Homology Medicines - nach Pfizer Invest von 60 Mio USD ein Übernahmekandidat? - 500 Beiträge pro Seite
eröffnet am 15.01.21 08:32:42 von
neuester Beitrag 19.10.21 10:18:57 von
neuester Beitrag 19.10.21 10:18:57 von
Beiträge: 17
ID: 1.338.767
ID: 1.338.767
Aufrufe heute: 1
Gesamt: 688
Gesamt: 688
Aktive User: 0
ISIN: US7469641051 · WKN: A4ZZ0Z
24,800
EUR
-0,20 %
-0,050 EUR
Letzter Kurs 12:26:07 Lang & Schwarz
Neuigkeiten
18.03.24 · globenewswire |
27.02.24 · Business Wire (engl.) |
03.01.24 · globenewswire |
18.11.23 · Business Wire (engl.) |
16.11.23 · Business Wire (engl.) |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,9000 | +59,66 | |
| 0,6000 | +57,48 | |
| 1,9200 | +23,87 | |
| 5,4500 | +19,00 | |
| 6,9300 | +17,46 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 4,3600 | -13,49 | |
| 1,6900 | -14,50 | |
| 2,8150 | -15,21 | |
| 2,2900 | -17,63 | |
| 2,2600 | -30,25 |
Hallo zusammen,
ich bin seit der Meldung, dass Pfizer mit 60 Mio USD in diesen Wert eingestiegen ist überzeugt, dass es hier eine sehr gute Chance gibt, dass es ein zukünftiges Buyout geben wird.
Ich habe die Meldung mal verlinkt: https://newsfilter.io/a/539ed9f8fca740c00c03c98d070bb1bc
"Pfizer Invests $120 Million in Biotechnology Innovation Through the Pfizer Breakthrough Growth Initiative
....
$60 million in Bedford, Mass.-based Homology Medicines (Nasdaq: FIXX), a clinical-stage genetic medicines company focused on treatments for rare genetic diseases with significant unmet medical needs. In addition, Seng Cheng, Senior Vice President and Chief Scientific Officer of Pfizer’s Rare Disease Research Unit, has joined Homology’s Scientific Advisory Board for matters related to Homology’s gene therapy and gene editing programs for phenylketonuria (PKU). "
Sehr gute Bewertungen von den üblichen Analysten:
https://www.marketbeat.com/stocks/NASDAQ/FIXX/?RegistrationC…
besonders auch:
https://www.tipranks.com/news/article/oppenheimer-says-these…
"Homology Medicines works on the research and creation of new genetic medicines. The company uses gene editing and gene therapy as the basis for treating disease caused by genetic mutations. Homology’s technology, using human hematopoietic stem cell derived adeno-associated virus vectors, aims to treat these diseases through gene correction and insertion.
Along with its recent third quarter earnings, Homology also announced a $60 million investment from Pfizer. This was a strategic investment on Pfizer’s part, and includes the larger company purchasing 5 million shares of FIXX. The announcement helped to stabilize Homology’s stock value after the Q3 net loss of 62 cents per share. Under the agreement, Pfizer purchased 5 million common shares of FIXX at a set price of $12 each.
Coinciding with the Pfizer agreement, Homology also announced that it will be progressing with the pheNIX gene therapy clinical trial for PKU treatment in adults. The new phase will include dose expansion, and comes after early trials showed that the drug candidate, HMI-102, was well tolerated by patients and positively affected the Phe/Tyr ratio at two doses. Moving to the next step, Homology will be conducting randomized concurrently controlled trials.
Oppenheimer analyst Matthew Biegler noted Pfizer’s cash investment into Homology, and its importance as a vote of confidence. The analyst wrote, “Proceeds from the investment will be used in further development of Homology’s phenylketonuria (PKU) gene therapy franchise, which includes HMI-102 and as well as its preclinical in vivo gene editing program, HMI-103. We believe Pfizer’s decision was spurred by updated clinical data from the PheNIX trial of HMI-102 presented last week, which showed encouraging signs of phenylalanine (Phe) reduction in two PKU patients. Homology plans to advance the trial into dose expansion cohorts in early 2021.”
Biegler is optimistic on Homology, as is clear from his $27 price target. At the current share price of $10.17, that target suggests an upside of 165% and fully supports his Outperform (i.e. Buy) rating. (To watch Biegler’s track record, click here)
Other analysts are on the same page. With 3 Buys received in the last three months, the word on the Street is that FIXX is a Strong Buy. On top of this, the $29.50 average price target brings the potential twelve-month gain to 190%."
Schaut es Euch an.
Freue mich auf Eure Beiträge.
Herzlichen Gruß
Sven
ich bin seit der Meldung, dass Pfizer mit 60 Mio USD in diesen Wert eingestiegen ist überzeugt, dass es hier eine sehr gute Chance gibt, dass es ein zukünftiges Buyout geben wird.
Ich habe die Meldung mal verlinkt: https://newsfilter.io/a/539ed9f8fca740c00c03c98d070bb1bc
"Pfizer Invests $120 Million in Biotechnology Innovation Through the Pfizer Breakthrough Growth Initiative
....
$60 million in Bedford, Mass.-based Homology Medicines (Nasdaq: FIXX), a clinical-stage genetic medicines company focused on treatments for rare genetic diseases with significant unmet medical needs. In addition, Seng Cheng, Senior Vice President and Chief Scientific Officer of Pfizer’s Rare Disease Research Unit, has joined Homology’s Scientific Advisory Board for matters related to Homology’s gene therapy and gene editing programs for phenylketonuria (PKU). "
Sehr gute Bewertungen von den üblichen Analysten:
https://www.marketbeat.com/stocks/NASDAQ/FIXX/?RegistrationC…
besonders auch:
https://www.tipranks.com/news/article/oppenheimer-says-these…
"Homology Medicines works on the research and creation of new genetic medicines. The company uses gene editing and gene therapy as the basis for treating disease caused by genetic mutations. Homology’s technology, using human hematopoietic stem cell derived adeno-associated virus vectors, aims to treat these diseases through gene correction and insertion.
Along with its recent third quarter earnings, Homology also announced a $60 million investment from Pfizer. This was a strategic investment on Pfizer’s part, and includes the larger company purchasing 5 million shares of FIXX. The announcement helped to stabilize Homology’s stock value after the Q3 net loss of 62 cents per share. Under the agreement, Pfizer purchased 5 million common shares of FIXX at a set price of $12 each.
Coinciding with the Pfizer agreement, Homology also announced that it will be progressing with the pheNIX gene therapy clinical trial for PKU treatment in adults. The new phase will include dose expansion, and comes after early trials showed that the drug candidate, HMI-102, was well tolerated by patients and positively affected the Phe/Tyr ratio at two doses. Moving to the next step, Homology will be conducting randomized concurrently controlled trials.
Oppenheimer analyst Matthew Biegler noted Pfizer’s cash investment into Homology, and its importance as a vote of confidence. The analyst wrote, “Proceeds from the investment will be used in further development of Homology’s phenylketonuria (PKU) gene therapy franchise, which includes HMI-102 and as well as its preclinical in vivo gene editing program, HMI-103. We believe Pfizer’s decision was spurred by updated clinical data from the PheNIX trial of HMI-102 presented last week, which showed encouraging signs of phenylalanine (Phe) reduction in two PKU patients. Homology plans to advance the trial into dose expansion cohorts in early 2021.”
Biegler is optimistic on Homology, as is clear from his $27 price target. At the current share price of $10.17, that target suggests an upside of 165% and fully supports his Outperform (i.e. Buy) rating. (To watch Biegler’s track record, click here)
Other analysts are on the same page. With 3 Buys received in the last three months, the word on the Street is that FIXX is a Strong Buy. On top of this, the $29.50 average price target brings the potential twelve-month gain to 190%."
Schaut es Euch an.
Freue mich auf Eure Beiträge.
Herzlichen Gruß
Sven
Hallo zusammen,
und noch ein paar intersaante Videos zur Firma:
Ein schönes Wochenende!
Sven
und noch ein paar intersaante Videos zur Firma:
Ein schönes Wochenende!
Sven
Hallo zusammen,
für mich sehr vielversprechend!!!
LG
Sven
Homology Medicines Announces Plans for Three Clinical Programs in 2021 Spanning Phenylketonuria (PKU) and Hunter Syndrome (MPS II)
- Clinical Data from pheNIX Gene Therapy Phase 2 Dose Expansion Trial Expected by End of Year; Trial Currently Recruiting Patients -
- Company Plans to Nominate Additional Development Candidate in New Therapeutic Area -
- Management Highlights Anticipated 2021 Milestones in Webcast Available on Homology’s Website -
BEDFORD, Mass., January 6, 2021 – Homology Medicines, Inc. (Nasdaq: FIXX), a genetic medicines company, announced today plans to progress its gene therapy and gene editing platform, and unveiled plans to have three clinical programs and a development candidate in a new therapeutic area during 2021.
“We ended 2020 with positive data from the dose-escalation phase of the world’s first PKU gene therapy clinical trial, pheNIX, and validation of both our PKU gene therapy and gene editing programs with an equity investment from Pfizer, a leader in genetic medicines,” stated Arthur Tzianabos, Ph.D., President and Chief Executive Officer of Homology Medicines. “The pheNIX clinical trial sites are recruiting for the Phase 2 dose expansion phase of the trial, and we anticipate clinical data by the end of the year. Continuing this momentum, we outlined today our plans to initiate two additional Phase 1/2 dose-escalation trials in 2021 with our gene therapy candidate for Hunter syndrome and our first gene editing candidate, which is for PKU.”
Dr. Tzianabos continued, “We are also leveraging our technology platform to expand our pipeline and plan to name another development candidate focused on a new therapeutic indication in 2021. In addition, we aim to advance our pipeline, which includes optimizing our metachromatic leukodystrophy (MLD) gene therapy development candidate, as well as expanding our fully characterized family of 15 AAVHSC vectors through capsid shuffling.”
Company management discussed its 2021 plans in a webcast that is now available on Homology’s website in the Investors section. Homology also plans to participate in the following events at the upcoming H.C. Wainwright Virtual BioConnect Conference on January 11, 2021:
Virtual fireside chat (webcast will be available at 6:00 a.m. ET on Homology’s website)
Panel titled, “Clinical Trials - Considerations in the Current Environment and its Impact on Future Design and Maintenance,” hosted by former Commissioner of the U.S. Food and Drug Administration (FDA) Scott Gottlieb, M.D., at 12:00 p.m. ET.
Today’s webcast and next week’s fireside chat will be available on Homology’s website for 90 days.
https://www.homologymedicines.com/news-story/homology-medici…
für mich sehr vielversprechend!!!
LG
Sven
Homology Medicines Announces Plans for Three Clinical Programs in 2021 Spanning Phenylketonuria (PKU) and Hunter Syndrome (MPS II)
- Clinical Data from pheNIX Gene Therapy Phase 2 Dose Expansion Trial Expected by End of Year; Trial Currently Recruiting Patients -
- Company Plans to Nominate Additional Development Candidate in New Therapeutic Area -
- Management Highlights Anticipated 2021 Milestones in Webcast Available on Homology’s Website -
BEDFORD, Mass., January 6, 2021 – Homology Medicines, Inc. (Nasdaq: FIXX), a genetic medicines company, announced today plans to progress its gene therapy and gene editing platform, and unveiled plans to have three clinical programs and a development candidate in a new therapeutic area during 2021.
“We ended 2020 with positive data from the dose-escalation phase of the world’s first PKU gene therapy clinical trial, pheNIX, and validation of both our PKU gene therapy and gene editing programs with an equity investment from Pfizer, a leader in genetic medicines,” stated Arthur Tzianabos, Ph.D., President and Chief Executive Officer of Homology Medicines. “The pheNIX clinical trial sites are recruiting for the Phase 2 dose expansion phase of the trial, and we anticipate clinical data by the end of the year. Continuing this momentum, we outlined today our plans to initiate two additional Phase 1/2 dose-escalation trials in 2021 with our gene therapy candidate for Hunter syndrome and our first gene editing candidate, which is for PKU.”
Dr. Tzianabos continued, “We are also leveraging our technology platform to expand our pipeline and plan to name another development candidate focused on a new therapeutic indication in 2021. In addition, we aim to advance our pipeline, which includes optimizing our metachromatic leukodystrophy (MLD) gene therapy development candidate, as well as expanding our fully characterized family of 15 AAVHSC vectors through capsid shuffling.”
Company management discussed its 2021 plans in a webcast that is now available on Homology’s website in the Investors section. Homology also plans to participate in the following events at the upcoming H.C. Wainwright Virtual BioConnect Conference on January 11, 2021:
Virtual fireside chat (webcast will be available at 6:00 a.m. ET on Homology’s website)
Panel titled, “Clinical Trials - Considerations in the Current Environment and its Impact on Future Design and Maintenance,” hosted by former Commissioner of the U.S. Food and Drug Administration (FDA) Scott Gottlieb, M.D., at 12:00 p.m. ET.
Today’s webcast and next week’s fireside chat will be available on Homology’s website for 90 days.
https://www.homologymedicines.com/news-story/homology-medici…

https://clinicaltrials.gov/ct2/show/NCT03952156?cond=hmi-102
Pfizer investiert 60 Mio. $ (12$/Aktie) und der Kurs fällt unter 10$.
Da konnte ich auch nicht widerstehen und bin seit dem 11.11.20 dabei.
Studienergebnisse noch dieses Jahr.
Viel Erfolg
HAllo zusammen,
tja.. da wird der Kurs erstmal durch Leerverkäufe nach unten geprügelt...
http://shortvolumes.com/?t=FIXX
Sollte aber nicht lange anhalten (können)
LG
Sven
tja.. da wird der Kurs erstmal durch Leerverkäufe nach unten geprügelt...
http://shortvolumes.com/?t=FIXX
Sollte aber nicht lange anhalten (können)
LG
Sven
Antwort auf Beitrag Nr.: 66.479.666 von Sven_Bonn am 17.01.21 19:47:54Hallo Sven,
der größte Teil der Aktien ist in festen Händen, deshalb mache ich mir keine Sorgen über die Leerverkäufe.
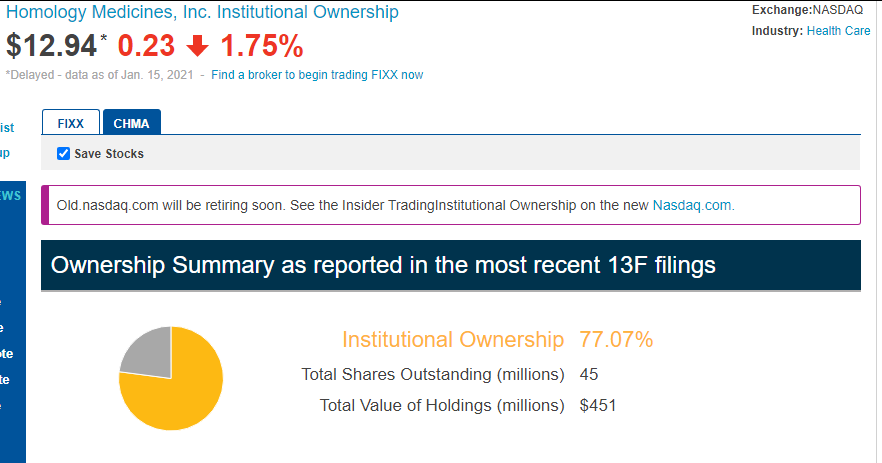
https://old.nasdaq.com/symbol/fixx/institutional-holdings
Gruß
der größte Teil der Aktien ist in festen Händen, deshalb mache ich mir keine Sorgen über die Leerverkäufe.

https://old.nasdaq.com/symbol/fixx/institutional-holdings
Gruß
Antwort auf Beitrag Nr.: 66.482.348 von pako21 am 18.01.21 08:31:44yes :-) Das sehe ich genaus. Ausserdem wird Phizer sicher das Unternehmen und deren Produkte durchleuchtet haben, bevor man hier investiert... Geduld sollte hier der Schlüssel sein.
Einen schönen Start in die Woche!
LG
Sven
Einen schönen Start in die Woche!
LG
Sven
Guten Morgen zusammen,
nun ist auch die PMORGAN CHASE & CO mit 7,6 % drin....
http://www.conferencecalltranscripts.org/summary/?id=8805601
Wird immer besser :-)
LG
Sven
nun ist auch die PMORGAN CHASE & CO mit 7,6 % drin....
http://www.conferencecalltranscripts.org/summary/?id=8805601
Wird immer besser :-)
LG
Sven
Hallo zusammen,
so.. das Potenzial wird immer größer...
Homology Medicines Announces First Presentation of Data with HMI-203 In Vivo Gene Therapy Development Candidate for Hunter Syndrome
- IND-Enabling Studies Demonstrated Potential of a Single I.V. Administration of HMI-203 to Address Peripheral and CNS Components of Disease -
- Data at WORLDSymposium™ Support Plans to Initiate a HMI-203 Phase 1/2 Clinical Trial
This Year -
BEDFORD, Mass., Feb. 08, 2021 (GLOBE NEWSWIRE) -- Homology Medicines, Inc. (Nasdaq: FIXX), a clinical-stage genetic medicines company, announced today the first scientific presentation of data from IND-enabling studies with its HMI-203 gene therapy development candidate for Hunter syndrome (MPS II). The results from Homology’s in vivo approach demonstrated the potential of HMI-203 to address peripheral manifestations in the murine disease model and showed that HMI-203 crossed the blood-brain-barrier following a single I.V. administration. These data will be presented during a poster presentation at the 17th Annual WORLDSymposium™ Meeting, where Homology will also present long-term data from its HMI-202 in vivo gene therapy program for metachromatic leukodystrophy (MLD).
“By leveraging our family of AAVHSC vectors to target both peripheral organs and the central and peripheral nervous systems in preclinical models of Hunter syndrome and MLD, debilitating diseases that have high unmet medical need, we also demonstrated the continued expansion of our CNS platform,” stated Albert Seymour, Ph.D., Chief Scientific Officer of Homology Medicines. “This is our first presentation of preclinical data from our Hunter syndrome gene therapy program, and these IND-enabling studies showed that a single I.V. administration produced high levels of enzymatic expression across disease-relevant tissues and achieved phenotypic correction of skeletal deformities. These results support our plans to move this program forward into a Phase 1/2 clinical trial this year.”
In the poster titled, “HMI-203: Investigational Gene Therapy for Mucopolysaccharidosis Type II (MPS II), or Hunter Syndrome,” a single I.V. administration of HMI-203 in the adult murine model:
Led to robust biodistribution and sustained human I2S (hI2S) enzyme expression, which resulted in:
Significant reductions in key Hunter syndrome biomarkers of heparin sulfate glycosaminoglycans (GAGs) and lysosomal-associated membrane protein 1 (LAMP-1) in the brain, liver, heart, spleen, lung and kidneys compared with vehicle.
Significant reductions in heparan sulfate GAGs in the cerebrospinal fluid (CSF) compared with vehicle.
Ameliorated paw deformities, as shown by significant changes in measurements of ankle depth, paw width, paw depth and ankle width compared with vehicle.
Led to uptake of hI2S from the serum of the HMI-203-treated model in human cell lines, demonstrating potential for cell cross-correction.
In an additional poster titled, “HMI-202: Gene Therapy Development Candidate for Metachromatic Leukodystrophy (MLD),” a single I.V. administration of HMI-202:
Crossed the blood-brain-barrier and blood-nerve-barrier in the murine MLD disease model and in non-human primates (NHPs), with human ARSA (hARSA) detected in neuronal and glial cells.
Showed durable hARSA activity in the central nervous system of the disease model, with distribution levels resembling those of Arsa in normal age-matched controls.
Demonstrated significant changes in key MLD biomarkers of LAMP-1, glial fibrillary acidic protein (GFAP), MAL transcript and neuronal sulfatides in the disease model compared with vehicle, similar to age-matched wild type controls.
Homology’s e-poster presentations will take place on February 11, 2021 at 2:30 p.m. ET. For more information, visit www.homologymedicines.com/publications.
About Mucopolysaccharidosis Type II (MPS II), Hunter Syndrome
MPS II, or Hunter syndrome, is a rare, X-linked lysosomal storage disorder caused by mutations in the iduronate-2-sulfatase (IDS) gene, which is responsible for producing the I2S enzyme that breaks down large sugar molecules, or cellular waste, called glycosaminoglycans (GAGs). Severe Hunter syndrome results in toxic lysosomal accumulation of GAGs that causes progressive debilitation and decline in intellectual function. Hunter syndrome occurs in approximately 1 in 100,000 to 1 in 170,000 males, and the severe form leads to life expectancy of 10 to 20 years.
About Metachromatic Leukodystrophy (MLD)
MLD is a rare lysosomal storage disorder caused by mutations in the ARSA gene. ARSA is responsible for the creation of the arylsulfatase A (ARSA) protein, which is required for the breakdown of cellular components. In MLD, these cellular components accumulate and destroy myelin-producing cells in the peripheral and central nervous system leading to progressive and serious neurological deterioration. The late infantile form of the disorder is estimated to affect 1 in 40,000 people, and it is fatal within five to ten years after onset.
About Homology Medicines, Inc.
Homology Medicines, Inc. is a clinical-stage genetic medicines company dedicated to transforming the lives of patients suffering from rare genetic diseases with significant unmet medical needs by curing the underlying cause of the disease. Homology’s proprietary platform is designed to utilize its human hematopoietic stem cell-derived adeno-associated virus vectors (AAVHSCs) to precisely and efficiently deliver genetic medicines in vivo either through a gene therapy or nuclease-free gene editing modality across a broad range of genetic disorders. Homology has a management team with a successful track record of discovering, developing and commercializing therapeutics with a particular focus on rare diseases, and intellectual property covering its suite of 15 AAVHSCs. Homology believes that its compelling preclinical data, scientific expertise, product development strategy, manufacturing capabilities and intellectual property position it as a leader in the development of genetic medicines. For more information, please visit www.homologymedicines.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding our expectations surrounding the potential, safety, efficacy, and regulatory and clinical progress of our product candidates; our plans to move forward into a Phase 1/2 clinical trial for HMI-203 this year; our position as a leader in the development of genetic medicines; and our participation in upcoming presentations and conferences. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the impact of the COVID-19 pandemic on our business and operations, including our preclinical studies and clinical trials, and on general economic conditions; we have and expect to continue to incur significant losses; our need for additional funding, which may not be available; failure to identify additional product candidates and develop or commercialize marketable products; the early stage of our development efforts; potential unforeseen events during clinical trials could cause delays or other adverse consequences; risks relating to the capabilities of our manufacturing facility; risks relating to the regulatory approval process; interim, topline and preliminary data may change as more patient data become available, and are subject to audit and verification procedures that could result in material changes in the final data; our product candidates may cause serious adverse side effects; inability to maintain our collaborations, or the failure of these collaborations; our reliance on third parties; failure to obtain U.S. or international marketing approval; ongoing regulatory obligations; effects of significant competition; unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives; product liability lawsuits; failure to attract, retain and motivate qualified personnel; the possibility of system failures or security breaches; risks relating to intellectual property and significant costs as a result of operating as a public company. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2020 and our other filings with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change.
Company Contacts:
Theresa McNeely
Chief Communications Officer
and Patient Advocate
tmcneely@homologymedicines.com
781-301-7277
https://investors.homologymedicines.com/news-releases/news-r…
LG
Sven
so.. das Potenzial wird immer größer...
Homology Medicines Announces First Presentation of Data with HMI-203 In Vivo Gene Therapy Development Candidate for Hunter Syndrome
- IND-Enabling Studies Demonstrated Potential of a Single I.V. Administration of HMI-203 to Address Peripheral and CNS Components of Disease -
- Data at WORLDSymposium™ Support Plans to Initiate a HMI-203 Phase 1/2 Clinical Trial
This Year -
BEDFORD, Mass., Feb. 08, 2021 (GLOBE NEWSWIRE) -- Homology Medicines, Inc. (Nasdaq: FIXX), a clinical-stage genetic medicines company, announced today the first scientific presentation of data from IND-enabling studies with its HMI-203 gene therapy development candidate for Hunter syndrome (MPS II). The results from Homology’s in vivo approach demonstrated the potential of HMI-203 to address peripheral manifestations in the murine disease model and showed that HMI-203 crossed the blood-brain-barrier following a single I.V. administration. These data will be presented during a poster presentation at the 17th Annual WORLDSymposium™ Meeting, where Homology will also present long-term data from its HMI-202 in vivo gene therapy program for metachromatic leukodystrophy (MLD).
“By leveraging our family of AAVHSC vectors to target both peripheral organs and the central and peripheral nervous systems in preclinical models of Hunter syndrome and MLD, debilitating diseases that have high unmet medical need, we also demonstrated the continued expansion of our CNS platform,” stated Albert Seymour, Ph.D., Chief Scientific Officer of Homology Medicines. “This is our first presentation of preclinical data from our Hunter syndrome gene therapy program, and these IND-enabling studies showed that a single I.V. administration produced high levels of enzymatic expression across disease-relevant tissues and achieved phenotypic correction of skeletal deformities. These results support our plans to move this program forward into a Phase 1/2 clinical trial this year.”
In the poster titled, “HMI-203: Investigational Gene Therapy for Mucopolysaccharidosis Type II (MPS II), or Hunter Syndrome,” a single I.V. administration of HMI-203 in the adult murine model:
Led to robust biodistribution and sustained human I2S (hI2S) enzyme expression, which resulted in:
Significant reductions in key Hunter syndrome biomarkers of heparin sulfate glycosaminoglycans (GAGs) and lysosomal-associated membrane protein 1 (LAMP-1) in the brain, liver, heart, spleen, lung and kidneys compared with vehicle.
Significant reductions in heparan sulfate GAGs in the cerebrospinal fluid (CSF) compared with vehicle.
Ameliorated paw deformities, as shown by significant changes in measurements of ankle depth, paw width, paw depth and ankle width compared with vehicle.
Led to uptake of hI2S from the serum of the HMI-203-treated model in human cell lines, demonstrating potential for cell cross-correction.
In an additional poster titled, “HMI-202: Gene Therapy Development Candidate for Metachromatic Leukodystrophy (MLD),” a single I.V. administration of HMI-202:
Crossed the blood-brain-barrier and blood-nerve-barrier in the murine MLD disease model and in non-human primates (NHPs), with human ARSA (hARSA) detected in neuronal and glial cells.
Showed durable hARSA activity in the central nervous system of the disease model, with distribution levels resembling those of Arsa in normal age-matched controls.
Demonstrated significant changes in key MLD biomarkers of LAMP-1, glial fibrillary acidic protein (GFAP), MAL transcript and neuronal sulfatides in the disease model compared with vehicle, similar to age-matched wild type controls.
Homology’s e-poster presentations will take place on February 11, 2021 at 2:30 p.m. ET. For more information, visit www.homologymedicines.com/publications.
About Mucopolysaccharidosis Type II (MPS II), Hunter Syndrome
MPS II, or Hunter syndrome, is a rare, X-linked lysosomal storage disorder caused by mutations in the iduronate-2-sulfatase (IDS) gene, which is responsible for producing the I2S enzyme that breaks down large sugar molecules, or cellular waste, called glycosaminoglycans (GAGs). Severe Hunter syndrome results in toxic lysosomal accumulation of GAGs that causes progressive debilitation and decline in intellectual function. Hunter syndrome occurs in approximately 1 in 100,000 to 1 in 170,000 males, and the severe form leads to life expectancy of 10 to 20 years.
About Metachromatic Leukodystrophy (MLD)
MLD is a rare lysosomal storage disorder caused by mutations in the ARSA gene. ARSA is responsible for the creation of the arylsulfatase A (ARSA) protein, which is required for the breakdown of cellular components. In MLD, these cellular components accumulate and destroy myelin-producing cells in the peripheral and central nervous system leading to progressive and serious neurological deterioration. The late infantile form of the disorder is estimated to affect 1 in 40,000 people, and it is fatal within five to ten years after onset.
About Homology Medicines, Inc.
Homology Medicines, Inc. is a clinical-stage genetic medicines company dedicated to transforming the lives of patients suffering from rare genetic diseases with significant unmet medical needs by curing the underlying cause of the disease. Homology’s proprietary platform is designed to utilize its human hematopoietic stem cell-derived adeno-associated virus vectors (AAVHSCs) to precisely and efficiently deliver genetic medicines in vivo either through a gene therapy or nuclease-free gene editing modality across a broad range of genetic disorders. Homology has a management team with a successful track record of discovering, developing and commercializing therapeutics with a particular focus on rare diseases, and intellectual property covering its suite of 15 AAVHSCs. Homology believes that its compelling preclinical data, scientific expertise, product development strategy, manufacturing capabilities and intellectual property position it as a leader in the development of genetic medicines. For more information, please visit www.homologymedicines.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding our expectations surrounding the potential, safety, efficacy, and regulatory and clinical progress of our product candidates; our plans to move forward into a Phase 1/2 clinical trial for HMI-203 this year; our position as a leader in the development of genetic medicines; and our participation in upcoming presentations and conferences. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the impact of the COVID-19 pandemic on our business and operations, including our preclinical studies and clinical trials, and on general economic conditions; we have and expect to continue to incur significant losses; our need for additional funding, which may not be available; failure to identify additional product candidates and develop or commercialize marketable products; the early stage of our development efforts; potential unforeseen events during clinical trials could cause delays or other adverse consequences; risks relating to the capabilities of our manufacturing facility; risks relating to the regulatory approval process; interim, topline and preliminary data may change as more patient data become available, and are subject to audit and verification procedures that could result in material changes in the final data; our product candidates may cause serious adverse side effects; inability to maintain our collaborations, or the failure of these collaborations; our reliance on third parties; failure to obtain U.S. or international marketing approval; ongoing regulatory obligations; effects of significant competition; unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives; product liability lawsuits; failure to attract, retain and motivate qualified personnel; the possibility of system failures or security breaches; risks relating to intellectual property and significant costs as a result of operating as a public company. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2020 and our other filings with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change.
Company Contacts:
Theresa McNeely
Chief Communications Officer
and Patient Advocate
tmcneely@homologymedicines.com
781-301-7277
https://investors.homologymedicines.com/news-releases/news-r…
LG
Sven
ggf. merken:
https://www.homologymedicines.com/images/uploads/pdf/WORLDSy…
WORLDSymposium™ 2021 Annual MeetingPoster PresentationFebruary 11, 2021; 2:30 p.m. ETHMI-203: Investigational Gene Therapy for Mucopolysaccharidosis Type II (MPS II), or Hunter Syndrome Patel K, Smith L, Gingras J, Tzianabos A, , Schulman L, Zhivich V, Seabrook T, Kivaa M, Faulkner D, Sengooba A, Behmoiras L, Dollive S, Avila N, Zhao J, White Y, Newman J, Woodcock S, Smith S, Elliott M, Francone O and Seymour A Homology Medicines, Inc., Bedford, MA 01730 Mucopolysaccharidosis type II (MPS II), or Hunter syndrome, is a rare X-linked lysosomal storage disorder (LSD) caused by mutations inthe iduronate-2-sulfatase IDS gene, resulting in loss of I2S enzyme activity leading to subsequent systemic (peripheral organs and CNS) toxic lysosomal accumulation of glycosaminoglycans (GAGs), large polysaccharides made of repeating disaccharide units responsible for providing structure and hydration to the cell1. The disease results in skeletal dysplasia, joint stiffness, hepatosplenomegaly and airway obstruction and in severe cases, neurocognitive deficits2. Hunter syndrome occurs in approximately 1 in100,000 to 1 in 170,000 males, and the severe form leads to life expectancy of 10 to 20 years. We report preclinical gene therapy data where a single intravenous (I.V.) dose of an investigational gene therapy construct (AAVHSC-HMI-203) delivering human IDS (hIDS) in the MPS II murine model3,4 resulted in systemic and CNS transduction and IDS expression. Significant levels of functionally active hI2S protein were observed in serum within a week post-administration and were found to be stable out to 28 weeks (last time point evaluated). The robust hIDS tissue expression significantly reduced murine GAG and LAMP-1 levels in the brain, liver, heart, spleen, lung and kidney tissue when compared to vehicle-treated MPS II mice. Furthermore, in vivo circulatingfunctional hI2S protein demonstrated the ability to cross-correct in vitro. Sustained levels of hI2S demonstrated amelioration of phenotypic symptoms associated with joints and digits in the MPS II mouse model following administration. Based on these nonclinical data, HMI-203 IND-enabling studies are ongoing to support the development of HMI-203 as a gene therapy for the treatment of MPS II. 1. Wraith et. al, 2008 2. Scarpa M., 2018 3. Muenzer J. et. al, 2002 4. Garcia et. al. 2007
https://www.homologymedicines.com/images/uploads/pdf/WORLDSy…
WORLDSymposium™ 2021 Annual MeetingPoster PresentationFebruary 11, 2021; 2:30 p.m. ETHMI-203: Investigational Gene Therapy for Mucopolysaccharidosis Type II (MPS II), or Hunter Syndrome Patel K, Smith L, Gingras J, Tzianabos A, , Schulman L, Zhivich V, Seabrook T, Kivaa M, Faulkner D, Sengooba A, Behmoiras L, Dollive S, Avila N, Zhao J, White Y, Newman J, Woodcock S, Smith S, Elliott M, Francone O and Seymour A Homology Medicines, Inc., Bedford, MA 01730 Mucopolysaccharidosis type II (MPS II), or Hunter syndrome, is a rare X-linked lysosomal storage disorder (LSD) caused by mutations inthe iduronate-2-sulfatase IDS gene, resulting in loss of I2S enzyme activity leading to subsequent systemic (peripheral organs and CNS) toxic lysosomal accumulation of glycosaminoglycans (GAGs), large polysaccharides made of repeating disaccharide units responsible for providing structure and hydration to the cell1. The disease results in skeletal dysplasia, joint stiffness, hepatosplenomegaly and airway obstruction and in severe cases, neurocognitive deficits2. Hunter syndrome occurs in approximately 1 in100,000 to 1 in 170,000 males, and the severe form leads to life expectancy of 10 to 20 years. We report preclinical gene therapy data where a single intravenous (I.V.) dose of an investigational gene therapy construct (AAVHSC-HMI-203) delivering human IDS (hIDS) in the MPS II murine model3,4 resulted in systemic and CNS transduction and IDS expression. Significant levels of functionally active hI2S protein were observed in serum within a week post-administration and were found to be stable out to 28 weeks (last time point evaluated). The robust hIDS tissue expression significantly reduced murine GAG and LAMP-1 levels in the brain, liver, heart, spleen, lung and kidney tissue when compared to vehicle-treated MPS II mice. Furthermore, in vivo circulatingfunctional hI2S protein demonstrated the ability to cross-correct in vitro. Sustained levels of hI2S demonstrated amelioration of phenotypic symptoms associated with joints and digits in the MPS II mouse model following administration. Based on these nonclinical data, HMI-203 IND-enabling studies are ongoing to support the development of HMI-203 as a gene therapy for the treatment of MPS II. 1. Wraith et. al, 2008 2. Scarpa M., 2018 3. Muenzer J. et. al, 2002 4. Garcia et. al. 2007
Homology Medicines Regains Worldwide Rights to Ophthalmology Program Based on its In Vivo Nuclease-Free Gene Editing Platform
BEDFORD, Mass., March 01, 2021 (GLOBE NEWSWIRE) -- Homology Medicines, Inc. (Nasdaq: FIXX), a clinical-stage genetic medicines company, announced today it has regained worldwide exclusive rights from Novartis to research, develop, manufacture and commercialize Homology’s proprietary nuclease-free gene editing technology platform for an ophthalmic target. Following a portfolio review, Novartis decided to conclude the collaboration and licensing agreement with Homology to pursue other opportunities in their pipeline.
“Our nuclease-free gene editing platform is applicable across a broad range of genetic disorders, including ophthalmic diseases where we have generated positive in vivo data in two different eye targets,” said Arthur Tzianabos, Ph.D., President and Chief Executive Officer of Homology Medicines. “Our work with Novartis has been productive in demonstrating that our AAVHSC vectors can edit the back of the eye, and we plan to share additional data from the ophthalmology program at a scientific meeting in May. The data are promising and support advancing this program, which we now intend to do on our own as we drive toward naming a development candidate.”
“Our collaboration with Homology has generated data that support gene editing in retinal cells in a rare ophthalmic disease, providing early proof-of-principle for further research using this approach.” said Jay Bradner, M.D., President of the Novartis Institutes for BioMedical Research (NIBR). “While we have made the decision to reallocate our resources to support other programs, we look forward to tracking Homology’s continued progress on this technology.”
The companies are co-authors on an upcoming scientific abstract that highlights the results of studies which showed that human hematopoietic stem cell-derived adeno-associated virus vectors (AAVHSCs) transduced relevant cell types after sub-retinal injection, supporting a nuclease-independent approach to editing across two targets. In addition, the data showed AAVHSCs crossed the blood-brain and blood-retinal barriers in non-human primates following I.V. administration.
In 2017, Homology granted Novartis worldwide exclusive rights to the Company’s proprietary AAVHSCs using its nuclease-free gene editing platform for certain ophthalmic targets, which are now fully owned by Homology.
About Homology Medicines, Inc.
Homology Medicines, Inc. is a clinical-stage genetic medicines company dedicated to transforming the lives of patients suffering from rare genetic diseases with significant unmet medical needs by curing the underlying cause of the disease. Homology’s proprietary platform is designed to utilize its human hematopoietic stem cell-derived adeno-associated virus vectors (AAVHSCs) to precisely and efficiently deliver genetic medicines in vivo either through a gene therapy or nuclease-free gene editing modality across a broad range of genetic disorders. Homology has a management team with a successful track record of discovering, developing and commercializing therapeutics with a particular focus on rare diseases, and intellectual property covering its family of 15 AAVHSCs. Homology believes that its compelling preclinical data, scientific expertise, product development strategy, manufacturing capabilities and intellectual property position it as a leader in the development of genetic medicines. For more information, please visit www.homologymedicines.com .
das wird nett.
LG
Sven
BEDFORD, Mass., March 01, 2021 (GLOBE NEWSWIRE) -- Homology Medicines, Inc. (Nasdaq: FIXX), a clinical-stage genetic medicines company, announced today it has regained worldwide exclusive rights from Novartis to research, develop, manufacture and commercialize Homology’s proprietary nuclease-free gene editing technology platform for an ophthalmic target. Following a portfolio review, Novartis decided to conclude the collaboration and licensing agreement with Homology to pursue other opportunities in their pipeline.
“Our nuclease-free gene editing platform is applicable across a broad range of genetic disorders, including ophthalmic diseases where we have generated positive in vivo data in two different eye targets,” said Arthur Tzianabos, Ph.D., President and Chief Executive Officer of Homology Medicines. “Our work with Novartis has been productive in demonstrating that our AAVHSC vectors can edit the back of the eye, and we plan to share additional data from the ophthalmology program at a scientific meeting in May. The data are promising and support advancing this program, which we now intend to do on our own as we drive toward naming a development candidate.”
“Our collaboration with Homology has generated data that support gene editing in retinal cells in a rare ophthalmic disease, providing early proof-of-principle for further research using this approach.” said Jay Bradner, M.D., President of the Novartis Institutes for BioMedical Research (NIBR). “While we have made the decision to reallocate our resources to support other programs, we look forward to tracking Homology’s continued progress on this technology.”
The companies are co-authors on an upcoming scientific abstract that highlights the results of studies which showed that human hematopoietic stem cell-derived adeno-associated virus vectors (AAVHSCs) transduced relevant cell types after sub-retinal injection, supporting a nuclease-independent approach to editing across two targets. In addition, the data showed AAVHSCs crossed the blood-brain and blood-retinal barriers in non-human primates following I.V. administration.
In 2017, Homology granted Novartis worldwide exclusive rights to the Company’s proprietary AAVHSCs using its nuclease-free gene editing platform for certain ophthalmic targets, which are now fully owned by Homology.
About Homology Medicines, Inc.
Homology Medicines, Inc. is a clinical-stage genetic medicines company dedicated to transforming the lives of patients suffering from rare genetic diseases with significant unmet medical needs by curing the underlying cause of the disease. Homology’s proprietary platform is designed to utilize its human hematopoietic stem cell-derived adeno-associated virus vectors (AAVHSCs) to precisely and efficiently deliver genetic medicines in vivo either through a gene therapy or nuclease-free gene editing modality across a broad range of genetic disorders. Homology has a management team with a successful track record of discovering, developing and commercializing therapeutics with a particular focus on rare diseases, and intellectual property covering its family of 15 AAVHSCs. Homology believes that its compelling preclinical data, scientific expertise, product development strategy, manufacturing capabilities and intellectual property position it as a leader in the development of genetic medicines. For more information, please visit www.homologymedicines.com .
das wird nett.
LG
Sven
Hallo zusammen,
nach dem Verkauf von Trillium kann es jetzt beim Buyout von FIXX durch Pfizer auch ganz schnell gehen.. Identische Voraussetzungen, allerdings hat Pfizer im Rahmen der Growth Innitiative noch mehr investiert.. Bei TRILL 25 Mio USD, bei FIXX waren es 60 Mio USD...
Noch einmal die Meldung:
https://investors.pfizer.com/investor-news/press-release-det…
LG
Sven
nach dem Verkauf von Trillium kann es jetzt beim Buyout von FIXX durch Pfizer auch ganz schnell gehen.. Identische Voraussetzungen, allerdings hat Pfizer im Rahmen der Growth Innitiative noch mehr investiert.. Bei TRILL 25 Mio USD, bei FIXX waren es 60 Mio USD...
Noch einmal die Meldung:
https://investors.pfizer.com/investor-news/press-release-det…
LG
Sven
nur kurz die Info.. nachbörslich fast 10% im Plus.. Volumen hoch.. da wird ordentlich gekauft... Pfizer ist hier ja bereits mit 60 MIO USD drin... Spannung....
Homology Medicines Announces World’s First Gene Editing Clinical Trial for PKU
Guten Morgen,das sieht immer besser aus.. Im Advirory Board ein Pfizer Director....
Das wird aus meiner Sicht ein klarer Buyout...
Schöner, aktueller Artikel:
Quelle:
https://www.globenewswire.com/news-release/2021/10/12/231296…
- IND Clearance of pheEDIT Study to Evaluate a One-Time Dose of Investigational HMI-103 Incorporating a Novel Nuclease-Free Gene Editing Approach -
- Company Provides Update on Enrollment in Ongoing pheNIX Trial of HMI-102 Gene Therapy for Adults with PKU -
BEDFORD, Mass., Oct. 12, 2021 (GLOBE NEWSWIRE) -- Homology Medicines, Inc. (Nasdaq: FIXX), a genetic medicines company, announced today the pheEDIT Phase 1 clinical trial for HMI-103, a one-time, in vivo product candidate that utilizes a gene editing approach for phenylketonuria (PKU), based on the Investigational New Drug Application (IND) clearance from the U.S. Food and Drug Administration (FDA). HMI-103 will be the world’s first gene editing candidate for PKU to enter clinical trials from Homology’s dual gene therapy and gene editing technology platform, and with the launch of pheEDIT Homology moves closer to its goal of offering solutions for both adults and pediatric patients with PKU.
“Today’s milestone is the culmination of our team’s tireless work to translate our gene editing technology from an academic discovery into a clinical program for people with PKU,” said Albert Seymour, Ph.D., Chief Scientific Officer of Homology Medicines. “Our positive preclinical data in the PKU model demonstrated phenotypic correction, and the precision of HMI-103 genome integration was confirmed in a humanized liver model, which showed no evidence of off-target mutations or unwanted on-target changes to the genome. These nonclinical data give us great confidence in initiating our Phase 1 pheEDIT trial, and we look forward to continuing to work with the PKU community on our clinical programs.”
The HMI-103 pheEDIT trial is expected to enroll up to nine patients ages 18-55 years old who have been diagnosed with PKU due to PAH deficiency. Once positive safety and efficacy results are established in the adult population, Homology plans to enroll younger patients in clinical trials. The Phase 1 dose-escalation trial is designed to evaluate three doses of HMI-103 to determine the recommended dose(s) for a future trial. In addition to safety endpoints, the trial will measure serum phenylalanine (Phe) changes.
HMI-103 is designed as a one-time administration to maximize the expression of functional phenylalanine hydroxylase (PAH) in liver cells and thus restore the natural biochemical pathway that metabolizes Phe. The product candidate was developed to specifically integrate a functional PAH gene into the genome using the natural DNA repair process of homologous recombination. In addition to expression, the integration is designed to correct the cell by inactivating at least one of the mutated genes and enable that correction to persist through cell division.
Homology expects that the first patient in the pheEDIT clinical trial will be dosed following requisite Institutional Biosafety Committee and Institutional Review Board approvals at the clinical sites. The trial will include an 82-day screening/run-in period prior to HMI-103 administration.
Homology also announced today an update from its ongoing Phase 2 pheNIX clinical trial evaluating HMI-102 gene therapy in adults with PKU. As of September 30, 2021, both doses in the trial have been generally well-tolerated and have shown evidence of biological activity, including clinically meaningful reductions in Phe levels, increases in Tyr and reductions in the Phe-to-Tyr ratio. Several new clinical trials sites have also recently been added to pheNIX for a total of 13 with more sites expected shortly. Despite increased interest, enrollment is slower than anticipated due in part to COVID-19 resurgence, and the Company anticipates providing a more detailed pheNIX update in mid-2022 when it expects to have a larger dataset.
“We continue to be encouraged by the data from our pheNIX trial and to hasten enrollment, we have expanded our Medical Affairs, Clinical Development and Operations teams to support not only pheNIX, but now pheEDIT and our Hunter syndrome gene therapy trial expected this year,” said Arthur Tzianabos, Ph.D., President and Chief Executive Officer of Homology Medicines. “The flexibility of our technology platform enables us to develop gene therapy and gene editing product candidates as potential one-time treatments, and news today of our first trial that incorporates gene editing for PKU demonstrates our ability to employ both approaches in an effort to support the PKU community.”
PKU is caused by mutations in the PAH gene, which is responsible for producing the enzyme that metabolizes Phe from dietary protein. As a result, Phe accumulates to toxic levels in the blood and brain and does not convert to melanin or the amino acid tyrosine (Tyr), a precursor to neurotransmitters. Homology’s approach to PKU with gene therapy and gene editing candidates is designed to treat both the adult and pediatric communities with one-time treatments that address the genetic cause of PKU. Since gene therapy does not integrate into the genome, it can be used in cells that are not rapidly dividing, such as an adult liver. Gene editing is designed to make a permanent correction in cells, including those that are rapidly dividing, such as a child’s liver.
About HMI-102 Gene Therapy and HMI-103 Gene Editing Product Candidates
HMI-102 is an investigational gene therapy in clinical development for the treatment of phenylketonuria (PKU) in adults. HMI-102 is designed to encode the PAH gene, which is mutated in people with PKU, and delivered via the liver-tropic AAVHSC15 vector. Homology has received Fast Track Designation and orphan drug designation for HMI-102 from the U.S. Food and Drug Administration (FDA), and orphan drug designation from the European Medicines Agency (EMA).
HMI-103 is an investigational, nuclease-free gene editing product candidate ultimately designed to treat pediatric patients with PKU, whose livers are rapidly dividing, following initial clinical trials in adults. HMI-103 also uses AAVHSC15 and is designed to encode the PAH gene flanked by homology arms, or long stretches of DNA, to target the PAH region of the genome. Using the body’s natural DNA repair process of homologous recombination, the PAH gene integrates into the genome. HMI-103 is designed to express phenylalanine hydroxylase (PAH) and integrate into the PAH gene to restore the natural biochemical pathway that metabolizes phenylalanine (Phe).
About Phenylketonuria (PKU)
PKU is a rare inborn error of metabolism caused by a mutation in the PAH gene. PKU results in a loss of function of the enzyme phenylalanine hydroxylase (PAH), which is responsible for the metabolism of phenylalanine (Phe), an amino acid obtained exclusively from the diet. If left untreated, toxic levels of Phe can accumulate in the blood and result in progressive and severe neurological impairment. Currently, there are no treatment options for PKU that target the underlying genetic cause of the disease. According to the National PKU Alliance, PKU affects nearly 16,500 people in the U.S. with approximately 350 newborns diagnosed each year. The worldwide prevalence of PKU is estimated to be 50,000 people.
Quelle: https://www.globenewswire.com/news-release/2021/10/12/231296…
Neue Präsentation online
https://investors.homologymedicines.com/static-files/79945b6…Sehr interessant.. Pfizer als Sahrholder wird meiner Meinung bald noch Weiteres bekanntgeben :-)
jetzt auch gegen das Hunter-Virus
Homology Medicines Initiates Clinical Trial for HMI-203, a One-Time Investigational Gene Therapy Candidate for Adults with MPS II (Hunter Syndrome)- juMPStart Trial to Evaluate First Systemic Gene Therapy Candidate for Patients with Hunter Syndrome -
https://newsfilter.io/a/55648cbbadc0f3e4b4f3bd6157fedde9
Da bin ich sicher, dass die ersten Tests bereits gut verlaufen sind und Pfizer schon entsprechend mehr weiss...
Vielleicht wird so dann der BO und der Premiumzuschlag gerechtfertigt...
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +0,79 | |
| -0,90 | |
| -2,56 | |
| -0,37 | |
| -1,48 | |
| +1,08 | |
| -0,10 | |
| -0,40 | |
| +1,46 | |
| 0,00 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 220 | ||
| 112 | ||
| 108 | ||
| 76 | ||
| 44 | ||
| 43 | ||
| 39 | ||
| 35 | ||
| 33 | ||
| 32 |





















