3D Signatures und es macht Boom - 500 Beiträge pro Seite
eröffnet am 17.03.17 11:38:06 von
neuester Beitrag 24.09.18 22:35:59 von
neuester Beitrag 24.09.18 22:35:59 von
Beiträge: 78
ID: 1.248.985
ID: 1.248.985
Aufrufe heute: 0
Gesamt: 6.944
Gesamt: 6.944
Aktive User: 0
ISIN: CA87975M2085 · WKN: A2PV0Q · Symbol: 3D0A
0,1340
EUR
+7,20 %
+0,0090 EUR
Letzter Kurs 22.04.24 Tradegate
Neuigkeiten
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 4,0000 | +67,36 | |
| 1,4800 | +33,82 | |
| 6,1000 | +31,38 | |
| 10,625 | +26,94 | |
| 2,9200 | +18,70 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 6,5500 | -18,13 | |
| 3,3500 | -18,89 | |
| 5,6050 | -29,85 | |
| 0,6021 | -35,26 | |
| 3,9400 | -43,31 |
Was haltet ihr von diesem Geheimtipp ?
Antwort auf Beitrag Nr.: 54.554.917 von Wohnwunsch am 17.03.17 11:38:06
wattttttt machen Die denn????????????????
wattttttt machen Die denn????????????????
Antwort auf Beitrag Nr.: 54.557.188 von Popeye82 am 17.03.17 15:57:20was heißt hier geheim…
iss mir bekannt
und is auf meiner Watch
iss mir bekannt
und is auf meiner Watch

Wunschwohnen
Antwort auf Beitrag Nr.: 54.557.188 von Popeye82 am 17.03.17 15:57:20das steht doch in der Überschrift es macht Boom 

Antwort auf Beitrag Nr.: 54.561.580 von bmuesli am 18.03.17 12:37:25
und Warum soll dit juuuuut sein???
und Warum soll dit juuuuut sein???
Antwort auf Beitrag Nr.: 54.562.084 von Popeye82 am 18.03.17 14:25:50
Booom?
http://www.reuters.com/article/brief-3d-signatures-software-…
Interessante Meldung. Ich warte auf das nächste pp.
seit ein paar Tagen auch Xetra Handel - da kommt wohl was Größeres.
Antwort auf Beitrag Nr.: 54.592.515 von techinvestor69 am 22.03.17 20:14:59
Vermutlich eine größere Push-Aktion...
"On December 16, 2016, the Company issued 5,187,618 units (the “Units) as part of the first tranche of its brokered private placement (the “Private Placement”) at a price per unit of $0.75 per share for gross proceeds of $3,890,714."
Das ist in etwa auch Alles, was die per 31.12.2016 an Cash besaßen.
"Each unit consists of one common share of the Company, and one purchase warrant (a “Warrant”). Each purchase warrant entitles the holder to purchase one additional common share at a price of $0.92 until December 16, 2018. In the event that, any time after June 16, 2017, the closing price of the Company’s common shares on the TSX Venture Exchange, exceeds $1.35 for a period of 20
consecutive trading days, the Company may accelerate the expiry date of the Warrants to that date that is 30 days following the date on which the Company sends notice to the holds of the Warrants of the new expiry date."
Haltefrist Warrants 4 Monate, d.h. die können nun bald gewandelt werden. Muss nur noch der Kurs gepusht werden.
Bin gespannt, WER hier dafür bezahlt wird, Werbung für diese Company in Deutschland zu machen. Dafür vermutlich auch Xetra.
In Kanada keiner interessiert von den Herren Investoren ? Nur meine Meinung.
Quelle: Finanzbericht 3M/6M per 31.12.2016
Mar 1 2017 - 16:22:10 ET - Interim financial statements/report - English
http://www.sedar.com/DisplayCompanyDocuments.do?lang=EN&issu…
Zitat von techinvestor69: seit ein paar Tagen auch Xetra Handel - da kommt wohl was Größeres.
Vermutlich eine größere Push-Aktion...

"On December 16, 2016, the Company issued 5,187,618 units (the “Units) as part of the first tranche of its brokered private placement (the “Private Placement”) at a price per unit of $0.75 per share for gross proceeds of $3,890,714."
Das ist in etwa auch Alles, was die per 31.12.2016 an Cash besaßen.

"Each unit consists of one common share of the Company, and one purchase warrant (a “Warrant”). Each purchase warrant entitles the holder to purchase one additional common share at a price of $0.92 until December 16, 2018. In the event that, any time after June 16, 2017, the closing price of the Company’s common shares on the TSX Venture Exchange, exceeds $1.35 for a period of 20
consecutive trading days, the Company may accelerate the expiry date of the Warrants to that date that is 30 days following the date on which the Company sends notice to the holds of the Warrants of the new expiry date."
Haltefrist Warrants 4 Monate, d.h. die können nun bald gewandelt werden. Muss nur noch der Kurs gepusht werden.

Bin gespannt, WER hier dafür bezahlt wird, Werbung für diese Company in Deutschland zu machen. Dafür vermutlich auch Xetra.

In Kanada keiner interessiert von den Herren Investoren ? Nur meine Meinung.
Quelle: Finanzbericht 3M/6M per 31.12.2016
Mar 1 2017 - 16:22:10 ET - Interim financial statements/report - English
http://www.sedar.com/DisplayCompanyDocuments.do?lang=EN&issu…
Das Thema warrants hast Du noch nicht verstanden, oder? Es macht keinen Sinn zu wandeln, wenn der Kurs unter dem Ausübungspreis ist...
Antwort auf Beitrag Nr.: 54.665.171 von techinvestor69 am 03.04.17 14:24:03"..wenn der Kurs unter dem Ausübungspreis ist..."
NOCH ist der das nicht. Ich habe sehr gut verstanden. Du meinen Wink mit dem Zaunpfahl wohl nicht.
NOCH ist der das nicht. Ich habe sehr gut verstanden. Du meinen Wink mit dem Zaunpfahl wohl nicht.

Der Ausübungspreis der warrants ist 0.92 CAD
Aktienkurs ist 0,70.
Also was soll Dein Quatsch?
Aktienkurs ist 0,70.
Also was soll Dein Quatsch?
Antwort auf Beitrag Nr.: 54.665.210 von tbhomy am 03.04.17 14:27:15Noch ist er nicht drüber, war gemeint. Dazu braucht es hier in Deutschland vermutlich einen Börsenbrief oder Ähnliches.
Nur meine Meinung.
Nur meine Meinung.
Schaun wir mal. Sechsstelliges Handelsvolumen in Kanada, also das Interesse an der Heimatbörse ist durchaus da.
Antwort auf Beitrag Nr.: 54.665.423 von techinvestor69 am 03.04.17 14:52:23
News
https://www.equities.com/companies/dxd-ca !
Dieser Beitrag wurde von MODelfin moderiert. Grund: Provokation
Antwort auf Beitrag Nr.: 54.719.949 von Popeye82 am 11.04.17 19:27:54
1. Hat 3DS den Antrag auf Börsennotierung im deutschen Freiverkehr selbst gestellt und ist verpflichteter MTF-Emittent nach MMVO 2016 ?
2. Wird es Unternehmensmeldungen in deutscher Sprache geben ?
3. Hat jemand für mich eine Liste mit den Investorenkonferenzen, an denen 3DS in 2016/17 teilgenommen hatte bzw. noch teilnimmt ?
Ich möchte vor einem Invest gerne wissen, mit wem oder was ich es bei der Company zu tun habe. Ich mag keine unliebsamen Überraschungen bei kleinen kanadischen Butzen. Vor Allem im Bereich der Unternehmensfinanzierung nicht. Danke.
Qualität des Emittenten 3D Signatures
Warum hat ein Unternehmen wie 3DS es nötig, sich über Kleinaktionäre in Deutschland zu finanzieren ?1. Hat 3DS den Antrag auf Börsennotierung im deutschen Freiverkehr selbst gestellt und ist verpflichteter MTF-Emittent nach MMVO 2016 ?
2. Wird es Unternehmensmeldungen in deutscher Sprache geben ?
3. Hat jemand für mich eine Liste mit den Investorenkonferenzen, an denen 3DS in 2016/17 teilgenommen hatte bzw. noch teilnimmt ?
Ich möchte vor einem Invest gerne wissen, mit wem oder was ich es bei der Company zu tun habe. Ich mag keine unliebsamen Überraschungen bei kleinen kanadischen Butzen. Vor Allem im Bereich der Unternehmensfinanzierung nicht. Danke.
Antwort auf Beitrag Nr.: 54.723.429 von tbhomy am 12.04.17 10:23:59
Ich möchte vor einem Invest gerne wissen, mit wem oder was ich es bei der Company zu tun habe. Ich mag keine unliebsamen Überraschungen bei kleinen kanadischen Butzen. Vor Allem im Bereich der Unternehmensfinanzierung nicht
_________________________________________________________________________
das ist verständlich, legetim -und auch richtig so-,
aber -wenn man sich Deine sonstige Ergüsse ansieht-
beim Verstehen wird es denke ich hapern
Ich möchte vor einem Invest gerne wissen, mit wem oder was ich es bei der Company zu tun habe. Ich mag keine unliebsamen Überraschungen bei kleinen kanadischen Butzen. Vor Allem im Bereich der Unternehmensfinanzierung nicht
_________________________________________________________________________
das ist verständlich, legetim -und auch richtig so-,
aber -wenn man sich Deine sonstige Ergüsse ansieht-
beim Verstehen wird es denke ich hapern
Antwort auf Beitrag Nr.: 54.729.234 von Popeye82 am 12.04.17 22:49:17Meine Worte lauteten: "Haltefrist Warrants 4 Monate, d.h. die können nun bald gewandelt werden. Muss nur noch der Kurs gepusht werden."
Wer sich mit Warrants bzw. der Unternehmensfinanzierung bei kanadischen Aktiengesellschaften nicht auskennt, sollte die Finger von diesen Werten lassen. Es sei denn, er ist mit Blindflug und Kasino bereits zufrieden.
War das etwas verständlicher ?
Nur meine Meinung.
Wer sich mit Warrants bzw. der Unternehmensfinanzierung bei kanadischen Aktiengesellschaften nicht auskennt, sollte die Finger von diesen Werten lassen. Es sei denn, er ist mit Blindflug und Kasino bereits zufrieden.
War das etwas verständlicher ?
Nur meine Meinung.
Antwort auf Beitrag Nr.: 54.731.004 von tbhomy am 13.04.17 10:07:15
Herr Mister Homy,
Das hatte ich schon im vorletzten Jahrhundert verstanden.
Sie sollten sich darum kümmern Die Sachen zu verstehen, die Sie hier nicht verstehen.
Oder den Thread (inkl. BEE(Wo sie genauso planlos sind)) bitte mit Ihren idiotischen Ergüssen verschonen.
Ich hoffe deutlich genug gesprochen zu haben.
Die freie Westliche Welt DANKT Ihnen
Herr Mister Homy,
Das hatte ich schon im vorletzten Jahrhundert verstanden.
Sie sollten sich darum kümmern Die Sachen zu verstehen, die Sie hier nicht verstehen.
Oder den Thread (inkl. BEE(Wo sie genauso planlos sind)) bitte mit Ihren idiotischen Ergüssen verschonen.
Ich hoffe deutlich genug gesprochen zu haben.
Die freie Westliche Welt DANKT Ihnen
Antwort auf Beitrag Nr.: 54.731.931 von Popeye82 am 13.04.17 11:53:13
Ich war davon ausgegangen, dass es sich hier um ein Diskussionsforum zu Börsenthemen handelt und es deutliche und für alle lesbare Forenregeln gibt.
Wer wirklich planlos zu kanadischen Reverse-Mergern ist, habe ich innerhalb der letzten 5 Jahre ausreichend recherchieren können.
Wer im Glashaus sitzt, sollte zudem nicht mit Steinen werfen...
Habe die Ehre.
Das ist eine Beleidigung, Popeye82.
Ich nehme die Beleidigung hier im öffentlichen Forum von Wallstreet-Onlone.de zur Kenntnis. Und lasse sie als schlechtes Beispiel für alle lesbar stehen.Ich war davon ausgegangen, dass es sich hier um ein Diskussionsforum zu Börsenthemen handelt und es deutliche und für alle lesbare Forenregeln gibt.
Wer wirklich planlos zu kanadischen Reverse-Mergern ist, habe ich innerhalb der letzten 5 Jahre ausreichend recherchieren können.
Wer im Glashaus sitzt, sollte zudem nicht mit Steinen werfen...
Habe die Ehre.
Antwort auf Beitrag Nr.: 54.733.368 von tbhomy am 13.04.17 15:05:36
Hochverehrter Sir Homy,
Ich gebe Ihnen noch Eine Chance, ansonsten geht es in Den Ignore Kerker.
Ich bin mir immer öfter unschlüssig ob es sich für User und Menschen lohnt >2,3 Zeilen Postings abzufassen, und komme ehrlich gesagt immer, immer öfter zu: NEIN.
Ich werde bei Ihnen Kleinen Lernverweigerer die Tage vielleicht nochmal eine Ausnahme machen,
und ein paar mehr Zeilen schreiben(die Einen wirklich auskömmlichen gemeinsamen Nenner darstellen sollten),
wenn dann aber von Ihnen, wieder mal, Nichts sinnvolles kommt,
und Sie ein bisschen FARBE bekennen; in Diesem Falle: Adios, forever.
HOChachtungsvoll,
Ihr P.
Hochverehrter Sir Homy,
Ich gebe Ihnen noch Eine Chance, ansonsten geht es in Den Ignore Kerker.
Ich bin mir immer öfter unschlüssig ob es sich für User und Menschen lohnt >2,3 Zeilen Postings abzufassen, und komme ehrlich gesagt immer, immer öfter zu: NEIN.
Ich werde bei Ihnen Kleinen Lernverweigerer die Tage vielleicht nochmal eine Ausnahme machen,
und ein paar mehr Zeilen schreiben(die Einen wirklich auskömmlichen gemeinsamen Nenner darstellen sollten),
wenn dann aber von Ihnen, wieder mal, Nichts sinnvolles kommt,
und Sie ein bisschen FARBE bekennen; in Diesem Falle: Adios, forever.
HOChachtungsvoll,
Ihr P.
Antwort auf Beitrag Nr.: 54.734.004 von Popeye82 am 13.04.17 16:13:48
Ich bin an Ihrem "gemeinsamen Nenner" nicht interessiert, denn ich stelle mich nicht mit Pöblern auf eine Stufe.
Ich poste meine Meinung in diesem öffentlichen Forum, wie es mir nach meiner Meinungsfreiheit beliebt. OHNE andere User zu beleidigen. Falls Sie mit Sachlichkeit in der Argumentation nicht mehr weiterkommen, haben Sie noch lange nicht das Recht, andere User oder mich zu beleidigen. Siehe auch Thread zu Bee Vectoring.
Ich bleibe dabei: 3D Signatures hat in meinen Augen nichts im deutschen Freiverkehr zu suchen. Und ich hoffe, dass man in Brüssel seitens der ESMA auch zu derart Schlüssen kommen wird bis 2018. SCALE z.B. ist ein guter weiterer Schritt zu mehr Transparenz an deutschen Börsen.
Zitat von Popeye82: Hochverehrter Sir Homy,
Ich gebe Ihnen noch Eine Chance, ansonsten geht es in Den Ignore Kerker.
Ich bin mir immer öfter unschlüssig ob es sich für User und Menschen lohnt >2,3 Zeilen Postings abzufassen, und komme ehrlich gesagt immer, immer öfter zu: NEIN.
Ich werde bei Ihnen Kleinen Lernverweigerer die Tage vielleicht nochmal eine Ausnahme machen,
und ein paar mehr Zeilen schreiben(die Einen wirklich auskömmlichen gemeinsamen Nenner darstellen sollten),
wenn dann aber von Ihnen, wieder mal, Nichts sinnvolles kommt,
und Sie ein bisschen FARBE bekennen; in Diesem Falle: Adios, forever.
HOChachtungsvoll,
Ihr P.
Ich bin an Ihrem "gemeinsamen Nenner" nicht interessiert, denn ich stelle mich nicht mit Pöblern auf eine Stufe.
Ich poste meine Meinung in diesem öffentlichen Forum, wie es mir nach meiner Meinungsfreiheit beliebt. OHNE andere User zu beleidigen. Falls Sie mit Sachlichkeit in der Argumentation nicht mehr weiterkommen, haben Sie noch lange nicht das Recht, andere User oder mich zu beleidigen. Siehe auch Thread zu Bee Vectoring.
Ich bleibe dabei: 3D Signatures hat in meinen Augen nichts im deutschen Freiverkehr zu suchen. Und ich hoffe, dass man in Brüssel seitens der ESMA auch zu derart Schlüssen kommen wird bis 2018. SCALE z.B. ist ein guter weiterer Schritt zu mehr Transparenz an deutschen Börsen.
Dann viel Erfolg beim weiterhin im Dunkeln tappen.
Ahnungslosigkeit UNLIMITED, das können Sie ja so gut.
Auf Nimmerwiedersehen
Ahnungslosigkeit UNLIMITED, das können Sie ja so gut.
Auf Nimmerwiedersehen
so, ich hab jetzt mal bei DXD und BEE
~60% der user auf Ignore gesetzt.
haben Die hart für gearbeitet.
300 (Ignores)schaff in diesem board höchstwahrscheinlich noch.
~60% der user auf Ignore gesetzt.
haben Die hart für gearbeitet.
300 (Ignores)schaff in diesem board höchstwahrscheinlich noch.
wer einen kleinen Überblick bekommen will SOLLTE es mal lesen:
http://investingnews.com/company-profiles/3d-signatures-canc…

http://investingnews.com/company-profiles/3d-signatures-canc…
sehr gute meldung iMo.
MaRS DD kenne ich, nämlich auch, ein bisschen,
hab von Denen -schon lange-auch eine pre-IPO Firma auf der Liste.
geht dabei mehr um das ENVIRONMENT,
sollte Ihnen operativ nochmal richtigen Schub geben:
www.thelifesciencesreport.com/pub/qmdata/50638?utm_source=de…
MaRS DD kenne ich, nämlich auch, ein bisschen,
hab von Denen -schon lange-auch eine pre-IPO Firma auf der Liste.
geht dabei mehr um das ENVIRONMENT,
sollte Ihnen operativ nochmal richtigen Schub geben:
www.thelifesciencesreport.com/pub/qmdata/50638?utm_source=de…
Dieses Bild ist nicht SSL-verschlüsselt: [url]http://tailwindsresearch.com/wp-content/uploads/2017/04/3DS-696x391.png
[/url]http://tailwindsresearch.com/2017/04/3d-signatures-disruptiv…
------>
Antwort auf Beitrag Nr.: 54.835.851 von Popeye82 am 29.04.17 23:43:20
http://www.sedar.com/GetFile.do?lang=EN&docClass=1&issuerNo=…
http://www.sedar.com/GetFile.do?lang=EN&docClass=1&issuerNo=…
Antwort auf Beitrag Nr.: 54.835.865 von Popeye82 am 30.04.17 00:04:40
“R&D” means research and development
“R&D” means research and development
first EVER large-scale multi-center study, focused on men with a clinical suspicion of prostate cancer
------> http://tailwindsresearch.com/2017/04/3d-signatures-receives-…
Dieses Bild ist nicht SSL-verschlüsselt: [url]http://tailwindsresearch.com/wp-content/uploads/2017/04/bigstock-160961438-300x200.jpg
[/url]------> http://tailwindsresearch.com/2017/04/3d-signatures-receives-…
3DS’ genomic imaging for precision medicine
May 02, 2017
http://www.biotuesdays.com/features/2017/4/26/3ds-genomic-im…
May 02, 2017
http://www.biotuesdays.com/features/2017/4/26/3ds-genomic-im…
Ich habe 3D Signatures schon eine Weile auf der Watchlist. Die Aktie leidet unter den früheren Finanzierungen, insbesondere der "Plicit Transaction".
Die Technologie ist extrem spannend, wobei es im Bereich der Erkennung von Krebs u.a. sehr viele neue Ansätze gibt, die allesamt vielversprechend sind.
Ich denke, dass die Aktie noch eine Weile unter Druck bleiben wird bzw. bei entsprechenden News auch immer mal wieder "Hüpfer" nach oben vollzieht - aber tendenziell ist es hier m.E. noch zu früh für ein Investment:
"Are you getting close to commercializing your tests in the U.S.?
We’re getting a lot of interest about bringing our tests to the U.S. market and we’re currently evaluating a variety of partnership models with lab companies that already have existing infrastructure and their own CLIA-approved lab. Our goal is to bring our first test for Hodgkin’s lymphoma to market in the first quarter next year and over the course of this year, we’ll determine whom to partner with. Our tests for prostate cancer, multiple myeloma and lung cancer are still two to three years away and longer-term, the game plan includes possibly having our own CLIA-approved lab."
http://www.biotuesdays.com/features/2017/4/26/3ds-genomic-im…
Bis dahin werden sie noch weiteres Kapital brauchen, es wird also weitere Verwässerung geben. Und wenn der Kurs weiter abwärts driftet, wird die Verwässerung leider umso größer ausfallen.
Ab Herbst nehme ich die Aktie wieder ins Visier - oder wenn es wichtige News gibt (z.B. Partnerschaft mit Kostenübernahme des Partners).
M@trix
Die Technologie ist extrem spannend, wobei es im Bereich der Erkennung von Krebs u.a. sehr viele neue Ansätze gibt, die allesamt vielversprechend sind.
Ich denke, dass die Aktie noch eine Weile unter Druck bleiben wird bzw. bei entsprechenden News auch immer mal wieder "Hüpfer" nach oben vollzieht - aber tendenziell ist es hier m.E. noch zu früh für ein Investment:
"Are you getting close to commercializing your tests in the U.S.?
We’re getting a lot of interest about bringing our tests to the U.S. market and we’re currently evaluating a variety of partnership models with lab companies that already have existing infrastructure and their own CLIA-approved lab. Our goal is to bring our first test for Hodgkin’s lymphoma to market in the first quarter next year and over the course of this year, we’ll determine whom to partner with. Our tests for prostate cancer, multiple myeloma and lung cancer are still two to three years away and longer-term, the game plan includes possibly having our own CLIA-approved lab."
http://www.biotuesdays.com/features/2017/4/26/3ds-genomic-im…
Bis dahin werden sie noch weiteres Kapital brauchen, es wird also weitere Verwässerung geben. Und wenn der Kurs weiter abwärts driftet, wird die Verwässerung leider umso größer ausfallen.
Ab Herbst nehme ich die Aktie wieder ins Visier - oder wenn es wichtige News gibt (z.B. Partnerschaft mit Kostenübernahme des Partners).
M@trix
Antwort auf Beitrag Nr.: 54.937.403 von Popeye82 am 13.05.17 14:28:54

- By the time signs of Alzheimer’s disease manifests in the form of memory problems, behaviorial changes, or loss of executive function, years of irreversible damage to the brain has already occurred. In the latest annual report from the Alzheimer’s Association, the organization looks at the potential for biomarkers to diagnose the disease at its earliest appearance and allow for treatments to hold the disease in check. We spoke to Heather Snyder, senior director of medical and scientific operations for the Alzheimer’s Association about the report, where efforts to validate biomarkers of early-stage Alzhimer’s disease stand, and why this holds the potential to change the way Alzheimer’s disease is viewed and treated much in the way diagnostic tools have turned heart disease into a chronic condition -
------> https://soundcloud.com/levine-media-group/how-biomarkers-can…
- By the time signs of Alzheimer’s disease manifests in the form of memory problems, behaviorial changes, or loss of executive function, years of irreversible damage to the brain has already occurred. In the latest annual report from the Alzheimer’s Association, the organization looks at the potential for biomarkers to diagnose the disease at its earliest appearance and allow for treatments to hold the disease in check. We spoke to Heather Snyder, senior director of medical and scientific operations for the Alzheimer’s Association about the report, where efforts to validate biomarkers of early-stage Alzhimer’s disease stand, and why this holds the potential to change the way Alzheimer’s disease is viewed and treated much in the way diagnostic tools have turned heart disease into a chronic condition -
------> https://soundcloud.com/levine-media-group/how-biomarkers-can…
Was hatte ich am 4. April noch gleich gepostet ? Da stand der Kurs im Schluss bei 0,511 Euro.
Und heute ? 0,379 Euro.
In Kanada keiner interessiert von den Herren Investoren ? Nur meine Meinung. Oh, dass sagte ich ja bereits schon einmal.
Und heute ? 0,379 Euro.
In Kanada keiner interessiert von den Herren Investoren ? Nur meine Meinung. Oh, dass sagte ich ja bereits schon einmal.

!
Dieser Beitrag wurde von CommunitySupport moderiert. Grund: Beleidigung
Antwort auf Beitrag Nr.: 54.959.135 von Popeye82 am 17.05.17 03:33:49User, die mich beleidigen, kann ich nicht ernt nehmen. Selbst meine Kinder weisen ein besseres Benehmen auf.
Ich weiß sehr gut, was es mit kanadischen Companies im deutschen Freiverkehr auf sich hat und wie diese zu bewerten sind. Und ich bin über diese Company hier ebenfalls ausreichend informiert.
Mir war klar, dass es den Kursverlauf wie erlebt nehmen würde. Daher stehe ich noch immer an der Seitenlinie. Habe hier noch nicht einmal einen einzigen Trade gesetzt.
Wer hier wann diese Aktie gekauft hat, will ich nicht bewerten. Das hat sicherlich jeder mit sich selbst ausgemacht. Jeder ist für sein Handeln selbst verantwortlich.
Es ist übrigend auch gut, zu wissen, wie die Kanadier ticken.
Ich frage mich zudem immer wieder, ob sich alle Forenuser überhaupt darüber im Klaren sind, dass Beleidigungen in Börsenforen kein Kavaliersdelikt sind ? Kennen die Pöbler eigentlich die Forenregeln ?
Nur meine kritische Meinung.
Ich weiß sehr gut, was es mit kanadischen Companies im deutschen Freiverkehr auf sich hat und wie diese zu bewerten sind. Und ich bin über diese Company hier ebenfalls ausreichend informiert.
Mir war klar, dass es den Kursverlauf wie erlebt nehmen würde. Daher stehe ich noch immer an der Seitenlinie. Habe hier noch nicht einmal einen einzigen Trade gesetzt.
Wer hier wann diese Aktie gekauft hat, will ich nicht bewerten. Das hat sicherlich jeder mit sich selbst ausgemacht. Jeder ist für sein Handeln selbst verantwortlich.
Es ist übrigend auch gut, zu wissen, wie die Kanadier ticken.

Ich frage mich zudem immer wieder, ob sich alle Forenuser überhaupt darüber im Klaren sind, dass Beleidigungen in Börsenforen kein Kavaliersdelikt sind ? Kennen die Pöbler eigentlich die Forenregeln ?
Nur meine kritische Meinung.
!
Dieser Beitrag wurde von FairMOD moderiert. Grund: themenfremder Inhalt
Placement bei 0,40 CAD - 5 Mio. sollen eingesammelt werden
hab heute paar bei 0,37 CAD aufs Auge genagelt bekommen
hab heute paar bei 0,37 CAD aufs Auge genagelt bekommen
Antwort auf Beitrag Nr.: 54.960.953 von tbhomy am 17.05.17 10:12:21
Am 17.5. lag der Schlusskurs bei 0,375 Euro (Tageshoch war 0,414 Euro). Heute wurde die Aktie auf TDG bei 0,236 Euro gehandelt. Es nahm den Verlauf, den ich auf Grund meiner Recherche in den SEDAR-Filings vermutet habe.
Schade, dass ich im Mai hier dauernd angemacht wurde, obwohl ich nur eine fundierte Meinung vertreten habe. Mir sagt das Alles, was ich wissen muss.
Viel Glück allen.
Zitat von tbhomy: User, die mich beleidigen, kann ich nicht ernt nehmen. Selbst meine Kinder weisen ein besseres Benehmen auf.
Ich weiß sehr gut, was es mit kanadischen Companies im deutschen Freiverkehr auf sich hat und wie diese zu bewerten sind. Und ich bin über diese Company hier ebenfalls ausreichend informiert.
Mir war klar, dass es den Kursverlauf wie erlebt nehmen würde. Daher stehe ich noch immer an der Seitenlinie. Habe hier noch nicht einmal einen einzigen Trade gesetzt.
Wer hier wann diese Aktie gekauft hat, will ich nicht bewerten. Das hat sicherlich jeder mit sich selbst ausgemacht. Jeder ist für sein Handeln selbst verantwortlich.
Es ist übrigend auch gut, zu wissen, wie die Kanadier ticken.
Ich frage mich zudem immer wieder, ob sich alle Forenuser überhaupt darüber im Klaren sind, dass Beleidigungen in Börsenforen kein Kavaliersdelikt sind ? Kennen die Pöbler eigentlich die Forenregeln ?
Nur meine kritische Meinung.
Am 17.5. lag der Schlusskurs bei 0,375 Euro (Tageshoch war 0,414 Euro). Heute wurde die Aktie auf TDG bei 0,236 Euro gehandelt. Es nahm den Verlauf, den ich auf Grund meiner Recherche in den SEDAR-Filings vermutet habe.
Schade, dass ich im Mai hier dauernd angemacht wurde, obwohl ich nur eine fundierte Meinung vertreten habe. Mir sagt das Alles, was ich wissen muss.
Viel Glück allen.
Antwort auf Beitrag Nr.: 55.370.498 von tbhomy am 21.07.17 13:12:51
obwohl ich nur eine fundierte Meinung vertreten habe
_______________________________________





obwohl ich nur eine fundierte Meinung vertreten habe
_______________________________________





Antwort auf Beitrag Nr.: 55.373.954 von Popeye82 am 21.07.17 20:06:48
Genau, im Gegensatz zu dir:
Popeye82 schrieb am 17.05.17 03:33:49 Beitrag Nr. 4

Zitat von Popeye82: obwohl ich nur eine fundierte Meinung vertreten habe
_______________________________________
Genau, im Gegensatz zu dir:
Popeye82 schrieb am 17.05.17 03:33:49 Beitrag Nr. 4

"Greetings,
Theratechnologies (TSX:TH) is the subject of our Feature this week as Luc Tanguay, president and CEO, and Philippe Dubuc, SVP and CFO, discuss the potential of the company’s ibalizumab drug for multidrug resistant HIV, which has a PDUFA date of Jan. 3.
Of special interest in our Briefs section was Titan Pharmaceuticals (NASDAQ:TTNP) treating the first Parkinson’s patient in a Phase 1/2 trial of its ropinirole implant; closely-held ZeptoMetrix withdrawing patent infringement claims against Microbix Biosystems (TSX:MBX); 3D Signatures (TSXV XD; OTCQB:TDSGF; FSE:3D0) completing the clinical trial component of its Hodgkin’s lymphoma test validation program; Flex Pharma (NASDAQ:FLKS) starting a Phase 2 trial in patients with Charcot-Marie-Tooth disease who suffer from cramping; and Medicenna Therapeutics (TSX:MDNA) presenting safety and efficacy data for its lead immunotherapy in brain cancer. In addition, BioTime (NYSE MKT, TASE: BTX) successfully treated its first patient in a study of Premvia for the treatment of age-related volume loss in the face, and also priced a $25-million stock offering.
XD; OTCQB:TDSGF; FSE:3D0) completing the clinical trial component of its Hodgkin’s lymphoma test validation program; Flex Pharma (NASDAQ:FLKS) starting a Phase 2 trial in patients with Charcot-Marie-Tooth disease who suffer from cramping; and Medicenna Therapeutics (TSX:MDNA) presenting safety and efficacy data for its lead immunotherapy in brain cancer. In addition, BioTime (NYSE MKT, TASE: BTX) successfully treated its first patient in a study of Premvia for the treatment of age-related volume loss in the face, and also priced a $25-million stock offering.
In Wall Street news, Echelon ascribed 2017 top pick status to Immunovaccine (TSX:IMV; OTCQX:IMMVF), while maintaining a "speculative buy" rating with a price target of $2.75, and BTIG raised its price target for KalVista Pharmaceuticals (NASDAQ:KALV) to $27 from $18."
Theratechnologies (TSX:TH) is the subject of our Feature this week as Luc Tanguay, president and CEO, and Philippe Dubuc, SVP and CFO, discuss the potential of the company’s ibalizumab drug for multidrug resistant HIV, which has a PDUFA date of Jan. 3.
Of special interest in our Briefs section was Titan Pharmaceuticals (NASDAQ:TTNP) treating the first Parkinson’s patient in a Phase 1/2 trial of its ropinirole implant; closely-held ZeptoMetrix withdrawing patent infringement claims against Microbix Biosystems (TSX:MBX); 3D Signatures (TSXV
 XD; OTCQB:TDSGF; FSE:3D0) completing the clinical trial component of its Hodgkin’s lymphoma test validation program; Flex Pharma (NASDAQ:FLKS) starting a Phase 2 trial in patients with Charcot-Marie-Tooth disease who suffer from cramping; and Medicenna Therapeutics (TSX:MDNA) presenting safety and efficacy data for its lead immunotherapy in brain cancer. In addition, BioTime (NYSE MKT, TASE: BTX) successfully treated its first patient in a study of Premvia for the treatment of age-related volume loss in the face, and also priced a $25-million stock offering.
XD; OTCQB:TDSGF; FSE:3D0) completing the clinical trial component of its Hodgkin’s lymphoma test validation program; Flex Pharma (NASDAQ:FLKS) starting a Phase 2 trial in patients with Charcot-Marie-Tooth disease who suffer from cramping; and Medicenna Therapeutics (TSX:MDNA) presenting safety and efficacy data for its lead immunotherapy in brain cancer. In addition, BioTime (NYSE MKT, TASE: BTX) successfully treated its first patient in a study of Premvia for the treatment of age-related volume loss in the face, and also priced a $25-million stock offering.In Wall Street news, Echelon ascribed 2017 top pick status to Immunovaccine (TSX:IMV; OTCQX:IMMVF), while maintaining a "speculative buy" rating with a price target of $2.75, and BTIG raised its price target for KalVista Pharmaceuticals (NASDAQ:KALV) to $27 from $18."
Antwort auf Beitrag Nr.: 56.443.559 von Popeye82 am 14.12.17 03:35:59http://business.financialpost.com/investing/podcast-3d-signa…
For more information about 3D Signatures, visit 3DSignatures.com and tune in to Fox Business Network as sponsored content on Saturday, February 17, 2018 at 5:30pm EST and Bloomberg International on Sunday, February 25, 2018 at 7:00am GMT and 10:00am D.F
"3D Signatures (OTCQB:TDSGF; TSXV XD; FSE:3D0) is the subject of our Feature this week as CEO, Jason Flowerday, discusses positive study results from the company’s Telo-HL genetic test to predict which Hodgkin’s lymphoma patients will respond to standard chemotherapy and which will relapse within the first 12 months.
XD; FSE:3D0) is the subject of our Feature this week as CEO, Jason Flowerday, discusses positive study results from the company’s Telo-HL genetic test to predict which Hodgkin’s lymphoma patients will respond to standard chemotherapy and which will relapse within the first 12 months.
Of special interest in our Briefs section was Evotec (Frankfurt Stock Exchange:EVT) and MaRS Innovation collaborating to develop topical drugs for severe inflammatory skin diseases; Hamilton Thorne (TSXV:HTL) posting a revenue gain of 109% for 2017; and closely-held Scientus Pharma completing a $15.8-million private placement.
In Wall Street news, William Blair launched coverage of Acer Therapeutics (NASDAQ:ACER) at “outperform” with a fair value estimate of $47. Maxim lowered its price targets for Anavex Life Sciences (NASDAQ:AVXL) to $5 from $14 and Quantum Genomics (Euronext Growth - FR0011648971- ALQGC) to €6 from €12. Maxim also downgraded Galmed Pharmaceuticals (NASDAQ:GLMD) to “hold” from “buy” and removed its previous $14 price target. Finally, New York investment community veteran, Miranda Toledano, joined HCW’s investment banking team in Israel."
 XD; FSE:3D0) is the subject of our Feature this week as CEO, Jason Flowerday, discusses positive study results from the company’s Telo-HL genetic test to predict which Hodgkin’s lymphoma patients will respond to standard chemotherapy and which will relapse within the first 12 months.
XD; FSE:3D0) is the subject of our Feature this week as CEO, Jason Flowerday, discusses positive study results from the company’s Telo-HL genetic test to predict which Hodgkin’s lymphoma patients will respond to standard chemotherapy and which will relapse within the first 12 months.Of special interest in our Briefs section was Evotec (Frankfurt Stock Exchange:EVT) and MaRS Innovation collaborating to develop topical drugs for severe inflammatory skin diseases; Hamilton Thorne (TSXV:HTL) posting a revenue gain of 109% for 2017; and closely-held Scientus Pharma completing a $15.8-million private placement.
In Wall Street news, William Blair launched coverage of Acer Therapeutics (NASDAQ:ACER) at “outperform” with a fair value estimate of $47. Maxim lowered its price targets for Anavex Life Sciences (NASDAQ:AVXL) to $5 from $14 and Quantum Genomics (Euronext Growth - FR0011648971- ALQGC) to €6 from €12. Maxim also downgraded Galmed Pharmaceuticals (NASDAQ:GLMD) to “hold” from “buy” and removed its previous $14 price target. Finally, New York investment community veteran, Miranda Toledano, joined HCW’s investment banking team in Israel."
Antwort auf Beitrag Nr.: 57.071.748 von Popeye82 am 20.02.18 17:01:54http://www.biotuesdays.com/features/2018/2/16/3d-signatures
"3DS Telo-HL test aims to personalize cancer treatment
February 20, 2018
Jason Flowerday, CEO

3D Signatures (OTCQB:TDSGF; TSXV XD; FSE:3D0) has reported positive results from a preliminary analysis of trial data for Telo-HL, its test for Hodgkin's lymphoma (HL) that is designed to personalize treatment for newly diagnosed patients.
XD; FSE:3D0) has reported positive results from a preliminary analysis of trial data for Telo-HL, its test for Hodgkin's lymphoma (HL) that is designed to personalize treatment for newly diagnosed patients.
"The results showed that the company's TeloView platform is able to distinguish, with a high degree of statistical significance, multiple differences between a patient group that responds to standard ABVD chemotherapy, and a group that relapses or is refractory to treatment within the first 12 months," CEO, Jason Flowerday, says in an interview with BioTuesdays.
ABVD is the standard-of-care, first-line chemotherapy regimen used in the treatment of HL that consists of doxorubicin, bleomycin, vinblastine and dacarbazine.
"We believe these results from the application of our TeloView platform to HL are so strongly positive, we can now even more confidently proceed with developing the final multivariate scoring model for the Telo-HL test to predict response at the individual patient level,” he contends.
“The ability to determine, via a blood test, which cancer patients should be prioritized for treatment would be a true game changer.”
— Jason Flowerday
The company remains on track to complete all phases of the test development and analytical validation by April 2018, he adds.
Mr. Flowerday says 3DS is considering making the test available broadly for research use and including it in HL clinical trials as part of a pharma services strategy that is in development.
"This milestone serves as an important proof of principle that our proprietary TeloView platform is highly robust and applicable to some tough cancers without effective biomarkers, and others which affect much larger patient populations," he adds.
3DS focused on HL because the treatment plan for this form of cancer is currently based on a trial and error system of testing different therapies to explore which, if any, a patient responds to.
Mr. Flowerday also says the HL test is the basis for accelerating development of other tests with major collaborators interested in TeloView's potential to personalize treatment for prostate cancer, which is the furthest along, for lung cancer, and for multiple myeloma.
"We have ongoing as well as newly planned pilot studies and collaborations with world oncology leaders that we expect to kick off later this year to build on the HL work and accelerate our other tests in development," he adds. "The ability to determine, via a blood test, which cancer patients should be prioritized for treatment would be a true game changer."
The TeloView platform builds on the foundational work done in Dr. Sabine Mai's Winnipeg laboratory to establish 3D telomere profiling as a novel biomarker platform. Dr. Mai is a director of 3DS and chair of its clinical and scientific advisory board. TeloView is supported by 25 clinical studies involving more than 3,000 patients and 20 different cancers, plus Alzheimer's disease.
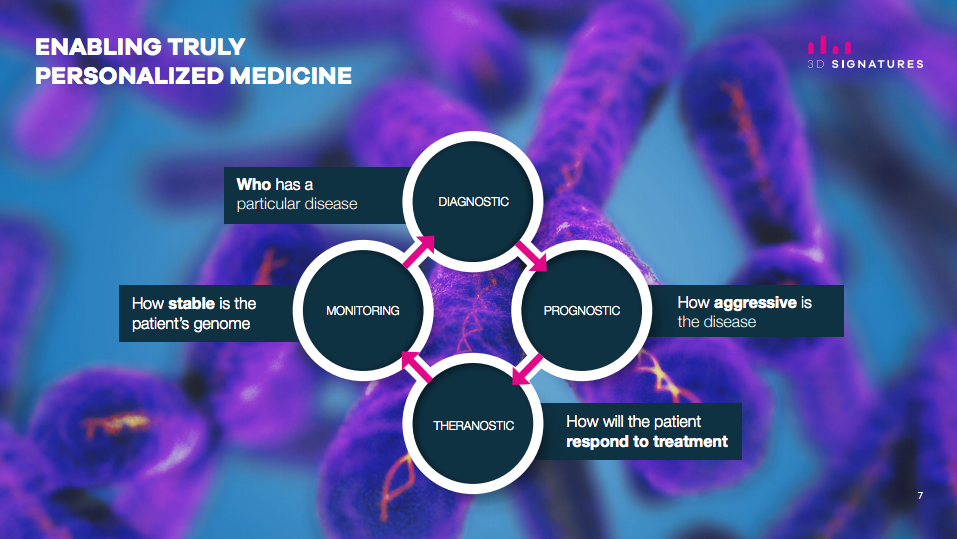
Mr. Flowerday explains that 3DS' technology is based on the three-dimensional analysis of telomeres, the protective caps at the ends of chromosomes. The software platform measures genomic instability based on a 3D analysis of telomeres. Unlike many other technologies, TeloView is not dependent on sequencing mutations in the DNA to measure the stability of the entire genome.

3D Telomere Analysis
As a result, the TeloView software platform measures the content and configuration of the genome in the cell nucleus to determine its stability, and its correspondence to either the stage of a given disease, the rate of progression of the disease, how different diseases will respond to various therapies, and a drug's efficacy and toxicity.
"Our proprietary imaging and analysis software is designed to go beyond identifying whether a patient suffers from a specific disease or condition," he contends. "Instead, the TeloView platform is designed to inform clinicians and patients how to personalize treatment and best manage an individual's disease based on their unique TeloView Score."

Proprietary Software Algorithm
Currently, there is no biomarker that can predict the 15% to 20% of patients that will fail standard ABVD chemotherapy when first diagnosed with Hodgkin's lymphoma, he adds.
For the past year, 3DS has analyzed tissue samples from some 400 HL patients, who were subsequently treated with ABVD chemotherapy, contributed from hospitals in Canada and Europe. During the study, Mr. Flowerday says the company perfected its chemical assay, microscopy and processed all cell types through its TeloView algorithm.
Blinded data from the study were shared with 3DS' statistical partner, BioStat Solutions, who was unblinded and compared the TeloView data with the corresponding clinical outcomes for patients, and identified highly significant group differences across multiple TeloView parameters, Mr. Flowerday says. "BSSI confirmed our analysis as being extremely accurate."
Ronald Bromley, CEO of BioStat Solutions, says his company is excited to be collaborating with 3DS, "helping them ensure the quality of the data being used is to the highest standards, and that they are poised to deliver the best possible analysis of this predictive technology for HL treatment."
BioStat Solutions is now building a final predictive scoring model to test and determine the sensitivity and specificity of Telo-HL's performance in predicting ABVD response on further of HL patient samples based on their telomere profile.
"We have now reached a point where the data coming out of our TeloView platform is significant in telling us which patients are responders to ABVD chemotherapy and which patients will relapse to therapy," he adds.
In addition, 3DS intends to submit the completed test development and validation work for peer-review and publication in a top-tier medical journal in the latter half of 2018.
"We expect Telo-HL to benefit patients seeking personalized treatment and to provide significant cost savings to payers and insurers that are currently burdened with expensive treatments and procedures that may not be necessary if patients could be considered for more targeted and effective therapies at the outset of treatment," he adds.

Pipeline"
"3DS Telo-HL test aims to personalize cancer treatment
February 20, 2018
Jason Flowerday, CEO

3D Signatures (OTCQB:TDSGF; TSXV
 XD; FSE:3D0) has reported positive results from a preliminary analysis of trial data for Telo-HL, its test for Hodgkin's lymphoma (HL) that is designed to personalize treatment for newly diagnosed patients.
XD; FSE:3D0) has reported positive results from a preliminary analysis of trial data for Telo-HL, its test for Hodgkin's lymphoma (HL) that is designed to personalize treatment for newly diagnosed patients."The results showed that the company's TeloView platform is able to distinguish, with a high degree of statistical significance, multiple differences between a patient group that responds to standard ABVD chemotherapy, and a group that relapses or is refractory to treatment within the first 12 months," CEO, Jason Flowerday, says in an interview with BioTuesdays.
ABVD is the standard-of-care, first-line chemotherapy regimen used in the treatment of HL that consists of doxorubicin, bleomycin, vinblastine and dacarbazine.
"We believe these results from the application of our TeloView platform to HL are so strongly positive, we can now even more confidently proceed with developing the final multivariate scoring model for the Telo-HL test to predict response at the individual patient level,” he contends.
“The ability to determine, via a blood test, which cancer patients should be prioritized for treatment would be a true game changer.”
— Jason Flowerday
The company remains on track to complete all phases of the test development and analytical validation by April 2018, he adds.
Mr. Flowerday says 3DS is considering making the test available broadly for research use and including it in HL clinical trials as part of a pharma services strategy that is in development.
"This milestone serves as an important proof of principle that our proprietary TeloView platform is highly robust and applicable to some tough cancers without effective biomarkers, and others which affect much larger patient populations," he adds.
3DS focused on HL because the treatment plan for this form of cancer is currently based on a trial and error system of testing different therapies to explore which, if any, a patient responds to.
Mr. Flowerday also says the HL test is the basis for accelerating development of other tests with major collaborators interested in TeloView's potential to personalize treatment for prostate cancer, which is the furthest along, for lung cancer, and for multiple myeloma.
"We have ongoing as well as newly planned pilot studies and collaborations with world oncology leaders that we expect to kick off later this year to build on the HL work and accelerate our other tests in development," he adds. "The ability to determine, via a blood test, which cancer patients should be prioritized for treatment would be a true game changer."
The TeloView platform builds on the foundational work done in Dr. Sabine Mai's Winnipeg laboratory to establish 3D telomere profiling as a novel biomarker platform. Dr. Mai is a director of 3DS and chair of its clinical and scientific advisory board. TeloView is supported by 25 clinical studies involving more than 3,000 patients and 20 different cancers, plus Alzheimer's disease.
Mr. Flowerday explains that 3DS' technology is based on the three-dimensional analysis of telomeres, the protective caps at the ends of chromosomes. The software platform measures genomic instability based on a 3D analysis of telomeres. Unlike many other technologies, TeloView is not dependent on sequencing mutations in the DNA to measure the stability of the entire genome.

3D Telomere Analysis
As a result, the TeloView software platform measures the content and configuration of the genome in the cell nucleus to determine its stability, and its correspondence to either the stage of a given disease, the rate of progression of the disease, how different diseases will respond to various therapies, and a drug's efficacy and toxicity.
"Our proprietary imaging and analysis software is designed to go beyond identifying whether a patient suffers from a specific disease or condition," he contends. "Instead, the TeloView platform is designed to inform clinicians and patients how to personalize treatment and best manage an individual's disease based on their unique TeloView Score."

Proprietary Software Algorithm
Currently, there is no biomarker that can predict the 15% to 20% of patients that will fail standard ABVD chemotherapy when first diagnosed with Hodgkin's lymphoma, he adds.
For the past year, 3DS has analyzed tissue samples from some 400 HL patients, who were subsequently treated with ABVD chemotherapy, contributed from hospitals in Canada and Europe. During the study, Mr. Flowerday says the company perfected its chemical assay, microscopy and processed all cell types through its TeloView algorithm.
Blinded data from the study were shared with 3DS' statistical partner, BioStat Solutions, who was unblinded and compared the TeloView data with the corresponding clinical outcomes for patients, and identified highly significant group differences across multiple TeloView parameters, Mr. Flowerday says. "BSSI confirmed our analysis as being extremely accurate."
Ronald Bromley, CEO of BioStat Solutions, says his company is excited to be collaborating with 3DS, "helping them ensure the quality of the data being used is to the highest standards, and that they are poised to deliver the best possible analysis of this predictive technology for HL treatment."
BioStat Solutions is now building a final predictive scoring model to test and determine the sensitivity and specificity of Telo-HL's performance in predicting ABVD response on further of HL patient samples based on their telomere profile.
"We have now reached a point where the data coming out of our TeloView platform is significant in telling us which patients are responders to ABVD chemotherapy and which patients will relapse to therapy," he adds.
In addition, 3DS intends to submit the completed test development and validation work for peer-review and publication in a top-tier medical journal in the latter half of 2018.
"We expect Telo-HL to benefit patients seeking personalized treatment and to provide significant cost savings to payers and insurers that are currently burdened with expensive treatments and procedures that may not be necessary if patients could be considered for more targeted and effective therapies at the outset of treatment," he adds.

Pipeline"
Antwort auf Beitrag Nr.: 57.071.748 von Popeye82 am 20.02.18 17:01:54http://www.3dsignatures.com/news/3d-signatures-inc-announces…
"3D Signatures Inc. Announces Positive Topline Results of Development Trial Assessing its Telo-HLᵀᴹ Test, for Hodgkin’s Lymphoma
TORONTO, February 20, 2018 − 3D Signatures Inc. (TSX-V XD) (OTCQB:TDSGF) (FSE:3D0) (the "Company" or "3DS") is pleased to announce that a preliminary third-party analysis of the trial data for Telo-HLTM, 3DS’ test in development for Hodgkin’s lymphoma (HL), shows that the Company’s TeloViewTM platform is able to distinguish, with a high degree of statistical significance, multiple differences between a patient group that responds to standard ABVD chemotherapy, and a group that relapses or is refractory to treatment within the first 12 months.
XD) (OTCQB:TDSGF) (FSE:3D0) (the "Company" or "3DS") is pleased to announce that a preliminary third-party analysis of the trial data for Telo-HLTM, 3DS’ test in development for Hodgkin’s lymphoma (HL), shows that the Company’s TeloViewTM platform is able to distinguish, with a high degree of statistical significance, multiple differences between a patient group that responds to standard ABVD chemotherapy, and a group that relapses or is refractory to treatment within the first 12 months.
Telo-HLTM is intended to provide clinicians with the first biomarker to identify the 15% - 20% of new HL patients who will likely fail standard chemotherapy, and who should immediately be considered for more advanced treatment or inclusion into clinical trials to access emerging treatments such as immunotherapies.
3DS has established a clinically-compliant methodology for application of its TeloViewTM process to diagnostic Hodgkin lymphoma biopsy samples, employing more sensitive imaging technology than its previous HL trials. Specimens from over 400 HL patients (who were subsequently treated with ABVD) were processed for this trial, from four contributing hospital sites across Canada and Europe. The 3DS three-step process was applied, comprising a wet lab co-immuno-telomeres FISH assay, 3-dimensional imaging (with identification of 30 Hodgkin and 30 Reed-Sternberg cells), followed by TeloView™ software analysis.
The multi-parametric telomeric analysis with TeloViewTM was performed by 3DS (blinded to patient status), and the results were then shared with statistical partner BioStat Solutions Inc. (“BSSI”), who compared the TeloViewTM data with the corresponding clinical outcomes for patients, and identified highly significant group differences across multiple TeloViewTM parameters.
"BSSI is excited to be collaborating with 3DS, helping them ensure the quality of the data being used is to the highest standards, and that they are poised to deliver the best possible analysis of this predictive technology for HL treatment,” said Ronald L. Bromley, CEO of BioStat Solutions, Inc.
“We believe that these results from the application of our TeloViewTM platform to Hodgkin’s lymphoma are so strong, the Company will now even more confidently proceed with developing the final scoring model for its Telo-HLTM test to predict response at the individual patient level,” said Jason Flowerday, CEO of 3D Signatures. “This is great news for the Telo-HLTM program, and we remain on track to complete all phases of the test development and analytical validation by April 2018.”
The Company intends to submit the completed test development and validation work to a highly reputable clinical journal for publication in the latter half of 2018.
“This trial builds on the foundational work done in Dr. Sabine Mai’s academic laboratory to establish 3D telomere profiling as a novel biomarker platform. We see this as a new genomic stability testing paradigm applicable broadly across clinical trial research and laboratory medicine”, says Dr. Kevin Little, CSO of 3D Signatures.
About Telo-HL™
Powered by 3DS’ proprietary TeloView™ software platform, Telo-HL™ is intended to provide clinicians with the first set of biomarkers that will distinguish between patients that will respond to standard ABVD chemotherapy, and the 15% - 20% of patients who will fail standard chemotherapy and be refractory or relapse within the first year. The Company expects Telo-HL™ to benefit patients seeking personalized treatment and to provide significant cost savings to payors and insurers that are currently burdened with expensive treatments and procedures that may not be necessary if patients could be considered for more targeted and effective therapies at the outset of treatment.
About BSSI
BioStat Solutions, Inc. (BSSI) is a privately held professional service corporation providing statistical and bioinformatics expertise to pharmaceutical and biotech companies as well as to the government and its contractors. BSSI’s diverse team of statisticians, bioinformaticists, epidemiologists and geneticists provides answers to complex and challenging analytical questions. Whether the client is facing big data or machine learning problems, or is looking for new biomarker or diagnostic device strategies, BSSI provides solid results towards effective decision-making. For more information, visit BSSI’s website at: http://www.biostatsolutions.com.
About 3DS
3DS (TSX-V XD; OTCQB:TDSGF; FSE:3D0) is a personalized medicine company with a proprietary software platform, TeloViewTM, that is designed to predict the course of certain diseases and to tailor treatment options for the individual patient. The technology is based on the three-dimensional analysis of telomeres, the protective caps at the ends of chromosomes. 3DS’ TeloViewTM software platform measures the organization of the genome and its correspondence to; the stage of a given disease, the rate of progression of the disease, how different diseases will respond to various therapies, and a drug’s efficacy and toxicity. 3DS’ proprietary imaging software is designed to go beyond identifying whether a patient suffers from a specific disease or condition. Instead, the TeloViewTM platform is designed to inform clinicians and patients with respect to how to personalize treatment and best manage an individual’s disease based on their unique TeloView ScoreTM. As healthcare moves increasingly toward better informed, patient-centric approaches, the Company intends for the TeloViewTM platform to deliver personalized medicine that allows for better treatments, leading to better outcomes.
XD; OTCQB:TDSGF; FSE:3D0) is a personalized medicine company with a proprietary software platform, TeloViewTM, that is designed to predict the course of certain diseases and to tailor treatment options for the individual patient. The technology is based on the three-dimensional analysis of telomeres, the protective caps at the ends of chromosomes. 3DS’ TeloViewTM software platform measures the organization of the genome and its correspondence to; the stage of a given disease, the rate of progression of the disease, how different diseases will respond to various therapies, and a drug’s efficacy and toxicity. 3DS’ proprietary imaging software is designed to go beyond identifying whether a patient suffers from a specific disease or condition. Instead, the TeloViewTM platform is designed to inform clinicians and patients with respect to how to personalize treatment and best manage an individual’s disease based on their unique TeloView ScoreTM. As healthcare moves increasingly toward better informed, patient-centric approaches, the Company intends for the TeloViewTM platform to deliver personalized medicine that allows for better treatments, leading to better outcomes.
The TeloViewTM platform is supported by 25 clinical studies involving more than 3,000 patients and 20 different cancers, plus Alzheimer’s disease. 3DS benefits from twenty years of research, $25M of non-dilutive investment into its platform and more than 130 supporting publications, and holds a portfolio of patents related to three-dimensional telomere analysis for proliferative diseases, including (but not limited to) hematological disorders such as Hodgkin's lymphoma, multiple myeloma, and chronic myeloid leukemia. 3DS’ intellectual property portfolio also covers prostate cancer, breast cancer, lung cancer, melanoma, colorectal cancer, and Alzheimer disease.
For more information, visit the Company’s website at: http://www.3dsignatures.com.
For further information, please contact:
Jason Flowerday
CEO & Director
416-673-8487
investors@3dsignatures.com
Cautionary Note Regarding Forward-Looking Statements
This news release contains forward looking statements which constitute “forward looking information” within the meaning of applicable Canadian securities legislation (“Forward-Looking Statements”). All statements included herein, other than statements of historical fact, are Forward Looking Statements and are subject to a variety of known and unknown risks and uncertainties which could cause actual events or results to differ materially from those reflected in the Forward Looking Statements. Often, but not always, these Forward-Looking Statements can be identified by the use of words such as "estimates", "potential", "open", "future", "assumes", "projects", “anticipates”, “believes”, “may”, “continues”, “expects”, "plans", "will", "to be", or statements that events "could" or "should" occur or be achieved, and similar expressions, including negative variations.
Such Forward Looking Statements reflect the Company’s current views with respect to future events, are subject to risks and uncertainties and are necessarily based upon a number of estimates and assumptions that, while considered reasonable by 3DS as of the date of such statements, are inherently subject to significant medical, scientific, business, economic, competitive, political and social uncertainties and contingencies. Many risk factors could cause the Company’s actual results, performance, achievements, prospects or opportunities to be materially different from any future results, performance or achievements that may be expressed or implied by such Forward Looking Statements, including risks related to 3DS’ management’s discretion over the actual application of the net proceeds and ability to allocate proceeds differently from what is described herein; the risk that the Telo-HLTM test may not be commercially launched as an LDT by the first quarter of 2018, or at all; uncertainties related to 3DS’ clinical trials and test development; risks related to the volatility of the price of 3DS’ common shares; risks related to the possibility that 3DS’ shareholders may experience dilution; risks related to 3DS’ requirements for additional financing and future access to capital, including the risk that the proceeds raised under the Private Placement may be insufficient to finance 3DS’ business objectives; the risk that a positive return on an investment in 3DS’ common shares is not guaranteed; risks related to 3DS’ intention to retain earnings and not pay cash dividends on its common shares in the foreseeable future; risks related to 3DS’ early stage of development; the risk that 3DS’ tests will not be successfully deployed; risks related to 3DS’ dependence on third parties, including collaborative partners, licensors and others; risks related to 3DS’ clinical trial recruitment; that there is currently no market for 3DS’ products and that such market may be slow to develop if at all; risks related to 3DS’ reliance on key personnel; risks related to the competitive nature of the biotechnology industry; risks related to 3DS’ limited operating history, lack of revenue, history of losses and inability to assure that it will earn profits in the future or that profitability will be sustained; risks related to government regulation; risks related to rapid technological change; risks related to the fact that 3DS’ software may now or in the future contain undetected errors, bugs or vulnerabilities; the risk that 3DS or its directors and officers may be subject to a variety of civil or other legal proceedings, with or without merit, including product liability claims; risks related to the protection of 3DS’ intellectual property rights; risks related to 3DS’ limited sales, marketing and distribution experience; risks related to the possibility that 3DS’ directors and officers may be placed in a conflict of interest as a result of their employment or affiliation with third parties, risks related to 3DS’ use and storage of personal information and compliance with applicable privacy laws, as well as those risks discussed under the heading "Risk Factors" in the Company's management’s discussion and analysis dated November 29, 2017 and filed on SEDAR. Although the Company has attempted to identify important factors that could cause actual actions, events or results to differ materially from those described in the Forward-Looking Statements, there may be other factors that cause actions, events or results to differ from those anticipated, estimated or intended.
In making the Forward-Looking Statements, the Company has made various material assumptions including, but not limited to, obtaining positive results from 3DS’ current and planned clinical trials and research and development initiatives; that the Telo-HLTM test will be commercially launched as an LDT by the first quarter of 2018; obtaining regulatory approvals with respect to 3DS’ clinical trials which are now ongoing or may in the future be commenced; 3DS’ ability to successfully develop its tests; assumptions regarding general business and economic conditions; that 3DS’ current positive relationship with third parties will be maintained; the availability of future financing on reasonable terms; 3DS’ ability to attract and retain skilled staff; assumptions regarding market competition and the products and technology offered by 3DS’ competitors; and 3DS’ ability to protect patents and proprietary rights.
3DS believes that the assumptions and expectations reflected in the Forward-Looking Statements in this press release are reasonable, but no assurance can be given that these expectations will prove to be correct. Forward Looking Statements should not be unduly relied upon. This information speaks only as of the date of this press release, and 3DS will not necessarily update this information, unless required to do so by securities laws.
Neither the TSX Venture Exchange nor its Regulation Service Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release."
"3D Signatures Inc. Announces Positive Topline Results of Development Trial Assessing its Telo-HLᵀᴹ Test, for Hodgkin’s Lymphoma
TORONTO, February 20, 2018 − 3D Signatures Inc. (TSX-V
 XD) (OTCQB:TDSGF) (FSE:3D0) (the "Company" or "3DS") is pleased to announce that a preliminary third-party analysis of the trial data for Telo-HLTM, 3DS’ test in development for Hodgkin’s lymphoma (HL), shows that the Company’s TeloViewTM platform is able to distinguish, with a high degree of statistical significance, multiple differences between a patient group that responds to standard ABVD chemotherapy, and a group that relapses or is refractory to treatment within the first 12 months.
XD) (OTCQB:TDSGF) (FSE:3D0) (the "Company" or "3DS") is pleased to announce that a preliminary third-party analysis of the trial data for Telo-HLTM, 3DS’ test in development for Hodgkin’s lymphoma (HL), shows that the Company’s TeloViewTM platform is able to distinguish, with a high degree of statistical significance, multiple differences between a patient group that responds to standard ABVD chemotherapy, and a group that relapses or is refractory to treatment within the first 12 months.Telo-HLTM is intended to provide clinicians with the first biomarker to identify the 15% - 20% of new HL patients who will likely fail standard chemotherapy, and who should immediately be considered for more advanced treatment or inclusion into clinical trials to access emerging treatments such as immunotherapies.
3DS has established a clinically-compliant methodology for application of its TeloViewTM process to diagnostic Hodgkin lymphoma biopsy samples, employing more sensitive imaging technology than its previous HL trials. Specimens from over 400 HL patients (who were subsequently treated with ABVD) were processed for this trial, from four contributing hospital sites across Canada and Europe. The 3DS three-step process was applied, comprising a wet lab co-immuno-telomeres FISH assay, 3-dimensional imaging (with identification of 30 Hodgkin and 30 Reed-Sternberg cells), followed by TeloView™ software analysis.
The multi-parametric telomeric analysis with TeloViewTM was performed by 3DS (blinded to patient status), and the results were then shared with statistical partner BioStat Solutions Inc. (“BSSI”), who compared the TeloViewTM data with the corresponding clinical outcomes for patients, and identified highly significant group differences across multiple TeloViewTM parameters.
"BSSI is excited to be collaborating with 3DS, helping them ensure the quality of the data being used is to the highest standards, and that they are poised to deliver the best possible analysis of this predictive technology for HL treatment,” said Ronald L. Bromley, CEO of BioStat Solutions, Inc.
“We believe that these results from the application of our TeloViewTM platform to Hodgkin’s lymphoma are so strong, the Company will now even more confidently proceed with developing the final scoring model for its Telo-HLTM test to predict response at the individual patient level,” said Jason Flowerday, CEO of 3D Signatures. “This is great news for the Telo-HLTM program, and we remain on track to complete all phases of the test development and analytical validation by April 2018.”
The Company intends to submit the completed test development and validation work to a highly reputable clinical journal for publication in the latter half of 2018.
“This trial builds on the foundational work done in Dr. Sabine Mai’s academic laboratory to establish 3D telomere profiling as a novel biomarker platform. We see this as a new genomic stability testing paradigm applicable broadly across clinical trial research and laboratory medicine”, says Dr. Kevin Little, CSO of 3D Signatures.
About Telo-HL™
Powered by 3DS’ proprietary TeloView™ software platform, Telo-HL™ is intended to provide clinicians with the first set of biomarkers that will distinguish between patients that will respond to standard ABVD chemotherapy, and the 15% - 20% of patients who will fail standard chemotherapy and be refractory or relapse within the first year. The Company expects Telo-HL™ to benefit patients seeking personalized treatment and to provide significant cost savings to payors and insurers that are currently burdened with expensive treatments and procedures that may not be necessary if patients could be considered for more targeted and effective therapies at the outset of treatment.
About BSSI
BioStat Solutions, Inc. (BSSI) is a privately held professional service corporation providing statistical and bioinformatics expertise to pharmaceutical and biotech companies as well as to the government and its contractors. BSSI’s diverse team of statisticians, bioinformaticists, epidemiologists and geneticists provides answers to complex and challenging analytical questions. Whether the client is facing big data or machine learning problems, or is looking for new biomarker or diagnostic device strategies, BSSI provides solid results towards effective decision-making. For more information, visit BSSI’s website at: http://www.biostatsolutions.com.
About 3DS
3DS (TSX-V
 XD; OTCQB:TDSGF; FSE:3D0) is a personalized medicine company with a proprietary software platform, TeloViewTM, that is designed to predict the course of certain diseases and to tailor treatment options for the individual patient. The technology is based on the three-dimensional analysis of telomeres, the protective caps at the ends of chromosomes. 3DS’ TeloViewTM software platform measures the organization of the genome and its correspondence to; the stage of a given disease, the rate of progression of the disease, how different diseases will respond to various therapies, and a drug’s efficacy and toxicity. 3DS’ proprietary imaging software is designed to go beyond identifying whether a patient suffers from a specific disease or condition. Instead, the TeloViewTM platform is designed to inform clinicians and patients with respect to how to personalize treatment and best manage an individual’s disease based on their unique TeloView ScoreTM. As healthcare moves increasingly toward better informed, patient-centric approaches, the Company intends for the TeloViewTM platform to deliver personalized medicine that allows for better treatments, leading to better outcomes.
XD; OTCQB:TDSGF; FSE:3D0) is a personalized medicine company with a proprietary software platform, TeloViewTM, that is designed to predict the course of certain diseases and to tailor treatment options for the individual patient. The technology is based on the three-dimensional analysis of telomeres, the protective caps at the ends of chromosomes. 3DS’ TeloViewTM software platform measures the organization of the genome and its correspondence to; the stage of a given disease, the rate of progression of the disease, how different diseases will respond to various therapies, and a drug’s efficacy and toxicity. 3DS’ proprietary imaging software is designed to go beyond identifying whether a patient suffers from a specific disease or condition. Instead, the TeloViewTM platform is designed to inform clinicians and patients with respect to how to personalize treatment and best manage an individual’s disease based on their unique TeloView ScoreTM. As healthcare moves increasingly toward better informed, patient-centric approaches, the Company intends for the TeloViewTM platform to deliver personalized medicine that allows for better treatments, leading to better outcomes.The TeloViewTM platform is supported by 25 clinical studies involving more than 3,000 patients and 20 different cancers, plus Alzheimer’s disease. 3DS benefits from twenty years of research, $25M of non-dilutive investment into its platform and more than 130 supporting publications, and holds a portfolio of patents related to three-dimensional telomere analysis for proliferative diseases, including (but not limited to) hematological disorders such as Hodgkin's lymphoma, multiple myeloma, and chronic myeloid leukemia. 3DS’ intellectual property portfolio also covers prostate cancer, breast cancer, lung cancer, melanoma, colorectal cancer, and Alzheimer disease.
For more information, visit the Company’s website at: http://www.3dsignatures.com.
For further information, please contact:
Jason Flowerday
CEO & Director
416-673-8487
investors@3dsignatures.com
Cautionary Note Regarding Forward-Looking Statements
This news release contains forward looking statements which constitute “forward looking information” within the meaning of applicable Canadian securities legislation (“Forward-Looking Statements”). All statements included herein, other than statements of historical fact, are Forward Looking Statements and are subject to a variety of known and unknown risks and uncertainties which could cause actual events or results to differ materially from those reflected in the Forward Looking Statements. Often, but not always, these Forward-Looking Statements can be identified by the use of words such as "estimates", "potential", "open", "future", "assumes", "projects", “anticipates”, “believes”, “may”, “continues”, “expects”, "plans", "will", "to be", or statements that events "could" or "should" occur or be achieved, and similar expressions, including negative variations.
Such Forward Looking Statements reflect the Company’s current views with respect to future events, are subject to risks and uncertainties and are necessarily based upon a number of estimates and assumptions that, while considered reasonable by 3DS as of the date of such statements, are inherently subject to significant medical, scientific, business, economic, competitive, political and social uncertainties and contingencies. Many risk factors could cause the Company’s actual results, performance, achievements, prospects or opportunities to be materially different from any future results, performance or achievements that may be expressed or implied by such Forward Looking Statements, including risks related to 3DS’ management’s discretion over the actual application of the net proceeds and ability to allocate proceeds differently from what is described herein; the risk that the Telo-HLTM test may not be commercially launched as an LDT by the first quarter of 2018, or at all; uncertainties related to 3DS’ clinical trials and test development; risks related to the volatility of the price of 3DS’ common shares; risks related to the possibility that 3DS’ shareholders may experience dilution; risks related to 3DS’ requirements for additional financing and future access to capital, including the risk that the proceeds raised under the Private Placement may be insufficient to finance 3DS’ business objectives; the risk that a positive return on an investment in 3DS’ common shares is not guaranteed; risks related to 3DS’ intention to retain earnings and not pay cash dividends on its common shares in the foreseeable future; risks related to 3DS’ early stage of development; the risk that 3DS’ tests will not be successfully deployed; risks related to 3DS’ dependence on third parties, including collaborative partners, licensors and others; risks related to 3DS’ clinical trial recruitment; that there is currently no market for 3DS’ products and that such market may be slow to develop if at all; risks related to 3DS’ reliance on key personnel; risks related to the competitive nature of the biotechnology industry; risks related to 3DS’ limited operating history, lack of revenue, history of losses and inability to assure that it will earn profits in the future or that profitability will be sustained; risks related to government regulation; risks related to rapid technological change; risks related to the fact that 3DS’ software may now or in the future contain undetected errors, bugs or vulnerabilities; the risk that 3DS or its directors and officers may be subject to a variety of civil or other legal proceedings, with or without merit, including product liability claims; risks related to the protection of 3DS’ intellectual property rights; risks related to 3DS’ limited sales, marketing and distribution experience; risks related to the possibility that 3DS’ directors and officers may be placed in a conflict of interest as a result of their employment or affiliation with third parties, risks related to 3DS’ use and storage of personal information and compliance with applicable privacy laws, as well as those risks discussed under the heading "Risk Factors" in the Company's management’s discussion and analysis dated November 29, 2017 and filed on SEDAR. Although the Company has attempted to identify important factors that could cause actual actions, events or results to differ materially from those described in the Forward-Looking Statements, there may be other factors that cause actions, events or results to differ from those anticipated, estimated or intended.
In making the Forward-Looking Statements, the Company has made various material assumptions including, but not limited to, obtaining positive results from 3DS’ current and planned clinical trials and research and development initiatives; that the Telo-HLTM test will be commercially launched as an LDT by the first quarter of 2018; obtaining regulatory approvals with respect to 3DS’ clinical trials which are now ongoing or may in the future be commenced; 3DS’ ability to successfully develop its tests; assumptions regarding general business and economic conditions; that 3DS’ current positive relationship with third parties will be maintained; the availability of future financing on reasonable terms; 3DS’ ability to attract and retain skilled staff; assumptions regarding market competition and the products and technology offered by 3DS’ competitors; and 3DS’ ability to protect patents and proprietary rights.
3DS believes that the assumptions and expectations reflected in the Forward-Looking Statements in this press release are reasonable, but no assurance can be given that these expectations will prove to be correct. Forward Looking Statements should not be unduly relied upon. This information speaks only as of the date of this press release, and 3DS will not necessarily update this information, unless required to do so by securities laws.
Neither the TSX Venture Exchange nor its Regulation Service Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release."



das muss mann erstmal checken

Versteh ich das jetzt richtig, dass Toyota sich nochmal nen dicken batzen holt?
Hallo ? Noch jemand da?
Wie tief befinden wir uns unterm Radar ?
Im Kanadischen " Stockhouse " und "Yahoo Board " ist alles ruhig.
3D hebt ab und keiner merkt es. Wer sich aktuell drüben eindeckt weis auch kein Mensch.
Wie tief befinden wir uns unterm Radar ?
Im Kanadischen " Stockhouse " und "Yahoo Board " ist alles ruhig.
3D hebt ab und keiner merkt es. Wer sich aktuell drüben eindeckt weis auch kein Mensch.
Ewigkeiten hat fast Keiner Was zusagen, aber wenn Es hoch geht kommen die ganzen Spinner raus. Ist fast jedes Mal Das Gleiche.



Dieses Portal ist richtig traurig geworden.




Dieses Portal ist richtig traurig geworden.

http://www.streetwisereports.com/pub/na/fund-manager-names-t…
http://www.streetwisereports.com/pub/na/can-a-simple-test-re…
http://www.streetwisereports.com/pub/co/8941
"The annual AlphaNorth Capital Conference features small-cap non-resource companies with high growth prospects. AlphaNorth's founder and chief investment officer, Steve Palmer, profiles several companies that he believes have bright prospects.
bluefinancial-graph630
The Life Sciences Report: Steve, you are a co-organizer of the annual AlphaNorth Capital Conference, which is attended by about 40 small-cap companies and major investors. How do you select the companies you invite?
Steve Palmer: We like to have some diversification among the companies that are there. It's a non-resource conference. We didn't want it to turn into a cannabis show or a crypto conference or anything like that. We tried to select some of the better companies from each category and have a wide variety at the conference. One of the key things I look for in an investment is a company that has something that is proprietary and a strong growth profile.
TLSR: Do you focus exclusively on Canadian equities?
SP: Mostly Canadian. There are several thousand junior Canadian companies, so there are more than enough opportunities to pick from on the Canadian side. We do have a couple U.S.-listed companies in the portfolios. We don't go looking for companies in other countries, but if opportunities are presented that make sense, we don't exclude those.
TLSR: Would you tell us about a couple of the companies that you're really excited about?
"3D Signatures' timeline to have a commercial product is much shorter than a traditional biotech company developing a new drug."
SP: 3D Signatures Inc. (DXD:TSX.V; TDSGF:OTCQB; 3D0:FSE) is one. It has a platform technology that looks at a component of a person's DNA and is potentially able to predict the outcome of various medical treatments. It just announced some results from a Hodgkin's lymphoma study. It has also done work on prostate cancer, Alzheimer's, etc.
I think it's relatively low risk. On the previous work that it has done, it has shown very strong results and predictive ability. It is currently doing a much larger confirmatory trial in Hodgkin's lymphoma. I like the fact that it's a platform. It has applications for various diseases and medical conditions. The timeline to have a commercial product is much shorter than a traditional biotech company developing a new drug.
TLSR: What is another company you like?
SP: Another one on the healthcare side is Antibe Therapeutics Inc. (ATE:TSX.V; ATBPG:OTCQX), which is developing a pain medication. It's doing a Phase 2 trial right now which will hopefully demonstrate equivalent or better efficacy but with greatly reduced negative side effects. The results are expected on that within several weeks.
I like 3D Signatures and Antibe because they each have a short-term potential catalyst that we view as having relatively high odds of success.
The pain market is huge. There's a lot of talk in the press about opioid use, etc. The existing drugs for osteoarthritis cause a high frequency of stomach ulcers and other side effects such as high blood pressure. Antibe is trying to show that its product has many fewer side effects but the same or better efficacy.
The company also owns a subsidiary that sells products for bone grafts in the dental area. Those products are expected to do roughly $10 million this year in revenue. So it's good that it has a base business. It's not an all-or-nothing-type situation like many biotech companies.
TLSR: Would you tell us about one more company?
SP: There's a company called ClearBlue Technologies Inc. that's going public shortly. It recently had a very good response by investors at our conference. It has products for what it calls smart off-grid solutions. It uses solar and wind energy to manage things like streetlights and security and broadcast Internet, etc. It has revenue, which is expected to more than double in 2018 from around $4 million in 2017. it's a high-growth business. It is the leader in the space. It has a huge sales funnel. I really like the CEO, Miriam Tuerk. She used to be in a senior role at BCE Emergis. I'm pretty excited about the prospects for that company.
The company is in the process of doing a reverse takeover. The shares should be trading in March on the TSX Venture exchange.
TLSR: Thanks, Steve, for sharing these with us.
The AlphaNorth Capital Conference is one of seven annual conferences produced by Vancouver-based Capital Event Management (CEM); the conference mandate is to connect capital with opportunity. In addition to the AlphaNorth Bahamas event, CEM conferences include stops in Whistler, BC; Scottsdale, AZ; Montreal, QC; Kelowna, BC; Muskoka, ON and Palm Beach, FL. In each case issuers from all sectors meet and establish relationships with top investors in the field, leading to financings and after market support. Full details at www.capitalevent.ca.
Steve Palmer is a founding partner, president and chief investment officer of AlphaNorth Asset Management and currently manages the award-winning AlphaNorth Partners Fund, AlphaNorth Growth Fund and AlphaNorth Resource Fund. Prior to founding AlphaNorth in 2007, Palmer was employed as vice president at one of the world's largest financial institutions, where he managed equity assets of approximately CA$350M. Palmer managed a pooled fund, which focused on Canadian small-capitalization companies, from its inception to August 2007, achieving returns of 35.8% annualized over a nine-year period, which ranked it No. 1 in performance by a major fund ranking service in its small-cap, pooled-fund category. Palmer earned a bachelor's degree in economics from the University of Western Ontario and is a Chartered Financial Analyst.
Read what other experts are saying about:
3D Signatures Inc.
Want to read more about Life Sciences Tools & Diagnostics, Biotechnology / Pharmaceuticals, Technology and Alternative Energy? Sign up to receive the FREE Streetwise Reports' newsletter.
Newsletter Sign-Up
Disclosure:
1) Patrice Fusillo conducted this interview for Streetwise Reports LLC and provides services to Streetwise Reports as an employee. She owns, or members of her immediate household or family own, shares of the following companies mentioned in this article: None. She is, or members of her immediate household or family are, paid by the following companies mentioned in this article: None.
2) The following companies mentioned in this interview are billboard sponsors of Streetwise Reports: 3D Signatures. Streetwise Reports does not accept stock in exchange for its services. Click here for important disclosures about sponsor fees. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
3) Steve Palmer: I, or members of my immediate household or family, own shares of the following companies mentioned in this article: None. I, or members of my immediate household or family, are paid by the following companies mentioned in this article: None. My company has a financial relationship with the following companies mentioned in this interview: None. AlphaNorth funds hold shares of the following companies mentioned in this article: All. I determined which companies would be included in this article based on my research and understanding of the sector. I had the opportunity to review the interview for accuracy as of the date of the interview and am responsible for the content of the interview.
4) The interview does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their immediate families are prohibited from making purchases and/or sales of those securities in the open market or otherwise from the time of the interview or the decision to write an article, until one week after the publication of the interview r article. As of the date of this interview, officers and/or employees of Streetwise Reports LLC (including members of their household) own securities of 3D Signatures, a company mentioned in this article."
Sein Wort in Gottes Ohr.
Antwort auf Beitrag Nr.: 57.163.599 von techinvestor69 am 01.03.18 20:11:34als Nächstes ist erstmal der grossangelegte Test wichtig.
p.S.
"Investoren, die diesen Wert beobachten, informieren sich auch über: Wertpapier Perf. %
Alexion Pharmaceuticals -1,45
Gilead Sciences -0,55 Incyte -1,95
BB Biotech -1,27
361 Degrees International -3,10
co.don -4,74 Invitae -0,65
Cidara Therapeutics +0,18
Adaptimmune Therapeutics (Spons. ADR) -1,17
Abivax"
spricht für Einen "bisschen Fortgeschrittenen Interessentenkreis", würde ich sagen.
wie Die Degrees Bude da raufkommt wundert mich aber.
"Investoren, die diesen Wert beobachten, informieren sich auch über: Wertpapier Perf. %
Alexion Pharmaceuticals -1,45
Gilead Sciences -0,55 Incyte -1,95
BB Biotech -1,27
361 Degrees International -3,10
co.don -4,74 Invitae -0,65
Cidara Therapeutics +0,18
Adaptimmune Therapeutics (Spons. ADR) -1,17
Abivax"
spricht für Einen "bisschen Fortgeschrittenen Interessentenkreis", würde ich sagen.
wie Die Degrees Bude da raufkommt wundert mich aber.
interessante Chart Analyse aus dem SH:
http://www.stockhouse.com/companies/bullboard?symbol=v.dxd&p…
und das kam gestern auf Bloomberg:
http://www.digitaljournal.com/pr/3680134
http://www.stockhouse.com/companies/bullboard?symbol=v.dxd&p…
und das kam gestern auf Bloomberg:
http://www.digitaljournal.com/pr/3680134
news!!!
https://finance.yahoo.com/news/3d-signatures-inc-linstitut-u…
3D Signatures Inc. and l'Institut Universitaire de Cardiologie et de Pneumologie de Québec (IUCPQ) Announce Collaboration to Combine TeloView(TM) with Genome Sequence Analysis in Lung Cancer
GlobeNewswire•March 6, 2018
TORONTO, March 06, 2018 (GLOBE NEWSWIRE) -- 3D Signatures Inc. (DXD.V) (TDSGF) (3D0.F) (the "Company" or "3DS"), a personalized medicine company with a proprietary software platform based on the three-dimensional analysis of chromosomal signatures, is pleased to announce that it has signed a collaboration agreement with l`Institut Universitaire de Cardiologie et de Pneumologie de Québec ("IUCPQ") to evaluate the clinical application of 3DS` proprietary TeloView(TM) software platform alongside DNA sequence analysis in lung cancer.
The study will be conducted at 3DS` laboratory in Toronto, Canada, through a collaboration with Dr. Philippe Joubert (MD, PhD, FRCPC), anatomic pathologist at the IUCPQ, researcher at the IUCPQ Research Center, and assistant clinical professor in the Department of Molecular Biology, Medical Biochemistry, and Pathology at Laval University. IUCPQ will conduct whole exome sequence analysis, and provide 3DS with matching tissue samples from the IUCPQ Tumor Bank, from patients with non-small cell lung cancer (NSCLC).
"Pathologists have a variety of emerging technologies available that may offer new insights into optimal disease management", states Dr. Joubert. "This pilot is an exciting opportunity to evaluate how three-dimensional telomere analysis may complement other new tools at our disposal to offer potential benefits to healthcare providers, lung cancer patients, and the healthcare system".
According to the World Health Organization, lung cancer is the most common cancer in the world, accounting for over 1.8 million new cases annually, and remains the leading cause of cancer deaths each year.1 Targeted therapies against specific gene mutations have had an important but limited effect on the total lung cancer population. The recent introduction of immunotherapies has been highly effective in only a minority of patients because clinicians lack robust biomarkers to predict which patients will benefit from these expensive treatments.
"We look forward to this additional collaboration with Dr. Joubert and the IUCPQ, to further expand TeloView`s potential application as a universal clinical biomarker in cancer," commented Jason Flowerday, CEO of 3DS. "We hope this pilot study will be an important precedent for demonstrating how our TeloView(TM) platform may integrate with genomic sequencing with the aim of supporting clinical decisions for the precision care of such a large patient population as lung cancer."
About the IUCPQ
Since its creation in 1918, the Institut Universitaire De Cardiologie et de Pneumologie de Quebec has stood on equal footing with leading North American establishments for ultra-specialized care. Specializing in the health of persons with cardiopulmonary diseases, the Institute is renowned and recognized internationally as a leader. What distinguishes the Institute is its expertise and innovative practices, its focus on research and teaching, and its technological advances. The Institute is where specialized expertise and the sharing of knowledge come together in an ongoing quest for excellence. The close and continuing synergy between clinical activities, research, teaching and the evaluation of health technologies and interventions ensures a care approach guided by best practices and forms the cornerstone of the Institute`s mission.
https://finance.yahoo.com/news/3d-signatures-inc-linstitut-u…
3D Signatures Inc. and l'Institut Universitaire de Cardiologie et de Pneumologie de Québec (IUCPQ) Announce Collaboration to Combine TeloView(TM) with Genome Sequence Analysis in Lung Cancer
GlobeNewswire•March 6, 2018
TORONTO, March 06, 2018 (GLOBE NEWSWIRE) -- 3D Signatures Inc. (DXD.V) (TDSGF) (3D0.F) (the "Company" or "3DS"), a personalized medicine company with a proprietary software platform based on the three-dimensional analysis of chromosomal signatures, is pleased to announce that it has signed a collaboration agreement with l`Institut Universitaire de Cardiologie et de Pneumologie de Québec ("IUCPQ") to evaluate the clinical application of 3DS` proprietary TeloView(TM) software platform alongside DNA sequence analysis in lung cancer.
The study will be conducted at 3DS` laboratory in Toronto, Canada, through a collaboration with Dr. Philippe Joubert (MD, PhD, FRCPC), anatomic pathologist at the IUCPQ, researcher at the IUCPQ Research Center, and assistant clinical professor in the Department of Molecular Biology, Medical Biochemistry, and Pathology at Laval University. IUCPQ will conduct whole exome sequence analysis, and provide 3DS with matching tissue samples from the IUCPQ Tumor Bank, from patients with non-small cell lung cancer (NSCLC).
"Pathologists have a variety of emerging technologies available that may offer new insights into optimal disease management", states Dr. Joubert. "This pilot is an exciting opportunity to evaluate how three-dimensional telomere analysis may complement other new tools at our disposal to offer potential benefits to healthcare providers, lung cancer patients, and the healthcare system".
According to the World Health Organization, lung cancer is the most common cancer in the world, accounting for over 1.8 million new cases annually, and remains the leading cause of cancer deaths each year.1 Targeted therapies against specific gene mutations have had an important but limited effect on the total lung cancer population. The recent introduction of immunotherapies has been highly effective in only a minority of patients because clinicians lack robust biomarkers to predict which patients will benefit from these expensive treatments.
"We look forward to this additional collaboration with Dr. Joubert and the IUCPQ, to further expand TeloView`s potential application as a universal clinical biomarker in cancer," commented Jason Flowerday, CEO of 3DS. "We hope this pilot study will be an important precedent for demonstrating how our TeloView(TM) platform may integrate with genomic sequencing with the aim of supporting clinical decisions for the precision care of such a large patient population as lung cancer."
About the IUCPQ
Since its creation in 1918, the Institut Universitaire De Cardiologie et de Pneumologie de Quebec has stood on equal footing with leading North American establishments for ultra-specialized care. Specializing in the health of persons with cardiopulmonary diseases, the Institute is renowned and recognized internationally as a leader. What distinguishes the Institute is its expertise and innovative practices, its focus on research and teaching, and its technological advances. The Institute is where specialized expertise and the sharing of knowledge come together in an ongoing quest for excellence. The close and continuing synergy between clinical activities, research, teaching and the evaluation of health technologies and interventions ensures a care approach guided by best practices and forms the cornerstone of the Institute`s mission.
http://www.3dsignatures.com/news/3d-signatures-inc-announces…
"3D Signatures Inc. Announces Successful Scoring Model Development and Analytical Validation, of the Telo-HL™ Test for Hodgkin’s Lymphoma
Print
Email
2018, Company
April 3, 2018
TORONTO, April 3, 2018
3D Signatures Inc. (TSX-V XD) (OTCQB:TDSGF) (FSE:3D0) (the “Company” or “3DS“), a personalized medicine company with a proprietary software platform (TeloView™) based on the three-dimensional analysis of chromosomal signatures, is pleased to announce the successful and on-time development of the scoring model for Telo-HL™, the Company’s lead test for Hodgkin’s lymphoma (“HL”), as well as completion of an analytical validation study to confirm the reproducibility of its Telo-HL™ test.
XD) (OTCQB:TDSGF) (FSE:3D0) (the “Company” or “3DS“), a personalized medicine company with a proprietary software platform (TeloView™) based on the three-dimensional analysis of chromosomal signatures, is pleased to announce the successful and on-time development of the scoring model for Telo-HL™, the Company’s lead test for Hodgkin’s lymphoma (“HL”), as well as completion of an analytical validation study to confirm the reproducibility of its Telo-HL™ test.
Powered by the Company’s proprietary TeloView™ platform, Telo-HL™ is a predictive test performed on diagnostic lymph node biopsy specimens, intended to provide clinicians with the first biomarker capable of identifying the 15% – 20% of HL patients who will fail standard ABVD chemotherapy, and who should immediately be considered for more advanced treatment or inclusion into clinical trials with an emerging immunotherapy.
The study data from the Company’s multi-parametric telomeric analysis withTeloView™ was analyzed by an independent statistical provider, BioStat Solutions Inc. (“BSSI”), to develop the Telo-HL™ scoring model from over 200 potential predictors that included different combinations of the telomeric nuclear organization, cell type, and clinical parameters. BSSI identified that a combination of at least three of the parameters analyzed by TeloView™ contributed to the scoring model with highly predictive characteristics. This included measures unique to 3DS’s platform, which can only be evaluated through three-dimensional analysis of telomeres, and for which current clinical data alone is insufficient to predict risk of relapse.
In addition, the Company reports it has successfully run an internal analytical validation of the test by processing and analyzing, in triplicate, archived samples from the same patients. This important step demonstrates the consistency of the Telo-HL™ test and reproducibility of TeloView™ results under a variety of conditions.
“In keeping with best practices, external scientific peer-review is now essential to confirm our own evaluation of Telo-HL™’s strong performance and reproducibility,” notes Dr. Kevin Little, CSO of 3DS. “The detailed findings will be submitted as quickly as possible for presentation in clinician meetings, and then publication in a top-level clinical journal in the latter half of 2018. This will build awareness with key opinion leaders and pharmaceutical companies that Telo-HL™ is ready and available to be incorporated into clinical trials as a correlative biomarker alongside new therapeutic interventions.”
“This is the most significant accomplishment for the Company yet, and I congratulate everyone involved for achieving this critical milestone as per our plan,” commented Jason Flowerday, CEO of 3DS. “This highly successful study is an important culmination of the work by Dr. Sabine Mai and the 3DS team, to develop the [b]first clinically-compliant and validated test based on telomeric profiling, which can uniquely inform treatment decisions in Hodgkin’s lymphoma[/b]. Telo-HL™ represents a critical proof-of-principle for the Company’s TeloView™ platform that we believe may establish an entirely new clinical paradigm for genome organization, and accelerate the development of our broader platform of TeloView™-based tests in prostate cancer, lung cancer and multiple myeloma.”
About 3DS
3DS (TSX-V XD; OTCQB:TDSGF; FSE:3D0) is a personalized medicine company with a proprietary software platform, TeloView™, that is designed to predict the course of certain diseases and to tailor treatment options for the individual patient. The technology is based on the three-dimensional analysis of telomeres, the protective caps at the ends of chromosomes. 3DS’ TeloView™ software platform measures the organization of the genome and its correspondence to; the stage of a given disease, the rate of progression of the disease, how different diseases will respond to various therapies, and a drug’s efficacy and toxicity. 3DS’ proprietary imaging software is designed to go beyond identifying whether a patient suffers from a specific disease or condition. Instead, the TeloView™ platform is designed to inform clinicians and patients with respect to how to personalize treatment and best manage an individual’s disease based on their unique TeloView Score™. As healthcare moves increasingly toward better informed, patient-centric approaches, the Company intends for the TeloView™ platform to deliver personalized medicine that allows for better treatments, leading to better outcomes.
XD; OTCQB:TDSGF; FSE:3D0) is a personalized medicine company with a proprietary software platform, TeloView™, that is designed to predict the course of certain diseases and to tailor treatment options for the individual patient. The technology is based on the three-dimensional analysis of telomeres, the protective caps at the ends of chromosomes. 3DS’ TeloView™ software platform measures the organization of the genome and its correspondence to; the stage of a given disease, the rate of progression of the disease, how different diseases will respond to various therapies, and a drug’s efficacy and toxicity. 3DS’ proprietary imaging software is designed to go beyond identifying whether a patient suffers from a specific disease or condition. Instead, the TeloView™ platform is designed to inform clinicians and patients with respect to how to personalize treatment and best manage an individual’s disease based on their unique TeloView Score™. As healthcare moves increasingly toward better informed, patient-centric approaches, the Company intends for the TeloView™ platform to deliver personalized medicine that allows for better treatments, leading to better outcomes.
The TeloView™ platform is supported by 25 clinical studies involving more than 3,000 patients and 20 different cancers, plus Alzheimer’s disease. 3DS benefits from twenty years of research, $25M of non-dilutive investment into its platform and more than 130 supporting publications, and holds a portfolio of patents related to three-dimensional telomere analysis for proliferative diseases, including (but not limited to) hematological disorders such as Hodgkin’s lymphoma, multiple myeloma, and chronic myeloid leukemia. 3DS’ intellectual property portfolio also covers prostate cancer, breast cancer, lung cancer, melanoma, colorectal cancer, and Alzheimer’s disease.
For more information, visit the Company’s website at: http://www.3dsignatures.com.
For further information, please contact:
Jason Flowerday
CEO & Director
416-673-8487
investors@3dsignatures.com"
"3D Signatures Inc. Announces Successful Scoring Model Development and Analytical Validation, of the Telo-HL™ Test for Hodgkin’s Lymphoma
2018, Company
April 3, 2018
TORONTO, April 3, 2018
3D Signatures Inc. (TSX-V
 XD) (OTCQB:TDSGF) (FSE:3D0) (the “Company” or “3DS“), a personalized medicine company with a proprietary software platform (TeloView™) based on the three-dimensional analysis of chromosomal signatures, is pleased to announce the successful and on-time development of the scoring model for Telo-HL™, the Company’s lead test for Hodgkin’s lymphoma (“HL”), as well as completion of an analytical validation study to confirm the reproducibility of its Telo-HL™ test.
XD) (OTCQB:TDSGF) (FSE:3D0) (the “Company” or “3DS“), a personalized medicine company with a proprietary software platform (TeloView™) based on the three-dimensional analysis of chromosomal signatures, is pleased to announce the successful and on-time development of the scoring model for Telo-HL™, the Company’s lead test for Hodgkin’s lymphoma (“HL”), as well as completion of an analytical validation study to confirm the reproducibility of its Telo-HL™ test.Powered by the Company’s proprietary TeloView™ platform, Telo-HL™ is a predictive test performed on diagnostic lymph node biopsy specimens, intended to provide clinicians with the first biomarker capable of identifying the 15% – 20% of HL patients who will fail standard ABVD chemotherapy, and who should immediately be considered for more advanced treatment or inclusion into clinical trials with an emerging immunotherapy.
The study data from the Company’s multi-parametric telomeric analysis withTeloView™ was analyzed by an independent statistical provider, BioStat Solutions Inc. (“BSSI”), to develop the Telo-HL™ scoring model from over 200 potential predictors that included different combinations of the telomeric nuclear organization, cell type, and clinical parameters. BSSI identified that a combination of at least three of the parameters analyzed by TeloView™ contributed to the scoring model with highly predictive characteristics. This included measures unique to 3DS’s platform, which can only be evaluated through three-dimensional analysis of telomeres, and for which current clinical data alone is insufficient to predict risk of relapse.
In addition, the Company reports it has successfully run an internal analytical validation of the test by processing and analyzing, in triplicate, archived samples from the same patients. This important step demonstrates the consistency of the Telo-HL™ test and reproducibility of TeloView™ results under a variety of conditions.
“In keeping with best practices, external scientific peer-review is now essential to confirm our own evaluation of Telo-HL™’s strong performance and reproducibility,” notes Dr. Kevin Little, CSO of 3DS. “The detailed findings will be submitted as quickly as possible for presentation in clinician meetings, and then publication in a top-level clinical journal in the latter half of 2018. This will build awareness with key opinion leaders and pharmaceutical companies that Telo-HL™ is ready and available to be incorporated into clinical trials as a correlative biomarker alongside new therapeutic interventions.”
“This is the most significant accomplishment for the Company yet, and I congratulate everyone involved for achieving this critical milestone as per our plan,” commented Jason Flowerday, CEO of 3DS. “This highly successful study is an important culmination of the work by Dr. Sabine Mai and the 3DS team, to develop the [b]first clinically-compliant and validated test based on telomeric profiling, which can uniquely inform treatment decisions in Hodgkin’s lymphoma[/b]. Telo-HL™ represents a critical proof-of-principle for the Company’s TeloView™ platform that we believe may establish an entirely new clinical paradigm for genome organization, and accelerate the development of our broader platform of TeloView™-based tests in prostate cancer, lung cancer and multiple myeloma.”
About 3DS
3DS (TSX-V
 XD; OTCQB:TDSGF; FSE:3D0) is a personalized medicine company with a proprietary software platform, TeloView™, that is designed to predict the course of certain diseases and to tailor treatment options for the individual patient. The technology is based on the three-dimensional analysis of telomeres, the protective caps at the ends of chromosomes. 3DS’ TeloView™ software platform measures the organization of the genome and its correspondence to; the stage of a given disease, the rate of progression of the disease, how different diseases will respond to various therapies, and a drug’s efficacy and toxicity. 3DS’ proprietary imaging software is designed to go beyond identifying whether a patient suffers from a specific disease or condition. Instead, the TeloView™ platform is designed to inform clinicians and patients with respect to how to personalize treatment and best manage an individual’s disease based on their unique TeloView Score™. As healthcare moves increasingly toward better informed, patient-centric approaches, the Company intends for the TeloView™ platform to deliver personalized medicine that allows for better treatments, leading to better outcomes.
XD; OTCQB:TDSGF; FSE:3D0) is a personalized medicine company with a proprietary software platform, TeloView™, that is designed to predict the course of certain diseases and to tailor treatment options for the individual patient. The technology is based on the three-dimensional analysis of telomeres, the protective caps at the ends of chromosomes. 3DS’ TeloView™ software platform measures the organization of the genome and its correspondence to; the stage of a given disease, the rate of progression of the disease, how different diseases will respond to various therapies, and a drug’s efficacy and toxicity. 3DS’ proprietary imaging software is designed to go beyond identifying whether a patient suffers from a specific disease or condition. Instead, the TeloView™ platform is designed to inform clinicians and patients with respect to how to personalize treatment and best manage an individual’s disease based on their unique TeloView Score™. As healthcare moves increasingly toward better informed, patient-centric approaches, the Company intends for the TeloView™ platform to deliver personalized medicine that allows for better treatments, leading to better outcomes.The TeloView™ platform is supported by 25 clinical studies involving more than 3,000 patients and 20 different cancers, plus Alzheimer’s disease. 3DS benefits from twenty years of research, $25M of non-dilutive investment into its platform and more than 130 supporting publications, and holds a portfolio of patents related to three-dimensional telomere analysis for proliferative diseases, including (but not limited to) hematological disorders such as Hodgkin’s lymphoma, multiple myeloma, and chronic myeloid leukemia. 3DS’ intellectual property portfolio also covers prostate cancer, breast cancer, lung cancer, melanoma, colorectal cancer, and Alzheimer’s disease.
For more information, visit the Company’s website at: http://www.3dsignatures.com.
For further information, please contact:
Jason Flowerday
CEO & Director
416-673-8487
investors@3dsignatures.com"
Halloooooo?
Noch jemand dabei ?? Noch jemand da?
Noch jemand dabei ?? Noch jemand da?
Insolvenz in Rekordzeit 


3D Signatures Announces Intention to File for Bankruptcy
2018-05-31 12:35 ET - News Release
TORONTO, May 31, 2018 (GLOBE NEWSWIRE) -- 3D Signatures Inc. (TSX-V DXD) (OTCQB:TDSGF) (FSE:3D0) (the "Company" or "3DS"), a personalized medicine company with a proprietary software platform (TeloViewTM) based on the three-dimensional analysis of chromosomal signatures, today announced its intention to assign itself into bankruptcy under the Bankruptcy and Insolvency Act (the “BIA”) and the layoff of its employees and contractors.
As a research and development stage company, 3DS is primarily dependent on funding from investors to continue as a going concern. While 3DS has been successful in reducing its overall cost structure, it has been unable to secure the additional financing required to fund its operations. As a result, the Company intends to assign itself into bankruptcy under the BIA. In addition, the Company has conducted a layoff of its employees and contractors. Subsequent to the filing of the assignment into bankruptcy, the Licensed Insolvency Trustee may request certain key employees to provide assistance with its administration. The Board of Directors of 3DS has not resigned and will cooperate fully with the Licensed Insolvency Trustee once they are retained in accordance with the resolution passed by the Board of Directors on May 30, 2018.
Third Quarter Financial Summary
The Company significantly reduced its cash monthly burn rate and recorded a net loss of $920,758 ($0.01 per Common Share) for the three months ended March 31, 2018 compared to $2,171,822 ($0.04 per Common Share) for the three months ended March 31, 2017.
As at March 31, 2018, the Company had cash resources of $618,411 compared to $1,200,395 as at June 30, 2017 and $2,552,822 at March 31, 2017. As at March 31, 2018 the Company had working capital of $390,689 compared to working capital of $1,329,408 as at June 30, 2017 and $3,215,197 at March 31, 2017.
The Company’s financial statements and management’s discussion and analysis are available on www.sedar.com.



3D Signatures Announces Intention to File for Bankruptcy
2018-05-31 12:35 ET - News Release
TORONTO, May 31, 2018 (GLOBE NEWSWIRE) -- 3D Signatures Inc. (TSX-V DXD) (OTCQB:TDSGF) (FSE:3D0) (the "Company" or "3DS"), a personalized medicine company with a proprietary software platform (TeloViewTM) based on the three-dimensional analysis of chromosomal signatures, today announced its intention to assign itself into bankruptcy under the Bankruptcy and Insolvency Act (the “BIA”) and the layoff of its employees and contractors.
As a research and development stage company, 3DS is primarily dependent on funding from investors to continue as a going concern. While 3DS has been successful in reducing its overall cost structure, it has been unable to secure the additional financing required to fund its operations. As a result, the Company intends to assign itself into bankruptcy under the BIA. In addition, the Company has conducted a layoff of its employees and contractors. Subsequent to the filing of the assignment into bankruptcy, the Licensed Insolvency Trustee may request certain key employees to provide assistance with its administration. The Board of Directors of 3DS has not resigned and will cooperate fully with the Licensed Insolvency Trustee once they are retained in accordance with the resolution passed by the Board of Directors on May 30, 2018.
Third Quarter Financial Summary
The Company significantly reduced its cash monthly burn rate and recorded a net loss of $920,758 ($0.01 per Common Share) for the three months ended March 31, 2018 compared to $2,171,822 ($0.04 per Common Share) for the three months ended March 31, 2017.
As at March 31, 2018, the Company had cash resources of $618,411 compared to $1,200,395 as at June 30, 2017 and $2,552,822 at March 31, 2017. As at March 31, 2018 the Company had working capital of $390,689 compared to working capital of $1,329,408 as at June 30, 2017 and $3,215,197 at March 31, 2017.
The Company’s financial statements and management’s discussion and analysis are available on www.sedar.com.
manchmal macht realisieren Keinen Spass.
da geht Jetzt ein bisschen Was über den Jordan bei Mir.
da geht Jetzt ein bisschen Was über den Jordan bei Mir.
ist doch noch nicht tot
3D Signatures arranges $105K loan; CEO, CFO resign2018-09-17 20:52 ET - News Release
Mr. Hugh Rogers reports
3D SIGNATURES INC. ANNOUNCES $105,000 LOAN AND CHANGES TO ITS BOARD OF DIRECTORS AND MANAGEMENT
3D Signatures Inc. has secured a $105,000 senior secured promissory note from shareholders of the company for the purpose of financially stabilizing 3DS in the short term. The promissory note bears an annual interest rate of 10 per cent and matures on Sept. 12, 2019.
The company also announces that John Swift, Keith Cassidy, Ian Fodie and Jason Flowerday have resigned from the board of directors, effective immediately. Dr. Sabine Mai, co-founder and inventor, will remain on the board of directors. Hugh Rogers, LLB, and Ryan Cheung, CPA, CA, have been appointed to the board of directors, effective immediately.
Mr. Flowerday has resigned as chief executive officer of the company pursuant to a negotiated severance settlement of two million common shares of 3DS, which are subject to a two-year escrow period. He will retain his existing options for a period of five years.
Mr. Cassidy has resigned as chief financial officer of the company and will also retain his existing options for a period of five years.
Mr. Rogers will act as interim chairman. Mr. Cheung has been appointed chief financial officer of the company.
Certain founders and insiders who purchased shares of the company at inception at a nominal price or who exercised options with a strike price of less than 10 cents have also agreed to a full escrow of their shares for two years. The total number of shares in escrow for two years pursuant to this arrangement is 15,272,437, representing 22.4 per cent of the issued and outstanding shares of the company on a fully diluted basis.
"The company has done exceptional work over the past two years, and we appreciate the efforts of all team members. As we transition operations and budget to reflect a more focused business model, we will assess all current clinical programs, including Hodgkin's lymphoma, prostate cancer, lung cancer and multiple myeloma. As a priority, the company is pursuing a clinical trial for Alzheimer's disease, which is a large and rapidly growing market with a critical unmet need for an accurate, convenient and non-invasive diagnostic tool," said Mr. Rogers.
About 3D Signatures Inc.
3DS is a personalized medicine company with a proprietary software platform, TeloView, that is designed to predict the course of certain diseases and to tailor treatment options for the individual patient. 3DS's TeloView software platform measures the organization of the genome and its correspondence to: the stage of a given disease, the rate of progression of the disease, how different diseases will respond to various therapies, and a drug's efficacy and toxicity. 3DS's proprietary imaging software is designed to go beyond identifying whether a patient suffers from a specific disease or condition. Instead, the TeloView platform is designed to inform clinicians and patients with respect to how to personalize treatment and best manage an individual's disease based on their unique TeloViewScore.
...
Quelle: www.stockwatch.com
Antwort auf Beitrag Nr.: 58.768.456 von techinvestor69 am 22.09.18 08:35:51ist doch noch nicht tot
_________________
Techinvestor
Glaubst Du wirklich dass Sie Das Ruder nochmal rumgerissen bekommen?
105k loan ist ganz süss,
aber ich glaube da nicht so richtig dran.
_________________
Techinvestor
Glaubst Du wirklich dass Sie Das Ruder nochmal rumgerissen bekommen?
105k loan ist ganz süss,
aber ich glaube da nicht so richtig dran.
Antwort auf Beitrag Nr.: 58.770.088 von Popeye82 am 22.09.18 13:15:18105k halten die Firma erstmal über Wasser
ist natürlich klar, dass danach noch paar Mio. geraised werden müssen
Ex-CEO hat nochmal 2 Mio. Aktien als Abfindung abgegriffen - schamlos
aber ich sehe zumindest eine Chance, dass die Aktie irgendwann demnächst wieder handelbar ist, dann könnte man zumindest die steuerlichen Verluste realisieren
ist natürlich klar, dass danach noch paar Mio. geraised werden müssen
Ex-CEO hat nochmal 2 Mio. Aktien als Abfindung abgegriffen - schamlos
aber ich sehe zumindest eine Chance, dass die Aktie irgendwann demnächst wieder handelbar ist, dann könnte man zumindest die steuerlichen Verluste realisieren
C XD 3D Signatures to resume at Sept. 26 open
XD 3D Signatures to resume at Sept. 26 open
 XD 3D Signatures to resume at Sept. 26 open
XD 3D Signatures to resume at Sept. 26 open Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +18,18 | |
| -2,86 | |
| +0,10 | |
| 0,00 | |
| -0,98 | |
| +106,67 | |
| -0,55 | |
| -0,23 | |
| -20,16 | |
| +2,91 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 185 | ||
| 126 | ||
| 90 | ||
| 66 | ||
| 65 | ||
| 50 | ||
| 41 | ||
| 32 | ||
| 32 | ||
| 31 |






















