IsoRay Inc - Entwickler von isotopbasierten Medizinprodukten - 500 Beiträge pro Seite
eröffnet am 28.10.20 23:37:41 von
neuester Beitrag 08.04.24 19:55:04 von
neuester Beitrag 08.04.24 19:55:04 von
Beiträge: 117
ID: 1.333.129
ID: 1.333.129
Aufrufe heute: 2
Gesamt: 8.345
Gesamt: 8.345
Aktive User: 0
ISIN: US46489V1044 · WKN: A0MQNM · Symbol: CATX
1,6800
USD
+9,80 %
+0,1500 USD
Letzter Kurs 18:33:33 AMEX
Meistbewertete Beiträge
Werte aus der Branche Gesundheitswesen
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 2,4800 | +93,75 | |
| 0,7670 | +27,83 | |
| 1,3700 | +19,13 | |
| 0,5501 | +18,33 | |
| 12,130 | +16,75 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,7000 | -11,46 | |
| 0,5925 | -15,36 | |
| 1,5500 | -19,27 | |
| 399,40 | -22,28 | |
| 0,7500 | -48,98 |
Kurzporträt:
IsoRay, Inc. ist ein Medizintechnikunternehmen. Das Unternehmen entwickelt, produziert und verkauft über seine Tochtergesellschaft IsoRay Medical, Inc. isotopbasierte Medizinprodukte und -geräte zur Behandlung von Krebs und anderen bösartigen Erkrankungen. Das Unternehmen beschäftigt sich mit der Produktion und dem Vertrieb von Cesium-131 (Cs-131) Brachytherapie-Samen. Brachytherapiesamen sind kleine Geräte, die eine therapeutische Strahlendosis enthalten, die bei einem interstitiellen Bestrahlungsverfahren verwendet wird. Das Brachytherapieverfahren platziert radioaktive Samen so nah wie möglich am (in oder in der Nähe) des krebsartigen Tumors. Das Cs-131 beinhaltet Radioisotop in der Behandlung aller bösartigen Tumore, wie Prostatakrebs, Hirnkrebs, Brustkrebs, Darmkrebs, gynäkologischen Krebs, Lungenkrebs, Leberkrebs, Augenmelanom und Bauchspeicheldrüsenkrebs. Der Samen von Proxcelan Cesium-131 des Unternehmens ist als Gerät der Klasse II eingestuft.
Mitarbeiteranzahl : 53 Personen.
Quelle: https://de.marketscreener.com/kurs/aktie/ISORAY-INC-15515/un…
Produkte:
https://isoray.com/clinicians/products/
Chart:
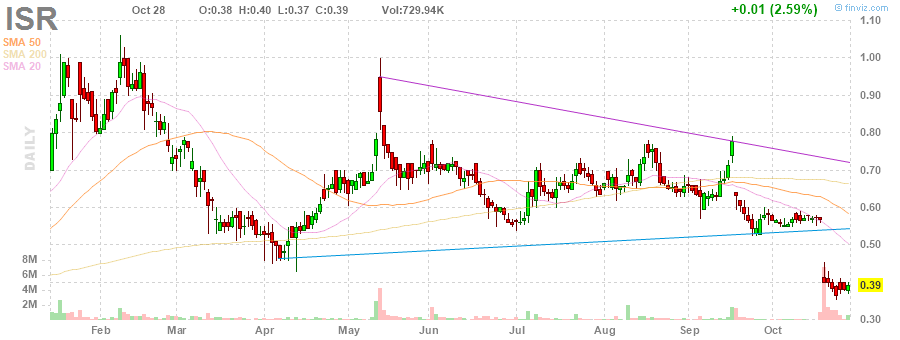
Zwei Kurslücken nach oben sind vorhanden.
Logo:

IsoRay, Inc. ist ein Medizintechnikunternehmen. Das Unternehmen entwickelt, produziert und verkauft über seine Tochtergesellschaft IsoRay Medical, Inc. isotopbasierte Medizinprodukte und -geräte zur Behandlung von Krebs und anderen bösartigen Erkrankungen. Das Unternehmen beschäftigt sich mit der Produktion und dem Vertrieb von Cesium-131 (Cs-131) Brachytherapie-Samen. Brachytherapiesamen sind kleine Geräte, die eine therapeutische Strahlendosis enthalten, die bei einem interstitiellen Bestrahlungsverfahren verwendet wird. Das Brachytherapieverfahren platziert radioaktive Samen so nah wie möglich am (in oder in der Nähe) des krebsartigen Tumors. Das Cs-131 beinhaltet Radioisotop in der Behandlung aller bösartigen Tumore, wie Prostatakrebs, Hirnkrebs, Brustkrebs, Darmkrebs, gynäkologischen Krebs, Lungenkrebs, Leberkrebs, Augenmelanom und Bauchspeicheldrüsenkrebs. Der Samen von Proxcelan Cesium-131 des Unternehmens ist als Gerät der Klasse II eingestuft.
Mitarbeiteranzahl : 53 Personen.
Quelle: https://de.marketscreener.com/kurs/aktie/ISORAY-INC-15515/un…
Produkte:
https://isoray.com/clinicians/products/
Chart:

Zwei Kurslücken nach oben sind vorhanden.
Logo:
Global Radiotherapy Market is Booming Worldwide with Varian Medical Systems, Inc., Elekta AB, Ion Beam Applications S.A., C.R. Bard, Inc., Isoray Medical, Inc., CIVCO Radiotherapy
By Product Type:
External Beam Radiation
Compact Advanced Radiotherapy Systems
Cyber Knife
Gamma Knife
Others
Proton Therapy Systems
Synchrotron
Cyclotron
High Energy Linear Accelerators (LINAC)
Internal Beam Radiation
Applicators
Afterloader
Seeds
Capnographs
Gas Analyzers
Systemic Radiation
By Therapy Type:
External Beam Radiation Therapy
Stereotactic Radiosurgery (SRS)
Stereotactic Body Radiation Therapy (SBRT)
Intensity-modulated Radiation Therapy (IMRT)
Proton Therapy
Image-guided Radiotherapy (IGRT)
Volumetric Modulated Arc Therapy (VMAT)
Others
Internal Beam Radiation Therapy
Low-dose Rate Brachytherapy (LDR)
High-dose Rate Brachytherapy (HDR)
Image-guided Brachytherapy (IGBT)
Pulse Dose Rate Brachytherapy (PDR)
Systemic Radiation Therapy
Oral Radiotherapy
Intravenous Radiotherapy
By Application:
Prostate Cancer
Breast Cancer
Lung Cancer
Colorectal Cancer
Cervical Cancer
Others
By End User:
Hospitals
Oncology Clinics
Ambulatory Radiotherapy Centers
Others
Quelle: https://dailyresearchcorrespondent.com/2020/12/02/global-rad…
By Product Type:
External Beam Radiation
Compact Advanced Radiotherapy Systems
Cyber Knife
Gamma Knife
Others
Proton Therapy Systems
Synchrotron
Cyclotron
High Energy Linear Accelerators (LINAC)
Internal Beam Radiation
Applicators
Afterloader
Seeds
Capnographs
Gas Analyzers
Systemic Radiation
By Therapy Type:
External Beam Radiation Therapy
Stereotactic Radiosurgery (SRS)
Stereotactic Body Radiation Therapy (SBRT)
Intensity-modulated Radiation Therapy (IMRT)
Proton Therapy
Image-guided Radiotherapy (IGRT)
Volumetric Modulated Arc Therapy (VMAT)
Others
Internal Beam Radiation Therapy
Low-dose Rate Brachytherapy (LDR)
High-dose Rate Brachytherapy (HDR)
Image-guided Brachytherapy (IGBT)
Pulse Dose Rate Brachytherapy (PDR)
Systemic Radiation Therapy
Oral Radiotherapy
Intravenous Radiotherapy
By Application:
Prostate Cancer
Breast Cancer
Lung Cancer
Colorectal Cancer
Cervical Cancer
Others
By End User:
Hospitals
Oncology Clinics
Ambulatory Radiotherapy Centers
Others
Quelle: https://dailyresearchcorrespondent.com/2020/12/02/global-rad…
Antwort auf Beitrag Nr.: 65.530.066 von Malecon am 28.10.20 23:37:41
Check nach einem Monat:
Noch nicht mal die erste Lücke geschlossen, obwohl zweimal versucht:
Die Lücke ist hartnäckig.
🎵
Zitat von Malecon: Zwei Kurslücken nach oben sind vorhanden.
Check nach einem Monat:
Noch nicht mal die erste Lücke geschlossen, obwohl zweimal versucht:

Die Lücke ist hartnäckig.
🎵
Die erste Lücke könnte bald geschlossen werden, wenn es so weitergeht.
Ob es dann gleich weiter nach oben zur zweiten Lücke (aus dem Monat September) geht, das weiß ich nicht.
Ob es dann gleich weiter nach oben zur zweiten Lücke (aus dem Monat September) geht, das weiß ich nicht.
Die Lückenschließung fast vollendet.
Antwort auf Beitrag Nr.: 66.091.468 von Malecon am 16.12.20 17:55:16Es sieht bullisch aus, gucke aber noch zu 

Antwort auf Beitrag Nr.: 65.530.066 von Malecon am 28.10.20 23:37:41
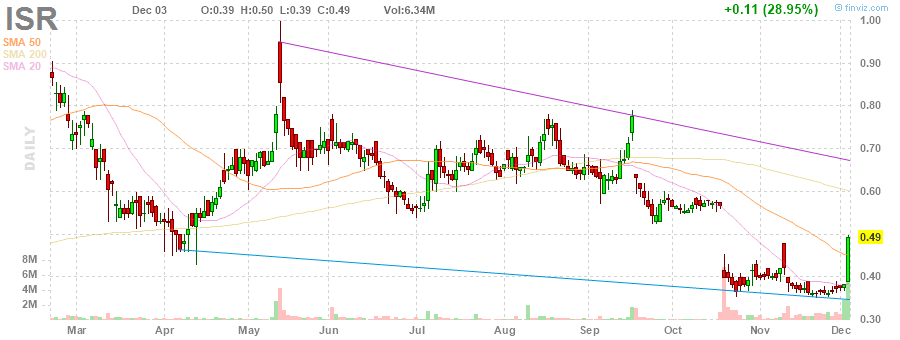
Nach 2 Monaten läuft die Lückenschließung volle Pulle:
🎵
Zitat von Malecon: Zwei Kurslücken nach oben sind vorhanden.
Nach 2 Monaten läuft die Lückenschließung volle Pulle:

🎵
Antwort auf Beitrag Nr.: 65.530.066 von Malecon am 28.10.20 23:37:41
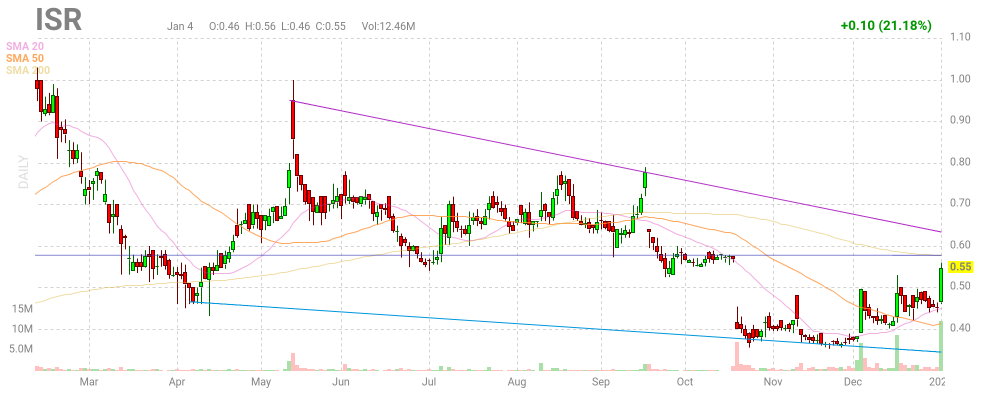
So, Leute, nach 2 Monate ist die erste Lückenschließung im Grunde genommen gelungen:
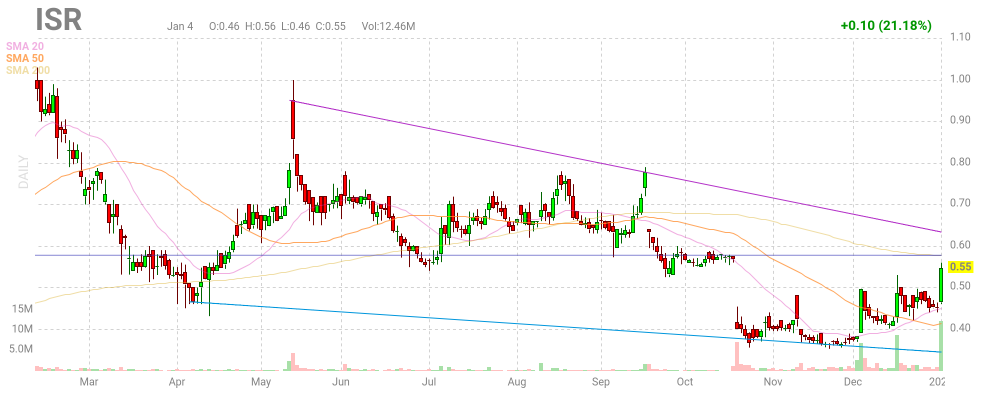
Ob auch die obere Lücke geschloßen wird, kann ich im Moment nicht beurteilen.
🎵
Zitat von Malecon: Kurzporträt:
IsoRay, Inc.
Zitat von Malecon: Zwei Kurslücken nach oben sind vorhanden.
So, Leute, nach 2 Monate ist die erste Lückenschließung im Grunde genommen gelungen:

Ob auch die obere Lücke geschloßen wird, kann ich im Moment nicht beurteilen.
🎵
Antwort auf Beitrag Nr.: 66.301.763 von Malecon am 05.01.21 23:30:21Guten Morgen @ all
1 min chart afters hours....
1,56 $
+194 %
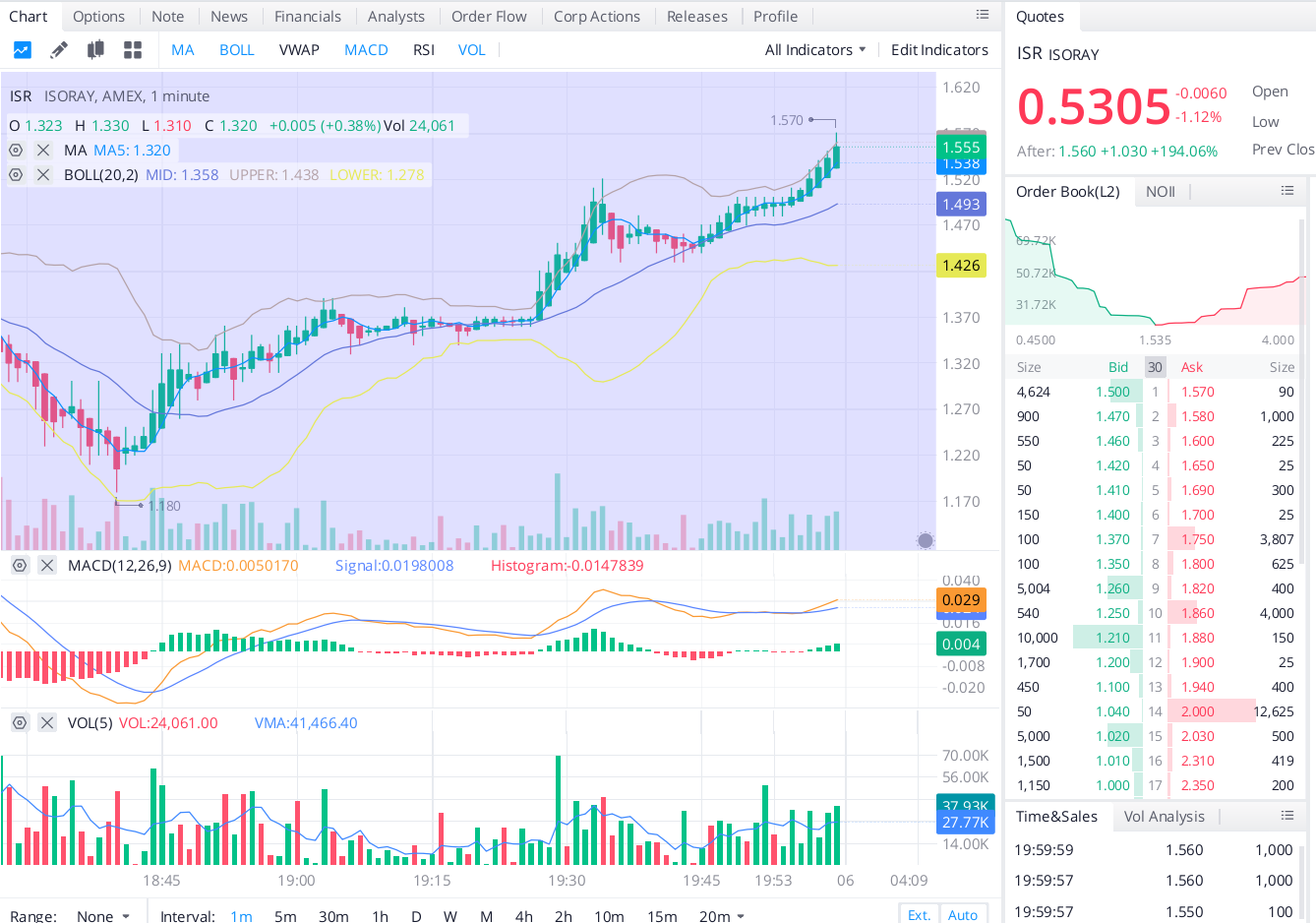
1 min chart afters hours....

1,56 $
+194 %

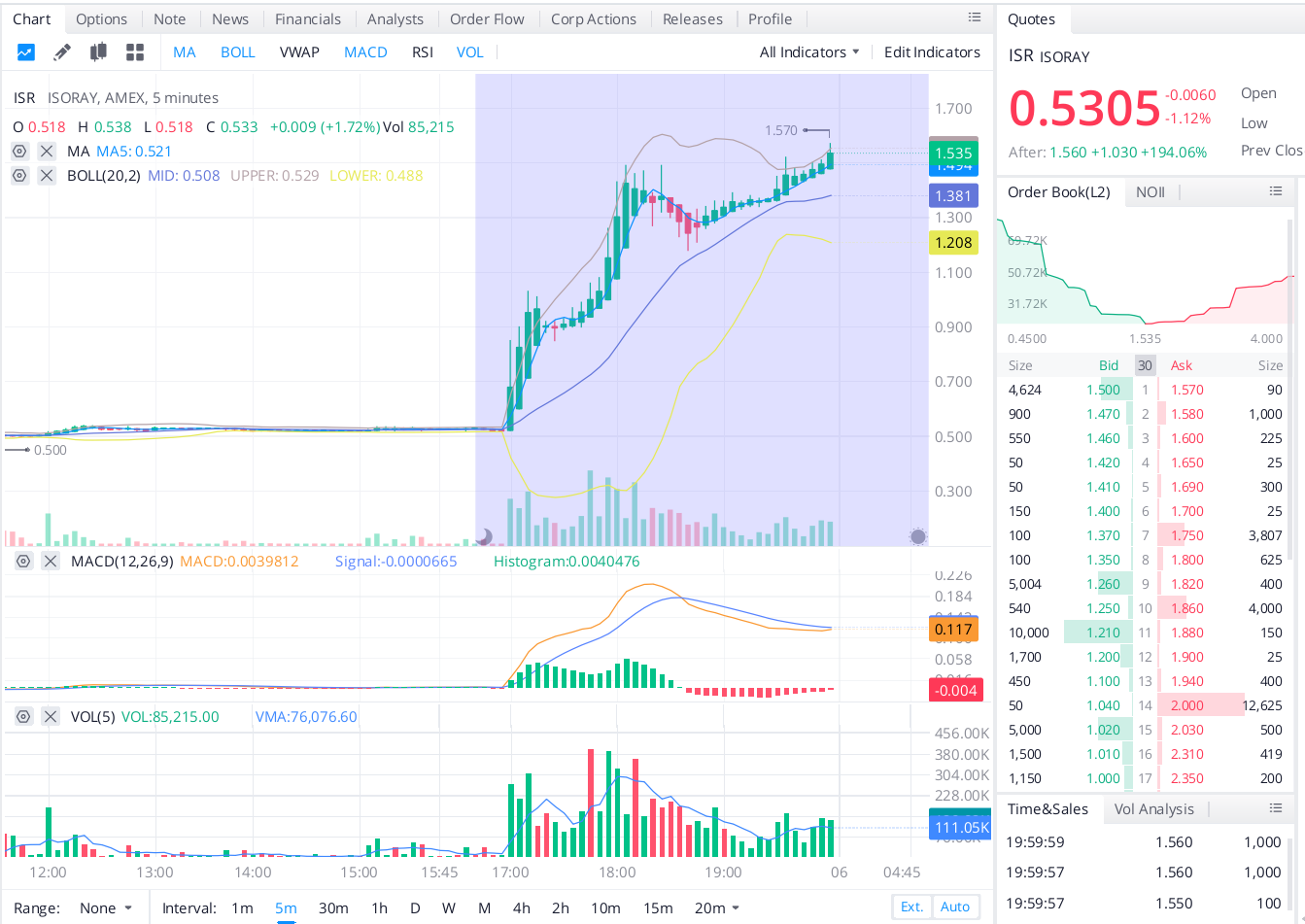
5 min chart bessere blick 


Auf Tradegate in €:
🎵

🎵
das schmerzt, wollte gestern nach malecon´s post einsteigen...
naja...hätte hätte
naja...hätte hätte

Ich habe die Aktie vor 2 Monaten vorgestellt und den Thread eröffnet.
Zeit einzusteigen war genug.
Zeit einzusteigen war genug.
Wieviel Luft nach oben könnte denn hier noch sein?
hoffe auf mindestens 5 bagger
wenn die USA mitmachen, könnte das gehen imo
wenn die USA mitmachen, könnte das gehen imo
Antwort auf Beitrag Nr.: 66.311.627 von price-of-success am 06.01.21 16:17:32https://isoray.com/about/news/
Es ging bis $ 2.
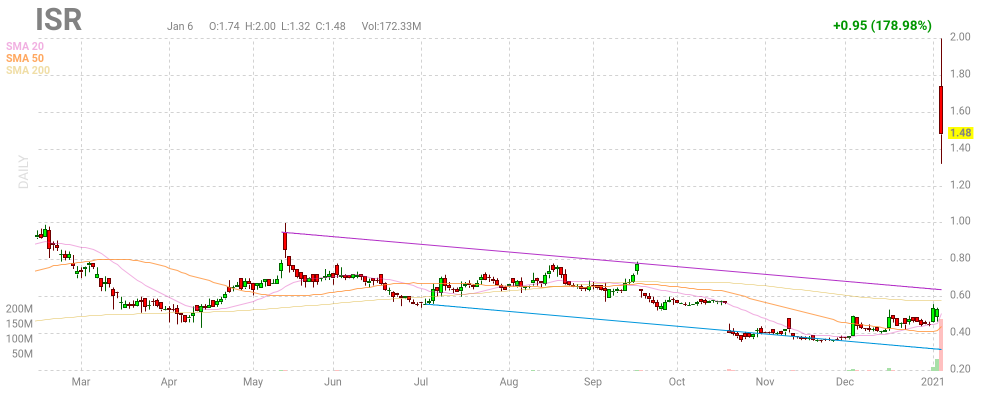
Das war eine Kursver-5-fachung seit der Threaderöffnung vor zwei Monaten.
🎵

Das war eine Kursver-5-fachung seit der Threaderöffnung vor zwei Monaten.
🎵
Antwort auf Beitrag Nr.: 66.314.465 von Malecon am 06.01.21 18:46:26Schön für dich!!!
Angenehmes Wachstum hier,
gefällt mir sehr gut.
Dieses Anwendungsprinzip ist phänomenal und kann viel Leid ersparen.
Ich bin sehr überzeugt von dieser Therapieform und der Kurs scheint nach dem sprunghaften Anstieg schon konsolidiert.
gefällt mir sehr gut.
Dieses Anwendungsprinzip ist phänomenal und kann viel Leid ersparen.
Ich bin sehr überzeugt von dieser Therapieform und der Kurs scheint nach dem sprunghaften Anstieg schon konsolidiert.
Hey Malecon, hat mich dann doch gejuckt und bin mit ner Miniposi am 21.12. eingestiegen, aktuell 454%. DANKE
Antwort auf Beitrag Nr.: 66.509.006 von deropa am 19.01.21 17:42:22
Bitte.
Zitat von deropa: aktuell 454%. DANKE
Bitte.
mann geht das ab
Antwort auf Beitrag Nr.: 66.525.620 von price-of-success am 20.01.21 16:22:20..wärts !?? 😀
Ich weiß garnicht, wieso sich hier grade so ne Verkaufs-Hysterie breitmacht ?
Wegen dem ebenso außerordentlichen Anstieg von heute vormittag !?..
Ich weiß garnicht, wieso sich hier grade so ne Verkaufs-Hysterie breitmacht ?
Wegen dem ebenso außerordentlichen Anstieg von heute vormittag !?..
jetzt Mittagspause an der Wallstreet
schafft die Aktie weitere 500%
heute nicht wg KE
Antwort auf Beitrag Nr.: 66.818.971 von price-of-success am 04.02.21 14:22:28Halper Sadeh LLC, an investor rights law firm, is investigating whether the merger of Isoray, Inc. (NYSE: ISR) and Viewpoint Molecular Targeting, Inc. is fair to Isoray shareholders.
Halper Sadeh encourages Isoray shareholders to click here to learn more about their legal rights and options or contact Daniel Sadeh or Zachary Halper at (212) 763-0060 or sadeh@halpersadeh.com or zhalper@halpersadeh.com.
The investigation concerns whether Isoray and its board violated the federal securities laws and/or breached their fiduciary duties to shareholders by failing to, among other things: (1) obtain the best possible consideration for Isoray shareholders; and (2) disclose all material information necessary for Isoray shareholders to adequately assess and value the merger consideration. On behalf of Isoray shareholders, Halper Sadeh LLC may seek increased consideration for shareholders, additional disclosures and information concerning the proposed transaction, or other relief and benefits.
Halper Sadeh encourages Isoray shareholders to click here to learn more about their legal rights and options or contact Daniel Sadeh or Zachary Halper at (212) 763-0060 or sadeh@halpersadeh.com or zhalper@halpersadeh.com.
The investigation concerns whether Isoray and its board violated the federal securities laws and/or breached their fiduciary duties to shareholders by failing to, among other things: (1) obtain the best possible consideration for Isoray shareholders; and (2) disclose all material information necessary for Isoray shareholders to adequately assess and value the merger consideration. On behalf of Isoray shareholders, Halper Sadeh LLC may seek increased consideration for shareholders, additional disclosures and information concerning the proposed transaction, or other relief and benefits.
Sieht ja aktuell bei absolut niedrigem Handelsvolumen recht vielversprechend aus. The only way is up oder wie ?
Was siehst du für ein Potential?
Antwort auf Beitrag Nr.: 73.208.746 von Crowww am 01.02.23 20:26:26Es ist ein US Biotech Crowww das kennst du ja das Spiel. Narketcap peanuts aber richtig Cash $ in der Kasse. Ich halte das Produkt für recht vielversprechend, da muss man halt etwas Geduld mitbringen.
Da ist ja heute nochmal ein bissl Handel an den diversen Handelsplätzen in den Staaten
Ach was wäre das mal fein die 50 cent Marke zu durchbrechen. Ein bissl Handel ist ja auch heute wieder vorhanden.
Isoray
newsroom
23.2.23
Initial Results From First Patient Dosed With Perspective Therapeutics’ Lead Drug VMT-α-NET Presented at The Treatment of Neuroendocrine Disorders at the 2023 PET/RTRC Annual Workshop Scientific Session
Feb 23, 2023
https://isoray.com/2023/02/initial-results-from-first-patien…
newsroom
23.2.23
Initial Results From First Patient Dosed With Perspective Therapeutics’ Lead Drug VMT-α-NET Presented at The Treatment of Neuroendocrine Disorders at the 2023 PET/RTRC Annual Workshop Scientific Session
Feb 23, 2023
https://isoray.com/2023/02/initial-results-from-first-patien…
Antwort auf Beitrag Nr.: 73.425.648 von Edelweiss70 am 07.03.23 20:22:13Heute wäre ein schöner Tag um die 0,50$ Marke zu knacken
Komm schon........
OOHOOOO ,da meint es aber jemand ernst mit 188.000 Stücken im Geld.
Zufall ?????
Zufall ?????
Perspective Therapeutics, Inc. CATX on NYSE
SEC Fillings
13.3.13
Latest Filings (excluding insider transactions
https://www.sec.gov/ix?doc=/Archives/edgar/data/0000728387/0…" target="_blank" rel="nofollow ugc noopener">https://www.sec.gov/ix?doc=/Archives/edgar/data/0000728387/0…
SEC Fillings
13.3.13
Latest Filings (excluding insider transactions
https://www.sec.gov/ix?doc=/Archives/edgar/data/0000728387/0…" target="_blank" rel="nofollow ugc noopener">https://www.sec.gov/ix?doc=/Archives/edgar/data/0000728387/0…
Perspective Therapeutics
IR
8K Filling
15.3.23
https://www.sec.gov/ix?doc=/Archives/edgar/data/0000728387/0…
IR
8K Filling
15.3.23
https://www.sec.gov/ix?doc=/Archives/edgar/data/0000728387/0…
Corporate Presentation
March23
Perspective Therapeutics IR
15.3.23
https://www.sec.gov/Archives/edgar/data/728387/0001437749230…
March23
Perspective Therapeutics IR
15.3.23
https://www.sec.gov/Archives/edgar/data/728387/0001437749230…
Antwort auf Beitrag Nr.: 73.484.665 von Edelweiss70 am 15.03.23 22:17:26Sehr gespannt auf den heutigen US Handel.50 Cents Marke übersprungen und Kaufsignal generiert, das kann lustig werden.
Perspective Therapeutics
IR
17.3.23
Corporate Presentation March 23
https://www.sec.gov/Archives/edgar/data/728387/0001437749230…
IR
17.3.23
Corporate Presentation March 23
https://www.sec.gov/Archives/edgar/data/728387/0001437749230…
Das sieht doch recht ansprechend aus und die Alpha Particle therapy halte ich auch für sehr vielversprechend. Hoffe das man da zukünftig mal etwas an Medungen mit ersten Abschlüssen liest $. Ich würde mir wünschen das es die Onkologie so weit unterstüzt,das man diese Krankheit irgendwann mal eindämmen kann.
Mit so wenigen Stücken gehandelt durch die 60 cents Marke ist schon beachtlich. Es bleibt spannend....
Auf das was da noch kommt ............
Auf das was da noch kommt ............
Glückwunsch!!!
Danked Crowww. Mal sehen was hier noch alles möglich wird
Ich habe auf deine Empfehlung hin gekauft, ...🍺
Aber leider viel zu wenige!!!🍾
Aber leider viel zu wenige!!!🍾
Glücklich ist der,der zufrieden ist Crowww 

Antwort auf Beitrag Nr.: 73.501.783 von Edelweiss70 am 17.03.23 21:06:36War doch eine ganz erfolgreiche Woche und macht Lust auf mehr. Bin schon sehr gespannt auf nächste Woche. Irgendetwas köchelt doch da gerade im Hintergrund
Schönes Volumen gehandelt heute auf AMEX und auf Tageshoch geschlossen.Herrlich
Im Brief ist wird es sehr dünn,da kann schnell mal ne Fahnenstange winken.
Antwort auf Beitrag Nr.: 73.533.931 von Edelweiss70 am 22.03.23 22:47:22Trotz wieder mal minimalen Umsätzen beim Handel kennt der Kurs nur eine Richtung 💪
Antwort auf Beitrag Nr.: 73.541.809 von Edelweiss70 am 23.03.23 21:37:26Auf eine gute nächste Woche , vielleicht gelingt ja mal der große Ausbruch.
Perspective Therapeutics
Investor Center
IR
3.4.23
RICHLAND, WASHINGTON & CORALVILLE, IOWA – April 3, 2023 Per…
Investor Center
IR
3.4.23
RICHLAND, WASHINGTON & CORALVILLE, IOWA – April 3, 2023 Per…
RICHLAND, WASHINGTON & CORALVILLE, IOWA – April 3, 2023 Perspective Therapeutics, Inc. (“Perspective”) (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy powering expanding treatment options for multiple cancers, expands management team and names Andrew Bright as Executive Vice-President of Brachytherapy. The appointment advances Perspective Therapeutics’ aspirations as a global leader in the manufacturing and distribution of Cesium-131 brachytherapy radioisotope seeds. Brachytherapy seeds have been designed to treat a variety of cancers including prostate, head & neck, lung and brain.
Thijs Spoor, Perspective Therapeutics’ CEO commented on the appointment: “We are delighted to have a senior leader of Andrew Bright’s caliber helm Perspective Therapeutics’ commercial brachytherapy business. The greater brachytherapy team and sales force equally share in my enthusiasm and look forward to mobilizing the commercial arm to new heights under his leadership. As a brachytherapy industry veteran, pioneer and advocate, we have no doubt Andrew will be able to move the needle in order to promote our catalog of Cesium-131 products which currently serve as a highly customized and effective treatment for the rapid recovery of patients in the fight against prostate cancer and other cancers throughout the body.”
Mr. Bright brings to this role over 30 years in leadership roles within the medical device industry, including over 20 years in brachytherapy. Mr. Bright was part of the commercial enterprise responsible for initial widespread adoption and growth of brachytherapy in the 1990’s and was involved in the development and introduction of some of the most successful and clinically relevant products in the industry. Additionally he has extensive US, European, South American and Pacific Rim experience. He also has prior commercial experience, launching therapeutic radiopharmaceuticals in both Europe and North America.
“Perspective Therapeutics’ brachytherapy team is second to none and I’m excited to be part of a group dedicated to bringing the benefits of Cesium-131 therapy to as many patients and physicians as possible,” said Mr. Bright. “I’ve long believed brachytherapy is an underutilized option for treating a variety of cancers; one that delivers precisely targeted therapy from the inside, with minimal side effects and unsurpassed cancer cure rates.”
Investor Relations Contact:
LifeSci Advisors
Chuck Padala
Thijs Spoor, Perspective Therapeutics’ CEO commented on the appointment: “We are delighted to have a senior leader of Andrew Bright’s caliber helm Perspective Therapeutics’ commercial brachytherapy business. The greater brachytherapy team and sales force equally share in my enthusiasm and look forward to mobilizing the commercial arm to new heights under his leadership. As a brachytherapy industry veteran, pioneer and advocate, we have no doubt Andrew will be able to move the needle in order to promote our catalog of Cesium-131 products which currently serve as a highly customized and effective treatment for the rapid recovery of patients in the fight against prostate cancer and other cancers throughout the body.”
Mr. Bright brings to this role over 30 years in leadership roles within the medical device industry, including over 20 years in brachytherapy. Mr. Bright was part of the commercial enterprise responsible for initial widespread adoption and growth of brachytherapy in the 1990’s and was involved in the development and introduction of some of the most successful and clinically relevant products in the industry. Additionally he has extensive US, European, South American and Pacific Rim experience. He also has prior commercial experience, launching therapeutic radiopharmaceuticals in both Europe and North America.
“Perspective Therapeutics’ brachytherapy team is second to none and I’m excited to be part of a group dedicated to bringing the benefits of Cesium-131 therapy to as many patients and physicians as possible,” said Mr. Bright. “I’ve long believed brachytherapy is an underutilized option for treating a variety of cancers; one that delivers precisely targeted therapy from the inside, with minimal side effects and unsurpassed cancer cure rates.”
Investor Relations Contact:
LifeSci Advisors
Chuck Padala
Entschuldigung aber der Text wollte infach nicht kopiert werden. Final hat es ja jetzt funktioniert und es freut mich , das in den Staaten heute ein wenig Handel in unserem Wert stattfindet. Natürlich kann das noch viel besser .......
Hallo, ich war irritiert, als ich bei isoray nach meiner alten sehr verlustbehafteten Aktie gesucht habe. Wurde isoray von Perspective Therapeutics übernommen? Und die Kursentwicklung sieht recht positiv aus. Gibt es hier gute Nachrichten? Kann mir jemand ein kurzes Update geben?
Wer nur auf die HP verweist, braucht mir nicht antworten, danke. Geht auch per PN. Kann im Gegenzug auch gerne ein Update zu einem ähnlichen Unternemen geben, falls jemand eine Gegenleistung braucht. ASXC
Wer nur auf die HP verweist, braucht mir nicht antworten, danke. Geht auch per PN. Kann im Gegenzug auch gerne ein Update zu einem ähnlichen Unternemen geben, falls jemand eine Gegenleistung braucht. ASXC
Antwort auf Beitrag Nr.: 73.645.145 von Mafieder am 10.04.23 20:21:11Guten Abend Mafieder
Hier können Sie es nochmal nachlesen. Nach der Übernahme von VMT wurde die Unternehmensgründungsurkunde in Perspective Therapeutics abgeändert.Die letzten 2 Monate fand ich die Entwicklung auch recht ansprechend. Die Cashsituation ist angesichts der Marketcap noch sehr gut und die aktuelle cashburnrate ist auch so ,das ich mir mit etwas Fantasie gut vorstellen kann,das eines der Produkte/Projekte Früchte tragen wird.
On February 3, 2023, Isoray, Inc. completed the merger with privately held Viewpoint Molecular Targeting, Inc. Isoray, Inc. amended its Certificate of Incorporation on February 14, 2023, to change its name to Perspective Therapeutics, Inc. The Company currently trades on the NYSE American as Isoray, Inc. under ticker symbol ISR, which is expected to change to Perspective Therapeutics, Inc. with the ticker symbol of CATX on February 21, 2023.
On January 31, 2023, the Board of Directors of Isoray, Inc. approved a change in the fiscal year end of the Company from June 30 to December 31. The Company’s fiscal year will now be the calendar year pursuant to such change. The Company will file a transition report on Form 10-KT covering the transition period from July 1, 2022, to December 31, 2022 and expects to do so by May 2023. As a result, the Company will not be filing a Form 10-Q for the three-month period ended December 31, 2022 but expects to file a Form 10-Q for the three-month period ending March 31, 2023 by mid May 2023.
Hier können Sie es nochmal nachlesen. Nach der Übernahme von VMT wurde die Unternehmensgründungsurkunde in Perspective Therapeutics abgeändert.Die letzten 2 Monate fand ich die Entwicklung auch recht ansprechend. Die Cashsituation ist angesichts der Marketcap noch sehr gut und die aktuelle cashburnrate ist auch so ,das ich mir mit etwas Fantasie gut vorstellen kann,das eines der Produkte/Projekte Früchte tragen wird.
On February 3, 2023, Isoray, Inc. completed the merger with privately held Viewpoint Molecular Targeting, Inc. Isoray, Inc. amended its Certificate of Incorporation on February 14, 2023, to change its name to Perspective Therapeutics, Inc. The Company currently trades on the NYSE American as Isoray, Inc. under ticker symbol ISR, which is expected to change to Perspective Therapeutics, Inc. with the ticker symbol of CATX on February 21, 2023.
On January 31, 2023, the Board of Directors of Isoray, Inc. approved a change in the fiscal year end of the Company from June 30 to December 31. The Company’s fiscal year will now be the calendar year pursuant to such change. The Company will file a transition report on Form 10-KT covering the transition period from July 1, 2022, to December 31, 2022 and expects to do so by May 2023. As a result, the Company will not be filing a Form 10-Q for the three-month period ended December 31, 2022 but expects to file a Form 10-Q for the three-month period ending March 31, 2023 by mid May 2023.
Antwort auf Beitrag Nr.: 73.645.283 von Edelweiss70 am 10.04.23 21:00:21Danke Eldeweiss. Werde mir die Tage mal durchlesen, was es an News gab, ob deren Produkte positive Nachrichten erfahren haben. Ich war vor ca. 2 Jahren hier drin und bin mit Verlust raus. Krebszellen wurden meine ich durch ihre Technik durch einen "Beschuss" gezielt zerstört.
Perspective Therapeutics
IR
15.4.23
RICHLAND, WA & CORALVILLE, IA, May 15, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy powering expanding treatment options for multiple cancers, announced the following changes to the Board of Directors: the appointment of Heidi Henson to the Board of Directors and named Audit Committee Chair; taking over from Rob Williamson, III, who will continue to serve as a member of the Committee. Additionally, Michael McCormick announced his resignation and amicable departure from the Board of Directors
“We are living in exciting times as science, technology and medicine converge — creating new treatment paradigms and therapies for unmet medical needs. I can already clearly see Perspective Therapeutics’ strong team and scientific foundation, energizing the Company’s mission and driving forces towards changing the landscape of cancer treatments through precision targeted medicine,” noted Heidi Henson, incoming member of the Board.
Lori Woods, Chair of the Board commented, “On behalf of Perspective Therapeutics, we wish Heidi a warm welcome and look forward to her active presence on the board and leadership as Audit Committee Chair.”
Thijs Spoor, Perspective Therapeutics’ CEO added, “We are truly delighted to have Heidi join the board of directors at this important juncture in the Company’s evolutionary lifecycle. Her deep leadership experience, while at the helm of life sciences companies, complements the synergies that are coming following on from the combination of Isoray and Viewpoint Molecular Targeting. As a commercial stage brachytherapy company, also focused on early stage development of precision targeted therapies; we are committed to transforming the lives of cancer patients by advancing the field of theranostic and alpha-particle radiotherapies. In this spirit, we are certain that Heidi’s guidance will help us to move further along in our objectives.”
As incoming Chair of the Audit Committee, Ms. Henson is a financial professional with over 25 years of executive leadership roles in the life sciences. In past executive leadership roles, Ms. Henson has served as the Chief Financial Officer of Pardes Biosciences, Imbria Pharmaceuticals, Respivant Sciences, Kura Oncology, Wellspring Biosciences, and Araxes Pharma. Ms. Henson is also currently serving on the board of directors of PepGen and Lista Therapeutics.
Ms. Woods also commented on Mr. McCormick’s departure, “As a longstanding member of the board, we hold Michael in the highest esteem. He has been both a wonderful colleague and advisor to the organization these last 8 years and we are sad to see him depart. On behalf of the whole board, we would like to thank him for his leadership and truly wish him all the best in his endeavors.”
IR
15.4.23
RICHLAND, WA & CORALVILLE, IA, May 15, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy powering expanding treatment options for multiple cancers, announced the following changes to the Board of Directors: the appointment of Heidi Henson to the Board of Directors and named Audit Committee Chair; taking over from Rob Williamson, III, who will continue to serve as a member of the Committee. Additionally, Michael McCormick announced his resignation and amicable departure from the Board of Directors
“We are living in exciting times as science, technology and medicine converge — creating new treatment paradigms and therapies for unmet medical needs. I can already clearly see Perspective Therapeutics’ strong team and scientific foundation, energizing the Company’s mission and driving forces towards changing the landscape of cancer treatments through precision targeted medicine,” noted Heidi Henson, incoming member of the Board.
Lori Woods, Chair of the Board commented, “On behalf of Perspective Therapeutics, we wish Heidi a warm welcome and look forward to her active presence on the board and leadership as Audit Committee Chair.”
Thijs Spoor, Perspective Therapeutics’ CEO added, “We are truly delighted to have Heidi join the board of directors at this important juncture in the Company’s evolutionary lifecycle. Her deep leadership experience, while at the helm of life sciences companies, complements the synergies that are coming following on from the combination of Isoray and Viewpoint Molecular Targeting. As a commercial stage brachytherapy company, also focused on early stage development of precision targeted therapies; we are committed to transforming the lives of cancer patients by advancing the field of theranostic and alpha-particle radiotherapies. In this spirit, we are certain that Heidi’s guidance will help us to move further along in our objectives.”
As incoming Chair of the Audit Committee, Ms. Henson is a financial professional with over 25 years of executive leadership roles in the life sciences. In past executive leadership roles, Ms. Henson has served as the Chief Financial Officer of Pardes Biosciences, Imbria Pharmaceuticals, Respivant Sciences, Kura Oncology, Wellspring Biosciences, and Araxes Pharma. Ms. Henson is also currently serving on the board of directors of PepGen and Lista Therapeutics.
Ms. Woods also commented on Mr. McCormick’s departure, “As a longstanding member of the board, we hold Michael in the highest esteem. He has been both a wonderful colleague and advisor to the organization these last 8 years and we are sad to see him depart. On behalf of the whole board, we would like to thank him for his leadership and truly wish him all the best in his endeavors.”
Nach dem ernüchternden Zahlenwerk war ein Stühlerücken vielleicht auch unabdingbar, bei der Mega Technologie bedarf es einfach Leute ,die die PS auf die Strasse bringen können
Perspective Therapeutics IR
15.5.23
Perspective Therapeutics Reports First Quarter Fiscal 2023 Results
RICHLAND, WASHINGTON & CORALVILLE, IOWA, May 15, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy powering expanding treatment options for multiple cancers, reports first quarter financial results for the period ended March 31, 2023.
“Perspective Therapeutics is at an exciting juncture of our journey as we advance our clinical alpha-particle radiopharmaceutical pipeline. Simultaneously, we look to further support revenue expansion of our commercial brachytherapy program,” said Thijs Spoor, Perspective Therapeutics’ CEO. “In 2023, we expect to report provisional results from ongoing compassionate use of VMT-α-NET for neuroendocrine tumors. We also continue to move forward with our Phase I/IIa Dose Escalation Studies for VMT-α-NET for neuroendocrine tumors and VMT-01 for metastatic melanoma. In turn, we look forward to providing further program and pipeline updates to the market and we also expect incremental data to come out during major medical meetings over 2023.
“The first quarter of 2023 was an exciting one for the Company as we effected our merger, renamed the Company and initiated integration activities. Drafting off the excitement around the merger, we have also been able to attract some extraordinary talent and have added several key hires to the executive team in brachytherapy commercial leadership as well as an industry veteran in the radioactive materials supply chain.” Mr. Spoor concluded, “I’d like to give my express thanks to the entire Perspective Therapeutics team for leaning in during such a dynamic time for the Company while maintaining a focus on providing and developing treatments for cancer patients with an unshakeable focus on safety and quality.”
First Quarter 2023 Financial Summary
Revenue - Revenue for the three months ended March 31, 2023 was $2.1 million, as compared to $2.9 million in the same period in 2022, a decline of 29%. The year over year decline in revenue was primarily a result of the loss of a large customer partially offset by grant revenue from Viewpoint.
Gross Profit - Gross profit was $487,000 for the three months ended March 31, 2023 as compared to $1.4 million for the same period in 2022, a decline of 66%. The year over year decline was primarily a result of the decrease in sales due to the loss of a large customer along with higher production costs which were partially offset by grant revenue.
Research and development (R&D) expenses - R&D for the three months ended March 31, 2023 increased by $3.3 million compared to the three months ended March 31, 2022. The increase was due to $2.9 million related to the development of the Company’s alpha therapy drug products gained through the merger with Viewpoint along with increases in the Company’s legacy research and development expenses related to payroll costs, share based compensation, and brachytherapy trial protocol expense.
Net loss - For the first quarter of 2023, the Company reported a net loss of $371,000, compared to a net loss of $1.3 million in the same period in 2022. The lower net loss for the quarter ended March 31, 2023 was due to a deferred income tax benefit related to the acquisition of Viewpoint.
Cash and cash equivalents - As of March 31, 2023 cash and cash equivalents was $36.5 million as compared to cash, cash equivalents and short-term investments of $43.8 million as of December 31, 2022.
Investor Relations Contact:
LifeSci Advisors
Chuck Padala
E: chuck@lifesciadvisors.com
15.5.23
Perspective Therapeutics Reports First Quarter Fiscal 2023 Results
RICHLAND, WASHINGTON & CORALVILLE, IOWA, May 15, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy powering expanding treatment options for multiple cancers, reports first quarter financial results for the period ended March 31, 2023.
“Perspective Therapeutics is at an exciting juncture of our journey as we advance our clinical alpha-particle radiopharmaceutical pipeline. Simultaneously, we look to further support revenue expansion of our commercial brachytherapy program,” said Thijs Spoor, Perspective Therapeutics’ CEO. “In 2023, we expect to report provisional results from ongoing compassionate use of VMT-α-NET for neuroendocrine tumors. We also continue to move forward with our Phase I/IIa Dose Escalation Studies for VMT-α-NET for neuroendocrine tumors and VMT-01 for metastatic melanoma. In turn, we look forward to providing further program and pipeline updates to the market and we also expect incremental data to come out during major medical meetings over 2023.
“The first quarter of 2023 was an exciting one for the Company as we effected our merger, renamed the Company and initiated integration activities. Drafting off the excitement around the merger, we have also been able to attract some extraordinary talent and have added several key hires to the executive team in brachytherapy commercial leadership as well as an industry veteran in the radioactive materials supply chain.” Mr. Spoor concluded, “I’d like to give my express thanks to the entire Perspective Therapeutics team for leaning in during such a dynamic time for the Company while maintaining a focus on providing and developing treatments for cancer patients with an unshakeable focus on safety and quality.”
First Quarter 2023 Financial Summary
Revenue - Revenue for the three months ended March 31, 2023 was $2.1 million, as compared to $2.9 million in the same period in 2022, a decline of 29%. The year over year decline in revenue was primarily a result of the loss of a large customer partially offset by grant revenue from Viewpoint.
Gross Profit - Gross profit was $487,000 for the three months ended March 31, 2023 as compared to $1.4 million for the same period in 2022, a decline of 66%. The year over year decline was primarily a result of the decrease in sales due to the loss of a large customer along with higher production costs which were partially offset by grant revenue.
Research and development (R&D) expenses - R&D for the three months ended March 31, 2023 increased by $3.3 million compared to the three months ended March 31, 2022. The increase was due to $2.9 million related to the development of the Company’s alpha therapy drug products gained through the merger with Viewpoint along with increases in the Company’s legacy research and development expenses related to payroll costs, share based compensation, and brachytherapy trial protocol expense.
Net loss - For the first quarter of 2023, the Company reported a net loss of $371,000, compared to a net loss of $1.3 million in the same period in 2022. The lower net loss for the quarter ended March 31, 2023 was due to a deferred income tax benefit related to the acquisition of Viewpoint.
Cash and cash equivalents - As of March 31, 2023 cash and cash equivalents was $36.5 million as compared to cash, cash equivalents and short-term investments of $43.8 million as of December 31, 2022.
Investor Relations Contact:
LifeSci Advisors
Chuck Padala
E: chuck@lifesciadvisors.com
Antwort auf Beitrag Nr.: 73.860.934 von Edelweiss70 am 16.05.23 20:06:45Da hat es aber gestern jemand sehr ernst gemeint Späthandel AMEX knapp 3,5 Millionen Stücke 🐿. Das könnte dann Montag wieder eine verdammt spannende Woche werden.
Perspective Therapeutics Initiates Phase 1/2a Clinical Trials for Two Targeted Alpha Therapy Oncology Product Candidates
VMT-α-NET for neuroendocrine cancers and VMT01 for melanoma are both entering therapeutic trials under IND at leading US institutions.
First melanoma patient screened and imaged with [203Pb]VMT01 to determine eligibility for [212Pb]VMT01 treatment.
Preliminary results from initial cohorts expected by end of 2023.
RICHLAND, WASHINGTON & CORALVILLE, IOWA , June 21, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy treatment options for multiple cancers, today announced that it has initiated clinical trial sites and commenced the enrollment period for its 212Pb-labeled therapeutic product candidates, VMT-α-NET and VMT01.
“We are excited to have our first clinical sites on board for our two lead development programs and to commence the enrollment period for these promising product candidates in patients with melanoma and neuroendocrine tumors,” said Thijs Spoor, Perspective’s Chief Executive Officer.
The first product candidate, VMT-α-NET, is a result of Perspective’s intensive in-house research and development efforts to develop a best-in-class radiopeptide. The molecule incorporates the Company’s proprietary chelator technology platform that enables radiolabeling with isotopes of lead (Pb) and shows superior preclinical results as compared to other ligands in its class. On the basis of this promising preclinical data, VMT-α-NET was awarded Fast Track designation under the FDA’s expedited development program. Targeting the somatostatin type receptor 2 (SSTR2) which is over-expressed on a range of different cancer types including neuroendocrine tumors, VMT-α-NET was designed to deliver potent alpha-particle radiation directly to the tumor. Investigators at Fortis Memorial Research Institute (FMRI) located in Gurgaon, India are excited about initial patient experiences in their investigator initiated compassionate use of VMT-α-NET, results of which are expected to be presented at a major medical meeting in Q3 of 2023. An IND-enabled imaging and radiation dosimetry trial utilizing VMT-α-NET is also currently enrolling patients at the University of Iowa, IA, and similar imaging work is occurring at University Hospital Carl Gustav Carus in Dresden, Germany.
Perspective Therapeutics’ trial is a Phase 1/2a Dose Escalation of [212Pb]VMT-α-NET Targeted Alpha-Particle Therapy for Advanced SSTR2 Positive Neuroendocrine Tumors (NCT05636618).
The second product candidate, VMT01, also delivers alpha-particle radiation to tumors by targeting the melanocortin 1 receptor (MC1R) present on melanoma cells. This product candidate has completed clinical imaging studies at Mayo Clinic, Rochester, and results will be presented at the Society of Nuclear Medicine and Molecular Imaging Annual Meeting in Chicago being held from June 24 to 27, 2023. Recent published preclinical data from Perspective’s scientists show its potential to deliver durable complete responses in treatment-resistant models when combined with existing immunotherapy drugs used to treat melanoma.
Perspective Therapeutics has initiated a Phase 1/2a Dose Escalation trial of [212Pb]VMT01 Targeted Alpha-Particle Therapy for patients with MC1R Positive unresectable and metastatic melanoma (NCT05655312).
Both US trials will be supplied with finished drug product from Perspective’s GMP manufacturing facility in Coralville, IA. Additional CDMO manufacturing sites are expected to be brought online in the coming months to enable broader coverage for sites across the US. Isotope will be supplied using Perspective’s proprietary VMT- α-GEN 212Pb benchtop generator.
“These clinical trials represent a significant advancement in our relentless pursuit of innovative and potent treatments for cancer," said Markus Puhlmann, Chief Medical Officer at Perspective. "We know that despite recent improvements in therapies for neuroendocrine cancers and melanoma, many patients become refractory to treatment. The targeted alpha-particle therapies that are being developed at Perspective have the potential to revolutionize the treatment of these and many other cancers. We look forward to seeing the results emerge over the coming months.”
Preliminary results from the initial cohorts for both trials are expected to be available by the end of 2023.
About Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc., is a medical technology and radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body. The Company is the sole producer of Cesium-131 brachytherapy seeds and has a proprietary technology that utilizes the isotope Lead-212 to deliver powerful alpha radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides which provide the opportunity to personalize treatment and optimize patient outcomes. This “theranostic” approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments.
Unless required to do so by law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
VMT-α-NET for neuroendocrine cancers and VMT01 for melanoma are both entering therapeutic trials under IND at leading US institutions.
First melanoma patient screened and imaged with [203Pb]VMT01 to determine eligibility for [212Pb]VMT01 treatment.
Preliminary results from initial cohorts expected by end of 2023.
RICHLAND, WASHINGTON & CORALVILLE, IOWA , June 21, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy treatment options for multiple cancers, today announced that it has initiated clinical trial sites and commenced the enrollment period for its 212Pb-labeled therapeutic product candidates, VMT-α-NET and VMT01.
“We are excited to have our first clinical sites on board for our two lead development programs and to commence the enrollment period for these promising product candidates in patients with melanoma and neuroendocrine tumors,” said Thijs Spoor, Perspective’s Chief Executive Officer.
The first product candidate, VMT-α-NET, is a result of Perspective’s intensive in-house research and development efforts to develop a best-in-class radiopeptide. The molecule incorporates the Company’s proprietary chelator technology platform that enables radiolabeling with isotopes of lead (Pb) and shows superior preclinical results as compared to other ligands in its class. On the basis of this promising preclinical data, VMT-α-NET was awarded Fast Track designation under the FDA’s expedited development program. Targeting the somatostatin type receptor 2 (SSTR2) which is over-expressed on a range of different cancer types including neuroendocrine tumors, VMT-α-NET was designed to deliver potent alpha-particle radiation directly to the tumor. Investigators at Fortis Memorial Research Institute (FMRI) located in Gurgaon, India are excited about initial patient experiences in their investigator initiated compassionate use of VMT-α-NET, results of which are expected to be presented at a major medical meeting in Q3 of 2023. An IND-enabled imaging and radiation dosimetry trial utilizing VMT-α-NET is also currently enrolling patients at the University of Iowa, IA, and similar imaging work is occurring at University Hospital Carl Gustav Carus in Dresden, Germany.
Perspective Therapeutics’ trial is a Phase 1/2a Dose Escalation of [212Pb]VMT-α-NET Targeted Alpha-Particle Therapy for Advanced SSTR2 Positive Neuroendocrine Tumors (NCT05636618).
The second product candidate, VMT01, also delivers alpha-particle radiation to tumors by targeting the melanocortin 1 receptor (MC1R) present on melanoma cells. This product candidate has completed clinical imaging studies at Mayo Clinic, Rochester, and results will be presented at the Society of Nuclear Medicine and Molecular Imaging Annual Meeting in Chicago being held from June 24 to 27, 2023. Recent published preclinical data from Perspective’s scientists show its potential to deliver durable complete responses in treatment-resistant models when combined with existing immunotherapy drugs used to treat melanoma.
Perspective Therapeutics has initiated a Phase 1/2a Dose Escalation trial of [212Pb]VMT01 Targeted Alpha-Particle Therapy for patients with MC1R Positive unresectable and metastatic melanoma (NCT05655312).
Both US trials will be supplied with finished drug product from Perspective’s GMP manufacturing facility in Coralville, IA. Additional CDMO manufacturing sites are expected to be brought online in the coming months to enable broader coverage for sites across the US. Isotope will be supplied using Perspective’s proprietary VMT- α-GEN 212Pb benchtop generator.
“These clinical trials represent a significant advancement in our relentless pursuit of innovative and potent treatments for cancer," said Markus Puhlmann, Chief Medical Officer at Perspective. "We know that despite recent improvements in therapies for neuroendocrine cancers and melanoma, many patients become refractory to treatment. The targeted alpha-particle therapies that are being developed at Perspective have the potential to revolutionize the treatment of these and many other cancers. We look forward to seeing the results emerge over the coming months.”
Preliminary results from the initial cohorts for both trials are expected to be available by the end of 2023.
About Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc., is a medical technology and radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body. The Company is the sole producer of Cesium-131 brachytherapy seeds and has a proprietary technology that utilizes the isotope Lead-212 to deliver powerful alpha radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides which provide the opportunity to personalize treatment and optimize patient outcomes. This “theranostic” approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments.
Unless required to do so by law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
Antwort auf Beitrag Nr.: 74.038.521 von Edelweiss70 am 21.06.23 16:18:03Trotz total flauem Handel kennt der Kurs nur eine Richtung. Stark 💪
Globenewswire
27.6.23
Perspective Therapeutics’ Cesium-131 Featured at the American Brachytherapy Society’s Annual Conference
Perspective Therapeutics’ Cesium-131 Featured at the American Brachytherapy Society’s Annual Conference
RICHLAND, WASHINGTON & CORALVILLE, IOWA, June 27, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy treatment options for multiple cancers, today announced that long term data regarding Cesium-131 brachytherapy in the treatment of prostate cancer was presented at the recent American Brachytherapy Society’s (ABS) annual conference held in Vancouver, British Columbia, Canada from June 21-24, 2023.
The presentation entitled Prostate Brachytherapy with Cs-131: Long Term Results Compared to Published SBRT Data, by authors Mohammed A. Mohammed, MD, Ronald M. Benoit, Sushil Beriwal, MD, and Ryan P. Smith, MD was presented by Ryan P. Smith, MD, Department of Radiation Oncology, UPMC Hillman Cancer Center, Pittsburgh PA. The authors reported that Cesium-131 prostate brachytherapy provided disease-free survival rates of 97.8% and 96%, at 5 and 10 years respectively.
“We’re excited that a presentation of long-term clinical data on Cesium-131 brachytherapy for prostate cancer was included at this year’s ABS meeting,” said Thijs Spoor, Perspective’s Chief Executive Officer. He added, “The data shows Cesium-131 brachytherapy offers prostate cancer patients the prospect of disease-free survival of 96% ten years after treatment. This coupled with a favorable side effect profile affirms the value of Cesium-131 brachytherapy as a leading option for prostate cancer treatment.”
Commenting on the meeting, Perspective’s EVP of Brachytherapy, Andrew Bright, said, “We were thrilled to be a part of this year’s meeting and to be able to re-emphasize our belief in the clinical value of brachytherapy, and the American Brachytherapy Society’s objectives.” He also remarked “It was an excellent meeting, based on the theme of Delivering the Right Care for Everyone: Advancing Brachytherapy Access for All. Peter Rossi MD, the out-going President of ABS, as well as the entire organization committee should be commended for a very successful meeting.”
Perspective Therapeutics is the world’s only producer of Cesium-131 brachytherapy which is powering expanding internal radiation treatment options throughout the body for prostate cancer as well as difficult to treat lung, brain, gynecological, head and neck, pelvic, and colorectal cancers.
27.6.23
Perspective Therapeutics’ Cesium-131 Featured at the American Brachytherapy Society’s Annual Conference
Perspective Therapeutics’ Cesium-131 Featured at the American Brachytherapy Society’s Annual Conference
RICHLAND, WASHINGTON & CORALVILLE, IOWA, June 27, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy treatment options for multiple cancers, today announced that long term data regarding Cesium-131 brachytherapy in the treatment of prostate cancer was presented at the recent American Brachytherapy Society’s (ABS) annual conference held in Vancouver, British Columbia, Canada from June 21-24, 2023.
The presentation entitled Prostate Brachytherapy with Cs-131: Long Term Results Compared to Published SBRT Data, by authors Mohammed A. Mohammed, MD, Ronald M. Benoit, Sushil Beriwal, MD, and Ryan P. Smith, MD was presented by Ryan P. Smith, MD, Department of Radiation Oncology, UPMC Hillman Cancer Center, Pittsburgh PA. The authors reported that Cesium-131 prostate brachytherapy provided disease-free survival rates of 97.8% and 96%, at 5 and 10 years respectively.
“We’re excited that a presentation of long-term clinical data on Cesium-131 brachytherapy for prostate cancer was included at this year’s ABS meeting,” said Thijs Spoor, Perspective’s Chief Executive Officer. He added, “The data shows Cesium-131 brachytherapy offers prostate cancer patients the prospect of disease-free survival of 96% ten years after treatment. This coupled with a favorable side effect profile affirms the value of Cesium-131 brachytherapy as a leading option for prostate cancer treatment.”
Commenting on the meeting, Perspective’s EVP of Brachytherapy, Andrew Bright, said, “We were thrilled to be a part of this year’s meeting and to be able to re-emphasize our belief in the clinical value of brachytherapy, and the American Brachytherapy Society’s objectives.” He also remarked “It was an excellent meeting, based on the theme of Delivering the Right Care for Everyone: Advancing Brachytherapy Access for All. Peter Rossi MD, the out-going President of ABS, as well as the entire organization committee should be commended for a very successful meeting.”
Perspective Therapeutics is the world’s only producer of Cesium-131 brachytherapy which is powering expanding internal radiation treatment options throughout the body for prostate cancer as well as difficult to treat lung, brain, gynecological, head and neck, pelvic, and colorectal cancers.
Es wäre sehr schön wenn die Investoren auch zeitnah über die guten Ergebnisse der klinischen Studien informiert würden.Der Kurs ist ja weiterhin auf stabilem ,interessanten Niveau
Globe Newswire
9.8.23
Perspective Therapeutics and GT Medical Technologies Collaborate to Expand Access to Unique Cesium-131 Based Brain Cancer Treatment
RICHLAND, Wash. & CORALVILLE, Iowa, Aug. 09, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy treatment options for multiple cancers, today announced a collaborative initiative focused on increasing access to Cesium-131 brachytherapy for the treatment of certain brain cancers in the form of GT Medical Technologies, Inc.’s (“GT MedTech”) GammaTile Therapy.
“We are delighted to announce the expansion of our collaboration with GT MedTech to increase our manufacturing in support of their objective of bringing GammaTile Therapy to more patients with difficult to treat brain cancers,” said Thijs Spoor, Chief Executive Officer of Perspective. “As the sole producer of Cesium-131 brachytherapy seeds, the therapeutic agent in GammaTile, we value our long-term partnership with GT MedTech and will increase seed production to allow them the capacity to address short notice orders with increased confidence.”
GammaTile Therapy is a radiation treatment option implanted during the last five minutes of brain tumor resection surgery. It is composed of bioresorbable collagen tiles embedded with Cesium-131 radiation seeds supplied by Perspective Therapeutics. GammaTile delivers targeted Cesium-131 radiation to help prevent brain tumor cell regrowth in newly diagnosed and recurrent brain tumors, including glioblastomas, metastatic brain tumors, aggressive meningiomas, and other brain tumor types.
Matthew Likens, CEO of GT MedTech, commented, "We are thrilled that Perspective is offering to increase production of Cesium-131 brachytherapy sources for our short notice orders, this will allow us to increase access to GammaTile Therapy, especially in cases where a last-minute opportunity to improve clinical outcomes presents itself. I know our customers will appreciate this increased commitment to patient care.”
Perspective Therapeutics is the world’s only producer of Cesium-131 brachytherapy which powers expanded internal radiation treatment options throughout the body, for prostate cancer as well as difficult to treat lung, brain, gynecological, head and neck, pelvic, and colorectal cancers.
9.8.23
Perspective Therapeutics and GT Medical Technologies Collaborate to Expand Access to Unique Cesium-131 Based Brain Cancer Treatment
RICHLAND, Wash. & CORALVILLE, Iowa, Aug. 09, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy treatment options for multiple cancers, today announced a collaborative initiative focused on increasing access to Cesium-131 brachytherapy for the treatment of certain brain cancers in the form of GT Medical Technologies, Inc.’s (“GT MedTech”) GammaTile Therapy.
“We are delighted to announce the expansion of our collaboration with GT MedTech to increase our manufacturing in support of their objective of bringing GammaTile Therapy to more patients with difficult to treat brain cancers,” said Thijs Spoor, Chief Executive Officer of Perspective. “As the sole producer of Cesium-131 brachytherapy seeds, the therapeutic agent in GammaTile, we value our long-term partnership with GT MedTech and will increase seed production to allow them the capacity to address short notice orders with increased confidence.”
GammaTile Therapy is a radiation treatment option implanted during the last five minutes of brain tumor resection surgery. It is composed of bioresorbable collagen tiles embedded with Cesium-131 radiation seeds supplied by Perspective Therapeutics. GammaTile delivers targeted Cesium-131 radiation to help prevent brain tumor cell regrowth in newly diagnosed and recurrent brain tumors, including glioblastomas, metastatic brain tumors, aggressive meningiomas, and other brain tumor types.
Matthew Likens, CEO of GT MedTech, commented, "We are thrilled that Perspective is offering to increase production of Cesium-131 brachytherapy sources for our short notice orders, this will allow us to increase access to GammaTile Therapy, especially in cases where a last-minute opportunity to improve clinical outcomes presents itself. I know our customers will appreciate this increased commitment to patient care.”
Perspective Therapeutics is the world’s only producer of Cesium-131 brachytherapy which powers expanded internal radiation treatment options throughout the body, for prostate cancer as well as difficult to treat lung, brain, gynecological, head and neck, pelvic, and colorectal cancers.
Perspective Therapeutics Announces First Patient Dosed in Phase 1/2a Dose Escalation Trial of VMT01 of its Targeted Alpha-Particle Therapy (TAT), for Treatment of MC1R-positive Metastatic Melanoma
RICHLAND, Wash. & CORALVILLE, Iowa, Aug. 11, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), announces the first patient was dosed at the University of Wisconsin in the Company’s Phase 1/2a dose escalation trial evaluating the safety and efficacy of 212Pb-VMT01 in patients with MC1R+ metastatic melanoma. The trial is a first-in-human, non-randomized, multi-center open-label dose escalation, dose expansion study of 212Pb-VMT01 in patients with histologically confirmed melanoma and a positive MC1R imaging scan.
“MC1R is implicated in the development of melanoma making it a promising target for potential treatment using radionuclide therapy,” said Thijs Spoor, Chief Executive Officer at Perspective Therapeutics. “Today we achieved an important milestone as we work to access the potential of VMT01 as a targeted alpha-particle therapy for MC1R+ metastatic melanoma."
“We are excited to work with Perspective and announce that the first patient with metastatic melanoma has been dosed in the first-in-human study of 212Pb-VMT01," said Zachary S. Morris, M.D., Ph.D., Vice Chair and Endowed Professor of Human Oncology, Program Director for the University of Wisconsin Bentson Research Fellowship, and Principal Investigator for the VMT01 clinical study. "With Perspective’s targeted radionuclide therapy, 212Pb-VMT01, we have the advantage of treating all disease sites following intravenous injection.”
VMT01 has recently completed clinical imaging studies at Mayo Clinic, Rochester. Results were presented at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting in Chicago. In addition, published preclinical data demonstrated durable complete responses in treatment-resistant models when combined with existing immunotherapy drugs used to treat melanoma.
“With the successful conclusion of our imaging study, we are excited to proceed with Perspective’s first-in-human dosing of 212Pb-VMT01,” said Markus Puhlmann, Chief Medical Officer of Perspective Therapeutics. “As we advance through development milestones, our goal is to demonstrate 212Pb-VMT01 can deliver systemic, precision, cytotoxic alpha particle radiation in order to selectively kill cancer cells. We look forward to presenting preliminary data from the study later in the year.”
The Phase 1/2a study consists of a dose-escalation part designed to determine the Maximum Tolerated Dose (MTD) or Maximum Feasible Dose (MFD) following a single administration of 212Pb-VMT01 followed by a dose expansion based on the identified MTD/MFD for the selection of dose(s) for further clinical development. Patients may be eligible to receive up to 3 administrations of 212Pb-VMT01 approximately 8 weeks apart. A dosimetry sub-study utilizing the SPECT imaging surrogate, 203Pb-VMT01, has been added to assess normal organ biodistribution, tumor uptake, radiation dosimetry, and correlation of uptake with observed toxicities and efficacy.
Clinical trial sites include Yale University, University of Iowa, Mayo Clinic in Rochester, Saint Louis University, Washington University, and University of Wisconsin.
212Pb was supplied using Perspective’s proprietary VMT-α-GEN 212Pb benchtop generator and final manufacturing was performed at Perspective’s GMP facility in Coralville, IA. Additional CDMO manufacturing sites are expected to be brought online in the coming months to enable broader coverage for sites across the US.
RICHLAND, Wash. & CORALVILLE, Iowa, Aug. 11, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), announces the first patient was dosed at the University of Wisconsin in the Company’s Phase 1/2a dose escalation trial evaluating the safety and efficacy of 212Pb-VMT01 in patients with MC1R+ metastatic melanoma. The trial is a first-in-human, non-randomized, multi-center open-label dose escalation, dose expansion study of 212Pb-VMT01 in patients with histologically confirmed melanoma and a positive MC1R imaging scan.
“MC1R is implicated in the development of melanoma making it a promising target for potential treatment using radionuclide therapy,” said Thijs Spoor, Chief Executive Officer at Perspective Therapeutics. “Today we achieved an important milestone as we work to access the potential of VMT01 as a targeted alpha-particle therapy for MC1R+ metastatic melanoma."
“We are excited to work with Perspective and announce that the first patient with metastatic melanoma has been dosed in the first-in-human study of 212Pb-VMT01," said Zachary S. Morris, M.D., Ph.D., Vice Chair and Endowed Professor of Human Oncology, Program Director for the University of Wisconsin Bentson Research Fellowship, and Principal Investigator for the VMT01 clinical study. "With Perspective’s targeted radionuclide therapy, 212Pb-VMT01, we have the advantage of treating all disease sites following intravenous injection.”
VMT01 has recently completed clinical imaging studies at Mayo Clinic, Rochester. Results were presented at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting in Chicago. In addition, published preclinical data demonstrated durable complete responses in treatment-resistant models when combined with existing immunotherapy drugs used to treat melanoma.
“With the successful conclusion of our imaging study, we are excited to proceed with Perspective’s first-in-human dosing of 212Pb-VMT01,” said Markus Puhlmann, Chief Medical Officer of Perspective Therapeutics. “As we advance through development milestones, our goal is to demonstrate 212Pb-VMT01 can deliver systemic, precision, cytotoxic alpha particle radiation in order to selectively kill cancer cells. We look forward to presenting preliminary data from the study later in the year.”
The Phase 1/2a study consists of a dose-escalation part designed to determine the Maximum Tolerated Dose (MTD) or Maximum Feasible Dose (MFD) following a single administration of 212Pb-VMT01 followed by a dose expansion based on the identified MTD/MFD for the selection of dose(s) for further clinical development. Patients may be eligible to receive up to 3 administrations of 212Pb-VMT01 approximately 8 weeks apart. A dosimetry sub-study utilizing the SPECT imaging surrogate, 203Pb-VMT01, has been added to assess normal organ biodistribution, tumor uptake, radiation dosimetry, and correlation of uptake with observed toxicities and efficacy.
Clinical trial sites include Yale University, University of Iowa, Mayo Clinic in Rochester, Saint Louis University, Washington University, and University of Wisconsin.
212Pb was supplied using Perspective’s proprietary VMT-α-GEN 212Pb benchtop generator and final manufacturing was performed at Perspective’s GMP facility in Coralville, IA. Additional CDMO manufacturing sites are expected to be brought online in the coming months to enable broader coverage for sites across the US.
Perspective Therapeutics Reports Second Quarter Fiscal 2023 Results and Recent Business Highlights
RICHLAND, Wash. and CORALVILLE, Iowa, Aug. 11, 2023 (GLOBE NEWSWIRE) -- Perspective, Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy powering expanding treatment options for multiple cancers, reports second quarter financial results for the period ended June 30, 2023 and recent business highlights.
“We made substantial progress on our pipeline during Q2 2023 with the initiation of Phase 1/2a clinical trials for VMT-α-NET for neuroendocrine tumors and VMT01 for melanoma and we expect preliminary results from initial cohorts by end of the year,” said Thijs Spoor, Perspective Therapeutics’ CEO. “We are supplying both U.S. trials with drug product from our GMP manufacturing facility in Coralville, Iowa. We expect to bring additional CDMO manufacturing sites online in the coming months to enable broader coverage of sites across the U.S. Isotope will be supplied using Perspective’s proprietary VMT-α-GEN 212Pb benchtop generator.”
Andrew Bright, Perspective Therapeutics’ EVP of Brachytherapy noted, “Our brachytherapy business has emerged from the recent merger with an engaged and enthusiastic team that is focused on expanding the use of Cesium-131 brachytherapy. This quarter we have supplemented the team with talent in several key leadership areas. With a strong team in place, we’re focused on securing new business and re-gaining old business. We have a renewed focus on our strategic collaboration with GT Medical Technologies based on a shared belief that Cesium-131 is ideal for delivering radiotherapy to a key group of brain cancer patients. Our sales efforts will be bolstered by long-term Cesium-131 data in prostate cancer and additional peer reviewed data of Cesium-131 use in other cancers.”
Key Operation Highlights
The appointment of Heidi Henson as Audit Committee Chair of the Board of Directors. Ms. Henson brings over 25 years of executive leadership roles in the life sciences. In past executive leadership roles, Ms. Henson has served as the Chief Financial Officer of Pardes Biosciences, Imbria Pharmaceuticals, Respivant Sciences, Kura Oncology, Wellspring Biosciences, and Araxes Pharma.
The appointment of Andrew Bright as Executive Vice-President of Brachytherapy. Mr. Bright brings to Perspective over 30 years in leadership within the medical device industry, including over 20 years in brachytherapy. Mr. Bright was part of the commercial enterprise responsible for initial widespread adoption and growth of brachytherapy in the 1990’s and was involved in the development and introduction of some of the most successful and clinically relevant products in the industry.
The appointment of Shane Cobb as Executive Vice President of Operations. Mr. Cobb brings a wealth of operational nuclear pharmacy industry experience to Perspective. He worked for 35 years in roles of increasing responsibility at Roche, Medi+Physics, Amersham, Nycomed Amersham, GE Healthcare, and RLS. Adding to his extensive knowledge of the space, during his time at GE Healthcare, he led multiple global scale projects including bringing a new Mo-99 generator offering to the US, spearheading safety improvement initiatives, developing production workflow systems for 31 cleanrooms to meet USP 797 compliance, and leading the first nuclear pharmacies in the US to receive Joint Commission accreditation for quality standards.
Launch of Australian subsidiary to accelerate the commencement of early-stage clinical R&D work. The launch of this entity is intended to accelerate Perspective’s translational pipeline products from preclinical into early phase proof of concept human clinical trials.
VMT01 Clinical Highlights
Initiated clinical trial sites and commenced the enrollment period for 212Pb labeled therapeutic product candidate VMT01 for the treatment of melanoma. VMT01 is entering therapeutic trials at leading U.S. institutions.
Multiple patients screened and first patient dosed in Phase 1/2a Dose Escalation Trial for VMT01 in the treatment of metastatic melanoma expressing MC1R. Preliminary data readout from dose escalation study is expected by the end of 2023.
First in human imaging data from VMT01 were presented at the 2023 Annual Society for Nuclear Medicine and Molecular Imaging (SNMMI) by Geoffrey Johnson, MD, PhD, the Chair of Nuclear Medicine at Mayo Clinic. Dr. Johnson noted that both the SPECT and PET imaging versions of VMT01 showed favorable uptake in melanoma tumors in a subset of patients, confirming VMT01/VMT02 as selection tools for patients to receive targeted alpha therapy with 212Pb-VMT01.
Preclinical combination data with checkpoint inhibitors were presented at the 2023 International Symposium on Radiopharmaceutical Sciences (iSRS). Data displayed synergy between targeted alpha therapy with 212Pb and immune checkpoint inhibitors.
VMT-α-NET Recent Milestones
Continued administrations of multiple cycles of administering VMT-α-NET in 10 patients in an investigator-initiated trial at Fortis Memorial Research Institute. We expect to disclose preliminary results in Q3 2023.
Initiated clinical trial sites and commenced the enrollment period for 212Pb labeled therapeutic product candidate VMT-α-NET. VMT-α-NET for neuroendocrine cancers is entering therapeutic trials at leading US institutions.
Continued recruitment in University of Iowa imaging and dosimetry study with 203Pb VMT-α-NET.
Brachytherapy Recent Highlights
A favorable comparison between Cesium-131 brachytherapy and Stereotactic Body Radiation Therapy, along with long-term Cesium-131 data, was presented at the American Brachytherapy Society annual meeting. The data showed that Cesium-131 brachytherapy offers prostate cancer patients the prospect of disease-free survival of 96% measured at ten years after treatment.
Bander et al. published a retrospective review of brain tumor treatment using surgery and Cesium-131 brachytherapy in the Journal of Neuro-Oncology. The study highlighted the superior local tumor control and lower complication rates of Cesium-131 compared to Iodine-125 data. (Bander, ED, et al., Safety and efficacy of Cesium-131 brachytherapy for brain tumors. Journal of Neurosurgery 163(2): 355-365. (2023)).
Established an initiative with GT Medical Technologies, Inc. (GT Medical) to broaden access to their GammaTile Therapy product, a unique delivery method for placing Cesium-131 during the last five minutes of brain tumor resection surgery.
The Centers for Medicare & Medicaid Services announced in July 2023 its proposed Medicare Hospital Outpatient Prospective Payment and Ambulatory Surgical Center Payment Systems payment rates for 2024. Under these rates, Perspective’s stranded pre-loaded Cesium-131 seeds would see an increase in reimbursement per seed. The final rule will be issued in November 2023.
Manufacturing and Supply Milestones
Updated all regulatory, operational, and related compliance activities to ensure all FDA related labeling, documentation, packaging, and customer facing materials reflects Perspective Therapeutics, with no disruption or inconvenience to our customers.
Multiple clinical GMP products have now been manufactured and shipped to clinical sites from Perspective’s GMP facility in Coralville.
Substantial progress towards transfer of manufacturing to multiple third party contract development and manufacturing organization sites well underway.
212Pb generators manufactured and shipped worldwide have shown robustness of supply.
Additional supplies of Th-228 feedstock have been identified to enable plentiful quantities of 212Pb to be purified as development of Perspective’s pipeline of products progresses towards commercialization.
Continued to increase its manufacturing in support of GT Medical’s objective of delivering GammaTile Therapy to more patients with difficult to treat brain cancers.
Second Quarter and Half Year 2023 Financial Summary
Revenue - Revenue for the three months ended June 30, 2023 was $2.1 million, as compared to $2.5 million in the same period in 2022, a decline of 17%. The year-over-year decline in revenue was primarily a result of the loss of a large brachytherapy customer partially offset by grant revenue related to our alpha-particle therapy operations.
Gross Profit - Gross profit was $248,000 for the three months ended June 30, 2023, as compared to $926,000 for the same period in 2022, a decline of 73%. The year over year decline was primarily a result of the decrease in sales due to the loss of a large brachytherapy customer, higher brachytherapy production costs, and a $298,000 write-off of Blu Build inventory as the Company discontinued selling its loader, and were partially offset by grant revenue from our alpha-particle therapy business.
Research and development (R&D) expenses - R&D for the three months ended June 30, 2023 and 2022 comparison was an increase in costs of $4.9 million related to the development of the Company’s alpha-particle therapy drug products gained through the merger with Viewpoint, partially offset by a decrease in the Company’s legacy research and development expenses.
Management believes that research and development expenses will increase as we continue to invest in the development of new drugs and products.
Net loss - For the second quarter of 2023, the Company reported a net loss of $11.1 million, compared to a net loss of $2.1 million in the same period in 2022.
For the first half of fiscal 2023 ended June 30, 2023, revenue decreased 23% to $4.2 million versus $5.4 million in the prior year comparable period. Prostate brachytherapy represented 42% of total revenue for the first six months of fiscal 2023 compared to 73% for the first six months of fiscal 2022. Total operating expenses for the first six months of fiscal 2023 increased 299% to $23.3 million, versus $5.8 million in the prior year comparable period. The net loss for the first six months of fiscal 2023 was $11.5 million, or ($0.05) per basic and diluted share, compared to a net loss of $3.4 million, or ($0.02) per basic and diluted share, in the prior year comparable period. Basic and diluted per share results are based on weighted average shares outstanding of approximately 254 million for the six months ended June 30, 2023, versus 142 million in the comparable prior year period.
Cash and cash equivalents - As of June 30, 2023, cash, cash equivalents, and restricted cash was $28.5 million as compared to cash, cash equivalents, restricted cash, and short-term investments totaled $43.9 million as of December 31, 2022.
About Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc., is a medical technology and radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body. The Company is the sole producer of Cesium-131 brachytherapy seeds and has a proprietary technology that utilizes the isotope Lead-212 to deliver powerful alpha radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides which provide the opportunity to personalize treatment and optimize patient outcomes. This “theranostic” approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments.
The Company’s melanoma (VMT-01) and neuroendocrine tumor (VMT-α-NET) programs are entering Phase 1/2a imaging and therapy trials for the treatment of metastatic melanoma and neuroendocrine tumors at several leading academic institutions. The Company has also developed a proprietary lead-212 generator to secure isotope supply for clinical trial and commercial operations.
For more information, please visit the Company’s website at www.perspectivetherapeutics.com.
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Statements in this press release that are not statements of historical fact are forward-looking statements. Words such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements in this press release include statements concerning, among other things, the Company's clinical development plans and the expected timing thereof; the expected timing for availability and release of data; the Company’s timing and expectations regarding regulatory communications, submissions and approvals; expectations regarding the potential market opportunities for the Company’s product candidates; the potential functionality, capabilities, and benefits of the Company’s product candidates; the potential size of the commercial market for the Company’s product candidates; the Company’s expectations, beliefs, intentions, and strategies regarding the future; the Company’s expectations regarding the addition of manufacturing sites; the Company’s expectations regarding supplying isotope using its proprietary benchtop generator; the Company’s expectations regarding potential competitors; the Company’s plans for bolstering sales efforts; the Company’s expectations regarding changes in reimbursement amounts for its products; the Company’s ability to transfer its manufacturing to third parties; and other statements that are not historical fact.
The Company may not actually achieve the plans, intentions or expectations disclosed in the forward-looking statements and you should not place undue reliance on the forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause the Company’s actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation, the potential that regulatory authorities may not grant or may delay approval for the Company’s product candidates; uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; early clinical trials may not be indicative of the results in later clinical trials; clinical trial results may not support regulatory approval or further development in a specified indication or at all; actions or advice of regulatory authorities may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company’s ability to obtain and maintain regulatory approval for the Company’s product candidates; delays, interruptions or failures in the manufacture and supply of the Company’s product candidates; the size and growth potential of the markets for the Company’s product candidates, and the Company’s ability to service those markets; the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company’s expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company’s ability to obtain additional funding to support its clinical development programs; the availability or potential availability of alternative products or treatments for conditions targeted by the Company that could affect the availability or commercial potential of its product candidates; the ability of the Company to manage growth and successfully integrate its businesses; whether the Company can maintain its key employees; whether there is sufficient training and use of the Company’s products and product candidates; the market acceptance and recognition of the Company’s products and product candidates; the Company’s ability to maintain and enforce its intellectual property rights; whether the Company can maintain its therapeutic isotope supply agreement with the Department of Energy; whether the Company will continue to comply with the procedures and regulatory requirements mandated by the FDA for additional trials, Phase 1 and 2 approvals, Fast Track approvals, and 510(k) approval and reimbursement codes; and any changes in applicable laws and regulations. Other factors that may cause the Company’s actual results to differ materially from those expressed or implied in the forward-looking statements in this press release are described under the heading “Risk Factors” in the Company’s most recent Transition Report on Form 10-KT and the Company’s most recent Quarterly Report on Form 10-Q, each filed with the Securities and Exchange Commission (the “SEC”), in the Company’s other filings with the SEC, and in the Company’s future reports to be filed with the SEC and available at www.sec.gov.
Forward-looking statements contained in this press release are made as of this date, and the Company undertakes no duty to update such information whether as a result of new information, future events or otherwise, except as required under applicable law.
Investor Relations Contact:
LifeSci Advisors
Chuck Padala
E: chuck@lifesciadvisors.com
Perspective Therapeutics, Inc. and Subsidiaries
Condensed Consolidated Balance Sheets (Unaudited)
(In thousands, except shares)
June 30, December 31,
2023 2022
ASSETS
Current assets:
Cash and cash equivalents $ 28,319 $ 20,993
Short-term investments - 22,764
Accounts receivable, net 1,113 1,363
Inventory 1,094 1,409
Note receivable - 6,109
Prepaid expenses and other current assets 1,428 577
Total current assets 31,954 53,215
Property and equipment, net 7,043 1,684
Right of use asset, net 805 378
Restricted cash 182 182
Inventory, non-current 2,269 2,396
Intangible assets 50,000 -
Goodwill 27,319 -
Other assets, net 573 236
Total assets $ 120,145 $ 58,091
LIABILITIES AND STOCKHOLDERS' EQUITY
Current liabilities:
Accounts payable and accrued expenses $ 4,906 $ 1,541
Lease liability 262 276
Accrued protocol expense 387 233
Accrued radioactive Accrued radioactive waste disposal 20 129
Accrued payroll and related taxes 2,259 212
Accrued vacation 684 285
Other notes payable, current 71 -
Total current liabilities 8,589 2,676
Non-current liabilities:
Lease liability, non-current 543 116
Note payable 1,701 -
Asset retirement obligation 659 657
Total liabilities 11,492 3,449
Commitments and contingencies
Stockholders' equity:
Preferred stock, $.001 par value; 7,000,000 shares authorized: Series B: 5,000,000 shares allocated; no shares issued and outstanding - -
Common stock, $.001 par value; 750,000,000 shares authorized; 280,479,421 and 142,112,766 shares issued and outstanding 280 142
Additional paid-in capital 225,782 160,432
Accumulated deficit (117,409 ) (105,932 )
Total stockholders' equity 108,653 54,642
Total liabilities and stockholders' equity $ 120,145 $ 58,091
Perspective Therapeutics, Inc. and Subsidiaries
Condensed Consolidated Statements of Operations (Unaudited)
(Dollars and shares in thousands, except for per-share amounts)
Three months ended Six months ended
June 30, June 30,
2023 2022 2023 2022
Sales, net $ 1,500 $ 2,505 $ 3,330 $ 5,415
Grant revenue 588 - 821 -
Total revenue 2,088 2,505 4,151 5,415
Cost of sales 1,840 1,579 3,416 3,048
Gross profit 248 926 735 2,367
Operating expenses:
Research and development 5,653 796 9,510 1,345
Sales and marketing 911 654 1,723 1,341
General and administrative 5,073 1,582 12,096 3,163
Change in estimate of asset retirement obligation (15 ) - (15 ) -
Loss on disposal of property and equipment - - 22 -
Total operating expenses 11,622 3,032 23,336 5,849
Operating loss (11,374 ) (2,106 ) (22,601 ) (3,482 )
Non-operating income (expense):
Interest income 294 28 668 57
Interest expense (28 ) - (46 ) -
Other income 2 2 -
Non-operating income, net 268 28 624 57
Net loss before deferred income tax benefit (11,106 ) (2,078 ) (21,977 ) (3,425 )
Deferred income tax benefit - - 10,500 -
Net loss $ (11,106 ) $ (2,078 ) $ (11,477 ) $ (3,425 )
Basic and diluted loss per share $ (0.04 ) $ (0.01 ) $ (0.05 ) $ (0.02 )
Weighted average shares used in computing net loss per share:
Basic and diluted 279,988 142,040 254,432 142,040
RICHLAND, Wash. and CORALVILLE, Iowa, Aug. 11, 2023 (GLOBE NEWSWIRE) -- Perspective, Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a precision oncology company developing alpha-particle therapies and complementary diagnostic imaging agents and an innovator in seed brachytherapy powering expanding treatment options for multiple cancers, reports second quarter financial results for the period ended June 30, 2023 and recent business highlights.
“We made substantial progress on our pipeline during Q2 2023 with the initiation of Phase 1/2a clinical trials for VMT-α-NET for neuroendocrine tumors and VMT01 for melanoma and we expect preliminary results from initial cohorts by end of the year,” said Thijs Spoor, Perspective Therapeutics’ CEO. “We are supplying both U.S. trials with drug product from our GMP manufacturing facility in Coralville, Iowa. We expect to bring additional CDMO manufacturing sites online in the coming months to enable broader coverage of sites across the U.S. Isotope will be supplied using Perspective’s proprietary VMT-α-GEN 212Pb benchtop generator.”
Andrew Bright, Perspective Therapeutics’ EVP of Brachytherapy noted, “Our brachytherapy business has emerged from the recent merger with an engaged and enthusiastic team that is focused on expanding the use of Cesium-131 brachytherapy. This quarter we have supplemented the team with talent in several key leadership areas. With a strong team in place, we’re focused on securing new business and re-gaining old business. We have a renewed focus on our strategic collaboration with GT Medical Technologies based on a shared belief that Cesium-131 is ideal for delivering radiotherapy to a key group of brain cancer patients. Our sales efforts will be bolstered by long-term Cesium-131 data in prostate cancer and additional peer reviewed data of Cesium-131 use in other cancers.”
Key Operation Highlights
The appointment of Heidi Henson as Audit Committee Chair of the Board of Directors. Ms. Henson brings over 25 years of executive leadership roles in the life sciences. In past executive leadership roles, Ms. Henson has served as the Chief Financial Officer of Pardes Biosciences, Imbria Pharmaceuticals, Respivant Sciences, Kura Oncology, Wellspring Biosciences, and Araxes Pharma.
The appointment of Andrew Bright as Executive Vice-President of Brachytherapy. Mr. Bright brings to Perspective over 30 years in leadership within the medical device industry, including over 20 years in brachytherapy. Mr. Bright was part of the commercial enterprise responsible for initial widespread adoption and growth of brachytherapy in the 1990’s and was involved in the development and introduction of some of the most successful and clinically relevant products in the industry.
The appointment of Shane Cobb as Executive Vice President of Operations. Mr. Cobb brings a wealth of operational nuclear pharmacy industry experience to Perspective. He worked for 35 years in roles of increasing responsibility at Roche, Medi+Physics, Amersham, Nycomed Amersham, GE Healthcare, and RLS. Adding to his extensive knowledge of the space, during his time at GE Healthcare, he led multiple global scale projects including bringing a new Mo-99 generator offering to the US, spearheading safety improvement initiatives, developing production workflow systems for 31 cleanrooms to meet USP 797 compliance, and leading the first nuclear pharmacies in the US to receive Joint Commission accreditation for quality standards.
Launch of Australian subsidiary to accelerate the commencement of early-stage clinical R&D work. The launch of this entity is intended to accelerate Perspective’s translational pipeline products from preclinical into early phase proof of concept human clinical trials.
VMT01 Clinical Highlights
Initiated clinical trial sites and commenced the enrollment period for 212Pb labeled therapeutic product candidate VMT01 for the treatment of melanoma. VMT01 is entering therapeutic trials at leading U.S. institutions.
Multiple patients screened and first patient dosed in Phase 1/2a Dose Escalation Trial for VMT01 in the treatment of metastatic melanoma expressing MC1R. Preliminary data readout from dose escalation study is expected by the end of 2023.
First in human imaging data from VMT01 were presented at the 2023 Annual Society for Nuclear Medicine and Molecular Imaging (SNMMI) by Geoffrey Johnson, MD, PhD, the Chair of Nuclear Medicine at Mayo Clinic. Dr. Johnson noted that both the SPECT and PET imaging versions of VMT01 showed favorable uptake in melanoma tumors in a subset of patients, confirming VMT01/VMT02 as selection tools for patients to receive targeted alpha therapy with 212Pb-VMT01.
Preclinical combination data with checkpoint inhibitors were presented at the 2023 International Symposium on Radiopharmaceutical Sciences (iSRS). Data displayed synergy between targeted alpha therapy with 212Pb and immune checkpoint inhibitors.
VMT-α-NET Recent Milestones
Continued administrations of multiple cycles of administering VMT-α-NET in 10 patients in an investigator-initiated trial at Fortis Memorial Research Institute. We expect to disclose preliminary results in Q3 2023.
Initiated clinical trial sites and commenced the enrollment period for 212Pb labeled therapeutic product candidate VMT-α-NET. VMT-α-NET for neuroendocrine cancers is entering therapeutic trials at leading US institutions.
Continued recruitment in University of Iowa imaging and dosimetry study with 203Pb VMT-α-NET.
Brachytherapy Recent Highlights
A favorable comparison between Cesium-131 brachytherapy and Stereotactic Body Radiation Therapy, along with long-term Cesium-131 data, was presented at the American Brachytherapy Society annual meeting. The data showed that Cesium-131 brachytherapy offers prostate cancer patients the prospect of disease-free survival of 96% measured at ten years after treatment.
Bander et al. published a retrospective review of brain tumor treatment using surgery and Cesium-131 brachytherapy in the Journal of Neuro-Oncology. The study highlighted the superior local tumor control and lower complication rates of Cesium-131 compared to Iodine-125 data. (Bander, ED, et al., Safety and efficacy of Cesium-131 brachytherapy for brain tumors. Journal of Neurosurgery 163(2): 355-365. (2023)).
Established an initiative with GT Medical Technologies, Inc. (GT Medical) to broaden access to their GammaTile Therapy product, a unique delivery method for placing Cesium-131 during the last five minutes of brain tumor resection surgery.
The Centers for Medicare & Medicaid Services announced in July 2023 its proposed Medicare Hospital Outpatient Prospective Payment and Ambulatory Surgical Center Payment Systems payment rates for 2024. Under these rates, Perspective’s stranded pre-loaded Cesium-131 seeds would see an increase in reimbursement per seed. The final rule will be issued in November 2023.
Manufacturing and Supply Milestones
Updated all regulatory, operational, and related compliance activities to ensure all FDA related labeling, documentation, packaging, and customer facing materials reflects Perspective Therapeutics, with no disruption or inconvenience to our customers.
Multiple clinical GMP products have now been manufactured and shipped to clinical sites from Perspective’s GMP facility in Coralville.
Substantial progress towards transfer of manufacturing to multiple third party contract development and manufacturing organization sites well underway.
212Pb generators manufactured and shipped worldwide have shown robustness of supply.
Additional supplies of Th-228 feedstock have been identified to enable plentiful quantities of 212Pb to be purified as development of Perspective’s pipeline of products progresses towards commercialization.
Continued to increase its manufacturing in support of GT Medical’s objective of delivering GammaTile Therapy to more patients with difficult to treat brain cancers.
Second Quarter and Half Year 2023 Financial Summary
Revenue - Revenue for the three months ended June 30, 2023 was $2.1 million, as compared to $2.5 million in the same period in 2022, a decline of 17%. The year-over-year decline in revenue was primarily a result of the loss of a large brachytherapy customer partially offset by grant revenue related to our alpha-particle therapy operations.
Gross Profit - Gross profit was $248,000 for the three months ended June 30, 2023, as compared to $926,000 for the same period in 2022, a decline of 73%. The year over year decline was primarily a result of the decrease in sales due to the loss of a large brachytherapy customer, higher brachytherapy production costs, and a $298,000 write-off of Blu Build inventory as the Company discontinued selling its loader, and were partially offset by grant revenue from our alpha-particle therapy business.
Research and development (R&D) expenses - R&D for the three months ended June 30, 2023 and 2022 comparison was an increase in costs of $4.9 million related to the development of the Company’s alpha-particle therapy drug products gained through the merger with Viewpoint, partially offset by a decrease in the Company’s legacy research and development expenses.
Management believes that research and development expenses will increase as we continue to invest in the development of new drugs and products.
Net loss - For the second quarter of 2023, the Company reported a net loss of $11.1 million, compared to a net loss of $2.1 million in the same period in 2022.
For the first half of fiscal 2023 ended June 30, 2023, revenue decreased 23% to $4.2 million versus $5.4 million in the prior year comparable period. Prostate brachytherapy represented 42% of total revenue for the first six months of fiscal 2023 compared to 73% for the first six months of fiscal 2022. Total operating expenses for the first six months of fiscal 2023 increased 299% to $23.3 million, versus $5.8 million in the prior year comparable period. The net loss for the first six months of fiscal 2023 was $11.5 million, or ($0.05) per basic and diluted share, compared to a net loss of $3.4 million, or ($0.02) per basic and diluted share, in the prior year comparable period. Basic and diluted per share results are based on weighted average shares outstanding of approximately 254 million for the six months ended June 30, 2023, versus 142 million in the comparable prior year period.
Cash and cash equivalents - As of June 30, 2023, cash, cash equivalents, and restricted cash was $28.5 million as compared to cash, cash equivalents, restricted cash, and short-term investments totaled $43.9 million as of December 31, 2022.
About Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc., is a medical technology and radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body. The Company is the sole producer of Cesium-131 brachytherapy seeds and has a proprietary technology that utilizes the isotope Lead-212 to deliver powerful alpha radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides which provide the opportunity to personalize treatment and optimize patient outcomes. This “theranostic” approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments.
The Company’s melanoma (VMT-01) and neuroendocrine tumor (VMT-α-NET) programs are entering Phase 1/2a imaging and therapy trials for the treatment of metastatic melanoma and neuroendocrine tumors at several leading academic institutions. The Company has also developed a proprietary lead-212 generator to secure isotope supply for clinical trial and commercial operations.
For more information, please visit the Company’s website at www.perspectivetherapeutics.com.
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Statements in this press release that are not statements of historical fact are forward-looking statements. Words such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements in this press release include statements concerning, among other things, the Company's clinical development plans and the expected timing thereof; the expected timing for availability and release of data; the Company’s timing and expectations regarding regulatory communications, submissions and approvals; expectations regarding the potential market opportunities for the Company’s product candidates; the potential functionality, capabilities, and benefits of the Company’s product candidates; the potential size of the commercial market for the Company’s product candidates; the Company’s expectations, beliefs, intentions, and strategies regarding the future; the Company’s expectations regarding the addition of manufacturing sites; the Company’s expectations regarding supplying isotope using its proprietary benchtop generator; the Company’s expectations regarding potential competitors; the Company’s plans for bolstering sales efforts; the Company’s expectations regarding changes in reimbursement amounts for its products; the Company’s ability to transfer its manufacturing to third parties; and other statements that are not historical fact.
The Company may not actually achieve the plans, intentions or expectations disclosed in the forward-looking statements and you should not place undue reliance on the forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause the Company’s actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation, the potential that regulatory authorities may not grant or may delay approval for the Company’s product candidates; uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; early clinical trials may not be indicative of the results in later clinical trials; clinical trial results may not support regulatory approval or further development in a specified indication or at all; actions or advice of regulatory authorities may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company’s ability to obtain and maintain regulatory approval for the Company’s product candidates; delays, interruptions or failures in the manufacture and supply of the Company’s product candidates; the size and growth potential of the markets for the Company’s product candidates, and the Company’s ability to service those markets; the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company’s expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company’s ability to obtain additional funding to support its clinical development programs; the availability or potential availability of alternative products or treatments for conditions targeted by the Company that could affect the availability or commercial potential of its product candidates; the ability of the Company to manage growth and successfully integrate its businesses; whether the Company can maintain its key employees; whether there is sufficient training and use of the Company’s products and product candidates; the market acceptance and recognition of the Company’s products and product candidates; the Company’s ability to maintain and enforce its intellectual property rights; whether the Company can maintain its therapeutic isotope supply agreement with the Department of Energy; whether the Company will continue to comply with the procedures and regulatory requirements mandated by the FDA for additional trials, Phase 1 and 2 approvals, Fast Track approvals, and 510(k) approval and reimbursement codes; and any changes in applicable laws and regulations. Other factors that may cause the Company’s actual results to differ materially from those expressed or implied in the forward-looking statements in this press release are described under the heading “Risk Factors” in the Company’s most recent Transition Report on Form 10-KT and the Company’s most recent Quarterly Report on Form 10-Q, each filed with the Securities and Exchange Commission (the “SEC”), in the Company’s other filings with the SEC, and in the Company’s future reports to be filed with the SEC and available at www.sec.gov.
Forward-looking statements contained in this press release are made as of this date, and the Company undertakes no duty to update such information whether as a result of new information, future events or otherwise, except as required under applicable law.
Investor Relations Contact:
LifeSci Advisors
Chuck Padala
E: chuck@lifesciadvisors.com
Perspective Therapeutics, Inc. and Subsidiaries
Condensed Consolidated Balance Sheets (Unaudited)
(In thousands, except shares)
June 30, December 31,
2023 2022
ASSETS
Current assets:
Cash and cash equivalents $ 28,319 $ 20,993
Short-term investments - 22,764
Accounts receivable, net 1,113 1,363
Inventory 1,094 1,409
Note receivable - 6,109
Prepaid expenses and other current assets 1,428 577
Total current assets 31,954 53,215
Property and equipment, net 7,043 1,684
Right of use asset, net 805 378
Restricted cash 182 182
Inventory, non-current 2,269 2,396
Intangible assets 50,000 -
Goodwill 27,319 -
Other assets, net 573 236
Total assets $ 120,145 $ 58,091
LIABILITIES AND STOCKHOLDERS' EQUITY
Current liabilities:
Accounts payable and accrued expenses $ 4,906 $ 1,541
Lease liability 262 276
Accrued protocol expense 387 233
Accrued radioactive Accrued radioactive waste disposal 20 129
Accrued payroll and related taxes 2,259 212
Accrued vacation 684 285
Other notes payable, current 71 -
Total current liabilities 8,589 2,676
Non-current liabilities:
Lease liability, non-current 543 116
Note payable 1,701 -
Asset retirement obligation 659 657
Total liabilities 11,492 3,449
Commitments and contingencies
Stockholders' equity:
Preferred stock, $.001 par value; 7,000,000 shares authorized: Series B: 5,000,000 shares allocated; no shares issued and outstanding - -
Common stock, $.001 par value; 750,000,000 shares authorized; 280,479,421 and 142,112,766 shares issued and outstanding 280 142
Additional paid-in capital 225,782 160,432
Accumulated deficit (117,409 ) (105,932 )
Total stockholders' equity 108,653 54,642
Total liabilities and stockholders' equity $ 120,145 $ 58,091
Perspective Therapeutics, Inc. and Subsidiaries
Condensed Consolidated Statements of Operations (Unaudited)
(Dollars and shares in thousands, except for per-share amounts)
Three months ended Six months ended
June 30, June 30,
2023 2022 2023 2022
Sales, net $ 1,500 $ 2,505 $ 3,330 $ 5,415
Grant revenue 588 - 821 -
Total revenue 2,088 2,505 4,151 5,415
Cost of sales 1,840 1,579 3,416 3,048
Gross profit 248 926 735 2,367
Operating expenses:
Research and development 5,653 796 9,510 1,345
Sales and marketing 911 654 1,723 1,341
General and administrative 5,073 1,582 12,096 3,163
Change in estimate of asset retirement obligation (15 ) - (15 ) -
Loss on disposal of property and equipment - - 22 -
Total operating expenses 11,622 3,032 23,336 5,849
Operating loss (11,374 ) (2,106 ) (22,601 ) (3,482 )
Non-operating income (expense):
Interest income 294 28 668 57
Interest expense (28 ) - (46 ) -
Other income 2 2 -
Non-operating income, net 268 28 624 57
Net loss before deferred income tax benefit (11,106 ) (2,078 ) (21,977 ) (3,425 )
Deferred income tax benefit - - 10,500 -
Net loss $ (11,106 ) $ (2,078 ) $ (11,477 ) $ (3,425 )
Basic and diluted loss per share $ (0.04 ) $ (0.01 ) $ (0.05 ) $ (0.02 )
Weighted average shares used in computing net loss per share:
Basic and diluted 279,988 142,040 254,432 142,040
Wallstreet-online
news
11.8.23
Hier nochmal der link zu den Quartalszahlen
https://www.wallstreet-online.de/nachricht/17234880-perspect…
news
11.8.23
Hier nochmal der link zu den Quartalszahlen
https://www.wallstreet-online.de/nachricht/17234880-perspect…
RICHLAND, WASH. & CORALVILLE, IOWA, Aug. 28, 2023 (GLOBE NEWSWIRE)
-- – Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), today announced that it will present new clinical and preclinical data for 212Pb-VMT-α-NET, a Targeted Alpha-Particle Therapy, at the upcoming 36th Annual Congress of the European Association of Nuclear Medicine (EANM). The conference is being held in Vienna, Austria, from September 9-13, 2023.
“Our team at Perspective Therapeutics continues to work diligently to advance much needed targeted alpha therapy using 212Pb,” said Thijs Spoor, Chief Executive Officer at Perspective Therapeutics. “We are particularly excited about the early clinical results from 212Pb-VMT-α-NET in patients suffering from different types of difficult-to-treat neuroendocrine tumors. These results support our belief that targeted alpha therapies have the potential to revolutionize our approach to eliminating cancers and intractable tumors.”
“We are excited to have early results presented by Dr. Dharmender Malik of Fortis Memorial research institute on the safety and efficacy of 212Pb-VMT-α-NET especially in patients with somatostatin receptor positive, metastatic neuroendocrine and medullary thyroid tumors,” said Markus Puhlmann, MD, MBA, Chief Medical Officer at Perspective Therapeutics. “Additionally, we will present data from our metastatic mouse model of neuroblastoma exploring the efficacy of 203/212Pb-VMT-α-NET for image-guided treatment in this metastatic neuroblastoma tumor model.”
Details about these presentations can be found below and on the EANM website (eanm23.eanm.org). Additionally, copies of the abstracts can be found in the EANM ’23 Abstract Book on the EANM website.
Presentation One:
This presentation will discuss early clinical results from the study of 212Pb-VMT-α-NET in ten patients with somatostatin receptor (SSTR)-expressing progressive neuroendocrine tumors and metastatic medullary thyroid carcinomas. 212Pb-VMT-α-NET demonstrated an encouraging safety profile and treatment resulted in partial responses in a number of patients.
Title: Early results of 212Pb-VMT-α-NET Targeted Alpha Therapy in Metastatic Gastro-entero-pancreatic Neuroendocrine Tumors: First in Human Clinical Experience on Safety and Efficacy
Session: Theranostics Track – What’s New in Endocrine Tumors
Abstract Number: OP-673
Presenter: D. Malik, Consultant Nuclear Medicine and PET-CT, MBBS, DNB, FANMB & RSO-II at Fortis Memorial research institute (FMRI), Gurugram, India at Fortis Healthcare Pvt.
-- – Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), today announced that it will present new clinical and preclinical data for 212Pb-VMT-α-NET, a Targeted Alpha-Particle Therapy, at the upcoming 36th Annual Congress of the European Association of Nuclear Medicine (EANM). The conference is being held in Vienna, Austria, from September 9-13, 2023.
“Our team at Perspective Therapeutics continues to work diligently to advance much needed targeted alpha therapy using 212Pb,” said Thijs Spoor, Chief Executive Officer at Perspective Therapeutics. “We are particularly excited about the early clinical results from 212Pb-VMT-α-NET in patients suffering from different types of difficult-to-treat neuroendocrine tumors. These results support our belief that targeted alpha therapies have the potential to revolutionize our approach to eliminating cancers and intractable tumors.”
“We are excited to have early results presented by Dr. Dharmender Malik of Fortis Memorial research institute on the safety and efficacy of 212Pb-VMT-α-NET especially in patients with somatostatin receptor positive, metastatic neuroendocrine and medullary thyroid tumors,” said Markus Puhlmann, MD, MBA, Chief Medical Officer at Perspective Therapeutics. “Additionally, we will present data from our metastatic mouse model of neuroblastoma exploring the efficacy of 203/212Pb-VMT-α-NET for image-guided treatment in this metastatic neuroblastoma tumor model.”
Details about these presentations can be found below and on the EANM website (eanm23.eanm.org). Additionally, copies of the abstracts can be found in the EANM ’23 Abstract Book on the EANM website.
Presentation One:
This presentation will discuss early clinical results from the study of 212Pb-VMT-α-NET in ten patients with somatostatin receptor (SSTR)-expressing progressive neuroendocrine tumors and metastatic medullary thyroid carcinomas. 212Pb-VMT-α-NET demonstrated an encouraging safety profile and treatment resulted in partial responses in a number of patients.
Title: Early results of 212Pb-VMT-α-NET Targeted Alpha Therapy in Metastatic Gastro-entero-pancreatic Neuroendocrine Tumors: First in Human Clinical Experience on Safety and Efficacy
Session: Theranostics Track – What’s New in Endocrine Tumors
Abstract Number: OP-673
Presenter: D. Malik, Consultant Nuclear Medicine and PET-CT, MBBS, DNB, FANMB & RSO-II at Fortis Memorial research institute (FMRI), Gurugram, India at Fortis Healthcare Pvt.
Antwort auf Beitrag Nr.: 74.393.831 von Edelweiss70 am 28.08.23 21:10:56Ist hier irgendetwas verkündet worden was wir noch nicht wussten ???? Dieser Kursaufschlag von 120% ist schon sehr spannend. Hatte schon mit einem neuen Couverage von Oppenheimer gerechnet aber so bleibt es einfach nur spannend
https://perspectivetherapeutics.com/press-releases/perspecti…
https://perspectivetherapeutics.com/press-releases/perspecti…
Perspective Therapeutics Completes Recruitment for First Patient Cohort in Phase 1/2a Dose Escalation Trial of VMT01 in Malignant Melanoma
SEATTLE, Oct. 16, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), today announced the completion of recruitment for the first patient cohort in its Phase 1/2a dose escalation study of 212Pb-VMT01 (clinicaltrials.gov identifier NCT05655312), its targeted alpha-particle therapy, in development for the treatment of MC1R-positive metastatic melanoma.
“We are encouraged by the pace of enrollment in the trial which we believe is indicative of the tremendous unmet need for patients with unresectable and intractable melanomas,” said Chief Medical Officer Markus Puhlmann, MD MBA, of Perspective Therapeutics.
“We are grateful to our clinical collaborators for their diligence in working closely with patients to deliver our targeted alpha-particle therapy, and we look forward to providing an update in the coming months.”
Perspective Therapeutics Completes Recruitment for First Patient Cohort in Phase 1/2a Dose Escalation Trial of VMT01 in Malignant Melanoma
SEATTLE, Oct. 16, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), today announced the completion of recruitment for the first patient cohort in its Phase 1/2a dose escalation study of 212Pb-VMT01 (clinicaltrials.gov identifier NCT05655312), its targeted alpha-particle therapy, in development for the treatment of MC1R-positive metastatic melanoma.
“We are encouraged by the pace of enrollment in the trial which we believe is indicative of the tremendous unmet need for patients with unresectable and intractable melanomas,” said Chief Medical Officer Markus Puhlmann, MD MBA, of Perspective Therapeutics. “We are grateful to our clinical collaborators for their diligence in working closely with patients to deliver our targeted alpha-particle therapy, and we look forward to providing an update in the coming months.”
powered by finative
“MC1R is a receptor that is displayed on the surface of melanoma
About the study
This ongoing trial (clinicaltrials.gov identifier NCT05655312) is a multi-center open-label dose escalation, dose expansion study of 212Pb-VMT01 in subjects with histologically confirmed melanoma and positive MC1R imaging scans. The first part of the study is a dose escalation phase to determine the Maximum Tolerated radioactivity Dose (MTD) or Maximum Feasible radioactivity Dose (MFD) following a single administration of 212Pb-VMT01. Patients with stage IV or unresectable stage III metastatic melanoma who have progressed on at least 1 approved first-line therapy will be scheduled to receive up to 3 administrations of 212Pb-VMT01 approximately 8 weeks apart. The first patient cohort is scheduled to receive 111 MBq (3mCi) per dose. The second cohort will receive administered activities of 185 MBq (5mCi), with cohorts 3 and 4 receiving 370 MBq (10 mCi) and 555 MBq (15 mCi) respectively, if the MTD or MFD is not reached during escalation. According to the Modified Toxicity Probability Interval 2 (mTPI-2) study design, intermediate de-escalation doses are also possible to allow selection of the optimal activity dose to take forward into the dose expansion part of the study.
SEATTLE, Oct. 16, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), today announced the completion of recruitment for the first patient cohort in its Phase 1/2a dose escalation study of 212Pb-VMT01 (clinicaltrials.gov identifier NCT05655312), its targeted alpha-particle therapy, in development for the treatment of MC1R-positive metastatic melanoma.
“We are encouraged by the pace of enrollment in the trial which we believe is indicative of the tremendous unmet need for patients with unresectable and intractable melanomas,” said Chief Medical Officer Markus Puhlmann, MD MBA, of Perspective Therapeutics.
“We are grateful to our clinical collaborators for their diligence in working closely with patients to deliver our targeted alpha-particle therapy, and we look forward to providing an update in the coming months.”
Perspective Therapeutics Completes Recruitment for First Patient Cohort in Phase 1/2a Dose Escalation Trial of VMT01 in Malignant Melanoma
SEATTLE, Oct. 16, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), today announced the completion of recruitment for the first patient cohort in its Phase 1/2a dose escalation study of 212Pb-VMT01 (clinicaltrials.gov identifier NCT05655312), its targeted alpha-particle therapy, in development for the treatment of MC1R-positive metastatic melanoma.
“We are encouraged by the pace of enrollment in the trial which we believe is indicative of the tremendous unmet need for patients with unresectable and intractable melanomas,” said Chief Medical Officer Markus Puhlmann, MD MBA, of Perspective Therapeutics. “We are grateful to our clinical collaborators for their diligence in working closely with patients to deliver our targeted alpha-particle therapy, and we look forward to providing an update in the coming months.”
powered by finative
“MC1R is a receptor that is displayed on the surface of melanoma
About the study
This ongoing trial (clinicaltrials.gov identifier NCT05655312) is a multi-center open-label dose escalation, dose expansion study of 212Pb-VMT01 in subjects with histologically confirmed melanoma and positive MC1R imaging scans. The first part of the study is a dose escalation phase to determine the Maximum Tolerated radioactivity Dose (MTD) or Maximum Feasible radioactivity Dose (MFD) following a single administration of 212Pb-VMT01. Patients with stage IV or unresectable stage III metastatic melanoma who have progressed on at least 1 approved first-line therapy will be scheduled to receive up to 3 administrations of 212Pb-VMT01 approximately 8 weeks apart. The first patient cohort is scheduled to receive 111 MBq (3mCi) per dose. The second cohort will receive administered activities of 185 MBq (5mCi), with cohorts 3 and 4 receiving 370 MBq (10 mCi) and 555 MBq (15 mCi) respectively, if the MTD or MFD is not reached during escalation. According to the Modified Toxicity Probability Interval 2 (mTPI-2) study design, intermediate de-escalation doses are also possible to allow selection of the optimal activity dose to take forward into the dose expansion part of the study.
Antwort auf Beitrag Nr.: 74.642.747 von Edelweiss70 am 16.10.23 18:31:01Perspektive therapeutics
Newswire
8.11.23
Perspective Therapeutics Announces First Patient Dosed in Phase 1/2a Study of [(212)Pb]VMT-α-NET for Treatment of Advanced SSTR2-Positive Neuroendocrine Tumors
November 8, 2023
SEATTLE, Nov. 08, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. ("Perspective" or "the Company") (NYSE AMERICAN: CATX), announced today that the first patient was dosed at Washington University in St Louis in the Company's Phase 1/2a trial evaluating the safety and efficacy of [212Pb]VMT-α-NET, a targeted alpha-particle therapy (TAT), in patients with unresectable or metastatic somatostatin receptor type 2 (SSTR2) expressing neuroendocrine tumors (NETs). The trial is a multi-center, open-label dose escalation, dose expansion study of [212Pb]VMT-α-NET in patients who have not received prior peptide receptor radionuclide therapy (PRRT).
"We are proud of our team's commitment and progress in bringing our radionuclide therapy to patients suffering from these difficult-to-treat neuroendocrine tumors," said Thijs Spoor, Chief Executive Officer of Perspective Therapeutics. "Based on the promising clinical data observed in the investigator-led study in India that was presented at the recent European Association of Nuclear Medicine meeting, we believe [212Pb]VMT-α-NET has the potential to provide significant improvements in clinical outcomes compared to standard of care [177Lu]DOTATATE. With our FDA Fast Track Designation for first-line treatment in all neuroendocrine tumors, we plan to rapidly advance this product through clinical development."
"Although [177Lu]DOTATATE is available to a subset of patients with NETs, we know that few develop objective anatomic responses to therapy and ultimately many patients progress on treatment," commented Richard Wahl, M.D., Professor of Radiology & Radiation Oncology, Mallinckrodt Institute of Radiology, Washington University School of Medicine in St Louis. "The development of Perspective's [212Pb]VMT-α-NET targeted alpha particle therapy is an important next step in the evolution of treatments for SSTR2-expressing tumors. We are excited to participate in the trial."
"We are excited about our collaboration with investigators from top-tier research centers who are evaluating [212Pb]VMT-α-NET in patients with intractable SSTR2-expressing neuroendocrine tumors," said Markus Puhlmann, Chief Medical Officer of Perspective Therapeutics. "Our goal is to demonstrate that systemic delivery of [212Pb]VMT-α-NET is safe and has the potential to selectively target tumor cells while sparing healthy tissues. We expect to present preliminary findings in early 2024."
Newswire
8.11.23
Perspective Therapeutics Announces First Patient Dosed in Phase 1/2a Study of [(212)Pb]VMT-α-NET for Treatment of Advanced SSTR2-Positive Neuroendocrine Tumors
November 8, 2023
SEATTLE, Nov. 08, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. ("Perspective" or "the Company") (NYSE AMERICAN: CATX), announced today that the first patient was dosed at Washington University in St Louis in the Company's Phase 1/2a trial evaluating the safety and efficacy of [212Pb]VMT-α-NET, a targeted alpha-particle therapy (TAT), in patients with unresectable or metastatic somatostatin receptor type 2 (SSTR2) expressing neuroendocrine tumors (NETs). The trial is a multi-center, open-label dose escalation, dose expansion study of [212Pb]VMT-α-NET in patients who have not received prior peptide receptor radionuclide therapy (PRRT).
"We are proud of our team's commitment and progress in bringing our radionuclide therapy to patients suffering from these difficult-to-treat neuroendocrine tumors," said Thijs Spoor, Chief Executive Officer of Perspective Therapeutics. "Based on the promising clinical data observed in the investigator-led study in India that was presented at the recent European Association of Nuclear Medicine meeting, we believe [212Pb]VMT-α-NET has the potential to provide significant improvements in clinical outcomes compared to standard of care [177Lu]DOTATATE. With our FDA Fast Track Designation for first-line treatment in all neuroendocrine tumors, we plan to rapidly advance this product through clinical development."
"Although [177Lu]DOTATATE is available to a subset of patients with NETs, we know that few develop objective anatomic responses to therapy and ultimately many patients progress on treatment," commented Richard Wahl, M.D., Professor of Radiology & Radiation Oncology, Mallinckrodt Institute of Radiology, Washington University School of Medicine in St Louis. "The development of Perspective's [212Pb]VMT-α-NET targeted alpha particle therapy is an important next step in the evolution of treatments for SSTR2-expressing tumors. We are excited to participate in the trial."
"We are excited about our collaboration with investigators from top-tier research centers who are evaluating [212Pb]VMT-α-NET in patients with intractable SSTR2-expressing neuroendocrine tumors," said Markus Puhlmann, Chief Medical Officer of Perspective Therapeutics. "Our goal is to demonstrate that systemic delivery of [212Pb]VMT-α-NET is safe and has the potential to selectively target tumor cells while sparing healthy tissues. We expect to present preliminary findings in early 2024."
Seatle Newswire
IR Perspective Therapeutics
14.11.23
Perspective Therapeutics Reports Third Quarter Fiscal 2023 Results and Recent Business Highlights
SEATTLE, Nov. 14, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (NYSE AMERICAN: CATX), today announced third quarter financial results for the period ended September 30, 2023 and provided recent business highlights..
“Our commitment to advancing targeted alpha-particle therapies against neuroendocrine tumors remains steadfast as we have completed dosing of the first patient in the Phase 1/2a clinical trial of [212Pb]VMT-α-NET,” said Thijs Spoor, Perspective Therapeutics' CEO. “In addition, we completed recruitment for the first patient cohort in the Company-sponsored Phase 1/2a dose escalation/dose expansion clinical trial evaluating [212Pb]VMT01 for the treatment of histologically confirmed melanoma. We are nearing completion of the imaging portion of the clinical trial evaluating the suitability of [203Pb]VMT01 for SPECT/CT imaging.”
“We are pleased with our execution this quarter and continue to see positive momentum in the number of patients being treated with Cesium-131 each month, marking a return to growth as compared to the same period in the prior year,” stated Andrew Bright, Perspective Therapeutics’ Executive Vice President of Brachytherapy. “The rebound in the number of patients treated was driven by engagement with current customers, as well as new customers adopting Cesium-131 in their brachytherapy programs. Additionally, increased utilization of Cesium-131 is evident in our core prostate business, as well as in other disease areas, notably lung and brain brachytherapy.”
VMT-α-NET Recent Highlights
Company-sponsored Phase 1/2a clinical trial of [212Pb]VMT-α-NET
Dosed first patient in the Phase 1/2a clinical trial of [212Pb]VMT-α-NET for unresectable or metastatic SSTR2-expressing neuroendocrine tumors (NETs) at Washington University in St. Louis.
The Company currently has two active sites for the clinical trial and anticipates that two additional clinical trial sites will become operational in December.
The Company remains on track to launch an additional 14 clinical trial sites.
Investigator-Initiated clinical trials of [212Pb]VMT-α-NET
Presented early clinical findings at the 36th Annual Congress of the European Association of Nuclear Medicine (EANM) for the Phase 2a clinical trial of [212Pb]VMT-α-NET in pre- and post-Lutathera gastroenteropancreatic (GEP)-NET patients, being conducted at Fortis Healthcare, India. Dr. Dharmender Malik of Fortis Memorial Research Institute presented data on [212Pb]VMT-α-NET therapy in 10 patients who had failed prior treatment, including standard care. Initial data readout demonstrated high objective response rate for 7 of 9 evaluable patients, spanning peptide receptor radionuclide therapy (PRRT)-naïve and PRRT-refractory cases. Two additional patients were enrolled in the third quarter for a total of 9 GEP-NET patients and 2 medullary thyroid cancer (MTC) patients. All ongoing patients are expected to complete their 4th treatment cycle by the end of February 2024. Updated clinical results are expected to be presented at an upcoming scientific meeting in 2024.
Two patients were screened in the Phase 1/2a clinical trial for post-Lutathera GEP-NET patients at the University of Iowa. A third patient is scheduled for screening mid-November. If eligible, all three patients will receive treatment in December for the completion of the first cohort. The end of fourth cycle of treatment is expected in June 2024.
End-of-life compassionate use program for patients with advanced NETs and lack of further treatment options is underway at the University Dresden in Germany. Four patients were treated during the third quarter, and investigators are planning additional treatments before the end of the year.
IND submissions are expected in November with subsequent trial activation in the first half of 2024 for NIH-sponsored studies in pre- and post-Lutathera NET patients.
VMT01 Recent Highlights
Completed recruitment for the first patient cohort in the Company-sponsored Phase 1/2a dose escalation/dose expansion clinical trial of [212Pb]VMT01 in patients with histologically confirmed melanoma and positive MC1R imaging scans. Three patients have been dosed at 111 MBq (3mCi), closing cohort 1. No serious adverse events or dose-limiting toxicities have been observed. The Company anticipates opening the second patient cohort, which will receive 185 MBq (5mCi) per dose, in December 2023 and anticipates the completion of screening in the first quarter of 2024.
Nearing completion of analysis of the dosimetry portion of the Company’s TIMAR-1 clinical trial, a first-in-human study evaluating the suitability of [203Pb]VMT01 for SPECT/CT imaging and [68Ga]VMT02 for PET/CT imaging of MC1R-expressing metastatic melanoma. Perspective is actively working on the Clinical Study Report and anticipates it will be released in the first half of 2024.
Brachytherapy Recent Highlights
Third quarter 2023 revenue was the highest since the second quarter of 2022, with a return to growth over the third quarter of 2022.
Appointed sales, marketing and clinical team members along with a senior customer service representative to supplement Perspective’s dedicated commercial team.
Launched marketing initiatives to increase direct patient communication and engagement on Cesium-131 brachytherapy.
Facilitated timely access to GT Medical’s GammaTile for challenging brain cancers through increased production of Cesium-131 brachytherapy seeds that allowed GT Medical to address short notice orders with increased confidence.
Presented long-term data for Cesium-131 brachytherapy in the treatment of prostate cancer at the American Society for Radiation Oncology (ASTRO) annual conference. In his presentation, Dr. Mohamed Abdelhakiem of the UPMC Hillman Cancer Center described urinary data collected from 341 patients treated with Cesium-131 between 2006 and 2022.
Presented preliminary long-term data for Cesium-131 utilized in salvage treatment of recurrent cervical and uterine cancers at the ASTRO annual conference. Dr. Zeta Chow of the University of Kentucky showed data that indicated Cesium-131 brachytherapy is a promising alternative to pelvic exenteration, a significant surgical procedure.
Publication of peer reviewed article in the journal of Brachytherapy highlighting Cesium-131 clinical data from Dr. Benoit and colleagues at the University of Pittsburgh School of Medicine. The results support Cesium-131 and provide additional data on dose parameters for improved urinary side effect profile without affecting disease free survival. [https://www.brachyjournal.com/article/S1538-4721(23)01632-X/…
Discovery Program Milestones
Presented data at EANM. Dijie Liu, Principal Research Scientist at Perspective, presented mouse model data highlighting the efficacy of [203/212Pb]VMT-α-NET in treating metastatic neuroblastoma tumors. The study showed successful tumor uptake via sequential SPECT imaging and demonstrated a maximum tolerated dose of [212Pb]VMT-α-NET as 2.22 MBq without acute toxicity, with a 100% overall survival rate at 90-days observed in the group receiving three fractionated doses of 740 kBq of [212Pb]VMT-α-NET.
Presented collaborative data at the World Molecular Imaging Congress. Dr. Michael K. Schultz, Chief Science Officer of Perspective presented data highlighting the effectiveness of [212Pb]VMT-α-NET in treating neuroendocrine tumors in a tumor xenograft mouse model. The results highlighted the significant therapeutic efficacy of treatment with three fractionated doses of [212Pb]VMT-α-NET, which resulted in a 70% complete response rate and 80% survival at 120 days.
Presented encouraging data on novel guest/host platform for effective in vivo image-guided pre-targeted alpha particle therapy at the World Molecular Imaging Congress. Dr. Amritjyot Kaur of Stony Brook University showed that the radioligand [203Pb]PSC-PEG3-Adma demonstrated extended lag times, impressive tumor-to-tissue ratios and has the potential to further reduce radiation toxicity using [203Pb]PSC-PEG3-Adma as a guide.
Publication of a peer-reviewed article in Nuclear Medicine and Biology reporting on collaborative research with the University of Alberta, to accelerate the availability of imaging surrogate isotope Pb-203 for image-guided dosimetry planning of Pb-212 labeled radiopeptide conjugates. The collaboration has established an approach to production that can be readily adopted by other cyclotron facilities that produce high yields and excellent specific activity. [https://pubmed.ncbi.nlm.nih.gov/36708660/]
Third Quarter 2023 Financial Summary
Total revenue for the three months ended September 30, 2023 was $2.19 million, compared to $1.72 million for the same period in 2022, an increase of 27%.
Sales revenues for the quarter ended September 30, 2023, was $1.9 million, compared to $1.7 million for the same period in 2022, an increase of 11%.
Prostate brachytherapy represented 55% of total revenue for the quarter ended September 30, 2023 compared to 68% for the same period in 2022.
Gross profit was $738,000 for the three months ended September 30, 2023 compared to $414,000 for the same period in 2022.
Research and development expenses were $5.7 million for the three months ended September 30, 2023 compared to $708,000 for the same period in 2022, an increase of 708%. Management continues to believe that research and development expenses may increase as the Company continues to advance its clinical programs.
Total operating expenses for the quarter ended September 30, 2023 were $11.3 million, compared to $4.6 million for the same period in 2022, an increase of 144%.
Net loss for the three months ended September 30, 2023 was $10.4 million or $0.04 per share basic and diluted, compared to $4.1 million or $0.03 per share basic and diluted for the same period in 2022.
Cash and cash equivalents as of September 30, 2023 was $18.0 million as compared to $43.9 million on December 31, 2022. Based on the Company's current operating plan, the Company believes that its existing cash and cash equivalents will be sufficient to fund its operating expenses and capital expenditure requirements into late second quarter of 2024.
About Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc., is a diversified medical technology and radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body. The Company has a proprietary technology that utilizes the alpha emitting isotope Lead-212 to deliver powerful radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides which provide the opportunity to personalize treatment and optimize patient outcomes. This "theranostic" approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments.
The Company's melanoma (VMT01) and neuroendocrine tumor (VMT-α-NET) programs have entered Phase 1/2a imaging and therapy trials for the treatment of metastatic melanoma and neuroendocrine tumors at several leading academic institutions in the United States. The Company has also developed a proprietary Lead-212 generator to secure key isotopes for clinical trial and commercial operations.
In addition to its targeted alpha therapy programs, Perspective is the sole producer of Cesium-131 brachytherapy seeds which is commercially available in the United States for the treatment of prostate cancer and other solid tumors.
For more information, please visit the Company's website at www.perspectivetherapeutics.com.
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Statements in this press release that are not statements of historical fact are forward-looking statements. Words such as "may," "will," "should," "expect," "plan," "anticipate," "could," "intend," "target," "project," "estimate," "believe," "predict," "potential" or "continue" or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements in this press release include statements concerning, among other things: the Company’s belief regarding the sufficiency of its cash resources to fund its operating expenses and capital expenditure requirements; the Company's clinical development plans and the expected timing thereof; the expected timing for availability and release of data; the Company’s timing and expectations regarding regulatory communications, submissions and approvals; expectations regarding the potential market opportunities for the Company's product candidates; the potential functionality, capabilities, and benefits of the Company's product candidates and the potential application of these product candidates for other disease indications; the Company's expectations, beliefs, intentions, and strategies regarding the future; the Company's expectations regarding the addition of clinical trial sites; the Company’s belief that research and development expenses may increase as the Company continues to advance its clinical programs; the Company's expectations regarding potential competitors; the Company's plans for bolstering sales efforts; the Company's expectations the impact of changes in reimbursement amounts for its products; and other statements that are not historical fact.
The Company may not actually achieve the plans, intentions or expectations disclosed in the forward-looking statements and you should not place undue reliance on the forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause the Company's actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation: the potential that regulatory authorities may not grant or may delay approval for the Company's product candidates; uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; the Company’s ability to continue as a going concern; early clinical trials may not be indicative of the results in later clinical trials; clinical trial results may not support regulatory approval or further development in a specified indication or at all; actions or advice of regulatory authorities may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company's ability to obtain and maintain regulatory approval for the Company's product candidates; delays, interruptions or failures in the manufacture and supply of the Company's product candidates; the size and growth potential of the markets for the Company's product candidates, and the Company's ability to service those markets; the Company's cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company's expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company's ability to obtain additional funding to support its clinical development programs; the availability or potential availability of alternative products or treatments for conditions targeted by the Company that could affect the availability or commercial potential of its product candidates; the ability of the Company to manage growth and successfully integrate its businesses; the Company's ability to maintain its key employees; whether there is sufficient training and use of the Company's products and product candidates; the market acceptance and recognition of the Company's products and product candidates; the Company's ability to maintain and enforce its intellectual property rights; the Company's ability to maintain its therapeutic isotope supply agreement with the Department of Energy; the Company's ability to continue to comply with the procedures and regulatory requirements mandated by the FDA for additional trials, Phase 1 and 2 approvals, Fast Track approvals, and 510(k) approval and reimbursement codes; and any changes in applicable laws and regulations. Other factors that may cause the Company's actual results to differ materially from those expressed or implied in the forward-looking statements in this press release are described under the heading "Risk Factors" in the Company's most recent Transition Report on Form 10-KT and the Company's most recent Quarterly Report on Form 10-Q, each filed with the Securities and Exchange Commission (the "SEC"), in the Company's other filings with the SEC, and in the Company's future reports to be filed with the SEC and available at www.sec.gov.
Forward-looking statements contained in this press release are made as of this date, and the Company undertakes no duty to update such information whether as a result of new information, future events or otherwise, except as required under applicable law.
https://www.wallstreet-online.de/nachricht/17546462-perspect…
IR Perspective Therapeutics
14.11.23
Perspective Therapeutics Reports Third Quarter Fiscal 2023 Results and Recent Business Highlights
SEATTLE, Nov. 14, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (NYSE AMERICAN: CATX), today announced third quarter financial results for the period ended September 30, 2023 and provided recent business highlights..
“Our commitment to advancing targeted alpha-particle therapies against neuroendocrine tumors remains steadfast as we have completed dosing of the first patient in the Phase 1/2a clinical trial of [212Pb]VMT-α-NET,” said Thijs Spoor, Perspective Therapeutics' CEO. “In addition, we completed recruitment for the first patient cohort in the Company-sponsored Phase 1/2a dose escalation/dose expansion clinical trial evaluating [212Pb]VMT01 for the treatment of histologically confirmed melanoma. We are nearing completion of the imaging portion of the clinical trial evaluating the suitability of [203Pb]VMT01 for SPECT/CT imaging.”
“We are pleased with our execution this quarter and continue to see positive momentum in the number of patients being treated with Cesium-131 each month, marking a return to growth as compared to the same period in the prior year,” stated Andrew Bright, Perspective Therapeutics’ Executive Vice President of Brachytherapy. “The rebound in the number of patients treated was driven by engagement with current customers, as well as new customers adopting Cesium-131 in their brachytherapy programs. Additionally, increased utilization of Cesium-131 is evident in our core prostate business, as well as in other disease areas, notably lung and brain brachytherapy.”
VMT-α-NET Recent Highlights
Company-sponsored Phase 1/2a clinical trial of [212Pb]VMT-α-NET
Dosed first patient in the Phase 1/2a clinical trial of [212Pb]VMT-α-NET for unresectable or metastatic SSTR2-expressing neuroendocrine tumors (NETs) at Washington University in St. Louis.
The Company currently has two active sites for the clinical trial and anticipates that two additional clinical trial sites will become operational in December.
The Company remains on track to launch an additional 14 clinical trial sites.
Investigator-Initiated clinical trials of [212Pb]VMT-α-NET
Presented early clinical findings at the 36th Annual Congress of the European Association of Nuclear Medicine (EANM) for the Phase 2a clinical trial of [212Pb]VMT-α-NET in pre- and post-Lutathera gastroenteropancreatic (GEP)-NET patients, being conducted at Fortis Healthcare, India. Dr. Dharmender Malik of Fortis Memorial Research Institute presented data on [212Pb]VMT-α-NET therapy in 10 patients who had failed prior treatment, including standard care. Initial data readout demonstrated high objective response rate for 7 of 9 evaluable patients, spanning peptide receptor radionuclide therapy (PRRT)-naïve and PRRT-refractory cases. Two additional patients were enrolled in the third quarter for a total of 9 GEP-NET patients and 2 medullary thyroid cancer (MTC) patients. All ongoing patients are expected to complete their 4th treatment cycle by the end of February 2024. Updated clinical results are expected to be presented at an upcoming scientific meeting in 2024.
Two patients were screened in the Phase 1/2a clinical trial for post-Lutathera GEP-NET patients at the University of Iowa. A third patient is scheduled for screening mid-November. If eligible, all three patients will receive treatment in December for the completion of the first cohort. The end of fourth cycle of treatment is expected in June 2024.
End-of-life compassionate use program for patients with advanced NETs and lack of further treatment options is underway at the University Dresden in Germany. Four patients were treated during the third quarter, and investigators are planning additional treatments before the end of the year.
IND submissions are expected in November with subsequent trial activation in the first half of 2024 for NIH-sponsored studies in pre- and post-Lutathera NET patients.
VMT01 Recent Highlights
Completed recruitment for the first patient cohort in the Company-sponsored Phase 1/2a dose escalation/dose expansion clinical trial of [212Pb]VMT01 in patients with histologically confirmed melanoma and positive MC1R imaging scans. Three patients have been dosed at 111 MBq (3mCi), closing cohort 1. No serious adverse events or dose-limiting toxicities have been observed. The Company anticipates opening the second patient cohort, which will receive 185 MBq (5mCi) per dose, in December 2023 and anticipates the completion of screening in the first quarter of 2024.
Nearing completion of analysis of the dosimetry portion of the Company’s TIMAR-1 clinical trial, a first-in-human study evaluating the suitability of [203Pb]VMT01 for SPECT/CT imaging and [68Ga]VMT02 for PET/CT imaging of MC1R-expressing metastatic melanoma. Perspective is actively working on the Clinical Study Report and anticipates it will be released in the first half of 2024.
Brachytherapy Recent Highlights
Third quarter 2023 revenue was the highest since the second quarter of 2022, with a return to growth over the third quarter of 2022.
Appointed sales, marketing and clinical team members along with a senior customer service representative to supplement Perspective’s dedicated commercial team.
Launched marketing initiatives to increase direct patient communication and engagement on Cesium-131 brachytherapy.
Facilitated timely access to GT Medical’s GammaTile for challenging brain cancers through increased production of Cesium-131 brachytherapy seeds that allowed GT Medical to address short notice orders with increased confidence.
Presented long-term data for Cesium-131 brachytherapy in the treatment of prostate cancer at the American Society for Radiation Oncology (ASTRO) annual conference. In his presentation, Dr. Mohamed Abdelhakiem of the UPMC Hillman Cancer Center described urinary data collected from 341 patients treated with Cesium-131 between 2006 and 2022.
Presented preliminary long-term data for Cesium-131 utilized in salvage treatment of recurrent cervical and uterine cancers at the ASTRO annual conference. Dr. Zeta Chow of the University of Kentucky showed data that indicated Cesium-131 brachytherapy is a promising alternative to pelvic exenteration, a significant surgical procedure.
Publication of peer reviewed article in the journal of Brachytherapy highlighting Cesium-131 clinical data from Dr. Benoit and colleagues at the University of Pittsburgh School of Medicine. The results support Cesium-131 and provide additional data on dose parameters for improved urinary side effect profile without affecting disease free survival. [https://www.brachyjournal.com/article/S1538-4721(23)01632-X/…
Discovery Program Milestones
Presented data at EANM. Dijie Liu, Principal Research Scientist at Perspective, presented mouse model data highlighting the efficacy of [203/212Pb]VMT-α-NET in treating metastatic neuroblastoma tumors. The study showed successful tumor uptake via sequential SPECT imaging and demonstrated a maximum tolerated dose of [212Pb]VMT-α-NET as 2.22 MBq without acute toxicity, with a 100% overall survival rate at 90-days observed in the group receiving three fractionated doses of 740 kBq of [212Pb]VMT-α-NET.
Presented collaborative data at the World Molecular Imaging Congress. Dr. Michael K. Schultz, Chief Science Officer of Perspective presented data highlighting the effectiveness of [212Pb]VMT-α-NET in treating neuroendocrine tumors in a tumor xenograft mouse model. The results highlighted the significant therapeutic efficacy of treatment with three fractionated doses of [212Pb]VMT-α-NET, which resulted in a 70% complete response rate and 80% survival at 120 days.
Presented encouraging data on novel guest/host platform for effective in vivo image-guided pre-targeted alpha particle therapy at the World Molecular Imaging Congress. Dr. Amritjyot Kaur of Stony Brook University showed that the radioligand [203Pb]PSC-PEG3-Adma demonstrated extended lag times, impressive tumor-to-tissue ratios and has the potential to further reduce radiation toxicity using [203Pb]PSC-PEG3-Adma as a guide.
Publication of a peer-reviewed article in Nuclear Medicine and Biology reporting on collaborative research with the University of Alberta, to accelerate the availability of imaging surrogate isotope Pb-203 for image-guided dosimetry planning of Pb-212 labeled radiopeptide conjugates. The collaboration has established an approach to production that can be readily adopted by other cyclotron facilities that produce high yields and excellent specific activity. [https://pubmed.ncbi.nlm.nih.gov/36708660/]
Third Quarter 2023 Financial Summary
Total revenue for the three months ended September 30, 2023 was $2.19 million, compared to $1.72 million for the same period in 2022, an increase of 27%.
Sales revenues for the quarter ended September 30, 2023, was $1.9 million, compared to $1.7 million for the same period in 2022, an increase of 11%.
Prostate brachytherapy represented 55% of total revenue for the quarter ended September 30, 2023 compared to 68% for the same period in 2022.
Gross profit was $738,000 for the three months ended September 30, 2023 compared to $414,000 for the same period in 2022.
Research and development expenses were $5.7 million for the three months ended September 30, 2023 compared to $708,000 for the same period in 2022, an increase of 708%. Management continues to believe that research and development expenses may increase as the Company continues to advance its clinical programs.
Total operating expenses for the quarter ended September 30, 2023 were $11.3 million, compared to $4.6 million for the same period in 2022, an increase of 144%.
Net loss for the three months ended September 30, 2023 was $10.4 million or $0.04 per share basic and diluted, compared to $4.1 million or $0.03 per share basic and diluted for the same period in 2022.
Cash and cash equivalents as of September 30, 2023 was $18.0 million as compared to $43.9 million on December 31, 2022. Based on the Company's current operating plan, the Company believes that its existing cash and cash equivalents will be sufficient to fund its operating expenses and capital expenditure requirements into late second quarter of 2024.
About Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc., is a diversified medical technology and radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body. The Company has a proprietary technology that utilizes the alpha emitting isotope Lead-212 to deliver powerful radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides which provide the opportunity to personalize treatment and optimize patient outcomes. This "theranostic" approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments.
The Company's melanoma (VMT01) and neuroendocrine tumor (VMT-α-NET) programs have entered Phase 1/2a imaging and therapy trials for the treatment of metastatic melanoma and neuroendocrine tumors at several leading academic institutions in the United States. The Company has also developed a proprietary Lead-212 generator to secure key isotopes for clinical trial and commercial operations.
In addition to its targeted alpha therapy programs, Perspective is the sole producer of Cesium-131 brachytherapy seeds which is commercially available in the United States for the treatment of prostate cancer and other solid tumors.
For more information, please visit the Company's website at www.perspectivetherapeutics.com.
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Statements in this press release that are not statements of historical fact are forward-looking statements. Words such as "may," "will," "should," "expect," "plan," "anticipate," "could," "intend," "target," "project," "estimate," "believe," "predict," "potential" or "continue" or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements in this press release include statements concerning, among other things: the Company’s belief regarding the sufficiency of its cash resources to fund its operating expenses and capital expenditure requirements; the Company's clinical development plans and the expected timing thereof; the expected timing for availability and release of data; the Company’s timing and expectations regarding regulatory communications, submissions and approvals; expectations regarding the potential market opportunities for the Company's product candidates; the potential functionality, capabilities, and benefits of the Company's product candidates and the potential application of these product candidates for other disease indications; the Company's expectations, beliefs, intentions, and strategies regarding the future; the Company's expectations regarding the addition of clinical trial sites; the Company’s belief that research and development expenses may increase as the Company continues to advance its clinical programs; the Company's expectations regarding potential competitors; the Company's plans for bolstering sales efforts; the Company's expectations the impact of changes in reimbursement amounts for its products; and other statements that are not historical fact.
The Company may not actually achieve the plans, intentions or expectations disclosed in the forward-looking statements and you should not place undue reliance on the forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause the Company's actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation: the potential that regulatory authorities may not grant or may delay approval for the Company's product candidates; uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; the Company’s ability to continue as a going concern; early clinical trials may not be indicative of the results in later clinical trials; clinical trial results may not support regulatory approval or further development in a specified indication or at all; actions or advice of regulatory authorities may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company's ability to obtain and maintain regulatory approval for the Company's product candidates; delays, interruptions or failures in the manufacture and supply of the Company's product candidates; the size and growth potential of the markets for the Company's product candidates, and the Company's ability to service those markets; the Company's cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company's expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company's ability to obtain additional funding to support its clinical development programs; the availability or potential availability of alternative products or treatments for conditions targeted by the Company that could affect the availability or commercial potential of its product candidates; the ability of the Company to manage growth and successfully integrate its businesses; the Company's ability to maintain its key employees; whether there is sufficient training and use of the Company's products and product candidates; the market acceptance and recognition of the Company's products and product candidates; the Company's ability to maintain and enforce its intellectual property rights; the Company's ability to maintain its therapeutic isotope supply agreement with the Department of Energy; the Company's ability to continue to comply with the procedures and regulatory requirements mandated by the FDA for additional trials, Phase 1 and 2 approvals, Fast Track approvals, and 510(k) approval and reimbursement codes; and any changes in applicable laws and regulations. Other factors that may cause the Company's actual results to differ materially from those expressed or implied in the forward-looking statements in this press release are described under the heading "Risk Factors" in the Company's most recent Transition Report on Form 10-KT and the Company's most recent Quarterly Report on Form 10-Q, each filed with the Securities and Exchange Commission (the "SEC"), in the Company's other filings with the SEC, and in the Company's future reports to be filed with the SEC and available at www.sec.gov.
Forward-looking statements contained in this press release are made as of this date, and the Company undertakes no duty to update such information whether as a result of new information, future events or otherwise, except as required under applicable law.
https://www.wallstreet-online.de/nachricht/17546462-perspect…
Antwort auf Beitrag Nr.: 74.807.612 von Edelweiss70 am 15.11.23 09:06:18Perspective Therapeutics Completes Initial Dose Escalation Cohort for Second Novel Targeted Alpha Therapy
November 16, 2023
SEATTLE, Nov. 16, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (NYSE AMERICAN: CATX), today announced the completion of cohort 1 dosing in the Phase 1/2a clinical trial of [212Pb]VMT-α-NET in patients with unresectable or metastatic somatostatin receptor type 2 (SSTR2)-expressing neuroendocrine tumors (NETs). This milestone follows the recent completion of the first dose escalation cohort for Perspective's melanoma therapeutic [212Pb]VMT01.
"We are pleased with the progress of our Company-sponsored clinical evaluation of [212Pb]VMT-α-NET in patients with difficult-to-treat SSTR2-expressing NETs. The rapid recruitment into this study reflects the demand for potent alpha-particle therapies that exists in this patient population," commented Markus Puhlmann, Chief Medical Officer of Perspective Therapeutics. "We are grateful to our collaborator, Nikolaos Trikalinos, MD, Medical Oncologist at Washington University in St. Louis, who serves as the referring medical oncologist for the study and ensures that patients can access our potentially life-changing therapeutic. With both of our investigational therapies, [212Pb]VMT-α-NET and [212Pb]VMT01, now having completed their first cohorts, we look forward to generating data at higher doses early in 2024."
The Company currently has two active clinical trial sites for the trial and anticipates that two additional sites will become operational in December, coinciding with the launch of cohort 2. Overall, the Company remains on track to launch an additional 14 clinical trial sites throughout the U.S. including the Mayo Clinic, the University of Iowa and more.
About The Study
This is a multi-center open-label clinical trial (clinicaltrials.gov identifier NCT05636618) of [212Pb]VMT-α-NET, a targeted alpha-particle therapy, for patients with advanced SSTR2-positive neuroendocrine tumors. The first part of this Phase 1/2a trial is a dose-escalation study designed to determine the Maximum Tolerated Dose ("MTD") or Maximum Feasible Dose ("MFD") following a single administration of [212Pb]VMT-α-NET. Patients who have not received prior peptide receptor radionuclide therapy will be scheduled to receive up to 4 administrations of [212Pb]VMT-α-NET approximately 8 weeks apart. The first patient cohort will receive 111 MBq (3mCi) per dose. The second cohort will receive administered activities of 185 MBq (5mCi), with cohorts 3 and 4 receiving 370 MBq (10 mCi) and 555 MBq (15 mCi), respectively, if the MTD or MFD is not reached during escalation. According to the Modified Toxicity Probability Interval 2 (mTPI-2) study design, intermediate de-escalation doses are also possible to allow selection of the optimal activity dose to take forward into the dose expansion part of the study.
The second part of the trial is a dose expansion phase based on the identified MTD/MFD. Patients with positive uptake on FDA-approved SSTR2 PET/CT will receive a fixed dose of [212Pb]VMT-α-NET IV administered at the recommended Phase 2 dose and schedule determined in the Phase I dose escalation.
About Neuroendocrine Tumors
Neuroendocrine tumors form in cells that interact with the nervous system or in glands that produce hormones. They can originate in various parts of the body, most often in the gut or the lungs and can be benign or malignant. Neuroendocrine tumors are typically classified as pancreatic neuroendocrine tumors or non-pancreatic neuroendocrine tumors. According to cancer.net, it is estimated that more than 12,000 people in the United States are diagnosed with a NET each year. Importantly, neuroendocrine tumors are associated with a relatively long duration of survival compared to other tumors and as a result, there are approximately 175,000 people living with this diagnosis.
About VMT-α-NET
VMT-α-NET is a clinical stage targeted alpha particle therapy (TAT) radiopharmaceutical being developed for the treatment and diagnosis of somatostatin receptor subtype 2 (SSTR2) expressing neuroendocrine tumors, which are a rare and difficult-to-treat type of cancer. VMT-α-NET incorporates Perspective Therapeutics' proprietary lead-specific chelator (PSC) to bind 203Pb for SPECT imaging, and 212Pb for alpha particle therapy.
About Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc., is a diversified medical technology and radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body. The Company has a proprietary technology that utilizes the alpha emitting isotope Lead-212 to deliver powerful radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides which provide the opportunity to personalize treatment and optimize patient outcomes. This "theranostic" approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments.
The Company's melanoma (VMT01) and neuroendocrine tumor (VMT-α-NET) programs have entered Phase 1/2a imaging and therapy trials for the treatment of metastatic melanoma and neuroendocrine tumors at several leading academic institutions in the United States. The Company has also developed a proprietary Lead-212 generator to secure key isotopes for clinical trial and commercial operations.
In addition to its targeted alpha therapy programs, Perspective is the sole producer of Cesium-131 brachytherapy seeds which is commercially available in the United States for the treatment of prostate cancer and other solid tumors.
For more information, please visit the Company's website at www.perspectivetherapeutics.com.
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Statements in this press release that are not statements of historical fact are forward-looking statements. Words such as "may," "will," "should," "expect," "plan," "anticipate," "could," "intend," "target," "project," "estimate," "believe," "predict," "potential" or "continue" or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements in this press release include statements concerning, among other things: the potential for [212Pb]VMT-α-NET to be a life-changing therapeutic for patients with difficult-to-treat SSTR2-expressing NETs, the Company's expectations regarding the addition of clinical trial sites; the Company's clinical development plans and the expected timing thereof; the expected timing for availability and release of data; expectations regarding the potential market opportunities for the Company's product candidates; the potential functionality, capabilities, and benefits of the Company's product candidates and the potential application of these product candidates for other disease indications; the Company's expectations, beliefs, intentions, and strategies regarding the future; and other statements that are not historical fact.
The Company may not actually achieve the plans, intentions or expectations disclosed in the forward-looking statements and you should not place undue reliance on the forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause the Company's actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation: the potential that regulatory authorities may not grant or may delay approval for the Company's product candidates; uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; the Company's ability to continue as a going concern; early clinical trials may not be indicative of the results in later clinical trials; clinical trial results may not support regulatory approval or further development in a specified indication or at all; actions or advice of regulatory authorities may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company's ability to obtain and maintain regulatory approval for the Company's product candidates; delays, interruptions or failures in the manufacture and supply of the Company's product candidates; the size and growth potential of the markets for the Company's product candidates, and the Company's ability to service those markets; the Company's cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company's expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company's ability to obtain additional funding to support its clinical development programs; the availability or potential availability of alternative products or treatments for conditions targeted by the Company that could affect the availability or commercial potential of its product candidates; the ability of the Company to manage growth and successfully integrate its businesses; the Company's ability to maintain its key employees; whether there is sufficient training and use of the Company's products and product candidates; the market acceptance and recognition of the Company's products and product candidates; the Company's ability to maintain and enforce its intellectual property rights; the Company's ability to maintain its therapeutic isotope supply agreement with the Department of Energy; the Company's ability to continue to comply with the procedures and regulatory requirements mandated by the FDA for additional trials, Phase 1 and 2 approvals, Fast Track approvals, and 510(k) approval and reimbursement codes; and any changes in applicable laws and regulations. Other factors that may cause the Company's actual results to differ materially from those expressed or implied in the forward-looking statements in this press release are described under the heading "Risk Factors" in the Company's most recent Transition Report on Form 10-KT and the Company's most recent Quarterly Report on Form 10-Q, each filed with the Securities and Exchange Commission (the "SEC"), in the Company's other filings with the SEC, and in the Company's future reports
Investor Relations Contact:
LifeSci Advisors
Chuck Padala
E: chuck@lifesciadvisors.com
Primary Logo
Company
Home
About us
Newsroom
Investor center
Contact us
Brachytherapy
Why Brachytherapy?
Why Cesium-131?
Find a Doctor
Theranostics
Technology
a-Particle Radiotherapy
VMT-a-GEN
Pipeline
Sign up for newsletter
Want to keep up-to-date on news and information?
Terms & Privacy Privacy Policy Expanded Access Policy
Investor Relations Website By VendorGroup
November 16, 2023
SEATTLE, Nov. 16, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (NYSE AMERICAN: CATX), today announced the completion of cohort 1 dosing in the Phase 1/2a clinical trial of [212Pb]VMT-α-NET in patients with unresectable or metastatic somatostatin receptor type 2 (SSTR2)-expressing neuroendocrine tumors (NETs). This milestone follows the recent completion of the first dose escalation cohort for Perspective's melanoma therapeutic [212Pb]VMT01.
"We are pleased with the progress of our Company-sponsored clinical evaluation of [212Pb]VMT-α-NET in patients with difficult-to-treat SSTR2-expressing NETs. The rapid recruitment into this study reflects the demand for potent alpha-particle therapies that exists in this patient population," commented Markus Puhlmann, Chief Medical Officer of Perspective Therapeutics. "We are grateful to our collaborator, Nikolaos Trikalinos, MD, Medical Oncologist at Washington University in St. Louis, who serves as the referring medical oncologist for the study and ensures that patients can access our potentially life-changing therapeutic. With both of our investigational therapies, [212Pb]VMT-α-NET and [212Pb]VMT01, now having completed their first cohorts, we look forward to generating data at higher doses early in 2024."
The Company currently has two active clinical trial sites for the trial and anticipates that two additional sites will become operational in December, coinciding with the launch of cohort 2. Overall, the Company remains on track to launch an additional 14 clinical trial sites throughout the U.S. including the Mayo Clinic, the University of Iowa and more.
About The Study
This is a multi-center open-label clinical trial (clinicaltrials.gov identifier NCT05636618) of [212Pb]VMT-α-NET, a targeted alpha-particle therapy, for patients with advanced SSTR2-positive neuroendocrine tumors. The first part of this Phase 1/2a trial is a dose-escalation study designed to determine the Maximum Tolerated Dose ("MTD") or Maximum Feasible Dose ("MFD") following a single administration of [212Pb]VMT-α-NET. Patients who have not received prior peptide receptor radionuclide therapy will be scheduled to receive up to 4 administrations of [212Pb]VMT-α-NET approximately 8 weeks apart. The first patient cohort will receive 111 MBq (3mCi) per dose. The second cohort will receive administered activities of 185 MBq (5mCi), with cohorts 3 and 4 receiving 370 MBq (10 mCi) and 555 MBq (15 mCi), respectively, if the MTD or MFD is not reached during escalation. According to the Modified Toxicity Probability Interval 2 (mTPI-2) study design, intermediate de-escalation doses are also possible to allow selection of the optimal activity dose to take forward into the dose expansion part of the study.
The second part of the trial is a dose expansion phase based on the identified MTD/MFD. Patients with positive uptake on FDA-approved SSTR2 PET/CT will receive a fixed dose of [212Pb]VMT-α-NET IV administered at the recommended Phase 2 dose and schedule determined in the Phase I dose escalation.
About Neuroendocrine Tumors
Neuroendocrine tumors form in cells that interact with the nervous system or in glands that produce hormones. They can originate in various parts of the body, most often in the gut or the lungs and can be benign or malignant. Neuroendocrine tumors are typically classified as pancreatic neuroendocrine tumors or non-pancreatic neuroendocrine tumors. According to cancer.net, it is estimated that more than 12,000 people in the United States are diagnosed with a NET each year. Importantly, neuroendocrine tumors are associated with a relatively long duration of survival compared to other tumors and as a result, there are approximately 175,000 people living with this diagnosis.
About VMT-α-NET
VMT-α-NET is a clinical stage targeted alpha particle therapy (TAT) radiopharmaceutical being developed for the treatment and diagnosis of somatostatin receptor subtype 2 (SSTR2) expressing neuroendocrine tumors, which are a rare and difficult-to-treat type of cancer. VMT-α-NET incorporates Perspective Therapeutics' proprietary lead-specific chelator (PSC) to bind 203Pb for SPECT imaging, and 212Pb for alpha particle therapy.
About Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc., is a diversified medical technology and radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body. The Company has a proprietary technology that utilizes the alpha emitting isotope Lead-212 to deliver powerful radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides which provide the opportunity to personalize treatment and optimize patient outcomes. This "theranostic" approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments.
The Company's melanoma (VMT01) and neuroendocrine tumor (VMT-α-NET) programs have entered Phase 1/2a imaging and therapy trials for the treatment of metastatic melanoma and neuroendocrine tumors at several leading academic institutions in the United States. The Company has also developed a proprietary Lead-212 generator to secure key isotopes for clinical trial and commercial operations.
In addition to its targeted alpha therapy programs, Perspective is the sole producer of Cesium-131 brachytherapy seeds which is commercially available in the United States for the treatment of prostate cancer and other solid tumors.
For more information, please visit the Company's website at www.perspectivetherapeutics.com.
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Statements in this press release that are not statements of historical fact are forward-looking statements. Words such as "may," "will," "should," "expect," "plan," "anticipate," "could," "intend," "target," "project," "estimate," "believe," "predict," "potential" or "continue" or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements in this press release include statements concerning, among other things: the potential for [212Pb]VMT-α-NET to be a life-changing therapeutic for patients with difficult-to-treat SSTR2-expressing NETs, the Company's expectations regarding the addition of clinical trial sites; the Company's clinical development plans and the expected timing thereof; the expected timing for availability and release of data; expectations regarding the potential market opportunities for the Company's product candidates; the potential functionality, capabilities, and benefits of the Company's product candidates and the potential application of these product candidates for other disease indications; the Company's expectations, beliefs, intentions, and strategies regarding the future; and other statements that are not historical fact.
The Company may not actually achieve the plans, intentions or expectations disclosed in the forward-looking statements and you should not place undue reliance on the forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause the Company's actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation: the potential that regulatory authorities may not grant or may delay approval for the Company's product candidates; uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; the Company's ability to continue as a going concern; early clinical trials may not be indicative of the results in later clinical trials; clinical trial results may not support regulatory approval or further development in a specified indication or at all; actions or advice of regulatory authorities may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company's ability to obtain and maintain regulatory approval for the Company's product candidates; delays, interruptions or failures in the manufacture and supply of the Company's product candidates; the size and growth potential of the markets for the Company's product candidates, and the Company's ability to service those markets; the Company's cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company's expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company's ability to obtain additional funding to support its clinical development programs; the availability or potential availability of alternative products or treatments for conditions targeted by the Company that could affect the availability or commercial potential of its product candidates; the ability of the Company to manage growth and successfully integrate its businesses; the Company's ability to maintain its key employees; whether there is sufficient training and use of the Company's products and product candidates; the market acceptance and recognition of the Company's products and product candidates; the Company's ability to maintain and enforce its intellectual property rights; the Company's ability to maintain its therapeutic isotope supply agreement with the Department of Energy; the Company's ability to continue to comply with the procedures and regulatory requirements mandated by the FDA for additional trials, Phase 1 and 2 approvals, Fast Track approvals, and 510(k) approval and reimbursement codes; and any changes in applicable laws and regulations. Other factors that may cause the Company's actual results to differ materially from those expressed or implied in the forward-looking statements in this press release are described under the heading "Risk Factors" in the Company's most recent Transition Report on Form 10-KT and the Company's most recent Quarterly Report on Form 10-Q, each filed with the Securities and Exchange Commission (the "SEC"), in the Company's other filings with the SEC, and in the Company's future reports
Investor Relations Contact:
LifeSci Advisors
Chuck Padala
E: chuck@lifesciadvisors.com
Primary Logo
Company
Home
About us
Newsroom
Investor center
Contact us
Brachytherapy
Why Brachytherapy?
Why Cesium-131?
Find a Doctor
Theranostics
Technology
a-Particle Radiotherapy
VMT-a-GEN
Pipeline
Sign up for newsletter
Want to keep up-to-date on news and information?
Terms & Privacy Privacy Policy Expanded Access Policy
Investor Relations Website By VendorGroup
Antwort auf Beitrag Nr.: 74.816.756 von Edelweiss70 am 16.11.23 15:31:59
SEATTLE, Nov. 27, 2023 (GLOBE NEWSWIRE) --
Perspective, Therapeutics, Inc. (NYSE AMERICAN: CATX), today announced the publication of four preclinical studies in support of the Company’s discovery pipeline. The studies were published in the European Journal of Nuclear Medicine and Molecular Imaging, Journal of Nuclear Medicine, and Pharmaceuticals.
“The results from these preclinical studies are compelling and continue to support our ongoing clinical efforts to advance our novel lead-based targeted alpha-particle therapies. Overall, these data demonstrate a dose-dependent therapeutic benefit of our treatment candidate [212Pb]VMT-α-NET in mouse models, and also demonstrate the high radiolabeling yields and high radiochemical purity and stability that are achievable using our radiopharmaceutical production process,” commented Michael Schultz, PhD, Chief Science Officer of Perspective Therapeutics. “Additionally, our collaborative investigations with our academic research partners are enhancing our understanding of biomarkers (e.g., NGal) that can be used in optimizing the therapeutic window for not only our alpha-particle therapies, but all tumor targeted radiopharmaceutical therapies. The use of these biomarkers has the potential to improve our ability to monitor patient safety and optimize the effectiveness of treatment. We are grateful to all our collaborators and look forward to generating and contributing additional data to our community in 2024.”
SEATTLE, Nov. 27, 2023 (GLOBE NEWSWIRE) --
Perspective, Therapeutics, Inc. (NYSE AMERICAN: CATX), today announced the publication of four preclinical studies in support of the Company’s discovery pipeline. The studies were published in the European Journal of Nuclear Medicine and Molecular Imaging, Journal of Nuclear Medicine, and Pharmaceuticals.
“The results from these preclinical studies are compelling and continue to support our ongoing clinical efforts to advance our novel lead-based targeted alpha-particle therapies. Overall, these data demonstrate a dose-dependent therapeutic benefit of our treatment candidate [212Pb]VMT-α-NET in mouse models, and also demonstrate the high radiolabeling yields and high radiochemical purity and stability that are achievable using our radiopharmaceutical production process,” commented Michael Schultz, PhD, Chief Science Officer of Perspective Therapeutics. “Additionally, our collaborative investigations with our academic research partners are enhancing our understanding of biomarkers (e.g., NGal) that can be used in optimizing the therapeutic window for not only our alpha-particle therapies, but all tumor targeted radiopharmaceutical therapies. The use of these biomarkers has the potential to improve our ability to monitor patient safety and optimize the effectiveness of treatment. We are grateful to all our collaborators and look forward to generating and contributing additional data to our community in 2024.”
Publication One
Title: Influence of the Molar Activity of 203/212Pb-PSC-PEG2-TOC on Somatostatin Receptor Type 2-Binding and Cell Uptake
Summary: This study in Pharmaceuticals (November 2023, 16(11), 1605) notes that the molar activity (AM) can play a crucial role in tumor uptake, especially in receptor-mediated uptake, such as in neuroendocrine tumors. In this work, the influence of the AM-to-cell uptake of 203/212Pb-PSC-TOC was investigated in multiple different cell lines to develop a more detailed understanding of the tracer in preparation for clinical use. This study provides independent confirmatory evidence of the stability of the 212Bi daughter radionuclide in formulation upon the decay of 212Pb, generating >95% radiochemical purity in their study.
Key Highlights:
The quality control showed a radiochemical yield greater than 95% in most cases for 203/212Pb-PSC-TOC, confirming stability of the 212Bi in the Perspective chelator.
Higher AM, correlated positively with cell uptake. A moderate AM of 15–40 MBq/nmol showed the highest cell uptake.
No uptake limitation was found in the first 24 to 48 hours. However, authors suggest that further escalation experiments with higher AM should be performed.
Author Affiliations: University Hospital Carl Gustav Carus, Technical University Dresden, Dresden, Germany; The University of Iowa, Iowa City, Iowa, USA.
Publication Two
Title: Structural Modifications Toward Improved Lead-203/Lead-212 Peptide-Based Image-Guided Alpha-Particle Radiopharmaceutical Therapies for Neuroendocrine Tumors
Summary: This study in the European Journal of Nuclear Medicine and Molecular Imaging (in press as of November 2023) aims to improve the performance of somatostatin receptor subtype 2 (SSTR2)-targeted radionuclide imaging and therapy through structural modifications to Tyr3-octreotide (TOC)-based radiopharmaceuticals. The findings suggest that PSC-PEG2-TOC (i.e., VMT-a-NET) is a promising candidate for Pb-based targeted therapy for SSTR2 positive tumors.
Key Highlights:
The data demonstrated that the modified radiopeptide drug conjugate (RPDC), [203Pb]PSC-PEG2-TOC, significantly improved tumor-targeting properties, including receptor binding, tumor accumulation and retention, and exhibited faster renal clearance as compared to [203Pb]-DOTATOC.
Treatment resulted in a dose-dependent therapeutic effect with minimal signs of toxicity in a tumor xenograft mouse model.
Fractionated administrations of 3.7 MBq [212Pb]PSC-PEG2-TOC over three doses further improved antitumor effectiveness, resulting in 80% survival (70% complete response) over 120 days in the mouse model.
Author Affiliations: Korea Military Academy, Seoul, Republic of Korea; Perspective Therapeutics, Coralville, Iowa, USA; The University of Iowa, Iowa City, Iowa, USA.
Publication Three
Title: Optimized Methods for the Production of High-Purity 203Pb Using Electroplated Thallium Targets
Summary: This study published in the Journal of Nuclear Medicine (November 2023, 64 (11) 1791-1797) aims to optimize the production and separation of high specific activity 203Pb using electroplated Thallium targets suitable for cyclotron irradiation. This work contributes to the broader body of knowledge to satisfy the growing interest in clinical applications for theranostic match pair (203Pb/212Pb).
Key Highlights:
The data confirmed the development of an efficient electroplating method for routine 203Pb production.
The data demonstrated that 203Pb production was fast and could be achieved in under 1.5 hours, the method employed a simple separation process that resulted in high recovery yields of approximately 95%, and high purity.
The study showed that the novel electroplating method is compatible with a 24 MeV incident beam and high current without using a degrader.
Results corroborated evidence published in a recent article by our colleagues at the University of Alberta (Nelson et al., Nuclear Medicine and Biology 116–117 (2023) 108314) that the production of 203Pb is relatively straightforward and scalable.
Author Affiliations: University of Alabama at Birmingham, Alabama, USA; Perspective Therapeutics, Coralville, Iowa, USA; The University of Iowa, Iowa City, Iowa, USA.
Publication Four
Title: Pre-clinical Evaluation of Biomarkers for Early Detection of Nephrotoxicity Following Alpha-Particle Radioligand Therapy
Summary:
This publication in the European Journal of Nuclear Medicine and Molecular Imaging 2023 (in press as of November 2023) highlights findings from a pre-clinical study evaluating biomarkers for early detection of nephrotoxicity following alpha-particle radioligand therapy (α-RLT). The results suggest that increased biomarker urinal neutrophil gelatinase-associated lipocalin (NGAL) secretion could be an additional diagnostic tool to improve our understanding of the potential for subclinical acute kidney injury (AKI) arising from radionuclide therapies and have the potential to guide more effective therapies with a lower risk of chronic kidney disease (CKD).
Key Highlights:
Early secretion of urinary biomarker NGAL may be a valuable tool for early detection of kidney injury in response to radioligand therapies and has the potential to assist in optimizing therapies.
Identification and feasibility of the use of urinary epidermal growth factor as a biomarker response readout that may provide evidence of early tubular damage in response to radioligand therapies.
Author Affiliations: Nationwide Children's Hospital, Columbus, Ohio, USA; The University of Iowa, Iowa City, Iowa, USA; Korea Military Academy, Seoul, Republic of Korea; and The Ohio State University College of Veterinary Medicine, Columbus, Ohio, USA.
so jetzt ist alles komplett ,habe eben nicht gesehen das die Meldung noch weiter ging
Title: Influence of the Molar Activity of 203/212Pb-PSC-PEG2-TOC on Somatostatin Receptor Type 2-Binding and Cell Uptake
Summary: This study in Pharmaceuticals (November 2023, 16(11), 1605) notes that the molar activity (AM) can play a crucial role in tumor uptake, especially in receptor-mediated uptake, such as in neuroendocrine tumors. In this work, the influence of the AM-to-cell uptake of 203/212Pb-PSC-TOC was investigated in multiple different cell lines to develop a more detailed understanding of the tracer in preparation for clinical use. This study provides independent confirmatory evidence of the stability of the 212Bi daughter radionuclide in formulation upon the decay of 212Pb, generating >95% radiochemical purity in their study.
Key Highlights:
The quality control showed a radiochemical yield greater than 95% in most cases for 203/212Pb-PSC-TOC, confirming stability of the 212Bi in the Perspective chelator.
Higher AM, correlated positively with cell uptake. A moderate AM of 15–40 MBq/nmol showed the highest cell uptake.
No uptake limitation was found in the first 24 to 48 hours. However, authors suggest that further escalation experiments with higher AM should be performed.
Author Affiliations: University Hospital Carl Gustav Carus, Technical University Dresden, Dresden, Germany; The University of Iowa, Iowa City, Iowa, USA.
Publication Two
Title: Structural Modifications Toward Improved Lead-203/Lead-212 Peptide-Based Image-Guided Alpha-Particle Radiopharmaceutical Therapies for Neuroendocrine Tumors
Summary: This study in the European Journal of Nuclear Medicine and Molecular Imaging (in press as of November 2023) aims to improve the performance of somatostatin receptor subtype 2 (SSTR2)-targeted radionuclide imaging and therapy through structural modifications to Tyr3-octreotide (TOC)-based radiopharmaceuticals. The findings suggest that PSC-PEG2-TOC (i.e., VMT-a-NET) is a promising candidate for Pb-based targeted therapy for SSTR2 positive tumors.
Key Highlights:
The data demonstrated that the modified radiopeptide drug conjugate (RPDC), [203Pb]PSC-PEG2-TOC, significantly improved tumor-targeting properties, including receptor binding, tumor accumulation and retention, and exhibited faster renal clearance as compared to [203Pb]-DOTATOC.
Treatment resulted in a dose-dependent therapeutic effect with minimal signs of toxicity in a tumor xenograft mouse model.
Fractionated administrations of 3.7 MBq [212Pb]PSC-PEG2-TOC over three doses further improved antitumor effectiveness, resulting in 80% survival (70% complete response) over 120 days in the mouse model.
Author Affiliations: Korea Military Academy, Seoul, Republic of Korea; Perspective Therapeutics, Coralville, Iowa, USA; The University of Iowa, Iowa City, Iowa, USA.
Publication Three
Title: Optimized Methods for the Production of High-Purity 203Pb Using Electroplated Thallium Targets
Summary: This study published in the Journal of Nuclear Medicine (November 2023, 64 (11) 1791-1797) aims to optimize the production and separation of high specific activity 203Pb using electroplated Thallium targets suitable for cyclotron irradiation. This work contributes to the broader body of knowledge to satisfy the growing interest in clinical applications for theranostic match pair (203Pb/212Pb).
Key Highlights:
The data confirmed the development of an efficient electroplating method for routine 203Pb production.
The data demonstrated that 203Pb production was fast and could be achieved in under 1.5 hours, the method employed a simple separation process that resulted in high recovery yields of approximately 95%, and high purity.
The study showed that the novel electroplating method is compatible with a 24 MeV incident beam and high current without using a degrader.
Results corroborated evidence published in a recent article by our colleagues at the University of Alberta (Nelson et al., Nuclear Medicine and Biology 116–117 (2023) 108314) that the production of 203Pb is relatively straightforward and scalable.
Author Affiliations: University of Alabama at Birmingham, Alabama, USA; Perspective Therapeutics, Coralville, Iowa, USA; The University of Iowa, Iowa City, Iowa, USA.
Publication Four
Title: Pre-clinical Evaluation of Biomarkers for Early Detection of Nephrotoxicity Following Alpha-Particle Radioligand Therapy
Summary:
This publication in the European Journal of Nuclear Medicine and Molecular Imaging 2023 (in press as of November 2023) highlights findings from a pre-clinical study evaluating biomarkers for early detection of nephrotoxicity following alpha-particle radioligand therapy (α-RLT). The results suggest that increased biomarker urinal neutrophil gelatinase-associated lipocalin (NGAL) secretion could be an additional diagnostic tool to improve our understanding of the potential for subclinical acute kidney injury (AKI) arising from radionuclide therapies and have the potential to guide more effective therapies with a lower risk of chronic kidney disease (CKD).
Key Highlights:
Early secretion of urinary biomarker NGAL may be a valuable tool for early detection of kidney injury in response to radioligand therapies and has the potential to assist in optimizing therapies.
Identification and feasibility of the use of urinary epidermal growth factor as a biomarker response readout that may provide evidence of early tubular damage in response to radioligand therapies.
Author Affiliations: Nationwide Children's Hospital, Columbus, Ohio, USA; The University of Iowa, Iowa City, Iowa, USA; Korea Military Academy, Seoul, Republic of Korea; and The Ohio State University College of Veterinary Medicine, Columbus, Ohio, USA.
so jetzt ist alles komplett ,habe eben nicht gesehen das die Meldung noch weiter ging
Perspective Therapeutics Announces First Ever Human SPECT Imaging of Pb-212 with VMT-α-NET by Clinical Collaborators in Germany
Imaging was conducted as part of a clinical study at Technical University of Dresden in Germany
Image was selected as “Image of the Month” in the European Journal of Nuclear Medicine and Molecular Imaging, November 2023
Radiolabeling was performed on-site using a Company-provided generator
SEATTLE, Nov. 28, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), today announced the publication of the first human SPECT images utilizing the alpha-emitting isotope of 212Pb, which was labeled to the Company’s proprietary theranostic VMT-α-NET product. The imaging was conducted as part of a series of four neuroendocrine tumor (NET) patients who were administered VMT-α-NET at a clinical study site in Germany. The Company is developing VMT-α-NET for the treatment and diagnosis of somatostatin receptor subtype 2 (SSTR2)-expressing neuroendocrine tumors.
VMT-α-NET is being administered under the supervision of Prof. Dr. Jörg Kotzerke, Director of the Department of Nuclear Medicine at the Technical University of Dresden in Germany. The patient received 90 MBq (2.4mCi) of [212Pb]VMT-α-NET intravenously, and whole-body scintigraphy and SPECT/CT images were acquired 2 hours, 5 hours, and 19 hours after injection. Images were collected on a Symbia Intevo T6 (Siemens Healthineers) using a high-energy collimator. The SPECT/CT images showed high accumulation of [212Pb]VMT-α-NET in liver metastases and were consistent with the previously acquired [68Ga]DOTATATE PET/CT. High tumor retention can be observed in the planar and SPECT/CT images over time. As expected, due to the short half-life of 212Pb (10.6 hours), the images acquired after 19 hours showed a high level of noise due to the low count statistics. The patient showed no early or acute side effects.
“We are pleased to be able to provide our patients with Perspective’s novel targeted alpha-particle therapy. This imaging study represents a world-first and shows that [212Pb]VMT-α-NET can be imaged after administration,” commented Dr. Kotzerke. “Particularly encouraging is the uptake and the retention in the tumors, and the concordance of this data with existing standard of care imaging. NET patients are in desperate need for new treatment options that are safe and effective.”
Under certain expanded access circumstances, VMT-α-NET may be made available to qualified doctors in some countries. Perspective Therapeutics supplies a generator, drug precursors and isotopes for the local production of its proprietary radiotherapeutic, VMT-α-NET.
"Expanding our collaboration with Prof. Dr. Kotzerke and his colleagues at the Technical University of Dresden is a key milestone as we work to improve access to VMT-α-NET, our potentially life-changing treatment, to patients with burdensome NETs,” said Thijs Spoor, Chief Executive Officer at Perspective Therapeutics. “The use of post-therapy SPECT imaging to confirm tumor uptake and clearance from healthy organs is of particular interest moving forward, as it means that post-therapy dosimetry can be used in next-cycle treatment planning by physicians using our products. We trust the healthcare systems that value dosimetry and patient safety will work with us to be able to have this covered as a routine part of patient care.”
Dr. Kotzerke and his team will continue to monitor patients’ progress over the coming months. The publication is entitled “First-in-human SPECT/CT imaging of [212Pb]Pb-VMT-α-NET in a patient with metastatic neuroendocrine tumor” and is available in the European Journal of Nuclear Medicine and Molecular Imaging at https://link.springer.com/article/10.1007/s00259-023-06529-1… It follows a similar publication in Clinical Nuclear Medicine from earlier this year showing successful SPECT imaging of Perspective’s surrogate diagnostic isotope 203Pb which was also linked to VMT-α-NET.
Recruitment is ongoing for the use of VMT-α-NET in imaging and therapeutic trials at leading U.S. institutions. VMT-α-NET is categorized as an investigational new drug by the U.S. Food and Drug Administration. The use of VMT-α-NET in qualifying expanded access situations is entirely separate from and outside the scope of the Company's Phase 1 trial.
Michler, E., Kästner, D., Brogsitter, C. et al. First-in-human SPECT/CT imaging of [212Pb]Pb-VMT-α-NET in a patient with metastatic neuroendocrine tumor. Eur J Nucl Med Mol Imaging (2023). https://doi.org/10.1007/s00259-023-06529-1
About Neuroendocrine Tumors
Neuroendocrine tumors form in cells that interact with the nervous system or in glands that produce hormones. They can originate in various parts of the body, most often in the gut or the lungs and can be benign or malignant. Neuroendocrine tumors are typically classified as pancreatic neuroendocrine tumors or non-pancreatic neuroendocrine tumors. According to cancer.net, it is estimated that more than 12,000 people in the United States are diagnosed with a NET each year. Importantly, neuroendocrine tumors are associated with a relatively long duration of survival compared to other tumors and as a result, there are approximately 175,000 people living with this diagnosis
About VMT-α-NET
VMT-α-NET is a clinical stage targeted alpha particle therapy (TAT) radiopharmaceutical being developed for the treatment and diagnosis of somatostatin receptor subtype 2 (SSTR2) expressing neuroendocrine tumors, which are a rare and difficult-to-treat type of cancer. VMT-α-NET incorporates Perspective Therapeutics' proprietary lead-specific chelator (PSC) to bind Pb-203 for SPECT imaging, and Pb-212 for alpha particle therapy.
Nachrichtenquelle: globenewswire | 28.11.2023, 14:00
Imaging was conducted as part of a clinical study at Technical University of Dresden in Germany
Image was selected as “Image of the Month” in the European Journal of Nuclear Medicine and Molecular Imaging, November 2023
Radiolabeling was performed on-site using a Company-provided generator
SEATTLE, Nov. 28, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), today announced the publication of the first human SPECT images utilizing the alpha-emitting isotope of 212Pb, which was labeled to the Company’s proprietary theranostic VMT-α-NET product. The imaging was conducted as part of a series of four neuroendocrine tumor (NET) patients who were administered VMT-α-NET at a clinical study site in Germany. The Company is developing VMT-α-NET for the treatment and diagnosis of somatostatin receptor subtype 2 (SSTR2)-expressing neuroendocrine tumors.
VMT-α-NET is being administered under the supervision of Prof. Dr. Jörg Kotzerke, Director of the Department of Nuclear Medicine at the Technical University of Dresden in Germany. The patient received 90 MBq (2.4mCi) of [212Pb]VMT-α-NET intravenously, and whole-body scintigraphy and SPECT/CT images were acquired 2 hours, 5 hours, and 19 hours after injection. Images were collected on a Symbia Intevo T6 (Siemens Healthineers) using a high-energy collimator. The SPECT/CT images showed high accumulation of [212Pb]VMT-α-NET in liver metastases and were consistent with the previously acquired [68Ga]DOTATATE PET/CT. High tumor retention can be observed in the planar and SPECT/CT images over time. As expected, due to the short half-life of 212Pb (10.6 hours), the images acquired after 19 hours showed a high level of noise due to the low count statistics. The patient showed no early or acute side effects.
“We are pleased to be able to provide our patients with Perspective’s novel targeted alpha-particle therapy. This imaging study represents a world-first and shows that [212Pb]VMT-α-NET can be imaged after administration,” commented Dr. Kotzerke. “Particularly encouraging is the uptake and the retention in the tumors, and the concordance of this data with existing standard of care imaging. NET patients are in desperate need for new treatment options that are safe and effective.”
Under certain expanded access circumstances, VMT-α-NET may be made available to qualified doctors in some countries. Perspective Therapeutics supplies a generator, drug precursors and isotopes for the local production of its proprietary radiotherapeutic, VMT-α-NET.
"Expanding our collaboration with Prof. Dr. Kotzerke and his colleagues at the Technical University of Dresden is a key milestone as we work to improve access to VMT-α-NET, our potentially life-changing treatment, to patients with burdensome NETs,” said Thijs Spoor, Chief Executive Officer at Perspective Therapeutics. “The use of post-therapy SPECT imaging to confirm tumor uptake and clearance from healthy organs is of particular interest moving forward, as it means that post-therapy dosimetry can be used in next-cycle treatment planning by physicians using our products. We trust the healthcare systems that value dosimetry and patient safety will work with us to be able to have this covered as a routine part of patient care.”
Dr. Kotzerke and his team will continue to monitor patients’ progress over the coming months. The publication is entitled “First-in-human SPECT/CT imaging of [212Pb]Pb-VMT-α-NET in a patient with metastatic neuroendocrine tumor” and is available in the European Journal of Nuclear Medicine and Molecular Imaging at https://link.springer.com/article/10.1007/s00259-023-06529-1… It follows a similar publication in Clinical Nuclear Medicine from earlier this year showing successful SPECT imaging of Perspective’s surrogate diagnostic isotope 203Pb which was also linked to VMT-α-NET.
Recruitment is ongoing for the use of VMT-α-NET in imaging and therapeutic trials at leading U.S. institutions. VMT-α-NET is categorized as an investigational new drug by the U.S. Food and Drug Administration. The use of VMT-α-NET in qualifying expanded access situations is entirely separate from and outside the scope of the Company's Phase 1 trial.
Michler, E., Kästner, D., Brogsitter, C. et al. First-in-human SPECT/CT imaging of [212Pb]Pb-VMT-α-NET in a patient with metastatic neuroendocrine tumor. Eur J Nucl Med Mol Imaging (2023). https://doi.org/10.1007/s00259-023-06529-1
About Neuroendocrine Tumors
Neuroendocrine tumors form in cells that interact with the nervous system or in glands that produce hormones. They can originate in various parts of the body, most often in the gut or the lungs and can be benign or malignant. Neuroendocrine tumors are typically classified as pancreatic neuroendocrine tumors or non-pancreatic neuroendocrine tumors. According to cancer.net, it is estimated that more than 12,000 people in the United States are diagnosed with a NET each year. Importantly, neuroendocrine tumors are associated with a relatively long duration of survival compared to other tumors and as a result, there are approximately 175,000 people living with this diagnosis
About VMT-α-NET
VMT-α-NET is a clinical stage targeted alpha particle therapy (TAT) radiopharmaceutical being developed for the treatment and diagnosis of somatostatin receptor subtype 2 (SSTR2) expressing neuroendocrine tumors, which are a rare and difficult-to-treat type of cancer. VMT-α-NET incorporates Perspective Therapeutics' proprietary lead-specific chelator (PSC) to bind Pb-203 for SPECT imaging, and Pb-212 for alpha particle therapy.
Nachrichtenquelle: globenewswire | 28.11.2023, 14:00
Antwort auf Beitrag Nr.: 74.875.686 von Edelweiss70 am 28.11.23 17:07:29Mal schauen was noch geht im Jahresendspurt
Antwort auf Beitrag Nr.: 74.995.265 von Edelweiss70 am 20.12.23 15:36:07Ein bissl Volumen wäre jetzt wünschenswert 🔥
PT
IR 12.12.23
Perspective Therapeutics Divests Brachytherapy Business
December 12, 2023
SEATTLE, Dec. 12, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. ("Perspective" or "the Company") (NYSE AMERICAN: CATX), today announced it agreed to fully divest its brachytherapy business, including its radioactive Cesium-131 seed assets and related business infrastructure, to GT Medical Technologies, Inc. ("GT Medical"). The transaction is expected to close in the first quarter of 2024 and is subject to customary closing conditions.
Under the terms of the transaction, Isoray Medical, Inc. ("Isoray"), a wholly-owned subsidiary of the Company, agreed to sell, and GT Medical agreed to purchase, the Company's commercial Cesium-131 brachytherapy division and certain related assets including inventory and intellectual property. The assets to be sold consist primarily of customer and supplier lists, production line equipment, intellectual property associated with the brachytherapy division, computer equipment and software used in the brachytherapy division, and the assignment of the brachytherapy manufacturing facility lease in Richland, WA, along with the assignment of other vendor contracts. The Company will retain most liabilities that exist as of the closing date including environmental, warranty, taxes, accrued payroll and vacation, and accounts payable. The Company will retain all the accounts receivable as of the date of closing. As consideration for the transaction, Perspective will receive an equity interest in GT Medical and will have the right to receive certain cash royalties on net sales of Cesium-131 seeds and GT Medical's GammaTile therapy utilizing Cesium-131 over the four-year period following the closing of the transaction.
The sale strengthens Perspective Therapeutics' position in the lead (Pb)-based targeted alpha-particle therapeutic space with all Company resources now dedicated to early discovery, and clinical development of the Company's melanoma (VMT01) and neuroendocrine tumor (VMT-α-NET) clinical programs.
"At Perspective Therapeutics, we are relentlessly focused on realizing the therapeutic potential for the best possible isotopes that can be used to benefit patients with difficult-to-treat oncological indications," said Perspective Therapeutics CEO Thijs Spoor. "The sale of our brachytherapy business marks a key strategic re-prioritization that allows us to focus on and dedicate resources towards accelerating the clinical development of our proprietary alpha-particle therapy portfolio. We believe that this focused allocation of resources will enable us to unlock shareholder value, with the added confidence that Cesium-131 as a brachytherapy technology, and the incredibly talented team dedicated to its production and commercialization, is in excellent hands."
GT Medical has been a long-time customer of Perspective Therapeutics as their patented innovation of the GammaTile product contains Cesium-131 radioactive seeds. This Surgically Targeted Radiation Therapy (STaRT) starts radiation immediately after tumor removal to help eradicate residual tumor cells in patients with brain tumors. The acquisition strengthens its position in the market by ensuring access to and control over radioactive seed production. In addition, it expands GT Medical's customer base to include multiple facilities treating patients with prostate, lung, head & neck, and gynecological tumors.
"As a longstanding customer, we are impressed by the infrastructure that Perspective Therapeutics established for its Cesium-131 brachytherapy business," said GT Medical Technologies CEO Matthew Likens. "Our acquisition of these Cesium-131 related assets makes GT Medical Technologies well positioned to enhance the delivery of GammaTile Therapy in the U.S. market, building on our commitment to excellence for both patients and the clinicians. We are excited about integrating the Cesium-131 brachytherapy team following the closing of the transaction."
About Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc., is a diversified medical technology and radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body. The Company has a proprietary technology that utilizes the alpha emitting isotope 212Pb to deliver powerful radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides which provide the opportunity to personalize treatment and optimize patient outcomes. This "theranostic" approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments.
The Company's melanoma (VMT01) and neuroendocrine tumor (VMT-α-NET) programs have entered Phase 1/2a imaging and therapy trials for the treatment of metastatic melanoma and neuroendocrine tumors at several leading academic institutions. The Company has also developed a proprietary 212Pb generator to secure key isotopes for clinical trial and commercial operations.
For more information, please visit the Company's website at www.perspectivetherapeutics.com .
IR 12.12.23
Perspective Therapeutics Divests Brachytherapy Business
December 12, 2023
SEATTLE, Dec. 12, 2023 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. ("Perspective" or "the Company") (NYSE AMERICAN: CATX), today announced it agreed to fully divest its brachytherapy business, including its radioactive Cesium-131 seed assets and related business infrastructure, to GT Medical Technologies, Inc. ("GT Medical"). The transaction is expected to close in the first quarter of 2024 and is subject to customary closing conditions.
Under the terms of the transaction, Isoray Medical, Inc. ("Isoray"), a wholly-owned subsidiary of the Company, agreed to sell, and GT Medical agreed to purchase, the Company's commercial Cesium-131 brachytherapy division and certain related assets including inventory and intellectual property. The assets to be sold consist primarily of customer and supplier lists, production line equipment, intellectual property associated with the brachytherapy division, computer equipment and software used in the brachytherapy division, and the assignment of the brachytherapy manufacturing facility lease in Richland, WA, along with the assignment of other vendor contracts. The Company will retain most liabilities that exist as of the closing date including environmental, warranty, taxes, accrued payroll and vacation, and accounts payable. The Company will retain all the accounts receivable as of the date of closing. As consideration for the transaction, Perspective will receive an equity interest in GT Medical and will have the right to receive certain cash royalties on net sales of Cesium-131 seeds and GT Medical's GammaTile therapy utilizing Cesium-131 over the four-year period following the closing of the transaction.
The sale strengthens Perspective Therapeutics' position in the lead (Pb)-based targeted alpha-particle therapeutic space with all Company resources now dedicated to early discovery, and clinical development of the Company's melanoma (VMT01) and neuroendocrine tumor (VMT-α-NET) clinical programs.
"At Perspective Therapeutics, we are relentlessly focused on realizing the therapeutic potential for the best possible isotopes that can be used to benefit patients with difficult-to-treat oncological indications," said Perspective Therapeutics CEO Thijs Spoor. "The sale of our brachytherapy business marks a key strategic re-prioritization that allows us to focus on and dedicate resources towards accelerating the clinical development of our proprietary alpha-particle therapy portfolio. We believe that this focused allocation of resources will enable us to unlock shareholder value, with the added confidence that Cesium-131 as a brachytherapy technology, and the incredibly talented team dedicated to its production and commercialization, is in excellent hands."
GT Medical has been a long-time customer of Perspective Therapeutics as their patented innovation of the GammaTile product contains Cesium-131 radioactive seeds. This Surgically Targeted Radiation Therapy (STaRT) starts radiation immediately after tumor removal to help eradicate residual tumor cells in patients with brain tumors. The acquisition strengthens its position in the market by ensuring access to and control over radioactive seed production. In addition, it expands GT Medical's customer base to include multiple facilities treating patients with prostate, lung, head & neck, and gynecological tumors.
"As a longstanding customer, we are impressed by the infrastructure that Perspective Therapeutics established for its Cesium-131 brachytherapy business," said GT Medical Technologies CEO Matthew Likens. "Our acquisition of these Cesium-131 related assets makes GT Medical Technologies well positioned to enhance the delivery of GammaTile Therapy in the U.S. market, building on our commitment to excellence for both patients and the clinicians. We are excited about integrating the Cesium-131 brachytherapy team following the closing of the transaction."
About Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc., is a diversified medical technology and radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body. The Company has a proprietary technology that utilizes the alpha emitting isotope 212Pb to deliver powerful radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides which provide the opportunity to personalize treatment and optimize patient outcomes. This "theranostic" approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments.
The Company's melanoma (VMT01) and neuroendocrine tumor (VMT-α-NET) programs have entered Phase 1/2a imaging and therapy trials for the treatment of metastatic melanoma and neuroendocrine tumors at several leading academic institutions. The Company has also developed a proprietary 212Pb generator to secure key isotopes for clinical trial and commercial operations.
For more information, please visit the Company's website at www.perspectivetherapeutics.com .
Antwort auf Beitrag Nr.: 75.020.273 von Edelweiss70 am 27.12.23 17:05:36SEATTLE, Jan. 02, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), today announced that Thijs Spoor, the Company’s Chief Executive Officer, will present at the 42nd Annual J.P. Morgan Healthcare Conference in San Francisco, CA, on Thursday, January 11, 2024, at 12 p.m. PT. Management will also participate at investor conferences alongside the J.P. Morgan Healthcare Conference in San Francisco.
42nd Annual J.P. Morgan Healthcare Conference – Company Presentation Date: Thursday, January 11, 2024
Na dann schauen wir mal ob da einer mal ein Invest riskieren wird.
42nd Annual J.P. Morgan Healthcare Conference – Company Presentation Date: Thursday, January 11, 2024
Na dann schauen wir mal ob da einer mal ein Invest riskieren wird.
Antwort auf Beitrag Nr.: 75.039.861 von Edelweiss70 am 02.01.24 19:10:06IR PT
5.1.24
SEATTLE, Jan. 05, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), announced today that it has entered into a patent license agreement with Mayo Clinic for the rights to the PSMA Alpha-PET DoubLET platform technology for the treatment of PSMA-expressing cancers, with an initial focus on prostate.
5.1.24
SEATTLE, Jan. 05, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), announced today that it has entered into a patent license agreement with Mayo Clinic for the rights to the PSMA Alpha-PET DoubLET platform technology for the treatment of PSMA-expressing cancers, with an initial focus on prostate.
Sieht ja ganz interessant aus. Wenn man jetzt noch die 0,65$ nachhaltig kanckt kann das was werden. Wobei ich denke die richtigen Impulse könnten erst nächste Woche am 11 Januar von der MS Konferenz kommen.Bleibt spannend....
SEATTLE, Jan. 05, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), announced today that it has entered into a patent license agreement with Mayo Clinic for the rights to the PSMA Alpha-PET DoubLET platform technology for the treatment of PSMA-expressing cancers, with an initial focus on prostate.
Hier nach mehrfachem Wunsch nochmal der ganze Artikel
The PSMA Alpha-PET DoubLET platform technology represents a potential leap forward in the field of prostate cancer diagnostics and treatment. This leading radiopharmaceutical platform provides detailed PET imaging-based diagnosis and dosimetry using long-lived copper-64 (64Cu) for imaging and alpha-particle targeted RPT using lead-212 (212Pb). It can also be used for beta-particle targeted RPT using copper isotopes. Preclinical studies demonstrated a high degree of radiation delivered to tumors while minimizing exposure to critical organs and tissues.
“This innovative approach developed by Mayo Clinic allows for more precise and personalized treatment plans,” said Thijs Spoor, Chief Executive Officer at Perspective Therapeutics. “This new license furthers our goal to bring new best-in-class products to the clinic that improve efficacy and minimize side effects.”
“It is not enough to develop a more powerful therapy. We focused on diagnostic accuracy, patient tolerance and convenience. PSMA Alpha-PET aims to improve the overall experience of individuals undergoing prostate cancer radiopharmaceutical therapy,” commented co-inventor Geoff Johnson, MD, PhD, Chair of Nuclear Medicine, Associate Director of the Mayo Clinic Comprehensive Cancer Center and Director of the Radiopharmaceutical Trial Disease Team. “The platform’s innovative design provides rapid and high-quality quantitative PET imaging to guide alpha and/or beta-particle RPT, with a reasonable workflow that patients and clinics can follow.
Co-inventor Mukesh Pandey, PhD, FRSC, Professor of Radiology and Director of Mayo Clinic Molecular Imaging Research Program said, “The team at Mayo Clinic conducted rigorous scientific experiments to validate the specific targeting of PSMA Alpha-PET to prostate tumors compared to other normal tissues. We are pleased to report that this compound performed very favorably in the preclinical setting where it had significantly less salivary gland uptake and improved kidney clearance than comparators. PSMA Alpha-PET was also effective in treating mice with prostate cancer tumor implants and was well tolerated.”
Dr. Markus Puhlmann, Chief Medical Officer of Perspective Therapeutics added, “PSMA Alpha-PET is a potentially groundbreaking solution that addresses some of the limitations of existing products in prostate cancer imaging and treatment. We are committed to advancing medical technology that we believe will make a meaningful difference in the lives of those affected by prostate cancer.”
Mayo Clinic has a financial interest in the technology referenced in this press release. Mayo Clinic will use any revenue it receives to support its not-for-profit mission in patient care, education and research
Hier nach mehrfachem Wunsch nochmal der ganze Artikel
The PSMA Alpha-PET DoubLET platform technology represents a potential leap forward in the field of prostate cancer diagnostics and treatment. This leading radiopharmaceutical platform provides detailed PET imaging-based diagnosis and dosimetry using long-lived copper-64 (64Cu) for imaging and alpha-particle targeted RPT using lead-212 (212Pb). It can also be used for beta-particle targeted RPT using copper isotopes. Preclinical studies demonstrated a high degree of radiation delivered to tumors while minimizing exposure to critical organs and tissues.
“This innovative approach developed by Mayo Clinic allows for more precise and personalized treatment plans,” said Thijs Spoor, Chief Executive Officer at Perspective Therapeutics. “This new license furthers our goal to bring new best-in-class products to the clinic that improve efficacy and minimize side effects.”
“It is not enough to develop a more powerful therapy. We focused on diagnostic accuracy, patient tolerance and convenience. PSMA Alpha-PET aims to improve the overall experience of individuals undergoing prostate cancer radiopharmaceutical therapy,” commented co-inventor Geoff Johnson, MD, PhD, Chair of Nuclear Medicine, Associate Director of the Mayo Clinic Comprehensive Cancer Center and Director of the Radiopharmaceutical Trial Disease Team. “The platform’s innovative design provides rapid and high-quality quantitative PET imaging to guide alpha and/or beta-particle RPT, with a reasonable workflow that patients and clinics can follow.
Co-inventor Mukesh Pandey, PhD, FRSC, Professor of Radiology and Director of Mayo Clinic Molecular Imaging Research Program said, “The team at Mayo Clinic conducted rigorous scientific experiments to validate the specific targeting of PSMA Alpha-PET to prostate tumors compared to other normal tissues. We are pleased to report that this compound performed very favorably in the preclinical setting where it had significantly less salivary gland uptake and improved kidney clearance than comparators. PSMA Alpha-PET was also effective in treating mice with prostate cancer tumor implants and was well tolerated.”
Dr. Markus Puhlmann, Chief Medical Officer of Perspective Therapeutics added, “PSMA Alpha-PET is a potentially groundbreaking solution that addresses some of the limitations of existing products in prostate cancer imaging and treatment. We are committed to advancing medical technology that we believe will make a meaningful difference in the lives of those affected by prostate cancer.”
Mayo Clinic has a financial interest in the technology referenced in this press release. Mayo Clinic will use any revenue it receives to support its not-for-profit mission in patient care, education and research
EIn wenig Volumen war ja gestern schon mal zu verzeichnen mit kanpp 600k Stücken an den verschiedenen Handelsplätzen. Würde mich nun über ein positives feedback auf den Auftritt bei der Morgan Stanley Investorenkonferenz am Donnerstag freuen.
Umsätze bisher an den Handelsplätzen zwar etwas flau aber die 0,65$ sind genommen und im Geld aktuell etwas untermauert. Auf eine spannende Woche
Antwort auf Beitrag Nr.: 75.068.565 von Edelweiss70 am 08.01.24 16:34:34SEATTLE, Jan. 09, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (NYSE AMERICAN: CATX), today announced it entered into strategic agreements with Lantheus Holdings, Inc., the leading radiopharmaceutical-focused company, and its affiliates (Lantheus) (NASDAQ: LNTH). For an upfront payment of $28 million in cash, Lantheus will obtain an exclusive option to negotiate for an exclusive license to Perspective’s [212Pb]VMT-α-NET, a clinical stage alpha therapy developed for the treatment of neuroendocrine tumors, and a right to co-fund the IND-enabling studies for early-stage therapeutic candidates targeting prostate-specific membrane antigen (PSMA) and gastrin releasing peptide receptor (GRPR) and, prior to IND filing, a right to negotiate for an exclusive license to such candidates. Lantheus has also agreed to purchase an equity stake of up to 19.9% (56,342,355) shares of Perspective's outstanding shares of common stock for up to approximately $33 million, subject to completion of a qualified third-party financing transaction and certain other closing conditions. Additionally, Perspective has agreed to acquire the assets and associated lease of Lantheus’ radiopharmaceutical manufacturing facility in Somerset, New Jersey for an undisclosed price.
Thijs Spoor, Chief Executive Officer of Perspective, stated, "At Perspective Therapeutics, our goal is to leverage the best isotopes and provide effective therapeutic choices for patients with difficult-to-treat cancers. We stand at the forefront of innovation in precision oncology, particularly in the development of lead-based alpha therapies and complementary diagnostic imaging agents. With the strategic acquisition of the Somerset radiopharmaceutical manufacturing facility, we will strengthen our operational capabilities by expanding our in-house manufacturing footprint that allows us to advance our leading portfolio of radiotherapies.”
Thijs Spoor, Chief Executive Officer of Perspective, stated, "At Perspective Therapeutics, our goal is to leverage the best isotopes and provide effective therapeutic choices for patients with difficult-to-treat cancers. We stand at the forefront of innovation in precision oncology, particularly in the development of lead-based alpha therapies and complementary diagnostic imaging agents. With the strategic acquisition of the Somerset radiopharmaceutical manufacturing facility, we will strengthen our operational capabilities by expanding our in-house manufacturing footprint that allows us to advance our leading portfolio of radiotherapies.”
Investorenkonferenz bei MS einfach so verpufft als nonevent oder kommt da jetzt mal was ???
Antwort auf Beitrag Nr.: 75.095.799 von Edelweiss70 am 12.01.24 22:32:16NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or the “Company”) (NYSE AMERICAN: CATX), a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced the closing of its previously announced underwritten public offering of (i) 156,399,542 shares of its common stock at the public offering price of $0.37 per share, including the exercise in full by the underwriters of their option to purchase up to an additional 24,324,324 shares of common stock and (ii) to certain investors in lieu of common stock, pre-funded warrants to purchase 30,086,944 shares of its common stock at a price of $0.369 per pre-funded warrant. The purchase price per share of each pre-funded warrant represents the per share public offering price for the common stock, minus the $0.001 per share exercise price of such pre-funded warrant. All of the shares of common stock and pre-funded warrants sold in the public offering were sold by Perspective. Gross proceeds to Perspective in the public offering were approximately $69.0 million, before underwriting discounts and commissions and estimated expenses of the public offering.
Oppenheimer & Co. and B. Riley Securities acted as joint book-running managers for the public offering.
Oppenheimer & Co. and B. Riley Securities acted as joint book-running managers for the public offering.
Ich gestehe meine Neugierde, wie wir den ehute die Woche beenden werden.
Perspektive Therapeutik
Der Handel:Perspektive Therapeutika, Inc. CATXÄrztlicher Leiter Markus Puhlmann insgesamt 280.000 Aktien zu bei einem Durchschnittspreis von 0,50 Dollar. Um diese Aktien zu erwerben, kostete es etwa 139.188 Dollar. Der Direktor des Unternehmens Robert F. Williamson III kaufte auch 51.996 Aktien des Unternehmens.
Was passiert : B. Riley Securities initiierte die Abdeckung der Aktie mit einem Buy-Rating und einem Kursziel von 1,20 US-Dollar.
Was Perspective Therapeutics tut : Perspective Therapeutics Inc ist ein Medizintechnik- und radiopharmazeutisches Unternehmen, das verschiedene Behandlungsanwendungen für Krebs im ganzen Körper entwickelt.
Der Handel:Perspektive Therapeutika, Inc. CATXÄrztlicher Leiter Markus Puhlmann insgesamt 280.000 Aktien zu bei einem Durchschnittspreis von 0,50 Dollar. Um diese Aktien zu erwerben, kostete es etwa 139.188 Dollar. Der Direktor des Unternehmens Robert F. Williamson III kaufte auch 51.996 Aktien des Unternehmens.
Was passiert : B. Riley Securities initiierte die Abdeckung der Aktie mit einem Buy-Rating und einem Kursziel von 1,20 US-Dollar.
Was Perspective Therapeutics tut : Perspective Therapeutics Inc ist ein Medizintechnik- und radiopharmazeutisches Unternehmen, das verschiedene Behandlungsanwendungen für Krebs im ganzen Körper entwickelt.
Perspective Therapeutics kündigt Schließung von 69,0 Millionen US-Dollar Öffentlichen Angeboten und 20,8 Millionen US-Dollar an Privatplatzierung an
22. Januar 2024 16:05 ET | Quelle:Perspektive Therapeutika, Inc.
Aktie
SEATTLE, Jan. 22, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. („Perspective“ oder das „Unternehmen“) (NYSE AMERICAN: CATX), ein radiopharmazeutisches Unternehmen, das bahnbrechende fortgeschrittene Behandlungsanwendungen für Krebserkrankungen im gesamten Körper ist, gab heute den Abschluss seines zuvor angekündigten öffentlichen Angebots von 156.399.542 Aktien seiner Stammaktien zum öffentlichen Angebotskurs von 0,37 Dollar bekannt.bis zu 24.324.324 Stammaktien und (ii) an bestimmte Anleger anstelle von Stammaktien vorfinanzierte Optionsscheine zum Kauf von 30.086.944 Aktien ihrer Stammaktien zu einem Preis von 0,369 Dollar pro vorfinanziertem Warrant. Der Kaufpreis pro Aktie jedes vorfinanzierten Warrants entspricht dem öffentlichen Angebotspreis pro Aktie für die Stammaktie, abzüglich des Ausübungspreises von 0,001 Dollar pro Aktie des solchen vorfinanzierten Optionsscheins. Alle Aktien von Stammaktien und vorfinanzierten Warrants, die im öffentlichen Angebot verkauft wurden, wurden perspective verkauft. Der Bruttoerlös aus der Perspektive des öffentlichen Angebots betrug etwa 69,0 Millionen US-Dollar, bevor er Rabatte und Provisionen und geschätzte Ausgaben des öffentlichen Angebots versicherungstechnische.
Oppenheimer & Co. und B. Riley Securities fungierte als gemeinsame Book-Rollunning-Manager für das öffentliche Angebot.
Gleichzeitig mit dem öffentlichen Angebot schloss Perspective seine zuvor angekündigte Privatplatzierung von 56.342.335 Aktien seiner Stammaktien an Lantheus Alpha Therapy, LLC zu einem Preis von 0,37 Dollar pro Aktie für einen Bruttoerlös von etwa 20,8 Millionen Dollar ab.
Der Gesamtumsatz aus dem öffentlichen Angebot und der gleichzeitigen Privatplatzierung beliefen sich auf etwa 89,8 Millionen US-Dollar, bevor Rabatte und Provisionen und andere Angebotsaufwendungen, die perspective zu zahlen sind, versicherungstechnische Rabatte und andere Angebotsauflagen lagen.
Die Perspektive beabsichtigt, den Nettoerlös aus dem öffentlichen Angebot und der gleichzeitigen Privatplatzierung für allgemeine Unternehmenszwecke zu verwenden, zu denen Forschungs- und Entwicklungsausgaben, präklinische Studien und Ausgaben für klinische Studien, Produktionsausgaben, Gewerbekapital, Investitionsinvestitionen, Kapitalinvestitionen, Akquisitionen neuer Technologien, Produkte oder Unternehmen und Investitionen umfassen können.
Die oben im öffentlichen Angebot beschriebenen Wertpapiere wurden per Perspective gemäß einer Registrierungserklärung auf Formular S-3 angeboten (Akte Nr. 333-275638) wurde ursprünglich am 17. November 2023 bei der Securities and Exchange Commission (die „SEC“) eingereicht und am 14. Dezember 2023 von der SEC für wirksam erklärt.
Die Wertpapiere im öffentlichen Angebot wurden mittels eines Prospektnachtrags und eines begleitenden Prospekts im Zusammenhang mit dem öffentlichen Angebot angeboten, das Teil der Registrierungserklärung ist. Am 17. Januar 2024 wurde bei der SEC ein vorläufiger Prospektnachtrag in Bezug auf das öffentliche Angebot eingereicht. Ein abschließender Prospektnachtrag in Bezug auf das öffentliche Angebot wurde bei der SEC eingereicht und ist auf der Website der SEC unter www.sec.gov verfügbar. Kopien des endgültigen Prospektnachtrags und des begleitenden Prospekts im Zusammenhang mit dem öffentlichen Angebot können von Oppenheimer & Co. Inc., Attention: Syndicate Prospectus Department, 85 Broad Street, 26th Floor, New York, NY 10004, oder per Telefon unter (212) 667-8055, oder per E-Mail an EquityProspectus-opco.com oder von B. Riley Securities, Inc., Achtung: Prospectus Department, 1300 17th Street North, Suite 1300, Arlington, Virginia 22209, Telefon: (703) 312-9580, E-Mail: prospectuses-brileyfin.com.
Die in der gleichzeitigen Privatplatzierung verkauften Aktien wurden nicht nach dem Securities Act oder einem staatlichen oder anderen geltenden Recht der Gerichtsbarkeit registriert und dürfen in den Vereinigten Staaten ohne Registrierung oder eine anwendbare Ausnahme von den Registrierungsanforderungen des Securities Act und den geltenden Wertpapiergesetzen oder der geltenden staatlichen oder sonstigen Gerichtsbarkeiten nicht angeboten oder verkauft werden.
Diese Pressemitteilung stellt weder ein Angebot zum Verkauf noch eine Aufforderung zur Aufforderung an ein Angebot zum Kauf einer der hierin beschriebenen Wertpapiere dar, noch darf es einen Verkauf dieser Wertpapiere in einem Staat oder einer Jurisdiktion geben, in dem ein solches Angebot, eine solche Aufforderung oder ein solcher Verkauf vor der Registrierung oder Qualifizierung nach den Wertpapiergesetzen eines solchen Staates rechtswidrig wäre.
Über Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc. ist ein radiopharmazeutisches Unternehmen, das bahnbrechenden fortschrittliche Behandlungsanwendungen für Krebs im ganzen Körper ist. Das Unternehmen verfügt über eine proprietäre Technologie, die das Alpha-Emitte-isotop Lead-212 nutzt, um leistungsstarke Strahlung speziell an Krebszellen über spezialisierte Targeting-Peptide zu liefern. Das Unternehmen entwickelt auch eine komplementäre Bildgebungsdiagnostik, die die gleichen Targeting-Peptide enthält, die die Möglichkeit bieten, die Behandlung zu personalisieren und die Patientenergebnisse zu optimieren. Dieser „theranostische“ Ansatz ermöglicht es, den spezifischen Tumor zu sehen und ihn dann zu behandeln, um die Wirksamkeit zu verbessern und die Toxizität im Zusammenhang mit vielen anderen Arten von Krebsbehandlungen zu minimieren.
Die Programme Melanom (VMT01) und der neuroendokrine Tumor (VMT-NET) des Unternehmens haben Phase 1/2a-Bildgebungs- und Therapiestudien zur Behandlung von metastasierendem Melanom und neuroendokrinen Tumoren an mehreren führenden akademischen Institutionen in den Vereinigten Staaten durchgeführt. Das Unternehmen hat auch einen proprietären Lead-212-Generator entwickelt, um wichtige Azidtome für klinische Studien und kommerzielle Operationen zu sichern.
SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION ACT OF 1995: Insofern befassen sich alle in dieser Pressemitteilung gemachten Aussagen mit Informationen, die nicht historisch sind, zukunftsgerichtete Aussagen gemäß dem Private Securities Litigation Reform Act von 1995. Solche Aussagen beinhalten, sind aber nicht beschränkt auf Aussagen über die erwartete Verwendung von Erlösen für das öffentliche Angebot und die gleichzeitige Privatplatzierung und andere Aussagen, die durch Wörter wie "Willen", "Potenzial", "könnte", "kann", "glauben", "beabsichtigen", "plant", "erwartet", "erwartet", "schätzt", "kann" Zukunftsgerichtete Aussagen beziehen sich naturgemäß auf Fragen, die in unterschiedlichem Maße unsicher sind. Unsicherheiten und Risiken können dazu führen, dass die tatsächlichen Ergebnisse der Perspektive wesentlich abweichen als die in den zukunftsgerichteten Aussagen von Perspective geäußerten oder implizierten. Für Perspective beinhaltet dies die Volatilität der Aktienkurse und Unsicherheiten in Bezug auf die Finanzmärkte, die medizinische Gemeinschaft und die Weltwirtschaft sowie die Auswirkungen der Instabilität in den allgemeinen Geschäfts- und Wirtschaftsbedingungen, einschließlich Veränderungen der Inflation, der Zinssätze und des Arbeitsmarktes. Detailliertere Informationen zu diesen und zusätzlichen Faktoren, die die tatsächlichen Ergebnisse der Perspektive beeinflussen könnten, werden in den Einreichungen von Perspective bei der SEC beschrieben, einschließlich ihres Übergangsberichts auf Formular 10-KT für die Übergangsfrist, die am 31. Dezember 2022 endete, wie sie durch ihre Quartalsberichte auf Formular 10-Q und andere bei der SEC eingereichten Dokumente überarbeitet oder ergänzt wurde. Alle zukunftsgerichteten Aussagen in dieser Pressemitteilung beziehen sich nur auf das Datum dieser Pressemitteilung. Perspective ist nicht verpflichtet, zukunftsgerichtete Aussagen zu aktualisieren oder zu revidieren, sei es aufgrund neuer Informationen, zukünftiger Ereignisse oder aus anderen Gründen.
Kontaktdaten
Medien und Investor Relations Kontakte:
Russo Partners, LLC
Nic Johnson oder Harrison Seidner, Ph.D.
E: Nic.johnson-russopartnersllc.com
E: Harrison.seidner-russopartnersllc.com
Kontakt
FirmenprofilÜber Perspective Therapeutics, Inc.Website:
http://www.isoray.com/
Pressemitteilungen
Druck
PDF herunterladen
Abonnieren über RSS
Abonnieren über ATOM
Javascript
Empfohlene Lesung
1. Februar, 2024 08:00 ET
Quelle:
Perspektive Therapeutika, Inc.
Perspektive Therapeutika führt „Pre-Targeting“ Theranostic Technology Platform ein
Unterzeichnet eine weltumrungene exklusive Lizenz mit der Stony Brook University für eine neue Plattform, die Radionuclide Targeting um bis zu 2,4 Millionen Dollar NIH Grant erweitern kann, um...
Perspective Therapeutics Introduces “Pre-Targeting...
18. Januar 2024 07:00 ET
Quelle:
Perspektive Therapeutika, Inc.
Perspective Therapeutics kündigt Preisfestsetzung von 60,0 Millionen US-Dollar an öffentliches Angebot und 20,8 Millionen US-Dollar an Privatplatzierung an
SEATTLE, Jan. 18, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. („Perspektive“ oder das „Unternehmen“) (NYSE AMERICAN: CATX), ein funkpharmazeutisches Unternehmen, das Pioniere der fortschrittlichen...
Perspective Therapeutics Announces Pricing of $6...
Erkunden
GlobeNewswire
Über uns
GlobeNewswire ist eines der weltweit größten Nachrichtensender-Vertriebsnetze, das sich auf die Bereitstellung von Pressemitteilungen, Finanzmitteilungen und Multimedia-Inhalten an Medien, Investoren und Verbraucher weltweit spezialisiert hat.
Folgen Sie uns in den sozialen Medien:
Newswire Distribution Netzwerk & Management
Home
Newsroom
RSS Feeds
Notified, Inc.
Rechtliche
Kontakt
22. Januar 2024 16:05 ET | Quelle:Perspektive Therapeutika, Inc.
Aktie
SEATTLE, Jan. 22, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. („Perspective“ oder das „Unternehmen“) (NYSE AMERICAN: CATX), ein radiopharmazeutisches Unternehmen, das bahnbrechende fortgeschrittene Behandlungsanwendungen für Krebserkrankungen im gesamten Körper ist, gab heute den Abschluss seines zuvor angekündigten öffentlichen Angebots von 156.399.542 Aktien seiner Stammaktien zum öffentlichen Angebotskurs von 0,37 Dollar bekannt.bis zu 24.324.324 Stammaktien und (ii) an bestimmte Anleger anstelle von Stammaktien vorfinanzierte Optionsscheine zum Kauf von 30.086.944 Aktien ihrer Stammaktien zu einem Preis von 0,369 Dollar pro vorfinanziertem Warrant. Der Kaufpreis pro Aktie jedes vorfinanzierten Warrants entspricht dem öffentlichen Angebotspreis pro Aktie für die Stammaktie, abzüglich des Ausübungspreises von 0,001 Dollar pro Aktie des solchen vorfinanzierten Optionsscheins. Alle Aktien von Stammaktien und vorfinanzierten Warrants, die im öffentlichen Angebot verkauft wurden, wurden perspective verkauft. Der Bruttoerlös aus der Perspektive des öffentlichen Angebots betrug etwa 69,0 Millionen US-Dollar, bevor er Rabatte und Provisionen und geschätzte Ausgaben des öffentlichen Angebots versicherungstechnische.
Oppenheimer & Co. und B. Riley Securities fungierte als gemeinsame Book-Rollunning-Manager für das öffentliche Angebot.
Gleichzeitig mit dem öffentlichen Angebot schloss Perspective seine zuvor angekündigte Privatplatzierung von 56.342.335 Aktien seiner Stammaktien an Lantheus Alpha Therapy, LLC zu einem Preis von 0,37 Dollar pro Aktie für einen Bruttoerlös von etwa 20,8 Millionen Dollar ab.
Der Gesamtumsatz aus dem öffentlichen Angebot und der gleichzeitigen Privatplatzierung beliefen sich auf etwa 89,8 Millionen US-Dollar, bevor Rabatte und Provisionen und andere Angebotsaufwendungen, die perspective zu zahlen sind, versicherungstechnische Rabatte und andere Angebotsauflagen lagen.
Die Perspektive beabsichtigt, den Nettoerlös aus dem öffentlichen Angebot und der gleichzeitigen Privatplatzierung für allgemeine Unternehmenszwecke zu verwenden, zu denen Forschungs- und Entwicklungsausgaben, präklinische Studien und Ausgaben für klinische Studien, Produktionsausgaben, Gewerbekapital, Investitionsinvestitionen, Kapitalinvestitionen, Akquisitionen neuer Technologien, Produkte oder Unternehmen und Investitionen umfassen können.
Die oben im öffentlichen Angebot beschriebenen Wertpapiere wurden per Perspective gemäß einer Registrierungserklärung auf Formular S-3 angeboten (Akte Nr. 333-275638) wurde ursprünglich am 17. November 2023 bei der Securities and Exchange Commission (die „SEC“) eingereicht und am 14. Dezember 2023 von der SEC für wirksam erklärt.
Die Wertpapiere im öffentlichen Angebot wurden mittels eines Prospektnachtrags und eines begleitenden Prospekts im Zusammenhang mit dem öffentlichen Angebot angeboten, das Teil der Registrierungserklärung ist. Am 17. Januar 2024 wurde bei der SEC ein vorläufiger Prospektnachtrag in Bezug auf das öffentliche Angebot eingereicht. Ein abschließender Prospektnachtrag in Bezug auf das öffentliche Angebot wurde bei der SEC eingereicht und ist auf der Website der SEC unter www.sec.gov verfügbar. Kopien des endgültigen Prospektnachtrags und des begleitenden Prospekts im Zusammenhang mit dem öffentlichen Angebot können von Oppenheimer & Co. Inc., Attention: Syndicate Prospectus Department, 85 Broad Street, 26th Floor, New York, NY 10004, oder per Telefon unter (212) 667-8055, oder per E-Mail an EquityProspectus-opco.com oder von B. Riley Securities, Inc., Achtung: Prospectus Department, 1300 17th Street North, Suite 1300, Arlington, Virginia 22209, Telefon: (703) 312-9580, E-Mail: prospectuses-brileyfin.com.
Die in der gleichzeitigen Privatplatzierung verkauften Aktien wurden nicht nach dem Securities Act oder einem staatlichen oder anderen geltenden Recht der Gerichtsbarkeit registriert und dürfen in den Vereinigten Staaten ohne Registrierung oder eine anwendbare Ausnahme von den Registrierungsanforderungen des Securities Act und den geltenden Wertpapiergesetzen oder der geltenden staatlichen oder sonstigen Gerichtsbarkeiten nicht angeboten oder verkauft werden.
Diese Pressemitteilung stellt weder ein Angebot zum Verkauf noch eine Aufforderung zur Aufforderung an ein Angebot zum Kauf einer der hierin beschriebenen Wertpapiere dar, noch darf es einen Verkauf dieser Wertpapiere in einem Staat oder einer Jurisdiktion geben, in dem ein solches Angebot, eine solche Aufforderung oder ein solcher Verkauf vor der Registrierung oder Qualifizierung nach den Wertpapiergesetzen eines solchen Staates rechtswidrig wäre.
Über Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc. ist ein radiopharmazeutisches Unternehmen, das bahnbrechenden fortschrittliche Behandlungsanwendungen für Krebs im ganzen Körper ist. Das Unternehmen verfügt über eine proprietäre Technologie, die das Alpha-Emitte-isotop Lead-212 nutzt, um leistungsstarke Strahlung speziell an Krebszellen über spezialisierte Targeting-Peptide zu liefern. Das Unternehmen entwickelt auch eine komplementäre Bildgebungsdiagnostik, die die gleichen Targeting-Peptide enthält, die die Möglichkeit bieten, die Behandlung zu personalisieren und die Patientenergebnisse zu optimieren. Dieser „theranostische“ Ansatz ermöglicht es, den spezifischen Tumor zu sehen und ihn dann zu behandeln, um die Wirksamkeit zu verbessern und die Toxizität im Zusammenhang mit vielen anderen Arten von Krebsbehandlungen zu minimieren.
Die Programme Melanom (VMT01) und der neuroendokrine Tumor (VMT-NET) des Unternehmens haben Phase 1/2a-Bildgebungs- und Therapiestudien zur Behandlung von metastasierendem Melanom und neuroendokrinen Tumoren an mehreren führenden akademischen Institutionen in den Vereinigten Staaten durchgeführt. Das Unternehmen hat auch einen proprietären Lead-212-Generator entwickelt, um wichtige Azidtome für klinische Studien und kommerzielle Operationen zu sichern.
SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION ACT OF 1995: Insofern befassen sich alle in dieser Pressemitteilung gemachten Aussagen mit Informationen, die nicht historisch sind, zukunftsgerichtete Aussagen gemäß dem Private Securities Litigation Reform Act von 1995. Solche Aussagen beinhalten, sind aber nicht beschränkt auf Aussagen über die erwartete Verwendung von Erlösen für das öffentliche Angebot und die gleichzeitige Privatplatzierung und andere Aussagen, die durch Wörter wie "Willen", "Potenzial", "könnte", "kann", "glauben", "beabsichtigen", "plant", "erwartet", "erwartet", "schätzt", "kann" Zukunftsgerichtete Aussagen beziehen sich naturgemäß auf Fragen, die in unterschiedlichem Maße unsicher sind. Unsicherheiten und Risiken können dazu führen, dass die tatsächlichen Ergebnisse der Perspektive wesentlich abweichen als die in den zukunftsgerichteten Aussagen von Perspective geäußerten oder implizierten. Für Perspective beinhaltet dies die Volatilität der Aktienkurse und Unsicherheiten in Bezug auf die Finanzmärkte, die medizinische Gemeinschaft und die Weltwirtschaft sowie die Auswirkungen der Instabilität in den allgemeinen Geschäfts- und Wirtschaftsbedingungen, einschließlich Veränderungen der Inflation, der Zinssätze und des Arbeitsmarktes. Detailliertere Informationen zu diesen und zusätzlichen Faktoren, die die tatsächlichen Ergebnisse der Perspektive beeinflussen könnten, werden in den Einreichungen von Perspective bei der SEC beschrieben, einschließlich ihres Übergangsberichts auf Formular 10-KT für die Übergangsfrist, die am 31. Dezember 2022 endete, wie sie durch ihre Quartalsberichte auf Formular 10-Q und andere bei der SEC eingereichten Dokumente überarbeitet oder ergänzt wurde. Alle zukunftsgerichteten Aussagen in dieser Pressemitteilung beziehen sich nur auf das Datum dieser Pressemitteilung. Perspective ist nicht verpflichtet, zukunftsgerichtete Aussagen zu aktualisieren oder zu revidieren, sei es aufgrund neuer Informationen, zukünftiger Ereignisse oder aus anderen Gründen.
Kontaktdaten
Medien und Investor Relations Kontakte:
Russo Partners, LLC
Nic Johnson oder Harrison Seidner, Ph.D.
E: Nic.johnson-russopartnersllc.com
E: Harrison.seidner-russopartnersllc.com
Kontakt
FirmenprofilÜber Perspective Therapeutics, Inc.Website:
http://www.isoray.com/
Pressemitteilungen
Druck
PDF herunterladen
Abonnieren über RSS
Abonnieren über ATOM
Javascript
Empfohlene Lesung
1. Februar, 2024 08:00 ET
Quelle:
Perspektive Therapeutika, Inc.
Perspektive Therapeutika führt „Pre-Targeting“ Theranostic Technology Platform ein
Unterzeichnet eine weltumrungene exklusive Lizenz mit der Stony Brook University für eine neue Plattform, die Radionuclide Targeting um bis zu 2,4 Millionen Dollar NIH Grant erweitern kann, um...
Perspective Therapeutics Introduces “Pre-Targeting...
18. Januar 2024 07:00 ET
Quelle:
Perspektive Therapeutika, Inc.
Perspective Therapeutics kündigt Preisfestsetzung von 60,0 Millionen US-Dollar an öffentliches Angebot und 20,8 Millionen US-Dollar an Privatplatzierung an
SEATTLE, Jan. 18, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. („Perspektive“ oder das „Unternehmen“) (NYSE AMERICAN: CATX), ein funkpharmazeutisches Unternehmen, das Pioniere der fortschrittlichen...
Perspective Therapeutics Announces Pricing of $6...
Erkunden
GlobeNewswire
Über uns
GlobeNewswire ist eines der weltweit größten Nachrichtensender-Vertriebsnetze, das sich auf die Bereitstellung von Pressemitteilungen, Finanzmitteilungen und Multimedia-Inhalten an Medien, Investoren und Verbraucher weltweit spezialisiert hat.
Folgen Sie uns in den sozialen Medien:
Newswire Distribution Netzwerk & Management
Home
Newsroom
RSS Feeds
Notified, Inc.
Rechtliche
Kontakt
SEATTLE, Feb. 12, 2024 (GLOBE NEWSWIRE) --
Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX),
a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced that Thijs Spoor, Chief Executive Officer, will present virtually at the Oppenheimer 34th Annual Healthcare Life Sciences Conference on Wednesday, February 14, 2024, at 11:20 a.m. ET. Management will also be available for one-on-one meetings.
The live webcast can be accessed at https://wsw.com/webcast/oppenheimer33/catx/2781216 and replays will be available on the Company's website for 90 days.
Na mal sehen ob wir da nochmal etwas Rückenwind bekommen am Mittwoch.
Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX),
a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced that Thijs Spoor, Chief Executive Officer, will present virtually at the Oppenheimer 34th Annual Healthcare Life Sciences Conference on Wednesday, February 14, 2024, at 11:20 a.m. ET. Management will also be available for one-on-one meetings.
The live webcast can be accessed at https://wsw.com/webcast/oppenheimer33/catx/2781216 and replays will be available on the Company's website for 90 days.
Na mal sehen ob wir da nochmal etwas Rückenwind bekommen am Mittwoch.
Antwort auf Beitrag Nr.: 75.265.374 von Edelweiss70 am 12.02.24 18:13:17So dann schauen wir mal was unser CEO heute so bei Oppenheimer präsentiert und ob es interessierte Investoren gibt.
Antwort auf Beitrag Nr.: 75.276.201 von Edelweiss70 am 14.02.24 09:26:30Globelnewswire
26.2.24
Perspective Therapeutics take part at Investor Conferences in Feb and March
SEATTLE, Feb. 26, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced that management will participate at upcoming discussions and investor conferences in February and March, 2024.
• Fireside Chat with JonesResearch – February 28, virtual Time: 11 a.m. ET Participant: Michael K. Schultz, Ph.D., Chief Science Officer Please RSVP to your JonesResearch representative. Additional details will be provided upon registration.
• B. Riley Securities Inaugural Radiopharma Day – March 1, New York, NY
26.2.24
Perspective Therapeutics take part at Investor Conferences in Feb and March
SEATTLE, Feb. 26, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced that management will participate at upcoming discussions and investor conferences in February and March, 2024.
• Fireside Chat with JonesResearch – February 28, virtual Time: 11 a.m. ET Participant: Michael K. Schultz, Ph.D., Chief Science Officer Please RSVP to your JonesResearch representative. Additional details will be provided upon registration.
• B. Riley Securities Inaugural Radiopharma Day – March 1, New York, NY
SEATTLE, March 04, 2024 (GLOBE NEWSWIRE) -
- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced that it has entered into an investment agreement with a select group of institutional accredited investors, to sell securities in a private placement for aggregate gross proceeds of approximately $87.4 million, before deducting placement agent fees and other offering expenses.
In the private placement, the Company is selling 92,009,981 shares of its common stock at a price of $0.95 per share, representing the closing price per share of the Company’s common stock on the NYSE American as of March 1, 2024. The private placement is expected to close on March 6, 2024, subject to the satisfaction of customary closing conditions.
Oppenheimer & Co. is acting as lead placement agent for the private placement. B. Riley Securities is acting as a placement agent, and JonesTrading Institutional Services LLC and LifeSci Capital are acting as co-placement agents for the private placement.
Perspective intends to use the net proceeds for general corporate and working capital purposes, which may include research and development expenditures, preclinical study and clinical trial expenditures, manufacturing expenditures, commercialization expenditures, capital expenditures, acquisitions of new technologies, products or businesses and investments.
The shares of common stock to be sold in the private placement have not been registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state or other applicable jurisdiction's securities laws, and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements of the Securities Act and applicable state or other jurisdictions' securities laws. The Company has agreed to file a registration statement with the U.S. Securities and Exchange Commission (the “SEC”) registering the resale of the shares of common stock issued in the private placement within a specified period of time after the closing of the private placement.
The shares of common stock to be sold in the private placement have not been registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state or other applicable jurisdiction's securities laws, and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements of the Securities Act and applicable state or other jurisdictions' securities laws. The Company has agreed to file a registration statement with the U.S. Securities and Exchange Commission (the “SEC”) registering the resale of the shares of common stock issued in the private placement within a specified period of time after the closing of the private placement.
- Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced that it has entered into an investment agreement with a select group of institutional accredited investors, to sell securities in a private placement for aggregate gross proceeds of approximately $87.4 million, before deducting placement agent fees and other offering expenses.
In the private placement, the Company is selling 92,009,981 shares of its common stock at a price of $0.95 per share, representing the closing price per share of the Company’s common stock on the NYSE American as of March 1, 2024. The private placement is expected to close on March 6, 2024, subject to the satisfaction of customary closing conditions.
Oppenheimer & Co. is acting as lead placement agent for the private placement. B. Riley Securities is acting as a placement agent, and JonesTrading Institutional Services LLC and LifeSci Capital are acting as co-placement agents for the private placement.
Perspective intends to use the net proceeds for general corporate and working capital purposes, which may include research and development expenditures, preclinical study and clinical trial expenditures, manufacturing expenditures, commercialization expenditures, capital expenditures, acquisitions of new technologies, products or businesses and investments.
The shares of common stock to be sold in the private placement have not been registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state or other applicable jurisdiction's securities laws, and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements of the Securities Act and applicable state or other jurisdictions' securities laws. The Company has agreed to file a registration statement with the U.S. Securities and Exchange Commission (the “SEC”) registering the resale of the shares of common stock issued in the private placement within a specified period of time after the closing of the private placement.
The shares of common stock to be sold in the private placement have not been registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state or other applicable jurisdiction's securities laws, and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements of the Securities Act and applicable state or other jurisdictions' securities laws. The Company has agreed to file a registration statement with the U.S. Securities and Exchange Commission (the “SEC”) registering the resale of the shares of common stock issued in the private placement within a specified period of time after the closing of the private placement.
Na da könnte sich doch was tun......
Übersetzung ins Deutsche(ohne Gewähr)
Perspective Therapeutics, Inc. („Perspective“ oder „das Unternehmen“) (NYSE AMERICAN: CATX), ein radiopharmazeutisches Unternehmen, das fortschrittliche Behandlungsanwendungen für Krebserkrankungen im gesamten Körper entwickelt, gab heute bekannt, dass es eine Investitionsvereinbarung mit einem ausgewählten Unternehmen abgeschlossen hat Gruppe institutioneller akkreditierter Anleger, Wertpapiere im Rahmen einer Privatplatzierung für einen Gesamtbruttoerlös von etwa 87,4 Millionen US-Dollar zu verkaufen, vor Abzug der Gebühren der Platzierungsstelle und anderer Angebotskosten.
Im Rahmen der Privatplatzierung verkauft das Unternehmen 92.009.981 Stammaktien zu einem Preis von 0,95 US-Dollar pro Aktie, was dem Schlusskurs pro Stammaktie des Unternehmens an der NYSE American am 1. März 2024 entspricht. Die Privatplatzierung wird erwartet Abschluss am 6. März 2024, vorbehaltlich der Erfüllung der üblichen Abschlussbedingungen.
Oppenheimer & Co. fungiert als Lead Placement Agent für die Privatplatzierung. B. Riley Securities fungiert als Platzierungsagent und JonesTrading Institutional Services LLC und LifeSci Capital fungieren als Co-Placement-Agenten für die Privatplatzierung.
Perspective beabsichtigt, den Nettoerlös für allgemeine Unternehmens- und Betriebskapitalzwecke zu verwenden, wozu Forschungs- und Entwicklungsausgaben, Ausgaben für präklinische Studien und klinische Studien, Herstellungsausgaben, Kommerzialisierungsausgaben, Kapitalausgaben, Akquisitionen neuer Technologien, Produkte oder Unternehmen und Investitionen gehören können.
Die im Rahmen der Privatplatzierung zu verkaufenden Stammaktien wurden nicht gemäß dem Securities Act von 1933 in der jeweils gültigen Fassung (der „Securities Act“) oder den Wertpapiergesetzen eines Staates oder einer anderen anwendbaren Gerichtsbarkeit registriert und dürfen nicht angeboten oder verkauft werden in den Vereinigten Staaten ohne Registrierung oder eine anwendbare Ausnahme von den Registrierungsanforderungen des Securities Act und den Wertpapiergesetzen der anwendbaren Bundesstaaten oder anderen Gerichtsbarkeiten. Das Unternehmen hat zugestimmt, bei der U.S. Securities and Exchange Commission (die „SEC“) eine Registrierungserklärung einzureichen, in der der Weiterverkauf der im Rahmen der Privatplatzierung ausgegebenen Stammaktien innerhalb eines bestimmten Zeitraums nach Abschluss der Privatplatzierung registriert wird.
Die im Rahmen der Privatplatzierung zu verkaufenden Stammaktien wurden nicht gemäß dem Securities Act von 1933 in der jeweils gültigen Fassung (der „Securities Act“) oder den Wertpapiergesetzen eines Staates oder einer anderen anwendbaren Gerichtsbarkeit registriert und dürfen nicht angeboten oder verkauft werden in den Vereinigten Staaten ohne Registrierung oder eine anwendbare Ausnahme von den Registrierungsanforderungen des Securities Act und den Wertpapiergesetzen der anwendbaren Bundesstaaten oder anderen Gerichtsbarkeiten. Das Unternehmen hat zugestimmt, eine Registrierungserklärung bei der U.S. Securities and Exchange Commission (die „SEC“) einzureichen, in der der Weiterverkauf der ausgegebenen Stammaktien
Übersetzung ins Deutsche(ohne Gewähr)
Perspective Therapeutics, Inc. („Perspective“ oder „das Unternehmen“) (NYSE AMERICAN: CATX), ein radiopharmazeutisches Unternehmen, das fortschrittliche Behandlungsanwendungen für Krebserkrankungen im gesamten Körper entwickelt, gab heute bekannt, dass es eine Investitionsvereinbarung mit einem ausgewählten Unternehmen abgeschlossen hat Gruppe institutioneller akkreditierter Anleger, Wertpapiere im Rahmen einer Privatplatzierung für einen Gesamtbruttoerlös von etwa 87,4 Millionen US-Dollar zu verkaufen, vor Abzug der Gebühren der Platzierungsstelle und anderer Angebotskosten.
Im Rahmen der Privatplatzierung verkauft das Unternehmen 92.009.981 Stammaktien zu einem Preis von 0,95 US-Dollar pro Aktie, was dem Schlusskurs pro Stammaktie des Unternehmens an der NYSE American am 1. März 2024 entspricht. Die Privatplatzierung wird erwartet Abschluss am 6. März 2024, vorbehaltlich der Erfüllung der üblichen Abschlussbedingungen.
Oppenheimer & Co. fungiert als Lead Placement Agent für die Privatplatzierung. B. Riley Securities fungiert als Platzierungsagent und JonesTrading Institutional Services LLC und LifeSci Capital fungieren als Co-Placement-Agenten für die Privatplatzierung.
Perspective beabsichtigt, den Nettoerlös für allgemeine Unternehmens- und Betriebskapitalzwecke zu verwenden, wozu Forschungs- und Entwicklungsausgaben, Ausgaben für präklinische Studien und klinische Studien, Herstellungsausgaben, Kommerzialisierungsausgaben, Kapitalausgaben, Akquisitionen neuer Technologien, Produkte oder Unternehmen und Investitionen gehören können.
Die im Rahmen der Privatplatzierung zu verkaufenden Stammaktien wurden nicht gemäß dem Securities Act von 1933 in der jeweils gültigen Fassung (der „Securities Act“) oder den Wertpapiergesetzen eines Staates oder einer anderen anwendbaren Gerichtsbarkeit registriert und dürfen nicht angeboten oder verkauft werden in den Vereinigten Staaten ohne Registrierung oder eine anwendbare Ausnahme von den Registrierungsanforderungen des Securities Act und den Wertpapiergesetzen der anwendbaren Bundesstaaten oder anderen Gerichtsbarkeiten. Das Unternehmen hat zugestimmt, bei der U.S. Securities and Exchange Commission (die „SEC“) eine Registrierungserklärung einzureichen, in der der Weiterverkauf der im Rahmen der Privatplatzierung ausgegebenen Stammaktien innerhalb eines bestimmten Zeitraums nach Abschluss der Privatplatzierung registriert wird.
Die im Rahmen der Privatplatzierung zu verkaufenden Stammaktien wurden nicht gemäß dem Securities Act von 1933 in der jeweils gültigen Fassung (der „Securities Act“) oder den Wertpapiergesetzen eines Staates oder einer anderen anwendbaren Gerichtsbarkeit registriert und dürfen nicht angeboten oder verkauft werden in den Vereinigten Staaten ohne Registrierung oder eine anwendbare Ausnahme von den Registrierungsanforderungen des Securities Act und den Wertpapiergesetzen der anwendbaren Bundesstaaten oder anderen Gerichtsbarkeiten. Das Unternehmen hat zugestimmt, eine Registrierungserklärung bei der U.S. Securities and Exchange Commission (die „SEC“) einzureichen, in der der Weiterverkauf der ausgegebenen Stammaktien
News ALert !!!!!!!!!!
SEATTLE, March 05, 2024 (GLOBE NEWSWIRE) --
Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced the consummation of the acquisition of a state-of-the-art manufacturing facility and associated equipment and systems for the production of its 203Pb- and 212Pb-labeled radiopharmaceuticals.
Previously operated by Lantheus Holdings, Inc. (“Lantheus”) (NASDAQ: LNTH), the leading radiopharmaceutical-focused company, this 11,500 square foot facility, located in Somerset, New Jersey, has operated in compliance with both Part 212 and Part 211 of Title 21 of the Code of Federal Regulations (CFR) in the U.S., meeting Current Good Manufacturing Practice (cGMP) requirements. As a cGMP compliant facility, Perspective intends to utilize the facility to manufacture clinical supply of high quality 203Pb-labeled tumor-specific peptides to visualize and diagnose tumors, and 212Pb-labeled radiopharmaceuticals to treat target tumors with targeted alpha therapies (TAT). Moreover, with its three cGMP suites at the facility, Perspective expects to have the capacity to meet future clinical trial and commercial demands at major cancer treatment centers throughout the Northeastern U.S.
The facility acquisition is part of a series of strategic agreements with Lantheus that Perspective announced on January 9, 2024, which included an initial equity investment in Perspective. In addition, on March 4, 2024, Perspective announced it had entered into an investment agreement to sell shares of the Company’s common stock to certain accredited institutional investors for gross proceeds of $87.4 million, $57.4 million of which will be purchased by Lantheus upon the consummation of the transaction.
“We look forward to welcoming the exceptional talent at this facility and integrating them into our team,” said Shane Cobb, Executive Vice President of Operations, Perspective Therapeutics. “The acquisition of this facility much earlier than initially planned is expected to allow us to move forward more quickly to deliver clinical and, if approved for commercialization, commercial doses. Given the 10.6-hour half-life for 212Pb lead, it is important that we have the infrastructure and personnel to support production of our radiopharmaceuticals, all within the vicinity of the patients we hope to impact. This is a significant milestone for Perspective as we build upon the progress we have made in establishing clinical manufacturing.”
“We are thrilled to expand our manufacturing and supply chain infrastructure to include this facility capable of operating in compliance with Parts 212 and 211,” said Thijs Spoor, Chief Executive Officer, Perspective Therapeutics. “The acquisition is a testament to our commitment to making our targeted alpha-particle therapies available to patients in critical need, and builds on our strategic expansion as we look to establish facilities in proximity to major treatment hubs in the U.S.”
“We are excited for the opportunity to strengthen our strategic collaboration with Perspective Therapeutics,” said Brian Markison, Chief Executive Officer, Lantheus. “We recognize the transformative potential of Perspective’s alpha therapies and platform which we believe have the potential to improve efficacy, while minimizing toxicity across a range of tumor settings. We are pleased the Somerset facility will be used to manufacture these innovative product candidates.”
About Part 211 and Part 212
Part 211 of Title 21 of the Code of Federal Regulations (CFR) outlines the Current Good Manufacturing Practice (cGMP) requirements for finished pharmaceutical products. It covers various aspects of pharmaceutical manufacturing, including facilities, equipment, controls, and processes to ensure the quality and safety of the final products. Part 212 of Title 21 of the CFR specifically pertains to the cGMP requirements for the manufacturing of positron emission tomography (PET) drugs. PET drugs are radiopharmaceuticals used in medical imaging, and Part 212 sets forth requirements tailored to their unique production processes and quality control measures. The main difference between Part 211 and Part 212 lies in their focus areas, with Part 212 catering to the specialized needs of PET drug manufacturing. Like drugs used for PET, Perspective anticipates that its 203Pb-labelled peptides will be regulated under Part 212. Like other manufactured drugs, Perspective anticipates that 212Pb-labelled peptides will be regulated under Part 211.
SEATTLE, March 05, 2024 (GLOBE NEWSWIRE) --
Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced the consummation of the acquisition of a state-of-the-art manufacturing facility and associated equipment and systems for the production of its 203Pb- and 212Pb-labeled radiopharmaceuticals.
Previously operated by Lantheus Holdings, Inc. (“Lantheus”) (NASDAQ: LNTH), the leading radiopharmaceutical-focused company, this 11,500 square foot facility, located in Somerset, New Jersey, has operated in compliance with both Part 212 and Part 211 of Title 21 of the Code of Federal Regulations (CFR) in the U.S., meeting Current Good Manufacturing Practice (cGMP) requirements. As a cGMP compliant facility, Perspective intends to utilize the facility to manufacture clinical supply of high quality 203Pb-labeled tumor-specific peptides to visualize and diagnose tumors, and 212Pb-labeled radiopharmaceuticals to treat target tumors with targeted alpha therapies (TAT). Moreover, with its three cGMP suites at the facility, Perspective expects to have the capacity to meet future clinical trial and commercial demands at major cancer treatment centers throughout the Northeastern U.S.
The facility acquisition is part of a series of strategic agreements with Lantheus that Perspective announced on January 9, 2024, which included an initial equity investment in Perspective. In addition, on March 4, 2024, Perspective announced it had entered into an investment agreement to sell shares of the Company’s common stock to certain accredited institutional investors for gross proceeds of $87.4 million, $57.4 million of which will be purchased by Lantheus upon the consummation of the transaction.
“We look forward to welcoming the exceptional talent at this facility and integrating them into our team,” said Shane Cobb, Executive Vice President of Operations, Perspective Therapeutics. “The acquisition of this facility much earlier than initially planned is expected to allow us to move forward more quickly to deliver clinical and, if approved for commercialization, commercial doses. Given the 10.6-hour half-life for 212Pb lead, it is important that we have the infrastructure and personnel to support production of our radiopharmaceuticals, all within the vicinity of the patients we hope to impact. This is a significant milestone for Perspective as we build upon the progress we have made in establishing clinical manufacturing.”
“We are thrilled to expand our manufacturing and supply chain infrastructure to include this facility capable of operating in compliance with Parts 212 and 211,” said Thijs Spoor, Chief Executive Officer, Perspective Therapeutics. “The acquisition is a testament to our commitment to making our targeted alpha-particle therapies available to patients in critical need, and builds on our strategic expansion as we look to establish facilities in proximity to major treatment hubs in the U.S.”
“We are excited for the opportunity to strengthen our strategic collaboration with Perspective Therapeutics,” said Brian Markison, Chief Executive Officer, Lantheus. “We recognize the transformative potential of Perspective’s alpha therapies and platform which we believe have the potential to improve efficacy, while minimizing toxicity across a range of tumor settings. We are pleased the Somerset facility will be used to manufacture these innovative product candidates.”
About Part 211 and Part 212
Part 211 of Title 21 of the Code of Federal Regulations (CFR) outlines the Current Good Manufacturing Practice (cGMP) requirements for finished pharmaceutical products. It covers various aspects of pharmaceutical manufacturing, including facilities, equipment, controls, and processes to ensure the quality and safety of the final products. Part 212 of Title 21 of the CFR specifically pertains to the cGMP requirements for the manufacturing of positron emission tomography (PET) drugs. PET drugs are radiopharmaceuticals used in medical imaging, and Part 212 sets forth requirements tailored to their unique production processes and quality control measures. The main difference between Part 211 and Part 212 lies in their focus areas, with Part 212 catering to the specialized needs of PET drug manufacturing. Like drugs used for PET, Perspective anticipates that its 203Pb-labelled peptides will be regulated under Part 212. Like other manufactured drugs, Perspective anticipates that 212Pb-labelled peptides will be regulated under Part 211.
Hier wächst ja nun augenscheinlich etwas und ich würde mir nun mal eine Meldung zu einem Auftrag aus dem Pharma oder Militärsektor wünschen. Die Möglichkeiten können mannigfaltiger beinahe nicht sein.
Antwort auf Beitrag Nr.: 75.398.111 von Edelweiss70 am 05.03.24 16:01:54Wäre doch mal ein schöner Wochenabschluss über 1,10$
globalnewswire 11.3.24
Perspective Therapeutics to Host In-Person and Virtual Analyst Day to Discuss Targeted Alpha-Particle Radiotherapy for Cancer on March 18, 2024
SEATTLE, March 11, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. ("Perspective" or "the Company") (NYSE AMERICAN: CATX), a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced that it will host an in-person and virtual analyst day at the New York Stock Exchange on Monday, March 18, 2024 at 10:00 AM ET. To register, click here.
The event will feature presentations from the following members of the Company’s management:
Thijs Spoor, Chief Executive Officer
Michael K. Schultz, PhD, Chief Science Officer
Markus Puhlman, MD, Chief Medical Officer
In addition, key opinion leader Richard L. Wahl, MD (Professor of Radiology, Mallinckrodt Institute of Radiology at Washington University School of Medicine) will provide an overview of the use of specialized targeting peptides and personalized alpha-particle radiopharmaceuticals such as 212Pb to diagnose tumors and deliver powerful radiation specifically to cancer cells.
The event will highlight the Company's theranostic approach and proprietary technology, including the development of complementary imaging diagnostics that enable the ability to see the specific tumor and then treat it, to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments. In addition, the Company’s management will discuss its next radiotherapy compound in development and provide an overview of its promising novel pretargeting technology.
A live question and answer session will follow the formal presentations.
About Richard L. Wahl, MD
Richard L. Wahl, MD, is a professor at Mallinckrodt Institute of Radiology (MIR) at Washington University School of Medicine in St. Louis and served as the director of MIR from 2014 to 2023. An alumnus, Wahl completed a diagnostic radiology residency and nuclear medicine fellowship at MIR. Prior to being named director at MIR, he was director of the Division of Nuclear Medicine at Johns Hopkins University and the first recipient of the Henry N. Wagner Jr. Professorship.
Wahl is a leader in the use of PET scans to diagnose human cancers and other diseases and was among the first to harness the power of the immune system to precisely target radiation therapy to cancers, a technique that has become known as radioimmunotherapy. He has been at the forefront of efforts to combine quantitative data from multiple kinds of scans to form so-called fusion images that can help physicians more precisely diagnose and characterize cancers.
Wahl has received numerous awards, including the 2018 Georg Charles de Hevesy Award from the Society of Nuclear Medicine and Molecular Imaging, where he served as president. He is an elected member of the American Society for Clinical Investigation, the American Association of Physicians and the National Academy of Medicine. A fellow of the American College of Radiology, Wahl holds 18 radiology patents and has published more than 450 peer-reviewed scientific manuscripts. He completed a research fellowship in immunology and earned his medical degree from Washington University in St. Louis.
Perspective Therapeutics to Host In-Person and Virtual Analyst Day to Discuss Targeted Alpha-Particle Radiotherapy for Cancer on March 18, 2024
SEATTLE, March 11, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. ("Perspective" or "the Company") (NYSE AMERICAN: CATX), a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced that it will host an in-person and virtual analyst day at the New York Stock Exchange on Monday, March 18, 2024 at 10:00 AM ET. To register, click here.
The event will feature presentations from the following members of the Company’s management:
Thijs Spoor, Chief Executive Officer
Michael K. Schultz, PhD, Chief Science Officer
Markus Puhlman, MD, Chief Medical Officer
In addition, key opinion leader Richard L. Wahl, MD (Professor of Radiology, Mallinckrodt Institute of Radiology at Washington University School of Medicine) will provide an overview of the use of specialized targeting peptides and personalized alpha-particle radiopharmaceuticals such as 212Pb to diagnose tumors and deliver powerful radiation specifically to cancer cells.
The event will highlight the Company's theranostic approach and proprietary technology, including the development of complementary imaging diagnostics that enable the ability to see the specific tumor and then treat it, to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments. In addition, the Company’s management will discuss its next radiotherapy compound in development and provide an overview of its promising novel pretargeting technology.
A live question and answer session will follow the formal presentations.
About Richard L. Wahl, MD
Richard L. Wahl, MD, is a professor at Mallinckrodt Institute of Radiology (MIR) at Washington University School of Medicine in St. Louis and served as the director of MIR from 2014 to 2023. An alumnus, Wahl completed a diagnostic radiology residency and nuclear medicine fellowship at MIR. Prior to being named director at MIR, he was director of the Division of Nuclear Medicine at Johns Hopkins University and the first recipient of the Henry N. Wagner Jr. Professorship.
Wahl is a leader in the use of PET scans to diagnose human cancers and other diseases and was among the first to harness the power of the immune system to precisely target radiation therapy to cancers, a technique that has become known as radioimmunotherapy. He has been at the forefront of efforts to combine quantitative data from multiple kinds of scans to form so-called fusion images that can help physicians more precisely diagnose and characterize cancers.
Wahl has received numerous awards, including the 2018 Georg Charles de Hevesy Award from the Society of Nuclear Medicine and Molecular Imaging, where he served as president. He is an elected member of the American Society for Clinical Investigation, the American Association of Physicians and the National Academy of Medicine. A fellow of the American College of Radiology, Wahl holds 18 radiology patents and has published more than 450 peer-reviewed scientific manuscripts. He completed a research fellowship in immunology and earned his medical degree from Washington University in St. Louis.
Antwort auf Beitrag Nr.: 75.433.719 von Edelweiss70 am 11.03.24 18:26:28
Globenewswire
18.3.24
Full article
„SEATTLE , March 18, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (NYSE AMERICAN: CATX), a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced a clinical trial collaboration agreement with Bristol Myers Squibb (NYSE: BMY) to evaluate the safety and tolerability of Perspective’s targeted alpha-particle therapy [212Pb]VMT01 in combination with Bristol Myers Squibb’s nivolumab in patients with histologically confirmed melanoma and positive melanocortin 1 receptor (MC1R) imaging scans. This combination study is an amendment to the Company’s ongoing Phase1/2a study of [212Pb]VMT01 in patients with metastatic melanoma. The study has completed enrollment in the first cohort and has commenced dosing in the second cohort.
Under the terms of the collaboration, Perspective will sponsor and fund the combination study and Bristol Myers Squibb will provide nivolumab for use in the study. „
Globenewswire
18.3.24
Full article
„SEATTLE , March 18, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. (NYSE AMERICAN: CATX), a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced a clinical trial collaboration agreement with Bristol Myers Squibb (NYSE: BMY) to evaluate the safety and tolerability of Perspective’s targeted alpha-particle therapy [212Pb]VMT01 in combination with Bristol Myers Squibb’s nivolumab in patients with histologically confirmed melanoma and positive melanocortin 1 receptor (MC1R) imaging scans. This combination study is an amendment to the Company’s ongoing Phase1/2a study of [212Pb]VMT01 in patients with metastatic melanoma. The study has completed enrollment in the first cohort and has commenced dosing in the second cohort.
Under the terms of the collaboration, Perspective will sponsor and fund the combination study and Bristol Myers Squibb will provide nivolumab for use in the study. „
Antwort auf Beitrag Nr.: 75.473.007 von Edelweiss70 am 18.03.24 13:45:41Der Kurs zieht vorbörslich ja schon ganz gut an , mal sehen was da geht
Antwort auf Beitrag Nr.: 75.473.016 von Edelweiss70 am 18.03.24 13:46:24So langsam geht ‘r d’r Peeeter 🐿.
Antwort auf Beitrag Nr.: 75.496.794 von Edelweiss70 am 21.03.24 18:49:31Fast auf Tageshoch bei neuem 52Wochen Hoch geschlossen.
Stabil 💪
Stabil 💪
Glückwunsch Edelweiss 😍
Danke Crowww, ich hoffe das ist erst der Anfang einer großen Story.
Auf das was da noch kommt …..
Auf das was da noch kommt …..
Antwort auf Beitrag Nr.: 75.499.050 von Edelweiss70 am 22.03.24 08:25:00Herrlich 🎉. Das sieht doch sehr gut aus. Hier muss doch zeitnah was vermeldet werden, das ist doch mehr als ein Brodeln.
Antwort auf Beitrag Nr.: 75.554.596 von Edelweiss70 am 02.04.24 22:53:17So dann mal auf in eine hoffentlich ereignisreiche und erfolgreiche Woche
So die 1,50 soll wohl nicht fallen. Morgen die Teilnahem an der 3 Goldman Sachs Healthruption Conference und am 11 April auf der Jefferies Radiopharma. Mal sehen ob man hier weitere Investoren überzeugen kann.
Beitrag zu dieser Diskussion schreiben
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| -0,59 | |
| +4,11 | |
| +1,79 | |
| -0,81 | |
| +0,29 | |
| -49,30 | |
| +1,39 | |
| +0,59 | |
| -2,17 | |
| +3,21 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 210 | ||
| 94 | ||
| 77 | ||
| 66 | ||
| 55 | ||
| 36 | ||
| 34 | ||
| 30 | ||
| 30 | ||
| 29 |



















