SQI Diagnostics Plattform vor dem Durchbruch - 500 Beiträge pro Seite
eröffnet am 31.10.13 15:06:40 von
neuester Beitrag 22.11.17 14:16:01 von
neuester Beitrag 22.11.17 14:16:01 von
Beiträge: 120
ID: 1.187.833
ID: 1.187.833
Aufrufe heute: 0
Gesamt: 36.582
Gesamt: 36.582
Aktive User: 0
ISIN: CA78466B1085 · WKN: A0N9K5 · Symbol: SQIDF
0,0149
USD
+14,62 %
+0,0019 USD
Letzter Kurs 15.06.23 Nasdaq OTC
Neuigkeiten
Werte aus der Branche Gesundheitswesen
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,2800 | +73,21 | |
| 0,7400 | +38,58 | |
| 3,2100 | +24,90 | |
| 0,5501 | +18,33 | |
| 0,6080 | +18,06 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,5925 | -15,36 | |
| 1,5500 | -19,27 | |
| 2,9500 | -20,05 | |
| 0,9102 | -42,03 | |
| 0,7500 | -48,98 |
Heute vermeldet SQI (http://www.sqidiagnostics.com/) eine Entwicklungskooperation mit ISIS Pharma, was der erste relevante Kunde für die über 5 Jahre lang entwickelte Plattform-Technologie ist.
Hintergrund:
SQI Diagnostics (SQD) reduces the development costs for large pharmaceuticals, labs and clinics. Using SQI’s multiplex and automation technology, it allows labs to generate test results faster, more accurately and with far less labor than current methods allow
The company has been running like a private deal for the last 4-5 years, as they have worked to develop their proprietary technology. Their technology has been approved by the FDA, Health Canada and has been CE Marked for Europe. This same, approved technology is the basis for the tests sold to drug companies and that is now ready to be sold. The time has finally come to market their products and start building some real revenues for the company and most importantly, increase valuations for shareholders.
This is a huge market. Industry analysts estimate that it takes well over $4 billion and many years to develop a new drug. Over this time, a pharma company will run 10,000s of tests examining 100s of blood-based biomarkers. As per the company’s latest news releases[1], they are currently undergoing talks and due diligence processes with several large global pharmaceutical companies. It has signed two master services agreements and appears to be poised to sign more in the coming quarters. One very large pharma company, Bristol Myers Squibb, invited SQI to its vendor only trade show (September 10-11, 2013) (2) and BMS is also presenting the results of evaluating SQI’s technology at an important industry trade show taking place October 7 to 9, 2013(2).
Hier die heutige Meldung:
SQI Diagnostics Announces Agreement with Isis Pharmaceuticals
http://finance.yahoo.com/news/sqi-diagnostics-announces-agre…
SQI ist somit nun in die Vermarktungsphase eingetreten und steht damit vor dem Durchbruch. Die Aktie erscheint entsprechend langfristig aussichtsreich.
xqx
Hintergrund:
SQI Diagnostics (SQD) reduces the development costs for large pharmaceuticals, labs and clinics. Using SQI’s multiplex and automation technology, it allows labs to generate test results faster, more accurately and with far less labor than current methods allow
The company has been running like a private deal for the last 4-5 years, as they have worked to develop their proprietary technology. Their technology has been approved by the FDA, Health Canada and has been CE Marked for Europe. This same, approved technology is the basis for the tests sold to drug companies and that is now ready to be sold. The time has finally come to market their products and start building some real revenues for the company and most importantly, increase valuations for shareholders.
This is a huge market. Industry analysts estimate that it takes well over $4 billion and many years to develop a new drug. Over this time, a pharma company will run 10,000s of tests examining 100s of blood-based biomarkers. As per the company’s latest news releases[1], they are currently undergoing talks and due diligence processes with several large global pharmaceutical companies. It has signed two master services agreements and appears to be poised to sign more in the coming quarters. One very large pharma company, Bristol Myers Squibb, invited SQI to its vendor only trade show (September 10-11, 2013) (2) and BMS is also presenting the results of evaluating SQI’s technology at an important industry trade show taking place October 7 to 9, 2013(2).
Hier die heutige Meldung:
SQI Diagnostics Announces Agreement with Isis Pharmaceuticals
http://finance.yahoo.com/news/sqi-diagnostics-announces-agre…
SQI ist somit nun in die Vermarktungsphase eingetreten und steht damit vor dem Durchbruch. Die Aktie erscheint entsprechend langfristig aussichtsreich.
xqx
Antwort auf Beitrag Nr.: 45.733.187 von M@trix am 31.10.13 15:06:40
Hört sich 'at a first glance' nicht uninteressant an(soweit ich weiss gibt es da auch noch einige ähnliche Firmen/Entwickler). Frage ist dann aber immer auch was/ob sich damit wirklich verdienen lässt.
Gruß
P.
Hört sich 'at a first glance' nicht uninteressant an(soweit ich weiss gibt es da auch noch einige ähnliche Firmen/Entwickler). Frage ist dann aber immer auch was/ob sich damit wirklich verdienen lässt.
Gruß
P.
SQI hat einen neuen Vertrag mit einem „globalen Pharmaunternehmen“ abgeschlossen:
http://finance.yahoo.com/news/sqi-diagnostics-wins-commercia…
Ich denke mal, es wird sich dabei um Allergan [AGN] handeln. Aus meiner Sicht ein weiterer wichtiger Schritt zur Kommerzialisierung der Produkte. 2014 könnte – wie im Titel des Threads geschrieben – der Durchbruch erfolgen. Das sieht auch der CEO so:
[...] "We believe that when we successfully complete this project, during the first calendar quarter of 2014, we will be in an excellent position to win the on-going consumable and instrument business for the human trials for this drug as well as additional ADA projects and biomarker business with this customer." The Company believes that the on-going conversion of previously announced major pharmaceutical customers in its sales pipeline that have evaluated, or who are in the process of evaluating SQI's custom and off-the-shelf biomarker products to be a catalyst leading to wider market adoption of its Diagnostics Tools and Services products. [...]
Mit einem Börsenwert von gerade mal 15 Mio. EUR ist das Unternehmen aus meiner Sicht recht günstig bewertet, wenn man sich klar macht, welche Zeit- und Kosteneinsparungen die marktreife Technologie den großen Pharma-Unternehmen ermöglicht. Näheres dazu auch in der Unternehmenspräsentation:
http://www.sqidiagnostics.com/about/files/sqi-ir-deck.pdf
Ich habe heute meine Position um 5.000 St. ausgebaut. Der Wert wird extrem dünn gehandelt, sodass bei Interesse in jedem Fall limitiert geordert werden sollte.
M@trix
http://finance.yahoo.com/news/sqi-diagnostics-wins-commercia…
Ich denke mal, es wird sich dabei um Allergan [AGN] handeln. Aus meiner Sicht ein weiterer wichtiger Schritt zur Kommerzialisierung der Produkte. 2014 könnte – wie im Titel des Threads geschrieben – der Durchbruch erfolgen. Das sieht auch der CEO so:
[...] "We believe that when we successfully complete this project, during the first calendar quarter of 2014, we will be in an excellent position to win the on-going consumable and instrument business for the human trials for this drug as well as additional ADA projects and biomarker business with this customer." The Company believes that the on-going conversion of previously announced major pharmaceutical customers in its sales pipeline that have evaluated, or who are in the process of evaluating SQI's custom and off-the-shelf biomarker products to be a catalyst leading to wider market adoption of its Diagnostics Tools and Services products. [...]
Mit einem Börsenwert von gerade mal 15 Mio. EUR ist das Unternehmen aus meiner Sicht recht günstig bewertet, wenn man sich klar macht, welche Zeit- und Kosteneinsparungen die marktreife Technologie den großen Pharma-Unternehmen ermöglicht. Näheres dazu auch in der Unternehmenspräsentation:
http://www.sqidiagnostics.com/about/files/sqi-ir-deck.pdf
Ich habe heute meine Position um 5.000 St. ausgebaut. Der Wert wird extrem dünn gehandelt, sodass bei Interesse in jedem Fall limitiert geordert werden sollte.
M@trix
Edison Investment Research has published an report on SQI Diagnostics (SQD.V). Edison is one of Europe’s largest independent equity research firms with worldwide distribution.
Edison puts a valuation on the stock. According to the report:
“We [Edison] value SQI at C$70m, or $1.55 per share, based on a five-year (2014-18), risk adjusted, sum-of-the-parts DCF valuation model.” The author later goes on to explain that “The C$1.55 per share value is not a price target but a fair value for the stock today. Upside would come from a more rapid uptake of assays and machines from pharma customers and centralized laboratories than we [Edison] currently model. This would also increase the probabilities of success for securing new business in later years, resulting in a higher valuation.”
http://my.alphastox.com/wp-content/uploads/2014/01/Edison-Re…
Weniger erfreulich:
"but a ~C$4m funding requirement is an overhang"
Dennoch sollte 2014 für SQI den Durchbruch und für die Aktie eine Neubewertung bringen.
Edison puts a valuation on the stock. According to the report:
“We [Edison] value SQI at C$70m, or $1.55 per share, based on a five-year (2014-18), risk adjusted, sum-of-the-parts DCF valuation model.” The author later goes on to explain that “The C$1.55 per share value is not a price target but a fair value for the stock today. Upside would come from a more rapid uptake of assays and machines from pharma customers and centralized laboratories than we [Edison] currently model. This would also increase the probabilities of success for securing new business in later years, resulting in a higher valuation.”
http://my.alphastox.com/wp-content/uploads/2014/01/Edison-Re…
Weniger erfreulich:
"but a ~C$4m funding requirement is an overhang"
Dennoch sollte 2014 für SQI den Durchbruch und für die Aktie eine Neubewertung bringen.
Aus dem MD&A von SQI:
"We anticipate that the successful evaluation projects completed in fiscal 2013 and early 2014 will proceed to the next expected commercial phase, where SQI will produce and sell test kits for either its fully automated sqidlite or semi-automated sqid-X platform. One case could result in the purchase of the custom product for use on an SQI platform in a portion of the customer’s human clinical trials and, in the other case, we believe that this customer has initiated the internal processes to acquire a sqidlite platform and will likely purchase the custom developed kits to process samples generated by their clinical testing studies."
Mit anderen Worten: wir stehen nun unmittelbar vor den ersten "echten Umsätzen". Leider ist der Wert selbst in Kanada so gut wie unbekannt und zudem sehr markteng. Umso fulminanter könnte jedoch die Entwicklung in 2014 werden...
Weniger schön aber auch nicht anders zu erwarten:
"As a result of the financing and additional cost reductions the Company now has funds sufficient to meet our anticipated cash requirements for approximately the next four months."
Mal sehen - ich bleibe zuversichtlich.
"We anticipate that the successful evaluation projects completed in fiscal 2013 and early 2014 will proceed to the next expected commercial phase, where SQI will produce and sell test kits for either its fully automated sqidlite or semi-automated sqid-X platform. One case could result in the purchase of the custom product for use on an SQI platform in a portion of the customer’s human clinical trials and, in the other case, we believe that this customer has initiated the internal processes to acquire a sqidlite platform and will likely purchase the custom developed kits to process samples generated by their clinical testing studies."
Mit anderen Worten: wir stehen nun unmittelbar vor den ersten "echten Umsätzen". Leider ist der Wert selbst in Kanada so gut wie unbekannt und zudem sehr markteng. Umso fulminanter könnte jedoch die Entwicklung in 2014 werden...
Weniger schön aber auch nicht anders zu erwarten:
"As a result of the financing and additional cost reductions the Company now has funds sufficient to meet our anticipated cash requirements for approximately the next four months."
Mal sehen - ich bleibe zuversichtlich.
Ich war so frei und hab mal wieder was für meinen Blog geschrieben, das ich hier gerne (mit)teile:
SQI Diagnostics vor Neubewertung
Wenn ein Unternehmen mit seinen Produkten unmittelbar vor der erfolgreichen Kommerzialisierung steht, ist das oft ein guter Moment, zu investieren. Ist die Aktie dazu noch nahezu unbekannt, ist die Chance auf einen günstigen Einstieg besonders hoch. Diesen seltenen Fall haben wir derzeit bei SQI Diagnostics, einem kanadischen Medizintechnik-Unternehmen. Kernkompetenz sind vollautomatische Analysen aus einer einzigen biologischen Probe (z.B. Blut), sogenannte Multiplex-Tests.
SQI hat über fünf Jahre an seiner Technologie gearbeitet und über 45 Millionen USD darin investiert. Mögliche Einsatzbereiche sind Diagnostische Labore sowie die Produktentwicklung von Pharma-Unternehmen, die durch den Einsatz der SQI-Produkte bis zu 40% ihrer Labor-Kosten reduzieren und schnellere sowie bessere Daten generieren können. SQI schätzt das Marktvolumen für Multiplex-Tests allein in Europa und den USA auf 2 Mrd. USD.
Im vergangenen Jahr wurden vier “Master Service Agreements” mit großen Pharma-Unternehmen geschlossen, um diese von den Produkten zu überzeugen. Prototypen wurden entwickelt und binnen weniger Wochen sollten nun daraus konkrete Abschlüsse hervorgehen. Allein diese vier Pharma-Unternehmen, darunter ISIS Pharmaceuticals und Allergan, haben 112 Medikamente in der Entwicklung. Für jedes dieser Medikamente liegt das Umsatzpotenzial von SQI zwischen einigen hunderttausend bis zu über einer Millionen US-Dollar. Und man kann mit einer gewissen Wahrscheinlichkeit davon ausgehen, dass nach Abschluss der ersten Deals weitere wesentlich leichter zu erreichen sein werden.
Die großen Pharma-Unternehmen müssen Kosten sparen. Und sie müssen auch alles tun, um die Entwicklung neuer Medikamente zu beschleunigen. Wenn ein Gigant wie Bristol-Myers Squibb öffentlich bekannt macht, dass die SQI-Technologie überlegen ist, dann hat das schon etwas von einem “Ritter-Schlag”.
Mit einem Börsenwert von gerade mal 25 Mio. CAD erscheint mir das Unternehmen recht günstig bewertet. Zwar wird SQI in 2014 noch Verluste ausweisen, doch es dürften erstmals seit Gründung relevante Umsätze verzeichnet werden. 2015 sollte dann der “große Sprung” einsetzen, den die Börse jedoch schon in 2014 antizipieren dürfte. Ich kann der (bezahlten) Bewertung von Edison Research einiges abgewinnen, die den fairen Unternehmenswert bei 70 Mio. CAD sehen. Das würde einem Kurs von 1,45 CAD entsprechen.
Ich habe entsprechend in den vergangenen Wochen eine mittelfristig ausgerichtete Position in Aktien von SQI Diagnostics aufgebaut und übe mich nun in Geduld. Die Aktie ist extrem markteng, sodass relevante Neuigkeiten sehr schnell zu einer Neubewertung führen können.
Daten:
SQI Diagnostics
Kurs: 0,52 CAD
Kürzel/ISIN: SQD.V / CA78466B1085
Bitte beachten Sie: Dies ist keine Anlageberatung. Sie sind für Ihre Wertpapiertransaktionen selbst verantwortlich.
---
Feedback immer willkommen!
M@trix
SQI Diagnostics vor Neubewertung
Wenn ein Unternehmen mit seinen Produkten unmittelbar vor der erfolgreichen Kommerzialisierung steht, ist das oft ein guter Moment, zu investieren. Ist die Aktie dazu noch nahezu unbekannt, ist die Chance auf einen günstigen Einstieg besonders hoch. Diesen seltenen Fall haben wir derzeit bei SQI Diagnostics, einem kanadischen Medizintechnik-Unternehmen. Kernkompetenz sind vollautomatische Analysen aus einer einzigen biologischen Probe (z.B. Blut), sogenannte Multiplex-Tests.
SQI hat über fünf Jahre an seiner Technologie gearbeitet und über 45 Millionen USD darin investiert. Mögliche Einsatzbereiche sind Diagnostische Labore sowie die Produktentwicklung von Pharma-Unternehmen, die durch den Einsatz der SQI-Produkte bis zu 40% ihrer Labor-Kosten reduzieren und schnellere sowie bessere Daten generieren können. SQI schätzt das Marktvolumen für Multiplex-Tests allein in Europa und den USA auf 2 Mrd. USD.
Im vergangenen Jahr wurden vier “Master Service Agreements” mit großen Pharma-Unternehmen geschlossen, um diese von den Produkten zu überzeugen. Prototypen wurden entwickelt und binnen weniger Wochen sollten nun daraus konkrete Abschlüsse hervorgehen. Allein diese vier Pharma-Unternehmen, darunter ISIS Pharmaceuticals und Allergan, haben 112 Medikamente in der Entwicklung. Für jedes dieser Medikamente liegt das Umsatzpotenzial von SQI zwischen einigen hunderttausend bis zu über einer Millionen US-Dollar. Und man kann mit einer gewissen Wahrscheinlichkeit davon ausgehen, dass nach Abschluss der ersten Deals weitere wesentlich leichter zu erreichen sein werden.
Die großen Pharma-Unternehmen müssen Kosten sparen. Und sie müssen auch alles tun, um die Entwicklung neuer Medikamente zu beschleunigen. Wenn ein Gigant wie Bristol-Myers Squibb öffentlich bekannt macht, dass die SQI-Technologie überlegen ist, dann hat das schon etwas von einem “Ritter-Schlag”.
Mit einem Börsenwert von gerade mal 25 Mio. CAD erscheint mir das Unternehmen recht günstig bewertet. Zwar wird SQI in 2014 noch Verluste ausweisen, doch es dürften erstmals seit Gründung relevante Umsätze verzeichnet werden. 2015 sollte dann der “große Sprung” einsetzen, den die Börse jedoch schon in 2014 antizipieren dürfte. Ich kann der (bezahlten) Bewertung von Edison Research einiges abgewinnen, die den fairen Unternehmenswert bei 70 Mio. CAD sehen. Das würde einem Kurs von 1,45 CAD entsprechen.
Ich habe entsprechend in den vergangenen Wochen eine mittelfristig ausgerichtete Position in Aktien von SQI Diagnostics aufgebaut und übe mich nun in Geduld. Die Aktie ist extrem markteng, sodass relevante Neuigkeiten sehr schnell zu einer Neubewertung führen können.
Daten:
SQI Diagnostics
Kurs: 0,52 CAD
Kürzel/ISIN: SQD.V / CA78466B1085
Bitte beachten Sie: Dies ist keine Anlageberatung. Sie sind für Ihre Wertpapiertransaktionen selbst verantwortlich.
---
Feedback immer willkommen!
M@trix
Antwort auf Beitrag Nr.: 46.542.443 von M@trix am 28.02.14 15:16:28
Der Blog ist ma abgespeichert.
Ich kann zu den meisten nichts konkret sagen(RGX eine Ausnahme), aber mach weiter so.

Gruß
P.
Der Blog ist ma abgespeichert.
Ich kann zu den meisten nichts konkret sagen(RGX eine Ausnahme), aber mach weiter so.


Gruß
P.
Antwort auf Beitrag Nr.: 46.496.037 von M@trix am 20.02.14 14:40:33
MD &A - Feb 14, 2014
http://sedar.com/GetFile.do?lang=EN&docClass=7&issuerNo=0002…
MD &A - Feb 14, 2014
http://sedar.com/GetFile.do?lang=EN&docClass=7&issuerNo=0002…
Antwort auf Beitrag Nr.: 46.239.061 von M@trix am 16.01.14 11:06:23
Das Edison Teil ist interessant.
Gruß
P.
Das Edison Teil ist interessant.
Gruß
P.
Antwort auf Beitrag Nr.: 46.239.061 von M@trix am 16.01.14 11:06:23
da kommt es.
SQI Diagnostics Announces Filing of Preliminary Prospectus - Mar 12, 2014
http://finance.yahoo.com/news/sqi-diagnostics-announces-fili…
" TORONTO , March 12, 2014 /CNW/ - SQI Diagnostics Inc. ("SQI" or the "Company") (SQD.V), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced it has filed and received a receipt for a preliminary short form prospectus in connection with a proposed offering of units of the Company. Each unit will be comprised of one common share and a fraction of up to one common share purchase warrant. The offering will be pursued on a best efforts basis pursuant to an agency agreement to be entered into between the Company and Euro Pacific Canada Inc. (the "Agent") in each of the provinces of Ontario , British Columbia and Alberta . The number of units to be distributed under the offering, the price and composition of each unit and the exercise price of each common share purchase warrant will be determined in the context of the market by the Company and the Agent prior to filing the final short form prospectus.
SQI intends to use the net proceeds to fund the Company's product development and commercialization programs, sales and marketing and for general working capital purposes.
The securities described herein have not been, and will not be, registered under the United States Securities Act of 1933, as amended (the "U.S. Securities Act") or any state securities laws and accordingly may not be offered or sold within the United States or to "U.S. persons", as such term is defined in Regulation S promulgated under the U.S. Securities Act ("U.S. Persons"), except in compliance with the registration requirements of the U.S. Securities Act and applicable state securities requirements or pursuant to exemptions therefrom. This news release does not constitute an offer to sell or a solicitation of an offer to buy and of the Company's securities to, or for the account of benefit of, persons in the United States or U.S. Persons.
About SQI Diagnostics
SQI Diagnostics is a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. The Company's proprietary microarray tests and fully-automated systems are designed to simplify protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. For more information, please visit www.sqidiagnostics.com. "
da kommt es.
SQI Diagnostics Announces Filing of Preliminary Prospectus - Mar 12, 2014
http://finance.yahoo.com/news/sqi-diagnostics-announces-fili…
" TORONTO , March 12, 2014 /CNW/ - SQI Diagnostics Inc. ("SQI" or the "Company") (SQD.V), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced it has filed and received a receipt for a preliminary short form prospectus in connection with a proposed offering of units of the Company. Each unit will be comprised of one common share and a fraction of up to one common share purchase warrant. The offering will be pursued on a best efforts basis pursuant to an agency agreement to be entered into between the Company and Euro Pacific Canada Inc. (the "Agent") in each of the provinces of Ontario , British Columbia and Alberta . The number of units to be distributed under the offering, the price and composition of each unit and the exercise price of each common share purchase warrant will be determined in the context of the market by the Company and the Agent prior to filing the final short form prospectus.
SQI intends to use the net proceeds to fund the Company's product development and commercialization programs, sales and marketing and for general working capital purposes.
The securities described herein have not been, and will not be, registered under the United States Securities Act of 1933, as amended (the "U.S. Securities Act") or any state securities laws and accordingly may not be offered or sold within the United States or to "U.S. persons", as such term is defined in Regulation S promulgated under the U.S. Securities Act ("U.S. Persons"), except in compliance with the registration requirements of the U.S. Securities Act and applicable state securities requirements or pursuant to exemptions therefrom. This news release does not constitute an offer to sell or a solicitation of an offer to buy and of the Company's securities to, or for the account of benefit of, persons in the United States or U.S. Persons.
About SQI Diagnostics
SQI Diagnostics is a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. The Company's proprietary microarray tests and fully-automated systems are designed to simplify protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. For more information, please visit www.sqidiagnostics.com. "
SQI Diagnostics Signs Commercial Agreement, with "Global Pharma Customer", SQI to Develop Custom 21-Plex Protein Microarray for Drug Epitope Mapping - Mar 19, 2014
http://sqidiagnostics.com/media/2014/sqi-diagnostics-signs-c…
"SQI Diagnostics Inc. (TSX-V: SQD), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced that it has expanded its previously announced product development relationship with a global pharmaceutical company (“Global Pharma 1”) to develop a custom multiplex test to support the pharmaceutical company’s clinical drug development activities, through the entering into of a revenue-generating agreement with Global Pharma 1 to develop a 21-plex protein microarray.
Pharmaceutical companies expend substantial resources to better understand potential immune responses to the novel therapeutics they are developing. Under this new commercial agreement, SQI will develop a 21-plex protein microarray, based on the already completed prototype, for use in identifying specific immunogenic regions within a specific drug (also known as “epitope mapping”) during Global Pharma 1’s human clinical trials. The agreement for the second phase of the project includes both payment for services and for the consumables used during development and sample testing.
SQI recently successfully completed development of a series of multiplex anti-drug antibody (“ADA”) assays for Global Pharma 1 to detect and measure immunogenic responses to the drug during its pre-clinical development as part of the first phase of the project.
About SQI Diagnostics
SQI Diagnostics is a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. The Company’s proprietary microarray tests and fully-automated systems are designed to simplify protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. For more information, please visit www.sqidiagnostics.com.
SQI’s custom Ig_plex® tests and sqidlite™ automated testing equipment provide significant benefits to drug developers. SQI’s Ig_plex® products have been shown to significantly improve the sensitivity and other important performance metrics of ADA testing, ultimately resulting higher quality data. By decreasing the total number of tests performed through multiplexing, the total time required to process tests is reduced, resulting in reduced labour and testing timelines. In addition, considerably less blood volume is required from patient samples. These benefits can potentially have a significant impact on a customer’s total cost of testing in the clinical phases of the customer’s drug development programs.
Sales and Marketing Contact
Russ Peloquin
Vice President, Global Commercial Operations
913.484.9022
rpeloquin@sqidiagnostics.com
Investor Relations Contact
Andrew Morris
Chief Executive Officer
416.674.9500 ext. 229
amorris@sqidiagnostics.com
James Smith
VP Corporate Development
416.674.9500 ext. 241
jsmith@sqidiagnostics.com "
http://sqidiagnostics.com/media/2014/sqi-diagnostics-signs-c…
"SQI Diagnostics Inc. (TSX-V: SQD), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced that it has expanded its previously announced product development relationship with a global pharmaceutical company (“Global Pharma 1”) to develop a custom multiplex test to support the pharmaceutical company’s clinical drug development activities, through the entering into of a revenue-generating agreement with Global Pharma 1 to develop a 21-plex protein microarray.
Pharmaceutical companies expend substantial resources to better understand potential immune responses to the novel therapeutics they are developing. Under this new commercial agreement, SQI will develop a 21-plex protein microarray, based on the already completed prototype, for use in identifying specific immunogenic regions within a specific drug (also known as “epitope mapping”) during Global Pharma 1’s human clinical trials. The agreement for the second phase of the project includes both payment for services and for the consumables used during development and sample testing.
SQI recently successfully completed development of a series of multiplex anti-drug antibody (“ADA”) assays for Global Pharma 1 to detect and measure immunogenic responses to the drug during its pre-clinical development as part of the first phase of the project.
About SQI Diagnostics
SQI Diagnostics is a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. The Company’s proprietary microarray tests and fully-automated systems are designed to simplify protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. For more information, please visit www.sqidiagnostics.com.
SQI’s custom Ig_plex® tests and sqidlite™ automated testing equipment provide significant benefits to drug developers. SQI’s Ig_plex® products have been shown to significantly improve the sensitivity and other important performance metrics of ADA testing, ultimately resulting higher quality data. By decreasing the total number of tests performed through multiplexing, the total time required to process tests is reduced, resulting in reduced labour and testing timelines. In addition, considerably less blood volume is required from patient samples. These benefits can potentially have a significant impact on a customer’s total cost of testing in the clinical phases of the customer’s drug development programs.
Sales and Marketing Contact
Russ Peloquin
Vice President, Global Commercial Operations
913.484.9022
rpeloquin@sqidiagnostics.com
Investor Relations Contact
Andrew Morris
Chief Executive Officer
416.674.9500 ext. 229
amorris@sqidiagnostics.com
James Smith
VP Corporate Development
416.674.9500 ext. 241
jsmith@sqidiagnostics.com "
Ja, es geht voran - nur nicht beim Kurs. Indes ist die Aktie sehr illiquide. Ich nehme an, es wird irgendwann ein Tag kommen, an dem eine sehr publikumswirksame Empfehlung die Aktie binnen weniger Tage auf deutlich über 1,00 CAD steigen lassen wird.
Generell wäre m.E. für SQI ein Listing an der Nasdaq sehr vorteilhaft. Ich frage mal nach, ob so etwas geplant ist.
Auf sedar wurden übrigens "Marketing-Unterlagen" veröffentlicht. SQI sucht offensichtlich einen Investor...
Generell wäre m.E. für SQI ein Listing an der Nasdaq sehr vorteilhaft. Ich frage mal nach, ob so etwas geplant ist.
Auf sedar wurden übrigens "Marketing-Unterlagen" veröffentlicht. SQI sucht offensichtlich einen Investor...
Antwort auf Beitrag Nr.: 46.674.345 von M@trix am 21.03.14 14:17:56
Ich hab mir Edison mal reingehauen und die sind ja, kurszieltechnisch, von einigen ANnahmen ausgegangen/von abhängig.
Bis dahin wäre es noch ein gutes STück Weg. Da muss man schon bisschen rechnen, mit den 3 verschiedenen Geräten.
Für mich, bis jetzt, nichts.
"Publikumswirksame" EMpfehlungen sind mir Sch....nurzegal, bin eigentlich sogar eher dagegen.
Entweder ne Firma schafft es, oder das ist eh nur stupid money.
Gruß
P.
Ich hab mir Edison mal reingehauen und die sind ja, kurszieltechnisch, von einigen ANnahmen ausgegangen/von abhängig.
Bis dahin wäre es noch ein gutes STück Weg. Da muss man schon bisschen rechnen, mit den 3 verschiedenen Geräten.
Für mich, bis jetzt, nichts.
"Publikumswirksame" EMpfehlungen sind mir Sch....nurzegal, bin eigentlich sogar eher dagegen.
Entweder ne Firma schafft es, oder das ist eh nur stupid money.
Gruß
P.
Hallo Popeye82,
die Empfehlungen sind nicht unwichtig, denn sie schaffen Liquidität und Bekanntheit. Beides ist auch für Institutionelle wichtig. Zudem vereinfacht es die Aufnahme von Kapital, ohne extrem verwässern zu müssen.
Wir werden sehen - ich bleibe dabei. Das Unternehmen hat Top Leute an Board und dass sie auch Abschlüsse erreichen können, hat sich nun auch gezeigt. Ich denke, in 12-18 Monaten wird SQI völlig anders bewertet als jetzt.
Beste Grüße,
M@trix
die Empfehlungen sind nicht unwichtig, denn sie schaffen Liquidität und Bekanntheit. Beides ist auch für Institutionelle wichtig. Zudem vereinfacht es die Aufnahme von Kapital, ohne extrem verwässern zu müssen.
Wir werden sehen - ich bleibe dabei. Das Unternehmen hat Top Leute an Board und dass sie auch Abschlüsse erreichen können, hat sich nun auch gezeigt. Ich denke, in 12-18 Monaten wird SQI völlig anders bewertet als jetzt.
Beste Grüße,
M@trix
Antwort auf Beitrag Nr.: 46.623.675 von Popeye82 am 13.03.14 16:49:14
SQI Diagnostics Completes $4.200.000 Equity Financing - Apr 10, 2014
http://finance.yahoo.com/news/sqi-diagnostics-completes-4-2-…
"TORONTO, April 10, 2014 /PRNewswire/ - SQI Diagnostics Inc. ("SQI" or the "Company") (SQD.V), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced that it has closed its previously announced public offering (the "Offering"). Pursuant to the Offering, SQI issued 8,400,000 units of the Company ("Units") at a price of C$0.50 per Unit for gross proceeds of C$4.2 million. Each Unit is comprised of one common share of the Company (a "Common Share") and one Common Share purchase warrant (a "Warrant"). Each Warrant is exercisable at a price of C$0.65 and entitles the holder thereof to acquire one Common Share until April 10, 2016.
The Units were issued pursuant to an agency agreement the Company entered into with Euro Pacific Canada Inc. (the "Agent"). H.C. Wainwright & Co., LLC and Kingsdale Capital Markets Inc. formed part of the selling group, and H.C. Wainwright & Co., LLC acted as lead U.S. placement agent. The Company paid the Agent a fee equal to 7% of the gross proceeds raised ($0.035 per unit) and issued compensation options to acquire up to that number of Common Shares as is equal to 7% of the number of Units issued pursuant to the Offering at the offering price until April 10, 2016.
SQI intends to use the net proceeds to fund the Company's product development and commercialization programs, sales and marketing and for general working capital purposes.
The securities described herein have not been, and will not be, registered under the United States Securities Act of 1933, as amended (the "U.S. Securities Act") or any state securities laws and accordingly may not be offered or sold within the United States or to "U.S. persons", as such term is defined in Regulation S promulgated under the U.S. Securities Act ("U.S. Persons"), except in compliance with the registration requirements of the U.S. Securities Act and applicable state securities requirements or pursuant to exemptions therefrom. This news release does not constitute an offer to sell or a solicitation of an offer to buy any of the Company's securities to, or for the account of benefit of, persons in the United States or U.S. Persons.
About SQI Diagnostics
SQI Diagnostics is a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. The Company's proprietary microarray tests and fully-automated systems are designed to simplify protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. For more information, please visit www.sqidiagnostics.com. "
SQI Diagnostics Completes $4.200.000 Equity Financing - Apr 10, 2014
http://finance.yahoo.com/news/sqi-diagnostics-completes-4-2-…
"TORONTO, April 10, 2014 /PRNewswire/ - SQI Diagnostics Inc. ("SQI" or the "Company") (SQD.V), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced that it has closed its previously announced public offering (the "Offering"). Pursuant to the Offering, SQI issued 8,400,000 units of the Company ("Units") at a price of C$0.50 per Unit for gross proceeds of C$4.2 million. Each Unit is comprised of one common share of the Company (a "Common Share") and one Common Share purchase warrant (a "Warrant"). Each Warrant is exercisable at a price of C$0.65 and entitles the holder thereof to acquire one Common Share until April 10, 2016.
The Units were issued pursuant to an agency agreement the Company entered into with Euro Pacific Canada Inc. (the "Agent"). H.C. Wainwright & Co., LLC and Kingsdale Capital Markets Inc. formed part of the selling group, and H.C. Wainwright & Co., LLC acted as lead U.S. placement agent. The Company paid the Agent a fee equal to 7% of the gross proceeds raised ($0.035 per unit) and issued compensation options to acquire up to that number of Common Shares as is equal to 7% of the number of Units issued pursuant to the Offering at the offering price until April 10, 2016.
SQI intends to use the net proceeds to fund the Company's product development and commercialization programs, sales and marketing and for general working capital purposes.
The securities described herein have not been, and will not be, registered under the United States Securities Act of 1933, as amended (the "U.S. Securities Act") or any state securities laws and accordingly may not be offered or sold within the United States or to "U.S. persons", as such term is defined in Regulation S promulgated under the U.S. Securities Act ("U.S. Persons"), except in compliance with the registration requirements of the U.S. Securities Act and applicable state securities requirements or pursuant to exemptions therefrom. This news release does not constitute an offer to sell or a solicitation of an offer to buy any of the Company's securities to, or for the account of benefit of, persons in the United States or U.S. Persons.
About SQI Diagnostics
SQI Diagnostics is a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. The Company's proprietary microarray tests and fully-automated systems are designed to simplify protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. For more information, please visit www.sqidiagnostics.com. "
.....hi.....
vom Hoch bei ca.2.- E runter auf 0,20.- der Markt zeigt was er bisher von dem Wert hält......Nichts.......Dabei ist das was SQI hat durchaus vorzeigbar......jetzt sollte mal definitif eine den Markt überzeugende Kooperation mit einem der Marktgrossen der Branche kommen,die sich auch in klingender Münze ausdrückt....das wäre doch ein Aufhorchsignal...
M.
vom Hoch bei ca.2.- E runter auf 0,20.- der Markt zeigt was er bisher von dem Wert hält......Nichts.......Dabei ist das was SQI hat durchaus vorzeigbar......jetzt sollte mal definitif eine den Markt überzeugende Kooperation mit einem der Marktgrossen der Branche kommen,die sich auch in klingender Münze ausdrückt....das wäre doch ein Aufhorchsignal...
M.
SQI Diagnostics Hired by 'Global Pharmaceutical Company', "one of the ten largest pharmaceutical companies in the world", to Develop Two Prototype Multiplex Immunoassays for Evaluation - May 20, 2014
http://sqidiagnostics.com/media/2014/sqi-diagnostics-hired-g…
"TORONTO, May 20, 2014 /CNW/ - SQI Diagnostics Inc. (TSX-V: SQD), a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced it is being paid by one of the ten largest pharmaceutical companies in the world to develop and validate two custom multiplex anti-drug-antibody ("ADA") assays. This new customer will evaluate these two products using an established drug from its portfolio in order to assess the capabilities and performance of SQI's technologies.
"This is an important opportunity to establish SQI's capabilities with one of the largest customers in our industry, and highlights the growing interest for our technology and its ability to make drug development faster and more efficient," said Andrew Morris, CEO of SQI Diagnostics. "To date, our customer base includes four of the fifty largest pharmaceutical and biotech companies which have contracted SQI to develop custom assays for evaluation to facilitate their immunogenicity testing."
About SQI Diagnostics
SQI Diagnostics is a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. The Company's proprietary microarray tests and fully-automated systems are designed to simplify protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. For more information, please visit www.sqidiagnostics.com. "
http://sqidiagnostics.com/media/2014/sqi-diagnostics-hired-g…
"TORONTO, May 20, 2014 /CNW/ - SQI Diagnostics Inc. (TSX-V: SQD), a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced it is being paid by one of the ten largest pharmaceutical companies in the world to develop and validate two custom multiplex anti-drug-antibody ("ADA") assays. This new customer will evaluate these two products using an established drug from its portfolio in order to assess the capabilities and performance of SQI's technologies.
"This is an important opportunity to establish SQI's capabilities with one of the largest customers in our industry, and highlights the growing interest for our technology and its ability to make drug development faster and more efficient," said Andrew Morris, CEO of SQI Diagnostics. "To date, our customer base includes four of the fifty largest pharmaceutical and biotech companies which have contracted SQI to develop custom assays for evaluation to facilitate their immunogenicity testing."
About SQI Diagnostics
SQI Diagnostics is a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. The Company's proprietary microarray tests and fully-automated systems are designed to simplify protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. For more information, please visit www.sqidiagnostics.com. "
Ich habe meine Position in SQI gestern erneut aufgestockt. Das Unternehmen ist auf dem richtigen Weg und die Aktie noch völlig unentdeckt. Insofern steigt der Wert des Unternehmens und es ist nur eine Frage der Zeit, bis der Kurs dies reflektiert.
Hier die m.E. sehr gelungene Investorenpräsentation:
http://sqidiagnostics.com/sites/default/files/SQI%20Website%…
Man achte vor allem auf Folie 13. Da steckt noch immenses Potenzial bei den bereits bestehenden Kunden und natürlich sollte SQI auch weitere Neukunden gewinnen können.
Für die kommenden 12 Monate ist keine Kapitalerhöhung mehr zu erwarten. Demnach sollte von nun an jede positive Nachricht den Unternehmenswert weiter steigen lassen.
Ich halte auf Sicht eines Jahres durchaus Kursregionen um 1,00 CAD für möglich. Aktuell 0,40 CAD...
M@trix
Hier die m.E. sehr gelungene Investorenpräsentation:
http://sqidiagnostics.com/sites/default/files/SQI%20Website%…
Man achte vor allem auf Folie 13. Da steckt noch immenses Potenzial bei den bereits bestehenden Kunden und natürlich sollte SQI auch weitere Neukunden gewinnen können.
Für die kommenden 12 Monate ist keine Kapitalerhöhung mehr zu erwarten. Demnach sollte von nun an jede positive Nachricht den Unternehmenswert weiter steigen lassen.
Ich halte auf Sicht eines Jahres durchaus Kursregionen um 1,00 CAD für möglich. Aktuell 0,40 CAD...
M@trix
SQI building value - now best time to invest
Imho now is the best time to invest - I just bought again last Friday. Reasons:
1. $45 million invested in the superior technology - current market value of the whole company $22.5 million.
2. First revenues and gaining momentum – good indicator is hiring (current opportunities from website):
Life Sciences Business Manager, Northeast US
Development Associate
Assay Development Scientist
Manufacturing Technologist
3. Full pipeline with already existing customers (see investor presentation slide 13):
http://sqidiagnostics.com/sites/default/files/SQI%20Website%…
4. Market for multiplexed testing by North American and European pharmaceutical companies $2.0 billion => lots of space to grow.
5. Break even next year.
6. No need for capital for the next 12 months (= no further dilution).
7. SQI seeks a listing on the Nasdaq. I don't think this will happen this year, but 2015 seems possible.
8. Stock unknown and undiscovered - yet.
So at $0,40 it's an absolute bargain! You will remember my words...
http://www.stockhouse.com/companies/bullboard/v.sqd/sqi-diag…
Imho now is the best time to invest - I just bought again last Friday. Reasons:
1. $45 million invested in the superior technology - current market value of the whole company $22.5 million.
2. First revenues and gaining momentum – good indicator is hiring (current opportunities from website):
Life Sciences Business Manager, Northeast US
Development Associate
Assay Development Scientist
Manufacturing Technologist
3. Full pipeline with already existing customers (see investor presentation slide 13):
http://sqidiagnostics.com/sites/default/files/SQI%20Website%…
4. Market for multiplexed testing by North American and European pharmaceutical companies $2.0 billion => lots of space to grow.
5. Break even next year.
6. No need for capital for the next 12 months (= no further dilution).
7. SQI seeks a listing on the Nasdaq. I don't think this will happen this year, but 2015 seems possible.
8. Stock unknown and undiscovered - yet.
So at $0,40 it's an absolute bargain! You will remember my words...
http://www.stockhouse.com/companies/bullboard/v.sqd/sqi-diag…
Antwort auf Beitrag Nr.: 47.085.114 von M@trix am 02.06.14 11:51:38
Dieser Einschätzung schliesse ich mich, so, nicht an.
Gruß
P.
Dieser Einschätzung schliesse ich mich, so, nicht an.
Gruß
P.
Hallo Popeye82,
kannst du vielleicht kurz begründen, warum nicht?
Bin immer offen für Argumente, die meine Bewertung hinterfragen.
Grüße,
M@trix
kannst du vielleicht kurz begründen, warum nicht?
Bin immer offen für Argumente, die meine Bewertung hinterfragen.
Grüße,
M@trix
Antwort auf Beitrag Nr.: 47.090.802 von M@trix am 03.06.14 08:48:44
Weil Du an SQI Diagnostics interessiert bist, die Butze kannst Du Dir auch mal anschauen.
Von SeeThruEquity weiss ich auf jeden Fall dass sie über einige interessante bis hochinteressante (meist "development stage")Buden Recherche machen, bin da eingetragen.
"COTI is a leading-edge bioinformatics company specializing in accelerating the discovery and development of small molecules - dramatically reducing the time and cost to bring new drugs to market. COTI's proprietary artificial intelligence system, CHEMSAS(R), utilizes a series of predictive computer models to identify compounds with a high probability of being successfully developed from disease specific drug discovery through chemical optimization and preclinical testing. These compounds are targeted for a variety of diseases, particularly those for which current treatments are either lacking or ineffective. "
Critical Outcome Technologies - SeeThruEquity Initiates Research Coverage - Jun 13, 2014
http://media.wix.com/ugd/a15970_a457b590c6ed465a8ebeccfc812c…
Gruß
P.
Weil Du an SQI Diagnostics interessiert bist, die Butze kannst Du Dir auch mal anschauen.
Von SeeThruEquity weiss ich auf jeden Fall dass sie über einige interessante bis hochinteressante (meist "development stage")Buden Recherche machen, bin da eingetragen.
"COTI is a leading-edge bioinformatics company specializing in accelerating the discovery and development of small molecules - dramatically reducing the time and cost to bring new drugs to market. COTI's proprietary artificial intelligence system, CHEMSAS(R), utilizes a series of predictive computer models to identify compounds with a high probability of being successfully developed from disease specific drug discovery through chemical optimization and preclinical testing. These compounds are targeted for a variety of diseases, particularly those for which current treatments are either lacking or ineffective. "
Critical Outcome Technologies - SeeThruEquity Initiates Research Coverage - Jun 13, 2014
http://media.wix.com/ugd/a15970_a457b590c6ed465a8ebeccfc812c…
Gruß
P.
Hallo Matrix, Hallo Popeye82 schliessen sich die beiden Technologien SQI/COTI aus ?
Popeye / Matrix auf der ersten Seite sprecht ihr von Matrixs Blog...ähem wo kann ich denn den finden ? leider war da auf der ersten Seite kein Link.....
Popeye / Matrix auf der ersten Seite sprecht ihr von Matrixs Blog...ähem wo kann ich denn den finden ? leider war da auf der ersten Seite kein Link.....
Antwort auf Beitrag Nr.: 47.196.144 von boersehp am 23.06.14 20:22:27
Hallo boersehp,
Also ob sich die beiden 'ausschliessen' kann ich Dir aus dem Stand nicht sagen.
Matrix Blog müsste der hier sein, kann man auch in seinem Profil nachlesen.
http://boerseninfo.wordpress.com
Gruß´
P.
Hallo boersehp,
Also ob sich die beiden 'ausschliessen' kann ich Dir aus dem Stand nicht sagen.
Matrix Blog müsste der hier sein, kann man auch in seinem Profil nachlesen.
http://boerseninfo.wordpress.com
Gruß´
P.
Ich habe meinen Bestand in den letzten Tagen weiter ausgebaut. Sehr aufmerksam verfolge ich seit einigen Wochen die für das Unternehmen auffällig hohe Zahl an Stellenanzeigen (aktuell 4, folgende 2 kürzlich noch offene Stellen scheinen schon besetzt worden zu sein "Assay Development Scientist" und "Manufacturing Technologist"):
http://sqidiagnostics.com/about/careers
Mein Eindruck ist, dass hier bereits Aufträge "sicher" sind, aber noch nicht kommuniziert werden können/sollen. Man lese dazu auch den jüngsten Brief an die Investoren:
http://sqidiagnostics.com/sites/default/files/SQI%20Sharehol…
Auszüge:
"This new customer will evaluate these two products using one of its already established drugs in order to assess the capabilities and performance of SQI’s technologies. As such, this is an initial entry point into a much larger opportunity to provide ADA testing for one or more of the numerous early stage drug candidates in their development pipeline. It’s also an opportunity, should we prove ourselves, to sell other types of product and services to them."
"For another Pharma customer, SQI has progressed from their evaluation of ADA tests to a request from them to build a higher value “epitope mapping” test prototype. We recently delivered our initial data resulting from the prototype test development to the customer and we now await the results of their assessment. For a third Pharma customer, our relationship recently expanded in that they asked us to provide data reporting services to them in addition to the assay development work we are doing, providing additional revenue from them as we move forward with them to commercialize the prototype."
"With many programs ongoing with a range of customers, we have a substantial volume of work ahead of us to deliver on – it will not be a quiet summer at SQI!"
Nach meiner Einschätzung könnte es hier bald "Schlag auf Schlag" gehen - und das bei einer Aktie, die extrem markteng ist... Die Bewertung kann sich dann sehr schnell verdoppeln, zumal für die nächsten 11 Monate auch keine weitere Verwässerung droht.
Ergo: SQI ist nun meine größte Position überhaupt. Ich bin davon überzeugt, dass hier eine unentdeckte Perle liegt deren Entdeckung nur noch eine Frage von Monaten ist.
M@trix
http://sqidiagnostics.com/about/careers
Mein Eindruck ist, dass hier bereits Aufträge "sicher" sind, aber noch nicht kommuniziert werden können/sollen. Man lese dazu auch den jüngsten Brief an die Investoren:
http://sqidiagnostics.com/sites/default/files/SQI%20Sharehol…
Auszüge:
"This new customer will evaluate these two products using one of its already established drugs in order to assess the capabilities and performance of SQI’s technologies. As such, this is an initial entry point into a much larger opportunity to provide ADA testing for one or more of the numerous early stage drug candidates in their development pipeline. It’s also an opportunity, should we prove ourselves, to sell other types of product and services to them."
"For another Pharma customer, SQI has progressed from their evaluation of ADA tests to a request from them to build a higher value “epitope mapping” test prototype. We recently delivered our initial data resulting from the prototype test development to the customer and we now await the results of their assessment. For a third Pharma customer, our relationship recently expanded in that they asked us to provide data reporting services to them in addition to the assay development work we are doing, providing additional revenue from them as we move forward with them to commercialize the prototype."
"With many programs ongoing with a range of customers, we have a substantial volume of work ahead of us to deliver on – it will not be a quiet summer at SQI!"
Nach meiner Einschätzung könnte es hier bald "Schlag auf Schlag" gehen - und das bei einer Aktie, die extrem markteng ist... Die Bewertung kann sich dann sehr schnell verdoppeln, zumal für die nächsten 11 Monate auch keine weitere Verwässerung droht.
Ergo: SQI ist nun meine größte Position überhaupt. Ich bin davon überzeugt, dass hier eine unentdeckte Perle liegt deren Entdeckung nur noch eine Frage von Monaten ist.
M@trix
Antwort auf Beitrag Nr.: 47.262.478 von M@trix am 04.07.14 16:27:27
Ich wäre mit Deinen Now best time to invest Kommentaren ein bisschen vorsichtig.
Ich denke die sich einstellende Erfahrung wird mir Recht geben.
Gruß
P.
Ich wäre mit Deinen Now best time to invest Kommentaren ein bisschen vorsichtig.
Ich denke die sich einstellende Erfahrung wird mir Recht geben.
Gruß
P.
Antwort auf Beitrag Nr.: 47.196.144 von boersehp am 23.06.14 20:22:27
Also hab jetzt mal beide Recherchen durch.
Die verfolgen, technisch, ganz andere Ansätze und -bin ich mir nicht gaaanz sicher- dürften sich daher eher keine Zielgruppen wegnehmen.
Und selbst wenn, der Markt -der (Pharma)R&D Auslagerung, Abnahme/Erleichterung von Arbeiten- ist riesengroß, dass wäre denke ich praktisch eher so wenn man 2 Lachse in der Ostsee hat, und befürchtet dass die sich gegenseitig dass Futter wegnehmen.
Zwischen ich finde was, eine Anwendung, interessant und ist ein (klarer)Kauf liegen bei mir aber oft gewaaaltige Unterschiede.
Ich denke, wenn, führt kein Weg dran vorbei sich die Sachen an Denen sie arbeiten, wie CHEMSAS(R), genauer angucken.
Gruß
P.
Also hab jetzt mal beide Recherchen durch.
Die verfolgen, technisch, ganz andere Ansätze und -bin ich mir nicht gaaanz sicher- dürften sich daher eher keine Zielgruppen wegnehmen.
Und selbst wenn, der Markt -der (Pharma)R&D Auslagerung, Abnahme/Erleichterung von Arbeiten- ist riesengroß, dass wäre denke ich praktisch eher so wenn man 2 Lachse in der Ostsee hat, und befürchtet dass die sich gegenseitig dass Futter wegnehmen.
Zwischen ich finde was, eine Anwendung, interessant und ist ein (klarer)Kauf liegen bei mir aber oft gewaaaltige Unterschiede.
Ich denke, wenn, führt kein Weg dran vorbei sich die Sachen an Denen sie arbeiten, wie CHEMSAS(R), genauer angucken.
Gruß
P.
Danke und noch ein schönes Wochenende!
Antwort auf Beitrag Nr.: 47.196.144 von boersehp am 23.06.14 20:22:27@boersehyp
Critical Outcome Technologies ist ein Bioinformatik-Unternehmen, SQI eher ein hochspezialisierter Maschinenbauer. Aus meiner Sicht sind die beiden Unternehmen überhaupt nicht vergleichbar. COTI KÖNNTE längerfristig ein größerer Hit werden, ich sehe aber keine kurzfristigen Trigger und COTI hat nach meiner Einschätzung noch einen langen Weg vor sich. Bei SQI ist die Entwicklungsphase weitestgehend abgeschlossen und die Kommerzialisierungsphase hat begonnen. Der Nutzen des SQI-Produkte ist evident und durch große Pharma-Unternehmen verifiziert: geringere Kosten und schnelleres Vorankommen. Jetzt geht es eben drum, wirkliche Abschlüsse zu erzielen. Was mir bei SQI sehr gut gefällt ist, dass wenn sie einen Kunden gewinnen, man recht sicher davon ausgehen kann, dass das Geschäft sich nach und nach deutlich ausweiten lassen wird. Offen ist noch, wie profitabel das Geschäftsmodell ist. Ich erwarte den break-even auf Quartalsbasis in der zweiten Hälfte des kommenden Jahres. Sollte ich mit der Annahme richtig liegen, dürfte der Börsenwert 2-3 mal höher liegen als jetzt.
@Popeye82
Mal sehen. Ich habe rund 20 Jahre Börsenerfahrung, davon etwa 15 mit Small Caps. Insofern bin ich mir der Risiken/Unwägbarkeiten durchaus bewusst.
M@trix
Critical Outcome Technologies ist ein Bioinformatik-Unternehmen, SQI eher ein hochspezialisierter Maschinenbauer. Aus meiner Sicht sind die beiden Unternehmen überhaupt nicht vergleichbar. COTI KÖNNTE längerfristig ein größerer Hit werden, ich sehe aber keine kurzfristigen Trigger und COTI hat nach meiner Einschätzung noch einen langen Weg vor sich. Bei SQI ist die Entwicklungsphase weitestgehend abgeschlossen und die Kommerzialisierungsphase hat begonnen. Der Nutzen des SQI-Produkte ist evident und durch große Pharma-Unternehmen verifiziert: geringere Kosten und schnelleres Vorankommen. Jetzt geht es eben drum, wirkliche Abschlüsse zu erzielen. Was mir bei SQI sehr gut gefällt ist, dass wenn sie einen Kunden gewinnen, man recht sicher davon ausgehen kann, dass das Geschäft sich nach und nach deutlich ausweiten lassen wird. Offen ist noch, wie profitabel das Geschäftsmodell ist. Ich erwarte den break-even auf Quartalsbasis in der zweiten Hälfte des kommenden Jahres. Sollte ich mit der Annahme richtig liegen, dürfte der Börsenwert 2-3 mal höher liegen als jetzt.
@Popeye82
Mal sehen. Ich habe rund 20 Jahre Börsenerfahrung, davon etwa 15 mit Small Caps. Insofern bin ich mir der Risiken/Unwägbarkeiten durchaus bewusst.
M@trix
SQI Diagnostics Appoints Industry Veteran to its Board of Directors
TORONTO, July 15, 2014 /PRNewswire/ - SQI Diagnostics Inc. (SQD.V), a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced it has appointed Dr. Allan Pronovost to its Board of Directors. SQI will also retain the services of Dr. Pronovost, as strategic advisor, where he will assist the Company in its efforts to secure strategic partnerships and/or additional capital to further advance the Company's IVD and Pharma business units. Dr. Pronovost has more than thirty years of experience in the field of innovative diagnostic testing.
"The extensive experience Allan brings to SQI is well-aligned with our strategic objectives. He has led the invention, development and commercialization of numerous innovative medical diagnostic tests, and also has a track record of success in providing diagnostic development services to Pharma and biotech companies," said Andrew Morris, CEO of SQI Diagnostics. "Dr. Pronovost will be an important advisor to SQI as we commercialize our products and services in the US and work to expand our current base of Pharma customers."
Dr. Pronovost is currently the President and CSO of Red Lion Chem Tech, a consortium of health related companies he founded over the past ten years, based in San Diego, California. He is also currently the Founder, Chairman and CEO of Stone Investments, LLC, an investment holding company. From 2003-2006, he was the President of the NID Division of Quest Diagnostics, the largest clinical diagnostic reference laboratory network in the world. Prior to this, from 1995-2006, he was Chairman and CEO of BioAlliances Group, LLC, a contract R&D and manufacturing company serving the Pharma and biotech industry, primarily in the development of novel diagnostic tests. From 1986-1995 he was the Vice President R&D at Quidel Corporation, San Diego and has also contributed in various roles at Ortho Clinical Diagnostics-Johnson & Johnson, DuPont, Eastman Kodak, Ansys Diagnostics and Trinity Biotech Holding PLC.
Dr. Pronovost has advanced 123 products from concept to market with commercial sales, including 74 FDA 510(K) Approved Medical Device products. He is credited with 63 issued patents or applications, and is an author of more than 80 publications including two books. Dr. Pronovost obtained his Ph.D. in Pathology at the University of Rhode Island and completed a Post-Doctoral Fellowship in Clinical Virology & Immunology at the Yale University School of Medicine and is certified in Clinical Laboratory Medicine.
Dr. Pronovost replaces Dr. Peter Lea who has resigned from the Board, effective immediately. Dr. Lea will continue as an active, non-voting senior advisor to the Board. Dr. Lea founded SQI Diagnostics in 1999 and invented and patented the core technology underpinning SQI's multiplexing microarray technology and is actively involved day to day in the role of Founder.
Dr. Pronovost will be granted options (the "Options") to purchase an aggregate of 300,000 common shares of the Corporation (the "Shares") at a price which is the greater of $0.40 per Share or the closing price per Share on the date hereof, pursuant to the Corporation's incentive stock option plan.
In addition, SQI has retained the services of Renmark Financial Communications Inc. ("Renmark") to provide the Company with investor relations services such as roadshow management, the organization of investor events and the distribution of corporate information. Renmark does not have any interest, directly or indirectly, in SQI Diagnostics or its securities, or any right or intent to acquire such an interest. The Company will pay to Renmark a monthly retainer of $5,000. The term of the agreement with Renmark is on a month-to-month basis, and the agreement can be terminated by either party by giving 30 days' written notice to the other party.
http://finance.yahoo.com/news/sqi-diagnostics-appoints-indus…
Wie mir scheint eine hochkarätige Ergänzung mit gutem Netzwerk und großem Erfahrungsschatz.
TORONTO, July 15, 2014 /PRNewswire/ - SQI Diagnostics Inc. (SQD.V), a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced it has appointed Dr. Allan Pronovost to its Board of Directors. SQI will also retain the services of Dr. Pronovost, as strategic advisor, where he will assist the Company in its efforts to secure strategic partnerships and/or additional capital to further advance the Company's IVD and Pharma business units. Dr. Pronovost has more than thirty years of experience in the field of innovative diagnostic testing.
"The extensive experience Allan brings to SQI is well-aligned with our strategic objectives. He has led the invention, development and commercialization of numerous innovative medical diagnostic tests, and also has a track record of success in providing diagnostic development services to Pharma and biotech companies," said Andrew Morris, CEO of SQI Diagnostics. "Dr. Pronovost will be an important advisor to SQI as we commercialize our products and services in the US and work to expand our current base of Pharma customers."
Dr. Pronovost is currently the President and CSO of Red Lion Chem Tech, a consortium of health related companies he founded over the past ten years, based in San Diego, California. He is also currently the Founder, Chairman and CEO of Stone Investments, LLC, an investment holding company. From 2003-2006, he was the President of the NID Division of Quest Diagnostics, the largest clinical diagnostic reference laboratory network in the world. Prior to this, from 1995-2006, he was Chairman and CEO of BioAlliances Group, LLC, a contract R&D and manufacturing company serving the Pharma and biotech industry, primarily in the development of novel diagnostic tests. From 1986-1995 he was the Vice President R&D at Quidel Corporation, San Diego and has also contributed in various roles at Ortho Clinical Diagnostics-Johnson & Johnson, DuPont, Eastman Kodak, Ansys Diagnostics and Trinity Biotech Holding PLC.
Dr. Pronovost has advanced 123 products from concept to market with commercial sales, including 74 FDA 510(K) Approved Medical Device products. He is credited with 63 issued patents or applications, and is an author of more than 80 publications including two books. Dr. Pronovost obtained his Ph.D. in Pathology at the University of Rhode Island and completed a Post-Doctoral Fellowship in Clinical Virology & Immunology at the Yale University School of Medicine and is certified in Clinical Laboratory Medicine.
Dr. Pronovost replaces Dr. Peter Lea who has resigned from the Board, effective immediately. Dr. Lea will continue as an active, non-voting senior advisor to the Board. Dr. Lea founded SQI Diagnostics in 1999 and invented and patented the core technology underpinning SQI's multiplexing microarray technology and is actively involved day to day in the role of Founder.
Dr. Pronovost will be granted options (the "Options") to purchase an aggregate of 300,000 common shares of the Corporation (the "Shares") at a price which is the greater of $0.40 per Share or the closing price per Share on the date hereof, pursuant to the Corporation's incentive stock option plan.
In addition, SQI has retained the services of Renmark Financial Communications Inc. ("Renmark") to provide the Company with investor relations services such as roadshow management, the organization of investor events and the distribution of corporate information. Renmark does not have any interest, directly or indirectly, in SQI Diagnostics or its securities, or any right or intent to acquire such an interest. The Company will pay to Renmark a monthly retainer of $5,000. The term of the agreement with Renmark is on a month-to-month basis, and the agreement can be terminated by either party by giving 30 days' written notice to the other party.
http://finance.yahoo.com/news/sqi-diagnostics-appoints-indus…
Wie mir scheint eine hochkarätige Ergänzung mit gutem Netzwerk und großem Erfahrungsschatz.
Antwort auf Beitrag Nr.: 47.324.344 von M@trix am 17.07.14 09:45:59
Lebensläufe ist immer so eine Sache.
Ich habe keine Ahnung von diesem Mann, aber schätze 123 concept to sales, und davon 74 bis zur FDA Genehmigung, duuuuurch, ist schon hammerhart, gut.
Gruß
P.
Lebensläufe ist immer so eine Sache.
Ich habe keine Ahnung von diesem Mann, aber schätze 123 concept to sales, und davon 74 bis zur FDA Genehmigung, duuuuurch, ist schon hammerhart, gut.
Gruß
P.
.....wieso der Absturz heute????
M.
M.
Leider ist dies ALLEIN der Marktenge geschuldet:
Transaction Volume: 80,130
Das ist ein Witz. Ich habe heute noch mal das Management angeschrieben, wann ein Nasdaq-Listing ansteht. Ohne Liquidität packt diese Aktie kein institutioneller Investor an.
Aus meiner Sicht jedenfalls eher eine Kaufchance. Mal sehen, was ich als Antwort bekomme. Je nach dem kaufe ich nach. Übrigens erneut neue Stellenausschreibung ("Quality Control Associate").
M@trix
Transaction Volume: 80,130
Das ist ein Witz. Ich habe heute noch mal das Management angeschrieben, wann ein Nasdaq-Listing ansteht. Ohne Liquidität packt diese Aktie kein institutioneller Investor an.
Aus meiner Sicht jedenfalls eher eine Kaufchance. Mal sehen, was ich als Antwort bekomme. Je nach dem kaufe ich nach. Übrigens erneut neue Stellenausschreibung ("Quality Control Associate").
M@trix
Antwort auf Beitrag Nr.: 47.196.144 von boersehp am 23.06.14 20:22:27
an Denen reizt es mich auch schon, geraume Zeit, mal dran zu knabbern. Aber eher eine 'medical device' Firma.
Aber einige Dinge abzuschätzen ist nicht so ganz einfach.
http://media.wix.com/ugd/a15970_7ed8b1f6bb0441628123c9e6fc35…
www.marketwired.com/press-release/oxysure-oxys-announces-cor…
www.oxysure.com/OxySure_Earnings_Presentation_1Q2014%20v2.pd…
Gruß
P.
an Denen reizt es mich auch schon, geraume Zeit, mal dran zu knabbern. Aber eher eine 'medical device' Firma.
Aber einige Dinge abzuschätzen ist nicht so ganz einfach.
http://media.wix.com/ugd/a15970_7ed8b1f6bb0441628123c9e6fc35…
www.marketwired.com/press-release/oxysure-oxys-announces-cor…
www.oxysure.com/OxySure_Earnings_Presentation_1Q2014%20v2.pd…
Gruß
P.
Aus dem aktuellen MD&A:
"The Company is currently in the process of finalizing a commercial contract with Isis’s CRO to implement the SQI-developed test in clinical testing, which could result in material revenues to the Company in fiscal 2014 with the CRO’s validation work expected to start in August, 2014"
"We anticipate that many of the successful evaluation projects completed over the last nine months will proceed to the next expected commercial phases, where SQI will produce and sell test kits to these customers for either its fully automated sqidlite or semi-automated sqid-X platforms. Within each Pharma customer, we are also seeking opportunities to expand the scope of ongoing programs and to develop new product opportunities."
"Management expects to reduce losses later in fiscal 2014 as it generates revenues and margin from a variety of Diagnostic Tools and Services’ customers. Successful US FDA clearance of its IVD Celiac test could result in initial revenue from that product in late fiscal 2014, further reducing overall losses."
http://www.sedar.com/DisplayCompanyDocuments.do?lang=EN&issu…
"The Company is currently in the process of finalizing a commercial contract with Isis’s CRO to implement the SQI-developed test in clinical testing, which could result in material revenues to the Company in fiscal 2014 with the CRO’s validation work expected to start in August, 2014"
"We anticipate that many of the successful evaluation projects completed over the last nine months will proceed to the next expected commercial phases, where SQI will produce and sell test kits to these customers for either its fully automated sqidlite or semi-automated sqid-X platforms. Within each Pharma customer, we are also seeking opportunities to expand the scope of ongoing programs and to develop new product opportunities."
"Management expects to reduce losses later in fiscal 2014 as it generates revenues and margin from a variety of Diagnostic Tools and Services’ customers. Successful US FDA clearance of its IVD Celiac test could result in initial revenue from that product in late fiscal 2014, further reducing overall losses."
http://www.sedar.com/DisplayCompanyDocuments.do?lang=EN&issu…
SQI Diagnostics Contracted to Automate Pathogen Detection Assays
TORONTO, Aug. 22, 2014 /PRNewswire/ - SQI Diagnostics Inc. (SQD.V), a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced that it has entered into a Master Service Agreement to contract with a UK-based company to automate their DNA-based pathogen detection assays. During the initial phase of this agreement, SQI will be paid to deliver an automated working prototype of one of the customer's assays, operational on SQI's sqidlite™ system. The initial phase is scheduled for completion by mid-September 2014. It is anticipated that other molecular diagnostic tests will be commissioned in a separate agreement following successful implementation of the initial phase.
The first assay being automated is used to identify pathogens in raw milk from dairy cows. Currently, dairy cows are routinely tested for health through a global network of laboratories and when symptoms of bacterial infections appear, pathogens are commonly detected using traditional plate cultures. The new application can be used for much faster and more accurate identification of multiple pathogens simultaneously. The UK-based company is developing additional assays for agriculture, food safety and human pathogen testing, intended to screen high volumes of samples on a regular basis.
"As we've explored this opportunity, we've been impressed by the compatibility and synergy of our new customer's high-performance molecular diagnostic technology with our automated multiplexing assay technologies," said Andrew Morris, CEO of SQI Diagnostics. "Although our automated systems have been developed to run our own protein-based IVD and pharma assays, our technology solution is highly adaptable to other types of tests and this presents a valuable additional market opportunity where our technologies can be applied with the potential for significant test volumes and revenue."
About SQI Diagnostics
SQI Diagnostics is a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. The Company's proprietary microarray tests and fully-automated systems are designed to simplify protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. For more information, please visit www.sqidiagnostics.com.
---
Heute findet zudem ein Investor Call statt, zudem ich einige Fragen eingereicht habe. Mal gespannt, wie die Antworten ausfallen. Der Call wird auch auf der Homepage von SQI veröffentlicht werden.
Generell bin ich weiterhin der Ansicht, dass die Aktie vollkommen unterbewertet ist. Die Zeit wird zeigen, ob ich da im Irrtum bin.
M@trix
TORONTO, Aug. 22, 2014 /PRNewswire/ - SQI Diagnostics Inc. (SQD.V), a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced that it has entered into a Master Service Agreement to contract with a UK-based company to automate their DNA-based pathogen detection assays. During the initial phase of this agreement, SQI will be paid to deliver an automated working prototype of one of the customer's assays, operational on SQI's sqidlite™ system. The initial phase is scheduled for completion by mid-September 2014. It is anticipated that other molecular diagnostic tests will be commissioned in a separate agreement following successful implementation of the initial phase.
The first assay being automated is used to identify pathogens in raw milk from dairy cows. Currently, dairy cows are routinely tested for health through a global network of laboratories and when symptoms of bacterial infections appear, pathogens are commonly detected using traditional plate cultures. The new application can be used for much faster and more accurate identification of multiple pathogens simultaneously. The UK-based company is developing additional assays for agriculture, food safety and human pathogen testing, intended to screen high volumes of samples on a regular basis.
"As we've explored this opportunity, we've been impressed by the compatibility and synergy of our new customer's high-performance molecular diagnostic technology with our automated multiplexing assay technologies," said Andrew Morris, CEO of SQI Diagnostics. "Although our automated systems have been developed to run our own protein-based IVD and pharma assays, our technology solution is highly adaptable to other types of tests and this presents a valuable additional market opportunity where our technologies can be applied with the potential for significant test volumes and revenue."
About SQI Diagnostics
SQI Diagnostics is a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. The Company's proprietary microarray tests and fully-automated systems are designed to simplify protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. For more information, please visit www.sqidiagnostics.com.
---
Heute findet zudem ein Investor Call statt, zudem ich einige Fragen eingereicht habe. Mal gespannt, wie die Antworten ausfallen. Der Call wird auch auf der Homepage von SQI veröffentlicht werden.
Generell bin ich weiterhin der Ansicht, dass die Aktie vollkommen unterbewertet ist. Die Zeit wird zeigen, ob ich da im Irrtum bin.
M@trix
Aber was die Frankfurter Börse da wieder für einen spread hat ist ja schon brutal......
"[...] The company's multiplexing technology, IgPlex, enables users to assay proteins on planar arrays printed on 96-well plates. With regards to competitors in the space, he said that SQI's assays "appear to be more impervious to the confounding effects of free drug remaining in the blood sample that interacts with the antibodies you are trying to detect."
Working to serve pharma clients' needs also develops SQI's skill set. Morris said that SQI's partners have asked it to tackle new problems, resulting in new assays.
"For example, for one customer we are developing an epitope mapping assay that breaks down their biological molecule into sub-units that become the basis for a multiplex test that can identify specific regions of their drug candidate which are immunoreactive," he said.
SQI is also seeing some interest in using its capabilities to follow-on the drug development work with the possibility for companion diagnostics products, Morris said. "Though these opportunities are not necessarily immediate, we believe that there is good potential here to use our systems and assay development/clearance experience to develop longer-term and potentially very large volume revenue business."
He noted that the assays SQI is developing for pharma are specific to the drugs they are developing and used primarily to guide drug development, so that their individual commercial potential is tied to the scope of the specific drug development program. However, with each project, the firm is expanding the functionality and capabilities of its technology and Morris believes SQI can bring these product lines to its overall customer base down the road.
While SQI's core focus is on generating revenue via various pharma relationships, it is still advancing its diagnostics menu, including obtaining US regulatory clearance for an updated, higher throughput Ig_plex Celiac DGP Panel while preparing a test for vasculitis for launch. The US Food and Drug Administration cleared SQI's original 4-plex celiac assay in 2011. The agency also cleared a test for rheumatoid arthritis in 2009. SQI has also received Health Canada licenses for both tests.
Earlier this year, SQI received a Health Canada license for the Ig_plex Celiac DGP Panel. The test is intended for use in clinical laboratories to help diagnose celiac disease and provides semi-quantitative determination of the IgG and IgA immunoglobulin classes of deamidated gliadin peptide and anti-tissue transglutaminase antibodies in a single test that requires only one human sample, SQI said at the time.
"We are still very focused on our IVD business as well," said Morris. He said the company has submitted its newest celiac panel to the FDA and is still working on the vasculitis test.
http://cdnwww.genomeweb.com/arrays/sqi-diagnostics-attractin…
Working to serve pharma clients' needs also develops SQI's skill set. Morris said that SQI's partners have asked it to tackle new problems, resulting in new assays.
"For example, for one customer we are developing an epitope mapping assay that breaks down their biological molecule into sub-units that become the basis for a multiplex test that can identify specific regions of their drug candidate which are immunoreactive," he said.
SQI is also seeing some interest in using its capabilities to follow-on the drug development work with the possibility for companion diagnostics products, Morris said. "Though these opportunities are not necessarily immediate, we believe that there is good potential here to use our systems and assay development/clearance experience to develop longer-term and potentially very large volume revenue business."
He noted that the assays SQI is developing for pharma are specific to the drugs they are developing and used primarily to guide drug development, so that their individual commercial potential is tied to the scope of the specific drug development program. However, with each project, the firm is expanding the functionality and capabilities of its technology and Morris believes SQI can bring these product lines to its overall customer base down the road.
While SQI's core focus is on generating revenue via various pharma relationships, it is still advancing its diagnostics menu, including obtaining US regulatory clearance for an updated, higher throughput Ig_plex Celiac DGP Panel while preparing a test for vasculitis for launch. The US Food and Drug Administration cleared SQI's original 4-plex celiac assay in 2011. The agency also cleared a test for rheumatoid arthritis in 2009. SQI has also received Health Canada licenses for both tests.
Earlier this year, SQI received a Health Canada license for the Ig_plex Celiac DGP Panel. The test is intended for use in clinical laboratories to help diagnose celiac disease and provides semi-quantitative determination of the IgG and IgA immunoglobulin classes of deamidated gliadin peptide and anti-tissue transglutaminase antibodies in a single test that requires only one human sample, SQI said at the time.
"We are still very focused on our IVD business as well," said Morris. He said the company has submitted its newest celiac panel to the FDA and is still working on the vasculitis test.
http://cdnwww.genomeweb.com/arrays/sqi-diagnostics-attractin…
hi,
langsam scheint was zu gehen hier.........wird Dornröschen endlichvon seinem Fluch befreit und wachgeküsst??
M.
langsam scheint was zu gehen hier.........wird Dornröschen endlichvon seinem Fluch befreit und wachgeküsst??
M.
Der Call war m.E. sehr erhellend und kompetent:
http://podcast.newswire.ca/media/sqi20140822.mp3
Ich denke, SQI ist auf dem richtigen Weg und fühle mich in meiner Einschätzung bestätigt. Das Kernproblem für SQI ist (noch) die Finanzierung, das kann sich aber schon im kommenden Jahr markant ändern. Selbst der break-even wird nicht ausgeschlossen, was ja auch meiner Erwartung entspricht (auf Quartalsbasis zum Ende 2015).
Die Aktie wird leider weiter sehr dünn gehandelt, ich bin jedoch zuversichtlich, dass bei weiteren News (die bereits angekündigt wurden) neue Investoren kommen werden und die Aktie sehr schnell wieder alte Bewertungsniveaus erreichen wird.
Insbesondere Dr. Allan Pronovost hat auf mich einen Überzeugenden Eindruck gemacht. Er gibt eine realistische Einschätzung des Unternehmens und ich traue ihm einiges für die Zukunft zu.
Geduld sollte belohnt werden.
M@trix
http://podcast.newswire.ca/media/sqi20140822.mp3
Ich denke, SQI ist auf dem richtigen Weg und fühle mich in meiner Einschätzung bestätigt. Das Kernproblem für SQI ist (noch) die Finanzierung, das kann sich aber schon im kommenden Jahr markant ändern. Selbst der break-even wird nicht ausgeschlossen, was ja auch meiner Erwartung entspricht (auf Quartalsbasis zum Ende 2015).
Die Aktie wird leider weiter sehr dünn gehandelt, ich bin jedoch zuversichtlich, dass bei weiteren News (die bereits angekündigt wurden) neue Investoren kommen werden und die Aktie sehr schnell wieder alte Bewertungsniveaus erreichen wird.
Insbesondere Dr. Allan Pronovost hat auf mich einen Überzeugenden Eindruck gemacht. Er gibt eine realistische Einschätzung des Unternehmens und ich traue ihm einiges für die Zukunft zu.
Geduld sollte belohnt werden.
M@trix
SQI ist seit heute am OTCQX® handelbar:
http://finance.yahoo.com/news/otc-markets-group-welcomes-sqi…
"We are pleased to be traded on the OTCQX marketplace," said Andrew Morris, CEO of SQI Diagnostics Inc. "Trading on the OTCQX will help increase awareness of SQI in the U.S. investment community and is an important step for SQI's continued growth."
Ich denke nicht, dass das dem Kurs helfen wird. Wichtig sind Deals, Deals, Deals.
M@trix
http://finance.yahoo.com/news/otc-markets-group-welcomes-sqi…
"We are pleased to be traded on the OTCQX marketplace," said Andrew Morris, CEO of SQI Diagnostics Inc. "Trading on the OTCQX will help increase awareness of SQI in the U.S. investment community and is an important step for SQI's continued growth."
Ich denke nicht, dass das dem Kurs helfen wird. Wichtig sind Deals, Deals, Deals.
M@trix
Antwort auf Beitrag Nr.: 47.326.384 von Popeye82 am 17.07.14 14:24:15
EIR - Sep 29, 2014
www.edisoninvestmentresearch.com/serve_pdf.php?d=researchrep…
EIR - Sep 29, 2014
www.edisoninvestmentresearch.com/serve_pdf.php?d=researchrep…
Danke!
Ich bin weiterhin sehr zuversichtlich. Die kommenden Wochen werden einiges an News bringen. Und mir ist zuletzt am Orderbuch aufgefallen, dass endlich die Brief-Seite ausgedünnt ist. Heißt: bei den nächsten guten News sollte es einen ordentlichen Schub nach oben geben.
Das EIR-Research war mir bisher etwas zu optimistisch. Jetzt ist es m.E. realistisch. Sollte SQI eine Vertriebs-Kooperation schließen (was ich als wahrscheinlich erachte im 2. Hj. 2015), könnte die Zeit nach 2015 dynamischer werden als erwartet.
Ich bin weiterhin sehr zuversichtlich. Die kommenden Wochen werden einiges an News bringen. Und mir ist zuletzt am Orderbuch aufgefallen, dass endlich die Brief-Seite ausgedünnt ist. Heißt: bei den nächsten guten News sollte es einen ordentlichen Schub nach oben geben.
Das EIR-Research war mir bisher etwas zu optimistisch. Jetzt ist es m.E. realistisch. Sollte SQI eine Vertriebs-Kooperation schließen (was ich als wahrscheinlich erachte im 2. Hj. 2015), könnte die Zeit nach 2015 dynamischer werden als erwartet.
Man achte auf das Orderbuch. Es scheint kaum noch Verkäufer zu geben. Wenn jetzt noch "good news" kommen, sollte die Wende geschafft sein.
Das Chartbild sieht jedenfalls nach einem langfristigen Doppel-Boden aus und wir sind nicht weit weg davon, den langfristigen Abwärtstrend seit 2011 zu brechen. Lediglich die Gesamtmarktsituation erscheint gerade alles andere als erbaulich.
M@trix

Das Chartbild sieht jedenfalls nach einem langfristigen Doppel-Boden aus und wir sind nicht weit weg davon, den langfristigen Abwärtstrend seit 2011 zu brechen. Lediglich die Gesamtmarktsituation erscheint gerade alles andere als erbaulich.
M@trix
Die höchsten Umsätze seit 3 Monaten (jedoch immer noch ein Witz). Dennoch: Die Aktie scheint die Wende begonnen zu haben.
Hier der neueste Aktionärsbrief:
http://sqidiagnostics.com/sites/default/files/SQI%20Sharehol…
Für meinen Geschmack steh ein bißchen viel die Formulierung "Ich glaube / Wir glauben" drin, doch grundsätzlich liest sich das recht positiv. Grundtenor: die ersten signifikanten Aufträge stehen kurz bevor.
"At the onset of the new fiscal year, we are excited to look forward with the expectation of building on our first commercial successes and revenues. With a growing list of customers and exciting projects in our pharma and custom development business, we anticipate strong growth in the number of customers and revenue-generating projects. While the conversion of paid development projects to full commercial adoption has been somewhat slower than initially expected, we are confident that our technology and custom solutions are being very well-received and that we will continue to validate and commercialize a number of these projects."
Das sehe ich auch so. 2015 wird für SQI DAS Jahr.
M@trix
Hier der neueste Aktionärsbrief:
http://sqidiagnostics.com/sites/default/files/SQI%20Sharehol…
Für meinen Geschmack steh ein bißchen viel die Formulierung "Ich glaube / Wir glauben" drin, doch grundsätzlich liest sich das recht positiv. Grundtenor: die ersten signifikanten Aufträge stehen kurz bevor.
"At the onset of the new fiscal year, we are excited to look forward with the expectation of building on our first commercial successes and revenues. With a growing list of customers and exciting projects in our pharma and custom development business, we anticipate strong growth in the number of customers and revenue-generating projects. While the conversion of paid development projects to full commercial adoption has been somewhat slower than initially expected, we are confident that our technology and custom solutions are being very well-received and that we will continue to validate and commercialize a number of these projects."
Das sehe ich auch so. 2015 wird für SQI DAS Jahr.
M@trix
SQI DIAGNOSTICS Receives FDA Clearance for its Ig_plex Celiac DGP Panel for Sale in the United States
TORONTO , Nov. 7, 2014 /CNW/ - SQI Diagnostics Inc. (the "Company" or "SQI Diagnostics") (SQD.V) (SQIDF) today announced that it has received notice that the United States Food and Drug Administration (FDA) has cleared the Company to market its proprietary Celiac Panel in the United States (US).
"We are very excited to have received FDA clearance," said Andrew Morris , CEO, SQI Diagnostics. "This clearance lays the foundation for sales in the US market of our newest in vitro diagnostic (IVD) autoimmune test." In addition to its suite of FDA cleared assays for celiac disease and rheumatoid arthritis, SQI is building a pipeline of other high-demand IVD autoimmune assays, including a quantitative 12-plex panel for lupus, a quantitative 3-plex panel for vasculitis, and an 8-plex panel for Crohn's disease. "Our revenue growth during the last fiscal year was achieved through the development of custom multiplexed assays for pharmaceutical companies and their clinical research organizations and most recently, molecular testing in the animal and human health markets. This clearance demonstrates the Company's ability to achieve success in the third multiplexing market in which it is involved: IVD autoimmune test development," said Morris. "The clearance is also an important element in marketing our products and services with leading global pharmaceutical companies, and diagnostic partners in our newest area of molecular testing."
Celiac disease is a medical condition in which the absorptive surface of the small intestine is damaged by gluten, resulting in an inability of the body to absorb nutrients necessary for good health. According to the New England Journal of Medicine1, it is estimated that 1 in 100 people in the United States is affected by celiac disease.
The SQI Celiac Panel is an Ig_plex Celiac DGP in vitro diagnostic test for the semi-quantitative detection of the IgA and IgG immunoglobulin classes of antibodies to deamidated gliadin peptide (DGP) and tissue transglutaminase (tTG) in human serum. The test is intended for use in clinical laboratories as an aid in the diagnosis of celiac disease in conjunction with other laboratory and clinical findings, and requires the use of a sqid-XTM system.
The American Journal of Gastroenterology has reported that as many as 97% of celiac sufferers were undiagnosed2. The 2013 American College of Gastroenterology guidelines and the American Gastroenterology Association recommend that tests for anti-tTG and anti-DPG should be part of routine testing for celiac disease3. Further, in a recent study by Health Quality Ontario, it was revealed that serologic testing for tTg has increased by 500% from 2004 to 20114.
For additional product information on the Ig_plex Celiac DGP Panel, please visit: http://sqidiagnostics.com/applications/ivd-and-clinical
Celiac Disease, Alessio Fasano , M.D., and Carlo Catassi , M.D., M.P.H., N Engl J Med 2012; 367:2419-2426, December 20, 2012 .
Characteristics of adult celiac disease in the USA : results of a national survey. Green, P.H. et.al. American Journal of Gastroenterology, 2001, 2006.
Am J Gastroenterol, 2013; 108:656–676; doi: 10.1038/ajg.2013.79; published online 16 April 2013 .
Health Quality Ontario Report, 2014.
http://finance.yahoo.com/news/sqi-diagnostics-receives-fda-c…
TORONTO , Nov. 7, 2014 /CNW/ - SQI Diagnostics Inc. (the "Company" or "SQI Diagnostics") (SQD.V) (SQIDF) today announced that it has received notice that the United States Food and Drug Administration (FDA) has cleared the Company to market its proprietary Celiac Panel in the United States (US).
"We are very excited to have received FDA clearance," said Andrew Morris , CEO, SQI Diagnostics. "This clearance lays the foundation for sales in the US market of our newest in vitro diagnostic (IVD) autoimmune test." In addition to its suite of FDA cleared assays for celiac disease and rheumatoid arthritis, SQI is building a pipeline of other high-demand IVD autoimmune assays, including a quantitative 12-plex panel for lupus, a quantitative 3-plex panel for vasculitis, and an 8-plex panel for Crohn's disease. "Our revenue growth during the last fiscal year was achieved through the development of custom multiplexed assays for pharmaceutical companies and their clinical research organizations and most recently, molecular testing in the animal and human health markets. This clearance demonstrates the Company's ability to achieve success in the third multiplexing market in which it is involved: IVD autoimmune test development," said Morris. "The clearance is also an important element in marketing our products and services with leading global pharmaceutical companies, and diagnostic partners in our newest area of molecular testing."
Celiac disease is a medical condition in which the absorptive surface of the small intestine is damaged by gluten, resulting in an inability of the body to absorb nutrients necessary for good health. According to the New England Journal of Medicine1, it is estimated that 1 in 100 people in the United States is affected by celiac disease.
The SQI Celiac Panel is an Ig_plex Celiac DGP in vitro diagnostic test for the semi-quantitative detection of the IgA and IgG immunoglobulin classes of antibodies to deamidated gliadin peptide (DGP) and tissue transglutaminase (tTG) in human serum. The test is intended for use in clinical laboratories as an aid in the diagnosis of celiac disease in conjunction with other laboratory and clinical findings, and requires the use of a sqid-XTM system.
The American Journal of Gastroenterology has reported that as many as 97% of celiac sufferers were undiagnosed2. The 2013 American College of Gastroenterology guidelines and the American Gastroenterology Association recommend that tests for anti-tTG and anti-DPG should be part of routine testing for celiac disease3. Further, in a recent study by Health Quality Ontario, it was revealed that serologic testing for tTg has increased by 500% from 2004 to 20114.
For additional product information on the Ig_plex Celiac DGP Panel, please visit: http://sqidiagnostics.com/applications/ivd-and-clinical
Celiac Disease, Alessio Fasano , M.D., and Carlo Catassi , M.D., M.P.H., N Engl J Med 2012; 367:2419-2426, December 20, 2012 .
Characteristics of adult celiac disease in the USA : results of a national survey. Green, P.H. et.al. American Journal of Gastroenterology, 2001, 2006.
Am J Gastroenterol, 2013; 108:656–676; doi: 10.1038/ajg.2013.79; published online 16 April 2013 .
Health Quality Ontario Report, 2014.
http://finance.yahoo.com/news/sqi-diagnostics-receives-fda-c…
Antwort auf Beitrag Nr.: 47.899.472 von M@trix am 29.09.14 14:55:04
+55%
Q.E.D.
Leider bei nur 6.000 St. Handelsvolumen... Heißt also leider nicht viel. Aber das zeigt, wie markteng diese Aktie ist und dass die Aktie ohne weiteres alte Hochs erreichen kann, sobald die Geschäftsentwicklung dies triggert.
M@trix
Zitat von M@trix: Danke!
Ich bin weiterhin sehr zuversichtlich. Die kommenden Wochen werden einiges an News bringen. Und mir ist zuletzt am Orderbuch aufgefallen, dass endlich die Brief-Seite ausgedünnt ist. Heißt: bei den nächsten guten News sollte es einen ordentlichen Schub nach oben geben.
Das EIR-Research war mir bisher etwas zu optimistisch. Jetzt ist es m.E. realistisch. Sollte SQI eine Vertriebs-Kooperation schließen (was ich als wahrscheinlich erachte im 2. Hj. 2015), könnte die Zeit nach 2015 dynamischer werden als erwartet.
+55%
Q.E.D.
Leider bei nur 6.000 St. Handelsvolumen... Heißt also leider nicht viel. Aber das zeigt, wie markteng diese Aktie ist und dass die Aktie ohne weiteres alte Hochs erreichen kann, sobald die Geschäftsentwicklung dies triggert.
M@trix
von heute - Alphastox
Finally, the time has come. I introduced you to this company about 9 months ago, with the company’s progress since then I feel it’s important to refresh your memory. Founded in 1999, SQI Diagnostics (TSX.V: SQD) was built on the idea that reducing the number of blood tests performed to diagnose a patient would be significantly beneficial. The two markets the company is currently targeting are diagnostic testing (clinical and veterinary) and drug development companies.SQI’s products provide significant labour and time saving capabilities to their clients with the potential of saving them millions down the road. The standard ELISA (manual operation) takes 32 minutes per patient compared to SQI’s SQiDlite which only takes 30 seconds per patient. This saves time, reduces costs and produces results sooner, while still maintaining superior technical performance. SQI offers multiplexing and automated analyzers that deliver full panels of up to 3,000 quantified results per kit.
The Company markets these custom tests under its Ig_plex brand. It is the world’s first multiplexing methodology that allows simultaneous quantitation of immunoglobulin isotype and subclass for multiple proteins. More simply, the company provides all the tests needed by the pharma for this one part of its testing, in a single test; previously, the pharma would have had to develop and run many individual tests – the largest number tests SQI has multiplexed for a pharma customer to date is 21. The positive results of this project were recently presented at a large pharmaceutical conference (THE 15TH ANNUAL IMMUNOGENICITY FOR BIOTHERAPEUTICS CONFERENCE)
The products address critical issues of Global Pharmaceutical companies and provide tangible benefits such as customized and outsourced test development. The company technologies are used to provide pharmaceutical companies reports on multiple proteins, multiple immunoglobulin isotypes and subclasses simultaneously as well as conducts side-by-side analysis of therapeutic proteins and drug metabolites. Plainly speaking, SQI’s products are used to reduce the amount of work pharma companies need to get this required testing done. One analysis for a large study shows that the total time reduced could be well over 1,000 man days; this translates into possible savings of over several hundred thousand dollars per large study and could drive significant, high margin revenue to SQI. SQI has previously announced 5 evaluation agreements with global pharma and has since updated on positive traction with a majority of these projects. Simply speaking again, while SQI may not win every evaluation, when it does, each could result in large and broad agreements to sell its sqidlite platforms and many test kits on top of revenue generated from the evaluation agreements.
Also, you should check out the Company’s press release about a U.K.-based customer that hired SQI to automate a molecular (DNA) test. It has since completed this project and management has reported that it expects to enter into additional agreements to manufacture and supply all kits and the automated equipment – the tests target a very interesting market where DNA tests replace very labourious and time-consuming tests that are currently done in basically the same petri dishes we all used in high school – the current method takes DAYS to complete. Using the automated platforms and SQI’s customer’s test this is reduced to hours. This is important as it would reduce the time a patient waits with a possibly serious infection by days. SQI and its customer are targeting infectious disease applications in both the human and animal health (milk-testing) markets...stay-tuned, as there are millions of these tests run each year in the US and Canada.
In its other business area of focus,SQI continually develops and files for approval of their in vitro diagnostic tests that are targeted to meet the market’s unmet needs for multiplexed tests for large reference labs that run tests in autoimmune disease – when a doctor requires a diagnostic blood test to assist them in making a diagnosis.
SQI Receives FDA Approval for Celiac Diagnostic Tests
The Company recently announced that it had received clearance to market and sell one of the important tests in its IVD development pipeline – a test for the standard and recognized blood markers for celiac disease. Using the Company’s celiac test and sqid-X system is estimated by the Company to result in a nearly 4x reduction in labour, and hence labour cost to produce test results.
Celiac disease affects approximately 1 in 100 people in the US, with more than 90% of undiagnosed celiac sufferers according to the American Journal of Gastroenterology. SQI Diagnostics has received clearance from the United States Food and Drug Administration (FDA) to market its proprietary Celiac Panel in the United States. The clearance lays the foundation for sales in the US market of the company’s in vitro diagnostic (IVD) autoimmune test. This is absolutely huge news.
So what does this mean to you? Very simple. FDA approval gives SQI the opportunity to go and sell their tests to labratories. With SQI’s Celiac 4-plex test, they estimate could potentially make around $468,000/per year per laboratory. How did I get to that number? Once a laboratory decides to use SQI’s Celiac 4-Plex panel, they will most likely conduct around 23,400 tests per year based on estimates of lab-testing volumes of about 450 celiac panels per week. With each test generating SQI around $20/test, SQI could possibly generate over $468,000 with a gross margin of about 80%...not too bad. Even if they only convert 10-15 laboratories per year, we could potentially see SQD.V being worth a lot more than their current market cap of $25 million
.
SQI is building a pipeline of other high-demand IVD autoimmune assays in addition to its suite of FDA cleared assays for celiac disease and rheumatoid arthritis. The company has seen revenue growth during the last fiscal year due to development of custom multiplexed assays for pharmaceutical companies and molecular testing in the animal and human health markets.
Led by Andrew Morris, the company and its experienced management team is well positioned within the pharmaceutical market, and the FDA clearance will be an important element in marketing the company’s products and services with leading global pharmaceutical companies.
Make sure you keep SQD.V on watch right now! I know it’s been a while since we last reported on the company but I truly feel that now is the time where we could potentially see the stock start moving a lot higher. The company now has the goods to start marketing and telling their story to new investors, something that hasn’t been done in a while. A lot of investors aren’t even aware of the story, so once the deal gets out there and investors understand the potential of the play and how significant this FDA approval really is to the company, the market should react positively and demand should build for the stock. The stock is still very tightly held among some very well-heeled investors, so any new demand should be well demonstrated in the market.
SQI hat auch die Investoren-Präsentation aktualisiert:
http://sqidiagnostics.com/sites/default/files/SQI%20Investor…
Besonderes Augenmerk sollte auf Folie 20 gerichtet werden. Diese verdeutlicht das immense Potenzial allein schon bei den bestehenden sechs Kunden. Auch die Folien 23 und 26 verdienen aufmerksame Beachtung.
Beim Start dieser Diskussion habe ich stark unterschätzt, wie viel Zeit vergehen würde, bis man wirkliche Umsätze generiert. Daher war das Jahr 2014 auch kein Erfolg, was die Aktienkursentwicklung betrifft. Nun steht SQI aber mit mehreren marktreifen, technisch überlegenen, kostensparenden Produkten da und baut sein Sales Team aus.
2015 dürfte daher die nachhaltige Wende bringen und "richtig Spaß" sollte dann das Jahr 2016 machen. Ich halte es nicht für abwegig, dass SQI bis Ende 2016 auf 15-20 Kunden kommt und mit jedem dieser Kunden Umsätze im niedrigen ein- bis zweistelligen Millionenbereich erzielt.
Der Umsatz Ende 2016 könnte also durchaus bei ca. 15-20 Mio. $ liegen. Bei einer Marge von 80% (die wahrscheinlich aber eher bei ca. 60% wegen mehr Personalaufwand liegen wird) kann durchaus ein Gewinn von ca. 10 Mio. $ hängen bleiben. Ein recht konservatives KGV von 15 unterstellt errechnet sich somit ein Kursziel von ca. 2,66 CAD. Aktuell notieren wir bei 0,38 CAD.
Selbst wenn die Aktie nochmal verwässert wird: SQI ist auf diesem Niveau stark unterbewertet.
Das Problem liegt m.E. in der Illiquidität der Aktie. Das schreckt leider jeden institutionellen Investor ab. Ich gehe jedoch davon aus, dass wenn die Zahlen "anfangen für sich zu sprechen", auch die Aktie liquider werden wird.
Für mich weiterhin ein klares langfristiges Investment mit Vervielfachungspotenzial.
M@trix, der gerade weitere 3.500 St. in Kanada zu 0,38 CAD zugekauft hat
http://sqidiagnostics.com/sites/default/files/SQI%20Investor…
Besonderes Augenmerk sollte auf Folie 20 gerichtet werden. Diese verdeutlicht das immense Potenzial allein schon bei den bestehenden sechs Kunden. Auch die Folien 23 und 26 verdienen aufmerksame Beachtung.
Beim Start dieser Diskussion habe ich stark unterschätzt, wie viel Zeit vergehen würde, bis man wirkliche Umsätze generiert. Daher war das Jahr 2014 auch kein Erfolg, was die Aktienkursentwicklung betrifft. Nun steht SQI aber mit mehreren marktreifen, technisch überlegenen, kostensparenden Produkten da und baut sein Sales Team aus.
2015 dürfte daher die nachhaltige Wende bringen und "richtig Spaß" sollte dann das Jahr 2016 machen. Ich halte es nicht für abwegig, dass SQI bis Ende 2016 auf 15-20 Kunden kommt und mit jedem dieser Kunden Umsätze im niedrigen ein- bis zweistelligen Millionenbereich erzielt.
Der Umsatz Ende 2016 könnte also durchaus bei ca. 15-20 Mio. $ liegen. Bei einer Marge von 80% (die wahrscheinlich aber eher bei ca. 60% wegen mehr Personalaufwand liegen wird) kann durchaus ein Gewinn von ca. 10 Mio. $ hängen bleiben. Ein recht konservatives KGV von 15 unterstellt errechnet sich somit ein Kursziel von ca. 2,66 CAD. Aktuell notieren wir bei 0,38 CAD.
Selbst wenn die Aktie nochmal verwässert wird: SQI ist auf diesem Niveau stark unterbewertet.
Das Problem liegt m.E. in der Illiquidität der Aktie. Das schreckt leider jeden institutionellen Investor ab. Ich gehe jedoch davon aus, dass wenn die Zahlen "anfangen für sich zu sprechen", auch die Aktie liquider werden wird.
Für mich weiterhin ein klares langfristiges Investment mit Vervielfachungspotenzial.
M@trix, der gerade weitere 3.500 St. in Kanada zu 0,38 CAD zugekauft hat
SQI DIAGNOSTICS ADVANCES COMMERCIAL OPPORTUNITY WITH LEADING GLOBAL PHARMACEUTICAL CUSTOMER
SQI Diagnostics Inc. has expanded its existing commercial relationship with one of the world's largest pharmaceutical companies. This final step in commercializing the Company's Ig_plex(TM) platform could lead to significant on-going revenue opportunities as the customer continues further development of its drug.SQI Diagnostics recently completed the successful development of a series of custom products to measure safety-related responses to this customer's development-stage drug ("immunogenicity"). Based on the performance of prior tests completed, additional development has been contracted, including the Company's proprietary Ig_plex characterization of antibodies produced in response to the drug ("anti-drug antibodies").
The agreement includes payment for services and consumables used during development and sample testing.
"We are very pleased with the momentum we have achieved in this commercial relationship," said Andrew Morris, CEO of SQI Diagnostics. "We believe the follow-on business further validates the need for our services in the marketplace and highlights customer satisfaction with the results achieved thus far. We expect this final phase will lead to the sale of products and instruments, which will further drive on-going revenues from anti-drug antibody tests. It could also lead to the broader adoption of other, potentially high volume, tests that can be run on our sqidlite(TM) system, supporting other aspects of the customer's safety and efficacy testing during drug development."
Global pharmaceutical companies invest significant funds to better understand potential immune responses to the novel drugs they are developing and the formation of anti-drug antibodies in study subjects is a concern for their effect on both drug efficacy and safety. Information provided by SQI Diagnostics' tests on the anti-drug antibodies present in a sample provides further characterization of the immune response during both pre-clinical and clinical studies.
Earlier in November, SQI Diagnostics received clearance from the Food and Drug Administration for its Ig_plex Celiac DGP Panel allowing it to be sold in the United States. In October, the Company presented comprehensive data describing the superior performance of multiple Ig_plex tests performed for a variety of customers and compounds at the 14th Annual Immunogenicity Conference in Boston, Massachusetts. All of these tests were developed and run on the Company's patented diagnostic platforms.
The Company continues to focus on selling products and services to pharmaceutical and biotech customers and on converting products initially used for evaluation by these customers to higher volume kit sales for use in clinical trials. A portion of business development effort is focused on pharmaceutical, biotech, and vaccine companies that currently use labour-intensive, expensive, low throughput "single-plex" tests in their product development activities and on the contract research organizations that service the testing needs of such companies.
We seek Safe Harbor.
© 2014 Canjex Publishing Ltd.
Alphastox Provides an Update on SQI Diagnostics (TSXV:SQD)
We’ve featured SQI Diagnostics (TSX-V: SQD; OTCQX: SQIDF ) before and unfortunately the stock hadn’t moved much since we first initiated coverage on the story, I’ll be the first to admit it…however, there’s a reason why were showing it to you now. We feel the company has the necessary catalysts coming down the pipeline that could potentially start getting the market very excited. It’s taken a number of months for this to happen but the company has enough cash to take them well into next year and has been progressing tremendously well operationally to the point where we could see the company generating substantial revenues in early 2015.SQI Diagnostics (TSXV:SQD) just announced yesterday that it has taken the final step in commercializing its Ig_plexTM platform by expanding its existing commercial relationship with a leading global pharmaceutical company. The agreement includes payment for services and consumables used during development and sample. This step could lead to significant on-going revenue opportunities as the customer continues to develop its drug, which is something that could obviously be very accretive to SQD shareholders.
The company recently developed and tested a series of custom products to measure safety-related responses to this customer’s development-stage drug which all passed with flying colours. Based on the successful performance, additional development has been contracted which could lead to more contracts later down the road.
Most importantly, this further validates the need for SQD’s services in the marketplace and highlights customer satisfaction with the results they’ve achieved thus far. Andrew Morris, SQI Diagnostics’ CEO expects this final phase to lead to a position where his customers are buying (on-going revenue) kits for their pre-clinical and clinical programs following the placement of a sqidlite system:
“we expect this final phase will lead to the sale of products and instruments, which will further drive on-going revenues from anti-drug antibody tests. It could also lead to the broader adoption of other, potentially high volume, tests that can be run on our sqidliteTM system, supporting other aspects of the customer’s safety and efficacy testing during drug development.”
This is a wildly bullish statement coming from the CEO which I wanted to clarify for myself. After speaking with Andrew, I don’t feel the statement was made loosely and unjustly. Morris and his team truly feel that they are well on their way towards obtaining a paying contract which could turn the company into a high margin revenue business leading to additional sources of revenue from each one of their customers. These sorts of companies take time to build but once they’ve gotten a stranglehold on a niche market, they can turn in an instant and that’s what were betting on with SQI Diagnostics (TSXV:SQD).
At $0.385 and a $21 million market cap, SQD.V needs to be kept on your radar screens. The company has the potential to quickly move to the upside once they start announcing a few revenue generating contracts which I am hoping will happen sometime in early 2015. As the company keeps successfully executing their business plan, there should be a lot more interest from investors to participate in taking a position in the deal in the secondary market. As I mentioned in my earlier pieces, the company has some very deep pocketed investors with long-term visions for the company…that’s why you don’t see any selling pressure on the stock after so many months of little volume. Therefore, once the next catalyst hits the marketplace and interest builds among investors, we should see SQI potentially skyrocket and early investors reap tremendous rewards for their patience. Make sure you keep the ticker on your screens… the time is now.
--------------
Eine sehr schöne Darstellung des Potenzials, die ich uneingeschränkt teile. SQI ist inzwischen mit Abstand meine größte Position und ich bin mir sicher, dass die Geduld in den nächsten beiden Jahren deutlich belohnt werden wird.
M@trix
SQI Diagnostics Wins Additional Business from UK-based Customer
CNW Group SQI Diagnostics Inc.
Technology Transfer Agreement Signals Revenue Growth Opportunities
TORONTO , Dec 15, 2014 /CNW/ - SQI Diagnostics Inc. ("SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF) today announced that it has advanced its existing commercial relationship with a UK-based customer, enabling the Company to manufacture a 19-plex infectious disease, DNA-based test in its state-of-the-art manufacturing facility in Toronto.
The technology transfer and the partnership on the project that has developed, will allow SQI to play a more strategic role for this customer, who is rapidly advancing their high-throughput diagnostic tests for agriculture, food safety and human infectious diseases. This DNA diagnostic test is intended to screen large volumes of samples on a recurring basis. The DNA-based products that SQI is being paid to commercialize will significantly reduce the time required to deliver a diagnostic result compared to current methods, which include traditional plate cultures. DNA-based tests for pathogens increase the accuracy of the identification of the source of the infection compared to traditional methods.
SQI Diagnostics previously announced a Master Service Agreement with this customer in August 2014 , to automate their DNA-based pathogen detection tests on the Company's sqidlite-dh platform. This initial automation project was successfully completed and demonstrated in September 2014.
"This additional business win is yet another positive development for us, highlighting that we continue to be successful in our commercialization strategies and that we are meeting the needs of our customers," said Andrew Morris , CEO of SQI. "This technology transfer agreement is important as it enables us to manufacture our customer's test kits in a controlled production environment. In turn, this enables us to produce these highly advanced multiplexed infectious disease tests for this customer's animal health business, for on-going human clinical validation programs and future sales, as well as for other end-user customers in multiple molecular diagnostic markets."
The Company expects to continue to build the business with this customer with subsequent agreements employing SQI's expertise in multiplexing, high volume automated processing, detection and analysis of patient samples. The market for DNA-based tests, particularly in infectious diagnostics is large and growing. It is estimated that dairy testing for mastitis in North America generates as many as 60 million tests annually. Sepsis, a serious infectious disease affecting humans that can be detected using the same technology, affects approximately 1,000,000 people in North America annually and is estimated to cost the healthcare system over US$1 billion each year – earlier diagnosis and treatment could significantly improve outcomes and reduce costs.
http://finance.yahoo.com/news/sqi-diagnostics-wins-additiona…
CNW Group SQI Diagnostics Inc.
Technology Transfer Agreement Signals Revenue Growth Opportunities
TORONTO , Dec 15, 2014 /CNW/ - SQI Diagnostics Inc. ("SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF) today announced that it has advanced its existing commercial relationship with a UK-based customer, enabling the Company to manufacture a 19-plex infectious disease, DNA-based test in its state-of-the-art manufacturing facility in Toronto.
The technology transfer and the partnership on the project that has developed, will allow SQI to play a more strategic role for this customer, who is rapidly advancing their high-throughput diagnostic tests for agriculture, food safety and human infectious diseases. This DNA diagnostic test is intended to screen large volumes of samples on a recurring basis. The DNA-based products that SQI is being paid to commercialize will significantly reduce the time required to deliver a diagnostic result compared to current methods, which include traditional plate cultures. DNA-based tests for pathogens increase the accuracy of the identification of the source of the infection compared to traditional methods.
SQI Diagnostics previously announced a Master Service Agreement with this customer in August 2014 , to automate their DNA-based pathogen detection tests on the Company's sqidlite-dh platform. This initial automation project was successfully completed and demonstrated in September 2014.
"This additional business win is yet another positive development for us, highlighting that we continue to be successful in our commercialization strategies and that we are meeting the needs of our customers," said Andrew Morris , CEO of SQI. "This technology transfer agreement is important as it enables us to manufacture our customer's test kits in a controlled production environment. In turn, this enables us to produce these highly advanced multiplexed infectious disease tests for this customer's animal health business, for on-going human clinical validation programs and future sales, as well as for other end-user customers in multiple molecular diagnostic markets."
The Company expects to continue to build the business with this customer with subsequent agreements employing SQI's expertise in multiplexing, high volume automated processing, detection and analysis of patient samples. The market for DNA-based tests, particularly in infectious diagnostics is large and growing. It is estimated that dairy testing for mastitis in North America generates as many as 60 million tests annually. Sepsis, a serious infectious disease affecting humans that can be detected using the same technology, affects approximately 1,000,000 people in North America annually and is estimated to cost the healthcare system over US$1 billion each year – earlier diagnosis and treatment could significantly improve outcomes and reduce costs.
http://finance.yahoo.com/news/sqi-diagnostics-wins-additiona…
SQI Diagnostics Reports Fourth Quarter and Fiscal 2014 Results
http://sqidiagnostics.com/media/2014/sqi-diagnostics-reports…
Auszug:
Fiscal 2015 Objectives and Outlook
Looking out into 2015, the Company’s main objectives include:
Building on the commercial success achieved thus far in demonstrating the superior value proposition that SQI’s proprietary technologies offer, we plan to win new customers and place sqidlite™ platforms within existing customers. These platforms are the basis for sustainable and on-going revenue growth through the sale of custom and other high volume test products run on these platforms. The Company currently estimates that each platform could generate up to $2 million in revenue when fully utilized.
Producing the best multiplexing products for our customers in each of our strategic markets. This includes demonstrating the service excellence achieved to date that has established SQI as a partner of choice for our drug development customers.
Growing the business within existing customers, who on a combined basis have over 130 drugs currently in development and are adding approximately 10 new products annually into their drug development pipelines.
Continuing to validate and clear tests in our autoimmune business to achieve a critical mass of tests and to open the door to large reference lab customers.
Selling more prototype products and services to drug developers and diagnostic companies as the entry point to position our technology as a platform they can use across their businesses.
Focusing on winning ancillary, high volume product business alongside our high margin, entry-point products.
Keeping the top of our sales funnel refreshed with new customer opportunities across all lines of business.
http://sqidiagnostics.com/media/2014/sqi-diagnostics-reports…
Auszug:
Fiscal 2015 Objectives and Outlook
Looking out into 2015, the Company’s main objectives include:
Building on the commercial success achieved thus far in demonstrating the superior value proposition that SQI’s proprietary technologies offer, we plan to win new customers and place sqidlite™ platforms within existing customers. These platforms are the basis for sustainable and on-going revenue growth through the sale of custom and other high volume test products run on these platforms. The Company currently estimates that each platform could generate up to $2 million in revenue when fully utilized.
Producing the best multiplexing products for our customers in each of our strategic markets. This includes demonstrating the service excellence achieved to date that has established SQI as a partner of choice for our drug development customers.
Growing the business within existing customers, who on a combined basis have over 130 drugs currently in development and are adding approximately 10 new products annually into their drug development pipelines.
Continuing to validate and clear tests in our autoimmune business to achieve a critical mass of tests and to open the door to large reference lab customers.
Selling more prototype products and services to drug developers and diagnostic companies as the entry point to position our technology as a platform they can use across their businesses.
Focusing on winning ancillary, high volume product business alongside our high margin, entry-point products.
Keeping the top of our sales funnel refreshed with new customer opportunities across all lines of business.
Ich habe mir jetzt noch den Conference Call angehört. Wichtigste Infos:
burn rate ca. 400.000 $ pro Monat, d.h. sie werden wohl nochmal Kapital aufnehmen müssen, wenn nicht große Aufträge gleich im ersten Quartal 2015 kommen.
Morris schätzt, dass sie pro Zielkunde (aktuell 6) binnen 18 bis 24 Monaten 3-5 Plattformen verkaufen können und damit Margen von bis zu 80% erzielen können.
Angenommen sie verkaufen nur 3 Plattformen an nur 4 Kunden und kommen pro Plattform auf ein Volumen von 2 Mio. $ (siehe Pressemitteilung), so kommen wir demnach auf einen Umsatz von rund 25 Mio. $. Wenn sie da tatsächlich Margen von 80% erreichen, sollte nach Kosten ein netter Gewinn übrig bleiben. Wenn man nur mal etwa ein fünftel annimmt und dann ein KGV von 20 anlegt, kommt man auf ein Kursziel, das fünf mal höher als der aktuelle Kurs liegt.
Insofern lag ich mit meiner Schätzung von vor ein paar Wochen recht gut:
http://www.wallstreet-online.de/diskussion/1187833-41-50/sqi…
Warten wir es ab. Ich denke, wir sind am Tief und sehen gerade exzellente Kaufkurse (wobei ich das zugegebenermaßen auch schon bei höheren Kursen gedacht habe).
M@trix
burn rate ca. 400.000 $ pro Monat, d.h. sie werden wohl nochmal Kapital aufnehmen müssen, wenn nicht große Aufträge gleich im ersten Quartal 2015 kommen.
Morris schätzt, dass sie pro Zielkunde (aktuell 6) binnen 18 bis 24 Monaten 3-5 Plattformen verkaufen können und damit Margen von bis zu 80% erzielen können.
Angenommen sie verkaufen nur 3 Plattformen an nur 4 Kunden und kommen pro Plattform auf ein Volumen von 2 Mio. $ (siehe Pressemitteilung), so kommen wir demnach auf einen Umsatz von rund 25 Mio. $. Wenn sie da tatsächlich Margen von 80% erreichen, sollte nach Kosten ein netter Gewinn übrig bleiben. Wenn man nur mal etwa ein fünftel annimmt und dann ein KGV von 20 anlegt, kommt man auf ein Kursziel, das fünf mal höher als der aktuelle Kurs liegt.
Insofern lag ich mit meiner Schätzung von vor ein paar Wochen recht gut:
http://www.wallstreet-online.de/diskussion/1187833-41-50/sqi…
Warten wir es ab. Ich denke, wir sind am Tief und sehen gerade exzellente Kaufkurse (wobei ich das zugegebenermaßen auch schon bei höheren Kursen gedacht habe).
M@trix
Ergänzung:
Aus dem MD&A: "Looking forward, we expect that we could have over 10 drug development customers with 1 or more systems in place by the end of 2015. Each of these systems is capable of processing enough product to generate well in excess of $2 million in revenue for SQI when operating at capacity."
Das wären demnach schon 20 Mio. $ Umsatz Ende 2015!! Dann wäre meine Schätzung noch zu konservativ... Diese MD&A ist generell sehr erhellend:
http://www.sedar.com/DisplayCompanyDocuments.do?lang=EN&issu…
Leider wird jedoch auch die nächste Finanzierung nicht mehr lange auf sich warten lassen:
The Company has funds sufficient to meet our anticipated cash requirements for approximately the next two months. The Company is actively reviewing its forecast expenditures, capital needs and financing options.
Das ist besonders ärgerlich, wenn man das aktuell sehr niedrige Kursniveau bedenkt. Vielleicht wäre es jetzt ratsamer (sofern möglich), einen Kredit aufzunehmen statt wieder Aktien auszugeben.
M@trix
Aus dem MD&A: "Looking forward, we expect that we could have over 10 drug development customers with 1 or more systems in place by the end of 2015. Each of these systems is capable of processing enough product to generate well in excess of $2 million in revenue for SQI when operating at capacity."
Das wären demnach schon 20 Mio. $ Umsatz Ende 2015!! Dann wäre meine Schätzung noch zu konservativ... Diese MD&A ist generell sehr erhellend:
http://www.sedar.com/DisplayCompanyDocuments.do?lang=EN&issu…
Leider wird jedoch auch die nächste Finanzierung nicht mehr lange auf sich warten lassen:
The Company has funds sufficient to meet our anticipated cash requirements for approximately the next two months. The Company is actively reviewing its forecast expenditures, capital needs and financing options.
Das ist besonders ärgerlich, wenn man das aktuell sehr niedrige Kursniveau bedenkt. Vielleicht wäre es jetzt ratsamer (sofern möglich), einen Kredit aufzunehmen statt wieder Aktien auszugeben.
M@trix
Denke, in Q1 sehen wir ein pp.
Marge bei den Plattformen dürfte nicht 80 Prozent, aber trotzdem sehr gut sein.
Marge bei den Plattformen dürfte nicht 80 Prozent, aber trotzdem sehr gut sein.
convertible debenture mit höherem strike price könnte Sinn machen, wenn sich Investoren darauf einlassen
in einem ääähnlichem Zusammenhang kann man sich vielleicht auch mal diese Firma anschauen -Genetic Signatures Limited
ein anstehendes IPO
http://geneticsignatures.com/
http://geneticsignatures.com/wp-content/uploads/Genetic-Sign…
Gruß
P.
ein anstehendes IPO
http://geneticsignatures.com/
http://geneticsignatures.com/wp-content/uploads/Genetic-Sign…
Gruß
P.
Kurs heute 0,60 CAD. Leider auch wieder nur bei niedrigem Volumen, aber mit meiner Einschätzung, dass wir kaum noch Verkäufer haben, scheine ich richtig gelegen zu haben. Und für ein mögliches Private Placement ist es mir auch lieber, wenn das Kursniveau höher ist. :-)
M@trix
M@trix
Trotz Kursverdopplung keine nennenswerte Abgabebereitschaft. Das ist sicher kein schlechtes Zeichen. :-)
Aus meiner Sicht nun wieder gute Kaufkurse bei SQI, die gestern bei lediglich 3.660 gehandelten Aktien um 34% eingebrochen ist. Die Marktenge ist bei Anstiegen ein Segen, aber wie man sieht ebenso ein Fluch, wenn mal jemand ohne Limit verkauft.
Gestern wurde die neue Investorenpräsentation online gestellt:
http://sqidiagnostics.com/sites/default/files/SQI%20Investor…
Außerdem habe ich gesehen, dass SQI inzwischen acht Stellen ausgeschrieben hat. Das ist ein Rekord und spricht dafür, dass sich die Dinge weiter positiv entwickeln:
http://sqidiagnostics.com/about/careers
Mit wirklich wichtigen News rechne ich indes nicht vor Ende Februar.
Gestern wurde die neue Investorenpräsentation online gestellt:
http://sqidiagnostics.com/sites/default/files/SQI%20Investor…
Außerdem habe ich gesehen, dass SQI inzwischen acht Stellen ausgeschrieben hat. Das ist ein Rekord und spricht dafür, dass sich die Dinge weiter positiv entwickeln:
http://sqidiagnostics.com/about/careers
Mit wirklich wichtigen News rechne ich indes nicht vor Ende Februar.
Kein Volumen in der Aktie. Riesenspread. Katastrophe.
In der Tat... das Problem ist bekannt, weshalb man "mittelfristig" ein Listing an der Nasdaq anstrebt. Leider sehe ich das nicht vor 2016...
Bis dahin sollten dann eben die Fundamentals überzeugen.
Bis dahin sollten dann eben die Fundamentals überzeugen.
Es ist doch nicht so schwierig, einen Market Maker anzuheuern, der vernünftig quotiert?
Damit habe ich JETZT nicht gerechnet, aber immerhin hält sich die Verwässerung in Grenzen und wie es scheint steht der größte Teil der Finanzierung schon (was ich sehr positive sehe):
SQI Diagnostics Announces Debt Financing of up to $4 Million
TORONTO, Jan. 21, 2015 /CNW/ - SQI Diagnostics Inc. ("SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced it intends to complete a non-brokered private placement (the "Offering") of secured debentures of up to $4 million (collectively, the "Debentures").
The Debentures will bear interest at a rate of 10% per annum on the principal amount outstanding and will be repayable 60 months from the date issued. The Debentures will be secured by a general security agreement over all the present and future assets of the Company including intangibles. In consideration for the Debentures, the Company is issuing an aggregate of up on four million common share purchase warrants (collectively, the "Warrants"). Each Warrant will entitle the holder to purchase one common share of the Corporation (a "Share") at a price of $0.60 and is exercisable at any time up to 60 months after the date of issue. The securities being issued pursuant to the Offering will be subject to a four month hold period in accordance with applicable Canadian securities law.
The Debentures may be redeemed in whole or in part, at par and without premium or penalty, at the option of the Company if at any time following the first anniversary of the date of issuance of the Debentures, and prior to the maturity date of such Debentures, the volume weighted average closing price of the Company's Shares on the TSXV (or any other stock exchange on which such Shares are then traded) is equal to or greater than $1.00 per share for twenty (20) consecutive trading days.
SQI intends to use the net proceeds to fund the Company's product development and commercialization programs, sales and marketing and for general working capital purposes.
The Company expects an initial closing of the Offering on or about January 23, 2015, with additional closings in February, 2015.
In connection with the Offering, the Company will pay a finder's fee in cash equal to 6% of the gross proceeds of the Offering and will issue compensation warrants equal to 10% of the aggregate warrants issued in the Offering. The finder's warrants will exercisable at a price of $0.60 at any time up to 60 months after the date of issue.
The issuance of the Debentures is subject to the execution of the debenture agreements and a general security agreement and the approval of the TSX Venture Exchange. The Offering is also subject to all necessary regulatory and stock exchange approvals.
The securities described herein have not been, and will not be, registered under the United States Securities Act of 1933, as amended (the "U.S. Securities Act") or any state securities laws and accordingly may not be offered or sold within the United States or to "U.S. persons", as such term is defined in Regulation S promulgated under the U.S. Securities Act ("U.S. Persons"), except in compliance with the registration requirements of the U.S. Securities Act and applicable state securities requirements or pursuant to exemptions therefrom. This news release does not constitute an offer to sell or a solicitation of an offer to buy and of the Company's securities to, or for the account of benefit of, persons in the United States or U.S. Persons.
http://www.newswire.ca/en/story/1475785/sqi-diagnostics-anno…
SQI Diagnostics Announces Debt Financing of up to $4 Million
TORONTO, Jan. 21, 2015 /CNW/ - SQI Diagnostics Inc. ("SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced it intends to complete a non-brokered private placement (the "Offering") of secured debentures of up to $4 million (collectively, the "Debentures").
The Debentures will bear interest at a rate of 10% per annum on the principal amount outstanding and will be repayable 60 months from the date issued. The Debentures will be secured by a general security agreement over all the present and future assets of the Company including intangibles. In consideration for the Debentures, the Company is issuing an aggregate of up on four million common share purchase warrants (collectively, the "Warrants"). Each Warrant will entitle the holder to purchase one common share of the Corporation (a "Share") at a price of $0.60 and is exercisable at any time up to 60 months after the date of issue. The securities being issued pursuant to the Offering will be subject to a four month hold period in accordance with applicable Canadian securities law.
The Debentures may be redeemed in whole or in part, at par and without premium or penalty, at the option of the Company if at any time following the first anniversary of the date of issuance of the Debentures, and prior to the maturity date of such Debentures, the volume weighted average closing price of the Company's Shares on the TSXV (or any other stock exchange on which such Shares are then traded) is equal to or greater than $1.00 per share for twenty (20) consecutive trading days.
SQI intends to use the net proceeds to fund the Company's product development and commercialization programs, sales and marketing and for general working capital purposes.
The Company expects an initial closing of the Offering on or about January 23, 2015, with additional closings in February, 2015.
In connection with the Offering, the Company will pay a finder's fee in cash equal to 6% of the gross proceeds of the Offering and will issue compensation warrants equal to 10% of the aggregate warrants issued in the Offering. The finder's warrants will exercisable at a price of $0.60 at any time up to 60 months after the date of issue.
The issuance of the Debentures is subject to the execution of the debenture agreements and a general security agreement and the approval of the TSX Venture Exchange. The Offering is also subject to all necessary regulatory and stock exchange approvals.
The securities described herein have not been, and will not be, registered under the United States Securities Act of 1933, as amended (the "U.S. Securities Act") or any state securities laws and accordingly may not be offered or sold within the United States or to "U.S. persons", as such term is defined in Regulation S promulgated under the U.S. Securities Act ("U.S. Persons"), except in compliance with the registration requirements of the U.S. Securities Act and applicable state securities requirements or pursuant to exemptions therefrom. This news release does not constitute an offer to sell or a solicitation of an offer to buy and of the Company's securities to, or for the account of benefit of, persons in the United States or U.S. Persons.
http://www.newswire.ca/en/story/1475785/sqi-diagnostics-anno…
Sehr gute Finanzierung. Das eine Finanzierung kommen muss, war bei der Burnrate absehbar. Keine Wandlungsmöglichkeit, also "nur" Verwässerung durch die paar warrants, die wiederum 2,4 Mio. extra in die Kasse spülen könnten.
SQI Diagnostics Completes Tranche of Debenture Financing
January 30, 2015 3:01 PM
TORONTO , Jan. 30, 2015 /CNW/ - SQI Diagnostics Inc. ("SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced that it has closed the first tranche of its previously announced non-brokered private placement (the "Offering") of secured debentures for gross proceeds of $1.95 million.
The Debentures will bear interest at a rate of 10% per annum on the principal amount outstanding and will be repayable 60 months from the date issued. The Debentures will be secured by a general security agreement over all the present and future assets of the Company including intangibles. In consideration for the Debentures, the Company issued of 1.95 million common share purchase warrants (collectively, the "Warrants"). Each Warrant will entitle the holder to purchase one common share of the Corporation (a "Share") at a price of $0.60 and is exercisable at any time up to 60 months after the date of issue. The securities being issued pursuant to the Offering will be subject to a four month hold period in accordance with applicable Canadian securities law.
The Debentures may be redeemed in whole or in part, at par and without premium or penalty, at the option of the Company if at any time following the first anniversary of the date of issuance of the Debentures, and prior to the maturity date of such Debentures, the volume weighted average closing price of the Company's Shares on the TSXV (or any other stock exchange on which such Shares are then traded) is equal to or greater than $1.00 per share for twenty (20) consecutive trading days.
SQI intends to use the net proceeds to fund the Company's product development and commercialization programs, sales and marketing and for general working capital purposes.
The Company expects an additional closing in February, 2015.[...]
http://finance.yahoo.com/news/sqi-diagnostics-completes-tran…
January 30, 2015 3:01 PM
TORONTO , Jan. 30, 2015 /CNW/ - SQI Diagnostics Inc. ("SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced that it has closed the first tranche of its previously announced non-brokered private placement (the "Offering") of secured debentures for gross proceeds of $1.95 million.
The Debentures will bear interest at a rate of 10% per annum on the principal amount outstanding and will be repayable 60 months from the date issued. The Debentures will be secured by a general security agreement over all the present and future assets of the Company including intangibles. In consideration for the Debentures, the Company issued of 1.95 million common share purchase warrants (collectively, the "Warrants"). Each Warrant will entitle the holder to purchase one common share of the Corporation (a "Share") at a price of $0.60 and is exercisable at any time up to 60 months after the date of issue. The securities being issued pursuant to the Offering will be subject to a four month hold period in accordance with applicable Canadian securities law.
The Debentures may be redeemed in whole or in part, at par and without premium or penalty, at the option of the Company if at any time following the first anniversary of the date of issuance of the Debentures, and prior to the maturity date of such Debentures, the volume weighted average closing price of the Company's Shares on the TSXV (or any other stock exchange on which such Shares are then traded) is equal to or greater than $1.00 per share for twenty (20) consecutive trading days.
SQI intends to use the net proceeds to fund the Company's product development and commercialization programs, sales and marketing and for general working capital purposes.
The Company expects an additional closing in February, 2015.[...]
http://finance.yahoo.com/news/sqi-diagnostics-completes-tran…
!
Dieser Beitrag wurde von CloudMOD moderiert. Grund: auf eigenen Wunsch des Users
Link funzt nicht
So, hier nochmal:
Gaining momentum
Outlook | Pharmaceutical & Healthcare | 06/02/2015
SQI has continued its positive momentum over the last year, signing five service agreements with drug development companies. The company also broadened its commercial focus in 2014 with a deal to automate DNA-based pathogen detection assays, a new application for its technology. We now look towards the conversion of customers from early contract work/validation to full commercialisation. Despite the encouraging deal progress, we reduce our valuation to C$55m vs C$60m previously (C$0.98 per share vs C$1.07), mainly due to the more protracted time frame for the phasing of contracts to reach significant revenue recognition.
http://www.edisoninvestmentresearch.com/research/report/sqi-…
Gaining momentum
Outlook | Pharmaceutical & Healthcare | 06/02/2015
SQI has continued its positive momentum over the last year, signing five service agreements with drug development companies. The company also broadened its commercial focus in 2014 with a deal to automate DNA-based pathogen detection assays, a new application for its technology. We now look towards the conversion of customers from early contract work/validation to full commercialisation. Despite the encouraging deal progress, we reduce our valuation to C$55m vs C$60m previously (C$0.98 per share vs C$1.07), mainly due to the more protracted time frame for the phasing of contracts to reach significant revenue recognition.
http://www.edisoninvestmentresearch.com/research/report/sqi-…
SQI Diagnostics Reports First Quarter Financial Results
Continues to Build on Fiscal 2014 Achievements
Toronto, OntarioFebruary 26, 2015SQI Diagnostics Inc. (“SQI” or the “Company”) (TSX-V: SQD; OTCQX: SQIDF), a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today reported its financial and operational results for the fiscal first quarter ended December 31, 2014. All figures in this press release are in Canadian dollars (CAD), unless otherwise stated.
“During the quarter the Company continued to win new business from our existing customers and add to our sales pipeline,” said Andrew Morris, President and CEO of SQI. “We expect this additional business to result in the installation of multiple platforms which are the basis for sustainable and on-going revenue growth through the sale of custom and other high volume test products.”
Highlights for the Quarter
Our first global pharmaceutical customer has agreed to a consignment sqidlite instrument planned for March 2015 delivery. This global pharma client will evaluate the performance of the sqidlite instrument and two immunogenicity tests developed by SQI, one of which was completed during our first quarter of fiscal 2015. We believe successful evaluation of the instrument and test kits will lead to the purchase of the sqidlite instrument and on-going purchases of test kits.
We announced a follow-on agreement and technology transfer agreement that expands our partnership with our diagnostic customer in the infectious disease-DNA market.
We secured a second project from a global pharmaceutical company previously referred to as Global Pharma 3. This second project is meaningful as it is our third global-sized drug development customer that has initiated repeat business.
We completed the development work for our fourth global pharmaceutical customer. We are confident that we will sell this customer one or more SQI platform(s), as well as kits for human studies for this project during the second half of fiscal 2015. We are in discussions with this customer to develop tests for additional projects relating to other new drugs in their pipeline. Subsequent to the period end, this customer presented select data from this development project at the Immunogenicity and Immunotoxicity conference.
We re-established our IVD, regulated product capability by attaining FDA clearance for our newest multiplexed celiac diagnostic test. This diagnostic test comprises four individual tests that we have multiplexed into a single test and that is used to aid in the diagnosis of celiac disease. Celiac disease is a leading gastrointestinal illness, reported to occur in 1 in 130 North Americans.
We continued to expand our pipeline of customers and are in various stages of negotiation with customers for multiple new development projects including: a cardiac biomarker assay; a veterinary assay; and, an immunogenicity assay for use by a US-based CRO. Subsequent to the quarter end, we brought one of these opportunities to fruition and are in active discussions with numerous remaining prospects.
On January 30, 2015 and on February 20, 2015 the Company completed two tranches of a debenture financing resulting in gross proceeds of $3,236,000.
Financial Results Overview
During the first quarter of fiscal 2015 the Company continued to record revenue from product and services sales in our DDTS business. Revenue for the three months ended December 31, 2014 was $15,000 compared to $2,000 for the same period last year. Revenue in the first quarter of fiscal 2015 includes fess earned for the technology transfer phase of the project to manufacture a DNA-based pathogen detection test as well as for additional work contracted by one of our global pharma customers.
For the quarter-ended December 31, 2014, the Company recorded a net loss of $1,365,000 ($0.02 net loss per share) compared to a net loss of $1,501,000 ($0.03 net loss per share) for the quarter-ended December 31, 2013. The net loss was lower for the three months ended December 31, 2014 as compared to the same period last year due mainly to decreases in R&D expenditures as discussed below.
R&D expenditures, excluding amortization and stock based compensation, for the three months ended December 31, 2014 were $684,000 compared to $840,000 for the three months ended December 31, 2013. R&D costs were lower for the three months ended December 31, 2014 as compared to the same period last year. In the quarter-ended December 31, 2013 the Company incurred significant costs related to the verification and validation of Celiac DGP. There were no assays in the validation or verification stages in the current quarter. In addition the Company incurred lower R&D salary and related costs in the first quarter of fiscal 2015 as compared to the first quarter of fiscal 2014 due to staff reductions made in February of 2014 and other temporary staff changes during the quarter.
Corporate and general expenses, excluding stock-based compensation, were $359,000 for the quarter-ended December 31, 2014 compared to $298,000 for the quarter-ended December 31, 2013. The increase is due to fees incurred in the OTCQX listing process and as a result of investor relations initiatives pursued to increase investor awareness.
Sales and marketing expenses, excluding stock based compensation, totalled $160,000 for the three months ended December 31, 2014 compared to $150,000 for the three months ended December 31, 2013. The increase in sales and marketing expenses is primarily due to the addition of one individual to the sales and marketing area. Sales and marketing expenses in the quarter-ended December 31, 2013 include a bonus paid for the execution of 3 commercial contracts, there were no bonuses paid in the quarter-ended December 31, 2014.
At December 31, 2014, current assets were $744,000 compared to $2,058,000 at September 30, 2014. As at December 31, 2014 the Company has a $342,000 working capital surplus compared to a surplus of $1,625,000 at September 30, 2014. As noted above, the Company completed a financing shortly after the quarter end.
http://sqidiagnostics.com/media/2015/sqi-diagnostics-reports…
Ergo: Es geht langsam voran, aber es geht voran. Durch die erfolgreiche Finanzierung ohne Verwässerung ist die Basis nun gelegt, um in diesem Jahr die operative Wende zu erreichen. Aus meiner Sicht weiterhin ein aussichtsreicher Wert.
M@trix
Continues to Build on Fiscal 2014 Achievements
Toronto, OntarioFebruary 26, 2015SQI Diagnostics Inc. (“SQI” or the “Company”) (TSX-V: SQD; OTCQX: SQIDF), a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today reported its financial and operational results for the fiscal first quarter ended December 31, 2014. All figures in this press release are in Canadian dollars (CAD), unless otherwise stated.
“During the quarter the Company continued to win new business from our existing customers and add to our sales pipeline,” said Andrew Morris, President and CEO of SQI. “We expect this additional business to result in the installation of multiple platforms which are the basis for sustainable and on-going revenue growth through the sale of custom and other high volume test products.”
Highlights for the Quarter
Our first global pharmaceutical customer has agreed to a consignment sqidlite instrument planned for March 2015 delivery. This global pharma client will evaluate the performance of the sqidlite instrument and two immunogenicity tests developed by SQI, one of which was completed during our first quarter of fiscal 2015. We believe successful evaluation of the instrument and test kits will lead to the purchase of the sqidlite instrument and on-going purchases of test kits.
We announced a follow-on agreement and technology transfer agreement that expands our partnership with our diagnostic customer in the infectious disease-DNA market.
We secured a second project from a global pharmaceutical company previously referred to as Global Pharma 3. This second project is meaningful as it is our third global-sized drug development customer that has initiated repeat business.
We completed the development work for our fourth global pharmaceutical customer. We are confident that we will sell this customer one or more SQI platform(s), as well as kits for human studies for this project during the second half of fiscal 2015. We are in discussions with this customer to develop tests for additional projects relating to other new drugs in their pipeline. Subsequent to the period end, this customer presented select data from this development project at the Immunogenicity and Immunotoxicity conference.
We re-established our IVD, regulated product capability by attaining FDA clearance for our newest multiplexed celiac diagnostic test. This diagnostic test comprises four individual tests that we have multiplexed into a single test and that is used to aid in the diagnosis of celiac disease. Celiac disease is a leading gastrointestinal illness, reported to occur in 1 in 130 North Americans.
We continued to expand our pipeline of customers and are in various stages of negotiation with customers for multiple new development projects including: a cardiac biomarker assay; a veterinary assay; and, an immunogenicity assay for use by a US-based CRO. Subsequent to the quarter end, we brought one of these opportunities to fruition and are in active discussions with numerous remaining prospects.
On January 30, 2015 and on February 20, 2015 the Company completed two tranches of a debenture financing resulting in gross proceeds of $3,236,000.
Financial Results Overview
During the first quarter of fiscal 2015 the Company continued to record revenue from product and services sales in our DDTS business. Revenue for the three months ended December 31, 2014 was $15,000 compared to $2,000 for the same period last year. Revenue in the first quarter of fiscal 2015 includes fess earned for the technology transfer phase of the project to manufacture a DNA-based pathogen detection test as well as for additional work contracted by one of our global pharma customers.
For the quarter-ended December 31, 2014, the Company recorded a net loss of $1,365,000 ($0.02 net loss per share) compared to a net loss of $1,501,000 ($0.03 net loss per share) for the quarter-ended December 31, 2013. The net loss was lower for the three months ended December 31, 2014 as compared to the same period last year due mainly to decreases in R&D expenditures as discussed below.
R&D expenditures, excluding amortization and stock based compensation, for the three months ended December 31, 2014 were $684,000 compared to $840,000 for the three months ended December 31, 2013. R&D costs were lower for the three months ended December 31, 2014 as compared to the same period last year. In the quarter-ended December 31, 2013 the Company incurred significant costs related to the verification and validation of Celiac DGP. There were no assays in the validation or verification stages in the current quarter. In addition the Company incurred lower R&D salary and related costs in the first quarter of fiscal 2015 as compared to the first quarter of fiscal 2014 due to staff reductions made in February of 2014 and other temporary staff changes during the quarter.
Corporate and general expenses, excluding stock-based compensation, were $359,000 for the quarter-ended December 31, 2014 compared to $298,000 for the quarter-ended December 31, 2013. The increase is due to fees incurred in the OTCQX listing process and as a result of investor relations initiatives pursued to increase investor awareness.
Sales and marketing expenses, excluding stock based compensation, totalled $160,000 for the three months ended December 31, 2014 compared to $150,000 for the three months ended December 31, 2013. The increase in sales and marketing expenses is primarily due to the addition of one individual to the sales and marketing area. Sales and marketing expenses in the quarter-ended December 31, 2013 include a bonus paid for the execution of 3 commercial contracts, there were no bonuses paid in the quarter-ended December 31, 2014.
At December 31, 2014, current assets were $744,000 compared to $2,058,000 at September 30, 2014. As at December 31, 2014 the Company has a $342,000 working capital surplus compared to a surplus of $1,625,000 at September 30, 2014. As noted above, the Company completed a financing shortly after the quarter end.
http://sqidiagnostics.com/media/2015/sqi-diagnostics-reports…
Ergo: Es geht langsam voran, aber es geht voran. Durch die erfolgreiche Finanzierung ohne Verwässerung ist die Basis nun gelegt, um in diesem Jahr die operative Wende zu erreichen. Aus meiner Sicht weiterhin ein aussichtsreicher Wert.
M@trix
Ich habe meine Position in SQI in den letzten Wochen etwas abgebaut. Zum einen war die schnelle Verdopplung "einladend", zum anderen geht es geschäftlich doch langsamer voran als ich dachte und ich wollte andere Opportunitäten nutzen (z.B. habe ich eine markante Position in Telesta und jüngst Microvision aufgebaut).
An meinem grundsätzlichen Szenario halte ich fest: SQI wird in diesem Jahr im letzten Quartal den break-even erreichen und sollte mit ca. 5 Mio. $ Umsatz die Entwicklungsphase hinter sich lassen. 2016 könnte ein RICHTIG gutes Jahr werden - da sind m.E. 15-20 Mio. $ Umsatz möglich.
Indes denke ich, dass das Potenzial der Aktie noch bis zum Sommer relativ bescheiden bleibt. Daher habe ich meinen Bestand auf eine solide Basis reduziert.
M@trix
An meinem grundsätzlichen Szenario halte ich fest: SQI wird in diesem Jahr im letzten Quartal den break-even erreichen und sollte mit ca. 5 Mio. $ Umsatz die Entwicklungsphase hinter sich lassen. 2016 könnte ein RICHTIG gutes Jahr werden - da sind m.E. 15-20 Mio. $ Umsatz möglich.
Indes denke ich, dass das Potenzial der Aktie noch bis zum Sommer relativ bescheiden bleibt. Daher habe ich meinen Bestand auf eine solide Basis reduziert.
M@trix
Antwort auf Beitrag Nr.: 49.285.130 von M@trix am 10.03.15 09:50:16Dann schau mal jetzt: 33% plus!
Ja, gesehen. Leider wieder nur geringe Umsätze. Indes: im Hintergrund läuft es wohl gut. Insofern sehe ich meine Einschätzung (siehe oben) bestätigt.
Grüßend
M@trix
Grüßend
M@trix
Antwort auf Beitrag Nr.: 49.285.130 von M@trix am 10.03.15 09:50:16
Wie es scheint bin ich nicht der einzige schon lange engagierte Investor, der die Geduld verliert:
SQI Diagnostics Inc. Announces New Nominees to SQI Diagnostics Board and Adjournment of Meeting
Auszug: "SQI Diagnostics Inc. ("SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF) announced that, as a result of recent discussions with certain shareholders representing approximately 45% of the outstanding voting common shares of the Company, after dealing with certain matters including the appointment of auditors at its annual and special meeting of shareholders on March 26, 2015 (the "Meeting") it intends to adjourn the Meeting such that the election of directors will take place when the Meeting reconvenes. The Meeting will be reconvened on April 7, 2015 at 11:00 a.m. (Toronto time) at the offices of the Company's counsel, Gowling Lafleur Henderson LLP, located at 100 King Street West, Suite 1600, Toronto, Ontario.
SQI has been in discussion with the above noted group of shareholders regarding the election of directors following the deposit of proxies in connection with the Meeting earlier this week. These discussions have resulted in an agreement to put forth four new nominees (Messrs. Clive J. Beddoe, Gerald R. Connor, Wilmot L. Matthews and Cameron R. Prange) proposed by these shareholders to stand for election as directors, together with three nominees proposed in the Company's management proxy circular (Messrs. Andrew Morris, Eric Schneider and Claude Ricks). It is anticipated that if the four new nominees, together with Messers. Morris, Schneider and Ricks are elected at the reconvened Meeting, the new Board will be chaired by Clive Beddoe, who is currently Chairman and principal founder of WestJet Airlines. The remaining management nominees have withdrawn from the director election process. The Company would like to thank Messrs. Peter Winkley, Paul Mountain, David Williams and Dr. Allan Pronovost for their contributions to the Company and past service to the Board."
Bemerkenswert finde ich, dass man sich selbst von Dr. Allan Pronovost schon wieder trennt.
Aus meiner Sicht kann die Meldung kurzfristig kaum positiv interpretiert werden, denn es lässt sich daraus ableiten, dass der Vertrieb noch immer nicht richtig voran kommt. Entsprechend dürfte die Aktie erst mal weiter seitwärts laufen. Ob die neuen Board-Mitglieder die Wende erreichen wird sich erst noch zeigen.
Ich habe meine Position erst mal weiter abgebaut.
M@trix
Zitat von M@trix: Ich habe meine Position in SQI in den letzten Wochen etwas abgebaut. Zum einen war die schnelle Verdopplung "einladend", zum anderen geht es geschäftlich doch langsamer voran als ich dachte und ich wollte andere Opportunitäten nutzen (z.B. habe ich eine markante Position in Telesta und jüngst Microvision aufgebaut).
An meinem grundsätzlichen Szenario halte ich fest: SQI wird in diesem Jahr im letzten Quartal den break-even erreichen und sollte mit ca. 5 Mio. $ Umsatz die Entwicklungsphase hinter sich lassen. 2016 könnte ein RICHTIG gutes Jahr werden - da sind m.E. 15-20 Mio. $ Umsatz möglich.
Indes denke ich, dass das Potenzial der Aktie noch bis zum Sommer relativ bescheiden bleibt. Daher habe ich meinen Bestand auf eine solide Basis reduziert.
M@trix
Wie es scheint bin ich nicht der einzige schon lange engagierte Investor, der die Geduld verliert:
SQI Diagnostics Inc. Announces New Nominees to SQI Diagnostics Board and Adjournment of Meeting
Auszug: "SQI Diagnostics Inc. ("SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF) announced that, as a result of recent discussions with certain shareholders representing approximately 45% of the outstanding voting common shares of the Company, after dealing with certain matters including the appointment of auditors at its annual and special meeting of shareholders on March 26, 2015 (the "Meeting") it intends to adjourn the Meeting such that the election of directors will take place when the Meeting reconvenes. The Meeting will be reconvened on April 7, 2015 at 11:00 a.m. (Toronto time) at the offices of the Company's counsel, Gowling Lafleur Henderson LLP, located at 100 King Street West, Suite 1600, Toronto, Ontario.
SQI has been in discussion with the above noted group of shareholders regarding the election of directors following the deposit of proxies in connection with the Meeting earlier this week. These discussions have resulted in an agreement to put forth four new nominees (Messrs. Clive J. Beddoe, Gerald R. Connor, Wilmot L. Matthews and Cameron R. Prange) proposed by these shareholders to stand for election as directors, together with three nominees proposed in the Company's management proxy circular (Messrs. Andrew Morris, Eric Schneider and Claude Ricks). It is anticipated that if the four new nominees, together with Messers. Morris, Schneider and Ricks are elected at the reconvened Meeting, the new Board will be chaired by Clive Beddoe, who is currently Chairman and principal founder of WestJet Airlines. The remaining management nominees have withdrawn from the director election process. The Company would like to thank Messrs. Peter Winkley, Paul Mountain, David Williams and Dr. Allan Pronovost for their contributions to the Company and past service to the Board."
Bemerkenswert finde ich, dass man sich selbst von Dr. Allan Pronovost schon wieder trennt.
Aus meiner Sicht kann die Meldung kurzfristig kaum positiv interpretiert werden, denn es lässt sich daraus ableiten, dass der Vertrieb noch immer nicht richtig voran kommt. Entsprechend dürfte die Aktie erst mal weiter seitwärts laufen. Ob die neuen Board-Mitglieder die Wende erreichen wird sich erst noch zeigen.
Ich habe meine Position erst mal weiter abgebaut.
M@trix
Das erklärt wohl den jüngsten Anstieg... wobei es noch immer KEIN sicherer Deal ist:
SQI Delivers Sqidlite System to Global Pharmaceutical Customer
1 hour ago
TORONTO , April 15, 2015 /CNW/ - SQI Diagnostics Inc. (TSX-V: SQD; OTCQX: SQIDF), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics ("SQI"), today announced the delivery and installation of a sqidlite™ system under the terms of an agreement with a top tier global pharmaceutical customer. As previously disclosed, two custom immunogenicity assays have been successfully developed for this customer.
SQI has executed an agreement to install the fully automated sqidlite system at one of the customer's US-based facilities. Under the terms of the agreement, the customer will use the equipment primarily in order to validate the performance of a 21-plex test developed for this customer to run on SQI's sqidlite system. Successful, final evaluation is expected to result in the purchase of the sqidlite system and the on-going sales of initial products for use in the customer's clinical programs. The Company is currently in discussions to initiate development services for one or more additional products in 2015.
"We are pleased to have advanced to this last stage of the sales process with this customer who is a key opinion leader in this important and growing market of immunogenicity and bioanalytical testing and who has a multitude of drugs in clinical development that could benefit from our advanced multiplexed diagnostic testing," said Andrew Morris , CEO of SQI Diagnostics.
The economic benefits of using SQI's custom tests with Ig_plex™ technology and sqidlite automated testing equipment in drug development are to decrease the total number of tests performed through multiplexing while at the same time requiring significantly less volume of valuable blood samples; reducing the total time required to process the tests resulting in significantly reduced labour costs and testing timelines. All of this has a significant impact on the customer's total cost of testing in the related clinical phases of their drug development programs. SQI's Ig_plex products have been shown to significantly improve the drug tolerance, sensitivity and other important performance metrics through the use of its custom anti-drug-antibody (ADA) tests, ultimately resulting in better, more cost effective, and easier to obtain data.
http://finance.yahoo.com/news/sqi-delivers-sqidlite-system-g…
SQI Delivers Sqidlite System to Global Pharmaceutical Customer
1 hour ago
TORONTO , April 15, 2015 /CNW/ - SQI Diagnostics Inc. (TSX-V: SQD; OTCQX: SQIDF), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics ("SQI"), today announced the delivery and installation of a sqidlite™ system under the terms of an agreement with a top tier global pharmaceutical customer. As previously disclosed, two custom immunogenicity assays have been successfully developed for this customer.
SQI has executed an agreement to install the fully automated sqidlite system at one of the customer's US-based facilities. Under the terms of the agreement, the customer will use the equipment primarily in order to validate the performance of a 21-plex test developed for this customer to run on SQI's sqidlite system. Successful, final evaluation is expected to result in the purchase of the sqidlite system and the on-going sales of initial products for use in the customer's clinical programs. The Company is currently in discussions to initiate development services for one or more additional products in 2015.
"We are pleased to have advanced to this last stage of the sales process with this customer who is a key opinion leader in this important and growing market of immunogenicity and bioanalytical testing and who has a multitude of drugs in clinical development that could benefit from our advanced multiplexed diagnostic testing," said Andrew Morris , CEO of SQI Diagnostics.
The economic benefits of using SQI's custom tests with Ig_plex™ technology and sqidlite automated testing equipment in drug development are to decrease the total number of tests performed through multiplexing while at the same time requiring significantly less volume of valuable blood samples; reducing the total time required to process the tests resulting in significantly reduced labour costs and testing timelines. All of this has a significant impact on the customer's total cost of testing in the related clinical phases of their drug development programs. SQI's Ig_plex products have been shown to significantly improve the drug tolerance, sensitivity and other important performance metrics through the use of its custom anti-drug-antibody (ADA) tests, ultimately resulting in better, more cost effective, and easier to obtain data.
http://finance.yahoo.com/news/sqi-delivers-sqidlite-system-g…
warrant extension - sowas mag ich nicht...
http://www.stockwatch.com/News/Item.aspx?bid=Z-C%3aSQD-22686…
Antwort auf Beitrag Nr.: 49.719.972 von techinvestor69 am 06.05.15 11:20:48Ich auch nicht. Allerdings ist der Ausübungskurs hier deutlich vom aktuellen Kurs entfernt. Insofern sehe ich es hier eher als neutral.
Wichtig sind jetzt Deals, Deals, Deals. Erst wenn da endlich was konkretes kommt, wird die Aktie wieder interessant.
M@trix
Wichtig sind jetzt Deals, Deals, Deals. Erst wenn da endlich was konkretes kommt, wird die Aktie wieder interessant.
M@trix
erst wenn SQD mal irgendwas an Investor Relations Arbeit entfaltet, wird hier was passieren
......Hi......
wasn los heute............?
sauber im plus....
M.
wasn los heute............?
sauber im plus....
M.
Kaum Umsatz bei SQD in Kanada. Mit 1.000 Stk die Briefseite geprinted, daher das zweistellige Kursplus. Schau Dir lieber mal das an: http://www.wallstreet-online.de/diskussion/1214676-31-40/ser…
Meinen Ausstieg habe ich bisher nicht bereut. Es geht leider alles sehr langsam voran. Aber es geht voran:
"During and subsequent to the quarter-end, we continued our discussions with this customer for the expansion of our revenue base to include multiple new drugs and the SQI products to be designed for testing these drugs. We expect that we will add at least two new drug projects for this customer in the current year -- and, if we continue with our current levels of customer satisfaction, to continue to add to the number of drugs for which we are building products during 2016."
[...]
"Our current-quarter revenue also includes milestone payments earned at the endpoint of multiple development projects. This represents a significant achievement in that SQI has shown it can win business, complete complex multiplexing projects, use the final product to produce data, and earn income.
During the quarter we had six ongoing paid development and testing service projects. Our two largest pharma customers -- one now at the platform and one going through their internal approval process to acquire a system -- together have four products now through development and moving into evaluation, with up to five additional products being discussed that could lead to additional development and product revenues. We believe that platform installations will also advance the additional projects under discussion and may cause the development timelines to shorten somewhat." [...]
http://finance.yahoo.com/news/edited-transcript-sqd-v-earnin…
Indes nur noch Cash für fünf Monate. Ich denke, es wird wieder eine Finanzierung geben im ersten Quartal 2016. Danach schaue ich mir die Aktie noch mal an.
M@trix
"During and subsequent to the quarter-end, we continued our discussions with this customer for the expansion of our revenue base to include multiple new drugs and the SQI products to be designed for testing these drugs. We expect that we will add at least two new drug projects for this customer in the current year -- and, if we continue with our current levels of customer satisfaction, to continue to add to the number of drugs for which we are building products during 2016."
[...]
"Our current-quarter revenue also includes milestone payments earned at the endpoint of multiple development projects. This represents a significant achievement in that SQI has shown it can win business, complete complex multiplexing projects, use the final product to produce data, and earn income.
During the quarter we had six ongoing paid development and testing service projects. Our two largest pharma customers -- one now at the platform and one going through their internal approval process to acquire a system -- together have four products now through development and moving into evaluation, with up to five additional products being discussed that could lead to additional development and product revenues. We believe that platform installations will also advance the additional projects under discussion and may cause the development timelines to shorten somewhat." [...]
http://finance.yahoo.com/news/edited-transcript-sqd-v-earnin…
Indes nur noch Cash für fünf Monate. Ich denke, es wird wieder eine Finanzierung geben im ersten Quartal 2016. Danach schaue ich mir die Aktie noch mal an.
M@trix
Director Clive Beddoe bought 50,000 shares of the firm’s stock in a transaction dated Monday, August 31st. The stock was purchased at an average price of C$0.49 per share, for a total transaction of C$24,500.00.
SQI Diagnostics (TSE:SQD) insider Lennie Ryer purchased 21,639 shares of the stock in a transaction that occurred on Wednesday, August 26th. The shares were acquired at an average price of C$0.40 per share, for a total transaction of C$8,655.60.
Hmmm.... die Insider haben schon bei der letzten Kapitalerhöhung fast alles genommen. Ein solches Vertrauen in das Unternehmen ist beachtlich.
Ich habe mir daher auch wieder ein paar Stücke zu 0,45 CAD ins Depot gelegt. Vielleicht kommt ja bald was positives...
M@trix
SQI Diagnostics (TSE:SQD) insider Lennie Ryer purchased 21,639 shares of the stock in a transaction that occurred on Wednesday, August 26th. The shares were acquired at an average price of C$0.40 per share, for a total transaction of C$8,655.60.
Hmmm.... die Insider haben schon bei der letzten Kapitalerhöhung fast alles genommen. Ein solches Vertrauen in das Unternehmen ist beachtlich.
Ich habe mir daher auch wieder ein paar Stücke zu 0,45 CAD ins Depot gelegt. Vielleicht kommt ja bald was positives...
M@trix
Die mangelnde Liquidität schreckt mich hier ab. Da kaufe ich lieber SVA.
Antwort auf Beitrag Nr.: 50.552.423 von techinvestor69 am 03.09.15 17:55:00Ja, die Liquidität ist SEHR bescheiden, wobei ich denke, dass der fundamentale Trend bei SQI stimmt. Es geht eben sehr langsam voran, wenn jedoch die ersten Orders kommen, kann man relativ sicher von weiteren ausgehen.
Börsenwert 27 Mio. CAD erscheint mir nicht überhöht, sondern lässt ausreichend Raum für Zugewinne, wenn die entsprechenden News kommen.
Und die Insiderkäufe finde ich an sich schon beachtlich - beobachte den Wert immerhin schon seit Jahren (mit zwischenzeitlichem positiv abgeschlossenem Investment).
Sernova habe ich auf der Watchlist, konnte mich aber noch nicht eingehender damit befassen. Ich kaufe jedoch ungern irgendwo ein, wenn die Haltefrist für Warrants endet. Oft wird dann vorher "gepusht" und dann abverkauft. Und erst Phase I/II. Solche Werte fasse ich per se ungern an. Bis das EVENTUELL zur Marktreife kommt, vergehen noch Jahre, in denen i.d.R. die Aktienzahl ständig wächst...
SQI ist aus meiner Sicht solide und ich glaube, dass die nächsten Monate ENDLICH relevante vertriebliche News bringen werden. Hat sicher nicht das Potenzial einer Telesta (meine größte Position), aber eben auch weniger Risiko im Geschäftsmodell.
M@trix
Börsenwert 27 Mio. CAD erscheint mir nicht überhöht, sondern lässt ausreichend Raum für Zugewinne, wenn die entsprechenden News kommen.
Und die Insiderkäufe finde ich an sich schon beachtlich - beobachte den Wert immerhin schon seit Jahren (mit zwischenzeitlichem positiv abgeschlossenem Investment).
Sernova habe ich auf der Watchlist, konnte mich aber noch nicht eingehender damit befassen. Ich kaufe jedoch ungern irgendwo ein, wenn die Haltefrist für Warrants endet. Oft wird dann vorher "gepusht" und dann abverkauft. Und erst Phase I/II. Solche Werte fasse ich per se ungern an. Bis das EVENTUELL zur Marktreife kommt, vergehen noch Jahre, in denen i.d.R. die Aktienzahl ständig wächst...
SQI ist aus meiner Sicht solide und ich glaube, dass die nächsten Monate ENDLICH relevante vertriebliche News bringen werden. Hat sicher nicht das Potenzial einer Telesta (meine größte Position), aber eben auch weniger Risiko im Geschäftsmodell.
M@trix
OT: Bei SVA laufen warrants erst nächstes Jahr aus.
10,000,000 - $0.40 - February 19, 2016.
Und ehe die warrants im Geld sind, liege ich schon gut vorne.
10,000,000 - $0.40 - February 19, 2016.
Und ehe die warrants im Geld sind, liege ich schon gut vorne.

Alphastoxx-Update, das ich inhaltlich vollkommen teile:
Dear Alphastox Subscribers,
I wanted to make sure you had a chance to review SQI Diagnostics (TSX-V: SQD; OTCQX: SQIDF)’s very busy last quarter. It looks like they’ve laid the foundation to what could be a very exciting new year so make sure you stay tuned! Over the last three months, the company was busy developing their sqidlite system, closing contracts, and raising funds (at a 25% premium to today’s price) for further research and growth.
The life sciences company recently released their results from their last quarter and I wanted to share some of their highlights. The most important thing to note is that their revenues for the last nine months grew over 4X for the same period last year which means, things are definitely progressing significantly. The growth has been consistent over the past three quarters and I truly think the next one could be a show-stopper if they’re able to close some of the major deals they currently have in the pipeline.
Other highlights from the quarter included:
- One of the big pharma customers in the US installed a fully automated sqidlite system to validate the performance of a 21-plex test developed for this customer. This customer is expected to purchase the system, as well as kits for use in the customer’s clinical programs. SQI expects to add a least two new drug projects (each new drug project could potentially add $1-$2 million in revenue) over the life of the drug development program for this customer as well as additional products in 2016.
- At the 9th Workshop on Recent Issues in Bioanalysis in Miami, Florida in April 2015, Bristol-Myers Squibb presented SQI’s immunogenicity testing.
- Processing/Testing of pre-clinical samples at SQI’s facility for its Global Pharma 3 company generated highly positive results, continuing to prove to the client the benefits of using the SQI-developed multiplexed test.
- A contract was completed on behalf of a customer in the animal health market to convert three existing veterinary ELISA test to the SQI platform. The successful results are expected to generate additional revenue in fiscal 2015 to develop a second multiplexed test The project represents the opportunity for SQI to convert all single-plex tests in the animal health market to their multiplex technology representing millions in potential revenue.
- With SQI’s DNA customer, a 31-plex DNA-based test is being developed to detect infections in human blood, and a second test targeted at detecting infections in dairy cattle could be developed next year.
One thing I need to highlight again is how dedicated SQI’s team is to building a successful company. At the end of July, SQI tried to raise up to $5 million at $0.50/share. At the time, the stock was sitting around those levels but a few weeks into their roadshow, the market took a hit and the stock came off 10 cents. When investors shied away from investing in the deal, it never fazed their team who came up with the money themselves and wrote a cheque for $2.7 million at a 25% premium to the market. This should add a ton of confidence for investors knowing that the deal isn’t going anywhere. They have a constant funding source who believe in the project and can see the progress Andrew and his team are making. With their latest results, they’ve set the stage for a very exciting and promising 2016.
Revenues in Q3 grew over 460% to $180,000 compared to last year which may seem like a modest number but is significant when you take into account that more than 60% of that revenue came from pre-existing customers who were only testing the product. None of them actually came in with major orders because they were all in the testing phase, but these significant orders could hopefully all be coming in next year. Even if they were only able to close on half of the customers they currently have testing the product, their revenues would jump significantly and would dwarf expectations.
As noted during the Q3 conference call held on August 19, the ongoing success of the company proves that there is a large market demand and a long-term business for SQI’s multiplexing products. The ongoing revenue validates the value of SQI’s multiplexing technology to the pharmaceutical industry. Even though the company’s initial work is in immunogenicity testing, they’re looking for ways to expand the core technology by looking at other opportunities within the pharma industry which could easily be another major revenue driver.
Make sure you keep SQD.V on watch right now. Having insiders write another cheque for $2.7 million at a premium is nothing to shy away from and I can guarantee you these guys that wrote the cheques are no dummies…there’s a reason why they continue to fund the deal and it ultimately comes down to Andrew’s vision, his ability to succeed and grow this company into a couple hundred million dollar enterprise in short order.
As always, if you have any questions, please do not hesitate to get in touch with me anytime. I look forward to hearing from you.
Best,
Etienne
Dear Alphastox Subscribers,
I wanted to make sure you had a chance to review SQI Diagnostics (TSX-V: SQD; OTCQX: SQIDF)’s very busy last quarter. It looks like they’ve laid the foundation to what could be a very exciting new year so make sure you stay tuned! Over the last three months, the company was busy developing their sqidlite system, closing contracts, and raising funds (at a 25% premium to today’s price) for further research and growth.
The life sciences company recently released their results from their last quarter and I wanted to share some of their highlights. The most important thing to note is that their revenues for the last nine months grew over 4X for the same period last year which means, things are definitely progressing significantly. The growth has been consistent over the past three quarters and I truly think the next one could be a show-stopper if they’re able to close some of the major deals they currently have in the pipeline.
Other highlights from the quarter included:
- One of the big pharma customers in the US installed a fully automated sqidlite system to validate the performance of a 21-plex test developed for this customer. This customer is expected to purchase the system, as well as kits for use in the customer’s clinical programs. SQI expects to add a least two new drug projects (each new drug project could potentially add $1-$2 million in revenue) over the life of the drug development program for this customer as well as additional products in 2016.
- At the 9th Workshop on Recent Issues in Bioanalysis in Miami, Florida in April 2015, Bristol-Myers Squibb presented SQI’s immunogenicity testing.
- Processing/Testing of pre-clinical samples at SQI’s facility for its Global Pharma 3 company generated highly positive results, continuing to prove to the client the benefits of using the SQI-developed multiplexed test.
- A contract was completed on behalf of a customer in the animal health market to convert three existing veterinary ELISA test to the SQI platform. The successful results are expected to generate additional revenue in fiscal 2015 to develop a second multiplexed test The project represents the opportunity for SQI to convert all single-plex tests in the animal health market to their multiplex technology representing millions in potential revenue.
- With SQI’s DNA customer, a 31-plex DNA-based test is being developed to detect infections in human blood, and a second test targeted at detecting infections in dairy cattle could be developed next year.
One thing I need to highlight again is how dedicated SQI’s team is to building a successful company. At the end of July, SQI tried to raise up to $5 million at $0.50/share. At the time, the stock was sitting around those levels but a few weeks into their roadshow, the market took a hit and the stock came off 10 cents. When investors shied away from investing in the deal, it never fazed their team who came up with the money themselves and wrote a cheque for $2.7 million at a 25% premium to the market. This should add a ton of confidence for investors knowing that the deal isn’t going anywhere. They have a constant funding source who believe in the project and can see the progress Andrew and his team are making. With their latest results, they’ve set the stage for a very exciting and promising 2016.
Revenues in Q3 grew over 460% to $180,000 compared to last year which may seem like a modest number but is significant when you take into account that more than 60% of that revenue came from pre-existing customers who were only testing the product. None of them actually came in with major orders because they were all in the testing phase, but these significant orders could hopefully all be coming in next year. Even if they were only able to close on half of the customers they currently have testing the product, their revenues would jump significantly and would dwarf expectations.
As noted during the Q3 conference call held on August 19, the ongoing success of the company proves that there is a large market demand and a long-term business for SQI’s multiplexing products. The ongoing revenue validates the value of SQI’s multiplexing technology to the pharmaceutical industry. Even though the company’s initial work is in immunogenicity testing, they’re looking for ways to expand the core technology by looking at other opportunities within the pharma industry which could easily be another major revenue driver.
Make sure you keep SQD.V on watch right now. Having insiders write another cheque for $2.7 million at a premium is nothing to shy away from and I can guarantee you these guys that wrote the cheques are no dummies…there’s a reason why they continue to fund the deal and it ultimately comes down to Andrew’s vision, his ability to succeed and grow this company into a couple hundred million dollar enterprise in short order.
As always, if you have any questions, please do not hesitate to get in touch with me anytime. I look forward to hearing from you.
Best,
Etienne
Irgendetwas sagt mir, dass das eine vorgezogene "Belohnung" ist und wir schon bald "good news" erhalten werden. Kapitalerhöhung mit erheblicher Beteiligung des Managements, Insiderkäufe, IR-Update und nun die Optionen:
SQI Diagnostics Announces Stock Option Grant
TORONTO , Sept. 18, 2015 /CNW/ - SQI Diagnostics Inc. ("SQI Diagnostics" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced that effective September 16, 2015 , they have granted 340,000 stock options of which 200,000 has been granted to certain officers and directors. Independent directors did not receive options in this grant.
The options were granted at an exercise price of $0.42. Pursuant to the previously approved stock option plan, the options have a term of 5 years and vest over a 24 month period. Following the grant of these options there will be 2,422,000 options outstanding. Twelve months from the date of this grant 420,000 currently outstanding options will have expired. There are currently 61,716,000 common shares outstanding.
Mal sehen, ob ich Recht behalte.
M@trix
SQI Diagnostics Announces Stock Option Grant
TORONTO , Sept. 18, 2015 /CNW/ - SQI Diagnostics Inc. ("SQI Diagnostics" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced that effective September 16, 2015 , they have granted 340,000 stock options of which 200,000 has been granted to certain officers and directors. Independent directors did not receive options in this grant.
The options were granted at an exercise price of $0.42. Pursuant to the previously approved stock option plan, the options have a term of 5 years and vest over a 24 month period. Following the grant of these options there will be 2,422,000 options outstanding. Twelve months from the date of this grant 420,000 currently outstanding options will have expired. There are currently 61,716,000 common shares outstanding.
Mal sehen, ob ich Recht behalte.

M@trix
Tapping into considerable market opportunities
SQI has made steady progress over recent months in signing new agreements with drug development companies in its high-volume assay business. Encouragingly, service revenues are now booked for custommade tests early on in development, albeit at modest levels. While the conversion of customers from early contract work/validation to meaningful revenue-generating accounts is slower than expected, industry interest
continues to grow steadily for SQI’s multiplexing technology capabilities, as does its pipeline of potential customers.
[...]
We value SQI at C$46m or C$0.75, adjusted from C$55m or C$0.98 per share, with the change primarily due to the removal of the IVD franchise from our forecasts as management shifts all current investment to its high-volume assay diagnostics tools and services business. In July 2015, SQI completed a nonbrokered private placement of 5.33m shares grossing proceeds of $2.7m at a price of $0.50 per unit. Estimated cash holdings of approximately $1.7m at end September 2015 should be sufficient to fund operation into CY16.
http://www.edisoninvestmentresearch.com/research/report/sqi-…
SQI has made steady progress over recent months in signing new agreements with drug development companies in its high-volume assay business. Encouragingly, service revenues are now booked for custommade tests early on in development, albeit at modest levels. While the conversion of customers from early contract work/validation to meaningful revenue-generating accounts is slower than expected, industry interest
continues to grow steadily for SQI’s multiplexing technology capabilities, as does its pipeline of potential customers.
[...]
We value SQI at C$46m or C$0.75, adjusted from C$55m or C$0.98 per share, with the change primarily due to the removal of the IVD franchise from our forecasts as management shifts all current investment to its high-volume assay diagnostics tools and services business. In July 2015, SQI completed a nonbrokered private placement of 5.33m shares grossing proceeds of $2.7m at a price of $0.50 per unit. Estimated cash holdings of approximately $1.7m at end September 2015 should be sufficient to fund operation into CY16.
http://www.edisoninvestmentresearch.com/research/report/sqi-…
Wichtige News und weiterer Insiderkauf:
SQI Diagnostics Formalizes Multiple Contracts With Global Pharmaceutical Firm
http://finance.yahoo.com/news/sqi-diagnostics-formalizes-mul…
SQI Diagnostics (TSE:SQD) insider Lennie Ryer bought 35,000 shares of the company’s stock in a transaction that occurred on Monday, October 26th. The stock was purchased at an average price of C$0.35 per share, for a total transaction of C$12,250.00.
http://www.dakotafinancialnews.com/new-insider-buying-for-oc…
SQI Diagnostics Formalizes Multiple Contracts With Global Pharmaceutical Firm
http://finance.yahoo.com/news/sqi-diagnostics-formalizes-mul…
SQI Diagnostics (TSE:SQD) insider Lennie Ryer bought 35,000 shares of the company’s stock in a transaction that occurred on Monday, October 26th. The stock was purchased at an average price of C$0.35 per share, for a total transaction of C$12,250.00.
http://www.dakotafinancialnews.com/new-insider-buying-for-oc…
Position heute weiter ausgebaut. Man lese sich die Mitschrift zum jüngsten Conference Call durch - da sind einige positive Hammer drin. Das bekommt nur (noch) niemand mit, weil die Aktie keiner mehr auf dem Schirm hat...
http://finance.yahoo.com/news/edited-transcript-sqd-v-earnin…
"In 2016, we see SQI selling ongoing consumable kits for the 21-plex product and completing development for the next two products. We expect all of the products to be generating recurring revenue in 2016 and beyond. We will continue to develop the relationship, and our success will allow us to add more new products to this customer's menu of SQI consumables."
"We continue development work with our DNA or molecular customer to automate and scale up the manufacturing for their infectious disease test panels. The first test for this customer started as a 19-plex animal health test demonstration; morphed into a 31-plex DNA test for humans; and is currently a 67-plex test used to detect infections in human blood. We expect that the successful completion of this project to lead to a long-term agreement to supply consumables and sqidlite [VH] platforms to this customer. The estimate for the one core product is about 1 million tests per year, with future products to include other human infectious disease and animal health panels."
"We can say that we have not faced a custom development multiplexing project that we could not solve. Our science is solid. Our value proposition is being touted by our customers. We believe that in aggregate, our potential consumable sales for the projects we have in hand, or will soon launch with agreements, represent about 2,700 consumable kits per month. We also estimate that we need to sell about 300 kits a month to be cash flow breakeven."
Ich rechne im kommenden Jahr mit einer Fülle an positiven News und dem endgültigen Durchbruch der Technologie sowie einer Neubewertung der Aktie.
Kurs: 0,32 CAD
M@trix
http://finance.yahoo.com/news/edited-transcript-sqd-v-earnin…
"In 2016, we see SQI selling ongoing consumable kits for the 21-plex product and completing development for the next two products. We expect all of the products to be generating recurring revenue in 2016 and beyond. We will continue to develop the relationship, and our success will allow us to add more new products to this customer's menu of SQI consumables."
"We continue development work with our DNA or molecular customer to automate and scale up the manufacturing for their infectious disease test panels. The first test for this customer started as a 19-plex animal health test demonstration; morphed into a 31-plex DNA test for humans; and is currently a 67-plex test used to detect infections in human blood. We expect that the successful completion of this project to lead to a long-term agreement to supply consumables and sqidlite [VH] platforms to this customer. The estimate for the one core product is about 1 million tests per year, with future products to include other human infectious disease and animal health panels."
"We can say that we have not faced a custom development multiplexing project that we could not solve. Our science is solid. Our value proposition is being touted by our customers. We believe that in aggregate, our potential consumable sales for the projects we have in hand, or will soon launch with agreements, represent about 2,700 consumable kits per month. We also estimate that we need to sell about 300 kits a month to be cash flow breakeven."
Ich rechne im kommenden Jahr mit einer Fülle an positiven News und dem endgültigen Durchbruch der Technologie sowie einer Neubewertung der Aktie.
Kurs: 0,32 CAD
M@trix
Antwort auf Beitrag Nr.: 48.351.214 von M@trix am 18.11.14 16:50:18
Das ist jetzt etwas mehr als ein Jahr her und leider war ich abermals zu optimistisch. Positiv ist zu vermerken, DASS 2015 erstmals Umsätze erzielt wurden und auch die erste Plattform bei einem Kunden installiert werden konnte (was jedoch noch immer kein Verkauf ist).
Für 2016 erwartet SQI laut aktuellem MD&A, dass sie weitere 5 sqidlite Plattformen installieren können und weitere Servicevereinbarungen erreichen. Generell liest sich die aktuelle MD&A recht zuversichtlich, indes geht es weiter sehr langsam voran.
2016 könnte m.E. der Umsatz auf ca. 5 Mio. CAD steigen. Börsenwert aktuell 21 Mio. CAD. Demnach m.E nicht allzuviel Luft nach oben - wenn es nicht doch positiver kommt.
Andererseits haben wieder die Insider in hohem Maße Geld hineingesteckt und ich habe keine Zweifel daran, dass SQI technologisch ausgereifte und ökonomisch überzeugende Leistungen offeriert. Der Markterfolg wird kommen und wenn er kommt, dann nachhaltig sein.
Insofern weiter eine Frage der Geduld. Den Kurs sehe ich Ende 2016 jedenfalls wieder deutlich höher als jetzt. Daher nutze ich die aktuellen Kurse wieder zum Positionsaufbau.
M@trix
Zitat von M@trix: SQI hat auch die Investoren-Präsentation aktualisiert:
http://sqidiagnostics.com/sites/default/files/SQI%20Investor…
Besonderes Augenmerk sollte auf Folie 20 gerichtet werden. Diese verdeutlicht das immense Potenzial allein schon bei den bestehenden sechs Kunden. Auch die Folien 23 und 26 verdienen aufmerksame Beachtung.
Beim Start dieser Diskussion habe ich stark unterschätzt, wie viel Zeit vergehen würde, bis man wirkliche Umsätze generiert. Daher war das Jahr 2014 auch kein Erfolg, was die Aktienkursentwicklung betrifft. Nun steht SQI aber mit mehreren marktreifen, technisch überlegenen, kostensparenden Produkten da und baut sein Sales Team aus.
2015 dürfte daher die nachhaltige Wende bringen und "richtig Spaß" sollte dann das Jahr 2016 machen. Ich halte es nicht für abwegig, dass SQI bis Ende 2016 auf 15-20 Kunden kommt und mit jedem dieser Kunden Umsätze im niedrigen ein- bis zweistelligen Millionenbereich erzielt.
Der Umsatz Ende 2016 könnte also durchaus bei ca. 15-20 Mio. $ liegen. Bei einer Marge von 80% (die wahrscheinlich aber eher bei ca. 60% wegen mehr Personalaufwand liegen wird) kann durchaus ein Gewinn von ca. 10 Mio. $ hängen bleiben. Ein recht konservatives KGV von 15 unterstellt errechnet sich somit ein Kursziel von ca. 2,66 CAD. Aktuell notieren wir bei 0,38 CAD.
Selbst wenn die Aktie nochmal verwässert wird: SQI ist auf diesem Niveau stark unterbewertet.
Das Problem liegt m.E. in der Illiquidität der Aktie. Das schreckt leider jeden institutionellen Investor ab. Ich gehe jedoch davon aus, dass wenn die Zahlen "anfangen für sich zu sprechen", auch die Aktie liquider werden wird.
Für mich weiterhin ein klares langfristiges Investment mit Vervielfachungspotenzial.
M@trix, der gerade weitere 3.500 St. in Kanada zu 0,38 CAD zugekauft hat
Das ist jetzt etwas mehr als ein Jahr her und leider war ich abermals zu optimistisch. Positiv ist zu vermerken, DASS 2015 erstmals Umsätze erzielt wurden und auch die erste Plattform bei einem Kunden installiert werden konnte (was jedoch noch immer kein Verkauf ist).
Für 2016 erwartet SQI laut aktuellem MD&A, dass sie weitere 5 sqidlite Plattformen installieren können und weitere Servicevereinbarungen erreichen. Generell liest sich die aktuelle MD&A recht zuversichtlich, indes geht es weiter sehr langsam voran.
2016 könnte m.E. der Umsatz auf ca. 5 Mio. CAD steigen. Börsenwert aktuell 21 Mio. CAD. Demnach m.E nicht allzuviel Luft nach oben - wenn es nicht doch positiver kommt.
Andererseits haben wieder die Insider in hohem Maße Geld hineingesteckt und ich habe keine Zweifel daran, dass SQI technologisch ausgereifte und ökonomisch überzeugende Leistungen offeriert. Der Markterfolg wird kommen und wenn er kommt, dann nachhaltig sein.
Insofern weiter eine Frage der Geduld. Den Kurs sehe ich Ende 2016 jedenfalls wieder deutlich höher als jetzt. Daher nutze ich die aktuellen Kurse wieder zum Positionsaufbau.
M@trix
I'm STILL learning
Was lange währt...
SQI Sells Sqidlite™-dh System to a Major Diagnostics Customer
March 29, 2016 2:59 PM
Major Milestone in Commercialization
TORONTO , March 29, 2016 /CNW/ - SQI Diagnostics Inc. ("SQI Diagnostics" "SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), today announced that it has sold its first sqidlite system in its diagnostic business segment. Management believes that this is the first of multiple systems that it will sell to this customer to run an estimated 1 million samples annually to test for infections in human blood in the US market.
"At SQI, we believe that our technology benefits our diagnostic and pharmaceutical customers by generating superior test results in less time and at a substantially reduced cost. Our business model is focused on the long term recurring sale of technologically advanced multiplexed test kits that will allow our customers to achieve these substantial benefits," said Andrew Morris CEO of SQI Diagnostics. "In order to be successful in generating long term recurring revenue streams from the sale of our multiplexed test kits, we need to first sell our sqidlite systems and this first sale in our diagnostics business segment is an important milestone for us."
SQI's relationship with this diagnostic customer began over a year ago with a contract to automate their DNA-based pathogen detection assays. Since then we have been working to automate and scale-up the manufacturing of an 80-plex test kit to detect infections in human blood. This 80-plex test creates large saving for the customer because it eliminates the work needed to develop, run, and purchase materials for 80 separate single tests.
Current technologies for diagnosing infections in human blood typically takes multiple days using microbiology (petri-dish-like tests) or "real-time" DNA based tests that are typically many multiples of the cost of microbiology tests. The value-drivers of this 80-plex product, being developed under contract at SQI, are to provide results in time-frames that are days faster than microbiology tests and that can be deployed at a target cost of delivery that is much less than "real-time" DNA-based tests.
This is our first sqidlite sale in our diagnostic business segment.
In our pharma business segment, the Company has one sqidlite system being commercially operated at a customer to whom we have recently sold and delivered our first substantial commercial order of test kits. We previously announced signing a multi-year, multi-product agreement with this customer following the development and evaluation of multiple custom products designed to run on the sqidlite system. We believe the success with the completed evaluation will result in the imminent sale of this sqidlite.
We have completed an additional agreement with another global pharma customer to install a sqidlite system for a 60 day evaluation period that follows the development of two custom, multiplexed test kit products. This system is currently scheduled for delivery to this customer in May, 2016.
During fiscal 2015 and 2016 we have also sold a limited number of kits that were run on sqidlite systems at SQI and that were done in conjunction with separate development contracts.
http://finance.yahoo.com/news/sqi-sells-sqidlite-dh-system-1…
SQI Sells Sqidlite™-dh System to a Major Diagnostics Customer
March 29, 2016 2:59 PM
Major Milestone in Commercialization
TORONTO , March 29, 2016 /CNW/ - SQI Diagnostics Inc. ("SQI Diagnostics" "SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), today announced that it has sold its first sqidlite system in its diagnostic business segment. Management believes that this is the first of multiple systems that it will sell to this customer to run an estimated 1 million samples annually to test for infections in human blood in the US market.
"At SQI, we believe that our technology benefits our diagnostic and pharmaceutical customers by generating superior test results in less time and at a substantially reduced cost. Our business model is focused on the long term recurring sale of technologically advanced multiplexed test kits that will allow our customers to achieve these substantial benefits," said Andrew Morris CEO of SQI Diagnostics. "In order to be successful in generating long term recurring revenue streams from the sale of our multiplexed test kits, we need to first sell our sqidlite systems and this first sale in our diagnostics business segment is an important milestone for us."
SQI's relationship with this diagnostic customer began over a year ago with a contract to automate their DNA-based pathogen detection assays. Since then we have been working to automate and scale-up the manufacturing of an 80-plex test kit to detect infections in human blood. This 80-plex test creates large saving for the customer because it eliminates the work needed to develop, run, and purchase materials for 80 separate single tests.
Current technologies for diagnosing infections in human blood typically takes multiple days using microbiology (petri-dish-like tests) or "real-time" DNA based tests that are typically many multiples of the cost of microbiology tests. The value-drivers of this 80-plex product, being developed under contract at SQI, are to provide results in time-frames that are days faster than microbiology tests and that can be deployed at a target cost of delivery that is much less than "real-time" DNA-based tests.
This is our first sqidlite sale in our diagnostic business segment.
In our pharma business segment, the Company has one sqidlite system being commercially operated at a customer to whom we have recently sold and delivered our first substantial commercial order of test kits. We previously announced signing a multi-year, multi-product agreement with this customer following the development and evaluation of multiple custom products designed to run on the sqidlite system. We believe the success with the completed evaluation will result in the imminent sale of this sqidlite.
We have completed an additional agreement with another global pharma customer to install a sqidlite system for a 60 day evaluation period that follows the development of two custom, multiplexed test kit products. This system is currently scheduled for delivery to this customer in May, 2016.
During fiscal 2015 and 2016 we have also sold a limited number of kits that were run on sqidlite systems at SQI and that were done in conjunction with separate development contracts.
http://finance.yahoo.com/news/sqi-sells-sqidlite-dh-system-1…
Antwort auf Beitrag Nr.: 52.090.781 von M@trix am 31.03.16 13:47:08Nachdem ich mich da eingelesen habe, wundere ich mich nur, warum dieses SQI-System nicht schon ein Kassenschlager ist.
Offeriert es doch
-schnellere, bessere Ergebnisse,
-die Möglichkeit, viele Testresultate synchron zu erhalten
-und all das zu bedeutend günstigeren Preisen.
Die Methode sollte sich bei all diesen Vorteilen durchsetzen.
Was ich nicht verstehe: Es ist die Rede von sqidlite und von Kits. Ist mit sqidlite die Plattform gemeint, auf der dann all jene kits angewendet werden oder wie ist das zu verstehen?
Gibt es da nicht eine 'laboratory-lobby', die sich gegen Einführung einer solch erfolgreichen Systems wehrt?
Danke für Eure Beiträge.
J.
Offeriert es doch
-schnellere, bessere Ergebnisse,
-die Möglichkeit, viele Testresultate synchron zu erhalten
-und all das zu bedeutend günstigeren Preisen.
Die Methode sollte sich bei all diesen Vorteilen durchsetzen.
Was ich nicht verstehe: Es ist die Rede von sqidlite und von Kits. Ist mit sqidlite die Plattform gemeint, auf der dann all jene kits angewendet werden oder wie ist das zu verstehen?
Gibt es da nicht eine 'laboratory-lobby', die sich gegen Einführung einer solch erfolgreichen Systems wehrt?
Danke für Eure Beiträge.
J.
Antwort auf Beitrag Nr.: 52.103.453 von BelaDavid am 01.04.16 21:34:10Hallo BelaDavid,
das Problem dürfte sein, dass SQI eine "Klitsche" aus Kanada ist (Börsenwert mal gerade 13 Mio. €), die rote Zahlen schreibt. Als potenzieller Käufer sucht man Stabilität und eine nachhaltig sicher Zusammenarbeit. Zudem denke ich, dass die Entscheider bei den Pharma-Unternehmen oft genau die sein dürften, die ggf. auch die Entlassungen begründen müssen, die ein solches System ermöglichen könnte... Dennoch: perspektivisch kann es sich kaum ein Unternehmen leisten, solche Systeme zu ignorieren. Weniger wegen der Kostenersparnis, sondern wegen der beschleunigten Produktentwicklung.
Ja, sqidlite ist so was hier:

Dafür verkaufen sie dann für die Tests die Ausrüstung (kits). Dazu:
"The next step with our customers is to establish SQI platforms at their laboratories for final evaluation, which once achieved will lead to our ultimate objective of selling them significant volumes of customized kits for their on-going testing needs. We would estimate that each platform would generate between $1 and $2 million of revenue per year from kit sales and that the larger customers could acquire several platforms as we broaden the application of our technology."
Das Geschäftsmodell ist daher sehr spannend, wenn sie den Markt durchdringen können. Denn WENN die Systeme mal installiert sind, klingelt die Kasse und die Kunden sind mehr oder weniger an SQI gebunden.
Eine neue Investorenpräsentation ist online:
http://sqidiagnostics.com/sites/default/files/sqi_investor_p…
Ziele für 2016:

Sollten sie die Ziele so erreichen, müsste SQI im kommenden Jahr bereits ca. 15-20 Mio. $ Umsatz generieren. Dann sollte der Börsenwert aus meiner Sicht mindestens bei 30-40 Mio. $ liegen, was wiederum eine Kursverdoppelung bis -verdreifachung implizieren würde.
Leider hat die Marktdurchdringung bisher viel länger als gedauert als gedacht. Ich bin daher eher zurückhaltend, wenngleich die Chancen definitiv da sind. Ich halte es aber für durchaus denkbar, dass SQI von einem Wettbewerber aufgekauft wird. Derzeit wäre das ja geradezu nur ein "Taschengeld" für die Branchengrößen...
M@trix
das Problem dürfte sein, dass SQI eine "Klitsche" aus Kanada ist (Börsenwert mal gerade 13 Mio. €), die rote Zahlen schreibt. Als potenzieller Käufer sucht man Stabilität und eine nachhaltig sicher Zusammenarbeit. Zudem denke ich, dass die Entscheider bei den Pharma-Unternehmen oft genau die sein dürften, die ggf. auch die Entlassungen begründen müssen, die ein solches System ermöglichen könnte... Dennoch: perspektivisch kann es sich kaum ein Unternehmen leisten, solche Systeme zu ignorieren. Weniger wegen der Kostenersparnis, sondern wegen der beschleunigten Produktentwicklung.
Ja, sqidlite ist so was hier:

Dafür verkaufen sie dann für die Tests die Ausrüstung (kits). Dazu:
"The next step with our customers is to establish SQI platforms at their laboratories for final evaluation, which once achieved will lead to our ultimate objective of selling them significant volumes of customized kits for their on-going testing needs. We would estimate that each platform would generate between $1 and $2 million of revenue per year from kit sales and that the larger customers could acquire several platforms as we broaden the application of our technology."
Das Geschäftsmodell ist daher sehr spannend, wenn sie den Markt durchdringen können. Denn WENN die Systeme mal installiert sind, klingelt die Kasse und die Kunden sind mehr oder weniger an SQI gebunden.
Eine neue Investorenpräsentation ist online:
http://sqidiagnostics.com/sites/default/files/sqi_investor_p…
Ziele für 2016:

Sollten sie die Ziele so erreichen, müsste SQI im kommenden Jahr bereits ca. 15-20 Mio. $ Umsatz generieren. Dann sollte der Börsenwert aus meiner Sicht mindestens bei 30-40 Mio. $ liegen, was wiederum eine Kursverdoppelung bis -verdreifachung implizieren würde.
Leider hat die Marktdurchdringung bisher viel länger als gedauert als gedacht. Ich bin daher eher zurückhaltend, wenngleich die Chancen definitiv da sind. Ich halte es aber für durchaus denkbar, dass SQI von einem Wettbewerber aufgekauft wird. Derzeit wäre das ja geradezu nur ein "Taschengeld" für die Branchengrößen...
M@trix
Ich habe in einer Investoren-Mail den Preis für ein Kit erfahren: $1,600 per kit
Nochmal aus der letzte Pressemitteilung:
Management believes that this is the first of multiple systems that it will sell to this customer to run an estimated 1 million samples annually to test for infections in human blood in the US market.
Da kann man sich also ausmalen, was die kits an Margen bringen können. Wie gesagt: Je mehr Plattformen sie verkaufen können, desto mehr klingelt die Kasse - nachhaltig.
M@trix
Nochmal aus der letzte Pressemitteilung:
Management believes that this is the first of multiple systems that it will sell to this customer to run an estimated 1 million samples annually to test for infections in human blood in the US market.
Da kann man sich also ausmalen, was die kits an Margen bringen können. Wie gesagt: Je mehr Plattformen sie verkaufen können, desto mehr klingelt die Kasse - nachhaltig.
M@trix
Antwort auf Beitrag Nr.: 52.140.519 von M@trix am 07.04.16 16:33:15Danke, M@trix für deine Ausführungen.
Ja, die Planzahlen sind eine Sache, das Tempo der Marktpenetration eine andere.
Bleibt die Frage, was SQI effektiv abzusetzen fähig sein wird.
'Management believes that...' ein erster Verkauf mit angetönten Folgekäufen derselben Firma.
Es sind ja viele Tests am Laufen mit mehreren (potentiellen) Kunden.
Ist es nicht ein schlechtes Zeichen, dass effektive Käufe so schleppend sind oder ist es effektiv rein zeitlich bedingt, dass Endresultate & Evaluation noch ausstehen?
Du scheinst nicht so überzeugt zu sein?
Grüsse,
J.
Ja, die Planzahlen sind eine Sache, das Tempo der Marktpenetration eine andere.
Bleibt die Frage, was SQI effektiv abzusetzen fähig sein wird.
'Management believes that...' ein erster Verkauf mit angetönten Folgekäufen derselben Firma.
Es sind ja viele Tests am Laufen mit mehreren (potentiellen) Kunden.
Ist es nicht ein schlechtes Zeichen, dass effektive Käufe so schleppend sind oder ist es effektiv rein zeitlich bedingt, dass Endresultate & Evaluation noch ausstehen?
Du scheinst nicht so überzeugt zu sein?
Grüsse,
J.
Antwort auf Beitrag Nr.: 52.143.039 von BelaDavid am 07.04.16 21:00:56Von der Technologie an sich bin ich überzeugt, ebenso vom Nutzen. Wäre SQI ein US-Unternehmen, dürfe die Marktdurchdringung wesentlich schneller gehen und wir hätten sicher schon einen Börsenwert von um die 100 Mio. $.
Bisher verlief die Geschäftsentwicklung jedenfalls regelmäßig langsamer als ich es erwartet hatte - was jedoch nicht heißt, dass sie nicht dennoch "Momentum aufnehmen" kann. ich weiß aus eigener Erfahrung wie wichtig der erste große Deal ist. Mit jedem weiteren Deal werden dann Folgedeals einfacher und irgendwann entsteht dann sogar ein Sog. Da sind wir aber noch nicht. Ich rechne mit dem Verkauf von vier Plattformen in 2016, 2017 könnte es dann jedoch um einiges zügiger voran gehen.
Aus meiner Sicht ist die Aktie "günstig". Die wichtigere Frage ist, wie lange sie das noch sein wird.
M@trix
Bisher verlief die Geschäftsentwicklung jedenfalls regelmäßig langsamer als ich es erwartet hatte - was jedoch nicht heißt, dass sie nicht dennoch "Momentum aufnehmen" kann. ich weiß aus eigener Erfahrung wie wichtig der erste große Deal ist. Mit jedem weiteren Deal werden dann Folgedeals einfacher und irgendwann entsteht dann sogar ein Sog. Da sind wir aber noch nicht. Ich rechne mit dem Verkauf von vier Plattformen in 2016, 2017 könnte es dann jedoch um einiges zügiger voran gehen.
Aus meiner Sicht ist die Aktie "günstig". Die wichtigere Frage ist, wie lange sie das noch sein wird.
M@trix
Antwort auf Beitrag Nr.: 52.145.031 von M@trix am 08.04.16 08:41:30Aleae iactae sunt. Grosses Volumen gestern, nicht...?
Ein Teil ging auf mein Konto resp. in mein Depot; hab mich zu meinem Geburi damit beschenkt.
's ist immer hart, antizyklisch zu kaufen. So gut wie niemand ausser dem Management scheint an das Produkt zu glauben.
Dieses Produkt zur Blutanalyse kann doch mittel-/ langfristig in der ganzen Welt vertrieben werden, nicht nur in den USA? Ein Markt dafür ist doch überall vorhanden...
Was meinst du zu dieser Stossrichtung?
Grüsse,
J.
Ein Teil ging auf mein Konto resp. in mein Depot; hab mich zu meinem Geburi damit beschenkt.
's ist immer hart, antizyklisch zu kaufen. So gut wie niemand ausser dem Management scheint an das Produkt zu glauben.
Dieses Produkt zur Blutanalyse kann doch mittel-/ langfristig in der ganzen Welt vertrieben werden, nicht nur in den USA? Ein Markt dafür ist doch überall vorhanden...
Was meinst du zu dieser Stossrichtung?
Grüsse,
J.
Allergan to Highlight SQI Diagnostics' Technology at 10th WRIB Conference
TORONTO, April 18, 2016 /PRNewswire/ - SQI Diagnostics Inc. . ("SQI Diagnostics" "SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics today announced that its immunogenicity testing technology is being presented by Allergan at the 10th Workshop on Recent Issues in Bioanalysis in Orlando, Florida ("10th WRIB"), on April 19, 2016.
The case study presentation will be given by Dr. Swati Gupta, PhD, Director of Immunology for Allergan. The title of the talk is "SQI Ig_plex Dual-Layer Multiplexing Capabilities in Immunogenicity Assays" and will provide novel case studies from products that were developed on SQI's multiplexing platform used in immunogenicity testing. The presentation will provide comparison of SQI tests to traditional bridging assays using a competitor's "electrochemiluminescent" technology. The presentation will provide data emphasizing SQI's improved drug tolerance, sensitivity, the ability to run all targets in one test and how to use the technology to profile for safety and efficacy of therapeutics.
"SQI is excited to see its immunogenicity products showcased again this year at the WRIB conference. The repeated validation and market exposure gained from the presentation and case study by Allergan is important," said Andrew Morris, CEO of SQI Diagnostics. "We believe that our technology provides industry-leading performance and benefits for the testing of multiple anti-drug antibody targets in a single test; significantly improved work-flow and reduced labor costs; and, it enables drug developers to align their processes with the emerging regulatory guidelines surrounding immunogenicity testing worldwide."
Immunogenicity testing is just one of a series of assay types that are being run on SQI's automated multiplexing technology.
SQI representatives will be at the presentation to answers questions and will be available throughout the conference and we encourage you to come to booth 31 for more information about our custom assay development services, products and fully automated systems.
TORONTO, April 18, 2016 /PRNewswire/ - SQI Diagnostics Inc. . ("SQI Diagnostics" "SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics today announced that its immunogenicity testing technology is being presented by Allergan at the 10th Workshop on Recent Issues in Bioanalysis in Orlando, Florida ("10th WRIB"), on April 19, 2016.
The case study presentation will be given by Dr. Swati Gupta, PhD, Director of Immunology for Allergan. The title of the talk is "SQI Ig_plex Dual-Layer Multiplexing Capabilities in Immunogenicity Assays" and will provide novel case studies from products that were developed on SQI's multiplexing platform used in immunogenicity testing. The presentation will provide comparison of SQI tests to traditional bridging assays using a competitor's "electrochemiluminescent" technology. The presentation will provide data emphasizing SQI's improved drug tolerance, sensitivity, the ability to run all targets in one test and how to use the technology to profile for safety and efficacy of therapeutics.
"SQI is excited to see its immunogenicity products showcased again this year at the WRIB conference. The repeated validation and market exposure gained from the presentation and case study by Allergan is important," said Andrew Morris, CEO of SQI Diagnostics. "We believe that our technology provides industry-leading performance and benefits for the testing of multiple anti-drug antibody targets in a single test; significantly improved work-flow and reduced labor costs; and, it enables drug developers to align their processes with the emerging regulatory guidelines surrounding immunogenicity testing worldwide."
Immunogenicity testing is just one of a series of assay types that are being run on SQI's automated multiplexing technology.
SQI representatives will be at the presentation to answers questions and will be available throughout the conference and we encourage you to come to booth 31 for more information about our custom assay development services, products and fully automated systems.
SQI Diagnostics Reports Second Quarter 2016 Results
TORONTO , May 13, 2016 /CNW/ - SQI Diagnostics Inc. ("SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), today reported its financial and operational results for the three and six months ended March 31, 2016 .
SQI is a Toronto -based life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced multiplexed diagnostics.
"The first six months have been pivotal in SQI's commercial development," said Andrew Morris , SQI's President and CEO. "We completed our first commercial sale of test kits to one of our global Pharma customers and received our first order for the purchase of a sqidlite system and we have surpassed an annualized revenue run-rate of $1M ."
SQI's proprietary technology is used to create custom tests for clients in the drug development market that can now deliver as many as 30 unique results – with the potential for more – from a single SQI test. In doing this, it removes the work needed to develop, run, and purchase materials for 30 separate single tests. Our customers benefit from a significantly reduced cost per result with superior quality data.
Financial and Business Highlights for the Quarter
In the first quarter of F16 we entered into a three year, multiproduct agreement with an existing Global Pharma customer. In the second quarter of F16 we delivered our first substantial commercial order of test kits to this customer, which was run on the sqidlite system in their lab. We believe the success from the completed evaluations and use of commercial products this quarter will result in the imminent sale of this sqidlite and recurring kit sales for three products that are ready now, plus several more that are in the planning phase.
During the quarter we continued the development work with our DNA customer to complete the automation and scale-up of manufacturing for their proprietary infectious disease test panels. The customer's first test has grown to an approximately 80-plex DNA-based test. As we recently announced, this customer has ordered its first sqidlite–DH platform and we are working with them to meet the project timelines with the first platform delivery to be in June of 2016. Validation and evaluation of the platform is expected to take several months following delivery.
We continue to convert our early-stage customer relationships into ongoing development and product sales revenues. Overall revenues were $477,000 for the six months ended March 31, 2016 (three months - $280,000 ) as compared to $85,000 for the six months ended March 31, 2015 (three months - $70,000 ).
We have also completed an additional agreement with another Global Pharma customer to install a sqidlite system for a 60 day evaluation period that follows the development of two custom, multiplexed test kit products. This system is scheduled for delivery to this customer in June, 2016 and will coincide with a new product launch.
In our Animal Health sector we have negotiated the development of a second test with a global veterinary products company that conducted a site visit and audit of our development and manufacturing centre in May, 2016.
We are also expecting to launch and deliver xPlex kits to our two largest Global Pharma customers in the third quarter of fiscal 2016. The xPlex product includes kits and software that will allow our pharmaceutical customers to develop multiplexed tests for themselves using their in-development drugs and our kits to automate these tests on our platforms installed at their facilities, thus eliminating the need to share their proprietary drugs.
On April 18, 2016 , we announced that Dr. Swati Gupta , PhD, Director of Immunology for Allergan Inc., will present a case study at the 10th Annual Workshop on Recent Issues in Bioanalysis in Orlando , Florida. The title of the talk was "SQI Ig_plex Dual-Layer Multiplexing Capabilities in Immunogenicity Assays" and it provided novel case studies from products that were developed on SQI's multiplexing platform used in immunogenicity testing. The presentation provided comparison of SQI tests to traditional bridging assays using a competitor's technology. The presentation provided data emphasizing SQI's improved drug tolerance, sensitivity, the ability to run all targets in one test and how to use the technology to profile for safety and efficacy of therapeutics. This added validation speaks to SQI's position and winning value proposition.
Q2 2016 Financial Results Overview
Revenue for the three months ended March 31, 2016 was $280,000 compared to $70,000 for the same period last year. Revenue for the six months ended March 31, 2016 was $477,000 compared to $85,000 for the same period last year. The Company continued to earn revenue from several development projects in its drug development business and its diagnostic sector. Significantly, revenue in the current quarter included our first sale of commercial kits to one of our global pharma customers.
The net loss for the quarter ended March 31, 2016 was $987,000 ( $0.01 net loss per share) as compared to the net loss of $1,613,000 ( $0.02 net loss per share) for the quarter-ended March 31, 2015 . The loss for the six months ended March 31, 2016 was $2,345,000 as compared to $2,978,000 for the same period last year. The decrease in the loss is attributable to the increases in revenues and decreased in R&D costs over the relevant periods as a result of SR&ED credits booked in the current quarter.
R&D expenditures, excluding amortization, SR&ED investment tax credits recoverable and stock based compensation, for the three months ended March 31, 2016 were $812,000 (six months –$1,525,000) compared to $788,000 for the three months ended March 31, 2015 (six months –$1,472,000). R&D costs remained consistent for the three and six months ended March 31, 2016 compared to the same periods in 2015. During the current quarter the Company engaged consultants to review and file SR&ED claims for fiscal 2014 and 2015 this has resulted in an investment tax credit recoverable of $360,000 being recorded in the current quarter.
Corporate and general expenses, excluding stock-based compensation, totaled $392,000 for the three months ended March 31, 2016 (six months – $787,000 ) compared to $459,000 for the three months ended March 31, 2015 (six months – $818,000 ). The decrease in corporate and general expenses over the three and six month periods is attributable to staffing changes and the favorable change in the US dollar exchange rate.
Sales and marketing expenses, excluding stock based compensation, for the three months ended March 31, 2016 totaled $174,000 (six months – $327,000 ) compared to $198,000 for the three months ended March 31, 2015 (six months – $358,000 ). Sales and marketing expenses were lower over the three and six month periods due to staffing changes. The Company lost one sales contractor and is currently in the process of filling that position. During the current quarter the Company hired a customer solutions manager.
At March 31, 2016 , current assets were $3,412,000 compared to $2,555,000 at September 30, 2015 . As at March 31, 2016 the Company has a $2,676,000 working capital surplus compared to a surplus of $1,787,000 at September 30, 2015
http://finance.yahoo.com/news/sqi-diagnostics-reports-second…
Zahlen sind gut, aber nicht überragend. Es geht voran, aber eben langsam. Schön ist, dass die Kosten sinken. Wenn sich das Geschäft so entwickelt wie SQI es erwartet, könnte im kommenden Jahr der break-even erreicht werden. Indes sehe ich derzeit kaum Kurspotenzial, solange nicht mehr Speed in den Vertrieb kommt.
M@trix
TORONTO , May 13, 2016 /CNW/ - SQI Diagnostics Inc. ("SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), today reported its financial and operational results for the three and six months ended March 31, 2016 .
SQI is a Toronto -based life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced multiplexed diagnostics.
"The first six months have been pivotal in SQI's commercial development," said Andrew Morris , SQI's President and CEO. "We completed our first commercial sale of test kits to one of our global Pharma customers and received our first order for the purchase of a sqidlite system and we have surpassed an annualized revenue run-rate of $1M ."
SQI's proprietary technology is used to create custom tests for clients in the drug development market that can now deliver as many as 30 unique results – with the potential for more – from a single SQI test. In doing this, it removes the work needed to develop, run, and purchase materials for 30 separate single tests. Our customers benefit from a significantly reduced cost per result with superior quality data.
Financial and Business Highlights for the Quarter
In the first quarter of F16 we entered into a three year, multiproduct agreement with an existing Global Pharma customer. In the second quarter of F16 we delivered our first substantial commercial order of test kits to this customer, which was run on the sqidlite system in their lab. We believe the success from the completed evaluations and use of commercial products this quarter will result in the imminent sale of this sqidlite and recurring kit sales for three products that are ready now, plus several more that are in the planning phase.
During the quarter we continued the development work with our DNA customer to complete the automation and scale-up of manufacturing for their proprietary infectious disease test panels. The customer's first test has grown to an approximately 80-plex DNA-based test. As we recently announced, this customer has ordered its first sqidlite–DH platform and we are working with them to meet the project timelines with the first platform delivery to be in June of 2016. Validation and evaluation of the platform is expected to take several months following delivery.
We continue to convert our early-stage customer relationships into ongoing development and product sales revenues. Overall revenues were $477,000 for the six months ended March 31, 2016 (three months - $280,000 ) as compared to $85,000 for the six months ended March 31, 2015 (three months - $70,000 ).
We have also completed an additional agreement with another Global Pharma customer to install a sqidlite system for a 60 day evaluation period that follows the development of two custom, multiplexed test kit products. This system is scheduled for delivery to this customer in June, 2016 and will coincide with a new product launch.
In our Animal Health sector we have negotiated the development of a second test with a global veterinary products company that conducted a site visit and audit of our development and manufacturing centre in May, 2016.
We are also expecting to launch and deliver xPlex kits to our two largest Global Pharma customers in the third quarter of fiscal 2016. The xPlex product includes kits and software that will allow our pharmaceutical customers to develop multiplexed tests for themselves using their in-development drugs and our kits to automate these tests on our platforms installed at their facilities, thus eliminating the need to share their proprietary drugs.
On April 18, 2016 , we announced that Dr. Swati Gupta , PhD, Director of Immunology for Allergan Inc., will present a case study at the 10th Annual Workshop on Recent Issues in Bioanalysis in Orlando , Florida. The title of the talk was "SQI Ig_plex Dual-Layer Multiplexing Capabilities in Immunogenicity Assays" and it provided novel case studies from products that were developed on SQI's multiplexing platform used in immunogenicity testing. The presentation provided comparison of SQI tests to traditional bridging assays using a competitor's technology. The presentation provided data emphasizing SQI's improved drug tolerance, sensitivity, the ability to run all targets in one test and how to use the technology to profile for safety and efficacy of therapeutics. This added validation speaks to SQI's position and winning value proposition.
Q2 2016 Financial Results Overview
Revenue for the three months ended March 31, 2016 was $280,000 compared to $70,000 for the same period last year. Revenue for the six months ended March 31, 2016 was $477,000 compared to $85,000 for the same period last year. The Company continued to earn revenue from several development projects in its drug development business and its diagnostic sector. Significantly, revenue in the current quarter included our first sale of commercial kits to one of our global pharma customers.
The net loss for the quarter ended March 31, 2016 was $987,000 ( $0.01 net loss per share) as compared to the net loss of $1,613,000 ( $0.02 net loss per share) for the quarter-ended March 31, 2015 . The loss for the six months ended March 31, 2016 was $2,345,000 as compared to $2,978,000 for the same period last year. The decrease in the loss is attributable to the increases in revenues and decreased in R&D costs over the relevant periods as a result of SR&ED credits booked in the current quarter.
R&D expenditures, excluding amortization, SR&ED investment tax credits recoverable and stock based compensation, for the three months ended March 31, 2016 were $812,000 (six months –$1,525,000) compared to $788,000 for the three months ended March 31, 2015 (six months –$1,472,000). R&D costs remained consistent for the three and six months ended March 31, 2016 compared to the same periods in 2015. During the current quarter the Company engaged consultants to review and file SR&ED claims for fiscal 2014 and 2015 this has resulted in an investment tax credit recoverable of $360,000 being recorded in the current quarter.
Corporate and general expenses, excluding stock-based compensation, totaled $392,000 for the three months ended March 31, 2016 (six months – $787,000 ) compared to $459,000 for the three months ended March 31, 2015 (six months – $818,000 ). The decrease in corporate and general expenses over the three and six month periods is attributable to staffing changes and the favorable change in the US dollar exchange rate.
Sales and marketing expenses, excluding stock based compensation, for the three months ended March 31, 2016 totaled $174,000 (six months – $327,000 ) compared to $198,000 for the three months ended March 31, 2015 (six months – $358,000 ). Sales and marketing expenses were lower over the three and six month periods due to staffing changes. The Company lost one sales contractor and is currently in the process of filling that position. During the current quarter the Company hired a customer solutions manager.
At March 31, 2016 , current assets were $3,412,000 compared to $2,555,000 at September 30, 2015 . As at March 31, 2016 the Company has a $2,676,000 working capital surplus compared to a surplus of $1,787,000 at September 30, 2015
http://finance.yahoo.com/news/sqi-diagnostics-reports-second…
Zahlen sind gut, aber nicht überragend. Es geht voran, aber eben langsam. Schön ist, dass die Kosten sinken. Wenn sich das Geschäft so entwickelt wie SQI es erwartet, könnte im kommenden Jahr der break-even erreicht werden. Indes sehe ich derzeit kaum Kurspotenzial, solange nicht mehr Speed in den Vertrieb kommt.
M@trix
SQI hat ein weiteres sqidlite™ system verkauft:
http://www.stockhouse.com/news/press-releases/2016/08/30/sqi…
"The sale of this platform is the culmination of a long evaluation process that sets the stage for the ongoing sale of test kits to this global pharmaceutical customer."
Leider geht es immer noch recht langsam voran, was sich auch in der Aktie widerspiegelt. Dennoch denke ich, dass man SQI nun auf dem Schirm haben sollte. Der Kurs hat m.E. bei 0,24 CAD einen Boden gebildet, d.h. ich sehe kaum noch Risiko nach unten, wohl aber Potenzial nach oben.
Gemäß der letzten MD&A werden sie im laufenden Quartal noch ein weiteres System verkaufen und bis Jahresende sollen es dann insgesamt sechs sein. Wenn dies gelingt, wird SQI im kommenden Jahr 4-6 Mio. CAD Umsatz generieren und der break-even näher rücken. Ab 2018 dürfte sich das Geschäft dann deutlich beschleunigen - dann könnten wir bei 12-15 Mio. CAD Umsatz und einem positiven Ergebnis von 5-7 Mio. CAD rauslaufen. Bei einem KGV von 25 könnte der Börsenwert also dann bei einem Vielfachen des heutigen (20 Mio. CAD) liegen - sofern es nicht zu weiterer signifikanter Verwässerung kommt.
Was mir bei SQI weiterhin gut gefällt ist, dass die Insider weiter an das Unternehmen glauben und jedes Mal weiteres Kapital reinstecken. Zudem ist das Geschäftsmodell sehr profitabel, wenn es sich mal durchgesetzt hat.
Ich bin bei SQI nicht investiert, habe die Aktie jedoch auf der Watchlist und halte sie für längerfristig vielversprechend. Sollte SQI weitere Deals abschließen, werde ich wieder investieren.
M@trix
http://www.stockhouse.com/news/press-releases/2016/08/30/sqi…
"The sale of this platform is the culmination of a long evaluation process that sets the stage for the ongoing sale of test kits to this global pharmaceutical customer."
Leider geht es immer noch recht langsam voran, was sich auch in der Aktie widerspiegelt. Dennoch denke ich, dass man SQI nun auf dem Schirm haben sollte. Der Kurs hat m.E. bei 0,24 CAD einen Boden gebildet, d.h. ich sehe kaum noch Risiko nach unten, wohl aber Potenzial nach oben.
Gemäß der letzten MD&A werden sie im laufenden Quartal noch ein weiteres System verkaufen und bis Jahresende sollen es dann insgesamt sechs sein. Wenn dies gelingt, wird SQI im kommenden Jahr 4-6 Mio. CAD Umsatz generieren und der break-even näher rücken. Ab 2018 dürfte sich das Geschäft dann deutlich beschleunigen - dann könnten wir bei 12-15 Mio. CAD Umsatz und einem positiven Ergebnis von 5-7 Mio. CAD rauslaufen. Bei einem KGV von 25 könnte der Börsenwert also dann bei einem Vielfachen des heutigen (20 Mio. CAD) liegen - sofern es nicht zu weiterer signifikanter Verwässerung kommt.
Was mir bei SQI weiterhin gut gefällt ist, dass die Insider weiter an das Unternehmen glauben und jedes Mal weiteres Kapital reinstecken. Zudem ist das Geschäftsmodell sehr profitabel, wenn es sich mal durchgesetzt hat.
Ich bin bei SQI nicht investiert, habe die Aktie jedoch auf der Watchlist und halte sie für längerfristig vielversprechend. Sollte SQI weitere Deals abschließen, werde ich wieder investieren.
M@trix
hi,
plus 18%............
anything new???
M.
plus 18%............
anything new???
M.
Antwort auf Beitrag Nr.: 54.017.837 von manffreddoo am 05.01.17 14:55:53Nein, noch nicht. Aber die jüngste MD&A liest sich sehr gut. Ich habe hier vor, noch im ersten Quartal wieder eine kleine Startposition zu erwerben. Der aktuelle Börsenwert ist m.E. zu niedrig im Hinblick auf die Aussichten.
M@trix
M@trix
....stand bisher an der Seitenlinie.....
.....denke über Einstieg nach.......
M.
.....denke über Einstieg nach.......
M.
Die Insider stehen nach wie vor vollkommen hinter SQI. Was es braucht sind indes Sales. Die jüngste MD&A war dahingehend eher enttäuschend für mich, sodass ich mein für Q1 geplantes Investment erst mal weiter zurückgestellt habe. SQI muss einfach mal auf der business-Seite liefern. Technologisch halte ich das Unternehmen nach wie vor für spannend und im Hinblick auf das Potenzial für sehr niedrig bewertet.
M@trix
SQI Diagnostics Inc. Announces Private Placement with Insider Participation
TORONTO , March 6, 2017 /CNW/ - SQI Diagnostics Inc. ("SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced that it intends to complete a non-brokered private placement (the "Private Placement") of up to 21,875,000 units ("Units") of the Company at a price of $0.16 per Unit for gross proceeds of up to $3.5 million , subject to regulatory and stock exchange approval. Each Unit will consist of one common share and one common share purchase warrant. Each common share purchase warrant will entitle the holder to purchase one common share at a price of $0.21 for a period of five years from the date of issuance, subject to accelerated expiry in certain circumstances.
It is anticipated that insiders of the Company will subscribe for up to 18,750,000 Units for gross proceeds of $3,000,000 under the Private Placement. The issuances of Units to insiders pursuant to the Private Placement will be considered related party transactions within the meaning of TSX Venture Exchange Policy 5.9 and Multilateral Instrument 61-101 Protection of Minority Security Holders in Special Transactions ("MI 61-101"). SQI intends to rely on exemptions from the formal valuation and minority approval requirements in sections 5.5(g) and 5.7(1)(e) of MI 61-101 in respect of such insider participation on the basis of financial hardship. Further details will be provided in the Company's material change report to be filed on SEDAR.
In connection with the Private Placement of Units to non-insiders, the Company may pay a finder's fee in cash, subject to regulatory and stock exchange approval.
The Company expects to close the Private Placement on or about March 10, 2017 .
The Private Placement is subject to all necessary regulatory and stock exchange approvals. The securities being issued pursuant to the Private Placement will be subject to a hold period expiring four months and one day from the date of issuance in accordance with applicable Canadian securities law.
SQI intends to use the net proceeds of the Private Placement to fund the Company's product commercialization and manufacturing programs, sales and marketing and for general working capital purposes.
The Company expects to file a material change report in respect of the related party transaction less than 21 days prior to the closing of the Private Placement, which the Company deems reasonable in the circumstances so as to be able to avail itself of the proceeds of the Private Placement in an expeditious manner.
The securities described herein have not been, and will not be, registered under the United States Securities Act of 1933, as amended (the "U.S. Securities Act") or any state securities laws and accordingly may not be offered or sold within the United States or to "U.S. persons", as such term is defined in Regulation S promulgated under the U.S. Securities Act ("U.S. Persons"), except in compliance with the registration requirements of the U.S. Securities Act and applicable state securities requirements or pursuant to exemptions therefrom. This news release does not constitute an offer to sell or a solicitation of an offer to buy any of the Company's securities to, or for the account of benefit of, persons in the United States or U.S. Persons.
http://finance.yahoo.com/news/sqi-diagnostics-inc-announces-…
M@trix
SQI Diagnostics Inc. Announces Private Placement with Insider Participation
TORONTO , March 6, 2017 /CNW/ - SQI Diagnostics Inc. ("SQI" or the "Company") (TSX-V: SQD; OTCQX: SQIDF), a life sciences and diagnostics company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics, today announced that it intends to complete a non-brokered private placement (the "Private Placement") of up to 21,875,000 units ("Units") of the Company at a price of $0.16 per Unit for gross proceeds of up to $3.5 million , subject to regulatory and stock exchange approval. Each Unit will consist of one common share and one common share purchase warrant. Each common share purchase warrant will entitle the holder to purchase one common share at a price of $0.21 for a period of five years from the date of issuance, subject to accelerated expiry in certain circumstances.
It is anticipated that insiders of the Company will subscribe for up to 18,750,000 Units for gross proceeds of $3,000,000 under the Private Placement. The issuances of Units to insiders pursuant to the Private Placement will be considered related party transactions within the meaning of TSX Venture Exchange Policy 5.9 and Multilateral Instrument 61-101 Protection of Minority Security Holders in Special Transactions ("MI 61-101"). SQI intends to rely on exemptions from the formal valuation and minority approval requirements in sections 5.5(g) and 5.7(1)(e) of MI 61-101 in respect of such insider participation on the basis of financial hardship. Further details will be provided in the Company's material change report to be filed on SEDAR.
In connection with the Private Placement of Units to non-insiders, the Company may pay a finder's fee in cash, subject to regulatory and stock exchange approval.
The Company expects to close the Private Placement on or about March 10, 2017 .
The Private Placement is subject to all necessary regulatory and stock exchange approvals. The securities being issued pursuant to the Private Placement will be subject to a hold period expiring four months and one day from the date of issuance in accordance with applicable Canadian securities law.
SQI intends to use the net proceeds of the Private Placement to fund the Company's product commercialization and manufacturing programs, sales and marketing and for general working capital purposes.
The Company expects to file a material change report in respect of the related party transaction less than 21 days prior to the closing of the Private Placement, which the Company deems reasonable in the circumstances so as to be able to avail itself of the proceeds of the Private Placement in an expeditious manner.
The securities described herein have not been, and will not be, registered under the United States Securities Act of 1933, as amended (the "U.S. Securities Act") or any state securities laws and accordingly may not be offered or sold within the United States or to "U.S. persons", as such term is defined in Regulation S promulgated under the U.S. Securities Act ("U.S. Persons"), except in compliance with the registration requirements of the U.S. Securities Act and applicable state securities requirements or pursuant to exemptions therefrom. This news release does not constitute an offer to sell or a solicitation of an offer to buy any of the Company's securities to, or for the account of benefit of, persons in the United States or U.S. Persons.
http://finance.yahoo.com/news/sqi-diagnostics-inc-announces-…
Ein Going Private würde für die Firma mehr Sinn machen.
Die neue Investorenpräsentation ist online:
http://sqidiagnostics.com/sites/default/files/sqi_investor_p…
Sie haben die Planzahlen endlich auf ein realistisches Niveau angepasst:
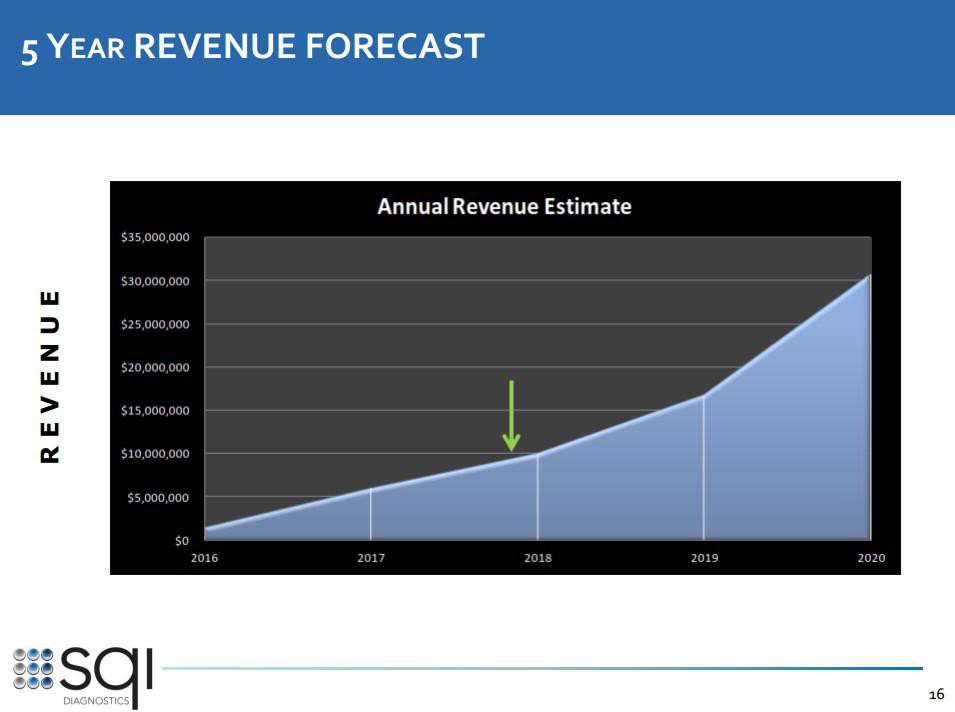
Eile ist hier nach wie vor nicht geboten, aber spätestens wenn einer der zwei "pending contracts" abgeschlossen wird, sollte man hier m.E. einen Fuß in die Tür stellen.
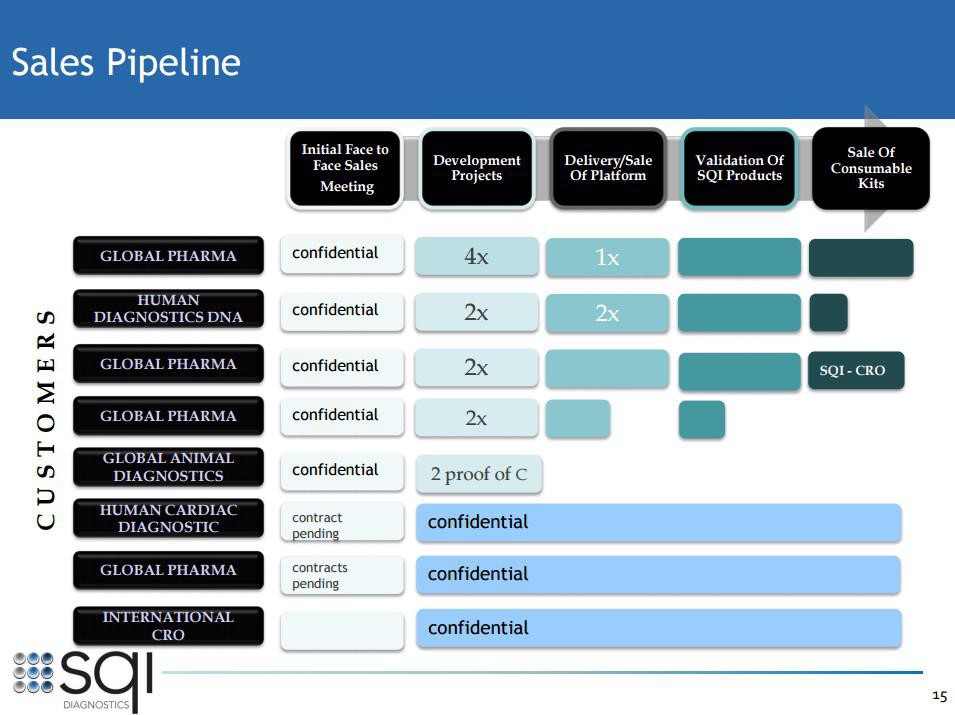
Das Geschäftsmodell wird mit zunehmendem Volumen immer lukrativer. Und mit einem Börsenwert von 14 Mio. CAD ist hier viel Luft nach oben, sobald der break-even erreicht ist.
M@trix
http://sqidiagnostics.com/sites/default/files/sqi_investor_p…
Sie haben die Planzahlen endlich auf ein realistisches Niveau angepasst:

Eile ist hier nach wie vor nicht geboten, aber spätestens wenn einer der zwei "pending contracts" abgeschlossen wird, sollte man hier m.E. einen Fuß in die Tür stellen.

Das Geschäftsmodell wird mit zunehmendem Volumen immer lukrativer. Und mit einem Börsenwert von 14 Mio. CAD ist hier viel Luft nach oben, sobald der break-even erreicht ist.
M@trix
Antwort auf Beitrag Nr.: 54.512.967 von M@trix am 10.03.17 17:42:41Aber der Chart entspricht einem Chart des Grauens... Das sieht für mich gar nichts gut aus....
Kurs berappelt sich......kaufe heute Anfangsposition...
M.
M.
Antwort auf Beitrag Nr.: 54.512.967 von M@trix am 10.03.17 17:42:41
SQI hat 104 Mio. Aktien ausstehend, Börsenwert also 17,7 Mio. CAD. bzw. rund 13 Mio. USD.
Auch bei der jüngsten Finanzierung haben die Insider zugegriffen - sie stehen also weiterhin voll hinter dem Unternehmen. Außerdem hält hier ein sehr bekannter Unternehmen nun eine große Position:
https://en.wikipedia.org/wiki/Clive_Beddoe
Ich denke, wir sind nun nahe dem Wendepunkt. Bei einer derart niedrigen Bewertung in Verbindung mit den geschlossenen Deals und der Pipeline sehe ich hier deutlich Aufwertungspotenzial in den kommenden Monaten bei so gut wie keinem Abwärtspotenzial. Das ist genau der richtige Moment um einzusteigen. Zudem sollten wir hierzu in den kommenden Wochen etwas hören:
"We advanced several customers along our sales pipeline. These include one who has advanced from the planning stage in Q2 of 2016 to an evaluation being completed in Q2 2017, as well as a new global pharma company who we are now negotiating contracts and work plans for a new custom project to be launched in 2017."
Ich habe an den CEO einige Fragen insbesondere zur Beschleunigung des Vertriebs gerichtet und werde hier ggf. in naher Zukunft wieder investieren.
M@trix
Zitat von M@trix: Die neue Investorenpräsentation ist online:
http://sqidiagnostics.com/sites/default/files/sqi_investor_p…
Sie haben die Planzahlen endlich auf ein realistisches Niveau angepasst:
Eile ist hier nach wie vor nicht geboten, aber spätestens wenn einer der zwei "pending contracts" abgeschlossen wird, sollte man hier m.E. einen Fuß in die Tür stellen.
Das Geschäftsmodell wird mit zunehmendem Volumen immer lukrativer. Und mit einem Börsenwert von 14 Mio. CAD ist hier viel Luft nach oben, sobald der break-even erreicht ist.
M@trix
SQI hat 104 Mio. Aktien ausstehend, Börsenwert also 17,7 Mio. CAD. bzw. rund 13 Mio. USD.
Auch bei der jüngsten Finanzierung haben die Insider zugegriffen - sie stehen also weiterhin voll hinter dem Unternehmen. Außerdem hält hier ein sehr bekannter Unternehmen nun eine große Position:
https://en.wikipedia.org/wiki/Clive_Beddoe
Ich denke, wir sind nun nahe dem Wendepunkt. Bei einer derart niedrigen Bewertung in Verbindung mit den geschlossenen Deals und der Pipeline sehe ich hier deutlich Aufwertungspotenzial in den kommenden Monaten bei so gut wie keinem Abwärtspotenzial. Das ist genau der richtige Moment um einzusteigen. Zudem sollten wir hierzu in den kommenden Wochen etwas hören:
"We advanced several customers along our sales pipeline. These include one who has advanced from the planning stage in Q2 of 2016 to an evaluation being completed in Q2 2017, as well as a new global pharma company who we are now negotiating contracts and work plans for a new custom project to be launched in 2017."
Ich habe an den CEO einige Fragen insbesondere zur Beschleunigung des Vertriebs gerichtet und werde hier ggf. in naher Zukunft wieder investieren.
M@trix
Ergänzung:
Zu den jüngsten News (https://finance.yahoo.com/news/sqi-diagnostics-signs-agreeme…) auch lesen:
http://www.telegraph.co.uk/science/2016/06/19/blood-test-sho…
Nochmal aus der PM:
"In addition to running the test in their own laboratory, SQI's customer also plans to sell both the test kit and SQI automation systems to its ever-expanding global customer base. These customers are renowned cardiologists, hospitals, and reference laboratories around the world."
Das ist also jetzt ein echter Hebel für SQI, was Sales betrifft.
M@trix
Zu den jüngsten News (https://finance.yahoo.com/news/sqi-diagnostics-signs-agreeme…) auch lesen:
http://www.telegraph.co.uk/science/2016/06/19/blood-test-sho…
Nochmal aus der PM:
"In addition to running the test in their own laboratory, SQI's customer also plans to sell both the test kit and SQI automation systems to its ever-expanding global customer base. These customers are renowned cardiologists, hospitals, and reference laboratories around the world."
Das ist also jetzt ein echter Hebel für SQI, was Sales betrifft.
M@trix
Antwort auf Beitrag Nr.: 54.798.385 von M@trix am 25.04.17 12:17:50 WELL DONE M@TRIX..........
Chart sieht auch gut aus.......schöne Bodenbildung........... und baldiger upmove??
M.
Chart sieht auch gut aus.......schöne Bodenbildung........... und baldiger upmove??
M.
Antwort auf Beitrag Nr.: 54.856.135 von manffreddoo am 03.05.17 16:36:47Ich erwarte spätestens im Juni Top News. Der Kurs hat gedreht und ich denke, bis Jahresende sehen wir Kurse um 0,40-0,50 CAD.
M@trix
M@trix
Nach dem ersten Upmove von 0,17 CAD auf 0,22 CAD seit meinem letzten Posting gab es nach den Zahlen nun Gewinnmitnahmen. Offenbar hatten einige hier noch mehr erwartet:
SQI Diagnostics Reports Second Quarter 2017 Results
https://finance.yahoo.com/news/sqi-diagnostics-reports-secon…
Es gibt auch eine neue Investorenpräsentation:
http://sqidiagnostics.com/sites/default/files/sqi_investor_p…
Die wohl wichtigste Folie daraus:
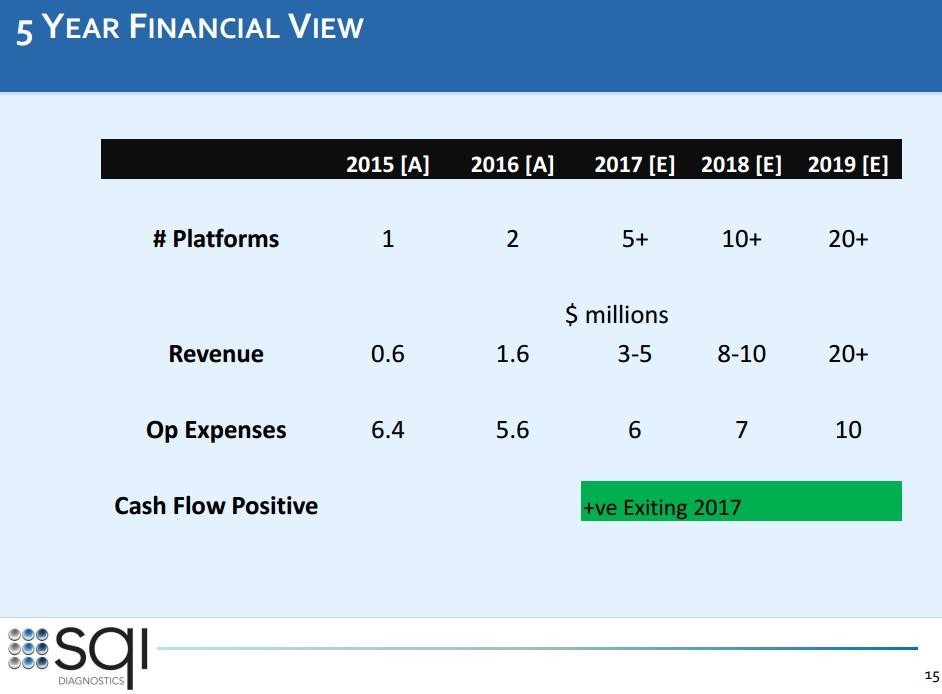
Man sieht hier schön, dass sie die Erwartungen endlich auf ein realistisches Niveau gesenkt haben. Zentral ist indes der Ausblick "cash flow positive" ab dem vierten Quartal. Um das zu erreichen müssen sie noch einige Deals abschließen.
Noch mal aus dem Q2-Report:
• We advanced the integration of our human diagnostics DNA customer at the large US reference lab where a large comparative study is to be launched in mid-May. For over a year, we have been working on product development, assessment, automation and integration of the sqidlite™ platform to address this customer's US reference lab testing needs. We are now at the point where our customer is launching a large product testing trial that we believe to be a final step prior to commercial launch. We believe that this will be completed in fiscal 2017 and that successful completion of this study will lead to commercial production of this product and recurring sales of kits in fiscal 2017.
• We completed the development program for a new pharma customer reported last quarter. We launched a new customer's product development program last quarter and completed it during the current quarter. This program is based on SQI's "off the shelf" test kit for testing 8 cytokines which are common markers tested during drug development. During the quarter, we established the performance of the SQI-developed test against a set of unknown challenge test samples. We are currently anticipating the customer's approval to begin production of the product for use in their commercial operations.
Es gibt also bei zwei großen Projekten eine höhere Wahrscheinlichkeit für einen Abschluss.
Insofern bleibe ich dabei: die Aktie hat m.E. nun gedreht und sollte - so die skizzierten Pläne umgesetzt werden - für deutliche Gewinne im weiteren Jahresverlauf und darüber hinaus gut sein.
Kurs: 0,185 CAD
M@trix
SQI Diagnostics Reports Second Quarter 2017 Results
https://finance.yahoo.com/news/sqi-diagnostics-reports-secon…
Es gibt auch eine neue Investorenpräsentation:
http://sqidiagnostics.com/sites/default/files/sqi_investor_p…
Die wohl wichtigste Folie daraus:

Man sieht hier schön, dass sie die Erwartungen endlich auf ein realistisches Niveau gesenkt haben. Zentral ist indes der Ausblick "cash flow positive" ab dem vierten Quartal. Um das zu erreichen müssen sie noch einige Deals abschließen.
Noch mal aus dem Q2-Report:
• We advanced the integration of our human diagnostics DNA customer at the large US reference lab where a large comparative study is to be launched in mid-May. For over a year, we have been working on product development, assessment, automation and integration of the sqidlite™ platform to address this customer's US reference lab testing needs. We are now at the point where our customer is launching a large product testing trial that we believe to be a final step prior to commercial launch. We believe that this will be completed in fiscal 2017 and that successful completion of this study will lead to commercial production of this product and recurring sales of kits in fiscal 2017.
• We completed the development program for a new pharma customer reported last quarter. We launched a new customer's product development program last quarter and completed it during the current quarter. This program is based on SQI's "off the shelf" test kit for testing 8 cytokines which are common markers tested during drug development. During the quarter, we established the performance of the SQI-developed test against a set of unknown challenge test samples. We are currently anticipating the customer's approval to begin production of the product for use in their commercial operations.
Es gibt also bei zwei großen Projekten eine höhere Wahrscheinlichkeit für einen Abschluss.
Insofern bleibe ich dabei: die Aktie hat m.E. nun gedreht und sollte - so die skizzierten Pläne umgesetzt werden - für deutliche Gewinne im weiteren Jahresverlauf und darüber hinaus gut sein.
Kurs: 0,185 CAD
M@trix
HI,
DING DONG............Zeit mal einzusteigen. Kurs nimmt Fahrt auf..............
Letzte Zahlen sehen , laut Post von M@trix recht ordentlich aus......Turnaround!
M.
DING DONG............Zeit mal einzusteigen. Kurs nimmt Fahrt auf..............
Letzte Zahlen sehen , laut Post von M@trix recht ordentlich aus......Turnaround!
M.
Antwort auf Beitrag Nr.: 55.038.118 von manffreddoo am 29.05.17 21:44:28...noch irgendwer hier ?...
President and CEO, Andrew Morris, along with Company management, will host a conference call on Thursday, August 24, 2017 at 10:00 a.m. ET to review financial results and discuss business developments for the period.
Alles findbare gelesen, meiner Meinung nach 60/40 dass es (endlich) schwarze Zahlen gibt...sollte es so sein...und noch 1-2 Deals (als Sahnehäubchen) in Aussicht gestellt werden...knallt`s mega !
in diesem Sinne...!

President and CEO, Andrew Morris, along with Company management, will host a conference call on Thursday, August 24, 2017 at 10:00 a.m. ET to review financial results and discuss business developments for the period.
Alles findbare gelesen, meiner Meinung nach 60/40 dass es (endlich) schwarze Zahlen gibt...sollte es so sein...und noch 1-2 Deals (als Sahnehäubchen) in Aussicht gestellt werden...knallt`s mega !
in diesem Sinne...!
Antwort auf Beitrag Nr.: 55.537.056 von BIO777 am 16.08.17 16:35:55...das war wohl eher ein Luftballon... 

Antwort auf Beitrag Nr.: 55.597.534 von BIO777 am 25.08.17 12:45:00http://www.stockhouse.com/news/press-releases/2017/11/20/sqi…
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| 0,00 | |
| +1,43 | |
| -2,25 | |
| -0,65 | |
| +0,43 | |
| +3,64 | |
| +86,67 | |
| +1,77 | |
| -0,39 | |
| 0,00 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 202 | ||
| 127 | ||
| 81 | ||
| 69 | ||
| 51 | ||
| 40 | ||
| 37 | ||
| 37 | ||
| 35 | ||
| 33 |





















