to multiple acetaminophen products being taken at once.
About Zogenix
Zogenix, Inc. (ZGNX), with offices in San Diego and Emeryville, California, is a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain. Zogenix's first commercial product, SUMAVEL® DosePro® (sumatriptan injection) Needle-free Delivery System, was launched in January 2010 for the acute treatment of migraine and cluster headache. Zogenix's lead investigational product candidate, Zohydro™ (hydrocodone bitartrate), is a novel, oral, single-entity (without acetaminophen) extended-release formulation of various strengths of hydrocodone intended for administration every 12 hours for around the clock management of moderate to severe chronic pain. Zogenix submitted an NDA to the FDA for Zohydro in May 2012. Zogenix's second DosePro investigational product candidate, Relday™, is a proprietary, long-acting injectable formulation of risperidone for the treatment of schizophrenia.
For additional information, please visit www.zogenix.com.
Forward Looking Statements
Zogenix cautions you that statements included in this press release that are not a description of historical facts are forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests," "assuming," "designed" and similar expressions are intended to identify forward-looking statements. These statements are based on the company's current beliefs and expectations. These forward-looking statements include statements regarding: the potential for Zohydro to be the first approved oral, single-entity extended-release formulation of hydrocodone; and the size of the chronic pain market and the potential of Zohydro to provide a significant new treatment alternative and be well positioned in that market. The inclusion of forward-looking statements should not be regarded as a representation by Zogenix that any of its plans will be achieved. Actual results may differ from those set forth in this release due to the risk and uncertainties inherent in Zogenix's business, including, without limitation: the top-line data Zogenix has reported for Zohydro is based on preliminary analysis of key efficacy and safety data, and such data may change following a more comprehensive review of the data related to the clinical trial, and may also change in connection with the continued review of such data as part of Zogenix's submission and the FDA's review of the NDA for Zohydro; the potential for delays associated with any additional data required to be submitted by Zogenix in support of the NDA; the potential for Zohydro to receive regulatory approval on a timely basis or at all; the potential for adverse safety findings relating to Zohydro to delay or prevent regulatory approval or commercialization; the impact of any inability to raise sufficient capital to fund ongoing operations; the ability of Zogenix and its licensors to obtain, maintain and successfully enforce adequate patent and other intellectual property protection of its products and product candidates and the ability to operate its business without infringing the intellectual property rights of others; and other risks described in Zogenix's filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Zogenix undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.
SODAS® is a trademark of Alkermes Pharma Ireland Limited.
SUMAVEL ®, DosePro ®, Relday™ and Zohydro™ are trademarks of Zogenix, Inc.
INVESTORS:
MEDIA:
Zack Kubow | The Ruth Group
Emily Poe | WCG
646.536.7020 | zkubow@theruthgroup.com
212.301.7183 | epoe@wcgworld.com
Zogenix Submits New Drug Application (NDA) to U.S. Food and Drug Administration (FDA) for Zohydro™ for Treatment of Chronic Pain
SAN DIEGO, May 2, 2012 /PRNewswire/ -- Zogenix, Inc. (ZGNX), a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain, announced today that the Company has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for Zohydro™ (hydrocodone bitartrate extended-release capsules), Zogenix's lead investigational product candidate for the treatment of chronic pain.
Zohydro is a novel, oral, single-entity (without acetaminophen) extended-release formulation of various strengths of hydrocodone intended for administration every 12 hours for around the clock management of moderate to severe chronic pain. If approved, Zohydro could be the first hydrocodone product to offer the benefit of less frequent dosing and the ability to treat patients with chronic pain without the risk of acetaminophen-related liver injury. Currently, hydrocodone is only available in immediate-release, combination products, most commonly with the analgesic acetaminophen, and requires dosing every 4 to 6 hours. Zohydro, classified as a Drug Enforcement Agency (DEA) Schedule II drug product, would carry more strict prescription and dispensing rules as compared to the currently available hydrocodone combination products. In addition, Zogenix has included in the NDA a comprehensive Risk Evaluation and Mitigation Strategy (REMS) that is consistent with current FDA and industry-wide guidelines for extended-release opioid products. The REMS is intended to control inappropriate prescribing, misuse and abuse of extended-release opioids while maintaining patient access to essential pain medications.
"The NDA submission for Zohydro is a significant milestone, bringing us another step closer to making this important acetaminophen-free hydrocodone treatment option available to patients in need of around the clock therapy for chronic pain," said Stephen Farr, Ph.D., president and chief operating officer of Zogenix. "Hydrocodone is often a physician's first opioid recommendation for treating acute, moderate or moderately severe pain. However, many patients are being treated with hydrocodone combination products that include acetaminophen and, when used in high dosages or over long periods of time, put themselves at risk for developing liver injury. Zohydro could provide a significant new treatment alternative that does not contribute to this health risk."
The NDA submission is based on data from over 1,100 patients with chronic pain participating in the pivotal Phase 3 efficacy study (Study 801), and an open-label Phase 3 safety study (Study 802) of Zohydro. Study 801 successfully met its primary efficacy endpoint, demonstrating that Zohydro resulted in significantly (p=0.008) improved chronic pain relief compared to placebo. The two key secondary endpoints in this study - the proportion of patients with at least 30% improvement in pain intensity and the improvement of overall satisfaction of medication - were also met. Additional study endpoints were supportive of the efficacy of Zohydro compared to placebo. The study demonstrated that Zohydro was generally safe and well tolerated. Overall, the most commonly reported adverse events (greater than or equal to 2%) in the placebo-controlled pivotal Phase 3 efficacy Study 801 in opioid-experienced patients were consistent with those typically seen with chronic opioid therapy and were constipation, nausea, somnolence, fatigue, headache, dizziness, dry mouth, vomiting and pruritus. Study 802, in which patients received Zohydro for up to 12 months, further demonstrated that Zohydro was generally safe and well tolerated, and the incidence of adverse events was consistent with that seen in the pivotal Phase 3 efficacy study.
In conjunction with Zohydro's NDA submission, Zogenix is required to make a milestone payment of $1.0 million to Alkermes Pharma Ireland Limited (APIL), a subsidiary of Alkermes, plc, under the Company's exclusive license agreement with APIL in the U.S. for Zohydro.
About Zohydro
Zohydro is a novel, oral, single-entity extended-release formulation of hydrocodone without acetaminophen for the management of moderate to severe chronic pain in patients requiring around the clock opioid therapy. If approved, Zohydro could be the first single-entity hydrocodone therapy, avoiding the potential for liver injury associated with the use of acetaminophen in high doses or over long periods of time.
Zohydro uses APIL's patented Spheroidal Oral Drug Absorption System (SODAS®) drug delivery technology which serves to enhance the release profile of hydrocodone to provide extended-release pain relief relative to existing immediate-release combination products.
About Chronic Pain
Chronic pain is defined as ongoing or recurrent pain that adversely affects an individual's well-being. An estimated 116 million people in the United States are burdened with chronic pain, at an estimated national economic cost of $560 to $635 billion annually.
Chronic pain can be treated with both immediate-release and extended-release opioids. Currently marketed hydrocodone products are only immediate-release and contain an analgesic combination ingredient, primarily acetaminophen. Acetaminophen may cause liver injury when used in high dosages, over long periods of time or in accidental overdoses due to multiple acetaminophen products being taken at once.
About Zogenix
Zogenix, Inc. (ZGNX), with offices in San Diego and Emeryville, California, is a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain. Zogenix's first commercial product, SUMAVEL® DosePro® (sumatriptan injection) Needle-free Delivery System, was launched in January 2010 for the acute treatment of migraine and cluster headache. Zogenix's lead investigational product candidate, Zohydro™ (hydrocodone bitartrate), is a novel, oral, single-entity (without acetaminophen) extended-release formulation of various strengths of hydrocodone intended for administration every 12 hours for around the clock management of moderate to severe chronic pain. Zogenix submitted an NDA to the FDA for Zohydro in May 2012. Zogenix's second DosePro investigational product candidate, Relday™, is a proprietary, long-acting injectable formulation of risperidone for the treatment of schizophrenia.
For additional information, please visit www.zogenix.com.
Forward Looking Statements
Zogenix cautions you that statements included in this press release that are not a description of historical facts are forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests," "assuming," "designed" and similar expressions are intended to identify forward-looking statements. These statements are based on the company's current beliefs and expectations. These forward-looking statements include statements regarding: the potential for Zohydro to be the first approved oral, single-entity extended-release formulation of hydrocodone; and the size of the chronic pain market and the potential of Zohydro to provide a significant new treatment alternative and be well positioned in that market. The inclusion of forward-looking statements should not be regarded as a representation by Zogenix that any of its plans will be achieved. Actual results may differ from those set forth in this release due to the risk and uncertainties inherent in Zogenix's business, including, without limitation: the top-line data Zogenix has reported for Zohydro is based on preliminary analysis of key efficacy and safety data, and such data may change following a more comprehensive review of the data related to the clinical trial, and may also change in connection with the continued review of such data as part of Zogenix's submission and the FDA's review of the NDA for Zohydro; the potential for delays associated with any additional data required to be submitted by Zogenix in support of the NDA; the potential for Zohydro to receive regulatory approval on a timely basis or at all; the potential for adverse safety findings relating to Zohydro to delay or prevent regulatory approval or commercialization; the impact of any inability to raise sufficient capital to fund ongoing operations; the ability of Zogenix and its licensors to obtain, maintain and successfully enforce adequate patent and other intellectual property protection of its products and product candidates and the ability to operate its business without infringing the intellectual property rights of others; and other risks described in Zogenix's filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Zogenix undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.
SODAS® is a trademark of Alkermes Pharma Ireland Limited.
SUMAVEL ®, DosePro ®, Relday™ and Zohydro™ are trademarks of Zogenix, Inc.
INVESTORS:
MEDIA:
Zack Kubow | The Ruth Group
Emily Poe | WCG
646.536.7020 | zkubow@theruthgroup.com
212.301.7183 | epoe@wcgworld.com
Antwort auf Beitrag Nr.: 43.111.003 von Kursziel1000 am 02.05.12 15:12:10Vielleicht muss ich öfter über Zogenix schimpfen, dann geht sie
vielleicht schneller nach oben

vielleicht schneller nach oben

Zogenix ist mittlerweile ein sehr enttäuschendes Invest
LEIDER
LEIDER
ZGNX hat ein Problem - sie verbrennen viel zu schnell die mühsam eingesammelten Cashbestände. Ende Q1 waren nur noch 40 Mio Dollar übrig. Das bedeutet, dass ZGNX Ende 2012 ohne Mittel dastehen wird. Also droht in der nächsten Zeit eine weitere Kapitalerhöhung. Erst 2013 ist mit einer Zulassung von Zohydro zu rechnen...
Antwort auf Beitrag Nr.: 43.188.382 von Der.Eroberer am 20.05.12 13:08:48Hier ist wohl ein Ausstieg zur Schmerzbegrenzung ratsam
LEIDER, Hoffnung stirbt eben zuletzt


LEIDER, Hoffnung stirbt eben zuletzt


Momentan ist unsere Zogenix wohl eher eine Geldverbrennungsmaschine
als ein lohnendes Invest...
Ob Zogenix ein Erfolg wird wage ich im Moment eher stark zu bezweifeln
LEIDER ......
als ein lohnendes Invest...
Ob Zogenix ein Erfolg wird wage ich im Moment eher stark zu bezweifeln
LEIDER ......
Antwort auf Beitrag Nr.: 43.217.204 von Wohnwunsch am 28.05.12 11:39:41Ich kann Dir nicht folgen. Erklär mir bitte mal woran Du es festmachst, dass ZGNX eine Geldverbrennungsmaschine wird.
Mein Kursziel steht weiterhin bei $5.00
Mein Kursziel steht weiterhin bei $5.00
Aktueller Kurs $1.85
Antwort auf Beitrag Nr.: 43.228.196 von Kursziel1000 am 30.05.12 17:47:30Ich wollte mal wieder schimpfen damit sie steigt, ist eben wie beim
letzten mal.
Sage ich nichts fällt Zogenix, schimpfe ich steit Zogenix

letzten mal.
Sage ich nichts fällt Zogenix, schimpfe ich steit Zogenix

Antwort auf Beitrag Nr.: 43.229.047 von Wohnwunsch am 30.05.12 20:39:56die läuft die zeit weg für dein mio traum depot deshalb jaulst du doch



Antwort auf Beitrag Nr.: 43.229.056 von Peederwoogn2 am 30.05.12 20:41:53Das glaub ich eher nicht, hier bist eher du der Ungeduldige


$2.04 sieht doch mal wieder ganz nett aus
Item 1.01 Material Definitive Agreement
On June 6, 2012, Zogenix, Inc. (“Zogenix”) and Mallinckrodt LLC (“Mallinckrodt”) entered into a co-promotion agreement. Under the terms of the co-promotion agreement (the “Agreement”), Mallinckrodt was granted a co-exclusive right (with Zogenix) to promote Sumavel DosePro to a mutually agreed prescriber audience in the United States. Under the Agreement, Mallinckrodt’s sales team will begin selling Sumavel DosePro to its customer base of prescribers no later than August 20, 2012. Mallinckrodt has committed to a minimum number of sales representatives for the initial term of the Agreement, which runs through June 30, 2014, and can be extended by mutual agreement of the parties in additional six month increments. Zogenix remains responsible for the manufacture, supply and distribution of commercial product for sale in the United States. In addition, Zogenix will supply product samples to Mallinckrodt at an agreed upon transfer price and Mallinckrodt will reimburse Zogenix for all other promotional materials used.
In partial consideration of Mallinckrodt’s sales efforts, Zogenix will pay Mallinckrodt a service fee on a quarterly basis that represents a specified fixed percentage of net sales of prescriptions generated from Mallinckrodt’s prescriber audience over a baseline amount of net sales to the same prescriber audience (the “Baseline Net Sales”). In addition, upon completion of the co-promotion term in June 30, 2014 (unless otherwise extended), and only if the Agreement is not terminated as a result of certain circumstances, Zogenix will be required to pay Mallinckrodt an additional tail payment calculated as a fixed percentage of the Mallinckrodt net sales over the Baseline Net Sales during the first full twelve (12) months following the last day of the term.
Mallinckrodt may terminate the Agreement with sixty (60) days notice in the event a material change is made to the net sales price of Sumavel DosePro that would result in a material adverse effect to Mallinckrodt’s financial return (as defined in the Agreement). Mallinckrodt may also terminate the Agreement if its request for the inclusion on its call list of a certain number of additional prescribers is not mutually agreed upon. Lastly, Mallinckrodt may terminate the Agreement if a governmental authority takes action or raises an objection that prevents or would reasonably be expected to make it unlawful for Mallinckrodt to perform, or subject Mallinckrodt to any penalty or claim, investigation or similar action related to, its obligations under the Agreement, in the event of Zogenix’s inability to meet trade demand for commercial product or where a third party files an action alleging that the making or selling of Sumavel DosePro infringes the intellectual property rights of such third party.
Zogenix may terminate the Agreement with sixty (60) days notice if Mallinckrodt does not achieve an agreed-upon minimum sales effort. Either party may terminate the agreement if certain minimum net sales thresholds are not met for any quarter ending after December 31, 2012 or certain levels of prescriptions are not met in a specified period. In addition, either party may terminate the Agreement related to safety concerns, in the event of a change of control of itself or the other party (excluding with respect to Mallinckrodt, any public spin-off of Mallinckrodt from its corporate parent Covidien plc), upon the introduction of a generic product, in connection with the material breach of the other party’s obligations or if a bankruptcy event occurs under certain circumstances.
* * *
http://www.otcmarkets.com/edgar/GetFilingHtml?FilingID=86620…
On June 6, 2012, Zogenix, Inc. (“Zogenix”) and Mallinckrodt LLC (“Mallinckrodt”) entered into a co-promotion agreement. Under the terms of the co-promotion agreement (the “Agreement”), Mallinckrodt was granted a co-exclusive right (with Zogenix) to promote Sumavel DosePro to a mutually agreed prescriber audience in the United States. Under the Agreement, Mallinckrodt’s sales team will begin selling Sumavel DosePro to its customer base of prescribers no later than August 20, 2012. Mallinckrodt has committed to a minimum number of sales representatives for the initial term of the Agreement, which runs through June 30, 2014, and can be extended by mutual agreement of the parties in additional six month increments. Zogenix remains responsible for the manufacture, supply and distribution of commercial product for sale in the United States. In addition, Zogenix will supply product samples to Mallinckrodt at an agreed upon transfer price and Mallinckrodt will reimburse Zogenix for all other promotional materials used.
In partial consideration of Mallinckrodt’s sales efforts, Zogenix will pay Mallinckrodt a service fee on a quarterly basis that represents a specified fixed percentage of net sales of prescriptions generated from Mallinckrodt’s prescriber audience over a baseline amount of net sales to the same prescriber audience (the “Baseline Net Sales”). In addition, upon completion of the co-promotion term in June 30, 2014 (unless otherwise extended), and only if the Agreement is not terminated as a result of certain circumstances, Zogenix will be required to pay Mallinckrodt an additional tail payment calculated as a fixed percentage of the Mallinckrodt net sales over the Baseline Net Sales during the first full twelve (12) months following the last day of the term.
Mallinckrodt may terminate the Agreement with sixty (60) days notice in the event a material change is made to the net sales price of Sumavel DosePro that would result in a material adverse effect to Mallinckrodt’s financial return (as defined in the Agreement). Mallinckrodt may also terminate the Agreement if its request for the inclusion on its call list of a certain number of additional prescribers is not mutually agreed upon. Lastly, Mallinckrodt may terminate the Agreement if a governmental authority takes action or raises an objection that prevents or would reasonably be expected to make it unlawful for Mallinckrodt to perform, or subject Mallinckrodt to any penalty or claim, investigation or similar action related to, its obligations under the Agreement, in the event of Zogenix’s inability to meet trade demand for commercial product or where a third party files an action alleging that the making or selling of Sumavel DosePro infringes the intellectual property rights of such third party.
Zogenix may terminate the Agreement with sixty (60) days notice if Mallinckrodt does not achieve an agreed-upon minimum sales effort. Either party may terminate the agreement if certain minimum net sales thresholds are not met for any quarter ending after December 31, 2012 or certain levels of prescriptions are not met in a specified period. In addition, either party may terminate the Agreement related to safety concerns, in the event of a change of control of itself or the other party (excluding with respect to Mallinckrodt, any public spin-off of Mallinckrodt from its corporate parent Covidien plc), upon the introduction of a generic product, in connection with the material breach of the other party’s obligations or if a bankruptcy event occurs under certain circumstances.
* * *
http://www.otcmarkets.com/edgar/GetFilingHtml?FilingID=86620…
$2.34 Aktie steigt weiter 

Die Aktie hält sich in einem sehr schwachen Gesamtmarkt fantastisch!
Schlusskurs am Freitag lag bei $2.45
Schlusskurs am Freitag lag bei $2.45
Der Kursverlauf von Zogenix nervt nur noch ab....
Mal dann dann wieder fallen lassen, hier wird Zeit sich langsam zu verabschieden.
Es sieht wohl nach Nullnummer aus
LEIDER
Mal dann dann wieder fallen lassen, hier wird Zeit sich langsam zu verabschieden.
Es sieht wohl nach Nullnummer aus
LEIDER
SK $2.21 heute
Diese Aktie ist ein völliger Flopp, jeder Anstieg wird am Folgetag zum Verkauf genutzt
Hier wird nicht mehr viel kommen, das wir Kurse über 2€ sehen
Schade war wohl ein Fehlinvest
Hier wird nicht mehr viel kommen, das wir Kurse über 2€ sehen
Schade war wohl ein Fehlinvest
Heut kommen ja die Zahlen oder????
Antwort auf Beitrag Nr.: 43.468.366 von faxe3 am 08.08.12 06:14:41nichts wird kommen, mindestens nix positives


Und wieder ab in den Keller......
Diese Aktie ist zum kotzen mittlerweile
Diese Aktie ist zum kotzen mittlerweile
Was ist denn hier plötzlich los, warum rennt die Aktie auf einmal ?
Gibt es von Zogenix was Neues ?
Gibt es von Zogenix was Neues ?
Antwort auf Beitrag Nr.: 43.671.542 von Wohnwunsch am 02.10.12 22:11:21Warum läuft Zogenix aktuell so steil nach oben ?
Was ist hier im Busch ? Ich kann keine aktuelle News auf
der Homepage finden ausser die vom 27.09
Hat hier jemand eine Meinung dazu ?
Was ist hier im Busch ? Ich kann keine aktuelle News auf
der Homepage finden ausser die vom 27.09
Hat hier jemand eine Meinung dazu ?
War ja zu erwarten das der Dreck heute wieder stark fällt
Was ist den hier jetzt los
Also wenn es nach WellsFargo geht soll man jetzt nachkaufen, trotz der negativen Abstimmung!Wünsche dir viel Glück, das Zogenix wieder hoch geht!!
"Wells Fargo believes the Federal Drug Administration’s decision concerning Zogenix’s Zohydro ER could be different than the panel’s negative vote.
They think that approval of the drug is a probable outcome and recommend buying shares of Zogenix, Inc. on any weakness from the negative panel vote"
"Wells Fargo believes the Federal Drug Administration’s decision concerning Zogenix’s Zohydro ER could be different than the panel’s negative vote.
They think that approval of the drug is a probable outcome and recommend buying shares of Zogenix, Inc. on any weakness from the negative panel vote"
Antwort auf Beitrag Nr.: 43.912.065 von sebirem am 10.12.12 20:44:47Also ich konnte bis jetzt nichts finden, was ist passiert bei Zogenix ?
Leider bin ich die letzten Wochen krankheitsbedingt ausgefallen und konnte
nicht folgen...
Was gab es hier für News ?
Leider bin ich die letzten Wochen krankheitsbedingt ausgefallen und konnte
nicht folgen...
Was gab es hier für News ?
Also das nenne ich mal einen massiven Kurseinbruch
Pfffffffff
Pfffffffff
Antwort auf Beitrag Nr.: 43.912.102 von Wohnwunsch am 10.12.12 20:53:05du solltest dich schon regelmäßig über deine Investments belesen, wenn du dein Geld reinsteckst!!
"Es wurde gegen eine Zulassung von Zohydro (Schmerzmittel) vom FDA Anesthetic and Analgesic Drug Products Advisory Committee gestimmt
2pro 11contra 1Enthaltung"
Aber das sagt noch nicht soo viel aus!Es kann trotzdem klappen!
Wir lassen und überraschen!
Bin ab this week wieder in CBRX und Cardiome stärker investiert!!
Viel Glück, bis dann!
Zogenix kommt wieder!!
"Es wurde gegen eine Zulassung von Zohydro (Schmerzmittel) vom FDA Anesthetic and Analgesic Drug Products Advisory Committee gestimmt
2pro 11contra 1Enthaltung"
Aber das sagt noch nicht soo viel aus!Es kann trotzdem klappen!
Wir lassen und überraschen!
Bin ab this week wieder in CBRX und Cardiome stärker investiert!!
Viel Glück, bis dann!
Zogenix kommt wieder!!
Antwort auf Beitrag Nr.: 43.931.435 von sebirem am 15.12.12 14:09:06
3 Healthcare Stocks With Recent Intensive Insider Buying
December 19, 2012 | includes: AVEO, NBY, ZGNX
Disclosure: I am long NBY, ZGNX. (More...)
When insiders accumulate a stock intensively, the stock can be expected to outperform the market during the next six months. Insiders tend to buy more often than usual before large price increases.
Intensive insider buying can be defined by the following three criteria:
The stock is purchased by three or more insiders within one month.
The stock is sold by no insiders in the month of intensive purchasing.
At least two purchasers increase their holdings by more than 10 percent.
In this article I will feature three healthcare stocks that met these three criteria of intensive insider buying during the last 30 days.
1. NovaBay Pharmaceuticals (NBY), a clinical-stage biotechnology company, engages in the development of various product candidates for the therapeutic needs of the anti-infective market.
(click to enlarge)
Insider buys
David Stroman purchased 10,000 shares on December 6 pursuant to a public offering. David Stroman currently holds 22,000 shares or less than 0.1% of the company. Dr. David Stroman joined NovaBay in October 2011 as Senior Vice President of Ophthalmic Drug Development.
Thomas Paulson purchased 10,000 shares on December 6 pursuant to a public offering. Thomas Paulson currently holds 63,855 shares or 0.2% of the company. Thomas Paulson has served as NovaBay's Chief Financial Officer since January 2008.
Ramin Najafi purchased 20,000 shares on December 6 pursuant to a public offering. Ramin Najafi currently controls 3,217,695 shares or 10.8% of the company. Ramin Najafi is the founder and Chairman of NovaBay. He has served as President since July 2002, and as Chief Executive Officer since November 2004.
Financials
The company reported the third-quarter financial results on November 1 with the following highlights:
Revenue $3.6 million
Net income $0.1 million
Cash $11.9 million
NovaBay received approximately $6.34 million from the public offering which closed on December 6.
Upcoming milestones
Ophthalmology
• Viral conjunctivitis (unmet medical need)
- Phase 2b global 450 patient trial. Results from the global trial expected 2H13.
• Bacterial conjunctivitis (new clinical target)
- Phase 2a proof-of-concept bacterial conjunctivitis trial, facilitating ultimate goal of one product for the treatment of both viral and bacterial conjunctivitis.
Dermatology - Impetigo
• Partner Galderma S.A. initiated Phase 2b global trial of NVC-422 for treatment of impetigo, a highly contagious skin infection, in September 2012. Galderma expects to report results in mid 2013.
Urology - UCBE
• Ongoing Phase 2 clinical trial for treatment of urinary catheter blockage and encrustation [UCBE]. Clinical data expected 1H13.
Chronic Non-Healing Wounds - NeutroPhase
• Secured agreement with Pioneer Pharma to market NeutroPhase throughout China in January 2012; expanded agreement to include Southeast Asia in September 2012. NeutroPhase recently utilized as part of successful new therapeutic technique for management of necrotizing fasciitis (NF, or "flesh-eating bacteria"). First commercial shipment of NeutroPhase to Southeast Asia completed in December 2012. Additional marketing agreements expected worldwide in 2013.
My analysis
The stock is currently trading below its 200-day moving average. There have been three insider buy transactions and there have not been any insider sell transactions during the last 30 days. There is one analyst buy rating, 0 neutral ratings and 0 sell ratings with a average target price of $3.50. The company is going to present results from three different phase 2b trials during 2013. The outlook for these results is positive based on the insider trading activity. I would expect each of these three results to move the stock at least 20% up or down. I have a long position in the stock currently.
2. Zogenix (ZGNX) is a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain. Zogenix's first commercial product, Sumavel DosePro (sumatriptan injection) Needle-free Delivery System, was launched in January 2010 for the acute treatment of migraine and cluster headache. Zogenix's lead investigational product candidate, Zohydro ER (hydrocodone bitartrate) is an oral, novel extended-release formulation of various strengths of hydrocodone without acetaminophen intended for administration every 12 hours for around the clock management of moderate to severe chronic pain. Zogenix's second DosePro investigational product candidate, Relday, is a proprietary, long-acting injectable formulation of risperidone for the treatment of schizophrenia. In May 2012, Zogenix submitted to the FDA a New Drug Application for Zohydro ER and an Investigational New Drug Application for Relday. The FDA assigned a PDUFA target action date of March 1, 2013 for the Zohydro ER NDA.
(click to enlarge)
Insider buys
Cynthia Robinson purchased 50,000 shares on December 13 and currently holds 52,746 shares or less than 0.1% of the company. Cynthia Robinson has served as the company's Chief Development Officer since April 2008.
Cam Garner purchased 70,000 shares on December 12 and currently holds 296,750 shares or 0.3% of the company. Cam Garner is one of the company's co-founders and has served as chairman of the company's board of directors since August 2006.
Ann Rhoads purchased 17,500 shares on December 12 and currently holds 150,742 shares or 0.2% of the company. Ann Rhoads has served as Executive Vice President, Chief Financial Officer, Treasurer and Secretary since March 2010.
Erle Mast purchased 10,000 shares on December 11 and currently holds 16,721 shares or less than 0.1% of the company. Erle Mast has served as a member of the company's board of directors since May 2008.
Financials
The company reported the third-quarter financial results on November 8 with the following highlights:
Revenue $8.5 million
Net loss $19.3 million
Cash $49.6 million
Debt $28.4 million
Outlook
The company's full year 2012 financial guidance is as follows:
Total revenue expected to be approximately $45.5 million
Net product revenue expected to be approximately $37 million
Product gross margin expected to be approximately 48% - 49%
Research and development expenses expected to be approximately $22 million
Selling, general and administrative expenses expected to be approximately $50 million
Net interest expense to be approximately $10 million for 2012
News
Zogenix announced on December 7 that the U.S. Food and Drug Administration's Anesthetic and Analgesic Drug Products Advisory Committee voted 2-11 [with 1 abstention] against the approval of Zohydro ER.
My analysis
The stock dipped on December 10 after the negative vote for Zohydro ER. There have been four insider buy transactions and there have not been any insider sell transactions during the last 30 days. It is important to note that all the insider transactions were made after the negative vote for Zohydro. There are four analyst buy ratings, one neutral rating and 0 sell ratings with a average target price of $6.00. I have a long position in the stock currently.
3. AVEO Oncology (AVEO) is a cancer therapeutics company committed to discovering, developing and commercializing targeted therapies to impact patients' lives.
(click to enlarge)
Insider buys
Henri Termeer purchased 44,501 shares on December 12-13 and currently holds 44,501 shares or 0.1% of the company. Henri Termeer has served as a director of AVEO since April 2011 and is Chairman of the Board.
Robert Young purchased 3,621 shares on December 3-13 and currently holds 8,010 shares or less than 0.1% of the company. Robert Young has served as a director of the company since July 2009.
Tuan Ha-Ngoc purchased 75,000 shares on December 12-13 and currently holds 314,640 shares or 0.7% of the company. Tuan Ha-Ngoc has served as president and chief executive officer of AVEO and as a member of the board of directors since June 2002.
Financials
The company reported the third-quarter financial results on October 30 with the following highlights:
Revenue $1.0 million
Net loss $30.1 million
Cash $189.7 million
Debt $25.9 million
Outlook
AVEO expects to end 2012 with approximately $135 million in cash, cash equivalents and marketable securities. Based on its revised operating plan, AVEO anticipates that this capital is sufficient to fund its operations through 2013.
News
AVEO Oncology and Astellas Pharma announced on November 28 that the U.S. Food and Drug Administration had accepted for filing the New Drug Application for tivozanib with the proposed indication for the treatment of patients with advanced renal cell carcinoma. Tivozanib is an investigational medicine and is not currently approved in any country. According to the timelines established by the Prescription Drug User Fee Act, the review of the NDA is expected to be complete by July 28, 2013.
My analysis
The stock made a new 52 -week low last week. There have been four insider buy transactions and there have not been any insider sell transactions during the last 30 days. There are two analyst buy ratings, four neutral ratings and one sell rating with a average target price of $12.50. The next major catalyst for the stock will be the July 28, 2013 PDUFA date. I am expecting the stock to start trending higher as we move closer to the PDUFA date based on the insider trading activity.
706 people decided to get ZGNX articles by email alert
3 Healthcare Stocks With Recent Intensive Insider Buying
December 19, 2012 | includes: AVEO, NBY, ZGNX
Disclosure: I am long NBY, ZGNX. (More...)
When insiders accumulate a stock intensively, the stock can be expected to outperform the market during the next six months. Insiders tend to buy more often than usual before large price increases.
Intensive insider buying can be defined by the following three criteria:
The stock is purchased by three or more insiders within one month.
The stock is sold by no insiders in the month of intensive purchasing.
At least two purchasers increase their holdings by more than 10 percent.
In this article I will feature three healthcare stocks that met these three criteria of intensive insider buying during the last 30 days.
1. NovaBay Pharmaceuticals (NBY), a clinical-stage biotechnology company, engages in the development of various product candidates for the therapeutic needs of the anti-infective market.
(click to enlarge)
Insider buys
David Stroman purchased 10,000 shares on December 6 pursuant to a public offering. David Stroman currently holds 22,000 shares or less than 0.1% of the company. Dr. David Stroman joined NovaBay in October 2011 as Senior Vice President of Ophthalmic Drug Development.
Thomas Paulson purchased 10,000 shares on December 6 pursuant to a public offering. Thomas Paulson currently holds 63,855 shares or 0.2% of the company. Thomas Paulson has served as NovaBay's Chief Financial Officer since January 2008.
Ramin Najafi purchased 20,000 shares on December 6 pursuant to a public offering. Ramin Najafi currently controls 3,217,695 shares or 10.8% of the company. Ramin Najafi is the founder and Chairman of NovaBay. He has served as President since July 2002, and as Chief Executive Officer since November 2004.
Financials
The company reported the third-quarter financial results on November 1 with the following highlights:
Revenue $3.6 million
Net income $0.1 million
Cash $11.9 million
NovaBay received approximately $6.34 million from the public offering which closed on December 6.
Upcoming milestones
Ophthalmology
• Viral conjunctivitis (unmet medical need)
- Phase 2b global 450 patient trial. Results from the global trial expected 2H13.
• Bacterial conjunctivitis (new clinical target)
- Phase 2a proof-of-concept bacterial conjunctivitis trial, facilitating ultimate goal of one product for the treatment of both viral and bacterial conjunctivitis.
Dermatology - Impetigo
• Partner Galderma S.A. initiated Phase 2b global trial of NVC-422 for treatment of impetigo, a highly contagious skin infection, in September 2012. Galderma expects to report results in mid 2013.
Urology - UCBE
• Ongoing Phase 2 clinical trial for treatment of urinary catheter blockage and encrustation [UCBE]. Clinical data expected 1H13.
Chronic Non-Healing Wounds - NeutroPhase
• Secured agreement with Pioneer Pharma to market NeutroPhase throughout China in January 2012; expanded agreement to include Southeast Asia in September 2012. NeutroPhase recently utilized as part of successful new therapeutic technique for management of necrotizing fasciitis (NF, or "flesh-eating bacteria"). First commercial shipment of NeutroPhase to Southeast Asia completed in December 2012. Additional marketing agreements expected worldwide in 2013.
My analysis
The stock is currently trading below its 200-day moving average. There have been three insider buy transactions and there have not been any insider sell transactions during the last 30 days. There is one analyst buy rating, 0 neutral ratings and 0 sell ratings with a average target price of $3.50. The company is going to present results from three different phase 2b trials during 2013. The outlook for these results is positive based on the insider trading activity. I would expect each of these three results to move the stock at least 20% up or down. I have a long position in the stock currently.
2. Zogenix (ZGNX) is a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain. Zogenix's first commercial product, Sumavel DosePro (sumatriptan injection) Needle-free Delivery System, was launched in January 2010 for the acute treatment of migraine and cluster headache. Zogenix's lead investigational product candidate, Zohydro ER (hydrocodone bitartrate) is an oral, novel extended-release formulation of various strengths of hydrocodone without acetaminophen intended for administration every 12 hours for around the clock management of moderate to severe chronic pain. Zogenix's second DosePro investigational product candidate, Relday, is a proprietary, long-acting injectable formulation of risperidone for the treatment of schizophrenia. In May 2012, Zogenix submitted to the FDA a New Drug Application for Zohydro ER and an Investigational New Drug Application for Relday. The FDA assigned a PDUFA target action date of March 1, 2013 for the Zohydro ER NDA.
(click to enlarge)
Insider buys
Cynthia Robinson purchased 50,000 shares on December 13 and currently holds 52,746 shares or less than 0.1% of the company. Cynthia Robinson has served as the company's Chief Development Officer since April 2008.
Cam Garner purchased 70,000 shares on December 12 and currently holds 296,750 shares or 0.3% of the company. Cam Garner is one of the company's co-founders and has served as chairman of the company's board of directors since August 2006.
Ann Rhoads purchased 17,500 shares on December 12 and currently holds 150,742 shares or 0.2% of the company. Ann Rhoads has served as Executive Vice President, Chief Financial Officer, Treasurer and Secretary since March 2010.
Erle Mast purchased 10,000 shares on December 11 and currently holds 16,721 shares or less than 0.1% of the company. Erle Mast has served as a member of the company's board of directors since May 2008.
Financials
The company reported the third-quarter financial results on November 8 with the following highlights:
Revenue $8.5 million
Net loss $19.3 million
Cash $49.6 million
Debt $28.4 million
Outlook
The company's full year 2012 financial guidance is as follows:
Total revenue expected to be approximately $45.5 million
Net product revenue expected to be approximately $37 million
Product gross margin expected to be approximately 48% - 49%
Research and development expenses expected to be approximately $22 million
Selling, general and administrative expenses expected to be approximately $50 million
Net interest expense to be approximately $10 million for 2012
News
Zogenix announced on December 7 that the U.S. Food and Drug Administration's Anesthetic and Analgesic Drug Products Advisory Committee voted 2-11 [with 1 abstention] against the approval of Zohydro ER.
My analysis
The stock dipped on December 10 after the negative vote for Zohydro ER. There have been four insider buy transactions and there have not been any insider sell transactions during the last 30 days. It is important to note that all the insider transactions were made after the negative vote for Zohydro. There are four analyst buy ratings, one neutral rating and 0 sell ratings with a average target price of $6.00. I have a long position in the stock currently.
3. AVEO Oncology (AVEO) is a cancer therapeutics company committed to discovering, developing and commercializing targeted therapies to impact patients' lives.
(click to enlarge)
Insider buys
Henri Termeer purchased 44,501 shares on December 12-13 and currently holds 44,501 shares or 0.1% of the company. Henri Termeer has served as a director of AVEO since April 2011 and is Chairman of the Board.
Robert Young purchased 3,621 shares on December 3-13 and currently holds 8,010 shares or less than 0.1% of the company. Robert Young has served as a director of the company since July 2009.
Tuan Ha-Ngoc purchased 75,000 shares on December 12-13 and currently holds 314,640 shares or 0.7% of the company. Tuan Ha-Ngoc has served as president and chief executive officer of AVEO and as a member of the board of directors since June 2002.
Financials
The company reported the third-quarter financial results on October 30 with the following highlights:
Revenue $1.0 million
Net loss $30.1 million
Cash $189.7 million
Debt $25.9 million
Outlook
AVEO expects to end 2012 with approximately $135 million in cash, cash equivalents and marketable securities. Based on its revised operating plan, AVEO anticipates that this capital is sufficient to fund its operations through 2013.
News
AVEO Oncology and Astellas Pharma announced on November 28 that the U.S. Food and Drug Administration had accepted for filing the New Drug Application for tivozanib with the proposed indication for the treatment of patients with advanced renal cell carcinoma. Tivozanib is an investigational medicine and is not currently approved in any country. According to the timelines established by the Prescription Drug User Fee Act, the review of the NDA is expected to be complete by July 28, 2013.
My analysis
The stock made a new 52 -week low last week. There have been four insider buy transactions and there have not been any insider sell transactions during the last 30 days. There are two analyst buy ratings, four neutral ratings and one sell rating with a average target price of $12.50. The next major catalyst for the stock will be the July 28, 2013 PDUFA date. I am expecting the stock to start trending higher as we move closer to the PDUFA date based on the insider trading activity.
706 people decided to get ZGNX articles by email alert
Antwort auf Beitrag Nr.: 43.945.898 von fortuna924 am 19.12.12 19:52:56http://seekingalpha.com/article/1073231-3-healthcare-stocks-…
Antwort auf Beitrag Nr.: 43.945.903 von fortuna924 am 19.12.12 19:54:22 The stock dipped on December 10 after the negative vote for Zohydro ER. There have been four insider buy transactions and there have not been any insider sell transactions during the last 30 days. It is important to note that all the insider transactions were made after the negative vote for Zohydro. There are four analyst buy ratings, one neutral rating and 0 sell ratings with a average target price of $6.00. I have a long position in the stock currently.
The stock dipped on December 10 after the negative vote for Zohydro ER. There have been four insider buy transactions and there have not been any insider sell transactions during the last 30 days. It is important to note that all the insider transactions were made after the negative vote for Zohydro. There are four analyst buy ratings, one neutral rating and 0 sell ratings with a average target price of $6.00. I have a long position in the stock currently.
 The stock dipped on December 10 after the negative vote for Zohydro ER. There have been four insider buy transactions and there have not been any insider sell transactions during the last 30 days. It is important to note that all the insider transactions were made after the negative vote for Zohydro. There are four analyst buy ratings, one neutral rating and 0 sell ratings with a average target price of $6.00. I have a long position in the stock currently.
The stock dipped on December 10 after the negative vote for Zohydro ER. There have been four insider buy transactions and there have not been any insider sell transactions during the last 30 days. It is important to note that all the insider transactions were made after the negative vote for Zohydro. There are four analyst buy ratings, one neutral rating and 0 sell ratings with a average target price of $6.00. I have a long position in the stock currently.
Antwort auf Beitrag Nr.: 43.945.913 von fortuna924 am 19.12.12 19:57:45Insiderkäufe nachdem die schlechten Nachrichten rauskamen.
Rechne bis ende der Woche mit Kursen um die 2$
Rechne bis ende der Woche mit Kursen um die 2$

Antwort auf Beitrag Nr.: 43.945.925 von fortuna924 am 19.12.12 20:00:27http://www.benzinga.com/news/12/12/3184155/zogenix-shares-ra…
Bis 24.-25.01.2013 sollte es stetig weiter hochgehen.
Bis 24.-25.01.2013 sollte es stetig weiter hochgehen.
Antwort auf Beitrag Nr.: 43.945.939 von fortuna924 am 19.12.12 20:04:20http://seekingalpha.com/article/1065231-is-zogenix-a-buy-aft…
Is Zogenix A Buy After FDA Panel Says 'No' To Zohydro?
December 14, 2012 | 10 comments | about: ZGNX, includes: ABT, ACUR, ALKS, COV, DRRX, JNJ, MAPP, NKTR, PTIE, TEVA
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. (More...)
Zogenix Inc., (ZGNX) is the first pharmaceutical company to face a U.S. Food and Drug Administration (FDA) panel seeking approval for pure hydrocodone, a painkilling drug that can be 10 times stronger than Vicodin.
Hydrocodone was first synthesized in Germany in 1920 and approved by the FDA in 1943. Hydrocodone is a semi-synthetic opioid derived from either codeine or thebaine. Hydrocodone compounded with acetaminophen produced the popular drug, Vicodin. Due to concerns about liver damage from prolonged use of acetaminophen at high doses, four pharmaceutical companies are developing extended-release versions of pure hydrocodone.
On December 10, 2012, shares of Zogenix sank to an all-time low after the FDA's Anesthetic and Analgesic Drug Products Advisory Committee ((AADPAC)) voted 2 to 11 (with one abstention) against the approval of Zohydro, the company's hydrocodone-based chronic pain reliever.
Zohydro ER
Zohydro ER (hydrocodone bitartrate extended-release capsules) is an extended-release formulation of hydrocodone without acetaminophen, formulated for the management of moderate-to-severe chronic pain when a continuous opioid analgesic is needed for an extended period of time. If approved by the FDA, Zohydro would be the first extended-release (ER) novel formulation hydrocodone therapy without acetaminophen for the management of chronic pain.
Zogenix claims a chief advantage of Zohydro is that the drug avoids the potential for liver injury associated with the use of acetaminophen. Acetaminophen can cause liver damage when taken in high doses or over long periods of time.
An estimated 100 million people in the United States suffer from chronic pain. Chronic pain can be managed with both immediate-release and extended-release opioids. Currently marketed hydrocodone products are limited to immediate-release compounds that contain an analgesic combination ingredient, which is usually acetaminophen.
Acetaminophen, also known as paracetamol, may cause liver injury when used in high dosages, over long periods of time or in accidental overdoses due to multiple acetaminophen products being taken at once.
Zohydro uses Alkermes's (ALKS) Spheroidal Oral Drug Absorption System (SODAS) drug delivery technology "to enhance the release profile of hydrocodone to provide extended-release pain relief relative to existing immediate-release combination products." Alkermes earned a $1 million milestone payment from Zogenix when the company filed Zohydro's new drug application (NDA) in May 2012.
Liver Toxicity
Acetaminophen is the most widely used medication in the United States. Acetaminophen overdose is the most common drug overdose reported to poison control centers. Acetaminophen toxicity is the most common cause of liver toxicity in the United States, replacing viral hepatitis as the most common cause of acute hepatic failure.
Acetaminophen is the active ingredient in Johnson & Johnson's (JNJ) Tylenol, and is a component of many over-the-counter cold and analgesic medications and prescription combinations, including codeine-acetaminophen (Tylenol #3), oxycodone-acetaminophen (Percocet) and or Vicodin (acetaminophen and hydrocodone).
In 2005, University of Washington researchers found that 63% of all accidental acetaminophen overdoses used narcotic-containing compounds. Over 80% of patients in the study reported taking an acetaminophen or other analgesics for acute or chronic pain syndromes. Of the 178 acetaminophen overdose patients surveyed, 65% survived, 27% died without transplantation, and 8% underwent liver transplantation.
In 2011, the FDA acted by "asking" drug manufacturers to limit the strength of acetaminophen in prescription drug products to 325 mg per unit. In addition, all prescription drug products that contain acetaminophen must include a "Boxed Warning" highlighting the potential for severe liver injury and a warning emphasizing the potential for allergic reactions.
Over-the-counter products containing acetaminophen were already required to contain a label warning consumers about these drugs' potential for liver damage.
Although Zohydro would provide patients with an effective dose of hydrocodone without acetaminophen toxicity potential, the drug could also increase illicit opioid use which some authorities believe is a "public health crisis."
Abuse Concerns
Some experts believe that the nation's sharp increase in painkiller addiction stems from a loophole in the 1970 Controlled Substances Act that classified pure hydrocodone as a strictly controlled Schedule II drug. However, hydrocodone combination products, such as vicodin, which contains hydrocodone and acetaminophen, into the less strict Schedule III classification. As a Schedule III drug, combination drugs such as vicodin can be refilled as many as five times, while Schedule II drugs can be filled only once.
Since it is relatively easy to obtain, vicodin use and abuse has significantly increased. Some authorities estimate that nearly two million people in the United States are addicted to vicodin. Despite increasing attention on Vicodin's potential for abuse and addiction, physicians prescribed hydocodone combination drugs approximately 136 million times in 2008.
Drug abuse experts fear that the approval of Zohydro and other pure hydrocodone drugs in development could bring on a new "epidemic" similar to the widespread oxycontin abuse seen during the 1990s.
According to the U.S. Drug and Enforcement Agency (DEA), hydrocodone is the most prescribed opioid in the United States and is responsible for more drug abuse and diversion than any other licit or illicit opioid.
The U.S. Centers for Disease Control and Prevention (CDC) believes overdoses involving prescription painkillers, a class of drugs that includes hydrocodone are a "public health epidemic."
A 2008 study conducted by the International Narcotics Control Board found the United States consumes 99% of the hydrocodone and 83% of the oxycodone use in the world.
The FDA's Office of Surveillance and Epidemiology estimated that for hydrocodone combination products, the abuse ratio was 14 emergency department visits per one million tablets dispensed. For oxycodone combination products, the ratio was 24 emergency department visits per one million tablets dispensed, but for pure oxycodone extended-release products, the ratio increased to 85 emergency department visits per one million tablets dispensed. FDA staff concluded that it is likely that similar patterns will be observed between combination hydrocodone products and the extended-release, single-ingredient hydrocodone product, Zohydro.
FDA staff advised that Zohydro may have a higher risk of abuse than other painkillers. Zohydro contains up to 50 mg of pure hydrocodone. Vicodin has only 5 mg and is combined with acetaminophen. Zohydro is a twice-daily pain reliever while combination products are used every four to six hours.
Opioid drug abusers prefer extended-release (ER) drugs over immediate-release (IR) formulations because ER versions have higher drug concentrations that can be manipulated for illicit drug use. Zogenix is an extended release product.
During the hearing, AADPAC members were noticeably moved by the testimony from parents whose children died from prescription pain reliever abuse and overdose. They pleaded with the committee to vote against recommending FDA approval for Zohydro.
AADPAC members said they could not recommend FDA approval for Zohydro for a number of reasons. Members were concerned that the drug could be abused because there were insufficient plans to limit the number of potential Zohyro customers. They also questioned why Zohydro was not formulated as a tamper resistant drug. There were also questions about the potential effectiveness of Zohydro's Risk Evaluation and Mitigation Strategy (REMS).
Tamper Resistance Issues
Committee members were concerned that Zohydro was not tamper resistant and that illicit drug users could crush the pills in order to snort or inject them for a greater high.
Zogenix President and Chief Operating Officer Stephen Farr told the committee that the company was in the early stages of studying a tamper-resistant pill. Farr claimed Zogenix did not develop a tamper-resistant version of the drug because there were choking and reduced effectiveness issues.
Several other pharmaceutical companies are believed to be in the final stages of developing a tamper resistant version of hydrocodone.
Purdue Pharma, a private company that introduced OxyContin in 1995, received a patent applying extended-release technology to hydrocodone in May 2011 for a once-daily tamper resistant hydrocodone tablet.
Teva Pharmaceuticals (TEVA) is also anxious to get a tamper deterrent hydrocodone drug on the market. In May 2011, Teva purchased the biopharmaceutical company, Cephalon, for $6.8 billion. One of Cephalon's key assets was their late stage investigational "tamper deterrent" (TD) hydrocodone pill called "TD Hydrocodone." Teva believes sales from the drug could be worth as much as $500 million annually. Teva executives have stated the drug could be on the market in the "relatively near future," hoping that revenue from TD Hydrocodone can replace the sales lost when the company's patent on Copaxone, Teva's mega-selling multiple sclerosis drug, expires in 2014. TD Hydrocodone is in the "final stage of testing."
The Danish pharmaceutical firm, Egalet Ltd., is also developing a tamper resistant instant release hydrocodone product, EGP-121, and an extended release hydrocodone pill EGP-113. Egalet also has tamper resistant morphine, oxycodone, and hydromorphone pills in its late stage clinical pipeline. The company could have their pure hydrocone drug on the market as soon as 2015.
Acura Pharmaceuticals(ACUR) has patented Aversion Technology in an attempt to decrease the abuse of common oral opioid analgesics. Remoxy (oxycodone) is being developed in a strateguc alliance between Pfizer (PFE) and Pain Therapeutics (PTIE). Nektar Therapeutics (NKTR) also has tamper resistant opioids in development.
REMS
On July 9, 2012, the FDA approved a standard Risk Evaluation and Mitigation Strategy (REMS) for extended-release (ER) and long-acting (LA) opioids, The FDA's REMS is part of a federal initiative to address the prescription drug abuse, misuse, and overdose epidemic. The FDA's REMS introduces new safety measures designed to reduce risks and improve the safe use of ER/LA opioids, while ensuring access to needed medications for patients in pain.
The new ER/LA opioid REMS will affect more than 20 companies that manufacture opioid analgesics. Under the new REMS, companies will be required to make education programs available to prescribers based on an FDA Blueprint.
Key components of the ER/LA opioid analgesics REMS include:
Training for prescribers, based on an FDA Blueprint, will include information on weighing the risks and benefits of opioid therapy, choosing patients appropriately, managing and monitoring patients, and counseling patients on the safe use of these drugs. These educational programs will also include information on how to recognize opioid misuse, abuse, and addiction, as well as general and specific drug information for ER/LA opioid analgesics.
Updated Medication Guide and patient counseling documents must contain consumer-friendly information on the safe use, storage and disposal of ER/ LA opioid analgesics.
Assessment/auditing. Companies will be expected to achieve certain FDA-established goals for the percentage of prescribers of ER/ LA opioids who complete the training, as well as assess prescribers' understanding of important risk information over time. The assessments also cover whether the REMS is adversely affecting patient access to necessary pain medications, which manufacturers must report to FDA as part of periodic required assessments.
The FDA expects the first continuing education activities under the REMS will be offered to prescribers by March 1, 2013.
While there is no mandatory requirement that prescribers take the training and no precondition to prescribing ER/LA opioids to patients, the Obama Administration has endorsed a mandatory training program on responsible opioid prescribing practices in April 2011 as part of its comprehensive plan to address the epidemic of prescription drug abuse. The program, which would be linked to DEA registration by providers, would require legislative changes that are being pursued by the White House.
Zogenix claimed that they submitted a REMS that is consistent with current FDA and industry-wide guidelines for extended-release opioid products.
"We have clearly put a lot of time and effort into considering risk mitigation initiatives for the Zohydro ER product. The good news is that you know that there is now a standard REMS that was being recently introduced for all extended release opioid products and clearly our product will fit in line with that. We will become members of the REMS program companies and that we will be able to introduce education and other activities that's in the REMS for hydrocodone, I think for the first time," Zogenix President and COO Stephen Farr stated during the company's Third Quarter 2012 earnings call. "In addition to the REMS, we are looking at other activities working with experts in the field to really supplement the REMS with what we think will be meaningful activities around education as well as surveillance in order to really try and pick up any signals of misuse and abuse of the product early so that we can react to it. So we will be presenting a lot of that information at the advisory committee."
Zogenix promised that their Zohydro sales force would receive training to recognize signs of inappropriate prescribing and would not interact with physicians engaging in this behavior.
Financials
On November 8, 2012 Zogenix reported financial results for the third quarter ended September 30, 2012.
Net product revenues for the third quarter 2012 were $8.5 million, compared to $8.8 million in the third quarter 2011. During the third quarter 2011, total revenues of $10.4 million also included $1.6 million in contract revenue related to the company's previous co-promotion agreement with Astellas Pharma (ALPMF) which ended March 31, 2012.
Cost of sales for the third quarter 2012 was $4.2 million, compared to $5.5 million in the third quarter 2011. Product gross margin was 50% in the third quarter 2012, compared to 38% in the third quarter 2011. This improvement in gross product margin is primarily due to a decrease in excess capacity charges.
Royalty expense for the third quarter 2012 was $325,000, compared to $343,000 in the third quarter 2011, reflecting the impact of decreased net product revenue.
Research and development expenses for the third quarter 2012 were $3.7 million, representing a 64% decrease from $10.1 million in the third quarter 2011. The decrease in research and development expenses was the result of lower costs associated with the Phase 3 clinical trials for Zohydro ER, which were completed in 2011. The third quarter 2011 also included a one-time $2.25 million up-front license payment to Durect made in connection with the execution of the Relday development and license agreement and a $750,000 milestone payment to Alkermes made in connection with the completion of the Phase 3 study (801) for Zohydro ER.
Selling, general and administrative expenses for the third quarter 2012 were $10.9 million, representing a 26% decrease from $14.7 million in the third quarter 2011. The decrease in selling, general and administrative expenses was primarily the result of a $3.7 million decrease in service fees to Astellas due to the termination of the co-promotion agreement on March 31, 2012 and a decrease in advertising and promotional expenses. This was partially offset by an increase in product sampling costs and field sales force costs.
Other expenses for the third quarter 2012 were $8.6 million, which includes a $3.6 million non-cash mark to market adjustment in the fair value of the company's outstanding warrants. Also included are non-recurring costs associated with repayment of the company's debt obligations and issuance of warrants in its July 2012 public equity offering.
Net loss for the third quarter 2012 was $19.3 million, or $0.21 per share, compared to a net loss of $22.0 million, or $0.59 per share, for the third quarter 2011. The weighted average shares outstanding were 90,370,000 for the third quarter 2012. Non-GAAP net loss adjusted for certain non-cash or non-recurring items for the third quarter 2012 was $0.16 per share as detailed in the non-GAAP financial results table included in this release.
Cash and cash equivalents as of September 30, 2012, were $49.6 million. During the third quarter 2012, Zogenix raised net proceeds of $65.4 million in an equity offering and repaid $21.2 million of debt obligations to Oxford Finance LLC and Silicon Valley Bank.
Ann Rhoads, chief financial officer of Zogenix, commented, "We have reduced our loss from operations by nearly half as compared to the third quarter of 2011. Following the successful equity raise and debt repayment early in this quarter, we are advancing our development programs while continuing to invest in the growth of our commercial product, Sumavel DosePro, with the support of our new co-promotion partner."
In November 2012, Zogenix announced that the company had refined its full year 2012 financial guidance. The company now believes that total revenue to be approximately $45.5 million, at the low end of the previous guidance range. Net product revenue is expected to be approximately $37 million, at the low end of the previous guidance range due to the disruption in sales focus during the co-promotion transition and prescription growth expected to be approximately 20% over 2011. Product gross margin are expected to be approximately 48% - 49%, towards the high end of the previous guidance range. Research and development expenses are expected to be approximately $22 million, at the high end of the previous guidance range primarily due to expenses associated with preparing for the FDA Advisory Committee meeting for Zohydro ER. Selling, general and administrative expenses are expected to be approximately $50 million, at the high end of the previous guidance range. Zogenix expects the net interest expense to be approximately $10 million for 2012, unchanged from the previous guidance, reflecting the payoff of the debt obligation with Oxford Finance LLC and Silicon Valley Bank
Conclusion: Buy
As a result of the FDA action, Oppenheimer downgraded Zogenix to "Perform" from "Outperform" and removed its $3 price target. The firm predicted that Zogenix would not receive FDA approval on March 1, 2013, and approval could be delayed at least one year. Stifel Nicolaus downgraded the stock to "Buy" from "Hold," but Wells Fargo & Co. reaffirmed their "Outperform" rating on Zogenix. The firm predicts that FDA approval of Zohydro is a probable outcome and recommends buying shares of Zogenix on any weakness after the negative panel vote.
I think the odds of the FDA approving Zohydro in March 2013 are highly unlikely, but there is more to Zogenix than Zohydro. At current share prices under $1.20, I think Zogenix is undervalued as it is now selling at nearly one-third of its 52 week high share price of $3.30.
Zogenix's first commercial product, Sumavel DosePro (sumatriptan injection) Needle-free Delivery System, was launched in January 2010 for the acute treatment of migraine and cluster headache. Sumavel DosePro is the first and only needle-free delivery system for subcutaneous sumatriptan for the treatment of acute migraine and cluster headache.
In June 2012, Zogenix and Mallinckrodt LLC, the pharmaceuticals business of Covidien (COV), announced that the two companies had entered into a co-promotion agreement for Sumavel DosePro.
Under terms of the agreement, Mallinckrodt's 95 salesperson strong U.S. sales force will sell Sumavel DosePro to its customer base of prescribers. Zogenix will continue to record all product revenues and Mallinckrodt will be compensated based on apercentage of net sales from prescriptions generated by Mallinckrodt. The agreement runs through June 30, 2014, and can be extended. Covidien's Pharmaceuticals business is the largest U.S. supplier of opioid pain medications and among the top 10 generic pharmaceuticals manufacturers in the United States.
For the third quarter of 2012, Sumavel DosePro generated $8.5 million net product revenue, a 5% increase over the second quarter 2012, and maintained consistent refill rate for SUMAVEL DosePro at 43% from the second quarter 2012.
Decision Resources, a research and advisory firms for pharmaceutical and healthcare issues, finds that Zogenix/Astellas Pharma/Desitin Pharmaceuticals' needle-free Sumavel DosePro, which launched earlier this month in the United States, and MAP Pharmaceuticals' (MAPP) orally-inhaled Levadex will earn approximately $400 million in combined sales by 2018 in the migraine drug market.
Zogenix's second DosePro investigational product candidate, Relday, is a proprietary, long-acting injectable formulation of risperidone for the treatment of schizophrenia. In May 2012, Zogenix submitted an Investigational New Drug Application for Relday. A Phase 1 study completion is expected by end of 2012.
The company's validated, proprietary DosePro needle-free technology platform has a broad range of applications, and can be a solution for self administration of subcutaneous pharmaceuticals and biologics.
The AADPAC provides FDA with independent expert advice and recommendations. The FDA usually agrees with AADAC decisions, but the final decision regarding approval of a medication is made by FDA.
In June 2009, a different FDA advisory panel recommended that the agency ban Vicodin (hydrocodone and acetaminophen) and Percocet (oxycodone and acetaminophen). The FDA has yet to do so. The FDA has also refused to approve other opioids such as Pfizer's and Pain Therapeutics' Remoxy formulated with Durect's (DRRX) ORADUR technology, and Abbott Laboratories' (ABT) Vicodin CR.
Those favoring FDA approval for Zohydro argue that it is a safe and effective drug that works without the potential for liver damage. Those opposing the approval of the drug contend Zohydro is likely to produce the next wave of substance abuse. Do the benefits of allowing Zohydro on the market outweigh the risks? I think the FDA is likely to say "no."
This article was sent to 706 people who get email alerts on ZGNX.
Is Zogenix A Buy After FDA Panel Says 'No' To Zohydro?
December 14, 2012 | 10 comments | about: ZGNX, includes: ABT, ACUR, ALKS, COV, DRRX, JNJ, MAPP, NKTR, PTIE, TEVA
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. (More...)
Zogenix Inc., (ZGNX) is the first pharmaceutical company to face a U.S. Food and Drug Administration (FDA) panel seeking approval for pure hydrocodone, a painkilling drug that can be 10 times stronger than Vicodin.
Hydrocodone was first synthesized in Germany in 1920 and approved by the FDA in 1943. Hydrocodone is a semi-synthetic opioid derived from either codeine or thebaine. Hydrocodone compounded with acetaminophen produced the popular drug, Vicodin. Due to concerns about liver damage from prolonged use of acetaminophen at high doses, four pharmaceutical companies are developing extended-release versions of pure hydrocodone.
On December 10, 2012, shares of Zogenix sank to an all-time low after the FDA's Anesthetic and Analgesic Drug Products Advisory Committee ((AADPAC)) voted 2 to 11 (with one abstention) against the approval of Zohydro, the company's hydrocodone-based chronic pain reliever.
Zohydro ER
Zohydro ER (hydrocodone bitartrate extended-release capsules) is an extended-release formulation of hydrocodone without acetaminophen, formulated for the management of moderate-to-severe chronic pain when a continuous opioid analgesic is needed for an extended period of time. If approved by the FDA, Zohydro would be the first extended-release (ER) novel formulation hydrocodone therapy without acetaminophen for the management of chronic pain.
Zogenix claims a chief advantage of Zohydro is that the drug avoids the potential for liver injury associated with the use of acetaminophen. Acetaminophen can cause liver damage when taken in high doses or over long periods of time.
An estimated 100 million people in the United States suffer from chronic pain. Chronic pain can be managed with both immediate-release and extended-release opioids. Currently marketed hydrocodone products are limited to immediate-release compounds that contain an analgesic combination ingredient, which is usually acetaminophen.
Acetaminophen, also known as paracetamol, may cause liver injury when used in high dosages, over long periods of time or in accidental overdoses due to multiple acetaminophen products being taken at once.
Zohydro uses Alkermes's (ALKS) Spheroidal Oral Drug Absorption System (SODAS) drug delivery technology "to enhance the release profile of hydrocodone to provide extended-release pain relief relative to existing immediate-release combination products." Alkermes earned a $1 million milestone payment from Zogenix when the company filed Zohydro's new drug application (NDA) in May 2012.
Liver Toxicity
Acetaminophen is the most widely used medication in the United States. Acetaminophen overdose is the most common drug overdose reported to poison control centers. Acetaminophen toxicity is the most common cause of liver toxicity in the United States, replacing viral hepatitis as the most common cause of acute hepatic failure.
Acetaminophen is the active ingredient in Johnson & Johnson's (JNJ) Tylenol, and is a component of many over-the-counter cold and analgesic medications and prescription combinations, including codeine-acetaminophen (Tylenol #3), oxycodone-acetaminophen (Percocet) and or Vicodin (acetaminophen and hydrocodone).
In 2005, University of Washington researchers found that 63% of all accidental acetaminophen overdoses used narcotic-containing compounds. Over 80% of patients in the study reported taking an acetaminophen or other analgesics for acute or chronic pain syndromes. Of the 178 acetaminophen overdose patients surveyed, 65% survived, 27% died without transplantation, and 8% underwent liver transplantation.
In 2011, the FDA acted by "asking" drug manufacturers to limit the strength of acetaminophen in prescription drug products to 325 mg per unit. In addition, all prescription drug products that contain acetaminophen must include a "Boxed Warning" highlighting the potential for severe liver injury and a warning emphasizing the potential for allergic reactions.
Over-the-counter products containing acetaminophen were already required to contain a label warning consumers about these drugs' potential for liver damage.
Although Zohydro would provide patients with an effective dose of hydrocodone without acetaminophen toxicity potential, the drug could also increase illicit opioid use which some authorities believe is a "public health crisis."
Abuse Concerns
Some experts believe that the nation's sharp increase in painkiller addiction stems from a loophole in the 1970 Controlled Substances Act that classified pure hydrocodone as a strictly controlled Schedule II drug. However, hydrocodone combination products, such as vicodin, which contains hydrocodone and acetaminophen, into the less strict Schedule III classification. As a Schedule III drug, combination drugs such as vicodin can be refilled as many as five times, while Schedule II drugs can be filled only once.
Since it is relatively easy to obtain, vicodin use and abuse has significantly increased. Some authorities estimate that nearly two million people in the United States are addicted to vicodin. Despite increasing attention on Vicodin's potential for abuse and addiction, physicians prescribed hydocodone combination drugs approximately 136 million times in 2008.
Drug abuse experts fear that the approval of Zohydro and other pure hydrocodone drugs in development could bring on a new "epidemic" similar to the widespread oxycontin abuse seen during the 1990s.
According to the U.S. Drug and Enforcement Agency (DEA), hydrocodone is the most prescribed opioid in the United States and is responsible for more drug abuse and diversion than any other licit or illicit opioid.
The U.S. Centers for Disease Control and Prevention (CDC) believes overdoses involving prescription painkillers, a class of drugs that includes hydrocodone are a "public health epidemic."
A 2008 study conducted by the International Narcotics Control Board found the United States consumes 99% of the hydrocodone and 83% of the oxycodone use in the world.
The FDA's Office of Surveillance and Epidemiology estimated that for hydrocodone combination products, the abuse ratio was 14 emergency department visits per one million tablets dispensed. For oxycodone combination products, the ratio was 24 emergency department visits per one million tablets dispensed, but for pure oxycodone extended-release products, the ratio increased to 85 emergency department visits per one million tablets dispensed. FDA staff concluded that it is likely that similar patterns will be observed between combination hydrocodone products and the extended-release, single-ingredient hydrocodone product, Zohydro.
FDA staff advised that Zohydro may have a higher risk of abuse than other painkillers. Zohydro contains up to 50 mg of pure hydrocodone. Vicodin has only 5 mg and is combined with acetaminophen. Zohydro is a twice-daily pain reliever while combination products are used every four to six hours.
Opioid drug abusers prefer extended-release (ER) drugs over immediate-release (IR) formulations because ER versions have higher drug concentrations that can be manipulated for illicit drug use. Zogenix is an extended release product.
During the hearing, AADPAC members were noticeably moved by the testimony from parents whose children died from prescription pain reliever abuse and overdose. They pleaded with the committee to vote against recommending FDA approval for Zohydro.
AADPAC members said they could not recommend FDA approval for Zohydro for a number of reasons. Members were concerned that the drug could be abused because there were insufficient plans to limit the number of potential Zohyro customers. They also questioned why Zohydro was not formulated as a tamper resistant drug. There were also questions about the potential effectiveness of Zohydro's Risk Evaluation and Mitigation Strategy (REMS).
Tamper Resistance Issues
Committee members were concerned that Zohydro was not tamper resistant and that illicit drug users could crush the pills in order to snort or inject them for a greater high.
Zogenix President and Chief Operating Officer Stephen Farr told the committee that the company was in the early stages of studying a tamper-resistant pill. Farr claimed Zogenix did not develop a tamper-resistant version of the drug because there were choking and reduced effectiveness issues.
Several other pharmaceutical companies are believed to be in the final stages of developing a tamper resistant version of hydrocodone.
Purdue Pharma, a private company that introduced OxyContin in 1995, received a patent applying extended-release technology to hydrocodone in May 2011 for a once-daily tamper resistant hydrocodone tablet.
Teva Pharmaceuticals (TEVA) is also anxious to get a tamper deterrent hydrocodone drug on the market. In May 2011, Teva purchased the biopharmaceutical company, Cephalon, for $6.8 billion. One of Cephalon's key assets was their late stage investigational "tamper deterrent" (TD) hydrocodone pill called "TD Hydrocodone." Teva believes sales from the drug could be worth as much as $500 million annually. Teva executives have stated the drug could be on the market in the "relatively near future," hoping that revenue from TD Hydrocodone can replace the sales lost when the company's patent on Copaxone, Teva's mega-selling multiple sclerosis drug, expires in 2014. TD Hydrocodone is in the "final stage of testing."
The Danish pharmaceutical firm, Egalet Ltd., is also developing a tamper resistant instant release hydrocodone product, EGP-121, and an extended release hydrocodone pill EGP-113. Egalet also has tamper resistant morphine, oxycodone, and hydromorphone pills in its late stage clinical pipeline. The company could have their pure hydrocone drug on the market as soon as 2015.
Acura Pharmaceuticals(ACUR) has patented Aversion Technology in an attempt to decrease the abuse of common oral opioid analgesics. Remoxy (oxycodone) is being developed in a strateguc alliance between Pfizer (PFE) and Pain Therapeutics (PTIE). Nektar Therapeutics (NKTR) also has tamper resistant opioids in development.
REMS
On July 9, 2012, the FDA approved a standard Risk Evaluation and Mitigation Strategy (REMS) for extended-release (ER) and long-acting (LA) opioids, The FDA's REMS is part of a federal initiative to address the prescription drug abuse, misuse, and overdose epidemic. The FDA's REMS introduces new safety measures designed to reduce risks and improve the safe use of ER/LA opioids, while ensuring access to needed medications for patients in pain.
The new ER/LA opioid REMS will affect more than 20 companies that manufacture opioid analgesics. Under the new REMS, companies will be required to make education programs available to prescribers based on an FDA Blueprint.
Key components of the ER/LA opioid analgesics REMS include:
Training for prescribers, based on an FDA Blueprint, will include information on weighing the risks and benefits of opioid therapy, choosing patients appropriately, managing and monitoring patients, and counseling patients on the safe use of these drugs. These educational programs will also include information on how to recognize opioid misuse, abuse, and addiction, as well as general and specific drug information for ER/LA opioid analgesics.
Updated Medication Guide and patient counseling documents must contain consumer-friendly information on the safe use, storage and disposal of ER/ LA opioid analgesics.
Assessment/auditing. Companies will be expected to achieve certain FDA-established goals for the percentage of prescribers of ER/ LA opioids who complete the training, as well as assess prescribers' understanding of important risk information over time. The assessments also cover whether the REMS is adversely affecting patient access to necessary pain medications, which manufacturers must report to FDA as part of periodic required assessments.
The FDA expects the first continuing education activities under the REMS will be offered to prescribers by March 1, 2013.
While there is no mandatory requirement that prescribers take the training and no precondition to prescribing ER/LA opioids to patients, the Obama Administration has endorsed a mandatory training program on responsible opioid prescribing practices in April 2011 as part of its comprehensive plan to address the epidemic of prescription drug abuse. The program, which would be linked to DEA registration by providers, would require legislative changes that are being pursued by the White House.
Zogenix claimed that they submitted a REMS that is consistent with current FDA and industry-wide guidelines for extended-release opioid products.
"We have clearly put a lot of time and effort into considering risk mitigation initiatives for the Zohydro ER product. The good news is that you know that there is now a standard REMS that was being recently introduced for all extended release opioid products and clearly our product will fit in line with that. We will become members of the REMS program companies and that we will be able to introduce education and other activities that's in the REMS for hydrocodone, I think for the first time," Zogenix President and COO Stephen Farr stated during the company's Third Quarter 2012 earnings call. "In addition to the REMS, we are looking at other activities working with experts in the field to really supplement the REMS with what we think will be meaningful activities around education as well as surveillance in order to really try and pick up any signals of misuse and abuse of the product early so that we can react to it. So we will be presenting a lot of that information at the advisory committee."
Zogenix promised that their Zohydro sales force would receive training to recognize signs of inappropriate prescribing and would not interact with physicians engaging in this behavior.
Financials
On November 8, 2012 Zogenix reported financial results for the third quarter ended September 30, 2012.
Net product revenues for the third quarter 2012 were $8.5 million, compared to $8.8 million in the third quarter 2011. During the third quarter 2011, total revenues of $10.4 million also included $1.6 million in contract revenue related to the company's previous co-promotion agreement with Astellas Pharma (ALPMF) which ended March 31, 2012.
Cost of sales for the third quarter 2012 was $4.2 million, compared to $5.5 million in the third quarter 2011. Product gross margin was 50% in the third quarter 2012, compared to 38% in the third quarter 2011. This improvement in gross product margin is primarily due to a decrease in excess capacity charges.
Royalty expense for the third quarter 2012 was $325,000, compared to $343,000 in the third quarter 2011, reflecting the impact of decreased net product revenue.
Research and development expenses for the third quarter 2012 were $3.7 million, representing a 64% decrease from $10.1 million in the third quarter 2011. The decrease in research and development expenses was the result of lower costs associated with the Phase 3 clinical trials for Zohydro ER, which were completed in 2011. The third quarter 2011 also included a one-time $2.25 million up-front license payment to Durect made in connection with the execution of the Relday development and license agreement and a $750,000 milestone payment to Alkermes made in connection with the completion of the Phase 3 study (801) for Zohydro ER.
Selling, general and administrative expenses for the third quarter 2012 were $10.9 million, representing a 26% decrease from $14.7 million in the third quarter 2011. The decrease in selling, general and administrative expenses was primarily the result of a $3.7 million decrease in service fees to Astellas due to the termination of the co-promotion agreement on March 31, 2012 and a decrease in advertising and promotional expenses. This was partially offset by an increase in product sampling costs and field sales force costs.
Other expenses for the third quarter 2012 were $8.6 million, which includes a $3.6 million non-cash mark to market adjustment in the fair value of the company's outstanding warrants. Also included are non-recurring costs associated with repayment of the company's debt obligations and issuance of warrants in its July 2012 public equity offering.
Net loss for the third quarter 2012 was $19.3 million, or $0.21 per share, compared to a net loss of $22.0 million, or $0.59 per share, for the third quarter 2011. The weighted average shares outstanding were 90,370,000 for the third quarter 2012. Non-GAAP net loss adjusted for certain non-cash or non-recurring items for the third quarter 2012 was $0.16 per share as detailed in the non-GAAP financial results table included in this release.
Cash and cash equivalents as of September 30, 2012, were $49.6 million. During the third quarter 2012, Zogenix raised net proceeds of $65.4 million in an equity offering and repaid $21.2 million of debt obligations to Oxford Finance LLC and Silicon Valley Bank.
Ann Rhoads, chief financial officer of Zogenix, commented, "We have reduced our loss from operations by nearly half as compared to the third quarter of 2011. Following the successful equity raise and debt repayment early in this quarter, we are advancing our development programs while continuing to invest in the growth of our commercial product, Sumavel DosePro, with the support of our new co-promotion partner."
In November 2012, Zogenix announced that the company had refined its full year 2012 financial guidance. The company now believes that total revenue to be approximately $45.5 million, at the low end of the previous guidance range. Net product revenue is expected to be approximately $37 million, at the low end of the previous guidance range due to the disruption in sales focus during the co-promotion transition and prescription growth expected to be approximately 20% over 2011. Product gross margin are expected to be approximately 48% - 49%, towards the high end of the previous guidance range. Research and development expenses are expected to be approximately $22 million, at the high end of the previous guidance range primarily due to expenses associated with preparing for the FDA Advisory Committee meeting for Zohydro ER. Selling, general and administrative expenses are expected to be approximately $50 million, at the high end of the previous guidance range. Zogenix expects the net interest expense to be approximately $10 million for 2012, unchanged from the previous guidance, reflecting the payoff of the debt obligation with Oxford Finance LLC and Silicon Valley Bank
Conclusion: Buy
As a result of the FDA action, Oppenheimer downgraded Zogenix to "Perform" from "Outperform" and removed its $3 price target. The firm predicted that Zogenix would not receive FDA approval on March 1, 2013, and approval could be delayed at least one year. Stifel Nicolaus downgraded the stock to "Buy" from "Hold," but Wells Fargo & Co. reaffirmed their "Outperform" rating on Zogenix. The firm predicts that FDA approval of Zohydro is a probable outcome and recommends buying shares of Zogenix on any weakness after the negative panel vote.
I think the odds of the FDA approving Zohydro in March 2013 are highly unlikely, but there is more to Zogenix than Zohydro. At current share prices under $1.20, I think Zogenix is undervalued as it is now selling at nearly one-third of its 52 week high share price of $3.30.
Zogenix's first commercial product, Sumavel DosePro (sumatriptan injection) Needle-free Delivery System, was launched in January 2010 for the acute treatment of migraine and cluster headache. Sumavel DosePro is the first and only needle-free delivery system for subcutaneous sumatriptan for the treatment of acute migraine and cluster headache.
In June 2012, Zogenix and Mallinckrodt LLC, the pharmaceuticals business of Covidien (COV), announced that the two companies had entered into a co-promotion agreement for Sumavel DosePro.
Under terms of the agreement, Mallinckrodt's 95 salesperson strong U.S. sales force will sell Sumavel DosePro to its customer base of prescribers. Zogenix will continue to record all product revenues and Mallinckrodt will be compensated based on apercentage of net sales from prescriptions generated by Mallinckrodt. The agreement runs through June 30, 2014, and can be extended. Covidien's Pharmaceuticals business is the largest U.S. supplier of opioid pain medications and among the top 10 generic pharmaceuticals manufacturers in the United States.
For the third quarter of 2012, Sumavel DosePro generated $8.5 million net product revenue, a 5% increase over the second quarter 2012, and maintained consistent refill rate for SUMAVEL DosePro at 43% from the second quarter 2012.
Decision Resources, a research and advisory firms for pharmaceutical and healthcare issues, finds that Zogenix/Astellas Pharma/Desitin Pharmaceuticals' needle-free Sumavel DosePro, which launched earlier this month in the United States, and MAP Pharmaceuticals' (MAPP) orally-inhaled Levadex will earn approximately $400 million in combined sales by 2018 in the migraine drug market.
Zogenix's second DosePro investigational product candidate, Relday, is a proprietary, long-acting injectable formulation of risperidone for the treatment of schizophrenia. In May 2012, Zogenix submitted an Investigational New Drug Application for Relday. A Phase 1 study completion is expected by end of 2012.
The company's validated, proprietary DosePro needle-free technology platform has a broad range of applications, and can be a solution for self administration of subcutaneous pharmaceuticals and biologics.
The AADPAC provides FDA with independent expert advice and recommendations. The FDA usually agrees with AADAC decisions, but the final decision regarding approval of a medication is made by FDA.
In June 2009, a different FDA advisory panel recommended that the agency ban Vicodin (hydrocodone and acetaminophen) and Percocet (oxycodone and acetaminophen). The FDA has yet to do so. The FDA has also refused to approve other opioids such as Pfizer's and Pain Therapeutics' Remoxy formulated with Durect's (DRRX) ORADUR technology, and Abbott Laboratories' (ABT) Vicodin CR.
Those favoring FDA approval for Zohydro argue that it is a safe and effective drug that works without the potential for liver damage. Those opposing the approval of the drug contend Zohydro is likely to produce the next wave of substance abuse. Do the benefits of allowing Zohydro on the market outweigh the risks? I think the FDA is likely to say "no."
This article was sent to 706 people who get email alerts on ZGNX.
Antwort auf Beitrag Nr.: 43.945.925 von fortuna924 am 19.12.12 20:00:27http://seekingalpha.com/article/1064931-fda-advisory-meeting…
Antwort auf Beitrag Nr.: 43.945.954 von fortuna924 am 19.12.12 20:08:18Warum sollte man Aktien kaufen, wenn man nicht von seiner eigenen Firma überzeugt ist?!
Ist noch irgendwer investiert?
Ist noch irgendwer investiert?
Antwort auf Beitrag Nr.: 43.945.961 von fortuna924 am 19.12.12 20:10:05http://finance.yahoo.com/mb/ZGNX/
auch zu empfehlen. Im Yahoo message board übertreiben sie schon
auch zu empfehlen. Im Yahoo message board übertreiben sie schon

Antwort auf Beitrag Nr.: 43.945.966 von fortuna924 am 19.12.12 20:12:17Insider buys
Henri Termeer purchased 44,501 shares on December 12-13 and currently holds 44,501 shares or 0.1% of the company. Henri Termeer has served as a director of AVEO since April 2011 and is Chairman of the Board.
Robert Young purchased 3,621 shares on December 3-13 and currently holds 8,010 shares or less than 0.1% of the company. Robert Young has served as a director of the company since July 2009.
Tuan Ha-Ngoc purchased 75,000 shares on December 12-13 and currently holds 314,640 shares or 0.7% of the company. Tuan Ha-Ngoc has served as president and chief executive officer of AVEO and as a member of the board of directors since June 2002.
Henri Termeer purchased 44,501 shares on December 12-13 and currently holds 44,501 shares or 0.1% of the company. Henri Termeer has served as a director of AVEO since April 2011 and is Chairman of the Board.
Robert Young purchased 3,621 shares on December 3-13 and currently holds 8,010 shares or less than 0.1% of the company. Robert Young has served as a director of the company since July 2009.
Tuan Ha-Ngoc purchased 75,000 shares on December 12-13 and currently holds 314,640 shares or 0.7% of the company. Tuan Ha-Ngoc has served as president and chief executive officer of AVEO and as a member of the board of directors since June 2002.

Antwort auf Beitrag Nr.: 43.946.028 von me_2 am 19.12.12 20:33:49 ... war ich auch ...
Antwort auf Beitrag Nr.: 43.946.788 von fortuna924 am 20.12.12 06:48:19http://seekingalpha.com/article/1073531-3-biotech-stocks-to-…

Antwort auf Beitrag Nr.: 43.946.788 von fortuna924 am 20.12.12 06:48:19
3 Biotech Stocks To Consider In The Wake Of Recent Developments
December 19, 2012 | 2 comments | includes: IDRA, OREX, ZGNX
Disclosure: I am long OREX, ZGNX. (More...)
When it comes to the biotech sector, many stocks that trade around $5/share are considered to be very speculative. As a result, I wanted to focus on companies that could benefit from developments within their specific markets. In this article I've chosen three companies that have met the follow criteria:
Each company must trade below $10/share
Each company must have a Market Cap under $450 million
Each company must have announced significant developments in the last 24 hours
Idera Pharmaceuticals (IDRA) which is based in Cambridge, Massachusetts operates as "a clinical stage biotechnology company that engages in the discovery and development of novel synthetic DNA- and RNA- based drug candidates. It is developing drug candidates designed to modulate immune responses mediated through Toll-like Receptors (TLRs); and evaluating gene silencing oligonucleotides (GSOs), which inhibit the production of disease-associated proteins by targeting RNA". (Yahoo! Finance)
From a fundamental perspective, shares of IDRA currently carry a market cap of $27 million, have traded down 17.17% since July 1st and are currently trading at an 18.98% premium to their 50 DMA and in-line with their 200 DMA.
IDRA Chart
(Click to enlarge)
IDRA data by YCharts
On Wednesday December 19th it was announced that its lead drug candidate IMO-3100, a treatment for moderate-to-severe plaque psoriasis, was more effective than a placebo in a Phase IIa clinical trial of two dose levels. The company thinks the trial data for the injectable treatment provides clinical proof-of-concept for the mechanism that suppresses certain factors that underlie the skin disorder. If Idera can continue to demonstrate such significant developments and meet certain endpoint requirements there's no reason why shares shouldn't be trading at much higher levels.
Zogenix Pharmaceuticals (ZGNX) which is based in San Diego, California operates as "a pharmaceutical company, engages in the development and commercialization of products for the treatment of central nervous system disorders and pain. Its commercial product includes Sumavel DosePro (sumatriptan injection), a delivery system that offers needle-free subcutaneous administration of sumatriptan for the acute treatment of migraine and cluster headache". (Yahoo! Finance)
From a fundamental perspective, shares of ZGNX currently carry a market cap of $135 million, have traded down 47.06% since July 1st and are currently trading at a 40.35% discount to their 50 DMA and at a 43.04% discount to their 200 DMA.
ZGNX Chart
(Click to enlarge)
ZGNX data by YCharts
On Tuesday December 18th it was announced that the FDA has scheduled two days of hearings February 7-8 after receiving "comments, petitions, and informal inquiries concerning the extent to which opioid drugs should be used in the treatment of pain." Hydrocodone is a semi-synthetic opioid derived from either codeine or thebaine. Such a forum will allow Zogenix to thoroughly explain how and why such drugs are needed especially in the concentration of central nervous system disorders. Although a non-FDA panel shot down ZGNX's Zohydro, which is a hydrocodone pill for chronic pain, this may give them a better chance to explain the positive catalysts associated with the drug.
Orexigen Therapeutics (OREX) which is based in La Jolla, California operates as "a biopharmaceutical company, focuses on the development of pharmaceutical product candidates for the treatment of obesity. Its lead combination product candidates include Contrave, which has completed Phase III clinical trials; and Empatic that has completed phase II clinical trials for the treatment of obesity". (Yahoo! Finance)
Shares of OREX currently carry a market cap of $435 million, have traded up just 0.85% since July 1st and are currently trading at a 10.45% premium to their 50 DMA and at a 5.79% premium to their 200 DMA.
OREX Chart
(Click to enlarge)
OREX data by YCharts
On December 18th it was announced that the company has completed the screening of new patients for its Light Study trial to evaluate its obesity drug Contrave. There are about 9,000 patients who will be randomized in the study within the course of the next few weeks. OREX also anticipates that it will be resubmitting the New Drug Application for its obesity drug Contrave as well as conducting the interim analysis during the first half of 2013, with the drug's potential approval coming as early as fourth quarter 2013. If such approval by the FDA occurs share could trade much higher than their current price of $5.36/share.
Final Analysis
Are there any negative catalysts potential investors should consider before establishing a position in either of the three companies? As is the case with any biotech company, potential investors need to keep in mind some of the negative catalysts that go hand-in-hand with Idera Pharmaceuticals, Zogenix Pharmaceuticals and Orexigen Therapeutics. On one hand, any negative indication by the FDA with regard to Idera's IMO-3100, Zogenix's Zohydro or Orixigen'ss Contrave could result in the sell-off of either stock. On the other hand, weaker than expected earnings at any point over the course of the next 12-18 months could also send shares of these companies down an unfavorable path.
For potential investors looking to establish a position in Idera, Zogenix or Orexigen, I'd take a closer look at each company and keep in mind the primary positive and negative catalysts moving forward. Given the fact that both companies are making considerable strides, I'd look to establish a small to medium position at current levels and add to that position once future developments are announced.
3 Biotech Stocks To Consider In The Wake Of Recent Developments
December 19, 2012 | 2 comments | includes: IDRA, OREX, ZGNX
Disclosure: I am long OREX, ZGNX. (More...)
When it comes to the biotech sector, many stocks that trade around $5/share are considered to be very speculative. As a result, I wanted to focus on companies that could benefit from developments within their specific markets. In this article I've chosen three companies that have met the follow criteria:
Each company must trade below $10/share
Each company must have a Market Cap under $450 million
Each company must have announced significant developments in the last 24 hours
Idera Pharmaceuticals (IDRA) which is based in Cambridge, Massachusetts operates as "a clinical stage biotechnology company that engages in the discovery and development of novel synthetic DNA- and RNA- based drug candidates. It is developing drug candidates designed to modulate immune responses mediated through Toll-like Receptors (TLRs); and evaluating gene silencing oligonucleotides (GSOs), which inhibit the production of disease-associated proteins by targeting RNA". (Yahoo! Finance)
From a fundamental perspective, shares of IDRA currently carry a market cap of $27 million, have traded down 17.17% since July 1st and are currently trading at an 18.98% premium to their 50 DMA and in-line with their 200 DMA.
IDRA Chart
(Click to enlarge)
IDRA data by YCharts
On Wednesday December 19th it was announced that its lead drug candidate IMO-3100, a treatment for moderate-to-severe plaque psoriasis, was more effective than a placebo in a Phase IIa clinical trial of two dose levels. The company thinks the trial data for the injectable treatment provides clinical proof-of-concept for the mechanism that suppresses certain factors that underlie the skin disorder. If Idera can continue to demonstrate such significant developments and meet certain endpoint requirements there's no reason why shares shouldn't be trading at much higher levels.
Zogenix Pharmaceuticals (ZGNX) which is based in San Diego, California operates as "a pharmaceutical company, engages in the development and commercialization of products for the treatment of central nervous system disorders and pain. Its commercial product includes Sumavel DosePro (sumatriptan injection), a delivery system that offers needle-free subcutaneous administration of sumatriptan for the acute treatment of migraine and cluster headache". (Yahoo! Finance)
From a fundamental perspective, shares of ZGNX currently carry a market cap of $135 million, have traded down 47.06% since July 1st and are currently trading at a 40.35% discount to their 50 DMA and at a 43.04% discount to their 200 DMA.
ZGNX Chart
(Click to enlarge)
ZGNX data by YCharts
On Tuesday December 18th it was announced that the FDA has scheduled two days of hearings February 7-8 after receiving "comments, petitions, and informal inquiries concerning the extent to which opioid drugs should be used in the treatment of pain." Hydrocodone is a semi-synthetic opioid derived from either codeine or thebaine. Such a forum will allow Zogenix to thoroughly explain how and why such drugs are needed especially in the concentration of central nervous system disorders. Although a non-FDA panel shot down ZGNX's Zohydro, which is a hydrocodone pill for chronic pain, this may give them a better chance to explain the positive catalysts associated with the drug.
Orexigen Therapeutics (OREX) which is based in La Jolla, California operates as "a biopharmaceutical company, focuses on the development of pharmaceutical product candidates for the treatment of obesity. Its lead combination product candidates include Contrave, which has completed Phase III clinical trials; and Empatic that has completed phase II clinical trials for the treatment of obesity". (Yahoo! Finance)
Shares of OREX currently carry a market cap of $435 million, have traded up just 0.85% since July 1st and are currently trading at a 10.45% premium to their 50 DMA and at a 5.79% premium to their 200 DMA.
OREX Chart
(Click to enlarge)
OREX data by YCharts
On December 18th it was announced that the company has completed the screening of new patients for its Light Study trial to evaluate its obesity drug Contrave. There are about 9,000 patients who will be randomized in the study within the course of the next few weeks. OREX also anticipates that it will be resubmitting the New Drug Application for its obesity drug Contrave as well as conducting the interim analysis during the first half of 2013, with the drug's potential approval coming as early as fourth quarter 2013. If such approval by the FDA occurs share could trade much higher than their current price of $5.36/share.
Final Analysis
Are there any negative catalysts potential investors should consider before establishing a position in either of the three companies? As is the case with any biotech company, potential investors need to keep in mind some of the negative catalysts that go hand-in-hand with Idera Pharmaceuticals, Zogenix Pharmaceuticals and Orexigen Therapeutics. On one hand, any negative indication by the FDA with regard to Idera's IMO-3100, Zogenix's Zohydro or Orixigen'ss Contrave could result in the sell-off of either stock. On the other hand, weaker than expected earnings at any point over the course of the next 12-18 months could also send shares of these companies down an unfavorable path.
For potential investors looking to establish a position in Idera, Zogenix or Orexigen, I'd take a closer look at each company and keep in mind the primary positive and negative catalysts moving forward. Given the fact that both companies are making considerable strides, I'd look to establish a small to medium position at current levels and add to that position once future developments are announced.
fortuna,
worauf spekulierst du hier?
Zulassung entgegen dem Votum des FDA advisory committee (nicht eines "non-FDA" panel wie im Artikel oben suggeriert)? wohl recht unwahrscheinlich.
Neue Aspekt oder Argumente beim Hearing im Feb/2012?
technische Reaktion?
Wert der (quasi nicht vorhandenen) Rest-Pipeline? Sumavel-umsätze sind ja auch nicht gerade hoch (welche Bewertung wäre ohne Zohydro gerechtfertigt?).
Gruß
me_2
worauf spekulierst du hier?
Zulassung entgegen dem Votum des FDA advisory committee (nicht eines "non-FDA" panel wie im Artikel oben suggeriert)? wohl recht unwahrscheinlich.
Neue Aspekt oder Argumente beim Hearing im Feb/2012?
technische Reaktion?
Wert der (quasi nicht vorhandenen) Rest-Pipeline? Sumavel-umsätze sind ja auch nicht gerade hoch (welche Bewertung wäre ohne Zohydro gerechtfertigt?).
Gruß
me_2
"Hearing im Feb/2013" natürlich
Antwort auf Beitrag Nr.: 43.947.284 von me_2 am 20.12.12 10:26:00ich spekuliere hier nur auf die Insiderkäufe und auf das Hearing im Februar. Vielleicht kommt es ja doch zur Zulassung.
Reines Roulette. Bin mit ner kleinen spekulativen Position dabei.
Antwort auf Beitrag Nr.: 43.947.301 von fortuna924 am 20.12.12 10:28:36FDA taking on pain drugs' knotty issues
Meeting to consider if pain drugs should come with dose limits
December 19, 2012 | By Eric Palmer
Share
Email
Tools
Comment
Print
Contact Author
Reprint
When an FDA expert panel recommended against a stronger form of hydrocodone from Zogenix ($ZGNX) this month, it said it was best if the FDA would first consider requiring ways to discourage abuse of opioids. The agency intends to do just that.
The FDA will hold a two-day meeting in February to take a look at "scientific evidence, such as study data or peer-reviewed analyses, on issues pertaining to the use of opioid drugs in the treatment of chronic pain." As Regulatory Focus points out, that will encompass questions such as whether pain drugs should be prescribed for limited durations, whether those durations should be different for various kinds of pain, say back pain versus cancer pain, and whether there should be a maximum daily dose.
At $9 billion a year, the prescription pain drug market can be quite lucrative, but the use, and abuse, of pain meds means the category is fraught with strong opinions. Purdue Pharma reworked its OxyContin formula to make the powerful opioid pills more difficult to crush or dissolve by addicts. Endo Pharmaceuticals ($ENDP) did something similar with its Opana painkiller. Congress has been considering whether to give the FDA authority to yank regulatory approval for generic drugs that aren't redesigned with such safety features. The patents are set to expire on both of those drugs next year, and the companies see the new formulations as a potential patent protector. Endo recently sued the FDA to have it classify its original formulation unsafe and require generics makers to go the tamper-resistant route, a move that would delay competition.
FierceBiotech Executive Breakfast: Big Data Biopharma Forum
Date: January 9, 2013
Location: San Francisco, CA
Join FierceBiotech Executive Editor, Ryan McBride, and a panel of industry experts from AstraZeneca, New Enterprise Associates (NEA) and Third Rock Ventures as they discuss the impact of the much-hyped Big Data phenomenon on their biopharma operations and investment plans. Register Today!
Sign up for our FREE newsletter for more news like this sent to your inbox!
Meanwhile, Zogenix is hoping the agency will still approve its Zohydro, a drug that contains 50 mg of hydrocodone, 10 times the dose of Vicodin.
Read more: FDA taking on pain drugs' knotty issues - FiercePharma http://www.fiercepharma.com/story/fda-taking-pain-drugs-knot…
Subscribe: http://www.fiercepharma.com/signup?sourceform=Viral-Tynt-Fie…
Meeting to consider if pain drugs should come with dose limits
December 19, 2012 | By Eric Palmer
Share
Tools
Comment
Contact Author
Reprint
When an FDA expert panel recommended against a stronger form of hydrocodone from Zogenix ($ZGNX) this month, it said it was best if the FDA would first consider requiring ways to discourage abuse of opioids. The agency intends to do just that.
The FDA will hold a two-day meeting in February to take a look at "scientific evidence, such as study data or peer-reviewed analyses, on issues pertaining to the use of opioid drugs in the treatment of chronic pain." As Regulatory Focus points out, that will encompass questions such as whether pain drugs should be prescribed for limited durations, whether those durations should be different for various kinds of pain, say back pain versus cancer pain, and whether there should be a maximum daily dose.
At $9 billion a year, the prescription pain drug market can be quite lucrative, but the use, and abuse, of pain meds means the category is fraught with strong opinions. Purdue Pharma reworked its OxyContin formula to make the powerful opioid pills more difficult to crush or dissolve by addicts. Endo Pharmaceuticals ($ENDP) did something similar with its Opana painkiller. Congress has been considering whether to give the FDA authority to yank regulatory approval for generic drugs that aren't redesigned with such safety features. The patents are set to expire on both of those drugs next year, and the companies see the new formulations as a potential patent protector. Endo recently sued the FDA to have it classify its original formulation unsafe and require generics makers to go the tamper-resistant route, a move that would delay competition.
FierceBiotech Executive Breakfast: Big Data Biopharma Forum
Date: January 9, 2013
Location: San Francisco, CA
Join FierceBiotech Executive Editor, Ryan McBride, and a panel of industry experts from AstraZeneca, New Enterprise Associates (NEA) and Third Rock Ventures as they discuss the impact of the much-hyped Big Data phenomenon on their biopharma operations and investment plans. Register Today!
Sign up for our FREE newsletter for more news like this sent to your inbox!
Meanwhile, Zogenix is hoping the agency will still approve its Zohydro, a drug that contains 50 mg of hydrocodone, 10 times the dose of Vicodin.

Read more: FDA taking on pain drugs' knotty issues - FiercePharma http://www.fiercepharma.com/story/fda-taking-pain-drugs-knot…
Subscribe: http://www.fiercepharma.com/signup?sourceform=Viral-Tynt-Fie…
Antwort auf Beitrag Nr.: 43.947.770 von fortuna924 am 20.12.12 12:10:37http://www.fiercepharma.com/story/fda-taking-pain-drugs-knot…
link dazu ...
link dazu ...
Antwort auf Beitrag Nr.: 43.947.772 von fortuna924 am 20.12.12 12:11:32http://meegle.blogspot.de/2012/12/2013-fda-drug-approval-dec…
noch alles möglich
Thursday, December 20, 2012
2013 FDA Drug Approval Decision Calendar
Here's an updated list of biotech and pharmaceutical companies with FDA drug approval decisions expected in 2013.
Biotech and drug stocks below are listed in chronological order based on the closest regulatory catalyst -- FDA drug approval decisions and advisory panels.
Johnson & Johnson (JNJ)Drug/indication: Canagliflozin for diabetes.FDA advisory panel: Jan. 10Approval decision date: March 29
See if (PATH) is in our portfolio
Theravance (THRX)Drug/indication: Vibativ for hospital acquired pneumoniaApproval decision date: Jan. 11Santarus (SNTS)Drug/indication: Uceris for ulcerative colitisApproval decision date: Jan. 16NuPathe (PATH)Drug/indication: Zelrix for migraineApproval decision date: Jan. 17, 2013Impax Labs (IPXL)Drug/indication: IPX066 for Parkinson's diseaseApproval decision date: Jan. 21Hyperion Therapeutics (HPTX)Drug/indication: Ravicti for urea cycle disorderApproval decision date: Jan. 23Sanofi (SNY) and Isis Pharmaceuticals (ISIS)Drug/indication: Kynamro for dyslipidemia/hypercholesterolemiaApproval decision date: Jan. 29, 2013Raptor Pharmaceutical (RPTP)Drug/indication: RP103 for cystinosisApproval decision date: Jan. 30, 2013Hemispherx Biopharma (HEB)Drug/indication: Ampligen for chronic fatigue syndromeApproval decision date: Feb. 1, 2013Celgene (CELG)Drug/indication: Pomalidomide for multiple myelomaApproval decision date: Feb. 8, 2013Dynavax (DVAX)Drug/indication: Heplisav for hepatitis B preventionApproval decision date: Feb. 22, 2013Roche (RHHBY) and Immunogen (IMGN)Drug/indication: T-DM1 for breast cancerApproval decision date Feb. 26Zogenix (ZGNX)Drug/indication: Zohydro for chronic painApproval decision date: March 1, 2013Depomed (DEPO)Drug/indication: Serada for menopauseFDA advisory panel: March 4Approval decision date: May 31Bristol-Myers Squibb (BMY)Drug/indication: Eliquis for blood clot preventionApproval decision date: March 15A.P. Pharma (APPA)Drug/indication: APF530 for chemotherapy induced nauseaApproval decision date: March 27Biogen Idec (BIIB)Drug/indication: BG-12 for multiple sclerosisApproval decision date: March 28MAP Pharma (MAPP)Drug/indication: Levadex for migraineApproval decision date: April 15Sucampo Pharmaceuticals (SCMP)Drug/indication: Amitzia for opioid-induced constipationApproval decision date: April 26Navidea Biopharmaceuticals (NAVB)Drug/indication: Lymphoseek, a radioactive tracing agent for lymph node mappingApproval decision date: April 30GlaxoSmithKline (GSK)Drug/indication: Dabrefenib for melanomaFDA advisory panel: June 3Delcath Systems (DCTH)Drug/indication: ChemoSat for liver metastases due to ocular melanomaApproval decision date: June 14AVEO Pharmaceuticals (AVEO)Drug/indication: Tivozanib for kidney cancerApproval decision date: July 28Companies with drugs filed to FDA but no assigned approval decision dates:Auxillium Pharmaceuticals (AUXL): Xiaflex for Peyronie's diseaseBayer and Algeta: Alpharadin for prostate cancerAntares Pharma (ATRS): Otrexup for rheumatoid arthritisGlaxoSmithKline: Dolutegravir for HIVSources: Company reports, TheStreet research, BioMedTracker>To follow the writer on Twitter, go to http://twitter.com/adamfeuerstein. Follow TheStreet on Twitter and become a fan on Facebook.
>To order reprints of this article, click here: Reprints FREE for a limited time only: Get TheStreet Ratings #1 Stock Report NOW!
noch alles möglich

Thursday, December 20, 2012
2013 FDA Drug Approval Decision Calendar
Here's an updated list of biotech and pharmaceutical companies with FDA drug approval decisions expected in 2013.
Biotech and drug stocks below are listed in chronological order based on the closest regulatory catalyst -- FDA drug approval decisions and advisory panels.
Johnson & Johnson (JNJ)Drug/indication: Canagliflozin for diabetes.FDA advisory panel: Jan. 10Approval decision date: March 29
See if (PATH) is in our portfolio
Theravance (THRX)Drug/indication: Vibativ for hospital acquired pneumoniaApproval decision date: Jan. 11Santarus (SNTS)Drug/indication: Uceris for ulcerative colitisApproval decision date: Jan. 16NuPathe (PATH)Drug/indication: Zelrix for migraineApproval decision date: Jan. 17, 2013Impax Labs (IPXL)Drug/indication: IPX066 for Parkinson's diseaseApproval decision date: Jan. 21Hyperion Therapeutics (HPTX)Drug/indication: Ravicti for urea cycle disorderApproval decision date: Jan. 23Sanofi (SNY) and Isis Pharmaceuticals (ISIS)Drug/indication: Kynamro for dyslipidemia/hypercholesterolemiaApproval decision date: Jan. 29, 2013Raptor Pharmaceutical (RPTP)Drug/indication: RP103 for cystinosisApproval decision date: Jan. 30, 2013Hemispherx Biopharma (HEB)Drug/indication: Ampligen for chronic fatigue syndromeApproval decision date: Feb. 1, 2013Celgene (CELG)Drug/indication: Pomalidomide for multiple myelomaApproval decision date: Feb. 8, 2013Dynavax (DVAX)Drug/indication: Heplisav for hepatitis B preventionApproval decision date: Feb. 22, 2013Roche (RHHBY) and Immunogen (IMGN)Drug/indication: T-DM1 for breast cancerApproval decision date Feb. 26Zogenix (ZGNX)Drug/indication: Zohydro for chronic painApproval decision date: March 1, 2013Depomed (DEPO)Drug/indication: Serada for menopauseFDA advisory panel: March 4Approval decision date: May 31Bristol-Myers Squibb (BMY)Drug/indication: Eliquis for blood clot preventionApproval decision date: March 15A.P. Pharma (APPA)Drug/indication: APF530 for chemotherapy induced nauseaApproval decision date: March 27Biogen Idec (BIIB)Drug/indication: BG-12 for multiple sclerosisApproval decision date: March 28MAP Pharma (MAPP)Drug/indication: Levadex for migraineApproval decision date: April 15Sucampo Pharmaceuticals (SCMP)Drug/indication: Amitzia for opioid-induced constipationApproval decision date: April 26Navidea Biopharmaceuticals (NAVB)Drug/indication: Lymphoseek, a radioactive tracing agent for lymph node mappingApproval decision date: April 30GlaxoSmithKline (GSK)Drug/indication: Dabrefenib for melanomaFDA advisory panel: June 3Delcath Systems (DCTH)Drug/indication: ChemoSat for liver metastases due to ocular melanomaApproval decision date: June 14AVEO Pharmaceuticals (AVEO)Drug/indication: Tivozanib for kidney cancerApproval decision date: July 28Companies with drugs filed to FDA but no assigned approval decision dates:Auxillium Pharmaceuticals (AUXL): Xiaflex for Peyronie's diseaseBayer and Algeta: Alpharadin for prostate cancerAntares Pharma (ATRS): Otrexup for rheumatoid arthritisGlaxoSmithKline: Dolutegravir for HIVSources: Company reports, TheStreet research, BioMedTracker>To follow the writer on Twitter, go to http://twitter.com/adamfeuerstein. Follow TheStreet on Twitter and become a fan on Facebook.
>To order reprints of this article, click here: Reprints FREE for a limited time only: Get TheStreet Ratings #1 Stock Report NOW!
Könnte es sehr schöner Zock wieder werden
DURECT Corp announced that its licensee, Zogenix Inc, reported positive single-dose pharmacokinetic (PK) results from the Phase 1 clinical trial of Relday, an investigational candidate of a , once-monthly subcutaneous formulation of risperidone for the treatment of schizophrenia. According to Zogenix, adverse events in the Phase 1 trial in patients diagnosed with schizophrenia were generally mild to moderate and consistent with other risperidone products. The Phase 1 clinical trial for Relday was conducted as a single-center, open-label, safety and PK trial of 30 patients with chronic, stable schizophrenia or schizoaffective disorder. Per Zogenix, based on the favorable safety and PK profile demonstrated with the 25 mg and 50 mg once-monthly doses tested in the Phase 1 trial, Zogenix has extended the study to include a 100 mg dose of the same formulation. The addition of this dose arm to the study will enable evaluation of dose proportionality across the full dose range that would be anticipated to be used in clinical practice. Zogenix expects to complete the extension of the Phase 1 clinical trial during the second quarter of 2013.
http://www.reuters.com/finance/stocks/ZGNX.O/key-development…
http://www.reuters.com/finance/stocks/ZGNX.O/key-development…
Antwort auf Beitrag Nr.: 43.982.182 von Aurum2010 am 04.01.13 15:19:38Zogenix ist gut und wird auch wieder an die 5$ Marke laufen, sie
benötigen jetzt Zeit um die Forschung voran zu treiben und das
Medikament an den Markt zu bringen.
Ich habe Zeit bei Zogenix auch wenn ich aktuell massiv im Minus bin
benötigen jetzt Zeit um die Forschung voran zu treiben und das
Medikament an den Markt zu bringen.
Ich habe Zeit bei Zogenix auch wenn ich aktuell massiv im Minus bin
SAN DIEGO, Jan. 7, 2013 (GLOBE NEWSWIRE) -- Zogenix, Inc. (ZGNX), a pharmaceutical company commercializing and developing products for the treatment of central nervous system disorders and pain, announced today preliminary unaudited gross product sales for the quarter ended December 31, 2012. Zogenix expects to report fourth quarter 2012 gross product sales of approximately $13.5 million on 145,200 units shipped, with unit volume up approximately 9% sequentially from third quarter 2012 and 26% from fourth quarter 2011.
For the full year 2012, Zogenix announced preliminary unaudited net product revenue of approximately $36 million, up approximately 18% over 2011 and slightly below previously issued guidance of $37 million for 2012.
Preliminary unaudited cash and cash equivalents as of December 31, 2012 were approximately $41.2 million.
Roger Hawley, chief executive officer of Zogenix, stated, "In 2012 we continued to drive SUMAVEL DosePro prescription growth and added a new co-promotion partner that provides incremental growth potential in 2013 and beyond. We expect our net product revenue for the year to be slightly below our guidance primarily due to the impact from Hurricane Sandy in the Northeast region. However, we did achieve 9% unit volume growth in the fourth quarter and believe we are well positioned to continue expanding utilization of SUMAVEL DosePro as an important attack specific treatment choice for migraine patients."
Mr. Hawley added, "During the year we also made good progress with our new product initiatives. The December 7th FDA Advisory Committee meeting resulted in a negative vote for Zohydro ER, but we remain engaged with the FDA in our ongoing efforts to gain approval. We remain confident in the benefit/risk balance of Zohydro ER as a new treatment option that addresses unmet medical needs for patients suffering with chronic pain. Separately, we recently announced positive single-dose pharmacokinetic (PK) results from our Phase 1 clinical trial for Relday and the extension of the study to include a 100 mg dose arm, which results we believe will accelerate the development timeline and partnering activities for this new potential treatment option for the long-acting antipsychotic market."
The preliminary estimates discussed above are subject to the completion of financial closing procedures, including final adjustment of allowances for sales returns and discounts, and other developments that may arise between now and the time the financial results for the fourth quarter are finalized, as well as the completion of the audit of the 2012 financial statements. Therefore, actual results may differ materially from these estimates.In addition, the above estimates do not present all information necessary for an understanding of Zogenix's financial condition as of December 31, 2012. Zogenix expects to report full financial results for the fourth quarter and full year ended December 31, 2012 in early March 2013.
http://finance.yahoo.com/news/zogenix-announces-preliminary-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/zogenix-announces-preliminary-…
For the full year 2012, Zogenix announced preliminary unaudited net product revenue of approximately $36 million, up approximately 18% over 2011 and slightly below previously issued guidance of $37 million for 2012.
Preliminary unaudited cash and cash equivalents as of December 31, 2012 were approximately $41.2 million.
Roger Hawley, chief executive officer of Zogenix, stated, "In 2012 we continued to drive SUMAVEL DosePro prescription growth and added a new co-promotion partner that provides incremental growth potential in 2013 and beyond. We expect our net product revenue for the year to be slightly below our guidance primarily due to the impact from Hurricane Sandy in the Northeast region. However, we did achieve 9% unit volume growth in the fourth quarter and believe we are well positioned to continue expanding utilization of SUMAVEL DosePro as an important attack specific treatment choice for migraine patients."
Mr. Hawley added, "During the year we also made good progress with our new product initiatives. The December 7th FDA Advisory Committee meeting resulted in a negative vote for Zohydro ER, but we remain engaged with the FDA in our ongoing efforts to gain approval. We remain confident in the benefit/risk balance of Zohydro ER as a new treatment option that addresses unmet medical needs for patients suffering with chronic pain. Separately, we recently announced positive single-dose pharmacokinetic (PK) results from our Phase 1 clinical trial for Relday and the extension of the study to include a 100 mg dose arm, which results we believe will accelerate the development timeline and partnering activities for this new potential treatment option for the long-acting antipsychotic market."
The preliminary estimates discussed above are subject to the completion of financial closing procedures, including final adjustment of allowances for sales returns and discounts, and other developments that may arise between now and the time the financial results for the fourth quarter are finalized, as well as the completion of the audit of the 2012 financial statements. Therefore, actual results may differ materially from these estimates.In addition, the above estimates do not present all information necessary for an understanding of Zogenix's financial condition as of December 31, 2012. Zogenix expects to report full financial results for the fourth quarter and full year ended December 31, 2012 in early March 2013.
http://finance.yahoo.com/news/zogenix-announces-preliminary-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/zogenix-announces-preliminary-…
Hi, noch jemand hier investiert ?
Ich bin am Mittwoch hier eingestiegen......
Gibt ein neues SEC Filling :
http://secfilings.nasdaq.com/filingFrameset.asp?FileName=000…
Schönes WE
Ich bin am Mittwoch hier eingestiegen......
Gibt ein neues SEC Filling :
http://secfilings.nasdaq.com/filingFrameset.asp?FileName=000…
Schönes WE
Zitat von Earthfire: Hi, noch jemand hier investiert ?
Ich bin am Mittwoch hier eingestiegen......
Gibt ein neues SEC Filling :
http://secfilings.nasdaq.com/filingFrameset.asp?FileName=000…
Schönes WE
Sorry der Link funktioniert nicht :
hier mal der Auszug :
Item 1.01 Material Definitive Agreement.
On February 28, 2013, Zogenix, Inc. (“Zogenix” or the “Company”) and Patheon UK Limited (“Patheon”) entered into a manufacturing services agreement (the “Services Agreement”). Under the Services Agreement, Patheon will serve as the Company’s exclusive manufacturer for the aseptic capsule assembly, filling and inspection, final device assembly and packaging of Sumavel® DosePro®, as well as other manufacturing and support services. The Services Agreement will replace the Company’s prior manufacturing services agreement with Patheon upon its expiration on October 31, 2013.
Under the Services Agreement, Zogenix is not required to have a minimum quantity of Sumavel® DosePro® manufactured, but is required to provide Patheon with forecasts of the required volumes of Sumavel® DosePro® the Company needs and is required to pay Patheon a monthly manufacturing fee over the term of the Services Agreement. The Company is also required to pay support and service fees, with the level of service fees increasing if annual production exceeds a specified volume. The term of the Services Agreement will commence on November 1, 2013 and expire on April 30, 2015. The parties may mutually agree in writing to renew the term for additional terms prior to the expiration of the then-current term.
Zogenix may terminate the Services Agreement upon specified written notice to Patheon. Either party may terminate the Services Agreement (1) upon written notice if the other party has failed to remedy a material breach of any of its representations, warranties or other obligations under the agreement within a specified period following receipt of written notice of such breach, and (2) immediately upon written notice to the other party in the event that the other party is declared insolvent or bankrupt by a court of competent jurisdiction, a voluntary petition of bankruptcy is filed in any court of competent jurisdiction by such other party or the agreement is assigned by such other party for the benefit of creditors. Patheon may also terminate the Services Agreement upon specified written notice if the Company assigns the agreement to certain specified parties.
* * *
The foregoing description of the Services Agreement does not purport to be complete and is qualified in its entirety by the Services Agreement, a copy of which Zogenix intends to file with its Quarterly Report on Form 10-Q for the period ending March 31, 2013.
Antwort auf Beitrag Nr.: 44.206.410 von Earthfire am 02.03.13 11:14:26Nicht mehr. War ein paar Wochen dabei bin aber wg. Langeweile wieder ausgestiegen. Leider zu früh (?)
Antwort auf Beitrag Nr.: 44.206.435 von me_2 am 02.03.13 11:29:20Der aktuelle Kursansteig ist (nur) durch die Verzögerung der FDA Entscheidung bedingt. Wie hoch schätzt du die Chance für ein Approval ?
Zitat von me_2: Der aktuelle Kursansteig ist (nur) durch die Verzögerung der FDA Entscheidung bedingt. Wie hoch schätzt du die Chance für ein Approval ?
Hi, ich weiss....ein wenig hatte ich vor dem Einstieg schon recherchiert

die Chance...das ist schwierig zu beantworten !
Sagen wir mal so.....es kommt immer wieder zu einem Approval gegen eine negative Abstimmung....oft mit gewissen Einschränkungen...wie dir bekannt sein dürfte.
Aber die Instis halten noch !
Es gab immer DEZ 2012 auch einige neue Instis
Dann die Insiderkäufe im Dezember 2012
Das hohe Volumen bei dem Anstieg der letzten Woche..vergleichbar mit den Verkäufen bei der neg Abstimmung.
Ich denke das eine große Mehrzahl der Käufer noch an ein gutes Ende glauben.
Die Verzögerung hat ja auch ihre Gründe !
Ich würde eine eine 50/50 Chance tippen....daher bin ich auch rein.
Wir werden sehen....sollte die Entscheidung positiv ausfallen....werden die Shorts covern wie verrückt und es wird wahrscheinlich zu einem Squeeze kommen.
Bei einem Approval tippe ich auf 4-5 $ kurzfristig...evtl. mehr.
Aber es ist und bleibt beim derzeitigen Stand eine Wette mit dem entsprechenden Risiko !
Antwort auf Beitrag Nr.: 44.206.769 von Earthfire am 02.03.13 14:24:50Hi, du bist sicher besser informiert als ich, ich hatte mich ja vom ZGNX- Roulettetisch bald wieder zum Einstandskurs verabschiedet.
Mich überrascht nur, dass die Verzögerung als hohe Approval-Chance gehandelt wird. Ich sehs auch eher in deinem Bereich von 50%, das ist mir beim aktuellen Preis zu viel, zumal ich im Erfolgsfall eher erst mal 2-2,5 $ sehe. Gibt ja noch ne Menge weiterer Hürden (FDA-Auflagen, DEA class II vermutlich, und ne Menge anderer Opiate am Markt).
Gruß
me_2
Mich überrascht nur, dass die Verzögerung als hohe Approval-Chance gehandelt wird. Ich sehs auch eher in deinem Bereich von 50%, das ist mir beim aktuellen Preis zu viel, zumal ich im Erfolgsfall eher erst mal 2-2,5 $ sehe. Gibt ja noch ne Menge weiterer Hürden (FDA-Auflagen, DEA class II vermutlich, und ne Menge anderer Opiate am Markt).
Gruß
me_2
Zitat von me_2: Hi, du bist sicher besser informiert als ich, ich hatte mich ja vom ZGNX- Roulettetisch bald wieder zum Einstandskurs verabschiedet.
Mich überrascht nur, dass die Verzögerung als hohe Approval-Chance gehandelt wird. Ich sehs auch eher in deinem Bereich von 50%, das ist mir beim aktuellen Preis zu viel, zumal ich im Erfolgsfall eher erst mal 2-2,5 $ sehe. Gibt ja noch ne Menge weiterer Hürden (FDA-Auflagen, DEA class II vermutlich, und ne Menge anderer Opiate am Markt).
Gruß
me_2
Na dann schauen wir mal,2-2,5$ sehe ich nicht...eher das doppelte.
Ich glaube nicht, das die Zulasssung zum derzeitigen Zeitpunkt eingepreist ist....da ist das Risiko eines Flops zu hoch für.
Viele Instis liegen ja in diesem Bereich schon......
Schönes WE
Gruß Earthfire
Was ist denn heute bei Zogenix los ?
Antwort auf Beitrag Nr.: 45.698.693 von Wohnwunsch am 25.10.13 20:35:43FDA approval
http://www.bloomberg.com/news/2013-10-25/zogenix-wins-u-s-ap…
http://www.bloomberg.com/news/2013-10-25/zogenix-wins-u-s-ap…
Antwort auf Beitrag Nr.: 45.699.761 von lamona am 26.10.13 07:04:43http://www.zogenix.com/content/products/zohydro.htm
Zohydro abailable 2014

Zohydro abailable 2014

Das war es dann wohl wieder bei Zogenix
Ist hier ausser mir noch jemand investiert ?
Warum steigt die Aktie plötzlich so stark ?
Warum steigt die Aktie plötzlich so stark ?
Ich bin hier ebenfalls investiert. Denke es ist ein sehr interessanter Wert, denn Zogenix hat aufgrund seiner Produkte/Pipeline sehr viel Potential. Eine vervielfachung in den nächsten 1-2 Jahren ist sehr wahrscheinlich...
Hier ein paar Infos zum Unternehmen.
Allgemeneine Informationen:

Quelle: http://www.zogenix.com/index.php

http://www.reuters.com/finance/stocks/companyProfile?rpc=66&…
Pipeline:
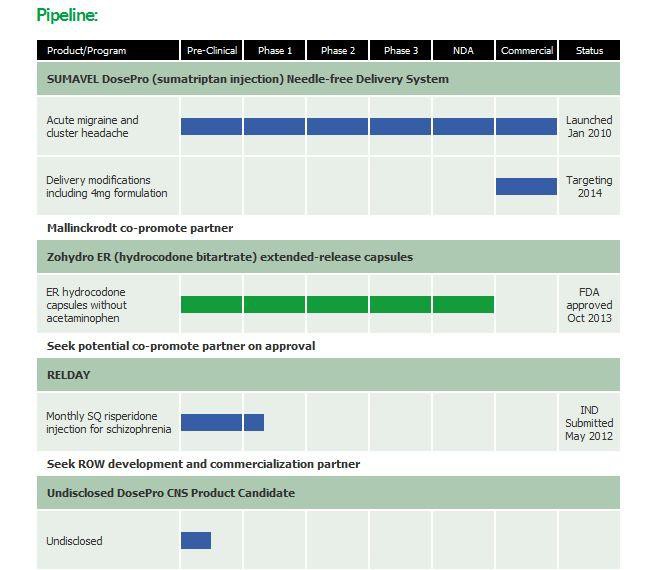
Quelle: http://www.zogenix.com/content/pipeline/overview.htm
6-Monatschart:
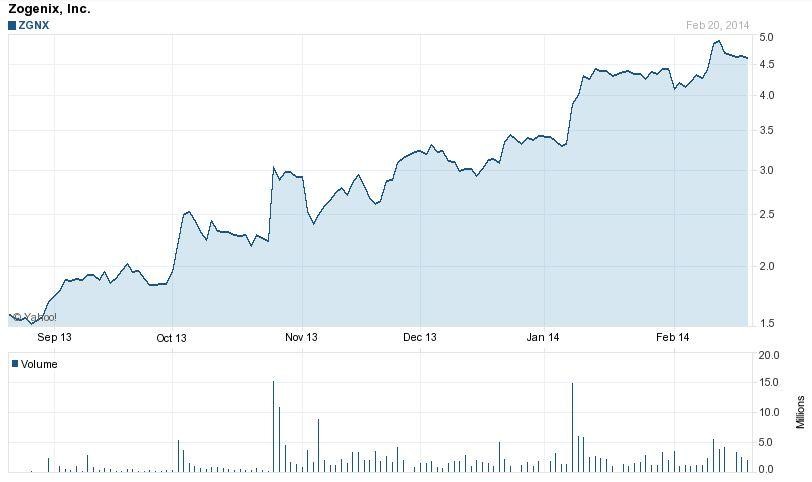
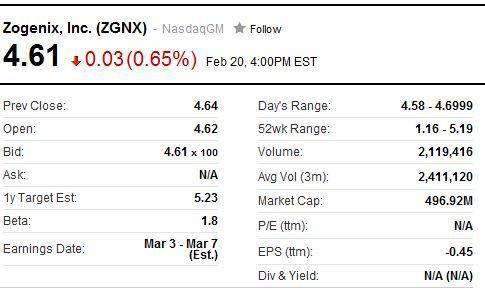
Holdings/Ownership:
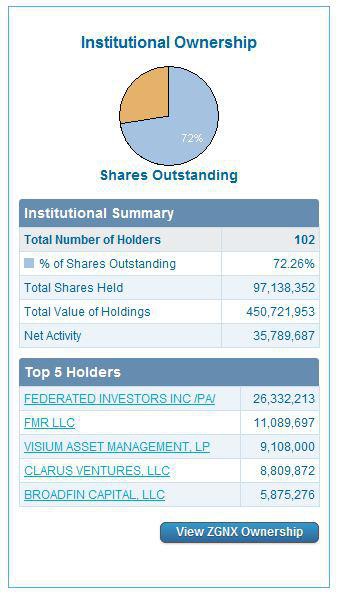
http://www.nasdaq.com/symbol/zgnx/ownership-summary
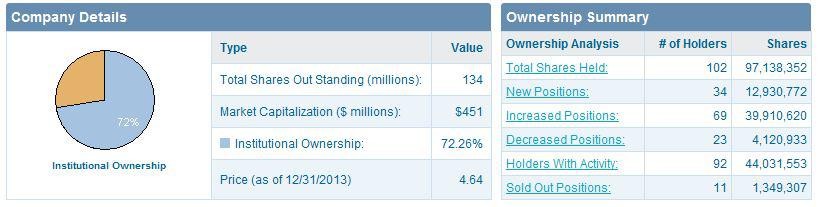
http://www.nasdaq.com/symbol/zgnx/institutional-holdings
Shortquote:
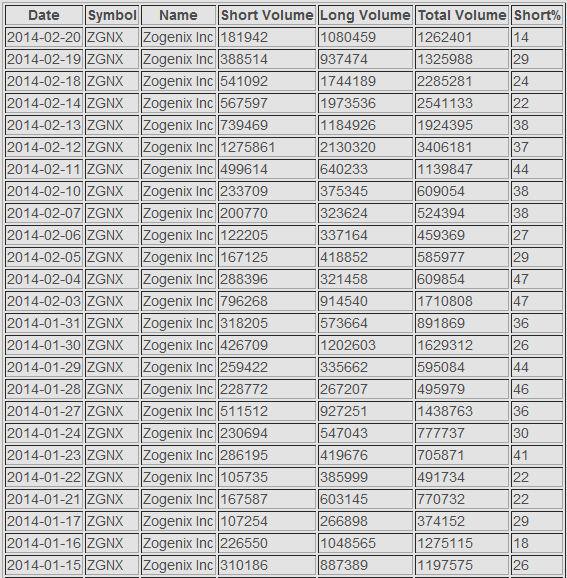
Targetprice/Analystenmeinungen:
http://247wallst.com/healthcare-business/2014/02/13/top-phar…" target="_blank" rel="nofollow ugc noopener">http://247wallst.com/healthcare-business/2014/02/13/top-phar…
http://247wallst.com/healthcare-business/2014/02/07/eight-bi…
http://www.4-traders.com/ZOGENIX-INC-6880640/consensus/
http://www.streetinsider.com/rating_history.php?q=ZGNX
http://www.marketwatch.com/investing/stock/zgnx/analystestim…
http://www.nasdaq.com/symbol/zgnx/analyst-research

Hier ein paar Infos zum Unternehmen.
Allgemeneine Informationen:

Quelle: http://www.zogenix.com/index.php

http://www.reuters.com/finance/stocks/companyProfile?rpc=66&…
Pipeline:

Quelle: http://www.zogenix.com/content/pipeline/overview.htm
6-Monatschart:


Holdings/Ownership:

http://www.nasdaq.com/symbol/zgnx/ownership-summary

http://www.nasdaq.com/symbol/zgnx/institutional-holdings
Shortquote:

Targetprice/Analystenmeinungen:
http://247wallst.com/healthcare-business/2014/02/13/top-phar…" target="_blank" rel="nofollow ugc noopener">http://247wallst.com/healthcare-business/2014/02/13/top-phar…
http://247wallst.com/healthcare-business/2014/02/07/eight-bi…
http://www.4-traders.com/ZOGENIX-INC-6880640/consensus/
http://www.streetinsider.com/rating_history.php?q=ZGNX
http://www.marketwatch.com/investing/stock/zgnx/analystestim…
http://www.nasdaq.com/symbol/zgnx/analyst-research
Ich erläutere kurz was für und gegen ein Invest in Zogenix spricht.
Vorteile
+Zogenix hat mit Sumavel bereits ein Produkt auf dem Markt.
+Das zweite Produkt Zydrohydro (Es handelt sich um ein Schmerzmittel) wird bereits vermarktet und steht kurz vor dem Verkauf. Das Medikament ist wesentlich wirkasamer als Vorgänger und Konkurrenzprodukte. Der Markt ist sehr groß. (Ein Grund warum die Aktie in den letzten Monaten so stark anstieg)
+Es befinden sich noch zwei weitere Produkte in der Pipeline. Allerdings befinden sich diese noch im Anfangsstadium.
+Chrattechnisch spricht nichts gegen einen weiteren Aufstieg.
+Die Marktkapitalisierung ist (noch) nicht so hoch.
+Es handelt sich um einen potentiellen Übernahmekandidat.
+Ein sehr großer Teil der Shares wird von Institutionen gehalten.
+Die Shortquote ist nicht so hoch.
Nachteile
-Produkte sind noch nicht lange auf dem Markt
-Einnahmen steigen zwar, es kann jedoch noch ein paar Wochen/Monate dauern bis deutlich mehr Kapital geniert wird (vermutlich ab Q2 2014)
Für Verbesserungen und Vervollständigungen wäre ich sehr dankbar. Hoffe diese Infos helfen einigen von euch.
Vorteile
+Zogenix hat mit Sumavel bereits ein Produkt auf dem Markt.
+Das zweite Produkt Zydrohydro (Es handelt sich um ein Schmerzmittel) wird bereits vermarktet und steht kurz vor dem Verkauf. Das Medikament ist wesentlich wirkasamer als Vorgänger und Konkurrenzprodukte. Der Markt ist sehr groß. (Ein Grund warum die Aktie in den letzten Monaten so stark anstieg)
+Es befinden sich noch zwei weitere Produkte in der Pipeline. Allerdings befinden sich diese noch im Anfangsstadium.
+Chrattechnisch spricht nichts gegen einen weiteren Aufstieg.
+Die Marktkapitalisierung ist (noch) nicht so hoch.
+Es handelt sich um einen potentiellen Übernahmekandidat.
+Ein sehr großer Teil der Shares wird von Institutionen gehalten.
+Die Shortquote ist nicht so hoch.
Nachteile
-Produkte sind noch nicht lange auf dem Markt
-Einnahmen steigen zwar, es kann jedoch noch ein paar Wochen/Monate dauern bis deutlich mehr Kapital geniert wird (vermutlich ab Q2 2014)
Für Verbesserungen und Vervollständigungen wäre ich sehr dankbar. Hoffe diese Infos helfen einigen von euch.
Sumavel gleich Sumatriptan. Ist schon sehr lange auf dem Markt. Patentschutz abgelaufen, viele Konkurrenzprodukte.
Gutes Medikament gegen Migräne
Hydrocodon in retardierter Form gibt es auch schon auf em Markt.
Sind also sogenannte Generika.
Das Umsatzpotential sollte daher auch nicht zu hoch eingeschätzt werden.
Wenn ich hier lese (und vertraue) dass Instis in er Aktie drin sind und sehe, dass der Kurs im letzten Jahr schön zugelegt hat, würde ich eher einen kursschonenden Ausstieg der Instis erwarten.
Samit will ich den Wert nicht schlecht machen. Hab nicht recherchiert, sondern nur hier gelesen, den Chart angesehen, meine Medizinischen Kenntnisse und meine Erfahrung an der Börse zusammengebracht.
Mein Fazit: Vorsicht, hier läuft man vielleicht in einen Abverkauf rein.
Viel Glück!
Gutes Medikament gegen Migräne
Hydrocodon in retardierter Form gibt es auch schon auf em Markt.
Sind also sogenannte Generika.
Das Umsatzpotential sollte daher auch nicht zu hoch eingeschätzt werden.
Wenn ich hier lese (und vertraue) dass Instis in er Aktie drin sind und sehe, dass der Kurs im letzten Jahr schön zugelegt hat, würde ich eher einen kursschonenden Ausstieg der Instis erwarten.
Samit will ich den Wert nicht schlecht machen. Hab nicht recherchiert, sondern nur hier gelesen, den Chart angesehen, meine Medizinischen Kenntnisse und meine Erfahrung an der Börse zusammengebracht.
Mein Fazit: Vorsicht, hier läuft man vielleicht in einen Abverkauf rein.
Viel Glück!
Antwort auf Beitrag Nr.: 46.567.903 von dottore am 05.03.14 12:48:41Hast recht dottore, es gibt sehr viel Konkurrenz in diesem Bereich. Das neue Produkt Zydrohydro ist seit Montag auf dem Markt und das könnte aus rein psychologischen Gründen (Phantasie) den Kurs ansteigen lassen. Unabhängig von den aktuellen Verkaufszahlen.
Die Q-Zahlen, welche gerstern veröffentlicht wurden, waren nur knapp unter den Analystenschätzungen. Alles in Ordnung.
Zydrohydro wird wie ein Nasensrpay verwendet, soweit ich weiß. Es soll außerdem sehr effektiv sein im Vergleich zu Konkurrenzprodukten.
Es wurde in den USA sehr viel über Zydrohydro diskutiert, da bestimmte Personen wollen, dass die FDA es wieder vom Markt nimmt. Sie meinen es könnte süchtig machen usw. Selbst auf CNN und anderen großen Sendern wurde diskutiert. Das heißt also dieses Medikament ist nun sehr bekannt. Eine bessere Werbeplattform gibt es nicht.
Ich denke der Kurs könnte bis Ende Q2 um 40-50% auf $6.75 steigen.
Heute in Deutschland im Plus. Mal sehen wie sich der Kurs ab 15.30 verhält.
Die Q-Zahlen, welche gerstern veröffentlicht wurden, waren nur knapp unter den Analystenschätzungen. Alles in Ordnung.
Zydrohydro wird wie ein Nasensrpay verwendet, soweit ich weiß. Es soll außerdem sehr effektiv sein im Vergleich zu Konkurrenzprodukten.
Es wurde in den USA sehr viel über Zydrohydro diskutiert, da bestimmte Personen wollen, dass die FDA es wieder vom Markt nimmt. Sie meinen es könnte süchtig machen usw. Selbst auf CNN und anderen großen Sendern wurde diskutiert. Das heißt also dieses Medikament ist nun sehr bekannt. Eine bessere Werbeplattform gibt es nicht.
Ich denke der Kurs könnte bis Ende Q2 um 40-50% auf $6.75 steigen.
Heute in Deutschland im Plus. Mal sehen wie sich der Kurs ab 15.30 verhält.
Was ist denn jetzt wieder los, warum bricht der Kurs völlig ein ?
Man ist das ein scheiss Invest
Man ist das ein scheiss Invest
Antwort auf Beitrag Nr.: 47.280.870 von Wohnwunsch am 08.07.14 21:30:25bin heut mal reingehuscht zu 1,20usd spekuliere auf eine ordentliche gegenbewegung
spekuliere auf eine ordentliche gegenbewegung nach diesem heftigen kurssturz.für mich nicht logisch diese kursabschläge in letzter zeit oder gibts dafür gründe?
nach diesem heftigen kurssturz.für mich nicht logisch diese kursabschläge in letzter zeit oder gibts dafür gründe?


 spekuliere auf eine ordentliche gegenbewegung
spekuliere auf eine ordentliche gegenbewegung nach diesem heftigen kurssturz.für mich nicht logisch diese kursabschläge in letzter zeit oder gibts dafür gründe?
nach diesem heftigen kurssturz.für mich nicht logisch diese kursabschläge in letzter zeit oder gibts dafür gründe?


Bin gestern eingestiegen.
Der Verkauf von Zohydro steigt von Monat zu Monat an, das sollte sich dann langfristig in den Quartalsberichten wiederspiegeln.
Ich hoffe bis Mitte 2015 auf USD 2,00 - 2,50 die Aktie.
Der Verkauf von Zohydro steigt von Monat zu Monat an, das sollte sich dann langfristig in den Quartalsberichten wiederspiegeln.
Ich hoffe bis Mitte 2015 auf USD 2,00 - 2,50 die Aktie.
Antwort auf Beitrag Nr.: 47.619.087 von rust12 am 27.08.14 09:14:05die 2,00usd werden wir noch in diesem jahr sehen
 ..
..

 ..
..
Antwort auf Beitrag Nr.: 47.623.104 von androlyt am 27.08.14 14:39:03und ganz neben bei ist zgnx ein übernahmekandidat auf diesem niveau ..
..
 ..
..
Bin auch dabei ... Hoffe das tief ist vorbei...
Huhuuu erwachen.... :-)
http://www.nasdaq.com/press-release/zogenix-2015-expectation…
Hört sich doch gut an !
Zogenix bekommt immer mehr Aufmerksamkeit.
Der Tiefpunkt der Aktie sollte bei USD 1,03 gewesen sein.
Bis USD 1,70 - 1,80 kann man die Aktie noch zukaufen.
August 2015 wenn der Quartalsbericht 2/2015 rauskommt, sollten wir die
USD 2,00 überschreiten.
Hört sich doch gut an !
Zogenix bekommt immer mehr Aufmerksamkeit.
Der Tiefpunkt der Aktie sollte bei USD 1,03 gewesen sein.
Bis USD 1,70 - 1,80 kann man die Aktie noch zukaufen.
August 2015 wenn der Quartalsbericht 2/2015 rauskommt, sollten wir die
USD 2,00 überschreiten.
http://microcapresearch.com/zogenix/
Hier noch ein interessanter Bericht.
Der sagt eigentlich alles aus wohin die Reise geht.
Hier noch ein interessanter Bericht.
Der sagt eigentlich alles aus wohin die Reise geht.
interessante Produktportfolio.. Heute Nachmittag mal eine kleine Posi gekauft. Mal abwarten, was die FDA morgen sagt 

Hallo, wann kommt die veräffentlichung von der FDA?
Antwort auf Beitrag Nr.: 48.935.195 von Henry168 am 30.01.15 15:29:58jetzt haben wir es schwarz auf weiss 
Zogenix Receives FDA Approval of New Formulation of Zohydro(R) ER
(Quelle Yahoo)
nachbörslich wurden schon 1.45 $ bezahlt und der Entscheid war noch nicht draussen!!
bin jetzt schon auf die Eröffnung am Montag gespannt
für die shortis wird es ein Blutbad geben aber für uns viel Kohle
aber für uns viel Kohle 


Grüsse aus der Schweiz
Zubi

Zogenix Receives FDA Approval of New Formulation of Zohydro(R) ER
(Quelle Yahoo)
nachbörslich wurden schon 1.45 $ bezahlt und der Entscheid war noch nicht draussen!!
bin jetzt schon auf die Eröffnung am Montag gespannt

für die shortis wird es ein Blutbad geben
 aber für uns viel Kohle
aber für uns viel Kohle 


Grüsse aus der Schweiz
Zubi
Hier nochmal der Link mit der FDA Meldung von Zogenix
http://www.nasdaq.com/press-release/zogenix-receives-fda-app…
Jetzt sollte es in den nächsten Monaten eigentlich Richtung USD 2,00 gehen.
Im dritten Quartal 2015 sollten wir dann deutliche Umsatzsteigerungen sehen.
http://www.nasdaq.com/press-release/zogenix-receives-fda-app…
Jetzt sollte es in den nächsten Monaten eigentlich Richtung USD 2,00 gehen.
Im dritten Quartal 2015 sollten wir dann deutliche Umsatzsteigerungen sehen.
Hallo, habe mir Freitag nachbörslich auch eine erste Position Ins Depot gelegt. Mal sehen was sie morgen draus machen werden unser lieben wallstreetler 
Mfg

Mfg
Moin, das vorbörsliche Volumen ist heute sehr stark, mal schauen ob sie heute im Modus sell on good News agieren werden...
Gleich geht es los
Gleich geht es los

http://www.nasdaq.com/press-release/zogenix-announces-confer…
Am 10.03.2015 kommen die Zahlen für das 4Quartal.
Ich gehe von einem Umsatz Richtung USD 9,5 - 10 Mio. aus, alles darüber wäre eine Überraschug.
Die von mir anvisierten USD 2,00 im Kurs, scheinen schon früher erreichbar zu sein.
Am 10.03.2015 kommen die Zahlen für das 4Quartal.
Ich gehe von einem Umsatz Richtung USD 9,5 - 10 Mio. aus, alles darüber wäre eine Überraschug.
Die von mir anvisierten USD 2,00 im Kurs, scheinen schon früher erreichbar zu sein.
http://www.nasdaq.com/symbol/zgnx/premarket
Premarket sieht gut aus. Zeitweise hatten wir schon die USD 2,00.
Der Markt erwartet wohl morgen gute Zahlen.
Premarket sieht gut aus. Zeitweise hatten wir schon die USD 2,00.
Der Markt erwartet wohl morgen gute Zahlen.
• Privately-held Collegium Pharmaceutical raises $50M from institutional investors to fund the advancement of its abuse-deterrent product candidate, Xtampa ER (oxycodone extended-release capsules) through FDA clearance and commercial launch as well as fund operational growth and other pipeline programs.
• The FDA accepted the company's New Drug Application (NDA) for Xtampa ER in February. Its abuse-deterrent technology is called DETERx, which combines oxycodone with fatty acid and waxes to form small spherical beads which resist particle size reduction and dose dumping when subjected to breaking, crushing, chewing and dissolving.
Quelle: seekingAlpha News
• The FDA accepted the company's New Drug Application (NDA) for Xtampa ER in February. Its abuse-deterrent technology is called DETERx, which combines oxycodone with fatty acid and waxes to form small spherical beads which resist particle size reduction and dose dumping when subjected to breaking, crushing, chewing and dissolving.
Quelle: seekingAlpha News
Antwort auf Beitrag Nr.: 49.288.124 von chriz86 am 10.03.15 14:25:35Zogenix, Inc. (ZGNX) Nachbörslich Trading -
1.90
 bei sehr grossem Volumen bereits 842,000 Aktien gehandelt
bei sehr grossem Volumen bereits 842,000 Aktien gehandelt
Grüsse aus der Schweiz
Zubi
1.90

 bei sehr grossem Volumen bereits 842,000 Aktien gehandelt
bei sehr grossem Volumen bereits 842,000 Aktien gehandelt Grüsse aus der Schweiz
Zubi
nice
Zogenix Announces Agreement of Sale of Zohydro(R) ER Business to Pernix for $100 Million at Closing Plus Potential Milestones of $283.5 Millionhttp://ir.zogenix.com/phoenix.zhtml?c=220862&p=irol-newsArti…
Zogenix Announces Agreement of Sale of Zohydro(R) ER Business to Pernix for $100 Million at Closing Plus Potential Milestones of $283.5 Million
War das keine gute Nachricht? Wieso ist sie so abgestürzt?
War das keine gute Nachricht? Wieso ist sie so abgestürzt?
Antwort auf Beitrag Nr.: 49.294.979 von Henry168 am 11.03.15 08:45:32
Wird schon werden
Medikament bzw Lizensen wurde verkauft ...gab zwar ne Menge Kohle aber kein Medikament mehr auf Markt dadurch !Zogenix will sich auf ihre 2 Medikamente in der Pipline konzerntrieren die in Phase 2 und 3stecken !Dürfte trotzdem weiterhin viel Fantasie drinn stecken in dem Wert
Antwort auf Beitrag Nr.: 49.299.545 von Fiasko78 am 11.03.15 14:07:52Phase III? Wenn ich mir die Pipeline anschaue, haben sie kein PIII. Eins soll 1.Hj fertig sein und dann noch PII.
Sie müssten ja bei dem Preis mehr Cash haben, als ihre MK beträgt.
Sie müssten ja bei dem Preis mehr Cash haben, als ihre MK beträgt.
Antwort auf Beitrag Nr.: 49.300.904 von Jagubert am 11.03.15 15:50:41
Sorry stimmt...
Die werden sich da schon was bei gedacht haben warum sie das Medikament verkauft haben ...Das Medikament stand in der Öffentlich sehr schlecht da aufgrund von Missbrauch.Aber mit dieser Meldung konnte wirklich keiner rechnen.Da wird sicher in den nächsten Tagen noch was kommen im positiven Sinne und wenns ne Übernahme oder positive Studien zu den 2 verbleibende Medikamente ist .
Antwort auf Beitrag Nr.: 49.301.573 von Fiasko78 am 11.03.15 16:31:44So hab mir mal den Chart angeschaut, da ich mit der Aktie Fett im Minus bin.

Habe fest gestellt: alles halb so schlimm. Im Moment ist Markttechnisch alles Okey. Die Aktie hat Ihren Boden bei 1,08€ gefunden. Korrekter Anstieg an 161ér Fibo bzw. bissl drüber, anschließend schöne Korrektur auf 38´er Fibo. Sehe auf diesem Niveau Einkaufpreise. Auf lange Sicht meine nächsten Ziel:
2,50/3,38/4,81 sieht gut aus.
Man sieht auch sehr schön die Fraktale im Chartbild, vorallem den immer wieder kehrenden Abverkauf und darauf folgenden Anstieg. Bei dieser Aktie sollte man sich daher immer auf schnelle tiefe Korekturen oder starke anstiege nach erreichen bestimmter Marken einstellen. Fraktale können auch gespiegelt oder verkleinert bzw. vergrößert vorkommen.
Wünsche euch allen schöne Gewinne.


Habe fest gestellt: alles halb so schlimm. Im Moment ist Markttechnisch alles Okey. Die Aktie hat Ihren Boden bei 1,08€ gefunden. Korrekter Anstieg an 161ér Fibo bzw. bissl drüber, anschließend schöne Korrektur auf 38´er Fibo. Sehe auf diesem Niveau Einkaufpreise. Auf lange Sicht meine nächsten Ziel:
2,50/3,38/4,81 sieht gut aus.
Man sieht auch sehr schön die Fraktale im Chartbild, vorallem den immer wieder kehrenden Abverkauf und darauf folgenden Anstieg. Bei dieser Aktie sollte man sich daher immer auf schnelle tiefe Korekturen oder starke anstiege nach erreichen bestimmter Marken einstellen. Fraktale können auch gespiegelt oder verkleinert bzw. vergrößert vorkommen.
Wünsche euch allen schöne Gewinne.
Wir werden die USD 2,00 dieses Jahr noch sehen.
[urlhttp://seekingalpha.com/article/3076046-two-sub-2-biotech-stocks-on-the-move?source=nasdaq][/url]
[urlhttp://seekingalpha.com/article/3076046-two-sub-2-biotech-stocks-on-the-move?source=nasdaq][/url]
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +0,37 | |
| +0,66 | |
| +0,09 | |
| +0,12 | |
| +3,43 | |
| -5,57 | |
| 0,00 | |
| -0,32 | |
| +0,63 | |
| +20,00 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 201 | ||
| 109 | ||
| 78 | ||
| 69 | ||
| 51 | ||
| 36 | ||
| 34 | ||
| 34 | ||
| 32 | ||
| 28 |



















