ARNA - Arena Pharmaceuticals - 500 Beiträge pro Seite
eröffnet am 07.10.06 21:02:16 von
neuester Beitrag 02.02.09 16:13:28 von
neuester Beitrag 02.02.09 16:13:28 von
Beiträge: 45
ID: 1.086.374
ID: 1.086.374
Aufrufe heute: 0
Gesamt: 8.134
Gesamt: 8.134
Aktive User: 0
ISIN: US0400476075 · WKN: A2DR4A
99,98
USD
+0,06 %
+0,06 USD
Letzter Kurs 11.03.22 NYSE
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 5,0000 | +99.999,00 | |
| 0,9998 | +122,18 | |
| 0,8970 | +75,20 | |
| 1,8200 | +34,81 | |
| 0,8680 | +31,54 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 49,24 | -9,67 | |
| 765,45 | -9,95 | |
| 2,9800 | -13,12 | |
| 4,8500 | -14,31 | |
| 0,6460 | -19,25 |
da es für meine zweite Lieblingsposition neben GPC Biotech noch keinen Thread gibt... hier ein kleiner Info-Thread...
Arena Pharmaceuticals (ARNA)
http://www.arenapharm.com/

Arena Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company, focuses on the research and development of small molecule drugs in the therapeutic areas of metabolic, central nervous system, cardiovascular, and inflammatory diseases. It is developing a pipeline of compounds targeting a class of drug targets called G protein- coupled receptors, or GPCRs, using its technologies, including CART (Constitutively Activated Receptor Technology) and Melanophore. The company has four internally discovered, clinical-stage drug candidates for major diseases. The most advanced candidate is lorcaserin, a selective 5-HT2C serotonin receptor agonist that is under investigation for the treatment of obesity. Arena’s lead drug candidate for the treatment of insomnia, APD125, is a selective 5-HT2A receptor inverse agonist. The company also has two clinical-stage collaborations with Merck & Co., Inc. and Ortho-McNeil, Inc. MK-0354, an Arena-discovered, orally administered drug candidate is under development by Merck for the treatment of atherosclerosis and related disorders. APD668, an Arena-discovered, orally administered drug candidate for the treatment of Type 2 diabetes, is under development by Ortho-McNeil. Arena was incorporated in 1997 and is based in San Diego, California.


von yahoo (7.10. Kurs 14.93 USD):
Market Cap (intraday): 707.01M
Enterprise Value (7-Oct-06)3: 452.09M
Revenue (ttm): 34.76M
Total Cash (mrq): 268.52M
Total Debt (mrq): 13.58M
Pipeline:
http://www.arenapharm.com/wt/page/prod_pipeline
* Lorcaserin, PIII, Fettsucht(obesity), 5-HT2C serotonin receptor, kein Partner, BLA u.U. 2009, mögliche peak sales 5+ Mrd USD
* APD125, PI (PII in Vorbereitung), Schlafstörungen(insomnia), 5-HT2A serotonin receptor, kein Partner, mögliche peak sales 2+ Mrd USD
* APD791, P0, Blutgerinnungshemmer (wie Plavix), 5-HT2A serotonin receptor, kein Partner, mögliche peak sales 6+ Mrd USD
* APD668, PI, Type 2 Diabetis, GDIR, Ortho-McNeil (J&J)
* Niacin Receptor Agonisten, P0, Atherosklerose/HDL, Merck
mfg ipollit
Arena Pharmaceuticals (ARNA)
http://www.arenapharm.com/

Arena Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company, focuses on the research and development of small molecule drugs in the therapeutic areas of metabolic, central nervous system, cardiovascular, and inflammatory diseases. It is developing a pipeline of compounds targeting a class of drug targets called G protein- coupled receptors, or GPCRs, using its technologies, including CART (Constitutively Activated Receptor Technology) and Melanophore. The company has four internally discovered, clinical-stage drug candidates for major diseases. The most advanced candidate is lorcaserin, a selective 5-HT2C serotonin receptor agonist that is under investigation for the treatment of obesity. Arena’s lead drug candidate for the treatment of insomnia, APD125, is a selective 5-HT2A receptor inverse agonist. The company also has two clinical-stage collaborations with Merck & Co., Inc. and Ortho-McNeil, Inc. MK-0354, an Arena-discovered, orally administered drug candidate is under development by Merck for the treatment of atherosclerosis and related disorders. APD668, an Arena-discovered, orally administered drug candidate for the treatment of Type 2 diabetes, is under development by Ortho-McNeil. Arena was incorporated in 1997 and is based in San Diego, California.
von yahoo (7.10. Kurs 14.93 USD):
Market Cap (intraday): 707.01M
Enterprise Value (7-Oct-06)3: 452.09M
Revenue (ttm): 34.76M
Total Cash (mrq): 268.52M
Total Debt (mrq): 13.58M
Pipeline:
http://www.arenapharm.com/wt/page/prod_pipeline
* Lorcaserin, PIII, Fettsucht(obesity), 5-HT2C serotonin receptor, kein Partner, BLA u.U. 2009, mögliche peak sales 5+ Mrd USD
* APD125, PI (PII in Vorbereitung), Schlafstörungen(insomnia), 5-HT2A serotonin receptor, kein Partner, mögliche peak sales 2+ Mrd USD
* APD791, P0, Blutgerinnungshemmer (wie Plavix), 5-HT2A serotonin receptor, kein Partner, mögliche peak sales 6+ Mrd USD
* APD668, PI, Type 2 Diabetis, GDIR, Ortho-McNeil (J&J)
* Niacin Receptor Agonisten, P0, Atherosklerose/HDL, Merck
mfg ipollit

das Übergewicht heutzutage ein großes Problem darstellt dürfte bekannt sein...
For drug companies, the stakes couldn't be higher. In a world where a blockbuster drug is one with $1 billion a year in sales, analysts give $5 billion as the low estimate for sales of an important obesity drug. If a company developed a truly safe, effective weight-loss drug and sold it for $3 a day to one-quarter of the 97 million American adults estimated to be overweight, sales would exceed $26 billion a year in this country alone.
zur Zeit gibt es nur zwei zugelassene Medikamente, die z.B. die Aufnahme von Fett vermindern. In den 90er-Jahren gab es u.a. das wirkungsvolles Medikament "FenPhen", dass als Blockbuster allerdings wegen schwerer Nebenwirkungen vom Markt genommen werden musste:
24.05.2006 21:47
Wyeth erhält FDA-Genehmigung für Fen-Phen-Fonds
Der amerikanische Pharmakonzern Wyeth (ISIN US9830241009 (Nachrichten/Aktienkurs)/ WKN 850229) hat am Mittwoch von der US-Gesundheitsbehörde FDA für die Ergänzung des Diätpräparate-Vergleichs die abschließende Zulassung erhalten.
Demnach wird Wyeth im Zusammenhang mit dem Diätpräparate-Mix "Fen-Phen" 1,275 Mrd. Dollar in einen neuen Fonds zahlen. Um Schadenzahlungen begleichen zu können wird der Konzern zunächst 400 Mio. Dollar einzahlen. Der Medikamente-Mix war 1997 aufgrund von Problemen mit Herzklappen vom Markt genommen worden.
FenPhen wirkt auf Serotonin Rezeptoren im Gehirn, die das Hungergefühl unterdrücken können. Allerdings nicht selektiv genug, so dass ähnliche Rezeptoren u.a. im Herz auch aktiviert werden, was wahrscheinlich zu den schweren Nebenwirkungen führt.
Lorcaserin (APD356) unterdrückt als praktisch selektive Variante von FenPhen über den 5-HT2C Serotonin Rezeptor das Hungergefühl. Da Lorca aber nur diese aktivieren soll, sind theoretisch die Nebenwirkungen nicht zu erwarten. Eine Wirksamkeit als Variante von FenPhen ist sehr wahrscheinlich, was in der PII bereits gezeigt werden konnte. Das Problem stellen die Nebenwirkungen dar, die bei FenPhen aufgetreten sind. Ich denke, dass dieses Risiko bis jetzt die großen Pharmas abgeschreckt hat. In der PII konnten keine Cardio-Probleme festgestellt werden, so dass die FDA die Durchführung der PIII genehmigt hat. Sollten auch hier bei 6000 Patienten die Nebenwirkungen nicht auftreten, so werden sich wohl alle um Lorca reißen. Nächstes Jahr gibt es eine erste Sicherheits-Analyse von 3000 Patienten, dann wird man vielleicht mehr wissen.
Obesity afflicts a large and increasing proportion of the population. More than 30 percent of Americans are obese, a figure that has risen sharply over the past decade, according to the Centers for Disease Control and Prevention. Substantial health risks are associated with overweight and obesity, leading to increased morbidity and mortality. Obesity is a leading cause of diabetes and accounts for more than 2.6 million deaths annually worldwide. The U.S. Surgeon General has estimated the direct and indirect economic costs of obesity at $117 billion per year. There are only two approved drugs to treat the condition. Concerns about side effects and efficacy have limited the use of these drugs, presenting an opportunity to help millions of patients with a new, safe and effective therapy.
mfg ipollit
For drug companies, the stakes couldn't be higher. In a world where a blockbuster drug is one with $1 billion a year in sales, analysts give $5 billion as the low estimate for sales of an important obesity drug. If a company developed a truly safe, effective weight-loss drug and sold it for $3 a day to one-quarter of the 97 million American adults estimated to be overweight, sales would exceed $26 billion a year in this country alone.
zur Zeit gibt es nur zwei zugelassene Medikamente, die z.B. die Aufnahme von Fett vermindern. In den 90er-Jahren gab es u.a. das wirkungsvolles Medikament "FenPhen", dass als Blockbuster allerdings wegen schwerer Nebenwirkungen vom Markt genommen werden musste:
24.05.2006 21:47
Wyeth erhält FDA-Genehmigung für Fen-Phen-Fonds
Der amerikanische Pharmakonzern Wyeth (ISIN US9830241009 (Nachrichten/Aktienkurs)/ WKN 850229) hat am Mittwoch von der US-Gesundheitsbehörde FDA für die Ergänzung des Diätpräparate-Vergleichs die abschließende Zulassung erhalten.
Demnach wird Wyeth im Zusammenhang mit dem Diätpräparate-Mix "Fen-Phen" 1,275 Mrd. Dollar in einen neuen Fonds zahlen. Um Schadenzahlungen begleichen zu können wird der Konzern zunächst 400 Mio. Dollar einzahlen. Der Medikamente-Mix war 1997 aufgrund von Problemen mit Herzklappen vom Markt genommen worden.
FenPhen wirkt auf Serotonin Rezeptoren im Gehirn, die das Hungergefühl unterdrücken können. Allerdings nicht selektiv genug, so dass ähnliche Rezeptoren u.a. im Herz auch aktiviert werden, was wahrscheinlich zu den schweren Nebenwirkungen führt.
Lorcaserin (APD356) unterdrückt als praktisch selektive Variante von FenPhen über den 5-HT2C Serotonin Rezeptor das Hungergefühl. Da Lorca aber nur diese aktivieren soll, sind theoretisch die Nebenwirkungen nicht zu erwarten. Eine Wirksamkeit als Variante von FenPhen ist sehr wahrscheinlich, was in der PII bereits gezeigt werden konnte. Das Problem stellen die Nebenwirkungen dar, die bei FenPhen aufgetreten sind. Ich denke, dass dieses Risiko bis jetzt die großen Pharmas abgeschreckt hat. In der PII konnten keine Cardio-Probleme festgestellt werden, so dass die FDA die Durchführung der PIII genehmigt hat. Sollten auch hier bei 6000 Patienten die Nebenwirkungen nicht auftreten, so werden sich wohl alle um Lorca reißen. Nächstes Jahr gibt es eine erste Sicherheits-Analyse von 3000 Patienten, dann wird man vielleicht mehr wissen.
Obesity afflicts a large and increasing proportion of the population. More than 30 percent of Americans are obese, a figure that has risen sharply over the past decade, according to the Centers for Disease Control and Prevention. Substantial health risks are associated with overweight and obesity, leading to increased morbidity and mortality. Obesity is a leading cause of diabetes and accounts for more than 2.6 million deaths annually worldwide. The U.S. Surgeon General has estimated the direct and indirect economic costs of obesity at $117 billion per year. There are only two approved drugs to treat the condition. Concerns about side effects and efficacy have limited the use of these drugs, presenting an opportunity to help millions of patients with a new, safe and effective therapy.
mfg ipollit
Antwort auf Beitrag Nr.: 24.479.657 von ipollit am 07.10.06 22:28:05ARNA :2Q Update-Details On Lorcaserin Phase III Design
2006-07-26 06:11 (New York)
Piper Jaffray & Co. Company Note
(ARNA - $10.52)
Outperform Volatility: Medium
2Q Update-Details On Lorcaserin Phase III Design
Thomas Wei, Senior Research Analyst Reason for Report:
212 284-9305, thomas.a.wei@pjc.com
Gur A. Roshwalb, M.D., Research Analyst
212-284-9314, gur.a.roshwalb@pjc.com
KEY POINTS:
Larger Phase III Trials For Lorcaserin But No Change To Timeline.
* Details On Lorcaserin Phase III Trial Design. ARNA provided an update on the
expected Phase III trial design for lorcaserin for obesity. As a reminder,
lorcaserin is a 5-HT2c agonist, similar in target but more selective than
fenfluramine, a popular diet drug which was withdrawn from the market for
causing valvular defects. ARNA disclosed that it expects the lorcaserin
Phase III program to be at the high end of its original guidance, involving
6,000 patients in three separate trials and expected to cost $125 million
over 2-3 years. Given the company's agreement with the FDA on key safety and
valvulopathy endpoints, and as such, ARNA did not see the need for a Special
Protocol Assessment (SPA). While the program size and cost is higher than
our original expectations, we are encouraged that there is no change to the
timeline for a 2009 filing, and we believe that the size is unlikely to
deter potential pharmaceutical partners for lorcaserin. We also believe that
the larger number of patients may help mitigate the potential event rate
risk stemming from the lack of data available to estimate the expected
background rate of valvulopathy in the control arm, and should provide the
appropriate statistical power to eliminate even a very small cardiac safety
risk with the drug and to convince physicians of its safety profile.
* Phase III Program To Assess Echocardiograms In 3,000 Patient Trial Over Two
Years. The initial Phase III trial will assess 10 mg of lorcaserin taken
twice daily vs. placebo in 3,000 obese patients. Echocardiograms will be
done at baseline and at 6, 12, 18, and 24 months. Echocardiograms will be
reviewed centrally and a Data Safety Monitoring Board (DSMB) will provide an
interim safety analysis of the echos at 6 and 12 months. The first DSMB
review will likely occur in mid-2007. Given the data for fenfluramine
showing separation in valvulopathy rates at month six, we believe that this
first interim analysis will be an important risk reduction event for the
drug. Assuming a positive safety outcome at the 6-month DSMB analysis, ARNA
will initiate two additional 1,500 patient trials, including one in
diabetes, testing 10 mg taken once or twice daily vs. placebo.
* Changes To Estimates. We have adjusted our model to account for updated
guidance and the size of the expected Phase III program. Our new model also
conservatively assumes that a pharma partnership for lorcaserin is signed in
2H07 following the first DSMB analysis. We are revising our EPS estimates
from ($1.91) to ($2.04) in 2006 and from ($0.02) to ($1.37) in 2007. We are
also adjusting our price target methodology for our coverage universe for
one-half fewer discount periods. Our price target remains unchanged at $20
based on a 35x 2013E EPS, discounted at 30% for 5.5 periods.
INVESTMENT RECOMMENDATION:
We are encouraged about the prospects for lorcaserin for obesity and the
company's platform technology for generating new drug candidates.
RISKS TO ACHIEVEMENT OF TARGET PRICE:
Risks include but are not limited to: (1) setbacks in lorcaserin partnership
discussions; and (2) cardiac safety concerns with lorcaserin.
COMPANY DESCRIPTION:
Arena is a leader in developing drugs to target GPCRs.
mfg ipollit
2006-07-26 06:11 (New York)
Piper Jaffray & Co. Company Note
(ARNA - $10.52)
Outperform Volatility: Medium
2Q Update-Details On Lorcaserin Phase III Design
Thomas Wei, Senior Research Analyst Reason for Report:
212 284-9305, thomas.a.wei@pjc.com
Gur A. Roshwalb, M.D., Research Analyst
212-284-9314, gur.a.roshwalb@pjc.com
KEY POINTS:
Larger Phase III Trials For Lorcaserin But No Change To Timeline.
* Details On Lorcaserin Phase III Trial Design. ARNA provided an update on the
expected Phase III trial design for lorcaserin for obesity. As a reminder,
lorcaserin is a 5-HT2c agonist, similar in target but more selective than
fenfluramine, a popular diet drug which was withdrawn from the market for
causing valvular defects. ARNA disclosed that it expects the lorcaserin
Phase III program to be at the high end of its original guidance, involving
6,000 patients in three separate trials and expected to cost $125 million
over 2-3 years. Given the company's agreement with the FDA on key safety and
valvulopathy endpoints, and as such, ARNA did not see the need for a Special
Protocol Assessment (SPA). While the program size and cost is higher than
our original expectations, we are encouraged that there is no change to the
timeline for a 2009 filing, and we believe that the size is unlikely to
deter potential pharmaceutical partners for lorcaserin. We also believe that
the larger number of patients may help mitigate the potential event rate
risk stemming from the lack of data available to estimate the expected
background rate of valvulopathy in the control arm, and should provide the
appropriate statistical power to eliminate even a very small cardiac safety
risk with the drug and to convince physicians of its safety profile.
* Phase III Program To Assess Echocardiograms In 3,000 Patient Trial Over Two
Years. The initial Phase III trial will assess 10 mg of lorcaserin taken
twice daily vs. placebo in 3,000 obese patients. Echocardiograms will be
done at baseline and at 6, 12, 18, and 24 months. Echocardiograms will be
reviewed centrally and a Data Safety Monitoring Board (DSMB) will provide an
interim safety analysis of the echos at 6 and 12 months. The first DSMB
review will likely occur in mid-2007. Given the data for fenfluramine
showing separation in valvulopathy rates at month six, we believe that this
first interim analysis will be an important risk reduction event for the
drug. Assuming a positive safety outcome at the 6-month DSMB analysis, ARNA
will initiate two additional 1,500 patient trials, including one in
diabetes, testing 10 mg taken once or twice daily vs. placebo.
* Changes To Estimates. We have adjusted our model to account for updated
guidance and the size of the expected Phase III program. Our new model also
conservatively assumes that a pharma partnership for lorcaserin is signed in
2H07 following the first DSMB analysis. We are revising our EPS estimates
from ($1.91) to ($2.04) in 2006 and from ($0.02) to ($1.37) in 2007. We are
also adjusting our price target methodology for our coverage universe for
one-half fewer discount periods. Our price target remains unchanged at $20
based on a 35x 2013E EPS, discounted at 30% for 5.5 periods.
INVESTMENT RECOMMENDATION:
We are encouraged about the prospects for lorcaserin for obesity and the
company's platform technology for generating new drug candidates.
RISKS TO ACHIEVEMENT OF TARGET PRICE:
Risks include but are not limited to: (1) setbacks in lorcaserin partnership
discussions; and (2) cardiac safety concerns with lorcaserin.
COMPANY DESCRIPTION:
Arena is a leader in developing drugs to target GPCRs.
mfg ipollit
und was macht die Konkurrenz?
z.B. die Wunderpille "Acomplia", der potentielle Multi-Mrd-Blockbuster
********
Neuartige Abspeckpille auf dem Markt
Der Arzneimittelkonzern Lilly hat gleich fünf Mittel in der Pipeline, und seit drei Wochen ist die erste biologische Abspeckpille auf dem deutschen Markt. Acomplia von Sanofi-Aventis könnte ein Blockbuster werden. Der Analyst Gbola Amusa von Sanford C Bernstein geht von einem Umsatz im Jahr 2010 von 3,9 Milliarden Euro aus – und nennt diese Prognose „konservativ“.
Die neuen Abspeckpillen wirken – anders als bisherige Produkte – dort, wo Schwarz die Regulation des Hungergefühl lokalisiert: Im Gehirn. Acomplia greift am so genannten Endocannabinoid-System. Es bedient sich Substanzen, die ähnlich wirken wie Cannabis. Sie machen Lust auf Naschereien und steigern die Geschmackssinne. Acomplia blockiert die Rezeptoren, an denen die Endocannabinoide andocken – und drosselt so den Appetit.
*******
Acomplia – Wunderpille bekämpft Fettsucht
von Prof. Oliver Reiser
Fett- und Nikotinsucht ade. Ein neues Arzneimittel, das nach einem revolutionären Mechanismus wirkt, könnte schon bald in Deutschland zugelassen werden.
Übergewicht ist eines der größten Probleme in der westlichen Welt. Etwa die Hälfte der erwachsenen Bevölkerung ab 18 Jahren in Deutschland hat Übergewicht, und trotz Diät und Sport scheint der Kampf gegen die Pfunde oftmals aussichtslos.
Immer wieder tauchen daher neue Diäten und Wunderpillen auf, die in kürzester Zeit ein dramatisches Abspecken versprechen. Doch ist der Erfolg meistens nur von kurzer Dauer, und der Jo Jo Effekt bringt oftmals mehr Pfunde wieder zurück als vor der Diät vorhanden waren.
Essen macht glücklich!
Erfolgreich sind langfristig nur zwei Maßnahmen: Eine Umstellung auf eine Kalorien bewusste Ernährung und ausreichend Bewegung. Leichter gesagt als getan: Denn die Lust gerade auf die zuckerhaltigen Kalorienbömbchen ist in der Regel nicht auf Bedarf an Nahrung zurückzuführen, sondern darauf, dass bei deren Genuss ins Gehirn Stoffe gelangen, die an Rezeptoren im so genannten „Wohlfühlzentrum“ binden und letztere so aktivieren. Das hierdurch ausgelöste Wohlbefinden wird mit Essen assoziiert, und das Gehirn verlangt durch Auslösen von Appetit in der Folge immer wieder nach der Nahrung, die dieses Wohlbefinden auslösen konnte. Es kommt zu einem Suchtverhalten, und ganz analog wird auf diesem Wege auch das Verlangen nach Nikotin oder Alkohol erzeugt.
Coca Cola light macht nicht glücklich!
Wer zur Kalorienreduktion seine Trinkgewohnheiten von zuckerhaltiger auf süßstoffhaltige Limonade umgestellt, kennt vielleicht diesen Effekt: Zunächst lässt sich das Gehirn austricksen und nimmt etwa Coca Cola light, die ja auch süß schmeckt, als Ersatz für Coca Cola an. Doch schon nach kurzer Zeit unterscheidet das Gehirn, trotz gleichen Geschmacks, zwischen Coca Cola und Coca Cola light, da das süßstoffhaltige Getränk die Wohlfühlrezeptoren im Gehirn nicht stimulieren kann.
Acomplia – ein Arzneimittel wirkt auf das Wohlfühlzentrum
Das von der Firma Sanofi-Aventis sich gegenwärtig in weit fortgeschrittener Evaluierung, in so genannter klinischer Phase III befindliche Medikament Acomplia (Wirkstoff Rimonabant) könnte in revolutionärer Weise unser Suchtverhalten – Essen, Nikotin und eventuell auch Alkohol – positiv beeinflussen. Acomplia wirkt als so genannter Antagonist am Wohlfühlzentrum im Gehirn, das heißt es blockiert die Rezeptoren, ohne sie dabei zu aktivieren und damit deren Funktion, in diesem Fall Wohlfühlen gekoppelt mit Appetit, auszulösen. Nimmt man also dieses Medikament ein, stellt sich bei anschließender Nahrungsaufnahme oder auch beim Rauchen der befriedigende Effekt, aber dadurch auch das Auslösen von Appetit nach weiterer Nahrung oder Nikotin, nicht mehr ein.
Klinische Studie in Europa, USA und Canada vielversprechend
Kürzliche Studien in Europa und Nordamerika, die je 3000 übergewichtige Probanden und einen Zeitraum von zwei Jahren umfassten, zeigen eindrucksvolle Ergebnisse: Bei fast zwei Dritteln der Testpersonen, die eine Dosis von 20 mg Acomplia pro Tag erhielten, wurde eine Gewichtsreduktion von fünf Prozent des Körpergewichts festgestellt. Nur ein Drittel der Testpersonen, die ein Placebo oder eine Dosis von nur 5 mg Acomplia erhielten, erzielten ein vergleichbares Ergebnis. Auch der Cholesterinspiegel wurde durch Acomplia positiv reguliert: Während das „gute“ HDL Cholesterin im Schnitt um 25 Prozent durch die höhere Dosis bedingt anstieg, fielen gleichermaßen die Werte für das „schlechte“ LDL Cholesterin. Alle Testpersonen erhielten zu Beginn der Studie eine Ernährungsberatung und die Empfehlung, 600 Kalorien pro Tag in ihrer Nahrung einzusparen. Keiner der Testpersonen wusste jedoch ob und welche Dosis Acomplia sie erhielt.
Keine bedenklichen Nebenwirkungen
Ein besonders wichtiges Ergebnis der Studie war die gute Verträglichkeit des neuen Medikaments. Einige Testpersonen klagten kurzzeitig über Übelkeit, doch in keinem bedenklichen Ausmaß. Depressionen und Angstzustände waren nicht stärker ausgeprägt als in den Kontrollgruppen.
Bislang mussten immer wieder gewichtsreduzierende Arzneimittel aufgrund von ernsten Nebenwirkungen für vom Markt genommen werden. Nur zwei Medikamente sind gegenwärtig zur langfristigen Behandlung von Fettsucht zugelassen, der Lipase Inhibitor Orlistat und Sibutramin, die aber beide nicht unproblematisch aufgrund ihres Nebenwirkungprofil sind.
Der neue Wirkmechanismus durch Blockierung der Suchtrezeptoren von Acomplia könnte in der Tat die Problematik herkömmlicher Medikamente auf Herz und Kreislauf umgehen. Die durch Acomplia bewirkte Umstellung der Essgewohnheiten ist darüber hinaus ein innovativer Weg, nachhaltig und langfristig Übergewicht zu bekämpfen.
Sollten die klinischen Studien weiterhin gute Ergebnisse zeigen, könnte bereits 2006/2007 Acomplia auf den deutschen Markt kommen. Eine Einführung in USA war für die erste Jahreshälfte 2006 geplant. Mit einer Entscheidung der Food and Drug Administration (FDA, Zulassungsbehörde in USA) wird Anfang März, evtl. sogar schon Ende Januar 2006 gerechnet.
******
DECEMBER 27, 2004
EUROPEAN BUSINESS
Back to Main Story
The End Of Obesity As We Know It
Sanofi-Aventis' Acomplia promises that and more. But approval isn't a slam dunk
Ever wonder why marijuana smokers get the munchies? So did a team of scientists at Sanofi Recherche lab in Montpelier, France. Fifteen years ago they began investigating marijuana's effects on the brain, including the well-known fact that cannabis makes users hungry. "We set out to try and create an anti-marijuana," a drug that could suppress appetite by blocking the same switch in the brain activated by cannabis, says Gérard Le Fur, senior executive vice-president and board member at newly merged French pharmaceutical giant Sanofi-Aventis (SNY ).
They succeeded beyond their wildest dreams, discovering a medicine that not only helps people lose weight but also shrinks abdominal fat, helps people stop smoking, improves cholesterol levels, and helps patients better regulate blood sugar.
Talk about a potential blockbuster. Initially, though, Sanofi will take it slow. It will seek approval of the drug, Acomplia, in Europe and the U.S. by the second quarter of 2005 as a treatment for just two of the conditions: obesity and tobacco addiction. Because patients in Acomplia trials regained weight after stopping treatment, the company hopes regulators will approve it for long-term use.
Researchers say side effects such as nausea and depression are relatively minor and short-lived. But as the first in an entirely new class of drugs that affect a pleasure center in the brain, even the slightest hint of psychiatric side effects may lead regulators to demand more long-term safety data, potentially delaying Acomplia's launch beyond 2006 as planned. Still, Sanofi-Aventis is confident that the drug's impressive efficacy will assuage any such worries. "It's a product that takes aim at two of the great maladies of the century," says Sanofi-Aventis CEO Jean-François Dehecq.
DEPRESSION QUESTION
Acomplia is the first in a new class of compounds under development to block receptors found in the brain and in fat tissue known as cannabinoid type 1 (CB1). These receptors control hunger and tobacco addiction. Chronic overeating and smoking sends them into overdrive. Blocking the CB1 receptors dramatically reduces such cravings. Results of a two-year clinical trial in the U.S. showed patients given Acomplia lost an average of 19 pounds, compared with five pounds for patients given a placebo. Those on Acomplia also reported higher levels of HDL, the good cholesterol, lower levels of triglycerides, and improved sensitivity to insulin. All are important in keeping heart disease at bay. "This could be a paradigm-shifting drug," says Dr. Louis J. Aronne, president of the North American Society for the Study of Obesity.
The market potential is huge. More than a third of Americans are clinically obese, or 30% above their ideal body weight. And it's not just an American phenomenon. Dr. Gbola Amusa, senior research analyst for Sanford C. Bernstein & Co. in London, estimates that as much as 10% of health-care costs in other industrialized countries are related to being overweight. The two leading obesity drugs, Xenical and Meridia, have unpleasant side effects such as diarrhea or high blood pressure, so analysts think Acomplia will quickly win market share if approved. Amusa estimates sales will reach $5.6 billion a year by the end of the decade.
Accomplia's real potential may go well beyond eating and smoking. The company hopes it will also become the first drug approved for the treatment of metabolic syndrome, a combination of abdominal fat, high blood pressure, high blood-fat levels, low levels of HDL cholesterol, and high blood-sugar levels, all of which contribute to cardiovascular disease. "This is not just a diet drug but a significant advancement in cardiovascular treatment," says Amusa.
Still, there is reason for caution. There was a noticeable rate of withdrawal in Acomplia's clinical trials due to depression. Researchers involved in the trials say that might be because people taking the drug went in with unrealistic weight-loss expectations or were more susceptible to depression to begin with.
Either way, there are no long-term studies yet of the effects of interfering with this part of the brain. And given the increased regulatory scrutiny on new drugs after the Vioxx and antidepressant controversies, the company may find it needs to submit more data than anticipated to secure approval. Is Acomplia too good to be true? "There has never been a diet drug approved that has had more benefits than risks," says Dr. Larry D. Sasich of Public Citizen's health research group in Washington. Dehecq and Le Fur are determined to prove doubters like him wrong.
************
ROCKVILLE, Md., Feb. 17 - The FDA issued an approvable letter on weight management today for Acomplia (rimonabant), spelling out the steps Sanofi-aventis needs to take to win final approval for its much-heralded diet drug.
Neither the company nor the FDA revealed the specifics of the letter.
In a press release issued in Paris, the company said "Sanofi-aventis will continue to work in close collaboration with the FDA."
Sanofi-aventis also said the FDA issued a non-approvable letter for the indication of smoking cessation for Acomplia.
*******
However, Aitken highlights the potential of several new medicines launched in the past year, including diabetes treatment Byetta, co-marketed by Eli Lilly and Amylin Pharmaceuticals, and Lunesta, an insomnia drug made by Sepracor. And there are more on the way that he says are worth watching. The two key drug launches this year are of Sutent, Pfizer's first big entry into cancer drugs, and Acomplia, the anti-obesity pill being developed by Sanofi-Aventis.
Sutent is already on the market, although sales data are not yet available. Acomplia has been delayed at the U.S. Food and Drug Administration and rejected as a stop-smoking drug. Some cardiologists, who are excited about the drug because of its potential to reduce the risk of heart disease, are also worried about side effects.
Acomplia works by blocking the same brain receptor that makes pot smokers hungry; psychiatric symptoms like anxiety are one of the most common reasons patients stopped taking Acomplia in clinical trials. "It's a pill that blocks the 'happy receptor,' " says Prediman K. Shah of Cedars Sinai Medical Center. "The main reason for concern is that it might have an adverse impact on depression or suicide." He is nonetheless very excited about the pill.

************
The Inscrutable French
Posted by Derek
Since I was asking the same musical question just the other day, I wanted to refer people to this article by Matthew Herper over at Forbes, who also wants to know: where is Acomplia/rimonabant, anyway?
It's amazed me for months now that Sanofi-Aventis can get away with saying nothing at all about the prospects for their potential biggest-selling drug ever. Back when the first FDA action came, I predicted, with miserable inaccuracy, that the company would have something to say within days. It's been months, and no one knows anything more than we did back in February.
As the Forbes piece makes clear, analysts and institutional investors seem to be losing patience. I'm not sure what it is about the Sanofi corporate culture that makes this strategy seem like a good idea, but they might want to reexamine it. What might appear like calm and steadfast behavior from their perspective is starting to look, from the outside, like the actions of a company with something to hide. This is America, guys. We talk about things over here; you can't shut us up. Join the party.
*******
praveen, The issue with rimonabant, I believe, is a mechanism-based increase in depressive symptoms. Consider that smoking marijuana containing THC (a mixed CB1, CB2 agonist) induces euphoria and hunger. The CB1 antagonist rimonabant decreases hunger but apparently also causes the opposite of euphoria, or depression. In the RIO trials of rimonabant in Europe and North America, both showed a dose-dependent increase in depressive symptoms. If rimonabant were used widely, it's not clear at this point whether the net effects; the decrease in morbidity and mortality due to weight loss, less the increase in morbiditiy and mortality due to depression, would be positive. How this will be handled by the FDA is entirely unclear at this point. The risk of depression was predictable from the mechanism and SNY should have addressed it earlier.
Of course, lorcaserin has a completely different mechanism of action and we know this isn't going to happen to ARNA. I remain upbeat about ARNA.
mfg ipollit
z.B. die Wunderpille "Acomplia", der potentielle Multi-Mrd-Blockbuster
********
Neuartige Abspeckpille auf dem Markt
Der Arzneimittelkonzern Lilly hat gleich fünf Mittel in der Pipeline, und seit drei Wochen ist die erste biologische Abspeckpille auf dem deutschen Markt. Acomplia von Sanofi-Aventis könnte ein Blockbuster werden. Der Analyst Gbola Amusa von Sanford C Bernstein geht von einem Umsatz im Jahr 2010 von 3,9 Milliarden Euro aus – und nennt diese Prognose „konservativ“.
Die neuen Abspeckpillen wirken – anders als bisherige Produkte – dort, wo Schwarz die Regulation des Hungergefühl lokalisiert: Im Gehirn. Acomplia greift am so genannten Endocannabinoid-System. Es bedient sich Substanzen, die ähnlich wirken wie Cannabis. Sie machen Lust auf Naschereien und steigern die Geschmackssinne. Acomplia blockiert die Rezeptoren, an denen die Endocannabinoide andocken – und drosselt so den Appetit.
*******
Acomplia – Wunderpille bekämpft Fettsucht
von Prof. Oliver Reiser
Fett- und Nikotinsucht ade. Ein neues Arzneimittel, das nach einem revolutionären Mechanismus wirkt, könnte schon bald in Deutschland zugelassen werden.
Übergewicht ist eines der größten Probleme in der westlichen Welt. Etwa die Hälfte der erwachsenen Bevölkerung ab 18 Jahren in Deutschland hat Übergewicht, und trotz Diät und Sport scheint der Kampf gegen die Pfunde oftmals aussichtslos.
Immer wieder tauchen daher neue Diäten und Wunderpillen auf, die in kürzester Zeit ein dramatisches Abspecken versprechen. Doch ist der Erfolg meistens nur von kurzer Dauer, und der Jo Jo Effekt bringt oftmals mehr Pfunde wieder zurück als vor der Diät vorhanden waren.
Essen macht glücklich!
Erfolgreich sind langfristig nur zwei Maßnahmen: Eine Umstellung auf eine Kalorien bewusste Ernährung und ausreichend Bewegung. Leichter gesagt als getan: Denn die Lust gerade auf die zuckerhaltigen Kalorienbömbchen ist in der Regel nicht auf Bedarf an Nahrung zurückzuführen, sondern darauf, dass bei deren Genuss ins Gehirn Stoffe gelangen, die an Rezeptoren im so genannten „Wohlfühlzentrum“ binden und letztere so aktivieren. Das hierdurch ausgelöste Wohlbefinden wird mit Essen assoziiert, und das Gehirn verlangt durch Auslösen von Appetit in der Folge immer wieder nach der Nahrung, die dieses Wohlbefinden auslösen konnte. Es kommt zu einem Suchtverhalten, und ganz analog wird auf diesem Wege auch das Verlangen nach Nikotin oder Alkohol erzeugt.
Coca Cola light macht nicht glücklich!
Wer zur Kalorienreduktion seine Trinkgewohnheiten von zuckerhaltiger auf süßstoffhaltige Limonade umgestellt, kennt vielleicht diesen Effekt: Zunächst lässt sich das Gehirn austricksen und nimmt etwa Coca Cola light, die ja auch süß schmeckt, als Ersatz für Coca Cola an. Doch schon nach kurzer Zeit unterscheidet das Gehirn, trotz gleichen Geschmacks, zwischen Coca Cola und Coca Cola light, da das süßstoffhaltige Getränk die Wohlfühlrezeptoren im Gehirn nicht stimulieren kann.
Acomplia – ein Arzneimittel wirkt auf das Wohlfühlzentrum
Das von der Firma Sanofi-Aventis sich gegenwärtig in weit fortgeschrittener Evaluierung, in so genannter klinischer Phase III befindliche Medikament Acomplia (Wirkstoff Rimonabant) könnte in revolutionärer Weise unser Suchtverhalten – Essen, Nikotin und eventuell auch Alkohol – positiv beeinflussen. Acomplia wirkt als so genannter Antagonist am Wohlfühlzentrum im Gehirn, das heißt es blockiert die Rezeptoren, ohne sie dabei zu aktivieren und damit deren Funktion, in diesem Fall Wohlfühlen gekoppelt mit Appetit, auszulösen. Nimmt man also dieses Medikament ein, stellt sich bei anschließender Nahrungsaufnahme oder auch beim Rauchen der befriedigende Effekt, aber dadurch auch das Auslösen von Appetit nach weiterer Nahrung oder Nikotin, nicht mehr ein.
Klinische Studie in Europa, USA und Canada vielversprechend
Kürzliche Studien in Europa und Nordamerika, die je 3000 übergewichtige Probanden und einen Zeitraum von zwei Jahren umfassten, zeigen eindrucksvolle Ergebnisse: Bei fast zwei Dritteln der Testpersonen, die eine Dosis von 20 mg Acomplia pro Tag erhielten, wurde eine Gewichtsreduktion von fünf Prozent des Körpergewichts festgestellt. Nur ein Drittel der Testpersonen, die ein Placebo oder eine Dosis von nur 5 mg Acomplia erhielten, erzielten ein vergleichbares Ergebnis. Auch der Cholesterinspiegel wurde durch Acomplia positiv reguliert: Während das „gute“ HDL Cholesterin im Schnitt um 25 Prozent durch die höhere Dosis bedingt anstieg, fielen gleichermaßen die Werte für das „schlechte“ LDL Cholesterin. Alle Testpersonen erhielten zu Beginn der Studie eine Ernährungsberatung und die Empfehlung, 600 Kalorien pro Tag in ihrer Nahrung einzusparen. Keiner der Testpersonen wusste jedoch ob und welche Dosis Acomplia sie erhielt.
Keine bedenklichen Nebenwirkungen
Ein besonders wichtiges Ergebnis der Studie war die gute Verträglichkeit des neuen Medikaments. Einige Testpersonen klagten kurzzeitig über Übelkeit, doch in keinem bedenklichen Ausmaß. Depressionen und Angstzustände waren nicht stärker ausgeprägt als in den Kontrollgruppen.
Bislang mussten immer wieder gewichtsreduzierende Arzneimittel aufgrund von ernsten Nebenwirkungen für vom Markt genommen werden. Nur zwei Medikamente sind gegenwärtig zur langfristigen Behandlung von Fettsucht zugelassen, der Lipase Inhibitor Orlistat und Sibutramin, die aber beide nicht unproblematisch aufgrund ihres Nebenwirkungprofil sind.
Der neue Wirkmechanismus durch Blockierung der Suchtrezeptoren von Acomplia könnte in der Tat die Problematik herkömmlicher Medikamente auf Herz und Kreislauf umgehen. Die durch Acomplia bewirkte Umstellung der Essgewohnheiten ist darüber hinaus ein innovativer Weg, nachhaltig und langfristig Übergewicht zu bekämpfen.
Sollten die klinischen Studien weiterhin gute Ergebnisse zeigen, könnte bereits 2006/2007 Acomplia auf den deutschen Markt kommen. Eine Einführung in USA war für die erste Jahreshälfte 2006 geplant. Mit einer Entscheidung der Food and Drug Administration (FDA, Zulassungsbehörde in USA) wird Anfang März, evtl. sogar schon Ende Januar 2006 gerechnet.
******
DECEMBER 27, 2004
EUROPEAN BUSINESS
Back to Main Story
The End Of Obesity As We Know It
Sanofi-Aventis' Acomplia promises that and more. But approval isn't a slam dunk
Ever wonder why marijuana smokers get the munchies? So did a team of scientists at Sanofi Recherche lab in Montpelier, France. Fifteen years ago they began investigating marijuana's effects on the brain, including the well-known fact that cannabis makes users hungry. "We set out to try and create an anti-marijuana," a drug that could suppress appetite by blocking the same switch in the brain activated by cannabis, says Gérard Le Fur, senior executive vice-president and board member at newly merged French pharmaceutical giant Sanofi-Aventis (SNY ).
They succeeded beyond their wildest dreams, discovering a medicine that not only helps people lose weight but also shrinks abdominal fat, helps people stop smoking, improves cholesterol levels, and helps patients better regulate blood sugar.
Talk about a potential blockbuster. Initially, though, Sanofi will take it slow. It will seek approval of the drug, Acomplia, in Europe and the U.S. by the second quarter of 2005 as a treatment for just two of the conditions: obesity and tobacco addiction. Because patients in Acomplia trials regained weight after stopping treatment, the company hopes regulators will approve it for long-term use.
Researchers say side effects such as nausea and depression are relatively minor and short-lived. But as the first in an entirely new class of drugs that affect a pleasure center in the brain, even the slightest hint of psychiatric side effects may lead regulators to demand more long-term safety data, potentially delaying Acomplia's launch beyond 2006 as planned. Still, Sanofi-Aventis is confident that the drug's impressive efficacy will assuage any such worries. "It's a product that takes aim at two of the great maladies of the century," says Sanofi-Aventis CEO Jean-François Dehecq.
DEPRESSION QUESTION
Acomplia is the first in a new class of compounds under development to block receptors found in the brain and in fat tissue known as cannabinoid type 1 (CB1). These receptors control hunger and tobacco addiction. Chronic overeating and smoking sends them into overdrive. Blocking the CB1 receptors dramatically reduces such cravings. Results of a two-year clinical trial in the U.S. showed patients given Acomplia lost an average of 19 pounds, compared with five pounds for patients given a placebo. Those on Acomplia also reported higher levels of HDL, the good cholesterol, lower levels of triglycerides, and improved sensitivity to insulin. All are important in keeping heart disease at bay. "This could be a paradigm-shifting drug," says Dr. Louis J. Aronne, president of the North American Society for the Study of Obesity.
The market potential is huge. More than a third of Americans are clinically obese, or 30% above their ideal body weight. And it's not just an American phenomenon. Dr. Gbola Amusa, senior research analyst for Sanford C. Bernstein & Co. in London, estimates that as much as 10% of health-care costs in other industrialized countries are related to being overweight. The two leading obesity drugs, Xenical and Meridia, have unpleasant side effects such as diarrhea or high blood pressure, so analysts think Acomplia will quickly win market share if approved. Amusa estimates sales will reach $5.6 billion a year by the end of the decade.
Accomplia's real potential may go well beyond eating and smoking. The company hopes it will also become the first drug approved for the treatment of metabolic syndrome, a combination of abdominal fat, high blood pressure, high blood-fat levels, low levels of HDL cholesterol, and high blood-sugar levels, all of which contribute to cardiovascular disease. "This is not just a diet drug but a significant advancement in cardiovascular treatment," says Amusa.
Still, there is reason for caution. There was a noticeable rate of withdrawal in Acomplia's clinical trials due to depression. Researchers involved in the trials say that might be because people taking the drug went in with unrealistic weight-loss expectations or were more susceptible to depression to begin with.
Either way, there are no long-term studies yet of the effects of interfering with this part of the brain. And given the increased regulatory scrutiny on new drugs after the Vioxx and antidepressant controversies, the company may find it needs to submit more data than anticipated to secure approval. Is Acomplia too good to be true? "There has never been a diet drug approved that has had more benefits than risks," says Dr. Larry D. Sasich of Public Citizen's health research group in Washington. Dehecq and Le Fur are determined to prove doubters like him wrong.
************
ROCKVILLE, Md., Feb. 17 - The FDA issued an approvable letter on weight management today for Acomplia (rimonabant), spelling out the steps Sanofi-aventis needs to take to win final approval for its much-heralded diet drug.
Neither the company nor the FDA revealed the specifics of the letter.
In a press release issued in Paris, the company said "Sanofi-aventis will continue to work in close collaboration with the FDA."
Sanofi-aventis also said the FDA issued a non-approvable letter for the indication of smoking cessation for Acomplia.
*******
However, Aitken highlights the potential of several new medicines launched in the past year, including diabetes treatment Byetta, co-marketed by Eli Lilly and Amylin Pharmaceuticals, and Lunesta, an insomnia drug made by Sepracor. And there are more on the way that he says are worth watching. The two key drug launches this year are of Sutent, Pfizer's first big entry into cancer drugs, and Acomplia, the anti-obesity pill being developed by Sanofi-Aventis.
Sutent is already on the market, although sales data are not yet available. Acomplia has been delayed at the U.S. Food and Drug Administration and rejected as a stop-smoking drug. Some cardiologists, who are excited about the drug because of its potential to reduce the risk of heart disease, are also worried about side effects.
Acomplia works by blocking the same brain receptor that makes pot smokers hungry; psychiatric symptoms like anxiety are one of the most common reasons patients stopped taking Acomplia in clinical trials. "It's a pill that blocks the 'happy receptor,' " says Prediman K. Shah of Cedars Sinai Medical Center. "The main reason for concern is that it might have an adverse impact on depression or suicide." He is nonetheless very excited about the pill.

************
The Inscrutable French
Posted by Derek
Since I was asking the same musical question just the other day, I wanted to refer people to this article by Matthew Herper over at Forbes, who also wants to know: where is Acomplia/rimonabant, anyway?
It's amazed me for months now that Sanofi-Aventis can get away with saying nothing at all about the prospects for their potential biggest-selling drug ever. Back when the first FDA action came, I predicted, with miserable inaccuracy, that the company would have something to say within days. It's been months, and no one knows anything more than we did back in February.
As the Forbes piece makes clear, analysts and institutional investors seem to be losing patience. I'm not sure what it is about the Sanofi corporate culture that makes this strategy seem like a good idea, but they might want to reexamine it. What might appear like calm and steadfast behavior from their perspective is starting to look, from the outside, like the actions of a company with something to hide. This is America, guys. We talk about things over here; you can't shut us up. Join the party.
*******
praveen, The issue with rimonabant, I believe, is a mechanism-based increase in depressive symptoms. Consider that smoking marijuana containing THC (a mixed CB1, CB2 agonist) induces euphoria and hunger. The CB1 antagonist rimonabant decreases hunger but apparently also causes the opposite of euphoria, or depression. In the RIO trials of rimonabant in Europe and North America, both showed a dose-dependent increase in depressive symptoms. If rimonabant were used widely, it's not clear at this point whether the net effects; the decrease in morbidity and mortality due to weight loss, less the increase in morbiditiy and mortality due to depression, would be positive. How this will be handled by the FDA is entirely unclear at this point. The risk of depression was predictable from the mechanism and SNY should have addressed it earlier.
Of course, lorcaserin has a completely different mechanism of action and we know this isn't going to happen to ARNA. I remain upbeat about ARNA.
mfg ipollit
Tue Dec 13, 2005 05:29 PM ET
By Bill Berkrot
NEW YORK, Dec 13 (Reuters) - Arena Pharmaceuticals Inc. ( ARNA ) on Tuesday said its experimental obesity drug was effective in promoting weight loss after three months in a mid-stage clinical trial, pushing its shares up by 19 percent in after-hours trading.
The effect of the drug, known as APD356, was highly statistically significant compared with a placebo, with patients taking the highest of three tested doses -- 20 milligrams a day -- losing almost 8 pounds (3.6 kg), or about 3.6 percent of total body weight.
Arena Chief Executive Jack Lief told Reuters the results were better than he anticipated and that the company plans to begin a series of pivotal late-stage clinical trials of the drug around the middle of 2006.
"I am one of the most bullish analysts on Arena, and their data surpassed even my expectations," said Adam Noah, an analyst with Merriman Curhan Ford, who predicted it could become a $1 billion a year drug.
The study did not include any diet or exercise programs, so presumably weight loss could have been even greater with those elements included.
"This trial demonstrated excellent weight loss, particularly considering there was no diet or exercise component in this trial, and the emerging safety and tolerability profile compares favorably with other weight loss drugs," Dr Steven Smith, the study's lead investigator, said in a statement.
It is expected that U.S. regulators will require a large 12-month efficacy study with a goal of 5 percent weight loss, and two years of safety data before considering approval.
If all goes well, Lief anticipates Arena will apply for approval with the U.S. Food and Drug Administration in 2009 with a 2010 launch, if approved for sale.
By that time, APD356 will likely be competing with Acomplia, Sanofi-Aventis's ( SNY ) highly anticipated weight loss drug, which is expected to win U.S. approval next year.
The 469-patient study tested the drug at 10 mg, 15 mg and 20 mg with the high dose group getting 10 mg twice a day. Patients weighed an average of 220 pounds (100 kg).
After 12 weeks, the 20 mg patients lost an average of 7.9 pounds, the 15 mg patients lost 5.7 pounds, or 2.6 percent of body weight, and the lowest dose group lost an average of 4 pounds, or 1.8 percent of body weight. All were considered statistically significant compared with the placebo group, which lost less than 1 pound.
Common side effects included headache, nausea and dizziness.
ADP356 works on certain serotonin receptors in the brain, which are believed to play a pivotal role in regulating food intake and metabolism. It is a similar mechanism to Wyeth's ( WYE ) notorious diet drug combination known as fen-phen, which was pulled from the market after it was found to cause serious heart valve damage.
Arena said its drug is many times more selective than was Wyeth's and appeared to have no effect on heart valves or pulmonary artery pressure.
Noah said the data and apparent safety should help Arena attract a partner to help market the medicine at very lucrative terms.
Arena shares rose to $13.60 in extended trading on the Inet electronic brokerage from their Nasdaq close at $11.41. They jumped more than 30 percent just after the data was released.
mfg ipollit
By Bill Berkrot
NEW YORK, Dec 13 (Reuters) - Arena Pharmaceuticals Inc. ( ARNA ) on Tuesday said its experimental obesity drug was effective in promoting weight loss after three months in a mid-stage clinical trial, pushing its shares up by 19 percent in after-hours trading.
The effect of the drug, known as APD356, was highly statistically significant compared with a placebo, with patients taking the highest of three tested doses -- 20 milligrams a day -- losing almost 8 pounds (3.6 kg), or about 3.6 percent of total body weight.
Arena Chief Executive Jack Lief told Reuters the results were better than he anticipated and that the company plans to begin a series of pivotal late-stage clinical trials of the drug around the middle of 2006.
"I am one of the most bullish analysts on Arena, and their data surpassed even my expectations," said Adam Noah, an analyst with Merriman Curhan Ford, who predicted it could become a $1 billion a year drug.
The study did not include any diet or exercise programs, so presumably weight loss could have been even greater with those elements included.
"This trial demonstrated excellent weight loss, particularly considering there was no diet or exercise component in this trial, and the emerging safety and tolerability profile compares favorably with other weight loss drugs," Dr Steven Smith, the study's lead investigator, said in a statement.
It is expected that U.S. regulators will require a large 12-month efficacy study with a goal of 5 percent weight loss, and two years of safety data before considering approval.
If all goes well, Lief anticipates Arena will apply for approval with the U.S. Food and Drug Administration in 2009 with a 2010 launch, if approved for sale.
By that time, APD356 will likely be competing with Acomplia, Sanofi-Aventis's ( SNY ) highly anticipated weight loss drug, which is expected to win U.S. approval next year.
The 469-patient study tested the drug at 10 mg, 15 mg and 20 mg with the high dose group getting 10 mg twice a day. Patients weighed an average of 220 pounds (100 kg).
After 12 weeks, the 20 mg patients lost an average of 7.9 pounds, the 15 mg patients lost 5.7 pounds, or 2.6 percent of body weight, and the lowest dose group lost an average of 4 pounds, or 1.8 percent of body weight. All were considered statistically significant compared with the placebo group, which lost less than 1 pound.
Common side effects included headache, nausea and dizziness.
ADP356 works on certain serotonin receptors in the brain, which are believed to play a pivotal role in regulating food intake and metabolism. It is a similar mechanism to Wyeth's ( WYE ) notorious diet drug combination known as fen-phen, which was pulled from the market after it was found to cause serious heart valve damage.
Arena said its drug is many times more selective than was Wyeth's and appeared to have no effect on heart valves or pulmonary artery pressure.
Noah said the data and apparent safety should help Arena attract a partner to help market the medicine at very lucrative terms.
Arena shares rose to $13.60 in extended trading on the Inet electronic brokerage from their Nasdaq close at $11.41. They jumped more than 30 percent just after the data was released.
mfg ipollit
A DIET PILL DEVELOPER'S market value plumped up nicely.
Shares of Arena Pharmaceuticals (ARNA: 12.22, -0.31, -2.5%) jumped 18% to $13.48 Wednesday after the drug maker's treatment for obesity showed promise in midstage trials. Participants on a higher-dose regimen lost nearly eight pounds apiece over three months.
"We believe Arena is developing a competitive anti-obesity compound, with 31% of patients losing more than 5% body weight at 12 weeks, without a diet or exercise program," wrote George Fulop, an analyst at New York investment bank Needham & Co. "Hence, we anticipate seeing additional efficacy in future trials." (Needham & Co. has an investment-banking relationship with the company.)
During the Phase II clinical trial, subjects received APD356, Arena's experimental oral obesity treatment, for 12 weeks. Patients taking a 10 milligram dose of the drug experienced an average weight loss of 4.0 pounds, compared with 0.7 pounds in the placebo group. Patients taking the 15 mg and 20 mg doses lost 5.7 pounds and 7.9 pounds, respectively. All are considered statistically significant losses, according to the company.
The trial enrolled 469 male and female patients with an average age of 41.5 years, an average weight of 220 pounds and average body mass index, or BMI, of 36.4. Eighty-seven percent of the participants were women, 53% were Caucasian, 29% were African-American and 18% were Hispanic. Patients didn't receive any diet or exercise advice, but were required to abstain from alcohol during the study.
"I'm very bullish on this, and the data surprised even me," says Adam Noah, an analyst at San Francisco investment bank Merriman Curhan Ford & Co. "We like the company — and not just because of this drug. It has a deep pipeline of drugs with very large markets. And the obesity program is the proof of principle on the entire pipeline, not just this one indication."
And the obesity program is the proof of principle on the entire pipeline, not just this one indication."
APD356 works on serotonin receptors in the hypothalamus, an area of the brain that helps control food intake and metabolism. It's a similar mechanism to that of fen-phen, Wyeth's (WYE: 50.05, -0.54, -1.1%) infamous diet drug combination. Fen-phen was withdrawn from the market in 1997 after the discovery that it caused damage to heart valves. Arena's compound was well tolerated at all doses and showed no apparent effects on heart valves or pulmonary artery pressures. Side effects included headaches, nausea and dizziness.
According to the Centers for Disease Control, approximately two-thirds of all adults in the U.S. considered obese or overweight, suggesting a huge market awaits Arena. Excessive pounds increase a person's risk of heart disease, diabetes, stroke, cancer and osteoarthritis. And even though medical treatments are limited for obese and overweight people, more than $114 billion in government funds was spent in 2000 in the U.S. on obesity-related medical costs tied to the condition, says the CDC.
Currently, there are only two obesity drugs on the market: Meridia by Abbott Laboratories (ABT: 47.90, -0.30, -0.6%) and Xenical by Switzerland's Roche. Both have serious side effects. Still, shorting Weight Watchers (WTW: 44.42, -0.17, -0.4%) shares may prove premature. Arena, which expects the APD356 program to enter Phase III trials next year, doesn't plan to submit the drug for approval until 2009. Even if everything goes perfectly, APD356 will likely compete with Acomplia, Sanofi-Aventis's (SNY: 43.95, -0.34, -0.8%) highly anticipated obesity drug, which is expected to be approved next year.
"While it's too hard to predict the market, I don't see why this can't be a blockbuster," says Merriman's Noah, who reiterated his Buy rating Wednesday. "Arena's results were just as good as Acomplia's and maybe slightly better. If you're a big pharmaceutical company that wants to get into the obesity market with a partner, then you get Arena. And it's a seller's market. Arena is in a position to negotiate this partnership very carefully and get very good numbers. We have an $18 price target and consider that conservative."
If you're a big pharmaceutical company that wants to get into the obesity market with a partner, then you get Arena. And it's a seller's market. Arena is in a position to negotiate this partnership very carefully and get very good numbers. We have an $18 price target and consider that conservative."
Jack Lief, Arena's chief executive and founder, says he's open to partnership discussions. "We are prepared to move to a new drug application by ourselves and ultimately commercialization by ourselves," says Lief. "But, we will join with a partner with the right economics."
While it has no drugs on the market, the eight-year-old company has several promising ones in its pipeline. Its insomnia treatment is scheduled to begin a Phase II trial by the end of this year. An atherosclerosis treatment developed in collaboration with Merck (MRK: 42.04, +0.09, +0.2%) is currently in a Phase I trial. Arena is also partnered with Johnson & Johnson (JNJ: 63.96, -0.27, -0.4%) on a treatment for type-2 diabetes.
For the nine months ended Sept. 30, the San Diego company posted a net loss of $48.8 million, or $1.69 a share, on revenues of $17.4 million. As of Sept. 30, Arena had $148.1 million in cash. Merriman's Noah says the company is burning through about $50 million in cash a year.
Quote:
"Arena's stock price has shown solid gains in 2005, yet we continue to believe the stock price still does not appropriately reflect Arena's pipeline potential, the positive proof of concept data announced to date, or the potential for partnership announcements," wrote Needham's Fulop, who reiterated his Buy rating and boosted his target price to $18 from $12. "In our opinion, Arena's stock remains attractive at these levels. Arena is one of our top picks for the fourth quarter of 2005, first quarter of 2006."
mfg ipollit
Shares of Arena Pharmaceuticals (ARNA: 12.22, -0.31, -2.5%) jumped 18% to $13.48 Wednesday after the drug maker's treatment for obesity showed promise in midstage trials. Participants on a higher-dose regimen lost nearly eight pounds apiece over three months.
"We believe Arena is developing a competitive anti-obesity compound, with 31% of patients losing more than 5% body weight at 12 weeks, without a diet or exercise program," wrote George Fulop, an analyst at New York investment bank Needham & Co. "Hence, we anticipate seeing additional efficacy in future trials." (Needham & Co. has an investment-banking relationship with the company.)
During the Phase II clinical trial, subjects received APD356, Arena's experimental oral obesity treatment, for 12 weeks. Patients taking a 10 milligram dose of the drug experienced an average weight loss of 4.0 pounds, compared with 0.7 pounds in the placebo group. Patients taking the 15 mg and 20 mg doses lost 5.7 pounds and 7.9 pounds, respectively. All are considered statistically significant losses, according to the company.
The trial enrolled 469 male and female patients with an average age of 41.5 years, an average weight of 220 pounds and average body mass index, or BMI, of 36.4. Eighty-seven percent of the participants were women, 53% were Caucasian, 29% were African-American and 18% were Hispanic. Patients didn't receive any diet or exercise advice, but were required to abstain from alcohol during the study.
"I'm very bullish on this, and the data surprised even me," says Adam Noah, an analyst at San Francisco investment bank Merriman Curhan Ford & Co. "We like the company — and not just because of this drug. It has a deep pipeline of drugs with very large markets.
 And the obesity program is the proof of principle on the entire pipeline, not just this one indication."
And the obesity program is the proof of principle on the entire pipeline, not just this one indication." APD356 works on serotonin receptors in the hypothalamus, an area of the brain that helps control food intake and metabolism. It's a similar mechanism to that of fen-phen, Wyeth's (WYE: 50.05, -0.54, -1.1%) infamous diet drug combination. Fen-phen was withdrawn from the market in 1997 after the discovery that it caused damage to heart valves. Arena's compound was well tolerated at all doses and showed no apparent effects on heart valves or pulmonary artery pressures. Side effects included headaches, nausea and dizziness.
According to the Centers for Disease Control, approximately two-thirds of all adults in the U.S. considered obese or overweight, suggesting a huge market awaits Arena. Excessive pounds increase a person's risk of heart disease, diabetes, stroke, cancer and osteoarthritis. And even though medical treatments are limited for obese and overweight people, more than $114 billion in government funds was spent in 2000 in the U.S. on obesity-related medical costs tied to the condition, says the CDC.
Currently, there are only two obesity drugs on the market: Meridia by Abbott Laboratories (ABT: 47.90, -0.30, -0.6%) and Xenical by Switzerland's Roche. Both have serious side effects. Still, shorting Weight Watchers (WTW: 44.42, -0.17, -0.4%) shares may prove premature. Arena, which expects the APD356 program to enter Phase III trials next year, doesn't plan to submit the drug for approval until 2009. Even if everything goes perfectly, APD356 will likely compete with Acomplia, Sanofi-Aventis's (SNY: 43.95, -0.34, -0.8%) highly anticipated obesity drug, which is expected to be approved next year.
"While it's too hard to predict the market, I don't see why this can't be a blockbuster," says Merriman's Noah, who reiterated his Buy rating Wednesday. "Arena's results were just as good as Acomplia's and maybe slightly better.
 If you're a big pharmaceutical company that wants to get into the obesity market with a partner, then you get Arena. And it's a seller's market. Arena is in a position to negotiate this partnership very carefully and get very good numbers. We have an $18 price target and consider that conservative."
If you're a big pharmaceutical company that wants to get into the obesity market with a partner, then you get Arena. And it's a seller's market. Arena is in a position to negotiate this partnership very carefully and get very good numbers. We have an $18 price target and consider that conservative." Jack Lief, Arena's chief executive and founder, says he's open to partnership discussions. "We are prepared to move to a new drug application by ourselves and ultimately commercialization by ourselves," says Lief. "But, we will join with a partner with the right economics."
While it has no drugs on the market, the eight-year-old company has several promising ones in its pipeline. Its insomnia treatment is scheduled to begin a Phase II trial by the end of this year. An atherosclerosis treatment developed in collaboration with Merck (MRK: 42.04, +0.09, +0.2%) is currently in a Phase I trial. Arena is also partnered with Johnson & Johnson (JNJ: 63.96, -0.27, -0.4%) on a treatment for type-2 diabetes.
For the nine months ended Sept. 30, the San Diego company posted a net loss of $48.8 million, or $1.69 a share, on revenues of $17.4 million. As of Sept. 30, Arena had $148.1 million in cash. Merriman's Noah says the company is burning through about $50 million in cash a year.
Quote:
"Arena's stock price has shown solid gains in 2005, yet we continue to believe the stock price still does not appropriately reflect Arena's pipeline potential, the positive proof of concept data announced to date, or the potential for partnership announcements," wrote Needham's Fulop, who reiterated his Buy rating and boosted his target price to $18 from $12. "In our opinion, Arena's stock remains attractive at these levels. Arena is one of our top picks for the fourth quarter of 2005, first quarter of 2006."
mfg ipollit
Antwort auf Beitrag Nr.: 24.481.237 von ipollit am 07.10.06 23:48:45Obese People Seen As Opportunity For Arena
UBS Investment Research initiated coverage on Arena Pharmaceuticals and said the specialty pharmaceutical company's anti-obesity drug lorcaserin shows promise while collaborations with big pharma validate Arena's approach.
"Obesity represents a significant market opportunity for an effective agent with a relatively clean side effect profile, and therefore we are hopeful about lorcaserin's prospects," wrote UBS analyst Maged Shenouda, in research report Thursday.
"We note that over 65% of Americans are currently overweight, with 32% being obese, a material commercial opportunity."
The analyst said the company's other main drug candidate APD125, an experimental insomnia treatment, is taking on a large but crowded market.
"Because it inhibits a CNS [central nervous system] activating system rather than acting as a CNS depressant, APD125 has the potential to avoid hangover effects seen with several marketed agents," the UBS analyst said. Arena could see a U.S. launch as early as 2011, Shenouda added.
In addition, Arena (nasdaq: ARNA - news - people ) has ongoing developmental partnerships with Merck (nyse: MRK - news - people ) and Johnson & Johnson (nyse: JNJ - news - people ) unit Ortho-McNeil to develop cardiovascular and diabetes drugs, respectively.
"Beyond their significant economic potential, we view these collaborations as external validation of Arena's discovery capabilities."
The research analyst has a price target of $20 on the stock.
mfg ipollit
UBS Investment Research initiated coverage on Arena Pharmaceuticals and said the specialty pharmaceutical company's anti-obesity drug lorcaserin shows promise while collaborations with big pharma validate Arena's approach.
"Obesity represents a significant market opportunity for an effective agent with a relatively clean side effect profile, and therefore we are hopeful about lorcaserin's prospects," wrote UBS analyst Maged Shenouda, in research report Thursday.
"We note that over 65% of Americans are currently overweight, with 32% being obese, a material commercial opportunity."
The analyst said the company's other main drug candidate APD125, an experimental insomnia treatment, is taking on a large but crowded market.
"Because it inhibits a CNS [central nervous system] activating system rather than acting as a CNS depressant, APD125 has the potential to avoid hangover effects seen with several marketed agents," the UBS analyst said. Arena could see a U.S. launch as early as 2011, Shenouda added.
In addition, Arena (nasdaq: ARNA - news - people ) has ongoing developmental partnerships with Merck (nyse: MRK - news - people ) and Johnson & Johnson (nyse: JNJ - news - people ) unit Ortho-McNeil to develop cardiovascular and diabetes drugs, respectively.
"Beyond their significant economic potential, we view these collaborations as external validation of Arena's discovery capabilities."
The research analyst has a price target of $20 on the stock.
mfg ipollit
@ipollit,
ich setze auf METABOLIC PHARM. Übergewichtspille! Hat einzigartige Vorteile, s. thrad hier auf W bzw. Firmenhomepage.
bzw. Firmenhomepage.
ich setze auf METABOLIC PHARM. Übergewichtspille! Hat einzigartige Vorteile, s. thrad hier auf W
 bzw. Firmenhomepage.
bzw. Firmenhomepage.
Antwort auf Beitrag Nr.: 24.483.945 von Fruehrentner am 08.10.06 01:30:03@Frührentner
Metabolic Pharm kommt mit der MK so gerade an 100 Mio USD dran, wie hoch ist denn der Cash (dürfte nicht viel sein), kein Partner... und dann angeblich ein potentieller Blockbuster in PIII (die Lorca PIII ist für ARNA recht günstig gerechnet und benötigt trotzdem min 125 Mio USD) - das passt nicht zusammen... wenn die MK bei 500 und deutlich mehr als 100 Mio USD Cash vorhanden ist, dann könnte ich mir die nochmal ansehen. Außerdem wären Lorca und AOD9604 zwei völlig unterschiedliche Ansätze... AOD9604 ist angeblich so etwas wie Xenical, also hemmt die Aufnahme von Fett.
sorry, solche Firmen sind mir zu suspekt... es gibt zuviel faule Kandidaten da draußen...
mfg ipollit
Metabolic Pharm kommt mit der MK so gerade an 100 Mio USD dran, wie hoch ist denn der Cash (dürfte nicht viel sein), kein Partner... und dann angeblich ein potentieller Blockbuster in PIII (die Lorca PIII ist für ARNA recht günstig gerechnet und benötigt trotzdem min 125 Mio USD) - das passt nicht zusammen... wenn die MK bei 500 und deutlich mehr als 100 Mio USD Cash vorhanden ist, dann könnte ich mir die nochmal ansehen. Außerdem wären Lorca und AOD9604 zwei völlig unterschiedliche Ansätze... AOD9604 ist angeblich so etwas wie Xenical, also hemmt die Aufnahme von Fett.
sorry, solche Firmen sind mir zu suspekt... es gibt zuviel faule Kandidaten da draußen...
mfg ipollit
Antwort auf Beitrag Nr.: 24.487.309 von ipollit am 08.10.06 11:48:16Pharmaceuticals
Sanofi Remains Tight-Lipped
Matthew Herper, 10.05.06, 11:30 AM ET
Sanofi-Aventis shareholders have plenty of reason to be nervous--and the silent treatment they are getting from the world's third-largest drug company isn't likely to calm their anxious stomachs.
U.S. approval of Sanoifi's (nyse: SNY - news - people ) weight-loss pill rimonabant, which some analysts and doctors said could be one of the biggest drugs ever, has been delayed for seven months and counting. And the U.S. Food and Drug Administration outright rejected its drug Multaq, a treatment for irregular heart rhythms, on Aug. 31.
Sanofi has also had to lower its 2006 earnings-per-share forecast by 9% because of disastrous patent negotiations surrounding the blood thinner Plavix, which it sells with Bristol-Myers Squibb (nyse: BMY - news - people ). Peter Dolan lost his job as Bristol's chief executive over the botched negotiation that allowed Canadian drugmaker Apotex to launch a generic version of Plavix at almost no financial risk. Sales have been halted for now as the companies battle in court to determine if Apotex can sell its generic version.
Add to this the feeling from some that the drug giant isn't addressing investors' questions. Gbola Amusa, an analyst at Sanford C. Bernstein, has been a big booster of Rimonabant, and he predicts Sanofi shares will outperform the market. But on Tuesday, he sent a note to investors saying that Sanofi had gone silent to most investors, analysts and the media, and will remain quiet until it announces its results on Oct. 31.
Questions regarding this assertion were referred to Sanofi's Paris headquarters, where spokesman Jean-Marc Podvin said he was not aware of any new policy of silence. "I don't think company policy has changed," says Podvin. But Amusa insists that Sanofi isn't addressing important issues, such as the Plavix litigation. "It's clear there's a difference between the way they communicate, compared to other companies," he says.
Sanofi's stock has retreated from a July high of $50.05, trading on Thursday at $44.42, down 19 cents on the day.
One issue Sanofi has barely addressed is why the FDA delayed rimonabant, which underpins Amusa's growth forecasts for Sanofi stock. Rimonabant was launched in the United Kingdom and some European countries under the brand name Acomplia. Sanofi had said that it expected to bring it to the U.S. market in the second half of 2006--but the second half is half over.
Rimonabant is seen as having potential not only because it takes off pounds, but also because of its effect on cholesterol--it cuts the bad kind and increases the good. However, the drug, which works by turning off the same brain receptors that marijuana turns on to give pot smokers the munchies, also seems to cause psychiatric side effects: a subset of patients seemed to quit taking their medicine because of anxiety or depression.
Obesity represents "a desperate need," says Jaideep Bajaj of consultancy ZS Associates, which advises drug firms on marketing matters. "But you will have to convince the market that it's not hocus-pocus." Previous obesity drugs, he says, have been "riddled with side effects."
The last big weight-loss treatment was the diet combo fen-phen, which was linked to heart valve damage and led Wyeth (nyse: WYE - news - people ) to pay out $21 billion in legal damages. And the FDA is still scarred by the scandal of Merck's (nyse: MRK - news - people ) painkiller Vioxx. Neurological side effects seem the most likely reason for the long delay--and they could conceivably prevent Acomplia from joining the ranks of blockbuster drugs like Plavix and Lipitor, the Pfizer (nyse: PFE - news - people ) cholesterol pill.
Amusa, for his part, thinks the depression problem represents a "red herring," partly because the effects seem to go away when patients stop the drug and seem to occur in other obesity trials. But many investors would probably prefer to get their reassurance from Sanofi itself.
**********
Wenn Acomplia Probleme wegen Depressionen bekommt und nicht zum erwarteten Mega-Blockbuster wird... dann wäre mit Lorca schnell ein Ersatz gefunden - die Frage ist nur... ist Loca zu sehr ein FenPhen oder ein FenPhen ohne Risko von Herzschäden.
mfg ipollit
Sanofi Remains Tight-Lipped
Matthew Herper, 10.05.06, 11:30 AM ET
Sanofi-Aventis shareholders have plenty of reason to be nervous--and the silent treatment they are getting from the world's third-largest drug company isn't likely to calm their anxious stomachs.
U.S. approval of Sanoifi's (nyse: SNY - news - people ) weight-loss pill rimonabant, which some analysts and doctors said could be one of the biggest drugs ever, has been delayed for seven months and counting. And the U.S. Food and Drug Administration outright rejected its drug Multaq, a treatment for irregular heart rhythms, on Aug. 31.
Sanofi has also had to lower its 2006 earnings-per-share forecast by 9% because of disastrous patent negotiations surrounding the blood thinner Plavix, which it sells with Bristol-Myers Squibb (nyse: BMY - news - people ). Peter Dolan lost his job as Bristol's chief executive over the botched negotiation that allowed Canadian drugmaker Apotex to launch a generic version of Plavix at almost no financial risk. Sales have been halted for now as the companies battle in court to determine if Apotex can sell its generic version.
Add to this the feeling from some that the drug giant isn't addressing investors' questions. Gbola Amusa, an analyst at Sanford C. Bernstein, has been a big booster of Rimonabant, and he predicts Sanofi shares will outperform the market. But on Tuesday, he sent a note to investors saying that Sanofi had gone silent to most investors, analysts and the media, and will remain quiet until it announces its results on Oct. 31.
Questions regarding this assertion were referred to Sanofi's Paris headquarters, where spokesman Jean-Marc Podvin said he was not aware of any new policy of silence. "I don't think company policy has changed," says Podvin. But Amusa insists that Sanofi isn't addressing important issues, such as the Plavix litigation. "It's clear there's a difference between the way they communicate, compared to other companies," he says.
Sanofi's stock has retreated from a July high of $50.05, trading on Thursday at $44.42, down 19 cents on the day.
One issue Sanofi has barely addressed is why the FDA delayed rimonabant, which underpins Amusa's growth forecasts for Sanofi stock. Rimonabant was launched in the United Kingdom and some European countries under the brand name Acomplia. Sanofi had said that it expected to bring it to the U.S. market in the second half of 2006--but the second half is half over.
Rimonabant is seen as having potential not only because it takes off pounds, but also because of its effect on cholesterol--it cuts the bad kind and increases the good. However, the drug, which works by turning off the same brain receptors that marijuana turns on to give pot smokers the munchies, also seems to cause psychiatric side effects: a subset of patients seemed to quit taking their medicine because of anxiety or depression.
Obesity represents "a desperate need," says Jaideep Bajaj of consultancy ZS Associates, which advises drug firms on marketing matters. "But you will have to convince the market that it's not hocus-pocus." Previous obesity drugs, he says, have been "riddled with side effects."
The last big weight-loss treatment was the diet combo fen-phen, which was linked to heart valve damage and led Wyeth (nyse: WYE - news - people ) to pay out $21 billion in legal damages. And the FDA is still scarred by the scandal of Merck's (nyse: MRK - news - people ) painkiller Vioxx. Neurological side effects seem the most likely reason for the long delay--and they could conceivably prevent Acomplia from joining the ranks of blockbuster drugs like Plavix and Lipitor, the Pfizer (nyse: PFE - news - people ) cholesterol pill.
Amusa, for his part, thinks the depression problem represents a "red herring," partly because the effects seem to go away when patients stop the drug and seem to occur in other obesity trials. But many investors would probably prefer to get their reassurance from Sanofi itself.
**********
Wenn Acomplia Probleme wegen Depressionen bekommt und nicht zum erwarteten Mega-Blockbuster wird... dann wäre mit Lorca schnell ein Ersatz gefunden - die Frage ist nur... ist Loca zu sehr ein FenPhen oder ein FenPhen ohne Risko von Herzschäden.
mfg ipollit
Antwort auf Beitrag Nr.: 24.487.309 von ipollit am 08.10.06 11:48:16@ipollit,
leider falsch. AOD9604 hemmt nicht die Fettaufnahme sondern regt die Verbrennung an, völlig anderer und neuer, und vor allem einzigartiger Ansatz, den bisher kein anderer Wirkstoff bei dieser Indikation hat, also Alleinstellungsmerkmal.
Und das mit wenig bis fast keinen Nebenwirkungen.
Mit der geringen MArktkapitalisierung und (noch) fehlendem Partner hast Du natürlich Recht, das ist nachteilig, aber das kann sich sehr schnell ändern bei positiven Ergebnissen.
leider falsch. AOD9604 hemmt nicht die Fettaufnahme sondern regt die Verbrennung an, völlig anderer und neuer, und vor allem einzigartiger Ansatz, den bisher kein anderer Wirkstoff bei dieser Indikation hat, also Alleinstellungsmerkmal.
Und das mit wenig bis fast keinen Nebenwirkungen.
Mit der geringen MArktkapitalisierung und (noch) fehlendem Partner hast Du natürlich Recht, das ist nachteilig, aber das kann sich sehr schnell ändern bei positiven Ergebnissen.
Wegen dem Deppen von Cramer ist mir das Ding davongelaufen... Naja kannma nix machen, evtl. wird das Gap noch geschlossen...
Grüße
blb
Grüße
blb
die Pipeline... Stand 05.08.2006
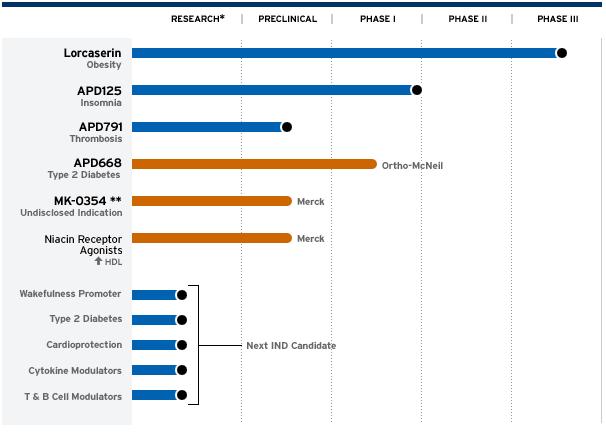
und die zu erwartenden nächsten Meilensteine (von der UBS-Präsentation am 25.09.2006)
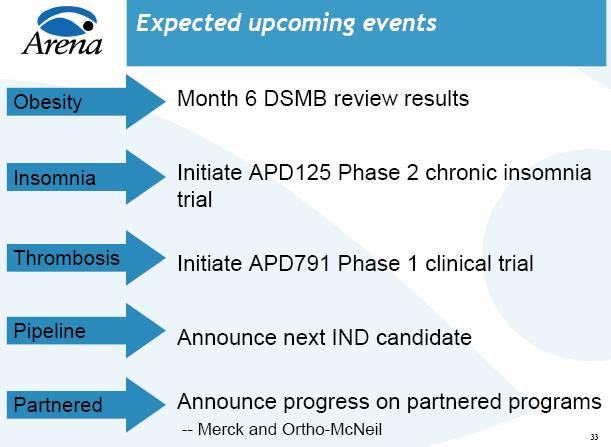
mfg ipollit
und die zu erwartenden nächsten Meilensteine (von der UBS-Präsentation am 25.09.2006)
mfg ipollit
Lorcaserin soll laut ARNA selektiv gegen den Rezeptor 5-HT2C wirken. Dies ist insofern wichtig, weil wahrscheinlich die unspezifische Wirkung von FenPhen auf 5-HT2A und 5-HT2B im Herz zu den schweren Nebenwirkungen führte...

mfg ipollit
mfg ipollit
die erfolgreiche Phase PIIb von Lorca zeigt eine dosisabhängige Gewichtsabnahme ohne eine zusätzliche Diät! Schweren Herzprobleme sind nicht aufgetreten...
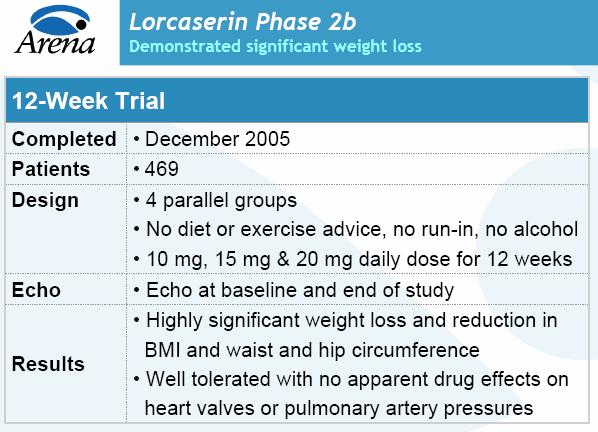
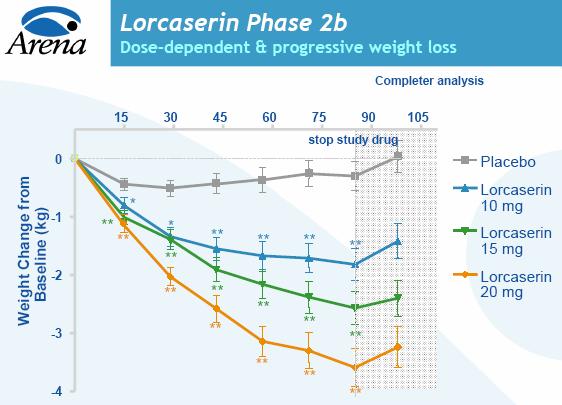
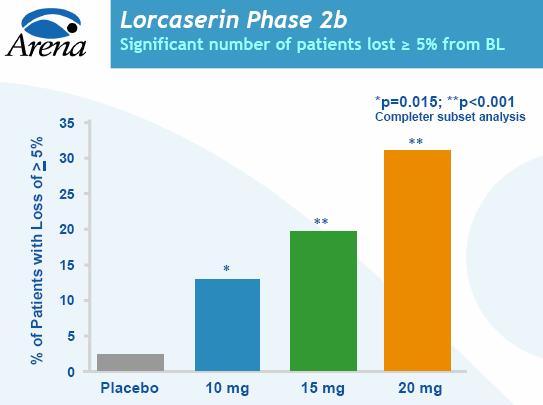
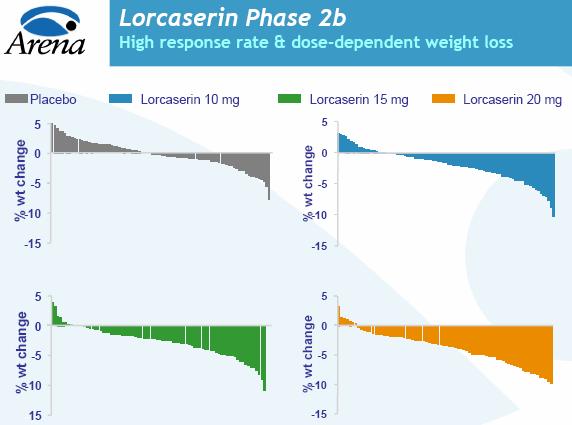
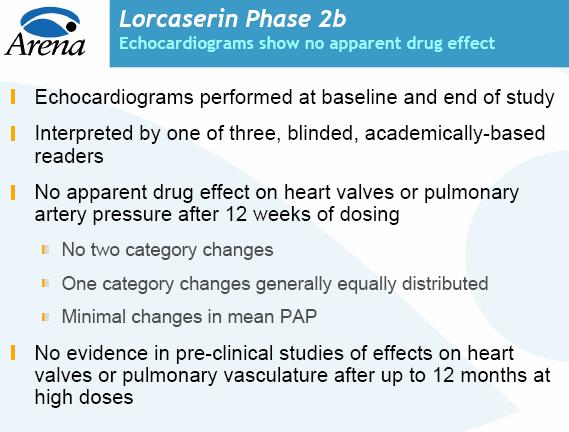
mfg ipollit
mfg ipollit
@ipollit,
ist Lorcaserin eigentlich schon "verpartnert"?
Grüsse
Frührentner
ist Lorcaserin eigentlich schon "verpartnert"?
Grüsse
Frührentner
Antwort auf Beitrag Nr.: 24.503.417 von Fruehrentner am 08.10.06 20:52:35nein, noch gibt es kein Partner, was meiner Meinung aber einen einfachen Grund hat. Lorca als FenPhen Variante ist ein heißes Eisen, da Wyeth wohl für FenPhen schlappe 21 Mrd USD Entschädigung zahlen musste und die FDA wohl dreimal hinsehen wird, bis Lorca ein OK bekommt. Ich denke aber auch, dass die BigPharmas nach einem ersten erfolgreichen DSMB-Check von 3000 Patienten der aktuellen PIII nicht mehr um Lorca herumkommen werden - ARNA wird es dann schwer haben, nicht übernommen zu werden... die Frage ist nur, zu welchem Preis?
mfg ipollit
mfg ipollit
ipollit,
zur Info: Metabolic Pharmaceuticals heute + 52 %!!!
Da ist was im Busch!
zur Info: Metabolic Pharmaceuticals heute + 52 %!!!
Da ist was im Busch!
Antwort auf Beitrag Nr.: 24.527.067 von Fruehrentner am 09.10.06 22:20:15ich weiß im Moment nicht was da los ist... könnte wieder dieser Cramer sein... aber AH wird anscheinend lustig gehandelt... über 6% im Plus und steigend...
mfg ipollit
mfg ipollit

Antwort auf Beitrag Nr.: 24.613.268 von ipollit am 14.10.06 01:21:26"Cramer pumped ARNA hard in the last segment on Friday's show. He had the CEO on, let him imply that lorcaserin could have $10 billion market and called it the spec play of the year."


Posted date: 10/16/2006
Arena’s New Obesity Drug Could Fatten Company’s Stance in the Market
Bio-Pharmaceutical Firm to Go After Piece of Multi-Billion-Dollar Pie
By KATIE WEEKS
San Diego Business Journal Staff
Scientist Mark Macias works in the laboratory at Arena Pharmaceuticals. Analysts are praising the company’s obesity drug.
Arena Pharmaceuticals Inc. founder and Chief Executive Officer Jack Lief earns almost a million dollars a year.
If Arena’s highly touted obesity drug garners much of what analysts say could be the biggest pharmaceutical market ever, Lief’s wallet could get even fatter.
Just two drugs are on the market for obesity, Roche’s Zenical and Abbott Laboratories’ Meridia, and both have side effects and poor patient retention, say analysts. Together, they pull in around $500 million worldwide, but the U.S. market potential for a drug that treats obesity effectively with few or no side effects is at least $2 billion to $5 billion, analysts say.
With more than 64 percent of adults overweight in America — 35 percent in California — some imagine sales could be even higher.
Arena’s drug Lorcaserin recently began the third and last stage of human clinical trials before the Food and Drug Administration considers it for market approval. Lorcaserin works by stimulating a serotonin receptor in the brain, which helps regulate appetite and metabolism. Lief said it targets the receptor more selectively than obesity drugs that have more side effects. The third phase is expected to last two years and involves 6,000 obese or overweight patients.
Tests have shown Lorcaserin has no apparent effects on the heart, unlike diet drugs containing fenfluramine. The FDA pulled “Fhen phen” diet drugs from the market in 1997 amid reports of the drug causing heart valve damage. Tens of thousands of patients sued drug maker Wyeth.
“We’re going to dispel all that fear,” said Lief, 60.
Sales Talk
He said Arena is talking with large, multinational pharmaceutical companies about partnering to create a sales force to market Lorcaserin, should it be approved.
“We’re prepared to sell it by ourselves without a partner,” he said, adding that 750 salespeople will be needed to reach the 6 percent of doctors who prescribe 51 percent of all obesity drugs. He said if the company sells Lorcaserin alone, Arena could grow to 1,000 employees in five years — which is when Lorcaserin would hit the market if approved.
Lief’s independence and determination have brought him a long way. Local venture capitalists did not see Arena’s early promise, and Lief used his own money and that of angel investors to get Arena, which now has 340 employees, off the ground.
“Our technology was disruptive. It’s just the opposite of what textbooks taught,” Lief said. “We didn’t even bother with the VCs.”
It’s difficult to find anyone saying anything nasty about the company these days.
mfg ipollit
Antwort auf Beitrag Nr.: 24.659.539 von ipollit am 16.10.06 16:00:11Arena Pharmaceuticals (ARNA - commentary - Cramer's Take): "Going right to 22, 23."
... danke Mr Cramer, das wollte ich hören!

... danke Mr Cramer, das wollte ich hören!


Antwort auf Beitrag Nr.: 24.711.792 von ipollit am 18.10.06 22:20:22Entscheidung über Schlankmacherpille
Kassen müssen nicht für "Acomplia" zahlen
Der Gemeinsame Bundesausschuss hat entschieden, das Medikament Acomplia als so genanntes Lifestyle-Präparat einzuordnen. Damit kann es zwar weiterhin von Ärzten verschrieben, muss aber nicht mehr von den Krankenkassen erstattet werden. Ein Ausschuss-Sprecher bestätigte einen entsprechenden Bericht von NDR info. Als Gründe für die Entscheidung wurden der hohe Preis des Mittels und der im Vergleich dazu geringe Nutzen genannt. Das Mittel sei nur zum Abnehmen zugelassen und falle damit in den Bereich Lifestyle. Das Medikament, als "Pille gegen Fettpolster", "bahnbrechendes neues Mittel" oder "neue Wunderpille" von der "Bunten" bis hin zum "Spiegel" gefeiert, ist seit etwa einem Monat auf dem Markt. Analysten hatten einen möglichen Jahresumsatz von bis zu fünf Milliarden Dollar für das Medikament vorhergesagt.
Von Christian Baars und Adrian Feuerbacher
"Bin Apotheker, 53 Jahre, und habe versuchsweise 14 Tage lang Acomplia eingenommen. Der Appetit verschwindet. Persönlichkeitsänderungen traten ein. Bin sehr ernst geworden. Habe nach 14 Tagen wegen Unwohlsein, Halsschmerzen, etc. das Produkt abgesetzt."
"Ich wurde immer depressiver. Oft dachte ich an Selbstmord. Ich konnte nicht mehr schlafen und hatte fürchterliche Alpträume."
Das schreiben Menschen im Internet, die Acomplia eingenommen haben. Aber auch einige Wissenschaftler halten das Mittel für riskant. So der Arzt und Apotheker Wolfgang Becker-Brüser, der Herausgeber der unabhängigen Fachzeitschrift Arznei-Telegramm. "In klinischen Studien haben ungefähr 40 bis 50 Prozent der Beteiligten die Studie vorzeitig abgebrochen", erzählt er. "Unter anderem auch wegen unerwünschter Wirkungen, beispielsweise auch sehr häufig wegen Depressionen oder Angstzuständen."
Acomplia wirkt im Zentralen Nervensystem
Eigentlich soll das Medikament stark übergewichtigen Menschen beim Abnehmen helfen. Rimonabant, der Wirkstoff von Acomplia, hemmt den Hunger. Das Mittel greift direkt ins Zentrale Nervensystem ein, blockiert einen bestimmten Rezeptor. Genau das ist aber der Grund, warum auch der Neurowissenschaftler Andreas Zimmer von der Universität Bonn skeptisch ist.
Zimmer hat in Tierversuchen Mäuse ohne diesen Rezeptor gezüchtet. Das Ergebnis: "Diese Mäuse leider unter einer erheblich erhöhten Mortalität. Das heißt: Tiere sterben viel rascher als normale Tiere eines natürlichen Todes", berichtet er von seinen Erfahrungen. Auch litten die Tiere verstärkt unter epileptischen Anfällen. Zudem macht Zimmer und seinen Kollegen eines besonders Sorgen: "Das Hirn der Tiere zeigt im Verlaufe des Alterns starke Nervenzellverluste."
Ärzte sollten genau kontrollierenZimmers Ergebnisse lassen sich allerdings nicht ohne weiteres auf den Menschen übertragen. Das erwidert auch der Hersteller von Acomplia, der Pharmakonzern Sanofi-Aventis: In den Zulassungsstudien für das Medikament seien solche Nebenwirkungen nicht aufgetreten. Doch der Neurowissenschaftler Zimmer warnt: Es handele sich um Effekte, die in dem Zeitraum von ein oder zwei Jahren, über den sich die klinischen Studien erstreckt haben, möglicherweise noch nicht auftreten. Vielleicht komme es zu den Nebenwirkungen erst, wenn man das Medikament über viele Jahre eingenommen hat. Zimmer rät Ärzten daher, ihre Patienten genau zu kontrollieren, wenn sie denn Acomplia verschreiben.
Hoher Preis und geringer EffektDer Schlankmacher hat aber nicht nur Nebenwirkungen, die zum Teil noch gar nicht richtig erforscht sind, sondern er kostet auch viel Geld: "Für 1000 Euro pro Jahr nehme ich im Schnitt fünf Kilo ab - und das auch nur so lange, wie ich das Mittel einnehme", fasst der Pharmakologe Becker-Brüser das Ergebnis der Studien zusammen. "Das ist doch absurd."
Hersteller sieht milde und vorübergehende NebenwirkungenSo sah das nun auch der Gemeinsame Bundesausschuss. Das Medikament Acomplia wurde als so genanntes Lifestyle-Präparat eingeordnet. Damit kann es zwar weiterhin von Ärzten verschrieben, muss aber nicht mehr von den Krankenkassen erstattet werden. Ein Sprecher des Ausschusses erklärte, das Mittel sei nur zum Abnehmen zugelassen. Gewichtsreduzierungen fielen in den Bereich Lifestyle. Er bestätigte einen entsprechenden Bericht von NDR info.
Sanofi-Aventis hatte im Vorfeld die Kritik an seinem Medikament zurückgewiesen: Das Mittel sei überzeugend und die Nebenwirkungen mild und vorübergehend. Marktanalysten hatten einen möglichen Jahresumsatz von bis zu fünf Milliarden Dollar für das Medikament vorhergesagt.
Stand: 17.10.2006 18:45 Uhr
*******
Lorcaserin hat zumindestens diese Nebenwirkungen nicht. Acomplia aber als LifeStyle-Mittelchen einzustufen, ist vollkommend bescheuert... wenn man so anfängt, kann man fast alles in Frage stellen.
mfg ipollit
Kassen müssen nicht für "Acomplia" zahlen
Der Gemeinsame Bundesausschuss hat entschieden, das Medikament Acomplia als so genanntes Lifestyle-Präparat einzuordnen. Damit kann es zwar weiterhin von Ärzten verschrieben, muss aber nicht mehr von den Krankenkassen erstattet werden. Ein Ausschuss-Sprecher bestätigte einen entsprechenden Bericht von NDR info. Als Gründe für die Entscheidung wurden der hohe Preis des Mittels und der im Vergleich dazu geringe Nutzen genannt. Das Mittel sei nur zum Abnehmen zugelassen und falle damit in den Bereich Lifestyle. Das Medikament, als "Pille gegen Fettpolster", "bahnbrechendes neues Mittel" oder "neue Wunderpille" von der "Bunten" bis hin zum "Spiegel" gefeiert, ist seit etwa einem Monat auf dem Markt. Analysten hatten einen möglichen Jahresumsatz von bis zu fünf Milliarden Dollar für das Medikament vorhergesagt.
Von Christian Baars und Adrian Feuerbacher
"Bin Apotheker, 53 Jahre, und habe versuchsweise 14 Tage lang Acomplia eingenommen. Der Appetit verschwindet. Persönlichkeitsänderungen traten ein. Bin sehr ernst geworden. Habe nach 14 Tagen wegen Unwohlsein, Halsschmerzen, etc. das Produkt abgesetzt."
"Ich wurde immer depressiver. Oft dachte ich an Selbstmord. Ich konnte nicht mehr schlafen und hatte fürchterliche Alpträume."
Das schreiben Menschen im Internet, die Acomplia eingenommen haben. Aber auch einige Wissenschaftler halten das Mittel für riskant. So der Arzt und Apotheker Wolfgang Becker-Brüser, der Herausgeber der unabhängigen Fachzeitschrift Arznei-Telegramm. "In klinischen Studien haben ungefähr 40 bis 50 Prozent der Beteiligten die Studie vorzeitig abgebrochen", erzählt er. "Unter anderem auch wegen unerwünschter Wirkungen, beispielsweise auch sehr häufig wegen Depressionen oder Angstzuständen."
Acomplia wirkt im Zentralen Nervensystem
Eigentlich soll das Medikament stark übergewichtigen Menschen beim Abnehmen helfen. Rimonabant, der Wirkstoff von Acomplia, hemmt den Hunger. Das Mittel greift direkt ins Zentrale Nervensystem ein, blockiert einen bestimmten Rezeptor. Genau das ist aber der Grund, warum auch der Neurowissenschaftler Andreas Zimmer von der Universität Bonn skeptisch ist.
Zimmer hat in Tierversuchen Mäuse ohne diesen Rezeptor gezüchtet. Das Ergebnis: "Diese Mäuse leider unter einer erheblich erhöhten Mortalität. Das heißt: Tiere sterben viel rascher als normale Tiere eines natürlichen Todes", berichtet er von seinen Erfahrungen. Auch litten die Tiere verstärkt unter epileptischen Anfällen. Zudem macht Zimmer und seinen Kollegen eines besonders Sorgen: "Das Hirn der Tiere zeigt im Verlaufe des Alterns starke Nervenzellverluste."
Ärzte sollten genau kontrollierenZimmers Ergebnisse lassen sich allerdings nicht ohne weiteres auf den Menschen übertragen. Das erwidert auch der Hersteller von Acomplia, der Pharmakonzern Sanofi-Aventis: In den Zulassungsstudien für das Medikament seien solche Nebenwirkungen nicht aufgetreten. Doch der Neurowissenschaftler Zimmer warnt: Es handele sich um Effekte, die in dem Zeitraum von ein oder zwei Jahren, über den sich die klinischen Studien erstreckt haben, möglicherweise noch nicht auftreten. Vielleicht komme es zu den Nebenwirkungen erst, wenn man das Medikament über viele Jahre eingenommen hat. Zimmer rät Ärzten daher, ihre Patienten genau zu kontrollieren, wenn sie denn Acomplia verschreiben.
Hoher Preis und geringer EffektDer Schlankmacher hat aber nicht nur Nebenwirkungen, die zum Teil noch gar nicht richtig erforscht sind, sondern er kostet auch viel Geld: "Für 1000 Euro pro Jahr nehme ich im Schnitt fünf Kilo ab - und das auch nur so lange, wie ich das Mittel einnehme", fasst der Pharmakologe Becker-Brüser das Ergebnis der Studien zusammen. "Das ist doch absurd."
Hersteller sieht milde und vorübergehende NebenwirkungenSo sah das nun auch der Gemeinsame Bundesausschuss. Das Medikament Acomplia wurde als so genanntes Lifestyle-Präparat eingeordnet. Damit kann es zwar weiterhin von Ärzten verschrieben, muss aber nicht mehr von den Krankenkassen erstattet werden. Ein Sprecher des Ausschusses erklärte, das Mittel sei nur zum Abnehmen zugelassen. Gewichtsreduzierungen fielen in den Bereich Lifestyle. Er bestätigte einen entsprechenden Bericht von NDR info.
Sanofi-Aventis hatte im Vorfeld die Kritik an seinem Medikament zurückgewiesen: Das Mittel sei überzeugend und die Nebenwirkungen mild und vorübergehend. Marktanalysten hatten einen möglichen Jahresumsatz von bis zu fünf Milliarden Dollar für das Medikament vorhergesagt.
Stand: 17.10.2006 18:45 Uhr
*******
Lorcaserin hat zumindestens diese Nebenwirkungen nicht. Acomplia aber als LifeStyle-Mittelchen einzustufen, ist vollkommend bescheuert... wenn man so anfängt, kann man fast alles in Frage stellen.
mfg ipollit
Bei uns wird immer über Frick lamentiert. Wer sich den Cramer anschaut, weiß, dass Frick dagegen ein kleienr Zirkusclown ist...
In the early nineties, Redux was the original miracle diet pill. A predecessor to fenphen, Redux was just the “fen”, fenfluramine in the form dexfenfluramine. The drug increased the production of seratonin, which gave the brain feelings of satiety, or fullness. Overweight people felt a great responsibility lifted from their shoulders. In Redux, the drug industry promised a thin, healthy body without painful exercise or unpleasant diet restrictions! Within three months, doctors were writing 85,000 prescriptions for Redux every week.
We now know how this story ends. Like most miracle drugs, Redux was too good to be true. The increase of seratonin caused people using Redux to be moody and disconsolate. Soon the fenfluramine cocktail drug fenphen replaced Redux on the shelves. This is not the end of the story, however. Soon after thousands of Americans had swallowed fenphen pills, the mortality reports began coming in.
Redux and fenphen work the same way, by flooding the brain with chemicals that trigger that full feeling. Because the patient feels full, they do not feel the need to eat. The drug imparts a comfortable form of anorexia. While this is unhealthy in and of itself, denying the patient essential nutrients, it was not the worst aspect of the drug. The drug did not simply elevate seratonin levels in the brain, it flooded the whole blood stream with seratonin. When this chemical reached the heart it weakened the heart valves, causing massive heart valve disease in people using either fenfluramine drug, Redux or fenphen.
When this deadly side effect became known the drugs were immediately recalled, but the damage was already done. Thousands of people developed heart disease, underwent surgery, or died as a result of their exposure to the chemical. Those patients who did not change their habits to include healthy lifestyle choices like exercise and diet restrictions gained the weight back. Nothing very good came of America’s brief affair with fenfluramine.
mfg ipollit
We now know how this story ends. Like most miracle drugs, Redux was too good to be true. The increase of seratonin caused people using Redux to be moody and disconsolate. Soon the fenfluramine cocktail drug fenphen replaced Redux on the shelves. This is not the end of the story, however. Soon after thousands of Americans had swallowed fenphen pills, the mortality reports began coming in.
Redux and fenphen work the same way, by flooding the brain with chemicals that trigger that full feeling. Because the patient feels full, they do not feel the need to eat. The drug imparts a comfortable form of anorexia. While this is unhealthy in and of itself, denying the patient essential nutrients, it was not the worst aspect of the drug. The drug did not simply elevate seratonin levels in the brain, it flooded the whole blood stream with seratonin. When this chemical reached the heart it weakened the heart valves, causing massive heart valve disease in people using either fenfluramine drug, Redux or fenphen.
When this deadly side effect became known the drugs were immediately recalled, but the damage was already done. Thousands of people developed heart disease, underwent surgery, or died as a result of their exposure to the chemical. Those patients who did not change their habits to include healthy lifestyle choices like exercise and diet restrictions gained the weight back. Nothing very good came of America’s brief affair with fenfluramine.
mfg ipollit
ARNAs Taschen füllen sich weiter... ca. 350 Mio USD Cash am Ende des Jahres...
08.12.2006 16:16
Arena Pharma prices stock offer at $13.21
SAN DIEGO (AFX) - Arena Pharmaceuticals Inc., (Nachrichten) a developer of treatments for obesity, cardiovascular and inflammatory diseases, said Friday it priced its public offering of 11.5 million common shares at $13.21 each.
The purchase price is flat with the stock's Thursday closing price. Shares rose 59 cents, or 4.5 percent, to $13.80 in morning trading on the Nasdaq.
All of the shares are being offered by Arena. The underwriters have a 30-day option to purchase up to an additional 1.73 million common shares to cover over-allotments, if any.
***********
älterer report...
Cash is King
- Our Initial Thoughts on the Current Secondary Offering
What should investors do now. We continue to recommend ARNA to investors with a long-term investment horizon. However, near term, we expect the secondary offering announced yesterday (after the market close) to likely be dilutive and the stock may be range - bound over the next 6 months. The timing of this secondary offering came earlier than we expected. We believe that ARNA will continue to be opportunistic and rely on public market to finance its mid-late stage product development effort until the next significant partnership is forged. Recall that ARNA expects to end the year with $213-$220 million in cash/cash equivalent ($251 million or $5.30/share in cash as of the end of 3Q’07). On a per share basis, we expect that the upcoming offering could modestly increase the cash position of ARNA to about $5.80/share (assuming 8 million shares are priced at $13.50/share).
?? We continue to expect that the next key stock moving event for ARNA will take place around mid '07 (6 months heart valve safety data of lorcaserin – obesity, phase III BLOOM trial; we expect the 6 month safety profile to be clean). While we believe that this offering is likely dilutive for the stock, the fundamentals of ARNA do not change, in our opinion. More importantly, with this offering, ARNA will have a stronger cash reserve to realistically carry forward on its own a mid-late stage pipeline that continues to mature, has more bargaining power during its future partnership discussions, and could weather potential clinical setbacks down the road. While having bumps on the road is the nature of drug development, we do not expect any negative events with ARNA over the coming 3-6 months.
?? Historically, ARNA has been conservative (and wise enough) to maintain over $100 million of cash on the balance sheet. By doing so, the Company was able to sustain its drug development effort and stay in the drivers seat in choosing what to develop on its own and having the leverage to negotiate attractive partnerships.
We expect that ARNA will continue to tap into public market financing opportunistically over the coming 2 years until a significant partnership for Lorcaserin (obesity, phase III) is in place. We continue to expect that a potential partnership on lorcaserin would be an end of phase III event. This is Arena's 2nd financing of the year (8 million shares with potentially 1.2 million shares of over allotment) following its last financing in January (an over subscribed deal; 10.6 million shares, including the over-allotments of 1.5 million shares @ $16.90 per share). The financing needs of ARNA are a result of the Company’s commitment to carry out the costly phase III trials of its obesity program (we expect that 6,000 patients and $125 million in cost is likely needed at a minimum; we expect the cost of phase III to be fully financed by ARNA with much of the cost front-end loaded in 2006 and 2007). It is important to recognize that while this deal is likely dilutive near term, ARNA will preserve the upside of the economics of a potentially billion-dollar drug and be in a position to demand attractive deal terms in its future partnership discussions.
In addition to the obesity phase III trial that was started in September of this year, ARNA has 2 other clinical compounds that it is developing on its own: (1) an insomnia program that is expected to enter phase II by early '07 and (2) an anti-thrombosis program that is expected to enter phase I by mid '07. In addition, the J&J partnership (diabetes program) is expected to enter phase II in '07 and we continue to expect ARNA to deliver one IND a year going forward.
mfg ipollit
08.12.2006 16:16
Arena Pharma prices stock offer at $13.21
SAN DIEGO (AFX) - Arena Pharmaceuticals Inc., (Nachrichten) a developer of treatments for obesity, cardiovascular and inflammatory diseases, said Friday it priced its public offering of 11.5 million common shares at $13.21 each.
The purchase price is flat with the stock's Thursday closing price. Shares rose 59 cents, or 4.5 percent, to $13.80 in morning trading on the Nasdaq.
All of the shares are being offered by Arena. The underwriters have a 30-day option to purchase up to an additional 1.73 million common shares to cover over-allotments, if any.
***********
älterer report...
Cash is King
- Our Initial Thoughts on the Current Secondary Offering
What should investors do now. We continue to recommend ARNA to investors with a long-term investment horizon. However, near term, we expect the secondary offering announced yesterday (after the market close) to likely be dilutive and the stock may be range - bound over the next 6 months. The timing of this secondary offering came earlier than we expected. We believe that ARNA will continue to be opportunistic and rely on public market to finance its mid-late stage product development effort until the next significant partnership is forged. Recall that ARNA expects to end the year with $213-$220 million in cash/cash equivalent ($251 million or $5.30/share in cash as of the end of 3Q’07). On a per share basis, we expect that the upcoming offering could modestly increase the cash position of ARNA to about $5.80/share (assuming 8 million shares are priced at $13.50/share).
?? We continue to expect that the next key stock moving event for ARNA will take place around mid '07 (6 months heart valve safety data of lorcaserin – obesity, phase III BLOOM trial; we expect the 6 month safety profile to be clean). While we believe that this offering is likely dilutive for the stock, the fundamentals of ARNA do not change, in our opinion. More importantly, with this offering, ARNA will have a stronger cash reserve to realistically carry forward on its own a mid-late stage pipeline that continues to mature, has more bargaining power during its future partnership discussions, and could weather potential clinical setbacks down the road. While having bumps on the road is the nature of drug development, we do not expect any negative events with ARNA over the coming 3-6 months.
?? Historically, ARNA has been conservative (and wise enough) to maintain over $100 million of cash on the balance sheet. By doing so, the Company was able to sustain its drug development effort and stay in the drivers seat in choosing what to develop on its own and having the leverage to negotiate attractive partnerships.
We expect that ARNA will continue to tap into public market financing opportunistically over the coming 2 years until a significant partnership for Lorcaserin (obesity, phase III) is in place. We continue to expect that a potential partnership on lorcaserin would be an end of phase III event. This is Arena's 2nd financing of the year (8 million shares with potentially 1.2 million shares of over allotment) following its last financing in January (an over subscribed deal; 10.6 million shares, including the over-allotments of 1.5 million shares @ $16.90 per share). The financing needs of ARNA are a result of the Company’s commitment to carry out the costly phase III trials of its obesity program (we expect that 6,000 patients and $125 million in cost is likely needed at a minimum; we expect the cost of phase III to be fully financed by ARNA with much of the cost front-end loaded in 2006 and 2007). It is important to recognize that while this deal is likely dilutive near term, ARNA will preserve the upside of the economics of a potentially billion-dollar drug and be in a position to demand attractive deal terms in its future partnership discussions.
In addition to the obesity phase III trial that was started in September of this year, ARNA has 2 other clinical compounds that it is developing on its own: (1) an insomnia program that is expected to enter phase II by early '07 and (2) an anti-thrombosis program that is expected to enter phase I by mid '07. In addition, the J&J partnership (diabetes program) is expected to enter phase II in '07 and we continue to expect ARNA to deliver one IND a year going forward.
mfg ipollit
und Merrill Lynch ist jetzt auch an Board... und dem entsprechend fleißig am pushen
ML Initiates ARNA with TP 20
Battle of the Bulge: Initiating with Buy
�� Initiating with Buy and $20 price objective
We are initiating coverage of Arena Pharmaceuticals with a Buy rating and $20
price objective. Arena is a small cap biotech featuring: (1) Lorcaserin, a potential $1bn+ drug in phase III for obesity; (2) early pipeline candidates for insomnia, diabetes, and cardiovascular disease; and (3) a novel drug discovery platform.
Lorcaserin for obesity is the key driver
Lorcaserin is an unpartnered oral drug candidate in a large phase III trial for
obesity that selectively targets serotonin (5-HT2C) receptors in the brain that
regulate feeding. It has shown efficacy in reducing weight in earlier trials and we believe it could be a $1bn (or more) drug. Two-thirds of American adults are obese or overweight and, but there are few drug options available for long-term treatment, providing a large market opportunity for Lorcaserin.
It must overcome “Fen-Phen” … but so far so good
Fenfluramine and dexfenfluramine were two nonspecific 5-HT receptor agonist
drugs used in conjunction with phentermine (so called “Fen-Phen”) for weight loss in the 90’s, but they were withdrawn from the market after being linked to heart valve damage (HVD). So far, Lorcaserin has shown no evidence of drug-related HVD. A data safety monitoring board (DSMB) review of 6-month heart
ultrasounds from a large phase III trial is expected in 3Q07.
ARNA undervalued; favorable risk reward profile
We believe ARNA stock is undervalued at current levels based our sum-of-theparts NPV model. The key risk is whether Arena can successfully commercialize Lorcaserin for obesity, but we believe there is favorable risk reward profile for biotech investors with high risk tolerance
mfg ipollit
ML Initiates ARNA with TP 20
Battle of the Bulge: Initiating with Buy
�� Initiating with Buy and $20 price objective
We are initiating coverage of Arena Pharmaceuticals with a Buy rating and $20
price objective. Arena is a small cap biotech featuring: (1) Lorcaserin, a potential $1bn+ drug in phase III for obesity; (2) early pipeline candidates for insomnia, diabetes, and cardiovascular disease; and (3) a novel drug discovery platform.
Lorcaserin for obesity is the key driver
Lorcaserin is an unpartnered oral drug candidate in a large phase III trial for
obesity that selectively targets serotonin (5-HT2C) receptors in the brain that
regulate feeding. It has shown efficacy in reducing weight in earlier trials and we believe it could be a $1bn (or more) drug. Two-thirds of American adults are obese or overweight and, but there are few drug options available for long-term treatment, providing a large market opportunity for Lorcaserin.
It must overcome “Fen-Phen” … but so far so good
Fenfluramine and dexfenfluramine were two nonspecific 5-HT receptor agonist
drugs used in conjunction with phentermine (so called “Fen-Phen”) for weight loss in the 90’s, but they were withdrawn from the market after being linked to heart valve damage (HVD). So far, Lorcaserin has shown no evidence of drug-related HVD. A data safety monitoring board (DSMB) review of 6-month heart
ultrasounds from a large phase III trial is expected in 3Q07.
ARNA undervalued; favorable risk reward profile
We believe ARNA stock is undervalued at current levels based our sum-of-theparts NPV model. The key risk is whether Arena can successfully commercialize Lorcaserin for obesity, but we believe there is favorable risk reward profile for biotech investors with high risk tolerance
mfg ipollit
von ihub...
Upcoming Milestones:
H1:07E Submit IND for APD791
Q2:07E Initiate Phase I studies with APD791
Q2:07E Initiate Phase II trials with APD125 for the treatment of insomnia
Q2:07E Approval of Sanofi-Aventis' rimonabant may provide visibility to Arena's Phase III obesity program
H1:07E Phase I data and advancement of J&J partnered compound, APD668, into Phase II studies for T2DM
Q3:07E Results of Phase III 6 month echocardiogram safety evaluation by DSMB
Q4:07E Initiation of 2 additional Phase III studies for lorcaserin
2007/2008E Enter Global Collaboration with lorcaserin
Q1:08E Results of Phase III 12 month echocardiogram safety evaluation by DSMB
2009E Completion of Phase III clinical trials
mfg ipollit
Upcoming Milestones:
H1:07E Submit IND for APD791
Q2:07E Initiate Phase I studies with APD791
Q2:07E Initiate Phase II trials with APD125 for the treatment of insomnia
Q2:07E Approval of Sanofi-Aventis' rimonabant may provide visibility to Arena's Phase III obesity program
H1:07E Phase I data and advancement of J&J partnered compound, APD668, into Phase II studies for T2DM
Q3:07E Results of Phase III 6 month echocardiogram safety evaluation by DSMB
Q4:07E Initiation of 2 additional Phase III studies for lorcaserin
2007/2008E Enter Global Collaboration with lorcaserin
Q1:08E Results of Phase III 12 month echocardiogram safety evaluation by DSMB
2009E Completion of Phase III clinical trials
mfg ipollit
Arena Pharmaceuticals Completes Enrollment with 3,182 Patients in Lorcaserin Phase 3 BLOOM Trial for Obesity
05 Feb 2007
Announced that it completed patient enrollment in the first of three planned Phase 3 pivotal trials evaluating the efficacy and safety of its lead drug candidate, lorcaserin hydrochloride, for the treatment of obesity.
SAN DIEGO, CA, USA | Feb 05, 2007 | Arena Pharmaceuticals, Inc. (Nasdaq: ARNA) today announced that it completed patient enrollment in the first of three planned Phase 3 pivotal trials evaluating the efficacy and safety of its lead drug candidate, lorcaserin hydrochloride, for the treatment of obesity. Known as BLOOM (Behavioral modification and Lorcaserin for Overweight and Obesity Management), this double-blinded, randomized, and placebo-controlled trial enrolled 3,182 patients at approximately 100 centers in the United States. Arena continues to expect BLOOM's independent Data Safety Monitoring Board (DSMB) to perform its month-six review of echocardiograms in the third quarter of 2007 and make a judgment as to whether it is appropriate to continue the trial.
"Completing the enrollment of more than 3,100 patients in the BLOOM trial in a little over four months reflects the significant need for additional options in the treatment of obesity," commented William Shanahan, M.D., Arena's Vice President and Chief Medical Officer. "We are looking forward to the DSMB's review of the month-six echocardiographic data in the third quarter and the initiation of two additional one-year pivotal Phase 3 studies later this year."
The BLOOM trial is evaluating a 20 mg daily dose (10 mg dosed twice daily) of lorcaserin versus placebo over a two-year treatment period in obese patients (BMI 30 to 45) with or without co-morbid conditions and overweight patients (BMI 27 to 30) with at least one co-morbid condition. The proportion of patients with a 5% or greater weight reduction from baseline at week 52 is the primary efficacy endpoint. All patients received echocardiograms at screening and will receive follow-up echocardiograms at 6, 12, 18 and 24 months after starting the trial. Echocardiograms will be reviewed by an independent DSMB at 6 and 12 months. The DSMB will review echocardiographic data, and based upon predetermined criteria will make a judgment as to whether it is appropriate to continue the trial at the time of each review.
The complete lorcaserin Phase 3 program is designed to enroll a total of approximately 6,000 overweight and obese patients in three pivotal trials. In addition to the BLOOM trial, two additional Phase 3 pivotal trials enrolling a total of approximately 3,000 patients will be initiated. In these additional pivotal trials Arena plans to evaluate the 20 mg and 10 mg daily doses versus placebo over a one-year treatment period, with one of the trials likely evaluating patients who also have type 2 diabetes. Diet and exercise will be part of each of the pivotal trials in accordance with the FDA guideline. In addition to the above planned pivotal trial program, several other small studies, such as drug interaction and abuse potential studies, will be conducted.
"Keeping the BLOOM trial on track is a top priority, and we are pleased to have completed enrollment of Arena's first Phase 3 clinical trial on schedule," commented Jack Lief, Arena's President and Chief Executive Officer.
About Lorcaserin
Lorcaserin, Arena's orally administered, internally discovered drug candidate for the treatment of obesity, is in an ongoing Phase 3 program. The compound stimulates the 5-HT2C serotonin receptor, located in the hypothalamus, an area of the brain which helps regulate satiety and influences metabolic rate. Results from a Phase 2 study demonstrated that treatment with lorcaserin produced highly statistically significant, progressive and dose- dependent weight loss over a 12-week period. In the study, which excluded diet and exercise, patients taking a daily 20 mg dose of lorcaserin realized a mean weight loss of 7.9 pounds, while patients on placebo lost less than one pound. Lorcaserin was generally well tolerated at all doses and had no apparent effects on heart valves or pulmonary artery pressure.
About Obesity
Obesity affects tens of millions of adults and children in the United States and poses a serious long-term threat to their health and welfare. The number of overweight and obese people has substantially increased over the past several decades. Approximately two-thirds of all adults in the United States are obese or overweight, and medical and related costs of obesity were more than $117 billion in 2000. Being obese or overweight is associated with a number of conditions, including heart disease, stroke, diabetes, cancer and osteoarthritis. Medical treatment options for obese and overweight people are currently limited.
About Arena Pharmaceuticals
Arena is a clinical-stage biopharmaceutical company focused on discovering, developing and commercializing oral drugs in four major therapeutic areas: cardiovascular, central nervous system, inflammatory, and metabolic diseases. Arena's most advanced product candidate, lorcaserin, is being investigated in a Phase 3 clinical trial program for the treatment of obesity. Arena's broad pipeline of novel compounds targeting G protein- coupled receptors, an important class of validated drug targets, includes compounds being evaluated independently and with its partners, Merck & Co., Inc. and Ortho-McNeil Pharmaceutical, Inc.
Arena Pharmaceuticals(R) and Arena(R) are registered service marks of the company. "APD" is an abbreviation for Arena Pharmaceuticals Development.
Forward-Looking Statements
Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include statements about the timing, protocol, design, scope and other aspects of the current and planned Phase 3 clinical trials and other studies of lorcaserin, the potential efficacy and tolerability of lorcaserin, the need for additional options in the treatment of obesity, the expected role and acts of the DSMB, the timing of DSMB reviews and other statements about Arena's strategy, preclinical and internal and partnered clinical programs, and ability to develop compounds and commercialize drugs. For such statements, Arena claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Arena's expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, Arena's planned clinical trials may not proceed at the time Arena expects or at all, the results of preclinical studies or clinical trials may not be predictive of future results, Arena's ability to partner lorcaserin, APD125 or other of its compounds or programs, the timing, success and cost of Arena's research, out-licensing endeavors and clinical trials, Arena's ability to obtain additional financing, Arena's ability to obtain and defend its patents, and the timing and receipt of payments and fees, if any, from Arena's collaborators. Additional factors that could cause actual results to differ materially from those stated or implied by Arena's forward-looking statements are disclosed in Arena's filings with the Securities and Exchange Commission. These forward-looking statements represent Arena's judgment as of the time of this release. Arena disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
Contacts: Jack Lief
President and CEO
David Walsey
Director, Corporate Communications
Arena Pharmaceuticals, Inc.
858.453.7200, ext. 1479
www.arenapharm.com
E. Blair Schoeb
WeissComm Partners
Media Relations
760.365.1857
SOURCE Arena Pharmaceuticals, Inc
http://www.pipelinereview.com/joomla/content/view/9685/109/
05 Feb 2007
Announced that it completed patient enrollment in the first of three planned Phase 3 pivotal trials evaluating the efficacy and safety of its lead drug candidate, lorcaserin hydrochloride, for the treatment of obesity.
SAN DIEGO, CA, USA | Feb 05, 2007 | Arena Pharmaceuticals, Inc. (Nasdaq: ARNA) today announced that it completed patient enrollment in the first of three planned Phase 3 pivotal trials evaluating the efficacy and safety of its lead drug candidate, lorcaserin hydrochloride, for the treatment of obesity. Known as BLOOM (Behavioral modification and Lorcaserin for Overweight and Obesity Management), this double-blinded, randomized, and placebo-controlled trial enrolled 3,182 patients at approximately 100 centers in the United States. Arena continues to expect BLOOM's independent Data Safety Monitoring Board (DSMB) to perform its month-six review of echocardiograms in the third quarter of 2007 and make a judgment as to whether it is appropriate to continue the trial.
"Completing the enrollment of more than 3,100 patients in the BLOOM trial in a little over four months reflects the significant need for additional options in the treatment of obesity," commented William Shanahan, M.D., Arena's Vice President and Chief Medical Officer. "We are looking forward to the DSMB's review of the month-six echocardiographic data in the third quarter and the initiation of two additional one-year pivotal Phase 3 studies later this year."
The BLOOM trial is evaluating a 20 mg daily dose (10 mg dosed twice daily) of lorcaserin versus placebo over a two-year treatment period in obese patients (BMI 30 to 45) with or without co-morbid conditions and overweight patients (BMI 27 to 30) with at least one co-morbid condition. The proportion of patients with a 5% or greater weight reduction from baseline at week 52 is the primary efficacy endpoint. All patients received echocardiograms at screening and will receive follow-up echocardiograms at 6, 12, 18 and 24 months after starting the trial. Echocardiograms will be reviewed by an independent DSMB at 6 and 12 months. The DSMB will review echocardiographic data, and based upon predetermined criteria will make a judgment as to whether it is appropriate to continue the trial at the time of each review.
The complete lorcaserin Phase 3 program is designed to enroll a total of approximately 6,000 overweight and obese patients in three pivotal trials. In addition to the BLOOM trial, two additional Phase 3 pivotal trials enrolling a total of approximately 3,000 patients will be initiated. In these additional pivotal trials Arena plans to evaluate the 20 mg and 10 mg daily doses versus placebo over a one-year treatment period, with one of the trials likely evaluating patients who also have type 2 diabetes. Diet and exercise will be part of each of the pivotal trials in accordance with the FDA guideline. In addition to the above planned pivotal trial program, several other small studies, such as drug interaction and abuse potential studies, will be conducted.
"Keeping the BLOOM trial on track is a top priority, and we are pleased to have completed enrollment of Arena's first Phase 3 clinical trial on schedule," commented Jack Lief, Arena's President and Chief Executive Officer.
About Lorcaserin
Lorcaserin, Arena's orally administered, internally discovered drug candidate for the treatment of obesity, is in an ongoing Phase 3 program. The compound stimulates the 5-HT2C serotonin receptor, located in the hypothalamus, an area of the brain which helps regulate satiety and influences metabolic rate. Results from a Phase 2 study demonstrated that treatment with lorcaserin produced highly statistically significant, progressive and dose- dependent weight loss over a 12-week period. In the study, which excluded diet and exercise, patients taking a daily 20 mg dose of lorcaserin realized a mean weight loss of 7.9 pounds, while patients on placebo lost less than one pound. Lorcaserin was generally well tolerated at all doses and had no apparent effects on heart valves or pulmonary artery pressure.
About Obesity
Obesity affects tens of millions of adults and children in the United States and poses a serious long-term threat to their health and welfare. The number of overweight and obese people has substantially increased over the past several decades. Approximately two-thirds of all adults in the United States are obese or overweight, and medical and related costs of obesity were more than $117 billion in 2000. Being obese or overweight is associated with a number of conditions, including heart disease, stroke, diabetes, cancer and osteoarthritis. Medical treatment options for obese and overweight people are currently limited.
About Arena Pharmaceuticals
Arena is a clinical-stage biopharmaceutical company focused on discovering, developing and commercializing oral drugs in four major therapeutic areas: cardiovascular, central nervous system, inflammatory, and metabolic diseases. Arena's most advanced product candidate, lorcaserin, is being investigated in a Phase 3 clinical trial program for the treatment of obesity. Arena's broad pipeline of novel compounds targeting G protein- coupled receptors, an important class of validated drug targets, includes compounds being evaluated independently and with its partners, Merck & Co., Inc. and Ortho-McNeil Pharmaceutical, Inc.
Arena Pharmaceuticals(R) and Arena(R) are registered service marks of the company. "APD" is an abbreviation for Arena Pharmaceuticals Development.
Forward-Looking Statements
Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include statements about the timing, protocol, design, scope and other aspects of the current and planned Phase 3 clinical trials and other studies of lorcaserin, the potential efficacy and tolerability of lorcaserin, the need for additional options in the treatment of obesity, the expected role and acts of the DSMB, the timing of DSMB reviews and other statements about Arena's strategy, preclinical and internal and partnered clinical programs, and ability to develop compounds and commercialize drugs. For such statements, Arena claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Arena's expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, Arena's planned clinical trials may not proceed at the time Arena expects or at all, the results of preclinical studies or clinical trials may not be predictive of future results, Arena's ability to partner lorcaserin, APD125 or other of its compounds or programs, the timing, success and cost of Arena's research, out-licensing endeavors and clinical trials, Arena's ability to obtain additional financing, Arena's ability to obtain and defend its patents, and the timing and receipt of payments and fees, if any, from Arena's collaborators. Additional factors that could cause actual results to differ materially from those stated or implied by Arena's forward-looking statements are disclosed in Arena's filings with the Securities and Exchange Commission. These forward-looking statements represent Arena's judgment as of the time of this release. Arena disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
Contacts: Jack Lief
President and CEO
David Walsey
Director, Corporate Communications
Arena Pharmaceuticals, Inc.
858.453.7200, ext. 1479
www.arenapharm.com
E. Blair Schoeb
WeissComm Partners
Media Relations
760.365.1857
SOURCE Arena Pharmaceuticals, Inc
http://www.pipelinereview.com/joomla/content/view/9685/109/
heute ein wenig Bewegung nach oben, aber immer noch recht schwach...


1Q07 EPS loss ($0.53); More clarity on DSMB review timing
Arena reported a 1Q07 EPS loss of ($0.53), narrower than our est. of ($0.68) and
consensus of ($0.67) due to lower than expected expenses. Importantly, the
company refined the timing of the 6-month DSMB review for the Lorcaserin phase
III trial to September (from 3Q07 previously). Maintain Buy.
Lorcaserin DSMB 6-month echo review in September
The 3,182-patient 2-year phase III “BLOOM” trial for Lorcaserin in the treatment
of obesity completed enrollment in Q1 and Arena now expects the DSMB (data
safety monitoring board) review of 6-month echocardiograms in September. The
company is reviewing the echos on an ongoing blinded basis with specific (but
undisclosed) criteria for what would constitute unusual incidence of heart valve
disease, and it would alert the DSMB if its expectations were exceeded, but so far
so good. The DSMB will review the unblinded data in its totality in Sept and
assuming no signs of drug-related valvulopathy, Arena plans to begin two 1-year
phase III trials for Lorcaserin. A 12-month DSMB review is expected in 1Q08.
Other pipeline candidates moving along
The phase II trial for APD125 in chronic insomnia enrolled faster than expected
and was expanded to 150 from 100 patients. We expect phase II data possibly by
3Q. Also, Arena’s partner, JNJ, has APD668 in several phase I trials for type 2
diabetes and we expect an update on the program in late 2Q or 3Q.
Favorable risk/reward profile, Maintain Buy
Lorcaserin represents a potentially important new treatment for obesity with $1bn
potential and we like the risk reward profile for ARNA at current levels.
mfg ipollit
1Q07 EPS loss ($0.53); More clarity on DSMB review timing
Arena reported a 1Q07 EPS loss of ($0.53), narrower than our est. of ($0.68) and
consensus of ($0.67) due to lower than expected expenses. Importantly, the
company refined the timing of the 6-month DSMB review for the Lorcaserin phase
III trial to September (from 3Q07 previously). Maintain Buy.
Lorcaserin DSMB 6-month echo review in September
The 3,182-patient 2-year phase III “BLOOM” trial for Lorcaserin in the treatment
of obesity completed enrollment in Q1 and Arena now expects the DSMB (data
safety monitoring board) review of 6-month echocardiograms in September. The
company is reviewing the echos on an ongoing blinded basis with specific (but
undisclosed) criteria for what would constitute unusual incidence of heart valve
disease, and it would alert the DSMB if its expectations were exceeded, but so far
so good. The DSMB will review the unblinded data in its totality in Sept and
assuming no signs of drug-related valvulopathy, Arena plans to begin two 1-year
phase III trials for Lorcaserin. A 12-month DSMB review is expected in 1Q08.
Other pipeline candidates moving along
The phase II trial for APD125 in chronic insomnia enrolled faster than expected
and was expanded to 150 from 100 patients. We expect phase II data possibly by
3Q. Also, Arena’s partner, JNJ, has APD668 in several phase I trials for type 2
diabetes and we expect an update on the program in late 2Q or 3Q.
Favorable risk/reward profile, Maintain Buy
Lorcaserin represents a potentially important new treatment for obesity with $1bn
potential and we like the risk reward profile for ARNA at current levels.
mfg ipollit
nur am Rande...
Arena sees $1 bln annual sales for obesity drug
NEW YORK, May 24 (Reuters) - Arena Pharmaceuticals Inc.'s (ARNA.O: Quote, Profile , Research) research chief expressed confidence on Thursday that the company's experimental obesity drug will eventually garner annual sales in excess of $1 billion.
Arena research director Dominic Behan said in an interview that the drug, lorcaserin, was showing impressive safety and efficacy in clinical studies and that the development program remains on track to file for review by U.S. health regulators in 2009 and for a 2010 approval for sale.
"Absolutely I think it could be a billion dollar a year drug or more, and how much more will depend on uptake," Behan told Reuters.
Behan said he expects that the company will likely enter into a partnership deal on the drug sometime after this September, when updated safety data is expected to become available, and before the planned 2009 filing with the Food and Drug Administration. The later a partnership deal is completed, the more lucrative it is likely to be for Arena.
"Most potential partners have been waiting to see the longer term safety profile of the compound, so as we kind of de-risk through these milestones, the price goes up," Behan said.
mfg ipollit
Arena sees $1 bln annual sales for obesity drug
NEW YORK, May 24 (Reuters) - Arena Pharmaceuticals Inc.'s (ARNA.O: Quote, Profile , Research) research chief expressed confidence on Thursday that the company's experimental obesity drug will eventually garner annual sales in excess of $1 billion.
Arena research director Dominic Behan said in an interview that the drug, lorcaserin, was showing impressive safety and efficacy in clinical studies and that the development program remains on track to file for review by U.S. health regulators in 2009 and for a 2010 approval for sale.
"Absolutely I think it could be a billion dollar a year drug or more, and how much more will depend on uptake," Behan told Reuters.
Behan said he expects that the company will likely enter into a partnership deal on the drug sometime after this September, when updated safety data is expected to become available, and before the planned 2009 filing with the Food and Drug Administration. The later a partnership deal is completed, the more lucrative it is likely to be for Arena.
"Most potential partners have been waiting to see the longer term safety profile of the compound, so as we kind of de-risk through these milestones, the price goes up," Behan said.
mfg ipollit
From Montgomery
Investment Highlights
* Acomplia problems not related to lorcaserin. Yesterday, an FDA advisory panel unanimously voted not to approve Sanofi-Aventis' obesity drug, Acomplia. However, we do not believe there are any fundamental implications for Arena Pharmaceuticals, Inc.'s lorcaserin from this dissappointing panel on Acomplia. In our opinion, the key reason for the negative vote on Acomplia was a high incidence of severe psychiatric side-effects, including suicidal ideation and depression, not because of a general attitude of the FDA about obesity drugs. Side-effects seen in patients taking lorcaserin in Phase II trials included nausea and headache, which were both transient. In our opinion, a high hurdle rate for approval of lorcaserin (due to possible cardiovascular side-effects) is already built into Arena's stock price. However, unlike Sanofi, with six-monthly echocardiograms, we believe Arena is collecting the right pieces of data to go to the FDA.
* Arena is more than lorcaserin; expect positive news flow. We continue to believe Arena's pipeline is underestimated by the Street, and we expect three positive datasets from three different Arena drugs in the next several months. Specifically, we believe Johnson & Johnson could progress APD668 into later stage trials by Q3. Therefore, we believe
underperformance likely as a result of Sanofi's disappointment creates a buying opportunity. We are maintaining our BUY rating and $25 price target on Arena Pharmaceuticals shares based on DCF valuation.
We currently consider Arena shares attractive for the following reasons:
* We believe investors are overlooking the value of Arena's pipeline due to overemphasis on lorcaserin and the DSMB safety review expected in 3Q07.
* We believe that by the end of 2007, we will have one additional confirmation of proof-of-concept and two new proof-of-concepts from Arena's pipeline.
* We believe it is highly likely that two of these results will be positive. We expect to see key data from Phase I studies of J&J-partnered diabetes drug candidate APD668 in Q2/Q3 and from a Phase II study of unpartnered insomnia drug candidate APD125 in Q3.
* The probability that lorcaserin's DSMB review in Q3 will be negative is substantially low, in our view, based on historical data on valvular abnormalities with fenfluramine-phentermine between three and six months.
* Even if lorcaserin's DSMB review is negative, we believe the value of cash and pipeline would be greater than $16.50 per share, which is approximately 28% higher than yesterday's closing price ($12.94).
* Also, in case of a failure, we believe Arena could be an ideal acquisition candidate for J&J or Merck as well as most other pharma companies because of its pipeline and drug development expertise.
* In our view, Arena is likely to manage expenses well, and the company has a substantial cash stockpile ($356M at the close of 1Q07, which does not include $50M from the sale of buildings).
Powerful drug development engine. In our view, Arena has an exceptional drug development platform focusing on G-protein coupled receptors and a sharp focus on high unmet need in medicine. The top four drugs in Arena's pipeline target the large and growing markets of obesity, insomnia, diabetes, and
cholesterol. If Arena or its partners are able to bring any of these drugs to the market in the next three to five years, we believe investors at current levels should realize a good return on Arena shares. Partnerships with J&J and Merck and multiple clinical successes of internal candidates testify to
the high quality of Arena's drug development engine.
Insomnia drug underestimated. We believe the potential of Arena's lead insomnia compound APD125 is underestimated by the market. In our view, there are four reasons for the underestimation: (1) too much emphasis on lead-compound lorcaserin; (2) early delays in APD125; (3) uncertainty about the reporting time for the Phase II study; and (4) uncertainty about the market need for a sleep maintenance agent that does not help with sleep onset. We believe that a better understanding of a more conservative value for lorcaserin and anticipation of positive Phase II data from APD125 this year build a strong investment case for Arena shares.
Because of progressing pipeline, lorcaserin is now less important. In our opinion, investors are too focused on Arena's lead compound, lorcaserin for obesity. This has been clear to us from the questioning on conference calls, the average buyside opinion on Arena, and commentary on public message boards. Since the upcoming DSMB review expected in 3Q07 is a binary event that is difficult to predict, and since there will be three more DSMB reviews at six-month intervals, we believe longer-term investors should focus more on Arena's pipeline and regard lorcaserin's success as an upside. Our confidence with regard to lorcaserin has actually marginally increased with the passage of time and lack of reports of serious side-effects; still, we believe this
is a more conservative way to model Arena's prospects given the difficulty in predicting the outcomes of DSMB reviews. We have therefore lowered our estimate of approval probability for lorcaserin from 50% to 30% in our model. As a result, we believe lorcaserin accounts for approximately 34% of Arena's
intrinsic value.
APD125 trial enrolling well despite early hiccups. Uncertainty about APD125 had increased when the Phase II trial was postponed from 4Q06 to 1Q07 pending resolution of what the company called "minor issues" relating to
qualification of an impurity in stability studies. These issues were resolved and Arena initiated the Phase II trial in 1Q07. We expected this study to be a crossover study involving approximately 100 insomniacs and randomized to at
least three arms (10mg, 40mg, and placebo). Because the enrollment proceeded more quickly than expected, Arena is likely to eventually enroll close to 150 patients in this trial.
Clinical risk is low. We believe the risk of development of APD125 is relatively low because of positive Phase I data from Arena and positive Phase II results on other compounds in the same class from Sanofi-Aventis. APD125's safety and efficacy are likely to be similar to or better than those of
Sanofi-Aventis' drug, in our opinion. We are encouraged by early results showing almost twice as much improvement in slow-wave sleep with APD125 than with Sanofi-Aventis' eplivanserin. As shown by ACADIA Pharmaceuticals in
March 2007, another compound from the same class exhibited statistically significant results in the co-treatment of schizophrenia; there could be additional clinical utility of APD125, in our view.
Market and need underestimated. We believe that some investors underestimate the market for APD125, as this drug does not help initiate sleep but only helps maintain sleep. Even our initial hypothesis had been that the speed of
onset would be very important in determining competitive positioning. However, experts on insomnia with whom we have conferred have intimated to us the dire need for new drug agents that can help maintain sleep. Sanofi-Aventis confirmed on its 4Q06 earnings conference call that older people do
not often have difficulty falling sleep (sleep onset) but do wake up in the middle of the night (measured usually by WASO, or wakenings after sleep,which is the number of times one awakens after falling asleep). Expeditious enrollment of Arena's recently commenced Phase II trial affirms for us the
need for a safe agent that can help with sleep maintenance.
Expecting results from APD668 in Q2/Q3. In a collaboration, Arena and J&J are developing drug candidates for type II diabetes. The first drug candidate from this collaboration, APD668, is an oral drug targeting the glucose-dependent insulinotropic receptor (GDIR), a novel receptor discovered by
Arena that has the potential to simulate insulin production in response to increases in blood glucose. In February 2006, J&J initiated a Phase I clinical trial of APD668, triggering a $5M milestone payment to Arena. We were under the impression that J&J was running a typical Phase I study and
were somewhat concerned that it was taking a long time to be completed (studies are ongoing since February 2006). We recently learned that J&J has conducted an extensive Phase I program, including multiple placebo-controlled studies, results of which will be announced either later in this quarter or early next quarter. Based on this drug's mechanism of action and the absence of any negative safety commentary by management, we expect these results to be positive. If APD668 enters Phase II studies in 2007, another milestone
payment could be made to Arena.
Downside limited. It is difficult to place a bet on the probability of a successful DSMB review for lorcaserin and even more difficult to place a bet entirely on that data point, although we believe the safety results should be
positive. We are encouraged by the lack of any serious adverse events as of yet. While the stock could react negatively to a negative DSMB outcome, we estimate Arena's value without lorcaserin to be approximately $14 per share.
Therefore, given the current share price of approximately $12, we believe Arena's pipeline is meaningfully undervalued. According to our estimates, if lorcaserin fails to cross the first DSMB review, which we believe is unlikely, Arena could end up with more than $310M in cash at the end of 2007.
Key Updates from 1Q07 Call
* Lorcaserin for obesity. The Phase III for obesity drug candidate lorcaserin is on track, with the first 6-month DSMB review expected in Q3 (around September). Arena will report these data through a press release and is likely to hold a conference call. Arena expects to start two pivotal trial following a positive DSMB review.
* APD125 for insomnia. We expect Arena to report results from the Phase II study of APD125 in Q3, versus prior expectations for the end of the year.
* Early drug candidates. We expect Arena to initiate a Phase I study to test APD791, a novel oral anti-thrombotic compound. We also expect Arena to nominate a research compound for preclinical studies, to be followed by possible entry into the clinic in 2008. Collaborations with Merck and J&J are progressing well, in our view. We expect to see Phase I results from APD668 (partnered with J&J) in Q2. We could see one or two Phase II initiations from partnered programs. These could include a Phase II with a GDIR agonist in diabetes in collaboration with J&J and a Phase II with MK-0354 in an undisclosed indication in collaboration with Merck. Such trial initiations would trigger milestone payments to Arena.
* Cash flow. Cash and equivalents totaled 356M at the end of 1Q07, versus 389M at the start of 2007. Arena reported Q1 results after the market close yesterday, and operating expenses and cash flows were in line with our expectations. We believe Arena is likely to end 2007 with approximately $270M in cash, which is higher than the company's guidance of 261-273M (including approximately 50M from the sale of buildings). According to our estimates, if lorcaserin is dropped, Arena could end up with more than $310M in cash at the end of 2007.
* Key events/milestones in 2007: data from APD668 Phase I in Q2; lorcaserin 6-month DSMB review in Q3; data from APD125 Phase II study in Q3; and data from APD791 Phase I study in Q4. Arena plans to start two additional Phase III studies after the DSMB review in Q3. We expect Arena to announce its next IND candidate in mid-2007 and we anticipate a Phase I study in 2008. If the diabetes collaboration with J&J progresses, we could expect the initiation of a Phase II study by J&J to be announced. The research phase of Arena's collaboration with Merck will end in October; we expect more clarity on this collaboration later in the year. We believe Arena has a strong pipeline that could lead to more in-licensing deals (lorcaserin, APD125, or APD791) and even an outright acquisition.
Key Driver 1: APD125 for Insomnia
APD125 is a novel and orally bioavailable highly selective inverse agonist ofhe 5-HT2A serotonin receptor developed to treat insomnia.
Timing
Arena has initiated a Phase II trial of APD125 and we expect results in 3Q07. Following this study, there could be additional Phase II studies or a Phase
III study. We assume that APD125 will be launched in 2011.
Approvability
The approvability of an insomnia drug depends on its efficacy and safety profile. While APD125 is early in its cycle, it has shown some promise to date. The Phase I program consisted of three randomized, double-blinded, placebo-controlled, dose-ranging studies. During Phase I trials, dose-limiting toxicity was not observed. The two highest doses, 80mg and 160mg, showed similar pharmacokinetics. Phase I results also demonstrated a robust, statistically significant increase in slow-wave sleep at a 40mg dose. There were also improvements in wake after sleep onset. In our opinion, there was no improvement in speed of onset of sleep.
The ongoing Phase II study is a randomized, double-blinded, placebo-controlled study evaluating the safety of night-time dosing with chronic insomnia patients. This is a crossover study with three arms: placebo, 10mg, and 40mg APD125. Every patient receives both active doses of APD125 and placebo in random order for one week, separated by at least a week to allow for drug washout. Due to better than expected enrollment, Arena will enroll 150 patients at approximately 25 clinical sites.
Since Sanofi-Aventis and Eli Lilly are developing drugs with a similar mechanism of action and are farther along, we believe clinical risks are limited. In addition, presented Phase I results give us confidence that the drug is both efficacious and safe.
Market
According to an NIH consensus statement in June 2006, population-based studies suggest that approximately 30% of the US population complains of sleep disruption, and that approximately 10% has insomnia-associated symptoms of daytime functional impairment. According to Espicom Business Intelligence, the market for treatment of insomnia was about $2.5B in 2004. We expect this market to grow to approximately $4.8B in 2010 based on the launch of new products and the resulting increase in patient awareness, especially among
the elderly.
Competition
By selectively targeting the 5-HT2A receptor, APD125 acts through a different mechanism than marketed insomnia drugs. Current treatments, including Ambien, Indiplon, Lunesta, and Sonata, are focused on a GABA receptor. While these drugs have good efficacy, they induce morning hangover and have abuse potential. We believe that APD125 could have efficacy similar to that of current agents without the limitations of the GABA mechanism.
We believe that Sanofi-Aventis and Eli Lilly are interested in the 5-HT2A pathway. Sanofi-Aventis has the lead with an ongoing Phase III program for eplivanserin. In November 2004, Eli Lilly acquired the rights to Merck's KGaA 5-HT2A antagonist, and is likely in late-stage trials. We are encouraged by early results showing almost twice as much improvement in slow-wave sleep with APD125 than with Sanofi-Aventis' eplivanserin.
Profitability
We believe Arena could sign a partnership for APD125 as early as 2008. Since Arena is likely to have completed its Phase II program by the time it can partner APD125, we believe Arena will retain significant economics from this drug. We assume Arena will retain 50% of the profits from APD125.
Key Driver 2: Lorcaserin Hydrochloride for Obesity
Lorcaserin hydrochloride is Arena's most advanced clinical candidate. It is a novel, oral 5-HT2C receptor agonist in the clinic to treat obesity.
Approvability
We are encouraged by the efficacy of this compound because there was a statistically significant placebo-adjusted mean weight loss of 3.3% in patients taking a 10mg BID dose of lorcaserin after three months.
The longer-term safety profile of APD356 will still need to be verified because of its similarity to fenfluramine. In the BLOOM trial (Arena's Phase III study), all patients receive echocardiographs at screening and follow-up echocardiograms at 6, 12, 18, and 24 months after the start of the trial to assess heart valve function over time. We believe the first review will occur in early 3Q07 (around September).
According to a large prospective study that assessed the relationship between the length of treatment with fenfluramine-phentermine and the prevalence of valvular abnormalities, valvular abnormalities were far more significant in patients who took these drugs for the longer term (Circulation 101 (2000):2071). This study examined 1,163 patients who had taken fenfluramine-phentermine and 672 control patients who had not taken the drug combination within five years. After five years, at least mild aortic regurgitation was present in 8.8% of treated patients versus 3.6% of control patients (p<0.001). Adjusted odds ratios show that the comparatively significant aortic regurgitation increased with the duration of treatment: 90 to 180 days, 1.5 (p=0.23); 181 to 360 days, 2.4 (p=0.002); 361 to 720 days, 4.6 (p<0.001); and >720 days, 6.2 (p<0.001).
Timing
We believe that lorcaserin could launch in 2010 with an NDA in 2009. The lorcaserin Phase III program comprises three trials. The BLOOM trial is a double-blinded, randomized, placebo-controlled trial evaluating two doses (20mg and 10mg) of lorcaserin versus placebo over a two-year treatment period in obese and overweight patients. BLOOM completed enrollment of 3,182 patients in 1Q07. We expect the first DSMB review in 3Q07, followed by DSMB reviews in 1Q08, 3Q08, and 1Q09. Top-line data from the trial should be available in 1H09.
Market
Obesity affects a large and growing percentage of the US population. We believe there is both a lack of safe and effective drugs to control obesity and a growing number of motivated patients.
Competition
Current long-term drugs including Meridia and Xenical have side-effect and efficacy limitations. Drug development for obesity is difficult because of obesity's multifactorial etiology. Sanofi-Aventis' Acomplia has experienced numerous regulatory hiccups. Phase IIb results show that lorcaserin has competitive efficacy against other drugs at the 12-week time point.
Profitability
Due to the late-stage nature of this program, we believe it will be possible for Arena to retain 50% profitability from this compound through a large pharmaceutical collaboration. Even without taking into account any patent-term extensions, lorcaserin patents will not begin to expire until 2023.
mfg ipollit
Investment Highlights
* Acomplia problems not related to lorcaserin. Yesterday, an FDA advisory panel unanimously voted not to approve Sanofi-Aventis' obesity drug, Acomplia. However, we do not believe there are any fundamental implications for Arena Pharmaceuticals, Inc.'s lorcaserin from this dissappointing panel on Acomplia. In our opinion, the key reason for the negative vote on Acomplia was a high incidence of severe psychiatric side-effects, including suicidal ideation and depression, not because of a general attitude of the FDA about obesity drugs. Side-effects seen in patients taking lorcaserin in Phase II trials included nausea and headache, which were both transient. In our opinion, a high hurdle rate for approval of lorcaserin (due to possible cardiovascular side-effects) is already built into Arena's stock price. However, unlike Sanofi, with six-monthly echocardiograms, we believe Arena is collecting the right pieces of data to go to the FDA.
* Arena is more than lorcaserin; expect positive news flow. We continue to believe Arena's pipeline is underestimated by the Street, and we expect three positive datasets from three different Arena drugs in the next several months. Specifically, we believe Johnson & Johnson could progress APD668 into later stage trials by Q3. Therefore, we believe
underperformance likely as a result of Sanofi's disappointment creates a buying opportunity. We are maintaining our BUY rating and $25 price target on Arena Pharmaceuticals shares based on DCF valuation.
We currently consider Arena shares attractive for the following reasons:
* We believe investors are overlooking the value of Arena's pipeline due to overemphasis on lorcaserin and the DSMB safety review expected in 3Q07.
* We believe that by the end of 2007, we will have one additional confirmation of proof-of-concept and two new proof-of-concepts from Arena's pipeline.
* We believe it is highly likely that two of these results will be positive. We expect to see key data from Phase I studies of J&J-partnered diabetes drug candidate APD668 in Q2/Q3 and from a Phase II study of unpartnered insomnia drug candidate APD125 in Q3.
* The probability that lorcaserin's DSMB review in Q3 will be negative is substantially low, in our view, based on historical data on valvular abnormalities with fenfluramine-phentermine between three and six months.
* Even if lorcaserin's DSMB review is negative, we believe the value of cash and pipeline would be greater than $16.50 per share, which is approximately 28% higher than yesterday's closing price ($12.94).
* Also, in case of a failure, we believe Arena could be an ideal acquisition candidate for J&J or Merck as well as most other pharma companies because of its pipeline and drug development expertise.
* In our view, Arena is likely to manage expenses well, and the company has a substantial cash stockpile ($356M at the close of 1Q07, which does not include $50M from the sale of buildings).
Powerful drug development engine. In our view, Arena has an exceptional drug development platform focusing on G-protein coupled receptors and a sharp focus on high unmet need in medicine. The top four drugs in Arena's pipeline target the large and growing markets of obesity, insomnia, diabetes, and
cholesterol. If Arena or its partners are able to bring any of these drugs to the market in the next three to five years, we believe investors at current levels should realize a good return on Arena shares. Partnerships with J&J and Merck and multiple clinical successes of internal candidates testify to
the high quality of Arena's drug development engine.
Insomnia drug underestimated. We believe the potential of Arena's lead insomnia compound APD125 is underestimated by the market. In our view, there are four reasons for the underestimation: (1) too much emphasis on lead-compound lorcaserin; (2) early delays in APD125; (3) uncertainty about the reporting time for the Phase II study; and (4) uncertainty about the market need for a sleep maintenance agent that does not help with sleep onset. We believe that a better understanding of a more conservative value for lorcaserin and anticipation of positive Phase II data from APD125 this year build a strong investment case for Arena shares.
Because of progressing pipeline, lorcaserin is now less important. In our opinion, investors are too focused on Arena's lead compound, lorcaserin for obesity. This has been clear to us from the questioning on conference calls, the average buyside opinion on Arena, and commentary on public message boards. Since the upcoming DSMB review expected in 3Q07 is a binary event that is difficult to predict, and since there will be three more DSMB reviews at six-month intervals, we believe longer-term investors should focus more on Arena's pipeline and regard lorcaserin's success as an upside. Our confidence with regard to lorcaserin has actually marginally increased with the passage of time and lack of reports of serious side-effects; still, we believe this
is a more conservative way to model Arena's prospects given the difficulty in predicting the outcomes of DSMB reviews. We have therefore lowered our estimate of approval probability for lorcaserin from 50% to 30% in our model. As a result, we believe lorcaserin accounts for approximately 34% of Arena's
intrinsic value.
APD125 trial enrolling well despite early hiccups. Uncertainty about APD125 had increased when the Phase II trial was postponed from 4Q06 to 1Q07 pending resolution of what the company called "minor issues" relating to
qualification of an impurity in stability studies. These issues were resolved and Arena initiated the Phase II trial in 1Q07. We expected this study to be a crossover study involving approximately 100 insomniacs and randomized to at
least three arms (10mg, 40mg, and placebo). Because the enrollment proceeded more quickly than expected, Arena is likely to eventually enroll close to 150 patients in this trial.
Clinical risk is low. We believe the risk of development of APD125 is relatively low because of positive Phase I data from Arena and positive Phase II results on other compounds in the same class from Sanofi-Aventis. APD125's safety and efficacy are likely to be similar to or better than those of
Sanofi-Aventis' drug, in our opinion. We are encouraged by early results showing almost twice as much improvement in slow-wave sleep with APD125 than with Sanofi-Aventis' eplivanserin. As shown by ACADIA Pharmaceuticals in
March 2007, another compound from the same class exhibited statistically significant results in the co-treatment of schizophrenia; there could be additional clinical utility of APD125, in our view.
Market and need underestimated. We believe that some investors underestimate the market for APD125, as this drug does not help initiate sleep but only helps maintain sleep. Even our initial hypothesis had been that the speed of
onset would be very important in determining competitive positioning. However, experts on insomnia with whom we have conferred have intimated to us the dire need for new drug agents that can help maintain sleep. Sanofi-Aventis confirmed on its 4Q06 earnings conference call that older people do
not often have difficulty falling sleep (sleep onset) but do wake up in the middle of the night (measured usually by WASO, or wakenings after sleep,which is the number of times one awakens after falling asleep). Expeditious enrollment of Arena's recently commenced Phase II trial affirms for us the
need for a safe agent that can help with sleep maintenance.
Expecting results from APD668 in Q2/Q3. In a collaboration, Arena and J&J are developing drug candidates for type II diabetes. The first drug candidate from this collaboration, APD668, is an oral drug targeting the glucose-dependent insulinotropic receptor (GDIR), a novel receptor discovered by
Arena that has the potential to simulate insulin production in response to increases in blood glucose. In February 2006, J&J initiated a Phase I clinical trial of APD668, triggering a $5M milestone payment to Arena. We were under the impression that J&J was running a typical Phase I study and
were somewhat concerned that it was taking a long time to be completed (studies are ongoing since February 2006). We recently learned that J&J has conducted an extensive Phase I program, including multiple placebo-controlled studies, results of which will be announced either later in this quarter or early next quarter. Based on this drug's mechanism of action and the absence of any negative safety commentary by management, we expect these results to be positive. If APD668 enters Phase II studies in 2007, another milestone
payment could be made to Arena.
Downside limited. It is difficult to place a bet on the probability of a successful DSMB review for lorcaserin and even more difficult to place a bet entirely on that data point, although we believe the safety results should be
positive. We are encouraged by the lack of any serious adverse events as of yet. While the stock could react negatively to a negative DSMB outcome, we estimate Arena's value without lorcaserin to be approximately $14 per share.
Therefore, given the current share price of approximately $12, we believe Arena's pipeline is meaningfully undervalued. According to our estimates, if lorcaserin fails to cross the first DSMB review, which we believe is unlikely, Arena could end up with more than $310M in cash at the end of 2007.
Key Updates from 1Q07 Call
* Lorcaserin for obesity. The Phase III for obesity drug candidate lorcaserin is on track, with the first 6-month DSMB review expected in Q3 (around September). Arena will report these data through a press release and is likely to hold a conference call. Arena expects to start two pivotal trial following a positive DSMB review.
* APD125 for insomnia. We expect Arena to report results from the Phase II study of APD125 in Q3, versus prior expectations for the end of the year.
* Early drug candidates. We expect Arena to initiate a Phase I study to test APD791, a novel oral anti-thrombotic compound. We also expect Arena to nominate a research compound for preclinical studies, to be followed by possible entry into the clinic in 2008. Collaborations with Merck and J&J are progressing well, in our view. We expect to see Phase I results from APD668 (partnered with J&J) in Q2. We could see one or two Phase II initiations from partnered programs. These could include a Phase II with a GDIR agonist in diabetes in collaboration with J&J and a Phase II with MK-0354 in an undisclosed indication in collaboration with Merck. Such trial initiations would trigger milestone payments to Arena.
* Cash flow. Cash and equivalents totaled 356M at the end of 1Q07, versus 389M at the start of 2007. Arena reported Q1 results after the market close yesterday, and operating expenses and cash flows were in line with our expectations. We believe Arena is likely to end 2007 with approximately $270M in cash, which is higher than the company's guidance of 261-273M (including approximately 50M from the sale of buildings). According to our estimates, if lorcaserin is dropped, Arena could end up with more than $310M in cash at the end of 2007.
* Key events/milestones in 2007: data from APD668 Phase I in Q2; lorcaserin 6-month DSMB review in Q3; data from APD125 Phase II study in Q3; and data from APD791 Phase I study in Q4. Arena plans to start two additional Phase III studies after the DSMB review in Q3. We expect Arena to announce its next IND candidate in mid-2007 and we anticipate a Phase I study in 2008. If the diabetes collaboration with J&J progresses, we could expect the initiation of a Phase II study by J&J to be announced. The research phase of Arena's collaboration with Merck will end in October; we expect more clarity on this collaboration later in the year. We believe Arena has a strong pipeline that could lead to more in-licensing deals (lorcaserin, APD125, or APD791) and even an outright acquisition.
Key Driver 1: APD125 for Insomnia
APD125 is a novel and orally bioavailable highly selective inverse agonist ofhe 5-HT2A serotonin receptor developed to treat insomnia.
Timing
Arena has initiated a Phase II trial of APD125 and we expect results in 3Q07. Following this study, there could be additional Phase II studies or a Phase
III study. We assume that APD125 will be launched in 2011.
Approvability
The approvability of an insomnia drug depends on its efficacy and safety profile. While APD125 is early in its cycle, it has shown some promise to date. The Phase I program consisted of three randomized, double-blinded, placebo-controlled, dose-ranging studies. During Phase I trials, dose-limiting toxicity was not observed. The two highest doses, 80mg and 160mg, showed similar pharmacokinetics. Phase I results also demonstrated a robust, statistically significant increase in slow-wave sleep at a 40mg dose. There were also improvements in wake after sleep onset. In our opinion, there was no improvement in speed of onset of sleep.
The ongoing Phase II study is a randomized, double-blinded, placebo-controlled study evaluating the safety of night-time dosing with chronic insomnia patients. This is a crossover study with three arms: placebo, 10mg, and 40mg APD125. Every patient receives both active doses of APD125 and placebo in random order for one week, separated by at least a week to allow for drug washout. Due to better than expected enrollment, Arena will enroll 150 patients at approximately 25 clinical sites.
Since Sanofi-Aventis and Eli Lilly are developing drugs with a similar mechanism of action and are farther along, we believe clinical risks are limited. In addition, presented Phase I results give us confidence that the drug is both efficacious and safe.
Market
According to an NIH consensus statement in June 2006, population-based studies suggest that approximately 30% of the US population complains of sleep disruption, and that approximately 10% has insomnia-associated symptoms of daytime functional impairment. According to Espicom Business Intelligence, the market for treatment of insomnia was about $2.5B in 2004. We expect this market to grow to approximately $4.8B in 2010 based on the launch of new products and the resulting increase in patient awareness, especially among
the elderly.
Competition
By selectively targeting the 5-HT2A receptor, APD125 acts through a different mechanism than marketed insomnia drugs. Current treatments, including Ambien, Indiplon, Lunesta, and Sonata, are focused on a GABA receptor. While these drugs have good efficacy, they induce morning hangover and have abuse potential. We believe that APD125 could have efficacy similar to that of current agents without the limitations of the GABA mechanism.
We believe that Sanofi-Aventis and Eli Lilly are interested in the 5-HT2A pathway. Sanofi-Aventis has the lead with an ongoing Phase III program for eplivanserin. In November 2004, Eli Lilly acquired the rights to Merck's KGaA 5-HT2A antagonist, and is likely in late-stage trials. We are encouraged by early results showing almost twice as much improvement in slow-wave sleep with APD125 than with Sanofi-Aventis' eplivanserin.
Profitability
We believe Arena could sign a partnership for APD125 as early as 2008. Since Arena is likely to have completed its Phase II program by the time it can partner APD125, we believe Arena will retain significant economics from this drug. We assume Arena will retain 50% of the profits from APD125.
Key Driver 2: Lorcaserin Hydrochloride for Obesity
Lorcaserin hydrochloride is Arena's most advanced clinical candidate. It is a novel, oral 5-HT2C receptor agonist in the clinic to treat obesity.
Approvability
We are encouraged by the efficacy of this compound because there was a statistically significant placebo-adjusted mean weight loss of 3.3% in patients taking a 10mg BID dose of lorcaserin after three months.
The longer-term safety profile of APD356 will still need to be verified because of its similarity to fenfluramine. In the BLOOM trial (Arena's Phase III study), all patients receive echocardiographs at screening and follow-up echocardiograms at 6, 12, 18, and 24 months after the start of the trial to assess heart valve function over time. We believe the first review will occur in early 3Q07 (around September).
According to a large prospective study that assessed the relationship between the length of treatment with fenfluramine-phentermine and the prevalence of valvular abnormalities, valvular abnormalities were far more significant in patients who took these drugs for the longer term (Circulation 101 (2000):2071). This study examined 1,163 patients who had taken fenfluramine-phentermine and 672 control patients who had not taken the drug combination within five years. After five years, at least mild aortic regurgitation was present in 8.8% of treated patients versus 3.6% of control patients (p<0.001). Adjusted odds ratios show that the comparatively significant aortic regurgitation increased with the duration of treatment: 90 to 180 days, 1.5 (p=0.23); 181 to 360 days, 2.4 (p=0.002); 361 to 720 days, 4.6 (p<0.001); and >720 days, 6.2 (p<0.001).
Timing
We believe that lorcaserin could launch in 2010 with an NDA in 2009. The lorcaserin Phase III program comprises three trials. The BLOOM trial is a double-blinded, randomized, placebo-controlled trial evaluating two doses (20mg and 10mg) of lorcaserin versus placebo over a two-year treatment period in obese and overweight patients. BLOOM completed enrollment of 3,182 patients in 1Q07. We expect the first DSMB review in 3Q07, followed by DSMB reviews in 1Q08, 3Q08, and 1Q09. Top-line data from the trial should be available in 1H09.
Market
Obesity affects a large and growing percentage of the US population. We believe there is both a lack of safe and effective drugs to control obesity and a growing number of motivated patients.
Competition
Current long-term drugs including Meridia and Xenical have side-effect and efficacy limitations. Drug development for obesity is difficult because of obesity's multifactorial etiology. Sanofi-Aventis' Acomplia has experienced numerous regulatory hiccups. Phase IIb results show that lorcaserin has competitive efficacy against other drugs at the 12-week time point.
Profitability
Due to the late-stage nature of this program, we believe it will be possible for Arena to retain 50% profitability from this compound through a large pharmaceutical collaboration. Even without taking into account any patent-term extensions, lorcaserin patents will not begin to expire until 2023.
mfg ipollit
Seattle (aktiencheck.de AG) - Die Analysten von Montgomery & Co stufen die Aktie von Arena Pharmaceuticals (ISIN US0400471027/ WKN 939027) unverändert mit "buy" ein. Das Kursziel werde bei 25 USD gesehen. (18.06.2007/ac/a/u) Analyse-Datum: 18.06.2007
Auf diesem Niveau bietet sich die Aufstockung von ARNA an:
Marktkapitalisierung: $ 660 Mio
Cash: ca. 370 Mio.
Die Pipeline mit doppeltem Blockbusterpotenzial wird somit mit
mageren $ 290 Mio. bewertet. Zudem liegt die Vermeldung einer
Partnerschaft in Bezug auf Lorcaserin in der Luft, was ARNA in ganz
andere Sphären bringen würde. Je später die Partnerschaft, umso
lukrativer wird sie für ARNA!









Marktkapitalisierung: $ 660 Mio
Cash: ca. 370 Mio.
Die Pipeline mit doppeltem Blockbusterpotenzial wird somit mit
mageren $ 290 Mio. bewertet. Zudem liegt die Vermeldung einer
Partnerschaft in Bezug auf Lorcaserin in der Luft, was ARNA in ganz
andere Sphären bringen würde. Je später die Partnerschaft, umso
lukrativer wird sie für ARNA!









Antwort auf Beitrag Nr.: 30.478.052 von Der.Eroberer am 03.07.07 23:03:05ich glaube nicht, dass eine Partnerschaft in der nächsten Zeit zu erwarten ist... im September kommen die ersten Sicherheitsdaten zu Locaserin (das ja ein direkter Nachfolger einer der größten Pharmakatastrophen FenPhen ist). Diese geben einen ersten Hinweis, ob Lorca auch das Herz schädigt... allerdings nur einen ersten. Eine bessere Aussage lässt sich Anfang 2008 machen, wenn die 12M Daten kommen. Erst in diesem Zeitraum könnten Pharmas anbeißen, falls alles glatt läuft.
meinetwegen kann der Kurs noch bis September da unten bleiben...
mfg ipollit
meinetwegen kann der Kurs noch bis September da unten bleiben...
mfg ipollit
Antwort auf Beitrag Nr.: 30.478.462 von ipollit am 03.07.07 23:59:29Ich rechne auch nicht kurzfristig mit einer Partnerschaft, aber
dieses Bonbon macht ARNA fast unwiderstehlich. Möglicherweise trauen
sie sich auch den Direktvertrieb zu. Das würde erstmal viel Geld
kosten, aber in der Folge üppige Gewinne sprudeln lassen.
Was hälst Du von einer direkten Vermarktung?
dieses Bonbon macht ARNA fast unwiderstehlich. Möglicherweise trauen
sie sich auch den Direktvertrieb zu. Das würde erstmal viel Geld
kosten, aber in der Folge üppige Gewinne sprudeln lassen.
Was hälst Du von einer direkten Vermarktung?
Antwort auf Beitrag Nr.: 30.479.243 von Der.Eroberer am 04.07.07 08:25:55da es sich um einen sehr großen Markt handelt und nicht um ein paar Spezialisten, die Locaserin einsetzen werden, fände ich eine Eigenvermarktung nicht so gut. Besser ein großer Pharma, der ordentlich Werbung macht und seine ganze Vertriebsmacht nutzen kann... ich denke auch, dass es so kommen wird, wenn die Sicherheitsdaten okay sind.
mfg ipollit
mfg ipollit
Antwort auf Beitrag Nr.: 30.513.386 von ipollit am 05.07.07 22:07:31Da stimme ich Dir natürlich zu. Ein großer Partner wäre die besser
Lösung. Vor dem Hintergrund des heutigen Alnylam-Deals stellt sich mir
natürlich die Frage welche Milestones ARNA innerhalb einer Partnerschaft
erringen könnte.
In Anbetracht der möglichen Umsätze für lorcaserin (1 Mrd +) kann
ich mir gut vorstellen, dass die upfront-payments mindestens 150-200 Mio $
betragen müssten, wenn man sich alleine die Phase III Kosten in Höhe
von 125 Mio. $ vor Augen hält, die ARNA vorschiesst. Ich denke, dass
ist ein relativ konservativer Ansatz. Sollten nach den 12 Monats-Daten noch keine
gravierenden Gründe gegen das Mittel sprechen, dann sollte sich die erste
Zahlung auf mindestens 300 Mio $ belaufen, denn das Risiko wäre
dann für den Partner relativ überschaubar.
Wenn ich mir aber die heutigen Zahlen des Alnylam-Roche-Deals anschaue,
deren potentielle Produkte noch nicht einmal Phase I - Status haben,
dann kommen mir Zweifel, ob ARNA sich mit 300 Mio zufrieden geben sollte.
Mir ist schon klar, dass Alnylam in einem gänzlich anderen Bereich tätig
ist, allerdings muss Alnylam es zuerst mal schaffen einen Produktkandidaten
mit Blockbusterpotenzial zu entwickeln, was ARNA bereits geschafft hat.
Lösung. Vor dem Hintergrund des heutigen Alnylam-Deals stellt sich mir
natürlich die Frage welche Milestones ARNA innerhalb einer Partnerschaft
erringen könnte.
In Anbetracht der möglichen Umsätze für lorcaserin (1 Mrd +) kann
ich mir gut vorstellen, dass die upfront-payments mindestens 150-200 Mio $
betragen müssten, wenn man sich alleine die Phase III Kosten in Höhe
von 125 Mio. $ vor Augen hält, die ARNA vorschiesst. Ich denke, dass
ist ein relativ konservativer Ansatz. Sollten nach den 12 Monats-Daten noch keine
gravierenden Gründe gegen das Mittel sprechen, dann sollte sich die erste
Zahlung auf mindestens 300 Mio $ belaufen, denn das Risiko wäre
dann für den Partner relativ überschaubar.
Wenn ich mir aber die heutigen Zahlen des Alnylam-Roche-Deals anschaue,
deren potentielle Produkte noch nicht einmal Phase I - Status haben,
dann kommen mir Zweifel, ob ARNA sich mit 300 Mio zufrieden geben sollte.
Mir ist schon klar, dass Alnylam in einem gänzlich anderen Bereich tätig
ist, allerdings muss Alnylam es zuerst mal schaffen einen Produktkandidaten
mit Blockbusterpotenzial zu entwickeln, was ARNA bereits geschafft hat.
Arena Pharmaceuticals to Report Second Quarter 2007 Financial Results and to Host Conference Call and Webcast on Thursday, July 19, 2007
Thursday July 12, 8:00 am ET
SAN DIEGO, July 12 /PRNewswire-FirstCall/ -- Arena Pharmaceuticals, Inc. (Nasdaq: ARNA - News) today announced it will report financial results for the second quarter 2007 after the Nasdaq Global Market closes on Thursday, July 19, 2007. That same afternoon, Jack Lief, Arena's President and Chief Executive Officer, and Robert E. Hoffman, Arena's Vice President, Finance and Chief Financial Officer, will host a conference call at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time) to discuss the financial results for the second quarter 2007 and to provide a corporate update.
Thursday July 12, 8:00 am ET
SAN DIEGO, July 12 /PRNewswire-FirstCall/ -- Arena Pharmaceuticals, Inc. (Nasdaq: ARNA - News) today announced it will report financial results for the second quarter 2007 after the Nasdaq Global Market closes on Thursday, July 19, 2007. That same afternoon, Jack Lief, Arena's President and Chief Executive Officer, and Robert E. Hoffman, Arena's Vice President, Finance and Chief Financial Officer, will host a conference call at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time) to discuss the financial results for the second quarter 2007 and to provide a corporate update.
Arena Keeps Burning the Cash
By Brian Orelli July 20, 2007
Clinical-stage drug developer Arena Pharmaceuticals (Nasdaq: ARNA) reported its second-quarter earnings yesterday. With the company's cash-burn rate having more than doubled over the past year, from $19 million to $39.1 million, I have to use the word "earnings" pretty loosely, but given what the money is being used for, it's hard for investors to complain.
Specifically, most of the increase was due to increased research and development costs associated with two trials -- phase 3 clinical trials for lorcaserin in treating obesity, and phase 2 trial testing for APD125 in treating insomnia. Investors won't have to wait too long to see what they've been paying for, either: Results from both trials are expected in the third quarter.
Early indications from locaserin's phase 2 trial are that the drug works quite well in reducing weight in obese patients. The company plans to start two additional clinical trials later this year, so it will be a while before Arena files a New Drug Application with the FDA.
Arena has collaborations with Johnson & Johnson's (NYSE: JNJ) Ortho-McNeil Pharmaceutical subsidiary and with Merck (NYSE: MRK), from which it receives some revenue from milestone payments. But those collaborations are for compounds in early-stage development. Its two most promising drugs, lorcaserin and APD125, were developed in-house and don't currently have partners, so Arena is footing the entire bill for their development.
Fortunately, the company does have some time before it runs out of money. It has $371 million in the bank from two follow-on stock offerings in 2006, resulting in a $334 million cash infusion. It also increased its cash position this quarter by selling and leasing back a building it owned for $48 million. At the end of the year, Arena expects to have $259 million to $271 million in cash and equivalents -- sill plenty of money to get it through the development of lorcaserin.
That's crucial, because Arena will live and die by the results of the lorcaserin trial. If the first trial is successful, the company should consider bringing in a marketing partner -- not so much because it needs the cash, but because it will allow Arena to share the financial risk of later trials and the FDA approval process. Sanofi-Aventis' (NYSE: SNY) recent FDA troubles with its anti-obesity drug rimonabant should stand as a warning. While Sanofi can afford to take a hit, Arena is too small to play that game alone.
As Motley Fool CAPS player skat5 sums it up, "I expect [more than a] double [in the stock price] if it gets approval and a similar drop if it does not."
By Brian Orelli July 20, 2007
Clinical-stage drug developer Arena Pharmaceuticals (Nasdaq: ARNA) reported its second-quarter earnings yesterday. With the company's cash-burn rate having more than doubled over the past year, from $19 million to $39.1 million, I have to use the word "earnings" pretty loosely, but given what the money is being used for, it's hard for investors to complain.
Specifically, most of the increase was due to increased research and development costs associated with two trials -- phase 3 clinical trials for lorcaserin in treating obesity, and phase 2 trial testing for APD125 in treating insomnia. Investors won't have to wait too long to see what they've been paying for, either: Results from both trials are expected in the third quarter.
Early indications from locaserin's phase 2 trial are that the drug works quite well in reducing weight in obese patients. The company plans to start two additional clinical trials later this year, so it will be a while before Arena files a New Drug Application with the FDA.
Arena has collaborations with Johnson & Johnson's (NYSE: JNJ) Ortho-McNeil Pharmaceutical subsidiary and with Merck (NYSE: MRK), from which it receives some revenue from milestone payments. But those collaborations are for compounds in early-stage development. Its two most promising drugs, lorcaserin and APD125, were developed in-house and don't currently have partners, so Arena is footing the entire bill for their development.
Fortunately, the company does have some time before it runs out of money. It has $371 million in the bank from two follow-on stock offerings in 2006, resulting in a $334 million cash infusion. It also increased its cash position this quarter by selling and leasing back a building it owned for $48 million. At the end of the year, Arena expects to have $259 million to $271 million in cash and equivalents -- sill plenty of money to get it through the development of lorcaserin.
That's crucial, because Arena will live and die by the results of the lorcaserin trial. If the first trial is successful, the company should consider bringing in a marketing partner -- not so much because it needs the cash, but because it will allow Arena to share the financial risk of later trials and the FDA approval process. Sanofi-Aventis' (NYSE: SNY) recent FDA troubles with its anti-obesity drug rimonabant should stand as a warning. While Sanofi can afford to take a hit, Arena is too small to play that game alone.
As Motley Fool CAPS player skat5 sums it up, "I expect [more than a] double [in the stock price] if it gets approval and a similar drop if it does not."
Für die aktuelle Diskussion enthistorisiert.
MfG MaatMOD
MfG MaatMOD
Antwort auf Beitrag Nr.: 36.495.022 von MaatMod am 02.02.09 15:04:30Danke an MaatMOD 
Nun, freue mich auf neue Beiträge.
Bin selber am 14.01 wieder mit 1.000 Stk eingestiegen

Nun, freue mich auf neue Beiträge.
Bin selber am 14.01 wieder mit 1.000 Stk eingestiegen

Analyse-Datum: 26.01.2009
Endingen (aktiencheck.de AG) - Für die Experten von "Global Biotech Investing" ist die Aktie von Arena Pharmaceuticals (ISIN US0400471027/ WKN 939027) ein glasklarer Kandidat für ihr Musterdepot.
Für die Gesellschaft und ihren Hoffnungsträger Lorcaserin gehe es 2009 in die heiße Phase. Das Unternehmen konzentriere sich voll und ganz auf einen erfolgreichen Ausgang der Testreihen zu Lorcaserin.
Für Ende März sei die Veröffentlichung der Phase III-Tests datiert.
Bei einem positiven Bescheid könne nach Ansicht der Experten ein entsprechender NDA bereits für Ende 2009 bei der US-Zulassungsstelle beantragt werden.
Die Spanne der Kurszieleinschätzungen für die Aktie liege zwischen 4 USD und 20 USD.
Zuletzt hätten die wenig überzeugenden Phase III-Daten des Konkurrenzproduktes Contrave vom Mitbewerber Orexigen die Aktie von Arena beflügelt. Aber auch wenn das Konkurrenzprodukt ebenfalls die Marktzulassung erhalte, würden die Analysten von Oppenheimer das Marktpotenzial von Lorcaserin für Arena Pharmaceuticals immer noch auf über eine Milliarde USD schätzen.
Für die Experten von "Global Biotech Investing" ist die Aktie von Arena Pharmaceuticals ein glasklarer Kandidat für ihr Musterdepot. (Ausgabe 2 vom 26.01.2009) (26.01.2009/ac/a/a)
Analyse-Datum: 26.01.2009
Endingen (aktiencheck.de AG) - Für die Experten von "Global Biotech Investing" ist die Aktie von Arena Pharmaceuticals (ISIN US0400471027/ WKN 939027) ein glasklarer Kandidat für ihr Musterdepot.
Für die Gesellschaft und ihren Hoffnungsträger Lorcaserin gehe es 2009 in die heiße Phase. Das Unternehmen konzentriere sich voll und ganz auf einen erfolgreichen Ausgang der Testreihen zu Lorcaserin.
Für Ende März sei die Veröffentlichung der Phase III-Tests datiert.
Bei einem positiven Bescheid könne nach Ansicht der Experten ein entsprechender NDA bereits für Ende 2009 bei der US-Zulassungsstelle beantragt werden.
Die Spanne der Kurszieleinschätzungen für die Aktie liege zwischen 4 USD und 20 USD.
Zuletzt hätten die wenig überzeugenden Phase III-Daten des Konkurrenzproduktes Contrave vom Mitbewerber Orexigen die Aktie von Arena beflügelt. Aber auch wenn das Konkurrenzprodukt ebenfalls die Marktzulassung erhalte, würden die Analysten von Oppenheimer das Marktpotenzial von Lorcaserin für Arena Pharmaceuticals immer noch auf über eine Milliarde USD schätzen.
Für die Experten von "Global Biotech Investing" ist die Aktie von Arena Pharmaceuticals ein glasklarer Kandidat für ihr Musterdepot. (Ausgabe 2 vom 26.01.2009) (26.01.2009/ac/a/a)
Analyse-Datum: 26.01.2009
Antwort auf Beitrag Nr.: 36.495.115 von josema am 02.02.09 15:15:00Hast du direkt in USA geordert?
Antwort auf Beitrag Nr.: 36.495.602 von Fruehrentner am 02.02.09 16:10:00nein.
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| -0,29 | |
| -0,15 | |
| +1,36 | |
| +66,67 | |
| -0,06 | |
| -8,53 | |
| 0,00 | |
| +0,62 | |
| 0,00 | |
| 0,00 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 35 | ||
| 28 | ||
| 21 | ||
| 19 | ||
| 19 | ||
| 16 | ||
| 14 | ||
| 13 | ||
| 12 | ||
| 11 |





















