Amarin - The Science Of Lipid Therapy - 500 Beiträge pro Seite (Seite 2)
eröffnet am 03.01.14 20:10:32 von
neuester Beitrag 04.04.24 15:47:54 von
neuester Beitrag 04.04.24 15:47:54 von
Beiträge: 1.840
ID: 1.190.027
ID: 1.190.027
Aufrufe heute: 4
Gesamt: 156.369
Gesamt: 156.369
Aktive User: 0
ISIN: US0231112063 · WKN: A0NBNG · Symbol: AMRN
0,8557
USD
-2,61 %
-0,0229 USD
Letzter Kurs 21:20:53 Nasdaq
Neuigkeiten
24.04.24 · globenewswire |
22.04.24 · globenewswire |
15.04.24 · globenewswire |
Amarin Highlights Key Data Providing Mechanistic Insights into Eicosapentaenoic Acid (EPA) at ACC.24 08.04.24 · globenewswire |
06.04.24 · globenewswire |
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,7296 | +25,04 | |
| 6,0000 | +25,00 | |
| 9,5000 | +18,75 | |
| 0,6400 | +18,52 | |
| 1,0900 | +14,74 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,8410 | -17,06 | |
| 9,7200 | -19,60 | |
| 4,0000 | -27,27 | |
| 2,7280 | -29,14 | |
| 14,510 | -32,32 |
Amarin Jumps After Diabetes Association Adds Drug to Its 'Must Have' List
The American Diabetes Association adds Amarin's Vascepa drug to its standard of care recommendation list.
Tony Owusu
Mar 28, 2019 8:11 AM EDT
Istock
Shares of pharmaceutical company Amarin (AMRN) were up more than 6% in premarket Thursday after the company's Vascepa drug was added to the American Diabetes Association's Standards of Medical Care in Diabetes list for 2019.
The drug, along with a specialized diet, has been shown to reduce triglyceride levels in adult patients with severe hypertriglyceridemia. Amarin has agreed to give the Food and Drug Administration data to support the ADA's finding in order expand Vascepa's FDA label to include its recommendation.
"As we have commenced transmission of data to the FDA for the submission of our sNDA seeking an expansion of the Vascepa label based on the landmark REDUCE-IT results, we are pleased by ADA's acknowledgement of the importance of the REDUCE-IT results in its 2019 update of the Standards of Care," said Dr. Craig B. Granowitz, senior vice president and chief medical officer of Amarin.
Amarin said that Vascepa works without raising bad cholesterol when a four-gram dose is taken daily.
The American Diabetes Association adds Amarin's Vascepa drug to its standard of care recommendation list.
Tony Owusu
Mar 28, 2019 8:11 AM EDT
Istock
Shares of pharmaceutical company Amarin (AMRN) were up more than 6% in premarket Thursday after the company's Vascepa drug was added to the American Diabetes Association's Standards of Medical Care in Diabetes list for 2019.
The drug, along with a specialized diet, has been shown to reduce triglyceride levels in adult patients with severe hypertriglyceridemia. Amarin has agreed to give the Food and Drug Administration data to support the ADA's finding in order expand Vascepa's FDA label to include its recommendation.
"As we have commenced transmission of data to the FDA for the submission of our sNDA seeking an expansion of the Vascepa label based on the landmark REDUCE-IT results, we are pleased by ADA's acknowledgement of the importance of the REDUCE-IT results in its 2019 update of the Standards of Care," said Dr. Craig B. Granowitz, senior vice president and chief medical officer of Amarin.
Amarin said that Vascepa works without raising bad cholesterol when a four-gram dose is taken daily.
Amarin takes application to expand Vascepa label to the FDA as M&A chatter heats up
by Natalie Grover
— on March 29, 2019 06:16 AM EDT
Updated: 08:26 AM
PDF
Having unveiled an unexpected set of heart protective results for their fish oil-derived pill Vascepa, Amarin is marching ahead to fulfill blockbuster expectations for its cholesterol-lowering drug by submitting an application to expand Vascepa’s label to include the reduction in cardiovascular risk.
The company sparked a flurry of M&A chatter after revealing data from the REDUCE-IT study last year — a 25% reduction in the risk for the first occurrence of a major cardio event, and a 26% reduction for 3-point MACE, a composite of cardiovascular death, nonfatal heart attack and nonfatal stroke — although concerns about the impact of the mineral oil placebo on the results prompted lingering questions. Earlier this month, Amarin debuted its exploratory analysis of the trial, with researchers suggesting a 30% reduction in cardiovascular events compared to the placebo arm.
John Thero
“The REDUCE-IT results support that approximately 1 fewer major cardiovascular adverse event would occur on average for every 6 patients treated with Vascepa for 5 years on top of statin therapy compared to placebo,” Amarin chief John Thero said in a statement.
On Thursday, Amarin said it was operating under the assumption that the sNDA will be reviewed over a standard ten months resulting in a PDUFA date near the end of January 2020, and that it expects the regulator with organize an advisory committee meeting of outside experts to deliberate and recommend whether Vascepa’s label should be expanded before the FDA makes its final decision.
Earlier in the week, the American Diabetes Association issued fresh “standards of care” guidelines to include Vascepa. They recommended that Vascepa “be considered for patients with diabetes and atherosclerotic cardiovascular disease or other cardiac risk factors on a statin with controlled LDL-C, but with elevated triglycerides to reduce cardiovascular risk.”
Vascepa, which generated about $229 million in 2018 sales, comprises omega-3 acid called EPA derived from fish — was originally approved in 2012 for patients with severe hypertriglyceridemia. It is reasonably priced with an annual price tag of roughly $2400, Amarin contends, adding that the majority of insured patients insurance who obtain Vascepa prescriptions pay a monthly co-pay charge of $9.99 or less.
Meanwhile, PCSK9 inhibitors that were approved in 2015 carried a price close to $14,000 and were pegged to attain blockbuster status for their ability to dramatically lower levels of LDL cholesterol, facing pushback from insurers for their high sticker prices that led to lower adoption than expected, despite later trials that demonstrated they also significantly cut the risk of heart attacks and stroke. Following Amgen’s decision to slash the price of its PCSK9 drug Repatha by 60% to $5,850 last year, the team behind their main rival treatment, Praluent — Regeneron $REGN and Sanofi $SNY — have followed suit with the same discount, beginning early March.
In 2016, Amarin $AMRN won a landmark ruling against the FDA, which allowed the drugmaker to exercise its first amendment rights by promoting Vascepa for off-label uses as long as it does so ‘truthfully.’ The company was also seeking to market the drug to patients with not just severe triglyceride levels, but those considered to have ‘high’ levels of the blood fat.
by Natalie Grover
— on March 29, 2019 06:16 AM EDT
Updated: 08:26 AM
Having unveiled an unexpected set of heart protective results for their fish oil-derived pill Vascepa, Amarin is marching ahead to fulfill blockbuster expectations for its cholesterol-lowering drug by submitting an application to expand Vascepa’s label to include the reduction in cardiovascular risk.
The company sparked a flurry of M&A chatter after revealing data from the REDUCE-IT study last year — a 25% reduction in the risk for the first occurrence of a major cardio event, and a 26% reduction for 3-point MACE, a composite of cardiovascular death, nonfatal heart attack and nonfatal stroke — although concerns about the impact of the mineral oil placebo on the results prompted lingering questions. Earlier this month, Amarin debuted its exploratory analysis of the trial, with researchers suggesting a 30% reduction in cardiovascular events compared to the placebo arm.
John Thero
“The REDUCE-IT results support that approximately 1 fewer major cardiovascular adverse event would occur on average for every 6 patients treated with Vascepa for 5 years on top of statin therapy compared to placebo,” Amarin chief John Thero said in a statement.
On Thursday, Amarin said it was operating under the assumption that the sNDA will be reviewed over a standard ten months resulting in a PDUFA date near the end of January 2020, and that it expects the regulator with organize an advisory committee meeting of outside experts to deliberate and recommend whether Vascepa’s label should be expanded before the FDA makes its final decision.
Earlier in the week, the American Diabetes Association issued fresh “standards of care” guidelines to include Vascepa. They recommended that Vascepa “be considered for patients with diabetes and atherosclerotic cardiovascular disease or other cardiac risk factors on a statin with controlled LDL-C, but with elevated triglycerides to reduce cardiovascular risk.”
Vascepa, which generated about $229 million in 2018 sales, comprises omega-3 acid called EPA derived from fish — was originally approved in 2012 for patients with severe hypertriglyceridemia. It is reasonably priced with an annual price tag of roughly $2400, Amarin contends, adding that the majority of insured patients insurance who obtain Vascepa prescriptions pay a monthly co-pay charge of $9.99 or less.
Meanwhile, PCSK9 inhibitors that were approved in 2015 carried a price close to $14,000 and were pegged to attain blockbuster status for their ability to dramatically lower levels of LDL cholesterol, facing pushback from insurers for their high sticker prices that led to lower adoption than expected, despite later trials that demonstrated they also significantly cut the risk of heart attacks and stroke. Following Amgen’s decision to slash the price of its PCSK9 drug Repatha by 60% to $5,850 last year, the team behind their main rival treatment, Praluent — Regeneron $REGN and Sanofi $SNY — have followed suit with the same discount, beginning early March.
In 2016, Amarin $AMRN won a landmark ruling against the FDA, which allowed the drugmaker to exercise its first amendment rights by promoting Vascepa for off-label uses as long as it does so ‘truthfully.’ The company was also seeking to market the drug to patients with not just severe triglyceride levels, but those considered to have ‘high’ levels of the blood fat.
Heute haben wir einen schönen Anstieg, $23.30 wäre das nächste Ziel, schönes Wochenende.
Jefferies-Potential Beat On Growing Q1 Vascepa Sales - Debate on Expectations $30 target Price
Potential Beat On Growing Q1 Vascepa Sales - Debate on Expectations
Michael J. Yee, Andrew Tsai, Kelechi Chikere, Ph.D., Arshad Haider
April 24, 2019
Key Takeaway
AMRN could report Q1 EPS results in the first week of May and we note IMS scripts have reached all-time highs over the past 9 of 10 weeks, tracking to perhaps ~$75-80M and possibly well above consensus $67M (4 ests). 2019 guidance of $350M would then look conservative and too low. For 2019, we are above at $385M and above cons $365M and guidance could eventually move higher towards our estimate.
Insights
Weekly scripts appear to reach record highs, setting up AMRN for a pot'l Q1 sales beat
Despite management cautioning to expect seasonality in Q1, scripts are up +16% Q/Q (accelerated even more than the +13-15% in Q4) - appearing to track to ~$75-80M in Q1 vs cons $67M (4 ests) and Jefferies at $79M. Historically, Vascepa sales have declined in Q1 over the past couple of years, but we see an increase this year instead due to 1) major new CVOT data during Q4 now published in the NEJM, 2) huge salesforce increase to 400 from 150 recently, and 3) positive doc channel checks suggesting reimbursement has not been much issue. We also note Q4 reported sales were even higher than third-party data (+40% vs +13%) suggesting Q1 IMS script data could be inaccurate (under-capturing) as well although management recently confirmed weekly scripts are tracking fairly accurately to AMRN's internal datapoints. Bigger picture, 2019 guidance of $350M appears conservative and only suggests an $80M+$85M+$90M+$95M trajectory (i.e. modest growth), even though sales grew by a whopping $20M+ Q/Q to $77M in Q4.
Additional Q1 dynamics and thoughts
We acknowledge some buysiders think a beat is already expected given third-party data is available though we argue confirming these strong results after last quarters doubt (huge beat in Q4) would be positive and it means consensus is going up. We also understand the stock has been in a trading range from teens to low $20s and investors seem to want to debate "M&A takeout or bust" more than anything with bears saying no takeout until litigation is settled and FDA label expansion confirmed.
Our estimated $79M in Q1 considers 1) a +9% price increase Dec 2018, 2) offset by increased gross to net typical of Q1, and 3) higher capture of 75% vs 72% for Q4 to be conservative. We can derive Q1 sales of ~$80M were we to assume a G2N of 55% (vs 50% for other quarters). Alternatively, adjusting the capture to historical of 83% (our est) would yield Q sales closer to the $70-75M range, which would still be above consensus estimates of $67M.
Our doctors consistently provide positive feedback on Vascepa
Anecdotally, docs inform us they have "accelerated their prescriptions" post the Nov 2018 AHA conference. The LDL increase in the placebo arm (mineral oil) has not deterred docs from prescribing Vascepa. From our NYC Doc Panel day last month, post the data, one doc increased his prescriptions by 3x-4x to 10/month, while the other doctor now prescribes it several times a month. The latter was formerly not a strong believer in triglyceride (TG) therapies unless patients exceeded the >500mg/dL threshold, but he now envisions his overall usage for "elevated" TG patients to increase as he becomes more familiar and comfortable and reimbursement has been easy.
Company Description
Amarin Corporation
Amarin Corporation, headquartered in Dublin, Ireland and Bedminster, New Jersey is a biopharmaceutical company focused on the commercialization and development of therapeutics to improve cardiovascular health. Amarin's approved drug, Vascepa, is an ultra-pure, EPA-only, omega-3 fatty acid, oral product for the treatment of severe high triglycerides and mixed dyslipidemia. The company’s cardiovascular programs capitalize on Amarin's expertise in the field of lipid science and the known therapeutic benefits of essential fatty acids in treating cardiovascular disease. AMRN reported a positive REDUCE-IT CVOT result in September 2018.
Company Valuation/Risks
Amarin Corporation
Our $30 PT is DCF-based. Risks: clinical, regulatory, a
Potential Beat On Growing Q1 Vascepa Sales - Debate on Expectations
Michael J. Yee, Andrew Tsai, Kelechi Chikere, Ph.D., Arshad Haider
April 24, 2019
Key Takeaway
AMRN could report Q1 EPS results in the first week of May and we note IMS scripts have reached all-time highs over the past 9 of 10 weeks, tracking to perhaps ~$75-80M and possibly well above consensus $67M (4 ests). 2019 guidance of $350M would then look conservative and too low. For 2019, we are above at $385M and above cons $365M and guidance could eventually move higher towards our estimate.
Insights
Weekly scripts appear to reach record highs, setting up AMRN for a pot'l Q1 sales beat
Despite management cautioning to expect seasonality in Q1, scripts are up +16% Q/Q (accelerated even more than the +13-15% in Q4) - appearing to track to ~$75-80M in Q1 vs cons $67M (4 ests) and Jefferies at $79M. Historically, Vascepa sales have declined in Q1 over the past couple of years, but we see an increase this year instead due to 1) major new CVOT data during Q4 now published in the NEJM, 2) huge salesforce increase to 400 from 150 recently, and 3) positive doc channel checks suggesting reimbursement has not been much issue. We also note Q4 reported sales were even higher than third-party data (+40% vs +13%) suggesting Q1 IMS script data could be inaccurate (under-capturing) as well although management recently confirmed weekly scripts are tracking fairly accurately to AMRN's internal datapoints. Bigger picture, 2019 guidance of $350M appears conservative and only suggests an $80M+$85M+$90M+$95M trajectory (i.e. modest growth), even though sales grew by a whopping $20M+ Q/Q to $77M in Q4.
Additional Q1 dynamics and thoughts
We acknowledge some buysiders think a beat is already expected given third-party data is available though we argue confirming these strong results after last quarters doubt (huge beat in Q4) would be positive and it means consensus is going up. We also understand the stock has been in a trading range from teens to low $20s and investors seem to want to debate "M&A takeout or bust" more than anything with bears saying no takeout until litigation is settled and FDA label expansion confirmed.
Our estimated $79M in Q1 considers 1) a +9% price increase Dec 2018, 2) offset by increased gross to net typical of Q1, and 3) higher capture of 75% vs 72% for Q4 to be conservative. We can derive Q1 sales of ~$80M were we to assume a G2N of 55% (vs 50% for other quarters). Alternatively, adjusting the capture to historical of 83% (our est) would yield Q sales closer to the $70-75M range, which would still be above consensus estimates of $67M.
Our doctors consistently provide positive feedback on Vascepa
Anecdotally, docs inform us they have "accelerated their prescriptions" post the Nov 2018 AHA conference. The LDL increase in the placebo arm (mineral oil) has not deterred docs from prescribing Vascepa. From our NYC Doc Panel day last month, post the data, one doc increased his prescriptions by 3x-4x to 10/month, while the other doctor now prescribes it several times a month. The latter was formerly not a strong believer in triglyceride (TG) therapies unless patients exceeded the >500mg/dL threshold, but he now envisions his overall usage for "elevated" TG patients to increase as he becomes more familiar and comfortable and reimbursement has been easy.
Company Description
Amarin Corporation
Amarin Corporation, headquartered in Dublin, Ireland and Bedminster, New Jersey is a biopharmaceutical company focused on the commercialization and development of therapeutics to improve cardiovascular health. Amarin's approved drug, Vascepa, is an ultra-pure, EPA-only, omega-3 fatty acid, oral product for the treatment of severe high triglycerides and mixed dyslipidemia. The company’s cardiovascular programs capitalize on Amarin's expertise in the field of lipid science and the known therapeutic benefits of essential fatty acids in treating cardiovascular disease. AMRN reported a positive REDUCE-IT CVOT result in September 2018.
Company Valuation/Risks
Amarin Corporation
Our $30 PT is DCF-based. Risks: clinical, regulatory, a
New Drug Submission Filed for Vascepa® with Health Canada
GlobeNewswire•April 29, 2019
BEDMINSTER, N.J., and DUBLIN, Ireland, April 29, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, today announced that its licensee in Canada, HLS Therapeutics Inc. (HLS.TO), has filed a New Drug Submission (“NDS”) with Health Canada for Vascepa® (icosapent ethyl) capsules. The NDS seeks an indication for promotion of Vascepa in Canada to reduce the risk of major adverse cardiovascular events (“MACE”) in statin-treated patients with elevated triglycerides and other risk factors. This is the first submission of Vascepa to Health Canada for any indication and, if approved, Vascepa will be the first drug approved in Canada for this important indication.
As previously announced, Vascepa has been granted priority review status by Health Canada. Priority review could accelerate the launch of Vascepa in the Canadian market by up to four-and-a-half months if the product is ultimately approved by Health Canada. Priority review status may be granted to regulatory filings in Canada for new treatments that potentially address serious, life-threatening conditions for which no drug is currently marketed in Canada, and for which there is substantial evidence of clinical effectiveness of that new treatment.
While the NDS includes results from the REDUCE-IT™ cardiovascular outcomes study of Vascepa, review of this regulatory submission in Canada is anticipated to be independent of the review of the supplemental new drug application which Amarin recently filed with the U.S. Food and Drug Administration regarding Vascepa based on the same clinical study results.
“We are hopeful that Vascepa will soon be approved to treat at-risk patients in Canada and we applaud HLS Therapeutics for moving rapidly with this filing,” commented Amarin’s president and chief executive officer, John F. Thero. “Cardiovascular disease is a major health issue in Canada as it is throughout the world. Vascepa provides physicians with a new treatment option to address cardiovascular risks beyond cholesterol management for at-risk patients.”
GlobeNewswire•April 29, 2019
BEDMINSTER, N.J., and DUBLIN, Ireland, April 29, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, today announced that its licensee in Canada, HLS Therapeutics Inc. (HLS.TO), has filed a New Drug Submission (“NDS”) with Health Canada for Vascepa® (icosapent ethyl) capsules. The NDS seeks an indication for promotion of Vascepa in Canada to reduce the risk of major adverse cardiovascular events (“MACE”) in statin-treated patients with elevated triglycerides and other risk factors. This is the first submission of Vascepa to Health Canada for any indication and, if approved, Vascepa will be the first drug approved in Canada for this important indication.
As previously announced, Vascepa has been granted priority review status by Health Canada. Priority review could accelerate the launch of Vascepa in the Canadian market by up to four-and-a-half months if the product is ultimately approved by Health Canada. Priority review status may be granted to regulatory filings in Canada for new treatments that potentially address serious, life-threatening conditions for which no drug is currently marketed in Canada, and for which there is substantial evidence of clinical effectiveness of that new treatment.
While the NDS includes results from the REDUCE-IT™ cardiovascular outcomes study of Vascepa, review of this regulatory submission in Canada is anticipated to be independent of the review of the supplemental new drug application which Amarin recently filed with the U.S. Food and Drug Administration regarding Vascepa based on the same clinical study results.
“We are hopeful that Vascepa will soon be approved to treat at-risk patients in Canada and we applaud HLS Therapeutics for moving rapidly with this filing,” commented Amarin’s president and chief executive officer, John F. Thero. “Cardiovascular disease is a major health issue in Canada as it is throughout the world. Vascepa provides physicians with a new treatment option to address cardiovascular risks beyond cholesterol management for at-risk patients.”
Amarin selloff creates attractive entry point, says Citi Citi analyst Joel Beatty believes the selloff yesterday in shares of Amarin (AMRN) brings an attractive entry point. He raised his price target for the stock to $23 from $20 and keeps a Buy rating on the name. The analyst sees five potential upside catalysts this year, including a likely supportive ICER cost-effectiveness review and potential priority review from the FDA. Further, while an acquisition of Amarin is possible, the company acquiring The Medicines Co. (MDCO) or Esperion (ESPR) "could also be a viable path," says Beatty.
Read more at:
https://thefly.com/landingPageNews.php?id=2901824
Read more at:
https://thefly.com/landingPageNews.php?id=2901824
Amarin to seek European approval for heart drug Vascepa
Clinical trials show Irish company’s fish-oil drug cut incidence of heart attacks and strokes
about 20 hours ago
Dominic Coyle
Amarin chief executive John Thero: ‘Our guidance is for 50 per cent greater growth over the prior year’
Amarin chief executive John Thero: ‘Our guidance is for 50 per cent greater growth over the prior year’
Irish drug company Amarin will seek European approval for its cardiovascular drug Vascepa before the end of the year.
The drug is in the process of seeking broader FDA approval in the US as well, following clinical trial results last year which showed its fish-oil drug dramatically cut the incidence of heart attack and strokes in high-risk patients.
Until now,it has been approved only for the treatment of patients with very high levels of blood fats – indicators of cardiovascular risk – as a treatment to reduce those levels, but without making any claims on cardiovascular events.
Chief executive John Thero said the company was “hoping to have submitted in Europe sometime towards the end of the year”. Amarin, which is based in Dublin but operates largely in the United States, has yet to decide whether it will manage the European rollout in-house or through partnerships with other companies.
‘Death benefits’
Citing the 25 per cent reduction in risk of an adverse cardiovascular event – such as a heart attack or stroke – and a 20 per cent reduction in deaths among high-risk patients, Mr Thero said: “You don’t even see those death benefits with statins. It is rare to see a reduction in these outcome studies in death.
“And we have more recently shown that not only do we reduce the first occurrence of a cardiovascular event but, even if someone has had a heart attack, we help to prevent the second occurrence, a third occurrence. So essentially, over a five-year period, we have one fewer cardiovascular event for every six patients treated. And that’s a number I’ve not seen before,” he said, noting that the equivalent number for statins is around one in 45.
The company said it has seen a strong increase in prescribing of Vascepa by both cardiologists and endocrinologists, as well as GPs, since its clinical trial results were published.
“In the first four months after we had the new data, we had more new physicians prescribing the product than we had in the previous nine months on aggregate,” said Mr Thero.
Bullish expectations
But he cautioned against overly bullish expectations against the company’s target for sales this year of $350 million.
“We don’t yet have an [expanded] label for the product and our sales force, two-thirds of it is brand new, and we only have one reported quarter at this point,” Mr Thero said. “That quarter did go better than expected, but it is only one quarter . Our guidance is for 50 per cent greater growth over the prior year.
“Each quarter last year got better [in sales] and we would expect each quarter this year to get better,” he said. “I do really think of this year as a step [in the company’s development]. It’s sort of that second phase and it is the third phase that really excites.
One in three adults have some risk for cardiovascular disease and we want them to be asking their doctors about it
“From the beginning, we recognised that what we needed was a label approved for cardiovascular risk prevention and it is [now] right around the corner. And that’s really where things begin.”
Mr Thero said an expanded permission on its label would see a further significant increase in its sales force and, for the first time, would allow the company to start promoting the drug directly to consumers for the first time as a therapy that can help prevent heart attacks and strokes.
“One in three adults have some risk for cardiovascular disease and we want them to be asking their doctors about it,” he said.
Cardiovascular disease is also the single biggest cause of death in Ireland, accounting for up to 10,000 deaths a year, according to the Irish Heart Foundation. That is one-third of all deaths, it says, and one in five of all premature deaths.
Clinical trials show Irish company’s fish-oil drug cut incidence of heart attacks and strokes
about 20 hours ago
Dominic Coyle
Amarin chief executive John Thero: ‘Our guidance is for 50 per cent greater growth over the prior year’
Amarin chief executive John Thero: ‘Our guidance is for 50 per cent greater growth over the prior year’
Irish drug company Amarin will seek European approval for its cardiovascular drug Vascepa before the end of the year.
The drug is in the process of seeking broader FDA approval in the US as well, following clinical trial results last year which showed its fish-oil drug dramatically cut the incidence of heart attack and strokes in high-risk patients.
Until now,it has been approved only for the treatment of patients with very high levels of blood fats – indicators of cardiovascular risk – as a treatment to reduce those levels, but without making any claims on cardiovascular events.
Chief executive John Thero said the company was “hoping to have submitted in Europe sometime towards the end of the year”. Amarin, which is based in Dublin but operates largely in the United States, has yet to decide whether it will manage the European rollout in-house or through partnerships with other companies.
‘Death benefits’
Citing the 25 per cent reduction in risk of an adverse cardiovascular event – such as a heart attack or stroke – and a 20 per cent reduction in deaths among high-risk patients, Mr Thero said: “You don’t even see those death benefits with statins. It is rare to see a reduction in these outcome studies in death.
“And we have more recently shown that not only do we reduce the first occurrence of a cardiovascular event but, even if someone has had a heart attack, we help to prevent the second occurrence, a third occurrence. So essentially, over a five-year period, we have one fewer cardiovascular event for every six patients treated. And that’s a number I’ve not seen before,” he said, noting that the equivalent number for statins is around one in 45.
The company said it has seen a strong increase in prescribing of Vascepa by both cardiologists and endocrinologists, as well as GPs, since its clinical trial results were published.
“In the first four months after we had the new data, we had more new physicians prescribing the product than we had in the previous nine months on aggregate,” said Mr Thero.
Bullish expectations
But he cautioned against overly bullish expectations against the company’s target for sales this year of $350 million.
“We don’t yet have an [expanded] label for the product and our sales force, two-thirds of it is brand new, and we only have one reported quarter at this point,” Mr Thero said. “That quarter did go better than expected, but it is only one quarter . Our guidance is for 50 per cent greater growth over the prior year.
“Each quarter last year got better [in sales] and we would expect each quarter this year to get better,” he said. “I do really think of this year as a step [in the company’s development]. It’s sort of that second phase and it is the third phase that really excites.
One in three adults have some risk for cardiovascular disease and we want them to be asking their doctors about it
“From the beginning, we recognised that what we needed was a label approved for cardiovascular risk prevention and it is [now] right around the corner. And that’s really where things begin.”
Mr Thero said an expanded permission on its label would see a further significant increase in its sales force and, for the first time, would allow the company to start promoting the drug directly to consumers for the first time as a therapy that can help prevent heart attacks and strokes.
“One in three adults have some risk for cardiovascular disease and we want them to be asking their doctors about it,” he said.
Cardiovascular disease is also the single biggest cause of death in Ireland, accounting for up to 10,000 deaths a year, according to the Irish Heart Foundation. That is one-third of all deaths, it says, and one in five of all premature deaths.
"Halbzeitbericht" -Händler Jon Najarian entdeckt ungewöhnliche Optionsaktivitäten in Aktien von Square und Amarin
https://finance.yahoo.com/video/options-traders-hip-square-1…
https://finance.yahoo.com/video/options-traders-hip-square-1…
Supergeil, burn shorties burn:
U.S. FDA Grants Priority Review for Vascepa® (Icosapent Ethyl) Supplemental New Drug Application Seeking Cardiovascular Risk Reduction Indication
GlobeNewswire•May 29, 2019
- PDUFA date assigned is September 28, 2019, four months sooner than expected
- Vascepa, assuming approval, will be first drug indicated to reduce residual cardiovascular risk in patients with statin-managed LDL-C cholesterol, but persistent elevated triglycerides, as studied in the landmark REDUCE-IT™ cardiovascular outcomes study
- Cardiovascular disease is the No. 1 cause of death for U.S. men and women
- Amarin is accelerating plans for commercial expansion, based on Priority Review designation
BEDMINSTER, N.J. and DUBLIN, Ireland, May 29, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN) announced today that its supplemental new drug application (sNDA) for Vascepa® (icosapent ethyl) capsules has been accepted for filing and granted Priority Review designation by the U.S. Food and Drug Administration (FDA). The Prescription Drug User Fee Act (PDUFA) goal date assigned by the FDA for this sNDA is September 28, 2019. Because of the Priority Review designation, the timing of this PDUFA date is four months earlier than the anticipated standard ten-month review for applications.
Assuming FDA approval, Vascepa will be the first drug indicated to reduce residual cardiovascular risk in patients with statin-managed LDL-C cholesterol, but persistent elevated triglycerides, an important indicator of cardiovascular disease. This is a serious health challenge experienced by millions of people.
The FDA grants Priority Review designation to applications for drugs that, if approved, have the potential to offer significant improvements in the effectiveness and safety of the treatment of serious conditions when compared to standard applications.
“We expect earlier approval of an expanded indication for Vascepa to lead to faster improvements in care for millions of patients with residual cardiovascular risk after statin therapy,” said John F. Thero, president and chief executive officer of Amarin. “These patients will be the focus of our planned expanded REDUCE-ITTM promotional efforts. We are very pleased that the FDA has accepted our application and granted it priority review. We believe the unprecedented REDUCE-IT results position Amarin to lead a transformative change in clinical practice for preventative treatment of cardiovascular disease, the leading cause of death for both men and women in the United States. Our plans to significantly expand promotion of Vascepa following label expansion are being accelerated to reflect the upcoming PDUFA date."
sNDA Based on Landmark REDUCE-IT Trial
The sNDA for Vascepa is based on the landmark REDUCE-IT cardiovascular outcomes study, primary results of which were published in The New England Journal of Medicine in November 2018.1 Additional results and analysis of total recurrent events observed were subsequently published in the Journal of American College of Cardiology in March 2019.2 Vascepa is currently indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (TG >500 mg/dL) hypertriglyceridemia, an important but much smaller patient population than can be addressed with an approval of this sNDA.
In REDUCE-IT, Vascepa achieved the primary endpoint with a 25% relative risk reduction compared to placebo (95% confidence interval [CI], 0.68-0.83; p<0.001) in the first occurrence of a major adverse cardiovascular event (MACE) in the intent-to-treat population. In REDUCE-IT, MACE consisted of a composite of cardiovascular death, nonfatal myocardial infarction (MI or heart attack), nonfatal stroke, coronary revascularization (procedures such as stents and by-pass) and unstable angina requiring hospitalization.
As further evidence of the robustness of the REDUCE-IT results, Vascepa achieved the study’s key secondary endpoint with a 26% relative risk reduction (HR, 0.74; 95% CI, 0.65-0.83; p<0.001) in 3-point MACE in the intent-to-treat population consisting of a composite of cardiovascular death, nonfatal heart attack and nonfatal stroke. Vascepa also achieved seven other secondary endpoints in the pre-specified hierarchical order below the key secondary endpoint, including a 20% relative risk reduction in cardiovascular death compared to placebo (HR, 0.80; 95% CI, 0.66-0.98; p=0.03). REDUCE-IT, a global study of 8,179 statin-treated adults with elevated CV risk, was performed based on a special protocol assessment (SPA) agreement with the FDA.
In REDUCE-IT, adverse events occurring with Vascepa use at greater than 5% and greater than placebo were: peripheral edema (6.5% Vascepa versus 5.0%), although there was no increase in the rate of heart failure in Vascepa patients; constipation (5.4% Vascepa versus 3.6%), although mineral oil, as used as placebo, is known to lower constipation; and atrial fibrillation (5.3% Vascepa versus 3.9%), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients. More information on safety data associated with REDUCE-IT is provided below and in the published results.
FDA Advisory Committee Update
In its sNDA filing acceptance communication to Amarin, the FDA did not indicate whether it plans to hold an advisory committee (AdCom) meeting to discuss this application. Amarin previously expressed that it believes an AdCom meeting organized by the FDA in conjunction with its review of the expanded label for Vascepa is likely. It is not uncommon for clarification on this topic to be provided by the FDA later in its review process.
About Amarin
Amarin Corporation plc. is a rapidly growing, innovative pharmaceutical company focused on developing therapeutics to improve cardiovascular health. Amarin’s product development program leverages its extensive experience in polyunsaturated fatty acids and lipid science. Vascepa (icosapent ethyl) is Amarin's first FDA-approved drug and is available by prescription in the United States, Lebanon and the United Arab Emirates. Amarin’s commercial partners are pursuing additional regulatory approvals for Vascepa in Canada, China and the Middle East. For more information about Amarin, visit www.amarincorp.com.
More About REDUCE-IT
REDUCE-IT1, an 8,179-patient cardiovascular outcomes study, was completed in 2018. REDUCE-IT was the first multinational cardiovascular outcomes study that evaluated the effect of prescription pure EPA therapy as an add-on to statins in patients with high cardiovascular risk who, despite stable statin therapy, had elevated triglyceride levels (at least 135 mg/dL). A large portion of the male and female patients enrolled in this outcomes study were diagnosed with type 2 diabetes.
More information on the REDUCE-IT study results can be found at www.amarincorp.com.
About Cardiovascular Disease
Worldwide, cardiovascular disease (CVD) remains the No. 1 killer of men and women. In the United States CVD leads to one in every three deaths – one death approximately every 38 seconds – with annual treatment cost in excess of $500 billion.3, 4
Multiple primary and secondary prevention trials have shown a significant reduction of 25% to 35% in the risk of cardiovascular events with statin therapy, leaving significant persistent residual risk despite the achievement of target LDL-C levels.5
Beyond the cardiovascular risk associated with LDL-C, genetic, epidemiologic, clinical and real-world data suggest that patients with elevated triglycerides (TG) (fats in the blood), and TG-rich lipoproteins, are at increased risk for cardiovascular disease.6, 7, 8, 9
About Vascepa (icosapent ethyl) Capsules
Vascepa (icosapent ethyl) capsules are a single-molecule prescription product consisting of the omega-3 acid commonly known as EPA in ethyl-ester form. Vascepa is not fish oil, but is derived from fish through a stringent and complex FDA-regulated manufacturing process designed to effectively eliminate impurities and isolate and protect the single molecule active ingredient from degradation. Vascepa, known in scientific literature as AMR101, has been designated a new chemical entity by the FDA. Amarin has been issued multiple patents internationally based on the unique clinical profile of Vascepa, including the drug’s ability to lower triglyceride levels in relevant patient populations without raising LDL-cholesterol levels.
Indication and Usage Based on Current FDA-Approved Label (not including REDUCE-IT results)
Vascepa (icosapent ethyl) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
The effect of Vascepa on the risk for pancreatitis and cardiovascular mortality and morbidity in patients with severe hypertriglyceridemia has not been determined.
Important Safety Information for Vascepa Based on Current FDA-Approved Label (not including REDUCE-IT results) (Includes Data from Two 12-Week Studies (n=622) (MARINE and ANCHOR) of Patients with Triglycerides Values of 200 to 2000 mg/dL)
Vascepa is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vascepa or any of its components.
In patients with hepatic impairment, monitor ALT and AST levels periodically during therapy.
Use with caution in patients with known hypersensitivity to fish and/or shellfish.
The most common reported adverse reaction (incidence >2% and greater than placebo) was arthralgia (2.3% for Vascepa, 1.0% for placebo). There was no reported adverse reaction >3% and greater than placebo.
Adverse events and product complaints may be reported by calling 1-855-VASCEPA or the FDA at 1-800-FDA-1088.
Patients receiving treatment with Vascepa and other drugs affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
Patients should be advised to swallow Vascepa capsules whole; not to break open, crush, dissolve, or chew Vascepa.
FULL VASCEPA PRESCRIBING INFORMATION CAN BE FOUND AT WWW.VASCEPA.COM.
Important Safety Information for Vascepa based on REDUCE-IT, as previously reported in The New England Journal of Medicine1 publication of the primary results of the REDUCE-IT study:
Excluding the major adverse cardiovascular events (MACE) results described above, overall adverse event rates in REDUCE-IT were similar across the statin plus Vascepa and the statin plus placebo treatment groups.
There were no significant differences between treatments in the overall rate of treatment emergent adverse events or serious adverse events leading to withdrawal of study drug.
There was no serious adverse event (SAE) occurring at a frequency of >2% which occurred at a numerically higher rate in the statin plus Vascepa treatment group than in the statin plus placebo treatment group.
Adverse events (AEs) occurring in 5% or greater of patients and more frequently with Vascepa than placebo were:
– peripheral edema (6.5% Vascepa patients versus 5.0% placebo patients), although there was no increase in the rate of heart failure in Vascepa patients
– constipation (5.4% Vascepa patients versus 3.6% placebo patients), although mineral oil, as used as placebo, is known to lower constipation, and
– atrial fibrillation (5.3% Vascepa patients versus 3.9% placebo patients), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients
There were numerically more SAEs related to bleeding in the statin plus Vascepa treatment group although overall rates were low with no fatal bleeding observed in either group and no significant difference in adjudicated hemorrhagic stroke or serious central nervous system or gastrointestinal bleeding events between treatments.
In summary, Vascepa was well tolerated with a safety profile generally consistent with clinical experience associated with omega-3 fatty acids and current FDA-approved labeling of such products.
Vascepa has been approved for use by the United States Food and Drug Administration (FDA) as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. FDA has not reviewed and opined on a supplemental new drug application related to REDUCE-IT. FDA has not reviewed the information herein or determined whether to approve Vascepa for use to reduce the risk of MACE. Nothing in this press release should be construed as promoting the use of Vascepa in any indication that has not been approved by the FDA.
Important Cautionary Information About These Data
Further REDUCE-IT data assessment and data release could yield additional useful information to inform greater understanding of the trial outcome. For example, detailed data assessment by regulatory authorities, such as the FDA and Health Canada, will continue and take several months to complete and announce. The final evaluation by regulatory authorities of the totality of efficacy and safety data from REDUCE-IT may include some or all of the following, as well as other considerations: new information or analyses affecting the degree of treatment benefit on studied endpoints; study conduct and data robustness, quality, integrity and consistency; additional safety data considerations and risk/benefit considerations; and consideration of REDUCE-IT results in the context of other clinical studies. Because regulatory reviews are typically fluid and not definitive interactions between sponsor and agency on individual elements of an application and related information, Amarin does not plan to update investors on ongoing communications with regulatory authorities. Amarin plans to announce the final outcome of such regulatory reviews when appropriate.
Recurrent event analyses for the total primary endpoint events and for the total key secondary endpoint in REDUCE-IT as published in the Journal of the American College of Cardiology were conducted using a series of statistical models. These analyses were tertiary or exploratory endpoints; most of the models used were prespecified and one was post hoc. Each recurrent event statistical model has inherent strengths and weaknesses, with no single model considered definitive or outperforming the other models, and this is an evolving field of science. Nonetheless, results from the total primary and total key secondary endpoint events analyses are consistent across the various recurrent event statistical models and are also consistent with the original primary and secondary endpoint results. Together, the REDUCE-IT recurrent event analyses and the original primary and key secondary endpoint analyses support the robustness of the clinical benefit of Vascepa therapy in reducing cardiovascular risk.
Forward-Looking Statements
This press release contains forward-looking statements, including expectations regarding FDA regulatory review, the applicability and reliability of REDUCE-IT results, expected outcome and timing of review elements and market dynamics for Vascepa. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. In addition, Amarin's ability to effectively commercialize Vascepa will depend in part on its ability to continue to effectively finance its business, efforts of third parties, its ability to gain regulatory approvals, create market demand for Vascepa through education, marketing and sales activities, to achieve market acceptance of Vascepa, to receive adequate levels of reimbursement from third-party payers, to develop and maintain a consistent source of commercial supply at a competitive price, to comply with legal and regulatory requirements in connection with the sale and promotion of Vascepa and to maintain patent protection for Vascepa. Among the factors that could cause actual results to differ materially from those described or projected herein include the following: uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals; the risk that sales may not meet expectations and related cost may increase beyond expectations; the risk that patents may not be upheld in patent litigation and applications may not result in issued patents sufficient to protect the Vascepa franchise. A further list and description of these risks, uncertainties and other risks associated with an investment in Amarin can be found in Amarin's filings with the U.S. Securities and Exchange Commission, including its most recent quarterly report on Form 10-Q. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Amarin undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Availability of Other Information About Amarin
Investors and others should note that Amarin communicates with its investors and the public using the company website (www.amarincorp.com), the investor relations website (investor.amarincorp.com), including but not limited to investor presentations and investor FAQs, Securities and Exchange Commission filings, press releases, public conference calls and webcasts. The information that Amarin posts on these channels and websites could be deemed to be material information. As a result, Amarin encourages investors, the media, and others interested in Amarin to review the information that is posted on these channels, including the investor relations website, on a regular basis. This list of channels may be updated from time to time on Amarin’s investor relations website and may include social media channels. The contents of Amarin’s website or these channels, or any other website that may be accessed from its website or these channels, shall not be deemed incorporated by reference in any filing under the Securities Act of 1933.
References
1 Bhatt DL, Steg PG, Miller M, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med 2019;380:11-22.
2 Bhatt DL, Steg PG, Miller M, et al. Effects of Icosapent Ethyl on Total Ischemic Events: From REDUCE-IT. J Am Coll Cardiol 2019. Epub ahead of print. https://doi.org/10.1016/j.jacc.2019.02.032.
3 American Heart Association. 2018. Disease and Stroke Statistics-2018 Update.
4 American Heart Association. 2017. Cardiovascular disease: A costly burden for America projections through 2035.
5 Ganda OP, Bhatt DL, Mason RP, et al. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72(3):330-343.
6 Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118:138-145.
7 Toth PP, Granowitz C, Hull M, et al. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: A real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7(15):e008740.
8 Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease - New insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547-563.
9 Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635.
Amarin Contact Information
Investor Relations:
Elisabeth Schwartz
Investor Relations
Amarin Corporation plc
In U.S.: +1 (908) 719-1315
investor.relations@amarincorp.com
Lee M. Stern
Trout Group
In U.S.: +1 (646) 378-2992
lstern@troutgroup.com
Media Inquiries:
Gwen Fisher
Corporate Communications
Amarin Corporation plc
In U.S.: +1 (908) 325-0735
PR@amarincorp.com
Contact:
U.S. FDA Grants Priority Review for Vascepa® (Icosapent Ethyl) Supplemental New Drug Application Seeking Cardiovascular Risk Reduction Indication
GlobeNewswire•May 29, 2019
- PDUFA date assigned is September 28, 2019, four months sooner than expected
- Vascepa, assuming approval, will be first drug indicated to reduce residual cardiovascular risk in patients with statin-managed LDL-C cholesterol, but persistent elevated triglycerides, as studied in the landmark REDUCE-IT™ cardiovascular outcomes study
- Cardiovascular disease is the No. 1 cause of death for U.S. men and women
- Amarin is accelerating plans for commercial expansion, based on Priority Review designation
BEDMINSTER, N.J. and DUBLIN, Ireland, May 29, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN) announced today that its supplemental new drug application (sNDA) for Vascepa® (icosapent ethyl) capsules has been accepted for filing and granted Priority Review designation by the U.S. Food and Drug Administration (FDA). The Prescription Drug User Fee Act (PDUFA) goal date assigned by the FDA for this sNDA is September 28, 2019. Because of the Priority Review designation, the timing of this PDUFA date is four months earlier than the anticipated standard ten-month review for applications.
Assuming FDA approval, Vascepa will be the first drug indicated to reduce residual cardiovascular risk in patients with statin-managed LDL-C cholesterol, but persistent elevated triglycerides, an important indicator of cardiovascular disease. This is a serious health challenge experienced by millions of people.
The FDA grants Priority Review designation to applications for drugs that, if approved, have the potential to offer significant improvements in the effectiveness and safety of the treatment of serious conditions when compared to standard applications.
“We expect earlier approval of an expanded indication for Vascepa to lead to faster improvements in care for millions of patients with residual cardiovascular risk after statin therapy,” said John F. Thero, president and chief executive officer of Amarin. “These patients will be the focus of our planned expanded REDUCE-ITTM promotional efforts. We are very pleased that the FDA has accepted our application and granted it priority review. We believe the unprecedented REDUCE-IT results position Amarin to lead a transformative change in clinical practice for preventative treatment of cardiovascular disease, the leading cause of death for both men and women in the United States. Our plans to significantly expand promotion of Vascepa following label expansion are being accelerated to reflect the upcoming PDUFA date."
sNDA Based on Landmark REDUCE-IT Trial
The sNDA for Vascepa is based on the landmark REDUCE-IT cardiovascular outcomes study, primary results of which were published in The New England Journal of Medicine in November 2018.1 Additional results and analysis of total recurrent events observed were subsequently published in the Journal of American College of Cardiology in March 2019.2 Vascepa is currently indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (TG >500 mg/dL) hypertriglyceridemia, an important but much smaller patient population than can be addressed with an approval of this sNDA.
In REDUCE-IT, Vascepa achieved the primary endpoint with a 25% relative risk reduction compared to placebo (95% confidence interval [CI], 0.68-0.83; p<0.001) in the first occurrence of a major adverse cardiovascular event (MACE) in the intent-to-treat population. In REDUCE-IT, MACE consisted of a composite of cardiovascular death, nonfatal myocardial infarction (MI or heart attack), nonfatal stroke, coronary revascularization (procedures such as stents and by-pass) and unstable angina requiring hospitalization.
As further evidence of the robustness of the REDUCE-IT results, Vascepa achieved the study’s key secondary endpoint with a 26% relative risk reduction (HR, 0.74; 95% CI, 0.65-0.83; p<0.001) in 3-point MACE in the intent-to-treat population consisting of a composite of cardiovascular death, nonfatal heart attack and nonfatal stroke. Vascepa also achieved seven other secondary endpoints in the pre-specified hierarchical order below the key secondary endpoint, including a 20% relative risk reduction in cardiovascular death compared to placebo (HR, 0.80; 95% CI, 0.66-0.98; p=0.03). REDUCE-IT, a global study of 8,179 statin-treated adults with elevated CV risk, was performed based on a special protocol assessment (SPA) agreement with the FDA.
In REDUCE-IT, adverse events occurring with Vascepa use at greater than 5% and greater than placebo were: peripheral edema (6.5% Vascepa versus 5.0%), although there was no increase in the rate of heart failure in Vascepa patients; constipation (5.4% Vascepa versus 3.6%), although mineral oil, as used as placebo, is known to lower constipation; and atrial fibrillation (5.3% Vascepa versus 3.9%), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients. More information on safety data associated with REDUCE-IT is provided below and in the published results.
FDA Advisory Committee Update
In its sNDA filing acceptance communication to Amarin, the FDA did not indicate whether it plans to hold an advisory committee (AdCom) meeting to discuss this application. Amarin previously expressed that it believes an AdCom meeting organized by the FDA in conjunction with its review of the expanded label for Vascepa is likely. It is not uncommon for clarification on this topic to be provided by the FDA later in its review process.
About Amarin
Amarin Corporation plc. is a rapidly growing, innovative pharmaceutical company focused on developing therapeutics to improve cardiovascular health. Amarin’s product development program leverages its extensive experience in polyunsaturated fatty acids and lipid science. Vascepa (icosapent ethyl) is Amarin's first FDA-approved drug and is available by prescription in the United States, Lebanon and the United Arab Emirates. Amarin’s commercial partners are pursuing additional regulatory approvals for Vascepa in Canada, China and the Middle East. For more information about Amarin, visit www.amarincorp.com.
More About REDUCE-IT
REDUCE-IT1, an 8,179-patient cardiovascular outcomes study, was completed in 2018. REDUCE-IT was the first multinational cardiovascular outcomes study that evaluated the effect of prescription pure EPA therapy as an add-on to statins in patients with high cardiovascular risk who, despite stable statin therapy, had elevated triglyceride levels (at least 135 mg/dL). A large portion of the male and female patients enrolled in this outcomes study were diagnosed with type 2 diabetes.
More information on the REDUCE-IT study results can be found at www.amarincorp.com.
About Cardiovascular Disease
Worldwide, cardiovascular disease (CVD) remains the No. 1 killer of men and women. In the United States CVD leads to one in every three deaths – one death approximately every 38 seconds – with annual treatment cost in excess of $500 billion.3, 4
Multiple primary and secondary prevention trials have shown a significant reduction of 25% to 35% in the risk of cardiovascular events with statin therapy, leaving significant persistent residual risk despite the achievement of target LDL-C levels.5
Beyond the cardiovascular risk associated with LDL-C, genetic, epidemiologic, clinical and real-world data suggest that patients with elevated triglycerides (TG) (fats in the blood), and TG-rich lipoproteins, are at increased risk for cardiovascular disease.6, 7, 8, 9
About Vascepa (icosapent ethyl) Capsules
Vascepa (icosapent ethyl) capsules are a single-molecule prescription product consisting of the omega-3 acid commonly known as EPA in ethyl-ester form. Vascepa is not fish oil, but is derived from fish through a stringent and complex FDA-regulated manufacturing process designed to effectively eliminate impurities and isolate and protect the single molecule active ingredient from degradation. Vascepa, known in scientific literature as AMR101, has been designated a new chemical entity by the FDA. Amarin has been issued multiple patents internationally based on the unique clinical profile of Vascepa, including the drug’s ability to lower triglyceride levels in relevant patient populations without raising LDL-cholesterol levels.
Indication and Usage Based on Current FDA-Approved Label (not including REDUCE-IT results)
Vascepa (icosapent ethyl) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
The effect of Vascepa on the risk for pancreatitis and cardiovascular mortality and morbidity in patients with severe hypertriglyceridemia has not been determined.
Important Safety Information for Vascepa Based on Current FDA-Approved Label (not including REDUCE-IT results) (Includes Data from Two 12-Week Studies (n=622) (MARINE and ANCHOR) of Patients with Triglycerides Values of 200 to 2000 mg/dL)
Vascepa is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vascepa or any of its components.
In patients with hepatic impairment, monitor ALT and AST levels periodically during therapy.
Use with caution in patients with known hypersensitivity to fish and/or shellfish.
The most common reported adverse reaction (incidence >2% and greater than placebo) was arthralgia (2.3% for Vascepa, 1.0% for placebo). There was no reported adverse reaction >3% and greater than placebo.
Adverse events and product complaints may be reported by calling 1-855-VASCEPA or the FDA at 1-800-FDA-1088.
Patients receiving treatment with Vascepa and other drugs affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
Patients should be advised to swallow Vascepa capsules whole; not to break open, crush, dissolve, or chew Vascepa.
FULL VASCEPA PRESCRIBING INFORMATION CAN BE FOUND AT WWW.VASCEPA.COM.
Important Safety Information for Vascepa based on REDUCE-IT, as previously reported in The New England Journal of Medicine1 publication of the primary results of the REDUCE-IT study:
Excluding the major adverse cardiovascular events (MACE) results described above, overall adverse event rates in REDUCE-IT were similar across the statin plus Vascepa and the statin plus placebo treatment groups.
There were no significant differences between treatments in the overall rate of treatment emergent adverse events or serious adverse events leading to withdrawal of study drug.
There was no serious adverse event (SAE) occurring at a frequency of >2% which occurred at a numerically higher rate in the statin plus Vascepa treatment group than in the statin plus placebo treatment group.
Adverse events (AEs) occurring in 5% or greater of patients and more frequently with Vascepa than placebo were:
– peripheral edema (6.5% Vascepa patients versus 5.0% placebo patients), although there was no increase in the rate of heart failure in Vascepa patients
– constipation (5.4% Vascepa patients versus 3.6% placebo patients), although mineral oil, as used as placebo, is known to lower constipation, and
– atrial fibrillation (5.3% Vascepa patients versus 3.9% placebo patients), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients
There were numerically more SAEs related to bleeding in the statin plus Vascepa treatment group although overall rates were low with no fatal bleeding observed in either group and no significant difference in adjudicated hemorrhagic stroke or serious central nervous system or gastrointestinal bleeding events between treatments.
In summary, Vascepa was well tolerated with a safety profile generally consistent with clinical experience associated with omega-3 fatty acids and current FDA-approved labeling of such products.
Vascepa has been approved for use by the United States Food and Drug Administration (FDA) as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. FDA has not reviewed and opined on a supplemental new drug application related to REDUCE-IT. FDA has not reviewed the information herein or determined whether to approve Vascepa for use to reduce the risk of MACE. Nothing in this press release should be construed as promoting the use of Vascepa in any indication that has not been approved by the FDA.
Important Cautionary Information About These Data
Further REDUCE-IT data assessment and data release could yield additional useful information to inform greater understanding of the trial outcome. For example, detailed data assessment by regulatory authorities, such as the FDA and Health Canada, will continue and take several months to complete and announce. The final evaluation by regulatory authorities of the totality of efficacy and safety data from REDUCE-IT may include some or all of the following, as well as other considerations: new information or analyses affecting the degree of treatment benefit on studied endpoints; study conduct and data robustness, quality, integrity and consistency; additional safety data considerations and risk/benefit considerations; and consideration of REDUCE-IT results in the context of other clinical studies. Because regulatory reviews are typically fluid and not definitive interactions between sponsor and agency on individual elements of an application and related information, Amarin does not plan to update investors on ongoing communications with regulatory authorities. Amarin plans to announce the final outcome of such regulatory reviews when appropriate.
Recurrent event analyses for the total primary endpoint events and for the total key secondary endpoint in REDUCE-IT as published in the Journal of the American College of Cardiology were conducted using a series of statistical models. These analyses were tertiary or exploratory endpoints; most of the models used were prespecified and one was post hoc. Each recurrent event statistical model has inherent strengths and weaknesses, with no single model considered definitive or outperforming the other models, and this is an evolving field of science. Nonetheless, results from the total primary and total key secondary endpoint events analyses are consistent across the various recurrent event statistical models and are also consistent with the original primary and secondary endpoint results. Together, the REDUCE-IT recurrent event analyses and the original primary and key secondary endpoint analyses support the robustness of the clinical benefit of Vascepa therapy in reducing cardiovascular risk.
Forward-Looking Statements
This press release contains forward-looking statements, including expectations regarding FDA regulatory review, the applicability and reliability of REDUCE-IT results, expected outcome and timing of review elements and market dynamics for Vascepa. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. In addition, Amarin's ability to effectively commercialize Vascepa will depend in part on its ability to continue to effectively finance its business, efforts of third parties, its ability to gain regulatory approvals, create market demand for Vascepa through education, marketing and sales activities, to achieve market acceptance of Vascepa, to receive adequate levels of reimbursement from third-party payers, to develop and maintain a consistent source of commercial supply at a competitive price, to comply with legal and regulatory requirements in connection with the sale and promotion of Vascepa and to maintain patent protection for Vascepa. Among the factors that could cause actual results to differ materially from those described or projected herein include the following: uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals; the risk that sales may not meet expectations and related cost may increase beyond expectations; the risk that patents may not be upheld in patent litigation and applications may not result in issued patents sufficient to protect the Vascepa franchise. A further list and description of these risks, uncertainties and other risks associated with an investment in Amarin can be found in Amarin's filings with the U.S. Securities and Exchange Commission, including its most recent quarterly report on Form 10-Q. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Amarin undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Availability of Other Information About Amarin
Investors and others should note that Amarin communicates with its investors and the public using the company website (www.amarincorp.com), the investor relations website (investor.amarincorp.com), including but not limited to investor presentations and investor FAQs, Securities and Exchange Commission filings, press releases, public conference calls and webcasts. The information that Amarin posts on these channels and websites could be deemed to be material information. As a result, Amarin encourages investors, the media, and others interested in Amarin to review the information that is posted on these channels, including the investor relations website, on a regular basis. This list of channels may be updated from time to time on Amarin’s investor relations website and may include social media channels. The contents of Amarin’s website or these channels, or any other website that may be accessed from its website or these channels, shall not be deemed incorporated by reference in any filing under the Securities Act of 1933.
References
1 Bhatt DL, Steg PG, Miller M, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med 2019;380:11-22.
2 Bhatt DL, Steg PG, Miller M, et al. Effects of Icosapent Ethyl on Total Ischemic Events: From REDUCE-IT. J Am Coll Cardiol 2019. Epub ahead of print. https://doi.org/10.1016/j.jacc.2019.02.032.
3 American Heart Association. 2018. Disease and Stroke Statistics-2018 Update.
4 American Heart Association. 2017. Cardiovascular disease: A costly burden for America projections through 2035.
5 Ganda OP, Bhatt DL, Mason RP, et al. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72(3):330-343.
6 Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118:138-145.
7 Toth PP, Granowitz C, Hull M, et al. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: A real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7(15):e008740.
8 Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease - New insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547-563.
9 Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635.
Amarin Contact Information
Investor Relations:
Elisabeth Schwartz
Investor Relations
Amarin Corporation plc
In U.S.: +1 (908) 719-1315
investor.relations@amarincorp.com
Lee M. Stern
Trout Group
In U.S.: +1 (646) 378-2992
lstern@troutgroup.com
Media Inquiries:
Gwen Fisher
Corporate Communications
Amarin Corporation plc
In U.S.: +1 (908) 325-0735
PR@amarincorp.com
Contact:
Die Meldung FDA Grants Priority Review, für das überdurchschnittliche Volumen
von Call Optionen.
Opinion: These 6 biotech stocks are promising takeover targets
by Micheal Brush
Amarin AMRN, + 8,70% hat ein Medikament gegen Herz-Kreislauf-Erkrankungen namens Vascepa. Es ist eine verschreibungspflichtige Omega-3-Fettsäure-Kapsel, die Triglycerid bei Menschen mit erhöhten Blutspiegel reduziert. Eine im vergangenen September veröffentlichte Studie zeigte, dass Vascepa das Risiko für Herz-Kreislauf-Erkrankungen um 25% senkte. Amarin hat bei der FDA die Ausweitung der Bezeichnung Vascepa auf Patienten mit einem Risiko für Herz-Kreislauf-Probleme, Herzinfarkt und Schlaganfall beantragt.
Vascepa ist kein Fischöl, das als rezeptfreies Mittel gegen Herz-Kreislauf-Probleme diskreditiert wurde. Vascepa wird jedoch durch einen Prozess aus Fisch gewonnen, der Verunreinigungen beseitigt, einen Wirkstoff isoliert und ihn vor Abbau schützt. Yee beschreibt Amarin als einen der offensichtlichen Übernahmekandidaten. "Wir sind weiterhin der Meinung, dass Vascepa viele Kisten als potenzieller Blockbuster betrachtet, der sich in den Händen der großen weltweiten Pharmadistribution noch besser behaupten würde", sagt er.
von Call Optionen.
Opinion: These 6 biotech stocks are promising takeover targets
by Micheal Brush
Amarin AMRN, + 8,70% hat ein Medikament gegen Herz-Kreislauf-Erkrankungen namens Vascepa. Es ist eine verschreibungspflichtige Omega-3-Fettsäure-Kapsel, die Triglycerid bei Menschen mit erhöhten Blutspiegel reduziert. Eine im vergangenen September veröffentlichte Studie zeigte, dass Vascepa das Risiko für Herz-Kreislauf-Erkrankungen um 25% senkte. Amarin hat bei der FDA die Ausweitung der Bezeichnung Vascepa auf Patienten mit einem Risiko für Herz-Kreislauf-Probleme, Herzinfarkt und Schlaganfall beantragt.
Vascepa ist kein Fischöl, das als rezeptfreies Mittel gegen Herz-Kreislauf-Probleme diskreditiert wurde. Vascepa wird jedoch durch einen Prozess aus Fisch gewonnen, der Verunreinigungen beseitigt, einen Wirkstoff isoliert und ihn vor Abbau schützt. Yee beschreibt Amarin als einen der offensichtlichen Übernahmekandidaten. "Wir sind weiterhin der Meinung, dass Vascepa viele Kisten als potenzieller Blockbuster betrachtet, der sich in den Händen der großen weltweiten Pharmadistribution noch besser behaupten würde", sagt er.
H.C. Wainwright Survey Shows Substantial Runway For Amarin Corporation's (AMRN) Vascepa
June 26, 2019 8:21 AM
H.C. Wainwright analyst Andrew Fein reiterated a Buy rating and $51.00 price target on Amarin Corporation (NASDAQ: AMRN) after conducted ...
on Amarin Corporation (NASDAQ: AMRN) after conducted ...
(Premium-only article. Please sign in or upgrade to SI Premium to view.)
Categories
Analyst Comments
June 26, 2019 8:21 AM
H.C. Wainwright analyst Andrew Fein reiterated a Buy rating and $51.00 price target
 on Amarin Corporation (NASDAQ: AMRN) after conducted ...
on Amarin Corporation (NASDAQ: AMRN) after conducted ...(Premium-only article. Please sign in or upgrade to SI Premium to view.)
Categories
Analyst Comments
$51 Full Report
H.C. Wainwright analyst Andrew Fein raised the price target on Amarin Corporation (NASDAQ: AMRN) to $51.00 (from $31.00) while maintaining a Buy rating.
Fein commented, "The detailed data presentation of the REDUCE-IT trial of Vascepa at this year’s American Heart Association (AHA) conference in conjunction with the publication in the New English Journal of Medicine (NEJM) provided much anticipated color on Vascepa’s clinical benefits and commercial potential, which in our view, landed in the best scenario and surpassed our expectation. We believe that beyond the topline of 25% reduction of MACE score, the robustness of the data is highlighted by the consistency across pre-specified analyses. A key secondary composite, namely 3-point MACE score (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke), which assesses the major CV events (and reflects the most important clinical relevance), showed an even higher reduction of risks at 26% compared to placebo. The clinical benefits as measured by the primary endpoint of MACE score and the key secondary 3-point MACE score are observed across subgroups (statistically significant in the vast majority of the subgroups), which speaks to the validity and robustness of the data. We echo the sentiment expressed by some KOLs present at the meeting that the REDUCE-IT trial data may be viewed as “unprecedented” and “paradigm-changing” in the context of minimal or modest benefits historically achieved by various therapies on top of statins. We point out that the subgroup analysis may give the first peek into the commercial potential of Vascepta: regardless of baseline triglycerides levels (≥150 vs. <150 mg/dL), diabetes status, baseline LDL-C, Vascepta provides similarly robust CV benefit. This, compared to the current label of Vascepa which is only indicated in adult patients with severe hypertriglyceridemia (≥ 500 mg/dL), should significantly broaden Vascepa’s target patient population if the FDA grants the label expansion. With regard to the placebo (mineral oil) controversy, we view it as temporary noise that has been given an oversized weight by the bears. We are not aware of any strong evidence supporting that mineral oil contributed to the worsened outcome on the placebo arm. At most, we believe it mounts to speculation and hypothesis of the potential physiological activity of mineral oil without any clinical validation of such claims. In any case, the possibly minimal biological activity of the placebo arm would not negate the robustness of the results, in our view. We expect the placebo issue to be raised during the FDA review, but do not believe it would negatively impact the regulatory outcome. As far as the safety signals go, the increased risk of atrial fibrillation should be offset by the reduction in stroke, according to our KOL, since atrial fibrillation often leads to stroke which was actually decreased with Vascepa’s treatment. We think perhaps a more relevant question for Vascepa now is how the data is received by physicians and how the REDUCE-IT results would change the uptake of the drug and physicians’ prescription regimen. If there is any indication, we point to the survey conducted at AHA after the presentation where it asked physicians how they plan to change practice after the REDUCE-IT data, and 87% of the audience said they would prescribe Vascepa to all high risk patients with moderate TG levels."
H.C. Wainwright analyst Andrew Fein raised the price target on Amarin Corporation (NASDAQ: AMRN) to $51.00 (from $31.00) while maintaining a Buy rating.
Fein commented, "The detailed data presentation of the REDUCE-IT trial of Vascepa at this year’s American Heart Association (AHA) conference in conjunction with the publication in the New English Journal of Medicine (NEJM) provided much anticipated color on Vascepa’s clinical benefits and commercial potential, which in our view, landed in the best scenario and surpassed our expectation. We believe that beyond the topline of 25% reduction of MACE score, the robustness of the data is highlighted by the consistency across pre-specified analyses. A key secondary composite, namely 3-point MACE score (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke), which assesses the major CV events (and reflects the most important clinical relevance), showed an even higher reduction of risks at 26% compared to placebo. The clinical benefits as measured by the primary endpoint of MACE score and the key secondary 3-point MACE score are observed across subgroups (statistically significant in the vast majority of the subgroups), which speaks to the validity and robustness of the data. We echo the sentiment expressed by some KOLs present at the meeting that the REDUCE-IT trial data may be viewed as “unprecedented” and “paradigm-changing” in the context of minimal or modest benefits historically achieved by various therapies on top of statins. We point out that the subgroup analysis may give the first peek into the commercial potential of Vascepta: regardless of baseline triglycerides levels (≥150 vs. <150 mg/dL), diabetes status, baseline LDL-C, Vascepta provides similarly robust CV benefit. This, compared to the current label of Vascepa which is only indicated in adult patients with severe hypertriglyceridemia (≥ 500 mg/dL), should significantly broaden Vascepa’s target patient population if the FDA grants the label expansion. With regard to the placebo (mineral oil) controversy, we view it as temporary noise that has been given an oversized weight by the bears. We are not aware of any strong evidence supporting that mineral oil contributed to the worsened outcome on the placebo arm. At most, we believe it mounts to speculation and hypothesis of the potential physiological activity of mineral oil without any clinical validation of such claims. In any case, the possibly minimal biological activity of the placebo arm would not negate the robustness of the results, in our view. We expect the placebo issue to be raised during the FDA review, but do not believe it would negatively impact the regulatory outcome. As far as the safety signals go, the increased risk of atrial fibrillation should be offset by the reduction in stroke, according to our KOL, since atrial fibrillation often leads to stroke which was actually decreased with Vascepa’s treatment. We think perhaps a more relevant question for Vascepa now is how the data is received by physicians and how the REDUCE-IT results would change the uptake of the drug and physicians’ prescription regimen. If there is any indication, we point to the survey conducted at AHA after the presentation where it asked physicians how they plan to change practice after the REDUCE-IT data, and 87% of the audience said they would prescribe Vascepa to all high risk patients with moderate TG levels."
Amarin Is A Long-Term Buy With Potential Blockbuster Drug Vascepa
Jul. 1, 2019
Summary
Amarin is preparing for a monumental label expansion for their flagship product Vascepa with a PDUFA date in September.
I have been waiting patiently for an opportunity to buy some cheap shares but the market is not providing me a chance. I am looking for a speculative buy.
With an expanded label, Vascepa could be a blockbuster drug that is a mainstay in the cardiac patient paradigm.
https://seekingalpha.com/article/4272946-amarin-long-term-bu…
Jul. 1, 2019
Summary
Amarin is preparing for a monumental label expansion for their flagship product Vascepa with a PDUFA date in September.
I have been waiting patiently for an opportunity to buy some cheap shares but the market is not providing me a chance. I am looking for a speculative buy.
With an expanded label, Vascepa could be a blockbuster drug that is a mainstay in the cardiac patient paradigm.
https://seekingalpha.com/article/4272946-amarin-long-term-bu…
Guiadance erhöht, Amarin wie geil:
Amarin's stock surges after raised revenue outlook, increased commercial expansion of Vascepa
Published: July 2, 2019 6:47 a.m. ET
Author photo
By
Tomi
Kilgore
Reporter and editor
Shares of Amarin Corp. AMRN, -0.77% surged 9.2% in premarket trading Tuesday, after the pharmaceutical company raised its full-year revenue outlook and announced plans to double its U.S. sales force to further increase commercial expansion of Vascepa for the treatment of cardiovascular disease. The company now expects 2019 revenue of $380 million to $420 million, above previous guidance of $350 million, and above the FactSet consensus of $364.3 million. Amarin said it increasing the size of its U.S. sales force to about 800 representatives, with the aim of having the expanded team deployed by October. The accelerated expansion followed the priority review designation of the supplemental new drug application (sNDA) for Vascepa. "We anticipate Vascepa revenue growth to accelerate further after label expansion approval and with a larger sales team, and then again after we commence promotion of Vascepa for cardiovascular risk reduction on television and through other media," said Chief Executive John Thero. Amarin's stock has soared 41.4% year to date through Monday, while the S&P 500 SPX, +0.77% has climbed 18.3%.
Amarin's stock surges after raised revenue outlook, increased commercial expansion of Vascepa
Published: July 2, 2019 6:47 a.m. ET
Author photo
By
Tomi
Kilgore
Reporter and editor
Shares of Amarin Corp. AMRN, -0.77% surged 9.2% in premarket trading Tuesday, after the pharmaceutical company raised its full-year revenue outlook and announced plans to double its U.S. sales force to further increase commercial expansion of Vascepa for the treatment of cardiovascular disease. The company now expects 2019 revenue of $380 million to $420 million, above previous guidance of $350 million, and above the FactSet consensus of $364.3 million. Amarin said it increasing the size of its U.S. sales force to about 800 representatives, with the aim of having the expanded team deployed by October. The accelerated expansion followed the priority review designation of the supplemental new drug application (sNDA) for Vascepa. "We anticipate Vascepa revenue growth to accelerate further after label expansion approval and with a larger sales team, and then again after we commence promotion of Vascepa for cardiovascular risk reduction on television and through other media," said Chief Executive John Thero. Amarin's stock has soared 41.4% year to date through Monday, while the S&P 500 SPX, +0.77% has climbed 18.3%.
Hier die komplette Pressemeldung, super kann ich nur sagen:
Amarin Provides Mid-2019 Update, Including Commercialization Plans for Vascepa® and Updates Full Year 2019 Revenue Guidance
GlobeNewswire•July 2, 2019
Record Revenue Achieved in 1H19 Primarily Due to Increased Demand for Vascepa
Guidance for Total 2019 Revenue Increased to a Range of $380 to $420 Million from $350 Million Following Unaudited Second Quarter Results Estimated Between $97 and $101 Million, or Between $170 and $174 Million for the First Half of 2019
U.S. Sales Force to Double in Size; Recruiting Commenced
Vascepa sNDA PDUFA Goal Date On-Track for September 28, 2019; Potential for Therapy to Become First Prescription Product Available for Cardiovascular Risk Reduction in Patients with Elevated Triglyceride Levels, Despite Statin Therapy
BEDMINSTER, N.J. and DUBLIN, Ireland, July 02, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, today provided a business update, including that the company is increasing revenue guidance for 2019 and planning to further increase its commercial expansion efforts to align with its progress and outlook that the realizable opportunity for Vascepa® (icosapent ethyl) is larger than previously believed. These revised plans follow estimated record revenues for the quarter ended June 30, 2019 and assume expanded FDA labelling for Vascepa.
sNDA Update
As previously announced, Amarin submitted an sNDA to the U.S. Food and Drug Administration (FDA) on March 28, 2019, seeking to expand the indication for Vascepa. The sNDA was based on the positive results of the landmark REDUCE-IT™ cardiovascular outcomes study. If approved, the expanded label is expected to allow for considerably broader promotion of Vascepa in the United States. As announced in May 2019, the FDA accepted the sNDA for filing and granted Priority Review designation with an assigned PDUFA goal date of September 28, 2019.
The FDA grants Priority Review designation to applications for drugs that, if approved, have the potential to offer significant improvements in the effectiveness and safety of the treatment of serious conditions when compared to standard applications. Assuming the FDA approves this sNDA, Vascepa is anticipated to be the first drug with an indication to reduce residual cardiovascular risk in patients with statin-managed LDL-cholesterol, but persistent elevated triglyceride (TG) levels, an important indicator of cardiovascular disease.
The results of the REDUCE-IT study were published in The New England Journal of Medicine in November 2018.1 Additional results and analysis of total recurrent events observed in REDUCE-IT were published in the Journal of American College of Cardiology in March 2019.2
Vascepa is currently indicated as an adjunct to diet to reduce TG levels in adults with severe (TG ≥500 mg/dL) hypertriglyceridemia, an important but much smaller patient population than can be addressed with an approval of this sNDA.
To date, the FDA has not informed Amarin as to whether it plans to convene an Advisory Committee meeting (AdCom) to review the sNDA. As previously disclosed, Amarin continues to prepare in the event that an AdCom is convened. If Amarin is informed definitively that there will or will not be an AdCom, the company plans to update investors accordingly.
Preliminary (Unaudited) First Half 2019 Financial Results
Record Revenue Levels Achieved: Net total revenue for the three and six months ended June 30, 2019 are estimated to have reached between $97 and $101 million and between $170 and $174 million, respectively. Both the second quarter and first half of 2019 results represent record revenue levels for Amarin. These results, which are subject to auditor review, represent an increase of approximately $44 to $48 million (approximately 84% to 92%) for the second quarter of 2019 over the corresponding period of 2018 and an increase of approximately $73 to $77 million (approximately 76% to 80%) for the first half of 2019 over the corresponding period of 2018. These results consist predominantly of U.S. sales-driven increases in prescriptions for Vascepa. Wholesaler inventory levels of Vascepa were within normal industry ranges at the end June 2019.
Current Assets: Amarin ended June 2019 with cash and cash equivalents of approximately $221 million (compared to $211 million at March 31, 2019), approximately $94 million in net accounts receivable and approximately $47 million in inventory.
No Debt, Except Remaining Balance of Royalty Bearing Instrument: Amarin ended June 2019 with no debt except the remaining balance on its royalty bearing instrument which is repaid at a rate of 10% of Vascepa revenues; aggregate repayment of less than $74 million remains until this royalty-like obligation is fully extinguished.
2019 Financial and Operational Guidance
Amarin initially provided guidance for 2019 in its press release dated January 4, 2019. Amarin now makes the following updates to that guidance:
2019 Revenue Guidance: Forecasting Vascepa revenue levels at this early stage remains difficult. Based on estimated total revenue results for the first half of 2019 which exceeded prior expectations, Amarin increases its guidance for 2019 net total revenue to a range of $380 to $420 million. While Amarin remains optimistic that Vascepa will generate billions of dollars in revenue in the years to come, the history of other therapies for chronic conditions suggests that growth builds over multiple years, and thus, the company is not prepared to provide quantified guidance regarding revenue levels beyond 2019.
Commercial Expansion in United States: Amarin has accelerated and further expanded its commercialization plans for Vascepa in the United States. Amarin intends to increase the size of its U.S. sales force to approximately 800 sales representatives with the aim of having its expanded team hired, trained and deployed by October 2019. This increase would represent a doubling of the size of Amarin’s current sales force.
The timing of such expansion had been accelerated in part due to the priority review designation of the sNDA for Vascepa. Assuming label expansion for Vascepa on the September 28, 2019 PDUFA date, Amarin expects to have educational and promotional materials available by early October 2019 to promote Vascepa based on the new label.
The size of the planned expansion reflects the result of evaluations involving multiple contributing factors. Previously Amarin had estimated the potential expansion of its sales force to reach between 600 and 800 sales representatives and for the expansion to potentially occur in phases. The decision to expand the sales force to approximately 800 sales representatives by October 2019 was based on new information including the encouraging progress being made by sales representatives hired at the start of 2019, positive feedback from physicians with deep understanding of the REDUCE-IT data, additional data on the commercial opportunity that exists in detailing physicians who have not yet been educated about Vascepa and data suggesting that education of healthcare professionals regarding Vascepa will be improved if our sales representatives call on physicians with greater frequency.
As a reminder, in late 2018, Amarin hired, trained and deployed 265 new and qualified sales representatives within approximately three months after learning the results of REDUCE-IT. At the time, more than 20,000 applications were received for the 265 open sales positions. Based on this track record and the robust results of Vascepa clinical trials, Amarin is confident it can double its sales force to 800 sales representatives by October 2019.
While Amarin is confident that expanding its sales force will result in meaningful revenue growth beyond 2019, the company’s revenue guidance for 2019 as described above anticipates little incremental contribution in 2019 from these newly hired sales representatives. Based on available data, it typically requires multiple months for newly hired sales representatives to become meaningfully productive particularly if they are calling on healthcare professionals who also are new to Vascepa, which will be the case for a large part of such sales force expansion.
Amarin intends to support its expanded U.S. sales team with multiple promotional and educational programs. All such initiatives will be aimed at providing truthful and non-misleading information to lead to improved patient care. Included in Amarin’s promotion plans is a direct-to-consumer advertising campaign, subject to review by the FDA’s Office of Prescription Drug Promotion (OPDP). The company anticipates submitting proposed Vascepa advertisements to OPDP in October 2019. Based on the timeline for other companies getting consumer advertising reviewed by FDA following new labelling, assuming new Vascepa labelling, launch of such branded advertising is anticipated in the second quarter of 2020.
Plans for commercialization of Vascepa outside of the United States remain unchanged, including intentions to submit for regulatory approval of Vascepa in the European Union near the end of 2019.
Comment from Amarin’s President and CEO
“We are pleased with the progress made to date, including the significant revenue growth we’ve achieved for Vascepa,” commented John Thero, president and chief executive officer of Amarin. “We anticipate Vascepa revenue growth to accelerate further after label expansion approval and with a larger sales team, and then again after we commence promotion of Vascepa for cardiovascular risk reduction on television and through other media. We are preparing for a robust launch of REDUCE-IT data with the aim of helping physicians improve patient care for millions of patients with residual cardiovascular risk after their cholesterol is controlled, as identified by elevated triglycerides.”
About Amarin
Amarin Corporation plc. is a rapidly growing, innovative pharmaceutical company focused on developing therapeutics to improve cardiovascular health. Amarin’s product development program leverages its extensive experience in polyunsaturated fatty acids and lipid science. Vascepa (icosapent ethyl) is Amarin's first FDA-approved drug and is available by prescription in the United States, Lebanon and the United Arab Emirates. Amarin’s commercial partners are pursuing additional regulatory approvals for Vascepa in Canada, China and the Middle East. For more information about Amarin, visit www.amarincorp.com.
About REDUCE-IT™
REDUCE-IT1 was an 8,179-patient multinational cardiovascular outcomes study completed in 2018. REDUCE-IT evaluated the effect of prescription pure EPA therapy as an add-on to statins in patients with high cardiovascular risk who, despite stable statin therapy, had elevated triglyceride levels (at least 135 mg/dL). A large portion of the male and female patients enrolled in this outcomes study were diagnosed with type 2 diabetes.
More information on the REDUCE-IT study results can be found at www.amarincorp.com.
About Cardiovascular Disease
Worldwide, cardiovascular disease (CVD) remains the #1 killer of men and women. In the United States CVD leads to one in every three deaths – one death approximately every 38 seconds – with annual treatment cost in excess of $500 billion.3, 4
Multiple primary and secondary prevention trials have shown a significant reduction of 25% to 35% in the risk of cardiovascular events with statin therapy, leaving significant persistent residual risk despite the achievement of target LDL-C levels.5
Beyond the cardiovascular risk associated with LDL-C, genetic, epidemiologic, clinical and real-world data suggest that patients with elevated triglycerides (TG) (fats in the blood), and TG-rich lipoproteins, are at increased risk for cardiovascular disease.6-9
About Vascepa® (icosapent ethyl) Capsules
Vascepa (icosapent ethyl) capsules are a single-molecule prescription product consisting of the omega-3 acid commonly known as EPA in ethyl-ester form. Vascepa is not fish oil, but is derived from fish through a stringent and complex FDA-regulated manufacturing process designed to effectively eliminate impurities and isolate and protect the single molecule active ingredient from degradation. Vascepa, known in scientific literature as AMR101, has been designated a new chemical entity by the FDA. Amarin has been issued multiple patents internationally based on the unique clinical profile of Vascepa, including the drug’s ability to lower triglyceride levels in relevant patient populations without raising LDL-cholesterol levels.
Indication and Usage Based on Current FDA-Approved Label (not including REDUCE-IT results)
Vascepa (icosapent ethyl) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
The effect of Vascepa on the risk for pancreatitis and cardiovascular mortality and morbidity in patients with severe hypertriglyceridemia has not been determined.
Important Safety Information for Vascepa Based on Current FDA-Approved Label (not including REDUCE-IT results) (Includes Data from Two 12-Week Studies (n=622) (MARINE and ANCHOR) of Patients with Triglycerides Values of 200 to 2000 mg/dL)
Vascepa is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vascepa or any of its components.
In patients with hepatic impairment, monitor ALT and AST levels periodically during therapy.
Use with caution in patients with known hypersensitivity to fish and/or shellfish.
The most common reported adverse reaction (incidence >2% and greater than placebo) was arthralgia (2.3% for Vascepa, 1.0% for placebo). There was no reported adverse reaction >3% and greater than placebo.
Adverse events and product complaints may be reported by calling 1-855-VASCEPA or the FDA at 1-800-FDA-1088.
Patients receiving treatment with Vascepa and other drugs affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
Patients should be advised to swallow Vascepa capsules whole; not to break open, crush, dissolve, or chew Vascepa.
FULL VASCEPA PRESCRIBING INFORMATION CAN BE FOUND AT WWW.VASCEPA.COM.
Important Safety Information for Vascepa based on REDUCE-IT, as previously reported in The New England Journal of Medicine1 publication of the primary results of the REDUCE-IT study:
Excluding the major adverse cardiovascular events (MACE) results described above, overall adverse event rates in REDUCE-IT were similar across the statin plus Vascepa and the statin plus placebo treatment groups.
There were no significant differences between treatments in the overall rate of treatment emergent adverse events or serious adverse events leading to withdrawal of study drug.
There was no serious adverse event (SAE) occurring at a frequency of >2% which occurred at a numerically higher rate in the statin plus Vascepa treatment group than in the statin plus placebo treatment group.
Adverse events (AEs) occurring in 5% or greater of patients and more frequently with Vascepa than placebo were:
– peripheral edema (6.5% Vascepa patients versus 5.0% placebo patients), although there was no increase in the rate of heart failure in Vascepa patients
– constipation (5.4% Vascepa patients versus 3.6% placebo patients), although mineral oil, as used as placebo, is known to lower constipation, and
– atrial fibrillation (5.3% Vascepa patients versus 3.9% placebo patients), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients
There were numerically more SAEs related to bleeding in the statin plus Vascepa treatment group although overall rates were low with no fatal bleeding observed in either group and no significant difference in adjudicated hemorrhagic stroke or serious central nervous system or gastrointestinal bleeding events between treatments.
In summary, Vascepa was well tolerated with a safety profile generally consistent with clinical experience associated with omega-3 fatty acids and current FDA-approved labeling of such products.
Vascepa has been approved for use by the United States Food and Drug Administration (FDA) as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. FDA has not reviewed and opined on a supplemental new drug application related to REDUCE-IT. FDA has not reviewed the information herein or determined whether to approve Vascepa for use to reduce the risk of MACE. Nothing in this press release should be construed as promoting the use of Vascepa in any indication that has not been approved by the FDA.
Important Cautionary Information About These Data
Further REDUCE-IT data assessment and data release could yield additional useful information to inform greater understanding of the trial outcome. For example, detailed data assessment by regulatory authorities, such as the FDA and Health Canada, will continue and take several months to complete and announce. The final evaluation by regulatory authorities of the totality of efficacy and safety data from REDUCE-IT may include some or all of the following, as well as other considerations: new information or analyses affecting the degree of treatment benefit on studied endpoints; study conduct and data robustness, quality, integrity and consistency; additional safety data considerations and risk/benefit considerations; and consideration of REDUCE-IT results in the context of other clinical studies. Because regulatory reviews are typically fluid and not definitive interactions between sponsor and agency on individual elements of an application and related information, Amarin does not plan to update investors on ongoing communications with regulatory authorities. Amarin plans to announce the final outcome of such regulatory reviews when appropriate.
Recurrent event analyses for the total primary endpoint events and for the total key secondary endpoint in REDUCE-IT as published in the Journal of the American College of Cardiology were conducted using a series of statistical models. These analyses were tertiary or exploratory endpoints; most of the models used were prespecified and one was post hoc. Each recurrent event statistical model has inherent strengths and weaknesses, with no single model considered definitive or outperforming the other models, and this is an evolving field of science. Nonetheless, results from the total primary and total key secondary endpoint events analyses are consistent across the various recurrent event statistical models and are also consistent with the original primary and secondary endpoint results. Together, the REDUCE-IT recurrent event analyses and the original primary and key secondary endpoint analyses support the robustness of the clinical benefit of Vascepa therapy in reducing cardiovascular risk.
Forward-Looking Statements
This press release contains forward-looking statements, including expectations regarding revenue and prescription growth, including updated revenue guidance for 2019; sales force expansion and marketing initiatives expected in 2019 and beyond; FDA regulatory review, including the timing and outcome of such review; the applicability and reliability of REDUCE-IT results; and the expected outcome and timing of review elements and market dynamics for Vascepa. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. In addition, Amarin's ability to effectively commercialize Vascepa will depend in part on its ability to continue to effectively finance its business, efforts of third parties, its ability to gain regulatory approvals, create market demand for Vascepa through education, marketing and sales activities, to achieve market acceptance of Vascepa, to receive adequate levels of reimbursement from third-party payers, to develop and maintain a consistent source of commercial supply at a competitive price, to comply with legal and regulatory requirements in connection with the sale and promotion of Vascepa and to maintain patent protection for Vascepa. Among the factors that could cause actual results to differ materially from those described or projected herein include the following: uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals; the risk that sales may not meet expectations and related cost may increase beyond expectations; the risk that patents may not be upheld in patent litigation and applications may not result in issued patents sufficient to protect the Vascepa franchise. A further list and description of these risks, uncertainties and other risks associated with an investment in Amarin can be found in Amarin's filings with the U.S. Securities and Exchange Commission, including its most recent quarterly report on Form 10-Q. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Amarin undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Availability of Other Information About Amarin
Investors and others should note that Amarin communicates with its investors and the public using the company website (www.amarincorp.com), the investor relations website (investor.amarincorp.com), including but not limited to investor presentations and investor FAQs, Securities and Exchange Commission filings, press releases, public conference calls and webcasts. The information that Amarin posts on these channels and websites could be deemed to be material information. As a result, Amarin encourages investors, the media, and others interested in Amarin to review the information that is posted on these channels, including the investor relations website, on a regular basis. This list of channels may be updated from time to time on Amarin’s investor relations website and may include social media channels. The contents of Amarin’s website or these channels, or any other website that may be accessed from its website or these channels, shall not be deemed incorporated by reference in any filing under the Securities Act of 1933.
References
Amarin Provides Mid-2019 Update, Including Commercialization Plans for Vascepa® and Updates Full Year 2019 Revenue Guidance
GlobeNewswire•July 2, 2019
Record Revenue Achieved in 1H19 Primarily Due to Increased Demand for Vascepa
Guidance for Total 2019 Revenue Increased to a Range of $380 to $420 Million from $350 Million Following Unaudited Second Quarter Results Estimated Between $97 and $101 Million, or Between $170 and $174 Million for the First Half of 2019
U.S. Sales Force to Double in Size; Recruiting Commenced
Vascepa sNDA PDUFA Goal Date On-Track for September 28, 2019; Potential for Therapy to Become First Prescription Product Available for Cardiovascular Risk Reduction in Patients with Elevated Triglyceride Levels, Despite Statin Therapy
BEDMINSTER, N.J. and DUBLIN, Ireland, July 02, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, today provided a business update, including that the company is increasing revenue guidance for 2019 and planning to further increase its commercial expansion efforts to align with its progress and outlook that the realizable opportunity for Vascepa® (icosapent ethyl) is larger than previously believed. These revised plans follow estimated record revenues for the quarter ended June 30, 2019 and assume expanded FDA labelling for Vascepa.
sNDA Update
As previously announced, Amarin submitted an sNDA to the U.S. Food and Drug Administration (FDA) on March 28, 2019, seeking to expand the indication for Vascepa. The sNDA was based on the positive results of the landmark REDUCE-IT™ cardiovascular outcomes study. If approved, the expanded label is expected to allow for considerably broader promotion of Vascepa in the United States. As announced in May 2019, the FDA accepted the sNDA for filing and granted Priority Review designation with an assigned PDUFA goal date of September 28, 2019.
The FDA grants Priority Review designation to applications for drugs that, if approved, have the potential to offer significant improvements in the effectiveness and safety of the treatment of serious conditions when compared to standard applications. Assuming the FDA approves this sNDA, Vascepa is anticipated to be the first drug with an indication to reduce residual cardiovascular risk in patients with statin-managed LDL-cholesterol, but persistent elevated triglyceride (TG) levels, an important indicator of cardiovascular disease.
The results of the REDUCE-IT study were published in The New England Journal of Medicine in November 2018.1 Additional results and analysis of total recurrent events observed in REDUCE-IT were published in the Journal of American College of Cardiology in March 2019.2
Vascepa is currently indicated as an adjunct to diet to reduce TG levels in adults with severe (TG ≥500 mg/dL) hypertriglyceridemia, an important but much smaller patient population than can be addressed with an approval of this sNDA.
To date, the FDA has not informed Amarin as to whether it plans to convene an Advisory Committee meeting (AdCom) to review the sNDA. As previously disclosed, Amarin continues to prepare in the event that an AdCom is convened. If Amarin is informed definitively that there will or will not be an AdCom, the company plans to update investors accordingly.
Preliminary (Unaudited) First Half 2019 Financial Results
Record Revenue Levels Achieved: Net total revenue for the three and six months ended June 30, 2019 are estimated to have reached between $97 and $101 million and between $170 and $174 million, respectively. Both the second quarter and first half of 2019 results represent record revenue levels for Amarin. These results, which are subject to auditor review, represent an increase of approximately $44 to $48 million (approximately 84% to 92%) for the second quarter of 2019 over the corresponding period of 2018 and an increase of approximately $73 to $77 million (approximately 76% to 80%) for the first half of 2019 over the corresponding period of 2018. These results consist predominantly of U.S. sales-driven increases in prescriptions for Vascepa. Wholesaler inventory levels of Vascepa were within normal industry ranges at the end June 2019.
Current Assets: Amarin ended June 2019 with cash and cash equivalents of approximately $221 million (compared to $211 million at March 31, 2019), approximately $94 million in net accounts receivable and approximately $47 million in inventory.
No Debt, Except Remaining Balance of Royalty Bearing Instrument: Amarin ended June 2019 with no debt except the remaining balance on its royalty bearing instrument which is repaid at a rate of 10% of Vascepa revenues; aggregate repayment of less than $74 million remains until this royalty-like obligation is fully extinguished.
2019 Financial and Operational Guidance
Amarin initially provided guidance for 2019 in its press release dated January 4, 2019. Amarin now makes the following updates to that guidance:
2019 Revenue Guidance: Forecasting Vascepa revenue levels at this early stage remains difficult. Based on estimated total revenue results for the first half of 2019 which exceeded prior expectations, Amarin increases its guidance for 2019 net total revenue to a range of $380 to $420 million. While Amarin remains optimistic that Vascepa will generate billions of dollars in revenue in the years to come, the history of other therapies for chronic conditions suggests that growth builds over multiple years, and thus, the company is not prepared to provide quantified guidance regarding revenue levels beyond 2019.
Commercial Expansion in United States: Amarin has accelerated and further expanded its commercialization plans for Vascepa in the United States. Amarin intends to increase the size of its U.S. sales force to approximately 800 sales representatives with the aim of having its expanded team hired, trained and deployed by October 2019. This increase would represent a doubling of the size of Amarin’s current sales force.
The timing of such expansion had been accelerated in part due to the priority review designation of the sNDA for Vascepa. Assuming label expansion for Vascepa on the September 28, 2019 PDUFA date, Amarin expects to have educational and promotional materials available by early October 2019 to promote Vascepa based on the new label.
The size of the planned expansion reflects the result of evaluations involving multiple contributing factors. Previously Amarin had estimated the potential expansion of its sales force to reach between 600 and 800 sales representatives and for the expansion to potentially occur in phases. The decision to expand the sales force to approximately 800 sales representatives by October 2019 was based on new information including the encouraging progress being made by sales representatives hired at the start of 2019, positive feedback from physicians with deep understanding of the REDUCE-IT data, additional data on the commercial opportunity that exists in detailing physicians who have not yet been educated about Vascepa and data suggesting that education of healthcare professionals regarding Vascepa will be improved if our sales representatives call on physicians with greater frequency.
As a reminder, in late 2018, Amarin hired, trained and deployed 265 new and qualified sales representatives within approximately three months after learning the results of REDUCE-IT. At the time, more than 20,000 applications were received for the 265 open sales positions. Based on this track record and the robust results of Vascepa clinical trials, Amarin is confident it can double its sales force to 800 sales representatives by October 2019.
While Amarin is confident that expanding its sales force will result in meaningful revenue growth beyond 2019, the company’s revenue guidance for 2019 as described above anticipates little incremental contribution in 2019 from these newly hired sales representatives. Based on available data, it typically requires multiple months for newly hired sales representatives to become meaningfully productive particularly if they are calling on healthcare professionals who also are new to Vascepa, which will be the case for a large part of such sales force expansion.
Amarin intends to support its expanded U.S. sales team with multiple promotional and educational programs. All such initiatives will be aimed at providing truthful and non-misleading information to lead to improved patient care. Included in Amarin’s promotion plans is a direct-to-consumer advertising campaign, subject to review by the FDA’s Office of Prescription Drug Promotion (OPDP). The company anticipates submitting proposed Vascepa advertisements to OPDP in October 2019. Based on the timeline for other companies getting consumer advertising reviewed by FDA following new labelling, assuming new Vascepa labelling, launch of such branded advertising is anticipated in the second quarter of 2020.
Plans for commercialization of Vascepa outside of the United States remain unchanged, including intentions to submit for regulatory approval of Vascepa in the European Union near the end of 2019.
Comment from Amarin’s President and CEO
“We are pleased with the progress made to date, including the significant revenue growth we’ve achieved for Vascepa,” commented John Thero, president and chief executive officer of Amarin. “We anticipate Vascepa revenue growth to accelerate further after label expansion approval and with a larger sales team, and then again after we commence promotion of Vascepa for cardiovascular risk reduction on television and through other media. We are preparing for a robust launch of REDUCE-IT data with the aim of helping physicians improve patient care for millions of patients with residual cardiovascular risk after their cholesterol is controlled, as identified by elevated triglycerides.”
About Amarin
Amarin Corporation plc. is a rapidly growing, innovative pharmaceutical company focused on developing therapeutics to improve cardiovascular health. Amarin’s product development program leverages its extensive experience in polyunsaturated fatty acids and lipid science. Vascepa (icosapent ethyl) is Amarin's first FDA-approved drug and is available by prescription in the United States, Lebanon and the United Arab Emirates. Amarin’s commercial partners are pursuing additional regulatory approvals for Vascepa in Canada, China and the Middle East. For more information about Amarin, visit www.amarincorp.com.
About REDUCE-IT™
REDUCE-IT1 was an 8,179-patient multinational cardiovascular outcomes study completed in 2018. REDUCE-IT evaluated the effect of prescription pure EPA therapy as an add-on to statins in patients with high cardiovascular risk who, despite stable statin therapy, had elevated triglyceride levels (at least 135 mg/dL). A large portion of the male and female patients enrolled in this outcomes study were diagnosed with type 2 diabetes.
More information on the REDUCE-IT study results can be found at www.amarincorp.com.
About Cardiovascular Disease
Worldwide, cardiovascular disease (CVD) remains the #1 killer of men and women. In the United States CVD leads to one in every three deaths – one death approximately every 38 seconds – with annual treatment cost in excess of $500 billion.3, 4
Multiple primary and secondary prevention trials have shown a significant reduction of 25% to 35% in the risk of cardiovascular events with statin therapy, leaving significant persistent residual risk despite the achievement of target LDL-C levels.5
Beyond the cardiovascular risk associated with LDL-C, genetic, epidemiologic, clinical and real-world data suggest that patients with elevated triglycerides (TG) (fats in the blood), and TG-rich lipoproteins, are at increased risk for cardiovascular disease.6-9
About Vascepa® (icosapent ethyl) Capsules
Vascepa (icosapent ethyl) capsules are a single-molecule prescription product consisting of the omega-3 acid commonly known as EPA in ethyl-ester form. Vascepa is not fish oil, but is derived from fish through a stringent and complex FDA-regulated manufacturing process designed to effectively eliminate impurities and isolate and protect the single molecule active ingredient from degradation. Vascepa, known in scientific literature as AMR101, has been designated a new chemical entity by the FDA. Amarin has been issued multiple patents internationally based on the unique clinical profile of Vascepa, including the drug’s ability to lower triglyceride levels in relevant patient populations without raising LDL-cholesterol levels.
Indication and Usage Based on Current FDA-Approved Label (not including REDUCE-IT results)
Vascepa (icosapent ethyl) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
The effect of Vascepa on the risk for pancreatitis and cardiovascular mortality and morbidity in patients with severe hypertriglyceridemia has not been determined.
Important Safety Information for Vascepa Based on Current FDA-Approved Label (not including REDUCE-IT results) (Includes Data from Two 12-Week Studies (n=622) (MARINE and ANCHOR) of Patients with Triglycerides Values of 200 to 2000 mg/dL)
Vascepa is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vascepa or any of its components.
In patients with hepatic impairment, monitor ALT and AST levels periodically during therapy.
Use with caution in patients with known hypersensitivity to fish and/or shellfish.
The most common reported adverse reaction (incidence >2% and greater than placebo) was arthralgia (2.3% for Vascepa, 1.0% for placebo). There was no reported adverse reaction >3% and greater than placebo.
Adverse events and product complaints may be reported by calling 1-855-VASCEPA or the FDA at 1-800-FDA-1088.
Patients receiving treatment with Vascepa and other drugs affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
Patients should be advised to swallow Vascepa capsules whole; not to break open, crush, dissolve, or chew Vascepa.
FULL VASCEPA PRESCRIBING INFORMATION CAN BE FOUND AT WWW.VASCEPA.COM.
Important Safety Information for Vascepa based on REDUCE-IT, as previously reported in The New England Journal of Medicine1 publication of the primary results of the REDUCE-IT study:
Excluding the major adverse cardiovascular events (MACE) results described above, overall adverse event rates in REDUCE-IT were similar across the statin plus Vascepa and the statin plus placebo treatment groups.
There were no significant differences between treatments in the overall rate of treatment emergent adverse events or serious adverse events leading to withdrawal of study drug.
There was no serious adverse event (SAE) occurring at a frequency of >2% which occurred at a numerically higher rate in the statin plus Vascepa treatment group than in the statin plus placebo treatment group.
Adverse events (AEs) occurring in 5% or greater of patients and more frequently with Vascepa than placebo were:
– peripheral edema (6.5% Vascepa patients versus 5.0% placebo patients), although there was no increase in the rate of heart failure in Vascepa patients
– constipation (5.4% Vascepa patients versus 3.6% placebo patients), although mineral oil, as used as placebo, is known to lower constipation, and
– atrial fibrillation (5.3% Vascepa patients versus 3.9% placebo patients), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients
There were numerically more SAEs related to bleeding in the statin plus Vascepa treatment group although overall rates were low with no fatal bleeding observed in either group and no significant difference in adjudicated hemorrhagic stroke or serious central nervous system or gastrointestinal bleeding events between treatments.
In summary, Vascepa was well tolerated with a safety profile generally consistent with clinical experience associated with omega-3 fatty acids and current FDA-approved labeling of such products.
Vascepa has been approved for use by the United States Food and Drug Administration (FDA) as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. FDA has not reviewed and opined on a supplemental new drug application related to REDUCE-IT. FDA has not reviewed the information herein or determined whether to approve Vascepa for use to reduce the risk of MACE. Nothing in this press release should be construed as promoting the use of Vascepa in any indication that has not been approved by the FDA.
Important Cautionary Information About These Data
Further REDUCE-IT data assessment and data release could yield additional useful information to inform greater understanding of the trial outcome. For example, detailed data assessment by regulatory authorities, such as the FDA and Health Canada, will continue and take several months to complete and announce. The final evaluation by regulatory authorities of the totality of efficacy and safety data from REDUCE-IT may include some or all of the following, as well as other considerations: new information or analyses affecting the degree of treatment benefit on studied endpoints; study conduct and data robustness, quality, integrity and consistency; additional safety data considerations and risk/benefit considerations; and consideration of REDUCE-IT results in the context of other clinical studies. Because regulatory reviews are typically fluid and not definitive interactions between sponsor and agency on individual elements of an application and related information, Amarin does not plan to update investors on ongoing communications with regulatory authorities. Amarin plans to announce the final outcome of such regulatory reviews when appropriate.
Recurrent event analyses for the total primary endpoint events and for the total key secondary endpoint in REDUCE-IT as published in the Journal of the American College of Cardiology were conducted using a series of statistical models. These analyses were tertiary or exploratory endpoints; most of the models used were prespecified and one was post hoc. Each recurrent event statistical model has inherent strengths and weaknesses, with no single model considered definitive or outperforming the other models, and this is an evolving field of science. Nonetheless, results from the total primary and total key secondary endpoint events analyses are consistent across the various recurrent event statistical models and are also consistent with the original primary and secondary endpoint results. Together, the REDUCE-IT recurrent event analyses and the original primary and key secondary endpoint analyses support the robustness of the clinical benefit of Vascepa therapy in reducing cardiovascular risk.
Forward-Looking Statements
This press release contains forward-looking statements, including expectations regarding revenue and prescription growth, including updated revenue guidance for 2019; sales force expansion and marketing initiatives expected in 2019 and beyond; FDA regulatory review, including the timing and outcome of such review; the applicability and reliability of REDUCE-IT results; and the expected outcome and timing of review elements and market dynamics for Vascepa. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. In addition, Amarin's ability to effectively commercialize Vascepa will depend in part on its ability to continue to effectively finance its business, efforts of third parties, its ability to gain regulatory approvals, create market demand for Vascepa through education, marketing and sales activities, to achieve market acceptance of Vascepa, to receive adequate levels of reimbursement from third-party payers, to develop and maintain a consistent source of commercial supply at a competitive price, to comply with legal and regulatory requirements in connection with the sale and promotion of Vascepa and to maintain patent protection for Vascepa. Among the factors that could cause actual results to differ materially from those described or projected herein include the following: uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals; the risk that sales may not meet expectations and related cost may increase beyond expectations; the risk that patents may not be upheld in patent litigation and applications may not result in issued patents sufficient to protect the Vascepa franchise. A further list and description of these risks, uncertainties and other risks associated with an investment in Amarin can be found in Amarin's filings with the U.S. Securities and Exchange Commission, including its most recent quarterly report on Form 10-Q. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Amarin undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Availability of Other Information About Amarin
Investors and others should note that Amarin communicates with its investors and the public using the company website (www.amarincorp.com), the investor relations website (investor.amarincorp.com), including but not limited to investor presentations and investor FAQs, Securities and Exchange Commission filings, press releases, public conference calls and webcasts. The information that Amarin posts on these channels and websites could be deemed to be material information. As a result, Amarin encourages investors, the media, and others interested in Amarin to review the information that is posted on these channels, including the investor relations website, on a regular basis. This list of channels may be updated from time to time on Amarin’s investor relations website and may include social media channels. The contents of Amarin’s website or these channels, or any other website that may be accessed from its website or these channels, shall not be deemed incorporated by reference in any filing under the Securities Act of 1933.
References
Hier nochmal die wichtigsten Eckdaten im Überblick:
Amarin Provides Mid-2019 Update
> Record Revenue Achieved in 1H19 Primarily Due to Increased Demand for Vascepa
> Guidance for Total 2019 Revenue Increased to a Range of $380 to $420 Million from $350 Million Following Unaudited Second Quarter Results Estimated Between $97 and $101 Million, or Between $170 and $174 Million for the First Half of 2019
> U.S. Sales Force to Double in Size; Recruiting Commenced
> Vascepa sNDA PDUFA Goal Date On-Track for September 28, 2019; Potential for Therapy to Become First Prescription Product Available for Cardiovascular Risk Reduction in Patients with Elevated Triglyceride Levels, Despite Statin Therapy
Amarin Provides Mid-2019 Update
> Record Revenue Achieved in 1H19 Primarily Due to Increased Demand for Vascepa
> Guidance for Total 2019 Revenue Increased to a Range of $380 to $420 Million from $350 Million Following Unaudited Second Quarter Results Estimated Between $97 and $101 Million, or Between $170 and $174 Million for the First Half of 2019
> U.S. Sales Force to Double in Size; Recruiting Commenced
> Vascepa sNDA PDUFA Goal Date On-Track for September 28, 2019; Potential for Therapy to Become First Prescription Product Available for Cardiovascular Risk Reduction in Patients with Elevated Triglyceride Levels, Despite Statin Therapy
Sogar Adam Feuerstein schreibt nichts Negatives:
Amarin raises sales guidance for heart drug Vascepa, hires more sales reps for larger marketing push
By Adam Feuerstein @adamfeuerstein
July 2, 2019
Vascepa pill handout
Amarin
Amarin (AMRN) pre-announced record quarterly sales of its heart drug Vascepa on Tuesday and raised its financial guidance for the remainder of the year, citing strong patient demand for the drug, derived from fish oil, now that it has been shown to significantly lower the risk of death and other adverse cardiovascular events.
The Bedminster, New Jersey-based drug maker is also doubling the size of its commercial salesforce to prepare for the highly expected expansion of Vascepa’s label by the Food and Drug Administration in late September, the company said.
Sales of Vascepa in the just-ended second quarter reached a record range of $97 million to $101 million, Amarin said. The final sales tally will be announced after the company’s auditors review the results.
advertisement
At the $99 million midpoint, Vascepa sales in the second quarter nearly doubled sales from the year-ago period. Sell-side analysts, on average, were expecting Vascepa sales of $91 million in the third quarter, according to Koyfin.
Related:
The biotech scorecard for the third quarter: 12 stock-moving events to watch
Amarin attributes the accelerated sales growth of Vascepa to the publication last year of results from the REDUCE-IT clinical trial, which found that Vascepa provides significant cardiovascular benefits to a much broader swath of people than previously believed.
Based on surging demand for Vascepa prescriptions, Amarin now expects 2019 sales in the range of $380 to $420 million, the company said. Its previous Vascepa sales guidance, offered last January, was $350 million.
The current analyst consensus has Vascepa sales reaching $390 million in 2019, so investors, to some extent, were already anticipating Tuesday’s guidance hike.
The FDA is expected to rule by Sept. 28 on Amarin’s request to expand the Vascepa label to include the heart benefit claims resulting from the REDUCE-IT clinical trial. So far, the FDA has still not informed Amarin about any plans to convene an outside advisory committee meeting to review the Vascepa data, the company said Tuesday.
Typically, the FDA gives a drugmaker 60 days advance notice to prepare for an advisory committee meeting. With the calendar turning over to July, the odds that the FDA will schedule a Vascepa meeting are growing smaller, which in turn, makes it more likely that the agency will approve the Vascepa label expansion without delay.
Doctors are already prescribing more Vascepa based on the publication of the heart benefits seen in the REDUCE-IT study. Amarin’s marketing efforts, however, must wait for an official green light from the FDA. In anticipation of that happening, the company said Tuesday it has begun hiring new salespeople to market Vascepa. Once fully hired, Amarin’s commercial team will total 800 people, double its current size.
Amarin also intends to submit a marketing application for Vascepa in Europe by the end of the year.
Shares of Amarin rose 9% to $21 in early trading.
Amarin raises sales guidance for heart drug Vascepa, hires more sales reps for larger marketing push
By Adam Feuerstein @adamfeuerstein
July 2, 2019
Vascepa pill handout
Amarin
Amarin (AMRN) pre-announced record quarterly sales of its heart drug Vascepa on Tuesday and raised its financial guidance for the remainder of the year, citing strong patient demand for the drug, derived from fish oil, now that it has been shown to significantly lower the risk of death and other adverse cardiovascular events.
The Bedminster, New Jersey-based drug maker is also doubling the size of its commercial salesforce to prepare for the highly expected expansion of Vascepa’s label by the Food and Drug Administration in late September, the company said.
Sales of Vascepa in the just-ended second quarter reached a record range of $97 million to $101 million, Amarin said. The final sales tally will be announced after the company’s auditors review the results.
advertisement
At the $99 million midpoint, Vascepa sales in the second quarter nearly doubled sales from the year-ago period. Sell-side analysts, on average, were expecting Vascepa sales of $91 million in the third quarter, according to Koyfin.
Related:
The biotech scorecard for the third quarter: 12 stock-moving events to watch
Amarin attributes the accelerated sales growth of Vascepa to the publication last year of results from the REDUCE-IT clinical trial, which found that Vascepa provides significant cardiovascular benefits to a much broader swath of people than previously believed.
Based on surging demand for Vascepa prescriptions, Amarin now expects 2019 sales in the range of $380 to $420 million, the company said. Its previous Vascepa sales guidance, offered last January, was $350 million.
The current analyst consensus has Vascepa sales reaching $390 million in 2019, so investors, to some extent, were already anticipating Tuesday’s guidance hike.
The FDA is expected to rule by Sept. 28 on Amarin’s request to expand the Vascepa label to include the heart benefit claims resulting from the REDUCE-IT clinical trial. So far, the FDA has still not informed Amarin about any plans to convene an outside advisory committee meeting to review the Vascepa data, the company said Tuesday.
Typically, the FDA gives a drugmaker 60 days advance notice to prepare for an advisory committee meeting. With the calendar turning over to July, the odds that the FDA will schedule a Vascepa meeting are growing smaller, which in turn, makes it more likely that the agency will approve the Vascepa label expansion without delay.
Doctors are already prescribing more Vascepa based on the publication of the heart benefits seen in the REDUCE-IT study. Amarin’s marketing efforts, however, must wait for an official green light from the FDA. In anticipation of that happening, the company said Tuesday it has begun hiring new salespeople to market Vascepa. Once fully hired, Amarin’s commercial team will total 800 people, double its current size.
Amarin also intends to submit a marketing application for Vascepa in Europe by the end of the year.
Shares of Amarin rose 9% to $21 in early trading.
Eigentlich nur noch eine Frage der Zeit, bis Big Pharma hier zugreift, oder was meint ihr?!
Revolutionäres Produkt/Medi ohne Nebenwirkungen, Topp-Wachstumschancen etc.
Revolutionäres Produkt/Medi ohne Nebenwirkungen, Topp-Wachstumschancen etc.
Glaub ich auch, der Preis muss stimmen, unter US$ 50 wird John Thero nicht verkaufen, hoffentlich!
Bloomberg:
Amarin Surges After Drugmaker Says Heart Pill Sales Are Booming
[Bloomberg]
Myah Ward
,Bloomberg•July 2, 2019
(Bloomberg) -- Amarin Corp.’s American depositary receipts surged to their highest intraday price since March 12 after the drugmaker said that its new heart pill was selling faster than projected and that it plans to double the size of its sales force.
The company now expects 2019 revenue to be $380 million to $420 million, up from a May 1 prediction of $350 million. Amarin will also increase its sales force to approximately 800 people, it said in a statement Tuesday.
The success of the heart drug, called Vascepa, had at one time made the Dublin-based company the subject of takeover speculation.
“We have great confidence in our ability to successfully market and sell Vascepa in the U.S. on our own,” Chief Executive Officer John Thero said in an email to Bloomberg.
The depositary receipts were up 13% to $21.79 at 1:01 p.m. in New York, after rising as much as 14%.
Vascepa is approved for sale in the U.S. to lower patients’ triglyceride levels. Triglycerides are a type of fat in the blood, and too high a level can increase the risk of heart disease and stroke.
The company has asked the U.S. Food and Drug Administration to let it market Vascepa as helping reduce the risk of cardiovascular events, following a successful clinical trial. The FDA’s decision is expected by late September.
To contact the reporter on this story: Myah Ward in New York at mward174@bloomberg.net
To contact the editors responsible for this story: Drew Armstrong at darmstrong17@bloomberg.net, Timothy Annett
For more articles like this, please visit us at bloomberg.com
©2019 Bloomberg L.P.
Amarin Surges After Drugmaker Says Heart Pill Sales Are Booming
[Bloomberg]
Myah Ward
,Bloomberg•July 2, 2019
(Bloomberg) -- Amarin Corp.’s American depositary receipts surged to their highest intraday price since March 12 after the drugmaker said that its new heart pill was selling faster than projected and that it plans to double the size of its sales force.
The company now expects 2019 revenue to be $380 million to $420 million, up from a May 1 prediction of $350 million. Amarin will also increase its sales force to approximately 800 people, it said in a statement Tuesday.
The success of the heart drug, called Vascepa, had at one time made the Dublin-based company the subject of takeover speculation.
“We have great confidence in our ability to successfully market and sell Vascepa in the U.S. on our own,” Chief Executive Officer John Thero said in an email to Bloomberg.
The depositary receipts were up 13% to $21.79 at 1:01 p.m. in New York, after rising as much as 14%.
Vascepa is approved for sale in the U.S. to lower patients’ triglyceride levels. Triglycerides are a type of fat in the blood, and too high a level can increase the risk of heart disease and stroke.
The company has asked the U.S. Food and Drug Administration to let it market Vascepa as helping reduce the risk of cardiovascular events, following a successful clinical trial. The FDA’s decision is expected by late September.
To contact the reporter on this story: Myah Ward in New York at mward174@bloomberg.net
To contact the editors responsible for this story: Drew Armstrong at darmstrong17@bloomberg.net, Timothy Annett
For more articles like this, please visit us at bloomberg.com
©2019 Bloomberg L.P.
Amarin guidance highlights continued Vascepa traction, says H.C. Wainwright Amarin's updated revenue guidance yesterday highlights the continued U.S. prescription traction of Vascepa, H.C. Wainwright analyst Andrew Fein tells investors in a research note. The analyst finds the guidance updates in line with his 100 physician survey, which suggests Amarin "remains in the early stages of Vascepa launch with significant upside ahead coinciding with growing physician awareness." Fein reiterates a Buy rating on the shares with a $51 price target.
Read more at:
https://thefly.com/landingPageNews.php?id=2929191
Read more at:
https://thefly.com/landingPageNews.php?id=2929191
Amarin guidance highlights continued Vascepa traction, says H.C. Wainwright Amarin's updated revenue guidance yesterday highlights the continued U.S. prescription traction of Vascepa, H.C. Wainwright analyst Andrew Fein tells investors in a research note. The analyst finds the guidance updates in line with his 100 physician survey, which suggests Amarin "remains in the early stages of Vascepa launch with significant upside ahead coinciding with growing physician awareness." Fein reiterates a Buy rating on the shares with a $51
 price target.
price target.
Read more at:
https://thefly.com/landingPageNews.php?id=2929191

 price target.
price target.Read more at:
https://thefly.com/landingPageNews.php?id=2929191
Überblick zu wichtigen Fakten, Visionen und Zitate aus den letzten Wochen.
- Amarin hat die Zulassung zum Verkauf des Medikaments Vascepa
in den USA erhalten. Vascepa ist in der Lage, den Triglyceridspiegel von Patienten zu senken. Die Triglyceride sind eine Art Fett im Blut - ein zu hoher T.Spiegel kann das Risiko für Herzkrankheiten und Schlaganfälle erhöhen.
- Amarin hat nach einer „erfolgreichen“ klinischen Studie ( Ergebnisse der REDUCE-IT-Studie wurden im November 2018 und im März 2019 veröffentlicht) bei der FDA einen Antrag auf Erweiterung des Vascepa-Labels zur möglichen Risikominderung von kardiovaskulären Ereignissen gestellt.
- Ob vor der FDA Entscheidung eine Sitzung des Advisory Committee (AdCom) einberufen wird , um die NDA zu überprüfen, bleibt abzuwarten. Zumindest hat die FDA bisher Amarin noch nichts mitgeteilt.
In diesem Zusammenhang sagt Adam Feuerstein:
Normalerweise teilt die FDA einem Arzneimittelhersteller 60 Tage im voraus mit, dass er sich auf eine Sitzung des Beratungsausschusses vorbereiten soll. Mit der Umstellung des Kalenders auf Juli sinken die Chancen, dass die FDA ein Vascepa-Meeting einberufen wird, was wiederum die Wahrscheinlichkeit erhöht, dass die Agentur die Erweiterung des Vascepa-Labels unverzüglich genehmigt.
Die Entscheidung der FDA wird am 28.09.19 erwartet.
Nach der Bekanntgabe ( 02.07.19 ) der Steigerung der Umsatzzahlen von Vascepa sagte CEO John Thero:
“We have great confidence in our ability to successfully market and sell Vascepa in the U.S. on our own”
Ende 2018 stellte Amarin nach Bekanntgabe der ersten Ergebnisse von REDUCE-IT bereits 265 neu-geschulte und qualifizierte Vertriebsmitarbeiter ein. Basierend auf dieser Erfolgsbilanz und den guten Ergebnissen der klinischen Studien mit Vascepa ist Amarin zuversichtlich, dass das Verkaufsteam bis Oktober 2019 auf 800 Vertriebsmitarbeiter verdoppelt werden kann.
Das zeugt von einer klaren, überzeugenden Strategie , das Verkaufs-Procedere selbst managen zu können - und damit vielleicht ersten Buyoutgerüchten entgegen zu treten.
https://www.statnews.com/2019/07/02/amarin-raises-sales-guid…" target="_blank" rel="nofollow ugc noopener">https://www.statnews.com/2019/07/02/amarin-raises-sales-guid…
https://finance.yahoo.com/news/amarin-provides-mid-2019-incl…
https://finance.yahoo.com/news/amarin-surges-drugmaker-says-…
- Amarin hat die Zulassung zum Verkauf des Medikaments Vascepa
in den USA erhalten. Vascepa ist in der Lage, den Triglyceridspiegel von Patienten zu senken. Die Triglyceride sind eine Art Fett im Blut - ein zu hoher T.Spiegel kann das Risiko für Herzkrankheiten und Schlaganfälle erhöhen.
- Amarin hat nach einer „erfolgreichen“ klinischen Studie ( Ergebnisse der REDUCE-IT-Studie wurden im November 2018 und im März 2019 veröffentlicht) bei der FDA einen Antrag auf Erweiterung des Vascepa-Labels zur möglichen Risikominderung von kardiovaskulären Ereignissen gestellt.
- Ob vor der FDA Entscheidung eine Sitzung des Advisory Committee (AdCom) einberufen wird , um die NDA zu überprüfen, bleibt abzuwarten. Zumindest hat die FDA bisher Amarin noch nichts mitgeteilt.
In diesem Zusammenhang sagt Adam Feuerstein:
Normalerweise teilt die FDA einem Arzneimittelhersteller 60 Tage im voraus mit, dass er sich auf eine Sitzung des Beratungsausschusses vorbereiten soll. Mit der Umstellung des Kalenders auf Juli sinken die Chancen, dass die FDA ein Vascepa-Meeting einberufen wird, was wiederum die Wahrscheinlichkeit erhöht, dass die Agentur die Erweiterung des Vascepa-Labels unverzüglich genehmigt.
Die Entscheidung der FDA wird am 28.09.19 erwartet.
Nach der Bekanntgabe ( 02.07.19 ) der Steigerung der Umsatzzahlen von Vascepa sagte CEO John Thero:
“We have great confidence in our ability to successfully market and sell Vascepa in the U.S. on our own”
Ende 2018 stellte Amarin nach Bekanntgabe der ersten Ergebnisse von REDUCE-IT bereits 265 neu-geschulte und qualifizierte Vertriebsmitarbeiter ein. Basierend auf dieser Erfolgsbilanz und den guten Ergebnissen der klinischen Studien mit Vascepa ist Amarin zuversichtlich, dass das Verkaufsteam bis Oktober 2019 auf 800 Vertriebsmitarbeiter verdoppelt werden kann.
Das zeugt von einer klaren, überzeugenden Strategie , das Verkaufs-Procedere selbst managen zu können - und damit vielleicht ersten Buyoutgerüchten entgegen zu treten.
https://www.statnews.com/2019/07/02/amarin-raises-sales-guid…" target="_blank" rel="nofollow ugc noopener">https://www.statnews.com/2019/07/02/amarin-raises-sales-guid…
https://finance.yahoo.com/news/amarin-provides-mid-2019-incl…
https://finance.yahoo.com/news/amarin-surges-drugmaker-says-…
Antwort auf Beitrag Nr.: 58.247.820 von Cyberhexe am 18.07.18 22:09:58
...am 28.9.2019 wird VASCEPA spätestens von der FDA zugelassen --> Prescription Drug User Fee Act (PDUFA). Dann gibts nicht nur Sekt!
Zitat von Cyberhexe:Zitat von lobberland: Ich hoffe auf Sekt... bisherigen Ergebnisse lassen es zumindest erhoffen
ich bin ebenfalls vorsichtig optimistisch , dass REDUCE-IT den Beweis liefern wird, dass hochdosiertes Fischöl in Form von Vascepa kardiovaskuläre Erkrankungen gegenüber Plazebo stat. signifikant um 15% senkt!
Ich habe deswegen heute eine erste Position aufgebaut, die möglicherweise in den nächsten Wochen noch aufgestockt wird - Ergebnisbekanntgabe von REDUCE-IT ist auf Ende 3q2018 angekündigt.
Ich würde meinen: Sekt, je nach Ergebnis vielleicht sogar Champagner!
...am 28.9.2019 wird VASCEPA spätestens von der FDA zugelassen --> Prescription Drug User Fee Act (PDUFA). Dann gibts nicht nur Sekt!
Gibt es hier Meinungen zu ACST? Dort stehen später im Jahr noch P3 Daten zum Krillöl Projekt an. Logischerweise kein CVOT aber sie könnten sowohl von AMRN profitieren als auch in Antizipation der P3 Ergebnisse weiter steigen. Marktkapitalisierung liegt bei 100 Mio.
Why Amarin Stock Is Up 63% So Far in 2019
[Motley Fool]
Beth McKenna, The Motley Fool
,Motley Fool•July 10, 2019
What happened
Shares of Irish biotech Amarin (NASDAQ: AMRN) are up 14.2% so far this month, through Tuesday, July 9.
This rise follows the stock's 42.5% gain in the first half of 2019, according to data from S&P Global Market Intelligence. The S&P 500 returned 18.4% over this period.
Amarin's year-to-date gain is a hearty 62.7% through July 9, versus the broader market's 20.2% return.
A pile of six see-through, light-gold capsules on a flat surface.
A pile of six see-through, light-gold capsules on a flat surface.
Image source: Getty Images.
So what
July
On July 2, Amarin shares surged 16.3% following the company increasing its full-year 2019 revenue guidance to a midpoint of $400 million, up from $350 million. The raise was driven by strong demand for Vascepa, its omega-3 fatty-acid drug derived from fish oil that's currently approved to treat patients with high triglyceride levels.
This demand could be only a start, however, as Amarin and its investors are eagerly anticipating the U.S. Food and Drug Administration's decision on a huge label expansion. In late September, investors should learn whether the FDA has given the green light to Vascepa for the treatment of patients at risk for major adverse cardiovascular events, including heart attacks or strokes.
As background: Amarin stock skyrocketed last September after the company released robust results of its Reduce-It cardiovascular outcomes study. As I previously wrote, "The study showed that Vascepa ... reduced the risk of major adverse cardiovascular events by 25% in patients already taking statin drugs compared to those receiving a placebo, which is a highly statistically significant result."
AMRN Chart
AMRN Chart
Data by YCharts.
First Half of 2019
Amarin stock likely got a tailwind in the first half of the year from the strong overall market. That said, we can attribute its outperformance of the market to several catalysts, including:
Jan. 9: Shares popped 8.1% following the company's optimistic presentation at the J.P. Morgan Healthcare Conference.
Jan. 10: Shares soared 22.1% on market chatter about pharmaceutical giant Pfizer being interested in making a bid for Amarin.
Feb. 22-27: Shares gained 21.5% in the four market days leading up to and including the company's release of its fourth-quarter and full-year 2018 results, driven by investor optimism about Vascepa sales on the heels of the Reduce-It study. Indeed, investors were right to be hopeful, as Vascepa's Q4 revenue rose 44% year over year to $77.3 million.
May 29: Shares surged 11.6% after Amarin announced that the FDA had granted an expedited review of Vascepa for patients at risk of major adverse cardiovascular events. This moved the timetable for the agency's decision to six months after the application submission rather than the more usual 10 months.
Now what
Take heart, investors: The FDA's decision on Vascepa's label expansion should be less than three months away, in late September. Look for Amarin stock to react accordingly.
[Motley Fool]
Beth McKenna, The Motley Fool
,Motley Fool•July 10, 2019
What happened
Shares of Irish biotech Amarin (NASDAQ: AMRN) are up 14.2% so far this month, through Tuesday, July 9.
This rise follows the stock's 42.5% gain in the first half of 2019, according to data from S&P Global Market Intelligence. The S&P 500 returned 18.4% over this period.
Amarin's year-to-date gain is a hearty 62.7% through July 9, versus the broader market's 20.2% return.
A pile of six see-through, light-gold capsules on a flat surface.
A pile of six see-through, light-gold capsules on a flat surface.
Image source: Getty Images.
So what
July
On July 2, Amarin shares surged 16.3% following the company increasing its full-year 2019 revenue guidance to a midpoint of $400 million, up from $350 million. The raise was driven by strong demand for Vascepa, its omega-3 fatty-acid drug derived from fish oil that's currently approved to treat patients with high triglyceride levels.
This demand could be only a start, however, as Amarin and its investors are eagerly anticipating the U.S. Food and Drug Administration's decision on a huge label expansion. In late September, investors should learn whether the FDA has given the green light to Vascepa for the treatment of patients at risk for major adverse cardiovascular events, including heart attacks or strokes.
As background: Amarin stock skyrocketed last September after the company released robust results of its Reduce-It cardiovascular outcomes study. As I previously wrote, "The study showed that Vascepa ... reduced the risk of major adverse cardiovascular events by 25% in patients already taking statin drugs compared to those receiving a placebo, which is a highly statistically significant result."
AMRN Chart
AMRN Chart
Data by YCharts.
First Half of 2019
Amarin stock likely got a tailwind in the first half of the year from the strong overall market. That said, we can attribute its outperformance of the market to several catalysts, including:
Jan. 9: Shares popped 8.1% following the company's optimistic presentation at the J.P. Morgan Healthcare Conference.
Jan. 10: Shares soared 22.1% on market chatter about pharmaceutical giant Pfizer being interested in making a bid for Amarin.
Feb. 22-27: Shares gained 21.5% in the four market days leading up to and including the company's release of its fourth-quarter and full-year 2018 results, driven by investor optimism about Vascepa sales on the heels of the Reduce-It study. Indeed, investors were right to be hopeful, as Vascepa's Q4 revenue rose 44% year over year to $77.3 million.
May 29: Shares surged 11.6% after Amarin announced that the FDA had granted an expedited review of Vascepa for patients at risk of major adverse cardiovascular events. This moved the timetable for the agency's decision to six months after the application submission rather than the more usual 10 months.
Now what
Take heart, investors: The FDA's decision on Vascepa's label expansion should be less than three months away, in late September. Look for Amarin stock to react accordingly.
Planet Fitness, Allergan, Amarin: 'Mad Money' Lightning Round
Jim Cramer weighs in on Planet Fitness, Allergan, Amarin, MPLX, Zynerba Pharmaceuticals, Schnieder National, United Parcel Service and more.
Scott Rutt
Jul 10, 2019 8:40 PM EDT
Portfolio Advice: Apple & Walmart
Here's what Jim Cramer had to say about some of the stocks during the Mad Money Lightning Round:
Planet Fitness (PLNT - Get Report) : "I think Planet Fitness is terrific and they're winners."
Allergan (AGN - Get Report) : "This one has gotten a takeover bid. It's done."
Amarin (AMRN - Get Report) : "This is pretty good. It's a buy."
MPLX (MPLX - Get Report) : "I'll say it's OK to own, but pipelines have been a tough place. "
Zynerba Pharmaceuticals (ZYNE - Get Report) : "Very interesting, but I'll stick with GW Pharmaceuticals (GWPH) ."
Schneider National (SNDR - Get Report) : "Let's go with United Parcel Service (UPS - Get Report) ."
Cramer and the AAP team are redeploying some cash to one of their recent initiations, Caterpillar (CAT - Get Report) . Find out what they're telling their investment club members and get in on the conversation with a free trial subscription to Action Alerts Plus.
On Real Money, Cramer says it looks like America is overrun with denim right now. Get more of his insights with a free trial subscription to Real Money.
Introducing TheStreet Courses: Financial titans Jim Cramer and Robert Powell are bringing their market savvy and investing strategies to you. Learn how to create tax-efficient income, avoid top mistakes, reduce risk and more. With our courses, you will have the tools and knowledge needed to achieve your financial goals. Learn more about TheStreet Courses on investing and personal finance here.
Search Jim Cramer's "Mad Money" trading recommendations using our exclusive "Mad Money" Stock Screener.
To read a full recap of this episode of "Mad Money," click here.
To watch replays of Cramer's video segments, visit the Mad Money page on CNBC.
To sign up for Jim Cramer's free Booyah! newsletter with all of his latest articles and videos please click here.
At the time of publication, Cramer's Action Alerts PLUS had a position in CAT.
Jim Cramer weighs in on Planet Fitness, Allergan, Amarin, MPLX, Zynerba Pharmaceuticals, Schnieder National, United Parcel Service and more.
Scott Rutt
Jul 10, 2019 8:40 PM EDT
Portfolio Advice: Apple & Walmart
Here's what Jim Cramer had to say about some of the stocks during the Mad Money Lightning Round:
Planet Fitness (PLNT - Get Report) : "I think Planet Fitness is terrific and they're winners."
Allergan (AGN - Get Report) : "This one has gotten a takeover bid. It's done."
Amarin (AMRN - Get Report) : "This is pretty good. It's a buy."
MPLX (MPLX - Get Report) : "I'll say it's OK to own, but pipelines have been a tough place. "
Zynerba Pharmaceuticals (ZYNE - Get Report) : "Very interesting, but I'll stick with GW Pharmaceuticals (GWPH) ."
Schneider National (SNDR - Get Report) : "Let's go with United Parcel Service (UPS - Get Report) ."
Cramer and the AAP team are redeploying some cash to one of their recent initiations, Caterpillar (CAT - Get Report) . Find out what they're telling their investment club members and get in on the conversation with a free trial subscription to Action Alerts Plus.
On Real Money, Cramer says it looks like America is overrun with denim right now. Get more of his insights with a free trial subscription to Real Money.
Introducing TheStreet Courses: Financial titans Jim Cramer and Robert Powell are bringing their market savvy and investing strategies to you. Learn how to create tax-efficient income, avoid top mistakes, reduce risk and more. With our courses, you will have the tools and knowledge needed to achieve your financial goals. Learn more about TheStreet Courses on investing and personal finance here.
Search Jim Cramer's "Mad Money" trading recommendations using our exclusive "Mad Money" Stock Screener.
To read a full recap of this episode of "Mad Money," click here.
To watch replays of Cramer's video segments, visit the Mad Money page on CNBC.
To sign up for Jim Cramer's free Booyah! newsletter with all of his latest articles and videos please click here.
At the time of publication, Cramer's Action Alerts PLUS had a position in CAT.
Antwort auf Beitrag Nr.: 60.947.566 von bernie55 am 03.07.19 17:02:19
Auf dem Advisory Committee Calendar ist für Juli 2019 soweit nichts angekündigt
https://www.fda.gov/advisory-committees/advisory-committee-c…
Zitat von bernie55: - Ob vor der FDA Entscheidung eine Sitzung des Advisory Committee (AdCom) einberufen wird , um die sNDA zu überprüfen, bleibt abzuwarten. Zumindest hat die FDA bisher Amarin noch nichts mitgeteilt.
In diesem Zusammenhang sagt Adam Feuerstein:
Normalerweise teilt die FDA einem Arzneimittelhersteller 60 Tage im voraus mit, dass er sich auf eine Sitzung des Beratungsausschusses vorbereiten soll. Mit der Umstellung des Kalenders auf Juli sinken die Chancen, dass die FDA ein Vascepa-Meeting einberufen wird, was wiederum die Wahrscheinlichkeit erhöht, dass die Agentur die Erweiterung des Vascepa-Labels unverzüglich genehmigt.
Auf dem Advisory Committee Calendar ist für Juli 2019 soweit nichts angekündigt
https://www.fda.gov/advisory-committees/advisory-committee-c…
Verschreibungen von VASCEPA von Februar 2015 - Juli 2019 ( CaptBeer auf stocktwits.com )


Wichtiger Schwerpunkt der 36. Tagung für die Prävention von Herz-Kreislauf-Erkrankungen -
die Ergebnisse von REDUCE-IT werden vorgestellt und diskutiert
ASPC 2019 (The American Society for Preventive Cardiology)
July 19-21, 2019
La Cantera Resort & Spa
San Antonio, TX
https://www.aspconline.org/congress2019/
CONGRESS OVERVIEW
Important advances in prevention and treatment of cardiovascular disease continue to emerge, and these advances must be consistently incorporated into clinical practice to provide the best care for patients.
Opening day Agenda
19.07.19
3:30–3:40 PM Welcoming Remarks
3:50–4:30 PM
Keynote Lecture: REDUCE-IT and Treating Beyond LDL-C Lowering Deepak Bhatt, MD
Seite 3 auf https://www.aspconline.org/wp-content/uploads/2019/05/ASPC-…
die Ergebnisse von REDUCE-IT werden vorgestellt und diskutiert
ASPC 2019 (The American Society for Preventive Cardiology)
July 19-21, 2019
La Cantera Resort & Spa
San Antonio, TX
https://www.aspconline.org/congress2019/
CONGRESS OVERVIEW
Important advances in prevention and treatment of cardiovascular disease continue to emerge, and these advances must be consistently incorporated into clinical practice to provide the best care for patients.
Opening day Agenda
19.07.19
3:30–3:40 PM Welcoming Remarks
3:50–4:30 PM
Keynote Lecture: REDUCE-IT and Treating Beyond LDL-C Lowering Deepak Bhatt, MD
Seite 3 auf https://www.aspconline.org/wp-content/uploads/2019/05/ASPC-…
Moin zusammen
Warum sackt sie so ein 🤔
Warum sackt sie so ein 🤔
Antwort auf Beitrag Nr.: 61.051.915 von lobberland am 18.07.19 08:54:43Guten Morgen,
das liegt an 2 Dingen (meiner Meinung nach)
einmal das hier:
https://investor.amarincorp.com/news-releases/news-release-d…
das führt dazu sich die Frage zu stellen warum Amarin 54 Tage vor spätester FDA Bekanntgabe ob zugelassen oder nicht bis zu 460 Mio in Cash umwandelt und mit diesem Schritt nicht erst das Ergebnis abgewartet hat. Daher "psychologischer Nebeneffekt" denken jetzt viele, Amarin vergoldet sich den zuletzt hingelegten Höhenflug der Aktie um für "härtere Zeiten" Cash zu haben, sprich härtere Zeiten bedeutet Sie sind selbst nicht vollkommen überzeugt davon das Sie diesmal durchkommen. Da Amarin ausschliesslich auf das eine Medikament setzt hängt eben extrem viel von dieser anstehenden Entscheidung ab..
Andere Interpretationen sind, Amarin glaubt dass sie "sicher" die Zulassung erhalten und will mit dem Cash wirklich schon die Markterweiterung angehen so dass Sie sofort im grösseren Masstab loslegen können.
Ein kleiner negativer Effekt zusätzlich aus dieser zunächst positiven Erkenntnis ist, Sie machen ernst damit alles alleine weiter stemmen zu wollen, sprich sind nicht an Übernahmeangeboten interessiert... Sprich kurzfristige Kurssprünge da Übernahme ansteht bleibt somit aus..
Zum anderen ist auch die Zwischenprüfung die immer noch nicht vom FDA an- oder abgekündigt wurde, vor "finalem" Entscheid weiter im Spiel. Eigentlich gehört das zum normalen Prozedere und keine durchzuführen wäre aus meiner Sicht absolut einzigartig, aber wenn sie kommt wäre das absolut normal und kein negatives Zeichen...
Naja all diese Punkte geben den Skeptikern kräftig Aufwind und ein weiteres Absinken des Kurses ist wahrscheinlich...
Jetzt heisst entweder daran glauben und am besten die nächsten Wochen einfach nicht so genau auf den Kurs achten oder alles raus mit Verlusten so schnell wie möglich...
oder alles raus mit Verlusten so schnell wie möglich...
Einige spielen auch das Spiel so, jetzt raus und wenn die Aktie noch weiter unten ist wieder alles rein, mit entsprechendem Geld also mit mehr Anteilen dann bis zum Finale dabei sein...
Die FDA Finalentscheidung kann aber "jederzeit" kommen dass heisst man spielt egal wie nicht "sicher" und kann bestimmte Kursbewegungen egal wie man sich entscheidet verpassen.
Ich gehe für meinen Teil auf Risiko da mich das Medikament die Studie und die Aussage der Fachärzte als auch Patienten sehr überzeugt haben. Ich glaube an einen Gamechanger, aber eines ist sicher die Aktie braucht insgesamt Zeit um sich auch später in sehr profitable Höhen zu schwingen, da Amarin das nun selbst ankurbelt und nicht die Ressourcen eines Grosskonzerns besitzt...
Hoffe diese Einschätzung hat Dir etwas geholfen ?
das liegt an 2 Dingen (meiner Meinung nach)
einmal das hier:
https://investor.amarincorp.com/news-releases/news-release-d…
das führt dazu sich die Frage zu stellen warum Amarin 54 Tage vor spätester FDA Bekanntgabe ob zugelassen oder nicht bis zu 460 Mio in Cash umwandelt und mit diesem Schritt nicht erst das Ergebnis abgewartet hat. Daher "psychologischer Nebeneffekt" denken jetzt viele, Amarin vergoldet sich den zuletzt hingelegten Höhenflug der Aktie um für "härtere Zeiten" Cash zu haben, sprich härtere Zeiten bedeutet Sie sind selbst nicht vollkommen überzeugt davon das Sie diesmal durchkommen. Da Amarin ausschliesslich auf das eine Medikament setzt hängt eben extrem viel von dieser anstehenden Entscheidung ab..
Andere Interpretationen sind, Amarin glaubt dass sie "sicher" die Zulassung erhalten und will mit dem Cash wirklich schon die Markterweiterung angehen so dass Sie sofort im grösseren Masstab loslegen können.
Ein kleiner negativer Effekt zusätzlich aus dieser zunächst positiven Erkenntnis ist, Sie machen ernst damit alles alleine weiter stemmen zu wollen, sprich sind nicht an Übernahmeangeboten interessiert... Sprich kurzfristige Kurssprünge da Übernahme ansteht bleibt somit aus..
Zum anderen ist auch die Zwischenprüfung die immer noch nicht vom FDA an- oder abgekündigt wurde, vor "finalem" Entscheid weiter im Spiel. Eigentlich gehört das zum normalen Prozedere und keine durchzuführen wäre aus meiner Sicht absolut einzigartig, aber wenn sie kommt wäre das absolut normal und kein negatives Zeichen...
Naja all diese Punkte geben den Skeptikern kräftig Aufwind und ein weiteres Absinken des Kurses ist wahrscheinlich...
Jetzt heisst entweder daran glauben und am besten die nächsten Wochen einfach nicht so genau auf den Kurs achten
 oder alles raus mit Verlusten so schnell wie möglich...
oder alles raus mit Verlusten so schnell wie möglich... Einige spielen auch das Spiel so, jetzt raus und wenn die Aktie noch weiter unten ist wieder alles rein, mit entsprechendem Geld also mit mehr Anteilen dann bis zum Finale dabei sein...
Die FDA Finalentscheidung kann aber "jederzeit" kommen dass heisst man spielt egal wie nicht "sicher" und kann bestimmte Kursbewegungen egal wie man sich entscheidet verpassen.
Ich gehe für meinen Teil auf Risiko da mich das Medikament die Studie und die Aussage der Fachärzte als auch Patienten sehr überzeugt haben. Ich glaube an einen Gamechanger, aber eines ist sicher die Aktie braucht insgesamt Zeit um sich auch später in sehr profitable Höhen zu schwingen, da Amarin das nun selbst ankurbelt und nicht die Ressourcen eines Grosskonzerns besitzt...
Hoffe diese Einschätzung hat Dir etwas geholfen ?

Antwort auf Beitrag Nr.: 61.052.095 von flyingbeef am 18.07.19 09:16:34Danke für deine ausführliche Einschätzung
Dabei bleibe ich auch auf jeden Fall, bin mir sicher das hier das eine Pferd auch ins Ziel läuft. 🙋♂️
Dabei bleibe ich auch auf jeden Fall, bin mir sicher das hier das eine Pferd auch ins Ziel läuft. 🙋♂️
Amarin ist höchstseriös und treibt die Markteinführung mit der Kapitalerhöhung voran, für mich ist die Zulassung in trockenen Tüchern, 31 % weniger Herzinfarkte, 28 % weniger Schlaganfälle und 20 % weniger Todesfälle beim Reduce-It Trial, sensationell und so gut wie ohne Nebenwirkungen!
Amarin stockt auf 800 Reps auf und macht Vascepa zum Megablockbuster, Nachkaufchance!
Wir wissen ja noch nicht den Ausgabekurs, ich tipppe um die 20 US Dollar und die Fonds die kaufen wollen ja auch Ihren Gewinn machen!
Amarin stockt auf 800 Reps auf und macht Vascepa zum Megablockbuster, Nachkaufchance!
Wir wissen ja noch nicht den Ausgabekurs, ich tipppe um die 20 US Dollar und die Fonds die kaufen wollen ja auch Ihren Gewinn machen!

Antwort auf Beitrag Nr.: 61.052.953 von Magnetfeldfredy am 18.07.19 10:28:25Hi sehe ich auch so.
Amarin steht für preiswerte Medikamente, keine Gewinnmaximierung, die haben auch meiner Meinung nach Anstand. Daher sehe ich diesen Cash-Move auch in keinster Weise als Bereicherung, dass würde einfach nicht passen, weder zu Ihrem Chef noch zur Firma selbst.
Ich denke auch dass Sie wissen das Sie in der Zielgeraden stehen. Dass Sie die Unabhängigkeit von raffgierigen Übernahmefirmen weiter bewahren wollen finde ich im Gegenteil auch sehr sympatisch.
Ich hab gerne in diese Aktie investiert, es fühlt sich einfach "gut" an und ist im Idealfall auch eine echte Bereicherung für die Menschheit. Da dann ein wenig mit Anteil gehabt zu haben und wenn es auch nur ein bischen Geld ist why not
Amarin steht für preiswerte Medikamente, keine Gewinnmaximierung, die haben auch meiner Meinung nach Anstand. Daher sehe ich diesen Cash-Move auch in keinster Weise als Bereicherung, dass würde einfach nicht passen, weder zu Ihrem Chef noch zur Firma selbst.
Ich denke auch dass Sie wissen das Sie in der Zielgeraden stehen. Dass Sie die Unabhängigkeit von raffgierigen Übernahmefirmen weiter bewahren wollen finde ich im Gegenteil auch sehr sympatisch.
Ich hab gerne in diese Aktie investiert, es fühlt sich einfach "gut" an und ist im Idealfall auch eine echte Bereicherung für die Menschheit. Da dann ein wenig mit Anteil gehabt zu haben und wenn es auch nur ein bischen Geld ist why not

@flyingbee
@Magnetfeldfredy
Euren Ausführungen ist nichts mehr hinzuzufügen.
 Super vielen Dank , klasse gemacht !
Super vielen Dank , klasse gemacht !
Die dargestellten möglichen Szenarien und Hypothesen decken sich soweit auch mit den Beiträgen in den amerikanischen Foren ( stocktwits, yahoo).
> ...sobald die Anteile von den Instituten gekauft wurden, werden die großen Namen gezwungen sein, mit der Berichterstattung zu beginnen.
> ...sobald die FDA-Zulassung vorliegt, wird Amarin in viele Investmentfonds mit Biotech-Beteiligungen aufgenommen.
> Amarins Plan ….dass Kapital verwendet wird, um strategische Vermögenswerte zu erwerben und das kommerzielle Angebot von Vascepa zu erhöhen..... Amarin muss viele Fischfarmen besitzen und möglicherweise sogar EPA-produzierende Mikroalgenfirmen oder -produkte kaufen.
> ..einem möglichen Buyout entgegenwirken...
> ….FDA-Nachrichten können jetzt jeden Tag sein!
> J. P. Morgan Securities LLC, Goldman Sachs & Co. LLC, Jefferies LLC und Cantor Fitzgerald & Co. agieren als gemeinsame Book-Running-Manager im Angebot.Diese „Institute“ werden die „Angebots-Anteile“ in Rekordzeit verschlingen
> ....geht davon aus, dass der Kurs zwischen 19 und 20 US-Dollar liegen wird.
Let´s hope the best....so time will tell
@Magnetfeldfredy
Euren Ausführungen ist nichts mehr hinzuzufügen.
 Super vielen Dank , klasse gemacht !
Super vielen Dank , klasse gemacht !
Die dargestellten möglichen Szenarien und Hypothesen decken sich soweit auch mit den Beiträgen in den amerikanischen Foren ( stocktwits, yahoo).
> ...sobald die Anteile von den Instituten gekauft wurden, werden die großen Namen gezwungen sein, mit der Berichterstattung zu beginnen.
> ...sobald die FDA-Zulassung vorliegt, wird Amarin in viele Investmentfonds mit Biotech-Beteiligungen aufgenommen.
> Amarins Plan ….dass Kapital verwendet wird, um strategische Vermögenswerte zu erwerben und das kommerzielle Angebot von Vascepa zu erhöhen..... Amarin muss viele Fischfarmen besitzen und möglicherweise sogar EPA-produzierende Mikroalgenfirmen oder -produkte kaufen.
> ..einem möglichen Buyout entgegenwirken...
> ….FDA-Nachrichten können jetzt jeden Tag sein!
> J. P. Morgan Securities LLC, Goldman Sachs & Co. LLC, Jefferies LLC und Cantor Fitzgerald & Co. agieren als gemeinsame Book-Running-Manager im Angebot.Diese „Institute“ werden die „Angebots-Anteile“ in Rekordzeit verschlingen
> ....geht davon aus, dass der Kurs zwischen 19 und 20 US-Dollar liegen wird.
Let´s hope the best....so time will tell
Amarin Prices Public Offering of American Depositary Shares
GlobeNewswire•July 19, 2019
BEDMINSTER, N.J. and DUBLIN, Ireland, July 18, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN) today announced the pricing of the underwritten public offering of 22,222,223 American Depositary Shares ("ADSs") at a price to the public of $18.00 per ADS.
The gross proceeds of this offering are expected to be approximately $400.0 million, before deducting the underwriting discounts and commissions and other estimated offering expenses payable by Amarin. The offering is expected to close on or about July 23, 2019, subject to customary closing conditions.
https://finance.yahoo.com/news/amarin-prices-public-offering…
GlobeNewswire•July 19, 2019
BEDMINSTER, N.J. and DUBLIN, Ireland, July 18, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN) today announced the pricing of the underwritten public offering of 22,222,223 American Depositary Shares ("ADSs") at a price to the public of $18.00 per ADS.
The gross proceeds of this offering are expected to be approximately $400.0 million, before deducting the underwriting discounts and commissions and other estimated offering expenses payable by Amarin. The offering is expected to close on or about July 23, 2019, subject to customary closing conditions.
https://finance.yahoo.com/news/amarin-prices-public-offering…
Reduce-IT
Hallo zusammen,würde gerne eure Meinung dazu hören. Ich habe Interviews von Fachärzten die sich die Studie angesehen haben gelesen.
Fazit: Grundtenor war positiv = tolles Potential
Aber: Natürlich gibt es auch immer die andere Seite der Medallie.
Negative Aspekte waren:
- Placebo war Mineralölbasierend, es könnte also sein dass die sehr positiven Ergebnisse im Vergleich zur Placebogruppe auf negativen Gesundheitseffekten beruhen die das Placebo ausgelöst haben könnte. (Dazu muss msn wissen das FDA hat explizit ein mineralölbasiertes Placebo für die Studie akzeptiert)
Hier geben Experten zu dass mutmasslich wenn das einen Effekt hat aber nicht so sehr ins Gewicht fällt so dass Vascepa keine Wirkung mehr hätte. Aber eben keine 25% mehr...
- Ein anderer Aspekt war das die ausgewählten Testpersonen mit nicht genug Zufall zu den Gruppen zugeteilt wurden und somit ebenfalls evtl. eine positivere Darstellung der Ergebnisse möglich waren als wenn das Verfahren mehr Zufall gehabt hätte
- Letzter Aspekt war dass zwar eine positive Wirkung statistisch nachvollziehbar nachgewiesen wurde, aber andere Fischölbasierte Produkttest nie eine auch nur annähernd vergleichbare Wirkung erzielt hätten. Dazu wurde angemerkt dass man viel zu wenig darüber weiss warum Vascepa wirkt und das noch genauer untersucht werden müsste.
Das sind alles Dinge die auch heute wohl auf dem Kongress wieder Thema sein werden. Kennt sich jemand von euch mit Pharma aus und kann das einschätzen ob diese Argumente Roadblocker sind oder das zwar valide Anmerkungen sind dass aber eine Zulassung nicht gefährden dürfte ? Auch würde mich interessieren ob diese Argumente trotz Zulassung den Marktantritt und die Geschwindigkeit der Medikamentenverschreibung weiter hemmen könnte ? Wäre an euren Meinungen sehr interessiert.
Einschätzung zur aktuellen Situation
Hallo, evtl. helfen diese Gedanken anderen auch ein wenig, die vom Kurssturz etwas mitgenkommen sind...
 (Ich selbst habe aktuell ordentlich Verluste...)
(Ich selbst habe aktuell ordentlich Verluste...) Ich hatte mir überlegt wenn ich John Thero wäre und so handeln würde wie er es getan hat, warum hätte ich das getan.
Eigentlich sind diese ganzen Spekulationen, bald kommt die FDA Entscheidung oder Amarin selsbt hat nun große Zweifel (sie wissen was...) totaler Blödsinn.
JT weiss genausowenig wie wir wie sich das FDA entscheiden wird und hofft das beste für sich und die Firma. Ganz sicher glaubt er an Ihr jahrelang erprobtes Produkt und die Studie besätigt Sie ja auch immens.
Also warum dann nochmal so eine Finanzspritze ?
Ganz einfach, es ist eine klare Entscheidung für seine Leute / seine Firma. Er hat wie viele andere Firmen die überleben wollen die Möglichkeit gesehen zu einem aktuell ganz guten Preis neue finanzielle Mittel zu erhalten.
Wozu ?
Naja für 2 Dinge:
1. Egal ob die FDA Entscheidung jetzt kommt später oder gar nicht, seine Firma kann erst mal ein paar Jahre weiter machen und gegebenenfalls auch ein neues Produkt / neue Anwendungen testen. Sprich er sichert erst mal die mittelfristige Zukunft ab, ganz unabhängig davon ob er an einen Erfolg glaubt (wovon ich überzeugt bin das er es glaubt) oder nicht. Ganz nüchterne Entscheidung eben. Und es ist besser 600 Mio auf der Hohen Kante zu haben als 200 Mio würdet ihr ähnlich sehen wenn es eure Firma wäre

2. Wie im Aktienverkaufsvohrhaben beschrieben können die zusätzlichen Finanzen wirklich helfen schneller zu expandieren. Er wird aber mit grösseren Ausgaben warten bis die Entscheidung da ist. Er bekommt keine Insidertips vom FDA usw. hier ist keine Verschwörung im Gange weder im positiven noch im negativen Sinne.... Siehe Stellenanzeigen sind aktuell immer noch die knapp 40 Stellen drin. Trotzdem kann man mit dem nun gewonnen Finanzmitteln schneller den Markt bedienen und auch dem FDA gegenüber besser zusichern auf benötigte Kapazitäten in absehbarer Zeit für den Markt upgraden zu können. Hilft also dem ganzen Prozess nur und ist evtl. sogar ein wichtiger Baustein, da Amarin ja plant erst mal alleine loszulegen und dass FDA eine gesunde finanzielle Basis sehen muss um den Markt der dieses Medikament erfordert auch schnellstmöglich dieses liefern zu können.
Was hat sich nun durch dieses nochmalige Geldholen verändert ?
Nichts, rein gar nichts... Die Chancen sind genauso hoch oder niedrig wie Sie vor der Finanzspritze waren, die Aktion hat "nichts" mit irgendwelchen bösen Vorahnungen das alles schief geht zu tun oder ähnliches... JT handelt absolut rational im Sinne seiner Firma.. und dessen weiterer Zukunft.
Das er da dann wissentlich bei Berufs-Skeptikern usw. unter den Aktionären (naja manche haben ja auch Grund dazu es gibt auch wirklich kriminelle Firmen oder Chefs...) den Kurs ins Trudeln bringt kann ihm egal sein. Die Firma wäre sowieso innerhalb kürzester Zeit extrem viel oder nicht sonderlich viel wert, ganz nachdem wie die FDA Entscheidung eben ausfällt.
Ja es ist keine Entscheidung wo wir als Anleger gehätschelt werden.. Er scheint sich zu sagen, die die überzeugt sind wie Amarin selbst die bleiben und wissen nun dass es um den Zieleinlauf geht, dass er seine Beweggründe wie hier beschrieben nicht klar und offen formulieren kann ist natürlich selbsterklärend

Antwort auf Beitrag Nr.: 61.070.479 von flyingbeef am 20.07.19 13:35:21
Das Unternehmen beabsichtigt, die finanziellen Mittel von 400 Mio. USD zu verwenden, um
- die Größe der vorhandenen Vertriebsmitarbeiter zu verdoppeln,
- die Marketinginfrastruktur zu verbessern ( u.a. mehr Werbung) und
- das Produktionspotential auszuweiten.
Amarin hat derzeit eine NCE-Exklusivität für die Vermarktung von Vascepa - diese läuft bis zum 20 Januar 2020.
Es ist also nur eine begrenzte Zeit vorhanden, bis die FDA mit dem Zulassungsverfahren für alternative Arzneimittel / generische Namen beginnen kann.
Amarin steht derzeit auf eigenen Füßen und verfügt noch nicht über die Maschinenproduktion, die die größere mögliche Konkurrenz sofort einsetzen könnte.
Amarin hat keine ZEIT zu verlieren.
ZEIT ist GELD – spielt eine sehr wichtige Rolle.
Amarin muss die aktuelle Situation somit ausnutzen, um Vascepa an die Verbraucher zu bringen und sich zugleich einen Markennamen aufzubauen.
https://seekingalpha.com/article/4276637-amarin-valuations-s…
https://www.fiercepharma.com/pharma/after-460m-cash-raise-am…
Zitat von flyingbeef: Und es ist besser 600 Mio auf der Hohen Kante zu haben als 200 Mio.....
Trotzdem kann man mit dem nun gewonnenen Finanzmitteln schneller den Markt bedienen …...
…...da Amarin ja plant erst mal alleine loszulegen und dass die FDA eine gesunde finanzielle Basis sehen muss, zur Vermarktung des Produktes...
Das Unternehmen beabsichtigt, die finanziellen Mittel von 400 Mio. USD zu verwenden, um
- die Größe der vorhandenen Vertriebsmitarbeiter zu verdoppeln,
- die Marketinginfrastruktur zu verbessern ( u.a. mehr Werbung) und
- das Produktionspotential auszuweiten.
Amarin hat derzeit eine NCE-Exklusivität für die Vermarktung von Vascepa - diese läuft bis zum 20 Januar 2020.
Es ist also nur eine begrenzte Zeit vorhanden, bis die FDA mit dem Zulassungsverfahren für alternative Arzneimittel / generische Namen beginnen kann.
Amarin steht derzeit auf eigenen Füßen und verfügt noch nicht über die Maschinenproduktion, die die größere mögliche Konkurrenz sofort einsetzen könnte.
Amarin hat keine ZEIT zu verlieren.
ZEIT ist GELD – spielt eine sehr wichtige Rolle.
Amarin muss die aktuelle Situation somit ausnutzen, um Vascepa an die Verbraucher zu bringen und sich zugleich einen Markennamen aufzubauen.
https://seekingalpha.com/article/4276637-amarin-valuations-s…
https://www.fiercepharma.com/pharma/after-460m-cash-raise-am…
Antwort auf Beitrag Nr.: 61.089.148 von bernie55 am 23.07.19 18:07:48Hallo,
Danke sehr interessant und hilfreich !
Über diese Exklusivität wusste ich nichts. Soweit ich weiss gibt es ein paar Marktbegleiter die sich auch auf Basis der erfolgreichen Reduce-IT Studie erhoffen schnell eigene Daseinsberechtigungen zu erhalten.
Insbesondere wird hier ja ständig in den amerikanischen Foren ja z.B. über Acasti Pharma und deren krill oil-derived mixture diskutiert.
Ich kann dass nicht richtig einschätzen ob eine große Chance (nach Erfolg Vascepa) besteht das dass FDA deren CaPre wirklich auch bis Anfang 2021 zulassen würden.
Halte das im Moment eher für Hype Propaganda...
Ich kann auch nicht einschätzen ob deren Medikament wirklich vergleichbar ist mit Vascepa, die beziehen sich ja, schlau wie sie sind, nur auf vermeintlich vergleichbare Teilaspekte in Ihrer Präsentation und behaupten natürlich Marketing-tauglich Sie können das auch nur "besser"
Außerdem kann ich zuletzt nicht sagen ob Sie das Krill basierte Öl auch in den benötigten Mengen und Zeitumfängen herstellen können, da habe ich eher meine Zweifel + mögliche Umwelteffekte.
Meines Wissens nach kann man Krill nicht effizient und umweltverträglich züchten, wie es weitestgehend eben mit Fischen funktioniert, zumindest ist mir keine Methode bekannt.
Außerdem ist Krill "die" Grundlage für das Leben im Meer schlechthin, keine Ahnung welche Mengen sie dann für Medikamente umwandeln müssten, daher die Idee ist nett aber die Umsetzung könnte alles andere als nett werden.
Zurück zum Thema:
Ich finde die Argumentation dass Amarin sich darüber bewusst ist Gas geben zu müssen, um möglichst ein großes Stück vom Kuchen sich sichern zu können schlüssig. Um aber das derart aus eigener Kraft weiter vorantreiben zu können, müssen die evtl. ab Mitte 2020 wieder Kapital abgreifen um deren Expansionskurs weiter voranzutreiben, für wie wahrscheinlich hältst du dass ?
Danke sehr interessant und hilfreich !
Über diese Exklusivität wusste ich nichts. Soweit ich weiss gibt es ein paar Marktbegleiter die sich auch auf Basis der erfolgreichen Reduce-IT Studie erhoffen schnell eigene Daseinsberechtigungen zu erhalten.
Insbesondere wird hier ja ständig in den amerikanischen Foren ja z.B. über Acasti Pharma und deren krill oil-derived mixture diskutiert.
Ich kann dass nicht richtig einschätzen ob eine große Chance (nach Erfolg Vascepa) besteht das dass FDA deren CaPre wirklich auch bis Anfang 2021 zulassen würden.
Halte das im Moment eher für Hype Propaganda...
Ich kann auch nicht einschätzen ob deren Medikament wirklich vergleichbar ist mit Vascepa, die beziehen sich ja, schlau wie sie sind, nur auf vermeintlich vergleichbare Teilaspekte in Ihrer Präsentation und behaupten natürlich Marketing-tauglich Sie können das auch nur "besser"

Außerdem kann ich zuletzt nicht sagen ob Sie das Krill basierte Öl auch in den benötigten Mengen und Zeitumfängen herstellen können, da habe ich eher meine Zweifel + mögliche Umwelteffekte.
Meines Wissens nach kann man Krill nicht effizient und umweltverträglich züchten, wie es weitestgehend eben mit Fischen funktioniert, zumindest ist mir keine Methode bekannt.
Außerdem ist Krill "die" Grundlage für das Leben im Meer schlechthin, keine Ahnung welche Mengen sie dann für Medikamente umwandeln müssten, daher die Idee ist nett aber die Umsetzung könnte alles andere als nett werden.
Zurück zum Thema:
Ich finde die Argumentation dass Amarin sich darüber bewusst ist Gas geben zu müssen, um möglichst ein großes Stück vom Kuchen sich sichern zu können schlüssig. Um aber das derart aus eigener Kraft weiter vorantreiben zu können, müssen die evtl. ab Mitte 2020 wieder Kapital abgreifen um deren Expansionskurs weiter voranzutreiben, für wie wahrscheinlich hältst du dass ?
Amarin to Report Second Quarter 2019 Results and Host Conference Call on July 31, 2019
BEDMINSTER, N.J., and DUBLIN, Ireland, July 24, 2019 (GLOBE NEWSWIRE) --
Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, today announced that it will host a conference call with members of Amarin senior management to discuss the company's second quarter 2019 financial results and provide an operational update on Wednesday, July 31, 2019 at 7:30 a.m. ET. The conference call will follow the anticipated release of the company's financial results earlier that day.
Event Details:
The conference call can be heard live on the investor relations section of the company's website at www.amarincorp.com, or via telephone by dialing 877-407-8033 within the United States or 201-689-8033 from outside the United States. A replay of the call will be made available for a period of two weeks following the conference call. To hear a replay of the call, dial 877-481-4010, PIN: 51652. A replay of the call will also be available through the company's website shortly after the call.
https://finance.yahoo.com/news/amarin-report-second-quarter…
BEDMINSTER, N.J., and DUBLIN, Ireland, July 24, 2019 (GLOBE NEWSWIRE) --
Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, today announced that it will host a conference call with members of Amarin senior management to discuss the company's second quarter 2019 financial results and provide an operational update on Wednesday, July 31, 2019 at 7:30 a.m. ET. The conference call will follow the anticipated release of the company's financial results earlier that day.
Event Details:
The conference call can be heard live on the investor relations section of the company's website at www.amarincorp.com, or via telephone by dialing 877-407-8033 within the United States or 201-689-8033 from outside the United States. A replay of the call will be made available for a period of two weeks following the conference call. To hear a replay of the call, dial 877-481-4010, PIN: 51652. A replay of the call will also be available through the company's website shortly after the call.
https://finance.yahoo.com/news/amarin-report-second-quarter…
Amarin up 3% premarket on draft ICER report confirming Vascepa value
Jul. 24, 2019 9:07 AM ET|About: Amarin Corporation plc (AMRN)|By: Douglas W. House, SA News Editor
Amarin (NASDAQ:AMRN) perks up 3% premarket on light volume on the heels of a draft report from the non-profit Institute for Clinical and Economic Review (ICER) concluding that Vascepa (icosapent ethyl) and Johnson & Johnson's (NYSE:JNJ) blood thinner Xarelto (rivaroxaban) provide clinical benefit, reduce cardiovascular risk and "fall below commonly cited thresholds for cost-effectiveness." In other words, the products are priced appropriately for the value delivered.
JNJ is off a fraction premarket.
See all stocks on the move »
Jul. 24, 2019 9:07 AM ET|About: Amarin Corporation plc (AMRN)|By: Douglas W. House, SA News Editor
Amarin (NASDAQ:AMRN) perks up 3% premarket on light volume on the heels of a draft report from the non-profit Institute for Clinical and Economic Review (ICER) concluding that Vascepa (icosapent ethyl) and Johnson & Johnson's (NYSE:JNJ) blood thinner Xarelto (rivaroxaban) provide clinical benefit, reduce cardiovascular risk and "fall below commonly cited thresholds for cost-effectiveness." In other words, the products are priced appropriately for the value delivered.

JNJ is off a fraction premarket.
See all stocks on the move »
Amarin Is Just Running Up That Hill; Peak Sales Could Reach $10B , Says Analyst
, Says Analyst
[TipRanks]
TipRanks
,TipRanks•July 24, 2019
Biotech Amarin (AMRN) is one of Wall Street’s biggest standouts, with shares skyrocketing 566% in just the last year. Its fish-oil derivative drug Vascepa is already clinically proven to lower very high triglycerides without raising bad cholesterol. What’s more, recent trials revealed it could also cut cardiovascular risks by 25% in patients with abnormally high triglyceride levels. The FDA will either approve or reject this potentially very lucrative label expansion on September 28, and approval should send shares soaring.
An approval would make Vascepa the first therapy, as an adjunct to diet, proven to reduce cardiovascular events when used to treat patients with persistent elevated triglyceride levels and other cardiovascular risk factors. It is this large, unmet medical need for potentially tens of millions of patients which Vascepa has been targeting for many years.
Ahead of this key date, Cantor Fitzgerald analyst Louise Chen has just released a very bullish report on Amarin. She made the call following the company's recent equity raise. “The proceeds should help accelerate the growth of Vascepa, and we think the peak sales potential of this drug could be $5B-$10B” writes Chen. FactSet forecasts $2.1B of sales by 2024.
Indeed Amarin currently shows a ‘Strong Buy’ Street consensus with all six analysts covering the stock bullish. Meanwhile the average analyst price target of $33 suggests shares can surge over 80% from current levels.
On July 18, AMRN announced the pricing of the underwritten public offering of 22.2 million shares at a price of $18 per shares. The gross proceeds of this offering are expected to be ~$400 million, before deducting expenses. Note that Amarin has also granted the underwriters a 30-day option to purchase up to an aggregate of 3.3 million additional shares.
As the company’s press release reveals, AMRN intends to use the net proceeds from the offering for multiple purposes. These include: (1) doubling its existing sales force for Vascepa to 800, increasing advertising, and supporting expanded commercial operations; (2) increasing the commercial supply of Vascepa from third-party drug product suppliers; and (3) for general corporate purposes.
“Amarin also may use a portion of the net proceeds to acquire strategic assets, although it currently has no agreements or commitments in this regard” the statement says.
Looking ahead the next catalysts to keep an eye on are as follows: 1) as you would expect, the potential FDA approval AMRN's supplemental new drug application (sNDA) seeking an expanded indication for Vascepa in the US based on the positive results of AMRN's REDUCE-IT study, 2) pick up in sales from expanded label as well as increased sales and promotional efforts, and 3) Amarin becoming profitable by 2020 (according to the analyst’s estimate).
Net-net, Chen reiterates her buy rating with a $35 price target (93% upside potential). “We continue to think that AMRN is an interesting asset in a consolidating space” she says.
 , Says Analyst
, Says Analyst[TipRanks]
TipRanks
,TipRanks•July 24, 2019
Biotech Amarin (AMRN) is one of Wall Street’s biggest standouts, with shares skyrocketing 566% in just the last year. Its fish-oil derivative drug Vascepa is already clinically proven to lower very high triglycerides without raising bad cholesterol. What’s more, recent trials revealed it could also cut cardiovascular risks by 25% in patients with abnormally high triglyceride levels. The FDA will either approve or reject this potentially very lucrative label expansion on September 28, and approval should send shares soaring.
An approval would make Vascepa the first therapy, as an adjunct to diet, proven to reduce cardiovascular events when used to treat patients with persistent elevated triglyceride levels and other cardiovascular risk factors. It is this large, unmet medical need for potentially tens of millions of patients which Vascepa has been targeting for many years.
Ahead of this key date, Cantor Fitzgerald analyst Louise Chen has just released a very bullish report on Amarin. She made the call following the company's recent equity raise. “The proceeds should help accelerate the growth of Vascepa, and we think the peak sales potential of this drug could be $5B-$10B” writes Chen. FactSet forecasts $2.1B of sales by 2024.
Indeed Amarin currently shows a ‘Strong Buy’ Street consensus with all six analysts covering the stock bullish. Meanwhile the average analyst price target of $33 suggests shares can surge over 80% from current levels.
On July 18, AMRN announced the pricing of the underwritten public offering of 22.2 million shares at a price of $18 per shares. The gross proceeds of this offering are expected to be ~$400 million, before deducting expenses. Note that Amarin has also granted the underwriters a 30-day option to purchase up to an aggregate of 3.3 million additional shares.
As the company’s press release reveals, AMRN intends to use the net proceeds from the offering for multiple purposes. These include: (1) doubling its existing sales force for Vascepa to 800, increasing advertising, and supporting expanded commercial operations; (2) increasing the commercial supply of Vascepa from third-party drug product suppliers; and (3) for general corporate purposes.
“Amarin also may use a portion of the net proceeds to acquire strategic assets, although it currently has no agreements or commitments in this regard” the statement says.
Looking ahead the next catalysts to keep an eye on are as follows: 1) as you would expect, the potential FDA approval AMRN's supplemental new drug application (sNDA) seeking an expanded indication for Vascepa in the US based on the positive results of AMRN's REDUCE-IT study, 2) pick up in sales from expanded label as well as increased sales and promotional efforts, and 3) Amarin becoming profitable by 2020 (according to the analyst’s estimate).
Net-net, Chen reiterates her buy rating with a $35 price target (93% upside potential). “We continue to think that AMRN is an interesting asset in a consolidating space” she says.
Amarin's Recent Negative News And Why It Doesn't Matter
We have a well-disguised short attack basing ....... just read 
https://seekingalpha.com/article/4277525-amarins-recent-nega…
Antwort auf Beitrag Nr.: 60.965.512 von kmastra am 05.07.19 22:03:13

ACST ist jetzt sehr gut gelaufen. Von daher habe ich mal die Häfte verkauft. Ich denke aber nach wie vor, dass sie sehr von der erweiterten Zulassung von VASCEPA profitieren könnten...
Zitat von kmastra: Gibt es hier Meinungen zu ACST? Dort stehen später im Jahr noch P3 Daten zum Krillöl Projekt an. Logischerweise kein CVOT aber sie könnten sowohl von AMRN profitieren als auch in Antizipation der P3 Ergebnisse weiter steigen. Marktkapitalisierung liegt bei 100 Mio.
ACST ist jetzt sehr gut gelaufen. Von daher habe ich mal die Häfte verkauft. Ich denke aber nach wie vor, dass sie sehr von der erweiterten Zulassung von VASCEPA profitieren könnten...
Antwort auf Beitrag Nr.: 61.107.949 von kmastra am 25.07.19 21:56:07Hallo, wegen Acasti, ich denke dass es zwar einen kleinen Push Effekt nochmal geben könnte wenn es hier eine erweiterte Zulassung gibt, aber eigentlich realistisch betrachtet mehr als psychologischen Wert hat dass ja sonst nicht für deren Medikament.
Die müssen sich erst einmal auch über eine mehrere Jahre lange Studie beweisen ob Ihre Mischung / Medikament wirklich eine Wirkung erzielt usw. das FDA wird die nicht einfach durchwinken. d.h. meine Meinung ist, der Kurs wird sich jetzt normalisieren evtl. so auf etwas über einem Dollar einpendeln und kann im Glücksfall dann nochmals über 2 Dollar nach positivem Ergebnis steigen.
Aber man weiss nie wir die Börse reagiert, richtig ? Zumindest ist jetzt ein guter Zeitpunkt um erst mal rauszugehen würde ich sagen...
? Zumindest ist jetzt ein guter Zeitpunkt um erst mal rauszugehen würde ich sagen...
Die müssen sich erst einmal auch über eine mehrere Jahre lange Studie beweisen ob Ihre Mischung / Medikament wirklich eine Wirkung erzielt usw. das FDA wird die nicht einfach durchwinken. d.h. meine Meinung ist, der Kurs wird sich jetzt normalisieren evtl. so auf etwas über einem Dollar einpendeln und kann im Glücksfall dann nochmals über 2 Dollar nach positivem Ergebnis steigen.
Aber man weiss nie wir die Börse reagiert, richtig
 ? Zumindest ist jetzt ein guter Zeitpunkt um erst mal rauszugehen würde ich sagen...
? Zumindest ist jetzt ein guter Zeitpunkt um erst mal rauszugehen würde ich sagen...
Was du für einen Blödsinn schreibst. Die reduce it Studie ging über fast 5 Jahre.
Eine Outcome Studie ala Reduce It kostet ca. 500 Millionen US Dollar mit 8000 Probanden weltweit, dann die genialen Ergebnisse, kaum zu toppen, vielleicht bald eine Übernahme für 50 US Dollar?
Antwort auf Beitrag Nr.: 61.111.432 von Optimist73 am 26.07.19 12:59:13@Optimist73
Meinst du damit meine Antwort ?
Es ist sicher kein Blödsinn zu sagen die brauchen schon mehr als nur "Trittbrettfahrerargumente" um sich ebenfalls am Markt mit Ihrem Wirkstoff erfolgreich platzieren zu können. Ergo sie müssten eine eigene Studie anstreben und ja die dauert... siehe Amarin... habe ja von "jahrelang" geschrieben.
Es ist im Gegenteil Blödsinn zu glauben dass Sie dadurch beim FDA auch gleich einen erweiterten Zulassungsspielraum bekämen, dass passiert 100% nicht...
Wo jetzt der zitierte Blödsinn deiner Meinung nach sein soll darfst du gerne mit entsprechenden Argumenten erläutern oder war es nur ein Missverständnis meiner Worte ? 🙃
Meinst du damit meine Antwort ?
Es ist sicher kein Blödsinn zu sagen die brauchen schon mehr als nur "Trittbrettfahrerargumente" um sich ebenfalls am Markt mit Ihrem Wirkstoff erfolgreich platzieren zu können. Ergo sie müssten eine eigene Studie anstreben und ja die dauert... siehe Amarin... habe ja von "jahrelang" geschrieben.
Es ist im Gegenteil Blödsinn zu glauben dass Sie dadurch beim FDA auch gleich einen erweiterten Zulassungsspielraum bekämen, dass passiert 100% nicht...
Wo jetzt der zitierte Blödsinn deiner Meinung nach sein soll darfst du gerne mit entsprechenden Argumenten erläutern oder war es nur ein Missverständnis meiner Worte ? 🙃
Antwort auf Beitrag Nr.: 61.112.482 von flyingbeef am 26.07.19 15:01:48Geht es bei dir um Amarin?
Ich will hier doch gar nicht AMRN schlecht reden! AMRN hat definitiv ein sehr gutes Produkt und vor allem eines der wenigen Produkte, das ziemlich sicher ein Blockbuster wird.
ACST wird Ende des Jahres Ergebnisse der P3 veröffentlichen. Da geht es um Biomarker Daten. Einen CVOT werden sie nie und nimmer machen - richtig. Ein solches Label wie (hoffentlich demnächst) AMRN auch dann nicht bekommen, wenn die P3 überhaupt erstmal erfolgreich ist und das Produkt zugelassen wird. Trotzdem könnten sie vom Erfolg AMRNs durchaus am Markt profitieren. Bei einer Bewertung von 100 Mio. fand ich es eine Kauf und einen Hinweis in diesem Forum Wert...
ACST wird Ende des Jahres Ergebnisse der P3 veröffentlichen. Da geht es um Biomarker Daten. Einen CVOT werden sie nie und nimmer machen - richtig. Ein solches Label wie (hoffentlich demnächst) AMRN auch dann nicht bekommen, wenn die P3 überhaupt erstmal erfolgreich ist und das Produkt zugelassen wird. Trotzdem könnten sie vom Erfolg AMRNs durchaus am Markt profitieren. Bei einer Bewertung von 100 Mio. fand ich es eine Kauf und einen Hinweis in diesem Forum Wert...
Antwort auf Beitrag Nr.: 61.112.824 von Optimist73 am 26.07.19 15:43:35Hallo , siehste dacht ich es mir doch es war ein Missverständniss, nein es ging um eine andere Aktie da gab es eine Rückfrage und dazu meine Antwort....
Zum Thema Acasti nochmal, nein hatte nicht den Eindruck das du hier Amarin schlecht machen wolltest keineswegs...
Es gibt nicht wenige die Aktien bei beiden haben eben wegen von vielen vermuteten Effekten die aus dem potentiellen Erfolg von Amarin resultieren werden. Was die Aktie für spekulierende „longs“ noch interessanter macht ist das ein grosser Pharmariese die als Konkurrenzprodukt zu Amarin aufkaufen könnte. Das ist aktuell der Hauptgrund warum die so steigt. PS ich habe mich wohl geirrt da geht noch was die Aktie steigt wieder kein Downgrade in Sicht.... wenn du Zeit hast würde ich bis Ende des Jahres abwarten wenn deren weitere Studienergebnisse vorliegen, sollten die positiv ausfallen und Amarin inzwuschen rocken dann sxhiesst die Aktie auch nochmal deutlich hoch... Daher viel Glück 😀
Zum Thema Acasti nochmal, nein hatte nicht den Eindruck das du hier Amarin schlecht machen wolltest keineswegs...
Es gibt nicht wenige die Aktien bei beiden haben eben wegen von vielen vermuteten Effekten die aus dem potentiellen Erfolg von Amarin resultieren werden. Was die Aktie für spekulierende „longs“ noch interessanter macht ist das ein grosser Pharmariese die als Konkurrenzprodukt zu Amarin aufkaufen könnte. Das ist aktuell der Hauptgrund warum die so steigt. PS ich habe mich wohl geirrt da geht noch was die Aktie steigt wieder kein Downgrade in Sicht.... wenn du Zeit hast würde ich bis Ende des Jahres abwarten wenn deren weitere Studienergebnisse vorliegen, sollten die positiv ausfallen und Amarin inzwuschen rocken dann sxhiesst die Aktie auch nochmal deutlich hoch... Daher viel Glück 😀
AMRN ab Minute 19. - deepdive zur aktuellen Situation!
https://players.brightcove.net/4090876629001/H1gCx5zEVb_defa…
https://players.brightcove.net/4090876629001/H1gCx5zEVb_defa…
Hallo zusammen,
der Abverkauf lief noch bis Gestern daher dieses Verharren auf den 18 Dollar Es gab noch ein Zusatzkontingent das nun auch weg ist.
siehe Pressemitteilung:
https://investor.amarincorp.com/news-releases/news-release-d…
Ab sofort sollte der Kurs wieder steigen und nun wieder etwas mehr Freude bringen
Trittbrettfahrerthema:
Acasti Kurs ging übrigens weiter durch die Decke, hier könnte auch bald eine Ausschüttung neuer Aktien zu einer leichten Abwertung und einem ersten Abverkauf führen, sehe aber aktuell bis Amarin News da sind auch hier eine weitere ordentliche steigende Tendenz. 🙃
der Abverkauf lief noch bis Gestern daher dieses Verharren auf den 18 Dollar Es gab noch ein Zusatzkontingent das nun auch weg ist.
siehe Pressemitteilung:
https://investor.amarincorp.com/news-releases/news-release-d…
Ab sofort sollte der Kurs wieder steigen und nun wieder etwas mehr Freude bringen

Trittbrettfahrerthema:
Acasti Kurs ging übrigens weiter durch die Decke, hier könnte auch bald eine Ausschüttung neuer Aktien zu einer leichten Abwertung und einem ersten Abverkauf führen, sehe aber aktuell bis Amarin News da sind auch hier eine weitere ordentliche steigende Tendenz. 🙃
https://finance.yahoo.com/news/amarin-reports-second-quarter…
-Vascepa Umsatz: 100,4 Mio
-AMRN hält einen AdCom mittlerweile für unwahrscheinlich
Klingt insgesamt doch alles recht zuversichtlich!
-Vascepa Umsatz: 100,4 Mio
-AMRN hält einen AdCom mittlerweile für unwahrscheinlich
Klingt insgesamt doch alles recht zuversichtlich!
Top Zahlen, top Aussichten:
Amarin Reports Second Quarter 2019 Financial Results and Operational Update
GlobeNewswire•July 31, 2019
Record Total Revenue of $100.8 Million Achieved in Q2 2019
Commercial Expansion Plans on Track in Anticipation of September 28, 2019 PDUFA Date for Vascepa®
If Approved, Vascepa to Become First Prescription Therapy to Treat Patients with Underlying Cardiovascular Risk Beyond Cholesterol Management as Demonstrated in the REDUCE-IT™ Cardiovascular Outcomes Study
Millions of Patients in the U.S. Have Underlying Cardiovascular Risk Beyond Cholesterol Management
Increased Cash Balance to More Than $600 Million on a Proforma Basis to Ensure Robust Promotion and Education for Vascepa
Management to Host Conference Call Today at 7:30 a.m. ET
BEDMINSTER, N.J., and DUBLIN, Ireland, July 31, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on the commercialization and development of therapeutics to improve cardiovascular health, today announced financial results for the three and six months ended June 30, 2019, and provided an update on company operations.
Key Amarin achievements since its last quarterly report include:
U.S. regulatory review progressing: The Priority Review of Amarin’s supplemental new drug application (sNDA) seeking to expand the indication for Vascepa® (icosapent ethyl) appears to be progressing in an orderly and timely manner toward the September 28, 2019 PDUFA goal date.
Second quarter net product revenue growth increased by 91%: Recognized $100.8 million in total revenue and $100.4 million in net product revenue from Vascepa sales in Q2 2019 compared to $52.5 million in Q2 2018, an increase of 91%. The total revenue result is at the upper end of the company’s previously estimated revenue of between $97 and $101 million announced on July 2, 2019.
U.S. prescriptions grew by more than 70%: Increased normalized prescriptions for Vascepa by 76% and 73% compared to Q2 2018 based on data from Symphony Health Solutions and IQVIA, respectively.
Commercial expansion preparation under way: Actively hiring additional sales managers and sales representatives to double the size of Amarin’s U.S. sales force to approximately 800 sales representatives by October 2019, while also executing on other plans to effectively educate healthcare professionals and consumers regarding the cardiovascular risk reduction profile of Vascepa and the significant unmet need for this disease, following an anticipated label expansion in late September.
International plans on track: Progressing through Amarin’s licensee, HLS Therapeutics Inc. (HLS.TO), towards anticipated approval of Vascepa in Canada in the fourth quarter of 2019. As previously disclosed, the application for Vascepa was granted a priority review designation by Health Canada. Other international progress is continuing, including Amarin’s plans to submit an application seeking approval for Vascepa in Europe before the end of 2019.
Increased cash balance to ensure robust launch of Vascepa: As of June 30, 2019, Amarin had a cash balance of $221.8 million. In July 2019, Amarin completed a $460.0 million equity offering resulting in an increase in Amarin’s cash balance to more than $600 million on a pro forma basis.
“Amarin made tremendous progress in the first half of 2019, including achieving $100 million in quarterly revenue which is a record for Vascepa sales,” stated John F. Thero, president and chief executive officer, Amarin. “We believe this is just the start of realizing the significant commercial opportunity for Vascepa, which will be driven by our passion to potentially help millions of at-risk patients and our ability to broadly communicate to healthcare professionals and patients the cost-effective value of Vascepa based on the FDA-approved expanded indication we’re anticipating in September. Our focus right now is ensuring we are prepared to robustly launch Vascepa based on that expanded indication.”
Regulatory Update
As previously announced, Amarin submitted an sNDA to the FDA on March 28, 2019, seeking to expand the indication for Vascepa. The sNDA was based on the positive results of the landmark REDUCE-IT™ cardiovascular outcomes study. If approved, the expanded label is anticipated to allow for considerably broader promotion of Vascepa in the United States. As announced in May 2019, the FDA accepted the sNDA for filing and granted Priority Review designation with an assigned PDUFA goal date of September 28, 2019.
To date, the FDA has not informed Amarin as to whether it plans to convene an Advisory Committee (AdCom) to review the sNDA. The FDA is not required to inform sponsor companies that it does not intend to hold an AdCom. While it remains possible that the FDA may elect to convene an AdCom, with less than two months remaining prior to the PDUFA date Amarin is now assuming that an AdCom is unlikely. If Amarin is informed definitively that there will or will not be an AdCom, the company plans to update investors accordingly.
Label negotiations in the sNDA process could commence as early as one month prior to the PDUFA date under FDA’s typical processes. As such, a final version of the updated Vascepa label is not available at this time. It would therefore be unproductive for Amarin to speculate on the wording of such a label before a final determination by the FDA except to express that Amarin is seeking a cardiovascular risk reduction label for Vascepa which is consistent with the results of the REDUCE-IT study.
Prescription Growth
Vascepa prescription growth in Q2 2019 stemmed from both prior prescribers and new prescribers. Normalized prescriptions for Vascepa (prescription of 120 grams of Vascepa representing a one-month supply) increased by approximately 76% and 73% in Q2 2019 compared to Q2 2018 based on data from Symphony Health and IQVIA, respectively. Estimated normalized Vascepa prescriptions, based on data from Symphony Health and IQVIA, totaled approximately 756,000 and 683,000 in the second quarter of 2019.
As there has been a trend of an increasing number of 90-day prescriptions (or scripts) vs. 30-day prescriptions, reporting from Symphony and IQVIA on prescription counts may not reflect the true demand for the product. Script counts from these services do not account for whether a script is for 90 days or 30 days as both are counted as one script. Accordingly, Amarin believes that pill counts reported as extended units by Symphony and IQVIA show a more accurate reflection of the demand for Vascepa. Normalized prescriptions, as referenced above, take this into account by using the extended unit number and dividing by the pill count in the bottle. Nonetheless, even when normalized, estimates from these independent sources for Vascepa and other drugs have historically been most accurate over longer periods of time, such as annually, while directionally informative over shorter periods of time.
As described more fully in Amarin’s Quarterly Report on Form 10-Q, Amarin recognizes product revenue when its customers, consisting mostly of independent commercial distributors, take possession of the product they order and Amarin ships to them. Amarin revenue is not recognized when individual patients fill prescriptions.
Commercial Update
Upon FDA approval of an expanded indication for Vascepa, Amarin’s goal is to be ready to launch a robust educational and promotional campaign aimed at healthcare professionals and consumers on the efficacy and safety profile of Vascepa as well as on the significant unmet need to help patients with underlying cardiovascular risks beyond cholesterol management.
When physicians become knowledgeable about the results of the REDUCE-IT study, it has been Amarin’s experience that they appreciate the importance of Vascepa and how it can be used to help the health of their patients. However, the vast majority of healthcare professionals have little knowledge of Vascepa. Amarin views this as an opportunity to be improved through expanded Vascepa promotion supported by an expanded label.
With the benefit of funds from recent financing, the company plans to create “surround sound” in its promotion of Vascepa. This surround sound will include more sales representatives, various forms of digital outreach, medical education, scientific presentations at industry meetings, direct-to-consumer advertising and other means of effective and responsible communications.
Most of the sales representatives hired at the start of 2019 are performing well and showing promise for greater contribution in the future. Their progress, together with the anticipated value of the expanded label for Vascepa, gives Amarin the confidence to double the size of its sales force.
To date, Amarin has hired most of the additional sales managers required for this expansion and is confident that it will have approximately 400 new sales representatives hired, trained and in the field promoting Vascepa by early October.
While the expanded sales team will reach a larger number of healthcare professionals, it is equally important to achieve more frequent interactions with targeted healthcare professionals. The current sales team calls on approximately 50,000 healthcare professionals. With the doubling of the sales force, Amarin expects to reach approximately 70,000 to 80,000 healthcare professionals. As of the end of June 2019, consistent with previously communicated projections, Amarin sales representatives called on approximately half of its current target physicians five or more times with the published results of the REDUCE-IT study.
Direct-to-consumer (DTC) promotion is likely to be a phased process. Upon label expansion, Amarin plans to increase promotion through more placement of Vascepa advertisement in platforms currently used. In parallel, new messaging for branded promotion based on the anticipated expanded label for Vascepa will be submitted to the FDA for review. Such submission for promotional review cannot be made until after the label is expanded. Subject to FDA review, Amarin anticipates launching that DTC campaign in the second quarter of 2020.
Managed care coverage for Vascepa continues to be favorable overall. Amarin anticipates marginal further improvement to such coverage in Q3 2019, prior to label expansion, and plans to pursue even broader managed care coverage following assumed label expansion.
The Institute for Clinical and Economic Review (ICER), an independent non-profit organization, released its draft evidence report regarding clinical effectiveness and economic impacts of Vascepa on July 24, 2019. ICER’s draft report concluded that Vascepa is cost effectiveness across all the non-profit organization’s analyses, based on its quality-adjusted life year (QALY) metrics Despite the draft report’s positive conclusion regarding cost effectiveness, Amarin believes that ICER understates the value of Vascepa. For example, the ICER base-case analyses reflect only the costs of heart attack, stroke and cardiovascular death and appear to exclude high costs associated with other cardiovascular events that were demonstrated to be lowered by Vascepa in the REDUCE-IT cardiovascular outcomes study (e.g., revascularization procedures and hospitalizations for unstable angina). Amarin believes this draft report, while not perfect in its value assessment, provides additional support for why medical insurance should broadly cover Vascepa for the population of patients studied in REDUCE-IT. While many payors already broadly cover Vascepa, upon anticipated label expansion, Amarin plans to use the results of the REDUCE-IT study, pharmaco-economic analysis, such as presented by ICER, and other medical information and data in negotiations with payers seeking expanded Vascepa insurance coverage.
Financial Update
Total revenue for the three months ended June 30, 2019 and 2018 was $100.8 million and $52.6 million, respectively. Net product revenue for the three months ended June 30, 2019 and 2018 was $100.4 million and $52.5 million, respectively. Total revenue for the six months ended June 30, 2019 and 2018, was $174.1 million and $96.6 million, respectively. Net product revenue for the six months ended June 30, 2019 and 2018 was $173.1 million and $96.3 million, respectively. The increase in net product revenue was primarily attributable to increases in new and recurring prescriptions of Vascepa as net selling price remained relatively unchanged for the six months ended June 30, 2019 as compared to the same period in 2018.
During the second quarter, based on data from Symphony Health Solutions and IQVIA, Amarin experienced continued prescription growth and an increase in Vascepa market share, particularly among physicians called on by Amarin’s sales professionals. Symphony Health Solutions and IQVIA reported estimated normalized total Vascepa prescriptions of approximately 756,000 and 683,000, respectfully, for the three months ended June 30, 2019, representing growth of approximately 76% and 73%, respectively, over levels estimated by these sources for the same three months of the prior year.
Licensing revenues recognized by the company were $1.0 million and $0.2 million in the six months ended June 30, 2019 and 2018, respectively, related to timing of milestones and other factors impacting revenue recognition for licensing fees under agreements for the commercialization of Vascepa outside the United States.
Cost of goods sold for the three months ended June 30, 2019 and 2018 was $22.8 million and $12.8 million, respectively. Cost of goods sold for the six months ended June 30, 2019 and 2018 was $39.9 million and $23.5 million, respectively. Gross margin on net product revenue for the three and six months ended June 30, 2019 and 2018 was 77% and 76%, respectively.
Selling, general and administrative (SG&A) expenses in the six months ended June 30, 2019 and 2018 were $145.0 and $97.4 million, respectively, an increase of 49%. This increase is due primarily to increased promotional activities, including commercial spend for expansion following successful REDUCE-IT results, as well as costs for sales force expansion, partially offset by elimination of expenses associated with the company’s prior co-promotion partner. As previously disclosed, the level of anticipated SG&A spending will increase as Amarin doubles the size of its sales force and increases its promotional and educational spending for Vascepa in conjunction with the anticipated label expansion.
Research and development (R&D) expenses in the six months ended June 30, 2019 and 2018 were $14.4 and $29.9 million, respectively, a decrease of 52%. This decrease in expense is primarily driven by a decline in REDUCE-IT related costs. Following the completion of the REDUCE-IT trial, costs consisted primarily of the clinical study’s wrap-up activities, regulatory support and publications. As previously disclosed, Amarin anticipates the level of spending on R&D will continue to decline as it has completed the REDUCE-IT study and initial publication of results from this important study.
Under U.S. GAAP, Amarin reported a net loss of $1.8 million in the three months ended June 30, 2019, or basic and diluted loss per share of $0.01. This net loss included $7.9 million in non-cash stock-based compensation expense. Amarin reported a net loss of $34.2 million in the second quarter of 2018, or basic and diluted loss per share of $0.12. This net loss included $3.6 million in non-cash stock-based compensation expense.
Under GAAP, Amarin reported a net loss of $26.3 million in the six months ended June 30, 2019, or basic and diluted loss per share of $0.08. This net loss included $14.8 million in non-cash stock-based compensation expense. For the six months ended June 30, 2018, Amarin reported a net loss of $58.3 million, or basic and diluted loss per share of $0.20. This net loss included $7.4 million in non-cash stock-based compensation expense.
Excluding non-cash gains or losses for stock-based compensation, non-GAAP adjusted net income was $6.1 million for the second quarter of 2019, or non-GAAP adjusted basic and diluted earnings per share of $0.02, compared to non-GAAP adjusted net loss of $30.6 million for the second quarter of 2018, or non-GAAP adjusted basic and diluted loss per share of $0.10.
Excluding non-cash gains or losses for stock-based compensation, non-GAAP adjusted net loss was $11.5 million for the six months ended June 30, 2019, or non-GAAP adjusted basic and diluted loss per share of $0.03, compared to non-GAAP adjusted net loss of $50.9 million for the six months ended June 30, 2018, or non-GAAP adjusted basic and diluted loss per share of $0.18.
As of June 30, 2019, Amarin reported cash and cash equivalents of $221.8 million, $95.4 million in net accounts receivable ($116.3 million in gross accounts receivable before allowances and reserves) and $46.3 million in inventory. As noted above, the company completed an equity offering in July 2019 for gross proceeds of approximately $460.0 million and issued approximately 25.5 million ADSs, including the full exercise of the underwriters’ 30-day over-allotment option to purchase up to an additional 15% of the ADSs issued in the offering. Net proceeds after fees and expenses from this financing were approximately $439.5 million. While Amarin was net cash flow positive in the three months ended June 30, 2019, the company expects net cash flow to be negative in the second half of 2019 reflecting planning increased spending for Vascepa promotion and anticipated increased purchases of Vascepa inventory.
As of June 30, 2019, prior to the above described financing, Amarin had approximately 331.3 million American Depository Shares (ADSs) and ordinary shares outstanding, 28.9 million common share equivalents of Series A Convertible Preferred Shares outstanding and approximately 16.6 million equivalent shares underlying stock options at a weighted-average exercise price of $5.86, as well as 9.3 million equivalent shares underlying restricted or deferred stock units.
Conference Call and Webcast Information
Amarin Reports Second Quarter 2019 Financial Results and Operational Update
GlobeNewswire•July 31, 2019
Record Total Revenue of $100.8 Million Achieved in Q2 2019
Commercial Expansion Plans on Track in Anticipation of September 28, 2019 PDUFA Date for Vascepa®
If Approved, Vascepa to Become First Prescription Therapy to Treat Patients with Underlying Cardiovascular Risk Beyond Cholesterol Management as Demonstrated in the REDUCE-IT™ Cardiovascular Outcomes Study
Millions of Patients in the U.S. Have Underlying Cardiovascular Risk Beyond Cholesterol Management
Increased Cash Balance to More Than $600 Million on a Proforma Basis to Ensure Robust Promotion and Education for Vascepa
Management to Host Conference Call Today at 7:30 a.m. ET
BEDMINSTER, N.J., and DUBLIN, Ireland, July 31, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on the commercialization and development of therapeutics to improve cardiovascular health, today announced financial results for the three and six months ended June 30, 2019, and provided an update on company operations.
Key Amarin achievements since its last quarterly report include:
U.S. regulatory review progressing: The Priority Review of Amarin’s supplemental new drug application (sNDA) seeking to expand the indication for Vascepa® (icosapent ethyl) appears to be progressing in an orderly and timely manner toward the September 28, 2019 PDUFA goal date.
Second quarter net product revenue growth increased by 91%: Recognized $100.8 million in total revenue and $100.4 million in net product revenue from Vascepa sales in Q2 2019 compared to $52.5 million in Q2 2018, an increase of 91%. The total revenue result is at the upper end of the company’s previously estimated revenue of between $97 and $101 million announced on July 2, 2019.
U.S. prescriptions grew by more than 70%: Increased normalized prescriptions for Vascepa by 76% and 73% compared to Q2 2018 based on data from Symphony Health Solutions and IQVIA, respectively.
Commercial expansion preparation under way: Actively hiring additional sales managers and sales representatives to double the size of Amarin’s U.S. sales force to approximately 800 sales representatives by October 2019, while also executing on other plans to effectively educate healthcare professionals and consumers regarding the cardiovascular risk reduction profile of Vascepa and the significant unmet need for this disease, following an anticipated label expansion in late September.
International plans on track: Progressing through Amarin’s licensee, HLS Therapeutics Inc. (HLS.TO), towards anticipated approval of Vascepa in Canada in the fourth quarter of 2019. As previously disclosed, the application for Vascepa was granted a priority review designation by Health Canada. Other international progress is continuing, including Amarin’s plans to submit an application seeking approval for Vascepa in Europe before the end of 2019.
Increased cash balance to ensure robust launch of Vascepa: As of June 30, 2019, Amarin had a cash balance of $221.8 million. In July 2019, Amarin completed a $460.0 million equity offering resulting in an increase in Amarin’s cash balance to more than $600 million on a pro forma basis.
“Amarin made tremendous progress in the first half of 2019, including achieving $100 million in quarterly revenue which is a record for Vascepa sales,” stated John F. Thero, president and chief executive officer, Amarin. “We believe this is just the start of realizing the significant commercial opportunity for Vascepa, which will be driven by our passion to potentially help millions of at-risk patients and our ability to broadly communicate to healthcare professionals and patients the cost-effective value of Vascepa based on the FDA-approved expanded indication we’re anticipating in September. Our focus right now is ensuring we are prepared to robustly launch Vascepa based on that expanded indication.”
Regulatory Update
As previously announced, Amarin submitted an sNDA to the FDA on March 28, 2019, seeking to expand the indication for Vascepa. The sNDA was based on the positive results of the landmark REDUCE-IT™ cardiovascular outcomes study. If approved, the expanded label is anticipated to allow for considerably broader promotion of Vascepa in the United States. As announced in May 2019, the FDA accepted the sNDA for filing and granted Priority Review designation with an assigned PDUFA goal date of September 28, 2019.
To date, the FDA has not informed Amarin as to whether it plans to convene an Advisory Committee (AdCom) to review the sNDA. The FDA is not required to inform sponsor companies that it does not intend to hold an AdCom. While it remains possible that the FDA may elect to convene an AdCom, with less than two months remaining prior to the PDUFA date Amarin is now assuming that an AdCom is unlikely. If Amarin is informed definitively that there will or will not be an AdCom, the company plans to update investors accordingly.
Label negotiations in the sNDA process could commence as early as one month prior to the PDUFA date under FDA’s typical processes. As such, a final version of the updated Vascepa label is not available at this time. It would therefore be unproductive for Amarin to speculate on the wording of such a label before a final determination by the FDA except to express that Amarin is seeking a cardiovascular risk reduction label for Vascepa which is consistent with the results of the REDUCE-IT study.
Prescription Growth
Vascepa prescription growth in Q2 2019 stemmed from both prior prescribers and new prescribers. Normalized prescriptions for Vascepa (prescription of 120 grams of Vascepa representing a one-month supply) increased by approximately 76% and 73% in Q2 2019 compared to Q2 2018 based on data from Symphony Health and IQVIA, respectively. Estimated normalized Vascepa prescriptions, based on data from Symphony Health and IQVIA, totaled approximately 756,000 and 683,000 in the second quarter of 2019.
As there has been a trend of an increasing number of 90-day prescriptions (or scripts) vs. 30-day prescriptions, reporting from Symphony and IQVIA on prescription counts may not reflect the true demand for the product. Script counts from these services do not account for whether a script is for 90 days or 30 days as both are counted as one script. Accordingly, Amarin believes that pill counts reported as extended units by Symphony and IQVIA show a more accurate reflection of the demand for Vascepa. Normalized prescriptions, as referenced above, take this into account by using the extended unit number and dividing by the pill count in the bottle. Nonetheless, even when normalized, estimates from these independent sources for Vascepa and other drugs have historically been most accurate over longer periods of time, such as annually, while directionally informative over shorter periods of time.
As described more fully in Amarin’s Quarterly Report on Form 10-Q, Amarin recognizes product revenue when its customers, consisting mostly of independent commercial distributors, take possession of the product they order and Amarin ships to them. Amarin revenue is not recognized when individual patients fill prescriptions.
Commercial Update
Upon FDA approval of an expanded indication for Vascepa, Amarin’s goal is to be ready to launch a robust educational and promotional campaign aimed at healthcare professionals and consumers on the efficacy and safety profile of Vascepa as well as on the significant unmet need to help patients with underlying cardiovascular risks beyond cholesterol management.
When physicians become knowledgeable about the results of the REDUCE-IT study, it has been Amarin’s experience that they appreciate the importance of Vascepa and how it can be used to help the health of their patients. However, the vast majority of healthcare professionals have little knowledge of Vascepa. Amarin views this as an opportunity to be improved through expanded Vascepa promotion supported by an expanded label.
With the benefit of funds from recent financing, the company plans to create “surround sound” in its promotion of Vascepa. This surround sound will include more sales representatives, various forms of digital outreach, medical education, scientific presentations at industry meetings, direct-to-consumer advertising and other means of effective and responsible communications.
Most of the sales representatives hired at the start of 2019 are performing well and showing promise for greater contribution in the future. Their progress, together with the anticipated value of the expanded label for Vascepa, gives Amarin the confidence to double the size of its sales force.
To date, Amarin has hired most of the additional sales managers required for this expansion and is confident that it will have approximately 400 new sales representatives hired, trained and in the field promoting Vascepa by early October.
While the expanded sales team will reach a larger number of healthcare professionals, it is equally important to achieve more frequent interactions with targeted healthcare professionals. The current sales team calls on approximately 50,000 healthcare professionals. With the doubling of the sales force, Amarin expects to reach approximately 70,000 to 80,000 healthcare professionals. As of the end of June 2019, consistent with previously communicated projections, Amarin sales representatives called on approximately half of its current target physicians five or more times with the published results of the REDUCE-IT study.
Direct-to-consumer (DTC) promotion is likely to be a phased process. Upon label expansion, Amarin plans to increase promotion through more placement of Vascepa advertisement in platforms currently used. In parallel, new messaging for branded promotion based on the anticipated expanded label for Vascepa will be submitted to the FDA for review. Such submission for promotional review cannot be made until after the label is expanded. Subject to FDA review, Amarin anticipates launching that DTC campaign in the second quarter of 2020.
Managed care coverage for Vascepa continues to be favorable overall. Amarin anticipates marginal further improvement to such coverage in Q3 2019, prior to label expansion, and plans to pursue even broader managed care coverage following assumed label expansion.
The Institute for Clinical and Economic Review (ICER), an independent non-profit organization, released its draft evidence report regarding clinical effectiveness and economic impacts of Vascepa on July 24, 2019. ICER’s draft report concluded that Vascepa is cost effectiveness across all the non-profit organization’s analyses, based on its quality-adjusted life year (QALY) metrics Despite the draft report’s positive conclusion regarding cost effectiveness, Amarin believes that ICER understates the value of Vascepa. For example, the ICER base-case analyses reflect only the costs of heart attack, stroke and cardiovascular death and appear to exclude high costs associated with other cardiovascular events that were demonstrated to be lowered by Vascepa in the REDUCE-IT cardiovascular outcomes study (e.g., revascularization procedures and hospitalizations for unstable angina). Amarin believes this draft report, while not perfect in its value assessment, provides additional support for why medical insurance should broadly cover Vascepa for the population of patients studied in REDUCE-IT. While many payors already broadly cover Vascepa, upon anticipated label expansion, Amarin plans to use the results of the REDUCE-IT study, pharmaco-economic analysis, such as presented by ICER, and other medical information and data in negotiations with payers seeking expanded Vascepa insurance coverage.
Financial Update
Total revenue for the three months ended June 30, 2019 and 2018 was $100.8 million and $52.6 million, respectively. Net product revenue for the three months ended June 30, 2019 and 2018 was $100.4 million and $52.5 million, respectively. Total revenue for the six months ended June 30, 2019 and 2018, was $174.1 million and $96.6 million, respectively. Net product revenue for the six months ended June 30, 2019 and 2018 was $173.1 million and $96.3 million, respectively. The increase in net product revenue was primarily attributable to increases in new and recurring prescriptions of Vascepa as net selling price remained relatively unchanged for the six months ended June 30, 2019 as compared to the same period in 2018.
During the second quarter, based on data from Symphony Health Solutions and IQVIA, Amarin experienced continued prescription growth and an increase in Vascepa market share, particularly among physicians called on by Amarin’s sales professionals. Symphony Health Solutions and IQVIA reported estimated normalized total Vascepa prescriptions of approximately 756,000 and 683,000, respectfully, for the three months ended June 30, 2019, representing growth of approximately 76% and 73%, respectively, over levels estimated by these sources for the same three months of the prior year.
Licensing revenues recognized by the company were $1.0 million and $0.2 million in the six months ended June 30, 2019 and 2018, respectively, related to timing of milestones and other factors impacting revenue recognition for licensing fees under agreements for the commercialization of Vascepa outside the United States.
Cost of goods sold for the three months ended June 30, 2019 and 2018 was $22.8 million and $12.8 million, respectively. Cost of goods sold for the six months ended June 30, 2019 and 2018 was $39.9 million and $23.5 million, respectively. Gross margin on net product revenue for the three and six months ended June 30, 2019 and 2018 was 77% and 76%, respectively.
Selling, general and administrative (SG&A) expenses in the six months ended June 30, 2019 and 2018 were $145.0 and $97.4 million, respectively, an increase of 49%. This increase is due primarily to increased promotional activities, including commercial spend for expansion following successful REDUCE-IT results, as well as costs for sales force expansion, partially offset by elimination of expenses associated with the company’s prior co-promotion partner. As previously disclosed, the level of anticipated SG&A spending will increase as Amarin doubles the size of its sales force and increases its promotional and educational spending for Vascepa in conjunction with the anticipated label expansion.
Research and development (R&D) expenses in the six months ended June 30, 2019 and 2018 were $14.4 and $29.9 million, respectively, a decrease of 52%. This decrease in expense is primarily driven by a decline in REDUCE-IT related costs. Following the completion of the REDUCE-IT trial, costs consisted primarily of the clinical study’s wrap-up activities, regulatory support and publications. As previously disclosed, Amarin anticipates the level of spending on R&D will continue to decline as it has completed the REDUCE-IT study and initial publication of results from this important study.
Under U.S. GAAP, Amarin reported a net loss of $1.8 million in the three months ended June 30, 2019, or basic and diluted loss per share of $0.01. This net loss included $7.9 million in non-cash stock-based compensation expense. Amarin reported a net loss of $34.2 million in the second quarter of 2018, or basic and diluted loss per share of $0.12. This net loss included $3.6 million in non-cash stock-based compensation expense.
Under GAAP, Amarin reported a net loss of $26.3 million in the six months ended June 30, 2019, or basic and diluted loss per share of $0.08. This net loss included $14.8 million in non-cash stock-based compensation expense. For the six months ended June 30, 2018, Amarin reported a net loss of $58.3 million, or basic and diluted loss per share of $0.20. This net loss included $7.4 million in non-cash stock-based compensation expense.
Excluding non-cash gains or losses for stock-based compensation, non-GAAP adjusted net income was $6.1 million for the second quarter of 2019, or non-GAAP adjusted basic and diluted earnings per share of $0.02, compared to non-GAAP adjusted net loss of $30.6 million for the second quarter of 2018, or non-GAAP adjusted basic and diluted loss per share of $0.10.
Excluding non-cash gains or losses for stock-based compensation, non-GAAP adjusted net loss was $11.5 million for the six months ended June 30, 2019, or non-GAAP adjusted basic and diluted loss per share of $0.03, compared to non-GAAP adjusted net loss of $50.9 million for the six months ended June 30, 2018, or non-GAAP adjusted basic and diluted loss per share of $0.18.
As of June 30, 2019, Amarin reported cash and cash equivalents of $221.8 million, $95.4 million in net accounts receivable ($116.3 million in gross accounts receivable before allowances and reserves) and $46.3 million in inventory. As noted above, the company completed an equity offering in July 2019 for gross proceeds of approximately $460.0 million and issued approximately 25.5 million ADSs, including the full exercise of the underwriters’ 30-day over-allotment option to purchase up to an additional 15% of the ADSs issued in the offering. Net proceeds after fees and expenses from this financing were approximately $439.5 million. While Amarin was net cash flow positive in the three months ended June 30, 2019, the company expects net cash flow to be negative in the second half of 2019 reflecting planning increased spending for Vascepa promotion and anticipated increased purchases of Vascepa inventory.
As of June 30, 2019, prior to the above described financing, Amarin had approximately 331.3 million American Depository Shares (ADSs) and ordinary shares outstanding, 28.9 million common share equivalents of Series A Convertible Preferred Shares outstanding and approximately 16.6 million equivalent shares underlying stock options at a weighted-average exercise price of $5.86, as well as 9.3 million equivalent shares underlying restricted or deferred stock units.
Conference Call and Webcast Information
Guten Abend zusammen,
würde gerne eure Meinung zu den Kursbewegungen wissen und mal meine "naive" Sicht auf die akutuelle Situation und was hier passiert darstellen...
Heute hat die Aktie meiner Meinung nach leider mal wieder bewiesen dass sie extrem unter Druck ist von sehr grossen Playern mit eigenen Interessen bestimmte Kurshöhen beizubehalten.
Leider ist auf der "dunklen" Seite der Macht sehr viel Geld im Spiel bei der 50/50 Chance mit Amarin wird alles gut oder es geht in den Keller... kann man gut auf beide Ausgänge spekulieren.
Wenn die Player die hier am Werk sind die Kursgewinne von uns Kleinanlegern so drücken können dass selbst mal 300 oder mehr Millionen nach oben locker runtergeschraubt werden können, sind wir wohl nur zum zuschauen verdammt..
Solche Player können sogar im Falle einer positiven Amarin Entscheidung schnell zu einem bis dahin immer noch sehr moderaten Kurs (den sie selbst ja verteidigt haben) Ihre Shorts auflösen und sich dann bei steigenden Kursen mit positiven Aktien eindecken und so dennoch profitieren. Sprich egal wie das Spiel ausgeht die machen Ihren Cash...
Wenn wir die Geldmauer nicht mit erheblich viel mehr Geld durchbrechen können so dass Sie schon vorher zum Rückkauf gezuwungen werden dann bleiben wir quasi bis zur Entscheidung festgenagelt... Sprich unsere einzige Chance wäre auf der positiven Seite große Geldgeber zu haben die nun einspringen.
Ich denke seit der Entscheidung sich eine große Menge Cash zu besorgen haben hier diese "dunklen" Player nun die Zügel des Spiels in der Hand und man kann wirklich nur noch von Kursmanipulation mit viel Geld sprechen, was meint Ihr könnte ich damit richtig liegen ?`
Hat jemand eine andere Theorie ?
An Mangel an positiven und Zuversichtlichen Aussichten kann es zumindest nicht liegen wir Kleinanleger sind ja eigentlich gesammelt sehr positiv und zuversichtlich eingestellt, sprich würde es von uns abhängen würde das Ding ordentlich steigen...
würde gerne eure Meinung zu den Kursbewegungen wissen und mal meine "naive" Sicht auf die akutuelle Situation und was hier passiert darstellen...
Heute hat die Aktie meiner Meinung nach leider mal wieder bewiesen dass sie extrem unter Druck ist von sehr grossen Playern mit eigenen Interessen bestimmte Kurshöhen beizubehalten.
Leider ist auf der "dunklen" Seite der Macht sehr viel Geld im Spiel bei der 50/50 Chance mit Amarin wird alles gut oder es geht in den Keller... kann man gut auf beide Ausgänge spekulieren.
Wenn die Player die hier am Werk sind die Kursgewinne von uns Kleinanlegern so drücken können dass selbst mal 300 oder mehr Millionen nach oben locker runtergeschraubt werden können, sind wir wohl nur zum zuschauen verdammt..
Solche Player können sogar im Falle einer positiven Amarin Entscheidung schnell zu einem bis dahin immer noch sehr moderaten Kurs (den sie selbst ja verteidigt haben) Ihre Shorts auflösen und sich dann bei steigenden Kursen mit positiven Aktien eindecken und so dennoch profitieren. Sprich egal wie das Spiel ausgeht die machen Ihren Cash...
Wenn wir die Geldmauer nicht mit erheblich viel mehr Geld durchbrechen können so dass Sie schon vorher zum Rückkauf gezuwungen werden dann bleiben wir quasi bis zur Entscheidung festgenagelt... Sprich unsere einzige Chance wäre auf der positiven Seite große Geldgeber zu haben die nun einspringen.
Ich denke seit der Entscheidung sich eine große Menge Cash zu besorgen haben hier diese "dunklen" Player nun die Zügel des Spiels in der Hand und man kann wirklich nur noch von Kursmanipulation mit viel Geld sprechen, was meint Ihr könnte ich damit richtig liegen ?`
Hat jemand eine andere Theorie ?
An Mangel an positiven und Zuversichtlichen Aussichten kann es zumindest nicht liegen wir Kleinanleger sind ja eigentlich gesammelt sehr positiv und zuversichtlich eingestellt, sprich würde es von uns abhängen würde das Ding ordentlich steigen...
Das ist Börse, bei Amarin ist alles im Grünen Bereich, Zulassung steht vor der Tür!
Die Spielchen der shorts muss man aushalten!
Die Spielchen der shorts muss man aushalten!
Antwort auf Beitrag Nr.: 61.148.489 von Magnetfeldfredy am 01.08.19 07:13:44Hi habe auf Yahoo Stock discussion folgende Erklärung für die 400 Millionen Finanzspritze die sich Amarin geholt hatte gesehen.
Ich finde der Poster hat absolut recht. Das passt und würde alle "Zweifler" hier ziemlich blöd aussehen lassen:
Why did Amarin need $400 M now?
If a broad label expansion is coming, if doctors are indicating an interest in an add on that focuses on the 75% of CVD risk that remains after statin use, if Insurance is looking at the ICER reprort and seeing a very positive benefit to cost when adding Vascepa maybe next year has a lot of potential.
Likely $500M in sales this year and $1500M (1.5 billion) next year is doable. That means you need $350 to $400 million in product to support those sales.
I'm in the 75% chance that at least a comarketer is coming soon after label expansion. In the recent conference call it was mentioned 3000 drug reps would be needed if you wanted to reach volume sales to come on at a faster clip
The recent fund raise was needed. We all had hoped for a rise at a much higher price. Amarin had to get the deal at what the market offered at this time. 100% think it had to be done before label expansion.
Für mich heisst es ich bin dabei Amarin glaubt voll an Ihren Erfolg und sie haben meiner Meinung nach auch beste Argumente und Gründe dafür. Besser kann es eigentlich nicht aussehen.
Natürlich weiss man nicht wie gross die Label Expansion am Schluss ausfallen wird, aber egal es wird eine Label Expansion geben und dass heisst der Kurs jetzt spiegelt ganz sicher nicht den Wert der Firma wieder. Ich denke bis 30 Dollar oder mehr sind selbst bei einer sehr eingeschränkten Label Expansion "sicher" drin.
Bleibt dabei, kauft am besten noch nach wenn ihr könnt und wollt, ich bin mittlwerweile sehr zuversichtlich !
Ich finde der Poster hat absolut recht. Das passt und würde alle "Zweifler" hier ziemlich blöd aussehen lassen:
Why did Amarin need $400 M now?
If a broad label expansion is coming, if doctors are indicating an interest in an add on that focuses on the 75% of CVD risk that remains after statin use, if Insurance is looking at the ICER reprort and seeing a very positive benefit to cost when adding Vascepa maybe next year has a lot of potential.
Likely $500M in sales this year and $1500M (1.5 billion) next year is doable. That means you need $350 to $400 million in product to support those sales.
I'm in the 75% chance that at least a comarketer is coming soon after label expansion. In the recent conference call it was mentioned 3000 drug reps would be needed if you wanted to reach volume sales to come on at a faster clip
The recent fund raise was needed. We all had hoped for a rise at a much higher price. Amarin had to get the deal at what the market offered at this time. 100% think it had to be done before label expansion.
Für mich heisst es ich bin dabei Amarin glaubt voll an Ihren Erfolg und sie haben meiner Meinung nach auch beste Argumente und Gründe dafür. Besser kann es eigentlich nicht aussehen.
Natürlich weiss man nicht wie gross die Label Expansion am Schluss ausfallen wird, aber egal es wird eine Label Expansion geben und dass heisst der Kurs jetzt spiegelt ganz sicher nicht den Wert der Firma wieder. Ich denke bis 30 Dollar oder mehr sind selbst bei einer sehr eingeschränkten Label Expansion "sicher" drin.
Bleibt dabei, kauft am besten noch nach wenn ihr könnt und wollt, ich bin mittlwerweile sehr zuversichtlich !
Biotech Stocks to Watch Through the 2nd Half of 2019
[GuruFocus.com]
GuruFocus.com
,GuruFocus.com•August 6, 2019
The biotechnology industry is an exciting one. A mix of technology and a better understanding of the human body has led to several very exciting breakthroughs over the past several years, and we're seeing improved quality of life, less abrasive therapies and longer lifespans among patients than ever before.
Of course, good health is highly valuable. Let's face it, we all want to live with a high quality of life for as long as possible. In fact, it's the human sense of self preservation that led to a market that's expected to rise to generate more than $10 trillion in annual value by 2022.
As you would expect, where there's this kind of market size, there are opportunities for investors. In fact, small biotechnology companies quite often become monsters after inventing breakthrough treatments. Here are a few that I've been following closely:
Amarin Corp. (NASDAQ:AMRN): Vascepa could quickly become a blockbuster
Amarin's claim to fame is a product known as Vascepa. The treatment is a single-molecule product that consists of an omega-3 fatty acid. Derived from fish through a stringent and complex Food and Drug Administration-regulated process, Vascepa is free of impurities and provides an isolated, single-molecule active ingredient.
At the moment, Vascepa is approved as an adjunct to diet to reduce triglyceride levels in adult patients with severe hypertriglyceridemia. While this indication has led to sales, it is a relatively narrow indication with a limited market potential. Nonetheless, the company may break into the big leagues in September.
Amarin is currently awaiting a decision from the FDA with regard to a supplemental New Drug Application. The application was submitted in hopes of expanding the label to include a blockbuster indication.
If it is approved, Vascepa will be the first drug that is indicated to reduce residual cardiovascular risk in patients with statin-managed LDL-C cholesterol, but experience persistent elevated triglycerides. That's a big deal for two reasons:
If the sNDA is approved, it will greatly expand the patient population that could benefit from treatment with Vascepa. Of course, with a greatly expanded addressable patient population comes a greatly expanded revenue opportunity.
Moreover, the American Heart Association used to recommend patients that fall into this category take a baby aspirin daily to mitigate cardiovascular risk. However, the organization recently pulled this recommendation, leaving the patient population with no available options. So demand is likely to be very high from the very beginning.
It's also worth mentioning that the Prescription Drug User Fee Act date, or the date that the FDA will make a decision, for the sNDA is Sept. 28. That's just around the corner! Considering this, Amarin is a compelling opportunity to pay attention to.
[GuruFocus.com]
GuruFocus.com
,GuruFocus.com•August 6, 2019
The biotechnology industry is an exciting one. A mix of technology and a better understanding of the human body has led to several very exciting breakthroughs over the past several years, and we're seeing improved quality of life, less abrasive therapies and longer lifespans among patients than ever before.
Of course, good health is highly valuable. Let's face it, we all want to live with a high quality of life for as long as possible. In fact, it's the human sense of self preservation that led to a market that's expected to rise to generate more than $10 trillion in annual value by 2022.
As you would expect, where there's this kind of market size, there are opportunities for investors. In fact, small biotechnology companies quite often become monsters after inventing breakthrough treatments. Here are a few that I've been following closely:
Amarin Corp. (NASDAQ:AMRN): Vascepa could quickly become a blockbuster
Amarin's claim to fame is a product known as Vascepa. The treatment is a single-molecule product that consists of an omega-3 fatty acid. Derived from fish through a stringent and complex Food and Drug Administration-regulated process, Vascepa is free of impurities and provides an isolated, single-molecule active ingredient.
At the moment, Vascepa is approved as an adjunct to diet to reduce triglyceride levels in adult patients with severe hypertriglyceridemia. While this indication has led to sales, it is a relatively narrow indication with a limited market potential. Nonetheless, the company may break into the big leagues in September.
Amarin is currently awaiting a decision from the FDA with regard to a supplemental New Drug Application. The application was submitted in hopes of expanding the label to include a blockbuster indication.
If it is approved, Vascepa will be the first drug that is indicated to reduce residual cardiovascular risk in patients with statin-managed LDL-C cholesterol, but experience persistent elevated triglycerides. That's a big deal for two reasons:
If the sNDA is approved, it will greatly expand the patient population that could benefit from treatment with Vascepa. Of course, with a greatly expanded addressable patient population comes a greatly expanded revenue opportunity.
Moreover, the American Heart Association used to recommend patients that fall into this category take a baby aspirin daily to mitigate cardiovascular risk. However, the organization recently pulled this recommendation, leaving the patient population with no available options. So demand is likely to be very high from the very beginning.
It's also worth mentioning that the Prescription Drug User Fee Act date, or the date that the FDA will make a decision, for the sNDA is Sept. 28. That's just around the corner! Considering this, Amarin is a compelling opportunity to pay attention to.
Antwort auf Beitrag Nr.: 61.141.730 von kmastra am 31.07.19 11:12:53
Jetzt also doch ein AdCom und zwar am 14.11. und damit einhergehend eine Verzögerung der Zulassung. Irgendwie merkwürdig! Kommunikation von AMRN ist jetzt natürlich mehr als unglücklich.
Ist da jetzt AMRN oder FDA verantwortlich?
Zitat von kmastra: https://finance.yahoo.com/news/amarin-reports-second-quarter…
-Vascepa Umsatz: 100,4 Mio
-AMRN hält einen AdCom mittlerweile für unwahrscheinlich
Klingt insgesamt doch alles recht zuversichtlich!
Jetzt also doch ein AdCom und zwar am 14.11. und damit einhergehend eine Verzögerung der Zulassung. Irgendwie merkwürdig! Kommunikation von AMRN ist jetzt natürlich mehr als unglücklich.
Ist da jetzt AMRN oder FDA verantwortlich?
Hui, da geht‘s nachbörslich aber ganz schön in den Keller...😦
Wollte demnächst ein paar Anteile erwerben, da ich von einer Übernahme in naher Auskunft ausgehe...
Wollte demnächst ein paar Anteile erwerben, da ich von einer Übernahme in naher Auskunft ausgehe...
Antwort auf Beitrag Nr.: 61.211.336 von kmastra am 08.08.19 22:41:32Korrupte Drecks FDA!
Antwort auf Beitrag Nr.: 61.211.603 von Magnetfeldfredy am 08.08.19 23:53:37Ich glaube die hatten seit dem letzten Rechtsstreit mit AMRN noch eine Rechnung offen... 

Ok Gestern war etwas emotional für alle...
Eigentlich wäre "kein Adcom" quasi ein Zeichen gewesen die werden das ablehnen.
Grund:
Ihr Entscheid für eine grosse Label Expansion wäre sehr angreifbar gewesen wenn sie kein Adcom einberufen hätten.
Da hätten alle Markbegleiter/Konkurrenten zurecht weiter auf den bisherigen Arguementen rumhacken können sprich Mineral-Öl Debatte usw. wenn das vorher nicht im Detail diskutiert worden wäre.
Für mich ist die Zulassung quais sicher, dass das FDA sich so spät und zögerlich entschlossen hat ist natürlich maximales Pech... Bleibt drin begrenzt den Schaden kauft am besten noch etwas nach und habt Geduld...
Jetzt verkaufen wäre eigentlich ein riesen Fehler, die Investoren werden spätestens nach ein paar Wochen merken dass sich nichts verändert hat und die Ausgangslage immer noch sehr gut aussieht...
Eigentlich wäre "kein Adcom" quasi ein Zeichen gewesen die werden das ablehnen.
Grund:
Ihr Entscheid für eine grosse Label Expansion wäre sehr angreifbar gewesen wenn sie kein Adcom einberufen hätten.
Da hätten alle Markbegleiter/Konkurrenten zurecht weiter auf den bisherigen Arguementen rumhacken können sprich Mineral-Öl Debatte usw. wenn das vorher nicht im Detail diskutiert worden wäre.
Für mich ist die Zulassung quais sicher, dass das FDA sich so spät und zögerlich entschlossen hat ist natürlich maximales Pech... Bleibt drin begrenzt den Schaden kauft am besten noch etwas nach und habt Geduld...
Jetzt verkaufen wäre eigentlich ein riesen Fehler, die Investoren werden spätestens nach ein paar Wochen merken dass sich nichts verändert hat und die Ausgangslage immer noch sehr gut aussieht...
Antwort auf Beitrag Nr.: 61.211.603 von Magnetfeldfredy am 08.08.19 23:53:37
aber Freddy, ein AdCom ist doch kein Weltuntergang - und zudem daraus Korruptionsvorwüfe abzuleiten ist mehr als töricht. Eine grössere Transparenz als bei einem AdCom ist gar nicht möglich!
Zitat von Magnetfeldfredy: Korrupte Drecks FDA!
aber Freddy, ein AdCom ist doch kein Weltuntergang - und zudem daraus Korruptionsvorwüfe abzuleiten ist mehr als töricht. Eine grössere Transparenz als bei einem AdCom ist gar nicht möglich!
Antwort auf Beitrag Nr.: 61.213.244 von Cyberhexe am 09.08.19 10:00:28Servsu Hexe, aber das PADUFA date war 28.09.2019, jetzt ADCOM am 14. November, das stinkt doch zum Himmel, oder wie siehst Du die Lage?
Antwort auf Beitrag Nr.: 61.213.283 von Magnetfeldfredy am 09.08.19 10:03:29
...ok Freddy, in diesem Punkt gebe ich dir zu 100% recht - das hätte vor dem PDUFA-Termin organisiert werden müssen. Aber sehr wahrscheinlich sind hierfür ganz banale Gründe wie "Volle Agenda" verantwortlich - an Verschwörungstheorien der FDA-Mitarbeiter kann und WILL ich nicht glauben.
Zitat von Magnetfeldfredy: Servsu Hexe, aber das PADUFA date war 28.09.2019, jetzt ADCOM am 14. November, das stinkt doch zum Himmel, oder wie siehst Du die Lage?
...ok Freddy, in diesem Punkt gebe ich dir zu 100% recht - das hätte vor dem PDUFA-Termin organisiert werden müssen. Aber sehr wahrscheinlich sind hierfür ganz banale Gründe wie "Volle Agenda" verantwortlich - an Verschwörungstheorien der FDA-Mitarbeiter kann und WILL ich nicht glauben.
Ok, oder denkst Du dass das Mineralöl Placebo wieder aufgewärmt wird oder vielleicht die Reichweite von Vascepa, 1/3-1/4 der US Bevölkerung ist potentielle Zielgruppe, der Grund ist?
Auf alle Fälle wird sich der Kurs bis zur Aufklärung nicht erholen, die Börse haßt Unsicherheit und das ist hier der Fall bis zum ADCOM Briefing also bis zum 12. November, 2 Tage vor dem Adcom werden die Fragen der FDA veröffentlicht!
Auf alle Fälle wird sich der Kurs bis zur Aufklärung nicht erholen, die Börse haßt Unsicherheit und das ist hier der Fall bis zum ADCOM Briefing also bis zum 12. November, 2 Tage vor dem Adcom werden die Fragen der FDA veröffentlicht!
Antwort auf Beitrag Nr.: 61.180.032 von flyingbeef am 05.08.19 20:44:46
ich vermerke Mich mal Hier.
Zitat von flyingbeef: Hi habe auf Yahoo Stock discussion folgende Erklärung für die 400 Millionen Finanzspritze die sich Amarin geholt hatte gesehen.
Ich finde der Poster hat absolut recht. Das passt und würde alle "Zweifler" hier ziemlich blöd aussehen lassen:
Why did Amarin need $400 M now?
If a broad label expansion is coming, if doctors are indicating an interest in an add on that focuses on the 75% of CVD risk that remains after statin use, if Insurance is looking at the ICER reprort and seeing a very positive benefit to cost when adding Vascepa maybe next year has a lot of potential.
Likely $500M in sales this year and $1500M (1.5 billion) next year is doable. That means you need $350 to $400 million in product to support those sales.
I'm in the 75% chance that at least a comarketer is coming soon after label expansion. In the recent conference call it was mentioned 3000 drug reps would be needed if you wanted to reach volume sales to come on at a faster clip
The recent fund raise was needed. We all had hoped for a rise at a much higher price. Amarin had to get the deal at what the market offered at this time. 100% think it had to be done before label expansion.
Für mich heisst es ich bin dabei Amarin glaubt voll an Ihren Erfolg und sie haben meiner Meinung nach auch beste Argumente und Gründe dafür. Besser kann es eigentlich nicht aussehen.
Natürlich weiss man nicht wie gross die Label Expansion am Schluss ausfallen wird, aber egal es wird eine Label Expansion geben und dass heisst der Kurs jetzt spiegelt ganz sicher nicht den Wert der Firma wieder. Ich denke bis 30 Dollar oder mehr sind selbst bei einer sehr eingeschränkten Label Expansion "sicher" drin.
Bleibt dabei, kauft am besten noch nach wenn ihr könnt und wollt, ich bin mittlwerweile sehr zuversichtlich !
ich vermerke Mich mal Hier.
Kursschwäche als Kaufchance:
Don’t Say Bye Bye to Amarin (AMRN) Stock, Say Buy Buy
[TipRanks]
TipRanks
,TipRanks•August 13, 2019
One week ago, Stifel analyst Derek Archila reiterated his "buy" rating on anti-cholesterol drugmaker Amarin (AMRN). He did this partly because he liked how Amarin's Q2 earnings report showed sales soaring, and losses lessening -- but also because he thought it unlikely the Food and Drug Administration would empanel an Advisory Committee to review Amarin's supplemental New Drug Application for expanded usage of "Vascepa," thus speeding the "sNDA" towards approval.
Last Thursday, Amarin announced that the FDA will be holding an AdCom after all, "tentatively scheduled for November 14, 2019, to review the data from Amarin's "REDUCE-IT cardiovascular outcomes study" and recommend whether the FDA should approve the expanded usage of Vascepa to treat "borderline" to "high" levels of triglycerides in the bloodstream. (I.e. levels from ranging from 151 mg/dL to 499 mg/dL).
Because of the late date of the AdCom, Amarin warned that it now "does not expect the FDA to take action on the sNDA by the previously announced September 28, 2019" deadline. Instead, it expects the deadline for approval under the Prescription Drug User Fee Act to be pushed back to "late December" -- postponing by three months the date at which Amarin might expect to begin booking new revenues under the expanded usage.
Because of that, Amarin stock plunged more nearly 17% in Friday trading, another 6% today, and at least one law firm filed a shareholders' class action lawsuit against Amarin, implying the company did something shady when it advised investors last week that "it was unlikely that the FDA would proceed with an advisory panel review."
Therein, says Archila, lies an opportunity.
Although frustrated by the FDA's decision, so at odds with his own guess, Archila urged investors to take advantage of Amarin's newly discounted stock price and buy some Amarin stock. The analyst reiterates a Buy rating on AMRN with a $26 price target, which implies nearly 90% upside from current levels. (To watch Archila's track record, click here)
His reasoning: "It's difficult to say why the FDA has chosen to convene an adcom this late in the game." Regardless, "our research and checks ... underlie our confidence that Vascepa's dataset will stand up to the scrutiny of a panel." But even more than that:
Under one possible scenario, Archila mused, it's possible the FDA might offer Amarin the option of labeling Vascepa for use "for cardiovascular risk reduction regardless of triglyceride levels" -- in other words, permitting the drug to be used by patients no matter how low, or how high, their triglycerides might be. No restrictions at all!
Such a labeling (if it's in fact on the table) would obviously be good news for Amarin. As Archila explains, it "would increase the commercial opportunity for the drug," meaning more sales and more profits for Amarin.
So as a pure question of risk and reward, Archila now believes the odds favor investing in Amarin.
All in all, AMRN has one of the best ratings by the Street. TipRanks reveals that the stock has a Strong Buy analyst consensus rating with 6 back-to-back buy ratings in the last three months. Meanwhile the average analyst price target of $32.67 suggests the stock has upside potential of nearly 135% from the current share price for the next 12 months. (See AMRN's price targets and analyst ratings on TipRanks)
Don’t Say Bye Bye to Amarin (AMRN) Stock, Say Buy Buy
[TipRanks]
TipRanks
,TipRanks•August 13, 2019
One week ago, Stifel analyst Derek Archila reiterated his "buy" rating on anti-cholesterol drugmaker Amarin (AMRN). He did this partly because he liked how Amarin's Q2 earnings report showed sales soaring, and losses lessening -- but also because he thought it unlikely the Food and Drug Administration would empanel an Advisory Committee to review Amarin's supplemental New Drug Application for expanded usage of "Vascepa," thus speeding the "sNDA" towards approval.
Last Thursday, Amarin announced that the FDA will be holding an AdCom after all, "tentatively scheduled for November 14, 2019, to review the data from Amarin's "REDUCE-IT cardiovascular outcomes study" and recommend whether the FDA should approve the expanded usage of Vascepa to treat "borderline" to "high" levels of triglycerides in the bloodstream. (I.e. levels from ranging from 151 mg/dL to 499 mg/dL).
Because of the late date of the AdCom, Amarin warned that it now "does not expect the FDA to take action on the sNDA by the previously announced September 28, 2019" deadline. Instead, it expects the deadline for approval under the Prescription Drug User Fee Act to be pushed back to "late December" -- postponing by three months the date at which Amarin might expect to begin booking new revenues under the expanded usage.
Because of that, Amarin stock plunged more nearly 17% in Friday trading, another 6% today, and at least one law firm filed a shareholders' class action lawsuit against Amarin, implying the company did something shady when it advised investors last week that "it was unlikely that the FDA would proceed with an advisory panel review."
Therein, says Archila, lies an opportunity.
Although frustrated by the FDA's decision, so at odds with his own guess, Archila urged investors to take advantage of Amarin's newly discounted stock price and buy some Amarin stock. The analyst reiterates a Buy rating on AMRN with a $26 price target, which implies nearly 90% upside from current levels. (To watch Archila's track record, click here)
His reasoning: "It's difficult to say why the FDA has chosen to convene an adcom this late in the game." Regardless, "our research and checks ... underlie our confidence that Vascepa's dataset will stand up to the scrutiny of a panel." But even more than that:
Under one possible scenario, Archila mused, it's possible the FDA might offer Amarin the option of labeling Vascepa for use "for cardiovascular risk reduction regardless of triglyceride levels" -- in other words, permitting the drug to be used by patients no matter how low, or how high, their triglycerides might be. No restrictions at all!
Such a labeling (if it's in fact on the table) would obviously be good news for Amarin. As Archila explains, it "would increase the commercial opportunity for the drug," meaning more sales and more profits for Amarin.
So as a pure question of risk and reward, Archila now believes the odds favor investing in Amarin.
All in all, AMRN has one of the best ratings by the Street. TipRanks reveals that the stock has a Strong Buy analyst consensus rating with 6 back-to-back buy ratings in the last three months. Meanwhile the average analyst price target of $32.67 suggests the stock has upside potential of nearly 135% from the current share price for the next 12 months. (See AMRN's price targets and analyst ratings on TipRanks)
Roth confident Amarin's Vascepa will be 'well-received' by FDA panel Roth Capital analyst Yasmeen Rahimi noted that the FDA's upcoming Advisory Committee reviewing the sNDA for Amarin's (AMRN) Vascepa will include ten voting and one non-voting committee members, including five endocrinologists, three cardiologists, a biostatistician, a pharmacoepidemiologist, a consumer representative and a non-voting nephrologist. Given that the American Diabetes Association has included Vascepa for CV risk reduction in its recommended treatment guidelines, and the panel has a majority of endocrinologists, she believes that "the committee is primed for a positive reception of Vascepa," Rahimi tells investors. Additionally, the AdCom Chairperson, Dr. Kenneth Burman, previously voted in favor of Novo Nordisk's (NVO) insulin degludec and insulin degludec/aspart when chairing a prior AdCom. Rahimi reads this as a strong sign that he is receptive to new therapies and as a positive for Vascepa's review, she said. The analyst keeps a Buy rating on Amarin shares with a price target of $31.
Read more at:
https://thefly.com/landingPageNews.php?id=2950505
Read more at:
https://thefly.com/landingPageNews.php?id=2950505
Trotz des angekündigten Adcoms weitere positive Analysteneinschätzungen:
Amarin initiated with an Outperform at SVB Leerink SVB Leerink analyst Ami Fadia initiated coverage of Amarin with an Outperform rating and $26 price target. She sees the recent 20% pullback in the stock following news that the FDA will hold an advisory committee meeting to review the supplemental new drug application for Vascepa as offering a good entry point, as she believes that Vascepa's label expansion will be approved by the agency. If approved, she thinks Vascepa sales can grow to over $4B in the U.S. at their peak. Fadia added that the November 14 FDA committee meeting and PDUFA date, which she thinks is likely to be rescheduled for late December, are two catalysts that could drive greater than 20% upside in Amarin shares.
Read more at:
https://thefly.com/landingPageNews.php?id=2950858
Amarin initiated with an Outperform at SVB Leerink SVB Leerink analyst Ami Fadia initiated coverage of Amarin with an Outperform rating and $26 price target. She sees the recent 20% pullback in the stock following news that the FDA will hold an advisory committee meeting to review the supplemental new drug application for Vascepa as offering a good entry point, as she believes that Vascepa's label expansion will be approved by the agency. If approved, she thinks Vascepa sales can grow to over $4B in the U.S. at their peak. Fadia added that the November 14 FDA committee meeting and PDUFA date, which she thinks is likely to be rescheduled for late December, are two catalysts that could drive greater than 20% upside in Amarin shares.
Read more at:
https://thefly.com/landingPageNews.php?id=2950858
Prescription Omega-3 Fatty Acids Effectively Reduce Triglycerides, AHA Says in Advisory
Mary Caffrey
Interest in omega-3 fatty acids have increased since publication of the results of REDUCE-IT.
Today’s prescription omega-3 fatty acid drugs effectively lower triglycerides, the American Heart Association (AHA) said today in an advisory report,1 which warned that patients should avoid treating themselves with fish oil supplements not approved by the FDA.
Prescription forms of omega-3 fatty acids can reduce triglyceride levels 20% to 30% for those who are diagnosed with high levels. Data from CDC show that about 25% of Americans have triglycerides above 150 mg/dL, which is above what is recommended;2 those with levels from 200 to 400 mg/dL are considered to have high triglycerides, and a those with 500 mg/dL have very high triglycerides.
Triglycerides are fats that circulate in the bloodstream. Once triglycerides reach 200 mg/dL, patients are at risk of atherosclerosis (narrowing of the arteries), heart attacks, and strokes. Those with triglycerides above 500 mg/dL are also at risk of pancreatitis, an inflammation of the pancreas.
“From our review of the evidence from 17 randomized, controlled clinical trials on high triglyceride levels, we concluded that treatment with 4 grams daily of any of the available prescription choices is effective and can be used safely in conjunction with statin medicines that lower cholesterol,” said Ann Skulas-Ray, PhD, an author of the advisory published in AHA journal Circulation,1 said in a statement.
The advisory comes as evidence accumulates that a formulation of omega-3 fatty acid, icosapent ethyl, sold as Vascepa, offers benefits beyond lowering triglycerides. The medication, a highly purified eicosapentaenoic acid (EPA), sold in a 4-gram capsule, has been shown to reduce the risk of heart attacks, strokes, and cardiovascular events by 25% among patients with high triglycerides when taken with a statin.3
FDA first approved Vascepa in 2012 to treat patient with high triglycerides. Amarin, which makes Vascepa, is seeking a cardiovascular indication for the medication from FDA. A decision had been expected in late September; however, FDA has now scheduled an advisory committee meeting for November 14, 2019, and a decision is not expected before December.
Highlights from today’s advisory include:
Two forms of omega-3 fatty acids are sold and no studies comparing them have been published, so the advisory does not recommend one over the other. Besides EPA, the other formulation combines EPA with docosahexaenoic acid (DHA).
The advisory said that “contrary to perception,” the EPA + DHA combination does not elevate low-density lipoprotein (LDL) cholesterol for most people with high triglycerides, although those with very high triglycerides (above 500 mg/dL) may see higher LDL cholesterol while taking this formulation.
Physicians should rule out other causes of high triglycerides, such as hypothyroidism and poorly managed type 2 diabetes, before prescribing medication, and urge patients to adopt a healthy lifestyle as well.
The review panel that issued the advisory found that prescription omega-3 drugs can reduce triglyceride levels with or without statin therapy.
In a statement, the AHA warns, “People should not try to treat the condition themselves with non-prescription fish oil supplements, since they are not reviewed or approved,” by FDA.
“Dietary supplements containing omega-3 fatty acids are not regulated by the FDA. They should not be used in place of prescription medication for the long-term management of high triglycerides,” said Skulas-Ray, who is an assistant professor in the Department of Nutritional Sciences at the University of Arizona in Tucson.
In an earlier advisory released in 2017, AHA said there was not enough evidence to support using omega-3 fatty acid supplements to prevent heart disease in the general population.
Interest in omega-3 fatty acids has increased with publication of results from REDUCE-IT, the study that identified Vascepa’s ability to reduce major cardiovascular events. REDUCE-IT was presented at the 2018 annual meeting of AHA, and additional results were presented at the 2019 meeting of the American College of Cardiology. The advisory notes that results from a trial involving a EPA + DHA medication are due in 2020.
References
Skulas-Ray AC, Wilson PWF, Harris WS et al on behalf of the American Heart Association. Omega-3 fatty acids for the management of hypertriglyceridemia. Circulation. 2019;140.
Carroll MD, Kit BK, Lacher DA. Trends in elevated triglyceride in adults: United States, 2001–2012. NCHS data brief, no 198. Hyattsville, MD: National Center for Health Statistics. 2015. https://www.cdc.gov/nchs/products/databriefs/db198.htm.
Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. doi: 10.1056/NEJMoa1812792.
Mary Caffrey
Interest in omega-3 fatty acids have increased since publication of the results of REDUCE-IT.
Today’s prescription omega-3 fatty acid drugs effectively lower triglycerides, the American Heart Association (AHA) said today in an advisory report,1 which warned that patients should avoid treating themselves with fish oil supplements not approved by the FDA.
Prescription forms of omega-3 fatty acids can reduce triglyceride levels 20% to 30% for those who are diagnosed with high levels. Data from CDC show that about 25% of Americans have triglycerides above 150 mg/dL, which is above what is recommended;2 those with levels from 200 to 400 mg/dL are considered to have high triglycerides, and a those with 500 mg/dL have very high triglycerides.
Triglycerides are fats that circulate in the bloodstream. Once triglycerides reach 200 mg/dL, patients are at risk of atherosclerosis (narrowing of the arteries), heart attacks, and strokes. Those with triglycerides above 500 mg/dL are also at risk of pancreatitis, an inflammation of the pancreas.
“From our review of the evidence from 17 randomized, controlled clinical trials on high triglyceride levels, we concluded that treatment with 4 grams daily of any of the available prescription choices is effective and can be used safely in conjunction with statin medicines that lower cholesterol,” said Ann Skulas-Ray, PhD, an author of the advisory published in AHA journal Circulation,1 said in a statement.
The advisory comes as evidence accumulates that a formulation of omega-3 fatty acid, icosapent ethyl, sold as Vascepa, offers benefits beyond lowering triglycerides. The medication, a highly purified eicosapentaenoic acid (EPA), sold in a 4-gram capsule, has been shown to reduce the risk of heart attacks, strokes, and cardiovascular events by 25% among patients with high triglycerides when taken with a statin.3
FDA first approved Vascepa in 2012 to treat patient with high triglycerides. Amarin, which makes Vascepa, is seeking a cardiovascular indication for the medication from FDA. A decision had been expected in late September; however, FDA has now scheduled an advisory committee meeting for November 14, 2019, and a decision is not expected before December.
Highlights from today’s advisory include:
Two forms of omega-3 fatty acids are sold and no studies comparing them have been published, so the advisory does not recommend one over the other. Besides EPA, the other formulation combines EPA with docosahexaenoic acid (DHA).
The advisory said that “contrary to perception,” the EPA + DHA combination does not elevate low-density lipoprotein (LDL) cholesterol for most people with high triglycerides, although those with very high triglycerides (above 500 mg/dL) may see higher LDL cholesterol while taking this formulation.
Physicians should rule out other causes of high triglycerides, such as hypothyroidism and poorly managed type 2 diabetes, before prescribing medication, and urge patients to adopt a healthy lifestyle as well.
The review panel that issued the advisory found that prescription omega-3 drugs can reduce triglyceride levels with or without statin therapy.
In a statement, the AHA warns, “People should not try to treat the condition themselves with non-prescription fish oil supplements, since they are not reviewed or approved,” by FDA.
“Dietary supplements containing omega-3 fatty acids are not regulated by the FDA. They should not be used in place of prescription medication for the long-term management of high triglycerides,” said Skulas-Ray, who is an assistant professor in the Department of Nutritional Sciences at the University of Arizona in Tucson.
In an earlier advisory released in 2017, AHA said there was not enough evidence to support using omega-3 fatty acid supplements to prevent heart disease in the general population.
Interest in omega-3 fatty acids has increased with publication of results from REDUCE-IT, the study that identified Vascepa’s ability to reduce major cardiovascular events. REDUCE-IT was presented at the 2018 annual meeting of AHA, and additional results were presented at the 2019 meeting of the American College of Cardiology. The advisory notes that results from a trial involving a EPA + DHA medication are due in 2020.
References
Skulas-Ray AC, Wilson PWF, Harris WS et al on behalf of the American Heart Association. Omega-3 fatty acids for the management of hypertriglyceridemia. Circulation. 2019;140.
Carroll MD, Kit BK, Lacher DA. Trends in elevated triglyceride in adults: United States, 2001–2012. NCHS data brief, no 198. Hyattsville, MD: National Center for Health Statistics. 2015. https://www.cdc.gov/nchs/products/databriefs/db198.htm.
Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. doi: 10.1056/NEJMoa1812792.
Sorry, nochmals mit der heutigen Empfehlung der AHA, das ist mal eine Ansage für Amarin und Vascepa, Topmeldung:
Published on: August 19, 2019
Prescription Omega-3 Fatty Acids Effectively Reduce Triglycerides, AHA Says in Advisory
Mary Caffrey
Interest in omega-3 fatty acids have increased since publication of the results of REDUCE-IT.
Today’s prescription omega-3 fatty acid drugs effectively lower triglycerides, the American Heart Association (AHA) said today in an advisory report,1 which warned that patients should avoid treating themselves with fish oil supplements not approved by the FDA.
Prescription forms of omega-3 fatty acids can reduce triglyceride levels 20% to 30% for those who are diagnosed with high levels. Data from CDC show that about 25% of Americans have triglycerides above 150 mg/dL, which is above what is recommended;2 those with levels from 200 to 400 mg/dL are considered to have high triglycerides, and a those with 500 mg/dL have very high triglycerides.
Triglycerides are fats that circulate in the bloodstream. Once triglycerides reach 200 mg/dL, patients are at risk of atherosclerosis (narrowing of the arteries), heart attacks, and strokes. Those with triglycerides above 500 mg/dL are also at risk of pancreatitis, an inflammation of the pancreas.
“From our review of the evidence from 17 randomized, controlled clinical trials on high triglyceride levels, we concluded that treatment with 4 grams daily of any of the available prescription choices is effective and can be used safely in conjunction with statin medicines that lower cholesterol,” said Ann Skulas-Ray, PhD, an author of the advisory published in AHA journal Circulation,1 said in a statement.
The advisory comes as evidence accumulates that a formulation of omega-3 fatty acid, icosapent ethyl, sold as Vascepa, offers benefits beyond lowering triglycerides. The medication, a highly purified eicosapentaenoic acid (EPA), sold in a 4-gram capsule, has been shown to reduce the risk of heart attacks, strokes, and cardiovascular events by 25% among patients with high triglycerides when taken with a statin.3
FDA first approved Vascepa in 2012 to treat patient with high triglycerides. Amarin, which makes Vascepa, is seeking a cardiovascular indication for the medication from FDA. A decision had been expected in late September; however, FDA has now scheduled an advisory committee meeting for November 14, 2019, and a decision is not expected before December.
Highlights from today’s advisory include:
Two forms of omega-3 fatty acids are sold and no studies comparing them have been published, so the advisory does not recommend one over the other. Besides EPA, the other formulation combines EPA with docosahexaenoic acid (DHA).
The advisory said that “contrary to perception,” the EPA + DHA combination does not elevate low-density lipoprotein (LDL) cholesterol for most people with high triglycerides, although those with very high triglycerides (above 500 mg/dL) may see higher LDL cholesterol while taking this formulation.
Physicians should rule out other causes of high triglycerides, such as hypothyroidism and poorly managed type 2 diabetes, before prescribing medication, and urge patients to adopt a healthy lifestyle as well.
The review panel that issued the advisory found that prescription omega-3 drugs can reduce triglyceride levels with or without statin therapy.
In a statement, the AHA warns, “People should not try to treat the condition themselves with non-prescription fish oil supplements, since they are not reviewed or approved,” by FDA.
“Dietary supplements containing omega-3 fatty acids are not regulated by the FDA. They should not be used in place of prescription medication for the long-term management of high triglycerides,” said Skulas-Ray, who is an assistant professor in the Department of Nutritional Sciences at the University of Arizona in Tucson.
In an earlier advisory released in 2017, AHA said there was not enough evidence to support using omega-3 fatty acid supplements to prevent heart disease in the general population.
Interest in omega-3 fatty acids has increased with publication of results from REDUCE-IT, the study that identified Vascepa’s ability to reduce major cardiovascular events. REDUCE-IT was presented at the 2018 annual meeting of AHA, and additional results were presented at the 2019 meeting of the American College of Cardiology. The advisory notes that results from a trial involving a EPA + DHA medication are due in 2020.
References
Skulas-Ray AC, Wilson PWF, Harris WS et al on behalf of the American Heart Association. Omega-3 fatty acids for the management of hypertriglyceridemia. Circulation. 2019;140.
Carroll MD, Kit BK, Lacher DA. Trends in elevated triglyceride in adults: United States, 2001–2012. NCHS data brief, no 198. Hyattsville, MD: National Center for Health Statistics. 2015. https://www.cdc.gov/nchs/products/databriefs/db198.htm.
Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. doi: 10.1056/NEJMoa1812792.
Published on: August 19, 2019
Prescription Omega-3 Fatty Acids Effectively Reduce Triglycerides, AHA Says in Advisory
Mary Caffrey
Interest in omega-3 fatty acids have increased since publication of the results of REDUCE-IT.
Today’s prescription omega-3 fatty acid drugs effectively lower triglycerides, the American Heart Association (AHA) said today in an advisory report,1 which warned that patients should avoid treating themselves with fish oil supplements not approved by the FDA.
Prescription forms of omega-3 fatty acids can reduce triglyceride levels 20% to 30% for those who are diagnosed with high levels. Data from CDC show that about 25% of Americans have triglycerides above 150 mg/dL, which is above what is recommended;2 those with levels from 200 to 400 mg/dL are considered to have high triglycerides, and a those with 500 mg/dL have very high triglycerides.
Triglycerides are fats that circulate in the bloodstream. Once triglycerides reach 200 mg/dL, patients are at risk of atherosclerosis (narrowing of the arteries), heart attacks, and strokes. Those with triglycerides above 500 mg/dL are also at risk of pancreatitis, an inflammation of the pancreas.
“From our review of the evidence from 17 randomized, controlled clinical trials on high triglyceride levels, we concluded that treatment with 4 grams daily of any of the available prescription choices is effective and can be used safely in conjunction with statin medicines that lower cholesterol,” said Ann Skulas-Ray, PhD, an author of the advisory published in AHA journal Circulation,1 said in a statement.
The advisory comes as evidence accumulates that a formulation of omega-3 fatty acid, icosapent ethyl, sold as Vascepa, offers benefits beyond lowering triglycerides. The medication, a highly purified eicosapentaenoic acid (EPA), sold in a 4-gram capsule, has been shown to reduce the risk of heart attacks, strokes, and cardiovascular events by 25% among patients with high triglycerides when taken with a statin.3
FDA first approved Vascepa in 2012 to treat patient with high triglycerides. Amarin, which makes Vascepa, is seeking a cardiovascular indication for the medication from FDA. A decision had been expected in late September; however, FDA has now scheduled an advisory committee meeting for November 14, 2019, and a decision is not expected before December.
Highlights from today’s advisory include:
Two forms of omega-3 fatty acids are sold and no studies comparing them have been published, so the advisory does not recommend one over the other. Besides EPA, the other formulation combines EPA with docosahexaenoic acid (DHA).
The advisory said that “contrary to perception,” the EPA + DHA combination does not elevate low-density lipoprotein (LDL) cholesterol for most people with high triglycerides, although those with very high triglycerides (above 500 mg/dL) may see higher LDL cholesterol while taking this formulation.
Physicians should rule out other causes of high triglycerides, such as hypothyroidism and poorly managed type 2 diabetes, before prescribing medication, and urge patients to adopt a healthy lifestyle as well.
The review panel that issued the advisory found that prescription omega-3 drugs can reduce triglyceride levels with or without statin therapy.
In a statement, the AHA warns, “People should not try to treat the condition themselves with non-prescription fish oil supplements, since they are not reviewed or approved,” by FDA.
“Dietary supplements containing omega-3 fatty acids are not regulated by the FDA. They should not be used in place of prescription medication for the long-term management of high triglycerides,” said Skulas-Ray, who is an assistant professor in the Department of Nutritional Sciences at the University of Arizona in Tucson.
In an earlier advisory released in 2017, AHA said there was not enough evidence to support using omega-3 fatty acid supplements to prevent heart disease in the general population.
Interest in omega-3 fatty acids has increased with publication of results from REDUCE-IT, the study that identified Vascepa’s ability to reduce major cardiovascular events. REDUCE-IT was presented at the 2018 annual meeting of AHA, and additional results were presented at the 2019 meeting of the American College of Cardiology. The advisory notes that results from a trial involving a EPA + DHA medication are due in 2020.
References
Skulas-Ray AC, Wilson PWF, Harris WS et al on behalf of the American Heart Association. Omega-3 fatty acids for the management of hypertriglyceridemia. Circulation. 2019;140.
Carroll MD, Kit BK, Lacher DA. Trends in elevated triglyceride in adults: United States, 2001–2012. NCHS data brief, no 198. Hyattsville, MD: National Center for Health Statistics. 2015. https://www.cdc.gov/nchs/products/databriefs/db198.htm.
Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. doi: 10.1056/NEJMoa1812792.
Antwort auf Beitrag Nr.: 61.293.085 von Magnetfeldfredy am 19.08.19 18:23:53Nun normalisiert sich der Kurs langsam wieder, leider ist noch ein laanger Weg zu gehen bis der Kurs wieder bei über 22 Dollar ist, aber auch ich komme wieder in die grüne Zone 🙃.
Und 22 Dollar ist laange noch nicht das Schlusslicht, sonst hätte ich damals ja nicht investiert 📈, also alle die noch Geld übrig haben, meinen Segen habt ihr... 😜.
Nein Scherz beiseite meine Empfehlung gebe ich daher da sich die grundsätzliche Ausgangslage seit dem Höhenflug Ende Juli eigentlich nicht geändert hat ausser dass die Zulassung später kommt und Amarin nun viel Cash auf der hohen Kante hat.
Gerüchteküchen warum Amarin sich Cash holte oder warum das FDA nun so spät einen Adcom Termin doch noch anberaumt hat in Kombination mit 👍👎 Entscheidungen bei Pharmaaktien sind halt einfach Gift und bringen immer wieder grosse Schwankungen mit sich.
Durchhalten und gemeinsam gewinnen heisst hier die Devise, super Produkt, Vision, Management, und Marktchancen haben sich nicht im geringsten verändert !
Und 22 Dollar ist laange noch nicht das Schlusslicht, sonst hätte ich damals ja nicht investiert 📈, also alle die noch Geld übrig haben, meinen Segen habt ihr... 😜.
Nein Scherz beiseite meine Empfehlung gebe ich daher da sich die grundsätzliche Ausgangslage seit dem Höhenflug Ende Juli eigentlich nicht geändert hat ausser dass die Zulassung später kommt und Amarin nun viel Cash auf der hohen Kante hat.
Gerüchteküchen warum Amarin sich Cash holte oder warum das FDA nun so spät einen Adcom Termin doch noch anberaumt hat in Kombination mit 👍👎 Entscheidungen bei Pharmaaktien sind halt einfach Gift und bringen immer wieder grosse Schwankungen mit sich.
Durchhalten und gemeinsam gewinnen heisst hier die Devise, super Produkt, Vision, Management, und Marktchancen haben sich nicht im geringsten verändert !
Top Meldung, Empfehlung für Europa:
New 2019 Updates to the European Society of Cardiology’s and European Atherosclerosis Society’s Guidelines for the Management of Dyslipidaemias Incorporate Findings from the REDUCE-IT™ Cardiovascular Outcomes Study
GlobeNewswire•September 3, 2019
Clinical Practice Guidelines address how best to prevent cardiovascular events in high-risk patients with elevated triglycerides on statin treatment, among millions of people globally
BEDMINSTER, N.J., and DUBLIN, Ireland, Sept. 03, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, today announced that the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) have updated their Clinical Practice Guidelines for the Management of Dyslipidaemias. This 2019 update incorporates findings from the REDUCE-IT™1,2 cardiovascular outcomes study and includes the recommendation that icosapent ethyl, 2g twice a day, should be considered for patients with cardiovascular disease who have triglyceride levels 135 mg/dL to 499 mg/dL despite statin treatment, which places them at high risk of cardiovascular events, such as heart attack, stroke or death.3 This new recommendation incorporates icosapent ethyl specifically.

Icosapent ethyl, studied in a series of clinical trials, including the globally conducted REDUCE-IT study, was developed by Amarin and is exclusively marketed by Amarin and its commercial partners in capsule form under the brand name Vascepa® (icosapent ethyl). In the United States, Vascepa is currently approved as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. Amarin has submitted a supplemental new drug application (sNDA) to the U.S. FDA for an expansion of the Vascepa U.S. FDA label based on the landmark REDUCE-IT results showing reduction of cardiovascular events in high risk patients. The company plans to submit an application seeking approval for icosapent ethyl in Europe for reducing cardiovascular events before the end of 2019. A similar application has already been submitted and is under priority review by Health Canada.
ESC and EAS do not provide endorsements or any form of certification for brand name commercial products. Accordingly, the inclusion of icosapent ethyl in the Clinical Practice Guidelines for the Management of Dyslipidaemias should not be understood as an endorsement or approval by ESC or EAS of Vascepa.
“We are pleased by ESC and EAS’s acknowledgement of the significance of the REDUCE-IT results as evidenced by their 2019 updates of the Clinical Practice Guidelines for the Management of Dyslipidaemias,” says Craig B. Granowitz, M.D., Ph.D., senior vice president and chief medical officer of Amarin. “Amarin believes strongly in the potential for icosapent ethyl to be an important treatment option for the millions of high-risk patients who are on statin therapy with controlled cholesterol levels, yet have elevated triglycerides and other cardiovascular risk factors. This update comes just weeks after the American Heart Association issued an advisory statement referencing REDUCE-IT and the cardiovascular risk-lowering effects of icosapent ethyl and months after the American Diabetes Association included the REDUCE-IT results as part of its updates to the Standards of Medical Care in Diabetes for 2019.4, [5] All of these updates further support the use of icosapent ethyl as an important treatment option for appropriate patients at high risk of cardiovascular events.”
Based on the results of REDUCE-IT, the 2019 updates to the Clinical Practice Guidelines for the Management of Dyslipidaemias specifically recommend that:
In high-risk (or above) patients with TG [triglycerides] levels between 1.5 – 5.6 mmol/L (135-499 mg/dL) despite statin treatment, n-3 PUFAs [polyunsaturated fatty acids] (icosapent ethyl 2x2 g/day) should be considered with a statin.6
The ESC and EAS recommendation is classified as a IIa recommendation denoting that icosapent ethyl should be considered for treatment of such patients. The classification is a Level B recommendation which reflects a relatively high weight of scientific evidence under ESC and EAS standards. Such recommendations are supported by the results of the REDUCE-IT cardiovascular outcomes study.
In the United States, approximately 15 million people match the criteria of the REDUCE-IT studied population, with triglycerides ≥ 135 mg/dL and other cardiovascular risk factors, despite statin treatment.7 About 25 percent of a representative sample survey of more than 7,800 patients from 27 European countries with coronary heart disease and controlled cholesterol levels had triglyceride levels of 150 mg/dL or greater, illustrating the potential pervasiveness of high-risk cardiovascular disease in Europe.8
“These updated guidelines from such prestigious organizations reaffirm the importance of the REDUCE-IT findings to patients globally, not only in enhancing care, but also in broadening awareness of the need for treatment among patients who may have their cholesterol controlled with a statin, but remain at risk because of elevated triglycerides,” says Deepak L. Bhatt, M.D., M.P.H., executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital, professor of medicine at Harvard Medical School, and principal investigator and steering committee chair for REDUCE-IT. “Based on what we’re learning from REDUCE-IT and the related guidance from ESC and EAS, I foresee the beginning of a global change in clinical practice in how best to treat patients with multifactorial risks of cardiovascular events beyond cholesterol management.”
Amarin acknowledges the rigor with which the Clinical Practice Guidelines for the Management of Dyslipidaemias are crafted and approved by the ESC’s and EAS’s task force, which is comprised of more than 15 leading professionals in the European Union who specialize in the care of patients with dyslipidaemias.
The complete 2019 updates to the Clinical Practice Guidelines for the Management of Dyslipidaemias can be accessed online by clicking here.
About Amarin
Amarin Corporation plc. is a rapidly growing, innovative pharmaceutical company focused on developing therapeutics to improve cardiovascular health. Amarin’s product development program leverages its extensive experience in polyunsaturated fatty acids and lipid science. Vascepa (icosapent ethyl) is Amarin's first FDA-approved drug and is available by prescription in the United States, Lebanon and the United Arab Emirates. Amarin’s commercial partners are pursuing additional regulatory approvals for Vascepa in Canada, China and the Middle East. For more information about Amarin, visit www.amarincorp.com.
REDUCE-IT™ Study
REDUCE-IT, an 8,179-patient cardiovascular outcomes study, was completed in 2018. REDUCE-IT was the first multinational cardiovascular outcomes study that evaluated the effect of prescription pure EPA therapy as an add-on to statins in patients with high cardiovascular risk who, despite stable statin therapy, had elevated triglyceride levels (at least 135 mg/dL). A large portion of the male and female patients enrolled in this outcomes study were diagnosed with type 2 diabetes.
More information on the REDUCE-IT study results can be found at www.amarincorp.com.
About Cardiovascular Disease
Worldwide, cardiovascular disease (CVD) remains the #1 killer of men and women. In the United States CVD leads to one in every three deaths – one death approximately every 38 seconds – with annual treatment cost in excess of $500 billion.9,[10]
Multiple primary and secondary prevention trials have shown a significant reduction of 25% to 35% in the risk of cardiovascular events with statin therapy, leaving significant persistent residual risk despite the achievement of target LDL-C levels.11
Beyond the cardiovascular risk associated with LDL-C, genetic, epidemiologic, clinical and real-world data suggest that patients with elevated triglycerides (TG) (fats in the blood), and TG-rich lipoproteins, are at increased risk for cardiovascular disease.12, 13,[14],[15]
About Vascepa® (icosapent ethyl) Capsules
Vascepa (icosapent ethyl) capsules are a single-molecule prescription product consisting of the omega-3 acid commonly known as EPA in ethyl-ester form. Vascepa is not fish oil, but is derived from fish through a stringent and complex FDA-regulated manufacturing process designed to effectively eliminate impurities and isolate and protect the single molecule active ingredient from degradation. Vascepa, known in scientific literature as AMR101, has been designated a new chemical entity by the FDA. Amarin has been issued multiple patents internationally based on the unique clinical profile of Vascepa, including the drug’s ability to lower triglyceride levels in relevant patient populations without raising LDL-cholesterol levels.
The FDA has not reviewed and opined on a supplemental new drug application related to REDUCE IT. FDA has thus not reviewed the information herein or determined whether to approve Vascepa for use to reduce the risk of major adverse cardiovascular events in the REDUCE-IT patient population
Indication and Usage Based on Current FDA-Approved Label (not including REDUCE-IT results)
Vascepa (icosapent ethyl) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
The effect of Vascepa on the risk for pancreatitis and cardiovascular mortality and morbidity in patients with severe hypertriglyceridemia has not been determined.
Important Safety Information for Vascepa Based on Current FDA-Approved Label (not including REDUCE-IT results) (Includes Data from Two 12-Week Studies (n=622) (MARINE and ANCHOR) of Patients with Triglycerides Values of 200 to 2000 mg/dL)
Vascepa is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vascepa or any of its components.
In patients with hepatic impairment, monitor ALT and AST levels periodically during therapy.
Use with caution in patients with known hypersensitivity to fish and/or shellfish.
The most common reported adverse reaction (incidence >2% and greater than placebo) was arthralgia (2.3% for Vascepa, 1.0% for placebo). There was no reported adverse reaction >3% and greater than placebo.
Adverse events and product complaints may be reported by calling 1-855-VASCEPA or the FDA at 1-800-FDA-1088. Patients receiving treatment with Vascepa and other drugs affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
Patients should be advised to swallow Vascepa capsules whole; not to break open, crush, dissolve, or chew Vascepa.
FULL VASCEPA PRESCRIBING INFORMATION CAN BE FOUND AT WWW.VASCEPA.COM.
Important Safety Information for Vascepa based on REDUCE-IT, as previously reported in The New England Journal of Medicine publication of the primary results of the REDUCE-IT study:
Excluding the major adverse cardiovascular events (MACE) results described above, overall adverse event rates in REDUCE-IT were similar across the statin plus Vascepa and the statin plus placebo treatment groups.
There were no significant differences between treatments in the overall rate of treatment emergent adverse events or serious adverse events leading to withdrawal of study drug.
There was no serious adverse event (SAE) occurring at a frequency of >2% which occurred at a numerically higher rate in the statin plus Vascepa treatment group than in the statin plus placebo treatment group.
Adverse events (AEs) occurring in 5% or greater of patients and more frequently with Vascepa than placebo were:
peripheral edema (6.5% Vascepa patients versus 5.0% placebo patients), although there was no increase in the rate of heart failure in Vascepa patients
constipation (5.4% Vascepa patients versus 3.6% placebo patients), although mineral oil, as used as placebo, is known to lower constipation, and
atrial fibrillation (5.3% Vascepa patients versus 3.9% placebo patients), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients
There were numerically more SAEs related to bleeding in the statin plus Vascepa treatment group although overall rates were low with no fatal bleeding observed in either group and no significant difference in adjudicated hemorrhagic stroke or serious central nervous system or gastrointestinal bleeding events between treatments.
In summary, Vascepa was well tolerated with a safety profile generally consistent with clinical experience associated with omega-3 fatty acids and current FDA-approved labeling of such products.
Important Cautionary Information About These Data
Further REDUCE-IT data assessment and data release could yield additional useful information to inform greater understanding of the trial outcome. For example, detailed data assessment by regulatory authorities, such as the FDA and Health Canada, will continue and take several months to complete and announce. The FDA advisory committee process and the final evaluation by regulatory authorities of the totality of efficacy and safety data from REDUCE-IT may include some or all of the following, as well as other considerations: new information or analyses affecting the degree of treatment benefit on studied endpoints; study conduct and data robustness, quality, integrity and consistency; additional safety data considerations and risk/benefit considerations; and consideration of REDUCE-IT results in the context of other clinical studies. Because regulatory reviews are typically fluid and not definitive interactions between sponsor and agency on individual elements of an application and related information, Amarin does not plan to update investors on ongoing communications with regulatory authorities. Amarin plans to announce the final outcome of such regulatory reviews when appropriate.

New 2019 Updates to the European Society of Cardiology’s and European Atherosclerosis Society’s Guidelines for the Management of Dyslipidaemias Incorporate Findings from the REDUCE-IT™ Cardiovascular Outcomes Study
GlobeNewswire•September 3, 2019
Clinical Practice Guidelines address how best to prevent cardiovascular events in high-risk patients with elevated triglycerides on statin treatment, among millions of people globally
BEDMINSTER, N.J., and DUBLIN, Ireland, Sept. 03, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, today announced that the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) have updated their Clinical Practice Guidelines for the Management of Dyslipidaemias. This 2019 update incorporates findings from the REDUCE-IT™1,2 cardiovascular outcomes study and includes the recommendation that icosapent ethyl, 2g twice a day, should be considered for patients with cardiovascular disease who have triglyceride levels 135 mg/dL to 499 mg/dL despite statin treatment, which places them at high risk of cardiovascular events, such as heart attack, stroke or death.3 This new recommendation incorporates icosapent ethyl specifically.


Icosapent ethyl, studied in a series of clinical trials, including the globally conducted REDUCE-IT study, was developed by Amarin and is exclusively marketed by Amarin and its commercial partners in capsule form under the brand name Vascepa® (icosapent ethyl). In the United States, Vascepa is currently approved as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. Amarin has submitted a supplemental new drug application (sNDA) to the U.S. FDA for an expansion of the Vascepa U.S. FDA label based on the landmark REDUCE-IT results showing reduction of cardiovascular events in high risk patients. The company plans to submit an application seeking approval for icosapent ethyl in Europe for reducing cardiovascular events before the end of 2019. A similar application has already been submitted and is under priority review by Health Canada.
ESC and EAS do not provide endorsements or any form of certification for brand name commercial products. Accordingly, the inclusion of icosapent ethyl in the Clinical Practice Guidelines for the Management of Dyslipidaemias should not be understood as an endorsement or approval by ESC or EAS of Vascepa.
“We are pleased by ESC and EAS’s acknowledgement of the significance of the REDUCE-IT results as evidenced by their 2019 updates of the Clinical Practice Guidelines for the Management of Dyslipidaemias,” says Craig B. Granowitz, M.D., Ph.D., senior vice president and chief medical officer of Amarin. “Amarin believes strongly in the potential for icosapent ethyl to be an important treatment option for the millions of high-risk patients who are on statin therapy with controlled cholesterol levels, yet have elevated triglycerides and other cardiovascular risk factors. This update comes just weeks after the American Heart Association issued an advisory statement referencing REDUCE-IT and the cardiovascular risk-lowering effects of icosapent ethyl and months after the American Diabetes Association included the REDUCE-IT results as part of its updates to the Standards of Medical Care in Diabetes for 2019.4, [5] All of these updates further support the use of icosapent ethyl as an important treatment option for appropriate patients at high risk of cardiovascular events.”
Based on the results of REDUCE-IT, the 2019 updates to the Clinical Practice Guidelines for the Management of Dyslipidaemias specifically recommend that:
In high-risk (or above) patients with TG [triglycerides] levels between 1.5 – 5.6 mmol/L (135-499 mg/dL) despite statin treatment, n-3 PUFAs [polyunsaturated fatty acids] (icosapent ethyl 2x2 g/day) should be considered with a statin.6
The ESC and EAS recommendation is classified as a IIa recommendation denoting that icosapent ethyl should be considered for treatment of such patients. The classification is a Level B recommendation which reflects a relatively high weight of scientific evidence under ESC and EAS standards. Such recommendations are supported by the results of the REDUCE-IT cardiovascular outcomes study.
In the United States, approximately 15 million people match the criteria of the REDUCE-IT studied population, with triglycerides ≥ 135 mg/dL and other cardiovascular risk factors, despite statin treatment.7 About 25 percent of a representative sample survey of more than 7,800 patients from 27 European countries with coronary heart disease and controlled cholesterol levels had triglyceride levels of 150 mg/dL or greater, illustrating the potential pervasiveness of high-risk cardiovascular disease in Europe.8
“These updated guidelines from such prestigious organizations reaffirm the importance of the REDUCE-IT findings to patients globally, not only in enhancing care, but also in broadening awareness of the need for treatment among patients who may have their cholesterol controlled with a statin, but remain at risk because of elevated triglycerides,” says Deepak L. Bhatt, M.D., M.P.H., executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital, professor of medicine at Harvard Medical School, and principal investigator and steering committee chair for REDUCE-IT. “Based on what we’re learning from REDUCE-IT and the related guidance from ESC and EAS, I foresee the beginning of a global change in clinical practice in how best to treat patients with multifactorial risks of cardiovascular events beyond cholesterol management.”
Amarin acknowledges the rigor with which the Clinical Practice Guidelines for the Management of Dyslipidaemias are crafted and approved by the ESC’s and EAS’s task force, which is comprised of more than 15 leading professionals in the European Union who specialize in the care of patients with dyslipidaemias.
The complete 2019 updates to the Clinical Practice Guidelines for the Management of Dyslipidaemias can be accessed online by clicking here.
About Amarin
Amarin Corporation plc. is a rapidly growing, innovative pharmaceutical company focused on developing therapeutics to improve cardiovascular health. Amarin’s product development program leverages its extensive experience in polyunsaturated fatty acids and lipid science. Vascepa (icosapent ethyl) is Amarin's first FDA-approved drug and is available by prescription in the United States, Lebanon and the United Arab Emirates. Amarin’s commercial partners are pursuing additional regulatory approvals for Vascepa in Canada, China and the Middle East. For more information about Amarin, visit www.amarincorp.com.
REDUCE-IT™ Study
REDUCE-IT, an 8,179-patient cardiovascular outcomes study, was completed in 2018. REDUCE-IT was the first multinational cardiovascular outcomes study that evaluated the effect of prescription pure EPA therapy as an add-on to statins in patients with high cardiovascular risk who, despite stable statin therapy, had elevated triglyceride levels (at least 135 mg/dL). A large portion of the male and female patients enrolled in this outcomes study were diagnosed with type 2 diabetes.
More information on the REDUCE-IT study results can be found at www.amarincorp.com.
About Cardiovascular Disease
Worldwide, cardiovascular disease (CVD) remains the #1 killer of men and women. In the United States CVD leads to one in every three deaths – one death approximately every 38 seconds – with annual treatment cost in excess of $500 billion.9,[10]
Multiple primary and secondary prevention trials have shown a significant reduction of 25% to 35% in the risk of cardiovascular events with statin therapy, leaving significant persistent residual risk despite the achievement of target LDL-C levels.11
Beyond the cardiovascular risk associated with LDL-C, genetic, epidemiologic, clinical and real-world data suggest that patients with elevated triglycerides (TG) (fats in the blood), and TG-rich lipoproteins, are at increased risk for cardiovascular disease.12, 13,[14],[15]
About Vascepa® (icosapent ethyl) Capsules
Vascepa (icosapent ethyl) capsules are a single-molecule prescription product consisting of the omega-3 acid commonly known as EPA in ethyl-ester form. Vascepa is not fish oil, but is derived from fish through a stringent and complex FDA-regulated manufacturing process designed to effectively eliminate impurities and isolate and protect the single molecule active ingredient from degradation. Vascepa, known in scientific literature as AMR101, has been designated a new chemical entity by the FDA. Amarin has been issued multiple patents internationally based on the unique clinical profile of Vascepa, including the drug’s ability to lower triglyceride levels in relevant patient populations without raising LDL-cholesterol levels.
The FDA has not reviewed and opined on a supplemental new drug application related to REDUCE IT. FDA has thus not reviewed the information herein or determined whether to approve Vascepa for use to reduce the risk of major adverse cardiovascular events in the REDUCE-IT patient population
Indication and Usage Based on Current FDA-Approved Label (not including REDUCE-IT results)
Vascepa (icosapent ethyl) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
The effect of Vascepa on the risk for pancreatitis and cardiovascular mortality and morbidity in patients with severe hypertriglyceridemia has not been determined.
Important Safety Information for Vascepa Based on Current FDA-Approved Label (not including REDUCE-IT results) (Includes Data from Two 12-Week Studies (n=622) (MARINE and ANCHOR) of Patients with Triglycerides Values of 200 to 2000 mg/dL)
Vascepa is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vascepa or any of its components.
In patients with hepatic impairment, monitor ALT and AST levels periodically during therapy.
Use with caution in patients with known hypersensitivity to fish and/or shellfish.
The most common reported adverse reaction (incidence >2% and greater than placebo) was arthralgia (2.3% for Vascepa, 1.0% for placebo). There was no reported adverse reaction >3% and greater than placebo.
Adverse events and product complaints may be reported by calling 1-855-VASCEPA or the FDA at 1-800-FDA-1088. Patients receiving treatment with Vascepa and other drugs affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
Patients should be advised to swallow Vascepa capsules whole; not to break open, crush, dissolve, or chew Vascepa.
FULL VASCEPA PRESCRIBING INFORMATION CAN BE FOUND AT WWW.VASCEPA.COM.
Important Safety Information for Vascepa based on REDUCE-IT, as previously reported in The New England Journal of Medicine publication of the primary results of the REDUCE-IT study:
Excluding the major adverse cardiovascular events (MACE) results described above, overall adverse event rates in REDUCE-IT were similar across the statin plus Vascepa and the statin plus placebo treatment groups.
There were no significant differences between treatments in the overall rate of treatment emergent adverse events or serious adverse events leading to withdrawal of study drug.
There was no serious adverse event (SAE) occurring at a frequency of >2% which occurred at a numerically higher rate in the statin plus Vascepa treatment group than in the statin plus placebo treatment group.
Adverse events (AEs) occurring in 5% or greater of patients and more frequently with Vascepa than placebo were:
peripheral edema (6.5% Vascepa patients versus 5.0% placebo patients), although there was no increase in the rate of heart failure in Vascepa patients
constipation (5.4% Vascepa patients versus 3.6% placebo patients), although mineral oil, as used as placebo, is known to lower constipation, and
atrial fibrillation (5.3% Vascepa patients versus 3.9% placebo patients), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients
There were numerically more SAEs related to bleeding in the statin plus Vascepa treatment group although overall rates were low with no fatal bleeding observed in either group and no significant difference in adjudicated hemorrhagic stroke or serious central nervous system or gastrointestinal bleeding events between treatments.
In summary, Vascepa was well tolerated with a safety profile generally consistent with clinical experience associated with omega-3 fatty acids and current FDA-approved labeling of such products.
Important Cautionary Information About These Data
Further REDUCE-IT data assessment and data release could yield additional useful information to inform greater understanding of the trial outcome. For example, detailed data assessment by regulatory authorities, such as the FDA and Health Canada, will continue and take several months to complete and announce. The FDA advisory committee process and the final evaluation by regulatory authorities of the totality of efficacy and safety data from REDUCE-IT may include some or all of the following, as well as other considerations: new information or analyses affecting the degree of treatment benefit on studied endpoints; study conduct and data robustness, quality, integrity and consistency; additional safety data considerations and risk/benefit considerations; and consideration of REDUCE-IT results in the context of other clinical studies. Because regulatory reviews are typically fluid and not definitive interactions between sponsor and agency on individual elements of an application and related information, Amarin does not plan to update investors on ongoing communications with regulatory authorities. Amarin plans to announce the final outcome of such regulatory reviews when appropriate.
Antwort auf Beitrag Nr.: 61.400.003 von Magnetfeldfredy am 03.09.19 15:27:59Hallo,
kurz mal was zur aktuellen Presse zum Thema Polypille zu generellen kardiovaskulären Erkrankungen.
Heute zufällig auf diesen Artikel gestossen:
https://www.spiegel.de/gesundheit/diagnose/herz-kreislauf-er…
Falls dies sich durchsetzt würde es meinem Verständnis nach in Zukunft den potentiellen Markt für Vascepa (und anderen bisher bewährten Behandlungen) deutlich reduzieren, da wenn viele Menschen prohylaktisch mit solchen Polypillen behandelt werden das deutlich Herz Kreislauferkrankungen reduzieren würde, was ja grundsätzlich keine schlechte Nachricht ist...
Was meint Ihr dazu ? Kennt sich jemand damit näher aus und wie wahrscheinlich hier ein Durchbruch in näherer Zukunft ist ?
Oder bin ich hier auf dem Holzweg, wer kann hierzu evtl. was beitragen, Danke schonmal im voraus !
kurz mal was zur aktuellen Presse zum Thema Polypille zu generellen kardiovaskulären Erkrankungen.
Heute zufällig auf diesen Artikel gestossen:
https://www.spiegel.de/gesundheit/diagnose/herz-kreislauf-er…
Falls dies sich durchsetzt würde es meinem Verständnis nach in Zukunft den potentiellen Markt für Vascepa (und anderen bisher bewährten Behandlungen) deutlich reduzieren, da wenn viele Menschen prohylaktisch mit solchen Polypillen behandelt werden das deutlich Herz Kreislauferkrankungen reduzieren würde, was ja grundsätzlich keine schlechte Nachricht ist...
Was meint Ihr dazu ? Kennt sich jemand damit näher aus und wie wahrscheinlich hier ein Durchbruch in näherer Zukunft ist ?
Oder bin ich hier auf dem Holzweg, wer kann hierzu evtl. was beitragen, Danke schonmal im voraus !
11:25 Amarin call volume above normal and directionally bullish Bullish option flow detected in Amarin with 9,202 calls trading, 1.2x expected, and implied vol increasing almost 4 points to 65.82%. Mar-20 20 calls and Sep-19 16 calls are the most active options, with total volume in those strikes near 4,300 contracts. The Put/Call Ratio is 0.05. Earnings are expected on October 30th. Read more at: https://thefly.com/landingPageNews.php?id=2958517
Read more at:
https://thefly.com/landingPageNews.php?id=2958517
Read more at:
https://thefly.com/landingPageNews.php?id=2958517
Antwort auf Beitrag Nr.: 61.404.386 von flyingbeef am 03.09.19 23:44:59Ich denke da bist du auf dem Holzweg. 😉
Es ist ja nicht so, dass es momentan keine Medikamente gegen Herzkreislauferkrankungen bzw. die Prophylaxe gibt. Die werden ja alle massenhaft jetzt schon eingesetzt. Viele schlucken da mehrere Pillen an einem Tag. Aspirin, Statine, Blutverdünner usw. Das ist aber überwiegend generisch, also billig. Eine Kombipille ist da auch m.E. kein großer Vorteil. Das können die Ärzte individuell sicher besser zusammenstellen. Nebenwirkungen spielen ja auch eine Rolle.
Vascepa ist eine weitere Alternative, mit anderem Wirkansatz. Wahrscheinlich nehmen die meisten Vascepa Patienten jetzt auch noch weitere Medikamente ein. Von daher sehe ich da keine kommerzielle Gefahr.
Es ist ja nicht so, dass es momentan keine Medikamente gegen Herzkreislauferkrankungen bzw. die Prophylaxe gibt. Die werden ja alle massenhaft jetzt schon eingesetzt. Viele schlucken da mehrere Pillen an einem Tag. Aspirin, Statine, Blutverdünner usw. Das ist aber überwiegend generisch, also billig. Eine Kombipille ist da auch m.E. kein großer Vorteil. Das können die Ärzte individuell sicher besser zusammenstellen. Nebenwirkungen spielen ja auch eine Rolle.
Vascepa ist eine weitere Alternative, mit anderem Wirkansatz. Wahrscheinlich nehmen die meisten Vascepa Patienten jetzt auch noch weitere Medikamente ein. Von daher sehe ich da keine kommerzielle Gefahr.
Verschreibungen pro Woche erstmals übeer 70.000
 , das wird ein Megablockbuster:
, das wird ein Megablockbuster:
NORMALIZED Scripts Update for Week Ending 13/09
ATH Across the Board in Numbers & Market Share
TRx > 70,000 & NRx ~ 33,000
Sector’s TRx & NRx & Refills at levels not seen since June-2013
V
TRx: 70,559 {vs 63,318; +11.44%} Sector +9.31% --- (8,467,050 vs 7,598,119) --- ATH
NRx: 32,840 {vs 27,532; +19.28%} Sector +16.18% --- (3,940,842 vs 3,303,855) --- ATH
Ref: 37,718 {vs 35,786; +5.40%} Sector +3.87% --- (4,526,208 vs 4,294,264) --- ATH
GenL
TRx: 62,111 {vs 58,040; +7.01%} (7,453,301 vs 6,964,784)
NRx: 29,544 {vs 26,210; +12.72%} (3,545,228 vs 3,145,165)
L
TRx: 1,000 {vs 926; +8.02%} (119,966 vs 111,060)
NRx: 419 {vs 316; +32.52%} (50,318 vs 37,969)
V TRx Market Share: 52.79% vs 51.78% ---ATH
V NRx Market Share: 52.29% vs 50.93% ---ATH
V Ref Market Share: 53.22% vs 52.45% ---ATH
For those interested in Old Scripts Numbers ATH Across the Board in Numbers & Market Share except NRx 2nd ATH in Market Share;
V TRx 53,972 vs GL TRx 46,942 & L 653 -- ATH
V NRx 21,521 vs GL NRx 19,964 & L 245 -- ATH
V Refills 32,451 vs GL Refills 26,978 & L 408 -- ATH

 , das wird ein Megablockbuster:
, das wird ein Megablockbuster:NORMALIZED Scripts Update for Week Ending 13/09
ATH Across the Board in Numbers & Market Share
TRx > 70,000 & NRx ~ 33,000
Sector’s TRx & NRx & Refills at levels not seen since June-2013
V
TRx: 70,559 {vs 63,318; +11.44%} Sector +9.31% --- (8,467,050 vs 7,598,119) --- ATH
NRx: 32,840 {vs 27,532; +19.28%} Sector +16.18% --- (3,940,842 vs 3,303,855) --- ATH
Ref: 37,718 {vs 35,786; +5.40%} Sector +3.87% --- (4,526,208 vs 4,294,264) --- ATH
GenL
TRx: 62,111 {vs 58,040; +7.01%} (7,453,301 vs 6,964,784)
NRx: 29,544 {vs 26,210; +12.72%} (3,545,228 vs 3,145,165)
L
TRx: 1,000 {vs 926; +8.02%} (119,966 vs 111,060)
NRx: 419 {vs 316; +32.52%} (50,318 vs 37,969)
V TRx Market Share: 52.79% vs 51.78% ---ATH
V NRx Market Share: 52.29% vs 50.93% ---ATH
V Ref Market Share: 53.22% vs 52.45% ---ATH
For those interested in Old Scripts Numbers ATH Across the Board in Numbers & Market Share except NRx 2nd ATH in Market Share;
V TRx 53,972 vs GL TRx 46,942 & L 653 -- ATH
V NRx 21,521 vs GL NRx 19,964 & L 245 -- ATH
V Refills 32,451 vs GL Refills 26,978 & L 408 -- ATH
$AMRN Dr. Budoff confirmed that EVAPORATE stopped at 9 months. Now AMRN confirms interim. Why is that important? Interim had an efficacy readout that had EVAPORATE stopping at 9 months if it hit endpoints (P value of ≤0.006). *ht to eightisenough at ST
Antwort auf Beitrag Nr.: 61.549.263 von Magnetfeldfredy am 23.09.19 19:03:46@Magnetfeldfredy
Würdest Du bitte zukünftig entsprechende Quellenangaben posten damit der geneigte Leser
derartige Aussagen nachvollziehen kann? Betrifft z. B. die Beiträge Nr. 585 und 586.
Danke und Gruß,
holgi-w
Würdest Du bitte zukünftig entsprechende Quellenangaben posten damit der geneigte Leser
derartige Aussagen nachvollziehen kann? Betrifft z. B. die Beiträge Nr. 585 und 586.
Danke und Gruß,
holgi-w
INVESTOR RELATIONS
AMARIN TO PARTICIPATE IN THE CANTOR GLOBAL HEALTHCARE CONFERENCE
Sep 23, 2019
DUBLIN, Ireland and BRIDGEWATER, N.J., Sept. 23, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (NASDAQ:AMRN), a pharmaceutical company focused on the commercialization and development of therapeutics to improve cardiovascular health, announced today that John F. Thero, Amarin's president and chief executive officer, is scheduled to present a general company update at the Cantor Global Healthcare Conference on
Thursday, October 3, 2019 from 10:05-10:35 a.m. Eastern Time in New York City.
A live audio webcast of the presentation will be available at: http://www.amarincorp.com, and will be accessible at the same link for 30 days.
https://investor.amarincorp.com/news-releases/news-release-d…
AMARIN TO PARTICIPATE IN THE CANTOR GLOBAL HEALTHCARE CONFERENCE
Sep 23, 2019
DUBLIN, Ireland and BRIDGEWATER, N.J., Sept. 23, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (NASDAQ:AMRN), a pharmaceutical company focused on the commercialization and development of therapeutics to improve cardiovascular health, announced today that John F. Thero, Amarin's president and chief executive officer, is scheduled to present a general company update at the Cantor Global Healthcare Conference on
Thursday, October 3, 2019 from 10:05-10:35 a.m. Eastern Time in New York City.
A live audio webcast of the presentation will be available at: http://www.amarincorp.com, and will be accessible at the same link for 30 days.
https://investor.amarincorp.com/news-releases/news-release-d…
Antwort auf Beitrag Nr.: 61.552.923 von holgi-w am 24.09.19 09:24:04@Magnetfeldfredy 
@holgi-w

https://stocktwits.com/Sam81/message/178114073

@holgi-w

https://stocktwits.com/Sam81/message/178114073

Große Hoffnung für diesen Late Braker:


Independent Drug-Pricing Assessment Finds Vascepa® (icosapent ethyl) Cost-Effective as an Adjunct to Statins in Treating Patients at High Risk of Cardiovascular Events, such as Heart Attack, Stroke and Cardiac Death
GlobeNewswire•October 17, 2019
DUBLIN, Ireland and BRIDGEWATER, N.J., Oct. 17, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, today announced that an independent organization that evaluates pricing of prescription drugs has issued its final report assessing the cost-effectiveness of Vascepa® (icosapent ethyl) capsules. The final assessment confirms the cost-effectiveness of Vascepa across all the non-profit organization’s analyses, including its most stringent criteria.1
The review was derived from results of the landmark phase 3 clinical trial REDUCE-IT®2, focusing on the clinical benefit-risk profile of Vascepa and its value as an additive therapy for cardiovascular disease. The conclusion from the report is that Vascepa easily meets “commonly cited thresholds for cost-effectiveness and therefore represent a high long-term value for money,” based on the organization’s value assessment framework.3
“We are proud to have priced Vascepa to be cost-effective, and we appreciate this positioning being recognized by third-parties,” said Craig Granowitz, M.D., Ph.D., chief medical officer, Amarin. “However, despite the report’s positive conclusion that Vascepa is cost-effective, we believe that it understates the true value of Vascepa. For example, the report’s base-case analyses reflect only the costs of heart attack, stroke and cardiovascular death and exclude other high costs associated with other cardiovascular events demonstrated to be lowered by Vascepa in the REDUCE-IT cardiovascular outcomes study (e.g., revascularization procedures and hospitalization for unstable angina) as well as lower rates of recurring cardiovascular events in patients treated with Vascepa during the study.”
Another Cost-Effectiveness Analysis of Vascepa to Be Presented at AHA
A separate independent, academic cost-effectiveness analysis of Vascepa based on patient-specific data from the REDUCE-IT study will be presented at the American Heart Association Scientific Sessions 2019 on November 16 by William S. Weintraub, M.D., Director of Outcomes Research, MedStar Cardiovascular Research Network. Amarin provided an unrestricted grant for this academically driven analysis, titled “Cost-Effectiveness of Icosapent Ethyl in REDUCE-IT.”
Cardiovascular Disease, an Urgent Public Health Issue
“There is no doubt that cardiovascular disease is an urgent and growing public health issue, with more than half of U.S. adults impacted,” Dr. Granowitz added. “The U.S. spends approximately $555 billion on cardiovascular disease each year, and it is projected that these direct and indirect costs could increase to more than $1.1 trillion by 2035.4 Amarin has worked for over a decade to develop and test Vascepa to create a new preventative care solution that will potentially help many of our family, friends and neighbors within this population. Our goal is to make Vascepa accessible to as many patients as possible who can benefit.”
Vascepa is currently approved in the United States as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. Amarin has submitted a supplemental new drug application (sNDA) to the U.S. FDA for a label expansion based on the REDUCE-IT study results showing reduction of cardiovascular events in high-risk patients. The Prescription Drug User Fee Act (PDUFA) target action date set by the FDA to act on the sNDA is December 28, 2019. Assuming FDA approval, Vascepa is positioned to become the first drug indicated to reduce persistent residual cardiovascular risk in statin-managed patients with elevated triglycerides (135 mg/dL or greater) and other risk factors for cardiovascular disease. The approval of such label expansion and the wording of the indication statement is under regulatory review.
Today, many insurance plans, or payers, already broadly cover Vascepa. Upon assumed FDA label expansion, Amarin plans to use the results of the REDUCE-IT study, further objective health-economic analyses, and other medical information and data in negotiations with payers to seek expanded Vascepa insurance coverage.
About Amarin
Amarin Corporation plc. is a rapidly growing, innovative pharmaceutical company focused on developing therapeutics to improve cardiovascular health. Amarin’s product development program leverages its extensive experience in polyunsaturated fatty acids and lipid science. Vascepa (icosapent ethyl) is Amarin's first FDA-approved drug and is available by prescription in the United States, Lebanon and the United Arab Emirates. Amarin’s commercial partners are pursuing additional regulatory approvals for Vascepa in Canada, China and the Middle East. For more information about Amarin, visit www.amarincorp.com.
REDUCE-IT® Study
REDUCE-IT, an 8,179-patient cardiovascular outcomes study, was completed in 2018. REDUCE-IT was the first multinational cardiovascular outcomes study that evaluated the effect of prescription pure EPA therapy as an add-on to statins in patients with high cardiovascular risk who, despite stable statin therapy, had elevated triglyceride levels (at least 135 mg/dL). A large portion of the male and female patients enrolled in this outcomes study were diagnosed with type 2 diabetes.
More information on the REDUCE-IT study results can be found at www.amarincorp.com.
About Cardiovascular Disease
Worldwide, cardiovascular disease (CVD) remains the #1 killer of men and women. In the United States CVD leads to one in every three deaths – one death approximately every 38 seconds – with annual treatment cost in excess of $500 billion.5,6
Multiple primary and secondary prevention trials have shown a significant reduction of 25% to 35% in the risk of cardiovascular events with statin therapy, leaving significant persistent residual risk despite the achievement of target LDL-C levels.7
Beyond the cardiovascular risk associated with LDL-C, genetic, epidemiologic, clinical and real-world data suggest that patients with elevated triglycerides (TG) (fats in the blood), and TG-rich lipoproteins, are at increased risk for cardiovascular disease.8,9,10,11
About Vascepa® (icosapent ethyl) Capsules
Vascepa (icosapent ethyl) capsules are a single-molecule prescription product consisting of the omega-3 acid commonly known as EPA in ethyl-ester form. Vascepa is not fish oil, but is derived from fish through a stringent and complex FDA-regulated manufacturing process designed to effectively eliminate impurities and isolate and protect the single molecule active ingredient from degradation. Vascepa, known in scientific literature as AMR101, has been designated a new chemical entity by the FDA. Amarin has been issued multiple patents internationally based on the unique clinical profile of Vascepa, including the drug’s ability to lower triglyceride levels in relevant patient populations without raising LDL-cholesterol levels.
The FDA has not completed review and opined on a supplemental new drug application related to REDUCE IT. FDA has not reviewed the information herein or determined whether to approve Vascepa for use to reduce the risk of major adverse cardiovascular events in the REDUCE-IT patient population.
Indication and Usage Based on Current FDA-Approved Label (not including REDUCE-IT results)
Vascepa (icosapent ethyl) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
The effect of Vascepa on the risk for pancreatitis and cardiovascular mortality and morbidity in patients with severe hypertriglyceridemia has not been determined.
Important Safety Information for Vascepa Based on Current FDA-Approved Label (not including REDUCE-IT results) (Includes Data from Two 12-Week Studies (n=622) (MARINE and ANCHOR) of Patients with Triglycerides Values of 200 to 2000 mg/dL)
Vascepa is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vascepa or any of its components.
In patients with hepatic impairment, monitor ALT and AST levels periodically during therapy.
Use with caution in patients with known hypersensitivity to fish and/or shellfish.
The most common reported adverse reaction (incidence >2% and greater than placebo) was arthralgia (2.3% for Vascepa, 1.0% for placebo). There was no reported adverse reaction >3% and greater than placebo.
Adverse events and product complaints may be reported by calling 1-855-VASCEPA or the FDA at 1-800-FDA-1088. Patients receiving treatment with Vascepa and other drugs affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
Patients should be advised to swallow Vascepa capsules whole; not to break open, crush, dissolve, or chew Vascepa.
FULL VASCEPA PRESCRIBING INFORMATION CAN BE FOUND AT WWW.VASCEPA.COM.
Important Safety Information for Vascepa based on REDUCE-IT, as previously reported in The New England Journal of Medicine publication of the primary results of the REDUCE-IT study:
Excluding the major adverse cardiovascular events (MACE) results described above, overall adverse event rates in REDUCE-IT were similar across the statin plus Vascepa and the statin plus placebo treatment groups.
There were no significant differences between treatments in the overall rate of treatment emergent adverse events or serious adverse events leading to withdrawal of study drug.
There was no serious adverse event (SAE) occurring at a frequency of >2% which occurred at a numerically higher rate in the statin plus Vascepa treatment group than in the statin plus placebo treatment group.
Adverse events (AEs) occurring in 5% or greater of patients and more frequently with Vascepa than placebo were:
- peripheral edema (6.5% Vascepa patients versus 5.0% placebo patients), although there was no increase in the rate of heart failure in Vascepa patients
- constipation (5.4% Vascepa patients versus 3.6% placebo patients), although mineral oil, as used as placebo, is known to lower constipation, and
- atrial fibrillation (5.3% Vascepa patients versus 3.9% placebo patients), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients
There were numerically more SAEs related to bleeding in the statin plus Vascepa treatment group although overall rates were low with no fatal bleeding observed in either group and no significant difference in adjudicated hemorrhagic stroke or serious central nervous system or gastrointestinal bleeding events between treatments.
In summary, Vascepa was well tolerated with a safety profile generally consistent with clinical experience associated with omega-3 fatty acids and current FDA-approved labeling of such products.
Important Cautionary Information About These Data
Further REDUCE-IT data assessment and data release could yield additional useful information to inform greater understanding of the trial outcome. For example, detailed data assessment by regulatory authorities, such as the FDA and Health Canada, will continue and take months to complete and announce. The FDA advisory committee process and the final evaluation by regulatory authorities of the totality of efficacy and safety data from REDUCE-IT may include some or all of the following, as well as other considerations: new information or analyses affecting the degree of treatment benefit on studied endpoints; study conduct and data robustness, quality, integrity and consistency; additional safety data considerations and risk/benefit considerations; and consideration of REDUCE-IT results in the context of other clinical studies. Because regulatory reviews are typically fluid and not definitive interactions between sponsor and agency on individual elements of an application and related information, Amarin does not plan to update investors on ongoing communications with regulatory authorities. Amarin plans to announce the final outcome of such regulatory reviews when appropriate.
Forward-Looking Statements
This press release contains forward-looking statements, including statements regarding the use of Vascepa to potentially help millions of patients. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected herein include the following: uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals; the risk that data interpretations or other information from third parties, the regulatory review process, regulatory authorities and in connection with an advisory committee could be made public that are negative or may delay approval or limit Vascepa’s marketability; the risk that special protocol assessment (SPA) agreements with the FDA are not a guarantee that FDA will approve a product candidate; the risk associated with the FDA's rescinding the REDUCE-IT SPA agreement; the risk related to FDA advisory committee meetings; and the risk that the FDA may not complete its review of the REDUCE-IT sNDA within the timing expected. A further list and description of these risks, uncertainties and other risks associated with an investment in Amarin can be found in Amarin's filings with the U.S. Securities and Exchange Commission, including its most recent Quarterly Report on Form 10-Q. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Amarin undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
GlobeNewswire•October 17, 2019
DUBLIN, Ireland and BRIDGEWATER, N.J., Oct. 17, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, today announced that an independent organization that evaluates pricing of prescription drugs has issued its final report assessing the cost-effectiveness of Vascepa® (icosapent ethyl) capsules. The final assessment confirms the cost-effectiveness of Vascepa across all the non-profit organization’s analyses, including its most stringent criteria.1
The review was derived from results of the landmark phase 3 clinical trial REDUCE-IT®2, focusing on the clinical benefit-risk profile of Vascepa and its value as an additive therapy for cardiovascular disease. The conclusion from the report is that Vascepa easily meets “commonly cited thresholds for cost-effectiveness and therefore represent a high long-term value for money,” based on the organization’s value assessment framework.3

“We are proud to have priced Vascepa to be cost-effective, and we appreciate this positioning being recognized by third-parties,” said Craig Granowitz, M.D., Ph.D., chief medical officer, Amarin. “However, despite the report’s positive conclusion that Vascepa is cost-effective, we believe that it understates the true value of Vascepa. For example, the report’s base-case analyses reflect only the costs of heart attack, stroke and cardiovascular death and exclude other high costs associated with other cardiovascular events demonstrated to be lowered by Vascepa in the REDUCE-IT cardiovascular outcomes study (e.g., revascularization procedures and hospitalization for unstable angina) as well as lower rates of recurring cardiovascular events in patients treated with Vascepa during the study.”
Another Cost-Effectiveness Analysis of Vascepa to Be Presented at AHA
A separate independent, academic cost-effectiveness analysis of Vascepa based on patient-specific data from the REDUCE-IT study will be presented at the American Heart Association Scientific Sessions 2019 on November 16 by William S. Weintraub, M.D., Director of Outcomes Research, MedStar Cardiovascular Research Network. Amarin provided an unrestricted grant for this academically driven analysis, titled “Cost-Effectiveness of Icosapent Ethyl in REDUCE-IT.”
Cardiovascular Disease, an Urgent Public Health Issue
“There is no doubt that cardiovascular disease is an urgent and growing public health issue, with more than half of U.S. adults impacted,” Dr. Granowitz added. “The U.S. spends approximately $555 billion on cardiovascular disease each year, and it is projected that these direct and indirect costs could increase to more than $1.1 trillion by 2035.4 Amarin has worked for over a decade to develop and test Vascepa to create a new preventative care solution that will potentially help many of our family, friends and neighbors within this population. Our goal is to make Vascepa accessible to as many patients as possible who can benefit.”
Vascepa is currently approved in the United States as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. Amarin has submitted a supplemental new drug application (sNDA) to the U.S. FDA for a label expansion based on the REDUCE-IT study results showing reduction of cardiovascular events in high-risk patients. The Prescription Drug User Fee Act (PDUFA) target action date set by the FDA to act on the sNDA is December 28, 2019. Assuming FDA approval, Vascepa is positioned to become the first drug indicated to reduce persistent residual cardiovascular risk in statin-managed patients with elevated triglycerides (135 mg/dL or greater) and other risk factors for cardiovascular disease. The approval of such label expansion and the wording of the indication statement is under regulatory review.
Today, many insurance plans, or payers, already broadly cover Vascepa. Upon assumed FDA label expansion, Amarin plans to use the results of the REDUCE-IT study, further objective health-economic analyses, and other medical information and data in negotiations with payers to seek expanded Vascepa insurance coverage.
About Amarin
Amarin Corporation plc. is a rapidly growing, innovative pharmaceutical company focused on developing therapeutics to improve cardiovascular health. Amarin’s product development program leverages its extensive experience in polyunsaturated fatty acids and lipid science. Vascepa (icosapent ethyl) is Amarin's first FDA-approved drug and is available by prescription in the United States, Lebanon and the United Arab Emirates. Amarin’s commercial partners are pursuing additional regulatory approvals for Vascepa in Canada, China and the Middle East. For more information about Amarin, visit www.amarincorp.com.
REDUCE-IT® Study
REDUCE-IT, an 8,179-patient cardiovascular outcomes study, was completed in 2018. REDUCE-IT was the first multinational cardiovascular outcomes study that evaluated the effect of prescription pure EPA therapy as an add-on to statins in patients with high cardiovascular risk who, despite stable statin therapy, had elevated triglyceride levels (at least 135 mg/dL). A large portion of the male and female patients enrolled in this outcomes study were diagnosed with type 2 diabetes.
More information on the REDUCE-IT study results can be found at www.amarincorp.com.
About Cardiovascular Disease
Worldwide, cardiovascular disease (CVD) remains the #1 killer of men and women. In the United States CVD leads to one in every three deaths – one death approximately every 38 seconds – with annual treatment cost in excess of $500 billion.5,6
Multiple primary and secondary prevention trials have shown a significant reduction of 25% to 35% in the risk of cardiovascular events with statin therapy, leaving significant persistent residual risk despite the achievement of target LDL-C levels.7
Beyond the cardiovascular risk associated with LDL-C, genetic, epidemiologic, clinical and real-world data suggest that patients with elevated triglycerides (TG) (fats in the blood), and TG-rich lipoproteins, are at increased risk for cardiovascular disease.8,9,10,11
About Vascepa® (icosapent ethyl) Capsules
Vascepa (icosapent ethyl) capsules are a single-molecule prescription product consisting of the omega-3 acid commonly known as EPA in ethyl-ester form. Vascepa is not fish oil, but is derived from fish through a stringent and complex FDA-regulated manufacturing process designed to effectively eliminate impurities and isolate and protect the single molecule active ingredient from degradation. Vascepa, known in scientific literature as AMR101, has been designated a new chemical entity by the FDA. Amarin has been issued multiple patents internationally based on the unique clinical profile of Vascepa, including the drug’s ability to lower triglyceride levels in relevant patient populations without raising LDL-cholesterol levels.
The FDA has not completed review and opined on a supplemental new drug application related to REDUCE IT. FDA has not reviewed the information herein or determined whether to approve Vascepa for use to reduce the risk of major adverse cardiovascular events in the REDUCE-IT patient population.
Indication and Usage Based on Current FDA-Approved Label (not including REDUCE-IT results)
Vascepa (icosapent ethyl) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
The effect of Vascepa on the risk for pancreatitis and cardiovascular mortality and morbidity in patients with severe hypertriglyceridemia has not been determined.
Important Safety Information for Vascepa Based on Current FDA-Approved Label (not including REDUCE-IT results) (Includes Data from Two 12-Week Studies (n=622) (MARINE and ANCHOR) of Patients with Triglycerides Values of 200 to 2000 mg/dL)
Vascepa is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vascepa or any of its components.
In patients with hepatic impairment, monitor ALT and AST levels periodically during therapy.
Use with caution in patients with known hypersensitivity to fish and/or shellfish.
The most common reported adverse reaction (incidence >2% and greater than placebo) was arthralgia (2.3% for Vascepa, 1.0% for placebo). There was no reported adverse reaction >3% and greater than placebo.
Adverse events and product complaints may be reported by calling 1-855-VASCEPA or the FDA at 1-800-FDA-1088. Patients receiving treatment with Vascepa and other drugs affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
Patients should be advised to swallow Vascepa capsules whole; not to break open, crush, dissolve, or chew Vascepa.
FULL VASCEPA PRESCRIBING INFORMATION CAN BE FOUND AT WWW.VASCEPA.COM.
Important Safety Information for Vascepa based on REDUCE-IT, as previously reported in The New England Journal of Medicine publication of the primary results of the REDUCE-IT study:
Excluding the major adverse cardiovascular events (MACE) results described above, overall adverse event rates in REDUCE-IT were similar across the statin plus Vascepa and the statin plus placebo treatment groups.
There were no significant differences between treatments in the overall rate of treatment emergent adverse events or serious adverse events leading to withdrawal of study drug.
There was no serious adverse event (SAE) occurring at a frequency of >2% which occurred at a numerically higher rate in the statin plus Vascepa treatment group than in the statin plus placebo treatment group.
Adverse events (AEs) occurring in 5% or greater of patients and more frequently with Vascepa than placebo were:
- peripheral edema (6.5% Vascepa patients versus 5.0% placebo patients), although there was no increase in the rate of heart failure in Vascepa patients
- constipation (5.4% Vascepa patients versus 3.6% placebo patients), although mineral oil, as used as placebo, is known to lower constipation, and
- atrial fibrillation (5.3% Vascepa patients versus 3.9% placebo patients), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients
There were numerically more SAEs related to bleeding in the statin plus Vascepa treatment group although overall rates were low with no fatal bleeding observed in either group and no significant difference in adjudicated hemorrhagic stroke or serious central nervous system or gastrointestinal bleeding events between treatments.
In summary, Vascepa was well tolerated with a safety profile generally consistent with clinical experience associated with omega-3 fatty acids and current FDA-approved labeling of such products.
Important Cautionary Information About These Data
Further REDUCE-IT data assessment and data release could yield additional useful information to inform greater understanding of the trial outcome. For example, detailed data assessment by regulatory authorities, such as the FDA and Health Canada, will continue and take months to complete and announce. The FDA advisory committee process and the final evaluation by regulatory authorities of the totality of efficacy and safety data from REDUCE-IT may include some or all of the following, as well as other considerations: new information or analyses affecting the degree of treatment benefit on studied endpoints; study conduct and data robustness, quality, integrity and consistency; additional safety data considerations and risk/benefit considerations; and consideration of REDUCE-IT results in the context of other clinical studies. Because regulatory reviews are typically fluid and not definitive interactions between sponsor and agency on individual elements of an application and related information, Amarin does not plan to update investors on ongoing communications with regulatory authorities. Amarin plans to announce the final outcome of such regulatory reviews when appropriate.
Forward-Looking Statements
This press release contains forward-looking statements, including statements regarding the use of Vascepa to potentially help millions of patients. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected herein include the following: uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals; the risk that data interpretations or other information from third parties, the regulatory review process, regulatory authorities and in connection with an advisory committee could be made public that are negative or may delay approval or limit Vascepa’s marketability; the risk that special protocol assessment (SPA) agreements with the FDA are not a guarantee that FDA will approve a product candidate; the risk associated with the FDA's rescinding the REDUCE-IT SPA agreement; the risk related to FDA advisory committee meetings; and the risk that the FDA may not complete its review of the REDUCE-IT sNDA within the timing expected. A further list and description of these risks, uncertainties and other risks associated with an investment in Amarin can be found in Amarin's filings with the U.S. Securities and Exchange Commission, including its most recent Quarterly Report on Form 10-Q. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Amarin undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Antwort auf Beitrag Nr.: 61.712.266 von Magnetfeldfredy am 17.10.19 15:57:36Cantor Fitzgerald analyst Louise Chen reiterated an Overweight rating and $35.00 price target on Amarin Corporation (NASDAQ: AMRN) after the company announced that an independent organization that evaluates pricing of prescription drugs, ICER, has issued its final report assessing the cost-effectiveness of Vascepa.
The final assessment confirms the cost-effectiveness of Vascepa across all the non-profit organization’s analyses, including its most stringent criteria, the analyst notes.
https://www.streetinsider.com/dr/news.php?id=16020750
The final assessment confirms the cost-effectiveness of Vascepa across all the non-profit organization’s analyses, including its most stringent criteria, the analyst notes.
https://www.streetinsider.com/dr/news.php?id=16020750
2 Stocks That Could Double Your Money Money20
These two biotech stocks are poised for big gains next year.
George Budwell
Oct 19, 2019 at 3:49PM
Buying growth stocks in an election year is generally ill-advised. During the last presidential election cycle in 2016, growth stocks, on balance, dramatically underperformed the broader markets, whereas value stocks as a whole produced market-beating returns on capital. Going one step further, some growth-oriented subsectors such as biotech were flat out taken to the woodshed over the course of 2016, thanks to the economic and regulatory uncertainties stirred up by the rancor of presidential politics.
The point is that investors arguably shouldn't initiate a new position in a growth-heavy equity in the midst of a politically charged market, without a well-defined major catalyst firmly etched into the calendar. This group of equities, after all, comes with the inherent disadvantage of having to swim against the current of presidential politics and all that comes with it.
A hand arranging wooden blocks with upward pointing arrows in stair-step fashion.
Image Source: Getty Images.
Fortunately, there are a select few growth plays that meet this rather high bar. The biotech stocks Amarin (NASDAQ:AMRN) and Heron Therapeutics (NASDAQ:HRTX) both have a strong chance of doubling in value in 2020, regardless of how the political landscape evolves in the meantime. Here's a brief rundown of the essential points investors need to consider about each of these promising growth plays.
Amarin: One last hurdle
Wall Street's current consensus 2020 price target for Amarin has its shares rising by an astounding 86% over the next 12 months. However, this stately price target might still be missing the mark by a country mile. Underscoring this point, investment banking firm H.C. Wainwright has significantly deviated from its peers on this equity by issuing a gob-smacking $51 price target on Amarin's shares. For those of you keeping score at home, Wainwright is basically calling for the biotech's shares to more than quadruple in value in 2020.
What's all the fuss about? Amarin is slated to do battle with the Food and Drug Administration's Endocrinologic and Metabolic Drugs Advisory Committee on Nov. 14. The question at hand is whether the company's prescription omega-3 treatment, Vascepa, is truly a quantum leap forward in the treatment of patients at risk of cardiovascular disease, despite being on statin therapy. The FDA is expected to hand down its final ruling on the matter by Dec. 28, according to a recent investor presentation.
While the FDA is unpredictable by its very nature, Vascepa seems to have an excellent shot at grabbing this high-dollar indication based on its unprecedented cardiovascular outcome trial results. The big deal is that lipid-lowering medications -- especially those aimed at treating underlying cardiovascular disease -- are some of the best-selling pharmaceutical products of all time. Pfizer's Lipitor, for instance, has racked up well over $100 billion in cumulative sales over its life cycle. Vascepa, for its part, may even surpass Lipitor from a total sales standpoint.
Stated bluntly, Amarin's shares will surely be off to the races in 2020 if Vascepa clears this last regulatory hurdle.
Heron: A small-cap stock with mid-cap potential
Every year there's a biotech stock that mounts a furious comeback. Heron Therapeutics is poised to be that stock in 2020.
The lowdown is that Heron's shares crumbled this year in response to the FDA rejecting its experimental pain medication HTX-011. On Oct. 1, Heron reported that it successfully refiled the drug's regulatory application with the FDA and that it is slated for a truncated six-month review cycle. In short, HTX-011 could be on the market by the third quarter of 2020.
Why should investors stand up and take notice? Although Heron's anti-nausea medications have started to show signs of life after a sluggish start, HTX-011 is the drug that should put the company on the map. Novel pain medications are worth their weight in gold because of their long commercial shelf lives and the well-documented need for non-opioid painkillers in the acute setting. At a minimum, HTX-011 should be able to generate several hundred million in sales per year and might even achieve blockbuster states late in the next decade.
Wall Street, in turn, expects Heron's shares to more than double in value over the next year, with some analysts even calling for the biotech's stock to rise by a jaw-dropping 306% from current levels. While naysayers may call these price targets unrealistic, the fact is that Heron will almost certainly turn into a buyout target if HTX-011 gets the green light from regulators next year. There is a huge demand for these types of drugs in the world of big pharma, and it's no easy feat to development one on your own. It's far easier to simply pay up to acquire one that's already on the market.
10 stocks we like better than Heron Therapeutics
When investing geniuses David and Tom Gardner have a stock tip, it can pay to listen. After all, the newsletter they have run for over a decade, Motley Fool Stock Advisor, has quadrupled the market.*
David and Tom just revealed what they believe are the ten best stocks for investors to buy right now… and Heron Therapeutics wasn't one of them! That's right -- they think these 10 stocks are even better buys.
These two biotech stocks are poised for big gains next year.
George Budwell
Oct 19, 2019 at 3:49PM
Buying growth stocks in an election year is generally ill-advised. During the last presidential election cycle in 2016, growth stocks, on balance, dramatically underperformed the broader markets, whereas value stocks as a whole produced market-beating returns on capital. Going one step further, some growth-oriented subsectors such as biotech were flat out taken to the woodshed over the course of 2016, thanks to the economic and regulatory uncertainties stirred up by the rancor of presidential politics.
The point is that investors arguably shouldn't initiate a new position in a growth-heavy equity in the midst of a politically charged market, without a well-defined major catalyst firmly etched into the calendar. This group of equities, after all, comes with the inherent disadvantage of having to swim against the current of presidential politics and all that comes with it.
A hand arranging wooden blocks with upward pointing arrows in stair-step fashion.
Image Source: Getty Images.
Fortunately, there are a select few growth plays that meet this rather high bar. The biotech stocks Amarin (NASDAQ:AMRN) and Heron Therapeutics (NASDAQ:HRTX) both have a strong chance of doubling in value in 2020, regardless of how the political landscape evolves in the meantime. Here's a brief rundown of the essential points investors need to consider about each of these promising growth plays.
Amarin: One last hurdle
Wall Street's current consensus 2020 price target for Amarin has its shares rising by an astounding 86% over the next 12 months. However, this stately price target might still be missing the mark by a country mile. Underscoring this point, investment banking firm H.C. Wainwright has significantly deviated from its peers on this equity by issuing a gob-smacking $51 price target on Amarin's shares. For those of you keeping score at home, Wainwright is basically calling for the biotech's shares to more than quadruple in value in 2020.
What's all the fuss about? Amarin is slated to do battle with the Food and Drug Administration's Endocrinologic and Metabolic Drugs Advisory Committee on Nov. 14. The question at hand is whether the company's prescription omega-3 treatment, Vascepa, is truly a quantum leap forward in the treatment of patients at risk of cardiovascular disease, despite being on statin therapy. The FDA is expected to hand down its final ruling on the matter by Dec. 28, according to a recent investor presentation.
While the FDA is unpredictable by its very nature, Vascepa seems to have an excellent shot at grabbing this high-dollar indication based on its unprecedented cardiovascular outcome trial results. The big deal is that lipid-lowering medications -- especially those aimed at treating underlying cardiovascular disease -- are some of the best-selling pharmaceutical products of all time. Pfizer's Lipitor, for instance, has racked up well over $100 billion in cumulative sales over its life cycle. Vascepa, for its part, may even surpass Lipitor from a total sales standpoint.
Stated bluntly, Amarin's shares will surely be off to the races in 2020 if Vascepa clears this last regulatory hurdle.
Heron: A small-cap stock with mid-cap potential
Every year there's a biotech stock that mounts a furious comeback. Heron Therapeutics is poised to be that stock in 2020.
The lowdown is that Heron's shares crumbled this year in response to the FDA rejecting its experimental pain medication HTX-011. On Oct. 1, Heron reported that it successfully refiled the drug's regulatory application with the FDA and that it is slated for a truncated six-month review cycle. In short, HTX-011 could be on the market by the third quarter of 2020.
Why should investors stand up and take notice? Although Heron's anti-nausea medications have started to show signs of life after a sluggish start, HTX-011 is the drug that should put the company on the map. Novel pain medications are worth their weight in gold because of their long commercial shelf lives and the well-documented need for non-opioid painkillers in the acute setting. At a minimum, HTX-011 should be able to generate several hundred million in sales per year and might even achieve blockbuster states late in the next decade.
Wall Street, in turn, expects Heron's shares to more than double in value over the next year, with some analysts even calling for the biotech's stock to rise by a jaw-dropping 306% from current levels. While naysayers may call these price targets unrealistic, the fact is that Heron will almost certainly turn into a buyout target if HTX-011 gets the green light from regulators next year. There is a huge demand for these types of drugs in the world of big pharma, and it's no easy feat to development one on your own. It's far easier to simply pay up to acquire one that's already on the market.
10 stocks we like better than Heron Therapeutics
When investing geniuses David and Tom Gardner have a stock tip, it can pay to listen. After all, the newsletter they have run for over a decade, Motley Fool Stock Advisor, has quadrupled the market.*
David and Tom just revealed what they believe are the ten best stocks for investors to buy right now… and Heron Therapeutics wasn't one of them! That's right -- they think these 10 stocks are even better buys.
Antwort auf Beitrag Nr.: 61.570.521 von Magnetfeldfredy am 26.09.19 07:44:03

Zitat von Magnetfeldfredy: Große Hoffnung für diesen Late Braker:

In den USA Foren erscheinen immer wieder sehr positive Experten-Kommentare zu AMRN, VASCEPA und zu der REDUCE IT Studie.
Hier mal einige aktuelle Links:
ACC says Vascepa & not fish oil delays ASCVD
Oct 21, 2019
Lipid Management for Prevention of ASCVD
Melvyn Rubenfire, MD, FACC
Die kürzlich durchgeführte REDUCE-IT-Studie hat gezeigt, dass Personen mit hohem Risiko für ASCVD oder Diabetes mit einem zusätzlichen vaskulären Risikofaktor und einem Triglyceridspiegel von 135-500 mg / dl mit gut kontrolliertem LDL-C auf einem Statin, das 4 g Icosapent Ethyl erhielt, 4 g Icosapent erhielten hatten eine 25% ige Reduktion der wichtigsten CV-Ereignisse, die nichts mit der Wirkung auf Triglyceride zu tun hatten.
Icosapent Ethyl ist eine geschützte und hochgereinigte Form von EPA. Die Ergebnisse der REDUCE-IT-Studie sollten nicht verallgemeinert werden, um ein Nahrungsergänzungsmittel mit Fischöl zu empfehlen.
Es wird angenommen, dass der Wert von icosapent Ethyl mit anderen Mechanismen zusammenhängt, einschließlich entzündungshemmender und antithrombotischer Wirkstoff
https://www.investorvillage.com/smbd.asp?mb=2294&mn=5215&pt=…
Comment on the Food and Drug Administration (FDA) Notice:
Oct 21, 2019
Endocrinologic and Metabolic Drugs Advisory Committee; Notice of Meeting; Establishment of a Public Docket; Request for Comments
John R. Nelson MD,FACC,FNLA,FASNC
Director,California Cardiovascular Institute
Zusammenfassend lässt sich festhalten, dass die Hinzufügung des Etiketts von Vascepa zur Verringerung von kardiovaskulären Ereignissen, einschließlich des Todes durch CV bei Patienten mit CVD oder DM mit einem oder mehreren Risikofaktoren für eine Statintherapie mit Tg 135-499, Leben retten und die CV-Morbidität verringern sowie die Kosten senken wird.
Wie ich eingangs sagte, ist dies ein wahrer historischer Moment im Bereich der Reduzierung / Prävention von kardiovaskulären Risiken, und ich bedanke mich bei der FDA für all ihre Bemühungen und dafür, dass sie mir die Gelegenheit gibt, zu einem so wichtigen Thema Stellung zu beziehen.
https://www.regulations.gov/document?D=FDA-2019-N-3936-0030
Hier mal einige aktuelle Links:
ACC says Vascepa & not fish oil delays ASCVD
Oct 21, 2019
Lipid Management for Prevention of ASCVD
Melvyn Rubenfire, MD, FACC
Die kürzlich durchgeführte REDUCE-IT-Studie hat gezeigt, dass Personen mit hohem Risiko für ASCVD oder Diabetes mit einem zusätzlichen vaskulären Risikofaktor und einem Triglyceridspiegel von 135-500 mg / dl mit gut kontrolliertem LDL-C auf einem Statin, das 4 g Icosapent Ethyl erhielt, 4 g Icosapent erhielten hatten eine 25% ige Reduktion der wichtigsten CV-Ereignisse, die nichts mit der Wirkung auf Triglyceride zu tun hatten.
Icosapent Ethyl ist eine geschützte und hochgereinigte Form von EPA. Die Ergebnisse der REDUCE-IT-Studie sollten nicht verallgemeinert werden, um ein Nahrungsergänzungsmittel mit Fischöl zu empfehlen.
Es wird angenommen, dass der Wert von icosapent Ethyl mit anderen Mechanismen zusammenhängt, einschließlich entzündungshemmender und antithrombotischer Wirkstoff
https://www.investorvillage.com/smbd.asp?mb=2294&mn=5215&pt=…
Comment on the Food and Drug Administration (FDA) Notice:
Oct 21, 2019
Endocrinologic and Metabolic Drugs Advisory Committee; Notice of Meeting; Establishment of a Public Docket; Request for Comments
John R. Nelson MD,FACC,FNLA,FASNC
Director,California Cardiovascular Institute
Zusammenfassend lässt sich festhalten, dass die Hinzufügung des Etiketts von Vascepa zur Verringerung von kardiovaskulären Ereignissen, einschließlich des Todes durch CV bei Patienten mit CVD oder DM mit einem oder mehreren Risikofaktoren für eine Statintherapie mit Tg 135-499, Leben retten und die CV-Morbidität verringern sowie die Kosten senken wird.
Wie ich eingangs sagte, ist dies ein wahrer historischer Moment im Bereich der Reduzierung / Prävention von kardiovaskulären Risiken, und ich bedanke mich bei der FDA für all ihre Bemühungen und dafür, dass sie mir die Gelegenheit gibt, zu einem so wichtigen Thema Stellung zu beziehen.
https://www.regulations.gov/document?D=FDA-2019-N-3936-0030
Einige Bilder, die den Effekt seit Reduce-it nochmal schön illustrieren.








Good news vom Patentstreit:
Roth reiterates $31 target.
Comments on patent litigation:
AMRN scores another win in its battle against Vascepa imitators. In its ongoing litigation with potential generic competitor drugs to Vascepa (named in the court docket as West-Ward Pharma, Hikma Pharma, and Dr. Reddy's Labs), yesterday's news brought a summary judgment that we see as ruling favorably for AMRN. Let's break down the litigation update piece-by-piece: 1) First, remember that AMRN (plaintiffs) and the generic drug developers (defendants) are engaged in a court dispute over ANDA patent litigation and what the generic developers are allowed IP-wise; 2) In this situation, the generic developers typically will try to get an early end to the litigation, known as a summary judgment, to limit the scope of the case and bring an early end to the trial; 3) However, in this update the Court ruled against the ANDA filers' (generics) summary judgment, and narrowed several potential lines of generic argument from the case, thus benefiting AMRN; and 4) As discussed in a press release on its website (see here), the ruling also brings the possibility of ANDA case settlement with generics, should the option be in AMRN's best interest. We see this litigation news, which limits the scope of IP the case will focus on, as reaffirming the robust patent protection (100+) for Vascepa and confirming the strength of its Orange Book patents, which don't expire until 2030. In our view, AMRN is set up to achieve a dominant market position as a CV-risk reducing drug for the long-term vs. the horde of generic EPA drug developers.
Roth reiterates $31 target.
Comments on patent litigation:
AMRN scores another win in its battle against Vascepa imitators. In its ongoing litigation with potential generic competitor drugs to Vascepa (named in the court docket as West-Ward Pharma, Hikma Pharma, and Dr. Reddy's Labs), yesterday's news brought a summary judgment that we see as ruling favorably for AMRN. Let's break down the litigation update piece-by-piece: 1) First, remember that AMRN (plaintiffs) and the generic drug developers (defendants) are engaged in a court dispute over ANDA patent litigation and what the generic developers are allowed IP-wise; 2) In this situation, the generic developers typically will try to get an early end to the litigation, known as a summary judgment, to limit the scope of the case and bring an early end to the trial; 3) However, in this update the Court ruled against the ANDA filers' (generics) summary judgment, and narrowed several potential lines of generic argument from the case, thus benefiting AMRN; and 4) As discussed in a press release on its website (see here), the ruling also brings the possibility of ANDA case settlement with generics, should the option be in AMRN's best interest. We see this litigation news, which limits the scope of IP the case will focus on, as reaffirming the robust patent protection (100+) for Vascepa and confirming the strength of its Orange Book patents, which don't expire until 2030. In our view, AMRN is set up to achieve a dominant market position as a CV-risk reducing drug for the long-term vs. the horde of generic EPA drug developers.
Cantor Fitzgerald Positive on Amarin (AMRN) As Generics Lose On Summary Judgement
October 29, 2019 3:04 PM EDT Send to a Friend
Cantor Fitzgerald analyst Louise Chen raised the price target on Amarin Corporation (NASDAQ: AMRN) to $35.00 while maintaining an Overweight ...
October 29, 2019 3:04 PM EDT Send to a Friend
Cantor Fitzgerald analyst Louise Chen raised the price target on Amarin Corporation (NASDAQ: AMRN) to $35.00 while maintaining an Overweight ...

Amarin to Report Third Quarter 2019 Results and Host Conference Call on November 5, 2019
GlobeNewswire•October 24, 2019
DUBLIN, Ireland and BRIDGEWATER, N.J., Oct. 24, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, today announced that it will host a conference call with members of Amarin senior management to discuss the company's third quarter 2019 financial results and provide an operational update on Tuesday, November 5, 2019 at 7:30 a.m. ET.
The conference call will follow the anticipated release of the company's financial results earlier that day.
https://finance.yahoo.com/news/amarin-report-third-quarter-2…" target="_blank" rel="nofollow ugc noopener">
https://finance.yahoo.com/news/amarin-report-third-quarter-2…
GlobeNewswire•October 24, 2019
DUBLIN, Ireland and BRIDGEWATER, N.J., Oct. 24, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, today announced that it will host a conference call with members of Amarin senior management to discuss the company's third quarter 2019 financial results and provide an operational update on Tuesday, November 5, 2019 at 7:30 a.m. ET.
The conference call will follow the anticipated release of the company's financial results earlier that day.
https://finance.yahoo.com/news/amarin-report-third-quarter-2…" target="_blank" rel="nofollow ugc noopener">
https://finance.yahoo.com/news/amarin-report-third-quarter-2…
Antwort auf Beitrag Nr.: 61.570.521 von Magnetfeldfredy am 26.09.19 07:44:03
Seven Data Presentations Relevant to Vascepa® (Icosapent Ethyl) Capsules and Persistent Cardiovascular Risk to be Presented at American Heart Association’s 2019 Scientific Sessions, November 16 – 18
Amarin to Webcast Discussion of Presented Data November 18, 4:30 - 5:30 p.m., Eastern Time
Upcoming Featured Presentations
Session FS.AOS.01 – Featured Science Population Science, November 16, 7:30 – 7:40 a.m., Eastern U.S. Time
“Cost-Effectiveness of Icosapent Ethyl in REDUCE-IT,” – presented by William S. Weintraub, M.D., MedStar Washington Hospital.
Session FS.MDP.05 – Follow-up of Landmark Trials, Moderated Science Poster, November 17, 4:20 – 4:25 p.m., Eastern U.S. Time
MDP479, “REDUCE-IT USA: Results from the 3,146 Patients Randomized in the United States,” – presented by Deepak L. Bhatt, M.D., M.P.H., Brigham and Women’s Hospital and Harvard Medical School.
Session LBS.06 – Late Breaking Science VI: New Frontiers in Lipid Therapy, November 18, 9:00 – 9:10 a.m., Eastern U.S. Time
“Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis in Patients with Elevated Triglycerides (200 – 499 mg/dL) on Statin Therapy (EVAPORATE study),” – presented by Matthew J. Budoff, M.D., Los Angeles Biomedical Research.
Other Upcoming Data Presentations
Presentation Sa1056,
“Purified Eicosapentaenoic Acid Ameliorates Cardiac Fibrosis and Tissue Inflammation on Spontaneously Hypertensive Rats,” – Giselle C. Meléndez, M.D., Danielle Medina-Hernandez, Adam Pflum, M.D., Vivian Xu, David M. Herrington, M.D., presented Saturday, November 16., 1:30 – 2 p.m., Eastern U.S. Time.
Presentation Su3237,
“Improving Appropriate Use Of Omega-3 Fatty Acids In Primary Care: Success Of Online CME,” – Jelena Spyropoulos, Medscape Education, New York, NY; George Boutsalis, David Anderson – presented Sunday, November 17, 12:30 – 1 p.m., Eastern U.S. Time.
Presentation Mo1016,
“Influence of Eicosapentaenoic and Arachidonic Acid on Cholesterol Crystal Formation in Membranes under Hyperglycemic Conditions,” – Samuel C.R. Sherratt, B.S., R. Preston Mason, Ph.D. -- presented Monday, November 18, 11:30 a.m. – noon, Eastern U.S. Time.
Moderated Poster Presentation MDP414,
“Many Statin Treated Persons with Borderline Triglyceride Levels are at Risk of ASCVD,” – Nathan D. Wong, Wenjun Fan, Sephy Philip, Peter P. Toth, and Craig Granowitz – presented Monday, November 18, 1:20 -1:25 p.m., Eastern U.S. Time.
http://www.globenewswire.com/news-release/2019/11/04/1940073…
Zitat von Magnetfeldfredy: Große Hoffnung für diesen Late Braker:
Seven Data Presentations Relevant to Vascepa® (Icosapent Ethyl) Capsules and Persistent Cardiovascular Risk to be Presented at American Heart Association’s 2019 Scientific Sessions, November 16 – 18
Amarin to Webcast Discussion of Presented Data November 18, 4:30 - 5:30 p.m., Eastern Time
Upcoming Featured Presentations
Session FS.AOS.01 – Featured Science Population Science, November 16, 7:30 – 7:40 a.m., Eastern U.S. Time
“Cost-Effectiveness of Icosapent Ethyl in REDUCE-IT,” – presented by William S. Weintraub, M.D., MedStar Washington Hospital.
Session FS.MDP.05 – Follow-up of Landmark Trials, Moderated Science Poster, November 17, 4:20 – 4:25 p.m., Eastern U.S. Time
MDP479, “REDUCE-IT USA: Results from the 3,146 Patients Randomized in the United States,” – presented by Deepak L. Bhatt, M.D., M.P.H., Brigham and Women’s Hospital and Harvard Medical School.
Session LBS.06 – Late Breaking Science VI: New Frontiers in Lipid Therapy, November 18, 9:00 – 9:10 a.m., Eastern U.S. Time
“Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis in Patients with Elevated Triglycerides (200 – 499 mg/dL) on Statin Therapy (EVAPORATE study),” – presented by Matthew J. Budoff, M.D., Los Angeles Biomedical Research.
Other Upcoming Data Presentations
Presentation Sa1056,
“Purified Eicosapentaenoic Acid Ameliorates Cardiac Fibrosis and Tissue Inflammation on Spontaneously Hypertensive Rats,” – Giselle C. Meléndez, M.D., Danielle Medina-Hernandez, Adam Pflum, M.D., Vivian Xu, David M. Herrington, M.D., presented Saturday, November 16., 1:30 – 2 p.m., Eastern U.S. Time.
Presentation Su3237,
“Improving Appropriate Use Of Omega-3 Fatty Acids In Primary Care: Success Of Online CME,” – Jelena Spyropoulos, Medscape Education, New York, NY; George Boutsalis, David Anderson – presented Sunday, November 17, 12:30 – 1 p.m., Eastern U.S. Time.
Presentation Mo1016,
“Influence of Eicosapentaenoic and Arachidonic Acid on Cholesterol Crystal Formation in Membranes under Hyperglycemic Conditions,” – Samuel C.R. Sherratt, B.S., R. Preston Mason, Ph.D. -- presented Monday, November 18, 11:30 a.m. – noon, Eastern U.S. Time.
Moderated Poster Presentation MDP414,
“Many Statin Treated Persons with Borderline Triglyceride Levels are at Risk of ASCVD,” – Nathan D. Wong, Wenjun Fan, Sephy Philip, Peter P. Toth, and Craig Granowitz – presented Monday, November 18, 1:20 -1:25 p.m., Eastern U.S. Time.
http://www.globenewswire.com/news-release/2019/11/04/1940073…
Antwort auf Beitrag Nr.: 61.830.312 von bernie55 am 04.11.19 12:31:27Moin,
endlich die 17 USD geknackt😀
und ein ganz herzliches dankeschön an alle hier. Ohne euch wäre ich ich nicht darüber gestolpert.
endlich die 17 USD geknackt😀
und ein ganz herzliches dankeschön an alle hier. Ohne euch wäre ich ich nicht darüber gestolpert.
Amarin EPS beats by $0.05, beats on revenue
Nov. 5, 2019 5:03 AM ET|About: Amarin Corporation plc (AMRN)|By: Gaurav Batavia, SA News Editor
Amarin (NASDAQ:AMRN): Q3 Non-GAAP EPS of $0.01 beats by $0.05; GAAP EPS of -$0.01 beats by $0.03.
Revenue of $112.4M (+103.3% Y/Y) beats by $2.36M.
Shares +0.6% PM.
Press Release
Nov. 5, 2019 5:03 AM ET|About: Amarin Corporation plc (AMRN)|By: Gaurav Batavia, SA News Editor
Amarin (NASDAQ:AMRN): Q3 Non-GAAP EPS of $0.01 beats by $0.05; GAAP EPS of -$0.01 beats by $0.03.
Revenue of $112.4M (+103.3% Y/Y) beats by $2.36M.
Shares +0.6% PM.
Press Release
Antwort auf Beitrag Nr.: 61.839.303 von Magnetfeldfredy am 05.11.19 11:33:48Die Q3 / 19 -Zahlen lesen sich doch mehr als gut. 
Apropos Zahlen -
hier eine wirklich sehr gute Übersicht von Sam81 aus dem stocktwits Forum ( Q.zahlen 19 im Vergleich zu den Q.zahlen 18 )


Apropos Zahlen -
hier eine wirklich sehr gute Übersicht von Sam81 aus dem stocktwits Forum ( Q.zahlen 19 im Vergleich zu den Q.zahlen 18 )
Der Kurs fällt von vorbörslich US Dollar 18 auf fast US Dollar 16, höchst manipuliert!
Das wird noch hochvolatil beim Erhalt der Adcom Fragen am 12.11.2019, evtl. werde ich stark nachkaufen!
Das wird noch hochvolatil beim Erhalt der Adcom Fragen am 12.11.2019, evtl. werde ich stark nachkaufen!
Goldman Sachs, die DrecKsshorter, haben die Aktie unter Kontrolle seitdem sie das lächerliche KZ US Dollar 17 ausgegeben haben, ich hoffe sie fallen gewaltig auf Ihre Schnauze und verlieren hunderte Millionen, die sie den Amarin Aktionären abknüpfen!
Draft für die Adcom Fragen nicht dramatisch wie es scheint:
Amarin is ready....
They got an advanced copy of the FDA briefing book, folks!
Here is Amarin's assessment on the FDA's perspective on the mineral oil...
Page 39-40 in the Q32019 10Q
https://investor.amarincorp.com/node/18261/html
"Based on our current understanding of FDA’s focus and perspectives due in part to our review of an ADVANCED COPY OF A DRAFT of FDA’s EMDAC briefing document received from FDA at the end of October 2019 as part of the agency’s typical procedures, we expect the final FDA EMDAC briefing document and the EMDAC meeting to cover the following topics, among others, related to our REDUCE-IT sNDA, which topics are among the broad range of topics typically explored at FDA advisory committee meetings and cover matters consistent with prior disclosures and available data:
* analyses related to study conduct and data robustness, quality, integrity and consistency; for example, as previously disclosed, REDUCE-IT was a single clinical trial conducted based on a special protocol assessment (SPA) agreement with the FDA and, as is typical for all CV outcomes studies, related analyses are expected on the overall strength and limitations of study data to support the indications sought, as described above, considering factors including, but not limited to, the use of mineral oil placebo used in the study and questions explored in past FDA reviews and raised publicly since then over the plausibility of an interaction of the placebo with statins that some have argued could have led to reduced absorption of statin medications, which we do not believe occurred for reasons previously disclosed, among others to be presented in our briefing document; but, consistent with the publication of REDUCE-IT results in The New England Journal of Medicine, we believe FDA has assessed that even if plausible based on indirect evidence of a potential effect, that an exploratory analysis indicates the effect, if any, of mineral oil on LDL cholesterol values on the time to the primary endpoint is NUMERICALLY SMALL and UNLIKELY TO CHANGE the overall conclusion of treatment benefit"
--------
Now the bearish case has no more merit! haha
I'm even more confident than ever.
Amarin is ready....
They got an advanced copy of the FDA briefing book, folks!
Here is Amarin's assessment on the FDA's perspective on the mineral oil...
Page 39-40 in the Q32019 10Q
https://investor.amarincorp.com/node/18261/html
"Based on our current understanding of FDA’s focus and perspectives due in part to our review of an ADVANCED COPY OF A DRAFT of FDA’s EMDAC briefing document received from FDA at the end of October 2019 as part of the agency’s typical procedures, we expect the final FDA EMDAC briefing document and the EMDAC meeting to cover the following topics, among others, related to our REDUCE-IT sNDA, which topics are among the broad range of topics typically explored at FDA advisory committee meetings and cover matters consistent with prior disclosures and available data:
* analyses related to study conduct and data robustness, quality, integrity and consistency; for example, as previously disclosed, REDUCE-IT was a single clinical trial conducted based on a special protocol assessment (SPA) agreement with the FDA and, as is typical for all CV outcomes studies, related analyses are expected on the overall strength and limitations of study data to support the indications sought, as described above, considering factors including, but not limited to, the use of mineral oil placebo used in the study and questions explored in past FDA reviews and raised publicly since then over the plausibility of an interaction of the placebo with statins that some have argued could have led to reduced absorption of statin medications, which we do not believe occurred for reasons previously disclosed, among others to be presented in our briefing document; but, consistent with the publication of REDUCE-IT results in The New England Journal of Medicine, we believe FDA has assessed that even if plausible based on indirect evidence of a potential effect, that an exploratory analysis indicates the effect, if any, of mineral oil on LDL cholesterol values on the time to the primary endpoint is NUMERICALLY SMALL and UNLIKELY TO CHANGE the overall conclusion of treatment benefit"
--------
Now the bearish case has no more merit! haha
I'm even more confident than ever.
FUD Artikel:
Mann kann alles schlechreden bzw. schlechtrechnen, Fakt ist, Amarin hat alle primären Endpunkte souverän erreicht und ein Placebo ähnliches Nebenwirkungsprofil.
Die shorts versuchen alles, big pharma steckt auch dahinter, hoffentlich nicht auch noch die korrupte
FDA?
6 Reasons Investors Are Nervous About Amarin's Big Date With the FDA
A positive outcome from an upcoming FDA advisory committee meeting is far from guaranteed.
Cory Renauer
Cory Renauer
(TMFang4apples)
Nov 7, 2019 at 6:16AM
Author Bio
Follow @@coryrenauer
Shares of America's favorite fish-oil stock, Amarin (NASDAQ:AMRN), have risen sixfold since the company released surprisingly positive results from the Reduce-It study last September. In a nutshell, the company's only product, a fish-oil capsule called Vascepa, appears to lower the risk of heart attacks for high-risk patients.
On Nov. 14, the company's proprietary formulation of fish oil will be the subject of an advisory committee meeting at the Food and Drug Administration. Here are six reasons investors are increasingly worried about the outcome of that meeting.
Man looking at a laptop and biting his thumb
Image source: Getty Images.
1. There's a lot on the line
Vascepa is officially approved for the treatment of a fairly small group of people with dangerously high triglyceride levels. Year over year, third-quarter sales of the drug more than doubled to an annualized $449 million, but the company still lost $0.01 per share.
Amarin's recent market cap is up around $6 billion -- in anticipation of an enormous sales bump, which won't happen unless the FDA approves an application to expand Vascepa's drug label to include patients at risk of a heart attack and other major adverse cardiovascular events (MACE). The FDA doesn't have to follow advisory committee suggestions, but it usually does. If the panel of independent experts votes against approving Vascepa for high-risk patients, Amarin's shares could lose half their value overnight.
2. The great unveiling
Reduce-It was a long-term clinical trial with 8,179 high-risk patients on statin therapy. Patients who added Vascepa to their statin regimen were 25% less likely to experience a MACE than patients treated with a placebo. With these results, approval to treat an enormous group of high-risk patients might seem like a slam dunk. Just below the surface, though, there are several concerns that could delay Vascepa's label expansion indefinitely.
About a week ahead of advisory committee meetings, the FDA distributes briefing documents that highlight the agency's concerns in detail. Since communications between drugmakers and the agency are confidential, we only know what Amarin has chosen to disclose.
Amarin received an advance copy of the soon-to-be-unveiled briefing document in October. In an unusual act of transparency, the company described several topics of concern that are likely to be discussed. While there could be more troublesome issues the company forgot to mention, the ones Amarin disclosed are serious enough to make investors sweat.
A wooden balance beam with rolled-up cash on one side, and blocks reading RISK on the other
Image source: Getty Images.
3. Different populations
Around 70% of patients enrolled in Reduce-It had already suffered their first MACE; within this group, investigators found risk of a second MACE reduced by 27% among patients treated with Vascepa versus a placebo. Unfortunately, Vascepa led to a less exciting 12% relative risk reduction among the patients who hadn't experienced their first MACE yet.
Genau diese Patientengruppe sollte untersucht werden, so ein Depp!
The observed difference between the secondary prevention group and the primary prevention group wasn't statistically significant, but it has raised eyebrows. That's because Amarin's seeking approval for all high-risk patients on statin therapy, not just those who have already suffered through their first MACE.
4. Mineral oil
Randomized, placebo-controlled studies are the best way to provide evidence of efficacy, but creating a placebo that fools patients and healthcare professionals can be a real challenge. For Reduce-It, Amarin used capsules filled with mineral oil as a placebo, and in hindsight, it was a terrible idea.
Mineral oil is an old-fashioned laxative that affects the absorption of some drugs, including statins. This could explain why investigators reported slightly increased blood concentrations of low-density lipoprotein (LDL) cholesterol among patients in the placebo group, even though they were continuing their regular statin therapy.
Although the placebo group's LDL cholesterol increase was probably too small to account for the overall 25% risk reduction in the group treated with Vascepa, we can't be absolutely certain it didn't play an important role without another long outcome study that employs a different placebo.
5. Bleeding events
The mineral-oil dilemma is the biggest problem facing Amarin, but it's not the only issue slated for discussion. The advisory panel will also consider the fact that 11.8% of patients taking Vascepa in the Reduce-It study experienced bleeding events, compared to just 9.9% of the placebo group.
Slightly increased risk of bleeding associated with Vascepa and other fish-oil capsules isn't a new revelation. In light of questionable efficacy results, though, it could be significant.
Person with eyes closed and fingers crossed
Image source: Getty Images.
6. Irregular heartbeats
During Reduce-It, 3.1% of patients given Vascepa were hospitalized for an irregular heartbeat or atrial fibrillation, compared to 2.1% of the placebo group.
Fish-oil capsules, including Vascepa, have already been associated with an increased risk of hospitalization due to atrial fibrillation, but a 25% MACE risk reduction is well worth the slightly elevated risk. If the panel questions those efficacy results because of the mineral-oil placebo, though, the FDA could end up asking Amarin for more data.
The most likely scenario
Drug approvals are always made with the assumption that the observed benefits outweigh potential risks. The 12% relative risk reduction among people on statin therapy who haven't already experienced their first MACE is not a huge benefit, when measured against the increased risk of atrial fibrillation and bleeding events that come with long-term Vascepa treatment.
There's a chance that Vascepa will receive the giant indication Amarin has asked for, but more likely than not, the FDA will limit any approval to patients trying to prevent their second heart attack. That would hardly be the end of the world for Amarin, but it would almost certainly result in a severe market beatdown.
Er erwähnt weder 28 % weniger Schlaganfälle, über 30 % weniger Angina-Pectonis bedingte Krankenhausaufenthalte.......
Mann kann alles schlechreden bzw. schlechtrechnen, Fakt ist, Amarin hat alle primären Endpunkte souverän erreicht und ein Placebo ähnliches Nebenwirkungsprofil.
Die shorts versuchen alles, big pharma steckt auch dahinter, hoffentlich nicht auch noch die korrupte
FDA?
6 Reasons Investors Are Nervous About Amarin's Big Date With the FDA
A positive outcome from an upcoming FDA advisory committee meeting is far from guaranteed.
Cory Renauer
Cory Renauer
(TMFang4apples)
Nov 7, 2019 at 6:16AM
Author Bio
Follow @@coryrenauer
Shares of America's favorite fish-oil stock, Amarin (NASDAQ:AMRN), have risen sixfold since the company released surprisingly positive results from the Reduce-It study last September. In a nutshell, the company's only product, a fish-oil capsule called Vascepa, appears to lower the risk of heart attacks for high-risk patients.
On Nov. 14, the company's proprietary formulation of fish oil will be the subject of an advisory committee meeting at the Food and Drug Administration. Here are six reasons investors are increasingly worried about the outcome of that meeting.
Man looking at a laptop and biting his thumb
Image source: Getty Images.
1. There's a lot on the line
Vascepa is officially approved for the treatment of a fairly small group of people with dangerously high triglyceride levels. Year over year, third-quarter sales of the drug more than doubled to an annualized $449 million, but the company still lost $0.01 per share.
Amarin's recent market cap is up around $6 billion -- in anticipation of an enormous sales bump, which won't happen unless the FDA approves an application to expand Vascepa's drug label to include patients at risk of a heart attack and other major adverse cardiovascular events (MACE). The FDA doesn't have to follow advisory committee suggestions, but it usually does. If the panel of independent experts votes against approving Vascepa for high-risk patients, Amarin's shares could lose half their value overnight.
2. The great unveiling
Reduce-It was a long-term clinical trial with 8,179 high-risk patients on statin therapy. Patients who added Vascepa to their statin regimen were 25% less likely to experience a MACE than patients treated with a placebo. With these results, approval to treat an enormous group of high-risk patients might seem like a slam dunk. Just below the surface, though, there are several concerns that could delay Vascepa's label expansion indefinitely.
About a week ahead of advisory committee meetings, the FDA distributes briefing documents that highlight the agency's concerns in detail. Since communications between drugmakers and the agency are confidential, we only know what Amarin has chosen to disclose.
Amarin received an advance copy of the soon-to-be-unveiled briefing document in October. In an unusual act of transparency, the company described several topics of concern that are likely to be discussed. While there could be more troublesome issues the company forgot to mention, the ones Amarin disclosed are serious enough to make investors sweat.
A wooden balance beam with rolled-up cash on one side, and blocks reading RISK on the other
Image source: Getty Images.
3. Different populations
Around 70% of patients enrolled in Reduce-It had already suffered their first MACE; within this group, investigators found risk of a second MACE reduced by 27% among patients treated with Vascepa versus a placebo. Unfortunately, Vascepa led to a less exciting 12% relative risk reduction among the patients who hadn't experienced their first MACE yet.
Genau diese Patientengruppe sollte untersucht werden, so ein Depp!
The observed difference between the secondary prevention group and the primary prevention group wasn't statistically significant, but it has raised eyebrows. That's because Amarin's seeking approval for all high-risk patients on statin therapy, not just those who have already suffered through their first MACE.
4. Mineral oil
Randomized, placebo-controlled studies are the best way to provide evidence of efficacy, but creating a placebo that fools patients and healthcare professionals can be a real challenge. For Reduce-It, Amarin used capsules filled with mineral oil as a placebo, and in hindsight, it was a terrible idea.
Mineral oil is an old-fashioned laxative that affects the absorption of some drugs, including statins. This could explain why investigators reported slightly increased blood concentrations of low-density lipoprotein (LDL) cholesterol among patients in the placebo group, even though they were continuing their regular statin therapy.
Although the placebo group's LDL cholesterol increase was probably too small to account for the overall 25% risk reduction in the group treated with Vascepa, we can't be absolutely certain it didn't play an important role without another long outcome study that employs a different placebo.
5. Bleeding events
The mineral-oil dilemma is the biggest problem facing Amarin, but it's not the only issue slated for discussion. The advisory panel will also consider the fact that 11.8% of patients taking Vascepa in the Reduce-It study experienced bleeding events, compared to just 9.9% of the placebo group.
Slightly increased risk of bleeding associated with Vascepa and other fish-oil capsules isn't a new revelation. In light of questionable efficacy results, though, it could be significant.
Person with eyes closed and fingers crossed
Image source: Getty Images.
6. Irregular heartbeats
During Reduce-It, 3.1% of patients given Vascepa were hospitalized for an irregular heartbeat or atrial fibrillation, compared to 2.1% of the placebo group.
Fish-oil capsules, including Vascepa, have already been associated with an increased risk of hospitalization due to atrial fibrillation, but a 25% MACE risk reduction is well worth the slightly elevated risk. If the panel questions those efficacy results because of the mineral-oil placebo, though, the FDA could end up asking Amarin for more data.
The most likely scenario
Drug approvals are always made with the assumption that the observed benefits outweigh potential risks. The 12% relative risk reduction among people on statin therapy who haven't already experienced their first MACE is not a huge benefit, when measured against the increased risk of atrial fibrillation and bleeding events that come with long-term Vascepa treatment.
There's a chance that Vascepa will receive the giant indication Amarin has asked for, but more likely than not, the FDA will limit any approval to patients trying to prevent their second heart attack. That would hardly be the end of the world for Amarin, but it would almost certainly result in a severe market beatdown.
Er erwähnt weder 28 % weniger Schlaganfälle, über 30 % weniger Angina-Pectonis bedingte Krankenhausaufenthalte.......
Ganz zu Schweigen von 20 % weniger CVD Todesfälle, eine Schande so einseitig zu schreiben, FUD pur😡
REDUCE-IT® USA Results, in Prespecified Subgroup Analyses of Landmark REDUCE-IT Global Study, Showed Robust Cardiovascular Risk Reductions Across a Variety of Study Endpoints, Including Cardiovascular Death and All-Cause Mortality
Mon November 11, 2019 6:30 AM|GlobeNewswire
EPS of $-0.01 beats by $0.04 Revenue of $112.41M (103.18% Y/Y) beats by $2.36M
Prespecified Analysis of 3,146 Patients in the USA Enrolled in REDUCE-IT Showed 31% Relative Risk Reductions for First Occurrence of Both 5-Point MACE and 3-Point MACE
Significant Reductions Shown in All Predefined Composite and Individual Cardiovascular Endpoints, Including Cardiovascular Death and All-Cause Mortality
Tolerability and Safety Findings Consistent with Full Study
https://seekingalpha.com/pr/17693881-reduce-usa-results-pres…
Mon November 11, 2019 6:30 AM|GlobeNewswire
EPS of $-0.01 beats by $0.04 Revenue of $112.41M (103.18% Y/Y) beats by $2.36M
Prespecified Analysis of 3,146 Patients in the USA Enrolled in REDUCE-IT Showed 31% Relative Risk Reductions for First Occurrence of Both 5-Point MACE and 3-Point MACE
Significant Reductions Shown in All Predefined Composite and Individual Cardiovascular Endpoints, Including Cardiovascular Death and All-Cause Mortality
Tolerability and Safety Findings Consistent with Full Study
https://seekingalpha.com/pr/17693881-reduce-usa-results-pres…
Antwort auf Beitrag Nr.: 61.890.437 von bernie55 am 11.11.19 13:34:33Amarin to Present at the Jefferies 2019 London Healthcare Conference
DUBLIN, Ireland and BRIDGEWATER, N.J., Nov. 11, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health announced today that John F. Thero, Amarin's president and chief executive officer, is scheduled to present a general company update at the Jefferies 2019 London Healthcare Conference on Thursday, November 21, 2019, at 8:40 a.m. Greenwich Mean Time (GMT) / 3:40 a.m. Eastern Time (ET) in London, UK.
A live audio webcast of the presentation will be available at: http://www.amarincorp.com, and will be accessible at the same link for 30 days.
https://finance.yahoo.com/news/amarin-present-jefferies-2019…
DUBLIN, Ireland and BRIDGEWATER, N.J., Nov. 11, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health announced today that John F. Thero, Amarin's president and chief executive officer, is scheduled to present a general company update at the Jefferies 2019 London Healthcare Conference on Thursday, November 21, 2019, at 8:40 a.m. Greenwich Mean Time (GMT) / 3:40 a.m. Eastern Time (ET) in London, UK.
A live audio webcast of the presentation will be available at: http://www.amarincorp.com, and will be accessible at the same link for 30 days.
https://finance.yahoo.com/news/amarin-present-jefferies-2019…
Mal schauen was uns die korrupte FDA beschert............
Amarin: Matters Of The Heart
Nov. 12, 2019 5:30 AM ET
Summary
The recommendation as the standard of care by the American Diabetes Association and the American Heart Association is paying off in terms of strong sales growth.
The company is doubling its sales force from 400 to 800 professionals by early 2020. I believe this will aggressively ramp up sales in the next few quarters.
The biggest catalyst to catapult Amarin shares to a new high is the upcoming supplemental new drug application (sNDA) for Vascepa.
https://seekingalpha.com/article/4305633-amarin-matters-hear…
Nov. 12, 2019 5:30 AM ET
Summary
The recommendation as the standard of care by the American Diabetes Association and the American Heart Association is paying off in terms of strong sales growth.
The company is doubling its sales force from 400 to 800 professionals by early 2020. I believe this will aggressively ramp up sales in the next few quarters.
The biggest catalyst to catapult Amarin shares to a new high is the upcoming supplemental new drug application (sNDA) for Vascepa.
https://seekingalpha.com/article/4305633-amarin-matters-hear…
Antwort auf Beitrag Nr.: 61.890.437 von bernie55 am 11.11.19 13:34:33Deepak L. Bhatt, MD, MPH
Executive Director of Interventional Cardiovascular Programs
Professor, Harvard Medical School
Cardiovascular Medicine

Executive Director of Interventional Cardiovascular Programs
Professor, Harvard Medical School
Cardiovascular Medicine

ADVISORY COMMITTEE MEETING
Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee Meeting Announcement
Date:
November 14, 2019
Time:
08:00 AM - 05:00 PM EST
LINK Conference:
https://www.fda.gov/advisory-committees/november-14-2019-mee…
Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee Meeting Announcement
Date:
November 14, 2019
Time:
08:00 AM - 05:00 PM EST
LINK Conference:
https://www.fda.gov/advisory-committees/november-14-2019-mee…
Fragen von ADCOM sind raus
VOTE:
Has the applicant provided sufficient evidence of efficacy and safety to support the approval of Vascepa for an indication to reduce the risk of cardiovascular events?
a. If yes, provide your recommendations regarding the indicated population and components of the primary endpoint to include in labeling.
b. If no, provide your rationale and comment on what additional data would be needed to support approval.
https://www.fda.gov/advisory-committees/november-14-2019-mee…
VOTE:
Has the applicant provided sufficient evidence of efficacy and safety to support the approval of Vascepa for an indication to reduce the risk of cardiovascular events?
a. If yes, provide your recommendations regarding the indicated population and components of the primary endpoint to include in labeling.
b. If no, provide your rationale and comment on what additional data would be needed to support approval.
https://www.fda.gov/advisory-committees/november-14-2019-mee…
Antwort auf Beitrag Nr.: 61.900.814 von bernie55 am 12.11.19 14:09:49 Fragestellung sind doch sehr gut aus...
Fragestellung sind doch sehr gut aus...
 Fragestellung sind doch sehr gut aus...
Fragestellung sind doch sehr gut aus...
Antwort auf Beitrag Nr.: 61.900.829 von bernie55 am 12.11.19 14:12:00
...mehr als gut.
Zitat von bernie55:Fragestellung sieht
doch sehr gut aus...
...mehr als gut.

Antwort auf Beitrag Nr.: 61.900.847 von bernie55 am 12.11.19 14:14:19ja sehr gut🙂🙃🙂
jetzt nur noch bis Jahresende ein positiver Bescheid der FDA
jetzt nur noch bis Jahresende ein positiver Bescheid der FDA
Geiiiiiiiiiiiiiiiiiiiiiiiiiiiillllllllllllllllllllllllllllllllllllllllllllll!
Short squeeeeeeeeeeeeeeezzzzzzzzzzzzzzzzzzzzzeeeeeeeeeeeeeeee, 55 Millionen Drecksshorts müssen covern, Buyout jederzeit für 30-40 US Dollar und mehr möglich!


Short squeeeeeeeeeeeeeeezzzzzzzzzzzzzzzzzzzzzeeeeeeeeeeeeeeee, 55 Millionen Drecksshorts müssen covern, Buyout jederzeit für 30-40 US Dollar und mehr möglich!



Who the fuck ist Goldmann Sachs with US Dollar 17,00😋









Antwort auf Beitrag Nr.: 61.901.264 von bernie55 am 12.11.19 14:46:48

Antwort auf Beitrag Nr.: 61.901.663 von Magnetfeldfredy am 12.11.19 15:19:34Die von der FDA veröffentlichten AC-Dokumente deuten auf eine erweiterte Anwendung von Vascepa. Obwohl die FDA einige Mängel in den kardiovaskulären Studien festgestellt hat, scheint keines ernst genug zu sein, um eine erweiterte Zulassung zu verhindern. Diese Mängel werden am Donnerstag von einer externen Expertenrunde (Advisory Committee) diskutiert, die dann darüber abstimmen wird, ob eine Erweiterung der Vascepa-Anwendung empfohlen wird. Amarin (AMRN) beantragt die FDA-Zulassung für eine Erweiterung des Vascepa-Labels um Daten, die eine 25-prozentige Verringerung des Risikos für ernste kardiovaskuläre Ereignisse wie Herzinfarkte, Schlaganfälle mit Todesfolge belegen. Vascepa ist derzeit auf dem besten Weg, Amarin einen Jahresumsatz von rund 500 Millionen US-Dollar zu liefern. Wenn das Vascepa-Label um diese Indikation bei kardiovaskulären Risikofaktoren erweitert wird und das Medikament häufiger verschrieben wird, dürfte Blockbusterpotenzial erreicht werden.
Gratulation an alle "noch" investierten !
Antwort auf Beitrag Nr.: 61.906.793 von flyingbeef am 12.11.19 23:46:13Bin jetzt erst wieder im grünen seit 3 Monaten 🤷♀️
Wenn man diesen Wert in den letzten kontinuierlich verfolgt hat, entsprechend die Wichtigkeit des Medikaments erkannte, dann wird man belohnt. Der Umsatz wird für Amarin enorm sein, es wird sicherlich eine Menge in der Übernahmegerüchteküche brodeln. Ich habe vor wenigen Wochen einem Vortrag beiwohnen dürfen. Kurz angerissen ging es um die Entwicklung auf dem Pharmamarkt, wo sog. bahnbrechende Ereignisse anstehen. Amarin kaman diesem Abend hervorragend weg, so dass ich hier eine kurzfristige Verdoppelung fast als zu konservativ eingeschätzt betrachte. Das Jahresende wird fulminant. Fonds werden anspringen - notgedrungen!
Antwort auf Beitrag Nr.: 61.901.168 von Magnetfeldfredy am 12.11.19 14:37:42
Amarin Corporation (AMRN)
PT Raised to $51 at H.C. Wainwright; REDUCE-IT Update is a 'Home run'
H.C. Wainwright analyst Andrew Fein raised the price target on Amarin Corporation (NASDAQ: AMRN) to $51.00 (from $31.00) while maintaining a Buy rating.
https://www.streetinsider.com/Analyst+Comments/Amarin+Corpor…
Zitat von Magnetfeldfredy: Who the fuck ist Goldmann Sachs with US Dollar 17,00 USD
Amarin Corporation (AMRN)
PT Raised to $51 at H.C. Wainwright; REDUCE-IT Update is a 'Home run'
H.C. Wainwright analyst Andrew Fein raised the price target on Amarin Corporation (NASDAQ: AMRN) to $51.00 (from $31.00) while maintaining a Buy rating.
https://www.streetinsider.com/Analyst+Comments/Amarin+Corpor…
Antwort auf Beitrag Nr.: 61.912.040 von bernie55 am 13.11.19 14:35:35

Das ist auch mein persönliches Kursziel bei einer Übernahme!

Antwort auf Beitrag Nr.: 61.900.148 von bernie55 am 12.11.19 12:58:22
AMARIN shares halted ahead of FDA committee meeting for its only drug
Jaimy Lee - Nov.14 2019
https://finance.yahoo.com/m/c771f894-61f0-3661-b64f-9bd05498…
 Der Tag der ( Vor) Entscheidung - let's hope the BEST
Der Tag der ( Vor) Entscheidung - let's hope the BEST 
Zitat von bernie55: ADVISORY COMMITTEE MEETING
Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee Meeting Announcement
Date:
November 14, 2019
Time:
08:00 AM - 05:00 PM EST
LINK Conference:
https://www.fda.gov/advisory-committees/november-14-2019-mee…
AMARIN shares halted ahead of FDA committee meeting for its only drug
Jaimy Lee - Nov.14 2019
https://finance.yahoo.com/m/c771f894-61f0-3661-b64f-9bd05498…
 Der Tag der ( Vor) Entscheidung - let's hope the BEST
Der Tag der ( Vor) Entscheidung - let's hope the BEST 
Let the show begin........

Antwort auf Beitrag Nr.: 61.922.312 von Magnetfeldfredy am 14.11.19 14:15:33das liest sich doch schon mal sehr gut hinsichtlich Mineralölaktivität:
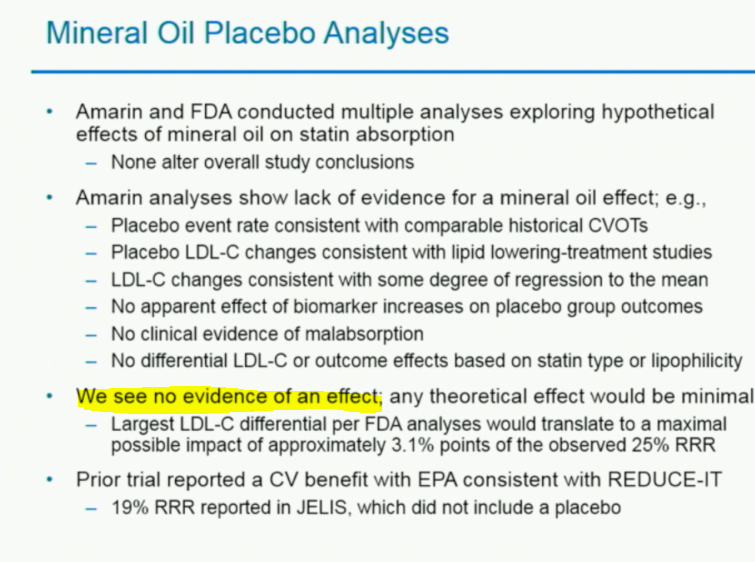



Dr Bhatt just finished and did an amazing job even for this layman. Everything was very positive. Only slight concerns were over increased bleeding which was not significAnt and a slight increase in AFib. He stated that both could be addressed within labeling It sounds like they took the FDA’s concerns or requests and have addressed each one with very positive results
Könnte ein home run werden?

Antwort auf Beitrag Nr.: 61.923.074 von Magnetfeldfredy am 14.11.19 15:36:55
HOPE THE BEST....
Zitat von Magnetfeldfredy: Könnte ein home run werden?
HOPE THE BEST....

Antwort auf Beitrag Nr.: 61.923.083 von bernie55 am 14.11.19 15:37:38Hi Bernie - sitzen wir wieder einmal im selben Boot?
Ich glaube nicht, dass wir absaufen!
wie immer: TIME WILL TELL!
direkt vom AC:

Ich glaube nicht, dass wir absaufen!
wie immer: TIME WILL TELL!
direkt vom AC:

@cyberhexe 
@magnetfeldfredy
@AMARIANS
Ich muss nun los - ich habe jetzt leider keine Zeit mehr, mit euch gemeinsam durch die Entscheidungsphase zu gehen.😢
Also, haltet die INFO-Stellung -
-
let`s hope together - for the best of best.
Bis dann
Grüße
bernie55

@magnetfeldfredy

@AMARIANS

Ich muss nun los - ich habe jetzt leider keine Zeit mehr, mit euch gemeinsam durch die Entscheidungsphase zu gehen.😢
Also, haltet die INFO-Stellung
 -
- let`s hope together - for the best of best.
Bis dann
Grüße
bernie55

Wenn
Wenn mich nicht alles täuscht ist der Kurs im Moment ausgesetzt... 😆
Hallo hier https://stocktwits.com/symbol/AMRN
in den Kommentaren werden auch die ganzen Folien gezeigt.
auf ein gutes Ergebnis😀
in den Kommentaren werden auch die ganzen Folien gezeigt.
auf ein gutes Ergebnis😀
Antwort auf Beitrag Nr.: 61.923.302 von esaposse am 14.11.19 15:55:10Marvin Konstam vom Tufts Medical Center stellte mehrere gezielte Fragen:
Konstam erkundigte sich nach Schlaganfällen, die durch Blutungen verursacht wurden und als Wirksamkeit und nicht als Sicherheitsendpunkt eingestuft wurden. Es gab 13 solcher Schlaganfälle in der Vascepa-Gruppe gegenüber zehn in der Placebo-Gruppe. Das Ergebnis war statistisch nicht unterschiedlich.
Dann übernahm Konstam den von Amarin vorgeschlagenen Risiko-Score, um Patienten mit geringerem Risiko für die Primärprävention auszuwählen. Konstam sagte, dass mit Statinen Risikorechner funktionieren, da der relative Nutzen unabhängig vom Ausgangsrisiko in etwa gleich ist. Unabhängig davon, welches Risiko für einen Herzinfarkt besteht, verringert ein Statin das Risiko um etwa den gleichen Betrag (etwa ein Drittel). Die von Amarin vorgelegten Daten scheinen jedoch zu belegen, dass der relative Nutzen bei Vascepa mit zunehmendem Grundrisiko zunimmt. Dies erschwere es, herauszufinden, bei welchen Patienten mit geringem Risiko das Medikament angewendet werden soll, argumentierte Konstam.
Konstam erkundigte sich nach Schlaganfällen, die durch Blutungen verursacht wurden und als Wirksamkeit und nicht als Sicherheitsendpunkt eingestuft wurden. Es gab 13 solcher Schlaganfälle in der Vascepa-Gruppe gegenüber zehn in der Placebo-Gruppe. Das Ergebnis war statistisch nicht unterschiedlich.
Dann übernahm Konstam den von Amarin vorgeschlagenen Risiko-Score, um Patienten mit geringerem Risiko für die Primärprävention auszuwählen. Konstam sagte, dass mit Statinen Risikorechner funktionieren, da der relative Nutzen unabhängig vom Ausgangsrisiko in etwa gleich ist. Unabhängig davon, welches Risiko für einen Herzinfarkt besteht, verringert ein Statin das Risiko um etwa den gleichen Betrag (etwa ein Drittel). Die von Amarin vorgelegten Daten scheinen jedoch zu belegen, dass der relative Nutzen bei Vascepa mit zunehmendem Grundrisiko zunimmt. Dies erschwere es, herauszufinden, bei welchen Patienten mit geringem Risiko das Medikament angewendet werden soll, argumentierte Konstam.
Antwort auf Beitrag Nr.: 61.924.259 von Cyberhexe am 14.11.19 17:18:44Die FDA hat viel Zeit darauf verwendet, das Risiko zu erörtern, dass das im Kontrollarm der REDUCE-IT-Studie verwendete Mineralöl die Absorption von Statinen beeinträchtigt und möglicherweise den kardiovaskulären Nutzen in Vascepas Richtung verschiebt.
In der Studie zeigten Patienten am Kontrollarm einen Anstieg des LDL (schlechten) Cholesterins, was darauf hindeutet, dass die Mineralölpillen, die sie täglich schlucken, möglicherweise die Statinabsorption beeinflusst haben.
Die FDA-Analyse ergab indirekte Beweise dafür, dass Mineralöl die Absorption von Statinen beeinträchtigen kann:
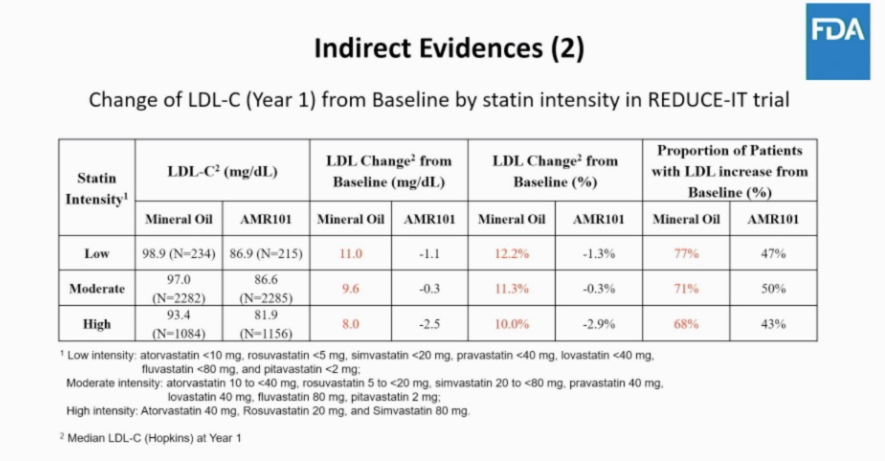
Die Auswirkungen auf den primären Endpunkt von REDUCE-IT waren jedoch gering und beeinträchtigten daher nicht den kardiovaskulären Gesamtnutzen von Vascepa, so die FDA-Gutachter:
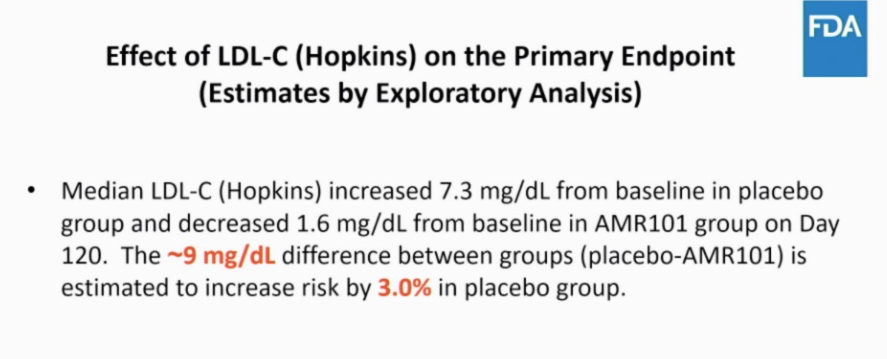
BINGO!
In der Studie zeigten Patienten am Kontrollarm einen Anstieg des LDL (schlechten) Cholesterins, was darauf hindeutet, dass die Mineralölpillen, die sie täglich schlucken, möglicherweise die Statinabsorption beeinflusst haben.
Die FDA-Analyse ergab indirekte Beweise dafür, dass Mineralöl die Absorption von Statinen beeinträchtigen kann:

Die Auswirkungen auf den primären Endpunkt von REDUCE-IT waren jedoch gering und beeinträchtigten daher nicht den kardiovaskulären Gesamtnutzen von Vascepa, so die FDA-Gutachter:

BINGO!
Antwort auf Beitrag Nr.: 61.924.856 von Cyberhexe am 14.11.19 17:58:38Danke für deine Upgrades hier👍🏻
das o.g. stimmt einen doch zuversichtlich
das o.g. stimmt einen doch zuversichtlich
Antwort auf Beitrag Nr.: 61.925.138 von lobberland am 14.11.19 18:19:19
sehr zuversichtlich - das dürfte ein ziemlich eindeutiges Votum geben!
Zitat von lobberland: Danke für deine Upgrades hier👍🏻
das o.g. stimmt einen doch zuversichtlich
sehr zuversichtlich - das dürfte ein ziemlich eindeutiges Votum geben!
Antwort auf Beitrag Nr.: 61.925.282 von Cyberhexe am 14.11.19 18:33:42allerdings gab es vor der Mittagspause, in der vorangegangenen Diskussion, auch einige Bedenken. Einige der Mitglieder des Gremiums shaben Kommentare abgegeben, die darauf hindeuten, die Anwendung von Vascepa auf eine engere Patientenpopulation als von Amarin gewünscht, zu beschränken.
Dr. Marvin Konstam vom Tufts Medical Center stellte FDA-Mitarbeitern weitere Fragen zum potenziellen Anstieg des kardiovaskulären Risikos im Zusammenhang mit einem Anstieg von Lipid-Biomarkern wie CRP und LDL-Cholesterin, insbesondere bei Patienten, bei denen sich diese Biomarker-Veränderungen möglicherweise überschneiden. Er stellte auch einen geringen Anstieg des Blutdrucks fest, der in der REDUCE-IT-Studie beobachtet wurde.
Als Reaktion darauf wiederholte John Sharretts, der stellvertretende Direktor der FDA-Abteilung, welche Vascepa geprüft hat, die Ansicht der Agentur, dass diese Veränderungen der Lipid-Biomarker, die einzeln bewertet wurden, den in der Vascepa-Studie berichteten Gesamtnutzen nicht beeinträchtigten. Er räumte jedoch auch ein, dass diese Schlussfolgerung umstritten sei, und erinnerte die Experten daran, dass sie heute zur Teilnahme aufgefordert wurden, gerade weil die FDA ihre Meinung einholen möchte.
Der Vorsitzende des Gremiums, Dr. Ken Burman von der Georgetown University, erklärte, dass eine klinische Studie zur Untersuchung des Einflusses von Mineralöl auf die Statinabsorption noch nie durchgeführt wurde. Eine solche Studie wäre relativ einfach und würde nicht so lange dauern.

Dr. Marvin Konstam vom Tufts Medical Center stellte FDA-Mitarbeitern weitere Fragen zum potenziellen Anstieg des kardiovaskulären Risikos im Zusammenhang mit einem Anstieg von Lipid-Biomarkern wie CRP und LDL-Cholesterin, insbesondere bei Patienten, bei denen sich diese Biomarker-Veränderungen möglicherweise überschneiden. Er stellte auch einen geringen Anstieg des Blutdrucks fest, der in der REDUCE-IT-Studie beobachtet wurde.
Als Reaktion darauf wiederholte John Sharretts, der stellvertretende Direktor der FDA-Abteilung, welche Vascepa geprüft hat, die Ansicht der Agentur, dass diese Veränderungen der Lipid-Biomarker, die einzeln bewertet wurden, den in der Vascepa-Studie berichteten Gesamtnutzen nicht beeinträchtigten. Er räumte jedoch auch ein, dass diese Schlussfolgerung umstritten sei, und erinnerte die Experten daran, dass sie heute zur Teilnahme aufgefordert wurden, gerade weil die FDA ihre Meinung einholen möchte.
Der Vorsitzende des Gremiums, Dr. Ken Burman von der Georgetown University, erklärte, dass eine klinische Studie zur Untersuchung des Einflusses von Mineralöl auf die Statinabsorption noch nie durchgeführt wurde. Eine solche Studie wäre relativ einfach und würde nicht so lange dauern.

Antwort auf Beitrag Nr.: 61.925.390 von Cyberhexe am 14.11.19 18:43:23Ich bin mir nahezu sicher, dass die Anwendung vin VASCEPA heute erheblich erweitert wird. Die Frage ist jedoch, wie weit?
Der größte Teil der Patienten in der REDUCE-IT-Studie ist für die sogenannte Sekundärprävention bestimmt. Dies bedeutet, dass diese Patienten bereits einen Herzinfarkt oder Schlaganfall hatten. Die Erweiterung um diese Indikation halte ich für sehr wahrscheinlich.
Die Frage ist jedoch, gibt es eine Erweiterung um Risikopatienten, die bisher noch kein kardiovaskuläres Ereignis hatten (Herzinfarkt, Schlaganfall, Stent).
Amarin schlug auf der Grundlage neuer Post-hoc-Analysen vor, diese Patienten mit einem Risiko-Score auszuwählen, der das kardiovaskuläre Gesamtrisiko des Patienten berechnet. Marvin Konstam von Tufts protestierte, dass eine Risikobewertung hier möglicherweise nicht angemessen sei.
Hier könnten auch Probleme wie die Sorgen um das Mineralöl-Placebo eine Rolle spielen. Die FDA rechnet damit, dass nur etwa 3% der insgesamt um 25% verringerten Ereignisse mit REDUCE-IT von Bedeutung sein könnten. Das Gremium arbeitet sehr hart daran, die Daten auf eine übersichtliche Tabelle mit Vor- und Nachteilen für diese Gruppen zu reduzieren, und bittet um Informationen zu Nebenwirkungen, die gegen die geringere Wirksamkeit in der Primärprävention abgewogen werden können. Thomas Weber von der Duke University bat um Einzelheiten für seine eigene Analyse, die darauf hindeutete, dass 33 Patienten behandelt werden müssten, nicht 22, wie zuvor vorgeschlagen.
Folgende Empfehlungen sind denkbar:
1.) das Gremium empfiehlt, Vascepa nur bei Patienten mit bereits bestehenden Herz-Kreislauf-Erkrankungen zu verschreiben
2.) es empfiehlt, dass Patienten einen höheren Triglyceridspiegel als die 135 Milligramm pro Deziliter haben, die Amarin vor der Verschreibung von Vascepa vorschlägt,
3.) allen von Amarin beantragten Indikationen wird zugestimmt
Alle Varianten sind denkbar und keines dieser Szenarien ist eine Katastrophe. Einige werden sogar argumentieren, dass, wenn es um die Größe des Marktes geht, nichts davon zählt. In einer Mitteilung an die Anleger argumentierte Michael Yee von Jefferies, dass die Beschränkung des Marktes auf sekundäre und nicht primäre Patienten immer noch einen Markt von mindestens 5 Millionen Patienten in den USA darstelle und Amarin nur 1 Million Patienten erreichen müsse für 2,5 Milliarden US-Dollar Umsatz.
Not too bad!
Der größte Teil der Patienten in der REDUCE-IT-Studie ist für die sogenannte Sekundärprävention bestimmt. Dies bedeutet, dass diese Patienten bereits einen Herzinfarkt oder Schlaganfall hatten. Die Erweiterung um diese Indikation halte ich für sehr wahrscheinlich.
Die Frage ist jedoch, gibt es eine Erweiterung um Risikopatienten, die bisher noch kein kardiovaskuläres Ereignis hatten (Herzinfarkt, Schlaganfall, Stent).
Amarin schlug auf der Grundlage neuer Post-hoc-Analysen vor, diese Patienten mit einem Risiko-Score auszuwählen, der das kardiovaskuläre Gesamtrisiko des Patienten berechnet. Marvin Konstam von Tufts protestierte, dass eine Risikobewertung hier möglicherweise nicht angemessen sei.
Hier könnten auch Probleme wie die Sorgen um das Mineralöl-Placebo eine Rolle spielen. Die FDA rechnet damit, dass nur etwa 3% der insgesamt um 25% verringerten Ereignisse mit REDUCE-IT von Bedeutung sein könnten. Das Gremium arbeitet sehr hart daran, die Daten auf eine übersichtliche Tabelle mit Vor- und Nachteilen für diese Gruppen zu reduzieren, und bittet um Informationen zu Nebenwirkungen, die gegen die geringere Wirksamkeit in der Primärprävention abgewogen werden können. Thomas Weber von der Duke University bat um Einzelheiten für seine eigene Analyse, die darauf hindeutete, dass 33 Patienten behandelt werden müssten, nicht 22, wie zuvor vorgeschlagen.
Folgende Empfehlungen sind denkbar:
1.) das Gremium empfiehlt, Vascepa nur bei Patienten mit bereits bestehenden Herz-Kreislauf-Erkrankungen zu verschreiben
2.) es empfiehlt, dass Patienten einen höheren Triglyceridspiegel als die 135 Milligramm pro Deziliter haben, die Amarin vor der Verschreibung von Vascepa vorschlägt,
3.) allen von Amarin beantragten Indikationen wird zugestimmt
Alle Varianten sind denkbar und keines dieser Szenarien ist eine Katastrophe. Einige werden sogar argumentieren, dass, wenn es um die Größe des Marktes geht, nichts davon zählt. In einer Mitteilung an die Anleger argumentierte Michael Yee von Jefferies, dass die Beschränkung des Marktes auf sekundäre und nicht primäre Patienten immer noch einen Markt von mindestens 5 Millionen Patienten in den USA darstelle und Amarin nur 1 Million Patienten erreichen müsse für 2,5 Milliarden US-Dollar Umsatz.
Not too bad!
Approved, 16 - 0 Voting for yes
Antwort auf Beitrag Nr.: 61.927.334 von asthmamoah am 14.11.19 22:09:19
Amarin
Wie geil👍👍👍👍👍💪💵💵💵💵
Antwort auf Beitrag Nr.: 61.927.400 von Magnetfeldfredy am 14.11.19 22:16:02😮😮😮 wow...
Amazing stuff, jetzt noch positive Evaporate am Montag als Late braker und wir könnten US Dollar 50 erreichen:
Amarin's Stock Could Reach $50 Per Share Soon
Here's where a de-risked Amarin could be headed over the next few months.
George Budwell
George Budwell
(TMFGBudwell)
Nov 13, 2019 at 9:37AM
Author Bio
Yesterday, Amarin's (NASDAQ:AMRN) shares jumped by a whopping 20.9% during normal trading hours. The spark behind this eye-catching move northward was the release of the U.S. Food and Drug Administration's (FDA) briefing materials for the company's upcoming advisory-committee meeting set for this Thursday.
Come Thursday, Amarin and the FDA's panel of experts will mull over the putative cardioprotective benefits of the company's prescription omega-3 treatment known as Vascepa. The big deal is that Vascepa's addressable market would expand to upwards of around 9.5 million Americans who are currently on statin therapy but still suffer from elevated triglyceride levels.
Businessman holding risk and rewards blocks.
Image Source: Getty Images.
That's a $15 billion per-year commercial opportunity right now -- based on the drug's estimated annual net price of $1,625, per the cost-effectiveness watchdog ICER. Future price hikes, combined with the continued growth of this target market as a whole, though, could push Vascepa's sales into truly rarefied air.
Keeping with this theme, Vascepa has the potential to generate annual sales larger than those of Pfizer's (NYSE:PFE) megablockbuster cholesterol medicine Lipitor, as well as AbbVie's anti-inflammatory behemoth Humira. For those new to the world of biopharma, Lipitor and Humira are the two bestselling pharmaceutical products of all time. That's just how big of a deal Vascepa's proposed cardioprotective indication is from a commercial standpoint.
With this background in mind, it's arguably the perfect time to consider if Amarin's stock can climb even higher in the days and weeks ahead. Here's a look at Amarin's potential risks and rewards in light of yesterday's market-moving event.
Amarin: The risks and rewards
Amarin's stock sports two clear-cut risk factors: the regulatory risk associated with Vascepa's proposed label expansion and the company's real world ability to fully capitalize on this ginormous commercial opportunity, post-approval.
Now, the risk emanating from the upcoming label-expansion decision appears to be minimal in the wake of yesterday's briefing-document release. The most critical issue heading into this advisory-committee meeting was the potentially confounding effect of the mineral oil placebo on the magnitude of Vascepa's observed cardioprotective benefit in the Reduce-It trial. However, the FDA's own reviewers admitted that they couldn't explain away Vascepa's cardioprotective benefit simply as a function of a non-inert placebo.
Two key statements in the briefing documents made this point crystal clear:
FDA analyses attempting to differentiate whether increases in LDL-C and other biomarkers were due to the mineral oil placebo are inconclusive...
FDA's exploratory analyses to assess the effect of these markers suggested that the difference in LDLC between the study groups could not account for the positive CV outcomes.
So, unless something truly extraordinary happens at Thursday's meeting, the FDA will more than likely approve Vascepa's label expansion.
Amarin's key risk thus boils down to the company's ability to maximize Vascepa's commercial potential. Although Amarin is planning on significantly beefing up its sales force once the FDA formally approves this label expansion, the reality of the situation is that the company simply cannot tackle this vast market by itself.
Amarin doesn't have the commercial infrastructure or the marketing clout to cover all the bases, so to speak. There's an inherent structure reason, after all, why all the bestselling drugs in the world are either marketed directly by a pharma titan or at least partnered with one of the industry's biggest names.
What this means is that Vascepa's peak sales would probably top out at the $2 billion mark under a go-it-alone approach and could take several years to reach this high-water mark. In that case, Amarin's shares would arguably be fairly valued at something along the lines of $27.8 per share (five times peak sales), representing a 33% upside potential from current levels.
For the uber-bulls out there, the plain truth is that Amarin's shares would almost certainly be trading at much higher levels if the market thought the company could realistically push Vascepa's sales significantly beyond the $2 billion mark within the next four years. So $2 billion does appear to be the market's working peak sales estimate -- at least as things stand now.
The wildcard
There is a wildcard at play here, however. Amarin's management hasn't even attempted to build out a broader clinical pipeline to complement Vascepa, and that's a big tell in regards to the company's value-creation strategy. Cutting to the chase, Amarin has probably already fielded a few tender offers in the event the FDA greenlights Vascepa's Reduce-It indication.
Amgen (NASDAQ:AMGN) and Pfizer have both been rumored to have interest in Amarin in the recent past, after all. Making this situation even more intriguing is that both Amgen and Pfizer have been been gobbling up high-value assets of late in order to finally move beyond the patent cliff. Vascepa, for its part, could turn into a crown jewel of the pharma industry under the umbrella of either Amgen or Pfizer, making it a highly desirable asset for these titans of the industry.
How much could Amarin fetch in a buyout? With two gold-star players vying for Vascepa, Amarin's buyout price could easily range from $36 to $42 per share (assuming a tender offer in the range of $13 billion to $15 billion). Now, if a bidding war broke out -- a scenario that played out when AbbVie had to pay top dollar to acquire Imbruvica, the low $50s might even be possible.
When would a buyout most likely occur? Big pharma has a tendency to announce needle-moving business development moves early in the year -- frequently in January around the time of the J.P. Morgan Healthcare Conference. The reason is that these early-year announcements help to set the stage for investors' expectations for the full year.
Final thoughts
The big picture is that investors shouldn't be surprised if Amarin indeed gets this all-important label expansion for Vascepa before the end of the year, and this regulatory win, in turn, leads to a buyout at a healthy premium within the first quarter of 2020. From a more conservative viewpoint, Amarin's shares should at least appreciate by another 10% to 20% before the end of 2019, especially with its biggest risk factor -- a complete response letter -- seemingly off the table.
Amarin's Stock Could Reach $50 Per Share Soon
Here's where a de-risked Amarin could be headed over the next few months.
George Budwell
George Budwell
(TMFGBudwell)
Nov 13, 2019 at 9:37AM
Author Bio
Yesterday, Amarin's (NASDAQ:AMRN) shares jumped by a whopping 20.9% during normal trading hours. The spark behind this eye-catching move northward was the release of the U.S. Food and Drug Administration's (FDA) briefing materials for the company's upcoming advisory-committee meeting set for this Thursday.
Come Thursday, Amarin and the FDA's panel of experts will mull over the putative cardioprotective benefits of the company's prescription omega-3 treatment known as Vascepa. The big deal is that Vascepa's addressable market would expand to upwards of around 9.5 million Americans who are currently on statin therapy but still suffer from elevated triglyceride levels.
Businessman holding risk and rewards blocks.
Image Source: Getty Images.
That's a $15 billion per-year commercial opportunity right now -- based on the drug's estimated annual net price of $1,625, per the cost-effectiveness watchdog ICER. Future price hikes, combined with the continued growth of this target market as a whole, though, could push Vascepa's sales into truly rarefied air.
Keeping with this theme, Vascepa has the potential to generate annual sales larger than those of Pfizer's (NYSE:PFE) megablockbuster cholesterol medicine Lipitor, as well as AbbVie's anti-inflammatory behemoth Humira. For those new to the world of biopharma, Lipitor and Humira are the two bestselling pharmaceutical products of all time. That's just how big of a deal Vascepa's proposed cardioprotective indication is from a commercial standpoint.
With this background in mind, it's arguably the perfect time to consider if Amarin's stock can climb even higher in the days and weeks ahead. Here's a look at Amarin's potential risks and rewards in light of yesterday's market-moving event.
Amarin: The risks and rewards
Amarin's stock sports two clear-cut risk factors: the regulatory risk associated with Vascepa's proposed label expansion and the company's real world ability to fully capitalize on this ginormous commercial opportunity, post-approval.
Now, the risk emanating from the upcoming label-expansion decision appears to be minimal in the wake of yesterday's briefing-document release. The most critical issue heading into this advisory-committee meeting was the potentially confounding effect of the mineral oil placebo on the magnitude of Vascepa's observed cardioprotective benefit in the Reduce-It trial. However, the FDA's own reviewers admitted that they couldn't explain away Vascepa's cardioprotective benefit simply as a function of a non-inert placebo.
Two key statements in the briefing documents made this point crystal clear:
FDA analyses attempting to differentiate whether increases in LDL-C and other biomarkers were due to the mineral oil placebo are inconclusive...
FDA's exploratory analyses to assess the effect of these markers suggested that the difference in LDLC between the study groups could not account for the positive CV outcomes.
So, unless something truly extraordinary happens at Thursday's meeting, the FDA will more than likely approve Vascepa's label expansion.
Amarin's key risk thus boils down to the company's ability to maximize Vascepa's commercial potential. Although Amarin is planning on significantly beefing up its sales force once the FDA formally approves this label expansion, the reality of the situation is that the company simply cannot tackle this vast market by itself.
Amarin doesn't have the commercial infrastructure or the marketing clout to cover all the bases, so to speak. There's an inherent structure reason, after all, why all the bestselling drugs in the world are either marketed directly by a pharma titan or at least partnered with one of the industry's biggest names.
What this means is that Vascepa's peak sales would probably top out at the $2 billion mark under a go-it-alone approach and could take several years to reach this high-water mark. In that case, Amarin's shares would arguably be fairly valued at something along the lines of $27.8 per share (five times peak sales), representing a 33% upside potential from current levels.
For the uber-bulls out there, the plain truth is that Amarin's shares would almost certainly be trading at much higher levels if the market thought the company could realistically push Vascepa's sales significantly beyond the $2 billion mark within the next four years. So $2 billion does appear to be the market's working peak sales estimate -- at least as things stand now.
The wildcard
There is a wildcard at play here, however. Amarin's management hasn't even attempted to build out a broader clinical pipeline to complement Vascepa, and that's a big tell in regards to the company's value-creation strategy. Cutting to the chase, Amarin has probably already fielded a few tender offers in the event the FDA greenlights Vascepa's Reduce-It indication.
Amgen (NASDAQ:AMGN) and Pfizer have both been rumored to have interest in Amarin in the recent past, after all. Making this situation even more intriguing is that both Amgen and Pfizer have been been gobbling up high-value assets of late in order to finally move beyond the patent cliff. Vascepa, for its part, could turn into a crown jewel of the pharma industry under the umbrella of either Amgen or Pfizer, making it a highly desirable asset for these titans of the industry.
How much could Amarin fetch in a buyout? With two gold-star players vying for Vascepa, Amarin's buyout price could easily range from $36 to $42 per share (assuming a tender offer in the range of $13 billion to $15 billion). Now, if a bidding war broke out -- a scenario that played out when AbbVie had to pay top dollar to acquire Imbruvica, the low $50s might even be possible.
When would a buyout most likely occur? Big pharma has a tendency to announce needle-moving business development moves early in the year -- frequently in January around the time of the J.P. Morgan Healthcare Conference. The reason is that these early-year announcements help to set the stage for investors' expectations for the full year.
Final thoughts
The big picture is that investors shouldn't be surprised if Amarin indeed gets this all-important label expansion for Vascepa before the end of the year, and this regulatory win, in turn, leads to a buyout at a healthy premium within the first quarter of 2020. From a more conservative viewpoint, Amarin's shares should at least appreciate by another 10% to 20% before the end of 2019, especially with its biggest risk factor -- a complete response letter -- seemingly off the table.
Antwort auf Beitrag Nr.: 61.923.179 von Cyberhexe am 14.11.19 15:45:47
YES - you know - you`ll never walk alone..
...sorry.... you `ll never sail alone.
Zitat von Cyberhexe: Hi Bernie - sitzen wir wieder einmal im selben Boot?
Ich glaube nicht, dass wir absaufen!
wie immer: TIME WILL TELL!
YES - you know - you`ll never walk alone..

...sorry.... you `ll never sail alone.

Antwort auf Beitrag Nr.: 61.927.334 von asthmamoah am 14.11.19 22:09:19

Zitat von asthmamoah: Approved, 16 - 0 Voting for yes

Antwort auf Beitrag Nr.: 61.927.859 von bernie55 am 14.11.19 23:15:43Amarin Announced FDA Advisory Committee Voted Unanimously (16-0) to Recommend Approval of Vascepa® (icosapent ethyl) Capsules Label Expansion to Reduce Cardiovascular Risk Based on Landmark REDUCE-IT® Outcomes Trial
•November 14, 2019
Cardiovascular disease events like heart attacks, stroke and death affect millions of patients in the United States and are estimated to cost $500 billion annually
Millions of high-risk patients with cardiovascular disease could benefit from this cost-effective therapy if expanded label receives FDA approval; PDUFA date is December 28
DUBLIN, Ireland and BRIDGEWATER, N.J., Nov. 14, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN) today announced that the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) of the U.S. Food and Drug Administration (FDA) has voted unanimously (16-0) to recommend approval of an indication and label expansion for Vascepa® (icosapent ethyl) capsules to reduce the risk of cardiovascular events in high-risk patients based on results from the landmark REDUCE-IT®1 cardiovascular outcomes trial.
The FDA is not bound by the recommendations of an advisory committee. Amarin plans to work with the agency as it completes its review of the company’s application seeking an appropriate label expansion for Vascepa to reflect REDUCE-IT results.
Cardiovascular disease is the number one cause of death for men and women in the United States and the nation’s costliest disease, with direct and indirect expenses in excess of $500 billion each year.2 An independent drug pricing watchdog group concluded that Vascepa is cost effective for cardiovascular risk reduction as demonstrated in REDUCE-IT even under the most stringent standards of that group, a result rarely achieved in its analyses.3
“Today we moved an important step closer to potentially helping millions of patients who are at risk for cardiovascular events despite being on standard-of-care statin therapy,” said John F. Thero, president and chief executive officer of Amarin. “Vascepa is positioned to be the first approved treatment to reduce cardiovascular events in the group of at-risk patients studied in the landmark REDUCE-IT clinical trial. We appreciate both the opportunity to present these results and the committee’s strong vote of confidence. We look forward to anticipated labeling discussions with the FDA, and we continue to prepare for the launch of Vascepa assuming FDA approval of our sNDA on or before the target PDUFA date of December 28.”
Deepak L. Bhatt, M.D., M.P.H., executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital Heart and Vascular Center, and professor of medicine at Harvard Medical School, said: “The REDUCE-IT results are quite remarkable and illustrate how icosapent ethyl could transform the treatment of cardiovascular disease in the United States and worldwide. From my perspective as not only a researcher but also a practicing physician, icosapent ethyl represents one of the most important developments in the prevention and treatment of cardiovascular disease since statins and, if FDA approved, will be a critical tool for physicians to use to help prevent cardiovascular events such as heart attack and stroke, including fatal ones, in high-risk patients.”
“Amarin thanks the patients, advocacy groups, physicians, researchers, and others who expressed overwhelming support for Vascepa through their written and in person comments at the advisory committee meeting,” Thero said. “We also recognize the contributions of the 8,179 patients who participated in REDUCE-IT, some for over six years. Thousands of patients and professionals contributed to the REDUCE-IT results. We look forward to having their sacrifices and contributions reflected in an expanded indication for Vascepa that has the potential to benefit millions of patients.”
https://finance.yahoo.com/news/amarin-announced-fda-advisory…
•November 14, 2019
Cardiovascular disease events like heart attacks, stroke and death affect millions of patients in the United States and are estimated to cost $500 billion annually
Millions of high-risk patients with cardiovascular disease could benefit from this cost-effective therapy if expanded label receives FDA approval; PDUFA date is December 28
DUBLIN, Ireland and BRIDGEWATER, N.J., Nov. 14, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN) today announced that the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) of the U.S. Food and Drug Administration (FDA) has voted unanimously (16-0) to recommend approval of an indication and label expansion for Vascepa® (icosapent ethyl) capsules to reduce the risk of cardiovascular events in high-risk patients based on results from the landmark REDUCE-IT®1 cardiovascular outcomes trial.
The FDA is not bound by the recommendations of an advisory committee. Amarin plans to work with the agency as it completes its review of the company’s application seeking an appropriate label expansion for Vascepa to reflect REDUCE-IT results.
Cardiovascular disease is the number one cause of death for men and women in the United States and the nation’s costliest disease, with direct and indirect expenses in excess of $500 billion each year.2 An independent drug pricing watchdog group concluded that Vascepa is cost effective for cardiovascular risk reduction as demonstrated in REDUCE-IT even under the most stringent standards of that group, a result rarely achieved in its analyses.3
“Today we moved an important step closer to potentially helping millions of patients who are at risk for cardiovascular events despite being on standard-of-care statin therapy,” said John F. Thero, president and chief executive officer of Amarin. “Vascepa is positioned to be the first approved treatment to reduce cardiovascular events in the group of at-risk patients studied in the landmark REDUCE-IT clinical trial. We appreciate both the opportunity to present these results and the committee’s strong vote of confidence. We look forward to anticipated labeling discussions with the FDA, and we continue to prepare for the launch of Vascepa assuming FDA approval of our sNDA on or before the target PDUFA date of December 28.”
Deepak L. Bhatt, M.D., M.P.H., executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital Heart and Vascular Center, and professor of medicine at Harvard Medical School, said: “The REDUCE-IT results are quite remarkable and illustrate how icosapent ethyl could transform the treatment of cardiovascular disease in the United States and worldwide. From my perspective as not only a researcher but also a practicing physician, icosapent ethyl represents one of the most important developments in the prevention and treatment of cardiovascular disease since statins and, if FDA approved, will be a critical tool for physicians to use to help prevent cardiovascular events such as heart attack and stroke, including fatal ones, in high-risk patients.”
“Amarin thanks the patients, advocacy groups, physicians, researchers, and others who expressed overwhelming support for Vascepa through their written and in person comments at the advisory committee meeting,” Thero said. “We also recognize the contributions of the 8,179 patients who participated in REDUCE-IT, some for over six years. Thousands of patients and professionals contributed to the REDUCE-IT results. We look forward to having their sacrifices and contributions reflected in an expanded indication for Vascepa that has the potential to benefit millions of patients.”
https://finance.yahoo.com/news/amarin-announced-fda-advisory…
Amarin erhält FDA-Nominierung zur Erweiterung des Labels von Heart Drug Vascepa, von Blomberg
https://finance.yahoo.com/news/amarin-wins-fda-panel-nod-213…
Auszug:
Alle 16 Diskussionsteilnehmer stimmten zu, dass das Medikament als Medikament verkauft werden sollte, um das Risiko für kardiovaskuläre Ereignisse wie Schlaganfall und Herzinfarkt zu verringern, und stimmten darin überein, dass das Medikament sowohl sicher als auch wirksam ist. Die Zulassung könnte die potenzielle Patientenpopulation von derzeit 600.000 auf 10 Millionen erhöhen, schätzte SVB Leerink Anfang dieses Monats.
Der Diskussionsteilnehmer James de Lemos äußerte sich skeptischer und sagte, Vascepa sollte "nur für die Sekundärprävention zugelassen werden", wobei das Unternehmen Vascepa gegen ein Placebo bei diesen Patienten mit geringerem Risiko untersuchen musste.
Jefferies Analytiker Michael Yee schrieb am Donnerstag zuvor, dass die potenzielle Patientenpopulation in den USA fünf bis 15 Millionen erreichen könnte, obwohl nur eine Million Patienten, die das Medikament einnehmen, es noch zu einem Medikament von 2,5 Milliarden Dollar machen würden.
Es wird erwartet, dass die US-Aufsichtsbehörden bis zum 28. Dezember eine endgültige Entscheidung treffen. Die Agentur befolgt in der Regel den Rat ihrer Expertengremien, ist jedoch nicht dazu verpflichtet. Die Aktien des Drogenkonsumenten wurden am Donnerstag während des Panels gestoppt, beendeten den Mittwoch jedoch mit einem 4-Monats-Hoch.
Gruss RS😎
https://finance.yahoo.com/news/amarin-wins-fda-panel-nod-213…
Auszug:
Alle 16 Diskussionsteilnehmer stimmten zu, dass das Medikament als Medikament verkauft werden sollte, um das Risiko für kardiovaskuläre Ereignisse wie Schlaganfall und Herzinfarkt zu verringern, und stimmten darin überein, dass das Medikament sowohl sicher als auch wirksam ist. Die Zulassung könnte die potenzielle Patientenpopulation von derzeit 600.000 auf 10 Millionen erhöhen, schätzte SVB Leerink Anfang dieses Monats.
Der Diskussionsteilnehmer James de Lemos äußerte sich skeptischer und sagte, Vascepa sollte "nur für die Sekundärprävention zugelassen werden", wobei das Unternehmen Vascepa gegen ein Placebo bei diesen Patienten mit geringerem Risiko untersuchen musste.
Jefferies Analytiker Michael Yee schrieb am Donnerstag zuvor, dass die potenzielle Patientenpopulation in den USA fünf bis 15 Millionen erreichen könnte, obwohl nur eine Million Patienten, die das Medikament einnehmen, es noch zu einem Medikament von 2,5 Milliarden Dollar machen würden.
Es wird erwartet, dass die US-Aufsichtsbehörden bis zum 28. Dezember eine endgültige Entscheidung treffen. Die Agentur befolgt in der Regel den Rat ihrer Expertengremien, ist jedoch nicht dazu verpflichtet. Die Aktien des Drogenkonsumenten wurden am Donnerstag während des Panels gestoppt, beendeten den Mittwoch jedoch mit einem 4-Monats-Hoch.
Gruss RS😎
Antwort auf Beitrag Nr.: 61.927.859 von bernie55 am 14.11.19 23:15:43👍👍👍 Sehr, sehr geiles Ergebnis. Mit solch einer Deutlichkeit hätte ich das nie erwartet😀😀
Fühlt sich an wie das 7:1 für Deutschland gegen Brasilien bei der WM 2014!













Antwort auf Beitrag Nr.: 61.928.510 von Magnetfeldfredy am 15.11.19 06:55:20
So kann man es auch sehen - auf jeden Fall irgendwie noch nicht zu "begreifen". 👍
Um aber bei dem gestrigen ADCOM-Voting zu bleiben - das einstimmige Votum ( 100 % Zustimmung ) fühlt sich eher an wie eine 1000 % ige Zustimmung
Zitat von Magnetfeldfredy: Fühlt sich an wie das 7:1 für Deutschland gegen Brasilien bei der WM 2014!
So kann man es auch sehen - auf jeden Fall irgendwie noch nicht zu "begreifen". 👍

Um aber bei dem gestrigen ADCOM-Voting zu bleiben - das einstimmige Votum ( 100 % Zustimmung ) fühlt sich eher an wie eine 1000 % ige Zustimmung

Wann wird der Handel denn ungefähr wieder aufgenommen?
Antwort auf Beitrag Nr.: 61.930.208 von Matze292 am 15.11.19 10:08:15Die Experten sind sich einig, dass bei Patienten mit Herzinfarkt, Schlaganfall oder bestehender kardiovaskulärer Erkrankung und hohem Triglyceridgehalt, einem Bluttestmarker, der auf ein höheres kardiovaskuläres Risiko hinweist, der Nutzen unbestreitbar ist. Für diese Menschen - die sogenannten Sekundärpräventionspatienten - spricht einfach nichts gegen einen sinnvollen und wichtigen kardiovaskulären Nutzen.
Das Panel war war jedoch längst nicht so positiv, wenn es um den Nutzen für Patienten ging, bei denen keine Herzvorerkrankung festgestellt wurde --> Primärpräventionspatienten. Obwohl diese Personen den größten potenziellen Markt darstellen, waren sie in der 8.000-Patienten-Studie von Amarin in der Minderheit.
Die AC-Experten hielten sich nicht an eine strukturierte Auflistung ihrer Bedenken, und einige ihrer Antworten waren so differenziert, dass sie auf verschiedene Arten interpretiert werden können. Auf alle Fälle äußerten einige Experten Bedenken, weil bei Patienten mit geringem Risiko für Herz-Kreislauf-Erkrankungen Nebenwirkungen in Form von Blutungen zu befürchten seien. Einige äußerten auch Bedenken, dass das in der Studie verwendete Mineralöl-Placebo die Vorteile von Vascepa verstärkt haben könnte.
Da die FDA keine konkreten Abstimmungen zu diesen Fragen beantragt hat, hat sie einen weiten Spielraum bei der Berücksichtigung der Expertenmeinungen. Es ist deswegen nicht ausgeschlossen, dass Vascepa auf die Sekundärpräventionspatienten beschränkt bleibt, zumal sich die beiden Kardiologen im AC auch dahingehend geäussert haben. Jedoch wäre auch diese maximale Beschränkung keine Katastrophe, weil allein dieser Markt schon sehr viel Umsatz-Phantasie hergibt.
Ich hoffe dennoch auf ein Kursfeuerwerk, und würde bei Kursen jenseits von 30$ die Hälfte meiner AMRN-Aktien verkaufen.
Das Panel war war jedoch längst nicht so positiv, wenn es um den Nutzen für Patienten ging, bei denen keine Herzvorerkrankung festgestellt wurde --> Primärpräventionspatienten. Obwohl diese Personen den größten potenziellen Markt darstellen, waren sie in der 8.000-Patienten-Studie von Amarin in der Minderheit.
Die AC-Experten hielten sich nicht an eine strukturierte Auflistung ihrer Bedenken, und einige ihrer Antworten waren so differenziert, dass sie auf verschiedene Arten interpretiert werden können. Auf alle Fälle äußerten einige Experten Bedenken, weil bei Patienten mit geringem Risiko für Herz-Kreislauf-Erkrankungen Nebenwirkungen in Form von Blutungen zu befürchten seien. Einige äußerten auch Bedenken, dass das in der Studie verwendete Mineralöl-Placebo die Vorteile von Vascepa verstärkt haben könnte.
Da die FDA keine konkreten Abstimmungen zu diesen Fragen beantragt hat, hat sie einen weiten Spielraum bei der Berücksichtigung der Expertenmeinungen. Es ist deswegen nicht ausgeschlossen, dass Vascepa auf die Sekundärpräventionspatienten beschränkt bleibt, zumal sich die beiden Kardiologen im AC auch dahingehend geäussert haben. Jedoch wäre auch diese maximale Beschränkung keine Katastrophe, weil allein dieser Markt schon sehr viel Umsatz-Phantasie hergibt.
Ich hoffe dennoch auf ein Kursfeuerwerk, und würde bei Kursen jenseits von 30$ die Hälfte meiner AMRN-Aktien verkaufen.
Antwort auf Beitrag Nr.: 61.930.208 von Matze292 am 15.11.19 10:08:15
Guckst Du hier:
Nasdaq Current Trading Halts
Demnach 15.11.2019, 07.10 h Ostküstenzeit. Also in wenigen Minuten.
Zitat von Matze292: Wann wird der Handel denn ungefähr wieder aufgenommen?
Guckst Du hier:
Nasdaq Current Trading Halts
Demnach 15.11.2019, 07.10 h Ostküstenzeit. Also in wenigen Minuten.
Antwort auf Beitrag Nr.: 61.930.724 von Cyberhexe am 15.11.19 10:46:29so warten auf die Eröffnung 

was meint Ihr erstmal gewinne mitnehmen?
bei https://stocktwits.com/symbol/AMRN spekulieren sie eröffnug zwischen 28-30 USD
MFG


was meint Ihr erstmal gewinne mitnehmen?
bei https://stocktwits.com/symbol/AMRN spekulieren sie eröffnug zwischen 28-30 USD
MFG
Antwort auf Beitrag Nr.: 61.932.332 von esaposse am 15.11.19 13:10:01
...nie und nimmer! Mit 23$ wäre ich schon zufrieden. Aber falls es im Laufe des Tages zu einer Übertreibung kommen sollte - mir soll es recht sein.
Die Hälfte steht zum Verkauf bei $30,25.
Zitat von esaposse: so warten auf die Eröffnung
was meint Ihr erstmal gewinne mitnehmen?
bei https://stocktwits.com/symbol/AMRN spekulieren sie eröffnug zwischen 28-30 USD
MFG
...nie und nimmer! Mit 23$ wäre ich schon zufrieden. Aber falls es im Laufe des Tages zu einer Übertreibung kommen sollte - mir soll es recht sein.
Die Hälfte steht zum Verkauf bei $30,25.
Antwort auf Beitrag Nr.: 61.932.386 von Cyberhexe am 15.11.19 13:14:26Handel läuft wieder
Ich würde sagen bei aktuell schon fast 8% nur im pre-market, so kann es weiter gehen einverstanden ? 
Viel Vergnügen, nach den letzten doch sehr Nervenzehrenden Wochen !
Das einzige was mir aufgefallen ist das Thero und Eckman vor dem Meeting Gestern noch einige Anteile verkauft hatten... So ganz sicher waren die sich auch nicht was passieren wird.
https://fintel.io/n/us/amrn

Viel Vergnügen, nach den letzten doch sehr Nervenzehrenden Wochen !
Das einzige was mir aufgefallen ist das Thero und Eckman vor dem Meeting Gestern noch einige Anteile verkauft hatten... So ganz sicher waren die sich auch nicht was passieren wird.
https://fintel.io/n/us/amrn
Evaporate am Montag als Latebraker könnte noch das Sahnehäubchen werden, wenn gezeigt wird das Vascepa Plaque statistisch signifikant in den Adern reduziert, was ja eine ähnliche Studie, geannt Cherry, schon zeigte!
Das wäre der letzte Beweis für alle Zweifler und könnte einen massiven Short-Squeeeezzzzzzzeee
auslösen!
Das wäre der letzte Beweis für alle Zweifler und könnte einen massiven Short-Squeeeezzzzzzzeee
auslösen!
Antwort auf Beitrag Nr.: 61.933.085 von flyingbeef am 15.11.19 14:15:30John Thero hat nur 5 % seiner Aktien verkauft, das hat mich nicht beunruhight, 16:0 und Evaportate vor der Tür lassen auf viel mehr hoffen, bzw. eine Übernahme für US Dollar 50!
Antwort auf Beitrag Nr.: 61.933.103 von Magnetfeldfredy am 15.11.19 14:16:43Hehe das wäre ganz grosses Kino und ja der nächste Squeeze darf gerne kommen 😎
Antwort auf Beitrag Nr.: 61.933.145 von Magnetfeldfredy am 15.11.19 14:19:44
Wenn ich mich richtig erinnere:
Amarin hat aber immer gesagt Übernahme "nicht mit uns" daher ich glaube die wollen die Zügel auch langfristig in der Hand haben sollte trotzdem ordentlich nach oben rauschen.
Zitat von Magnetfeldfredy: John Thero hat nur 5 % seiner Aktien verkauft, das hat mich nicht beunruhight, 16:0 und Evaportate vor der Tür lassen auf viel mehr hoffen, bzw. eine Übernahme für US Dollar 50!
Wenn ich mich richtig erinnere:
Amarin hat aber immer gesagt Übernahme "nicht mit uns" daher ich glaube die wollen die Zügel auch langfristig in der Hand haben sollte trotzdem ordentlich nach oben rauschen.
Antwort auf Beitrag Nr.: 61.933.181 von flyingbeef am 15.11.19 14:22:08
Sollte es zu einem BuyOut-Bidding von verschiedensten BPs ( Pfizer ? Amgen ? xxxx ? etc. ) kommen und ein sehr hohes Buyoutangebot vorliegen , dann würde ich das " nicht mit uns " nicht mehr unterschreiben.
Zitat von flyingbeef:Zitat von Magnetfeldfredy: John Thero hat nur 5 % seiner Aktien verkauft, das hat mich nicht beunruhight, 16:0 und Evaportate vor der Tür lassen auf viel mehr hoffen, bzw. eine Übernahme für US Dollar 50!
Wenn ich mich richtig erinnere:
Amarin hat aber immer gesagt Übernahme "nicht mit uns" daher ich glaube die wollen die Zügel auch langfristig in der Hand haben sollte trotzdem ordentlich nach oben rauschen.
Sollte es zu einem BuyOut-Bidding von verschiedensten BPs ( Pfizer ? Amgen ? xxxx ? etc. ) kommen und ein sehr hohes Buyoutangebot vorliegen , dann würde ich das " nicht mit uns " nicht mehr unterschreiben.
(Bloomberg) -- The unanimous panel backing to expand the label for Amarin Corp.’s heart drug Vascepa sparked debate across Wall Street as analysts and investors weighed whether the drug could be sold to as many as 15 million Americans.
The divide comes after members of an advisory panel to the U.S. Food and Drug Administration “offered different opinions when it came to specific recommendations for a potential Vascepa label,” according to analysts at Roth Capital Partners. That may leave billions in sales hanging in the balance. But Jefferies’ Michael Yee disagreed, saying the “label debate doesn’t really matter to the thesis” for investors. He believes doctors will use the drug “widely across patients.”
Amarin shares climbed as much as 11% Friday after being halted all day Thursday. With the stock briefly nearing $24 a share, it traded about a nickel short of a July peak.
Here’s what analysts had to say about the news.
Roth Capital Partners, Yasmeen Rahimi
“With unanimously favorable response for label expansion, question moves to which patients make it on label, with 10/16 docs voting in favor of both” atherosclerotic cardiovascular disease groups. Roth highlighted that some committee members “were caught up with analysis that showed a 12% relative CV risk reduction” without significant benefit for patients who had not had something like a heart attack or stroke and are at a lower risk.
Counted 10 of the 16 panelists “who were receptive to Vascepa label expansion including primary and secondary prevention patients” with the six others wanting the drug’s label to include patients who have had a stroke, or have cardiovascular disease.
Says there was “lots of safety talk, but vote indicated AdCom members felt the two signals could be adequately managed through label, thus meaning no patient restrictions.”
Maintains buy rating with $31 price target.
Jefferies, Michael Yee
“Our thesis and valuation are not based on specific ‘details’ of a label as we think docs will use this widely across patients...if stock was down, we think it bounces since label details don’t matter in big picture.”
Notes that while Amarin got the positive vote for approval, “investors will debate whether the labeled indication will include a more broad ‘primary prevention’ (high-risk) population which is patients w/o established CV disease but have diabetes and at least one other major” cardiovascular risk factor.
Highlights that the “stock has already run-up a lot and we wouldn’t be surprised if it’s volatile on this labeling discussion but we aren’t changing numbers as the populations are huge already.”
Maintains buy rating, $30 price target.
Cantor Fitzgerald, Louise Chen
“There were few-to-no surprises brought up during the meeting, in our view, and we think Vascepa is well on-track now for expected approval by year-end.”
Notes that most of the meeting “focused on concerns about the impact of mineral oil, as well as on which patient populations should be included in the label. Although some AdCom members believe mineral oil may have affected results somewhat, they agreed that the REDUCE-IT data are meaningful enough to warrant approval.”
Highlighted that panelist discussions on how high patients’ triglycerides, a type of fat in the blood, must be to benefit were “less clear-cut compared to the mineral oil debate.”
Maintains overweight rating with a $35 price target.
Citi, Joel Beatty
Amarin is unlikely to reach a market value above $10 billion over the next several months with its market value at $7.7 billion as of Wednesday’s close.
Sees the drug as “likely to be used in high-risk primary prevention patients regardless of” the exact label wording, though there will likely be some debate over the coming weeks regarding the label.
“We believe the exact wording of the indication matters relatively little compared to other drugs, because we believe physicians will still use Vascepa in very high risk” primary prevention patients.
Maintains buy rating and $23 price target.
Stifel, Derek Archila
“While the panel was in agreement Vascepa should be approved for secondary prevention patients (our base case), the key question that remains is whether or not Vascepa will receive a broader label inclusive of primary prevention patients, which would be upside to our model.”
“We believe approval in secondary prevention alone offers an addressable market that is at least ~10-15 million patients for Vascepa, making us comfortable with our ~$3 billion in peak sales estimate.”
Rates shares at buy with a $26 price target.
(Updates share movement in third paragraph.)
To contact the reporter on this story: Bailey Lipschultz in New York at blipschultz@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Scott Schnipper, Richard Richtmyer
For more articles like this, please visit us at bloomberg.com
The divide comes after members of an advisory panel to the U.S. Food and Drug Administration “offered different opinions when it came to specific recommendations for a potential Vascepa label,” according to analysts at Roth Capital Partners. That may leave billions in sales hanging in the balance. But Jefferies’ Michael Yee disagreed, saying the “label debate doesn’t really matter to the thesis” for investors. He believes doctors will use the drug “widely across patients.”
Amarin shares climbed as much as 11% Friday after being halted all day Thursday. With the stock briefly nearing $24 a share, it traded about a nickel short of a July peak.
Here’s what analysts had to say about the news.
Roth Capital Partners, Yasmeen Rahimi
“With unanimously favorable response for label expansion, question moves to which patients make it on label, with 10/16 docs voting in favor of both” atherosclerotic cardiovascular disease groups. Roth highlighted that some committee members “were caught up with analysis that showed a 12% relative CV risk reduction” without significant benefit for patients who had not had something like a heart attack or stroke and are at a lower risk.
Counted 10 of the 16 panelists “who were receptive to Vascepa label expansion including primary and secondary prevention patients” with the six others wanting the drug’s label to include patients who have had a stroke, or have cardiovascular disease.
Says there was “lots of safety talk, but vote indicated AdCom members felt the two signals could be adequately managed through label, thus meaning no patient restrictions.”
Maintains buy rating with $31 price target.
Jefferies, Michael Yee
“Our thesis and valuation are not based on specific ‘details’ of a label as we think docs will use this widely across patients...if stock was down, we think it bounces since label details don’t matter in big picture.”
Notes that while Amarin got the positive vote for approval, “investors will debate whether the labeled indication will include a more broad ‘primary prevention’ (high-risk) population which is patients w/o established CV disease but have diabetes and at least one other major” cardiovascular risk factor.
Highlights that the “stock has already run-up a lot and we wouldn’t be surprised if it’s volatile on this labeling discussion but we aren’t changing numbers as the populations are huge already.”
Maintains buy rating, $30 price target.
Cantor Fitzgerald, Louise Chen
“There were few-to-no surprises brought up during the meeting, in our view, and we think Vascepa is well on-track now for expected approval by year-end.”
Notes that most of the meeting “focused on concerns about the impact of mineral oil, as well as on which patient populations should be included in the label. Although some AdCom members believe mineral oil may have affected results somewhat, they agreed that the REDUCE-IT data are meaningful enough to warrant approval.”
Highlighted that panelist discussions on how high patients’ triglycerides, a type of fat in the blood, must be to benefit were “less clear-cut compared to the mineral oil debate.”
Maintains overweight rating with a $35 price target.
Citi, Joel Beatty
Amarin is unlikely to reach a market value above $10 billion over the next several months with its market value at $7.7 billion as of Wednesday’s close.
Sees the drug as “likely to be used in high-risk primary prevention patients regardless of” the exact label wording, though there will likely be some debate over the coming weeks regarding the label.
“We believe the exact wording of the indication matters relatively little compared to other drugs, because we believe physicians will still use Vascepa in very high risk” primary prevention patients.
Maintains buy rating and $23 price target.
Stifel, Derek Archila
“While the panel was in agreement Vascepa should be approved for secondary prevention patients (our base case), the key question that remains is whether or not Vascepa will receive a broader label inclusive of primary prevention patients, which would be upside to our model.”
“We believe approval in secondary prevention alone offers an addressable market that is at least ~10-15 million patients for Vascepa, making us comfortable with our ~$3 billion in peak sales estimate.”
Rates shares at buy with a $26 price target.
(Updates share movement in third paragraph.)
To contact the reporter on this story: Bailey Lipschultz in New York at blipschultz@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Scott Schnipper, Richard Richtmyer
For more articles like this, please visit us at bloomberg.com
Meinung von "Sam" aus Investorshub, toller Mensch, der jeden Freitag die scripts meldet:
Sam81 Friday, 11/15/19 04:19:07 AM
Re: None 0
Post # of 226871
Dear all,
I wasn't able to post last night after i reached my limit.
Congrats to all
Anyhow, what a day, up and down with all discussions but finally science prevailed and we got our 16-0 vote
In addition with regard to primary indication, i would say panelists were 10 for - 4 against & 2 undecided (10-4-2) and that bodes well for us.
Personally, and after watching and listening to all panelists after the vote, i would say we will get a label for both primary & secondary, TG 150 and above, 40 Years onward & of course diabetic and with one or more CVD risk factors.
And as with statins, the prerequisites with time will be loosened after Real World Data & follow up
Finally, and all my personal opinion, i am strongly against a BO. Why burn your cards and miss opportunities for your shareholders by taking a premium NOW of 100% - 200% (based on Wednesday close of $21.49) when in the future (2021-2022) your company will be generating billions if not tens of billions of income and your valuation will be at the level on its own and then you can decide to get a premium on top of that or remain an independent big firm just like others.
Congrats to all once again and let's enjoy 2020 and more💰
Sam81 Friday, 11/15/19 04:19:07 AM
Re: None 0
Post # of 226871
Dear all,
I wasn't able to post last night after i reached my limit.
Congrats to all
Anyhow, what a day, up and down with all discussions but finally science prevailed and we got our 16-0 vote

In addition with regard to primary indication, i would say panelists were 10 for - 4 against & 2 undecided (10-4-2) and that bodes well for us.

Personally, and after watching and listening to all panelists after the vote, i would say we will get a label for both primary & secondary, TG 150 and above, 40 Years onward & of course diabetic and with one or more CVD risk factors.
And as with statins, the prerequisites with time will be loosened after Real World Data & follow up
Finally, and all my personal opinion, i am strongly against a BO. Why burn your cards and miss opportunities for your shareholders by taking a premium NOW of 100% - 200% (based on Wednesday close of $21.49) when in the future (2021-2022) your company will be generating billions if not tens of billions of income and your valuation will be at the level on its own and then you can decide to get a premium on top of that or remain an independent big firm just like others.
Congrats to all once again and let's enjoy 2020 and more💰
Antwort auf Beitrag Nr.: 61.935.875 von Magnetfeldfredy am 15.11.19 18:43:48Gute Zusammenfassung, ich denke auch, dass wir mit der gestrigen Entscheidung stetig steigen werden. Es wird auch zu Rücksetzern kommen, gerade wenn der Gesamtmarkt in die Knie geht, aber die Richtung ist jetzt klar aufwärts
Antwort auf Beitrag Nr.: 61.935.875 von Magnetfeldfredy am 15.11.19 18:43:48Hier die ScriptZahlen von Sam81:

Ich sitze hier mit Popcorn echt wahr 

Sorry war jetzt nicht sehr informativ aber an solchen Tagen darf man auch mal etwas albern sein 🙃


Sorry war jetzt nicht sehr informativ aber an solchen Tagen darf man auch mal etwas albern sein 🙃
Antwort auf Beitrag Nr.: 61.933.355 von bernie55 am 15.11.19 14:40:50
Ich denke das Amarin natürlich weiss das sie ihr Marktpotential nur dann umfangreich nutzen können wenn sie eine Partnerschafr mit BP eingehen. Es ist wichtig in möglichst kurzer Zeit einen grossen Fussabdruck im Markt zu hinterlassen sichert höhere Einnahmen über Jahre. Ausserdem brauchen sie einen grossen Partner für Produktion usw. jedoch wie gesagt Buyout sehe ich nicht, Amarin kann so die Zügel in der Hand behalten und hat eine ideale Verhandlungsposition. Denke das auch die Nachricht einer Partnerschaft in Kombination mit hoffentlich full label expansion nochmal ordentlich Dampf macht. Sehe im ersten Quartal nächsten Jahres solch eine Entscheidung als realistisch. Sie müssen nichts überstürzen und können ideal verhandeln...
Zitat von bernie55:Zitat von flyingbeef: ...
Wenn ich mich richtig erinnere:
Amarin hat aber immer gesagt Übernahme "nicht mit uns" daher ich glaube die wollen die Zügel auch langfristig in der Hand haben sollte trotzdem ordentlich nach oben rauschen.
Sollte es zu einem BuyOut-Bidding von verschiedensten BPs ( Pfizer ? Amgen ? xxxx ? etc. ) kommen und ein sehr hohes Buyoutangebot vorliegen , dann würde ich das " nicht mit uns " nicht mehr unterschreiben.
Ich denke das Amarin natürlich weiss das sie ihr Marktpotential nur dann umfangreich nutzen können wenn sie eine Partnerschafr mit BP eingehen. Es ist wichtig in möglichst kurzer Zeit einen grossen Fussabdruck im Markt zu hinterlassen sichert höhere Einnahmen über Jahre. Ausserdem brauchen sie einen grossen Partner für Produktion usw. jedoch wie gesagt Buyout sehe ich nicht, Amarin kann so die Zügel in der Hand behalten und hat eine ideale Verhandlungsposition. Denke das auch die Nachricht einer Partnerschaft in Kombination mit hoffentlich full label expansion nochmal ordentlich Dampf macht. Sehe im ersten Quartal nächsten Jahres solch eine Entscheidung als realistisch. Sie müssen nichts überstürzen und können ideal verhandeln...
Antwort auf Beitrag Nr.: 61.933.181 von flyingbeef am 15.11.19 14:22:08
die Mehrheit der Aktien befinden sich im Streubesitz bzw. bei institutionellen Anlegern. Wenn diese mit einer Übernahme einverstanden sind, dann wird das Management nur wenig dagegen ausrichten können!
Für das Ausmass des wirtschaftlichen Erfolg ist entscheidend, wie "breit" das Label bei der Anwendung sein wird. Dies werden wir spätestens am 28.12.2019 erfahren, wenn die FDA ihre Zulassungsentscheidung einschliesslich Label bekanntgeben wird. Die Sekundärpräventionspatienten dürften sicher sein - das ist fast schon 100%ig....obschon es ein solch sicheres Szenario an der Börse eigentlich nicht gibt. Bei den Primärpräventionspatienten erscheint mir alles offen - ich persönlich rechne nicht damit, dass alle Risikopatienten mit eingeschlossen werden, sondern nur solche mit bestehender Herz-Kreislaufsymptomatik. Aber das ist reine Spekulation.
Es gilt wie immer: TIME WILL TELL!
Zitat von flyingbeef:Zitat von Magnetfeldfredy: John Thero hat nur 5 % seiner Aktien verkauft, das hat mich nicht beunruhight, 16:0 und Evaportate vor der Tür lassen auf viel mehr hoffen, bzw. eine Übernahme für US Dollar 50!
Wenn ich mich richtig erinnere:
Amarin hat aber immer gesagt Übernahme "nicht mit uns" daher ich glaube die wollen die Zügel auch langfristig in der Hand haben sollte trotzdem ordentlich nach oben rauschen.
die Mehrheit der Aktien befinden sich im Streubesitz bzw. bei institutionellen Anlegern. Wenn diese mit einer Übernahme einverstanden sind, dann wird das Management nur wenig dagegen ausrichten können!
Für das Ausmass des wirtschaftlichen Erfolg ist entscheidend, wie "breit" das Label bei der Anwendung sein wird. Dies werden wir spätestens am 28.12.2019 erfahren, wenn die FDA ihre Zulassungsentscheidung einschliesslich Label bekanntgeben wird. Die Sekundärpräventionspatienten dürften sicher sein - das ist fast schon 100%ig....obschon es ein solch sicheres Szenario an der Börse eigentlich nicht gibt. Bei den Primärpräventionspatienten erscheint mir alles offen - ich persönlich rechne nicht damit, dass alle Risikopatienten mit eingeschlossen werden, sondern nur solche mit bestehender Herz-Kreislaufsymptomatik. Aber das ist reine Spekulation.
Es gilt wie immer: TIME WILL TELL!
Antwort auf Beitrag Nr.: 61.936.895 von flyingbeef am 15.11.19 20:41:53
das darf doch mal sein, insbesondere nach einem positiven AC...ich habe mir gestern ein Fläschchen Markgräfler Gutedel gegönnt - Isteiner Churchhill
Zitat von flyingbeef: Ich sitze hier mit Popcorn echt wahr

Sorry war jetzt nicht sehr informativ aber an solchen Tagen darf man auch mal etwas albern sein 🙃
das darf doch mal sein, insbesondere nach einem positiven AC...ich habe mir gestern ein Fläschchen Markgräfler Gutedel gegönnt - Isteiner Churchhill

Evaportate mogen wird hoffentlich noch mehr Positives hervorbringen, sollte sich bestätigen, dass Vascepa u.a. auch Plaque statistisch signifikant reduziert, ist das der Sargnagel für 55 Millionen shorts!



Evaporate heißt die Studie!

What's Next for Amarin's Stock?
Amarin's days could be numbered after a positive adcom vote.
George Budwell
George Budwell
(TMFGBudwell)
Nov 15, 2019 at 9:00AM
Author Bio
Yesterday, Amarin (NASDAQ:AMRN) scored a major win. All 16 panelists on Vascepa's advisory committee voted in favor of the drug's cardioprotective benefit based on the unprecedented results of the cardiovascular outcomes study Reduce-It.
Wall Street, for its part, immediately chimed in on the potential impact of this favorable outcome. Analysts quickly noted that this label expansion could broaden the drug's target market to anywhere between 5 million to 15 million patients in the United States, depending on how broad of a label the FDA chooses to go with.
Wooden blocks that spell out "buy-out".
Image Source: Getty Images.
The quirky part of the story is that these same analysts suggested that Vascepa's peak sales could vary from a low of $1.5 billion to a high of $4 billion following this positive advisory committee vote -- again, reflecting the huge discrepancy in the drug's potential target market based on the uncertainty of its final label.
What the Street is really saying
What's odd about these peak sales figures is that they simply don't make mathematical sense. To square this circle, we have to assume that Wall Street is either predicting that Amarin will significantly lower Vascepa's net selling price (it won't), or that the company will have a hard time servicing this massive target market (it might).
Breaking this down further, most industry insiders agree that Vascepa's Reduce-It indication should cover at least around 10 million people in the United States (i.e., patients presently on statin therapy but who still have trouble controlling their triglyceride levels). A $4 billion peak sales estimate thus implies a 25% penetration rate.
That's crazy low for a drug aimed at patients with an elevated risk of heart attack or stoke, and that are already receiving care in the form of statin therapy. Stated plainly, pharma reps aren't going to have to beat the bushes to identify the vast majority of these patients.
What gives? Wall Street is basically saying that Amarin won't be able to handle the volume associated with this market -- and there's good reason to believe that a bottleneck effect could come into play.
What's next?
To remedy this situation, Amarin has three clear choices to maximize shareholder value in the near-term:
Aggressively build out its commercial infrastructure. Amarin has announced a doubling of its sales force, but it still has a long ways to go before it can fully cover this enormous patient population. This option also runs the risk of jacking up expenses well before the surge in demand fully materializes. That's not exactly prudent for a company finally on the cusp of becoming cash flow positive.
Enter into a co-promotional partnership with a big pharma like Pfizer (NYSE:PFE). Pfizer has the connections and commercial bandwidth to transform Vascepa into a juggernaut within a very short period of time. A co-promotional deal might also involve a significant equity stake from the likes of Pfizer or perhaps a large up-front cash infusion. Either way, Amarin's shareholders would benefit immensely from a partnering deal.
Put the company up for sale. Amgen, Pfizer, and Novartis have all been rumored to have interest in Amarin. An outright sale eliminates the inherent commercialization risk associated with a go-it-alone strategy, and it would almost certainly come at a healthy premium.
What's the most likely outcome? A buyout. These types of megablockbuster drugs do not come around often, and there's just no way big pharma is going to let this opportunity pass them by. In fact, Pfizer has a Vascepa-sized hole in its product portfolio right now with the loss of exclusivity for its nerve pain medication Lyrica. Pfizer's management has also openly admitted to an interest in pursuing more bolt-on acquisitions. Amarin, in many ways, would be an ideal pick-up for Pfizer, especially with the big pharma's ongoing metamorphosis into a growth-oriented company.
Amarin's days could be numbered after a positive adcom vote.
George Budwell
George Budwell
(TMFGBudwell)
Nov 15, 2019 at 9:00AM
Author Bio
Yesterday, Amarin (NASDAQ:AMRN) scored a major win. All 16 panelists on Vascepa's advisory committee voted in favor of the drug's cardioprotective benefit based on the unprecedented results of the cardiovascular outcomes study Reduce-It.
Wall Street, for its part, immediately chimed in on the potential impact of this favorable outcome. Analysts quickly noted that this label expansion could broaden the drug's target market to anywhere between 5 million to 15 million patients in the United States, depending on how broad of a label the FDA chooses to go with.
Wooden blocks that spell out "buy-out".
Image Source: Getty Images.
The quirky part of the story is that these same analysts suggested that Vascepa's peak sales could vary from a low of $1.5 billion to a high of $4 billion following this positive advisory committee vote -- again, reflecting the huge discrepancy in the drug's potential target market based on the uncertainty of its final label.
What the Street is really saying
What's odd about these peak sales figures is that they simply don't make mathematical sense. To square this circle, we have to assume that Wall Street is either predicting that Amarin will significantly lower Vascepa's net selling price (it won't), or that the company will have a hard time servicing this massive target market (it might).
Breaking this down further, most industry insiders agree that Vascepa's Reduce-It indication should cover at least around 10 million people in the United States (i.e., patients presently on statin therapy but who still have trouble controlling their triglyceride levels). A $4 billion peak sales estimate thus implies a 25% penetration rate.
That's crazy low for a drug aimed at patients with an elevated risk of heart attack or stoke, and that are already receiving care in the form of statin therapy. Stated plainly, pharma reps aren't going to have to beat the bushes to identify the vast majority of these patients.
What gives? Wall Street is basically saying that Amarin won't be able to handle the volume associated with this market -- and there's good reason to believe that a bottleneck effect could come into play.
What's next?
To remedy this situation, Amarin has three clear choices to maximize shareholder value in the near-term:
Aggressively build out its commercial infrastructure. Amarin has announced a doubling of its sales force, but it still has a long ways to go before it can fully cover this enormous patient population. This option also runs the risk of jacking up expenses well before the surge in demand fully materializes. That's not exactly prudent for a company finally on the cusp of becoming cash flow positive.
Enter into a co-promotional partnership with a big pharma like Pfizer (NYSE:PFE). Pfizer has the connections and commercial bandwidth to transform Vascepa into a juggernaut within a very short period of time. A co-promotional deal might also involve a significant equity stake from the likes of Pfizer or perhaps a large up-front cash infusion. Either way, Amarin's shareholders would benefit immensely from a partnering deal.
Put the company up for sale. Amgen, Pfizer, and Novartis have all been rumored to have interest in Amarin. An outright sale eliminates the inherent commercialization risk associated with a go-it-alone strategy, and it would almost certainly come at a healthy premium.
What's the most likely outcome? A buyout. These types of megablockbuster drugs do not come around often, and there's just no way big pharma is going to let this opportunity pass them by. In fact, Pfizer has a Vascepa-sized hole in its product portfolio right now with the loss of exclusivity for its nerve pain medication Lyrica. Pfizer's management has also openly admitted to an interest in pursuing more bolt-on acquisitions. Amarin, in many ways, would be an ideal pick-up for Pfizer, especially with the big pharma's ongoing metamorphosis into a growth-oriented company.
Antwort auf Beitrag Nr.: 61.939.715 von Cyberhexe am 16.11.19 12:26:23
POPCORN...

ISTEINER CHURCHHILL..




Zitat von Cyberhexe:Zitat von flyingbeef: Ich sitze hier mit Popcorn echt wahr
....das darf doch mal sein, insbesondere nach einem positiven AC...ich habe mir gestern ein Fläschchen Markgräfler Gutedel gegönnt - Isteiner Churchhill
POPCORN...


ISTEINER CHURCHHILL..





Antwort auf Beitrag Nr.: 61.942.841 von Magnetfeldfredy am 17.11.19 10:42:03
Kurz zur Info:
Cherry Study using 1.8g /day of EPA for 7-8 months got ~ 18% reduction in plaque on healthier sushi eating Japanese population.
EVAPORATE will have ~9 month interim data with 4g/EPA a day.
Welche Plaque-Regression wird morgen am 18.11.19 um 15.00 MEZ in der EVAPORATE - Studie als Zwischenergebnis bekanntgegeben ????
Zitat von Magnetfeldfredy: Evaportate mogen wird hoffentlich noch mehr Positives hervorbringen, sollte sich bestätigen, dass Vascepa u.a. auch Plaque statistisch signifikant reduziert, ist das der Sargnagel für 55 Millionen shorts!
Kurz zur Info:
Cherry Study using 1.8g /day of EPA for 7-8 months got ~ 18% reduction in plaque on healthier sushi eating Japanese population.
EVAPORATE will have ~9 month interim data with 4g/EPA a day.
Welche Plaque-Regression wird morgen am 18.11.19 um 15.00 MEZ in der EVAPORATE - Studie als Zwischenergebnis bekanntgegeben ????

Zeitplan für November -Dezember
Monday, November 18, 9:00 – 9:10 a.m., Eastern U.S. Time
Session LBS.06 –
Late Breaking Science VI: New Frontiers in Lipid Therapy,
“Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis in Patients with Elevated Triglycerides (200 – 499 mg/dL) on Statin Therapy (EVAPORATE study),” – presented by Matthew J. Budoff, M.D., Los Angeles Biomedical Research.
Thursday, November 21, 2019, at 8:40 a.m. Greenwich Mean Time (GMT) / 3:40 a.m. Eastern Time (ET) in London, UK.
A general company update at the Jefferies 2019 London Healthcare Conference on
02.12 - 06.12
CANADA approval ?
28.12.19
PDUFA - FDA
Monday, November 18, 9:00 – 9:10 a.m., Eastern U.S. Time
Session LBS.06 –
Late Breaking Science VI: New Frontiers in Lipid Therapy,
“Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis in Patients with Elevated Triglycerides (200 – 499 mg/dL) on Statin Therapy (EVAPORATE study),” – presented by Matthew J. Budoff, M.D., Los Angeles Biomedical Research.
Thursday, November 21, 2019, at 8:40 a.m. Greenwich Mean Time (GMT) / 3:40 a.m. Eastern Time (ET) in London, UK.
A general company update at the Jefferies 2019 London Healthcare Conference on
02.12 - 06.12
CANADA approval ?
28.12.19
PDUFA - FDA
Leider sind es nur Interim results, aber als Late Braker tituliert, d.h. bahnbrechende news bzw. game-changer, yes we hope, alla Obama und nicht Twitter Depp Trump!

 > 9,000,000 Total Capsules/week
> 9,000,000 Total Capsules/week 

And this is first the beginning, so Jon Thero, CEO Amarin!

Antwort auf Beitrag Nr.: 61.943.798 von bernie55 am 17.11.19 14:27:33
Isteiner Churchhill - das ist nicht geflunkert, weil richtig übersetzt:

Zitat von bernie55:Zitat von Cyberhexe: ...
....das darf doch mal sein, insbesondere nach einem positiven AC...ich habe mir gestern ein Fläschchen Markgräfler Gutedel gegönnt - Isteiner Churchhill
POPCORN...
ISTEINER CHURCHHILL..
Isteiner Churchhill - das ist nicht geflunkert, weil richtig übersetzt:

Antwort auf Beitrag Nr.: 61.950.698 von Cyberhexe am 18.11.19 15:17:44...und hier das Original: Isteiner Churchhill mit Isteiner Klotz im Hintergrund 
hier wachsen edle Tropfen!


hier wachsen edle Tropfen!

Antwort auf Beitrag Nr.: 61.946.360 von Magnetfeldfredy am 18.11.19 06:51:33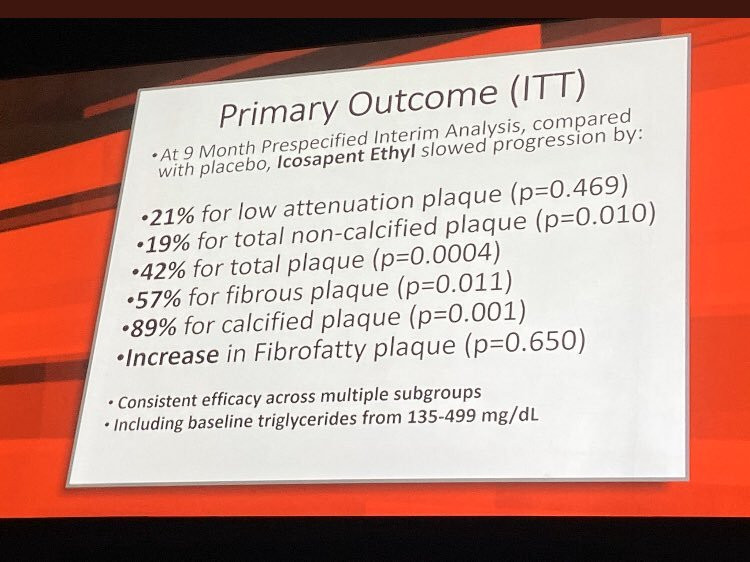
sieht doch erstmal gut aus
VG Christian

sieht doch erstmal gut aus
VG Christian
Antwort auf Beitrag Nr.: 61.950.929 von esaposse am 18.11.19 15:38:35commentator (Stephen nicholls) says that study failed to achieve primary endpoint at 9 months. Says there was no evaporation of plaque but a slowing of plaque progression in a number of secondary endpoints. Says further studies are needed.
D.h. nicht der erwartete Durchbruch, wir müssen die finalen Ergebnisse abwarten, heute wohl eher kein Short Squeeze
D.h. nicht der erwartete Durchbruch, wir müssen die finalen Ergebnisse abwarten, heute wohl eher kein Short Squeeze

 Für mich sehr gute Ergebnisse, Evaporate zeigt den mechanism of action mit der Plaque Reduktion eindrucksvoll, und dass nach 9 Monaten noch nicht alles optimal ist beweisen auch die Reduce-It Studie, je länger man Vascepa nimmt desto besser werden die Ergebnisse!
Für mich sehr gute Ergebnisse, Evaporate zeigt den mechanism of action mit der Plaque Reduktion eindrucksvoll, und dass nach 9 Monaten noch nicht alles optimal ist beweisen auch die Reduce-It Studie, je länger man Vascepa nimmt desto besser werden die Ergebnisse!Auch wurde das leidige Mineralöl-Placebo mit Evoporate beerdigt, super Interview, big pharma hat jetzt mit diesen Ergebnissen eindrucksvolle Beweise wie Vascepa wirkt und wird zur Übernahme führen:
Antwort auf Beitrag Nr.: 61.950.758 von Cyberhexe am 18.11.19 15:22:27Hast Du den Weinberg gekauft?😂
Antwort auf Beitrag Nr.: 61.953.158 von asthmamoah am 18.11.19 19:08:33

...nur einen ganz kleinen Teil davon!
Zitat von asthmamoah: Hast Du den Weinberg gekauft?😂

...nur einen ganz kleinen Teil davon!

Antwort auf Beitrag Nr.: 61.951.982 von Magnetfeldfredy am 18.11.19 17:07:19
Die Ergebnisse, die nur auf vorläufigen Daten basieren, zeigen, dass die Studie ihren primären Endpunkt, nämlich verringertes Plaquevolumen- (noch) nicht erreicht hat.
Bei den veröffentlichten Daten handelt es sich um einen neunmonatigen "Zwischenblick" auf eine kleine Studie mit 80 Patienten, die eigentlich für eine doppelt so lange Laufzeit ausgelegt ist.
Die Daten beinhalten auch Auswertungen auf andere Plaque-Wachstumsmaße, wobei die Plaque-Progressionsraten in der Mineralöl-Placebo-Gruppe identisch mit denen eines Placebos auf Cellulosebasis waren. Diese anderen Placebo-Daten stammen aus einer anderen Studie, sind jedoch genau auf die Daten der Placebo-Kohorte der neuen Vascepa-Studie abgestimmt.
"Bei vier verschiedenen Plaque-Markern, einschließlich der Gesamtplaque, wurde eine signifikante Verlangsamung der Progression festgestellt", sagte Matthew Budoff, UCLA-Professor an der School of Medicine.
Diese Verlangsamung von 42% im Vergleich zu Placebo war bei den bisherigen Wirksamkeitsdaten besonders bemerkenswert. Beruhigend ist vor allem "dass die verlangsamten Fortschrittsraten, die wir bei fünf der sechs von uns gemessenen Marker sahen, auf die Vorteile von Icosapent ethyl und nicht auf die Schäden von Mineralöl zurückzuführen sind", fügte er hinzu.
Zitat von Magnetfeldfredy:Für mich sehr gute Ergebnisse, Evaporate zeigt den mechanism of action mit der Plaque Reduktion eindrucksvoll, und dass nach 9 Monaten noch nicht alles optimal ist beweisen auch die Reduce-It Studie, je länger man Vascepa nimmt desto besser werden die Ergebnisse!
Auch wurde das leidige Mineralöl-Placebo mit Evoporate beerdigt, super Interview, big pharma hat jetzt mit diesen Ergebnissen eindrucksvolle Beweise wie Vascepa wirkt und wird zur Übernahme führen:
Die Ergebnisse, die nur auf vorläufigen Daten basieren, zeigen, dass die Studie ihren primären Endpunkt, nämlich verringertes Plaquevolumen- (noch) nicht erreicht hat.
Bei den veröffentlichten Daten handelt es sich um einen neunmonatigen "Zwischenblick" auf eine kleine Studie mit 80 Patienten, die eigentlich für eine doppelt so lange Laufzeit ausgelegt ist.
Die Daten beinhalten auch Auswertungen auf andere Plaque-Wachstumsmaße, wobei die Plaque-Progressionsraten in der Mineralöl-Placebo-Gruppe identisch mit denen eines Placebos auf Cellulosebasis waren. Diese anderen Placebo-Daten stammen aus einer anderen Studie, sind jedoch genau auf die Daten der Placebo-Kohorte der neuen Vascepa-Studie abgestimmt.
"Bei vier verschiedenen Plaque-Markern, einschließlich der Gesamtplaque, wurde eine signifikante Verlangsamung der Progression festgestellt", sagte Matthew Budoff, UCLA-Professor an der School of Medicine.
Diese Verlangsamung von 42% im Vergleich zu Placebo war bei den bisherigen Wirksamkeitsdaten besonders bemerkenswert. Beruhigend ist vor allem "dass die verlangsamten Fortschrittsraten, die wir bei fünf der sechs von uns gemessenen Marker sahen, auf die Vorteile von Icosapent ethyl und nicht auf die Schäden von Mineralöl zurückzuführen sind", fügte er hinzu.
Wir haben einen absoluten Gewinner mit Amarin, hier die Zusammenfassung der AHA2019 mit unglaublich guten Ergebnissen, dazu das historische 16:0 beim Adcom:
Amarin Highlights Key REDUCE-IT®-Related Data Presented at American Heart Association 2019 Scientific Sessions
[GlobeNewswire]
GlobeNewswire•November 18, 2019
REDUCE-IT USA results, in prespecified subgroup analyses, showed cardiovascular risk reductions across all endpoints, including 30% relative risk reduction in all-cause mortality
New analysis determined icosapent ethyl (Vascepa®) is highly cost-effective in patients from the REDUCE-IT study and, as is rarely found, may result in net healthcare cost-savings to patients, payers and society
Data showed prevalence of elevated risk of major cardiovascular events (mean 10-year ASCVD risk score greater than 20%) in more than 20% of patients on statins with triglycerides below 150 mg/dL
Interim EVAPORATE study provides important mechanistic data with relevance to the reduction in cardiovascular events seen in the REDUCE-IT clinical trial; final study results likely in early 2020
DUBLIN, Ireland and BRIDGEWATER, N.J., Nov. 18, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, hosted a webcast today to discuss important data with study authors who presented at the American Heart Association 2019 Scientific Sessions, November 16-18. The data covered related to Vascepa® (icosapent ethyl) capsules, the landmark clinical outcomes study REDUCE-IT®[1] , as well as the cardiovascular risk of patients with elevated triglycerides, a type of fat in the blood.
“The more we study the REDUCE-IT data and the at-risk conditions of the patients studied in this important clinical trial, the better we understand the nature and extent of persistent cardiovascular risk among patients on statins and with elevated triglycerides, and how to address it,” said Craig Granowitz, M.D., Ph.D., chief medical officer, Amarin. “At Amarin, we are proud to have played a role in supporting and sharing data with the scientific and medical communities that could make a major difference in cardiovascular care, an area where the need for new and innovative treatment options is urgent and growing.”
About Cardiovascular Risk
The number of deaths in the United States attributed to cardiovascular disease continues to rise.2,[3] There are 605,000 new and 200,000 recurrent heart attacks per year (approximately 1 every 40 seconds), in the United States. Stroke rates are similar, accounting for 1 of every 19 U.S. deaths (approximately 1 every 40 seconds).4
Controlling bad cholesterol, also known as LDL-C, is one way to reduce a patient’s risk for cardiovascular events, such as heart attack, stroke or death. However, even with the achievement of target LDL-C levels, millions of patients still have significant and persistent risk of cardiovascular events, especially those patients with high triglycerides. Statin therapy has been shown to control LDL-C, thereby reducing the risk of cardiovascular events by 25-35% – but that still leaves 65-75% risk remaining.5 People with high triglycerides have 35% more cardiovascular events compared to people with normal (in range) triglycerides taking statins.6,[7],[8]
Key Data Presented at AHA and Reviewed During Amarin’s Webcast
“REDUCE-IT USA: Results from the 3,146 Patients Randomized in the United States,” – presented by Deepak L. Bhatt, M.D., M.P.H., Brigham and Women’s Hospital and Harvard Medical School.
Highlights: This prespecified REDUCE-IT subgroup analysis showed substantial risk reductions in the USA patients treated with icosapent ethyl 4 g/day versus placebo across all prespecified composite and individual primary and secondary endpoints, including 31% relative risk reduction and 6.5% absolute risk reduction in first occurrence of 5-point major adverse cardiovascular events (MACE), corresponding to a number needed to treat of 15 (NNT=15), and a significant 30% relative and 2.6% absolute risk reduction (NNT=39) in all-cause mortality in the USA subgroup.
Additional prespecified cardiovascular endpoints in which the REDUCE-IT USA subgroup showed significant relative risk reduction included myocardial infarction, cardiovascular death, and stroke, similar to the full cohort in the overall REDUCE-IT global results. These results were incremental to the cardiovascular risk reduction achieved by conventional therapy administered to the high-risk patients studied, including incremental to statin therapy.
The REDUCE-IT USA subgroup consisted of 3,146 patients (nearly 40%) of the previously reported full trial cohort. REDUCE-IT was not specifically powered to examine individual subgroups. P-values presented for the USA subgroup are nominal and exploratory with no adjustment for multiple comparisons. Differences in efficacy outcomes for the USA patients are best viewed as qualitative and not quantitative; nevertheless, the data are useful and provide reassurance that the results in the USA are at least as strong as the results seen outside the USA and in the trial overall.
The REDUCE-IT USA study results were also published in Circulation, AHA’s official scientific journal.9
“Cost-Effectiveness of Icosapent Ethyl in REDUCE-IT,” – presented by William S. Weintraub, M.D., MedStar Washington Hospital.
Highlights: In this combined patient-level and simulation lifetime cost-effectiveness analysis, icosapent ethyl in high cardiovascular risk patients shows exceptional benefit with cardiovascular event reduction as well as cost-savings in-trial and over patients’ lifetime in many simulations. Findings of potential cost effectiveness of a medical therapy are rare. This analysis considered the current market cost for Vascepa and the potential savings from avoiding major adverse cardiovascular events, such as strokes and heart attacks, the cost of which can be high. This cost-effectiveness analysis was conducted by MedStar. As is typical, the cost-effectiveness analyses were not prespecified as part of the REDUCE-IT clinical trial design but did rely on the results of this landmark outcomes study.
“Many Statin Treated Persons with Borderline Triglyceride Levels are at Risk of ASCVD,” – presented by Nathan D. Wong, Ph.D., M.P.H, University of California, Irvine.
Highlights: This study showed prevalence of elevated risk of major cardiovascular events (a mean 10-year atherosclerotic cardiovascular disease (ASCVD) risk score greater than 20%) in more than 20% of patients on statins with triglycerides below 150 mg/dL. This suggests the need for greater lifestyle and other therapies to address remaining residual ASCVD risk.
“Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis in Patients with Elevated Triglycerides (200 – 499 mg/dL) on Statin Therapy (EVAPORATE) study,” – presented by Matthew J. Budoff, M.D., Los Angeles Biomedical Research.
Highlights: At the prespecified 9-month interim analysis, there was slowing of total non-calcified plaque (sum of LAP, fibrofatty, and fibrous plaque) (35% v. 43%, p=0.010), total plaque (non-calcified + calcified plaque) (p=0.0004), fibrous plaque (15% v. 26%, p=0.011) and calcified plaque (-1% v. 9%, p=0.001), after adjustment by baseline plaque, age, sex, diabetes status, baseline triglyceride levels, and statin use. However, there was no significant change in the primary endpoint of low attenuation plaque between active and placebo groups (74% vs 94%, p=0.469) at this 9-month interim look. This investigator-initiated study is continuing to its designed completion of an 18-month review.
EVAPORATE is the first study in the United States, which enrolled a total of 80 patients, to use multidetector computed tomography (MDCT) to evaluate the effects of icosapent ethyl as an adjunct to statin therapy on plaque characteristics in a high cardiovascular-risk population with persistent high triglyceride levels. Patients underwent interim scans at 9 months and are currently being followed for an additional 9 months with MDCT. Final results from this study are anticipated in early 2020.
Arterial plaque and coronary atherosclerosis are key factors leading to significant increases in the probability of acute obstructions and angina or other coronary artery disease signs and symptoms.
All of the analyses highlighted above were funded by Amarin.
A replay of the webcast will be available for two weeks following the webcast. To hear a replay of the webcast, dial 877-481-4010 (inside the United States) or 919-882-2331 (outside the United States). A replay of the webcast is also be available through the company's website, Amarincorp.com, in the Investor section. For both dial-in numbers please use conference ID 55923.
About Amarin
Amarin Corporation plc. is a rapidly growing, innovative pharmaceutical company focused on developing therapeutics to improve cardiovascular health. Amarin’s product development program leverages its extensive experience in polyunsaturated fatty acids and lipid science. Vascepa (icosapent ethyl) is Amarin's first FDA-approved drug and is available by prescription in the United States, Lebanon and the United Arab Emirates. Amarin’s commercial partners are pursuing additional regulatory approvals for Vascepa in Canada, China and the Middle East. For more information about Amarin, visit www.amarincorp.com.
About REDUCE-IT®
REDUCE-IT, an 8,179-patient cardiovascular outcomes study, was completed in 2018. REDUCE-IT was the first multinational cardiovascular outcomes study that evaluated the cardioprotective effect of icosapent ethyl, a unique prescription therapy, as an add-on to statins in patients with high cardiovascular risk. As defined in the published results of the study, the high cardiovascular risk patients, despite stable statin therapy, had elevated triglyceride levels (lower enrollment target of TG >135 mg/dL). As per the study’s design, approximately 71% of the enrolled patients had established cardiovascular disease and the other patients were diagnosed, as per trial enrollment requirements, as having diabetes and other cardiovascular risk factors.
More information on the REDUCE-IT study results can be found at www.amarincorp.com.
About Vascepa® (icosapent ethyl) Capsules
Vascepa (icosapent ethyl) capsules are a single-molecule prescription product which has been developed and studied for more than a decade and has been prescribed more than 8 million times in the United States. Vascepa, known in scientific literature as AMR101, has been designated a new chemical entity by the FDA. Amarin has been issued multiple patents in the United States and internationally based on the unique clinical profile of Vascepa, including the drug’s ability to lower triglyceride levels in relevant patient populations without raising LDL-cholesterol levels.
The FDA has not completed its review or made a final determination on a supplemental new drug application related to REDUCE-IT. FDA has not reviewed the information herein or determined whether to approve Vascepa for use to reduce the risk of major adverse cardiovascular events in the REDUCE-IT patient population.
Indication and Usage Based on Current FDA-Approved Label (not including REDUCE-IT results)
Vascepa (icosapent ethyl) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
The effect of Vascepa on the risk for pancreatitis and cardiovascular mortality and morbidity in patients with severe hypertriglyceridemia has not been determined.
Important Safety Information for Vascepa Based on Current FDA-Approved Label (not including REDUCE-IT results) (Includes Data from Two 12-Week Studies (n=622) (MARINE and ANCHOR) of Patients with Triglycerides Values of 200 to 2000 mg/dL)
Vascepa is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vascepa or any of its components.
In patients with hepatic impairment, monitor ALT and AST levels periodically during therapy.
Use with caution in patients with known hypersensitivity to fish and/or shellfish.
The most common reported adverse reaction (incidence >2% and greater than placebo) was arthralgia (2.3% for Vascepa, 1.0% for placebo). There was no reported adverse reaction >3% and greater than placebo.
Adverse events and product complaints may be reported by calling 1-855-VASCEPA or the FDA at 1-800-FDA-1088. Patients receiving treatment with Vascepa and other drugs affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
Patients should be advised to swallow Vascepa capsules whole; not to break open, crush, dissolve, or chew Vascepa.
FULL VASCEPA PRESCRIBING INFORMATION CAN BE FOUND AT WWW.VASCEPA.COM.
Important Safety Information for Vascepa based on REDUCE-IT, as previously reported in The New England Journal of Medicine publication of the primary results of the REDUCE-IT study:
Excluding the major adverse cardiovascular events (MACE) results described above, overall adverse event rates in REDUCE-IT were similar across the statin plus Vascepa and the statin plus placebo treatment groups.
There were no significant differences between treatments in the overall rate of treatment emergent adverse events or serious adverse events leading to withdrawal of study drug.
There was no serious adverse event (SAE) occurring at a frequency of >2% which occurred at a numerically higher rate in the statin plus Vascepa treatment group than in the statin plus placebo treatment group.
Adverse events (AEs) occurring in 5% or greater of patients and more frequently with Vascepa than placebo were:
peripheral edema (6.5% Vascepa patients versus 5.0% placebo patients), although there was no increase in the rate of heart failure in Vascepa patients
constipation (5.4% Vascepa patients versus 3.6% placebo patients), although mineral oil, as used as placebo, is known to lower constipation, and
atrial fibrillation (5.3% Vascepa patients versus 3.9% placebo patients), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients
There were numerically more SAEs related to bleeding in the statin plus Vascepa treatment group although overall rates were low with no fatal bleeding observed in either group and no significant difference in adjudicated hemorrhagic stroke or serious central nervous system or gastrointestinal bleeding events between treatments.
In summary, Vascepa was well tolerated with a safety profile generally consistent with clinical experience associated with omega-3 fatty acids and current FDA-approved labeling of such products.
Important Cautionary Information About These Data
Further REDUCE-IT data assessment and data release are expected to yield additional useful information to inform greater understanding of the trial outcome. For example, detailed data assessment by regulatory authorities, such as the FDA and Health Canada, will continue and take time to complete and announce. The FDA advisory committee process and the final evaluation by regulatory authorities of the totality of efficacy and safety data from REDUCE-IT is anticipated to include some or all of the following, as well as other considerations: new information or analyses affecting the degree of treatment benefit on studied endpoints; study conduct and data robustness, quality, integrity and consistency; additional safety data considerations and risk/benefit considerations; and consideration of REDUCE-IT results in the context of other clinical studies. More detailed presentation of such considerations is set forth in the risk factors section of Amarin’s Quarterly Report on Form 10-Q filed with the U.S. Securities and Exchange Commission. Because regulatory reviews are typically fluid and not definitive interactions between sponsor and agency on individual elements of an application and related information, Amarin does not plan to update investors further on ongoing communications with regulatory authorities. Amarin plans to announce the final outcome of such regulatory reviews when appropriate.
Forward-Looking Statements
This press release contains forward-looking statements, including statements regarding the use of Vascepa to potentially help millions of patients. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected herein include the following: uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals; the risk that data interpretations or other information from third parties, the regulatory review process, regulatory authorities and in connection with an advisory committee could be made public that are negative or may delay approval or limit Vascepa’s marketability; the risk that special protocol assessment (SPA) agreements with the FDA are not a guarantee that FDA will approve a product candidate; the risk associated with the FDA's rescinding the REDUCE-IT SPA agreement; the risk related to FDA advisory committee meetings; and the risk that the FDA may not complete its review of the REDUCE-IT sNDA within the timing expected. A further list and description of these risks, uncertainties and other risks associated with an investment in Amarin can be found in Amarin's filings with the U.S. Securities and Exchange Commission, including its most recent Quarterly Report on Form 10-Q. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Amarin undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Availability of Other Information About Amarin
Investors and others should note that Amarin communicates with its investors and the public using the company website (www.amarincorp.com), the investor relations website (investor.amarincorp.com), including but not limited to investor presentations and investor FAQs, Securities and Exchange Commission filings, press releases, public conference calls and webcasts. The information that Amarin posts on these channels and websites could be deemed to be material information. As a result, Amarin encourages investors, the media, and others interested in Amarin to review the information that is posted on these channels, including the investor relations website, on a regular basis. This list of channels may be updated from time to time on Amarin’s investor relations website and may include social media channels. The contents of Amarin’s website or these channels, or any other website that may be accessed from its website or these channels, shall not be deemed incorporated by reference in any filing under the Securities Act of 1933.
Amarin Contact Information
Investor Inquiries:
Elisabeth Schwartz
Investor Relations
Amarin Corporation plc
In U.S.: +1 (908) 719-1315
investor.relations@amarincorp.com
Lee M. Stern
Solebury Trout
In U.S.: +1 (646) 378-2992
lstern@soleburytrout.com
Media Inquiries:
Gwen Fisher
Corporate Communications
Amarin Corporation plc
In U.S.: +1 (908) 325-0735
pr@amarincorp.com
References
1 Bhatt DL, Steg PG, Miller M, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med 2019;380:11-22.
2 American Heart Association. Heart Disease and Stroke Statistics – 2019 Update: A Report from the American Heart Association. Published January 31, 2019.
3 American Heart Association / American Stroke Association. 2017. Cardiovascular disease: A costly burden for America projections through 2035.
4 American Heart Association: Heart Disease and Stroke Statistics -- 2019 At-a-Glance.
5 Ganda OP, Bhatt DL, Mason RP, et al. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72(3):330-343.
6 Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118:138-145.
7 Toth PP, Granowitz C, Hull M, et al. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: A real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7(15):e008740.
8 Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease - New insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547-563.
9 Bhatt DL, Miller M, Brinton EA, et al. REDUCE-IT USA: Results from the 3,146 Patients Randomized in the United States. Circulation 2019. DOI: 10.1161/CIRCULATIONAHA.119.044440.
Contact:
Sign in to post a message.
Amarin Highlights Key REDUCE-IT®-Related Data Presented at American Heart Association 2019 Scientific Sessions
[GlobeNewswire]
GlobeNewswire•November 18, 2019
REDUCE-IT USA results, in prespecified subgroup analyses, showed cardiovascular risk reductions across all endpoints, including 30% relative risk reduction in all-cause mortality
New analysis determined icosapent ethyl (Vascepa®) is highly cost-effective in patients from the REDUCE-IT study and, as is rarely found, may result in net healthcare cost-savings to patients, payers and society
Data showed prevalence of elevated risk of major cardiovascular events (mean 10-year ASCVD risk score greater than 20%) in more than 20% of patients on statins with triglycerides below 150 mg/dL
Interim EVAPORATE study provides important mechanistic data with relevance to the reduction in cardiovascular events seen in the REDUCE-IT clinical trial; final study results likely in early 2020
DUBLIN, Ireland and BRIDGEWATER, N.J., Nov. 18, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN), a pharmaceutical company focused on improving cardiovascular health, hosted a webcast today to discuss important data with study authors who presented at the American Heart Association 2019 Scientific Sessions, November 16-18. The data covered related to Vascepa® (icosapent ethyl) capsules, the landmark clinical outcomes study REDUCE-IT®[1] , as well as the cardiovascular risk of patients with elevated triglycerides, a type of fat in the blood.
“The more we study the REDUCE-IT data and the at-risk conditions of the patients studied in this important clinical trial, the better we understand the nature and extent of persistent cardiovascular risk among patients on statins and with elevated triglycerides, and how to address it,” said Craig Granowitz, M.D., Ph.D., chief medical officer, Amarin. “At Amarin, we are proud to have played a role in supporting and sharing data with the scientific and medical communities that could make a major difference in cardiovascular care, an area where the need for new and innovative treatment options is urgent and growing.”
About Cardiovascular Risk
The number of deaths in the United States attributed to cardiovascular disease continues to rise.2,[3] There are 605,000 new and 200,000 recurrent heart attacks per year (approximately 1 every 40 seconds), in the United States. Stroke rates are similar, accounting for 1 of every 19 U.S. deaths (approximately 1 every 40 seconds).4
Controlling bad cholesterol, also known as LDL-C, is one way to reduce a patient’s risk for cardiovascular events, such as heart attack, stroke or death. However, even with the achievement of target LDL-C levels, millions of patients still have significant and persistent risk of cardiovascular events, especially those patients with high triglycerides. Statin therapy has been shown to control LDL-C, thereby reducing the risk of cardiovascular events by 25-35% – but that still leaves 65-75% risk remaining.5 People with high triglycerides have 35% more cardiovascular events compared to people with normal (in range) triglycerides taking statins.6,[7],[8]
Key Data Presented at AHA and Reviewed During Amarin’s Webcast
“REDUCE-IT USA: Results from the 3,146 Patients Randomized in the United States,” – presented by Deepak L. Bhatt, M.D., M.P.H., Brigham and Women’s Hospital and Harvard Medical School.
Highlights: This prespecified REDUCE-IT subgroup analysis showed substantial risk reductions in the USA patients treated with icosapent ethyl 4 g/day versus placebo across all prespecified composite and individual primary and secondary endpoints, including 31% relative risk reduction and 6.5% absolute risk reduction in first occurrence of 5-point major adverse cardiovascular events (MACE), corresponding to a number needed to treat of 15 (NNT=15), and a significant 30% relative and 2.6% absolute risk reduction (NNT=39) in all-cause mortality in the USA subgroup.
Additional prespecified cardiovascular endpoints in which the REDUCE-IT USA subgroup showed significant relative risk reduction included myocardial infarction, cardiovascular death, and stroke, similar to the full cohort in the overall REDUCE-IT global results. These results were incremental to the cardiovascular risk reduction achieved by conventional therapy administered to the high-risk patients studied, including incremental to statin therapy.
The REDUCE-IT USA subgroup consisted of 3,146 patients (nearly 40%) of the previously reported full trial cohort. REDUCE-IT was not specifically powered to examine individual subgroups. P-values presented for the USA subgroup are nominal and exploratory with no adjustment for multiple comparisons. Differences in efficacy outcomes for the USA patients are best viewed as qualitative and not quantitative; nevertheless, the data are useful and provide reassurance that the results in the USA are at least as strong as the results seen outside the USA and in the trial overall.
The REDUCE-IT USA study results were also published in Circulation, AHA’s official scientific journal.9
“Cost-Effectiveness of Icosapent Ethyl in REDUCE-IT,” – presented by William S. Weintraub, M.D., MedStar Washington Hospital.
Highlights: In this combined patient-level and simulation lifetime cost-effectiveness analysis, icosapent ethyl in high cardiovascular risk patients shows exceptional benefit with cardiovascular event reduction as well as cost-savings in-trial and over patients’ lifetime in many simulations. Findings of potential cost effectiveness of a medical therapy are rare. This analysis considered the current market cost for Vascepa and the potential savings from avoiding major adverse cardiovascular events, such as strokes and heart attacks, the cost of which can be high. This cost-effectiveness analysis was conducted by MedStar. As is typical, the cost-effectiveness analyses were not prespecified as part of the REDUCE-IT clinical trial design but did rely on the results of this landmark outcomes study.
“Many Statin Treated Persons with Borderline Triglyceride Levels are at Risk of ASCVD,” – presented by Nathan D. Wong, Ph.D., M.P.H, University of California, Irvine.
Highlights: This study showed prevalence of elevated risk of major cardiovascular events (a mean 10-year atherosclerotic cardiovascular disease (ASCVD) risk score greater than 20%) in more than 20% of patients on statins with triglycerides below 150 mg/dL. This suggests the need for greater lifestyle and other therapies to address remaining residual ASCVD risk.
“Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis in Patients with Elevated Triglycerides (200 – 499 mg/dL) on Statin Therapy (EVAPORATE) study,” – presented by Matthew J. Budoff, M.D., Los Angeles Biomedical Research.
Highlights: At the prespecified 9-month interim analysis, there was slowing of total non-calcified plaque (sum of LAP, fibrofatty, and fibrous plaque) (35% v. 43%, p=0.010), total plaque (non-calcified + calcified plaque) (p=0.0004), fibrous plaque (15% v. 26%, p=0.011) and calcified plaque (-1% v. 9%, p=0.001), after adjustment by baseline plaque, age, sex, diabetes status, baseline triglyceride levels, and statin use. However, there was no significant change in the primary endpoint of low attenuation plaque between active and placebo groups (74% vs 94%, p=0.469) at this 9-month interim look. This investigator-initiated study is continuing to its designed completion of an 18-month review.
EVAPORATE is the first study in the United States, which enrolled a total of 80 patients, to use multidetector computed tomography (MDCT) to evaluate the effects of icosapent ethyl as an adjunct to statin therapy on plaque characteristics in a high cardiovascular-risk population with persistent high triglyceride levels. Patients underwent interim scans at 9 months and are currently being followed for an additional 9 months with MDCT. Final results from this study are anticipated in early 2020.
Arterial plaque and coronary atherosclerosis are key factors leading to significant increases in the probability of acute obstructions and angina or other coronary artery disease signs and symptoms.
All of the analyses highlighted above were funded by Amarin.
A replay of the webcast will be available for two weeks following the webcast. To hear a replay of the webcast, dial 877-481-4010 (inside the United States) or 919-882-2331 (outside the United States). A replay of the webcast is also be available through the company's website, Amarincorp.com, in the Investor section. For both dial-in numbers please use conference ID 55923.
About Amarin
Amarin Corporation plc. is a rapidly growing, innovative pharmaceutical company focused on developing therapeutics to improve cardiovascular health. Amarin’s product development program leverages its extensive experience in polyunsaturated fatty acids and lipid science. Vascepa (icosapent ethyl) is Amarin's first FDA-approved drug and is available by prescription in the United States, Lebanon and the United Arab Emirates. Amarin’s commercial partners are pursuing additional regulatory approvals for Vascepa in Canada, China and the Middle East. For more information about Amarin, visit www.amarincorp.com.
About REDUCE-IT®
REDUCE-IT, an 8,179-patient cardiovascular outcomes study, was completed in 2018. REDUCE-IT was the first multinational cardiovascular outcomes study that evaluated the cardioprotective effect of icosapent ethyl, a unique prescription therapy, as an add-on to statins in patients with high cardiovascular risk. As defined in the published results of the study, the high cardiovascular risk patients, despite stable statin therapy, had elevated triglyceride levels (lower enrollment target of TG >135 mg/dL). As per the study’s design, approximately 71% of the enrolled patients had established cardiovascular disease and the other patients were diagnosed, as per trial enrollment requirements, as having diabetes and other cardiovascular risk factors.
More information on the REDUCE-IT study results can be found at www.amarincorp.com.
About Vascepa® (icosapent ethyl) Capsules
Vascepa (icosapent ethyl) capsules are a single-molecule prescription product which has been developed and studied for more than a decade and has been prescribed more than 8 million times in the United States. Vascepa, known in scientific literature as AMR101, has been designated a new chemical entity by the FDA. Amarin has been issued multiple patents in the United States and internationally based on the unique clinical profile of Vascepa, including the drug’s ability to lower triglyceride levels in relevant patient populations without raising LDL-cholesterol levels.
The FDA has not completed its review or made a final determination on a supplemental new drug application related to REDUCE-IT. FDA has not reviewed the information herein or determined whether to approve Vascepa for use to reduce the risk of major adverse cardiovascular events in the REDUCE-IT patient population.
Indication and Usage Based on Current FDA-Approved Label (not including REDUCE-IT results)
Vascepa (icosapent ethyl) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
The effect of Vascepa on the risk for pancreatitis and cardiovascular mortality and morbidity in patients with severe hypertriglyceridemia has not been determined.
Important Safety Information for Vascepa Based on Current FDA-Approved Label (not including REDUCE-IT results) (Includes Data from Two 12-Week Studies (n=622) (MARINE and ANCHOR) of Patients with Triglycerides Values of 200 to 2000 mg/dL)
Vascepa is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vascepa or any of its components.
In patients with hepatic impairment, monitor ALT and AST levels periodically during therapy.
Use with caution in patients with known hypersensitivity to fish and/or shellfish.
The most common reported adverse reaction (incidence >2% and greater than placebo) was arthralgia (2.3% for Vascepa, 1.0% for placebo). There was no reported adverse reaction >3% and greater than placebo.
Adverse events and product complaints may be reported by calling 1-855-VASCEPA or the FDA at 1-800-FDA-1088. Patients receiving treatment with Vascepa and other drugs affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
Patients should be advised to swallow Vascepa capsules whole; not to break open, crush, dissolve, or chew Vascepa.
FULL VASCEPA PRESCRIBING INFORMATION CAN BE FOUND AT WWW.VASCEPA.COM.
Important Safety Information for Vascepa based on REDUCE-IT, as previously reported in The New England Journal of Medicine publication of the primary results of the REDUCE-IT study:
Excluding the major adverse cardiovascular events (MACE) results described above, overall adverse event rates in REDUCE-IT were similar across the statin plus Vascepa and the statin plus placebo treatment groups.
There were no significant differences between treatments in the overall rate of treatment emergent adverse events or serious adverse events leading to withdrawal of study drug.
There was no serious adverse event (SAE) occurring at a frequency of >2% which occurred at a numerically higher rate in the statin plus Vascepa treatment group than in the statin plus placebo treatment group.
Adverse events (AEs) occurring in 5% or greater of patients and more frequently with Vascepa than placebo were:
peripheral edema (6.5% Vascepa patients versus 5.0% placebo patients), although there was no increase in the rate of heart failure in Vascepa patients
constipation (5.4% Vascepa patients versus 3.6% placebo patients), although mineral oil, as used as placebo, is known to lower constipation, and
atrial fibrillation (5.3% Vascepa patients versus 3.9% placebo patients), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients
There were numerically more SAEs related to bleeding in the statin plus Vascepa treatment group although overall rates were low with no fatal bleeding observed in either group and no significant difference in adjudicated hemorrhagic stroke or serious central nervous system or gastrointestinal bleeding events between treatments.
In summary, Vascepa was well tolerated with a safety profile generally consistent with clinical experience associated with omega-3 fatty acids and current FDA-approved labeling of such products.
Important Cautionary Information About These Data
Further REDUCE-IT data assessment and data release are expected to yield additional useful information to inform greater understanding of the trial outcome. For example, detailed data assessment by regulatory authorities, such as the FDA and Health Canada, will continue and take time to complete and announce. The FDA advisory committee process and the final evaluation by regulatory authorities of the totality of efficacy and safety data from REDUCE-IT is anticipated to include some or all of the following, as well as other considerations: new information or analyses affecting the degree of treatment benefit on studied endpoints; study conduct and data robustness, quality, integrity and consistency; additional safety data considerations and risk/benefit considerations; and consideration of REDUCE-IT results in the context of other clinical studies. More detailed presentation of such considerations is set forth in the risk factors section of Amarin’s Quarterly Report on Form 10-Q filed with the U.S. Securities and Exchange Commission. Because regulatory reviews are typically fluid and not definitive interactions between sponsor and agency on individual elements of an application and related information, Amarin does not plan to update investors further on ongoing communications with regulatory authorities. Amarin plans to announce the final outcome of such regulatory reviews when appropriate.
Forward-Looking Statements
This press release contains forward-looking statements, including statements regarding the use of Vascepa to potentially help millions of patients. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected herein include the following: uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals; the risk that data interpretations or other information from third parties, the regulatory review process, regulatory authorities and in connection with an advisory committee could be made public that are negative or may delay approval or limit Vascepa’s marketability; the risk that special protocol assessment (SPA) agreements with the FDA are not a guarantee that FDA will approve a product candidate; the risk associated with the FDA's rescinding the REDUCE-IT SPA agreement; the risk related to FDA advisory committee meetings; and the risk that the FDA may not complete its review of the REDUCE-IT sNDA within the timing expected. A further list and description of these risks, uncertainties and other risks associated with an investment in Amarin can be found in Amarin's filings with the U.S. Securities and Exchange Commission, including its most recent Quarterly Report on Form 10-Q. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Amarin undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Availability of Other Information About Amarin
Investors and others should note that Amarin communicates with its investors and the public using the company website (www.amarincorp.com), the investor relations website (investor.amarincorp.com), including but not limited to investor presentations and investor FAQs, Securities and Exchange Commission filings, press releases, public conference calls and webcasts. The information that Amarin posts on these channels and websites could be deemed to be material information. As a result, Amarin encourages investors, the media, and others interested in Amarin to review the information that is posted on these channels, including the investor relations website, on a regular basis. This list of channels may be updated from time to time on Amarin’s investor relations website and may include social media channels. The contents of Amarin’s website or these channels, or any other website that may be accessed from its website or these channels, shall not be deemed incorporated by reference in any filing under the Securities Act of 1933.
Amarin Contact Information
Investor Inquiries:
Elisabeth Schwartz
Investor Relations
Amarin Corporation plc
In U.S.: +1 (908) 719-1315
investor.relations@amarincorp.com
Lee M. Stern
Solebury Trout
In U.S.: +1 (646) 378-2992
lstern@soleburytrout.com
Media Inquiries:
Gwen Fisher
Corporate Communications
Amarin Corporation plc
In U.S.: +1 (908) 325-0735
pr@amarincorp.com
References
1 Bhatt DL, Steg PG, Miller M, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med 2019;380:11-22.
2 American Heart Association. Heart Disease and Stroke Statistics – 2019 Update: A Report from the American Heart Association. Published January 31, 2019.
3 American Heart Association / American Stroke Association. 2017. Cardiovascular disease: A costly burden for America projections through 2035.
4 American Heart Association: Heart Disease and Stroke Statistics -- 2019 At-a-Glance.
5 Ganda OP, Bhatt DL, Mason RP, et al. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72(3):330-343.
6 Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118:138-145.
7 Toth PP, Granowitz C, Hull M, et al. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: A real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7(15):e008740.
8 Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease - New insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547-563.
9 Bhatt DL, Miller M, Brinton EA, et al. REDUCE-IT USA: Results from the 3,146 Patients Randomized in the United States. Circulation 2019. DOI: 10.1161/CIRCULATIONAHA.119.044440.
Contact:
Sign in to post a message.
..ohne Kommentar:


Antwort auf Beitrag Nr.: 61.950.698 von Cyberhexe am 18.11.19 15:17:44
Hi cyberhexe,
hi cyberwitch
das hatte ich mir schon gedacht, dass es diesen Isteiner Kirchberg gibt. Ich fand die wortwörtliche Deutsch - Englisch - Kreativ - Übersetzung einfach nur zum Kringeln, du Spaßvogel.... you Jokebird
Grüße
bernie55
Zitat von Cyberhexe:Zitat von bernie55: ...
ISTEINER CHURCHHILL..
Isteiner Churchhill - das ist nicht geflunkert, weil richtig übersetzt:
Hi cyberhexe,
hi cyberwitch

das hatte ich mir schon gedacht, dass es diesen Isteiner Kirchberg gibt. Ich fand die wortwörtliche Deutsch - Englisch - Kreativ - Übersetzung einfach nur zum Kringeln, du Spaßvogel.... you Jokebird

Grüße
bernie55

Antwort auf Beitrag Nr.: 61.958.078 von bernie55 am 19.11.19 10:50:06https://thefly.com/landingPageNews.php?id=2995848&headline=A…
😀
citi analyst hat vorher bei Pfizer gearbeitet
noch Fragen🙄🙄🙄
😀
citi analyst hat vorher bei Pfizer gearbeitet
noch Fragen🙄🙄🙄
Antwort auf Beitrag Nr.: 61.954.658 von Cyberhexe am 18.11.19 21:57:36
Superprima Zusammenfassung - THX
Zitat von Cyberhexe:Zitat von Magnetfeldfredy: Für mich sehr gute Ergebnisse, Evaporate zeigt den mechanism of action mit der Plaque Reduktion eindrucksvoll, und dass nach 9 Monaten noch nicht alles optimal ist beweisen auch die Reduce-It Studie, je länger man Vascepa nimmt desto besser werden die Ergebnisse!
Auch wurde das leidige Mineralöl-Placebo mit Evoporate beerdigt, super Interview, big pharma hat jetzt mit diesen Ergebnissen eindrucksvolle Beweise wie Vascepa wirkt und wird zur Übernahme führen:
Die Ergebnisse, die nur auf vorläufigen Daten basieren, zeigen, dass die Studie ihren primären Endpunkt, nämlich verringertes Plaquevolumen- (noch) nicht erreicht hat.
Bei den veröffentlichten Daten handelt es sich um einen neunmonatigen "Zwischenblick" auf eine kleine Studie mit 80 Patienten, die eigentlich für eine doppelt so lange Laufzeit ausgelegt ist.
Die Daten beinhalten auch Auswertungen auf andere Plaque-Wachstumsmaße, wobei die Plaque-Progressionsraten in der Mineralöl-Placebo-Gruppe identisch mit denen eines Placebos auf Cellulosebasis waren. Diese anderen Placebo-Daten stammen aus einer anderen Studie, sind jedoch genau auf die Daten der Placebo-Kohorte der neuen Vascepa-Studie abgestimmt.
"Bei vier verschiedenen Plaque-Markern, einschließlich der Gesamtplaque, wurde eine signifikante Verlangsamung der Progression festgestellt", sagte Matthew Budoff, UCLA-Professor an der School of Medicine.
Diese Verlangsamung von 42% im Vergleich zu Placebo war bei den bisherigen Wirksamkeitsdaten besonders bemerkenswert. Beruhigend ist vor allem "dass die verlangsamten Fortschrittsraten, die wir bei fünf der sechs von uns gemessenen Marker sahen, auf die Vorteile von Icosapent ethyl und nicht auf die Schäden von Mineralöl zurückzuführen sind", fügte er hinzu.
Superprima Zusammenfassung - THX

Antwort auf Beitrag Nr.: 61.958.477 von esaposse am 19.11.19 11:21:59
Dass diese Herabstufung von buy auf neutral absolut unsinnig ist, zeigt sich auch daran, dass das vorherige Kursziel von 23 USD gleichzeitig auf 27 USD erhöht wurde.
Quasi ein soft- downgrading.

Zitat von esaposse: https://thefly.com/landingPageNews.php?id=2995848&headline=A…
😀
citi analyst hat vorher bei Pfizer gearbeitet
noch Fragen🙄🙄🙄
Dass diese Herabstufung von buy auf neutral absolut unsinnig ist, zeigt sich auch daran, dass das vorherige Kursziel von 23 USD gleichzeitig auf 27 USD erhöht wurde.
Quasi ein soft- downgrading.


Antwort auf Beitrag Nr.: 61.958.585 von bernie55 am 19.11.19 11:30:31Ich glaube das ist Absicht, !
Der will Investoren die Botschaften "zwischen" den Zeilen finden können etwas sagen, nämlich jetzt das als no-buy einzustufen ist wie Geld aus dem Fenster werfen
Daher hat er das Kursziel nach oben angepasst bei gleichzeitiger "hey jetzt aber Vorsicht !" Meldung
Der denkt um 2 oder 3 Ecken der gute Mann uns ist ein schlauer Fuchs... naja oder auch nicht
Der will Investoren die Botschaften "zwischen" den Zeilen finden können etwas sagen, nämlich jetzt das als no-buy einzustufen ist wie Geld aus dem Fenster werfen

Daher hat er das Kursziel nach oben angepasst bei gleichzeitiger "hey jetzt aber Vorsicht !" Meldung
Der denkt um 2 oder 3 Ecken der gute Mann uns ist ein schlauer Fuchs... naja oder auch nicht

Korrupte Wallstreet, die uns Lemminge verarschen will, mich nicht mehr!
dited Transcript of AMRN conference call or presentation 18-Nov-19 9:30pm GMT
Amarin Corporation PLC O Webcast Discussion Of Presented Data at American Heart Assocition's 2019 Scientific Sessions
Dublin Nov 19, 2019 (Thomson StreetEvents) -- Edited Transcript of Amarin Corporation PLC conference call or presentation Monday, November 18, 2019 at 9:30:00pm GMT
TEXT version of Transcript
================================================================================
Corporate Participants
================================================================================
* Craig B. Granowitz
Amarin Corporation plc - Chief Medical Officer & Senior VP
* Elisabeth Schwartz
Amarin Corporation plc - Senior Director of IR
================================================================================
Conference Call Participants
================================================================================
* Deepak Bhatt
* Matthew Budoff
* William Weintraub
================================================================================
Presentation
--------------------------------------------------------------------------------
Operator [1]
--------------------------------------------------------------------------------
Greetings. Welcome to the AHA investor discussion conference call. (Operator Instructions) Please note this conference is being recorded.
I would now like to turn the conference over to your host, Elisabeth Schwartz. Thank you. You may begin.
--------------------------------------------------------------------------------
Elisabeth Schwartz, Amarin Corporation plc - Senior Director of IR [2]
--------------------------------------------------------------------------------
Welcome to today's discussion to explore for our investment community the several important new data sets and analyses related to Vascepa and REDUCE-IT as presented at the 2019 American Heart Association's Scientific Sessions here in Philadelphia.
Please be aware that this conference call will contain forward-looking statements that are intended to be covered under the safe harbor provided by the Private Securities Litigation Reform Act. Examples of such statements include, but are not limited to, our current expectations regarding Vascepa clinical data and cost-effectiveness analyses and expectations regarding the application of these data to clinical use, in reimbursement and regulatory settings and to commercial prospects for Vascepa. And these statements are based on information available to us today, November 18, 2019.
We may not actually meet the expectations disclosed in our forward-looking statements. Actual results or events could differ materially. We assume no obligation to update these statements as circumstances change. For additional information concerning these factors that could cause actual results to differ materially, please see the Forward-Looking Statements section and the Risk Factors section of our most recent Form 10-Q filed with the SEC.
This call is intended for investors in Amarin and is not intended to promote the use of Amarin's Vascepa outside its approved indication.
I will now turn the call over to Dr. Craig Granowitz, the Chief Medical Officer of Amarin.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [3]
--------------------------------------------------------------------------------
Thanks, Elisabeth, and thank you all to our panelists and listeners today for joining us. We're excited here -- to be here in Philadelphia at the conclusion of the American Heart Association's Scientific Session. We're fortunate to have 3 clinicians/researchers that are immersed in Vascepa-related sciences and have presented here at AHA to speak with us today: Doctors Matthew Budoff, Deepak Bhatt and William Weintraub. I'll give a quick recap of the presentations by each of these 3 research physicians, and then we'll move into a discussion of some of the key issues with the results.
Dr. Budoff presented this morning on the interim 9-month results of the 18-month EVAPORATE study. EVAPORATE is a first study in the U.S. to use multidetector computed tomography to evaluate the effects of icosapent ethyl as an adjunct to statin therapy on plaque characteristics in a high cardiovascular-risk population with persistently high triglyceride level. Patients underwent interim scans at 9 months and are currently being followed for an additional 9 months. Final results of this investigator-initiated study are anticipated in early 2020.
Dr. Bhatt spoke yesterday about REDUCE-IT USA. These prespecified REDUCE-IT subgroup analyses showed substantial risk reduction in the U.S. patients treated with icosapent ethyl 4 grams a day versus placebo across all predefined composite and individual primary and secondary endpoint, including a 31% relative risk reduction and a 6.5% absolute risk reduction in first occurrence of 5-point MACE. This corresponds to a number needed to treat of 15 and a significant 30% relative and 2.6% absolute risk reduction or a number needed to treat of 39 in all-cause mortality in this U.S. subgroup. I want to emphasize that these analyses of this data in the U.S. population in most studies are usually not as pronounced as what we say in the REDUCE-IT USA analysis.
Finally, we will speak to Dr. Weintraub about the preliminary analysis of the cost effectiveness of icosapent ethyl. In this combined patient level and simulation lifetime cost-effectiveness analysis, icosapent ethyl in high cardiovascular-risk patients showed an exceptional benefit with cardiovascular event reduction as well as cost savings in trial and over patients' lifetime in many simulations.
Finding of cost effectiveness of medical therapies are rare. This analysis considered the current market cost for Vascepa and the potential savings from avoiding major MACE events such as stroke and heart attack, the cost of which can often be very high. As is typical, the cost-effectiveness analysis were not prespecified as part of reducing clinical study design but did rely on the landmark results.
So Matt, I'd like to start with you. You're Professor of Medicine and Endowed Chair at the UCLA School of Medicine and Program Director of the Lundquist Institute. So Matt, if you could just give a summary of some of the key points that you wanted to convey this morning at the late-breaker. And I do want to note that you were the first on the agenda for the morning. So clearly, there was a high degree of interest in the sponsors of the session to hear what you had to say in the EVAPORATE study.
--------------------------------------------------------------------------------
Matthew Budoff, [4]
--------------------------------------------------------------------------------
Yes. And I think it was very, very well received by the physicians in the audience. Basically, we demonstrated that, over just a short 9-month analysis, that there was generally a uniform plaque regression in the patients who received Vascepa as compared to those patients who received placebo. Because it was a small number of patients, it was an 80-person trial, and we only had follow-up in 67 patients at the interim analysis, the power was a little diminished because we powered it based on an 18-month trial. But I have to say that while the primary endpoint was 21% reduced, which was not enough to stop the trial, it was very concordant with all the other parameters. For example, total plaque went down by 42% with a p-value of 0.004, and that's -- we have not seen that type of plaque stabilization or slowing of plaque progression with other therapies that we've studied, especially over a short interim time frame of only 9 months.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [5]
--------------------------------------------------------------------------------
Now, Matt, that's a really helpful summary. And I guess there are some questions that I've heard this morning about what does the mechanistic study actually mean, why do the study -- what -- where do you think that's going to add to the body of literature. And I'll note that Dr. Bhatt was also an investigator on this trial as well. But how, Matt, do you see this kind of fitting in? What role does this play in terms of our understanding of the field?
--------------------------------------------------------------------------------
Matthew Budoff, [6]
--------------------------------------------------------------------------------
Well, I think a lot of clinicians were surprised, to say the least, at the robustness of the REDUCE-IT study and how large the benefit was, and they want to understand better. We're telling them it's not just triglycerides, that it's not only different things, and we don't know what it is. So when you look at the primary endpoints of REDUCE-IT, all of them that could be artherosclerotic line up and show benefit. So I think it suggests that this is a direct anti-artherosclerotic benefit, but you can't derive that solely from the REDUCE-IT study. Now we can say with confidence, based on the results of EVAPORATE, that there is an anti-atherosclerotic property at play here that's supplemental to all of the other benefits that we've already seen with Vascepa.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [7]
--------------------------------------------------------------------------------
Dr. Bhatt, I know that there have been a number of questions asked of you, including even last week at the FDA meeting, about time to curve separation. And I think sort of putting that in context with the mechanistic results of Matt's study might be very helpful context for people to understand sort of these intermediate signals in the context of a clinical event. Could you shed some light on that?
--------------------------------------------------------------------------------
Deepak Bhatt, [8]
--------------------------------------------------------------------------------
I think that's a great question. And in the REDUCE-IT trial, we saw benefit in the 2 major cohorts, which were the patients enrolled with secondary prevention. By that, I mean stable patients with coronary artery disease or cerebrovascular disease or peripheral artery disease. As well, we saw benefits in patients who are high-risk primary prevention. By that, specifically, what we enrolled were people with diabetes and at least one other cardiovascular risk factor. So in that population of patients, there was significant benefit. The drug worked a little bit differently in terms of timing of that benefit kicking in. In the secondary prevention cohort, it looked like the curves were really starting to separate at about a year, whereas it took a couple of years in the primary prevention cohort. And that makes sense because that's what we saw in the statin trials. And in fact, if we had studied really high-risk patients, like people coming in with heart attacks or acute strokes, then I think the curves would have separated even sooner. So it really just has to do with the baseline risk.
But what I think Dr. Budoff's study has nicely done, it's done a lot of things. I mean, it's shown for those doctors that really want to see, what's the mechanism of action, if shown, will look. The plaque in the arteries looks likely to progress. So that's a really powerful visual image. But beyond that, there's another message that is a bit more subtle that, at least to my knowledge, no one has explicitly stated. This was an interim analysis. It was just 9 months. It's meant to be an 18-month study. But I'm really glad Dr. Budoff took a look, prespecified all or part of the plan that he'd laid out because it tells me that there are benefits that are kicking in early. So if you're a physician and you see the patients that we enrolled in REDUCE-IT were stable patients, again, they're not in the throes of a heart attack or that sort of patient, you're going to throw the kitchen sink at them in terms of risk reduction. They're pretty stable. They're in the office. They're not actually complaining of anything. They're doing okay. It'd be easy to just say, look, I'll see you back in a year. But the problem with that approach now we see with a REDUCE-IT-like patient that's out there in practice, their plaque is progressing in the course of that year. So this really provides an impetus for why a physician needs to pull the trigger and act sooner with respect to prescribe it.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [9]
--------------------------------------------------------------------------------
No, I think that's really important context. One of the other sessions I was at yesterday was talking about the effects both on the particles and on inflammation. And Matt, I don't know if you want to comment at all because that might tie into -- one of the questions I think comes up is, why are there so many endpoints that are looked at in this? What are all these different surrogates of total plaque and low attenuation plaque and calcified plaque? Maybe you could help give, again, some of our investors some context of what all of that means because it's just some terminology that might be confusing.
--------------------------------------------------------------------------------
Matthew Budoff, [10]
--------------------------------------------------------------------------------
Well, absolutely. So I think when we look towards therapies that have both presumed the anti-atherosclerotic effect and an anti-inflammatory effect, then we start to think about things like the low attenuation plaque, which what we think of kind of is the necrotic core, the really vulnerable plaque, that might be most likely to rupture.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [11]
--------------------------------------------------------------------------------
And what happens when the plaque ruptures, Matt?
--------------------------------------------------------------------------------
Matthew Budoff, [12]
--------------------------------------------------------------------------------
When a plaque ruptures, patients have an acute heart attack or an acute stroke. The artery gets occluded, and they end up with an immediate event. 1/3 of people die suddenly when these plaques rupture.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [13]
--------------------------------------------------------------------------------
And that really was the endpoint, right, Deepak, of REDUCE-IT? That's sort of the clinical consequence of a plaque gone bad.
--------------------------------------------------------------------------------
Deepak Bhatt, [14]
--------------------------------------------------------------------------------
Look, it's a great one-two punch when you think about EVAPORATE and REDUCE-IT. You've got basic mechanistic data now by imaging and a clinical trial that shows clear-cut benefits. So it's a really nice package scientifically. For physicians out there that want mechanism of action, now we've got one. Now there are several other mechanisms of action at play too, I think, with icosapent ethyl with the ability to retard plaque progression. That's pretty important.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [15]
--------------------------------------------------------------------------------
Right. So Matt, I'm sorry I interrupted you talking about some of the different endpoints of the study.
--------------------------------------------------------------------------------
Matthew Budoff, [16]
--------------------------------------------------------------------------------
Yes. So obviously, when you think about just overall progression in your coronary arteries, you would use an endpoint like total plaque, and that was very significantly reduced in the EVAPORATE trial. We focused on this more vulnerable plaque because of some of the evidence from REDUCE-IT that sudden death was reduced. So there was a signal there that maybe this plaque ruptures. There's some data from abroad, from Japan, the CHERRY study, and other studies that have also suggested that, that would be a good target to look at specifically because of a drug that has these pleiotropic or multiple effects on the vasculature.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [17]
--------------------------------------------------------------------------------
And I think you mentioned at least one point to me that there've been a number of these imaging studies done, mostly in Japan, not in the U.S., and that's one of the unique aspects of EVAPORATE. But has there been a difference that have been seen in the Japanese studies with different omega-3 preparations?
--------------------------------------------------------------------------------
Matthew Budoff, [18]
--------------------------------------------------------------------------------
Yes. So there's been a number of trials. There's been 2 large-scale trials, 1 in the U.S. and 1 outside the U.S., that looked at 4 grams of Lovaza without showing significant benefits.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [19]
--------------------------------------------------------------------------------
And Lovaza is what part of...
--------------------------------------------------------------------------------
Matthew Budoff, [20]
--------------------------------------------------------------------------------
EPA and DHA, mixed -- combination pill. And both of those trials failed to show benefit even out to 2 years. So I think this is much different than the EPA-DHA mix data that we've seen from imaging. And the Japanese data did line up very nicely with 1.8 grams of EPA. But again, Japanese populations, invasive imaging, where they're putting catheters into people's coronary arteries, things that would be really hard to emulate in the United States.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [21]
--------------------------------------------------------------------------------
Yes. And that's very helpful context. I think one of the other questions that people have asked me is the interim analysis threshold and the statistical benchmark for that, which seems awfully high, right? If you could talk a little bit about how the statistics might be. And I don't want to put you on the spot. I know you're not a statistician, but I'm not sure that everyone really understands that the hurdles at an interim analysis are quite high to stop a study.
--------------------------------------------------------------------------------
Matthew Budoff, [22]
--------------------------------------------------------------------------------
Yes. And basically, what you're saying is if there's overwhelming efficacy, if the drug is so good at an early time point, then you can almost argue it's unethical to continue the study, just like we stop clinical trials early when there's overwhelming benefit because we don't want to keep people on placebo or keep people off of this therapy. So we didn't hit that interim analysis based on the primary endpoint that we chose. But again, had we chosen total plaque, which is a very reasonable endpoint in many trials, we would have actually stopped the trial early because total plaque came in at P 0.004, which is less than the necessary stopping parameter. So we can -- the Steering Committee can obviously take some responsibility for picking the wrong endpoint, but hindsight is 2020. But the trial is still positive, and the trial is still ongoing, so I think that it's good news. And I think based on my conversations at the American Heart Association after the presentation, many physicians feel much more comfortable now, not people who just live and die based on clinical trials but those that want to understand the mechanism, that this adds a lot to their confidence in moving forward with the use of icosapent ethyl in their practice.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [23]
--------------------------------------------------------------------------------
No, that's helpful. There was one other question. I know you showed a slide at the meeting today about the placebo controls, and I know you covered a tremendous amount of data very quickly on that. And this might be a point to help clarify. What was actually done? And why is it meaningful?
--------------------------------------------------------------------------------
Matthew Budoff, [24]
--------------------------------------------------------------------------------
Yes. So since the REDUCE-IT trial was published and after we started EVAPORATE, there were some questions about the mineral oil placebo that was used and whether that was contributing to harm rather than -- or offsetting some of the benefit of icosapent ethyl. We had already started EVAPORATE, so EVAPORATE was using the same mineral oil placebo that we used in REDUCE-IT. So what we did is we matched that placebo group to another group of patients who are on a different placebo, one that's made out of cellulose that everybody acknowledges is completely inert and has no adverse properties to it. And we showed that the rate of progression on patients on mineral oil was identical to the rate of progression of those patients on an inert cellulose placebo. So hopefully, laying any concerns about mineral oil being bad, rather now all the benefit is on icosapent ethyl being good.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [25]
--------------------------------------------------------------------------------
No, that's very helpful. And again, I just want to clarify one other point before we left for Dr. Weintraub out of the conversation. But -- so you showed a curve, and it looked like there was a 45-degree line, and there were all these dots on the curve. Maybe you can just sort of put that in context of what that meant and why did that give you comfort and confidence because I'm not sure everyone is so familiar with looking at that kind of scatter plot.
--------------------------------------------------------------------------------
Matthew Budoff, [26]
--------------------------------------------------------------------------------
Yes. No, absolutely. So this is basically just putting all of the rates of progression among both cohorts, the -- there were blue dots on the curve, if you saw it on the slide, that were represented the cellulose placebo from another randomized trial, and there were red dots that represented EVAPORATE placebo patients. And the line of identity went -- was identical for the two. In other words, we graphed out what is the rate of progression among those 2 cohorts, and they were -- the lines were superimposed.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [27]
--------------------------------------------------------------------------------
Right. So each one of those dots was an individual patient over that time course and sort of seeing that there was an equal distribution across both sides, which said, statistically, there was absolutely no difference in the rate of change between those 2 of the parameters you're looking at.
--------------------------------------------------------------------------------
Matthew Budoff, [28]
--------------------------------------------------------------------------------
Absolutely. And what people need to understand is when we're up there presenting, as we all do fairly frequently, there's literally a giant clock that only we can see that is counting down towards 0. And when it hit 0, you have to stop talking. So...
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [29]
--------------------------------------------------------------------------------
And it's flashing red so...
--------------------------------------------------------------------------------
Matthew Budoff, [30]
--------------------------------------------------------------------------------
Yes, yes. Yes, yes.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [31]
--------------------------------------------------------------------------------
And they turn off the microphone.
--------------------------------------------------------------------------------
Matthew Budoff, [32]
--------------------------------------------------------------------------------
But -- so we are under some time pressure. So yes, so we couldn't put in all of the data, obviously, that we would have liked to in a more open presentation. I was limited to 10 minutes to present the entire spectrum of data. But yes, that line basically shows that there's -- with all the individual data points, that there's no difference. And we did multiple analyses with all different types of plaque. And regardless of what we looked at, their rates of progression on cellulose placebo, inert placebo was identical to mineral oil placebo. And I did, I think, hopefully, to at least the scientists and the clinicians in the room, effectively convey that mineral oil, therefore, is not the bad player that some people have accused it of being.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [33]
--------------------------------------------------------------------------------
No, thanks. Thank you. And I think that was another important analysis that came out of the study. Bill, I wanted to turn our attention to you. We're very fortunate to have William Weintraub here from the Medstar Institute, who has a long history of doing cost effectiveness analyses as well. And Bill, you're also a cardiologist also?
--------------------------------------------------------------------------------
William Weintraub, [34]
--------------------------------------------------------------------------------
I am.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [35]
--------------------------------------------------------------------------------
So he's a clinical cardiologist and a health economist with a lot of publications in this particular area and really an acknowledged expert. And I give Dr. Boden and the Steering Committee a lot of credit for reaching out and engaging with Bill on the cost effectiveness that was presented here at the meeting. And again, this was done with the support of Amarin but really independent of Amarin, knowing all the sensitivities, particularly around cost effectiveness analyses.
Bill, you might want to just share just in a minute or 2 your perspective on the study and its design. And again, this was done in the overall total population, not the U.S. population. One of the reasons I wanted to go to the total population first before asking Dr. Bhatt to comment on the U.S. population is I don't want to confuse the 2 because I think having them presented at the same meeting could be potentially confusing to people. So you studied the overall REDUCE-IT population.
--------------------------------------------------------------------------------
William Weintraub, [36]
--------------------------------------------------------------------------------
Right. We did. We looked at the U.S. population too, but we started out with the total population. We used U.S. prices, but we used the total population, which is the typical way this is done. Now -- and with REDUCE-IT, we found a 25% relative risk reduction for the first or primary endpoint but 30% overall. In the cost-effective analysis, we used all events, not just the first event. So here, we have a relatively inexpensive pharmaceutical, (inaudible) pharmaceutical. And we have a remarkable reduction in event rates, 30%. This is an unusual situation.
Now what we did is we have to use a number of different costs inputs. We have to look at the cost of the drug. We used what is called SSR net pricing, which we strongly believe that's the right price to use in cost-effective analyses. We used different input values for the cost of events. We used Optum cost, which gives a very broad distribution of costs in patients under the age of 65 and Medicare costs over the age of 65 for our base-case analysis.
Then the other thing we have to look at is utility, how well patients are functioning on scale 0 to 1, with 1 being perfect health and functioning, 0 being generally mortality. We use utility values from the literature, and then we have to estimate survival. Now the remarkable thing about what we did here is that we had 5 full years of in-trial data. And with that, we could look in-trial not using a simulation, not using estimation but using real data. And with that, we found that icosapent ethyl in our base-case analysis is a dominant strategy and as we offer better outcomes at lower cost.
For (inaudible) pharmaceutical, none of us have ever seen anything quite like it before, very unusual and very important. This carried over into our long-term survival analysis as well. But there always has to be a partial simulation because we have to estimate event rates that we haven't measured. We have to estimate survival. Nonetheless, both of these work together to suggest that we have remarkably efficacious therapy, which offers great value. And at least, our preliminary results do suggest that it is dominant, that we went for better outcome at lower cost.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [37]
--------------------------------------------------------------------------------
Thanks, Bob. A very good summary. And again, some of the questions that I've been asked on a number of occasions, is there was an independent group called ICER, that also did an analysis. They came up with somewhat different findings, even though I think they use the same drug cost. Maybe you could talk a bit about the ICER analysis, what are the strengths and weaknesses of each one of them because ultimately, it's sort of this constellation of models that are out there, right?
--------------------------------------------------------------------------------
William Weintraub, [38]
--------------------------------------------------------------------------------
All right. So I mean, they've also found that the cost of icosapent ethyl is highly cost-effective is using most benchmarks. They did not find it to be a dominant strategy. But still, we've got a relatively remarkable incremental cost effectiveness ratio of $18,000 for quality adjusted life year gain, but still very good by almost any measure. The difference is to shift from dominant to $18,000. It's actually not that big. It sounds a lot, but it's actually a very small shift that can result in that. What do they do that was different from what we did. First of all, that was a total simulation. They did not have access to the clinical trial data.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [39]
--------------------------------------------------------------------------------
And so, Bill, for -- I'm sorry to interrupt, but what does that mean the simulation versus actual clinical data, because I think these are concepts, and not everyone is so familiar with.
--------------------------------------------------------------------------------
William Weintraub, [40]
--------------------------------------------------------------------------------
Well, thank you for interrupting. Allow me to clarify that. So a simulation would mean you take results of the trial. It's not that it's made up. I don't want you people to think that this is some kind of fantasy, that's not the case. What they did is they took the overall results of the trial as published in the first -- REDUCE-IT paper and then develop a mathematical model from that, that allows them to estimate the cost effectiveness. That can be -- that works pretty well, but it's not as good as having the actual data in your hands. The other thing is that there's going to be variation. We can look at the distribution of things a lot better when we have the data. So everyone understands that not all patients are the same. You have the distribution of patients. But if you only have the sort of the bottom line results, you can't look at that distribution the same way you can get all of your data.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [41]
--------------------------------------------------------------------------------
Now I also understand that they only looked at some of the endpoints, not all of the primary endpoints of the study as well?
--------------------------------------------------------------------------------
William Weintraub, [42]
--------------------------------------------------------------------------------
Right. So they don't have access to all of the endpoints. We have actual data. So we could look at heart failure hospitalization, AFib hospitalizations. We could look at things that they didn't have access to.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [43]
--------------------------------------------------------------------------------
All right. That's helpful context of how you could get the range. I think another term that isn't familiar to many, is what is dominant actually mean and how to try to understand what dominant actually would represent.
--------------------------------------------------------------------------------
William Weintraub, [44]
--------------------------------------------------------------------------------
All right. So most of the time in a cost-effective analysis for a new therapy, it'll be -- if it's effective, you'll find that it increases costs. And when that happens, you calculate as the ICER group did, you calculate what's called an incremental cost effectiveness ratio, which is generally measured in cost per quality adjusted life year gained. You don't calculate that ratio for technical reasons, you don't calculate that ratio when you have improved outcome at lower cost, improved the outcome at lower cost is called dominant. And our analysis strongly suggests that we have a dominant strategy here that has improved the outcome at low cost.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [45]
--------------------------------------------------------------------------------
So really, a dominant strategy today in the U.S. health care system would be what would be a fully integrated payer where they're actually paying for and bearing all of the risk, including not only the drug cost, but also the downstream bad outcomes, which is the hospitalization costs as well as the provider costs and all of the other downstream implications of the disease.
--------------------------------------------------------------------------------
William Weintraub, [46]
--------------------------------------------------------------------------------
Now what we aim at is a societal study in which we reflect everything across society, that's almost impossible to do because you can't make those pull of those measurements. So we use various proxies to allow you to get at that. But your point is this is looking broadly rather than just considering a hospital or a doctor's office or something, or something like that. So we're trying -- we aim to look as broadly across the society as we can.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [47]
--------------------------------------------------------------------------------
Right. And I think in our U.S. system, there are very few groups that really are tasked with or have that obligation, look at total costs. So I think probably, if the health care system is changing over time. There are more and more of those that will be both bearing the risk of all the costs as well as the economic potential gains of being able to deliver care below that cost threshold, whatever that is, whether they're reimbursed.
--------------------------------------------------------------------------------
William Weintraub, [48]
--------------------------------------------------------------------------------
Yes, that's very true. But people who do cost-effectiveness analysis, this is not uncommon for people to attempt to think as broadly as we can. But I think more and more as we consider health care economics, people are going to expect that we will own broadly.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [49]
--------------------------------------------------------------------------------
Now that's all very, very helpful context of this. When you said that some of the differences are subtle between the different models, what are the things that the model is most sensitive to?
--------------------------------------------------------------------------------
William Weintraub, [50]
--------------------------------------------------------------------------------
Right. So that's very important. It's most sensitive to the cost of the pharmaceutical -- there's very little difference between what we did and what the ICER did. They use SSR costs, we use SSR costs. The real difficulty is the cost of events, and there's uncertainty about what a hospitalization for heart attack or stroke for cardiac catheterization for percutaneous coronary intervention or coronary surgery costs. And because of that, that could have a big effect on the results of the analysis. And we were very sensitive to that, we'll be looking to that in very great detail.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [51]
--------------------------------------------------------------------------------
Now U.S. also had a unique asset that probably has not been made available very often before from commercial payers, you might want to just comment a bit on that and why those costs might be much more reflective of what is actually being paid in the real world.
--------------------------------------------------------------------------------
William Weintraub, [52]
--------------------------------------------------------------------------------
So I'm not sure what you're referring to.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [53]
--------------------------------------------------------------------------------
Well, you had some of the real commercial costs as opposed to the projected cost?
--------------------------------------------------------------------------------
William Weintraub, [54]
--------------------------------------------------------------------------------
Yes. Right. So you're thinking about the cost from the Optum Group?
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [55]
--------------------------------------------------------------------------------
Exactly.
--------------------------------------------------------------------------------
William Weintraub, [56]
--------------------------------------------------------------------------------
So Optum looks broadly across payers in the United States. And this is a huge database of -- I don't remember the exact number. But it's on the order of 160 million people in this database, broadly across payers, allowing what we believe is actually a far more accurate assessment of costs than Medicare costs. I think that people recognize that Medicare costs really do not cover the cost of events. And so if you use Medicare cost alone, you're going to underestimate the value of prevention. And so I think that's very important for people to come to understand looking at cost-effective analysis that we have to work hard to fund the best estimates of cost. And so that we can appropriately evaluate the value of the therapies that we all paid for patients.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [57]
--------------------------------------------------------------------------------
And since you had the patient level data, those that were less than 65 years old were not yet in Medicare, you actually use the true commercial costs. And for those that are over 65, you use the Medicare costs.
--------------------------------------------------------------------------------
William Weintraub, [58]
--------------------------------------------------------------------------------
Yes. We've done that. And I think that's relatively conservative analysis. But it can also be criticized. Well, you might say, "Oh, you should use the Optum costs for everybody." Well, what would happen then if you use Optum costs for everybody? If we think that really reflects real cost, then the finding of a dominant strategy for a cost being implemented would be strengthened even more?
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [59]
--------------------------------------------------------------------------------
All right. I think that's helpful context. I think these are some of the details and that are important in really trying to reflect reality what truly are the cost and risk of the global population in the U.S. group. And as you mentioned, and we'll turn it back over to Dr. Bhatt and the talk about the REDUCE-IT USA, but to look really in the U.S. population with U.S. costs might give results that are potentially different.
--------------------------------------------------------------------------------
William Weintraub, [60]
--------------------------------------------------------------------------------
So we have a subgroup analysis. We have not done this as thoroughly as we plan to in -- for the U.S., but our subgroup analysis suggests that the value of icosapent ethyl is going to be even more strengthened with -- for the U.S. patients.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [61]
--------------------------------------------------------------------------------
Thank you, Bill. I appreciate that. So Dr. Bhatt, I know that there was a tremendous amount of interest in doing the U.S. subgroup out of the study. What were the findings from that analysis?
--------------------------------------------------------------------------------
Deepak Bhatt, [62]
--------------------------------------------------------------------------------
So this was a prespecified subgroup. And the reason we were interested in looking at in the first place was to provide some context because there's a number of these large international trials, for whatever reason in the past, show results in the U.S., they're just different from the rest of the world, usually not looking as good. And then it's hard to know, is it just that it's a small subgroup, is it just a spurious finding. But then the American physician is left in the challenging position of a trial where the data might look good, but then in the wrong country, it doesn't look that good. So that was the basis for prespecifying this and the scientific rationale. So we did go ahead with this. And of course, the timing of this analysis is useful as U.S. regulators and other physicians in the U.S. are considering how to apply the data in the U.S. But the analysis itself was prespecified. So the overall results, first, let me say, for both within the U.S. and outside the U.S. look spectacular. That is for the primary efficacy endpoint, the key secondary efficacy endpoint in both cases, both within the U.S., but also in the subgroup of non-U. S. patients, both of these are statistically significantly reduced. So I don't want to create the impression that the drug is working in the U.S. and working only in the U.S. That would be a mistake. So hopefully no one walks away with that impression. The drug works globally in all the regions we studied, and there's no reason to think it wouldn't work in the regions where we didn't include patients.
Now what we found in our U.S. subgroup analysis was for the primary and key sector endpoint, results that were at least as robust, at least as large in magnitude as in the overall trial. And then on top of that, for the endpoint of all-cause mortality, a significant 30% reduction in death in the U.S. as well as that translated to a 2.6% absolute risk reduction mortality. So that is really upping the ante, so to speak, we're showing not just reductions in things like heart attack and stroke, that's pretty good and good enough, but also now showing a reduction in dying.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [63]
--------------------------------------------------------------------------------
Now I know the paper had a, what I would call an exhaustive review in one of the supplemental tables of all of the differences in the population. It might be very helpful for you with your clinical judgment and sort of deep immersion in the database over the last year, and maybe pick out those criteria of the U.S. subgroup that was different than the non-U. S. subgroup.
--------------------------------------------------------------------------------
Deepak Bhatt, [64]
--------------------------------------------------------------------------------
It's a great question because, again, I wouldn't want anyone in the audience walking away thinking, "Oh, the drug is, for some reason, magically just working in the U.S. and doesn't work as well outside the U.S." That's not true at all. It worked in all the populations we studied, whether it's the region, ethnicity, various other subgroups, the drug was consistent in its benefits. The reason I think the results look better in the U.S., and there's this reduction in mortality that's significant, and that the overall trial there was a trend towards a lower all-cause mortality. And mortality from cardiovascular causes and the overall trial was significantly reduced by 20%. But the reason, I think, we're seeing an amplification, you could say, a benefit in the U.S. subgroup isn't because there were people living in the U.S. per se, but rather, they had more risk factors. So I think a higher risk population, well, we saw more benefit and this is akin to what was observed in the statin literature, higher risk patients of the curves for events separate sooner, the degree of benefit is larger, but even in lower-risk patients with statins. If you follow them long enough, benefit emerges. So I think that's really the key that the U.S. patients were higher risk than the non-U. S. patients. And by higher risk, I mean, more obesity. For example, that's known from other studies, that's not a novel observation, there's more obesity in the U.S. in other regions of the world. That's unfortunate, but that's true. But there are other risk factors as well that were occurring with a higher degree of prevalence in the U.S. versus non-U. S. groups. But one other thing I'll mention, too, is that the EPA or eicosapentaenoic acid levels at baseline were a bit lower in the U.S. versus outside the U.S., so that might be another reason why it seems like the drug was performing even better than it did in the overall trial, where it performed quite well. I also want to point out, too, sometimes people have thought once they saw this data set that, "oh, maybe it's just -- there's a bunch more secondary prevention patients in the U.S.," that's really what's driving it. In fact, it's the opposite. There's a higher proportion of primary prevention patients from the U.S. So that's not what's driving it. It's just that there's more risk factors, a clustering of risk factors. So I think the take-home message isn't so much that all of the drug works there in the U.S., but rather, if you have higher risk patients, they derive even greater benefit, and that benefit kicks in sooner.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [65]
--------------------------------------------------------------------------------
Right. And so I think you raised what to me was a really important finding. And I think there's a lot of confusion about primary and secondary prevention in high-risk and low-risk in this perception that primary prevention is low risk and secondary prevention is high risk. The results of the REDUCE-IT USA seemed to be somewhat at odds with that common understanding or perhaps common misunderstanding?
--------------------------------------------------------------------------------
Deepak Bhatt, [66]
--------------------------------------------------------------------------------
Yes. The truth is this is an outdated paradigm talking about artificial universes of primary prevention and secondary prevention. And so your existing in different areas. When in fact, there's a lot of overlap. And Dr. Budoff, for example, spends a lot of his day doing CT angiography, that is imaging of hard arteries. So what is someone who is asymptomatic, but for whatever reason, they end up getting a CT angiogram, and they've got a ton of plaque in their arteries. Is that a primary prevention patient? Or is that a secondary prevention patient? That nomenclature isn't set up to capture that patient. To me, if they've got a ton of plaque in their arteries, they're at high risk. They may not know they're at high risk, they may not have symptoms or sort of an early warning system, where they're having chest pain or anything. To me, that's almost more dangerous. I'd rather have the secondary prevention patient who has stable angina. It knows when he or she's running to the bus and they're having chest pain, they stop running. But it's that asymptomatic patient, in many respects, is scarier because you don't really know what to do to reduce their risk. So it's an artificial classification. For the purposes of doing clinical trials, we enroll a bunch of different types of patients. We sort of guesstimate what their event rates might be so that we can do a trial with a particular drug or device in a finite period of time with a finite sample size. So it's a bit artificial. What we've shown in REDUCE-IT is across a variety of risk, the patients benefit substantially for icosapent ethyl.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [67]
--------------------------------------------------------------------------------
Yes. And I think, Matt, there's both a trial list, but also participating center in REDUCE-IT. I mean, what are your thoughts on this whole issue of primary prevention and secondary prevention? And when you think about the EVAPORATE study? Do you have both types of patients in EVAPORATE?
--------------------------------------------------------------------------------
Matthew Budoff, [68]
--------------------------------------------------------------------------------
Yes. No, I think as Dr. Bhatt already mentioned, they're not perfectly captured the way that we think of it today, but I always think of patients with a lot of atherosclerosis has, at least, what we call a secondary risk equivalent. In other words, we might not -- the guidelines may not quite put them into the secondary prevention bucket, but we treat them as aggressively as that group. And I think that's something else where EVAPORATE, I think, will help some clinicians. We're getting a lot of patients in the U.S. getting calcium scoring, which is another CT-based way of measuring risk, and we're getting a lot of patients getting CT angiography now for different reasons. And if you have a tool or a treatment that will actually help reduce the plaque burden that they see, that's yet -- going to be in their clinical eyes. Another indication to use this therapy is they use it -- I see a lot of plaque. I know this slows down plaque, I'm going to use this therapy. And we don't have that many therapies that have this level of evidence now.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [69]
--------------------------------------------------------------------------------
And regardless of whether it's primary prevention or secondary prevention. I guess, the guidelines have also tried to move away from some of these hard and fast benchmarks or numerically based thresholds. And perhaps, Dr. Bhatt, you could comment first on that, and maybe you, Dr. Budoff, on this sort of movement of the guideline to risk characterizations as opposed to primary prevention or secondary prevention around an arbitrary pivot?
--------------------------------------------------------------------------------
Deepak Bhatt, [70]
--------------------------------------------------------------------------------
Yes. Things are really changing the guidelines, as Dr. Budoff alluded to, in terms of risk stratification, even in terms of allocating statin therapy in the primary prevention world. Now we're saying, "Yes, you can use coronary calcium. It can be quite useful figure out who really does benefit from being in that group receiving statin therapy." So there's really an evolution in our thinking in terms of what those terms primary and secondary prevention mean. And I think the use of coronary calcium and to seek angiography in general is really poised to boom in this country. For a variety of different reasons, guideline related to non-guideline related. A lot of it's patient driven. Patients want to know, is there a trouble brewing in their coronary arteries. Is their plaque that's built up, and I think that will make these terms of primary and secondary prevention even more obsolete. The issue is, is a person at risk? That's the real issue. And you might define that by biomarkers, such as LDL elevation in terms of statin allocation, in terms of triglyceride elevation, in terms of consideration of icosapent ethyl. But on the other hand, you might move away from biomarker and say, "Oh, look, there's plaque in the left main, that's a problem regardless what the biomarker show."
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [71]
--------------------------------------------------------------------------------
Yes. I mean, I think, Matt, also and just in closing, before turning it back over to Elizabeth. I mean as both a clinical trial list, but really as a practicing physician, I think this, to me, is the science of medicine and the art of medicine, just like I think the EVAPORATE study is both the real hard-core science, but at the end of the day, it's about the outcomes, which is the quality of the science. Maybe you just want to comment a bit on that perspective with -- being a trial that's doing a lot of imaging. How do you sort of bring that into your discussion with patients in the context of primary prevention or secondary prevention?
--------------------------------------------------------------------------------
Matthew Budoff, [72]
--------------------------------------------------------------------------------
Yes. And I think it's really critical that people understand that whenever we put these buckets, the concept is primary prevention is lower risk and secondary prevention is higher risk. But what we're now seeing are high-risk primary prevention, which are really as high-risk as the other groups. So that's where I think we're starting to try to blur the lines as much as possible because as you said, it's one bad day. They had a heart attack, now they're secondary prevention. If they died of the heart attack, they never make it to secondary prevention, and we never treat them with these drugs that are only allocated to secondary prevention. It's too late to put the cholesterol pill under their tongue while we're doing CPR to try to get their cholesterol down. So we need better ways of identifying who's at risk of having a bad day in the near future. And we're going to turn to imaging. It's already happening. It's going to happen. I agree with Dr. Bhatt, I think it's going to happen faster, where we're going to want to take a look in our coronary arteries. And if they're all blocked up, we're going to be like, I'm high risk, I need to do things about it. And if we have tools that can help us either unblock the artery or lower our risk or do both, as I think we have evidence now with icosapent ethyl. I think that's going to be a therapy that doctors are going to turn to.
--------------------------------------------------------------------------------
Deepak Bhatt, [73]
--------------------------------------------------------------------------------
Yes, I agree. And even beyond just using imaging, there are still some simple clinical descriptors as if somebody has diabetes, essentially, that's an equivalent to someone that's had a prior heart attack, a number of observational and epidemiologic studies show that. So there are some things even without doing biomarkers or fancy imaging, you know that, that person is at risk.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [74]
--------------------------------------------------------------------------------
I think that's a great way to cap it because at the end, it really is about the individual interaction between the patient and the clinician about what's going to work best for them and work best in their life, no matter what the intervention is or not.
So I think with that, Elisabeth, did we cover many of the questions that you think are coming in?
--------------------------------------------------------------------------------
Elisabeth Schwartz, Amarin Corporation plc - Senior Director of IR [75]
--------------------------------------------------------------------------------
Yes, I think that was very helpful to put these results into context, and Craig, I want to thank you for leading this discussion today. Dr. Budoff, Dr. Bhatt, Dr. Weintraub, I'd like to thank you for being with us today, and I very much appreciate the contributions you're making to medical science.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [76]
--------------------------------------------------------------------------------
Thank you.
--------------------------------------------------------------------------------
Elisabeth Schwartz, Amarin Corporation plc - Senior Director of IR [77]
--------------------------------------------------------------------------------
Thank you.
--------------------------------------------------------------------------------
Operator [78]
--------------------------------------------------------------------------------
This concludes today's teleconference. You may now disconnect your lines at this time. Thank you for your participation, and have a wonderful day.
InvestorsHub NewsWire
WCVC Schedules Restaurant Franchise Outlook And Q3 Financial Performance Update • WCVC • Nov 19, 2019 10:34 AM
REDHAWK ANNOUNCES FIRST QUARTER FISCAL 2020 RESULTS • SNDD • Nov 19, 2019 10:00 AM
Target Group Inc. (OTCQB: CBDY) Breakout Alert $.10 Target • CBDY • Nov 19, 2019 10:00 AM
Progressive Care Reports October 2019 Performance: Q4 on Record Pace with Expanding Margins • RXMD • Nov 19, 2019 9:30 AM
Target Group, Inc. (OTCQB: CBDY) Breakout Alert $.10 Target • ACB • Nov 19, 2019 7:15 AM
NEW WAVE ESPORTS INVESTS IN TALON ESPORTS • NWES • Nov 18, 2019 5:05 PM
Freedom Leaf Announces Closing of a $5 Million Round of Growth Capital • FRLF • Nov 18, 2019 10:58 AM
What Is A Stream Worth? Nov 18, 2019 10:00 AM
Amarin Corporation PLC O Webcast Discussion Of Presented Data at American Heart Assocition's 2019 Scientific Sessions
Dublin Nov 19, 2019 (Thomson StreetEvents) -- Edited Transcript of Amarin Corporation PLC conference call or presentation Monday, November 18, 2019 at 9:30:00pm GMT
TEXT version of Transcript
================================================================================
Corporate Participants
================================================================================
* Craig B. Granowitz
Amarin Corporation plc - Chief Medical Officer & Senior VP
* Elisabeth Schwartz
Amarin Corporation plc - Senior Director of IR
================================================================================
Conference Call Participants
================================================================================
* Deepak Bhatt
* Matthew Budoff
* William Weintraub
================================================================================
Presentation
--------------------------------------------------------------------------------
Operator [1]
--------------------------------------------------------------------------------
Greetings. Welcome to the AHA investor discussion conference call. (Operator Instructions) Please note this conference is being recorded.
I would now like to turn the conference over to your host, Elisabeth Schwartz. Thank you. You may begin.
--------------------------------------------------------------------------------
Elisabeth Schwartz, Amarin Corporation plc - Senior Director of IR [2]
--------------------------------------------------------------------------------
Welcome to today's discussion to explore for our investment community the several important new data sets and analyses related to Vascepa and REDUCE-IT as presented at the 2019 American Heart Association's Scientific Sessions here in Philadelphia.
Please be aware that this conference call will contain forward-looking statements that are intended to be covered under the safe harbor provided by the Private Securities Litigation Reform Act. Examples of such statements include, but are not limited to, our current expectations regarding Vascepa clinical data and cost-effectiveness analyses and expectations regarding the application of these data to clinical use, in reimbursement and regulatory settings and to commercial prospects for Vascepa. And these statements are based on information available to us today, November 18, 2019.
We may not actually meet the expectations disclosed in our forward-looking statements. Actual results or events could differ materially. We assume no obligation to update these statements as circumstances change. For additional information concerning these factors that could cause actual results to differ materially, please see the Forward-Looking Statements section and the Risk Factors section of our most recent Form 10-Q filed with the SEC.
This call is intended for investors in Amarin and is not intended to promote the use of Amarin's Vascepa outside its approved indication.
I will now turn the call over to Dr. Craig Granowitz, the Chief Medical Officer of Amarin.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [3]
--------------------------------------------------------------------------------
Thanks, Elisabeth, and thank you all to our panelists and listeners today for joining us. We're excited here -- to be here in Philadelphia at the conclusion of the American Heart Association's Scientific Session. We're fortunate to have 3 clinicians/researchers that are immersed in Vascepa-related sciences and have presented here at AHA to speak with us today: Doctors Matthew Budoff, Deepak Bhatt and William Weintraub. I'll give a quick recap of the presentations by each of these 3 research physicians, and then we'll move into a discussion of some of the key issues with the results.
Dr. Budoff presented this morning on the interim 9-month results of the 18-month EVAPORATE study. EVAPORATE is a first study in the U.S. to use multidetector computed tomography to evaluate the effects of icosapent ethyl as an adjunct to statin therapy on plaque characteristics in a high cardiovascular-risk population with persistently high triglyceride level. Patients underwent interim scans at 9 months and are currently being followed for an additional 9 months. Final results of this investigator-initiated study are anticipated in early 2020.
Dr. Bhatt spoke yesterday about REDUCE-IT USA. These prespecified REDUCE-IT subgroup analyses showed substantial risk reduction in the U.S. patients treated with icosapent ethyl 4 grams a day versus placebo across all predefined composite and individual primary and secondary endpoint, including a 31% relative risk reduction and a 6.5% absolute risk reduction in first occurrence of 5-point MACE. This corresponds to a number needed to treat of 15 and a significant 30% relative and 2.6% absolute risk reduction or a number needed to treat of 39 in all-cause mortality in this U.S. subgroup. I want to emphasize that these analyses of this data in the U.S. population in most studies are usually not as pronounced as what we say in the REDUCE-IT USA analysis.
Finally, we will speak to Dr. Weintraub about the preliminary analysis of the cost effectiveness of icosapent ethyl. In this combined patient level and simulation lifetime cost-effectiveness analysis, icosapent ethyl in high cardiovascular-risk patients showed an exceptional benefit with cardiovascular event reduction as well as cost savings in trial and over patients' lifetime in many simulations.
Finding of cost effectiveness of medical therapies are rare. This analysis considered the current market cost for Vascepa and the potential savings from avoiding major MACE events such as stroke and heart attack, the cost of which can often be very high. As is typical, the cost-effectiveness analysis were not prespecified as part of reducing clinical study design but did rely on the landmark results.
So Matt, I'd like to start with you. You're Professor of Medicine and Endowed Chair at the UCLA School of Medicine and Program Director of the Lundquist Institute. So Matt, if you could just give a summary of some of the key points that you wanted to convey this morning at the late-breaker. And I do want to note that you were the first on the agenda for the morning. So clearly, there was a high degree of interest in the sponsors of the session to hear what you had to say in the EVAPORATE study.
--------------------------------------------------------------------------------
Matthew Budoff, [4]
--------------------------------------------------------------------------------
Yes. And I think it was very, very well received by the physicians in the audience. Basically, we demonstrated that, over just a short 9-month analysis, that there was generally a uniform plaque regression in the patients who received Vascepa as compared to those patients who received placebo. Because it was a small number of patients, it was an 80-person trial, and we only had follow-up in 67 patients at the interim analysis, the power was a little diminished because we powered it based on an 18-month trial. But I have to say that while the primary endpoint was 21% reduced, which was not enough to stop the trial, it was very concordant with all the other parameters. For example, total plaque went down by 42% with a p-value of 0.004, and that's -- we have not seen that type of plaque stabilization or slowing of plaque progression with other therapies that we've studied, especially over a short interim time frame of only 9 months.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [5]
--------------------------------------------------------------------------------
Now, Matt, that's a really helpful summary. And I guess there are some questions that I've heard this morning about what does the mechanistic study actually mean, why do the study -- what -- where do you think that's going to add to the body of literature. And I'll note that Dr. Bhatt was also an investigator on this trial as well. But how, Matt, do you see this kind of fitting in? What role does this play in terms of our understanding of the field?
--------------------------------------------------------------------------------
Matthew Budoff, [6]
--------------------------------------------------------------------------------
Well, I think a lot of clinicians were surprised, to say the least, at the robustness of the REDUCE-IT study and how large the benefit was, and they want to understand better. We're telling them it's not just triglycerides, that it's not only different things, and we don't know what it is. So when you look at the primary endpoints of REDUCE-IT, all of them that could be artherosclerotic line up and show benefit. So I think it suggests that this is a direct anti-artherosclerotic benefit, but you can't derive that solely from the REDUCE-IT study. Now we can say with confidence, based on the results of EVAPORATE, that there is an anti-atherosclerotic property at play here that's supplemental to all of the other benefits that we've already seen with Vascepa.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [7]
--------------------------------------------------------------------------------
Dr. Bhatt, I know that there have been a number of questions asked of you, including even last week at the FDA meeting, about time to curve separation. And I think sort of putting that in context with the mechanistic results of Matt's study might be very helpful context for people to understand sort of these intermediate signals in the context of a clinical event. Could you shed some light on that?
--------------------------------------------------------------------------------
Deepak Bhatt, [8]
--------------------------------------------------------------------------------
I think that's a great question. And in the REDUCE-IT trial, we saw benefit in the 2 major cohorts, which were the patients enrolled with secondary prevention. By that, I mean stable patients with coronary artery disease or cerebrovascular disease or peripheral artery disease. As well, we saw benefits in patients who are high-risk primary prevention. By that, specifically, what we enrolled were people with diabetes and at least one other cardiovascular risk factor. So in that population of patients, there was significant benefit. The drug worked a little bit differently in terms of timing of that benefit kicking in. In the secondary prevention cohort, it looked like the curves were really starting to separate at about a year, whereas it took a couple of years in the primary prevention cohort. And that makes sense because that's what we saw in the statin trials. And in fact, if we had studied really high-risk patients, like people coming in with heart attacks or acute strokes, then I think the curves would have separated even sooner. So it really just has to do with the baseline risk.
But what I think Dr. Budoff's study has nicely done, it's done a lot of things. I mean, it's shown for those doctors that really want to see, what's the mechanism of action, if shown, will look. The plaque in the arteries looks likely to progress. So that's a really powerful visual image. But beyond that, there's another message that is a bit more subtle that, at least to my knowledge, no one has explicitly stated. This was an interim analysis. It was just 9 months. It's meant to be an 18-month study. But I'm really glad Dr. Budoff took a look, prespecified all or part of the plan that he'd laid out because it tells me that there are benefits that are kicking in early. So if you're a physician and you see the patients that we enrolled in REDUCE-IT were stable patients, again, they're not in the throes of a heart attack or that sort of patient, you're going to throw the kitchen sink at them in terms of risk reduction. They're pretty stable. They're in the office. They're not actually complaining of anything. They're doing okay. It'd be easy to just say, look, I'll see you back in a year. But the problem with that approach now we see with a REDUCE-IT-like patient that's out there in practice, their plaque is progressing in the course of that year. So this really provides an impetus for why a physician needs to pull the trigger and act sooner with respect to prescribe it.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [9]
--------------------------------------------------------------------------------
No, I think that's really important context. One of the other sessions I was at yesterday was talking about the effects both on the particles and on inflammation. And Matt, I don't know if you want to comment at all because that might tie into -- one of the questions I think comes up is, why are there so many endpoints that are looked at in this? What are all these different surrogates of total plaque and low attenuation plaque and calcified plaque? Maybe you could help give, again, some of our investors some context of what all of that means because it's just some terminology that might be confusing.
--------------------------------------------------------------------------------
Matthew Budoff, [10]
--------------------------------------------------------------------------------
Well, absolutely. So I think when we look towards therapies that have both presumed the anti-atherosclerotic effect and an anti-inflammatory effect, then we start to think about things like the low attenuation plaque, which what we think of kind of is the necrotic core, the really vulnerable plaque, that might be most likely to rupture.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [11]
--------------------------------------------------------------------------------
And what happens when the plaque ruptures, Matt?
--------------------------------------------------------------------------------
Matthew Budoff, [12]
--------------------------------------------------------------------------------
When a plaque ruptures, patients have an acute heart attack or an acute stroke. The artery gets occluded, and they end up with an immediate event. 1/3 of people die suddenly when these plaques rupture.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [13]
--------------------------------------------------------------------------------
And that really was the endpoint, right, Deepak, of REDUCE-IT? That's sort of the clinical consequence of a plaque gone bad.
--------------------------------------------------------------------------------
Deepak Bhatt, [14]
--------------------------------------------------------------------------------
Look, it's a great one-two punch when you think about EVAPORATE and REDUCE-IT. You've got basic mechanistic data now by imaging and a clinical trial that shows clear-cut benefits. So it's a really nice package scientifically. For physicians out there that want mechanism of action, now we've got one. Now there are several other mechanisms of action at play too, I think, with icosapent ethyl with the ability to retard plaque progression. That's pretty important.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [15]
--------------------------------------------------------------------------------
Right. So Matt, I'm sorry I interrupted you talking about some of the different endpoints of the study.
--------------------------------------------------------------------------------
Matthew Budoff, [16]
--------------------------------------------------------------------------------
Yes. So obviously, when you think about just overall progression in your coronary arteries, you would use an endpoint like total plaque, and that was very significantly reduced in the EVAPORATE trial. We focused on this more vulnerable plaque because of some of the evidence from REDUCE-IT that sudden death was reduced. So there was a signal there that maybe this plaque ruptures. There's some data from abroad, from Japan, the CHERRY study, and other studies that have also suggested that, that would be a good target to look at specifically because of a drug that has these pleiotropic or multiple effects on the vasculature.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [17]
--------------------------------------------------------------------------------
And I think you mentioned at least one point to me that there've been a number of these imaging studies done, mostly in Japan, not in the U.S., and that's one of the unique aspects of EVAPORATE. But has there been a difference that have been seen in the Japanese studies with different omega-3 preparations?
--------------------------------------------------------------------------------
Matthew Budoff, [18]
--------------------------------------------------------------------------------
Yes. So there's been a number of trials. There's been 2 large-scale trials, 1 in the U.S. and 1 outside the U.S., that looked at 4 grams of Lovaza without showing significant benefits.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [19]
--------------------------------------------------------------------------------
And Lovaza is what part of...
--------------------------------------------------------------------------------
Matthew Budoff, [20]
--------------------------------------------------------------------------------
EPA and DHA, mixed -- combination pill. And both of those trials failed to show benefit even out to 2 years. So I think this is much different than the EPA-DHA mix data that we've seen from imaging. And the Japanese data did line up very nicely with 1.8 grams of EPA. But again, Japanese populations, invasive imaging, where they're putting catheters into people's coronary arteries, things that would be really hard to emulate in the United States.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [21]
--------------------------------------------------------------------------------
Yes. And that's very helpful context. I think one of the other questions that people have asked me is the interim analysis threshold and the statistical benchmark for that, which seems awfully high, right? If you could talk a little bit about how the statistics might be. And I don't want to put you on the spot. I know you're not a statistician, but I'm not sure that everyone really understands that the hurdles at an interim analysis are quite high to stop a study.
--------------------------------------------------------------------------------
Matthew Budoff, [22]
--------------------------------------------------------------------------------
Yes. And basically, what you're saying is if there's overwhelming efficacy, if the drug is so good at an early time point, then you can almost argue it's unethical to continue the study, just like we stop clinical trials early when there's overwhelming benefit because we don't want to keep people on placebo or keep people off of this therapy. So we didn't hit that interim analysis based on the primary endpoint that we chose. But again, had we chosen total plaque, which is a very reasonable endpoint in many trials, we would have actually stopped the trial early because total plaque came in at P 0.004, which is less than the necessary stopping parameter. So we can -- the Steering Committee can obviously take some responsibility for picking the wrong endpoint, but hindsight is 2020. But the trial is still positive, and the trial is still ongoing, so I think that it's good news. And I think based on my conversations at the American Heart Association after the presentation, many physicians feel much more comfortable now, not people who just live and die based on clinical trials but those that want to understand the mechanism, that this adds a lot to their confidence in moving forward with the use of icosapent ethyl in their practice.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [23]
--------------------------------------------------------------------------------
No, that's helpful. There was one other question. I know you showed a slide at the meeting today about the placebo controls, and I know you covered a tremendous amount of data very quickly on that. And this might be a point to help clarify. What was actually done? And why is it meaningful?
--------------------------------------------------------------------------------
Matthew Budoff, [24]
--------------------------------------------------------------------------------
Yes. So since the REDUCE-IT trial was published and after we started EVAPORATE, there were some questions about the mineral oil placebo that was used and whether that was contributing to harm rather than -- or offsetting some of the benefit of icosapent ethyl. We had already started EVAPORATE, so EVAPORATE was using the same mineral oil placebo that we used in REDUCE-IT. So what we did is we matched that placebo group to another group of patients who are on a different placebo, one that's made out of cellulose that everybody acknowledges is completely inert and has no adverse properties to it. And we showed that the rate of progression on patients on mineral oil was identical to the rate of progression of those patients on an inert cellulose placebo. So hopefully, laying any concerns about mineral oil being bad, rather now all the benefit is on icosapent ethyl being good.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [25]
--------------------------------------------------------------------------------
No, that's very helpful. And again, I just want to clarify one other point before we left for Dr. Weintraub out of the conversation. But -- so you showed a curve, and it looked like there was a 45-degree line, and there were all these dots on the curve. Maybe you can just sort of put that in context of what that meant and why did that give you comfort and confidence because I'm not sure everyone is so familiar with looking at that kind of scatter plot.
--------------------------------------------------------------------------------
Matthew Budoff, [26]
--------------------------------------------------------------------------------
Yes. No, absolutely. So this is basically just putting all of the rates of progression among both cohorts, the -- there were blue dots on the curve, if you saw it on the slide, that were represented the cellulose placebo from another randomized trial, and there were red dots that represented EVAPORATE placebo patients. And the line of identity went -- was identical for the two. In other words, we graphed out what is the rate of progression among those 2 cohorts, and they were -- the lines were superimposed.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [27]
--------------------------------------------------------------------------------
Right. So each one of those dots was an individual patient over that time course and sort of seeing that there was an equal distribution across both sides, which said, statistically, there was absolutely no difference in the rate of change between those 2 of the parameters you're looking at.
--------------------------------------------------------------------------------
Matthew Budoff, [28]
--------------------------------------------------------------------------------
Absolutely. And what people need to understand is when we're up there presenting, as we all do fairly frequently, there's literally a giant clock that only we can see that is counting down towards 0. And when it hit 0, you have to stop talking. So...
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [29]
--------------------------------------------------------------------------------
And it's flashing red so...
--------------------------------------------------------------------------------
Matthew Budoff, [30]
--------------------------------------------------------------------------------
Yes, yes. Yes, yes.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [31]
--------------------------------------------------------------------------------
And they turn off the microphone.
--------------------------------------------------------------------------------
Matthew Budoff, [32]
--------------------------------------------------------------------------------
But -- so we are under some time pressure. So yes, so we couldn't put in all of the data, obviously, that we would have liked to in a more open presentation. I was limited to 10 minutes to present the entire spectrum of data. But yes, that line basically shows that there's -- with all the individual data points, that there's no difference. And we did multiple analyses with all different types of plaque. And regardless of what we looked at, their rates of progression on cellulose placebo, inert placebo was identical to mineral oil placebo. And I did, I think, hopefully, to at least the scientists and the clinicians in the room, effectively convey that mineral oil, therefore, is not the bad player that some people have accused it of being.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [33]
--------------------------------------------------------------------------------
No, thanks. Thank you. And I think that was another important analysis that came out of the study. Bill, I wanted to turn our attention to you. We're very fortunate to have William Weintraub here from the Medstar Institute, who has a long history of doing cost effectiveness analyses as well. And Bill, you're also a cardiologist also?
--------------------------------------------------------------------------------
William Weintraub, [34]
--------------------------------------------------------------------------------
I am.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [35]
--------------------------------------------------------------------------------
So he's a clinical cardiologist and a health economist with a lot of publications in this particular area and really an acknowledged expert. And I give Dr. Boden and the Steering Committee a lot of credit for reaching out and engaging with Bill on the cost effectiveness that was presented here at the meeting. And again, this was done with the support of Amarin but really independent of Amarin, knowing all the sensitivities, particularly around cost effectiveness analyses.
Bill, you might want to just share just in a minute or 2 your perspective on the study and its design. And again, this was done in the overall total population, not the U.S. population. One of the reasons I wanted to go to the total population first before asking Dr. Bhatt to comment on the U.S. population is I don't want to confuse the 2 because I think having them presented at the same meeting could be potentially confusing to people. So you studied the overall REDUCE-IT population.
--------------------------------------------------------------------------------
William Weintraub, [36]
--------------------------------------------------------------------------------
Right. We did. We looked at the U.S. population too, but we started out with the total population. We used U.S. prices, but we used the total population, which is the typical way this is done. Now -- and with REDUCE-IT, we found a 25% relative risk reduction for the first or primary endpoint but 30% overall. In the cost-effective analysis, we used all events, not just the first event. So here, we have a relatively inexpensive pharmaceutical, (inaudible) pharmaceutical. And we have a remarkable reduction in event rates, 30%. This is an unusual situation.
Now what we did is we have to use a number of different costs inputs. We have to look at the cost of the drug. We used what is called SSR net pricing, which we strongly believe that's the right price to use in cost-effective analyses. We used different input values for the cost of events. We used Optum cost, which gives a very broad distribution of costs in patients under the age of 65 and Medicare costs over the age of 65 for our base-case analysis.
Then the other thing we have to look at is utility, how well patients are functioning on scale 0 to 1, with 1 being perfect health and functioning, 0 being generally mortality. We use utility values from the literature, and then we have to estimate survival. Now the remarkable thing about what we did here is that we had 5 full years of in-trial data. And with that, we could look in-trial not using a simulation, not using estimation but using real data. And with that, we found that icosapent ethyl in our base-case analysis is a dominant strategy and as we offer better outcomes at lower cost.
For (inaudible) pharmaceutical, none of us have ever seen anything quite like it before, very unusual and very important. This carried over into our long-term survival analysis as well. But there always has to be a partial simulation because we have to estimate event rates that we haven't measured. We have to estimate survival. Nonetheless, both of these work together to suggest that we have remarkably efficacious therapy, which offers great value. And at least, our preliminary results do suggest that it is dominant, that we went for better outcome at lower cost.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [37]
--------------------------------------------------------------------------------
Thanks, Bob. A very good summary. And again, some of the questions that I've been asked on a number of occasions, is there was an independent group called ICER, that also did an analysis. They came up with somewhat different findings, even though I think they use the same drug cost. Maybe you could talk a bit about the ICER analysis, what are the strengths and weaknesses of each one of them because ultimately, it's sort of this constellation of models that are out there, right?
--------------------------------------------------------------------------------
William Weintraub, [38]
--------------------------------------------------------------------------------
All right. So I mean, they've also found that the cost of icosapent ethyl is highly cost-effective is using most benchmarks. They did not find it to be a dominant strategy. But still, we've got a relatively remarkable incremental cost effectiveness ratio of $18,000 for quality adjusted life year gain, but still very good by almost any measure. The difference is to shift from dominant to $18,000. It's actually not that big. It sounds a lot, but it's actually a very small shift that can result in that. What do they do that was different from what we did. First of all, that was a total simulation. They did not have access to the clinical trial data.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [39]
--------------------------------------------------------------------------------
And so, Bill, for -- I'm sorry to interrupt, but what does that mean the simulation versus actual clinical data, because I think these are concepts, and not everyone is so familiar with.
--------------------------------------------------------------------------------
William Weintraub, [40]
--------------------------------------------------------------------------------
Well, thank you for interrupting. Allow me to clarify that. So a simulation would mean you take results of the trial. It's not that it's made up. I don't want you people to think that this is some kind of fantasy, that's not the case. What they did is they took the overall results of the trial as published in the first -- REDUCE-IT paper and then develop a mathematical model from that, that allows them to estimate the cost effectiveness. That can be -- that works pretty well, but it's not as good as having the actual data in your hands. The other thing is that there's going to be variation. We can look at the distribution of things a lot better when we have the data. So everyone understands that not all patients are the same. You have the distribution of patients. But if you only have the sort of the bottom line results, you can't look at that distribution the same way you can get all of your data.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [41]
--------------------------------------------------------------------------------
Now I also understand that they only looked at some of the endpoints, not all of the primary endpoints of the study as well?
--------------------------------------------------------------------------------
William Weintraub, [42]
--------------------------------------------------------------------------------
Right. So they don't have access to all of the endpoints. We have actual data. So we could look at heart failure hospitalization, AFib hospitalizations. We could look at things that they didn't have access to.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [43]
--------------------------------------------------------------------------------
All right. That's helpful context of how you could get the range. I think another term that isn't familiar to many, is what is dominant actually mean and how to try to understand what dominant actually would represent.
--------------------------------------------------------------------------------
William Weintraub, [44]
--------------------------------------------------------------------------------
All right. So most of the time in a cost-effective analysis for a new therapy, it'll be -- if it's effective, you'll find that it increases costs. And when that happens, you calculate as the ICER group did, you calculate what's called an incremental cost effectiveness ratio, which is generally measured in cost per quality adjusted life year gained. You don't calculate that ratio for technical reasons, you don't calculate that ratio when you have improved outcome at lower cost, improved the outcome at lower cost is called dominant. And our analysis strongly suggests that we have a dominant strategy here that has improved the outcome at low cost.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [45]
--------------------------------------------------------------------------------
So really, a dominant strategy today in the U.S. health care system would be what would be a fully integrated payer where they're actually paying for and bearing all of the risk, including not only the drug cost, but also the downstream bad outcomes, which is the hospitalization costs as well as the provider costs and all of the other downstream implications of the disease.
--------------------------------------------------------------------------------
William Weintraub, [46]
--------------------------------------------------------------------------------
Now what we aim at is a societal study in which we reflect everything across society, that's almost impossible to do because you can't make those pull of those measurements. So we use various proxies to allow you to get at that. But your point is this is looking broadly rather than just considering a hospital or a doctor's office or something, or something like that. So we're trying -- we aim to look as broadly across the society as we can.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [47]
--------------------------------------------------------------------------------
Right. And I think in our U.S. system, there are very few groups that really are tasked with or have that obligation, look at total costs. So I think probably, if the health care system is changing over time. There are more and more of those that will be both bearing the risk of all the costs as well as the economic potential gains of being able to deliver care below that cost threshold, whatever that is, whether they're reimbursed.
--------------------------------------------------------------------------------
William Weintraub, [48]
--------------------------------------------------------------------------------
Yes, that's very true. But people who do cost-effectiveness analysis, this is not uncommon for people to attempt to think as broadly as we can. But I think more and more as we consider health care economics, people are going to expect that we will own broadly.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [49]
--------------------------------------------------------------------------------
Now that's all very, very helpful context of this. When you said that some of the differences are subtle between the different models, what are the things that the model is most sensitive to?
--------------------------------------------------------------------------------
William Weintraub, [50]
--------------------------------------------------------------------------------
Right. So that's very important. It's most sensitive to the cost of the pharmaceutical -- there's very little difference between what we did and what the ICER did. They use SSR costs, we use SSR costs. The real difficulty is the cost of events, and there's uncertainty about what a hospitalization for heart attack or stroke for cardiac catheterization for percutaneous coronary intervention or coronary surgery costs. And because of that, that could have a big effect on the results of the analysis. And we were very sensitive to that, we'll be looking to that in very great detail.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [51]
--------------------------------------------------------------------------------
Now U.S. also had a unique asset that probably has not been made available very often before from commercial payers, you might want to just comment a bit on that and why those costs might be much more reflective of what is actually being paid in the real world.
--------------------------------------------------------------------------------
William Weintraub, [52]
--------------------------------------------------------------------------------
So I'm not sure what you're referring to.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [53]
--------------------------------------------------------------------------------
Well, you had some of the real commercial costs as opposed to the projected cost?
--------------------------------------------------------------------------------
William Weintraub, [54]
--------------------------------------------------------------------------------
Yes. Right. So you're thinking about the cost from the Optum Group?
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [55]
--------------------------------------------------------------------------------
Exactly.
--------------------------------------------------------------------------------
William Weintraub, [56]
--------------------------------------------------------------------------------
So Optum looks broadly across payers in the United States. And this is a huge database of -- I don't remember the exact number. But it's on the order of 160 million people in this database, broadly across payers, allowing what we believe is actually a far more accurate assessment of costs than Medicare costs. I think that people recognize that Medicare costs really do not cover the cost of events. And so if you use Medicare cost alone, you're going to underestimate the value of prevention. And so I think that's very important for people to come to understand looking at cost-effective analysis that we have to work hard to fund the best estimates of cost. And so that we can appropriately evaluate the value of the therapies that we all paid for patients.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [57]
--------------------------------------------------------------------------------
And since you had the patient level data, those that were less than 65 years old were not yet in Medicare, you actually use the true commercial costs. And for those that are over 65, you use the Medicare costs.
--------------------------------------------------------------------------------
William Weintraub, [58]
--------------------------------------------------------------------------------
Yes. We've done that. And I think that's relatively conservative analysis. But it can also be criticized. Well, you might say, "Oh, you should use the Optum costs for everybody." Well, what would happen then if you use Optum costs for everybody? If we think that really reflects real cost, then the finding of a dominant strategy for a cost being implemented would be strengthened even more?
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [59]
--------------------------------------------------------------------------------
All right. I think that's helpful context. I think these are some of the details and that are important in really trying to reflect reality what truly are the cost and risk of the global population in the U.S. group. And as you mentioned, and we'll turn it back over to Dr. Bhatt and the talk about the REDUCE-IT USA, but to look really in the U.S. population with U.S. costs might give results that are potentially different.
--------------------------------------------------------------------------------
William Weintraub, [60]
--------------------------------------------------------------------------------
So we have a subgroup analysis. We have not done this as thoroughly as we plan to in -- for the U.S., but our subgroup analysis suggests that the value of icosapent ethyl is going to be even more strengthened with -- for the U.S. patients.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [61]
--------------------------------------------------------------------------------
Thank you, Bill. I appreciate that. So Dr. Bhatt, I know that there was a tremendous amount of interest in doing the U.S. subgroup out of the study. What were the findings from that analysis?
--------------------------------------------------------------------------------
Deepak Bhatt, [62]
--------------------------------------------------------------------------------
So this was a prespecified subgroup. And the reason we were interested in looking at in the first place was to provide some context because there's a number of these large international trials, for whatever reason in the past, show results in the U.S., they're just different from the rest of the world, usually not looking as good. And then it's hard to know, is it just that it's a small subgroup, is it just a spurious finding. But then the American physician is left in the challenging position of a trial where the data might look good, but then in the wrong country, it doesn't look that good. So that was the basis for prespecifying this and the scientific rationale. So we did go ahead with this. And of course, the timing of this analysis is useful as U.S. regulators and other physicians in the U.S. are considering how to apply the data in the U.S. But the analysis itself was prespecified. So the overall results, first, let me say, for both within the U.S. and outside the U.S. look spectacular. That is for the primary efficacy endpoint, the key secondary efficacy endpoint in both cases, both within the U.S., but also in the subgroup of non-U. S. patients, both of these are statistically significantly reduced. So I don't want to create the impression that the drug is working in the U.S. and working only in the U.S. That would be a mistake. So hopefully no one walks away with that impression. The drug works globally in all the regions we studied, and there's no reason to think it wouldn't work in the regions where we didn't include patients.
Now what we found in our U.S. subgroup analysis was for the primary and key sector endpoint, results that were at least as robust, at least as large in magnitude as in the overall trial. And then on top of that, for the endpoint of all-cause mortality, a significant 30% reduction in death in the U.S. as well as that translated to a 2.6% absolute risk reduction mortality. So that is really upping the ante, so to speak, we're showing not just reductions in things like heart attack and stroke, that's pretty good and good enough, but also now showing a reduction in dying.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [63]
--------------------------------------------------------------------------------
Now I know the paper had a, what I would call an exhaustive review in one of the supplemental tables of all of the differences in the population. It might be very helpful for you with your clinical judgment and sort of deep immersion in the database over the last year, and maybe pick out those criteria of the U.S. subgroup that was different than the non-U. S. subgroup.
--------------------------------------------------------------------------------
Deepak Bhatt, [64]
--------------------------------------------------------------------------------
It's a great question because, again, I wouldn't want anyone in the audience walking away thinking, "Oh, the drug is, for some reason, magically just working in the U.S. and doesn't work as well outside the U.S." That's not true at all. It worked in all the populations we studied, whether it's the region, ethnicity, various other subgroups, the drug was consistent in its benefits. The reason I think the results look better in the U.S., and there's this reduction in mortality that's significant, and that the overall trial there was a trend towards a lower all-cause mortality. And mortality from cardiovascular causes and the overall trial was significantly reduced by 20%. But the reason, I think, we're seeing an amplification, you could say, a benefit in the U.S. subgroup isn't because there were people living in the U.S. per se, but rather, they had more risk factors. So I think a higher risk population, well, we saw more benefit and this is akin to what was observed in the statin literature, higher risk patients of the curves for events separate sooner, the degree of benefit is larger, but even in lower-risk patients with statins. If you follow them long enough, benefit emerges. So I think that's really the key that the U.S. patients were higher risk than the non-U. S. patients. And by higher risk, I mean, more obesity. For example, that's known from other studies, that's not a novel observation, there's more obesity in the U.S. in other regions of the world. That's unfortunate, but that's true. But there are other risk factors as well that were occurring with a higher degree of prevalence in the U.S. versus non-U. S. groups. But one other thing I'll mention, too, is that the EPA or eicosapentaenoic acid levels at baseline were a bit lower in the U.S. versus outside the U.S., so that might be another reason why it seems like the drug was performing even better than it did in the overall trial, where it performed quite well. I also want to point out, too, sometimes people have thought once they saw this data set that, "oh, maybe it's just -- there's a bunch more secondary prevention patients in the U.S.," that's really what's driving it. In fact, it's the opposite. There's a higher proportion of primary prevention patients from the U.S. So that's not what's driving it. It's just that there's more risk factors, a clustering of risk factors. So I think the take-home message isn't so much that all of the drug works there in the U.S., but rather, if you have higher risk patients, they derive even greater benefit, and that benefit kicks in sooner.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [65]
--------------------------------------------------------------------------------
Right. And so I think you raised what to me was a really important finding. And I think there's a lot of confusion about primary and secondary prevention in high-risk and low-risk in this perception that primary prevention is low risk and secondary prevention is high risk. The results of the REDUCE-IT USA seemed to be somewhat at odds with that common understanding or perhaps common misunderstanding?
--------------------------------------------------------------------------------
Deepak Bhatt, [66]
--------------------------------------------------------------------------------
Yes. The truth is this is an outdated paradigm talking about artificial universes of primary prevention and secondary prevention. And so your existing in different areas. When in fact, there's a lot of overlap. And Dr. Budoff, for example, spends a lot of his day doing CT angiography, that is imaging of hard arteries. So what is someone who is asymptomatic, but for whatever reason, they end up getting a CT angiogram, and they've got a ton of plaque in their arteries. Is that a primary prevention patient? Or is that a secondary prevention patient? That nomenclature isn't set up to capture that patient. To me, if they've got a ton of plaque in their arteries, they're at high risk. They may not know they're at high risk, they may not have symptoms or sort of an early warning system, where they're having chest pain or anything. To me, that's almost more dangerous. I'd rather have the secondary prevention patient who has stable angina. It knows when he or she's running to the bus and they're having chest pain, they stop running. But it's that asymptomatic patient, in many respects, is scarier because you don't really know what to do to reduce their risk. So it's an artificial classification. For the purposes of doing clinical trials, we enroll a bunch of different types of patients. We sort of guesstimate what their event rates might be so that we can do a trial with a particular drug or device in a finite period of time with a finite sample size. So it's a bit artificial. What we've shown in REDUCE-IT is across a variety of risk, the patients benefit substantially for icosapent ethyl.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [67]
--------------------------------------------------------------------------------
Yes. And I think, Matt, there's both a trial list, but also participating center in REDUCE-IT. I mean, what are your thoughts on this whole issue of primary prevention and secondary prevention? And when you think about the EVAPORATE study? Do you have both types of patients in EVAPORATE?
--------------------------------------------------------------------------------
Matthew Budoff, [68]
--------------------------------------------------------------------------------
Yes. No, I think as Dr. Bhatt already mentioned, they're not perfectly captured the way that we think of it today, but I always think of patients with a lot of atherosclerosis has, at least, what we call a secondary risk equivalent. In other words, we might not -- the guidelines may not quite put them into the secondary prevention bucket, but we treat them as aggressively as that group. And I think that's something else where EVAPORATE, I think, will help some clinicians. We're getting a lot of patients in the U.S. getting calcium scoring, which is another CT-based way of measuring risk, and we're getting a lot of patients getting CT angiography now for different reasons. And if you have a tool or a treatment that will actually help reduce the plaque burden that they see, that's yet -- going to be in their clinical eyes. Another indication to use this therapy is they use it -- I see a lot of plaque. I know this slows down plaque, I'm going to use this therapy. And we don't have that many therapies that have this level of evidence now.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [69]
--------------------------------------------------------------------------------
And regardless of whether it's primary prevention or secondary prevention. I guess, the guidelines have also tried to move away from some of these hard and fast benchmarks or numerically based thresholds. And perhaps, Dr. Bhatt, you could comment first on that, and maybe you, Dr. Budoff, on this sort of movement of the guideline to risk characterizations as opposed to primary prevention or secondary prevention around an arbitrary pivot?
--------------------------------------------------------------------------------
Deepak Bhatt, [70]
--------------------------------------------------------------------------------
Yes. Things are really changing the guidelines, as Dr. Budoff alluded to, in terms of risk stratification, even in terms of allocating statin therapy in the primary prevention world. Now we're saying, "Yes, you can use coronary calcium. It can be quite useful figure out who really does benefit from being in that group receiving statin therapy." So there's really an evolution in our thinking in terms of what those terms primary and secondary prevention mean. And I think the use of coronary calcium and to seek angiography in general is really poised to boom in this country. For a variety of different reasons, guideline related to non-guideline related. A lot of it's patient driven. Patients want to know, is there a trouble brewing in their coronary arteries. Is their plaque that's built up, and I think that will make these terms of primary and secondary prevention even more obsolete. The issue is, is a person at risk? That's the real issue. And you might define that by biomarkers, such as LDL elevation in terms of statin allocation, in terms of triglyceride elevation, in terms of consideration of icosapent ethyl. But on the other hand, you might move away from biomarker and say, "Oh, look, there's plaque in the left main, that's a problem regardless what the biomarker show."
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [71]
--------------------------------------------------------------------------------
Yes. I mean, I think, Matt, also and just in closing, before turning it back over to Elizabeth. I mean as both a clinical trial list, but really as a practicing physician, I think this, to me, is the science of medicine and the art of medicine, just like I think the EVAPORATE study is both the real hard-core science, but at the end of the day, it's about the outcomes, which is the quality of the science. Maybe you just want to comment a bit on that perspective with -- being a trial that's doing a lot of imaging. How do you sort of bring that into your discussion with patients in the context of primary prevention or secondary prevention?
--------------------------------------------------------------------------------
Matthew Budoff, [72]
--------------------------------------------------------------------------------
Yes. And I think it's really critical that people understand that whenever we put these buckets, the concept is primary prevention is lower risk and secondary prevention is higher risk. But what we're now seeing are high-risk primary prevention, which are really as high-risk as the other groups. So that's where I think we're starting to try to blur the lines as much as possible because as you said, it's one bad day. They had a heart attack, now they're secondary prevention. If they died of the heart attack, they never make it to secondary prevention, and we never treat them with these drugs that are only allocated to secondary prevention. It's too late to put the cholesterol pill under their tongue while we're doing CPR to try to get their cholesterol down. So we need better ways of identifying who's at risk of having a bad day in the near future. And we're going to turn to imaging. It's already happening. It's going to happen. I agree with Dr. Bhatt, I think it's going to happen faster, where we're going to want to take a look in our coronary arteries. And if they're all blocked up, we're going to be like, I'm high risk, I need to do things about it. And if we have tools that can help us either unblock the artery or lower our risk or do both, as I think we have evidence now with icosapent ethyl. I think that's going to be a therapy that doctors are going to turn to.
--------------------------------------------------------------------------------
Deepak Bhatt, [73]
--------------------------------------------------------------------------------
Yes, I agree. And even beyond just using imaging, there are still some simple clinical descriptors as if somebody has diabetes, essentially, that's an equivalent to someone that's had a prior heart attack, a number of observational and epidemiologic studies show that. So there are some things even without doing biomarkers or fancy imaging, you know that, that person is at risk.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [74]
--------------------------------------------------------------------------------
I think that's a great way to cap it because at the end, it really is about the individual interaction between the patient and the clinician about what's going to work best for them and work best in their life, no matter what the intervention is or not.
So I think with that, Elisabeth, did we cover many of the questions that you think are coming in?
--------------------------------------------------------------------------------
Elisabeth Schwartz, Amarin Corporation plc - Senior Director of IR [75]
--------------------------------------------------------------------------------
Yes, I think that was very helpful to put these results into context, and Craig, I want to thank you for leading this discussion today. Dr. Budoff, Dr. Bhatt, Dr. Weintraub, I'd like to thank you for being with us today, and I very much appreciate the contributions you're making to medical science.
--------------------------------------------------------------------------------
Craig B. Granowitz, Amarin Corporation plc - Chief Medical Officer & Senior VP [76]
--------------------------------------------------------------------------------
Thank you.
--------------------------------------------------------------------------------
Elisabeth Schwartz, Amarin Corporation plc - Senior Director of IR [77]
--------------------------------------------------------------------------------
Thank you.
--------------------------------------------------------------------------------
Operator [78]
--------------------------------------------------------------------------------
This concludes today's teleconference. You may now disconnect your lines at this time. Thank you for your participation, and have a wonderful day.
InvestorsHub NewsWire
WCVC Schedules Restaurant Franchise Outlook And Q3 Financial Performance Update • WCVC • Nov 19, 2019 10:34 AM
REDHAWK ANNOUNCES FIRST QUARTER FISCAL 2020 RESULTS • SNDD • Nov 19, 2019 10:00 AM
Target Group Inc. (OTCQB: CBDY) Breakout Alert $.10 Target • CBDY • Nov 19, 2019 10:00 AM
Progressive Care Reports October 2019 Performance: Q4 on Record Pace with Expanding Margins • RXMD • Nov 19, 2019 9:30 AM
Target Group, Inc. (OTCQB: CBDY) Breakout Alert $.10 Target • ACB • Nov 19, 2019 7:15 AM
NEW WAVE ESPORTS INVESTS IN TALON ESPORTS • NWES • Nov 18, 2019 5:05 PM
Freedom Leaf Announces Closing of a $5 Million Round of Growth Capital • FRLF • Nov 18, 2019 10:58 AM
What Is A Stream Worth? Nov 18, 2019 10:00 AM
Oppenheimer analyst Leland Gershell initiated coverage of Amarin with an Underperform rating and $7  price target.
price target.
https://thefly.com/landingPageNews.php?id=299&headline=AMRN
Mal jetzt im Ernst - ich weiß wirklich nicht was die Jungs geraucht haben. Eine Herabstufung im nachbörslichen Handel. Denen scheint der Arsch aber so was von volle Pulle auf Grundeis zu gehen.
 price target.
price target.https://thefly.com/landingPageNews.php?id=299&headline=AMRN
Mal jetzt im Ernst - ich weiß wirklich nicht was die Jungs geraucht haben. Eine Herabstufung im nachbörslichen Handel. Denen scheint der Arsch aber so was von volle Pulle auf Grundeis zu gehen.
Antwort auf Beitrag Nr.: 61.966.046 von bernie55 am 19.11.19 22:39:29News im nachbörslichen Handel werden durch den sogenannten "algo driven trading " automatisch berücksichtigt. Das heißt , dass Schlagzeilenleser integriert sind., die den Kurs automatisch steuern -entweder nach unten oder nach oben.
Also eine gute Möglichkeit für die ANALO - Combos , den Aktienkurs ein wenig zu senken und ordentlich zu kaufen.
Also eine gute Möglichkeit für die ANALO - Combos , den Aktienkurs ein wenig zu senken und ordentlich zu kaufen.
Antwort auf Beitrag Nr.: 61.966.208 von bernie55 am 19.11.19 22:59:03Habe kurz grob überschlagen vom heutigen Volumen was gehandelt wurde sind cirak 4 Millionen Dollar seit negativen Schlagzeigzeilen zumeist in abwertende Richtung gelaufen gab auch Zukäufe so ist es nicht... mal abwarten was der Tag Morgen so bringt.
Immer wieder interessant was man so alles "auslösen" kann sowohl in ungerechtfertigt positive wie auch in negative Richtung.
Amarin ist und bleibt eben für Achterbahnfahrfans ein muss
Achja noch ein wichtiger Hinweis "Nicht während der Fahrt aussteigen !"
Hoffe dieser hilft dem einen oder anderen Anleger hier zu "überleben", ist ein einfacher aber ganz sicher wohl überlegter und gut gemeinter Tipp von mir
Was den Analysten angeht nunja "schwarzmalen" ist dafür kein Ausdruck mehr das ist schon die Apokalypse kommen sehen, was der da anmeldet und für solche Weltuntergangsapostel kann ich nur sagen "der will provozieren" thats it... insofern pflichte bei das ist "gewollte" Manipulation nicht mehr und nicht weniger.
Immer wieder interessant was man so alles "auslösen" kann sowohl in ungerechtfertigt positive wie auch in negative Richtung.
Amarin ist und bleibt eben für Achterbahnfahrfans ein muss

Achja noch ein wichtiger Hinweis "Nicht während der Fahrt aussteigen !"
Hoffe dieser hilft dem einen oder anderen Anleger hier zu "überleben", ist ein einfacher aber ganz sicher wohl überlegter und gut gemeinter Tipp von mir

Was den Analysten angeht nunja "schwarzmalen" ist dafür kein Ausdruck mehr das ist schon die Apokalypse kommen sehen, was der da anmeldet und für solche Weltuntergangsapostel kann ich nur sagen "der will provozieren" thats it... insofern pflichte bei das ist "gewollte" Manipulation nicht mehr und nicht weniger.
Antwort auf Beitrag Nr.: 61.966.256 von flyingbeef am 19.11.19 23:10:02Ach ja und diesen kleinen Fun-Fact will ich euch nicht vorenthalten:
Posted by Lars Charter on Nov 18th, 2019
Amarin logoOppenheimer & Co. Inc. grew its stake in shares of Amarin Co. plc (NASDAQ:AMRN) by 19.7% in the 3rd quarter, according to the company in its most recent Form 13F filing with the Securities & Exchange Commission. The fund owned 27,779 shares of the biopharmaceutical company’s stock after purchasing an additional 4,570 shares during the period. Oppenheimer & Co. Inc.’s holdings in Amarin were worth $421,000 as of its most recent SEC filing
Sie kaufen also zu und wollen wohl mehr abgreifen aber am besten zu etwas günstigeren Konditionen
Posted by Lars Charter on Nov 18th, 2019
Amarin logoOppenheimer & Co. Inc. grew its stake in shares of Amarin Co. plc (NASDAQ:AMRN) by 19.7% in the 3rd quarter, according to the company in its most recent Form 13F filing with the Securities & Exchange Commission. The fund owned 27,779 shares of the biopharmaceutical company’s stock after purchasing an additional 4,570 shares during the period. Oppenheimer & Co. Inc.’s holdings in Amarin were worth $421,000 as of its most recent SEC filing
Sie kaufen also zu und wollen wohl mehr abgreifen aber am besten zu etwas günstigeren Konditionen

Antwort auf Beitrag Nr.: 61.966.046 von bernie55 am 19.11.19 22:39:29Lachnummer des Jahres und eigentlich korrupt, SEC!

Antwort auf Beitrag Nr.: 61.967.084 von Magnetfeldfredy am 20.11.19 07:32:32Schade, dass es nicht um den gesundheitlichen Nutzen des Medikamentes geht.
Hier geht es um richtig viel Geld und da ist denen alles recht.
Ich hoffe nur, dass da nicht noch was schlimmeres kommt.
Hier geht es um richtig viel Geld und da ist denen alles recht.
Ich hoffe nur, dass da nicht noch was schlimmeres kommt.
Antwort auf Beitrag Nr.: 61.968.662 von Stevo0815 am 20.11.19 09:58:17Tja,
so lange man rein und raus "hüpfen" kann wie man lustig ist, ist eine Aktie egal was dahinter steckt ein Spielball der Finanzjongleure das ist die Realität.
Klar gibt es Leute wie du und ich die auch aus gewissen Überzeugungen heraus sich für eine Investition entscheiden, jedoch muss klar sein wir mit unserem kleinen finanziellen Handlungsspielraum sind wenn wir mitmachen dazu verdammt uns das anzusehen, wir können wenig tun außer selbst nicht zu verkaufen und so weder unsere Aktien preiswert Shorts in den Rachen zu werfen oder den Kurs noch weiter zu drücken.
Wenn man grundsätzlich aber vom Erfolg überzeugt ist sollte man sich nicht dadurch aus der Ruhe bringen lassen es Bedarf einfach an Geduld und Zeit.
so lange man rein und raus "hüpfen" kann wie man lustig ist, ist eine Aktie egal was dahinter steckt ein Spielball der Finanzjongleure das ist die Realität.
Klar gibt es Leute wie du und ich die auch aus gewissen Überzeugungen heraus sich für eine Investition entscheiden, jedoch muss klar sein wir mit unserem kleinen finanziellen Handlungsspielraum sind wenn wir mitmachen dazu verdammt uns das anzusehen, wir können wenig tun außer selbst nicht zu verkaufen und so weder unsere Aktien preiswert Shorts in den Rachen zu werfen oder den Kurs noch weiter zu drücken.
Wenn man grundsätzlich aber vom Erfolg überzeugt ist sollte man sich nicht dadurch aus der Ruhe bringen lassen es Bedarf einfach an Geduld und Zeit.
Gezielte Shortattacke auf Amarin, mit BP in der Hinterhalt? um den Buy-Out Preis zu senken?, Korruption pur!
Weil eine Arschgeige bearisch ist und KZ US Dollar 7 ausgibt wird versucht alles Positive auszublenden?
Die Medien berichten darüber und verbreiten Angst und Panik, alles gut organisiert, traurig aber wahr, in welcher Welt leben wir?
30 % weniger Herzinfarkt, 30 % wengiger Schlaganfälle, 20 weniger Toesfälle....., 16:0 zugunsten Amarin und Vascepa beim Adcom......
Solch ein Analyst gehört eingesperrt und die Hintermänner mit!
Die Medien berichten darüber und verbreiten Angst und Panik, alles gut organisiert, traurig aber wahr, in welcher Welt leben wir?
30 % weniger Herzinfarkt, 30 % wengiger Schlaganfälle, 20 weniger Toesfälle....., 16:0 zugunsten Amarin und Vascepa beim Adcom......
Solch ein Analyst gehört eingesperrt und die Hintermänner mit!
Ich bleib dabei Korruption:
Street Fight: Oppenheimer breaks from Amarin bulls with Underperform rating
Market Mover
Laurie Pasternack Chan - email
Amarin's valuation is 'rich,' while its M&A thesis is 'stale,' Oppenheimer said
Shares of Amarin (AMRN) dropped in morning trading after Oppenheimer analyst Leland Gershell initiated the biopharmaceutical company with an Underperform rating and a price target of $7, citing a "stale" M&A thesis. The rating, a split from other bullish analysts, comes as Citi analyst Joel Beatty told investors that he prefers Amarin shares to those of The Medicines Co (MDCO) after this week's divergence in the two stocks.
AMARIN'S VALUATION 'RICH," M&A THESIS 'STALE': Oppenheimer analyst Leland Gershell initiated coverage of Amarin on Tuesday with an Underperform rating and $7 price target. Amarin's current valuation reflects expectations that, following near-term label expansion of sole omega-3 product Vascepa, the company's sales will inflect and grow to $2B-plus by 2024 and its operating margins will meaningfully improve, Gershell told investors in a research note. The analyst, however, forecasts Amarin's sales growth to "underwhelm" and that "heavy selling costs" will impede its profitability. Furthermore, a 12-month stream of late-stage competitor data starting next month will increasingly weigh on shares as these products will offer superior profiles, adds Gershell. He views Amarin's valuation as "rich" and the M&A thesis as "stale," adding that "while some may regard Amarin as a probable M&A target, we see the likelihood of this outcome as only shrinking with time."
Gershell says his bearish view is supported by sizable current off-label use that dampens growth potential following label expansion for Vascepa, as well as results from his firm's physician survey, indicating moderate patient adherence, hurdles to reimbursement, and a wide-ranging outlook on future prescribing. He also stated that he forecasts selling expense escalation for Vascepa above Street projections as Amarin struggles to meet revenue estimates in a category fraught with omega-3 generics, dietary supplements and foreseeable competition. "Competitive clouds are fast approaching," Gershell further said, stating that Epanova's N~13K STRENGTH trial threatens Vascepa's distinction among omega-3s of cardiovascular outcomes benefit.
As far as M&A goes, Gershell said he thinks the "ripest" time for Amarin to be acquired has already passed. From here, the analyst said he increasingly discounts this outcome as emerging competition layers onto a "murky" intellectual property position.
JEFFERIES, CANTOR MORE BULLISH: Following the data presented at the American Heart Association and last week's successful FDA panel vote, there are three-to-four potential drivers for Amarin shares over the next few months, Jefferies analyst Michael Yee said. He added that while some investors do not like the risk/reward debate on the December 28 potential FDA approval and label wording, he's not too concerned about the exact wording. Based on commentary by the FDA at the end of the panel, Yee thinks the agency "might be broader on the label than some investors expect." The analyst still expects Amarin to rise toward $30 per share based on these catalysts. Cantor Fitzgerald analyst Louise Chen said the most interesting takeaway from the conference call discussing its three presentations at the American Heart Association meeting was the physicians' viewpoint regarding the "outdated" paradigm of primary versus secondary risk patients. With a blurring of the line between these two cohorts, Chen thinks physicians will see a place for Vascepa in both patient types, regardless of label language. She continues to think the peak sales potential of Vascepa is underappreciated and that upward earnings revisions should drive Amarin shares higher.
CITI PREFERS AMARIN OVER THE MEDICINES CO: Amarin shares have declined 5% this week, while The Medicines Co. stock is up 35%, which has brought The Medicines Co.'s enterprise value to $7.5B, exceeding Amarin's enterprise value of $7.4B, Citi analyst Joel Beatty noted. The analyst said he does not see anything fundamental having changed that would support this "drastic of a change in the relative valuation of the two companies," and while keeping a Neutral rating on both stocks, he prefers owning Amarin over The Medicines Co. at this time. The Medicines Co. now looks expensive relative to Amarin, he contended, adding that that Bloomberg's report this week of Novartis (NVS) having expressed interest in The Medicines Co. does not provide new evidence that the company is attractive from a valuation standpoint to potential acquirers.
The analyst said he views the data presented at AHA over the weekend as "incrementally favorable" for Amarin.
PRICE ACTION: In morning trading, shares of Amarin are down about 8% to $20.91.
Symbols: AMRN MDCO NVS
Keywords: Street Fight, Vascepa, M&A, analyst, initiation, bull, bear
Street Fight: Oppenheimer breaks from Amarin bulls with Underperform rating
Market Mover
Laurie Pasternack Chan - email
Amarin's valuation is 'rich,' while its M&A thesis is 'stale,' Oppenheimer said
Shares of Amarin (AMRN) dropped in morning trading after Oppenheimer analyst Leland Gershell initiated the biopharmaceutical company with an Underperform rating and a price target of $7, citing a "stale" M&A thesis. The rating, a split from other bullish analysts, comes as Citi analyst Joel Beatty told investors that he prefers Amarin shares to those of The Medicines Co (MDCO) after this week's divergence in the two stocks.
AMARIN'S VALUATION 'RICH," M&A THESIS 'STALE': Oppenheimer analyst Leland Gershell initiated coverage of Amarin on Tuesday with an Underperform rating and $7 price target. Amarin's current valuation reflects expectations that, following near-term label expansion of sole omega-3 product Vascepa, the company's sales will inflect and grow to $2B-plus by 2024 and its operating margins will meaningfully improve, Gershell told investors in a research note. The analyst, however, forecasts Amarin's sales growth to "underwhelm" and that "heavy selling costs" will impede its profitability. Furthermore, a 12-month stream of late-stage competitor data starting next month will increasingly weigh on shares as these products will offer superior profiles, adds Gershell. He views Amarin's valuation as "rich" and the M&A thesis as "stale," adding that "while some may regard Amarin as a probable M&A target, we see the likelihood of this outcome as only shrinking with time."
Gershell says his bearish view is supported by sizable current off-label use that dampens growth potential following label expansion for Vascepa, as well as results from his firm's physician survey, indicating moderate patient adherence, hurdles to reimbursement, and a wide-ranging outlook on future prescribing. He also stated that he forecasts selling expense escalation for Vascepa above Street projections as Amarin struggles to meet revenue estimates in a category fraught with omega-3 generics, dietary supplements and foreseeable competition. "Competitive clouds are fast approaching," Gershell further said, stating that Epanova's N~13K STRENGTH trial threatens Vascepa's distinction among omega-3s of cardiovascular outcomes benefit.
As far as M&A goes, Gershell said he thinks the "ripest" time for Amarin to be acquired has already passed. From here, the analyst said he increasingly discounts this outcome as emerging competition layers onto a "murky" intellectual property position.
JEFFERIES, CANTOR MORE BULLISH: Following the data presented at the American Heart Association and last week's successful FDA panel vote, there are three-to-four potential drivers for Amarin shares over the next few months, Jefferies analyst Michael Yee said. He added that while some investors do not like the risk/reward debate on the December 28 potential FDA approval and label wording, he's not too concerned about the exact wording. Based on commentary by the FDA at the end of the panel, Yee thinks the agency "might be broader on the label than some investors expect." The analyst still expects Amarin to rise toward $30 per share based on these catalysts. Cantor Fitzgerald analyst Louise Chen said the most interesting takeaway from the conference call discussing its three presentations at the American Heart Association meeting was the physicians' viewpoint regarding the "outdated" paradigm of primary versus secondary risk patients. With a blurring of the line between these two cohorts, Chen thinks physicians will see a place for Vascepa in both patient types, regardless of label language. She continues to think the peak sales potential of Vascepa is underappreciated and that upward earnings revisions should drive Amarin shares higher.
CITI PREFERS AMARIN OVER THE MEDICINES CO: Amarin shares have declined 5% this week, while The Medicines Co. stock is up 35%, which has brought The Medicines Co.'s enterprise value to $7.5B, exceeding Amarin's enterprise value of $7.4B, Citi analyst Joel Beatty noted. The analyst said he does not see anything fundamental having changed that would support this "drastic of a change in the relative valuation of the two companies," and while keeping a Neutral rating on both stocks, he prefers owning Amarin over The Medicines Co. at this time. The Medicines Co. now looks expensive relative to Amarin, he contended, adding that that Bloomberg's report this week of Novartis (NVS) having expressed interest in The Medicines Co. does not provide new evidence that the company is attractive from a valuation standpoint to potential acquirers.
The analyst said he views the data presented at AHA over the weekend as "incrementally favorable" for Amarin.
PRICE ACTION: In morning trading, shares of Amarin are down about 8% to $20.91.
Symbols: AMRN MDCO NVS
Keywords: Street Fight, Vascepa, M&A, analyst, initiation, bull, bear
Antwort auf Beitrag Nr.: 61.968.956 von flyingbeef am 20.11.19 10:23:28
...das sehe ich genauso. Die Erweiterung des Labels wird "zwischen den Jahren" (28.12.2019) kommen - alles andere wäre eine Riesenüberraschung. Die Frage ist lediglich, wie "breit" diese Labelanpassung ausfallen wird.
Die MC mit derzeit 7Mrd$ ist schon recht üppig. Erst wenn die Labelerweiterung auf mehr als 1 Milliarde $ Jahresumsatz hindeutet, wird der Kurs wieder signifikant anziehen. Und das wird früher oder später der Fall sein. Den Verschwörungstheorien verschiedener Forumsteilnehmer kann ich hingegen nur wenig abgewinnen.
Zitat von flyingbeef: Tja,
Wenn man grundsätzlich aber vom Erfolg überzeugt ist sollte man sich nicht dadurch aus der Ruhe bringen lassen es Bedarf einfach an Geduld und Zeit.
...das sehe ich genauso. Die Erweiterung des Labels wird "zwischen den Jahren" (28.12.2019) kommen - alles andere wäre eine Riesenüberraschung. Die Frage ist lediglich, wie "breit" diese Labelanpassung ausfallen wird.
Die MC mit derzeit 7Mrd$ ist schon recht üppig. Erst wenn die Labelerweiterung auf mehr als 1 Milliarde $ Jahresumsatz hindeutet, wird der Kurs wieder signifikant anziehen. Und das wird früher oder später der Fall sein. Den Verschwörungstheorien verschiedener Forumsteilnehmer kann ich hingegen nur wenig abgewinnen.
"Den Verschwörungstheorien verschiedener Forumsteilnehmer kann ich hingegen nur wenig abgewinnen"
Was ist dann Deiner Meinung nach das Kursziel von Oppenheimer mit US Dollar 7?
Erst das Mineralöl-Thema, dann Adcom und wenn dann einem nichts mehr Negatives einfällt, Growth-Concerns zu vermelden......lachhaft und die Medien springen darauf an......., das stinkt zum Himmel!
Warum ist denn Amarin beim KZ US Dollar51 von Wainwright nicht explodiert, das sind die shorts die zusammen mit den Medien solche Szenarien kreieren!
Was ist dann Deiner Meinung nach das Kursziel von Oppenheimer mit US Dollar 7?
Erst das Mineralöl-Thema, dann Adcom und wenn dann einem nichts mehr Negatives einfällt, Growth-Concerns zu vermelden......lachhaft und die Medien springen darauf an......., das stinkt zum Himmel!
Warum ist denn Amarin beim KZ US Dollar51 von Wainwright nicht explodiert, das sind die shorts die zusammen mit den Medien solche Szenarien kreieren!
Egal, ich bin von Amarin und Vascepa voll überzeugt, sollen die Drecksshorts doch manipulieren wie sie wollen, am Ende wird eine Übernahme kommen, hoffentlich über Nacht und alle shorts zerstören!
Antwort auf Beitrag Nr.: 61.975.190 von Magnetfeldfredy am 20.11.19 19:15:12
...warum bist du denn so "angefressen"? Qualität hat sich noch immer an der Börse durchgesetzt, sofern die Liquidität gegeben ist. Und diese ist nach der Kapitalerhöhung vom 17.7.19 prächtig. Also keine Gefahr...und wenn sich der Trend fortsetzt, dann werden diese Schnäppchenkurse genutzt
und weitere Stücke ins Portfolio gelegt.
Jul 17, 2019
BEDMINSTER, N.J. and DUBLIN, Ireland, July 17, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (NASDAQ: AMRN) today announced that it has commenced an underwritten public offering of $400,000,000 of its American Depositary Shares pursuant to a shelf registration. All of the shares in the proposed offering are to be sold by Amarin.
Zitat von Magnetfeldfredy: Egal, ich bin von Amarin und Vascepa voll überzeugt, sollen die Drecksshorts doch manipulieren wie sie wollen, am Ende wird eine Übernahme kommen, hoffentlich über Nacht und alle shorts zerstören!
...warum bist du denn so "angefressen"? Qualität hat sich noch immer an der Börse durchgesetzt, sofern die Liquidität gegeben ist. Und diese ist nach der Kapitalerhöhung vom 17.7.19 prächtig. Also keine Gefahr...und wenn sich der Trend fortsetzt, dann werden diese Schnäppchenkurse genutzt

und weitere Stücke ins Portfolio gelegt.
Jul 17, 2019
BEDMINSTER, N.J. and DUBLIN, Ireland, July 17, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (NASDAQ: AMRN) today announced that it has commenced an underwritten public offering of $400,000,000 of its American Depositary Shares pursuant to a shelf registration. All of the shares in the proposed offering are to be sold by Amarin.
Hast Recht , ärgert mich halt immer wenn solche Dumpfbacken wie Oppenheimer mit einem absolut lächerlichen Kursziel und ohne Begründung solche negativen Reaktionen auslösen!
, ärgert mich halt immer wenn solche Dumpfbacken wie Oppenheimer mit einem absolut lächerlichen Kursziel und ohne Begründung solche negativen Reaktionen auslösen!
Ich bin zu emotional, das bringt Nichts!
 , ärgert mich halt immer wenn solche Dumpfbacken wie Oppenheimer mit einem absolut lächerlichen Kursziel und ohne Begründung solche negativen Reaktionen auslösen!
, ärgert mich halt immer wenn solche Dumpfbacken wie Oppenheimer mit einem absolut lächerlichen Kursziel und ohne Begründung solche negativen Reaktionen auslösen!Ich bin zu emotional, das bringt Nichts!

Die wollen nur die Schwachen rausschütteln, "weak hands" und billig unsere Aktien!💹💰
Immer die Ruhe Bewahren!!!!
" target="_blank" rel="nofollow ugc noopener">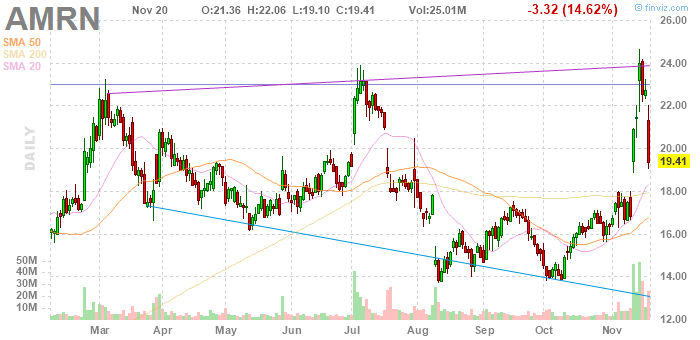
Wie Cyberhexe schon sagte:
Also keine Gefahr...und wenn sich der Trend fortsetzt, dann werden diese Schnäppchenkurse genutzt
und weitere Stücke ins Portfolio gelegt.
Die faulen Früchte fallen herunter,
ich bleibe im Titel.
Die fundamentalen Daten setzen sich durch.
Gruss RS 😎
" target="_blank" rel="nofollow ugc noopener">

Wie Cyberhexe schon sagte:
Also keine Gefahr...und wenn sich der Trend fortsetzt, dann werden diese Schnäppchenkurse genutzt

und weitere Stücke ins Portfolio gelegt.
Die faulen Früchte fallen herunter,
ich bleibe im Titel.
Die fundamentalen Daten setzen sich durch.
Gruss RS 😎
Rastelly, ich kenne auch einen Fusballrastelli:



Da fällt mir fast der Ball vom Nacken, bei unseren korrupten Freunden, Oppenheimer:
Amid Falling Profits, Advisors Bolt from Oppenheimer
By Andrew Welsch
February 04, 2016, 12:00 a.m. EST
Facebook
Twitter
LinkedIn
Email
Print
Show more sharing options
Profits are falling and the advisor force is shrinking at Oppenheimer & Co., a brokerage firm with a reputation for giving troubled advisors a second chance but which has fallen under intense regulatory scrutiny in recent years.
The New York firm said profits for its private client group plummeted 40% year-over-year, falling to $14 million for the quarter from $23 million for the same period a year ago. The firm blamed the slump on lower transaction-based revenue and higher legal and regulatory costs.
Meanwhile, advisors have been jumping ship from Oppenheimer; headcount at the brokerage firm fell to 1,233 for the fourth quarter, a decline of 91 from the year-ago period. Oppenheimer attributed the drop to the company's "ongoing review of financial advisor productivity, compliance, and client service," according to its earnings report.
White Paper Charting your own course: Discover five business models for the modern RIA
Advisors are choosing the independent RIA model now more than ever. Discover the 5 paths you can take to independence.
Sponsor content from
However, the firm's advisor force has actually been shrinking for some time. Five years ago, Oppenheimer employed about 1,500 advisors. The departures at Oppenheimer appear to be picking up steam.
Recruiters say some advisors have been leaving due to the company's outdated technology and its legal and compliance troubles. "Their recruiting deal stinks," says a recruiter who asked not to be named.
This recruiter says that the firm used to be a home for advisors looking for a second chance after having some kind of non-criminal issue at their past firm.
"The big advantage for them was that you had a lot of flexibility. It was for people who didn't fit the mold. Now, I don't know," the recruiter says.
Oppenheimer's run-ins with regulators, who have hit the firm with millions in fines, are also a contributing factor to advisor departures and weak recruiting.
"Oppenheimer's recent history of bad press, which followed bad hires, and mediocre recruiting deals makes it hard for them to attract A players. That makes the A players that they have nervous," says recruiter Danny Sarch.
LITIGATION EXPENSES
The company is overseen by a Wall Street veteran, Albert G. Lowenthal, who has served as chairman and CEO for Oppenheimer and its predecessor firm Fahnestock & Co., since 1985. He has been working in the securities industry since 1967, according to the company's website.
In 2003, Fahnestock acquired Oppenheimer private client and asset management units from CIBC World Markets, and then renamed itself.
In recent years, Oppenheimer has fallen under the scrutiny of regulators for allegedly lax compliance procedures and broker misconduct. In early 2015, FINRA hit Oppenheimer with $3.75 million in fines for supervisory failures related to an ex-broker who stole client money and excessively traded in client accounts. Oppenheimer neither admitted nor denied the charges, but consented to the entry of FINRA's findings. That fine was on top of $6 million Oppenheimer paid to resolve customer arbitration claims related to the ex-broker's actions.
Read more: Oppenheimer Fined for Penny Stocks Scheme and AML Violations
And a year ago, Oppenheimer paid $20 million to the SEC and the Treasury Department’s Financial Crimes Enforcement Network for improperly selling penny stocks in unregistered offerings on behalf of customers. The company agreed to admit to wrongdoing in that case – and narrowly avoided tougher penalties in a closed-door vote by SEC commissioners. Separately, the SEC also barred and fined Robert Okin, a former head of Oppenheimer's Private Client Division.
Two commissioners, Luis A. Aguilar and Kara M. Stein, dissented from their colleagues and sharply criticized Oppenheimer's operations.
Referring to recent regulatory violations by Oppenheimer, Commissioners Aguilar and Stein said in a dissenting letter dated a year ago that that these "are just the most recent chapter in a long and unfortunate history of regulatory failures, some more significant than others, but cumulatively indicative of a wholly failed compliance culture."
FUTURE CHALLENGES
Beyond its past regulatory problems, Oppenheimer still faces hurdles.
Client assets under administration for the company's private client group dropped 10% to $79 billion for the quarter, partially due to recent market turmoil.
Profits have also been on a downward trajectory in recent years. Companywide, annual profits dropped 77% year-over-year to $1.9 million from $8.8 million for last year. In 2013, the company reported $25 million in profits.
The company's stock was trading at just under $15 a share on Feb. 4, down from a high of $28 a share in June of last year. Ten years ago Oppenheimer stock was trading at about $21 a share.
Following his company's most recent earnings report, CEO Lowenthal said in a statement that Oppenheimer's technology investments would give it a more competitive platform to meet client and regulatory needs.
"The company and its management recognize the importance of effective supervision, appropriate systems and controls, and an ethical culture to ensure success in today's competitive environment," Lowenthal said.
Through a spokeswoman, the company did not respond to requests for comment.
Read more:
What to Expect Next on Fiduciary Rule
FINRA's New Target: Broker Culture
SEC to Intensify Probes Into Retirement Planning, Cybersecurity
Andrew Welsch
Senior Editor, On Wall Street
linkedin
Facebook
Twitter
LinkedIn
Email
Print
Show more sharing options
Reprint
For reprint and licensing requests for this article, click here.
RecruitingCareer movesOppenheimer
Amid Falling Profits, Advisors Bolt from Oppenheimer
By Andrew Welsch
February 04, 2016, 12:00 a.m. EST
Show more sharing options
Profits are falling and the advisor force is shrinking at Oppenheimer & Co., a brokerage firm with a reputation for giving troubled advisors a second chance but which has fallen under intense regulatory scrutiny in recent years.
The New York firm said profits for its private client group plummeted 40% year-over-year, falling to $14 million for the quarter from $23 million for the same period a year ago. The firm blamed the slump on lower transaction-based revenue and higher legal and regulatory costs.
Meanwhile, advisors have been jumping ship from Oppenheimer; headcount at the brokerage firm fell to 1,233 for the fourth quarter, a decline of 91 from the year-ago period. Oppenheimer attributed the drop to the company's "ongoing review of financial advisor productivity, compliance, and client service," according to its earnings report.
White Paper Charting your own course: Discover five business models for the modern RIA
Advisors are choosing the independent RIA model now more than ever. Discover the 5 paths you can take to independence.
Sponsor content from
However, the firm's advisor force has actually been shrinking for some time. Five years ago, Oppenheimer employed about 1,500 advisors. The departures at Oppenheimer appear to be picking up steam.
Recruiters say some advisors have been leaving due to the company's outdated technology and its legal and compliance troubles. "Their recruiting deal stinks," says a recruiter who asked not to be named.
This recruiter says that the firm used to be a home for advisors looking for a second chance after having some kind of non-criminal issue at their past firm.
"The big advantage for them was that you had a lot of flexibility. It was for people who didn't fit the mold. Now, I don't know," the recruiter says.
Oppenheimer's run-ins with regulators, who have hit the firm with millions in fines, are also a contributing factor to advisor departures and weak recruiting.
"Oppenheimer's recent history of bad press, which followed bad hires, and mediocre recruiting deals makes it hard for them to attract A players. That makes the A players that they have nervous," says recruiter Danny Sarch.
LITIGATION EXPENSES
The company is overseen by a Wall Street veteran, Albert G. Lowenthal, who has served as chairman and CEO for Oppenheimer and its predecessor firm Fahnestock & Co., since 1985. He has been working in the securities industry since 1967, according to the company's website.
In 2003, Fahnestock acquired Oppenheimer private client and asset management units from CIBC World Markets, and then renamed itself.
In recent years, Oppenheimer has fallen under the scrutiny of regulators for allegedly lax compliance procedures and broker misconduct. In early 2015, FINRA hit Oppenheimer with $3.75 million in fines for supervisory failures related to an ex-broker who stole client money and excessively traded in client accounts. Oppenheimer neither admitted nor denied the charges, but consented to the entry of FINRA's findings. That fine was on top of $6 million Oppenheimer paid to resolve customer arbitration claims related to the ex-broker's actions.
Read more: Oppenheimer Fined for Penny Stocks Scheme and AML Violations
And a year ago, Oppenheimer paid $20 million to the SEC and the Treasury Department’s Financial Crimes Enforcement Network for improperly selling penny stocks in unregistered offerings on behalf of customers. The company agreed to admit to wrongdoing in that case – and narrowly avoided tougher penalties in a closed-door vote by SEC commissioners. Separately, the SEC also barred and fined Robert Okin, a former head of Oppenheimer's Private Client Division.
Two commissioners, Luis A. Aguilar and Kara M. Stein, dissented from their colleagues and sharply criticized Oppenheimer's operations.
Referring to recent regulatory violations by Oppenheimer, Commissioners Aguilar and Stein said in a dissenting letter dated a year ago that that these "are just the most recent chapter in a long and unfortunate history of regulatory failures, some more significant than others, but cumulatively indicative of a wholly failed compliance culture."
FUTURE CHALLENGES
Beyond its past regulatory problems, Oppenheimer still faces hurdles.
Client assets under administration for the company's private client group dropped 10% to $79 billion for the quarter, partially due to recent market turmoil.
Profits have also been on a downward trajectory in recent years. Companywide, annual profits dropped 77% year-over-year to $1.9 million from $8.8 million for last year. In 2013, the company reported $25 million in profits.
The company's stock was trading at just under $15 a share on Feb. 4, down from a high of $28 a share in June of last year. Ten years ago Oppenheimer stock was trading at about $21 a share.
Following his company's most recent earnings report, CEO Lowenthal said in a statement that Oppenheimer's technology investments would give it a more competitive platform to meet client and regulatory needs.
"The company and its management recognize the importance of effective supervision, appropriate systems and controls, and an ethical culture to ensure success in today's competitive environment," Lowenthal said.
Through a spokeswoman, the company did not respond to requests for comment.
Read more:
What to Expect Next on Fiduciary Rule
FINRA's New Target: Broker Culture
SEC to Intensify Probes Into Retirement Planning, Cybersecurity
Andrew Welsch
Senior Editor, On Wall Street
Show more sharing options
Reprint
For reprint and licensing requests for this article, click here.
RecruitingCareer movesOppenheimer
And a year ago, Oppenheimer paid $20 million to the SEC and the Treasury Department’s Financial Crimes Enforcement Network for improperly selling penny stocks in unregistered offerings on behalf of customers. The company agreed to admit to wrongdoing in that case – and narrowly avoided tougher penalties in a closed-door vote by SEC commissioners. Separately, the SEC also barred and fined Robert Okin, a former head of Oppenheimer's Private Client Division.
Amarin - The Science Of Lipid Therapy | wallstreet-online.de - Vollständige Diskussion unter:
https://www.wallstreet-online.de/diskussion/1190027-721-730/…
Amarin - The Science Of Lipid Therapy | wallstreet-online.de - Vollständige Diskussion unter:
https://www.wallstreet-online.de/diskussion/1190027-721-730/…
Antwort auf Beitrag Nr.: 61.977.344 von Magnetfeldfredy am 20.11.19 23:17:07Ja, auch diesen Spitznamen rührt bei mir aus dem Fussball.
Allerdings schon ein paar Tage her und jetzt in Natur "eisgrau" .
Habe diesen damals einfach als User übernommen.
Gruss RS😎
Allerdings schon ein paar Tage her und jetzt in Natur "eisgrau" .
Habe diesen damals einfach als User übernommen.
Gruss RS😎
John Thero heute in London, geil, was er zum Fraud Analysten von Oppenheimer sagt:
Oasis2
14m
$AMRN the CEO has just slammed the Oppenheimer analyst saying that it’s basically untrue what he said and that he had never spoke to this analyst. JT basically says it’s rubbish about what the analyst says about the competition. He praises the more thoughtful analysts out there like Michael Yee at Jefferies.
WOW!!
Oasis2
14m
$AMRN the CEO has just slammed the Oppenheimer analyst saying that it’s basically untrue what he said and that he had never spoke to this analyst. JT basically says it’s rubbish about what the analyst says about the competition. He praises the more thoughtful analysts out there like Michael Yee at Jefferies.
WOW!!
Antwort auf Beitrag Nr.: 61.979.954 von Magnetfeldfredy am 21.11.19 10:37:18Der , der mit J.T. nie gesprochen hat.


Hier ein Leland Buy - Rating von 12 USD -
aktueller Kurs MGEM = 0,6285

aktueller Kurs MGEM = 0,6285

Antwort auf Beitrag Nr.: 61.977.344 von Magnetfeldfredy am 20.11.19 23:17:07




















Zitat von Magnetfeldfredy: Rastelly, ich kenne auch einen Fusballrastelli:




















Is Amarin Really a $7 Stock?
Amarin's shares got walloped yesterday by a bearish analyst report.
George Budwell
George Budwell
(TMFGBudwell)
Nov 21, 2019 at 9:25AM
Author Bio
Yesterday, Amarin (NASDAQ:AMRN) lost over 10% of its value in response to a rather harsh report by Oppenheimer analyst Leland Gershell. The key issue that triggered this double-digit sell-off appears to be Gershell's uberbearish prediction that Amarin's stock could drop by as much as 70% to a lowly $7 per share over the next year.
The analyst's reasoning is based on two underlying assumptions:
Vascepa's sales will fall well short of expectations following its label expansion for patients at risk of cardiovascular disease. Amarin's stock has recently been trading at a price-to-sales ratio north of 20, so it's definitely fair to say that the market expects Vascepa's sales to grow by leaps and bounds soon. The current consensus estimate stands at $2.2 billion, according to EvaluatePharma. However, a broad label could easily double -- or perhaps quadruple -- this figure, given the sheer size of the market.
Amarin's prospects as a buyout candidate will quickly fade due to the emergence of competition in the space from AstraZeneca's Epanova, Acasti Pharma's CaPre, Matinas BioPharma's MAT9001, among others.
Here's why investors shouldn't get overly worked up by this bearish analyst report.
Woman staring at an open laptop computer with a look of shock on her face.
Image source: Getty Images.
Amarin: King of the omega-3 hill
While Gershell's take is certainly audacious, it simply doesn't line up with the facts on the ground. First off, Vascepa is the only omega-3 treatment in history to show a statistically significant cardiovascular benefit in a large, placebo-controlled trial. So while it's easy to point to the emergence of "superior competition" as a potential risk factor, the reality of the situation is that most -- if not all -- of these rival candidates could very well flop in terms of conveying a cardiovascular benefit. In fact, it would be rather surprising if any of these putative competitors emerged as a direct rival to Vascepa during the drug's prime years.
Keeping with this theme, the candidate with the best chance of knocking off Vascepa -- Acasti's CaPre -- probably wouldn't get through a full-on cardiovascular trial until Vascepa was in its twilight years from a patent protection standpoint. That's important because doctors aren't going to readily prescribe a medicine off-label for a wide swath of Americans just because it seems to stack up well against the standard of care. Eyeball tests don't fly when it comes to patient care. CaPre will have to complete a time-consuming cardiovascular outcomes trial before it will ever be prescribed in lieu of Vascepa, and that won't happen until almost the end of the next decade.
The take-home point is that Vascepa should easily grab the lion's share of the omega-3 treatment space for the whole of the next decade, thanks to its highly anticipated label expansion as an add-on to statin therapy in patients with elevated triglyceride levels.
Is Amarin still a strong buyout candidate?
On the buyout front, Amarin's prospects haven't changed whatsoever since Vascepa's positive adcom vote. Long story short, there is no logical reason to believe that Astra's Epanova is going to perform any better than its mixed fish oil predecessors, much less upstage Vascepa's unprecedented Reduce-It results. Moreover, the biggest competitive threats to Vascepa are upwards of a decade from truly materializing.
So, given the fact that there isn't a single product with a proven cardiovascular benefit currently being marketed by a small biopharma, it's more than reasonable to assume that Amarin does indeed have a huge target on its back right now. In other words, Amarin's shares have a much better chance of rocketing to $70 than crashing to $7 over the next 12 months.
Amarin's shares got walloped yesterday by a bearish analyst report.
George Budwell
George Budwell
(TMFGBudwell)
Nov 21, 2019 at 9:25AM
Author Bio
Yesterday, Amarin (NASDAQ:AMRN) lost over 10% of its value in response to a rather harsh report by Oppenheimer analyst Leland Gershell. The key issue that triggered this double-digit sell-off appears to be Gershell's uberbearish prediction that Amarin's stock could drop by as much as 70% to a lowly $7 per share over the next year.
The analyst's reasoning is based on two underlying assumptions:
Vascepa's sales will fall well short of expectations following its label expansion for patients at risk of cardiovascular disease. Amarin's stock has recently been trading at a price-to-sales ratio north of 20, so it's definitely fair to say that the market expects Vascepa's sales to grow by leaps and bounds soon. The current consensus estimate stands at $2.2 billion, according to EvaluatePharma. However, a broad label could easily double -- or perhaps quadruple -- this figure, given the sheer size of the market.
Amarin's prospects as a buyout candidate will quickly fade due to the emergence of competition in the space from AstraZeneca's Epanova, Acasti Pharma's CaPre, Matinas BioPharma's MAT9001, among others.
Here's why investors shouldn't get overly worked up by this bearish analyst report.
Woman staring at an open laptop computer with a look of shock on her face.
Image source: Getty Images.
Amarin: King of the omega-3 hill
While Gershell's take is certainly audacious, it simply doesn't line up with the facts on the ground. First off, Vascepa is the only omega-3 treatment in history to show a statistically significant cardiovascular benefit in a large, placebo-controlled trial. So while it's easy to point to the emergence of "superior competition" as a potential risk factor, the reality of the situation is that most -- if not all -- of these rival candidates could very well flop in terms of conveying a cardiovascular benefit. In fact, it would be rather surprising if any of these putative competitors emerged as a direct rival to Vascepa during the drug's prime years.
Keeping with this theme, the candidate with the best chance of knocking off Vascepa -- Acasti's CaPre -- probably wouldn't get through a full-on cardiovascular trial until Vascepa was in its twilight years from a patent protection standpoint. That's important because doctors aren't going to readily prescribe a medicine off-label for a wide swath of Americans just because it seems to stack up well against the standard of care. Eyeball tests don't fly when it comes to patient care. CaPre will have to complete a time-consuming cardiovascular outcomes trial before it will ever be prescribed in lieu of Vascepa, and that won't happen until almost the end of the next decade.
The take-home point is that Vascepa should easily grab the lion's share of the omega-3 treatment space for the whole of the next decade, thanks to its highly anticipated label expansion as an add-on to statin therapy in patients with elevated triglyceride levels.
Is Amarin still a strong buyout candidate?
On the buyout front, Amarin's prospects haven't changed whatsoever since Vascepa's positive adcom vote. Long story short, there is no logical reason to believe that Astra's Epanova is going to perform any better than its mixed fish oil predecessors, much less upstage Vascepa's unprecedented Reduce-It results. Moreover, the biggest competitive threats to Vascepa are upwards of a decade from truly materializing.
So, given the fact that there isn't a single product with a proven cardiovascular benefit currently being marketed by a small biopharma, it's more than reasonable to assume that Amarin does indeed have a huge target on its back right now. In other words, Amarin's shares have a much better chance of rocketing to $70 than crashing to $7 over the next 12 months.

Antwort auf Beitrag Nr.: 61.987.463 von Magnetfeldfredy am 22.11.19 06:42:08Hi Magnetfeldredy,
siehste und das meine ich mit "nicht aus der Ruhe bringen lassen" .
Wenn man selbst überzeugt ist von dem Wert und seine eigenen Recherchen gemacht hat, bestätigt diese Sichtweise nur das was man selbst denkt.
Aber ich kann es nachvollziehen, bei dem Auf und Ab hier mit Amarin bisher und so vielen Gerüchten ... usw. kann man schonmal echt die Nerven verlieren.
Ich sehe aber sehr zuversichtlich in die nahe Zukunft "nächsten" Wochen und wenn dann klar ist wie weit die Label Expansion ist kann man sicher auch weiterhin hier ein sehr erfolgreicher long "bleiben" wenn man noch Sitzfleisch hat und nicht an ersten Profit ran muss/möchte.
siehste und das meine ich mit "nicht aus der Ruhe bringen lassen" .
Wenn man selbst überzeugt ist von dem Wert und seine eigenen Recherchen gemacht hat, bestätigt diese Sichtweise nur das was man selbst denkt.
Aber ich kann es nachvollziehen, bei dem Auf und Ab hier mit Amarin bisher und so vielen Gerüchten ... usw. kann man schonmal echt die Nerven verlieren.
Ich sehe aber sehr zuversichtlich in die nahe Zukunft "nächsten" Wochen und wenn dann klar ist wie weit die Label Expansion ist kann man sicher auch weiterhin hier ein sehr erfolgreicher long "bleiben" wenn man noch Sitzfleisch hat und nicht an ersten Profit ran muss/möchte.
Reduktion des Plaques hat Vascepa in der Evaporate Studie in 5 von 6 verschiedenen Parametern gezeigt, Vascepa wird auch bei Alzheimer in einer Studie erprobt, wenn dort die Gehirnarterien von Plaque befreit werden bzw. sich die Progression statistisch signifikant verringert haben wir eine 1000 Dollar Aktie:
Ich meine diese Studie
https://clinicaltrials.gov/ct2/show/NCT02719327
Wenn ich die Interimsergebnisse anschaue, hoffe ich auf einen ähnlichen Effekt in den Gehirnarterien:
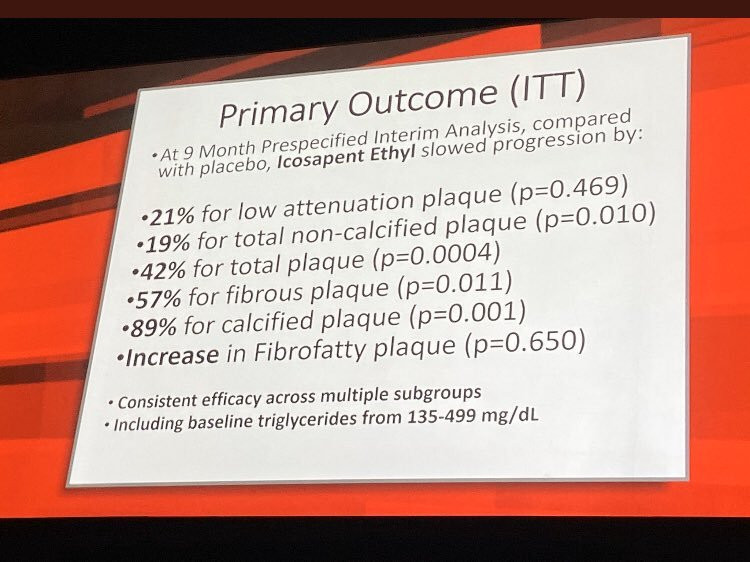
https://clinicaltrials.gov/ct2/show/NCT02719327
Wenn ich die Interimsergebnisse anschaue, hoffe ich auf einen ähnlichen Effekt in den Gehirnarterien:

Does Amarin Have a Dark Horse Suitor?
Gilead's decision to add Vascepa to an ongoing NASH study failed to grab much attention last week. But this development is quite intriguing for several reasons.
George Budwell
George Budwell
(TMFGBudwell)
Nov 24, 2019 at 6:38PM
Author Bio
Amarin (NASDAQ:AMRN), a mid-cap pharma company, has long been rumored to be a buyout candidate. The core reason is that the company's prescription omega-3 treatment, Vascepa (icosapent ethyl), hit the mark in a large, placebo-controlled cardiovascular outcomes trial -- a feat no other omega-3 therapy has ever accomplished. In fact, GlaxoSmithKline's competing omega-3 treatment, Lovaza, failed to show a similar cardiovascular benefit in its Ascend trial.
This novel, orally administered omega-3 pill could thus end up as a key component in the standard of care for patients at risk of cardiovascular disease, despite being on statin therapy. The big deal is that this target market is believed to encompass almost 10 million Americans at present. Even so, this already large patient population could grow significantly over the next decade because of the out-of-control obesity epidemic. So, conservatively speaking, Vascepa should rack up at least $2 billion in annual sales, depending on the scope of the drug's as-of-yet to be determined label for this indication.
Two men shaking hands over a table.
Image Source: Getty Images.
Buyout rumors aplenty
Amarin has repeatedly been linked to three pharma heavyweights as a possible takeover target. These not-so-secret names are Amgen, Pfizer, and Novartis (NYSE:NVS), and they have bubbled to the top of the M&A rumor mill because each company has an abiding interest in cardiovascular care, as well as the financial flexibility to pay top dollar for Amarin's hand.
This weekend, however, news broke that Novartis has decided to acquire The Medicines Company mainly for its experimental lipid-lowering treatment inclisiran. This $7 billion deal, in turn, probably spells the end of Novartis' long-rumored interest in Amarin.
Nonetheless, a new challenger may have quietly emerged from the shadows. Approximately two weeks ago, Gilead Sciences (NASDAQ:GILD) added Vascepa to an ongoing phase 2 study for patients with nonalcoholic steatohepatitis (NASH). The drug will be assessed as part of a triplet therapy that includes the biotech's non-steroidal farnesoid X receptor agonist cilofexor and the acetyl-CoA carboxylase inhibitor firsocostat. Top-line data from this ongoing trial is due out next May, according to clinicaltrials.gov.
Why would Gilead buy Amarin?
The lowdown is that Gilead is attempting to develop a top shelf combination treatment for NASH. By doing so, the biotech would be able to leap frog the first-generation of monotherapies set to hit the market as soon as 2020. And it might even be able to establish a competitive moat against the army of big pharmas and big biotechs racing to bring their own combo NASH treatments to market. Vascepa could be Gilead's ticket to unlocking the wide-open $65 billion NASH market.
Why might Vascepa be the missing ingredient in Gilead's NASH quest? Back in April, Gilead unleashed some promising data for the doublet therapy of cilofexor and firsocostat in NASH patients at the International Liver Congress. The drawback was that some of the patients evaluated in the trial exhibited a severe spike in triglyceride levels -- a known side-effect of firsocostat. Presumably, Gilead decided to add a Vascepa arm to explore the drug's ability to combat this potentially serious side effect.
What's more, Vascepa might also provide an all-important cardioprotective benefit in this at-risk patient population. And this second clinical benefit could be a game-changer for the therapy from a commercial standpoint. There is a well-established link between NASH and cardiovascular disease, after all.
What's the key takeaway? While this clinical trial news might turn out to be a big, fat nothingburger, it may also be the first sign that Gilead is considering an Amarin partnership, or perhaps a full-on buyout. The fact is that Gilead is one of the few biopharmas that could buy Amarin in cash without batting an eye. Moreover, Vascepa's potential dual purpose as an add-on to statin-therapy in patients with cardiovascular disease, and as part of a top-selling NASH cocktail, easily justifies the premium a buyout would entail.
Gilead's decision to add Vascepa to an ongoing NASH study failed to grab much attention last week. But this development is quite intriguing for several reasons.
George Budwell
George Budwell
(TMFGBudwell)
Nov 24, 2019 at 6:38PM
Author Bio
Amarin (NASDAQ:AMRN), a mid-cap pharma company, has long been rumored to be a buyout candidate. The core reason is that the company's prescription omega-3 treatment, Vascepa (icosapent ethyl), hit the mark in a large, placebo-controlled cardiovascular outcomes trial -- a feat no other omega-3 therapy has ever accomplished. In fact, GlaxoSmithKline's competing omega-3 treatment, Lovaza, failed to show a similar cardiovascular benefit in its Ascend trial.
This novel, orally administered omega-3 pill could thus end up as a key component in the standard of care for patients at risk of cardiovascular disease, despite being on statin therapy. The big deal is that this target market is believed to encompass almost 10 million Americans at present. Even so, this already large patient population could grow significantly over the next decade because of the out-of-control obesity epidemic. So, conservatively speaking, Vascepa should rack up at least $2 billion in annual sales, depending on the scope of the drug's as-of-yet to be determined label for this indication.
Two men shaking hands over a table.
Image Source: Getty Images.
Buyout rumors aplenty
Amarin has repeatedly been linked to three pharma heavyweights as a possible takeover target. These not-so-secret names are Amgen, Pfizer, and Novartis (NYSE:NVS), and they have bubbled to the top of the M&A rumor mill because each company has an abiding interest in cardiovascular care, as well as the financial flexibility to pay top dollar for Amarin's hand.
This weekend, however, news broke that Novartis has decided to acquire The Medicines Company mainly for its experimental lipid-lowering treatment inclisiran. This $7 billion deal, in turn, probably spells the end of Novartis' long-rumored interest in Amarin.
Nonetheless, a new challenger may have quietly emerged from the shadows. Approximately two weeks ago, Gilead Sciences (NASDAQ:GILD) added Vascepa to an ongoing phase 2 study for patients with nonalcoholic steatohepatitis (NASH). The drug will be assessed as part of a triplet therapy that includes the biotech's non-steroidal farnesoid X receptor agonist cilofexor and the acetyl-CoA carboxylase inhibitor firsocostat. Top-line data from this ongoing trial is due out next May, according to clinicaltrials.gov.
Why would Gilead buy Amarin?
The lowdown is that Gilead is attempting to develop a top shelf combination treatment for NASH. By doing so, the biotech would be able to leap frog the first-generation of monotherapies set to hit the market as soon as 2020. And it might even be able to establish a competitive moat against the army of big pharmas and big biotechs racing to bring their own combo NASH treatments to market. Vascepa could be Gilead's ticket to unlocking the wide-open $65 billion NASH market.
Why might Vascepa be the missing ingredient in Gilead's NASH quest? Back in April, Gilead unleashed some promising data for the doublet therapy of cilofexor and firsocostat in NASH patients at the International Liver Congress. The drawback was that some of the patients evaluated in the trial exhibited a severe spike in triglyceride levels -- a known side-effect of firsocostat. Presumably, Gilead decided to add a Vascepa arm to explore the drug's ability to combat this potentially serious side effect.
What's more, Vascepa might also provide an all-important cardioprotective benefit in this at-risk patient population. And this second clinical benefit could be a game-changer for the therapy from a commercial standpoint. There is a well-established link between NASH and cardiovascular disease, after all.
What's the key takeaway? While this clinical trial news might turn out to be a big, fat nothingburger, it may also be the first sign that Gilead is considering an Amarin partnership, or perhaps a full-on buyout. The fact is that Gilead is one of the few biopharmas that could buy Amarin in cash without batting an eye. Moreover, Vascepa's potential dual purpose as an add-on to statin-therapy in patients with cardiovascular disease, and as part of a top-selling NASH cocktail, easily justifies the premium a buyout would entail.
Antwort auf Beitrag Nr.: 61.991.558 von flyingbeef am 22.11.19 14:27:59

Grüße bernie55

Zitat von flyingbeef: Hi Magnetfeldredy,
...."nicht aus der Ruhe bringen lassen" .
....wenn man selbst überzeugt ist von dem Wert.........
......ich sehe aber sehr zuversichtlich in die nahe Zukunft "nächsten" Wochen..........
....wenn man noch Sitzfleisch hat und nicht an ersten Profit ran muss/möchte.

Grüße bernie55


Pharma
Amarin for $20B? Novartis' MedCo deal drives fresh buyout rumors for Vascepa's maker
by Kyle Blankenship | Nov 25, 2019 8:39am
Close-up of two people shaking hands with other people in the background
Could Amarin be next to get snapped up now that MedCo has agreed to sell itself to Novartis? Some investors seem to think so. (Pixabay)
Teilen
Facebook
Twitter
LinkedIn
Email
Print
Driven by a landmark cardiovascular outcomes trial that could spell blockbuster sales for its fish-oil derivative, Vascepa, Amarin has been a darling for pharma M&A speculators. Now, after Novartis' $9.7 billion agreement to pick up The Medicines Company, the rumor mill is back up and running.
Amarin, currently valued at around $21 per share, could be the target of a big acquisition with an FDA decision on a heart-helping label expansion for Vascepa coming late next month and prescriptions for the lipid-lowering drug on the rise.
Those buyout rumors are nothing new, but could the MedCo deal for PCSK9 candidate inclisiran last week spell a buying boom in the cardiology space? Some bullish investors seem to think so––and the most

Amarin for $20B? Novartis' MedCo deal drives fresh buyout rumors for Vascepa's maker
by Kyle Blankenship | Nov 25, 2019 8:39am
Close-up of two people shaking hands with other people in the background
Could Amarin be next to get snapped up now that MedCo has agreed to sell itself to Novartis? Some investors seem to think so. (Pixabay)
Teilen
Driven by a landmark cardiovascular outcomes trial that could spell blockbuster sales for its fish-oil derivative, Vascepa, Amarin has been a darling for pharma M&A speculators. Now, after Novartis' $9.7 billion agreement to pick up The Medicines Company, the rumor mill is back up and running.
Amarin, currently valued at around $21 per share, could be the target of a big acquisition with an FDA decision on a heart-helping label expansion for Vascepa coming late next month and prescriptions for the lipid-lowering drug on the rise.
Those buyout rumors are nothing new, but could the MedCo deal for PCSK9 candidate inclisiran last week spell a buying boom in the cardiology space? Some bullish investors seem to think so––and the most


Amarin Corporation plc's (NASDAQ:AMRN) Shift From Loss To Profit
,Simply Wall St.• December 2, 2019

https://finance.yahoo.com/news/amarin-corporation-plcs-nasda…
,Simply Wall St.• December 2, 2019

https://finance.yahoo.com/news/amarin-corporation-plcs-nasda…
INVESTOR RELATIONS / EUROPÄISCHE ARZNEIMITTELAGENTUR GENEHMIGT ZULASSUNGSANTRAG FÜR AMARINS ICOSAPENT ETHYL (VASCEPA®) ZUR REDUZIERUNG DES KARDIOVASKULÄREN RISIKOS BEI HOCHRISIKOPATIENTEN (SIEHE REDUCE-IT®-STUDIE)
EUROPEAN MEDICINES AGENCY ACCEPTS FOR REVIEW MARKETING AUTHORIZATION APPLICATION FOR AMARIN’S ICOSAPENT ETHYL (VASCEPA®) FOR REDUCTION OF CARDIOVASCULAR RISK IN HIGH-RISK PATIENTS, AS REFLECTED IN REDUCE-IT® STUDY
Dec 2, 2019
DUBLIN, Ireland and BRIDGEWATER, N.J., Dec. 02, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (NASDAQ: AMRN), a pharmaceutical company focused on improving cardiovascular health, announced today that the European Medicines Agency (EMA) has validated the marketing authorization application (MAA) seeking approval for icosapent ethyl (brand name Vascepa® in the United States) as a treatment to reduce the risk of cardiovascular events in high-risk patients who have their cholesterol levels controlled with statin treatment, but have elevated triglycerides,135 mg/dL or above, and other cardiovascular risk factors. The validation confirms the submission for Vascepa is sufficiently complete for the EMA to begin its review procedure, which is currently expected to be completed before the end of 2020.
“The prevalence in Europe of people with persistent cardiovascular risk beyond standard of care statin therapy is high, like it is in most of the world. This application moves us one step closer to being able to potentially help millions of high-risk patients with icosapent ethyl as studied in REDUCE-IT®,” says John Thero, president and CEO, Amarin. “We seek to make icosapent ethyl accessible to as many patients as possible who can benefit. If marketing authorization is granted by the EMA, icosapent ethyl could become the first and only EMA-approved, non-LDL lowering agent with a cardiovascular disease risk reduction indication as an adjunct to statin therapy in dyslipidemic patients in Europe.”
A recent survey showed that about 25 percent of a representative sample survey of more than 7,800 patients from 27 European countries with coronary heart disease and controlled LDL-cholesterol levels had elevated triglycerides levels (>150 mg/dL), illustrating the potential pervasiveness of high-risk cardiovascular disease in Europe beyond currently available therapies.1
Icosapent ethyl is in the late stages of review in the United States by the U.S. Food and Drug Administration (FDA) for an indication like the one being sought through a centralized review process in the Europe Union. In November 2019, an FDA advisory committee voted unanimously (16 – 0) to recommend to the FDA that icosapent ethyl be approved for an indication and label expansion to reduce the risk of cardiovascular events in high-risk patients. Although the FDA is not bound by the recommendation of its advisory committee, approval of icosapent ethyl in the United States consistent with such recommendation is anticipated on or before the FDA’s target Prescription Drug User Fee Act (PDUFA) date of December 28, 2019. In the United States, icosapent ethyl has the brand name Vascepa. If approved in Europe, the brand name may also be Vascepa, but this will be determined as part of the regulatory review process.
The MAA for icosapent ethyl is based on the global landmark clinical outcomes study, REDUCE-IT®.2 In the high-risk, statin-treated patient population studied in REDUCE-IT, as previously published, icosapent ethyl provided a highly statistically significant 25% relative risk reduction compared to placebo in the first occurrence of a major adverse CV event (MACE) in the intent-to-treat population consisting of a composite of cardiovascular death, nonfatal myocardial infarction (MI or heart attack), nonfatal stroke, coronary revascularization (procedures such as stents and by-pass) and unstable angina requiring hospitalization. For total (first and subsequent) cardiovascular events, icosapent ethyl showed a statistically significant 30% relative risk reduction compared to placebo in published exploratory analyses.3
In REDUCE-IT, adverse events occurring with icosapent ethyl use at greater than 5% and greater than placebo were: peripheral edema (6.5% Vascepa versus 5.0%); constipation (5.3% Vascepa versus 3.6%); and atrial fibrillation (5.3% Vascepa versus 3.9%). More information on safety data associated with REDUCE-IT is provided below.
Icosapent ethyl, which has been studied in a series of clinical trials, including REDUCE-IT, was developed by Amarin and is exclusively marketed by Amarin and its commercial partners in capsule form.
In the United States, Vascepa is currently approved as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. There have been more than 8 million prescriptions for Vascepa since it was launched in 2013 in the United States for this important niche indication. The drug is not currently available in Europe for any indication.
https://investor.amarincorp.com/news-releases/news-release-d…
Dec 2, 2019
DUBLIN, Ireland and BRIDGEWATER, N.J., Dec. 02, 2019 (GLOBE NEWSWIRE) -- Amarin Corporation plc (NASDAQ: AMRN), a pharmaceutical company focused on improving cardiovascular health, announced today that the European Medicines Agency (EMA) has validated the marketing authorization application (MAA) seeking approval for icosapent ethyl (brand name Vascepa® in the United States) as a treatment to reduce the risk of cardiovascular events in high-risk patients who have their cholesterol levels controlled with statin treatment, but have elevated triglycerides,135 mg/dL or above, and other cardiovascular risk factors. The validation confirms the submission for Vascepa is sufficiently complete for the EMA to begin its review procedure, which is currently expected to be completed before the end of 2020.
“The prevalence in Europe of people with persistent cardiovascular risk beyond standard of care statin therapy is high, like it is in most of the world. This application moves us one step closer to being able to potentially help millions of high-risk patients with icosapent ethyl as studied in REDUCE-IT®,” says John Thero, president and CEO, Amarin. “We seek to make icosapent ethyl accessible to as many patients as possible who can benefit. If marketing authorization is granted by the EMA, icosapent ethyl could become the first and only EMA-approved, non-LDL lowering agent with a cardiovascular disease risk reduction indication as an adjunct to statin therapy in dyslipidemic patients in Europe.”
A recent survey showed that about 25 percent of a representative sample survey of more than 7,800 patients from 27 European countries with coronary heart disease and controlled LDL-cholesterol levels had elevated triglycerides levels (>150 mg/dL), illustrating the potential pervasiveness of high-risk cardiovascular disease in Europe beyond currently available therapies.1
Icosapent ethyl is in the late stages of review in the United States by the U.S. Food and Drug Administration (FDA) for an indication like the one being sought through a centralized review process in the Europe Union. In November 2019, an FDA advisory committee voted unanimously (16 – 0) to recommend to the FDA that icosapent ethyl be approved for an indication and label expansion to reduce the risk of cardiovascular events in high-risk patients. Although the FDA is not bound by the recommendation of its advisory committee, approval of icosapent ethyl in the United States consistent with such recommendation is anticipated on or before the FDA’s target Prescription Drug User Fee Act (PDUFA) date of December 28, 2019. In the United States, icosapent ethyl has the brand name Vascepa. If approved in Europe, the brand name may also be Vascepa, but this will be determined as part of the regulatory review process.
The MAA for icosapent ethyl is based on the global landmark clinical outcomes study, REDUCE-IT®.2 In the high-risk, statin-treated patient population studied in REDUCE-IT, as previously published, icosapent ethyl provided a highly statistically significant 25% relative risk reduction compared to placebo in the first occurrence of a major adverse CV event (MACE) in the intent-to-treat population consisting of a composite of cardiovascular death, nonfatal myocardial infarction (MI or heart attack), nonfatal stroke, coronary revascularization (procedures such as stents and by-pass) and unstable angina requiring hospitalization. For total (first and subsequent) cardiovascular events, icosapent ethyl showed a statistically significant 30% relative risk reduction compared to placebo in published exploratory analyses.3
In REDUCE-IT, adverse events occurring with icosapent ethyl use at greater than 5% and greater than placebo were: peripheral edema (6.5% Vascepa versus 5.0%); constipation (5.3% Vascepa versus 3.6%); and atrial fibrillation (5.3% Vascepa versus 3.9%). More information on safety data associated with REDUCE-IT is provided below.
Icosapent ethyl, which has been studied in a series of clinical trials, including REDUCE-IT, was developed by Amarin and is exclusively marketed by Amarin and its commercial partners in capsule form.
In the United States, Vascepa is currently approved as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. There have been more than 8 million prescriptions for Vascepa since it was launched in 2013 in the United States for this important niche indication. The drug is not currently available in Europe for any indication.
https://investor.amarincorp.com/news-releases/news-release-d…
Antwort auf Beitrag Nr.: 61.943.903 von bernie55 am 17.11.19 14:49:02

Canadian Drug Expert Committee (CDEC) meeting > December 11, 2019
CDEC recommendation sent to sponsor and drug plans December 23, 2019 To January 02, 2020
https://www.cadth.ca/icosapent-ethyl
Zitat von bernie55: Zeitplan für November -Dezember
02.12 - 06.12CANADA approval ?
28.12.19
PDUFA - FDA

Canadian Drug Expert Committee (CDEC) meeting > December 11, 2019
CDEC recommendation sent to sponsor and drug plans December 23, 2019 To January 02, 2020
https://www.cadth.ca/icosapent-ethyl
National Lipid Association Scientific Statement on Icosapent Ethyl
Dec 03, 2019 | Sherrie R. Webb, PA-C
The following are key points to remember about this statement from the National Lipid Association on use of icosapent ethyl (IPE):
1. The National Lipid Association now makes a Class I recommendation for use of IPE (4 g/d) to reduce cardiovascular risk in patients
≥45 years of age with clinical atherosclerotic cardiovascular disease (ASCVD) or
≥50 years of age with diabetes mellitus requiring medication and ≥1 additional risk factor;
already on high-intensity or maximally tolerated statin therapy;
with fasting triglycerides 135-499 mg/dL;
and with or without ezetimibe.
2. Additional risk factors are defined as
men ≥55 years of age or women ≥65 years of age;
cigarette smoker or stopped within 3 months;
treated or untreated hypertension;
0high-density lipoprotein cholesterol (HDL-C) ≤40 mg/dl in men or ≤50 mg/dl in women;
high-sensitivity C-reactive protein (hs-CRP) >3.0 mg/dL;
creatinine clearance <60 mL/min and >30 mL/min;
retinopathy;
microalbuminuria or macroalbuminuria;
and ankle-brachial index <0.9 without symptoms of intermittent claudication.
https://www.acc.org/latest-in-cardiology/ten-points-to-remem…
Dec 03, 2019 | Sherrie R. Webb, PA-C
The following are key points to remember about this statement from the National Lipid Association on use of icosapent ethyl (IPE):
1. The National Lipid Association now makes a Class I recommendation for use of IPE (4 g/d) to reduce cardiovascular risk in patients
≥45 years of age with clinical atherosclerotic cardiovascular disease (ASCVD) or
≥50 years of age with diabetes mellitus requiring medication and ≥1 additional risk factor;
already on high-intensity or maximally tolerated statin therapy;
with fasting triglycerides 135-499 mg/dL;
and with or without ezetimibe.
2. Additional risk factors are defined as
men ≥55 years of age or women ≥65 years of age;
cigarette smoker or stopped within 3 months;
treated or untreated hypertension;
0high-density lipoprotein cholesterol (HDL-C) ≤40 mg/dl in men or ≤50 mg/dl in women;
high-sensitivity C-reactive protein (hs-CRP) >3.0 mg/dL;
creatinine clearance <60 mL/min and >30 mL/min;
retinopathy;
microalbuminuria or macroalbuminuria;
and ankle-brachial index <0.9 without symptoms of intermittent claudication.
https://www.acc.org/latest-in-cardiology/ten-points-to-remem…
!
Dieser Beitrag wurde von MadMod moderiert. Grund: falscher Thread
Antwort auf Beitrag Nr.: 62.082.500 von bernie55 am 04.12.19 22:43:15
So, dann schauen wir `mal was die FDA im Dezember verkünden wird.
 TIME WILL TELL
TIME WILL TELL 
Zitat von bernie55: National Lipid Association Scientific Statement on Icosapent Ethyl
Dec 03, 2019 | Sherrie R. Webb, PA-C
The following are key points to remember about this statement from the National Lipid Association on use of icosapent ethyl (IPE):
1. The National Lipid Association now makes a Class I recommendation for use of IPE (4 g/d) to reduce cardiovascular risk in patients
≥45 years of age with clinical atherosclerotic cardiovascular disease (ASCVD) or
≥50 years of age with diabetes mellitus requiring medication and ≥1 additional risk factor;
already on high-intensity or maximally tolerated statin therapy;
with fasting triglycerides 135-499 mg/dL;
and with or without ezetimibe.
2. Additional risk factors are defined as
men ≥55 years of age or women ≥65 years of age;
cigarette smoker or stopped within 3 months;
treated or untreated hypertension;
0high-density lipoprotein cholesterol (HDL-C) ≤40 mg/dl in men or ≤50 mg/dl in women;
high-sensitivity C-reactive protein (hs-CRP) >3.0 mg/dL;
creatinine clearance <60 mL/min and >30 mL/min;
retinopathy;
microalbuminuria or macroalbuminuria;
and ankle-brachial index <0.9 without symptoms of intermittent claudication.
https://www.acc.org/latest-in-cardiology/ten-points-to-remem…
So, dann schauen wir `mal was die FDA im Dezember verkünden wird.
 TIME WILL TELL
TIME WILL TELL 
Hätte nix dagegen:
Acquisition Rumors Aren’t the Only Thing Moving Amarin Stock
Amarin stock could more than double next year
By Will Ashworth, InvestorPlace Contributor Dec 6, 2019, 6:40 am EST
It’s been three weeks since the U.S. Food and Drug Administration recommended that Amarin’s (NASDAQ:AMRN) prescription-strength fish oil drug, Vascepa, be approved for broader use to help patients at risk for heart and stroke problems. Amarin stock jumped on the news but has since fallen back into the low $20s.
Acquisition Rumors Aren't the Only Thing Moving Amarin Stock
Source: Pavel Kapysh / Shutterstock.com
The word on the street is that some of the biggest players in pharmaceuticals are sniffing around Amarin’s business. Some speculate that the Amarin stock price could be worth as much as $56 a share in the hands of a strategic buyer.
With M&A heating up in the biotech industry, could Amarin be worth $20 billion to a strategic buyer?
Amarin is currently valued at $7.8 billion by investors. A $20-billion buy would mean paying a 160% premium to its current stock price. Maybe I’m old fashioned, but that strikes me as just a little too rich for a drug that’s only been approved for sale since July 2012 and then only to lower patients’ triglyceride levels.
Are investors getting a little ahead of themselves?
A Closer Look at Amarin Stock
Amarin released its Q3 2019 earnings in early November. Revenues grew 103% to $112.4 million. Through the first nine months, business was so good it exceeded company sales for all of 2018.
As for Vascepa, Amarin reported that the total number of prescriptions at the end of September was 826,000 at the midpoint of estimates from third-party healthcare data providers, up 89% from the same period a year earlier.
“Growth in net product revenue was supported by increased prescription levels of Vascepa® (icosapent ethyl) capsules. The increased prescription levels reflect both a higher number of Vascepa prescribers and an increase in the average prescriptions per prescriber,” stated the Q3 2019 press release.
Not bad for a drug that’s limited to treating patients with high triglycerides. Imagine what it could do with a broader application to lower fat levels for a significant number of Americans at risk for heart problems. Currently, these people are using drugs such as Lipitor and Zocor to lower their cholesterol.
The FDA believes Vascepa can reduce the number of people with heart problems. It’s expected to make a final decision by Dec. 28. The recent findings by the regulatory body suggest Amarin will receive good news by the end of this month.
Acquisition Rumors Aren’t the Only Thing Moving Amarin Stock
Amarin stock could more than double next year
By Will Ashworth, InvestorPlace Contributor Dec 6, 2019, 6:40 am EST
It’s been three weeks since the U.S. Food and Drug Administration recommended that Amarin’s (NASDAQ:AMRN) prescription-strength fish oil drug, Vascepa, be approved for broader use to help patients at risk for heart and stroke problems. Amarin stock jumped on the news but has since fallen back into the low $20s.
Acquisition Rumors Aren't the Only Thing Moving Amarin Stock
Source: Pavel Kapysh / Shutterstock.com
The word on the street is that some of the biggest players in pharmaceuticals are sniffing around Amarin’s business. Some speculate that the Amarin stock price could be worth as much as $56 a share in the hands of a strategic buyer.
With M&A heating up in the biotech industry, could Amarin be worth $20 billion to a strategic buyer?
Amarin is currently valued at $7.8 billion by investors. A $20-billion buy would mean paying a 160% premium to its current stock price. Maybe I’m old fashioned, but that strikes me as just a little too rich for a drug that’s only been approved for sale since July 2012 and then only to lower patients’ triglyceride levels.
Are investors getting a little ahead of themselves?
A Closer Look at Amarin Stock
Amarin released its Q3 2019 earnings in early November. Revenues grew 103% to $112.4 million. Through the first nine months, business was so good it exceeded company sales for all of 2018.
As for Vascepa, Amarin reported that the total number of prescriptions at the end of September was 826,000 at the midpoint of estimates from third-party healthcare data providers, up 89% from the same period a year earlier.
“Growth in net product revenue was supported by increased prescription levels of Vascepa® (icosapent ethyl) capsules. The increased prescription levels reflect both a higher number of Vascepa prescribers and an increase in the average prescriptions per prescriber,” stated the Q3 2019 press release.
Not bad for a drug that’s limited to treating patients with high triglycerides. Imagine what it could do with a broader application to lower fat levels for a significant number of Americans at risk for heart problems. Currently, these people are using drugs such as Lipitor and Zocor to lower their cholesterol.
The FDA believes Vascepa can reduce the number of people with heart problems. It’s expected to make a final decision by Dec. 28. The recent findings by the regulatory body suggest Amarin will receive good news by the end of this month.
BPs auf vorweihnachtliche Shopping-Tour :
XBiotech Announces Agreement to Sell True Human Antibody Bermekimab Targeting IL-1a to Janssen
GlobeNewswire•December 7, 2019
https://finance.yahoo.com/news/xbiotech-announces-agreement-…
Sanofi to Buy U.S. Biotech Firm for $2.5 Billion in Cancer Push
,Bloomberg•December 9, 2019
https://finance.yahoo.com/news/sanofi-buy-synthorx-2-5-06451…
Merck to acquire ArQule for $20 per share in cash or about $2.7 billion
MarketWatch•December 9, 2019
https://finance.yahoo.com/m/4b3125eb-204d-3393-a6f1-63bed2ad…
XBiotech Announces Agreement to Sell True Human Antibody Bermekimab Targeting IL-1a to Janssen
GlobeNewswire•December 7, 2019
https://finance.yahoo.com/news/xbiotech-announces-agreement-…
Sanofi to Buy U.S. Biotech Firm for $2.5 Billion in Cancer Push
,Bloomberg•December 9, 2019
https://finance.yahoo.com/news/sanofi-buy-synthorx-2-5-06451…
Merck to acquire ArQule for $20 per share in cash or about $2.7 billion
MarketWatch•December 9, 2019
https://finance.yahoo.com/m/4b3125eb-204d-3393-a6f1-63bed2ad…
Fehtl nur noch unsere Amarin, aber das wird deutlich teurer, hatte auch mal Arqule......
Antwort auf Beitrag Nr.: 62.113.787 von Magnetfeldfredy am 09.12.19 15:24:39
Will Meade
Former PM at Goldman Sachs founded $1.4 billion hedge fund. Educated at the University of Chicago and Johns Hopkins SAIS. Former Edito

Will Meade
Former PM at Goldman Sachs founded $1.4 billion hedge fund. Educated at the University of Chicago and Johns Hopkins SAIS. Former Edito
Amarin's Litigation Landscape: Mostly Successful, Clearing The Field For A Decade
Dec. 10, 2019 3:00 PM
Amarin Corporation plc (AMRN)
Summary
- Amarin has successfully cleared the market of various Vascepa competitors.
- Barring a few items, its strategy has been mainly successful.
- As such, it has a clear playing field for Vascepa for the next decade.
https://seekingalpha.com/amp/article/4311700-amarins-litigat…
Dec. 10, 2019 3:00 PM
Amarin Corporation plc (AMRN)
Summary
- Amarin has successfully cleared the market of various Vascepa competitors.
- Barring a few items, its strategy has been mainly successful.
- As such, it has a clear playing field for Vascepa for the next decade.
https://seekingalpha.com/amp/article/4311700-amarins-litigat…
Überragende Scripts, Über 80.000 pro Woche und dass ohne CV Label, grandios:
NORMALIZED Scripts Update for Week Ending 06/12
ATH Across the Board in Numbers
2nd ATH in TRx & Refills Market Share, 3rd ATH in Refills Market Share
V
TRx: 83,269 {vs 67,887; +22.70%} Sector +24.12% --- (9,995,466 vs 8,146,400) -- ATH

NRx: 37,735 {vs 29,849; +26.42%} Sector +28.84% --- (4,528,247 vs 3,581,885) -- ATH
Ref: 45,560 {vs 38,038; +19.78%} Sector +20.43% --- (5,467,219 vs 4,564,515) -- ATH
GenL
TRx: 65,439 {vs 51,932; +26.01%} (7,852,629 vs 6,231,865)
NRx: 30,129 {vs 22,878; +31.70%} (3,615,518 vs 2,745,325)
L
TRx: 993 {vs 810; +22.61%} (119,172 vs 97,196)
NRx: 432 {vs 284; +52.22%} (51,826 vs 34,047)
V TRx Market Share: 55.63% vs 56.28% --- 2nd ATH Market Share
V NRx Market Share: 55.25% vs 56.31% --- 3rd ATH Market Share
V Ref Market Share: 55.95% vs 56.25% --- 2nd ATH Market Share
For those interested in Old Scripts Numbers ATH in TRx & Refills Numbers– 2nd ATH in NRx Numbers;
V TRx 63,459 vs GL TRx 48,673 & L 627 -- ATH
V NRx 24,267 vs GL NRx 19,868 & L 233 -- 2nd ATH
V Refills 39,192 vs GL Refills 28,805 & L 394 -- ATH
NORMALIZED Scripts Update for Week Ending 06/12
ATH Across the Board in Numbers
2nd ATH in TRx & Refills Market Share, 3rd ATH in Refills Market Share
V
TRx: 83,269 {vs 67,887; +22.70%} Sector +24.12% --- (9,995,466 vs 8,146,400) -- ATH


NRx: 37,735 {vs 29,849; +26.42%} Sector +28.84% --- (4,528,247 vs 3,581,885) -- ATH
Ref: 45,560 {vs 38,038; +19.78%} Sector +20.43% --- (5,467,219 vs 4,564,515) -- ATH
GenL
TRx: 65,439 {vs 51,932; +26.01%} (7,852,629 vs 6,231,865)
NRx: 30,129 {vs 22,878; +31.70%} (3,615,518 vs 2,745,325)
L
TRx: 993 {vs 810; +22.61%} (119,172 vs 97,196)
NRx: 432 {vs 284; +52.22%} (51,826 vs 34,047)
V TRx Market Share: 55.63% vs 56.28% --- 2nd ATH Market Share
V NRx Market Share: 55.25% vs 56.31% --- 3rd ATH Market Share
V Ref Market Share: 55.95% vs 56.25% --- 2nd ATH Market Share
For those interested in Old Scripts Numbers ATH in TRx & Refills Numbers– 2nd ATH in NRx Numbers;
V TRx 63,459 vs GL TRx 48,673 & L 627 -- ATH
V NRx 24,267 vs GL NRx 19,868 & L 233 -- 2nd ATH
V Refills 39,192 vs GL Refills 28,805 & L 394 -- ATH
Antwort auf Beitrag Nr.: 62.155.625 von bernie55 am 13.12.19 19:13:50AMARIN HALTED !!!!!!!!!!!!

Antwort auf Beitrag Nr.: 62.155.625 von bernie55 am 13.12.19 19:13:50T1 Halt - News Pending
Trading is halted pending the release of material news.
Trading is halted pending the release of material news.
Antwort auf Beitrag Nr.: 62.155.649 von bernie55 am 13.12.19 19:14:43 Oha...😃
Uiuiui die lassen sich aber Zeit, mein Popcorn ist bald alle menno und was mach ich dann 

Antwort auf Beitrag Nr.: 62.156.621 von flyingbeef am 13.12.19 21:21:59
Morgen einfach weiteres Popcorn kaufen.
News können auch erst am Montag rauskommen - vielleicht mit Pressekonferenz ?
Zitat von flyingbeef: Uiuiui die lassen sich aber Zeit, mein Popcorn ist bald alle menno und was mach ich dann
Morgen einfach weiteres Popcorn kaufen.

News können auch erst am Montag rauskommen - vielleicht mit Pressekonferenz ?
FDA approves use of drug to reduce risk of cardiovascular events in certain adult patient groups
https://www.fda.gov/news-events/press-announcements/fda-appr…
https://www.fda.gov/news-events/press-announcements/fda-appr…
The U.S. Food and Drug Administration today approved the use of Vascepa (icosapent ethyl) as an adjunctive (secondary) therapy to reduce the risk of cardiovascular events among adults with elevated triglyceride levels (a type of fat in the blood) of 150 milligrams per deciliter or higher.
Patients must also have either established cardiovascular disease or diabetes and two or more additional risk factors for cardiovascular disease.
Patients are advised to continue physical activity and maintain a healthy diet.
Vascepa is the first FDA approved drug to reduce cardiovascular risk among patients with elevated triglyceride levels as an add-on to maximally tolerated statin therapy. Statins are drugs used to treat elevated cholesterol levels and reduce the risk of cardiovascular events.
“The FDA recognizes there is a need for additional medical treatments for cardiovascular disease,” said John Sharretts, M.D., acting deputy director of the Division of Metabolism and Endocrinology Products in the FDA’s Center for Drug Evaluation and Research. “Today’s approval will give patients with elevated triglycerides and other important risk factors, including heart disease, stroke and diabetes, an adjunctive treatment option that can help decrease their risk of cardiovascular events.”
High levels of triglycerides can play a role in the hardening of arteries or thickening of the artery wall, which can increase the risk of a heart attack or stroke; however, the mechanisms of action that contribute to reduced cardiovascular events among patients taking Vascepa are not completely understood.
Vascepa’s efficacy and safety were established in a study with 8,179 patients who were either 45 years and older with a documented history of coronary artery, cerebrovascular, carotid artery and peripheral artery disease, or 50 years and older with diabetes and additional risk factors for cardiovascular disease. Patients who received Vascepa were significantly less likely to experience a cardiovascular event, such as a stroke or heart attack. Vascepa’s active ingredient is the omega-3 fatty acid, eicosapentaenoic acid, derived from fish oil. Vascepa is taken orally.
In clinical trials, Vascepa was associated with an increased risk of atrial fibrillation or atrial flutter (irregular heart rhythms) requiring hospitalization. The incidence of atrial fibrillation was greater among patients with a history of atrial fibrillation or atrial flutter. Vascepa was also associated with an increased risk of bleeding events. The incidence of bleeding was higher among patients who were also taking other medications that increase the risk of bleeding, such as aspirin, clopidogrel or warfarin at the same time.
Patients with allergies to fish or shellfish should be advised about the potential for allergic reactions. They should discontinue treatment and seek medical attention if any allergic reactions occur.
The most common side effects reported in the clinical trials for Vascepa were musculoskeletal pain, peripheral edema (swelling of legs and hands), atrial fibrillation and arthralgia (joint pain).
Vascepa was initially approved in 2012 for adults with severe triglyceride levels. This supplement application received Priority Review. The FDA grants priority review to applications for drugs that, if approved, would improve the safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions.
The approval of Vascepa was granted to Amarin Pharma Inc.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Patients must also have either established cardiovascular disease or diabetes and two or more additional risk factors for cardiovascular disease.
Patients are advised to continue physical activity and maintain a healthy diet.
Vascepa is the first FDA approved drug to reduce cardiovascular risk among patients with elevated triglyceride levels as an add-on to maximally tolerated statin therapy. Statins are drugs used to treat elevated cholesterol levels and reduce the risk of cardiovascular events.
“The FDA recognizes there is a need for additional medical treatments for cardiovascular disease,” said John Sharretts, M.D., acting deputy director of the Division of Metabolism and Endocrinology Products in the FDA’s Center for Drug Evaluation and Research. “Today’s approval will give patients with elevated triglycerides and other important risk factors, including heart disease, stroke and diabetes, an adjunctive treatment option that can help decrease their risk of cardiovascular events.”
High levels of triglycerides can play a role in the hardening of arteries or thickening of the artery wall, which can increase the risk of a heart attack or stroke; however, the mechanisms of action that contribute to reduced cardiovascular events among patients taking Vascepa are not completely understood.
Vascepa’s efficacy and safety were established in a study with 8,179 patients who were either 45 years and older with a documented history of coronary artery, cerebrovascular, carotid artery and peripheral artery disease, or 50 years and older with diabetes and additional risk factors for cardiovascular disease. Patients who received Vascepa were significantly less likely to experience a cardiovascular event, such as a stroke or heart attack. Vascepa’s active ingredient is the omega-3 fatty acid, eicosapentaenoic acid, derived from fish oil. Vascepa is taken orally.
In clinical trials, Vascepa was associated with an increased risk of atrial fibrillation or atrial flutter (irregular heart rhythms) requiring hospitalization. The incidence of atrial fibrillation was greater among patients with a history of atrial fibrillation or atrial flutter. Vascepa was also associated with an increased risk of bleeding events. The incidence of bleeding was higher among patients who were also taking other medications that increase the risk of bleeding, such as aspirin, clopidogrel or warfarin at the same time.
Patients with allergies to fish or shellfish should be advised about the potential for allergic reactions. They should discontinue treatment and seek medical attention if any allergic reactions occur.
The most common side effects reported in the clinical trials for Vascepa were musculoskeletal pain, peripheral edema (swelling of legs and hands), atrial fibrillation and arthralgia (joint pain).
Vascepa was initially approved in 2012 for adults with severe triglyceride levels. This supplement application received Priority Review. The FDA grants priority review to applications for drugs that, if approved, would improve the safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions.
The approval of Vascepa was granted to Amarin Pharma Inc.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Antwort auf Beitrag Nr.: 62.157.113 von bernie55 am 13.12.19 22:39:40Somit stehen sowohl die Primär- als auch die Sekundärprävention auf dem Label, wobei sich die Primärprävention auf Diabetiker plus 2 zusätzliche Faktoren ( Alter und Triggern über 150 ) bezieht.
Sekundärprävention bezieht sich auf die Patienten, die bereits Statine einnehmen.
Sekundärprävention bezieht sich auf die Patienten, die bereits Statine einnehmen.
AMARIN RECEIVES FDA APPROVAL OF VASCEPA® (ICOSAPENT ETHYL) TO REDUCE CARDIOVASCULAR RISK
Dec 13, 2019
VASCEPA becomes the first and only FDA-approved medication for reducing cardiovascular risk beyond cholesterol lowering therapy in high-risk patients approved for treatment
Millions of people in the United States qualify as treatment candidates for VASCEPA
Cardiovascular disease events, including heart attack, stroke and cardiovascular death, occur in the United States every 14 seconds and are economically, physically and emotionally costly
VASCEPA has been assessed by independent bodies as priced cost effectively as a cardiovascular risk reduction treatment
VASCEPA total net revenue guidance increased for 2019 to a range of $410 to $425 million and for 2020 is newly guided to a projected range of $650 to $700 million
Amarin to host webcast on Monday, December 16 at 7:30 a.m., Eastern Time
https://investor.amarincorp.com/news-releases/news-release-d…
Dec 13, 2019
VASCEPA becomes the first and only FDA-approved medication for reducing cardiovascular risk beyond cholesterol lowering therapy in high-risk patients approved for treatment
Millions of people in the United States qualify as treatment candidates for VASCEPA
Cardiovascular disease events, including heart attack, stroke and cardiovascular death, occur in the United States every 14 seconds and are economically, physically and emotionally costly
VASCEPA has been assessed by independent bodies as priced cost effectively as a cardiovascular risk reduction treatment
VASCEPA total net revenue guidance increased for 2019 to a range of $410 to $425 million and for 2020 is newly guided to a projected range of $650 to $700 million
Amarin to host webcast on Monday, December 16 at 7:30 a.m., Eastern Time
https://investor.amarincorp.com/news-releases/news-release-d…
Antwort auf Beitrag Nr.: 62.157.203 von bernie55 am 13.12.19 22:52:03Das heisst es ist nicht das gaaanz große Label, wurde also entsprechend eingeschränkt, es ist aber auch nicht die Minimallösung = mittleres Endergebnis
Für mich eine realistische und nachvollziehbare Zulassung. Vascepa als Wunderheilmittel für alles anzupreisen was in manchen Foren so geschrieben wurde war ja immer schon deutlich übertrieben.
Denke wir können zufrieden sein, bin mal gespannt wie das im Aktienmarkt ankommt und wohin die Reise die nächsten Tage führt.
Was meint ihr ist eine Partnerschaft/BO damit eher realistischer geworden da nun Amarin Potential sehr gut aber ist aber auch nicht zuu teuer werden wird so dass ein solcher Schritt eher wahrscheinlicher wird als unwahrscheinlicher ?
Für mich eine realistische und nachvollziehbare Zulassung. Vascepa als Wunderheilmittel für alles anzupreisen was in manchen Foren so geschrieben wurde war ja immer schon deutlich übertrieben.
Denke wir können zufrieden sein, bin mal gespannt wie das im Aktienmarkt ankommt und wohin die Reise die nächsten Tage führt.
Was meint ihr ist eine Partnerschaft/BO damit eher realistischer geworden da nun Amarin Potential sehr gut aber ist aber auch nicht zuu teuer werden wird so dass ein solcher Schritt eher wahrscheinlicher wird als unwahrscheinlicher ?
Super geil, freut mich für alle Patienten und Aktionäre, Buyout wird 2020 kommen, US Dollar 50!
Antwort auf Beitrag Nr.: 62.157.326 von Magnetfeldfredy am 13.12.19 23:21:09



Zitat von Magnetfeldfredy: Super geil, freut mich für alle Patienten und Aktionäre, Buyout wird 2020 kommen, US Dollar 50!



Antwort auf Beitrag Nr.: 62.157.335 von bernie55 am 13.12.19 23:26:10

Antwort auf Beitrag Nr.: 62.157.077 von bernie55 am 13.12.19 22:33:56

Zitat von bernie55: FDA approves use of drug to reduce risk of cardiovascular events in certain adult patient groups
https://www.fda.gov/news-events/press-announcements/fda-appr…

Nur so ein Gefühl zu später Stunde aber ich denke der nächste Squeeze steht am Montag an. Ich sollte dafür aber zur gebührenden Begleitung dieses Ereignisses mal auf was anderes umsteigen, mir ist schon ganz schlecht vom ganzen Popcorn *grins*, hat irgendjemand konstruktive Vorschläge 🙃 ?
Closing Nasdaq nachbörslich 26,70 USD 👍😃
Antwort auf Beitrag Nr.: 62.157.644 von asthmamoah am 14.12.19 06:25:06Vascepa wird für Patienten zugelassen, bei denen bereits eine Herz-Kreislauf-Erkrankung festgestellt wurde (Herzinfarkt, Angina pectoris ...) oder für diejenigen, die Diabetes und zwei Risikofaktoren für Herzinfarkte haben. Vascepa ist für Patienten zugelassen, deren Triglycerid im Blut haben 150 Milligramm pro Deziliter übersteigt - Amarin hatte auf eine Schwelle oberhalb 135 mg/dL gehofft.
"Die FDA erkennt an, dass es einen Bedarf an zusätzlichen medizinischen Behandlungen für Herz-Kreislauf-Erkrankungen gibt", sagte Dr. John Sharretts, stellvertretender Direktor der Division of Metabolism and Endocrinology Products im Center for Drug Evaluation and Research der FDA, in einer Erklärung. "Die heutige Zulassung wird Patienten mit erhöhten Triglyceriden und anderen wichtigen Risikofaktoren, einschließlich Herzerkrankungen, Schlaganfall und Diabetes, eine ergänzende Behandlungsmöglichkeit bieten, die dazu beitragen kann, das Risiko von Herz-Kreislauf-Erkrankungen zu verringern."
Trotz dieser Einschränkungen könnte die Verwendung von Vascepa dramatisch zunehmen. Vor der Zulassung haben die Ergebnisse einer im vergangenen November veröffentlichten wissenschaftlichen Studie dazu geführt, dass sich der Umsatz von Amarin im dritten Quartal dieses Jahres gegenüber dem Vorjahr auf 112 Millionen US-Dollar verdoppelt hat. Ich rechne mit einer weiteren signifikanten Umsatzsteigerung. Vascepa wird relativ schnell ein Blockbuster werden, also > 1 Milliarde$ Jahresumsatz generieren.
"Die FDA erkennt an, dass es einen Bedarf an zusätzlichen medizinischen Behandlungen für Herz-Kreislauf-Erkrankungen gibt", sagte Dr. John Sharretts, stellvertretender Direktor der Division of Metabolism and Endocrinology Products im Center for Drug Evaluation and Research der FDA, in einer Erklärung. "Die heutige Zulassung wird Patienten mit erhöhten Triglyceriden und anderen wichtigen Risikofaktoren, einschließlich Herzerkrankungen, Schlaganfall und Diabetes, eine ergänzende Behandlungsmöglichkeit bieten, die dazu beitragen kann, das Risiko von Herz-Kreislauf-Erkrankungen zu verringern."
Trotz dieser Einschränkungen könnte die Verwendung von Vascepa dramatisch zunehmen. Vor der Zulassung haben die Ergebnisse einer im vergangenen November veröffentlichten wissenschaftlichen Studie dazu geführt, dass sich der Umsatz von Amarin im dritten Quartal dieses Jahres gegenüber dem Vorjahr auf 112 Millionen US-Dollar verdoppelt hat. Ich rechne mit einer weiteren signifikanten Umsatzsteigerung. Vascepa wird relativ schnell ein Blockbuster werden, also > 1 Milliarde$ Jahresumsatz generieren.
Antwort auf Beitrag Nr.: 62.159.558 von Cyberhexe am 14.12.19 14:36:21kleine Korrektur:
Vascepa wird für Patienten zugelassen, bei denen bereits eine Herz-Kreislauf-Erkrankung festgestellt wurde (Herzinfarkt, Angina pectoris ...) oder für diejenigen, die Diabetes und zwei Risikofaktoren für Herzinfarkte haben. Vascepa ist nun für Patienten zugelassen, deren Triglyceridgehalt im Blut 150 Milligramm pro Deziliter übersteigt - Amarin hatte auf einen Minimalwert von 135 mg/dL gehofft.
Vascepa wird für Patienten zugelassen, bei denen bereits eine Herz-Kreislauf-Erkrankung festgestellt wurde (Herzinfarkt, Angina pectoris ...) oder für diejenigen, die Diabetes und zwei Risikofaktoren für Herzinfarkte haben. Vascepa ist nun für Patienten zugelassen, deren Triglyceridgehalt im Blut 150 Milligramm pro Deziliter übersteigt - Amarin hatte auf einen Minimalwert von 135 mg/dL gehofft.
Sehe ich auch so Hexe, geil, jetzt kommt noch die Kanada Zulassung und es kann jederzeit ein Buyout kommen, was glaubst Du?
Sehr guter Artikel:
Amarin eyes blockbuster Vascepa sales as FDA grants heart-helping label expansion
by Kyle Blankenship | Dec 13, 2019 4:45pm
Amarin Vascepa pill
The Vascepa label expansion will likely spur even more buyout speculation for Amarin. (Amarin)
Teilen
Facebook
Twitter
LinkedIn
Email
Print
Amarin has been on a scorching run with its fish-oil derivative Vascepa after a major cardiovascular outcomes trial last year set a possible blockbuster trajectory for the drug. Now, the FDA has voted to give Vascepa a major label boost with the addition of CV risk reduction––and those blockbuster sales could be on the near horizon.
The FDA approved Vascepa as an add-on to statins to reduce the risk of cardiovascular events in patients with elevated triglycerides who have either established CV disease or diabetes with two additional CV risk factors, the administration said Friday.
The indication settles a lingering question around Vascepa's expanded label of whether the FDA would limit the drug's patient pool to only those with a preexisting CV disease or expand it to the much larger population of patients at high risk of disease.
With a broader label in hand, Vascepa is now poised to become the blockbuster drug Amarin hoped it would become after a major outcomes trial last year showed the drug on top of statins cut the risk of CV events by 25% in patients with abnormally high triglyceride levels.
RELATED: Blockbuster in sight? Amarin's Vascepa sails through FDA panel vote toward new CV nod
Amarin raised its 2020 sales forecast to between $600 million and $700 million as it plans to roll out an expanded sales team of 800 by mid-January, CEO John Thero said Friday. With the new label, Thero said Amarin was expecting peak sales estimates in the "multiple billions."
"We are creating a market here and going where people have not gone before," he said. "Our focus is on improving patient care and executing on our plan."
Vascepa has now been approved for a possible patient population that runs into the tens of millions, Thero said.
"This is an indication that gives physicians tools to treat their high-risk patients, whether those are patients with an established CV disease or diabetes with other CV risk factors," he said. "This is a lot of patients."
The FDA's approval follows an advisory committee's unanimous vote in November to recommend the drug's expanded label. In that review, committee members were tasked with investigating concerns that the control arm of Vascepa's outcomes trial, dubbed Reduce-It, may have had a skewing effect on the drug's results.
The trial tested Vascepa as an add-on to statin therapy, compared with the mineral oil placebo paired with statins. The FDA examined whether that mineral oil interfered with patients' ability to absorb statin therapy and artificially raised patients' cholesterol levels, but it called the study "inconclusive," John Sharretts, acting deputy director of the FDA's division of metabolism and endocrinology products, said in a briefing at the time.
RELATED: Amarin for $20B? Novartis' MedCo deal drives fresh buyout rumors for Vascepa's maker
With Vascepa now a likely blockbuster candidate, already sky-high speculation about Amarin's acquisition by a major player will likely continue apace.
Following the committee vote and Novartis' $9.7 billion buyout of The Medicines Company and its PCSK9 cholesterol buster candidate, inclisiran, rumors––however fanciful––of a possible $20 billion Amarin acquisition made the rounds among investors.
And Vascepa's label expansion made Amarin "an even more interesting asset in a consolidating space," Cantor analyst Louise Chen said in a Friday note to investors.
In the meantime, Amarin has expressed its intention to go it alone without a major partner.
In July, Thero told investors Amarin was prepared to take on the increased demand for Vascepa with a sales hiring goal of 800 employees and a planned direct-to-consumer advertising campaign upon expansion. The drugmaker said the company expects to reach 70,000 to 80,000 healthcare professionals when its sales force is fully fleshed out and is seeing higher-than-expected access to doctors in the meantime.
Read more on
cardiovascular outcomes cardiovascular disease fish oil drug labels Amarin Vascepa
Amarin eyes blockbuster Vascepa sales as FDA grants heart-helping label expansion
by Kyle Blankenship | Dec 13, 2019 4:45pm
Amarin Vascepa pill
The Vascepa label expansion will likely spur even more buyout speculation for Amarin. (Amarin)
Teilen
Amarin has been on a scorching run with its fish-oil derivative Vascepa after a major cardiovascular outcomes trial last year set a possible blockbuster trajectory for the drug. Now, the FDA has voted to give Vascepa a major label boost with the addition of CV risk reduction––and those blockbuster sales could be on the near horizon.
The FDA approved Vascepa as an add-on to statins to reduce the risk of cardiovascular events in patients with elevated triglycerides who have either established CV disease or diabetes with two additional CV risk factors, the administration said Friday.
The indication settles a lingering question around Vascepa's expanded label of whether the FDA would limit the drug's patient pool to only those with a preexisting CV disease or expand it to the much larger population of patients at high risk of disease.
With a broader label in hand, Vascepa is now poised to become the blockbuster drug Amarin hoped it would become after a major outcomes trial last year showed the drug on top of statins cut the risk of CV events by 25% in patients with abnormally high triglyceride levels.
RELATED: Blockbuster in sight? Amarin's Vascepa sails through FDA panel vote toward new CV nod
Amarin raised its 2020 sales forecast to between $600 million and $700 million as it plans to roll out an expanded sales team of 800 by mid-January, CEO John Thero said Friday. With the new label, Thero said Amarin was expecting peak sales estimates in the "multiple billions."
"We are creating a market here and going where people have not gone before," he said. "Our focus is on improving patient care and executing on our plan."
Vascepa has now been approved for a possible patient population that runs into the tens of millions, Thero said.
"This is an indication that gives physicians tools to treat their high-risk patients, whether those are patients with an established CV disease or diabetes with other CV risk factors," he said. "This is a lot of patients."
The FDA's approval follows an advisory committee's unanimous vote in November to recommend the drug's expanded label. In that review, committee members were tasked with investigating concerns that the control arm of Vascepa's outcomes trial, dubbed Reduce-It, may have had a skewing effect on the drug's results.
The trial tested Vascepa as an add-on to statin therapy, compared with the mineral oil placebo paired with statins. The FDA examined whether that mineral oil interfered with patients' ability to absorb statin therapy and artificially raised patients' cholesterol levels, but it called the study "inconclusive," John Sharretts, acting deputy director of the FDA's division of metabolism and endocrinology products, said in a briefing at the time.
RELATED: Amarin for $20B? Novartis' MedCo deal drives fresh buyout rumors for Vascepa's maker
With Vascepa now a likely blockbuster candidate, already sky-high speculation about Amarin's acquisition by a major player will likely continue apace.
Following the committee vote and Novartis' $9.7 billion buyout of The Medicines Company and its PCSK9 cholesterol buster candidate, inclisiran, rumors––however fanciful––of a possible $20 billion Amarin acquisition made the rounds among investors.

And Vascepa's label expansion made Amarin "an even more interesting asset in a consolidating space," Cantor analyst Louise Chen said in a Friday note to investors.
In the meantime, Amarin has expressed its intention to go it alone without a major partner.
In July, Thero told investors Amarin was prepared to take on the increased demand for Vascepa with a sales hiring goal of 800 employees and a planned direct-to-consumer advertising campaign upon expansion. The drugmaker said the company expects to reach 70,000 to 80,000 healthcare professionals when its sales force is fully fleshed out and is seeing higher-than-expected access to doctors in the meantime.
Read more on
cardiovascular outcomes cardiovascular disease fish oil drug labels Amarin Vascepa
Antwort auf Beitrag Nr.: 62.159.984 von Magnetfeldfredy am 14.12.19 16:27:41..und auch ein Artikel im Wallstreet-Journal
FDA Approves Fish-Oil-Derived Drug for Use Preventing Heart Attacks, Strokes
Vascepa becomes new agent to help millions of heart-disease patients who have high cholesterol despite taking medicine for it
By Jared S. Hopkins
Dec. 13, 2019 5:48 pm ET
The U.S. Food and Drug Administration on Friday expanded the approved use of a fish-oil-derived drug to reduce the likelihood of heart attacks and strokes in high-risk patients.
The drug, Vascepa from Amarin Corp. PLC, now becomes a new tool for reducing the risk of heart attacks, strokes and deaths in millions of heart-disease or diabetes patients with elevated triglycerides while opening up a multibillion-dollar commercial opportunity for its maker. The expanded label could mean Vascepa sales surpass $3 billion, analysts say. Last year’s sales approached $230 million.
analysts say. Last year’s sales approached $230 million.
Vascepa was approved in the U.S. in 2012 to treat adults with severe hypertriglyceridemia, or very high levels of triglycerides, which are fats that circulate in the blood k of heart disease.
High triglyceride levels can signal the presence of metabolic abnormalities that can damage the heart and blood vessels. About four million Americans have severe hypertriglyceridemia, according to Amarin, and 70 million have elevated triglyceride levels.
The FDA said Vascepa could be taken on top of cholesterol drugs known as statins. Patients must have either heart disease, or diabetes and at least two additional risk factors for cardiovascular disease.
“For the first time, physicians, patients and payers have an FDA-approved treatment option beyond cholesterol lowering that has been demonstrated to significantly reduce major adverse cardiovascular events when used on top of a statin,” Amarin Chief Executive John Thero said.
A large late-stage study, commissioned by Amarin, showed that Vascepa can reduce the risk of cardiovascular-disease events in patients with elevated triglyceride levels who are at high risk and on cholesterol-lowering drugs.
Most patients in the study had already had a heart attack, stroke or other cardiovascular event. The rest were at high risk: They had Type 2 diabetes and at least one other risk factor, such as tobacco use.
The FDA acted earlier than the expected Dec. 28 date, the latest in a series of recent earlier-than-expected decisions from the FDA.
In November, a panel of outside advisers voted 16 to 0 in recommending the agency enlarge the approved use of the drug. The FDA wasn’t bound to follow the advice of its advisory committee, but it normally does.
The FDA rejected Amarin’s first request for a broader label in 2013, prompting the company to sponsor the latest study. In 2018, Amarin announced data from that study, which showed high-risk patients who took Vascepa in addition to a statin experienced a 25% reduction in risk of a heart attack, stroke or other serious cardiac event, compared with patients who took a placebo.
The company’s shares surged on the news in 2018. The shares closed up 4.9% at $24.12 after Friday’s announcement. Amarin is based in Dublin, Ireland, but operates out of Bridgewater, N.J.
A monthly supply of the gram dose of Vascepa carries a list price of $303.65 before insurance kicks in.
https://www.wsj.com/articles/fda-approves-fish-oil-derived-d…
FDA Approves Fish-Oil-Derived Drug for Use Preventing Heart Attacks, Strokes
Vascepa becomes new agent to help millions of heart-disease patients who have high cholesterol despite taking medicine for it
By Jared S. Hopkins
Dec. 13, 2019 5:48 pm ET
The U.S. Food and Drug Administration on Friday expanded the approved use of a fish-oil-derived drug to reduce the likelihood of heart attacks and strokes in high-risk patients.
The drug, Vascepa from Amarin Corp. PLC, now becomes a new tool for reducing the risk of heart attacks, strokes and deaths in millions of heart-disease or diabetes patients with elevated triglycerides while opening up a multibillion-dollar commercial opportunity for its maker. The expanded label could mean Vascepa sales surpass $3 billion,
 analysts say. Last year’s sales approached $230 million.
analysts say. Last year’s sales approached $230 million.Vascepa was approved in the U.S. in 2012 to treat adults with severe hypertriglyceridemia, or very high levels of triglycerides, which are fats that circulate in the blood k of heart disease.
High triglyceride levels can signal the presence of metabolic abnormalities that can damage the heart and blood vessels. About four million Americans have severe hypertriglyceridemia, according to Amarin, and 70 million have elevated triglyceride levels.
The FDA said Vascepa could be taken on top of cholesterol drugs known as statins. Patients must have either heart disease, or diabetes and at least two additional risk factors for cardiovascular disease.
“For the first time, physicians, patients and payers have an FDA-approved treatment option beyond cholesterol lowering that has been demonstrated to significantly reduce major adverse cardiovascular events when used on top of a statin,” Amarin Chief Executive John Thero said.
A large late-stage study, commissioned by Amarin, showed that Vascepa can reduce the risk of cardiovascular-disease events in patients with elevated triglyceride levels who are at high risk and on cholesterol-lowering drugs.
Most patients in the study had already had a heart attack, stroke or other cardiovascular event. The rest were at high risk: They had Type 2 diabetes and at least one other risk factor, such as tobacco use.
The FDA acted earlier than the expected Dec. 28 date, the latest in a series of recent earlier-than-expected decisions from the FDA.
In November, a panel of outside advisers voted 16 to 0 in recommending the agency enlarge the approved use of the drug. The FDA wasn’t bound to follow the advice of its advisory committee, but it normally does.
The FDA rejected Amarin’s first request for a broader label in 2013, prompting the company to sponsor the latest study. In 2018, Amarin announced data from that study, which showed high-risk patients who took Vascepa in addition to a statin experienced a 25% reduction in risk of a heart attack, stroke or other serious cardiac event, compared with patients who took a placebo.
The company’s shares surged on the news in 2018. The shares closed up 4.9% at $24.12 after Friday’s announcement. Amarin is based in Dublin, Ireland, but operates out of Bridgewater, N.J.
A monthly supply of the gram dose of Vascepa carries a list price of $303.65 before insurance kicks in.
https://www.wsj.com/articles/fda-approves-fish-oil-derived-d…
Hier CaptBeer auf stockwits, wie ich finde mit beeindruckenden Statements bzgl. möglicher Patientenanzahl für die neue VASCEPA Indikation:
Good Morning Friends! I've been trying to figure out the potential patient population for the new VASCEPA Indication.
The language is very broad and flexible allowing doctors plenty of interpretative license. I'll be presenting statistics from various sources (referenced in the illustrations).
PART # 1: The Indication as "Adjunct to Statin Therapy" :
Here's the Indication: “as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels (≥150 mg/dL) and established cardiovascular disease or diabetes mellitus and two or more additional risk factors for cardiovascular disease.”
In 2014 there were approximately 64 million adult patients on statins or eligible for the use.
This chart groups those patients as illustrated:

Good Morning Friends! I've been trying to figure out the potential patient population for the new VASCEPA Indication.
The language is very broad and flexible allowing doctors plenty of interpretative license. I'll be presenting statistics from various sources (referenced in the illustrations).
PART # 1: The Indication as "Adjunct to Statin Therapy" :
Here's the Indication: “as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels (≥150 mg/dL) and established cardiovascular disease or diabetes mellitus and two or more additional risk factors for cardiovascular disease.”
In 2014 there were approximately 64 million adult patients on statins or eligible for the use.
This chart groups those patients as illustrated:

Antwort auf Beitrag Nr.: 62.160.674 von bernie55 am 14.12.19 19:17:33PART # 2: The secondary prevention part of the indication has a High TG level (TG>150 mg/dl) criteria. The illustration below is from 2012 and shows the age adjusted and sex differentiated percentages of adult patients with elevated TG's (>=150 mg/dl). The chart indicates that approximately 25% of adults >= 20 years of age have "High" TG's. According to unspecified sources the population of adults >=20 years of age is 245.4 million. 25% = 61.3 million have TG's >= 150 mg/dl.
Keep in mind that the two populations overlap each other and that both of these stats are several years old. The High Statin eligibility Group has been trending much higher while the High TG Group has been slightly trending lower.
Conclusion: it's easy to see 90-100 million eligible patients for this indication.

Keep in mind that the two populations overlap each other and that both of these stats are several years old. The High Statin eligibility Group has been trending much higher while the High TG Group has been slightly trending lower.
Conclusion: it's easy to see 90-100 million eligible patients for this indication.

Am Montag, 16.12.19 ist wohl nicht nur der conference call von AMRN.
Gibt es auch eine Konferenz bzgl. der Abwicklung von Genericaverfahren ? Das würde für Amarin bzgl. Vascepa sehr wichtig sein > Patentschutz für Vascepa bis 2030 ?
Monday is another big day against Generics.
JT is confident and he is not known to over promise, just look at the 2020 forecast and the initial 2019 forecast. "In our view, AMRN is set up to achieve a dominant market position as a CV-risk reducing drug for the long-term vs. the horde of generic EPA drug developers."
https://www.medtechy.com/message-boards/boards/companies/ama…
Gibt es auch eine Konferenz bzgl. der Abwicklung von Genericaverfahren ? Das würde für Amarin bzgl. Vascepa sehr wichtig sein > Patentschutz für Vascepa bis 2030 ?
Monday is another big day against Generics.
JT is confident and he is not known to over promise, just look at the 2020 forecast and the initial 2019 forecast. "In our view, AMRN is set up to achieve a dominant market position as a CV-risk reducing drug for the long-term vs. the horde of generic EPA drug developers."
https://www.medtechy.com/message-boards/boards/companies/ama…
Antwort auf Beitrag Nr.: 62.160.947 von bernie55 am 14.12.19 20:38:38Can Amarin’s patents protect Vascepa from generics?
https://www.markmanadvisors.com/blog/2019/12/3/can-amarins-p…
https://www.markmanadvisors.com/blog/2019/12/3/can-amarins-p…
Dr Bhatt's Kommentar ( a very broad label) bzgl. Vascepa-Zulassung auf Twitter:


Feuerstein's Kommentar ( label is restricted ) auf Twitter :


Antwort auf Beitrag Nr.: 62.159.771 von Magnetfeldfredy am 14.12.19 15:44:52
Nach der FDA Entscheidung vom 13.12.19 kann ich mir aktuell irgendwie auch nicht mehr vorstellen, dass sich die BPs zurücklehnen und sich die Entwicklung von AMRN von der Seitenlinie anschauen werden.
Ich glaube vielmehr, dass einige BPs an der Tür von AMRN anklopfen werden.
DESHALB riecht es m.M.n. ganz stark nach..........

Zitat von Magnetfeldfredy: .......jetzt kommt noch die Kanada Zulassung und es kann jederzeit ein Buyout kommen.......
Nach der FDA Entscheidung vom 13.12.19 kann ich mir aktuell irgendwie auch nicht mehr vorstellen, dass sich die BPs zurücklehnen und sich die Entwicklung von AMRN von der Seitenlinie anschauen werden.
Ich glaube vielmehr, dass einige BPs an der Tür von AMRN anklopfen werden.
DESHALB riecht es m.M.n. ganz stark nach..........

Antwort auf Beitrag Nr.: 62.163.009 von bernie55 am 15.12.19 13:29:41Falls kein Buyout zustande kommen sollte ( aus welchem Grund auch immer ) , wäre alternativ natürlich auch noch eine Partnerschaft mit einem BP denkbar....
Auf jeden Fall fühlt sich nach der FDA Entscheidung aktuell alles an wie eine WinWin Situation .
Und wie immer: TIME WILL TELL
Auf jeden Fall fühlt sich nach der FDA Entscheidung aktuell alles an wie eine WinWin Situation .

Und wie immer: TIME WILL TELL

Antwort auf Beitrag Nr.: 62.157.203 von bernie55 am 13.12.19 22:52:03
Hierzu habe ich eine tolles statement von dem User Mike im Finance - Yahoo Board gefunden, der den ganzen Sachverhalt nochmal kurz , knackig und sachlich aus der Sicht eines Mediziners sehr gut erklärt.
Hier die von mir überarbeitete Übersetzung :
Ich lese ständig von Leuten, die sagen, dass Vascepa kein primäres Etikett bekommen hat. Ich bin Arzt und möchte diese Begriffe erklären.
Hier ist ein kurzer Überblick über primäre, sekundäre Prävention und ein völlig unabhängiges Konzept, "sekundärer Zusatz".
Primärprävention
Verhindern, dass eine Krankheit überhaupt erst auftritt.
Sekundärprävention:
Vorbeugung weiterer Probleme, nachdem eine Krankheit bereits festgestellt wurde.
AMRN wurde für Diabetiker mit TG > 150 auf Statine mit 2 Risikofaktoren (Rauchen, Adipositas, Bluthochdruck, Familiengeschichte, hoher Cholesterinspiegel) gewährt, die NIE ein unerwünschtes kardiovaskuläres Ereignis (Herzinfarkt, Schlaganfall, etc.) hatten. Dies wird als Primärprävention bezeichnet.
AMRN wurde auch für Patienten mit TG > 150 auf Statinen gekennzeichnet, die in der Vergangenheit bereits unerwünschte kardiovaskuläre Ereignisse (Herzinfarkt, Schlaganfall, etc.) hatten. Dies wird als Sekundäreprävention bezeichnet.
Der Begriff sekundärer Zusatz bedeutet einfach, dass das Medikament bei Patienten gestartet wird, die bereits auf einem Statin sind. Das hat also nichts mit primärer oder sekundärer Prävention zu tun.
Zusammenfassend lässt sich sagen, dass Amarin eine enorme Etikettenerweiterung erhalten hat, die sogar die Primärprävention bei Diabetikern miteinschließt, was die Patientenpopulation deutlich vervielfacht.
Zitat von bernie55: Somit stehen sowohl die Primär- als auch die Sekundärprävention auf dem Label, wobei sich die Primärprävention auf Diabetiker plus 2 zusätzliche Faktoren ( Alter und Triggern über 150 ) bezieht.
Sekundärprävention bezieht sich auf die Patienten, die bereits Statine einnehmen.
Hierzu habe ich eine tolles statement von dem User Mike im Finance - Yahoo Board gefunden, der den ganzen Sachverhalt nochmal kurz , knackig und sachlich aus der Sicht eines Mediziners sehr gut erklärt.
Hier die von mir überarbeitete Übersetzung :
Ich lese ständig von Leuten, die sagen, dass Vascepa kein primäres Etikett bekommen hat. Ich bin Arzt und möchte diese Begriffe erklären.
Hier ist ein kurzer Überblick über primäre, sekundäre Prävention und ein völlig unabhängiges Konzept, "sekundärer Zusatz".
Primärprävention
Verhindern, dass eine Krankheit überhaupt erst auftritt.
Sekundärprävention:
Vorbeugung weiterer Probleme, nachdem eine Krankheit bereits festgestellt wurde.
AMRN wurde für Diabetiker mit TG > 150 auf Statine mit 2 Risikofaktoren (Rauchen, Adipositas, Bluthochdruck, Familiengeschichte, hoher Cholesterinspiegel) gewährt, die NIE ein unerwünschtes kardiovaskuläres Ereignis (Herzinfarkt, Schlaganfall, etc.) hatten. Dies wird als Primärprävention bezeichnet.
AMRN wurde auch für Patienten mit TG > 150 auf Statinen gekennzeichnet, die in der Vergangenheit bereits unerwünschte kardiovaskuläre Ereignisse (Herzinfarkt, Schlaganfall, etc.) hatten. Dies wird als Sekundäreprävention bezeichnet.
Der Begriff sekundärer Zusatz bedeutet einfach, dass das Medikament bei Patienten gestartet wird, die bereits auf einem Statin sind. Das hat also nichts mit primärer oder sekundärer Prävention zu tun.
Zusammenfassend lässt sich sagen, dass Amarin eine enorme Etikettenerweiterung erhalten hat, die sogar die Primärprävention bei Diabetikern miteinschließt, was die Patientenpopulation deutlich vervielfacht.
Antwort auf Beitrag Nr.: 62.162.952 von bernie55 am 15.12.19 13:11:59
Das Posting von A. "Fuckelstone" - der !! Vetreter der Big Short Fraktion schlechthin - sollte einfach noch einmal die Diskrepanz zwischen Realität und Interpretation bzgl. der Labelerweiterung von Vascepa aufzeigen.
Zitat von bernie55: Feuerstein's Kommentar auf Twitter zur Vascepa Zulassung - ( label is restricted ):
Das Posting von A. "Fuckelstone" - der !! Vetreter der Big Short Fraktion schlechthin - sollte einfach noch einmal die Diskrepanz zwischen Realität und Interpretation bzgl. der Labelerweiterung von Vascepa aufzeigen.

Antwort auf Beitrag Nr.: 62.162.907 von bernie55 am 15.12.19 13:02:25
...und auf der AMRN Webseite:
“The FDA approval of icosapent ethyl as an addition to statin therapy to reduce the risk of cardiovascular events is a major milestone in cardiovascular prevention,” said Deepak L. Bhatt, M.D., M.P.H., executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital, professor of medicine at Harvard Medical School, and lead investigator of the REDUCE-IT study which served as the basis for the supplemental New Drug Application to the FDA for VASCEPA. “Nothing this significant has happened in the world of cardiovascular prevention since the introduction of statins nearly three decades ago. Many patients stand to benefit from this historic advance in care.”
https://investor.amarincorp.com/news-releases/news-release-d…
Zitat von bernie55: Dr Bhatt's Kommentar ( a very broad label) bzgl. Vascepa-Zulassung auf Twitter:
...und auf der AMRN Webseite:
“The FDA approval of icosapent ethyl as an addition to statin therapy to reduce the risk of cardiovascular events is a major milestone in cardiovascular prevention,” said Deepak L. Bhatt, M.D., M.P.H., executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital, professor of medicine at Harvard Medical School, and lead investigator of the REDUCE-IT study which served as the basis for the supplemental New Drug Application to the FDA for VASCEPA. “Nothing this significant has happened in the world of cardiovascular prevention since the introduction of statins nearly three decades ago. Many patients stand to benefit from this historic advance in care.”
https://investor.amarincorp.com/news-releases/news-release-d…
Die armen Kerle, die heute zu 27 bis 28 abgezockt wurden vorbörslich.
Das sind noch billige Kurse, die Übernahme wird im kommenden Jahr das Doppelte kosten, Megablockbuster weltweit!
Antwort auf Beitrag Nr.: 62.166.255 von Magnetfeldfredy am 16.12.19 09:20:14Das stimmt!!
Billig?
Die MK ist 9 milliarden Dollar.
Die MK ist 9 milliarden Dollar.
Bei zu erwartenden Umsätzen von bis zu 10 Milliarden weltweit in ein paar Jahren, ist das ein Schnäppchen, Börse handelt Zukunft, und die Übernahme wird es nicht unter 50 US Dollar geben!
Dazu hat Amarin noch mit Vascepa eine Trial laufen für Demenz, Gilead hat Vascepa zur Fettleber in sein Programm mit aufgenommen......
Dazu hat Amarin noch mit Vascepa eine Trial laufen für Demenz, Gilead hat Vascepa zur Fettleber in sein Programm mit aufgenommen......
Antwort auf Beitrag Nr.: 62.166.159 von Magictrader am 16.12.19 09:12:35
Wenn du als MAGIC - Trader denkst und handelst, dann wirst du recht haben.
Wenn du als MAGIC-Longie denkst und handelst , dann glaube ich eher nicht.
Zitat von Magictrader: Die armen Kerle, die heute zu 27 bis 28 abgezockt wurden vorbörslich.
Wenn du als MAGIC - Trader denkst und handelst, dann wirst du recht haben.

Wenn du als MAGIC-Longie denkst und handelst , dann glaube ich eher nicht.

Hat jemand eine Meinung zum Konkurrenten Acasti?
Dafür werden heute morgen irre Preise bezahlt.
Dafür werden heute morgen irre Preise bezahlt.
Vielleicht alles längst im Kurs enthalten und fällt nach den News erstmal 20 % in den kommenden Tagen? Geheimtipp nun wirklich nicht, sell on good news nicht auszuschließen.
H.C. Wainwright keeps $51 target on Amarin with label meeting expectations The FDA on Friday approved Amarin's Vascepa for an expanded label for cardiovascular risk reduction in elevated triglyceride patients. "We now have further clarity on the breakdown of labeling, which we interpret positively in terms of our expectations," H.C. Wainwright analyst Andrew Fein tells investors in a research note. He believes that characterization of triglyceride levels at 150mg/dL or higher did not come as a surprise, despite incorporation of patients with triglyceride levels as low as 135mg/dL in the REDUCE-IT trial. The analyst continues to think physicians can utilize their best judgment in assessing whether to assign the proven benefits of Vascepa in cardiovascular risk reduction when evaluating at-risk patients. Fein points out that his omega-3 proprietary physician survey indicated broad physician support for utilizing Vascepa with an expanded label in CV risk reduction. He reiterates a Buy rating on Amarin shares with a $51 price target. Fein maintains his current Vascepa sales revenue estimates of $416M in 2019 and $663M in 2020. The stock in premarket trading is up 11% to $26.75.
target on Amarin with label meeting expectations The FDA on Friday approved Amarin's Vascepa for an expanded label for cardiovascular risk reduction in elevated triglyceride patients. "We now have further clarity on the breakdown of labeling, which we interpret positively in terms of our expectations," H.C. Wainwright analyst Andrew Fein tells investors in a research note. He believes that characterization of triglyceride levels at 150mg/dL or higher did not come as a surprise, despite incorporation of patients with triglyceride levels as low as 135mg/dL in the REDUCE-IT trial. The analyst continues to think physicians can utilize their best judgment in assessing whether to assign the proven benefits of Vascepa in cardiovascular risk reduction when evaluating at-risk patients. Fein points out that his omega-3 proprietary physician survey indicated broad physician support for utilizing Vascepa with an expanded label in CV risk reduction. He reiterates a Buy rating on Amarin shares with a $51 price target. Fein maintains his current Vascepa sales revenue estimates of $416M in 2019 and $663M in 2020. The stock in premarket trading is up 11% to $26.75.
Read more at:
https://thefly.com/landingPageNews.php?id=3007085
 target on Amarin with label meeting expectations The FDA on Friday approved Amarin's Vascepa for an expanded label for cardiovascular risk reduction in elevated triglyceride patients. "We now have further clarity on the breakdown of labeling, which we interpret positively in terms of our expectations," H.C. Wainwright analyst Andrew Fein tells investors in a research note. He believes that characterization of triglyceride levels at 150mg/dL or higher did not come as a surprise, despite incorporation of patients with triglyceride levels as low as 135mg/dL in the REDUCE-IT trial. The analyst continues to think physicians can utilize their best judgment in assessing whether to assign the proven benefits of Vascepa in cardiovascular risk reduction when evaluating at-risk patients. Fein points out that his omega-3 proprietary physician survey indicated broad physician support for utilizing Vascepa with an expanded label in CV risk reduction. He reiterates a Buy rating on Amarin shares with a $51 price target. Fein maintains his current Vascepa sales revenue estimates of $416M in 2019 and $663M in 2020. The stock in premarket trading is up 11% to $26.75.
target on Amarin with label meeting expectations The FDA on Friday approved Amarin's Vascepa for an expanded label for cardiovascular risk reduction in elevated triglyceride patients. "We now have further clarity on the breakdown of labeling, which we interpret positively in terms of our expectations," H.C. Wainwright analyst Andrew Fein tells investors in a research note. He believes that characterization of triglyceride levels at 150mg/dL or higher did not come as a surprise, despite incorporation of patients with triglyceride levels as low as 135mg/dL in the REDUCE-IT trial. The analyst continues to think physicians can utilize their best judgment in assessing whether to assign the proven benefits of Vascepa in cardiovascular risk reduction when evaluating at-risk patients. Fein points out that his omega-3 proprietary physician survey indicated broad physician support for utilizing Vascepa with an expanded label in CV risk reduction. He reiterates a Buy rating on Amarin shares with a $51 price target. Fein maintains his current Vascepa sales revenue estimates of $416M in 2019 and $663M in 2020. The stock in premarket trading is up 11% to $26.75.Read more at:
https://thefly.com/landingPageNews.php?id=3007085

So ein lächerlicher Arschlochanalyst, die Agenda ist so offensichtlich, dass es schon peinlich wird!😡
Schon sehr entäuschend was da heute manipuliert abläuft, ich bleibe dabei:
Why Amarin Stock Is Jumping Today
Vascepa is now in position to become a blockbuster medication.
George Budwell
George Budwell
(TMFGBudwell)
Dec 16, 2019 at 9:16AM
Author Bio
What happened
Shares of Amarin (NASDAQ:AMRN) jumped by as much as 11.7% in pre-market trading Monday morning. What's driving this sizable move higher ahead of the opening bell?
On Friday, Amarin announced that the Food and Drug Administration had approved Vascepa as an add-on to statin therapy in patients with elevated triglyceride levels who either have established cardiovascular disease or diabetes mellitus with two additional risk factors for cardiovascular disease.
Wooden blocks that spell out the word "billion" arranged on a wooden table top.
Image Source: Getty Images.
So what
While the FDA didn't exactly grant Vascepa the broadest label expansion possible, the drug's new target market still encompasses tens of millions of patients. This sizable commercial opportunity, in fact, could make Vascepa one of the best-selling drugs in the world by the middle of the next decade.
Reflecting this news, Amarin also rolled out its 2020 sales forecast on Friday, guiding for net revenue of between $650 million and $700 million next year. Based on the forecast, Vascepa is on track to achieve blockbuster status (i.e., more than $1 billion in annual sales) as soon as 2021.
Now what
The burning question now is whether Amarin will choose to promote Vascepa's new label by itself, bring in a big-time partner, or sell itself outright. Based on the information available at the moment, Amarin appears set to take a go-it-alone approach -- at least initially.
As proof, the company has been aggressively hiring new pharma reps over the last few months in anticipation of this landmark event. But that doesn't mean that a partnering deal or a buyout won't ultimately materialize. Put simply, shareholders may want to think twice about taking profits on this promising mid-cap biotech stock right now. Another big leg up could be right around the corner.
Why Amarin Stock Is Jumping Today
Vascepa is now in position to become a blockbuster medication.
George Budwell
George Budwell
(TMFGBudwell)
Dec 16, 2019 at 9:16AM
Author Bio
What happened
Shares of Amarin (NASDAQ:AMRN) jumped by as much as 11.7% in pre-market trading Monday morning. What's driving this sizable move higher ahead of the opening bell?
On Friday, Amarin announced that the Food and Drug Administration had approved Vascepa as an add-on to statin therapy in patients with elevated triglyceride levels who either have established cardiovascular disease or diabetes mellitus with two additional risk factors for cardiovascular disease.
Wooden blocks that spell out the word "billion" arranged on a wooden table top.
Image Source: Getty Images.
So what
While the FDA didn't exactly grant Vascepa the broadest label expansion possible, the drug's new target market still encompasses tens of millions of patients. This sizable commercial opportunity, in fact, could make Vascepa one of the best-selling drugs in the world by the middle of the next decade.
Reflecting this news, Amarin also rolled out its 2020 sales forecast on Friday, guiding for net revenue of between $650 million and $700 million next year. Based on the forecast, Vascepa is on track to achieve blockbuster status (i.e., more than $1 billion in annual sales) as soon as 2021.
Now what
The burning question now is whether Amarin will choose to promote Vascepa's new label by itself, bring in a big-time partner, or sell itself outright. Based on the information available at the moment, Amarin appears set to take a go-it-alone approach -- at least initially.
As proof, the company has been aggressively hiring new pharma reps over the last few months in anticipation of this landmark event. But that doesn't mean that a partnering deal or a buyout won't ultimately materialize. Put simply, shareholders may want to think twice about taking profits on this promising mid-cap biotech stock right now. Another big leg up could be right around the corner.
Hier noch einmal - schwarz auf weiß- zu sehen.
Das Gesamtvolumen und die Shortquote vom 16.12.19

Das Gesamtvolumen und die Shortquote vom 16.12.19

Antwort auf Beitrag Nr.: 62.176.650 von bernie55 am 17.12.19 09:37:39Hier sind die ungewöhnliche Aktivitäten bei AMRN am gestrigen Handelstag zu sehen.
Zwei große Blockaufträge kamen um 11:15 Uhr (Pazifikzeit) innerhalb von zwei Sekunden vom Dark Pool, die sich auf rund 800.000 Aktien
vom Dark Pool, die sich auf rund 800.000 Aktien  im Wert von 18 Millionen Dollar
im Wert von 18 Millionen Dollar  beliefen.
beliefen.

https://www.guerillastocktrading.com/stock-trading/dark-pool…
Zwei große Blockaufträge kamen um 11:15 Uhr (Pazifikzeit) innerhalb von zwei Sekunden
 vom Dark Pool, die sich auf rund 800.000 Aktien
vom Dark Pool, die sich auf rund 800.000 Aktien  im Wert von 18 Millionen Dollar
im Wert von 18 Millionen Dollar  beliefen.
beliefen.
https://www.guerillastocktrading.com/stock-trading/dark-pool…
Naja leider so ungewöhnlich ist das alles wahrscheinlich nicht, "selling on good news" habe ich öfter gelesen und ja alle die eher kurzfristigen Profit haben wollten sind jetzt einfach raus da es nun wieder ganz anders gelagerte News geben muss um dem Kurs "kurzfristig" kräftigen Aufwind zu geben.
Klar könnte eine Partnerschaft oder BO jederzeit bald Fakt sein, aber die meisten lesen immer noch heraus Amarin geht erst mal "bewusst" alleine weiter und bis sie nicht bewiesen haben das Ihre Sales zahlen extrem signifikant nun weiter steigen könnte es auch sein das potentielle Übernahme oder Partnerschaftszenarien sich noch in weiterer Ferne befinden.
Die Gefahr das der Kurs zu hoch wird und damit solche Übernahmen nicht mehr finanziell möglich wären ist wohl eher gering wenn man das Verhalten des Kurses über die Monate / Jahre so ansieht.
Ich hätte jetzt auch nicht gedacht das der Abverkauf solche Ausmaße annimmt, aber ich denke der Kurs wird wohl jetzt eine ganze Weile so zwischen 20 und 26 Dollar herumdümpeln außer natürlich es zündet bald wieder eine positive Pressemitteilung... Jedoch denke ich das dass Kanada Approval usw. auch nur 1 bis 2 Dollar Upside bringen wird wie gesagt erst muss mal Amarin zeigen das sie nun das Geschäft auch im grösseren Stil beherrschen und die Verschreibungen weiterhin extrem zunehmen.
Für longs ist alles weiterhin gut und perspektivisch wird hier alles immer besser werden langsam aber stetig
Klar könnte eine Partnerschaft oder BO jederzeit bald Fakt sein, aber die meisten lesen immer noch heraus Amarin geht erst mal "bewusst" alleine weiter und bis sie nicht bewiesen haben das Ihre Sales zahlen extrem signifikant nun weiter steigen könnte es auch sein das potentielle Übernahme oder Partnerschaftszenarien sich noch in weiterer Ferne befinden.
Die Gefahr das der Kurs zu hoch wird und damit solche Übernahmen nicht mehr finanziell möglich wären ist wohl eher gering wenn man das Verhalten des Kurses über die Monate / Jahre so ansieht.
Ich hätte jetzt auch nicht gedacht das der Abverkauf solche Ausmaße annimmt, aber ich denke der Kurs wird wohl jetzt eine ganze Weile so zwischen 20 und 26 Dollar herumdümpeln außer natürlich es zündet bald wieder eine positive Pressemitteilung... Jedoch denke ich das dass Kanada Approval usw. auch nur 1 bis 2 Dollar Upside bringen wird wie gesagt erst muss mal Amarin zeigen das sie nun das Geschäft auch im grösseren Stil beherrschen und die Verschreibungen weiterhin extrem zunehmen.
Für longs ist alles weiterhin gut und perspektivisch wird hier alles immer besser werden langsam aber stetig

Antwort auf Beitrag Nr.: 62.177.370 von flyingbeef am 17.12.19 10:58:39ich würde zu mehr Gelassenheit raten; die jetzigen Kurskapriolen können in einigen Tagen bereits vergessen sein, wenn der Kurs langsam aber stetig zu steigen beginnt. Das Unrternehmen hat ja bereits mitgeteilt, dass die Produktionskapazitäten derzeit genügen, um den Markt mit Vascepa in der Grössenordnung von 5 Milliarden$/Jahr bedienen zu können. Wenn die Verkaufserlöse nun stetig zuehmen, wird der Kurs nicht zurückbleiben.
Deswegen: KEPP COOL!
Deswegen: KEPP COOL!
Antwort auf Beitrag Nr.: 62.177.556 von Cyberhexe am 17.12.19 11:16:29KEEP COOL...natürlich 

Recht hast!
haha ist ja verrückt, dachte steige nach den positiven News bei knapp 30 aus und nehme Gewinne mit, nix da  jetzt habe ich nochmal bisschen nachgekauft... bei den peak sales bin ich eher konservativ ($2-3 Mrd.), ausserdem kommen ab 2029 Generikas auf den Markt, darum finde ich eine MK von ca. $10 Mrd. ok (=$28), bei einem Buyout könnte es natürlich noch deutlich mehr geben
jetzt habe ich nochmal bisschen nachgekauft... bei den peak sales bin ich eher konservativ ($2-3 Mrd.), ausserdem kommen ab 2029 Generikas auf den Markt, darum finde ich eine MK von ca. $10 Mrd. ok (=$28), bei einem Buyout könnte es natürlich noch deutlich mehr geben
 jetzt habe ich nochmal bisschen nachgekauft... bei den peak sales bin ich eher konservativ ($2-3 Mrd.), ausserdem kommen ab 2029 Generikas auf den Markt, darum finde ich eine MK von ca. $10 Mrd. ok (=$28), bei einem Buyout könnte es natürlich noch deutlich mehr geben
jetzt habe ich nochmal bisschen nachgekauft... bei den peak sales bin ich eher konservativ ($2-3 Mrd.), ausserdem kommen ab 2029 Generikas auf den Markt, darum finde ich eine MK von ca. $10 Mrd. ok (=$28), bei einem Buyout könnte es natürlich noch deutlich mehr geben
Antwort auf Beitrag Nr.: 62.181.744 von paul81 am 17.12.19 18:44:05Ich kann mir vorstellen, dass das Gap bei ca. 17$ noch geschlossen wird.
Ich bin bei 19,2 € eingestiegen und bei 25,5 € wieder alles verkauft. Da ich jetzt bei LYNX bin , habe ich dann bei 20,8 DOLLAR wieder anständig eingekauft. Aktuell 4 % Verlust, aber das ist zu verschmerzen. Das Wellen reiten mit AMARIN macht Spaß. :-) Spannender ist nur TRANSCANNA
Hätte nix dagegen, Amarin für 50 US Dollar übernommen zu sehen:
10 Biotech M&A Targets Under The Scanner For 2020
[Benzinga]
Shanthi Rexaline
,Benzinga•December 24, 2019
Biopharma M&A activity scaled record highs in 2019, with a few mega mergers announced involving names such as Bristol-Myers Squibb Co (NYSE: BMY), Roche Holdings AG Basel ADR (OTC: RHHBY) and AbbVie Inc (NYSE: ABBV).
It's feared that the frenetic pace of M&A in the sector could slow down in the coming year.
Yet there are fundamental reasons to think otherwise: there could at least be many more bolt-on acquisitions as companies, especially cash-rich big pharma companies with an ageing pipeline, strive to reinvigorate slowing growth.
The upcoming year is likely to see many hostile deals, Evaluate Pharma reported, citing Steven Slaughter, managing director of Manulife Investment Management.
"There are enough interesting targets where management teams don't want to sell, but in the large-cap world balance sheets are becoming so large they just have to deploy some capital," Slaughter was quoted as saying at the Jefferies Healthcare Conference.
Increased scrutiny by regulators could serve as a dampener. In 2019, the FTC made several second requests, requiring additional information for vetting M&A transactions.
10 Biotech M&A Targets For 2020
The following are potential targets that could find themselves in the M&A mix in the coming year.
Gene editing company Crispr Therapeutics AG (NASDAQ: CRSP) is speculated to be a potential target for Vertex Pharmaceuticals Incorporated (NASDAQ: VRTX), given the licensing agreement in place between the companies. Incidentally, Vertex itself is considered an M&A target.
Alnylam Pharmaceuticals, Inc. (NASDAQ: ALNY), which has the first-ever FDA-approved RNAi therapeutic in Onpattro, is also a potential M&A target. Analysts single out Sanofi SA (NASDAQ: SNY) as the likely pursuer, given the stake it has in Alnylam. It should be noted here that Sanofi recently agreed to buy Synthorx Inc (NASDAQ: THOR) for $2.5 billion.
Amarin Corporation's (NASDAQ: AMRN) attraction as an M&A target has increased following the FDA nod for an expanded indication for its fish oil pill Vascepa.
Incyte Corporation (NASDAQ: INCY), which has a blockbuster commercial product in Jakafi.
Global Blood Therapeutics Inc (NASDAQ: GBT), which recently received the FDA nod for its sickle cell disease treatment Oxbryta
Several gene therapy companies are discussed as M&A prospects. Notable among them are:
Uniqure NV (NASDAQ: QURE).
Sarepta Therapeutics Inc (NASDAQ: SRPT). Following the recent licensing agreement with Roche, the probability of an outright sale has fallen.
Regenxbio Inc (NASDAQ: RGNX)
Solid Biosciences Inc (NASDAQ: SLDB)
BioMarin Pharmaceutical Inc. (NASDAQ: BMRN)
0
See more from Benzinga
A Perspective On Biopharma's Record M&A Run In 2019
The Daily Biotech Pulse: Roche Gets US Antitrust Clearance For Spark Purchase, An Orphan Drug Designation For Prevail, Dynavax Names CEO
After Amarin Snags Vascepa Label Expansion, Analyst Says Biopharma An Attractive M&A Target
© 2019 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
10 Biotech M&A Targets Under The Scanner For 2020
[Benzinga]
Shanthi Rexaline
,Benzinga•December 24, 2019
Biopharma M&A activity scaled record highs in 2019, with a few mega mergers announced involving names such as Bristol-Myers Squibb Co (NYSE: BMY), Roche Holdings AG Basel ADR (OTC: RHHBY) and AbbVie Inc (NYSE: ABBV).
It's feared that the frenetic pace of M&A in the sector could slow down in the coming year.
Yet there are fundamental reasons to think otherwise: there could at least be many more bolt-on acquisitions as companies, especially cash-rich big pharma companies with an ageing pipeline, strive to reinvigorate slowing growth.
The upcoming year is likely to see many hostile deals, Evaluate Pharma reported, citing Steven Slaughter, managing director of Manulife Investment Management.
"There are enough interesting targets where management teams don't want to sell, but in the large-cap world balance sheets are becoming so large they just have to deploy some capital," Slaughter was quoted as saying at the Jefferies Healthcare Conference.
Increased scrutiny by regulators could serve as a dampener. In 2019, the FTC made several second requests, requiring additional information for vetting M&A transactions.
10 Biotech M&A Targets For 2020
The following are potential targets that could find themselves in the M&A mix in the coming year.
Gene editing company Crispr Therapeutics AG (NASDAQ: CRSP) is speculated to be a potential target for Vertex Pharmaceuticals Incorporated (NASDAQ: VRTX), given the licensing agreement in place between the companies. Incidentally, Vertex itself is considered an M&A target.
Alnylam Pharmaceuticals, Inc. (NASDAQ: ALNY), which has the first-ever FDA-approved RNAi therapeutic in Onpattro, is also a potential M&A target. Analysts single out Sanofi SA (NASDAQ: SNY) as the likely pursuer, given the stake it has in Alnylam. It should be noted here that Sanofi recently agreed to buy Synthorx Inc (NASDAQ: THOR) for $2.5 billion.
Amarin Corporation's (NASDAQ: AMRN) attraction as an M&A target has increased following the FDA nod for an expanded indication for its fish oil pill Vascepa.
Incyte Corporation (NASDAQ: INCY), which has a blockbuster commercial product in Jakafi.
Global Blood Therapeutics Inc (NASDAQ: GBT), which recently received the FDA nod for its sickle cell disease treatment Oxbryta
Several gene therapy companies are discussed as M&A prospects. Notable among them are:
Uniqure NV (NASDAQ: QURE).
Sarepta Therapeutics Inc (NASDAQ: SRPT). Following the recent licensing agreement with Roche, the probability of an outright sale has fallen.
Regenxbio Inc (NASDAQ: RGNX)
Solid Biosciences Inc (NASDAQ: SLDB)
BioMarin Pharmaceutical Inc. (NASDAQ: BMRN)
0
See more from Benzinga
A Perspective On Biopharma's Record M&A Run In 2019
The Daily Biotech Pulse: Roche Gets US Antitrust Clearance For Spark Purchase, An Orphan Drug Designation For Prevail, Dynavax Names CEO
After Amarin Snags Vascepa Label Expansion, Analyst Says Biopharma An Attractive M&A Target
© 2019 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Biotech In 2020: M&A And Gene Therapy In Focus
Dec. 27, 2019 7:30 AM ET |
About: Amarin Corporation plc (AMRN), ATHX, ICPT, MYOV, RCKT, IBB, XBI, Includes: ACHN, ALXN, ARQL, AVRO, BOLD, CRSP, EDIT, GLW, MRK, NTLA, THMO, TMDX
SA Marketplace
SA Marketplace
Liver Therapy Forum (LTF)
A liver biomedical scientist’s insight into the liver therapeutics market
(11,909 followers)
Summary
Our biotech Roundtable looks at the growth sector that has had a strong finish to 2019.
Gene therapy is one topic that gets a lot of attention, with the sector interest backed up by M&A.
But our panel also talks about the importance of patience and staying focused in a volatile space.
2019 finishes with an extra kick for investors. Part of that is the continued strong run of the market, erasing memories of last year's near bear market and extending the decade-long bull. There also are plenty of market headlines and events that have sprung over the end of the year, from Phase 1 trade deals to M&A to political whirlwinds and more.
More trivially, the 2010s are ending and the 2020s begin. It's been quite a decade for equity investors, with the S&P 500 returning more than 250% in that time. Even underperformance could leave a portfolio in good shape, and any alpha that was found would really leave investors well off.
Where does that leave investors and the markets for 2020, at the start of a new decade? That's what we try to answer in our annual Marketplace Roundtable series. We are publishing roundtable discussions featuring more than 80 authors from across the spectrum of investing styles and focuses you find on Seeking Alpha: Macro to value investing, small cap to energy, gold to quant and alternative strategies, and more.
Today's discussion focuses on the Biotech sector, which finished the year strong after a slow start. We're featuring the following panel:
Jonathan Faison, author of ROTY
Bhavneesh Sharma, author of Vasuda Healthcare Analytics
Stephen Ayers, author of The Formula
Terry Chrisomalis, author of Biotech Analysis Central
First Genesis Consulting, author of Liver Therapy Forum (LTF)
Wall Street Titan, author of Stem Cell News and Analysis
Questions are in bold header font, disclosures are available at the end of the article.
The stock market has had another strong year, recovering from last year’s Q4 correction. The S&P 500 has returned 10% annualized over the last two years, as of early December. Yet there’s still a sense that the music is due to stop among many investors (see the theme of "most hated bull market"). Where do you fall out?
Bhavneesh Sharma: Expecting 3400 PT for SPX in 2020.
Stephen Ayers: I agree that some stocks are looking quite expensive now. That's why it's especially important to always have a keen eye out for value.
Terry Chrisomalis: I believe that the market will continue to head higher. This is on the basis of the strong economic reports that have been coming in terms of jobs, unemployment rate falling, and many other positive developments like phase 1 of the China trade deal. I still see a bullish sentiment for the markets heading into 2020.
First Genesis Consulting: Ongoing innovation is an important tool for remaining competitive in the biopharmaceutical arena. The trailblazers are cognizant of the risks associated with moving paradigm-shifting drug concepts into clinical trials. I manage the fallout by discerning those trailblazers, foundational goals and the varying paths to clinical success. I avoid going with the flow but I make my flow based on my insights. Drug candidates are never identical but could have a similar pharmacological target.
Wall Street Titan: Having been a young employee in the trading room of Bear Stearns during the crash of 1987, I have experienced many market cycles. That experience puts me in the group with the cautious. One can never know when a lingering problem in the basement of concerns will rise into a perceived crisis. The growing national debt and exploding budget deficit during a healthy economy should be a cause for concern for everyone. When the Republican Party, once hawks on deficit spending, now ignore this issue, it's something to be concerned about. What happens to this structural deficit when the economy weakens? The music will stop when the reality of the exploding national debt raises its ugly head, until then its still party time.
What’s a lesson you learned in 2019?
Jonathan Faison: Slow down: You don't have to nail them all. Focus on just a few ideas that interest you for deep due diligence, establishing positions where you believe you have an edge or advantage.
Bhavneesh Sharma: Markets can stay irrational for long and don't fight the Fed.
Stephen Ayers: Patience pays off! If you are convicted in an idea, stay with it until the story plays out.
First Genesis Consulting: Developed a bridge/gap plan during the long drought associated with clinical trials to reduce investor impatience. It can be very challenging to encourage investors on focus on the long-term rather than the short-term goals.
Biotech is finishing the year on a strong note, with Q4 providing most of its performance this year. What's going on?
Jonathan Faison: Perfect storm of funds and momentum money chasing the sector, combined with big pharmaceutical companies buying up small companies and drugs to replenish their pipelines as patent cliffs approach.
Bhavneesh Sharma: Strong M&A activity is fueling the surge in the sector.
Stephen Ayers: There are a few things going on that are positively influencing the sector at the moment. For one, drugs are getting approved faster than ever by the FDA. Two, big pharma is paying big premium for acquisitions (e.g. ASrQule (NASDAQ:ARQL), Audentes (NASDAQ:BOLD)). And three, we are seeing a lot of technological advancement that's just now beginning to change the landscape of biotechnology. Ideas like "targeted oncology" and "gene therapy" are beginning to enter their heydays.
Terry Chrisomalis: The strong surge in biotech has come about because of strong M&A deals. Especially, in the last few months. Alexion Pharmaceuticals (ALXN) paid $930 million to acquire Achillion pharmaceuticals (ACHN) for the treatment of diseases affected by the complement system. Merck (MRK) wanted to get its hands on a precision oncology biotech by the name of ArQule (ARQL) and it spent $2.7 billion to do so. Besides outright acquisitions, there also have been an increase of licensing deals as well. For example, XBiotech out-licensed its antibody Bermekimab for dermatological indications for $1.35 billion. I expect more deals like these to continue in 2020.
First Genesis Consulting: Many factors are at play from therapeutic innovation from biopharmaceutical/biotech companies to emerging solutions to the global conflict between countries and continents.
Healthcare remains one of the hottest topics in the political arena as we enter an election year. What are you watching for or expecting?
Bhavneesh Sharma: I don't expect much progress to be made in drug price control, so I'm bullish on the sector.
First Genesis Consulting: Politicians are not known for being truthful. However, I would like to see the specific workable plans to lowering prescription drug prices. This would have significant bearing on biotech stocks.
Wall Street Titan: The affordability of healthcare is the hot button issue of our time. It's well established that the United States has the highest level of healthcare spending per GDP of any other country. Politics plays the biggest role in how we got here and the lobbying power of the healthcare industry has been effective in getting keeping the status quo. Some of the 2020 Democratic presidential candidates have put out plans that basically socialize parts of the healthcare system. In the end, I expect that someone from the moderate branch of the Democratic party to move ahead against President Trump in 2020. However, this is an area that must be watched closely.
What technologies or areas of study have your attention currently?
Jonathan Faison: Gene therapy, "picks and shovels" plays, large orphan markets, drugs with a clear leg up over competition.
Bhavneesh Sharma: Gene therapy, allogeneic cell therapies in cancer.
First Genesis Consulting: Therapeutics for liver and rare diseases.
Wall Street Titan: Immunotherapy harnesses the immune system of a patient and re-engineers it as a weapon. The greatest success seen is in the clinic is a therapy called CAR-T where a T-cell is modified using chimeric antigen receptors to create CAR-T cells that target cancer. However, the manufacturing cost of CAR-T therapies is astronomical due to the personalized nature of the therapy. ThermoGenesis Holdings, Inc. (THMO) is a company I recently added to my portfolio that has developed a platform that can reduce the manufacturing costs of CAR-T therapies called CAR-TXpress. The key to the platform is a proprietary technology called Buoyancy-Activated Cell Sorting (BACS) whereby lipid microbubbles can be made to bind to targeted immune cells for more efficient cell separation during centrifugation. The company claims CAR-TXpress can reduce CAR-T manufacturing costs by 2/3. ThermoGenesis recently signed a distribution agreement with Corning (GLW) and that should make 2020 an interesting year for them.
What’s a story you think the market will be paying more attention to in the next year in biotech?
Jonathan Faison: This is more of a medical device story, but TransMedics (TMDX) is of interest due to the possibility of addressing the huge problem of low supply of available donor organs and low utilization rates (65% to 80% of donated organs are wasted). Clinical trial data for Organ Care System (OCS) has shown double to triple utilization rates for donor organs and improved outcomes (50% or more reduction in post-transplant complications). They have key regulatory decisions for OCS Heart and pivotal data for OCS liver in 2020. We also have a couple ROTY members visiting the CFO to interview him with questions from our community. The movie John Q comes to mind.
Bhavneesh Sharma: Gene therapy.
Stephen Ayers: AVROBIO (AVRO) is developing a lentiviral-based gene therapy for Fabry disease. Fabry disease is a genetic disorder characterized by an enzyme deficiency, alpha-galactosidase A. The lack of alpha-galactosidase A causes a chronic buildup in globotriaosylceramide, a type of fat. Over time, the disease wreaks havoc on the entire body and decreases life expectancy by nearly 20 years (compared to the average, healthy male). Current treatments are limited to enzyme replacement therapies, but these permit bouts of severe disease activity and require chronic dosing. RD-01 is a one-time dose that appears safe and, based on phase 1/2 data, to deliver consistent control of the disease. Continued success could see a quick entry into the market, which would, assuredly, change the treatment landscape of Fabry disease. The company is expected to slowly leak more data over the course of the next several months. The market has been slow to catch on, as AVROBIO's valuation is pinned under $500M.
First Genesis Consulting: NASH. The reason being that we could see the first ever FDA approved therapeutics for this chronic progressive liver disease.
Wall Street Titan: As gene editing moves forward into the clinic, the companies focused on CRISPR-CAS9 (What are genome editing and CRISPR-Cas9?) should garner more attention. The idea that mankind may be able to successfully repair defective DNA is still mind blowing. It wasn't that long ago when this would have been considered science fiction, not science. Charles Darwin would have surely laughed at the notion. However, CRISPR-CAS9 is still in its early stages and editing the human genome is fraught with risk such as off target edits. The market will be paying attention to see how leaders, Editas Medicine (EDIT), CRISPR Therapeutics AG (CRSP) and Intellia Therapeutics (NTLA) progress and advance their pipelines in 2020.
What do you think the most significant event in the sector this year was?
Bhavneesh Sharma: Gene therapy came back as a focus sub-sector after being beaten down for almost two decades.
First Genesis Consulting: FDA approval of innovative gene therapies for rare pediatric diseases.
What’s a favorite idea for 2020, and what’s the story?
Jonathan Faison: After $3 billion buyout of Audentes Therapeutics (BOLD), gene therapy continues to be in focus. We've held Rocket Pharmaceuticals (RCKT) since $12ish, and initial data sets in Fanconi Anemia and LAD:I have not disappointed. Danon Disease phase 1 data in 2020 will be a key catalyst to look forward to as well.
Bhavneesh Sharma: Small caps and biotechs have been underperforming the market in 2019 and recovering now. Expecting them to outperform in 2020.
Stephen Ayers: I am still really bullish on Amarin Corporation (AMRN). Their drug, Vascepa, could be the first of its kind to secure a cardiovascular risk reduction label. Because it can be used in combination with other drugs that also reduce cardiovascular risk (e.g. Lipitor), it will not run into any relevant competition. Additionally, its label should be quite inclusive. And especially because the drug has been on the market for years already, the market uptake following broad label approval (the drug is, currently, only approved for very high triglyceride levels) should boom. Even without the extended indication, we are already seeing high demands as scripts continue to grow. For the quarter ending Sept. 31, Amarin saw 100%-plus product revenue growth, compared to the same period in 2018.
All in all, Vascepa is a blockbuster drug bound to change the treatment landscape of cardiovascular disease that should easily procure over $3B in peak annual revenue. Amarin's current enterprise value of $7.75B (as of December 11, 2019) is yet to fully recognize the potential. Do not be surprised to see Amarin acquired in 2020.
Terry Chrisomalis: My favorite idea for 2020 would be a biotech by the name of Myovant Sciences (MYOV). That's because it had surprisingly solid results in its phase 3 HERO study treating men with advanced prostate cancer. It was noted that 96.7% of men who took relugolix had achieved sustained testosterone suppression to castrate levels, which was the primary endpoint of the study. With the primary endpoint met, the biotech expects to file a NDA to the FDA for relugolix approval in the treatment of men with advanced prostate cancer. The filing of this NDA is expected in Q2 of 2020. Relugolix is being evaluated for two other large market indications. These other indications are Uterine fibroids and endometriosis. Positive results were already reported for both LIBERTY studies (LIBERTY-1 and LIBERTY-2) using relugolix combination regimen in women with uterine fibroids and menstrual bleeding. These two positive studies, along with another bioequivalence study, which is expected in the company's calendar Q1 2020, will allow an NDA filing for relugolix of this program as well by April of 2020. Another catalyst for this program is a marketing authorization application (NYSE:MAA) for the relugolix combination for treating women with uterine fibroids and menstrual bleeding by Q1 2020. Lastly, another set of catalysts involve readouts from additional late-stage studies known as SPIRIT-2 and SPIRIT-1. For both of these studies, relugolix combination therapy is being explored in women with endometriosis pain. The data readout for SPIRIT-2 is expected Q1 of calendar year 2020 and then SPIRIT-1 is expected Q2 of calendar year 2020. With a strong pipeline and multitude of upcoming catalysts in the early part of next year, I believe there is substantial upside for investors. Which is why I believe it is a top idea for 2020.
First Genesis Consulting: Obeticholic acid(OCA) by Intercept (ICPT) granted FDA approval as the first-ever approved therapeutics for NASH an emerging global epidemic. As a company, Intercept has done clinical wonders for the scientific and clinical communities by bringing liver research and therapeutics to the forefront. Intercept gave a clinical voice to orphan liver diseases and non-viral liver diseases with great unmet needs, an area of medicine that big pharma typically stayed away from due to perceived lack of profitability. This is reflected in the Breakthrough Designation by the FDA for OCA in NASH.
Wall Street Titan: I have covered Athersys (ATHX) on SA for a number of years and, like all long-term shareholders, have been disappointed by a clinical timeline that has extended out much longer than anyone anticipated. However, this should be the year that we see partner data out of Japan in the huge unmet medical need of ischemic stroke using the company's regenerative stem cell product called MultiStem. MultiStem is a type of stem cell that has been shown to modulate the hyperinflammation in the brain that arises after a stroke and does great damage beyond the clot. MultiStem also creates a more favorable environment to promote neurogenesis, angiogenesis and healing. I did a deep dive into the science behind this program in a 2016 article that reviews the Phase II results and my rationale for optimism. We should see results from stroke in 2020 and they could be a game changer. Athersys also has an advanced program in Japan in Acute Respiratory Distress Syndrome that has shown great promise in a Phase II trial. Both Japan trials are funded by partner Healios KK, (Healios K.K.) a leading Japan based stem cell biotech that's betting its entire future on MultiStem's success and has made a substantial financial commitment to the Athersys programs. Healios is the largest shareholder of Athersys.
Dec. 27, 2019 7:30 AM ET |
About: Amarin Corporation plc (AMRN), ATHX, ICPT, MYOV, RCKT, IBB, XBI, Includes: ACHN, ALXN, ARQL, AVRO, BOLD, CRSP, EDIT, GLW, MRK, NTLA, THMO, TMDX
SA Marketplace
SA Marketplace
Liver Therapy Forum (LTF)
A liver biomedical scientist’s insight into the liver therapeutics market
(11,909 followers)
Summary
Our biotech Roundtable looks at the growth sector that has had a strong finish to 2019.
Gene therapy is one topic that gets a lot of attention, with the sector interest backed up by M&A.
But our panel also talks about the importance of patience and staying focused in a volatile space.
2019 finishes with an extra kick for investors. Part of that is the continued strong run of the market, erasing memories of last year's near bear market and extending the decade-long bull. There also are plenty of market headlines and events that have sprung over the end of the year, from Phase 1 trade deals to M&A to political whirlwinds and more.
More trivially, the 2010s are ending and the 2020s begin. It's been quite a decade for equity investors, with the S&P 500 returning more than 250% in that time. Even underperformance could leave a portfolio in good shape, and any alpha that was found would really leave investors well off.
Where does that leave investors and the markets for 2020, at the start of a new decade? That's what we try to answer in our annual Marketplace Roundtable series. We are publishing roundtable discussions featuring more than 80 authors from across the spectrum of investing styles and focuses you find on Seeking Alpha: Macro to value investing, small cap to energy, gold to quant and alternative strategies, and more.
Today's discussion focuses on the Biotech sector, which finished the year strong after a slow start. We're featuring the following panel:
Jonathan Faison, author of ROTY
Bhavneesh Sharma, author of Vasuda Healthcare Analytics
Stephen Ayers, author of The Formula
Terry Chrisomalis, author of Biotech Analysis Central
First Genesis Consulting, author of Liver Therapy Forum (LTF)
Wall Street Titan, author of Stem Cell News and Analysis
Questions are in bold header font, disclosures are available at the end of the article.
The stock market has had another strong year, recovering from last year’s Q4 correction. The S&P 500 has returned 10% annualized over the last two years, as of early December. Yet there’s still a sense that the music is due to stop among many investors (see the theme of "most hated bull market"). Where do you fall out?
Bhavneesh Sharma: Expecting 3400 PT for SPX in 2020.
Stephen Ayers: I agree that some stocks are looking quite expensive now. That's why it's especially important to always have a keen eye out for value.
Terry Chrisomalis: I believe that the market will continue to head higher. This is on the basis of the strong economic reports that have been coming in terms of jobs, unemployment rate falling, and many other positive developments like phase 1 of the China trade deal. I still see a bullish sentiment for the markets heading into 2020.
First Genesis Consulting: Ongoing innovation is an important tool for remaining competitive in the biopharmaceutical arena. The trailblazers are cognizant of the risks associated with moving paradigm-shifting drug concepts into clinical trials. I manage the fallout by discerning those trailblazers, foundational goals and the varying paths to clinical success. I avoid going with the flow but I make my flow based on my insights. Drug candidates are never identical but could have a similar pharmacological target.
Wall Street Titan: Having been a young employee in the trading room of Bear Stearns during the crash of 1987, I have experienced many market cycles. That experience puts me in the group with the cautious. One can never know when a lingering problem in the basement of concerns will rise into a perceived crisis. The growing national debt and exploding budget deficit during a healthy economy should be a cause for concern for everyone. When the Republican Party, once hawks on deficit spending, now ignore this issue, it's something to be concerned about. What happens to this structural deficit when the economy weakens? The music will stop when the reality of the exploding national debt raises its ugly head, until then its still party time.
What’s a lesson you learned in 2019?
Jonathan Faison: Slow down: You don't have to nail them all. Focus on just a few ideas that interest you for deep due diligence, establishing positions where you believe you have an edge or advantage.
Bhavneesh Sharma: Markets can stay irrational for long and don't fight the Fed.
Stephen Ayers: Patience pays off! If you are convicted in an idea, stay with it until the story plays out.
First Genesis Consulting: Developed a bridge/gap plan during the long drought associated with clinical trials to reduce investor impatience. It can be very challenging to encourage investors on focus on the long-term rather than the short-term goals.
Biotech is finishing the year on a strong note, with Q4 providing most of its performance this year. What's going on?
Jonathan Faison: Perfect storm of funds and momentum money chasing the sector, combined with big pharmaceutical companies buying up small companies and drugs to replenish their pipelines as patent cliffs approach.
Bhavneesh Sharma: Strong M&A activity is fueling the surge in the sector.
Stephen Ayers: There are a few things going on that are positively influencing the sector at the moment. For one, drugs are getting approved faster than ever by the FDA. Two, big pharma is paying big premium for acquisitions (e.g. ASrQule (NASDAQ:ARQL), Audentes (NASDAQ:BOLD)). And three, we are seeing a lot of technological advancement that's just now beginning to change the landscape of biotechnology. Ideas like "targeted oncology" and "gene therapy" are beginning to enter their heydays.
Terry Chrisomalis: The strong surge in biotech has come about because of strong M&A deals. Especially, in the last few months. Alexion Pharmaceuticals (ALXN) paid $930 million to acquire Achillion pharmaceuticals (ACHN) for the treatment of diseases affected by the complement system. Merck (MRK) wanted to get its hands on a precision oncology biotech by the name of ArQule (ARQL) and it spent $2.7 billion to do so. Besides outright acquisitions, there also have been an increase of licensing deals as well. For example, XBiotech out-licensed its antibody Bermekimab for dermatological indications for $1.35 billion. I expect more deals like these to continue in 2020.
First Genesis Consulting: Many factors are at play from therapeutic innovation from biopharmaceutical/biotech companies to emerging solutions to the global conflict between countries and continents.
Healthcare remains one of the hottest topics in the political arena as we enter an election year. What are you watching for or expecting?
Bhavneesh Sharma: I don't expect much progress to be made in drug price control, so I'm bullish on the sector.
First Genesis Consulting: Politicians are not known for being truthful. However, I would like to see the specific workable plans to lowering prescription drug prices. This would have significant bearing on biotech stocks.
Wall Street Titan: The affordability of healthcare is the hot button issue of our time. It's well established that the United States has the highest level of healthcare spending per GDP of any other country. Politics plays the biggest role in how we got here and the lobbying power of the healthcare industry has been effective in getting keeping the status quo. Some of the 2020 Democratic presidential candidates have put out plans that basically socialize parts of the healthcare system. In the end, I expect that someone from the moderate branch of the Democratic party to move ahead against President Trump in 2020. However, this is an area that must be watched closely.
What technologies or areas of study have your attention currently?
Jonathan Faison: Gene therapy, "picks and shovels" plays, large orphan markets, drugs with a clear leg up over competition.
Bhavneesh Sharma: Gene therapy, allogeneic cell therapies in cancer.
First Genesis Consulting: Therapeutics for liver and rare diseases.
Wall Street Titan: Immunotherapy harnesses the immune system of a patient and re-engineers it as a weapon. The greatest success seen is in the clinic is a therapy called CAR-T where a T-cell is modified using chimeric antigen receptors to create CAR-T cells that target cancer. However, the manufacturing cost of CAR-T therapies is astronomical due to the personalized nature of the therapy. ThermoGenesis Holdings, Inc. (THMO) is a company I recently added to my portfolio that has developed a platform that can reduce the manufacturing costs of CAR-T therapies called CAR-TXpress. The key to the platform is a proprietary technology called Buoyancy-Activated Cell Sorting (BACS) whereby lipid microbubbles can be made to bind to targeted immune cells for more efficient cell separation during centrifugation. The company claims CAR-TXpress can reduce CAR-T manufacturing costs by 2/3. ThermoGenesis recently signed a distribution agreement with Corning (GLW) and that should make 2020 an interesting year for them.
What’s a story you think the market will be paying more attention to in the next year in biotech?
Jonathan Faison: This is more of a medical device story, but TransMedics (TMDX) is of interest due to the possibility of addressing the huge problem of low supply of available donor organs and low utilization rates (65% to 80% of donated organs are wasted). Clinical trial data for Organ Care System (OCS) has shown double to triple utilization rates for donor organs and improved outcomes (50% or more reduction in post-transplant complications). They have key regulatory decisions for OCS Heart and pivotal data for OCS liver in 2020. We also have a couple ROTY members visiting the CFO to interview him with questions from our community. The movie John Q comes to mind.
Bhavneesh Sharma: Gene therapy.
Stephen Ayers: AVROBIO (AVRO) is developing a lentiviral-based gene therapy for Fabry disease. Fabry disease is a genetic disorder characterized by an enzyme deficiency, alpha-galactosidase A. The lack of alpha-galactosidase A causes a chronic buildup in globotriaosylceramide, a type of fat. Over time, the disease wreaks havoc on the entire body and decreases life expectancy by nearly 20 years (compared to the average, healthy male). Current treatments are limited to enzyme replacement therapies, but these permit bouts of severe disease activity and require chronic dosing. RD-01 is a one-time dose that appears safe and, based on phase 1/2 data, to deliver consistent control of the disease. Continued success could see a quick entry into the market, which would, assuredly, change the treatment landscape of Fabry disease. The company is expected to slowly leak more data over the course of the next several months. The market has been slow to catch on, as AVROBIO's valuation is pinned under $500M.
First Genesis Consulting: NASH. The reason being that we could see the first ever FDA approved therapeutics for this chronic progressive liver disease.
Wall Street Titan: As gene editing moves forward into the clinic, the companies focused on CRISPR-CAS9 (What are genome editing and CRISPR-Cas9?) should garner more attention. The idea that mankind may be able to successfully repair defective DNA is still mind blowing. It wasn't that long ago when this would have been considered science fiction, not science. Charles Darwin would have surely laughed at the notion. However, CRISPR-CAS9 is still in its early stages and editing the human genome is fraught with risk such as off target edits. The market will be paying attention to see how leaders, Editas Medicine (EDIT), CRISPR Therapeutics AG (CRSP) and Intellia Therapeutics (NTLA) progress and advance their pipelines in 2020.
What do you think the most significant event in the sector this year was?
Bhavneesh Sharma: Gene therapy came back as a focus sub-sector after being beaten down for almost two decades.
First Genesis Consulting: FDA approval of innovative gene therapies for rare pediatric diseases.
What’s a favorite idea for 2020, and what’s the story?
Jonathan Faison: After $3 billion buyout of Audentes Therapeutics (BOLD), gene therapy continues to be in focus. We've held Rocket Pharmaceuticals (RCKT) since $12ish, and initial data sets in Fanconi Anemia and LAD:I have not disappointed. Danon Disease phase 1 data in 2020 will be a key catalyst to look forward to as well.
Bhavneesh Sharma: Small caps and biotechs have been underperforming the market in 2019 and recovering now. Expecting them to outperform in 2020.
Stephen Ayers: I am still really bullish on Amarin Corporation (AMRN). Their drug, Vascepa, could be the first of its kind to secure a cardiovascular risk reduction label. Because it can be used in combination with other drugs that also reduce cardiovascular risk (e.g. Lipitor), it will not run into any relevant competition. Additionally, its label should be quite inclusive. And especially because the drug has been on the market for years already, the market uptake following broad label approval (the drug is, currently, only approved for very high triglyceride levels) should boom. Even without the extended indication, we are already seeing high demands as scripts continue to grow. For the quarter ending Sept. 31, Amarin saw 100%-plus product revenue growth, compared to the same period in 2018.
All in all, Vascepa is a blockbuster drug bound to change the treatment landscape of cardiovascular disease that should easily procure over $3B in peak annual revenue. Amarin's current enterprise value of $7.75B (as of December 11, 2019) is yet to fully recognize the potential. Do not be surprised to see Amarin acquired in 2020.

Terry Chrisomalis: My favorite idea for 2020 would be a biotech by the name of Myovant Sciences (MYOV). That's because it had surprisingly solid results in its phase 3 HERO study treating men with advanced prostate cancer. It was noted that 96.7% of men who took relugolix had achieved sustained testosterone suppression to castrate levels, which was the primary endpoint of the study. With the primary endpoint met, the biotech expects to file a NDA to the FDA for relugolix approval in the treatment of men with advanced prostate cancer. The filing of this NDA is expected in Q2 of 2020. Relugolix is being evaluated for two other large market indications. These other indications are Uterine fibroids and endometriosis. Positive results were already reported for both LIBERTY studies (LIBERTY-1 and LIBERTY-2) using relugolix combination regimen in women with uterine fibroids and menstrual bleeding. These two positive studies, along with another bioequivalence study, which is expected in the company's calendar Q1 2020, will allow an NDA filing for relugolix of this program as well by April of 2020. Another catalyst for this program is a marketing authorization application (NYSE:MAA) for the relugolix combination for treating women with uterine fibroids and menstrual bleeding by Q1 2020. Lastly, another set of catalysts involve readouts from additional late-stage studies known as SPIRIT-2 and SPIRIT-1. For both of these studies, relugolix combination therapy is being explored in women with endometriosis pain. The data readout for SPIRIT-2 is expected Q1 of calendar year 2020 and then SPIRIT-1 is expected Q2 of calendar year 2020. With a strong pipeline and multitude of upcoming catalysts in the early part of next year, I believe there is substantial upside for investors. Which is why I believe it is a top idea for 2020.
First Genesis Consulting: Obeticholic acid(OCA) by Intercept (ICPT) granted FDA approval as the first-ever approved therapeutics for NASH an emerging global epidemic. As a company, Intercept has done clinical wonders for the scientific and clinical communities by bringing liver research and therapeutics to the forefront. Intercept gave a clinical voice to orphan liver diseases and non-viral liver diseases with great unmet needs, an area of medicine that big pharma typically stayed away from due to perceived lack of profitability. This is reflected in the Breakthrough Designation by the FDA for OCA in NASH.
Wall Street Titan: I have covered Athersys (ATHX) on SA for a number of years and, like all long-term shareholders, have been disappointed by a clinical timeline that has extended out much longer than anyone anticipated. However, this should be the year that we see partner data out of Japan in the huge unmet medical need of ischemic stroke using the company's regenerative stem cell product called MultiStem. MultiStem is a type of stem cell that has been shown to modulate the hyperinflammation in the brain that arises after a stroke and does great damage beyond the clot. MultiStem also creates a more favorable environment to promote neurogenesis, angiogenesis and healing. I did a deep dive into the science behind this program in a 2016 article that reviews the Phase II results and my rationale for optimism. We should see results from stroke in 2020 and they could be a game changer. Athersys also has an advanced program in Japan in Acute Respiratory Distress Syndrome that has shown great promise in a Phase II trial. Both Japan trials are funded by partner Healios KK, (Healios K.K.) a leading Japan based stem cell biotech that's betting its entire future on MultiStem's success and has made a substantial financial commitment to the Athersys programs. Healios is the largest shareholder of Athersys.
@alle

HLS Therapeutics Announces Health Canada Approval for Vascepa® to Reduce the Risk of Cardiovascular Events
December 31, 2019, 7:00 PM GMT+1
- Health Canada approval follows priority review for Vascepa
- Vascepa becomes the first and only HC-approved medication for reducing cardiovascular risk beyond cholesterol lowering therapy in the studied high-risk patients approved for treatment
- Commercial launch expected to take place in the mid-February 2020 timeframe
- Vascepa is supported by data from the REDUCE-IT® trial, an international, 8,179 patient outcomes study that showed a 25% placebo-controlled risk reduction in the first occurrence of major adverse cardiovascular events
- Vascepa is the subject of numerous Canadian issued and pending patents with expiration dates which could extend to 2039.
https://finance.yahoo.com/quote/AMRN/community?p=AMRN

HLS Therapeutics Announces Health Canada Approval for Vascepa® to Reduce the Risk of Cardiovascular Events

December 31, 2019, 7:00 PM GMT+1
- Health Canada approval follows priority review for Vascepa
- Vascepa becomes the first and only HC-approved medication for reducing cardiovascular risk beyond cholesterol lowering therapy in the studied high-risk patients approved for treatment
- Commercial launch expected to take place in the mid-February 2020 timeframe
- Vascepa is supported by data from the REDUCE-IT® trial, an international, 8,179 patient outcomes study that showed a 25% placebo-controlled risk reduction in the first occurrence of major adverse cardiovascular events
- Vascepa is the subject of numerous Canadian issued and pending patents with expiration dates which could extend to 2039.
https://finance.yahoo.com/quote/AMRN/community?p=AMRN
Antwort auf Beitrag Nr.: 62.258.021 von bernie55 am 01.01.20 15:05:34Dir Auch und allen Amarinos🥂🍀
Ich hoffe suf eine Übernahme💵
Ich hoffe suf eine Übernahme💵
Hier nochmals die aktuelle Pressemeldung von heute, geil, Vascepa von Amarin wird Millionen Menschen helfen
VASCEPA® Approved in Canada to Reduce the Risk of Cardiovascular Events
[GlobeNewswire]
GlobeNewswire•January 2, 2020
VASCEPA becomes the first and only Health Canada approved medication for reducing cardiovascular risk beyond cholesterol lowering therapy in the studied high-risk patients approved for treatment

Commercial launch expected in mid-February 2020 by Amarin’s commercial partner for Canada
VASCEPA approval was supported by clinical results from the REDUCE-IT® trial which included a 25% placebo-controlled relative risk reduction in the first occurrence of major adverse cardiovascular events such as heart attack and stroke
DUBLIN, Ireland and BRIDGEWATER, N.J., Jan. 02, 2020 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN) is pleased to announce that Health Canada has approved the use of VASCEPA® (icosapent ethyl) to reduce the risk of cardiovascular events (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization or hospitalization for unstable angina) in statin-treated patients with elevated triglycerides, who are at high risk of cardiovascular events due to established cardiovascular disease, or diabetes, and at least one other cardiovascular risk factor. This is the first and only approval by Health Canada of any drug for this important indication. The approval was achieved through Amarin’s commercial licensee for VASCEPA in Canada, HLS Therapeutics, Inc. (“HLS”). Pursuant to an agreement announced in 2017, HLS has exclusive commercial rights to VASCEPA in the Canadian market.
“We congratulate HLS on securing this important approval of VASCEPA. We are confident that HLS will work to educate healthcare professionals across Canada on the life-saving and risk-reducing effects of VASCEPA. HLS is managed by an experienced and capable team of pharmaceutical industry professionals and we look forward to witnessing their progress,” stated John F. Thero, president and chief executive officer of Amarin.
The approval of VASCEPA by Health Canada positions this important drug to address a large unmet medical need. In clinical study of VASCEPA over approximately five years of patient follow-up, approximately 28 percent of patients treated with statins and other contemporary therapy but not treated with VASCEPA experienced a major adverse cardiovascular event (MACE), defined as the first occurrence of either myocardial infarction (heart attack), stroke, coronary revascularization, unstable angina requiring hospitalization or cardiovascular death.1 As evidenced by this MACE occurrence, there is a group of patients who, despite controlling their cholesterol on statin therapy, continue to have persistent high cardiovascular risk. As reflected in the Health Canada approved label, VASCEPA demonstrated a 25% relative risk reduction in the first occurrence of MACE in these high-risk patients. More information regarding clinical studies of VASCEPA can be found at www.amarincorp.com.
The approval of VASCEPA by Health Canada follows the recent approval of a similar indication for VASCEPA in the United States. On December 13, 2019, the United States Food and Drug Administration (FDA) expanded the indicated use of VASCEPA to include use “as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels (≥150 mg/dL) and established cardiovascular disease or diabetes mellitus and two or more additional risk factors for cardiovascular disease.”
Under the previously announced terms of the Amarin-HLS’s agreement, in 2017 HLS paid Amarin $5.0 million to in-license the exclusive Canadian rights to VASCEPA and in 2018 paid an additional $2.5 million following reporting of successful clinical outcomes study (REDUCE-IT) results for VASCEPA. Furthermore, under the terms of this agreement, Amarin will receive $2.5 million as a result of today's approval by Health Canada and is eligible to receive more than $50.0 million in additional milestone payments, most of which are sales-based milestones. Amarin is responsible for supplying VASCEPA for sale in Canada on a cost-plus basis. In addition to milestone and supply payments, the agreement for commercialization in Canada provides for payment to Amarin of double-digit royalties on VASCEPA net sales in Canada. HLS currently expects Canadian sales of VASCEPA to reach between CAD $150 and $250 million per year.
Pricing of VASCEPA in Canada will be jointly established by the parties. In the United States, two separate health economic studies presented in recent months conclude that VASCEPA is cost-effective. Such analyses suggested that the price of VASCEPA in the United States could be considerably higher and still remain cost-effective. These findings reflect the affordable pricing of VASCEPA in the United States and the relatively high costs of MACE, such as heart attacks and stroke, which VASCEPA helps avoid.
Vascepa is the subject of numerous Canadian issued patents and pending patents with expiration dates which could extend to 2039. The eligible patents will be added to Health Canada’s Patent Register following receipt of NOC and in accordance with Health Canada’s process.
Pursuant to this approval, VASCEPA is now approved in three countries in addition to the United States, Canada, United Arab Emirates and Lebanon. Amarin, together with its commercial partners in select geographies, is pursuing additional regulatory approvals for VASCEPA in China, the European Union, the Middle East and North Africa, and is evaluating other regions for potential regulatory filings.
VASCEPA® Approved in Canada to Reduce the Risk of Cardiovascular Events
[GlobeNewswire]
GlobeNewswire•January 2, 2020
VASCEPA becomes the first and only Health Canada approved medication for reducing cardiovascular risk beyond cholesterol lowering therapy in the studied high-risk patients approved for treatment


Commercial launch expected in mid-February 2020 by Amarin’s commercial partner for Canada
VASCEPA approval was supported by clinical results from the REDUCE-IT® trial which included a 25% placebo-controlled relative risk reduction in the first occurrence of major adverse cardiovascular events such as heart attack and stroke
DUBLIN, Ireland and BRIDGEWATER, N.J., Jan. 02, 2020 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN) is pleased to announce that Health Canada has approved the use of VASCEPA® (icosapent ethyl) to reduce the risk of cardiovascular events (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization or hospitalization for unstable angina) in statin-treated patients with elevated triglycerides, who are at high risk of cardiovascular events due to established cardiovascular disease, or diabetes, and at least one other cardiovascular risk factor. This is the first and only approval by Health Canada of any drug for this important indication. The approval was achieved through Amarin’s commercial licensee for VASCEPA in Canada, HLS Therapeutics, Inc. (“HLS”). Pursuant to an agreement announced in 2017, HLS has exclusive commercial rights to VASCEPA in the Canadian market.
“We congratulate HLS on securing this important approval of VASCEPA. We are confident that HLS will work to educate healthcare professionals across Canada on the life-saving and risk-reducing effects of VASCEPA. HLS is managed by an experienced and capable team of pharmaceutical industry professionals and we look forward to witnessing their progress,” stated John F. Thero, president and chief executive officer of Amarin.
The approval of VASCEPA by Health Canada positions this important drug to address a large unmet medical need. In clinical study of VASCEPA over approximately five years of patient follow-up, approximately 28 percent of patients treated with statins and other contemporary therapy but not treated with VASCEPA experienced a major adverse cardiovascular event (MACE), defined as the first occurrence of either myocardial infarction (heart attack), stroke, coronary revascularization, unstable angina requiring hospitalization or cardiovascular death.1 As evidenced by this MACE occurrence, there is a group of patients who, despite controlling their cholesterol on statin therapy, continue to have persistent high cardiovascular risk. As reflected in the Health Canada approved label, VASCEPA demonstrated a 25% relative risk reduction in the first occurrence of MACE in these high-risk patients. More information regarding clinical studies of VASCEPA can be found at www.amarincorp.com.
The approval of VASCEPA by Health Canada follows the recent approval of a similar indication for VASCEPA in the United States. On December 13, 2019, the United States Food and Drug Administration (FDA) expanded the indicated use of VASCEPA to include use “as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels (≥150 mg/dL) and established cardiovascular disease or diabetes mellitus and two or more additional risk factors for cardiovascular disease.”
Under the previously announced terms of the Amarin-HLS’s agreement, in 2017 HLS paid Amarin $5.0 million to in-license the exclusive Canadian rights to VASCEPA and in 2018 paid an additional $2.5 million following reporting of successful clinical outcomes study (REDUCE-IT) results for VASCEPA. Furthermore, under the terms of this agreement, Amarin will receive $2.5 million as a result of today's approval by Health Canada and is eligible to receive more than $50.0 million in additional milestone payments, most of which are sales-based milestones. Amarin is responsible for supplying VASCEPA for sale in Canada on a cost-plus basis. In addition to milestone and supply payments, the agreement for commercialization in Canada provides for payment to Amarin of double-digit royalties on VASCEPA net sales in Canada. HLS currently expects Canadian sales of VASCEPA to reach between CAD $150 and $250 million per year.
Pricing of VASCEPA in Canada will be jointly established by the parties. In the United States, two separate health economic studies presented in recent months conclude that VASCEPA is cost-effective. Such analyses suggested that the price of VASCEPA in the United States could be considerably higher and still remain cost-effective. These findings reflect the affordable pricing of VASCEPA in the United States and the relatively high costs of MACE, such as heart attacks and stroke, which VASCEPA helps avoid.
Vascepa is the subject of numerous Canadian issued patents and pending patents with expiration dates which could extend to 2039. The eligible patents will be added to Health Canada’s Patent Register following receipt of NOC and in accordance with Health Canada’s process.
Pursuant to this approval, VASCEPA is now approved in three countries in addition to the United States, Canada, United Arab Emirates and Lebanon. Amarin, together with its commercial partners in select geographies, is pursuing additional regulatory approvals for VASCEPA in China, the European Union, the Middle East and North Africa, and is evaluating other regions for potential regulatory filings.
@magnetfeldfredy, trotzdem läuft es gerade richtig schlecht mit der Aktie! 😲
Richtig schlecht ist was Anderes, meiner Meinung nach versuchen Oppenheimer und Co. durch Kursmanipulationen billig reinzukommen um bei der fetten Übernahme dabeizusein!
Das sind Jahrhundertergebnisse von Vascepa, die weltweit zu 5-10 Milliarden Umsätzen pro Jahr führen werden, mich schüttelt hier keiner mehr raus!

Das sind Jahrhundertergebnisse von Vascepa, die weltweit zu 5-10 Milliarden Umsätzen pro Jahr führen werden, mich schüttelt hier keiner mehr raus!

Amarin to Present at 38th Annual J.P. Morgan Healthcare Conference
January 6, 2020, 12:00 PM GMT+1
DUBLIN, Ireland and BRIDGEWATER, N.J., Jan. 06, 2020 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN) announced today that John F. Thero, Amarin's president and chief executive officer, is scheduled to present at the 38th Annual J.P. Morgan Healthcare Conference in San Francisco, CA on Wednesday, January 15, 2020 at 10:30 a.m. Pacific Time / 1:30 p.m. Eastern Time.
A live audio webcast of the presentation will be available at: http://www.amarincorp.com and will be accessible at the same link for 30 days.
https://finance.yahoo.com/news/amarin-present-38th-annual-j-…
January 6, 2020, 12:00 PM GMT+1
DUBLIN, Ireland and BRIDGEWATER, N.J., Jan. 06, 2020 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN) announced today that John F. Thero, Amarin's president and chief executive officer, is scheduled to present at the 38th Annual J.P. Morgan Healthcare Conference in San Francisco, CA on Wednesday, January 15, 2020 at 10:30 a.m. Pacific Time / 1:30 p.m. Eastern Time.
A live audio webcast of the presentation will be available at: http://www.amarincorp.com and will be accessible at the same link for 30 days.
https://finance.yahoo.com/news/amarin-present-38th-annual-j-…
HLS Therapeutics Announces 8 Years of Data Protection for Vascepa® 
CNW Group•January 6, 2020
Peak-year sales estimate increases to CAD$200 -300 million, from CAD$150 -250 million
Commercial launch expected to take place in the mid-February 2020 timeframe
Vascepa is the subject of numerous Canadian issued and pending patents with expiration dates which could extend to 2039
https://finance.yahoo.com/news/hls-therapeutics-announces-8-…

CNW Group•January 6, 2020
Peak-year sales estimate increases to CAD$200 -300 million, from CAD$150 -250 million
Commercial launch expected to take place in the mid-February 2020 timeframe
Vascepa is the subject of numerous Canadian issued and pending patents with expiration dates which could extend to 2039
https://finance.yahoo.com/news/hls-therapeutics-announces-8-…
Amarin updates preliminary 2019 results and 2020 outlook
Amarin updates preliminary 2019 results and 2020 outlookAmarin (NASDAQ:AMRN) provides an update of preliminary 2019 results and additional 2020 guidance.
FY 2019 net total revenues are expected to be at or slightly above the upper-end of the previous guidance of $410M to $425M.
In 2020, Amarin expects to increase its U.S. sales force to 800 sales representatives, up from 400 in 2019. Health professional targets will be expanded from approx. 50,000 to a planned 75,000 physicians.
FY 2020 total net revenue is estimated in a range of $650M to $700M, mostly from sales of VASCEPA in the U.S.
The company intends to spend ~$250M on inventory purchases in 2020, approx. twice the amount spent in 2019.
Operating expenses in 2020 are expected to increase ~$200M to $250M over 2019 levels.
Quelle: Seeking Alpha, Jan. 7, 2020 6:26 AM ET
Was ist bloß los mit AMARIN?! Mein Englisch ist nicht gut, aber ich meine keine negativen Nachrichten zu lesen. Trotzdem fällt die Aktie ständig.
Antwort auf Beitrag Nr.: 62.306.072 von PC-DIDI am 07.01.20 18:29:25Hier wird leider herummanipuliert...
Muss man aushalten 🤷♂️ ich geh hier nicht weg ✌️
Muss man aushalten 🤷♂️ ich geh hier nicht weg ✌️
Cool bleiben, auch wenns schwer fällt, Verdoppelung innerhalb eines Jahres möglich:
Bullish Looking Amarin Is Set to More Than Double From Current Levels
Pharmaceutical firm AMRN developments therapeutics for the treatment of cardiovascular diseases.
By BRUCE KAMICH
Jan 07, 2020 | 01:45 PM EST
Stocks quotes in this article: AMRN
During the 'Lightning Round' segment of Mad Money Moneyrs can call in about stocks they are interested in. Monday night one caller asked about Amarin Corp. plc (AMRN) : "This one is a rocket ship and it has more to go," said Jim Cramer. Let's check out the charts of AMRN.
In this daily bar chart of AMRN, below, we can see that the prices were in a wide trading range pattern until October when prices began to strengthen. AMRN is testing the rising 50-day moving average line today and is above the rising 200-day moving average line.
The daily On-Balance-Volume (OBV) line has been rising from early October telling us that buyers of AMRN have turned more aggressive. Heavier trading volume in November and December as prices rallied is another positive sign.
The Moving Average Convergence Divergence (MACD) oscillator is above the zero line in positive territory and could soon cross to the upside for a fresh buy signal.
In this weekly bar chart of AMRN, below, we can see the past three years of price activity. Prices made a line formation into 2018 before a huge upside price gap changed the whole picture. A $12-$24 trading range developed for about 12 months but prices made a higher high and higher low from September. AMRN above the rising 40-week moving average line.
The weekly OBV line has been strengthening in recent months and the MACD oscillator is in a bullish configuration above the zero line.
In this weekly close only Point and Figure chart of AMRN, below, the software is projecting a potential upside price target in the $44 area. More than double from current levels.
Bottom line strategy: Traders can probe the long side of AMRN at current levels risking a close below $17 for now. $44 is our upside Point and Figure price target.
Get an email alert each time I write an article for Real Money. Click the "+Follow" next to my byline to this article.
Employees of TheStreet are prohibited from trading individual securities.
TAGS: Investing | Stocks | Technical Analysis | Trading | Pharmaceuticals | Mad Money
Bullish Looking Amarin Is Set to More Than Double From Current Levels
Pharmaceutical firm AMRN developments therapeutics for the treatment of cardiovascular diseases.
By BRUCE KAMICH
Jan 07, 2020 | 01:45 PM EST
Stocks quotes in this article: AMRN
During the 'Lightning Round' segment of Mad Money Moneyrs can call in about stocks they are interested in. Monday night one caller asked about Amarin Corp. plc (AMRN) : "This one is a rocket ship and it has more to go," said Jim Cramer. Let's check out the charts of AMRN.
In this daily bar chart of AMRN, below, we can see that the prices were in a wide trading range pattern until October when prices began to strengthen. AMRN is testing the rising 50-day moving average line today and is above the rising 200-day moving average line.
The daily On-Balance-Volume (OBV) line has been rising from early October telling us that buyers of AMRN have turned more aggressive. Heavier trading volume in November and December as prices rallied is another positive sign.
The Moving Average Convergence Divergence (MACD) oscillator is above the zero line in positive territory and could soon cross to the upside for a fresh buy signal.
In this weekly bar chart of AMRN, below, we can see the past three years of price activity. Prices made a line formation into 2018 before a huge upside price gap changed the whole picture. A $12-$24 trading range developed for about 12 months but prices made a higher high and higher low from September. AMRN above the rising 40-week moving average line.
The weekly OBV line has been strengthening in recent months and the MACD oscillator is in a bullish configuration above the zero line.
In this weekly close only Point and Figure chart of AMRN, below, the software is projecting a potential upside price target in the $44 area. More than double from current levels.
Bottom line strategy: Traders can probe the long side of AMRN at current levels risking a close below $17 for now. $44 is our upside Point and Figure price target.
Get an email alert each time I write an article for Real Money. Click the "+Follow" next to my byline to this article.
Employees of TheStreet are prohibited from trading individual securities.
TAGS: Investing | Stocks | Technical Analysis | Trading | Pharmaceuticals | Mad Money
Vielleicht ist es jetzt der Dip vor der Übernahme ....
Hoffe ich auch, wenn auch langfristig viel höhere Kurse, bis zur Dreistelligkeit meiner Meinung nach, drin sind, ich bleibe, so oder so.....
Vascepa redzuiert zu den Statinen das Herzinfarktrisiko um 31 %, 28 % Schlaganfälle und 20 % Todesfälle mit kaum Nebenwirkungen, megagenial....
Big pharma weiß das und basht und manipuliert was das Zeug hält, aber wer zuletzt lacht, lacht am Besten!
Vascepa redzuiert zu den Statinen das Herzinfarktrisiko um 31 %, 28 % Schlaganfälle und 20 % Todesfälle mit kaum Nebenwirkungen, megagenial....

Big pharma weiß das und basht und manipuliert was das Zeug hält, aber wer zuletzt lacht, lacht am Besten!

In Amarin sind viele Fonds investiert.
Quelle: Yahoo!finance (InsiderMoney)
As you can see these stocks had an average of 24.75 hedge funds with bullish positions and the average amount invested in these stocks was $791 million. That figure was $1393 million in AMRN's case. L Brands Inc (NYSE:LB) is the most popular stock in this table. On the other hand Grupo Aeroportuario del Pacifico (NYSE:PAC) is the least popular one with only 3 bullish hedge fund positions. Amarin Corporation plc (NASDAQ:AMRN) is not the most popular stock in this group but hedge fund interest is still above average. Our calculations showed that top 20 most popular stocks among hedge funds returned 41.3% in 2019 and outperformed the S&P 500 ETF (SPY) by 10.1 percentage points. Hedge funds were also right about betting on AMRN as the stock returned 57.5% in 2019 and outperformed the market. Hedge funds were rewarded for their relative bullishness.
Quelle: Yahoo!finance (InsiderMoney)
As you can see these stocks had an average of 24.75 hedge funds with bullish positions and the average amount invested in these stocks was $791 million. That figure was $1393 million in AMRN's case. L Brands Inc (NYSE:LB) is the most popular stock in this table. On the other hand Grupo Aeroportuario del Pacifico (NYSE:PAC) is the least popular one with only 3 bullish hedge fund positions. Amarin Corporation plc (NASDAQ:AMRN) is not the most popular stock in this group but hedge fund interest is still above average. Our calculations showed that top 20 most popular stocks among hedge funds returned 41.3% in 2019 and outperformed the S&P 500 ETF (SPY) by 10.1 percentage points. Hedge funds were also right about betting on AMRN as the stock returned 57.5% in 2019 and outperformed the market. Hedge funds were rewarded for their relative bullishness.
13 January 2020 07:00 GMT
 AZN beendet Epanova Trial III
AZN beendet Epanova Trial III 
Independent Data Monitoring Committee has recommended to discontinue the trial as Epanova is unlikely to demonstrate a benefit to patients
Following the recommendation from an independent Data Monitoring Committee, AstraZeneca has decided to close the Phase III STRENGTH trial for Epanova (omega-3 carboxylic acids) due to its low likelihood of demonstrating a benefit to patients with mixed dyslipidaemia (MDL) who are at increased risk of cardiovascular (CV) disease.
https://www.astrazeneca.com/media-centre/press-releases/2020…
 AZN beendet Epanova Trial III
AZN beendet Epanova Trial III 
Independent Data Monitoring Committee has recommended to discontinue the trial as Epanova is unlikely to demonstrate a benefit to patients
Following the recommendation from an independent Data Monitoring Committee, AstraZeneca has decided to close the Phase III STRENGTH trial for Epanova (omega-3 carboxylic acids) due to its low likelihood of demonstrating a benefit to patients with mixed dyslipidaemia (MDL) who are at increased risk of cardiovascular (CV) disease.
https://www.astrazeneca.com/media-centre/press-releases/2020…
Wie geil, damit ist Vascepa von Amarin konkurrenzlos bei der Reduktion von Herzinfarkt, Schlaganfällen....weil nur hochreines EPA ohne DHA, das ist 5 US Dollar pro Aktie alleine wert:
Update on Phase III STRENGTH trial for Epanova in mixed dyslipidaemia
PUBLISHED 13 January 2020
13 January 2020 07:00 GMT
Independent Data Monitoring Committee has recommended to discontinue
the trial as Epanova is unlikely to demonstrate a benefit to patients
Following the recommendation from an independent Data Monitoring Committee, AstraZeneca has decided to close the Phase III STRENGTH trial for Epanova (omega-3 carboxylic acids) due to its low likelihood of demonstrating a benefit to patients with mixed dyslipidaemia (MDL) who are at increased risk of cardiovascular (CV) disease.
STRENGTH is a large-scale, global CV outcomes trial designed to evaluate the safety and efficacy of Epanova compared to placebo, both in combination with standard-of-care statin medicines.
Mene Pangalos, Executive Vice President, BioPharmaceuticals R&D, said: “It was important to assess the potential benefit of Epanova in mixed dyslipidaemia. We are disappointed by these results, but we remain committed to addressing the needs of patients in the cardiovascular space where we have an extensive pipeline.”
Steven E. Nissen MD, Study Chair for the STRENGTH trial and Chief Academic Officer for the Heart and Vascular Institute, Cleveland Clinic, US, said: “The academic leadership of the STRENGTH trial is obviously disappointed in this result, but we are very proud to have had the opportunity to answer this important scientific question. We are also grateful for the opportunity to conduct the STRENGTH trial as an exemplary collaboration between academic physicians and industry.”
This trial will now be closed in an orderly fashion, and full data will be presented at a forthcoming medical meeting.
Financial considerations
A review is being undertaken of the ongoing value of the $533m Epanova intangible asset. Any impairment will be treated as a non-Core item in the fourth quarter of 2019. A write down of up to $100m relating to inventories is also anticipated to impact the Core earnings in the fourth quarter of 2019.
STRENGTH
STRENGTH is a large-scale CV outcomes trial evaluating the effect of Epanova 4g daily compared to placebo (corn oil) on reducing the risk of major adverse cardiovascular events (MACE) in patients on optimal statin therapy with mixed dyslipidaemia and at high risk for CV disease. A total of 13,086 patients were enrolled at 675 sites in 22 countries.
Mixed dyslipidaemia
MDL includes patients with raised triglyceride levels (moderate hypertriglyceridemia) between 175-499mg/dL mg/dL, and low HDL cholesterol. Elevated triglycerides affect a growing number of patients and is often worsened by other factors such as diabetes or obesity. Lifestyle changes and potentially treating the underlying cause is likely to at least partly improve the condition and reduce cardiovascular risk.
Epanova
Epanova is a fish oil-derived mixture of free fatty acids primarily composed of EPA and DHA. It is approved in the US and indicated as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridaemia, and this indication is not impacted by the data from the STRENGTH trial.
AstraZeneca in CVRM
Cardiovascular, Renal and Metabolism (CVRM) together forms one of AstraZeneca’s three therapy areas and is a key growth driver for the Company. By following the science to understand more clearly the underlying links between the heart, kidneys and pancreas, AstraZeneca is investing in a portfolio of medicines to protect organs and improve outcomes by slowing disease progression, reducing risks and tackling comorbidities. The Company’s ambition is to modify or halt the natural course of CVRM diseases and potentially regenerate organs and restore function, by continuing to deliver transformative science that improves treatment practices and cardiovascular health for millions of patients worldwide.
AstraZeneca
AstraZeneca (LSE/STO/NYSE: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialisation of prescription medicines, primarily for the treatment of diseases in three therapy areas - Oncology, Cardiovascular, Renal and Metabolism, and Respiratory. AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. Please visit astrazeneca.com and follow the Company on Twitter @AstraZeneca.
Update on Phase III STRENGTH trial for Epanova in mixed dyslipidaemia
PUBLISHED 13 January 2020
13 January 2020 07:00 GMT
Independent Data Monitoring Committee has recommended to discontinue
the trial as Epanova is unlikely to demonstrate a benefit to patients
Following the recommendation from an independent Data Monitoring Committee, AstraZeneca has decided to close the Phase III STRENGTH trial for Epanova (omega-3 carboxylic acids) due to its low likelihood of demonstrating a benefit to patients with mixed dyslipidaemia (MDL) who are at increased risk of cardiovascular (CV) disease.
STRENGTH is a large-scale, global CV outcomes trial designed to evaluate the safety and efficacy of Epanova compared to placebo, both in combination with standard-of-care statin medicines.
Mene Pangalos, Executive Vice President, BioPharmaceuticals R&D, said: “It was important to assess the potential benefit of Epanova in mixed dyslipidaemia. We are disappointed by these results, but we remain committed to addressing the needs of patients in the cardiovascular space where we have an extensive pipeline.”
Steven E. Nissen MD, Study Chair for the STRENGTH trial and Chief Academic Officer for the Heart and Vascular Institute, Cleveland Clinic, US, said: “The academic leadership of the STRENGTH trial is obviously disappointed in this result, but we are very proud to have had the opportunity to answer this important scientific question. We are also grateful for the opportunity to conduct the STRENGTH trial as an exemplary collaboration between academic physicians and industry.”
This trial will now be closed in an orderly fashion, and full data will be presented at a forthcoming medical meeting.
Financial considerations
A review is being undertaken of the ongoing value of the $533m Epanova intangible asset. Any impairment will be treated as a non-Core item in the fourth quarter of 2019. A write down of up to $100m relating to inventories is also anticipated to impact the Core earnings in the fourth quarter of 2019.
STRENGTH
STRENGTH is a large-scale CV outcomes trial evaluating the effect of Epanova 4g daily compared to placebo (corn oil) on reducing the risk of major adverse cardiovascular events (MACE) in patients on optimal statin therapy with mixed dyslipidaemia and at high risk for CV disease. A total of 13,086 patients were enrolled at 675 sites in 22 countries.
Mixed dyslipidaemia
MDL includes patients with raised triglyceride levels (moderate hypertriglyceridemia) between 175-499mg/dL mg/dL, and low HDL cholesterol. Elevated triglycerides affect a growing number of patients and is often worsened by other factors such as diabetes or obesity. Lifestyle changes and potentially treating the underlying cause is likely to at least partly improve the condition and reduce cardiovascular risk.
Epanova
Epanova is a fish oil-derived mixture of free fatty acids primarily composed of EPA and DHA. It is approved in the US and indicated as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridaemia, and this indication is not impacted by the data from the STRENGTH trial.
AstraZeneca in CVRM
Cardiovascular, Renal and Metabolism (CVRM) together forms one of AstraZeneca’s three therapy areas and is a key growth driver for the Company. By following the science to understand more clearly the underlying links between the heart, kidneys and pancreas, AstraZeneca is investing in a portfolio of medicines to protect organs and improve outcomes by slowing disease progression, reducing risks and tackling comorbidities. The Company’s ambition is to modify or halt the natural course of CVRM diseases and potentially regenerate organs and restore function, by continuing to deliver transformative science that improves treatment practices and cardiovascular health for millions of patients worldwide.
AstraZeneca
AstraZeneca (LSE/STO/NYSE: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialisation of prescription medicines, primarily for the treatment of diseases in three therapy areas - Oncology, Cardiovascular, Renal and Metabolism, and Respiratory. AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. Please visit astrazeneca.com and follow the Company on Twitter @AstraZeneca.
Oh man, diese Aktie kostet Nerven. Ich hoffe, dass es jetzt mal ein wenig gen Norden geht.
Dann noch die Klage gut durchbringen und dann geht es ans Ernten.
Dann noch die Klage gut durchbringen und dann geht es ans Ernten.
Acasti Pharma's krill oil-derived drug fails late-stage study
1 MIN READ
(Reuters) - Acasti Pharma Inc said on Monday its krill oil-derived drug candidate, CaPre, was not more effective than placebo in reducing high levels of triglycerides in a late-stage study.
U.S.-listed shares of Acasti were down 54% at $1 before the bell.
The Canada-based drug developer said CaPre failed to show a statistically significant reduction in blood triglycerides compared to placebo after 12 weeks and 26 weeks of treatment.
Triglycerides are a type of fat found in blood that contributes to heart disease alongside cholesterol.
1 MIN READ
(Reuters) - Acasti Pharma Inc said on Monday its krill oil-derived drug candidate, CaPre, was not more effective than placebo in reducing high levels of triglycerides in a late-stage study.
U.S.-listed shares of Acasti were down 54% at $1 before the bell.
The Canada-based drug developer said CaPre failed to show a statistically significant reduction in blood triglycerides compared to placebo after 12 weeks and 26 weeks of treatment.
Triglycerides are a type of fat found in blood that contributes to heart disease alongside cholesterol.
Antwort auf Beitrag Nr.: 62.357.180 von Newark am 13.01.20 12:42:40
Both the placebo and CaPre study groups experienced significant reductions in triglycerides within the first four weeks from baseline, and even though the difference at 12 and 26 weeks was in favor of CaPre, due to the unexpectedly large placebo response, TRILOGY 1 did not reach statistical significance.
...schlecht für AZN und ACST....gut für AMRN!
Zitat von Newark: Acasti Pharma's krill oil-derived drug fails late-stage study
1 MIN READ
(Reuters) - Acasti Pharma Inc said on Monday its krill oil-derived drug candidate, CaPre, was not more effective than placebo in reducing high levels of triglycerides in a late-stage study.
U.S.-listed shares of Acasti were down 54% at $1 before the bell.
The Canada-based drug developer said CaPre failed to show a statistically significant reduction in blood triglycerides compared to placebo after 12 weeks and 26 weeks of treatment.
Triglycerides are a type of fat found in blood that contributes to heart disease alongside cholesterol.
Both the placebo and CaPre study groups experienced significant reductions in triglycerides within the first four weeks from baseline, and even though the difference at 12 and 26 weeks was in favor of CaPre, due to the unexpectedly large placebo response, TRILOGY 1 did not reach statistical significance.
...schlecht für AZN und ACST....gut für AMRN!
Antwort auf Beitrag Nr.: 62.357.336 von Cyberhexe am 13.01.20 12:56:17
Dazu der Kommentar von A.F.....



Zitat von Cyberhexe:Zitat von Newark: (Reuters) - Acasti Pharma Inc said on Monday its krill oil-derived drug candidate, CaPre, was not more effective than placebo in reducing high levels of triglycerides in a late-stage study.The Canada-based drug developer said CaPre failed to show a statistically significant reduction in blood triglycerides compared to placebo after 12 weeks and 26 weeks of treatmentBoth the placebo and CaPre study groups experienced significant reductions in triglycerides within the first four weeks from baseline, and even though the difference at 12 and 26 weeks was in favor of CaPre, due to the unexpectedly large placebo response, TRILOGY 1 did not reach statistical significance.
....schlecht für AZN und ACST....gut für AMRN!
Dazu der Kommentar von A.F.....




Two Pieces of Good News for Amarin (AMRN) - Cantor Fitzgerald
Article
Related Articles (1)
Stock Quotes (1)
Comments (0)
FREE Breaking News Alerts from StreetInsider.com!
E-mail Address
StreetInsider.com Top Tickers, 1/13/2020
1. INFY
2. NBIX
3. PFE
4. TERP
5. RARE
6. APO
7. TDOC
8. LLY
9. AMZN
10. LK
Top News
Most Read
Special Reports
Futures near record high ahead of trade agreement, earnings
Woodward (WWD), Hexcel Corp. (HXL) Enter Merger of Equals
China's yuan rallies, yen slides ahead of U.S. trade deal
lululemon athletica (LULU) Raises Prelim. Q4 EPS/Revenue Guidance Above Consensus
Oil steady as U.S.-Iran tensions ease, trade deal details elusive
January 13, 2020 7:23 AM EST
Tweet Share E-mail
Get Alerts
AMRN Hot Sheet
Price: $20.39 +7.60%
Rating Summary:
8 Buy, 10 Hold, 1 Sell
Rating Trend: Down Down
Today's Overall Ratings:
Up: 28 | Down: 30 | New: 15
Trade AMRN Now!
Join SI Premium – FREE
Get instant alerts when news breaks on your stocks. Claim your 1-week free trial to StreetInsider Premium here.
Cantor Fitzgerald analyst Louise Chen reiterated an Overweight rating and $35.00 price target on Amarin Corporation (NASDAQ: AMRN) as two pieces of good news further support their positive rating:
1) ACST announced that due to the unexpectedly large placebo response, TRILOGY 1 did not reach statistical significance.
2) Following the recommendation from an independent Data Monitoring Committee, AZN has decided to close the Phase III STRENGTH trial for Epanova (omega-3 carboxylic acids) due to its low likelihood of demonstrating a benefit to patients with mixed dyslipidaemia (MDL) who are at increased risk of cardiovascular (CV) disease.
"Therefore, AMRN has been the only company in its class with an outcomes study (REDUCE-IT) that has shown a statistically significant benefit in reducing CV disease," Chen commented. "We think the news today underscores our view that AMRN is an interesting asset in a consolidating space."
For an analyst ratings summary and ratings history on Amarin Corporation click here. For more ratings news on Amarin Corporation click here.
Shares of Amarin Corporation closed at $19.75 yesterday.
Article
Related Articles (1)
Stock Quotes (1)
Comments (0)
FREE Breaking News Alerts from StreetInsider.com!
E-mail Address
StreetInsider.com Top Tickers, 1/13/2020
1. INFY
2. NBIX
3. PFE
4. TERP
5. RARE
6. APO
7. TDOC
8. LLY
9. AMZN
10. LK
Top News
Most Read
Special Reports
Futures near record high ahead of trade agreement, earnings
Woodward (WWD), Hexcel Corp. (HXL) Enter Merger of Equals
China's yuan rallies, yen slides ahead of U.S. trade deal
lululemon athletica (LULU) Raises Prelim. Q4 EPS/Revenue Guidance Above Consensus
Oil steady as U.S.-Iran tensions ease, trade deal details elusive
January 13, 2020 7:23 AM EST
Tweet Share E-mail
Get Alerts
AMRN Hot Sheet
Price: $20.39 +7.60%
Rating Summary:
8 Buy, 10 Hold, 1 Sell
Rating Trend: Down Down
Today's Overall Ratings:
Up: 28 | Down: 30 | New: 15
Trade AMRN Now!
Join SI Premium – FREE
Get instant alerts when news breaks on your stocks. Claim your 1-week free trial to StreetInsider Premium here.
Cantor Fitzgerald analyst Louise Chen reiterated an Overweight rating and $35.00 price target on Amarin Corporation (NASDAQ: AMRN) as two pieces of good news further support their positive rating:
1) ACST announced that due to the unexpectedly large placebo response, TRILOGY 1 did not reach statistical significance.
2) Following the recommendation from an independent Data Monitoring Committee, AZN has decided to close the Phase III STRENGTH trial for Epanova (omega-3 carboxylic acids) due to its low likelihood of demonstrating a benefit to patients with mixed dyslipidaemia (MDL) who are at increased risk of cardiovascular (CV) disease.
"Therefore, AMRN has been the only company in its class with an outcomes study (REDUCE-IT) that has shown a statistically significant benefit in reducing CV disease," Chen commented. "We think the news today underscores our view that AMRN is an interesting asset in a consolidating space."
For an analyst ratings summary and ratings history on Amarin Corporation click here. For more ratings news on Amarin Corporation click here.
Shares of Amarin Corporation closed at $19.75 yesterday.
Antwort auf Beitrag Nr.: 62.358.557 von Magnetfeldfredy am 13.01.20 15:02:39
Diesen tollen Meldungen nach, hätte die Aktie doch heute richtig hoch knallen müssen. Aktuell ist sie seit gestern nur um 4,5 % hoch gegangen.
Zitat von Magnetfeldfredy: Two Pieces of Good News for Amarin (AMRN) - Cantor Fitzgerald
Article
Cantor Fitzgerald analyst Louise Chen reiterated an Overweight rating and $35.00 price target on Amarin Corporation (NASDAQ: AMRN) as two pieces of good news further support their positive rating:
1) ACST announced that due to the unexpectedly large placebo response, TRILOGY 1 did not reach statistical significance.
2) Following the recommendation from an independent Data Monitoring Committee, AZN has decided to close the Phase III STRENGTH trial for Epanova (omega-3 carboxylic acids) due to its low likelihood of demonstrating a benefit to patients with mixed dyslipidaemia (MDL) who are at increased risk of cardiovascular (CV) disease.
"Therefore, AMRN has been the only company in its class with an outcomes study (REDUCE-IT) that has shown a statistically significant benefit in reducing CV disease," Chen commented. "We think the news today underscores our view that AMRN is an interesting asset in a consolidating space."
Diesen tollen Meldungen nach, hätte die Aktie doch heute richtig hoch knallen müssen. Aktuell ist sie seit gestern nur um 4,5 % hoch gegangen.
Ja, ist schon etwas schwach, aber der Sektor lief gestern nicht sehr gut. Auf jeden Fall geht den Pessimisten so langsam die Munition aus.
Was spricht jetzt noch gegen AMRN?
Was spricht jetzt noch gegen AMRN?
Das sind manipulierte koordinierte Aktionen von den shorts, big pharma... um den Übernahmepreis nicht zu hoch werden zu lassen!
Vascepa von Amarin hat jetzt durch das Scheitern von Epanova und Acasti ein weltweites Alleinstellungsmerkmal, jetzt fehlt nur noch der Generikarechtsstreit, bei dem sich ja Amarin schon mit TEVA geeinigt hat.
Vascepa von Amarin hat jetzt durch das Scheitern von Epanova und Acasti ein weltweites Alleinstellungsmerkmal, jetzt fehlt nur noch der Generikarechtsstreit, bei dem sich ja Amarin schon mit TEVA geeinigt hat.
----muss wohl noch mal aufstocken.
Die Patentklage soll sich noch bis ende März hinziehen,laut CEO
*schnarch*
*schnarch*
Ich habe mal Analysten Meinungen zu Amarin gelesen bei Consors und auch bei Lynx. Oje, teilweise ja echt übel.
Antwort auf Beitrag Nr.: 62.412.659 von PC-DIDI am 17.01.20 23:49:02Alles Schmierfinken...
Antwort auf Beitrag Nr.: 62.413.193 von lobberland am 18.01.20 08:18:20
Das mag stimmen, aber leider reagieren Menschen schneller und intensiver auf negative Aussagen, als auf positive. Ich habe nicht gerade wenig Geld in Amarin gesteckt und bisher schon 8 % Verlust. Überlege bei - 10 % zu verkaufen.
Zitat von lobberland: Alles Schmierfinken...
Das mag stimmen, aber leider reagieren Menschen schneller und intensiver auf negative Aussagen, als auf positive. Ich habe nicht gerade wenig Geld in Amarin gesteckt und bisher schon 8 % Verlust. Überlege bei - 10 % zu verkaufen.
Welche lächerliche Analysten, Oppenheimer der ein Kursziel von 8 US Dollar ausgegeben hat und Acasti hochgejubelt hat und nun in Phase III nicht einmal den primary endpoint erreicht hat, das ich nicht lache, der hat sich sowas von lächerlich gemacht!


Die seriösen Analysten bewerten Amarin mit 30-35 US Dollar Wainwright mit 51 US Dollar, ich sehe 50 US Dollar bei einer Übernahme, ansosnten auch 35 US Dollar innerhalb von 12 Monaten!
Amarin hat die Konkurrenten Astra Zeneca nach Scheitern der großen Phase III vom Hals und die kleinen Acasti.....
Jetzt sind die Patentklagen im Fokus, da hat TEVA schon vor Jahren das Handtuch geworfen....
Das sind Spielchen die man ertragen muß, bevor ein short squeeze auf 30-35 stattfinden wird, danach die Übernahme..., nur meine bescheidene Meinung!
Amarin hat mit Vascepa den Goldbarren den Alle haben möchten, weltweit!



Die seriösen Analysten bewerten Amarin mit 30-35 US Dollar Wainwright mit 51 US Dollar, ich sehe 50 US Dollar bei einer Übernahme, ansosnten auch 35 US Dollar innerhalb von 12 Monaten!
Amarin hat die Konkurrenten Astra Zeneca nach Scheitern der großen Phase III vom Hals und die kleinen Acasti.....

Jetzt sind die Patentklagen im Fokus, da hat TEVA schon vor Jahren das Handtuch geworfen....
Das sind Spielchen die man ertragen muß, bevor ein short squeeze auf 30-35 stattfinden wird, danach die Übernahme..., nur meine bescheidene Meinung!
Amarin hat mit Vascepa den Goldbarren den Alle haben möchten, weltweit!

Antwort auf Beitrag Nr.: 62.414.996 von Magnetfeldfredy am 18.01.20 13:30:32
Du hast es sicherlich sehr gut zusammengefasst. Aber das meine ich NICHT. EGAL, wie die Realität aussieht. Nicht -Börsen-Profis wie ich lesen die Analysen z.B. bei Consors und Lynx, oder wo auch immer. Und wenn da Negatives geschrieben wird, EGAL, ob es stimmt, beeinflusst das Denken und Handeln, leider.
Zitat von Magnetfeldfredy: Welche lächerliche Analysten, Oppenheimer der ein Kursziel von 8 US Dollar ausgegeben hat und Acasti hochgejubelt hat und nun in Phase III nicht einmal den primary endpoint erreicht hat, das ich nicht lache, der hat sich sowas von lächerlich gemacht!
Die seriösen Analysten bewerten Amarin mit 30-35 US Dollar Wainwright mit 51 US Dollar, ich sehe 50 US Dollar bei einer Übernahme, ansosnten auch 35 US Dollar innerhalb von 12 Monaten!
Amarin hat die Konkurrenten Astra Zeneca nach Scheitern der großen Phase III vom Hals und die kleinen Acasti.....
Jetzt sind die Patentklagen im Fokus, da hat TEVA schon vor Jahren das Handtuch geworfen....
Das sind Spielchen die man ertragen muß, bevor ein short squeeze auf 30-35 stattfinden wird, danach die Übernahme..., nur meine bescheidene Meinung!
Amarin hat mit Vascepa den Goldbarren den Alle haben möchten, weltweit!
Du hast es sicherlich sehr gut zusammengefasst. Aber das meine ich NICHT. EGAL, wie die Realität aussieht. Nicht -Börsen-Profis wie ich lesen die Analysen z.B. bei Consors und Lynx, oder wo auch immer. Und wenn da Negatives geschrieben wird, EGAL, ob es stimmt, beeinflusst das Denken und Handeln, leider.
Die Frage ist, Wie viel ist im aktuellen Kurs bereits eingereist?
Antwort auf Beitrag Nr.: 62.415.473 von PC-DIDI am 18.01.20 15:09:58
Zum Glück werden aber die Kurse bei solchen Werten nicht von den Nicht-Börsen-Profis gemacht, dafür ist Amarin schon etwas zu groß, dass man da "push & dump" spielen könnte. Die Profis lassen sich aber nicht von solchen Analysen beeindrucken.
Klar, wenn die Aktie jetzt immer weiter fallen würde, steigen vielleicht die Profis aus, aber derzeit sehe ich nur normale Schwankungen und 10 % sind bei Biotec-Werten eher wenig. Da schwankt der Kurs einfach stärker.
Zitat von PC-DIDI: Du hast es sicherlich sehr gut zusammengefasst. Aber das meine ich NICHT. EGAL, wie die Realität aussieht. Nicht -Börsen-Profis wie ich lesen die Analysen z.B. bei Consors und Lynx, oder wo auch immer. Und wenn da Negatives geschrieben wird, EGAL, ob es stimmt, beeinflusst das Denken und Handeln, leider.
Zum Glück werden aber die Kurse bei solchen Werten nicht von den Nicht-Börsen-Profis gemacht, dafür ist Amarin schon etwas zu groß, dass man da "push & dump" spielen könnte. Die Profis lassen sich aber nicht von solchen Analysen beeindrucken.
Klar, wenn die Aktie jetzt immer weiter fallen würde, steigen vielleicht die Profis aus, aber derzeit sehe ich nur normale Schwankungen und 10 % sind bei Biotec-Werten eher wenig. Da schwankt der Kurs einfach stärker.
https://truetoyourheart.com/commercial
Super Werbespot für unseren kommenden Megablockbuster, yes, we can!
Super Werbespot für unseren kommenden Megablockbuster, yes, we can!
Amarin geht ja gerade super ab. Siehe Nasdaq
Amarin hat sehr gute Versicherungsabdeckung für das neue Reduce-It Label:
Amarin says payers response on new Vascepa indication 'very positive' In a statement to the Fly, Amarin (AMRN) said: "We do not generally comment on changes in specific plans, as the access landscape can be very fluid. With that said, Vascepa's new FDA-approved cardiovascular risk reduction indication has received a significant amount of attention with payers. While we are early in the process of our discussions with payers regarding the new indication, the response has been very positive. Vascepa is covered by the vast majority of Commercial and Medicare Part D payers, and we anticipate that patient access will continue to improve overall as payers adapt to the new indication."
Vascepa is covered by the vast majority of Commercial and Medicare Part D payers, and we anticipate that patient access will continue to improve overall as payers adapt to the new indication."
Read more at:
https://thefly.com/landingPageNews.php?id=3021466
Amarin says payers response on new Vascepa indication 'very positive' In a statement to the Fly, Amarin (AMRN) said: "We do not generally comment on changes in specific plans, as the access landscape can be very fluid. With that said, Vascepa's new FDA-approved cardiovascular risk reduction indication has received a significant amount of attention with payers. While we are early in the process of our discussions with payers regarding the new indication, the response has been very positive.
 Vascepa is covered by the vast majority of Commercial and Medicare Part D payers, and we anticipate that patient access will continue to improve overall as payers adapt to the new indication."
Vascepa is covered by the vast majority of Commercial and Medicare Part D payers, and we anticipate that patient access will continue to improve overall as payers adapt to the new indication."Read more at:
https://thefly.com/landingPageNews.php?id=3021466
Antwort auf Beitrag Nr.: 62.423.070 von Magnetfeldfredy am 20.01.20 07:34:02
...und die Werbespots laufen in den USA auf immer mehr TV -Kanälen.
Siehe stocktwits:
just saw V commercial on History channel while Vikings was on! Very cool!!
Just saw a commercial on HGTV!
Seeing VASCEPA commercials on Fox Business !!!
Zitat von Magnetfeldfredy: https://truetoyourheart.com/commercial
Super Werbespot für unseren kommenden Megablockbuster, yes, we can!
...und die Werbespots laufen in den USA auf immer mehr TV -Kanälen.

Siehe stocktwits:
just saw V commercial on History channel while Vikings was on! Very cool!!
Just saw a commercial on HGTV!
Seeing VASCEPA commercials on Fox Business !!!
Antwort auf Beitrag Nr.: 62.413.193 von lobberland am 18.01.20 08:18:20
..und einer dieser Spezies kommt aus dem Hause Oppenheimer und heißt L.Gershell.
Gestern hat er einen Verkauf von AMRN noch einmal bestätigt und - jetzt Achtung !! - das Kursziel auf 13 Dollar erhöht !
Heute senkt er die EPS für das Jahr 2020 von 0,08 $ auf 0,04 $!!! - aber für 2022 schätzt er die EPS auf 0,56 $.
Faktencheck : DER TYP hat sie doch nicht mehr alle !!!!
https://slatersentinel.com/news/2020/01/23/amarin-co-plc-to-…
Zitat von lobberland: Alles Schmierfinken...
..und einer dieser Spezies kommt aus dem Hause Oppenheimer und heißt L.Gershell.
Gestern hat er einen Verkauf von AMRN noch einmal bestätigt und - jetzt Achtung !! - das Kursziel auf 13 Dollar erhöht !
Heute senkt er die EPS für das Jahr 2020 von 0,08 $ auf 0,04 $!!! - aber für 2022 schätzt er die EPS auf 0,56 $.
Faktencheck : DER TYP hat sie doch nicht mehr alle !!!!
https://slatersentinel.com/news/2020/01/23/amarin-co-plc-to-…
Der hat Acasti mit KZ 7 US Dollar gepusht, und unser Superkonkurrent hat dann nicht einmal die Phase 3 für Triglyceridsenkung erreicht!







Antwort auf Beitrag Nr.: 62.465.799 von Magnetfeldfredy am 23.01.20 15:50:09
Der Oberchecker oder eher der Oberabzocker.
oder eher der Oberabzocker. 
Der Typ gehört ordentlich in die Pfanne "gekloppt
DESHALB :
BUYOUT hier und jetzt !!!


Zitat von Magnetfeldfredy: Der hat Acasti mit KZ 7 US Dollar gepusht, und unser Superkonkurrent hat dann nicht einmal die Phase 3 für Triglyceridsenkung erreicht!
Der Oberchecker
 oder eher der Oberabzocker.
oder eher der Oberabzocker. 
Der Typ gehört ordentlich in die Pfanne "gekloppt

DESHALB :
BUYOUT hier und jetzt !!!



Meine Hoffnung ist, dass sich die Beurteilungen/ Empfehlungen dieser ANALysten irgendwann mal abnutzen und die Bewertungen bei einer großen Anzahl von Aktionären nur noch ein müdes Lächeln hervorrufen....
HLS Therapeutics Provides Investor Update on the Canadian Launch of Vascepa® (Icosapent Ethyl)
CNW GroupJanuary 28, 2020, 12:30 PM GMT+1
Vascepa to be available on or about February 18, 2020 , supported by a national Cardiovascular salesforce
Vascepa becomes the first and only Health Canada-approved medication for reducing cardiovascular risk beyond cholesterol-lowering therapy in the studied high-risk patients approved for treatment
Vascepa is supported by data from the REDUCE-IT® trial, an international 8,179 patient outcomes study that showed a 25% placebo-controlled risk reduction in the first occurrence of major adverse cardiovascular events
Cardiovascular disease is the number one killer globally1 and HLS is taking steps to make Vascepa accessible and affordable to the Canadian population
https://finance.yahoo.com/news/hls-therapeutics-provides-inv…
CNW GroupJanuary 28, 2020, 12:30 PM GMT+1
Vascepa to be available on or about February 18, 2020 , supported by a national Cardiovascular salesforce
Vascepa becomes the first and only Health Canada-approved medication for reducing cardiovascular risk beyond cholesterol-lowering therapy in the studied high-risk patients approved for treatment
Vascepa is supported by data from the REDUCE-IT® trial, an international 8,179 patient outcomes study that showed a 25% placebo-controlled risk reduction in the first occurrence of major adverse cardiovascular events
Cardiovascular disease is the number one killer globally1 and HLS is taking steps to make Vascepa accessible and affordable to the Canadian population
https://finance.yahoo.com/news/hls-therapeutics-provides-inv…
Antwort auf Beitrag Nr.: 62.507.858 von bernie55 am 28.01.20 13:08:12
Mein bescheidenes Englisch sagt mir, dass das gute Nachrichten sind?!
Zitat von bernie55: HLS Therapeutics Provides Investor Update on the Canadian Launch of Vascepa® (Icosapent Ethyl)
CNW GroupJanuary 28, 2020, 12:30 PM GMT+1
Vascepa to be available on or about February 18, 2020 , supported by a national Cardiovascular salesforce
Vascepa becomes the first and only Health Canada-approved medication for reducing cardiovascular risk beyond cholesterol-lowering therapy in the studied high-risk patients approved for treatment
Vascepa is supported by data from the REDUCE-IT® trial, an international 8,179 patient outcomes study that showed a 25% placebo-controlled risk reduction in the first occurrence of major adverse cardiovascular events
Cardiovascular disease is the number one killer globally1 and HLS is taking steps to make Vascepa accessible and affordable to the Canadian population
https://finance.yahoo.com/news/hls-therapeutics-provides-inv…
Mein bescheidenes Englisch sagt mir, dass das gute Nachrichten sind?!
Antwort auf Beitrag Nr.: 62.508.062 von PC-DIDI am 28.01.20 13:25:45Yepp - nach der Zulassung in Kanada wird Vascepa ab ca. dem 18. Februar 2020 dort in den Verkauf kommen - unterstützt wird die Einführung von einer nationalen kardiovaskulären Verriebsorganisation.
Efficacy of Ethyl Icosapentate in Patients With Severe Hypertriglyceridemia
Actual Study Start Date : January 20, 2020
Estimated Primary Completion Date : March 2022
Estimated Study Completion Date : May 2022
Locations
China Mochida Investigational sitesRecruitingChangsha, China
Sponsors and Collaborators
Mochida Pharmaceutical Company, Ltd.
Sumitomo Pharmaceutical (Suzhou) Co., Ltd.
https://clinicaltrials.gov/ct2/show/NCT04239950?term=Icosape…
Actual Study Start Date : January 20, 2020
Estimated Primary Completion Date : March 2022
Estimated Study Completion Date : May 2022
Locations
China Mochida Investigational sitesRecruitingChangsha, China
Sponsors and Collaborators
Mochida Pharmaceutical Company, Ltd.
Sumitomo Pharmaceutical (Suzhou) Co., Ltd.
https://clinicaltrials.gov/ct2/show/NCT04239950?term=Icosape…
Antwort auf Beitrag Nr.: 62.160.989 von bernie55 am 14.12.19 20:48:07
Trial Proceedings Complete, Decision by March 31 per Judge
Die Verhandlungstage bzgl. des Patantschutzes für Vascepa sind abgeschlossen.
Die Entscheidung des Gerichts wird spätestens am 31.03.20 erwartet.
https://www.investorvillage.com/smbd.asp?mb=2294&mn=6538&pt=…
Zitat von bernie55: Can Amarin’s patents protect Vascepa from generics?
https://www.markmanadvisors.com/blog/2019/12/3/can-amarins-p…
Trial Proceedings Complete, Decision by March 31 per Judge
Die Verhandlungstage bzgl. des Patantschutzes für Vascepa sind abgeschlossen.
Die Entscheidung des Gerichts wird spätestens am 31.03.20 erwartet.
https://www.investorvillage.com/smbd.asp?mb=2294&mn=6538&pt=…
Jefferies confident Amarin will win patent trial, sees up to 20% rally Court proceedings for the Amarin (AMRN) versus Dr. Reddy's (RDY) and Hikma Pharmaceuticals (HKMPF) case have ended, following seven days of expert witness testimony, Jefferies analyst Michael Yee tells investors in a research note. The judge did not provide a preliminary ruling or hint at which way she is ruling, but she did state the written ruling will be made by March 31, Yee points out. With the proceedings completed, and Dr. Reddy's and Hikma having presented their best case, Yee is now more confident that Amarin is likely to prevail, "removing the chief overhang on the stock." Amarin shares have been relatively range bound the last month despite positive news on the competitive front with two key competitor trials having failed, Yee contends. He attributes this to uncertainty associated with the patent trial and the potential risk for Reddy's and Hikma, two prospective generics for Amarin's Vascepa, to present some "bombshell" evidence. However, the analyst now thinks it will be hard for the judge to not rule in favor of Amarin. Yee sees the stock up 10%-20% on a positive ruling and keeps a Buy rating on Amarin with a $30 price target. The stock closed Tuesday at $20.26.
Read more at:
https://thefly.com/landingPageNews.php?id=3024775
Read more at:
https://thefly.com/landingPageNews.php?id=3024775
So viele gute Nachrichten und Amarin geht RUNTER! 😥
Mani - pu- lation!
Habe ich heute von meinem Broker bekommen:


Acasti Pharma Provides Update on TRILOGY 1 and TRILOGY 2 Phase 3 Trials of CaPre
February 10, 2020
Investigation underway into unexpected and inconsistent findings that may have negatively impacted results reported in TRILOGY 1
Announces plans to seek FDA guidance prior to unblinding TRILOGY 2 data, which is expected to delay reporting of TRILOGY 2 topline results until calendar Q3, 2020
https://www.acastipharma.com/en/investors/news-events/press-…
February 10, 2020
Investigation underway into unexpected and inconsistent findings that may have negatively impacted results reported in TRILOGY 1
Announces plans to seek FDA guidance prior to unblinding TRILOGY 2 data, which is expected to delay reporting of TRILOGY 2 topline results until calendar Q3, 2020
https://www.acastipharma.com/en/investors/news-events/press-…
VASCEPA Scripts For Week Ending February 7, 2020 : 👍
Vascepa
TRx: 81,064 (+72.31% y/y, +3.74% w/w)
NRx: 39,331 (+64.56% y/y, +3.10% w/w)
Refills: 41,733 (+80.31% y/y, +4.34% w/w)
Lovaza (Generic & Branded )
TRx: 59,587 (-7.13% y/y, + 1.61% w/w)
NRx: 29,782 (-3.16% y/y, -0.42% w/w)
Refills: 29,805 (-10.78% y/y, +3.72% w/w)
Graphische Darstellung:
https://mobile.twitter.com/ra_fun/status/1228333367013335045…
Vascepa
TRx: 81,064 (+72.31% y/y, +3.74% w/w)
NRx: 39,331 (+64.56% y/y, +3.10% w/w)
Refills: 41,733 (+80.31% y/y, +4.34% w/w)
Lovaza (Generic & Branded )
TRx: 59,587 (-7.13% y/y, + 1.61% w/w)
NRx: 29,782 (-3.16% y/y, -0.42% w/w)
Refills: 29,805 (-10.78% y/y, +3.72% w/w)
Graphische Darstellung:
https://mobile.twitter.com/ra_fun/status/1228333367013335045…
Antwort auf Beitrag Nr.: 62.684.437 von bernie55 am 14.02.20 16:16:40Aktueller Überblick bzgl. TRx pro Quartal - von Captbeer ( auf stocktwits) - 👍 🙂


Vascepa ist ja noch ganz frisch auf dem Markt. Aber wenn ich so sehe, was heute passiert ist, scheinen die Leute zu erkennen, welches Potential noch darin steckt.
Hier wird zu Kursen geworfen 🙄 Denke mal so kommen wir einer Übernahme näher... Schnäppchen für den Übernehmer
So, nochmal Amarin nachgekauft. :-)
Kennt sich jemand hier mit den lawsuit - Geschichten bzgl. den Patentstreitigkeiten näher aus...es wäre nett wenn ich etwas Info bekommen könnte-Danke!
Antwort auf Beitrag Nr.: 62.979.338 von Gustl24 am 12.03.20 14:42:58Hallo Gustl24,
ich bin kein Jurist , ich habe keine Checkung von patenrechtlichen Dingen. Jedoch habe ich aus dem Artikel -
An Update On Amarin's Generic Lawsuit - mal wichtige Aspekte des Patentstreites mit Deepl übersetzt :
https://seekingalpha.com/article/4330873-update-on-amarins-g…
Es gibt hier zwei verschiedene Aspekte - Verletzung und Offensichtlichkeit.
Der Gesichtspunkt der Verletzung ist irrelevant, und ich denke, es ist eine Tatsache, dass generisches Vascepa auf verschiedene Weise gegen Marken-Vascepa verstoßen wird.
Das einzige verbleibende Argument des Prozesses ist der Aspekt der Offensichtlichkeit, was bedeutet, dass Vascepa aus dem Stand der Technik offensichtlich war, und dass seine Patente daher ungültig sind.
Der zentrale wissenschaftliche Fortschritt bei Vascepa besteht jedoch darin, dass DHA sich nachteilig auf die Lebenslaufrisiken auswirkt, während reine EPA für die Lebenslaufrisiken wirksam ist. Dieses Wissen war im Stand der Technik nicht vorhanden und ist Amarins eigene Entdeckung.
Aus dem Amarin-Brief:
Die Beklagten versuchen auch, die klaffenden Löcher in ihrem Offensichtlichkeitsangriff zu überdecken, indem sie behaupten ,dass es der Stand der Technik sei (sagen sie), auf den sich die Erfinder bei der Konzeption ihrer Erfindung gestützt hätten.
Dies ist sowohl faktisch als auch rechtlich falsch.
Was die Tatsachen betrifft, so sagte der namentlich genannte Erfinder Dr. Manku aus, dass die Erfindung auf geschützten klinischen Daten aus von Amarin (und seinem Vorgänger Laxdale) gesponserten Versuchen bei neuropsychiatrischen Erkrankungen beruhte. PPF ¶ 63.
Diese Daten trugen zur Aufklärung der biochemischen Wirkungen von EPA bei und zeigten den Erfindern - eine Lehre, die im Stand der Technik fehlte -, dass DHA die Wirkungen von EPA zu beeinträchtigen schien, was zu ihren Erkenntnissen über den klinischen Nutzen von gereinigter EPA führte.
Dieser Nutzenunterschied zwischen EPA und DHA ist also die Innovation bei Vascepa, und diese Schlussfolgerung wurde durch die entscheidende REDUCE-IT-Studie und andere frühere Amarin-Studien gezogen. Diese Schlussfolgerung - und die dafür notwendigen Daten - waren im Stand der Technik nicht vorhanden. Daher war sie nicht offensichtlich, und als solche sind Vascepa-Patente nicht ungültig.
Das gibt mir die Zuversicht, dass Amarin auf einen Sieg hofft. Wenn das geschieht, werden die Aktien wieder steigen und die derzeitigen Tiefststände aufgrund des Coronavirus zum Kaufen bringen.
Und wie immer TIME WILL TELL und LEt´S HOPE THE BEST.
Grüße
bernie55
ich bin kein Jurist , ich habe keine Checkung von patenrechtlichen Dingen. Jedoch habe ich aus dem Artikel -
An Update On Amarin's Generic Lawsuit - mal wichtige Aspekte des Patentstreites mit Deepl übersetzt :
https://seekingalpha.com/article/4330873-update-on-amarins-g…
Es gibt hier zwei verschiedene Aspekte - Verletzung und Offensichtlichkeit.
Der Gesichtspunkt der Verletzung ist irrelevant, und ich denke, es ist eine Tatsache, dass generisches Vascepa auf verschiedene Weise gegen Marken-Vascepa verstoßen wird.
Das einzige verbleibende Argument des Prozesses ist der Aspekt der Offensichtlichkeit, was bedeutet, dass Vascepa aus dem Stand der Technik offensichtlich war, und dass seine Patente daher ungültig sind.
Der zentrale wissenschaftliche Fortschritt bei Vascepa besteht jedoch darin, dass DHA sich nachteilig auf die Lebenslaufrisiken auswirkt, während reine EPA für die Lebenslaufrisiken wirksam ist. Dieses Wissen war im Stand der Technik nicht vorhanden und ist Amarins eigene Entdeckung.
Aus dem Amarin-Brief:
Die Beklagten versuchen auch, die klaffenden Löcher in ihrem Offensichtlichkeitsangriff zu überdecken, indem sie behaupten ,dass es der Stand der Technik sei (sagen sie), auf den sich die Erfinder bei der Konzeption ihrer Erfindung gestützt hätten.
Dies ist sowohl faktisch als auch rechtlich falsch.
Was die Tatsachen betrifft, so sagte der namentlich genannte Erfinder Dr. Manku aus, dass die Erfindung auf geschützten klinischen Daten aus von Amarin (und seinem Vorgänger Laxdale) gesponserten Versuchen bei neuropsychiatrischen Erkrankungen beruhte. PPF ¶ 63.
Diese Daten trugen zur Aufklärung der biochemischen Wirkungen von EPA bei und zeigten den Erfindern - eine Lehre, die im Stand der Technik fehlte -, dass DHA die Wirkungen von EPA zu beeinträchtigen schien, was zu ihren Erkenntnissen über den klinischen Nutzen von gereinigter EPA führte.
Dieser Nutzenunterschied zwischen EPA und DHA ist also die Innovation bei Vascepa, und diese Schlussfolgerung wurde durch die entscheidende REDUCE-IT-Studie und andere frühere Amarin-Studien gezogen. Diese Schlussfolgerung - und die dafür notwendigen Daten - waren im Stand der Technik nicht vorhanden. Daher war sie nicht offensichtlich, und als solche sind Vascepa-Patente nicht ungültig.
Das gibt mir die Zuversicht, dass Amarin auf einen Sieg hofft. Wenn das geschieht, werden die Aktien wieder steigen und die derzeitigen Tiefststände aufgrund des Coronavirus zum Kaufen bringen.
Und wie immer TIME WILL TELL und LEt´S HOPE THE BEST.
Grüße
bernie55

Antwort auf Beitrag Nr.: 62.982.224 von bernie55 am 12.03.20 16:53:25Ganz herzlichen Dank Bernie 👍😀!!
Antwort auf Beitrag Nr.: 62.986.064 von Gustl24 am 12.03.20 20:09:18Gern geschehen....

Amarin upgraded to Buy from Neutral
Goldman Sachs Goldman Sachs analyst Paul Choi upgraded Amarin (AMRN) to Buy from Neutral with a $28 price target. The shares closed Thursday down $1.06 to $11.50. The 52% pullback in Amarin shares since December 13, 2019, which has been driven by both idiosyncratic and macro factors, makes the valuation "compelling" in the midst of the company's ongoing launch of Vascepa in the REDUCE-IT population, Choi tells investors in a research note. The analyst, who says ongoing patent litigation between Amarin and Dr. Reddy's (RDY) / Hikma has been an overhang on the stock, expects the ongoing launch of Vascepa to be successful over the near-term.
He estimates approximately 8.7M patients are now eligible for Vascape in addition to the 3.5M previously eligible under the initial label.
Read more at:
https://thefly.com/landingPageNews.php?id=3050882
Goldman Sachs Goldman Sachs analyst Paul Choi upgraded Amarin (AMRN) to Buy from Neutral with a $28 price target. The shares closed Thursday down $1.06 to $11.50. The 52% pullback in Amarin shares since December 13, 2019, which has been driven by both idiosyncratic and macro factors, makes the valuation "compelling" in the midst of the company's ongoing launch of Vascepa in the REDUCE-IT population, Choi tells investors in a research note. The analyst, who says ongoing patent litigation between Amarin and Dr. Reddy's (RDY) / Hikma has been an overhang on the stock, expects the ongoing launch of Vascepa to be successful over the near-term.
He estimates approximately 8.7M patients are now eligible for Vascape in addition to the 3.5M previously eligible under the initial label.
Read more at:
https://thefly.com/landingPageNews.php?id=3050882
Matinas BioPharma hört nicht auf Amarin ans Bein zu pinkeln:
Matinas BioPharma Announces Initiation of ENHANCE-IT Study of MAT9001 Against Vascepa®
https://www.biospace.com/article/releases/matinas-biopharma-…
Es handelt sich scheinbar um eine Studie der Phase II. Ansonsten ist mir als Laie das Studiendesign unklar:
"The primary endpoint is the percent change from baseline to end-of-treatment in plasma triglycerides."
Was immer das heißen mag...
Aus meiner Sicht muss dennoch eine Phase 3 erfolgen und das FDA Zulassungsverfahren durchlaufen werden. Meiner bescheidenen Meinung nach ist hier in den nächsten 2 Jahren keinesfalls mit einem Ergebnis zu rechnen.
Sehr wahrscheinlich spekuliert MATINAS auf eine Übernahme durch BigPharma.
Matinas BioPharma Announces Initiation of ENHANCE-IT Study of MAT9001 Against Vascepa®
https://www.biospace.com/article/releases/matinas-biopharma-…
Es handelt sich scheinbar um eine Studie der Phase II. Ansonsten ist mir als Laie das Studiendesign unklar:
"The primary endpoint is the percent change from baseline to end-of-treatment in plasma triglycerides."
Was immer das heißen mag...

Aus meiner Sicht muss dennoch eine Phase 3 erfolgen und das FDA Zulassungsverfahren durchlaufen werden. Meiner bescheidenen Meinung nach ist hier in den nächsten 2 Jahren keinesfalls mit einem Ergebnis zu rechnen.
Sehr wahrscheinlich spekuliert MATINAS auf eine Übernahme durch BigPharma.
Antwort auf Beitrag Nr.: 63.132.535 von tippse am 25.03.20 21:19:14
GOOD FINDING ,tippse 👍
Analysis predicts purified fish oil could prevent thousands of cardiovascular events
Abstract to be presented at American College of Cardiology/World Congress of Cardiology
Irvine, CA - March 25, 2020 - Researchers from the University of California, Irvine have conducted a statistical analysis that predicts more than 70,000 heart attacks, strokes and other adverse cardiovascular events could be prevented each year in the U.S. through the use of a highly purified fish oil therapy.
Led by Nathan D. Wong, PhD, professor and director of the Heart Disease Prevention Program in the Division of Cardiology at the UCI School of Medicine, the abstract of the statistical analysis was accepted by the American College of Cardiology and is slated to be presented at the upcoming ACC.20/World Congress of Cardiology virtual conference taking place March 28-30. The analysis utilizes data from the Centers for Disease Control and Prevention's National Health and Nutrition Examination Survey (NHANES), and inclusion criteria from a multinational clinical trial led by investigators from Harvard University called REDUCE-IT, which was published in the New England Journal of Medicine in January of 2019.
The REDUCE-IT trial showed patients with known cardiovascular disease or diabetes and multiple risk factors who have elevated triglyceride levels and are at increased risk for ischemic events benefitted substantially from icosapent ethyl, a highly purified fish oil therapy, which lowered cardiovascular events, including heart attacks and strokes, by 25 percent. Positive results were not found in other trials, possibly due to mixtures with other omega-3 fatty acids such as DHA, or inadequate dosages according to Wong.
"Our analysis extends the findings of the REDUCE-IT trial by estimating its potential impact on the U.S. population," said Wong. "By using inclusion criteria and cardiovascular disease event rates from the REDUCE-IT trial and applying it to data on US adults from NHANES, we were able to estimate the beneficial impact icosapent ethyl could have on preventing initial and total cardiovascular events in eligible U.S. adults with cardiovascular disease or diabetes and multiple risk factors."
Wong's analysis is the first to project the findings of REDUCE-IT to the overall U.S. population.
"When you consider that for every 21 patients treated with icosapent ethyl you can spare a cardiovascular event, you begin to see the implications of our results," said Wong.
Icosapent ethyl is a purified stable eicosapentaenoic acid (EPA) which was recently approved by the Federal Drug Administration (FDA) in conjunction with maximally tolerated statin therapy to reduce the risk of cardiovascular events in certain adults with elevated triglyceride levels. The only drug of its kind to show such an effect, icosapent ethyl, is currently marketed under the name Vascepa® by Amarin Pharma. The EPA therapy has also gained the support of several major societies, which have incorporated it in various guidelines, scientific statements and advisories, including the American Diabetes Association, American Heart Association, National Lipid Association, and the European Society of Cardiology/European Atherosclerosis Society.
https://www.eurekalert.org/pub_releases/2020-03/uoc--app0325…
Zitat von tippse: https://www.eurekalert.org/pub_releases/2020-03/uoc--app0325…
GOOD FINDING ,tippse 👍
Analysis predicts purified fish oil could prevent thousands of cardiovascular events
Abstract to be presented at American College of Cardiology/World Congress of Cardiology
Irvine, CA - March 25, 2020 - Researchers from the University of California, Irvine have conducted a statistical analysis that predicts more than 70,000 heart attacks, strokes and other adverse cardiovascular events could be prevented each year in the U.S. through the use of a highly purified fish oil therapy.
Led by Nathan D. Wong, PhD, professor and director of the Heart Disease Prevention Program in the Division of Cardiology at the UCI School of Medicine, the abstract of the statistical analysis was accepted by the American College of Cardiology and is slated to be presented at the upcoming ACC.20/World Congress of Cardiology virtual conference taking place March 28-30. The analysis utilizes data from the Centers for Disease Control and Prevention's National Health and Nutrition Examination Survey (NHANES), and inclusion criteria from a multinational clinical trial led by investigators from Harvard University called REDUCE-IT, which was published in the New England Journal of Medicine in January of 2019.
The REDUCE-IT trial showed patients with known cardiovascular disease or diabetes and multiple risk factors who have elevated triglyceride levels and are at increased risk for ischemic events benefitted substantially from icosapent ethyl, a highly purified fish oil therapy, which lowered cardiovascular events, including heart attacks and strokes, by 25 percent. Positive results were not found in other trials, possibly due to mixtures with other omega-3 fatty acids such as DHA, or inadequate dosages according to Wong.
"Our analysis extends the findings of the REDUCE-IT trial by estimating its potential impact on the U.S. population," said Wong. "By using inclusion criteria and cardiovascular disease event rates from the REDUCE-IT trial and applying it to data on US adults from NHANES, we were able to estimate the beneficial impact icosapent ethyl could have on preventing initial and total cardiovascular events in eligible U.S. adults with cardiovascular disease or diabetes and multiple risk factors."
Wong's analysis is the first to project the findings of REDUCE-IT to the overall U.S. population.
"When you consider that for every 21 patients treated with icosapent ethyl you can spare a cardiovascular event, you begin to see the implications of our results," said Wong.
Icosapent ethyl is a purified stable eicosapentaenoic acid (EPA) which was recently approved by the Federal Drug Administration (FDA) in conjunction with maximally tolerated statin therapy to reduce the risk of cardiovascular events in certain adults with elevated triglyceride levels. The only drug of its kind to show such an effect, icosapent ethyl, is currently marketed under the name Vascepa® by Amarin Pharma. The EPA therapy has also gained the support of several major societies, which have incorporated it in various guidelines, scientific statements and advisories, including the American Diabetes Association, American Heart Association, National Lipid Association, and the European Society of Cardiology/European Atherosclerosis Society.
https://www.eurekalert.org/pub_releases/2020-03/uoc--app0325…
Antwort auf Beitrag Nr.: 63.141.604 von bernie55 am 26.03.20 16:09:47Eight Data Presentations Relevant to VASCEPA® (Icosapent Ethyl) Capsules and Persistent Cardiovascular Risk to be Presented at the American College of Cardiology’s 69th Annual Scientific Session Together With World Congress of Cardiology (ACC.20/WCC), March 28 – 30
https://finance.yahoo.com/news/eight-data-presentations-rele…" target="_blank" rel="nofollow ugc noopener">https://finance.yahoo.com/news/eight-data-presentations-rele…
https://finance.yahoo.com/news/eight-data-presentations-rele…" target="_blank" rel="nofollow ugc noopener">https://finance.yahoo.com/news/eight-data-presentations-rele…
Antwort auf Beitrag Nr.: 63.141.949 von bernie55 am 26.03.20 16:37:36Das "Live"-Meeting in Chicago ist längst abgesagt. Inwieweit Präsentationen virtuell stattfinden muss man sehen. Ich denke den Amerikanern stehen harte Tage bevor... 

Antwort auf Beitrag Nr.: 63.149.680 von tippse am 27.03.20 10:11:15
Dass das meeting wegen CV 19 abgesagt wurde, ist wohl klar.
Ein virtuell durchgeführtes meeting wurde zumindest angekündigt.......is slated to be presented at the upcoming ACC.20/World Congress of Cardiology virtual conference taking place March 28-30
Zitat von tippse: Das "Live"-Meeting in Chicago ist längst abgesagt. Inwieweit Präsentationen virtuell stattfinden muss man sehen. Ich denke den Amerikanern stehen harte Tage bevor...
Dass das meeting wegen CV 19 abgesagt wurde, ist wohl klar.
Ein virtuell durchgeführtes meeting wurde zumindest angekündigt.......is slated to be presented at the upcoming ACC.20/World Congress of Cardiology virtual conference taking place March 28-30
Die 120-Tage-EMA-Entscheidung für Amarin wird nicht verzögert 
Sie steht auf der Tagesordnung des Treffens, das vom 23. bis 26. März stattgefunden hat.
Eine Liste mit Fragen an Amarin wurde herausgegeben , und es wird etwa 2 Monate dauern, bis wir eine weitere Aktualisierung des EMA-Status erhalten.
3.3. Initial applications; List of questions (Day 120; Day 90 for procedures with accelerated assessment timetable)
3.3.3. icosapent ethyl - EMEA/H/C/005398
indicated to reduce cardiovascular risk as an adjunct to statin therapy.
Scope: List of questions
Action: For adoption
https://www.ema.europa.eu/en/committees/chmp/chmp-agendas-mi…

Sie steht auf der Tagesordnung des Treffens, das vom 23. bis 26. März stattgefunden hat.
Eine Liste mit Fragen an Amarin wurde herausgegeben , und es wird etwa 2 Monate dauern, bis wir eine weitere Aktualisierung des EMA-Status erhalten.
3.3. Initial applications; List of questions (Day 120; Day 90 for procedures with accelerated assessment timetable)
3.3.3. icosapent ethyl - EMEA/H/C/005398
indicated to reduce cardiovascular risk as an adjunct to statin therapy.
Scope: List of questions
Action: For adoption
https://www.ema.europa.eu/en/committees/chmp/chmp-agendas-mi…
Antwort auf Beitrag Nr.: 63.141.949 von bernie55 am 26.03.20 16:37:36
EICOSAPENTAENOIC ACID (EPA) LEVELS FROM VASCEPA® (ICOSAPENT ETHYL) IN REDUCE-IT® STRONGLY CORRELATED WITH CARDIOVASCULAR OUTCOMES
Mar 30, 2020
> Serum EPA Levels Showed Approximately 400% Increase Following Administration of VASCEPA High EPA Levels
> Associated with Substantial Reductions in Multiple Cardiovascular Endpoints Including Heart Failure and Total Mortality
> Die EPA-Spiegel im Serum zeigten nach der Verabreichung von VASCEPA einen ca. 400%igen Anstieg.
> Hohe EPA-Spiegel sind mit einer substanziellen Reduktion mehrerer kardiovaskulärer Endpunkte, einschließlich Herzinsuffizienz und Gesamtsterblichkeit, verbunden.
Amarin Corporation plc (NASDAQ:AMRN), today announced that data presented by Deepak L. Bhatt, M.D., M.P.H., Executive Director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital and Professor of Medicine at Harvard Medical School, at the American College of Cardiology’s 69th Annual Scientific Session Together With World Congress of Cardiology (ACC.20/WCC), demonstrated that on-treatment serum EPA levels from VASCEPA® (icosapent ethyl), administered at 4 g/day, strongly correlated with reductions in cardiovascular events in the REDUCE-IT® study.
As part of a prespecified analysis, serum EPA, DHA (docosahexaenoic acid,) and other biomarkers were measured at baseline and across several visits. Following administration of VASCEPA, a pure, stable, prescription EPA therapy, serum EPA levels showed an approximately 400% increase across the study from baseline (26.1 μg/mL) versus placebo, including to year 1 (144 μg/mL; p=1x10-30). DHA levels were measured and showed a decrease of 2.9% (p=0.002).
On-treatment EPA levels in the VASCEPA group were associated strongly with reduced cardiovascular events, including benefits observed in the primary and key secondary endpoints, each component of these endpoints such as cardiovascular death, as well as benefit in heart failure and total mortality with high on-treatment EPA levels.
These analyses also suggest that achieved EPA with 4g/day of VASCEPA is a marker for the majority of the relative risk reduction observed in REDUCE-IT. The EPA levels achieved in REDUCE-IT were well above levels that can be achieved with diet or with dietary supplements.
Biomarker analyses suggest substantially less contribution of changes in measured lipid, lipoprotein, and inflammatory biomarkers to the cardiovascular benefit observed in REDUCE-IT.
“Following the approval of icosapent ethyl as an adjunct to maximally tolerated statin therapy for a broad range of patients at risk for cardiovascular disease, a common question from physicians has been, what is the mechanism behind the large reductions in cardiovascular events such as heart attacks and strokes seen in REDUCE-IT?” commented Deepak L. Bhatt, M.D., M.P.H., lead investigator of the REDUCE-IT trial. “Now we see that the benefits appear to be driven primarily by on-treatment EPA levels with icosapent ethyl, whereas changes in triglycerides levels and other cardiovascular risk markers, including LDL, HDL, apoB and CRP, appear to be responsible for a significantly lesser proportion of the overall observed benefits.”
Importantly, REDUCE-IT administered an ethyl ester form of EPA. Until the complexities of different chemical forms of EPA are better understood, the benefits of icosapent ethyl in REDUCE-IT cannot be assumed to apply to EPA levels achieved by other chemical compositions.
“This analysis was conducted to better understand the impact of serum EPA levels as delivered through pure, stable, prescription VASCEPA and provides additional insight into the unprecedented results seen in REDUCE-IT,” said Craig Granowitz, M.D., Ph.D., Amarin’s senior vice president and chief medical officer. “These findings further enhance our understanding that results achieved with VASCEPA in the REDUCE-IT study cannot be extrapolated to other agents that continue to be routinely used to reduce cardiovascular risk, despite not having outcomes data nor being FDA approved for this use. Such agents include fibrates, niacin, DHA-containing omega-3 prescription products, or ‘fish oil dietary supplements.’”
Slides from the presentation are available at virtual.acc.org.
https://investor.amarincorp.com/news-releases/news-release-d…
Zitat von bernie55: Eight Data Presentations Relevant to VASCEPA® (Icosapent Ethyl) Capsules and Persistent Cardiovascular Risk to be Presented at the American College of Cardiology’s 69th Annual Scientific Session Together With World Congress of Cardiology (ACC.20/WCC), March 28 – 30
https://finance.yahoo.com/news/eight-data-presentations-rele…" target="_blank" rel="nofollow ugc noopener">https://finance.yahoo.com/news/eight-data-presentations-rele…
EICOSAPENTAENOIC ACID (EPA) LEVELS FROM VASCEPA® (ICOSAPENT ETHYL) IN REDUCE-IT® STRONGLY CORRELATED WITH CARDIOVASCULAR OUTCOMES
Mar 30, 2020
> Serum EPA Levels Showed Approximately 400% Increase Following Administration of VASCEPA High EPA Levels
> Associated with Substantial Reductions in Multiple Cardiovascular Endpoints Including Heart Failure and Total Mortality
> Die EPA-Spiegel im Serum zeigten nach der Verabreichung von VASCEPA einen ca. 400%igen Anstieg.
> Hohe EPA-Spiegel sind mit einer substanziellen Reduktion mehrerer kardiovaskulärer Endpunkte, einschließlich Herzinsuffizienz und Gesamtsterblichkeit, verbunden.
Amarin Corporation plc (NASDAQ:AMRN), today announced that data presented by Deepak L. Bhatt, M.D., M.P.H., Executive Director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital and Professor of Medicine at Harvard Medical School, at the American College of Cardiology’s 69th Annual Scientific Session Together With World Congress of Cardiology (ACC.20/WCC), demonstrated that on-treatment serum EPA levels from VASCEPA® (icosapent ethyl), administered at 4 g/day, strongly correlated with reductions in cardiovascular events in the REDUCE-IT® study.
As part of a prespecified analysis, serum EPA, DHA (docosahexaenoic acid,) and other biomarkers were measured at baseline and across several visits. Following administration of VASCEPA, a pure, stable, prescription EPA therapy, serum EPA levels showed an approximately 400% increase across the study from baseline (26.1 μg/mL) versus placebo, including to year 1 (144 μg/mL; p=1x10-30). DHA levels were measured and showed a decrease of 2.9% (p=0.002).
On-treatment EPA levels in the VASCEPA group were associated strongly with reduced cardiovascular events, including benefits observed in the primary and key secondary endpoints, each component of these endpoints such as cardiovascular death, as well as benefit in heart failure and total mortality with high on-treatment EPA levels.
These analyses also suggest that achieved EPA with 4g/day of VASCEPA is a marker for the majority of the relative risk reduction observed in REDUCE-IT. The EPA levels achieved in REDUCE-IT were well above levels that can be achieved with diet or with dietary supplements.
Biomarker analyses suggest substantially less contribution of changes in measured lipid, lipoprotein, and inflammatory biomarkers to the cardiovascular benefit observed in REDUCE-IT.
“Following the approval of icosapent ethyl as an adjunct to maximally tolerated statin therapy for a broad range of patients at risk for cardiovascular disease, a common question from physicians has been, what is the mechanism behind the large reductions in cardiovascular events such as heart attacks and strokes seen in REDUCE-IT?” commented Deepak L. Bhatt, M.D., M.P.H., lead investigator of the REDUCE-IT trial. “Now we see that the benefits appear to be driven primarily by on-treatment EPA levels with icosapent ethyl, whereas changes in triglycerides levels and other cardiovascular risk markers, including LDL, HDL, apoB and CRP, appear to be responsible for a significantly lesser proportion of the overall observed benefits.”
Importantly, REDUCE-IT administered an ethyl ester form of EPA. Until the complexities of different chemical forms of EPA are better understood, the benefits of icosapent ethyl in REDUCE-IT cannot be assumed to apply to EPA levels achieved by other chemical compositions.
“This analysis was conducted to better understand the impact of serum EPA levels as delivered through pure, stable, prescription VASCEPA and provides additional insight into the unprecedented results seen in REDUCE-IT,” said Craig Granowitz, M.D., Ph.D., Amarin’s senior vice president and chief medical officer. “These findings further enhance our understanding that results achieved with VASCEPA in the REDUCE-IT study cannot be extrapolated to other agents that continue to be routinely used to reduce cardiovascular risk, despite not having outcomes data nor being FDA approved for this use. Such agents include fibrates, niacin, DHA-containing omega-3 prescription products, or ‘fish oil dietary supplements.’”
Slides from the presentation are available at virtual.acc.org.
https://investor.amarincorp.com/news-releases/news-release-d…
Mir ist die Lust an der Börse vergangen, etliche Patente vom US-Patentamt wurden Amarin gewährt,
und ein lokales Gericht macht mit einem Urteil alles platt, an was sollst Du da noch glauben bzw. man kann sich auf Nichts mehr verlassen, Top Produkt, 10 Jahre Entwickling für fast 1 Milliarde US Dollar und dann kommt die Lokalrichterin und zerstört vorerst Alles, scheiß USA!
und ein lokales Gericht macht mit einem Urteil alles platt, an was sollst Du da noch glauben bzw. man kann sich auf Nichts mehr verlassen, Top Produkt, 10 Jahre Entwickling für fast 1 Milliarde US Dollar und dann kommt die Lokalrichterin und zerstört vorerst Alles, scheiß USA!
https://www.sharedeals.de/amarin-crash-wegen-fragwuerdiger-g…
Da fragt man sich, wer hier eigentlich einen an der Waffel hat:
Das Patentamt oder die Justiz, denn zuerst 6 Patente zu erteilen, die danach gleichzeitig alle für offensichtlich für ungültig erklärt werden grenzt in gewisser Weise an Schizophrenie.
Firmen treffen existentielle Investitionsentscheidungen u.a. in Abhängigkeit von erteilten Patenten.
Das ist für sogar ein Grund auf Schadenersatz.
Für mich war/ist AMRN mit über 20$ überbewertet, weswegen ich jetzt die erste Position bei ca. 11,30€ aufgebaut hatte - erstmal ein Griff ins Klo
Da fragt man sich, wer hier eigentlich einen an der Waffel hat:
Das Patentamt oder die Justiz, denn zuerst 6 Patente zu erteilen, die danach gleichzeitig alle für offensichtlich für ungültig erklärt werden grenzt in gewisser Weise an Schizophrenie.
Firmen treffen existentielle Investitionsentscheidungen u.a. in Abhängigkeit von erteilten Patenten.
Das ist für sogar ein Grund auf Schadenersatz.
Für mich war/ist AMRN mit über 20$ überbewertet, weswegen ich jetzt die erste Position bei ca. 11,30€ aufgebaut hatte - erstmal ein Griff ins Klo

Antwort auf Beitrag Nr.: 63.186.978 von Magnetfeldfredy am 31.03.20 11:41:29Ich hätte/ habe auch mit einem anderen Urteil gerechnet. 
Das ist ein Schlag ins Gesicht des Patentrechts und der medizinischen Forschung.
Die jahrelangen Studien ( u.a. Reduce it) von Amarin und die daraus gezogenen Schlussfolgerungen aus ihren Ergebnissen ( siehe unten) waren im „Stand der Technik“ nicht vorhanden( nicht offensichtlich).
Der Nutzenunterschied zwischen EPA und DHA ist also die Innovation bei Vascepa, und diese Schlussfolgerung wurde durch die entscheidende REDUCE-IT-Studie und andere frühere Amarin-Studien gezogen. Diese Schlussfolgerung - und die dafür notwendigen Daten - waren im Stand der Technik nicht vorhanden.
Daher war sie nicht offensichtlich, und als solche sind Vascepa-Patente nicht ungültig.
Das Gericht hat diese Meinung nicht geteilt.
AMARIN COMMENTS ON RULING IN VASCEPA® ANDA LITIGATION
Mar 30, 2020
https://investor.amarincorp.com/news-releases/news-release-d…
Hier die Übersetzung/ Antwort des Artikels
Amarin Corporation plc (NASDAQ:AMRN) kommentierte heute die Entscheidung des US-Bezirksgerichts für den Bezirk Nevada zugunsten der Generikahersteller im Patentrechtsstreit des Unternehmens gegen zwei Einreicher von verkürzten Anträgen auf neue Arzneimittel oder ANDAs für Amarins VASCEPA® (icosapent ethyl) Kapsel-Franchise. Auf der Grundlage von Amarins Überprüfung der Website der US Food and Drug Administration (FDA) wurde kein ANDA für VASCEPA genehmigt, das für die Einführung eines Generikums in den Vereinigten Staaten erforderlich wäre.
Das Unternehmen ist daher nicht der Ansicht, dass die Einführung eines Generikums durch die Prozessbeteiligten, die mit VASCEPA konkurrieren würden, zu diesem Zeitpunkt bevorsteht.
"Amarin stimmt der Entscheidung nicht zu und wird alle verfügbaren Rechtsmittel energisch verfolgen, einschließlich einer Berufung gegen die Entscheidung des Gerichtshofs und einer vorläufigen einstweiligen Verfügung, um, falls ein ANDA von der FDA zugelassen wird, die Einführung von generischen Versionen von VASCEPA in den Vereinigten Staaten zu verhindern", sagte John F. Thero, Präsident und Vorstandsvorsitzender von Amarin. "Wir bei Amarin verfügen über eine starke Bilanz mit Kapazität und Flexibilität und wir planen, zum Wohle unserer Patienten, Ärzte, der breiteren Gesundheitsbranche und unserer Investoren für den Schutz unserer VASCEPA-Franchise zu kämpfen. Wir glauben, dass wir in einer günstigen Position sind, um eine Unterlassungsverfügung gegen eine anhängige Berufung gegen die Einführung von Generika zu erwirken, vorausgesetzt, dass wir eine Kaution zur Sicherung der entgangenen Gewinne von Generika stellen, falls diese in der Berufung obsiegen sollten. Während wir daran arbeiten, alle notwendigen rechtlichen Schritte zu unternehmen, um unser geistiges Eigentum zu verteidigen und zu schützen, werden wir weiterhin unsere Aufklärungs- und Werbemaßnahmen für VASCEPA bei der Behandlung von indizierten Patienten mit hohem Risiko für kardiovaskuläre Ereignisse wie Herzinfarkt und Schlaganfall vorantreiben. Nachdem wir das Ergebnis unserer Bemühungen zur Verhinderung der Markteinführung eines Generikums ermittelt haben (falls eine ANDA-Zulassung erteilt wird), erwarten wir ein Update darüber, wie wir bestimmte Werbeaktivitäten für VASCEPA in den Vereinigten Staaten anpassen würden".

Das ist ein Schlag ins Gesicht des Patentrechts und der medizinischen Forschung.

Die jahrelangen Studien ( u.a. Reduce it) von Amarin und die daraus gezogenen Schlussfolgerungen aus ihren Ergebnissen ( siehe unten) waren im „Stand der Technik“ nicht vorhanden( nicht offensichtlich).
Der Nutzenunterschied zwischen EPA und DHA ist also die Innovation bei Vascepa, und diese Schlussfolgerung wurde durch die entscheidende REDUCE-IT-Studie und andere frühere Amarin-Studien gezogen. Diese Schlussfolgerung - und die dafür notwendigen Daten - waren im Stand der Technik nicht vorhanden.
Daher war sie nicht offensichtlich, und als solche sind Vascepa-Patente nicht ungültig.
Das Gericht hat diese Meinung nicht geteilt.

AMARIN COMMENTS ON RULING IN VASCEPA® ANDA LITIGATION
Mar 30, 2020
https://investor.amarincorp.com/news-releases/news-release-d…
Hier die Übersetzung/ Antwort des Artikels
Amarin Corporation plc (NASDAQ:AMRN) kommentierte heute die Entscheidung des US-Bezirksgerichts für den Bezirk Nevada zugunsten der Generikahersteller im Patentrechtsstreit des Unternehmens gegen zwei Einreicher von verkürzten Anträgen auf neue Arzneimittel oder ANDAs für Amarins VASCEPA® (icosapent ethyl) Kapsel-Franchise. Auf der Grundlage von Amarins Überprüfung der Website der US Food and Drug Administration (FDA) wurde kein ANDA für VASCEPA genehmigt, das für die Einführung eines Generikums in den Vereinigten Staaten erforderlich wäre.
Das Unternehmen ist daher nicht der Ansicht, dass die Einführung eines Generikums durch die Prozessbeteiligten, die mit VASCEPA konkurrieren würden, zu diesem Zeitpunkt bevorsteht.
"Amarin stimmt der Entscheidung nicht zu und wird alle verfügbaren Rechtsmittel energisch verfolgen, einschließlich einer Berufung gegen die Entscheidung des Gerichtshofs und einer vorläufigen einstweiligen Verfügung, um, falls ein ANDA von der FDA zugelassen wird, die Einführung von generischen Versionen von VASCEPA in den Vereinigten Staaten zu verhindern", sagte John F. Thero, Präsident und Vorstandsvorsitzender von Amarin. "Wir bei Amarin verfügen über eine starke Bilanz mit Kapazität und Flexibilität und wir planen, zum Wohle unserer Patienten, Ärzte, der breiteren Gesundheitsbranche und unserer Investoren für den Schutz unserer VASCEPA-Franchise zu kämpfen. Wir glauben, dass wir in einer günstigen Position sind, um eine Unterlassungsverfügung gegen eine anhängige Berufung gegen die Einführung von Generika zu erwirken, vorausgesetzt, dass wir eine Kaution zur Sicherung der entgangenen Gewinne von Generika stellen, falls diese in der Berufung obsiegen sollten. Während wir daran arbeiten, alle notwendigen rechtlichen Schritte zu unternehmen, um unser geistiges Eigentum zu verteidigen und zu schützen, werden wir weiterhin unsere Aufklärungs- und Werbemaßnahmen für VASCEPA bei der Behandlung von indizierten Patienten mit hohem Risiko für kardiovaskuläre Ereignisse wie Herzinfarkt und Schlaganfall vorantreiben. Nachdem wir das Ergebnis unserer Bemühungen zur Verhinderung der Markteinführung eines Generikums ermittelt haben (falls eine ANDA-Zulassung erteilt wird), erwarten wir ein Update darüber, wie wir bestimmte Werbeaktivitäten für VASCEPA in den Vereinigten Staaten anpassen würden".
Antwort auf Beitrag Nr.: 63.186.978 von Magnetfeldfredy am 31.03.20 11:41:29Es ist diese grottige, alberne, bornierte, dumme(!) und von objektiven Rechtssätzen weit entfernte Sprechung amerikanischen Rechts, die einem Roulette ähnlicher sind. Wer (in Texas) nach wie vor die Todesstrafe für Pferdediebstahl aussprechen kann, die Todesstrafe als solches überhaupt (an menschenverachtendem Verhalten nicht zu überbieten) und für "gebrühte Eier bei McDoof" 2 Mio $ Schadenssersatz rechtens spricht, der hat als System(!) nicht mehr alle ander Waffel! So gern ich in die USA reise, so gern ich die Ami´s als solche auch mag (weil in vielen Bereichen las liebenswert und nett kennen gelernt), so klar sage ich auch, dass deren Rechtsprechung dekadent, überzeitigt und dumm ist! Dem Einwurf der "Schizophrenie" von @tippse ist nicht mehr viel hinzu zu fügen!
Mein Beileid Leute ist ein harter Schlag für alle die an Amarin geglaubt haben...…..echt elend......
Neben der aktuell belastenden Coronasituation kommt jetzt auch noch dieses Gerichtsurteil dazu, von denen wir überrollt werden und uns sowohl emotional hart trifft als auch finanziell so richtig "abledert".
Was kann kommen ?
Mal einige Fragen, die im Raum stehen bzw. diskutiert werden
1- Wird/Kann das US - Patentamt ( als eigenständige Institut) dieses Gerichsturteil so stehen lassen ?
Hat das Patentamt nicht eigene schlüssige Tatsachen/ Fakten zugrunde gelegt für die Bewilligung der AMRN -Patente ?
2 - Amarin wird in die Berufung gehen - welches Gericht wird sich dieses Falls annehmen ? Wie ist dieses Gericht ( Richter) politisch ausgerichtet ?
3 - Auf der Grundlage von Amarins Überprüfung der Website der US Food and Drug Administration (FDA) wurde kein ANDA für VASCEPA genehmigt, das für die Einführung eines Generikums in den Vereinigten Staaten erforderlich wäre.
Müssten dann nicht erst Studien durchgeführt werden und diese von der FDA geprüft und dann genehmigt werden ?
Ab wann dürfte überhaupt dann mit einer möglichen Einführung von Generika gerechnet werden ?
Wäre super, eure Meinungen/ Einschätzungen zu lesen .

Was kann kommen ?
Mal einige Fragen, die im Raum stehen bzw. diskutiert werden
1- Wird/Kann das US - Patentamt ( als eigenständige Institut) dieses Gerichsturteil so stehen lassen ?
Hat das Patentamt nicht eigene schlüssige Tatsachen/ Fakten zugrunde gelegt für die Bewilligung der AMRN -Patente ?
2 - Amarin wird in die Berufung gehen - welches Gericht wird sich dieses Falls annehmen ? Wie ist dieses Gericht ( Richter) politisch ausgerichtet ?
3 - Auf der Grundlage von Amarins Überprüfung der Website der US Food and Drug Administration (FDA) wurde kein ANDA für VASCEPA genehmigt, das für die Einführung eines Generikums in den Vereinigten Staaten erforderlich wäre.
Müssten dann nicht erst Studien durchgeführt werden und diese von der FDA geprüft und dann genehmigt werden ?
Ab wann dürfte überhaupt dann mit einer möglichen Einführung von Generika gerechnet werden ?
Wäre super, eure Meinungen/ Einschätzungen zu lesen .

Antwort auf Beitrag Nr.: 63.191.466 von bernie55 am 31.03.20 17:05:58Hier steht eigentlich alles drin.
Neben" target="_blank" rel="nofollow ugc noopener">https://www.sharedeals.de/amarin-crash-wegen-fragwuerdiger-gerichtsentscheidung/
Was kann kommen ?
Mal einige Fragen, die im Raum stehen bzw. diskutiert werden
1- Wird/Kann das US - Patentamt ( als eigenständige Institut) dieses Gerichsturteil so stehen lassen ?
Hat das Patentamt nicht eigene schlüssige Tatsachen/ Fakten zugrunde gelegt für die Bewilligung der AMRN -Patente ?
2 - Amarin wird in die Berufung gehen - welches Gericht wird sich dieses Falls annehmen ? Wie ist dieses Gericht ( Richter) politisch ausgerichtet ?
3 - Auf der Grundlage von Amarins Überprüfung der Website der US Food and Drug Administration (FDA) wurde kein ANDA für VASCEPA genehmigt, das für die Einführung eines Generikums in den Vereinigten Staaten erforderlich wäre.
Müssten dann nicht erst Studien durchgeführt werden und diese von der FDA geprüft und dann genehmigt werden ?
Ab wann dürfte überhaupt dann mit einer möglichen Einführung von Generika gerechnet werden ?
Wäre super, eure Meinungen/ Einschätzungen zu lesen .
Neben" target="_blank" rel="nofollow ugc noopener">https://www.sharedeals.de/amarin-crash-wegen-fragwuerdiger-gerichtsentscheidung/
Zitat von bernie55: Nebender aktuell belastenden Coronasituation kommt jetzt auch noch dieses Gerichtsurteil dazu, von denen wir überrollt werden und uns sowohl emotional hart trifft als auch finanziell so richtig "abledert".

Was kann kommen ?
Mal einige Fragen, die im Raum stehen bzw. diskutiert werden
1- Wird/Kann das US - Patentamt ( als eigenständige Institut) dieses Gerichsturteil so stehen lassen ?
Hat das Patentamt nicht eigene schlüssige Tatsachen/ Fakten zugrunde gelegt für die Bewilligung der AMRN -Patente ?
2 - Amarin wird in die Berufung gehen - welches Gericht wird sich dieses Falls annehmen ? Wie ist dieses Gericht ( Richter) politisch ausgerichtet ?
3 - Auf der Grundlage von Amarins Überprüfung der Website der US Food and Drug Administration (FDA) wurde kein ANDA für VASCEPA genehmigt, das für die Einführung eines Generikums in den Vereinigten Staaten erforderlich wäre.
Müssten dann nicht erst Studien durchgeführt werden und diese von der FDA geprüft und dann genehmigt werden ?
Ab wann dürfte überhaupt dann mit einer möglichen Einführung von Generika gerechnet werden ?
Wäre super, eure Meinungen/ Einschätzungen zu lesen .

Antwort auf Beitrag Nr.: 63.191.679 von Frhstck am 31.03.20 17:27:54Das sind alles berechtigte Fragen.
Ich halte das Urteil für absurd, wenn man berücksichtigt, wie viel Geld man erst in Studien stecken musste, um dann von einer Richterin erzählt zu bekommen, das ganze sei nichts wert, weil "offensichtlich". Trotzdem ist es erst mal in der Welt und wird auf längere Sicht den Kurs weit unten halten. Wo er sich einpendeln wird, weiß keiner. Vermutlich ist auch der heutige Absturz übertrieben - aber kurzfristig sind solche Übertreibungen nun mal möglich, wenn hoher verkaufsdruck besteht.
Man steht ja nicht vor der Pleite, sondern hat noch ca 500. Mio auf dem Konto, das ist heute 1 Drittel der Mkt-Kap.
Ich halte das Urteil für absurd, wenn man berücksichtigt, wie viel Geld man erst in Studien stecken musste, um dann von einer Richterin erzählt zu bekommen, das ganze sei nichts wert, weil "offensichtlich". Trotzdem ist es erst mal in der Welt und wird auf längere Sicht den Kurs weit unten halten. Wo er sich einpendeln wird, weiß keiner. Vermutlich ist auch der heutige Absturz übertrieben - aber kurzfristig sind solche Übertreibungen nun mal möglich, wenn hoher verkaufsdruck besteht.
Man steht ja nicht vor der Pleite, sondern hat noch ca 500. Mio auf dem Konto, das ist heute 1 Drittel der Mkt-Kap.
Antwort auf Beitrag Nr.: 63.191.466 von bernie55 am 31.03.20 17:05:58@tippse
@Frhstk
Danke für euren Linkhinweis - ich stelle diesen Artikel jetzt mal ins Forum, da dieser einige meiner Fragen "beleuchtet".
Sascha / 31.03.20 / 9:20
Amarin: Crash – wegen fragwürdiger Gerichtsentscheidung!
In dem Gerichtsverfahren vor dem District Court for the District of Nevada ging es um insgesamt sechs Patente des Unternehmens in Bezug auf sein wichtigstes Produkt Vascepa. Bei Vascepa handelt es sich um eine auf Fischöl basierende Pille, die bei zu hohen Blutfettwerten (Stichwort: LDL-Cholesterin) zum Einsatz kommt. Die US-amerikanische Arzneimittelaufsicht FDA hatte dieses Medikament grundsätzlich bereits vor einigen Jahren zugelassen, wobei man jedoch den Anwendungsbereich zunächst noch eng begrenzte.
Amarin verliert Patentrechtsstreit in erster Instanz...
Erst nachdem weitere jahrelange klinische Studien zu einem positiven Abschluss gebracht werden konnten, kam es dann zu einer sogenannten Label-Ausweitung. Dadurch war Vascepa zuletzt auf dem besten Weg ein Blockbuster-Medikament zu werden. Als Blockbuster werden dabei in der Branche Medikamente bezeichnet, die einen Jahresumsatz in Höhe von mindestens einer Milliarde US-Dollar einspielen. Angesichts solcher Aussichten also kein Wunder, dass sich auch andere Biopharma-Unternehmen grundsätzlich für diesen Markt interessieren.
Daher hatten die britische Hikma Pharmaceuticals sowie die indische Dr. Reddy's Laboratories gegen Patente von Amarin zum Schutz von Vascepa geklagt. Experten hatten diesen Klagen jedoch wenig Chancen eingeräumt. So empfahlen beispielsweise die Analysten von Goldman Sachs die Aktie noch vor rund zwei Wochen mit Kursziel 28 USD zum Kauf. Doch gestern urteilte die Richterin Miranda Du, dass die Ungültigkeit von Amarins Vascepa-Patenten „offensichtlich“ sei.
Aktie crasht, Unternehmen kündigt Ausschöpfung aller Rechtsmittel an!
Heute Nacht bestätigte Amarin dann in einer Pressemitteilung die Meldungen, kündigte jedoch zugleich an alle Rechtsmittel gegen diese Entscheidung der Richterin ausschöpfen zu wollen. So plane man nicht nur in Berufung zu gehen, sondern zum Schutz von Unternehmen und Medikament auch eine einstweilige Verfügung zur Aussetzung des Urteils bis zu einer Entscheidung des Berufungsgerichts zu beantragen.
Ferner habe man in der Datenbank der FDA geprüft, ob es dort einen Antrag auf Zulassung eines Generikums (ANDA) gebe, was jedoch bis dato nicht der Fall sei. Daher gehe man derzeit nicht davon aus, dass irgendein anderes Unternehmen kurzfristig ein solches Generikum auf den Markt bringen wird. Denn bevor dies möglich sei, müsse zunächst einmal ein Antrag auf Zulassung bei der FDA eingereicht und positiv von dieser beschieden werden.
„Vor Gericht und auf hoher See immer in Gottes Hand“
Wie ist diese ganze Entwicklung nun zu bewerten? Nun, zunächst einmal ist diese Entscheidung der Richterin eine negative Überraschung, weshalb der heftige Ausverkauf der Aktie logisch erscheint. Die Kernfrage ist jedoch gar nicht mal so sehr, ob ein Berufungsgericht diese Entscheidung kippt. Die Kernfrage ist, ob es einem anderen Unternehmen gelingt ein Generikum zu Vascepa möglichst kurzfristig auf den Markt zu bringen.
Solange der Rechtsstreit um die Patente noch nicht abschließend entschieden ist, halte ich diese Gefahr jedoch für recht gering. Denn kein Unternehmen wird sich der Gefahr aussetzen wollen hinterher dann möglicherweise Schadenersatz in Millionenhöhe leisten zu müssen. Zumal man ja gerade erst wieder gezeigt bekommen hat, dass man „vor Gericht und auf hoher See in Gottes Hand ist“.
Fazit: Sehr mutige Anleger greifen zu, aber...
US-amerikanische Trader kommentierten das Urteil der Richterin im Internet mit den Worten, dass „es wieder einmal zeige wie eine Frau ganze Leben zerstören könne“ beziehungsweise das „diese Richterin wohl auf fallende Kurse bei Amarin gewettet habe“. In der Tat halte auch ich diese Entscheidung für äußerst fragwürdig. Denn das US-Patentamt mag durchaus mal bei der Vergabe eines einzelnen Patents eine Fehlentscheidung treffen. Aber bei gleich sechs Patenten?
Da es jedoch kurzfristig ohnehin noch kein Generikum gibt, Amarin die Zeit bis zur Markteinführung eines solchen sicherlich noch hinauszögern kann und letztlich durchaus gute Chancen bestehen, dass ein Berufungsgericht diese Entscheidung der Richterin Miranda Du noch kippt, können sehr mutige Anleger die Aktie in den aktuellen Crash hinein durchaus kaufen. Optimalerweise bezahlt man dabei weniger als 4,50 USD für die Aktie.
Denn schon kurzfristig halte ich eine deutliche Kurserholung in Richtung sechs bis sieben USD für möglich. Sollte Amarin seine Patente vor Gericht doch noch verteidigen können, wären mittelfristig auch wieder zweistellige Kursziele möglich. Aber selbst wenn nicht, dürfte sich Vascepa im Laufe der nächsten zwei, drei Jahre zu einem Blockbuster entwickeln. Konkurrenz muss nicht immer schlecht sein, sondern kann schließlich auch das Geschäft beleben!
https://www.sharedeals.de/amarin-crash-wegen-fragwuerdiger-g…
@Frhstk
Danke für euren Linkhinweis - ich stelle diesen Artikel jetzt mal ins Forum, da dieser einige meiner Fragen "beleuchtet".
Sascha / 31.03.20 / 9:20
Amarin: Crash – wegen fragwürdiger Gerichtsentscheidung!
In dem Gerichtsverfahren vor dem District Court for the District of Nevada ging es um insgesamt sechs Patente des Unternehmens in Bezug auf sein wichtigstes Produkt Vascepa. Bei Vascepa handelt es sich um eine auf Fischöl basierende Pille, die bei zu hohen Blutfettwerten (Stichwort: LDL-Cholesterin) zum Einsatz kommt. Die US-amerikanische Arzneimittelaufsicht FDA hatte dieses Medikament grundsätzlich bereits vor einigen Jahren zugelassen, wobei man jedoch den Anwendungsbereich zunächst noch eng begrenzte.
Amarin verliert Patentrechtsstreit in erster Instanz...
Erst nachdem weitere jahrelange klinische Studien zu einem positiven Abschluss gebracht werden konnten, kam es dann zu einer sogenannten Label-Ausweitung. Dadurch war Vascepa zuletzt auf dem besten Weg ein Blockbuster-Medikament zu werden. Als Blockbuster werden dabei in der Branche Medikamente bezeichnet, die einen Jahresumsatz in Höhe von mindestens einer Milliarde US-Dollar einspielen. Angesichts solcher Aussichten also kein Wunder, dass sich auch andere Biopharma-Unternehmen grundsätzlich für diesen Markt interessieren.
Daher hatten die britische Hikma Pharmaceuticals sowie die indische Dr. Reddy's Laboratories gegen Patente von Amarin zum Schutz von Vascepa geklagt. Experten hatten diesen Klagen jedoch wenig Chancen eingeräumt. So empfahlen beispielsweise die Analysten von Goldman Sachs die Aktie noch vor rund zwei Wochen mit Kursziel 28 USD zum Kauf. Doch gestern urteilte die Richterin Miranda Du, dass die Ungültigkeit von Amarins Vascepa-Patenten „offensichtlich“ sei.
Aktie crasht, Unternehmen kündigt Ausschöpfung aller Rechtsmittel an!
Heute Nacht bestätigte Amarin dann in einer Pressemitteilung die Meldungen, kündigte jedoch zugleich an alle Rechtsmittel gegen diese Entscheidung der Richterin ausschöpfen zu wollen. So plane man nicht nur in Berufung zu gehen, sondern zum Schutz von Unternehmen und Medikament auch eine einstweilige Verfügung zur Aussetzung des Urteils bis zu einer Entscheidung des Berufungsgerichts zu beantragen.
Ferner habe man in der Datenbank der FDA geprüft, ob es dort einen Antrag auf Zulassung eines Generikums (ANDA) gebe, was jedoch bis dato nicht der Fall sei. Daher gehe man derzeit nicht davon aus, dass irgendein anderes Unternehmen kurzfristig ein solches Generikum auf den Markt bringen wird. Denn bevor dies möglich sei, müsse zunächst einmal ein Antrag auf Zulassung bei der FDA eingereicht und positiv von dieser beschieden werden.
„Vor Gericht und auf hoher See immer in Gottes Hand“
Wie ist diese ganze Entwicklung nun zu bewerten? Nun, zunächst einmal ist diese Entscheidung der Richterin eine negative Überraschung, weshalb der heftige Ausverkauf der Aktie logisch erscheint. Die Kernfrage ist jedoch gar nicht mal so sehr, ob ein Berufungsgericht diese Entscheidung kippt. Die Kernfrage ist, ob es einem anderen Unternehmen gelingt ein Generikum zu Vascepa möglichst kurzfristig auf den Markt zu bringen.
Solange der Rechtsstreit um die Patente noch nicht abschließend entschieden ist, halte ich diese Gefahr jedoch für recht gering. Denn kein Unternehmen wird sich der Gefahr aussetzen wollen hinterher dann möglicherweise Schadenersatz in Millionenhöhe leisten zu müssen. Zumal man ja gerade erst wieder gezeigt bekommen hat, dass man „vor Gericht und auf hoher See in Gottes Hand ist“.
Fazit: Sehr mutige Anleger greifen zu, aber...
US-amerikanische Trader kommentierten das Urteil der Richterin im Internet mit den Worten, dass „es wieder einmal zeige wie eine Frau ganze Leben zerstören könne“ beziehungsweise das „diese Richterin wohl auf fallende Kurse bei Amarin gewettet habe“. In der Tat halte auch ich diese Entscheidung für äußerst fragwürdig. Denn das US-Patentamt mag durchaus mal bei der Vergabe eines einzelnen Patents eine Fehlentscheidung treffen. Aber bei gleich sechs Patenten?
Da es jedoch kurzfristig ohnehin noch kein Generikum gibt, Amarin die Zeit bis zur Markteinführung eines solchen sicherlich noch hinauszögern kann und letztlich durchaus gute Chancen bestehen, dass ein Berufungsgericht diese Entscheidung der Richterin Miranda Du noch kippt, können sehr mutige Anleger die Aktie in den aktuellen Crash hinein durchaus kaufen. Optimalerweise bezahlt man dabei weniger als 4,50 USD für die Aktie.
Denn schon kurzfristig halte ich eine deutliche Kurserholung in Richtung sechs bis sieben USD für möglich. Sollte Amarin seine Patente vor Gericht doch noch verteidigen können, wären mittelfristig auch wieder zweistellige Kursziele möglich. Aber selbst wenn nicht, dürfte sich Vascepa im Laufe der nächsten zwei, drei Jahre zu einem Blockbuster entwickeln. Konkurrenz muss nicht immer schlecht sein, sondern kann schließlich auch das Geschäft beleben!
https://www.sharedeals.de/amarin-crash-wegen-fragwuerdiger-g…
Lieber Bernie,
die Kurziele gehen weiter von US Dollar 4 bis US Dollar 51, hier Cantor:
Cantor Fitzgerald analyst Louise Chen said Amarin's loss of its Vascepa patent case was surprising, but she expects the stock to "over-correct" and then potentially bounce back from there as she contends that it is "not game over yet." After catching up with Amarin's management, she noted that the company will appeal the ruling and said that the decision does not affect international opportunities in the EU, China and rest of the world, which "could still total billions of dollars in sales." Chen keeps an Overweight rating and $35 price target on Amarin shares.
Read more at:
https://thefly.com/landingPageNews.php?id=3063794
Auch ich bleibe dabei nach einer schlaflosen Nacht, Vascepa wird in der EU eine 10-jährige Exklusivität haben in Canada 8 Jahre und im Rest der Welt auch sehr lange!
Das Urteil ist eine Frechheit und Amarin hat sehr gute Anwälte und schon einmal gegen die FDA gewonnnen, ich glaube das Spiel ist noch nicht aus und es werden hinter den Kulissen einige Schweinereien ablaufen!
Für mich persönlich bedeutet es Riesenverluste und Zweifel am System, aber am Ende wird sich Amarin mit Vascepa durchsetzen, wir brauchen halt wieder Zeit und viel Nerven! Machs guad!
die Kurziele gehen weiter von US Dollar 4 bis US Dollar 51, hier Cantor:
Cantor Fitzgerald analyst Louise Chen said Amarin's loss of its Vascepa patent case was surprising, but she expects the stock to "over-correct" and then potentially bounce back from there as she contends that it is "not game over yet." After catching up with Amarin's management, she noted that the company will appeal the ruling and said that the decision does not affect international opportunities in the EU, China and rest of the world, which "could still total billions of dollars in sales." Chen keeps an Overweight rating and $35 price target on Amarin shares.
Read more at:
https://thefly.com/landingPageNews.php?id=3063794
Auch ich bleibe dabei nach einer schlaflosen Nacht, Vascepa wird in der EU eine 10-jährige Exklusivität haben in Canada 8 Jahre und im Rest der Welt auch sehr lange!
Das Urteil ist eine Frechheit und Amarin hat sehr gute Anwälte und schon einmal gegen die FDA gewonnnen, ich glaube das Spiel ist noch nicht aus und es werden hinter den Kulissen einige Schweinereien ablaufen!
Für mich persönlich bedeutet es Riesenverluste und Zweifel am System, aber am Ende wird sich Amarin mit Vascepa durchsetzen, wir brauchen halt wieder Zeit und viel Nerven! Machs guad!
Antwort auf Beitrag Nr.: 63.192.435 von bernie55 am 31.03.20 18:33:00
Klingt logisch! Ich HATTE bis vor kurzem noch Amarin Aktien. War sehr davon überzeugt. Wegen Corona habe ich alle Amerika Aktien verkauft. Außer Abbott, weil die gerade einen Corona Schnelltest rausgebracht haben.
Trotz des fiesen Schadens, den die Amarin Aktie nun abbekommen hat, werde ich jetzt mal vorsichtig nachkaufen. Weniger geht kaum, mehr kann viel passieren.
Zitat von bernie55: @tippse
@Frhstk
Danke für euren Linkhinweis - ich stelle diesen Artikel jetzt mal ins Forum, da dieser einige meiner Fragen "beleuchtet".
Sascha / 31.03.20 / 9:20
Amarin: Crash – wegen fragwürdiger Gerichtsentscheidung!
In dem Gerichtsverfahren vor dem District Court for the District of Nevada ging es um insgesamt sechs Patente des Unternehmens in Bezug auf sein wichtigstes Produkt Vascepa. Bei Vascepa handelt es sich um eine auf Fischöl basierende Pille, die bei zu hohen Blutfettwerten (Stichwort: LDL-Cholesterin) zum Einsatz kommt. Die US-amerikanische Arzneimittelaufsicht FDA hatte dieses Medikament grundsätzlich bereits vor einigen Jahren zugelassen, wobei man jedoch den Anwendungsbereich zunächst noch eng begrenzte.
Amarin verliert Patentrechtsstreit in erster Instanz...
Erst nachdem weitere jahrelange klinische Studien zu einem positiven Abschluss gebracht werden konnten, kam es dann zu einer sogenannten Label-Ausweitung. Dadurch war Vascepa zuletzt auf dem besten Weg ein Blockbuster-Medikament zu werden. Als Blockbuster werden dabei in der Branche Medikamente bezeichnet, die einen Jahresumsatz in Höhe von mindestens einer Milliarde US-Dollar einspielen. Angesichts solcher Aussichten also kein Wunder, dass sich auch andere Biopharma-Unternehmen grundsätzlich für diesen Markt interessieren.
Daher hatten die britische Hikma Pharmaceuticals sowie die indische Dr. Reddy's Laboratories gegen Patente von Amarin zum Schutz von Vascepa geklagt. Experten hatten diesen Klagen jedoch wenig Chancen eingeräumt. So empfahlen beispielsweise die Analysten von Goldman Sachs die Aktie noch vor rund zwei Wochen mit Kursziel 28 USD zum Kauf. Doch gestern urteilte die Richterin Miranda Du, dass die Ungültigkeit von Amarins Vascepa-Patenten „offensichtlich“ sei.
Aktie crasht, Unternehmen kündigt Ausschöpfung aller Rechtsmittel an!
Heute Nacht bestätigte Amarin dann in einer Pressemitteilung die Meldungen, kündigte jedoch zugleich an alle Rechtsmittel gegen diese Entscheidung der Richterin ausschöpfen zu wollen. So plane man nicht nur in Berufung zu gehen, sondern zum Schutz von Unternehmen und Medikament auch eine einstweilige Verfügung zur Aussetzung des Urteils bis zu einer Entscheidung des Berufungsgerichts zu beantragen.
Ferner habe man in der Datenbank der FDA geprüft, ob es dort einen Antrag auf Zulassung eines Generikums (ANDA) gebe, was jedoch bis dato nicht der Fall sei. Daher gehe man derzeit nicht davon aus, dass irgendein anderes Unternehmen kurzfristig ein solches Generikum auf den Markt bringen wird. Denn bevor dies möglich sei, müsse zunächst einmal ein Antrag auf Zulassung bei der FDA eingereicht und positiv von dieser beschieden werden.
„Vor Gericht und auf hoher See immer in Gottes Hand“
Wie ist diese ganze Entwicklung nun zu bewerten? Nun, zunächst einmal ist diese Entscheidung der Richterin eine negative Überraschung, weshalb der heftige Ausverkauf der Aktie logisch erscheint. Die Kernfrage ist jedoch gar nicht mal so sehr, ob ein Berufungsgericht diese Entscheidung kippt. Die Kernfrage ist, ob es einem anderen Unternehmen gelingt ein Generikum zu Vascepa möglichst kurzfristig auf den Markt zu bringen.
Solange der Rechtsstreit um die Patente noch nicht abschließend entschieden ist, halte ich diese Gefahr jedoch für recht gering. Denn kein Unternehmen wird sich der Gefahr aussetzen wollen hinterher dann möglicherweise Schadenersatz in Millionenhöhe leisten zu müssen. Zumal man ja gerade erst wieder gezeigt bekommen hat, dass man „vor Gericht und auf hoher See in Gottes Hand ist“.
Fazit: Sehr mutige Anleger greifen zu, aber...
US-amerikanische Trader kommentierten das Urteil der Richterin im Internet mit den Worten, dass „es wieder einmal zeige wie eine Frau ganze Leben zerstören könne“ beziehungsweise das „diese Richterin wohl auf fallende Kurse bei Amarin gewettet habe“. In der Tat halte auch ich diese Entscheidung für äußerst fragwürdig. Denn das US-Patentamt mag durchaus mal bei der Vergabe eines einzelnen Patents eine Fehlentscheidung treffen. Aber bei gleich sechs Patenten?
Da es jedoch kurzfristig ohnehin noch kein Generikum gibt, Amarin die Zeit bis zur Markteinführung eines solchen sicherlich noch hinauszögern kann und letztlich durchaus gute Chancen bestehen, dass ein Berufungsgericht diese Entscheidung der Richterin Miranda Du noch kippt, können sehr mutige Anleger die Aktie in den aktuellen Crash hinein durchaus kaufen. Optimalerweise bezahlt man dabei weniger als 4,50 USD für die Aktie.
Denn schon kurzfristig halte ich eine deutliche Kurserholung in Richtung sechs bis sieben USD für möglich. Sollte Amarin seine Patente vor Gericht doch noch verteidigen können, wären mittelfristig auch wieder zweistellige Kursziele möglich. Aber selbst wenn nicht, dürfte sich Vascepa im Laufe der nächsten zwei, drei Jahre zu einem Blockbuster entwickeln. Konkurrenz muss nicht immer schlecht sein, sondern kann schließlich auch das Geschäft beleben!
https://www.sharedeals.de/amarin-crash-wegen-fragwuerdiger-g…
Klingt logisch! Ich HATTE bis vor kurzem noch Amarin Aktien. War sehr davon überzeugt. Wegen Corona habe ich alle Amerika Aktien verkauft. Außer Abbott, weil die gerade einen Corona Schnelltest rausgebracht haben.
Trotz des fiesen Schadens, den die Amarin Aktie nun abbekommen hat, werde ich jetzt mal vorsichtig nachkaufen. Weniger geht kaum, mehr kann viel passieren.
Super Info auf Amarins Homepage unter FAQ, Vascepa hat 10 Jahre Exklusivität in Europa ohne das Generika hergestellt werden dürfen, das alleine ist ein Milliardenmarkt, der Kurssturz ist total übertrieben und wird hoffentlich bald korrigiert:
Will the European Medicines Agency grant regulatory exclusivity to Amarin® for Icosapent Ethyl (VASCEPA®) if the marketing authorization application is approved?The European Medicines Agency (EMA) has conveyed in their Day 120 communication to Amarin for the ongoing centralized procedure that the EMA consider the active substance icosapent ethyl contained in the medicinal product VASCEPA to be qualified as a new active substance in comparison to the known mixture of “omega-3-acid ethyl esters 90” previously authorized in the European Union. Per regulations published by the European Medicines Agency, ‘the period of eight years from the initial authorisation of a medicine during which the marketing authorisation holder benefits from the exclusive rights to the results of preclinical tests and clinical trials on the medicine.’1, 2 The regulations also state ‘Extra market protection if new indication is registered in first 8 years and brings significant clinical benefit over existing therapies.’ Additional approval is granted per the summary of the European regulatory timeline below:New active substance (NAS): 10 years of market protection•8 years of data protection (no generic pre-approval activity) •2 additional years of market protection (10 years total market protection)•Potential for 1 additional year of market protectionVASCEPA (icosapent ethyl) is not currently approved for marketing or sale in Europe. As is one of the the purposes of the Day 120 letter from the EMA, it included various questions for which the EMA seeks adequate response before it will decide on such approval. There are risks involved with any such approval which risks are described in the Risk Factors section of Amarin’s Annual Report on Form 10-K. As is typical in a regulatory review process, such questions are between the regulatory authorities and the sponsor and outside the scope of this response.Dated: March 31, 2020 1https://www.ema.europa.eu/en/documents/presentation/presenta…
•2 additional years of market protection (10 years total market protection)•Potential for 1 additional year of market protectionVASCEPA (icosapent ethyl) is not currently approved for marketing or sale in Europe. As is one of the the purposes of the Day 120 letter from the EMA, it included various questions for which the EMA seeks adequate response before it will decide on such approval. There are risks involved with any such approval which risks are described in the Risk Factors section of Amarin’s Annual Report on Form 10-K. As is typical in a regulatory review process, such questions are between the regulatory authorities and the sponsor and outside the scope of this response.Dated: March 31, 2020 1https://www.ema.europa.eu/en/documents/presentation/presenta…
Da sieht man halt, dass Europa Medikamentenhersteller viel besser schützt als die degenerierte USA, Amarin hat fast 1 Milliarde an Kosten gehabt mit drei erfolgreichen Phase 3 Ergebnissen die in sensationellen Reduce It Ergebnissen mündeten und nun macht eine Lokalrichterin alles kapput, ignoriert das Patentamt........, das glaube ich nicht!
Will the European Medicines Agency grant regulatory exclusivity to Amarin® for Icosapent Ethyl (VASCEPA®) if the marketing authorization application is approved?The European Medicines Agency (EMA) has conveyed in their Day 120 communication to Amarin for the ongoing centralized procedure that the EMA consider the active substance icosapent ethyl contained in the medicinal product VASCEPA to be qualified as a new active substance in comparison to the known mixture of “omega-3-acid ethyl esters 90” previously authorized in the European Union. Per regulations published by the European Medicines Agency, ‘the period of eight years from the initial authorisation of a medicine during which the marketing authorisation holder benefits from the exclusive rights to the results of preclinical tests and clinical trials on the medicine.’1, 2 The regulations also state ‘Extra market protection if new indication is registered in first 8 years and brings significant clinical benefit over existing therapies.’ Additional approval is granted per the summary of the European regulatory timeline below:New active substance (NAS): 10 years of market protection•8 years of data protection (no generic pre-approval activity)
 •2 additional years of market protection (10 years total market protection)•Potential for 1 additional year of market protectionVASCEPA (icosapent ethyl) is not currently approved for marketing or sale in Europe. As is one of the the purposes of the Day 120 letter from the EMA, it included various questions for which the EMA seeks adequate response before it will decide on such approval. There are risks involved with any such approval which risks are described in the Risk Factors section of Amarin’s Annual Report on Form 10-K. As is typical in a regulatory review process, such questions are between the regulatory authorities and the sponsor and outside the scope of this response.Dated: March 31, 2020 1https://www.ema.europa.eu/en/documents/presentation/presenta…
•2 additional years of market protection (10 years total market protection)•Potential for 1 additional year of market protectionVASCEPA (icosapent ethyl) is not currently approved for marketing or sale in Europe. As is one of the the purposes of the Day 120 letter from the EMA, it included various questions for which the EMA seeks adequate response before it will decide on such approval. There are risks involved with any such approval which risks are described in the Risk Factors section of Amarin’s Annual Report on Form 10-K. As is typical in a regulatory review process, such questions are between the regulatory authorities and the sponsor and outside the scope of this response.Dated: March 31, 2020 1https://www.ema.europa.eu/en/documents/presentation/presenta…Da sieht man halt, dass Europa Medikamentenhersteller viel besser schützt als die degenerierte USA, Amarin hat fast 1 Milliarde an Kosten gehabt mit drei erfolgreichen Phase 3 Ergebnissen die in sensationellen Reduce It Ergebnissen mündeten und nun macht eine Lokalrichterin alles kapput, ignoriert das Patentamt........, das glaube ich nicht!

Welche Chancen hat Amarin in zweiter Instanz zu gewinnen?
https://www.markmanadvisors.com/blog/2020/3/31/can-amarin-wi…
Was mich an der Situation ankotzt, ist die Tatsache, dass durch dieses dubiose Urteil "Alles" in Frage gestellt wird was Amarin in einer Dekade erreicht hat, ganz zu Schweigen vom individuellen Schaden an die Aktionäre!
Mich würde nicht wunderen wenn big pharma dahintersteckt und Amarin auf diese Weise zerstören will.....
Jedoch haben wir mit der zu erwartenden Exklusivität in Europa, Kanada und dem Rest der Welt immer noch Blockbusterpotential!
Mich würde nicht wunderen wenn big pharma dahintersteckt und Amarin auf diese Weise zerstören will.....
Jedoch haben wir mit der zu erwartenden Exklusivität in Europa, Kanada und dem Rest der Welt immer noch Blockbusterpotential!
Antwort auf Beitrag Nr.: 63.197.019 von paul81 am 01.04.20 09:07:19
Was sagen eigentlich die USA -Patentämter zu dem Gerichtsurteil ?
Das sind doch Institutionen , die Patentrechte erteilen und - ich gehe mal davon aus - eigentlich vom FACHwissen her wissen was sie tun.
Zitat von paul81: Welche Chancen hat Amarin in zweiter Instanz zu gewinnen?
https://www.markmanadvisors.com/blog/2020/3/31/can-amarin-wi…
Was sagen eigentlich die USA -Patentämter zu dem Gerichtsurteil ?
Das sind doch Institutionen , die Patentrechte erteilen und - ich gehe mal davon aus - eigentlich vom FACHwissen her wissen was sie tun.
Laut "The honorable Chief Judge Miranda M. Du" ...
... ist das hier alles Klopapier:https://patents.justia.com/assignee/amarin-pharmaceuticals-i…
Danke, Anke

When the going gets tough...
... the tough gets going!Die Wissenschaftler und Biomediziner bei Amarin haben bewiesen, dass sie abliefern können.
Nun muss es auch das Management tun! Eine Idee hierzu wäre die seit 5 Jahren bestehnde Kooperation mit Eddingpharma
https://investor.amarincorp.com/news-releases/news-release-d…
Mit den Chinesen zusammen in einem Jointventure das Original zum Preis der Generika anbieten und die Dr. Reddys Nachmacherbuden dieser Welt einfach davonblasen
Mit einem Messer im Rücken gehen wir noch lange nicht nach Hause.
Ein Elon Musk würde jetzt erst langsam warm werden.
GO AMRN f**k them All

So, habe erstmal Amarin gekauft. Günstiger kommt man wohl kaum an die Aktie
Bin auch seit heute mit einer Position drin. Denke hier kann man wenig verkehrt machen.
Es können 2 Fälle eintreten:
1. In der Berufung wird das Urteil bestätigt. Entsprechend verdient die Firma außerhalb der USA jede Menge Geld.
2. Die Berufung kippt das Urteil. Entsprechend back to Start und wir stehen im zweistelligen Bereich.
Haltedauer mal auf 2-3 Jahre ausgelegt.
Es können 2 Fälle eintreten:
1. In der Berufung wird das Urteil bestätigt. Entsprechend verdient die Firma außerhalb der USA jede Menge Geld.
2. Die Berufung kippt das Urteil. Entsprechend back to Start und wir stehen im zweistelligen Bereich.
Haltedauer mal auf 2-3 Jahre ausgelegt.
!
Dieser Beitrag wurde von MODelfin moderiert. Grund: Bitte hinterfragen Sie Ihre Inhalte, Danke.
Diese "Dame" hat Amarin zum Zockerwert verkümmern lassen, siehe heute, aber noch ist nicht aller Abend Tage, Europa und der Rest der Welt wartet auf Vascepa ohne Generika in den nächsten 10 Jahren, dass macht Amarin allein zu einem 20 US Dollar Wert!
Zockerwerte müssen ja nicht unbedingt schlecht sein. 🙂
Denke hier besteht auch die Möglichleit eines Shortsqueezes.
Und ansonsten gehe ich auch davon aus, günstiger kommt man hier nicht rein.
Alle die sich teuer eingekauft haben, bleibt nur warten oder verbilligten.
Mal gewinnt man , mal verliert man.
Denke hier besteht auch die Möglichleit eines Shortsqueezes.
Und ansonsten gehe ich auch davon aus, günstiger kommt man hier nicht rein.
Alle die sich teuer eingekauft haben, bleibt nur warten oder verbilligten.
Mal gewinnt man , mal verliert man.
Man bedenke, dass AMRN unter einigermaßen "normalen" Umständen dieses Jahr in der Produktion an die 1 Mrd 1-Gramm-Kapseln herankommen könnte, und das bei einem Gross-Profit mittlerweile über 80% Richtung 85%! Ich habe mir den 10K 2019 sehr genau durchgelesen und hatte Tränen der Begeisterung in den Augen - das ist eine gute Ausgangsbasis. Jetzt muss man nur kreativ werden 

Is Amarin Stock a Buy?
Amarin's stock is stupid cheap after yesterday's dramatic downturn. Here's why.
George Budwell
George Budwell
(TMFGBudwell)
Apr 1, 2020 at 10:00AM
Author Bio
Yesterday, Amarin Corp. (NASDAQ:AMRN) lost 70% of its value in a single trading session. The biopharma's shares collapsed after the U.S. District Court for the District of Nevada invalidated Vascepa's patent firewall against generic competitors inside the United States.
Vascepa is a highly refined omega-3 treatment approved by the U.S. Food and Drug Administration for patients with high triglyceride levels who are at risk for cardiovascular disease, despite being on statin therapy. The drug was widely expected by Wall Street analysts and industry insiders to achieve megablockbuster status (greater than $5 billion in annual sales) before the end of the decade. But this adverse patent ruling puts that enormous peak sales figure in serious jeopardy.
A young woman grabbing the side of her head with a look of shock on her face while staring at a laptop.
Image source: Getty Images.
Did investors overreact to this news? Let's break down the company's near-term outlook to find out.
Is Amarin's stock cheap?
After yesterday's bloodbath, Amarin's market cap now stands at $1.44 billion. Amarin is therefore trading at just a little over two times its last stated cash position. Under normal circumstances, commercial-stage biopharmas trade at no less than three times cash on hand. So, from this point of view, Amarin's stock is definitely in bargain territory.
What about the company's near-term top-line outlook? We already know that Amarin's first-quarter numbers weren't going to be great thanks to the COVID-19 pandemic grounding its sales force, and the company's Q2 sales will likely take a big hit as well.
So assuming no generic magically pops up in 2020 (no generic is approved, and Amarin has legal options to stop one from entering the market while an appeal is under review), Amarin should thus haul in somewhere between $450 million and $650 million in annual sales this year. That's a wide range to be sure, but there's no way to accurately gauge how much growth the company can generate during a viral pandemic, or how this pandemic will impact patients filling their prescriptions in the months ahead.
The key takeaway, though, is that Amarin's shares are most likely trading at under three times 2020 sales right now. That definitely qualifies as bargain territory for a commercial-stage biopharma stock.
What's next?
The big unknown -- and arguably the main reason Amarin's stock plunged yesterday -- is Vascepa's commercial opportunity in 2021 and beyond. The bad news is that these patent decisions are rarely overturned on appeal -- a point echoed by multiple analysts yesterday in the wake of this unexpected ruling. So generics in the all-important U.S. market are probably going to come into play by the summer of 2021.
There is a silver lining, however. This patent ruling doesn't impact the drug's international IP, and there is no generic litigation pending outside the United States. So once Amarin gains widespread approval for Vascepa outside of the U.S., the drug should still be able to generate a decent amount of sales overall. What's more, Vascepa won't lose all of its U.S. market share to generics.
What this all boils down to that Vascepa is likely going to end up as a fringe blockbuster ($1 billion in annual sales) after this devastating blow to its IP in the United States. Stated bluntly, Vascepa's megablockbuster aspirations are probably out the window, and so too are Amarin's prospects as a buyout candidate. That's the new reality, barring a miracle on the legal front.
The good news is that Amarin's shares are undeniably undervalued on the heels of this emotionally charged downturn. In short, this biopharma should eventually rebound to the $8 range (about three times peak sales), which is roughly double from where it closed Tuesday. Driving this point home, Amarin's shares are now trading as if Vascepa won't perform well internationally and it will eventually lose around 90% of its U.S. market share. Neither of those dire outcomes is likely for a long list of reasons.
Amarin's stock is stupid cheap after yesterday's dramatic downturn. Here's why.
George Budwell
George Budwell
(TMFGBudwell)
Apr 1, 2020 at 10:00AM
Author Bio
Yesterday, Amarin Corp. (NASDAQ:AMRN) lost 70% of its value in a single trading session. The biopharma's shares collapsed after the U.S. District Court for the District of Nevada invalidated Vascepa's patent firewall against generic competitors inside the United States.
Vascepa is a highly refined omega-3 treatment approved by the U.S. Food and Drug Administration for patients with high triglyceride levels who are at risk for cardiovascular disease, despite being on statin therapy. The drug was widely expected by Wall Street analysts and industry insiders to achieve megablockbuster status (greater than $5 billion in annual sales) before the end of the decade. But this adverse patent ruling puts that enormous peak sales figure in serious jeopardy.
A young woman grabbing the side of her head with a look of shock on her face while staring at a laptop.
Image source: Getty Images.
Did investors overreact to this news? Let's break down the company's near-term outlook to find out.
Is Amarin's stock cheap?
After yesterday's bloodbath, Amarin's market cap now stands at $1.44 billion. Amarin is therefore trading at just a little over two times its last stated cash position. Under normal circumstances, commercial-stage biopharmas trade at no less than three times cash on hand. So, from this point of view, Amarin's stock is definitely in bargain territory.
What about the company's near-term top-line outlook? We already know that Amarin's first-quarter numbers weren't going to be great thanks to the COVID-19 pandemic grounding its sales force, and the company's Q2 sales will likely take a big hit as well.
So assuming no generic magically pops up in 2020 (no generic is approved, and Amarin has legal options to stop one from entering the market while an appeal is under review), Amarin should thus haul in somewhere between $450 million and $650 million in annual sales this year. That's a wide range to be sure, but there's no way to accurately gauge how much growth the company can generate during a viral pandemic, or how this pandemic will impact patients filling their prescriptions in the months ahead.
The key takeaway, though, is that Amarin's shares are most likely trading at under three times 2020 sales right now. That definitely qualifies as bargain territory for a commercial-stage biopharma stock.
What's next?
The big unknown -- and arguably the main reason Amarin's stock plunged yesterday -- is Vascepa's commercial opportunity in 2021 and beyond. The bad news is that these patent decisions are rarely overturned on appeal -- a point echoed by multiple analysts yesterday in the wake of this unexpected ruling. So generics in the all-important U.S. market are probably going to come into play by the summer of 2021.
There is a silver lining, however. This patent ruling doesn't impact the drug's international IP, and there is no generic litigation pending outside the United States. So once Amarin gains widespread approval for Vascepa outside of the U.S., the drug should still be able to generate a decent amount of sales overall. What's more, Vascepa won't lose all of its U.S. market share to generics.
What this all boils down to that Vascepa is likely going to end up as a fringe blockbuster ($1 billion in annual sales) after this devastating blow to its IP in the United States. Stated bluntly, Vascepa's megablockbuster aspirations are probably out the window, and so too are Amarin's prospects as a buyout candidate. That's the new reality, barring a miracle on the legal front.
The good news is that Amarin's shares are undeniably undervalued on the heels of this emotionally charged downturn. In short, this biopharma should eventually rebound to the $8 range (about three times peak sales), which is roughly double from where it closed Tuesday. Driving this point home, Amarin's shares are now trading as if Vascepa won't perform well internationally and it will eventually lose around 90% of its U.S. market share. Neither of those dire outcomes is likely for a long list of reasons.
Antwort auf Beitrag Nr.: 63.203.253 von Semjasa88 am 01.04.20 16:17:24
WARTEN - YEPP 👍
VERBILLIGEN - YEPP - heute damit angefangen 👍
Zitat von Semjasa88: Zockerwerte müssen ja nicht unbedingt schlecht sein. 🙂
Denke hier besteht auch die Möglichleit eines Shortsqueezes.
Und ansonsten gehe ich auch davon aus, günstiger kommt man hier nicht rein.
Alle die sich teuer eingekauft haben, bleibt nur warten oder verbilligen.
Mal gewinnt man , mal verliert man.
WARTEN - YEPP 👍
VERBILLIGEN - YEPP - heute damit angefangen 👍
Das wird eine gute Erholung...
Amarin (AMRN +18.0%) is up on massive turnover of over 63M shares in apparent reaction to a call between a patent lawyer and Jefferies' Jared Holz about the adverse patent ruling on Vascepa (icosapent ethyl) that caused the stock to plummet almost 71% yesterday. Quelle vergessen...
https://seekingalpha.com/news/3557411-amarin-jumps-18-on-pos… 
 Auch nicht schlecht, Analyst belässt Price Target bei 51$ !!! Das nenne ich mal bullish- über 1000%
Auch nicht schlecht, Analyst belässt Price Target bei 51$ !!! Das nenne ich mal bullish- über 1000% 
„…The next step, naturally, involves an appeal. With this in mind, H.C. Wainwright’s Andrew Fein, a long standing Amarin bull, sought clarity on the probability of a successful turnaround following the biotech’s appeal…While the ANDA filers (Dr. Reddy’s and Hikma Pharmaceuticals) are now free to move forward with their generic versions, such a move is currently unlikely and would be considered a “launch at-risk,” so long as Amarin is still pursuing a reversal of the verdict.
Due to “absent clarity on full ramifications of Judge Du's decision and the full extent of the appellate options available to Amarin,” Fein keeps his Buy rating intact, along with the very bullish $51 price target. The contrarian act here, could yield the risk-tolerant investor a massive 1076% gain in the next 12 months, should Fein’s optimistic scenario play out.…“ Quelle TipRanks
Was ist das für ein trauriges Schauspiel auf Kosten der Aktionäre und Firma?
Erst Urteil, dann evtl. Fehler dabei, Kurs unterirdisch, USA schäme Dich samt Richterin und Präsident, hier unsere Hoffnung vom Patentanwalt:
https://centurylink.cwebcast.com/ses/1rvwqrkLXyk6VlfZ0WkY5w~…
Erst Urteil, dann evtl. Fehler dabei, Kurs unterirdisch, USA schäme Dich samt Richterin und Präsident, hier unsere Hoffnung vom Patentanwalt:
https://centurylink.cwebcast.com/ses/1rvwqrkLXyk6VlfZ0WkY5w~…
Antwort auf Beitrag Nr.: 63.208.710 von easymueller am 02.04.20 01:35:23Denke die 8$ werden wir die nächsten Monate wiedersehen
Zitat von easymueller: https://seekingalpha.com/news/3557411-amarin-jumps-18-on-pos…
Jefferies post Harvard expert report
Good luck guys hope you win appeal
Yee put this out 10:20 pm eastern Weds night FYI after the call
Amarin Corporation
Target Change
USA | Biotechnology
RATING HOLD
PRICE $4.98^
MARKET CAP $2.1B
PRICE TARGET (PT) $6.00 (FROM $4.00)
UPSIDE SCENARIO PT $10.00
DOWNSIDE SCENARIO PT $2.00
Patent Expert Thinks ~50% Chance of Winning Appeal Next Year - Raise PT to $6
April 1, 2020
Key Takeaway
Per our patent lawyer expert, there was a pot'l procedural error in the patent ruling, and AMRN has a pretty decent shot at appeal (and good likelihood of preliminary injunction in a few weeks as well). That said, it could take 12 mos and won't be expedited. Good sum of the parts on US + EU oppt'y (latter has 10+ yr exclusivity) for long-term investors and.call option on appeal win makes this interesting. But it will take time to next year's ruling.Our patent expert thinks the AMRN case was one of the most unusual and surprising
District Court decisions he's witnessed and there is a 50% chance of overturning it in AMRN's favor (much higher than traditional appeals). Our expert believes Judge Du made a "clearly erroneous" methodology of assessment on determining obviousness. Many of the Federal Appeal Circuit judges are big "sticklers" for correct procedure and because AMRN already was supposed to have a good chance when looking at obviousness regardless (hence why we thought good chance of winning originally), this should give AMRN a decent shot for an appeal. Our PT moves to $6 to now include even a modest chance of appeal win in 2021 plus modest US and conservative EU sales.
The Judge may have made a "major procedural error" as she shifted the burden of proof on AMRN when looking at Secondary considerations for obviousness. Primary considerations refer to 'prior art' like old patents and scientific literature, whereas Secondary considerations are more objective measures (e.g. unexpected benefit, long felt need, commercial success, skepticism, praise). Our expert believes Judge Du should have placed the burden of proof entirely on the generics (defendants) when assessing primary and secondary considerations. He said there are many examples of this and the Supreme Court has clearly recommended that method to District courts. He provided a couple of famous cases in which the Judges had applied the wrong burden-shifting framework, which the Federal appellate court later viewed to be not permissible.
Our patent expert thinks AMRN has a good chance of winning appeal due to a
combination of: (1) This was Judge Du's first patent bench trial and there appears to be a major procedural error, (2) the Federal Circuit of Appeals favors the patent holder (e.g. AMRN) 64% of the time (although only reverses invalidity/validity cases 18% of the time), (3) there are 12 active judges on the Federal Circuit and at least half are pro-procedural or pro-patentee, (4) obviousness issue will likely be reassessed and may come to the original held view the patent is novel. However, the expert noted it does partially depend on which 3 judges are assigned to the panel.
Timelines: AMRN will appeal and file an injunction to prevent generics from launching at-risk within weeks. He sees an 80% chance AMRN secures an injunction by early May, although a final appeal decision is a year out in April/May 2021. He is doubtful generics will launch at-risk until the appeal decision, but our discussions with generic companies suggest they would like to launch. Hikma appears confident with its manufacturing and has been stockpiling raw material.
Investment Thesis / Where We Differ
We maintain HOLD and believe the stock will be range-bound until there is more clarity on an appeal and scenarios where generics may not immediately hit.
For value folks, we see long-term value due to appeal decision later, no Gx immediate launch, manufacturing challenges, - and AMRN isn't really burning cash anymore, but the Street will not likely care in the near/medium term.
While generics should be expected to come, the stock mostly reflects this now. We do think generics will face challenges in reality - which creates upside value for longer-term non-consensus investors, but the Street won't likely care for now.
In the meantime, AMRN will (1) seek an injunction (2+ wks for ruling) and appeal the case (average 9+ months for decision), (2) continue to execute on commercial sales this year (will likely cut DTC and some expenses) in US, and (3) continue to interrogate EU where it is up for approval YE:20 and does not currently face any patent issues and should have 10 years exclusivity due to CVOT label, not the initial US trig label.
Scenarios
Base Case
Our base case of $6/share is based on a DCF analysis that considers a 15-20% chance AMRN wins its appeal in 2021.
In this scenario, we assume US and EU Vascepa sales both extend out to at least 2030.
We consider peak sales of $3-4B on a 4x multiple (probability adjusted).
Upside Scenario
Our upside scenario of $10/share considers a higher 30% chance AMRN wins its appeal in 2021.
In this scenario, we consider peak sales of $3-4B on a 4x multiple (probability adjusted).
Downside Scenario
Our downside scenario of $2/share assumes AMRN loses its appeal, implying generic entry as early as 2021.
In this scenario, we assume USA sales into YE:20/early 2021 and no revenues thereafter. We do not consider EU sales as well.
However, we do consider net cash of ~1/share.
Catalysts
2020: Quarterly results and sales growth of Vascepa
Mid:20: File an appeal and seek injunction ruling
Q4:20: EU approval of Vascepa
Mid:21: Potential appeal decision on the HIkma/Reddy's cases
Good luck guys hope you win appeal
Yee put this out 10:20 pm eastern Weds night FYI after the call
Amarin Corporation
Target Change
USA | Biotechnology
RATING HOLD
PRICE $4.98^
MARKET CAP $2.1B
PRICE TARGET (PT) $6.00 (FROM $4.00)
UPSIDE SCENARIO PT $10.00
DOWNSIDE SCENARIO PT $2.00
Patent Expert Thinks ~50% Chance of Winning Appeal Next Year - Raise PT to $6
April 1, 2020
Key Takeaway
Per our patent lawyer expert, there was a pot'l procedural error in the patent ruling, and AMRN has a pretty decent shot at appeal (and good likelihood of preliminary injunction in a few weeks as well). That said, it could take 12 mos and won't be expedited. Good sum of the parts on US + EU oppt'y (latter has 10+ yr exclusivity) for long-term investors and.call option on appeal win makes this interesting. But it will take time to next year's ruling.Our patent expert thinks the AMRN case was one of the most unusual and surprising
District Court decisions he's witnessed and there is a 50% chance of overturning it in AMRN's favor (much higher than traditional appeals). Our expert believes Judge Du made a "clearly erroneous" methodology of assessment on determining obviousness. Many of the Federal Appeal Circuit judges are big "sticklers" for correct procedure and because AMRN already was supposed to have a good chance when looking at obviousness regardless (hence why we thought good chance of winning originally), this should give AMRN a decent shot for an appeal. Our PT moves to $6 to now include even a modest chance of appeal win in 2021 plus modest US and conservative EU sales.
The Judge may have made a "major procedural error" as she shifted the burden of proof on AMRN when looking at Secondary considerations for obviousness. Primary considerations refer to 'prior art' like old patents and scientific literature, whereas Secondary considerations are more objective measures (e.g. unexpected benefit, long felt need, commercial success, skepticism, praise). Our expert believes Judge Du should have placed the burden of proof entirely on the generics (defendants) when assessing primary and secondary considerations. He said there are many examples of this and the Supreme Court has clearly recommended that method to District courts. He provided a couple of famous cases in which the Judges had applied the wrong burden-shifting framework, which the Federal appellate court later viewed to be not permissible.
Our patent expert thinks AMRN has a good chance of winning appeal due to a
combination of: (1) This was Judge Du's first patent bench trial and there appears to be a major procedural error, (2) the Federal Circuit of Appeals favors the patent holder (e.g. AMRN) 64% of the time (although only reverses invalidity/validity cases 18% of the time), (3) there are 12 active judges on the Federal Circuit and at least half are pro-procedural or pro-patentee, (4) obviousness issue will likely be reassessed and may come to the original held view the patent is novel. However, the expert noted it does partially depend on which 3 judges are assigned to the panel.
Timelines: AMRN will appeal and file an injunction to prevent generics from launching at-risk within weeks. He sees an 80% chance AMRN secures an injunction by early May, although a final appeal decision is a year out in April/May 2021. He is doubtful generics will launch at-risk until the appeal decision, but our discussions with generic companies suggest they would like to launch. Hikma appears confident with its manufacturing and has been stockpiling raw material.
Investment Thesis / Where We Differ
We maintain HOLD and believe the stock will be range-bound until there is more clarity on an appeal and scenarios where generics may not immediately hit.
For value folks, we see long-term value due to appeal decision later, no Gx immediate launch, manufacturing challenges, - and AMRN isn't really burning cash anymore, but the Street will not likely care in the near/medium term.
While generics should be expected to come, the stock mostly reflects this now. We do think generics will face challenges in reality - which creates upside value for longer-term non-consensus investors, but the Street won't likely care for now.
In the meantime, AMRN will (1) seek an injunction (2+ wks for ruling) and appeal the case (average 9+ months for decision), (2) continue to execute on commercial sales this year (will likely cut DTC and some expenses) in US, and (3) continue to interrogate EU where it is up for approval YE:20 and does not currently face any patent issues and should have 10 years exclusivity due to CVOT label, not the initial US trig label.
Scenarios
Base Case
Our base case of $6/share is based on a DCF analysis that considers a 15-20% chance AMRN wins its appeal in 2021.
In this scenario, we assume US and EU Vascepa sales both extend out to at least 2030.
We consider peak sales of $3-4B on a 4x multiple (probability adjusted).
Upside Scenario
Our upside scenario of $10/share considers a higher 30% chance AMRN wins its appeal in 2021.
In this scenario, we consider peak sales of $3-4B on a 4x multiple (probability adjusted).
Downside Scenario
Our downside scenario of $2/share assumes AMRN loses its appeal, implying generic entry as early as 2021.
In this scenario, we assume USA sales into YE:20/early 2021 and no revenues thereafter. We do not consider EU sales as well.
However, we do consider net cash of ~1/share.
Catalysts
2020: Quarterly results and sales growth of Vascepa
Mid:20: File an appeal and seek injunction ruling
Q4:20: EU approval of Vascepa
Mid:21: Potential appeal decision on the HIkma/Reddy's cases
Ich bin kein Jurist, aber die Bitch hat einen Fehler gemacht:
Markman Attorney downplays Major procedural mistake that Jeffries attorney claims b/c even if include secondary considerations TOGETHER with primary considerations Du could still show obvious since she negates the 2 secondary considerations with 3 that go against us. The main/real point is that judge made procedural mistake by weighing secondary considerations against each other.
Markman Attorney downplays Major procedural mistake that Jeffries attorney claims b/c even if include secondary considerations TOGETHER with primary considerations Du could still show obvious since she negates the 2 secondary considerations with 3 that go against us. The main/real point is that judge made procedural mistake by weighing secondary considerations against each other.
Antwort auf Beitrag Nr.: 63.211.086 von Magnetfeldfredy am 02.04.20 10:14:39Das hätte man auch anders umschreiben können. 🙄
"Das richtige Wort am richtigen Ort, das ist die wahre Definition von Stil."
Das im Urteil Fehler sind , sollte nach dem Conference Call allen bewusst sein.
Die Geier mit den Law Suits kreisen auch schon. 🤣
"Das richtige Wort am richtigen Ort, das ist die wahre Definition von Stil."
Das im Urteil Fehler sind , sollte nach dem Conference Call allen bewusst sein.
Die Geier mit den Law Suits kreisen auch schon. 🤣
Nein, das kann man in diesem Fall nur härtest ausdrücken, was dieses Miststück angerichtet hat,
ich bin seit 10 Jahren in Amarin dabei und habe alle Höhen und Tiefen mitgemacht, Vascepa ist ein lebensrettendes Medikament und hat der Firma fast 1 Milliarde US Dollar Entwicklungskosten gekostet,was wir Aktionäre finaziert haben!
Diese bitch hat über Nacht Milliarden vernichtet, Familien in den Ruin gebracht, die in Amarin investiert waren/sind, die Firma an die Wand gefahren.......
Armes Amerika, mit solchen unfähigen Richtern und dazu Trump und Corona, schlimmer gehts nimmmer!
ich bin seit 10 Jahren in Amarin dabei und habe alle Höhen und Tiefen mitgemacht, Vascepa ist ein lebensrettendes Medikament und hat der Firma fast 1 Milliarde US Dollar Entwicklungskosten gekostet,was wir Aktionäre finaziert haben!
Diese bitch hat über Nacht Milliarden vernichtet, Familien in den Ruin gebracht, die in Amarin investiert waren/sind, die Firma an die Wand gefahren.......
Armes Amerika, mit solchen unfähigen Richtern und dazu Trump und Corona, schlimmer gehts nimmmer!
Antwort auf Beitrag Nr.: 63.211.647 von Magnetfeldfredy am 02.04.20 10:58:34Genauso sehe ich das auch 👍
Antwort auf Beitrag Nr.: 63.211.647 von Magnetfeldfredy am 02.04.20 10:58:34
Und deshalb, wer kann.... KAUFEN... zweistellig, sollte rasch wieder drin sein
Zitat von Magnetfeldfredy: Nein, das kann man in diesem Fall nur härtest ausdrücken, was dieses Miststück angerichtet hat,
ich bin seit 10 Jahren in Amarin dabei und habe alle Höhen und Tiefen mitgemacht, Vascepa ist ein lebensrettendes Medikament und hat der Firma fast 1 Milliarde US Dollar Entwicklungskosten gekostet,was wir Aktionäre finaziert haben!
Diese bitch hat über Nacht Milliarden vernichtet, Familien in den Ruin gebracht, die in Amarin investiert waren/sind, die Firma an die Wand gefahren.......
Armes Amerika, mit solchen unfähigen Richtern und dazu Trump und Corona, schlimmer gehts nimmmer!
Und deshalb, wer kann.... KAUFEN... zweistellig, sollte rasch wieder drin sein
Wurde gestern auf Amarins Homepage unter FAQ eingestellt:
Where can I find information on the ongoing MARINE patent ANDA litigation appeal and what is Amarin’s policy regarding updates as the litigation and related events progress? As announced on March 30, 2020, the United States District Court for the District of Nevada ruled in favor of the generic companies in Amarin’s patent litigation against two filers of abbreviated new drug applications, or ANDAs, for Amarin’s VASCEPA® (icosapent ethyl) capsule franchise. For a generic product to be launched in the United State the generic company’s ANDA must be approved by the U.S. Food and Drug Administration, or FDA. Based on a review of FDA’s website, https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?ev… no ANDA for VASCEPA has been reported to be approved as of the date of this web-posting. For the latest updates on this matter, investors are encouraged to monitor the FDA website and public statements from the generic companies in the litigation. While Amarin plans to update investors as appropriate, it does not undertake to provide the most current information available from FDA’s website or what limited information may otherwise become known to it on the status of ANDA applications at FDA related to VASCEPA. If a generic company’s ANDA is approved at FDA, and if the generic company has sufficient commercial supply available, that generic company may choose to launch its generic product in the United States. If a generic product is launched and the district court judgment is later overruled by a Federal Circuit judgment in Amarin’s favor, the generic company would then be subject to liability to Amarin for patent infringement. Damages in such cases are typically equal to at least the brand’s lost profits caused by the generic sales. Because a branded company’s lost profit is typically higher than a generic company’s profit, such a launch is considered risky for the generic company. Due to such risk, more often than not, generic companies choose to not undertake such a launch at risk. There can be no guarantee against a generic launch. If a generic launch occurs, Amarin does not believe generic companies have made the investment of resources, know-how and time to develop sufficient quantities of quality supply to meet current and growing demand at or near the level supplied by Amarin. Amarin’s belief is based on its understanding of the investment, know-how and time required to manufacture large quantities of such supply, the complex nature of manufacturing VASCEPA to the required standards and our assessment of currently available information (as informed by our suppliers’ knowledge of market dynamics) regarding the general lack of such large quantities of such supply available on short notice. It has required many years and significant investment and commitments by Amarin to develop high capacity, consistent, cost effective, high-quality, stable supply of VASCEPA. The active ingredient in VASCEPA is a fragile molecule. VASCEPA clinical results were achieved through use of product manufactured to the high quality standards developed by Amarinover years, which include high purity levels, protection against oxidation and product stability through active ingredient manufacturing, encapsulation and distribution. Starting with raw material, the various steps taken to produce VASCEPA to high standards at commercial capacity typically takes several months including international shipping. Amarin strongly disagrees with the district court ruling and will vigorously pursue an appeal of the lower court’s decision. Amarin believes it has a strong balance sheet with capacity and flexibility, and plans to fight to protect the VASCEPA franchise for the benefit of our patients, physicians, the broader healthcare community and our investors. Based on current market dynamics, as Amarin works to take appropriate legal actions to defend and protect its intellectual property, it plans to continue to press forward with
educational and promotional efforts for VASCEPA in treating indicated patients at high risk of cardiovascular events, such as heart attack and stroke. Accordingly, on April 2, 2020, Amarin filed an appeal of the district court ruling. Amarin has no immediateplan to file a preliminary injunction against launch, due to a variety of factors, including that no ANDA has yet been approved and Amarin’s expectation that, in the meantime, it is able to move expeditiously to Federal Circuit review. Amarin believes it is favorably situated to prevail on appeal and restore its exclusivity position in the United States. The timing of such appeal proceedings and an outcome on the merits is difficult to predict. It is not uncommon for such an appeal to take from several months to approximately one year until judgment. It can sometimes be longer. If approval of a generic ANDA is granted by FDA and an ANDA filer is able to supply the product in significant commercial quantities and the status quo in the market for VASCEPA is not maintained, the introduction of generic versions of VASCEPA could significantly affect the market for Amarin’s VASCEPA, subject to patent infringement liability such as lost profit damages in favor of Amarin should Amarin prevail on appeal. At this time, Amarin is giving legal priority to the appeal. If after an ANDA approval Amarin believes that the ANDA filer has meaningful supply capacity and is likely to launch at risk, such factors may convince Amarin that an injunction should be sought. Amarin is prepared to rapidly move foran injunction if, and when, it believes that doing so is necessary. Updates on litigation proceedings are available through legal systems such as PACER. Amarin plans to update stakeholders with the results of the litigation once they are published by the Court. For more information on the ANDA litigation process please see Amarin’s latest 10-K/10-Q on file with the U.S. Securities and Exchange Commission, as updated by subsequent reports such as form 8-Ks.
Where can I find information on the ongoing MARINE patent ANDA litigation appeal and what is Amarin’s policy regarding updates as the litigation and related events progress? As announced on March 30, 2020, the United States District Court for the District of Nevada ruled in favor of the generic companies in Amarin’s patent litigation against two filers of abbreviated new drug applications, or ANDAs, for Amarin’s VASCEPA® (icosapent ethyl) capsule franchise. For a generic product to be launched in the United State the generic company’s ANDA must be approved by the U.S. Food and Drug Administration, or FDA. Based on a review of FDA’s website, https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?ev… no ANDA for VASCEPA has been reported to be approved as of the date of this web-posting. For the latest updates on this matter, investors are encouraged to monitor the FDA website and public statements from the generic companies in the litigation. While Amarin plans to update investors as appropriate, it does not undertake to provide the most current information available from FDA’s website or what limited information may otherwise become known to it on the status of ANDA applications at FDA related to VASCEPA. If a generic company’s ANDA is approved at FDA, and if the generic company has sufficient commercial supply available, that generic company may choose to launch its generic product in the United States. If a generic product is launched and the district court judgment is later overruled by a Federal Circuit judgment in Amarin’s favor, the generic company would then be subject to liability to Amarin for patent infringement. Damages in such cases are typically equal to at least the brand’s lost profits caused by the generic sales. Because a branded company’s lost profit is typically higher than a generic company’s profit, such a launch is considered risky for the generic company. Due to such risk, more often than not, generic companies choose to not undertake such a launch at risk. There can be no guarantee against a generic launch. If a generic launch occurs, Amarin does not believe generic companies have made the investment of resources, know-how and time to develop sufficient quantities of quality supply to meet current and growing demand at or near the level supplied by Amarin. Amarin’s belief is based on its understanding of the investment, know-how and time required to manufacture large quantities of such supply, the complex nature of manufacturing VASCEPA to the required standards and our assessment of currently available information (as informed by our suppliers’ knowledge of market dynamics) regarding the general lack of such large quantities of such supply available on short notice. It has required many years and significant investment and commitments by Amarin to develop high capacity, consistent, cost effective, high-quality, stable supply of VASCEPA. The active ingredient in VASCEPA is a fragile molecule. VASCEPA clinical results were achieved through use of product manufactured to the high quality standards developed by Amarinover years, which include high purity levels, protection against oxidation and product stability through active ingredient manufacturing, encapsulation and distribution. Starting with raw material, the various steps taken to produce VASCEPA to high standards at commercial capacity typically takes several months including international shipping. Amarin strongly disagrees with the district court ruling and will vigorously pursue an appeal of the lower court’s decision. Amarin believes it has a strong balance sheet with capacity and flexibility, and plans to fight to protect the VASCEPA franchise for the benefit of our patients, physicians, the broader healthcare community and our investors. Based on current market dynamics, as Amarin works to take appropriate legal actions to defend and protect its intellectual property, it plans to continue to press forward with
educational and promotional efforts for VASCEPA in treating indicated patients at high risk of cardiovascular events, such as heart attack and stroke. Accordingly, on April 2, 2020, Amarin filed an appeal of the district court ruling. Amarin has no immediateplan to file a preliminary injunction against launch, due to a variety of factors, including that no ANDA has yet been approved and Amarin’s expectation that, in the meantime, it is able to move expeditiously to Federal Circuit review. Amarin believes it is favorably situated to prevail on appeal and restore its exclusivity position in the United States. The timing of such appeal proceedings and an outcome on the merits is difficult to predict. It is not uncommon for such an appeal to take from several months to approximately one year until judgment. It can sometimes be longer. If approval of a generic ANDA is granted by FDA and an ANDA filer is able to supply the product in significant commercial quantities and the status quo in the market for VASCEPA is not maintained, the introduction of generic versions of VASCEPA could significantly affect the market for Amarin’s VASCEPA, subject to patent infringement liability such as lost profit damages in favor of Amarin should Amarin prevail on appeal. At this time, Amarin is giving legal priority to the appeal. If after an ANDA approval Amarin believes that the ANDA filer has meaningful supply capacity and is likely to launch at risk, such factors may convince Amarin that an injunction should be sought. Amarin is prepared to rapidly move foran injunction if, and when, it believes that doing so is necessary. Updates on litigation proceedings are available through legal systems such as PACER. Amarin plans to update stakeholders with the results of the litigation once they are published by the Court. For more information on the ANDA litigation process please see Amarin’s latest 10-K/10-Q on file with the U.S. Securities and Exchange Commission, as updated by subsequent reports such as form 8-Ks.
Hallo zusammen,
ich war bei Amarin jetzt ne Weile draussen, habe mit Verlust bei 20 Dollar die Reissleine gezogen gehabt.
Das was jetzt passiert ist , ist eine legendär überzogene Reaktion gewesen. Daher investiere ich gerne wieder und wünsche allen "longs" die bisher nicht raus sind dass sich das alles komplett erholt.
Die Chancen dafür wenn man dem ganzen etwas Zeit gibt stehen ganz sicher nicht schlecht.
Gebt nicht auf und ich weiss nervenaufreibend ist Amarin wirklich immer aber derzeit gilt das ja quasi fast für alle Investments. Die Welt wird sich wieder normalisieren und auch dieser Blödsinn hier relativiert sich wieder.
LG an alle !
ich war bei Amarin jetzt ne Weile draussen, habe mit Verlust bei 20 Dollar die Reissleine gezogen gehabt.
Das was jetzt passiert ist , ist eine legendär überzogene Reaktion gewesen. Daher investiere ich gerne wieder und wünsche allen "longs" die bisher nicht raus sind dass sich das alles komplett erholt.
Die Chancen dafür wenn man dem ganzen etwas Zeit gibt stehen ganz sicher nicht schlecht.
Gebt nicht auf und ich weiss nervenaufreibend ist Amarin wirklich immer aber derzeit gilt das ja quasi fast für alle Investments. Die Welt wird sich wieder normalisieren und auch dieser Blödsinn hier relativiert sich wieder.
LG an alle !
Antwort auf Beitrag Nr.: 63.225.360 von flyingbeef am 03.04.20 10:56:53man wird aber wohl Geduld mitbringen müssen.
Antwort auf Beitrag Nr.: 63.227.466 von xylophon am 03.04.20 13:32:06Geduld wird wahrscheinlich jeder haben, die Frage ist ja, wo ist das Geld momentan besser "angelegt" ?
Denke hier hat man nur noch sehr geringes Abwärtspotential. Cash ist mehr als ausreichend vorhanden und im letzten Quartal hat die Firma angefangen Profit zu machen.
Auf der Basis von 1-2 Jahren gewinnt man hier zwangsläufig.
Denke hier hat man nur noch sehr geringes Abwärtspotential. Cash ist mehr als ausreichend vorhanden und im letzten Quartal hat die Firma angefangen Profit zu machen.
Auf der Basis von 1-2 Jahren gewinnt man hier zwangsläufig.
!
Dieser Beitrag wurde von CloudMOD moderiert. Grund: Kopie aus fremden Medien- bitte nachlesen unter 9.5. Nutzungsbedingungen: "wie zitiere ich richtig"
Antwort auf Beitrag Nr.: 63.227.595 von Semjasa88 am 03.04.20 13:40:31simmt schon, zumal man ja finanziell gut genug da steht, dass keine Pleite droht. Trotzdem habe ich den Eindruck, dass heute Geduld nicht so stark vertreten ist an den Börsen. Viele Trader, wenige Anleger.
Mit Sicht auf 1 Jahr werden die Kurse sehr wahrscheinlich weit höher stehen - aber wer wartet so lange, wenn die Aktie jetzt erst mal vielleicht eine Weile stagniert zwischen 4 und 5 Dollar?
Mit Sicht auf 1 Jahr werden die Kurse sehr wahrscheinlich weit höher stehen - aber wer wartet so lange, wenn die Aktie jetzt erst mal vielleicht eine Weile stagniert zwischen 4 und 5 Dollar?
...für mich sieht das so aus, dass die Fischbüchse Präparate verkauft, die patentrechtlich nicht schutzwürdig sind, dies haben die Mitbewerber rechtlich feststellen lassen, ist nun die Frage, zu welchem Preis die Allerwelts-Fischkapseln nun über den Ladentisch verramscht werden. Ich war nie investiert und werde nicht investieren. Die warnenden Stimmen vor Amarin gab es ja zu hauf!
2 Berichte auf Deutsch, die zeigen, dass es eben doch ein wenig mehr ist als "Fischölkapseln".
https://www.onvista.de/news/amarin-implosion!-343529361
https://www.onvista.de/news/amarin-folgt-nach-der-explosion-…
Der erste war skeptisch vor der Zulassung (die FDA hat auch lange geprüft, die Zulassung dann aber erteilt, was erst mal positiv zu werten ist). Der zweite ist kritisch nach dem Urteil.
Vom Kursverlauf her kann man wohl festhalten, dass vor der Zulassung viele "gezockt" haben und dann auf die guten News verkauft - mit teilweise enttäuschendem Resultat. Aber die Zulassung bringt eben den Umsatz nicht am nächsten Tag.
Alle, die nur auf schnellen Gewinn aus waren, dürften dieser Tage aussteigen. Der Umsatz für dieses Jahr ist nicht in großer Gefahr, fürs nächste Jahr mag er niedriger ausfallen, aber es bleibt eine Chance, dass ein großes Unternehmen AMRN ganz gern zu diesem Schnäppchenpreis übernimmt und eine ausreichend starke Rechtsabteilung hat, die Risiken abzuschätzen. Ich gehe davon aus, dass zB für 7-8 Dollar viele Aktionäre ja sagen würden - das wäre für die meisten Großen der Branche ein Witzbetrag. Und Vascepa passt für viele Anbieter im Bereich "Healthcare".
https://www.onvista.de/news/amarin-implosion!-343529361
https://www.onvista.de/news/amarin-folgt-nach-der-explosion-…
Der erste war skeptisch vor der Zulassung (die FDA hat auch lange geprüft, die Zulassung dann aber erteilt, was erst mal positiv zu werten ist). Der zweite ist kritisch nach dem Urteil.
Vom Kursverlauf her kann man wohl festhalten, dass vor der Zulassung viele "gezockt" haben und dann auf die guten News verkauft - mit teilweise enttäuschendem Resultat. Aber die Zulassung bringt eben den Umsatz nicht am nächsten Tag.
Alle, die nur auf schnellen Gewinn aus waren, dürften dieser Tage aussteigen. Der Umsatz für dieses Jahr ist nicht in großer Gefahr, fürs nächste Jahr mag er niedriger ausfallen, aber es bleibt eine Chance, dass ein großes Unternehmen AMRN ganz gern zu diesem Schnäppchenpreis übernimmt und eine ausreichend starke Rechtsabteilung hat, die Risiken abzuschätzen. Ich gehe davon aus, dass zB für 7-8 Dollar viele Aktionäre ja sagen würden - das wäre für die meisten Großen der Branche ein Witzbetrag. Und Vascepa passt für viele Anbieter im Bereich "Healthcare".
Antwort auf Beitrag Nr.: 63.240.069 von xylophon am 04.04.20 22:27:36sehr schöne Einschätzung. Danke
Kurs wird zu Wochenanfang weiter steigen. Da wurde so einiges in den USA Leerverkauft. Mit den aktuellen Marktvorgaben +4% sollten wir hier einen schönen Squeeze sehen. Rechne mit +15% für heute und +25% bis zur Mitte der Woche
Antwort auf Beitrag Nr.: 63.235.992 von dpdwvz am 04.04.20 08:45:08Lächerlicher Beitrag ohne fundamentales Wissen!
!
Dieser Beitrag wurde von CloudMOD moderiert. Grund: Kopie aus fremden Medien- bitte nachlesen unter 9.5. Nutzungsbedingungen: "wie zitiere ich richtig"
Hatte den Wert schon eine Weile auf der Watch, und seit letzter Woche bin ich dabei. Die beiden Artikel von Bastian Galuschka sind oberflächlich stimmig, jedoch gehen diese nicht in die Tiefe.
Richterin Du hat schon viele aufgeschreckt, nachdem nur 6 Patente als "offensichtlich" bewertet wurden (diese basieren lediglich auf die MARINE Studie):
U.S. Patent No. 8,293,728 (“the ‘728 Patent”),
U.S. Patent No. 8,318,715 (“the ‘715 Patent”),
U.S. Patent No. 8,357,677 (“the ‘677 Patent”),
U.S. Patent No. 8,367,652 (“the ‘652 Patent”),
U.S. Patent No. 8,431,560 (“the ‘560 Patent”),
U.S. Patent No. 8,518,929 (“the ‘929 Patent”)
Patente aus der REDUCE-IT Studie sollten mehr Wert sein.
Was ich jedoch interessant finde ist, dass in US-Foren das Urteil genau unter die Lupe genommen wird. Dieses hat man gefunden https://twitter.com/ra_fun/status/1246978588504621056 und IR mitgeteilt.
Nicht ganz fair von Hikma & Dr. Reddy
Richterin Du hat schon viele aufgeschreckt, nachdem nur 6 Patente als "offensichtlich" bewertet wurden (diese basieren lediglich auf die MARINE Studie):
U.S. Patent No. 8,293,728 (“the ‘728 Patent”),
U.S. Patent No. 8,318,715 (“the ‘715 Patent”),
U.S. Patent No. 8,357,677 (“the ‘677 Patent”),
U.S. Patent No. 8,367,652 (“the ‘652 Patent”),
U.S. Patent No. 8,431,560 (“the ‘560 Patent”),
U.S. Patent No. 8,518,929 (“the ‘929 Patent”)
Patente aus der REDUCE-IT Studie sollten mehr Wert sein.
Was ich jedoch interessant finde ist, dass in US-Foren das Urteil genau unter die Lupe genommen wird. Dieses hat man gefunden https://twitter.com/ra_fun/status/1246978588504621056 und IR mitgeteilt.
Nicht ganz fair von Hikma & Dr. Reddy

Fairness gibt es nicht im Milliardengeschäft, Amarin hat in einer Dekade über 700 Millionen für Reduce-It investiert und sensationelle Ergebnisse erzielt, ca. 30 % weniger Herzinfarkt, Schlaganfälle und Todesfälle, die EU gibt 10 Jahre Exklusivität und diese bitch stellt alles in Frag, ich wittere Verschwörung bzw. Korruption!
Antwort auf Beitrag Nr.: 63.248.599 von Magnetfeldfredy am 06.04.20 10:21:06Es scheint, dass neben den möglichen juristischen Verfahrensfehlern auch statistische Erhebungsfehler von Du gemacht worden sind - ich hoffe, dass diese grundsätzlich so schwer wiegen, dass die Berufung vor Gericht Erfolg hat.
Ein anderer wichtiger Aspekt sollte aber mit in die Überlegung einbezogen werden.
Das ganze Patentsystem in den USA wird durch diese fragwürdige Entscheidung in Frage gestellt.
Warum sollte eine Biotechfirma zukünftig also überhaupt noch Patente bzw. Patentschutz anstreben und Geld für zeitintensive Studien investieren, wenn der Schutz der ausgesprochenen Patente durch das Patentamt ( FACHinstitution !! ) nicht mehr gewährleistet ist ?
Allein schon dieser Gedanke lässt mich hoffen, dass diese Gerichtsentscheidung NICHT aufrechterhalten werden kann.
Ein anderer wichtiger Aspekt sollte aber mit in die Überlegung einbezogen werden.
Das ganze Patentsystem in den USA wird durch diese fragwürdige Entscheidung in Frage gestellt.
Warum sollte eine Biotechfirma zukünftig also überhaupt noch Patente bzw. Patentschutz anstreben und Geld für zeitintensive Studien investieren, wenn der Schutz der ausgesprochenen Patente durch das Patentamt ( FACHinstitution !! ) nicht mehr gewährleistet ist ?
Allein schon dieser Gedanke lässt mich hoffen, dass diese Gerichtsentscheidung NICHT aufrechterhalten werden kann.
Antwort auf Beitrag Nr.: 63.247.315 von Magnetfeldfredy am 06.04.20 08:56:07Wo ist der Inhalt in deinem Beitrag?
Antwort auf Beitrag Nr.: 63.249.550 von Magnetfeldfredy am 06.04.20 11:30:47Die Durchführung der Tests und somit die positiven Ergebnisse für Amarin sind umstritten!
...irgendwie scheinen sich hier alle Mut zuzusprechen, dass das Urteil der achso bösen und inkompetentin Richterin doch hoffentlich aufgehoben wird, zu einer seriösen Anlageentscheidung gehört aber auch seine Invests kritisch zu hinterfragen....ich sehe sehr gute Chancen für die Mitbewerber, das Amarin keine patentrechtlich besonders schutzwürdigen Fischölkapseln herstellt.
Antwort auf Beitrag Nr.: 63.254.881 von dpdwvz am 06.04.20 18:49:13Das kann man natürlich einfach so in den Raum stellen. Es geht aber eben nicht nur um die üblichen Kapseln und die kritischen Punkte wurden ja auch in dem ersten von mir angesprochenen Artikel VOR der Zulassung erwähnt und die FDA hat auch gezögert und eine weitere Anhörung angesetzt.
Sie hat dann aber die Zulassung erteilt - und zwar nicht als "Nahrungsergänzungsmittel", das in jedem Drogeriemarkt als "Fischölkapsel" erhältlich ist, sondern eben für eines spezielle Aufbereitung, die über die normalen Kapseln hinausgeht. Aus dem durchaus kritischen Artikel:
Die hierzulande vertriebenen Kapseln enthalten Eicosapentaensäure (EPA) und Docosahexaensäure (DHA). Auch für diese Kombination testete Amarin in der Studie VITAL bei über 25.000 über 50-Jährigen Anwendern den Nutzen. Und sie fiel enttäuschend aus. Einzig beim sekundären Endpunkt "Häufigkeit von Herzinfarkten" gab es ein Signal eines möglichen Nutzens. Vascepa wiederum enthält EPA in reiner Form, also nicht, wie sonst üblich, in Kombination mit DHA.
Zu unterstellen, das wäre ohne gründliche Auseinanderesetzung mit dem Thema passiert, verkennt die Dauer und Kompetenz der FDA. Es wäre schon lustig, wenn man über eine halbe Milliarde für Prüfungen ausgeben muss und dann sagt eine Richterin, dass das Mittel doch offensichtlich nützlich und ohne schädliche Nebenwirkungen ist, sie hat als Kind auch immer Lebertran gegesssen oder so.....
Damit unterminiert man das komplette System der Zulassung.
Und zuletzt: ich bin nur mit einer ganz kleinen Summe dabei, deren Verlust mir absolut nicht weh tun würde. Und bei 8 Euro wäre ich im Gewinn.
Sie hat dann aber die Zulassung erteilt - und zwar nicht als "Nahrungsergänzungsmittel", das in jedem Drogeriemarkt als "Fischölkapsel" erhältlich ist, sondern eben für eines spezielle Aufbereitung, die über die normalen Kapseln hinausgeht. Aus dem durchaus kritischen Artikel:
Die hierzulande vertriebenen Kapseln enthalten Eicosapentaensäure (EPA) und Docosahexaensäure (DHA). Auch für diese Kombination testete Amarin in der Studie VITAL bei über 25.000 über 50-Jährigen Anwendern den Nutzen. Und sie fiel enttäuschend aus. Einzig beim sekundären Endpunkt "Häufigkeit von Herzinfarkten" gab es ein Signal eines möglichen Nutzens. Vascepa wiederum enthält EPA in reiner Form, also nicht, wie sonst üblich, in Kombination mit DHA.
Zu unterstellen, das wäre ohne gründliche Auseinanderesetzung mit dem Thema passiert, verkennt die Dauer und Kompetenz der FDA. Es wäre schon lustig, wenn man über eine halbe Milliarde für Prüfungen ausgeben muss und dann sagt eine Richterin, dass das Mittel doch offensichtlich nützlich und ohne schädliche Nebenwirkungen ist, sie hat als Kind auch immer Lebertran gegesssen oder so.....
Damit unterminiert man das komplette System der Zulassung.
Und zuletzt: ich bin nur mit einer ganz kleinen Summe dabei, deren Verlust mir absolut nicht weh tun würde. Und bei 8 Euro wäre ich im Gewinn.
Antwort auf Beitrag Nr.: 63.254.881 von dpdwvz am 06.04.20 18:49:13Keine Ahnung und große Klappe , Reduce It- hat weltweit einzigartige Ergebnisse gezeigt, die patentrechtlicht ganz anders gestaltet sind als Marine, aber was soll ich jemanden erläutern der von der Materie null Ahnung hat, setzen 6!
, Reduce It- hat weltweit einzigartige Ergebnisse gezeigt, die patentrechtlicht ganz anders gestaltet sind als Marine, aber was soll ich jemanden erläutern der von der Materie null Ahnung hat, setzen 6!
 , Reduce It- hat weltweit einzigartige Ergebnisse gezeigt, die patentrechtlicht ganz anders gestaltet sind als Marine, aber was soll ich jemanden erläutern der von der Materie null Ahnung hat, setzen 6!
, Reduce It- hat weltweit einzigartige Ergebnisse gezeigt, die patentrechtlicht ganz anders gestaltet sind als Marine, aber was soll ich jemanden erläutern der von der Materie null Ahnung hat, setzen 6!
Amarin: Did the district court commit error by misinterpreting Kurabayashi?
https://www.markmanadvisors.com/blog/2020/4/7/amarin-did-the…
Antwort auf Beitrag Nr.: 63.254.881 von dpdwvz am 06.04.20 18:49:13ABSOLUT!
Deswegen würde ich Dir dringend raten, angesichts Deines Risikoprofils und Deiner Einschätzung der Situation hier weiterhin auf der "Seitenlinie zu bleiben", wie man so schön zu sagen pflegt
Deswegen würde ich Dir dringend raten, angesichts Deines Risikoprofils und Deiner Einschätzung der Situation hier weiterhin auf der "Seitenlinie zu bleiben", wie man so schön zu sagen pflegt
Antwort auf Beitrag Nr.: 63.235.992 von dpdwvz am 04.04.20 08:45:08 👍 gehört aber auch seine Invests kritisch zu hinterfragen
👍 gehört aber auch seine Invests kritisch zu hinterfragen  ....ich sehe sehr gute Chancen für die Mitbewerber, das Amarin keine patentrechtlich besonders schutzwürdigen Fischölkapseln herstellt.
....ich sehe sehr gute Chancen für die Mitbewerber, das Amarin keine patentrechtlich besonders schutzwürdigen Fischölkapseln herstellt. 
UPS..
KEIN KOMMENTAR !
Zitat von dpdwvz: ...für mich sieht das so aus, dass die Fischbüchse..irgendwie scheinen sich hier alle Mut zuzusprechen, dass das Urteil der achso bösen und inkompetentin Richterin doch hoffentlich aufgehoben wird, zu einer seriösen AnlageentscheidungPräparate verkauft, die patentrechtlich nicht schutzwürdig
sind, dies haben die Mitbewerber
rechtlich feststellen lassen, ist nun die Frage, zu welchem Preis die Allerwelts-Fischkapseln
nun über den Ladentisch verramscht
werden. Ich war nie investiert und werde nicht investieren. Die warnenden Stimmen vor Amarin
gab es ja zu hauf!
 👍 gehört aber auch seine Invests kritisch zu hinterfragen
👍 gehört aber auch seine Invests kritisch zu hinterfragen  ....ich sehe sehr gute Chancen für die Mitbewerber, das Amarin keine patentrechtlich besonders schutzwürdigen Fischölkapseln herstellt.
....ich sehe sehr gute Chancen für die Mitbewerber, das Amarin keine patentrechtlich besonders schutzwürdigen Fischölkapseln herstellt. 
UPS..

KEIN KOMMENTAR !

Hmm hier werden eher kritische Kommentare geäußert und der Kurs geht gen Norden... wo habe ich einen Denkfehler?
Sieht doch ganz gut aus heute! :-) Wer weiß, vielleicht hat unsere Frau Richterin einen schlauen Plan?!
Mit einem bewussten Fehlurteil eine Aktie in Grund und Boden zu stampfen, sie dann kaufen und mit einem neuen Urteil hochzupushen...
Mit einem bewussten Fehlurteil eine Aktie in Grund und Boden zu stampfen, sie dann kaufen und mit einem neuen Urteil hochzupushen...
Eine Büchse Thunfisch essen bringt wahrscheinlich mehr als die Amarin Pillen 😅...Kurs gen Norden?...da wurde aber ein recht kurzes Zeitintervall gewählt 🙈
Antwort auf Beitrag Nr.: 63.278.164 von dpdwvz am 08.04.20 18:08:05Bin ich VÖLLIG bei Dir.
Ich könnte mir bei Dir auch gut vorstellen, dass uns hier an leckeren Rezepten für Nudelsalate teilhaben lässt.
Ich könnte mir bei Dir auch gut vorstellen, dass uns hier an leckeren Rezepten für Nudelsalate teilhaben lässt.
...also wirklich, wer einen Kurs von 5 Mark 30 feiert, wo doch schon 30 Euro aufgerufen wurden, der hat echt Humor 😂
Antwort auf Beitrag Nr.: 63.278.539 von dpdwvz am 08.04.20 18:44:54
Und was ist mit Denen, die bei 4 "Mark" gekauft haben und bei 5,35 "Mark" verkauft haben. Humor? Glück?
Zitat von dpdwvz: ...also wirklich, wer einen Kurs von 5 Mark 30 feiert, wo doch schon 30 Euro aufgerufen wurden, der hat echt Humor 😂
Und was ist mit Denen, die bei 4 "Mark" gekauft haben und bei 5,35 "Mark" verkauft haben. Humor? Glück?
AMARIN TO HOST CONFERENCE CALL ON APRIL 13, 2020 TO PROVIDE PRELIMINARY FIRST QUARTER 2020 RESULTS AND OPERATIONAL UPDATE
Apr 8, 2020
DUBLIN, Ireland and BRIDGEWATER, N.J., April 08, 2020 (GLOBE NEWSWIRE) -- Amarin Corporation plc (NASDAQ:AMRN) today announced that it will host a conference call with management on Monday, April 13, 2020, at 4:30 p.m. ET to discuss the company's preliminary first quarter 2020 financial results and provide an operational update on matters including COVID-19, the company’s planned appeal in the ongoing patent litigation related to VASCEPA® (icosapent ethyl) in the United States and plans for international expansion.
A live question and answer period is not planned for the April 13th call as analysts and investors have already submitted questions to the company covering a wide range of topics, responses to which management plans to cover during the call. This conference call is not intended to replace the regularly scheduled call Amarin plans to hold in connection with its reporting of first quarter 2020 results. During that quarterly call management plans to respond to additional questions from analysts and investors.
Conference Call and Webcast Information:
The conference call will take place on April 13, 2020, at 4:30 p.m. ET. The call can be heard live on the investor relations section of the company's website at www.amarincorp.com, or via telephone by dialing 877-869-3847 within the United States, 201-689-8261 from outside the United States. A replay of the call will be made available for a period of two weeks following the conference call. To hear a replay of the call, dial 877-481-4010, PIN: 34132. A replay of the call will also be available through the company's website shortly after the call.
https://investor.amarincorp.com/news-releases/news-release-d…
Apr 8, 2020
DUBLIN, Ireland and BRIDGEWATER, N.J., April 08, 2020 (GLOBE NEWSWIRE) -- Amarin Corporation plc (NASDAQ:AMRN) today announced that it will host a conference call with management on Monday, April 13, 2020, at 4:30 p.m. ET to discuss the company's preliminary first quarter 2020 financial results and provide an operational update on matters including COVID-19, the company’s planned appeal in the ongoing patent litigation related to VASCEPA® (icosapent ethyl) in the United States and plans for international expansion.
A live question and answer period is not planned for the April 13th call as analysts and investors have already submitted questions to the company covering a wide range of topics, responses to which management plans to cover during the call. This conference call is not intended to replace the regularly scheduled call Amarin plans to hold in connection with its reporting of first quarter 2020 results. During that quarterly call management plans to respond to additional questions from analysts and investors.
Conference Call and Webcast Information:
The conference call will take place on April 13, 2020, at 4:30 p.m. ET. The call can be heard live on the investor relations section of the company's website at www.amarincorp.com, or via telephone by dialing 877-869-3847 within the United States, 201-689-8261 from outside the United States. A replay of the call will be made available for a period of two weeks following the conference call. To hear a replay of the call, dial 877-481-4010, PIN: 34132. A replay of the call will also be available through the company's website shortly after the call.
https://investor.amarincorp.com/news-releases/news-release-d…
Antwort auf Beitrag Nr.: 63.278.164 von dpdwvz am 08.04.20 18:08:05Bei Dir hilft nur Ibidum

Prescription trends positive for Amarin's Vascepa, says Cantor Fitzgerald 07:09 AMRN Prescription trends for Amarin's Vascepa have grown meaningfully since before the REDUCE-IT study results were announced, Cantor Fitzgerald analyst Louise Chen tells investors in a research note, citing Symphony Health data. The analyst says the actual number of prescriptions for Vascepa is underrepresented by data sources such as Symphony as they count 30-day and 90-day as one prescription. Comparing the normalized and non-normalized prescription counts, Chen has seen a positive trend for Vascepa since June 30, 2017. The analyst's relative percentages for normalized TRx and NRx data reported April 3 are showing increases of 138% and 163%, respectively. Chen keeps an Overweight rating on Amarin with a $35 price target.
 The stock closed Thursday up 24c to $6.05
The stock closed Thursday up 24c to $6.05
Read more at:
https://thefly.com/landingPageNews.php?id=3070783

 The stock closed Thursday up 24c to $6.05
The stock closed Thursday up 24c to $6.05Read more at:
https://thefly.com/landingPageNews.php?id=3070783
Antwort auf Beitrag Nr.: 63.281.167 von bernie55 am 08.04.20 22:46:49heute wird es dann mal interessant. 16:30 E.T. heißt nach Börsenschluss.
Aber morgen wird man dann sehen, ob man die Investoren überzeugen konnte.
Heute werde ich die Füße still halten, das ist pures Lottospiel, keiner kann wissen, wie die Konferenz am Abend ankommen wird.
Aber morgen wird man dann sehen, ob man die Investoren überzeugen konnte.
Heute werde ich die Füße still halten, das ist pures Lottospiel, keiner kann wissen, wie die Konferenz am Abend ankommen wird.
Antwort auf Beitrag Nr.: 63.311.509 von Magnetfeldfredy am 13.04.20 13:53:25Ich hatte auf untenstehender Webseite einen Beitrag von Mitchel L.Zola zu einem Update der America Heart Association ( AHA) gesichtet - leider ist der Zugang zu diesem Artikel nur noch über Registierung möglich.
Titel : AHA Updates management when CAD und T2DM conincide
Es war u.a. eine Erklärung zu lesen , die eine stärkere Beteiligung von Herz-Kreislauf-Spezialisten beim Umgang mit Patienten mit Koronarerkrankung und Typ-2-Diabetes "einfordert".
Und - das ist/ wäre der Hammer -
ein Aufruf , VASCEPA als first line treatment zu berücksichtigen.
Alles ohne Gewähr.
https://profreg.medscape.com/px/sso/oauthlogin?oauth=1&clien…
Titel : AHA Updates management when CAD und T2DM conincide
Es war u.a. eine Erklärung zu lesen , die eine stärkere Beteiligung von Herz-Kreislauf-Spezialisten beim Umgang mit Patienten mit Koronarerkrankung und Typ-2-Diabetes "einfordert".
Und - das ist/ wäre der Hammer -
ein Aufruf , VASCEPA als first line treatment zu berücksichtigen.
Alles ohne Gewähr.
https://profreg.medscape.com/px/sso/oauthlogin?oauth=1&clien…
Antwort auf Beitrag Nr.: 63.314.842 von bernie55 am 13.04.20 19:55:21AHA Statement: "Icosapent ethyl is first non–LDL-focused lipid therapy to demonstrate cardiovascular benefit and should be con- sidered first-line therapy for patients with T2DM and CAD whose triglycerides remain elevated (>135 mg/dL) despite max tolerated statins.."

https://mobile.twitter.com/terrapharma1?lang=de

https://mobile.twitter.com/terrapharma1?lang=de
Amarin reports Q1 Vascepa sales of $150M 
Apr. 13, 2020 4:53 PM
- Preliminary Amarin (NASDAQ:AMRN) Q1 numbers show revenue of about $150M vs. estimates for roughly $130M.
- Cash and equivalents of $620M, with less than $50M still due on its royalty-like debt instrument.
https://seekingalpha.com/news/3560317-amarin-reports-q1-vasc…

Apr. 13, 2020 4:53 PM
- Preliminary Amarin (NASDAQ:AMRN) Q1 numbers show revenue of about $150M vs. estimates for roughly $130M.
- Cash and equivalents of $620M, with less than $50M still due on its royalty-like debt instrument.
https://seekingalpha.com/news/3560317-amarin-reports-q1-vasc…
Antwort auf Beitrag Nr.: 63.281.167 von bernie55 am 08.04.20 22:46:49KOMMENTARE....ZITATE...von der Conference Call:
.....Continued Vascepa sales growth
......Vascepa testing for lowering cardiovascular risk in COVID patients!
......help for covid patients!!!
..... the judge has no clue about science
.......I care about shareholders...
......All experienced commentators agree the judgment should have been for AMRN
.........We have added new lead counsel
......Insurance coverage for Vascepa increased in April
........preferred drug status under Anthem Blue Plan in 14 more states"
......Uniqueness of Vascepa has been known for years"
.....100% CVS preferred brand Vascepa....
.....Trial in China started...
.......AMRN is well capitalized
.....Continued Vascepa sales growth
......Vascepa testing for lowering cardiovascular risk in COVID patients!
......help for covid patients!!!
..... the judge has no clue about science
.......I care about shareholders...
......All experienced commentators agree the judgment should have been for AMRN
.........We have added new lead counsel
......Insurance coverage for Vascepa increased in April
........preferred drug status under Anthem Blue Plan in 14 more states"
......Uniqueness of Vascepa has been known for years"
.....100% CVS preferred brand Vascepa....
.....Trial in China started...
.......AMRN is well capitalized
Antwort auf Beitrag Nr.: 63.316.114 von bernie55 am 13.04.20 23:01:56

Zum Glück habe ich vorhin nochmal nachgekauft bei 6,4 Dollar. Aktueller Kurs NASDAQ 6,9 Dollar.
Mal sehen, was sich morgen noch tut.
Mal sehen, was sich morgen noch tut.
Super Zusammenfassung Bernie, um so unglaublicher, dass die drecks korrupten Wallstreet-Gangster den Kurs auf US Dollar 4 geprügelt haben!
Jetzt nach super Q1 und tollen Aussichten, 6,9 US Dollar📈, wir kommen von 14 US Dollar, das steckt System dahinter und die kurrpte Vietnam Richterin Du.......(Schlizauge sei wachsam......!😡)
Ich tippe mal, die oberkorrupten Goldman-Sucks Banker organisieren im Hintergrund eine feindliche Übernahme und haben mit Jefferies den Kurs runtergeprügelt um billig einzusammeln......
Jetzt nach super Q1 und tollen Aussichten, 6,9 US Dollar📈, wir kommen von 14 US Dollar, das steckt System dahinter und die kurrpte Vietnam Richterin Du.......(Schlizauge sei wachsam......!😡)
Ich tippe mal, die oberkorrupten Goldman-Sucks Banker organisieren im Hintergrund eine feindliche Übernahme und haben mit Jefferies den Kurs runtergeprügelt um billig einzusammeln......
We have come too far to stop fighting now...
Lesen und auswendig lernen! Jeder zweite Satz ist zitierenswert, deshalb nur der Link.https://www.fool.com/earnings/call-transcripts/2020/04/13/am…
Antwort auf Beitrag Nr.: 63.319.684 von tippse am 14.04.20 11:18:39
..und schon damit angefangen ?

..good finding..thx 👍
Grüße
bernie55
Zitat von tippse: Lesen und auswendig lernen! Jeder zweite Satz ist zitierenswert, deshalb nur der Link.
https://www.fool.com/earnings/call-transcripts/2020/04/13/am…
..und schon damit angefangen ?


..good finding..thx 👍
Grüße
bernie55

Antwort auf Beitrag Nr.: 63.319.684 von tippse am 14.04.20 11:18:39Hier nun ein paar statements von J.T. bzgl. des Berufungsverfahrens. - mit Translator
Es sei daran erinnert, dass erfolgreiche Ergebnisse der MARINE-Studie von der FDA als Unterstützung für die in der ANCHOR-Studie angestrebte Indikation und für den Zugang zur REDUCE-IT-Studie benötigt wurden, die alle vor mehr als einem Jahrzehnt mit der FDA abgestimmt wurden. Ich erkenne an, dass es für Menschen schwierig ist, für eine solche Analyse in der Zeit zurückzugehen. Aus diesen und anderen Gründen glaube ich nicht, dass das US-Patentamt bei der Beurteilung, dass die Patente für VASCEPA nicht offensichtlich waren, hätte überstimmt werden dürfen. Die Einsicht ist leider eine Macht im menschlichen Geist. Vieles, was zu einem bestimmten Zeitpunkt nicht offensichtlich ist, kann dann, nachdem es entdeckt wurde, offensichtlich erscheinen. Das Berufungsverfahren wird sich wahrscheinlich nicht auf einen Großteil des Inhalts konzentrieren, den ich gerade zum Ausdruck gebracht habe, und daher spielen solche Argumente kaum eine Rolle, außer dass sie Ihnen einen Kontext liefern, der unsere breitere Überzeugung unterstützt, dass unsere Patente als erfinderisch und nicht als offensichtlich hätten aufrechterhalten werden sollen.
Anstatt den Fall erneut zu verhandeln, wird sich die Berufung auf Argumente eher rechtlicher Natur und wichtige sachliche Fehler konzentrieren. Das Ziel einer Berufung ist es, durch ein juristisches Argument ein Gremium von drei spezialisierten Bundesgerichtsrichtern davon zu überzeugen, dass die ANDA-Entscheidung aufgehoben oder neu überdacht werden sollte, wenn das Gesetz richtig angewendet und sachliche Fehler korrigiert werden. Bitte erwarten Sie daher nicht, dass Berufungen eine Möglichkeit zur Wiederaufnahme des Verfahrens darstellen. Bitte erwarten Sie nicht, dass gegen jedes Element, das Sie in der Entscheidung des Richters für falsch halten könnten, Berufung eingelegt wird. Das liegt nicht in der Natur einer Berufung in diesem Rahmen.
Um Erfolg zu haben, müssen wir die Bundesrichter wirksam davon überzeugen, dass die Meinung des Bezirksgerichts aufgrund von Rechts- und Tatsachenfehlern, die sich auf seine Meinung stützen, falsch ist. Wir glauben, dass wir zahlreiche Argumente haben, die zu einer starken inhaltlichen Berufung beitragen werden. Viele von Ihnen haben uns Beispiele von Argumenten genannt, die zu Punkten führen, die in der Berufung vorgebracht werden könnten. Zum jetzigen Zeitpunkt werden wir nicht mitteilen, welche Argumente hervorgehoben werden sollen.
Wenn unser Appell eingereicht wird, wahrscheinlich Anfang Mai, werden solche Argumente öffentlich sein. Wir glauben, dass unser externer Anwalt in der Sache vor dem Bezirksgericht ein überzeugendes Argument vorgebracht hat.
Alle erfahrenen unabhängigen Kommentatoren haben sich die Akten ernsthaft angesehen und sind sich einig, dass der Prozess zu einem Urteil für Amarin hätte führen müssen. Mit Blick auf die Zukunft haben wir festgestellt, dass die Bedeutung dieses Falles für die Berufung die Ergänzung des Teams um eine neue Sichtweise auf die Akten erfordert.
Die Art und Weise, wie man eine Berufung in diesem Bereich argumentiert, ist spezialisiert, und dementsprechend haben wir unserem Rechtsteam einen neuen Hauptanwalt für diese Angelegenheit hinzugefügt, der wie unser Prozessanwalt ein anerkannter Führer auf diesem Gebiet ist und Fälle dieser Art am Bundesgerichtshof gewonnen hat. Unser neuer Chefsyndikus wird mit unserem internen Team und dem Team zusammenarbeiten, das die Angelegenheit vor dem Bezirksgericht vertreten hat. Wir glauben, dass unser Team gut aufgestellt ist, um das Beste aus dem Fall zu machen, und wir glauben, dass wir in einer günstigen Position sind, um einen starken Fall in der Berufung vorzubringen.
Unser Ziel ist es, das Berufungsverfahren so schnell wie möglich zu einer Anhörung und einem Urteil zu beschleunigen. Wir streben an, dass die Angelegenheit im Sommer in einer mündlichen Verhandlung gehört wird, nachdem die Briefings mit einem Urteil so bald wie möglich danach abgeschlossen sind. Wir können und bemühen uns um eine Beschleunigung, aber wir haben keinen Einfluss auf den Zeitplan des Gerichts. Wir hoffen jedoch, dass wir in den nächsten ein bis zwei Wochen mehr Einzelheiten über den Zeitplan für die Briefings erfahren werden. Wenn für diese Berufung beim Bundesberufungsgericht wesentliche Schriftsätze eingereicht werden, werden sie über PACER, die internetbasierte Plattform für die Veröffentlichung von Schriftsätzen, öffentlich zugänglich sein.
Unsere Absicht ist es, bei der Berufung energisch zu kämpfen, und falls wir gewinnen, werden wir energisch für Schadenersatz kämpfen, falls die Markteinführung von Generika durch eine oder mehrere Generikahersteller erfolgt.
Es sei daran erinnert, dass erfolgreiche Ergebnisse der MARINE-Studie von der FDA als Unterstützung für die in der ANCHOR-Studie angestrebte Indikation und für den Zugang zur REDUCE-IT-Studie benötigt wurden, die alle vor mehr als einem Jahrzehnt mit der FDA abgestimmt wurden. Ich erkenne an, dass es für Menschen schwierig ist, für eine solche Analyse in der Zeit zurückzugehen. Aus diesen und anderen Gründen glaube ich nicht, dass das US-Patentamt bei der Beurteilung, dass die Patente für VASCEPA nicht offensichtlich waren, hätte überstimmt werden dürfen. Die Einsicht ist leider eine Macht im menschlichen Geist. Vieles, was zu einem bestimmten Zeitpunkt nicht offensichtlich ist, kann dann, nachdem es entdeckt wurde, offensichtlich erscheinen. Das Berufungsverfahren wird sich wahrscheinlich nicht auf einen Großteil des Inhalts konzentrieren, den ich gerade zum Ausdruck gebracht habe, und daher spielen solche Argumente kaum eine Rolle, außer dass sie Ihnen einen Kontext liefern, der unsere breitere Überzeugung unterstützt, dass unsere Patente als erfinderisch und nicht als offensichtlich hätten aufrechterhalten werden sollen.
Anstatt den Fall erneut zu verhandeln, wird sich die Berufung auf Argumente eher rechtlicher Natur und wichtige sachliche Fehler konzentrieren. Das Ziel einer Berufung ist es, durch ein juristisches Argument ein Gremium von drei spezialisierten Bundesgerichtsrichtern davon zu überzeugen, dass die ANDA-Entscheidung aufgehoben oder neu überdacht werden sollte, wenn das Gesetz richtig angewendet und sachliche Fehler korrigiert werden. Bitte erwarten Sie daher nicht, dass Berufungen eine Möglichkeit zur Wiederaufnahme des Verfahrens darstellen. Bitte erwarten Sie nicht, dass gegen jedes Element, das Sie in der Entscheidung des Richters für falsch halten könnten, Berufung eingelegt wird. Das liegt nicht in der Natur einer Berufung in diesem Rahmen.
Um Erfolg zu haben, müssen wir die Bundesrichter wirksam davon überzeugen, dass die Meinung des Bezirksgerichts aufgrund von Rechts- und Tatsachenfehlern, die sich auf seine Meinung stützen, falsch ist. Wir glauben, dass wir zahlreiche Argumente haben, die zu einer starken inhaltlichen Berufung beitragen werden. Viele von Ihnen haben uns Beispiele von Argumenten genannt, die zu Punkten führen, die in der Berufung vorgebracht werden könnten. Zum jetzigen Zeitpunkt werden wir nicht mitteilen, welche Argumente hervorgehoben werden sollen.
Wenn unser Appell eingereicht wird, wahrscheinlich Anfang Mai, werden solche Argumente öffentlich sein. Wir glauben, dass unser externer Anwalt in der Sache vor dem Bezirksgericht ein überzeugendes Argument vorgebracht hat.
Alle erfahrenen unabhängigen Kommentatoren haben sich die Akten ernsthaft angesehen und sind sich einig, dass der Prozess zu einem Urteil für Amarin hätte führen müssen. Mit Blick auf die Zukunft haben wir festgestellt, dass die Bedeutung dieses Falles für die Berufung die Ergänzung des Teams um eine neue Sichtweise auf die Akten erfordert.
Die Art und Weise, wie man eine Berufung in diesem Bereich argumentiert, ist spezialisiert, und dementsprechend haben wir unserem Rechtsteam einen neuen Hauptanwalt für diese Angelegenheit hinzugefügt, der wie unser Prozessanwalt ein anerkannter Führer auf diesem Gebiet ist und Fälle dieser Art am Bundesgerichtshof gewonnen hat. Unser neuer Chefsyndikus wird mit unserem internen Team und dem Team zusammenarbeiten, das die Angelegenheit vor dem Bezirksgericht vertreten hat. Wir glauben, dass unser Team gut aufgestellt ist, um das Beste aus dem Fall zu machen, und wir glauben, dass wir in einer günstigen Position sind, um einen starken Fall in der Berufung vorzubringen.
Unser Ziel ist es, das Berufungsverfahren so schnell wie möglich zu einer Anhörung und einem Urteil zu beschleunigen. Wir streben an, dass die Angelegenheit im Sommer in einer mündlichen Verhandlung gehört wird, nachdem die Briefings mit einem Urteil so bald wie möglich danach abgeschlossen sind. Wir können und bemühen uns um eine Beschleunigung, aber wir haben keinen Einfluss auf den Zeitplan des Gerichts. Wir hoffen jedoch, dass wir in den nächsten ein bis zwei Wochen mehr Einzelheiten über den Zeitplan für die Briefings erfahren werden. Wenn für diese Berufung beim Bundesberufungsgericht wesentliche Schriftsätze eingereicht werden, werden sie über PACER, die internetbasierte Plattform für die Veröffentlichung von Schriftsätzen, öffentlich zugänglich sein.
Unsere Absicht ist es, bei der Berufung energisch zu kämpfen, und falls wir gewinnen, werden wir energisch für Schadenersatz kämpfen, falls die Markteinführung von Generika durch eine oder mehrere Generikahersteller erfolgt.
sehr enttäuschender Kursverlauf heute, vom Tageshoch hat man ca 10 % verloren.
Ja, die shorts haben uns nach der "grandiosen Fehlentscheidung" solange in den Händen bis wir das Urteil revidiert bekommen, leider.....
Ich habe Hoffung, Unrechtsrichterin Du (Vietkong) hat wegen "obviousness" gegen Amarin entschieden, d.h. Patente sind ungültig weil man wußte das EPA Triclyzride und die damit verbundenen Krankheiten wie Herzinfarkt, Schlaganfall.... reduziert.....
Hier die beste These,dass es nicht so ist, warum hat dann big pharma mit AZN, GSK und Konsorten neue Trials mit EPA und DHA aufgelegt, wenn es offensichtlich war das hochreines EPA hilft.....
Denkfehler, Prozessfehler, scheiss egal, das ist eine korrupte oder verblödete Richterin:
cont. JT: Will bring tremendous shareholder value. Will vigorously fight. With all due respect, current court decision doesn't take into the value of Vascepa and goes against the finding of the USPTO. If it were obvious GSK, AZN would have gone with pure epa and not mixed epa/dha
Ich habe Hoffung, Unrechtsrichterin Du (Vietkong) hat wegen "obviousness" gegen Amarin entschieden, d.h. Patente sind ungültig weil man wußte das EPA Triclyzride und die damit verbundenen Krankheiten wie Herzinfarkt, Schlaganfall.... reduziert.....
Hier die beste These,dass es nicht so ist, warum hat dann big pharma mit AZN, GSK und Konsorten neue Trials mit EPA und DHA aufgelegt, wenn es offensichtlich war das hochreines EPA hilft.....
Denkfehler, Prozessfehler, scheiss egal, das ist eine korrupte oder verblödete Richterin:
cont. JT: Will bring tremendous shareholder value. Will vigorously fight. With all due respect, current court decision doesn't take into the value of Vascepa and goes against the finding of the USPTO. If it were obvious GSK, AZN would have gone with pure epa and not mixed epa/dha
Amarin gearing up to push back against ANDA filers ruling, says Roth Capital 08:36 AMRN Roth Capital analyst Yasmeen Rahimi notes that ahead of COVID-19-associated disruptions, Amarin reported a picture for Q1 that pointed toward strong revenue growth, uptick in scripts, and substantial execution on part of its comprehensive sales strategy. On the closely-watched ANDA litigation front, the next few weeks should provide more insight into the company's appeal strategy, as it aims to file its arguments by early May. Rahimi has a Buy rating and a $31 price target on the shares.
price target on the shares.
Read more at:
https://thefly.com/landingPageNews.php?id=3071738
 price target on the shares.
price target on the shares.Read more at:
https://thefly.com/landingPageNews.php?id=3071738
Antwort auf Beitrag Nr.: 63.331.030 von Magnetfeldfredy am 15.04.20 09:28:59
Auch wenn ich persönlich diesen Überlegungsansatz für überaus logisch und überzeugend halte 👍, müssen wir davon ausgehen, dass AMRN sich in der Berufung schwerpunktmäßig nicht auf medizinisch-relevante Inhalte konzentrieren wird , sondern vielmehr auf " Argumente eher rechtlicher Natur und wichtiger sachlicher Fehler".
Juristen ticken und argumentieren einfach anders.
Siehe J.T. - auf der C.C. : Um Erfolg zu haben, müssen wir die Bundesrichter wirksam davon überzeugen, dass die Meinung des Bezirksgerichts aufgrund von Rechts- und Tatsachenfehlern, die sich auf seine Meinung stützen, falsch ist.
Zitat von Magnetfeldfredy: Ja, die shorts haben uns nach der "grandiosen Fehlentscheidung" solange in den Händen bis wir das Urteil revidiert bekommen, leider.....
Ich habe Hoffung, Unrechtsrichterin Du (Vietkong) hat wegen "obviousness" gegen Amarin entschieden, d.h. Patente sind ungültig weil man wußte das EPA Triclyzride und die damit verbundenen Krankheiten wie Herzinfarkt, Schlaganfall.... reduziert
Hier die beste These,dass es nicht so ist, warum hat dann big pharma mit AZN, GSK und Konsorten neue Trials mit EPA und DHA aufgelegt, wenn es offensichtlich war das hochreines EPA hilft.....
Denkfehler, Prozessfehler, scheiss egal, das ist eine korrupte oder verblödete Richterin:
cont. JT: Will bring tremendous shareholder value. Will vigorously fight. With all due respect, current court decision doesn't take into the value of Vascepa and goes against the finding of the USPTO. If it were obvious GSK, AZN would have gone with pure epa and not mixed epa/dha
Auch wenn ich persönlich diesen Überlegungsansatz für überaus logisch und überzeugend halte 👍, müssen wir davon ausgehen, dass AMRN sich in der Berufung schwerpunktmäßig nicht auf medizinisch-relevante Inhalte konzentrieren wird , sondern vielmehr auf " Argumente eher rechtlicher Natur und wichtiger sachlicher Fehler".
Juristen ticken und argumentieren einfach anders.

Siehe J.T. - auf der C.C. : Um Erfolg zu haben, müssen wir die Bundesrichter wirksam davon überzeugen, dass die Meinung des Bezirksgerichts aufgrund von Rechts- und Tatsachenfehlern, die sich auf seine Meinung stützen, falsch ist.
!
Dieser Beitrag wurde von CloudMOD moderiert. Grund: Kopie, Inhalt aus fremden Medien, Urheberrechtsverletzung, zudem ohne Quellenangabe
Roth Capital glaubt an Amarin, Patentanwalt gibt Amarin große Chance den Appeal zu gewinnen:
Patent lawyer puts high PoS on Amarin appeal, says Roth Capital 09:07 AMRN Roth Capital analyst Yasmeen Rahimi hosted a call with leading patent lawyer Shashank Upadhye to dive into the nuances behind the recent District Court ruling in favor of generics, as well as strategy and prospects surrounding Amarin's intended appeal to the Federal Court. The analyst notes that Upadhye puts high Probability of Success on Amarin's appeal, with "a winning appeal argument" likely focusing on District Court's take on primary versus secondary considerations. Rahimi has a Buy rating and a $31 price target on the shares.
Read more at:
https://thefly.com/landingPageNews.php?id=3073495
Patent lawyer puts high PoS on Amarin appeal, says Roth Capital 09:07 AMRN Roth Capital analyst Yasmeen Rahimi hosted a call with leading patent lawyer Shashank Upadhye to dive into the nuances behind the recent District Court ruling in favor of generics, as well as strategy and prospects surrounding Amarin's intended appeal to the Federal Court. The analyst notes that Upadhye puts high Probability of Success on Amarin's appeal, with "a winning appeal argument" likely focusing on District Court's take on primary versus secondary considerations. Rahimi has a Buy rating and a $31 price target on the shares.
Read more at:
https://thefly.com/landingPageNews.php?id=3073495
...dürfen die Fisch-Bonbons nun als Medizin verkauft werden oder nicht? 😂👍
!
Dieser Beitrag wurde von FairMOD moderiert. Grund: Beleidigung!
Dieser Beitrag wurde von FairMOD moderiert. Grund: persönlicher Angriff!
Dieser Beitrag wurde von CloudMOD moderiert. Grund: unnötige Provokation, bleiben Sie bitte sachlich, persönliche Angriffe bitte unterlassen
Das macht Hoffung, der hier beschriebene Patentanwalt ist lawyer des Jahres und gibt Amarin große Chancen beim Appeal:
Shashank Upadhye ist tatsächlich hochqualifiziert, lawyer of the year 19-20, sowie ein
"prolific writer and frequent speaker, Shashank is the author of numerous articles and the leading treatise on navigating complex U.S. laws of FDA brand/generic ...
Amin Talati & Upadhye is a boutique IP and FDA firm. We specialize in IP (patent and trademark) work, predominantly in the medical device and pharmaceutical ...
Patent lawyer specializing in pharmaceuticals, medical devices and related FDA law.
Formerly, chief IP and regulatory affairs counsel for several global drug ..."
Generic Pharmaceutical Patent and FDA Law...
Patent lawyer puts high PoS on Amarin appeal, says Roth Capital 09:07 AMRN Roth Capital analyst Yasmeen Rahimi hosted a call with leading patent lawyer Shashank Upadhye to dive into the nuances behind the recent District Court ruling in favor of generics, as well as strategy and prospects surrounding Amarin's intended appeal to the Federal Court. The analyst notes that Upadhye puts high Probability of Success on Amarin's appeal, with "a winning appeal argument" likely focusing on District Court's take on primary versus secondary considerations. Rahimi has a Buy rating and a $31 price target on the shares.
Read more at:
https://thefly.com/landingPageNews.php?id=3073495
Shashank Upadhye ist tatsächlich hochqualifiziert, lawyer of the year 19-20, sowie ein
"prolific writer and frequent speaker, Shashank is the author of numerous articles and the leading treatise on navigating complex U.S. laws of FDA brand/generic ...
Amin Talati & Upadhye is a boutique IP and FDA firm. We specialize in IP (patent and trademark) work, predominantly in the medical device and pharmaceutical ...
Patent lawyer specializing in pharmaceuticals, medical devices and related FDA law.
Formerly, chief IP and regulatory affairs counsel for several global drug ..."
Generic Pharmaceutical Patent and FDA Law...
Patent lawyer puts high PoS on Amarin appeal, says Roth Capital 09:07 AMRN Roth Capital analyst Yasmeen Rahimi hosted a call with leading patent lawyer Shashank Upadhye to dive into the nuances behind the recent District Court ruling in favor of generics, as well as strategy and prospects surrounding Amarin's intended appeal to the Federal Court. The analyst notes that Upadhye puts high Probability of Success on Amarin's appeal, with "a winning appeal argument" likely focusing on District Court's take on primary versus secondary considerations. Rahimi has a Buy rating and a $31 price target on the shares.
Read more at:
https://thefly.com/landingPageNews.php?id=3073495
Antwort auf Beitrag Nr.: 63.381.194 von Magnetfeldfredy am 20.04.20 09:18:41👍 alles klar 

Die Fehler der Richterin sind so offensichtlich dass ich Korruption annehme:
Procedural error: it looks like that Judge Du committed at least two errors
- Applied USPTO approach, determined prima facie obviousness and see secondary considerations (SCs) after that
- Weighted SCs against each others (plus gave a “negative” to some non-existing SCs)
Could it be enough?
The factual error – looks like it is important … the Apo-B / Kurabayashi (Kura):
- In case of Claim 8 of 677 patent: if Kura does not TSM and is not “obvious to try” the prima facie obviousness of the claim is weaker
- In case of ALL claims: the “unexpected benefit” exists … more doubt about obviousness
So, procedural error could be enough for reversal but with factual error the chance is definitely higher.
Procedural error: it looks like that Judge Du committed at least two errors
- Applied USPTO approach, determined prima facie obviousness and see secondary considerations (SCs) after that
- Weighted SCs against each others (plus gave a “negative” to some non-existing SCs)
Could it be enough?
The factual error – looks like it is important … the Apo-B / Kurabayashi (Kura):
- In case of Claim 8 of 677 patent: if Kura does not TSM and is not “obvious to try” the prima facie obviousness of the claim is weaker
- In case of ALL claims: the “unexpected benefit” exists … more doubt about obviousness
So, procedural error could be enough for reversal but with factual error the chance is definitely higher.
Antwort auf Beitrag Nr.: 63.415.532 von Magnetfeldfredy am 22.04.20 16:25:14
Also wenn dem so ist, dass Verfahrensfehler für eine Umkehrung bzw. einen möglichen Erfolg ausreichen, aber mit einem faktischen Fehler die Chance definitiv höher sind , dann würde ich doch als Juristenlaie sagen :
Just take these two arguments for the court proceedings
Zitat von Magnetfeldfredy: Die Fehler der Richterin sind so offensichtlich dass ich Korruption annehme:
Procedural error: it looks like that Judge Du committed at least two errors
Applied USPTO approach, determined prima facie obviousness and see secondary considerations (SCs) after that
- Weighted SCs against each others (plus gave a “negative” to some non-existing SCs)
Could it be enough?
The factual error – looks like it is important … the Apo-B / Kurabayashi (Kura):
- In case of Claim 8 of 677 patent: if Kura does not TSM and is not “obvious to try” the prima facie obviousness of the claim is weaker
- In case of ALL claims: the “unexpected benefit” exists … more doubt about obviousness
So, procedural error could be enough for reversal but with factual error the chance is definitely higher.
Also wenn dem so ist, dass Verfahrensfehler für eine Umkehrung bzw. einen möglichen Erfolg ausreichen, aber mit einem faktischen Fehler die Chance definitiv höher sind , dann würde ich doch als Juristenlaie sagen :
Just take these two arguments for the court proceedings
Hi Bernie,
das Urteil schreit zum Himmel vor Ungerechtigkeit, Juristen geben dem Appeal sehr gute Gewinnchancen, hast Du Dich schon mal gefragt warum sofort von Goldmann Sachs, Jefferies Kursziele von nie gerechtfertigten US Dollar 4 gegeben wurden?
Ich vermute Richterin Du wurde nicht nur von den Generika geschmiert sondern auch von Goldmann...., da wurde zum Teufel manipuliert um Klienten billigst Amarin Aktien zu beschaffen, das ist meine Theorie.......
Time will tell..........
das Urteil schreit zum Himmel vor Ungerechtigkeit, Juristen geben dem Appeal sehr gute Gewinnchancen, hast Du Dich schon mal gefragt warum sofort von Goldmann Sachs, Jefferies Kursziele von nie gerechtfertigten US Dollar 4 gegeben wurden?
Ich vermute Richterin Du wurde nicht nur von den Generika geschmiert sondern auch von Goldmann...., da wurde zum Teufel manipuliert um Klienten billigst Amarin Aktien zu beschaffen, das ist meine Theorie.......
Time will tell..........
Es geht Schlag auf Schlag:
Markman Advisors LLC
@MarkmanAdvisors
Amarin files motion for expedited briefing schedule for the appeal -- with the consent of generics. If granted, Amarin's brief due by May 12, and oral argument will likely happen in July or August. $ARMN #vascepa
Markman Advisors LLC
@MarkmanAdvisors
Amarin files motion for expedited briefing schedule for the appeal -- with the consent of generics. If granted, Amarin's brief due by May 12, and oral argument will likely happen in July or August. $ARMN #vascepa
Antwort auf Beitrag Nr.: 63.418.472 von Magnetfeldfredy am 22.04.20 19:36:04Servus, man das ganze verkommt zu einem Krimi hier.
Ich hoffe auf Gerechtigkeit.
Ich hoffe auf Gerechtigkeit.
2 Top Biotech Stocks Under $10
Amarin and Atara Biotherapeutics could both be diamonds in the rough.
George Budwell
George Budwell
(TMFGBudwell)
Apr 27, 2020 at 7:56AM
Author Bio
Stocks priced below $10 a share can be a mixed bag. Low share prices are often indicative of companies with poor fundamentals, a weak near-term outlook, and a large number of outstanding shares. These types of equities, in turn, tend to be exceptionally risky and highly volatile from a price standpoint. But there are a few significant upsides to buying stocks in the $5 to $10 range.
Low-priced equities, on occasion, can deliver substantial returns on capital within an exceedingly short period. What's more, it's far easier to build a position in round lots of 100 shares at a time. By doing so, investors can reduce their risk profile and generate immediate cash from the position by selling call options. While selling calls can cap the maximum return from a low-priced equity, this strategy also ensures that you won't walk away empty-handed in a worst-case scenario.
A man wearing sunglasses while throwing money into the air.
Image source: Getty Images.
Which companies with share prices under $10 should investors be paying attention to right now? Two of the best low-priced equities at the moment are Amarin (NASDAQ:AMRN) and Atara Biotherapeutics. (NASDAQ:ATRA). These two biotech stocks have been heating up over the course of April and both are seemingly undervalued relative to their long-term outlook. Here's a snapshot of the pros and cons of each company.
Amarin: A bet on a full-blown comeback
Amarin is a single-product, commercial-stage biopharma. The company's value proposition thus begins and ends with its prescription omega-3 treatment Vascepa. Last year, the drug gained a major label expansion as a treatment for patients taking statins but who are still at risk of cardiovascular disease. This new indication was thought to be worth at least $2 billion in annual sales, and possibly quite a bit more. Unfortunately, a U.S. District Court stripped Vascepa of its patent protection in March, causing Amarin's shares to collapse.
Thanks to the growing optimism that this decision will be overturned upon appeal, Amarin's shares have gained 88% in the nearly four weeks since this adverse ruling. This rapid recovery has also been fueled by the fact that Vascepa could still achieve blockbuster status, regardless of this patent ruling, due to its healthy commercial opportunity in former U.S. territories.
What are the risks and rewards? With a successful appeal in hand, Amarin's shares should rise by at least another 70% from current levels in light of where this stock was trading prior to this black swan event. If this appeal fails, however, Amarin should ultimately settle at around $8 a share -- based on Vascepa's commercial opportunity in the former territories and the average pre-pandemic premium for a commercial-stage biopharma stock. So, even in a worst-case scenario, Amarin's shares are arguably still undervalued right now.
Atara: A make-or-break moment is on the way
Atara is a cell-based immunotherapy company. The company's main selling point to investors is its leadership position in the field of off-the-shelf T-cell therapies for cancer, autoimmune disorders, and viral infections. Atara's strategy centers around launching the first off-the-shelf T-cell therapy, dubbed tab-cel, for patients with Epstein-Barr virus associated post-transplant lymphoproliferative disease as soon as next year. A regulatory filing for this first-of-its-kind therapy is slated for the second half of 2020.
Although tab-cel's peak sales are expected to be rather modest at around $140 million a year, the company's second product candidate, ATA188, could be a megablockbuster as a novel treatment for patients with progressive multiple sclerosis. What's more, Atara's buyout value should skyrocket if its cell-based platform is validated with a successful regulatory filing for tab-cel. There are several big pharmas and blue-chip biotech companies racing to develop similar technologies after all.
What's the risk? While Atara does have the inside track for becoming the first company to bring an off-the-shelf T-cell therapy to market, there's also no guarantee that this novel therapy will get a green light from regulators. That's a significant risk factor to be sure. Atara's shares, in short, will surely take a huge step backward if tab-cel stumbles in the clinic or fails to win over regulators. On the flip side, this small-cap biotech stock does have the potential to double (or possibly triple) in value in the event that tab-cel does live up to expectations.
Bottom line: Atara is the epitome of a high-risk, high-reward clinical-stage biotech stock. Invest accordingly.
Amarin and Atara Biotherapeutics could both be diamonds in the rough.
George Budwell
George Budwell
(TMFGBudwell)
Apr 27, 2020 at 7:56AM
Author Bio
Stocks priced below $10 a share can be a mixed bag. Low share prices are often indicative of companies with poor fundamentals, a weak near-term outlook, and a large number of outstanding shares. These types of equities, in turn, tend to be exceptionally risky and highly volatile from a price standpoint. But there are a few significant upsides to buying stocks in the $5 to $10 range.
Low-priced equities, on occasion, can deliver substantial returns on capital within an exceedingly short period. What's more, it's far easier to build a position in round lots of 100 shares at a time. By doing so, investors can reduce their risk profile and generate immediate cash from the position by selling call options. While selling calls can cap the maximum return from a low-priced equity, this strategy also ensures that you won't walk away empty-handed in a worst-case scenario.
A man wearing sunglasses while throwing money into the air.
Image source: Getty Images.
Which companies with share prices under $10 should investors be paying attention to right now? Two of the best low-priced equities at the moment are Amarin (NASDAQ:AMRN) and Atara Biotherapeutics. (NASDAQ:ATRA). These two biotech stocks have been heating up over the course of April and both are seemingly undervalued relative to their long-term outlook. Here's a snapshot of the pros and cons of each company.
Amarin: A bet on a full-blown comeback
Amarin is a single-product, commercial-stage biopharma. The company's value proposition thus begins and ends with its prescription omega-3 treatment Vascepa. Last year, the drug gained a major label expansion as a treatment for patients taking statins but who are still at risk of cardiovascular disease. This new indication was thought to be worth at least $2 billion in annual sales, and possibly quite a bit more. Unfortunately, a U.S. District Court stripped Vascepa of its patent protection in March, causing Amarin's shares to collapse.
Thanks to the growing optimism that this decision will be overturned upon appeal, Amarin's shares have gained 88% in the nearly four weeks since this adverse ruling. This rapid recovery has also been fueled by the fact that Vascepa could still achieve blockbuster status, regardless of this patent ruling, due to its healthy commercial opportunity in former U.S. territories.
What are the risks and rewards? With a successful appeal in hand, Amarin's shares should rise by at least another 70% from current levels in light of where this stock was trading prior to this black swan event. If this appeal fails, however, Amarin should ultimately settle at around $8 a share -- based on Vascepa's commercial opportunity in the former territories and the average pre-pandemic premium for a commercial-stage biopharma stock. So, even in a worst-case scenario, Amarin's shares are arguably still undervalued right now.
Atara: A make-or-break moment is on the way
Atara is a cell-based immunotherapy company. The company's main selling point to investors is its leadership position in the field of off-the-shelf T-cell therapies for cancer, autoimmune disorders, and viral infections. Atara's strategy centers around launching the first off-the-shelf T-cell therapy, dubbed tab-cel, for patients with Epstein-Barr virus associated post-transplant lymphoproliferative disease as soon as next year. A regulatory filing for this first-of-its-kind therapy is slated for the second half of 2020.
Although tab-cel's peak sales are expected to be rather modest at around $140 million a year, the company's second product candidate, ATA188, could be a megablockbuster as a novel treatment for patients with progressive multiple sclerosis. What's more, Atara's buyout value should skyrocket if its cell-based platform is validated with a successful regulatory filing for tab-cel. There are several big pharmas and blue-chip biotech companies racing to develop similar technologies after all.
What's the risk? While Atara does have the inside track for becoming the first company to bring an off-the-shelf T-cell therapy to market, there's also no guarantee that this novel therapy will get a green light from regulators. That's a significant risk factor to be sure. Atara's shares, in short, will surely take a huge step backward if tab-cel stumbles in the clinic or fails to win over regulators. On the flip side, this small-cap biotech stock does have the potential to double (or possibly triple) in value in the event that tab-cel does live up to expectations.
Bottom line: Atara is the epitome of a high-risk, high-reward clinical-stage biotech stock. Invest accordingly.
Amarin to Report First Quarter 2020 Results and Host Conference Call on April 30, 2020
Apr. 28, 2020 7:00 AM ETGlobeNewswireAmarin Corporation plc (AMRN)
DUBLIN, Ireland and BRIDGEWATER, N.J., April 28, 2020 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN) today announced that it will host a conference call with members of Amarin senior management to discuss the company's first quarter 2020 financial results and provide an operational update on Thursday, April 30, 2020, at 7:30 a.m. ET.
The conference call will follow the anticipated release of the company's financial results earlier that day
https://seekingalpha.com/pr/17850387-amarin-to-report-first-…
Apr. 28, 2020 7:00 AM ETGlobeNewswireAmarin Corporation plc (AMRN)
DUBLIN, Ireland and BRIDGEWATER, N.J., April 28, 2020 (GLOBE NEWSWIRE) -- Amarin Corporation plc (AMRN) today announced that it will host a conference call with members of Amarin senior management to discuss the company's first quarter 2020 financial results and provide an operational update on Thursday, April 30, 2020, at 7:30 a.m. ET.
The conference call will follow the anticipated release of the company's financial results earlier that day
https://seekingalpha.com/pr/17850387-amarin-to-report-first-…
Wenn das so weitergeht lache ich Ende des Jahres über meinen Griff ins Klo beim Einstieg hier vor ein paar Wochen... Börse, Leute ich sag´s Euch ...
Aber jetzt wieder rüber in den WDI Thread - hier geht´s mir viel zu kultiviert zu: lauter Leute, die Ahnung haben - bis auf unseren kleinen Stinker User Nudelsalat .. oder war´s Thunfisch? egal - unwichtig
Aber jetzt wieder rüber in den WDI Thread - hier geht´s mir viel zu kultiviert zu: lauter Leute, die Ahnung haben - bis auf unseren kleinen Stinker User Nudelsalat .. oder war´s Thunfisch? egal - unwichtig

Antwort auf Beitrag Nr.: 63.507.299 von tippse am 29.04.20 22:05:50...in allen Qualitätstiteln vertreten unsere tippse, liest eher die Börsen-Bild als das Handelsblatt 🤠
...wer den Absturz zum Einstieg genutzt hat, fährt tatsächlich gerade gute Gewinne, die Hardcore-Fans dürften aber noch auf locker 50% Miese sitzen und alles auf eine verrückte Richterin zu schieben ist zu kurz gedacht, aber jeder ist seines Glückes Schmied
Ich bin durch Zufall auf Amarin aufmerksam geworden, habe mich eingelesen und bin mit einer Position bei 3,75 eingstiegen. Mit einem so guten Verlauf habe ich nicht gerechnet und daher ist die Position leider zu klein gewesen. Aber trotzdem macht es Spaß :-).
Und wenn die Gerichtsentscheidung revidiert wird, gibt es nochmal einen schönen Schub.
Und wenn die Gerichtsentscheidung revidiert wird, gibt es nochmal einen schönen Schub.
Ich sitze auf 50% Miesen und die Richterin ist verrückt.
Antwort auf Beitrag Nr.: 63.514.106 von Newark am 30.04.20 11:29:29Die Richterin ist geschmiert und nicht verrückt, aber der Appeal hat gute Chancen die Ungerechtigkeit aufzuheben🍀
Die Anal -ysten erhöhen das Kursziel von US Dollar 6 auf US Dollar 12, es scheint als ob sich die Klienten eingedeckt haben und Kurs steigen darf, Dreckspack:
Citi doubles Amarin price target to $12 ahead of appeal decision Citi analyst Joel Beatty doubled the firm's price target on Amarin to $12 from $6 and keeps a Buy rating on the shares. The stock closed Thursday down 65c to $7.61. Beatty sees a "relatively high likelihood" of share upside from a European partnership in the second half of 2020, and also believes competitors are unlikely to launch a generic version of Vascepa before the appeal decision expected by the end of the year. While acknowledging a high risk to the stock from the appeal decision, he believes his 20%-40% probability of success on appeal for Amarin still provides a "favorable enough risk/reward" to buy the stock at the current price. Shares of Amarin are worth $24 on an appeal win, and $7 on a loss, Beatty tells investors in a research note.
Read more at:
https://thefly.com/landingPageNews.php?id=3083795
Citi doubles Amarin price target to $12 ahead of appeal decision Citi analyst Joel Beatty doubled the firm's price target on Amarin to $12 from $6 and keeps a Buy rating on the shares. The stock closed Thursday down 65c to $7.61. Beatty sees a "relatively high likelihood" of share upside from a European partnership in the second half of 2020, and also believes competitors are unlikely to launch a generic version of Vascepa before the appeal decision expected by the end of the year. While acknowledging a high risk to the stock from the appeal decision, he believes his 20%-40% probability of success on appeal for Amarin still provides a "favorable enough risk/reward" to buy the stock at the current price. Shares of Amarin are worth $24 on an appeal win, and $7 on a loss, Beatty tells investors in a research note.
Read more at:
https://thefly.com/landingPageNews.php?id=3083795
Roth Capital sieht Amarin als strong buy mit Kursziel US Dollar 31:
Amarin a 'strong buy' ahead of federal circuit ruling, says Roth Capital Roth Capital analyst Yasmeen Rahimi sees COVID-19 disruptions as only a "minor distraction" from Amarin's continued commercial rollout of Vascepa. Although the pandemic has made it difficult for the company to fully take advantage of its sales expansion started in 2019, Amarin has taken innovative steps to keep interactions between sales reps and doctors, Rahimi tells investors in a research note. Further, the analyst notes that Amarin bumped up the timeline for a Vascepa appeals decision, with a hearing as early as Q3 and ruling thereafter by the end of 2020 or early 2021. With an expedited timeframe for its appeal, Amarin is a "strong buy" ahead of a potential federal circuit ruling, contends Rahimi. Following a deep dive with a "patent law guru," the analyst sees a high probability of success for the company on appeal. Alongside the "strategic optionality" being explored for Vascepa commercialization in Europe and a "strong resiliency" in the COVID crisis, Amarin shares offer "significant upside" potential for 2020, says Rahimi. The analyst keeps a Buy rating on the name with a $31 price target. The stock closed Friday at $7.34.
The stock closed Friday at $7.34.
Read more at:
https://thefly.com/landingPageNews.php?id=3084953
Amarin a 'strong buy' ahead of federal circuit ruling, says Roth Capital Roth Capital analyst Yasmeen Rahimi sees COVID-19 disruptions as only a "minor distraction" from Amarin's continued commercial rollout of Vascepa. Although the pandemic has made it difficult for the company to fully take advantage of its sales expansion started in 2019, Amarin has taken innovative steps to keep interactions between sales reps and doctors, Rahimi tells investors in a research note. Further, the analyst notes that Amarin bumped up the timeline for a Vascepa appeals decision, with a hearing as early as Q3 and ruling thereafter by the end of 2020 or early 2021. With an expedited timeframe for its appeal, Amarin is a "strong buy" ahead of a potential federal circuit ruling, contends Rahimi. Following a deep dive with a "patent law guru," the analyst sees a high probability of success for the company on appeal. Alongside the "strategic optionality" being explored for Vascepa commercialization in Europe and a "strong resiliency" in the COVID crisis, Amarin shares offer "significant upside" potential for 2020, says Rahimi. The analyst keeps a Buy rating on the name with a $31 price target.
 The stock closed Friday at $7.34.
The stock closed Friday at $7.34.Read more at:
https://thefly.com/landingPageNews.php?id=3084953
Antwort auf Beitrag Nr.: 63.555.807 von Magnetfeldfredy am 04.05.20 15:13:04
Unseriös, da sich ein Kursziel immer auf einen Zeitraum von 12 Monaten bezieht.
Rein rechnerisch (Bierdeckel) ginge es so:
KZ 31
KGV 20 = 1,50$ eps * 350 Mio shares = 500 Mio EBIT
500 Mio EBIT + 500 Mio SGA + 500 Mio Tax = 1,5 Mrd.
Grossprofit 80% => 1,8 bis 2 Mrd$ Umsatz
Dieses Jahr wahrscheinlich 650 Mio Umsatz ...
Mit Canada und Europa in 2023 denkbar ... vorher nicht!
Asien und ROW kenne ich den Stand der Planungen nicht...
Zitat von Magnetfeldfredy: The analyst keeps a Buy rating on the name with a $31 price target
Unseriös, da sich ein Kursziel immer auf einen Zeitraum von 12 Monaten bezieht.
Rein rechnerisch (Bierdeckel) ginge es so:
KZ 31
KGV 20 = 1,50$ eps * 350 Mio shares = 500 Mio EBIT
500 Mio EBIT + 500 Mio SGA + 500 Mio Tax = 1,5 Mrd.
Grossprofit 80% => 1,8 bis 2 Mrd$ Umsatz
Dieses Jahr wahrscheinlich 650 Mio Umsatz ...
Mit Canada und Europa in 2023 denkbar ... vorher nicht!
Asien und ROW kenne ich den Stand der Planungen nicht...
Antwort auf Beitrag Nr.: 63.582.237 von tippse am 06.05.20 16:00:00Das passt von Dir, jedoch musst Du die Übernahmeprovision addieren, dann könnte das hinkommen!
Jedoch müssen wir zurvor den Appeal gewinnen ansonsten gehe ich von der Hälfte aus,
ca. US Dollar 15!
Die Richterin hat meiner Meinung nach Unrecht gesprochen und Amarin samt Aktionäre an die Wand gedrückt, jedoch wird das nicht lange währen, es geht um zu viel, welche Pharmafirma würde noch für Milliarden forschen und entwickeln wenn eine Richterin gewährte Patente aufhebt, die Weiterentwicklung nicht berücksichtigt......
Traurig aber wahr, wir haben eine Schlacht verloren, aber noch nicht den Krieg!
Jedoch müssen wir zurvor den Appeal gewinnen ansonsten gehe ich von der Hälfte aus,
ca. US Dollar 15!
Die Richterin hat meiner Meinung nach Unrecht gesprochen und Amarin samt Aktionäre an die Wand gedrückt, jedoch wird das nicht lange währen, es geht um zu viel, welche Pharmafirma würde noch für Milliarden forschen und entwickeln wenn eine Richterin gewährte Patente aufhebt, die Weiterentwicklung nicht berücksichtigt......
Traurig aber wahr, wir haben eine Schlacht verloren, aber noch nicht den Krieg!
Why an Appeals Court May Hold Key to Amarin Stock Future
Carla Baranauckas
May 8, 2020 2:02 pm
Last Updated: May 8, 2020 2:07 pm
Amarin Corp. PLC (NASDAQ: AMRN) plans to mount a vigorous and speedy appeal of a U.S. District Court ruling that blocked its patents on the medication Vascepa, the company’s chief executive says.
In a conference call last week about first-quarter earnings, president and CEO John Thero said Amarin had filed a notice of appeal. It will submit legal briefs next week.
01:25 / 01:25
00:00
Vascepa is a synthetic formulation of fish oil. It is prescribed to help reduce the risk of heart attack and stroke in patients with cardiovascular disease or diabetes. When used in conjunction with a low-fat, low-cholesterol diet, it can help reduce high triglyceride levels, Amarin says.
Amarin holds six patents on Vascepa, which delivers high doses of omega-3 fatty acids. The company has put most of its muscle behind this one drug and it appeared to have limited competition. Acasti Pharma Inc. (NASDAQ: ACST) and AstraZeneca PLC (NYSE: AZN) invested millions of dollars trying to develop drugs that could take market share from Amarin. Neither was successful.
‘Invalid as Obvious’
Generic competitors Dr. Reddy’s Laboratories Inc. (NYSE: RDY) and Hikma Pharmaceuticals notified the Food and Drug Administration of their intent to develop generic alternatives. Amarin then filed suit in U.S. District Court in Nevada claiming patent infringement.
Chief Judge Miranda Du ruled on March 30 that the drugmakers would be infringing on Amarin’s claims in its patents if they brought generic drugs to market but that those claims were “invalid as obvious” and should not have been granted by the U.S. Patent Office.
“We are convinced that the invention of Vascepa was not obvious,” Thero insisted on April 30. He said that Amarin’s competitors were benefiting from hindsight.
“It remains astonishing that the invention of Vascepa can now be seen as obvious by everyone,” Thero said. “During our more than 10 years of developing and testing Vascepa, this was not obvious to our competitors or others in the industry. I appreciate that the elegance of our solution seems obvious after the fact. However, this is often due to the nature of innovation.”
Amarin and the defendants have agreed to expedite the appeal, Thero said. Arguments may be heard as early as the first week of September or October.
The company is bolstering its legal team by bringing in high-power attorney Jonathan Singer of Fish & Richardson as lead counsel. Singer heads the life sciences practice for the law firm, which is a leader in patent infringement litigation.
He successfully argued an appeal for Cephalon Inc. to protect its patent on Amrix, a muscle relaxant. A lower court had deemed it “obvious.” Cephalon is now part of Teva Pharmaceutical Industries Ltd. (NYSE: TEVA).
Potential Major Impact for Drug Industry
Thero said the Vascepa case had the potential to have a major impact on the pharmaceutical industry.
If the district court’s decision in the appeal process is not overturned, undermining the local Vascepa patent will prevent companies from taking the risk of developing future innovative therapies to meet unmet medical needs.
The development of new drugs is expensive and takes many years. Developers must be able to rely on properly granted patents from the United States Patent Office. A precedent in which patents are revoked in circumstances such as ours will keep developers from taking such risks for fear that they will not be able to cover their costs or benefit from their risks.
24/7 Wall St.
Why Partial Reopenings Are Set to Boost Disney Stock
Amarin has made a significant investment in Vascepa and has doubled its sales force to 800, Thero said. It is also investigating whether the drug can reduce the cardiovascular risks associated with COVID-19.
The litigation has been a drag on Amarin’s share price. The stock fell to a 52-week low of $3.95 on March 31, the day after the district court ruling. It’s 52-week high had been near $25 anticipating FDA approval of Vascepa, which came in December. In today’s trading, Amarin was up about 3% at midday at $7.76.
Carla Baranauckas
May 8, 2020 2:02 pm
Last Updated: May 8, 2020 2:07 pm
Amarin Corp. PLC (NASDAQ: AMRN) plans to mount a vigorous and speedy appeal of a U.S. District Court ruling that blocked its patents on the medication Vascepa, the company’s chief executive says.
In a conference call last week about first-quarter earnings, president and CEO John Thero said Amarin had filed a notice of appeal. It will submit legal briefs next week.
01:25 / 01:25
00:00
Vascepa is a synthetic formulation of fish oil. It is prescribed to help reduce the risk of heart attack and stroke in patients with cardiovascular disease or diabetes. When used in conjunction with a low-fat, low-cholesterol diet, it can help reduce high triglyceride levels, Amarin says.
Amarin holds six patents on Vascepa, which delivers high doses of omega-3 fatty acids. The company has put most of its muscle behind this one drug and it appeared to have limited competition. Acasti Pharma Inc. (NASDAQ: ACST) and AstraZeneca PLC (NYSE: AZN) invested millions of dollars trying to develop drugs that could take market share from Amarin. Neither was successful.
‘Invalid as Obvious’
Generic competitors Dr. Reddy’s Laboratories Inc. (NYSE: RDY) and Hikma Pharmaceuticals notified the Food and Drug Administration of their intent to develop generic alternatives. Amarin then filed suit in U.S. District Court in Nevada claiming patent infringement.
Chief Judge Miranda Du ruled on March 30 that the drugmakers would be infringing on Amarin’s claims in its patents if they brought generic drugs to market but that those claims were “invalid as obvious” and should not have been granted by the U.S. Patent Office.
“We are convinced that the invention of Vascepa was not obvious,” Thero insisted on April 30. He said that Amarin’s competitors were benefiting from hindsight.
“It remains astonishing that the invention of Vascepa can now be seen as obvious by everyone,” Thero said. “During our more than 10 years of developing and testing Vascepa, this was not obvious to our competitors or others in the industry. I appreciate that the elegance of our solution seems obvious after the fact. However, this is often due to the nature of innovation.”
Amarin and the defendants have agreed to expedite the appeal, Thero said. Arguments may be heard as early as the first week of September or October.
The company is bolstering its legal team by bringing in high-power attorney Jonathan Singer of Fish & Richardson as lead counsel. Singer heads the life sciences practice for the law firm, which is a leader in patent infringement litigation.
He successfully argued an appeal for Cephalon Inc. to protect its patent on Amrix, a muscle relaxant. A lower court had deemed it “obvious.” Cephalon is now part of Teva Pharmaceutical Industries Ltd. (NYSE: TEVA).
Potential Major Impact for Drug Industry
Thero said the Vascepa case had the potential to have a major impact on the pharmaceutical industry.
If the district court’s decision in the appeal process is not overturned, undermining the local Vascepa patent will prevent companies from taking the risk of developing future innovative therapies to meet unmet medical needs.
The development of new drugs is expensive and takes many years. Developers must be able to rely on properly granted patents from the United States Patent Office. A precedent in which patents are revoked in circumstances such as ours will keep developers from taking such risks for fear that they will not be able to cover their costs or benefit from their risks.
24/7 Wall St.
Why Partial Reopenings Are Set to Boost Disney Stock
Amarin has made a significant investment in Vascepa and has doubled its sales force to 800, Thero said. It is also investigating whether the drug can reduce the cardiovascular risks associated with COVID-19.

The litigation has been a drag on Amarin’s share price. The stock fell to a 52-week low of $3.95 on March 31, the day after the district court ruling. It’s 52-week high had been near $25 anticipating FDA approval of Vascepa, which came in December. In today’s trading, Amarin was up about 3% at midday at $7.76.
Antwort auf Beitrag Nr.: 63.613.938 von Magnetfeldfredy am 08.05.20 21:44:26The development of new drugs is expensive and takes many years. Developers must be able to rely on properly granted patents from the United States Patent Office.
Danke Fredy, genau meine Meinung. Wenn es obvious ist, dass ein vom Patent Office erteiltes Patent (in diesem Fall sogar mehrere!) ungültig ist, da obvious, dann ist die Unsinnigkeit eines Patent Offices auch obvious ...
Von daher sehe ich mehr als gute Chancen, dass dem appeal stattgegeben wird.
Danke Fredy, genau meine Meinung. Wenn es obvious ist, dass ein vom Patent Office erteiltes Patent (in diesem Fall sogar mehrere!) ungültig ist, da obvious, dann ist die Unsinnigkeit eines Patent Offices auch obvious ...

Von daher sehe ich mehr als gute Chancen, dass dem appeal stattgegeben wird.
Der Appeal von Amarin ist da, es wurden vorsätzliche Fehler gemacht, kurze Zusammenfassung:
Robust ..... Holistic ..... Hindsight!!!!!
We have a new favorite Amarin word, hindsight. No other word so perfectly addresses the anguish, disgust, contempt, and disbelief experienced by Amarin longs over the past six weeks since the Du-debacle. The lower court had a duty as a matter of law to not use hindsight, yet that is precisely what she inexcusably did in ruling so monstrously.
My favorite part of Singer's masterpiece is at pages 46-47, where he lays out the concepts that must be rigorously applied to avoid hindsight based obviousness analysis, and then slams the learned jurist in a sneering, contemptuous, but poetically beautiful way, "The district court's analysis of both these concepts was anything but rigorous." Boom!
Right below that, Singer goes for the jugular with the argument that I thought was the low-hanging fruit, namely that there is no way that any reasonable, objective person, much less an experienced judge, could rationally conclude that the generics met their elevated burden of proving obviousness by clear and convincing evidence. (clear and convincing evidence in bold)
"The problem for Defendants, and the district court, is that there is nowhere near enough evidence in the record to meet that high burden, and the district court legally erred in concluding otherwise. Indeed, the only way the district court reached its conclusion was through legal and factual error that ignored the critical teachings in the art ....."
Nailed it. Could not have said it better myself. This is the most direct reason why this appeal should be won.
At first glance, my only issue with the Brief, is cosmetic. For some unbeknownst reason, the top patent lawyer in the country, working for a big sophisticated law firm, inexplicably formatted the Brief with a left justification instead of a full justification.
To someone like myself who writes Briefs for a living, this is a sloppy eyesore, but this cosmetic issue should not detract from the most important part, namely that the substance has been brilliantly and persuasively articulated.
Robust ..... Holistic ..... Hindsight!!!!!
We have a new favorite Amarin word, hindsight. No other word so perfectly addresses the anguish, disgust, contempt, and disbelief experienced by Amarin longs over the past six weeks since the Du-debacle. The lower court had a duty as a matter of law to not use hindsight, yet that is precisely what she inexcusably did in ruling so monstrously.
My favorite part of Singer's masterpiece is at pages 46-47, where he lays out the concepts that must be rigorously applied to avoid hindsight based obviousness analysis, and then slams the learned jurist in a sneering, contemptuous, but poetically beautiful way, "The district court's analysis of both these concepts was anything but rigorous." Boom!
Right below that, Singer goes for the jugular with the argument that I thought was the low-hanging fruit, namely that there is no way that any reasonable, objective person, much less an experienced judge, could rationally conclude that the generics met their elevated burden of proving obviousness by clear and convincing evidence. (clear and convincing evidence in bold)
"The problem for Defendants, and the district court, is that there is nowhere near enough evidence in the record to meet that high burden, and the district court legally erred in concluding otherwise. Indeed, the only way the district court reached its conclusion was through legal and factual error that ignored the critical teachings in the art ....."
Nailed it. Could not have said it better myself. This is the most direct reason why this appeal should be won.
At first glance, my only issue with the Brief, is cosmetic. For some unbeknownst reason, the top patent lawyer in the country, working for a big sophisticated law firm, inexplicably formatted the Brief with a left justification instead of a full justification.
To someone like myself who writes Briefs for a living, this is a sloppy eyesore, but this cosmetic issue should not detract from the most important part, namely that the substance has been brilliantly and persuasively articulated.
Antwort auf Beitrag Nr.: 63.656.350 von Magnetfeldfredy am 13.05.20 08:30:28"Methodische" Fehler - einverstanden, aber wie kommst Du auf "Vorsatz"?
Weißt Du, wo kann man Singers "masterpiece" einsehen kann?
Weißt Du, wo kann man Singers "masterpiece" einsehen kann?
Beitrag zu dieser Diskussion schreiben
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| -2,18 | |
| +0,01 | |
| +2,09 | |
| -0,32 | |
| -0,15 | |
| -2,29 | |
| +0,67 | |
| -2,45 | |
| -2,97 | |
| -17,06 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 239 | ||
| 83 | ||
| 82 | ||
| 77 | ||
| 68 | ||
| 55 | ||
| 49 | ||
| 47 | ||
| 34 | ||
| 34 |
Amarin - The Science Of Lipid Therapy



























