Benitec – Die Biotech-Hoffnung aus Australien - 500 Beiträge pro Seite | Diskussion im Forum
eröffnet am 30.05.14 12:05:35 von
neuester Beitrag 09.07.18 12:27:22 von
neuester Beitrag 09.07.18 12:27:22 von
Beiträge: 33
ID: 1.194.930
ID: 1.194.930
Aufrufe heute: 0
Gesamt: 7.503
Gesamt: 7.503
Aktive User: 0
ISIN: AU000000BLT8 · WKN: 722783
0,0162
USD
+1,50 %
+0,0002 USD
Letzter Kurs 10.04.20 Nasdaq OTC
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,0400 | +48,57 | |
| 50,80 | +40,72 | |
| 12,810 | +39,69 | |
| 1,5890 | +39,51 | |
| 0,5400 | +38,46 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,6610 | -24,02 | |
| 5,0000 | -26,36 | |
| 28,18 | -32,62 | |
| 0,5660 | -40,42 | |
| 2,8100 | -48,72 |
Benitec könnte vor einem medizinischen Durchbruch stehen, der nicht nur die eigenen Aktionäre beglücken sondern gleichzeitig den Biotech-Riesen Gilead bis ins Mark treffen würde. Unterdessen steht Pharma-Blue Chip Pfizer (u.a. Viagra) als …
Lesen sie den ganzen Artikel: Benitec – Die Biotech-Hoffnung aus Australien
Lesen sie den ganzen Artikel: Benitec – Die Biotech-Hoffnung aus Australien
Benitec mit neuer Meldung: Die Einführung von Sponsored ADRs vereinfacht den Handel am US-OTC-Markt. Das könnte für verstärkte Nachfrage von US-Investoren sorgen. Bisher war die Aktie z.B. über Interactive Brokers nicht handelbar.
Hier die komplette Meldung:
Sydney Australia, 30 May 2014 Benitec Biopharma Limited (ASX:BLT) is establishing a sponsored Level 1 American Depositary Receipt (ADR) program facility trading in the Over-The-Counter (OTC) market in the United States. Currently, Benitec has an unsponsored ADR program in the U.S.
Benitec has appointed the Bank of New York Mellon (BNYM) as its authorised U.S. representative and Depositary Bank to establish the ADR facility.
The primary benefit of an ADR program is to widen the secondary capital market for Benitec allowing Benitec shares to be traded more easily for U.S. investors. The ADRs will be tradeable via licensed U.S. brokers in the ordinary course of trading in the OTC market in the U.S.
Benitec will release further details on the ADR sponsored program as they become available.
For further information, please contact the persons below, or visit the Benitec website at www.benitec.com.
Hier die komplette Meldung:
Sydney Australia, 30 May 2014 Benitec Biopharma Limited (ASX:BLT) is establishing a sponsored Level 1 American Depositary Receipt (ADR) program facility trading in the Over-The-Counter (OTC) market in the United States. Currently, Benitec has an unsponsored ADR program in the U.S.
Benitec has appointed the Bank of New York Mellon (BNYM) as its authorised U.S. representative and Depositary Bank to establish the ADR facility.
The primary benefit of an ADR program is to widen the secondary capital market for Benitec allowing Benitec shares to be traded more easily for U.S. investors. The ADRs will be tradeable via licensed U.S. brokers in the ordinary course of trading in the OTC market in the U.S.
Benitec will release further details on the ADR sponsored program as they become available.
For further information, please contact the persons below, or visit the Benitec website at www.benitec.com.
Bisher enttäuschende Entwicklung der Aktie. Heute in Australien wieder -4,7 % auf 1,105 AUD.
Dabei gibt es gute News: Der erste Patient wurde inzwischen dosiert:
Benitec treats first patient in HCV trial
http://lifescientist.com.au/content/biotechnology/article/be…
Und die Aktie ist nun in den USA als Sponsored US ADR in den USA handelbar:
Sydney Australia, 2 June 2014 Benitec Biopharma Limited (ASX:BLT) (OTC:BTEBY) is pleased to announce the establishment of a sponsored, Level 1 American Depositary Receipt (ADR) program facility, trading in the Over-The-Counter (OTC) market in the United States. The ADR program was declared effective in the U.S. on Friday 30 May 2014. The ADR tickersymbol is BTEBY.
Benitec has appointed the Bank of New York Mellon (BNYM) as its authorised U.S. representative and Depositary Bank to administer the ADR facility.
Benitec has negotiated the ability for BNIKF shareholders to transition across to the sponsored ADR program without incurring issuance fees for a limited period. Should BNIKF shareholders wish to transition their holdings, they should contact BNY Mellon's issuance desk on +1 212 815 2037 / 2724 / 6933 / 2252 by 30 June 2014. After this period issuance fees will apply.
Eventuell sorgte die Meldung für die Enttäuschung:
http://www.news.com.au/finance/economy/sienna-cancer-diagnos…
Ich bleib weiter investiert.
Bitte unbedingt vor einem Kauf in Deutschland Schlusskurs in Australien http://bigcharts.marketwatch.com/quickchart/quickchart.asp?s…
und Umrechnungskurs EUR/AUD beachten.(aktuell 1,4665)
Dabei gibt es gute News: Der erste Patient wurde inzwischen dosiert:
Benitec treats first patient in HCV trial
http://lifescientist.com.au/content/biotechnology/article/be…
Und die Aktie ist nun in den USA als Sponsored US ADR in den USA handelbar:
Sydney Australia, 2 June 2014 Benitec Biopharma Limited (ASX:BLT) (OTC:BTEBY) is pleased to announce the establishment of a sponsored, Level 1 American Depositary Receipt (ADR) program facility, trading in the Over-The-Counter (OTC) market in the United States. The ADR program was declared effective in the U.S. on Friday 30 May 2014. The ADR tickersymbol is BTEBY.
Benitec has appointed the Bank of New York Mellon (BNYM) as its authorised U.S. representative and Depositary Bank to administer the ADR facility.
Benitec has negotiated the ability for BNIKF shareholders to transition across to the sponsored ADR program without incurring issuance fees for a limited period. Should BNIKF shareholders wish to transition their holdings, they should contact BNY Mellon's issuance desk on +1 212 815 2037 / 2724 / 6933 / 2252 by 30 June 2014. After this period issuance fees will apply.
Eventuell sorgte die Meldung für die Enttäuschung:
http://www.news.com.au/finance/economy/sienna-cancer-diagnos…
Ich bleib weiter investiert.
Bitte unbedingt vor einem Kauf in Deutschland Schlusskurs in Australien http://bigcharts.marketwatch.com/quickchart/quickchart.asp?s…
und Umrechnungskurs EUR/AUD beachten.(aktuell 1,4665)
Bin auch in Benitec investiert...hat Potenzial. Bin am überlegen aufzustocken...
BENITEC EXECUTES LICENSE AGREEMENT WITH CIRCUIT THERAPEUTICS FOR PAIN; PROVIDES TT-034 TRIAL COMMENTARY
Sydney Australia, 3 November 2014: ddRNAi therapeutics company Benitec Biopharma Limited (ASX: BLT, OTC: BTEBY)) is pleased to announce the execution of an exclusive licensing agreement with US-based biotech company Circuit Therapeutics (Circuit) for the use of Benitec's ddRNAi technology in the area of intractable pain. Intractable pain is a severe and constant pain which is not curable using current therapies. It causes adverse biologic affects for sufferers, and untreated, can lead to death.
The licensing agreement covers the application of ddRNAi to target the inhibition of a Nav1.7, a sodium channel that is exclusively produced in certain sensory nerves and is critical for generation of pain. Licensing ddRNAi technology will enable Circuit to use Benitec's novel gene silencing therapy to block Nav1.7 in particular neurons that control pain without the anticipated side effects of less specific and/or less targeted therapies.
Chief Executive Officer of Benitec Biopharma, Dr Peter French, commented, "We are very pleased to license ddRNAi to Circuit for these pain applications. We believe that ddRNAi can provide a unique solution to chronic intractable pain, and as we are focusing internally on our hepatitis C, hepatitis B, non-small cell lung cancer and age-related macular degeneration programs, this license allows us to advance the pain therapeutic potential of ddRNAi without taking resources from our other key programs. Success in this program will have implications for other forms of neuropathic pain, including cancer pain."
Dr Fred Moll, Chairman of Circuit Therapeutics, commented, "A major focus for Circuit has been the development of novel, gene-based therapies to treat intractable pain. Accessing Benitec's ddRNAi approach, we believe, will enable the advancement of our programs targeting the Nav1.7 sodium ion channel".
The terms of this license are commercial in confidence, however, they are broadly in line with previous license agreements, and are within expected guidelines for biotech initiatives in the early stage of development.
Discussions with other potential licensing partners are ongoing, with the expectation of further progressing Benitec Biopharma's technology in a range of other serious chronic diseases with unmet needs.
Response to questions received on TT034 trial progress
Benitec Biopharma has received a number of questions regarding one of the exclusion
criteria in the Company's Phase I/II(a) Clinical Trial for Hepatitis C.
The questions relate to the prevalence of neutralising antibodies to the AAV8 vector in patients being screened for recruitment in the Company's TT034 trial.
Benitec notes that neutralising antibodies have not been a major factor in the delays associated with dosing the second patient in the current clinical trial.
It is important to note that previous exposure to AAV vectors, which causes production of neutralising antibodies, is only one of many exclusion criteria under the TT-034 trial, and is not something that is expected to be material to the commercial prospects for TT034 upon entry to the market.
The Company wishes to reiterate that the criteria for trial inclusion are designed to ensure the safety and welfare of the trial subjects for TT-034, remembering that this is a first is man, non-withdrawable treatment.
Although the inclusion criteria for this Phase I/II(a) trial are appropriately strict due to the non-withdrawable nature of TT-034, once a suitable safety profile has been established for the therapeutic, many of the trial exclusion parameters will be relaxed, enabling the treatment of much larger groups of patients.
Interview with Dr Peter French on Circuit licensing agreement and TT-034 trial
Investors are invited to listen to an audio interview where Dr Peter French discusses the licencing agreement with Circuit Therapeutics and responds to recent shareholder interest in the progress of Benitec's TT-034 trial for Hepatitis C. To listen to the interview, please copy and paste the following link into your browser: http://www.brrmedia.com/event/129281.
For further information regarding Benitec and its activities, please contact the persons below, or visit the Benitec website at http://www.benitec.com/www.benitec.com
Sydney Australia, 3 November 2014: ddRNAi therapeutics company Benitec Biopharma Limited (ASX: BLT, OTC: BTEBY)) is pleased to announce the execution of an exclusive licensing agreement with US-based biotech company Circuit Therapeutics (Circuit) for the use of Benitec's ddRNAi technology in the area of intractable pain. Intractable pain is a severe and constant pain which is not curable using current therapies. It causes adverse biologic affects for sufferers, and untreated, can lead to death.
The licensing agreement covers the application of ddRNAi to target the inhibition of a Nav1.7, a sodium channel that is exclusively produced in certain sensory nerves and is critical for generation of pain. Licensing ddRNAi technology will enable Circuit to use Benitec's novel gene silencing therapy to block Nav1.7 in particular neurons that control pain without the anticipated side effects of less specific and/or less targeted therapies.
Chief Executive Officer of Benitec Biopharma, Dr Peter French, commented, "We are very pleased to license ddRNAi to Circuit for these pain applications. We believe that ddRNAi can provide a unique solution to chronic intractable pain, and as we are focusing internally on our hepatitis C, hepatitis B, non-small cell lung cancer and age-related macular degeneration programs, this license allows us to advance the pain therapeutic potential of ddRNAi without taking resources from our other key programs. Success in this program will have implications for other forms of neuropathic pain, including cancer pain."
Dr Fred Moll, Chairman of Circuit Therapeutics, commented, "A major focus for Circuit has been the development of novel, gene-based therapies to treat intractable pain. Accessing Benitec's ddRNAi approach, we believe, will enable the advancement of our programs targeting the Nav1.7 sodium ion channel".
The terms of this license are commercial in confidence, however, they are broadly in line with previous license agreements, and are within expected guidelines for biotech initiatives in the early stage of development.
Discussions with other potential licensing partners are ongoing, with the expectation of further progressing Benitec Biopharma's technology in a range of other serious chronic diseases with unmet needs.
Response to questions received on TT034 trial progress
Benitec Biopharma has received a number of questions regarding one of the exclusion
criteria in the Company's Phase I/II(a) Clinical Trial for Hepatitis C.
The questions relate to the prevalence of neutralising antibodies to the AAV8 vector in patients being screened for recruitment in the Company's TT034 trial.
Benitec notes that neutralising antibodies have not been a major factor in the delays associated with dosing the second patient in the current clinical trial.
It is important to note that previous exposure to AAV vectors, which causes production of neutralising antibodies, is only one of many exclusion criteria under the TT-034 trial, and is not something that is expected to be material to the commercial prospects for TT034 upon entry to the market.
The Company wishes to reiterate that the criteria for trial inclusion are designed to ensure the safety and welfare of the trial subjects for TT-034, remembering that this is a first is man, non-withdrawable treatment.
Although the inclusion criteria for this Phase I/II(a) trial are appropriately strict due to the non-withdrawable nature of TT-034, once a suitable safety profile has been established for the therapeutic, many of the trial exclusion parameters will be relaxed, enabling the treatment of much larger groups of patients.
Interview with Dr Peter French on Circuit licensing agreement and TT-034 trial
Investors are invited to listen to an audio interview where Dr Peter French discusses the licencing agreement with Circuit Therapeutics and responds to recent shareholder interest in the progress of Benitec's TT-034 trial for Hepatitis C. To listen to the interview, please copy and paste the following link into your browser: http://www.brrmedia.com/event/129281.
For further information regarding Benitec and its activities, please contact the persons below, or visit the Benitec website at http://www.benitec.com/www.benitec.com
mal wieder ein paar news zu benitec - durchaus positiv (und grade auch günstig zu haben, da lange ohne news).
Guckst Du hier Alder :
http://blt.live.hqi.com.au/IRM/Company/ShowPage.aspx/PDFs/14…
und guckst Du da auch nochmal, voll krass die news EY !
http://blt.live.hqi.com.au/IRM/Company/ShowPage.aspx/PDFs/15…
Guckst Du hier Alder :
http://blt.live.hqi.com.au/IRM/Company/ShowPage.aspx/PDFs/14…
und guckst Du da auch nochmal, voll krass die news EY !
http://blt.live.hqi.com.au/IRM/Company/ShowPage.aspx/PDFs/15…
Antwort auf Beitrag Nr.: 48.761.711 von DOOFbutHappy am 13.01.15 12:24:25Ich gehe davon aus, dass bald ein Übernahmeangebot für diese Firma kommt;
auf dem Biopharmasektor ist diesvezüglich ja im Moment einiges los

auf dem Biopharmasektor ist diesvezüglich ja im Moment einiges los

Antwort auf Beitrag Nr.: 48.763.610 von Beutelgote am 13.01.15 15:23:25Von wem? Warum? Zeigt sich nicht am Kurs.
Antwort auf Beitrag Nr.: 48.852.809 von justfun am 22.01.15 16:37:55Natürlich alles nur reine Spekulation ...

klar würde der Kurs (durch Insider) schon vorher kräftig einsteigen falls da was Konkretes im Busch wäre, ergo muss der Spekulant schon vor den Insidern investiert sein ...um maximal zu profitieren, oder etwa nicht ?

klar würde der Kurs (durch Insider) schon vorher kräftig einsteigen falls da was Konkretes im Busch wäre, ergo muss der Spekulant schon vor den Insidern investiert sein ...um maximal zu profitieren, oder etwa nicht ?
Überblick zur Studienlage:
Studienstart war 2014, nicht wie erhofft, schon 2013.
http://dcru.org/about-us/news/benitec-selects-dcru-site-hepa…
Benitec selects DCRU as site for Hepatitis C phase I/II clinical trial
RNAi-based therapeutics company Benitec Biopharma Limited (ASX Code: BLT) today announced the selection of the Duke Clinical Research Unit, the early phase unit of the Duke Clinical Research Institute (DCRI), Durham, North Carolina, USA as a site for its upcoming phase I/II first-in-man trial for TT-034 in Hepatitis C. TT-034 is being developed as a potential "one-shot-cure" for Hepatitis C (HCV).
"We are very excited to be working with Duke, a world renowned research institution with significant experience in this area," said Peter French , Ph.D., Chief Executive Officer of Benitec. "The TT-034 trial marks the transition of Benitec to a clinical stage company. We expect that positive results from the trial will provide a value inflection point for the company, and also be a validation for our ddRNAi technology as an effective platform for therapeutics."
The phase I/II clinical trial is an open-label dose escalation study to evaluate the safety and activity of single doses of TT-034 in patients with chronic HCV genotype 1 infection who have failed previous treatments. The trial is expected to involve 14 patients in 5 sequential dose cohorts. Additional consolidation cohorts may be added during the study to confirm the results of the trial. The primary safety endpoints are dose limiting adverse events. The primary activity end points are serum viral load reduction and degree of hepatocyte transduction (measured through liver biopsies). There is a pre-specified interim read on safety and activity within months of trial commencement. The clinical trial is expected to begin enrolling patients during the second half of 2013.
Duke's principal investigator for the study will be Keyur Patel , M.D. Dr Patel has previous experience with oligonucleotide therapeutics in HCV, is a recipient of the prestigious American Association for the Study of Liver Diseases (AASLD) Shelia Sherlock Clinical and Translational Research award and has over 100 citations in peer-reviewed publications.
"TT-034 is a potentially transformative new treatment," Dr. Patel commented. "A therapeutic that could cure an HCV patient with a single injection would obviously be a big step forward compared to even the best treatments that are currently on the horizon, as they all involve comparatively lengthy regimens with a combination of several drugs."
Studienstart war 2014, nicht wie erhofft, schon 2013.
http://dcru.org/about-us/news/benitec-selects-dcru-site-hepa…
Benitec selects DCRU as site for Hepatitis C phase I/II clinical trial
RNAi-based therapeutics company Benitec Biopharma Limited (ASX Code: BLT) today announced the selection of the Duke Clinical Research Unit, the early phase unit of the Duke Clinical Research Institute (DCRI), Durham, North Carolina, USA as a site for its upcoming phase I/II first-in-man trial for TT-034 in Hepatitis C. TT-034 is being developed as a potential "one-shot-cure" for Hepatitis C (HCV).
"We are very excited to be working with Duke, a world renowned research institution with significant experience in this area," said Peter French , Ph.D., Chief Executive Officer of Benitec. "The TT-034 trial marks the transition of Benitec to a clinical stage company. We expect that positive results from the trial will provide a value inflection point for the company, and also be a validation for our ddRNAi technology as an effective platform for therapeutics."
The phase I/II clinical trial is an open-label dose escalation study to evaluate the safety and activity of single doses of TT-034 in patients with chronic HCV genotype 1 infection who have failed previous treatments. The trial is expected to involve 14 patients in 5 sequential dose cohorts. Additional consolidation cohorts may be added during the study to confirm the results of the trial. The primary safety endpoints are dose limiting adverse events. The primary activity end points are serum viral load reduction and degree of hepatocyte transduction (measured through liver biopsies). There is a pre-specified interim read on safety and activity within months of trial commencement. The clinical trial is expected to begin enrolling patients during the second half of 2013.
Duke's principal investigator for the study will be Keyur Patel , M.D. Dr Patel has previous experience with oligonucleotide therapeutics in HCV, is a recipient of the prestigious American Association for the Study of Liver Diseases (AASLD) Shelia Sherlock Clinical and Translational Research award and has over 100 citations in peer-reviewed publications.
"TT-034 is a potentially transformative new treatment," Dr. Patel commented. "A therapeutic that could cure an HCV patient with a single injection would obviously be a big step forward compared to even the best treatments that are currently on the horizon, as they all involve comparatively lengthy regimens with a combination of several drugs."
Studienlage Hep C
http://www.prnewswire.com/news-releases/benitec-advances-hep…
Benitec Advances Hepatitis C Clinical Trial
SYDNEY, Jan. 7, 2015 /PRNewswire/ -- Benitec Biopharma Limited (ASX: BLT, OTC: BTEBY) is pleased to advise that the third patient in its Phase I/IIa clinical trial of TT-034 for hepatitis C was dosed earlier today at the Duke Clinical Research Unit (USA). This is a significant step for this "first in man" study, and follows review of the collective data from the first two patients by the independent Data Safety Monitoring Board (DSMB). The DSMB determined that the patients from the first dosing cohort were clear of any significant treatment-related adverse events.
The newly dosed patient is the first to receive the increased dose of TT-034 (1.25 x 10^11 vg/kg, a concentration that is a half log higher than the doses administered in the first cohort). While TT-034 is designed as a potential "one-shot" cure for hepatitis C, the current dose is still below that expected to inhibit viral replication and data from the second dosing cohort are therefore expected to serve primarily as a further safety assessment.
As with previous patients, the newly dosed patient will be monitored for six weeks and results will be reviewed by the DSMB. Should the results indicate appropriate safety outcomes, the DSMB is expected to recommend that the remaining two patients in the second cohort be dosed. It is aimed to dose both at approximately the same time. The trial sites at Duke Clinical Research Unit and University of California San Diego have identified a number of patients who have passed initial screening who can be prepared in anticipation of this outcome.
About TT-034
TT-034 is a ddRNAi-based therapeutic, designed to treat and potentially cure hepatitis C (HCV) with a single administration. TT-034 targets the hepatitis C viral RNA at three separate, highly conserved sites. As such it acts as a "triple therapy" even though it is a monotherapy, and minimizes the ability of the virus to mutate and escape the therapy. Once it reaches the liver cells, it enters the nucleus and produces three separate short hairpin RNAs continuously for the lifetime of the cell. Thus TT-034 has the potential to not only treat the existing HCV infection, but also to guard against reinfection for months to years without the need to re-treat. TT-034 safety and efficacy has been tested extensively in pre-clinical in vivo studies with no adverse effects observed at therapeutic doses.
http://www.prnewswire.com/news-releases/benitec-advances-hep…
Benitec Advances Hepatitis C Clinical Trial
SYDNEY, Jan. 7, 2015 /PRNewswire/ -- Benitec Biopharma Limited (ASX: BLT, OTC: BTEBY) is pleased to advise that the third patient in its Phase I/IIa clinical trial of TT-034 for hepatitis C was dosed earlier today at the Duke Clinical Research Unit (USA). This is a significant step for this "first in man" study, and follows review of the collective data from the first two patients by the independent Data Safety Monitoring Board (DSMB). The DSMB determined that the patients from the first dosing cohort were clear of any significant treatment-related adverse events.
The newly dosed patient is the first to receive the increased dose of TT-034 (1.25 x 10^11 vg/kg, a concentration that is a half log higher than the doses administered in the first cohort). While TT-034 is designed as a potential "one-shot" cure for hepatitis C, the current dose is still below that expected to inhibit viral replication and data from the second dosing cohort are therefore expected to serve primarily as a further safety assessment.
As with previous patients, the newly dosed patient will be monitored for six weeks and results will be reviewed by the DSMB. Should the results indicate appropriate safety outcomes, the DSMB is expected to recommend that the remaining two patients in the second cohort be dosed. It is aimed to dose both at approximately the same time. The trial sites at Duke Clinical Research Unit and University of California San Diego have identified a number of patients who have passed initial screening who can be prepared in anticipation of this outcome.
About TT-034
TT-034 is a ddRNAi-based therapeutic, designed to treat and potentially cure hepatitis C (HCV) with a single administration. TT-034 targets the hepatitis C viral RNA at three separate, highly conserved sites. As such it acts as a "triple therapy" even though it is a monotherapy, and minimizes the ability of the virus to mutate and escape the therapy. Once it reaches the liver cells, it enters the nucleus and produces three separate short hairpin RNAs continuously for the lifetime of the cell. Thus TT-034 has the potential to not only treat the existing HCV infection, but also to guard against reinfection for months to years without the need to re-treat. TT-034 safety and efficacy has been tested extensively in pre-clinical in vivo studies with no adverse effects observed at therapeutic doses.
Liebe Mitinvestierte:
der Kursverlauf ist ja zur Zeit trotz positiver Gesamtmärkte eher enttäuschend. Das hat sicher viele Gründe (von "Risikoaversion" bis zu "- wo anders kann man gerade einfacher Geld machen.."). Auch die eher zäh tröpfelnden Nachrichten tragen ihren Teil dazu bei.
Daher versuche ich einmal eine Prognose der Studiendauer:
optimistisch: Mitte/Ende Feb/15 können die beiden fehlenden Patienten der Kohorte II dosiert werden (erfreulich ist, dass offensichtlich genügend Patienten zu Auswahl stehen). Start Kohorte III März 15 (Pat. 1 --> 6 Wochen beobachten, Auswertung, restlichen Patienten dosieren = 2 Monate). Der Abschluss der Kohorte V wäre dann Ende August 2015 erreicht.
pessimistisch: die nachdosierten Patienten werden auch 6 Wochen beobachtet, bevor eine neue Kohorte startet --> dann kommen noch 4 Monate drauf und wir sind an Jahresende.
ABER: wir testen hier nicht "nur" ein Medikament, sondern ein Verfahren (zur Erinnerung: http://www.wallstreet-online.de/nachricht/6790734-benitec-bi… Benitec setzt dagegen auf dd-RNAi, eine Form der RNA-Interferenz bei der die RNA von der menschlichen DNA gesteuert wird. Mit Hilfe von klinisch erprobten Gentherapie-Vektoren wird die benötigte DNA in die Zelle eingeschleust. Anschließend wird der natürliche, körpereigene Prozess genutzt, um fortlaufend therapeutische siRNA-Moleküle innerhalb der Zelle zu produzieren.)
Bei dem kleinsten Anzeichen, dass die Methode sicher funktionieren könnte, stehen die Aufkäufer Schlange.
FAZIT: es ist wie immer bei den Biotech´s: flopp oder top. Ich persönlich gebe der Methode gute Chancen und bin dabei.
der Kursverlauf ist ja zur Zeit trotz positiver Gesamtmärkte eher enttäuschend. Das hat sicher viele Gründe (von "Risikoaversion" bis zu "- wo anders kann man gerade einfacher Geld machen.."). Auch die eher zäh tröpfelnden Nachrichten tragen ihren Teil dazu bei.
Daher versuche ich einmal eine Prognose der Studiendauer:
optimistisch: Mitte/Ende Feb/15 können die beiden fehlenden Patienten der Kohorte II dosiert werden (erfreulich ist, dass offensichtlich genügend Patienten zu Auswahl stehen). Start Kohorte III März 15 (Pat. 1 --> 6 Wochen beobachten, Auswertung, restlichen Patienten dosieren = 2 Monate). Der Abschluss der Kohorte V wäre dann Ende August 2015 erreicht.
pessimistisch: die nachdosierten Patienten werden auch 6 Wochen beobachtet, bevor eine neue Kohorte startet --> dann kommen noch 4 Monate drauf und wir sind an Jahresende.
ABER: wir testen hier nicht "nur" ein Medikament, sondern ein Verfahren (zur Erinnerung: http://www.wallstreet-online.de/nachricht/6790734-benitec-bi… Benitec setzt dagegen auf dd-RNAi, eine Form der RNA-Interferenz bei der die RNA von der menschlichen DNA gesteuert wird. Mit Hilfe von klinisch erprobten Gentherapie-Vektoren wird die benötigte DNA in die Zelle eingeschleust. Anschließend wird der natürliche, körpereigene Prozess genutzt, um fortlaufend therapeutische siRNA-Moleküle innerhalb der Zelle zu produzieren.)
Bei dem kleinsten Anzeichen, dass die Methode sicher funktionieren könnte, stehen die Aufkäufer Schlange.
FAZIT: es ist wie immer bei den Biotech´s: flopp oder top. Ich persönlich gebe der Methode gute Chancen und bin dabei.
Bin hier jüngst eingestiegen. Es ist ruhig um die Aktie geworden, dabei sind die letzten News recht positiv ausgefallen:
http://www.benitec.com/investor-centre/asx-announcements
Zudem strebt Benitec ein IPO in den USA an. Das könnte zu einer Neubewertung oder zumindest neuem Interesse führen.
Mit 25 Mio. AUD Cash droht wohl erst mal auch keine relevante Verwässerung. Die Story ist jedenfalls weiterhin sehr ansprechend:

M@trix
http://www.benitec.com/investor-centre/asx-announcements
Zudem strebt Benitec ein IPO in den USA an. Das könnte zu einer Neubewertung oder zumindest neuem Interesse führen.
Mit 25 Mio. AUD Cash droht wohl erst mal auch keine relevante Verwässerung. Die Story ist jedenfalls weiterhin sehr ansprechend:

M@trix
Ja, es geht sehr zäh voran.
Eigentlich sind alle Nachrichten gut, aber es scheint auf die lange Variante aus Beitrag 12 hinauszulaufen. Daher kann es schon noch ein paar Monate dauern, bis relevante Studiendaten bezüglich der Wirksamkeit der Therapie bei Hep. C bekannt werden. Die Verträglichkeit scheint ja bis jetzt gut zu sein.
Stay long.
Eigentlich sind alle Nachrichten gut, aber es scheint auf die lange Variante aus Beitrag 12 hinauszulaufen. Daher kann es schon noch ein paar Monate dauern, bis relevante Studiendaten bezüglich der Wirksamkeit der Therapie bei Hep. C bekannt werden. Die Verträglichkeit scheint ja bis jetzt gut zu sein.
Stay long.
UPS - Kapitalerhöhung für einen IPO an der Nasdaq.
Das ist deutlich früher, als ich erwartet hätte, aber hinsichtlich der Dauer der Weiterentwicklung der Wirkstoffe nicht verwunderlich. Bin auf die genauen finanziellen Einzelheiten gespannt.
Das ist deutlich früher, als ich erwartet hätte, aber hinsichtlich der Dauer der Weiterentwicklung der Wirkstoffe nicht verwunderlich. Bin auf die genauen finanziellen Einzelheiten gespannt.
Ich habe hier jüngst weiter zugekauft. Die Aktie steigt seit 4 Wochen in Australien unter höheren Umsätzen an und der Abwärtstrend wurde klar gebrochen. Das Nasdaq-Listig soll im Herbst erfolgen und dürfte weiteres Interesse generieren.
M@trix

M@trix
Benitec Biopharma: New And Old
Aug 5, 2015 5:34 AM | about stocks: BNTC, BNIKF, BTEBY
Benitec Biopharma (BNTC, BTEBY, BNIKF) is about to upgrade its US listing to the NASDQ in a fully underwritten IPO that should net the company US$60M or more. But what does this mean for both new and existing investors?
For new investors it represents one of the last few opportunities to buy into a clinical stage RNAi company (other notable exceptions would be Gradalis and Calimmune, should they ever list publicly) at a price well below what the NASDQ seems to see as normal for such companies. Yes, this is risky technology and not for the faint-hearted but the risk/reward ratio is very high. Furthermore, the risks have been very well explained in the prospectus.
Before the IPO, Benitec will have a market capitalisation of about AUD$100M. Some may say that this is low because the company's technology, ddRNAi, is unproven. My reply to this is that there are currently no RNAi or gene therapy drugs on the market that have gained worldwide approval yet this has not stopped Alynlam (NASDAQ:ALNY) having a market capitalisation of US$10.6B or Bluebird Bio (NASDAQ:BLUE) having a market capitalisation of US$5.4B. ddRNAi is just as ground breaking as either of these companies' technology and so it is not unrealistic to expect that Benitec has similar potential. It is also worth pointing out that both Alynlam and Bluebird have gained their financial strength on the back of targeting orphan or rare diseases whereas Benitec is generally focused on mainstream indications, such as Hepatitis C (HCV), Hepatitis B (HBV) and Age-Related Macular Degeneration (NASDAQ:AMD). The HCV treatment, TT-034, is already in a Pl/lla clinical trial and an early indication of its effectiveness is likely to be announced before the end of this calendar year. While the HCV field has seen some dramatic improvements in treatment in the last twelve months, one has to bear in mind that TT-034 is a one-shot treatment that, because it is based on DNA, provides an added protection against viral re-infection. This is unique to TT-034 and the ddRNAi technology. This technology should therefore be very high on the health payer's radar as the total cost of treatment per individual should be lower than treatments that require multiple doses and possible repeat treatments. Furthermore, the design of the treatment for HBV is based on the design of the HCV treatment and so a successful trial of TT-034, especially the performance of the AAV vector, will have a very significant impact on the development of Hepbarna, the ddRNAi treatment for HBV. HBV is a largely untapped market in term of effective treatments so Hepbarna should be worth even more than TT-034.
These two pipeline products, TT-034 and Hepbarna, alone have the potential to raise the value of the company to a level well above its current AUD$100M market capitalisation. Add to these new treatments for AMD and Non-Small Cell Lung Cancer, which are also heading for the clinical, and the out-licensed treatment for HIV which is already in the clinic and about to release preliminary results, and it is not difficult to see why a proposed issue price of US$13.06 represents good value for money.
For old/existing investors the IPO means a dilution of their holdings. Roughly, a fifty percent dilution. On the surface this is not good but if one looks at the Use of Proceeds section of the prospectus, then it is clear that additional funding is required in order to progress the current and future clinical trials. While some investors may not like this, the fact is that the underwritten IPO will guarantee the funds needed for the company to progress. Non-dilutive funds would have been much better but the reality is that the company has been unable to obtain such funding by way of a significant deal with a partner.
The release of preliminary results for Calimmune's ddRNAi HIV treatment or the preliminary indication of efficacy for TT-034 could change all that. Both of these sets of results will be available before the end of 2015 so there is plenty of potential upside in the very near term, even for existing investors.
It is also very reasonable and responsible of the company's management to issue the IPO now in order to reduce the risk associated with the clinical trial outcomes. All shareholders want the TT-034 trial to be successful but it is a high risk and securing funding now rather than hoping for higher IPO returns later is a sensible course of action.
I have said before that the success of the IPO will depend on what the company does with the new capital. Nothing has changed. Most of the capital will be spent on known programs but I notice that there will still be a significant amount of money available for new undertakings. I look forward to the company revealing what these will be.
I believe the company is undervalued and the NASDQ is a far better board than the ASX to determine the real value of the company. Hopefully, the IPO will be successful but, even if is less that spectacular, the results that we will hear about before the end of the year should make up for any shortcomings.
Additional disclosure: I am long on Benitec. This article is not intended to be investment advice. Readers should do their own research.
http://seekingalpha.com/instablog/19837781-pannobhaso/425184…
Aug 5, 2015 5:34 AM | about stocks: BNTC, BNIKF, BTEBY
Benitec Biopharma (BNTC, BTEBY, BNIKF) is about to upgrade its US listing to the NASDQ in a fully underwritten IPO that should net the company US$60M or more. But what does this mean for both new and existing investors?
For new investors it represents one of the last few opportunities to buy into a clinical stage RNAi company (other notable exceptions would be Gradalis and Calimmune, should they ever list publicly) at a price well below what the NASDQ seems to see as normal for such companies. Yes, this is risky technology and not for the faint-hearted but the risk/reward ratio is very high. Furthermore, the risks have been very well explained in the prospectus.
Before the IPO, Benitec will have a market capitalisation of about AUD$100M. Some may say that this is low because the company's technology, ddRNAi, is unproven. My reply to this is that there are currently no RNAi or gene therapy drugs on the market that have gained worldwide approval yet this has not stopped Alynlam (NASDAQ:ALNY) having a market capitalisation of US$10.6B or Bluebird Bio (NASDAQ:BLUE) having a market capitalisation of US$5.4B. ddRNAi is just as ground breaking as either of these companies' technology and so it is not unrealistic to expect that Benitec has similar potential. It is also worth pointing out that both Alynlam and Bluebird have gained their financial strength on the back of targeting orphan or rare diseases whereas Benitec is generally focused on mainstream indications, such as Hepatitis C (HCV), Hepatitis B (HBV) and Age-Related Macular Degeneration (NASDAQ:AMD). The HCV treatment, TT-034, is already in a Pl/lla clinical trial and an early indication of its effectiveness is likely to be announced before the end of this calendar year. While the HCV field has seen some dramatic improvements in treatment in the last twelve months, one has to bear in mind that TT-034 is a one-shot treatment that, because it is based on DNA, provides an added protection against viral re-infection. This is unique to TT-034 and the ddRNAi technology. This technology should therefore be very high on the health payer's radar as the total cost of treatment per individual should be lower than treatments that require multiple doses and possible repeat treatments. Furthermore, the design of the treatment for HBV is based on the design of the HCV treatment and so a successful trial of TT-034, especially the performance of the AAV vector, will have a very significant impact on the development of Hepbarna, the ddRNAi treatment for HBV. HBV is a largely untapped market in term of effective treatments so Hepbarna should be worth even more than TT-034.
These two pipeline products, TT-034 and Hepbarna, alone have the potential to raise the value of the company to a level well above its current AUD$100M market capitalisation. Add to these new treatments for AMD and Non-Small Cell Lung Cancer, which are also heading for the clinical, and the out-licensed treatment for HIV which is already in the clinic and about to release preliminary results, and it is not difficult to see why a proposed issue price of US$13.06 represents good value for money.
For old/existing investors the IPO means a dilution of their holdings. Roughly, a fifty percent dilution. On the surface this is not good but if one looks at the Use of Proceeds section of the prospectus, then it is clear that additional funding is required in order to progress the current and future clinical trials. While some investors may not like this, the fact is that the underwritten IPO will guarantee the funds needed for the company to progress. Non-dilutive funds would have been much better but the reality is that the company has been unable to obtain such funding by way of a significant deal with a partner.
The release of preliminary results for Calimmune's ddRNAi HIV treatment or the preliminary indication of efficacy for TT-034 could change all that. Both of these sets of results will be available before the end of 2015 so there is plenty of potential upside in the very near term, even for existing investors.
It is also very reasonable and responsible of the company's management to issue the IPO now in order to reduce the risk associated with the clinical trial outcomes. All shareholders want the TT-034 trial to be successful but it is a high risk and securing funding now rather than hoping for higher IPO returns later is a sensible course of action.
I have said before that the success of the IPO will depend on what the company does with the new capital. Nothing has changed. Most of the capital will be spent on known programs but I notice that there will still be a significant amount of money available for new undertakings. I look forward to the company revealing what these will be.
I believe the company is undervalued and the NASDQ is a far better board than the ASX to determine the real value of the company. Hopefully, the IPO will be successful but, even if is less that spectacular, the results that we will hear about before the end of the year should make up for any shortcomings.
Additional disclosure: I am long on Benitec. This article is not intended to be investment advice. Readers should do their own research.
http://seekingalpha.com/instablog/19837781-pannobhaso/425184…
Antwort auf Beitrag Nr.: 50.334.477 von M@trix am 05.08.15 10:30:34
Ich muss mich korrigieren: das Nasdaq-Listing erfolgt schon morgen!
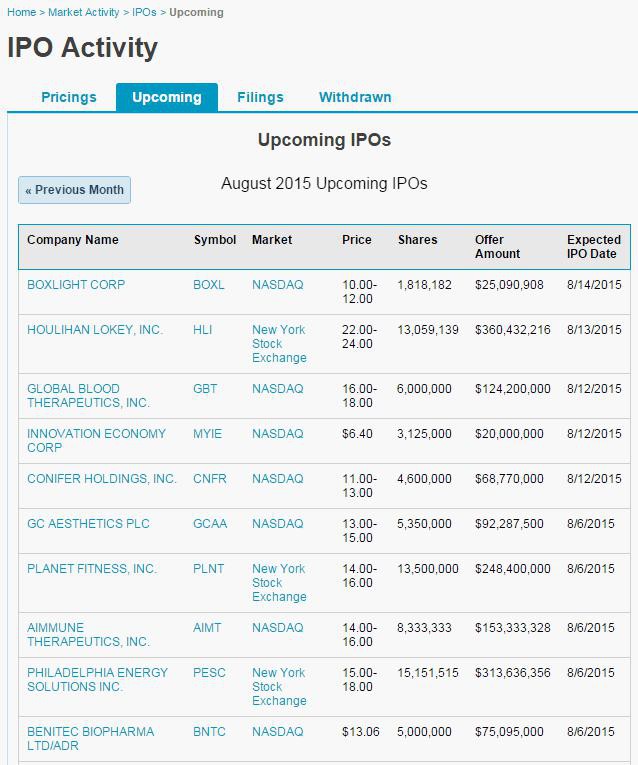
http://www.nasdaq.com/markets/ipos/activity.aspx?tab=upcomin…
Zitat von M@trix: Ich habe hier jüngst weiter zugekauft. Die Aktie steigt seit 4 Wochen in Australien unter höheren Umsätzen an und der Abwärtstrend wurde klar gebrochen. Das Nasdaq-Listig soll im Herbst erfolgen und dürfte weiteres Interesse generieren.
M@trix
Ich muss mich korrigieren: das Nasdaq-Listing erfolgt schon morgen!

http://www.nasdaq.com/markets/ipos/activity.aspx?tab=upcomin…
Antwort auf Beitrag Nr.: 50.338.881 von M@trix am 05.08.15 17:51:01Hallo m@trix, die neuen Shares in US sind ja ADRs, wie viele Shares sind in 1 ADR? Oder anders, wie ist der umgerechnete Preis von 13,05 USD im Verhältnis zum Preis bei uns (ca. 0,60 EUR) zu sehen? (Warum zeigt NASDAQ heute als "previous close" price 27,xx USD????)
Ich frage mich, ob die aktuelle Situation eine (für mich Wieder-,nach Verlusten) Einstiegschance darstellt, oder, wie ja auch einer der Kommentatoren zu pannobhaso schrieb, man erstmal die Auswirkungen des IPOs (=KE) auf den Share Price abwarten sollte.
Danke im voraus für deine Meinung
me_2
Ich frage mich, ob die aktuelle Situation eine (für mich Wieder-,nach Verlusten) Einstiegschance darstellt, oder, wie ja auch einer der Kommentatoren zu pannobhaso schrieb, man erstmal die Auswirkungen des IPOs (=KE) auf den Share Price abwarten sollte.
Danke im voraus für deine Meinung
me_2
Antwort auf Beitrag Nr.: 50.352.468 von me_2 am 07.08.15 12:00:19Hallo me_2,
hier findest du das Filing mit allen Erklärungen:
https://www.sec.gov/Archives/edgar/data/1552795/000119312515…
Wenn das Listing erfolgt, ist das aus meiner Sicht für Benitec ein Meilenstein und eindeutig positiv zu werten. Natürlich ist die Verwässerung unerfreulich, aber das Unternehmen generiert eben auch signifikant Cash. Wer weiß, wie das Umfeld für Biotechs in 12 Monaten ist...
Wichtig für den Kurs sind jedoch eher aktuelle Informationen zur laufenden Studie. Ich denke, diese werden erst nach dem Listing bekannt gegeben werden.
Grüßend
M@trix
hier findest du das Filing mit allen Erklärungen:
https://www.sec.gov/Archives/edgar/data/1552795/000119312515…
Wenn das Listing erfolgt, ist das aus meiner Sicht für Benitec ein Meilenstein und eindeutig positiv zu werten. Natürlich ist die Verwässerung unerfreulich, aber das Unternehmen generiert eben auch signifikant Cash. Wer weiß, wie das Umfeld für Biotechs in 12 Monaten ist...
Wichtig für den Kurs sind jedoch eher aktuelle Informationen zur laufenden Studie. Ich denke, diese werden erst nach dem Listing bekannt gegeben werden.
Grüßend
M@trix
Microcap biotech Benitec Biopharma decreases proposed IPO deal size to $15 million
8/13/15
Benitec Biopharma, which is developing a gene therapy platform based on DNA-directed RNA interference, reduced the number of ADSs it plans to offer for the second time and reduced the number of warrants offered.
The Balmain, Australia-based company now plans to raise $15 million by offering 2 million shares at a price of $10. The company had previously filed to offer 2 million shares at a price of $13.55. At the revised price, Benitec Biopharma will raise -41% fewer proceeds than previously anticipated.
Benitec Biopharma, which was founded in 1995 and booked $1 million in sales for the 12 months ended December 31, 2014, plans to list on the Nasdaq under the symbol BNTC. Maxim Group LLC is the sole bookrunner on the deal. It is expected to price during the week of August 31, 2015.
http://www.renaissancecapital.com/news/microcap-biotech-beni…
Also deutlich weniger Verwässerung, deutlich niedrigerer Preis, deutlich weniger Geld. Denke der Börse wird das nicht gefallen... Bin erst mal wieder raus.
M@trix
8/13/15
Benitec Biopharma, which is developing a gene therapy platform based on DNA-directed RNA interference, reduced the number of ADSs it plans to offer for the second time and reduced the number of warrants offered.
The Balmain, Australia-based company now plans to raise $15 million by offering 2 million shares at a price of $10. The company had previously filed to offer 2 million shares at a price of $13.55. At the revised price, Benitec Biopharma will raise -41% fewer proceeds than previously anticipated.
Benitec Biopharma, which was founded in 1995 and booked $1 million in sales for the 12 months ended December 31, 2014, plans to list on the Nasdaq under the symbol BNTC. Maxim Group LLC is the sole bookrunner on the deal. It is expected to price during the week of August 31, 2015.
http://www.renaissancecapital.com/news/microcap-biotech-beni…
Also deutlich weniger Verwässerung, deutlich niedrigerer Preis, deutlich weniger Geld. Denke der Börse wird das nicht gefallen... Bin erst mal wieder raus.
M@trix
Hallo M@trix,
da hast Du wohl recht gehabt. Gute 20% Minus in Down under.
Ich sehe Benitec zwar als Langfristinvestment, aber der niedriger als erwartete Emissionspreis der ADR´s gibt schon zu denken. Mehr war am Markt wohl nicht drin.
Der Schritt an die Nasdaq ist natürlich ein Fortschritt für die Entwicklung er Firma und der Preis kann schnell ins Laufen kommen, wenn ein paar gute Ergebnisse zu vermelden sind. Ob und zu welchem Preis ich allerdings nachkaufe, im die Verwässerung auszugleichen, weiß ich noch nicht.
Nebenbei: die "Marktlage" im Bereich Hepatitistherapie ist nich einfach. Auch Konkurrenten machen gute Fortschritte.
da hast Du wohl recht gehabt. Gute 20% Minus in Down under.
Ich sehe Benitec zwar als Langfristinvestment, aber der niedriger als erwartete Emissionspreis der ADR´s gibt schon zu denken. Mehr war am Markt wohl nicht drin.
Der Schritt an die Nasdaq ist natürlich ein Fortschritt für die Entwicklung er Firma und der Preis kann schnell ins Laufen kommen, wenn ein paar gute Ergebnisse zu vermelden sind. Ob und zu welchem Preis ich allerdings nachkaufe, im die Verwässerung auszugleichen, weiß ich noch nicht.
Nebenbei: die "Marktlage" im Bereich Hepatitistherapie ist nich einfach. Auch Konkurrenten machen gute Fortschritte.
News: Benitec Biopharma wind-down Hepatitis C program
ASX - Update on TT-034 Hepatitis C Clinical TrialBenitec Biopharma will wind-down its Hepatitis C program and terminate it upon completion of patients in Cohort 4 in its Phase I/IIa clinical tiral TT-034.
See announcement for further details: http://www.asx.com.au/asxpdf/20160226/pdf/435d6q0yyczywx.pdf
Source: http://www.asx.com.au
News?
Benitec's response to ASX price query.http://blt.live.irmau.com/irm/PDF/1691/ResponsetoASXpriceque…

Benitec announces appointment of new Director
Es tut sich was im Management... http://www.asx.com.au/asxpdf/20160816/pdf/439bq3clf85271.pdf Ankündigung: Benitec initiates a strategic engagement with NantVentures
• An oncology-focused research and development collaboration is planned that willreturn Benitec to the clinic with a Phase II gene-silencing asset that has achieved proof
of concept in a solid tumour-based indication
• Chief Investment Officer of NantVentures, Jerel A. Banks, M.D., Ph.D. will be
appointed to the Board
• Placement of 19.99% shares to Nant Capital, LLC

Mehr News unter: http://www.benitec.com/investor-centre/asx-announcements
Scheint wieder etwas in Fahrt zu kommen.
Re-Rating!

Hier hat sich ja leider schon etwas Staub angesammelt...

daher bringe ich kurz die News/Ankündigungen der letzten 2 Monate aufs Radar.
14/11/2016 Annual Report to shareholders @ http://www.asx.com.au/asx/statistics/displayAnnouncement.do?…
21/12/2016 asterix Benitec announces pivotal HBV in vivo data http://www.asx.com.au/asx/statistics/displayAnnouncement.do?…
22/12/2016 asterix MARKET UPDATE http://www.asx.com.au/asx/statistics/displayAnnouncement.do?…
23/12/2016 asterix Cancer program in-licensed from Nant http://www.asx.com.au/asx/statistics/displayAnnouncement.do?…
17/01/2017 asterix BENITEC ANNOUNCES EU ODD FOR BB301 http://www.asx.com.au/asx/statistics/displayAnnouncement.do?…
mMn Neubewertung voll im Gange!



Antwort auf Beitrag Nr.: 54.107.282 von freddyDD am 18.01.17 09:33:27Ich vermute, dass der Anstieg abverkauft werden wird. Es ist einfach noch zu früh:
"BB-301 is currently in preclinical development and Benitec plans to initiate IND-enabling studies later this year. Entry into the clinic with a Phase I/II study in OPMD patients is anticipated in 2018, subject to toxicity results and future regulatory review."
M@trix
"BB-301 is currently in preclinical development and Benitec plans to initiate IND-enabling studies later this year. Entry into the clinic with a Phase I/II study in OPMD patients is anticipated in 2018, subject to toxicity results and future regulatory review."
M@trix
Hepatitis B Presentation
Hier eine interessante Präsentation, die wohl am 19. February 2017 gehalten werden wird, zu ersten Erkenntnissen hinsichtlich der Wirksamkeit von BB-101, BB-102 und BB-103 beim Hepatitis B Virus (HBV) am chimärischen Maus-Model.APASL Hepatitis B Presentation
http://www.benitec.com/documents/presentation/20170219_Benit…
(PDF Size >8MB)

Antwort auf Beitrag Nr.: 54.337.090 von freddyDD am 16.02.17 15:17:13APASL = Asian Pacific Association for the Study of the Liver
Annual Meeting 2017 in Shanghai
Date: Feb 15-19, 2017
City: Shanghai, China
Website: http://www.apasl2017.org/
Annual Meeting 2017 in Shanghai
Date: Feb 15-19, 2017
City: Shanghai, China
Website: http://www.apasl2017.org/
Lesenswert:
After rocky road, Aussie firm Benitec gets new life via Nantworks deal
http://www.bioworld.com/content/after-rocky-road-aussie-firm…
Heute zudem wichtige positive News:
Key pre-clinical data on oculopharyngeal muscular dystrophy (OPMD) published
in Nature Communications
http://blt.live.irmau.com/irm/PDF/1828_0/OPMDKEYDATAPUBLISHE…
Wie es scheint ist Benitec wieder auf dem richtigen Weg. Nach dem jüngsten Anstieg dürfte es abermals eine Konsolidierung geben. Ggf. kaufe ich dann wieder eine neue längerfristige Position.
M@trix
After rocky road, Aussie firm Benitec gets new life via Nantworks deal
http://www.bioworld.com/content/after-rocky-road-aussie-firm…
Heute zudem wichtige positive News:
Key pre-clinical data on oculopharyngeal muscular dystrophy (OPMD) published
in Nature Communications
http://blt.live.irmau.com/irm/PDF/1828_0/OPMDKEYDATAPUBLISHE…
Wie es scheint ist Benitec wieder auf dem richtigen Weg. Nach dem jüngsten Anstieg dürfte es abermals eine Konsolidierung geben. Ggf. kaufe ich dann wieder eine neue längerfristige Position.
M@trix
Hoffentlich kommen wir bald mal wieder gute News ziemlich ruhig geworden
Endlich gute News
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +0,55 | |
| +3,43 | |
| +1,43 | |
| -1,45 | |
| +0,70 | |
| +8,72 | |
| -2,35 | |
| -50,00 | |
| 0,00 | |
| +9,52 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 229 | ||
| 113 | ||
| 81 | ||
| 77 | ||
| 57 | ||
| 50 | ||
| 45 | ||
| 40 | ||
| 37 | ||
| 37 |





















