Moderna (Seite 176)
eröffnet am 24.11.19 21:27:56 von
neuester Beitrag 05.05.23 10:23:01 von
neuester Beitrag 05.05.23 10:23:01 von
Beiträge: 1.856
ID: 1.315.946
ID: 1.315.946
Aufrufe heute: 1
Gesamt: 274.455
Gesamt: 274.455
Aktive User: 0
ISIN: US60770K1079 · WKN: A2N9D9 · Symbol: MRNA
100,54
EUR
+2,50 %
+2,45 EUR
Letzter Kurs 19:13:26 Tradegate
Neuigkeiten
| Moderna Aktien jetzt im kostenlosen Demokonto handeln!Anzeige |
22.04.24 · Accesswire |
17.04.24 · Accesswire |
10.04.24 · Sharedeals |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,4800 | +33,82 | |
| 0,7802 | +24,91 | |
| 4,5700 | +24,18 | |
| 0,7560 | +21,94 | |
| 0,5900 | +21,90 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,7603 | -15,56 | |
| 3,3500 | -16,25 | |
| 2,9400 | -16,95 | |
| 26,75 | -49,77 | |
| 3,4950 | -52,51 |
Beitrag zu dieser Diskussion schreiben
Antwort auf Beitrag Nr.: 63.355.369 von Gustl24 am 17.04.20 06:15:17ohje....
na dann pusht euch mal gegenseitig.
zumindest hat sich das stille mitlesen insofern gelohnt als dass das hier eine extrem lehrreiche stunde in marktpsychologie ist.
na dann pusht euch mal gegenseitig.
zumindest hat sich das stille mitlesen insofern gelohnt als dass das hier eine extrem lehrreiche stunde in marktpsychologie ist.
Guten Morgen,
sehe ich genauso, hätte, hätte bis 10:00 Uhr einen Vorlauf bis ca. 50% erwartet.
Na dann eben ab 10:00 Uhr wenn die Amis wach werden😉
Mal sehen was der Tag bringt...
sehe ich genauso, hätte, hätte bis 10:00 Uhr einen Vorlauf bis ca. 50% erwartet.
Na dann eben ab 10:00 Uhr wenn die Amis wach werden😉
Mal sehen was der Tag bringt...
Nasdaq Price 48,7$ - entspricht 44,9€ da werden gerade billig Aktien verkauft...
„…Award will fund development of mRNA-1273 to FDA licensure Award will fund manufacturing process scale-up to enable large-scale production in 2020 for pandemic response
NIH-led Phase 1 study of mRNA-1273 has completed enrollment of 3 dose cohorts (25 µg, 100 µg and 250 µg); expanding to an additional 6 cohorts of older adults and elderly adults
Phase 2 study expected to begin in Q2 2020, following safety data from ongoing Phase 1 study Moderna to hire up to 150 new team members to support efforts …“
Conference call to be held on Friday, April 17 at 8:00 a.m. ET
NIH-led Phase 1 study of mRNA-1273 has completed enrollment of 3 dose cohorts (25 µg, 100 µg and 250 µg); expanding to an additional 6 cohorts of older adults and elderly adults
Phase 2 study expected to begin in Q2 2020, following safety data from ongoing Phase 1 study Moderna to hire up to 150 new team members to support efforts …“
Conference call to be held on Friday, April 17 at 8:00 a.m. ET
Und nochmals eine Quelle: https://seekingalpha.com/pr/17840261-moderna-announces-award…
Antwort auf Beitrag Nr.: 63.353.137 von Griever am 16.04.20 20:59:32Du scheinst dich in Medizin/ Biotech fundamental wenig auszukennen...und mit ein bisschen Zahlenspielerei wirst du hier nicht weiterkommen...
Mir ist bekannt das aktuell ein paar Dutzend Firmen bzgl. Corona-Impfstoff forschen: https://t.co/RCyXTONoJk?amp=1
Und natürlich gibt es eine Menge Trittbrettfahrer...da wird man auch Ende des Jahres noch im preclinical Status verweilen
Aber durch die Meldung von heute Nacht hat es sich eigentlich erübrigt, Moderna wird das Feld bzgl. Corona-Impfstoff mit anführen...
Und eine MK von 12 Mrd. ist in wenigen Monaten sollten sie erfolgreich sein (!) ein Witz.
Dann wird sie bei 60-80 Mrd. stehen. Du kannst jetzt staunend den Kurs zur Dreistelligkeit die nächsten Monate verfolgen und weiterhin den Kopf schütteln.
P.S. Wenn man natürlich zur Fraktion -ich kann das alles nicht verstehen, ist doch nur ne Influenza, spanische Grippe, Verschwörungstheoretiker etc. gehört - kann man sich jedes Wort sparen...
Mir ist bekannt das aktuell ein paar Dutzend Firmen bzgl. Corona-Impfstoff forschen: https://t.co/RCyXTONoJk?amp=1
Und natürlich gibt es eine Menge Trittbrettfahrer...da wird man auch Ende des Jahres noch im preclinical Status verweilen

Aber durch die Meldung von heute Nacht hat es sich eigentlich erübrigt, Moderna wird das Feld bzgl. Corona-Impfstoff mit anführen...
Und eine MK von 12 Mrd. ist in wenigen Monaten sollten sie erfolgreich sein (!) ein Witz.
Dann wird sie bei 60-80 Mrd. stehen. Du kannst jetzt staunend den Kurs zur Dreistelligkeit die nächsten Monate verfolgen und weiterhin den Kopf schütteln.
P.S. Wenn man natürlich zur Fraktion -ich kann das alles nicht verstehen, ist doch nur ne Influenza, spanische Grippe, Verschwörungstheoretiker etc. gehört - kann man sich jedes Wort sparen...
Hier die E-mail press release Mitteilung die ich heute Nacht erhalten habe.
Zur information : Was ist BARDA ??
The Biomedical Advanced Research and Development Authority is a U.S. Department of Health and Human Services office responsible for procurement and development of countermeasures principally against ... Wikipedia
Founded: 2006
Parent agency: Office of the Assistant Secretary for Preparedness and Response
Agency executive: Rick A. Bright, Director of BARDA
https://www.phe.gov/about/barda/Pages/default.aspx
April 16, 2020 5:55 PM EDT
Moderna Announces Award from U.S. Government Agency BARDA for up to $483 Million to Accelerate Development of mRNA Vaccine (mRNA-1273) Against Novel Coronavirus
Award will fund development of mRNA-1273 to FDA licensure
Award will fund manufacturing process scale-up to enable large-scale production in 2020 for pandemic response
NIH-led Phase 1 study of mRNA-1273 has completed enrollment of 3 dose cohorts (25 µg, 100 µg and 250 µg); expanding to an additional 6 cohorts of older adults and elderly adults
Phase 2 study expected to begin in Q2 2020, following safety data from ongoing Phase 1 study
Moderna to hire up to 150 new team members to support efforts
Conference call to be held on Friday, April 17 at 8:00 a.m. ET
CAMBRIDGE, Mass.--(BUSINESS WIRE)--Apr. 16, 2020-- Moderna, Inc., (Nasdaq: MRNA) a clinical stage biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines to create a new generation of transformative medicines for patients, today announced an agreement for a commitment of up to $483 million from the Biomedical Advanced Research and Development Authority (BARDA), a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS), to accelerate development of the Company’s mRNA vaccine candidate (mRNA-1273) against the novel coronavirus (SARS-CoV-2).
Under the terms of the agreement, BARDA will fund the advancement of mRNA-1273 to FDA licensure. A Phase 1 study of mRNA-1273 is being conducted by the National Institutes of Health (NIH). The Phase 1 open-label study, which began on March 16, 2020 has completed enrollment of the original study: 45 healthy adult volunteers ages 18 to 55 years in three dose cohorts (25 µg, 100 µg and 250 µg). The NIH recently amended the Phase 1 protocol to include an additional six cohorts: three cohorts of older adults (ages 51-70) and three cohorts of elderly adults (age 71 and above). Enrollment for these cohorts is ongoing.
If supported by safety data from the Phase 1 study, the Company intends to begin a Phase 2 study of mRNA-1273 under its own Investigational New Drug (IND) application in the second quarter of 2020. Subject to data from these studies and discussions with regulators, a Phase 3 study could begin as soon as fall, 2020. BARDA funding will support these late-stage clinical development programs, as well as the scale-up of mRNA-1273 manufacture in 2020 to enable potential pandemic response.
To support the scale-up, Moderna plans to hire up to 150 new team members in the U.S. this year. This includes a significant increase in its skilled manufacturing staff to expand manufacturing capacity from two shifts per day, 5 days per week to three shifts per day, 7 days per week, engineers to manage process scale-up, and clinical and regulatory staff to support clinical development.
“We are thankful for BARDA’s support to fund the accelerated development of mRNA-1273, our vaccine candidate against SARS-CoV-2,” said Stéphane Bancel, Moderna’s Chief Executive Officer. “Time is of the essence to provide a vaccine against this pandemic virus. By investing now in our manufacturing process scale-up to enable large scale production for pandemic response, we believe that we would be able to supply millions of doses per month in 2020 and with further investments, tens of millions per month in 2021, if the vaccine candidate is successful in the clinic.”
“Vaccines are a critical tool for saving lives and stopping the spread of the SARS-CoV-2 virus,” said BARDA Director Rick Bright, Ph.D. “Delivering a safe and effective vaccine for a rapidly spreading virus requires accelerated action. BARDA’s goal is to have vaccine available as quickly as possible and preparing now for advanced stage clinical trials and production scale-up while the Phase 1 is underway could shave months off development of COVID-19 vaccines.”
Conference Call and Webcast Information
Moderna will host a live conference call and webcast at 8:00 a.m. ET on Friday, April 17, 2020. To access the live conference call, please dial 866-922-5184 (domestic) or 409-937-8950 (international) and refer to conference ID 5115809. A webcast of the call will also be available under “Events and Presentations” in the Investors section of the Moderna website at investors.modernatx.com. The archived webcast will be available on Moderna’s website approximately two hours after the conference call.
About mRNA-1273
mRNA-1273 is an mRNA vaccine against SARS-CoV-2 encoding for a prefusion stabilized form of the Spike (S) protein, which was selected by Moderna in collaboration with investigators from Vaccine Research Center (VRC) at the National Institute of Allergy and Infectious Diseases (NIAID), a part of the NIH. The first clinical batch, which was funded by the Coalition for Epidemic Preparedness Innovations, was completed on February 7, 2020 and underwent analytical testing; it was shipped to NIH on February 24, 42 days from sequence selection. The first participant in the NIH-led Phase 1 study of mRNA-1273 was dosed on March 16, 63 days from sequence selection to Phase 1 study dosing. A summary of the Company’s work to date on SARS-CoV-2 can be found here.
Zur information : Was ist BARDA ??
The Biomedical Advanced Research and Development Authority is a U.S. Department of Health and Human Services office responsible for procurement and development of countermeasures principally against ... Wikipedia
Founded: 2006
Parent agency: Office of the Assistant Secretary for Preparedness and Response
Agency executive: Rick A. Bright, Director of BARDA
https://www.phe.gov/about/barda/Pages/default.aspx
April 16, 2020 5:55 PM EDT
Moderna Announces Award from U.S. Government Agency BARDA for up to $483 Million to Accelerate Development of mRNA Vaccine (mRNA-1273) Against Novel Coronavirus
Award will fund development of mRNA-1273 to FDA licensure
Award will fund manufacturing process scale-up to enable large-scale production in 2020 for pandemic response
NIH-led Phase 1 study of mRNA-1273 has completed enrollment of 3 dose cohorts (25 µg, 100 µg and 250 µg); expanding to an additional 6 cohorts of older adults and elderly adults
Phase 2 study expected to begin in Q2 2020, following safety data from ongoing Phase 1 study
Moderna to hire up to 150 new team members to support efforts
Conference call to be held on Friday, April 17 at 8:00 a.m. ET
CAMBRIDGE, Mass.--(BUSINESS WIRE)--Apr. 16, 2020-- Moderna, Inc., (Nasdaq: MRNA) a clinical stage biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines to create a new generation of transformative medicines for patients, today announced an agreement for a commitment of up to $483 million from the Biomedical Advanced Research and Development Authority (BARDA), a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS), to accelerate development of the Company’s mRNA vaccine candidate (mRNA-1273) against the novel coronavirus (SARS-CoV-2).
Under the terms of the agreement, BARDA will fund the advancement of mRNA-1273 to FDA licensure. A Phase 1 study of mRNA-1273 is being conducted by the National Institutes of Health (NIH). The Phase 1 open-label study, which began on March 16, 2020 has completed enrollment of the original study: 45 healthy adult volunteers ages 18 to 55 years in three dose cohorts (25 µg, 100 µg and 250 µg). The NIH recently amended the Phase 1 protocol to include an additional six cohorts: three cohorts of older adults (ages 51-70) and three cohorts of elderly adults (age 71 and above). Enrollment for these cohorts is ongoing.
If supported by safety data from the Phase 1 study, the Company intends to begin a Phase 2 study of mRNA-1273 under its own Investigational New Drug (IND) application in the second quarter of 2020. Subject to data from these studies and discussions with regulators, a Phase 3 study could begin as soon as fall, 2020. BARDA funding will support these late-stage clinical development programs, as well as the scale-up of mRNA-1273 manufacture in 2020 to enable potential pandemic response.
To support the scale-up, Moderna plans to hire up to 150 new team members in the U.S. this year. This includes a significant increase in its skilled manufacturing staff to expand manufacturing capacity from two shifts per day, 5 days per week to three shifts per day, 7 days per week, engineers to manage process scale-up, and clinical and regulatory staff to support clinical development.
“We are thankful for BARDA’s support to fund the accelerated development of mRNA-1273, our vaccine candidate against SARS-CoV-2,” said Stéphane Bancel, Moderna’s Chief Executive Officer. “Time is of the essence to provide a vaccine against this pandemic virus. By investing now in our manufacturing process scale-up to enable large scale production for pandemic response, we believe that we would be able to supply millions of doses per month in 2020 and with further investments, tens of millions per month in 2021, if the vaccine candidate is successful in the clinic.”
“Vaccines are a critical tool for saving lives and stopping the spread of the SARS-CoV-2 virus,” said BARDA Director Rick Bright, Ph.D. “Delivering a safe and effective vaccine for a rapidly spreading virus requires accelerated action. BARDA’s goal is to have vaccine available as quickly as possible and preparing now for advanced stage clinical trials and production scale-up while the Phase 1 is underway could shave months off development of COVID-19 vaccines.”
Conference Call and Webcast Information
Moderna will host a live conference call and webcast at 8:00 a.m. ET on Friday, April 17, 2020. To access the live conference call, please dial 866-922-5184 (domestic) or 409-937-8950 (international) and refer to conference ID 5115809. A webcast of the call will also be available under “Events and Presentations” in the Investors section of the Moderna website at investors.modernatx.com. The archived webcast will be available on Moderna’s website approximately two hours after the conference call.
About mRNA-1273
mRNA-1273 is an mRNA vaccine against SARS-CoV-2 encoding for a prefusion stabilized form of the Spike (S) protein, which was selected by Moderna in collaboration with investigators from Vaccine Research Center (VRC) at the National Institute of Allergy and Infectious Diseases (NIAID), a part of the NIH. The first clinical batch, which was funded by the Coalition for Epidemic Preparedness Innovations, was completed on February 7, 2020 and underwent analytical testing; it was shipped to NIH on February 24, 42 days from sequence selection. The first participant in the NIH-led Phase 1 study of mRNA-1273 was dosed on March 16, 63 days from sequence selection to Phase 1 study dosing. A summary of the Company’s work to date on SARS-CoV-2 can be found here.
Ja stimmt, schaut euch den tTmes &Sales an wenn's keiner glaubt .
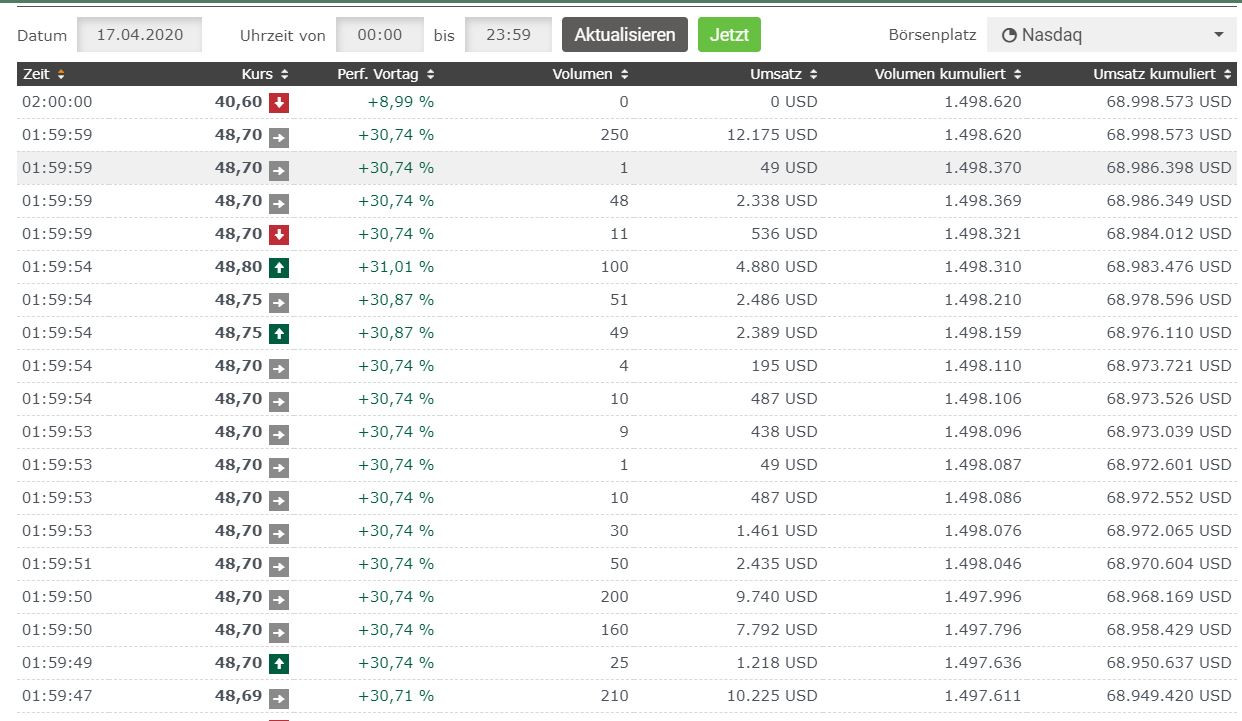

Antwort auf Beitrag Nr.: 63.353.137 von Griever am 16.04.20 20:59:32Nur mal so am Rande, wir haben im After Market +19,95% = 48,70 $ erreicht😉
Ab 09:00 Uhr geht bei uns die Post ab...
https://www.nasdaq.com/articles/moderna-receives-%24483-mill…
Gute Nacht😴
Ab 09:00 Uhr geht bei uns die Post ab...
https://www.nasdaq.com/articles/moderna-receives-%24483-mill…
Gute Nacht😴
ihr malt euch hier ein zeug zusammen....
"biotech", "revolution", "top 3".
leute...basierend auf was? welche zahlen? welche umsatzbringer? welche margen?
der kurs steht aktuell bei mehr als 12 milliarden (!) dollar.
https://www.cnbc.com/2020/04/14/there-are-now-70-coronavirus…
"biotech", "revolution", "top 3".
leute...basierend auf was? welche zahlen? welche umsatzbringer? welche margen?
der kurs steht aktuell bei mehr als 12 milliarden (!) dollar.
https://www.cnbc.com/2020/04/14/there-are-now-70-coronavirus…
Moderna



















