Catalyst Pharmaceuticals (CPRX) - Aktuell 1,39$ - Kursziel 4$ - 500 Beiträge pro Seite
eröffnet am 14.10.16 08:37:43 von
neuester Beitrag 19.10.16 20:54:00 von
neuester Beitrag 19.10.16 20:54:00 von
Beiträge: 15
ID: 1.239.831
ID: 1.239.831
Aufrufe heute: 0
Gesamt: 1.202
Gesamt: 1.202
Aktive User: 0
ISIN: US14888U1016 · WKN: A0LCUL · Symbol: CN2
14,135
EUR
+2,65 %
+0,365 EUR
Letzter Kurs 24.04.24 Tradegate
Neuigkeiten
22.04.24 · globenewswire |
28.03.24 · globenewswire |
27.03.24 · globenewswire |
13.03.24 · globenewswire |
05.03.24 · globenewswire |
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,3300 | +52,87 | |
| 6,0000 | +25,00 | |
| 9,2900 | +20,96 | |
| 111,75 | +18,87 | |
| 0,6400 | +18,52 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,8410 | -17,06 | |
| 9,7200 | -19,60 | |
| 2,7280 | -29,14 | |
| 14,510 | -32,32 | |
| 8,0000 | -36,76 |
5 Oktober: Piper Jaffray upgraded the stock to "overweight" from "neutral."
Das Kursziel wurde von 1$ auf 4$ angehoben.
Massive Käufe an diesem Tag. Dann hat sich der Kurs beruhigt und zieht nun wieder an.
Aktuell stehen wir bei 1,39$.
Die Begründung
Piper expects lower clinical risk for the company's investigational drug candidate Firdapse due to the finalized design of its second Phase III trial set to start this quarter, the Fly reports.
Firdapse is being investigated for the treatment of Lambert Eaton myasthenic syndrome (LEMS), which is an autoimmune disease.
Additionally, the firm believes regulatory risk has been eased due to close interaction with the FDA on the study's design.
Piper said the program will "soon be back on track and poised for 2017 value creation," according to a note cited by the Fly.
Der Chart
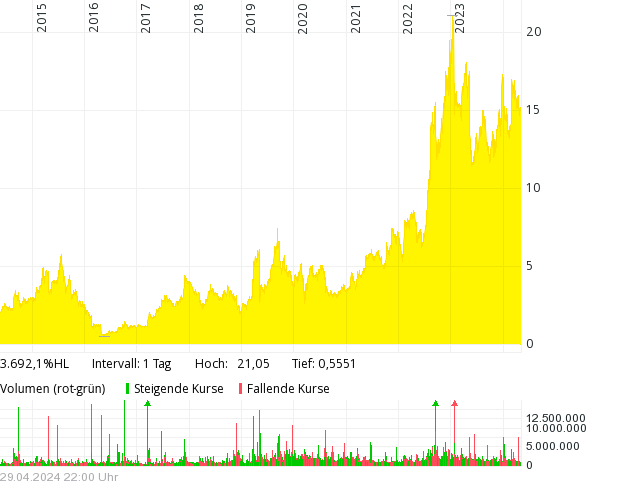
Das Kursziel wurde von 1$ auf 4$ angehoben.
Massive Käufe an diesem Tag. Dann hat sich der Kurs beruhigt und zieht nun wieder an.
Aktuell stehen wir bei 1,39$.
Die Begründung
Piper expects lower clinical risk for the company's investigational drug candidate Firdapse due to the finalized design of its second Phase III trial set to start this quarter, the Fly reports.
Firdapse is being investigated for the treatment of Lambert Eaton myasthenic syndrome (LEMS), which is an autoimmune disease.
Additionally, the firm believes regulatory risk has been eased due to close interaction with the FDA on the study's design.
Piper said the program will "soon be back on track and poised for 2017 value creation," according to a note cited by the Fly.
Der Chart
!
Dieser Beitrag wurde von CloudMOD moderiert. Grund: ohne nachvollziehbare Quellenangabe, ggf überarbeitet neu einstellenIndikation
Mal schauen wie es weitergeht...Catalyst Pharmaceuticals to Present at 15th Annual BIO Investor Forum
GlobeNewswire•October 13, 2016CORAL GABLES, Fla., Oct. 13, 2016 (GLOBE NEWSWIRE) -- Catalyst Pharmaceuticals, Inc. (CPRX), a biopharmaceutical company focused on developing and commercializing innovative therapies for people with rare debilitating diseases, today announced that Patrick J. McEnany, Chief Executive Officer, and Steven Miller, Ph.D., Chief Operating Officer and Chief Scientific Officer, will present at the 15th Annual BIO Investor Forum being held at the Westin St. Francis in San Francisco, October 18-19.
The presentation is scheduled for Tuesday, October 18th at 9:00 am (PDT). The presentation materials will be posted at www.catalystpharma.com in the Investors section under Events & Presentations.
About Catalyst Pharmaceuticals
Catalyst Pharmaceuticals is a biopharmaceutical company focused on developing and commercializing innovative therapies for people with rare debilitating diseases, including Lambert-Eaton myasthenic syndrome (LEMS), congenital myasthenic syndromes (CMS), infantile spasms, and Tourette's Disorder. Firdapse® for the treatment of LEMS has received Breakthrough Therapy Designation from the U.S. Food and Drug Administration (FDA) and orphan drug designation for LEMS, CMS and Mysthenia Gravis. Firdapse is the first and only approved drug in Europe for symptomatic treatment in adults with LEMS.
Catalyst is also developing CPP-115 to treat infantile spasms, epilepsy and other neurological conditions associated with reduced GABAergic signaling, like post-traumatic stress disorder and Tourette's Disorder. CPP-115 has been granted U.S. orphan drug designation for the treatment of infantile spasms by the FDA and has been granted E.U. orphan medicinal product designation for the treatment of West Syndrome by the European Commission. In addition, Catalyst is developing a generic version of Sabril® (vigabatrin).
Forward-Looking Statements
This press release contains forward-looking statements. Forward-looking statements involve known and unknown risks and uncertainties, which may cause Catalyst's actual results in future periods to differ materially from forecasted results. A number of factors, including whether the receipt of breakthrough therapy designation for Firdapse will expedite the development and review of Firdapse by the FDA or the likelihood that the product will be found to be safe and effective, what study design for a second trial evaluation of Firdapse for the treatment of LEMS will be acceptable to the FDA, the timing of such trial, and whether it will be successful, whether Catalyst’s assumptions in its updated business plan will be accurate and the impact of unanticipated events or delays in projected activities on Catalyst’s cash requirements and on Catalyst’s ability to get to an accepted NDA submission for Firdapse without the need for additional funding, what clinical trials and studies will be required before Catalyst can resubmit an NDA for Firdapse for the treatment of CMS and whether any such required clinical trials and studies will be successful, whether the investigator-sponsored study evaluating Firdapse for the treatment of MuSK-MG will be successful, whether any NDA for Firdapse resubmitted to the FDA will ever be accepted for filing, the timing of any such NDA filing or acceptance, whether, if an NDA for Firdapse is accepted for filing, such NDA will be given a priority review by the FDA, whether Firdapse will ever be approved for commercialization, whether Catalyst will be the first company to receive approval for amifampridine (3,4-DAP), giving it 7-year marketing exclusivity for its product, whether CPP-115 will be determined to be safe for humans, what additional testing will be required before CPP-115 is “Phase 2 ready”, whether CPP-115 will be determined to be effective for the treatment of infantile spasms, post-traumatic stress disorder, Tourette's Disorder or any other indications, whether Catalyst can successfully design and complete a bioequivalence study of its version of vigabatrin compared to Sabril that is acceptable to the FDA, whether any such bioequivalence study the design of which is acceptable to the FDA will be successful, whether any ANDA that Catalyst submits for a generic version of Sabril will be accepted for filing, whether any ANDA for Sabril accepted for filing by the FDA will be approved (and the timing of any such approval), whether any of Catalyst's product candidates will ever be approved for commercialization or successfully commercialized, and those other factors described in Catalyst's Annual Report on Form 10-K for the fiscal year 2015 and its other filings with the U.S. Securities and Exchange Commission (SEC), could adversely affect Catalyst. Copies of Catalyst's filings with the SEC are available from the SEC, may be found on Catalyst's website, or may be obtained upon request from Catalyst. Catalyst does not undertake any obligation to update the information contained herein, which speaks only as of this date.
Also mein Kursziel ist 40 USD
Ich hätte nichts dagegen... 

Der nächste Analyst mit Kursziel 2,50
Roth Capital Weighs in on Catalyst Pharmaceuticals Inc (CPRX) Following Meeting with ManagementSHARE ON:
Jason Cohen, Editor — October 13, 2016, 4:28 PM EDT
Roth Capital analyst Scott Henry is out today with a research note on shares of Catalyst Pharmaceuticals Inc (NASDAQ:CPRX), following recent meeting with the company’s senior management. Henry rates CPRX a Buy, with a price target of $2.50, which represents a potential upside of 81% from where the stock is currently trading.
The analyst points out that expectations remain for the second phase 3 Firdapse trial to commence in 4Q16. Firdapse is being developed as a treatment for LambertEaton Myasthenic Syndrome (LEMS), and data could be available by mid-year 2017, ahead of Henry’s 4Q17 target.
With respect to the trial design the analyst notes, “The trial will bring patients to two locations (Miami, Los Angeles – recruiting in the winter could be a benefit). Patients will be put up for 7-10 days at CPRX’s expense. The time commitment for patients may be ~4 hours on two separate days. The trial likely costs ~$15K/ patient.”
“No disclosure yet on potential Firdapse pricing, but we would assume that our $100K target is at (or below) the low end of the range. Nothing new on Jacobus as that company has been mostly silent per industry contacts,” the analyst added.
http://www.smarteranalyst.com/2016/10/13/roth-capital-weighs…
Alle 3 Analysten stufen die Aktie mit Kaufen ein. Das durchschnittliche Kursziel ist $3.25.


Antwort auf Beitrag Nr.: 53.477.871 von kosto1929 am 14.10.16 12:16:38Ich habe nochmal bei Yahoo nachgeschaut:
Aktuell gibt es sogar 4 Analysten für das Jahr 2017.
Dort reicht das Kursziel bis $6.00. Das Minimum liegt bei $2.50.
Das durchschnittliche Kursziel liegt bei $4.38
Auf geht´s!
http://finance.yahoo.com/quote/CPRX/analysts?p=CPRX
Aktuell gibt es sogar 4 Analysten für das Jahr 2017.
Dort reicht das Kursziel bis $6.00. Das Minimum liegt bei $2.50.
Das durchschnittliche Kursziel liegt bei $4.38
Auf geht´s!

http://finance.yahoo.com/quote/CPRX/analysts?p=CPRX
Die Pipeline

Premarket geht sie schon mal ab!
Antwort auf Beitrag Nr.: 53.479.191 von lunatics am 14.10.16 14:40:34Naja, $1.45 ist noch nicht die Welt. Bid steht auch "nur" bei $1.39
Mann, da hat sich doch glatt jemand 700 Stück zu $1.50 vorbörslich geschnappt.
Weiß der mehr?!
Weiß der mehr?!

Heute wieder schwach. Ich steige erstmal aus (Verlust 12%) und betrachte den Wert von der Seitenlinie.
Schade. Ich warte und sehe mal was da kommen mag.
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +0,19 | |
| -1,59 | |
| -0,15 | |
| -0,14 | |
| -0,70 | |
| 0,00 | |
| -0,96 | |
| +0,93 | |
| -3,67 | |
| -1,18 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 223 | ||
| 99 | ||
| 91 | ||
| 80 | ||
| 53 | ||
| 47 | ||
| 45 | ||
| 42 | ||
| 41 | ||
| 32 |
Catalyst Pharmaceuticals (CPRX) - Aktuell 1,39$ - Kursziel 4$





















