Theralase Technologies Inc ---> Cold Laser Manufacturer - 500 Beiträge pro Seite
eröffnet am 20.02.14 16:01:41 von
neuester Beitrag 15.11.18 21:37:21 von
neuester Beitrag 15.11.18 21:37:21 von
Beiträge: 53
ID: 1.191.797
ID: 1.191.797
Aufrufe heute: 0
Gesamt: 9.482
Gesamt: 9.482
Aktive User: 0
ISIN: CA88337V1004 · WKN: A0DLB7 · Symbol: TTX
0,1140
EUR
0,00 %
0,0000 EUR
Letzter Kurs 18.04.24 Tradegate
Neuigkeiten
05.04.24 · Accesswire |
27.03.24 · Accesswire |
26.03.24 · Accesswire |
30.11.23 · Accesswire |
Werte aus der Branche Gesundheitswesen
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,8000 | +99.999,00 | |
| 0,8486 | +34,00 | |
| 0,9399 | +17,49 | |
| 1,3700 | +15,13 | |
| 3,9100 | +14,66 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,1880 | -10,68 | |
| 2,5100 | -13,00 | |
| 4,5200 | -16,30 | |
| 0,5383 | -22,06 | |
| 1,0700 | -27,21 |
Über Theralase - Kalt-Laser Hersteller
Theralase Technologies Inc. entwirft, entwickelt und produziert gepulste super- , Kälte- Laser-Technologie für eine breite Palette von menschlichen , tierischen Begleiter und Pferde -Anwendungen. Bio- stimulierende Funktionen gehören : Heil neuralen Muskel-Skelett- Bedingungen durch Schmerzausschaltung , Entzündungen Reduzierung und erhöhte Geweberegeneration. Die Kombination Theralase Lasertechnik mit der photodynamischen Verbindungen ( PDC ) zu Bio- destruktiven Funktionen, einschließlich Zerstörung von Krebszellen , Bakterien , Viren und Fettzellen. Theralase Laser dringen tiefer - bis zu 10 cm ( 4 in) unter der Hautoberfläche - und heilen Gewebe schneller als jede andere Kaltlaser auf dem Markt. Theralase Kunden profitieren von Zugang zu kontinuierlichen Verbesserungen sowie Unterstützung und Ausbildung von hochqualifizierten Fachkräften .
Neben der Entwicklung und Bereitstellung von Lasertechnik, arbeitet Theralase ein Full-Service medizinischen Reha-Klinik in Toronto, Ontario für Schulung und Ausbildung von Gesundheits- Praktiker .
Theralase Technologies Inc. entwirft, entwickelt und produziert gepulste super- , Kälte- Laser-Technologie für eine breite Palette von menschlichen , tierischen Begleiter und Pferde -Anwendungen. Bio- stimulierende Funktionen gehören : Heil neuralen Muskel-Skelett- Bedingungen durch Schmerzausschaltung , Entzündungen Reduzierung und erhöhte Geweberegeneration. Die Kombination Theralase Lasertechnik mit der photodynamischen Verbindungen ( PDC ) zu Bio- destruktiven Funktionen, einschließlich Zerstörung von Krebszellen , Bakterien , Viren und Fettzellen. Theralase Laser dringen tiefer - bis zu 10 cm ( 4 in) unter der Hautoberfläche - und heilen Gewebe schneller als jede andere Kaltlaser auf dem Markt. Theralase Kunden profitieren von Zugang zu kontinuierlichen Verbesserungen sowie Unterstützung und Ausbildung von hochqualifizierten Fachkräften .
Neben der Entwicklung und Bereitstellung von Lasertechnik, arbeitet Theralase ein Full-Service medizinischen Reha-Klinik in Toronto, Ontario für Schulung und Ausbildung von Gesundheits- Praktiker .
Homepage:
http://www.theralase.com/www/index.php
Stockhouse:
http://www.stockhouse.com/companies/quote/v.tlt/theralase-te…
Shares Out: 65.8m
http://www.theralase.com/www/index.php
Stockhouse:
http://www.stockhouse.com/companies/quote/v.tlt/theralase-te…
Shares Out: 65.8m
Brot und Buttergeschäft
TLC-1000 Kaltlaser-Technologie für nicht-invasive Schmerztherapie
-> TLC-2000 wird das Upgarde!
Theralase erzeugt derzeit 2.000.000 $ an jährlichen Einnahmen aus der Laser-Therapie Abteilung des Unternehmens, und hat eine Strategie, diese Zahl von 300 bis 400% im Jahr 2014 erhöhen. Die Wachstumsstrategie umfasst Expansion in Kanada und aggressiv in wichtigen US-Märkten sowie die Einführung eines wiederkehrende Einnahmen-Modell mit der Veröffentlichung des TLC-2000. Das Unternehmen hat klinische Studien der Universität von Buffalo ausgelagert, um die Wirksamkeit der neuen Biofeedback-Technologie zu demonstrieren. Theralase erwartet eine installierte Basis von 400 Einheiten an jedem 16.500 $, die auf 6,6 Millionen Dollar Umsatz entspricht.
Lesen Sie mehr unter http://www.stockhouse.com/news/press-releases/2014/02/12/the…
----------------------------
Nettes Geschäft - wenn so kommt wie Management denkt.
Zur Fantasie in diesem Wert später - oder wer hat Zeit sie zu erklären
TLC-1000 Kaltlaser-Technologie für nicht-invasive Schmerztherapie
-> TLC-2000 wird das Upgarde!
Theralase erzeugt derzeit 2.000.000 $ an jährlichen Einnahmen aus der Laser-Therapie Abteilung des Unternehmens, und hat eine Strategie, diese Zahl von 300 bis 400% im Jahr 2014 erhöhen. Die Wachstumsstrategie umfasst Expansion in Kanada und aggressiv in wichtigen US-Märkten sowie die Einführung eines wiederkehrende Einnahmen-Modell mit der Veröffentlichung des TLC-2000. Das Unternehmen hat klinische Studien der Universität von Buffalo ausgelagert, um die Wirksamkeit der neuen Biofeedback-Technologie zu demonstrieren. Theralase erwartet eine installierte Basis von 400 Einheiten an jedem 16.500 $, die auf 6,6 Millionen Dollar Umsatz entspricht.
Lesen Sie mehr unter http://www.stockhouse.com/news/press-releases/2014/02/12/the…
----------------------------
Nettes Geschäft - wenn so kommt wie Management denkt.
Zur Fantasie in diesem Wert später - oder wer hat Zeit sie zu erklären

Antwort auf Beitrag Nr.: 46.496.779 von Jon_Schnee am 20.02.14 16:01:41
Hallo John,
Gibts da wirklich (bisher)nix, was da rankommt?
Gruß
P.
Zitat von Roger Dumoulin-White: Theralase's strong PDC pipeline has scientifically proven that our PDC technology is capable of consistently producing cell kill levels of 100%, even at very low concentrations, across a wide range of cancers and bacteria. In fact, our PDCs have been proven to be significantly more effective in destroying cancer cells and bacteria than currently FDA approved Photo Dynamic Compounds.
Hallo John,
Gibts da wirklich (bisher)nix, was da rankommt?
Gruß
P.
Antwort auf Beitrag Nr.: 46.498.535 von Popeye82 am 20.02.14 19:02:59Hallo Popeye
wir sind ja schon bei der Fantasie
Jedoch ein Krebs - hier gibt's x-Ansätze und Unternehmen.
Gut für Patienten schlecht für Investoren. Hier geht's um die Nadel im Heuhaufen.
schlecht für Investoren. Hier geht's um die Nadel im Heuhaufen.
Ansatz für den Blasenkrebs gefällt mir.
Wie die Chancen gegenüber der Konkurrenz ist weiss ich noch nicht.
Eigentlich investiere ich nicht in "Krebs Biotechs".
Hier finde ich einfach die Kombination zwischen Brot/Butter Gefschäft und Fantasie intressant. Daher Watch List und weiter sich über die Firma zu informieren.
Was ist Eure Meinung dazu??????????
ANTI- KREBS -Plattform-Technologie
Theralase als Foto Dynamische Verbindungen ( PDCs ) bekannten Anti -Krebs-Medikamente , die die DNA von Krebszellen zu lokalisieren, und wenn bestrahlt wird, zerstören die DNA , was zu Apoptose (natürlicher Zelltod) patentiert. Während PDCs sind in Photodynamische Therapie (PDT) als potenzielle Behandlung Regime für eine Reihe von Bedingungen, hat Theralase entdeckt, dass sie besonders vielversprechend halten, wie die Behandlung auf bestimmte Krebserkrankungen. Durch Studien intern und unabhängig durchgeführt Theralase der PDC im zerstören Brust-, Darm -, Gehirn -und Blasenkrebszellenbewährt. Eine aktuelle Mausmodell gezeigt, vollständige Zerstörung der subkutanen Blasenkrebstumoren . Die Studie war ein wichtiger Meilenstein, was bedeutet, dass das Unternehmen seine führende Wirkstoffkandidat ist wirksam bei der Zerstörung von Krebs in einem lebenden Tier -Modell und können die aus wiederkehrenden Krebs zu verhindern.
wir sind ja schon bei der Fantasie

Jedoch ein Krebs - hier gibt's x-Ansätze und Unternehmen.
Gut für Patienten
 schlecht für Investoren. Hier geht's um die Nadel im Heuhaufen.
schlecht für Investoren. Hier geht's um die Nadel im Heuhaufen.Ansatz für den Blasenkrebs gefällt mir.
Wie die Chancen gegenüber der Konkurrenz ist weiss ich noch nicht.
Eigentlich investiere ich nicht in "Krebs Biotechs".
Hier finde ich einfach die Kombination zwischen Brot/Butter Gefschäft und Fantasie intressant. Daher Watch List und weiter sich über die Firma zu informieren.
Was ist Eure Meinung dazu??????????
ANTI- KREBS -Plattform-Technologie
Theralase als Foto Dynamische Verbindungen ( PDCs ) bekannten Anti -Krebs-Medikamente , die die DNA von Krebszellen zu lokalisieren, und wenn bestrahlt wird, zerstören die DNA , was zu Apoptose (natürlicher Zelltod) patentiert. Während PDCs sind in Photodynamische Therapie (PDT) als potenzielle Behandlung Regime für eine Reihe von Bedingungen, hat Theralase entdeckt, dass sie besonders vielversprechend halten, wie die Behandlung auf bestimmte Krebserkrankungen. Durch Studien intern und unabhängig durchgeführt Theralase der PDC im zerstören Brust-, Darm -, Gehirn -und Blasenkrebszellenbewährt. Eine aktuelle Mausmodell gezeigt, vollständige Zerstörung der subkutanen Blasenkrebstumoren . Die Studie war ein wichtiger Meilenstein, was bedeutet, dass das Unternehmen seine führende Wirkstoffkandidat ist wirksam bei der Zerstörung von Krebs in einem lebenden Tier -Modell und können die aus wiederkehrenden Krebs zu verhindern.
So, habe mir den Wert nun näher angesehen und bin zu einem ersten Urteil gekommen: Langfristig sehr spannend, kurzfristig jedoch voraussichtlich ohne neue Impulse.
Speziell die am 7. März endende Haltefrist könnte noch mal etwas Druck auf die Aktie bringen, denn die Kapitalerhöhung wurde ja zu 0,20 CAD (= 85% über aktuellen Kursniveau) durchgeführt und die Warrants laufen bis November 2015. Warum also die Aktie halten? Ich denke, da werde noch einige die Gewinne einbuchen. NACH dem 7. März werde ich mir die Aktie dann noch mal ansehen und ggf. einen "längerfristigen Fuß in die Tür stellen".
Unternehmen mit "Durchbrüchen bei Krebs" gab es auch in den 1990ern schon viele. 99% der Unternehmen sind gescheitert... Und bei Theralase liegt das alles noch sehr weit weg (2017/2018). Mir gefällt aber, dass sie recht wenig Geld verbrennen, schon ein Produkt am Markt haben und somit bei wenig Verwässerung weitermachen können.
Beste Grüße,
M@trix
Speziell die am 7. März endende Haltefrist könnte noch mal etwas Druck auf die Aktie bringen, denn die Kapitalerhöhung wurde ja zu 0,20 CAD (= 85% über aktuellen Kursniveau) durchgeführt und die Warrants laufen bis November 2015. Warum also die Aktie halten? Ich denke, da werde noch einige die Gewinne einbuchen. NACH dem 7. März werde ich mir die Aktie dann noch mal ansehen und ggf. einen "längerfristigen Fuß in die Tür stellen".
Unternehmen mit "Durchbrüchen bei Krebs" gab es auch in den 1990ern schon viele. 99% der Unternehmen sind gescheitert... Und bei Theralase liegt das alles noch sehr weit weg (2017/2018). Mir gefällt aber, dass sie recht wenig Geld verbrennen, schon ein Produkt am Markt haben und somit bei wenig Verwässerung weitermachen können.
Beste Grüße,
M@trix
Antwort auf Beitrag Nr.: 46.502.439 von M@trix am 21.02.14 13:47:35
Ich hab keine Ahnung von Denen , aber ich halte viel von Leuten, bzw. sehe es als eine große Stärke, die (auch)sagen (können) "interessant, aber (für mich)noch nicht(s)". Und das wird, meiner Einschätzung nach, eine iiiiiiiiiiiiiiiiiiiiiiiiimmer wichtigere Eigenschaft.
, aber ich halte viel von Leuten, bzw. sehe es als eine große Stärke, die (auch)sagen (können) "interessant, aber (für mich)noch nicht(s)". Und das wird, meiner Einschätzung nach, eine iiiiiiiiiiiiiiiiiiiiiiiiimmer wichtigere Eigenschaft.
In dem Sinne, Danke für Deinen Kommentar.
Gruß
P.
Ich hab keine Ahnung von Denen
 , aber ich halte viel von Leuten, bzw. sehe es als eine große Stärke, die (auch)sagen (können) "interessant, aber (für mich)noch nicht(s)". Und das wird, meiner Einschätzung nach, eine iiiiiiiiiiiiiiiiiiiiiiiiimmer wichtigere Eigenschaft.
, aber ich halte viel von Leuten, bzw. sehe es als eine große Stärke, die (auch)sagen (können) "interessant, aber (für mich)noch nicht(s)". Und das wird, meiner Einschätzung nach, eine iiiiiiiiiiiiiiiiiiiiiiiiimmer wichtigere Eigenschaft.In dem Sinne, Danke für Deinen Kommentar.
Gruß
P.
Antwort auf Beitrag Nr.: 46.502.439 von M@trix am 21.02.14 13:47:35vielen dank für Deine Einschätzung.
Ja mit potentiellen Krebsmittel ist es eine Sache. Erfolgswahrscheinlichkeit ist klein.
Die andere Frage ist ob sie es schaffen mit der Schmerztherapie mind. 6 Mio zu erwirtschaften und das auch Nachhaltig.? Margen soll gut sein.
Kurs darf gerne noch ein gutes Stück zurück kommen.
Ja mit potentiellen Krebsmittel ist es eine Sache. Erfolgswahrscheinlichkeit ist klein.
Die andere Frage ist ob sie es schaffen mit der Schmerztherapie mind. 6 Mio zu erwirtschaften und das auch Nachhaltig.? Margen soll gut sein.
Kurs darf gerne noch ein gutes Stück zurück kommen.
Die Aktie könnte heute einen "Boost" bekommen durch diesen Artikel vom bekannten 321gold.com-Betreiber Bob Moriarty:
Cancer Cure at the Speed of Light
Bob Moriarty
Archives
Mar 3, 2014
After 13 years of writing about emerging companies both big and small, it amazes me how few have actually accomplished much. That’s why it’s refreshing to come across a company that has not only a real product but real management with skin in the game and a real future.
Someone contacted me a week or so back and insisted I take a look at a tiny company in the medical device field. That’s not really something I am an expert on but what I was told was intriguing enough that I did look into the company. It has a real product, real management and a real future. In fact, they seem to have a cure for cancer. At the speed of light.
The company is named Theralase Technology. (TLT-V) It is the lovechild of Roger White, company President and CEO. His mother was a former nurse and his grandfather was a doctor. Roger’s father was a watchmaker and jack-of-all-trades.
White started working for him when he was 7, learning clock repair and watchmaking. When White was 10 he took apart his own bike just to see how it was made. He was quite good at disassembling the bicycle but when it came time to putting it back together, he wasn’t so good. But as is so often the case in life, he learned a lot more from his mistakes than from his success.
Torn between the engineering side of his father and the medical side of his mother, Roger White graduated as an Electrical Engineer in 1986. But in 1993, his father called him to tell him about an interesting technology he had discovered while on travels in Belgium. It was an “invisible laser” that could double the speed of healing and decrease the pain of injuries.
Roger White traveled to Brussels to meet the inventor of this laser light. The man who invented the specific frequency of laser light explained that the building blocks of connective tissue, fibroblasts, have a 100% increase in cell division when exposed to this particular light. This cuts healing time in half and reduces pain without the use of medicine or surgery.
White fell in love with the concept and bought the rights to the technology in 1994 after meeting with physicians and scientists familiar with the process. He formed his own company to research the product and began manufacturing it in 1995. By 2000 he launched his first medical device product, the TLC-1000. 1200 of these units are in the field and are providing cash flow to the company today.
Theralase is the perfect company for a public financing. We forget that the real purpose of a company selling stock is for it to be able to grow at a faster rate than just by internal financing. A big investor heard of the company and convinced them to accept a big financing. That financing took place in November and added over $3 million to the company’s coffers.
Theralase plans on using the money to introduce a new model of the laser light medical device by September. In the past Theralase sold the devices to the medical industry for $16,000. They have made a deal with a leasing company. The leasing company will provide the units to doctors on a 3-year lease for $500 a month. That will make the decision to use far easier for doctors. The leasing company gives $16,500 to Theralase upon signing the deal with the doctor. When the 3-year lease is complete, Theralase gets the $500 a month.
It’s pretty much a no lose deal for everyone. Experience from units in the field suggests that the average doctor can generate $10,000 per month in revenue from the device. Theralase gets their money up front and has a back end cash flow once the three-year period is complete.
Doctors use the therapeutic cold laser technology to heal a wide variety of nerve, muscle and joint issues such as lower back pain, tendonitis and knee problems. In the US some 56 million patients suffer from chronic pain. The medical pain market in the US exceeds $100 billion a year. Our population is aging rapidly so there will be more demand in the future. The process is well known and proven with lasers used by thousands of doctors and nurses worldwide with a track record of millions of successful treatments.
The cold laser works in three ways. It eliminates pain, reduces inflammation and accelerates tissue healing. Now I must admit that like Roger White, when I first heard about this, I wondered if I needed to put on my aluminum foil beanie. It literally sounded too good to be true. But it’s been studied and tested in blind and controlled clinical studies and it works. No tin foil hat necessary.
If Theralase’s cold laser device was the only dog in the hunt frankly I wouldn’t be all that interested in working with them. They are doing a couple of million a year in sales now. The updated machine due out in September of 2014 combined with the new leasing scheme will triple their revenue. Ho, hum. But then Roger mentioned their cure for cancer. . .
That woke me up. I had to have him explain it to me carefully so I understood it. A cure for cancer without surgery or expensive medicine would be a game changer. Curing cancer at the speed of light would be a world class, perhaps universe class game changer.
In the late 1990s, Roger White read a short piece in an industry publication named Laser Focus World talking about a chemistry professor at a Virginia university who was working on molecules called Photo Dynamic Compounds (PDC) that were attracted to cancer cells. When activated by light, these compounds kill the cancer cells with no damage to healthy tissue surrounding the cancer.
White got in touch with the professor and bought the rights to the technology. Compared to the current standards of care for cancer patients using surgery, chemo or radiation, a simple and safe cure would be a revolutionary product.
Let’s talk about cancer for a minute so you understand how these PDCs work. Normal cells grow and eventually normal cells die. Cancer cells don’t die. They keep growing until they kill the host. There is literally a switch in the DNA of cancer cells that stays on when it should go off.
When the PDC is injected into the cancerous area of the body, healthy cells reject the PDC. Cancerous cells attract the PDC and it combines with the DNA of the cancer cell. When the cold laser light is activated on both healthy and cancerous cells, the healthy cells are not affected and the cancer cells die.
Naturally this process requires a lot of testing. The first test the company conducted is called a toxicity test. The test should measure both dark toxicity and light toxicity. With the PDC injected in the cancer area, the PDC should have no toxicity on any cells because it’s dark. When exposed to light, the PDC on the cancer cells should react. Best case is zero dark toxicity and 100% light toxicity. Theralase found this result in the lab studies of four of their patented PDCs.
The next test Theralase conducted was on a mouse. The company injected cancer cells into a mouse and successfully created a tumor in the mouse. Then they injected their patented PDC and activated it with a laser. This test showed a total eradication of the tumor and the mouse lived an additional 20 months, which is a normal mouse life span. Much to the surprise of the company, the PDC/Laser light treatment also took five strokes off the mouse’s golf game.
The treatment works on a variety of cancer cells including breast, colon, bladder and brain cancer. But Theralase had to pick one specific cancer to work on to get approval of the FDA and Health Canada for use in treatment.
Theralase chose bladder cancer. In the US there are 70,000 new cases yearly and 14,000 deaths from the disease. Spending on bladder cancer amounts to $3.9 billion yearly. There have been no new drugs to treat bladder cancer since 1998. Bladder cancer is the most expensive cancer to treat and has an 80% recurrence rate.
Theralase is in the process of completing a mouse model on dose toxicity, working on the Good Medical Procedures (GMP) manufacturing standards, and finishing the FDA Investigational New Drug application. The next phase will be achieving Breakthrough Status and partnering with a major pharmaceutical company.
Theralase will design the laser. They are ISO-13485 certified and have some 20 years experience with laser devices. Manufacture of the PDC will go out to a GMP facility. At this time there appears to be little regulatory risk. Since the PDC never enters the blood stream it has virtually zero toxicity. The FDA likes new cancer drugs and should love this procedure. Theralase's applications have been fast tracked through the approval process.
With a price of $.34 today and 65 million shares outstanding, the market gives a market cap of about $22 million for Theralase. The company has about $3 million in the bank today from the placement completed in November. Those shares were bought at $.15 with a full warrant attached for purchase of an additional share at $.20 for two years. The four month hold on the shares expires on March 7 which explains why the shares have been weak, peaking at $.56 a little over a month ago. With the warrants solidly in the money it would be perfectly natural for investors in the PP to sell short to lock in a high price, knowing their shares come free trading a few days from now so they can exercise their warrants.
This may be the most important company I have or ever will write about. This isn’t just a treatment for cancer; it’s a cure for cancer through what should be a medically benign method. The anticipated cash flow for the next three years from the already tested and sold cold laser devices would more than justify today’s share price but it’s simply not possible to anticipate the financial value for a cure of any form of cancer. It should cure bladder cancer; it may cure breast cancer and even lung cancer. The blue sky on this stock goes from Canada all the way to the moon.
And I really like talking to Roger White. Most of the people I talk to are figuring out in their minds what not to tell me. I know when I am talking to them that I am getting the part of the story they want me to know but I have to pry to find out what I really need to know. Roger’s story of having been a tool and die maker that morphed into president of a cancer cure company fascinated me. I asked him to tell me the story. It’s not something that you would find in a company brochure or on their website or written about by your typical financial writer but it was riveting.
He sat down and immediately wrote me an 1800-word missive that answered all my questions. This piece isn’t very much longer than that and I’ve worked on it for days. He’s not hiding anything. This is the real deal.
To the extent that I believe this is a game changer, I’m proud to be able to write this piece. This company could improve the lives of thousands or millions of people. I hope they succeed. I hope they make a giant fortune, I hope everyone who invests in them makes a giant fortune and I’m triple-thrilled to have them as a sponsor.
I don’t own shares; I’d like my contribution to their success to be doing a good job of telling the story. I f**king love this company.
I will not put in my typical mealy-mouth disclaimer required by the SEC on this write-up. Shut up and buy the stock if you can afford some. If they somehow manage to fail you have contributed more to the health of your fellow man and perhaps even yourself than any other stock I can think of. When they succeed, you have not only done good, you have done well.
Theralase Technology Inc.
TLT-V $.34 (Feb 28, 2014)
TLTFF-OTCBB 65.8 million shares
http://www.321gold.com/editorials/moriarty/moriarty030314.ht…
Cancer Cure at the Speed of Light
Bob Moriarty
Archives
Mar 3, 2014
After 13 years of writing about emerging companies both big and small, it amazes me how few have actually accomplished much. That’s why it’s refreshing to come across a company that has not only a real product but real management with skin in the game and a real future.
Someone contacted me a week or so back and insisted I take a look at a tiny company in the medical device field. That’s not really something I am an expert on but what I was told was intriguing enough that I did look into the company. It has a real product, real management and a real future. In fact, they seem to have a cure for cancer. At the speed of light.
The company is named Theralase Technology. (TLT-V) It is the lovechild of Roger White, company President and CEO. His mother was a former nurse and his grandfather was a doctor. Roger’s father was a watchmaker and jack-of-all-trades.
White started working for him when he was 7, learning clock repair and watchmaking. When White was 10 he took apart his own bike just to see how it was made. He was quite good at disassembling the bicycle but when it came time to putting it back together, he wasn’t so good. But as is so often the case in life, he learned a lot more from his mistakes than from his success.
Torn between the engineering side of his father and the medical side of his mother, Roger White graduated as an Electrical Engineer in 1986. But in 1993, his father called him to tell him about an interesting technology he had discovered while on travels in Belgium. It was an “invisible laser” that could double the speed of healing and decrease the pain of injuries.
Roger White traveled to Brussels to meet the inventor of this laser light. The man who invented the specific frequency of laser light explained that the building blocks of connective tissue, fibroblasts, have a 100% increase in cell division when exposed to this particular light. This cuts healing time in half and reduces pain without the use of medicine or surgery.
White fell in love with the concept and bought the rights to the technology in 1994 after meeting with physicians and scientists familiar with the process. He formed his own company to research the product and began manufacturing it in 1995. By 2000 he launched his first medical device product, the TLC-1000. 1200 of these units are in the field and are providing cash flow to the company today.
Theralase is the perfect company for a public financing. We forget that the real purpose of a company selling stock is for it to be able to grow at a faster rate than just by internal financing. A big investor heard of the company and convinced them to accept a big financing. That financing took place in November and added over $3 million to the company’s coffers.
Theralase plans on using the money to introduce a new model of the laser light medical device by September. In the past Theralase sold the devices to the medical industry for $16,000. They have made a deal with a leasing company. The leasing company will provide the units to doctors on a 3-year lease for $500 a month. That will make the decision to use far easier for doctors. The leasing company gives $16,500 to Theralase upon signing the deal with the doctor. When the 3-year lease is complete, Theralase gets the $500 a month.
It’s pretty much a no lose deal for everyone. Experience from units in the field suggests that the average doctor can generate $10,000 per month in revenue from the device. Theralase gets their money up front and has a back end cash flow once the three-year period is complete.
Doctors use the therapeutic cold laser technology to heal a wide variety of nerve, muscle and joint issues such as lower back pain, tendonitis and knee problems. In the US some 56 million patients suffer from chronic pain. The medical pain market in the US exceeds $100 billion a year. Our population is aging rapidly so there will be more demand in the future. The process is well known and proven with lasers used by thousands of doctors and nurses worldwide with a track record of millions of successful treatments.
The cold laser works in three ways. It eliminates pain, reduces inflammation and accelerates tissue healing. Now I must admit that like Roger White, when I first heard about this, I wondered if I needed to put on my aluminum foil beanie. It literally sounded too good to be true. But it’s been studied and tested in blind and controlled clinical studies and it works. No tin foil hat necessary.
If Theralase’s cold laser device was the only dog in the hunt frankly I wouldn’t be all that interested in working with them. They are doing a couple of million a year in sales now. The updated machine due out in September of 2014 combined with the new leasing scheme will triple their revenue. Ho, hum. But then Roger mentioned their cure for cancer. . .
That woke me up. I had to have him explain it to me carefully so I understood it. A cure for cancer without surgery or expensive medicine would be a game changer. Curing cancer at the speed of light would be a world class, perhaps universe class game changer.
In the late 1990s, Roger White read a short piece in an industry publication named Laser Focus World talking about a chemistry professor at a Virginia university who was working on molecules called Photo Dynamic Compounds (PDC) that were attracted to cancer cells. When activated by light, these compounds kill the cancer cells with no damage to healthy tissue surrounding the cancer.
White got in touch with the professor and bought the rights to the technology. Compared to the current standards of care for cancer patients using surgery, chemo or radiation, a simple and safe cure would be a revolutionary product.
Let’s talk about cancer for a minute so you understand how these PDCs work. Normal cells grow and eventually normal cells die. Cancer cells don’t die. They keep growing until they kill the host. There is literally a switch in the DNA of cancer cells that stays on when it should go off.
When the PDC is injected into the cancerous area of the body, healthy cells reject the PDC. Cancerous cells attract the PDC and it combines with the DNA of the cancer cell. When the cold laser light is activated on both healthy and cancerous cells, the healthy cells are not affected and the cancer cells die.
Naturally this process requires a lot of testing. The first test the company conducted is called a toxicity test. The test should measure both dark toxicity and light toxicity. With the PDC injected in the cancer area, the PDC should have no toxicity on any cells because it’s dark. When exposed to light, the PDC on the cancer cells should react. Best case is zero dark toxicity and 100% light toxicity. Theralase found this result in the lab studies of four of their patented PDCs.
The next test Theralase conducted was on a mouse. The company injected cancer cells into a mouse and successfully created a tumor in the mouse. Then they injected their patented PDC and activated it with a laser. This test showed a total eradication of the tumor and the mouse lived an additional 20 months, which is a normal mouse life span. Much to the surprise of the company, the PDC/Laser light treatment also took five strokes off the mouse’s golf game.
The treatment works on a variety of cancer cells including breast, colon, bladder and brain cancer. But Theralase had to pick one specific cancer to work on to get approval of the FDA and Health Canada for use in treatment.
Theralase chose bladder cancer. In the US there are 70,000 new cases yearly and 14,000 deaths from the disease. Spending on bladder cancer amounts to $3.9 billion yearly. There have been no new drugs to treat bladder cancer since 1998. Bladder cancer is the most expensive cancer to treat and has an 80% recurrence rate.
Theralase is in the process of completing a mouse model on dose toxicity, working on the Good Medical Procedures (GMP) manufacturing standards, and finishing the FDA Investigational New Drug application. The next phase will be achieving Breakthrough Status and partnering with a major pharmaceutical company.
Theralase will design the laser. They are ISO-13485 certified and have some 20 years experience with laser devices. Manufacture of the PDC will go out to a GMP facility. At this time there appears to be little regulatory risk. Since the PDC never enters the blood stream it has virtually zero toxicity. The FDA likes new cancer drugs and should love this procedure. Theralase's applications have been fast tracked through the approval process.
With a price of $.34 today and 65 million shares outstanding, the market gives a market cap of about $22 million for Theralase. The company has about $3 million in the bank today from the placement completed in November. Those shares were bought at $.15 with a full warrant attached for purchase of an additional share at $.20 for two years. The four month hold on the shares expires on March 7 which explains why the shares have been weak, peaking at $.56 a little over a month ago. With the warrants solidly in the money it would be perfectly natural for investors in the PP to sell short to lock in a high price, knowing their shares come free trading a few days from now so they can exercise their warrants.
This may be the most important company I have or ever will write about. This isn’t just a treatment for cancer; it’s a cure for cancer through what should be a medically benign method. The anticipated cash flow for the next three years from the already tested and sold cold laser devices would more than justify today’s share price but it’s simply not possible to anticipate the financial value for a cure of any form of cancer. It should cure bladder cancer; it may cure breast cancer and even lung cancer. The blue sky on this stock goes from Canada all the way to the moon.
And I really like talking to Roger White. Most of the people I talk to are figuring out in their minds what not to tell me. I know when I am talking to them that I am getting the part of the story they want me to know but I have to pry to find out what I really need to know. Roger’s story of having been a tool and die maker that morphed into president of a cancer cure company fascinated me. I asked him to tell me the story. It’s not something that you would find in a company brochure or on their website or written about by your typical financial writer but it was riveting.
He sat down and immediately wrote me an 1800-word missive that answered all my questions. This piece isn’t very much longer than that and I’ve worked on it for days. He’s not hiding anything. This is the real deal.
To the extent that I believe this is a game changer, I’m proud to be able to write this piece. This company could improve the lives of thousands or millions of people. I hope they succeed. I hope they make a giant fortune, I hope everyone who invests in them makes a giant fortune and I’m triple-thrilled to have them as a sponsor.
I don’t own shares; I’d like my contribution to their success to be doing a good job of telling the story. I f**king love this company.
I will not put in my typical mealy-mouth disclaimer required by the SEC on this write-up. Shut up and buy the stock if you can afford some. If they somehow manage to fail you have contributed more to the health of your fellow man and perhaps even yourself than any other stock I can think of. When they succeed, you have not only done good, you have done well.
Theralase Technology Inc.
TLT-V $.34 (Feb 28, 2014)
TLTFF-OTCBB 65.8 million shares
http://www.321gold.com/editorials/moriarty/moriarty030314.ht…
Antwort auf Beitrag Nr.: 46.554.523 von M@trix am 03.03.14 14:00:05danke!
+30% - jetzt pushen GoldBullen schon Biotech Klitschen. Ist der Biotech Hype schon im dunkelroten Bereich?
+30% - jetzt pushen GoldBullen schon Biotech Klitschen. Ist der Biotech Hype schon im dunkelroten Bereich?
Nur kurz: bin hier nun meiner ersten Position dabei. Werde den Wert noch mal in einem Beitrag "aufarbeiten" - wahrscheinlich aber erst nächste Woche.
Theralase CEO Roger White talks to Cantech Letter
APRIL 8, 2014 BY NICK WADDELL
"CEO Roger White says the company’s Photo Dynamic Compounds have the potential to be a game changer and disruptive in the war against cancer because they work by destroying only the cancerous tissue and leaving healthy tissue intact. Recently, Cantech Letter caught up with White to talk about 2014 and beyond. [...]"
http://www.cantechletter.com/2014/04/roger-white-ceo-therala…
APRIL 8, 2014 BY NICK WADDELL
"CEO Roger White says the company’s Photo Dynamic Compounds have the potential to be a game changer and disruptive in the war against cancer because they work by destroying only the cancerous tissue and leaving healthy tissue intact. Recently, Cantech Letter caught up with White to talk about 2014 and beyond. [...]"
http://www.cantechletter.com/2014/04/roger-white-ceo-therala…
Hatte heute die Empfehlung vom Boersenreporter im Postfach.Was denkt ihr lohnt sich ein Einstieg?
Ich habe meine Position heute aufgestockt. Das was Theralase gestern gemeldet hat, ist bahnbrechend:
Theralase Discovers Anti-Cancer Memory Response
http://finance.yahoo.com/news/theralase-discovers-anti-cance…
Das wäre also nicht nur eine Heilung für Krebs, sondern gar eine Art Impfung!
Natürlich ist es ein weiter Weg bis zu einem Produkt, doch wenn man sich den jüngsten Biotech-Hype vergegenwärtigt, sollte ein solches Unternehmen mehr als 27 Mio. CAD auf die Börsenwaage bringen. Ich könnte mir vorstellen, dass wir hier schnell weiter steigen in Richtung 100 Mio. CAD Börsenwert.
Theralase Discovers Anti-Cancer Memory Response
http://finance.yahoo.com/news/theralase-discovers-anti-cance…
Das wäre also nicht nur eine Heilung für Krebs, sondern gar eine Art Impfung!
Natürlich ist es ein weiter Weg bis zu einem Produkt, doch wenn man sich den jüngsten Biotech-Hype vergegenwärtigt, sollte ein solches Unternehmen mehr als 27 Mio. CAD auf die Börsenwaage bringen. Ich könnte mir vorstellen, dass wir hier schnell weiter steigen in Richtung 100 Mio. CAD Börsenwert.
Bob Moriarty told readers to “shut up +buy Theralase” - SHE/321g - May 30, 2014
www.stockhouse.com/news/newswire/2014/05/30/bob-moriarty-tol…
"When newsletter writer Bob Moriarty wrote a report on Theralase Technology Inc. (TSX: V.TLT, Stock Forum) in March, the stock was trading 34 cents, and Moriarty said he was not a shareholder.
However, in his 321gold newsletter, he said he wanted to help the company succeed by doing a good job of telling the story.
Moriarty published an update Friday, May 30, 2014, to remind his readers about how valuable a vaccine to cure cancer would be, but said they should do their own due diligence.
Ironically, the stock was trading Friday at 34 cents when Stockhouse was alerted to the reports, leaving a market cap of $22.6 million, based on 66.6 million shares outstanding. The 52-week range is 56 cents and 16.5 cents.
In his first report, Moriarty explains how Theralase President Roger Dumoulin-White read a short piece in an industry publication named Laser Focus World, talking about a chemistry professor at the Virginia University who was working on molecules called Photo Dynamic Compounds (PDC) that were attracted to cancer cells.
When activated by light, these compounds kill the cancer cells with no damage to healthy tissue, surrounding the cancer.
White got in touch with the professor and bought the rights to the technology.
Moriarty reports that the treatment works on a variety of cancer cells, including breast, colon, bladder and brain cancer.
But he said the company had to pick one specific cancer to work on to get approval of the FDA and Health Canada for use in treatment.
Earlier this week, the stock jumped 64% when the company announced results from pre-clinical trials for its flagship Photo Dynamic Compound (PDC). The trials were conducted at the Princess Margaret Cancer Centre in Toronto.
The company said it has discovered that PDC, has demonstrated an ability to render animals immune to repeated exposures of the same cancer.
“This initial data has been accepted for presentation at the 37th Annual American Society for Photobiology taking place in San Diego, California in June, 2014,’’ the company said in a press release.
“If our PDC technology is proven effective in cancer patients, the implications of this discovery are nothing short of game-changing for both Theralase and for cancer patients,’’ said Dumoulin-White.
Getting back to Moriarty, the respected scribe told his readers in March to “shut up and buy the stock if you can afford some.’’
“If they somehow manage to fail, you have contributed more to the health of your fellow man and perhaps even yourself than any other stock I can think of,’’ he wrote. “When they succeed, you have not only done good, you have done well.’’
FULL DISCLOSURE: Theralase Technology is a Stockhouse client. "
www.stockhouse.com/news/newswire/2014/05/30/bob-moriarty-tol…
"When newsletter writer Bob Moriarty wrote a report on Theralase Technology Inc. (TSX: V.TLT, Stock Forum) in March, the stock was trading 34 cents, and Moriarty said he was not a shareholder.
However, in his 321gold newsletter, he said he wanted to help the company succeed by doing a good job of telling the story.
Moriarty published an update Friday, May 30, 2014, to remind his readers about how valuable a vaccine to cure cancer would be, but said they should do their own due diligence.
Ironically, the stock was trading Friday at 34 cents when Stockhouse was alerted to the reports, leaving a market cap of $22.6 million, based on 66.6 million shares outstanding. The 52-week range is 56 cents and 16.5 cents.
In his first report, Moriarty explains how Theralase President Roger Dumoulin-White read a short piece in an industry publication named Laser Focus World, talking about a chemistry professor at the Virginia University who was working on molecules called Photo Dynamic Compounds (PDC) that were attracted to cancer cells.
When activated by light, these compounds kill the cancer cells with no damage to healthy tissue, surrounding the cancer.
White got in touch with the professor and bought the rights to the technology.
Moriarty reports that the treatment works on a variety of cancer cells, including breast, colon, bladder and brain cancer.
But he said the company had to pick one specific cancer to work on to get approval of the FDA and Health Canada for use in treatment.
Earlier this week, the stock jumped 64% when the company announced results from pre-clinical trials for its flagship Photo Dynamic Compound (PDC). The trials were conducted at the Princess Margaret Cancer Centre in Toronto.
The company said it has discovered that PDC, has demonstrated an ability to render animals immune to repeated exposures of the same cancer.
“This initial data has been accepted for presentation at the 37th Annual American Society for Photobiology taking place in San Diego, California in June, 2014,’’ the company said in a press release.
“If our PDC technology is proven effective in cancer patients, the implications of this discovery are nothing short of game-changing for both Theralase and for cancer patients,’’ said Dumoulin-White.
Getting back to Moriarty, the respected scribe told his readers in March to “shut up and buy the stock if you can afford some.’’
“If they somehow manage to fail, you have contributed more to the health of your fellow man and perhaps even yourself than any other stock I can think of,’’ he wrote. “When they succeed, you have not only done good, you have done well.’’
FULL DISCLOSURE: Theralase Technology is a Stockhouse client. "
So, noch ein paar nachgelegt zu 0,315 CAD. Nun sollte sich die Aktie fangen, wenn das Szenario aufgehen soll.
Antwort auf Beitrag Nr.: 47.101.894 von M@trix am 04.06.14 16:53:36.....geht doch....zumindest heute mit über 21% plus...
M.
M.
Antwort auf Beitrag Nr.: 47.210.670 von manffreddoo am 25.06.14 22:57:10hier die News dazu:
Theralase Releases Peer-Reviewed Research on Anti-Cancer Memory Response and Destruction of Cancerous Cells
http://www.theralase.com/www2/news
Theralase Releases Peer-Reviewed Research on Anti-Cancer Memory Response and Destruction of Cancerous Cells
http://www.theralase.com/www2/news
Theralase hält sich gut - schön wäre es wenn es über die 0,35 $ steigt - dann wäre -charttechnisch- der Weg nach weiter oben frei.
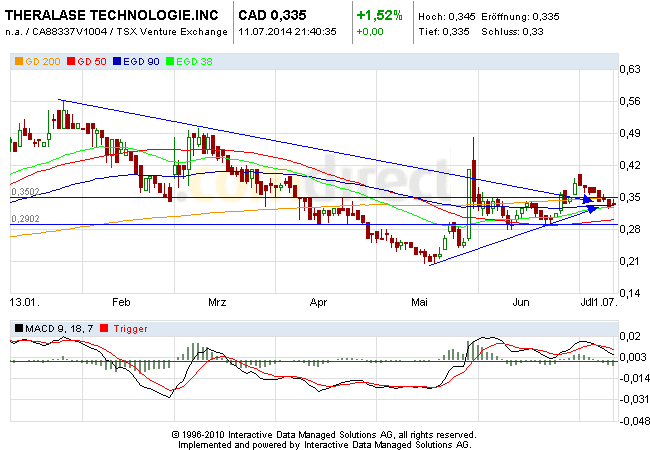

Theralase ist für mich eine Aktie, die irgendwann mal in unfassbare Höhen explodieren kann und doch nach unten relativ abgesichert ist. Heutige News:
Theralase Advances Intellectual Property of Anti-Cancer Technology
"[...] Theralase's PDCs are able to be activated by laser wavelengths ranging from the visible spectrum to the Near Infrared (NIR) spectrum. This newly conceived multi-wavelength laser system is able to activate the PDCs from a few hundred microns (millionths of a meter) to ten centimeters in depth allowing oncologists the ability to target surface as well as deep cancerous tumors.
Dr. Arkady Mandel, Chief Scientific Officer of Theralase stated, "The new multi-wavelength laser system combined with Theralase's PDCs presents the first opportunity for an oncologist to be able to provide a fully "patient specific" Photo Dynamic Therapy (PDT) treatment. The treatment is customizable for a patient, by optimizing the quantity of PDC delivered and the quantity and wavelength of laser light delivered based on the disease characterisation, which includes: the depth of the cancer, the localisation of the cancer in the tissues and the stage of the progression of the disease. In terms of bladder cancer, for example, patients diagnosed with a Non Muscle Invasive Bladder Cancer (NMIBC) that is fairly superficial would benefit from activation of the PDCs that has localized in their cancer cells in the lower visible range (i.e.: blue, green wavelengths). Patients; however, with more advanced Muscle Invasive Bladder Cancer (MIBC) that presents deeper in the organ, would best be served by having the PDCs in their cancer cells activated in the higher visible ranges (i.e.:red to near infrared wavelengths). The clear advantage of the combination of Theralase PDCs and the customized TLC-3000 laser delivery system is truly personalized care for the patient. As a result, Theralase's approach can eliminate unnecessary treatments, minimize the potential for adverse events and ultimately improve patient outcomes. [...]"
http://finance.yahoo.com/news/theralase-advances-intellectua…
Theralase Advances Intellectual Property of Anti-Cancer Technology
"[...] Theralase's PDCs are able to be activated by laser wavelengths ranging from the visible spectrum to the Near Infrared (NIR) spectrum. This newly conceived multi-wavelength laser system is able to activate the PDCs from a few hundred microns (millionths of a meter) to ten centimeters in depth allowing oncologists the ability to target surface as well as deep cancerous tumors.
Dr. Arkady Mandel, Chief Scientific Officer of Theralase stated, "The new multi-wavelength laser system combined with Theralase's PDCs presents the first opportunity for an oncologist to be able to provide a fully "patient specific" Photo Dynamic Therapy (PDT) treatment. The treatment is customizable for a patient, by optimizing the quantity of PDC delivered and the quantity and wavelength of laser light delivered based on the disease characterisation, which includes: the depth of the cancer, the localisation of the cancer in the tissues and the stage of the progression of the disease. In terms of bladder cancer, for example, patients diagnosed with a Non Muscle Invasive Bladder Cancer (NMIBC) that is fairly superficial would benefit from activation of the PDCs that has localized in their cancer cells in the lower visible range (i.e.: blue, green wavelengths). Patients; however, with more advanced Muscle Invasive Bladder Cancer (MIBC) that presents deeper in the organ, would best be served by having the PDCs in their cancer cells activated in the higher visible ranges (i.e.:red to near infrared wavelengths). The clear advantage of the combination of Theralase PDCs and the customized TLC-3000 laser delivery system is truly personalized care for the patient. As a result, Theralase's approach can eliminate unnecessary treatments, minimize the potential for adverse events and ultimately improve patient outcomes. [...]"
http://finance.yahoo.com/news/theralase-advances-intellectua…
Antwort auf Beitrag Nr.: 47.327.290 von M@trix am 17.07.14 16:34:26.......hi,
dazu bräuchten sie Abnehmer für ihre Podukte,wer immer das auch sei........ja dann könnte das ein Multi-Bagger werden....for sure
M.
dazu bräuchten sie Abnehmer für ihre Podukte,wer immer das auch sei........ja dann könnte das ein Multi-Bagger werden....for sure
M.
Lesenswert (wenngleich bezahlte IR):
Kocheta Junior Tech Report on Theralase (June 2014)
http://www.321gold.com/editorials/kotecha/kotecha061914.pdf
Kocheta Junior Tech Report on Theralase (June 2014)
http://www.321gold.com/editorials/kotecha/kotecha061914.pdf
Moin zusammen,
hier tut sich ja nicht mehr viel...
Ist hier wer investiert?
Überlege auch einzusteigen, nachdem die Aktie ja massiv gefallen ist.
Kennt jemand die Gründe hierfür?
hier tut sich ja nicht mehr viel...
Ist hier wer investiert?
Überlege auch einzusteigen, nachdem die Aktie ja massiv gefallen ist.
Kennt jemand die Gründe hierfür?
Theralase Partners with SAFC, in Manufacture of Anti-Cancer Drugs - Oct 7, 2014
http://app.quotemedia.com/quotetools/newsStoryPopup.go?story…
"Toronto, Ontario / TNW-ACCESSWIRE / October 7, 2014 / Theralase Technologies Inc. ("Theralase(R)") (TSXV: TLT) (TLTFF: OTC Link(R)) announced today that it has collaborated with Sigma-Aldrich Corporation's (NASDAQ: SIAL) custom manufacturing services business unit SAFC(R) Commercial , in the manufacture of the Photo Dynamic Compounds ("PDCs") being developed by Theralase for the destruction of cancer.
Under the terms of the agreement, SAFC will develop the Standard Operating Procedures ("SOPs") to manufacture initial quantities of each of Theralase's four lead PDCs. Once Theralase selects the final lead PDC at the end of the fourth quarter 2014, SAFC will manufacture both a 100-gram pre-Good Manufacturing Practice ("pre-GMP") batch and finally a 500-gram GMP batch of the PDC. This final 500-gram batch is intended to be suitable for final sterilization and packaging into individual patient doses for safety and efficacy evaluation through a Health Canada / FDA Phase I/IIa clinical trial, expected in early 2015.
SAFC is Theralase's partner of choice to provide a continuous supply of the lead PDC for future clinical trials and eventually commercialization of the PDC for human bladder cancer applications.
Roger Dumoulin-White, President and CEO of Theralase Inc. stated that, "Theralase is delighted that we have partnered with SAFC in the development and manufacture of our PDCs, as they have the depth and experience to execute on the manufacture of our PDCs, quickly, efficiently and in whatever quantity is required. SAFC brings a very sophisticated and experienced team of analytical and developmental chemists to the table, whose expertise will prove indispensable in the development and commercialization of our anti-cancer technology."
Dr. Arkady Mandel, Chief Scientific Officer of Theralase Inc. stated that, "SAFC has been selected as our chosen partner in the development of our PDC platform because of their manufacturing expertise. I was extremely impressed with the knowledge and experience that their team brings to the development of our PDCs."
About Theralase Technologies Inc.
Founded in 1994, Theralase Technologies Inc. ("Theralase(R)") (TSXV: TLT) (TLTFF: OTC Link(R)) designs, manufactures and markets patented super-pulsed laser technology used for the elimination of pain, reduction of inflammation and dramatic acceleration of tissue healing. Theralase has sold over 1,200 systems to licensed healthcare practitioners such as: medical doctors, chiropractors, physical therapists and athletic therapists. Theralase has been very successful in healing nerve, muscle and joint conditions in clinical practice and now Theralase's scientists are investigating the application of its lasers in the destruction of cancer using specially designed molecules called Photo Dynamic Compounds ("PDCs"), which localize to the DNA of cancer cells and then, when light activated, destroy the cancer cells.
Additional information is available at www.theralase.com and www.sedar.com. "
http://app.quotemedia.com/quotetools/newsStoryPopup.go?story…
"Toronto, Ontario / TNW-ACCESSWIRE / October 7, 2014 / Theralase Technologies Inc. ("Theralase(R)") (TSXV: TLT) (TLTFF: OTC Link(R)) announced today that it has collaborated with Sigma-Aldrich Corporation's (NASDAQ: SIAL) custom manufacturing services business unit SAFC(R) Commercial , in the manufacture of the Photo Dynamic Compounds ("PDCs") being developed by Theralase for the destruction of cancer.
Under the terms of the agreement, SAFC will develop the Standard Operating Procedures ("SOPs") to manufacture initial quantities of each of Theralase's four lead PDCs. Once Theralase selects the final lead PDC at the end of the fourth quarter 2014, SAFC will manufacture both a 100-gram pre-Good Manufacturing Practice ("pre-GMP") batch and finally a 500-gram GMP batch of the PDC. This final 500-gram batch is intended to be suitable for final sterilization and packaging into individual patient doses for safety and efficacy evaluation through a Health Canada / FDA Phase I/IIa clinical trial, expected in early 2015.
SAFC is Theralase's partner of choice to provide a continuous supply of the lead PDC for future clinical trials and eventually commercialization of the PDC for human bladder cancer applications.
Roger Dumoulin-White, President and CEO of Theralase Inc. stated that, "Theralase is delighted that we have partnered with SAFC in the development and manufacture of our PDCs, as they have the depth and experience to execute on the manufacture of our PDCs, quickly, efficiently and in whatever quantity is required. SAFC brings a very sophisticated and experienced team of analytical and developmental chemists to the table, whose expertise will prove indispensable in the development and commercialization of our anti-cancer technology."
Dr. Arkady Mandel, Chief Scientific Officer of Theralase Inc. stated that, "SAFC has been selected as our chosen partner in the development of our PDC platform because of their manufacturing expertise. I was extremely impressed with the knowledge and experience that their team brings to the development of our PDCs."
About Theralase Technologies Inc.
Founded in 1994, Theralase Technologies Inc. ("Theralase(R)") (TSXV: TLT) (TLTFF: OTC Link(R)) designs, manufactures and markets patented super-pulsed laser technology used for the elimination of pain, reduction of inflammation and dramatic acceleration of tissue healing. Theralase has sold over 1,200 systems to licensed healthcare practitioners such as: medical doctors, chiropractors, physical therapists and athletic therapists. Theralase has been very successful in healing nerve, muscle and joint conditions in clinical practice and now Theralase's scientists are investigating the application of its lasers in the destruction of cancer using specially designed molecules called Photo Dynamic Compounds ("PDCs"), which localize to the DNA of cancer cells and then, when light activated, destroy the cancer cells.
Additional information is available at www.theralase.com and www.sedar.com. "
Theralase(V.TLT) 'further demonstrates 'phenomenal cancer immunity response', in testing' - SH - Nov 12, 2014
- Gaalen Engen -
http://theralase.com/pressrelease/theralase-validates-anti-c…
www.stockhouse.com/news/newswire/2014/11/12/theralase-v-tlt-…
"Theralase Technologies (TSX: V.TLT, Stock Forum) celebrated today when the company released the results from new preclinical research carried out at the Princess Margaret Cancer Centre, University Health Network (“UHN”) located in Toronto, Canada.
According to the news release, trial results further confirmed the company's Photo Dynamic Compound's (“PDCs”) ability to destroy a primary host tumour and then render the animal immune to a repeated exposure of the same cancer.
Testing performed by UHN in March 2012 involved mice being injected with 350K colon cancer cells to generate cancerous tumours that were allowed to grow five millimetres in size before treating the tumours with PDCs and Near Infrared (“NIR”) light to activate the PDC. The vast majority of tumours were destroyed as a result and the mice remained cancer-free for 20 months post-treatment.
In May 2014, the UHN again treated mice with the same protocol and those mice who successfully responded to the Photo Dynamic Therapy (“PDT”) were re-injected with an equal amount of colon cancer cells 10-23 days later. With no further treatment intervention, 60% of the mice did not demonstrate tumour re-growth, while 40% had a small tumour regrowth that was quickly destroyed.
The aforementioned results pose the possibility of short-term immune-mediated “memory response” that destroys cancer cells with no further intervention.
Research in November 2014 at UHN, mice were once again treated according to the March 2012 protocol and all of the mice who received PDT were successfully treated and remained cancer-free. These mice were then injected with an equal amount of colon cancer cells 20 days later and with no further treatment intervention none of the mice demonstrated tumour re-growth. Further confirming an ability to destroy the primary tumour and strongly suggesting a protection of the animal via the immune system leading to a short-term immune-mediated “memory response”.
Incredibly good news for the company and the world in general as it suggests a major breakthrough in the treatment of cancer, providing substantial treatment benefits and survival advantages to cancer patients
The company's patented technology has so far shown itself able to rapidly and effectively destroy “patient-specific” cancer cells, prevent their recurrence and provide long-lasting protection against local and distant metastasis, an immense clinical benefit to cancer patients and the facilities that treat their disease.
Company Chief Scientific Officer, Dr. Arkady Mandel, commented on the landmark confirmatory findings, “Our primary focus since the initial findings of the PDT memory response in May 2014 has been to further understand and validate this phenomenal effect.”
He went on to illustrate, “Our research program has achieved this mandate by demonstrating that Theralase's NIR PDT destroys the primary tumour and suggests a short term memory response, even when the animal is further challenged with a new round of cancer cells 20 days later, thus preventing the animal from developing cancer.”
Then explained, “Our latest research suggests that our original hypothesis that "memory cells", such as memory T lymphocytes, part of the immune system, may be reprogrammed to "search and destroy" the threat posed by cancer cells.”
And finally concluded, “This is a very important step toward our long-term goal of developing an affordable and practical vaccine to prevent cancer recurrence.”
Roger Dumoulin-White, Company President and CEO, added, “I am delighted that our scientific and preclinical research teams continue to further understand and validate this effect to allow us to prepare for clinical validation in human patients. Iiiiif we are able to validate the effect in humans, then the implications of this discovery are nothing short of game changing for both Theralase and more importantly for cancer patients.”
Human trials are still in the distance, but these amazing results are building the necessary framework to establish those tests and Theralase has shown itself to have both the management and medical skills and relationships to take this process to commercialization and quite possibly radically impact the sector, giving cancer patients a far less invasive yet thoroughly effective life-saving treatment.
There are hurdles left to be cleared, but the company's path seems pointed toward success and smart investors should be taking note of its continued progress. I know I am.
Theralase was in the news recently when the Toronto-based company announced in the middle of October that it would present its latest research on the destruction of cancer and bacteria at 10th International Symposium on Photodynamic Therapy and Photodiagnosis in Clinical Practice.
Shares climbed 13.43% on the news to $0.38 per share.
Currently there are 82.8m outstanding shares with a market cap of $31.5 million. "
- Gaalen Engen -
http://theralase.com/pressrelease/theralase-validates-anti-c…
www.stockhouse.com/news/newswire/2014/11/12/theralase-v-tlt-…
"Theralase Technologies (TSX: V.TLT, Stock Forum) celebrated today when the company released the results from new preclinical research carried out at the Princess Margaret Cancer Centre, University Health Network (“UHN”) located in Toronto, Canada.
According to the news release, trial results further confirmed the company's Photo Dynamic Compound's (“PDCs”) ability to destroy a primary host tumour and then render the animal immune to a repeated exposure of the same cancer.
Testing performed by UHN in March 2012 involved mice being injected with 350K colon cancer cells to generate cancerous tumours that were allowed to grow five millimetres in size before treating the tumours with PDCs and Near Infrared (“NIR”) light to activate the PDC. The vast majority of tumours were destroyed as a result and the mice remained cancer-free for 20 months post-treatment.
In May 2014, the UHN again treated mice with the same protocol and those mice who successfully responded to the Photo Dynamic Therapy (“PDT”) were re-injected with an equal amount of colon cancer cells 10-23 days later. With no further treatment intervention, 60% of the mice did not demonstrate tumour re-growth, while 40% had a small tumour regrowth that was quickly destroyed.
The aforementioned results pose the possibility of short-term immune-mediated “memory response” that destroys cancer cells with no further intervention.
Research in November 2014 at UHN, mice were once again treated according to the March 2012 protocol and all of the mice who received PDT were successfully treated and remained cancer-free. These mice were then injected with an equal amount of colon cancer cells 20 days later and with no further treatment intervention none of the mice demonstrated tumour re-growth. Further confirming an ability to destroy the primary tumour and strongly suggesting a protection of the animal via the immune system leading to a short-term immune-mediated “memory response”.
Incredibly good news for the company and the world in general as it suggests a major breakthrough in the treatment of cancer, providing substantial treatment benefits and survival advantages to cancer patients
The company's patented technology has so far shown itself able to rapidly and effectively destroy “patient-specific” cancer cells, prevent their recurrence and provide long-lasting protection against local and distant metastasis, an immense clinical benefit to cancer patients and the facilities that treat their disease.
Company Chief Scientific Officer, Dr. Arkady Mandel, commented on the landmark confirmatory findings, “Our primary focus since the initial findings of the PDT memory response in May 2014 has been to further understand and validate this phenomenal effect.”
He went on to illustrate, “Our research program has achieved this mandate by demonstrating that Theralase's NIR PDT destroys the primary tumour and suggests a short term memory response, even when the animal is further challenged with a new round of cancer cells 20 days later, thus preventing the animal from developing cancer.”
Then explained, “Our latest research suggests that our original hypothesis that "memory cells", such as memory T lymphocytes, part of the immune system, may be reprogrammed to "search and destroy" the threat posed by cancer cells.”
And finally concluded, “This is a very important step toward our long-term goal of developing an affordable and practical vaccine to prevent cancer recurrence.”
Roger Dumoulin-White, Company President and CEO, added, “I am delighted that our scientific and preclinical research teams continue to further understand and validate this effect to allow us to prepare for clinical validation in human patients. Iiiiif we are able to validate the effect in humans, then the implications of this discovery are nothing short of game changing for both Theralase and more importantly for cancer patients.”
Human trials are still in the distance, but these amazing results are building the necessary framework to establish those tests and Theralase has shown itself to have both the management and medical skills and relationships to take this process to commercialization and quite possibly radically impact the sector, giving cancer patients a far less invasive yet thoroughly effective life-saving treatment.
There are hurdles left to be cleared, but the company's path seems pointed toward success and smart investors should be taking note of its continued progress. I know I am.
Theralase was in the news recently when the Toronto-based company announced in the middle of October that it would present its latest research on the destruction of cancer and bacteria at 10th International Symposium on Photodynamic Therapy and Photodiagnosis in Clinical Practice.
Shares climbed 13.43% on the news to $0.38 per share.
Currently there are 82.8m outstanding shares with a market cap of $31.5 million. "
Theralase Releases Annual General Meeting Video
...da geht was zur Zeit...........
M.
M.
Antwort auf Beitrag Nr.: 48.566.648 von manffreddoo am 14.12.14 11:25:07bis wie weit kann das ding noch steigen?
.......
hab keine Glaskugel...........
ausgehend von der momentanen MK und dem Marktpotential um einiges..........
Rücksetzer sind immer möglich bei Marktschwäche,auch zurück auf Anfang wenn der Markt das Produkt ignoriert
M.
hab keine Glaskugel...........
ausgehend von der momentanen MK und dem Marktpotential um einiges..........
Rücksetzer sind immer möglich bei Marktschwäche,auch zurück auf Anfang wenn der Markt das Produkt ignoriert
M.
...kleine Korrektur abgeschlossen??
Auf zu neuen HÖHEN???
M.
Auf zu neuen HÖHEN???
M.
Theralase(V.TLT) 'celebrates positive test results, from revolutionary bladder cancer treatment' - SHE - Dec 23, 2014
www.stockhouse.com/news/press-releases/2014/12/23/theralase-…
www.stockhouse.com/news/newswire/2014/12/23/theralase-v-tlt-…
"Theralase Technologies (TSX: V.TLT, Stock Forum) had reason to break out the champagne today when the company announced preliminary results from the use of Photo Dynamic Therapy (“PDT”) to destroy bladder cancer in an orthotopic (“occurring at the normal place in the body”) rat model.
According to the news release, the University of Toledo along with the Princess Margaret Cancer Centre, University Health Network and Theralase carried out the experiment in a well-established orthotopic model for bladder cancer in rats.
Results from the experiment showed that from visual inspection, TLD1433 appeared to localize to specific regions of the bladder, indicating an accumulation in the damaged and tumor urothelium with areas of tumor necrosis spotted in the treated bladders.
All of this suggested that TLD1433 used with visible red laser light has the potential to induce urothelial tumor destruction with an exceptionally high margin of safety for the subject.
The company will continue to evaluate these preliminary experiments for further confirmation that laser light activated TLD1433 creates exacting tumor necrosis while preserving the normal bladder wall, sparing the surrounding urothelium. As a result, this treatment may offer versatility beyond what is available today with traditional bladder cancer treatment methodologies.
TLD1433 is undergoing the final stages of pre-clinical optimization in preparation for a Health Canada and FDA human Phase I / IIa clinical study for this new class of PDCs in 2015.
Chair and Professor of Urology and Director of Urological Research, University of Toledo, Steve Selman, MD, FACS, commented on the results, “I am pleased to report that this preliminary study warrants further evaluation of the Theralase PDCs in their ability to localize to compromised urothelium and when laser light activated destroy the bladder cancer tumours via necrosis. This is early research, with non- optimized doses of PDC and laser light sources, but it is encouraging. Further evaluation with additional treated and control animals, along with additional blinded histopathological analysis will more definitely support these initial finding and provide us the scientific rigour required to strongly support out initial clinical findings.”
Roger Dumoulin-White, company President and CEO, added, “I am elated that Theralase is able to report the successful achievement of this very significant milestone in our ongoing collaborative research and development program for our state-of-the-art PDC technology.”
He went on to explain,”It has always been Theralase's strategic milestone to prove the safety and efficacy of our PDC technology in an orthotopic rat model to provide our shareholders and investors the assurance that our technology has been validated to be safe and effective in animals prior to the Theralase team turning their attention to evaluating this technology in a human clinical study.”
Then he summed up, “I am proud to be associated with such a strong scientific and clinical team that was able to accomplish this very significant milestone. 2015 is going to be extremely exciting year for the Company.”
Theralase Technologies was in the news recently when the Toronto-based company announced a week ago that Brian C. Wilson had joined its Medical and Scientific Advisory Board.
Currently there are 84.8m outstanding shares with a market cap of $60.2 million. "
www.stockhouse.com/news/press-releases/2014/12/23/theralase-…
www.stockhouse.com/news/newswire/2014/12/23/theralase-v-tlt-…
"Theralase Technologies (TSX: V.TLT, Stock Forum) had reason to break out the champagne today when the company announced preliminary results from the use of Photo Dynamic Therapy (“PDT”) to destroy bladder cancer in an orthotopic (“occurring at the normal place in the body”) rat model.
According to the news release, the University of Toledo along with the Princess Margaret Cancer Centre, University Health Network and Theralase carried out the experiment in a well-established orthotopic model for bladder cancer in rats.
Results from the experiment showed that from visual inspection, TLD1433 appeared to localize to specific regions of the bladder, indicating an accumulation in the damaged and tumor urothelium with areas of tumor necrosis spotted in the treated bladders.
All of this suggested that TLD1433 used with visible red laser light has the potential to induce urothelial tumor destruction with an exceptionally high margin of safety for the subject.
The company will continue to evaluate these preliminary experiments for further confirmation that laser light activated TLD1433 creates exacting tumor necrosis while preserving the normal bladder wall, sparing the surrounding urothelium. As a result, this treatment may offer versatility beyond what is available today with traditional bladder cancer treatment methodologies.
TLD1433 is undergoing the final stages of pre-clinical optimization in preparation for a Health Canada and FDA human Phase I / IIa clinical study for this new class of PDCs in 2015.
Chair and Professor of Urology and Director of Urological Research, University of Toledo, Steve Selman, MD, FACS, commented on the results, “I am pleased to report that this preliminary study warrants further evaluation of the Theralase PDCs in their ability to localize to compromised urothelium and when laser light activated destroy the bladder cancer tumours via necrosis. This is early research, with non- optimized doses of PDC and laser light sources, but it is encouraging. Further evaluation with additional treated and control animals, along with additional blinded histopathological analysis will more definitely support these initial finding and provide us the scientific rigour required to strongly support out initial clinical findings.”
Roger Dumoulin-White, company President and CEO, added, “I am elated that Theralase is able to report the successful achievement of this very significant milestone in our ongoing collaborative research and development program for our state-of-the-art PDC technology.”
He went on to explain,”It has always been Theralase's strategic milestone to prove the safety and efficacy of our PDC technology in an orthotopic rat model to provide our shareholders and investors the assurance that our technology has been validated to be safe and effective in animals prior to the Theralase team turning their attention to evaluating this technology in a human clinical study.”
Then he summed up, “I am proud to be associated with such a strong scientific and clinical team that was able to accomplish this very significant milestone. 2015 is going to be extremely exciting year for the Company.”
Theralase Technologies was in the news recently when the Toronto-based company announced a week ago that Brian C. Wilson had joined its Medical and Scientific Advisory Board.
Currently there are 84.8m outstanding shares with a market cap of $60.2 million. "
Theralase Announces Proposed Public Offering, of Units - Feb 24, 2015
www.marketwired.com/press-release/theralase-announces-propos…
"Theralase Technologies Inc. ("Theralase" or the "Corporation") (TSX VENTURE:TLT) (OTC PINK:TLFF) is pleased to announce that it proposes to offer for sale a minimum of 9,090,910 units of the Corporation (each, a "Unit") and a maximum of 18,181,819 Units at a price of $0.44 per Unit (the "Offering Price") for minimum aggregate gross proceeds of approximately $4,000,000 and maximum aggregate gross proceeds of approximately $8,000,000 (the "Offering").
Each Unit will consist of one common share of the Corporation (each, a "Common Share") and one common share purchase warrant (each, a "Warrant"). Each Warrant will entitle the holder to acquire an additional Common Share (each a "Warrant Share") at a price of $0.54 for a period of 60 months following the date of issuance.
In connection with the Offering, the Corporation has engaged Euro Pacific Canada Inc. (the "Agent") to offer the Units for sale to the public on a commercially reasonable efforts basis. For its services, the Agent will receive a cash commission equal to 8.0% of the gross proceeds raised under the Offering (exclusive of proceeds raised from the President's List) and that number of non-transferable broker warrants equal to 5.0% of the number of Units sold (exclusive of Units sold to subscribers on the President's List). Each broker warrant will be exercisable into one Unit for a period of 60 months from the closing of the Offering at a price of $0.54 per Unit.
The Offering is expected to close on or about March 2, 2015. The Offering is subject to a number of conditions, including, without limitation, receipt of all regulatory approvals (including TSX Venture Exchange approval). The Units will be qualified for sale by way of a prospectus supplement to the Corporation's short form base shelf prospectus dated January 9, 2015, which will be filed in the Provinces of British Columbia, Alberta and Ontario.
The net proceeds of the Offering will be used to fund research and development activities by Theralase's Photo Dynamic Therapy (PDT) Division, commercialization activities by Theralase's Therapeutic Laser Technology (TLT) Division and for working capital and general corporate purposes.
The securities offered have not been registered under the U.S. Securities Act of 1933, as amended, and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements. This press release shall not constitute an offer to sell or a solicitation of an offer to buy nor shall there be any sale of the securities offered in any jurisdiction in which such offer, solicitation or sale would be unlawful.
About Theralase
Founded in 1994, Theralase (TSX VENTURE:TLT)(OTC PINK:TLFF) designs, manufactures and markets patented super-pulsed laser technology used for the elimination of pain, reduction of inflammation and dramatic acceleration of tissue healing. Theralase has sold over 1,200 systems to licensed healthcare practitioners, including medical doctors, chiropractors, physical therapists and athletic therapists. Theralase has been so successful in healing nerve, muscle and joint conditions in clinical practice that Theralase's scientists and clinicians have now turned their attention to investigating the application of its lasers in the destruction of cancer, using specially designed molecules called Photo Dynamic Compounds, which are able to localize to the cancer cells and when light activated, destroy them.
Additional information is available at www.theralase.com and www.sedar.com.
Theralase was recognized as a TSX Venture 50® company in 2015. TSX Venture 50 is a trademark of TSX Inc. and is used under license. "
www.marketwired.com/press-release/theralase-announces-propos…
"Theralase Technologies Inc. ("Theralase" or the "Corporation") (TSX VENTURE:TLT) (OTC PINK:TLFF) is pleased to announce that it proposes to offer for sale a minimum of 9,090,910 units of the Corporation (each, a "Unit") and a maximum of 18,181,819 Units at a price of $0.44 per Unit (the "Offering Price") for minimum aggregate gross proceeds of approximately $4,000,000 and maximum aggregate gross proceeds of approximately $8,000,000 (the "Offering").
Each Unit will consist of one common share of the Corporation (each, a "Common Share") and one common share purchase warrant (each, a "Warrant"). Each Warrant will entitle the holder to acquire an additional Common Share (each a "Warrant Share") at a price of $0.54 for a period of 60 months following the date of issuance.
In connection with the Offering, the Corporation has engaged Euro Pacific Canada Inc. (the "Agent") to offer the Units for sale to the public on a commercially reasonable efforts basis. For its services, the Agent will receive a cash commission equal to 8.0% of the gross proceeds raised under the Offering (exclusive of proceeds raised from the President's List) and that number of non-transferable broker warrants equal to 5.0% of the number of Units sold (exclusive of Units sold to subscribers on the President's List). Each broker warrant will be exercisable into one Unit for a period of 60 months from the closing of the Offering at a price of $0.54 per Unit.
The Offering is expected to close on or about March 2, 2015. The Offering is subject to a number of conditions, including, without limitation, receipt of all regulatory approvals (including TSX Venture Exchange approval). The Units will be qualified for sale by way of a prospectus supplement to the Corporation's short form base shelf prospectus dated January 9, 2015, which will be filed in the Provinces of British Columbia, Alberta and Ontario.
The net proceeds of the Offering will be used to fund research and development activities by Theralase's Photo Dynamic Therapy (PDT) Division, commercialization activities by Theralase's Therapeutic Laser Technology (TLT) Division and for working capital and general corporate purposes.
The securities offered have not been registered under the U.S. Securities Act of 1933, as amended, and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements. This press release shall not constitute an offer to sell or a solicitation of an offer to buy nor shall there be any sale of the securities offered in any jurisdiction in which such offer, solicitation or sale would be unlawful.
About Theralase
Founded in 1994, Theralase (TSX VENTURE:TLT)(OTC PINK:TLFF) designs, manufactures and markets patented super-pulsed laser technology used for the elimination of pain, reduction of inflammation and dramatic acceleration of tissue healing. Theralase has sold over 1,200 systems to licensed healthcare practitioners, including medical doctors, chiropractors, physical therapists and athletic therapists. Theralase has been so successful in healing nerve, muscle and joint conditions in clinical practice that Theralase's scientists and clinicians have now turned their attention to investigating the application of its lasers in the destruction of cancer, using specially designed molecules called Photo Dynamic Compounds, which are able to localize to the cancer cells and when light activated, destroy them.
Additional information is available at www.theralase.com and www.sedar.com.
Theralase was recognized as a TSX Venture 50® company in 2015. TSX Venture 50 is a trademark of TSX Inc. and is used under license. "
Diese immense Verwässerung kommt offensichtlich nicht gut an... Mal sehen, ob sie so überhaupt noch das Closing schaffen. Die Aktie ist jedenfalls für's erste "tot". Schaue ich mir in 9-12 Monaten wieder an.
M@trix
M@trix
Theralase(V.TLT) produces 1st pre-commercial lot of lead anti-cancer drug - SHE - Apr 9, 2015
http://theralase.com/pressrelease/theralase-manufactures-fir…
www.stockhouse.com/news/newswire/2015/04/09/theralase-v-tlt-…
"Theralase Technologies (TSX: V.TLT, Stock Forum) made significant headway in bringing its lead anti-cancer drug to market when the company announced today that it had manufactured its first pre-commercial batch of Photo Dynamic Compound (“PDC”), TLD-1433.
According to the news release, Good Manufacturing Practices (“GMP”) must be followed when producing drugs intended for use in humans and as a result, said drugs have to go through a “scale up” process in production from a small quantity to validate manufacturing process to larger pre-commercial quantities produced under the GMP standards for human use.
The company has achieved pre-commercial production status and will be releasing the production lot to Health Canada for toxicology testing, a key component to Health Canada Clinical Trial Application (“CTA”).
A second pre-commercial batch is scheduled to be produced in Q2 2015 to continue optimizing the manufacturing process and provide research materials for testing of additional cancer indications of TLD-1433. Production of a commercial batch adhering to human use GMP standards is expected to be complete in Q2 2015.
Company Chief Scientific Officer, Dr. Arkady Mandel, commented, “We have been successful in establishing a robust scale-up manufacturing process for our anti-cancer drugs. We have managed to transfer the manufacturing process knowledge of TLD-1433 from the laboratory to pre-commercial production levels, able to fulfill requirements to initiate the toxicology program. It has been demonstrated repeatedly that our lead PDC is effective in the treatment of cancer in animal models and we look forward to effectively treating cancer in humans in a clinical setting.”
He explained, “The successful production of the first pre-commercial batch of our lead PDC, TLD-1433, represents one of the most important milestones to date in the history of Theralase's anti-cancer program, leading to commercial production of advanced biopharmaceuticals for the treatment of cancer.”
Roger Dumoulin-White, President and CEO of Theralase, added, “Theralase is extremely well positioned to commence our Health Canada Phase Ib human clinical studies in bladder cancer in 4Q2015, now that we have successfully assembled a top notch team consisting of: cutting-edge research scientists, world renowned uro-oncologists, world-class manufacturing, an internationally recognized toxicology lab and a strong clinical research organization. 2015 will be a landmark year for the Company, as we execute on our strategic initiatives.”
Theralase Technologies was in the news recently when the Toronto-based company announced holding a pre-CTA meeting with Health Canada a week ago.
Shares gained 3.03% on the news to $0.34 per share.
Currently there are 104.6m outstanding shares with a market cap of $35.6 million. "
http://theralase.com/pressrelease/theralase-manufactures-fir…
www.stockhouse.com/news/newswire/2015/04/09/theralase-v-tlt-…
"Theralase Technologies (TSX: V.TLT, Stock Forum) made significant headway in bringing its lead anti-cancer drug to market when the company announced today that it had manufactured its first pre-commercial batch of Photo Dynamic Compound (“PDC”), TLD-1433.
According to the news release, Good Manufacturing Practices (“GMP”) must be followed when producing drugs intended for use in humans and as a result, said drugs have to go through a “scale up” process in production from a small quantity to validate manufacturing process to larger pre-commercial quantities produced under the GMP standards for human use.
The company has achieved pre-commercial production status and will be releasing the production lot to Health Canada for toxicology testing, a key component to Health Canada Clinical Trial Application (“CTA”).
A second pre-commercial batch is scheduled to be produced in Q2 2015 to continue optimizing the manufacturing process and provide research materials for testing of additional cancer indications of TLD-1433. Production of a commercial batch adhering to human use GMP standards is expected to be complete in Q2 2015.
Company Chief Scientific Officer, Dr. Arkady Mandel, commented, “We have been successful in establishing a robust scale-up manufacturing process for our anti-cancer drugs. We have managed to transfer the manufacturing process knowledge of TLD-1433 from the laboratory to pre-commercial production levels, able to fulfill requirements to initiate the toxicology program. It has been demonstrated repeatedly that our lead PDC is effective in the treatment of cancer in animal models and we look forward to effectively treating cancer in humans in a clinical setting.”
He explained, “The successful production of the first pre-commercial batch of our lead PDC, TLD-1433, represents one of the most important milestones to date in the history of Theralase's anti-cancer program, leading to commercial production of advanced biopharmaceuticals for the treatment of cancer.”
Roger Dumoulin-White, President and CEO of Theralase, added, “Theralase is extremely well positioned to commence our Health Canada Phase Ib human clinical studies in bladder cancer in 4Q2015, now that we have successfully assembled a top notch team consisting of: cutting-edge research scientists, world renowned uro-oncologists, world-class manufacturing, an internationally recognized toxicology lab and a strong clinical research organization. 2015 will be a landmark year for the Company, as we execute on our strategic initiatives.”
Theralase Technologies was in the news recently when the Toronto-based company announced holding a pre-CTA meeting with Health Canada a week ago.
Shares gained 3.03% on the news to $0.34 per share.
Currently there are 104.6m outstanding shares with a market cap of $35.6 million. "
Theralase habe ich seit den ersten fantastischen Ergebnissen von PDT bei Mäusen auf der Watchlist. Nun wird es spannend. Wenn die Ergebnisse bei Menschen auch nur annähernd ähnlich erfolgreich ausfallen und sich keine markanten Nebenwirkungen zeigen, dann haben wir hier einen möglichen Vervielfacher.
M@trix
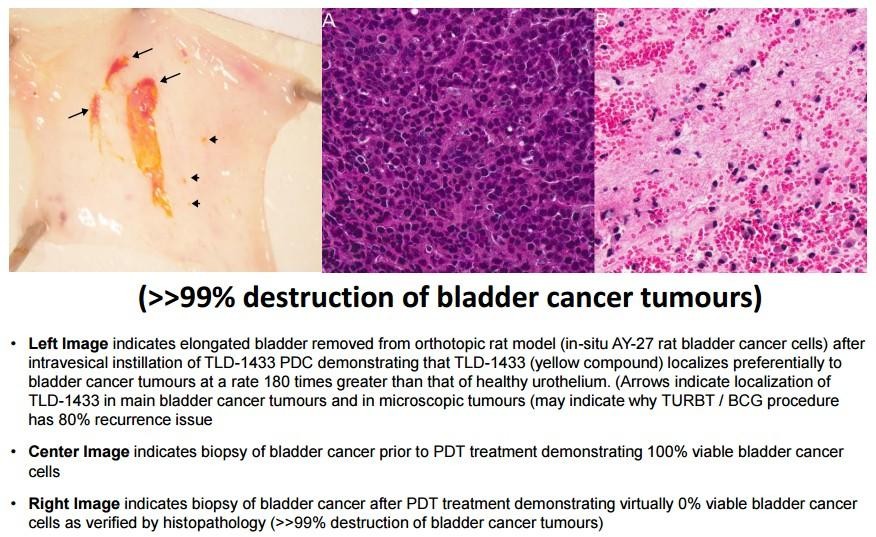
M@trix

Antwort auf Beitrag Nr.: 54.679.046 von M@trix am 05.04.17 09:53:04Hallo M@trix,
darf ich fragen ob du schon eingestiegen bist? Habe TLT bis vor ca. einem Jahr auch verfolgt und schon im Depot gehabt.
Übrigens, vielen Dank für deine sehr nützlichen Beiträge.
Barney-Gumble
darf ich fragen ob du schon eingestiegen bist? Habe TLT bis vor ca. einem Jahr auch verfolgt und schon im Depot gehabt.
Übrigens, vielen Dank für deine sehr nützlichen Beiträge.
Barney-Gumble
kommt der schöne Up-move der letzten Tage nur von den Insider Käufen oder kauft sich hier jemand anderes ein?
Meines Wissens werden so bald keine News erwartet, daher finde ich den up move eher ungewöhnlich.
Meines Wissens werden so bald keine News erwartet, daher finde ich den up move eher ungewöhnlich.
Antwort auf Beitrag Nr.: 54.799.282 von 2brix am 25.04.17 13:52:30Der Kurs steigt, weil nun einmal die klinischen Tests begonnen haben. Und wenn dieser Satz hier stimmt, dürften positive Ergebnisse sehr wahrscheinlich sein:
On the plus side of their potential success is that there have been two previously FDA approved PDC drugs; specifically: Aminolevulinic Acid (ALA) and Photofrin®, which have been FDA approved for actinic keratosis (Rough, scaly patches of skin that are considered precancerous) and esophageal cancer, respectively. Theralase to their credit has tested their new TLD-1400 series of PDCs against these two FDA approved drugs and have demonstrated in documented scientific research that they are 668,000x more effective than ALA and 198x more effective than Photofrin®, while delivering a treatment with virtually no side effects. This bodes well for a successful Phase 1/2a clinical trial. The second plus in their corner is that their TLD-1400 series of drugs are nuclear acting, meaning that they enter cancer cells and lock onto the DNA of the cell, unlike ALA and Photofrin®. When light activated, this new class of PDCs destroy the cancer cell by what is called apoptosis or natural cell death, basically the cell dies of natural causes. The beauty of nuclear acting drugs is that it doesn’t matter if the cancer cell came from a mouse, an ostrich, a dolphin or a human, they are all destroyed with the same efficacy and safety profile, as these are all mammallian cells, known collectively as eukaryotes.
So the multi-billion dollar question is not what if the Theralase technology doesn’t work as well on humans as it does on mice, but more appropriately when will Theralase deliver us positive clinical study results.
https://www.midasletter.com/2014/02/theralase-technologies-i…
Theralase zielt auf die dauerhafte Heilung von Krebs ab. Das ist etwas anderes als es zig andere Biotechs tun, deren primäres Ziel darin liegt, das Krebswachstum einzudämmen und somit das Leben des Patienten etwas zu verlängern.
Dazu auch lesenswert und recht aktuell:
http://www.stockhouse.com/news/newswire/2017/04/12/is-trojan…
"To be a cancer-killing Trojan Horse, able to destroy cancer cells from the inside requires two chemical-biological properties: the ability to slip past the tough cell walls of cancer cells plus the capacity to do damage once inside. This leads back to laser light.
Once the TLD-1433 has been given time to penetrate these cancer cells it’s time for the actual laser therapy – and the cancer-killing begins. When laser light targets cancer-diseased tissue with TLD-1433 inside, a photo-chemical reaction takes place."
"These ROS molecules are like tiny cancer-bombs. TLD-1433 generates enough of these ROS cancer-bombs to destroy cancer cells from the inside, with enormous success. Laboratory results indicate the destruction of more than 99% of cancer cells from Theralase’s PDT technology, with virtually no damage to surrounding tissue.
As impressive as this sounds, in the minds of many readers this will still not be seen as good enough. Part of cancer’s plague is its ability to recur and reproduce. Almost getting rid of cancer falls far short of a cure.
This leads to perhaps the most important aspect of PDT treatment of cancer. This form of laser therapy is a “smart” cancer treatment. Essentially, killing cancer cells with TLD-1433 + transferrin teaches the body how to fight the cancer.
Theralase’s PDT cancer treatment also functions like a cancer vaccine: permanently improving the body’s capacity to target and destroy cells of that particular type of cancer. PDT doesn’t merely fight cancer. If it can be perfected for human use, it will genuinely cure cancer – benignly. No surgery; no chemo; no radiation."
Darin liegt eine Menge Fantasie und daher der starke Kursanstieg.
Es ist indes noch viel zu früh für ein Urteil. Das ist noch immer ein sehr spekulatives Investment.
M@trix
On the plus side of their potential success is that there have been two previously FDA approved PDC drugs; specifically: Aminolevulinic Acid (ALA) and Photofrin®, which have been FDA approved for actinic keratosis (Rough, scaly patches of skin that are considered precancerous) and esophageal cancer, respectively. Theralase to their credit has tested their new TLD-1400 series of PDCs against these two FDA approved drugs and have demonstrated in documented scientific research that they are 668,000x more effective than ALA and 198x more effective than Photofrin®, while delivering a treatment with virtually no side effects. This bodes well for a successful Phase 1/2a clinical trial. The second plus in their corner is that their TLD-1400 series of drugs are nuclear acting, meaning that they enter cancer cells and lock onto the DNA of the cell, unlike ALA and Photofrin®. When light activated, this new class of PDCs destroy the cancer cell by what is called apoptosis or natural cell death, basically the cell dies of natural causes. The beauty of nuclear acting drugs is that it doesn’t matter if the cancer cell came from a mouse, an ostrich, a dolphin or a human, they are all destroyed with the same efficacy and safety profile, as these are all mammallian cells, known collectively as eukaryotes.
So the multi-billion dollar question is not what if the Theralase technology doesn’t work as well on humans as it does on mice, but more appropriately when will Theralase deliver us positive clinical study results.
https://www.midasletter.com/2014/02/theralase-technologies-i…
Theralase zielt auf die dauerhafte Heilung von Krebs ab. Das ist etwas anderes als es zig andere Biotechs tun, deren primäres Ziel darin liegt, das Krebswachstum einzudämmen und somit das Leben des Patienten etwas zu verlängern.
Dazu auch lesenswert und recht aktuell:
http://www.stockhouse.com/news/newswire/2017/04/12/is-trojan…
"To be a cancer-killing Trojan Horse, able to destroy cancer cells from the inside requires two chemical-biological properties: the ability to slip past the tough cell walls of cancer cells plus the capacity to do damage once inside. This leads back to laser light.
Once the TLD-1433 has been given time to penetrate these cancer cells it’s time for the actual laser therapy – and the cancer-killing begins. When laser light targets cancer-diseased tissue with TLD-1433 inside, a photo-chemical reaction takes place."
"These ROS molecules are like tiny cancer-bombs. TLD-1433 generates enough of these ROS cancer-bombs to destroy cancer cells from the inside, with enormous success. Laboratory results indicate the destruction of more than 99% of cancer cells from Theralase’s PDT technology, with virtually no damage to surrounding tissue.
As impressive as this sounds, in the minds of many readers this will still not be seen as good enough. Part of cancer’s plague is its ability to recur and reproduce. Almost getting rid of cancer falls far short of a cure.
This leads to perhaps the most important aspect of PDT treatment of cancer. This form of laser therapy is a “smart” cancer treatment. Essentially, killing cancer cells with TLD-1433 + transferrin teaches the body how to fight the cancer.
Theralase’s PDT cancer treatment also functions like a cancer vaccine: permanently improving the body’s capacity to target and destroy cells of that particular type of cancer. PDT doesn’t merely fight cancer. If it can be perfected for human use, it will genuinely cure cancer – benignly. No surgery; no chemo; no radiation."
Darin liegt eine Menge Fantasie und daher der starke Kursanstieg.
Es ist indes noch viel zu früh für ein Urteil. Das ist noch immer ein sehr spekulatives Investment.
M@trix
Theralase: Ersten drei Patienten nach 90 Tagen Krebsfrei!
Ich wollt euch auf die Errungenschaften der kleinen kanadische Firma , Theralase Technologies Ltd., aufmerksam machen. Es scheint dies ist in Deutschland noch nicht so richtig angekommen zu sein.....
Es werden derzeit klinische Studien an Menschen für Blasenkrebs geführt. Bisher wurden die ersten drei Patienten behandelt und am 25. Juli 2017 wurde die bahnbrechenden Nachricht veröffentlicht, dass aus diesen drei Patienten keiner Anzeichen von Blasenkrebs zeigten! Dies bedeutet, dass 90 Tage nach der Behandlung, 100% Effektivität erreicht wurde (d.h. alle Tumor Zellen wurden zerstört), und es wird schon von einer Art Immunität gesprochen die schon in Mäusen nachgewiesen werden konnte!
Das ist noch nie in der Geschichte der Krebsbehandlung passiert!
Ihre Technologie wird auch fuer andere Krebsarten (Hirntumor, Prostata, Lunge ...) vorbereitet und werden bald folgen. Als nächstes wird wohl Gehirntumor (GBM) getestet.
Alle ihre Technologie ist patentiert (oder zum Patent angemeldet) und sie arbeiten mit den top Krankenhäusern und Ärzte der Welt auf dem Gebiet Blasenkrebs.
Das Potenzial dieser Technologie ist mit dieser Wirksamkeit, die deutlich höher sind als jede andere bestehende Behandlung, unglaublich.
Ich habe einige Links hinzugefügt, die einen schnellen Einblick in das Unternehmen bieten:
Http://www.stockhouse.com/news/newswire/2017/04/12/is-trojan-horse-key-to-curing-cancer
Http://www.proactiveinvestors.com/companies/news/171615/laser-specialist-theralase-technologies-primed-for-a-pivotal-year-171615.html
Https: //www.midasletter.com/2014/02/theralase-technologies-inc-cure-cancer/
Https://web.tmxmoney.com/article.php?newsid=5439517071804507&qm_symbol=TLT
Https://web.tmxmoney.com/article.php?newsid=7747100758987441&qm_symbol=TLT
Unten die bedeutendste Nachricht, die am 25.Juli über die Wirksamkeit der ersten drei Patienten herausgegeben wurden:
Https://web.tmxmoney.com/article.php?newsid=7894676571202796&qm_symbol=TLT
Wir lesen derzeit nur über die Immuntherapie, die nur in wenigen Prozent der Bevölkerung funktioniert und auch extrem niedrige Wirksamkeitswerte hat ...
Theralase hat mit den Resultaten am 25. Juli Geschichte geschrieben und lässt dabei Big Pharma alt aussehen.....
Vorklinische Tests an Lungenkrebs erreichen 100% Effektivität
die guten Neuigkeiten hoeren nicht auf....https://web.tmxmoney.com/article.php?newsid=7562553431667150…
Antwort auf Beitrag Nr.: 55.480.260 von lonade am 08.08.17 15:12:32Und wieso kackt sie so ab ? Minus 40%
Die Entwicklung der klinischen Studien ist, wenn auch in einem frühen Stadium, absolut beeindruckend. Leider schlecht finanziert, daher warte ich noch mit den Einstieg. Bleibt nun aber auf watch 

Antwort auf Beitrag Nr.: 57.990.165 von DWL33 am 15.06.18 00:22:25Absolut beeindruckend, so sehe ich das auch, 
Die Frage ist wann erkennt der Markt das Potential?
TLT hat in der gerade beendeten klinischen Studie bei 6 Personen Blasenkrebs komplett geheilt!!
Das ist nach meinem Erkenntnisstand absolut einmalig.
Momentan scheint das noch niemanden zu interessieren, aber das ändert sich, hoffentlich bald.
Gruẞ goldin
.

Die Frage ist wann erkennt der Markt das Potential?
TLT hat in der gerade beendeten klinischen Studie bei 6 Personen Blasenkrebs komplett geheilt!!
Das ist nach meinem Erkenntnisstand absolut einmalig.

Momentan scheint das noch niemanden zu interessieren, aber das ändert sich, hoffentlich bald.
Gruẞ goldin
.
Patient nach 180 Tagen Krebsfrei!
http://www.4-traders.com/THERALASE-TECHNOLOGIES-IN-20768441/…
Unglaublich, was diese kleine Firma hier entwickelt!
http://www.4-traders.com/THERALASE-TECHNOLOGIES-IN-20768441/…
Unglaublich, was diese kleine Firma hier entwickelt!
zwei gute Links die alles ueber Theralase zusammenfassen:
http://www.stockhouse.com/news/newswire/2018/06/21/theralase…
http://www.stockhouse.com/news/newswire/2018/07/17/refined-a…
http://www.stockhouse.com/news/newswire/2018/06/21/theralase…
http://www.stockhouse.com/news/newswire/2018/07/17/refined-a…
gute kurze reportage ueber Theralase auf BTV
http://www.b-tv.com/theralase-technologies-feature-ep-329/
http://www.b-tv.com/theralase-technologies-feature-ep-329/
es wird eine laengere Reportage dieses Wochenende gesendet:
http://www.b-tv.com/btv-ep-329-press-release/
BTV Reveals Novel Cancer Treatment, Cannabis And Lithium Companies
News
By Editor Last updated Jul 25, 2018
Share
Thursday July 26, 2018 Vancouver, BC
On BNN Sat July 28 & Sun July 29, 2018 – BTV-Business Television goes on location with four small cap companies in popular sectors. Full Episode
BlissCo Cannabis Corp. (BLIS:CSE) – BTV shares how this CEO’s distribution background will help his company profit in the rapidly expanding cannabis market. See Feature
Critical Elements Corp. (CRE:TSX.V, CRECF:OTCQX) – Electric vehicle demand spells opportunity for the key ingredient needed: lithium. BTV visits this impressive Quebec lithium project. See Feature
FSD Pharma Inc. (HUGE:CSE) – Headquartered at the former Kraft plant in ON, in short order, this cannabis company surpassed one billion shares traded on the CSE. See Feature
Theralase Technologies Inc. (TLT:TSX.V, TLTFF:OTCQX) – BTV reveals their innovative medical technology for bladder cancer treatment. See Feature
Including comments from Managing Director at GMP Securities L.P., Martin Landry. See Feature
Plus, Junior Mining Monthly Editor, Gerardo Del Real, weighs on lithium. See Feature
BTV, a half-hour weekly investment program, profiles emerging companies across Canada and the US to bring investors information for their portfolio. With Host Taylor Thoen, BTV interviews experts, top analysts, plus features companies at their location for an insightful business perspective.
BTV BROADCAST TIMES:
CANADA: BNN – Saturday July 28 @ 8:00pm EST, Sunday July 29 @ 9:30pm EST
Bell Express Vu – Saturday July 28 @ 8:00pm EST, Sunday July 29 @ 9:30pm EST
Air Canada: TV Seatback: Business Channel
U.S. National:
Biz Television Network – Sun August 5 @ 10:00pm & 4:30pm PST, Sat August 11 @ 9:00pm PST
Submit a Company for upcoming BTV episodes:
Contact: (604) 664-7401 x3 info@...
http://www.b-tv.com/btv-ep-329-press-release/
BTV Reveals Novel Cancer Treatment, Cannabis And Lithium Companies
News
By Editor Last updated Jul 25, 2018
Share
Thursday July 26, 2018 Vancouver, BC
On BNN Sat July 28 & Sun July 29, 2018 – BTV-Business Television goes on location with four small cap companies in popular sectors. Full Episode
BlissCo Cannabis Corp. (BLIS:CSE) – BTV shares how this CEO’s distribution background will help his company profit in the rapidly expanding cannabis market. See Feature
Critical Elements Corp. (CRE:TSX.V, CRECF:OTCQX) – Electric vehicle demand spells opportunity for the key ingredient needed: lithium. BTV visits this impressive Quebec lithium project. See Feature
FSD Pharma Inc. (HUGE:CSE) – Headquartered at the former Kraft plant in ON, in short order, this cannabis company surpassed one billion shares traded on the CSE. See Feature
Theralase Technologies Inc. (TLT:TSX.V, TLTFF:OTCQX) – BTV reveals their innovative medical technology for bladder cancer treatment. See Feature
Including comments from Managing Director at GMP Securities L.P., Martin Landry. See Feature
Plus, Junior Mining Monthly Editor, Gerardo Del Real, weighs on lithium. See Feature
BTV, a half-hour weekly investment program, profiles emerging companies across Canada and the US to bring investors information for their portfolio. With Host Taylor Thoen, BTV interviews experts, top analysts, plus features companies at their location for an insightful business perspective.
BTV BROADCAST TIMES:
CANADA: BNN – Saturday July 28 @ 8:00pm EST, Sunday July 29 @ 9:30pm EST
Bell Express Vu – Saturday July 28 @ 8:00pm EST, Sunday July 29 @ 9:30pm EST
Air Canada: TV Seatback: Business Channel
U.S. National:
Biz Television Network – Sun August 5 @ 10:00pm & 4:30pm PST, Sat August 11 @ 9:00pm PST
Submit a Company for upcoming BTV episodes:
Contact: (604) 664-7401 x3 info@...
ein paar updates
Patient nach 9 Monaten immer noch Krebsfrei....nach nur einer Behandlung
http://www.stockhouse.com/news/newswire/2018/11/15/cancer-br…
https://mobile.tmxmoney.com/quote/readnews/?id=8084532108174…
Patient nach 9 Monaten immer noch Krebsfrei....nach nur einer Behandlung
http://www.stockhouse.com/news/newswire/2018/11/15/cancer-br…
https://mobile.tmxmoney.com/quote/readnews/?id=8084532108174…
Echt super, bin wirklich begeistert. Sowohl therapeutisch als auch kursmässig gesehen. Eine der besten Biotech Aktien die es gibt.
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| -1,44 | |
| -2,39 | |
| -0,07 | |
| +1,20 | |
| -0,08 | |
| 0,00 | |
| -6,12 | |
| -2,28 | |
| 0,00 | |
| -22,63 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 193 | ||
| 101 | ||
| 95 | ||
| 72 | ||
| 64 | ||
| 60 | ||
| 44 | ||
| 36 | ||
| 36 | ||
| 32 |
| Zeit | Titel |
|---|---|
| 07.02.24 |






















