ThermoDox (Leberkrebs) in PIII --Verdammt Billig !!!!! - 500 Beiträge pro Seite
eröffnet am 19.02.10 20:29:18 von
neuester Beitrag 12.02.13 12:27:28 von
neuester Beitrag 12.02.13 12:27:28 von
Beiträge: 113
ID: 1.156.076
ID: 1.156.076
Aufrufe heute: 1
Gesamt: 13.034
Gesamt: 13.034
Aktive User: 0
Top-Diskussionen
| Titel | letzter Beitrag | Aufrufe |
|---|---|---|
| vor 53 Minuten | 5416 | |
| heute 18:05 | 4214 | |
| vor 59 Minuten | 3560 | |
| heute 18:00 | 3048 | |
| vor 53 Minuten | 2186 | |
| heute 18:11 | 1845 | |
| heute 18:26 | 1557 | |
| vor 1 Stunde | 1402 |
Meistdiskutierte Wertpapiere
| Platz | vorher | Wertpapier | Kurs | Perf. % | Anzahl | ||
|---|---|---|---|---|---|---|---|
| 1. | 1. | 17.704,62 | -0,27 | 191 | |||
| 2. | 2. | 148,30 | -1,09 | 98 | |||
| 3. | 7. | 6,6600 | -1,01 | 71 | |||
| 4. | 8. | 3,7750 | +0,94 | 67 | |||
| 5. | 5. | 0,1795 | -2,71 | 67 | |||
| 6. | 17. | 7,3450 | +0,55 | 47 | |||
| 7. | 4. | 2.394,52 | +0,64 | 42 | |||
| 8. | Neu! | 734,47 | -20,90 | 33 |
Hab eben ein paar Cipher-Aktien verkauft und bin in diese kaum bekannte Perle eingestiegen .
In den nächsten tagen werde ich mehr über Celsion schreiben .
Celsion (CLSN)
Marktkap: 35 Mio$
Cash: 14,1 Mio$
Kurs: 2,90$
Shares Out: 12,1 Million
Insider
http://finance.yahoo.com/q/it?s=CLSN
Data Monitoring Committee Recommends Continuation of Celsion's Phase III ThermoDox(R) Study for Primary Liver Cancer
http://www.celsion.com/releasedetail.cfm?ReleaseID=444013
News
http://www.celsion.com/releases.cfm
Pipeline

In den nächsten tagen werde ich mehr über Celsion schreiben .
Celsion (CLSN)
Marktkap: 35 Mio$
Cash: 14,1 Mio$
Kurs: 2,90$
Shares Out: 12,1 Million
Insider
http://finance.yahoo.com/q/it?s=CLSN
Data Monitoring Committee Recommends Continuation of Celsion's Phase III ThermoDox(R) Study for Primary Liver Cancer
http://www.celsion.com/releasedetail.cfm?ReleaseID=444013
News
http://www.celsion.com/releases.cfm
Pipeline

Hmm..ok. Verfolgungswürdig.
Chart hatte ich noch vergessen ..Nun aber schönes Wochenende 

Ich denke hier sind 200-300% bis Jahresende möglich ...
In diesem Brief findet man viele Infos
http://www.celsion.com/letter.cfm
August 17, 2009
Dear Celsion Shareholders,
Celsion is pleased to report an exciting development in the second quarter of 2009; the expansion of our Phase III liver cancer clinical trial to include sites in Japan. This development comes as a result of an innovative partnership with our Japanese partner, Yakult Honsha. It represents what we believe is an unprecedented agreement with Japan's Regulatory Authority, the Pharmaceutical and Medical Devices Agency (PMDA), for the support of our trial without the typical clinical bridging studies normally required for Japanese registrational studies. This arrangement has the potential to bring ThermoDox®, Celsion's lead clinical candidate, to market two years earlier than would otherwise be expected in Japan. Japan has the highest incidence of primary liver cancer in the industrialized world. We believe this early market entry would translate into $30 million in added royalties and provide a cost savings of between $5 and $7 million for Celsion, as our agreement with Yakult provides that they fund all costs associated with studies in Japan.
I want to thank our dedicated employees at Celsion for helping to bring about this extraordinary agreement. I can find no similar example where the PMDA has allowed a global registrational study to include a Japanese cohort, the data from which can be immediately used to file for drug approval in Japan. This accomplishment is one of many that our team has achieved as we've successfully transformed Celsion from a device to an oncology focused drug development company during the past two years. The effort and commitment of our employees is consistent with the promise of ThermoDox and our platform technology to provide a significant treatment option for cancer patients and the medical community that supports them.
The expansion of our Phase III liver cancer trial, which now has 196 patients enrolled and 158 randomized, is just one of our many accomplishments this quarter. Celsion has also made significant progress in our pivotal Phase I/II Recurrent Chest Wall cancer study and our strategic initiative to study the potential of ThermoDox in combination with High Intensity Focused Ultrasound, or HIFU, to treat difficult cancers.
2nd Quarter Financials
Celsion is in an aggressive research and development phase. The resources required to bring about such important progress, such as the accelerated enrollment in our primary liver cancer clinical trial, has resulted in higher costs for the Company this quarter.
Our net loss for the second quarter ended June 30, 2009 was $4.6 million or $.45 per diluted share as compared to a net loss of $2.4 million or $.24 per diluted share for the second quarter of 2008. For the six months ended June 30, 2009, the Company's net loss was $8.2 million or $.81 per diluted share as compared to a net loss of $6.5 million or $.64 per diluted share for the first six months of 2008.
Total Operating Expenses for the second quarter of 2009 increased by $ 2.6 million over the prior year's second quarter, primarily due to an increase in Research and Development costs for the primary liver cancer clinical trial. Celsion has structured its clinical trial contracts with Contract Research Organizations (CROs) and other vendors such that levels of payments are directly tied to the progress made in patient enrollment. Thus, the acceleration in patient enrollment in the primary liver cancer study during the second quarter resulted in higher costs versus 2008.
Turning to the Balance Sheet, in June 2009, the Company received the final $15 million payment from Boston Scientific for total payments of $60 million from the sale of the Prolieve. The Company ended the quarter with $14.9 million in cash and investments.
Global Phase III Liver Cancer Study
We recently transitioned our Global Phase III Liver Cancer study to a new CRO. Under the terms of our engagement, we are aggressively adding some 30 sites to our trial over the next three months in three new countries: Malaysia, Thailand and the Philippines. We employed this new strategy to offset any delays we might see with our Clinical Trial Authorization (CTA) in China. Under the new agreement, we agreed to a milestone-based payment schedule and agreed to definitive dates for study enrollment. We designed the agreement so that our CRO would incur a financial penalty if the enrollment dates and targets are not met.
Our CTA in China is on track with our most recent projections. We expect to be enrolling patients in our first sites in China this year, assuming we receive a positive response to our submission from the State Food and Drug Administration (SFDA). China's Center for Drug Evaluation (CDE) reports that it has completed its review of our submission. Typically, a sponsor can expect a formal response from the SFDA within four to six weeks following CDE's review. We are expecting to receive a positive response within this time frame. We are seeking approximately 150 patients from 15 sites in China over a five to six month period. This would mean enrolling two to three patients to each site per month, which we believe is achievable.
We also have CTAs in the U.S., Hong Kong, Korea, Taiwan, Canada and Italy. We have 29 sites up and screening patients, which is on track with our projections. Korea and Taiwan are meeting or exceeding our expectations and Italy has all five of its sites up and began enrolling patients in February, proving to be quite productive.
Overall, patient accrual through July is ahead of our estimates. We currently have enrolled 196 patients in the trial. Of those, 158 have been randomized, two qualify and will be treated and 33 are not eligible.
With our new sites, the commitment of our new CRO and our recent accrual momentum, we continue to believe that we have the funds to complete enrollment of the trial. We believe we can achieve this within the first quarter of 2010. We may choose to extend the enrollment period, however, to accommodate a minimum number of patients required by Japan's Pharmaceuticals Medical Devices Agency for Japanese New Drug Application (NDA) submission. If this happens, we would anticipate it taking no longer than two to three months. This projection is reflected in our latest expense forecast and our management objectives.
I would like again to stress how excited we are to open up Japan to our liver cancer study. It has not been easy. It has required extraordinary coordination, meeting difficult time lines and the ability to find common ground to address issues unique to PMDA interests. But it has been well worth it.
We believe this is a precedent-setting arrangement that will, if supported by data, allow Yakult to file a NDA concurrently with the U.S. filing and to bring ThermoDox to market approximately two years sooner than the usual pathway would allow. We believe it will provide Celsion with milestone and royalty payments two years sooner and that it will extend the effective period of Japanese market exclusivity for Celsion and Yakult by two years.
We expect that the incremental revenue value of this earlier market introduction is conservatively in excess of $30 million in royalties. In addition, we could realize an $18 million approval milestone payment in 2012 rather than waiting until 2014; and because Yakult will fund the Japanese portion, Celsion will realize the immediate avoidance of between $5 and $7 million in Phase III clinical trial costs, depending upon the number of Japanese patients enrolled.
This innovative collaborative effort is a win-win-win any way you look at it. It further endorses the confidence we've seen from the FDA's collaborative approach to our study. It brings Celsion an earlier revenue stream from potentially the world's largest market for ThermoDox. It provides Yakult an earlier market entry and effectively longer exclusivity. However, the real winner will be the Japanese liver cancer patients who potentially will have accelerated access to the promise of ThermoDox.
Pivotal Phase I/II Recurrent Chest Wall Cancer Study
Our pivotal Phase I/II Recurrent Chest Wall Cancer (RCW) study is enrolling patients at four sites: New York University, St. Barnabus Medical Center in N.J., Rhode Island Hospital and Florida Cancer Institute. Duke University and Virginia Commonwealth University will be active this month. Along with Cancer Treatment Centers of America in Oklahoma, which will join the study at the 50 mg dosing, we will have initiated seven of 10 sites in August.
ThermoDox has shown remarkable evidence of clinical activity in treating this disease and we initiated our Phase I/II trial based largely on the FDA's written support that this study would be considered pivotal, pending a robust objective complete response rate, which is our primary endpoint. The data from our Phase 1 study at Duke show that ThermoDox, in combination with hyperthermia, has the potential to be an effective treatment option for patients with this devastating disease. To date, 100 percent of evaluable patients have shown evidence of clinical activity.
Across all dosing cohorts, the response rates reported through 2009 include six patients with stable disease, five patients with partial responses and two patients with a complete response. Two more patients have been added to Duke's study. We have yet to get the clinical data, but we do know that the patients have rounded out the study's 40 mg cohort.
Our Phase I/II study is designed to start at 40mg. If this dosing is supported by safety data from three to six patients, the dose will escalate to 50mg. Assuming no dose limiting toxicity (DLT) is found, 50mg will be declared the maximum tolerated dose (MTD) for the remainder of the study and will continue until 100 patients are enrolled. This is an open label study. Patients will be followed for safety, but a survival benefit will not be assessed.
We expect to have the information we need to report Phase I results by October or November this year and to have a first data set for Phase II early next year. Although our early experience suggests recruiting patients can be challenging, I want to stress that we have a clear plan to reach patients directly, to broadly publicize the study through traditional and electronic media, to engage support groups, and to establish awareness among the breast cancer medical community.
HIFU and ThermoDox
Besides its potential for treating liver cancer and recurrent chest wall cancer, we believe ThermoDox can be used in combination with high intensity focused ultrasound to treat two very important indications: metastatic bone cancer and pancreatic cancer.
We signed a joint research agreement last October with Royal Philips Electronics, the premier manufacturer of investigational magnetic resonance imaging (MRI)-guided high HIFU systems. Celsion is using this technology in combination with ThermoDox to deliver high concentrations of doxorubicin - the potent anticancer agent in ThermoDox - directly to tumor sites. By using Philips' HIFU technology to deliver targeted, localized activation temperatures, Philips' and Celsion's research will explore the potential for ThermoDox to non-invasively treat a number of solid tumor cancers that may be susceptible to the combination of a high concentration of doxorubicin and concurrent hyperthermia.
Our agreement with Philips provides that the funding for these studies will be principally borne by Philips under their existing research and development program. Celsion has amended its existing Cooperative Research and Development Agreement (CRADA) with the National Cancer Institute (NCI) to incorporate experiments that may be of particular interest to Celsion. Our immediate objective is to have preclinical work completed by year end, sufficient to support a pre-IND meeting with FDA.
This is novel research that is going to take some time to explore fully. Our early work with input from our medical consultants and the NCI indicates that ThermoDox with HIFU could someday be used to treat the cancer lesions and alleviate the pain of bone metastases, which affect more than 300,000 patients in the U.S. annually. We also believe it can be used in the treatment of pancreatic cancer, an indication with an extremely high mortality rate, affecting more than 37,000 people in the U.S. each year.
Our work on this research is progressing well. We have completed the Theoretical Thermal Modeling that establishes the optimum tissue heating algorithms. We've created thermal tissue temperature profiles and maps and we've compared different heating algorithms and drug administration regimes in an effort to understand their effect on drug concentration in tissue. Our phantom modeling is underway. Our phantom studies are designed to optimize HIFU power levels, heating trajectories and durations of sonications that keep heated areas at target temperature while avoiding near and far field heating. We expect to be analyzing mouse muscle tissue used in HIFU plus ThermoDox experiments in August and our protocols for experiments with rabbits are complete.
We have identified and are working with three sites on these studies. They include the NCI, Sunnybrook Health Sciences Centre, Toronto (for small animal bone & muscle studies) and Université de Bordeaux (for large animal studies). Additionally we have sponsored academic research at University of Oxford under the direction of Dr. Coussios, who will be presenting his work and data at the International Symposium Therapeutic Ultrasound.
Milestones
We have high expectations for this year. We continue to believe that we will have a CTA in China; we expect to complete the Phase I portion of our RCW trial; we expect to enroll more than 300 patients in our Phase III trial; and, lastly, we continue to believe that a second license agreement will be signed this year.
We are on target with our plans and have committed ourselves to delivering on milestones that reduce our development risk. We have substantially funded our ThermoDox clinical program, and provided a clear regulatory pathway. We have stabilized and focused Celsion, and built a strong clinical capability.
In closing, the second quarter of 2009 was extremely productive for Celsion. We are making significant progress against our clinical program milestones, expanding our platform through key alliances, all the while being careful to manage our expenses and cost.
Our fundamentals are solid and we will continue to work hard to deliver and build shareholder confidence and value. Enhancing the valuation of the Company is my primary responsibility, and one that I take seriously.
Sincerely,
Michael H. Tardugno
President and CEO
In diesem Brief findet man viele Infos
http://www.celsion.com/letter.cfm
August 17, 2009
Dear Celsion Shareholders,
Celsion is pleased to report an exciting development in the second quarter of 2009; the expansion of our Phase III liver cancer clinical trial to include sites in Japan. This development comes as a result of an innovative partnership with our Japanese partner, Yakult Honsha. It represents what we believe is an unprecedented agreement with Japan's Regulatory Authority, the Pharmaceutical and Medical Devices Agency (PMDA), for the support of our trial without the typical clinical bridging studies normally required for Japanese registrational studies. This arrangement has the potential to bring ThermoDox®, Celsion's lead clinical candidate, to market two years earlier than would otherwise be expected in Japan. Japan has the highest incidence of primary liver cancer in the industrialized world. We believe this early market entry would translate into $30 million in added royalties and provide a cost savings of between $5 and $7 million for Celsion, as our agreement with Yakult provides that they fund all costs associated with studies in Japan.
I want to thank our dedicated employees at Celsion for helping to bring about this extraordinary agreement. I can find no similar example where the PMDA has allowed a global registrational study to include a Japanese cohort, the data from which can be immediately used to file for drug approval in Japan. This accomplishment is one of many that our team has achieved as we've successfully transformed Celsion from a device to an oncology focused drug development company during the past two years. The effort and commitment of our employees is consistent with the promise of ThermoDox and our platform technology to provide a significant treatment option for cancer patients and the medical community that supports them.
The expansion of our Phase III liver cancer trial, which now has 196 patients enrolled and 158 randomized, is just one of our many accomplishments this quarter. Celsion has also made significant progress in our pivotal Phase I/II Recurrent Chest Wall cancer study and our strategic initiative to study the potential of ThermoDox in combination with High Intensity Focused Ultrasound, or HIFU, to treat difficult cancers.
2nd Quarter Financials
Celsion is in an aggressive research and development phase. The resources required to bring about such important progress, such as the accelerated enrollment in our primary liver cancer clinical trial, has resulted in higher costs for the Company this quarter.
Our net loss for the second quarter ended June 30, 2009 was $4.6 million or $.45 per diluted share as compared to a net loss of $2.4 million or $.24 per diluted share for the second quarter of 2008. For the six months ended June 30, 2009, the Company's net loss was $8.2 million or $.81 per diluted share as compared to a net loss of $6.5 million or $.64 per diluted share for the first six months of 2008.
Total Operating Expenses for the second quarter of 2009 increased by $ 2.6 million over the prior year's second quarter, primarily due to an increase in Research and Development costs for the primary liver cancer clinical trial. Celsion has structured its clinical trial contracts with Contract Research Organizations (CROs) and other vendors such that levels of payments are directly tied to the progress made in patient enrollment. Thus, the acceleration in patient enrollment in the primary liver cancer study during the second quarter resulted in higher costs versus 2008.
Turning to the Balance Sheet, in June 2009, the Company received the final $15 million payment from Boston Scientific for total payments of $60 million from the sale of the Prolieve. The Company ended the quarter with $14.9 million in cash and investments.
Global Phase III Liver Cancer Study
We recently transitioned our Global Phase III Liver Cancer study to a new CRO. Under the terms of our engagement, we are aggressively adding some 30 sites to our trial over the next three months in three new countries: Malaysia, Thailand and the Philippines. We employed this new strategy to offset any delays we might see with our Clinical Trial Authorization (CTA) in China. Under the new agreement, we agreed to a milestone-based payment schedule and agreed to definitive dates for study enrollment. We designed the agreement so that our CRO would incur a financial penalty if the enrollment dates and targets are not met.
Our CTA in China is on track with our most recent projections. We expect to be enrolling patients in our first sites in China this year, assuming we receive a positive response to our submission from the State Food and Drug Administration (SFDA). China's Center for Drug Evaluation (CDE) reports that it has completed its review of our submission. Typically, a sponsor can expect a formal response from the SFDA within four to six weeks following CDE's review. We are expecting to receive a positive response within this time frame. We are seeking approximately 150 patients from 15 sites in China over a five to six month period. This would mean enrolling two to three patients to each site per month, which we believe is achievable.
We also have CTAs in the U.S., Hong Kong, Korea, Taiwan, Canada and Italy. We have 29 sites up and screening patients, which is on track with our projections. Korea and Taiwan are meeting or exceeding our expectations and Italy has all five of its sites up and began enrolling patients in February, proving to be quite productive.
Overall, patient accrual through July is ahead of our estimates. We currently have enrolled 196 patients in the trial. Of those, 158 have been randomized, two qualify and will be treated and 33 are not eligible.
With our new sites, the commitment of our new CRO and our recent accrual momentum, we continue to believe that we have the funds to complete enrollment of the trial. We believe we can achieve this within the first quarter of 2010. We may choose to extend the enrollment period, however, to accommodate a minimum number of patients required by Japan's Pharmaceuticals Medical Devices Agency for Japanese New Drug Application (NDA) submission. If this happens, we would anticipate it taking no longer than two to three months. This projection is reflected in our latest expense forecast and our management objectives.
I would like again to stress how excited we are to open up Japan to our liver cancer study. It has not been easy. It has required extraordinary coordination, meeting difficult time lines and the ability to find common ground to address issues unique to PMDA interests. But it has been well worth it.
We believe this is a precedent-setting arrangement that will, if supported by data, allow Yakult to file a NDA concurrently with the U.S. filing and to bring ThermoDox to market approximately two years sooner than the usual pathway would allow. We believe it will provide Celsion with milestone and royalty payments two years sooner and that it will extend the effective period of Japanese market exclusivity for Celsion and Yakult by two years.
We expect that the incremental revenue value of this earlier market introduction is conservatively in excess of $30 million in royalties. In addition, we could realize an $18 million approval milestone payment in 2012 rather than waiting until 2014; and because Yakult will fund the Japanese portion, Celsion will realize the immediate avoidance of between $5 and $7 million in Phase III clinical trial costs, depending upon the number of Japanese patients enrolled.
This innovative collaborative effort is a win-win-win any way you look at it. It further endorses the confidence we've seen from the FDA's collaborative approach to our study. It brings Celsion an earlier revenue stream from potentially the world's largest market for ThermoDox. It provides Yakult an earlier market entry and effectively longer exclusivity. However, the real winner will be the Japanese liver cancer patients who potentially will have accelerated access to the promise of ThermoDox.
Pivotal Phase I/II Recurrent Chest Wall Cancer Study
Our pivotal Phase I/II Recurrent Chest Wall Cancer (RCW) study is enrolling patients at four sites: New York University, St. Barnabus Medical Center in N.J., Rhode Island Hospital and Florida Cancer Institute. Duke University and Virginia Commonwealth University will be active this month. Along with Cancer Treatment Centers of America in Oklahoma, which will join the study at the 50 mg dosing, we will have initiated seven of 10 sites in August.
ThermoDox has shown remarkable evidence of clinical activity in treating this disease and we initiated our Phase I/II trial based largely on the FDA's written support that this study would be considered pivotal, pending a robust objective complete response rate, which is our primary endpoint. The data from our Phase 1 study at Duke show that ThermoDox, in combination with hyperthermia, has the potential to be an effective treatment option for patients with this devastating disease. To date, 100 percent of evaluable patients have shown evidence of clinical activity.
Across all dosing cohorts, the response rates reported through 2009 include six patients with stable disease, five patients with partial responses and two patients with a complete response. Two more patients have been added to Duke's study. We have yet to get the clinical data, but we do know that the patients have rounded out the study's 40 mg cohort.
Our Phase I/II study is designed to start at 40mg. If this dosing is supported by safety data from three to six patients, the dose will escalate to 50mg. Assuming no dose limiting toxicity (DLT) is found, 50mg will be declared the maximum tolerated dose (MTD) for the remainder of the study and will continue until 100 patients are enrolled. This is an open label study. Patients will be followed for safety, but a survival benefit will not be assessed.
We expect to have the information we need to report Phase I results by October or November this year and to have a first data set for Phase II early next year. Although our early experience suggests recruiting patients can be challenging, I want to stress that we have a clear plan to reach patients directly, to broadly publicize the study through traditional and electronic media, to engage support groups, and to establish awareness among the breast cancer medical community.
HIFU and ThermoDox
Besides its potential for treating liver cancer and recurrent chest wall cancer, we believe ThermoDox can be used in combination with high intensity focused ultrasound to treat two very important indications: metastatic bone cancer and pancreatic cancer.
We signed a joint research agreement last October with Royal Philips Electronics, the premier manufacturer of investigational magnetic resonance imaging (MRI)-guided high HIFU systems. Celsion is using this technology in combination with ThermoDox to deliver high concentrations of doxorubicin - the potent anticancer agent in ThermoDox - directly to tumor sites. By using Philips' HIFU technology to deliver targeted, localized activation temperatures, Philips' and Celsion's research will explore the potential for ThermoDox to non-invasively treat a number of solid tumor cancers that may be susceptible to the combination of a high concentration of doxorubicin and concurrent hyperthermia.
Our agreement with Philips provides that the funding for these studies will be principally borne by Philips under their existing research and development program. Celsion has amended its existing Cooperative Research and Development Agreement (CRADA) with the National Cancer Institute (NCI) to incorporate experiments that may be of particular interest to Celsion. Our immediate objective is to have preclinical work completed by year end, sufficient to support a pre-IND meeting with FDA.
This is novel research that is going to take some time to explore fully. Our early work with input from our medical consultants and the NCI indicates that ThermoDox with HIFU could someday be used to treat the cancer lesions and alleviate the pain of bone metastases, which affect more than 300,000 patients in the U.S. annually. We also believe it can be used in the treatment of pancreatic cancer, an indication with an extremely high mortality rate, affecting more than 37,000 people in the U.S. each year.
Our work on this research is progressing well. We have completed the Theoretical Thermal Modeling that establishes the optimum tissue heating algorithms. We've created thermal tissue temperature profiles and maps and we've compared different heating algorithms and drug administration regimes in an effort to understand their effect on drug concentration in tissue. Our phantom modeling is underway. Our phantom studies are designed to optimize HIFU power levels, heating trajectories and durations of sonications that keep heated areas at target temperature while avoiding near and far field heating. We expect to be analyzing mouse muscle tissue used in HIFU plus ThermoDox experiments in August and our protocols for experiments with rabbits are complete.
We have identified and are working with three sites on these studies. They include the NCI, Sunnybrook Health Sciences Centre, Toronto (for small animal bone & muscle studies) and Université de Bordeaux (for large animal studies). Additionally we have sponsored academic research at University of Oxford under the direction of Dr. Coussios, who will be presenting his work and data at the International Symposium Therapeutic Ultrasound.
Milestones
We have high expectations for this year. We continue to believe that we will have a CTA in China; we expect to complete the Phase I portion of our RCW trial; we expect to enroll more than 300 patients in our Phase III trial; and, lastly, we continue to believe that a second license agreement will be signed this year.
We are on target with our plans and have committed ourselves to delivering on milestones that reduce our development risk. We have substantially funded our ThermoDox clinical program, and provided a clear regulatory pathway. We have stabilized and focused Celsion, and built a strong clinical capability.
In closing, the second quarter of 2009 was extremely productive for Celsion. We are making significant progress against our clinical program milestones, expanding our platform through key alliances, all the while being careful to manage our expenses and cost.
Our fundamentals are solid and we will continue to work hard to deliver and build shareholder confidence and value. Enhancing the valuation of the Company is my primary responsibility, and one that I take seriously.
Sincerely,
Michael H. Tardugno
President and CEO
Nächste Woche findet eine Presentation statt das eventuell für steigende kurse sorgen könnte .
Mit zwei Final-Studien für Brust und Leberkrebs in der Pipeline ist diese Aktie mit rund 35 Mio$ Marktkap vieeeeeeel zu billig .
Celsion to Host Research and Development Day in New York City on February 24, 2010
Key Opinion Leaders to address ThermoDox(R) and RFA in Patients with Primary Liver Cancer
http://www.celsion.com/releasedetail.cfm?ReleaseID=439610
Mit Yakult hat CLSN einen sehr guten Partner für Japan (von denen ist auch das joghurt das ich gerne trinke ) .Weitere Partnerschaften sollen bald folgen .
) .Weitere Partnerschaften sollen bald folgen .
Celsion Corporation Announces Exclusive Japan License Agreement with Yakult Honsha for ThermoDox(R)
Dec 15, 2008
http://www.celsion.com/releasedetail.cfm?ReleaseID=354312
Mit zwei Final-Studien für Brust und Leberkrebs in der Pipeline ist diese Aktie mit rund 35 Mio$ Marktkap vieeeeeeel zu billig .
Celsion to Host Research and Development Day in New York City on February 24, 2010
Key Opinion Leaders to address ThermoDox(R) and RFA in Patients with Primary Liver Cancer
http://www.celsion.com/releasedetail.cfm?ReleaseID=439610
Mit Yakult hat CLSN einen sehr guten Partner für Japan (von denen ist auch das joghurt das ich gerne trinke
 ) .Weitere Partnerschaften sollen bald folgen .
) .Weitere Partnerschaften sollen bald folgen .Celsion Corporation Announces Exclusive Japan License Agreement with Yakult Honsha for ThermoDox(R)
Dec 15, 2008
http://www.celsion.com/releasedetail.cfm?ReleaseID=354312
Die Woche beginnt gleich mit guten Nachrichten ...
http://finance.yahoo.com/news/Celsions-Technology-Is-the-prn…
Celsion's Technology Is the Focus of 6.4 Million EUR 'HIFU-CHEM' Program To Study ThermoDox(R) and MRI-guided HIFU
Project Will Focus on Early Development and Investigate Treatment Options for Two Indications: Liver and Bone Metastases
Press Release Source: Celsion Corporation On Monday February 22, 2010, 7:30 am

http://finance.yahoo.com/news/Celsions-Technology-Is-the-prn…
Celsion's Technology Is the Focus of 6.4 Million EUR 'HIFU-CHEM' Program To Study ThermoDox(R) and MRI-guided HIFU
Project Will Focus on Early Development and Investigate Treatment Options for Two Indications: Liver and Bone Metastases
Press Release Source: Celsion Corporation On Monday February 22, 2010, 7:30 am
Netter Start mal sehen wo wir enden 

Volumen so stark wie seit 3 Monaten nicht mehr, Kurs über 3$ was will man mehr ....
Insider Aktivitäten ....
http://finance.yahoo.com/q/it?s=CLSN
http://www.nasdaq.com/asp/quotes_sec.asp?symbol=CLSN&selecte…
http://finance.yahoo.com/q/it?s=CLSN
http://www.nasdaq.com/asp/quotes_sec.asp?symbol=CLSN&selecte…
Die Aktie will nach oben .....

Die 200-Tages-Linie wird in angriff genommen .....

Ist wirklich eine vielversprechende Technik die Celsion da anwendet .
ThermoDox Technologie (Video)
http://play.rbn.com/play.asx?url=shareholder/shareholder/wmd…
http://www.celsion.com/techInAction.cfm
ThermoDox Technologie (Video)
http://play.rbn.com/play.asx?url=shareholder/shareholder/wmd…
http://www.celsion.com/techInAction.cfm
News ....
Weitere Indikation für ThermoDox ...
http://finance.yahoo.com/news/Celsion-Plans-to-Launch-Phase-…
Celsion Plans to Launch Phase II Program to Study ThermoDox(R) in Combination with RFA for Colorectal Liver Metastases
Celsion focuses its tumor targeting technology on cancer indication with over half million global incidence
Weitere Indikation für ThermoDox ...
http://finance.yahoo.com/news/Celsion-Plans-to-Launch-Phase-…
Celsion Plans to Launch Phase II Program to Study ThermoDox(R) in Combination with RFA for Colorectal Liver Metastases
Celsion focuses its tumor targeting technology on cancer indication with over half million global incidence
Mal sehen ob wir die Woche noch die 4$ schaffen ...

Falls sich einer mit ThermoDox näher befassen will ...
R$D Präsentation 24 Feb
http://files.shareholder.com/downloads/CLN/362172103x0x35469…
R$D Präsentation 24 Feb
http://files.shareholder.com/downloads/CLN/362172103x0x35469…
Schaut euch mal Seite 5 und 6 an dort sind die erwarteten Umsatzzahlen angegeben  wenn nur die hälfte davon eintrifft wird sich Celsion ähnlich wie HGSI entwickeln !
wenn nur die hälfte davon eintrifft wird sich Celsion ähnlich wie HGSI entwickeln !
Präsentation Jan 2010
http://files.shareholder.com/downloads/CLN/602406864x0x34615…
•
First drug, ThermoDox®, is in 2 registrational trials with near term commercialization
•
ThermoDox addresses $ billion market potential
•
Strong IP patent protection to 2018 with extension potential, Orphan Drug designation in US
•
Commercialization plans maximize shareholder value
•
US strategy is to market and sell directly
•
Ex-US strategy is through license agreements with Pharma partners
Key Events Through 2010
Enroll >250 patients in Phase 3 HCC Trial 1/10
Sign 2nd license agreement for ThermoDox Q2/3 10
600 patients in Phase 3 HCC Trial Q2 10
Interim Analysis Phase III Q4 10
1st data set analysis from RCW Phase I/II trial 12/10
 wenn nur die hälfte davon eintrifft wird sich Celsion ähnlich wie HGSI entwickeln !
wenn nur die hälfte davon eintrifft wird sich Celsion ähnlich wie HGSI entwickeln !Präsentation Jan 2010
http://files.shareholder.com/downloads/CLN/602406864x0x34615…
•
First drug, ThermoDox®, is in 2 registrational trials with near term commercialization
•
ThermoDox addresses $ billion market potential
•
Strong IP patent protection to 2018 with extension potential, Orphan Drug designation in US
•
Commercialization plans maximize shareholder value
•
US strategy is to market and sell directly
•
Ex-US strategy is through license agreements with Pharma partners
Key Events Through 2010
Enroll >250 patients in Phase 3 HCC Trial 1/10
Sign 2nd license agreement for ThermoDox Q2/3 10
600 patients in Phase 3 HCC Trial Q2 10
Interim Analysis Phase III Q4 10
1st data set analysis from RCW Phase I/II trial 12/10
Die letzten Empfehlungen für CLSN....
Griffin Securities Updates Coverage on Celsion Corp. (CLSN)
2010-03-02 14:28:35.965 GMT
We are updating coverage on Celsion Corp.(NasdaqGM: CLSN) with a BUY rating and
maintaining our 12-month price target of $10.00 for CLSN shares.
-------
Needham & co. Mark Monane reiterated his "BUY" recommendation on Feb 25, 2010 for CLSN with a $7 price target
Griffin Securities Updates Coverage on Celsion Corp. (CLSN)
2010-03-02 14:28:35.965 GMT
We are updating coverage on Celsion Corp.(NasdaqGM: CLSN) with a BUY rating and
maintaining our 12-month price target of $10.00 for CLSN shares.
-------
Needham & co. Mark Monane reiterated his "BUY" recommendation on Feb 25, 2010 for CLSN with a $7 price target
Die 4$-Marke rückt näher  ..
..
 ..
..
So muss es sein ...Fair Value sehe ich zwischen 7-8$ natürlich vor den PIII-Daten .
RAALLLLLYYYYYYY  Widerstände sind durchbrochen
Widerstände sind durchbrochen
 Widerstände sind durchbrochen
Widerstände sind durchbrochen
Ich denke heute noch wird die 4$-Marke fallen....

Da war sie die 4$ 

5$ ist wohl nächste Woche dran 

Und nochmal 

Mal sehen ob heute schon die 5$ fällt ...

Seit gestern wieder Bewegung, macht Freude, glaub, da steckt viel Potential drin

Antwort auf Beitrag Nr.: 38.977.286 von BrauchGeld am 19.02.10 20:29:18Lieber Herr Brauchgeld,
Guten Tag. Ich heisse Odaat/Celsiodaat und komme aus den Vereignigten Staaten (USA.) Bitte, verzeihen Sie mich, weil my Deutsch schrecklich ist.
Ick kann genug Deutsch, aber, zu erweisen dass Sie Celsion's Technologie nicht nur verstehen, sondern auch sehr gern haben.
Doch, auch Ich Celsion liebe, and denke ich, dass Celsion das "Cancer Answer" habe.
Auf Yahoo gebrauchen wir ein schones Mesasage Board (auf Englisch) um Celsion zu diskutieren. Ich lade Sie ein, mit uns uber Celsion zu reden.
Das Website sei: http://messages.finance.yahoo.com/mb3?s=CLSN
Alles Gute beim investieren. Noch einmail, es tut mir leid, dass mein Deustch so schlecht ist.
Celsion kaufen...biz zum nicht mehr!!!
- celsiodaat (USA)
Guten Tag. Ich heisse Odaat/Celsiodaat und komme aus den Vereignigten Staaten (USA.) Bitte, verzeihen Sie mich, weil my Deutsch schrecklich ist.
Ick kann genug Deutsch, aber, zu erweisen dass Sie Celsion's Technologie nicht nur verstehen, sondern auch sehr gern haben.
Doch, auch Ich Celsion liebe, and denke ich, dass Celsion das "Cancer Answer" habe.
Auf Yahoo gebrauchen wir ein schones Mesasage Board (auf Englisch) um Celsion zu diskutieren. Ich lade Sie ein, mit uns uber Celsion zu reden.
Das Website sei: http://messages.finance.yahoo.com/mb3?s=CLSN
Alles Gute beim investieren. Noch einmail, es tut mir leid, dass mein Deustch so schlecht ist.
Celsion kaufen...biz zum nicht mehr!!!
- celsiodaat (USA)
600 Patienten werden in der phase 3 rekrutiert.
Mit der FDA wurde ein SPA (Special Protocol Assessment) vereinbart.
90% der Patienten sind bereits aufgenommen und bald sollen erste vorläufige Daten veröffentlicht werden.
Der Markt soll größer 1 Milliarde sein.
Bin heute mit ein paar tausend Aktien eingestiegen.
Hat die Firma sonst noch jemand auf dem Radar?
wachholder
Mit der FDA wurde ein SPA (Special Protocol Assessment) vereinbart.
90% der Patienten sind bereits aufgenommen und bald sollen erste vorläufige Daten veröffentlicht werden.
Der Markt soll größer 1 Milliarde sein.
Bin heute mit ein paar tausend Aktien eingestiegen.
Hat die Firma sonst noch jemand auf dem Radar?
wachholder
Diesen Freitag (Conference Call) wird es spannend.
Die Phase III steht kurz vor dem Abschluss.
Hier zwei gute Zusammenfassungen:
http://www.gekkowire.com/?p=7671
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
w
Die Phase III steht kurz vor dem Abschluss.
Hier zwei gute Zusammenfassungen:
http://www.gekkowire.com/?p=7671
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
w
Bin seit gestern auch mit am Start. Sehe eine gute Chance auf Kursgewinne bis zur Präsentation der Zwischendaten.
@all
nach der dilution vom freitag zu 3,15 usd ist nun der weg nach oben frei. erstes ziel in meinen augen nach vermeldung des vollst. enrollments 4-5 usd.
we will see....
take-care
nach der dilution vom freitag zu 3,15 usd ist nun der weg nach oben frei. erstes ziel in meinen augen nach vermeldung des vollst. enrollments 4-5 usd.
we will see....
take-care
@all
vorbild für den kursverlauf ist RPTP...ergebnisse werden hier in kürze
erwartet
lets go CLSN
take-care
vorbild für den kursverlauf ist RPTP...ergebnisse werden hier in kürze
erwartet
lets go CLSN
take-care
3,80 usd gerade...
das ist meiner meinung nach erst der anfang...
56 mio marketcap und das bei diesen aussichten
etliche insiderkäufe in den letzten monaten, verdoppler noch vor den zwischenergebnissen ?
ich denke durchaus realistisch....
bei guten ergebnissen lässt der kursverlauf von DNDN grüßen....
take-care
das ist meiner meinung nach erst der anfang...
56 mio marketcap und das bei diesen aussichten
etliche insiderkäufe in den letzten monaten, verdoppler noch vor den zwischenergebnissen ?
ich denke durchaus realistisch....
bei guten ergebnissen lässt der kursverlauf von DNDN grüßen....
take-care
Antwort auf Beitrag Nr.: 41.777.716 von take-care am 12.07.11 17:58:24aber nur bei guten ergebnissen,chance 50/50
nice close....
erstes ziel von 4 usd in reichweite.
@asics
bei guten ergebnissen sehen wir hier mittlere 2-stellige werte in den nächsten 12 monaten. die mc ist enorm niedrig .
erstes ziel von 4 usd in reichweite.
@asics
bei guten ergebnissen sehen wir hier mittlere 2-stellige werte in den nächsten 12 monaten. die mc ist enorm niedrig .
Antwort auf Beitrag Nr.: 41.786.006 von take-care am 14.07.11 08:12:21hallo take-care
ich bin seit 2,75$ auch dabei, und wenn es ihnen gelingt thermodox auf den markt zu bringen dann ist das die 2te dendreon.
aber immer im hinterkopf behalten, dass es sich hier um einen biotechwert handelt,sollte es da irgendwelche probleme geben bist du so schnell unten,so schnell kannst du gar nicht schauen.
hoffen wir mal das beste
asics
ich bin seit 2,75$ auch dabei, und wenn es ihnen gelingt thermodox auf den markt zu bringen dann ist das die 2te dendreon.
aber immer im hinterkopf behalten, dass es sich hier um einen biotechwert handelt,sollte es da irgendwelche probleme geben bist du so schnell unten,so schnell kannst du gar nicht schauen.
hoffen wir mal das beste
asics
Antwort auf Beitrag Nr.: 41.789.254 von asics01 am 14.07.11 16:05:04@asics
das ist richtig, deshalb "spiele" ich den "runup" vor den ergebnissen.
teil der gewinne bleibt in diesem fall aber auch drin bei verkündung der zwischenergebnisse.
heute kleine verschnaufpause....kann dem kurs/chart nur gut tun.
take-care
das ist richtig, deshalb "spiele" ich den "runup" vor den ergebnissen.
teil der gewinne bleibt in diesem fall aber auch drin bei verkündung der zwischenergebnisse.
heute kleine verschnaufpause....kann dem kurs/chart nur gut tun.
take-care
ja hier stept der bär,wenn da gute daten kommen gibt es kein halten mehr









ich hab schon die 4,37$ gesehen
Antwort auf Beitrag Nr.: 41.812.365 von asics01 am 19.07.11 21:17:27jau
und das schöne an der sache....die marketcap ist immer noch lächerlich...
ich glaube das CLSN die 6 vor den ergebnissen sehen wird...und danach ist dann alles offen...
take-care
und das schöne an der sache....die marketcap ist immer noch lächerlich...
ich glaube das CLSN die 6 vor den ergebnissen sehen wird...und danach ist dann alles offen...
take-care
Antwort auf Beitrag Nr.: 41.812.386 von take-care am 19.07.11 21:22:44lass mal positive ergebnisse kommen,dann sind wir 2 stellig,wenn nicht alles vorbei.
also lass uns das geniessen solange es geht


also lass uns das geniessen solange es geht



Antwort auf Beitrag Nr.: 41.812.410 von asics01 am 19.07.11 21:27:20@asics
werde nur mit einem kleinen teil der gewinne in die entscheidung gehen
spiele vorerst nur den "RUNUP"



macht spass...bist du bei DRRX investiert ?
take-care
werde nur mit einem kleinen teil der gewinne in die entscheidung gehen
spiele vorerst nur den "RUNUP"



macht spass...bist du bei DRRX investiert ?
take-care
Antwort auf Beitrag Nr.: 41.812.438 von take-care am 19.07.11 21:31:14nein DRRX habe ich nicht,ich bin voll dabei mit CLSN,CXM, ZGNX, SUPG.und meine long position LGND.
asics
asics
Antwort auf Beitrag Nr.: 41.812.476 von asics01 am 19.07.11 21:37:10CLSN enorm stark...trotz erneuter dilution wieder bei 4,20 usd
chart schaut super aus und mc nach wie vor unter 100 mio...
die dilution wurde zu ca.4,25 usd durchgeführt und von insidern mitgetragen...habe sowas selten erlebt...
die jungs scheinen dran zu glauben oder wissen durch die "interim looks" einfach mehr als wir...
nächstes ziel kurzfristig 5 - 6 usd
take-care
chart schaut super aus und mc nach wie vor unter 100 mio...
die dilution wurde zu ca.4,25 usd durchgeführt und von insidern mitgetragen...habe sowas selten erlebt...
die jungs scheinen dran zu glauben oder wissen durch die "interim looks" einfach mehr als wir...
nächstes ziel kurzfristig 5 - 6 usd
take-care
ja CLSN ist stark,ich mache mir zu zeit gedanken über einen teilverkauf,oder ob ich das blatt bis zum schluss ausreizen soll?????
wie gehst du vor???
aber eigentlich will ich gar nicht raus,nur wenn die das ding gegen die wand fahren sollten ärgere ich mich wieder grün und blau.
asics
wie gehst du vor???
aber eigentlich will ich gar nicht raus,nur wenn die das ding gegen die wand fahren sollten ärgere ich mich wieder grün und blau.
asics
mein depot momentan
CLSN
AMRN - übernahmespekulation...13,30 usd scheint der boden gewesen zu sein
DRRX - phase 3 ergebnisse in Q4
RNN - phase 2b - Q4
FEED - 380 mio umsatz 2011 - mc unter 100 mio...q-zahlen anfang august
take-care
watchlist
SGMO
ZGNX
YMI
CORT
AEZS
KERX
CLSN
AMRN - übernahmespekulation...13,30 usd scheint der boden gewesen zu sein
DRRX - phase 3 ergebnisse in Q4
RNN - phase 2b - Q4
FEED - 380 mio umsatz 2011 - mc unter 100 mio...q-zahlen anfang august
take-care
watchlist
SGMO
ZGNX
YMI
CORT
AEZS
KERX
Antwort auf Beitrag Nr.: 41.834.841 von take-care am 23.07.11 16:55:50reizt du CLSN bis zum schluss aus,oder verkaufst du vor den daten einen teil???
asics
asics
schau dir mal PPHM an
Antwort auf Beitrag Nr.: 41.834.847 von asics01 am 23.07.11 17:00:17@asics
ich werde nur mit einem kleinen teil meiner aktien in die entscheidung / ergebnisse gehen.
denke aber das wir im vorfeld bereits zwischen 5-6 usd laufen.
meiner meinung nach stehen die chancen sehr gut für pos. ergebnisse, but who knows ??
take-care
ich werde nur mit einem kleinen teil meiner aktien in die entscheidung / ergebnisse gehen.
denke aber das wir im vorfeld bereits zwischen 5-6 usd laufen.
meiner meinung nach stehen die chancen sehr gut für pos. ergebnisse, but who knows ??
take-care
Antwort auf Beitrag Nr.: 41.834.852 von asics01 am 23.07.11 17:01:49@asics
PPHM schau ich mir mal an, haben die irgendein "ereignis" / catalyst in den nächsten 1-4 monaten ??
take-care
PPHM schau ich mir mal an, haben die irgendein "ereignis" / catalyst in den nächsten 1-4 monaten ??
take-care
bei clsn lassen sie ja gerade gewaltig luft ab,bin mal gespannt wie weit sie clsn runterdrücken werden?????
Antwort auf Beitrag Nr.: 41.848.274 von asics01 am 26.07.11 20:11:45@asics
schaun mer mal....ich bleibe bis 5 usd voll dabei.
alle biotechs haben überproportional stark in den letzten 2 tagen verloren.
mache mir bei clsn eigentlich die wenigsten gedanken.
nächster uptrend spät. bei bekanntgabe des full-enrollments.
take-care
schaun mer mal....ich bleibe bis 5 usd voll dabei.
alle biotechs haben überproportional stark in den letzten 2 tagen verloren.
mache mir bei clsn eigentlich die wenigsten gedanken.
nächster uptrend spät. bei bekanntgabe des full-enrollments.
take-care
CLSN
voller vertrauensbeweis... director kauft 46.838 shares zu 4,27 usd
dieses gepaart mit den insiderkäufen aus den letzten monaten ist ein tolles zeichen.
http://xml.10kwizard.com/filing_raw.php?repo=tenk&ipage=7731…
take-care
voller vertrauensbeweis... director kauft 46.838 shares zu 4,27 usd
dieses gepaart mit den insiderkäufen aus den letzten monaten ist ein tolles zeichen.
http://xml.10kwizard.com/filing_raw.php?repo=tenk&ipage=7731…
take-care
JP Morgan - Kursziel 8,50 USD








Celsion Reaches Major Milestone With Enrollment of 600th Patient in Its Pivotal Phase III HEAT Study of ThermoDox(R) in Primary Liver Cancer
http://media.marketwire.com/attachments/201103/46158_CLSN.JP…
COLUMBIA, MD -- (Marketwire) -- 08/03/11 -- Celsion Corporation (NASDAQ: CLSN), a leading oncology drug development company, today announced that it has reached its pre-planned enrollment objective of 600 patients in the Company's pivotal, Phase III HEAT study, a multinational, randomized, double-blind, placebo-controlled clinical trial of ThermoDox in combination with radio frequency ablation (RFA) for the treatment of primary liver cancer. The enrollment objective was established to ensure that the study's primary end point, progression-free survival (PFS), can be achieved with adequate statistical power and is one of two triggers for an interim efficacy analysis by the study's independent Data Monitoring Committee (DMC). The second trigger is the confirmation of 190 PFS events in the study population. The HEAT study is being conducted under a U.S. Food and Drug Administration (FDA) Special Protocol Assessment, has received FDA Fast Track Designation, and has been designated as a Priority Trial for primary liver cancer by the National Institutes of Health.
"This milestone is a significant accomplishment for the Celsion team and our many collaborators in the global oncology community. Reaching our enrollment objective is also a critical step toward establishing ThermoDox's potential as a new standard of care in the first line treatment of non-resectable primary liver cancer," said Michael H. Tardugno, President and Chief Executive Officer of Celsion. "As this potential becomes clearer in the coming months, Celsion will take steps toward ensuring a seamless commercialization process for ThermoDox and the expansion of its clinical development into other indications where it may enhance the efficacy of thermal-based oncology therapies, including recurrent chest wall breast cancer as well as metastatic liver and bone cancers. With this accomplishment, Celsion remains firmly on a path toward its evolution into a leading oncology drug development company."
"The HEAT study is the largest clinical trial ever conducted in intermediate hepatocellular carcinoma (HCC) and its outcome is greatly anticipated within the medical community. HCC is an aggressive, deadly disease, and one of a few cancers that is growing at an alarming rate throughout the world," added Nicholas Borys, MD, Celsion's Chief Medical Officer. "We believe that adding ThermoDox to a RFA procedure will dramatically increase the hope for a cure."
The design and statistical plan of the HEAT study incorporate a pre-planned interim efficacy analysis. The HEAT study's independent Data Monitoring Committee will evaluate the unblinded data after the 600 patient target enrollment is reached and 190 PFS events are realized in the study population. The DMC is comprised of an independent group of medical and scientific experts with the responsibility for reviewing and evaluating patient safety and efficacy data from the HEAT study. The interim analysis is intended to evaluate safety, efficacy and futility to determine if there is overwhelming evidence of clinical benefit or a low probability of treatment success to continue, modify or terminate the study.
Consistent with the Company's global regulatory strategy, Celsion is continuing to enroll patients in the HEAT study in order to randomize at least 200 patients in China, a requirement for registrational filing in China. In addition to meeting the FDA enrollment objective, the HEAT study has also enrolled a sufficient number of patients to support, in Asia, registrational filings in S. Korea and Taiwan, two important markets for ThermoDox. Continued enrollment will have no effect on the timing for the pre-planned interim analysis, and has the potential to improve the time to final data.
About Primary Liver Cancer
Primary liver cancer is one of the most deadly forms of cancer and ranks as the fifth most common solid tumor cancer. The incidence of primary liver cancer is approximately 20,000 cases per year in the United States, approximately 40,000 cases per year in Europe and is rapidly growing worldwide at approximately 700,000 cases per year, due to the high prevalence of Hepatitis B and C in developing countries. The standard first-line treatment for liver cancer is surgical resection of the tumor; however, 90% of patients are ineligible for surgery. Radio frequency ablation (RFA) has increasingly become the standard of care for non-resectable liver tumors, but the treatment becomes less effective for larger tumors. There are few non-surgical therapeutic treatment options available as radiation therapy and chemotherapy are largely ineffective in the treatment of primary liver cancer.
About ThermoDox and the Phase III HEAT Study
ThermoDox is a proprietary heat-activated liposomal encapsulation of doxorubicin, an approved and frequently used oncology drug for the treatment of a wide range of cancers. In the HEAT study, ThermoDox is administered intravenously in combination with RFA. Localized mild hyperthermia (39.5 - 42 degrees Celsius) created by the RFA releases the entrapped doxorubicin from the liposome. This delivery technology enables high concentrations of doxorubicin to be deposited preferentially in a targeted tumor.
For primary liver cancer, ThermoDox is being evaluated in a 600 patient global Phase III study under an FDA Special Protocol Assessment. The study is designed to evaluate the efficacy of ThermoDox in combination with Radio Frequency Ablation (RFA) when compared to patients who receive RFA alone as the control. The primary endpoint for the study is progression-free survival (PFS) with a secondary confirmatory endpoint of overall survival. A pre-planned, unblinded interim efficacy analysis will be performed by the independent Data Monitoring Committee when enrollment in the HEAT study is complete and 190 PFS events are realized in the study population. Additional information on the Company's ThermoDox clinical studies may be found at www.clinicaltrials.gov.
About Celsion Corporation
Celsion is a leading oncology company dedicated to the development and commercialization of innovative cancer drugs including tumor-targeting treatments using focused heat energy in combination with heat-activated drug delivery systems. Celsion has research, license, or commercialization agreements with leading institutions such as the National Institutes of Health, Duke University Medical Center, University of Hong Kong, the University of Pisa, and the North Shore Long Island Jewish Health System.
For more information on Celsion, visit our website: http://www.celsion.com.
Celsion wishes to inform readers that forward-looking statements in this release are made pursuant to the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995. Readers are cautioned that such forward-looking statements involve risks and uncertainties including, without limitation, unforeseen changes in the course of research and development activities and in clinical trials by others; possible acquisitions of other technologies, assets or businesses; possible actions by customers, suppliers, competitors, regulatory authorities; and other risks detailed from time to time in the Company's periodic reports filed with the Securities and Exchange Commission.
Investor Contact
David Pitts
Argot Partners
212-600-1902
Email Contact
Source: Marketwire (August 3, 2011 - 8:00 AM EDT)
News by QuoteMedia
www.quotemedia.com
http://media.marketwire.com/attachments/201103/46158_CLSN.JP…
COLUMBIA, MD -- (Marketwire) -- 08/03/11 -- Celsion Corporation (NASDAQ: CLSN), a leading oncology drug development company, today announced that it has reached its pre-planned enrollment objective of 600 patients in the Company's pivotal, Phase III HEAT study, a multinational, randomized, double-blind, placebo-controlled clinical trial of ThermoDox in combination with radio frequency ablation (RFA) for the treatment of primary liver cancer. The enrollment objective was established to ensure that the study's primary end point, progression-free survival (PFS), can be achieved with adequate statistical power and is one of two triggers for an interim efficacy analysis by the study's independent Data Monitoring Committee (DMC). The second trigger is the confirmation of 190 PFS events in the study population. The HEAT study is being conducted under a U.S. Food and Drug Administration (FDA) Special Protocol Assessment, has received FDA Fast Track Designation, and has been designated as a Priority Trial for primary liver cancer by the National Institutes of Health.
"This milestone is a significant accomplishment for the Celsion team and our many collaborators in the global oncology community. Reaching our enrollment objective is also a critical step toward establishing ThermoDox's potential as a new standard of care in the first line treatment of non-resectable primary liver cancer," said Michael H. Tardugno, President and Chief Executive Officer of Celsion. "As this potential becomes clearer in the coming months, Celsion will take steps toward ensuring a seamless commercialization process for ThermoDox and the expansion of its clinical development into other indications where it may enhance the efficacy of thermal-based oncology therapies, including recurrent chest wall breast cancer as well as metastatic liver and bone cancers. With this accomplishment, Celsion remains firmly on a path toward its evolution into a leading oncology drug development company."
"The HEAT study is the largest clinical trial ever conducted in intermediate hepatocellular carcinoma (HCC) and its outcome is greatly anticipated within the medical community. HCC is an aggressive, deadly disease, and one of a few cancers that is growing at an alarming rate throughout the world," added Nicholas Borys, MD, Celsion's Chief Medical Officer. "We believe that adding ThermoDox to a RFA procedure will dramatically increase the hope for a cure."
The design and statistical plan of the HEAT study incorporate a pre-planned interim efficacy analysis. The HEAT study's independent Data Monitoring Committee will evaluate the unblinded data after the 600 patient target enrollment is reached and 190 PFS events are realized in the study population. The DMC is comprised of an independent group of medical and scientific experts with the responsibility for reviewing and evaluating patient safety and efficacy data from the HEAT study. The interim analysis is intended to evaluate safety, efficacy and futility to determine if there is overwhelming evidence of clinical benefit or a low probability of treatment success to continue, modify or terminate the study.
Consistent with the Company's global regulatory strategy, Celsion is continuing to enroll patients in the HEAT study in order to randomize at least 200 patients in China, a requirement for registrational filing in China. In addition to meeting the FDA enrollment objective, the HEAT study has also enrolled a sufficient number of patients to support, in Asia, registrational filings in S. Korea and Taiwan, two important markets for ThermoDox. Continued enrollment will have no effect on the timing for the pre-planned interim analysis, and has the potential to improve the time to final data.
About Primary Liver Cancer
Primary liver cancer is one of the most deadly forms of cancer and ranks as the fifth most common solid tumor cancer. The incidence of primary liver cancer is approximately 20,000 cases per year in the United States, approximately 40,000 cases per year in Europe and is rapidly growing worldwide at approximately 700,000 cases per year, due to the high prevalence of Hepatitis B and C in developing countries. The standard first-line treatment for liver cancer is surgical resection of the tumor; however, 90% of patients are ineligible for surgery. Radio frequency ablation (RFA) has increasingly become the standard of care for non-resectable liver tumors, but the treatment becomes less effective for larger tumors. There are few non-surgical therapeutic treatment options available as radiation therapy and chemotherapy are largely ineffective in the treatment of primary liver cancer.
About ThermoDox and the Phase III HEAT Study
ThermoDox is a proprietary heat-activated liposomal encapsulation of doxorubicin, an approved and frequently used oncology drug for the treatment of a wide range of cancers. In the HEAT study, ThermoDox is administered intravenously in combination with RFA. Localized mild hyperthermia (39.5 - 42 degrees Celsius) created by the RFA releases the entrapped doxorubicin from the liposome. This delivery technology enables high concentrations of doxorubicin to be deposited preferentially in a targeted tumor.
For primary liver cancer, ThermoDox is being evaluated in a 600 patient global Phase III study under an FDA Special Protocol Assessment. The study is designed to evaluate the efficacy of ThermoDox in combination with Radio Frequency Ablation (RFA) when compared to patients who receive RFA alone as the control. The primary endpoint for the study is progression-free survival (PFS) with a secondary confirmatory endpoint of overall survival. A pre-planned, unblinded interim efficacy analysis will be performed by the independent Data Monitoring Committee when enrollment in the HEAT study is complete and 190 PFS events are realized in the study population. Additional information on the Company's ThermoDox clinical studies may be found at www.clinicaltrials.gov.
About Celsion Corporation
Celsion is a leading oncology company dedicated to the development and commercialization of innovative cancer drugs including tumor-targeting treatments using focused heat energy in combination with heat-activated drug delivery systems. Celsion has research, license, or commercialization agreements with leading institutions such as the National Institutes of Health, Duke University Medical Center, University of Hong Kong, the University of Pisa, and the North Shore Long Island Jewish Health System.
For more information on Celsion, visit our website: http://www.celsion.com.
Celsion wishes to inform readers that forward-looking statements in this release are made pursuant to the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995. Readers are cautioned that such forward-looking statements involve risks and uncertainties including, without limitation, unforeseen changes in the course of research and development activities and in clinical trials by others; possible acquisitions of other technologies, assets or businesses; possible actions by customers, suppliers, competitors, regulatory authorities; and other risks detailed from time to time in the Company's periodic reports filed with the Securities and Exchange Commission.
Investor Contact
David Pitts
Argot Partners
212-600-1902
Email Contact
Source: Marketwire (August 3, 2011 - 8:00 AM EDT)
News by QuoteMedia
www.quotemedia.com
@asics
wollte auch gerade die news posten...
wunderbar, der kurs zieht vorbörslich schon an...hoffe auf interessante 4- 8 wochen bis zu den ergebnissen
take-care
wollte auch gerade die news posten...
wunderbar, der kurs zieht vorbörslich schon an...hoffe auf interessante 4- 8 wochen bis zu den ergebnissen
take-care
Antwort auf Beitrag Nr.: 41.885.322 von take-care am 03.08.11 14:34:26hoffentlich spielt der gesamtmarkt auch ein bischen mit
heute war wieder leben in der bude,runtergezogen bis auf 3,26$ bin bei 3,31$ nochmal rein,aber jetzt ist genug
clsn hätte man heute für 2.85$ kaufen können,echt der hammer
für 2,83$ gabs auch welche,die überm teich wollen es wissen.ich werde mit clsn untergehen wenn es sein muss,amen
@asics
unfassbar, aber wahr...hätte ich nicht für möglich gehalten, aber die biotechs bluten seit tagen überproportional.
und dann kam gestern auch noch DNDN´s big miss bei den Q-zahlen...
habe heute erneut zugeschlagen unter 3 usd...bin vollgesogen nun + amrn bis unters dach...
hoffe wir rebounden nun langsam...es hat sich nichts geändert an den fundamentals bei CLSN + insiderkäufen zu 4,27 usd
abwarten und hoffentlich bald freuen
take-care
unfassbar, aber wahr...hätte ich nicht für möglich gehalten, aber die biotechs bluten seit tagen überproportional.
und dann kam gestern auch noch DNDN´s big miss bei den Q-zahlen...
habe heute erneut zugeschlagen unter 3 usd...bin vollgesogen nun + amrn bis unters dach...
hoffe wir rebounden nun langsam...es hat sich nichts geändert an den fundamentals bei CLSN + insiderkäufen zu 4,27 usd
abwarten und hoffentlich bald freuen
take-care
Antwort auf Beitrag Nr.: 41.895.005 von take-care am 04.08.11 19:42:21das kann aber noch ein steiniger weg weden bis september,am besten nicht mehr nach dem kurs schauen die nächsten wochen.toi toi toi
Antwort auf Beitrag Nr.: 41.895.070 von asics01 am 04.08.11 19:51:59csln ist jetzt wohl aus dem winterschlaf erwacht,kommt da was die nächsten tage?????
zeit wäre es ja
asics
zeit wäre es ja
asics
Antwort auf Beitrag Nr.: 42.715.039 von asics01 am 07.02.12 20:26:21Am 14.2. Bio´s-CEO-Conference. Für einen simplen pre-conference-hype ist mir die entwicklung der letzten Tage zu heftig. Riesen Short-Position hier. Lassen wir uns überraschen. wenn´s news gibt wird es ´nen hübschen squeeze geben.
ich seh grad wie einsam du hier im board warst all die zeit. immerhin heute schon 3 (drei!) aufrufe. den vielen zuschauern daher mal bissl was buntes geboten hier:

OK, ist noch weit weg die 200MA, aber das wird schon



OK, ist noch weit weg die 200MA, aber das wird schon



Antwort auf Beitrag Nr.: 42.715.134 von ThumsUp am 07.02.12 20:41:45sehe ich genauso,na dann wollen wir mal hoffen dass die bei clsn ihre hausaufgaben gemacht haben.
oh mann das gibt dann aber ein grillfest





asics
oh mann das gibt dann aber ein grillfest






asics
Antwort auf Beitrag Nr.: 42.715.187 von asics01 am 07.02.12 20:50:03hoffentlich nicht nur heisse luft,und zockerei.clsn wurde aber auch heftig runtergeprügelt.
asics
asics
Celsion is eine gute Aktie der kursrückgang in den letzten Wochen hatte mit den zwei K.E. zu tun .
Thermodox hat gute chancen die Phase 3 Studie erfolgreich abzuschließen . Vorläufige Phase-3 daten sind gegen ende des Jahres zu erwarten .
Celsion (CLSN)
Marktkap: 77 M
Cash: 35 M
Kurs: 2.33
Shares Out : 33 M
Präsentation Dez 2011
http://files.shareholder.com/downloads/CLN/1667690300x0x5297…
CEO Letter
http://www.celsion.com/letter.cfm
Insiderkäufe
http://www.secform4.com/insider-trading/749647.htm
Ausblick 2012:
Lead ThermoDox Program - HCC
•
Ph III HCC Trial Enrollment Completion
•
Ph III HCC Blinded Interim Analysis (190 PFS events)
•
Rolling NDA Submission for HCC
•
Ph III HCC Final Analysis (380 PFS events)
•
2nd License Agreement for ThermoDox
Global commercialization plans maximize
shareholder value
U.S. strategy is to market and sell directly
Ex-U.S. strategy is through license agreements
with Pharma Partner(s)
Clinical strategy supports early license interest
Thermodox hat gute chancen die Phase 3 Studie erfolgreich abzuschließen . Vorläufige Phase-3 daten sind gegen ende des Jahres zu erwarten .
Celsion (CLSN)
Marktkap: 77 M
Cash: 35 M
Kurs: 2.33
Shares Out : 33 M
Präsentation Dez 2011
http://files.shareholder.com/downloads/CLN/1667690300x0x5297…
CEO Letter
http://www.celsion.com/letter.cfm
Insiderkäufe
http://www.secform4.com/insider-trading/749647.htm
Ausblick 2012:
Lead ThermoDox Program - HCC
•
Ph III HCC Trial Enrollment Completion
•
Ph III HCC Blinded Interim Analysis (190 PFS events)
•
Rolling NDA Submission for HCC
•
Ph III HCC Final Analysis (380 PFS events)
•
2nd License Agreement for ThermoDox
Global commercialization plans maximize
shareholder value
U.S. strategy is to market and sell directly
Ex-U.S. strategy is through license agreements
with Pharma Partner(s)
Clinical strategy supports early license interest
Celsion Corporation| CLSN
UP
Another stock that's setting up for a big breakout is Celsion(CLSN_), an oncology drug development company focused on the development of therapeutics for those suffering with difficult to treat forms of cancer. This is another stock off to a solid start in 2012, with shares up over 15%.
If you take a look at the chart for Celsion, you'll see that this stock was hammered by the bears from its November high of $3.84 to a recent low of $1.64 a share. After tagging that low last month, the stock has rebounded sharply and is now approaching a number of key breakout levels. What's bullish about shares of Celsion here is that the stock is coming up on those breakout levels and the upside volume is tracking in strong.
Market players should now watch for CLSN to break out above some near-term overhead resistance at $1.95 to $1.99 a share, and its 50-day moving average of $2.01 a share on solid volume. Look for volume on a move above those levels that registers near or well above its three-month average action of 385,810 shares. At last check, CLSN has hit a high of $2.07 today, and the volume is already well above its average.
One could be a buyer of CLSN off any close above the 50-day that's accompanied by strong volume. I would simply use a mental stop that's right below $1.95 in case this breakout fails. If we do get that action either today or soon, then I would target a spike back towards some near-term resistance at $2.40 a share, or possibly its 200-day moving average of $2.83 a share.
Keep in mind that CLSN has a decent short interest since 9.1% of the tradable float is currently sold short by the bears. Any future breakout could easily spark a big short-squeeze as the bears cover some of their positions to avoid short-term losses.
To see more breakout candidates, includingVonage Holdings(VG_), Glu Mobile(GLUU_) and Zoom Technologies(ZOOM_), check out the Breakout Stocks of the Week portfolio on Stockpickr.
-- Written by Roberto Pedone in Winderemere,
inShare.1
« First‹ Previous
1 2 3 4 56
At the time of publication, author had no positions in stocks mentioned.
Roberto Pedone, based out of Windermere, Fla., is an independent trader who focuses on stocks, options, futures, commodities and currencies. He is also an outside contributor to Beconequity.com and maintains the website Maddmoney.net, which he sold to Blue Wave Advisors in 2008. Roberto studied International Business at The Milwaukee School of Engineering, and he spent a year overseas studying business in Lubeck, Germany.
More from TheStreet ■
10 Stocks JPMorgan Says May Rise Up to 58%
■
5 Earnings Stocks Poised to Pop
■
Turn Off the Noise
■
How The Major Stock Indexes Fared On Friday
■
A Hopeful Diagnosis
From Around the Web ■
Economist Who Predicted the 2008 Crash Gives Chilling 2012 Forecast. See the Evidence. (Newsmax.com)
■
What Do They Mean by Overbought and Oversold? (DailyFX)
■
Warning! 7 Lies All Women Tell Men (MadeMan)
■
Jobs Data Not Good News For Gold, Silver (TradersHuddle.com)
■
11 Ways to Lower Cholesterol (Lifescript)
[?]
TheStreet Premium Services For Personal Service: 877-471-2967
Compare All Services
Jim Cramer's Action Alerts PLUS:
Trade right alongside a Wall Street pro — enjoy access to his Charitable Trust portfolio and be sent trade alerts BEFORE he makes a move. Learn More
Login
ETF Profits:
Get money-making ideas from the hottest investment vehicle on the planet. Our experts show you how to play various ETF sectors to help pump-up your portfolio. Learn More
Login
OptionsProfits:
Get 50+ trade ideas a week from the industry's top options experts. Plus — exclusive commentary on market trends and essential trading tools. Learn More
Login
Real Money:
Our team of professional Wall Street Pros — including Jim Cramer, Doug Kass, and Nicholas Vardy — delivers intelligent analysis, timely trade ideas, and colorful commentary. Learn More
Login
Stocks Under $10:
Break into the market with small- and mid-cap stocks... all $10 or less! David Peltier tells you exactly which low-priced stocks he's buying and selling. Learn More
Login
To begin commenting right away, you can log in below using your Disqus, Facebook, Twitter, OpenID or Yahoo login credentials. Alternatively, you can post a comment as a "guest" just by entering an email address. Your use of the commenting tool is subject to multiple terms of service/use and privacy policies - see here for more details.
blog comments powered by Disqus
Disqus
Like
Dislike
Add New Comment
Required: Please login below to comment.
Post as …
Showing 0 comments
Sort by Popular nowBest ratingNewest firstOldest first Subscribe by email Subscribe by RSS
Real-time updating is enabled. (Pause)
Dow Jones
S&P 500
NASDAQ
12,874.83
1,345.12
2,904.78
Oil *
116.20
UP
+29.70
UP
+0.79
UP
+2.79
10 Yr
1.97%
+0.23%
+0.06%
+0.10%
Data delayed 20 minutes
Top Stories and Tools
10 Dividend Stocks Most Likely to Outperform
10 Worst Cars of All Time
Ex-Dividend Date Calendar: Find Payout Dates
2012 Bank Ratings and Safety Screener
FREE NEWSLETTER: Dividend and Income Investor
Barrington's Best Stock Picks for 2012
Brokerage Partners
Free Newsletters from TheStreet
After the Bell
Before the Bell
Booyah! Newsletter
ETF Daily
Midday Bell
TheStreet Top 10 Stories
Winners & Losers
We respect your privacy.
Manage Newsletters
Podcasts
[Select Podcast]The Real StoryWall Street Confidential Podcast
Daily RSS Updates
TheStreet Mobile Edition
Connect with TheStreet
.
TheStreet Mobile | MainStreet | StockPickr | BankingMyWay | Jim Cramer | Doug Kass | Real Money | Try Action Alerts PLUS
Your Account
Log In
TheStreet Corporate | Home | About Us | Advertise | Reprints | Customer Service | Employment | Privacy Policy | Sitemap | Topic Archive | Video Archive | Stock Quotes Online | Terms of Use
TheStreet's enterprise databases running Oracle are professionally monitored and managed by Pythian Remote DBA.
Quotes delayed at least 20 minutes for all exchanges. Market Data provided by Interactive Data. Company fundamental data provided by Morningstar. Earnings and ratings provided by Zacks. Mutual fund data provided by Valueline. ETF data provided by Lipper. Terms & Conditions. Powered and implemented by Interactive Data Managed Solutions.
TheStreet Ratings updates stock ratings daily. However, if no rating change occurs, the data on this page does not update. The data does update after 90 days if no rating change occurs within that time period.
IDC calculates the Market Cap for the basic symbol to include common shares only. Year-to-date mutual fund returns are calculated on a monthly basis by Value Line and posted mid-month.
*Oil Data in Market Overview is Brent Crude Pricing
© 2012 TheStreet, Inc. All rights reserved.
Stock Game
Earnings Calendar
Latest News
Mad Money Screener
Dividend Calendar
Fan
Follow
Celsion Corporation| CLSN
UP
Another stock that's setting up for a big breakout is Celsion(CLSN_), an oncology drug development company focused on the development of therapeutics for those suffering with difficult to treat forms of cancer. This is another stock off to a solid start in 2012, with shares up over 15%.
If you take a look at the chart for Celsion, you'll see that this stock was hammered by the bears from its November high of $3.84 to a recent low of $1.64 a share. After tagging that low last month, the stock has rebounded sharply and is now approaching a number of key breakout levels. What's bullish about shares of Celsion here is that the stock is coming up on those breakout levels and the upside volume is tracking in strong.
Market players should now watch for CLSN to break out above some near-term overhead resistance at $1.95 to $1.99 a share, and its 50-day moving average of $2.01 a share on solid volume. Look for volume on a move above those levels that registers near or well above its three-month average action of 385,810 shares. At last check, CLSN has hit a high of $2.07 today, and the volume is already well above its average.
One could be a buyer of CLSN off any close above the 50-day that's accompanied by strong volume. I would simply use a mental stop that's right below $1.95 in case this breakout fails. If we do get that action either today or soon, then I would target a spike back towards some near-term resistance at $2.40 a share, or possibly its 200-day moving average of $2.83 a share.
Keep in mind that CLSN has a decent short interest since 9.1% of the tradable float is currently sold short by the bears. Any future breakout could easily spark a big short-squeeze as the bears cover some of their positions to avoid short-term losses.
To see more breakout candidates, includingVonage Holdings(VG_), Glu Mobile(GLUU_) and Zoom Technologies(ZOOM_), check out the Breakout Stocks of the Week portfolio on Stockpickr.
-- Written by Roberto Pedone in Winderemere, Fla.
RELATED LINKS:
>>5 Popular Twitter Stocks to Trade for Gains
>>6 Stocks Reaping the Benefits of Big Buybacks
>>5 Stocks Under $10 Set to Trigger Big Moves
Follow Stockpickr on Twitter and become a fan on Facebook.
>To order reprints of this article, click here: Reprints
Add Comment
inShare.1
« First‹ Previous
1 2 3 4 56
At the time of publication, author had no positions in stocks mentioned.
Roberto Pedone, based out of Windermere, Fla., is an independent trader who focuses on stocks, options, futures, commodities and currencies. He is also an outside contributor to Beconequity.com and maintains the website Maddmoney.net, which he sold to Blue Wave Advisors in 2008. Roberto studied International Business at The Milwaukee School of Engineering, and he spent a year overseas studying business in Lubeck, Germany.
More from TheStreet ■
10 Stocks JPMorgan Says May Rise Up to 58%
■
5 Earnings Stocks Poised to Pop
■
Turn Off the Noise
■
How The Major Stock Indexes Fared On Friday
■
A Hopeful Diagnosis
From Around the Web ■
Economist Who Predicted the 2008 Crash Gives Chilling 2012 Forecast. See the Evidence. (Newsmax.com)
■
What Do They Mean by Overbought and Oversold? (DailyFX)
■
Warning! 7 Lies All Women Tell Men (MadeMan)
■
Jobs Data Not Good News For Gold, Silver (TradersHuddle.com)
■
11 Ways to Lower Cholesterol (Lifescript)
[?]
TheStreet Premium Services For Personal Service: 877-471-2967
Compare All Services
Jim Cramer's Action Alerts PLUS:
Trade right alongside a Wall Street pro — enjoy access to his Charitable Trust portfolio and be sent trade alerts BEFORE he makes a move. Learn More
Login
ETF Profits:
Get money-making ideas from the hottest investment vehicle on the planet. Our experts show you how to play various ETF sectors to help pump-up your portfolio. Learn More
Login
OptionsProfits:
Get 50+ trade ideas a week from the industry's top options experts. Plus — exclusive commentary on market trends and essential trading tools. Learn More
Login
Real Money:
Our team of professional Wall Street Pros — including Jim Cramer, Doug Kass, and Nicholas Vardy — delivers intelligent analysis, timely trade ideas, and colorful commentary. Learn More
Login
Stocks Under $10:
Break into the market with small- and mid-cap stocks... all $10 or less! David Peltier tells you exactly which low-priced stocks he's buying and selling. Learn More
Login
To begin commenting right away, you can log in below using your Disqus, Facebook, Twitter, OpenID or Yahoo login credentials. Alternatively, you can post a comment as a "guest" just by entering an email address. Your use of the commenting tool is subject to multiple terms of service/use and privacy policies - see here for more details.
blog comments powered by Disqus
Disqus
Like
Dislike
Add New Comment
Required: Please login below to comment.
Post as …
Showing 0 comments
Sort by Popular nowBest ratingNewest firstOldest first Subscribe by email Subscribe by RSS
Real-time updating is enabled. (Pause)
Dow Jones
S&P 500
NASDAQ
12,874.83
1,345.12
2,904.78
Oil *
116.20
UP
+29.70
UP
+0.79
UP
+2.79
10 Yr
1.97%
+0.23%
+0.06%
+0.10%
Data delayed 20 minutes
Top Stories and Tools
10 Dividend Stocks Most Likely to Outperform
10 Worst Cars of All Time
Ex-Dividend Date Calendar: Find Payout Dates
2012 Bank Ratings and Safety Screener
FREE NEWSLETTER: Dividend and Income Investor
Barrington's Best Stock Picks for 2012
Brokerage Partners
Free Newsletters from TheStreet
After the Bell
Before the Bell
Booyah! Newsletter
ETF Daily
Midday Bell
TheStreet Top 10 Stories
Winners & Losers
We respect your privacy.
Manage Newsletters
Podcasts
[Select Podcast]The Real StoryWall Street Confidential Podcast
Daily RSS Updates
TheStreet Mobile Edition
Connect with TheStreet
.
TheStreet Mobile | MainStreet | StockPickr | BankingMyWay | Jim Cramer | Doug Kass | Real Money | Try Action Alerts PLUS
Your Account
Log In
TheStreet Corporate | Home | About Us | Advertise | Reprints | Customer Service | Employment | Privacy Policy | Sitemap | Topic Archive | Video Archive | Stock Quotes Online | Terms of Use
TheStreet's enterprise databases running Oracle are professionally monitored and managed by Pythian Remote DBA.
Quotes delayed at least 20 minutes for all exchanges. Market Data provided by Interactive Data. Company fundamental data provided by Morningstar. Earnings and ratings provided by Zacks. Mutual fund data provided by Valueline. ETF data provided by Lipper. Terms & Conditions. Powered and implemented by Interactive Data Managed Solutions.
TheStreet Ratings updates stock ratings daily. However, if no rating change occurs, the data on this page does not update. The data does update after 90 days if no rating change occurs within that time period.
IDC calculates the Market Cap for the basic symbol to include common shares only. Year-to-date mutual fund returns are calculated on a monthly basis by Value Line and posted mid-month.
*Oil Data in Market Overview is Brent Crude Pricing
© 2012 TheStreet, Inc. All rights reserved.
Stock Game
Earnings Calendar
Latest News
Mad Money Screener
Dividend Calendar
Fan
Follow
UP
Another stock that's setting up for a big breakout is Celsion(CLSN_), an oncology drug development company focused on the development of therapeutics for those suffering with difficult to treat forms of cancer. This is another stock off to a solid start in 2012, with shares up over 15%.
If you take a look at the chart for Celsion, you'll see that this stock was hammered by the bears from its November high of $3.84 to a recent low of $1.64 a share. After tagging that low last month, the stock has rebounded sharply and is now approaching a number of key breakout levels. What's bullish about shares of Celsion here is that the stock is coming up on those breakout levels and the upside volume is tracking in strong.
Market players should now watch for CLSN to break out above some near-term overhead resistance at $1.95 to $1.99 a share, and its 50-day moving average of $2.01 a share on solid volume. Look for volume on a move above those levels that registers near or well above its three-month average action of 385,810 shares. At last check, CLSN has hit a high of $2.07 today, and the volume is already well above its average.
One could be a buyer of CLSN off any close above the 50-day that's accompanied by strong volume. I would simply use a mental stop that's right below $1.95 in case this breakout fails. If we do get that action either today or soon, then I would target a spike back towards some near-term resistance at $2.40 a share, or possibly its 200-day moving average of $2.83 a share.
Keep in mind that CLSN has a decent short interest since 9.1% of the tradable float is currently sold short by the bears. Any future breakout could easily spark a big short-squeeze as the bears cover some of their positions to avoid short-term losses.
To see more breakout candidates, includingVonage Holdings(VG_), Glu Mobile(GLUU_) and Zoom Technologies(ZOOM_), check out the Breakout Stocks of the Week portfolio on Stockpickr.
-- Written by Roberto Pedone in Winderemere,
inShare.1
« First‹ Previous
1 2 3 4 56
At the time of publication, author had no positions in stocks mentioned.
Roberto Pedone, based out of Windermere, Fla., is an independent trader who focuses on stocks, options, futures, commodities and currencies. He is also an outside contributor to Beconequity.com and maintains the website Maddmoney.net, which he sold to Blue Wave Advisors in 2008. Roberto studied International Business at The Milwaukee School of Engineering, and he spent a year overseas studying business in Lubeck, Germany.
More from TheStreet ■
10 Stocks JPMorgan Says May Rise Up to 58%
■
5 Earnings Stocks Poised to Pop
■
Turn Off the Noise
■
How The Major Stock Indexes Fared On Friday
■
A Hopeful Diagnosis
From Around the Web ■
Economist Who Predicted the 2008 Crash Gives Chilling 2012 Forecast. See the Evidence. (Newsmax.com)
■
What Do They Mean by Overbought and Oversold? (DailyFX)
■
Warning! 7 Lies All Women Tell Men (MadeMan)
■
Jobs Data Not Good News For Gold, Silver (TradersHuddle.com)
■
11 Ways to Lower Cholesterol (Lifescript)
[?]
TheStreet Premium Services For Personal Service: 877-471-2967
Compare All Services
Jim Cramer's Action Alerts PLUS:
Trade right alongside a Wall Street pro — enjoy access to his Charitable Trust portfolio and be sent trade alerts BEFORE he makes a move. Learn More
Login
ETF Profits:
Get money-making ideas from the hottest investment vehicle on the planet. Our experts show you how to play various ETF sectors to help pump-up your portfolio. Learn More
Login
OptionsProfits:
Get 50+ trade ideas a week from the industry's top options experts. Plus — exclusive commentary on market trends and essential trading tools. Learn More
Login
Real Money:
Our team of professional Wall Street Pros — including Jim Cramer, Doug Kass, and Nicholas Vardy — delivers intelligent analysis, timely trade ideas, and colorful commentary. Learn More
Login
Stocks Under $10:
Break into the market with small- and mid-cap stocks... all $10 or less! David Peltier tells you exactly which low-priced stocks he's buying and selling. Learn More
Login
To begin commenting right away, you can log in below using your Disqus, Facebook, Twitter, OpenID or Yahoo login credentials. Alternatively, you can post a comment as a "guest" just by entering an email address. Your use of the commenting tool is subject to multiple terms of service/use and privacy policies - see here for more details.
blog comments powered by Disqus
Disqus
Like
Dislike
Add New Comment
Required: Please login below to comment.
Post as …
Showing 0 comments
Sort by Popular nowBest ratingNewest firstOldest first Subscribe by email Subscribe by RSS
Real-time updating is enabled. (Pause)
Dow Jones
S&P 500
NASDAQ
12,874.83
1,345.12
2,904.78
Oil *
116.20
UP
+29.70
UP
+0.79
UP
+2.79
10 Yr
1.97%
+0.23%
+0.06%
+0.10%
Data delayed 20 minutes
Top Stories and Tools
10 Dividend Stocks Most Likely to Outperform
10 Worst Cars of All Time
Ex-Dividend Date Calendar: Find Payout Dates
2012 Bank Ratings and Safety Screener
FREE NEWSLETTER: Dividend and Income Investor
Barrington's Best Stock Picks for 2012
Brokerage Partners
Free Newsletters from TheStreet
After the Bell
Before the Bell
Booyah! Newsletter
ETF Daily
Midday Bell
TheStreet Top 10 Stories
Winners & Losers
We respect your privacy.
Manage Newsletters
Podcasts
[Select Podcast]The Real StoryWall Street Confidential Podcast
Daily RSS Updates
TheStreet Mobile Edition
Connect with TheStreet
.
TheStreet Mobile | MainStreet | StockPickr | BankingMyWay | Jim Cramer | Doug Kass | Real Money | Try Action Alerts PLUS
Your Account
Log In
TheStreet Corporate | Home | About Us | Advertise | Reprints | Customer Service | Employment | Privacy Policy | Sitemap | Topic Archive | Video Archive | Stock Quotes Online | Terms of Use
TheStreet's enterprise databases running Oracle are professionally monitored and managed by Pythian Remote DBA.
Quotes delayed at least 20 minutes for all exchanges. Market Data provided by Interactive Data. Company fundamental data provided by Morningstar. Earnings and ratings provided by Zacks. Mutual fund data provided by Valueline. ETF data provided by Lipper. Terms & Conditions. Powered and implemented by Interactive Data Managed Solutions.
TheStreet Ratings updates stock ratings daily. However, if no rating change occurs, the data on this page does not update. The data does update after 90 days if no rating change occurs within that time period.
IDC calculates the Market Cap for the basic symbol to include common shares only. Year-to-date mutual fund returns are calculated on a monthly basis by Value Line and posted mid-month.
*Oil Data in Market Overview is Brent Crude Pricing
© 2012 TheStreet, Inc. All rights reserved.
Stock Game
Earnings Calendar
Latest News
Mad Money Screener
Dividend Calendar
Fan
Follow
Celsion Corporation| CLSN
UP
Another stock that's setting up for a big breakout is Celsion(CLSN_), an oncology drug development company focused on the development of therapeutics for those suffering with difficult to treat forms of cancer. This is another stock off to a solid start in 2012, with shares up over 15%.
If you take a look at the chart for Celsion, you'll see that this stock was hammered by the bears from its November high of $3.84 to a recent low of $1.64 a share. After tagging that low last month, the stock has rebounded sharply and is now approaching a number of key breakout levels. What's bullish about shares of Celsion here is that the stock is coming up on those breakout levels and the upside volume is tracking in strong.
Market players should now watch for CLSN to break out above some near-term overhead resistance at $1.95 to $1.99 a share, and its 50-day moving average of $2.01 a share on solid volume. Look for volume on a move above those levels that registers near or well above its three-month average action of 385,810 shares. At last check, CLSN has hit a high of $2.07 today, and the volume is already well above its average.
One could be a buyer of CLSN off any close above the 50-day that's accompanied by strong volume. I would simply use a mental stop that's right below $1.95 in case this breakout fails. If we do get that action either today or soon, then I would target a spike back towards some near-term resistance at $2.40 a share, or possibly its 200-day moving average of $2.83 a share.
Keep in mind that CLSN has a decent short interest since 9.1% of the tradable float is currently sold short by the bears. Any future breakout could easily spark a big short-squeeze as the bears cover some of their positions to avoid short-term losses.
To see more breakout candidates, includingVonage Holdings(VG_), Glu Mobile(GLUU_) and Zoom Technologies(ZOOM_), check out the Breakout Stocks of the Week portfolio on Stockpickr.
-- Written by Roberto Pedone in Winderemere, Fla.
RELATED LINKS:
>>5 Popular Twitter Stocks to Trade for Gains
>>6 Stocks Reaping the Benefits of Big Buybacks
>>5 Stocks Under $10 Set to Trigger Big Moves
Follow Stockpickr on Twitter and become a fan on Facebook.
>To order reprints of this article, click here: Reprints
Add Comment
inShare.1
« First‹ Previous
1 2 3 4 56
At the time of publication, author had no positions in stocks mentioned.
Roberto Pedone, based out of Windermere, Fla., is an independent trader who focuses on stocks, options, futures, commodities and currencies. He is also an outside contributor to Beconequity.com and maintains the website Maddmoney.net, which he sold to Blue Wave Advisors in 2008. Roberto studied International Business at The Milwaukee School of Engineering, and he spent a year overseas studying business in Lubeck, Germany.
More from TheStreet ■
10 Stocks JPMorgan Says May Rise Up to 58%
■
5 Earnings Stocks Poised to Pop
■
Turn Off the Noise
■
How The Major Stock Indexes Fared On Friday
■
A Hopeful Diagnosis
From Around the Web ■
Economist Who Predicted the 2008 Crash Gives Chilling 2012 Forecast. See the Evidence. (Newsmax.com)
■
What Do They Mean by Overbought and Oversold? (DailyFX)
■
Warning! 7 Lies All Women Tell Men (MadeMan)
■
Jobs Data Not Good News For Gold, Silver (TradersHuddle.com)
■
11 Ways to Lower Cholesterol (Lifescript)
[?]
TheStreet Premium Services For Personal Service: 877-471-2967
Compare All Services
Jim Cramer's Action Alerts PLUS:
Trade right alongside a Wall Street pro — enjoy access to his Charitable Trust portfolio and be sent trade alerts BEFORE he makes a move. Learn More
Login
ETF Profits:
Get money-making ideas from the hottest investment vehicle on the planet. Our experts show you how to play various ETF sectors to help pump-up your portfolio. Learn More
Login
OptionsProfits:
Get 50+ trade ideas a week from the industry's top options experts. Plus — exclusive commentary on market trends and essential trading tools. Learn More
Login
Real Money:
Our team of professional Wall Street Pros — including Jim Cramer, Doug Kass, and Nicholas Vardy — delivers intelligent analysis, timely trade ideas, and colorful commentary. Learn More
Login
Stocks Under $10:
Break into the market with small- and mid-cap stocks... all $10 or less! David Peltier tells you exactly which low-priced stocks he's buying and selling. Learn More
Login
To begin commenting right away, you can log in below using your Disqus, Facebook, Twitter, OpenID or Yahoo login credentials. Alternatively, you can post a comment as a "guest" just by entering an email address. Your use of the commenting tool is subject to multiple terms of service/use and privacy policies - see here for more details.
blog comments powered by Disqus
Disqus
Like
Dislike
Add New Comment
Required: Please login below to comment.
Post as …
Showing 0 comments
Sort by Popular nowBest ratingNewest firstOldest first Subscribe by email Subscribe by RSS
Real-time updating is enabled. (Pause)
Dow Jones
S&P 500
NASDAQ
12,874.83
1,345.12
2,904.78
Oil *
116.20
UP
+29.70
UP
+0.79
UP
+2.79
10 Yr
1.97%
+0.23%
+0.06%
+0.10%
Data delayed 20 minutes
Top Stories and Tools
10 Dividend Stocks Most Likely to Outperform
10 Worst Cars of All Time
Ex-Dividend Date Calendar: Find Payout Dates
2012 Bank Ratings and Safety Screener
FREE NEWSLETTER: Dividend and Income Investor
Barrington's Best Stock Picks for 2012
Brokerage Partners
Free Newsletters from TheStreet
After the Bell
Before the Bell
Booyah! Newsletter
ETF Daily
Midday Bell
TheStreet Top 10 Stories
Winners & Losers
We respect your privacy.
Manage Newsletters
Podcasts
[Select Podcast]The Real StoryWall Street Confidential Podcast
Daily RSS Updates
TheStreet Mobile Edition
Connect with TheStreet
.
TheStreet Mobile | MainStreet | StockPickr | BankingMyWay | Jim Cramer | Doug Kass | Real Money | Try Action Alerts PLUS
Your Account
Log In
TheStreet Corporate | Home | About Us | Advertise | Reprints | Customer Service | Employment | Privacy Policy | Sitemap | Topic Archive | Video Archive | Stock Quotes Online | Terms of Use
TheStreet's enterprise databases running Oracle are professionally monitored and managed by Pythian Remote DBA.
Quotes delayed at least 20 minutes for all exchanges. Market Data provided by Interactive Data. Company fundamental data provided by Morningstar. Earnings and ratings provided by Zacks. Mutual fund data provided by Valueline. ETF data provided by Lipper. Terms & Conditions. Powered and implemented by Interactive Data Managed Solutions.
TheStreet Ratings updates stock ratings daily. However, if no rating change occurs, the data on this page does not update. The data does update after 90 days if no rating change occurs within that time period.
IDC calculates the Market Cap for the basic symbol to include common shares only. Year-to-date mutual fund returns are calculated on a monthly basis by Value Line and posted mid-month.
*Oil Data in Market Overview is Brent Crude Pricing
© 2012 TheStreet, Inc. All rights reserved.
Stock Game
Earnings Calendar
Latest News
Mad Money Screener
Dividend Calendar
Fan
Follow
Antwort auf Beitrag Nr.: 42.715.400 von asics01 am 07.02.12 21:21:26 Boa ey, seitenlanges nichts! war das nicht zu kürzen??
Boa ey, seitenlanges nichts! war das nicht zu kürzen??
was mich neugierig gemacht hat waren folgende insiderkäufe kurz vor erreichen des fiesen tiefs:

http://www.nasdaq.com/symbol/clsn/insider-trades
das schaut doch irgendwie konzertiert aus.
 Boa ey, seitenlanges nichts! war das nicht zu kürzen??
Boa ey, seitenlanges nichts! war das nicht zu kürzen??
was mich neugierig gemacht hat waren folgende insiderkäufe kurz vor erreichen des fiesen tiefs:
http://www.nasdaq.com/symbol/clsn/insider-trades
das schaut doch irgendwie konzertiert aus.

Antwort auf Beitrag Nr.: 42.715.276 von Biohero am 07.02.12 21:02:42Oh sorry, du hast sie schon aufgeführt...
ja fehler von mir,habe beim kopieren und einstellen nicht aufgepasst













Antwort auf Beitrag Nr.: 42.715.276 von Biohero am 07.02.12 21:02:42Brean Murray mit Buy rating für CLSN und erwarten Positive P3 Daten .
http://www.streetinsider.com/New+Coverage/Brean+Murray+Carre…
Brean Murray Carret & Co. Starts Celsion Corp (CLSN) at Buy; Expectiing Positive Phase 3 Data This Year
Brean Murray Carret & Co. initiates coverage on Celsion Corp (NASDAQ: CLSN) with a Buy. PT $7.00.
Brean analyst says, "We believe ThermoDox will be broadly adopted once it is approved. Due to the potential ability of ThermoDox to reduce the recurrence rate in intermediate HCC patients, we are optimistic about its adoption as the future standard of care, especially given how seamlessly ThermoDox can be integrated with RFA. Its effectiveness should increase the number of RFA procedures and enable the RFA/ThermoDox combination to take market share from procedures like TACE with chemotherapy. We view the use of an approved active agent (doxorubicin) as a risk mitigating factor for Celsion."
http://www.streetinsider.com/New+Coverage/Brean+Murray+Carre…
Brean Murray Carret & Co. Starts Celsion Corp (CLSN) at Buy; Expectiing Positive Phase 3 Data This Year
Brean Murray Carret & Co. initiates coverage on Celsion Corp (NASDAQ: CLSN) with a Buy. PT $7.00.
Brean analyst says, "We believe ThermoDox will be broadly adopted once it is approved. Due to the potential ability of ThermoDox to reduce the recurrence rate in intermediate HCC patients, we are optimistic about its adoption as the future standard of care, especially given how seamlessly ThermoDox can be integrated with RFA. Its effectiveness should increase the number of RFA procedures and enable the RFA/ThermoDox combination to take market share from procedures like TACE with chemotherapy. We view the use of an approved active agent (doxorubicin) as a risk mitigating factor for Celsion."
Celsion notiert aktuell fast auf allzeittief obwohl die Insider sowie die Institutionen weiter aktien zugekauft haben .
Phase 3 daten werden vor jahresende veröffentlicht ,selbst das management glaubt an positive daten sollte das so eintreffen dann haben wir hier den nächsten verzehnfacher mit leichtigkeit wenn man bedenkt das die marktkap gerade mal 60 million dollar beträgt .
Insiderkäufe
http://www.secform4.com/insider-trading/749647.htm
CLSN ist für mich mit abstand die unterbewerteste aktie mit einem phase 3 kandidat im krebsbereich . Schaut euch z.b. die bewertung von müllaktien wie KERX,CVM,CTIC usw dann versteht ihr was ich meine .
Phase 3 daten werden vor jahresende veröffentlicht ,selbst das management glaubt an positive daten sollte das so eintreffen dann haben wir hier den nächsten verzehnfacher mit leichtigkeit wenn man bedenkt das die marktkap gerade mal 60 million dollar beträgt .
Insiderkäufe
http://www.secform4.com/insider-trading/749647.htm
CLSN ist für mich mit abstand die unterbewerteste aktie mit einem phase 3 kandidat im krebsbereich . Schaut euch z.b. die bewertung von müllaktien wie KERX,CVM,CTIC usw dann versteht ihr was ich meine .
Neues Buy-Rating mit Kursziel 10$ und weitere Insiderkäufe ...Ich denke die 10$ werden wir noch vor Jahresende sehen .
(CLSN) Celsion initiated with a Buy at Roth Capital
Target $10
Insiderkäufe
http://finance.yahoo.com/q/it?s=CLSN+Insider+Transactions
(CLSN) Celsion initiated with a Buy at Roth Capital
Target $10
Insiderkäufe
http://finance.yahoo.com/q/it?s=CLSN+Insider+Transactions
Weitere Insiderkäufe kurz vor der veröffentlichung der Phase 3 daten .
http://www.secform4.com/insider-trading/749647.htm
Celsion hat sich in den letzten 6 monate super entwickelt und das ist erst der anfang denn sollten die Phase 3 daten positiv sein dann sehe ich minimum $20++ weil die aktuelle marktkap von aktuell $160 M einfach nur lächerlich ist für ein Blockbuster Kandidat .

http://www.secform4.com/insider-trading/749647.htm
Celsion hat sich in den letzten 6 monate super entwickelt und das ist erst der anfang denn sollten die Phase 3 daten positiv sein dann sehe ich minimum $20++ weil die aktuelle marktkap von aktuell $160 M einfach nur lächerlich ist für ein Blockbuster Kandidat .
Sieht so aus als würden weitere institutionelle auf den fahrenden zug aufspringen ...

Antwort auf Beitrag Nr.: 43.617.811 von Biohero am 18.09.12 18:42:11Phase 3 Daten kommen in Januar bis dahin dürfte CLSN locker über $10 notieren ..
Celsion (CLSN_) is up first. Last week, the company announced the passing of the 380th progression event in its pivotal study of the liver cancer therapy Thermodox study. Celsion is now locking up the study and analyzing data, with results expected in January.
Celsion (CLSN_) is up first. Last week, the company announced the passing of the 380th progression event in its pivotal study of the liver cancer therapy Thermodox study. Celsion is now locking up the study and analyzing data, with results expected in January.
in zwei Wochen sind wir schlauer. die Chancen für die Zulassung sollen gut sein, aber wehe wenn nicht. mir ist das alles zu heiß, auch wenn die relative stärke der Aktie für weitaus höhere kurse spricht.
Wie heiß das ist kann man ja heute am Intradaychart sehen...
Hoffe für alle ivestierten, dass sie rechzeitig verkauft haben, denn:
http://finance.yahoo.com/news/celsion-announces-results-phas…
http://finance.yahoo.com/news/celsion-announces-results-phas…
 Ganz bestimmt! Selbst in Übersee gibt es total überraschte Gesichter!!! Pushen,pushen,pushen... und dann dann hoffen das alle verkauft haben!
Ganz bestimmt! Selbst in Übersee gibt es total überraschte Gesichter!!! Pushen,pushen,pushen... und dann dann hoffen das alle verkauft haben!
Antwort auf Beitrag Nr.: 44.087.502 von kmastra am 31.01.13 14:26:52


wenn die heute weiter fällt dann werd ich mal ne spass posi nehmen.



wenn die heute weiter fällt dann werd ich mal ne spass posi nehmen.
don´t catch a falling knife!!!
Viel Glück!
Viel Glück!
Antwort auf Beitrag Nr.: 44.090.482 von zet100 am 01.02.13 07:29:11Soll das ein Witz sein? Ich habe Celsion in keinster Weise gepusht. Sogar davor gewarnt wie heiß das Ganze hier ist! Im übrigen hatte ich Celsion auch nie im Depot. Hier nur die Information reingestellt!
Da musst du dich schon an den Helden persönlich wenden!!!
Da musst du dich schon an den Helden persönlich wenden!!!
Antwort auf Beitrag Nr.: 44.091.995 von ultrawuffsn am 01.02.13 12:26:37wurde in stuttgart eingestoppt.meine vermutung war richtig das der kurs bis zum 3. support weiterfällt.mal sehn was nächste woche geht.kurs ist ja charttechnisch massiv unterstützt.viel weiter runter wird es nicht gehn.

Antwort auf Beitrag Nr.: 44.095.011 von ultrawuffsn am 01.02.13 22:11:24 Up to $25,000,000 of Shares
Common Stock
We have entered into a sales agreement with Cantor Fitzgerald & Co. relating to shares of our common stock offered by this prospectus supplement and the accompanying prospectus. In accordance with the terms of the sales agreement, we may offer and sell shares of our common stock having an aggregate offering price of up to $25,000,000 from time to time through Cantor Fitzgerald & Co., acting as agent.
Our common stock is listed on The NASDAQ Capital Market under the symbol “CLSN”. On January 31, 2013, the last reported sale price of our common stock on The NASDAQ Capital Market was $1.51 per share.
Sales of our common stock, if any, under this prospectus supplement and the accompanying prospectus may be made in sales deemed to be “at-the-market” equity offerings as defined in Rule 415 promulgated under the Securities Act of 1933, as amended (Securities Act), including sales made directly on or through The NASDAQ Capital Market, the existing trading market for our common stock, sales made to or through a market maker other than on an exchange or otherwise, in negotiated transactions at market prices prevailing at the time of sale or at prices related to such prevailing market prices, and/or any other method permitted by law, including in privately negotiated transactions. Cantor Fitzgerald & Co. will act as sales agent on a best efforts basis and use commercially reasonable efforts to sell on our behalf all of the shares of common stock requested to be sold by us, consistent with its normal trading and sales practices, on mutually agreed terms between Cantor Fitzgerald & Co. and us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
Cantor Fitzgerald & Co. will be entitled to compensation at a fixed commission rate of 3.0% of the gross sales price per share sold. In connection with the sale of our common stock on our behalf, Cantor Fitzgerald & Co. will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of Cantor Fitzgerald & Co. will be deemed to be underwriting commissions or discounts.
Investing in our securities involves a high degree of risk. Before making an investment decision, please read “Risk Factors” beginning on page S-7 of this prospectus supplement, page 8 of the accompanying prospectus and in the documents incorporated by reference into this prospectus supplement and the accompanying prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Common Stock
We have entered into a sales agreement with Cantor Fitzgerald & Co. relating to shares of our common stock offered by this prospectus supplement and the accompanying prospectus. In accordance with the terms of the sales agreement, we may offer and sell shares of our common stock having an aggregate offering price of up to $25,000,000 from time to time through Cantor Fitzgerald & Co., acting as agent.
Our common stock is listed on The NASDAQ Capital Market under the symbol “CLSN”. On January 31, 2013, the last reported sale price of our common stock on The NASDAQ Capital Market was $1.51 per share.
Sales of our common stock, if any, under this prospectus supplement and the accompanying prospectus may be made in sales deemed to be “at-the-market” equity offerings as defined in Rule 415 promulgated under the Securities Act of 1933, as amended (Securities Act), including sales made directly on or through The NASDAQ Capital Market, the existing trading market for our common stock, sales made to or through a market maker other than on an exchange or otherwise, in negotiated transactions at market prices prevailing at the time of sale or at prices related to such prevailing market prices, and/or any other method permitted by law, including in privately negotiated transactions. Cantor Fitzgerald & Co. will act as sales agent on a best efforts basis and use commercially reasonable efforts to sell on our behalf all of the shares of common stock requested to be sold by us, consistent with its normal trading and sales practices, on mutually agreed terms between Cantor Fitzgerald & Co. and us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
Cantor Fitzgerald & Co. will be entitled to compensation at a fixed commission rate of 3.0% of the gross sales price per share sold. In connection with the sale of our common stock on our behalf, Cantor Fitzgerald & Co. will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of Cantor Fitzgerald & Co. will be deemed to be underwriting commissions or discounts.
Investing in our securities involves a high degree of risk. Before making an investment decision, please read “Risk Factors” beginning on page S-7 of this prospectus supplement, page 8 of the accompanying prospectus and in the documents incorporated by reference into this prospectus supplement and the accompanying prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Antwort auf Beitrag Nr.: 44.095.576 von ultrawuffsn am 02.02.13 09:51:05können also 25 millionen neue aktien auf den markt schmeißen um frisches kapital zu bekommen.jetzt erklärt sich auch das hier noch keine entscheidene gegenbewegung zustande gekommen ist. die werden schon einige teile auf den markt gebracht haben bei dem hammervolumen.laut einem anderen juser haben die noch 30 mille chash.also auf null wird es nicht gehn.
die werden schon einige teile auf den markt gebracht haben bei dem hammervolumen.laut einem anderen juser haben die noch 30 mille chash.also auf null wird es nicht gehn. läuft mindestens noch mal auf die 2 dollar hoch als gegenbewegung.
läuft mindestens noch mal auf die 2 dollar hoch als gegenbewegung.
 die werden schon einige teile auf den markt gebracht haben bei dem hammervolumen.laut einem anderen juser haben die noch 30 mille chash.also auf null wird es nicht gehn.
die werden schon einige teile auf den markt gebracht haben bei dem hammervolumen.laut einem anderen juser haben die noch 30 mille chash.also auf null wird es nicht gehn. läuft mindestens noch mal auf die 2 dollar hoch als gegenbewegung.
läuft mindestens noch mal auf die 2 dollar hoch als gegenbewegung.
Antwort auf Beitrag Nr.: 44.095.617 von ultrawuffsn am 02.02.13 10:12:27Our common stock is listed on The NASDAQ Capital Market under the symbol “CLSN”. On January 31, 2013, the last reported sale price of our common stock on The NASDAQ Capital Market was $1.51 per share.
letzte dillution bei 1,51. viele teile werden die nicht mehr haben zum schmeißen.steht ja auch drinn bei bedarf sollten die ausgegeben werden.wenn die fertig sind oder aufhören damit startet die gegenbewegung.montag vielleicht.
viele teile werden die nicht mehr haben zum schmeißen.steht ja auch drinn bei bedarf sollten die ausgegeben werden.wenn die fertig sind oder aufhören damit startet die gegenbewegung.montag vielleicht.
letzte dillution bei 1,51.
 viele teile werden die nicht mehr haben zum schmeißen.steht ja auch drinn bei bedarf sollten die ausgegeben werden.wenn die fertig sind oder aufhören damit startet die gegenbewegung.montag vielleicht.
viele teile werden die nicht mehr haben zum schmeißen.steht ja auch drinn bei bedarf sollten die ausgegeben werden.wenn die fertig sind oder aufhören damit startet die gegenbewegung.montag vielleicht.
Antwort auf Beitrag Nr.: 44.095.628 von ultrawuffsn am 02.02.13 10:15:36chash haben die also erst mal.also geht es hier weiter.wer auf null wartet kann wohl lange warten. werden sicherlich weitere news folgen was nun wird mit der gescheiterten phase 3.jede news kann zu neuer speku führen.nächste woche gehts über 2 dollar.
werden sicherlich weitere news folgen was nun wird mit der gescheiterten phase 3.jede news kann zu neuer speku führen.nächste woche gehts über 2 dollar.
 werden sicherlich weitere news folgen was nun wird mit der gescheiterten phase 3.jede news kann zu neuer speku führen.nächste woche gehts über 2 dollar.
werden sicherlich weitere news folgen was nun wird mit der gescheiterten phase 3.jede news kann zu neuer speku führen.nächste woche gehts über 2 dollar.
Antwort auf Beitrag Nr.: 44.095.632 von ultrawuffsn am 02.02.13 10:18:18VERBINDLICHKEITEN UND EIGENKAPITAL
Kurzfristige Verbindlichkeiten:
Verbindlichkeiten aus Lieferungen und
$ 2.944.956 $ 4.010.203
Passive Rechnungsabgrenzungen
2.715.509 2.031.934
Wechselobligo - kurzfristiger Anteil
945.433 110.287
Summe kurzfristige Verbindlichkeiten
6.605.898 6.152.424
Stammaktien Optionsschein Haftung
1.417.248 166.398
Wechselobligo - langfristige Teil
4.140.186 71.602
Andere langfristige Verbindlichkeiten
221.832 65.467
Summe der Passiva
12.385.164 6.455.891
Eigenkapital:
Stammaktien, $ 0,01 Nennwert; 75.000.000 Aktien genehmigt und 35.459.494 und 33.899.057 Aktien ausgegeben am 30. September 2012 und dem 31. Dezember 2011 und 34.792.207 und 33.186.325 Aktien im Umlauf am 30. September 2012 und 31. Dezember 2011, jeweils
354.595 338.991
Zusätzlich eingezahltes Kapital
158.121.079 153.237.225
Kumulierten übrigen Comprehensive Loss
(131,306 ) (276,700 )
Bilanzverlust
(142,620,295 ) (124,221,823 )
Zwischensumme
15.724.073 29.077.693
Eigene Aktien zu Anschaffungskosten (667.287 und 712.732 Aktien am 30. September 2012 und 31. Dezember 2011, jeweils)
(2,700,085 ) (2,884,125 )
Summe Eigenkapital
13.023.988 26.193.568
Total Verbindlichkeiten und Eigenkapital
$ 25.409.152 $ 32.649.459
Kurzfristige Verbindlichkeiten:
Verbindlichkeiten aus Lieferungen und
$ 2.944.956 $ 4.010.203
Passive Rechnungsabgrenzungen
2.715.509 2.031.934
Wechselobligo - kurzfristiger Anteil
945.433 110.287
Summe kurzfristige Verbindlichkeiten
6.605.898 6.152.424
Stammaktien Optionsschein Haftung
1.417.248 166.398
Wechselobligo - langfristige Teil
4.140.186 71.602
Andere langfristige Verbindlichkeiten
221.832 65.467
Summe der Passiva
12.385.164 6.455.891
Eigenkapital:
Stammaktien, $ 0,01 Nennwert; 75.000.000 Aktien genehmigt und 35.459.494 und 33.899.057 Aktien ausgegeben am 30. September 2012 und dem 31. Dezember 2011 und 34.792.207 und 33.186.325 Aktien im Umlauf am 30. September 2012 und 31. Dezember 2011, jeweils
354.595 338.991
Zusätzlich eingezahltes Kapital
158.121.079 153.237.225
Kumulierten übrigen Comprehensive Loss
(131,306 ) (276,700 )
Bilanzverlust
(142,620,295 ) (124,221,823 )
Zwischensumme
15.724.073 29.077.693
Eigene Aktien zu Anschaffungskosten (667.287 und 712.732 Aktien am 30. September 2012 und 31. Dezember 2011, jeweils)
(2,700,085 ) (2,884,125 )
Summe Eigenkapital
13.023.988 26.193.568
Total Verbindlichkeiten und Eigenkapital
$ 25.409.152 $ 32.649.459
Antwort auf Beitrag Nr.: 44.097.055 von ultrawuffsn am 03.02.13 08:48:17Total Verbindlichkeiten und Eigenkapital
$ 25.409.152 $ 32.649.459
25 mille eigenkapital haben die ja noch.die sind jetzt nicht pleite.
zusätzlich bringen die 25 mille aktien je nach notwendigkeit auf den markt um arbeiten zu können.
ich werde meine teile mal ganz doll festhalten.gehts montag noch weiter runter erwäge ich nachzukaufen.
$ 25.409.152 $ 32.649.459
25 mille eigenkapital haben die ja noch.die sind jetzt nicht pleite.

zusätzlich bringen die 25 mille aktien je nach notwendigkeit auf den markt um arbeiten zu können.
ich werde meine teile mal ganz doll festhalten.gehts montag noch weiter runter erwäge ich nachzukaufen.

Antwort auf Beitrag Nr.: 44.097.061 von ultrawuffsn am 03.02.13 08:51:31
Historische Kurse (Nasdaq)
Monat:
Datum Erster Hoch Tief Schluss Stücke Volumen
01.02.13 1,42 1,44 1,25 1,32 $ 17.034.220 21,7 M
31.01.13 1,59 1,72 1,41 1,51 $ 34.764.607 53,6 M
30.01.13 7,95 8,38 7,775 8,02 $ 2.810.618 21,6 M
29.01.13 8,05 8,33 7,60 7,68 $ 2.706.739 19,6 M
28.01.13 7,80 8,45 7,80 8,18 $ 3.973.928 26,5 M
25.01.13 7,56 7,84 7,44 7,72 $ 2.833.482 18,6 M
am freitag haben die zu 1,51 schon aktien auf den markt gebracht(siehe news).wie gesagt wokingkapital.deshalb auch das hammervolumen und natürlich wegen dem vorerst scheitern der phase 3.ist ja klar.die sind also nicht pleite.weiß jemand was die noch in der pipline haben???? gehe aber davon aus das die weiter forschen bei dem teil.mal sehn.weitere news werden die nächste woche sicher folgen.
Historische Kurse (Nasdaq)
Monat:
Datum Erster Hoch Tief Schluss Stücke Volumen
01.02.13 1,42 1,44 1,25 1,32 $ 17.034.220 21,7 M
31.01.13 1,59 1,72 1,41 1,51 $ 34.764.607 53,6 M
30.01.13 7,95 8,38 7,775 8,02 $ 2.810.618 21,6 M
29.01.13 8,05 8,33 7,60 7,68 $ 2.706.739 19,6 M
28.01.13 7,80 8,45 7,80 8,18 $ 3.973.928 26,5 M
25.01.13 7,56 7,84 7,44 7,72 $ 2.833.482 18,6 M
am freitag haben die zu 1,51 schon aktien auf den markt gebracht(siehe news).wie gesagt wokingkapital.deshalb auch das hammervolumen und natürlich wegen dem vorerst scheitern der phase 3.ist ja klar.die sind also nicht pleite.weiß jemand was die noch in der pipline haben???? gehe aber davon aus das die weiter forschen bei dem teil.mal sehn.weitere news werden die nächste woche sicher folgen.

Antwort auf Beitrag Nr.: 44.097.092 von ultrawuffsn am 03.02.13 09:26:11könnte heute soweit sein.
ziel 2 dollar.

ziel 2 dollar.
Antwort auf Beitrag Nr.: 44.109.699 von ultrawuffsn am 06.02.13 14:57:49trüben vorbörse 1,26/1,27
über tageshoch von gestern.gestern 1. grüne kerze,weitere sollten folgen bis zum wiederstand von 2 dollar.

über tageshoch von gestern.gestern 1. grüne kerze,weitere sollten folgen bis zum wiederstand von 2 dollar.

Antwort auf Beitrag Nr.: 44.109.735 von ultrawuffsn am 06.02.13 15:03:26hier der auszug.
Celsion und Hisun haben vereinbart, dass der Technology Development Contract eingegangen am 18. Januar, wird 2013 in Kraft bleiben, während die Parteien zur Zusammenarbeit fortsetzen und Auswertung nächsten Schritte in Bezug auf ThermoDox ®, das die Subgruppenanalyse der chinesischen Kohorte gehören Patienten in der Phase-III-Studie für primären Leberkrebs und andere Aktivitäten, die Entwicklung von ThermoDox ® für das Gebiet zu fördern.
heißt für mich.die bekommen die 5 millionen nicht aus china(ist ja klar da phase 3 gescheitert) aber der kontrakt bleibt in kraft.die versuchen also weiter phase 3 zu bekommen.

Celsion und Hisun haben vereinbart, dass der Technology Development Contract eingegangen am 18. Januar, wird 2013 in Kraft bleiben, während die Parteien zur Zusammenarbeit fortsetzen und Auswertung nächsten Schritte in Bezug auf ThermoDox ®, das die Subgruppenanalyse der chinesischen Kohorte gehören Patienten in der Phase-III-Studie für primären Leberkrebs und andere Aktivitäten, die Entwicklung von ThermoDox ® für das Gebiet zu fördern.
heißt für mich.die bekommen die 5 millionen nicht aus china(ist ja klar da phase 3 gescheitert) aber der kontrakt bleibt in kraft.die versuchen also weiter phase 3 zu bekommen.

Antwort auf Beitrag Nr.: 44.110.020 von ultrawuffsn am 06.02.13 15:45:28zur zeit 1,25 über 1,30 gehts scharf.
wenn die gescheiterte phase 3 weiter machen dann haben wir wieder ne gute speku und kurs wird sich in richtung 2-3 dollar bewegen.

wenn die gescheiterte phase 3 weiter machen dann haben wir wieder ne gute speku und kurs wird sich in richtung 2-3 dollar bewegen.
Antwort auf Beitrag Nr.: 44.110.392 von ultrawuffsn am 06.02.13 16:46:23
CLSN
$1.23
*
unch
negative
*Real-Time - data as of 2/6/2013 10:50:57 AM











CLSN
$1.23
*
unch
negative
*Real-Time - data as of 2/6/2013 10:50:57 AM












Antwort auf Beitrag Nr.: 44.110.432 von ThumsUp am 06.02.13 16:51:34Celsion Corp. (CLSN)
-NasdaqCM
1.24 Up 0.01(0.81%) 10:54AM EST - Nasdaq Real Time Price
-NasdaqCM
1.24 Up 0.01(0.81%) 10:54AM EST - Nasdaq Real Time Price
Antwort auf Beitrag Nr.: 44.110.442 von ultrawuffsn am 06.02.13 16:54:49fette bids auf der 1,20.über 100k sieht nach boden aus.
sieht nach boden aus.
 sieht nach boden aus.
sieht nach boden aus.
sieht gut aus auf Tageshoch bei 1,29$ geschlossen bin heute zu 1,22$ rein und hoffen auf eine t. Gegenbewegung bis in den Bereich von 1,8-1,95$ das wäre ja schonmal ein schönes +
LG Printi
LG Printi
nachbörslich 1,34$
das kann am Montag knallen
und nun ab ins Wochenende ein bischen Geld unters Volk bringen
LG Printi
das kann am Montag knallen

und nun ab ins Wochenende ein bischen Geld unters Volk bringen

LG Printi
Ein versöhnlicher Wochenhausklang.
Zielgerichtete Therapien wie diese von Celsion
bergen noch viel Potenzial. Diese neue Technologie, bin ich zuversichtlich, , was niemand sonst kann - Wärme zielgerichtete Therapien haben ein großes Potenzial für die Zukunft.
Die Daten muss man sich noch genauer anschauen um Erkenntnisse zu erlangen und Entscheidungen zu treffen wie so die Phase III fehlgeschlagen ist. Die Auswertungen laufen und
wer wer weiß welche interessanten Erkenntnisse hier noch raus kommen.
Also sind wir etwas geduldig, erste Kurziele sollten dann zur Schliesung des offenen GAP wie genannt zwischen 2 $- und 2, 75 $ sein.
Zielgerichtete Therapien wie diese von Celsion
bergen noch viel Potenzial. Diese neue Technologie, bin ich zuversichtlich, , was niemand sonst kann - Wärme zielgerichtete Therapien haben ein großes Potenzial für die Zukunft.
Die Daten muss man sich noch genauer anschauen um Erkenntnisse zu erlangen und Entscheidungen zu treffen wie so die Phase III fehlgeschlagen ist. Die Auswertungen laufen und
wer wer weiß welche interessanten Erkenntnisse hier noch raus kommen.
Also sind wir etwas geduldig, erste Kurziele sollten dann zur Schliesung des offenen GAP wie genannt zwischen 2 $- und 2, 75 $ sein.
vorbörslich bereits bei 1,4$
Das kann jetzt sehr schnell auf 2$ gehen.
LG Printi
Das kann jetzt sehr schnell auf 2$ gehen.
LG Printi


Das Volumen ist krass....nach noch nicht einmal einer Stunde!!! 
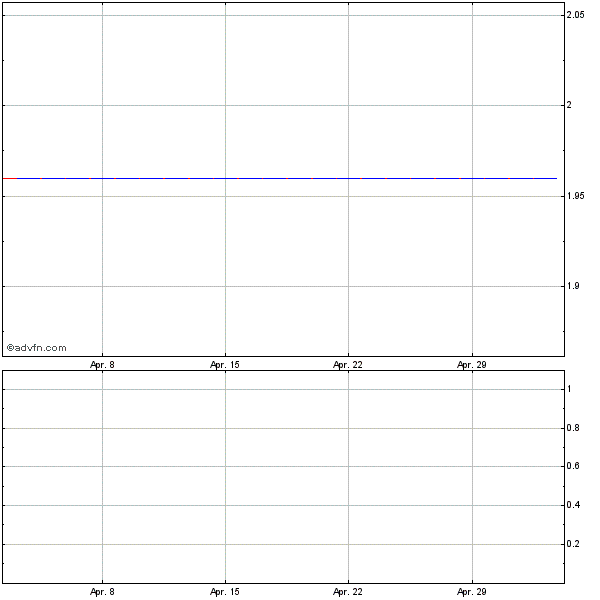

schaut schonmal nicht schlecht aus die Futures haben auch schön ins + gedreht

Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 191 | ||
| 98 | ||
| 71 | ||
| 67 | ||
| 67 | ||
| 47 | ||
| 42 | ||
| 33 | ||
| 28 | ||
| 27 |
| Wertpapier | Beiträge | |
|---|---|---|
| 27 | ||
| 24 | ||
| 22 | ||
| 20 | ||
| 20 | ||
| 19 | ||
| 19 | ||
| 18 | ||
| 16 | ||
| 16 |





















