Exelixis....ein schlafender Riese? USD 3.40 am 14.5.2014 - 500 Beiträge pro Seite (Seite 2)
eröffnet am 15.05.14 05:58:48 von
neuester Beitrag 29.06.21 21:28:56 von
neuester Beitrag 29.06.21 21:28:56 von
Beiträge: 832
ID: 1.194.406
ID: 1.194.406
Aufrufe heute: 0
Gesamt: 97.268
Gesamt: 97.268
Aktive User: 0
ISIN: US30161Q1040 · WKN: 936718 · Symbol: EXEL
22,700
USD
-0,48 %
-0,110 USD
Letzter Kurs 02:00:00 Nasdaq
Neuigkeiten
01.04.24 · wO Chartvergleich |
29.03.24 · wO Chartvergleich |
28.03.24 · wO Chartvergleich |
27.02.24 · Business Wire (engl.) |
06.02.24 · Business Wire (engl.) |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 3.000,00 | +74.900,00 | |
| 2,9400 | +73,96 | |
| 0,9575 | +65,80 | |
| 2,4700 | +33,51 | |
| 1,3500 | +32,35 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,3000 | -19,25 | |
| 6,5000 | -20,25 | |
| 1,8100 | -23,95 | |
| 1,4300 | -24,34 | |
| 1,3000 | -82,71 |
Die Preisgestaltung von Genentech bei der Behandlung von schwarzem Hautkrebs gibt Anlass zum Protest, weil Cotellic (Cobimetinib) pro Monat und Patient $6,600 kostet, jedoch für Zelboraf monatlich fast das Doppelte ($11,000) pro behandeltem Patienten in Rechnung gestellt werden. Da die Erlöse von Cotellic in den US mit EXEL nahezu geteilt werden, jedoch Zelboraf zu 100% von Genentech verbucht wird, ist die Vorteilnahme von Genentech bei der Preisgestaltung schon sehr deutlich. Falls innerhalb von 30 Tagen keine Einigung erfolgt, soll ein Schiedsgerichtsverfahren eingeleitet werden. Bin mal gespannt, ob Exelixis damit Aussicht auf Erfolg hat. Ich glaube, dass Genentech eine Rationale zur Preisgestaltung vorlegen wird, die juristisch sehr wahrscheinlich nicht anfechtbar sein wird. Nach der nun initialisierten ph3-Studie zur Behandlung von einer bestimmten Form von Darmkrebs mit Cobi+Atezolizumab dürfte jedoch die Übernahmewahrscheinlichkeit zugenommen haben. Ich halte es nämlich für durchaus realistisch, dass kurz-bis mittelfristig weitere Pivotalstudien mit Cobi initialisiert werden.
Primärquelle:
http://www.exelixis.com/investors-media/sec-filings
--> 05/04/16 10-Q Quarterly report which provides a continuing view of a company's financial position
"To date, we believe Genentech’s cost and revenue allocations for COTELLIC, as determined exclusively by Genentech, have been contrary to the applicable terms of the collaboration agreement. We have raised this concern with Genentech, along with other material concerns regarding Genentech’s performance under the collaboration agreement, but thus far have been unable to come to resolution on any of these issues. Accordingly, on May 3, 2016, we issued a formal notice of dispute to Genentech, per the collaboration agreement’s dispute resolution procedures. This notice asserts claims against Genentech related to its clinical development, pricing and commercialization of COTELLIC, and cost and revenue allocations arising from COTELLIC’s commercialization in the United States. If the dispute is not resolved within thirty days of Genentech’s receipt of this notice, we intend to initiate an arbitration."
Primärquelle:
http://www.exelixis.com/investors-media/sec-filings
--> 05/04/16 10-Q Quarterly report which provides a continuing view of a company's financial position
"To date, we believe Genentech’s cost and revenue allocations for COTELLIC, as determined exclusively by Genentech, have been contrary to the applicable terms of the collaboration agreement. We have raised this concern with Genentech, along with other material concerns regarding Genentech’s performance under the collaboration agreement, but thus far have been unable to come to resolution on any of these issues. Accordingly, on May 3, 2016, we issued a formal notice of dispute to Genentech, per the collaboration agreement’s dispute resolution procedures. This notice asserts claims against Genentech related to its clinical development, pricing and commercialization of COTELLIC, and cost and revenue allocations arising from COTELLIC’s commercialization in the United States. If the dispute is not resolved within thirty days of Genentech’s receipt of this notice, we intend to initiate an arbitration."
Bin mal gespannt, welch zusätzliche Info noch bekannt wird:
....oral presentation (Abstract #3502) today at the 2016 ASCO...
http://www.exelixis.com/investors-media/press-releases?cpurl…
http://www.exelixis.com/investors-media/press-releases?cpurl…
....oral presentation (Abstract #3502) today at the 2016 ASCO...
http://www.exelixis.com/investors-media/press-releases?cpurl…
http://www.exelixis.com/investors-media/press-releases?cpurl…
Antwort auf Beitrag Nr.: 52.546.172 von cyberhexe123 am 05.06.16 18:23:58

Conclusions:
Analysis of circulating cell biomarkers showed that cabo induces systemic changes consistent with activation of the immune system in metastatic TNBC patients. These hypothesis-generating data support consideration of studies of Cabozantib with immunotherapy in this patient population.
Warum nicht eine Studie von Atezolizumab mit Cabo bei TNBC?
Würde mich wundern, wenn man bei Genentech nicht darüber nachdenken würde!
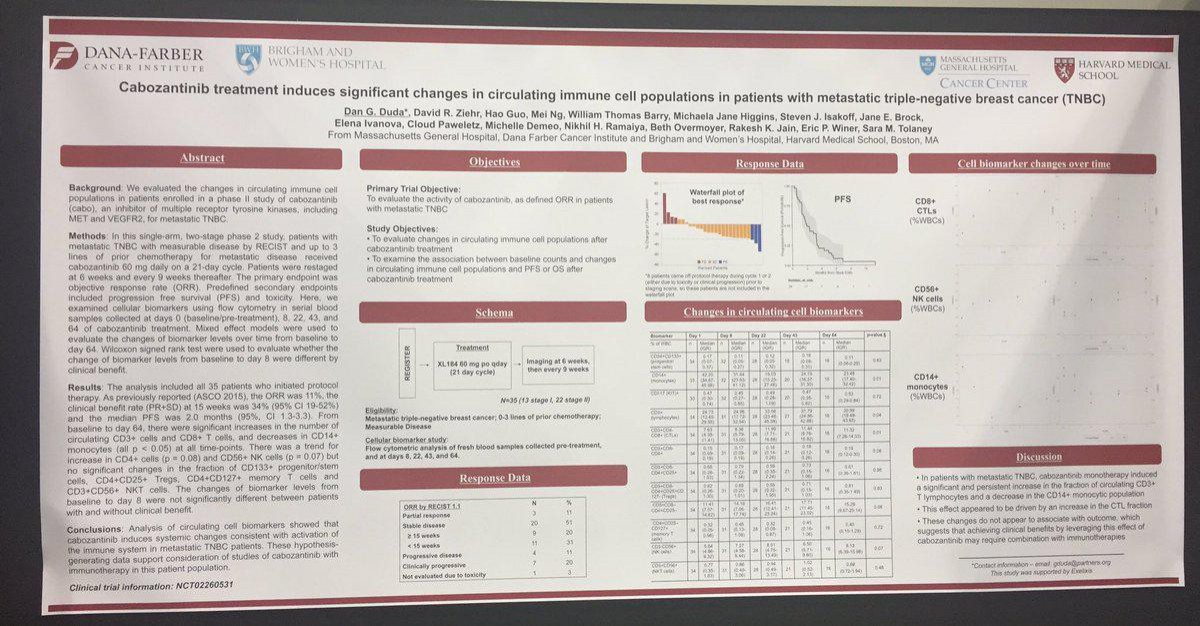
Analysis of circulating cell biomarkers showed that cabo induces systemic changes consistent with activation of the immune system in metastatic TNBC patients. These hypothesis-generating data support consideration of studies of Cabozantib with immunotherapy in this patient population.
Warum nicht eine Studie von Atezolizumab mit Cabo bei TNBC?
Würde mich wundern, wenn man bei Genentech nicht darüber nachdenken würde!

Die Beiträge in den "Journals" reissen nicht ab - Heute im Lancet:
Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial
Interpretation
Treatment with cabozantinib increased overall survival, delayed disease progression, and improved the objective response compared with everolimus. Based on these results, cabozantinib should be considered as a new standard-of-care treatment option for previously treated patients with advanced renal cell carcinoma. Patients should be monitored for adverse events that might require dose modifications.
http://www.thelancet.com/journals/lanonc/article/PIIS1470-20…
Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial
Interpretation
Treatment with cabozantinib increased overall survival, delayed disease progression, and improved the objective response compared with everolimus. Based on these results, cabozantinib should be considered as a new standard-of-care treatment option for previously treated patients with advanced renal cell carcinoma. Patients should be monitored for adverse events that might require dose modifications.
http://www.thelancet.com/journals/lanonc/article/PIIS1470-20…
Antwort auf Beitrag Nr.: 52.546.172 von cyberhexe123 am 05.06.16 18:23:58
Dieser Slide hat sehr viel "Zündstoff", untermauert diese doch die These, dass die kombinierte Hemmung von PDL1 und MEK besonders aussichtsreich ist bei der Behandlung von festen und zunächst örtlich begrenzten Tumoren. Und welches ist der einzige MEK-Hemmer in der Klinik von Roche/Genentech: COBIMETINIB.
Also, die übernahmewahrscheinlichkeit von Exelixis durch seinen Grossen Nachbarn in SouthCity wird dadurch nicht geringer!
Zitat von cyberhexe123:
Dieser Slide hat sehr viel "Zündstoff", untermauert diese doch die These, dass die kombinierte Hemmung von PDL1 und MEK besonders aussichtsreich ist bei der Behandlung von festen und zunächst örtlich begrenzten Tumoren. Und welches ist der einzige MEK-Hemmer in der Klinik von Roche/Genentech: COBIMETINIB.
Also, die übernahmewahrscheinlichkeit von Exelixis durch seinen Grossen Nachbarn in SouthCity wird dadurch nicht geringer!
Antwort auf Beitrag Nr.: 52.546.529 von cyberhexe123 am 05.06.16 19:35:54Die Synergien zwischen MEK-Hemmern und PDL1-Hemmern sind aussergewöhnlich....ich würde mich wirklich wundern: ...Cobi+Cabo...eine aktuelle Marktkapitaliserung von 1.5 Milliarden USD bei 2 zugelassenen Wirkstoffen in 3 verschiedenen und mit der Aussicht auf weitere Indikationen! Ungeachtet der Charttechnik - als Mittel- bis Langfristinvestor interessiert die mich eh nur am Rande - bin ich mehr und mehr davon überzeugt, dass tiefe zweistellige Kurse noch nicht das Ende der Fahnenstange  bedeuten.... es sei denn, es erfolgt demnächst eine Übernahme. Das wäre kurzfristig sicherlich von Vorteil, aber mittel- bis langfristig eher nicht.
bedeuten.... es sei denn, es erfolgt demnächst eine Übernahme. Das wäre kurzfristig sicherlich von Vorteil, aber mittel- bis langfristig eher nicht.
Der Entwicklungsaufwand für ein neues Medikament ist in den vergangenen Jahrzehnten stark gestiegen, vor allem aufgrund der hohen gesetzlichen Anforderungen an die Sicherheit. Während die erforderliche Teilnehmerzahl für klinische Studien früher wenige Hundert betrug, sind es heute in der Regel mehrere Tausend. Bis zur Markteinführung eines neuen Medikaments dauert es durchschnittlich 8 bis 12 Jahre. Durch die lange Entwicklungszeit bleibt den Pharmafirmen wenig Zeit, die hohen Kosten innerhalb der Laufzeit des Patentschutzes zu amortisieren. Eine 2012 publizierte Schätzung des renommierten Londoner Office of Health Economics geht für das Jahr 2000 von Forschungs- und Entwicklungskosten von rund 1.5 Milliarden US-Dollar (rund 1.3 Milliarden Franken) für ein erfolgreich auf den Markt gebrachtes Medikament mit neuem Wirkstoff aus.
http://www.interpharma.ch/fakten-statistiken/2093-viel-zeit-…
 bedeuten.... es sei denn, es erfolgt demnächst eine Übernahme. Das wäre kurzfristig sicherlich von Vorteil, aber mittel- bis langfristig eher nicht.
bedeuten.... es sei denn, es erfolgt demnächst eine Übernahme. Das wäre kurzfristig sicherlich von Vorteil, aber mittel- bis langfristig eher nicht.Der Entwicklungsaufwand für ein neues Medikament ist in den vergangenen Jahrzehnten stark gestiegen, vor allem aufgrund der hohen gesetzlichen Anforderungen an die Sicherheit. Während die erforderliche Teilnehmerzahl für klinische Studien früher wenige Hundert betrug, sind es heute in der Regel mehrere Tausend. Bis zur Markteinführung eines neuen Medikaments dauert es durchschnittlich 8 bis 12 Jahre. Durch die lange Entwicklungszeit bleibt den Pharmafirmen wenig Zeit, die hohen Kosten innerhalb der Laufzeit des Patentschutzes zu amortisieren. Eine 2012 publizierte Schätzung des renommierten Londoner Office of Health Economics geht für das Jahr 2000 von Forschungs- und Entwicklungskosten von rund 1.5 Milliarden US-Dollar (rund 1.3 Milliarden Franken) für ein erfolgreich auf den Markt gebrachtes Medikament mit neuem Wirkstoff aus.
http://www.interpharma.ch/fakten-statistiken/2093-viel-zeit-…
Antwort auf Beitrag Nr.: 52.546.529 von cyberhexe123 am 05.06.16 19:35:54Diese Folie überzeugt auch mich letztlich, nachdem Roche ja bereits die Pivotal Studie in CRC indirekt über clinicaltrials.gov bekannt gegeben hatte - das Comitment auf das ich gewartet habe.
Klar synergistische Effekte beider drugs!
Ich bin nun auch im Cobimentinib Bullen-Camp.
Klar synergistische Effekte beider drugs!
Ich bin nun auch im Cobimentinib Bullen-Camp.
Antwort auf Beitrag Nr.: 52.546.925 von Ville7 am 05.06.16 20:58:41...nicht das wir noch Freunde werden 

Antwort auf Beitrag Nr.: 52.547.048 von cyberhexe123 am 05.06.16 21:26:43Wird spannend, wie der Markt die nächsten Tage auf diesen sehr guten Newsflow reagiert.
Hi Hexe,
super Infos, was hast Du denn nach all den positiven news der letzten Zeit für ein persönliches Kursziel für Exelixis?
Gruß
Fredy
super Infos, was hast Du denn nach all den positiven news der letzten Zeit für ein persönliches Kursziel für Exelixis?
Gruß
Fredy
Antwort auf Beitrag Nr.: 52.547.360 von Magnetfeldfredy am 05.06.16 22:49:58hi freddy
hab leider auch keine Glaskugel! Die fundamentalen Daten werden jedoch fast wöchentlich besser, so dass einerseits die Übernahmewahrscheinlichkeit zunimmt - wie gross diese mittlerweile ist, ohne Glaskugel? ... und andereseits dadurch mittelfristig auch zweistellige Kurse immer wahrscheinlicher werden.
Ich werde jedenfalls den Teufel tun und wegen charttechnischen Bedenken mein derzeitiges Portfolio zu ändern - hab allerdings Exelixis in den letzten tagen zu Gunsten von Radius Health auf 50% meines Portfolios reduziert.
hab leider auch keine Glaskugel! Die fundamentalen Daten werden jedoch fast wöchentlich besser, so dass einerseits die Übernahmewahrscheinlichkeit zunimmt - wie gross diese mittlerweile ist, ohne Glaskugel? ... und andereseits dadurch mittelfristig auch zweistellige Kurse immer wahrscheinlicher werden.
Ich werde jedenfalls den Teufel tun und wegen charttechnischen Bedenken mein derzeitiges Portfolio zu ändern - hab allerdings Exelixis in den letzten tagen zu Gunsten von Radius Health auf 50% meines Portfolios reduziert.
Antwort auf Beitrag Nr.: 52.546.298 von cyberhexe123 am 05.06.16 18:50:14
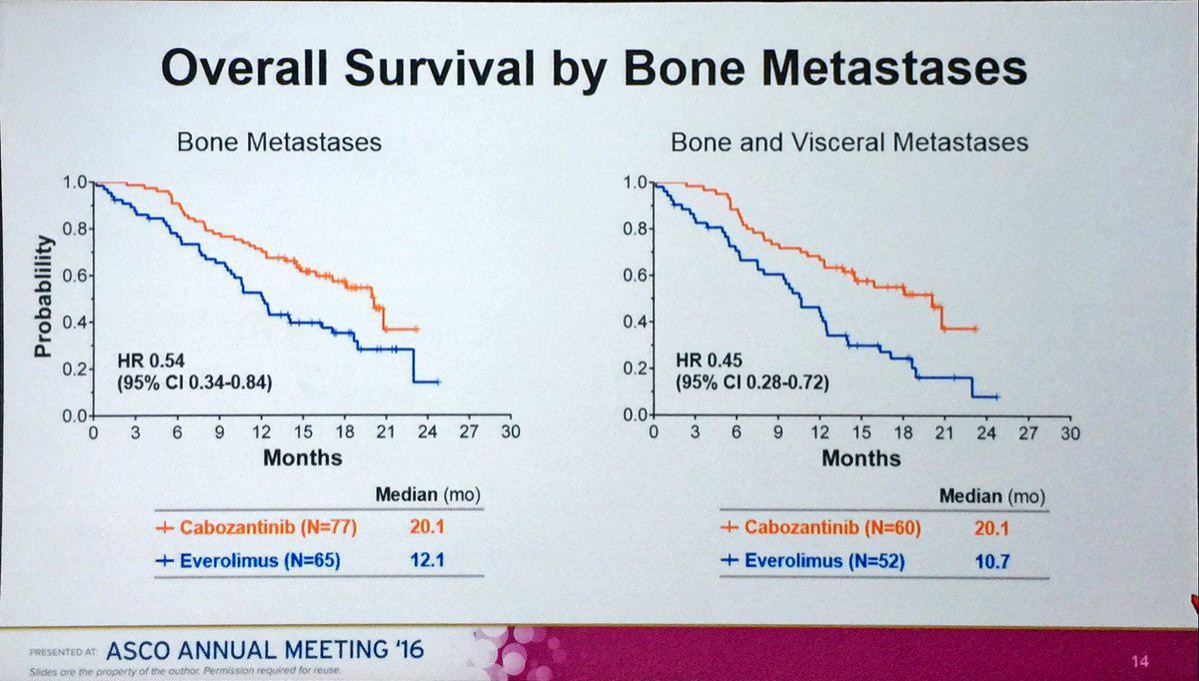
eindrucksvolle Bestätigung der Wirkung von Cabo bei Skelettmetastasen, die bei RCC relativ häufig sind:
"Generell kann sich ein Rezidiv eines Nierezellkarzinoms in allen anderen Organen des Körpers entwickeln. Die häufigsten Lokalisationen sind die Lunge (65-70%), die regionären Lymphknoten (60-65%), Knochen (39-40%), ..."
file:///C:/Users/CYBERH~1/AppData/Local/Temp/LOonko_1505_fin…
Zitat von cyberhexe123:
eindrucksvolle Bestätigung der Wirkung von Cabo bei Skelettmetastasen, die bei RCC relativ häufig sind:
"Generell kann sich ein Rezidiv eines Nierezellkarzinoms in allen anderen Organen des Körpers entwickeln. Die häufigsten Lokalisationen sind die Lunge (65-70%), die regionären Lymphknoten (60-65%), Knochen (39-40%), ..."
file:///C:/Users/CYBERH~1/AppData/Local/Temp/LOonko_1505_fin…
Die Zukunft in der Krebsbekämpfung gehört der Mehrfachtherapie. Anbei ein sehr einfaches aber anschauliches Bild, wshalb das so ist:

...aus einem Vortrag von Wiliam Kaelin auf ASCO2016.

...aus einem Vortrag von Wiliam Kaelin auf ASCO2016.
Exelixis auf 52 Wochen Hoch, supi, und noch Potential ohne Ende!
Antwort auf Beitrag Nr.: 52.552.457 von Magnetfeldfredy am 06.06.16 16:49:45Bisher schaut es sehr gut aus. Mit Breakaway gap und unter sehr hohem Volumen über die Resistance gegangen.
Antwort auf Beitrag Nr.: 52.554.368 von Ville7 am 06.06.16 20:38:19http://us.lrd.yahoo.com/_ylt=ApG_BdA2o3C83lgl_HqW0EOHH8V_;_y…
Zitat von Ville7: Bisher schaut es sehr gut aus. Mit Breakaway gap und unter sehr hohem Volumen über die Resistance gegangen.
Interessant ist die Tatsache, dass Roches OX40 den grossen Erwartungen nicht gerecht wurde, wodurch die Position von Cobimetinib innerhalb des Portfolios der Schweizer sicherlich an Bedeutung gewonnen hat. Die Synergie mit Atezolizumab in CRC ist nämlich deutlich. Obschon auch in anderen Indikationen ph1-Ergebnisse zu Cobi+Atezolizumab vorliegen müssten (--> A Phase 1b Study of the Safety and Pharmacology of Atezolizumab Administered With Cobimetinib in Patients With Locally Advanced or Metastatic Solid Tumors), werden diese (noch) nicht publiziert. Hält der Basler Pharmagigant den Ball absichtlich weit unten?
The Street Biotech expert AF am 6.6.2016:
OX40 is one of the most-hyped cancer immunotherapy targets for its theoretical ability to improve response to currently approved checkpoint inhibitors. But this weekend, Roche's (RHHBY) OX40 drug candidate, combined with the checkpoint inhibitor Tecentriq, showed decent safety but not much else.
The Street Biotech expert AF am 6.6.2016:
OX40 is one of the most-hyped cancer immunotherapy targets for its theoretical ability to improve response to currently approved checkpoint inhibitors. But this weekend, Roche's (RHHBY) OX40 drug candidate, combined with the checkpoint inhibitor Tecentriq, showed decent safety but not much else.
Die Versuche von Cyberhexe mich zu diskreditieren und mich als den absoluten Idioten hinzustellen, hören nicht auf - daher habe ich eine Antwort im anderen Thread geschrieben, die ich auch hier posten werde. Fazit: das Ziel von Cyberhexe, mich aus ihrem Thread zu verbannen, wird sie nicht erreichen. Ich schreibe weiterhin was und wo ich es für richtig erachte.
------------------
Wertfrei von jeglicher Hassirrade stelle ich weiterhin fest:
Abstracts re Cobi:
Aus den Abstracts heraus gab es keine (harten) Infos für eine Werterhöhung der Firma durch die dort vorab veröffentlichten Daten. Erst das Commitement von Roche zur Atezo+Cobi Kombi (mit dem Start der Phase 3, den überzeugenden präklinischen Daten und der Unterstreichung deren Bedeutung für die eigene Pipeline durch Roche) hat den Auslöser gegeben, dass das nun eingepreist wird. Übrigens war Roche selbst überascht durch den Effekt des MEK-Inhibitors, sie haben mehrfach erwähnt, dass der Effekt überaschend kommt, da der MEKi auch gegenteilige Effekte hervorruft, der für eine Kombi nachteilig ist. Siehe Roche Präsentation zu ASCO.
Abstracts re Cabo:
Die Daten waren weitgehend bekannt. Keine unerwarteten Entwicklungen. Es war davon auszugehen, dass die entsprechenden Subgruppen (bon/visceral) besonders gut funktionieren.
Somit war meine Einschätzung zum Zeitpunkt der abstract Releases auch aus heutiger Sicht -unter meinen Investitionsprinzipien- absolut korrekt.
Es gab in den letzten zwei Wochen aus meiner Sicht vier Effekte, die den Kurs ins Steigen gebracht haben, die ersten zwei besonders hervorgehoben:
1. Vermeldung positiver Endpunkt PFS gegen Sunitinib (CABOSUN) in 1st line
2. Verlautbarung durch Roche, dass Cobi in der weiteren Entwicklung ein bedeutender Zusatz zu Atezo (und evtl. anderen Produkten) sein wird.
3. gute Akzeptanz von Cabo durch KOLs auf ASCO
4. Kombi von Cabo+Nivo in BC anscheinend gut vertragen lt. Apolo, Spekulation auf gute Daten zu ESMO
Noch etwas Grundsätzliches:
Jeder hat an der Börse seinen eigenen Ansatz. Dir geht es darum stets Recht zu haben und dich als der große Experte im Bereich Biotech darzustellen. Nun ist Biotech und auch die Ausgänge von klin. Trials aber nicht vorherzusagen, nicht mal durch den allergrößten Experten, da es sich nicht um Mathematik mit klaren einfachen Regeln handelt, sondern der Mensch ein zu komplexes System ist. Du beanspruchst das in deiner Hybris aber. Es ist ein Zeichen von Größe zu erkennen, wo die eigenen Grenzen sind. Diese Größe hast du leider nicht.
Ich beanspruche für mich nicht ein fachlicher Experte in der tiefsten Tiefe zu sein. Es reicht, wenn ich die Entwicklung einzelner Unternehmen und deren Produkte und der Konkurrenzsituation auf dem für Investitionen notwendigen Level einschätzen kann. Ich bin lediglich ein branchenfremder Biotechinvestor, ein Autodidakt, der sich massiv selbst eingearbeitet und der seit mehr als 10 Jahren erfolgreich zahlreiche Winner (zum richtigem Timing) gepickt und daraus hervorragende Erfolge erzielt hat. Diese Winner habe ich mir alle ohne einen Frontrunner (wie du gerne einer sein möchtest) herausgesucht. Für dich ist mein Wissen dabei Halbwissen - ich spiele für dich in der Kreisliga. Und du in der Championsliga. Somit muss das alles Zufall gewesen sein.
Wenn dein exorbitantes Wissen sich dann auch (gegenüber meinen Erfolgen) in dem Faktor in Börsenerfolge umschlägt, dann müsstest du bestimmt schon Milliardär sein.
Was ich damit sagen möchte: Für mich zählt mein Erfolg im Depot. Dabei wäre es mir egal, ob ich die letzte wissenschaftliche Nuance verpasse. An der Börse ist das für einen Erfolg auch gar nicht notwendig. Bzw. manchmal ist es besser, wenn man nicht zu früh Spekulationen kauft oder wenn man sich nicht durch Hypes anstecken lässt. Man muss nicht nur einschätzen können, wieviel eingepreist werden kann, sondern auch wie viel eingepreist ist und wie der Markt max. ein- und auspreisen können wird um in Angstphasen das ausgesuchte Biotech kaufen zu können und in Hypephasen wieder abstossen zu können. Es spielen somit viel mehr Faktoren für einen Erfolg eine Rolle als reines Fachwissen, das teilweise auch falsches Wissen sein kann (der gegenwärtige Wissensstand wird regelmäßig durch empirische Untersuchungen (Studien) neu definiert.
Also langer Rede - kurzer Sinn. Werde du glücklich mit deinem Ansatz, ich bin es mit meinem. Aber höre auf damit, mir einen Maulkorb verpassen zu wollen. Ich schreibe in dem Thread in dem es mir passt. Ich schreibe was mir passt. Wenn es dir nicht gefällt, dann wähle doch ein ganz anderes Forum. Z.B. einen Blog ohne Kommentarfunktion. Das entspricht deine Persönlichkeit besser.
------------------
Wertfrei von jeglicher Hassirrade stelle ich weiterhin fest:
Abstracts re Cobi:
Aus den Abstracts heraus gab es keine (harten) Infos für eine Werterhöhung der Firma durch die dort vorab veröffentlichten Daten. Erst das Commitement von Roche zur Atezo+Cobi Kombi (mit dem Start der Phase 3, den überzeugenden präklinischen Daten und der Unterstreichung deren Bedeutung für die eigene Pipeline durch Roche) hat den Auslöser gegeben, dass das nun eingepreist wird. Übrigens war Roche selbst überascht durch den Effekt des MEK-Inhibitors, sie haben mehrfach erwähnt, dass der Effekt überaschend kommt, da der MEKi auch gegenteilige Effekte hervorruft, der für eine Kombi nachteilig ist. Siehe Roche Präsentation zu ASCO.
Abstracts re Cabo:
Die Daten waren weitgehend bekannt. Keine unerwarteten Entwicklungen. Es war davon auszugehen, dass die entsprechenden Subgruppen (bon/visceral) besonders gut funktionieren.
Somit war meine Einschätzung zum Zeitpunkt der abstract Releases auch aus heutiger Sicht -unter meinen Investitionsprinzipien- absolut korrekt.
Es gab in den letzten zwei Wochen aus meiner Sicht vier Effekte, die den Kurs ins Steigen gebracht haben, die ersten zwei besonders hervorgehoben:
1. Vermeldung positiver Endpunkt PFS gegen Sunitinib (CABOSUN) in 1st line
2. Verlautbarung durch Roche, dass Cobi in der weiteren Entwicklung ein bedeutender Zusatz zu Atezo (und evtl. anderen Produkten) sein wird.
3. gute Akzeptanz von Cabo durch KOLs auf ASCO
4. Kombi von Cabo+Nivo in BC anscheinend gut vertragen lt. Apolo, Spekulation auf gute Daten zu ESMO
Noch etwas Grundsätzliches:
Jeder hat an der Börse seinen eigenen Ansatz. Dir geht es darum stets Recht zu haben und dich als der große Experte im Bereich Biotech darzustellen. Nun ist Biotech und auch die Ausgänge von klin. Trials aber nicht vorherzusagen, nicht mal durch den allergrößten Experten, da es sich nicht um Mathematik mit klaren einfachen Regeln handelt, sondern der Mensch ein zu komplexes System ist. Du beanspruchst das in deiner Hybris aber. Es ist ein Zeichen von Größe zu erkennen, wo die eigenen Grenzen sind. Diese Größe hast du leider nicht.
Ich beanspruche für mich nicht ein fachlicher Experte in der tiefsten Tiefe zu sein. Es reicht, wenn ich die Entwicklung einzelner Unternehmen und deren Produkte und der Konkurrenzsituation auf dem für Investitionen notwendigen Level einschätzen kann. Ich bin lediglich ein branchenfremder Biotechinvestor, ein Autodidakt, der sich massiv selbst eingearbeitet und der seit mehr als 10 Jahren erfolgreich zahlreiche Winner (zum richtigem Timing) gepickt und daraus hervorragende Erfolge erzielt hat. Diese Winner habe ich mir alle ohne einen Frontrunner (wie du gerne einer sein möchtest) herausgesucht. Für dich ist mein Wissen dabei Halbwissen - ich spiele für dich in der Kreisliga. Und du in der Championsliga. Somit muss das alles Zufall gewesen sein.
Wenn dein exorbitantes Wissen sich dann auch (gegenüber meinen Erfolgen) in dem Faktor in Börsenerfolge umschlägt, dann müsstest du bestimmt schon Milliardär sein.
Was ich damit sagen möchte: Für mich zählt mein Erfolg im Depot. Dabei wäre es mir egal, ob ich die letzte wissenschaftliche Nuance verpasse. An der Börse ist das für einen Erfolg auch gar nicht notwendig. Bzw. manchmal ist es besser, wenn man nicht zu früh Spekulationen kauft oder wenn man sich nicht durch Hypes anstecken lässt. Man muss nicht nur einschätzen können, wieviel eingepreist werden kann, sondern auch wie viel eingepreist ist und wie der Markt max. ein- und auspreisen können wird um in Angstphasen das ausgesuchte Biotech kaufen zu können und in Hypephasen wieder abstossen zu können. Es spielen somit viel mehr Faktoren für einen Erfolg eine Rolle als reines Fachwissen, das teilweise auch falsches Wissen sein kann (der gegenwärtige Wissensstand wird regelmäßig durch empirische Untersuchungen (Studien) neu definiert.
Also langer Rede - kurzer Sinn. Werde du glücklich mit deinem Ansatz, ich bin es mit meinem. Aber höre auf damit, mir einen Maulkorb verpassen zu wollen. Ich schreibe in dem Thread in dem es mir passt. Ich schreibe was mir passt. Wenn es dir nicht gefällt, dann wähle doch ein ganz anderes Forum. Z.B. einen Blog ohne Kommentarfunktion. Das entspricht deine Persönlichkeit besser.
Ein neues US-Patent zum PI3K-Signalweg wurde gewährt. Allerdings wurde das PI3K-Programm 2011 an Merck auslizenziert, so dass die kaufmännische Bedeutung dessen wohl eher gering ist.
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
United States Patent 9,346,807
Kearney May 24, 2016
Inhibitors of PI3K-delta and methods of their use and manufacture
Abstract
The invention is directed to Compounds of Formula I: and pharmaceutically acceptable salts or solvates thereof, as well as methods of making and using the compounds. ##STR00001##
Inventors: Kearney; Patrick (San Francisco, CA)
Applicant:
Name City State Country Type
Kearney; Patrick
San Francisco
CA
US
Assignee: Exelixis, Inc. (South San Francisco, CA)
Family ID: 44898157
Appl. No.: 13/822,840
Filed: September 14, 2011
PCT Filed: September 14, 2011
Exelixis war bisher jedoch sehr sorgfältig darin, das geistige Eigentum sehr sorgfältig zu schützen - eine Grundvoraussetzung für wirtschaftlichen Erfolg in dieser Branche:
Approximately 1,790 results found in the Worldwide database for:
Exelixis as the applicant
Only the first 500 results are displayed.
http://worldwide.espacenet.com/searchResults?submitted=true&…
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
United States Patent 9,346,807
Kearney May 24, 2016
Inhibitors of PI3K-delta and methods of their use and manufacture
Abstract
The invention is directed to Compounds of Formula I: and pharmaceutically acceptable salts or solvates thereof, as well as methods of making and using the compounds. ##STR00001##
Inventors: Kearney; Patrick (San Francisco, CA)
Applicant:
Name City State Country Type
Kearney; Patrick
San Francisco
CA
US
Assignee: Exelixis, Inc. (South San Francisco, CA)
Family ID: 44898157
Appl. No.: 13/822,840
Filed: September 14, 2011
PCT Filed: September 14, 2011
Exelixis war bisher jedoch sehr sorgfältig darin, das geistige Eigentum sehr sorgfältig zu schützen - eine Grundvoraussetzung für wirtschaftlichen Erfolg in dieser Branche:
Approximately 1,790 results found in the Worldwide database for:
Exelixis as the applicant
Only the first 500 results are displayed.
http://worldwide.espacenet.com/searchResults?submitted=true&…
Antwort auf Beitrag Nr.: 52.547.912 von cyberhexe123 am 06.06.16 07:17:33

Zitat von cyberhexe123:Zitat von cyberhexe123:
eindrucksvolle Bestätigung der Wirkung von Cabo bei Skelettmetastasen, die bei RCC relativ häufig sind:
"Generell kann sich ein Rezidiv eines Nierezellkarzinoms in allen anderen Organen des Körpers entwickeln. Die häufigsten Lokalisationen sind die Lunge (65-70%), die regionären Lymphknoten (60-65%), Knochen (39-40%), ..."
file:///C:/Users/CYBERH~1/AppData/Local/Temp/LOonko_1505_fin…

Antwort auf Beitrag Nr.: 50.871.063 von Cyberhexe am 17.10.15 15:48:21ville schrieb Heute:
"Übrigens war Roche selbst überascht durch den Effekt des MEK-Inhibitors, sie haben mehrfach erwähnt, dass der Effekt überaschend kommt, da der MEKi auch gegenteilige Effekte hervorruft, der für eine Kombi nachteilig ist. Siehe Roche Präsentation zu ASCO."
...für diese Aussage solltest du einen Beweis erbringen. Aber ich rechne mit wenig bis gar nichts!
Cyberhexe schrieb am 17.10.15 15:48:21
Beitrag Nr. 338 (50.871.063)
....ich möchte empfehlen, den Roche-Hyperlink im Breitrag vom 17.10.15 zu öffnen. Vielleicht findet sich da eine Spur, dass Roche überrascht ist von der Wirkungsweise von Cobi.
"Übrigens war Roche selbst überascht durch den Effekt des MEK-Inhibitors, sie haben mehrfach erwähnt, dass der Effekt überaschend kommt, da der MEKi auch gegenteilige Effekte hervorruft, der für eine Kombi nachteilig ist. Siehe Roche Präsentation zu ASCO."
...für diese Aussage solltest du einen Beweis erbringen. Aber ich rechne mit wenig bis gar nichts!
Cyberhexe schrieb am 17.10.15 15:48:21
Beitrag Nr. 338 (50.871.063)
Zitat von Cyberhexe: Cobimetinib hemmt selektiv das MEK-Signal innerhalb des MAPK-Signalweges (mitogen activated protein kinase) bzw. der signalgebenden Enzymkaskade RAS-RAF-MEK-ERK, an deren Ende der Impuls zur Zellteilung gegeben wird.
DER MAPK-Signalweg hat in vielen Krebsformen eine zentrale Bedeutung:
Onkogene RAS-Mutationen sind am Entstehen von verschiedenartigen Krebsformen beteiligt, so bspw. bei ca. 50% der CRC-Erkrankungen (Dickdarmkrebs). Onkogene RAF-Mutationen, konkret BRAF, wurden ursächlich identifiziert bei ca. 50% Hautkrebserkrankungen, 40% Schilddrüsenkrebs, 30% Unterleibskrebs (eierstock) sowie ca. 10% CRC.
Durch die vielfache Beteiligung von MEK in diesen die Zellteilung auslösenden Signalwegen, ist es möglich, Cobi mit vielerlei anderen Wirkstoffen zu kombinieren, um somit eine anti-neoplastsche Wirkung zu optimieren
--> https://clinicaltrials.gov/ct2/results?term=cobimetinib&Sear…
Da die Akltivierung des MAPK-Signalwegs häufig ursächlich mit dem Therapieversagen von antitumoralen Wirkstoffen in Verbiundung gebracht wird, ist die Blockade dieses Signalweges ausserdem eine viel versprechende Strategie, um derartige Resistenzen zu vermeiden oder zumindest zu verzögern.
Die Kombination mit anderen Wirkstoffen erfolgt nach den folgenden Prinzipien:
- Hemmung innerhalb des gleichen Signalweges, also MAPK-Signalweg zB mit Vemurafenib zur Hemmung von RAF
- Hemmung von verschiedenen Signalwegen zB des Signalweges, der die Angiogenese, also die Neubildung von Blutgefässen, fördert --> Vascular Endothelial Growth Factor (VEGF) oder --> Epidermal growth factor receptor (EGFR)
- durch Anregung des programmierten Zelltods zB in Kombination mit Taxanen
- und "last but not least" das körpereigene Immunsystem auf die Krebszellen aufmerksam zu machen und es zu einer Immunantwort zu aktivieren. Es wird vermutet, dass Cobi eine durch andere Stoffe (wie zB monoklonale Antikörper) induzierte Immunantwort verstärkt --> https://clinicaltrials.gov/ct2/show/NCT01656642?term=cobimet…
- Thewrapieversagen wird durch Hemmung des MAPK-Signalweges verzögert
http://www.roche.com/research_and_development/what_we_are_wo…
Cobi könnte eine grosse Geschichte werden - Good Hunting!
ch
....ich möchte empfehlen, den Roche-Hyperlink im Breitrag vom 17.10.15 zu öffnen. Vielleicht findet sich da eine Spur, dass Roche überrascht ist von der Wirkungsweise von Cobi.

Antwort auf Beitrag Nr.: 52.563.932 von cyberhexe123 am 07.06.16 22:52:38Glückwunsch. Du hast die von mir in diesem Posting absichtlich für dich eingebaute Ente einwandfrei entdeckt. Wenigstens der Beweis, dass du meine Messages liest, bevor du deine endlosen, ständig wiederholenden Rants abziehst.
ASCO2016 ist Geschichte und nun werden die Ergebnisse nach und nach auch in den wissenschaftlichen Journals veröffentlicht. Für EXEL war ASCO eine überaus erfolgreiche Veranstaltung, die für sehr viel Aufmerksamkeit sorgt. Dem Aktienkurs hat ASCO2016 entgegen anderen Prognosen auch nicht geschadet. Das nächste kurstreibende Ereignis dürfte die Zulassung von Cabo bei RCC in der EU sein. Das nächste Treffen des Arzneimittelausschusses der EMA beginnt übernächste Woche: http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/l…
Falls es keinen Unterbruch ("Clock Stop") aufgrund von noch ausstehenden Fragen (der "List of Questions") gegeben hat, könnte am 24.6.2016 bereits eine Zulassungsempfehlung des Arzneimittelausschusses publiziert werden - die endgültige Entscheidung fällt die Kommission spätestens 67 Tage nach der CHMP-Edmpfehlung.
Cobimetinib/Atezolizumab Combo Achieves Response in Advanced Colorectal Cancer
Darcy Lewis
Published Online: 6:21 PM, Tue June 7, 2016
"This is consistent with the hypothesis that tumors with high mutation burden are more responsive to combined immunotherapy."
—Johanna C. Bendell, MD
Johanna C. Bendell, MD
The combination of cobimetinib (Cotellic) and atezolizumab (Tecentriq) is safe and clinically active in advanced colorectal cancer and resulted in a higher clinical response rate in patients with microsatellite stable (MSS) colorectal cancer patients than either agent alone, according to results from a phase Ib dose escalation and cohort expansion trial presented at the 2016 ASCO Annual Meeting.
The investigator-assessed observed response rate (ORR) was 17% and the 6-month overall survival (OS) was 72% among a cohort of 23 patients.
“These results suggest that cobimetinib can sensitize tumors to atezolizumab by increasing MHC I expression on tumor cells and promoting intratumoral CD8 T-cell accumulation,” said lead author Johanna C. Bendell, MD, director of the GI cancer research program and associate director of the drug development program at the Sarah Cannon Research Institute, and an associate with Tennessee Oncology in Nashville. “This is consistent with the hypothesis that tumors with high mutation burden are more responsive to combined immunotherapy.” She called the pairing of an anti-PD-L1 agent and a MEK inhibitor, such as that of the experimental combination regimen, a “rational combination.”
http://ht.ly/svUj3011j5z
...es ist sehr ruhig Heute im Forum - weshalb denn wohl?
Vielleicht halten sich nun alle an die Regeln: Falschmeldungen, Unterstellungen und Beleidigungen nicht in diesem Thread.
Falls es keinen Unterbruch ("Clock Stop") aufgrund von noch ausstehenden Fragen (der "List of Questions") gegeben hat, könnte am 24.6.2016 bereits eine Zulassungsempfehlung des Arzneimittelausschusses publiziert werden - die endgültige Entscheidung fällt die Kommission spätestens 67 Tage nach der CHMP-Edmpfehlung.
Cobimetinib/Atezolizumab Combo Achieves Response in Advanced Colorectal Cancer
Darcy Lewis
Published Online: 6:21 PM, Tue June 7, 2016
"This is consistent with the hypothesis that tumors with high mutation burden are more responsive to combined immunotherapy."
—Johanna C. Bendell, MD
Johanna C. Bendell, MD
The combination of cobimetinib (Cotellic) and atezolizumab (Tecentriq) is safe and clinically active in advanced colorectal cancer and resulted in a higher clinical response rate in patients with microsatellite stable (MSS) colorectal cancer patients than either agent alone, according to results from a phase Ib dose escalation and cohort expansion trial presented at the 2016 ASCO Annual Meeting.
The investigator-assessed observed response rate (ORR) was 17% and the 6-month overall survival (OS) was 72% among a cohort of 23 patients.
“These results suggest that cobimetinib can sensitize tumors to atezolizumab by increasing MHC I expression on tumor cells and promoting intratumoral CD8 T-cell accumulation,” said lead author Johanna C. Bendell, MD, director of the GI cancer research program and associate director of the drug development program at the Sarah Cannon Research Institute, and an associate with Tennessee Oncology in Nashville. “This is consistent with the hypothesis that tumors with high mutation burden are more responsive to combined immunotherapy.” She called the pairing of an anti-PD-L1 agent and a MEK inhibitor, such as that of the experimental combination regimen, a “rational combination.”
http://ht.ly/svUj3011j5z
...es ist sehr ruhig Heute im Forum - weshalb denn wohl?

Vielleicht halten sich nun alle an die Regeln: Falschmeldungen, Unterstellungen und Beleidigungen nicht in diesem Thread.
http://us.rd.yahoo.com/finance/external/mfool/SIG=12qmvlk1f/…
Der Kurs kommt nicht zurück, die Daten sind zu gut und nun wird Cabo schon first line diskutiert.....
Der Kurs kommt nicht zurück, die Daten sind zu gut und nun wird Cabo schon first line diskutiert.....

Antwort auf Beitrag Nr.: 52.573.403 von Magnetfeldfredy am 08.06.16 22:06:45MF zieht wohl die richtigen Schlussfolgerungen, dass das Ergebnis der 1b-Kombistudie von Cabo+Nivolumab abzuwarten sei, da dieser das Potenzial zur Standardtherapie zugetraut wird. Eine Cabo-Pivotalstudie 1L in RCC wäre somit verschwendete Ressourcen.
aus MF (Hyperlink von Freddy im letzten beitrag zur Verfügung gestellt)
Assuming Exelixis needs to run a phase 3 trial with overall survival as an endpoint to confirm the results, it would be a couple of years before Cabometyx would be approved as a first-line treatment. But the PFS phase 2 data could convince more doctors to use it now, either off-label as first-line treatment or as the preferred second-line treatment equal to, or perhaps in front of, Bristol-Myers Squibb's Opdivo.
The battle with Bristol-Myers Squibb is an interesting one, because the companies are testing the combination of Cabometyx and Opdivo in a phase 1b trial in genitourinary cancers including renal cell carcinoma. Data from that trial is expected this year, so it might make sense for Exelixis to wait for the data before starting a phase 3 trial in first-line renal cell carcinoma. If it's clear that the combination of Cabometyx and Opdivo works substantially better, there's no sense in getting the drug approved as a stand-alone first-line treatment.
aus MF (Hyperlink von Freddy im letzten beitrag zur Verfügung gestellt)
Assuming Exelixis needs to run a phase 3 trial with overall survival as an endpoint to confirm the results, it would be a couple of years before Cabometyx would be approved as a first-line treatment. But the PFS phase 2 data could convince more doctors to use it now, either off-label as first-line treatment or as the preferred second-line treatment equal to, or perhaps in front of, Bristol-Myers Squibb's Opdivo.
The battle with Bristol-Myers Squibb is an interesting one, because the companies are testing the combination of Cabometyx and Opdivo in a phase 1b trial in genitourinary cancers including renal cell carcinoma. Data from that trial is expected this year, so it might make sense for Exelixis to wait for the data before starting a phase 3 trial in first-line renal cell carcinoma. If it's clear that the combination of Cabometyx and Opdivo works substantially better, there's no sense in getting the drug approved as a stand-alone first-line treatment.
Es ist unglaublich, wieviele Patente sich Exelixis weltweit erarbeitet hat - bei der bzw. für die Grösse des Unternehmens eindeutig Champions League.
http://worldwide.espacenet.com/searchResults?submitted=true&…
Approximately 1,790 results found in the Worldwide database for:
Exelixis as the applicant
Only the first 500 results are displayed.
Ich bin schon der Meinung, Regelverstösse sollten geahndet werden
http://worldwide.espacenet.com/searchResults?submitted=true&…
Approximately 1,790 results found in the Worldwide database for:
Exelixis as the applicant
Only the first 500 results are displayed.
Ich bin schon der Meinung, Regelverstösse sollten geahndet werden

Notes from ASCO2016 published by Ohad Hammer
Zusammenfassung ASCO2016 von OH:http://www.orf-blog.com/notes-from-asco-2016/#more-1016
Cabo&Cobi haben auf ASCO2016 grosse Aufmerksamkeit genossen, dies wird auch in dieser Zusammenfassung mehr als deutlich.
"Exelixis – Cabo established as a potential market leader"
...bei Cabo wird die Chance auf Erstlinienbehandlung beim Nierenzellkarzinom nach ersten Daten von CABOSUN erwähnt, wodurch Blockbusterpotenzial erreicht werden könnte. Cabo übertrifft beim Progressionsfreien Überleben den Standard in 1L "Sutent", so dass der potenzielle (im Sinne von mögliche) Jahresumsatz auf ca. USD 1.5 Mrd beziffert wird.
Der synergistische Ansatz des MEK-Hemmers Cobimetinib mit Roches Checkpoint-Hemmer Atezolizumab lässt viel Raum für Spekulationen auf zukünftge Indikationen bzw. zukünftige Kombitherapien, denn
vorstellbar ist auch eine Synergie zu anderen Antikörpern? bspw. zu einem Angiogenesehemmer?
Es ist wie jedes Jahr auf ASCO bzw. in der Wissenschaft überhaupt: Die Beantwortung einer Frage induziert viele weitere Fragen.
die EXEL-Leerverkäufe sind um ca. 1Mio Anteile zurückgegangen:
http://www.nasdaq.com/symbol/exel/short-interest
http://www.nasdaq.com/symbol/exel/short-interest
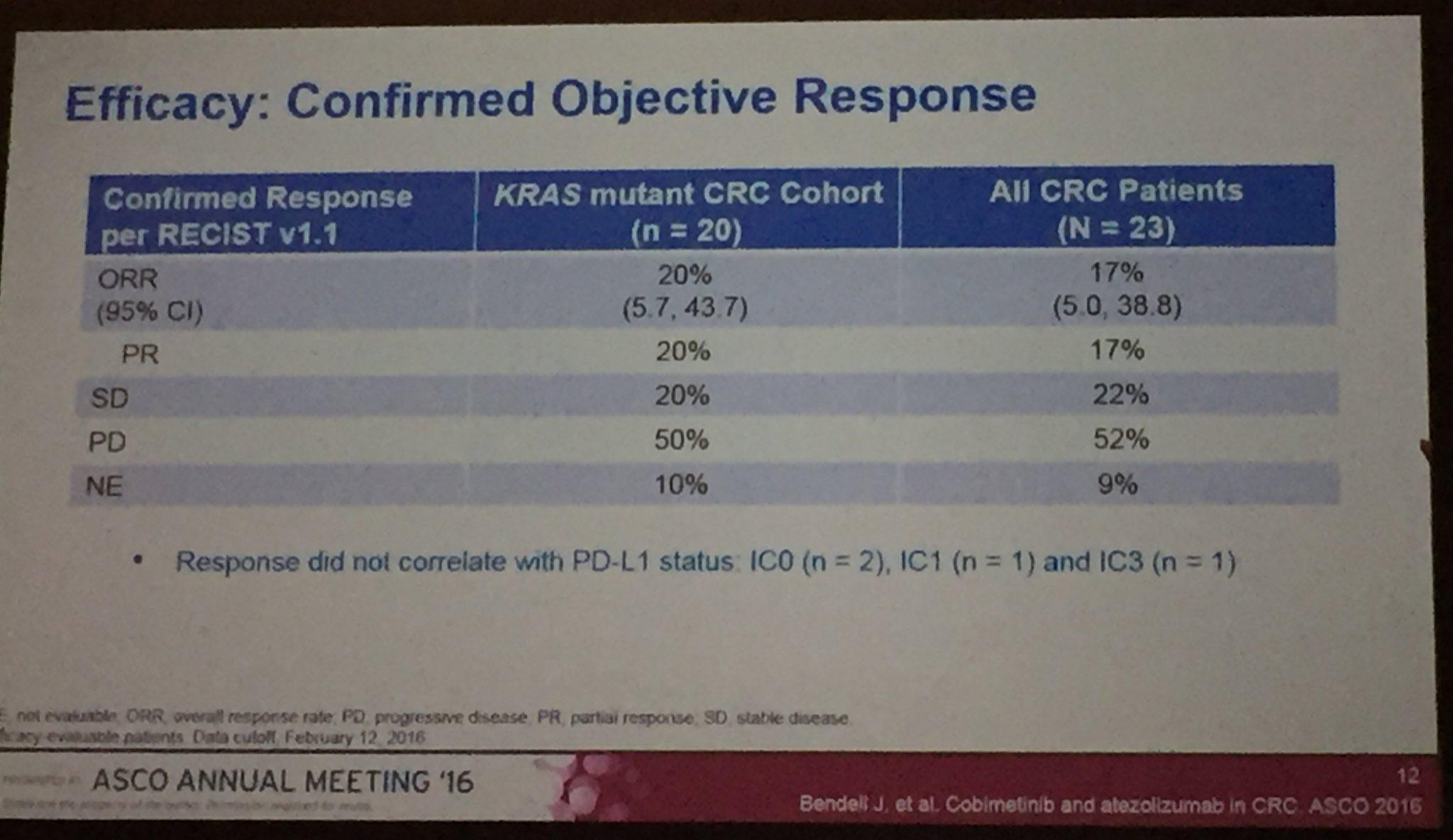
Die Ergebnisse der Kombistudie (Atezolizumab+Cobi) in 3L CRC (Darmkrebs), auf ASCO2016 vorgestellt, geben Anlass zu grossen Hoffnungen. Die Ansprechrate lag bei diesen fast austherapierten Patienten (Ansprechrate Chemo 3L ist, wenn ich mich recht erinnere, bei ca. 1%) bei immerhin 20% (KRAS mutiert) bzw. 17% bei der gesamten Stichprobe. Eine Phase3-Studie ist bereits initialisiert --> https://clinicaltrials.gov/ct2/show/NCT02788279?term=cobimet…. Es würde mich wundern, wenn nicht noch weitere (Pivotal-)Studien dieser Kombitherapie initialisiert würden, da in dieser ph1b-Studie ....
https://clinicaltrials.gov/ct2/show/NCT01988896?term=28363&r…
...nicht nur Darmkrebs-Patienten sondern auch Lungenkrebs- (NSCLC) und Hautkrebspatienten (Melanoma) teilnehmen:
Inclusion Criteria:
Signed Informed Consent Form.
Solid tumor that is metastatic, locally advanced or recurrent.
Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
Life expectancy greater than or equal to 12 weeks.
Measurable disease, as defined by Response Evaluation Criteria in Solid Tumors (RECIST) v 1.1.
Adequate hematologic and end organ function.
Use of highly effective contraception.
Histological tumor tissue specimen.
Participants enrolling in the indication-specific expansion cohorts in Stage 2 must consent to tumor biopsies and must have one of the following types of cancer:
Metatastic colorectal cancer
Non-small cell lung cancer
Melanoma.
Consent to undergo pre-treatment and/or on-treatment tumor biopsies for biomarker analysis for participants in biopsy-mandated cohorts.
Die Autoren sehen Cabo im Vorteil bei Patienten mit fortgeschrittener Erkrankung ("poor prognosis") während man Nivo aufgrund der verzögerten Wirkung der Immunaktivität bei weniger fortgeschrittenem Krankheitsverlauf den Vorzug gibt:
http://www.ncbi.nlm.nih.gov/pubmed/27271250
Nivolumab versus Cabozantinib: Comparing Overall Survival in Metastatic Renal Cell Carcinoma.
Wiecek W1, Karcher H1.
Author information
Abstract
Renal-cell carcinoma (RCC) affects over 330,000 new patients every year, of whom 1/3 present with metastatic RCC (mRCC) at diagnosis. Most mRCC patients treated with a first-line agent relapse within 1 year and need second-line therapy. The present study aims to compare overall survival (OS) between nivolumab and cabozantinib from two recent pivotal studies comparing, respectively, each one of the two emerging treatments against everolimus in patients who relapse following first-line treatment. Comparison is traditionally carried out using the Bucher method, which assumes proportional hazard. Since OS curves intersected in one of the pivotal studies, models not assuming proportional hazards were also considered to refine the comparison. Four Bayesian parametric survival network meta-analysis models were implemented on overall survival (OS) data digitized from the Kaplan-Meier curves reported in the studies. Three models allowing hazard ratios (HR) to vary over time were assessed against a fixed-HR model. The Bucher method favored cabozantinib, with a fixed HR for OS vs. nivolumab of 1.09 (95% confidence interval: [0.77, 1.54]). However, all models with time-varying HR showed better fits than the fixed-HR model. The log-logistic model fitted the data best, exhibiting a HR for OS initially favoring cabozantinib, the trend inverting to favor nivolumab after month 5 (95% credible interval <1 from 10 months). The initial probability of cabozantinib conferring superior OS was 54%, falling to 41.5% by month 24. Numerical differences in study-adjusted OS estimates between the two treatments remained small. This study evidences that HR for OS of nivolumab vs. cabozantinib varies over time, favoring cabozantinib in the first months of treatment but nivolumab afterwards, a possible indication that patients with poor prognosis benefit more from cabozantinib in terms of survival, nivolumab benefiting patients with better prognosis. More evidence, including real-world observational data, is needed to compare effectiveness between cabozantinib and nivolumab.
http://www.ncbi.nlm.nih.gov/pubmed/27271250
Nivolumab versus Cabozantinib: Comparing Overall Survival in Metastatic Renal Cell Carcinoma.
Wiecek W1, Karcher H1.
Author information
Abstract
Renal-cell carcinoma (RCC) affects over 330,000 new patients every year, of whom 1/3 present with metastatic RCC (mRCC) at diagnosis. Most mRCC patients treated with a first-line agent relapse within 1 year and need second-line therapy. The present study aims to compare overall survival (OS) between nivolumab and cabozantinib from two recent pivotal studies comparing, respectively, each one of the two emerging treatments against everolimus in patients who relapse following first-line treatment. Comparison is traditionally carried out using the Bucher method, which assumes proportional hazard. Since OS curves intersected in one of the pivotal studies, models not assuming proportional hazards were also considered to refine the comparison. Four Bayesian parametric survival network meta-analysis models were implemented on overall survival (OS) data digitized from the Kaplan-Meier curves reported in the studies. Three models allowing hazard ratios (HR) to vary over time were assessed against a fixed-HR model. The Bucher method favored cabozantinib, with a fixed HR for OS vs. nivolumab of 1.09 (95% confidence interval: [0.77, 1.54]). However, all models with time-varying HR showed better fits than the fixed-HR model. The log-logistic model fitted the data best, exhibiting a HR for OS initially favoring cabozantinib, the trend inverting to favor nivolumab after month 5 (95% credible interval <1 from 10 months). The initial probability of cabozantinib conferring superior OS was 54%, falling to 41.5% by month 24. Numerical differences in study-adjusted OS estimates between the two treatments remained small. This study evidences that HR for OS of nivolumab vs. cabozantinib varies over time, favoring cabozantinib in the first months of treatment but nivolumab afterwards, a possible indication that patients with poor prognosis benefit more from cabozantinib in terms of survival, nivolumab benefiting patients with better prognosis. More evidence, including real-world observational data, is needed to compare effectiveness between cabozantinib and nivolumab.
Exelixis ist unermüdlich bei der Sicherung des geistigen Eigentums - mit Datum Heute (14.6.2016) gibt es einen neuen Eintrag in der Datenbank für gewährte Patente:
United States Patent 9,365,516
Wilson , et al. June 14, 2016
Process for preparing quinoline derivatives
Abstract
A process for preparing a compound of Formula I is disclosed: ##STR00001## wherein: R.sup.1 is halo; R.sup.2 is halo; R.sup.3 is (C.sub.1-C.sub.6)alkyl or (C.sub.1-C.sub.6)alkyl optionally substituted with heterocycloalkyl; R.sup.4 is (C.sub.1-C.sub.6)alkyl; and Q is CH or N; comprising: (a) contacting 1,1-cyclopropane dicarboxylic acid with thionyl chloride in a polar aprotic solvent; (b) adding ##STR00002## and a tertiary amine base to the mixture of step (a) to form a compound of Formula A; and ##STR00003## (c) coupling a compound of Formula A with an amine of Formula B to form a compound of Formula I. ##STR00004##
Inventors: Wilson; Jo Ann (San Francisco, CA), Naganathan; Sriram (San Jose, CA), Andersen; Neil G. (Montara, CA), Pfeiffer; Matthew (Salt Lake City, UT)
Applicant:
Name City State Country Type
Exelixis, Inc.
South San Francisco
CA
US
Assignee: Exelixis, Inc. (South San Francisco, CA)
Family ID: 1000001906876
Appl. No.: 14/353,251
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
Natürlich hat ein Patent wie dieses kaum bis keinen Einfluss auf das Tagesgeschäft, und doch sind diese in der Summe sehr wichtig für den wirtschaftlichen Erfolg eines Unternehmens. Diesbezüglich ist Exelixis sehr vorbildlich, da das Unternehmen im Verhältnis zur eigenen Grösse über eine unglaubliche Anzahl von Patenten verfügt:
http://worldwide.espacenet.com/searchResults?submitted=true&…
United States Patent 9,365,516
Wilson , et al. June 14, 2016
Process for preparing quinoline derivatives
Abstract
A process for preparing a compound of Formula I is disclosed: ##STR00001## wherein: R.sup.1 is halo; R.sup.2 is halo; R.sup.3 is (C.sub.1-C.sub.6)alkyl or (C.sub.1-C.sub.6)alkyl optionally substituted with heterocycloalkyl; R.sup.4 is (C.sub.1-C.sub.6)alkyl; and Q is CH or N; comprising: (a) contacting 1,1-cyclopropane dicarboxylic acid with thionyl chloride in a polar aprotic solvent; (b) adding ##STR00002## and a tertiary amine base to the mixture of step (a) to form a compound of Formula A; and ##STR00003## (c) coupling a compound of Formula A with an amine of Formula B to form a compound of Formula I. ##STR00004##
Inventors: Wilson; Jo Ann (San Francisco, CA), Naganathan; Sriram (San Jose, CA), Andersen; Neil G. (Montara, CA), Pfeiffer; Matthew (Salt Lake City, UT)
Applicant:
Name City State Country Type
Exelixis, Inc.
South San Francisco
CA
US
Assignee: Exelixis, Inc. (South San Francisco, CA)
Family ID: 1000001906876
Appl. No.: 14/353,251
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
Natürlich hat ein Patent wie dieses kaum bis keinen Einfluss auf das Tagesgeschäft, und doch sind diese in der Summe sehr wichtig für den wirtschaftlichen Erfolg eines Unternehmens. Diesbezüglich ist Exelixis sehr vorbildlich, da das Unternehmen im Verhältnis zur eigenen Grösse über eine unglaubliche Anzahl von Patenten verfügt:
http://worldwide.espacenet.com/searchResults?submitted=true&…
...interessanter Beitrag zum Thema Blasenkrebs, in welchem der Tyrosin-Kinase-Hemmer Cabozantinib als vielversprechender Therapieansatz erwähnt ist. In 2 Studien (1ph1 + 1ph2) wird Cabo in dieser Indikation in der Klinik getestet, wobei beide Studien vom National Cancer Institute finanziert werden:
https://clinicaltrials.gov/ct2/results?term=cabozantinib+bla…
Oncology (Williston Park). 2016 Jun;30(6). pii: 217520.
New Agents for the Treatment of Advanced Bladder Cancer.
Cheetham PJ, Petrylak DP.
Abstract
Despite recent advances in the management of a wide variety of solid tumors, the outcomes for patients with metastatic urothelial carcinoma (MUC) remain extremely poor. Cisplatin-based combination chemotherapy remains the standard of care for first-line systemic treatment of MUC, and for more than 20 years there have been no other US Food and Drug Administration-approved treatment options available for these patients. Finally there appears to be hope on the horizon, with an ever-increasing number of precisely targeted agents being developed for use in MUC, resulting in improved survival rates. These targeted agents have now entered the cancer treatment arena, a direct result of a greater understanding of the genetic background of MUC. In this review article, we summarize the current state of development of these targeted agents, used either alone or in combination with traditional chemotherapy in MUC. Our discussion focuses on the most promising novel agents, including therapies targeting receptors for fibroblast growth factor and endothelial growth factor; antiangiogenesis agents (bevacizumab); tyrosine kinase inhibitors (cabozantinib); and immune checkpoint inhibitors that target proteins in the immune checkpoint-regulation pathway (anti-programmed death 1 and anti-programmed death ligand 1).
https://clinicaltrials.gov/ct2/results?term=cabozantinib+bla…
Oncology (Williston Park). 2016 Jun;30(6). pii: 217520.
New Agents for the Treatment of Advanced Bladder Cancer.
Cheetham PJ, Petrylak DP.
Abstract
Despite recent advances in the management of a wide variety of solid tumors, the outcomes for patients with metastatic urothelial carcinoma (MUC) remain extremely poor. Cisplatin-based combination chemotherapy remains the standard of care for first-line systemic treatment of MUC, and for more than 20 years there have been no other US Food and Drug Administration-approved treatment options available for these patients. Finally there appears to be hope on the horizon, with an ever-increasing number of precisely targeted agents being developed for use in MUC, resulting in improved survival rates. These targeted agents have now entered the cancer treatment arena, a direct result of a greater understanding of the genetic background of MUC. In this review article, we summarize the current state of development of these targeted agents, used either alone or in combination with traditional chemotherapy in MUC. Our discussion focuses on the most promising novel agents, including therapies targeting receptors for fibroblast growth factor and endothelial growth factor; antiangiogenesis agents (bevacizumab); tyrosine kinase inhibitors (cabozantinib); and immune checkpoint inhibitors that target proteins in the immune checkpoint-regulation pathway (anti-programmed death 1 and anti-programmed death ligand 1).
Ja, Cabo scheint eine Mehrfachwaffe gegen Krebs zu sein gut für die Menchen und gut uns Aktionäre, langfristig sehe ich zweistellige Kurse von 20-30 US Dollar, First line im Kampf gegen Nierenkrebs weltweit, Cobi gegen Hautkrebs, Cabo evtl. gegen Leberkrebs, Darmkrebs......
Übernahmeziel Nummer eins, hoffentlich nicht bei den lächerlichen Kursen wie derzeit!
Übernahmeziel Nummer eins, hoffentlich nicht bei den lächerlichen Kursen wie derzeit!
http://www.ema.europa.eu/docs/en_GB/document_library/Agenda/…
Cabo zur Behandlung von RCC ist nicht auf der Juni-Agenda (20. - 23.6.2016) des Arzneimittelausschusses CHMP. Das dauert dann halt doch wieder länger bei den Europäern!
Cabo zur Behandlung von RCC ist nicht auf der Juni-Agenda (20. - 23.6.2016) des Arzneimittelausschusses CHMP. Das dauert dann halt doch wieder länger bei den Europäern!
Die Kombistudie aus Cobi und Atezolizumab zur Behandlung von CRC (Darmkrebs) erfährt grosse Aufmerksamkeit: Der Median bei der Überlebenszeitanalyse nach Monaten erhöht sich von 1% auf 72% - "an incredible breakthrough" --> ein unglaublicher Durchbruch!
ASCO 2016 Update: Checkpoint Immunotherapy Advances in Solid Cancers June 06, 2016 | Arthur N. Brodsky, Ph.D.
Previously, CRC patients without heavily mutated tumors didn’t benefit from checkpoint immunotherapy. Fortunately, a new study presented today by Johanna Bendell, M.D., revealed that the combination of the checkpoint inhibitor atezolizumab that targets PD-L1 and cobimetinib, a MEK pathway inhibitor that targets growth pathways, produced responses that were higher than expected from either treatment alone based on results from past trials of the drugs as monotherapies. Importantly, the patients chosen for this had mutations that led to overactivity in the MEK-governed growth pathway. This combination also improved 6-month overall survival, from 1% in patients treated with the standard of care, to 72%. This represents an incredible breakthrough, and will hopefully pave the way for more effective approaches to treating these CRC patients.
http://www.cancerresearch.org/news-publications/our-blog/jun…
ASCO 2016 Update: Checkpoint Immunotherapy Advances in Solid Cancers June 06, 2016 | Arthur N. Brodsky, Ph.D.
Previously, CRC patients without heavily mutated tumors didn’t benefit from checkpoint immunotherapy. Fortunately, a new study presented today by Johanna Bendell, M.D., revealed that the combination of the checkpoint inhibitor atezolizumab that targets PD-L1 and cobimetinib, a MEK pathway inhibitor that targets growth pathways, produced responses that were higher than expected from either treatment alone based on results from past trials of the drugs as monotherapies. Importantly, the patients chosen for this had mutations that led to overactivity in the MEK-governed growth pathway. This combination also improved 6-month overall survival, from 1% in patients treated with the standard of care, to 72%. This represents an incredible breakthrough, and will hopefully pave the way for more effective approaches to treating these CRC patients.
http://www.cancerresearch.org/news-publications/our-blog/jun…
Antwort auf Beitrag Nr.: 52.672.694 von Cyberhexe am 22.06.16 19:28:59Die Kombistudie aus Cobi und Atezolizumab zur Behandlung von CRC (Darmkrebs) erfährt grosse Aufmerksamkeit: Der Median bei der Überlebenszeitanalyse nach 6 Monaten erhöht sich von 1% auf 72% - "an incredible breakthrough" --> ein unglaublicher Durchbruch!
Interessanter Beitrag zu Cabo aus einem wissenschaftlichen Journal:
Clinical Cancer Research, July 2016
Volume 22, Issue 13
Identification of existing drugs that effectively target NTRK1- and ROS1-rearrangements in lung cancer.
Curtis R. Chong, Magda Bahcall, Marzia Capelletti, Takayuki Kosaka, Dalia Ercan, Taebo Sim, Lynette M Sholl, Mizuki Nishino, Bruce E. Johnson, Nathanael S. Gray, Pasi A. Janne
PURPOSE:
Efforts to discover drugs that overcome resistance to targeted therapies in patients with rare oncogenic alterations, such as NTRK1- and ROS1-rearrangements, are complicated by the cost and protracted timeline of drug discovery.
EXPERIMENTAL DESIGN:
In an effort to identify inhibitors of NTRK1 and ROS1, which are aberrantly activated in some patients with non-small cell lung cancer (NSCLC), we created and screened a library of existing targeted drugs against Ba/F3 cells transformed with these oncogenes.
RESULTS:
This screen identified the FDA-approved drug cabozantinib as a potent inhibitor of CD74-ROS1 transformed Ba/F3, including the crizotinib-resistant mutants G2032R and L2026M (IC50 = 9, 26, and 11 nM, respectively). Cabozantinib inhibited CD74-ROS1-transformed Ba/F3 cells more potently than brigatinib (wild-type/G2032R/L2026M IC50 = 30/170/200 nM, respectively), entrectinib (IC50 = 6/2,200 /3,500 nM), and PF-06463922 (IC50 = 1/270/2 nM). Cabozantinib inhibited ROS1 autophosphorylation and downstream ERK activation in transformed Ba/F3 cells and in patient-derived tumor cell lines. The IGF-1R inhibitor BMS-536924, potently inhibited CD74-NTRK1 transformed compared to parental Ba/F3 cells (IC50 = 19 nM vs. > 470 nM). A patient with metastatic ROS1-rearranged NSCLC with progression on crizotinib was treated with cabozantinib and experienced a partial response.
CONCLUSIONS:
While acquired resistance to targeted therapies is challenging, this study highlights that existing agents may be repurposed to overcome drug resistance, and identifies cabozantinib as a promising treatment of ROS1-rearranged NSCLC after progression on crizotinib.
http://clincancerres.aacrjournals.org/content/early/2016/06/…
Clinical Cancer Research, July 2016
Volume 22, Issue 13
Identification of existing drugs that effectively target NTRK1- and ROS1-rearrangements in lung cancer.
Curtis R. Chong, Magda Bahcall, Marzia Capelletti, Takayuki Kosaka, Dalia Ercan, Taebo Sim, Lynette M Sholl, Mizuki Nishino, Bruce E. Johnson, Nathanael S. Gray, Pasi A. Janne
PURPOSE:
Efforts to discover drugs that overcome resistance to targeted therapies in patients with rare oncogenic alterations, such as NTRK1- and ROS1-rearrangements, are complicated by the cost and protracted timeline of drug discovery.
EXPERIMENTAL DESIGN:
In an effort to identify inhibitors of NTRK1 and ROS1, which are aberrantly activated in some patients with non-small cell lung cancer (NSCLC), we created and screened a library of existing targeted drugs against Ba/F3 cells transformed with these oncogenes.
RESULTS:
This screen identified the FDA-approved drug cabozantinib as a potent inhibitor of CD74-ROS1 transformed Ba/F3, including the crizotinib-resistant mutants G2032R and L2026M (IC50 = 9, 26, and 11 nM, respectively). Cabozantinib inhibited CD74-ROS1-transformed Ba/F3 cells more potently than brigatinib (wild-type/G2032R/L2026M IC50 = 30/170/200 nM, respectively), entrectinib (IC50 = 6/2,200 /3,500 nM), and PF-06463922 (IC50 = 1/270/2 nM). Cabozantinib inhibited ROS1 autophosphorylation and downstream ERK activation in transformed Ba/F3 cells and in patient-derived tumor cell lines. The IGF-1R inhibitor BMS-536924, potently inhibited CD74-NTRK1 transformed compared to parental Ba/F3 cells (IC50 = 19 nM vs. > 470 nM). A patient with metastatic ROS1-rearranged NSCLC with progression on crizotinib was treated with cabozantinib and experienced a partial response.
CONCLUSIONS:
While acquired resistance to targeted therapies is challenging, this study highlights that existing agents may be repurposed to overcome drug resistance, and identifies cabozantinib as a promising treatment of ROS1-rearranged NSCLC after progression on crizotinib.
http://clincancerres.aacrjournals.org/content/early/2016/06/…
man darf gespannt sein, ob der europ. Arzneimittelausschuss Cabo auf der Agenda der Heute beginnenden Juli-Sitzung hat:
http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/l…
Nachdem in der Mai-Sitzung die "List of questions" (D120) verabschiedet wurde, müsste eigentlich eine Entscheidung des CHMP anstehen, sofern der beschleunigte Ablauf beibehalten wurde und man nicht zum Standardablauf zurückgekehrt ist gemäss:
• CHMP list of questions to the applicant to address in writing and at an oral explanation if necessary with switch to a standard timetable (see 5.2)
http://www.ema.europa.eu/docs/en_GB/document_library/Scienti…
6. Timetable for the accelerated assessment procedure
6.1. Pre-submission phase
− 6 – 7 months before the actual submission of the marketing authorisation application: Notify the intention to submit a request for accelerated assessment as part of the letter of intent.
− Pre-submission meetings with the Rapporteurs and the EMA
− 2-3 months before the actual submission of the marketing authorisation application: Submission of request for accelerated assessment: ..
6.2. Accelerated assessment procedure
− Day 1 Start of the procedure.
− Days 1 – 90 First assessment phase:
• CHMP Rapporteurs’ assessment reports
• PRAC Rapporteur updated assessment report
• Peer-review
− Day 90 CHMP plenary meeting with adoption of either:
• CHMP positive opinion; or
• CHMP list of questions to the applicant to be addressed in writing and at an oral explanation if necessary with maintenance of the accelerated timetable. The CHMP may also adopt questions for a Scientific Advisory Group, as applicable; or
• CHMP list of questions to the applicant to address in writing and at an oral explanation if necessary with switch to a standard timetable (see 5.3).
− Stop of the clock: One month stop of the clock by default.
− Clarification meeting will be planned shortly after adoption of the list of questions.
− Day 91 Restart of the clock following submission of the applicant’s written responses.
− Days 91 – 120 Second assessment phase
• CHMP and PRAC assessment report of the responses
− Day 120 CHMP plenary meeting with adoption of either:
• CHMP positive opinion; or
• CHMP list of questions to the applicant to address in writing and at an oral explanation if necessary with maintenance of the accelerated timetable; or
• CHMP list of questions to the applicant to address in writing and at an oral explanation if necessary with switch to a standard timetable (see 5.2)
− No Stop of the clock: The CHMP would request the submission of the written responses without clock-stop
− D121 Submission of written responses
− Days 121 – 150 Third assessment phase:
• CHMP and PRAC assessment report of the responses
− Day 150 CHMP opinion
http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/l…
Nachdem in der Mai-Sitzung die "List of questions" (D120) verabschiedet wurde, müsste eigentlich eine Entscheidung des CHMP anstehen, sofern der beschleunigte Ablauf beibehalten wurde und man nicht zum Standardablauf zurückgekehrt ist gemäss:
• CHMP list of questions to the applicant to address in writing and at an oral explanation if necessary with switch to a standard timetable (see 5.2)
http://www.ema.europa.eu/docs/en_GB/document_library/Scienti…
6. Timetable for the accelerated assessment procedure
6.1. Pre-submission phase
− 6 – 7 months before the actual submission of the marketing authorisation application: Notify the intention to submit a request for accelerated assessment as part of the letter of intent.
− Pre-submission meetings with the Rapporteurs and the EMA
− 2-3 months before the actual submission of the marketing authorisation application: Submission of request for accelerated assessment: ..
6.2. Accelerated assessment procedure
− Day 1 Start of the procedure.
− Days 1 – 90 First assessment phase:
• CHMP Rapporteurs’ assessment reports
• PRAC Rapporteur updated assessment report
• Peer-review
− Day 90 CHMP plenary meeting with adoption of either:
• CHMP positive opinion; or
• CHMP list of questions to the applicant to be addressed in writing and at an oral explanation if necessary with maintenance of the accelerated timetable. The CHMP may also adopt questions for a Scientific Advisory Group, as applicable; or
• CHMP list of questions to the applicant to address in writing and at an oral explanation if necessary with switch to a standard timetable (see 5.3).
− Stop of the clock: One month stop of the clock by default.
− Clarification meeting will be planned shortly after adoption of the list of questions.
− Day 91 Restart of the clock following submission of the applicant’s written responses.
− Days 91 – 120 Second assessment phase
• CHMP and PRAC assessment report of the responses
− Day 120 CHMP plenary meeting with adoption of either:
• CHMP positive opinion; or
• CHMP list of questions to the applicant to address in writing and at an oral explanation if necessary with maintenance of the accelerated timetable; or
• CHMP list of questions to the applicant to address in writing and at an oral explanation if necessary with switch to a standard timetable (see 5.2)
− No Stop of the clock: The CHMP would request the submission of the written responses without clock-stop
− D121 Submission of written responses
− Days 121 – 150 Third assessment phase:
• CHMP and PRAC assessment report of the responses
− Day 150 CHMP opinion
CHMP Juli-Sitzung
Spätestens am Freitag werden wir erfahren, ob der Arzneimittelausschuss CHMP Cabo zur Behandlung von RCC 2L zur Zulassung empfiehlt. Alles andere als eine Zulassungsempfehlung wäre eine grosse Überraschung, weshalb der Kurs auch nicht sehr ausgeprägt auf eine positive Empfehlung reagieren sollte. Bei einer negativen Empfehlung bzw. Verzögerung allerdings würde der Kurs spürbar nachgeben.http://www.ema.europa.eu/docs/en_GB/document_library/Agenda/…
3.1.1. cabozantinib - EMEA/H/C/004163
Accelerated assessment
treatment of advanced renal cell carcinoma (RCC)
Scope: Opinion
Action: For adoption
List of Questions adopted on 26.05.2016.
Leerink Swann Reiterates “Buy” Rating for Exelixis Inc. (EXEL)
July 19th, 2016 • 0 comments • Filed Under • by ABMN Staff
Exelixis logoExelixis Inc. (NASDAQ:EXEL)‘s stock had its “buy” rating reiterated by Leerink Swann in a note issued to investors on Tuesday. They currently have a $10.00 target price on the biotechnology company’s stock. Leerink Swann’s target price suggests a potential upside of 18.76% from the stock’s previous close.
Möglicherweise sind die "double digits" bereits am Freitag fällig, nachdem der europ Arzneimittelausschuss (CHMP) Cabo zur Zulassung empfohlen hat und zwar zur Zweitlinienbehandlung von fortgeschrittenem Nierenkrebs.
July 19th, 2016 • 0 comments • Filed Under • by ABMN Staff
Exelixis logoExelixis Inc. (NASDAQ:EXEL)‘s stock had its “buy” rating reiterated by Leerink Swann in a note issued to investors on Tuesday. They currently have a $10.00 target price on the biotechnology company’s stock. Leerink Swann’s target price suggests a potential upside of 18.76% from the stock’s previous close.
Möglicherweise sind die "double digits" bereits am Freitag fällig, nachdem der europ Arzneimittelausschuss (CHMP) Cabo zur Zulassung empfohlen hat und zwar zur Zweitlinienbehandlung von fortgeschrittenem Nierenkrebs.
ESMO-Kongress in Kopenhagen (7.10. - 11.10.2016)
Cabozantinib gemeinsam mit Checkpoint-Hemmern - auf dem ESMO-Kongress gibts Ergebnisse:Genitourinary tumours, non-prostate
Session Type
Poster Discussion session
Details
ESMO 2016 Congress, 09.10.2016, 16:30 - 17:30, Athens
Download Pdf Calendar Export Share
774PD - A phase I study of cabozantinib plus nivolumab (CaboNivo) in patients (pts) refractory metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors
A. B. Apolo (Bethesda, United States of America) A. Mortazavi (Columbus, United States of America) M. Stein (New Brunswick, United States of America) S. K. Pal (Duarte, United States of America) N. Davarpanah (Bethesda, United States of America) H. L. Parnes (Bethesda, United States of America) Y. M. Ning (Bethesda, United States of America) D. C. Francis (Bethesda, United States of America) L. M. Cordes (Bethesda, United States of America) M. Berniger (Bethesda, United States of America) S. M. Steinberg (Bethesda, United States of America) P. Monk (Columbus, United States of America) T. Lancaster (Columbus, United States of America) T. Mayer (New Brunswick, United States of America) R. Costello (Bethesda, United States of America) D. P. Bottaro (Bethesda, United States of America) W. L. Dahut (Bethesda, United States of America)
...auserdem zu Cabo:
787P - A phase II study of cabozantinib in patients (pts) with relapsed/refractory metastatic urothelial carcinoma
814P - Efficacy of cabozantinib (cabo) vs everolimus (eve) by metastatic site and tumor burden in patients (pts) with advanced renal cell carcinoma (RCC) in the phase 3 METEOR trial
815P - Evaluation of the novel “trial within a trial” design of METEOR, a randomized phase 3 trial of cabozantinib versus everolimus in patients (pts) with advanced renal cell carcinoma (RCC)
816P - Quality of life (QoL) in the phase 3 METEOR trial of cabozantinib vs everolimus for advanced renal cell carcinoma (RCC)
818P - Analysis of regional differences in the phase 3 METEOR study of cabozantinib (cabo) versus everolimus (eve) in advanced renal cell carcinoma (RCC)
1421TiP - A randomized double-blind phase II study evaluating the role of maintenance therapy with cabozantinib in high grade undifferentiated uterine sarcoma (HGUS) after stabilization or response to doxorubicin +/- ifosfamide following surgery or in metastatic first line treatment
Cobimetinib gemeinsam mit Checkpoint-Hemmern - auf dem ESMO-Kongress gibts weitere Ergebnisse:
1109PD - Preliminary safety and clinical activity of atezolizumab combined with cobimetinib and vemurafenib in BRAF V600-mutant metastatic melanoma
ausserdem zu Cobi:
1111PD - Genomic features of complete responders (CR) versus fast progressors (PD) in patients with BRAFV600-mutated metastatic melanoma treated with cobimetinib + vemurafenib or vemurafenib alone
von grosser Bedeutung:
286P - First-line cobimetinib (C) + paclitaxel (P) in patients (pts) with advanced triple-negative breast cancer (TNBC): Updated results and tumoral immune cell infiltration data from the phase 2 COLET study
1142P - Prognostic subgroups and impact of treatment for post-progression overall survival (ppOS) in patients (pts) with BRAFV600-mutated metastatic melanoma treated with dacarbazine (DTIC) or vemurafenib (VEM) ± cobimetinib (COBI): A pooled analysis
...dazu noch late-breaking abstracts (LBA)!
offene Stellen bei Ipsen Deutschland:
https://ipsen-pharma.de/files/mannheim-freiburg_1.pdfIhre Aufgabe:
Mit Cabozantinib hat IPSEN einen innovativen Wirkstoff im Bereich Onkologie/solide Tumore erworben und bereitet nun die Markteinführung in der Indikation „Nierenzellkarzinom" vor.
https://ipsen-pharma.de/startseite/karriere/stellenangebote/…
...es wird aber auch höchste Zeit, da bereits Morgen mit grösster Wahrscheinlichkeit die Zulassungsempfehlung des Arzneimiitelausschusses CHMP bekanntgegeben wird. Die Kommission wird dann nach spätestens 67 Tagen die gesamteuropäische Marktzulassung erteilen - normalerweise folgt die Kommission der Empfehlung des CHMP.
Antwort auf Beitrag Nr.: 52.879.342 von Cyberhexe am 21.07.16 08:01:59...wie erwartet, der Arzneimittelausschuss der EMA (CHMP) hat eine Zulassungsempfehlung ausgesprochen:
http://www.ema.europa.eu/docs/en_GB/document_library/Summary…
http://www.ema.europa.eu/docs/en_GB/document_library/Summary…
EMA Newsletter
Two new medicines for advanced kidney cancer Cabometyx and Kisplyx provide additional treatment options for patients with unmet medical need
The European Medicines Agency (EMA) has recommended granting marketing authorisations in the
European Union (EU) for two medicines for the treatment of advanced
renal cell carcinoma (kidney cancer).
Cabometyx (cabozantinib) and Kisplyx (lenvatinib) are indicated for the treatment of adult patients with advanced renal cell carcinoma who have been previously treated with a
vascular endothelial growth factor (VEGF)inhibitor; Cabometyx is to be used as monotherapy while Kisplyx is for use in combination with everolimus.
Renal cell carcinoma is the most common form of kidney cancer in adults. Advanced renal cell
carcinoma includes both metastatic disease and locally advanced renal cell carcinoma that cannot be removed by surgery. Despite the recent approval of new therapies for advanced
renal cell carcinoma, many patients who do not respond to the existing treatments
have a poor prognosis. Therefore new treatment options are needed
Both Cabometyx and Kisplyx are tyrosine kinase inhibitors.
This means that they work by blocking certain enzymes known as tyrosine kinases. These enzymes can be found in some receptors on the surface of cancer cells and are involved in the growth and spread of cancer cells, and in the blood vessels that supply the tumours.
Both active substances are already approved in the EU as different medicines for the treatment of
thyroid cancer , an orphan condition.
In December 2013, EMA recommended for approval anothercabozantinib-
containing medicine (Cometriq) to treat adults with medullary thyroid cancer. Another lenvatinib-
containing medicine(Lenvima) was recommended for approval for the treatment of patients
with thyroid carcinoma in March 2015.
For the treatment of renal cell carcinoma, both medicines were reviewed under EMA’s accelerated
assessment programme, as they target patients with an unmet medical need.
The main study on which Cabometyx’s recommendation is based is a phase III trial involving 658
patients with metastatic renal cell carcinoma that had progressed after prior VEGF receptor
tyrosine kinase inhibitor therapy. In this study, patients treated with Cabometyx had a longer
period of time without their disease progressing(progression-free survival)compared to patients treated with everolimus (7.4 months compared to 3.8 months). In addition,preliminary results showed that patients treated with Cabometyx lived longer than patients treated with everolimus alone(median of 21.4 months compared to 16.5 months).
The most frequent adverse reactions associated with cabozantinib include diarrhoea, fatigue, nausea, decreased appetite, palmar-Plantar erythrodysaesthesia syndrome (hands and
feet redness, swelling and pain), hypertension and vomiting.
The Committee for Medicinal Products for Human Use (CHMP) concluded that the benefit/risk
balance of Cabometyx is positive .
Ich habe letzte Woche 3/4 meiner EXEL-Aktien zu Gunsten von Progenics liquidiert.
Progenics gibt es gar keinen aktuellen Thread bisher. Bin vorsichtig, wieviel da aktuell eingepreist ist. Hältst du einen Verkauf von Relistor an zB AGN in Höhe von 500mio für wahrscheinlich?
MFG
MFG
Antwort auf Beitrag Nr.: 53.055.787 von biopadawan am 14.08.16 09:24:11
Ich weiss nicht, wie realistisch ein Verkauf von Relistor derzeit ist, Valeant sollte jedoch sowohl das know how als auch die Grösse haben, damit einen Verkaufserfolg zu realisieren.
Dies wäre sicherlich im Interesse von PGNX, da das Unternehmen am Verkaufserfolg folgendermassen partizipiert:
...Under our Agreement with Valeant, we recognized in the third quarter of 2014 a $40 million development milestone for the chronic non-cancer pain indication approval, and remain eligible to receive (i) a development milestone of up to $50 million upon U.S. marketing approval of an oral formulation of RELISTOR (USD50m bezahlt am 26.7.16), and (ii)up to $200 million of commercialization milestone payments upon achievement of specified U.S. sales targets, ranging from $10 million when calendar-year U.S. net sales first exceed $100 million, to $75 million when such sales first exceed $1 billion. We also receive royalties of 15% of calendar-year worldwide net sales by Valeant and its affiliates up to $100 million, and are eligible to receive (i) 17% on the next $400 million of such sales, and 19% on such sales over $500 million, and (ii) 60% of any upfront, milestone, reimbursement or other revenue ...
Die Umsatzschätzungen für Relistor (oral+sc) reichen von USD 200m bis > 1b/y
Die Lizenzgebühren davon in Höhe von 15-19% sind sehr üppig, so dass das Unterhemen bei einer MC von derzeit USD 467.5m nach unten sehr gut abgesichert erscheint.
Die nicht uninteressante Pipeline mit viel versprechenden Aktivsubstanzen zur Behandlung und Diagnostik von Prostatakrebs sowie AZEDRA zur Behandlung sehr seltener Erkrankungen (pheochromocytoma und paragangoma) verspricht ebenfalls Wachstumspotenzial. Am 30.07.2015 hat die US Food and Drug Administration (FDA) für Azedra Breakthrough-Status gewährt, um Patienten mit iobenguane-avid metastasiertem oder rezidivierendem Phäochromozytom und Paragangliom zu behandeln. Die Daten aus der zulassungsrelevanten ph2-Studie werden Ende 2016/Anfang 2017 erwartet.
Positiv: Institutionelle Anleger halten derzeit über 80% des Unternehmens.
Zitat von biopadawan: Progenics gibt es gar keinen aktuellen Thread bisher. Bin vorsichtig, wieviel da aktuell eingepreist ist. Hältst du einen Verkauf von Relistor an zB AGN in Höhe von 500mio für wahrscheinlich?
MFG
Ich weiss nicht, wie realistisch ein Verkauf von Relistor derzeit ist, Valeant sollte jedoch sowohl das know how als auch die Grösse haben, damit einen Verkaufserfolg zu realisieren.
Dies wäre sicherlich im Interesse von PGNX, da das Unternehmen am Verkaufserfolg folgendermassen partizipiert:
...Under our Agreement with Valeant, we recognized in the third quarter of 2014 a $40 million development milestone for the chronic non-cancer pain indication approval, and remain eligible to receive (i) a development milestone of up to $50 million upon U.S. marketing approval of an oral formulation of RELISTOR (USD50m bezahlt am 26.7.16), and (ii)up to $200 million of commercialization milestone payments upon achievement of specified U.S. sales targets, ranging from $10 million when calendar-year U.S. net sales first exceed $100 million, to $75 million when such sales first exceed $1 billion. We also receive royalties of 15% of calendar-year worldwide net sales by Valeant and its affiliates up to $100 million, and are eligible to receive (i) 17% on the next $400 million of such sales, and 19% on such sales over $500 million, and (ii) 60% of any upfront, milestone, reimbursement or other revenue ...
Die Umsatzschätzungen für Relistor (oral+sc) reichen von USD 200m bis > 1b/y
Die Lizenzgebühren davon in Höhe von 15-19% sind sehr üppig, so dass das Unterhemen bei einer MC von derzeit USD 467.5m nach unten sehr gut abgesichert erscheint.
Die nicht uninteressante Pipeline mit viel versprechenden Aktivsubstanzen zur Behandlung und Diagnostik von Prostatakrebs sowie AZEDRA zur Behandlung sehr seltener Erkrankungen (pheochromocytoma und paragangoma) verspricht ebenfalls Wachstumspotenzial. Am 30.07.2015 hat die US Food and Drug Administration (FDA) für Azedra Breakthrough-Status gewährt, um Patienten mit iobenguane-avid metastasiertem oder rezidivierendem Phäochromozytom und Paragangliom zu behandeln. Die Daten aus der zulassungsrelevanten ph2-Studie werden Ende 2016/Anfang 2017 erwartet.
Positiv: Institutionelle Anleger halten derzeit über 80% des Unternehmens.
Hi Hexe,
interessanter Wert, Deine PGNX, ich fühl mich mit Exelixis sehr wohl, die Reisen nach oben ist noch lange nicht zu Ende, evtl. first line bei Nierenkrebs....., vielleicht eine zweite Medivation?
interessanter Wert, Deine PGNX, ich fühl mich mit Exelixis sehr wohl, die Reisen nach oben ist noch lange nicht zu Ende, evtl. first line bei Nierenkrebs....., vielleicht eine zweite Medivation?
Curr Opin Urol. 2016 Sep;26(5):439-46. doi: 10.1097/MOU.0000000000000319.
Advances in treatment of metastatic renal cell carcinoma.
Gong J1, Gerendash B, Dizman N, Khan A, Pal SK.
Author information
Abstract
PURPOSE OF REVIEW:
Multiple agents, including vascular endothelial growth factor (VEGF) inhibitors and mammalian target of rapamycin inhibitors have been approved over the past decade for the treatment of metastatic renal cell carcinoma (mRCC). Here, we focus on nivolumab, cabozantinib, and lenvatinib plus everolimus, agents that have recently emerged with positive clinical data leading to 'Food and Drug Administration approval or pending approval in mRCC. We also review the development of novel agents of interest showing promise in mRCC as part of combination therapy'.
RECENT FINDINGS:
Nivolumab and cabozantinib both offer improved survival over everolimus in the second-line treatment of mRCC. Lenvatinib plus everolimus has similarly shown encouraging survival benefits in a phase II trial for the second-line setting. Novel combinations in mRCC, including dual immune checkpoint blockade, VEGF and programmed death 1 inhibition, VEGF and vaccine therapy, dual angiogenic blockade, and VEGF-directed therapy with nanoparticle-containing camptothecin have shown promising activity in early-phase trials.
SUMMARY:
Multiple promising agents are available in the treatment of mRCC. The appropriate sequencing of agents in the treatment of mRCC may become further elucidated by future studies that prospectively analyze potential biomarkers to identify patients who will derive the greatest benefit from VEGF, mammalian target of rapamycin, or checkpoint inhibitors.
Advances in treatment of metastatic renal cell carcinoma.
Gong J1, Gerendash B, Dizman N, Khan A, Pal SK.
Author information
Abstract
PURPOSE OF REVIEW:
Multiple agents, including vascular endothelial growth factor (VEGF) inhibitors and mammalian target of rapamycin inhibitors have been approved over the past decade for the treatment of metastatic renal cell carcinoma (mRCC). Here, we focus on nivolumab, cabozantinib, and lenvatinib plus everolimus, agents that have recently emerged with positive clinical data leading to 'Food and Drug Administration approval or pending approval in mRCC. We also review the development of novel agents of interest showing promise in mRCC as part of combination therapy'.
RECENT FINDINGS:
Nivolumab and cabozantinib both offer improved survival over everolimus in the second-line treatment of mRCC. Lenvatinib plus everolimus has similarly shown encouraging survival benefits in a phase II trial for the second-line setting. Novel combinations in mRCC, including dual immune checkpoint blockade, VEGF and programmed death 1 inhibition, VEGF and vaccine therapy, dual angiogenic blockade, and VEGF-directed therapy with nanoparticle-containing camptothecin have shown promising activity in early-phase trials.
SUMMARY:
Multiple promising agents are available in the treatment of mRCC. The appropriate sequencing of agents in the treatment of mRCC may become further elucidated by future studies that prospectively analyze potential biomarkers to identify patients who will derive the greatest benefit from VEGF, mammalian target of rapamycin, or checkpoint inhibitors.
Exelixis Announces Outcome from First Planned Interim Analysis of the Phase 3 CELESTIAL Trial of Cabozantinib in Patients with Advanced Hepatocellular Carcinoma
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Sep. 6, 2016-- Exelixis, Inc. (NASDAQ:EXEL) today announced the outcome from the first planned interim analysis of CELESTIAL, a randomized global phase 3 trial of cabozantinib compared with placebo in patients with advanced hepatocellular carcinoma (HCC) who have been previously treated with sorafenib. Following this interim analysis, which was scheduled to take place when 50 percent of the events for the primary endpoint of overall survival (OS) had occurred, the trial’s Independent Data Monitoring Committee (IDMC) determined that the study should continue without modifications per the study protocol. The trial protocol calls for a second interim analysis to take place once 75 percent of events have been observed.
Ein Independent Data Monitoring Committee (IDMC) ist ein unabhängiges Gremium mit mindestens einem Statistiker, deren Mitglieder in keiner Weise in die betreffende klinische Studie involviert sind. Die Studie wird ausserhalb des Gremiums nicht entblindet, so dass keine Verletzung des Studienprotokolls stattfindet. Die im Studienprotokoll vereinbarten Zwischenauswertungen sollen in erster Linie verhindern, dass eine nicht wirksame Substanz weiterhin an Studienteilnehmer verabreicht wird sowie der Kontrolle, ob schwerwiegende Nebenwirkungen in einer grösseren Studie beobachtet werden. In den allerwenigsten Fällen wird bereits in diesen Zwischenauswertungen ein statistischer Nachweis über die Wirksamkeit erbracht . Hierfür sind die statistischen Hürden meistens viel zu hoch und die Power der Studie nicht ausreichend. Häufiger ist hingegen, dass der Abbruch einer Studie entweder aufgrund beobachteter Nebenwirkungen oder ober wegen ungenügender Wirksamkeit empfohlen wird. Dies ist bei CELESTIAL nicht der Fall. Dies ist positiv zu werten.
Passend zum Thema eine Studie veröffentlicht im "Expert Review of Anticancer Therapy", in welcher zu ergründen versucht wird, weshalb derart viele Studien bei Leberkrebs (HCC) nicht erfolgreich sind. Als Hauptgründe werden folgende Punkte aufgeführt:
Systemic treatment of hepatocellular carcinoma: why so many failures in the development of new drugs?
Key issues
Regorafenib has been the first drug, after sorafenib, to produce a positive result in a phase III trial conducted in patients with advanced hepatocellular carcinoma, while many drugs produced negative results despite the interesting background and rationale.
Heterogeneity of study populations, lack of understanding of critical drivers of tumor progression/dissemination, risk of liver toxicity associated with experimental agents, flaws in trial design and marginal antitumoral potency can be considered the main reasons for failure of phase III clinical trials in HCC.
Most of the ongoing trials are conducted without any molecular selection criteria. This is disappointing, because many drugs could be probably better tested in a molecularly selected population.
Hopefully, in the near future, the knowledge of potential predictive factors for drug efficacy in patients with advanced HCC will increase, and this could improve the chance of obtaining positive results in clinical trials.
The randomized phase III trial comparing nivolumab vs. sorafenib, that is currently ongoing, will provide evidence about the efficacy of the immune checkpoint inhibition strategy in advanced HCC.
4.4. Cabozantinib
Cabozantinib is a novel oral multiple tyrosine-kinase targeted agent that can inhibit both VEGFR2 and c-Met simultaneously. Cabozantinib can also downregulate KIT, RET, FLT3, and Tie-2 [42 Xiang Q, Chen W, Ren M, et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res. 2014;20:2959–2970.]. In the trial reported by Cohn et al. [43 Cohn AL, Kelley RK, Yang TS, et al. Activity of cabozantinib (XL184) in hepatocellular carcinoma patients (pts): results from a phase II randomized discontinuation trial (RDT). J Clin Oncol. 2012;30:261.], 41 patients with advanced HCC and Child–Pugh class A who received ≤1 prior systemic regimen were enrolled. Half of them had received prior sorafenib. In the lead-in stage, the patients received 100 mg daily over 12 weeks. According to the original RECIST criteria, 2 of 36 patients who were evaluable for the tumor assessment at 12 weeks achieved a confirmed partial response. The overall DCR (partial response + stable disease) at 12 weeks was 68%. Based on these results, a randomized phase III trial is currently comparing cabozantinib versus placebo as second-line treatment of patients with advanced HCC (ClinicalTrials.gov Identifier NCT01908426).
http://www.tandfonline.com/doi/abs/10.1080/14737140.2016.122…
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Sep. 6, 2016-- Exelixis, Inc. (NASDAQ:EXEL) today announced the outcome from the first planned interim analysis of CELESTIAL, a randomized global phase 3 trial of cabozantinib compared with placebo in patients with advanced hepatocellular carcinoma (HCC) who have been previously treated with sorafenib. Following this interim analysis, which was scheduled to take place when 50 percent of the events for the primary endpoint of overall survival (OS) had occurred, the trial’s Independent Data Monitoring Committee (IDMC) determined that the study should continue without modifications per the study protocol. The trial protocol calls for a second interim analysis to take place once 75 percent of events have been observed.
Ein Independent Data Monitoring Committee (IDMC) ist ein unabhängiges Gremium mit mindestens einem Statistiker, deren Mitglieder in keiner Weise in die betreffende klinische Studie involviert sind. Die Studie wird ausserhalb des Gremiums nicht entblindet, so dass keine Verletzung des Studienprotokolls stattfindet. Die im Studienprotokoll vereinbarten Zwischenauswertungen sollen in erster Linie verhindern, dass eine nicht wirksame Substanz weiterhin an Studienteilnehmer verabreicht wird sowie der Kontrolle, ob schwerwiegende Nebenwirkungen in einer grösseren Studie beobachtet werden. In den allerwenigsten Fällen wird bereits in diesen Zwischenauswertungen ein statistischer Nachweis über die Wirksamkeit erbracht . Hierfür sind die statistischen Hürden meistens viel zu hoch und die Power der Studie nicht ausreichend. Häufiger ist hingegen, dass der Abbruch einer Studie entweder aufgrund beobachteter Nebenwirkungen oder ober wegen ungenügender Wirksamkeit empfohlen wird. Dies ist bei CELESTIAL nicht der Fall. Dies ist positiv zu werten.
Passend zum Thema eine Studie veröffentlicht im "Expert Review of Anticancer Therapy", in welcher zu ergründen versucht wird, weshalb derart viele Studien bei Leberkrebs (HCC) nicht erfolgreich sind. Als Hauptgründe werden folgende Punkte aufgeführt:
Systemic treatment of hepatocellular carcinoma: why so many failures in the development of new drugs?
Key issues
Regorafenib has been the first drug, after sorafenib, to produce a positive result in a phase III trial conducted in patients with advanced hepatocellular carcinoma, while many drugs produced negative results despite the interesting background and rationale.
Heterogeneity of study populations, lack of understanding of critical drivers of tumor progression/dissemination, risk of liver toxicity associated with experimental agents, flaws in trial design and marginal antitumoral potency can be considered the main reasons for failure of phase III clinical trials in HCC.
Most of the ongoing trials are conducted without any molecular selection criteria. This is disappointing, because many drugs could be probably better tested in a molecularly selected population.
Hopefully, in the near future, the knowledge of potential predictive factors for drug efficacy in patients with advanced HCC will increase, and this could improve the chance of obtaining positive results in clinical trials.
The randomized phase III trial comparing nivolumab vs. sorafenib, that is currently ongoing, will provide evidence about the efficacy of the immune checkpoint inhibition strategy in advanced HCC.
4.4. Cabozantinib
Cabozantinib is a novel oral multiple tyrosine-kinase targeted agent that can inhibit both VEGFR2 and c-Met simultaneously. Cabozantinib can also downregulate KIT, RET, FLT3, and Tie-2 [42 Xiang Q, Chen W, Ren M, et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res. 2014;20:2959–2970.]. In the trial reported by Cohn et al. [43 Cohn AL, Kelley RK, Yang TS, et al. Activity of cabozantinib (XL184) in hepatocellular carcinoma patients (pts): results from a phase II randomized discontinuation trial (RDT). J Clin Oncol. 2012;30:261.], 41 patients with advanced HCC and Child–Pugh class A who received ≤1 prior systemic regimen were enrolled. Half of them had received prior sorafenib. In the lead-in stage, the patients received 100 mg daily over 12 weeks. According to the original RECIST criteria, 2 of 36 patients who were evaluable for the tumor assessment at 12 weeks achieved a confirmed partial response. The overall DCR (partial response + stable disease) at 12 weeks was 68%. Based on these results, a randomized phase III trial is currently comparing cabozantinib versus placebo as second-line treatment of patients with advanced HCC (ClinicalTrials.gov Identifier NCT01908426).
http://www.tandfonline.com/doi/abs/10.1080/14737140.2016.122…
spätestens 67 Tage nach der Zulassungsempfehlung des Arzneimittelausschusses CHMP sollte die Europäische Kommission ein Präparat zulassen und für den gesamteuropäischen Markt (beim zentralisierten Zulassungsverfahren) freigeben.
Im Falle von Cabometyx (cabozantinib) zur Behandlung von Nierenkrebs (RCC) ergibt sich folgendes potenzielles Zulassungsdatum:
Bekanntgabe der positiven Empfehlung durch CHMP am 22.07.2016
http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_e…
67 Tage --> spätestens am 27.09.2016 sollte Cabometyx (cabozantinib) für die Vermarktung zugelassen sein. Da IPSENs Vorbereitungen zur Marfkteinführung seit längerer Zeit lanciert werden, sollte Cabometyx (cabozantinib ) auch am europäischen Markt kurzfristig zur Verfügung stehen.
Im Falle von Cabometyx (cabozantinib) zur Behandlung von Nierenkrebs (RCC) ergibt sich folgendes potenzielles Zulassungsdatum:
Bekanntgabe der positiven Empfehlung durch CHMP am 22.07.2016
http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_e…
67 Tage --> spätestens am 27.09.2016 sollte Cabometyx (cabozantinib) für die Vermarktung zugelassen sein. Da IPSENs Vorbereitungen zur Marfkteinführung seit längerer Zeit lanciert werden, sollte Cabometyx (cabozantinib ) auch am europäischen Markt kurzfristig zur Verfügung stehen.
Die Leerverkäufe sind zurück gegangen...
...und zwar innerhalb von 16 Tagen von 10.136m auf 8.159m Aktien:8/31/2016 8,158,907 1,072,703 7.605933
8/15/2016 10,136,448 1,722,819 5.883641
7/29/2016 9,206,470 3,702,457 2.486584
Antwort auf Beitrag Nr.: 53.263.170 von Cyberhexe am 13.09.16 07:39:52sorry...da bin ich im falschen Thread!
Antwort auf Beitrag Nr.: 53.263.179 von Cyberhexe am 13.09.16 07:41:32
8/31/2016 23,864,839 4,772,863 5.000110
8/15/2016 34,290,608 7,107,573 4.824517
7/29/2016 48,805,957 4,393,298 11.109184
7/15/2016 50,251,265 6,647,956 7.558905
Read more: http://www.nasdaq.com/symbol/exel/short-interest#ixzz4K71R7J…
das sind die richtigen Zahlen der EXEL-Leerverkäufe
...und zwar hat sich die Anzahl der leerverkauften Aktien innerfhalb von 6 Wochen mehr als halbiert:8/31/2016 23,864,839 4,772,863 5.000110
8/15/2016 34,290,608 7,107,573 4.824517
7/29/2016 48,805,957 4,393,298 11.109184
7/15/2016 50,251,265 6,647,956 7.558905
Read more: http://www.nasdaq.com/symbol/exel/short-interest#ixzz4K71R7J…
Eisais LENVATINIB zur Behandlung von advRCC ebenfalls in der EU zugelassen
EISAI RECEIVES LICENSE FOR NEW INDICATION FOR ANTICANCER AGENT KISPLYX® ▼ (LENVATINIB MESYLATE) FOR TREATMENT OF ADVANCED RENAL CELL CARCINOMAEisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced that its European regional headquarters Eisai Europe Ltd. (Location: U.K.) has received license from the European Commission for anticancer agent Kisplyx® ▼ (generic name: lenvatinib mesylate, “lenvatinib”) in combination with everolimus for the treatment of adult patients with advanced renal cell carcinoma following one prior vascular endothelial growth factor (VEGF) targeted therapy. Following the United States, Europe marks the second region where lenvatinib has been licensed for the advanced renal cell carcinoma indication.
This license was based on a Phase II clinical study (Study 205)1 that evaluated the safety and efficacy of lenvatinib in combination with everolimus in patients with unresectable advanced or metastatic renal cell carcinoma following one prior VEGF-targeted therapy. From the results of the study, the lenvatinib plus everolimus group (n=51) demonstrated a significant extension in the study's primary endpoint of progression free survival (PFS) compared to the everolimus alone group (n=50) (median PFS for the lenvatinib plus everolimus group: 14.6 months vs median PFS for the everolimus alone group: 5.5 months; Hazard Ratio (HR) 0.40 [95% CI: 0.24-0.68], p=0.0005). Furthermore, updated median overall survival in the study population was 25.5 months in the lenvatinib plus everolimus group compared with 15.4 months in the everolimus alone group (HR 0.59 [95% CI: 0.36-0.97]).2 The most common treatment-emergent adverse events (TEAEs) reported in the lenvatinib plus everolimus group were diarrhea, decreased appetite and fatigue. The most common TEAEs of Grade 3 or higher (Common Terminology Criteria for Adverse Events) were diarrhea, hypertension and fatigue.
The number of patients with renal cancer is estimated to be approximately 338,000 worldwide, including approximately 115,000 in Europe, 58,000 in the United States and 17,000 in Japan.3 Renal cell carcinoma comprises more than 90% of all malignancies of the kidney,4 and originates from malignant cells in the lining of the tubules of the kidney. The incidence of renal cell carcinoma in people over 55 years of age is rising, and it is more likely to affect men than women. For advanced or metastatic renal cell carcinoma that is difficult to treat with surgery, the standard treatment is molecular targeted drug therapy, however with low 5-year survival rates, this remains a disease with significant unmet medical need.
In Europe, lenvatinib has been designated as an orphan drug for thyroid cancer and is marketed as Lenvima® for this indication. In Europe, renal cell carcinoma does not meet the criteria for orphan drug designation. Accordingly, under European regulations, any licensed medicine that previously received orphan drug designation for an indication and subsequently receives license for a non-orphan indication must be marketed under a different trade name. As such, lenvatinib will be marketed as Kisplyx® ▼ in the European Union for the indication covering renal cell carcinoma.
Eisai positions oncology as a key therapeutic area, and is aiming to discover revolutionary new medicines with the potential to cure cancer. Eisai remains committed to providing further clinical evidence for lenvatinib aimed at maximizing value of the drug as it seeks to contribute further to addressing the diverse needs of, and increasing the benefits provided to, patients with cancer, their families, and healthcare providers.
*Please refer to the following notes for the licensed indications in the United States, Japan and Europe
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
< Notes to editors >
1. About lenvatinib mesylate (generic name, “lenvatinib”)
Discovered and developed in-house, lenvatinib is an orally administered multiple receptor tyrosine kinase (RTK) inhibitor with a novel binding mode that selectively inhibits the kinase activities of vascular endothelial growth factor (VEGF) receptors (VEGFR1, VEGFR2 and VEGFR3) and fibroblast growth factor (FGF) receptors (FGFR1, FGFR2, FGFR3 and FGFR4) in addition to other proangiogenic and oncogenic pathway-related RTKs (including the platelet-derived growth factor (PDGF) receptor PDGFRα; KIT; and RET) involved in tumor proliferation.
Currently, Eisai has obtained license for lenvatinib as a treatment for refractory thyroid cancer in over 45 countries including in the United States, Japan, in Europe, Korea, Canada, and Mexico, and has submitted applications for regulatory review in countries throughout the world including South Africa and Malaysia. Specifically, Eisai has obtained license for the agent indicated in the United States for treatment for locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer, in Japan for the treatment of unresectable thyroid cancer, and in Europe for the treatment of adult patients with progressive, locally advanced or metastatic differentiated (papillary, follicular, Hürthle cell) thyroid carcinoma (DTC), refractory to radioactive iodine, respectively.
Furthermore, lenvatinib was also licensed in the United States in May 2016 for an additional indication in combination with everolimus for the treatment of patients with advanced renal cell carcinoma following one prior anti-angiogenic therapy.
Meanwhile, Eisai is conducting clinical studies of lenvatinib in several other tumor types such as hepatocellular carcinoma (Phase III), endometrial carcinoma (Phase II), biliary tract cancer (Phase II), and in combination with an immune checkpoint inhibitor for various types of cancer (Phase Ib/II).
Lenvatinib is marketed globally for use in the treatment of thyroid cancer and also in the United States for use in the treatment of renal cell carcinoma under the brand name Lenvima. Lenvatinib has been designated as an orphan drug for thyroid cancer by the regulatory authorities in Japan, the United States and Europe. Under European regulations, any licensed medicine that previously received orphan drug designation for an indication and now received license for a non-orphan indication must be marketed under a different trade name. As such, lenvatinib will be marketed as Kisplyx® ▼ in the European Union for the indication covering renal cell carcinoma.
2. About the Phase II Clinical Study (Study 205)1
Study 205 was a multicenter, randomized, open-label study of the combination of lenvatinib (18 mg) plus everolimus (5 mg), lenvatinib alone (24 mg), and everolimus alone (10 mg) in patients with unresectable advanced or metastatic renal cell carcinoma following one prior VEGF-targeted therapy, and was conducted in Europe and the United States. 153 patients were randomized in a 1:1:1 ratio to one of three treatment arms to compare the efficacy and safety of these three regimens.
From the results of the study, the combination of lenvatinib plus everolimus group demonstrated a significant extension in the study's primary endpoint of progression free survival (PFS) compared to the everolimus alone group (median PFS for the lenvatinib plus everolimus group: 14.6 months vs median PFS for the everolimus alone group: 5.5 months; Hazard Ratio (HR) 0.40 [95% CI: 0.24-0.68], p=0.0005). Additionally, median PFS for the lenvatinib alone group was 7.4 months, demonstrating an extension in PFS compared to the everolimus alone group (HR: 0.61 [95% CI: 0.38-0.98]).
The study also assessed objective response rate (ORR) and overall survival (OS) as secondary endpoints. Regarding ORR, both the lenvatinib plus everolimus group and the lenvatinib alone group showed an improvement in ORR compared to the everolimus alone group (lenvatinib plus everolimus: 43%, lenvatinib alone: 27%, everolimus alone: 6%). Furthermore, regarding OS, updated median overall survival in the study population was 25.5 months in the lenvatinib plus everolimus group compared with 15.4 months in the everolimus group (HR 0.59; 95% CI 0.36 - 0.97).2
The most common any-grade treatment-emergent adverse events (TEAEs) reported in the lenvatinib plus everolimus group were diarrhea, decreased appetite and fatigue. The most common TEAEs of Grade 3 or higher (Common Terminology Criteria for Adverse Events) were diarrhea, hypertension and fatigue.
Antwort auf Beitrag Nr.: 53.291.793 von Magnetfeldfredy am 16.09.16 19:26:06Exelixis ist mittlerweile jedoch sehr prominent bewertet, weshalb ich gestern weitere Anteile verkauft habe - von ursprünglich "all in" habe ich auf 15% reduziert. Der Rest bleibt im Depopt, weil es einerseits tatsächlich noch Luft nach oben gibt und andererseits die Aktie nach unten gut abgesichert erscheint....und zudem sieht die Performance dieser Position im Depot nicht schlecht aus.
Ich habe mittlerweile folgende (Risiko)positionen aufgebaut, bei denen die Verdoppelungswahrscheinlichkeit mE grösser ist als bei EXEL: PGNX, RDUS und IONS...wobei mehr als die Hälfte in PGNX investiert wurde. Natürlich ist die grössere Chance auch mit einem grösseren Risko verbunden.
Ich habe mittlerweile folgende (Risiko)positionen aufgebaut, bei denen die Verdoppelungswahrscheinlichkeit mE grösser ist als bei EXEL: PGNX, RDUS und IONS...wobei mehr als die Hälfte in PGNX investiert wurde. Natürlich ist die grössere Chance auch mit einem grösseren Risko verbunden.
CT-707, a novel FAK inhibitor, synergizes with Cabozantinib to suppress HCC
CT-707, a novel FAK inhibitor, synergizes with Cabozantinib to suppress hepatocellular carcinoma by blocking Cabozantinib-induced FAK activationAbstract
Hepatocellular carcinoma (HCC) is among the leading causes of cancer-related death worldwide, and the development of new treatment regimens is urgently needed to improve therapeutic approach. In our study, we found that the combination of a Met inhibitor, cabozantinib, and a novel FAK inhibitor, CT-707, exerted synergistic anti-tumor effects against HCC in vitro and in vivo. Interestingly, further studies showed that therapeutic concentrations of cabozantinib increased the phosphorylation of FAK, which might attenuate the anti-tumor activity of cabozantinib. The simultaneous exposure to CT-707 effectively inhibited the activation of FAK that was induced by cabozantinib, which contributes to the synergistic effect of the combination. Furthermore, cabozantinib increased the mRNA and protein levels of integrin α5, which is a canonical upstream of FAK, and the introduction of cilengitide to block integrin function could abrogate FAK activation by cabozantinib, indicating that cabozantinib up-regulated the phosphorylation of FAK in an integrin-dependent manner. Similar synergy was also observed on PHA-665752, another selective MET inhibitor, indicating that this observation might be a common characteristic of MET-targeting strategies. Our findings not only favor the development of the novel FAK inhibitor, CT-707, as a therapeutic agent against HCC but also provide a new strategy of combining MET and FAK inhibitors to potentiate the anticancer activities of these two types of agents for treating HCC patients.
http://mct.aacrjournals.org/content/early/2016/09/16/1535-71…
http://finance.yahoo.com/news/exelixis-provides-timing-key-c…
Das hört sich sehr gut an, prime time presentation, die Daten müssen sehr gut sein!
Das hört sich sehr gut an, prime time presentation, die Daten müssen sehr gut sein!

Cobimetinib/neue klinische Studien: NCT02908672 and NCT02902029
https://clinicaltrials.gov/ct2/show/NCT02908672?term=cobimet…
A Study of Atezolizumab Plus Cobimetinib and Vemurafenib Versus Placebo Plus Cobimetinib and Vemurafenib in Previously Untreated BRAFv600 Mutation-Positive Participants With Metastatic or Unresectable Locally Advanced Melanoma
This study is not yet open for participant recruitment. (see Contacts and Locations)
Verified September 2016 by Hoffmann-La Roche
Sponsor:
Hoffmann-La Roche
Information provided by (Responsible Party):
Hoffmann-La Roche
Phase 3
Estimated Enrollment:
500
Study Start Date: February 2017
Estimated Study Completion Date: August 2024
Evaluating the Efficacy and Safety of a Sequencing Schedule of Cobimetinib Plus Vemurafenib Followed by Immunotherapy With an Anti- PD-L1 Antibody in Patients With Unresectable or Metastatic BRAF V600 Mutant Melanoma (ImmunoCobiVem)
This study is not yet open for participant recruitment. (see Contacts and Locations)
Verified September 2016 by University Hospital, Essen
Sponsor:
University Hospital, Essen
Information provided by (Responsible Party):
Prof. Dr. med. Dirk Schadendorf, University Hospital, Essen
Malignant Melanoma
Drug: Vemurafenib
Drug: Cobimetinib
Drug: Atezolizumab
Phase 2
Estimated Enrollment: 176
Study Start Date: September 2016
Estimated Study Completion Date: June 2020
Estimated Primary Completion Date: March 2020 (Final data collection date for primary outcome measure)
https://clinicaltrials.gov/ct2/show/NCT02902029?term=cobimet…
https://clinicaltrials.gov/ct2/show/NCT02908672?term=cobimet…
A Study of Atezolizumab Plus Cobimetinib and Vemurafenib Versus Placebo Plus Cobimetinib and Vemurafenib in Previously Untreated BRAFv600 Mutation-Positive Participants With Metastatic or Unresectable Locally Advanced Melanoma
This study is not yet open for participant recruitment. (see Contacts and Locations)
Verified September 2016 by Hoffmann-La Roche
Sponsor:
Hoffmann-La Roche
Information provided by (Responsible Party):
Hoffmann-La Roche
Phase 3
Estimated Enrollment:
500
Study Start Date: February 2017
Estimated Study Completion Date: August 2024
Evaluating the Efficacy and Safety of a Sequencing Schedule of Cobimetinib Plus Vemurafenib Followed by Immunotherapy With an Anti- PD-L1 Antibody in Patients With Unresectable or Metastatic BRAF V600 Mutant Melanoma (ImmunoCobiVem)
This study is not yet open for participant recruitment. (see Contacts and Locations)
Verified September 2016 by University Hospital, Essen
Sponsor:
University Hospital, Essen
Information provided by (Responsible Party):
Prof. Dr. med. Dirk Schadendorf, University Hospital, Essen
Malignant Melanoma
Drug: Vemurafenib
Drug: Cobimetinib
Drug: Atezolizumab
Phase 2
Estimated Enrollment: 176
Study Start Date: September 2016
Estimated Study Completion Date: June 2020
Estimated Primary Completion Date: March 2020 (Final data collection date for primary outcome measure)
https://clinicaltrials.gov/ct2/show/NCT02902029?term=cobimet…
Exelixis auf dem Weg zur neuen Medivation:
http://finance.yahoo.com/news/exelixis-announces-collaborato…
http://finance.yahoo.com/news/exelixis-announces-collaborato…

Guidelines
European Association of Urology Guidelines for Clear Cell Renal Cancers That Are Resistant to Vascular Endothelial Growth Factor Receptor–Targeted Therapy
Nach Bekanntgabe der Studienergebnisse von CABOSUN auf ESMO am Montag, 10.10.16
vielen Dank Franca
--> http://www.investorvillage.com/smbd.asp?mb=1086&mn=3458&pt=m…
...könnte die bisherige Reihenfolge bei der Behandlung von RCC in Frage gestellt werden.
European Association of Urology Guidelines for Clear Cell Renal Cancers That Are Resistant to Vascular Endothelial Growth Factor Receptor–Targeted Therapy

Nach Bekanntgabe der Studienergebnisse von CABOSUN auf ESMO am Montag, 10.10.16
vielen Dank Franca

--> http://www.investorvillage.com/smbd.asp?mb=1086&mn=3458&pt=m…
...könnte die bisherige Reihenfolge bei der Behandlung von RCC in Frage gestellt werden.

Together against #kidneycancer @DrChoueiri @montypal #ESMO16 can't wait for the data of #cabosun today
Antwort auf Beitrag Nr.: 53.435.133 von Cyberhexe am 08.10.16 12:44:24
Der Tweet von IKCC KidneyCancer
"Together against #kidneycancer @DrChoueiri @montypal #ESMO16 can't wait for the data of #cabosun today"
steht im Widerspruch zur öffentlichen Ankündigung, dass CABOSUN erst am Montag publiziert wird!(?)
https://cslide.ctimeetingtech.com/library/esmo/browse/search…
Presidential Symposium 3 Chair(s) A. Cervantes (Valencia, Spain)F. Ciardiello (Napoli, Italy) Session Type Presidential Symposium Details ESMO 2016 Congress, 10.10.2016, 16:30 - 18:10, Copenhagen
LBA30_PR - CABOzantinib versus SUNitinib (CABOSUN) as initial targeted therapy for patients with metastatic renal cell carcinoma (mRCC) of poor and intermediate risk groups: Results from ALLIANCE A031203 trial
T. K. Choueiri (Boston, United States of America)S. Halabi (Durham, United States of America) B. Sanford (Durham, United States of America)O. Hahn (Chicago, United States of America) M. Michaelson (Boston, United States of America)M. Walsh (Boston, United States of America) T. Olencki (Columbus, United States of America)J. Picus (St Louis, United States of America) E. J. Small (San Francisco, United States of America)S. Dakhil (Wichita, United States of America) D. George (Durham, United States of America)M. J. Morris (New York, NY, United States of America)
Der Tweet von IKCC KidneyCancer
"Together against #kidneycancer @DrChoueiri @montypal #ESMO16 can't wait for the data of #cabosun today"
steht im Widerspruch zur öffentlichen Ankündigung, dass CABOSUN erst am Montag publiziert wird!(?)
https://cslide.ctimeetingtech.com/library/esmo/browse/search…
Presidential Symposium 3 Chair(s) A. Cervantes (Valencia, Spain)F. Ciardiello (Napoli, Italy) Session Type Presidential Symposium Details ESMO 2016 Congress, 10.10.2016, 16:30 - 18:10, Copenhagen
LBA30_PR - CABOzantinib versus SUNitinib (CABOSUN) as initial targeted therapy for patients with metastatic renal cell carcinoma (mRCC) of poor and intermediate risk groups: Results from ALLIANCE A031203 trial
T. K. Choueiri (Boston, United States of America)S. Halabi (Durham, United States of America) B. Sanford (Durham, United States of America)O. Hahn (Chicago, United States of America) M. Michaelson (Boston, United States of America)M. Walsh (Boston, United States of America) T. Olencki (Columbus, United States of America)J. Picus (St Louis, United States of America) E. J. Small (San Francisco, United States of America)S. Dakhil (Wichita, United States of America) D. George (Durham, United States of America)M. J. Morris (New York, NY, United States of America)
Antwort auf Beitrag Nr.: 53.435.205 von Cyberhexe am 08.10.16 13:06:45Montag ist bestätigt:
http://www.exelixis.com/investors-media/event-calendar
http://www.exelixis.com/investors-media/event-calendar
Was erwartest Du persönlich von den Daten, Kurs?
Antwort auf Beitrag Nr.: 53.436.015 von Magnetfeldfredy am 08.10.16 16:36:21Ausgangslage:
1.) Sunitinib
http://www.nejm.org/doi/full/10.1056/NEJMoa065044
Results
The median progression-free survival was significantly longer in the sunitinib group (11 months) than in the interferon alfa group (5 months), corresponding to a hazard ratio of 0.42 (95% confidence interval, 0.32 to 0.54; P<0.001). Sunitinib was also associated with a higher objective response rate than was interferon alfa (31% vs. 6%, P<0.001). The proportion of patients with grade 3 or 4 treatment-related fatigue was significantly higher in the group treated with interferon alfa, whereas diarrhea was more frequent in the sunitinib group (P<0.05). Patients in the sunitinib group reported a significantly better quality of life than did patients in the interferon alfa group (P<0.001).
Überlebenszeitanalyse:
Sunitinib: 26.4 Monate
IFVN: 21.8 Monate
HR= 0.82 (0.67 to 1.00)
p=0.05
ein Wechsel von der Kontrollarmgruppe in die Verumgruppe war erlaubt!
2.) RCC
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3176905/
Curr Oncol. 2011 Oct; 18(Suppl 2): S11–S19.
--> 4. DISCUSSION AND FUTURE STEPS
"Improvement in os from a single treatment is now almost impossible to demonstrate in mrcc."
Eine Verbesserung beim Überleben bei mRCC wird von den 17 Autoren als "fast unmöglich" bezeichnet.
3.) "clinically meaningful improvement"
hab auf die Schnelle zu RCC nichts gefunden, jedoch zu anderen Krebsarten Folgendes:
Targets for Meaningfulness
"The conclusions of the working groups are summarized below. All groups except the colon cancer group focused on patients with metastatic disease receiving first-line systemic treatment. All groups selected overall survival as the primary clinical endpoint, with all stipulating a hazard ratio (HR) ≤ 0.8 corresponding to improvement in median overall survival of 2.5 to 6 months, depending on setting, as the minimum incremental improvement over standard therapy that defines a clinically meaningful outcome.
Pancreatic Cancer—FOLFIRINOX-Eligible Patients: The current baseline median overall survival was estimated at 10 to 11 months. The minimum improvement over current overall survival considered clinically meaningful is 4 to 5 months, with a target hazard ratio of 0.67 to 0.69. As secondary endpoints, 1-year overall survival would be expected to improve to a minimum of 63% from the current rate of 48%, and progression-free survival is expected to improve by at least 4 to 5 months.
Pancreatic Cancer—Gemcitabine- or Gemcitabine/Nab-paclitaxel-Eligible Patients: The current baseline median overall survival was estimated at 8 to 9 months. The minimum improvement over current overall survival considered clinically meaningful is 3 to 4 months, with a target hazard ratio of 0.6 to 0.75. As secondary endpoints, 1-year overall survival would be expected to improve to a minimum of 50% from the current rate of 35%, and progression-free survival is expected to improve by at least 3 to 4 months.
Lung Cancer—Non–Squamous Cell Carcinoma: The current baseline median overall survival was estimated at 13 months. The minimum improvement over current overall survival considered clinically meaningful is 3.25 to 4 months, with a target hazard ratio of 0.76 to 0.8. As secondary endpoints, 1-year overall survival would be expected to improve to a minimum of 61% from the current rate of 53%, and progression-free survival is expected to improve by at least 4 months.
Lung Cancer—Squamous Cell Carcinoma: The current baseline median overall survival was estimated at 10 months. The minimum improvement over current overall survival considered clinically meaningful is 2.5 to 3 months, with a target hazard ratio of 0.77 to 0.8. As secondary endpoints, 1-year overall survival would be expected to improve to a minimum of 53% from the current rate of 44%, and progression-free survival is expected to improve by at least 3 months.
Breast Cancer—Metastatic Triple-Negative Disease, Previously Untreated for Metastatic Disease: The current baseline median overall survival was estimated at 18 months. The minimum improvement over current overall survival considered clinically meaningful is 4.5 to 6 months, with a target hazard ratio of 0.75 to 0.8. As secondary endpoints, 1-year overall survival would be expected to improve to a minimum of 71% from the current rate of 63%, and progression-free survival is expected to improve by at least 4 months.
Colon Cancer—Disease Progression With All Prior Therapies (or Not a Candidate for Standard Second- or Third-Line Options): The current baseline median overall survival was estimated at 4 to 6 months. The minimum improvement over current overall survival considered clinically meaningful is 3 to 5 months, with a target hazard ratio of 0.67. As secondary endpoints, 1-year overall survival would be expected to improve to a minimum of 35% from the current rate of 25%, and progression-free survival is expected to improve by at least 3 to 5 months."
4.) CABOSUN-Ergebnis erwartet:
beim Progressionsfreien Überleben eine HR < 0.8, sehr wahrscheinlich keine abschliessenden Daten zur Überlebenszeitanalyse
Ich rechne mal mit einer positiven Kursbewegung (--> hab deswegen meine freie Cashposition erneut (re)investiert), deren Umfang jedoch für mich nicht einschätzbar ist. Die involvierten Personen verbreiten jedoch durchweg positive Stimmung. Bin sehr gespannt.
1.) Sunitinib
http://www.nejm.org/doi/full/10.1056/NEJMoa065044
Results
The median progression-free survival was significantly longer in the sunitinib group (11 months) than in the interferon alfa group (5 months), corresponding to a hazard ratio of 0.42 (95% confidence interval, 0.32 to 0.54; P<0.001). Sunitinib was also associated with a higher objective response rate than was interferon alfa (31% vs. 6%, P<0.001). The proportion of patients with grade 3 or 4 treatment-related fatigue was significantly higher in the group treated with interferon alfa, whereas diarrhea was more frequent in the sunitinib group (P<0.05). Patients in the sunitinib group reported a significantly better quality of life than did patients in the interferon alfa group (P<0.001).
Überlebenszeitanalyse:
Sunitinib: 26.4 Monate
IFVN: 21.8 Monate
HR= 0.82 (0.67 to 1.00)
p=0.05
ein Wechsel von der Kontrollarmgruppe in die Verumgruppe war erlaubt!
2.) RCC
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3176905/
Curr Oncol. 2011 Oct; 18(Suppl 2): S11–S19.
--> 4. DISCUSSION AND FUTURE STEPS
"Improvement in os from a single treatment is now almost impossible to demonstrate in mrcc."
Eine Verbesserung beim Überleben bei mRCC wird von den 17 Autoren als "fast unmöglich" bezeichnet.
3.) "clinically meaningful improvement"
hab auf die Schnelle zu RCC nichts gefunden, jedoch zu anderen Krebsarten Folgendes:
Targets for Meaningfulness
"The conclusions of the working groups are summarized below. All groups except the colon cancer group focused on patients with metastatic disease receiving first-line systemic treatment. All groups selected overall survival as the primary clinical endpoint, with all stipulating a hazard ratio (HR) ≤ 0.8 corresponding to improvement in median overall survival of 2.5 to 6 months, depending on setting, as the minimum incremental improvement over standard therapy that defines a clinically meaningful outcome.
Pancreatic Cancer—FOLFIRINOX-Eligible Patients: The current baseline median overall survival was estimated at 10 to 11 months. The minimum improvement over current overall survival considered clinically meaningful is 4 to 5 months, with a target hazard ratio of 0.67 to 0.69. As secondary endpoints, 1-year overall survival would be expected to improve to a minimum of 63% from the current rate of 48%, and progression-free survival is expected to improve by at least 4 to 5 months.
Pancreatic Cancer—Gemcitabine- or Gemcitabine/Nab-paclitaxel-Eligible Patients: The current baseline median overall survival was estimated at 8 to 9 months. The minimum improvement over current overall survival considered clinically meaningful is 3 to 4 months, with a target hazard ratio of 0.6 to 0.75. As secondary endpoints, 1-year overall survival would be expected to improve to a minimum of 50% from the current rate of 35%, and progression-free survival is expected to improve by at least 3 to 4 months.
Lung Cancer—Non–Squamous Cell Carcinoma: The current baseline median overall survival was estimated at 13 months. The minimum improvement over current overall survival considered clinically meaningful is 3.25 to 4 months, with a target hazard ratio of 0.76 to 0.8. As secondary endpoints, 1-year overall survival would be expected to improve to a minimum of 61% from the current rate of 53%, and progression-free survival is expected to improve by at least 4 months.
Lung Cancer—Squamous Cell Carcinoma: The current baseline median overall survival was estimated at 10 months. The minimum improvement over current overall survival considered clinically meaningful is 2.5 to 3 months, with a target hazard ratio of 0.77 to 0.8. As secondary endpoints, 1-year overall survival would be expected to improve to a minimum of 53% from the current rate of 44%, and progression-free survival is expected to improve by at least 3 months.
Breast Cancer—Metastatic Triple-Negative Disease, Previously Untreated for Metastatic Disease: The current baseline median overall survival was estimated at 18 months. The minimum improvement over current overall survival considered clinically meaningful is 4.5 to 6 months, with a target hazard ratio of 0.75 to 0.8. As secondary endpoints, 1-year overall survival would be expected to improve to a minimum of 71% from the current rate of 63%, and progression-free survival is expected to improve by at least 4 months.
Colon Cancer—Disease Progression With All Prior Therapies (or Not a Candidate for Standard Second- or Third-Line Options): The current baseline median overall survival was estimated at 4 to 6 months. The minimum improvement over current overall survival considered clinically meaningful is 3 to 5 months, with a target hazard ratio of 0.67. As secondary endpoints, 1-year overall survival would be expected to improve to a minimum of 35% from the current rate of 25%, and progression-free survival is expected to improve by at least 3 to 5 months."
4.) CABOSUN-Ergebnis erwartet:
beim Progressionsfreien Überleben eine HR < 0.8, sehr wahrscheinlich keine abschliessenden Daten zur Überlebenszeitanalyse
Ich rechne mal mit einer positiven Kursbewegung (--> hab deswegen meine freie Cashposition erneut (re)investiert), deren Umfang jedoch für mich nicht einschätzbar ist. Die involvierten Personen verbreiten jedoch durchweg positive Stimmung. Bin sehr gespannt.
Danke Hexe, yes we hope!

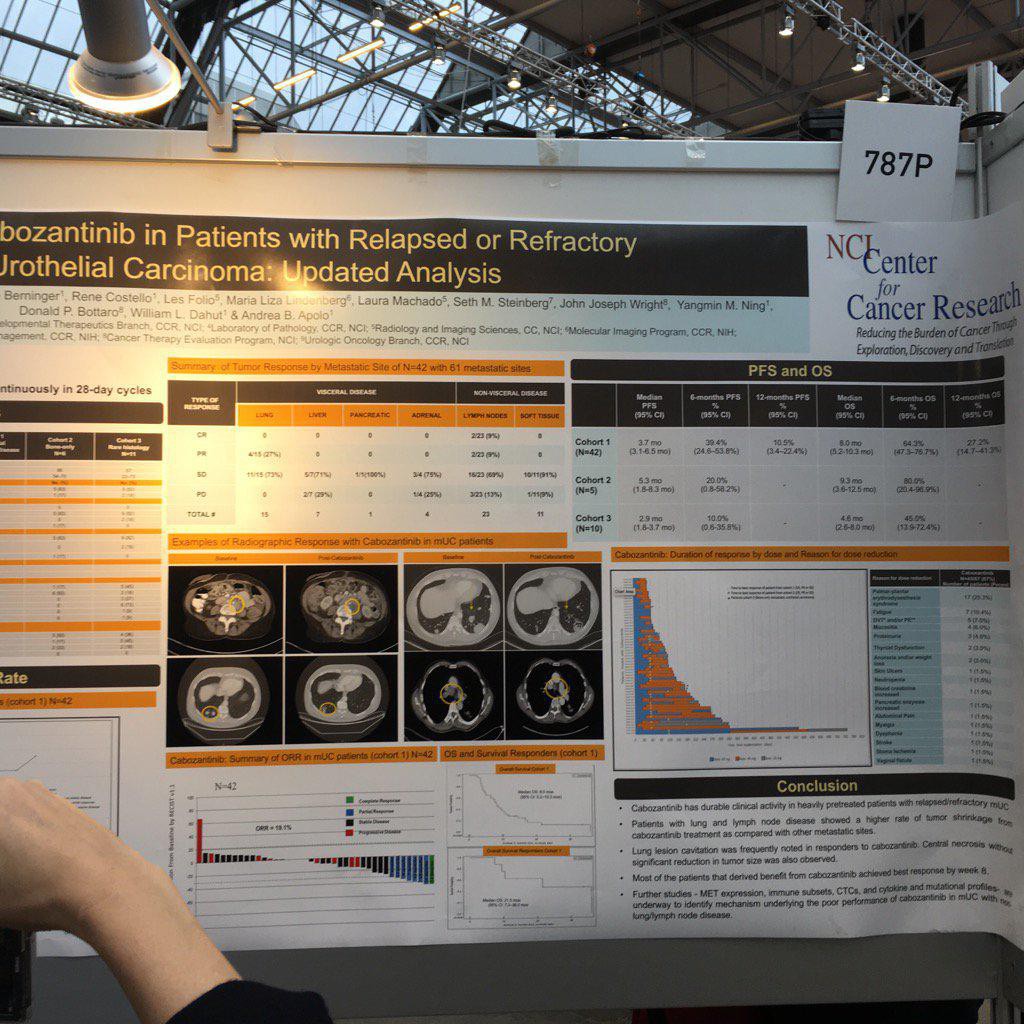
ESMO2016: Poster Display 9.10.2016, 13:00 - 14:00, Central Hall
787P - A phase II study of cabozantinib in patients (pts) with relapsed/refractory metastatic urothelial carcinoma (mUC)
Background
Hepatocyte growth factor (HGF) and its receptor (MET) are activated in UC. In translational studies, cabozantinib, a receptor tyrosine kinase inhibitor primarily targeting MET and VEGFR2, reversed HGF-driven UC cell growth and invasion.
Methods
In this phase II study, pts received cabozantinib 60 mg/day in 28-day cycles in 3 cohorts: 1) mUC, 2) bone-only mUC, and 3) metastatic rare bladder histology. Primary objective was overall response rate (ORR) by RECIST v1.1. In cohort 1, we also report on the tumor responses by site of metastases (mets). Secondary objectives were to assess the progression-free survival (PFS) and overall survival (OS) in all cohorts.
Results
Of 67 pts enrolled, 70% of male, median age: 63 (22–82), KPS 80% in 73% pts. Primary sites: 69% bladder, 25% upper tract, 6% both. No. prior therapies: 1/2/3/ ≥ 4 (range 1–6) in 34/39/16/9% of pts, respectively. Of 41 evaluable pts in cohort 1, there was 1 complete response (CR), 7 partial responses (PR) (ORR = 19.5%; 95% CI: 8.8–34.9%), 18 stable disease (SD), and 15 progressive disease (PD). Median progression-free survival (mPFS): 3.7 mos(95% CI: 2.3–6.5); 6-month PFS: 38.0%(95% CI: 23.3–52.6%). Median overall survival (mOS): 8.2 mos (95% CI: 5.2–10.3); 6-month OS: 65.1%(95% CI: 48.3–77.6%); 12-month OS: 25.7% (95% CI: 13.0–40.3%). ORR in cohort 1 was evaluated by site of mets in 63 target-lesions: 25.3%lung,/9.5%liver/6.3renal/1.6%kidney/1.6% pancreatic/39.8% LN/15.9% ST. Lung mets had 25% PR/75% SD/0%PD. Treatment resulted in lung lesion cavitation, which could not be interpreted as CR (at best PR). Liver mets had 83.3%SD/16.7%PD. There was no ORR in liver/adrenal/kidney/pancreatic mets. LN mets had 8%CR/8%PR/72%SD/12%PD. ST mets had 90%SD/10%PD. Of 5 pts in cohort 2, 60% had improvement by NaF FDG/PET. mOS: 9.3 mos (95% CI: 3.6–12.5). Of 10 evaluable pts in cohort 3, there were 0%CR/PR/50%SD/50%PD. mPFS: 2.9 mos (95% CI: 1.8–3.7); mOS: 4.6 mos (95% CI: 2.6–8.0).
Conclusions
Cabozantinib has clinical activity in relapsed/refractory pts with mUC. Lung and LN mets had higher ORR. Lung lesion cavitation was frequently noted in responders. Further studies are underway to correlate responses with MET expression, immune subsets, CTCs, and cytokine/mutational profiles.
Clinical trial identification
NCT01688999
Legal entity responsible for the study
NCI/NHI
Funding
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--
Exelixis, Inc. (EXEL) today announced detailed results from the CABOSUN randomized phase 2 trial of cabozantinib in patients with previously untreated advanced renal cell carcinoma (RCC) with intermediate- or poor-risk disease per the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC). Principal investigator Toni K. Choueiri, M.D. will present detailed data from late-breaking CABOSUN abstract [#LBA30_PR] today in the Presidential Symposium 3 session, starting at 16:30 CEST (local Copenhagen time) / 10:30 a.m. EDT / 7:30 a.m. PDT at the European Society for Medical Oncology (ESMO) 2016, which is being held October 7 – 11, 2016 in Copenhagen.
CABOSUN was conducted by The Alliance for Clinical Trials in Oncology as part of Exelixis’ collaboration with the National Cancer Institute’s Cancer Therapy Evaluation Program (NCI-CTEP).
In CABOSUN, with a median follow-up of 20.8 months, cabozantinib demonstrated a clinically meaningful and statistically significant 31 percent reduction in the rate of disease progression or death [HR 0.69, 95% CI (0.48-0.99), one-sided P=0.012]. The median progression-free survival (PFS) for cabozantinib was 8.2 months versus 5.6 months for sunitinib, corresponding to a 2.6 months (46 percent) improvement favoring cabozantinib over sunitinib. PFS benefits were independent of IMDC risk group (intermediate or poor risk) and presence or absence of bone metastases at baseline. The results for sunitinib were in line with a previously published retrospective analysis of 1,174 intermediate- and poor-risk RCC patients from the IMDC database, which documented a median PFS of 5.6 months with a first-line targeted therapy, mainly sunitinib, in this patient population.1
Objective response rate (ORR) was also significantly improved, at 46 percent (95% CI 34% – 57%) for cabozantinib versus 18 percent (95% CI 10% to 28%) for sunitinib. With a median follow up of 22.8 months, median overall survival was 30.3 months for cabozantinib versus 21.8 months for sunitinib [HR 0.80, 95% CI (0.50 - 1.26)].
“The results presented today support the potential of cabozantinib to become a new therapeutic option for previously untreated patients following their diagnosis with advanced kidney cancer,” said Toni K. Choueiri, M.D., Director, Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute and chair of the CABOSUN study. “Not only has cabozantinib surpassed sunitinib, the current standard of care, in progression-free survival and objective response rate, cabozantinib’s effects on progression-free survival were also consistently favorable across patient stratification subgroups including IMDC intermediate versus poor-risk groups and presence or absence of bone metastases.”
“We at the Alliance for Clinical Trials in Oncology are pleased that CABOSUN has successfully demonstrated that cabozantinib has the potential to benefit patients with advanced renal cell carcinoma as a first-line therapy,” said Michael J. Morris, M.D., Associate Member at Memorial Sloan Kettering Cancer Center, and Chair of the Alliance Genitourinary Committee. “We are grateful to everyone who has participated in the trial, especially the physicians, patients and their families.”
Based on these results, Exelixis plans to submit a Supplemental New Drug Application (sNDA) for cabozantinib as a treatment of first-line advanced renal cell carcinoma, and is working with the Alliance to transfer the complete CABOSUN clinical database to Exelixis.
“The past year has seen a tremendous level of progress in the treatment of kidney cancer, and we are excited to be at the forefront of bringing these advancements to patients,” said Michael M. Morrissey, Ph.D., president and chief executive officer of Exelixis. “Patients in the first-line setting with either intermediate- or poor-risk disease progress rapidly with sunitinib, a current standard of care; therefore, there is a clear need for new options that provide improved clinical benefit in this difficult to treat patient population. To that end, based on the CABOSUN results, we are planning to submit a supplemental New Drug Application in the United States for cabozantinib as a first-line treatment for advanced renal cell carcinoma.”
Exelixis, Inc. (EXEL) today announced detailed results from the CABOSUN randomized phase 2 trial of cabozantinib in patients with previously untreated advanced renal cell carcinoma (RCC) with intermediate- or poor-risk disease per the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC). Principal investigator Toni K. Choueiri, M.D. will present detailed data from late-breaking CABOSUN abstract [#LBA30_PR] today in the Presidential Symposium 3 session, starting at 16:30 CEST (local Copenhagen time) / 10:30 a.m. EDT / 7:30 a.m. PDT at the European Society for Medical Oncology (ESMO) 2016, which is being held October 7 – 11, 2016 in Copenhagen.
CABOSUN was conducted by The Alliance for Clinical Trials in Oncology as part of Exelixis’ collaboration with the National Cancer Institute’s Cancer Therapy Evaluation Program (NCI-CTEP).
In CABOSUN, with a median follow-up of 20.8 months, cabozantinib demonstrated a clinically meaningful and statistically significant 31 percent reduction in the rate of disease progression or death [HR 0.69, 95% CI (0.48-0.99), one-sided P=0.012]. The median progression-free survival (PFS) for cabozantinib was 8.2 months versus 5.6 months for sunitinib, corresponding to a 2.6 months (46 percent) improvement favoring cabozantinib over sunitinib. PFS benefits were independent of IMDC risk group (intermediate or poor risk) and presence or absence of bone metastases at baseline. The results for sunitinib were in line with a previously published retrospective analysis of 1,174 intermediate- and poor-risk RCC patients from the IMDC database, which documented a median PFS of 5.6 months with a first-line targeted therapy, mainly sunitinib, in this patient population.1
Objective response rate (ORR) was also significantly improved, at 46 percent (95% CI 34% – 57%) for cabozantinib versus 18 percent (95% CI 10% to 28%) for sunitinib. With a median follow up of 22.8 months, median overall survival was 30.3 months for cabozantinib versus 21.8 months for sunitinib [HR 0.80, 95% CI (0.50 - 1.26)].
“The results presented today support the potential of cabozantinib to become a new therapeutic option for previously untreated patients following their diagnosis with advanced kidney cancer,” said Toni K. Choueiri, M.D., Director, Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute and chair of the CABOSUN study. “Not only has cabozantinib surpassed sunitinib, the current standard of care, in progression-free survival and objective response rate, cabozantinib’s effects on progression-free survival were also consistently favorable across patient stratification subgroups including IMDC intermediate versus poor-risk groups and presence or absence of bone metastases.”
“We at the Alliance for Clinical Trials in Oncology are pleased that CABOSUN has successfully demonstrated that cabozantinib has the potential to benefit patients with advanced renal cell carcinoma as a first-line therapy,” said Michael J. Morris, M.D., Associate Member at Memorial Sloan Kettering Cancer Center, and Chair of the Alliance Genitourinary Committee. “We are grateful to everyone who has participated in the trial, especially the physicians, patients and their families.”
Based on these results, Exelixis plans to submit a Supplemental New Drug Application (sNDA) for cabozantinib as a treatment of first-line advanced renal cell carcinoma, and is working with the Alliance to transfer the complete CABOSUN clinical database to Exelixis.
“The past year has seen a tremendous level of progress in the treatment of kidney cancer, and we are excited to be at the forefront of bringing these advancements to patients,” said Michael M. Morrissey, Ph.D., president and chief executive officer of Exelixis. “Patients in the first-line setting with either intermediate- or poor-risk disease progress rapidly with sunitinib, a current standard of care; therefore, there is a clear need for new options that provide improved clinical benefit in this difficult to treat patient population. To that end, based on the CABOSUN results, we are planning to submit a supplemental New Drug Application in the United States for cabozantinib as a first-line treatment for advanced renal cell carcinoma.”
Antwort auf Beitrag Nr.: 53.442.033 von Magnetfeldfredy am 10.10.16 09:14:35sehr gut! 
Cabo vs. SOC Sutent
mPFS 8,2 vs 5,6... HR 0,69
ORR 46% vs 18%
mOS 30,3 vs 21,8... HR 0,8
Damit scheint der Weg für 1st-line RCC frei zu sein, EXEL will sogar auf dieser Basis eine offizielle 1st-Zulassung beantragen.
Selbst wenn PD1s irgendwann in RCC zum Einsatz kommen, so sehen die neusten Ergebnisse ja eher danach aus, als ob doch der PD1L-Status relevant für die Wirksamkeit ist (Nivo war unselektiert in 1st-line NSCLC ein deutlicher Fehlschlag). D.h. nur ein Teil der RCC-Patienten wird dann mit PD1 überhaupt behandelt werden können. Cabo sollte damit erstmal SOC werden in RCC, exellent!
Gruß
ipollit

Cabo vs. SOC Sutent
mPFS 8,2 vs 5,6... HR 0,69
ORR 46% vs 18%
mOS 30,3 vs 21,8... HR 0,8
Damit scheint der Weg für 1st-line RCC frei zu sein, EXEL will sogar auf dieser Basis eine offizielle 1st-Zulassung beantragen.
Selbst wenn PD1s irgendwann in RCC zum Einsatz kommen, so sehen die neusten Ergebnisse ja eher danach aus, als ob doch der PD1L-Status relevant für die Wirksamkeit ist (Nivo war unselektiert in 1st-line NSCLC ein deutlicher Fehlschlag). D.h. nur ein Teil der RCC-Patienten wird dann mit PD1 überhaupt behandelt werden können. Cabo sollte damit erstmal SOC werden in RCC, exellent!
Gruß
ipollit
Ein paar Kommentare von LR zu aktuellen Daten von EXEL...




Gruß
ipollit




Gruß
ipollit
Link fehlt!
Sorry, Fehler!
Sunitinib bei adjuvanter Therapie
...nach Operation eines lokal fortgeschrittenen Nierenzellkarzinoms:http://www.nejm.org/doi/full/10.1056/NEJMoa1611406?query=fea…
Price target 21 US Dollar:
Exelixis upgraded to Overweight after trial success at Piper Jaffray Piper Jaffray analyst Edward Tenthoff upgraded Exelixis to Overweight from Neutral after the company reported Phase II CABOSUN data in 157 front-line metastatic Renal Cell Carcinoma patients. The data are "good enough" to file a supplemental new drug application and could drive off-label front-line RCC use, Tenthoff tells investors in a research note. The analyst raised his price target for the shares to $21 from $8.
Read more at:
http://thefly.com/landingPageNews.php?id=2441996
Exelixis upgraded to Overweight after trial success at Piper Jaffray Piper Jaffray analyst Edward Tenthoff upgraded Exelixis to Overweight from Neutral after the company reported Phase II CABOSUN data in 157 front-line metastatic Renal Cell Carcinoma patients. The data are "good enough" to file a supplemental new drug application and could drive off-label front-line RCC use, Tenthoff tells investors in a research note. The analyst raised his price target for the shares to $21 from $8.
Read more at:
http://thefly.com/landingPageNews.php?id=2441996
Antwort auf Beitrag Nr.: 53.444.472 von Cyberhexe am 10.10.16 14:54:33"mRCC 1L: P3 nicht erforderlich", meint Sumanta Kumar Pal -->
http://www.cityofhope.org/people/pal-sumanta
Sumanta Pal @montypal
Sumanta Pal hat ESMO - Eur. Oncology retweetet
Disagree. P3 not needed. I would not put a pt on this trial; benefit of 1L Cabo too obvious in #CABOSUN @DrChoueiri @uretericbud @allaf_mo
Sumanta Pal hat hinzugefügt,
ESMO - Eur. Oncology @myESMO
"A phase III trial is warranted to confirm the CABOSUN results" B. Escudier #ESMO16
http://www.cityofhope.org/people/pal-sumanta
Sumanta Pal @montypal
Sumanta Pal hat ESMO - Eur. Oncology retweetet
Disagree. P3 not needed. I would not put a pt on this trial; benefit of 1L Cabo too obvious in #CABOSUN @DrChoueiri @uretericbud @allaf_mo
Sumanta Pal hat hinzugefügt,
ESMO - Eur. Oncology @myESMO
"A phase III trial is warranted to confirm the CABOSUN results" B. Escudier #ESMO16
Antwort auf Beitrag Nr.: 53.448.207 von Cyberhexe am 10.10.16 23:00:21

Product Name Information
Product: Sutent
Generic Name: sunitinib malate
Therapeutic Subcategory: Anti-angiogenics
WW sales
Product: Sutent
Generic Name: sunitinib malate
Therapeutic Subcategory: Anti-angiogenics
WW sales

Antwort auf Beitrag Nr.: 53.448.207 von Cyberhexe am 10.10.16 23:00:21"I would not put a pt on this trial; benefit of 1L Cabo too obvious in #CABOSUN"
Sehr nachvollziehbares Argument.
Das gilt ja nicht nur für einen möglichen weiteren Trial, sondern auch für die normale Behandlung neuer first-line-Patienten.
Jetzt einfach weiter Sutent verabreichen? Welcher Patient und welcher Arzt fühlt sich damit jetzt noch wirklich bestmöglich versorgt?
Andererseits: Wie sieht es mit der Kostenerstattung aus, solange es keine offizielle Zulassung gibt? Ich glaube, in den USA entscheidet das jede Kasse für sich, oder?
Wenn die FDA hier jetzt eine große P III mit Cabo vs. Sutent fordern sollte, haben wir ein erhebliches ethisches Dilemma.
Andererseits hat die FDA in der Vergangenheit schon unethische und sinnfreie Entscheidungen getroffen (bspw. bei T-DM1 in der third line BC).
Unterm Strich halte ich die Chancen für eine Zulassung von Cabo für die first line aber für recht gut.
Ich denke, dass aber bereits ab sofort ein gewisser "off label use" in der first line beginnen wird, so dass die Umsätze mit Cabo so und so erst mal dynamisch wachsen werden.
Ein Rätsel bleibt für mich, dass das Produkt in Japan nach wie vor nicht verpartnert wurde? Es gibt dafür eigentlich nur zwei Erklärungen: Man kann sich mit niemandem über die Konditionen einigen oder man prüft eben doch im Hintergrund die Möglichkeit einer Übernahme (möglicherweise sogar durch einen japanischen Pharma).
Es bleibt spannend.
Sehr nachvollziehbares Argument.
Das gilt ja nicht nur für einen möglichen weiteren Trial, sondern auch für die normale Behandlung neuer first-line-Patienten.
Jetzt einfach weiter Sutent verabreichen? Welcher Patient und welcher Arzt fühlt sich damit jetzt noch wirklich bestmöglich versorgt?
Andererseits: Wie sieht es mit der Kostenerstattung aus, solange es keine offizielle Zulassung gibt? Ich glaube, in den USA entscheidet das jede Kasse für sich, oder?
Wenn die FDA hier jetzt eine große P III mit Cabo vs. Sutent fordern sollte, haben wir ein erhebliches ethisches Dilemma.
Andererseits hat die FDA in der Vergangenheit schon unethische und sinnfreie Entscheidungen getroffen (bspw. bei T-DM1 in der third line BC).
Unterm Strich halte ich die Chancen für eine Zulassung von Cabo für die first line aber für recht gut.
Ich denke, dass aber bereits ab sofort ein gewisser "off label use" in der first line beginnen wird, so dass die Umsätze mit Cabo so und so erst mal dynamisch wachsen werden.
Ein Rätsel bleibt für mich, dass das Produkt in Japan nach wie vor nicht verpartnert wurde? Es gibt dafür eigentlich nur zwei Erklärungen: Man kann sich mit niemandem über die Konditionen einigen oder man prüft eben doch im Hintergrund die Möglichkeit einer Übernahme (möglicherweise sogar durch einen japanischen Pharma).
Es bleibt spannend.
Antwort auf Beitrag Nr.: 53.448.840 von HA_LUX am 11.10.16 07:33:38"Jetzt einfach weiter Sutent verabreichen? Welcher Patient und welcher Arzt fühlt sich damit jetzt noch wirklich bestmöglich versorgt?"
Sutent ist allerdings nicht irgendein Medikament, sondern in RCC die seit vielen Jahren etablierte Standardbehandlung (SOC) für fast alle Patienten. Alle Ärtze kennen Sutent und können es gut einschätzen. Cabo ist dagegen nicht etabliert, es ist ja erst vor Kurzen gegen RCC zugelassen, die Ärzte haben keine Erfahrung damit. Wird sich ein Arzt wirklich wohl fühlen, wenn er einem Patienten statt des Standard, dessen Wirkung und Nebenwirkung er gut kennt, ein neues Mittel gibt, das eventuell besser wirkt, dessen Nebenwirkungen er aber z.B. gar nicht kennt. Da geht man wahrscheinlich doch kein Risiko ein und behandelt so wie es zuvor auch gut war.
Zu bedenken ist auch, dass Sutent bald generisch wird und dann viel billiger sein wird.
Cabosun ist nur eine PII, die nicht mal selber von EXEL durchgeführt worden ist. Ich dachte eine PII reicht eventuell für eine Zulassung an austherapierten Patienten, die sowieso keine Alternative haben. Aber mit einer PII direkt den Standard bei der Ersthehaldung ändern? Das hört sich für mich sehr extrem an... ich hatte eine PIII erwartet.
Viele Krebsmittel werden in Indikationen Offlabel verabreicht, für die sie nie zugelassen worden sind, nur aufgrund von klinischen Daten und der Erfahrung der Ärzte. Wahrscheinlich ist es nach diesen Daten erstmal gar nicht so entscheidend, ob Cabo eine offizielle 1st-line Zulassung erhält. Wenn die Ärzte damit gut in 3rd-line klarkommen, werden sie es bestimmt auch 1st-line nach diesen Daten probieren.
Gruß
ipollit
Sutent ist allerdings nicht irgendein Medikament, sondern in RCC die seit vielen Jahren etablierte Standardbehandlung (SOC) für fast alle Patienten. Alle Ärtze kennen Sutent und können es gut einschätzen. Cabo ist dagegen nicht etabliert, es ist ja erst vor Kurzen gegen RCC zugelassen, die Ärzte haben keine Erfahrung damit. Wird sich ein Arzt wirklich wohl fühlen, wenn er einem Patienten statt des Standard, dessen Wirkung und Nebenwirkung er gut kennt, ein neues Mittel gibt, das eventuell besser wirkt, dessen Nebenwirkungen er aber z.B. gar nicht kennt. Da geht man wahrscheinlich doch kein Risiko ein und behandelt so wie es zuvor auch gut war.
Zu bedenken ist auch, dass Sutent bald generisch wird und dann viel billiger sein wird.
Cabosun ist nur eine PII, die nicht mal selber von EXEL durchgeführt worden ist. Ich dachte eine PII reicht eventuell für eine Zulassung an austherapierten Patienten, die sowieso keine Alternative haben. Aber mit einer PII direkt den Standard bei der Ersthehaldung ändern? Das hört sich für mich sehr extrem an... ich hatte eine PIII erwartet.
Viele Krebsmittel werden in Indikationen Offlabel verabreicht, für die sie nie zugelassen worden sind, nur aufgrund von klinischen Daten und der Erfahrung der Ärzte. Wahrscheinlich ist es nach diesen Daten erstmal gar nicht so entscheidend, ob Cabo eine offizielle 1st-line Zulassung erhält. Wenn die Ärzte damit gut in 3rd-line klarkommen, werden sie es bestimmt auch 1st-line nach diesen Daten probieren.
Gruß
ipollit
Antwort auf Beitrag Nr.: 53.451.618 von ipollit am 11.10.16 13:00:57ipolit schrieb:
Sutent ist allerdings nicht irgendein Medikament, sondern in RCC die seit vielen Jahren etablierte Standardbehandlung (SOC) für fast alle Patienten.
Ein neues Medikament muss besser sein (Wirkung vs. Nebenwirkung) als der bisherige Standard. Da in dieser ph2-Studie das progressionsfreie Überleben unter Cabo gegenüber dem Standard (Sunitinib) statistisch signifikant günstiger ist und sowohl Ansprechrate als auch die Tendenz beim Überleben ebenfalls Cabo begünstigt, ist der Beweis wissenschaftlich erbracht, dass Cabo in Erstlinienbehandlung bei RCC bei mittlerer bis mässiger Prognose gegenüber dem bisherigen Standard vorteilhaft ist.
Zudem wurde eine Lebenszeitverlängerung von den Experten als fast unmöglich bezeichnet.
Obwohl die Studie für einen nachzuweisenden Überlebensvorteil nicht ausgelegt war (zu geringe Mächtigkeith), ist die erzielte Tendenz mehr als bemerkenswert.
Curr Oncol. 2011 Oct; 18(Suppl 2): S11–S19.
--> 4. DISCUSSION AND FUTURE STEPS
"Improvement in os from a single treatment is now almost impossible to demonstrate in mrcc."
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3176905/
[/i]Alle Ärtze kennen Sutent und können es gut einschätzen. Cabo ist dagegen nicht etabliert, es ist ja erst vor Kurzen gegen RCC zugelassen, die Ärzte haben keine Erfahrung damit. Wird sich ein Arzt wirklich wohl fühlen, wenn er einem Patienten statt des Standard, dessen Wirkung und Nebenwirkung er gut kennt, ein neues Mittel gibt, das eventuell besser wirkt, dessen Nebenwirkungen er aber z.B. gar nicht kennt. Da geht man wahrscheinlich doch kein Risiko ein und behandelt so wie es zuvor auch gut war.[/i]
Wenn jeder Arzt so handeln würde, gebe es keine Innovation. Dass es natürlich eine gewisse Trägheit gibt und nicht jeder Arzt gerade die neuste Errungenschaft an den Patienten bringt, ist jedoch eine Tatsache, weshalb fast jedes Medikament "klein anzufangen hat". Der Voprteil bei Cabo ist jedoch, dass dieses Medi bereits bei 2 weiteren Indikationen (RCC 2L und Schilddrüsenkarzinom) am Markt eingeführt ist , so dass doch bereits etliche Patiebten damit behandelt wurden. Die Aufmerksamkeit in den wissenschaftlichen Medien wird zudem für Cabo drastisch zunehmen, so dass zumindest in den grossen onkologischen Zentren sicherlich zeitnah das innovative Medikament zur Anwendung kommt.
Zu bedenken ist auch, dass Sutent bald generisch wird und dann viel billiger sein wird.
Wenn jedoch ein wirksameres Medikament am Markt ist, werden nur die wenigsten Ärzte dieses den Patienten vorenthalten.
Cabosun ist nur eine PII, die nicht mal selber von EXEL durchgeführt worden ist. Ich dachte eine PII reicht eventuell für eine Zulassung an austherapierten Patienten, die sowieso keine Alternative haben. Aber mit einer PII direkt den Standard bei der Ersthehaldung ändern? Das hört sich für mich sehr extrem an... ich hatte eine PIII erwartet.
Von wem die Studie durchgeführt bzw. gesponsert wurde, spielt nur dann eine Rolle, wenn die Studienleitung bzw. die daran beteiligten Kliniken nicht vertrauenswürdig ist/sind. Das NCI geniesst jedoch die allerbeste Reputation , ebenso die daran beteiligten Kliniken, so dass dies überhaupt keine Rolle spielt.
Natürlich wäre eine ph2-Studie für ein vollwertiges NDA kaum ausreichend, da es sich jedoch um einen ergaenzenden Zulassungsantrag handelt, ist ein Zulassung bei einer entsprechenden Guete der Daten durchaus moeglich.
Sutent ist allerdings nicht irgendein Medikament, sondern in RCC die seit vielen Jahren etablierte Standardbehandlung (SOC) für fast alle Patienten.
Ein neues Medikament muss besser sein (Wirkung vs. Nebenwirkung) als der bisherige Standard. Da in dieser ph2-Studie das progressionsfreie Überleben unter Cabo gegenüber dem Standard (Sunitinib) statistisch signifikant günstiger ist und sowohl Ansprechrate als auch die Tendenz beim Überleben ebenfalls Cabo begünstigt, ist der Beweis wissenschaftlich erbracht, dass Cabo in Erstlinienbehandlung bei RCC bei mittlerer bis mässiger Prognose gegenüber dem bisherigen Standard vorteilhaft ist.
Zudem wurde eine Lebenszeitverlängerung von den Experten als fast unmöglich bezeichnet.
Obwohl die Studie für einen nachzuweisenden Überlebensvorteil nicht ausgelegt war (zu geringe Mächtigkeith), ist die erzielte Tendenz mehr als bemerkenswert.
Curr Oncol. 2011 Oct; 18(Suppl 2): S11–S19.
--> 4. DISCUSSION AND FUTURE STEPS
"Improvement in os from a single treatment is now almost impossible to demonstrate in mrcc."
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3176905/
[/i]Alle Ärtze kennen Sutent und können es gut einschätzen. Cabo ist dagegen nicht etabliert, es ist ja erst vor Kurzen gegen RCC zugelassen, die Ärzte haben keine Erfahrung damit. Wird sich ein Arzt wirklich wohl fühlen, wenn er einem Patienten statt des Standard, dessen Wirkung und Nebenwirkung er gut kennt, ein neues Mittel gibt, das eventuell besser wirkt, dessen Nebenwirkungen er aber z.B. gar nicht kennt. Da geht man wahrscheinlich doch kein Risiko ein und behandelt so wie es zuvor auch gut war.[/i]
Wenn jeder Arzt so handeln würde, gebe es keine Innovation. Dass es natürlich eine gewisse Trägheit gibt und nicht jeder Arzt gerade die neuste Errungenschaft an den Patienten bringt, ist jedoch eine Tatsache, weshalb fast jedes Medikament "klein anzufangen hat". Der Voprteil bei Cabo ist jedoch, dass dieses Medi bereits bei 2 weiteren Indikationen (RCC 2L und Schilddrüsenkarzinom) am Markt eingeführt ist , so dass doch bereits etliche Patiebten damit behandelt wurden. Die Aufmerksamkeit in den wissenschaftlichen Medien wird zudem für Cabo drastisch zunehmen, so dass zumindest in den grossen onkologischen Zentren sicherlich zeitnah das innovative Medikament zur Anwendung kommt.
Zu bedenken ist auch, dass Sutent bald generisch wird und dann viel billiger sein wird.
Wenn jedoch ein wirksameres Medikament am Markt ist, werden nur die wenigsten Ärzte dieses den Patienten vorenthalten.
Cabosun ist nur eine PII, die nicht mal selber von EXEL durchgeführt worden ist. Ich dachte eine PII reicht eventuell für eine Zulassung an austherapierten Patienten, die sowieso keine Alternative haben. Aber mit einer PII direkt den Standard bei der Ersthehaldung ändern? Das hört sich für mich sehr extrem an... ich hatte eine PIII erwartet.
Von wem die Studie durchgeführt bzw. gesponsert wurde, spielt nur dann eine Rolle, wenn die Studienleitung bzw. die daran beteiligten Kliniken nicht vertrauenswürdig ist/sind. Das NCI geniesst jedoch die allerbeste Reputation , ebenso die daran beteiligten Kliniken, so dass dies überhaupt keine Rolle spielt.
Natürlich wäre eine ph2-Studie für ein vollwertiges NDA kaum ausreichend, da es sich jedoch um einen ergaenzenden Zulassungsantrag handelt, ist ein Zulassung bei einer entsprechenden Guete der Daten durchaus moeglich.
Antwort auf Beitrag Nr.: 53.453.907 von Cyberhexe am 11.10.16 17:17:30Na, immer noch der Meinung, dass Cobi für EXEL das weit höhere Potential im Vgl. zu Cabo in sich birgt? Der gigantische Kurshüpfer seit Jahresbeginn ist fast ausschließlich auf Cabo zurückzuführen.
Antwort auf Beitrag Nr.: 53.454.174 von Ville7 am 11.10.16 17:42:16
Der Wahrheitsgehalt einer Lüge wird durch ständiges Wiederholen nicht grösser!
Zu deinen Unterstellungen nur so viel:
--> mein Beitrag Nr.401 auf deine Unwahrheiten:
Beitrag Nr. 401 (51.064.530)
Antwort auf Beitrag Nr.: 51.055.359 von Ville7 am 10.11.15 18:25:30
Ville7 schrieb:
"Und auch vom Potenzial her bist du viel zu bullish re Cobi. Es gibt einige Konkurrenzprodukte zu Cobi mit gleichem Wirkmechanismus. SL Garman und ipollit haben dich darüber bereits aufgeklärt, aber bei dir prallt so was ja ab wie Teflon."
Mein Kommentar:
"Es gibt praktisch zu jedem onkologisch wirksamen Wirkstoff Konkurrenzprodukte. Falls sich jedoch ein Hinauszögern des Therapieversagens sowie der synergistische Effekt zu Atezolizumab bestätigen sollte, dann bin ich nicht zu bullish. Bestätigen sich diese Thesen nicht, dann ist Cobi lediglich ein Kombiwirkstoff ähnlich wie Vemurafenib zur Behandlung von mMelanoma.
SLGraman und ipolt spielen nicht in deiner Liga - das solltest du so akzeptieren."
Ville7 schrieb:
"Exelixis steht und fällt mit Cabozantinib."
Mein Kommentar:
"das ist wieder schwarz-weiss; ein Unternehmen wird von vielen Faktoren beeinflusst, Exelixis im Wesentlichen von Cabo, Cobi sowie Cash Flow. Das grosse Problem von Exel ist, dass bei der Entwicklung von Cabo kein potenter Mitstreiter zur Verfügung steht, um alle viel versprechenden Indikationen in der Klinik zu testen. Aber vielleicht wird sich das noch ändern. Der Patentschutz dauert jedoch nicht ewig."
Ville7 schrieb:
"Wenn du nur wegen Cobi drin bist, wirst du mit deinem Investment wahrscheinlich sogar Erfolg haben, aber nicht weil Cobi der Erfolg für Exelixis wird, sondern Cabozantinib."
Mein Kommentar:
"Ich bin nicht nur wegen Cobi und auch nicht nur wegen Cabo in Exelixis investiert. Das Gesamtkonzept ist entscheidend. Hier sind wir dann wieder mal bei Adam&Eva."
SOLLTEN DIE LÜGEN UND UNTERSTELLUNGEN (versuch es doch mal mit Zitaten!) wieder häufiger werden, dann dürftest du wieder mal Nachricht bekommen.
Zitat von Ville7: Na, immer noch der Meinung, dass Cobi für EXEL das weit höhere Potential im Vgl. zu Cabo in sich birgt? Der gigantische Kurshüpfer seit Jahresbeginn ist fast ausschließlich auf Cabo zurückzuführen.
Der Wahrheitsgehalt einer Lüge wird durch ständiges Wiederholen nicht grösser!
Zu deinen Unterstellungen nur so viel:
--> mein Beitrag Nr.401 auf deine Unwahrheiten:
Beitrag Nr. 401 (51.064.530)
Antwort auf Beitrag Nr.: 51.055.359 von Ville7 am 10.11.15 18:25:30
Ville7 schrieb:
"Und auch vom Potenzial her bist du viel zu bullish re Cobi. Es gibt einige Konkurrenzprodukte zu Cobi mit gleichem Wirkmechanismus. SL Garman und ipollit haben dich darüber bereits aufgeklärt, aber bei dir prallt so was ja ab wie Teflon."
Mein Kommentar:
"Es gibt praktisch zu jedem onkologisch wirksamen Wirkstoff Konkurrenzprodukte. Falls sich jedoch ein Hinauszögern des Therapieversagens sowie der synergistische Effekt zu Atezolizumab bestätigen sollte, dann bin ich nicht zu bullish. Bestätigen sich diese Thesen nicht, dann ist Cobi lediglich ein Kombiwirkstoff ähnlich wie Vemurafenib zur Behandlung von mMelanoma.
SLGraman und ipolt spielen nicht in deiner Liga - das solltest du so akzeptieren."
Ville7 schrieb:
"Exelixis steht und fällt mit Cabozantinib."
Mein Kommentar:
"das ist wieder schwarz-weiss; ein Unternehmen wird von vielen Faktoren beeinflusst, Exelixis im Wesentlichen von Cabo, Cobi sowie Cash Flow. Das grosse Problem von Exel ist, dass bei der Entwicklung von Cabo kein potenter Mitstreiter zur Verfügung steht, um alle viel versprechenden Indikationen in der Klinik zu testen. Aber vielleicht wird sich das noch ändern. Der Patentschutz dauert jedoch nicht ewig."
Ville7 schrieb:
"Wenn du nur wegen Cobi drin bist, wirst du mit deinem Investment wahrscheinlich sogar Erfolg haben, aber nicht weil Cobi der Erfolg für Exelixis wird, sondern Cabozantinib."
Mein Kommentar:
"Ich bin nicht nur wegen Cobi und auch nicht nur wegen Cabo in Exelixis investiert. Das Gesamtkonzept ist entscheidend. Hier sind wir dann wieder mal bei Adam&Eva."
SOLLTEN DIE LÜGEN UND UNTERSTELLUNGEN (versuch es doch mal mit Zitaten!) wieder häufiger werden, dann dürftest du wieder mal Nachricht bekommen.
Antwort auf Beitrag Nr.: 53.456.754 von Cyberhexe am 11.10.16 23:11:48
Ein vollstaendiges Bild dieser "Luegen und Unterstellungen" erhaelt man, wenn man nicht nur die von dir selektierten Textschnippsel liest. Diese Muehe muss man sich schon machen..
Klaer mich bitte mal auf welche Nachricht ich von dir bekommen hatte. Willst du mich einschuechtern oder mit juristischem Schritten drohen? Laecherlich...
Meine Wahrnehmung sieht bei dir ein erstaunlich raumfuellendes Ego. Ein Nimbus der Unfehlbarkeit und immerwaehrender Wahrheit umgibt dich.. zumindest im Selbstbild...
Zitat von Cyberhexe: SOLLTEN DIE LÜGEN UND UNTERSTELLUNGEN (versuch es doch mal mit Zitaten!) wieder häufiger werden, dann dürftest du wieder mal Nachricht bekommen.
Ein vollstaendiges Bild dieser "Luegen und Unterstellungen" erhaelt man, wenn man nicht nur die von dir selektierten Textschnippsel liest. Diese Muehe muss man sich schon machen..
Klaer mich bitte mal auf welche Nachricht ich von dir bekommen hatte. Willst du mich einschuechtern oder mit juristischem Schritten drohen? Laecherlich...
Meine Wahrnehmung sieht bei dir ein erstaunlich raumfuellendes Ego. Ein Nimbus der Unfehlbarkeit und immerwaehrender Wahrheit umgibt dich.. zumindest im Selbstbild...
Antwort auf Beitrag Nr.: 53.457.075 von Ville7 am 12.10.16 02:48:58Der Informationsgehalt deiner Beiträge geht wieder mall gegen NULL. Es liegt zudem an demjenigen, der eine Behauptung aufstellt, diese, am besten mit Zitaten, auch zu beweisen. Aber das ist, so viel dürfte jedem klar sein, deine Sache nicht.
Toni K. Choueiri, MD,
http://doctors.dana-farber.org/directory/profile.asp?pict_id…
on Metastatic RCC: Results From the ALLIANCE A031203 Trial
2016 ESMO Congress
http://www.ascopost.com/videos/2016-esmo-congress/toni-k-cho…
Toni K. Choueiri, MD, hat die CABOSUN-Daten auf ESMO präsentiert und ist selbst sehr angetan von dessen Wirkung ..."does have the potential to change first line strategy"...weshalb er Cabo das Potenzial zugesteht, die bisherige Praxis der Erstlinienbehandlung in RCC zu verändern!
http://doctors.dana-farber.org/directory/profile.asp?pict_id…
on Metastatic RCC: Results From the ALLIANCE A031203 Trial
2016 ESMO Congress
http://www.ascopost.com/videos/2016-esmo-congress/toni-k-cho…
Toni K. Choueiri, MD, hat die CABOSUN-Daten auf ESMO präsentiert und ist selbst sehr angetan von dessen Wirkung ..."does have the potential to change first line strategy"...weshalb er Cabo das Potenzial zugesteht, die bisherige Praxis der Erstlinienbehandlung in RCC zu verändern!
Antwort auf Beitrag Nr.: 53.466.015 von Cyberhexe am 12.10.16 23:52:04Die EMA hat hestern zu Cabo einen neuen Assesssment Report veröffentlicht:
See the assessment report for Cabometyx (cabozantinib), a new medicine for patients with advanced kidney cancer:
http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/…
See the assessment report for Cabometyx (cabozantinib), a new medicine for patients with advanced kidney cancer:
http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/…
The 54th Annual Meeting of Japan Society of Clinical Oncology.
http://congress.jsco.or.jp/jsco2016/english/
Cabozantinib wird wohl auf folgender Veranstaltung einen wesentlichen Bestandteil darstellen:
October 20 (Thu) 16:00~18:00 Room 3 (Conference Center 3F 301+302)
International Session 4: Urological Cancer (Prostate and Renal Cancer)
Chair: Yukio Homma (Department of Urology, The University of Tokyo)
Clinical practice guideline for prostate cancer 2016 in Japan
Speaker: Yoichi Arai (Department of Urology, Tohoku University Graduate School of Medicine)
Year in review from US: Prostate cancer
Speaker: Anthony L. Zietman (Department of Radiation Oncology, Massachusetts General Hospital, USA)
ESMO guidelines and progress in renal cell cancer
Speaker: Alan Horwich (Clinical Oncology, The Institute of Cancer Research, UK)
Year in review from Japan: Renal cell carcinoma
Speaker: Hideaki Miyake (Urology, Faculty of Medicine, Hamamatsu University School of Medicine)
...da eine japanische Kooperation zum Vertrieb von Cabo im Land der aufgehenden Sonne noch auszuhandeln ist, wird Exelixis diese Veranstaltung sicherlich als "Roadschow" nutzen.
http://congress.jsco.or.jp/jsco2016/english/
Cabozantinib wird wohl auf folgender Veranstaltung einen wesentlichen Bestandteil darstellen:
October 20 (Thu) 16:00~18:00 Room 3 (Conference Center 3F 301+302)
International Session 4: Urological Cancer (Prostate and Renal Cancer)
Chair: Yukio Homma (Department of Urology, The University of Tokyo)
Clinical practice guideline for prostate cancer 2016 in Japan
Speaker: Yoichi Arai (Department of Urology, Tohoku University Graduate School of Medicine)
Year in review from US: Prostate cancer
Speaker: Anthony L. Zietman (Department of Radiation Oncology, Massachusetts General Hospital, USA)
ESMO guidelines and progress in renal cell cancer
Speaker: Alan Horwich (Clinical Oncology, The Institute of Cancer Research, UK)
Year in review from Japan: Renal cell carcinoma
Speaker: Hideaki Miyake (Urology, Faculty of Medicine, Hamamatsu University School of Medicine)
...da eine japanische Kooperation zum Vertrieb von Cabo im Land der aufgehenden Sonne noch auszuhandeln ist, wird Exelixis diese Veranstaltung sicherlich als "Roadschow" nutzen.
Antwort auf Beitrag Nr.: 53.488.614 von Cyberhexe am 16.10.16 14:03:10METEOR wird auf dem JSCO-Jahreskongress ein Thema sein:
http://www.myschedule.jp/jsco2016/search/result_list_multipl…
http://www.myschedule.jp/jsco2016/search/result_list_multipl…
Antwort auf Beitrag Nr.: 53.488.647 von Cyberhexe am 16.10.16 14:10:13The 54th Annual Meeting of JAPAN SOCITY of CLINICAL ONCOLOGY
Oct 20, 2016
IS4-3
ESMO guidelines and progress in renal cell cancer
Oct 20, 2016 16:00 - 18:00 Room 3 | Conference Center 3F 301+302
[Speaker] Alan Horwich:1
1:Clinical Oncology, The Institute of Cancer Research, UK
...ill be recommended with LOE I, SOR A and MCBS V. A trial of cabozantinib versus everolimus was in 658 patients who had failed a TKI....
Oct 20, 2016
IS4-3
ESMO guidelines and progress in renal cell cancer
Oct 20, 2016 16:00 - 18:00 Room 3 | Conference Center 3F 301+302
[Speaker] Alan Horwich:1
1:Clinical Oncology, The Institute of Cancer Research, UK
...ill be recommended with LOE I, SOR A and MCBS V. A trial of cabozantinib versus everolimus was in 658 patients who had failed a TKI....
Sorry Hexe, was bedeutet das?
Darum ist Exelixis ein Übernahmekandidat für mich:
https://finance.yahoo.com/m/04f5e658-0943-357c-a457-4645b68f…
https://finance.yahoo.com/m/04f5e658-0943-357c-a457-4645b68f…
Antwort auf Beitrag Nr.: 53.490.478 von Magnetfeldfredy am 17.10.16 07:18:47
auf dem Jahrestreffen der JAPAN SOCIETY of CLINICAL ONCOLOGY werden die METEOR-Daten präsentiert (Cabo 2L RCC vs. Everolimus) - das bringt natürlich Aufmerksamkeit, die beim Verhandeln mit potenziellen japanischen Interessenten über eine Partnerschaft nicht von Nachteil ist.
In February 2016, Exelixis and Ipsen announced an exclusive licensing agreement for the commercialization and further development of cabozantinib. Under the agreement, Ipsen will have exclusive commercialization rights for current and potential future cabozantinib indications outside of the United States, Canada and Japan.
METEOR
Cabozantinib is an oral inhibitor of tyrosine kinases including MET, VEGFR, and AXL. The randomised phase 3 METEOR trial compared the efficacy and safety of cabozantinib versus the mTOR inhibitor everolimus in patients with advanced renal cell carcinoma who progressed after previous VEGFR tyrosine-kinase inhibitor treatment.
Findings
Between Aug 8, 2013, and Nov 24, 2014, 658 patients were randomly assigned to receive cabozantinib (n=330) or everolimus (n=328). The median duration of follow-up for overall survival and safety was 18·7 months (IQR 16·1–21·1) in the cabozantinib group and 18·8 months (16·0–21·2) in the everolimus group. Median overall survival was 21·4 months (95% CI 18·7–not estimable) with cabozantinib and 16·5 months (14·7–18·8) with everolimus (hazard ratio [HR] 0·66 [95% CI 0·53–0·83]; p=0·00026). Cabozantinib treatment also resulted in improved progression-free survival (HR 0·51 [95% CI 0·41–0·62]; p<0·0001) and objective response (17% [13–22] with cabozantinib vs 3% [2–6] with everolimus; p<0·0001) per independent radiology review among all randomised patients. The most common grade 3 or 4 adverse events were hypertension (49 [15%] in the cabozantinib group vs 12 [4%] in the everolimus group), diarrhoea (43 [13%] vs 7 [2%]), fatigue (36 [11%] vs 24 [7%]), palmar-plantar erythrodysaesthesia syndrome (27 [8%] vs 3 [1%]), anaemia (19 [6%] vs 53 [17%]), hyperglycaemia (3 [1%] vs 16 [5%]), and hypomagnesaemia (16 [5%] vs none). Serious adverse events grade 3 or worse occurred in 130 (39%) patients in the cabozantinib group and in 129 (40%) in the everolimus group. One treatment-related death occurred in the cabozantinib group (death; not otherwise specified) and two occurred in the everolimus group (one aspergillus infection and one pneumonia aspiration).
Zitat von Magnetfeldfredy: Sorry Hexe, was bedeutet das?
auf dem Jahrestreffen der JAPAN SOCIETY of CLINICAL ONCOLOGY werden die METEOR-Daten präsentiert (Cabo 2L RCC vs. Everolimus) - das bringt natürlich Aufmerksamkeit, die beim Verhandeln mit potenziellen japanischen Interessenten über eine Partnerschaft nicht von Nachteil ist.
In February 2016, Exelixis and Ipsen announced an exclusive licensing agreement for the commercialization and further development of cabozantinib. Under the agreement, Ipsen will have exclusive commercialization rights for current and potential future cabozantinib indications outside of the United States, Canada and Japan.
METEOR
Cabozantinib is an oral inhibitor of tyrosine kinases including MET, VEGFR, and AXL. The randomised phase 3 METEOR trial compared the efficacy and safety of cabozantinib versus the mTOR inhibitor everolimus in patients with advanced renal cell carcinoma who progressed after previous VEGFR tyrosine-kinase inhibitor treatment.
Findings
Between Aug 8, 2013, and Nov 24, 2014, 658 patients were randomly assigned to receive cabozantinib (n=330) or everolimus (n=328). The median duration of follow-up for overall survival and safety was 18·7 months (IQR 16·1–21·1) in the cabozantinib group and 18·8 months (16·0–21·2) in the everolimus group. Median overall survival was 21·4 months (95% CI 18·7–not estimable) with cabozantinib and 16·5 months (14·7–18·8) with everolimus (hazard ratio [HR] 0·66 [95% CI 0·53–0·83]; p=0·00026). Cabozantinib treatment also resulted in improved progression-free survival (HR 0·51 [95% CI 0·41–0·62]; p<0·0001) and objective response (17% [13–22] with cabozantinib vs 3% [2–6] with everolimus; p<0·0001) per independent radiology review among all randomised patients. The most common grade 3 or 4 adverse events were hypertension (49 [15%] in the cabozantinib group vs 12 [4%] in the everolimus group), diarrhoea (43 [13%] vs 7 [2%]), fatigue (36 [11%] vs 24 [7%]), palmar-plantar erythrodysaesthesia syndrome (27 [8%] vs 3 [1%]), anaemia (19 [6%] vs 53 [17%]), hyperglycaemia (3 [1%] vs 16 [5%]), and hypomagnesaemia (16 [5%] vs none). Serious adverse events grade 3 or worse occurred in 130 (39%) patients in the cabozantinib group and in 129 (40%) in the everolimus group. One treatment-related death occurred in the cabozantinib group (death; not otherwise specified) and two occurred in the everolimus group (one aspergillus infection and one pneumonia aspiration).
Danke und viel Glück uns Longs!

Cobi wird derzeit zusammen mit Atezolizumab in 2 ph3-Studien (Melanom u. Dickdarm) sowie in ph2 mit Paclitaxel in Erstlinienbehandlung von TNBC (dreifach negativer Brustkrebs) in der Klinik getestet, wobei die bisherigen Tendenzen einer synergistischen Wirkung (Atezolizumab) sowie einer Verzögerung des Therapieversagens (Paclitaxel) bestätigt werden sollen.
https://clinicaltrials.gov/ct2/results?term=cobimetinib+atez…
Cobi ist für die mittel-bis längerfristige Perspektive Exelixis von grosser Bedeutung. Time will tell!
https://clinicaltrials.gov/ct2/results?term=cobimetinib+atez…
Cobi ist für die mittel-bis längerfristige Perspektive Exelixis von grosser Bedeutung. Time will tell!
Antwort auf Beitrag Nr.: 53.502.936 von Cyberhexe am 18.10.16 21:59:50es gibt bereits viel versprechnede Ergebnisse von Cabo mit dem Checkpoint-Hemmer Nivolumab (siehe untenstehender Bericht: "Nivo/Cabo Combo Shows Promise in GU Cancers" ) - ebenfalls vielversprechend ist die Kombi aus Cobi und Atezolizumab bei Dickdarmkrebs. Bin mal gespannt, ob irgendwann auch eine Kombi aus Cabo und Atezolizumab in der Klinik erscheint.
Nivolumab/Cabozantinib Combo Shows Promise in GU Cancers
Virginia Powers, PhD
Published Online: Tuesday, Oct 18, 2016
Print Button
Andrea B. Apolo, MD
Andrea B. Apolo, MD
Cabozantinib (Cabometyx) plus nivolumab (Opdivo) demonstrated promising activity in the second-line setting and beyond at all dose levels tested in patients with advanced/refractory genitourinary cancers, according to results from a phase I study reported at the 2016 ESMO Congress.1
Among 23 patients with evaluable data, the objective response rate (ORR) was 43%; 1 patient with bladder squamous cell carcinoma (SCC) showed complete response, and partial responses were observed in 9 patients. Among 6 patients with urothelial carcinoma, 4 achieved a response.
“Cabozantinib targets multiple tyrosine kinases, including VEGFR, MET, and AXL, and has also been reported to show immunomodulatory properties leading to a decrease in CD4+ T regulator cells, plus increased PD-1 expression, and decreased CTLA expression in these cells,” said principal investigator Andrea B. Apolo, MD, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute. “We recently reported that cabozantinib has durable clinical activity in heavily pretreated patients with relapsed or refractory metastatic urothelial carcinoma.2
“Cabozantinib has immunomodulary properties that may counteract tumor-induced immunosuppression, providing a rationale for combining it with the PD-1 inhibitor nivolumab,” added Apolo.
Apolo and colleagues conducted this phase I dose-escalation study to evaluate safety, and also to determine the dose-limiting toxicity and recommended phase II dose of cabozantinib plus nivolumab with or without ipilimumab.
The recommended phase II dose for the combination was determined as cabozantinib at 40 mg daily plus nivolumab at 3 mg/kg once every 2 weeks.
The study enrolled 6 patients with metastatic urothelial carcinoma, 4 with urachal bladder cancer, 3 with bladder SCC, 5 with germ cell tumor, 4 with castrate-resistant prostate cancer, 1 with renal cell carcinoma, and 1 with trophoblastic tumor. The median patient age was 55 years (range, 35-75) and 21 (88%) patients were male. Patients had received 1 to 6 (median, 3) prior treatments, with nearly half (n = 10) receiving 4 or more prior regimens. The Karnofsky Performance status was 90%, 80%, and 70% for 9, 12, and 3 patients, respectively.
“It is important to note that rare tumors, such as squamous cell carcinoma of the bladder, urachal adenocarcinoma, and penile cancer, demonstrated responses to the combination of cabozantinib and nivolumab,” Apolo said.
An additional 15 patients have been enrolled in part 2 of this study, which will evaluate the triplet of cabozantinib, nivolumab, and ipilimumab; those findings will be reported at a later date.
A rolling 6 design was used: In part 1, 6 patients received 4 dose levels (DL) of oral cabozantinib daily and nivolumab intravenously every 2 weeks for 28 days: DL1/2 comprised cabozantinib at 40 mg plus nivolumab at 1 mg/kg or 3 mg/kg, and DL3/4 comprised cabozantinib at 60 mg plus nivolumab at 1 mg/kg or 3 mg/kg. Tumors were assessed for ORR by RECIST 1.1 every 8 weeks.
“Activity was seen at all dose levels,” Apolo noted.
One patient left the study for an unrelated reason but 52% of patients remained on study at cutoff. Dose reductions were required for 9 patients. Mild-to-moderate fatigue was reported in 79% of patients, diarrhea in 71%, anorexia in 10%, and hoarseness in 10%.
Late adverse events (AEs) of any grade occurring in >40% of patients included increased alanine aminotransferase in 75% of patients, hypothyroidism in 63%, increased aspartase aminotransferase in 54%, and decreased neutrophil count in 42%. Grade 3 late AEs included decrease neutrophil count in 17% of patients. One grade 4 late AE of increased lipase was reported.
“The cabozantinib and nivolumab combination was well tolerated with no dose-limiting toxicities. Part 2 of this phase I study is ongoing and expansion studies in patients with metastatic urothelial and renal cell carcinoma are planned,” said Apolo.
References
Apolo AB, Mortazavi A, Stein M, et al. A phase I study of cabozantinib plus nivolumab (CaboNivo) in patients (pts) with refractory metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors. Presented at: 2016 ESMO Congress; October 7-11, 2016; Copenhagen, Denmark. Abstract 774PD.
Apolo AB, Parnes HL, Francis DC, et al. A phase II study of cabozantinib in patients (pts) with relapsed or refractory metastatic urothelial carcinoma (mUC). J Clin Oncol 34, 2016 (suppl; abstr 4534).
Nivolumab/Cabozantinib Combo Shows Promise in GU Cancers
Virginia Powers, PhD
Published Online: Tuesday, Oct 18, 2016
Print Button
Andrea B. Apolo, MD
Andrea B. Apolo, MD
Cabozantinib (Cabometyx) plus nivolumab (Opdivo) demonstrated promising activity in the second-line setting and beyond at all dose levels tested in patients with advanced/refractory genitourinary cancers, according to results from a phase I study reported at the 2016 ESMO Congress.1
Among 23 patients with evaluable data, the objective response rate (ORR) was 43%; 1 patient with bladder squamous cell carcinoma (SCC) showed complete response, and partial responses were observed in 9 patients. Among 6 patients with urothelial carcinoma, 4 achieved a response.
“Cabozantinib targets multiple tyrosine kinases, including VEGFR, MET, and AXL, and has also been reported to show immunomodulatory properties leading to a decrease in CD4+ T regulator cells, plus increased PD-1 expression, and decreased CTLA expression in these cells,” said principal investigator Andrea B. Apolo, MD, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute. “We recently reported that cabozantinib has durable clinical activity in heavily pretreated patients with relapsed or refractory metastatic urothelial carcinoma.2
“Cabozantinib has immunomodulary properties that may counteract tumor-induced immunosuppression, providing a rationale for combining it with the PD-1 inhibitor nivolumab,” added Apolo.
Apolo and colleagues conducted this phase I dose-escalation study to evaluate safety, and also to determine the dose-limiting toxicity and recommended phase II dose of cabozantinib plus nivolumab with or without ipilimumab.
The recommended phase II dose for the combination was determined as cabozantinib at 40 mg daily plus nivolumab at 3 mg/kg once every 2 weeks.
The study enrolled 6 patients with metastatic urothelial carcinoma, 4 with urachal bladder cancer, 3 with bladder SCC, 5 with germ cell tumor, 4 with castrate-resistant prostate cancer, 1 with renal cell carcinoma, and 1 with trophoblastic tumor. The median patient age was 55 years (range, 35-75) and 21 (88%) patients were male. Patients had received 1 to 6 (median, 3) prior treatments, with nearly half (n = 10) receiving 4 or more prior regimens. The Karnofsky Performance status was 90%, 80%, and 70% for 9, 12, and 3 patients, respectively.
“It is important to note that rare tumors, such as squamous cell carcinoma of the bladder, urachal adenocarcinoma, and penile cancer, demonstrated responses to the combination of cabozantinib and nivolumab,” Apolo said.
An additional 15 patients have been enrolled in part 2 of this study, which will evaluate the triplet of cabozantinib, nivolumab, and ipilimumab; those findings will be reported at a later date.
A rolling 6 design was used: In part 1, 6 patients received 4 dose levels (DL) of oral cabozantinib daily and nivolumab intravenously every 2 weeks for 28 days: DL1/2 comprised cabozantinib at 40 mg plus nivolumab at 1 mg/kg or 3 mg/kg, and DL3/4 comprised cabozantinib at 60 mg plus nivolumab at 1 mg/kg or 3 mg/kg. Tumors were assessed for ORR by RECIST 1.1 every 8 weeks.
“Activity was seen at all dose levels,” Apolo noted.
One patient left the study for an unrelated reason but 52% of patients remained on study at cutoff. Dose reductions were required for 9 patients. Mild-to-moderate fatigue was reported in 79% of patients, diarrhea in 71%, anorexia in 10%, and hoarseness in 10%.
Late adverse events (AEs) of any grade occurring in >40% of patients included increased alanine aminotransferase in 75% of patients, hypothyroidism in 63%, increased aspartase aminotransferase in 54%, and decreased neutrophil count in 42%. Grade 3 late AEs included decrease neutrophil count in 17% of patients. One grade 4 late AE of increased lipase was reported.
“The cabozantinib and nivolumab combination was well tolerated with no dose-limiting toxicities. Part 2 of this phase I study is ongoing and expansion studies in patients with metastatic urothelial and renal cell carcinoma are planned,” said Apolo.
References
Apolo AB, Mortazavi A, Stein M, et al. A phase I study of cabozantinib plus nivolumab (CaboNivo) in patients (pts) with refractory metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors. Presented at: 2016 ESMO Congress; October 7-11, 2016; Copenhagen, Denmark. Abstract 774PD.
Apolo AB, Parnes HL, Francis DC, et al. A phase II study of cabozantinib in patients (pts) with relapsed or refractory metastatic urothelial carcinoma (mUC). J Clin Oncol 34, 2016 (suppl; abstr 4534).
Roche berichtet für Cotellic Einnahmen (Jan - Sep) in Höhe von CHF 22m in Europa und CHF 8m
in US.
"Die Kombinationstherapie mit Cotellic
(CHF 30 Millionen) und Zelboraf bei metastasierendem Melanom wurde
gut vom Markt aufgenommen, insbesondere in Schlüsselmärkten wie
Frankreich, Deutschland und den USA."
http://www.roche.com/dam/jcr:d76d8b6b-09ff-4403-a664-452aae7…
in US.
"Die Kombinationstherapie mit Cotellic
(CHF 30 Millionen) und Zelboraf bei metastasierendem Melanom wurde
gut vom Markt aufgenommen, insbesondere in Schlüsselmärkten wie
Frankreich, Deutschland und den USA."
http://www.roche.com/dam/jcr:d76d8b6b-09ff-4403-a664-452aae7…
Antwort auf Beitrag Nr.: 53.513.352 von Cyberhexe am 20.10.16 07:25:39
Wenn man im Vergleich dazu die Verkaufszahlen von Zelboraf in Höhe von 160 Mio ansieht, ist das doch etwas enttäuschend. Auch wenn man berücksichtigt, dass Zelboraf natürlich noch weitere Umsätze ohne die Kombination mit Cotellic generiert...
Wie hoch sind nochmal die Royalties für EXEL?
Zitat von Cyberhexe: Roche berichtet für Cotellic Einnahmen (Jan - Sep) in Höhe von CHF 22m in Europa und CHF 8m
in US.
"Die Kombinationstherapie mit Cotellic
(CHF 30 Millionen) und Zelboraf bei metastasierendem Melanom wurde
gut vom Markt aufgenommen, insbesondere in Schlüsselmärkten wie
Frankreich, Deutschland und den USA."
http://www.roche.com/dam/jcr:d76d8b6b-09ff-4403-a664-452aae7…
Wenn man im Vergleich dazu die Verkaufszahlen von Zelboraf in Höhe von 160 Mio ansieht, ist das doch etwas enttäuschend. Auch wenn man berücksichtigt, dass Zelboraf natürlich noch weitere Umsätze ohne die Kombination mit Cotellic generiert...
Wie hoch sind nochmal die Royalties für EXEL?
Antwort auf Beitrag Nr.: 53.514.927 von Milestones am 20.10.16 10:14:51Royalties gibt es nur Ex-US.
US ist "profit share", aber auch "loss share". Und da sieht es so aus als ob EXEL in diesem Jahr 7-15 Mio USD am Verlust mittragen muss. Deswegen auch die Arbitration mit Roche, da Cotellic in der Kombi sehr billig angesetzt wurde und das sehr unfair erscheint.
US ist "profit share", aber auch "loss share". Und da sieht es so aus als ob EXEL in diesem Jahr 7-15 Mio USD am Verlust mittragen muss. Deswegen auch die Arbitration mit Roche, da Cotellic in der Kombi sehr billig angesetzt wurde und das sehr unfair erscheint.
Antwort auf Beitrag Nr.: 53.514.927 von Milestones am 20.10.16 10:14:51
Exelixis hat sich für eine gemeinsame Vermarktung von Cobimetinib mit Genentech in den US entschieden und trägt gemäss Vertrag 25% der beim Verkauf anfallenden Kosten für Vertrieb und Vermarktung. Ausserhalb der US erhält EXEL "low double digit" (ich schätze mal ca. 12-15%) Lizenzgebühren von den Netto-Umsätzen. Auch wenn kurzfristig sogar kleinere Verluste anfallen, dürfte mittel- bis langfristig dieses Vermarktungsmodell für EXEL weitaus lukrativer sein als dasjenige mit den Lizenzgebühren.
Allerdings wird bei der bisher einzigen Zulassung von Cobi (brand: Cotellic) dieses gemeinsam mit Vemurafenib (brand: Zelboraf) zur Behandlung des schwarzen Hautkrebs verabreicht. Die Kosten hierfür betragen ca. USD 17600/Behandlung - die Preisgestaltung von Genentech ist jedoch nicht im Sinne von Exelixis, da 2/3 dieser Kosten für Zelboraf und ca. nur 1/3 für Cotellic aufzuwenden sind. Exelixis hat deswegen ein Schiedsverfahren eingeleitet, um dieses Ungleichgewicht abzuschwächen.
Die heute von Roche berichteten 30mUSD für Cotellic sind ein erstes Ausrufezeichen, wobei die Wachstumsprognosen mit "sehr gut" bezeichnet werden.
EXELIXIS : Management's Discussion and Analysis of Financial Condition and Results of Operations. (form 10-Q)
05/04/2016 | 04:29pm EDT
The profit share has multiple tiers: we are entitled to 50% of profits and losses from the first
$200.0 million of U.S. actual sales, decreasing to 30% of profits and losses
from U.S. actual sales in excess of $400.0 million. We are entitled to low
double-digit royalties on ex-U.S. net sales. In November 2013, we exercised an
option under the collaboration agreement to co-promote in the United States, if
commercialized. Following the approval of COTELLIC in the United States in
November 2015, we began fielding 25% of the sales force promoting COTELLIC in
combination with vemurafenib as a treatment for patients with BRAF V600E or
V600K mutation-positive advanced melanoma.
NICE says Roche's Cotellic, Zelboraf combination for melanoma too costly
(Ref: London South East, NICE)
June 16th, 2016
"The UK list prices for Cotellic and Zelboraf are 4276 pounds ($6072) and 7000 pounds ($9941), respectively, for a 28-day supply."
Zitat von Milestones:Zitat von Cyberhexe: Roche berichtet für Cotellic Einnahmen (Jan - Sep) in Höhe von CHF 22m in Europa und CHF 8m
in US.
"Die Kombinationstherapie mit Cotellic
(CHF 30 Millionen) und Zelboraf bei metastasierendem Melanom wurde
gut vom Markt aufgenommen, insbesondere in Schlüsselmärkten wie
Frankreich, Deutschland und den USA."
http://www.roche.com/dam/jcr:d76d8b6b-09ff-4403-a664-452aae7…
Wenn man im Vergleich dazu die Verkaufszahlen von Zelboraf in Höhe von 160 Mio ansieht, ist das doch etwas enttäuschend. Auch wenn man berücksichtigt, dass Zelboraf natürlich noch weitere Umsätze ohne die Kombination mit Cotellic generiert...
Wie hoch sind nochmal die Royalties für EXEL?
Exelixis hat sich für eine gemeinsame Vermarktung von Cobimetinib mit Genentech in den US entschieden und trägt gemäss Vertrag 25% der beim Verkauf anfallenden Kosten für Vertrieb und Vermarktung. Ausserhalb der US erhält EXEL "low double digit" (ich schätze mal ca. 12-15%) Lizenzgebühren von den Netto-Umsätzen. Auch wenn kurzfristig sogar kleinere Verluste anfallen, dürfte mittel- bis langfristig dieses Vermarktungsmodell für EXEL weitaus lukrativer sein als dasjenige mit den Lizenzgebühren.
Allerdings wird bei der bisher einzigen Zulassung von Cobi (brand: Cotellic) dieses gemeinsam mit Vemurafenib (brand: Zelboraf) zur Behandlung des schwarzen Hautkrebs verabreicht. Die Kosten hierfür betragen ca. USD 17600/Behandlung - die Preisgestaltung von Genentech ist jedoch nicht im Sinne von Exelixis, da 2/3 dieser Kosten für Zelboraf und ca. nur 1/3 für Cotellic aufzuwenden sind. Exelixis hat deswegen ein Schiedsverfahren eingeleitet, um dieses Ungleichgewicht abzuschwächen.
Die heute von Roche berichteten 30mUSD für Cotellic sind ein erstes Ausrufezeichen, wobei die Wachstumsprognosen mit "sehr gut" bezeichnet werden.
EXELIXIS : Management's Discussion and Analysis of Financial Condition and Results of Operations. (form 10-Q)
05/04/2016 | 04:29pm EDT
The profit share has multiple tiers: we are entitled to 50% of profits and losses from the first
$200.0 million of U.S. actual sales, decreasing to 30% of profits and losses
from U.S. actual sales in excess of $400.0 million. We are entitled to low
double-digit royalties on ex-U.S. net sales. In November 2013, we exercised an
option under the collaboration agreement to co-promote in the United States, if
commercialized. Following the approval of COTELLIC in the United States in
November 2015, we began fielding 25% of the sales force promoting COTELLIC in
combination with vemurafenib as a treatment for patients with BRAF V600E or
V600K mutation-positive advanced melanoma.
NICE says Roche's Cotellic, Zelboraf combination for melanoma too costly
(Ref: London South East, NICE)
June 16th, 2016
"The UK list prices for Cotellic and Zelboraf are 4276 pounds ($6072) and 7000 pounds ($9941), respectively, for a 28-day supply."
Antwort auf Beitrag Nr.: 53.507.571 von Cyberhexe am 19.10.16 13:38:23Slides zur Kombi mit dem Checkpoint-Hemmer Nivo: sehr viel versprechend bei Blasenkrebs
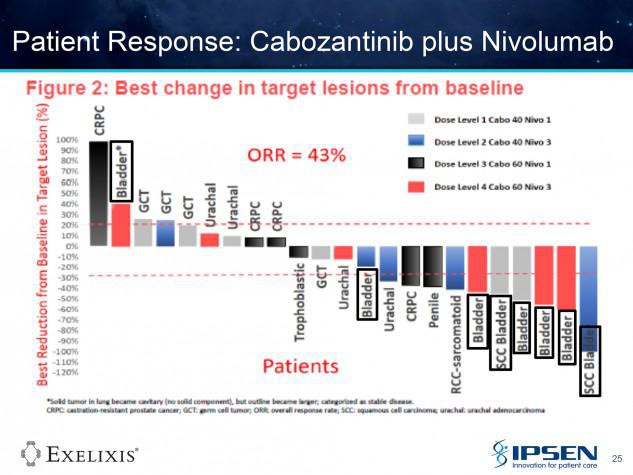

Antwort auf Beitrag Nr.: 53.522.130 von Cyberhexe am 21.10.16 05:43:46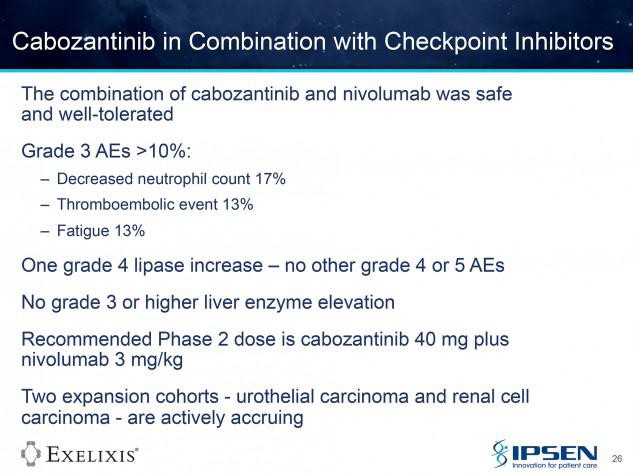

Diese kleine ph1b-Studie mit Cobi und Atezolizumab zur Behandlung von "microsatellite instability–high colorectal cancer" ist von grösserer Bedeutung, und zwar stützt diese die These, dass eine Hemmung des MAP-Kinase-Signalweges (bei Cobi die MEK-Hemmung) die Antitumoraktivität der Checkpointhemmer erweitert bzw. bei "Microsatellite Stable mCRC" erst möglich macht.
Anbei der Originalbeitrag von Franca
Es würde mich schon wundern, wenn Genentech den einzigen MEK-Hemmer in dessen Portfolio mit Exelixis teilen würde.
http://www.investorvillage.com/smbd.asp?mb=1086&mn=4699&pt=m…
Cobi + Atezolizumab in mCRC: it´s more than a study!
maybe this is a proof that inhibition of the MEK pathway can be synergistic with an PDL1 inhibitor promoting an infiltration of T-Cells into tumors triggering an anti-tumor activity with durable responses that had not been observed with the single checkpoint inhibitor - that sounds great. There is an ongoing resp. recruiting ph3 study (NCT02788279) evaluating both drugs in chemo-refractory metastatic Colrectal Adenocarcinoma:
https://clinicaltrials.gov/ct2/show/NCT02788279?term=NCT0278…
Cobimetinib Plus Atezolizumab Active in Microsatellite Stable mCRC
"The cobimetinib/atezolizumab combination showed an investigator-assessed observed response rate (ORR) of 17% in 23 patients overall. An ORR of 20% was observed in 22 patients with KRAS-mutant tumors."
http://www.onclive.com/conference-coverage/2016-world-gi/cob…
"So far, immunotherapy has only shown activity in patients with microsatellite instability–high colorectal cancer, which is only about 4% of the population,” said principal investigator Johanna C. Bendell, MD, director of the GI Cancer Research Program at the Sarah Cannon Research Institute and an associate with Tennessee Oncology in Nashville.
“Microsatellite instability–high colorectal cancers are associated with a deficiency in DNA mismatch repair and a high mutation burden which demonstrates response to single-agent therapy targeting the PD-L1/PD-1 axis; however, the majority of mCRC patients are MSS and have lower response rates to PD-L1/PD-1 blockade,” she continued. “So what do we do with the remaining 96% of patients?”
http://www.esmo.org/Press-Office/Press-Releases/Anti-PD-L1-I…
"Commenting on the findings, Professor Florian Lordick, Director of the University Cancer Centre Leipzig, Germany, says “this important phase 1b study now shows for the first time that metastatic colorectal cancer can be sensitized for immune therapy by inhibition of MEK-dependent intracellular signaling.”
Gruss Cyberhexe!
Anbei der Originalbeitrag von Franca

Es würde mich schon wundern, wenn Genentech den einzigen MEK-Hemmer in dessen Portfolio mit Exelixis teilen würde.
http://www.investorvillage.com/smbd.asp?mb=1086&mn=4699&pt=m…
Cobi + Atezolizumab in mCRC: it´s more than a study!
maybe this is a proof that inhibition of the MEK pathway can be synergistic with an PDL1 inhibitor promoting an infiltration of T-Cells into tumors triggering an anti-tumor activity with durable responses that had not been observed with the single checkpoint inhibitor - that sounds great. There is an ongoing resp. recruiting ph3 study (NCT02788279) evaluating both drugs in chemo-refractory metastatic Colrectal Adenocarcinoma:
https://clinicaltrials.gov/ct2/show/NCT02788279?term=NCT0278…
Cobimetinib Plus Atezolizumab Active in Microsatellite Stable mCRC
"The cobimetinib/atezolizumab combination showed an investigator-assessed observed response rate (ORR) of 17% in 23 patients overall. An ORR of 20% was observed in 22 patients with KRAS-mutant tumors."
http://www.onclive.com/conference-coverage/2016-world-gi/cob…
"So far, immunotherapy has only shown activity in patients with microsatellite instability–high colorectal cancer, which is only about 4% of the population,” said principal investigator Johanna C. Bendell, MD, director of the GI Cancer Research Program at the Sarah Cannon Research Institute and an associate with Tennessee Oncology in Nashville.
“Microsatellite instability–high colorectal cancers are associated with a deficiency in DNA mismatch repair and a high mutation burden which demonstrates response to single-agent therapy targeting the PD-L1/PD-1 axis; however, the majority of mCRC patients are MSS and have lower response rates to PD-L1/PD-1 blockade,” she continued. “So what do we do with the remaining 96% of patients?”
http://www.esmo.org/Press-Office/Press-Releases/Anti-PD-L1-I…
"Commenting on the findings, Professor Florian Lordick, Director of the University Cancer Centre Leipzig, Germany, says “this important phase 1b study now shows for the first time that metastatic colorectal cancer can be sensitized for immune therapy by inhibition of MEK-dependent intracellular signaling.”
Gruss Cyberhexe!
Antwort auf Beitrag Nr.: 53.529.567 von Cyberhexe am 21.10.16 23:48:20anbei noch eine ASCO-Grafik zu Cobimetinib (C) +Atezolizumab (A) bei Dickdarmkrebs (CRC):
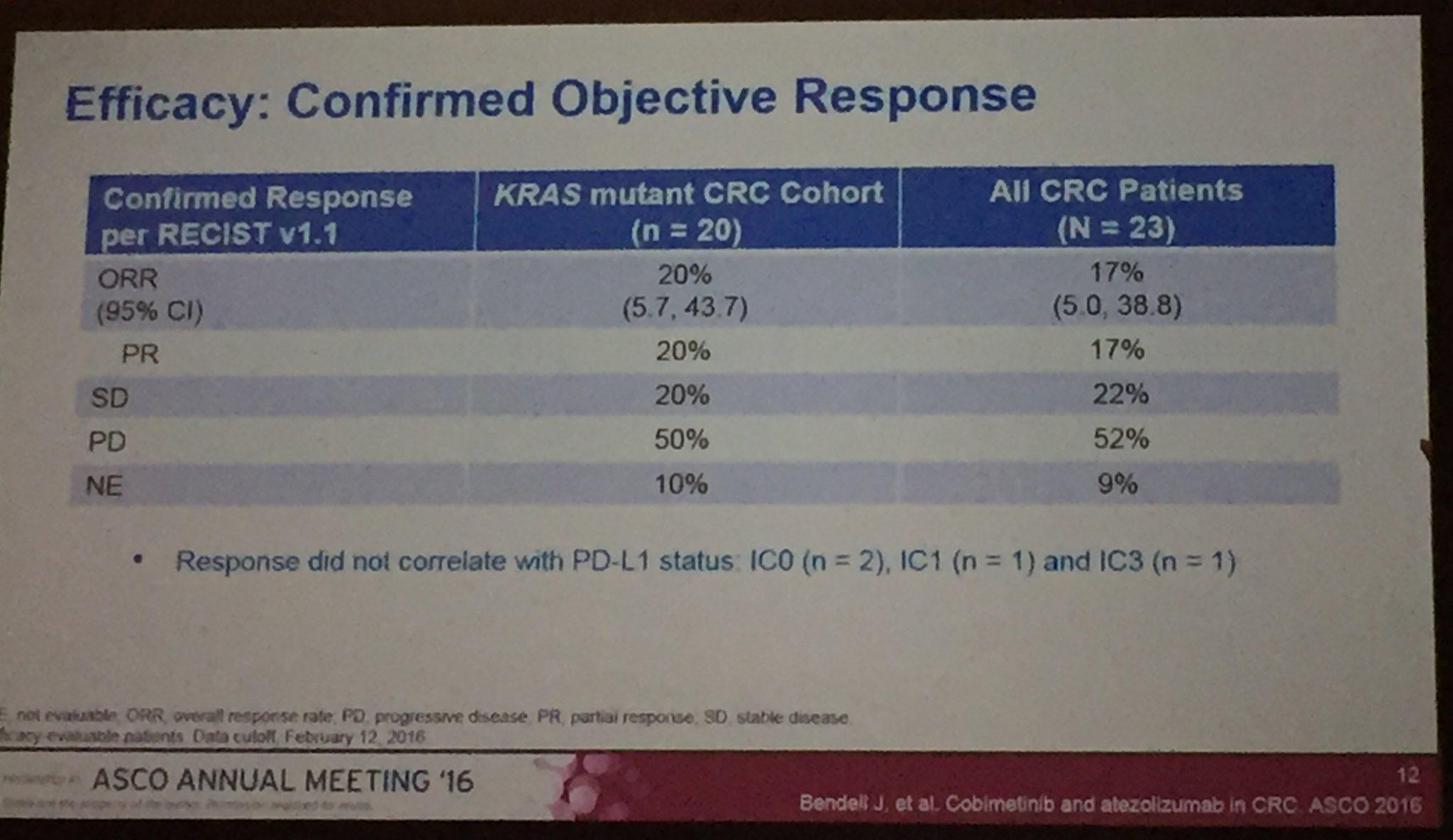
Durch Aktivierung der Wachstumsrezeptoren sowie durch Mutationen ist der MAP-Kinase-Signalweg einer der am häuifigsten disregulierten Signalwege bei Krebs und deswegen wohl auch ein viel versprechender Ansatzpunkt zu dessen Therapie.
Beim untersuchten CRC ist die antitumorale Aktivität bei der Kombitherapie (C+A) weitaus grösser als bei der Verabreichung der Einzelkomponenten, bspw. bei "Microsatellite stabilem metastasenbildendem Dickdarmkrebs" --> mit den Einzelkomponenten fast keine Wirkung nachweisbar, in der Kombitherapie jedoch eine Ansprechrate von 17% bzw. 20% (KRAS wild type).
Daduirch wird die These gestützt, dass Cobi in der Lage ist, den Tumor für den Checkpoint-Hemmer zu sensibilisieren.
Exelixis hätte als kleines Unternehmen nicht die Möglichkeit, das möglicherweise unendliche Potenzial eines MEK-Hemmers zu schöpfen, weshalb die Kooperation mit Genentech aus wissenschaftlicher Sicht optimal ist. Sollte sich die These der Synergie zwischen Checkpoint- und MEK-Hemmer in der ph3 bestätigen - davon ist auszugehen-, dann bin ich mal gespannt wie lange es dauert, bis die beiden Nachbarn in SSF ihren Zaun einreissen.

Durch Aktivierung der Wachstumsrezeptoren sowie durch Mutationen ist der MAP-Kinase-Signalweg einer der am häuifigsten disregulierten Signalwege bei Krebs und deswegen wohl auch ein viel versprechender Ansatzpunkt zu dessen Therapie.
Beim untersuchten CRC ist die antitumorale Aktivität bei der Kombitherapie (C+A) weitaus grösser als bei der Verabreichung der Einzelkomponenten, bspw. bei "Microsatellite stabilem metastasenbildendem Dickdarmkrebs" --> mit den Einzelkomponenten fast keine Wirkung nachweisbar, in der Kombitherapie jedoch eine Ansprechrate von 17% bzw. 20% (KRAS wild type).
Daduirch wird die These gestützt, dass Cobi in der Lage ist, den Tumor für den Checkpoint-Hemmer zu sensibilisieren.
Exelixis hätte als kleines Unternehmen nicht die Möglichkeit, das möglicherweise unendliche Potenzial eines MEK-Hemmers zu schöpfen, weshalb die Kooperation mit Genentech aus wissenschaftlicher Sicht optimal ist. Sollte sich die These der Synergie zwischen Checkpoint- und MEK-Hemmer in der ph3 bestätigen - davon ist auszugehen-, dann bin ich mal gespannt wie lange es dauert, bis die beiden Nachbarn in SSF ihren Zaun einreissen.
COLET ist eine weitere interssante Studie, bei der die Verzögerung des Therapieversagens durch Cobi bei Taxanen nachgewiesen werden soll.
"Cobimetinib (COBI) + Paclitaxel (PTX) as first-line treatment in patients (pts) with advanced triple-negative breast cancer (TNBC): Interim safety review of the ongoing phase 2 COLET study"
Diese Studie besteht aus 2 Teilen, und zwar einem ersten, bereits abgeschlossenen Teil (eine sogen run-in-Studie), bei welchem prioritär Verträglichkeit und Sicherheit der Wirkstoffkombination im Vordergrund stehen sowie einen 2. Teil, bei welchem randomisert und verblindet nachgewiesen werden soll, dass Cobi die Aktivität von Paclitaxel verlängert bzw. das Therapieversagen hinauszögert.
Die beobachtete Wirkung bei den 16 untersuchten Teilnehmern in Teil 1 (8 PR ="partial response" sowie 3 Teilnehmer mit "stable disease") ist sehr viel versprechend bei dieser sehr schwierig zu behandelnden Form von Brustkrebs. Die Nebenwirkungsprofile entsprechen denen der Einzelanwendung beider Wirkstoffe.
Sollte sich diese These des verzögerten Therapieversagens in dieser Studie bestätigen, sind der Phantasie keine Grenzen gesetzt. Ich weiss nicht, ob Genentech dann bereit sit, ein derartiges Schlüsselmolekül mit einem kleinen Partner wie Exelixis in den US zu teilen .
Preliminary efficacy from safety run-in included unconfirmed partial response (n = 8), stable disease (n = 3), and progressive disease (n = 3); 2 pts had not completed a tumor assessment. Mutation and copy number changes in oncogenes, tumor suppressors, and other genes associated with TNBC will be reported. Conclusions: This is the first study to evaluate C+P in TNBC. The safety profile of C+P is consistent with known safety profiles. Initial results are promising, and enrollment in the randomized stage is ongoing (primary efficacy outcome of progression-free survival)
"Cobimetinib (COBI) + Paclitaxel (PTX) as first-line treatment in patients (pts) with advanced triple-negative breast cancer (TNBC): Interim safety review of the ongoing phase 2 COLET study"
Diese Studie besteht aus 2 Teilen, und zwar einem ersten, bereits abgeschlossenen Teil (eine sogen run-in-Studie), bei welchem prioritär Verträglichkeit und Sicherheit der Wirkstoffkombination im Vordergrund stehen sowie einen 2. Teil, bei welchem randomisert und verblindet nachgewiesen werden soll, dass Cobi die Aktivität von Paclitaxel verlängert bzw. das Therapieversagen hinauszögert.
Die beobachtete Wirkung bei den 16 untersuchten Teilnehmern in Teil 1 (8 PR ="partial response" sowie 3 Teilnehmer mit "stable disease") ist sehr viel versprechend bei dieser sehr schwierig zu behandelnden Form von Brustkrebs. Die Nebenwirkungsprofile entsprechen denen der Einzelanwendung beider Wirkstoffe.
Sollte sich diese These des verzögerten Therapieversagens in dieser Studie bestätigen, sind der Phantasie keine Grenzen gesetzt. Ich weiss nicht, ob Genentech dann bereit sit, ein derartiges Schlüsselmolekül mit einem kleinen Partner wie Exelixis in den US zu teilen .
Preliminary efficacy from safety run-in included unconfirmed partial response (n = 8), stable disease (n = 3), and progressive disease (n = 3); 2 pts had not completed a tumor assessment. Mutation and copy number changes in oncogenes, tumor suppressors, and other genes associated with TNBC will be reported. Conclusions: This is the first study to evaluate C+P in TNBC. The safety profile of C+P is consistent with known safety profiles. Initial results are promising, and enrollment in the randomized stage is ongoing (primary efficacy outcome of progression-free survival)
Antwort auf Beitrag Nr.: 53.540.397 von Cyberhexe am 24.10.16 14:03:00Uebrigens wird COLET randPh2 TNBC 1L (randomisierte Phase 2 Studie zur Erstlinienbehandlung von dreifach negativem Brustkrebs) ausgewertet, wenn in beiden Armen 60 Ereignisse hinsichtlich PFS (Progression Free Survival ) beobachtet wurden. Die Strudie hat 77% power um eine Hazard Ratio von 0.5, also ein um 50% geringeres Risiko beim Fortschreiten der Erkrankung bei einem zweiseitigen Signifikanzlevel von unter 5 %, nachzuweisen.
In the randomized stage, pts will be followed up until 60 PFS events occur across the 2 arms. This provides 77% power to detect a hazard ratio of 0.5 at a two-sided significance level of 0.05. Recruitment into safety run-in is complete; randomization stage accrual has begun. Pts from sites across Europe, North America, and the Asia-Pacific region will be enrolled.
In the randomized stage, pts will be followed up until 60 PFS events occur across the 2 arms. This provides 77% power to detect a hazard ratio of 0.5 at a two-sided significance level of 0.05. Recruitment into safety run-in is complete; randomization stage accrual has begun. Pts from sites across Europe, North America, and the Asia-Pacific region will be enrolled.
Hyperlink zum Video von Toni Choueiri, MD of Dana-Farber Cancer Institute, Boston, MA bei seiner Präsentation der CABOSUN-Daten auf ESMO 2016 in Kopenhagen:
http://www.oncologytube.com/video/esmo-2016-press-brief-on-r…
Toni Choueiri ist davon überzeugt, dass Cabozantinib eine potenzielle Erstlinienbehandlung bei fortgeschrittenem Nierenkrebs darstellt.
Description: Toni Choueiri, MD of Dana-Farber Cancer Institute, Boston, MA gives a press brief about the results of the CABOSUN trial of cabozantinib compared to sunitinib in treatment naive poor and intermediate risk renal-cell carcinoma (RCC) patients (NCT01835158) at the 2016 annual meeting of the European Society of Medical Oncology (ESMO), held in Copenhagen, Denmark. Dr Choueiri explains that cabozantinib is an oral inhibitor of tyrosine kinases. The primary endpoint of the trial was progression-free survival (PFS) and secondary endpoint was overall survival (OS), objective response rate (ORR) and safety. He explains that treatment with cabozantinib resulted in median PFS of 8.2 months compared to 5.6 arms with the control arm of sunitinib. ORR was 46% for cabozantinib compared to 18% for sunitinib. OS is preliminary; median OS with cabozantinib is 30.3 months compared to 21.8 months for sunitinib. They intend to have an update on OS within the next months. In terms of safety, occurence of grade 3/4 events was not different and discontinuation around 20% for both drugs. There were no suprises in terms of side effects, which include hypertension, diarrhea, PPE and fatigue. Further, there was a greater exposure in the cabozantinib arm. He then explains the implications; sunitinib is a standard of care therapy in first-line in metastatic RCC and as cabozantinib improved PFS and ORR, he believes that it presents a potential first-line treatment in patients with advanced RCC.
http://www.oncologytube.com/video/esmo-2016-press-brief-on-r…
Toni Choueiri ist davon überzeugt, dass Cabozantinib eine potenzielle Erstlinienbehandlung bei fortgeschrittenem Nierenkrebs darstellt.
Description: Toni Choueiri, MD of Dana-Farber Cancer Institute, Boston, MA gives a press brief about the results of the CABOSUN trial of cabozantinib compared to sunitinib in treatment naive poor and intermediate risk renal-cell carcinoma (RCC) patients (NCT01835158) at the 2016 annual meeting of the European Society of Medical Oncology (ESMO), held in Copenhagen, Denmark. Dr Choueiri explains that cabozantinib is an oral inhibitor of tyrosine kinases. The primary endpoint of the trial was progression-free survival (PFS) and secondary endpoint was overall survival (OS), objective response rate (ORR) and safety. He explains that treatment with cabozantinib resulted in median PFS of 8.2 months compared to 5.6 arms with the control arm of sunitinib. ORR was 46% for cabozantinib compared to 18% for sunitinib. OS is preliminary; median OS with cabozantinib is 30.3 months compared to 21.8 months for sunitinib. They intend to have an update on OS within the next months. In terms of safety, occurence of grade 3/4 events was not different and discontinuation around 20% for both drugs. There were no suprises in terms of side effects, which include hypertension, diarrhea, PPE and fatigue. Further, there was a greater exposure in the cabozantinib arm. He then explains the implications; sunitinib is a standard of care therapy in first-line in metastatic RCC and as cabozantinib improved PFS and ORR, he believes that it presents a potential first-line treatment in patients with advanced RCC.
Beim Jahrestreffen 2016 der "Society for Melanoma Research" in Boston, Massachusetts vom 6.-9.11.2016 sind folgende interessante Veröffentlichungen angekündigt:
Plenary Session 2, 7. November 10h30 - 12h30
Safety and Clinical Activity of Atezolizumab + Cobimetinib + Vemurafenib in BRAFV600 mutant metastatic melanoma
Daten von knokurrierenden APIs
Plenary Session 10, 9. November 8h00 - 9h35
- Efficacy of nivolumab (NIVO) plus ipilimumab (IPI) combination in patients with
advanced melanoma (MEL)and elevated serum lactate dehydrogenase (LDH):
a pooled analysis
- Checkmate 037 Überlebenszeitanalyse in Zweitlinienbehandlung mMelanoma
- KEYNOTE-006 patients treated with ipilimumab im Anschluss an Pemprolizumab
Plenary Session 11 -LBA (Late Breaking Abstracts) - 9.November 10h00 - 12h00
- Results of COLUMBUS Part 1 --> ph3-Studie Encorafenib (ENCO) Plus Binimetinib
(BINI)Versus Vemurafenib (VEM) or ENCO in BRAF‐Mutant Melanoma
- neoadjuvant + adjuvant dabrafenib and trametinib (D+T) is associated
with improved relapse ‐free survival (RFS) versus standard of care (SOC)
http://www.melanomacongress.com/docs/2016_SMR_Agenda.pdf
Plenary Session 2, 7. November 10h30 - 12h30
Safety and Clinical Activity of Atezolizumab + Cobimetinib + Vemurafenib in BRAFV600 mutant metastatic melanoma
Daten von knokurrierenden APIs
Plenary Session 10, 9. November 8h00 - 9h35
- Efficacy of nivolumab (NIVO) plus ipilimumab (IPI) combination in patients with
advanced melanoma (MEL)and elevated serum lactate dehydrogenase (LDH):
a pooled analysis
- Checkmate 037 Überlebenszeitanalyse in Zweitlinienbehandlung mMelanoma
- KEYNOTE-006 patients treated with ipilimumab im Anschluss an Pemprolizumab
Plenary Session 11 -LBA (Late Breaking Abstracts) - 9.November 10h00 - 12h00
- Results of COLUMBUS Part 1 --> ph3-Studie Encorafenib (ENCO) Plus Binimetinib
(BINI)Versus Vemurafenib (VEM) or ENCO in BRAF‐Mutant Melanoma
- neoadjuvant + adjuvant dabrafenib and trametinib (D+T) is associated
with improved relapse ‐free survival (RFS) versus standard of care (SOC)
http://www.melanomacongress.com/docs/2016_SMR_Agenda.pdf
...konnte nicht widerstehen und hab meine EXEL-Position heute wieder ausgebaut.
Das Ergebnis entspricht in etwa meinen Erwartungen und verspricht sehr viel Wachstumspotenzial:
http://www.exelixis.com/investors-media/press-releases?cpurl…
Das Ergebnis entspricht in etwa meinen Erwartungen und verspricht sehr viel Wachstumspotenzial:
http://www.exelixis.com/investors-media/press-releases?cpurl…
Warum der Kursverfall......................
Antwort auf Beitrag Nr.: 53.616.369 von Cyberhexe am 03.11.16 21:26:31Börse ist nicht immer rational: da ist kein Haar in der Suppe - also, ich habe zumindest auf den ersten Blick keines entdeckt - und der Kurs wird einstellig. Dann wird die Cash-Position vollständig reinvestiert.
- Cabozantinib Franchise Net Product Revenues of $42.7 Million -
- Total Revenues of $62.2 Million -
- Conference Call and Webcast Today at 5:00 PM Eastern Time -
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)-- Exelixis, Inc. (EXEL) today reported financial results for the third quarter of 2016 and provided an update on progress toward delivering upon its key 2016 corporate objectives, as well as commercial and clinical development milestones.
Exelixis is focused on the U.S. launch of CABOMETYX™ (cabozantinib) tablets as a treatment for patients with advanced renal cell carcinoma (RCC) who have received prior anti-angiogenic therapy. CABOMETYX generated $31.2 million in net product revenue during the third quarter of 2016, which reflects the first full quarter of product sales. Net product revenues for the third quarter of 2016, including sales of COMETRIQ® (cabozantinib) capsules for the treatment of certain forms of thyroid cancer, were $42.7 million. While Exelixis focuses on commercialization in the United States, its partner Ipsen is in the process of launching CABOMETYX in the European Union, following the European Commission’s (EC) September 2016 approval of CABOMETYX for the treatment of adult patients with advanced RCC who have received prior vascular endothelial growth factor (VEGF)-targeted therapy. Exelixis is eligible to receive royalties on CABOMETYX sales by Ipsen outside of the United States, Canada and Japan.
“The third quarter of 2016 was an important inflection point for Exelixis. We recorded our first full quarter of CABOMETYX sales and also made significant progress on our path towards becoming a profitable, fully integrated, commercial biopharmaceutical company,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “Feedback from prescribers, as well as performance to date, suggest that clinicians treating advanced renal cell carcinoma see CABOMETYX as a differentiated therapy and are increasingly incorporating it into their practice. While we continued to execute on the U.S. CABOMETYX launch and pursue important clinical trials like CABOSUN that have the potential to further advance our business, we also demonstrated sound fiscal discipline, resulting in a significantly decreased net loss and cash burn. As we close out the year, we remain committed to maximizing our opportunity to improve the treatment of cancer while building a strong and nimble company.”
Cabozantinib Highlights
Presented Positive Results from Phase 2 CABOSUN Trial in Advanced RCC. At the European Society for Medical Oncology (ESMO) 2016 Congress (CACOX), detailed results were presented from CABOSUN, the randomized phase 2 trial of cabozantinib compared with sunitinib in patients with previously untreated advanced RCC with intermediate- or poor-risk disease per the International Metastatic Renal Carcinoma Database Consortium risk criteria. In this trial, cabozantinib demonstrated a statistically significant and clinically meaningful reduction in the rate of disease progression or death as compared to sunitinib. The CABOSUN results were the subject of a late-breaking abstract at ESMO, and were highlighted at one of the Congress’ Presidential Symposia and in its official media program. CABOSUN was conducted by The Alliance for Clinical Trials in Oncology with support from the National Cancer Institute’s Cancer Therapy Evaluation Program (NCI-CTEP).
Plans for Supplemental New Drug Application in First-Line Advanced RCC. Based on the CABOSUN results, Exelixis plans to submit a Supplemental New Drug Application (sNDA) for cabozantinib as a treatment for previously untreated advanced RCC. The company is working with The Alliance to transfer the complete CABOSUN clinical database to Exelixis and will facilitate an independent radiological review of the CABOSUN imaging data in preparation for filing.
Phase 1 Trial Results for Cabozantinib in Combination with Nivolumab in Advanced Genitourinary Tumors. Also at the ESMO 2016 Congress, positive results were presented from the NCI-CTEP-sponsored phase 1 trial of cabozantinib in combination with nivolumab in patients with previously treated genitourinary tumors. Part II of the study is evaluating the triplet combination of cabozantinib, nivolumab, and ipilimumab and thus far has enrolled 15 patients. Expansion cohorts assessing cabozantinib and nivolumab, including patients with bladder, renal, and rare genitourinary cancers, are also currently being accrued.
European Commission Approval of CABOMETYX for the Treatment of Advanced RCC. On September 9, 2016, the EC approved CABOMETYX for the treatment of advanced RCC in adults following prior VEGF-targeted therapy. The approval allows for the marketing of CABOMETYX in all 28 member states of the European Union, Norway and Iceland. Under the license agreement with Ipsen, the EC approval triggered a $60.0 million milestone payment from Ipsen to Exelixis, which is expected to be received in the fourth quarter of 2016.
Outcome from First Planned Interim Analysis of Phase 3 CELESTIAL Trial. On September 6, 2016, Exelixis announced the outcome from the first planned interim analysis of CELESTIAL, the randomized global phase 3 trial of cabozantinib compared with placebo in patients with advanced hepatocellular carcinoma who have been previously treated with sorafenib. Following the analysis, the trial’s Independent Data Monitoring Committee determined that the study should continue without modifications per the study protocol. The trial protocol calls for a second interim analysis to take place once 75 percent of planned events have been observed.
Cobimetinib Highlights
Results from Cobimetinib Combination Trials Support Further Advancement. Cobimetinib, the Exelixis-discovered MEK inhibitor now the subject of a worldwide collaboration with Genentech, a member of the Roche Group, was the subject of seven presentations at the ESMO 2016 Congress. For the first time, investigators presented preliminary results from the phase 1b clinical trial of the triple combination of cobimetinib, vemurafenib, and atezolizumab in patients with previously untreated BRAF V600 mutation-positive advanced melanoma. The regimen was associated with promising antitumor activity and a manageable safety profile; details of a subsequent Roche-sponsored phase 3 pivotal trial, TRILOGY, have been posted to www.ClinicalTrials.gov. Investigators also presented updated results from the phase 1 trial of cobimetinib plus atezolizumab in advanced colorectal cancer that provide a rationale for COTEZO, the ongoing phase 3 pivotal trial in the same disease setting. New data from the phase 1 part of COLET, the phase 1/2 trial of cobimetinib and paclitaxel in triple-negative breast cancer, were also the subject of a poster presentation at the meeting.
Third Quarter 2016 Financial Results
Total revenues for the quarter ended September 30, 2016 were $62.2 million, compared to $9.9 million for the comparable period in 2015. Total revenues for the third quarter of 2016 include $42.7 million of net product revenue compared to $6.9 million for the comparable period in 2015. The increase in net product revenues for the three months ended September 30, 2016, as compared to the same period in 2015, reflects the impact of the commercial launch of CABOMETYX in late April 2016, as well as an increase in COMETRIQ revenues. Net product revenues for CABOMETYX and COMETRIQ were $31.2 million and $11.5 million, respectively. Total revenues for the quarter ended September 30, 2016 include the recognition of $15.0 million of contract revenue from the Daiichi Sankyo CS-3150 milestone, $3.8 million of license revenues recognized under Exelixis' collaboration and license agreement with Ipsen and $0.7 million of royalties on ex-U.S. net sales of COTELLIC® (cobimetinib). There was $3.0 million of contract revenues for a milestone payment received from Merck (MRK) related to their worldwide license of Exelixis' PI3K-delta program during the comparable period in 2015.
Research and development expenses for the quarter ended September 30, 2016 were $20.3 million, compared to $26.1 million for the comparable period in 2015. The decrease was primarily related to decreases in share-based compensation, clinical trial costs and the allocation of general corporate costs; those decreases were partially offset by increases in personnel related expenses resulting from an increase in headcount predominantly associated with the build-out of the Exelixis Medical Affairs organization.
Selling, general and administrative expenses for the quarter ended September 30, 2016 were $32.5 million, compared to $17.8 million for the comparable period in 2015. The increase was primarily related to an increase in personnel related expenses resulting from an increase in headcount connected with the build-out of the Exelixis U.S. commercial organization, marketing and outside services to support the launch and commercialization of CABOMETYX.
Other income (expense), net for the quarter ended September 30, 2016 was a net expense of ($18.5) million compared to ($9.8) million for the comparable period in 2015. The increase in net expense was primarily due to the $13.8 million of loss associated with the conversion through September 30, 2016 of $285.3 million in aggregate principal amount of the company's 2019 Notes for 53,704,911 shares of our common stock. The net expense also includes interest expense which includes $3.9 million of non-cash expense related to the accretion of the discounts on both the 2019 Notes and the company’s indebtedness under its Secured Convertible Notes due 2018 held by entities associated with Deerfield for the quarter ended September 30, 2016, as compared to $4.9 million for the comparable period in 2015.
Net loss for the quarter ended September 30, 2016 was ($11.3) million, or ($0.04) per share, basic, compared to ($45.5) million, or ($0.21) per share, basic, for the comparable period in 2015. The decreased net loss for the quarter was primarily due to increases in net revenues and a decrease in research and development expenses, which were partially offset by increases in selling, general and administrative expenses and other income (expense), net.
Cash and cash equivalents, short- and long-term investments and long-term restricted cash and investments totaled $379.6 million at September 30, 2016, which increased from $253.3 million at December 31, 2015.
Basis of Presentation
Exelixis adopted a 52- or 53-week fiscal year that generally ends on the Friday closest to December 31st. For convenience, references in this press release as of and for the fiscal periods ended September 30, 2016, January 1, 2016 and October 2, 2015 are indicated as being as of and for the periods ended September 30, 2016, December 31, 2015 and September 30, 2015, respectively.
Correction of an Immaterial Error
Certain historical amounts in other income (expense), net, net loss and stockholders’ equity (deficit) presented herein have been revised to reflect the correction of the accounting for non-cash interest expense associated with the 2019 Notes. See “Note 1 - Organization and Summary of Significant Accounting Policies” to Exelixis' Condensed Consolidated Financial Statements included in Exelixis' quarterly report on Form 10-Q for the quarterly period ended September 30, 2016 for a further description of this error and the historical amounts which have been corrected.
Conference Call and Webcast
Exelixis management will discuss the company’s financial results for the third quarter of 2016 and provide a general business update during a conference call beginning at 5:00 p.m. EDT/2:00 p.m. PDT today, Thursday, November 3, 2016.
- Cabozantinib Franchise Net Product Revenues of $42.7 Million -
- Total Revenues of $62.2 Million -
- Conference Call and Webcast Today at 5:00 PM Eastern Time -
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)-- Exelixis, Inc. (EXEL) today reported financial results for the third quarter of 2016 and provided an update on progress toward delivering upon its key 2016 corporate objectives, as well as commercial and clinical development milestones.
Exelixis is focused on the U.S. launch of CABOMETYX™ (cabozantinib) tablets as a treatment for patients with advanced renal cell carcinoma (RCC) who have received prior anti-angiogenic therapy. CABOMETYX generated $31.2 million in net product revenue during the third quarter of 2016, which reflects the first full quarter of product sales. Net product revenues for the third quarter of 2016, including sales of COMETRIQ® (cabozantinib) capsules for the treatment of certain forms of thyroid cancer, were $42.7 million. While Exelixis focuses on commercialization in the United States, its partner Ipsen is in the process of launching CABOMETYX in the European Union, following the European Commission’s (EC) September 2016 approval of CABOMETYX for the treatment of adult patients with advanced RCC who have received prior vascular endothelial growth factor (VEGF)-targeted therapy. Exelixis is eligible to receive royalties on CABOMETYX sales by Ipsen outside of the United States, Canada and Japan.
“The third quarter of 2016 was an important inflection point for Exelixis. We recorded our first full quarter of CABOMETYX sales and also made significant progress on our path towards becoming a profitable, fully integrated, commercial biopharmaceutical company,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “Feedback from prescribers, as well as performance to date, suggest that clinicians treating advanced renal cell carcinoma see CABOMETYX as a differentiated therapy and are increasingly incorporating it into their practice. While we continued to execute on the U.S. CABOMETYX launch and pursue important clinical trials like CABOSUN that have the potential to further advance our business, we also demonstrated sound fiscal discipline, resulting in a significantly decreased net loss and cash burn. As we close out the year, we remain committed to maximizing our opportunity to improve the treatment of cancer while building a strong and nimble company.”
Cabozantinib Highlights
Presented Positive Results from Phase 2 CABOSUN Trial in Advanced RCC. At the European Society for Medical Oncology (ESMO) 2016 Congress (CACOX), detailed results were presented from CABOSUN, the randomized phase 2 trial of cabozantinib compared with sunitinib in patients with previously untreated advanced RCC with intermediate- or poor-risk disease per the International Metastatic Renal Carcinoma Database Consortium risk criteria. In this trial, cabozantinib demonstrated a statistically significant and clinically meaningful reduction in the rate of disease progression or death as compared to sunitinib. The CABOSUN results were the subject of a late-breaking abstract at ESMO, and were highlighted at one of the Congress’ Presidential Symposia and in its official media program. CABOSUN was conducted by The Alliance for Clinical Trials in Oncology with support from the National Cancer Institute’s Cancer Therapy Evaluation Program (NCI-CTEP).
Plans for Supplemental New Drug Application in First-Line Advanced RCC. Based on the CABOSUN results, Exelixis plans to submit a Supplemental New Drug Application (sNDA) for cabozantinib as a treatment for previously untreated advanced RCC. The company is working with The Alliance to transfer the complete CABOSUN clinical database to Exelixis and will facilitate an independent radiological review of the CABOSUN imaging data in preparation for filing.
Phase 1 Trial Results for Cabozantinib in Combination with Nivolumab in Advanced Genitourinary Tumors. Also at the ESMO 2016 Congress, positive results were presented from the NCI-CTEP-sponsored phase 1 trial of cabozantinib in combination with nivolumab in patients with previously treated genitourinary tumors. Part II of the study is evaluating the triplet combination of cabozantinib, nivolumab, and ipilimumab and thus far has enrolled 15 patients. Expansion cohorts assessing cabozantinib and nivolumab, including patients with bladder, renal, and rare genitourinary cancers, are also currently being accrued.
European Commission Approval of CABOMETYX for the Treatment of Advanced RCC. On September 9, 2016, the EC approved CABOMETYX for the treatment of advanced RCC in adults following prior VEGF-targeted therapy. The approval allows for the marketing of CABOMETYX in all 28 member states of the European Union, Norway and Iceland. Under the license agreement with Ipsen, the EC approval triggered a $60.0 million milestone payment from Ipsen to Exelixis, which is expected to be received in the fourth quarter of 2016.
Outcome from First Planned Interim Analysis of Phase 3 CELESTIAL Trial. On September 6, 2016, Exelixis announced the outcome from the first planned interim analysis of CELESTIAL, the randomized global phase 3 trial of cabozantinib compared with placebo in patients with advanced hepatocellular carcinoma who have been previously treated with sorafenib. Following the analysis, the trial’s Independent Data Monitoring Committee determined that the study should continue without modifications per the study protocol. The trial protocol calls for a second interim analysis to take place once 75 percent of planned events have been observed.
Cobimetinib Highlights
Results from Cobimetinib Combination Trials Support Further Advancement. Cobimetinib, the Exelixis-discovered MEK inhibitor now the subject of a worldwide collaboration with Genentech, a member of the Roche Group, was the subject of seven presentations at the ESMO 2016 Congress. For the first time, investigators presented preliminary results from the phase 1b clinical trial of the triple combination of cobimetinib, vemurafenib, and atezolizumab in patients with previously untreated BRAF V600 mutation-positive advanced melanoma. The regimen was associated with promising antitumor activity and a manageable safety profile; details of a subsequent Roche-sponsored phase 3 pivotal trial, TRILOGY, have been posted to www.ClinicalTrials.gov. Investigators also presented updated results from the phase 1 trial of cobimetinib plus atezolizumab in advanced colorectal cancer that provide a rationale for COTEZO, the ongoing phase 3 pivotal trial in the same disease setting. New data from the phase 1 part of COLET, the phase 1/2 trial of cobimetinib and paclitaxel in triple-negative breast cancer, were also the subject of a poster presentation at the meeting.
Third Quarter 2016 Financial Results
Total revenues for the quarter ended September 30, 2016 were $62.2 million, compared to $9.9 million for the comparable period in 2015. Total revenues for the third quarter of 2016 include $42.7 million of net product revenue compared to $6.9 million for the comparable period in 2015. The increase in net product revenues for the three months ended September 30, 2016, as compared to the same period in 2015, reflects the impact of the commercial launch of CABOMETYX in late April 2016, as well as an increase in COMETRIQ revenues. Net product revenues for CABOMETYX and COMETRIQ were $31.2 million and $11.5 million, respectively. Total revenues for the quarter ended September 30, 2016 include the recognition of $15.0 million of contract revenue from the Daiichi Sankyo CS-3150 milestone, $3.8 million of license revenues recognized under Exelixis' collaboration and license agreement with Ipsen and $0.7 million of royalties on ex-U.S. net sales of COTELLIC® (cobimetinib). There was $3.0 million of contract revenues for a milestone payment received from Merck (MRK) related to their worldwide license of Exelixis' PI3K-delta program during the comparable period in 2015.
Research and development expenses for the quarter ended September 30, 2016 were $20.3 million, compared to $26.1 million for the comparable period in 2015. The decrease was primarily related to decreases in share-based compensation, clinical trial costs and the allocation of general corporate costs; those decreases were partially offset by increases in personnel related expenses resulting from an increase in headcount predominantly associated with the build-out of the Exelixis Medical Affairs organization.
Selling, general and administrative expenses for the quarter ended September 30, 2016 were $32.5 million, compared to $17.8 million for the comparable period in 2015. The increase was primarily related to an increase in personnel related expenses resulting from an increase in headcount connected with the build-out of the Exelixis U.S. commercial organization, marketing and outside services to support the launch and commercialization of CABOMETYX.
Other income (expense), net for the quarter ended September 30, 2016 was a net expense of ($18.5) million compared to ($9.8) million for the comparable period in 2015. The increase in net expense was primarily due to the $13.8 million of loss associated with the conversion through September 30, 2016 of $285.3 million in aggregate principal amount of the company's 2019 Notes for 53,704,911 shares of our common stock. The net expense also includes interest expense which includes $3.9 million of non-cash expense related to the accretion of the discounts on both the 2019 Notes and the company’s indebtedness under its Secured Convertible Notes due 2018 held by entities associated with Deerfield for the quarter ended September 30, 2016, as compared to $4.9 million for the comparable period in 2015.
Net loss for the quarter ended September 30, 2016 was ($11.3) million, or ($0.04) per share, basic, compared to ($45.5) million, or ($0.21) per share, basic, for the comparable period in 2015. The decreased net loss for the quarter was primarily due to increases in net revenues and a decrease in research and development expenses, which were partially offset by increases in selling, general and administrative expenses and other income (expense), net.
Cash and cash equivalents, short- and long-term investments and long-term restricted cash and investments totaled $379.6 million at September 30, 2016, which increased from $253.3 million at December 31, 2015.
Basis of Presentation
Exelixis adopted a 52- or 53-week fiscal year that generally ends on the Friday closest to December 31st. For convenience, references in this press release as of and for the fiscal periods ended September 30, 2016, January 1, 2016 and October 2, 2015 are indicated as being as of and for the periods ended September 30, 2016, December 31, 2015 and September 30, 2015, respectively.
Correction of an Immaterial Error
Certain historical amounts in other income (expense), net, net loss and stockholders’ equity (deficit) presented herein have been revised to reflect the correction of the accounting for non-cash interest expense associated with the 2019 Notes. See “Note 1 - Organization and Summary of Significant Accounting Policies” to Exelixis' Condensed Consolidated Financial Statements included in Exelixis' quarterly report on Form 10-Q for the quarterly period ended September 30, 2016 for a further description of this error and the historical amounts which have been corrected.
Conference Call and Webcast
Exelixis management will discuss the company’s financial results for the third quarter of 2016 and provide a general business update during a conference call beginning at 5:00 p.m. EDT/2:00 p.m. PDT today, Thursday, November 3, 2016.
Antwort auf Beitrag Nr.: 53.616.585 von Magnetfeldfredy am 03.11.16 21:46:25das ist nicht rational Freddy, da wird im Hintergrund sehr washrscheinlich leer verfkauft was das Zeug hält. Aber mittelfristig werden die guten Zahlen/Aussichten den Kurs beflügeln - da bin ich mir ziemlich sicher. In dieser Zeit wird nachgekauft bis die "Munition" ausgeht.
Antwort auf Beitrag Nr.: 53.616.684 von Cyberhexe am 03.11.16 21:56:36
irrational, zumal die heutigen Nachrichten für die Konkurrenzprodukte der Checkpoint-Hemmer über relativ häufige Herzmuskelentzündungen alles andere als erfreulich sind:
http://www.nejm.org/doi/full/10.1056/NEJMoa1609214
Immune checkpoint inhibitors have improved clinical outcomes associated with numerous cancers, but high-grade, immune-related adverse events can occur, particularly with combination immunotherapy. We report the cases of two patients with melanoma in whom fatal myocarditis developed after treatment with ipilimumab and nivolumab. In both patients, there was development of myositis with rhabdomyolysis, early progressive and refractory cardiac electrical instability, and myocarditis with a robust presence of T-cell and macrophage infiltrates. Selective clonal T-cell populations infiltrating the myocardium were identical to those present in tumors and skeletal muscle. Pharmacovigilance studies show that myocarditis occurred in 0.27% of patients treated with a combination of ipilimumab and nivolumab, which suggests that our patients were having a rare, potentially fatal, T-cell–driven drug reaction.
Zitat von Cyberhexe: das ist nicht rational Freddy, da wird im Hintergrund sehr washrscheinlich leer verfkauft was das Zeug hält. Aber mittelfristig werden die guten Zahlen/Aussichten den Kurs beflügeln - da bin ich mir ziemlich sicher. In dieser Zeit wird nachgekauft bis die "Munition" ausgeht.
irrational, zumal die heutigen Nachrichten für die Konkurrenzprodukte der Checkpoint-Hemmer über relativ häufige Herzmuskelentzündungen alles andere als erfreulich sind:
http://www.nejm.org/doi/full/10.1056/NEJMoa1609214
Immune checkpoint inhibitors have improved clinical outcomes associated with numerous cancers, but high-grade, immune-related adverse events can occur, particularly with combination immunotherapy. We report the cases of two patients with melanoma in whom fatal myocarditis developed after treatment with ipilimumab and nivolumab. In both patients, there was development of myositis with rhabdomyolysis, early progressive and refractory cardiac electrical instability, and myocarditis with a robust presence of T-cell and macrophage infiltrates. Selective clonal T-cell populations infiltrating the myocardium were identical to those present in tumors and skeletal muscle. Pharmacovigilance studies show that myocarditis occurred in 0.27% of patients treated with a combination of ipilimumab and nivolumab, which suggests that our patients were having a rare, potentially fatal, T-cell–driven drug reaction.
Antwort auf Beitrag Nr.: 53.616.684 von Cyberhexe am 03.11.16 21:56:36
Fast alle Investoren sind auf Cobi & Cabo fokussiert, um so überraschender kam für viele die Meilensteinzahlungen in Höhe von $15M für die Initialisierung einer ph3-Studie von CS-3150 von Daiichi Sankyo zur Behandlung von Bluthochdruck in japanischen Probanden.
On September 26, 2016, Exelixis announced its partner Daiichi Sankyo initiated a phase 3 pivotal trial to evaluate CS-3150 (esaxerenone (r-INN)), an oral, non-steroidal, selective mineralocorticoid receptor antagonist, as a treatment for essential hypertension in Japanese patients. Enrollment of the trial’s first patient made Exelixis eligible to receive a $15.0 million milestone payment, which it received in the fourth quarter of 2016. CS-3150 is one of the compounds identified during Exelixis’ prior research collaboration with Daiichi Sankyo.
Zitat von Cyberhexe: das ist nicht rational Freddy, da wird im Hintergrund sehr washrscheinlich leer verfkauft was das Zeug hält. Aber mittelfristig werden die guten Zahlen/Aussichten den Kurs beflügeln - da bin ich mir ziemlich sicher. In dieser Zeit wird nachgekauft bis die "Munition" ausgeht.
Fast alle Investoren sind auf Cobi & Cabo fokussiert, um so überraschender kam für viele die Meilensteinzahlungen in Höhe von $15M für die Initialisierung einer ph3-Studie von CS-3150 von Daiichi Sankyo zur Behandlung von Bluthochdruck in japanischen Probanden.
On September 26, 2016, Exelixis announced its partner Daiichi Sankyo initiated a phase 3 pivotal trial to evaluate CS-3150 (esaxerenone (r-INN)), an oral, non-steroidal, selective mineralocorticoid receptor antagonist, as a treatment for essential hypertension in Japanese patients. Enrollment of the trial’s first patient made Exelixis eligible to receive a $15.0 million milestone payment, which it received in the fourth quarter of 2016. CS-3150 is one of the compounds identified during Exelixis’ prior research collaboration with Daiichi Sankyo.
Heute gibt`s eine verdiente Erholung, mal schaun wie`s weitergeht, noch keine Analystenkommentare nach Q3?
Antwort auf Beitrag Nr.: 53.623.197 von Cyberhexe am 04.11.16 18:32:46Ich weiß nicht ob das bekannt war, aber im CC wurde zudem bestätigt, dass EXEL double digit royaties bekäme:
Stephen Willey
Okay. And then the 3150 data looks pretty interesting, I guess. I'm not necessarily sure there's been much of a public disclosure with respect to the economics of the Daiichi Sankyo collaboration, I think because there's some mention maybe of double-digit royalties. But can you maybe provide some commentary around what the milestone flow might look like beyond just the initiation of the Phase III? Thanks.
Peter Lamb
Yes. This is Peter. I'm happy to speak to that. So, clearly we’re very excited to see another one of a partnered programs moving into like stage developments and we’re extremely happy with the support of the program has been getting from upon a Daiichi Sankyo.
With respect to the actual agreement one of the terms of the agreement Daiichi Sankyo was solely responsible for all developments any future, potential commercialization and Exelixis is eligible for a series of regulatory and commercial milestones and you’re correct we’d also eligible flow double-digit royalties on any sales in the future.
Stephen Willey
Okay. And then the 3150 data looks pretty interesting, I guess. I'm not necessarily sure there's been much of a public disclosure with respect to the economics of the Daiichi Sankyo collaboration, I think because there's some mention maybe of double-digit royalties. But can you maybe provide some commentary around what the milestone flow might look like beyond just the initiation of the Phase III? Thanks.
Peter Lamb
Yes. This is Peter. I'm happy to speak to that. So, clearly we’re very excited to see another one of a partnered programs moving into like stage developments and we’re extremely happy with the support of the program has been getting from upon a Daiichi Sankyo.
With respect to the actual agreement one of the terms of the agreement Daiichi Sankyo was solely responsible for all developments any future, potential commercialization and Exelixis is eligible for a series of regulatory and commercial milestones and you’re correct we’d also eligible flow double-digit royalties on any sales in the future.
http://www.smarteranalyst.com/2016/11/04/cowen-sings-praises…
Tolle Analysgtenzusammenfassung nach den überzeugenden Q3 Ergebnissen mit Durchschnittspreistarget von US Dollar 17,75!

Tolle Analysgtenzusammenfassung nach den überzeugenden Q3 Ergebnissen mit Durchschnittspreistarget von US Dollar 17,75!


Antwort auf Beitrag Nr.: 53.623.794 von kmastra am 04.11.16 19:59:09"you’re correct we’d also eligible flow double-digit royalties on any sales in the future"
Das soll wohl low double digit royalties heißen!?
Das soll wohl low double digit royalties heißen!?
Hallo! Auf >TheLancet.com< wurden gestern 2 Ergebnisse zu cabozantinib vorgestellt:
1. Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): a randomised, controlled, open-label, multicentre, phase 2 Trial
2. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm Trial
Liest sich ganz gut! Weiß jemand, ob diese Ergebnisse bereits schon publik bzw. bekannt waren?
Danke!
1. Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): a randomised, controlled, open-label, multicentre, phase 2 Trial
2. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm Trial
Liest sich ganz gut! Weiß jemand, ob diese Ergebnisse bereits schon publik bzw. bekannt waren?
Danke!
Antwort auf Beitrag Nr.: 53.625.846 von exel2016 am 05.11.16 09:28:04
Auf ASCO2014 wurde die ph2-Studie mit Erlotinib bereits thematisiert --> siehe allererster Beitrag in dieem Thread vom 15.05.14 05:58:48 Beitrag Nr. 1 ( 46.984.068):
Abstract 8014/Poster 28: “Phase II trial of XL184 (cabozantinib) plus erlotinib in patients (pts) with advanced EGFR-mutant non-small cell lung cancer (NSCLC) with progressive disease (PD) on epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy: a California Cancer Consortium phase II trial (NCI 9303).”
Obschon auch die andere Studie bekannt ist, sind derartige Veröffentlichungen in "peer-reviewed Journals" für die wissenschaftliche Verbreitung von grosser Bedeutung.
Zitat von exel2016: Hallo! Auf >TheLancet.com< wurden gestern 2 Ergebnisse zu cabozantinib vorgestellt:
1. Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): a randomised, controlled, open-label, multicentre, phase 2 Trial
2. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm Trial
Liest sich ganz gut! Weiß jemand, ob diese Ergebnisse bereits schon publik bzw. bekannt waren?
Danke!
Auf ASCO2014 wurde die ph2-Studie mit Erlotinib bereits thematisiert --> siehe allererster Beitrag in dieem Thread vom 15.05.14 05:58:48 Beitrag Nr. 1 ( 46.984.068):
Abstract 8014/Poster 28: “Phase II trial of XL184 (cabozantinib) plus erlotinib in patients (pts) with advanced EGFR-mutant non-small cell lung cancer (NSCLC) with progressive disease (PD) on epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy: a California Cancer Consortium phase II trial (NCI 9303).”
Obschon auch die andere Studie bekannt ist, sind derartige Veröffentlichungen in "peer-reviewed Journals" für die wissenschaftliche Verbreitung von grosser Bedeutung.
http://www.onclive.com/conference-coverage/kidney-2016/caboz…
Untergruppenanalyse bei METEOR zeigt erhöhte Wirfksamkeit von Cabozantinib bei MET-Überexpression.
Cabozantinib Benefits Similar in MET Expressing Advanced RCC
Ginny Vachon, PhD
Published Online: Saturday, Nov 05, 2016
Print Button
Dr.
Thomas Powles, MBBS
Cabozantinib (Cabometyx) demonstrated consistent benefits compared with everolimus (Afinitor) for patients with advanced, pretreated renal cell carcinoma (RCC) in a subgroup analysis of the phase III METEOR trial that explored MET expression, according to a presentation by Thomas Powles, MD, MBBS, MRCP, at the 2016 International Kidney Cancer Symposium.
In April 2016, cabozantinib was approved by the FDA as a treatment for patients with advanced RCC following prior anti–angiogenic therapy. The TKI blocks multiple VEGF receptors, making it a potent angiogenesis inhibitor. Additionally, the agent uniquely inhibits RET and MET, raising the potential for MET expression to act as a predictive biomarker.
The median overall survival (OS) in those with high MET expression was 22.0 months with cabozantinib versus 15.2 months with everolimus (HR, 0.55; 95% CI, 0.31-0.99). In those with low MET expression, the median OS with cabozantinib was 20.8 versus 18.4 months with everolimus (HR, 0.72; 95% CI, 0.52-1.00).
These data showed a pattern toward greater benefit in patients with high MET expressing tumors; however, this trend was neither clinically meaningful nor statistically significant and was largely driven by small sample size and imbalances in the patient populations, said Powles, leader of the genitourinary cancer group at Barts Cancer Institute (BCI), London, United Kingdom
METEOR was a randomized phase III trial directly comparing the efficacy and safety of cabozantinib and everolimus in 658 patients with advanced RCC who progressed following VEGFR TKI treatment. Patients received daily cabozantinib at 60 mg (n = 330) or everolimus at 10 mg (n = 328). Approximately 30% of patients had received ≥2 prior VEGFR TKIs.
Across the full study by independent radiology review, median OS was 21.4 months (95% CI 18.7-not estimable) with cabozantinib compared with 16.5 months (95% CI, 14.7-18.8) with everolimus (HR, 0.66; 95% CI, 0.53-0.83; P = .00026). Median progression-free survival (PFS) was 7.4 months with cabozantinib compared with 3.9 months with everolimus (HR, 0.51; 95% CI, 0.41-0.62; P <.0001). Furthermore, cabozantinib achieved a better objective response rate (ORR) of 17% versus 3% with everolimus (P <.0001).
In a secondary analysis of the study, MET expression was quantitated from archival or recently biopsied tumor tissue samples by immunohistochemistry using the SP44 antibody, and classified as MET high or low using a cut off of ≥50% for tumor tissue cells stained with an intensity of ≥2+ by immunohistochemistry. Overall, 15% of patients were classified as MET high, 59% as MET low, and 26% as MET unknown.
“I think we know more about [staining] with PD-L1 than we do in this particular area, and it’s a really hard area which I think we need to work on a lot more,” said Powles. “My gut feeling is we’re not going to be doing this in 20 years time,” he added.
When presenting the baseline characteristics of patients by MET status, Powles pointed out that the number of patients with high MET status was relatively small (cabozantinib, n = 50; everolimus, n = 51). In the MET low group, 150 patients were treated with cabozantinib and 162 were treated with everolimus.
The median PFS in those with high MET expression was 7.4 months with cabozantinib versus 3.7 months with everolimus (HR, 0.38; 95% CI, 0.23-0.62). In those with low MET expression, the median PFS with cabozantinib was 7.3 versus 4.2 months with everolimus (HR, 0.57; 95% CI, 0.43-0.76). The median PFS in those with unknown MET status was 9.1 months with cabozantinib versus 3.7 months with everolimus (HR, 0.52; 95% CI, 0.37-0.72).
“PFS is probably the more accurate endpoint that we have in VEGF-targeted therapy,” stated Powles. Overall, cabozantinib was superior for OS, PFS, and ORR regardless of MET status.
Untergruppenanalyse bei METEOR zeigt erhöhte Wirfksamkeit von Cabozantinib bei MET-Überexpression.
Cabozantinib Benefits Similar in MET Expressing Advanced RCC
Ginny Vachon, PhD
Published Online: Saturday, Nov 05, 2016
Print Button
Dr.
Thomas Powles, MBBS
Cabozantinib (Cabometyx) demonstrated consistent benefits compared with everolimus (Afinitor) for patients with advanced, pretreated renal cell carcinoma (RCC) in a subgroup analysis of the phase III METEOR trial that explored MET expression, according to a presentation by Thomas Powles, MD, MBBS, MRCP, at the 2016 International Kidney Cancer Symposium.
In April 2016, cabozantinib was approved by the FDA as a treatment for patients with advanced RCC following prior anti–angiogenic therapy. The TKI blocks multiple VEGF receptors, making it a potent angiogenesis inhibitor. Additionally, the agent uniquely inhibits RET and MET, raising the potential for MET expression to act as a predictive biomarker.
The median overall survival (OS) in those with high MET expression was 22.0 months with cabozantinib versus 15.2 months with everolimus (HR, 0.55; 95% CI, 0.31-0.99). In those with low MET expression, the median OS with cabozantinib was 20.8 versus 18.4 months with everolimus (HR, 0.72; 95% CI, 0.52-1.00).
These data showed a pattern toward greater benefit in patients with high MET expressing tumors; however, this trend was neither clinically meaningful nor statistically significant and was largely driven by small sample size and imbalances in the patient populations, said Powles, leader of the genitourinary cancer group at Barts Cancer Institute (BCI), London, United Kingdom
METEOR was a randomized phase III trial directly comparing the efficacy and safety of cabozantinib and everolimus in 658 patients with advanced RCC who progressed following VEGFR TKI treatment. Patients received daily cabozantinib at 60 mg (n = 330) or everolimus at 10 mg (n = 328). Approximately 30% of patients had received ≥2 prior VEGFR TKIs.
Across the full study by independent radiology review, median OS was 21.4 months (95% CI 18.7-not estimable) with cabozantinib compared with 16.5 months (95% CI, 14.7-18.8) with everolimus (HR, 0.66; 95% CI, 0.53-0.83; P = .00026). Median progression-free survival (PFS) was 7.4 months with cabozantinib compared with 3.9 months with everolimus (HR, 0.51; 95% CI, 0.41-0.62; P <.0001). Furthermore, cabozantinib achieved a better objective response rate (ORR) of 17% versus 3% with everolimus (P <.0001).
In a secondary analysis of the study, MET expression was quantitated from archival or recently biopsied tumor tissue samples by immunohistochemistry using the SP44 antibody, and classified as MET high or low using a cut off of ≥50% for tumor tissue cells stained with an intensity of ≥2+ by immunohistochemistry. Overall, 15% of patients were classified as MET high, 59% as MET low, and 26% as MET unknown.
“I think we know more about [staining] with PD-L1 than we do in this particular area, and it’s a really hard area which I think we need to work on a lot more,” said Powles. “My gut feeling is we’re not going to be doing this in 20 years time,” he added.
When presenting the baseline characteristics of patients by MET status, Powles pointed out that the number of patients with high MET status was relatively small (cabozantinib, n = 50; everolimus, n = 51). In the MET low group, 150 patients were treated with cabozantinib and 162 were treated with everolimus.
The median PFS in those with high MET expression was 7.4 months with cabozantinib versus 3.7 months with everolimus (HR, 0.38; 95% CI, 0.23-0.62). In those with low MET expression, the median PFS with cabozantinib was 7.3 versus 4.2 months with everolimus (HR, 0.57; 95% CI, 0.43-0.76). The median PFS in those with unknown MET status was 9.1 months with cabozantinib versus 3.7 months with everolimus (HR, 0.52; 95% CI, 0.37-0.72).
“PFS is probably the more accurate endpoint that we have in VEGF-targeted therapy,” stated Powles. Overall, cabozantinib was superior for OS, PFS, and ORR regardless of MET status.
Beim 15th internationalen KidneyCancer Symposium am 4. und 5. November in Miami, Florida wurde folgende Folie präsentiert:
Das wissenschaftliche Interesse an Cabo ist stetig steigend!

Das wissenschaftliche Interesse an Cabo ist stetig steigend!
Antwort auf Beitrag Nr.: 53.600.052 von Cyberhexe am 01.11.16 21:19:21
Das ist ein viel versprechendes Ergebnis, wenn auch bei einer sehr kleinen Stichprobe: Eine Ansprechrate von 83% - wenn ich mich recht erinnere, dann war die Ansprechrate in coBRIM bei 70% (cobi+vemurafenib) bzw. bei 50% (vemurafenib).
http://www.fiercepharma.com/pharma/roche-s-tecentriq-based-m…
In one study in 30 previously untreated patients with BRAF-positive melanoma, the PD-L1 inhibitor shone in combination with Roche’s MEK enzyme inhibitor Cotellic and BRAF blocker Zelboraf. The cocktail delivered an 83% response rate--or 24 patients--with a complete response in three of them, the Phase Ib study found.
Zitat von Cyberhexe: Beim Jahrestreffen 2016 der "Society for Melanoma Research" in Boston, Massachusetts vom 6.-9.11.2016 sind folgende interessante Veröffentlichungen angekündigt:
Plenary Session 2, 7. November 10h30 - 12h30
Safety and Clinical Activity of Atezolizumab + Cobimetinib + Vemurafenib in BRAFV600 mutant metastatic melanoma
http://www.melanomacongress.com/docs/2016_SMR_Agenda.pdf
Das ist ein viel versprechendes Ergebnis, wenn auch bei einer sehr kleinen Stichprobe: Eine Ansprechrate von 83% - wenn ich mich recht erinnere, dann war die Ansprechrate in coBRIM bei 70% (cobi+vemurafenib) bzw. bei 50% (vemurafenib).
http://www.fiercepharma.com/pharma/roche-s-tecentriq-based-m…
In one study in 30 previously untreated patients with BRAF-positive melanoma, the PD-L1 inhibitor shone in combination with Roche’s MEK enzyme inhibitor Cotellic and BRAF blocker Zelboraf. The cocktail delivered an 83% response rate--or 24 patients--with a complete response in three of them, the Phase Ib study found.
Neueson Ohad Hammer:
Exelixis off to a strong launch in RCC
At ESMO, Exelixis reported the long anticipated data in 1st line renal cancer (CABOSUN study), where Cabometyx beat standard of care drug, Sutent. This makes Cabometyx the only drug to show superiority over Sutent and although the trial was a P2 of 157 patients, it may be enough for approval. Two recent precedents for an approval in oncology based on P2 is Lenvima in RCC and Lilly’s Lartruvo in soft tissue sarcoma.
Last week, Exelixis reported its quarterly results which included the first full quarter since Cabometyx’s approval (April 2016). The cabo franchise (Cabometyx for RCC and Cometriq for medullary thyroid cancer) generated sales of $43M, of which $31M were for Cabometyx. According to Exelixis, in Q3 Cabometyx had a market share of 20% and 35% in new 2nd and 3rd line RCC patients, respectively. This is encouraging especially given Opdivo’s simultaneous launch in 2nd/3rd line RCC.
Cabometyx and Opdivo should continue to take market share from legacy products such as Afinitor and Inlyta but also expand the overall market (more patients receiving treatment, more treatment lines, longer treatment duration). The ultimate goal for both agents is 1st line approval. BMS is running a large P3 (1000+ patients) for Opdivo+Yervoy vs. Sutent that should have data in H2:17. Exelixis hopes data from CABOSUN will be enough for accelerated approval potentially towards the end 2017.
Regardless of 1st line market dynamics, it is clear Cabometyx is still far from saturating the market. Even if Opdivo+Yervoy becomes the dominant 1st line option, Cabometyx will probably dominate the 2nd/3rd line market as it will be regarded as superior to every other approved agent. Last quarter’s run rate (~$120M annually) demonstrates that Cabometyx can easily become a $500M product in the US alone without any label expansions. For the sake of comparison, Pfizer’s Inlyta, which demonstrated limited efficacy in 2nd line RCC (median PFS of 4.8 months and no survival benefit), had peak US sales of ~$210M.
The Cabometyx and Cotellic programs are starting to generate preliminary combination data with PD-1 agents. At ESMO, Exelixis reported encouraging signs of efficacy for Cabometyx+Opdivo in patients with various genitourinary tumors, including a response rate of 43%. The signal appears particularly strong in bladder cancer (both urothelial carcinoma and SCC of the bladder) but it is hard to distinguish between cabo’s contribution and that of Opdivo, especially without PD-L1 expression data.
For Cotellic, Genentech is pursuing combination studies with its PD-L1 agent, Tecentriq, across different indications. In 2017, there will be three pivotal trials in colon cancer and melanoma based on encouraging combination results. Most recently, Genentech reported a 50% response rate in BRAF-negative melanoma with a good duration of response. Genentech’s efforts are clearly a positive indication and provide potential upside for Exelixis, which has co-promotion rights for Cotellic in the US, but until P3 data are available in 2-3 years, the drug is unlikely to contribute significant sales to Exelixis.
http://www.orf-blog.com/biotech-portfolio-updates-esmo-2016-…
Exelixis off to a strong launch in RCC
At ESMO, Exelixis reported the long anticipated data in 1st line renal cancer (CABOSUN study), where Cabometyx beat standard of care drug, Sutent. This makes Cabometyx the only drug to show superiority over Sutent and although the trial was a P2 of 157 patients, it may be enough for approval. Two recent precedents for an approval in oncology based on P2 is Lenvima in RCC and Lilly’s Lartruvo in soft tissue sarcoma.
Last week, Exelixis reported its quarterly results which included the first full quarter since Cabometyx’s approval (April 2016). The cabo franchise (Cabometyx for RCC and Cometriq for medullary thyroid cancer) generated sales of $43M, of which $31M were for Cabometyx. According to Exelixis, in Q3 Cabometyx had a market share of 20% and 35% in new 2nd and 3rd line RCC patients, respectively. This is encouraging especially given Opdivo’s simultaneous launch in 2nd/3rd line RCC.
Cabometyx and Opdivo should continue to take market share from legacy products such as Afinitor and Inlyta but also expand the overall market (more patients receiving treatment, more treatment lines, longer treatment duration). The ultimate goal for both agents is 1st line approval. BMS is running a large P3 (1000+ patients) for Opdivo+Yervoy vs. Sutent that should have data in H2:17. Exelixis hopes data from CABOSUN will be enough for accelerated approval potentially towards the end 2017.
Regardless of 1st line market dynamics, it is clear Cabometyx is still far from saturating the market. Even if Opdivo+Yervoy becomes the dominant 1st line option, Cabometyx will probably dominate the 2nd/3rd line market as it will be regarded as superior to every other approved agent. Last quarter’s run rate (~$120M annually) demonstrates that Cabometyx can easily become a $500M product in the US alone without any label expansions. For the sake of comparison, Pfizer’s Inlyta, which demonstrated limited efficacy in 2nd line RCC (median PFS of 4.8 months and no survival benefit), had peak US sales of ~$210M.
The Cabometyx and Cotellic programs are starting to generate preliminary combination data with PD-1 agents. At ESMO, Exelixis reported encouraging signs of efficacy for Cabometyx+Opdivo in patients with various genitourinary tumors, including a response rate of 43%. The signal appears particularly strong in bladder cancer (both urothelial carcinoma and SCC of the bladder) but it is hard to distinguish between cabo’s contribution and that of Opdivo, especially without PD-L1 expression data.
For Cotellic, Genentech is pursuing combination studies with its PD-L1 agent, Tecentriq, across different indications. In 2017, there will be three pivotal trials in colon cancer and melanoma based on encouraging combination results. Most recently, Genentech reported a 50% response rate in BRAF-negative melanoma with a good duration of response. Genentech’s efforts are clearly a positive indication and provide potential upside for Exelixis, which has co-promotion rights for Cotellic in the US, but until P3 data are available in 2-3 years, the drug is unlikely to contribute significant sales to Exelixis.
http://www.orf-blog.com/biotech-portfolio-updates-esmo-2016-…
interessanter Vortrag von Nizar M. Tannir, MD, Professor and Deputy Chair, GU Medical Oncology, MD Anderson: "Cabozantinib is the best salvage therapy for RCC" auf dem "International Kidney Cancer Symposium" am 4.-5.11.2016, der davon ausgeht, dass Cabozantinib die derzeit beste Lösung ist bei der Erstlinienbehandlung von Nierenkrebs
Schlussfolgerungen ab 17´10:
- it delivers a trifecta of OS, PFS and ORR in a ph3 trial
- it has unique MOA targeting VEGFR, MET and AXL, which are important pathways in RCC
- it is particularly effective in patients with bone metastasis
- it was shown superior tu Sunitinib, a long standing 1L SOC (Standard of Care) agent in RCC (CaboSUN)
http://www.oncologytube.com/video/cabozantinib-is-the-best-s…
Schlussfolgerungen ab 17´10:
- it delivers a trifecta of OS, PFS and ORR in a ph3 trial
- it has unique MOA targeting VEGFR, MET and AXL, which are important pathways in RCC
- it is particularly effective in patients with bone metastasis
- it was shown superior tu Sunitinib, a long standing 1L SOC (Standard of Care) agent in RCC (CaboSUN)
http://www.oncologytube.com/video/cabozantinib-is-the-best-s…
Antwort auf Beitrag Nr.: 53.640.702 von Cyberhexe am 08.11.16 06:02:42...und noch ein weiterer Vortrag von diesem Nierenkrebs-Symposium abgehalten am 4. und 5. November in Miami, Florida und zwar werden die METEOR-Daten in Abhängigkeit der MET-Expression thematisiert:
Professor Thomas Powles
MBBS, MRCP, MD, Barts Cancer Institute presents Clinical outcomes based on MET expression level in METEOR, a randomized phase 3 trial of cabozantinib versus everolimus in advanced renal cell carcinoma (RCC) at the 2016 annual International Kidney Cancer Symposium.
Die Schnlussfolgerungen sind die Folgenden:
Die 3 entscheidenden Endpunkte, nämlich Überleben, progressionsfreies Überleben und Ansprechrate bei fortgeschrittenem Nierenkrebs sind bei Cabozantinib gegenüber Everolimus verbessert und zwar unabhängig vom Grad der Tumor-MET-Expression.
http://www.oncologytube.com/video/clinical-outcomes-based-on…
Professor Thomas Powles
MBBS, MRCP, MD, Barts Cancer Institute presents Clinical outcomes based on MET expression level in METEOR, a randomized phase 3 trial of cabozantinib versus everolimus in advanced renal cell carcinoma (RCC) at the 2016 annual International Kidney Cancer Symposium.
Die Schnlussfolgerungen sind die Folgenden:
Die 3 entscheidenden Endpunkte, nämlich Überleben, progressionsfreies Überleben und Ansprechrate bei fortgeschrittenem Nierenkrebs sind bei Cabozantinib gegenüber Everolimus verbessert und zwar unabhängig vom Grad der Tumor-MET-Expression.
http://www.oncologytube.com/video/clinical-outcomes-based-on…
Nivolumab bei RCC wird in Schottland nicht bezahlt!
Der schottische NHS (National Health Service) verweigert die Bezahlung von Novolumab in der Zweitlinienbehandlung bei fortgeschrittenem Nierenkrebs.On the downside, the SMC also issued guidance which will deny Scottish NHS patients with previously treated advanced kidney cancer the option of treatment with Opdivo.
The submission for kidney cancer included data from a pivotal Phase III trial showing that patients who received the drug as a second-line treatment lived an average of 5.4 months longer than those treated with a current standard therapy (everolimus), BMS said. But the SMC took the position that the the firm's justification of the treatment's cost in relation to its health benefits was not sufficient and that it failed to present a sufficiently robust economic analysis to gain acceptance.
http://www.pharmatimes.com/news/smc_endorses_use_of_five_med…
anbei eine sehr gute Übersicht zu den METEOR-Ergebnissen, publiziert von IKCC, einem internationalen Netzwerk zu Nierenkrebs:
http://iokidney.com/clinical-trials/results-meteor/infograph…
...übrigens, ich habe gestern meine EXEL-Position reduziert.
http://iokidney.com/clinical-trials/results-meteor/infograph…
...übrigens, ich habe gestern meine EXEL-Position reduziert.
CABOSUN wird nun in den wissenschaftlichen Journals veröffentlicht...zB im Journal of Clinical Oncology:
http://ascopubs.org/doi/abs/10.1200/JCO.2016.70.7398
Results
From July 2013 to April 2015, 157 patients were randomly assigned (cabozantinib, n = 79; sunitinib, n = 78). Compared with sunitinib, cabozantinib treatment significantly increased median PFS (8.2 v 5.6 months) and was associated with a 34% reduction in rate of progression or death (adjusted hazard ratio, 0.66; 95% CI, 0.46 to 0.95; one-sided P = .012). ORR was 46% (95% CI, 34 to 57) for cabozantinib versus 18% (95% CI, 10 to 28) for sunitinib. All-causality grade 3 or 4 adverse events were 67% for cabozantinib and 68% for sunitinib and included diarrhea (cabozantinib, 10% v sunitinib, 11%), fatigue (6% v 15%), hypertension (28% v 22%), palmar-plantar erythrodysesthesia (8% v 4%), and hematologic adverse events (3% v 22%).
Conclusion
Cabozantinib demonstrated a significant clinical benefit in PFS and ORR over standard-of-care sunitinib as first-line therapy in patients with intermediate- or poor-risk mRCC.
http://ascopubs.org/doi/abs/10.1200/JCO.2016.70.7398
Results
From July 2013 to April 2015, 157 patients were randomly assigned (cabozantinib, n = 79; sunitinib, n = 78). Compared with sunitinib, cabozantinib treatment significantly increased median PFS (8.2 v 5.6 months) and was associated with a 34% reduction in rate of progression or death (adjusted hazard ratio, 0.66; 95% CI, 0.46 to 0.95; one-sided P = .012). ORR was 46% (95% CI, 34 to 57) for cabozantinib versus 18% (95% CI, 10 to 28) for sunitinib. All-causality grade 3 or 4 adverse events were 67% for cabozantinib and 68% for sunitinib and included diarrhea (cabozantinib, 10% v sunitinib, 11%), fatigue (6% v 15%), hypertension (28% v 22%), palmar-plantar erythrodysesthesia (8% v 4%), and hematologic adverse events (3% v 22%).
Conclusion
Cabozantinib demonstrated a significant clinical benefit in PFS and ORR over standard-of-care sunitinib as first-line therapy in patients with intermediate- or poor-risk mRCC.
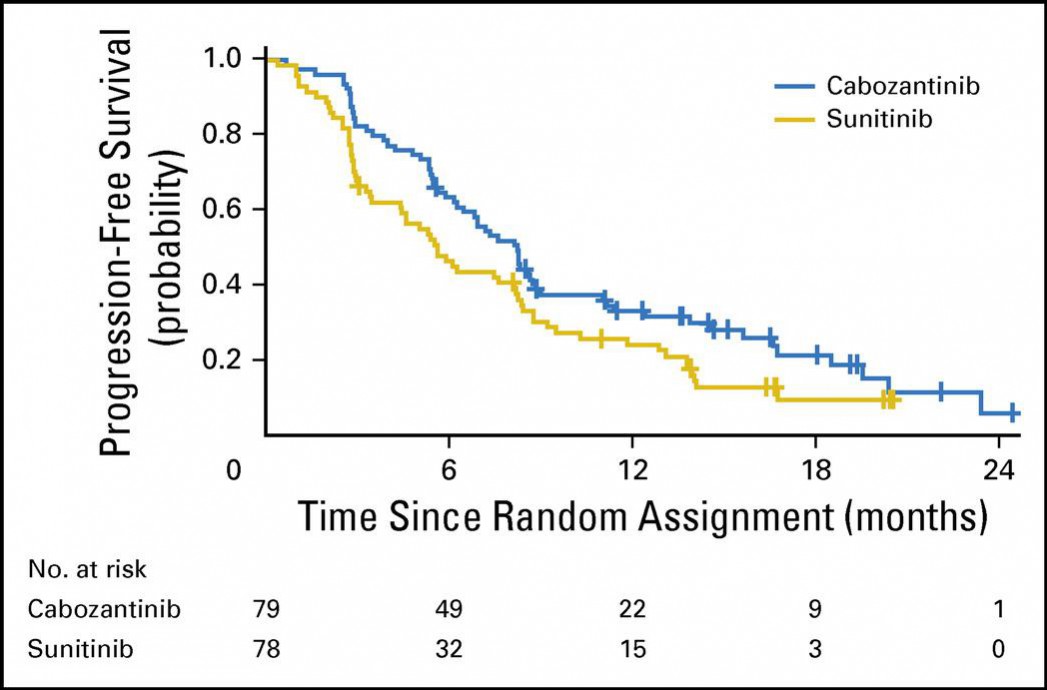
Diese Grafik zeigt sehr eindrücklich die bessere Wirksamkeit von Cabo (blau) gegenüber Sunitinib (gelb) in Erstlinienbehandlung bei Nierenkrebs (RCC):

http://www.thelancet.com/journals/lanonc/article/PIIS1470-20…
Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): a randomised, controlled, open-label, multicentre, phase 2 trial.
FINDINGS:
Between Feb 7, 2013, and July 1, 2014, we enrolled and randomly assigned 42 patients to erlotinib treatment, 40 patients to cabozantinib treatment, and 43 patients to erlotinib plus cabozantinib treatment, of whom 111 (89%) in total were included in the primary analysis (erlotinib [n=38], cabozantinib [n=38], erlotinib plus cabozantinib [n=35]). Compared with erlotinib alone (median 1·8 months [95% CI 1·7-2·2]), progression-free survival was significantly improved in the cabozantinib group (4·3 months [3·6-7·4]; hazard ratio [HR] 0·39, 80% CI 0·27-0·55; one-sided p=0·0003) and in the erlotinib plus cabozantinib group (4·7 months [2·4-7·4]; HR 0·37, 0·25-0·53; one-sided p=0·0003). Among participants included in the safety analysis of the erlotinib (n=40), cabozantinib (n=40), and erlotinib plus cabozantinib (n=39) groups, the most common grade 3 or 4 adverse events were diarrhoea (three [8%] cases in the erlotinib group vs three [8%] in the cabozantinib group vs 11 [28%] in the erlotinib plus cabozantinib group), hypertension (none vs ten [25%] vs one [3%]), fatigue (five [13%] vs six [15%] vs six [15%]), oral mucositis (none vs four [10%] vs one [3%]), and thromboembolic event (none vs three [8%] vs two [5%]). One death due to respiratory failure occurred in the cabozantinib group, deemed possibly related to either drug, and one death due to pneumonitis occurred in the erlotinib plus cabozantinib group, deemed related to either drug or the combination.
INTERPRETATION:
Despite its small sample size, this trial showed that, in patients with EGFR wild-type NSCLC, cabozantinib alone or combined with erlotinib has clinically meaningful, superior efficacy to that of erlotinib alone, with additional toxicity that was generally manageable. Cabozantinib-based regimens are promising for further investigation in this patient population.
interessant ist die Tatsache, dass Roche mit Erlotinib (brand: TARCEVA) in den ersten 9 Monaten in 2016 immer noch CHF765m erlöst hat - allerdings nicht nur NSCLC:
http://www.roche.com/dam/jcr:3c96267c-0ac9-4f4c-a9e0-b3fa18a…
Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): a randomised, controlled, open-label, multicentre, phase 2 trial.
FINDINGS:
Between Feb 7, 2013, and July 1, 2014, we enrolled and randomly assigned 42 patients to erlotinib treatment, 40 patients to cabozantinib treatment, and 43 patients to erlotinib plus cabozantinib treatment, of whom 111 (89%) in total were included in the primary analysis (erlotinib [n=38], cabozantinib [n=38], erlotinib plus cabozantinib [n=35]). Compared with erlotinib alone (median 1·8 months [95% CI 1·7-2·2]), progression-free survival was significantly improved in the cabozantinib group (4·3 months [3·6-7·4]; hazard ratio [HR] 0·39, 80% CI 0·27-0·55; one-sided p=0·0003) and in the erlotinib plus cabozantinib group (4·7 months [2·4-7·4]; HR 0·37, 0·25-0·53; one-sided p=0·0003). Among participants included in the safety analysis of the erlotinib (n=40), cabozantinib (n=40), and erlotinib plus cabozantinib (n=39) groups, the most common grade 3 or 4 adverse events were diarrhoea (three [8%] cases in the erlotinib group vs three [8%] in the cabozantinib group vs 11 [28%] in the erlotinib plus cabozantinib group), hypertension (none vs ten [25%] vs one [3%]), fatigue (five [13%] vs six [15%] vs six [15%]), oral mucositis (none vs four [10%] vs one [3%]), and thromboembolic event (none vs three [8%] vs two [5%]). One death due to respiratory failure occurred in the cabozantinib group, deemed possibly related to either drug, and one death due to pneumonitis occurred in the erlotinib plus cabozantinib group, deemed related to either drug or the combination.
INTERPRETATION:
Despite its small sample size, this trial showed that, in patients with EGFR wild-type NSCLC, cabozantinib alone or combined with erlotinib has clinically meaningful, superior efficacy to that of erlotinib alone, with additional toxicity that was generally manageable. Cabozantinib-based regimens are promising for further investigation in this patient population.
interessant ist die Tatsache, dass Roche mit Erlotinib (brand: TARCEVA) in den ersten 9 Monaten in 2016 immer noch CHF765m erlöst hat - allerdings nicht nur NSCLC:
http://www.roche.com/dam/jcr:3c96267c-0ac9-4f4c-a9e0-b3fa18a…
"New drug beats standard therapy in advanced kidney cancer"
“These results are very relevant to our practice and our kidney cancer patients – they may change the standard,” Choueiri said.mental-drug-beats-standard-therapy-in-advanced-kidney-cancer."
http://www.dana-farber.org/Newsroom/News-Releases/new-experi…
“These results are very relevant to our practice and our kidney cancer patients – they may change the standard,” Choueiri said.mental-drug-beats-standard-therapy-in-advanced-kidney-cancer."
http://www.dana-farber.org/Newsroom/News-Releases/new-experi…
Antwort auf Beitrag Nr.: 53.736.075 von Cyberhexe am 21.11.16 05:24:57First-Line Cabozantinib Superior to Sunitinib in mRCC
Jason M. Broderick @jasoncology
Published Online: Thursday, Nov 17, 2016
Cabozantinib (Cabometyx) reduced the risk of progression or death by 34% versus sunitinib (Sutent) as a first-line treatment for patients with metastatic renal cell carcinoma (RCC), according to results from the phase II CABOSUN trial published in the Journal of Clinical Oncology.
The median progression-free survival (PFS) was 2.6 months higher with cabozantinib versus sunitinib. The overall response rate (ORR) was 46% versus 18%, respectively.
http://ht.ly/5q9C306jXBE
Jason M. Broderick @jasoncology
Published Online: Thursday, Nov 17, 2016
Cabozantinib (Cabometyx) reduced the risk of progression or death by 34% versus sunitinib (Sutent) as a first-line treatment for patients with metastatic renal cell carcinoma (RCC), according to results from the phase II CABOSUN trial published in the Journal of Clinical Oncology.
The median progression-free survival (PFS) was 2.6 months higher with cabozantinib versus sunitinib. The overall response rate (ORR) was 46% versus 18%, respectively.
http://ht.ly/5q9C306jXBE
Antwort auf Beitrag Nr.: 53.711.430 von Cyberhexe am 16.11.16 23:57:27Cabozantinib Shows Activity in Advanced RET-Rearranged NSCLC
By Matthew Stenger
Posted: 11/18/2016 9:34:04 AM
Last Updated: 11/18/2016 9:34:04 AM
Key Points
Cabozantinib produced response in 28% of patients with advanced RET-rearranged NSCLC.
Response was observed in patients with KIF5B-RET, RIM33-RET, and CLIP1-RET fusions.
In a phase II trial reported in The Lancet Oncology, Drilon et al found that cabozantinib (Cabometyx) produced responses in some patients with advanced RET-rearranged non–small cell lung cancer (NSCLC). RET rearrangements are found in 1% to 2% of NSCLCs.
In the single-arm study, 25 evaluable patients from a single U.S. center with measurable metastatic or unresectable disease with a RET rearrangement were treated with cabozantinib at 60 mg daily. KIF5B-RET was the predominant fusion type, found in 16 patients.
Responses
Confirmed partial response was observed in 7 of 25 patients (overall response rate = 28%, 95% confidence interval [CI] = 12%–49%). Median duration of response was 7.0 months. Response was observed in 3 of 15 patients (20%) with KIF5B-RET fusions and 2 of 6 patients (33%) with unknown genes (fluorescence in situ hybridization–positive). Responses were observed in patients with RIM33-RET or CLIP1-RET fusions but not in those with CCDC6-RET or ERC1-RET fusions. Median progression-free survival was 5.5 months, and median overall survival was 9.9 months.
http://www.ascopost.com/News/44134?platform=hootsuite
By Matthew Stenger
Posted: 11/18/2016 9:34:04 AM
Last Updated: 11/18/2016 9:34:04 AM
Key Points
Cabozantinib produced response in 28% of patients with advanced RET-rearranged NSCLC.
Response was observed in patients with KIF5B-RET, RIM33-RET, and CLIP1-RET fusions.
In a phase II trial reported in The Lancet Oncology, Drilon et al found that cabozantinib (Cabometyx) produced responses in some patients with advanced RET-rearranged non–small cell lung cancer (NSCLC). RET rearrangements are found in 1% to 2% of NSCLCs.
In the single-arm study, 25 evaluable patients from a single U.S. center with measurable metastatic or unresectable disease with a RET rearrangement were treated with cabozantinib at 60 mg daily. KIF5B-RET was the predominant fusion type, found in 16 patients.
Responses
Confirmed partial response was observed in 7 of 25 patients (overall response rate = 28%, 95% confidence interval [CI] = 12%–49%). Median duration of response was 7.0 months. Response was observed in 3 of 15 patients (20%) with KIF5B-RET fusions and 2 of 6 patients (33%) with unknown genes (fluorescence in situ hybridization–positive). Responses were observed in patients with RIM33-RET or CLIP1-RET fusions but not in those with CCDC6-RET or ERC1-RET fusions. Median progression-free survival was 5.5 months, and median overall survival was 9.9 months.
http://www.ascopost.com/News/44134?platform=hootsuite
Die Aufmerksamkeit hinsichtlich Cabozantinib in den wissenschaftlichen Medien ist enorm:
http://www.futuremedicine.com/doi/abs/10.2217/fon-2016-0358?…
Review
Cabozantinib in genitourinary malignancies
Tian Zhang1, Se Eun Park1, Cierra Hong2 & Daniel J George*,1,3
*Author for correspondence: daniel.george@duke.edu
Cabozantinib inhibits a variety of cellular receptors including VEGFR1–3, MET, AXL, RET, FLT3 and KIT. These signaling pathways have been shown to be important in genitourinary malignancies. Along its clinical development, it has shown most activity in advanced renal cell carcinoma; the METEOR study compared cabozantinib to everolimus and showed clinically and statistically significant improvements in both progression-free survival and overall survival. Herein, we review the development of cabozantinib in the genitourinary malignancies of renal cell carcinoma, prostate adenocarcinoma and urothelial carcinoma.
Full Text PDF (1725 KB) PDF Plus (1763 KB)
Interessanterweise hat die Kombitherapie Binimetinib+Encorafenib vs. Vemurafenib in einer ph3-Studie erstaunlich gute Ergebnisse erzielt, so dass eine weitere Kombitherapie zur Behandlung von metMelanoma (BRAF) mittelfristig am Markt platziert wird. Hab mir deswegen mal einige Arra (ARRY) ins Depot gelegt.
In November 2016, new results from the pivotal Phase 3 COLUMBUS trial were presented at the Society for Melanoma Research Annual Congress. The study met its primary endpoint, with the combination of bini/enco significantly improving progression free survival (PFS) compared with vemurafenib, a BRAF inhibitor, alone. The combination of bini/enco was generally well-tolerated and reported adverse events (AEs) were overall consistent with previous published clinical trial results for the bini/enco combination in BRAF-mutant melanoma patients.
In the analysis of the primary endpoint, the median PFS (mPFS) for patients treated with the combination of bini/enco was 14.9 months versus 7.3 months for patients treated with vemurafenib; hazard ratio (HR) 0.54, (95% CI 0.41-0.71, p<0.001). As part of the trial design, the primary analysis was based on a Blinded Independent Central Review (BICR) of patient scans, while results by local review at the investigative site were also analyzed. The chart below outlines the mPFS results, as determined by both assessments, for the combination of bini/enco versus vemurafenib, bini/enco versus encorafenib, and encorafenib versus vemurafenib:
http://investor.arraybiopharma.com/phoenix.zhtml?c=123810&p=…
http://www.futuremedicine.com/doi/abs/10.2217/fon-2016-0358?…
Review
Cabozantinib in genitourinary malignancies
Tian Zhang1, Se Eun Park1, Cierra Hong2 & Daniel J George*,1,3
*Author for correspondence: daniel.george@duke.edu
Cabozantinib inhibits a variety of cellular receptors including VEGFR1–3, MET, AXL, RET, FLT3 and KIT. These signaling pathways have been shown to be important in genitourinary malignancies. Along its clinical development, it has shown most activity in advanced renal cell carcinoma; the METEOR study compared cabozantinib to everolimus and showed clinically and statistically significant improvements in both progression-free survival and overall survival. Herein, we review the development of cabozantinib in the genitourinary malignancies of renal cell carcinoma, prostate adenocarcinoma and urothelial carcinoma.
Full Text PDF (1725 KB) PDF Plus (1763 KB)
Interessanterweise hat die Kombitherapie Binimetinib+Encorafenib vs. Vemurafenib in einer ph3-Studie erstaunlich gute Ergebnisse erzielt, so dass eine weitere Kombitherapie zur Behandlung von metMelanoma (BRAF) mittelfristig am Markt platziert wird. Hab mir deswegen mal einige Arra (ARRY) ins Depot gelegt.
In November 2016, new results from the pivotal Phase 3 COLUMBUS trial were presented at the Society for Melanoma Research Annual Congress. The study met its primary endpoint, with the combination of bini/enco significantly improving progression free survival (PFS) compared with vemurafenib, a BRAF inhibitor, alone. The combination of bini/enco was generally well-tolerated and reported adverse events (AEs) were overall consistent with previous published clinical trial results for the bini/enco combination in BRAF-mutant melanoma patients.
In the analysis of the primary endpoint, the median PFS (mPFS) for patients treated with the combination of bini/enco was 14.9 months versus 7.3 months for patients treated with vemurafenib; hazard ratio (HR) 0.54, (95% CI 0.41-0.71, p<0.001). As part of the trial design, the primary analysis was based on a Blinded Independent Central Review (BICR) of patient scans, while results by local review at the investigative site were also analyzed. The chart below outlines the mPFS results, as determined by both assessments, for the combination of bini/enco versus vemurafenib, bini/enco versus encorafenib, and encorafenib versus vemurafenib:
http://investor.arraybiopharma.com/phoenix.zhtml?c=123810&p=…
Antwort auf Beitrag Nr.: 53.678.649 von Cyberhexe am 12.11.16 08:51:05
Wahres Timing Genie, alle Achtung!
Zitat von Cyberhexe: anbei eine sehr gute Übersicht zu den METEOR-Ergebnissen, publiziert von IKCC, einem internationalen Netzwerk zu Nierenkrebs:
http://iokidney.com/clinical-trials/results-meteor/infograph…
...übrigens, ich habe gestern meine EXEL-Position reduziert.
Wahres Timing Genie, alle Achtung!
First line or not first line,
das ist hier die Frage.https://deutsche-wirtschafts-nachrichten.de/2016/11/25/actel…
Dann kann man auch die Antwort bezüglich guten Timings geben... Ich hätte jedenfalls jetzt nicht reduziert.
interessante Erhebung, präsentiert am letzten Wochenende auf EMUC in Mailand:
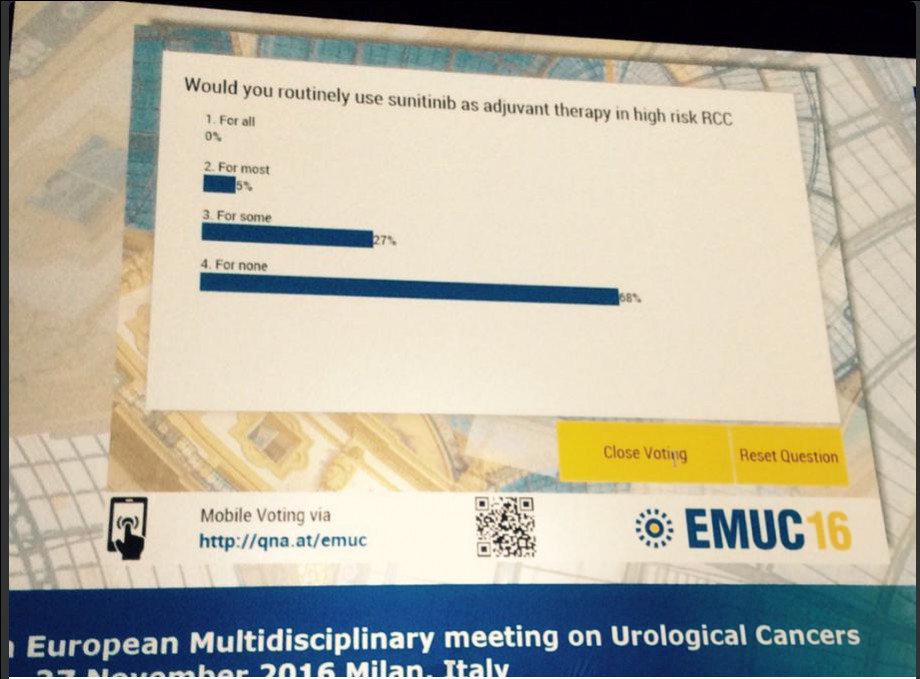

interessanter Beitrag:
http://ht.ly/4MAS306Apuu
TKI Sequencing in RCC Will Bring in New Paradigms, Outcomes
Brielle Urciuoli
Published Online: Sunday, Nov 27, 2016
Print Button
Robert A. Figlin, MD
Robert A. Figlin, MD
In the past decade, the addition of tyrosine kinase inhibitors (TKIs) has had a huge impact on the treatment of renal cell carcinoma (RCC) in the first- and second-line settings. Currently available agents include sorafenib (Nexavar), sunitinib (Sutent), pazopanib (Votrient), axitinib (Inlyta), and the recently-added cabozantinib (Cabometyx), and lenvatinib (Lenvima).
After the results of the CABOSUN and S-TRAC trials were presented last month at the 2016 European Society of Medical Oncology (ESMO) Congress, Robert A. Figlin, MD, FACP, is sure that TKI sequencing for kidney cancer will continue to evolve, ultimately shifting the treatment paradigms—and outcomes—for patients with the disease.
First, the phase II CABOSUN trial showed that cabozantinib significantly improved progression-free survival (PFS) over sunitinib, the current standard, in patients with high-risk newly diagnosed metastatic kidney cancer.
“CABOSUN is an interesting observation because it is the first illustration in a selected population of newly diagnosed metastatic patients where there may be an agent that has superior efficacy when it comes to the 10-year standard—sunitinib,” said Figlin, the Steven Spielberg Family Chair in Hematology-Oncology and associate director of academic program development in the Samuel Oschin Comprehensive Cancer Institute at Cedars-Sinai Medical Center.
In the trial, 157 patients with previously untreated clear-cell metastatic RCC with intermediate or poor prognosis were randomized to either cabozantinib or sunitinib for 4 weeks on, 2 weeks off. The cabozantinib group showed a 31% reduction in median rate of progress or death compared to the sunitinib group. “I think that this trial offers the opportunity for intermediate and poor-prognosis patients to be offered cabozantinib as the first treatment for their metastatic disease. So, it definitely has the capacity for a paradigm shift,” Figlin said.
However, Figlin is still unwilling to predict that cabozantinib will be a more viable option for all newly diagnosed patients with metastatic disease. “I’m a very evidence-based guy, so I’m not willing to think that cabozantinib would be appropriate for good-prognosis metastatic patients,” he said. “I’ll wait to see the data to make sure that the two populations of patients were equally balanced in terms of the risk groups in the intermediate population.”
Figlin emphasized that intermediate kidney cancer patients are a heterogeneous group. “They’re not all one group of patients, and they can often be divided by number of risk factors—one versus more than one,” Figlin said. These risk factors can include hemoglobin, LDH, weight loss, and performance status, and Figlin uses the Memorial Sloan Kettering Cancer Center Motzer score to stratify patients according to intermediate and poor risk.
“If you have one of those factors, we’ve shown that you do differently with sunitinib than if you have two of those risk factors, along with performance status. As such, I would hold that for the results of this trial to be applied to all patients, I’d like to be sure that the two groups were well-balanced,” Figlin said.
Also, now that cabozantinib was proven to be more effective than sunitinib in intermediate and poor prognosis metastatic patients, Figlin is hoping that the design of future clinical trials will reflect that. “Many of the pivotal trials that are taking place in the frontline setting with immuno-oncology approaches use sunitinib as the control arm. Then the question is, how are we going to be able to interpret that data when we now have an agent that may be superior to the control arms and the pivotal trials?” he said.
Different Mechanisms of Action
Cabozantinib and sunitinib, while both TKIs, have different mechanisms of action. Cabozantinib targets MET and AXL, whereas sunitinib targets VEGFR. This latter drug proved to be beneficial in the phase III S-TRAC trial. In the S-TRAC trial, sunitinib met its primary endpoint of disease-free survival when used as adjuvant therapy in patients with high-risk RCC patients after nephrectomy. The trial randomized 615 patients to either placebo or suntinib. After a year, the patients on sunitinib had a disease-free survival of 6.8 years, compared with the average of 5.6 years for those on a placebo.
This was the first trial to show that adjuvant therapy is helpful in this patient population, and it’s an important discovery, especially after the results of the ASSURE trial—which compared sorafenib to sunitinib with a placebo control arm—failed to demonstrate efficacy in any of the cohorts studied.
Figlin said that he is eager to see if sunitinib will prolong overall survival in addition to the extension in disease-free survival already demonstrated. If so, yet another question will arise. “So then the question becomes, if disease-free survival is enhanced by 1.2 years, is that worth the treatment when we have better treatments for those people at the time they were to progress? It becomes a ‘treat now or treat later’ phenomenon,” Figlin said.
Once these questions are answered, there likely will be some major changes in how oncologists are sequencing their TKIs for this group of patients. “One can imagine that for high-risk resected patients, the treatment could easily be sunitinib; and that for newly diagnosed intermediate and poor prognosis metastatic patients, the treatment could be cabozantinib; and for good prognosis patients with metastatic disease, the treatment would be sunitinib, which would be a major paradigm shift as compared to the last decade,” he said.
TKI Questions Linger
While trials such as CABOSUN and S-TRAC answer some questions, other questions linger regarding TKI sequencing in kidney cancer, such as how to treat patients who develop metastatic disease if they have already been on a TKI inhibitor in the past. Seeing what kind of role immunotherapy agents will eventually play in the frontline setting will help to answer this question, said Figlin.
“You could imagine a couple of scenarios,” Figlin said. “Suppose a person receives a TKI and then relapses very quickly, maybe a TKI isn’t appropriate at all. But, what if a person relapses a year later? A TKI might totally be appropriate.”
And while survival benefit is obviously key in determining which agents to use, it is not the only factor considered in the decision-making process.
“Every patient we treat, we think about comorbidities, how the person will tolerate the drug, whether theres are any drug-drug interactions, as well as overall endpoint of survival benefit,” Figlin said.
TKIs have been shown to present numerous adverse events, though their benefits will usually outweigh the hardship that patients undergo, according to Figlin.
“These agents are associated with effects such as hypertension, fatigue, hand-foot syndrome, and other things that are associated with changes in quality of life, but there are quality of life metrics that demonstrate that those are still superior over alternative placebos or less effective treatments,” Figlin said. “There are manipulations that doctors have become comfortable with to make sure the patient has the opportunity to benefit but doesn’t suffer any unacceptable side effects.”
TKIs, administered both simultaneously and in sequence, have changed the treatment landscape for patients with kidney cancer over the past decade. As these agents continue to be tested in different settings, and alongside immuno-oncology agents, the role of TKIs will continue to shift. -
See more at: http://www.onclive.com/web-exclusives/tki-sequencing-in-rcc-…
http://ht.ly/4MAS306Apuu
TKI Sequencing in RCC Will Bring in New Paradigms, Outcomes
Brielle Urciuoli
Published Online: Sunday, Nov 27, 2016
Print Button
Robert A. Figlin, MD
Robert A. Figlin, MD
In the past decade, the addition of tyrosine kinase inhibitors (TKIs) has had a huge impact on the treatment of renal cell carcinoma (RCC) in the first- and second-line settings. Currently available agents include sorafenib (Nexavar), sunitinib (Sutent), pazopanib (Votrient), axitinib (Inlyta), and the recently-added cabozantinib (Cabometyx), and lenvatinib (Lenvima).
After the results of the CABOSUN and S-TRAC trials were presented last month at the 2016 European Society of Medical Oncology (ESMO) Congress, Robert A. Figlin, MD, FACP, is sure that TKI sequencing for kidney cancer will continue to evolve, ultimately shifting the treatment paradigms—and outcomes—for patients with the disease.
First, the phase II CABOSUN trial showed that cabozantinib significantly improved progression-free survival (PFS) over sunitinib, the current standard, in patients with high-risk newly diagnosed metastatic kidney cancer.
“CABOSUN is an interesting observation because it is the first illustration in a selected population of newly diagnosed metastatic patients where there may be an agent that has superior efficacy when it comes to the 10-year standard—sunitinib,” said Figlin, the Steven Spielberg Family Chair in Hematology-Oncology and associate director of academic program development in the Samuel Oschin Comprehensive Cancer Institute at Cedars-Sinai Medical Center.
In the trial, 157 patients with previously untreated clear-cell metastatic RCC with intermediate or poor prognosis were randomized to either cabozantinib or sunitinib for 4 weeks on, 2 weeks off. The cabozantinib group showed a 31% reduction in median rate of progress or death compared to the sunitinib group. “I think that this trial offers the opportunity for intermediate and poor-prognosis patients to be offered cabozantinib as the first treatment for their metastatic disease. So, it definitely has the capacity for a paradigm shift,” Figlin said.
However, Figlin is still unwilling to predict that cabozantinib will be a more viable option for all newly diagnosed patients with metastatic disease. “I’m a very evidence-based guy, so I’m not willing to think that cabozantinib would be appropriate for good-prognosis metastatic patients,” he said. “I’ll wait to see the data to make sure that the two populations of patients were equally balanced in terms of the risk groups in the intermediate population.”
Figlin emphasized that intermediate kidney cancer patients are a heterogeneous group. “They’re not all one group of patients, and they can often be divided by number of risk factors—one versus more than one,” Figlin said. These risk factors can include hemoglobin, LDH, weight loss, and performance status, and Figlin uses the Memorial Sloan Kettering Cancer Center Motzer score to stratify patients according to intermediate and poor risk.
“If you have one of those factors, we’ve shown that you do differently with sunitinib than if you have two of those risk factors, along with performance status. As such, I would hold that for the results of this trial to be applied to all patients, I’d like to be sure that the two groups were well-balanced,” Figlin said.
Also, now that cabozantinib was proven to be more effective than sunitinib in intermediate and poor prognosis metastatic patients, Figlin is hoping that the design of future clinical trials will reflect that. “Many of the pivotal trials that are taking place in the frontline setting with immuno-oncology approaches use sunitinib as the control arm. Then the question is, how are we going to be able to interpret that data when we now have an agent that may be superior to the control arms and the pivotal trials?” he said.
Different Mechanisms of Action
Cabozantinib and sunitinib, while both TKIs, have different mechanisms of action. Cabozantinib targets MET and AXL, whereas sunitinib targets VEGFR. This latter drug proved to be beneficial in the phase III S-TRAC trial. In the S-TRAC trial, sunitinib met its primary endpoint of disease-free survival when used as adjuvant therapy in patients with high-risk RCC patients after nephrectomy. The trial randomized 615 patients to either placebo or suntinib. After a year, the patients on sunitinib had a disease-free survival of 6.8 years, compared with the average of 5.6 years for those on a placebo.
This was the first trial to show that adjuvant therapy is helpful in this patient population, and it’s an important discovery, especially after the results of the ASSURE trial—which compared sorafenib to sunitinib with a placebo control arm—failed to demonstrate efficacy in any of the cohorts studied.
Figlin said that he is eager to see if sunitinib will prolong overall survival in addition to the extension in disease-free survival already demonstrated. If so, yet another question will arise. “So then the question becomes, if disease-free survival is enhanced by 1.2 years, is that worth the treatment when we have better treatments for those people at the time they were to progress? It becomes a ‘treat now or treat later’ phenomenon,” Figlin said.
Once these questions are answered, there likely will be some major changes in how oncologists are sequencing their TKIs for this group of patients. “One can imagine that for high-risk resected patients, the treatment could easily be sunitinib; and that for newly diagnosed intermediate and poor prognosis metastatic patients, the treatment could be cabozantinib; and for good prognosis patients with metastatic disease, the treatment would be sunitinib, which would be a major paradigm shift as compared to the last decade,” he said.
TKI Questions Linger
While trials such as CABOSUN and S-TRAC answer some questions, other questions linger regarding TKI sequencing in kidney cancer, such as how to treat patients who develop metastatic disease if they have already been on a TKI inhibitor in the past. Seeing what kind of role immunotherapy agents will eventually play in the frontline setting will help to answer this question, said Figlin.
“You could imagine a couple of scenarios,” Figlin said. “Suppose a person receives a TKI and then relapses very quickly, maybe a TKI isn’t appropriate at all. But, what if a person relapses a year later? A TKI might totally be appropriate.”
And while survival benefit is obviously key in determining which agents to use, it is not the only factor considered in the decision-making process.
“Every patient we treat, we think about comorbidities, how the person will tolerate the drug, whether theres are any drug-drug interactions, as well as overall endpoint of survival benefit,” Figlin said.
TKIs have been shown to present numerous adverse events, though their benefits will usually outweigh the hardship that patients undergo, according to Figlin.
“These agents are associated with effects such as hypertension, fatigue, hand-foot syndrome, and other things that are associated with changes in quality of life, but there are quality of life metrics that demonstrate that those are still superior over alternative placebos or less effective treatments,” Figlin said. “There are manipulations that doctors have become comfortable with to make sure the patient has the opportunity to benefit but doesn’t suffer any unacceptable side effects.”
TKIs, administered both simultaneously and in sequence, have changed the treatment landscape for patients with kidney cancer over the past decade. As these agents continue to be tested in different settings, and alongside immuno-oncology agents, the role of TKIs will continue to shift. -
See more at: http://www.onclive.com/web-exclusives/tki-sequencing-in-rcc-…
Antwort auf Beitrag Nr.: 53.775.762 von Milestones am 26.11.16 09:06:05
obschon persönliche Eitelkeiten nicht zur Diskussion stehen ,sollte man bedenken, dass das Risk Management der Marktteilnehmer unterschiedlich sein kann. Mein persönliches Risk Management bestand darin, die "all in one"-Position Exelixis abzubauen und dafür andere vielversprechende Positionen aufzubauen wie z.B. PGNX, ARRY und RDUS. Die Risikostreuung stand hierbei im Vordergrund, obschon auch die Performance dieser Werte überzeugt.
Zitat von Milestones: das ist hier die Frage.
https://deutsche-wirtschafts-nachrichten.de/2016/11/25/actel…
Dann kann man auch die Antwort bezüglich guten Timings geben... Ich hätte jedenfalls jetzt nicht reduziert.
obschon persönliche Eitelkeiten nicht zur Diskussion stehen ,sollte man bedenken, dass das Risk Management der Marktteilnehmer unterschiedlich sein kann. Mein persönliches Risk Management bestand darin, die "all in one"-Position Exelixis abzubauen und dafür andere vielversprechende Positionen aufzubauen wie z.B. PGNX, ARRY und RDUS. Die Risikostreuung stand hierbei im Vordergrund, obschon auch die Performance dieser Werte überzeugt.
Antwort auf Beitrag Nr.: 53.751.219 von Cyberhexe am 22.11.16 22:09:52
Array ist übrigens direkter Konkurrent von Roche/Genentech/Exelixis bei der Therapie des BRAF mMelanom. Erstaunlicherweise ist das ph3-Ergebnis in COLUMBUS auf den ersten Blick sogar besser als jenes in coBRIM (Kombi cobi+vemurafenib) --> HR 0.54 vs. 0.56 beim progressionsfreien Überleben und dies bei signifikant geringerer Photosensibilität. Allerdings ist das Ergebnis der Überlebenszeitanalyse abzuwarten.
Zitat von Cyberhexe: Die Aufmerksamkeit hinsichtlich Cabozantinib in den wissenschaftlichen Medien ist enorm:
http://www.futuremedicine.com/doi/abs/10.2217/fon-2016-0358?…
Review
Cabozantinib in genitourinary malignancies
Tian Zhang1, Se Eun Park1, Cierra Hong2 & Daniel J George*,1,3
*Author for correspondence: daniel.george@duke.edu
Cabozantinib inhibits a variety of cellular receptors including VEGFR1–3, MET, AXL, RET, FLT3 and KIT. These signaling pathways have been shown to be important in genitourinary malignancies. Along its clinical development, it has shown most activity in advanced renal cell carcinoma; the METEOR study compared cabozantinib to everolimus and showed clinically and statistically significant improvements in both progression-free survival and overall survival. Herein, we review the development of cabozantinib in the genitourinary malignancies of renal cell carcinoma, prostate adenocarcinoma and urothelial carcinoma.
Full Text PDF (1725 KB) PDF Plus (1763 KB)
Interessanterweise hat die Kombitherapie Binimetinib+Encorafenib vs. Vemurafenib in einer ph3-Studie erstaunlich gute Ergebnisse erzielt, so dass eine weitere Kombitherapie zur Behandlung von metMelanoma (BRAF) mittelfristig am Markt platziert wird. Hab mir deswegen mal einige Arra (ARRY) ins Depot gelegt.
In November 2016, new results from the pivotal Phase 3 COLUMBUS trial were presented at the Society for Melanoma Research Annual Congress. The study met its primary endpoint, with the combination of bini/enco significantly improving progression free survival (PFS) compared with vemurafenib, a BRAF inhibitor, alone. The combination of bini/enco was generally well-tolerated and reported adverse events (AEs) were overall consistent with previous published clinical trial results for the bini/enco combination in BRAF-mutant melanoma patients.
In the analysis of the primary endpoint, the median PFS (mPFS) for patients treated with the combination of bini/enco was 14.9 months versus 7.3 months for patients treated with vemurafenib; hazard ratio (HR) 0.54, (95% CI 0.41-0.71, p<0.001). As part of the trial design, the primary analysis was based on a Blinded Independent Central Review (BICR) of patient scans, while results by local review at the investigative site were also analyzed. The chart below outlines the mPFS results, as determined by both assessments, for the combination of bini/enco versus vemurafenib, bini/enco versus encorafenib, and encorafenib versus vemurafenib:
http://investor.arraybiopharma.com/phoenix.zhtml?c=123810&p=…
Array ist übrigens direkter Konkurrent von Roche/Genentech/Exelixis bei der Therapie des BRAF mMelanom. Erstaunlicherweise ist das ph3-Ergebnis in COLUMBUS auf den ersten Blick sogar besser als jenes in coBRIM (Kombi cobi+vemurafenib) --> HR 0.54 vs. 0.56 beim progressionsfreien Überleben und dies bei signifikant geringerer Photosensibilität. Allerdings ist das Ergebnis der Überlebenszeitanalyse abzuwarten.
Interessanter Artikel
http://www.fool.com/investing/2016/11/30/whats-pfizers-excus…Wobei der Autor Exelixis 2018 rund 450 Mio Umsatz zugesteht. Hier liegt der Knackpunkt. Hat er damit recht oder deutet die Umsatzentwicklung heute schon darauf hin, dass wir ganz andere Zahlen sehen könnten? Das dürfte schlussendlich nicht unerheblich von den off-label Verschreibungen abhängen...
Antwort auf Beitrag Nr.: 53.806.308 von Milestones am 01.12.16 09:56:27
Fairer Wert dann eher 20 fach Umsatz 2018 ? Meinungen ?
lg
Der Unterschied
liegt aber vor allem an der Tatsache, dass Exelixis seinen Cabo Umsatz in US alleine behalten darf und nur Europa an Ipsen auslizenziert hat. Medivation muss Xtandi auch in den USA mit Astellas teilen....Fairer Wert dann eher 20 fach Umsatz 2018 ? Meinungen ?
lg
Exelixis könnte mit Cabo und Cobi wirklich die zweite Medivation werden, first line treatment mit Cabo bei RCC möglich, dann die Leberkrebsstudie 2017, möglicherweise Phase III für Lungen/Darmkrebs!
Natürlich ist Exel schon sportlich bewertet aber hat noch viel Potential, buy-out könnte im mittleren 20-er bis 30-er Bereich liegen, super Wert!
Natürlich ist Exel schon sportlich bewertet aber hat noch viel Potential, buy-out könnte im mittleren 20-er bis 30-er Bereich liegen, super Wert!
Immunotherapy Combos on Horizon in First-Line RCC
...interessanter Artikel zu Nierenkrebs in Erstlinienbehandlung:Immunotherapy combinations have significant potential as treatment for patients with renal cell carcinoma (RCC), says Robert J. Motzer, MD, a medical oncologist at Memorial Sloan Kettering Cancer Center.
“I am quite optimistic that these combinations will change our approach to kidney cancer in the first-line setting for years to come,” says Motzer.
Immunotherapy combinations have significant potential as treatment for patients with renal cell carcinoma (RCC), says Robert J. Motzer, MD, a medical oncologist at Memorial Sloan Kettering Cancer Center.
http://www.onclive.com/web-exclusives/immunotherapy-combos-o…
Hi Hexe,
wie siehst Du die Chancen für Exelixis und Cabo als first-line Behandlung, Übernahmechance?
Auf welche Aktien setzt Du 2017?
Lg
Fredy
wie siehst Du die Chancen für Exelixis und Cabo als first-line Behandlung, Übernahmechance?
Auf welche Aktien setzt Du 2017?
Lg
Fredy
Ist das ein guter Deal?
http://www.businesswire.com/news/home/20170130006192/en/Exel…
8K:
http://ih.advfn.com/p.php?pid=nmona&article=73733339&adw=112…
Exelixis will also receive royalties on net sales of cabozantinib in Japan at an initial tiered rate of 15% to 24% on net sales for the first $300.0 million of cumulative net sales. Thereafter, the royalty rate will be adjusted to 20% to 30% on annual net sales.
Upfront 50 Mio finde ich enttäuschend. Da hatte ich zwecks Schuldenabbau auf mehr gehofft.
Insgesamt aber o.k. was die Royalties und die Beteiligung an den Kosten zur Entwicklung betrifft.
Takeda ist sicherlich auch kein schlechter Partner mit einer zudem starken Präsenz in den USA (#Übernahme).
http://www.businesswire.com/news/home/20170130006192/en/Exel…
8K:
http://ih.advfn.com/p.php?pid=nmona&article=73733339&adw=112…
Exelixis will also receive royalties on net sales of cabozantinib in Japan at an initial tiered rate of 15% to 24% on net sales for the first $300.0 million of cumulative net sales. Thereafter, the royalty rate will be adjusted to 20% to 30% on annual net sales.
Upfront 50 Mio finde ich enttäuschend. Da hatte ich zwecks Schuldenabbau auf mehr gehofft.
Insgesamt aber o.k. was die Royalties und die Beteiligung an den Kosten zur Entwicklung betrifft.
Takeda ist sicherlich auch kein schlechter Partner mit einer zudem starken Präsenz in den USA (#Übernahme).
Antwort auf Beitrag Nr.: 54.203.056 von kmastra am 31.01.17 12:36:59Ich verstehe nicht, was es da zu meckern gibt - außer, dass Takeda kein Übernahmeangebot vorgelegt hat. 50Mio Upfront ist sehr gut, wenn man die weiteren Konditionen anschaut, die m.E. besser als der Ipsen Deal sind:
* 100% der zusätzlichen Entwicklungskosten für Japan (Stichwort: Bridging Studien; das macht es für Takeda halt teurer und rechtfertigt geringeren Upfront)
* 20% Beteiligung an den weiteren weltweiten Entwicklungskosten sind super (meiner Erinnerung hatte Ipsen da 35%, verbleiben also 45% bei Exelixis)
* 15-25% auf die ersten 300Mio; das ist besser als beim Ipsen Deal, wo es auf die ersten 100 Mio glaube ich nur 2% gibt.
* 20-30% Royalties sind auch sehr gut, ich glaube Ipsen hat im Peak nur 26% im Vertrag stehen.
* 100% der zusätzlichen Entwicklungskosten für Japan (Stichwort: Bridging Studien; das macht es für Takeda halt teurer und rechtfertigt geringeren Upfront)
* 20% Beteiligung an den weiteren weltweiten Entwicklungskosten sind super (meiner Erinnerung hatte Ipsen da 35%, verbleiben also 45% bei Exelixis)
* 15-25% auf die ersten 300Mio; das ist besser als beim Ipsen Deal, wo es auf die ersten 100 Mio glaube ich nur 2% gibt.
* 20-30% Royalties sind auch sehr gut, ich glaube Ipsen hat im Peak nur 26% im Vertrag stehen.
Antwort auf Beitrag Nr.: 54.208.102 von Ville7 am 31.01.17 20:31:27Verstehe auch nicht was es da jetzt zu meckern gibt. Ich denke ich habe das einigermaßen differenziert dargelegt...
Antwort auf Beitrag Nr.: 54.203.056 von kmastra am 31.01.17 12:36:59
mE ist dies eine gute Vereinbarung, die im Wesentlichen der Ipsen-Vereinbarung ähnlich ist, nämlich:
"In consideration for the exclusive license and other rights contained in the agreement, Ipsen will pay us an upfront payment of $200.0 million. We will be eligible to receive regulatory milestones, including a $60.0 million milestone payment upon approval of cabozantinib by the EMA in second-line RCC and milestone payments of $10.0 million upon the filing and $40.0 million upon the approval of cabozantinib in second-line HCC, as well as additional regulatory milestones payments for potential further indications. The agreement also provides that we will be eligible to receive payments of up to $545.0 million associated with potential commercial milestone payments, including two $10.0 million milestone payments upon the launch of the product in the first two of the following countries: Germany, France, Italy, Spain and the United Kingdom. Exelixis will also receive royalties on net sales of cabozantinib outside of the United States, Canada and Japan. We will receive a 2% royalty on the initial $50 million of net sales, and 12% royalty on the next $100 million of net sales. After this initial period, Exelixis will receive a tiered royalty of 22% to 26% on annual net sales. These tiers will reset each calendar year. Exelixis is responsible for funding cabozantinib related development costs for existing trials; development costs for potential future trials will be shared between the parties, with Ipsen to reimburse us for 35% of such costs. Pursuant to the terms of the agreement, we will remain responsible for the manufacture and supply of cabozantinib for all development and commercialization activities under the collaboration. As part of the collaboration, we entered into a supply agreement which provides that through the end of the second quarter of 2018, we will supply finished, labeled product to Ipsen for distribution in the territories outside of the United States, Canada and Japan, and from the end of the second quarter of 2018 forward, we will supply primary packaged bulk tablets to Ipsen."
Die Einmalzahlung beim Ipsen-Vertrag ist selbstverständlich höher, da es sich dabei um einen grösseren Absatzmarkt handelt. Bei den Lizenzgebühren ist anzumerken, dass beim Ipsen-Vertrag lediglich die ersten 50 Mio bzw. 150 Mio Umsatz mit einer tieferen Marge (2 bzw 12w%) abgegolten werden, jedoch nicht jedes Jahr sondern nur "...on the initial $50 million of net sales, and 12% royalty on the next $100 million of net sales".
Die aktuelle Marktkapitalisierung spiegelt den zukünftigen Erfolg von Cabozantinib, weitere Phantasie könnte vor allem aus der Kombi von Cobi+Atezolizumab genährt werden. Atezolizumab (brand: TECENTRIQ) wird bereits jetzt schon mit riesigen Umsätzen in den unterschiedlichsten Indikationen gehandelt (Jahresumsatz > 10 Milliarden USD), so dass eine nachgewiesene Synergie zwischen dem Checkpoint-Hemmer Atezoliuzumab und Roches einzigem MEK-Hemmer Cobimetinib eine Übernahme von Exelixis durch Roche/Genentech wahrscheinlicher werden lässt.
Zitat von kmastra: Ist das ein guter Deal?
http://www.businesswire.com/news/home/20170130006192/en/Exel…
8K:
http://ih.advfn.com/p.php?pid=nmona&article=73733339&adw=112…
Exelixis will also receive royalties on net sales of cabozantinib in Japan at an initial tiered rate of 15% to 24% on net sales for the first $300.0 million of cumulative net sales. Thereafter, the royalty rate will be adjusted to 20% to 30% on annual net sales.
Upfront 50 Mio finde ich enttäuschend. Da hatte ich zwecks Schuldenabbau auf mehr gehofft.
Insgesamt aber o.k. was die Royalties und die Beteiligung an den Kosten zur Entwicklung betrifft.
Takeda ist sicherlich auch kein schlechter Partner mit einer zudem starken Präsenz in den USA (#Übernahme).
mE ist dies eine gute Vereinbarung, die im Wesentlichen der Ipsen-Vereinbarung ähnlich ist, nämlich:
"In consideration for the exclusive license and other rights contained in the agreement, Ipsen will pay us an upfront payment of $200.0 million. We will be eligible to receive regulatory milestones, including a $60.0 million milestone payment upon approval of cabozantinib by the EMA in second-line RCC and milestone payments of $10.0 million upon the filing and $40.0 million upon the approval of cabozantinib in second-line HCC, as well as additional regulatory milestones payments for potential further indications. The agreement also provides that we will be eligible to receive payments of up to $545.0 million associated with potential commercial milestone payments, including two $10.0 million milestone payments upon the launch of the product in the first two of the following countries: Germany, France, Italy, Spain and the United Kingdom. Exelixis will also receive royalties on net sales of cabozantinib outside of the United States, Canada and Japan. We will receive a 2% royalty on the initial $50 million of net sales, and 12% royalty on the next $100 million of net sales. After this initial period, Exelixis will receive a tiered royalty of 22% to 26% on annual net sales. These tiers will reset each calendar year. Exelixis is responsible for funding cabozantinib related development costs for existing trials; development costs for potential future trials will be shared between the parties, with Ipsen to reimburse us for 35% of such costs. Pursuant to the terms of the agreement, we will remain responsible for the manufacture and supply of cabozantinib for all development and commercialization activities under the collaboration. As part of the collaboration, we entered into a supply agreement which provides that through the end of the second quarter of 2018, we will supply finished, labeled product to Ipsen for distribution in the territories outside of the United States, Canada and Japan, and from the end of the second quarter of 2018 forward, we will supply primary packaged bulk tablets to Ipsen."
Die Einmalzahlung beim Ipsen-Vertrag ist selbstverständlich höher, da es sich dabei um einen grösseren Absatzmarkt handelt. Bei den Lizenzgebühren ist anzumerken, dass beim Ipsen-Vertrag lediglich die ersten 50 Mio bzw. 150 Mio Umsatz mit einer tieferen Marge (2 bzw 12w%) abgegolten werden, jedoch nicht jedes Jahr sondern nur "...on the initial $50 million of net sales, and 12% royalty on the next $100 million of net sales".
Die aktuelle Marktkapitalisierung spiegelt den zukünftigen Erfolg von Cabozantinib, weitere Phantasie könnte vor allem aus der Kombi von Cobi+Atezolizumab genährt werden. Atezolizumab (brand: TECENTRIQ) wird bereits jetzt schon mit riesigen Umsätzen in den unterschiedlichsten Indikationen gehandelt (Jahresumsatz > 10 Milliarden USD), so dass eine nachgewiesene Synergie zwischen dem Checkpoint-Hemmer Atezoliuzumab und Roches einzigem MEK-Hemmer Cobimetinib eine Übernahme von Exelixis durch Roche/Genentech wahrscheinlicher werden lässt.
Cabozantinib (C) exposure-response (ER) modeling of safety endpoints in patients (pts) with renal cell carcinoma (RCC) in the phase III METEOR study.
http://abstracts.asco.org/197/AbstView_197_178499.html
http://abstracts.asco.org/197/AbstView_197_178499.html
Antwort auf Beitrag Nr.: 54.355.282 von Cyberhexe am 18.02.17 17:06:25
interessante Vorträge auf dem
Genitourinary Cancers Symposium 2017
Feb 16 - 18, 2017 | Orlando, Florida United States of America
Organized by : American Society of Clinical Oncology (ASCO)
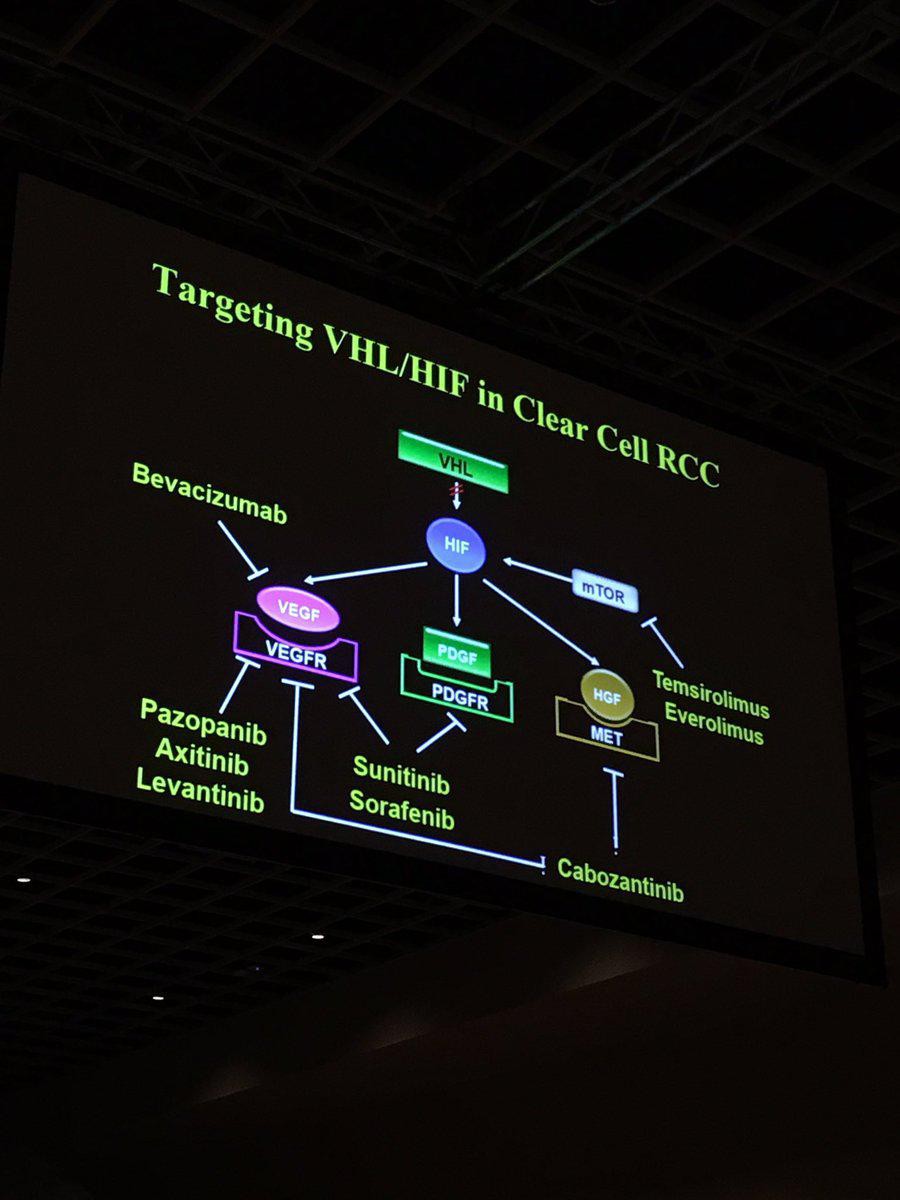
Zitat von Cyberhexe: Cabozantinib (C) exposure-response (ER) modeling of safety endpoints in patients (pts) with renal cell carcinoma (RCC) in the phase III METEOR study.
http://abstracts.asco.org/197/AbstView_197_178499.html
interessante Vorträge auf dem
Genitourinary Cancers Symposium 2017
Feb 16 - 18, 2017 | Orlando, Florida United States of America
Organized by : American Society of Clinical Oncology (ASCO)

Dr. Apolo on Study of Cabozantinib Plus Nivolumab and Ipilimumab in Urothelial Carcinoma
http://www.onclive.com/conference-coverage/gu-2017/dr-apolo-…
http://www.onclive.com/conference-coverage/gu-2017/dr-apolo-…
Roche hat Phase 2 Studienergebnisse zu RCC --> TECENTRIQ (atezolizumab) plus Avastin (bevacizumab) veröffentlicht und zwar gegenüber SOC Sunitinib.
http://www.roche.com/media/store/releases/med-cor-2017-02-18…
The study showed that people whose disease expressed PD-L1 (programmed death-ligand 1) and were treated with TECENTRIQ plus Avastin had a 36 percent reduction in the risk of their disease worsening or death compared to people treated with sunitinib alone (median progression-free survival [mPFS]: 14.7 vs. 7.8 months; HR= 0.64; 95% CI 0.38, 1.08). No PFS advantage was observed compared to sunitinib in the intention-to-treat [ITT] population (mPFS: 11.7 vs. 8.4 months; HR = 1.00; 95% CI 0.69, 1.45).
Erstaunlicherweise ist die Hazard Ratio bei der Kombitherapie aus Bevacizumab (AVASTIN) und Atezolizumab (TECENTRIQ) vs. Sunitinib nur unwesentlich günstiger (=0.64)
als diejenige von Cabozantinib vs. Sunitinib (0.66). In Anbetracht der horrenden Kosten einer Kombi aus Checkpoint-Inhibitor (Atezolizumab) und Angiogenesehemmer (Bevacizumab) würde ich behaupten --> Vorteil Cabozantinib-Monotherapie. Man darf auf weitere Resultate gespannt sein, zB. Cabo in Kombistudie mit Checkpoint-Hemmer.
http://ascopubs.org/doi/abs/10.1200/JCO.2016.70.7398?url_ver…
Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial
Results
From July 2013 to April 2015, 157 patients were randomly assigned (cabozantinib, n = 79; sunitinib, n = 78). Compared with sunitinib, cabozantinib treatment significantly increased median PFS (8.2 v 5.6 months) and was associated with a 34% reduction in rate of progression or death (adjusted hazard ratio, 0.66; 95% CI, 0.46 to 0.95; one-sided P = .012). ORR was 46% (95% CI, 34 to 57) for cabozantinib versus 18% (95% CI, 10 to 28) for sunitinib. All-causality grade 3 or 4 adverse events were 67% for cabozantinib and 68% for sunitinib and included diarrhea (cabozantinib, 10% v sunitinib, 11%), fatigue (6% v 15%), hypertension (28% v 22%), palmar-plantar erythrodysesthesia (8% v 4%), and hematologic adverse events (3% v 22%).
Conclusion
Cabozantinib demonstrated a significant clinical benefit in PFS and ORR over standard-of-care sunitinib as first-line therapy in patients with intermediate- or poor-risk mRCC.
http://www.roche.com/media/store/releases/med-cor-2017-02-18…
The study showed that people whose disease expressed PD-L1 (programmed death-ligand 1) and were treated with TECENTRIQ plus Avastin had a 36 percent reduction in the risk of their disease worsening or death compared to people treated with sunitinib alone (median progression-free survival [mPFS]: 14.7 vs. 7.8 months; HR= 0.64; 95% CI 0.38, 1.08). No PFS advantage was observed compared to sunitinib in the intention-to-treat [ITT] population (mPFS: 11.7 vs. 8.4 months; HR = 1.00; 95% CI 0.69, 1.45).
Erstaunlicherweise ist die Hazard Ratio bei der Kombitherapie aus Bevacizumab (AVASTIN) und Atezolizumab (TECENTRIQ) vs. Sunitinib nur unwesentlich günstiger (=0.64)
als diejenige von Cabozantinib vs. Sunitinib (0.66). In Anbetracht der horrenden Kosten einer Kombi aus Checkpoint-Inhibitor (Atezolizumab) und Angiogenesehemmer (Bevacizumab) würde ich behaupten --> Vorteil Cabozantinib-Monotherapie. Man darf auf weitere Resultate gespannt sein, zB. Cabo in Kombistudie mit Checkpoint-Hemmer.
http://ascopubs.org/doi/abs/10.1200/JCO.2016.70.7398?url_ver…
Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial
Results
From July 2013 to April 2015, 157 patients were randomly assigned (cabozantinib, n = 79; sunitinib, n = 78). Compared with sunitinib, cabozantinib treatment significantly increased median PFS (8.2 v 5.6 months) and was associated with a 34% reduction in rate of progression or death (adjusted hazard ratio, 0.66; 95% CI, 0.46 to 0.95; one-sided P = .012). ORR was 46% (95% CI, 34 to 57) for cabozantinib versus 18% (95% CI, 10 to 28) for sunitinib. All-causality grade 3 or 4 adverse events were 67% for cabozantinib and 68% for sunitinib and included diarrhea (cabozantinib, 10% v sunitinib, 11%), fatigue (6% v 15%), hypertension (28% v 22%), palmar-plantar erythrodysesthesia (8% v 4%), and hematologic adverse events (3% v 22%).
Conclusion
Cabozantinib demonstrated a significant clinical benefit in PFS and ORR over standard-of-care sunitinib as first-line therapy in patients with intermediate- or poor-risk mRCC.
Antwort auf Beitrag Nr.: 54.358.054 von Cyberhexe am 19.02.17 11:02:15Zumal HR=0,64 nur für PD-L1+ gilt (die Mehrzahl der Patienten dürfte eher PD-L1- sein... z.B. RCC "Six studies and 1323 cases were included ... PD-L1 was expressed in 24.2 % of clear cell tumors compared to 10.9 % of non-clear cell tumors"). In der unselektierten Gruppe hat diese Kombi keinen Effekt mit HR=1.0. Allerdings ist unklar, ob die Cabo-Studie wirklich vergleichbar ist, die PFS-Ergebnisse liegen ja ziemlich auseinander.
PD-L1+ sollte zu einer Verschlimmerung von RCC führen, so dass ein PD1-Hemmer durchaus Sinn macht in dieser Gruppe. Besser wäre aber wohl die Kombi aus Cabo + PD1 (Cabo ist wie Avastin auch ein VEGFR-Hemmer)... dazu laufen doch auch schon Studien, wenn ich mich richtig erinnere.
In RCC sind TKIs wie Cabo der Standard, so dass Cabo alleine dadurch schon einen Vorteil hat. Die Ärzte sind mit TKIs vertraut und können leicht switchen. Auf der LR-Konferenz letzte Woche http://wsw.com/webcast/leerink28/exel/?lobby=true&day=1 hat EXEL ja davon gesprochen, dass Cabo bei den neuen Verschreibungen bereits in Q3 einen 20% Anteil insgesamt (40% TKI) in 2nd-line hat sowie 35% insgesamt (55% TKI) in 3rd-line RCC. Die Zulassung 1st-line soll auf Basis der PII erfolgen. Q4-Zahlen gibt es noch nicht, aber nach Ergebnissen der Konkurrenten sollte der Marktanteil weiter gewachsen sein. Z.B. Pfizers Inlyta Q3 -25% und Q4 -40%.
Gut auch... die exUS-Partner beteiligen sich an den Entwicklungskosten (sofern sie ein Interesse an den entsprechenden neuen Indikationen haben), Ipsen zahlt 35% und Takeda 20%, d.h. EXEL muss weniger als die Hälfte der US-Entwicklungskosten zahlen bei 100% der US-Rechte. IO-Kombis sollen zudem von den IO-Partnern mitfinanziert werden. Dadurch kann EXEL einiges an Kosten sparen.
Gruß
ipollit
PD-L1+ sollte zu einer Verschlimmerung von RCC führen, so dass ein PD1-Hemmer durchaus Sinn macht in dieser Gruppe. Besser wäre aber wohl die Kombi aus Cabo + PD1 (Cabo ist wie Avastin auch ein VEGFR-Hemmer)... dazu laufen doch auch schon Studien, wenn ich mich richtig erinnere.
In RCC sind TKIs wie Cabo der Standard, so dass Cabo alleine dadurch schon einen Vorteil hat. Die Ärzte sind mit TKIs vertraut und können leicht switchen. Auf der LR-Konferenz letzte Woche http://wsw.com/webcast/leerink28/exel/?lobby=true&day=1 hat EXEL ja davon gesprochen, dass Cabo bei den neuen Verschreibungen bereits in Q3 einen 20% Anteil insgesamt (40% TKI) in 2nd-line hat sowie 35% insgesamt (55% TKI) in 3rd-line RCC. Die Zulassung 1st-line soll auf Basis der PII erfolgen. Q4-Zahlen gibt es noch nicht, aber nach Ergebnissen der Konkurrenten sollte der Marktanteil weiter gewachsen sein. Z.B. Pfizers Inlyta Q3 -25% und Q4 -40%.
Gut auch... die exUS-Partner beteiligen sich an den Entwicklungskosten (sofern sie ein Interesse an den entsprechenden neuen Indikationen haben), Ipsen zahlt 35% und Takeda 20%, d.h. EXEL muss weniger als die Hälfte der US-Entwicklungskosten zahlen bei 100% der US-Rechte. IO-Kombis sollen zudem von den IO-Partnern mitfinanziert werden. Dadurch kann EXEL einiges an Kosten sparen.
Gruß
ipollit
J Clin Oncol. 2017 Feb 20;35(6):591-597. doi: 10.1200/JCO.2016.70.7398. Epub 2016 Nov 14.
Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial.
http://ascopubs.org/doi/pdf/10.1200/JCO.2016.70.7398
Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial.
http://ascopubs.org/doi/pdf/10.1200/JCO.2016.70.7398
Ohad Hammer hat gestern Exelixis zum Verkauf empfohlen:
http://www.orf-blog.com/biotech-portfolio-updates-esperion-t…
http://www.orf-blog.com/biotech-portfolio-updates-esperion-t…
Das National Institute for Health and Care Excellence (NICE) hat entschieden, die Kosten für Cabozantinib als Zweitlinienbehandlung von Nierenkrebs nicht zu bezahlen. Die Alternative, der Checkpoint-Inhibitor Nivolumab (PD-L1), ist jedoch nicht günstiger...im Gegenteil:
Besonders rasant steigt derzeit der Einsatz von Immuntherapien, Vorreiter ist hier das Medikament Nivolumab. Das Mittel hat bei fortgeschrittenem schwarzem Hautkrebs in einigen Fällen verblüffende Erfolge erzielt und ist jetzt auch für die meisten Arten von Lungenkrebs zugelassen; wahrscheinlich dürfen Ärzte den Antikörper bald auch gegen bestimmte Nierentumore verordnen. Ein Jahr Behandlung kostet rund 100.000 Euro.
http://www.swr.de/swr2/wissen/krebs-teure-medikamente/-/id=6…
3. Nivolumab and cabozantinib as second-line therapy options
http://www.thepharmaletter.com/article/nice-draft-guidance-r…
Final NICE guidance is expected in June 2017; eligible patients will continue to receive access to cabozantinib via the Managed Access Programme until this time, according to Ipsen.
...ich gehe davon aus, dass man sich im Juni auf eine Kostenerstattung (mit entsprechenden Rabatten) einigen wird.
Besonders rasant steigt derzeit der Einsatz von Immuntherapien, Vorreiter ist hier das Medikament Nivolumab. Das Mittel hat bei fortgeschrittenem schwarzem Hautkrebs in einigen Fällen verblüffende Erfolge erzielt und ist jetzt auch für die meisten Arten von Lungenkrebs zugelassen; wahrscheinlich dürfen Ärzte den Antikörper bald auch gegen bestimmte Nierentumore verordnen. Ein Jahr Behandlung kostet rund 100.000 Euro.
http://www.swr.de/swr2/wissen/krebs-teure-medikamente/-/id=6…
3. Nivolumab and cabozantinib as second-line therapy options
http://www.thepharmaletter.com/article/nice-draft-guidance-r…
Final NICE guidance is expected in June 2017; eligible patients will continue to receive access to cabozantinib via the Managed Access Programme until this time, according to Ipsen.
...ich gehe davon aus, dass man sich im Juni auf eine Kostenerstattung (mit entsprechenden Rabatten) einigen wird.
DOI: 10.1200/JCO.2016.70.7398 Journal of Clinical Oncology 35, no. 6 (February 2017) 591-597.
Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial
....
Conclusion
Cabozantinib demonstrated a significant clinical benefit in PFS and ORR over standard-of-care sunitinib as first-line therapy in patients with intermediate- or poor-risk mRCC.
http://ascopubs.org/doi/abs/10.1200/JCO.2016.70.7398
Cabozantinib mit signifikantem klinischen Nutzen beim progressionsfreien Überleben als auch bei der Überlebenszeitanalyse gegenüber dem aktuellen Standard Sunitinib als Initial bzw. Erstlinienbehandlung!
Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial
....
Conclusion
Cabozantinib demonstrated a significant clinical benefit in PFS and ORR over standard-of-care sunitinib as first-line therapy in patients with intermediate- or poor-risk mRCC.
http://ascopubs.org/doi/abs/10.1200/JCO.2016.70.7398
Cabozantinib mit signifikantem klinischen Nutzen beim progressionsfreien Überleben als auch bei der Überlebenszeitanalyse gegenüber dem aktuellen Standard Sunitinib als Initial bzw. Erstlinienbehandlung!
Antwort auf Beitrag Nr.: 54.388.615 von Cyberhexe am 22.02.17 23:11:17Die Entscheidung über die Kostenerstattung in England (gilt nicht für den Rest of UK) erfolgt im Juni 2017.
Cabozantinib for treating renal cell carcinoma [ID931]
In development [GID-TA10075] Expected publication date: June 2017
Note that this document is not NICE's final guidance on this technology. The recommendations in section 1 may change after consultation.
After consultation:
· The appraisal committee will meet again to consider the evidence, this appraisal consultation document and comments from the consultees.
· At that meeting, the committee will also consider comments made by people who are not consultees.
· After considering these comments, the committee will prepare the final appraisal determination (FAD).
· Subject to any appeal by consultees, the FAD may be used as the basis for NICE’s guidance on using cabozantinib in the NHS in England.
For further details, see NICE’s guide to the processes of technology appraisal.
The key dates for this appraisal are:
Closing date for comments: 14 March 2017
Second appraisal committee meeting: 23 March 2017
Details of membership of the appraisal committee are given in section 8.
https://www.nice.org.uk/guidance/indevelopment/GID-TA10075/c…
https://www.nice.org.uk/guidance/GID-TA10075/documents/appra…
Cabozantinib for treating renal cell carcinoma [ID931]
In development [GID-TA10075] Expected publication date: June 2017
Note that this document is not NICE's final guidance on this technology. The recommendations in section 1 may change after consultation.
After consultation:
· The appraisal committee will meet again to consider the evidence, this appraisal consultation document and comments from the consultees.
· At that meeting, the committee will also consider comments made by people who are not consultees.
· After considering these comments, the committee will prepare the final appraisal determination (FAD).
· Subject to any appeal by consultees, the FAD may be used as the basis for NICE’s guidance on using cabozantinib in the NHS in England.
For further details, see NICE’s guide to the processes of technology appraisal.
The key dates for this appraisal are:
Closing date for comments: 14 March 2017
Second appraisal committee meeting: 23 March 2017
Details of membership of the appraisal committee are given in section 8.
https://www.nice.org.uk/guidance/indevelopment/GID-TA10075/c…
https://www.nice.org.uk/guidance/GID-TA10075/documents/appra…
sehr viel versprechende Ergebnisse in der Kombinationstherapie von Cabo mit den Checkpoint-Hemmern Nivolumab (PD-1-Hemmer) und Ipilimumab (CTLA-4-Hemmer)
Cabozantinib Regimens Active Across Multiple Advanced GU Malignancies
http://www.onclive.com/conference-coverage/gu-2017/cabozanti…
Dr. Apolo on Study of Cabozantinib Plus Nivolumab and Ipilimumab in Urothelial Carcinoma
Andrea B. Apolo, MD
Published Online: Thursday, Feb 16, 2017
http://www.onclive.com/conference-coverage/gu-2017/dr-apolo-…
Cabozantinib Regimens Active Across Multiple Advanced GU Malignancies
http://www.onclive.com/conference-coverage/gu-2017/cabozanti…
Dr. Apolo on Study of Cabozantinib Plus Nivolumab and Ipilimumab in Urothelial Carcinoma
Andrea B. Apolo, MD
Published Online: Thursday, Feb 16, 2017
http://www.onclive.com/conference-coverage/gu-2017/dr-apolo-…
Knochenmetastasen und Cabozantinib:
http://www.impactjournals.com/oncotarget/index.php?journal=o…
Cabozantinib targets bone microenvironment modulating human osteoclast and osteoblast functions
Our results show that non-cytotoxic doses of cabozantinib significantly inhibit osteoclast differentiation (p=0.0145) and bone resorption activity (p=0.0252). Moreover, cabozantinib down-modulates the expression of osteoclast marker genes, TRAP (p=0.006), CATHEPSIN K (p=0.004) and Receptor Activator of Nuclear Factor k B (RANK) (p=0.001). Cabozantinib treatment has no effect on osteoblast viability or differentiation, but increases osteoprotegerin mRNA (p=0.015) and protein levels (p=0.004) and down-modulates Receptor Activator of Nuclear Factor k B Ligand (RANKL) at both mRNA (p<0.001) and protein levels (p=0.043). Direct cell-to-cell contact between cabozantinib pre-treated osteoblasts and untreated osteoclasts confirmed the indirect anti-resorptive effect of cabozantinib.
Die Wirkung auf Knochenmetastasen wurde bereits in den COMET-Studien beobachtet.
"Cabozantinib was associated with improvements in CTC conversion, bone biomarkers, and post–random assignment incidence of SSEs but not PSA outcomes."
Quelle: http://ascopubs.org/doi/full/10.1200/JCO.2015.65.5597
http://www.impactjournals.com/oncotarget/index.php?journal=o…
Cabozantinib targets bone microenvironment modulating human osteoclast and osteoblast functions
Our results show that non-cytotoxic doses of cabozantinib significantly inhibit osteoclast differentiation (p=0.0145) and bone resorption activity (p=0.0252). Moreover, cabozantinib down-modulates the expression of osteoclast marker genes, TRAP (p=0.006), CATHEPSIN K (p=0.004) and Receptor Activator of Nuclear Factor k B (RANK) (p=0.001). Cabozantinib treatment has no effect on osteoblast viability or differentiation, but increases osteoprotegerin mRNA (p=0.015) and protein levels (p=0.004) and down-modulates Receptor Activator of Nuclear Factor k B Ligand (RANKL) at both mRNA (p<0.001) and protein levels (p=0.043). Direct cell-to-cell contact between cabozantinib pre-treated osteoblasts and untreated osteoclasts confirmed the indirect anti-resorptive effect of cabozantinib.
Die Wirkung auf Knochenmetastasen wurde bereits in den COMET-Studien beobachtet.
"Cabozantinib was associated with improvements in CTC conversion, bone biomarkers, and post–random assignment incidence of SSEs but not PSA outcomes."
Quelle: http://ascopubs.org/doi/full/10.1200/JCO.2015.65.5597
Antwort auf Beitrag Nr.: 54.408.856 von Cyberhexe am 25.02.17 08:33:33Azetolizumab und Cabozantinib...möglicherweise die ideale Kombination zur Behandlung von RCC:
http://www.exelixis.com/investors-media/press-releases?cpurl…
http://www.exelixis.com/investors-media/press-releases
Based on the dose-escalation results, the trial has the potential to enroll up to four expansion cohorts, including a cohort of patients with previously untreated advanced clear cell renal cell carcinoma (RCC) and three cohorts of urothelial carcinoma (UC), namely platinum eligible first-line patients, first- or second-line platinum ineligible patients, and patients previously treated with platinum-containing chemotherapy.
http://www.exelixis.com/investors-media/press-releases?cpurl…
http://www.exelixis.com/investors-media/press-releases
Based on the dose-escalation results, the trial has the potential to enroll up to four expansion cohorts, including a cohort of patients with previously untreated advanced clear cell renal cell carcinoma (RCC) and three cohorts of urothelial carcinoma (UC), namely platinum eligible first-line patients, first- or second-line platinum ineligible patients, and patients previously treated with platinum-containing chemotherapy.
Antwort auf Beitrag Nr.: 47.891.537 von Cyberhexe am 27.09.14 18:05:26
...und heute diskutiert ein Trittbrettfahrer und Denglish-Experte über Risikomanagement. Den grössten Schwätzer, der mir auf dieser WS-Plattform begegnet ist, wird ab heute ignoriert.
Zitat von Cyberhexe: bereits vor Bekanntgabe der COMET1-Ergebnisse (01.09.2014) wurden dieser Studie nur geringe Erfolgsaussichten bescheinigt (siehe Mail vom 24.8.2014), so dass der Kurseinbruch um fast 70% für viele nicht nachvollziehbar ist:
cyberhexe schrieb am 24.08.14 07:23:56
Beitrag Nr. 21 (47.594.053)
folgende 3 kursbewegenden Ereignisse stehen unmittelbar bevor:
coBRIM wird derzeit konsolidiert => ph3 Kombitherapie cobimetinib/vemurafenib vs vemurafenib zur Behandlung von Patienten mit einer BRAF V600 Mutation und einem nicht operierbaren fortgeschrittenen metastatischen Melanom
Kooperationspartner Roche/Genentech hat bereits bekanntgegeben, dass eine statistisch signifikante Lebensverlängerung erreicht wurde; detaillierte Ergebnisse werden auf ESMO (26.-30.9. in Madrid) veröffentlicht. Sehr wahrscheinlich erfolgt ein baldiger Zulassungsantrag in den USA und Europa (sehr wahrscheinlich noch in 2014)
COMET1 und COMET2 dürften sich mittlerweile auch im Readout-Modus befinden. Hierbei handelt es sich um 2 Studien bei kastrationsrefraktären Prostatakarzinoms --> CRPC (Castration Resistant Prostata Carzinom)
COMET1, mit dem primären Endpunkt Lebensverlängerung, ist eine Plazebo-kontrollierte ph3-Studie, in welcher 960 Männer mit CRPC - bereits mehrfach vorbehandelt - randomisiert entweder mit Cabozantinib oder Prednison behandelt werden.
COMET2, ebenfalls eine ph3-Studie, fokussiert sich beim primären Endpunkt auf die Schmerzreduzierung bei CRPC.
A Phase 3, Randomized, Double-blind, Controlled Trial of Cabozantinib (XL184) Versus Mitoxantrone Plus Prednisone in Men With Previously Treated Symptomatic Castration-resistant Prostate Cancer
Estimated Enrollment: 246
Study Start Date: March 2012
Während ein statistisch signifikanter Überlebensvorteil unter der Kombitherapie in coBRIM bereits mitgeteilt wurde, sind die beiden CRPC-ph3-Studien im Ergebnis noch völlig offen, wobei COMET-2 jedoch wahrscheinlich günstigere Erfolgsaussichten bescheinigt werden. Den überlebensvorteil in COMET1 nachzuweisen halten Experten in Anbetracht der vielfach vorbehandelten und schwer kranken Studienteilnehmer für die weitaus grössere Herausforderung.
Für mich ist dieser Kurs (USD 1.56 am 26.9.14) ein "strong buy" - habe gestern mein letztes trockenes Pulver in Exelixis investiert bei einem mittlerweile unvernünftigen Investitionsgrad...aber die Verlockung ist nun mal zu gross!
gltal
...und heute diskutiert ein Trittbrettfahrer und Denglish-Experte über Risikomanagement. Den grössten Schwätzer, der mir auf dieser WS-Plattform begegnet ist, wird ab heute ignoriert.
http://www.exelixis.com/investors-media/press-releases
Exelixis Announces Fourth Quarter and Full Year 2016 Financial Results and Provides Corporate Update
- Cabozantinib Franchise Net Product Revenues of $51.9 million for the Fourth Quarter 2016, $135.4 million for the Full Year 2016 -
- Total Revenues of $77.6 million for the Fourth Quarter 2016, $191.5 million for the Full Year 2016 -
- Conference Call and Webcast Today at 5:00 PM Eastern Time -
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Feb. 27, 2017-- Exelixis, Inc. (Nasdaq:EXEL) today reported financial results for the fourth quarter and full year of 2016 and provided an update on progress toward delivering upon its key corporate objectives, as well as commercial and clinical development milestones.
Exelixis is focused on maximizing the opportunity for its two internally-discovered compounds, cabozantinib and cobimetinib, each of which has the potential to help patients around the world fighting a variety of cancers. The company’s most immediate priority is continuing to execute on the U.S. launch of CABOMETYX™ (cabozantinib) tablets as a treatment for patients with advanced renal cell carcinoma (RCC) who have received prior anti-angiogenic therapy. CABOMETYX generated $44.7 million and $93.5 million in net product revenue during the fourth quarter and full year of 2016, respectively. COMETRIQ® (cabozantinib) capsules for the treatment of medullary thyroid cancer generated an additional $7.2 million and $41.9 million in net product revenue during the fourth quarter and full year of 2016, respectively. In addition, Exelixis is preparing a regulatory filing for cabozantinib as a treatment for previously-untreated patients with advanced RCC based on the positive data from the CABOSUN randomized phase 2 trial. Exelixis and its partner Genentech, a member of the Roche Group, are co-promoting Cotellic® (cobimetinib) in the United States, while Genentech continues to advance the cobimetinib clinical development program, which now includes three ongoing or planned phase 3 pivotal trials of combination regimens including cobimetinib for forms of colorectal cancer and advanced melanoma.
“2016 marked an inflection point for Exelixis, with the U.S. approval and launch of CABOMETYX, and the emergence of key data sets that have supported a significantly expanded late-stage clinical development program for cobimetinib. At the same time, we secured important partnerships and collaborations that will further advance the cabozantinib franchise on a global basis and improved our balance sheet, providing strength and flexibility as we move forward,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis.
“We started 2017 in a strong financial position with a focus on driving the business to generate free cash to reinvest in our pipeline. We are making progress towards a U.S. regulatory filing based on the CABOSUN results, targeted for the third quarter of this year, and have recently announced collaborations focused on conducting late-stage clinical trials of cabozantinib in combination with leading immunotherapies. Separately, our partner Genentech continues to expand its late-stage clinical development program for cobimetinib in areas of considerable therapeutic and commercial potential. The robust clinical development programs for both cabozantinib and cobimetinib form a solid foundation to build on in the year ahead as we and our partners work to improve cancer care for patients around the world.”
Cabozantinib Highlights
Strong Growth in Cabozantinib Franchise Net Revenues. Cabozantinib generated $51.9 million in net product revenue during the fourth quarter of 2016, an increase of 21 percent from the third quarter of 2016. Full year 2016 net product revenue was $135.4 million, an increase of 296 percent year-over-year. The year-over-year increase was driven primarily by the U.S. introduction of CABOMETYX following FDA approval in April 2016 as a treatment for patients with advanced RCC who have received prior anti-angiogenic therapy.
Presented Positive Results from Phase 2 CABOSUN Trial in Advanced RCC. At the European Society for Medical Oncology (ESMO) Congress in October 2016, detailed results were presented from CABOSUN, the randomized phase 2 trial of cabozantinib compared with sunitinib in patients with previously untreated advanced RCC with intermediate- or poor-risk disease per the International Metastatic Renal Carcinoma Database Consortium risk criteria. In this trial, cabozantinib demonstrated a statistically significant and clinically meaningful reduction in the rate of disease progression or death as compared to sunitinib. The CABOSUN results were the subject of a late-breaking abstract at ESMO, and were highlighted at one of the Congress’ Presidential Symposia and in its official media program. CABOSUN was conducted by The Alliance for Clinical Trials in Oncology (The Alliance) with support from the National Cancer Institute’s Cancer Therapy Evaluation Program (NCI-CTEP).
Advanced Filing Plans for Cabozantinib in Previously Untreated Advanced RCC. In the fourth quarter 2016, the transfer of the CABOSUN clinical database from The Alliance to Exelixis was completed, and Exelixis is preparing a Supplemental New Drug Application, which is targeted for submission in the third quarter of 2017.
Phase 1 Trial Results for Cabozantinib in Combination with Nivolumab in Advanced Genitourinary Tumors. Also at the ESMO 2016 Congress, encouraging results were presented from Part 1 of the two part NCI-CTEP-sponsored phase 1 trial of cabozantinib in combination with nivolumab in patients with previously treated genitourinary tumors. Expansion cohorts assessing cabozantinib and nivolumab, including patients with bladder, renal, and rare genitourinary cancers, are also currently being accrued.
At the ASCO Genitourinary Cancers Symposium in February 2017, investigators presented new data from Part 1 as well as Part 2 of the trial, which adds ipilimumab to the combination regimen of cabozantinib and nivolumab.
Collaborations for Late-Stage Development of Cabozantinib in Combination with Immunotherapies. After the year ended, Exelixis announced agreements with Bristol-Myers Squibb (BMS) and Roche to collaborate on the development of cabozantinib in combination with immunotherapy agents. Exelixis and BMS announced their intent to collaborate on the evaluation of cabozantinib in combination with Opdivo® (nivolumab) alone or in combination with Yervoy® (ipilimumab) in a phase 3 trial in first-line RCC, and potentially in other tumor types including hepatocellular carcinoma (HCC) and bladder cancer. Studies are anticipated to begin in 2017. The collaborations build upon previously published preclinical and clinical data that underscore the scientific rationale for combining cabozantinib with immunotherapies, and provide the resources and collaborative framework to evaluate the potential for cabozantinib combination regimens to benefit patients with a variety of cancers. Separately, Exelixis and Roche will collaborate to initiate testing of cabozantinib in combination with Tecentriq® (atezolizumab), an anti-PD-L1 antibody, in patients with advanced RCC or bladder cancer.
New and Amended Partnerships to Support the Global Cabozantinib Franchise. On December 21, 2016, Exelixis and Ipsen announced an amendment to their exclusive collaboration and licensing agreement for the commercialization and continued development of cabozantinib, to include commercialization rights in Canada for Ipsen. Exelixis received a $10.0 million upfront payment and is eligible to receive regulatory milestones for the approvals of cabozantinib in Canada for advanced RCC after prior treatment, for first-line advanced RCC, and advanced HCC, as well as additional regulatory milestones for potential further indications. In line with the prior transaction between the parties, the agreement also includes commercial milestones and provides for Exelixis to receive tiered royalties on Ipsen’s net sales of cabozantinib in Canada.
After the year ended, in January 2017 Exelixis and Takeda jointly announced an exclusive licensing agreement for the commercialization and further development of cabozantinib in Japan, including rights to CABOMETYX and COMETRIQ. Under the terms of the agreement, Exelixis received a $50.0 million upfront payment. Exelixis is eligible to receive development, regulatory, and first-sales milestones of $95.0 million for the first three planned indications. In addition, Exelixis will be eligible to receive royalties on sales by Takeda. Takeda will be responsible for 20 percent of the costs associated with the global cabozantinib development plan and 100 percent of costs associated with the cabozantinib development activities that are exclusively for the benefit of Japan.
Cobimetinib Highlights
Results Presented at ESMO 2016 from Cobimetinib Combination Trials Support Further Advancement. Cobimetinib, the Exelixis-discovered MEK inhibitor that is the subject of a worldwide collaboration with Genentech, a member of the Roche Group, was featured in seven presentations at the ESMO 2016 Congress. For the first time, investigators presented preliminary results from the phase 1b clinical trial of the triple combination of cobimetinib, vemurafenib, and atezolizumab in patients with previously untreated BRAF V600 mutation-positive advanced melanoma. The regimen was associated with promising antitumor activity and a manageable safety profile. These results provided the rationale for the Roche-sponsored phase 3 pivotal trial, IMspire150 TRILOGY, which began enrolling patients in January 2017.
Investigators also presented updated results from the phase 1 trial of cobimetinib plus atezolizumab in advanced colorectal cancer that provide the rationale for IMblaze370 (formerly known as COTEZO), the ongoing phase 3 pivotal trial in the same disease setting. New data from the phase 1 part of COLET, the phase 1/2 trial of cobimetinib and paclitaxel in triple-negative breast cancer, were also the subject of a poster presentation at the meeting.
Presentation of Cobimetinib Combination Therapy Data at the Society for Melanoma Research 2016 Congress. On November 7, 2016, Exelixis announced the presentation of data from the metastatic melanoma cohort of a phase 1b dose escalation trial of cobimetinib and atezolizumab in patients with solid tumors. Data from this trial will form the basis of a Genentech-sponsored phase 3 pivotal trial of the combination in patients with previously untreated BRAF wild-type advanced melanoma, which is also expected to start this year.
Update on Dispute between Exelixis and Genentech. Since the conclusion of the fourth quarter, Exelixis announced that Genentech, Inc., a member of the Roche Group, had withdrawn its counterclaim against Exelixis in the ongoing JAMS arbitration concerning alleged breaches of the parties’ collaboration agreement. Genentech had asserted a counterclaim for breach of contract, which sought monetary damages and interest related to cost allocations under the collaboration agreement. When notifying the arbitral panel, and Exelixis, of this unilateral action, Genentech further stated that it is changing the manner in which it allocates promotional expenses of the Cotellic plus Zelboraf® (vemurafenib) combination therapy. Genentech’s revised allocation applies retrospectively and prospectively and substantially reduces Exelixis’ exposure to costs associated with promotion of the Cotellic plus Zelboraf combination in the United States.
2017 Financial Guidance
The company is providing guidance that total costs and operating expenses for the full year will be between $290 million and $310 million. This guidance includes approximately $25 million of non-cash costs and expenses related primarily to stock-based compensation expense.
Fourth Quarter and Full Year 2016 Financial Results
Total revenues for the quarter ended December 31, 2016 were $77.6 million, compared to $9.9 million for the comparable period in 2015. Total revenues for the year ended December 31, 2016 were $191.5 million, compared to $37.2 million for the comparable period in 2015.
Total revenues for the quarter ended December 31, 2016 include $51.9 million of net product revenues compared to $9.9 million for the comparable period in 2015. Net product revenues for the year ended December 31, 2016 were $135.4 million, compared to $34.2 million for the comparable period in 2015. The increase in net product revenues for both the quarter and year ended December 31, 2016, as compared to the same periods in 2015, primarily reflects the impact of the commercial launch of CABOMETYX in late April 2016.
Total revenues for the quarter ended December 31, 2016 also include two $10.0 million milestones achieved for the first commercial sales of CABOMETYX by Ipsen in Germany and the United Kingdom. Total revenues for the year ended December 31, 2016 also include the recognition of $20.0 million of revenue for milestones from two of our collaboration partners, Daiichi Sankyo and Merck. Total revenues for the quarter and year ended December 31, 2016 also include $1.0 million and $2.8 million, respectively, of royalty revenues from Ipsen and Roche and $4.7 million and $13.3 million, respectively, of license revenues from Ipsen.
In comparison, during the year ended December 31, 2015, we recognized $3.0 million of contract revenues for a milestone payment received from Merck.
Research and development expenses for the quarter ended December 31, 2016 were $23.8 million, compared to $23.5 million for the comparable period in 2015. Research and development expenses for the year ended December 31, 2016 were $96.0 million, compared to $96.4 million for the comparable period in 2015. For both the quarter and year-ended December 31, 2016 as compared to the same periods in 2015, decreases in share-based compensation and the allocation of general corporate costs were offset by increases in personnel related expenses resulting from an increase in headcount predominantly associated with the build-out of the Exelixis Medical Affairs organization. For the year-ended December 31, 2016 as compared to the same period in 2015, there were also decreases in clinical trial costs for METEOR, the Company’s phase 3 trial in advanced RCC.
Selling, general and administrative expenses for the quarter ended December 31, 2016 were $13.0 million, compared to $17.1 million for the comparable period in 2015. Selling, general and administrative expenses for the year ended December 31, 2016 were $116.1 million, compared to $57.3 million for the comparable period in 2015. For both the quarter and year-ended December 31, 2016 as compared to the same periods in 2015, there were increases in personnel related expenses resulting from an increase in headcount connected with the build-out of the Exelixis U.S. commercial organization and outside services to support the launch and commercialization of CABOMETYX. These increases were offset by a decrease in marketing costs related to losses on our collaboration with Genentech.
As described above, in December 2016 Genentech stated that it changed, both retroactively and prospectively, the manner in which it allocates promotional expenses of the Cotellic plus Zelboraf combination therapy. As a result, Exelixis is relieved of $18.7 million of disputed costs previously recorded by Exelixis, and Exelixis has invoiced Genentech for expenses, with interest, that Exelixis had previously paid. Accordingly, during the quarter ended December 31, 2016, we offset selling, general and administrative expenses for a $23.1 million recovery of net losses, which had been recorded from 2013 through September 30, 2016, including $13.3 million for losses that we had recognized and recorded prior to 2016. During the quarter and year ended December 31, 2016, we also recognized a net gain of $0.6 million and a net loss of $4.5 million, respectively, for current U.S. activities in those periods under the collaboration agreement as computed under Genentech’s revised cost allocation approach.
Other expense, net for the quarter ended December 31, 2016 was a net expense of ($3.8) million compared to ($9.9) million for the comparable period in 2015. Other expense, net for the year ended December 31, 2016 was a net expense of ($42.1) million compared to ($40.3) million for the comparable period in 2015. The decrease in other expense, net for the quarter ended December 31, 2016 as compared to 2015 was primarily due to the reduction in interest expense as a result of the conversion and redemption of $287.5 million in aggregate principal amount of our 4.25% Convertible Senior Subordinated Notes due 2019 (2019 Notes). For the year ended December 31, 2016, the reduction in interest expense was offset by $13.9 million of loss associated with the conversion of our 2019 Notes for 54,009,279 shares of our common stock.
Net income (loss) for the quarter ended December 31, 2016 was net income of $35.1 million, or $0.12 per share, basic and fully diluted, compared to a net loss ($41.6) million, or ($0.18) per share, basic and fully diluted, for the comparable period in 2015. Net loss for the year ended December 31, 2016 was a net loss ($70.2) million, or ($0.28) per share, basic and fully diluted, compared to a net loss ($161.7) million, or ($0.77) per share, basic and fully diluted, for the comparable period in 2015. The decrease in net loss for the quarter and year ended December 31, 2016 was primarily due to increases in net product revenues; increases in collaboration revenues; the recovery of net losses previously recorded under our collaboration agreement with Genentech; and a decrease in interest expense; partially offset by increases in personnel expenses associated with the increase in headcount connected with the build-out of the Exelixis U.S. commercial and medical affairs organizations and other costs associated with the launch of CABOMETYX. For the year ended December 31, 2016, the decrease in net loss was also partially offset by the loss associated with the conversion of the 2019 Notes.
Cash and cash equivalents, short- and long-term investments and long-term restricted cash and investments totaled $479.6 million at December 31, 2016 as compared to $253.3 million at December 31, 2015.
Exelixis Announces Fourth Quarter and Full Year 2016 Financial Results and Provides Corporate Update
- Cabozantinib Franchise Net Product Revenues of $51.9 million for the Fourth Quarter 2016, $135.4 million for the Full Year 2016 -
- Total Revenues of $77.6 million for the Fourth Quarter 2016, $191.5 million for the Full Year 2016 -
- Conference Call and Webcast Today at 5:00 PM Eastern Time -
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Feb. 27, 2017-- Exelixis, Inc. (Nasdaq:EXEL) today reported financial results for the fourth quarter and full year of 2016 and provided an update on progress toward delivering upon its key corporate objectives, as well as commercial and clinical development milestones.
Exelixis is focused on maximizing the opportunity for its two internally-discovered compounds, cabozantinib and cobimetinib, each of which has the potential to help patients around the world fighting a variety of cancers. The company’s most immediate priority is continuing to execute on the U.S. launch of CABOMETYX™ (cabozantinib) tablets as a treatment for patients with advanced renal cell carcinoma (RCC) who have received prior anti-angiogenic therapy. CABOMETYX generated $44.7 million and $93.5 million in net product revenue during the fourth quarter and full year of 2016, respectively. COMETRIQ® (cabozantinib) capsules for the treatment of medullary thyroid cancer generated an additional $7.2 million and $41.9 million in net product revenue during the fourth quarter and full year of 2016, respectively. In addition, Exelixis is preparing a regulatory filing for cabozantinib as a treatment for previously-untreated patients with advanced RCC based on the positive data from the CABOSUN randomized phase 2 trial. Exelixis and its partner Genentech, a member of the Roche Group, are co-promoting Cotellic® (cobimetinib) in the United States, while Genentech continues to advance the cobimetinib clinical development program, which now includes three ongoing or planned phase 3 pivotal trials of combination regimens including cobimetinib for forms of colorectal cancer and advanced melanoma.
“2016 marked an inflection point for Exelixis, with the U.S. approval and launch of CABOMETYX, and the emergence of key data sets that have supported a significantly expanded late-stage clinical development program for cobimetinib. At the same time, we secured important partnerships and collaborations that will further advance the cabozantinib franchise on a global basis and improved our balance sheet, providing strength and flexibility as we move forward,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis.
“We started 2017 in a strong financial position with a focus on driving the business to generate free cash to reinvest in our pipeline. We are making progress towards a U.S. regulatory filing based on the CABOSUN results, targeted for the third quarter of this year, and have recently announced collaborations focused on conducting late-stage clinical trials of cabozantinib in combination with leading immunotherapies. Separately, our partner Genentech continues to expand its late-stage clinical development program for cobimetinib in areas of considerable therapeutic and commercial potential. The robust clinical development programs for both cabozantinib and cobimetinib form a solid foundation to build on in the year ahead as we and our partners work to improve cancer care for patients around the world.”
Cabozantinib Highlights
Strong Growth in Cabozantinib Franchise Net Revenues. Cabozantinib generated $51.9 million in net product revenue during the fourth quarter of 2016, an increase of 21 percent from the third quarter of 2016. Full year 2016 net product revenue was $135.4 million, an increase of 296 percent year-over-year. The year-over-year increase was driven primarily by the U.S. introduction of CABOMETYX following FDA approval in April 2016 as a treatment for patients with advanced RCC who have received prior anti-angiogenic therapy.
Presented Positive Results from Phase 2 CABOSUN Trial in Advanced RCC. At the European Society for Medical Oncology (ESMO) Congress in October 2016, detailed results were presented from CABOSUN, the randomized phase 2 trial of cabozantinib compared with sunitinib in patients with previously untreated advanced RCC with intermediate- or poor-risk disease per the International Metastatic Renal Carcinoma Database Consortium risk criteria. In this trial, cabozantinib demonstrated a statistically significant and clinically meaningful reduction in the rate of disease progression or death as compared to sunitinib. The CABOSUN results were the subject of a late-breaking abstract at ESMO, and were highlighted at one of the Congress’ Presidential Symposia and in its official media program. CABOSUN was conducted by The Alliance for Clinical Trials in Oncology (The Alliance) with support from the National Cancer Institute’s Cancer Therapy Evaluation Program (NCI-CTEP).
Advanced Filing Plans for Cabozantinib in Previously Untreated Advanced RCC. In the fourth quarter 2016, the transfer of the CABOSUN clinical database from The Alliance to Exelixis was completed, and Exelixis is preparing a Supplemental New Drug Application, which is targeted for submission in the third quarter of 2017.
Phase 1 Trial Results for Cabozantinib in Combination with Nivolumab in Advanced Genitourinary Tumors. Also at the ESMO 2016 Congress, encouraging results were presented from Part 1 of the two part NCI-CTEP-sponsored phase 1 trial of cabozantinib in combination with nivolumab in patients with previously treated genitourinary tumors. Expansion cohorts assessing cabozantinib and nivolumab, including patients with bladder, renal, and rare genitourinary cancers, are also currently being accrued.
At the ASCO Genitourinary Cancers Symposium in February 2017, investigators presented new data from Part 1 as well as Part 2 of the trial, which adds ipilimumab to the combination regimen of cabozantinib and nivolumab.
Collaborations for Late-Stage Development of Cabozantinib in Combination with Immunotherapies. After the year ended, Exelixis announced agreements with Bristol-Myers Squibb (BMS) and Roche to collaborate on the development of cabozantinib in combination with immunotherapy agents. Exelixis and BMS announced their intent to collaborate on the evaluation of cabozantinib in combination with Opdivo® (nivolumab) alone or in combination with Yervoy® (ipilimumab) in a phase 3 trial in first-line RCC, and potentially in other tumor types including hepatocellular carcinoma (HCC) and bladder cancer. Studies are anticipated to begin in 2017. The collaborations build upon previously published preclinical and clinical data that underscore the scientific rationale for combining cabozantinib with immunotherapies, and provide the resources and collaborative framework to evaluate the potential for cabozantinib combination regimens to benefit patients with a variety of cancers. Separately, Exelixis and Roche will collaborate to initiate testing of cabozantinib in combination with Tecentriq® (atezolizumab), an anti-PD-L1 antibody, in patients with advanced RCC or bladder cancer.
New and Amended Partnerships to Support the Global Cabozantinib Franchise. On December 21, 2016, Exelixis and Ipsen announced an amendment to their exclusive collaboration and licensing agreement for the commercialization and continued development of cabozantinib, to include commercialization rights in Canada for Ipsen. Exelixis received a $10.0 million upfront payment and is eligible to receive regulatory milestones for the approvals of cabozantinib in Canada for advanced RCC after prior treatment, for first-line advanced RCC, and advanced HCC, as well as additional regulatory milestones for potential further indications. In line with the prior transaction between the parties, the agreement also includes commercial milestones and provides for Exelixis to receive tiered royalties on Ipsen’s net sales of cabozantinib in Canada.
After the year ended, in January 2017 Exelixis and Takeda jointly announced an exclusive licensing agreement for the commercialization and further development of cabozantinib in Japan, including rights to CABOMETYX and COMETRIQ. Under the terms of the agreement, Exelixis received a $50.0 million upfront payment. Exelixis is eligible to receive development, regulatory, and first-sales milestones of $95.0 million for the first three planned indications. In addition, Exelixis will be eligible to receive royalties on sales by Takeda. Takeda will be responsible for 20 percent of the costs associated with the global cabozantinib development plan and 100 percent of costs associated with the cabozantinib development activities that are exclusively for the benefit of Japan.
Cobimetinib Highlights
Results Presented at ESMO 2016 from Cobimetinib Combination Trials Support Further Advancement. Cobimetinib, the Exelixis-discovered MEK inhibitor that is the subject of a worldwide collaboration with Genentech, a member of the Roche Group, was featured in seven presentations at the ESMO 2016 Congress. For the first time, investigators presented preliminary results from the phase 1b clinical trial of the triple combination of cobimetinib, vemurafenib, and atezolizumab in patients with previously untreated BRAF V600 mutation-positive advanced melanoma. The regimen was associated with promising antitumor activity and a manageable safety profile. These results provided the rationale for the Roche-sponsored phase 3 pivotal trial, IMspire150 TRILOGY, which began enrolling patients in January 2017.
Investigators also presented updated results from the phase 1 trial of cobimetinib plus atezolizumab in advanced colorectal cancer that provide the rationale for IMblaze370 (formerly known as COTEZO), the ongoing phase 3 pivotal trial in the same disease setting. New data from the phase 1 part of COLET, the phase 1/2 trial of cobimetinib and paclitaxel in triple-negative breast cancer, were also the subject of a poster presentation at the meeting.
Presentation of Cobimetinib Combination Therapy Data at the Society for Melanoma Research 2016 Congress. On November 7, 2016, Exelixis announced the presentation of data from the metastatic melanoma cohort of a phase 1b dose escalation trial of cobimetinib and atezolizumab in patients with solid tumors. Data from this trial will form the basis of a Genentech-sponsored phase 3 pivotal trial of the combination in patients with previously untreated BRAF wild-type advanced melanoma, which is also expected to start this year.
Update on Dispute between Exelixis and Genentech. Since the conclusion of the fourth quarter, Exelixis announced that Genentech, Inc., a member of the Roche Group, had withdrawn its counterclaim against Exelixis in the ongoing JAMS arbitration concerning alleged breaches of the parties’ collaboration agreement. Genentech had asserted a counterclaim for breach of contract, which sought monetary damages and interest related to cost allocations under the collaboration agreement. When notifying the arbitral panel, and Exelixis, of this unilateral action, Genentech further stated that it is changing the manner in which it allocates promotional expenses of the Cotellic plus Zelboraf® (vemurafenib) combination therapy. Genentech’s revised allocation applies retrospectively and prospectively and substantially reduces Exelixis’ exposure to costs associated with promotion of the Cotellic plus Zelboraf combination in the United States.
2017 Financial Guidance
The company is providing guidance that total costs and operating expenses for the full year will be between $290 million and $310 million. This guidance includes approximately $25 million of non-cash costs and expenses related primarily to stock-based compensation expense.
Fourth Quarter and Full Year 2016 Financial Results
Total revenues for the quarter ended December 31, 2016 were $77.6 million, compared to $9.9 million for the comparable period in 2015. Total revenues for the year ended December 31, 2016 were $191.5 million, compared to $37.2 million for the comparable period in 2015.
Total revenues for the quarter ended December 31, 2016 include $51.9 million of net product revenues compared to $9.9 million for the comparable period in 2015. Net product revenues for the year ended December 31, 2016 were $135.4 million, compared to $34.2 million for the comparable period in 2015. The increase in net product revenues for both the quarter and year ended December 31, 2016, as compared to the same periods in 2015, primarily reflects the impact of the commercial launch of CABOMETYX in late April 2016.
Total revenues for the quarter ended December 31, 2016 also include two $10.0 million milestones achieved for the first commercial sales of CABOMETYX by Ipsen in Germany and the United Kingdom. Total revenues for the year ended December 31, 2016 also include the recognition of $20.0 million of revenue for milestones from two of our collaboration partners, Daiichi Sankyo and Merck. Total revenues for the quarter and year ended December 31, 2016 also include $1.0 million and $2.8 million, respectively, of royalty revenues from Ipsen and Roche and $4.7 million and $13.3 million, respectively, of license revenues from Ipsen.
In comparison, during the year ended December 31, 2015, we recognized $3.0 million of contract revenues for a milestone payment received from Merck.
Research and development expenses for the quarter ended December 31, 2016 were $23.8 million, compared to $23.5 million for the comparable period in 2015. Research and development expenses for the year ended December 31, 2016 were $96.0 million, compared to $96.4 million for the comparable period in 2015. For both the quarter and year-ended December 31, 2016 as compared to the same periods in 2015, decreases in share-based compensation and the allocation of general corporate costs were offset by increases in personnel related expenses resulting from an increase in headcount predominantly associated with the build-out of the Exelixis Medical Affairs organization. For the year-ended December 31, 2016 as compared to the same period in 2015, there were also decreases in clinical trial costs for METEOR, the Company’s phase 3 trial in advanced RCC.
Selling, general and administrative expenses for the quarter ended December 31, 2016 were $13.0 million, compared to $17.1 million for the comparable period in 2015. Selling, general and administrative expenses for the year ended December 31, 2016 were $116.1 million, compared to $57.3 million for the comparable period in 2015. For both the quarter and year-ended December 31, 2016 as compared to the same periods in 2015, there were increases in personnel related expenses resulting from an increase in headcount connected with the build-out of the Exelixis U.S. commercial organization and outside services to support the launch and commercialization of CABOMETYX. These increases were offset by a decrease in marketing costs related to losses on our collaboration with Genentech.
As described above, in December 2016 Genentech stated that it changed, both retroactively and prospectively, the manner in which it allocates promotional expenses of the Cotellic plus Zelboraf combination therapy. As a result, Exelixis is relieved of $18.7 million of disputed costs previously recorded by Exelixis, and Exelixis has invoiced Genentech for expenses, with interest, that Exelixis had previously paid. Accordingly, during the quarter ended December 31, 2016, we offset selling, general and administrative expenses for a $23.1 million recovery of net losses, which had been recorded from 2013 through September 30, 2016, including $13.3 million for losses that we had recognized and recorded prior to 2016. During the quarter and year ended December 31, 2016, we also recognized a net gain of $0.6 million and a net loss of $4.5 million, respectively, for current U.S. activities in those periods under the collaboration agreement as computed under Genentech’s revised cost allocation approach.
Other expense, net for the quarter ended December 31, 2016 was a net expense of ($3.8) million compared to ($9.9) million for the comparable period in 2015. Other expense, net for the year ended December 31, 2016 was a net expense of ($42.1) million compared to ($40.3) million for the comparable period in 2015. The decrease in other expense, net for the quarter ended December 31, 2016 as compared to 2015 was primarily due to the reduction in interest expense as a result of the conversion and redemption of $287.5 million in aggregate principal amount of our 4.25% Convertible Senior Subordinated Notes due 2019 (2019 Notes). For the year ended December 31, 2016, the reduction in interest expense was offset by $13.9 million of loss associated with the conversion of our 2019 Notes for 54,009,279 shares of our common stock.
Net income (loss) for the quarter ended December 31, 2016 was net income of $35.1 million, or $0.12 per share, basic and fully diluted, compared to a net loss ($41.6) million, or ($0.18) per share, basic and fully diluted, for the comparable period in 2015. Net loss for the year ended December 31, 2016 was a net loss ($70.2) million, or ($0.28) per share, basic and fully diluted, compared to a net loss ($161.7) million, or ($0.77) per share, basic and fully diluted, for the comparable period in 2015. The decrease in net loss for the quarter and year ended December 31, 2016 was primarily due to increases in net product revenues; increases in collaboration revenues; the recovery of net losses previously recorded under our collaboration agreement with Genentech; and a decrease in interest expense; partially offset by increases in personnel expenses associated with the increase in headcount connected with the build-out of the Exelixis U.S. commercial and medical affairs organizations and other costs associated with the launch of CABOMETYX. For the year ended December 31, 2016, the decrease in net loss was also partially offset by the loss associated with the conversion of the 2019 Notes.
Cash and cash equivalents, short- and long-term investments and long-term restricted cash and investments totaled $479.6 million at December 31, 2016 as compared to $253.3 million at December 31, 2015.
EXELIXIS wird Cabo in Erstlinienbehandlung von Nierenkrebs zur Zulassung einreichen :


Antwort auf Beitrag Nr.: 54.425.557 von Cyberhexe am 28.02.17 01:36:57die Markteinführung von Cabo 2L RCC ist gelungen:
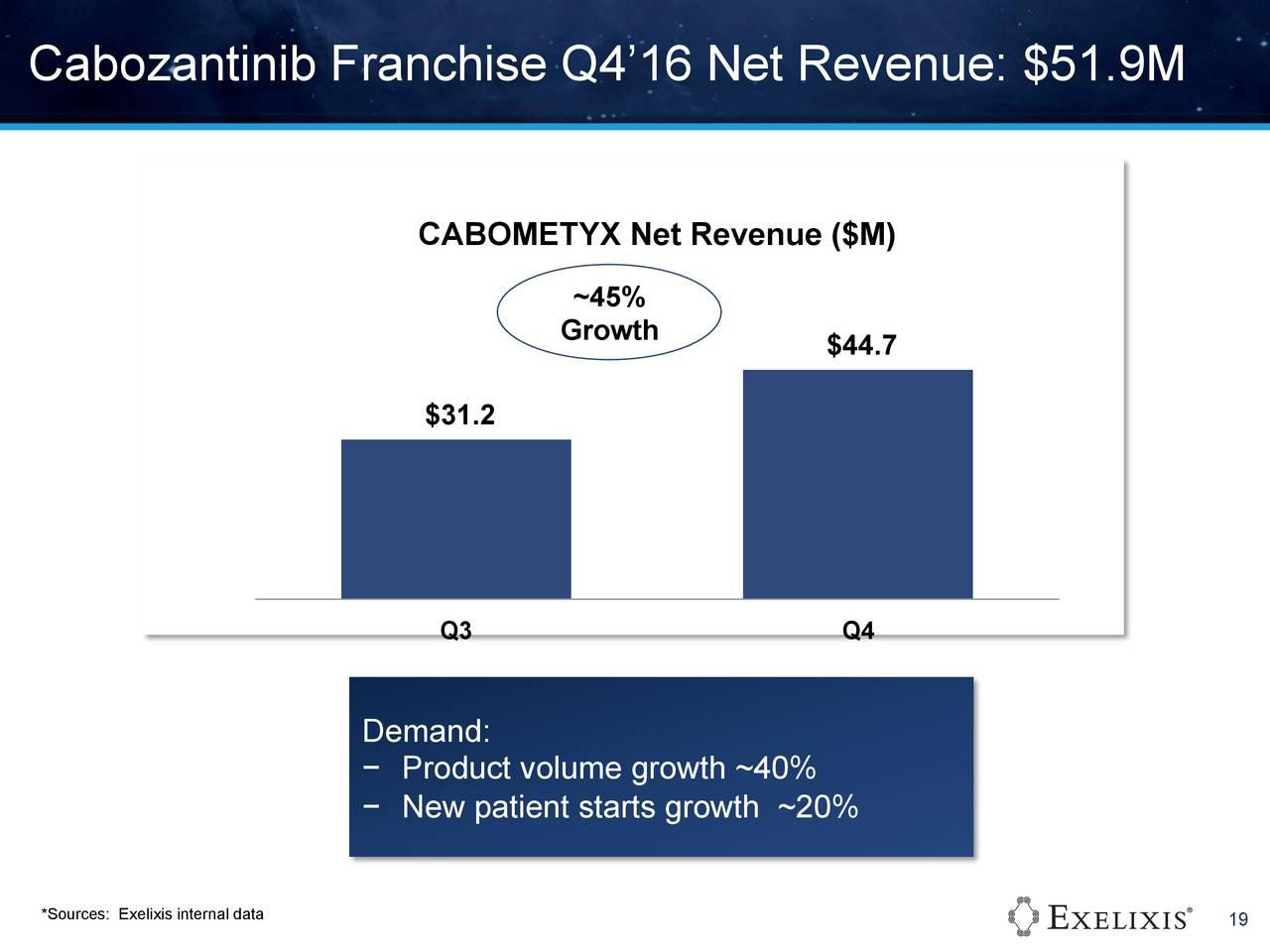

Antwort auf Beitrag Nr.: 54.425.566 von Cyberhexe am 28.02.17 01:43:04Zulassung zur Erstlinienbehandlung soll im 3q2017 eingereicht werden:


Antwort auf Beitrag Nr.: 54.425.572 von Cyberhexe am 28.02.17 01:45:33Ergebnisse zur Leberkrebs-ph3-Studie CELESTIAL werden noch in 2017 erwartet:
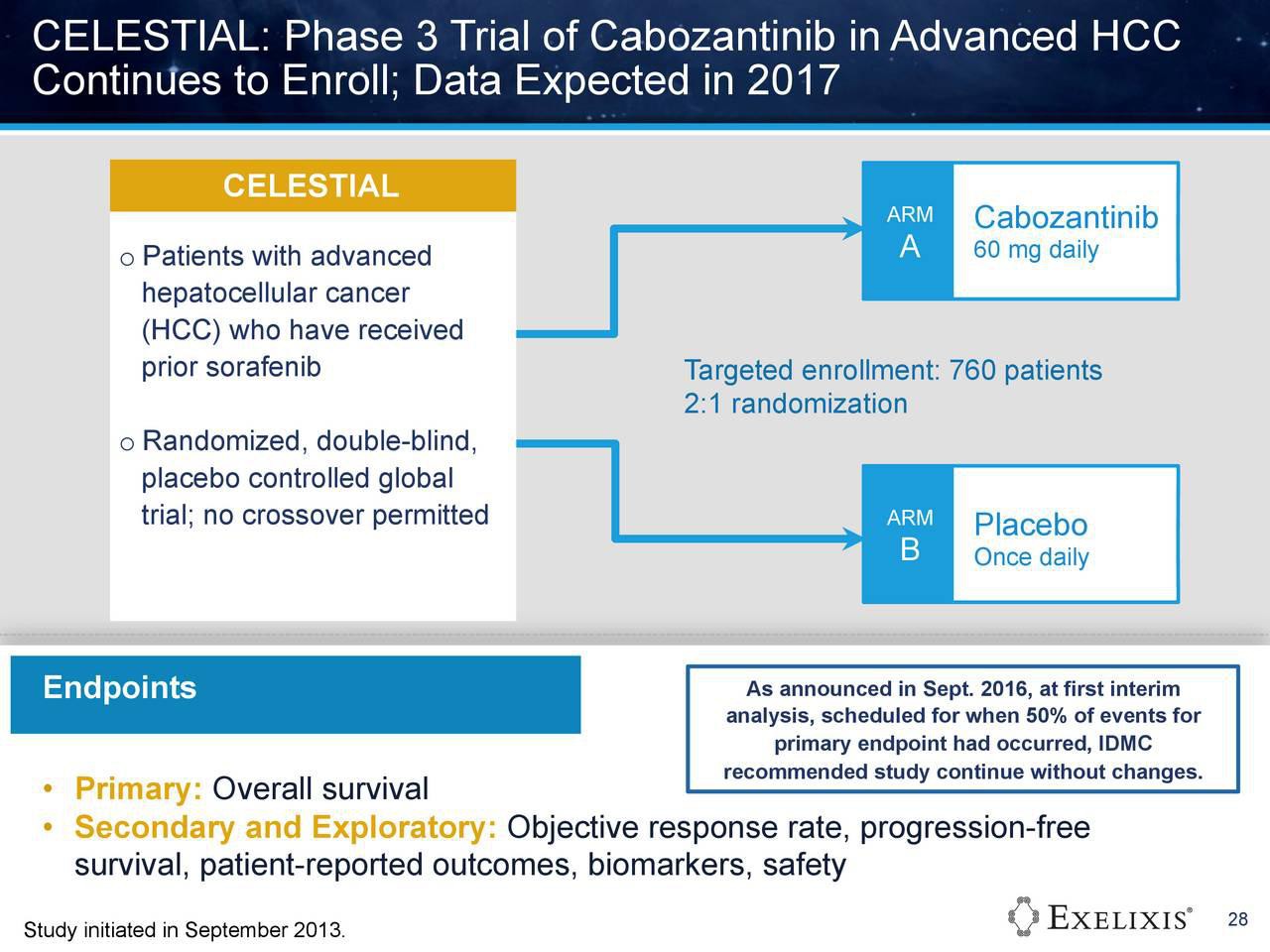

Antwort auf Beitrag Nr.: 54.425.578 von Cyberhexe am 28.02.17 01:47:59das Potenzial für zukünftige Studien ist überwältigend:
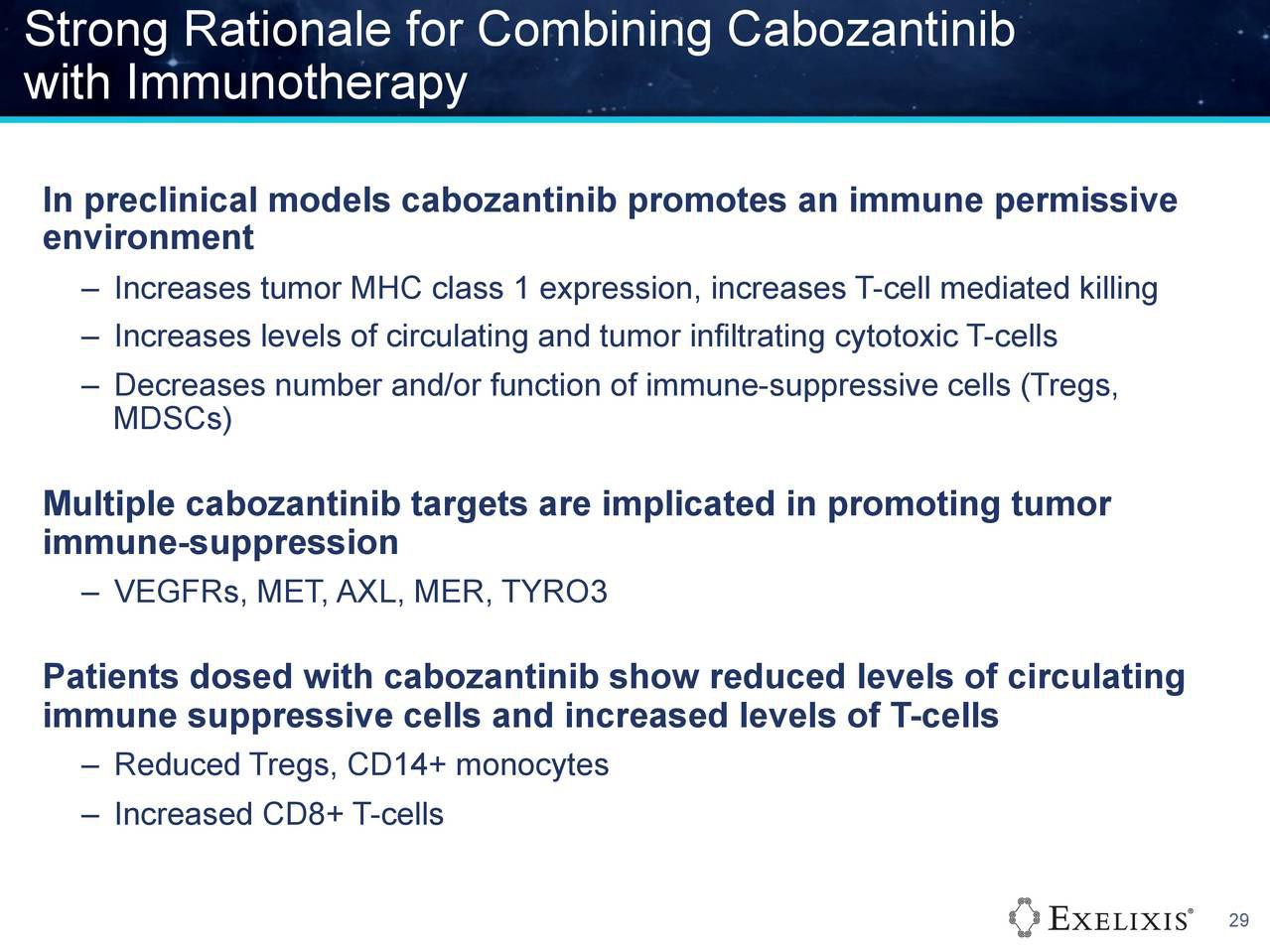

Antwort auf Beitrag Nr.: 54.425.581 von Cyberhexe am 28.02.17 01:50:13Cabozantinib eine Pipeline in sich:
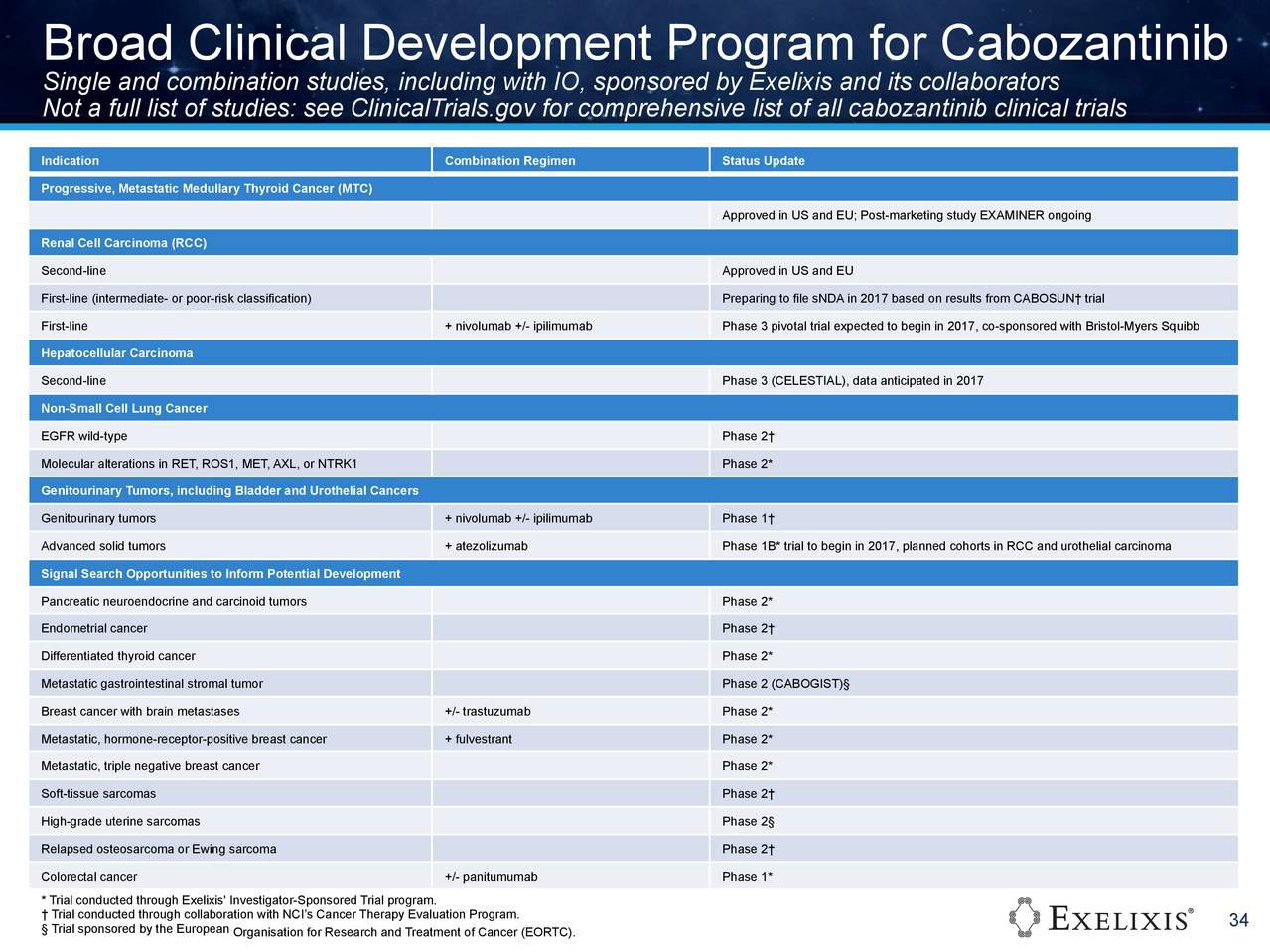

Cobimetinib...die 2. Pipeline:
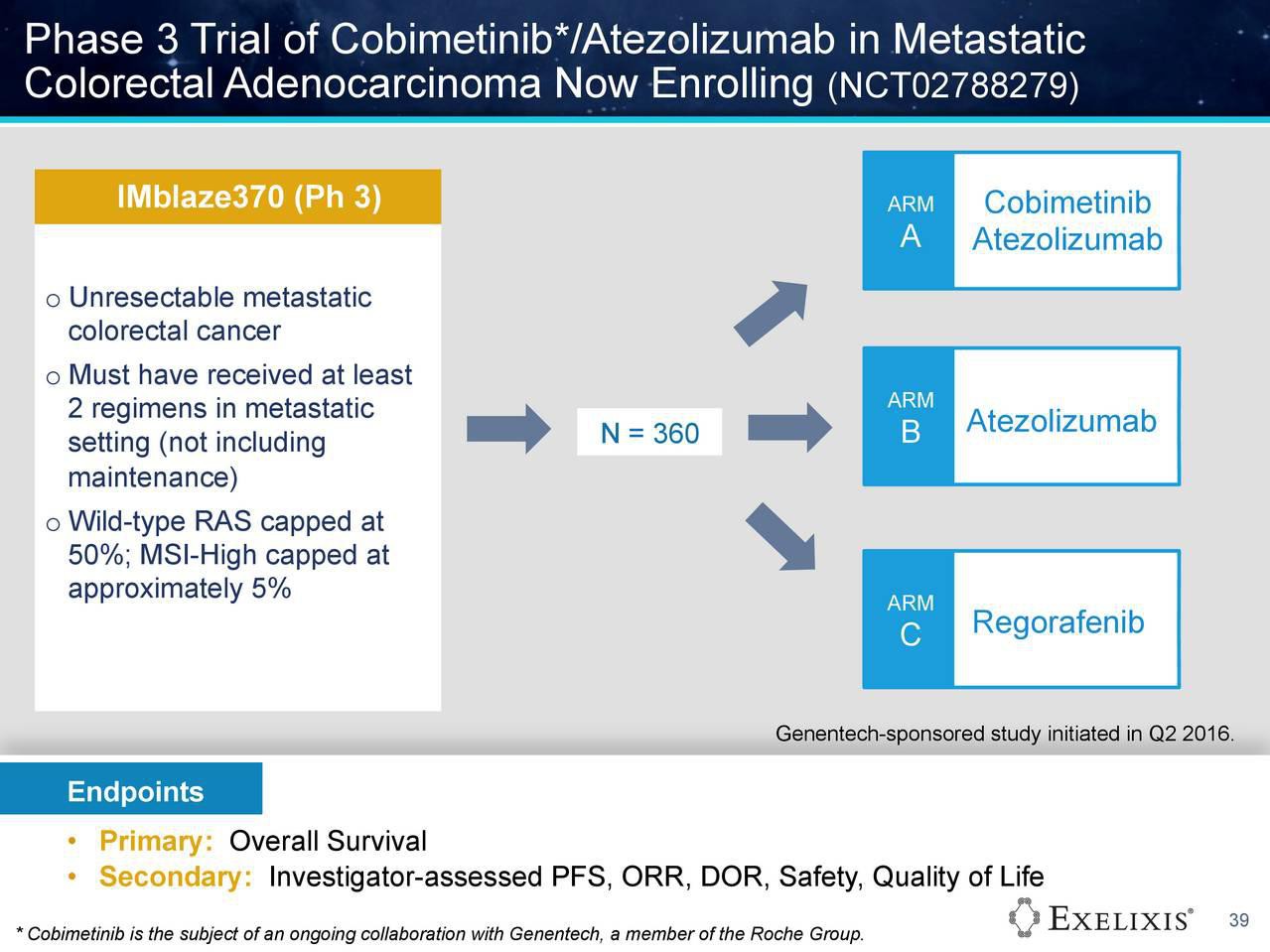


Antwort auf Beitrag Nr.: 54.425.587 von Cyberhexe am 28.02.17 01:52:42
Die "Cabo-Pipeline" ist sehr beeindruckend!
Zitat von Cyberhexe: Cabozantinib eine Pipeline in sich:
Die "Cabo-Pipeline" ist sehr beeindruckend!
Die FDA hat beim Leberkarzinom Cabo den Status für seltene Krankheiten zuerkannt.
http://www.exelixis.com/investors-media/press-releases?cpurl…
Exelixis' Cabozantinib Granted Orphan Drug Designation for the Treatment of Hepatocellular Carcinoma
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Mar. 6, 2017-- Exelixis, Inc. (Nasdaq: EXEL) today announced that the U.S. Food & Drug Administration (FDA) has granted orphan drug designation to cabozantinib for the treatment of hepatocellular carcinoma (HCC). This information was posted to FDA's website on March 4, 2017 and can be accessed at
http://www.accessdata.fda.gov/scripts/opdlisting/oopd/detail…
A pivotal phase 3 trial (CELESTIAL) of cabozantinib is ongoing in patients with advanced HCC, and Exelixis has guided that data from the trial are expected in 2017.
Orphan drug status is granted to treatments for diseases that affect fewer than 200,000 people in the U.S. and provides certain incentives for medications intended for the treatment, diagnosis or prevention of rare diseases. At present, these incentives include seven years of marketing exclusivity for the orphan indication, certain federal grants, tax credits and waiver of certain FDA fees.
About the CELESTIAL Trial
http://www.exelixis.com/investors-media/press-releases?cpurl…
Exelixis' Cabozantinib Granted Orphan Drug Designation for the Treatment of Hepatocellular Carcinoma
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Mar. 6, 2017-- Exelixis, Inc. (Nasdaq: EXEL) today announced that the U.S. Food & Drug Administration (FDA) has granted orphan drug designation to cabozantinib for the treatment of hepatocellular carcinoma (HCC). This information was posted to FDA's website on March 4, 2017 and can be accessed at
http://www.accessdata.fda.gov/scripts/opdlisting/oopd/detail…
A pivotal phase 3 trial (CELESTIAL) of cabozantinib is ongoing in patients with advanced HCC, and Exelixis has guided that data from the trial are expected in 2017.
Orphan drug status is granted to treatments for diseases that affect fewer than 200,000 people in the U.S. and provides certain incentives for medications intended for the treatment, diagnosis or prevention of rare diseases. At present, these incentives include seven years of marketing exclusivity for the orphan indication, certain federal grants, tax credits and waiver of certain FDA fees.
About the CELESTIAL Trial
erstaunlich auch die Wirkung von Cabo bei neuroendokrinen Tumoren - hab dennoch heute 1k EXEL verkauft.
Cabozantinib Elicits Responses in Neuroendocrine and Carcinoid Tumors
Median progression-free survival was 21.8 months (95% confidence interval [CI] = 8.5–32.0) in the pancreatic neuroendocrine tumor group and 31.4 months (95% CI = 8.5 to not reached) in the carcinoid group. “Progression-free survival appears very encouraging, in the context of historical results. Other drugs (sunitinib [Sutent], pazopanib [Votrient], everolimus, bevacizumab [Avastin]) have yielded median progression-free survival times of 8 to 16 months,” Dr. Chan noted.
http://www.ascopost.com/issues/february-25-2017/cabozantinib…
Cabozantinib Elicits Responses in Neuroendocrine and Carcinoid Tumors
Median progression-free survival was 21.8 months (95% confidence interval [CI] = 8.5–32.0) in the pancreatic neuroendocrine tumor group and 31.4 months (95% CI = 8.5 to not reached) in the carcinoid group. “Progression-free survival appears very encouraging, in the context of historical results. Other drugs (sunitinib [Sutent], pazopanib [Votrient], everolimus, bevacizumab [Avastin]) have yielded median progression-free survival times of 8 to 16 months,” Dr. Chan noted.
http://www.ascopost.com/issues/february-25-2017/cabozantinib…
EXELIXIS bedient langfristige Darlehen und reduziert dadurch den Schuldenstand sowie die hohen Kapitalkosten hierfür:
http://www.exelixis.com/investors-media/press-releases?cpurl…
http://www.exelixis.com/investors-media/press-releases?cpurl…
Für Leberkrebs wurde von der FDA der Status "Seltene Krankheiten" zuerkannt, wodurch verschiedene Erleichterungen bzw. Vergünstigungen beim Zulassungsprozess sowie eine befristete Exklusivität bei der Vermarktung und Steuererleichterungen möglich sind.
Die Ergebnisse von CELESTIAL (ph3/HCC) sind wohl das bedeutendste Ereignis für EXEL in 2017.
http://www.exelixis.com/investors-media/press-releases
Exelixis' Cabozantinib Granted Orphan Drug Designation for the Treatment of Hepatocellular Carcinoma
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Mar. 6, 2017-- Exelixis, Inc. (Nasdaq: EXEL) today announced that the U.S. Food & Drug Administration (FDA) has granted orphan drug designation to cabozantinib for the treatment of hepatocellular carcinoma (HCC). This information was posted to FDA's website on March 4, 2017 and can be accessed at
http://www.accessdata.fda.gov/scripts/opdlisting/oopd/detail…
A pivotal phase 3 trial (CELESTIAL) of cabozantinib is ongoing in patients with advanced HCC, and Exelixis has guided that data from the trial are expected in 2017.
Orphan drug status is granted to treatments for diseases that affect fewer than 200,000 people in the U.S. and provides certain incentives for medications intended for the treatment, diagnosis or prevention of rare diseases. At present, these incentives include seven years of marketing exclusivity for the orphan indication, certain federal grants, tax credits and waiver of certain FDA fees
Die Ergebnisse von CELESTIAL (ph3/HCC) sind wohl das bedeutendste Ereignis für EXEL in 2017.
http://www.exelixis.com/investors-media/press-releases
Exelixis' Cabozantinib Granted Orphan Drug Designation for the Treatment of Hepatocellular Carcinoma
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Mar. 6, 2017-- Exelixis, Inc. (Nasdaq: EXEL) today announced that the U.S. Food & Drug Administration (FDA) has granted orphan drug designation to cabozantinib for the treatment of hepatocellular carcinoma (HCC). This information was posted to FDA's website on March 4, 2017 and can be accessed at
http://www.accessdata.fda.gov/scripts/opdlisting/oopd/detail…
A pivotal phase 3 trial (CELESTIAL) of cabozantinib is ongoing in patients with advanced HCC, and Exelixis has guided that data from the trial are expected in 2017.
Orphan drug status is granted to treatments for diseases that affect fewer than 200,000 people in the U.S. and provides certain incentives for medications intended for the treatment, diagnosis or prevention of rare diseases. At present, these incentives include seven years of marketing exclusivity for the orphan indication, certain federal grants, tax credits and waiver of certain FDA fees
Antwort auf Beitrag Nr.: 54.640.181 von Cyberhexe am 30.03.17 06:12:58
Anbei die Ergebnisse der ph2-Studie von Cabo beim Leberkarzinom:
Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study
https://academic.oup.com/annonc/article/28/3/528/3058531/Cab…
Key Message
Cabozantinib has clinical activity in patients with hepatocellular carcinoma, with median PFS and OS of 5.2 and 11.5 months, respectively; 78% tumor regression rate, 5% objective response rate; and reductions in alpha-fetoprotein. Responses were independent of prior VEGFR-targeted therapy or ethnicity. The safety profile of cabozantinib was consistent with that observed in other tumor types.
Zitat von Cyberhexe: Für Leberkrebs wurde von der FDA der Status "Seltene Krankheiten" zuerkannt, wodurch verschiedene Erleichterungen bzw. Vergünstigungen beim Zulassungsprozess sowie eine befristete Exklusivität bei der Vermarktung und Steuererleichterungen möglich sind.
Die Ergebnisse von CELESTIAL (ph3/HCC) sind wohl das bedeutendste Ereignis für EXEL in 2017.
http://www.exelixis.com/investors-media/press-releases
Exelixis' Cabozantinib Granted Orphan Drug Designation for the Treatment of Hepatocellular Carcinoma
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Mar. 6, 2017-- Exelixis, Inc. (Nasdaq: EXEL) today announced that the U.S. Food & Drug Administration (FDA) has granted orphan drug designation to cabozantinib for the treatment of hepatocellular carcinoma (HCC). This information was posted to FDA's website on March 4, 2017 and can be accessed at
http://www.accessdata.fda.gov/scripts/opdlisting/oopd/detail…
A pivotal phase 3 trial (CELESTIAL) of cabozantinib is ongoing in patients with advanced HCC, and Exelixis has guided that data from the trial are expected in 2017.
Orphan drug status is granted to treatments for diseases that affect fewer than 200,000 people in the U.S. and provides certain incentives for medications intended for the treatment, diagnosis or prevention of rare diseases. At present, these incentives include seven years of marketing exclusivity for the orphan indication, certain federal grants, tax credits and waiver of certain FDA fees
Anbei die Ergebnisse der ph2-Studie von Cabo beim Leberkarzinom:
Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study
https://academic.oup.com/annonc/article/28/3/528/3058531/Cab…
Key Message
Cabozantinib has clinical activity in patients with hepatocellular carcinoma, with median PFS and OS of 5.2 and 11.5 months, respectively; 78% tumor regression rate, 5% objective response rate; and reductions in alpha-fetoprotein. Responses were independent of prior VEGFR-targeted therapy or ethnicity. The safety profile of cabozantinib was consistent with that observed in other tumor types.
interessante Podiumsdiskussion über Cabozantinib u.a. mit Toni K. Choueiri, MD, Dana-Farber Cancer Institute, einem der Protagonisten von CABOSUN:
"Overall Survival favoured Cabozantinib"
http://ht.ly/P4tr30b4tTr
"Overall Survival favoured Cabozantinib"
http://ht.ly/P4tr30b4tTr
Phase 2-Ergebnisse von Cabo bei Leberkrebs (HCC) veröffentlicht in einem Journal (Annals of Oncology):
Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study
Conclusions
Cabozantinib has clinical activity in HCC patients, including objective tumor responses, disease stabilization, and reductions in AFP. Adverse events were managed with dose reductions.
https://academic.oup.com/annonc/article-lookup/doi/10.1093/a…
Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study
Conclusions
Cabozantinib has clinical activity in HCC patients, including objective tumor responses, disease stabilization, and reductions in AFP. Adverse events were managed with dose reductions.
https://academic.oup.com/annonc/article-lookup/doi/10.1093/a…
Das Gesundheitsministerium der Briten empfiehlt, die Kosten von Cabo zur Behandlung bei Nierenkrebs in Zweitlinienbehandlung nicht zu erstatten :
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
Appraisal consultation document Cabozantinib for previously treated advanced
renal cell carcinoma
1.1 Cabozantinib is not recommended within its marketing authorisation for treating advanced renal cell carcinoma in adults after vascular endothelial growth factor (VEGF) targeted therapy.
1.2
This recommendation is not intended to affect treatment with cabozantinib that was started in the NHS before this guidance was published.
People having treatment outside this recommendation may continue without
change to the funding arrangements in place for them before this
guidance was published, until they and their NHS clinician consider it appropriate to stop.
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
Appraisal consultation document Cabozantinib for previously treated advanced
renal cell carcinoma
1.1 Cabozantinib is not recommended within its marketing authorisation for treating advanced renal cell carcinoma in adults after vascular endothelial growth factor (VEGF) targeted therapy.
1.2
This recommendation is not intended to affect treatment with cabozantinib that was started in the NHS before this guidance was published.
People having treatment outside this recommendation may continue without
change to the funding arrangements in place for them before this
guidance was published, until they and their NHS clinician consider it appropriate to stop.
Antwort auf Beitrag Nr.: 54.783.334 von Cyberhexe am 23.04.17 09:07:00
How Cabozantinib is Being Used in Practice for Patients With RCC
Daniel James George, MD
Published Online: 1:42 PM, Fri April 28, 201
http://www.targetedonc.com/conference/coa-2017/how-cabozanti…
".... a very large uptake on the use of it (Cabo)"
Bin gespannt auf die Umsätze für das 1q2017:
Exelixis to Release First Quarter 2017 Financial Results on Monday, May 1, 2017
- Conference Call and Webcast to Follow at 5:00 p.m. EDT/ 2:00 p.m. PDT -
How Cabozantinib is Being Used in Practice for Patients With RCC
Daniel James George, MD
Published Online: 1:42 PM, Fri April 28, 201
http://www.targetedonc.com/conference/coa-2017/how-cabozanti…
".... a very large uptake on the use of it (Cabo)"
Bin gespannt auf die Umsätze für das 1q2017:
Exelixis to Release First Quarter 2017 Financial Results on Monday, May 1, 2017
- Conference Call and Webcast to Follow at 5:00 p.m. EDT/ 2:00 p.m. PDT -
Antwort auf Beitrag Nr.: 54.836.215 von Cyberhexe am 30.04.17 09:09:43beeindruckendes Quartalsergebnis:
http://www.exelixis.com/investors-media/press-releases?cpurl…
http://www.exelixis.com/investors-media/press-releases?cpurl…
interessanter Artikel über Nierenkrebs aus dem World Journal of Clinical Oncology:
http://www.wjgnet.com/2218-4333/full/v8/i2/100.htm
The therapeutic options for patients with metastatic renal cell carcinoma (mRCC) have completely changed during the last ten years. With the sequential use of targeted therapies, median overall survival has increased in daily practice and now it is not uncommon to see patients surviving kidney cancer for more than four to five years. Once treatment fails with the first line targeted therapy, head to head comparisons have shown that cabozantinib, nivolumab and the combination of lenvatinib plus everolimus are more effective than everolimus alone and that axitinib is more active than sorafenib. Unfortunately, it is very unlikely that we will ever have prospective data comparing the activity of axitinib, cabozantinib, lenvatinib or nivolumab. It is frustrating to observe the lack of biomarkers that we have in this field, thus there is no firm recommendation about the optimal sequence of treatment in the second line. In the absence of reliable biomarkers, there are several clinical endpoints that can help physicians to make decisions for an individual patient, such as the tumor burden, the expected response rate and the time to achieve the response to each agent, the prior response to the agent administered, the toxicity profile of the different compounds and patient preference. Here, we propose the introduction of the tumor-growth rate (TGR) during first-line treatment as a new tool to be used to select the second line strategy in mRCC. The rapidness of TGR before the onset of the treatment reflects the variability between patients in terms of tumor growth kinetics and it could be a surrogate marker of tumor aggressiveness that may guide treatment decisions.
http://www.wjgnet.com/2218-4333/full/v8/i2/100.htm
The therapeutic options for patients with metastatic renal cell carcinoma (mRCC) have completely changed during the last ten years. With the sequential use of targeted therapies, median overall survival has increased in daily practice and now it is not uncommon to see patients surviving kidney cancer for more than four to five years. Once treatment fails with the first line targeted therapy, head to head comparisons have shown that cabozantinib, nivolumab and the combination of lenvatinib plus everolimus are more effective than everolimus alone and that axitinib is more active than sorafenib. Unfortunately, it is very unlikely that we will ever have prospective data comparing the activity of axitinib, cabozantinib, lenvatinib or nivolumab. It is frustrating to observe the lack of biomarkers that we have in this field, thus there is no firm recommendation about the optimal sequence of treatment in the second line. In the absence of reliable biomarkers, there are several clinical endpoints that can help physicians to make decisions for an individual patient, such as the tumor burden, the expected response rate and the time to achieve the response to each agent, the prior response to the agent administered, the toxicity profile of the different compounds and patient preference. Here, we propose the introduction of the tumor-growth rate (TGR) during first-line treatment as a new tool to be used to select the second line strategy in mRCC. The rapidness of TGR before the onset of the treatment reflects the variability between patients in terms of tumor growth kinetics and it could be a surrogate marker of tumor aggressiveness that may guide treatment decisions.
Antwort auf Beitrag Nr.: 54.843.859 von Cyberhexe am 02.05.17 09:02:33Bin in der Woche nach Pfingsten in SSF (South San Francisco) bei Exelixis. Bin mal gespannt, ob sich die Rechtsabteilung des Unternehmens für solche Verleumdungen interessiert. Wahrscheinlich nicht, aber ich werde dennoch mal nachfragen:
Zitiert aus dem Thread "Exelixis...ein schlafender Riese? USD 3.40 am 14.5.2014
Am 11.11.15 schrieb der Teilnehmer ville7 um 09:45:33 im Beitrag Nr. 399 (51.059.685) als Antwort auf Beitrag Nr. 398 (Nr. 51.058.935) von Franca_ole am11.11.15 um 09:00:03 die folgenden Sätze" "Im Übrigen stehe ich der Firma weiterhin sehr kritisch gegenüber, da sie schon oft gezeigt hat, dass sie mit ihren Finanzierungsrunden und -arten und eigenen Optionszuteilungen über die Leichen der Aktionäre geht ohne eine Wimper zu zucken. Never fall in love mit einem Produkt (Cobi) oder einer Firma (EXEL). Schaut so aus als hättest du noch das ein oder andere zu lernen"."
Zitiert aus dem Thread "Exelixis...ein schlafender Riese? USD 3.40 am 14.5.2014
Am 11.11.15 schrieb der Teilnehmer ville7 um 09:45:33 im Beitrag Nr. 399 (51.059.685) als Antwort auf Beitrag Nr. 398 (Nr. 51.058.935) von Franca_ole am11.11.15 um 09:00:03 die folgenden Sätze" "Im Übrigen stehe ich der Firma weiterhin sehr kritisch gegenüber, da sie schon oft gezeigt hat, dass sie mit ihren Finanzierungsrunden und -arten und eigenen Optionszuteilungen über die Leichen der Aktionäre geht ohne eine Wimper zu zucken. Never fall in love mit einem Produkt (Cobi) oder einer Firma (EXEL). Schaut so aus als hättest du noch das ein oder andere zu lernen"."
Antwort auf Beitrag Nr.: 54.881.533 von Cyberhexe am 06.05.17 18:05:22Ich hab jetzt richtig Angst!...


Ich bin übrigens nächste Woche bei der Bafin und werde mal fragen bis wohin Pushen und Frontrunning in Messageboards erlaubt ist...
Ehrlich, das von dir nun erreichte Niveau ist mir inzwischen zu far zu tief.
Aber du wirst mich sicher noch überraschen, dass du noch tiefer kannst!



Ich bin übrigens nächste Woche bei der Bafin und werde mal fragen bis wohin Pushen und Frontrunning in Messageboards erlaubt ist...

Ehrlich, das von dir nun erreichte Niveau ist mir inzwischen zu far zu tief.
Aber du wirst mich sicher noch überraschen, dass du noch tiefer kannst!
Antwort auf Beitrag Nr.: 54.881.860 von Ville7 am 06.05.17 19:21:22
Das hätte ich auch, wenn ich ein seriöses Unternehmen, dazu noch ein US amerikanisches, derart diffamiert hätte. Deswegen würde ich raten, erst Hirn einschalten und dann schreiben - nicht umgekehrt!
Zitat von Ville7: Ich hab jetzt richtig Angst!...
Ich bin übrigens nächste Woche bei der Bafin und werde mal fragen bis wohin Pushen und Frontrunning in Messageboards erlaubt ist...
Ehrlich, das von dir nun erreichte Niveau ist mir inzwischen zu far zu tief.
Aber du wirst mich sicher noch überraschen, dass du noch tiefer kannst!
Das hätte ich auch, wenn ich ein seriöses Unternehmen, dazu noch ein US amerikanisches, derart diffamiert hätte. Deswegen würde ich raten, erst Hirn einschalten und dann schreiben - nicht umgekehrt!
Antwort auf Beitrag Nr.: 54.883.597 von Cyberhexe am 07.05.17 09:27:28...gute Ergebnisse von Cabozantinib bei Minderjährigen mit Nierenkrebs:
“In both patients, disease control was prompt and durable, allowing for a return to normal activities of daily living, and the treatment was well tolerated,” the authors wrote in Pediatric Blood & Cancer.1"
Cabozantinib Treatment in Children With Recurrent Metastatic RCC
http://www.cancertherapyadvisor.com/renal-cell-carcinoma/cab…
“In both patients, disease control was prompt and durable, allowing for a return to normal activities of daily living, and the treatment was well tolerated,” the authors wrote in Pediatric Blood & Cancer.1"
Cabozantinib Treatment in Children With Recurrent Metastatic RCC
http://www.cancertherapyadvisor.com/renal-cell-carcinoma/cab…
Antwort auf Beitrag Nr.: 54.895.235 von Cyberhexe am 08.05.17 22:13:01Cabozantinib, Crizotinib, Volitinib, or
Sunitinib in Treating Patients With Locally
Advanced or Metastatic Papillary Kidney
Cancer (S1500)
Phase 2; 180 patients
Last updated: March 07, 2017
STATUS: Recruiting (accepting new patients) (per ClinicalTrials.gov/NCT02761057)
http://www.10forio.info/clinical-trials/s1500-cabozantinib-c…
Sunitinib in Treating Patients With Locally
Advanced or Metastatic Papillary Kidney
Cancer (S1500)
Phase 2; 180 patients
Last updated: March 07, 2017
STATUS: Recruiting (accepting new patients) (per ClinicalTrials.gov/NCT02761057)
http://www.10forio.info/clinical-trials/s1500-cabozantinib-c…
Antwort auf Beitrag Nr.: 54.906.233 von Cyberhexe am 10.05.17 07:34:40Laurence Albiges, MD, PhD, from the Gustave Roussy Institute, Villejuif, France: "Die Nebenwirkungen von Cabo sind beherrschbar, üblicherweise (oft) erfolgt eine Reduzierung der Dosis."
Managing side effects of cabozantinib treatment for metastatic renal cell carcinoma:
http://www.oncologytube.com/video/managing-side-effects-of-c…
Description: Laurence Albiges, MD, PhD, from the Gustave Roussy Institute, Villejuif, France discusses the safety of cabozantinib treatment in metastatic renal cell carcinoma at the European Cancer Congress of the European Cancer Organisation (ECCO) 2017 in Amsterdam, Netherlands. Cabozantinib is a potent VEGFR tyrosine kinase inhibitor (TKI), and is used for second-line or later treatment in patients in whom prior VEGFR inhibitor treatment failed. Dr Albiges describes common side effects of cabozantinib treatment, which can include hypertension, hand-foot syndrome (acral erythema), diarrhea, and fatigue. Management of these will often require a dose reduction, with 2 out of 3 patients in the Phase III METEOR trial of cabozantinib needing a dose reduction (NCT01865747). However, she argues that the positive effect of cabozantinib on overall survival (OS) and progression-free survival (PFS) speak for its use as a second-line treatment in metastatic renal cell carcinoma.
Managing side effects of cabozantinib treatment for metastatic renal cell carcinoma:
http://www.oncologytube.com/video/managing-side-effects-of-c…
Description: Laurence Albiges, MD, PhD, from the Gustave Roussy Institute, Villejuif, France discusses the safety of cabozantinib treatment in metastatic renal cell carcinoma at the European Cancer Congress of the European Cancer Organisation (ECCO) 2017 in Amsterdam, Netherlands. Cabozantinib is a potent VEGFR tyrosine kinase inhibitor (TKI), and is used for second-line or later treatment in patients in whom prior VEGFR inhibitor treatment failed. Dr Albiges describes common side effects of cabozantinib treatment, which can include hypertension, hand-foot syndrome (acral erythema), diarrhea, and fatigue. Management of these will often require a dose reduction, with 2 out of 3 patients in the Phase III METEOR trial of cabozantinib needing a dose reduction (NCT01865747). However, she argues that the positive effect of cabozantinib on overall survival (OS) and progression-free survival (PFS) speak for its use as a second-line treatment in metastatic renal cell carcinoma.
Antwort auf Beitrag Nr.: 54.906.293 von Cyberhexe am 10.05.17 07:43:59MAY 9, 2017
Is Cabozantinib a Standard of Care for Advanced RCC?
“There is a great deal of enthusiasm surrounding checkpoint inhibitors,” Dr. Sternberg told Clinical Oncology News. “However, cabozantinib is the only agent to demonstrate efficacy across three key end points—overall survival, progression-free survival and overall response rate.”
http://www.clinicaloncology.com/Kidney-Cancer/Article/05-17/…
Is Cabozantinib a Standard of Care for Advanced RCC?
“There is a great deal of enthusiasm surrounding checkpoint inhibitors,” Dr. Sternberg told Clinical Oncology News. “However, cabozantinib is the only agent to demonstrate efficacy across three key end points—overall survival, progression-free survival and overall response rate.”
http://www.clinicaloncology.com/Kidney-Cancer/Article/05-17/…
Antwort auf Beitrag Nr.: 54.915.710 von Cyberhexe am 11.05.17 06:35:29Neue Studie bei RCC mit Cabo und Pembrolizumab, finanziert durch
University of Colorado, Denver
Collaborator: Merck Sharp & Dohme Corp.
Information provided by (Responsible Party):
University of Colorado, Denver
https://clinicaltrials.gov/ct2/show/NCT03149822?term=cabozan…
Pembrolizumab bzw. KEYTRUDA ist derzeit wohl der vielversprechendste Checkpoint-Hemmer überhaupt.
Study of Pembrolizumab and Cabozantinib in Patients With Metastatic Renal Cell Carcinoma
Overview
This study is designed to evaluate the combination of pembrolizumab and cabozantinib in subjects with locally advanced, recurrent, or metastatic renal cell carcinoma that has progressed after treatment with at least one prior VEGF-targeted therapy, or for whom VEGF-targeted therapy has proven to be intolerable, or is considered inappropriate
Full Title of Study: “Phase I/II Study of Pembrolizumab and Cabozantinib in Patients With Metastatic Renal Cell Carcinoma”
Study Type
Study Type: Interventional
Study Design
Allocation: Non-Randomized
Intervention Model: Single Group Assignment
Primary Purpose: Treatment
Masking: No masking
Study Primary Completion Date: June 2020
https://trialbulletin.com/lib/entry/ct-03149822
University of Colorado, Denver
Collaborator: Merck Sharp & Dohme Corp.
Information provided by (Responsible Party):
University of Colorado, Denver
https://clinicaltrials.gov/ct2/show/NCT03149822?term=cabozan…
Pembrolizumab bzw. KEYTRUDA ist derzeit wohl der vielversprechendste Checkpoint-Hemmer überhaupt.
Study of Pembrolizumab and Cabozantinib in Patients With Metastatic Renal Cell Carcinoma
Overview
This study is designed to evaluate the combination of pembrolizumab and cabozantinib in subjects with locally advanced, recurrent, or metastatic renal cell carcinoma that has progressed after treatment with at least one prior VEGF-targeted therapy, or for whom VEGF-targeted therapy has proven to be intolerable, or is considered inappropriate
Full Title of Study: “Phase I/II Study of Pembrolizumab and Cabozantinib in Patients With Metastatic Renal Cell Carcinoma”
Study Type
Study Type: Interventional
Study Design
Allocation: Non-Randomized
Intervention Model: Single Group Assignment
Primary Purpose: Treatment
Masking: No masking
Study Primary Completion Date: June 2020
https://trialbulletin.com/lib/entry/ct-03149822
Antwort auf Beitrag Nr.: 54.906.233 von Cyberhexe am 10.05.17 07:34:40
Bull Cancer. 2017 May 3. pii: S0007-4551(17)30099-1. doi: 10.1016/j.bulcan.2017.03.013.
Cabozantinib: Mechanism of action, efficacy and indications
Cochin V1, Gross-Goupil M2, Ravaud A3, Godbert Y4, Le Moulec S4.
Author information
Abstract
Cabozantinib is an oral multiple tyrosine kinase receptor inhibitor (ITK): VEGFR2, c-MET and RET. Inhibition of VEGFR and c-MET decrease resistance of VEGFR inhibitor via c-MET axis. Cabozantinib improve progression-free survival (PFS) in progressive metastatic medullary thyroid cancer (MTC): 4 months in the placebo group and 11.2 months in the cabozantinib group (P<0.001) in all patient subgroups including those with or without prior ITK and RET mutation status. Cabozantinib increased overall survival (OS) compared with everolimus in patients with advanced renal cell carcinoma who progressed after previous VEGFR ITK treatment: 21.4 months in cabozantinib group and 16.5 months in everolimus group (P<0.0003). Cabozantinib obtained the AMM for the treatment of progressive metastatic MTC and advanced renal cell carcinoma. Cabozantinib is a new option in the treatment of MTC by inclusion in therapeutic trials (no payment in this indication) and advanced renal cell carcinoma (hospital delivery). Its tolerance is similar to anti-angiogenic therapies and justifies an optimal management of the secondary effect.
Bull Cancer. 2017 May 3. pii: S0007-4551(17)30099-1. doi: 10.1016/j.bulcan.2017.03.013.
Cabozantinib: Mechanism of action, efficacy and indications
Cochin V1, Gross-Goupil M2, Ravaud A3, Godbert Y4, Le Moulec S4.
Author information
Abstract
Cabozantinib is an oral multiple tyrosine kinase receptor inhibitor (ITK): VEGFR2, c-MET and RET. Inhibition of VEGFR and c-MET decrease resistance of VEGFR inhibitor via c-MET axis. Cabozantinib improve progression-free survival (PFS) in progressive metastatic medullary thyroid cancer (MTC): 4 months in the placebo group and 11.2 months in the cabozantinib group (P<0.001) in all patient subgroups including those with or without prior ITK and RET mutation status. Cabozantinib increased overall survival (OS) compared with everolimus in patients with advanced renal cell carcinoma who progressed after previous VEGFR ITK treatment: 21.4 months in cabozantinib group and 16.5 months in everolimus group (P<0.0003). Cabozantinib obtained the AMM for the treatment of progressive metastatic MTC and advanced renal cell carcinoma. Cabozantinib is a new option in the treatment of MTC by inclusion in therapeutic trials (no payment in this indication) and advanced renal cell carcinoma (hospital delivery). Its tolerance is similar to anti-angiogenic therapies and justifies an optimal management of the secondary effect.
Antwort auf Beitrag Nr.: 54.945.122 von Cyberhexe am 15.05.17 12:03:32Heute werden zu ASCO die Abstracts veröffentlicht. Exelixis ist selbstverständlich auch "am Start", und zwar mit Cabo unter anderem auch wieder beim Prostata Carcinom (da bin ich heute noch der Meinung, dass, vor allem bei den Knochenmetastasen, bei vorteilhaftem Studiendesign ein signifikanter Nutzen sichtbar wäre).
POSTER SESSION Tumor Biology
Jun 03
1:15 PM - 4:45 PM
Poster Board: #283 • Abstract 11583
Role of ERBB signaling in RET-rearranged lung cancer and contribution of EGFR amplification to cabozantinib resistance.
Roger Stephen Smith, BSc - First Author
Northwestern University Feinberg School of Medicine
POSTER SESSION Gynecologic Cancer
Jun 03
1:15 PM - 4:45 PM
Poster Board: #346 • Abstract 5524
Phase II study of cabozantinib (cabo) in patients (pts) with recurrent/metastatic endometrial cancer (EC): A study of the Princess Margaret, Chicago, and California phase II consortia.
Neesha C. Dhani, MD, FRCPC - First Author
Princess Margaret Cancer Centre, University Health Network
Discussed at the Poster Discussion Session on Saturday, June 3, 2017, 4:45 PM - 6:00 PM, at Arie Crown Theater
Poster Board: #409 • Abstract 5587
Exploratory phase II evaluation of cabozantinib in recurrent/metastatic uterine carcinosarcoma (CS): A study of the Princess Margaret, Chicago, and California phase II consortia.
Victoria Mandilaras, MD - First Author
McGill University Health Centre
POSTER SESSION Genitourinary (Nonprostate) Cancer
Jun 04
8:00 AM - 11:30 AM
Poster Board: #240 • Abstract 4562
A phase I study of cabozantinib plus nivolumab (CaboNivo) and cabonivo plus ipilimumab (CaboNivoIpi) in patients (pts) with refractory metastatic (m) urothelial carcinoma (UC) and other genitourinary (GU) tumors.
Andrea B. Apolo, M.D. - First Author
Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health
Poster Board: #248 • Abstract 4570
Clinical outcomes by nephrectomy status in METEOR, a randomized phase 3 trial of cabozantinib (cabo) vs everolimus (eve) in patients (pts) with advanced renal cell carcinoma (RCC).
Nizar M. Tannir, MD, FACP - First Author
The University of Texas MD Anderson Cancer Center
Jun 05
Poster Board: #105 • Abstract 5031
Circulating tumor cell subsets and macrophage polarization to predict efficacy of cabozantinib in advanced prostate cancer with visceral metastases.
Edwin M. Posadas, MD FACP - First Author
Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center
Bei Cobi sind vor allem die Ergebnisse mit Atezolizumab von Bedeutung:
Poster Board: #152 • Abstract 3057
Atezolizumab (A) + cobimetinib (C) in metastatic melanoma (mel): Updated safety and clinical activity.
Wilson H. Miller, MD, PhD - First Author
Segal Cancer Centre, Jewish General Hospital, McGill University
Poster Board: #158 • Abstract 3063
Atezolizumab (A) + cobimetinib (C) + vemurafenib (V) in BRAFV600-mutant metastatic melanoma (mel): Updated safety and clinical activity.
Ryan J. Sullivan, M.D. - First Author
Massachusetts General Hospital Cancer Center
POSTER SESSION Tumor Biology
Jun 03
1:15 PM - 4:45 PM
Poster Board: #283 • Abstract 11583
Role of ERBB signaling in RET-rearranged lung cancer and contribution of EGFR amplification to cabozantinib resistance.
Roger Stephen Smith, BSc - First Author
Northwestern University Feinberg School of Medicine
POSTER SESSION Gynecologic Cancer
Jun 03
1:15 PM - 4:45 PM
Poster Board: #346 • Abstract 5524
Phase II study of cabozantinib (cabo) in patients (pts) with recurrent/metastatic endometrial cancer (EC): A study of the Princess Margaret, Chicago, and California phase II consortia.
Neesha C. Dhani, MD, FRCPC - First Author
Princess Margaret Cancer Centre, University Health Network
Discussed at the Poster Discussion Session on Saturday, June 3, 2017, 4:45 PM - 6:00 PM, at Arie Crown Theater
Poster Board: #409 • Abstract 5587
Exploratory phase II evaluation of cabozantinib in recurrent/metastatic uterine carcinosarcoma (CS): A study of the Princess Margaret, Chicago, and California phase II consortia.
Victoria Mandilaras, MD - First Author
McGill University Health Centre
POSTER SESSION Genitourinary (Nonprostate) Cancer
Jun 04
8:00 AM - 11:30 AM
Poster Board: #240 • Abstract 4562
A phase I study of cabozantinib plus nivolumab (CaboNivo) and cabonivo plus ipilimumab (CaboNivoIpi) in patients (pts) with refractory metastatic (m) urothelial carcinoma (UC) and other genitourinary (GU) tumors.
Andrea B. Apolo, M.D. - First Author
Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health
Poster Board: #248 • Abstract 4570
Clinical outcomes by nephrectomy status in METEOR, a randomized phase 3 trial of cabozantinib (cabo) vs everolimus (eve) in patients (pts) with advanced renal cell carcinoma (RCC).
Nizar M. Tannir, MD, FACP - First Author
The University of Texas MD Anderson Cancer Center
Jun 05
Poster Board: #105 • Abstract 5031
Circulating tumor cell subsets and macrophage polarization to predict efficacy of cabozantinib in advanced prostate cancer with visceral metastases.
Edwin M. Posadas, MD FACP - First Author
Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center
Bei Cobi sind vor allem die Ergebnisse mit Atezolizumab von Bedeutung:
Poster Board: #152 • Abstract 3057
Atezolizumab (A) + cobimetinib (C) in metastatic melanoma (mel): Updated safety and clinical activity.
Wilson H. Miller, MD, PhD - First Author
Segal Cancer Centre, Jewish General Hospital, McGill University
Poster Board: #158 • Abstract 3063
Atezolizumab (A) + cobimetinib (C) + vemurafenib (V) in BRAFV600-mutant metastatic melanoma (mel): Updated safety and clinical activity.
Ryan J. Sullivan, M.D. - First Author
Massachusetts General Hospital Cancer Center
Antwort auf Beitrag Nr.: 54.959.246 von Cyberhexe am 17.05.17 06:13:00
A phase I study of cabozantinib plus nivolumab (CaboNivo) and ipilimumab (CaboNivoIpi) in patients (pts) with refractory metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors.
Meeting:
2017 Genitourinary Cancers Symposium
Category:
Genitourinary Cancer
Subcategory:
Urothelial Carcinoma
Session Type and Session Title:
Poster Session B: Prostate Cancer and Urothelial Carcinoma
Abstract Number:
293
Citation:
J Clin Oncol 35, 2017 (suppl 6S; abstract 293)
Author(s):
Andrea B. Apolo, Amir Mortazavi, Mark N. Stein, Sumanta K. Pal, Nicole N. Davarpanah, Howard L. Parnes, Yangmin M. Ning, Deneise C Francis, Lisa M Cordes, Paul Monk, Tiffany Lancaster, Rene Costello, Swati Nanda, Donald P. Bottaro, John Joseph Wright, Howard Streicher, Seth M. Steinberg, Marilise Berninger, Liza Lindenberg, William L. Dahut; Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD; Arthur G. James Cancer Hospital, The Ohio State University Wexner Medical Center, Columbus, OH; Rutgers Cancer Institute of New Jersey, New Brunswick, NJ; City of Hope, Duarte, CA; Division of Cancer Prevention, National Cancer Institute at the National Institutes of Health, Bethesda, MD; FDA, Silver Spring, MD; Genitourinary Malignancies Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD; National Institutes of Health, Bethesda, MD; The Ohio State University, Columbus, OH; Center for Cancer Research, National Cancer Institute, Bethesda, MD; Medical Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD; National Cancer Institute, Bethesda, MD; National Cancer Institute at the National Institutes of Health, Bethesda, MD; Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD; Biostatistics and Data Management Section, National Cancer Institute at the National Institutes of Health, Bethesda, MD; National Institutes of Health, Washington, DC
Background: We report the safety and clinical activity of CaboNivo and CaboNivoIpi in pts with mUC and other GU tumors. Methods: Part I included 4 dose levels (DLs) (Cabo PO daily and Nivo IV q2wk): DL1 Cabo40/Nivo1, DL2 Cabo40/Nivo3, DL3 Cabo60/Nivo1, DL4 Cabo 60/Nivo3. Part II included 3 DLs (Cabo PO daily, plus NivoIpi IV q3 wk x 4 doses then Nivo q2wk): DL5 Cabo 40/Nivo1/Ipi1, DL6 Cabo40/Nivo3/Ipi1, DL7 Cabo 60/Nivo3/Ipi 1. Tumors were assessed for overall response rate (ORR) by RECIST 1.1. Adverse events (AEs) were graded (G) by NCI-CTCAE v4.0. Results: From 7/22/15-10/14/16, 40pts [mUC N=14/(plasmacytoid N=1); bladder urachal N=4; bladder squamous cell carcinoma (bSCC) N=2; germ cell tumor (GCT) N=4; castrate-resistant prostate cancer (CRPC) N=9/(neuroendocrine prostate N=1); sarcomatoid renal cell carcinoma (sRCC) N=1; trophoblastic tumor N=1); sertoli cell tumor N=1; and penile SCC N=4] were treated. Median age was 58 (range 31-77); 36 (90%) were male. AEs related to study drugs with [1] CaboNivo included G3 hyponatremia 4/24 (17%), hypophosphatemia 4/24 (17%), lipase increase 3/24 (13%), dehydration 2/24 (8%), diarrhea 2/24 (8%), fatigue 2/24 (8%), HTN 2/24 (8%), thromboembolic event 1/24 (4%), rash 1/24 (4%), chest pain 1/24 (4%), amylase increase 1/24 (4%), hyperthyroid 1/24 (4%), proteinuria 1/24 (4%), thrombocytopenia 1/24 (4%); and G4 pyelonephritis 1/24 (4%); [2] CaboNivoIpi included G3 hypophosphatemia 2/16 (13%), lipase increase 2/16 (13%), fatigue 1/16 (6%), ALT increase 1/16 (6%), and HTN 1/16 (6%). There were 2/40 (5%) G3 immune-related AEs: 1 aseptic meningitis/CaboNivo; and 1 colitis/CaboNivoIpi. There were no G5 toxicities, or DLTs. 38 pts were evaluable for response. ORR was 12/38 (32%): 1 CR (bSCC); 11 PRs (5 mUC, 1 sRCC, 1 urachal, 1 bSCC, 1 CRPC, 2 penile). SD 20/38 (53%); 9/11 responses were ongoing and 26/39 (67%) pts remain on study. Conclusions: CaboNivo and CaboNivoIpi were well tolerated with no DLTs. Responses were seen at all DLs. The recommended dose for Part I is Cabo40/Nivo3 and for Part II is Cabo40/Nivo3/Ipi1. Rare tumors such as bSCC, urachal, and penile cancer demonstrated responses. Clinical trial information: NCT02496208
A phase I study of cabozantinib plus nivolumab (CaboNivo) and ipilimumab (CaboNivoIpi) in patients (pts) with refractory metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors.
Meeting:
2017 Genitourinary Cancers Symposium
Category:
Genitourinary Cancer
Subcategory:
Urothelial Carcinoma
Session Type and Session Title:
Poster Session B: Prostate Cancer and Urothelial Carcinoma
Abstract Number:
293
Citation:
J Clin Oncol 35, 2017 (suppl 6S; abstract 293)
Author(s):
Andrea B. Apolo, Amir Mortazavi, Mark N. Stein, Sumanta K. Pal, Nicole N. Davarpanah, Howard L. Parnes, Yangmin M. Ning, Deneise C Francis, Lisa M Cordes, Paul Monk, Tiffany Lancaster, Rene Costello, Swati Nanda, Donald P. Bottaro, John Joseph Wright, Howard Streicher, Seth M. Steinberg, Marilise Berninger, Liza Lindenberg, William L. Dahut; Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD; Arthur G. James Cancer Hospital, The Ohio State University Wexner Medical Center, Columbus, OH; Rutgers Cancer Institute of New Jersey, New Brunswick, NJ; City of Hope, Duarte, CA; Division of Cancer Prevention, National Cancer Institute at the National Institutes of Health, Bethesda, MD; FDA, Silver Spring, MD; Genitourinary Malignancies Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD; National Institutes of Health, Bethesda, MD; The Ohio State University, Columbus, OH; Center for Cancer Research, National Cancer Institute, Bethesda, MD; Medical Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD; National Cancer Institute, Bethesda, MD; National Cancer Institute at the National Institutes of Health, Bethesda, MD; Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD; Biostatistics and Data Management Section, National Cancer Institute at the National Institutes of Health, Bethesda, MD; National Institutes of Health, Washington, DC
Background: We report the safety and clinical activity of CaboNivo and CaboNivoIpi in pts with mUC and other GU tumors. Methods: Part I included 4 dose levels (DLs) (Cabo PO daily and Nivo IV q2wk): DL1 Cabo40/Nivo1, DL2 Cabo40/Nivo3, DL3 Cabo60/Nivo1, DL4 Cabo 60/Nivo3. Part II included 3 DLs (Cabo PO daily, plus NivoIpi IV q3 wk x 4 doses then Nivo q2wk): DL5 Cabo 40/Nivo1/Ipi1, DL6 Cabo40/Nivo3/Ipi1, DL7 Cabo 60/Nivo3/Ipi 1. Tumors were assessed for overall response rate (ORR) by RECIST 1.1. Adverse events (AEs) were graded (G) by NCI-CTCAE v4.0. Results: From 7/22/15-10/14/16, 40pts [mUC N=14/(plasmacytoid N=1); bladder urachal N=4; bladder squamous cell carcinoma (bSCC) N=2; germ cell tumor (GCT) N=4; castrate-resistant prostate cancer (CRPC) N=9/(neuroendocrine prostate N=1); sarcomatoid renal cell carcinoma (sRCC) N=1; trophoblastic tumor N=1); sertoli cell tumor N=1; and penile SCC N=4] were treated. Median age was 58 (range 31-77); 36 (90%) were male. AEs related to study drugs with [1] CaboNivo included G3 hyponatremia 4/24 (17%), hypophosphatemia 4/24 (17%), lipase increase 3/24 (13%), dehydration 2/24 (8%), diarrhea 2/24 (8%), fatigue 2/24 (8%), HTN 2/24 (8%), thromboembolic event 1/24 (4%), rash 1/24 (4%), chest pain 1/24 (4%), amylase increase 1/24 (4%), hyperthyroid 1/24 (4%), proteinuria 1/24 (4%), thrombocytopenia 1/24 (4%); and G4 pyelonephritis 1/24 (4%); [2] CaboNivoIpi included G3 hypophosphatemia 2/16 (13%), lipase increase 2/16 (13%), fatigue 1/16 (6%), ALT increase 1/16 (6%), and HTN 1/16 (6%). There were 2/40 (5%) G3 immune-related AEs: 1 aseptic meningitis/CaboNivo; and 1 colitis/CaboNivoIpi. There were no G5 toxicities, or DLTs. 38 pts were evaluable for response. ORR was 12/38 (32%): 1 CR (bSCC); 11 PRs (5 mUC, 1 sRCC, 1 urachal, 1 bSCC, 1 CRPC, 2 penile). SD 20/38 (53%); 9/11 responses were ongoing and 26/39 (67%) pts remain on study. Conclusions: CaboNivo and CaboNivoIpi were well tolerated with no DLTs. Responses were seen at all DLs. The recommended dose for Part I is Cabo40/Nivo3 and for Part II is Cabo40/Nivo3/Ipi1. Rare tumors such as bSCC, urachal, and penile cancer demonstrated responses. Clinical trial information: NCT02496208
food for thought:
"In 2017, pazopanib and sunitinib remain the mainstays of frontline therapy for advanced renal cell carcinoma. Independent review of frontline cabozantinib therapy may alter standard of care for patients at intermediate and poor risk. Multiple agents show a survival advantage in the second-line setting, including nivolumab, cabozantinib, and combination lenvatinib and everolimus. Selection of subsequent therapy will depend on patient disease status, comorbidities, and resource availability."
http://www.jnccn.org/content/15/5S/703.long
"In 2017, pazopanib and sunitinib remain the mainstays of frontline therapy for advanced renal cell carcinoma. Independent review of frontline cabozantinib therapy may alter standard of care for patients at intermediate and poor risk. Multiple agents show a survival advantage in the second-line setting, including nivolumab, cabozantinib, and combination lenvatinib and everolimus. Selection of subsequent therapy will depend on patient disease status, comorbidities, and resource availability."
http://www.jnccn.org/content/15/5S/703.long
Antwort auf Beitrag Nr.: 54.881.533 von Cyberhexe am 06.05.17 18:05:22
company is not amused about such slanderous comments!
Zitat von Cyberhexe: Bin in der Woche nach Pfingsten in SSF (South San Francisco) bei Exelixis. Bin mal gespannt, ob sich die Rechtsabteilung des Unternehmens für solche Verleumdungen interessiert. Wahrscheinlich nicht, aber ich werde dennoch mal nachfragen:
Zitiert aus dem Thread "Exelixis...ein schlafender Riese? USD 3.40 am 14.5.2014
Am 11.11.15 schrieb der Teilnehmer ville7 um 09:45:33 im Beitrag Nr. 399 (51.059.685) als Antwort auf Beitrag Nr. 398 (Nr. 51.058.935) von Franca_ole am11.11.15 um 09:00:03 die folgenden Sätze" "Im Übrigen stehe ich der Firma weiterhin sehr kritisch gegenüber, da sie schon oft gezeigt hat, dass sie mit ihren Finanzierungsrunden und -arten und eigenen Optionszuteilungen über die Leichen der Aktionäre geht ohne eine Wimper zu zucken. Never fall in love mit einem Produkt (Cobi) oder einer Firma (EXEL). Schaut so aus als hättest du noch das ein oder andere zu lernen"."

company is not amused about such slanderous comments!
Antwort auf Beitrag Nr.: 55.115.488 von Cyberhexe am 10.06.17 02:52:30
Leerink - EXEL 6Jun2017: Incremental Cotellic Melanoma Updates at ASCO Bode Well for IMblaze370 Trial
• Bottom Line: EXEL's partner Roche provided incremental updates at ASCO from its MEK inhibitor Cotellic (cobimetinib) which is being studied in combination with Tecentriq in metastatic melanoma. In addition, we attended the RCC session at ASCO which featured incremental (and somewhat disappointing) updates to several Phase I/II combination trials from competitors which did not have any impact on our view of the Cabometyx opportunity in RCC. We remain positive on EXEL's outlook and stock and are maintaining our OP rating.
• Updated Phase Ib data from RHHBY's Cotellic/Tecentriq combination in metastatic melanoma indicate responses are very durable. Twenty-two patients with metastatic melanoma were evaluable for safety and efficacy, including 20 patients with non-ocular melanoma (10 each with BRAF-wild type and BRAF V600-mutation positive disease). Among the 20 non-ocular melanoma patients, the objective response rate (ORR) was 45%, with similar response rate seen both in BRAFwt (50% ORR) and BRAF+ pts (40% ORR). The overall response rate was similar to data presented last year at SMR. Of note, median PFS and median duration of response had not yet been reached across both groups, potentially highlighting the benefits of combining MEK and PD1 inhibitors based on an immunologic rationale. Median PFS is currently estimated at 15.7 months, similar in both patient groups with follow-up still ongoing (compared to historic Tecentriq monotherapy mPFS of only 5.5 months). With the main caveat being the small patient numbers, these responses seem very durable, exceeding what has been seen previously with MEK/BRAF inhibitors and PD1 inhibitors alone. Updated Phase Ib data of the triple combination of Cotellic with Zelboraf and Tecentriq in BRAF+ melanoma also indicate responses (81.6%, 18% CRs) are still ongoing with mDOR and mPFS (12.9 months+) not yet reached.
• Pivotal trials evaluating the combination of Cotellic and Tecentriq are ongoing in several indications with data possible in early 2018 based on our estimates. The Phase III trial of Tecentriq combined with Cotellic "IMblaze370" in MSS colorectal cancer completed enrolling in 1Q17 with data likely early next year. Phase III trials "IMspire150" and "IMspire170" evaluating the combination in BRAFwt and BRAF+ melanoma, respectively, are underway or planned. Phase II trials of Cotellic/Tecentriq are ongoing in TNBC, GI tumors (w/ Avastin), and other rare tumors (basket trial). EXEL has a profit-share agreement on Cotellic in the US and receives double-digit royalties on ex-US sales (the drug is currently marketed in BRAF+ melanoma in combination with Zelboraf).
• MEK + PD1 IO-thesis catching on as BMY partners with NVS
in interesting move following a similar collaboration with ARRY,
but RHHYBY/EXEL have a significant head start. BMY announced yesterday that it has entered into a clinical collaboration with NVS to investigate the combination of Opdivo and Opdivo + Yervoy with NVS' Mekinist (MEK inhibitor) as a potential treatment for metastatic colorectal cancer in patients with microsatellite stable (MSS) tumors that are proficient in DNA mismatch repair. This follows an announcement from ARRY last week that it also has entered into a clinical trial collaboration with BMY with the goal to investigate their MEK inhibitor binimetini in combination with BMY's Opdivo with or without Yervoy in colorectal cancer (CRC) in a Phase I/II study run by ARRY. Earlier, ARRY announced a similar trial collaboration with MRK with the goal to investigate their binimetinib in combination with Keytruda in colorectal cancer. Why BMY choose to partner with NVS (which currently markets the leading MEK/BRAF inhibitor combination) in addition to ARRY is open to interpretation, but taken together, this partnering activity in our viewis supportive of RHHBY/EXEL's MEK strategy with CRC Phase III data potentially available in early 2018.
• Cabometyx well positioned in RCC in our view with front-line IO combo Phase III (w/ BMY, Takeda) to start this month. Updated results at ASCO were presented from 3 Phase I/II trials combining PD1 inhibitors with VEGF-targeted agents. (1) A follow-up analysis from AACR 2017 of RHHBY's Tecentriq + Avastin combo (IMmotion-150 Phase II trial) in frontline RCC looked very similar to the results previously presented at AACR’17 with the presenter noting that an improved PFS was not seen in the overall patient population, but post-hoc subgroup analyses teased out a signal, e.g., in PDL1-high and other patient subgroups (e.g., T-effector high of myeloid inflammation-high). (2) MRK’s Keytruda + Votrient Ph I combination data in advanced RCC reported significant hepatotoxicity and 90% Gr. 3/4 AEs; the presenter noted that the combination is not suitable for further evaluation. (3) PFE’s (MP) avelumab + Inlyta Ph Ib JAVELIN Renal 100 study in frontline RCC reported a confirmed ORR of 54.5% in 55 enrolled patients, less than the 83.3% ORR reported in a much smaller dataset previously at ESMO 2016. Inlyta appears to be better tolerated with PD1 inhibitors than Votrient and several Phase III trials (either with Keytruda or with avelumab) have recently initiated. (4) EXEL has identified a tolerable dose for the combination of Cabometyx and Opdivo/Yervoy and the Phase III trial in 1L RCC has been posted on clinicaltrials.gov. We're optimistic in the combination's potential given that Cabometyx has thus far generated the best single agent PFS and OS in RCC which could be significantly augmented by combining with Opdivo or Opdivo/Yervoy.
Leerink - EXEL 6Jun2017: Incremental Cotellic Melanoma Updates at ASCO Bode Well for IMblaze370 Trial
• Bottom Line: EXEL's partner Roche provided incremental updates at ASCO from its MEK inhibitor Cotellic (cobimetinib) which is being studied in combination with Tecentriq in metastatic melanoma. In addition, we attended the RCC session at ASCO which featured incremental (and somewhat disappointing) updates to several Phase I/II combination trials from competitors which did not have any impact on our view of the Cabometyx opportunity in RCC. We remain positive on EXEL's outlook and stock and are maintaining our OP rating.
• Updated Phase Ib data from RHHBY's Cotellic/Tecentriq combination in metastatic melanoma indicate responses are very durable. Twenty-two patients with metastatic melanoma were evaluable for safety and efficacy, including 20 patients with non-ocular melanoma (10 each with BRAF-wild type and BRAF V600-mutation positive disease). Among the 20 non-ocular melanoma patients, the objective response rate (ORR) was 45%, with similar response rate seen both in BRAFwt (50% ORR) and BRAF+ pts (40% ORR). The overall response rate was similar to data presented last year at SMR. Of note, median PFS and median duration of response had not yet been reached across both groups, potentially highlighting the benefits of combining MEK and PD1 inhibitors based on an immunologic rationale. Median PFS is currently estimated at 15.7 months, similar in both patient groups with follow-up still ongoing (compared to historic Tecentriq monotherapy mPFS of only 5.5 months). With the main caveat being the small patient numbers, these responses seem very durable, exceeding what has been seen previously with MEK/BRAF inhibitors and PD1 inhibitors alone. Updated Phase Ib data of the triple combination of Cotellic with Zelboraf and Tecentriq in BRAF+ melanoma also indicate responses (81.6%, 18% CRs) are still ongoing with mDOR and mPFS (12.9 months+) not yet reached.
• Pivotal trials evaluating the combination of Cotellic and Tecentriq are ongoing in several indications with data possible in early 2018 based on our estimates. The Phase III trial of Tecentriq combined with Cotellic "IMblaze370" in MSS colorectal cancer completed enrolling in 1Q17 with data likely early next year. Phase III trials "IMspire150" and "IMspire170" evaluating the combination in BRAFwt and BRAF+ melanoma, respectively, are underway or planned. Phase II trials of Cotellic/Tecentriq are ongoing in TNBC, GI tumors (w/ Avastin), and other rare tumors (basket trial). EXEL has a profit-share agreement on Cotellic in the US and receives double-digit royalties on ex-US sales (the drug is currently marketed in BRAF+ melanoma in combination with Zelboraf).
• MEK + PD1 IO-thesis catching on as BMY partners with NVS
in interesting move following a similar collaboration with ARRY,
but RHHYBY/EXEL have a significant head start. BMY announced yesterday that it has entered into a clinical collaboration with NVS to investigate the combination of Opdivo and Opdivo + Yervoy with NVS' Mekinist (MEK inhibitor) as a potential treatment for metastatic colorectal cancer in patients with microsatellite stable (MSS) tumors that are proficient in DNA mismatch repair. This follows an announcement from ARRY last week that it also has entered into a clinical trial collaboration with BMY with the goal to investigate their MEK inhibitor binimetini in combination with BMY's Opdivo with or without Yervoy in colorectal cancer (CRC) in a Phase I/II study run by ARRY. Earlier, ARRY announced a similar trial collaboration with MRK with the goal to investigate their binimetinib in combination with Keytruda in colorectal cancer. Why BMY choose to partner with NVS (which currently markets the leading MEK/BRAF inhibitor combination) in addition to ARRY is open to interpretation, but taken together, this partnering activity in our viewis supportive of RHHBY/EXEL's MEK strategy with CRC Phase III data potentially available in early 2018.
• Cabometyx well positioned in RCC in our view with front-line IO combo Phase III (w/ BMY, Takeda) to start this month. Updated results at ASCO were presented from 3 Phase I/II trials combining PD1 inhibitors with VEGF-targeted agents. (1) A follow-up analysis from AACR 2017 of RHHBY's Tecentriq + Avastin combo (IMmotion-150 Phase II trial) in frontline RCC looked very similar to the results previously presented at AACR’17 with the presenter noting that an improved PFS was not seen in the overall patient population, but post-hoc subgroup analyses teased out a signal, e.g., in PDL1-high and other patient subgroups (e.g., T-effector high of myeloid inflammation-high). (2) MRK’s Keytruda + Votrient Ph I combination data in advanced RCC reported significant hepatotoxicity and 90% Gr. 3/4 AEs; the presenter noted that the combination is not suitable for further evaluation. (3) PFE’s (MP) avelumab + Inlyta Ph Ib JAVELIN Renal 100 study in frontline RCC reported a confirmed ORR of 54.5% in 55 enrolled patients, less than the 83.3% ORR reported in a much smaller dataset previously at ESMO 2016. Inlyta appears to be better tolerated with PD1 inhibitors than Votrient and several Phase III trials (either with Keytruda or with avelumab) have recently initiated. (4) EXEL has identified a tolerable dose for the combination of Cabometyx and Opdivo/Yervoy and the Phase III trial in 1L RCC has been posted on clinicaltrials.gov. We're optimistic in the combination's potential given that Cabometyx has thus far generated the best single agent PFS and OS in RCC which could be significantly augmented by combining with Opdivo or Opdivo/Yervoy.
Antwort auf Beitrag Nr.: 55.115.488 von Cyberhexe am 10.06.17 02:52:30Was willst du damit eigentlich bezwecken? Dich einfach nur wichtig machen???
Wer bei Kursen von gut 12 USD die bestehenden Aktien kurze Zeit später zu gut 5 USD verwässert (wie 2011 !?!) der interessiert sich nicht sonderlich für seine Bestandsaktionäre...das ist nicht beleidigend, sondern einfach nur Fakt.
Ich denke außer der Firma selbst und dir wird mir hier jeder zustimmen können...
Wer bei Kursen von gut 12 USD die bestehenden Aktien kurze Zeit später zu gut 5 USD verwässert (wie 2011 !?!) der interessiert sich nicht sonderlich für seine Bestandsaktionäre...das ist nicht beleidigend, sondern einfach nur Fakt.
Ich denke außer der Firma selbst und dir wird mir hier jeder zustimmen können...
Zitat von Cyberhexe:Zitat von Cyberhexe: Bin in der Woche nach Pfingsten in SSF (South San Francisco) bei Exelixis. Bin mal gespannt, ob sich die Rechtsabteilung des Unternehmens für solche Verleumdungen interessiert. Wahrscheinlich nicht, aber ich werde dennoch mal nachfragen:
Zitiert aus dem Thread "Exelixis...ein schlafender Riese? USD 3.40 am 14.5.2014
Am 11.11.15 schrieb der Teilnehmer ville7 um 09:45:33 im Beitrag Nr. 399 (51.059.685) als Antwort auf Beitrag Nr. 398 (Nr. 51.058.935) von Franca_ole am11.11.15 um 09:00:03 die folgenden Sätze" "Im Übrigen stehe ich der Firma weiterhin sehr kritisch gegenüber, da sie schon oft gezeigt hat, dass sie mit ihren Finanzierungsrunden und -arten und eigenen Optionszuteilungen über die Leichen der Aktionäre geht ohne eine Wimper zu zucken. Never fall in love mit einem Produkt (Cobi) oder einer Firma (EXEL). Schaut so aus als hättest du noch das ein oder andere zu lernen"."
company is not amused about such slanderous comments!
neue ph1b-Studie mit Cabo und Roches Checkpoint-Hemmer Atezolizumab bei Blasen- und Nierenkrebs:
Exelixis Announces Initiation of Phase 1b Trial of Cabozantinib in Combination with Atezolizumab in Patients with Locally Advanced or Metastatic Solid Tumors
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Jun. 12, 2017-- Exelixis, Inc. (NASDAQ:EXEL) today announced the initiation of the dose-escalation stage of a phase 1b trial of cabozantinib in combination with atezolizumab (TECENTRIQ®) in patients with locally advanced or metastatic urothelial carcinoma (UC) or renal cell carcinoma (RCC). The primary objective is to determine the optimal dose and schedule of daily oral administration of cabozantinib when given in combination with atezolizumab to inform the trial’s subsequent expansion stage.
“Patients with locally advanced or metastatic urothelial or renal cell carcinoma are in need of additional therapies that can slow disease progression,” said Sumanta Kumar Pal, M.D., Co-director, Kidney Cancer Program at City of Hope, and Principal Investigator of the study. “As new investigational and approved therapies become available, research into their use in combination with other treatments may be a productive avenue for improving clinical outcomes in patients with these tumor types. Identifying the appropriate dose for cabozantinib when paired with the immunotherapy atezolizumab is the first step in examining this potential combination therapy.”
This multicenter phase 1b, open-label study is divided in two parts: a dose-escalation phase and an expansion cohort phase. The dose-escalation phase will enroll 9 to 36 patients with inoperable, locally advanced, metastatic or recurrent UC (including renal, pelvis, ureter, urinary bladder and urethra) after prior platinum-based therapy or RCC with or without prior systemic therapy. The starting dose of cabozantinib will be 40 mg daily and may be increased to 60 mg daily or decreased to 20 mg daily. All patients will receive the standard atezolizumab dosing regimen (1200 mg infusion once every 3 weeks).
Exelixis Announces Initiation of Phase 1b Trial of Cabozantinib in Combination with Atezolizumab in Patients with Locally Advanced or Metastatic Solid Tumors
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Jun. 12, 2017-- Exelixis, Inc. (NASDAQ:EXEL) today announced the initiation of the dose-escalation stage of a phase 1b trial of cabozantinib in combination with atezolizumab (TECENTRIQ®) in patients with locally advanced or metastatic urothelial carcinoma (UC) or renal cell carcinoma (RCC). The primary objective is to determine the optimal dose and schedule of daily oral administration of cabozantinib when given in combination with atezolizumab to inform the trial’s subsequent expansion stage.
“Patients with locally advanced or metastatic urothelial or renal cell carcinoma are in need of additional therapies that can slow disease progression,” said Sumanta Kumar Pal, M.D., Co-director, Kidney Cancer Program at City of Hope, and Principal Investigator of the study. “As new investigational and approved therapies become available, research into their use in combination with other treatments may be a productive avenue for improving clinical outcomes in patients with these tumor types. Identifying the appropriate dose for cabozantinib when paired with the immunotherapy atezolizumab is the first step in examining this potential combination therapy.”
This multicenter phase 1b, open-label study is divided in two parts: a dose-escalation phase and an expansion cohort phase. The dose-escalation phase will enroll 9 to 36 patients with inoperable, locally advanced, metastatic or recurrent UC (including renal, pelvis, ureter, urinary bladder and urethra) after prior platinum-based therapy or RCC with or without prior systemic therapy. The starting dose of cabozantinib will be 40 mg daily and may be increased to 60 mg daily or decreased to 20 mg daily. All patients will receive the standard atezolizumab dosing regimen (1200 mg infusion once every 3 weeks).
Antwort auf Beitrag Nr.: 55.124.399 von Ville7 am 12.06.17 13:49:45
natürlich will ich mich wichtig machen, und zwar auf deine KOSTEN!
Zitat von Ville7: Was willst du damit eigentlich bezwecken? Dich einfach nur wichtig machen???
Wer bei Kursen von gut 12 USD die bestehenden Aktien kurze Zeit später zu gut 5 USD verwässert (wie 2011 !?!) der interessiert sich nicht sonderlich für seine Bestandsaktionäre...das ist nicht beleidigend, sondern einfach nur Fakt.
Ich denke außer der Firma selbst und dir wird mir hier jeder zustimmen können...
Zitat von Cyberhexe: ...
company is not amused about such slanderous comments!
natürlich will ich mich wichtig machen, und zwar auf deine KOSTEN!

Antwort auf Beitrag Nr.: 55.128.668 von Cyberhexe am 13.06.17 06:38:40Jedem wie er es braucht. Schön wenn ich helfen konnte.
Neben 'wichtig' hast du dich auch ziemlich lächerlich gemacht. Aber das hast du bestimmt schon selbst bemerkt.
Neben 'wichtig' hast du dich auch ziemlich lächerlich gemacht. Aber das hast du bestimmt schon selbst bemerkt.
Antwort auf Beitrag Nr.: 55.128.668 von Cyberhexe am 13.06.17 06:38:40zurück zu den wichtigen Dingen:
Cabozantinib Eradicates Advanced Murine Prostate Cancer by Activating Antitumor Innate Immunity
http://cancerdiscovery.aacrjournals.org/content/early/2017/0…
Abstract
Several kinase inhibitors that target aberrant signaling pathways in tumor cells have been deployed in cancer therapy. However, their impact on the tumor immune microenvironment remains poorly understood. The tyrosine kinase inhibitor cabozantinib showed striking responses in cancer clinical trial patients across several malignancies. Here, we show that cabozantinib rapidly eradicates invasive, poorly differentiated PTEN/p53-deficient murine prostate cancer. This was associated with enhanced release of neutrophil chemotactic factors from tumor cells, including CXCL12 and HMGB1, resulting in robust infiltration of neutrophils into the tumor. Critically, cabozantinib-induced tumor clearance in mice was abolished by antibody-mediated granulocyte depletion or HMGB1 neutralization or blockade of neutrophil chemotaxis with the CXCR4 inhibitor plerixafor. Collectively, these data demonstrate that cabozantinib triggers a neutrophil-mediated anticancer innate immune response, resulting in tumor clearance.
SIGNIFICANCE: This study is the first to demonstrate that a tyrosine kinase inhibitor can activate neutrophil-mediated antitumor innate immunity, resulting in invasive cancer clearance. Cancer Discov; 7(7); 1–16. ©2017 AACR.
Cabozantinib Eradicates Advanced Murine Prostate Cancer by Activating Antitumor Innate Immunity
http://cancerdiscovery.aacrjournals.org/content/early/2017/0…
Abstract
Several kinase inhibitors that target aberrant signaling pathways in tumor cells have been deployed in cancer therapy. However, their impact on the tumor immune microenvironment remains poorly understood. The tyrosine kinase inhibitor cabozantinib showed striking responses in cancer clinical trial patients across several malignancies. Here, we show that cabozantinib rapidly eradicates invasive, poorly differentiated PTEN/p53-deficient murine prostate cancer. This was associated with enhanced release of neutrophil chemotactic factors from tumor cells, including CXCL12 and HMGB1, resulting in robust infiltration of neutrophils into the tumor. Critically, cabozantinib-induced tumor clearance in mice was abolished by antibody-mediated granulocyte depletion or HMGB1 neutralization or blockade of neutrophil chemotaxis with the CXCR4 inhibitor plerixafor. Collectively, these data demonstrate that cabozantinib triggers a neutrophil-mediated anticancer innate immune response, resulting in tumor clearance.
SIGNIFICANCE: This study is the first to demonstrate that a tyrosine kinase inhibitor can activate neutrophil-mediated antitumor innate immunity, resulting in invasive cancer clearance. Cancer Discov; 7(7); 1–16. ©2017 AACR.
Antwort auf Beitrag Nr.: 55.133.498 von Cyberhexe am 13.06.17 19:14:51wichtige Info für Schottland: das "Scottish Medicines Consortium" hat die Behandlung von fortgeschrittenem Nierenkrebs mit Nivo+Cabo genehmigt, wodurch eine Kostenerstattung durch die KV möglich wird.
SMC approve of nivolumab and cabozantinib
Statement from Kidney Cancer Scotland on the news of the SMC’s approval of nivolumab and cabozantinib for late stage kidney cancer patients in Scotland.
The news today that two important drugs for kidney cancer have been approved for patients in advanced stages of the disease is welcomed by Kidney Cancer Scotland. Their Regional Development Manager, Karen McNee, said; “To say the approval of these two drugs is wonderful news is a huge understatement. This is massive news for those patients who have been told their treatments are failing and there are no options. nivolumab is an immunotherapy drug which essentially use the body’s immune system to kill the cancerous cells. This is a relatively novel way of treating cancers and this is the first immunotherapy drugs to be approved in Scotland for kidney cancer. Cabozantinib has seen patient’s survival rates improve and the toleration of the drug offer a leap in reduced side-effects, delivering a far better quality of life. These are ground-breaking, life-extending treatments and to have both approve literally means the world to those who will receive them.”
Kidney cancer patient, Tom Wallace, said of the news: “As someone who has benefited greatly from nivolumab for the last 15 months through the Early Access Scheme, I know first-hand of the life changing benefits of this medicine and I am delighted at today’s SMC announcement. Access to this innovative immunotherapy medicine should significantly improve the quality of life, as well as life-expectations, of late-stage kidney cancer patients in Scotland and provide clinicians with a different treatment option”
Joe McCann added: “I know how it feels to be told that there are no more treatment options left – that there is no hope. I was extremely fortunate to be given the opportunity to receive one of these new treatments, cabozantinib, through an early access scheme. I was told after I received the new treatment that if I had not received the medicine my survival chances to Christmas 2016 would have been greatly reduced, and I am still here, this is why I’m so happy that this treatment option is now available for other people suffering with advanced kidney cancer. This is truly a great day for kidney cancer patients in Scotland!”
https://www.kcuk.org.uk/smc-approve-nivolumab-cabozantinib/
SMC approve of nivolumab and cabozantinib
Statement from Kidney Cancer Scotland on the news of the SMC’s approval of nivolumab and cabozantinib for late stage kidney cancer patients in Scotland.
The news today that two important drugs for kidney cancer have been approved for patients in advanced stages of the disease is welcomed by Kidney Cancer Scotland. Their Regional Development Manager, Karen McNee, said; “To say the approval of these two drugs is wonderful news is a huge understatement. This is massive news for those patients who have been told their treatments are failing and there are no options. nivolumab is an immunotherapy drug which essentially use the body’s immune system to kill the cancerous cells. This is a relatively novel way of treating cancers and this is the first immunotherapy drugs to be approved in Scotland for kidney cancer. Cabozantinib has seen patient’s survival rates improve and the toleration of the drug offer a leap in reduced side-effects, delivering a far better quality of life. These are ground-breaking, life-extending treatments and to have both approve literally means the world to those who will receive them.”
Kidney cancer patient, Tom Wallace, said of the news: “As someone who has benefited greatly from nivolumab for the last 15 months through the Early Access Scheme, I know first-hand of the life changing benefits of this medicine and I am delighted at today’s SMC announcement. Access to this innovative immunotherapy medicine should significantly improve the quality of life, as well as life-expectations, of late-stage kidney cancer patients in Scotland and provide clinicians with a different treatment option”
Joe McCann added: “I know how it feels to be told that there are no more treatment options left – that there is no hope. I was extremely fortunate to be given the opportunity to receive one of these new treatments, cabozantinib, through an early access scheme. I was told after I received the new treatment that if I had not received the medicine my survival chances to Christmas 2016 would have been greatly reduced, and I am still here, this is why I’m so happy that this treatment option is now available for other people suffering with advanced kidney cancer. This is truly a great day for kidney cancer patients in Scotland!”
https://www.kcuk.org.uk/smc-approve-nivolumab-cabozantinib/
Antwort auf Beitrag Nr.: 55.128.668 von Cyberhexe am 13.06.17 06:38:40Was ist dieser Ville eigentlich für ein Clown ?
Der wütet doch schon auf dem IV-Board wie ein
Inkompetenter herum. Was will der denn ?
Der wütet doch schon auf dem IV-Board wie ein
Inkompetenter herum. Was will der denn ?
Antwort auf Beitrag Nr.: 55.135.463 von uschi-buschi am 14.06.17 06:54:16aha, die Zweit-ID von Cyberhexe... oder der besagte Hardcore-Fan.
Goldig!
Goldig!

Antwort auf Beitrag Nr.: 55.135.463 von uschi-buschi am 14.06.17 06:54:16
http://theoncologist.alphamedpress.org/content/early/2017/06…
Cobimetinib und Duligotuzumab sind als Komitherapie OUT...in Ph1b wurde abgebrochen.
"Because of the generally poor tolerability of the combination
and limited efficacy seen in this limited patient population,
the dose expansion was not pursued and the study was halted."
http://theoncologist.alphamedpress.org/content/early/2017/06…
Cobimetinib und Duligotuzumab sind als Komitherapie OUT...in Ph1b wurde abgebrochen.
"Because of the generally poor tolerability of the combination
and limited efficacy seen in this limited patient population,
the dose expansion was not pursued and the study was halted."
Antwort auf Beitrag Nr.: 55.137.065 von Cyberhexe am 14.06.17 11:33:30viel versprechendes Ergebnis einer ph1-Studie bei Urogenitalkarzinomen:
Dr. Apolo on Immunotherapy Combinations With Cabozantinib in Genitourinary Tumors
Andrea Apolo, MD
Published Online: Tuesday, Jun 13, 2017
Andrea Apolo, MD, medical oncologist, chief of the bladder cancer section of the Genitourinary Malignancies Branch, National Cancer Institute, discusses immunotherapy combinations with cabozantinib (Cometriq) in genitourinary tumors. In a phase I study, cabozantinib plus nivolumab (Opdivo), as well as cabozantinib/nivolumab plus ipilimumab (Yervoy) was evaluated in patients with refractory metastatic urothelial carcinoma and other genitourinary tumors. Apolo reports that the overall response rate for the entire cohort is 37%, while the bladder cancer cohort is 44%.
http://www.onclive.com/onclive-tv/dr-apolo-on-immunotherapy-…
Dr. Apolo on Immunotherapy Combinations With Cabozantinib in Genitourinary Tumors
Andrea Apolo, MD
Published Online: Tuesday, Jun 13, 2017
Andrea Apolo, MD, medical oncologist, chief of the bladder cancer section of the Genitourinary Malignancies Branch, National Cancer Institute, discusses immunotherapy combinations with cabozantinib (Cometriq) in genitourinary tumors. In a phase I study, cabozantinib plus nivolumab (Opdivo), as well as cabozantinib/nivolumab plus ipilimumab (Yervoy) was evaluated in patients with refractory metastatic urothelial carcinoma and other genitourinary tumors. Apolo reports that the overall response rate for the entire cohort is 37%, while the bladder cancer cohort is 44%.
http://www.onclive.com/onclive-tv/dr-apolo-on-immunotherapy-…
Antwort auf Beitrag Nr.: 55.146.545 von Cyberhexe am 15.06.17 17:10:41bei Cotellic gab es eine Warnung zu Rhabdomyolysis und auftretenden Blutungen:
https://assets.publishing.service.gov.uk/media/59241d98e5274…
https://assets.publishing.service.gov.uk/media/59241d98e5274…
Die Erweiterung der Zulassung --> Erstlinienbehandlung bei Nierenkrebs soll im 3.Quartal beantragt werden:
http://www.exelixis.com/investors-media/press-releases?cpurl…
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Jun. 19, 2017-- Exelixis, Inc. (NASDAQ:EXEL) announced today that the analysis of the review by a blinded independent radiology review committee (IRC) has confirmed the primary efficacy endpoint results of investigator-assessed progression-free survival (PFS) from the CABOSUN randomized phase 2 trial of cabozantinib as compared with sunitinib in patients with previously untreated advanced renal cell carcinoma (RCC) with intermediate- or poor-risk disease per the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC). Per the IRC analysis, cabozantinib demonstrated a clinically meaningful and statistically significant reduction in the rate of disease progression or death as measured by PFS. Exelixis remains on target to complete a supplemental New Drug Application (sNDA) for cabozantinib as a treatment of first-line advanced renal cell carcinoma in the third quarter of 2017.
http://www.exelixis.com/investors-media/press-releases?cpurl…
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Jun. 19, 2017-- Exelixis, Inc. (NASDAQ:EXEL) announced today that the analysis of the review by a blinded independent radiology review committee (IRC) has confirmed the primary efficacy endpoint results of investigator-assessed progression-free survival (PFS) from the CABOSUN randomized phase 2 trial of cabozantinib as compared with sunitinib in patients with previously untreated advanced renal cell carcinoma (RCC) with intermediate- or poor-risk disease per the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC). Per the IRC analysis, cabozantinib demonstrated a clinically meaningful and statistically significant reduction in the rate of disease progression or death as measured by PFS. Exelixis remains on target to complete a supplemental New Drug Application (sNDA) for cabozantinib as a treatment of first-line advanced renal cell carcinoma in the third quarter of 2017.
Antwort auf Beitrag Nr.: 55.146.581 von Cyberhexe am 15.06.17 17:19:14
Die Erweiterung der Zulassung --> "Erstlinienbehandlung bei fortgeschrittenem Nierenkrebs" soll im 3.Quartal beantragt werden:
http://www.exelixis.com/investors-media/press-releases?cpurl…
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Jun. 19, 2017-- Exelixis, Inc. (NASDAQ:EXEL) announced today that the analysis of the review by a blinded independent radiology review committee (IRC) has confirmed the primary efficacy endpoint results of investigator-assessed progression-free survival (PFS) from the CABOSUN randomized phase 2 trial of cabozantinib as compared with sunitinib in patients with previously untreated advanced renal cell carcinoma (RCC) with intermediate- or poor-risk disease per the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC). Per the IRC analysis, cabozantinib demonstrated a clinically meaningful and statistically significant reduction in the rate of disease progression or death as measured by PFS. Exelixis remains on target to complete a supplemental New Drug Application (sNDA) for cabozantinib as a treatment of first-line advanced renal cell carcinoma in the third quarter of 2017.
Die Erweiterung der Zulassung --> "Erstlinienbehandlung bei fortgeschrittenem Nierenkrebs" soll im 3.Quartal beantragt werden:
http://www.exelixis.com/investors-media/press-releases?cpurl…
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Jun. 19, 2017-- Exelixis, Inc. (NASDAQ:EXEL) announced today that the analysis of the review by a blinded independent radiology review committee (IRC) has confirmed the primary efficacy endpoint results of investigator-assessed progression-free survival (PFS) from the CABOSUN randomized phase 2 trial of cabozantinib as compared with sunitinib in patients with previously untreated advanced renal cell carcinoma (RCC) with intermediate- or poor-risk disease per the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC). Per the IRC analysis, cabozantinib demonstrated a clinically meaningful and statistically significant reduction in the rate of disease progression or death as measured by PFS. Exelixis remains on target to complete a supplemental New Drug Application (sNDA) for cabozantinib as a treatment of first-line advanced renal cell carcinoma in the third quarter of 2017.
Antwort auf Beitrag Nr.: 55.165.574 von Cyberhexe am 19.06.17 17:33:24
Ipsen kündigt an, die Daten zu CABOSUN bei der europ. Arzneimittelbehörde EMA im 3q2017 einzureichen. Zweck ist eine Erweiterung der Indikation und zwar zur Erstlinienbehandlung von fortgeschrittenem Nierenkrebs.
Independent review affirms positive mid-stage results for Ipsen and Exelixis' cabozantinib
Jun. 19, 2017 4:09 PM ET|By: Douglas W. House, SA News Editor
Ipsen (OTCPK:IPSEY) and commercialization partner Exelixis (NASDAQ:EXEL) announce that an analysis by a independent radiology review committee has confirmed the positive results from the Phase 2 CABOSUN study assessing CABOMETYX (cabozantinib) compared to sunitinib (Pfizer's SUTENT) in treatment-naive patients with advanced renal cell carcinoma (RCC) with intermediate- or poor-risk disease.
The study met its primary endpoint showing treatment with cabozantinib produced a statistically significant improvement in progression-free survival compared to sunitinib as determined by investigator assessment.
Ipsen chief David Meek says his company will file the data with European regulators in Q3.
Ipsen kündigt an, die Daten zu CABOSUN bei der europ. Arzneimittelbehörde EMA im 3q2017 einzureichen. Zweck ist eine Erweiterung der Indikation und zwar zur Erstlinienbehandlung von fortgeschrittenem Nierenkrebs.
Independent review affirms positive mid-stage results for Ipsen and Exelixis' cabozantinib
Jun. 19, 2017 4:09 PM ET|By: Douglas W. House, SA News Editor
Ipsen (OTCPK:IPSEY) and commercialization partner Exelixis (NASDAQ:EXEL) announce that an analysis by a independent radiology review committee has confirmed the positive results from the Phase 2 CABOSUN study assessing CABOMETYX (cabozantinib) compared to sunitinib (Pfizer's SUTENT) in treatment-naive patients with advanced renal cell carcinoma (RCC) with intermediate- or poor-risk disease.
The study met its primary endpoint showing treatment with cabozantinib produced a statistically significant improvement in progression-free survival compared to sunitinib as determined by investigator assessment.
Ipsen chief David Meek says his company will file the data with European regulators in Q3.
Toni Choueiri ist im Feld der Onkologen bzw. der Onkouro- bzw. Onkonephrologen eine herausragende Persönlichkeit, er ist Direktor von "Dana-Farber/Brigham and Women’s Cancer Center where he is Director of the Kidney Cancer Center and Clinical Director of the Lank Center for Genitourinary Oncology". Er widerspricht Rini und Vogelzang in deren Meinung, dass eine positive ph2-Studie immer mit einer ph3-Studie zu bestätigen sei:
http://ascopubs.org/doi/pdf/10.1200/JCO.2017.72.2629
Toni K. Choueiri††, Susan Halabi†, Michael J. Morris†, and Daniel George†
"...(mRCC). The CABOSUN study met its primary end point of
progression-free survival (PFS) in a statistically and clinically
meaningful way....."
"...We appreciate that randomized phase II studies do not convey
the same level of confidence as a phase III study. By the same token,
Rini and Vogelzang imply that all randomized phase II studies are
equivalent in size, agents tested, population, and treatment effect,
and thereby require confirmation with a phase III study if they are
positive. Such an approach may not be financially or practically
feasible. A carefully designed and well-conducted randomized
phase II trial provides reliable answers to important questions
and estimates regarding treatment effect."
http://ascopubs.org/doi/pdf/10.1200/JCO.2017.72.2629
Toni K. Choueiri††, Susan Halabi†, Michael J. Morris†, and Daniel George†
"...(mRCC). The CABOSUN study met its primary end point of
progression-free survival (PFS) in a statistically and clinically
meaningful way....."
"...We appreciate that randomized phase II studies do not convey
the same level of confidence as a phase III study. By the same token,
Rini and Vogelzang imply that all randomized phase II studies are
equivalent in size, agents tested, population, and treatment effect,
and thereby require confirmation with a phase III study if they are
positive. Such an approach may not be financially or practically
feasible. A carefully designed and well-conducted randomized
phase II trial provides reliable answers to important questions
and estimates regarding treatment effect."
Antwort auf Beitrag Nr.: 55.188.427 von Cyberhexe am 23.06.17 06:30:45Cabometyx's Benefit in Kidney Cancer Is Confirmed
http://www.curetoday.com/articles/cabometyxs-benefit-in-kidn…
"Progression-free survival (PFS) was extended for patients with previously untreated, intermediate- or poor-risk advanced renal cell carcinoma (RCC) who were treated with Cabometyx (cabozantinib) compared to those treated with Sutent (sunitinib), according to results from a blinded, independent data review, which confirmed previous results from the CABOSUN trial.
Results from the randomized, open-label phase 2 CABOSUN trial were first presented at the 2016 ESMO Congress and published in the Journal of Clinical Oncology. Cabometyx reduced the risk of progression or death by 34 percent versus Sutent as a first-line treatment for patients with metastatic RCC. The median PFS was 2.6 months longer with Cabometyx versus Sutent, at 8.2 versus 5.6 months.
According to analysis by an independent radiology review committee, Cabometyx demonstrated a clinically meaningful and statistically significant reduction in the rate of disease progression or death as measured by PFS. Exelixis, manufacturer of cabozantinib, announced the findings in a press release.
...
Median OS was 30.3 months in the Cabometyx arm versus 21.8 months in the Sutent arm. Cabometyx was also superior for overall response rate (ORR), 46 percent versus 18 percent."
http://www.curetoday.com/articles/cabometyxs-benefit-in-kidn…
"Progression-free survival (PFS) was extended for patients with previously untreated, intermediate- or poor-risk advanced renal cell carcinoma (RCC) who were treated with Cabometyx (cabozantinib) compared to those treated with Sutent (sunitinib), according to results from a blinded, independent data review, which confirmed previous results from the CABOSUN trial.
Results from the randomized, open-label phase 2 CABOSUN trial were first presented at the 2016 ESMO Congress and published in the Journal of Clinical Oncology. Cabometyx reduced the risk of progression or death by 34 percent versus Sutent as a first-line treatment for patients with metastatic RCC. The median PFS was 2.6 months longer with Cabometyx versus Sutent, at 8.2 versus 5.6 months.
According to analysis by an independent radiology review committee, Cabometyx demonstrated a clinically meaningful and statistically significant reduction in the rate of disease progression or death as measured by PFS. Exelixis, manufacturer of cabozantinib, announced the findings in a press release.
...
Median OS was 30.3 months in the Cabometyx arm versus 21.8 months in the Sutent arm. Cabometyx was also superior for overall response rate (ORR), 46 percent versus 18 percent."
Antwort auf Beitrag Nr.: 55.188.439 von Cyberhexe am 23.06.17 06:36:29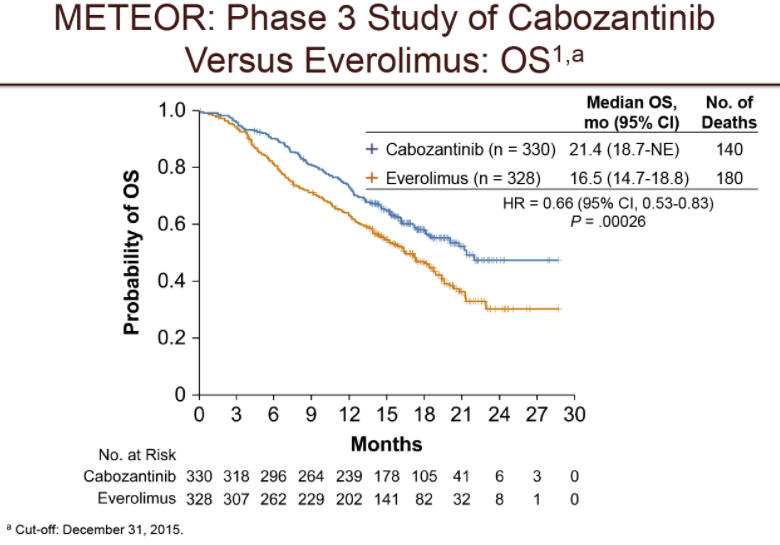
Vor allem bei Knochenmetastasen sowie bei Metastasen an den Eingeweide (innere Organe) ist der Überlebensvorteil unter Cabozantinib bei Nierenkrebs (RCC) maximal gegenüber Everolimus, ebenso bei Patienten mit guter bis mittlerer Prognose.
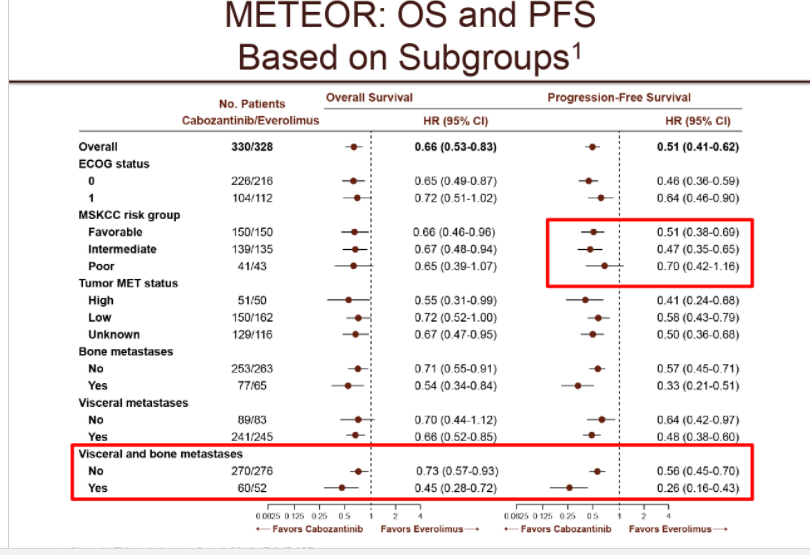

Vor allem bei Knochenmetastasen sowie bei Metastasen an den Eingeweide (innere Organe) ist der Überlebensvorteil unter Cabozantinib bei Nierenkrebs (RCC) maximal gegenüber Everolimus, ebenso bei Patienten mit guter bis mittlerer Prognose.

Antwort auf Beitrag Nr.: 55.191.720 von Cyberhexe am 23.06.17 13:33:59Der europ. Arzneimittelausschuss CHMP der EMA hat eine positive Zulassungsempfehlung zur Erstlinienbehandlung von fortgeschrittenem Nierenkrebs (RCC) mit Tivozanib (brand: Fotivda) ausgesprochen:
"On 22 June 2017, the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending the granting of a marketing authorisation for the medicinal product Fotivda, intended for the treatment of advanced renal cell carcinoma. The applicant for this medicinal product is EUSA Pharma.
Fotivda will be available as 890 µg and 1340 µg hard capsules. The active substance of Fotivda is tivozanib hydrochloride monohydrate, a protein kinase inhibitor (ATC code: L01XE34) which, by blocking vascular endothelial growth factor receptors (VEGFR), inhibits angiogenesis leading to inhibition of tumour growth.
The benefits with Fotivda are its ability to improve progression-free survival in patients with advanced disease. The most common side effects are hypertension, dysphonia, fatigue and diarrhoea.
The full indication is: "first line treatment of adult patients with advanced renal cell carcinoma (RCC) and for adult patients who are VEGFR and mTOR pathway inhibitor-naïve following disease progression after one prior treatment with cytokine therapy for advanced RCC."
It is proposed that Fotivda be prescribed by physicians experienced in the treatment of cancer."
"On 22 June 2017, the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending the granting of a marketing authorisation for the medicinal product Fotivda, intended for the treatment of advanced renal cell carcinoma. The applicant for this medicinal product is EUSA Pharma.
Fotivda will be available as 890 µg and 1340 µg hard capsules. The active substance of Fotivda is tivozanib hydrochloride monohydrate, a protein kinase inhibitor (ATC code: L01XE34) which, by blocking vascular endothelial growth factor receptors (VEGFR), inhibits angiogenesis leading to inhibition of tumour growth.
The benefits with Fotivda are its ability to improve progression-free survival in patients with advanced disease. The most common side effects are hypertension, dysphonia, fatigue and diarrhoea.
The full indication is: "first line treatment of adult patients with advanced renal cell carcinoma (RCC) and for adult patients who are VEGFR and mTOR pathway inhibitor-naïve following disease progression after one prior treatment with cytokine therapy for advanced RCC."
It is proposed that Fotivda be prescribed by physicians experienced in the treatment of cancer."
Antwort auf Beitrag Nr.: 55.193.313 von Cyberhexe am 23.06.17 16:42:41
FDA Daten zu Tivozanib in RCC:
https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesM…
Tivozanib Approval Status
FDA approved: No
Generic name: tivozanib
Company: AVEO Oncology and Astellas Pharma Inc.
Treatment for: Renal Cell Carcinoma
Tivozanib is an investigational tyrosine kinase inhibitor for the treatment of advanced renal cell carcinoma.
In June 2013, AVEO Oncology announced the receipt of a Complete Response Letter from the U.S. Food and Drug Administration (FDA), advising the company that the New Drug Application (NDA) for tivozanib had not been approved.
FDA Daten zu Tivozanib in RCC:
https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesM…
Tivozanib Approval Status
FDA approved: No
Generic name: tivozanib
Company: AVEO Oncology and Astellas Pharma Inc.
Treatment for: Renal Cell Carcinoma
Tivozanib is an investigational tyrosine kinase inhibitor for the treatment of advanced renal cell carcinoma.
In June 2013, AVEO Oncology announced the receipt of a Complete Response Letter from the U.S. Food and Drug Administration (FDA), advising the company that the New Drug Application (NDA) for tivozanib had not been approved.
Antwort auf Beitrag Nr.: 55.193.313 von Cyberhexe am 23.06.17 16:42:41Cabo und die Checkpoint-Hemmer Nivolumab bzw. Ipilimumab scheinen miteinander verträglich zu sein. Bisher wurden keine negativen Wechselwirkungen beobachtet.
"In the combination of cabozantinib plus nivolumab (Opdivo) and cabozantinib plus ipilimumab (Yervoy), the typical side effects of each agent were shown. There was no synergy between the toxicities though, which is an encouraging sign, says Apolo."
http://www.onclive.com/onclive-tv/dr-apolo-on-side-effects-o…
"In the combination of cabozantinib plus nivolumab (Opdivo) and cabozantinib plus ipilimumab (Yervoy), the typical side effects of each agent were shown. There was no synergy between the toxicities though, which is an encouraging sign, says Apolo."
http://www.onclive.com/onclive-tv/dr-apolo-on-side-effects-o…
Antwort auf Beitrag Nr.: 55.235.240 von Cyberhexe am 30.06.17 08:10:12VEGF-Hemmer bei wiederkehrendem Glioblastoma: Cabo an prominenter Stelle!
http://clincancerres.aacrjournals.org/content/early/2017/06/…
http://clincancerres.aacrjournals.org/content/early/2017/06/…
Antwort auf Beitrag Nr.: 55.297.270 von Cyberhexe am 10.07.17 23:55:02Cabozantinib use in renal cell carcinoma
Neuwelt, A.J., Mathur, S., Johnson, A.T., Kessler, E.R., Bowles, D.
In the last several years, many new drugs have been approved to treat metastatic renal cell carcinoma (RCC). Cabozantinib is a novel multikinase inhibitor with activity against vascular endothelial growth factor receptor (VEGFR), proto-oncogene tyrosine-protein kinase receptor Ret and other kinases that recently joined this impressive list of approved agents. Cabozantinib is an active agent in the preclinical and clinical setting, having recently demonstrated superiority over everolimus in a blinded, randomized phase III study of patients with progressive RCC after at least one prior line of antiangiogenic therapy. This agent’s toxicity profile is similar to those of other multikinase inhibitors approved to treat RCC. This review will explore cabozantinib's pharmacologic and safety profile and its preclinical and clinical activity in RCC.
Neuwelt, A.J., Mathur, S., Johnson, A.T., Kessler, E.R., Bowles, D.
In the last several years, many new drugs have been approved to treat metastatic renal cell carcinoma (RCC). Cabozantinib is a novel multikinase inhibitor with activity against vascular endothelial growth factor receptor (VEGFR), proto-oncogene tyrosine-protein kinase receptor Ret and other kinases that recently joined this impressive list of approved agents. Cabozantinib is an active agent in the preclinical and clinical setting, having recently demonstrated superiority over everolimus in a blinded, randomized phase III study of patients with progressive RCC after at least one prior line of antiangiogenic therapy. This agent’s toxicity profile is similar to those of other multikinase inhibitors approved to treat RCC. This review will explore cabozantinib's pharmacologic and safety profile and its preclinical and clinical activity in RCC.
Antwort auf Beitrag Nr.: 55.297.273 von Cyberhexe am 10.07.17 23:55:40Cabozantinib bei Nierenkrebs wird nun auch in England und Wales von den Krankenkassen bezahlt:
"New treatment option: Cabozantinib is now available for use in the NHS for advanced kidney cancer patients
Kidney Cancer UK is delighted that National Institute for Health and Care Excellence (NICE) has recommended the drug cabozantinib (Cabometyx) be made available to advanced kidney cancer patients throughout the NHS. The drug, which was recommended for use in Scotland last month by the Scottish Medicines Consortium (SMC), will now also be recommended for patients in England and Wales, on the NHS."
"New treatment option: Cabozantinib is now available for use in the NHS for advanced kidney cancer patients
Kidney Cancer UK is delighted that National Institute for Health and Care Excellence (NICE) has recommended the drug cabozantinib (Cabometyx) be made available to advanced kidney cancer patients throughout the NHS. The drug, which was recommended for use in Scotland last month by the Scottish Medicines Consortium (SMC), will now also be recommended for patients in England and Wales, on the NHS."
Antwort auf Beitrag Nr.: 55.314.640 von Cyberhexe am 13.07.17 06:55:18
sorry, hab den Link vergessen:
http://upflow.co/l/BdbS/kidney-cancer-uk-delighted-nice-reco…
Zitat von Cyberhexe: Cabozantinib bei Nierenkrebs wird nun auch in England und Wales von den Krankenkassen bezahlt:
"New treatment option: Cabozantinib is now available for use in the NHS for advanced kidney cancer patients
Kidney Cancer UK is delighted that National Institute for Health and Care Excellence (NICE) has recommended the drug cabozantinib (Cabometyx) be made available to advanced kidney cancer patients throughout the NHS. The drug, which was recommended for use in Scotland last month by the Scottish Medicines Consortium (SMC), will now also be recommended for patients in England and Wales, on the NHS."
sorry, hab den Link vergessen:
http://upflow.co/l/BdbS/kidney-cancer-uk-delighted-nice-reco…
Antwort auf Beitrag Nr.: 55.314.643 von Cyberhexe am 13.07.17 06:56:22NICE empfiehlt Cabo zur Behandlung von fortgeschrittenem Nierenkrebs, so dass die Kosten vom National Health Service übernommen werden:
"A new treatment option for people with kidney cancer"
https://www.nice.org.uk/news/article/a-new-treatment-option-…
"A new treatment option for people with kidney cancer"
https://www.nice.org.uk/news/article/a-new-treatment-option-…
Antwort auf Beitrag Nr.: 55.328.551 von Cyberhexe am 14.07.17 22:20:48
Falls das Ergebnis in Phase 3 bei Leberkrebs - Entblindung noch in 2017- auch noch positiv ausfällt, dann gehts mit dem Kurs in dieser Geschwindigkeit in Richtung $50.
Cabozantinib in the treatment of hepatocellular carcinoma
Mary Linton B Peters & Rebecca A Miksad
Published Online:July 13th, 2017https://doi.org/10.2217/fon-2017-0169
View Article
Tools
Share
Abstract
Despite clinical studies with different mechanisms of action, no new systemic therapies were approved for hepatocellular carcinoma (HCC) between sorafenib in 2007 and regorafenib in 2017. This is an area of interest to improve outcomes and quality of life. Cabozantinib is oral, small-molecule tyrosine kinase inhibitor that primarily targets MET, VEGFR2, AXL and RET, with additional effect on KIT and FLT3. Cabozantinib is approved for progressive metastatic medullary thyroid cancer and previously treated renal cell carcinoma, and is in development for multiple solid tumors. Given positive results from a Phase II study, cabozantinib is under evaluation in a Phase III randomized controlled trial for patients with advanced HCC previously treated with sorafenib. It has been granted orphan drug status in the USA for this indication. This review summarizes the development of cabozantinib in HCC.
Falls das Ergebnis in Phase 3 bei Leberkrebs - Entblindung noch in 2017- auch noch positiv ausfällt, dann gehts mit dem Kurs in dieser Geschwindigkeit in Richtung $50.
Cabozantinib in the treatment of hepatocellular carcinoma
Mary Linton B Peters & Rebecca A Miksad
Published Online:July 13th, 2017https://doi.org/10.2217/fon-2017-0169
View Article
Tools
Share
Abstract
Despite clinical studies with different mechanisms of action, no new systemic therapies were approved for hepatocellular carcinoma (HCC) between sorafenib in 2007 and regorafenib in 2017. This is an area of interest to improve outcomes and quality of life. Cabozantinib is oral, small-molecule tyrosine kinase inhibitor that primarily targets MET, VEGFR2, AXL and RET, with additional effect on KIT and FLT3. Cabozantinib is approved for progressive metastatic medullary thyroid cancer and previously treated renal cell carcinoma, and is in development for multiple solid tumors. Given positive results from a Phase II study, cabozantinib is under evaluation in a Phase III randomized controlled trial for patients with advanced HCC previously treated with sorafenib. It has been granted orphan drug status in the USA for this indication. This review summarizes the development of cabozantinib in HCC.
Antwort auf Beitrag Nr.: 55.328.704 von Cyberhexe am 14.07.17 22:46:34Roche warnt bei Cobimetinib (brand: COTELLIC) vor Nebenwirkungen, nämlich schweren Blutungen und Rhabdomyolyse (Auflösung von quergestreifter Muskulatur).
Important additional warnings for haemorrhage and rhabdomyolysis with Cotellic™
https://assets.publishing.service.gov.uk/media/59241d98e5274…
Important additional warnings for haemorrhage and rhabdomyolysis with Cotellic™
https://assets.publishing.service.gov.uk/media/59241d98e5274…
Cabo zur Behandlung von Leberkrebs - Ergebnisse bzw. 2. Zwischenergebnis wird noch in 2017 erwartet:
Cabozantinib in the treatment of hepatocellular carcinoma
Mary Linton B Peters & Rebecca A Miksad
Published Online:July 13th, 2017
Despite clinical studies with different mechanisms of action, no new systemic therapies were approved for hepatocellular carcinoma (HCC) between sorafenib in 2007 and regorafenib in 2017. This is an area of interest to improve outcomes and quality of life. Cabozantinib is oral, small-molecule tyrosine kinase inhibitor that primarily targets MET, VEGFR2, AXL and RET, with additional effect on KIT and FLT3. Cabozantinib is approved for progressive metastatic medullary thyroid cancer and previously treated renal cell carcinoma, and is in development for multiple solid tumors. Given positive results from a Phase II study, cabozantinib is under evaluation in a Phase III randomized controlled trial for patients with advanced HCC previously treated with sorafenib. It has been granted orphan drug status in the USA for this indication. This review summarizes the development of cabozantinib in HCC.
https://www.futuremedicine.com/doi/abs/10.2217/fon-2017-0169…
https://clinicaltrials.gov/ct2/show/NCT01908426?term=cabozan…
Cabozantinib in the treatment of hepatocellular carcinoma
Mary Linton B Peters & Rebecca A Miksad
Published Online:July 13th, 2017
Despite clinical studies with different mechanisms of action, no new systemic therapies were approved for hepatocellular carcinoma (HCC) between sorafenib in 2007 and regorafenib in 2017. This is an area of interest to improve outcomes and quality of life. Cabozantinib is oral, small-molecule tyrosine kinase inhibitor that primarily targets MET, VEGFR2, AXL and RET, with additional effect on KIT and FLT3. Cabozantinib is approved for progressive metastatic medullary thyroid cancer and previously treated renal cell carcinoma, and is in development for multiple solid tumors. Given positive results from a Phase II study, cabozantinib is under evaluation in a Phase III randomized controlled trial for patients with advanced HCC previously treated with sorafenib. It has been granted orphan drug status in the USA for this indication. This review summarizes the development of cabozantinib in HCC.
https://www.futuremedicine.com/doi/abs/10.2217/fon-2017-0169…
https://clinicaltrials.gov/ct2/show/NCT01908426?term=cabozan…
Antwort auf Beitrag Nr.: 55.362.356 von Cyberhexe am 20.07.17 13:31:43Das ist doch eine salomonische Lösung des Interessenkonfliktes zwischen Roche/Genentech und Exelixis:
bei den Kombitherapien, in welchen Cobimetinib gemeinsam mit einem anderen Roche/Genentech-Wirkstoff zur Anwendung kommt, werden nicht die jeweiligen Einzelpreise berücksichtigt, sondern für jeden Wirkstoff die Hälfte des Preises der Kombitherapie.
Dies hat bspw. für die Melanoma-Franchise die Konsequenz, dass bisher ...
"Allerdings wird bei der bisher einzigen Zulassung von Cobi (brand: Cotellic) dieses gemeinsam mit Vemurafenib (brand: Zelboraf) zur Behandlung des schwarzen Hautkrebs verabreicht. Die Kosten hierfür betragen ca. USD 17600/Behandlung - die Preisgestaltung von Genentech ist jedoch nicht im Sinne von Exelixis, da 2/3 dieser Kosten für Zelboraf und ca. nur 1/3 für Cotellic aufzuwenden sind. Exelixis hat deswegen ein Schiedsverfahren eingeleitet, um dieses Ungleichgewicht abzuschwächen."
...pro Patient vom Gesamtpreis der Kombitherapie 2/3 für Zelboraf und 1/3 für Cotellic verrechnet wurden.
Nach der neuen Abmachung werden die Erlöse für die Therapie gleichmässig verteilt, also 50% auf Zelboraf (oder jedes andere Kombipräparat) und 50% für Cotellic (Cobimetinib).
Exelixis partizipiert somit zur Hälfte und nicht nur zu einem Drittel an den Erlösen der Kombitherapie.
"Exelixis Announces Settlement of Dispute with Genentech Regarding Companies’ Collaboration Agreement for Cobimetinib
- Companies define new revenue and cost-sharing terms for all commercial applications of cobimetinib
- Cobimetinib’s clinical development program includes three ongoing or planned phase 3 pivotal trials
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Jul. 20, 2017-- Exelixis, Inc. (NASDAQ:EXEL) announced today a settlement of the company’s dispute with Genentech, a member of the Roche Group, concerning the parties’ collaboration for the development and commercialization of cobimetinib, which is marketed as COTELLIC®. Effective July 1, 2017, as part of the settlement the companies entered into an amendment (the “Amendment”) to the existing Collaboration Agreement, dated December 22, 2006, to revise the revenue and cost-sharing arrangements for the collaboration. The Amendment resolves the companies’ dispute pursuant to the arbitration demand filed on June 3, 2016, and aligns both companies’ interests in advancing cobimetinib as a promising therapy for patients with multiple forms of cancer.
The Amendment applies to COTELLIC®’s initial commercial application in combination with ZELBORAF® (vemurafenib), as well as future commercial uses of COTELLIC®, alone or in combination. Under its terms, Exelixis continues to be entitled to an initial equal share of U.S. profits and losses, which will decrease as sales increase as specified in the original 2006 agreement. However, effective as of July 1, 2017, the revenue applied to the profit and loss statement for the COTELLIC® collaboration (“the Collaboration P&L”) will be calculated using the average of the quarterly net selling prices of COTELLIC® and any additional branded Genentech product(s) prescribed with COTELLIC®. Exelixis will continue to share U.S. commercialization costs, while Genentech’s portion of these costs will now be allocated to the Collaboration P&L based on the number of products in the combination. Exelixis will continue to co-promote COTELLIC® in the U.S., providing up to 25 percent of the U.S. sales force. Outside of the U.S., Exelixis remains eligible for royalties on COTELLIC® sales according to the terms of the original 2006 agreement."
bei den Kombitherapien, in welchen Cobimetinib gemeinsam mit einem anderen Roche/Genentech-Wirkstoff zur Anwendung kommt, werden nicht die jeweiligen Einzelpreise berücksichtigt, sondern für jeden Wirkstoff die Hälfte des Preises der Kombitherapie.
Dies hat bspw. für die Melanoma-Franchise die Konsequenz, dass bisher ...
"Allerdings wird bei der bisher einzigen Zulassung von Cobi (brand: Cotellic) dieses gemeinsam mit Vemurafenib (brand: Zelboraf) zur Behandlung des schwarzen Hautkrebs verabreicht. Die Kosten hierfür betragen ca. USD 17600/Behandlung - die Preisgestaltung von Genentech ist jedoch nicht im Sinne von Exelixis, da 2/3 dieser Kosten für Zelboraf und ca. nur 1/3 für Cotellic aufzuwenden sind. Exelixis hat deswegen ein Schiedsverfahren eingeleitet, um dieses Ungleichgewicht abzuschwächen."
...pro Patient vom Gesamtpreis der Kombitherapie 2/3 für Zelboraf und 1/3 für Cotellic verrechnet wurden.
Nach der neuen Abmachung werden die Erlöse für die Therapie gleichmässig verteilt, also 50% auf Zelboraf (oder jedes andere Kombipräparat) und 50% für Cotellic (Cobimetinib).
Exelixis partizipiert somit zur Hälfte und nicht nur zu einem Drittel an den Erlösen der Kombitherapie.
"Exelixis Announces Settlement of Dispute with Genentech Regarding Companies’ Collaboration Agreement for Cobimetinib
- Companies define new revenue and cost-sharing terms for all commercial applications of cobimetinib
- Cobimetinib’s clinical development program includes three ongoing or planned phase 3 pivotal trials
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Jul. 20, 2017-- Exelixis, Inc. (NASDAQ:EXEL) announced today a settlement of the company’s dispute with Genentech, a member of the Roche Group, concerning the parties’ collaboration for the development and commercialization of cobimetinib, which is marketed as COTELLIC®. Effective July 1, 2017, as part of the settlement the companies entered into an amendment (the “Amendment”) to the existing Collaboration Agreement, dated December 22, 2006, to revise the revenue and cost-sharing arrangements for the collaboration. The Amendment resolves the companies’ dispute pursuant to the arbitration demand filed on June 3, 2016, and aligns both companies’ interests in advancing cobimetinib as a promising therapy for patients with multiple forms of cancer.
The Amendment applies to COTELLIC®’s initial commercial application in combination with ZELBORAF® (vemurafenib), as well as future commercial uses of COTELLIC®, alone or in combination. Under its terms, Exelixis continues to be entitled to an initial equal share of U.S. profits and losses, which will decrease as sales increase as specified in the original 2006 agreement. However, effective as of July 1, 2017, the revenue applied to the profit and loss statement for the COTELLIC® collaboration (“the Collaboration P&L”) will be calculated using the average of the quarterly net selling prices of COTELLIC® and any additional branded Genentech product(s) prescribed with COTELLIC®. Exelixis will continue to share U.S. commercialization costs, while Genentech’s portion of these costs will now be allocated to the Collaboration P&L based on the number of products in the combination. Exelixis will continue to co-promote COTELLIC® in the U.S., providing up to 25 percent of the U.S. sales force. Outside of the U.S., Exelixis remains eligible for royalties on COTELLIC® sales according to the terms of the original 2006 agreement."
Antwort auf Beitrag Nr.: 55.364.129 von Cyberhexe am 20.07.17 16:50:37Sehr gut: eine unabhängige Überprüfung bestätigt die CABOSUN-Ergebnisse:
http://ht.ly/YoSf30dN7Oe
http://ht.ly/YoSf30dN7Oe
Antwort auf Beitrag Nr.: 55.380.495 von Cyberhexe am 23.07.17 19:24:07nichts Neues, aber dennoch gut zu lesen:
https://www.healio.com/hematology-oncology/genitourinary-can…
"Cabozantinib appears safe, effective across age groups in renal cell carcinoma"
https://www.healio.com/hematology-oncology/genitourinary-can…
"Cabozantinib appears safe, effective across age groups in renal cell carcinoma"
Antwort auf Beitrag Nr.: 55.445.889 von Cyberhexe am 02.08.17 19:01:2010 Präsentationen zu Cabo und Cobi auf der ESMO in Madrid:
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Aug. 1, 2017-- Exelixis, Inc. (NASDAQ:EXEL) today announced that data from clinical trials of cabozantinib and cobimetinib will be the subject of 10 presentations at the European Society for Medical Oncology (ESMO) 2017 Congress in Madrid, September 8 – 12, 2017.
Quartalszahlen ohne grosse Überraschungen:
http://www.exelixis.com/investors-media/press-releases?cpurl…
kursbewegendes Ereignis evtl. im Herbst, falls bei der 2. Analyse von CELESTIAL beim fortgeschrittenen Leberkarzinom bereits statistisch signifikante Verbesserungen nachgewiesen werden:
"CELESTIAL Data Anticipated in the Second Half of 2017. CELESTIAL, the ongoing phase 3 pivotal trial of cabozantinib in patients with advanced hepatocellular carcinoma (HCC), continues to progress. Exelixis is tracking events closely and continues to anticipate that the second interim analysis at 75 percent of the required events will be completed in the second half of 2017."
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Aug. 1, 2017-- Exelixis, Inc. (NASDAQ:EXEL) today announced that data from clinical trials of cabozantinib and cobimetinib will be the subject of 10 presentations at the European Society for Medical Oncology (ESMO) 2017 Congress in Madrid, September 8 – 12, 2017.
Quartalszahlen ohne grosse Überraschungen:
http://www.exelixis.com/investors-media/press-releases?cpurl…
kursbewegendes Ereignis evtl. im Herbst, falls bei der 2. Analyse von CELESTIAL beim fortgeschrittenen Leberkarzinom bereits statistisch signifikante Verbesserungen nachgewiesen werden:
"CELESTIAL Data Anticipated in the Second Half of 2017. CELESTIAL, the ongoing phase 3 pivotal trial of cabozantinib in patients with advanced hepatocellular carcinoma (HCC), continues to progress. Exelixis is tracking events closely and continues to anticipate that the second interim analysis at 75 percent of the required events will be completed in the second half of 2017."
Antwort auf Beitrag Nr.: 55.449.480 von Cyberhexe am 03.08.17 10:35:57
A new treatment option for people with kidney cancer
People with advanced kidney cancer are set to benefit from a new treatment, after NICE has said cabozantinib should be available on the NHS.
https://www.nice.org.uk/news/article/a-new-treatment-option-…
A new treatment option for people with kidney cancer
People with advanced kidney cancer are set to benefit from a new treatment, after NICE has said cabozantinib should be available on the NHS.
https://www.nice.org.uk/news/article/a-new-treatment-option-…

Auf ESMO 2017 in Madrid werden zu Cabozantinib 7 unterschiedliche Studienergebnisse präsentiert, unter anderem dieser "Late-breaking abstract" zu CABOSUN:
10-09-2017
15:45 - 15:45 LBA38 - Progression-free survival (PFS) by independent review and updated overall survival (OS) results
from Alliance A031203 trial (CABOSUN): cabozantinib versus sunitinib as initial targeted therapy for
patients (pts) with metastatic renal cell carcinoma (mRCC)
T. Choueiri , C. Hessel , S. Halabi , B. Sanford , O. Hahn , M. Michaelson , M. Walsh , T. Olencki , J. 1 2 3 3 4 5 5 6
Picus , E. Small , S. Dakhil , C. Scheffold , D. George , M. Morris ; Boston, US, South San 7 8 9 2 3 10 1 2
Francisco, CA/US, Durham, NC/US, Chicago, IL/US, Boston, MA/US, Columbus, OH/US, St. 3 4 5 6 7
Louis, US, San Francisco, CA/US, Wichita, KS/US, New York, NY/US
10-09-2017
15:45 - 15:45 LBA38 - Progression-free survival (PFS) by independent review and updated overall survival (OS) results
from Alliance A031203 trial (CABOSUN): cabozantinib versus sunitinib as initial targeted therapy for
patients (pts) with metastatic renal cell carcinoma (mRCC)
T. Choueiri , C. Hessel , S. Halabi , B. Sanford , O. Hahn , M. Michaelson , M. Walsh , T. Olencki , J. 1 2 3 3 4 5 5 6
Picus , E. Small , S. Dakhil , C. Scheffold , D. George , M. Morris ; Boston, US, South San 7 8 9 2 3 10 1 2
Francisco, CA/US, Durham, NC/US, Chicago, IL/US, Boston, MA/US, Columbus, OH/US, St. 3 4 5 6 7
Louis, US, San Francisco, CA/US, Wichita, KS/US, New York, NY/US
interessante Studie zu Nierenkrebs:
Cabozantinib, Crizotinib, Volitinib, or
Sunitinib in Treating Patients With Locally
Advanced or Metastatic Papillary Kidney
Cancer (S1500)
http://www.10forio.info/clinical-trials/s1500-cabozantinib-c…
Cabozantinib, Crizotinib, Volitinib, or
Sunitinib in Treating Patients With Locally
Advanced or Metastatic Papillary Kidney
Cancer (S1500)
http://www.10forio.info/clinical-trials/s1500-cabozantinib-c…
sNDA für First line RCC
https://seekingalpha.com/news/3289762-exelixis-completes-snd…
positiv für exel:
https://seekingalpha.com/news/3289664-late-stage-study-brist…
https://seekingalpha.com/news/3289664-late-stage-study-brist…
Antwort auf Beitrag Nr.: 55.535.808 von paul81 am 16.08.17 14:20:29die FDA hat auf 19.9. ein Advisory Committee einberufen zur Risiko/Nutzen-Abwägung von Sunitinib als unterstützende medikamentöse Therapie nach Nierenentfernung bei RCC:
https://www.fda.gov/AdvisoryCommittees/Calendar/ucm570507.ht…
Checkmate-214-Daten http://iokidney.com/clinical-trials/checkmate-214
sind durchwachsen:
http://www.onclive.com/web-exclusives/frontline-nivolumabipi…
viel versprechendes Ergebnis --> objektive Ansprechrate aber keine stat. Signifikanz:
Median progression-free survival (PFS) was 11.56 months (95% CI, 8.71-15.51) for the combination versus 8.38 months (95% CI, 7.03-10.81) for sunitinib, but investigators reported that the difference was not statistically significant (hazard ratio, 0.82; 95% CI, 0.64-1.05; stratified 2-sided P = .03).
https://www.fda.gov/AdvisoryCommittees/Calendar/ucm570507.ht…
Checkmate-214-Daten http://iokidney.com/clinical-trials/checkmate-214
sind durchwachsen:
http://www.onclive.com/web-exclusives/frontline-nivolumabipi…
viel versprechendes Ergebnis --> objektive Ansprechrate aber keine stat. Signifikanz:
Median progression-free survival (PFS) was 11.56 months (95% CI, 8.71-15.51) for the combination versus 8.38 months (95% CI, 7.03-10.81) for sunitinib, but investigators reported that the difference was not statistically significant (hazard ratio, 0.82; 95% CI, 0.64-1.05; stratified 2-sided P = .03).
Antwort auf Beitrag Nr.: 55.546.101 von Cyberhexe am 17.08.17 18:35:54ESMO2017 --> Cobi
https://cslide.ctimeetingtech.com/library/esmo/browse/search…
1225PD - Prognostic impact of early complete metabolic response on FDG-PET, in BRAF V600 mutant metastatic melanoma patients treated with combination vemurafenib & cobimetinib
Background
Imaging with FDG-PET allows early recognition of metabolic response to targeted agents. We evaluated the timing of complete metabolic response (CMR) on PET as a predictor of clinical outcome in BRAF V600 mutant melanoma patients treated with vemurafenib and cobimetinb, as part of the BRIM-7 trial.
Methods
BRAF inhibitor naïve patients from BRIM-7 were included if they had evaluable PET scans at baseline, in cycle 1 (C1) (D10-15) and in C2 (D35-49). Metabolic response was evaluated by percentage injected dose (%ID). 52 of 63 BRAF-naïve patients were eligible for analysis (3 excluded - no C1 scan; 6 no C2 scan; 2 unevaluable scans due to excessive physiological muscle uptake). The primary aim was to evaluate the prognostic significance of an early CMR to combination vemurafenib and cobimetinib therapy. We divided patients into 3 groups, based on timing of CMR attainment.
Results
13 patients achieved CMR in cycle 1 (CMR1), 15 patients achieved CMR in cycle 2 (CMR2) and 24 patients did not achieve CMR within the first 2 cycles of treatment (noCMR). The median, 2 year and 3 year progression free survival (PFS) and overall survival (OS) of the 3 above groups are summarized in Table.rn
Table:
1225PD
rnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrn
CMR1 CMR2 No CMR
Median PFS (yrs) Not reached 1.1 1.0
2 yr PFS (%, 95% CI) 83.9 (65.7-100) 13.3 (3.7-48.4) 37.5 (22.4-62.9)
3 yr PFS (%, 95% CI) 71.9 (48.8-100) 13.3 (3.7-48.4) 18.8 (7.9-44.5)
Median OS (yrs) Not reached 2.4 2.5
2 yr OS (%, 95% CI) 84.6 (67.1-100) 63.0 (41.5-95.8) 58.3 (41.6-81.8)
3 yr OS (%, 95% CI) 74.0 (52.2-100) 21.0 (6.3-70.2) 30.9 (16.5-57.9)
rn
Patients achieving CMR1 had significantly better outcome than patients achieving CMR2 in terms of PFS (HR 0.18, 95% CI 0.05-0.62) and OS (HR 0.23, 95% CI 0.06-0.85). Similar results were observed comparing CMR1 over no CMR in PFS (HR 0.19, 95% CI 0.06-0.64) and in OS (HR 0.25, 95% CI 0.07-0.87). There was no difference between the CMR2 and noCMR groups in terms of PFS or OS.
rn
Conclusions
Attainment of CMR on an early D10-14 PET was highly predictive of long-term survival with BRAF and MEK inhibition. However, attainment of CMR at a later time point at D35-49 did not appear predictive of a survival benefit. In fact, no difference in PFS or OS could be observed in patients who achieved CMR at D35-49, compared to those patients who did not attain CMR. Correlative science analysis to investigate the mechanism of these observations are underway.
Clinical trial identification
number: NCT01271803
https://cslide.ctimeetingtech.com/library/esmo/browse/search…
1225PD - Prognostic impact of early complete metabolic response on FDG-PET, in BRAF V600 mutant metastatic melanoma patients treated with combination vemurafenib & cobimetinib
Background
Imaging with FDG-PET allows early recognition of metabolic response to targeted agents. We evaluated the timing of complete metabolic response (CMR) on PET as a predictor of clinical outcome in BRAF V600 mutant melanoma patients treated with vemurafenib and cobimetinb, as part of the BRIM-7 trial.
Methods
BRAF inhibitor naïve patients from BRIM-7 were included if they had evaluable PET scans at baseline, in cycle 1 (C1) (D10-15) and in C2 (D35-49). Metabolic response was evaluated by percentage injected dose (%ID). 52 of 63 BRAF-naïve patients were eligible for analysis (3 excluded - no C1 scan; 6 no C2 scan; 2 unevaluable scans due to excessive physiological muscle uptake). The primary aim was to evaluate the prognostic significance of an early CMR to combination vemurafenib and cobimetinib therapy. We divided patients into 3 groups, based on timing of CMR attainment.
Results
13 patients achieved CMR in cycle 1 (CMR1), 15 patients achieved CMR in cycle 2 (CMR2) and 24 patients did not achieve CMR within the first 2 cycles of treatment (noCMR). The median, 2 year and 3 year progression free survival (PFS) and overall survival (OS) of the 3 above groups are summarized in Table.rn
Table:
1225PD
rnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrnrn
CMR1 CMR2 No CMR
Median PFS (yrs) Not reached 1.1 1.0
2 yr PFS (%, 95% CI) 83.9 (65.7-100) 13.3 (3.7-48.4) 37.5 (22.4-62.9)
3 yr PFS (%, 95% CI) 71.9 (48.8-100) 13.3 (3.7-48.4) 18.8 (7.9-44.5)
Median OS (yrs) Not reached 2.4 2.5
2 yr OS (%, 95% CI) 84.6 (67.1-100) 63.0 (41.5-95.8) 58.3 (41.6-81.8)
3 yr OS (%, 95% CI) 74.0 (52.2-100) 21.0 (6.3-70.2) 30.9 (16.5-57.9)
rn
Patients achieving CMR1 had significantly better outcome than patients achieving CMR2 in terms of PFS (HR 0.18, 95% CI 0.05-0.62) and OS (HR 0.23, 95% CI 0.06-0.85). Similar results were observed comparing CMR1 over no CMR in PFS (HR 0.19, 95% CI 0.06-0.64) and in OS (HR 0.25, 95% CI 0.07-0.87). There was no difference between the CMR2 and noCMR groups in terms of PFS or OS.
rn
Conclusions
Attainment of CMR on an early D10-14 PET was highly predictive of long-term survival with BRAF and MEK inhibition. However, attainment of CMR at a later time point at D35-49 did not appear predictive of a survival benefit. In fact, no difference in PFS or OS could be observed in patients who achieved CMR at D35-49, compared to those patients who did not attain CMR. Correlative science analysis to investigate the mechanism of these observations are underway.
Clinical trial identification
number: NCT01271803
Exel heute 10 % im negativen Bereich. Warum, habe keine Meldungen gefunden.
Hoffe keine schlechten Vorboten und habe trotzdem einige Stücke gekauft.
Morgen ESMO in Madrid.
Hoffe keine schlechten Vorboten und habe trotzdem einige Stücke gekauft.
Morgen ESMO in Madrid.
Volumen der gehandelten Aktien auch deutlich über dem Durchschnitt.
Hoffe da kommt keine schlechte Meldung.
Einige haben meist vorab die entsprechenden Infos.
Hoffe da kommt keine schlechte Meldung.
Einige haben meist vorab die entsprechenden Infos.
CABOZANTINIB wird bei schlechter und mittelmässiger Prognose bei RCC Stadium IV als Erstlinienbehandlung empfohlen:

Antwort auf Beitrag Nr.: 55.704.012 von Cyberhexe am 09.09.17 12:27:45
das ist doch überaus erfreulich: Cabo zeigt in einer Metaanalyse sowohl beim progressionsfreien Überleben als auch in der Überlebenszeitanalyse fast durchweg die besten Ergebnisse in Zweitlinienbehandlung. Solche empirischen Daten im Direktvergleich sollten für weiteren Aufschwung sprich einen grösseren Marktanteil sorgen.
http://journals.plos.org/plosone/article?id=10.1371/journal.…
Cabozantinib versus everolimus, nivolumab, axitinib, sorafenib and best supportive care: A network meta-analysis of progression-free survival and overall survival in second line treatment of advanced renal cell carcinoma
Conclusion
With all five families of distributions, cabozantinib was superior to all its comparators with a higher probability of longer PFS and OS during the analyzed 3 years, except with the Gompertz model, where nivolumab was preferred after 24 months.
Zitat von Cyberhexe: CABOZANTINIB wird bei schlechter und mittelmässiger Prognose bei RCC Stadium IV als Erstlinienbehandlung empfohlen:
das ist doch überaus erfreulich: Cabo zeigt in einer Metaanalyse sowohl beim progressionsfreien Überleben als auch in der Überlebenszeitanalyse fast durchweg die besten Ergebnisse in Zweitlinienbehandlung. Solche empirischen Daten im Direktvergleich sollten für weiteren Aufschwung sprich einen grösseren Marktanteil sorgen.
http://journals.plos.org/plosone/article?id=10.1371/journal.…
Cabozantinib versus everolimus, nivolumab, axitinib, sorafenib and best supportive care: A network meta-analysis of progression-free survival and overall survival in second line treatment of advanced renal cell carcinoma
Conclusion
With all five families of distributions, cabozantinib was superior to all its comparators with a higher probability of longer PFS and OS during the analyzed 3 years, except with the Gompertz model, where nivolumab was preferred after 24 months.
Antwort auf Beitrag Nr.: 55.772.682 von Cyberhexe am 19.09.17 06:06:39und noch ein interessanter wissenschaftlicher Artikel zu Cabo, und zwar hinsichtlich Zweitlinienbehandlung von mRCC:
Second-line systemic therapy in metastatic renal-cell carcinoma: A review
http://www.sciencedirect.com/science/article/pii/S1078143917…
Cabozantinib in METEOR trial has demonstrated better ORR, mPFS, and OS benefit over everolimus [14] ; [19]. Cabozantinib has also shown activity in reversing acquired resistance to sunitinib through inhibition of MET and AXL in xenograft models [38]
It favors cabozantinib in the first 5 months of treatment and nivolumab afterwards which could indicate that cabozantinib could be more beneficial in patients with poor prognosis and nivolumab in patients with better prognosis [39]
Second-line systemic therapy in metastatic renal-cell carcinoma: A review
http://www.sciencedirect.com/science/article/pii/S1078143917…
Cabozantinib in METEOR trial has demonstrated better ORR, mPFS, and OS benefit over everolimus [14] ; [19]. Cabozantinib has also shown activity in reversing acquired resistance to sunitinib through inhibition of MET and AXL in xenograft models [38]
It favors cabozantinib in the first 5 months of treatment and nivolumab afterwards which could indicate that cabozantinib could be more beneficial in patients with poor prognosis and nivolumab in patients with better prognosis [39]
Antwort auf Beitrag Nr.: 55.781.265 von Cyberhexe am 20.09.17 08:18:20
Ipsen and Exelixis Announce Results from Phase 2 CABOSUN Trial of Cabozantinib versus Sunitinib in Previously Untreated Advanced Renal Cell Carcinoma at ESMO 2017
The updated overall survival (OS) analysis had a data cut-off of July 1, 2017, and showed a favorable trend for patients randomized to cabozantinib compared to sunitinib that was not statistically significant. Median overall survival was 26.6 months for patients receiving cabozantinib versus 21.2 months for those receiving sunitinib (HR= 0.80, 95% CI 0.53-1.21, two-sided P=0.29).
http://pipelinereview.com/index.php/2017091065806/Small-Mole…
Ipsen and Exelixis Announce Results from Phase 2 CABOSUN Trial of Cabozantinib versus Sunitinib in Previously Untreated Advanced Renal Cell Carcinoma at ESMO 2017
The updated overall survival (OS) analysis had a data cut-off of July 1, 2017, and showed a favorable trend for patients randomized to cabozantinib compared to sunitinib that was not statistically significant. Median overall survival was 26.6 months for patients receiving cabozantinib versus 21.2 months for those receiving sunitinib (HR= 0.80, 95% CI 0.53-1.21, two-sided P=0.29).
http://pipelinereview.com/index.php/2017091065806/Small-Mole…
Antwort auf Beitrag Nr.: 55.796.514 von Cyberhexe am 21.09.17 18:14:49interessanter Artikel zu Cabo und Checkpoint-Hemmer Nivolumab bei mUC:
http://www.onclive.com/web-exclusives/cabozantinib-nivolumab…
Cabozantinib, Nivolumab Active in Metastatic Urothelial Carcinoma
Wayne Kuznar
Published Online: Wednesday, Sep 20, 2017
Print Button
bladder Cabozantinib (Cabometyx) and nivolumab (Opdivo) with or without ipilimumab (Yervoy) show activity in genitourinary (GU) tumors, particularly urothelial carcinoma, and have manageable safety profiles. The objective response rate (ORR) for all GU tumor types was 33% in 42 patients treated in a phase I trial.1 Final results from the trial were reported by Rosa Nadal, MD, a medical oncologist at the Center for Cancer Research, National Cancer Institute, Bethesda, MD at the 2017 ESMO Congress in Madrid, Spain.
Cabozantinib has shown clinical activity in pretreated patients with refractory metastatic urothelial carcinoma (mUC). In the phase II CheckMate-275 trial, nivolumab demonstrated clinical activity in patients with mUC whose disease has progressed despite prior platinum-containing chemotherapy.2 The combination of nivolumab and ipilimumab also showed manageable toxicity and clinical activity in the phase I/II CheckMate-032 study.3
Cabozantinib has immunomodulatory properties that may counteract tumor-induced immunosuppression, providing the rationale for combining cabozantinib with immunotherapy, explained Nadal. Cabozantinib has been shown to reduce the percentage of regulatory T cells in CD4-positive T cells, and downregulate the T-reg population by acting on T-cell polarization. In patients with mUC, those with a lower percentage of T-reg in CD4-positive T cells at baseline had a better response than those who had a higher percentage at baseline.
The objective of the study presented here was to determine the dose-limiting toxicity (DLT) and a recommended phase II dose for combination cabozantinib and nivolumab and combination cabozantinib, nivolumab, and ipilimumab. Secondary endpoints included ORR, progression-free survival (PFS), and overall survival (OS).
The study included 42 patients with a metastatic GU malignancy who had disease progression on at least 1 standard therapy (or for whom no standard therapy has been shown to prolong survival): 15 with urothelial carcinoma of the bladder and 27 with non-urothelial carcinoma GU malignancies. Two-thirds of patients had ≥2 prior systemic regimens. Fifty-five percent of patients had visceral disease (liver and/or lung metastases).
Seven dose levels of cabozantinib and nivolumab were tested in part 1, and 3 dose levels of the cabozantinib, nivolumab, and ipilimumab combination were tested in part 2.
The ORR in the overall study population was 33%. “Please note that this is a very heterogeneous group of patients with many rare tumor types included,” Nadal said. With a median potential follow-up of 16.8 months, the median duration of response was not reached in patients receiving either regimen. Responses were ongoing at the time of database lock in 6 of 9 responders to cabozantinib and nivolumab, and 3 of 4 responders to cabozantinib, nivolumab, and ipilimumab. Median time to response appears to be about 2 months, said Nadal.
“We were delighted to see responses in rare tumors such as squamous cell carcinoma, sarcomatoid renal cell carcinoma, and penile cancer,” she said. “One patient with castration-resistant prostate cancer and 1 patient with urachal adenocarcinoma achieved partial responses.” The ORRs were 38% with cabozantinib and nivolumab and 22% with cabozantinib, nivolumab, and ipilimumab. Median PFS was 5.9 months, with a probability of PFS at 6 months of 50.0% and a probability at 12 months of 35.7%. Median OS was 20 months, with a 6-month survival of 83.3% and a 12-month survival of 63.6%.
The rate of grade 3 or 4 adverse events (AEs) was 67% with cabozantinib and nivolumab over a median follow-up of 18.4 months and 72% with cabozantinib, nivolumab, and ipilimumab over a median follow-up of 12.8 months. Treatment-related AEs leading to discontinuation occurred in 12% of the group receiving cabozantinib and nivolumab and 11.1% receiving cabozantinib, nivolumab, and ipilimumab. Fifty percent of patients in the cabozantinib, nivolumab, and ipilimumab group and 33% in the cabozantinib, nivolumab, and ipilimumab group required at least 1 dose reduction of cabozantinib.
The most common clinical AEs of any grade with cabozantinib and nivolumab were fatigue (71%), nausea/vomiting (71%), diarrhea (67%), skin disorders (67%), anorexia (62%), and oral mucositis or sore throat (62%). In the cabozantinib, nivolumab, and ipilimumab group, most common AEs were fatigue (83%), diarrhea (83%), skin disorders (78%), nausea/vomiting (67%), and anorexia (55%).
“When we started the protocol, we were concerned about overlapping toxicities such as diarrhea. Our concern was that people would not differentiate diarrhea from cabozantinib versus colitis from nivolumab, but we actually found that people were able to clinically differentiate these 2 toxicities,” said Nadal.
The most common potential immune-related adverse event was pneumonitis, experienced by 11% of patients receiving cabozantinib, nivolumab, and ipilimumab.
http://www.onclive.com/web-exclusives/cabozantinib-nivolumab…
Cabozantinib, Nivolumab Active in Metastatic Urothelial Carcinoma
Wayne Kuznar
Published Online: Wednesday, Sep 20, 2017
Print Button
bladder Cabozantinib (Cabometyx) and nivolumab (Opdivo) with or without ipilimumab (Yervoy) show activity in genitourinary (GU) tumors, particularly urothelial carcinoma, and have manageable safety profiles. The objective response rate (ORR) for all GU tumor types was 33% in 42 patients treated in a phase I trial.1 Final results from the trial were reported by Rosa Nadal, MD, a medical oncologist at the Center for Cancer Research, National Cancer Institute, Bethesda, MD at the 2017 ESMO Congress in Madrid, Spain.
Cabozantinib has shown clinical activity in pretreated patients with refractory metastatic urothelial carcinoma (mUC). In the phase II CheckMate-275 trial, nivolumab demonstrated clinical activity in patients with mUC whose disease has progressed despite prior platinum-containing chemotherapy.2 The combination of nivolumab and ipilimumab also showed manageable toxicity and clinical activity in the phase I/II CheckMate-032 study.3
Cabozantinib has immunomodulatory properties that may counteract tumor-induced immunosuppression, providing the rationale for combining cabozantinib with immunotherapy, explained Nadal. Cabozantinib has been shown to reduce the percentage of regulatory T cells in CD4-positive T cells, and downregulate the T-reg population by acting on T-cell polarization. In patients with mUC, those with a lower percentage of T-reg in CD4-positive T cells at baseline had a better response than those who had a higher percentage at baseline.
The objective of the study presented here was to determine the dose-limiting toxicity (DLT) and a recommended phase II dose for combination cabozantinib and nivolumab and combination cabozantinib, nivolumab, and ipilimumab. Secondary endpoints included ORR, progression-free survival (PFS), and overall survival (OS).
The study included 42 patients with a metastatic GU malignancy who had disease progression on at least 1 standard therapy (or for whom no standard therapy has been shown to prolong survival): 15 with urothelial carcinoma of the bladder and 27 with non-urothelial carcinoma GU malignancies. Two-thirds of patients had ≥2 prior systemic regimens. Fifty-five percent of patients had visceral disease (liver and/or lung metastases).
Seven dose levels of cabozantinib and nivolumab were tested in part 1, and 3 dose levels of the cabozantinib, nivolumab, and ipilimumab combination were tested in part 2.
The ORR in the overall study population was 33%. “Please note that this is a very heterogeneous group of patients with many rare tumor types included,” Nadal said. With a median potential follow-up of 16.8 months, the median duration of response was not reached in patients receiving either regimen. Responses were ongoing at the time of database lock in 6 of 9 responders to cabozantinib and nivolumab, and 3 of 4 responders to cabozantinib, nivolumab, and ipilimumab. Median time to response appears to be about 2 months, said Nadal.
“We were delighted to see responses in rare tumors such as squamous cell carcinoma, sarcomatoid renal cell carcinoma, and penile cancer,” she said. “One patient with castration-resistant prostate cancer and 1 patient with urachal adenocarcinoma achieved partial responses.” The ORRs were 38% with cabozantinib and nivolumab and 22% with cabozantinib, nivolumab, and ipilimumab. Median PFS was 5.9 months, with a probability of PFS at 6 months of 50.0% and a probability at 12 months of 35.7%. Median OS was 20 months, with a 6-month survival of 83.3% and a 12-month survival of 63.6%.
The rate of grade 3 or 4 adverse events (AEs) was 67% with cabozantinib and nivolumab over a median follow-up of 18.4 months and 72% with cabozantinib, nivolumab, and ipilimumab over a median follow-up of 12.8 months. Treatment-related AEs leading to discontinuation occurred in 12% of the group receiving cabozantinib and nivolumab and 11.1% receiving cabozantinib, nivolumab, and ipilimumab. Fifty percent of patients in the cabozantinib, nivolumab, and ipilimumab group and 33% in the cabozantinib, nivolumab, and ipilimumab group required at least 1 dose reduction of cabozantinib.
The most common clinical AEs of any grade with cabozantinib and nivolumab were fatigue (71%), nausea/vomiting (71%), diarrhea (67%), skin disorders (67%), anorexia (62%), and oral mucositis or sore throat (62%). In the cabozantinib, nivolumab, and ipilimumab group, most common AEs were fatigue (83%), diarrhea (83%), skin disorders (78%), nausea/vomiting (67%), and anorexia (55%).
“When we started the protocol, we were concerned about overlapping toxicities such as diarrhea. Our concern was that people would not differentiate diarrhea from cabozantinib versus colitis from nivolumab, but we actually found that people were able to clinically differentiate these 2 toxicities,” said Nadal.
The most common potential immune-related adverse event was pneumonitis, experienced by 11% of patients receiving cabozantinib, nivolumab, and ipilimumab.
Antwort auf Beitrag Nr.: 55.796.952 von Cyberhexe am 21.09.17 18:59:07yeahhh:
Cabozantinib scheint im Kampf gegen Krebs eine Wunderwaffe zu sein, da nun auch bei CELESTIAL --> "Study of Cabozantinib (XL184) vs Placebo in Subjects With Hepatocellular Carcinoma Who Have Received Prior Sorafenib" der primäre Endpunkt erreicht wurde.
Aber asl ob dies nicht genügen würde, hat die FDA auch noch Priority Review zugesichert, und zwar bei der Erstlinienbehandlung von Nierenkrebs mit Cabo.
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-news&ny…
Cabozantinib scheint im Kampf gegen Krebs eine Wunderwaffe zu sein, da nun auch bei CELESTIAL --> "Study of Cabozantinib (XL184) vs Placebo in Subjects With Hepatocellular Carcinoma Who Have Received Prior Sorafenib" der primäre Endpunkt erreicht wurde.
Aber asl ob dies nicht genügen würde, hat die FDA auch noch Priority Review zugesichert, und zwar bei der Erstlinienbehandlung von Nierenkrebs mit Cabo.
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-news&ny…
Antwort auf Beitrag Nr.: 55.960.713 von Cyberhexe am 16.10.17 16:39:58
Cabo hat eine lediglich bescheidene Ansprechrate bei Glioblastoma:
Conclusions
Cabozantinib treatment appeared to have modest clinical activity with a 4.3% response rate in patients who had received prior antiangiogenic therapy for GBM.
https://academic.oup.com/neuro-oncology/article-lookup/doi/1…
Cabo hat eine lediglich bescheidene Ansprechrate bei Glioblastoma:
Conclusions
Cabozantinib treatment appeared to have modest clinical activity with a 4.3% response rate in patients who had received prior antiangiogenic therapy for GBM.
https://academic.oup.com/neuro-oncology/article-lookup/doi/1…
Antwort auf Beitrag Nr.: 56.011.101 von Cyberhexe am 24.10.17 07:28:47....sehr gutes Q-Ergebnis von Exelixis - der Kurs dürfte wieder anziehen in Richtung $30.
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArt…
Exelixis Announces Third Quarter 2017 Financial Results and Provides Corporate Update
- Cabozantinib Franchise Net Product Revenue of $96.4 million, Total Revenue of $152.5 million -
- Net Income of $81.4 million, Diluted EPS of $0.26 per Share -
- Conference Call and Webcast Today at 5:00 PM Eastern Time -
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Nov. 1, 2017-- Exelixis, Inc. (NASDAQ: EXEL) today reported financial results for the third quarter of 2017 and provided an update on progress toward fulfilling its key corporate objectives, as well as commercial and clinical development milestones.
Exelixis is focused on maximizing the opportunity for its two internally discovered compounds, cabozantinib and cobimetinib, to improve care and outcomes for people with cancer around the world. The company’s top priority remains the ongoing commercialization of CABOMETYX® (cabozantinib) tablets as a treatment for patients with advanced renal cell carcinoma (RCC) who have received prior anti-angiogenic therapy. During the third quarter of 2017, CABOMETYX generated $90.4 million in net product revenue, while COMETRIQ® (cabozantinib) capsules for the treatment of patients with progressive, metastatic medullary thyroid cancer generated an additional $6.1 million in net product revenue, for a combined $96.4 million in net product revenue for the cabozantinib franchise.
“In addition to strong financial performance, the third quarter of 2017 was marked by significant clinical and regulatory milestones that continue to drive us forward in our mission to help cancer patients recover stronger and live longer,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “In August, we completed the filing for CABOMETYX in previously untreated advanced RCC, which has been accepted by the FDA and granted Priority Review. With an upcoming FDA action date of February 15, 2018, our commercial team is fully prepared for a potential launch of CABOMETYX in this expanded indication to bring this much needed option to even more patients with advanced RCC as quickly as possible. In addition, based on the positive results from the CELESTIAL pivotal trial, demonstrating that cabozantinib provided a statistically significant and clinically meaningful improvement in overall survival for patients with advanced hepatocellular carcinoma, we are moving rapidly to complete our U.S. regulatory filing in the first quarter of next year.”
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArt…
Exelixis Announces Third Quarter 2017 Financial Results and Provides Corporate Update
- Cabozantinib Franchise Net Product Revenue of $96.4 million, Total Revenue of $152.5 million -
- Net Income of $81.4 million, Diluted EPS of $0.26 per Share -
- Conference Call and Webcast Today at 5:00 PM Eastern Time -
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Nov. 1, 2017-- Exelixis, Inc. (NASDAQ: EXEL) today reported financial results for the third quarter of 2017 and provided an update on progress toward fulfilling its key corporate objectives, as well as commercial and clinical development milestones.
Exelixis is focused on maximizing the opportunity for its two internally discovered compounds, cabozantinib and cobimetinib, to improve care and outcomes for people with cancer around the world. The company’s top priority remains the ongoing commercialization of CABOMETYX® (cabozantinib) tablets as a treatment for patients with advanced renal cell carcinoma (RCC) who have received prior anti-angiogenic therapy. During the third quarter of 2017, CABOMETYX generated $90.4 million in net product revenue, while COMETRIQ® (cabozantinib) capsules for the treatment of patients with progressive, metastatic medullary thyroid cancer generated an additional $6.1 million in net product revenue, for a combined $96.4 million in net product revenue for the cabozantinib franchise.
“In addition to strong financial performance, the third quarter of 2017 was marked by significant clinical and regulatory milestones that continue to drive us forward in our mission to help cancer patients recover stronger and live longer,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “In August, we completed the filing for CABOMETYX in previously untreated advanced RCC, which has been accepted by the FDA and granted Priority Review. With an upcoming FDA action date of February 15, 2018, our commercial team is fully prepared for a potential launch of CABOMETYX in this expanded indication to bring this much needed option to even more patients with advanced RCC as quickly as possible. In addition, based on the positive results from the CELESTIAL pivotal trial, demonstrating that cabozantinib provided a statistically significant and clinically meaningful improvement in overall survival for patients with advanced hepatocellular carcinoma, we are moving rapidly to complete our U.S. regulatory filing in the first quarter of next year.”
Antwort auf Beitrag Nr.: 56.080.505 von Cyberhexe am 02.11.17 11:32:30Cabozantinib ist gegenüber Everolimus in allen Aspekten (QoL, PFS, OS, TTD...) im Vorteil:
Quality of Life Outcomes for Cabozantinib Versus Everolimus in Patients With Metastatic Renal Cell Carcinoma: METEOR Phase III Randomized Trial
Purpose
In the phase III METEOR trial (ClinicalTrials.gov identifier: NCT01865747), 658 previously treated patients with advanced renal cell carcinoma were randomly assigned 1:1 to receive cabozantinib or everolimus. The cabozantinib arm had improved progression-free survival, overall survival, and objective response rate compared with everolimus. Changes in quality of life (QoL), an exploratory end point, are reported here.
Patients and Methods
Patients completed the 19-item Functional Assessment of Cancer Therapy–Kidney Symptom Index (FKSI-19) and the five-level EuroQol (EQ-5D-5L) questionnaires at baseline and throughout the study. The nine-item FKSI–Disease-Related Symptoms (FKSI-DRS), a subset of FKSI-19, was also investigated. Data were summarized descriptively and by repeated-measures analysis (for which a clinically relevant difference was an effect size ≥ 0.3). Time to deterioration (TTD) was defined as the earlier of date of death, radiographic progressive disease, or ≥ 4-point decrease from baseline in FKSI-DRS.
Results
The QoL questionnaire completion rates remained ≥ 75% through week 48 in each arm. There was no difference over time for FKSI-19 Total, FKSI-DRS, or EQ-5D data between the cabozantinib and everolimus arms. Among the individual FKSI-19 items, cabozantinib was associated with worse diarrhea and nausea; everolimus was associated with worse shortness of breath. These differences are consistent with the adverse event profile of each drug. Cabozantinib improved TTD overall, with a marked improvement in patients with bone metastases at baseline.
Conclusion
In patients with advanced renal cell carcinoma, relative to everolimus, cabozantinib generally maintained QoL to a similar extent. Compared with everolimus, cabozantinib extended TTD overall and markedly improved TTD in patients with bone metastases
Quality of Life Outcomes for Cabozantinib Versus Everolimus in Patients With Metastatic Renal Cell Carcinoma: METEOR Phase III Randomized Trial
Purpose
In the phase III METEOR trial (ClinicalTrials.gov identifier: NCT01865747), 658 previously treated patients with advanced renal cell carcinoma were randomly assigned 1:1 to receive cabozantinib or everolimus. The cabozantinib arm had improved progression-free survival, overall survival, and objective response rate compared with everolimus. Changes in quality of life (QoL), an exploratory end point, are reported here.
Patients and Methods
Patients completed the 19-item Functional Assessment of Cancer Therapy–Kidney Symptom Index (FKSI-19) and the five-level EuroQol (EQ-5D-5L) questionnaires at baseline and throughout the study. The nine-item FKSI–Disease-Related Symptoms (FKSI-DRS), a subset of FKSI-19, was also investigated. Data were summarized descriptively and by repeated-measures analysis (for which a clinically relevant difference was an effect size ≥ 0.3). Time to deterioration (TTD) was defined as the earlier of date of death, radiographic progressive disease, or ≥ 4-point decrease from baseline in FKSI-DRS.
Results
The QoL questionnaire completion rates remained ≥ 75% through week 48 in each arm. There was no difference over time for FKSI-19 Total, FKSI-DRS, or EQ-5D data between the cabozantinib and everolimus arms. Among the individual FKSI-19 items, cabozantinib was associated with worse diarrhea and nausea; everolimus was associated with worse shortness of breath. These differences are consistent with the adverse event profile of each drug. Cabozantinib improved TTD overall, with a marked improvement in patients with bone metastases at baseline.
Conclusion
In patients with advanced renal cell carcinoma, relative to everolimus, cabozantinib generally maintained QoL to a similar extent. Compared with everolimus, cabozantinib extended TTD overall and markedly improved TTD in patients with bone metastases
Antwort auf Beitrag Nr.: 56.987.123 von Cyberhexe am 09.02.18 17:50:54gutes Ergebnis mit Cabozantinib bei metastasierendem Schilddrüsenkrebs in ph2, weshalb noch in 2018 der Beginn eine Pivotalstudie geplant ist:
Exelixis Announces Results from a Phase 2 Investigator-Sponsored Trial of Cabozantinib in the First-Line Treatment of Metastatic Radioiodine-Refractory Differentiated Thyroid Carcinoma
– 54 percent objective response rate and 43 percent stable disease observed among 35 evaluable patients –
– Exelixis plans to initiate a pivotal phase 3 trial later this year –
– Results to be presented during an oral session on February 16 at the 2018 Multidisciplinary Head and Neck Cancers Symposium –
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Feb. 13, 2018-- Exelixis, Inc. (NASDAQ:EXEL) today announced results from a phase 2 investigator-sponsored trial (IST) of cabozantinib for the first-line treatment of metastatic radioiodine (RAI)-refractory differentiated thyroid carcinoma (DTC). The results were the subject of a news briefing that took place earlier today and will be presented during an oral session on February 16 starting at 1:30 p.m. MT at the 2018 Multidisciplinary Head and Neck Cancers Symposium, which is being held in Scottsdale, Arizona, February 15–17, 2018.
Patients with metastatic, RAI-refractory DTC were enrolled in this single-arm, open-label trial, and were administered oral cabozantinib 60 mg once daily. The primary endpoint of the trial is objective response rate. Among the 35 patients who were evaluable for response, partial response was achieved by 54 percent of patients (n=19), and stable disease was reported in 43 percent of patients (n=15) per RECIST 1.1. All but one evaluated patient experienced a decrease in tumor target lesions. Secondary endpoints of the trial include progression-free survival (PFS), time to progression (TTP), duration of response (DOR) and clinical benefit rate (CBR) defined as the number of patients achieving an objective response or stable disease for at least 6 months. The CBR at six months was 80 percent (n=28). With a median follow up for the study of 35 weeks the median PFS has not been reached. The median TTP among those patients who progressed was 35 weeks.
“While many patients with differentiated thyroid cancer can be treated successfully with radioiodine, there are very few options for those patients whose tumors have become resistant to treatment,” said Marcia Brose, M.D., Ph.D., Associate Professor of Otorhinolaryngology: Head and Neck Surgery and Director of the Center for Rare Cancers at the Abramson Cancer Center of the University of Pennsylvania, and principal investigator of the trial. “These findings suggest that cabozantinib, which showed encouraging efficacy and a manageable safety profile in this phase 2 trial, may be a promising treatment option for this patient population and warrants further evaluation.”
Exelixis Announces Results from a Phase 2 Investigator-Sponsored Trial of Cabozantinib in the First-Line Treatment of Metastatic Radioiodine-Refractory Differentiated Thyroid Carcinoma
– 54 percent objective response rate and 43 percent stable disease observed among 35 evaluable patients –
– Exelixis plans to initiate a pivotal phase 3 trial later this year –
– Results to be presented during an oral session on February 16 at the 2018 Multidisciplinary Head and Neck Cancers Symposium –
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Feb. 13, 2018-- Exelixis, Inc. (NASDAQ:EXEL) today announced results from a phase 2 investigator-sponsored trial (IST) of cabozantinib for the first-line treatment of metastatic radioiodine (RAI)-refractory differentiated thyroid carcinoma (DTC). The results were the subject of a news briefing that took place earlier today and will be presented during an oral session on February 16 starting at 1:30 p.m. MT at the 2018 Multidisciplinary Head and Neck Cancers Symposium, which is being held in Scottsdale, Arizona, February 15–17, 2018.
Patients with metastatic, RAI-refractory DTC were enrolled in this single-arm, open-label trial, and were administered oral cabozantinib 60 mg once daily. The primary endpoint of the trial is objective response rate. Among the 35 patients who were evaluable for response, partial response was achieved by 54 percent of patients (n=19), and stable disease was reported in 43 percent of patients (n=15) per RECIST 1.1. All but one evaluated patient experienced a decrease in tumor target lesions. Secondary endpoints of the trial include progression-free survival (PFS), time to progression (TTP), duration of response (DOR) and clinical benefit rate (CBR) defined as the number of patients achieving an objective response or stable disease for at least 6 months. The CBR at six months was 80 percent (n=28). With a median follow up for the study of 35 weeks the median PFS has not been reached. The median TTP among those patients who progressed was 35 weeks.
“While many patients with differentiated thyroid cancer can be treated successfully with radioiodine, there are very few options for those patients whose tumors have become resistant to treatment,” said Marcia Brose, M.D., Ph.D., Associate Professor of Otorhinolaryngology: Head and Neck Surgery and Director of the Center for Rare Cancers at the Abramson Cancer Center of the University of Pennsylvania, and principal investigator of the trial. “These findings suggest that cabozantinib, which showed encouraging efficacy and a manageable safety profile in this phase 2 trial, may be a promising treatment option for this patient population and warrants further evaluation.”
Antwort auf Beitrag Nr.: 57.024.528 von Cyberhexe am 14.02.18 16:19:24zukünftige Konkurrenz von Cabozantinib bei mRCC:
Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial
http://www.thelancet.com/journals/lanonc/article/PIIS1470-20…
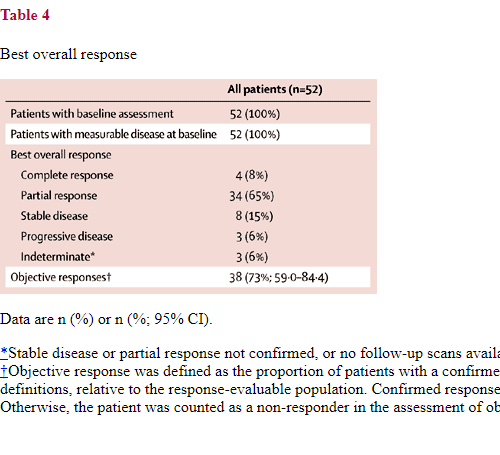
Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial
http://www.thelancet.com/journals/lanonc/article/PIIS1470-20…

Antwort auf Beitrag Nr.: 57.027.216 von Cyberhexe am 14.02.18 20:15:38Cabozantinib Proves Superior to Sunitinib in All Subgroups Examined in CABOSUN
Wayne Kuznar
Published: Monday, Feb 12, 2018
Print Button
http://www.onclive.com/web-exclusives/cabozantinib-proves-su…
Anbei ein interessanter Tweet von City of Hope Prof. Sumanta Kumar Pal, M.D.,
https://www.cityofhope.org/people/pal-sumanta
einem der renommiertesten US-Onko-/Urologen, in welchem er die Verwendung von Cabozantinib auch bei mRCC-Patienten mit günstigerer Prognose nicht ausschliesst bzw. nahezu gutheisst:
Wayne Kuznar
Published: Monday, Feb 12, 2018
Print Button
http://www.onclive.com/web-exclusives/cabozantinib-proves-su…
Anbei ein interessanter Tweet von City of Hope Prof. Sumanta Kumar Pal, M.D.,
https://www.cityofhope.org/people/pal-sumanta
einem der renommiertesten US-Onko-/Urologen, in welchem er die Verwendung von Cabozantinib auch bei mRCC-Patienten mit günstigerer Prognose nicht ausschliesst bzw. nahezu gutheisst:

"Wunderwaffe" Cabozantinib scheint auch bei Schiklddrüsenkrebs Aussergewöhnliches zu bewirken:
Cabozantinib Shows Promise as First Line Treatment for Differentiated Thyroid Cancer
February 13, 2018
Marcia Brose
Marcia S. Brose, MD PhD
PHILADELPHIA – A kinase inhibitor called cabozantinib could be a viable therapy option for patients with metastatic, radioactive iodine-resistant thyroid cancer. In a trial initiated and led by the Abramson Cancer Center and the Perelman School of Medicine at the University of Pennsylvania, tumors shrunk in 34 out of 35 patients who took the drug, and more than half of those patients saw the tumor size decrease by more than 30 percent. Researchers will present their findings at the 2018 Multidisciplinary Head and Neck Cancers Symposium in Scottsdale, Arizona this Friday.
https://www.pennmedicine.org/news/news-releases/2018/februar…
Cabozantinib Shows Promise as First Line Treatment for Differentiated Thyroid Cancer
February 13, 2018
Marcia Brose
Marcia S. Brose, MD PhD
PHILADELPHIA – A kinase inhibitor called cabozantinib could be a viable therapy option for patients with metastatic, radioactive iodine-resistant thyroid cancer. In a trial initiated and led by the Abramson Cancer Center and the Perelman School of Medicine at the University of Pennsylvania, tumors shrunk in 34 out of 35 patients who took the drug, and more than half of those patients saw the tumor size decrease by more than 30 percent. Researchers will present their findings at the 2018 Multidisciplinary Head and Neck Cancers Symposium in Scottsdale, Arizona this Friday.
https://www.pennmedicine.org/news/news-releases/2018/februar…
Antwort auf Beitrag Nr.: 57.086.217 von Cyberhexe am 21.02.18 23:39:43Cabozantinib nun auch bald bei Leberkrebs:
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArt…
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArt…
Antwort auf Beitrag Nr.: 57.422.447 von Cyberhexe am 29.03.18 13:07:04Axitinib ph3-Studie zur Behandlung von wiederkehrendem Nierenkrebs wird wegen fehlender Wirksamkeit abgebrochen. Schlecht für die Patienten, gut für EXELIXIS!
NEW YORK--(BUSINESS WIRE)--Pfizer Inc. today announced that the independent Data Monitoring Committee for the Phase 3 ATLAS trial evaluating INLYTA® (axitinib) as adjuvant therapy for patients at high risk of recurrent renal cell carcinoma (RCC) after nephrectomy recommended stopping the trial at a planned interim analysis due to futility. The recommendation was based on the study failing to demonstrate a clear improvement in the primary endpoint of extending disease-free survival (DFS) for patients treated with INLYTA compared with patients treated with placebo. No new safety signals were observed, and the safety profile was consistent with the known profile of INLYTA in advanced RCC.
“Forward-Looking Information and Factors That May Affect Future Results”
Tweet this
“We are disappointed by the outcome of this study as we had hoped the efficacy that INLYTA has demonstrated as a second-line treatment in patients with advanced renal cell carcinoma would carry over to patients with earlier stage disease, where it would delay or prevent disease relapse. That goal was not achieved. We will conduct additional analyses on the data that may provide insight into this result. Studies evaluating INLYTA in combination with immune checkpoint inhibitors for patients with a variety of advanced stage cancers, including RCC, will continue,” said Mace Rothenberg, M.D., Chief Development Officer, Oncology, Pfizer Global Product Development.
NEW YORK--(BUSINESS WIRE)--Pfizer Inc. today announced that the independent Data Monitoring Committee for the Phase 3 ATLAS trial evaluating INLYTA® (axitinib) as adjuvant therapy for patients at high risk of recurrent renal cell carcinoma (RCC) after nephrectomy recommended stopping the trial at a planned interim analysis due to futility. The recommendation was based on the study failing to demonstrate a clear improvement in the primary endpoint of extending disease-free survival (DFS) for patients treated with INLYTA compared with patients treated with placebo. No new safety signals were observed, and the safety profile was consistent with the known profile of INLYTA in advanced RCC.
“Forward-Looking Information and Factors That May Affect Future Results”
Tweet this
“We are disappointed by the outcome of this study as we had hoped the efficacy that INLYTA has demonstrated as a second-line treatment in patients with advanced renal cell carcinoma would carry over to patients with earlier stage disease, where it would delay or prevent disease relapse. That goal was not achieved. We will conduct additional analyses on the data that may provide insight into this result. Studies evaluating INLYTA in combination with immune checkpoint inhibitors for patients with a variety of advanced stage cancers, including RCC, will continue,” said Mace Rothenberg, M.D., Chief Development Officer, Oncology, Pfizer Global Product Development.
Antwort auf Beitrag Nr.: 57.524.582 von Cyberhexe am 12.04.18 09:56:29sorry, hab den Hyperlink vergessen:
https://investors.pfizer.com/investor-news/press-release-det…
https://investors.pfizer.com/investor-news/press-release-det…
Antwort auf Beitrag Nr.: 57.524.603 von Cyberhexe am 12.04.18 09:59:10Die Daten zu Cabozantinib bei RCC und deren Publikation sind sehr gut:
https://www.onclive.com/onclive-tv/dr-wei-on-the-use-of-cabo…
Dr. Wei on the Use of Cabozantinib in Advanced Kidney Cancer
Xiao X. Wei, MD
Published: Thursday, Apr 05, 2018
Xiao X. Wei, MD, MAS, instructor of medicine, Harvard Medical School, Dana-Farber Cancer Institute, discusses the side effect profile of cabozantinib (Cabometyx) in advanced kidney cancer.
Cabozantinib has a relatively similar side effect profile compared with the other tyrosine kinase inhibitors like sunitinib (Sutent) and pazopanib (Votrient). Data from the CABOSUN study compared cabozantinib with sunitinib, so physicians have an idea of how the 2 agents are side by side. The randomized phase II trial of 157 patients compared the 2 agents on the regular 4/2 dose schedule. The primary endpoint of investigator assessment response was met at 8.2 months versus 5.6 months respectively. The overall survival data, at a median follow-up of 20 months or more, favored cabozantinib.
https://www.onclive.com/onclive-tv/dr-wei-on-the-use-of-cabo…
Dr. Wei on the Use of Cabozantinib in Advanced Kidney Cancer
Xiao X. Wei, MD
Published: Thursday, Apr 05, 2018
Xiao X. Wei, MD, MAS, instructor of medicine, Harvard Medical School, Dana-Farber Cancer Institute, discusses the side effect profile of cabozantinib (Cabometyx) in advanced kidney cancer.
Cabozantinib has a relatively similar side effect profile compared with the other tyrosine kinase inhibitors like sunitinib (Sutent) and pazopanib (Votrient). Data from the CABOSUN study compared cabozantinib with sunitinib, so physicians have an idea of how the 2 agents are side by side. The randomized phase II trial of 157 patients compared the 2 agents on the regular 4/2 dose schedule. The primary endpoint of investigator assessment response was met at 8.2 months versus 5.6 months respectively. The overall survival data, at a median follow-up of 20 months or more, favored cabozantinib.
Antwort auf Beitrag Nr.: 57.524.732 von Cyberhexe am 12.04.18 10:08:45
...es geht was bei der Indikation "WNierenkrebs":
FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma
https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/u…
...es geht was bei der Indikation "WNierenkrebs":
FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma
https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/u…
Antwort auf Beitrag Nr.: 57.560.403 von Cyberhexe am 17.04.18 09:20:18
Ira Mellman präsentiert an der AACR2018 einen MEK-Effekt (Cobimetinib) in ph1 bei der Infiltration von Immunzellen in Tumorgewebe:
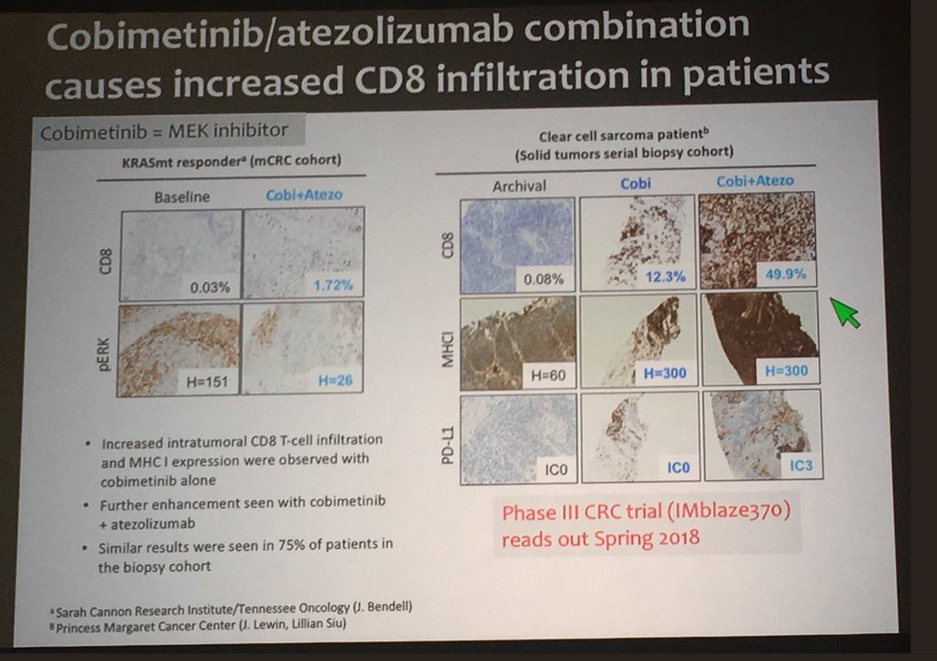
Ira Mellman präsentiert an der AACR2018 einen MEK-Effekt (Cobimetinib) in ph1 bei der Infiltration von Immunzellen in Tumorgewebe:

ASCO2018 und Exelixis ist mit 15 Präsentationen zu Cabo dabei:
Cabozantinib to Be Featured in 15 Presentations at ASCO 2018 Annual Meeting
– Cabozantinib data in a range of tumor types, including advanced hepatocellular carcinoma and advanced renal cell carcinoma, to be presented at ASCO –
– Follow-up data from the phase 1 trial of cabozantinib in combination with nivolumab with or without ipilimumab in metastatic genitourinary cancers to be highlighted –
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Apr. 26, 2018-- Exelixis, Inc. (NASDAQ:EXEL) today announced that data from clinical trials of cabozantinib will be the subject of 15 presentations at the American Society of Clinical Oncology (ASCO) 2018 Annual Meeting in Chicago, June 1-5, 2018.
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArt…
Cabozantinib to Be Featured in 15 Presentations at ASCO 2018 Annual Meeting
– Cabozantinib data in a range of tumor types, including advanced hepatocellular carcinoma and advanced renal cell carcinoma, to be presented at ASCO –
– Follow-up data from the phase 1 trial of cabozantinib in combination with nivolumab with or without ipilimumab in metastatic genitourinary cancers to be highlighted –
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Apr. 26, 2018-- Exelixis, Inc. (NASDAQ:EXEL) today announced that data from clinical trials of cabozantinib will be the subject of 15 presentations at the American Society of Clinical Oncology (ASCO) 2018 Annual Meeting in Chicago, June 1-5, 2018.
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArt…
Antwort auf Beitrag Nr.: 57.636.939 von Cyberhexe am 26.04.18 17:17:02und dazu noch den Jahresbericht 2017:
https://www.exelixis.com/wp-content/uploads/2018/04/2017-Exe…
https://www.exelixis.com/wp-content/uploads/2018/04/2017-Exe…
Antwort auf Beitrag Nr.: 57.637.215 von Cyberhexe am 26.04.18 17:34:23
Exelixis Announces First Quarter 2018 Financial Results and Provides Corporate Update
- Total Revenues of $212.3 million -
- Cabozantinib Franchise Net Product Revenues of $134.3 million -
- Net Income of $115.9 million, Diluted EPS of $0.37 -
- Conference Call and Webcast Today at 5:00 PM Eastern Time -
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--May 2, 2018-- Exelixis, Inc. (Nasdaq: EXEL) today reported financial results for the first quarter of 2018 and provided an update on progress toward fulfilling its key corporate objectives, as well as commercial and clinical development milestones.
“In the first quarter of 2018, Exelixis continued to make significant progress in the ongoing commercialization of CABOMETYX® (cabozantinib) for advanced renal cell carcinoma. Following FDA approval for its expanded indication in advanced first-line renal cell carcinoma, our team immediately began promoting CABOMETYX across all lines of therapy for this patient population, resulting in further uptake from prescribers at both major academic institutions and in the community setting,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “The resulting growth in U.S. sales, as well as the increasing collaboration revenues from our various partners, were important contributors to our strong financial performance during the quarter, leading to net income of $115.9 million or $0.37 per share on a fully diluted basis.”
Dr. Morrissey continued: “From its initial approval for a rare disease indication five years ago, cabozantinib has grown to become an oncology franchise with the potential for global impact. We and our collaboration partners are committed to maximizing its opportunity to help patients across multiple tumor types. This now includes our recent regulatory submissions for previously treated advanced hepatocellular carcinoma, an aggressive cancer with worldwide relevance. Our efforts in liver cancer, as well as our plans to start additional phase 3 trials in other forms of cancer later this year, are each reflective of the Exelixis corporate mission to help patients with cancer recover stronger and live longer.”
First Quarter 2018 Financial Results
Total revenues for the quarter ended March 31, 2018 were $212.3 million, compared to $80.9 million for the comparable period in 2017.
Total revenues include net product revenues of $134.3 million for the quarter ended March 31, 2018, compared to $68.9 million for the comparable period in 2017. The increase in net product revenues reflects the growth of our second and later-line advanced renal cell carcinoma (RCC) business and the impact of additional sales following the U.S. Food and Drug Administration’s (FDA) approval in December 2017 of the expanded indication for CABOMETYX, which now encompass all patients with advanced RCC.
Total revenues also include collaboration revenues of $78.1 million for the quarter ended March 31, 2018 compared to $12.0 million for the comparable period in 2017. The increase in collaboration revenues for the quarter ended March 31, 2018 was primarily the result of recording $45.8 million in revenue for a $50.0 million milestone from Ipsen Pharma SAS (Ipsen) we expect to earn in the second quarter of 2018 for the approval of cabozantinib for the first-line treatment of advanced RCC by the European Commission (EC). The determination to recognize the $45.8 million in revenue was made following the Committee for Medicinal Products for Human Use’s (CHMP) positive opinion of cabozantinib for the first-line treatment of advanced RCC. The increase in collaboration revenues was also a result of a $20.0 million milestone from our collaboration partner Daiichi Sankyo Company, Limited (Daiichi Sankyo), which was earned as a result of Daiichi Sankyo’s submission of a regulatory application to the Japanese Pharmaceutical and Medical Devices Agency for esaxerenone (CS-3150) as a treatment for patients with essential hypertension. These increases were partially offset by a decrease in the recognition of deferred revenue due to our adoption of Accounting Standards Update No. 2014-09 Revenue from Contracts with Customers (Accounting Standards Codification Topic 606) on January 1, 2018. As a result, $258.5 million was recorded in stockholders’ equity relating primarily to a reduction in the remaining unrecognized upfront and non-substantive milestone payments that had been received from our collaboration partners and was included in deferred revenue at December 31, 2017. For more information on our adoption of the new revenue standard, see “Note 1. Organization and Summary of Significant Accounting Policies - Revenue” contained in Part I, Item 1 of Exelixis’ Quarterly Report on Form 10-Q expected to be filed with the Securities and Exchange Commission (SEC) on May 2, 2018.
Research and development expenses for the quarter ended March 31, 2018 were $37.8 million, compared to $23.2 million for the comparable period in 2017. The increase in research and development expenses was primarily related to an increase in personnel-related expenses resulting from an increase in headcount in support of our development and discovery efforts and an increase in clinical trial costs. Clinical trial costs increased primarily due to start-up costs associated with CheckMate 9ER, an ongoing phase 3 pivotal trial of cabozantinib plus immunotherapy in patients with previously untreated RCC that is being conducted with Bristol-Myers Squibb Company, and start-up costs associated with our phase 1b trial of cabozantinib and atezolizumab in locally advanced or metastatic solid tumors; those increases were partially offset by decreases in costs related to METEOR, our completed phase 3 pivotal trial comparing CABOMETYX to everolimus in patients with advanced RCC. Research and development expenses for the quarter ended March 31, 2018 also included a $3.0 million upfront payment for our exclusive collaboration and license agreement with StemSynergy Therapeutics, Inc. (StemSynergy).
Selling, general and administrative expenses for the quarter ended March 31, 2018 were $52.6 million, compared to $34.3 million for the comparable period in 2017. The increase in selling, general and administrative expenses was primarily a result of increases in corporate giving, personnel expenses and marketing activities. The increase in personnel expense resulted from an increase in general and administrative headcount to support the company’s commercial and research and development organizations.
Net income for the quarter ended March 31, 2018 was $115.9 million, or $0.39 per share, basic and $0.37 per share, diluted, compared to a $16.7 million, or $0.06 per share, basic and $0.05 per share diluted, for the comparable period in 2017. The increase in net income was primarily the result of increases in net product revenues and collaboration revenues, which was partially offset by the increases in research and development and selling, general and administrative expenses.
Cash and cash equivalents, short- and long-term investments and short- and long-term restricted cash and investments totaled $525.6 million at March 31, 2018, as compared to $457.2 million at December 31, 2017.
2018 Financial Guidance
The company is maintaining its guidance that total costs and operating expenses for the full year will be between $430 million and $460 million. This guidance includes approximately $50 million of non-cash costs and expenses related primarily to stock-based compensation expense.
Exelixis Announces First Quarter 2018 Financial Results and Provides Corporate Update
- Total Revenues of $212.3 million -
- Cabozantinib Franchise Net Product Revenues of $134.3 million -
- Net Income of $115.9 million, Diluted EPS of $0.37 -
- Conference Call and Webcast Today at 5:00 PM Eastern Time -
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--May 2, 2018-- Exelixis, Inc. (Nasdaq: EXEL) today reported financial results for the first quarter of 2018 and provided an update on progress toward fulfilling its key corporate objectives, as well as commercial and clinical development milestones.
“In the first quarter of 2018, Exelixis continued to make significant progress in the ongoing commercialization of CABOMETYX® (cabozantinib) for advanced renal cell carcinoma. Following FDA approval for its expanded indication in advanced first-line renal cell carcinoma, our team immediately began promoting CABOMETYX across all lines of therapy for this patient population, resulting in further uptake from prescribers at both major academic institutions and in the community setting,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “The resulting growth in U.S. sales, as well as the increasing collaboration revenues from our various partners, were important contributors to our strong financial performance during the quarter, leading to net income of $115.9 million or $0.37 per share on a fully diluted basis.”
Dr. Morrissey continued: “From its initial approval for a rare disease indication five years ago, cabozantinib has grown to become an oncology franchise with the potential for global impact. We and our collaboration partners are committed to maximizing its opportunity to help patients across multiple tumor types. This now includes our recent regulatory submissions for previously treated advanced hepatocellular carcinoma, an aggressive cancer with worldwide relevance. Our efforts in liver cancer, as well as our plans to start additional phase 3 trials in other forms of cancer later this year, are each reflective of the Exelixis corporate mission to help patients with cancer recover stronger and live longer.”
First Quarter 2018 Financial Results
Total revenues for the quarter ended March 31, 2018 were $212.3 million, compared to $80.9 million for the comparable period in 2017.
Total revenues include net product revenues of $134.3 million for the quarter ended March 31, 2018, compared to $68.9 million for the comparable period in 2017. The increase in net product revenues reflects the growth of our second and later-line advanced renal cell carcinoma (RCC) business and the impact of additional sales following the U.S. Food and Drug Administration’s (FDA) approval in December 2017 of the expanded indication for CABOMETYX, which now encompass all patients with advanced RCC.
Total revenues also include collaboration revenues of $78.1 million for the quarter ended March 31, 2018 compared to $12.0 million for the comparable period in 2017. The increase in collaboration revenues for the quarter ended March 31, 2018 was primarily the result of recording $45.8 million in revenue for a $50.0 million milestone from Ipsen Pharma SAS (Ipsen) we expect to earn in the second quarter of 2018 for the approval of cabozantinib for the first-line treatment of advanced RCC by the European Commission (EC). The determination to recognize the $45.8 million in revenue was made following the Committee for Medicinal Products for Human Use’s (CHMP) positive opinion of cabozantinib for the first-line treatment of advanced RCC. The increase in collaboration revenues was also a result of a $20.0 million milestone from our collaboration partner Daiichi Sankyo Company, Limited (Daiichi Sankyo), which was earned as a result of Daiichi Sankyo’s submission of a regulatory application to the Japanese Pharmaceutical and Medical Devices Agency for esaxerenone (CS-3150) as a treatment for patients with essential hypertension. These increases were partially offset by a decrease in the recognition of deferred revenue due to our adoption of Accounting Standards Update No. 2014-09 Revenue from Contracts with Customers (Accounting Standards Codification Topic 606) on January 1, 2018. As a result, $258.5 million was recorded in stockholders’ equity relating primarily to a reduction in the remaining unrecognized upfront and non-substantive milestone payments that had been received from our collaboration partners and was included in deferred revenue at December 31, 2017. For more information on our adoption of the new revenue standard, see “Note 1. Organization and Summary of Significant Accounting Policies - Revenue” contained in Part I, Item 1 of Exelixis’ Quarterly Report on Form 10-Q expected to be filed with the Securities and Exchange Commission (SEC) on May 2, 2018.
Research and development expenses for the quarter ended March 31, 2018 were $37.8 million, compared to $23.2 million for the comparable period in 2017. The increase in research and development expenses was primarily related to an increase in personnel-related expenses resulting from an increase in headcount in support of our development and discovery efforts and an increase in clinical trial costs. Clinical trial costs increased primarily due to start-up costs associated with CheckMate 9ER, an ongoing phase 3 pivotal trial of cabozantinib plus immunotherapy in patients with previously untreated RCC that is being conducted with Bristol-Myers Squibb Company, and start-up costs associated with our phase 1b trial of cabozantinib and atezolizumab in locally advanced or metastatic solid tumors; those increases were partially offset by decreases in costs related to METEOR, our completed phase 3 pivotal trial comparing CABOMETYX to everolimus in patients with advanced RCC. Research and development expenses for the quarter ended March 31, 2018 also included a $3.0 million upfront payment for our exclusive collaboration and license agreement with StemSynergy Therapeutics, Inc. (StemSynergy).
Selling, general and administrative expenses for the quarter ended March 31, 2018 were $52.6 million, compared to $34.3 million for the comparable period in 2017. The increase in selling, general and administrative expenses was primarily a result of increases in corporate giving, personnel expenses and marketing activities. The increase in personnel expense resulted from an increase in general and administrative headcount to support the company’s commercial and research and development organizations.
Net income for the quarter ended March 31, 2018 was $115.9 million, or $0.39 per share, basic and $0.37 per share, diluted, compared to a $16.7 million, or $0.06 per share, basic and $0.05 per share diluted, for the comparable period in 2017. The increase in net income was primarily the result of increases in net product revenues and collaboration revenues, which was partially offset by the increases in research and development and selling, general and administrative expenses.
Cash and cash equivalents, short- and long-term investments and short- and long-term restricted cash and investments totaled $525.6 million at March 31, 2018, as compared to $457.2 million at December 31, 2017.
2018 Financial Guidance
The company is maintaining its guidance that total costs and operating expenses for the full year will be between $430 million and $460 million. This guidance includes approximately $50 million of non-cash costs and expenses related primarily to stock-based compensation expense.
Analysts at Cantor Fitzgerald Reiterate their “Buy” rating for Arena Pharma (ARNA) with $65 PT; Orchid Island Capital (ORC) Sentiment Is 1.31
May 9, 2018 - By Peter Erickson
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) Logo
Analysts at Cantor Fitzgerald have $65.0000 TP on Arena Pharma (NASDAQ:ARNA). Cantor Fitzgerald’s TP indicates a potential upside of 54.69% from the company’s last stock close. The rating was disclosed in analysts note on 9 May.
https://t.co/9WBuOEbSHi
Es gibt Teilnehmer, die biegen sich ihre eigene Wahrheit und, das Kuriose daran, retrospektive Korrekturen werden ignorant ausgeblendet. In amerikanischen Foren wie zB InvestorVillage wurde diese Vorgehensweise relativ schnell durchschaut. Die Reputation des Teilnehmers ging gegen Null, so dass erfreulicherweise auch die Null-Format-Beiträge in der Häufigkeit auf Null zurückgegangen sind.
Zitiert aus dem Thread "Exelixis...ein schlafender Riese? USD 3.40 am 14.5.2014
Am 11.11.15 schrieb der Teilnehmer ville7 um 09:45:33 im Beitrag Nr. 399 (51.059.685)
"Im Übrigen stehe ich der Firma weiterhin sehr kritisch gegenüber, da sie schon oft gezeigt hat, dass sie mit ihren Finanzierungsrunden und -arten und eigenen Optionszuteilungen über die Leichen der Aktionäre geht ohne eine Wimper zu zucken. Never fall in love mit einem Produkt (Cobi) oder einer Firma (EXEL). Schaut so aus als hättest du noch das ein oder andere zu lernen"."
May 9, 2018 - By Peter Erickson
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) Logo
Analysts at Cantor Fitzgerald have $65.0000 TP on Arena Pharma (NASDAQ:ARNA). Cantor Fitzgerald’s TP indicates a potential upside of 54.69% from the company’s last stock close. The rating was disclosed in analysts note on 9 May.
https://t.co/9WBuOEbSHi
Es gibt Teilnehmer, die biegen sich ihre eigene Wahrheit und, das Kuriose daran, retrospektive Korrekturen werden ignorant ausgeblendet. In amerikanischen Foren wie zB InvestorVillage wurde diese Vorgehensweise relativ schnell durchschaut. Die Reputation des Teilnehmers ging gegen Null, so dass erfreulicherweise auch die Null-Format-Beiträge in der Häufigkeit auf Null zurückgegangen sind.
Zitiert aus dem Thread "Exelixis...ein schlafender Riese? USD 3.40 am 14.5.2014
Am 11.11.15 schrieb der Teilnehmer ville7 um 09:45:33 im Beitrag Nr. 399 (51.059.685)
"Im Übrigen stehe ich der Firma weiterhin sehr kritisch gegenüber, da sie schon oft gezeigt hat, dass sie mit ihren Finanzierungsrunden und -arten und eigenen Optionszuteilungen über die Leichen der Aktionäre geht ohne eine Wimper zu zucken. Never fall in love mit einem Produkt (Cobi) oder einer Firma (EXEL). Schaut so aus als hättest du noch das ein oder andere zu lernen"."
Antwort auf Beitrag Nr.: 57.733.885 von Cyberhexe am 10.05.18 13:55:57
Cabozantinibs Presse ist hervorragend:
https://www.kcsn.org.uk/cabozantinib-significantly-improves-…
Cabozantinib significantly improves overall survival in people with previously treated advanced kidney cancer
May 10, 2018
Cabozantinib significantly improves overall survival in previously treated patients with advanced renal cell carcinoma (RCC) compared to everolimus, with consistent results after long-term follow-up.
Results from a second interim analysis of the phase 3 METEOR trial have been published in the British Journal of Cancer, showing cabozantinib significantly improved overall survival compared with everolimus in patients with advanced RCC after prior treatment with a vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor (TKI).
In the first interim analysis from the phase 3 METEOR trial, cabozantinib significantly improved progression-free survival, overall survival, and objective response rate compared with everolimus in previously treated patients with advanced RCC.
In the trial, 658 patients with advanced RCC who had received at least one prior VEGF receptor TKI were randomised to receive either cabozantinib or everolimus. The patients were followed to collect overall survival data.
Median overall survival was 21.4 months with cabozantinib and 17.1 months with everolimus. Safety profiles of cabozantinib and everolimus were consistent with those reported previously.
Cabozantinibs Presse ist hervorragend:
https://www.kcsn.org.uk/cabozantinib-significantly-improves-…
Cabozantinib significantly improves overall survival in people with previously treated advanced kidney cancer
May 10, 2018
Cabozantinib significantly improves overall survival in previously treated patients with advanced renal cell carcinoma (RCC) compared to everolimus, with consistent results after long-term follow-up.
Results from a second interim analysis of the phase 3 METEOR trial have been published in the British Journal of Cancer, showing cabozantinib significantly improved overall survival compared with everolimus in patients with advanced RCC after prior treatment with a vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor (TKI).
In the first interim analysis from the phase 3 METEOR trial, cabozantinib significantly improved progression-free survival, overall survival, and objective response rate compared with everolimus in previously treated patients with advanced RCC.
In the trial, 658 patients with advanced RCC who had received at least one prior VEGF receptor TKI were randomised to receive either cabozantinib or everolimus. The patients were followed to collect overall survival data.
Median overall survival was 21.4 months with cabozantinib and 17.1 months with everolimus. Safety profiles of cabozantinib and everolimus were consistent with those reported previously.
Warum "kackt der Kurs" so ab, ./. 11 %?
Antwort auf Beitrag Nr.: 57.735.937 von Magnetfeldfredy am 10.05.18 18:55:10
Kombistudie Cobi/Atezolizumab hat primären Endpunkt nicht erreicht ;-(
Exelixis Provides Update on IMblaze370 Phase 3 Pivotal Trial of Atezolizumab and Cobimetinib in Patients With Heavily Pretreated Locally Advanced or Metastatic Colorectal Cancer
– Study did not meet its primary endpoint of improving overall survival versus regorafenib –
– Results will be submitted for presentation at an upcoming medical meeting –
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--May 10, 2018-- Exelixis, Inc. (Nasdaq:EXEL) today announced that IMblaze370, the phase 3 pivotal trial of atezolizumab (TECENTRIQ®), an anti-PDL1 antibody discovered and developed by Genentech, a member of the Roche Group, and cobimetinib (COTELLIC®), an Exelixis-discovered MEK inhibitor, did not meet its primary endpoint. Genentech, Exelixis’ collaborator and sponsor of the IMblaze370 trial, informed the company that the combination of atezolizumab and cobimetinib did not deliver an improvement in overall survival (OS) versus regorafenib. The IMblaze370 trial evaluated the combination in patients with difficult-to-treat, locally advanced or metastatic colorectal cancer (CRC) whose disease had progressed or who were intolerant to at least two systemic chemotherapy regimens.
The safety profile for the combination appeared consistent with the known safety profile of each individual medicine, and no new safety signals were identified with the combination. Genentech will further examine results from IMblaze370 and plans to present the data at an upcoming medical meeting.
“We are disappointed that the IMblaze370 trial did not reach a positive conclusion,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “Metastatic colorectal cancer is an aggressive and difficult-to-treat disease, and patients and clinicians would be well served by additional treatment options. We will continue to work with Genentech on the evaluation of cobimetinib’s potential in other tumor types, including in melanoma, in which there are two ongoing phase 3 pivotal trials. Separately, Exelixis remains focused on and committed to maximizing the potential of the cabozantinib franchise through our commercial activities and ongoing clinical development program evaluating the compound alone, or in combination with immune checkpoint inhibitors, across numerous tumor types.”
Other ongoing late-stage clinical trials of cobimetinib include: IMspire150 TRILOGY, a fully enrolled study of cobimetinib, vemurafenib, and atezolizumab in previously untreated patients with BRAF V600-positive metastatic melanoma; and IMspire170, which is evaluating cobimetinib and atezolizumab in BRAF V600-wild type metastatic melanoma.
About the IMblaze370 Phase 3 Pivotal Trial
In early June 2016, shortly before the initial presentation of data from the phase 1b clinical trial of cobimetinib and atezolizumab at the 2016 Annual Meeting of the American Society of Clinical Oncology, Genentech initiated IMblaze370, a phase 3 pivotal trial of cobimetinib plus atezolizumab and atezolizumab monotherapy versus regorafenib in patients with locally advanced or metastatic CRC who had received at least two prior regimens of chemotherapy. The trial enrolled 363 patients who were randomized 2:1:1 to receive cobimetinib plus atezolizumab, atezolizumab alone, or regorafenib.
Zitat von Magnetfeldfredy: Warum "kackt der Kurs" so ab, ./. 11 %?
Kombistudie Cobi/Atezolizumab hat primären Endpunkt nicht erreicht ;-(
Exelixis Provides Update on IMblaze370 Phase 3 Pivotal Trial of Atezolizumab and Cobimetinib in Patients With Heavily Pretreated Locally Advanced or Metastatic Colorectal Cancer
– Study did not meet its primary endpoint of improving overall survival versus regorafenib –
– Results will be submitted for presentation at an upcoming medical meeting –
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--May 10, 2018-- Exelixis, Inc. (Nasdaq:EXEL) today announced that IMblaze370, the phase 3 pivotal trial of atezolizumab (TECENTRIQ®), an anti-PDL1 antibody discovered and developed by Genentech, a member of the Roche Group, and cobimetinib (COTELLIC®), an Exelixis-discovered MEK inhibitor, did not meet its primary endpoint. Genentech, Exelixis’ collaborator and sponsor of the IMblaze370 trial, informed the company that the combination of atezolizumab and cobimetinib did not deliver an improvement in overall survival (OS) versus regorafenib. The IMblaze370 trial evaluated the combination in patients with difficult-to-treat, locally advanced or metastatic colorectal cancer (CRC) whose disease had progressed or who were intolerant to at least two systemic chemotherapy regimens.
The safety profile for the combination appeared consistent with the known safety profile of each individual medicine, and no new safety signals were identified with the combination. Genentech will further examine results from IMblaze370 and plans to present the data at an upcoming medical meeting.
“We are disappointed that the IMblaze370 trial did not reach a positive conclusion,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “Metastatic colorectal cancer is an aggressive and difficult-to-treat disease, and patients and clinicians would be well served by additional treatment options. We will continue to work with Genentech on the evaluation of cobimetinib’s potential in other tumor types, including in melanoma, in which there are two ongoing phase 3 pivotal trials. Separately, Exelixis remains focused on and committed to maximizing the potential of the cabozantinib franchise through our commercial activities and ongoing clinical development program evaluating the compound alone, or in combination with immune checkpoint inhibitors, across numerous tumor types.”
Other ongoing late-stage clinical trials of cobimetinib include: IMspire150 TRILOGY, a fully enrolled study of cobimetinib, vemurafenib, and atezolizumab in previously untreated patients with BRAF V600-positive metastatic melanoma; and IMspire170, which is evaluating cobimetinib and atezolizumab in BRAF V600-wild type metastatic melanoma.
About the IMblaze370 Phase 3 Pivotal Trial
In early June 2016, shortly before the initial presentation of data from the phase 1b clinical trial of cobimetinib and atezolizumab at the 2016 Annual Meeting of the American Society of Clinical Oncology, Genentech initiated IMblaze370, a phase 3 pivotal trial of cobimetinib plus atezolizumab and atezolizumab monotherapy versus regorafenib in patients with locally advanced or metastatic CRC who had received at least two prior regimens of chemotherapy. The trial enrolled 363 patients who were randomized 2:1:1 to receive cobimetinib plus atezolizumab, atezolizumab alone, or regorafenib.
Danke, Übertreibung nach unten?
Antwort auf Beitrag Nr.: 57.738.973 von Magnetfeldfredy am 11.05.18 10:09:14Selber nachdenkenund nicht blind followen.
User Cyberhexe hat leider einen unglücklichen Trackrecord: MACK, TTPH, RDUS, zuvor dafür 80% seiner/ihrer EXEL zu 6USD vor dem run up verkauft.
User Cyberhexe hat leider einen unglücklichen Trackrecord: MACK, TTPH, RDUS, zuvor dafür 80% seiner/ihrer EXEL zu 6USD vor dem run up verkauft.
Antwort auf Beitrag Nr.: 57.744.067 von Ville7 am 11.05.18 23:04:57
wer solch falsche Behauptungen ins Netz stellt, darf man einen Lügner nennen!
Zitat von Ville7: Selber nachdenkenund nicht blind followen.
User Cyberhexe hat leider einen unglücklichen Trackrecord: MACK, TTPH, RDUS, zuvor dafür 80% seiner/ihrer EXEL zu 6USD vor dem run up verkauft.
wer solch falsche Behauptungen ins Netz stellt, darf man einen Lügner nennen!
Antwort auf Beitrag Nr.: 57.744.427 von Cyberhexe am 12.05.18 06:57:14Wer sich Mühe gibt findet die EXEL Verkaufsmeldungen von dir verstreut über die Threads: u.a. hier:
https://www.wallstreet-online.de/diskussion/1232720-1-10/rad…
Das Netz vergisst nicht. So viel zum Thema wer hier was ist.
https://www.wallstreet-online.de/diskussion/1232720-1-10/rad…
Das Netz vergisst nicht. So viel zum Thema wer hier was ist.
Ich bin bei Exel schon lange raus und habe deshalb nachgefragt!
Antwort auf Beitrag Nr.: 57.738.973 von Magnetfeldfredy am 11.05.18 10:09:14
ja, wie so oft bei solchen Meldungen. Ich denke, dass sich der Kurs spätestens nach den nächsten Quartalszahlen wieder kräftig erholt:
Halte immer noch 2000 Aktien!
Zitat von Magnetfeldfredy: Danke, Übertreibung nach unten?
ja, wie so oft bei solchen Meldungen. Ich denke, dass sich der Kurs spätestens nach den nächsten Quartalszahlen wieder kräftig erholt:

Halte immer noch 2000 Aktien!
Antwort auf Beitrag Nr.: 57.744.643 von Ville7 am 12.05.18 08:10:40
wer lesen kann, hat eindeutig Vorteile:
...habe diese Woche das Risiko etwas gestreut und meine übergewichtige Exelixis-Position (mittlerweile 80%) auf ca. 50% reduziert und dafür Radius Health erworben.
Wenn jedoch jemand schreibt, ich hätte 80% bei $6 verkauft, dann ist dieser jemand ein LÜGNER!
Zitat von Ville7: Wer sich Mühe gibt findet die EXEL Verkaufsmeldungen von dir verstreut über die Threads: u.a. hier:
https://www.wallstreet-online.de/diskussion/1232720-1-10/rad…
Das Netz vergisst nicht. So viel zum Thema wer hier was ist.
wer lesen kann, hat eindeutig Vorteile:
...habe diese Woche das Risiko etwas gestreut und meine übergewichtige Exelixis-Position (mittlerweile 80%) auf ca. 50% reduziert und dafür Radius Health erworben.
Wenn jedoch jemand schreibt, ich hätte 80% bei $6 verkauft, dann ist dieser jemand ein LÜGNER!
Antwort auf Beitrag Nr.: 53.795.601 von Cyberhexe am 29.11.16 21:25:21
....natürlich ist fast jede Börsenentscheidung retrospektiv suboptimal - die Entscheidung, den Investitionsgrad bei Exelixis ("all in one") nach einer Performance von über 100% zurückzufahren, würde ich so immer wieder tun.
Wenn dann Teilnehmer auch nach Jahren nichts Besseres mitzuteilen haben als genau dieses Risiko-Management zu kritisieren, dann ist das schon sehr belustigend.
Zitat von Cyberhexe:Zitat von Milestones: das ist hier die Frage.
https://deutsche-wirtschafts-nachrichten.de/2016/11/25/actel…
Dann kann man auch die Antwort bezüglich guten Timings geben... Ich hätte jedenfalls jetzt nicht reduziert.
obschon persönliche Eitelkeiten nicht zur Diskussion stehen ,sollte man bedenken, dass das Risk Management der Marktteilnehmer unterschiedlich sein kann. Mein persönliches Risk Management bestand darin, die "all in one"-Position Exelixis abzubauen und dafür andere vielversprechende Positionen aufzubauen wie z.B. PGNX, ARRY und RDUS. Die Risikostreuung stand hierbei im Vordergrund, obschon auch die Performance dieser Werte überzeugt.
....natürlich ist fast jede Börsenentscheidung retrospektiv suboptimal - die Entscheidung, den Investitionsgrad bei Exelixis ("all in one") nach einer Performance von über 100% zurückzufahren, würde ich so immer wieder tun.
Wenn dann Teilnehmer auch nach Jahren nichts Besseres mitzuteilen haben als genau dieses Risiko-Management zu kritisieren, dann ist das schon sehr belustigend.
!
Dieser Beitrag wurde von MODelfin moderiert. Grund: überwiegend persönliche Auseinandersetzung ohne echten Themenbezug die Sie bitte jetzt beenden bzw. außerhalb des Threads klären, Danke.
Antwort auf Beitrag Nr.: 57.744.694 von Cyberhexe am 12.05.18 08:23:09ASCO2018
Exel ist mit Cabo&Cobi sehr gut vertreten:
Poster Board: #354 • Abstract 4528
Clinical efficacy of cabozantinib plus nivolumab (CaboNivo) and CaboNivo plus ipilimumab (CaboNivoIpi) in patients (pts) with chemotherapy-refractory metastatic urothelial carcinoma (mUC) either naïve (n) or refractory (r) to checkpoint inhibitor (CPI).
Rosa Maria Nadal, MD, MD, PhD - First Author
National Cancer Institute, National Institutes of Health
Poster Board: #382 • Abstract 4556
Quality-adjusted time without symptoms or toxicity (Q-TWiST): Analysis of cabozantinib(Cabo) vs sunitinib (Sun) in patients with advanced renal cell carcinoma (aRCC) of intermediate or poor risk (Alliance A031203).
Ronald C. Chen, MD, MPH - First Author
University of North Carolina at Chapel Hill
Poster Board: #405 • Abstract 4579
Cabozantinib (Cabo) in advanced non-clear cell renal cell carcinoma (nccRCC): A retrospective multicenter analysis.
Nieves Martinez Chanza - First Author
Dana-Farber Cancer Institute
Poster Board: #415b • Abstract TPS4593
A phase I-II study to evaluate safety and efficacy of the combination of niraparib plus cabozantinib in patients with advanced kidney/urothelial carcinoma.
Daniel E. Castellano, MD - First Author
Hospital 12 de Octubre
Poster Board: #107 • Abstract 1026
A phase II study of cabozantinib (cabo) alone or in combination with trastuzumab (T) in patients (pts) with breast cancer brain metastases (BCBM).
Jose Pablo Leone, MD - First Author
Dana-Farber Cancer Institute
Poster Board: #190b • Abstract TPS1119
A phase II study of nivolumab in combination with cabozantinib for metastatic triple-negative breast cancer (mTNBC).
Romualdo Barroso-Sousa, MD, PhD - First Author
Dana-Farber Cancer Institute
Poster Board: #76 • Abstract 6088
A phase II trial of cabozantinib (CABO) for the treatment of radioiodine (RAI)-refractory differentiated thyroid carcinoma (DTC) in the first-line setting.
Marcia S. Brose, MD, PhD - First Author
Department of Otorhinolaryngology, Head and Neck Surgery and the Abramson Cancer Center of the University of Pennsylvania
Poster Board: #48 • Abstract 3555
A phase I/II trial of cabozantinib (C) with or without panitumumab (P) in patients (pts) with RAS wild-type (WT) metastatic colorectal cancer (mCRC): Clinical outcomes in pts with METamplification (amp) detected in blood.
Jingquan Jia, MD, PhD - First Author
Duke University Medical Center
Poster Board: #208 • Abstract 4019
Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase 3 CELESTIAL trial.
Ghassan K. Abou-Alfa, MD - First Author
Memorial Sloan Kettering Cancer Center
Discussed at the Poster Discussion Session on Sunday, June 3, 2018, 4:45 PM - 6:00 PM, at Hall D2
Poster Board: #277 • Abstract 4088
Outcomes in patients (pts) who had received sorafenib (S) as the only prior systemic therapy in the phase 3 CELESTIAL trial of cabozantinib(C) versus placebo (P) in advanced hepatocellular carcinoma (HCC).
Robin Kate Kelley, MD - First Author
University of California San Francisco
Poster Board: #279 • Abstract 4090
Outcomes based on age in the phase 3 CELESTIAL trial of cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC).
Lorenza Rimassa, MD - First Author
Humanitas Clinical and Research Center
Poster Board: #333a • Abstract TPS4157
A phase II trial of cabozantinib and erlotinib for patients with EGFR and c-MET co-expressing metastatic pancreatic adenocarcinoma.
Olumide B. Gbolahan, MBBS - First Author
Indiana University School of Medicine
Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase 3 CELESTIAL trial.
Ghassan K. Abou-Alfa, MD - First Author
Memorial Sloan Kettering Cancer Center
Discussed at the Poster Discussion Session on Sunday, June 3, 2018, 4:45 PM - 6:00 PM, at Hall D2
Poster Board: #349 • Abstract 9522
Efficacy and safety of cobimetinib (C) combined with vemurafenib (V) in patients (pts) with BRAFV600 mutation–positive metastatic melanoma: analysis from the 4-year extended follow-up of the phase 3 coBRIM study.
Brigitte Dreno, MD PhD - First Author
Dermatology Departement, CHU Nantes
Poster Board: #386 • Abstract 9559
Analysis of the kinetics and effects of vemurafenib (V) + cobimetinib (C) on intratumoral and host immunity in patients (pts) with BRAFV600 mutant melanoma (BRAFmM): Implications for combination with immunotherapy.
Suthee Rapisuwon, MD - First Author
Georgetown University, Lombardi Comprehensive Cancer Center
Exel ist mit Cabo&Cobi sehr gut vertreten:
Poster Board: #354 • Abstract 4528
Clinical efficacy of cabozantinib plus nivolumab (CaboNivo) and CaboNivo plus ipilimumab (CaboNivoIpi) in patients (pts) with chemotherapy-refractory metastatic urothelial carcinoma (mUC) either naïve (n) or refractory (r) to checkpoint inhibitor (CPI).
Rosa Maria Nadal, MD, MD, PhD - First Author
National Cancer Institute, National Institutes of Health
Poster Board: #382 • Abstract 4556
Quality-adjusted time without symptoms or toxicity (Q-TWiST): Analysis of cabozantinib(Cabo) vs sunitinib (Sun) in patients with advanced renal cell carcinoma (aRCC) of intermediate or poor risk (Alliance A031203).
Ronald C. Chen, MD, MPH - First Author
University of North Carolina at Chapel Hill
Poster Board: #405 • Abstract 4579
Cabozantinib (Cabo) in advanced non-clear cell renal cell carcinoma (nccRCC): A retrospective multicenter analysis.
Nieves Martinez Chanza - First Author
Dana-Farber Cancer Institute
Poster Board: #415b • Abstract TPS4593
A phase I-II study to evaluate safety and efficacy of the combination of niraparib plus cabozantinib in patients with advanced kidney/urothelial carcinoma.
Daniel E. Castellano, MD - First Author
Hospital 12 de Octubre
Poster Board: #107 • Abstract 1026
A phase II study of cabozantinib (cabo) alone or in combination with trastuzumab (T) in patients (pts) with breast cancer brain metastases (BCBM).
Jose Pablo Leone, MD - First Author
Dana-Farber Cancer Institute
Poster Board: #190b • Abstract TPS1119
A phase II study of nivolumab in combination with cabozantinib for metastatic triple-negative breast cancer (mTNBC).
Romualdo Barroso-Sousa, MD, PhD - First Author
Dana-Farber Cancer Institute
Poster Board: #76 • Abstract 6088
A phase II trial of cabozantinib (CABO) for the treatment of radioiodine (RAI)-refractory differentiated thyroid carcinoma (DTC) in the first-line setting.
Marcia S. Brose, MD, PhD - First Author
Department of Otorhinolaryngology, Head and Neck Surgery and the Abramson Cancer Center of the University of Pennsylvania
Poster Board: #48 • Abstract 3555
A phase I/II trial of cabozantinib (C) with or without panitumumab (P) in patients (pts) with RAS wild-type (WT) metastatic colorectal cancer (mCRC): Clinical outcomes in pts with METamplification (amp) detected in blood.
Jingquan Jia, MD, PhD - First Author
Duke University Medical Center
Poster Board: #208 • Abstract 4019
Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase 3 CELESTIAL trial.
Ghassan K. Abou-Alfa, MD - First Author
Memorial Sloan Kettering Cancer Center
Discussed at the Poster Discussion Session on Sunday, June 3, 2018, 4:45 PM - 6:00 PM, at Hall D2
Poster Board: #277 • Abstract 4088
Outcomes in patients (pts) who had received sorafenib (S) as the only prior systemic therapy in the phase 3 CELESTIAL trial of cabozantinib(C) versus placebo (P) in advanced hepatocellular carcinoma (HCC).
Robin Kate Kelley, MD - First Author
University of California San Francisco
Poster Board: #279 • Abstract 4090
Outcomes based on age in the phase 3 CELESTIAL trial of cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC).
Lorenza Rimassa, MD - First Author
Humanitas Clinical and Research Center
Poster Board: #333a • Abstract TPS4157
A phase II trial of cabozantinib and erlotinib for patients with EGFR and c-MET co-expressing metastatic pancreatic adenocarcinoma.
Olumide B. Gbolahan, MBBS - First Author
Indiana University School of Medicine
Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase 3 CELESTIAL trial.
Ghassan K. Abou-Alfa, MD - First Author
Memorial Sloan Kettering Cancer Center
Discussed at the Poster Discussion Session on Sunday, June 3, 2018, 4:45 PM - 6:00 PM, at Hall D2
Poster Board: #349 • Abstract 9522
Efficacy and safety of cobimetinib (C) combined with vemurafenib (V) in patients (pts) with BRAFV600 mutation–positive metastatic melanoma: analysis from the 4-year extended follow-up of the phase 3 coBRIM study.
Brigitte Dreno, MD PhD - First Author
Dermatology Departement, CHU Nantes
Poster Board: #386 • Abstract 9559
Analysis of the kinetics and effects of vemurafenib (V) + cobimetinib (C) on intratumoral and host immunity in patients (pts) with BRAFV600 mutant melanoma (BRAFmM): Implications for combination with immunotherapy.
Suthee Rapisuwon, MD - First Author
Georgetown University, Lombardi Comprehensive Cancer Center
Antwort auf Beitrag Nr.: 57.749.494 von Cyberhexe am 13.05.18 16:10:15EXEL-Umsatz und dessen Herkunft:

Antwort auf Beitrag Nr.: 57.753.580 von Cyberhexe am 14.05.18 12:25:38oh, da hab ich in meinen Internet-freien Ferien etwas verpasst:
Exelixis’ Partner Ipsen Announces European Commission Approval of CABOMETYX® (Cabozantinib) for Previously Untreated Intermediate- or Poor-Risk Advanced Renal Cell Carcinoma
– Approval based on statistically significant and clinically meaningful improvement in progression-free survival for CABOMETYX versus sunitinib in CABOSUN trial –
– Triggers $50 million milestone payment to Exelixis under licensing agreement with Ipsen –
SOUTH SAN FRANCISCO--(BUSINESS WIRE)--May 17, 2018-- Exelixis, Inc. (Nasdaq:EXEL) today announced that its partner Ipsen received approval from the European Commission (EC) for CABOMETYX® (cabozantinib) 20 mg, 40 mg and 60 mg for the first-line treatment of adults with intermediate- or poor-risk advanced renal cell carcinoma (RCC) in the European Union.
“The expanded marketing authorization of CABOMETYX to include previously untreated patients in Europe with intermediate- or poor-risk advanced kidney cancer is an exciting milestone for a patient population in need of more treatment options,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “We look forward to our continued collaboration with our partners Ipsen and Takeda to bring new options to more patients with difficult-to-treat cancers in Europe and around the world.”
Under the terms of the Collaboration and License Agreement with Ipsen, Exelixis will receive a milestone payment of $50 million for the EC approval, of which approximately $46 million was recognized as collaboration revenue in the first quarter of 2018. The payment will be made by Ipsen within the next 70 days.
“The expanded marketing authorization of CABOMETYX to include previously untreated patients in Europe with intermediate- or poor-risk advanced kidney cancer is an exciting milestone for a patient population in need of more treatment options,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “We look forward to our continued collaboration with our partners Ipsen and Takeda to bring new options to more patients with difficult-to-treat cancers in Europe and around the world.”
Under the terms of the Collaboration and License Agreement with Ipsen, Exelixis will receive a milestone payment of $50 million for the EC approval, of which approximately $46 million was recognized as collaboration revenue in the first quarter of 2018. The payment will be made by Ipsen within the next 70 days.
CABOMETYX was approved in the European Union in September 2016 for the treatment of advanced RCC in adults following prior vascular endothelial growth factor (VEGF)-targeted therapy. The expanded EC approval to include first-line treatment is based on results of the CABOSUN trial, which met its primary endpoint of improved progression-free survival (PFS) compared with sunitinib in patients with previously untreated advanced RCC determined to be intermediate- or poor-risk by the International Metastatic RCC Database Consortium (IMDC) criteria. In December 2017, the U.S. Food and Drug Administration (FDA) approved CABOMETYX for the expanded indication of patients with advanced RCC based on the results from the CABOSUN trial.
Exelixis’ Partner Ipsen Announces European Commission Approval of CABOMETYX® (Cabozantinib) for Previously Untreated Intermediate- or Poor-Risk Advanced Renal Cell Carcinoma
– Approval based on statistically significant and clinically meaningful improvement in progression-free survival for CABOMETYX versus sunitinib in CABOSUN trial –
– Triggers $50 million milestone payment to Exelixis under licensing agreement with Ipsen –
SOUTH SAN FRANCISCO--(BUSINESS WIRE)--May 17, 2018-- Exelixis, Inc. (Nasdaq:EXEL) today announced that its partner Ipsen received approval from the European Commission (EC) for CABOMETYX® (cabozantinib) 20 mg, 40 mg and 60 mg for the first-line treatment of adults with intermediate- or poor-risk advanced renal cell carcinoma (RCC) in the European Union.
“The expanded marketing authorization of CABOMETYX to include previously untreated patients in Europe with intermediate- or poor-risk advanced kidney cancer is an exciting milestone for a patient population in need of more treatment options,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “We look forward to our continued collaboration with our partners Ipsen and Takeda to bring new options to more patients with difficult-to-treat cancers in Europe and around the world.”
Under the terms of the Collaboration and License Agreement with Ipsen, Exelixis will receive a milestone payment of $50 million for the EC approval, of which approximately $46 million was recognized as collaboration revenue in the first quarter of 2018. The payment will be made by Ipsen within the next 70 days.
“The expanded marketing authorization of CABOMETYX to include previously untreated patients in Europe with intermediate- or poor-risk advanced kidney cancer is an exciting milestone for a patient population in need of more treatment options,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “We look forward to our continued collaboration with our partners Ipsen and Takeda to bring new options to more patients with difficult-to-treat cancers in Europe and around the world.”
Under the terms of the Collaboration and License Agreement with Ipsen, Exelixis will receive a milestone payment of $50 million for the EC approval, of which approximately $46 million was recognized as collaboration revenue in the first quarter of 2018. The payment will be made by Ipsen within the next 70 days.
CABOMETYX was approved in the European Union in September 2016 for the treatment of advanced RCC in adults following prior vascular endothelial growth factor (VEGF)-targeted therapy. The expanded EC approval to include first-line treatment is based on results of the CABOSUN trial, which met its primary endpoint of improved progression-free survival (PFS) compared with sunitinib in patients with previously untreated advanced RCC determined to be intermediate- or poor-risk by the International Metastatic RCC Database Consortium (IMDC) criteria. In December 2017, the U.S. Food and Drug Administration (FDA) approved CABOMETYX for the expanded indication of patients with advanced RCC based on the results from the CABOSUN trial.
Antwort auf Beitrag Nr.: 57.823.103 von Cyberhexe am 24.05.18 13:59:35interessant: die ph1b Kombi-Studie von Cabo mit Atezolizumab (von Roche/Genentech) zur Behandlung von "solid tumors" wurde mehrfach geändert, und zwar im Wesentlichen
- Anzahl Studienteilnehmer von n=156 auf 360
- Anzahl der Studienarme von 5 auf 9
- neue Kohorten: UC, CRPC (wow, Cabo wieder beim Prostatakarzinom), NSCLC
- Beobachtungszeit verlängert von 36 auf 41 Monate
Änderungen:
https://clinicaltrials.gov/ct2/history/NCT03170960?A=1&B=5&C…
Studie:
https://clinicaltrials.gov/ct2/show/NCT03170960
- Anzahl Studienteilnehmer von n=156 auf 360
- Anzahl der Studienarme von 5 auf 9
- neue Kohorten: UC, CRPC (wow, Cabo wieder beim Prostatakarzinom), NSCLC
- Beobachtungszeit verlängert von 36 auf 41 Monate
Änderungen:
https://clinicaltrials.gov/ct2/history/NCT03170960?A=1&B=5&C…
Studie:
https://clinicaltrials.gov/ct2/show/NCT03170960
...und ein weiterer Zulassungsantrag bzgl Cabo, und zwar zur Behandlung von fortgeschrittenem Leberkrebs. Die Entscheidung (PDUFA) ist auf Januar 2019 terminiert:
On May 29, the U.S. Food and Drug Administration (FDA) accepted for filing the supplemental new drug application (sNDA) for cabozantinib (Cabometyx) tablets as a treatment for patients with previously treated advanced hepatocellular carcinoma (HCC). The filing has been assigned a Prescription Drug User Fee Act (PDUFA) action date of January 14, 2019.
http://www.ascopost.com/News/58887
FDA Accepts Application for Cabozantinib in Advanced HCC
http://www.targetedonc.com/news/fda-accepts-application-for-…
On May 29, the U.S. Food and Drug Administration (FDA) accepted for filing the supplemental new drug application (sNDA) for cabozantinib (Cabometyx) tablets as a treatment for patients with previously treated advanced hepatocellular carcinoma (HCC). The filing has been assigned a Prescription Drug User Fee Act (PDUFA) action date of January 14, 2019.
http://www.ascopost.com/News/58887
FDA Accepts Application for Cabozantinib in Advanced HCC
http://www.targetedonc.com/news/fda-accepts-application-for-…
Antwort auf Beitrag Nr.: 57.883.449 von Cyberhexe am 01.06.18 08:51:13Cabo mit Atezolizuimab:
Exelixis Announces Further Expansion to Clinical Research Protocol for Phase 1b COSMIC-021 Trial of Cabozantinib in Combination with Anti-PD-L1 Immunotherapy in Patients with Locally Advanced or Metastatic Solid Tumors
– Ten new trial cohorts added to expansion phase of combination trial, bringing the total number of cohorts to 18 –
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Jun. 1, 2018-- Exelixis, Inc. (Nasdaq:EXEL) today announced an amendment to the protocol for COSMIC-021, the phase 1b trial of cabozantinib (CABOMETYX®) in combination with atezolizumab (TECENTRIQ®) in patients with locally advanced or metastatic solid tumors to add 10 new expansion cohorts to the trial. The primary objective in the expansion stage of this trial remains to determine the objective response rate in each cohort.
The 10 new expansion cohorts will evaluate the combination of cabozantinib and atezolizumab in patients with:
non-small cell lung cancer (NSCLC) with an EGFR mutation who have progressed following treatment with an EGFR-targeting tyrosine kinase inhibitor for metastatic disease
renal cell carcinoma (RCC) with non-clear cell histology who have not had prior systemic anticancer therapy for inoperable, locally advanced, recurrent or metastatic disease
triple-negative breast cancer who have progressed following treatment with at least one prior systemic therapy for inoperable, locally advanced, recurrent or metastatic disease
epithelial ovarian cancer who have platinum-resistant or refractory disease
endometrial cancer who have progressed following treatment with at least one prior systemic therapy for inoperable, locally advanced, recurrent or metastatic disease
advanced hepatocellular carcinoma (HCC) who have a Child-Pugh score of A and have not had prior systemic anticancer therapy for inoperable, locally advanced, recurrent or metastatic disease
gastric or gastroesophageal junction adenocarcinoma who have progressed following treatment with platinum-containing or fluoropyrimidine-containing chemotherapy for inoperable locally advanced, recurrent or metastatic disease
colorectal adenocarcinoma who have progressed following treatment with systemic chemotherapy that contained fluoropyrimidine in combination with oxaliplatin or irinotecan for metastatic disease
head and neck cancer of squamous cell histology who have progressed following treatment with platinum-containing chemotherapy for inoperable locally advanced, recurrent or metastatic disease
differentiated thyroid cancer who are radio-refractory or deemed ineligible for treatment with iodine-131
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArt…
Exelixis Announces Further Expansion to Clinical Research Protocol for Phase 1b COSMIC-021 Trial of Cabozantinib in Combination with Anti-PD-L1 Immunotherapy in Patients with Locally Advanced or Metastatic Solid Tumors
– Ten new trial cohorts added to expansion phase of combination trial, bringing the total number of cohorts to 18 –
SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Jun. 1, 2018-- Exelixis, Inc. (Nasdaq:EXEL) today announced an amendment to the protocol for COSMIC-021, the phase 1b trial of cabozantinib (CABOMETYX®) in combination with atezolizumab (TECENTRIQ®) in patients with locally advanced or metastatic solid tumors to add 10 new expansion cohorts to the trial. The primary objective in the expansion stage of this trial remains to determine the objective response rate in each cohort.
The 10 new expansion cohorts will evaluate the combination of cabozantinib and atezolizumab in patients with:
non-small cell lung cancer (NSCLC) with an EGFR mutation who have progressed following treatment with an EGFR-targeting tyrosine kinase inhibitor for metastatic disease
renal cell carcinoma (RCC) with non-clear cell histology who have not had prior systemic anticancer therapy for inoperable, locally advanced, recurrent or metastatic disease
triple-negative breast cancer who have progressed following treatment with at least one prior systemic therapy for inoperable, locally advanced, recurrent or metastatic disease
epithelial ovarian cancer who have platinum-resistant or refractory disease
endometrial cancer who have progressed following treatment with at least one prior systemic therapy for inoperable, locally advanced, recurrent or metastatic disease
advanced hepatocellular carcinoma (HCC) who have a Child-Pugh score of A and have not had prior systemic anticancer therapy for inoperable, locally advanced, recurrent or metastatic disease
gastric or gastroesophageal junction adenocarcinoma who have progressed following treatment with platinum-containing or fluoropyrimidine-containing chemotherapy for inoperable locally advanced, recurrent or metastatic disease
colorectal adenocarcinoma who have progressed following treatment with systemic chemotherapy that contained fluoropyrimidine in combination with oxaliplatin or irinotecan for metastatic disease
head and neck cancer of squamous cell histology who have progressed following treatment with platinum-containing chemotherapy for inoperable locally advanced, recurrent or metastatic disease
differentiated thyroid cancer who are radio-refractory or deemed ineligible for treatment with iodine-131
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArt…
Die neuen Wirkstoffe zur Behandlung des Leberkarzinoms werden in die wissenschaftlichen Lehrbücher aufgenommen:
Dtsch med Wochenschr 2018; 143(11): 815-819
DOI: 10.1055/s-0043-124158
Klinischer Fortschritt
Verdauungs- und Stoffwechselerkrankungen
© Georg Thieme Verlag KG Stuttgart · New York
Hepatozelluläres Karzinom: neue multimodale Therapiekonzepte
Hepatocellular Carcinoma: New multimodal therapy concepts
Michael Schultheiß, Dominik Bettinger, Stefan Fichtner-Feigl, Robert Thimme
Was ist neu?
Aktueller Stand und Allgemeines Die Leitlinien für das hepatozelluläre Karzinom (HCC) sind derzeit unter Revision, Neuauflagen werden 2018 erwartet. Patienten mit chronischen Lebererkrankungen oder Leberzirrhose müssen mittels Ultraschall alle 6 Monate in Bezug auf die Entwicklung eines HCC gescreent werden. Die chirurgische Resektion oder die Lebertransplantation sind kurative Optionen im frühen Stadium.
Lokoregionäre Therapien Die selektive interne Radiotherapie (SIRT) wird zunehmend häufiger als lokoregionäre Therapie eingesetzt. Die Studiendaten sprechen für eine gute Verträglichkeit, aber keine überlegene Wirksamkeit gegenüber transarterieller Chemoembolisation (TACE) oder Systemtherapie mit Sorafenib.
Neue zielgerichtete Therapien Regorafenib stellt bei Patienten mit Progress unter Sorafenib eine neu zugelassene Alternative in der Zweitlinientherapie dar. Positive Phase-III-Studien wurden für Lenvatinib in der Erst- und Cabozantinib in der Zweitlinientherapie publiziert.
Immuntherapie Der Checkpoint-Inhibitor Nivolumab ist in den USA in der Zweitlinientherapie aufgrund guter Phase-I/II-Daten zugelassen worden. Daten zu einer Phase-III-Studie in der Erstlinientherapie vs. Sorafenib werden 2018 erwartet.
https://www.thieme-connect.com/DOI/DOI?10.1055/s-0043-124158
Dtsch med Wochenschr 2018; 143(11): 815-819
DOI: 10.1055/s-0043-124158
Klinischer Fortschritt
Verdauungs- und Stoffwechselerkrankungen
© Georg Thieme Verlag KG Stuttgart · New York
Hepatozelluläres Karzinom: neue multimodale Therapiekonzepte
Hepatocellular Carcinoma: New multimodal therapy concepts
Michael Schultheiß, Dominik Bettinger, Stefan Fichtner-Feigl, Robert Thimme
Was ist neu?
Aktueller Stand und Allgemeines Die Leitlinien für das hepatozelluläre Karzinom (HCC) sind derzeit unter Revision, Neuauflagen werden 2018 erwartet. Patienten mit chronischen Lebererkrankungen oder Leberzirrhose müssen mittels Ultraschall alle 6 Monate in Bezug auf die Entwicklung eines HCC gescreent werden. Die chirurgische Resektion oder die Lebertransplantation sind kurative Optionen im frühen Stadium.
Lokoregionäre Therapien Die selektive interne Radiotherapie (SIRT) wird zunehmend häufiger als lokoregionäre Therapie eingesetzt. Die Studiendaten sprechen für eine gute Verträglichkeit, aber keine überlegene Wirksamkeit gegenüber transarterieller Chemoembolisation (TACE) oder Systemtherapie mit Sorafenib.
Neue zielgerichtete Therapien Regorafenib stellt bei Patienten mit Progress unter Sorafenib eine neu zugelassene Alternative in der Zweitlinientherapie dar. Positive Phase-III-Studien wurden für Lenvatinib in der Erst- und Cabozantinib in der Zweitlinientherapie publiziert.
Immuntherapie Der Checkpoint-Inhibitor Nivolumab ist in den USA in der Zweitlinientherapie aufgrund guter Phase-I/II-Daten zugelassen worden. Daten zu einer Phase-III-Studie in der Erstlinientherapie vs. Sorafenib werden 2018 erwartet.
https://www.thieme-connect.com/DOI/DOI?10.1055/s-0043-124158
Antwort auf Beitrag Nr.: 57.890.358 von Cyberhexe am 01.06.18 22:29:43
Cabo & Atezolizumab
Exelixis and Roche cast a much, much wider net in collaboration
Company: Exelixis (EXEL) and Roche (OTCQX:RHHBF)(OTCQX:RHHBY)
Therapy: Cabozantinib and atezolizumab
Disease: Various
News: EXEL announced that they would be amending the protocol for their COSMIC-021 study to include 10 new expansion cohorts. This study is assessing the combination of their flagship drug cabozantinib and RHHBF's atezolizumab in various solid tumors. The ten disease areas are in EGFR-mutant non-small cell lung cancer, first-line renal cell carcinoma, triple-negative breast cancer, platinum-resistant ovarian cancer, endometrial cancer, first-line hepatocellular carcinoma, gastric cancer, metastatic colorectal cancer, head and neck cancer, and differentiated thyroid cancer.
Looking forward: Reading down that list is like a what's what of hard-to-treat tumors. This is very interesting news for the collaboration, and that brings the entire study up to 18 expansion cohorts. This is a remarkably wide net for a phase 1b trial, and it makes me wonder whether they have seen anything of particular interest so far in their study. At any rate, it would seem that the various pieces of information we're likely to get out of this study will be worth watching in the years to come.
Cabo & Atezolizumab
Exelixis and Roche cast a much, much wider net in collaboration
Company: Exelixis (EXEL) and Roche (OTCQX:RHHBF)(OTCQX:RHHBY)
Therapy: Cabozantinib and atezolizumab
Disease: Various
News: EXEL announced that they would be amending the protocol for their COSMIC-021 study to include 10 new expansion cohorts. This study is assessing the combination of their flagship drug cabozantinib and RHHBF's atezolizumab in various solid tumors. The ten disease areas are in EGFR-mutant non-small cell lung cancer, first-line renal cell carcinoma, triple-negative breast cancer, platinum-resistant ovarian cancer, endometrial cancer, first-line hepatocellular carcinoma, gastric cancer, metastatic colorectal cancer, head and neck cancer, and differentiated thyroid cancer.
Looking forward: Reading down that list is like a what's what of hard-to-treat tumors. This is very interesting news for the collaboration, and that brings the entire study up to 18 expansion cohorts. This is a remarkably wide net for a phase 1b trial, and it makes me wonder whether they have seen anything of particular interest so far in their study. At any rate, it would seem that the various pieces of information we're likely to get out of this study will be worth watching in the years to come.
Kursziel bei Oppenheimer bei $40!
Oppenheimer Thinks Exelixis’ Stock is Going to Recover
Jason Carr 7 days ago Categories:Healthcare, Top Market News Tags:EXEL, Exelixis, Leah R. Cann, NASDAQ:EXEL
In a report released yesterday, Leah R. Cann from Oppenheimer assigned a Buy rating to Exelixis (NASDAQ: EXEL), with a price target of $40. The company’s shares closed yesterday at $20.31, close to its 52-week low of $18.03.
http://www.analystratings.com/articles/oppenheimer-thinks-ex…
Oppenheimer Thinks Exelixis’ Stock is Going to Recover
Jason Carr 7 days ago Categories:Healthcare, Top Market News Tags:EXEL, Exelixis, Leah R. Cann, NASDAQ:EXEL
In a report released yesterday, Leah R. Cann from Oppenheimer assigned a Buy rating to Exelixis (NASDAQ: EXEL), with a price target of $40. The company’s shares closed yesterday at $20.31, close to its 52-week low of $18.03.
http://www.analystratings.com/articles/oppenheimer-thinks-ex…
Antwort auf Beitrag Nr.: 57.913.761 von Cyberhexe am 05.06.18 18:00:29interessant: Cabo möglichweise auch bei Ovarian Cancer, also Eierstockkrebs, wirksam:
https://www.gynecologiconcology-online.net/article/S0090-825…
https://www.gynecologiconcology-online.net/article/S0090-825…
Antwort auf Beitrag Nr.: 58.083.715 von Cyberhexe am 28.06.18 07:00:24Let's start with oncology mid-cap Exelixis (EXEL) which is off a third from 52-week highs. Oppenheimer reissues their Buy rating and $40 price target on the stock. Oppenheimer's analyst cites an upcoming potential catalyst that might get the shares moving again in her commentary
“The CELESTIAL data for Cabometyx in hepatocellar carcinoma (HCC) were first presented in October 2017, as top-line results, and in detail in mid-January 2018. We anticipate Cabometyx will be approved for use in HCC by early January 2019. We believe the publication of these results in the prestigious Medicine will further increase the visibility of these data, and could lead to more rapid adoption of Cabometyx in this setting, once it is approved.”
“The CELESTIAL data for Cabometyx in hepatocellar carcinoma (HCC) were first presented in October 2017, as top-line results, and in detail in mid-January 2018. We anticipate Cabometyx will be approved for use in HCC by early January 2019. We believe the publication of these results in the prestigious Medicine will further increase the visibility of these data, and could lead to more rapid adoption of Cabometyx in this setting, once it is approved.”
Antwort auf Beitrag Nr.: 58.147.142 von Cyberhexe am 05.07.18 18:12:50Exelixis ist mittlerweile hochprofitabel:
Exelixis Announces Second Quarter 2018 Financial Results and Provides Corporate Update
- Total Revenues of $186.1 million, Net Income of $87.5 million, Diluted EPS of $0.28 -
- Cabozantinib Franchise Net Product Revenues of $145.8 million -
- Ipsen Royalty Rate Increased to 22 Percent upon Reaching $150.0 million in Cumulative Net Sales -
- Conference Call and Webcast Today at 5:00 P.M. Eastern Daylight Time -
ALAMEDA, Calif.--(BUSINESS WIRE)--Aug. 1, 2018-- Exelixis, Inc. (Nasdaq: EXEL) today reported financial results for the second quarter of 2018 and provided an update on progress toward fulfilling its key corporate objectives, as well as commercial and clinical development milestones.
“The second quarter of 2018 was highlighted by the strong commercial performance of CABOMETYX® (cabozantinib) in advanced renal cell carcinoma and continued regulatory progress for cabozantinib across multiple indications,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “We are pleased with our partner Ipsen’s progress as it launches CABOMETYX in the first-line setting following their recent label expansion in the European Union and the achievement of key commercial sales milestones. In advanced hepatocellular carcinoma, acceptance of our supplemental New Drug Application by the U.S. Food and Drug Administration brought us a step closer to offering CABOMETYX as a treatment to another patient population in need of new options.”
Dr. Morrissey continued: “Our strong financial performance in the second quarter was driven primarily by an increase in U.S. sales of CABOMETYX, as well as a milestone recognized from our collaborative partnerships, leading to net income of $87.5 million or $0.28 per share on a fully diluted basis. The progress we made in the second quarter put us in position for continued momentum across the business in the second half of 2018.”
Exelixis Announces Second Quarter 2018 Financial Results and Provides Corporate Update
- Total Revenues of $186.1 million, Net Income of $87.5 million, Diluted EPS of $0.28 -
- Cabozantinib Franchise Net Product Revenues of $145.8 million -
- Ipsen Royalty Rate Increased to 22 Percent upon Reaching $150.0 million in Cumulative Net Sales -
- Conference Call and Webcast Today at 5:00 P.M. Eastern Daylight Time -
ALAMEDA, Calif.--(BUSINESS WIRE)--Aug. 1, 2018-- Exelixis, Inc. (Nasdaq: EXEL) today reported financial results for the second quarter of 2018 and provided an update on progress toward fulfilling its key corporate objectives, as well as commercial and clinical development milestones.
“The second quarter of 2018 was highlighted by the strong commercial performance of CABOMETYX® (cabozantinib) in advanced renal cell carcinoma and continued regulatory progress for cabozantinib across multiple indications,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “We are pleased with our partner Ipsen’s progress as it launches CABOMETYX in the first-line setting following their recent label expansion in the European Union and the achievement of key commercial sales milestones. In advanced hepatocellular carcinoma, acceptance of our supplemental New Drug Application by the U.S. Food and Drug Administration brought us a step closer to offering CABOMETYX as a treatment to another patient population in need of new options.”
Dr. Morrissey continued: “Our strong financial performance in the second quarter was driven primarily by an increase in U.S. sales of CABOMETYX, as well as a milestone recognized from our collaborative partnerships, leading to net income of $87.5 million or $0.28 per share on a fully diluted basis. The progress we made in the second quarter put us in position for continued momentum across the business in the second half of 2018.”
Heute Position zu 14,65€ aufgebaut...Reaktion erscheint mir völlig übertrieben.
Erst Abstufung von Morgan St. die Tage, heute gute Daten einer fortgeschr. Studie von Merck/ Pfizer - wobei die "die Katze noch nicht ganz aus dem Sack gelassen haben"...
Erst Abstufung von Morgan St. die Tage, heute gute Daten einer fortgeschr. Studie von Merck/ Pfizer - wobei die "die Katze noch nicht ganz aus dem Sack gelassen haben"...
19.9.
https://investorplace.com/2018/09/7-biotech-stocks-to-buy-no…
Exelixis (NASDAQ:EXEL) lost half its value in 2018. The downtrend started when Roche and Exelixis ended Roche’s Phase 2 MODUL study. This included Exelixis’ COTELLIC (cobimetinib) for patients suffering from CRC or metastatic colorectal cancer. The negative developments will hurt future earnings, which is why the stock continued to fall with no “bottom” in sight.
In its second-quarter report, released on Aug. 1, the company reported earnings of 28 cents a share on revenue of $186.1 million, up 88%. Both numbers beat consensus estimates and yet the stock continued falling.
EXEL is looking to complement its cabozantinib development activities. Still, management sees healthy growth for this business, even after factoring increased competition. It is developing potential new indications for cabozantinib, be it single agent or in combination with immune checkpoint inhibitors. It is seeking collaborations with early stage biotech this year and beyond, keeping its financial risks low.
https://investorplace.com/2018/09/7-biotech-stocks-to-buy-no…
Exelixis (NASDAQ:EXEL) lost half its value in 2018. The downtrend started when Roche and Exelixis ended Roche’s Phase 2 MODUL study. This included Exelixis’ COTELLIC (cobimetinib) for patients suffering from CRC or metastatic colorectal cancer. The negative developments will hurt future earnings, which is why the stock continued to fall with no “bottom” in sight.
In its second-quarter report, released on Aug. 1, the company reported earnings of 28 cents a share on revenue of $186.1 million, up 88%. Both numbers beat consensus estimates and yet the stock continued falling.
EXEL is looking to complement its cabozantinib development activities. Still, management sees healthy growth for this business, even after factoring increased competition. It is developing potential new indications for cabozantinib, be it single agent or in combination with immune checkpoint inhibitors. It is seeking collaborations with early stage biotech this year and beyond, keeping its financial risks low.
Antwort auf Beitrag Nr.: 58.744.168 von faultcode am 19.09.18 23:52:53weitere Zulassung für Cabozantinib --> EMA erteilt Zulassung zur Zweitlinienbehandlung (nach Sorafenib) von Leberkrebs:
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArt…
Meilensteinzahlung: 40mUSD
Lizenzgebühren bis zu 26%
"The agreement also includes up to $545 million of potential commercial milestones and provides for Exelixis to receive tiered royalties up to 26% on Ipsen’s net sales of cabozantinib in its territories."
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArt…
Meilensteinzahlung: 40mUSD
Lizenzgebühren bis zu 26%
"The agreement also includes up to $545 million of potential commercial milestones and provides for Exelixis to receive tiered royalties up to 26% on Ipsen’s net sales of cabozantinib in its territories."

Bei Exelixis läufts wirklich rund: Esaxerenone-Zulassung in Japan!
Per the collaboration agreement between Exelixis and Daiichi Sankyo, Exelixis will receive a $20 million milestone payment upon the first commercial sale of MINNEBRO in Japan. Exelixis previously received a $20 million milestone payment in the first quarter of 2018 triggered by the filing of Daiichi Sankyo’s associated regulatory application. Exelixis is eligible for substantial commercialization milestones, as well as low double-digit royalties on sales of MINNEBRO. Since the conclusion of Exelixis and Daiichi Sankyo’s joint research period in November 2007, Daiichi Sankyo has been responsible for all subsequent preclinical and clinical development, and also oversees regulatory, manufacturing and commercialization activities for MINNEBRO.
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArt…
Per the collaboration agreement between Exelixis and Daiichi Sankyo, Exelixis will receive a $20 million milestone payment upon the first commercial sale of MINNEBRO in Japan. Exelixis previously received a $20 million milestone payment in the first quarter of 2018 triggered by the filing of Daiichi Sankyo’s associated regulatory application. Exelixis is eligible for substantial commercialization milestones, as well as low double-digit royalties on sales of MINNEBRO. Since the conclusion of Exelixis and Daiichi Sankyo’s joint research period in November 2007, Daiichi Sankyo has been responsible for all subsequent preclinical and clinical development, and also oversees regulatory, manufacturing and commercialization activities for MINNEBRO.
http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArt…
Antwort auf Beitrag Nr.: 59.583.080 von Cyberhexe am 08.01.19 12:41:42Am Kongress der amerikanischen Gesellschaft für Krebsforschung (AACR) in Atlanta wird Exelixis Ende März 2019 interessante Daten zu Cabo und Neurofibroma veröffentlichen:

AACR Annual Meeting 2019
Integrative Cancer Science • Global Impact • Individualized Patient Care
Friday, March 29-Wednesday, April 3, 2019
Georgia World Congress Center
Atlanta, Georgia

AACR Annual Meeting 2019
Integrative Cancer Science • Global Impact • Individualized Patient Care
Friday, March 29-Wednesday, April 3, 2019
Georgia World Congress Center
Atlanta, Georgia
GUTEN Tag.
Antwort auf Beitrag Nr.: 59.986.753 von Cyberhexe am 28.02.19 14:50:52...eine weitere "cash cow" für Exelixis: MINNEBRO® (esaxerenone)
- $20 million milestone payment
- low double-digit royalties on MINNEBRO sales
http://ir.exelixis.com/news-releases/news-release-details/ex…
- $20 million milestone payment
- low double-digit royalties on MINNEBRO sales
http://ir.exelixis.com/news-releases/news-release-details/ex…
Exelixis Updates Phase 1b COSMIC-021 Trial of Cabozantinib in Combination With Atezolizumab in Patients With Advanced Solid Tumors
Business Wire•July 15, 2019
ALAMEDA, Calif.-
– Original metastatic castration-resistant prostate cancer and immunotherapy-refractory non-small cell lung cancer cohorts expanded to 80 subjects –
– Four cohorts added for metastatic castration-resistant prostate cancer based on encouraging early data
https://finance.yahoo.com/news/exelixis-updates-phase-1b-cos…
Business Wire•July 15, 2019
ALAMEDA, Calif.-
– Original metastatic castration-resistant prostate cancer and immunotherapy-refractory non-small cell lung cancer cohorts expanded to 80 subjects –
– Four cohorts added for metastatic castration-resistant prostate cancer based on encouraging early data
https://finance.yahoo.com/news/exelixis-updates-phase-1b-cos…
"Cabozantinib Versus Sunitinib for Untreated Patients with Advanced Renal Cell Carcinoma of Intermediate or Poor Risk: Subgroup Analysis of the Alliance A031203 CABOSUN trial"
Cabozantinib scheint tatsächlich die Wunderwaffe zu sein, die sich sowohl Patienten und Investoren erhofft hatten. Sogar in Erstlinienbehandlung von RCC ist Cabo mittlerweile eine nicht zu ünterschätzende Option.
theoncologist.alphamedpress.org/content/early/2019/08/09/the…
Cabozantinib scheint tatsächlich die Wunderwaffe zu sein, die sich sowohl Patienten und Investoren erhofft hatten. Sogar in Erstlinienbehandlung von RCC ist Cabo mittlerweile eine nicht zu ünterschätzende Option.
theoncologist.alphamedpress.org/content/early/2019/08/09/the…
Exelixis to Present at the 2019 Cantor Global Healthcare Conference on October 2, 2019
Business WireSeptember 25, 2019
Exelixis, Inc. (EXEL) today announced that members of Exelixis management will provide a corporate overview at the 2019 Cantor Global Healthcare Conference, which is being held next week in New York. Exelixis’ presentation has been scheduled for Wednesday, October 2, 2019 at 8:55 AM EDT / 5:55 AM PDT.
To access the webcast link, log onto www.exelixis.com and proceed to the News & Events / Event Calendar page under the Investors & Media heading. Please connect to the company’s website at least 15 minutes prior to the presentation to ensure adequate time for any software download that may be required to listen to the webcast. A replay will also be available at the same location for 14 days.
https://finance.yahoo.com/news/exelixis-present-2019-cantor-…
Business WireSeptember 25, 2019
Exelixis, Inc. (EXEL) today announced that members of Exelixis management will provide a corporate overview at the 2019 Cantor Global Healthcare Conference, which is being held next week in New York. Exelixis’ presentation has been scheduled for Wednesday, October 2, 2019 at 8:55 AM EDT / 5:55 AM PDT.
To access the webcast link, log onto www.exelixis.com and proceed to the News & Events / Event Calendar page under the Investors & Media heading. Please connect to the company’s website at least 15 minutes prior to the presentation to ensure adequate time for any software download that may be required to listen to the webcast. A replay will also be available at the same location for 14 days.
https://finance.yahoo.com/news/exelixis-present-2019-cantor-…
Health Canada Approves Ipsen's CABOMETYX™ (cabozantinib) for the First-Line Treatment of Adults with Advanced Renal Cell Carcinoma
NEWS PROVIDED BY
Ipsen Biopharmaceuticals Canada Inc.
Oct 15, 2019, 08:00 ET
CABOMETYXTM demonstrated a statistically significant, 52 per cent reduction in the risk of progression or death compared with sunitinibi, giving Canadian patients with intermediate or poor risk a new oral first-line treatment option in their fight against advanced kidney cancer
https://www.newswire.ca/news-releases/health-canada-approves…
NEWS PROVIDED BY
Ipsen Biopharmaceuticals Canada Inc.
Oct 15, 2019, 08:00 ET
CABOMETYXTM demonstrated a statistically significant, 52 per cent reduction in the risk of progression or death compared with sunitinibi, giving Canadian patients with intermediate or poor risk a new oral first-line treatment option in their fight against advanced kidney cancer
https://www.newswire.ca/news-releases/health-canada-approves…
Exelixis’ Collaborator Daiichi Sankyo Announces Positive Results From Phase 3 Pivotal Trial of Esaxerenone in Patients With Diabetic Nephropathy
Business Wire Business Wire•November 8, 2019
Results being presented today in a late-breaking presentation (abstract TH-PO1201) at Kidney Week 2019, the annual meeting of the American Society of Nephrology in Washington, D.C.
https://finance.yahoo.com/news/exelixis-collaborator-daiichi…
Business Wire Business Wire•November 8, 2019
Results being presented today in a late-breaking presentation (abstract TH-PO1201) at Kidney Week 2019, the annual meeting of the American Society of Nephrology in Washington, D.C.
https://finance.yahoo.com/news/exelixis-collaborator-daiichi…
Exelixis’ Partner Ipsen Announces Health Canada’s Approval of CABOMETYX® (cabozantinib) Tablets for the Treatment of Patients With Previously Treated Advanced Hepatocellular Carcinoma
Business Wire Business Wire•November 12, 2019
ALAMEDA, Calif.--(BUSINESS WIRE)--
– Approval based on statistically significant and clinically meaningful overall survival benefit demonstrated in the CELESTIAL phase 3 pivotal trial –
https://finance.yahoo.com/news/exelixis-partner-ipsen-announ…
Business Wire Business Wire•November 12, 2019
ALAMEDA, Calif.--(BUSINESS WIRE)--
– Approval based on statistically significant and clinically meaningful overall survival benefit demonstrated in the CELESTIAL phase 3 pivotal trial –
https://finance.yahoo.com/news/exelixis-partner-ipsen-announ…
Exelixis to Present at the Stifel 2019 Healthcare Conference on November 19, 2019
Business Wire•November 12, 2019
ALAMEDA, Calif.--(BUSINESS WIRE)--
Exelixis, Inc. (EXEL) today announced that Michael M. Morrissey, Ph.D., the company’s President and Chief Executive Officer, will provide a corporate overview at the Stifel 2019 Healthcare Conference being held next week in New York. Exelixis’ presentation has been scheduled for Tuesday, November 19, 2019 at 9:10 AM EST / 6:10 AM PST.
To access the webcast link, log onto www.exelixis.com and proceed to the News & Events / Event Calendar page under the Investors & Media heading. Please connect to the company’s website at least 15 minutes prior to the presentation to ensure adequate time for any software download that may be required to listen to the webcast. A replay will also be available at the same location for 14 days
https://finance.yahoo.com/news/exelixis-present-stifel-2019-…
Business Wire•November 12, 2019
ALAMEDA, Calif.--(BUSINESS WIRE)--
Exelixis, Inc. (EXEL) today announced that Michael M. Morrissey, Ph.D., the company’s President and Chief Executive Officer, will provide a corporate overview at the Stifel 2019 Healthcare Conference being held next week in New York. Exelixis’ presentation has been scheduled for Tuesday, November 19, 2019 at 9:10 AM EST / 6:10 AM PST.
To access the webcast link, log onto www.exelixis.com and proceed to the News & Events / Event Calendar page under the Investors & Media heading. Please connect to the company’s website at least 15 minutes prior to the presentation to ensure adequate time for any software download that may be required to listen to the webcast. A replay will also be available at the same location for 14 days
https://finance.yahoo.com/news/exelixis-present-stifel-2019-…
Antwort auf Beitrag Nr.: 61.912.994 von bernie55 am 13.11.19 16:11:08...die "Wunderwaffe" Cabozantinib wird von Roche gemeinsam mit Atezolizumab in 12 weiteren Indikationen in ph1b getestet sowie in 3 weiteren ph3-Studien, und zwar in den "mächtigen" Indikationen nicht-kleinzelliger Lungenkrebs, kastrationsresistenter Prostatakrebs sowie Nierenkrebs ! Wow!!
https://ir.exelixis.com/news-releases/news-release-details/e…
Exelixis Enters into a Clinical Collaboration for Three Phase 3 Combination Trials for Patients with Advanced Solid Tumors
PDF Version
– New pivotal trials will evaluate the combination of cabozantinib and atezolizumab in patients with advanced non-small cell lung cancer, castration-resistant prostate cancer and renal cell carcinoma –
– Collaboration based on data from phase 1b COSMIC-021 trial –
ALAMEDA, Calif.--(BUSINESS WIRE)--Dec. 19, 2019-- Exelixis, Inc. (NASDAQ:EXEL) today announced a collaboration agreement with Roche to evaluate cabozantinib (CABOMETYX®), Exelixis’ small molecule inhibitor of receptor tyrosine kinases, in combination with atezolizumab (TECENTRIQ®), Roche’s PD-L1 immune checkpoint inhibitor, in patients with locally advanced or metastatic solid tumors. The clinical program, which will be co-funded by the companies, is expected to include three phase 3 pivotal trials in advanced non-small cell lung cancer (NSCLC), castration-resistant prostate cancer (CRPC) and renal cell carcinoma (RCC).
“Encouraging phase 1 data suggests this combination of cabozantinib and atezolizumab may improve outcomes for patients with prostate, lung and kidney cancers, and we look forward to collaborating with Roche to learn more in these pivotal trials,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “This clinical collaboration is an important further step in our committed efforts to maximize the value of the cabozantinib franchise through these cost-sharing clinical collaborations in additional high-impact indications, while building value with new compounds from internal and external sources in 2020 and beyond.”
The clinical development collaboration builds on encouraging activity observed in the phase 1b COSMIC-021 trial. The trial is currently enrolling 24 expansion cohorts in 12 tumor types including RCC, NSCLC and CRPC.
https://ir.exelixis.com/news-releases/news-release-details/e…
Exelixis Enters into a Clinical Collaboration for Three Phase 3 Combination Trials for Patients with Advanced Solid Tumors
PDF Version
– New pivotal trials will evaluate the combination of cabozantinib and atezolizumab in patients with advanced non-small cell lung cancer, castration-resistant prostate cancer and renal cell carcinoma –
– Collaboration based on data from phase 1b COSMIC-021 trial –
ALAMEDA, Calif.--(BUSINESS WIRE)--Dec. 19, 2019-- Exelixis, Inc. (NASDAQ:EXEL) today announced a collaboration agreement with Roche to evaluate cabozantinib (CABOMETYX®), Exelixis’ small molecule inhibitor of receptor tyrosine kinases, in combination with atezolizumab (TECENTRIQ®), Roche’s PD-L1 immune checkpoint inhibitor, in patients with locally advanced or metastatic solid tumors. The clinical program, which will be co-funded by the companies, is expected to include three phase 3 pivotal trials in advanced non-small cell lung cancer (NSCLC), castration-resistant prostate cancer (CRPC) and renal cell carcinoma (RCC).
“Encouraging phase 1 data suggests this combination of cabozantinib and atezolizumab may improve outcomes for patients with prostate, lung and kidney cancers, and we look forward to collaborating with Roche to learn more in these pivotal trials,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “This clinical collaboration is an important further step in our committed efforts to maximize the value of the cabozantinib franchise through these cost-sharing clinical collaborations in additional high-impact indications, while building value with new compounds from internal and external sources in 2020 and beyond.”
The clinical development collaboration builds on encouraging activity observed in the phase 1b COSMIC-021 trial. The trial is currently enrolling 24 expansion cohorts in 12 tumor types including RCC, NSCLC and CRPC.
Antwort auf Beitrag Nr.: 62.197.206 von Cyberhexe am 19.12.19 13:57:25Und? Meinst du, dass sich ein Einstieg hie nich lohnt? Du hast doch sicher jede Menge Informationen ausgewertet :-)
Antwort auf Beitrag Nr.: 62.205.057 von opportoni am 20.12.19 11:11:28hie nich sollte hier noch heißen. zu dicke Finger...
Antwort auf Beitrag Nr.: 62.205.057 von opportoni am 20.12.19 11:11:28
ich habe aus Nostalgiegründen noch ein paar Restaktien im Portfolio, würde diese momentan aber nicht zum Kauf empfehlen. Obschon Cabo tatsächlich eine Wunderwaffe zu sein scheint, fehlt die "Breite" in EXELs Portfolio. Cobimetinib bleibt nämlich hinter den wirtschaftlichen Erwartungen zurück.
Zitat von opportoni: Und? Meinst du, dass sich ein Einstieg hie nich lohnt? Du hast doch sicher jede Menge Informationen ausgewertet :-)
ich habe aus Nostalgiegründen noch ein paar Restaktien im Portfolio, würde diese momentan aber nicht zum Kauf empfehlen. Obschon Cabo tatsächlich eine Wunderwaffe zu sein scheint, fehlt die "Breite" in EXELs Portfolio. Cobimetinib bleibt nämlich hinter den wirtschaftlichen Erwartungen zurück.
Antwort auf Beitrag Nr.: 62.205.639 von Cyberhexe am 20.12.19 12:30:37Die Wunderwaffe Cabozantinib immer noch erfolgreich:
Exelixis Announces Results for Combination of Cabozantinib and Nivolumab With or Without Ipilimumab in Advanced Hepatocellular Carcinoma
PDF Version
– Data from the CheckMate 040 trial presented at the 2020 American Society of Clinical Oncology’s Gastrointestinal Cancers Symposium –
ALAMEDA, Calif.--(BUSINESS WIRE)--Jan. 24, 2020-- Exelixis, Inc. (NASDAQ: EXEL) today announced phase 1/2 clinical trial results from the combination of cabozantinib (CABOMETYX®) and nivolumab (Opdivo®) with or without ipilimumab (Yervoy®) in advanced hepatocellular carcinoma (HCC). Data from the cabozantinib combination cohort of the CheckMate 040 trial will be presented on Friday, January 24 during Rapid Abstract Session B from 7:00 – 7:45 a.m. PT at the 2020 American Society of Clinical Oncology’s Gastrointestinal Cancers Symposium (ASCO GI), which is being held in San Francisco, California, January 23-25, 2020. The data will also be included in Poster Session B from 12:00 – 1:30 p.m. PT and 4:30 – 5:30 p.m. PT on January 24.
Exelixis Announces Results for Combination of Cabozantinib and Nivolumab With or Without Ipilimumab in Advanced Hepatocellular Carcinoma
PDF Version
– Data from the CheckMate 040 trial presented at the 2020 American Society of Clinical Oncology’s Gastrointestinal Cancers Symposium –
ALAMEDA, Calif.--(BUSINESS WIRE)--Jan. 24, 2020-- Exelixis, Inc. (NASDAQ: EXEL) today announced phase 1/2 clinical trial results from the combination of cabozantinib (CABOMETYX®) and nivolumab (Opdivo®) with or without ipilimumab (Yervoy®) in advanced hepatocellular carcinoma (HCC). Data from the cabozantinib combination cohort of the CheckMate 040 trial will be presented on Friday, January 24 during Rapid Abstract Session B from 7:00 – 7:45 a.m. PT at the 2020 American Society of Clinical Oncology’s Gastrointestinal Cancers Symposium (ASCO GI), which is being held in San Francisco, California, January 23-25, 2020. The data will also be included in Poster Session B from 12:00 – 1:30 p.m. PT and 4:30 – 5:30 p.m. PT on January 24.
Gibt es eine 2. Chance bei Prostatakrebs: Cabzantinib gemeinsam mit dem Checkpoint-Hemmer von Roche Atezolizumab!
In 2014, the Bay Area biotech company Exelixis (EXEL) tried to develop a drug for prostate cancer; its clinical trial ended in failure and nearly shut down the company. A second attempt has delivered better, tumor-shrinking results, according to data released Monday, while still leaving some important questions unanswered.
In an interim look at the ongoing study, a combination regimen consisting of Exelixis’ targeted cancer drug Cabometyx and Roche’s checkpoint inhibitor Tecentriq demonstrated a tumor response rate of 32%. The median duration of response was 8.3 months.
The results were derived from 44 prostate cancer patients who still had growing, measurable tumors despite prior treatment with one more more novel hormone drugs. Twelve of the 44 patients also received prior treatment with docetaxel chemotherapy.
After Cabometyx (used on its own) failed in prostate cancer in 2014, Exelixis was forced to lay off 70% of its workforce. But development of the drug, which works by blocking certain cell-growth signals, continued and eventually paid off with approvals in kidney and liver cancer.
Related: Investor impatience with Gilead reclamation project sparks acquisition chatter
Cabometyx sales in the U.S. reached $765 million last year, but are now starting to level off due to competition. Exelixis believes the next leg of growth for Cabometyx will come from combination regimens in liver, lung, and prostate cancer. The company has set an aggressive revenue goal of $4 billion in sales in 2025.
Exelixis has already tripled patient enrollment in the Cabometyx/Tecentriq prostate cancer study to collect more data. The company intends to file with the Food and Drug Administration for accelerated approval in 2021, said Exelixis CEO Michael Morrissey.
Despite new treatment options approved in recent years, prostate cancer remains the most common cause of cancer death among U.S. men, behind lung cancer.
The 32% response rate for the Cabometyx/Tecentriq combination is “clinically meaningful” and should lead to an FDA approval because these men have an especially poor prognosis and limited treatment options, said Dr. Neeraj Agarwal, a prostate cancer expert and professor of medicine at the Huntsman Cancer Center at the University of Utah. Agarwal participated in the Exelixis clinical trial and is presenting the new data later this week at a cancer research meeting sponsored by the American Society of Clinical Oncology.
Dr. Ben Davies, a professor of urology at the University of Pittsburgh, called the Cabometyx/Tecentriq response rate “fine” but also “unclear” because nearly three-fourths of the patients were not treated previously with docetaxel chemotherapy, which could also confer a benefit.
“I would love to see what the data on the pretreated chemo patients looked like,” said Davies. “As it stands, it’s hard to consume the data with much zeal.” Davies was not involved in the Exelixis study.
Exelixis said patients with prior docetaxel treatment were counted among the responders to Cabometyx and Tecentriq, although details are not being disclosed at this time.
Agarwal said the docetaxel argument is moot because it is rarely used in actual clinical practice; side effects can be intolerable.
“These are older men with aggressive prostate cancer and a relatively short expectation for survival. These are not young people with melanoma. I am personally very excited for these [Cabometyx/Tecentriq] results because we need something new to offer to these patients,” he said.
In 2014, the Bay Area biotech company Exelixis (EXEL) tried to develop a drug for prostate cancer; its clinical trial ended in failure and nearly shut down the company. A second attempt has delivered better, tumor-shrinking results, according to data released Monday, while still leaving some important questions unanswered.
In an interim look at the ongoing study, a combination regimen consisting of Exelixis’ targeted cancer drug Cabometyx and Roche’s checkpoint inhibitor Tecentriq demonstrated a tumor response rate of 32%. The median duration of response was 8.3 months.
The results were derived from 44 prostate cancer patients who still had growing, measurable tumors despite prior treatment with one more more novel hormone drugs. Twelve of the 44 patients also received prior treatment with docetaxel chemotherapy.
After Cabometyx (used on its own) failed in prostate cancer in 2014, Exelixis was forced to lay off 70% of its workforce. But development of the drug, which works by blocking certain cell-growth signals, continued and eventually paid off with approvals in kidney and liver cancer.
Related: Investor impatience with Gilead reclamation project sparks acquisition chatter
Cabometyx sales in the U.S. reached $765 million last year, but are now starting to level off due to competition. Exelixis believes the next leg of growth for Cabometyx will come from combination regimens in liver, lung, and prostate cancer. The company has set an aggressive revenue goal of $4 billion in sales in 2025.
Exelixis has already tripled patient enrollment in the Cabometyx/Tecentriq prostate cancer study to collect more data. The company intends to file with the Food and Drug Administration for accelerated approval in 2021, said Exelixis CEO Michael Morrissey.
Despite new treatment options approved in recent years, prostate cancer remains the most common cause of cancer death among U.S. men, behind lung cancer.
The 32% response rate for the Cabometyx/Tecentriq combination is “clinically meaningful” and should lead to an FDA approval because these men have an especially poor prognosis and limited treatment options, said Dr. Neeraj Agarwal, a prostate cancer expert and professor of medicine at the Huntsman Cancer Center at the University of Utah. Agarwal participated in the Exelixis clinical trial and is presenting the new data later this week at a cancer research meeting sponsored by the American Society of Clinical Oncology.
Dr. Ben Davies, a professor of urology at the University of Pittsburgh, called the Cabometyx/Tecentriq response rate “fine” but also “unclear” because nearly three-fourths of the patients were not treated previously with docetaxel chemotherapy, which could also confer a benefit.
“I would love to see what the data on the pretreated chemo patients looked like,” said Davies. “As it stands, it’s hard to consume the data with much zeal.” Davies was not involved in the Exelixis study.
Exelixis said patients with prior docetaxel treatment were counted among the responders to Cabometyx and Tecentriq, although details are not being disclosed at this time.
Agarwal said the docetaxel argument is moot because it is rarely used in actual clinical practice; side effects can be intolerable.
“These are older men with aggressive prostate cancer and a relatively short expectation for survival. These are not young people with melanoma. I am personally very excited for these [Cabometyx/Tecentriq] results because we need something new to offer to these patients,” he said.
Antwort auf Beitrag Nr.: 62.652.697 von Cyberhexe am 12.02.20 06:56:45Vielleicht doch ein Investment wert?
Exelixis Outlines Key Priorities

https://finance.yahoo.com/news/exelixis-outlines-key-priorit…
Exelixis Outlines Key Priorities

https://finance.yahoo.com/news/exelixis-outlines-key-priorit…
Antwort auf Beitrag Nr.: 62.663.287 von pako21 am 12.02.20 22:17:14
KGV von 10. Keine Schulden. 1,3 Mrd. $ Cash in der Bilanz. Bei rund 6 Mrd. $ Marktkapitalisierung.
Wachstum von 1 Mrd. $ Umsatz auf 4 Mrd.$ Umsatz nicht eingepreist!
Ganz klarer Kauf!
Auch, weil jedes Jahr 0,6 Mrd.$ freeCashFlow hinzu kommen!
Zitat von pako21: Vielleicht doch ein Investment wert?
Exelixis Outlines Key Priorities
https://finance.yahoo.com/news/exelixis-outlines-key-priorit…
KGV von 10. Keine Schulden. 1,3 Mrd. $ Cash in der Bilanz. Bei rund 6 Mrd. $ Marktkapitalisierung.
Wachstum von 1 Mrd. $ Umsatz auf 4 Mrd.$ Umsatz nicht eingepreist!
Ganz klarer Kauf!
Auch, weil jedes Jahr 0,6 Mrd.$ freeCashFlow hinzu kommen!
Antwort auf Beitrag Nr.: 62.663.353 von Hugo-Ehon-Balder am 12.02.20 22:25:26Exelixis Announces First 100 Patients Enrolled in Phase 3 COSMIC-311 Pivotal Trial of Cabozantinib in Relapsed Radioiodine-Refractory Differentiated Thyroid Cancer
PDF Version
– Analysis for the co-primary endpoint of objective response rate and an interim analysis of progression-free survival expected in the second half of 2020 –
ALAMEDA, Calif.--(BUSINESS WIRE)--Feb. 25, 2020-- Exelixis, Inc. (NASDAQ: EXEL) today announced enrollment of the first 100 patients in COSMIC-311, a phase 3 pivotal trial evaluating cabozantinib (CABOMETYX®) versus placebo in patients with radioactive iodine-refractory differentiated thyroid cancer who have progressed after up to two vascular endothelial growth factor (VEGF) receptor-targeted therapies.
“Given the encouraging clinical activity observed for cabozantinib in phase 1 and 2 trials in differentiated thyroid cancer, and the poor prognosis for patients who have progressed after prior VEGF receptor-targeting therapy, it is exciting to reach this milestone for COSMIC-311,” said Gisela Schwab, M.D., President, Product Development and Medical Affairs and Chief Medical Officer, Exelixis. “This brings us one step closer to a first analysis that will help us better understand cabozantinib’s potential in treating patients with this intractable form of thyroid cancer. We look forward to sharing those initial results later this year.”
PDF Version
– Analysis for the co-primary endpoint of objective response rate and an interim analysis of progression-free survival expected in the second half of 2020 –
ALAMEDA, Calif.--(BUSINESS WIRE)--Feb. 25, 2020-- Exelixis, Inc. (NASDAQ: EXEL) today announced enrollment of the first 100 patients in COSMIC-311, a phase 3 pivotal trial evaluating cabozantinib (CABOMETYX®) versus placebo in patients with radioactive iodine-refractory differentiated thyroid cancer who have progressed after up to two vascular endothelial growth factor (VEGF) receptor-targeted therapies.
“Given the encouraging clinical activity observed for cabozantinib in phase 1 and 2 trials in differentiated thyroid cancer, and the poor prognosis for patients who have progressed after prior VEGF receptor-targeting therapy, it is exciting to reach this milestone for COSMIC-311,” said Gisela Schwab, M.D., President, Product Development and Medical Affairs and Chief Medical Officer, Exelixis. “This brings us one step closer to a first analysis that will help us better understand cabozantinib’s potential in treating patients with this intractable form of thyroid cancer. We look forward to sharing those initial results later this year.”
Antwort auf Beitrag Nr.: 63.070.771 von Cyberhexe am 19.03.20 21:41:21Cabozantinib erhält eine weitere Zulassung in Japan und zwar für Nierenkrebs (RCC), weshalb eine Meilensteinzahlung in Höhe von 31Mio$ fällig wird...und natürlich Lizenzgebühren auf den Umsatz!
Exelixis Announces Partner Takeda Receives Approval in Japan for CABOMETYX® (cabozantinib) Tablets for the Treatment of Curatively Unresectable or Metastatic Renal Cell Carcinoma
PDF Version
ALAMEDA, Calif.--(BUSINESS WIRE)--Mar. 25, 2020-- Exelixis, Inc. (NASDAQ: EXEL) today announced that Takeda Pharmaceutical Company Limited (Takeda), its partner responsible for the clinical development and commercialization of CABOMETYX® (cabozantinib) in Japan, received approval from the Japanese Ministry of Health, Labor and Welfare to manufacture and market CABOMETYX as a treatment for patients with curatively unresectable or metastatic renal cell carcinoma (RCC).
The approval is based on the results of three clinical trials: METEOR, the Exelixis-sponsored phase 3 pivotal trial of cabozantinib versus everolimus in patients with advanced RCC that experienced disease progression following treatment with at least one prior VEGF receptor tyrosine kinase inhibitor (VEGFR-TKI); CABOSUN, the Alliance for Clinical Trials in Oncology-sponsored phase 2 trial comparing cabozantinib with sunitinib in patients with previously untreated advanced RCC with intermediate- or poor-risk disease; and Cabozantinib-2001, a Takeda-sponsored phase 2 trial in 35 Japanese patients with advanced RCC who had progressed after prior VEGFR-TKI therapy.
“Nearly 17,000 new cases of renal cell carcinoma are estimated to be diagnosed in Japan annually, and since many cases are diagnosed at an advanced stage, the prognosis remains poor for these patients,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “The approval of CABOMETYX is an important milestone for people with kidney cancer in Japan, and we are excited to continue our collaboration with Takeda as we work to bring more options to patients who need novel therapies.”
Per the terms of Exelixis and Takeda’s collaboration and license agreement, Exelixis is eligible to receive a $31 million milestone payment from Takeda upon the first commercial sale of CABOMETYX for unresectable or metastatic RCC. In January 2020, Takeda applied for approval to manufacture and sell cabozantinib as a treatment for patients with unresectable hepatocellular carcinoma (HCC) that had progressed after prior systemic therapy in Japan, which triggered a $10 million milestone payment. Exelixis will also be eligible to receive further development, regulatory and first-sale milestone payments of up to $45 million from Takeda related both to previously treated and untreated RCC and previously treated HCC. Exelixis continues to be eligible to receive additional development, regulatory and first-sale milestones for potential future cabozantinib indications and is also eligible for sales revenue milestones and royalties on net sales of cabozantinib in Japan.
Takeda fully funds cabozantinib development activities that are exclusively for the benefit of Japan and is responsible for 20% of the costs associated with global cabozantinib clinical trials, providing the company opts into those trials.
https://ir.exelixis.com/news-releases/news-release-details/e…
Exelixis Announces Partner Takeda Receives Approval in Japan for CABOMETYX® (cabozantinib) Tablets for the Treatment of Curatively Unresectable or Metastatic Renal Cell Carcinoma
PDF Version
ALAMEDA, Calif.--(BUSINESS WIRE)--Mar. 25, 2020-- Exelixis, Inc. (NASDAQ: EXEL) today announced that Takeda Pharmaceutical Company Limited (Takeda), its partner responsible for the clinical development and commercialization of CABOMETYX® (cabozantinib) in Japan, received approval from the Japanese Ministry of Health, Labor and Welfare to manufacture and market CABOMETYX as a treatment for patients with curatively unresectable or metastatic renal cell carcinoma (RCC).
The approval is based on the results of three clinical trials: METEOR, the Exelixis-sponsored phase 3 pivotal trial of cabozantinib versus everolimus in patients with advanced RCC that experienced disease progression following treatment with at least one prior VEGF receptor tyrosine kinase inhibitor (VEGFR-TKI); CABOSUN, the Alliance for Clinical Trials in Oncology-sponsored phase 2 trial comparing cabozantinib with sunitinib in patients with previously untreated advanced RCC with intermediate- or poor-risk disease; and Cabozantinib-2001, a Takeda-sponsored phase 2 trial in 35 Japanese patients with advanced RCC who had progressed after prior VEGFR-TKI therapy.
“Nearly 17,000 new cases of renal cell carcinoma are estimated to be diagnosed in Japan annually, and since many cases are diagnosed at an advanced stage, the prognosis remains poor for these patients,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “The approval of CABOMETYX is an important milestone for people with kidney cancer in Japan, and we are excited to continue our collaboration with Takeda as we work to bring more options to patients who need novel therapies.”
Per the terms of Exelixis and Takeda’s collaboration and license agreement, Exelixis is eligible to receive a $31 million milestone payment from Takeda upon the first commercial sale of CABOMETYX for unresectable or metastatic RCC. In January 2020, Takeda applied for approval to manufacture and sell cabozantinib as a treatment for patients with unresectable hepatocellular carcinoma (HCC) that had progressed after prior systemic therapy in Japan, which triggered a $10 million milestone payment. Exelixis will also be eligible to receive further development, regulatory and first-sale milestone payments of up to $45 million from Takeda related both to previously treated and untreated RCC and previously treated HCC. Exelixis continues to be eligible to receive additional development, regulatory and first-sale milestones for potential future cabozantinib indications and is also eligible for sales revenue milestones and royalties on net sales of cabozantinib in Japan.
Takeda fully funds cabozantinib development activities that are exclusively for the benefit of Japan and is responsible for 20% of the costs associated with global cabozantinib clinical trials, providing the company opts into those trials.
https://ir.exelixis.com/news-releases/news-release-details/e…
Antwort auf Beitrag Nr.: 63.160.693 von Cyberhexe am 28.03.20 11:02:15Cabozantinib die Wunderwaffe hat gemeinsam mit Nivolumab wieder einen primären Endpunkt errreicht!
Bristol Myers Squibb and Exelixis Announce Positive Topline Results from Pivotal Phase 3 CheckMate -9ER Trial Evaluating Opdivo® (nivolumab) in Combination with CABOMETYX® (cabozantinib) in Previously Untreated Advanced Renal Cell Carcinoma
Study met primary endpoint of significantly improving progression-free survival, and secondary endpoints of overall survival and objective response rate vs. sunitinib
Opdivo in combination with CABOMETYX demonstrates clinically meaningful efficacy results across all endpoints and preliminary assessment showing a favorable safety profile
https://ir.exelixis.com/news-releases/news-release-details/b…
Bristol Myers Squibb and Exelixis Announce Positive Topline Results from Pivotal Phase 3 CheckMate -9ER Trial Evaluating Opdivo® (nivolumab) in Combination with CABOMETYX® (cabozantinib) in Previously Untreated Advanced Renal Cell Carcinoma
Study met primary endpoint of significantly improving progression-free survival, and secondary endpoints of overall survival and objective response rate vs. sunitinib
Opdivo in combination with CABOMETYX demonstrates clinically meaningful efficacy results across all endpoints and preliminary assessment showing a favorable safety profile
https://ir.exelixis.com/news-releases/news-release-details/b…
das neuste von 157 Patenten:
Results of Search in US Patent Collection db for:
AN/exelixis: 157 patents.
Hits 1 through 50 out of 157
Results of Search in US Patent Collection db for:
AN/exelixis: 157 patents.
Hits 1 through 50 out of 157

Antwort auf Beitrag Nr.: 63.399.185 von Cyberhexe am 21.04.20 13:36:06Exelixis Announces Submission of Supplemental New Drug Application to U.S. Food and Drug Administration for CABOMETYX® (cabozantinib) in Combination With Opdivo® (nivolumab) for Advanced Renal Cell Carcinoma
https://ir.exelixis.com/press-releases
...meine EXEL-Nostalgie-Position verbleibt im Portfolio....auf immer und ewig...so viel Sentimentalität muss einfach sein!
https://ir.exelixis.com/press-releases
...meine EXEL-Nostalgie-Position verbleibt im Portfolio....auf immer und ewig...so viel Sentimentalität muss einfach sein!

Antwort auf Beitrag Nr.: 65.007.909 von Cyberhexe am 07.09.20 12:05:10Phase I Study of Cabozantinib and Nivolumab Alone or With Ipilimumab for Advanced or Metastatic Urothelial Carcinoma and Other Genitourinary Tumors
RESULTS
Fifty-four patients were enrolled at eight dose levels with a median follow-up time of 44.6 months; data cutoff was January 20, 2020. Grade 3 or 4 treatment-related adverse events (AEs) occurred in 75% and 87% of patients treated with CaboNivo and CaboNivoIpi, respectively, and included fatigue (17% and 10%, respectively), diarrhea (4% and 7%, respectively), and hypertension (21% and 10%, respectively); grade 3 or 4 immune-related AEs included hepatitis (0% and 13%, respectively) and colitis (0% and 7%, respectively).
https://ascopubs.org/doi/abs/10.1200/JCO.20.01652?journalCod…
RESULTS
Fifty-four patients were enrolled at eight dose levels with a median follow-up time of 44.6 months; data cutoff was January 20, 2020. Grade 3 or 4 treatment-related adverse events (AEs) occurred in 75% and 87% of patients treated with CaboNivo and CaboNivoIpi, respectively, and included fatigue (17% and 10%, respectively), diarrhea (4% and 7%, respectively), and hypertension (21% and 10%, respectively); grade 3 or 4 immune-related AEs included hepatitis (0% and 13%, respectively) and colitis (0% and 7%, respectively).
https://ascopubs.org/doi/abs/10.1200/JCO.20.01652?journalCod…
Exelixis and Iconic Therapeutics Announce Promising Preclinical Data That Support Best-in-Class Potential for ICON-2 in Treatment of Solid Tumors
https://ir.exelixis.com/news-releases/news-release-details/e…
Cabo und Cobi erhalten Zuwachs!
https://ir.exelixis.com/news-releases/news-release-details/e…
Cabo und Cobi erhalten Zuwachs!
Cabozantinib hält wirklich fast alle Versprechen (bis auf mCRPC):
LUGANO, Switzerland – The results of the phase 3 CheckMate 9ER trial have provided a new first-line treatment option for patients with metastatic kidney cancer. The late breaking results are presented at ESMO 2020. (1)
The trial took two drugs used as monotherapies in the second line, nivolumab and cabozantinib, and combined them for use as a first-line treatment against standard of care, sunitinib. The combination was superior to sunitinib for progression-free survival, overall survival, and response rate.
Quelle:
https://www.esmo.org/newsroom/press-office/esmo2020-metastat…
LUGANO, Switzerland – The results of the phase 3 CheckMate 9ER trial have provided a new first-line treatment option for patients with metastatic kidney cancer. The late breaking results are presented at ESMO 2020. (1)
The trial took two drugs used as monotherapies in the second line, nivolumab and cabozantinib, and combined them for use as a first-line treatment against standard of care, sunitinib. The combination was superior to sunitinib for progression-free survival, overall survival, and response rate.
Quelle:
https://www.esmo.org/newsroom/press-office/esmo2020-metastat…
Antwort auf Beitrag Nr.: 65.203.861 von Cyberhexe am 27.09.20 07:22:47In der klinischen Phase-3-Studie führte ein Behandlungsschema, das die Opdivo-Immuntherapie von BMS und das zielgerichtete Krebsmedi Cabozantinib bzw. Cabometyx von Exelixis kombiniert, zu einer 40%igen Senkung des Sterberisikos im Vergleich zu Sutent, einem älteren Nierenkrebs-Medikament, das von Pfizer vertrieben wird. Die Studie mit dem Namen Checkmate-9ER wurde auf der Jahrestagung der Europäischen Gesellschaft für medizinische Onkologie (ESMO) vorgestellt. Die Opdivo-Cabometyx-Kombi reduzierte das Risiko des Fortschreitens der Krankheit um 49% im Vergleich zu Sutent. Im Median verstrichen bei den mit der Kombination behandelten Patienten mehr als 16 Monate, ohne dass sich ihre Krankheit verschlechterte, doppelt so lange wie bei den Patienten in der Sutent-Kohorte. Einundsechzig Prozent der Patienten berichteten über mittelschwere oder schwere Nebenwirkungen, die dem Kombinationsregime zugeschrieben wurden, obwohl die Abbruchrate mit 3% niedriger ist als in der Sutent-Armstudie. Toni Choueiri, ein Spezialist für Nierenkrebs am Dana-Farber Cancer Institute in Boston und einer der Prüfer der Checkmate-9ER-Studie, sagt, dass die starken, konsistenten Ergebnisse das Opdivo-Cabometyx-Schema zu einer "wichtigen Behandlungsoption" für Patienten mit fortgeschrittenem Nierenkrebs machen.
https://www.esmo.org/newsroom/press-office/esmo2020-metastat…
https://www.esmo.org/newsroom/press-office/esmo2020-metastat…
Antwort auf Beitrag Nr.: 65.239.159 von Cyberhexe am 30.09.20 16:53:04Cabozantinib übertrifft viele Erwartungen - meine nicht!
U.S. Food and Drug Administration Accepts for Priority Review Applications for OPDIVO® (nivolumab) in Combination with CABOMETYX® (cabozantinib) in Advanced Renal Cell Carcinoma
https://ir.exelixis.com/news-releases/news-release-details/u…
U.S. Food and Drug Administration Accepts for Priority Review Applications for OPDIVO® (nivolumab) in Combination with CABOMETYX® (cabozantinib) in Advanced Renal Cell Carcinoma
https://ir.exelixis.com/news-releases/news-release-details/u…
Die Aktie von Exelixis Inc ist in diesen Tagen günstiger als sonst zu haben:
Nach oben ist eine Kurslücke entstanden.
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| -0,62 | |
| -7,65 | |
| -0,47 | |
| -3,30 | |
| -0,70 | |
| +0,15 | |
| -1,38 | |
| +0,02 | |
| +0,19 | |
| +0,20 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 244 | ||
| 98 | ||
| 69 | ||
| 59 | ||
| 42 | ||
| 36 | ||
| 32 | ||
| 25 | ||
| 24 | ||
| 24 |
01.04.24 · wO Chartvergleich · Consolidated Water |
29.03.24 · wO Chartvergleich · Consolidated Water |
28.03.24 · wO Chartvergleich · Energiekontor |


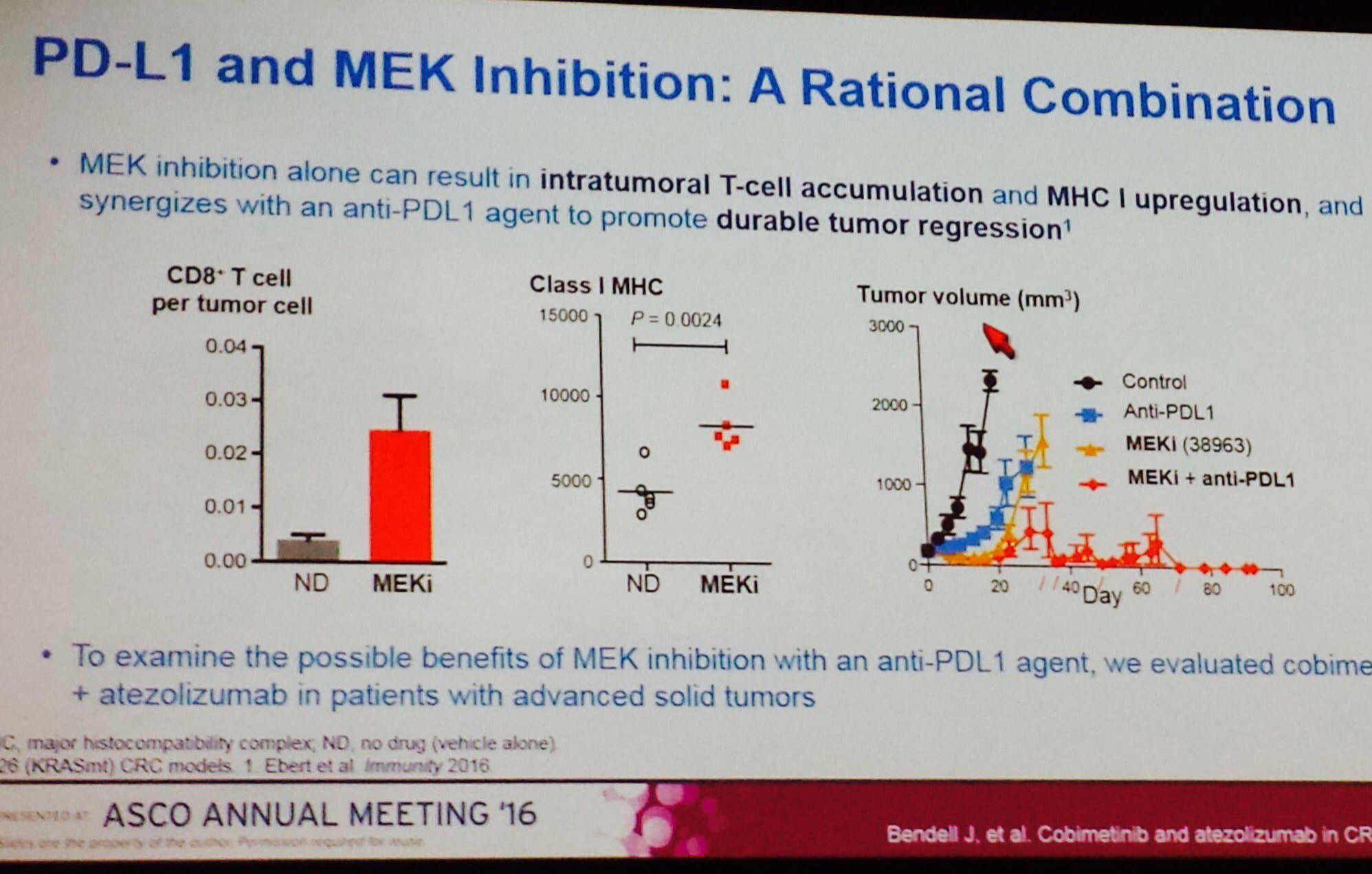
 ...aber die Verlockung ist nun mal zu gross!
...aber die Verlockung ist nun mal zu gross!


















