Mountain Valley MD Holdings Inc - building a world-class health and wellness organization
eröffnet am 09.03.20 09:52:14 von
neuester Beitrag 14.03.24 18:15:08 von
neuester Beitrag 14.03.24 18:15:08 von
Beiträge: 483
ID: 1.321.544
ID: 1.321.544
Aufrufe heute: 0
Gesamt: 47.985
Gesamt: 47.985
Aktive User: 0
ISIN: CA62430M1014 · WKN: A2P082 · Symbol: MVMD
0,0500
CAD
-9,09 %
-0,0050 CAD
Letzter Kurs 23.04.24 CSE
Neuigkeiten
08.04.24 · Business Wire (engl.) |
01.03.24 · Business Wire (engl.) |
15.12.23 · Business Wire (engl.) |
08.09.23 · Business Wire (engl.) |
Werte aus der Branche Gesundheitswesen
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 2,2300 | +74,22 | |
| 0,8500 | +41,67 | |
| 0,6868 | +24,85 | |
| 0,5799 | +15,93 | |
| 12,000 | +15,50 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,8450 | -10,10 | |
| 2,8200 | -11,18 | |
| 0,5925 | -15,36 | |
| 0,7600 | -15,65 | |
| 399,40 | -22,28 |
Beitrag zu dieser Diskussion schreiben
Irgendwas ist in Canada nun losgegangen
Der Kurs bewegt sich nach oben
Ich geh mal mit, bisschen kraxeln geht immer
Der Kurs bewegt sich nach oben
Ich geh mal mit, bisschen kraxeln geht immer
Neuer CFO auf neuen CFO
TjaDer erfahrene CFO ist schon Geschichte bevor er gestartet hat…
Weiß man was über den Rückzug?
Neuer CFO
…jemand mit Branchenerfahrung und InvestmenterfahrungSollte klappen
Option bei 0,05 d.h. alles unter dem Kurs ist für
Management uninteressant
Hat jemand was in Erfahrung bringen können was den Pump verursacht hat?
Die Woche haben wir äußerst positiv abgeschlossen. Von 0,035 auf 0,095 Cad$ sind mal eben 160%
Ganz ehrlich gesagt, ich halte dies nicht für nachhaltig und habe das Vertrauen in die Bude verloren.
Ich bin aber noch investiert und hoffe auf eine Wiederholung von 2021 mit knapp 3000% nach oben 😎
Aktuell Charttechnisch am starken Widerstand. Schauen wir mal was die Tage noch kommt ....
Chart ist im weekly
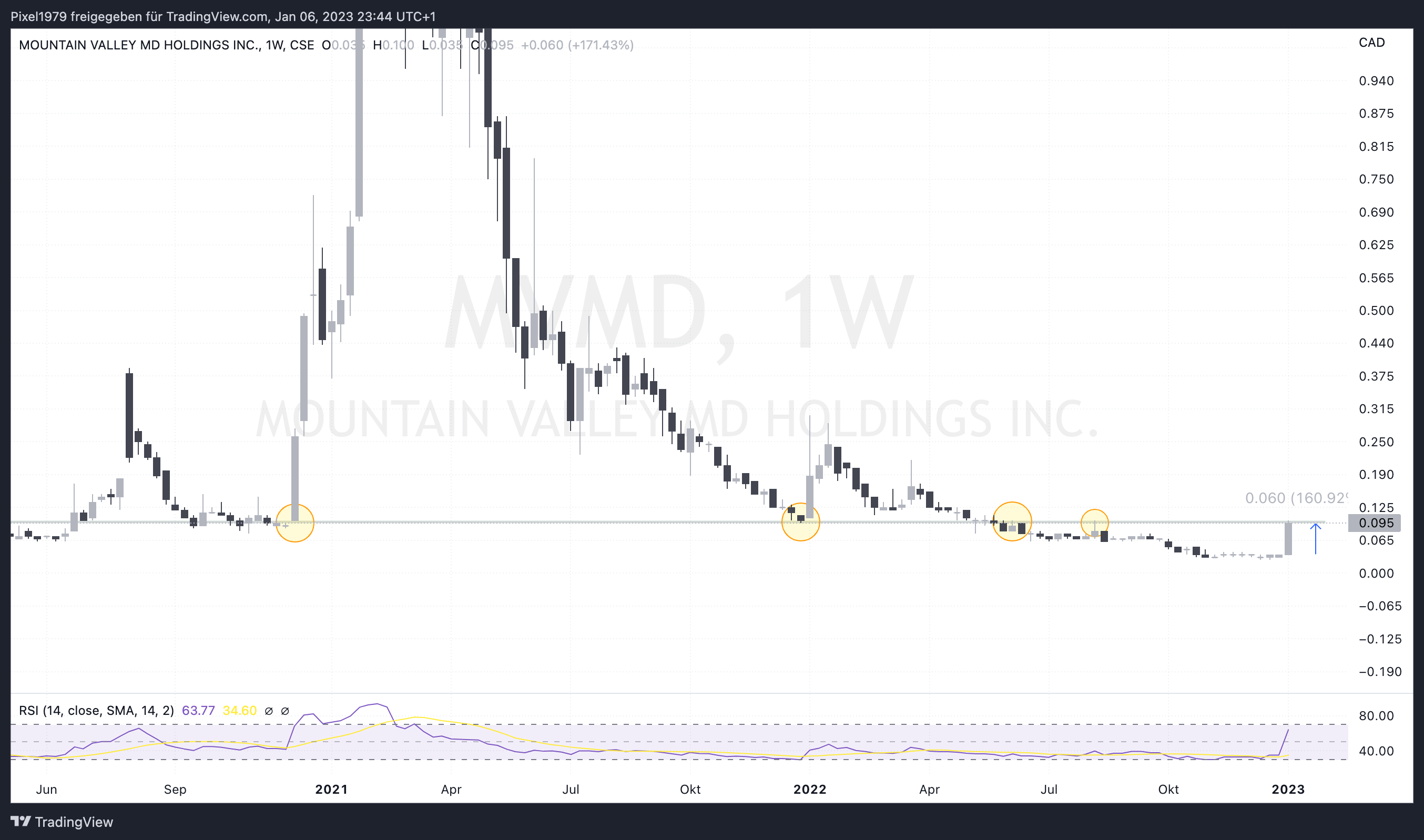
Die Woche haben wir äußerst positiv abgeschlossen. Von 0,035 auf 0,095 Cad$ sind mal eben 160%
Ganz ehrlich gesagt, ich halte dies nicht für nachhaltig und habe das Vertrauen in die Bude verloren.
Ich bin aber noch investiert und hoffe auf eine Wiederholung von 2021 mit knapp 3000% nach oben 😎
Aktuell Charttechnisch am starken Widerstand. Schauen wir mal was die Tage noch kommt ....
Chart ist im weekly

Was neues aus dem Kasperletheater 😂
MVMD-Kommerzialisierungsfokus
Es gibt drei Hauptgeschäftsbereiche, auf die sich MVMD konzentriert, um kommerziell voranzukommen:
(1) Neue Innovationen, die die Verabreichung und Wirksamkeit vonnutrazeutischen Gesundheits- und Wellnessprodukten verbessern;
(2) landwirtschaftliche Pflanzensignaltechnologie, die die Steigerung der Ernteerträge organisch antreibt und die Reduzierung des Düngemitteleinsatzes unterstützt; und
(3) die Anwendung von löslichen Arzneimitteln zur positiven Beeinflussungder Tiergesundheit.
https://www.mountainvalleymd.com/updates/mvmd-commercializat…
MVMD-Kommerzialisierungsfokus
Es gibt drei Hauptgeschäftsbereiche, auf die sich MVMD konzentriert, um kommerziell voranzukommen:
(1) Neue Innovationen, die die Verabreichung und Wirksamkeit vonnutrazeutischen Gesundheits- und Wellnessprodukten verbessern;
(2) landwirtschaftliche Pflanzensignaltechnologie, die die Steigerung der Ernteerträge organisch antreibt und die Reduzierung des Düngemitteleinsatzes unterstützt; und
(3) die Anwendung von löslichen Arzneimitteln zur positiven Beeinflussungder Tiergesundheit.
https://www.mountainvalleymd.com/updates/mvmd-commercializat…
... von der Krebsforschung zum Düngemittelvertrieb, was für ein Gurkenverein 😀
Mountain Valley MD Beginnt den kommerziellen Verkauf von Agrarius, einer Agro-Anlagentechnologie
13. September 2022 09:00 ET | Quelle: Mountain Valley MD Holdings Inc.
https://www.globenewswire.com/news-release/2022/09/13/251507…
Mountain Valley MD Beginnt den kommerziellen Verkauf von Agrarius, einer Agro-Anlagentechnologie
13. September 2022 09:00 ET | Quelle: Mountain Valley MD Holdings Inc.
https://www.globenewswire.com/news-release/2022/09/13/251507…
MVMD-Interview mit Gokul Kannan an der Stanford University
Letzte Woche war ich mit dem Chief Medical Officer von MVMD, Dr. Azhar Rana, und wir hatten die Gelegenheit, uns mit unserem Onkologieberater Gokul Kannan an der Stanford University zu treffen.
Wir besichtigten den Stanford-Campus und setzten uns dann mit Gokul in eine ungeschriebene Umgebung, um die Entwicklungen der onkologischen Forschung zu diskutieren. Stanford hat den Ruf, eine der weltweit führenden Forschungs- und Lehreinrichtungen zu sein, und war eine erstaunliche Kulisse für unsere Diskussion.
Azhar stellte einige großartige Fragen zur Krebsforschung, zur Präzisionsmedizin und "den nächsten großen Dingen, die in der Onkologie auftauchen". Als einen kurzen Blick können Sie sich hier einen einminütigen Clip dieser "auftauchenden" Frage ansehen, der von meinem iPhone aufgenommen wurde.
Es ist die Frage des "aufkommenden Weltraums", die unser Team bei MVMD begeistert - dies bezieht sich direkt auf unsere Mission "Mehr Leben" und die Vorstellung einer Zukunft, in der wir die verheerenden Auswirkungen von Krebs reduzieren können.
Wir sind stolz darauf, mit Gokul zusammenzuarbeiten - er ist wirklich ein aufstrebender Star in der Biotechnik- und Chemieingenieurforschung. Seine Forschung konzentrierte sich auf Arzneimittelabgabe, Biomaterialien, Tissue Engineering & regenerative Medizin sowie Physikalische & medizinische Chemie/Chemietechnik. In letzter Zeit konzentrierte sich seine Arbeit auf das Studium der Verwendung von Biomaterialien und Computerbiologie, um Mikroglia, Glioblastom- und Krebsbiologie sowie Gewebetechnik zu studieren, mit zahlreichen Publikationen in der Presse oder in der Überprüfung und mehreren Titelartikeln in prominenten Zeitschriften.
Ich freue mich darauf, in den kommenden Wochen mehr über unsere Aktivitäten im Bereich der Onkologie zu erfahren.
Mit freundlichen Grüßen,
Mit freundlichen Grüßen,
Dennis Hancock
Präsident und CEO, Mountain Valley MD
https://www.mountainvalleymd.com/updates/mvmd-interview-with…
Letzte Woche war ich mit dem Chief Medical Officer von MVMD, Dr. Azhar Rana, und wir hatten die Gelegenheit, uns mit unserem Onkologieberater Gokul Kannan an der Stanford University zu treffen.
Wir besichtigten den Stanford-Campus und setzten uns dann mit Gokul in eine ungeschriebene Umgebung, um die Entwicklungen der onkologischen Forschung zu diskutieren. Stanford hat den Ruf, eine der weltweit führenden Forschungs- und Lehreinrichtungen zu sein, und war eine erstaunliche Kulisse für unsere Diskussion.
Azhar stellte einige großartige Fragen zur Krebsforschung, zur Präzisionsmedizin und "den nächsten großen Dingen, die in der Onkologie auftauchen". Als einen kurzen Blick können Sie sich hier einen einminütigen Clip dieser "auftauchenden" Frage ansehen, der von meinem iPhone aufgenommen wurde.
Es ist die Frage des "aufkommenden Weltraums", die unser Team bei MVMD begeistert - dies bezieht sich direkt auf unsere Mission "Mehr Leben" und die Vorstellung einer Zukunft, in der wir die verheerenden Auswirkungen von Krebs reduzieren können.
Wir sind stolz darauf, mit Gokul zusammenzuarbeiten - er ist wirklich ein aufstrebender Star in der Biotechnik- und Chemieingenieurforschung. Seine Forschung konzentrierte sich auf Arzneimittelabgabe, Biomaterialien, Tissue Engineering & regenerative Medizin sowie Physikalische & medizinische Chemie/Chemietechnik. In letzter Zeit konzentrierte sich seine Arbeit auf das Studium der Verwendung von Biomaterialien und Computerbiologie, um Mikroglia, Glioblastom- und Krebsbiologie sowie Gewebetechnik zu studieren, mit zahlreichen Publikationen in der Presse oder in der Überprüfung und mehreren Titelartikeln in prominenten Zeitschriften.
Ich freue mich darauf, in den kommenden Wochen mehr über unsere Aktivitäten im Bereich der Onkologie zu erfahren.
Mit freundlichen Grüßen,
Mit freundlichen Grüßen,
Dennis Hancock
Präsident und CEO, Mountain Valley MD
https://www.mountainvalleymd.com/updates/mvmd-interview-with…
Antwort auf Beitrag Nr.: 71.491.325 von Pixel_Muc am 04.05.22 14:11:01Spekulativ sehr interessant, Bewertung aber sehr hoch...
Antwort auf Beitrag Nr.: 71.487.170 von ooy am 04.05.22 06:37:33Ich hab das Gefühl das ich immer dasselbe lese, aber letztendlich nichts bei rum kommt.
Wie ist deine Sicht der Dinge was MVMD betrifft?
Wie ist deine Sicht der Dinge was MVMD betrifft?
TORONTO, April 28, 2022 (GLOBE NEWSWIRE) -- Mountain Valley MD Holdings Inc. (the “Company” or “MVMD”) (CSE: MVMD) (OTCQX: MVMDF) (FRA: 20MP) is pleased to provide a business update on its technologies and key initiatives.
KEY TECHNOLOGIES:
MVMD’s primary technologies are being developed with the objective to improve the administration, efficacy and safety of new and existing medicines, therapies, and nutraceuticals.
The Company’s Quicksome™ desiccation technology utilizes advanced liposomal technology and other stabilizing molecules to encapsulate and formulate active ingredients into more efficient product formats that are delivered sublingually or that could be applied to novel applications to address cold chain distribution challenges. Where successfully applied, Quicksome™ supports a new generation of product formulations that could enable new supply chain methods and enhance efficacy and safety of vaccines, pharmaceuticals, and nutraceuticals.
The Company’s patented Quicksol™ technology covers the macrocyclic lactone class of anti-parasitic drugs, where proprietary solubilization techniques that use no harmful organic solvents have been initially applied to the drugs ivermectin and selamectin, which, if successful in pre-clinical trials, could be effectively applied in oncology and multiple anti-viral and anti-parasitic applications that could positively impact human and animal health globally.
The Company’s patent-pending Porous Aluminum Nanostructure Adjuvant (“PANA”) technology has high surface area for vaccine-antigen binding that the Company believes may provide vaccine dose sparing advantages with long-term stability in aqueous media, and greater stability in harsh environments.
SIGNIFICANT PROJECTS:
MVMD’s primary technologies are being researched across various initiatives in the health and wellness industry, including the following:
COLD CHAIN
Cold chain is a temperature-controlled supply chain that prescribes necessary conditions during the transport, storage, and handling of vaccines and drugs to minimize excessive heat or cold exposure that can render product ineffective. The World Health Organization’s (WHO) guideline for temperature requirements for three defined vaccine management categories* includes traditional cold chain between +2°C and +8°C, Extended Controlled Temperature Conditions (ECTC) above +8°C for a specified number of days to support vaccine distribution, and Controlled Temperature Chain (CTC) where the vaccine must be able to tolerate ambient temperatures of at least +40°C for a minimum of 3 days.
On July 31, 2020, the Company announced its results from a U.S. Food and Drug Administration (FDA) Polio Vaccine Lab evaluation that confirmed the Company had successfully preserved Polio D Antigen in its proprietary Quicksome™ rapid dissolve sublingual technology. In addition to its sublingual cold chain work, the Company studied in the first half of 2021, whether it could apply a thin Quicksome™ desiccated liposome layer of Trivalent Inactivated Poliovirus Vaccine (tIPV) inside a vial for five days of exposure at 40 degrees Celsius and then reconstituted for injection at the point of administration.
Based on the cold chain technology achievements announced in July of 2021, the Company has advanced additional characterization studies with its proprietary Quicksome™ technology to optimize application across different human and animal vaccines. The Company believes the technology would allow for long term stability and ease of global distribution, appropriate for pandemic preparedness, as well as other administration and distribution advantages, including elimination of cold chain storage requirements and the related expenses, while reducing instances of vaccine spoilage.
MVMD is finalizing agreements with third-party organizations who specialize in vaccine research and is initiating studies of the Company’s technologies across an expanded vaccine target list, including those that have broad commercialization potential.
Additionally, MVMD is in discussions with a potential partner in South America to apply the Company’s cold chain technology to targeted husbandry animal vaccines.
INSULIN
Diabetes is a disease in which the human or animal body either can't produce insulin or can't properly use the insulin it produces. Insulin is a hormone produced by the pancreas and its role is to regulate the amount of glucose circulating in the blood.
In MVMD’s view, despite significant innovation in recent years in relation to diabetes, insulin remains a cornerstone of treatment, and there continues to be an unmet need in the insulin space, resulting from challenges such as costs, the use of needles, hypoglycemia, and fear of injection.
The Company is advancing its exploration of the application of its technology to the needleless administration of insulin and is currently planning the execution of formulation experiments with the goal of optimizing the potential delivery of rapid-acting human insulin in a sublingual format. Additionally, the Company has initiated the selection process for a third party CRO and is engaging experts in insulin science to support planning of necessary trials to validate the potential delivery of rapid-acting human insulin in a sublingual format.
The Company expects to have further information on formulation developments and to complete testing of formulations in a Type 1 diabetes model in the second half of 2022.
TUBERCULOSIS
Tuberculosis (TB) is a disease that is caused by bacteria that spread from person to person through microscopic droplets released into the air. TB affects roughly 25% of the world’s population and is the leading infectious disease killer in the world, claiming approximately 1.5 million lives each year.**
In March of 2021, the Company announced that it had confirmed its ability to create a solubilized selamectin product using its patented Quicksol™ technology applied to the macrocyclic lactone drug class. Selamectin drug is characterized as a highly insoluble molecule and MVMD believes that there is significant potential in treating mycobacterium-based infections in humans and animals with its solubilized format of the drug, Selactosol™ 1.5%.
MVMD has completed its initial assessment and established the plan to optimize the formulation, the necessary testing assays, and related preclinical protocols required to advance studies for its novel Selactosol™ 1.5% targeting mycobacterium-based infections. MVMD intends to commence pre-clinical studies with its Selactosol™ 1.5% solution in the latter half of 2022.
ONCOLOGY
As announced on May 3, 2021, pre-clinical trials for Triple-negative Breast Cancer, Metastatic Melanoma and Lewis Lung Carcinoma were conducted to support MVMD’s exploration of application of its technology to potential cancer treatments. Preliminary murine cell model studies were completed, and the research conducted presented some noteworthy exploratory findings that have resulted in MVMD expanding its oncology work to explore human cell line tumors with further investigational cell viability and proliferation research studies being executed over the past two quarters.
MVMD is also reviewing additional pre-clinical models to explore combinations of its patented Soluvec™ with existing chemotherapeutic and immunomodulatory treatments across solid tumor and hematological malignancies.
On April 5, 2022, the Company was granted a Patent for ‘Novel Injectable, Infusable, Instillable Ivermectin Adjuvant for Cancer Therapies’ for its solubilized ivermectin (Soluvec™). The issuance of this patent verifies the novel approach of MVMD’s technology as applied to potential cancer types that the Company is exploring and assists in safeguarding the invention in support of potential commercial value in the future as the technology progresses through trials.
HUSBANDRY ANIMALS
Previously, the Company had disclosed the application of its Quicksol™ technology to the drug ivermectin and belief that a more solubilized format versus current in-market products would have novel applications across the broad husbandry animal marketplace.
The Company completed initial husbandry animal trials in Bangladesh, previously announced on March 16, 2021, that were conducted with MVMD’s injectable solubilized ivermectin technology, Soluvec™️ 1%. The studies were conducted under the supervision of The People's Republic of Bangladesh’s Ministry of Fisheries & Livestock and informed the requirements for final commercialization pathway inside of Bangladesh.
Over the past six months, MVMD has been coordinating dosing and formulation testing and conducting extended stability tests on GMP manufactured Soluvec™️ 1% in order to optimize the formulation for different conditions and environments. The results of some of the stability testing have now been received and have indicated that the base formula requires optimization and further stability testing in order to allow for the scaling of batch sizes for production and ensuring Soluvec™️ 1% product applications have a predictable shelf life. This work is expected to proceed through to the first quarter of 2023.
Concurrently, MVMD is working with its partner in Bangladesh to negotiate a commercial licensing agreement that would include management by the licensee of a local manufacturing partner and process for scaled Soluvec™️ 1% commercial production requirements and completion of market specific stability testing for the husbandry animal applications inside Bangladesh, in support of pursuing government approval inside Bangladesh necessary to enable Soluvec™️ 1% product to be commercialized.
It is currently anticipated that Soluvec™️ 1% will be ready for retail product introduction by the licensee in the first quarter of 2023, however this may be a longer or shorter period depending on various factors such as the results of the additional stability testing and the related timing of regulatory review and approval.
AQUACULTURE
The Company’s partner in Bangladesh is working with the local Ministry of Fisheries, which engaged MVMD to evaluate whether a combined application of both its Quicksol™ and Quicksome™ technologies to a novel fish food application would be able to reduce the effect of parasitic infections across a variety of farmed fish species. The Company is working with the Ministry to finalize key protocols in these different species and is completing the trial planning protocol and aims to commence the trials in the third quarter of 2022.
Additionally, MVMD is coordinating the research framework with an acclaimed international university to conduct a collaborative study of Soluvec™1% coated fish feed to study its health benefits in targeted aquatic species. It is anticipated the related studies will commence in the in the fourth quarter of 2022.
NUTRACEUTICALS
Following evaluation of North American GMP manufacturing options for MVMD’s nutraceutical product strategy, the Company is in the final stages of securing its third-party lead production partner.
The Company’s strategy will be to secure the selected lead manufacturing partner as a licensee, who will in turn produce nutraceutical products based on or embodying MVMD’s proprietary technologies for third parties approved by and who have an agreement with MVMD. The Company believes this strategy will help ensure product quality, support the ability to scale production, streamline audit process for royalty agreements, and provide the necessary protection of its technology and trade secrets versus having numerous licensed partners each replicating the manufacturing process for their own products.
MVMD’s current licensing arrangements with Circadian Wellness and Red White & Bloom Brands, and future such agreements, are anticipated to be supported by this strategy.
Securing the lead manufacturer and finalizing the scaled GMP production environment is intended to align with MVMD’s anticipated increased business development efforts in the latter half of 2022 to secure additional nutraceutical licensing partnerships.
DOSE SPARING ADJUVANT
The Company’s patent-pending PANA technology has high surface area for vaccine-antigen binding that the Company believes may provide vaccine dose sparing advantages with long-term stability in aqueous media, and greater stability in harsh environments.
In partnership with the Tulane University School of Medicine in New Orleans, Louisiana, as previously disclosed, the Company executed a study comparing an existing Alhydrogel adjuvant to the Company’s invented stable nano-particulate adjuvant by both intramuscular injection and intradermal injection immunization. The study evaluated the antibody responses following vaccination with fractional doses of IPV and compared delivery types with IPV alone or adjuvanted. The evaluation of MVMD’s novel aluminum nanoparticle adjuvant from this study demonstrated no toxicity or adverse reactions when combined with tIPV in intramuscular or intradermal injection. However, the initial results were not satisfactory in terms of producing a robust response or desired elevation in the immune response over IPV alone.
The Company continues its work with its key advisors at Tulane University to explore if changes in the adjuvant development and administration may support a positive research outcome across a broad spectrum of vaccines. MVMD anticipates exploratory progress over the third quarter of 2022, related to results from ongoing characterization work that will inform the plan forward and related timing for the technology evaluation.
R&D COVID-19
The Company had explored the application of a solubilized form of ivermectin in the potential treatment of COVID-19. In May 2021 the Company announced the results from its third-party Bio Safety Level 4 (“BSL-4”) COVID-19 viral clearance study conducted with its solubilized ivermectin technology, Soluvec™. In management’s view based on an internal review of publicly available literature, the positive indicators from the BSL-4 trials are consistent in general with various global trials and contribute to evidence for the effect of ivermectin on inhibition of viral replication and the potential application as a treatment for COVID-19.
MVMD also acknowledges the positions of certain regulatory bodies, for example Health Canada and the Food and Drug Administration in the United States, that ivermectin has not be authorized or approved for use in the treatment of COVID-19. The Company’s primary focus, in line with its “cold chain” work, has been on those jurisdictions which may have less access to vaccines and other treatments, where ivermectin has been studied for use in the treatment of COVID-19, and which might benefit from access to a solubilized form of the drug.
In addition to potential sublingual administration applications, MVMD believes that its solubilized form of ivermectin could be favourable for front line emergency use applications, including injection and intravenous uses, potentially for COVID-19 treatments where authorized or in research for future possible pandemics.
Due to the complexity and cost of human ivermectin trials, as well as the global and regulatory landscape for approval of treatments for COVID-19, the Company is currently working to evaluate the business case for further studies in humans, including stability of human grade GMP product, with academic and non-academic partnerships.
The Company intends to provide further updates on its trials and developments as they become available.
KEY TECHNOLOGIES:
MVMD’s primary technologies are being developed with the objective to improve the administration, efficacy and safety of new and existing medicines, therapies, and nutraceuticals.
The Company’s Quicksome™ desiccation technology utilizes advanced liposomal technology and other stabilizing molecules to encapsulate and formulate active ingredients into more efficient product formats that are delivered sublingually or that could be applied to novel applications to address cold chain distribution challenges. Where successfully applied, Quicksome™ supports a new generation of product formulations that could enable new supply chain methods and enhance efficacy and safety of vaccines, pharmaceuticals, and nutraceuticals.
The Company’s patented Quicksol™ technology covers the macrocyclic lactone class of anti-parasitic drugs, where proprietary solubilization techniques that use no harmful organic solvents have been initially applied to the drugs ivermectin and selamectin, which, if successful in pre-clinical trials, could be effectively applied in oncology and multiple anti-viral and anti-parasitic applications that could positively impact human and animal health globally.
The Company’s patent-pending Porous Aluminum Nanostructure Adjuvant (“PANA”) technology has high surface area for vaccine-antigen binding that the Company believes may provide vaccine dose sparing advantages with long-term stability in aqueous media, and greater stability in harsh environments.
SIGNIFICANT PROJECTS:
MVMD’s primary technologies are being researched across various initiatives in the health and wellness industry, including the following:
COLD CHAIN
Cold chain is a temperature-controlled supply chain that prescribes necessary conditions during the transport, storage, and handling of vaccines and drugs to minimize excessive heat or cold exposure that can render product ineffective. The World Health Organization’s (WHO) guideline for temperature requirements for three defined vaccine management categories* includes traditional cold chain between +2°C and +8°C, Extended Controlled Temperature Conditions (ECTC) above +8°C for a specified number of days to support vaccine distribution, and Controlled Temperature Chain (CTC) where the vaccine must be able to tolerate ambient temperatures of at least +40°C for a minimum of 3 days.
On July 31, 2020, the Company announced its results from a U.S. Food and Drug Administration (FDA) Polio Vaccine Lab evaluation that confirmed the Company had successfully preserved Polio D Antigen in its proprietary Quicksome™ rapid dissolve sublingual technology. In addition to its sublingual cold chain work, the Company studied in the first half of 2021, whether it could apply a thin Quicksome™ desiccated liposome layer of Trivalent Inactivated Poliovirus Vaccine (tIPV) inside a vial for five days of exposure at 40 degrees Celsius and then reconstituted for injection at the point of administration.
Based on the cold chain technology achievements announced in July of 2021, the Company has advanced additional characterization studies with its proprietary Quicksome™ technology to optimize application across different human and animal vaccines. The Company believes the technology would allow for long term stability and ease of global distribution, appropriate for pandemic preparedness, as well as other administration and distribution advantages, including elimination of cold chain storage requirements and the related expenses, while reducing instances of vaccine spoilage.
MVMD is finalizing agreements with third-party organizations who specialize in vaccine research and is initiating studies of the Company’s technologies across an expanded vaccine target list, including those that have broad commercialization potential.
Additionally, MVMD is in discussions with a potential partner in South America to apply the Company’s cold chain technology to targeted husbandry animal vaccines.
INSULIN
Diabetes is a disease in which the human or animal body either can't produce insulin or can't properly use the insulin it produces. Insulin is a hormone produced by the pancreas and its role is to regulate the amount of glucose circulating in the blood.
In MVMD’s view, despite significant innovation in recent years in relation to diabetes, insulin remains a cornerstone of treatment, and there continues to be an unmet need in the insulin space, resulting from challenges such as costs, the use of needles, hypoglycemia, and fear of injection.
The Company is advancing its exploration of the application of its technology to the needleless administration of insulin and is currently planning the execution of formulation experiments with the goal of optimizing the potential delivery of rapid-acting human insulin in a sublingual format. Additionally, the Company has initiated the selection process for a third party CRO and is engaging experts in insulin science to support planning of necessary trials to validate the potential delivery of rapid-acting human insulin in a sublingual format.
The Company expects to have further information on formulation developments and to complete testing of formulations in a Type 1 diabetes model in the second half of 2022.
TUBERCULOSIS
Tuberculosis (TB) is a disease that is caused by bacteria that spread from person to person through microscopic droplets released into the air. TB affects roughly 25% of the world’s population and is the leading infectious disease killer in the world, claiming approximately 1.5 million lives each year.**
In March of 2021, the Company announced that it had confirmed its ability to create a solubilized selamectin product using its patented Quicksol™ technology applied to the macrocyclic lactone drug class. Selamectin drug is characterized as a highly insoluble molecule and MVMD believes that there is significant potential in treating mycobacterium-based infections in humans and animals with its solubilized format of the drug, Selactosol™ 1.5%.
MVMD has completed its initial assessment and established the plan to optimize the formulation, the necessary testing assays, and related preclinical protocols required to advance studies for its novel Selactosol™ 1.5% targeting mycobacterium-based infections. MVMD intends to commence pre-clinical studies with its Selactosol™ 1.5% solution in the latter half of 2022.
ONCOLOGY
As announced on May 3, 2021, pre-clinical trials for Triple-negative Breast Cancer, Metastatic Melanoma and Lewis Lung Carcinoma were conducted to support MVMD’s exploration of application of its technology to potential cancer treatments. Preliminary murine cell model studies were completed, and the research conducted presented some noteworthy exploratory findings that have resulted in MVMD expanding its oncology work to explore human cell line tumors with further investigational cell viability and proliferation research studies being executed over the past two quarters.
MVMD is also reviewing additional pre-clinical models to explore combinations of its patented Soluvec™ with existing chemotherapeutic and immunomodulatory treatments across solid tumor and hematological malignancies.
On April 5, 2022, the Company was granted a Patent for ‘Novel Injectable, Infusable, Instillable Ivermectin Adjuvant for Cancer Therapies’ for its solubilized ivermectin (Soluvec™). The issuance of this patent verifies the novel approach of MVMD’s technology as applied to potential cancer types that the Company is exploring and assists in safeguarding the invention in support of potential commercial value in the future as the technology progresses through trials.
HUSBANDRY ANIMALS
Previously, the Company had disclosed the application of its Quicksol™ technology to the drug ivermectin and belief that a more solubilized format versus current in-market products would have novel applications across the broad husbandry animal marketplace.
The Company completed initial husbandry animal trials in Bangladesh, previously announced on March 16, 2021, that were conducted with MVMD’s injectable solubilized ivermectin technology, Soluvec™️ 1%. The studies were conducted under the supervision of The People's Republic of Bangladesh’s Ministry of Fisheries & Livestock and informed the requirements for final commercialization pathway inside of Bangladesh.
Over the past six months, MVMD has been coordinating dosing and formulation testing and conducting extended stability tests on GMP manufactured Soluvec™️ 1% in order to optimize the formulation for different conditions and environments. The results of some of the stability testing have now been received and have indicated that the base formula requires optimization and further stability testing in order to allow for the scaling of batch sizes for production and ensuring Soluvec™️ 1% product applications have a predictable shelf life. This work is expected to proceed through to the first quarter of 2023.
Concurrently, MVMD is working with its partner in Bangladesh to negotiate a commercial licensing agreement that would include management by the licensee of a local manufacturing partner and process for scaled Soluvec™️ 1% commercial production requirements and completion of market specific stability testing for the husbandry animal applications inside Bangladesh, in support of pursuing government approval inside Bangladesh necessary to enable Soluvec™️ 1% product to be commercialized.
It is currently anticipated that Soluvec™️ 1% will be ready for retail product introduction by the licensee in the first quarter of 2023, however this may be a longer or shorter period depending on various factors such as the results of the additional stability testing and the related timing of regulatory review and approval.
AQUACULTURE
The Company’s partner in Bangladesh is working with the local Ministry of Fisheries, which engaged MVMD to evaluate whether a combined application of both its Quicksol™ and Quicksome™ technologies to a novel fish food application would be able to reduce the effect of parasitic infections across a variety of farmed fish species. The Company is working with the Ministry to finalize key protocols in these different species and is completing the trial planning protocol and aims to commence the trials in the third quarter of 2022.
Additionally, MVMD is coordinating the research framework with an acclaimed international university to conduct a collaborative study of Soluvec™1% coated fish feed to study its health benefits in targeted aquatic species. It is anticipated the related studies will commence in the in the fourth quarter of 2022.
NUTRACEUTICALS
Following evaluation of North American GMP manufacturing options for MVMD’s nutraceutical product strategy, the Company is in the final stages of securing its third-party lead production partner.
The Company’s strategy will be to secure the selected lead manufacturing partner as a licensee, who will in turn produce nutraceutical products based on or embodying MVMD’s proprietary technologies for third parties approved by and who have an agreement with MVMD. The Company believes this strategy will help ensure product quality, support the ability to scale production, streamline audit process for royalty agreements, and provide the necessary protection of its technology and trade secrets versus having numerous licensed partners each replicating the manufacturing process for their own products.
MVMD’s current licensing arrangements with Circadian Wellness and Red White & Bloom Brands, and future such agreements, are anticipated to be supported by this strategy.
Securing the lead manufacturer and finalizing the scaled GMP production environment is intended to align with MVMD’s anticipated increased business development efforts in the latter half of 2022 to secure additional nutraceutical licensing partnerships.
DOSE SPARING ADJUVANT
The Company’s patent-pending PANA technology has high surface area for vaccine-antigen binding that the Company believes may provide vaccine dose sparing advantages with long-term stability in aqueous media, and greater stability in harsh environments.
In partnership with the Tulane University School of Medicine in New Orleans, Louisiana, as previously disclosed, the Company executed a study comparing an existing Alhydrogel adjuvant to the Company’s invented stable nano-particulate adjuvant by both intramuscular injection and intradermal injection immunization. The study evaluated the antibody responses following vaccination with fractional doses of IPV and compared delivery types with IPV alone or adjuvanted. The evaluation of MVMD’s novel aluminum nanoparticle adjuvant from this study demonstrated no toxicity or adverse reactions when combined with tIPV in intramuscular or intradermal injection. However, the initial results were not satisfactory in terms of producing a robust response or desired elevation in the immune response over IPV alone.
The Company continues its work with its key advisors at Tulane University to explore if changes in the adjuvant development and administration may support a positive research outcome across a broad spectrum of vaccines. MVMD anticipates exploratory progress over the third quarter of 2022, related to results from ongoing characterization work that will inform the plan forward and related timing for the technology evaluation.
R&D COVID-19
The Company had explored the application of a solubilized form of ivermectin in the potential treatment of COVID-19. In May 2021 the Company announced the results from its third-party Bio Safety Level 4 (“BSL-4”) COVID-19 viral clearance study conducted with its solubilized ivermectin technology, Soluvec™. In management’s view based on an internal review of publicly available literature, the positive indicators from the BSL-4 trials are consistent in general with various global trials and contribute to evidence for the effect of ivermectin on inhibition of viral replication and the potential application as a treatment for COVID-19.
MVMD also acknowledges the positions of certain regulatory bodies, for example Health Canada and the Food and Drug Administration in the United States, that ivermectin has not be authorized or approved for use in the treatment of COVID-19. The Company’s primary focus, in line with its “cold chain” work, has been on those jurisdictions which may have less access to vaccines and other treatments, where ivermectin has been studied for use in the treatment of COVID-19, and which might benefit from access to a solubilized form of the drug.
In addition to potential sublingual administration applications, MVMD believes that its solubilized form of ivermectin could be favourable for front line emergency use applications, including injection and intravenous uses, potentially for COVID-19 treatments where authorized or in research for future possible pandemics.
Due to the complexity and cost of human ivermectin trials, as well as the global and regulatory landscape for approval of treatments for COVID-19, the Company is currently working to evaluate the business case for further studies in humans, including stability of human grade GMP product, with academic and non-academic partnerships.
The Company intends to provide further updates on its trials and developments as they become available.





















