SGBX (Mkab 22M) Cash 19M / 100M Corona-Tests - 500 Beiträge pro Seite
eröffnet am 20.05.20 09:40:52 von
neuester Beitrag 13.03.21 09:03:39 von
neuester Beitrag 13.03.21 09:03:39 von
Beiträge: 22
ID: 1.325.059
ID: 1.325.059
Aufrufe heute: 0
Gesamt: 3.392
Gesamt: 3.392
Aktive User: 0
ISIN: US78418A5056 · WKN: A2PZPM · Symbol: SGBX
0,1505
USD
-2,97 %
-0,0046 USD
Letzter Kurs 02:00:00 NYSE
Neuigkeiten
19.04.24 · globenewswire |
15.04.24 · globenewswire |
11.04.24 · globenewswire |
04.04.24 · globenewswire |
21.03.24 · globenewswire |
Werte aus der Branche Baugewerbe
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 2,6400 | +129,57 | |
| 0,9799 | +58,05 | |
| 5,9000 | +27,16 | |
| 31.800,00 | +20,91 | |
| 158,90 | +19,97 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 6,4200 | -19,14 | |
| 0,8600 | -21,10 | |
| 2,1500 | -25,86 | |
| 1,6200 | -39,78 | |
| 10,510 | -48,56 |
Hallo an Alle
Ich habe gestern wieder etwas interessantes ausgegraben! Wichtig, das ist ein reiner Corona-Zock!!!
Bei SG-Blocks gab es eine Firmenumstrukturierung und die sind neu hauptsächlich in der Baubranche tätig. Trotzdem haben sie es hingekriegt, einen leider nicht exklusiven Vertriebsvertrag in der USA mit OSANG Healthcare abzuschliessen! OSANG Healthcare hat ein von der FDA zugelassener Corona-Schnelltest.
Nach der Ankündigung des Vertrags schoss das Teil durch die Decke! Der Grund für meinen Einstieg ist dieser Kommentar hier auf Twitter! 100 Millionen Test-Kits für die USA! Durch die KE zu 2.50$ haben sie gerade 17M eingenommen. Ich nehme an, sie werden nun die erste grosse Bestellung tätigen.
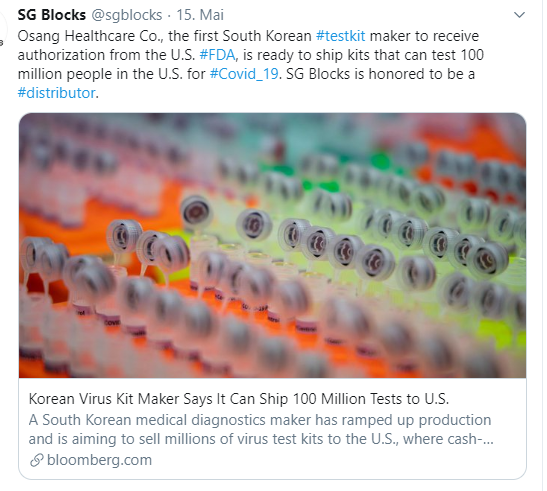
Link zum Vertriebsvertrag: https://ir.sgblocks.com/press-releases/detail/134/sg-blocks-…
Ich habe gestern wieder etwas interessantes ausgegraben! Wichtig, das ist ein reiner Corona-Zock!!!
Bei SG-Blocks gab es eine Firmenumstrukturierung und die sind neu hauptsächlich in der Baubranche tätig. Trotzdem haben sie es hingekriegt, einen leider nicht exklusiven Vertriebsvertrag in der USA mit OSANG Healthcare abzuschliessen! OSANG Healthcare hat ein von der FDA zugelassener Corona-Schnelltest.
Nach der Ankündigung des Vertrags schoss das Teil durch die Decke! Der Grund für meinen Einstieg ist dieser Kommentar hier auf Twitter! 100 Millionen Test-Kits für die USA! Durch die KE zu 2.50$ haben sie gerade 17M eingenommen. Ich nehme an, sie werden nun die erste grosse Bestellung tätigen.

Link zum Vertriebsvertrag: https://ir.sgblocks.com/press-releases/detail/134/sg-blocks-…

Bin auch mal mit einer Position rein, hast ja zur Zeit einen Lauf mit Deinen Empfehlungen...
Gute Firma an sich, nicht nur wegen Corona, kommen ja aus ganz anderen Regionen in Ihrer Bewertung. Danke für die Empfehlung, mal schauen was daraus wird...
Gute Firma an sich, nicht nur wegen Corona, kommen ja aus ganz anderen Regionen in Ihrer Bewertung. Danke für die Empfehlung, mal schauen was daraus wird...
https://ir.sgblocks.com/press-releases/detail/141/sg-blocks-…
Hier geht es nicht mehr lange und das Teil hebt ab💰
Hier geht es nicht mehr lange und das Teil hebt ab💰
Sofort im Kurs gesehen...Gut recherchiert.
Es geht vorwärts, die Tests können nun offiziell bestellt werden:
https://www.sgblocks.com/covid-19-testing-details
Sie haben auch eine Art Testcontainer im Angebot, wird im Juni fertig zusammengebaut sein:
https://ir.sgblocks.com/press-releases/detail/142/sg-blocks-…
Und was ich noch interessant finde in der Vereinbarung:
https://ir.sgblocks.com/press-releases/detail/134/sg-blocks-…
In connection with the entry into the distributorship agreement, subject to conditions the Company granted an affiliate of OHC the right to elect to participate, at its sole and exclusive option, in up to 19.9% of any stock offering by the Company during the next six months.
Für 6 Monate ist es OSANG möglich, an KE zu 19,9% teilzunehmen.
Und noch aus dem Opko Forum von Goldbarren999
From recent news reporting: 70% of Americans are demanding more tests. Congress has issued $25 billion explicitly for testing, this money is still filtering to states. Harvard researchers call for 20-100 million tests per day and Rockefeller Foundation projection calls for $100-500 billion investment in minimum 10 million PCR tests per week and possibly as many as 200-300 million tests per week to open the US economy. Morgan Stanley analyst anticipates that Amazon alone will spend $1 billion on employee testing. The Wall Street Journal reports more companies (including Walmart) plan to regularly test asymptomatic workers. PCR testing is the only logical fit for this type of application. The Trump administration vows 100 million swabs by the end of 2020 which implies demand for 100 million PCR tests. Analysts’ forecasts for Covid-19 testing market range from $52 billion 2021 to $10 billion in 2027.
Vielleicht fällt hier auch was für SGBX ab.
Habt ihr eine Idee, wie viel bei SGBX pro Test hängen bleiben könnte? Vielleicht 1$? Ich versuche ein bisschen rauszufinden, um wie viel der Kurs wohl steigen könnte.
https://www.sgblocks.com/covid-19-testing-details
Sie haben auch eine Art Testcontainer im Angebot, wird im Juni fertig zusammengebaut sein:
https://ir.sgblocks.com/press-releases/detail/142/sg-blocks-…
Und was ich noch interessant finde in der Vereinbarung:
https://ir.sgblocks.com/press-releases/detail/134/sg-blocks-…
In connection with the entry into the distributorship agreement, subject to conditions the Company granted an affiliate of OHC the right to elect to participate, at its sole and exclusive option, in up to 19.9% of any stock offering by the Company during the next six months.
Für 6 Monate ist es OSANG möglich, an KE zu 19,9% teilzunehmen.
Und noch aus dem Opko Forum von Goldbarren999
From recent news reporting: 70% of Americans are demanding more tests. Congress has issued $25 billion explicitly for testing, this money is still filtering to states. Harvard researchers call for 20-100 million tests per day and Rockefeller Foundation projection calls for $100-500 billion investment in minimum 10 million PCR tests per week and possibly as many as 200-300 million tests per week to open the US economy. Morgan Stanley analyst anticipates that Amazon alone will spend $1 billion on employee testing. The Wall Street Journal reports more companies (including Walmart) plan to regularly test asymptomatic workers. PCR testing is the only logical fit for this type of application. The Trump administration vows 100 million swabs by the end of 2020 which implies demand for 100 million PCR tests. Analysts’ forecasts for Covid-19 testing market range from $52 billion 2021 to $10 billion in 2027.
Vielleicht fällt hier auch was für SGBX ab.
Habt ihr eine Idee, wie viel bei SGBX pro Test hängen bleiben könnte? Vielleicht 1$? Ich versuche ein bisschen rauszufinden, um wie viel der Kurs wohl steigen könnte.
Antwort auf Beitrag Nr.: 63.891.335 von blpp am 03.06.20 23:01:00Hier sollte eine Verdoppler möglich sein! Ist wie bei TTOO man braucht etwas Geduld aber ich bleibe hier weiterhin sehr optimistisch!
Hallo zusammen,
so... mein erster Post... habe sehr viel Positives aus diesem Forum mitgenommen, da wollte ich auch kurz etwas zurückgeben :-)
Gefunden auf
https://finance.yahoo.com/quote/ZYNE/community?p=ZYNE
Entscheidungen stehen an..
The results will be released within 96 hours and I believe shareholders should consider the following: This post is not meant to imply one way or the other as to what the phase 3 results of fragile x will be since only people close to the situation know and are legally prohibited from publicizing it. Before the results are announced it is anyone's guess and of course there is considerable amount of risk. Also for those holding and waiting for the phase 3 results today's stock price of $6 is meaningless. After the results the stock price goes to either $40 on good news or $4 on bad news.
What this post is meant to be is that in the event that POSITIVE results are announced Monday morning longs who held on for so long shouldn't be shaken out of their investment to some newcomer who will reap the rewards intended for you.
At a phase 3 positive outcome these shares are worth gold and longs don't let yourself be shaken out of a goldmine. I have seen this many times over and over where longs sit on a stock for years and then when they strike gold they get impatient and dump their shares for a $2 or $3 profit and sell it to the vultures who capitalize and make a mint out of it. A year later when they see the company bought out for $50 a share all they can do is to try to rationalize their stupid move.
DO NOT MAKE THAT MISTAKE! Do not sell your shares below $40 on a positive phase 3.
Just remember the following before you consider surrendering your shares in this GOLDMINE at a fire sale price.
1- The average price target of the analysts TODAY is in the mid $20 range. This price target of the analysts values "only" the market of the upcoming drug for fragile x. It discounts "entirely" the rest of the pipeline which is a multi billion market. The mid $20 price also shaves off a chunk of the value for fragile x since the analysts included in their target the possibility of a non positive outcome. So in fact the true analyst price target is in the mid $40's
2- As mentioned in my prior post fragile x includes 70,000 patients. The analysts are figuring 20% as definite candidates or 14,000. 14,000 x $30000 a year = $420m. Even if I discount it by 50% I get $200m with a $100m net or $4 EPS. A multiple of 10 gives you $40 for fragile x alone.
3- Considering that fragile x has no other remedy. The rest of the 80% will benefit regardless of labeland doctors will have to subscribe it, be it off label if need be, as there is no alternative. If so, you have a market of over $500m with a share value of $100.
4- What about the rest of the portfolio. The epilepsy application has a million patient market. With this company's convenient gel application that has no side effects and is safe if the company has positive phase 3 for the epilepsy indication this drug will become a universal treatment even if it is in patients where it works better in conjunction with other drugs. The epilepsy indication if approved is a multi billion market and is worth several $100 per share. BTW the fragilex study if positive is a major indication to the efficacy of the compound in the company's other indications.
5- The company's patent for the delivery system with the gel is worth at least $6 a share or $150m. Even GW would benefit from it since it reduces side effects.
6- So even if the phase 3 results should disappoint the share are still worth $4-$5 a share.
7- When contemplating to sell your shares at $10 or $15 just remember that there are 6.8 million shorts that on a positive outcome will be desperate to buy you shares and if the longs hold on they may have to pay $50. Why give them YOUR shares on a silver plate.
8- These shares traded above $15 awhile back without positive results and are worth at least double on phase 3 positive results.
9- The shorts may unleash millions of shares at the open to dilute the positive effect of the results to surpress the stock price momentarily so that they can cover.
10- There are lined up a group of money managers who said they will take a stake on phase 3 positive results.
11- On phase 3 positive results this company is a TAKEOVER target.
Drücken wir uns die Daumen, dort geht man übrigens von einem Kurs
so... mein erster Post... habe sehr viel Positives aus diesem Forum mitgenommen, da wollte ich auch kurz etwas zurückgeben :-)
Gefunden auf
https://finance.yahoo.com/quote/ZYNE/community?p=ZYNE
Entscheidungen stehen an..
The results will be released within 96 hours and I believe shareholders should consider the following: This post is not meant to imply one way or the other as to what the phase 3 results of fragile x will be since only people close to the situation know and are legally prohibited from publicizing it. Before the results are announced it is anyone's guess and of course there is considerable amount of risk. Also for those holding and waiting for the phase 3 results today's stock price of $6 is meaningless. After the results the stock price goes to either $40 on good news or $4 on bad news.
What this post is meant to be is that in the event that POSITIVE results are announced Monday morning longs who held on for so long shouldn't be shaken out of their investment to some newcomer who will reap the rewards intended for you.
At a phase 3 positive outcome these shares are worth gold and longs don't let yourself be shaken out of a goldmine. I have seen this many times over and over where longs sit on a stock for years and then when they strike gold they get impatient and dump their shares for a $2 or $3 profit and sell it to the vultures who capitalize and make a mint out of it. A year later when they see the company bought out for $50 a share all they can do is to try to rationalize their stupid move.
DO NOT MAKE THAT MISTAKE! Do not sell your shares below $40 on a positive phase 3.
Just remember the following before you consider surrendering your shares in this GOLDMINE at a fire sale price.
1- The average price target of the analysts TODAY is in the mid $20 range. This price target of the analysts values "only" the market of the upcoming drug for fragile x. It discounts "entirely" the rest of the pipeline which is a multi billion market. The mid $20 price also shaves off a chunk of the value for fragile x since the analysts included in their target the possibility of a non positive outcome. So in fact the true analyst price target is in the mid $40's
2- As mentioned in my prior post fragile x includes 70,000 patients. The analysts are figuring 20% as definite candidates or 14,000. 14,000 x $30000 a year = $420m. Even if I discount it by 50% I get $200m with a $100m net or $4 EPS. A multiple of 10 gives you $40 for fragile x alone.
3- Considering that fragile x has no other remedy. The rest of the 80% will benefit regardless of labeland doctors will have to subscribe it, be it off label if need be, as there is no alternative. If so, you have a market of over $500m with a share value of $100.
4- What about the rest of the portfolio. The epilepsy application has a million patient market. With this company's convenient gel application that has no side effects and is safe if the company has positive phase 3 for the epilepsy indication this drug will become a universal treatment even if it is in patients where it works better in conjunction with other drugs. The epilepsy indication if approved is a multi billion market and is worth several $100 per share. BTW the fragilex study if positive is a major indication to the efficacy of the compound in the company's other indications.
5- The company's patent for the delivery system with the gel is worth at least $6 a share or $150m. Even GW would benefit from it since it reduces side effects.
6- So even if the phase 3 results should disappoint the share are still worth $4-$5 a share.
7- When contemplating to sell your shares at $10 or $15 just remember that there are 6.8 million shorts that on a positive outcome will be desperate to buy you shares and if the longs hold on they may have to pay $50. Why give them YOUR shares on a silver plate.
8- These shares traded above $15 awhile back without positive results and are worth at least double on phase 3 positive results.
9- The shorts may unleash millions of shares at the open to dilute the positive effect of the results to surpress the stock price momentarily so that they can cover.
10- There are lined up a group of money managers who said they will take a stake on phase 3 positive results.
11- On phase 3 positive results this company is a TAKEOVER target.
Drücken wir uns die Daumen, dort geht man übrigens von einem Kurs
... von 2 bis 3 USD aus, wenn es negativ ausgeht....
Antwort auf Beitrag Nr.: 64.207.030 von Sven_Bonn am 27.06.20 18:31:02Danke für deine Beitrag, jedoch sind wir hier bei SG Blocks  kopiere in doch in den ZYNE Thread! Danke!
kopiere in doch in den ZYNE Thread! Danke!
 kopiere in doch in den ZYNE Thread! Danke!
kopiere in doch in den ZYNE Thread! Danke!
"The first prototypes of the D-Tec 1 and D-Tec 2 units are set to be completed in early July, with Grimshaw currently working on a plan to combine these mobile, shipping-container units with permanent facilities."
https://www.dezeen.com/2020/06/03/grimshaw-shipping-containe…
Hoffe hier kommen in den nächsten beiden Wochen News dazu, nachdem auf deren Homepage ja sogar schon für Juni der erste Prototyp angekündigt wurde.
Ansonsten ist am 30. Juli noch das Annual Meeting of Stockholders.
Vor ein paar Tagen ist noch ein Artikel raus gekommen, der auch noch ein paar interessante Informationen liefert. "We’re spending a lot of time in concept development for recreating shipping containers for tele-education and learning labs,” Galvin said. This could be in the form of decentralizing the school with smaller classrooms, testing facilities, and lab spaces. Galvin told us he’s currently in conversation with a number of government officials, leaders in education, and employers across the country about deploying these facilities."
https://www.6sqft.com/brooklyn-based-company-repurposes-ship…
https://www.dezeen.com/2020/06/03/grimshaw-shipping-containe…
Hoffe hier kommen in den nächsten beiden Wochen News dazu, nachdem auf deren Homepage ja sogar schon für Juni der erste Prototyp angekündigt wurde.
Ansonsten ist am 30. Juli noch das Annual Meeting of Stockholders.
Vor ein paar Tagen ist noch ein Artikel raus gekommen, der auch noch ein paar interessante Informationen liefert. "We’re spending a lot of time in concept development for recreating shipping containers for tele-education and learning labs,” Galvin said. This could be in the form of decentralizing the school with smaller classrooms, testing facilities, and lab spaces. Galvin told us he’s currently in conversation with a number of government officials, leaders in education, and employers across the country about deploying these facilities."
https://www.6sqft.com/brooklyn-based-company-repurposes-ship…
NEW YORK--( BUSINESS WIRE )-- SG Blocks, Inc. (NASDAQ: SGBX ), a leading designer, fabricator and innovator of container-based structures, today announced management will present at the Sidoti Microcap Virtual Investor Conference on June 30, 2020 at 1:45pm ET and conduct one-on-one virtual meetings throughout the day.
To attend the virtual presentation and to schedule a virtual meeting, please visit https://www.sidoticonference.com or contact SGBX@HaydenIR.com .
To attend the virtual presentation and to schedule a virtual meeting, please visit https://www.sidoticonference.com or contact SGBX@HaydenIR.com .
Langsam aber sicher tut sich hier was! Bin gespannt ob morgen eine gute News zu den Testkits komm 

Er sagte sowas wie "test and lab services represents the bigger revenue than boxes", wenn ich das richtig verstanden habe. Cash reicht jedenfalls für 3-4 Jahre und nächste Woche werden die Grimshaw Produkte auf der Homepage vorgestellt.
Hier tut sich auch wieder etwas! Nicht die News welche ich wollte aber positiv ist es!
https://www.businesswire.com/news/home/20200723005228/en/SG-…
https://www.businesswire.com/news/home/20200723005228/en/SG-…
PRINCETON, N.J., July 28, 2020 /PRNewswire/ -- Soligenix, Inc. (Nasdaq: SNGX) (Soligenix or the Company), a late-stage biopharmaceutical company focused on developing and commercializing products to treat rare diseases where there is an unmet medical need, announced today publication of pre-clinical immunogenicity studies for its CiVax™ program (heat stable COVID-19 vaccine), demonstrating immunity of both broad-spectrum antibody and cell-mediated, rapid onset immunity is possible using the CoVaccine HT™ (CoVaccine) adjuvant. The article, authored by collaborators at the University of Hawai?i at Manoa (UHM), is titled, "CoVaccine HT™ adjuvant potentiates robust immune responses to recombinant SARS-CoV-2 spike-S1 immunization," and has been submitted for peer-review to the journal npj Vaccines. An accelerated preprint of the manuscript has been made available here.
CiVax™ is the Company's heat stable subunit vaccine candidate for the prevention of COVID-19, the infection caused by SARS-CoV-2. Ongoing collaborations with Axel Lehrer, PhD, Assistant Professor in the Department of Tropical Medicine, Medical Microbiology and Pharmacology, John A. Burns School of Medicine (JABSOM), UHM have demonstrated the feasibility of developing a broadly immunogenic vaccine for COVID-19. With significant research dedicated worldwide to the generation of COVID-19 vaccines, it is noteworthy that the essential attributes of a vaccine successful in controlling the ongoing pandemic are believed to include the ability to rapidly stimulate a balanced antibody response, including an enhanced Th1 response, which includes raising significant virus neutralizing antibodies and potent cell-mediated immunity, demonstrated by T-cell activation. Previous work with the CoVaccine adjuvant, which Soligenix licensed from BTG Specialty Pharmaceuticals, a division of Boston Scientific Corporation, has indicated that CoVaccine has these critical characteristics. In these results, Lehrer and his colleagues now demonstrate these attributes of CoVaccine, specifically in the context of SARS-CoV-2. Moreover, these results, using a prototype antigen, also demonstrate a rapid onset of immunity with antibody responses detected within 14 days after the first vaccination.
Many programs have been initiated seeking a vaccine for COVID-19, but the novelty of the virus and previous challenges developing potent vaccines for the related SARS-CoV (Severe Acute Respiratory Syndrome Coronavirus) and MERS-CoV (Middle East Respiratory Syndrome Coronavirus), suggest that a timely solution requires parallel approaches. The total number of vaccine doses required to control the ongoing pandemic also suggests that multiple vaccines, based on different manufacturing platforms, will be necessary to efficiently vaccinate the worldwide population. Subunit vaccines are considered the gold standard for vaccine safety, but are relatively under-represented in fast-tracked COVID-19 vaccine candidates to date. Unlike virally vectored vaccines, there is no limit to the number of times the adjuvant and antigen can be used and the typical safety profile of a subunit vaccine results in a vaccine that is suitable for immune compromised or elderly populations as well. In contrast, while RNA and DNA vaccines are rapid to manufacture, there is a lack of regulatory precedent (no RNA or DNA vaccine has been approved to date).
"Our work to date has demonstrated not only the feasibility of rapid and efficient manufacturing of the required vaccine antigens, but also the potential for a broadly applicable and easily distributed vaccine," stated Dr. Lehrer. "We are delighted with our earlier successes on development of filovirus and flavivirus vaccines with this platform. The results in our latest manuscript confirm that the advantages of our vaccine platform with the CoVaccine adjuvant can also be realized in the context of SARS-CoV-2, while we continue our work to rapidly advance development of a heat stable subunit COVID-19 vaccine in collaboration with Soligenix."
"We believe that creating a vaccine with enhanced stability at elevated temperatures that can obviate the costs and logistical burdens associated with cold chain storage and distribution has the potential to provide a distinct advantage over other COVID-19 vaccines currently in development and simplifies worldwide distribution," stated Christopher J. Schaber, PhD, President and Chief Executive Officer of Soligenix. "Our approach appears to be unique in its use of a well-established, well-understood, and safe, subunit platform coupled with a novel adjuvant and a thermostabilizing formulation. We are very encouraged with the latest results and look forward to continuing to advance development of CiVax™."
About Coronavirus Infection
Coronavirus infections can cause a wide spectrum of disease in humans, ranging from a common cold to a more severe respiratory infection, such as Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), which have a case mortality rate of approximately 10% and 30%, respectively. Similar to filoviruses, coronaviruses also are endemic in wildlife populations and can be transmitted to humans with close contact. The COVID-19 outbreak, caused by SARS-CoV-2, is the most recent example of a suspected species crossover seen with this virus family. Although the case fatality rate of COVID-19 is still under investigation, COVID-19 has been declared a global pandemic by the World Health Organization. The global impact of this emerging infection demonstrates the urgent need for robust technology platforms to rapidly develop new vaccines for novel diseases. The only FDA sanctioned treatments for COVID-19 are available under "Emergency Use Authorization." There is currently no approved vaccine.
About John A. Burns School of Medicine, University of Hawai'i at Manoa
The John A. Burns School of Medicine (JABSOM) at the UHM is one of the leading medical education institutions in the United States. For the last three years, JABSOM has been a leader in National Institutes of Health research awards among community-based public medical schools (i.e., public medical schools without a university hospital). JABSOM has also been a leader in the rate of MD graduates (who are also residency trained in state) retained as practitioners in-state. In addition, Hawai'i's cultural diversity and geographical setting affords JABSOM a unique research environment to excel in health disparity research. JABSOM faculty bring external funding of about $40 million annually into the state.
About Soligenix, Inc.
Soligenix is a late-stage biopharmaceutical company focused on developing and commercializing products to treat rare diseases where there is an unmet medical need. Our Specialized BioTherapeutics business segment is developing SGX301 as a novel photodynamic therapy utilizing safe visible light for the treatment of cutaneous T-cell lymphoma, our first-in-class innate defense regulator (IDR) technology, dusquetide (SGX942) for the treatment of oral mucositis in head and neck cancer, and proprietary formulations of oral beclomethasone 17,21-dipropionate (BDP) for the prevention/treatment of gastrointestinal (GI) disorders characterized by severe inflammation including pediatric Crohn's disease (SGX203) and acute radiation enteritis (SGX201).
Our Public Health Solutions business segment includes active development programs for RiVax®, our ricin toxin vaccine candidate, SGX943, our therapeutic candidate for antibiotic resistant and emerging infectious disease, and vaccine programs targeting both filoviruses (such as Marburg and Ebola) and coronaviruses. The development of our vaccine programs incorporates the use of our proprietary heat stabilization platform technology, known as ThermoVax®. To date, this business segment has been supported with government grant and contract funding from the National Institute of Allergy and Infectious Diseases (NIAID), the Biomedical Advanced Research and Development Authority (BARDA), and the Defense Threat Reduction Agency (DTRA).
For further information regarding Soligenix, Inc., please visit the Company's website at www.soligenix.com.
This press release may contain forward-looking statements that reflect Soligenix, Inc.'s current expectations about its future results, performance, prospects and opportunities, including but not limited to, potential market sizes, patient populations and clinical trial enrollment. Statements that are not historical facts, such as "anticipates," "estimates," "believes," "hopes," "intends," "plans," "expects," "goal," "may," "suggest," "will," "potential," or similar expressions, are forward-looking statements. These statements are subject to a number of risks, uncertainties and other factors that could cause actual events or results in future periods to differ materially from what is expressed in, or implied by, these statements, such as experienced with the COVID-19 outbreak. Soligenix cannot assure you that it will be able to successfully develop, achieve regulatory approval for or commercialize products based on its technologies, particularly in light of the significant uncertainty inherent in developing therapeutics and vaccines against bioterror threats, conducting preclinical and clinical trials of therapeutics and vaccines, obtaining regulatory approvals and manufacturing therapeutics and vaccines, that product development and commercialization efforts will not be reduced or discontinued due to difficulties or delays in clinical trials or due to lack of progress or positive results from research and development efforts, that it will be able to successfully obtain any further funding to support product development and commercialization efforts, including grants and awards, maintain its existing grants which are subject to performance requirements, enter into any biodefense procurement contracts with the US Government or other countries, that it will be able to compete with larger and better financed competitors in the biotechnology industry, that changes in health care practice, third party reimbursement limitations and Federal and/or state health care reform initiatives will not negatively affect its business, or that the US Congress may not pass any legislation that would provide additional funding for the Project BioShield program. In addition, there can be no assurance as to the timing or success of the Phase 3 clinical trial of SGX942 (dusquetide) as a treatment for oral mucositis in patients with head and neck cancer receiving chemoradiation therapy, or any of our other clinical/preclinical trials. Despite the statistically significant result achieved in the SGX301 Phase 3 clinical trial for the treatment of cutaneous T-cell lymphoma, there can be no assurance that a marketing authorization from the FDA or EMA will be successful. Further, there can be no assurance that RiVax® will qualify for a biodefense Priority Review Voucher (PRV) or that the prior sales of PRVs will be indicative of any potential sales price for a PRV for RiVax®. Also, no assurance can be provided that the Company will receive or continue to receive non-dilutive government funding from grants and contracts that have been or may be awarded or for which the Company will apply in the future. These and other risk factors are described from time to time in filings with the Securities and Exchange Commission, including, but not limited to, Soligenix's reports on Forms 10-Q and 10-K. Unless required by law, Soligenix assumes no obligation to update or revise any forward-looking statements as a result of new information or future events.
CiVax™ is the Company's heat stable subunit vaccine candidate for the prevention of COVID-19, the infection caused by SARS-CoV-2. Ongoing collaborations with Axel Lehrer, PhD, Assistant Professor in the Department of Tropical Medicine, Medical Microbiology and Pharmacology, John A. Burns School of Medicine (JABSOM), UHM have demonstrated the feasibility of developing a broadly immunogenic vaccine for COVID-19. With significant research dedicated worldwide to the generation of COVID-19 vaccines, it is noteworthy that the essential attributes of a vaccine successful in controlling the ongoing pandemic are believed to include the ability to rapidly stimulate a balanced antibody response, including an enhanced Th1 response, which includes raising significant virus neutralizing antibodies and potent cell-mediated immunity, demonstrated by T-cell activation. Previous work with the CoVaccine adjuvant, which Soligenix licensed from BTG Specialty Pharmaceuticals, a division of Boston Scientific Corporation, has indicated that CoVaccine has these critical characteristics. In these results, Lehrer and his colleagues now demonstrate these attributes of CoVaccine, specifically in the context of SARS-CoV-2. Moreover, these results, using a prototype antigen, also demonstrate a rapid onset of immunity with antibody responses detected within 14 days after the first vaccination.
Many programs have been initiated seeking a vaccine for COVID-19, but the novelty of the virus and previous challenges developing potent vaccines for the related SARS-CoV (Severe Acute Respiratory Syndrome Coronavirus) and MERS-CoV (Middle East Respiratory Syndrome Coronavirus), suggest that a timely solution requires parallel approaches. The total number of vaccine doses required to control the ongoing pandemic also suggests that multiple vaccines, based on different manufacturing platforms, will be necessary to efficiently vaccinate the worldwide population. Subunit vaccines are considered the gold standard for vaccine safety, but are relatively under-represented in fast-tracked COVID-19 vaccine candidates to date. Unlike virally vectored vaccines, there is no limit to the number of times the adjuvant and antigen can be used and the typical safety profile of a subunit vaccine results in a vaccine that is suitable for immune compromised or elderly populations as well. In contrast, while RNA and DNA vaccines are rapid to manufacture, there is a lack of regulatory precedent (no RNA or DNA vaccine has been approved to date).
"Our work to date has demonstrated not only the feasibility of rapid and efficient manufacturing of the required vaccine antigens, but also the potential for a broadly applicable and easily distributed vaccine," stated Dr. Lehrer. "We are delighted with our earlier successes on development of filovirus and flavivirus vaccines with this platform. The results in our latest manuscript confirm that the advantages of our vaccine platform with the CoVaccine adjuvant can also be realized in the context of SARS-CoV-2, while we continue our work to rapidly advance development of a heat stable subunit COVID-19 vaccine in collaboration with Soligenix."
"We believe that creating a vaccine with enhanced stability at elevated temperatures that can obviate the costs and logistical burdens associated with cold chain storage and distribution has the potential to provide a distinct advantage over other COVID-19 vaccines currently in development and simplifies worldwide distribution," stated Christopher J. Schaber, PhD, President and Chief Executive Officer of Soligenix. "Our approach appears to be unique in its use of a well-established, well-understood, and safe, subunit platform coupled with a novel adjuvant and a thermostabilizing formulation. We are very encouraged with the latest results and look forward to continuing to advance development of CiVax™."
About Coronavirus Infection
Coronavirus infections can cause a wide spectrum of disease in humans, ranging from a common cold to a more severe respiratory infection, such as Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), which have a case mortality rate of approximately 10% and 30%, respectively. Similar to filoviruses, coronaviruses also are endemic in wildlife populations and can be transmitted to humans with close contact. The COVID-19 outbreak, caused by SARS-CoV-2, is the most recent example of a suspected species crossover seen with this virus family. Although the case fatality rate of COVID-19 is still under investigation, COVID-19 has been declared a global pandemic by the World Health Organization. The global impact of this emerging infection demonstrates the urgent need for robust technology platforms to rapidly develop new vaccines for novel diseases. The only FDA sanctioned treatments for COVID-19 are available under "Emergency Use Authorization." There is currently no approved vaccine.
About John A. Burns School of Medicine, University of Hawai'i at Manoa
The John A. Burns School of Medicine (JABSOM) at the UHM is one of the leading medical education institutions in the United States. For the last three years, JABSOM has been a leader in National Institutes of Health research awards among community-based public medical schools (i.e., public medical schools without a university hospital). JABSOM has also been a leader in the rate of MD graduates (who are also residency trained in state) retained as practitioners in-state. In addition, Hawai'i's cultural diversity and geographical setting affords JABSOM a unique research environment to excel in health disparity research. JABSOM faculty bring external funding of about $40 million annually into the state.
About Soligenix, Inc.
Soligenix is a late-stage biopharmaceutical company focused on developing and commercializing products to treat rare diseases where there is an unmet medical need. Our Specialized BioTherapeutics business segment is developing SGX301 as a novel photodynamic therapy utilizing safe visible light for the treatment of cutaneous T-cell lymphoma, our first-in-class innate defense regulator (IDR) technology, dusquetide (SGX942) for the treatment of oral mucositis in head and neck cancer, and proprietary formulations of oral beclomethasone 17,21-dipropionate (BDP) for the prevention/treatment of gastrointestinal (GI) disorders characterized by severe inflammation including pediatric Crohn's disease (SGX203) and acute radiation enteritis (SGX201).
Our Public Health Solutions business segment includes active development programs for RiVax®, our ricin toxin vaccine candidate, SGX943, our therapeutic candidate for antibiotic resistant and emerging infectious disease, and vaccine programs targeting both filoviruses (such as Marburg and Ebola) and coronaviruses. The development of our vaccine programs incorporates the use of our proprietary heat stabilization platform technology, known as ThermoVax®. To date, this business segment has been supported with government grant and contract funding from the National Institute of Allergy and Infectious Diseases (NIAID), the Biomedical Advanced Research and Development Authority (BARDA), and the Defense Threat Reduction Agency (DTRA).
For further information regarding Soligenix, Inc., please visit the Company's website at www.soligenix.com.
This press release may contain forward-looking statements that reflect Soligenix, Inc.'s current expectations about its future results, performance, prospects and opportunities, including but not limited to, potential market sizes, patient populations and clinical trial enrollment. Statements that are not historical facts, such as "anticipates," "estimates," "believes," "hopes," "intends," "plans," "expects," "goal," "may," "suggest," "will," "potential," or similar expressions, are forward-looking statements. These statements are subject to a number of risks, uncertainties and other factors that could cause actual events or results in future periods to differ materially from what is expressed in, or implied by, these statements, such as experienced with the COVID-19 outbreak. Soligenix cannot assure you that it will be able to successfully develop, achieve regulatory approval for or commercialize products based on its technologies, particularly in light of the significant uncertainty inherent in developing therapeutics and vaccines against bioterror threats, conducting preclinical and clinical trials of therapeutics and vaccines, obtaining regulatory approvals and manufacturing therapeutics and vaccines, that product development and commercialization efforts will not be reduced or discontinued due to difficulties or delays in clinical trials or due to lack of progress or positive results from research and development efforts, that it will be able to successfully obtain any further funding to support product development and commercialization efforts, including grants and awards, maintain its existing grants which are subject to performance requirements, enter into any biodefense procurement contracts with the US Government or other countries, that it will be able to compete with larger and better financed competitors in the biotechnology industry, that changes in health care practice, third party reimbursement limitations and Federal and/or state health care reform initiatives will not negatively affect its business, or that the US Congress may not pass any legislation that would provide additional funding for the Project BioShield program. In addition, there can be no assurance as to the timing or success of the Phase 3 clinical trial of SGX942 (dusquetide) as a treatment for oral mucositis in patients with head and neck cancer receiving chemoradiation therapy, or any of our other clinical/preclinical trials. Despite the statistically significant result achieved in the SGX301 Phase 3 clinical trial for the treatment of cutaneous T-cell lymphoma, there can be no assurance that a marketing authorization from the FDA or EMA will be successful. Further, there can be no assurance that RiVax® will qualify for a biodefense Priority Review Voucher (PRV) or that the prior sales of PRVs will be indicative of any potential sales price for a PRV for RiVax®. Also, no assurance can be provided that the Company will receive or continue to receive non-dilutive government funding from grants and contracts that have been or may be awarded or for which the Company will apply in the future. These and other risk factors are described from time to time in filings with the Securities and Exchange Commission, including, but not limited to, Soligenix's reports on Forms 10-Q and 10-K. Unless required by law, Soligenix assumes no obligation to update or revise any forward-looking statements as a result of new information or future events.
Antwort auf Beitrag Nr.: 64.568.838 von wojetzt am 28.07.20 13:33:38Falscher Thread 

yep, sorry
https://finance.yahoo.com/news/sg-blocks-host-second-quarter…
SG Blocks, Inc. (NASDAQ: SGBX), a leading designer, innovator and fabricator of container-based structures, will hold a conference call on, August 13, 2020 at 4:30 p.m. Eastern time to discuss its results for the second quarter ended June 30, 2020. Financial results will be issued in a press release prior to the call.
Ich hoffe die Q2-Zahlen machen dem schön langsam aufsteigenden Kurs keine Probleme:
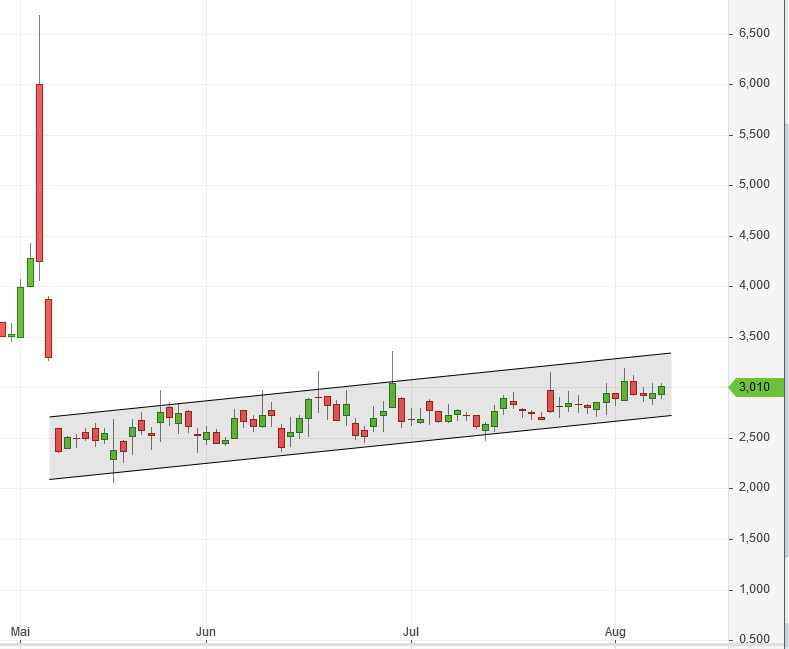
SG Blocks, Inc. (NASDAQ: SGBX), a leading designer, innovator and fabricator of container-based structures, will hold a conference call on, August 13, 2020 at 4:30 p.m. Eastern time to discuss its results for the second quarter ended June 30, 2020. Financial results will be issued in a press release prior to the call.
Ich hoffe die Q2-Zahlen machen dem schön langsam aufsteigenden Kurs keine Probleme:

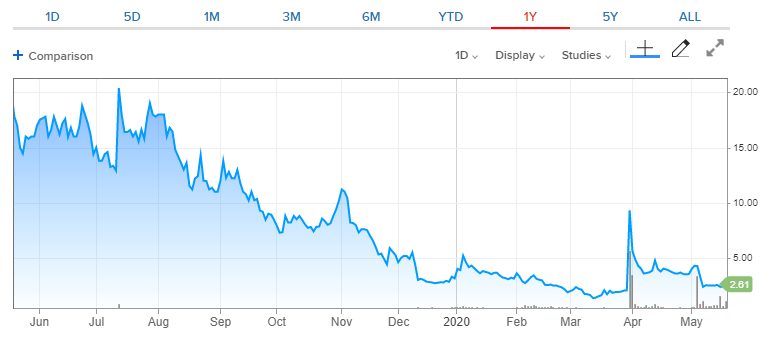
Tja, Kurs ist fett weggebrochen nach den Zahlen und hat noch immer keinen Halt gefunden. Schade eigentlich.
Ich habe mal IR angeschrieben und bzgl. der Covid Tests nachgefragt. Hier die erwartete Antwort, von Paul Galvin:
Good Morning XXX,
Thank you for the note.
In copy is James Carbanaro form Hayden IR who is available to speak.
We cannot give out any non public information. … just wanted to acknowledge your email and to let you know we are aware of the market opportunities and are working diligently to capitalize.
Have a nice day.
Paul
Ich habe mal IR angeschrieben und bzgl. der Covid Tests nachgefragt. Hier die erwartete Antwort, von Paul Galvin:
Good Morning XXX,
Thank you for the note.
In copy is James Carbanaro form Hayden IR who is available to speak.
We cannot give out any non public information. … just wanted to acknowledge your email and to let you know we are aware of the market opportunities and are working diligently to capitalize.
Have a nice day.
Paul
Am Freitag hat sich der Kurs recht brav benommen. Kein Wunder, denn diese Meldung hier war saugut:
BLINK AND SG BLOCKS ENTER INTO STRATEGIC MASTER DEVELOPMENT AND PRODUCTION AGREEMENT TO BRING SOLAR, OFF-GRID, MODULAR EV CHARGING SOLUTIONS TO MARKET
https://www.globenewswire.com/news-release/2020/10/16/210979…
Geht doch schön langsam bergauf  garkeiner investiert hier? Ungewöhnlich still im Forum.
garkeiner investiert hier? Ungewöhnlich still im Forum.
Aktuelle News: https://finance.yahoo.com/news/sg-blocks-announces-expansion…
Viel Erfolg uns allen!
 garkeiner investiert hier? Ungewöhnlich still im Forum.
garkeiner investiert hier? Ungewöhnlich still im Forum. Aktuelle News: https://finance.yahoo.com/news/sg-blocks-announces-expansion…
Viel Erfolg uns allen!
Neues impftestzentrum in Illinois plus 225 Eigentumswohnungen in Austin.
Jetzt fehlt nur noc h schnellladestationen von blink charging und wir sehen neue hoechstkurse
Jetzt fehlt nur noc h schnellladestationen von blink charging und wir sehen neue hoechstkurse
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +0,90 | |
| -8,27 | |
| -0,46 | |
| +1,48 | |
| -0,12 | |
| +0,35 | |
| +2,80 | |
| -0,68 | |
| +0,19 | |
| +1,03 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 201 | ||
| 109 | ||
| 78 | ||
| 69 | ||
| 51 | ||
| 36 | ||
| 34 | ||
| 34 | ||
| 32 | ||
| 28 |

















