Prolor Biotech - eine Biobetter-Plattform der Zukunft? - 500 Beiträge pro Seite
eröffnet am 22.07.11 15:10:37 von
neuester Beitrag 24.04.13 18:19:15 von
neuester Beitrag 24.04.13 18:19:15 von
Beiträge: 5
ID: 1.167.773
ID: 1.167.773
Aufrufe heute: 0
Gesamt: 823
Gesamt: 823
Aktive User: 0
Top-Diskussionen
| Titel | letzter Beitrag | Aufrufe |
|---|---|---|
| gestern 10:48 | 129 | |
| vor 1 Stunde | 61 | |
| gestern 23:10 | 55 | |
| vor 1 Stunde | 51 | |
| vor 1 Stunde | 50 | |
| vor 1 Stunde | 47 | |
| gestern 21:58 | 46 | |
| vor 1 Stunde | 45 |
Meistdiskutierte Wertpapiere
| Platz | vorher | Wertpapier | Kurs | Perf. % | Anzahl | ||
|---|---|---|---|---|---|---|---|
| 1. | 1. | 17.773,00 | -0,65 | 228 | |||
| 2. | 2. | 157,59 | -2,41 | 114 | |||
| 3. | 5. | 3,6700 | +6,84 | 101 | |||
| 4. | 3. | 6,7350 | -3,88 | 80 | |||
| 5. | 4. | 2.390,36 | +0,39 | 79 | |||
| 6. | 6. | 6,4580 | -2,87 | 60 | |||
| 7. | 7. | 22,780 | -4,96 | 49 | |||
| 8. | 8. | 7,4600 | -1,58 | 47 |
Ein Thread für eine interessante Technologie und ein interessantes Unternehmen:
PROLOR BIOTECH INC. (WKN A0RPQD), Ticker: PBTH
Ich will versuchen, hier relevante fundamentale Nachrichten zu Prolor zu sammeln, weil ich der Meinung bin, dass man dort einen interessanten Ansatz verfolgt, den es zu beobachten lohnt.
Kurz zur Einführung:
Prolor will sogenannte bio-betters entwickeln, das heißt, sie wollen bereits vorhandene biotechnologische Wirkstoffe aufpeppen.
Das ist insofern ein guter Ansatz, weil sie sich nur Targets und Wirkstoffe vornehmen, die bereits voll validiert sind.
Die Verbesserung soll in einer Verlängerung der Wirkzeit und damit in einer Senkung der Verabreichungshäufigkeit bestehen.
Erreichen will man dies, indem man den Abbau von Biotherapeutika in der Blutbahn verlangsamt.
Das Prinzip fußt auf Forschungen und Patenten der Washington University St. Louis. Die sogenannte CTP-Technologie hat Prolor von dort weitgehend exklusiv einlizensiert.
Was ist CTP?
An der Washington University hatte man entdeckt, dass die natürlichen weiblichen Hormone hCG und LH chemisch vollkommen identisch sind, abgesehen von einer kurzen Aminosäuresequenz, die CTP genannt wurde. Das Hormon hCG verfügt über die Zusatzsequenz, LH aber nicht.
Die Konsequenz ist, dass LH im Körper binnen 20 Minuten abgebaut wird. hCG hingegen hat eine Lebens- (und Wirk)dauer von 2 Tagen!
Man konnte nachweisen, dass es tatsächlich die CTP-Sequenz ist, die diesen Unterschied bewirkt.
Entscheidend ist aber, dass man CTP auch mit anderen therapeutischen Proteinen verknüpfen kann, um deren Lebensdauer zu verlängern.
Ebenfalls sehr wichtig ist, dass das Nebenwirkungsprofil von CTP an sich sehr günstig ist. Immerhin handelt es sich um einen natürlichen Baustein der menschlichen Biologie.
Dass das alles im Prinzip auch wirklich funktioniert, ist bereits bewiesen. Denn Schering-Plough (jetzt Merck) hat bereits den Wirkstoff ELONVA (ein Fertilitätshormon) am Markt, der auf CTP + FSH basiert. Dort wurde erreicht, dass die Einmalgabe von ELONVA sieben (!) Injektionen (täglich eine Injektion) normalen FSHs ersetzt.
(Wie schon geschrieben, haben sie die Patente nur weitgehend exklusiv, LH, TSH, FSH und hCG hat Merck, alles andere hat Prolor).
Ob und wie dies auch bei anderen Biotherapeutika funktionieren wird und wie groß die möglichen Vorteile im Einzelfall sein können, kann nur die Zukunft zeigen.
Prolor arbeitet jedenfalls erst mal an folgenden Indikationen:
Human Growth Hormone (hGH)
Factor IX
Anti-Obesity Peptide Oxyntomodulin
Factor VIIa
Interferon β and Erythropoietin (EPO)
Atherosclerosis and rheumatoid arthritis long-acting therapies
Sie geben an, dass sie damit einen Markt adressieren, der gegenwärtig 62 Milliarden Dollar schwer ist.
Natürlich darf man diese Zahl jetzt nicht zu ernst nehmen, aber dass in dem Ansatz auch kommerziell viel Potential stecken könnte, das kann man schon gut glauben.
Wer das vertiefen und weniger laienhaft beschrieben haben möchte, findet hier eine Präsentation (pdf):
http://www.modigeneinc.com/_Uploads/dbsAttachedFiles/PROLORP…
Und hier das 10-k für 2010:
http://www.modigeneinc.com/_Uploads/dbsAttachedFiles/proxy_f…
Der Kurs steht jetzt so ca. bei 6,40 Dollar. 54 Mio. Aktien stehen aus. Dazu gibts 6,8 Mio. Optionen.
Voll verwässert beträgt die Marktkapitalisierung also bereits beeindruckende 390 Mio. Dollar.
Für ein P II-Unternehmen eine ganze Menge. Zeigt m.E. auch, dass der Markt die Plattform nicht egal findet.
Für heute wars das erst mal.
PROLOR BIOTECH INC. (WKN A0RPQD), Ticker: PBTH
Ich will versuchen, hier relevante fundamentale Nachrichten zu Prolor zu sammeln, weil ich der Meinung bin, dass man dort einen interessanten Ansatz verfolgt, den es zu beobachten lohnt.
Kurz zur Einführung:
Prolor will sogenannte bio-betters entwickeln, das heißt, sie wollen bereits vorhandene biotechnologische Wirkstoffe aufpeppen.
Das ist insofern ein guter Ansatz, weil sie sich nur Targets und Wirkstoffe vornehmen, die bereits voll validiert sind.
Die Verbesserung soll in einer Verlängerung der Wirkzeit und damit in einer Senkung der Verabreichungshäufigkeit bestehen.
Erreichen will man dies, indem man den Abbau von Biotherapeutika in der Blutbahn verlangsamt.
Das Prinzip fußt auf Forschungen und Patenten der Washington University St. Louis. Die sogenannte CTP-Technologie hat Prolor von dort weitgehend exklusiv einlizensiert.
Was ist CTP?
An der Washington University hatte man entdeckt, dass die natürlichen weiblichen Hormone hCG und LH chemisch vollkommen identisch sind, abgesehen von einer kurzen Aminosäuresequenz, die CTP genannt wurde. Das Hormon hCG verfügt über die Zusatzsequenz, LH aber nicht.
Die Konsequenz ist, dass LH im Körper binnen 20 Minuten abgebaut wird. hCG hingegen hat eine Lebens- (und Wirk)dauer von 2 Tagen!
Man konnte nachweisen, dass es tatsächlich die CTP-Sequenz ist, die diesen Unterschied bewirkt.
Entscheidend ist aber, dass man CTP auch mit anderen therapeutischen Proteinen verknüpfen kann, um deren Lebensdauer zu verlängern.
Ebenfalls sehr wichtig ist, dass das Nebenwirkungsprofil von CTP an sich sehr günstig ist. Immerhin handelt es sich um einen natürlichen Baustein der menschlichen Biologie.
Dass das alles im Prinzip auch wirklich funktioniert, ist bereits bewiesen. Denn Schering-Plough (jetzt Merck) hat bereits den Wirkstoff ELONVA (ein Fertilitätshormon) am Markt, der auf CTP + FSH basiert. Dort wurde erreicht, dass die Einmalgabe von ELONVA sieben (!) Injektionen (täglich eine Injektion) normalen FSHs ersetzt.
(Wie schon geschrieben, haben sie die Patente nur weitgehend exklusiv, LH, TSH, FSH und hCG hat Merck, alles andere hat Prolor).
Ob und wie dies auch bei anderen Biotherapeutika funktionieren wird und wie groß die möglichen Vorteile im Einzelfall sein können, kann nur die Zukunft zeigen.
Prolor arbeitet jedenfalls erst mal an folgenden Indikationen:
Human Growth Hormone (hGH)
Factor IX
Anti-Obesity Peptide Oxyntomodulin
Factor VIIa
Interferon β and Erythropoietin (EPO)
Atherosclerosis and rheumatoid arthritis long-acting therapies
Sie geben an, dass sie damit einen Markt adressieren, der gegenwärtig 62 Milliarden Dollar schwer ist.
Natürlich darf man diese Zahl jetzt nicht zu ernst nehmen, aber dass in dem Ansatz auch kommerziell viel Potential stecken könnte, das kann man schon gut glauben.
Wer das vertiefen und weniger laienhaft beschrieben haben möchte, findet hier eine Präsentation (pdf):
http://www.modigeneinc.com/_Uploads/dbsAttachedFiles/PROLORP…
Und hier das 10-k für 2010:
http://www.modigeneinc.com/_Uploads/dbsAttachedFiles/proxy_f…
Der Kurs steht jetzt so ca. bei 6,40 Dollar. 54 Mio. Aktien stehen aus. Dazu gibts 6,8 Mio. Optionen.
Voll verwässert beträgt die Marktkapitalisierung also bereits beeindruckende 390 Mio. Dollar.
Für ein P II-Unternehmen eine ganze Menge. Zeigt m.E. auch, dass der Markt die Plattform nicht egal findet.
Für heute wars das erst mal.
Ich ergänze meine Einführung, indem ich zwei Texte von Steven Breazzano übernehme, die auf Seeking Alpha erschienen sind.
Er erklärt die Hintergründe des Unternehmens und die aktuellen klinischen bzw. vorklinischen Projekte:
Artikel 1:
Prolor Biotech (PBTH.OB) – based in Nes-Ziona, Israel engages in the development and commercialization of bio-better proteins and peptides. Utilizing their proprietary CTP enhanced recombinant proteins, the company aims to leverage its technology platform to provide new, enhanced versions of current recombinant proteins in a safe, efficacious, and reliable manner. By utilizing this strategy, Prolor hopes to achieve less expensive and faster clinical trials since endpoints and study protocols will be the same as those used for existing therapies. Additionally, Prolor believes its strategy of targeting therapeutic proteins already approved by the FDA, with proven safety and efficacy, allows them to lower the risk profile of their proprietary development portfolio compared to de novo therapeutic protein development.
Background: Originally, researchers at Washington University in St. Louis discovered a naturally occurring 28 amino acid long sequence (dubbed CTP) at the end of the protein that was responsible for the increase in hCG (human chorionic gonadotrophin, life span of up to 2 days) vs. LH (luteinizing hormone, life span of ~20 minutes) which have very similar chemical composition. Through numerous experiments, the researchers confirmed that CTP was responsible for the longer life span of hCG as compared to LH. Washington University in St. Louis now licenses the technology exclusively to Prolor [with the exception of 4 proteins (FSH, hCG, LH, TSH) licensed to Merck (MRK)]. Merck has gone ahead and developed FSH-CTP and received approval in the EU. This treatment (Elonva) replaces seven daily injections of FSH for a single one of FSH-CTP, providing a solid proof of concept.
Attention is currently focused on the lead drug candidate hGH-CTP, a modified form of human growth hormone which is currently in Phase 2 trials and has received orphan drug status in both adults and children. Recently, on Feb. 9, the Independent Data Safety Monitoring Board reviewed safety data and concluded the study was safe to continue as planned. The study has enrolled approximately 50 adult patients, split into 4 cohorts, given repeating injections with escalating doses weekly or 2x per month. Previously, all were on daily hGH treatment. IGF-1 (an accepted marker of hGH activity) levels will be measured to select proper dosing for a potential Phase 3 trial. While many drugs fail in Phase 2, I believe Prolor has an above-average chance of success given its promising Phase 1 results. In the Phase 1 trial, 24 healthy adults were given one of three doses of hGH-CTP or placebo, and their IGF-1 activity was measured.
The study results suggested that the daily injections required by patients using conventional hGH could potentially be replaced with just two monthly injections of hGH-CTP. While this by no means guarantees success in hGH deficient adults, the predictable behavior of the hGH-CTP (judged by the IGF-1 monitoring levels) provides reasonable predictive power in the current trial. The study is scheduled to be completed in 2Q 2011, with top-line data reported in 3Q 2011. Current treatment for hGH deficiency generally involves daily injections (2-7x per week), allowing Prolor to potentially corner large portions of the ~$3 Billion hGH market. However, the market is currently crowded with five producers of hGH, making this a potential challenge. The hGH-CTP program is scheduled to begin in children later this year, which presents a particularly significant market opportunity given the difficulty of multiple injections in children. Following approval, Prolor can be expected to take significant market share in this indication.
Competitive Landscape: Prolor is by no means the first pharmaceutical firm to take a look at long-lasting versions of recombinant proteins. A survey of the field reveals PEGylation (attachment of polyethylene glycol polymers to the protein of interest) and glycosylation (alterations of the structure generally required) as the preferred techniques. Schering-Plough’s (SGP) PEGIntron and Roche’s (RHHBY.PK) Pegasys are both PEGylated forms of interferon alpha for the treatment of Hepatitis C. Amgen’s (AMGN) Neulasta is a PEGylated form of G-CSF for the treatment of neutropenia and Amgen’s Aranesp is a glycosylated form of erythropoietin for the treatment of anemia. Enzon’s Oncaspar is PEGylated form of L-asparaginase for the treatment of acute lymphoblastic leukemia in patients who are hypersensitive to the native unmodified form of L-asparaginase. Collectively these drugs bring in annual revenues of over $8 Billion. For all their success, the above techniques have significant drawbacks. In general, PEGylation is only applicable to larger proteins and requires larger dosing due to lower activity (the protein is masked by the polyethylene glycol!). With glycosylation, development is a challenge (modification of protein structure and identification is not straightforward) and the new structures are sometimes targeted by the immune system (immunogenicity).
Novo Nordisk (NVO) announced this past fall that they would be terminating their PEGylated hGH program due to an administration profile requiring more than 1 injection per week. This further serves to highlight the challenges in dealing with long-lasting therapeutics. Ambrx and Merck-Serono are collaborating on a PEGylated hGH formulation as well, and while announcements were made about Phase 1 and 2 trials around 2007-2008, I have been unable to ascertain the status of the program.
Pipeline: While the lead candidate (hGH-CTP) is in Phase 2 trials, the pipeline contains various early-stage candidates. Most promising is the factor IX – CTP program for the treatment of hemophilia B (patients with this disease have low levels or are missing a critical blood-clotting protein, factor IX), a smaller (~$700 MM) but fast growing market (~14% annually) with clear room for improvement. In preclinical models, mice treated with Prolor’s long-lasting treatment lost half as much blood as the beneFIX (current marketed drug) treated mice did, and approximately 1/3 as much as the untreated group. In second bleeding events the beneFIX treated mice bleeding persisted over 3x as long compared to the Factor IX - CTP. Furthermore, the Factor IX - CTP group experienced no spontaneous rebleeding events 12 hours post-second bleeding event, compared to 50% for the beneFIX group. Prolor intends to begin roughly a 70 patient Phase 2 trial sometime in 2012. We believe these preclinical models are promising and bode well for this Phase 2 trial. From an investor standpoint, treatments for hemophilia can move through clinical trials quickly and inexpensively (smaller patient sizes required) because Phase 1 can be skipped.
Prolor also has a long-lasting protein in preclinical development for the treatment of obesity (by targeting the body’s natural hunger mechanism) with a new platform involving reversible PEGylation. While details are thin on this project, some of the preclinical and scientific publication data are promising (suppression of appetite without loss of energy levels). Overall, I view this project as highly speculative given the FDA’s strict approach to obesity drugs [ask Arena (ARNA), Vivus (VVUS), and Orexigen (OREX) investors!]. Any compound at this stage of development for an obesity indication must be viewed as extremely risky.
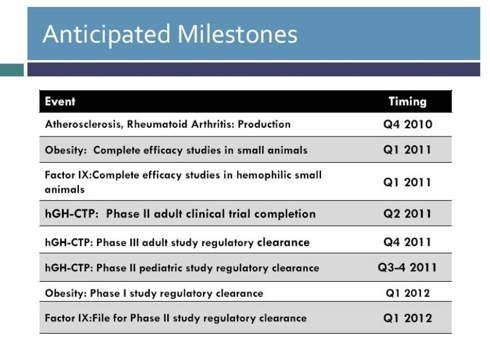
(Click to enlarge)
While the SEC Form 10-k filed in 2010 points an Epogen-CTP program, to the best of my knowledge this has been put on hold. This should not be viewed as a large negative due to the fact significant improvements over Amgen’s Aranesp would need to be realized in order to take significant market share. It also appears that at this time Prolor will not pursue development of its Interferon b program.
Fundamentals: According to the most recent 10-q filed with the SEC (9/30/10), and with its equity raise in early 2010 of $24. 4 MM, the company has a strong balance sheet and relatively small cash burn and expects this to last through 2012. The company has 54 MM shares outstanding, and 60 MM fully diluted.
Conclusion and Future Directions: Overall, I believe Prolor’s higher risk obesity program offers significant potential upside in addition to its (relatively) lower risk hemophilia and growth hormone programs. More globally, I view the approach of greatly improving existing biologics by increasing their therapeutic life as an attractive niche and believe Prolor possesses a respectable competitive advantage in this area (as judged by the competition abandoning their extended release projects). While questions remain to be answered concerning Prolor’s potential market share, the data suggest it could be significant given the large decreases in administration of hGH (and therefore, convenience) with what appears to be similar efficacy, especially in the potential pediatric indication. A strong marketing/distribution agreement might help in this regard. Over the long-term I believe that Prolor’s proprietary CTP system mitigates the high risk of drug development relative to completely novel formulations, given its improvements over the current technology and European approval of Merck’s Elonva.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Artikel 2:
Prolor Biotech: Interim Phase 2 Data Promising
Upcoming Events - Full Phase 2 human growth hormone dose ranging study (hGH-CTP) results Q3 2011, initiation of Phase 3 in adults (2012), and Phase 2 in children (Q3-Q4 2011).
Prolor Biotech (PBTH) – based in Nes-Ziona, Israel, engages in the development and commercialization of bio-better proteins and peptides. Utilizing their proprietary CTP enhanced recombinant proteins, the company aims to leverage its technology platform to provide new, enhanced versions of current recombinant proteins in a safe, efficacious, and reliable manner. By utilizing this strategy, Prolor hopes to achieve less expensive and faster clinical trials since endpoints and study protocols will be the same as those used for existing therapies. Additionally, Prolor believes its strategy of targeting therapeutic proteins already approved by the FDA, with proven safety and efficacy, allows them to lower the risk profile of their proprietary development portfolio compared to de novo therapeutic protein development. The lead candidate, long-lasting human growth hormone, is currently in Phase 2 trials for hGH deficient adults.
Interim Phase 2 Data - Recently, Prolor released interim data on their ongoing Phase 2 trial. The positive interim data from 34 patients follows PROLOR's announcement in February that hGH-CTP demonstrated a good safety profile, as confirmed in a review of interim data by the independent Data and Safety Monitoring Board. Still not complete, the study will enroll up to approximately 65 patients total.
Designed as an open label, switchover trial in responding adult patients (i.e. normal IGF-1 levels, the clinically accepted biomarker for hGH levels) currently on daily injections of hGH, patients were placed into one of 4 dosing cohorts. Receiving either 30%, 45%, or 100% of the cumulative weekly total of hGH, patients were injected once weekly for a month and their IGF-1 levels monitored. A fourth experimental cohort was given a 100% cumulative dose every other week. The goal of the study is to determine the dose ranges that provide hGH deficient adults with IGF-1 levels within the normal range (+/- 2 standard deviations, SD).
The preliminary data (30% and 45% cohorts) indicate that Prolor's hGH-CTP has a half-life approximately 10 fold longer than commercial hGH, providing the ability to replace daily injections with weekly, at a minimum. Delving more deeply into the numbers, at the 45% dosing range, patients' IGF-1 levels were within +/- 2 SD, the range endocrinologists look for, 100% of the time over the 7 days. Furthermore, IGF-1 levels stayed within a tighter range, +/- 1.5 SD 93% of the time. Based on these results alone, it is likely that Prolor could use this dose to initiate a larger, Phase 3 trial. In the 30% cumulative dosing arm, the results were quite informative and positive. While staying in the range of +/- 2 SD 64% of the time, and the narrower +/- 1 SD 43% of the time, the IGF-1 levels never dipped below – 2.5 SD. This provides effective information, as some endocrinologists prefer lower levels. Importantly, no spike of IGF-1 was observed at the start of the week in either cohort. As with the Phase 1 trial, safety and tolerability remained excellent with no antibodies formed.
Prolor is currently preparing for additional trials for its long-lasting hGH-CTP in both pediatric indications and adult indications. Later this year PBTH plans to initiate a 6 month trial in children in Europe, where the end point will be growth, as opposed to the previously used IGF-1 levels (additional details not released yet). While some investors may worry how relevant the biomarker IGF-1 is on the clinical outcome, it is a clinically validated biomarker and I believe this trial will also be successful, given the successful Phase 1 and 2 trials. In the first half of 2012, PBTH plans to initiate a larger, Phase 3 study in adults for hGH. I expect as the full trial results from the Phase 2 become available, additional details will be unveiled for these upcoming trials.
Pipeline: Recently, in addition to the interim Phase 2 data, Prolor announced favorable preclinical obesity data. While obesity is a forbidden word in biotech at the moment, this project is very promising and merits closer investigation. Injecting a long lasting version (not CTP, but a reversible pegylation technology, which is unproven) of the natural human appetite suppressant oxyntomodulin, Prolor aims to use this natural weight loss tool to help in the fight against obesity. Much of the preclinical and scientific publication data is promising (suppression of appetite without loss of energy levels), and a once weekly injection of a small amount of OXY-RPEG (reversible pegylation) would be reasonable.
Additionally, PBTH plans to initiate a Phase 2 trial for its factor 9 (F-IX-CTP), a long lasting version of the clotting factor needed in patients with hemophilia B. In preclinical models, mice treated with Prolor's long-lasting treatment lost half as much blood as the beneFIX (current marketed drug) treated mice did, and approximately 1/3rd as much as the untreated group. In second bleeding events the beneFIX treated mice bleeding persisted over 3x as long compared to the Factor IX - CTP. Furthermore, the Factor IX - CTP group experienced no spontaneous rebleeding events 12 hours post-second bleeding event, compared to 50% for the beneFIX group. Prolor intends to begin roughly a 70 patient Phase 2 trial sometime in 2012.
Risk Factors: While low-risk (relatively speaking) from a clinical success perspective, the risks around Prolor revolve primarily around the competitive landscape. There are multiple companies with hGH products currently on the market, including most of the large pharmaceutical players. In addition, clearly affirming the potential market size for the area, other firms have initiated programs in the long-lasting hGH space. While I believe Prolor is the current front-runner (they have obtained orphan drug designation in both adult and pediatric indications), major delays could prove fatal to PBTH. However, in the hemophilia B (factor 9) market, Biogen (BIIB) appears to be the current front runner. While it is hard to ascertain the dosing regimen from Biogen's presentation, there is reason to believe Prolor's candidate is superior. However, Biogen has Orphan Drug Designation in the indication and is currently ahead in trials.
Also a comparison of Prolor's milestones from February and the recent investor presentations reveal slight delays in some of the progress of Prolor's programs.
Valuation and Financials: With cash and cash equivalents of $22 MM expected to last through 2012, PBTH doesn't need to access the capital markets or ink a partnership in the near future. Given the fragmented and crowded hGH market however, I would not be surprised if Prolor inks a marketing deal or a partnership with a larger hGH player sometime in early 2012. Currently, there are 54 MM shares outstanding and 60 MM fully diluted.
With the hGH market standing at approximately $3 BB and growing at 7% annually, market penetration in 2016 (2 years after approval, possibly) of $400 MM is reasonable. Additional assumptions of 20% profit margin, 90% royalty rate, 65% success rate, and 80 MM shares outstanding discounted back 20% to 2012 and multiplied by a P/E ratio of 25 (consistent with newly profitable biotech companies) yields a NPV of $7/share. It should be noted that this NPV calculation excludes all other clinical programs. While of course these assumptions are simply my conservative estimates, every investor should evaluate these assumptions and perform their own due diligence.
Conclusion and Future Directions: Overall, I believe Prolor's higher risk obesity program offers significant potential upside in addition to its (relatively) lower risk hemophilia and growth hormone biweekly Phase 2 cohort. More globally, I view the approach of greatly improving existing biologics by increasing their therapeutic life as an attractive niche and believe Prolor possesses a respectable competitive advantage in this area. Financially, Prolor's stock has suffered greatly in 2011 and remains well below levels a year ago, making Prolor an attractive contrarian value play as well. A conservative model for future earnings discounted back to the present excluding all clinical programs except hGH gives a high net present value ($7/share) for the stock. Maybe this is why large owner and director Dr. Frost keeps buying shares on weakness.
Disclosure: I am long PBTH.
Er erklärt die Hintergründe des Unternehmens und die aktuellen klinischen bzw. vorklinischen Projekte:
Artikel 1:
Prolor Biotech (PBTH.OB) – based in Nes-Ziona, Israel engages in the development and commercialization of bio-better proteins and peptides. Utilizing their proprietary CTP enhanced recombinant proteins, the company aims to leverage its technology platform to provide new, enhanced versions of current recombinant proteins in a safe, efficacious, and reliable manner. By utilizing this strategy, Prolor hopes to achieve less expensive and faster clinical trials since endpoints and study protocols will be the same as those used for existing therapies. Additionally, Prolor believes its strategy of targeting therapeutic proteins already approved by the FDA, with proven safety and efficacy, allows them to lower the risk profile of their proprietary development portfolio compared to de novo therapeutic protein development.
Background: Originally, researchers at Washington University in St. Louis discovered a naturally occurring 28 amino acid long sequence (dubbed CTP) at the end of the protein that was responsible for the increase in hCG (human chorionic gonadotrophin, life span of up to 2 days) vs. LH (luteinizing hormone, life span of ~20 minutes) which have very similar chemical composition. Through numerous experiments, the researchers confirmed that CTP was responsible for the longer life span of hCG as compared to LH. Washington University in St. Louis now licenses the technology exclusively to Prolor [with the exception of 4 proteins (FSH, hCG, LH, TSH) licensed to Merck (MRK)]. Merck has gone ahead and developed FSH-CTP and received approval in the EU. This treatment (Elonva) replaces seven daily injections of FSH for a single one of FSH-CTP, providing a solid proof of concept.
Attention is currently focused on the lead drug candidate hGH-CTP, a modified form of human growth hormone which is currently in Phase 2 trials and has received orphan drug status in both adults and children. Recently, on Feb. 9, the Independent Data Safety Monitoring Board reviewed safety data and concluded the study was safe to continue as planned. The study has enrolled approximately 50 adult patients, split into 4 cohorts, given repeating injections with escalating doses weekly or 2x per month. Previously, all were on daily hGH treatment. IGF-1 (an accepted marker of hGH activity) levels will be measured to select proper dosing for a potential Phase 3 trial. While many drugs fail in Phase 2, I believe Prolor has an above-average chance of success given its promising Phase 1 results. In the Phase 1 trial, 24 healthy adults were given one of three doses of hGH-CTP or placebo, and their IGF-1 activity was measured.
The study results suggested that the daily injections required by patients using conventional hGH could potentially be replaced with just two monthly injections of hGH-CTP. While this by no means guarantees success in hGH deficient adults, the predictable behavior of the hGH-CTP (judged by the IGF-1 monitoring levels) provides reasonable predictive power in the current trial. The study is scheduled to be completed in 2Q 2011, with top-line data reported in 3Q 2011. Current treatment for hGH deficiency generally involves daily injections (2-7x per week), allowing Prolor to potentially corner large portions of the ~$3 Billion hGH market. However, the market is currently crowded with five producers of hGH, making this a potential challenge. The hGH-CTP program is scheduled to begin in children later this year, which presents a particularly significant market opportunity given the difficulty of multiple injections in children. Following approval, Prolor can be expected to take significant market share in this indication.
Competitive Landscape: Prolor is by no means the first pharmaceutical firm to take a look at long-lasting versions of recombinant proteins. A survey of the field reveals PEGylation (attachment of polyethylene glycol polymers to the protein of interest) and glycosylation (alterations of the structure generally required) as the preferred techniques. Schering-Plough’s (SGP) PEGIntron and Roche’s (RHHBY.PK) Pegasys are both PEGylated forms of interferon alpha for the treatment of Hepatitis C. Amgen’s (AMGN) Neulasta is a PEGylated form of G-CSF for the treatment of neutropenia and Amgen’s Aranesp is a glycosylated form of erythropoietin for the treatment of anemia. Enzon’s Oncaspar is PEGylated form of L-asparaginase for the treatment of acute lymphoblastic leukemia in patients who are hypersensitive to the native unmodified form of L-asparaginase. Collectively these drugs bring in annual revenues of over $8 Billion. For all their success, the above techniques have significant drawbacks. In general, PEGylation is only applicable to larger proteins and requires larger dosing due to lower activity (the protein is masked by the polyethylene glycol!). With glycosylation, development is a challenge (modification of protein structure and identification is not straightforward) and the new structures are sometimes targeted by the immune system (immunogenicity).
Novo Nordisk (NVO) announced this past fall that they would be terminating their PEGylated hGH program due to an administration profile requiring more than 1 injection per week. This further serves to highlight the challenges in dealing with long-lasting therapeutics. Ambrx and Merck-Serono are collaborating on a PEGylated hGH formulation as well, and while announcements were made about Phase 1 and 2 trials around 2007-2008, I have been unable to ascertain the status of the program.
Pipeline: While the lead candidate (hGH-CTP) is in Phase 2 trials, the pipeline contains various early-stage candidates. Most promising is the factor IX – CTP program for the treatment of hemophilia B (patients with this disease have low levels or are missing a critical blood-clotting protein, factor IX), a smaller (~$700 MM) but fast growing market (~14% annually) with clear room for improvement. In preclinical models, mice treated with Prolor’s long-lasting treatment lost half as much blood as the beneFIX (current marketed drug) treated mice did, and approximately 1/3 as much as the untreated group. In second bleeding events the beneFIX treated mice bleeding persisted over 3x as long compared to the Factor IX - CTP. Furthermore, the Factor IX - CTP group experienced no spontaneous rebleeding events 12 hours post-second bleeding event, compared to 50% for the beneFIX group. Prolor intends to begin roughly a 70 patient Phase 2 trial sometime in 2012. We believe these preclinical models are promising and bode well for this Phase 2 trial. From an investor standpoint, treatments for hemophilia can move through clinical trials quickly and inexpensively (smaller patient sizes required) because Phase 1 can be skipped.
Prolor also has a long-lasting protein in preclinical development for the treatment of obesity (by targeting the body’s natural hunger mechanism) with a new platform involving reversible PEGylation. While details are thin on this project, some of the preclinical and scientific publication data are promising (suppression of appetite without loss of energy levels). Overall, I view this project as highly speculative given the FDA’s strict approach to obesity drugs [ask Arena (ARNA), Vivus (VVUS), and Orexigen (OREX) investors!]. Any compound at this stage of development for an obesity indication must be viewed as extremely risky.

(Click to enlarge)
While the SEC Form 10-k filed in 2010 points an Epogen-CTP program, to the best of my knowledge this has been put on hold. This should not be viewed as a large negative due to the fact significant improvements over Amgen’s Aranesp would need to be realized in order to take significant market share. It also appears that at this time Prolor will not pursue development of its Interferon b program.
Fundamentals: According to the most recent 10-q filed with the SEC (9/30/10), and with its equity raise in early 2010 of $24. 4 MM, the company has a strong balance sheet and relatively small cash burn and expects this to last through 2012. The company has 54 MM shares outstanding, and 60 MM fully diluted.
Conclusion and Future Directions: Overall, I believe Prolor’s higher risk obesity program offers significant potential upside in addition to its (relatively) lower risk hemophilia and growth hormone programs. More globally, I view the approach of greatly improving existing biologics by increasing their therapeutic life as an attractive niche and believe Prolor possesses a respectable competitive advantage in this area (as judged by the competition abandoning their extended release projects). While questions remain to be answered concerning Prolor’s potential market share, the data suggest it could be significant given the large decreases in administration of hGH (and therefore, convenience) with what appears to be similar efficacy, especially in the potential pediatric indication. A strong marketing/distribution agreement might help in this regard. Over the long-term I believe that Prolor’s proprietary CTP system mitigates the high risk of drug development relative to completely novel formulations, given its improvements over the current technology and European approval of Merck’s Elonva.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Artikel 2:
Prolor Biotech: Interim Phase 2 Data Promising
Upcoming Events - Full Phase 2 human growth hormone dose ranging study (hGH-CTP) results Q3 2011, initiation of Phase 3 in adults (2012), and Phase 2 in children (Q3-Q4 2011).
Prolor Biotech (PBTH) – based in Nes-Ziona, Israel, engages in the development and commercialization of bio-better proteins and peptides. Utilizing their proprietary CTP enhanced recombinant proteins, the company aims to leverage its technology platform to provide new, enhanced versions of current recombinant proteins in a safe, efficacious, and reliable manner. By utilizing this strategy, Prolor hopes to achieve less expensive and faster clinical trials since endpoints and study protocols will be the same as those used for existing therapies. Additionally, Prolor believes its strategy of targeting therapeutic proteins already approved by the FDA, with proven safety and efficacy, allows them to lower the risk profile of their proprietary development portfolio compared to de novo therapeutic protein development. The lead candidate, long-lasting human growth hormone, is currently in Phase 2 trials for hGH deficient adults.
Interim Phase 2 Data - Recently, Prolor released interim data on their ongoing Phase 2 trial. The positive interim data from 34 patients follows PROLOR's announcement in February that hGH-CTP demonstrated a good safety profile, as confirmed in a review of interim data by the independent Data and Safety Monitoring Board. Still not complete, the study will enroll up to approximately 65 patients total.
Designed as an open label, switchover trial in responding adult patients (i.e. normal IGF-1 levels, the clinically accepted biomarker for hGH levels) currently on daily injections of hGH, patients were placed into one of 4 dosing cohorts. Receiving either 30%, 45%, or 100% of the cumulative weekly total of hGH, patients were injected once weekly for a month and their IGF-1 levels monitored. A fourth experimental cohort was given a 100% cumulative dose every other week. The goal of the study is to determine the dose ranges that provide hGH deficient adults with IGF-1 levels within the normal range (+/- 2 standard deviations, SD).
The preliminary data (30% and 45% cohorts) indicate that Prolor's hGH-CTP has a half-life approximately 10 fold longer than commercial hGH, providing the ability to replace daily injections with weekly, at a minimum. Delving more deeply into the numbers, at the 45% dosing range, patients' IGF-1 levels were within +/- 2 SD, the range endocrinologists look for, 100% of the time over the 7 days. Furthermore, IGF-1 levels stayed within a tighter range, +/- 1.5 SD 93% of the time. Based on these results alone, it is likely that Prolor could use this dose to initiate a larger, Phase 3 trial. In the 30% cumulative dosing arm, the results were quite informative and positive. While staying in the range of +/- 2 SD 64% of the time, and the narrower +/- 1 SD 43% of the time, the IGF-1 levels never dipped below – 2.5 SD. This provides effective information, as some endocrinologists prefer lower levels. Importantly, no spike of IGF-1 was observed at the start of the week in either cohort. As with the Phase 1 trial, safety and tolerability remained excellent with no antibodies formed.
Prolor is currently preparing for additional trials for its long-lasting hGH-CTP in both pediatric indications and adult indications. Later this year PBTH plans to initiate a 6 month trial in children in Europe, where the end point will be growth, as opposed to the previously used IGF-1 levels (additional details not released yet). While some investors may worry how relevant the biomarker IGF-1 is on the clinical outcome, it is a clinically validated biomarker and I believe this trial will also be successful, given the successful Phase 1 and 2 trials. In the first half of 2012, PBTH plans to initiate a larger, Phase 3 study in adults for hGH. I expect as the full trial results from the Phase 2 become available, additional details will be unveiled for these upcoming trials.
Pipeline: Recently, in addition to the interim Phase 2 data, Prolor announced favorable preclinical obesity data. While obesity is a forbidden word in biotech at the moment, this project is very promising and merits closer investigation. Injecting a long lasting version (not CTP, but a reversible pegylation technology, which is unproven) of the natural human appetite suppressant oxyntomodulin, Prolor aims to use this natural weight loss tool to help in the fight against obesity. Much of the preclinical and scientific publication data is promising (suppression of appetite without loss of energy levels), and a once weekly injection of a small amount of OXY-RPEG (reversible pegylation) would be reasonable.
Additionally, PBTH plans to initiate a Phase 2 trial for its factor 9 (F-IX-CTP), a long lasting version of the clotting factor needed in patients with hemophilia B. In preclinical models, mice treated with Prolor's long-lasting treatment lost half as much blood as the beneFIX (current marketed drug) treated mice did, and approximately 1/3rd as much as the untreated group. In second bleeding events the beneFIX treated mice bleeding persisted over 3x as long compared to the Factor IX - CTP. Furthermore, the Factor IX - CTP group experienced no spontaneous rebleeding events 12 hours post-second bleeding event, compared to 50% for the beneFIX group. Prolor intends to begin roughly a 70 patient Phase 2 trial sometime in 2012.
Risk Factors: While low-risk (relatively speaking) from a clinical success perspective, the risks around Prolor revolve primarily around the competitive landscape. There are multiple companies with hGH products currently on the market, including most of the large pharmaceutical players. In addition, clearly affirming the potential market size for the area, other firms have initiated programs in the long-lasting hGH space. While I believe Prolor is the current front-runner (they have obtained orphan drug designation in both adult and pediatric indications), major delays could prove fatal to PBTH. However, in the hemophilia B (factor 9) market, Biogen (BIIB) appears to be the current front runner. While it is hard to ascertain the dosing regimen from Biogen's presentation, there is reason to believe Prolor's candidate is superior. However, Biogen has Orphan Drug Designation in the indication and is currently ahead in trials.
Also a comparison of Prolor's milestones from February and the recent investor presentations reveal slight delays in some of the progress of Prolor's programs.
Valuation and Financials: With cash and cash equivalents of $22 MM expected to last through 2012, PBTH doesn't need to access the capital markets or ink a partnership in the near future. Given the fragmented and crowded hGH market however, I would not be surprised if Prolor inks a marketing deal or a partnership with a larger hGH player sometime in early 2012. Currently, there are 54 MM shares outstanding and 60 MM fully diluted.
With the hGH market standing at approximately $3 BB and growing at 7% annually, market penetration in 2016 (2 years after approval, possibly) of $400 MM is reasonable. Additional assumptions of 20% profit margin, 90% royalty rate, 65% success rate, and 80 MM shares outstanding discounted back 20% to 2012 and multiplied by a P/E ratio of 25 (consistent with newly profitable biotech companies) yields a NPV of $7/share. It should be noted that this NPV calculation excludes all other clinical programs. While of course these assumptions are simply my conservative estimates, every investor should evaluate these assumptions and perform their own due diligence.
Conclusion and Future Directions: Overall, I believe Prolor's higher risk obesity program offers significant potential upside in addition to its (relatively) lower risk hemophilia and growth hormone biweekly Phase 2 cohort. More globally, I view the approach of greatly improving existing biologics by increasing their therapeutic life as an attractive niche and believe Prolor possesses a respectable competitive advantage in this area. Financially, Prolor's stock has suffered greatly in 2011 and remains well below levels a year ago, making Prolor an attractive contrarian value play as well. A conservative model for future earnings discounted back to the present excluding all clinical programs except hGH gives a high net present value ($7/share) for the stock. Maybe this is why large owner and director Dr. Frost keeps buying shares on weakness.
Disclosure: I am long PBTH.
das ist eben amerika!
Prolor hat bisher ca. 40 mio USD investiert/ausgegeben, ca 20 mio USD bares in der Kasse und die Börse/NYSE bewertet das Unternehmen mit ca. 400.000.000 USD. Aus 60 auf 400!!!! Das ist eine geniale wertschöpfung!!!
bei den ausgangsdaten kann ich mir gut vorstellen, da können schnell eine 1/2 - 1 mia USD börsenwert noch daraus werden!!
klinisch haben die jungs erste daten mit einer wirksamkeitsstudie phase 2 bei einem produktkandidaten vor kurzem bekannt gegeben!
danke für den tipp, da spekulier ich mit. die party ist ja voll im gange
Prolor hat bisher ca. 40 mio USD investiert/ausgegeben, ca 20 mio USD bares in der Kasse und die Börse/NYSE bewertet das Unternehmen mit ca. 400.000.000 USD. Aus 60 auf 400!!!! Das ist eine geniale wertschöpfung!!!
bei den ausgangsdaten kann ich mir gut vorstellen, da können schnell eine 1/2 - 1 mia USD börsenwert noch daraus werden!!
klinisch haben die jungs erste daten mit einer wirksamkeitsstudie phase 2 bei einem produktkandidaten vor kurzem bekannt gegeben!
danke für den tipp, da spekulier ich mit. die party ist ja voll im gange

PROLOR Biotech Announces Positive Top-Line Results From Phase II Trial of its Long-Acting Human Growth Hormone That Achieved All Key Study Goals
--Trial of hGH-CTP in Growth Hormone Deficient Adults Achieved Key Efficacy and Safety Endpoints--
--Positive Results Set Stage for Initiating Phase III Trial Expected to Begin in 2012--
NES-ZIONA, Israel, Aug. 4, 2011 /PRNewswire/ -- PROLOR Biotech, Inc. (NYSE Amex: PBTH) today reported positive results from a Phase II clinical trial of its long-acting CTP-modified version of human growth hormone (hGH-CTP) in growth hormone deficient adults. The data show that a single weekly injection of hGH-CTP has the potential to replace seven consecutive daily injections of currently marketed human growth hormone (hGH).
"The findings from the Phase II trial are very promising for adults in need of growth hormone therapy and their physicians," said Dr. Avri Havron, Chief Executive Officer of PROLOR. "The results show that hGH-CTP can potentially provide an exceptional therapy for adults with growth hormone deficiency when given once weekly, while demonstrating an excellent safety and tolerability profile across all doses and for all patients in the trial. The Phase II trial results have enabled us to identify the most suitable dose range of hGH-CTP for our planned Phase III trial, and we look forward to its anticipated initiation in 2012."
"Based on these positive results, PROLOR is offering patients who participated in the Phase II trial the opportunity to continue treatment with single weekly injections of hGH-CTP for an additional four months," noted Shai Novik, President of PROLOR. "Based on feedback from patients and their physicians, we expect that the majority of patients who completed the Phase II study will elect to participate in the four-month extension period, which we view as a powerful endorsement of hGH-CTP in view of the fact that those patients who elect to participate will have to undergo continued clinical monitoring requirements such as routine blood collections. We expect that information from the voluntary extension period will provide additional data enabling us to further verify the anticipated doses and titration schedule of hGH-CTP for the planned Phase III trial."
The objectives of the randomized open-label, multicenter Phase II trial were to measure the safety and tolerability of hGH-CTP in growth hormone deficient adults and to assess dose ranging and dose response in order to identify the dose range that will be targeted in the planned Phase III trial.
Design of the Phase II Trial
The three main cohorts in the trial received a single weekly dose of hGH-CTP for a period of four weeks, containing 30%, 45% or 100% of the equivalent cumulative commercial hGH dose these patients would usually inject each day over the course of seven days (referred to as the "30%," "45%" and "100%" cohorts, respectively.) The top-line data reflect results from 39 patients, with 13 patients in each cohort comprised of 11 males and two females.
In addition to the three main cohorts, PROLOR researchers are also enrolling growth hormone deficient adults in an experimental fourth cohort, which is being conducted outside of the formal Phase II trial. The patients in the experimental fourth cohort are receiving a single injection of hGH-CTP once every two weeks that contains 50% of the cumulative commercial dose of hGH that they would usually inject each day during a two-week period. Enrollment in this experimental cohort is ongoing.
Efficacy for the three main cohorts receiving a single weekly injection of hGH-CTP is defined by measuring daily insulin-like growth factor 1 (IGF-1) levels within the desired therapeutic range over a period of seven days during the last week of treatment in the study. The desired therapeutic range is defined as an IGF-1 level that is between +2 standard deviations through -2 standard deviations from the average IGF-1 levels expected in a normal population stratified by age group and gender. In addition, the trial measured IGF-1 levels within a narrower range of +/- 1.5 standard deviations from the average normal population IGF-1 levels, for the purpose of learning more about the variance of patients within the normal range.
Phase II Trial Top-Line Data Analysis
As shown in the table below, the study data show that patients in all three cohorts (the "30%," "45%" and "100%" cohorts) achieved average IGF-1 levels that were within the normal range on 100% of the days when they were assessed. In addition, the data show that patients in each of the cohorts achieved average IGF-1 levels within the narrow definition of the normal range on many or most of the days when they were assessed, indicating a favorable variance profile within the normal range.
hGH-CTP demonstrated excellent safety and tolerability in all patients across all trial cohorts, with no apparent issues. In addition, there were no indications that hGH-CTP can induce excessive levels of IGF-1 in patients above the normal range when used in high doses.
Cohort
% Days Within
Normal Range
(+/- 2 SD)
Avg. Cmax
(preferred below
+2 SD)
Variance Measure:
% Days Within
Narrow Normal
Range
(+/-1.5 SD)
30%
100%
-0.9
57%
45%
100%
0.1
100%
100%
100%
0.4
86%
The table shows the average percent of days within the normal therapeutic range (+/- 2 SD), average percent of days within a narrower normal therapeutic range (+/- 1.5 SD), and average Cmax (highest concentration level) of IGF-1 for males, measured during the last treatment week, expressed in standard deviations from the mean IGF-1 levels expected in the normal population.
The incremental average elevated levels of IGF-1 of the two females included in each cohort during the last treatment week were dose proportional. The small number of females in each cohort does not allow for a statistical analysis, yet provides a positive indication of response.
Based on the Phase II study results, PROLOR researchers estimate that 2mg per week of hGH-CTP, containing 50% of the cumulative weekly hGH dose that an adult patient would usually be prescribed as the initial treatment dose, has a high likelihood of being defined as the starting dose for males and females in the adult Phase III trial.
--------------
Die Tabelle hats zerschossen. Ich mag die nicht neu formatieren. Ist auch egal. Daten sind gut. Nächstes Jahr beginnt die P III. Ich gehe davon aus, dass Prolor als P III-Unternehmen noch positiver wahrgenommen werden wird.
--Trial of hGH-CTP in Growth Hormone Deficient Adults Achieved Key Efficacy and Safety Endpoints--
--Positive Results Set Stage for Initiating Phase III Trial Expected to Begin in 2012--
NES-ZIONA, Israel, Aug. 4, 2011 /PRNewswire/ -- PROLOR Biotech, Inc. (NYSE Amex: PBTH) today reported positive results from a Phase II clinical trial of its long-acting CTP-modified version of human growth hormone (hGH-CTP) in growth hormone deficient adults. The data show that a single weekly injection of hGH-CTP has the potential to replace seven consecutive daily injections of currently marketed human growth hormone (hGH).
"The findings from the Phase II trial are very promising for adults in need of growth hormone therapy and their physicians," said Dr. Avri Havron, Chief Executive Officer of PROLOR. "The results show that hGH-CTP can potentially provide an exceptional therapy for adults with growth hormone deficiency when given once weekly, while demonstrating an excellent safety and tolerability profile across all doses and for all patients in the trial. The Phase II trial results have enabled us to identify the most suitable dose range of hGH-CTP for our planned Phase III trial, and we look forward to its anticipated initiation in 2012."
"Based on these positive results, PROLOR is offering patients who participated in the Phase II trial the opportunity to continue treatment with single weekly injections of hGH-CTP for an additional four months," noted Shai Novik, President of PROLOR. "Based on feedback from patients and their physicians, we expect that the majority of patients who completed the Phase II study will elect to participate in the four-month extension period, which we view as a powerful endorsement of hGH-CTP in view of the fact that those patients who elect to participate will have to undergo continued clinical monitoring requirements such as routine blood collections. We expect that information from the voluntary extension period will provide additional data enabling us to further verify the anticipated doses and titration schedule of hGH-CTP for the planned Phase III trial."
The objectives of the randomized open-label, multicenter Phase II trial were to measure the safety and tolerability of hGH-CTP in growth hormone deficient adults and to assess dose ranging and dose response in order to identify the dose range that will be targeted in the planned Phase III trial.
Design of the Phase II Trial
The three main cohorts in the trial received a single weekly dose of hGH-CTP for a period of four weeks, containing 30%, 45% or 100% of the equivalent cumulative commercial hGH dose these patients would usually inject each day over the course of seven days (referred to as the "30%," "45%" and "100%" cohorts, respectively.) The top-line data reflect results from 39 patients, with 13 patients in each cohort comprised of 11 males and two females.
In addition to the three main cohorts, PROLOR researchers are also enrolling growth hormone deficient adults in an experimental fourth cohort, which is being conducted outside of the formal Phase II trial. The patients in the experimental fourth cohort are receiving a single injection of hGH-CTP once every two weeks that contains 50% of the cumulative commercial dose of hGH that they would usually inject each day during a two-week period. Enrollment in this experimental cohort is ongoing.
Efficacy for the three main cohorts receiving a single weekly injection of hGH-CTP is defined by measuring daily insulin-like growth factor 1 (IGF-1) levels within the desired therapeutic range over a period of seven days during the last week of treatment in the study. The desired therapeutic range is defined as an IGF-1 level that is between +2 standard deviations through -2 standard deviations from the average IGF-1 levels expected in a normal population stratified by age group and gender. In addition, the trial measured IGF-1 levels within a narrower range of +/- 1.5 standard deviations from the average normal population IGF-1 levels, for the purpose of learning more about the variance of patients within the normal range.
Phase II Trial Top-Line Data Analysis
As shown in the table below, the study data show that patients in all three cohorts (the "30%," "45%" and "100%" cohorts) achieved average IGF-1 levels that were within the normal range on 100% of the days when they were assessed. In addition, the data show that patients in each of the cohorts achieved average IGF-1 levels within the narrow definition of the normal range on many or most of the days when they were assessed, indicating a favorable variance profile within the normal range.
hGH-CTP demonstrated excellent safety and tolerability in all patients across all trial cohorts, with no apparent issues. In addition, there were no indications that hGH-CTP can induce excessive levels of IGF-1 in patients above the normal range when used in high doses.
Cohort
% Days Within
Normal Range
(+/- 2 SD)
Avg. Cmax
(preferred below
+2 SD)
Variance Measure:
% Days Within
Narrow Normal
Range
(+/-1.5 SD)
30%
100%
-0.9
57%
45%
100%
0.1
100%
100%
100%
0.4
86%
The table shows the average percent of days within the normal therapeutic range (+/- 2 SD), average percent of days within a narrower normal therapeutic range (+/- 1.5 SD), and average Cmax (highest concentration level) of IGF-1 for males, measured during the last treatment week, expressed in standard deviations from the mean IGF-1 levels expected in the normal population.
The incremental average elevated levels of IGF-1 of the two females included in each cohort during the last treatment week were dose proportional. The small number of females in each cohort does not allow for a statistical analysis, yet provides a positive indication of response.
Based on the Phase II study results, PROLOR researchers estimate that 2mg per week of hGH-CTP, containing 50% of the cumulative weekly hGH dose that an adult patient would usually be prescribed as the initial treatment dose, has a high likelihood of being defined as the starting dose for males and females in the adult Phase III trial.
--------------
Die Tabelle hats zerschossen. Ich mag die nicht neu formatieren. Ist auch egal. Daten sind gut. Nächstes Jahr beginnt die P III. Ich gehe davon aus, dass Prolor als P III-Unternehmen noch positiver wahrgenommen werden wird.
Übernahme von Prolor:
OPKO Health ($OPK) announced today that it is poised to acquire Israel-based Prolor Biotech in an all-stock transaction valued at $480 million, or $7 per share of Prolor common stock.
Read more: OPKO Health to acquire Prolor Biotech for $480M - FierceBiotech http://www.fiercebiotech.com/story/opko-health-acquire-prolo…
Subscribe at FierceBiotech
-----------
Eigentlich etwas schade. Hätte vielleicht mal mehr draus werden können.
OPKO Health ($OPK) announced today that it is poised to acquire Israel-based Prolor Biotech in an all-stock transaction valued at $480 million, or $7 per share of Prolor common stock.
Read more: OPKO Health to acquire Prolor Biotech for $480M - FierceBiotech http://www.fiercebiotech.com/story/opko-health-acquire-prolo…
Subscribe at FierceBiotech
-----------
Eigentlich etwas schade. Hätte vielleicht mal mehr draus werden können.
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 229 | ||
| 115 | ||
| 99 | ||
| 80 | ||
| 78 | ||
| 60 | ||
| 48 | ||
| 47 | ||
| 41 | ||
| 35 |
| Wertpapier | Beiträge | |
|---|---|---|
| 33 | ||
| 32 | ||
| 29 | ||
| 25 | ||
| 25 | ||
| 24 | ||
| 23 | ||
| 20 | ||
| 18 | ||
| 17 |





















