ARENA PHARMACEUTICALS + BELVIQ - Wie geht es weiter ??? - 500 Beiträge pro Seite
eröffnet am 12.07.12 11:11:32 von
neuester Beitrag 11.07.17 13:41:55 von
neuester Beitrag 11.07.17 13:41:55 von
Beiträge: 377
ID: 1.175.491
ID: 1.175.491
Aufrufe heute: 0
Gesamt: 35.800
Gesamt: 35.800
Aktive User: 0
ISIN: US0400476075 · WKN: A2DR4A
99,98
USD
+0,06 %
+0,06 USD
Letzter Kurs 11.03.22 NYSE
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 6,0000 | +25,00 | |
| 56,69 | +20,00 | |
| 0,6400 | +18,52 | |
| 26,46 | +15,70 | |
| 1,1100 | +15,70 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 9,7200 | -19,60 | |
| 4,0000 | -27,27 | |
| 2,7280 | -29,14 | |
| 14,510 | -32,32 | |
| 0,7000 | -61,96 |
June 27, 2012 - Full FDA Press Release:
FDA approves




 to treat some overweight or obese adults
to treat some overweight or obese adults
The U.S. Food and Drug Administration today approved Belviq (lorcaserin hydrochloride), as an addition to a reduced-calorie diet and exercise, for chronic weight management.
The drug is approved for use in adults with a body mass index (BMI) of 30 or greater (obese), or adults with a BMI of 27 or greater (overweight) and who have at least one weight-related condition such as high blood pressure (hypertension), type 2 diabetes, or high cholesterol (dyslipidemia).
BMI, which measures body fat based on an individual’s weight and height, is used to define the obesity and overweight categories. According to the Centers for Disease Control and Prevention, more than one-third of adults in the United States are obese.
“Obesity threatens the overall well being of patients and is a major public health concern,” said Janet Woodcock, M.D., director of the FDA’s Center for Drug Evaluation and Research. “The approval of this drug, used responsibly in combination with a healthy diet and lifestyle, provides a treatment option for Americans who are obese or are overweight and have at least one weight-related comorbid condition.”
Belviq works by activating the serotonin 2C receptor in the brain. Activation of this receptor may help a person eat less and feel full after eating smaller amounts of food.
The safety and efficacy of Belviq were evaluated in three randomized, placebo-controlled trials that included nearly 8,000 obese and overweight patients, with and without type 2 diabetes, treated for 52 to 104 weeks. All participants received lifestyle modification that consisted of a reduced calorie diet and exercise counseling. Compared with placebo, treatment with Belviq for up to one year was associated with average weight loss ranging from 3 percent to 3.7 percent.
About 47 percent of patients without type 2 diabetes lost at least 5 percent of their body weight compared with about 23 percent of patients treated with placebo. In people with type 2 diabetes, about 38 percent of patients treated with Belviq and 16 percent treated with placebo lost at least 5 percent of their body weight. Belviq treatment was associated with favorable changes in glycemic control in those with type 2 diabetes. The approved labeling for Belviq recommends that the drug be discontinued in patients who fail to lose 5 percent of their body weight after 12 weeks of treatment, as these patients are unlikely to achieve clinically meaningful weight loss with continued treatment.
Belviq should not be used during pregnancy. Treatment with Belviq may cause serious side effects, including serotonin syndrome, particularly when taken with certain medicines that increase serotonin levels or activate serotonin receptors. These include, but are not limited to, drugs commonly used to treat depression and migraine. Belviq may also cause disturbances in attention or memory.
In 1997, the weight-loss drugs fenfluramine and dexfenfluramine were withdrawn from the market after evidence emerged that they caused heart valve damage. This effect is assumed to be related to activation of the serotonin 2B receptor on heart tissue. When used at the approved dose of 10 milligrams twice a day, Belviq does not appear to activate the serotonin 2B receptor.
Heart valve function was assessed by echocardiography in nearly 8,000 patients in the Belviq development program. There was no statistically significant difference in the development of FDA-defined valve abnormalities between Belviq and placebo-treated patients. Because preliminary data suggest that the number of serotonin 2B receptors may be increased in patients with congestive heart failure, Belviq should be used with caution in patients with this condition. Belviq has not been studied in patients with serious valvular heart disease.
The drug’s manufacturer will be required to conduct six postmarketing studies, including a long-term cardiovascular outcomes trial to assess the effect of Belviq on the risk for major adverse cardiac events such as heart attack and stroke.
The most common side effects of Belviq in non-diabetic patients are headache, dizziness, fatigue, nausea, dry mouth, and constipation, and in diabetic patients are low blood sugar (hypoglycemia), headache, back pain, cough, and fatigue.
Belviq is manufactured by Arena Pharmaceuticals GmbH of Zofingen, Switzerland, and distributed by Eisai Inc. of Woodcliff Lake, N.J.
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/uc…
FDA approves





 to treat some overweight or obese adults
to treat some overweight or obese adultsThe U.S. Food and Drug Administration today approved Belviq (lorcaserin hydrochloride), as an addition to a reduced-calorie diet and exercise, for chronic weight management.
The drug is approved for use in adults with a body mass index (BMI) of 30 or greater (obese), or adults with a BMI of 27 or greater (overweight) and who have at least one weight-related condition such as high blood pressure (hypertension), type 2 diabetes, or high cholesterol (dyslipidemia).
BMI, which measures body fat based on an individual’s weight and height, is used to define the obesity and overweight categories. According to the Centers for Disease Control and Prevention, more than one-third of adults in the United States are obese.
“Obesity threatens the overall well being of patients and is a major public health concern,” said Janet Woodcock, M.D., director of the FDA’s Center for Drug Evaluation and Research. “The approval of this drug, used responsibly in combination with a healthy diet and lifestyle, provides a treatment option for Americans who are obese or are overweight and have at least one weight-related comorbid condition.”
Belviq works by activating the serotonin 2C receptor in the brain. Activation of this receptor may help a person eat less and feel full after eating smaller amounts of food.
The safety and efficacy of Belviq were evaluated in three randomized, placebo-controlled trials that included nearly 8,000 obese and overweight patients, with and without type 2 diabetes, treated for 52 to 104 weeks. All participants received lifestyle modification that consisted of a reduced calorie diet and exercise counseling. Compared with placebo, treatment with Belviq for up to one year was associated with average weight loss ranging from 3 percent to 3.7 percent.
About 47 percent of patients without type 2 diabetes lost at least 5 percent of their body weight compared with about 23 percent of patients treated with placebo. In people with type 2 diabetes, about 38 percent of patients treated with Belviq and 16 percent treated with placebo lost at least 5 percent of their body weight. Belviq treatment was associated with favorable changes in glycemic control in those with type 2 diabetes. The approved labeling for Belviq recommends that the drug be discontinued in patients who fail to lose 5 percent of their body weight after 12 weeks of treatment, as these patients are unlikely to achieve clinically meaningful weight loss with continued treatment.
Belviq should not be used during pregnancy. Treatment with Belviq may cause serious side effects, including serotonin syndrome, particularly when taken with certain medicines that increase serotonin levels or activate serotonin receptors. These include, but are not limited to, drugs commonly used to treat depression and migraine. Belviq may also cause disturbances in attention or memory.
In 1997, the weight-loss drugs fenfluramine and dexfenfluramine were withdrawn from the market after evidence emerged that they caused heart valve damage. This effect is assumed to be related to activation of the serotonin 2B receptor on heart tissue. When used at the approved dose of 10 milligrams twice a day, Belviq does not appear to activate the serotonin 2B receptor.
Heart valve function was assessed by echocardiography in nearly 8,000 patients in the Belviq development program. There was no statistically significant difference in the development of FDA-defined valve abnormalities between Belviq and placebo-treated patients. Because preliminary data suggest that the number of serotonin 2B receptors may be increased in patients with congestive heart failure, Belviq should be used with caution in patients with this condition. Belviq has not been studied in patients with serious valvular heart disease.
The drug’s manufacturer will be required to conduct six postmarketing studies, including a long-term cardiovascular outcomes trial to assess the effect of Belviq on the risk for major adverse cardiac events such as heart attack and stroke.
The most common side effects of Belviq in non-diabetic patients are headache, dizziness, fatigue, nausea, dry mouth, and constipation, and in diabetic patients are low blood sugar (hypoglycemia), headache, back pain, cough, and fatigue.
Belviq is manufactured by Arena Pharmaceuticals GmbH of Zofingen, Switzerland, and distributed by Eisai Inc. of Woodcliff Lake, N.J.
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/uc…
Hier noch einmal einige Artikel aus der Presse:
 FDA approves Arena obesity drug; first in 13 years
FDA approves Arena obesity drug; first in 13 years
http://www.reuters.com/article/2012/06/27/us-arena-obesity-i…
 Arena Pharmaceuticals confirms FDA approval of diet drug lorcaserin
Arena Pharmaceuticals confirms FDA approval of diet drug lorcaserin
http://finance.yahoo.com/news/arena-pharmaceuticals-confirms…
 Arena’s Weight-Loss Pill Approved by U.S. Regulators
Arena’s Weight-Loss Pill Approved by U.S. Regulators
http://www.bloomberg.com/news/2012-06-27/arena-s-weight-loss…" target="_blank" rel="nofollow ugc noopener">
http://www.bloomberg.com/news/2012-06-27/arena-s-weight-loss…
 FDA approves Arena obesity drug; first in 13 years
FDA approves Arena obesity drug; first in 13 years
http://www.reuters.com/article/2012/06/27/us-arena-obesity-i…
 Arena Pharmaceuticals confirms FDA approval of diet drug lorcaserin
Arena Pharmaceuticals confirms FDA approval of diet drug lorcaserin
http://finance.yahoo.com/news/arena-pharmaceuticals-confirms…
 Arena’s Weight-Loss Pill Approved by U.S. Regulators
Arena’s Weight-Loss Pill Approved by U.S. Regulators
http://www.bloomberg.com/news/2012-06-27/arena-s-weight-loss…" target="_blank" rel="nofollow ugc noopener">
http://www.bloomberg.com/news/2012-06-27/arena-s-weight-loss…
Arena Pharmaceuticals Promotes Craig M. Audet to Executive Officer as Senior Vice President, Operations and Head of Global Regulatory Affairs
http://finance.yahoo.com/news/arena-pharmaceuticals-promotes…
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) has announced Craig M. Audet’s promotion to Senior VP, Operations and Head of Global Regulatory Affairs. This new role means Audet will serve as and executive officer for the company. He has over 25 years experience in the industry. Before joining Arena, he served as VP and Head of the US Regulatory Affairs Marketed Products Group for Sanofi-Aventis from 2003 to 2008.
http://wallstcheatsheet.com/stocks/jp-morgan-sued-by-ferc-an…
So wie ich obenstehenden Artikel verstehe, hat ARENA einen " klugen" Kopf an die Spitze gesetzt , der u.a. sehr viel Erfahrungen bei der Vermarktung von Produkten und damit zusammenhängenden gesetzlichen Regularien in diesem Bereich hat.
Alle Zeichen stehen auf "Expansion"
http://finance.yahoo.com/news/arena-pharmaceuticals-promotes…
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) has announced Craig M. Audet’s promotion to Senior VP, Operations and Head of Global Regulatory Affairs. This new role means Audet will serve as and executive officer for the company. He has over 25 years experience in the industry. Before joining Arena, he served as VP and Head of the US Regulatory Affairs Marketed Products Group for Sanofi-Aventis from 2003 to 2008.
http://wallstcheatsheet.com/stocks/jp-morgan-sued-by-ferc-an…
So wie ich obenstehenden Artikel verstehe, hat ARENA einen " klugen" Kopf an die Spitze gesetzt , der u.a. sehr viel Erfahrungen bei der Vermarktung von Produkten und damit zusammenhängenden gesetzlichen Regularien in diesem Bereich hat.
Alle Zeichen stehen auf "Expansion"

Hier ein sehr schöner Überblick über das Prozedere und die zeitliche Dimension für die mögliche Zula von Belviq in Europa durch die EMA ( European Medicines Agency)
Mit einem " Approval" seitens der EMA dürfte somit zeitmäßig ab Mitte/Ende Dezember 2012 zu rechnen sein - abhängig davon , in welchem Zeitraum ARENA auf die Fragen der EMA antworten wird.
Ich schätze die Chancen auf ein Approval seitens der FDA als sehr gut ein.
The general process:
A two-phase evaluation:
120 days and 90 days, leading to an opinion (approval or disapproval decision). So the entire process is AT LEAST 210 days.
You can break down the process as follow:
First Phase:
Day 0: EMA accepts ARNA application for (lor). > am 24.03.12 !!!
Day 80: EMA releases Co Rapporteurs Report (from 2 groups) - this is equivalent to FDA's Briefing doc.
Day 100: EMA Peer Review (of the Report).
Day 120: EMA sends ARNA a List of Questions.
Clock now stops.
The 1st phase of the process is finished. Once ARNA submits the responses to the List of Questions, the clocks start again for the 2nd phase. I don't know how long this would take between Day 120 and 121, but ARNA has maximum of 60 days to respond.
Second Phase
Day 121: ARNA responds to List of Questions.
Day 150: Join Report from the 2 groups.
Day 180: EMA sends ARNA a List of Issues.
Clock stops again.
ARNA has maximum of 30 days to respond.
Day 181: ARNA has Oral Explanation to List of Issues.
Day 210: Final Scientific Opinion <-- Approval Decision.
So if you add 210 days (total 2 phases) to the 2 idled periods of 60 and 30 days, it will be grand total of 310 which is about 10 months.
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…" target="_blank" rel="nofollow ugc noopener">
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
Mit einem " Approval" seitens der EMA dürfte somit zeitmäßig ab Mitte/Ende Dezember 2012 zu rechnen sein - abhängig davon , in welchem Zeitraum ARENA auf die Fragen der EMA antworten wird.
Ich schätze die Chancen auf ein Approval seitens der FDA als sehr gut ein.
The general process:
A two-phase evaluation:
120 days and 90 days, leading to an opinion (approval or disapproval decision). So the entire process is AT LEAST 210 days.
You can break down the process as follow:
First Phase:
Day 0: EMA accepts ARNA application for (lor). > am 24.03.12 !!!
Day 80: EMA releases Co Rapporteurs Report (from 2 groups) - this is equivalent to FDA's Briefing doc.
Day 100: EMA Peer Review (of the Report).
Day 120: EMA sends ARNA a List of Questions.
Clock now stops.
The 1st phase of the process is finished. Once ARNA submits the responses to the List of Questions, the clocks start again for the 2nd phase. I don't know how long this would take between Day 120 and 121, but ARNA has maximum of 60 days to respond.
Second Phase
Day 121: ARNA responds to List of Questions.
Day 150: Join Report from the 2 groups.
Day 180: EMA sends ARNA a List of Issues.
Clock stops again.
ARNA has maximum of 30 days to respond.
Day 181: ARNA has Oral Explanation to List of Issues.
Day 210: Final Scientific Opinion <-- Approval Decision.
So if you add 210 days (total 2 phases) to the 2 idled periods of 60 and 30 days, it will be grand total of 310 which is about 10 months.
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…" target="_blank" rel="nofollow ugc noopener">
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
Zitat von bernie55: Arena Pharmaceuticals Promotes Craig M. Audet to Executive Officer as Senior Vice President, Operations and Head of Global Regulatory Affairs
So wie ich obenstehenden Artikel verstehe, hat ARENA einen " klugen" Kopf an die Spitze gesetzt , der u.a. sehr viel Erfahrungen bei der Vermarktung von Produkten und damit zusammenhängenden gesetzlichen Regularien in diesem Bereich hat.
Alle Zeichen stehen auf "Expansion"
Soweit ich richtig informiert bin, besitzt ARENA die alleinigen Vertriebsrechte u.a. für Europa und China…

Ausgehend von einem möglichen Approval in Europa wird sich ARENA jetzt schon im Vorfeld positionieren und sich wohl auch nach europäischen Partnern umhören - die Ernennung von Craig M. Audet hat wohl den Zweck, diese Aufgabe für Europa zu übernehmen – zum einen um Partner zu finden, zum anderen Kooperationen abzuschließen, die es ARENA ermöglicht,die „ neuen“ Märkte zu erschließen….
BLOOMBERG > positive Presse über Perspektiven von ARENA und über mögliche Übernahme durch Big Pharmas  ...
...
@ AREANICS....it`s only a beginning ...
First Diet Pill in Decade Turns Arena Into Deal Bait: Real M&A
The first medication in more than a decade to help 78 million obese Americans slim down is turning into takeover bait.
Arena Pharmaceuticals Inc. (ARNA) won Food and Drug Administration approval last month for Belviq, a treatment affecting an area of the brain that helps a person feel full after consuming less food. With the first weight-loss drug to be cleared for sale in the U.S. in 13 years, Arena is projected by analysts to increase revenue 18-fold in the next four years, the fastest growth in the world among specialty pharmaceutical companies greater than $1 billion, according to data compiled by Bloomberg.
Enlarge image First Diet Pill in Decade Turns Arena Into Deal Bait
About 42 percent of the U.S. population may be obese by 2030, up from about a third currently. Photographer: David Paul Morris/Bloomberg
While Arena’s 494 percent stock gain this year makes it the most expensive U.S. specialty drugmaker relative to revenue, the San Diego-based company offers potential buyers a medication that Piper Jaffray Cos. estimates will reach $2 billion in annual sales. Arena, with a $2.2 billion market value, may draw takeover interest from GlaxoSmithKline Plc (GSK), which is divesting its diet pill Alli, said WBB Securities LLC. Tokyo-based Eisai Co. (4523), which has licensed the rights to sell Belviq in the U.S., could also be an acquirer, said Lazard Capital Markets LLC.
“The large pharmaceutical companies are all on the edge of their seats looking at Arena,” Stephen Brozak, president of WBB Securities in Clark, New Jersey, said in a telephone interview. Obesity “is a global pandemic. There are people that absolutely are in need of these types of products. Large pharma are wonderful at marketing drugs and this is a product that lends itself to marketing.”
Appetite Suppression
“Arena is focused on bringing Belviq to the market in the U.S. with Eisai and in obtaining approval of the drug in markets outside of the U.S.,” David Schull, a spokesman for Arena, said in an e-mailed statement when asked whether the company has been approached by potential acquirers about a deal.
The FDA approved Belviq, previously called lorcaserin, on June 27. The pill works in a similar way to fenfluramine, part of the fen-phen appetite-suppression drug combination pulled from pharmacies 15 years ago when it was linked to heart valve abnormalities.
Arena and Eisai agreed to conduct six post-market studies to assess the safety and efficacy of the drug, including determining the potential for major cardiac risks such as heart attack and stroke, the FDA said.
Belviq will be available after the Drug Enforcement Administration completes a review to classify the drug based on its potential for abuse, which Arena has said may take four to six months.
Obesity Rate
“It’s the single most important thing that’s happened to Arena in its entire history,” WBB Securities’ Brozak said. “This is the first approval they’ve ever gotten.”
Analysts project that by 2015 Arena’s sales will have risen faster than every other specialty drugmaker in the world with a market capitalization greater than $1 billion, data compiled by Bloomberg show. The company’s revenue may total $232 million that year, versus $13 million in 2011, according to analysts’ estimates compiled by Bloomberg.
About 42 percent of the U.S. population may be obese by 2030, up from about a third currently, according to a report presented at the Centers for Disease Control and Prevention’s obesity conference in May. Globally, about 500 million people are obese, according to a World Health Organization report. Obesity is defined as having a body mass index of more than 30.
Glaxo, the U.K.’s largest drugmaker, may be interested in purchasing Arena to bolster its presence in the market for weight-loss remedies after sales declined for Alli, its over- the-counter diet pill, according to WBB Securities’ Brozak.
‘Dress Rehearsal’
London-based Glaxo has said it plans to divest Alli, which contains orlistat, a chemical that blocks the intestines from absorbing fat when taken as many as three times a day with meals. Orlistat has been linked to reports of liver injury, prompting consumer advocacy groups to demand its removal from the market. The FDA announced new warnings on the pill’s label in mid-2010, and Glaxo has said that Alli is safe and effective when used as directed.
Glaxo has “done the dress rehearsal,” so it would now know how to best market Arena’s weight-loss drug, Brozak said.
Kevin Colgan, a spokesman for Glaxo, said the company doesn’t comment on speculation, when asked whether it wants to acquire Arena.
Other companies are also seeking approval for weight-loss drugs. Qnexa from Vivus Inc. (VVUS) is slated for an FDA decision by July 17. Orexigen Therapeutics Inc. (OREX), which is developing the pill Contrave with Takeda Pharmaceutical Co., agreed in September to conduct a two-year study of the drug’s heart risks.
Drug Partnership
Belviq is the first prescription obesity medicine to be approved for sale in the U.S. since Roche Holding AG (ROG)’s Xenical in 1999. Glaxo’s Alli is a half-dose version of Xenical’s active ingredient and received FDA clearance in 2007 as the first diet drug available without a prescription.
Once available, lorcaserin will be marketed in the U.S. by Eisai under the name Belviq and the company will pay Arena a portion of the drug’s revenue. The partnership may deter potential acquirers that don’t want to share Belviq’s sales, said Alan Carr, a New York-based analyst for Needham & Co.
“Arena is much more attractive from an M&A perspective if you’re getting worldwide rights,” he said in a phone interview.
Eisai may decide to buy Arena so that it collects all of the revenue from Belviq, said Bill Tanner, an analyst for Lazard in New York. The two companies have a so-called standstill agreement that prevents the Japanese drugmaker from purchasing Arena unless someone else tries to first. It’s intended to protect Arena from a hostile takeover and wouldn’t prevent the companies from negotiating a deal, Tanner said.
Eisai’s Interest
“If this is a big drug, it could be a fairly sizeable payment that they have to make to Arena,” he said in a phone interview. “It probably makes sense that they’d want to take a look at acquiring it.”
Marcia Diljak, a spokeswoman for Eisai, declined to comment on whether the company would be interested in buying Arena.
Eisai will probably want to first gauge Belviq’s commercial success so that it doesn’t risk overpaying for Arena in case sales are weaker than analysts expect, Tanner said.
Arena, which has posted an almost six-fold stock price increase this year, is valued at 52 times analysts’ estimates for fiscal 2012 sales. That’s higher than all 14 other specialty pharmaceutical companies in the U.S. that have a market capitalization greater than $1 billion, data compiled by Bloomberg show. Even based on revenue estimates for fiscal 2015, Arena trades at the second-richest multiple in the group.
“If you knew for a fact that there wasn’t a buyer for the company, I don’t think this stock would be where it is,” Lazard’s Tanner said. “It’s not overly expensive if you think it’s going to be a decent drug.”
Edward Tenthoff, a New York-based analyst for Piper Jaffray, estimates Belviq will reach annual sales of $2 billion just in the U.S. by 2020.
“The approval of a drug with the market potential of Belviq certainly makes Arena” an acquisition candidate, Tenthoff said in a phone interview.
“This could be a true blockbuster drug in an age when there are fewer and fewer blockbusters.”
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…" target="_blank" rel="nofollow ugc noopener">
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…
 ...
...@ AREANICS....it`s only a beginning ...

First Diet Pill in Decade Turns Arena Into Deal Bait: Real M&A
The first medication in more than a decade to help 78 million obese Americans slim down is turning into takeover bait.
Arena Pharmaceuticals Inc. (ARNA) won Food and Drug Administration approval last month for Belviq, a treatment affecting an area of the brain that helps a person feel full after consuming less food. With the first weight-loss drug to be cleared for sale in the U.S. in 13 years, Arena is projected by analysts to increase revenue 18-fold in the next four years, the fastest growth in the world among specialty pharmaceutical companies greater than $1 billion, according to data compiled by Bloomberg.
Enlarge image First Diet Pill in Decade Turns Arena Into Deal Bait
About 42 percent of the U.S. population may be obese by 2030, up from about a third currently. Photographer: David Paul Morris/Bloomberg
While Arena’s 494 percent stock gain this year makes it the most expensive U.S. specialty drugmaker relative to revenue, the San Diego-based company offers potential buyers a medication that Piper Jaffray Cos. estimates will reach $2 billion in annual sales. Arena, with a $2.2 billion market value, may draw takeover interest from GlaxoSmithKline Plc (GSK), which is divesting its diet pill Alli, said WBB Securities LLC. Tokyo-based Eisai Co. (4523), which has licensed the rights to sell Belviq in the U.S., could also be an acquirer, said Lazard Capital Markets LLC.
“The large pharmaceutical companies are all on the edge of their seats looking at Arena,” Stephen Brozak, president of WBB Securities in Clark, New Jersey, said in a telephone interview. Obesity “is a global pandemic. There are people that absolutely are in need of these types of products. Large pharma are wonderful at marketing drugs and this is a product that lends itself to marketing.”
Appetite Suppression
“Arena is focused on bringing Belviq to the market in the U.S. with Eisai and in obtaining approval of the drug in markets outside of the U.S.,” David Schull, a spokesman for Arena, said in an e-mailed statement when asked whether the company has been approached by potential acquirers about a deal.
The FDA approved Belviq, previously called lorcaserin, on June 27. The pill works in a similar way to fenfluramine, part of the fen-phen appetite-suppression drug combination pulled from pharmacies 15 years ago when it was linked to heart valve abnormalities.
Arena and Eisai agreed to conduct six post-market studies to assess the safety and efficacy of the drug, including determining the potential for major cardiac risks such as heart attack and stroke, the FDA said.
Belviq will be available after the Drug Enforcement Administration completes a review to classify the drug based on its potential for abuse, which Arena has said may take four to six months.
Obesity Rate
“It’s the single most important thing that’s happened to Arena in its entire history,” WBB Securities’ Brozak said. “This is the first approval they’ve ever gotten.”
Analysts project that by 2015 Arena’s sales will have risen faster than every other specialty drugmaker in the world with a market capitalization greater than $1 billion, data compiled by Bloomberg show. The company’s revenue may total $232 million that year, versus $13 million in 2011, according to analysts’ estimates compiled by Bloomberg.
About 42 percent of the U.S. population may be obese by 2030, up from about a third currently, according to a report presented at the Centers for Disease Control and Prevention’s obesity conference in May. Globally, about 500 million people are obese, according to a World Health Organization report. Obesity is defined as having a body mass index of more than 30.
Glaxo, the U.K.’s largest drugmaker, may be interested in purchasing Arena to bolster its presence in the market for weight-loss remedies after sales declined for Alli, its over- the-counter diet pill, according to WBB Securities’ Brozak.
‘Dress Rehearsal’
London-based Glaxo has said it plans to divest Alli, which contains orlistat, a chemical that blocks the intestines from absorbing fat when taken as many as three times a day with meals. Orlistat has been linked to reports of liver injury, prompting consumer advocacy groups to demand its removal from the market. The FDA announced new warnings on the pill’s label in mid-2010, and Glaxo has said that Alli is safe and effective when used as directed.
Glaxo has “done the dress rehearsal,” so it would now know how to best market Arena’s weight-loss drug, Brozak said.
Kevin Colgan, a spokesman for Glaxo, said the company doesn’t comment on speculation, when asked whether it wants to acquire Arena.
Other companies are also seeking approval for weight-loss drugs. Qnexa from Vivus Inc. (VVUS) is slated for an FDA decision by July 17. Orexigen Therapeutics Inc. (OREX), which is developing the pill Contrave with Takeda Pharmaceutical Co., agreed in September to conduct a two-year study of the drug’s heart risks.
Drug Partnership
Belviq is the first prescription obesity medicine to be approved for sale in the U.S. since Roche Holding AG (ROG)’s Xenical in 1999. Glaxo’s Alli is a half-dose version of Xenical’s active ingredient and received FDA clearance in 2007 as the first diet drug available without a prescription.
Once available, lorcaserin will be marketed in the U.S. by Eisai under the name Belviq and the company will pay Arena a portion of the drug’s revenue. The partnership may deter potential acquirers that don’t want to share Belviq’s sales, said Alan Carr, a New York-based analyst for Needham & Co.
“Arena is much more attractive from an M&A perspective if you’re getting worldwide rights,” he said in a phone interview.
Eisai may decide to buy Arena so that it collects all of the revenue from Belviq, said Bill Tanner, an analyst for Lazard in New York. The two companies have a so-called standstill agreement that prevents the Japanese drugmaker from purchasing Arena unless someone else tries to first. It’s intended to protect Arena from a hostile takeover and wouldn’t prevent the companies from negotiating a deal, Tanner said.
Eisai’s Interest
“If this is a big drug, it could be a fairly sizeable payment that they have to make to Arena,” he said in a phone interview. “It probably makes sense that they’d want to take a look at acquiring it.”
Marcia Diljak, a spokeswoman for Eisai, declined to comment on whether the company would be interested in buying Arena.
Eisai will probably want to first gauge Belviq’s commercial success so that it doesn’t risk overpaying for Arena in case sales are weaker than analysts expect, Tanner said.
Arena, which has posted an almost six-fold stock price increase this year, is valued at 52 times analysts’ estimates for fiscal 2012 sales. That’s higher than all 14 other specialty pharmaceutical companies in the U.S. that have a market capitalization greater than $1 billion, data compiled by Bloomberg show. Even based on revenue estimates for fiscal 2015, Arena trades at the second-richest multiple in the group.
“If you knew for a fact that there wasn’t a buyer for the company, I don’t think this stock would be where it is,” Lazard’s Tanner said. “It’s not overly expensive if you think it’s going to be a decent drug.”
Edward Tenthoff, a New York-based analyst for Piper Jaffray, estimates Belviq will reach annual sales of $2 billion just in the U.S. by 2020.
“The approval of a drug with the market potential of Belviq certainly makes Arena” an acquisition candidate, Tenthoff said in a phone interview.
“This could be a true blockbuster drug in an age when there are fewer and fewer blockbusters.”
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…" target="_blank" rel="nofollow ugc noopener">
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…
Das Medikament BELVIQ weckt Begehren.....die BIGS haben im Vorfeld der AC Entscheidung und der FDA Entscheidung immer gegen ARENA und ihr Medikament gewettert und immer wieder " bewusst" falsche Information veröffentlicht.
Diese Methode hat Schule !!!
Jim Cramer: "What's important when you're in that hedge-fund mode is to not do anything that's remotely truthful.
Because the truth is so against your view that it's important to create a new truth to develop a fiction."
http://www.youtube.com/watch?v=NSeTKuNyPr4" target="_blank" rel="nofollow ugc noopener">
http://www.youtube.com/watch?v=NSeTKuNyPr4
Erst gestern fing die "Basher- Konsorte" wieder an," kleine" Nebenwirkungen des Medikaments als " hyperproblematisch" darzustellen...
...hier der Stein des Anstosses.....
Arena Pharmaceuticals (ARNA), Vivus Pharmaceuticals (VVUS), Onyx Pharmaceutical (ONXX)
Arena Pharmaceuticals (ARNA) was chosen among followers of Jim Cramer on Twitter as the champion stock for the second half of 2012, but Cramer disagrees with this pick. The stock has rallied $225 on the potential approval of its obesity drug by the FDA; this will be the first major obesity drug for 10 years. The drug could generate $2 billion in revenue, equal to Arena's present market cap of $2 billion. In addition to the fact that Arena has already risen substantially, the story is not perfect. The obesity drug is not as effective as initially thought, and there are substantial cases of side effects, including hallucinations.
Vivus Pharmaceutical (VVUS) also has an obesity drug awaiting approval, and this drug has shown to be more effective than Arena's treatment.
In addition, Vivus owns all of its drug, while Arena just owns 40% of its obesity drug.
Cramer's pick among biotechs is Onyx (ONXX), which has a blood cancer drug which is expected to earn FDA approval, and already has treatments for blood and liver cancer.
http://seekingalpha.com/article/713341-cramer-s-mad-money-fi…
JIM CRAMER versucht mit allen nur erdenklichen Mitteln das Hedgefondsklientel zu bedienen..
...Leute, keep cool....keine SL setzen...
...was sind im übrigen aktuell Minus 5-10 %im Vergleich zu den knapp 500 %, die wir bis jetzt gemacht haben ??
Diese Methode hat Schule !!!
Jim Cramer: "What's important when you're in that hedge-fund mode is to not do anything that's remotely truthful.
Because the truth is so against your view that it's important to create a new truth to develop a fiction."
http://www.youtube.com/watch?v=NSeTKuNyPr4" target="_blank" rel="nofollow ugc noopener">
http://www.youtube.com/watch?v=NSeTKuNyPr4
Erst gestern fing die "Basher- Konsorte" wieder an," kleine" Nebenwirkungen des Medikaments als " hyperproblematisch" darzustellen...
...hier der Stein des Anstosses.....
Arena Pharmaceuticals (ARNA), Vivus Pharmaceuticals (VVUS), Onyx Pharmaceutical (ONXX)
Arena Pharmaceuticals (ARNA) was chosen among followers of Jim Cramer on Twitter as the champion stock for the second half of 2012, but Cramer disagrees with this pick. The stock has rallied $225 on the potential approval of its obesity drug by the FDA; this will be the first major obesity drug for 10 years. The drug could generate $2 billion in revenue, equal to Arena's present market cap of $2 billion. In addition to the fact that Arena has already risen substantially, the story is not perfect. The obesity drug is not as effective as initially thought, and there are substantial cases of side effects, including hallucinations.
Vivus Pharmaceutical (VVUS) also has an obesity drug awaiting approval, and this drug has shown to be more effective than Arena's treatment.
In addition, Vivus owns all of its drug, while Arena just owns 40% of its obesity drug.
Cramer's pick among biotechs is Onyx (ONXX), which has a blood cancer drug which is expected to earn FDA approval, and already has treatments for blood and liver cancer.
http://seekingalpha.com/article/713341-cramer-s-mad-money-fi…
JIM CRAMER versucht mit allen nur erdenklichen Mitteln das Hedgefondsklientel zu bedienen..
...Leute, keep cool....keine SL setzen...
...was sind im übrigen aktuell Minus 5-10 %im Vergleich zu den knapp 500 %, die wir bis jetzt gemacht haben ??
AP News
Arena files for approval of Belviq in Switzerland
Posted on July 11, 2012
SAN DIEGO (AP) — Arena Pharmaceuticals Inc. said Wednesday that it filed for marketing approval for its anti-obesity pill Belviq in Switzerland.
Arena said it expects Swissmedic, the country's health agency, to accept the filing later this month and begin its review process.
The Food and Drug Administration approved Belviq on June 27, making it the first long-term prescription weight loss drug approved in the U.S. in more than a decade. European Union regulators are reviewing the drug.
Shares of Arena Pharmaceuticals lost 68 cents, or 5.8 percent, to $11.13 on Wednesday, after competitor Orexigen Therapeutics Inc. said enrollment in a study of its potentially competing experimental weight-loss drug Contrave was going more quickly than expected. Arena picked up 4 cents to $11.17 in after-hours trading.
http://www.businessweek.com/ap/2012-07-11/arena-files-for-ap…
Arena files for approval of Belviq in Switzerland
Posted on July 11, 2012
SAN DIEGO (AP) — Arena Pharmaceuticals Inc. said Wednesday that it filed for marketing approval for its anti-obesity pill Belviq in Switzerland.
Arena said it expects Swissmedic, the country's health agency, to accept the filing later this month and begin its review process.
The Food and Drug Administration approved Belviq on June 27, making it the first long-term prescription weight loss drug approved in the U.S. in more than a decade. European Union regulators are reviewing the drug.
Shares of Arena Pharmaceuticals lost 68 cents, or 5.8 percent, to $11.13 on Wednesday, after competitor Orexigen Therapeutics Inc. said enrollment in a study of its potentially competing experimental weight-loss drug Contrave was going more quickly than expected. Arena picked up 4 cents to $11.17 in after-hours trading.
http://www.businessweek.com/ap/2012-07-11/arena-files-for-ap…
auch für den INFO-Thread:
hier werden mal ARNA und VVUS nebeneinander betrachtet:
Vivus: Getting Ready For FDA Approval
July 12, 2012 |
Vivus (VVUS) is a biopharmaceutical company, which mainly develops medicines for treatment of sleep apnea, diabetes, obesity and male sexual health. While its portfolio is relatively diversified, there is a great deal of interest in company's obesity treatment products. Vivus is currently awaiting final approval from FDA, for Qnexa, their most recent drug. Qnexa is a drug meant to help reduce weight. A few years ago, Qnexa was initially rejected by FDA at the first attempt. At the second presentation in this year, Qnexa got a vote of confidence by a whopping 20-2 margin by the committee.
Vivus will get a final decision about Qnexa on July, 17, 2012. If approved, Qnexa will be the second drug to be approved by FDA in weight loss category in two months. The company plans on marketing the drug by the end of year 2012. European authorities have delayed a decision on the approval of the drug. Vivus is entirely focusing on getting Qnexa approved by FDA at the moment. Currently, Lorcaserin is the only weight reduction drug approved by FDA. Arena Pharmaceuticals (ARNA) is the developer of Lorcaserin.
Clinical Background
Qnexa has already created a strong buzz in the market. Clinical studies have shown a significant weight loss in the participants. According to the results published by the company, average weight loss for top dose participants was 14.4%. Average weight loss for low dose Qnexa was 6.7% and 2.1% for placebo. Mean weight loss after week 56 was 10.9% for top dose and 5.1% for low dose. Participants who completed the top dose treatment, 83.5% lost ≥5%; 67.7% lost ≥10% and 48.1% lost ≥15% of their baseline weight. There are some adverse effect fears, due to which Vivus has to follow a labeling protocol. Qnexa will not be prescribed to the pregnant woman. Vivus plans on advising the patients to stop taking the medicine, if weight loss is less than 3% after three months.
Stock Performance
VVUS Chart
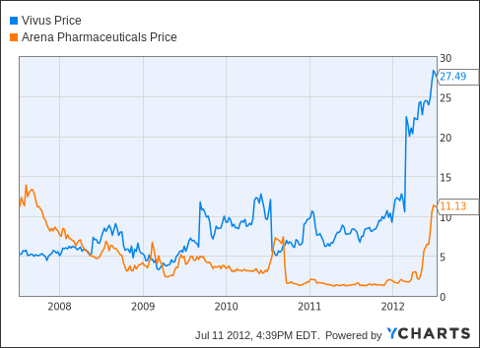
Both Vivus and Arena stocks are currently trading near its 52 week highs. Vivus stock closed at $28.47 on July, 09, 2010. The 52-week trading range for Vivus is $6.13 - $29.99. Even after the recent retreat, the stock is trading near its 52-week highs. Market cap of Vivus Inc. is $2.83 billion, slightly higher than the $2.15 billion market cap of Arena. Vivus stock has a relatively high Beta of 1.42. Trailing twelve month EPS is -0.63. One year analyst target for Vivus is $31.20.
Ratio Analysis
Estimates for the current year EPS are 40% more than the past 12 months EPS. One year forward estimate for EPS is to grow by 166%. Forward P/E is unusually high at 72.90. Current P/B ratio stands at 8.72. Firm is in an exceptionally strong liquidity position. Quick and current ratios are 25.70 and 25.92 respectively. Unlike Arena Pharmaceuticals, Vivus has no debt in its capital structure.
Pipeline Products
Vivus had Avanafil approved by FDA in April, 2012, a drug for men with erectile dysfunction. Vivus plans to sell Avanafil under the brand name Stendra. Stendra promises to work in fifteen minutes, half the time it takes the pills from rivals companies to work. That is a very short period by industry standards.
By getting Stendra approved, Vivus now stand to compete directly with giants of the industry like, Pfizer (PFE), GlaxoSmithKline (GSK) and Bayer. Analysts believe that the sales from Stendra can go up to $68 million in the next year and to $459 million by the year 2017. Erectile dysfunction market itself is currently worth $4.3 billion. Viagra, Pfizer's well-known super-charger, holds almost half of the market share. Vivus is working on getting a partnership to market Stendra as well.
Besides the existing products, Vivus wants to focus on the marketing of Qnexa, if approved. Vivus wants to preserve the entire budget to spend on the marketing of Qnexa. Obesity market is largely unexplored, and it is a large market. There will be less competition in this segment. Vivus has a good marketing network. Vivus has more than one product in the market. As such, the revenues will not be depending on only one drug in a hugely unexplored market.
If approved, Vivus plans to launch Qnexa in the later part of year 2012. On the other hand, Arena Pharmaceutical expects to market the drug in 2013. This will give Vivus Inc a head start on its main competitor, and the firm can capture a large chunk of the market. Vivus is planning to directly market the drug in the U.S. itself and seek partnerships outside the U.S. USA is an immense obesity market. Arena does not have the pure marketing rights for its own obesity drug. Only a certain portion of revenues from sales will go to Arena, whereas the rest will be taken by Aisai Pharmaceuticals, the Japanese partner. This I believe is again an advantage for Vivus over Arena.
Insider Sales
The biggest bearish argument made against Vivus is the sale of shares by three executives in the past two months. Insiders selling stock before the approval are sure to raise concerns among the investors. The main reason, which most people have been ignoring, is something less critical. Vivus executives have been selling stocks under the 10b5-1 trading plan to cover themselves legally against allegations of insider trading. CEO of Vivus recently sold 100,000 shares, but he still owns 4.3 million shares.
Summary
I think the FDA decision is almost certain, and Vivus is extremely hopeful on the approval of Qnexa. This drug will prove to be a catalyst for the success of the company. Analysts believe, after the approval, Vivus stock should be priced at $40. Vivus, currently trading below $30 presents an exciting opportunity to make tidy profits. Insider sales are worrisome, but it is hard to say that insiders are getting rid of their shares, as they hold substantial stake in the company. This still raises a red flag for interested investors. Therefore, as the stock has already made substantial gains, I would suggest a hold and buy at pullback strategy for Vivus.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/718321-vivus-getting-ready-f…
hier werden mal ARNA und VVUS nebeneinander betrachtet:
Vivus: Getting Ready For FDA Approval
July 12, 2012 |
Vivus (VVUS) is a biopharmaceutical company, which mainly develops medicines for treatment of sleep apnea, diabetes, obesity and male sexual health. While its portfolio is relatively diversified, there is a great deal of interest in company's obesity treatment products. Vivus is currently awaiting final approval from FDA, for Qnexa, their most recent drug. Qnexa is a drug meant to help reduce weight. A few years ago, Qnexa was initially rejected by FDA at the first attempt. At the second presentation in this year, Qnexa got a vote of confidence by a whopping 20-2 margin by the committee.
Vivus will get a final decision about Qnexa on July, 17, 2012. If approved, Qnexa will be the second drug to be approved by FDA in weight loss category in two months. The company plans on marketing the drug by the end of year 2012. European authorities have delayed a decision on the approval of the drug. Vivus is entirely focusing on getting Qnexa approved by FDA at the moment. Currently, Lorcaserin is the only weight reduction drug approved by FDA. Arena Pharmaceuticals (ARNA) is the developer of Lorcaserin.
Clinical Background
Qnexa has already created a strong buzz in the market. Clinical studies have shown a significant weight loss in the participants. According to the results published by the company, average weight loss for top dose participants was 14.4%. Average weight loss for low dose Qnexa was 6.7% and 2.1% for placebo. Mean weight loss after week 56 was 10.9% for top dose and 5.1% for low dose. Participants who completed the top dose treatment, 83.5% lost ≥5%; 67.7% lost ≥10% and 48.1% lost ≥15% of their baseline weight. There are some adverse effect fears, due to which Vivus has to follow a labeling protocol. Qnexa will not be prescribed to the pregnant woman. Vivus plans on advising the patients to stop taking the medicine, if weight loss is less than 3% after three months.
Stock Performance
VVUS Chart

Both Vivus and Arena stocks are currently trading near its 52 week highs. Vivus stock closed at $28.47 on July, 09, 2010. The 52-week trading range for Vivus is $6.13 - $29.99. Even after the recent retreat, the stock is trading near its 52-week highs. Market cap of Vivus Inc. is $2.83 billion, slightly higher than the $2.15 billion market cap of Arena. Vivus stock has a relatively high Beta of 1.42. Trailing twelve month EPS is -0.63. One year analyst target for Vivus is $31.20.
Ratio Analysis
Estimates for the current year EPS are 40% more than the past 12 months EPS. One year forward estimate for EPS is to grow by 166%. Forward P/E is unusually high at 72.90. Current P/B ratio stands at 8.72. Firm is in an exceptionally strong liquidity position. Quick and current ratios are 25.70 and 25.92 respectively. Unlike Arena Pharmaceuticals, Vivus has no debt in its capital structure.
Pipeline Products
Vivus had Avanafil approved by FDA in April, 2012, a drug for men with erectile dysfunction. Vivus plans to sell Avanafil under the brand name Stendra. Stendra promises to work in fifteen minutes, half the time it takes the pills from rivals companies to work. That is a very short period by industry standards.
By getting Stendra approved, Vivus now stand to compete directly with giants of the industry like, Pfizer (PFE), GlaxoSmithKline (GSK) and Bayer. Analysts believe that the sales from Stendra can go up to $68 million in the next year and to $459 million by the year 2017. Erectile dysfunction market itself is currently worth $4.3 billion. Viagra, Pfizer's well-known super-charger, holds almost half of the market share. Vivus is working on getting a partnership to market Stendra as well.
Besides the existing products, Vivus wants to focus on the marketing of Qnexa, if approved. Vivus wants to preserve the entire budget to spend on the marketing of Qnexa. Obesity market is largely unexplored, and it is a large market. There will be less competition in this segment. Vivus has a good marketing network. Vivus has more than one product in the market. As such, the revenues will not be depending on only one drug in a hugely unexplored market.
If approved, Vivus plans to launch Qnexa in the later part of year 2012. On the other hand, Arena Pharmaceutical expects to market the drug in 2013. This will give Vivus Inc a head start on its main competitor, and the firm can capture a large chunk of the market. Vivus is planning to directly market the drug in the U.S. itself and seek partnerships outside the U.S. USA is an immense obesity market. Arena does not have the pure marketing rights for its own obesity drug. Only a certain portion of revenues from sales will go to Arena, whereas the rest will be taken by Aisai Pharmaceuticals, the Japanese partner. This I believe is again an advantage for Vivus over Arena.
Insider Sales
The biggest bearish argument made against Vivus is the sale of shares by three executives in the past two months. Insiders selling stock before the approval are sure to raise concerns among the investors. The main reason, which most people have been ignoring, is something less critical. Vivus executives have been selling stocks under the 10b5-1 trading plan to cover themselves legally against allegations of insider trading. CEO of Vivus recently sold 100,000 shares, but he still owns 4.3 million shares.
Summary
I think the FDA decision is almost certain, and Vivus is extremely hopeful on the approval of Qnexa. This drug will prove to be a catalyst for the success of the company. Analysts believe, after the approval, Vivus stock should be priced at $40. Vivus, currently trading below $30 presents an exciting opportunity to make tidy profits. Insider sales are worrisome, but it is hard to say that insiders are getting rid of their shares, as they hold substantial stake in the company. This still raises a red flag for interested investors. Therefore, as the stock has already made substantial gains, I would suggest a hold and buy at pullback strategy for Vivus.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/718321-vivus-getting-ready-f…
Ist VVUS für ARNA eine Gefahr?:
Has Arena Pharmaceuticals Inc. (NASDAQ: ARNA) Peaked or will Biopharmaceutical Company See Greater Gains in the Near Future?
Posted on July 13, 2012 by Editor
To think that less than three months ago shares of Arena Pharmaceuticals Inc. (NASDAQ: ARNA) were trading as low as 2.00 is mind-numbing but with an FDA approval comes plenty of rewards and shareholders of the biopharmaceutical company experienced that first hand when the agency made lorcaserin, to be marked under the commercial name Belviq, the first obesity medication to win favor in 13 years.
That FDA approval came back in late June and shares climbed as high as 13.50 following the news but since that time ARNA has seen a noticeable pullback with shares falling as low as 10.08 on Thursday. While shares have managed to climb to the 11.20 mark on Friday there are concerns about where the price will go in the near future as the company could be facing some competition.
Just how likely it is that the FDA issues yet another approval for an obesity medication is yet to be seen but Vivus Inc. and their shareholders will find out next week with their Prescription Drug User Fee Act (PDUFA), or FDA `Action` date, for Qnexa, scheduled for July 17th. The overwhelming belief is that Vivus will win approval and that could spell trouble for ARNA and their shareholders.
Of course ARNA’s success isn’t dependent upon what happens on July 17th, the facts seem to support the belief that Belviq has fewer side effects than Qnexa and will be accessible to a larger market. That market is massive considering that the FDA approved lorcaserin for those who are obese or who have a body mass index (BMI) of 27 and have another risk factor like high blood pressure, high cholesterol, or diabetes. What that equates to is nearly two-thirds of the population in the United States.
As it stands there are still some concerns over what risks are presented by lorcaserin, namely how it could play a role in heart disease. While those concerns may have some merit the reality is if you are obese then you are already carrying risks of heart disease. That’s not to say that the risks of lorcaserin should be overlooked but common sense should prevail.
Whether or not Vivus and their Qnexa obesity medication can compete with ARNA’s Belviq remains to be seen but one thing is for certain, Qnexa doesn’t have the same market to work with. Even with an FDA approval Qnexa is facing tighter restrictions as IPQ Analytics, an independent research firm, has warned of “risks that exist which are not currently being factored into expectations.” This could lead to a risk management program that would accompany an approval for Qnexa, something that Belviq was able to avoid.
That could be of substantial benefit to ARNA as the risk management program for Qnexa could place restrictions on prescriptions for women of childbearing age and/or those with cardiovascular risk given the fact that the drug had been sent back for additional studies due to concerns about heart valve damage, a permanent and life-threatening side effect.
Not lost on investors is the fact that a number of insider transactions among executives at Vivus have taken place, giving some skeptics reason to believe that the company has its doubts about Qnexa and its impact on the market. While these transactions are well within the rights of executives and may simply be seen as an opportunity to grab profits the timing does appear odd.
Aside from the potential competition in the way of Qnexa, ARNA is also facing doubt from those who believe the company’s diet drug won’t earn as much as they expect. Jim Cramer has been among the most vocal on this front, predicting Arena will gain just below 40% of its revenue from treatment, substantially lower than what the company has predicted.
Without question there are legitimate concerns investors should be aware of when it comes to ARNA, namely that the company’s shares have already jumped more than 500% for the year. That leaves many to question whether or not ARNA has already peaked. With shares as high as they are right now it may make sense to wait for a pullback and if Vivus gets that approval next week there will almost certainly be a downturn for ARNA shares which could then provide an excellent opportunity for investors to build a position.
http://www.otcequity.com/?p=2331
Has Arena Pharmaceuticals Inc. (NASDAQ: ARNA) Peaked or will Biopharmaceutical Company See Greater Gains in the Near Future?
Posted on July 13, 2012 by Editor
To think that less than three months ago shares of Arena Pharmaceuticals Inc. (NASDAQ: ARNA) were trading as low as 2.00 is mind-numbing but with an FDA approval comes plenty of rewards and shareholders of the biopharmaceutical company experienced that first hand when the agency made lorcaserin, to be marked under the commercial name Belviq, the first obesity medication to win favor in 13 years.
That FDA approval came back in late June and shares climbed as high as 13.50 following the news but since that time ARNA has seen a noticeable pullback with shares falling as low as 10.08 on Thursday. While shares have managed to climb to the 11.20 mark on Friday there are concerns about where the price will go in the near future as the company could be facing some competition.
Just how likely it is that the FDA issues yet another approval for an obesity medication is yet to be seen but Vivus Inc. and their shareholders will find out next week with their Prescription Drug User Fee Act (PDUFA), or FDA `Action` date, for Qnexa, scheduled for July 17th. The overwhelming belief is that Vivus will win approval and that could spell trouble for ARNA and their shareholders.
Of course ARNA’s success isn’t dependent upon what happens on July 17th, the facts seem to support the belief that Belviq has fewer side effects than Qnexa and will be accessible to a larger market. That market is massive considering that the FDA approved lorcaserin for those who are obese or who have a body mass index (BMI) of 27 and have another risk factor like high blood pressure, high cholesterol, or diabetes. What that equates to is nearly two-thirds of the population in the United States.
As it stands there are still some concerns over what risks are presented by lorcaserin, namely how it could play a role in heart disease. While those concerns may have some merit the reality is if you are obese then you are already carrying risks of heart disease. That’s not to say that the risks of lorcaserin should be overlooked but common sense should prevail.
Whether or not Vivus and their Qnexa obesity medication can compete with ARNA’s Belviq remains to be seen but one thing is for certain, Qnexa doesn’t have the same market to work with. Even with an FDA approval Qnexa is facing tighter restrictions as IPQ Analytics, an independent research firm, has warned of “risks that exist which are not currently being factored into expectations.” This could lead to a risk management program that would accompany an approval for Qnexa, something that Belviq was able to avoid.
That could be of substantial benefit to ARNA as the risk management program for Qnexa could place restrictions on prescriptions for women of childbearing age and/or those with cardiovascular risk given the fact that the drug had been sent back for additional studies due to concerns about heart valve damage, a permanent and life-threatening side effect.
Not lost on investors is the fact that a number of insider transactions among executives at Vivus have taken place, giving some skeptics reason to believe that the company has its doubts about Qnexa and its impact on the market. While these transactions are well within the rights of executives and may simply be seen as an opportunity to grab profits the timing does appear odd.
Aside from the potential competition in the way of Qnexa, ARNA is also facing doubt from those who believe the company’s diet drug won’t earn as much as they expect. Jim Cramer has been among the most vocal on this front, predicting Arena will gain just below 40% of its revenue from treatment, substantially lower than what the company has predicted.
Without question there are legitimate concerns investors should be aware of when it comes to ARNA, namely that the company’s shares have already jumped more than 500% for the year. That leaves many to question whether or not ARNA has already peaked. With shares as high as they are right now it may make sense to wait for a pullback and if Vivus gets that approval next week there will almost certainly be a downturn for ARNA shares which could then provide an excellent opportunity for investors to build a position.
http://www.otcequity.com/?p=2331
wie hoch schätzt ihr die chance der zulassung für vvus ? bis jetzt hat man ja von fda bezüglich nachfragen, die zu einer verzögerung der zulassung führen, nichts gehört
sorry falsches thread
In diesem Schreiben wird noch einmal auf die bewusste " Manipulation" von JIM CRAMER eingegangen und seine Aussagen bzgl. Belviq kritisch beleuchtet und widerlegt...
REAL PHYSICIAN: CRAMER REBUTTAL
11-Jul-12 09:14 am
A very important (but never discussed) point about anti-obesity treatment is what real physicians will prescribe for their patients in coming years. I have discussed with many of my fellow M.D.s, and almost all of us are going with BELVIQ instead of QNEXA, regardless of a CRL delay with the latter.
1) We physicians all well remember the recurrent valvulopathies and pulmonary hypertension that occurred with Fen/Fen. QNEXA will contain phentermine (the first FEN). We have a right and sacred responsibility to assess risk vs. potential benefits for every patient for which we write prescriptions. Belviq causes none of these problems (less than placebo controls) in FDA data.
2) The media (and Jim Cramer) have it all wrong as far as Belviq efficacy in weight loss and he misrepresented Belviq last night.
*****The AVERAGE WEIGHT LOSS WITH BELVIQ IS 8% OF BODY WEIGHT AMONG RESPONDERS. About 50% of patients will lose MORE than 5% of BW in 12 weeks. If not, they need to stop it. Among those that do reach this threshold, 35% lost > 10% of BW, and the top 25% of responders lost > 16.7% of BW!
3) Cramer is mistaken regarding his interpretation of FDA data. Regarding weight loss efficacy, he related BELVIQ was not placebo-controlled, and QNEXA’s data was. Wrong! He has it just backwards. BELVIQ’s data was placebo-controlled, and QNEXA was not. This makes a huge difference in accuracy of drug efficacy.
4) Cramer stated that around 60-70% of patients will not respond to BLEVIQ. Wrong again. The real answer is 47.5% will not lose more than 5%, which makes them classified as non-responders. Nevertheless, do not ever underestimate the power of 5%-8% of weight loss. It can have dramatic effects on overall health, with significant reductions in incidence of Type II diabetes mellitus, coronary vascular disease, hyperlipidemias, sleep apnea, stroke , and even malignancy reduction. Our patients will feel much better about their overall health, and can use the psychological improvement to exercise more, with reduction in pain from osteoarthritis in weight bearing joints.
5) Cramer made another blunder in attempting to use scare tactics about “hallucinations” with BELVIQ. The incidence of hallucinations is ridiculously miniscule as to the absurd. We will not go into the significantly elevated risk of cleft lip incidence in babies born of QNEXA mothers, and the mind-numbing “dopey” side effect of topiramate (called “DOPAMAX”). Who needs that? None of this was discussed by Cramer.
6) Cramer misrepresented BELVIQ’s earnings potential because of “limited numbers of Easai drug reps in America”. How hard would it be to train any drug rep about a medication that will change America’s heath?
7) Cramer misrepresented BELVIQ ‘s earnings potential because of its marketing company taking about 40% of its revenues in the United States. He neglected to mention that outside the U.S (Europe, Latin America, and Asia), ARNA will get significantly more, if not all, of the earnings.
8) Cramer said QNEXA will not need to be a scheduled prescription, and BELVIQ will. He got this just backwards as well . It is highly likely QNEXA will be scheduled. BELVIQ has yet to be determined, but far less likely as highly classified as QNEXA, meaning more restrictions on it. QNEXA will have to be mail ordered, whereas most all pharmacies will actually stock BELVIQ.
9) Finally, the ultimate blunder is that Cramer blatantly said that NO INSURANCE COMPANY WILL EVER COVER AND ANTI-OBESITY MED? Why? Because he had asked one! This is preposterous, because as we all know, covered formulary changes take time. When insurance companies can actually SAVE $, by eliminating other costly meds by addressing the root health problem (obesity), instead of its sequellae, we will have stumbled on a major solution to the cost of healthcare in America!
Sentiment : Strong Buy
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…" target="_blank" rel="nofollow ugc noopener">
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…[/quote]
REAL PHYSICIAN: CRAMER REBUTTAL
11-Jul-12 09:14 am
A very important (but never discussed) point about anti-obesity treatment is what real physicians will prescribe for their patients in coming years. I have discussed with many of my fellow M.D.s, and almost all of us are going with BELVIQ instead of QNEXA, regardless of a CRL delay with the latter.
1) We physicians all well remember the recurrent valvulopathies and pulmonary hypertension that occurred with Fen/Fen. QNEXA will contain phentermine (the first FEN). We have a right and sacred responsibility to assess risk vs. potential benefits for every patient for which we write prescriptions. Belviq causes none of these problems (less than placebo controls) in FDA data.
2) The media (and Jim Cramer) have it all wrong as far as Belviq efficacy in weight loss and he misrepresented Belviq last night.
*****The AVERAGE WEIGHT LOSS WITH BELVIQ IS 8% OF BODY WEIGHT AMONG RESPONDERS. About 50% of patients will lose MORE than 5% of BW in 12 weeks. If not, they need to stop it. Among those that do reach this threshold, 35% lost > 10% of BW, and the top 25% of responders lost > 16.7% of BW!
3) Cramer is mistaken regarding his interpretation of FDA data. Regarding weight loss efficacy, he related BELVIQ was not placebo-controlled, and QNEXA’s data was. Wrong! He has it just backwards. BELVIQ’s data was placebo-controlled, and QNEXA was not. This makes a huge difference in accuracy of drug efficacy.
4) Cramer stated that around 60-70% of patients will not respond to BLEVIQ. Wrong again. The real answer is 47.5% will not lose more than 5%, which makes them classified as non-responders. Nevertheless, do not ever underestimate the power of 5%-8% of weight loss. It can have dramatic effects on overall health, with significant reductions in incidence of Type II diabetes mellitus, coronary vascular disease, hyperlipidemias, sleep apnea, stroke , and even malignancy reduction. Our patients will feel much better about their overall health, and can use the psychological improvement to exercise more, with reduction in pain from osteoarthritis in weight bearing joints.
5) Cramer made another blunder in attempting to use scare tactics about “hallucinations” with BELVIQ. The incidence of hallucinations is ridiculously miniscule as to the absurd. We will not go into the significantly elevated risk of cleft lip incidence in babies born of QNEXA mothers, and the mind-numbing “dopey” side effect of topiramate (called “DOPAMAX”). Who needs that? None of this was discussed by Cramer.
6) Cramer misrepresented BELVIQ’s earnings potential because of “limited numbers of Easai drug reps in America”. How hard would it be to train any drug rep about a medication that will change America’s heath?
7) Cramer misrepresented BELVIQ ‘s earnings potential because of its marketing company taking about 40% of its revenues in the United States. He neglected to mention that outside the U.S (Europe, Latin America, and Asia), ARNA will get significantly more, if not all, of the earnings.
8) Cramer said QNEXA will not need to be a scheduled prescription, and BELVIQ will. He got this just backwards as well . It is highly likely QNEXA will be scheduled. BELVIQ has yet to be determined, but far less likely as highly classified as QNEXA, meaning more restrictions on it. QNEXA will have to be mail ordered, whereas most all pharmacies will actually stock BELVIQ.
9) Finally, the ultimate blunder is that Cramer blatantly said that NO INSURANCE COMPANY WILL EVER COVER AND ANTI-OBESITY MED? Why? Because he had asked one! This is preposterous, because as we all know, covered formulary changes take time. When insurance companies can actually SAVE $, by eliminating other costly meds by addressing the root health problem (obesity), instead of its sequellae, we will have stumbled on a major solution to the cost of healthcare in America!
Sentiment : Strong Buy
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…" target="_blank" rel="nofollow ugc noopener">
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…[/quote]
The Time To Buy Arena Pharmaceuticals Is Now
July 16, 2012
One of the benefits for investors who focus in specific sectors is that new opportunities can come from research. While researching Theravance (THRX) and Valeant (VRX) over the last few quarters, Arena Pharmaceuticals (ARNA) came up recently. A number of readers bought Arena when shares traded below $6. This leaves investors noticing shares at the current price paying two-fold or more.
Theravance and its partner GlaxoSmithKline PLC (GSK) reported positive results from four Phase III tests for the treatment of chronic obstructive pulmonary disease ("COPD"). Theravance and GSK are testing LAMA/LABA, which is a mix of two molecules, in four 24-week long studies. LAMA/LABA are two molecules that dilate the bronchial passages.
Theravance rose from $18 to $29.49 in recent weeks, up over 60%. Theravance runs the risk of dropping as investors lock gains. The company is worth another look if Theravance files for a regulatory filing for COPD treatment in late-2012. By comparison, Arena is up 8-fold from its 52-week low: should investors new to Arena be initiating a position in the company?
As readers will already be aware, the Food and Drug Administration approved Arena's obesity drug, Belviq, the first weight-loss drug in 13 years. Belviq is known chemically as lorcaserin. Two other firms are trying to gain approval for the same kind of drug: Vivus Inc. (VVUS) and Orexigen Therapeutics (OREX).
There are six reasons why Arena is a company to consider buying:
1) Wall Street Wants Arena to Fall
When Arena gained approval, shares opened at $13.50, but ended the day slightly above $11, a gain of nearly 30%. Shares did not rally because 25% of the shares are institution-owned and 75% retail-owned. By comparison, VVUS is 68% institution-owned, which means Wall Street would favor VVUS over Arena. In addition, short-interest was 46 million shares as of June 29 2012.
A bias for negative news will give new investors an opportunity to start a position in the company at a discount.
2) Takeover Candidate
Arena filed for marketing approval in Switzerland on July 11, which means the company will need an European partner for distribution. A larger pharmaceutical company might want to buy Arena before the company finds a partner to share marketing and distribution costs. As Arena continues to establish global distribution, the value of the company will keep going up.
3) Threat of Qnexa Exaggerated
Qnexa is still awaiting further reviews due to safety concerns.
Qnexa is a combination of phentermine and topiramate. Topiramate, an appetite surpressor, is known to cause birth defects. Phentermine works by increasing metabolism, but is only for short-term use. The onset of tolerance may be followed by a rebound in weight gain.
The results of the FORTRESS (Fetal Outcome Retrospective Topiramte Exposure Study) is still interim, since VVUS did not perform data validation on the results.
4) Arena Insiders Are More Bullish than VVUS Insiders
In 2012, VVUS insiders sold 1.7 million shares for a cumulative market total of $37.4 million as of June 27 2012:
Cumulative Total of VVUS Insider Sales:
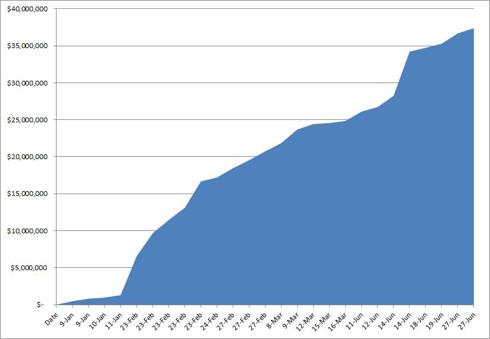
By comparison, Arena insiders sold just 177,495 shares valued at just $1.7 million.
5) Skepticism for Belviq Exaggerated
In the BLOSSOM ((Behavioral modification and Lorcaserin Second Study for Obesity Management) study, 63% of the participants who completed the trial lost at least 5% of their body weight. 35% lost at least 10% of their body weight. The long-term benefit was clear: patients lost an average of 17.0 pounds, or 7.9% of their body weight.
6) High Revenue Potential
There are a number of assumptions that need to be made to determine a range of values for Arena. Assuming Belviq will sell for between $2 and $4 per day, and taken twice daily, the revenue per patient per year is:
Revenue Per Patient / Year
Low
$2,920
High
$5,840
There are 78 million Americans who are considered obese, which investors might incorrectly think is be the sales size for the drug. The revenue range for Belviq is enormously wide, at between $228 million and $228 billion:
% Obesity Market (U.S.) # Receiving Treatment Market Size (Low-end)Market Size (High-end)
100% 78,000,000 $227,760,000,000 $455,520,000,000
75% 58,500,000 $170,820,000,000 $341,640,000,000
50% 39,000,000 $113,880,000,000 $227,760,000,000
25% 19,500,000 $56,940,000,000 $113,880,000,000
10% 7,800,000 $22,776,000,000 $45,552,000,000
5% 3,900,000 $11,388,000,000 $22,776,000,000
4% 3,120,000 $9,110,400,000 $18,220,800,000
3% 2,340,000 $6,832,800,000 $13,665,600,000
2% 1,560,000 $4,555,200,000 $9,110,400,000
1% 780,000 $2,277,600,000 $4,555,200,000
0.50% 390,000 $1,138,800,000 $2,277,600,000
0.25% 195,000 $569,400,000 $1,138,800,000
0.10% 78,000 $227,760,000 $455,520,000
Investors should assume worst case scenarios for Arena: no more than 5% (4 million) seek treatment for obesity, with 10% choosing Belviq. After 12-weeks of usage, assume only 33% continue treatment.
The resultant revenue would be between $387 - $774 million:
% Obesity Market (U.S.) # Receiving Treatment Revenue (Low-end) $ Revenue (High-end) $
0.20% 156,000 455,520,000 911,040,000
0.17% 132,600 387,192,000 774,384,000
0.15% 117,000 341,640,000 683,280,000
0.10% 78,000 227,760,000 455,520,000
The resulting earnings and forward P/E at a 25% margin for Arena would be:
% Obesity Market (U.S.)
EPS at 25% Margin (LOW) EPS at 25% Margin (High) P/E (High) P/E
0.25% 0.58 1.16 19.36 9.68
0.25% 0.49 0.98 22.77 11.39
0.25% 0.43 0.87 25.81 12.9
0.10% 0.29 0.58 38.71 19.36
Arena's partner, Eisai, will pay Arena between $40 and $60 million. The conservative payment amount from Eisai should temper investor expectations on the initial sales strength for Belviq and the valuation for Arena.
From its 10k filing on May 2012:
If lorcaserin is approved for US marketing, and upon the delivery of product supply for launch, we will also receive a milestone payment from Eisai of $40.0 million or $60.0 million, depending on the approved drug label.
Conclusion:
There is no doubt that Arena's obesity drug will be successful. The recent surge in shares makes Arena a riskier play than when shares traded below $6. Still, Arena has a product that is safe, and sales will grow as Arena and Eisai market and distribute the product. A share pull-back is likely to be minimal, but the long-term upside is enormous. More importantly, investors who want to start a position in Arena are paying an estimated forward-P/E of between 11.4 and 22.8.
Disclosure: I have no positions in any stocks mentioned, but may initiate a long position in ARNA over the next 72 hours.
http://seekingalpha.com/article/722811-the-time-to-buy-arena…
July 16, 2012
One of the benefits for investors who focus in specific sectors is that new opportunities can come from research. While researching Theravance (THRX) and Valeant (VRX) over the last few quarters, Arena Pharmaceuticals (ARNA) came up recently. A number of readers bought Arena when shares traded below $6. This leaves investors noticing shares at the current price paying two-fold or more.
Theravance and its partner GlaxoSmithKline PLC (GSK) reported positive results from four Phase III tests for the treatment of chronic obstructive pulmonary disease ("COPD"). Theravance and GSK are testing LAMA/LABA, which is a mix of two molecules, in four 24-week long studies. LAMA/LABA are two molecules that dilate the bronchial passages.
Theravance rose from $18 to $29.49 in recent weeks, up over 60%. Theravance runs the risk of dropping as investors lock gains. The company is worth another look if Theravance files for a regulatory filing for COPD treatment in late-2012. By comparison, Arena is up 8-fold from its 52-week low: should investors new to Arena be initiating a position in the company?
As readers will already be aware, the Food and Drug Administration approved Arena's obesity drug, Belviq, the first weight-loss drug in 13 years. Belviq is known chemically as lorcaserin. Two other firms are trying to gain approval for the same kind of drug: Vivus Inc. (VVUS) and Orexigen Therapeutics (OREX).
There are six reasons why Arena is a company to consider buying:
1) Wall Street Wants Arena to Fall
When Arena gained approval, shares opened at $13.50, but ended the day slightly above $11, a gain of nearly 30%. Shares did not rally because 25% of the shares are institution-owned and 75% retail-owned. By comparison, VVUS is 68% institution-owned, which means Wall Street would favor VVUS over Arena. In addition, short-interest was 46 million shares as of June 29 2012.
A bias for negative news will give new investors an opportunity to start a position in the company at a discount.
2) Takeover Candidate
Arena filed for marketing approval in Switzerland on July 11, which means the company will need an European partner for distribution. A larger pharmaceutical company might want to buy Arena before the company finds a partner to share marketing and distribution costs. As Arena continues to establish global distribution, the value of the company will keep going up.
3) Threat of Qnexa Exaggerated
Qnexa is still awaiting further reviews due to safety concerns.
Qnexa is a combination of phentermine and topiramate. Topiramate, an appetite surpressor, is known to cause birth defects. Phentermine works by increasing metabolism, but is only for short-term use. The onset of tolerance may be followed by a rebound in weight gain.
The results of the FORTRESS (Fetal Outcome Retrospective Topiramte Exposure Study) is still interim, since VVUS did not perform data validation on the results.
4) Arena Insiders Are More Bullish than VVUS Insiders
In 2012, VVUS insiders sold 1.7 million shares for a cumulative market total of $37.4 million as of June 27 2012:
Cumulative Total of VVUS Insider Sales:

By comparison, Arena insiders sold just 177,495 shares valued at just $1.7 million.
5) Skepticism for Belviq Exaggerated
In the BLOSSOM ((Behavioral modification and Lorcaserin Second Study for Obesity Management) study, 63% of the participants who completed the trial lost at least 5% of their body weight. 35% lost at least 10% of their body weight. The long-term benefit was clear: patients lost an average of 17.0 pounds, or 7.9% of their body weight.
6) High Revenue Potential
There are a number of assumptions that need to be made to determine a range of values for Arena. Assuming Belviq will sell for between $2 and $4 per day, and taken twice daily, the revenue per patient per year is:
Revenue Per Patient / Year
Low
$2,920
High
$5,840
There are 78 million Americans who are considered obese, which investors might incorrectly think is be the sales size for the drug. The revenue range for Belviq is enormously wide, at between $228 million and $228 billion:
% Obesity Market (U.S.) # Receiving Treatment Market Size (Low-end)Market Size (High-end)
100% 78,000,000 $227,760,000,000 $455,520,000,000
75% 58,500,000 $170,820,000,000 $341,640,000,000
50% 39,000,000 $113,880,000,000 $227,760,000,000
25% 19,500,000 $56,940,000,000 $113,880,000,000
10% 7,800,000 $22,776,000,000 $45,552,000,000
5% 3,900,000 $11,388,000,000 $22,776,000,000
4% 3,120,000 $9,110,400,000 $18,220,800,000
3% 2,340,000 $6,832,800,000 $13,665,600,000
2% 1,560,000 $4,555,200,000 $9,110,400,000
1% 780,000 $2,277,600,000 $4,555,200,000
0.50% 390,000 $1,138,800,000 $2,277,600,000
0.25% 195,000 $569,400,000 $1,138,800,000
0.10% 78,000 $227,760,000 $455,520,000
Investors should assume worst case scenarios for Arena: no more than 5% (4 million) seek treatment for obesity, with 10% choosing Belviq. After 12-weeks of usage, assume only 33% continue treatment.
The resultant revenue would be between $387 - $774 million:
% Obesity Market (U.S.) # Receiving Treatment Revenue (Low-end) $ Revenue (High-end) $
0.20% 156,000 455,520,000 911,040,000
0.17% 132,600 387,192,000 774,384,000
0.15% 117,000 341,640,000 683,280,000
0.10% 78,000 227,760,000 455,520,000
The resulting earnings and forward P/E at a 25% margin for Arena would be:
% Obesity Market (U.S.)
EPS at 25% Margin (LOW) EPS at 25% Margin (High) P/E (High) P/E
0.25% 0.58 1.16 19.36 9.68
0.25% 0.49 0.98 22.77 11.39
0.25% 0.43 0.87 25.81 12.9
0.10% 0.29 0.58 38.71 19.36
Arena's partner, Eisai, will pay Arena between $40 and $60 million. The conservative payment amount from Eisai should temper investor expectations on the initial sales strength for Belviq and the valuation for Arena.
From its 10k filing on May 2012:
If lorcaserin is approved for US marketing, and upon the delivery of product supply for launch, we will also receive a milestone payment from Eisai of $40.0 million or $60.0 million, depending on the approved drug label.
Conclusion:
There is no doubt that Arena's obesity drug will be successful. The recent surge in shares makes Arena a riskier play than when shares traded below $6. Still, Arena has a product that is safe, and sales will grow as Arena and Eisai market and distribute the product. A share pull-back is likely to be minimal, but the long-term upside is enormous. More importantly, investors who want to start a position in Arena are paying an estimated forward-P/E of between 11.4 and 22.8.
Disclosure: I have no positions in any stocks mentioned, but may initiate a long position in ARNA over the next 72 hours.
http://seekingalpha.com/article/722811-the-time-to-buy-arena…
Arena Pharmaceuticals Can Be An Interesting Bet Today
July 16, 2012 |
Tomorrow makes for an interesting set of circumstances that might make it worthy to buy Arena Pharmaceuticals (ARNA) today. Arena Pharmaceuticals has already received FDA approval for its main obesity drug, "Belviq". Now, tomorrow VIVUS (VVUS) will see the FDA decision on whether to grant approval to its "Qnexa" drug, also meant to treat obesity. Two things can happen:
Qnexa is not approved: If Qnexa is not approved, Arena will find itself with less competition in the obesity market. As such, even if VVUS takes a plunge, it's quite likely that ARNA will head up because of the lower competition to its already-approved medicine.
Qnexa is approved: If Qnexa is approved, then VVUS will rally strongly, and it is likely that ARNA will follow somewhat due to being in the same sector. Sure, competitive pressure can mean ARNA doesn't move much, but when an equity in a given sector jumps, many of peers also see some upside pressure.
The other stock in the sector
There is a third stock in the obesity drug sector. Orexigen Therapeutics (OREX). Here things aren't so clear cut. Although OREX's stock would benefit from a Qnexa approval, due to speculation that its own drug would also get approved, it's likely that OREX would also drop if Qnexa fails to get approval.
Conclusion
Due to this unique set of circumstances, it seems like buying ARNA today will make for a decent long trade going into FDA's decision about VVUS's Qnexa tomorrow, with either decision (approval/refusal) seemingly having a good chance of producing upside for ARNA stock over the short term.
As it stands, going into the decision ARNA's market capitalization, at $2.04 billion, is actually smaller than VVUS at $2.71 billion, and ARNA already has an approved obesity drug.
Disclosure: I have no positions in any stocks mentioned, but may initiate a long position in ARNA over the next 72 hours.
http://seekingalpha.com/article/723341-arena-pharmaceuticals…
July 16, 2012 |
Tomorrow makes for an interesting set of circumstances that might make it worthy to buy Arena Pharmaceuticals (ARNA) today. Arena Pharmaceuticals has already received FDA approval for its main obesity drug, "Belviq". Now, tomorrow VIVUS (VVUS) will see the FDA decision on whether to grant approval to its "Qnexa" drug, also meant to treat obesity. Two things can happen:
Qnexa is not approved: If Qnexa is not approved, Arena will find itself with less competition in the obesity market. As such, even if VVUS takes a plunge, it's quite likely that ARNA will head up because of the lower competition to its already-approved medicine.
Qnexa is approved: If Qnexa is approved, then VVUS will rally strongly, and it is likely that ARNA will follow somewhat due to being in the same sector. Sure, competitive pressure can mean ARNA doesn't move much, but when an equity in a given sector jumps, many of peers also see some upside pressure.
The other stock in the sector
There is a third stock in the obesity drug sector. Orexigen Therapeutics (OREX). Here things aren't so clear cut. Although OREX's stock would benefit from a Qnexa approval, due to speculation that its own drug would also get approved, it's likely that OREX would also drop if Qnexa fails to get approval.
Conclusion
Due to this unique set of circumstances, it seems like buying ARNA today will make for a decent long trade going into FDA's decision about VVUS's Qnexa tomorrow, with either decision (approval/refusal) seemingly having a good chance of producing upside for ARNA stock over the short term.
As it stands, going into the decision ARNA's market capitalization, at $2.04 billion, is actually smaller than VVUS at $2.71 billion, and ARNA already has an approved obesity drug.
Disclosure: I have no positions in any stocks mentioned, but may initiate a long position in ARNA over the next 72 hours.
http://seekingalpha.com/article/723341-arena-pharmaceuticals…
Arena Pharmaceuticals
Huge opportunity; just be careful.
3 Reasons to Buy
Belviq will launch into a humungous market – pun fully intended – with weak competition from drugs currently approved to treat obesity.
Having a marketing partner, Eisai, in place will help establish a quick launch once the Drug Enforcement Administration signs off on the drug.
A successful launch will trigger milestone payments from Eisai, which will provide funds for development of other drugs or an incentive for Eisai to purchase Arena.
3 Reasons to Sell
No obesity drug has reached the $1 billion blockbuster status; Arena and marketing partner Eisai will need to succeed where others have failed.
Post-marketing trials required by the FDA have the potential to highlight side effects that, in the worst-case scenario, could result in the drug being pulled off the market.
Arena only has one drug on the market, which makes its valuation dependent on the success and failure of a single revenue stream.
Arena Pharmaceuticals was founded in 1997 to study a class of receptors called G-protein coupled receptors. Lately, Arena has been mainly focused on its obesity drug lorcaserin, which targets a G-protein receptor responsible for making people feel content. The company has a few other programs, but they're in phase 1 or earlier. The near- and medium-term success of Arena is dependent on lorcaserin.
In 2010, the company went in front of an FDA advisory committee and the panel of outside experts shot down the drug. The agency followed the committee's advice, requesting additional information on the safety of lorcaserin.
After gathering the additional data, Arena went back to the FDA and finally managed to gain marketing approval in June 2012 for lorcaserin, which it named Belviq.
The Opportunity
The nation is getting fatter. Look at how many more people have become obese over the past 20 years, according to the Centers for Disease Control and Prevention.
U.S. obesity map, 1990

U.S. obesity map, 2000

U.S. obesity map, 2010

Source: CDC.
According to the CDC, more than 35% of U.S. adults are considered obese. That's more than 78 million potential customers. And there are another 12.5 million obese children and adolescents who aren't potential customers yet – Belviq isn't approved for children – but those kids will grow up and many, likely most, will be obese adults.
Arena's long-term market is actually less than half of that because the FDA recommended that doctors discontinue use of Belviq after 12 weeks if the patient hasn't lost at least 5% of his or her body weight. Only 42% of patients hit that mark in the clinical trials.
Patients' weight loss is a combination of Belviq's effect on the body and the patients' ability to change dieting and exercise habits. Only time will tell, but I think it's reasonable to assume that patients enrolled in a clinical trial are more motivated to lose weight than those looking for a quick fix from an FDA-approved drug. Assuming that's the case, the percentage of patients achieving the goal of losing 5% in 12 weeks in the real world could be considerably lower than what was seen in the clinical trials. On the flip side, doctors aren't required to follow the FDA's advice and might be inclined to keep patients on Belviq even if they haven't reached the goal if they're losing at least a little weight.
Belviq is also approved for patients with BMIs above 27, which is only considered overweight, as long as the patient has another comorbidity that would be exacerbated by the extra weight: high blood pressure, high cholesterol, or type 2 diabetes, for instance. Arena tested the drug in type 2 diabetics specifically, but the fraction of patients achieving the 5%-in-12-weeks goal was even lower; just 32% in that trial reached the early efficacy goal.
The potential market for Arena is huge, even factoring in that less than half of the patients will respond to the drug. In reality, Arena and marketing partner Eisai are in for an uphill battle to get doctors to prescribe the drug and patients to take it regularly. No obesity drug has ever reached blockbuster status, defined as annual sales of $1 billion or more.
Part of the problem has to do with side effects. The last obesity drug approved, Roche's Xenical, works by blocking the absorption of fat. That fat passes through the patient. You can imagine how that might affect bodily functions, but just in case it isn't clear, I'll turn to the adverse-reactions section of the label to explain: "Most common treatment emergent adverse reactions… include oily spotting, flatus with discharge, fecal urgency fatty/oily stool, oily evacuation, increased defecation and fecal incontinence."
With those types of issues, it's not surprising that doctors would be reluctant to prescribe the drug and patients might be reluctant to stay on it. The over-the-counter version of Xenical, GlaxoSmithKline's alli, has also been a relatively weak seller.
Belviq's biggest issue probably won't be getting patients onto the drug, since it has relatively mild side effects, but keeping them on it. People taking obesity drugs are looking for a quick fix and when that doesn't happen, they're not likely to stay on the medication. In the two pivotal phase 3 trials, 44% of patients taking Belviq dropped out of the trials within the first year. If that number increases further in a real-life setting, which seems possible given that people going into clinical trials are likely more motivated, Arena and marketing partner Eisai will have trouble producing blockbuster sales despite the blockbuster-sized market.
Arena is expecting $85 million from Eisai for achieving a variety of milestones between the FDA approval and when it delivers a supply of the drug for the launch. That cash, along with about $140 million Arena expected to have at the end of June, will tide the company over for a while.
Eisai is solely responsible for distributing and marketing the drug. Arena receives a cut for providing the drug, which starts at 31.5% of Eisai's aggregate annual net sales and increases to as much as 36.5% on the portion of Eisai's aggregate annual net sales exceeding $750 million.
Arena is due $1.2 billion in one-time purchase price adjustments and milestone payments when certain annual sales thresholds are reached. The payments will have an effect on Arena's valuation because they'll increase the cash balance, and could drive a takeout by Eisai to avoid making the payments. For investors, the payments won't have a major effect on the long-term value, which will solely be determined by the trajectory of Belviq's sales.
Four Key Developments You MUST Watch
Approval of VIVUS' Qnexa:
The rival biotech is set to receive an FDA decision for its obesity drug on or around July 17, 2012. I think an approval is likely, but I'm not convinced it's the worst thing in the world for Arena.
Sure, launching into a space with no real competition is an opportunity, but launching around the same time as Qnexa will help drive the overall obesity market faster than Eisai can do it alone. As both companies target doctors, it will keep obesity medications on doctors' minds, which will drive sales of both drugs regardless of which company's sales reps have visited the doctor's office more often.
For instance, any office visits by a sales rep selling Qnexa will get the doctor thinking about prescribing drugs for his or her obese patients. Since Qnexa isn't appropriate for women who might become pregnant, the doctor might be more likely to prescribe Belviq than if Qnexa's sales reps hadn't visited.
Determination of final scheduling:
Belviq was approved by the FDA, but Arena's marketing partner Eisai can't launch the drug yet because the FDA requested that the Drug Enforcement Administration give the drug a Schedule IV because of its potential for abuse. If the DEA agrees, or issues a less-severe schedule, Arena will be just fine. Selling a Schedule IV drug is mostly a recordkeeping issue and won't inhibit Eisai from marketing the drug. The DEA is expected to make its decision sometime toward the end of 2012.
Costs for post-approval trials required by the FDA:
Arena has six post-approval commitments that the FDA has requested. The biotech is only on the hook for 10% of the cost of the cardiovascular study, but it has to pay 50% of the cost of the pediatric studies requested by the FDA. The remaining costs are paid by Eisai, per their licensing agreement.
Arena expects to begin the trials in the middle of 2013, so it won't start incurring the extra cost until then. It's unlikely that the company will need to raise money to pay for the trials – at least not anytime in the near future – but the costs will eventually start cutting into Arena's profit, so investors should keep an eye on the exact expenses as they're disclosed.
Decision about selling the drug in the EU:
Arena has already submitted a marketing application in the EU, with an approval decision possible in late October, likely later as the clock stops every time EU officials ask questions and have to wait for data from the company. The EU tends to be a little more liberal about approving drugs than the FDA, but there are certainly examples where EU officials have turned down drugs that the FDA approved. Call it cautiously optimistic.
The opportunity in the EU is difficult to determine but certainly smaller than it is stateside. Europe is a smaller drug market in general and tends to be less obese than the U.S. Depending on pricing and other factors, Arena – or whichever company Arena sells the EU rights to – might have difficulty getting countries with centralized health care to cover the drug. Coverage is likely to be an issue in the U.S. as well, but Americans are more used to paying out of pocket for their health care than Europeans who generally pay for it indirectly through their taxes.
Risks
Beyond the risk that Belviq's sales won't hit investors' expectations, Arena has some additional risks that come from having a new drug on the market.
As part of the approval, the FDA required Arena to run multiple post-approval trials. Of those, the one measuring cardiovascular outcomes – heart attacks, strokes, and the like – is the most risky because if it doesn't go Arena's way, Belviq could be off the market.
Abbott Labs' Meridia was approved in 1997, but was pulled from the market in 2010 after a post-approval trial hinted that the obesity drug could be causing deleterious effects on patients' hearts.
Belviq is related to fenfluramine – the fen part of Wyeth's fen-phen that was also pulled from the market. Thus far, data suggest that Belviq is activating the receptor that causes patients to become content without affecting the receptor that would cause valve damage like fenfluramine does, but only a large trial will answer the question for sure.
There's also a risk that manufacturing issues could keep Arena from meeting demand. Arena's management has said that Belviq is easy to manufacture and that the company has 500 million doses of the active ingredient currently available to sell. But this is Arena's first product, so there's no history of the company successfully manufacturing a drug. The risk, however slight, shouldn't be ignored.
Leadership
Jack Lief is the co-founder, chairman, president, and CEO of Arena. The other co-founder, Dominic Behan, serves as a director, executive vice president, and chief scientific officer. After 15 years, you have to think there's a bit of love for the company; they're probably not in it (just) for the paycheck.
I'm impressed that the company was able to gain approval on time after having an FDA advisory panel meeting just a month before its decision date. The FDA ended up delaying the decision on VIVUS' Qnexa after its advisory panel meeting, but Arena's management was able to satisfy the agency's last-minute questions. Investors should have confidence that Arena's leadership knows what they're doing, at least on the regulatory side.
Sometimes leaders who are good at development of drugs aren't that effective on the commercial side, but it doesn't really matter for Arena because its marketing partner Eisai will be in full control there. The Japanese pharma isn't the biggest drug maker out there, but it does have experience in the U.S. market, having helped sell Aricept and AcipHex, which are sold in the large Alzheimer's and heartburn markets, respectively.
The Foolish Bottom Line
Arena has a lot of potential, but succeeding where other obesity drugs have failed won't be easy.
At the time of publication Brian Orelli owned no shares of the companies mentioned. The Motley Fool owned shares of Abbott Laboratories.
http://www.fool.com/reports/arna-f.aspx
Huge opportunity; just be careful.
3 Reasons to Buy
Belviq will launch into a humungous market – pun fully intended – with weak competition from drugs currently approved to treat obesity.
Having a marketing partner, Eisai, in place will help establish a quick launch once the Drug Enforcement Administration signs off on the drug.
A successful launch will trigger milestone payments from Eisai, which will provide funds for development of other drugs or an incentive for Eisai to purchase Arena.
3 Reasons to Sell
No obesity drug has reached the $1 billion blockbuster status; Arena and marketing partner Eisai will need to succeed where others have failed.
Post-marketing trials required by the FDA have the potential to highlight side effects that, in the worst-case scenario, could result in the drug being pulled off the market.
Arena only has one drug on the market, which makes its valuation dependent on the success and failure of a single revenue stream.
Arena Pharmaceuticals was founded in 1997 to study a class of receptors called G-protein coupled receptors. Lately, Arena has been mainly focused on its obesity drug lorcaserin, which targets a G-protein receptor responsible for making people feel content. The company has a few other programs, but they're in phase 1 or earlier. The near- and medium-term success of Arena is dependent on lorcaserin.
In 2010, the company went in front of an FDA advisory committee and the panel of outside experts shot down the drug. The agency followed the committee's advice, requesting additional information on the safety of lorcaserin.
After gathering the additional data, Arena went back to the FDA and finally managed to gain marketing approval in June 2012 for lorcaserin, which it named Belviq.
The Opportunity
The nation is getting fatter. Look at how many more people have become obese over the past 20 years, according to the Centers for Disease Control and Prevention.
U.S. obesity map, 1990

U.S. obesity map, 2000

U.S. obesity map, 2010

Source: CDC.
According to the CDC, more than 35% of U.S. adults are considered obese. That's more than 78 million potential customers. And there are another 12.5 million obese children and adolescents who aren't potential customers yet – Belviq isn't approved for children – but those kids will grow up and many, likely most, will be obese adults.
Arena's long-term market is actually less than half of that because the FDA recommended that doctors discontinue use of Belviq after 12 weeks if the patient hasn't lost at least 5% of his or her body weight. Only 42% of patients hit that mark in the clinical trials.
Patients' weight loss is a combination of Belviq's effect on the body and the patients' ability to change dieting and exercise habits. Only time will tell, but I think it's reasonable to assume that patients enrolled in a clinical trial are more motivated to lose weight than those looking for a quick fix from an FDA-approved drug. Assuming that's the case, the percentage of patients achieving the goal of losing 5% in 12 weeks in the real world could be considerably lower than what was seen in the clinical trials. On the flip side, doctors aren't required to follow the FDA's advice and might be inclined to keep patients on Belviq even if they haven't reached the goal if they're losing at least a little weight.
Belviq is also approved for patients with BMIs above 27, which is only considered overweight, as long as the patient has another comorbidity that would be exacerbated by the extra weight: high blood pressure, high cholesterol, or type 2 diabetes, for instance. Arena tested the drug in type 2 diabetics specifically, but the fraction of patients achieving the 5%-in-12-weeks goal was even lower; just 32% in that trial reached the early efficacy goal.
The potential market for Arena is huge, even factoring in that less than half of the patients will respond to the drug. In reality, Arena and marketing partner Eisai are in for an uphill battle to get doctors to prescribe the drug and patients to take it regularly. No obesity drug has ever reached blockbuster status, defined as annual sales of $1 billion or more.
Part of the problem has to do with side effects. The last obesity drug approved, Roche's Xenical, works by blocking the absorption of fat. That fat passes through the patient. You can imagine how that might affect bodily functions, but just in case it isn't clear, I'll turn to the adverse-reactions section of the label to explain: "Most common treatment emergent adverse reactions… include oily spotting, flatus with discharge, fecal urgency fatty/oily stool, oily evacuation, increased defecation and fecal incontinence."
With those types of issues, it's not surprising that doctors would be reluctant to prescribe the drug and patients might be reluctant to stay on it. The over-the-counter version of Xenical, GlaxoSmithKline's alli, has also been a relatively weak seller.
Belviq's biggest issue probably won't be getting patients onto the drug, since it has relatively mild side effects, but keeping them on it. People taking obesity drugs are looking for a quick fix and when that doesn't happen, they're not likely to stay on the medication. In the two pivotal phase 3 trials, 44% of patients taking Belviq dropped out of the trials within the first year. If that number increases further in a real-life setting, which seems possible given that people going into clinical trials are likely more motivated, Arena and marketing partner Eisai will have trouble producing blockbuster sales despite the blockbuster-sized market.
Arena is expecting $85 million from Eisai for achieving a variety of milestones between the FDA approval and when it delivers a supply of the drug for the launch. That cash, along with about $140 million Arena expected to have at the end of June, will tide the company over for a while.
Eisai is solely responsible for distributing and marketing the drug. Arena receives a cut for providing the drug, which starts at 31.5% of Eisai's aggregate annual net sales and increases to as much as 36.5% on the portion of Eisai's aggregate annual net sales exceeding $750 million.
Arena is due $1.2 billion in one-time purchase price adjustments and milestone payments when certain annual sales thresholds are reached. The payments will have an effect on Arena's valuation because they'll increase the cash balance, and could drive a takeout by Eisai to avoid making the payments. For investors, the payments won't have a major effect on the long-term value, which will solely be determined by the trajectory of Belviq's sales.
Four Key Developments You MUST Watch
Approval of VIVUS' Qnexa:
The rival biotech is set to receive an FDA decision for its obesity drug on or around July 17, 2012. I think an approval is likely, but I'm not convinced it's the worst thing in the world for Arena.
Sure, launching into a space with no real competition is an opportunity, but launching around the same time as Qnexa will help drive the overall obesity market faster than Eisai can do it alone. As both companies target doctors, it will keep obesity medications on doctors' minds, which will drive sales of both drugs regardless of which company's sales reps have visited the doctor's office more often.
For instance, any office visits by a sales rep selling Qnexa will get the doctor thinking about prescribing drugs for his or her obese patients. Since Qnexa isn't appropriate for women who might become pregnant, the doctor might be more likely to prescribe Belviq than if Qnexa's sales reps hadn't visited.
Determination of final scheduling:
Belviq was approved by the FDA, but Arena's marketing partner Eisai can't launch the drug yet because the FDA requested that the Drug Enforcement Administration give the drug a Schedule IV because of its potential for abuse. If the DEA agrees, or issues a less-severe schedule, Arena will be just fine. Selling a Schedule IV drug is mostly a recordkeeping issue and won't inhibit Eisai from marketing the drug. The DEA is expected to make its decision sometime toward the end of 2012.
Costs for post-approval trials required by the FDA:
Arena has six post-approval commitments that the FDA has requested. The biotech is only on the hook for 10% of the cost of the cardiovascular study, but it has to pay 50% of the cost of the pediatric studies requested by the FDA. The remaining costs are paid by Eisai, per their licensing agreement.
Arena expects to begin the trials in the middle of 2013, so it won't start incurring the extra cost until then. It's unlikely that the company will need to raise money to pay for the trials – at least not anytime in the near future – but the costs will eventually start cutting into Arena's profit, so investors should keep an eye on the exact expenses as they're disclosed.
Decision about selling the drug in the EU:
Arena has already submitted a marketing application in the EU, with an approval decision possible in late October, likely later as the clock stops every time EU officials ask questions and have to wait for data from the company. The EU tends to be a little more liberal about approving drugs than the FDA, but there are certainly examples where EU officials have turned down drugs that the FDA approved. Call it cautiously optimistic.
The opportunity in the EU is difficult to determine but certainly smaller than it is stateside. Europe is a smaller drug market in general and tends to be less obese than the U.S. Depending on pricing and other factors, Arena – or whichever company Arena sells the EU rights to – might have difficulty getting countries with centralized health care to cover the drug. Coverage is likely to be an issue in the U.S. as well, but Americans are more used to paying out of pocket for their health care than Europeans who generally pay for it indirectly through their taxes.
Risks
Beyond the risk that Belviq's sales won't hit investors' expectations, Arena has some additional risks that come from having a new drug on the market.
As part of the approval, the FDA required Arena to run multiple post-approval trials. Of those, the one measuring cardiovascular outcomes – heart attacks, strokes, and the like – is the most risky because if it doesn't go Arena's way, Belviq could be off the market.
Abbott Labs' Meridia was approved in 1997, but was pulled from the market in 2010 after a post-approval trial hinted that the obesity drug could be causing deleterious effects on patients' hearts.
Belviq is related to fenfluramine – the fen part of Wyeth's fen-phen that was also pulled from the market. Thus far, data suggest that Belviq is activating the receptor that causes patients to become content without affecting the receptor that would cause valve damage like fenfluramine does, but only a large trial will answer the question for sure.
There's also a risk that manufacturing issues could keep Arena from meeting demand. Arena's management has said that Belviq is easy to manufacture and that the company has 500 million doses of the active ingredient currently available to sell. But this is Arena's first product, so there's no history of the company successfully manufacturing a drug. The risk, however slight, shouldn't be ignored.
Leadership
Jack Lief is the co-founder, chairman, president, and CEO of Arena. The other co-founder, Dominic Behan, serves as a director, executive vice president, and chief scientific officer. After 15 years, you have to think there's a bit of love for the company; they're probably not in it (just) for the paycheck.
I'm impressed that the company was able to gain approval on time after having an FDA advisory panel meeting just a month before its decision date. The FDA ended up delaying the decision on VIVUS' Qnexa after its advisory panel meeting, but Arena's management was able to satisfy the agency's last-minute questions. Investors should have confidence that Arena's leadership knows what they're doing, at least on the regulatory side.
Sometimes leaders who are good at development of drugs aren't that effective on the commercial side, but it doesn't really matter for Arena because its marketing partner Eisai will be in full control there. The Japanese pharma isn't the biggest drug maker out there, but it does have experience in the U.S. market, having helped sell Aricept and AcipHex, which are sold in the large Alzheimer's and heartburn markets, respectively.
The Foolish Bottom Line
Arena has a lot of potential, but succeeding where other obesity drugs have failed won't be easy.
At the time of publication Brian Orelli owned no shares of the companies mentioned. The Motley Fool owned shares of Abbott Laboratories.
http://www.fool.com/reports/arna-f.aspx
Vivus Announces FDA Approval of Once Daily Qsymia™ (Phentermine and Topiramate Extended-release)
THE FIRST ONCE DAILY COMBINATION TREATMENT FOR CHRONIC WEIGHT MANAGEMENT IN ADULTS WHO ARE OBESE OR OVERWEIGHT WITH A WEIGHT-RELATED COMORBIDITY
It is expected that Qsymia will be available in the fourth quarter of 2012."
The most common adverse reactions for patients treated with Qsymia included tingling sensation of hands and feet, dizziness, altered taste, insomnia, constipation and dry mouth.
Qsymia was approved with a Risk Evaluation and Mitigation Strategy (REMS) with a goal of informing prescribers and female patients of reproductive potential about an increased risk of orofacial clefts in infants exposed to Qsymia during the first trimester of pregnancy, the importance of pregnancy prevention for females of reproductive potential receiving Qsymia and the need to discontinue Qsymia immediately if pregnancy occurs.
The Qsymia REMS program includes
>a Medication Guide,
>Healthcare Provider training,
>distribution through certified pharmacies,
>implementation system and
>a time table for assessments.
As part of the approval of Qsymia, VIVUS is committed to conduct post-marketing studies.
> The company will conduct a study to assess the long-term treatment effect of Qsymia on the incidence of major adverse cardiovascular events in overweight and obese subjects with confirmed cardiovascular disease,
> studies to assess the safety and efficacy of Qsymia for weight management in obese pediatric and adolescent subjects,
> studies to assess drug utilization and pregnancy exposure,
> a study to assess renal function, as well as animal and in vitro studies.
..das APPROVAL mit post approval studies und mit REMS ist nun doch noch gekommen.....
....wegen des Risikos möglicher Missbildungen bei Neugeborenen wird die Einnahme von Qsympia bei der " Risikogruppe" prophylaktisch sehr stark zu kontrollieren sein...
..wie " legitimierte" Ärzte mit dieser Ausgangssituation umgehen werden, wird sich zeigen.....
...ich kann mir einfach nicht vorstellen, dass erfahrene " Obesity-Docs" die damit verbundenen " Aufwendungen" durchführen zumal mit BELVIQ bzgl. Safety-Standards andere Möglichkeiten gegeben sind..
TIME WILL TELL
THE FIRST ONCE DAILY COMBINATION TREATMENT FOR CHRONIC WEIGHT MANAGEMENT IN ADULTS WHO ARE OBESE OR OVERWEIGHT WITH A WEIGHT-RELATED COMORBIDITY
It is expected that Qsymia will be available in the fourth quarter of 2012."
The most common adverse reactions for patients treated with Qsymia included tingling sensation of hands and feet, dizziness, altered taste, insomnia, constipation and dry mouth.
Qsymia was approved with a Risk Evaluation and Mitigation Strategy (REMS) with a goal of informing prescribers and female patients of reproductive potential about an increased risk of orofacial clefts in infants exposed to Qsymia during the first trimester of pregnancy, the importance of pregnancy prevention for females of reproductive potential receiving Qsymia and the need to discontinue Qsymia immediately if pregnancy occurs.
The Qsymia REMS program includes
>a Medication Guide,
>Healthcare Provider training,
>distribution through certified pharmacies,
>implementation system and
>a time table for assessments.
As part of the approval of Qsymia, VIVUS is committed to conduct post-marketing studies.
> The company will conduct a study to assess the long-term treatment effect of Qsymia on the incidence of major adverse cardiovascular events in overweight and obese subjects with confirmed cardiovascular disease,
> studies to assess the safety and efficacy of Qsymia for weight management in obese pediatric and adolescent subjects,
> studies to assess drug utilization and pregnancy exposure,
> a study to assess renal function, as well as animal and in vitro studies.
..das APPROVAL mit post approval studies und mit REMS ist nun doch noch gekommen.....
....wegen des Risikos möglicher Missbildungen bei Neugeborenen wird die Einnahme von Qsympia bei der " Risikogruppe" prophylaktisch sehr stark zu kontrollieren sein...
..wie " legitimierte" Ärzte mit dieser Ausgangssituation umgehen werden, wird sich zeigen.....
...ich kann mir einfach nicht vorstellen, dass erfahrene " Obesity-Docs" die damit verbundenen " Aufwendungen" durchführen zumal mit BELVIQ bzgl. Safety-Standards andere Möglichkeiten gegeben sind..
TIME WILL TELL
..der erste Artikel, der die Zulassung von QSYMIA wegen der großen Nebenwirkungen verurteilt....
Drugs
FDA approves weight loss drug Qsymia, but we say skip it
Jul 18, 2012 5:25 PM
The Food and Drug Administration Tuesday approved Qsymia, a combination of the stimulant phentermine and the anti-seizure drug topiramate extended-release, to help obese and overweight people lose weight. But our medical advisers say the pill should be avoided because it can cause several serious side effects.
According to the evidence submitted to the FDA, Qsymia appears to help people drop a few pounds. In studies, obese and overweight people who took Qsymia for one year lost 3.5 to 9.3 more pounds than those who took a placebo. But that small benefit is probably not worth the risks of birth defects, heart attacks, and strokes. In fact, two years ago the FDA rejected the drug, then called Qnexa, due to these concerns, and it is not clear why the FDA reversed course this time, since those side effects are still an issue.
The drug also carries a warning that it can increase heart rate and should not be used by people who have heart disease or have suffered a stroke. Due to the heart concern, Vivus, the manufacturer of Qsymia, is required to conduct a study to determine whether the drug poses a risk of major cardiovascular problems, including heart attack and stroke. Also too, pregnant women should not take Qsymia because it increases the risk of their children being born with a cleft lip or palate.
Qsymia will only be available through specially certified pharmacies under a Risk Evaluation and Mitigation Strategy, or REMS, which is intended to inform doctors and patients about the possibility of birth defects.
"The very idea that a post marketing risk evaluation strategy was a condition required by the FDA for approval of this combination drug product seems like putting the cart ahead of the horse," says Marvin Lipman, M.D., chief medical adviser for Consumer Reports. "Such a study may very well result in preventable mortality and morbidity, a high price to pay in exchange for a few pounds of flesh."
Qsymia contains several additional warnings, including that it can increase the risk of glaucoma, kidney stones, mood problems such as anxiety and depression, and suicidal behavior or thinking about suicide (ideation).
"This drug should only be used in the confines of a research trial in which consumers have been fully informed of its risks and benefits and, someone is responsible for the complications that occur. All the rest of us should wait until we know a lot more about its safety," says John Santa, M.D., director of the Consumer Reports Health Ratings Center.
Bottom Line: We recommend avoiding all weight-loss drugs and supplements. Their benefits are usually marginal. And even if they do help you to shed a few pounds, the side effects can be troublesome and even dangerous.
If you need to lose weight, increasing exercise and limiting portion size when it comes to food are better options.
Additional links:
How to control your weight [Consumer Reports]
Weight-loss drugs: Alli and Xenical (Orlistat):Slim benefits and embarrassing side effects [Consumer Reports]
Lose weight, stay active: Six small changes can help keep off pounds [Consumer Reports]
Sources:
FDA approves weight-management drug Qsymia [FDA]
Panel recommends FDA approve weight loss drug Qnexa [Consumer Reports]
—Steve Mitchell
http://news.consumerreports.org/health/2012/07/fda-approves-…" target="_blank" rel="nofollow ugc noopener">
http://news.consumerreports.org/health/2012/07/fda-approves-…
Drugs
FDA approves weight loss drug Qsymia, but we say skip it
Jul 18, 2012 5:25 PM
The Food and Drug Administration Tuesday approved Qsymia, a combination of the stimulant phentermine and the anti-seizure drug topiramate extended-release, to help obese and overweight people lose weight. But our medical advisers say the pill should be avoided because it can cause several serious side effects.
According to the evidence submitted to the FDA, Qsymia appears to help people drop a few pounds. In studies, obese and overweight people who took Qsymia for one year lost 3.5 to 9.3 more pounds than those who took a placebo. But that small benefit is probably not worth the risks of birth defects, heart attacks, and strokes. In fact, two years ago the FDA rejected the drug, then called Qnexa, due to these concerns, and it is not clear why the FDA reversed course this time, since those side effects are still an issue.
The drug also carries a warning that it can increase heart rate and should not be used by people who have heart disease or have suffered a stroke. Due to the heart concern, Vivus, the manufacturer of Qsymia, is required to conduct a study to determine whether the drug poses a risk of major cardiovascular problems, including heart attack and stroke. Also too, pregnant women should not take Qsymia because it increases the risk of their children being born with a cleft lip or palate.
Qsymia will only be available through specially certified pharmacies under a Risk Evaluation and Mitigation Strategy, or REMS, which is intended to inform doctors and patients about the possibility of birth defects.
"The very idea that a post marketing risk evaluation strategy was a condition required by the FDA for approval of this combination drug product seems like putting the cart ahead of the horse," says Marvin Lipman, M.D., chief medical adviser for Consumer Reports. "Such a study may very well result in preventable mortality and morbidity, a high price to pay in exchange for a few pounds of flesh."
Qsymia contains several additional warnings, including that it can increase the risk of glaucoma, kidney stones, mood problems such as anxiety and depression, and suicidal behavior or thinking about suicide (ideation).
"This drug should only be used in the confines of a research trial in which consumers have been fully informed of its risks and benefits and, someone is responsible for the complications that occur. All the rest of us should wait until we know a lot more about its safety," says John Santa, M.D., director of the Consumer Reports Health Ratings Center.
Bottom Line: We recommend avoiding all weight-loss drugs and supplements. Their benefits are usually marginal. And even if they do help you to shed a few pounds, the side effects can be troublesome and even dangerous.
If you need to lose weight, increasing exercise and limiting portion size when it comes to food are better options.
Additional links:
How to control your weight [Consumer Reports]
Weight-loss drugs: Alli and Xenical (Orlistat):Slim benefits and embarrassing side effects [Consumer Reports]
Lose weight, stay active: Six small changes can help keep off pounds [Consumer Reports]
Sources:
FDA approves weight-management drug Qsymia [FDA]
Panel recommends FDA approve weight loss drug Qnexa [Consumer Reports]
—Steve Mitchell
http://news.consumerreports.org/health/2012/07/fda-approves-…" target="_blank" rel="nofollow ugc noopener">
http://news.consumerreports.org/health/2012/07/fda-approves-…
Eisai: additional salespeople in the metabolic field
It seems that Eisai is preparing a launch all over the country:
http://us.eisai.com/job.asp?ID=145
Medical Science Liaison (MSL) / Senior MSL – Metabolic, South Central Field Based Medical Affairs 2520BR 11-Jul-2012
Medical Science Liaison (MSL) / Senior MSL – Metabolic, Great Lakes US Field Based Medical Affairs 2521BR 11-Jul-2012
Medical Science Liaison (MSL) / Senior MSL – Metabolic, Southeast US Field Based Medical Affairs 2537BR 11-Jul-2012
Medical Science Liaison (MSL) / Senior MSL – Metabolic, New England US Field Based Medical Affairs 2538BR 11-Jul-2012
Medical Science Liaison (MSL) / Senior MSL – Metabolic, Western US Field Based Medical Affairs 2528BR 11-Jul-2012
http://www.investorvillage.com/smbd.asp?mb=633&mn=32993&pt=m…
It seems that Eisai is preparing a launch all over the country:
http://us.eisai.com/job.asp?ID=145
Medical Science Liaison (MSL) / Senior MSL – Metabolic, South Central Field Based Medical Affairs 2520BR 11-Jul-2012
Medical Science Liaison (MSL) / Senior MSL – Metabolic, Great Lakes US Field Based Medical Affairs 2521BR 11-Jul-2012
Medical Science Liaison (MSL) / Senior MSL – Metabolic, Southeast US Field Based Medical Affairs 2537BR 11-Jul-2012
Medical Science Liaison (MSL) / Senior MSL – Metabolic, New England US Field Based Medical Affairs 2538BR 11-Jul-2012
Medical Science Liaison (MSL) / Senior MSL – Metabolic, Western US Field Based Medical Affairs 2528BR 11-Jul-2012
http://www.investorvillage.com/smbd.asp?mb=633&mn=32993&pt=m…
Arena Pharmaceuticals: Analyzing The Eisai Partnership, Financials, And The Way Forward
July 20, 2012
In a market of thousands of stocks, somehow a select minority generate a majority of debate and arguments. The passions surrounding these stocks often reach fevered levels, and it is important for investors to maintain calm while analyzing a stock. That is true for every company, including Arena Pharmaceuticals (ARNA).
Arena is a company that generates fierce reactions from investors on both sides. After all, when a stock rises by over 400% year-to-date, that is to be expected.
With Arena's first product, Belviq, now approved by the FDA for "chronic weight management," the company has entered a new phase. Going forward, Arena will rise and fall alongside Belviq's results. That is what is needed to mitigate the long-term losses in the stock. How much money you made in Arena from the approval of Belviq depends on when you first bough the shares. Though the stock may be up over 400% in 2012, it is down over 60% since going public.
In our view, the easy money has been made here. Now, it is time for Arena to prove that the drug it has spent years and enormous sums of money developing can be a blockbuster. So where exactly do we go from here? Arena is transitioning away from a developmental stage company into a commercial biotechnology company, and as such, now faces a different set of expectations.
Belviq: Manufacturing a Blockbuster and a Partnership
Belviq is set to be available in early 2013, and will be manufactured in Switzerland and sold to Eisai, which has marketing and distribution rights to Belviq in North America (because of the way Belviq works, it has the potential to be abused, which is why the DEA must rule on how to schedule the drug. Should the DEA process go faster than expected, Belviq may begin selling earlier than that). It is this partnership with Eisai, which was struck in 2010, that we would like to focus on, for it will be one of the most important aspects of the Arena story going forward. Under the terms of the deal, Arena will manufacture Belviq and then sell it to Eisai, which will then resell it in the United States, by far the biggest market for Belviq.
Arena is set to receive anywhere between 31.5% and 36.5% of Eisai's net Belviq sales in America. The rate rises to 36.5% above net Belviq sales of $750 million.
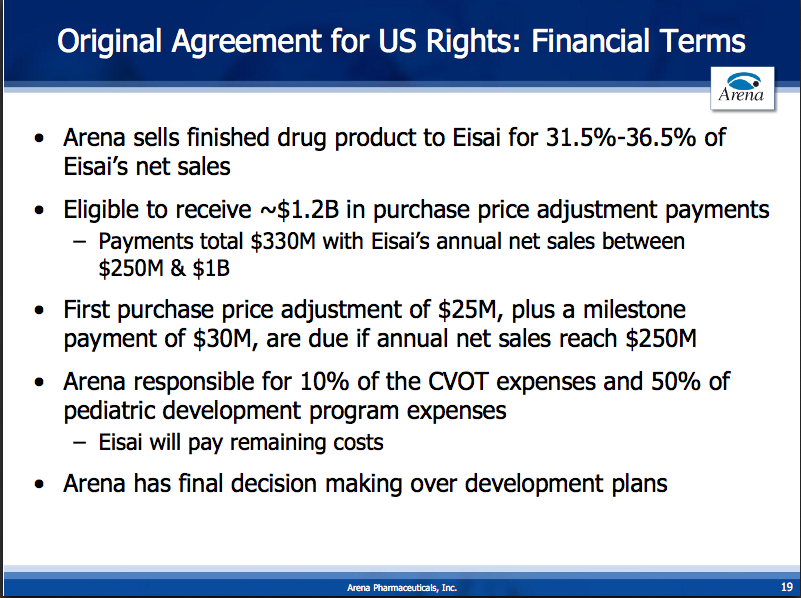
Arena is also set to receive over $1 billion in price adjustment payments, and Eisai bears the majority of post-approval clinical trial costs. It is responsible for 90% of cardiovascular outcome trials (CVOT) and half of the expenses for pediatric studies. After striking this agreement, Arena and Eisai expanded it to cover the rest of North and South America, with Arena's payment percentage set at 30.75%-35.75% of net sales in those countries.

Arena is once again set to bear only a minority of regulatory and post-approval expenses, be they marketing or clinical. Essentially, Arena is receiving an extremely high royalty rate on Belviq, as it will have little in the way of expenses to pay relating to Belviq. That will free up Arena to return focus to its pipeline, where there are several projects in the works. But first, we need to discuss road ahead for Belviq, as well as the company's financials.
Belviq Goes International
With Belviq now approved in the United States, Arena can turn its attention to expanding internationally. In March, the company filed for approval of the drug with the EMA (European Medicines Agency). England is the company's rapporteur and Sweden is the company's co-rapporteur. This is a Europe-specific requirement for drug approval. Rapporteurs are country-specific regulators within the Europan Union that help the CHMP (the Committee for Medicinal Products for Human Use), which reviews and evaluates marketing applications. Rapporteurs perform scientific evaluations of a marketing application and present an assessment of it the CHMP, which then takes it into account as part of its decision making process. The review process in the European Union will be centralized, meaning that approval will be valid throughout the entire European Union, as opposed to just a subset of member states. Arena has not given an timeline as to when it expects a decision regarding Belviq from the EMA, However, it has stated that Day 120 questions in late July. On July 11, Arena also filed for approval of Belviq in Switzerland, but declined to give a timeline for potential approval.
As Arena notes, obesity rates in many European countries have tripled in the past 30 years, which sets up an opportunity for Belviq. For the record, Vivus (VVUS) is set to present data to the CHMP in September regarding Qsymia. We expect to hear more about what is going on in Europe from Arena when it reports its second quarter results in August (an exact date has not yet been set). In addition, Arena has said that it is looking for a European partner for Belviq, and we expect an update on that front during the upcoming earnings conference call as well.
Financials: When the Rubber Meets the Road
Going forward, Arena's stock will not rise and fall simply on what happens with Belviq on the regulatory front. When the drug launches, Arena will be judged on how well it sells, and not if the company can get the drug approved. In the years to come, that is what will drive the stock.
Arena has lost well over $1 billion since it was founded due to the costs of developing Belviq. And in its last quarter, the company lost another $29.4 million, or 18 cents per share. However, with Belviq now approved in the United States, the company appears to be on a path to profitability. However, consensus estimates (based on data from Reuters) call for a loss of 33 cents per share in 2012, and 17 cents in 2013.
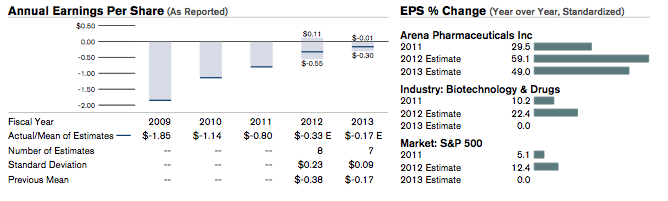
Bloomberg's consensus estimates for Arena are more pessimistic, with the consensus for 2012 being a loss of 46 cents per share, and 29 cents in 2013. While some analysts forecast 2014 as the year that Arena will post a profit (Merrill Lynch estimates a 1 cent per share profit for 2014), Wall Street is still pessimistic, with the Bloomberg consensus estimate calling for a loss of 8 cents in 2014.
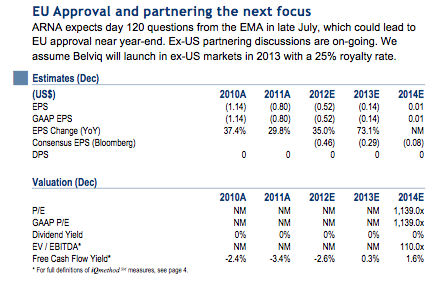
Wall Street is pessimistic about Belviq's potential. While Arena's supporters may see that pessimism as unfair, it is important to know that what analysts think matters. The reason why is not because they are correct, or offer a perfectly accurate forecast. The reason is that institutional investors, the ones that drive money flows in the market, buy into what analysts say. That is why if analysts are pessimistic about Arena, institutional investors are likely to be as well. Retail investors in Arena need to understand this. You may be correct in your profit estimates for Arena, but until analysts begin raising estimates for Arena, institutional investors will almost certainly maintain a skeptical view of the company, even if it is unwarranted.
With Arena posting consistent losses, its cash is being burned away. In the last quarter, Arena burned just over $22 million, and that is set to continue. However, Arena has over $88 milliion in cash, and raised $69.6 million in gross proceeds from a stock offering in May. At its current burn rate, Arena has about 7 quarters of cash left. However, as Belviq sales begin in early 2013, and payments from Eisai start to arrive, there will be less pressure on Arena's cash position. We would like to hear Arena's management talk about the company's capital plans when Q2 2012 earnings are released. In our view, what the loss is (the Reuters consensus calls for a loss of 8 cents per share) is not important. What Arena says on the conference call is much more important, and we will be listening closely.
Patents and Pipelines
Shares of Vivus were slammed on July 19 after Citron Research published a detailed report outlining the weak patent protection of Qsymia. While whether or not Citron's claims are valid is an issue for another article, it is important to note that Arena seems to have all of its ducks in line when it comes to Belviq's intellectual property. Arena has patents for Belviq in all major markets, including the United States, Japan, China, and Europe, with the first expiration set for 2023.
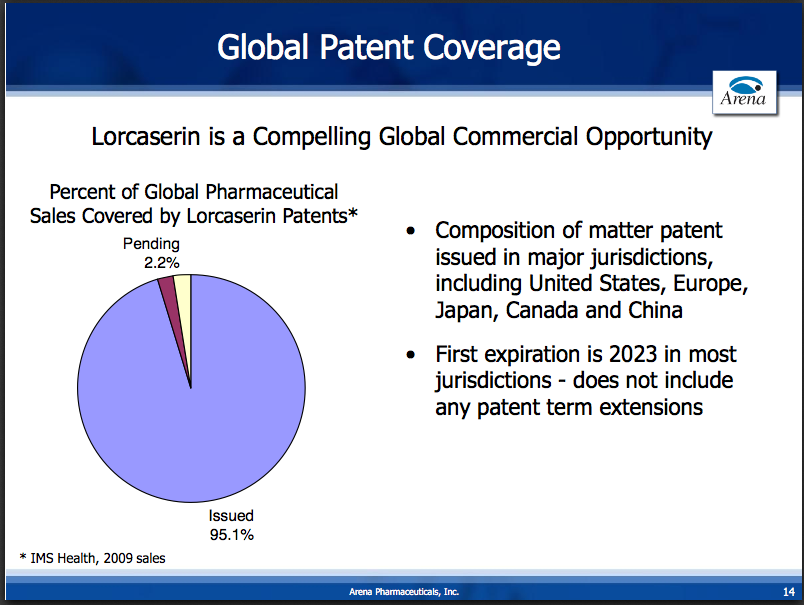
Now that Belviq has been approved, focus can return to the rest of Arena's pipeline. Arena has 4 compounds in various, albeit early stages of development, with APD811, for the treatment of pulmonary arterial hypertension being the furthest along. APD811 is in Phase I trials, and the other 3 compounds are in the research and preclinical phases. While these compounds are far from potential approval, focus can now return to them, from the perspectives of both Arena itself and its investors. We expect the company to provide some sort of pipeline update on its upcoming earnings call, as almost no company [aside from Alexion Pharmaceuticals (ALXN), which sells Soliris] can truly succeed by selling just one drug. Arena will, sooner or later, have to diversify away from Belviq. With Qsymia now approved and Orexigen's (OREX) Contrave in late-stage trials, competition will be fierce in the obesity drug space. Arena has 4 products in the pipeline, while Vivus and Orexigen each have 2. For Orexigen, however, Contrave is one of those 2 drugs, making the company even more reliant on its success than Arena or Vivus are on their obesity drugs (and Orexigen's other pipeline drug is for obesity as well). And Vivus also sells Stendra, for the treatment of erectile dysfunction, which was approved at the end of April by the FDA. Arena's pipeline is deeper than that of either Vivus or Orexigen, which gives investors more potential ways to profit, as there are more chances for a successful clinical trial.
Conclusions
Arena Pharmaceuticals has certainly seen a great deal of drama in the past few years. Now that Belviq is approved in the United States, the company is entering the next phase of its life. As Belviq begins selling, the focus will shift to Arena's financials, and the company will have to show that it and Eisai can make Belviq a success. In addition, a renewed focus on Arena's pipeline will bring more potential opportunities for both the company and its investors. Depending on how well Belviq sells, analysts may ratchet up earnings estimates, which would cause institutional investors to re-examine their view of Arena Pharmaceuticals. That could provide another catalyst for the shares to rise. The past few months and years have certainly been interesting ones for Arena Pharmaceuticals. And we expect that the road ahead will be just as interesting.
http://seekingalpha.com/article/734891-arena-pharmaceuticals…
July 20, 2012
In a market of thousands of stocks, somehow a select minority generate a majority of debate and arguments. The passions surrounding these stocks often reach fevered levels, and it is important for investors to maintain calm while analyzing a stock. That is true for every company, including Arena Pharmaceuticals (ARNA).
Arena is a company that generates fierce reactions from investors on both sides. After all, when a stock rises by over 400% year-to-date, that is to be expected.

With Arena's first product, Belviq, now approved by the FDA for "chronic weight management," the company has entered a new phase. Going forward, Arena will rise and fall alongside Belviq's results. That is what is needed to mitigate the long-term losses in the stock. How much money you made in Arena from the approval of Belviq depends on when you first bough the shares. Though the stock may be up over 400% in 2012, it is down over 60% since going public.

In our view, the easy money has been made here. Now, it is time for Arena to prove that the drug it has spent years and enormous sums of money developing can be a blockbuster. So where exactly do we go from here? Arena is transitioning away from a developmental stage company into a commercial biotechnology company, and as such, now faces a different set of expectations.
Belviq: Manufacturing a Blockbuster and a Partnership
Belviq is set to be available in early 2013, and will be manufactured in Switzerland and sold to Eisai, which has marketing and distribution rights to Belviq in North America (because of the way Belviq works, it has the potential to be abused, which is why the DEA must rule on how to schedule the drug. Should the DEA process go faster than expected, Belviq may begin selling earlier than that). It is this partnership with Eisai, which was struck in 2010, that we would like to focus on, for it will be one of the most important aspects of the Arena story going forward. Under the terms of the deal, Arena will manufacture Belviq and then sell it to Eisai, which will then resell it in the United States, by far the biggest market for Belviq.
Arena is set to receive anywhere between 31.5% and 36.5% of Eisai's net Belviq sales in America. The rate rises to 36.5% above net Belviq sales of $750 million.

Arena is also set to receive over $1 billion in price adjustment payments, and Eisai bears the majority of post-approval clinical trial costs. It is responsible for 90% of cardiovascular outcome trials (CVOT) and half of the expenses for pediatric studies. After striking this agreement, Arena and Eisai expanded it to cover the rest of North and South America, with Arena's payment percentage set at 30.75%-35.75% of net sales in those countries.

Arena is once again set to bear only a minority of regulatory and post-approval expenses, be they marketing or clinical. Essentially, Arena is receiving an extremely high royalty rate on Belviq, as it will have little in the way of expenses to pay relating to Belviq. That will free up Arena to return focus to its pipeline, where there are several projects in the works. But first, we need to discuss road ahead for Belviq, as well as the company's financials.
Belviq Goes International
With Belviq now approved in the United States, Arena can turn its attention to expanding internationally. In March, the company filed for approval of the drug with the EMA (European Medicines Agency). England is the company's rapporteur and Sweden is the company's co-rapporteur. This is a Europe-specific requirement for drug approval. Rapporteurs are country-specific regulators within the Europan Union that help the CHMP (the Committee for Medicinal Products for Human Use), which reviews and evaluates marketing applications. Rapporteurs perform scientific evaluations of a marketing application and present an assessment of it the CHMP, which then takes it into account as part of its decision making process. The review process in the European Union will be centralized, meaning that approval will be valid throughout the entire European Union, as opposed to just a subset of member states. Arena has not given an timeline as to when it expects a decision regarding Belviq from the EMA, However, it has stated that Day 120 questions in late July. On July 11, Arena also filed for approval of Belviq in Switzerland, but declined to give a timeline for potential approval.
As Arena notes, obesity rates in many European countries have tripled in the past 30 years, which sets up an opportunity for Belviq. For the record, Vivus (VVUS) is set to present data to the CHMP in September regarding Qsymia. We expect to hear more about what is going on in Europe from Arena when it reports its second quarter results in August (an exact date has not yet been set). In addition, Arena has said that it is looking for a European partner for Belviq, and we expect an update on that front during the upcoming earnings conference call as well.
Financials: When the Rubber Meets the Road
Going forward, Arena's stock will not rise and fall simply on what happens with Belviq on the regulatory front. When the drug launches, Arena will be judged on how well it sells, and not if the company can get the drug approved. In the years to come, that is what will drive the stock.
Arena has lost well over $1 billion since it was founded due to the costs of developing Belviq. And in its last quarter, the company lost another $29.4 million, or 18 cents per share. However, with Belviq now approved in the United States, the company appears to be on a path to profitability. However, consensus estimates (based on data from Reuters) call for a loss of 33 cents per share in 2012, and 17 cents in 2013.

Bloomberg's consensus estimates for Arena are more pessimistic, with the consensus for 2012 being a loss of 46 cents per share, and 29 cents in 2013. While some analysts forecast 2014 as the year that Arena will post a profit (Merrill Lynch estimates a 1 cent per share profit for 2014), Wall Street is still pessimistic, with the Bloomberg consensus estimate calling for a loss of 8 cents in 2014.

Wall Street is pessimistic about Belviq's potential. While Arena's supporters may see that pessimism as unfair, it is important to know that what analysts think matters. The reason why is not because they are correct, or offer a perfectly accurate forecast. The reason is that institutional investors, the ones that drive money flows in the market, buy into what analysts say. That is why if analysts are pessimistic about Arena, institutional investors are likely to be as well. Retail investors in Arena need to understand this. You may be correct in your profit estimates for Arena, but until analysts begin raising estimates for Arena, institutional investors will almost certainly maintain a skeptical view of the company, even if it is unwarranted.
With Arena posting consistent losses, its cash is being burned away. In the last quarter, Arena burned just over $22 million, and that is set to continue. However, Arena has over $88 milliion in cash, and raised $69.6 million in gross proceeds from a stock offering in May. At its current burn rate, Arena has about 7 quarters of cash left. However, as Belviq sales begin in early 2013, and payments from Eisai start to arrive, there will be less pressure on Arena's cash position. We would like to hear Arena's management talk about the company's capital plans when Q2 2012 earnings are released. In our view, what the loss is (the Reuters consensus calls for a loss of 8 cents per share) is not important. What Arena says on the conference call is much more important, and we will be listening closely.
Patents and Pipelines
Shares of Vivus were slammed on July 19 after Citron Research published a detailed report outlining the weak patent protection of Qsymia. While whether or not Citron's claims are valid is an issue for another article, it is important to note that Arena seems to have all of its ducks in line when it comes to Belviq's intellectual property. Arena has patents for Belviq in all major markets, including the United States, Japan, China, and Europe, with the first expiration set for 2023.

Now that Belviq has been approved, focus can return to the rest of Arena's pipeline. Arena has 4 compounds in various, albeit early stages of development, with APD811, for the treatment of pulmonary arterial hypertension being the furthest along. APD811 is in Phase I trials, and the other 3 compounds are in the research and preclinical phases. While these compounds are far from potential approval, focus can now return to them, from the perspectives of both Arena itself and its investors. We expect the company to provide some sort of pipeline update on its upcoming earnings call, as almost no company [aside from Alexion Pharmaceuticals (ALXN), which sells Soliris] can truly succeed by selling just one drug. Arena will, sooner or later, have to diversify away from Belviq. With Qsymia now approved and Orexigen's (OREX) Contrave in late-stage trials, competition will be fierce in the obesity drug space. Arena has 4 products in the pipeline, while Vivus and Orexigen each have 2. For Orexigen, however, Contrave is one of those 2 drugs, making the company even more reliant on its success than Arena or Vivus are on their obesity drugs (and Orexigen's other pipeline drug is for obesity as well). And Vivus also sells Stendra, for the treatment of erectile dysfunction, which was approved at the end of April by the FDA. Arena's pipeline is deeper than that of either Vivus or Orexigen, which gives investors more potential ways to profit, as there are more chances for a successful clinical trial.
Conclusions
Arena Pharmaceuticals has certainly seen a great deal of drama in the past few years. Now that Belviq is approved in the United States, the company is entering the next phase of its life. As Belviq begins selling, the focus will shift to Arena's financials, and the company will have to show that it and Eisai can make Belviq a success. In addition, a renewed focus on Arena's pipeline will bring more potential opportunities for both the company and its investors. Depending on how well Belviq sells, analysts may ratchet up earnings estimates, which would cause institutional investors to re-examine their view of Arena Pharmaceuticals. That could provide another catalyst for the shares to rise. The past few months and years have certainly been interesting ones for Arena Pharmaceuticals. And we expect that the road ahead will be just as interesting.
http://seekingalpha.com/article/734891-arena-pharmaceuticals…
...hier ein detailierter Bericht über VIVUS...
....da geht es u.a auch über Patentfragen und möglicherweise Verstöße gegen Patentrechte.....
Vivus (NASDAQ:VVUS): Why FDA Approval Is Not the Prescription
While the company has been busy furthering their FDA submission and selling stock, Vivus has simply avoided confronting the reality that they might not even be able to go to market.
The foundation stone of Vivus' IP protection is a patent licensing deal signed a decade ago with a Dr. Najarian, an obesity doctor and researcher who filed as sole inventor for Qsymia's two-drug combo. One question that has been hanging over the company for years is the possibility of patent infringement on one of Qsymia's two active ingredients, topiramate, a patent held by Johnson & Johnson, who used to sell the drug as an obesity treatment before it went generic. It affords patent protection until 2017.
It originally was filed by a researcher named Shank on June 29, 1996 (US PTO No. 6,071,537)[/i]
There is no evidence of the validity of this patent ever being challenged, either at the USPTO or the corresponding EP patent (EP0915697) filed here. There are no mentions in Vivus' filings of the Shank patent, and therefore no clues as to how Vivus plans to deal with it.
How exactly does Vivus overcome the Shank patent to sell topiramate for weight loss? Citron rates this unresolved question a substantial risk factor.
Vivus has had not just the last 5 months, but 5 years since questions about the Najarian, Shank and McElroy patents were posed by analysts, to create enough clarity to go to market.
Yet to this day, all we hear from the company is assurances that they have legal opinions in hand stating "they are OK". No joint ventures, no counterparty agreements, no releases.
Quite a cloud of uncertainty for a "multi-billion dollar opportunity" resting on the combination of two common generic drugs, don't you think?
If this were a solid pharma with high management credibility, maybe investors could give them the benefit of the doubt. But this management?
Conclusion
Despite years of lead time, Vivus' executives have failed to provide strong patent protection for Qsymia. Taken together, these points provide the best explanation yet why Vivus hasn't been able to recruit a high-profile deep-pocketed pharma partner to leverage its expertise while taking some of the risk of marketing its newly approved diet combo drug.
Instead of selling a stake to a credible pharma marketer, they sold stock – over $230 million to …. you guessed it. The hope that a big pharma company is going to ride in pay $3 billion for Vivus is ludicrous – the strategy of trying to pierce its patent protection for a few million in legal fees is a far lower risk.
The more marketing hype about the FDA approval of this combination of two well-known and inexpensive legacy generic drugs, the more Vivus sets up its own ambush – by legal challenges to its patent, and generic drug competition starting before they can even get to market.
http://www.citronresearch.com/index.php/2012/07/19/vivus-why…
....da geht es u.a auch über Patentfragen und möglicherweise Verstöße gegen Patentrechte.....
Vivus (NASDAQ:VVUS): Why FDA Approval Is Not the Prescription
While the company has been busy furthering their FDA submission and selling stock, Vivus has simply avoided confronting the reality that they might not even be able to go to market.
The foundation stone of Vivus' IP protection is a patent licensing deal signed a decade ago with a Dr. Najarian, an obesity doctor and researcher who filed as sole inventor for Qsymia's two-drug combo. One question that has been hanging over the company for years is the possibility of patent infringement on one of Qsymia's two active ingredients, topiramate, a patent held by Johnson & Johnson, who used to sell the drug as an obesity treatment before it went generic. It affords patent protection until 2017.
It originally was filed by a researcher named Shank on June 29, 1996 (US PTO No. 6,071,537)[/i]
There is no evidence of the validity of this patent ever being challenged, either at the USPTO or the corresponding EP patent (EP0915697) filed here. There are no mentions in Vivus' filings of the Shank patent, and therefore no clues as to how Vivus plans to deal with it.
How exactly does Vivus overcome the Shank patent to sell topiramate for weight loss? Citron rates this unresolved question a substantial risk factor.
Vivus has had not just the last 5 months, but 5 years since questions about the Najarian, Shank and McElroy patents were posed by analysts, to create enough clarity to go to market.
Yet to this day, all we hear from the company is assurances that they have legal opinions in hand stating "they are OK". No joint ventures, no counterparty agreements, no releases.
Quite a cloud of uncertainty for a "multi-billion dollar opportunity" resting on the combination of two common generic drugs, don't you think?
If this were a solid pharma with high management credibility, maybe investors could give them the benefit of the doubt. But this management?
Conclusion
Despite years of lead time, Vivus' executives have failed to provide strong patent protection for Qsymia. Taken together, these points provide the best explanation yet why Vivus hasn't been able to recruit a high-profile deep-pocketed pharma partner to leverage its expertise while taking some of the risk of marketing its newly approved diet combo drug.
Instead of selling a stake to a credible pharma marketer, they sold stock – over $230 million to …. you guessed it. The hope that a big pharma company is going to ride in pay $3 billion for Vivus is ludicrous – the strategy of trying to pierce its patent protection for a few million in legal fees is a far lower risk.
The more marketing hype about the FDA approval of this combination of two well-known and inexpensive legacy generic drugs, the more Vivus sets up its own ambush – by legal challenges to its patent, and generic drug competition starting before they can even get to market.
http://www.citronresearch.com/index.php/2012/07/19/vivus-why…
...und noch eine NEWS zu den möglichen Patentproblematiken sowohl bei VIVUS als auch bei OREXIGEN....
Vivus falls on concerns of patent protection for diet pill
Thu Jul 19, 2012 4:12pm EDT
(Reuters) - Shares of Vivus Inc fell sharply on Thursday afternoon after well-known short-seller Citron Research cast doubts on the drugmaker's ability to protect its weight-loss pill's patents.
Citron in a report on its website pointed out the possibility that Johnson & Johnson could challenge Vivus in court over patents that cover usage of anti-seizure drug topiramate -- one of the key ingredients in Vivus' freshly approved Qsymia.
The Citron report comes two days after U.S. health regulators approved Qsymia for use in obese and overweight adults who have at least one weight-related condition. The drug was expected to have the potential to bring in more than $1 billion in sales.
Citron is run by California-based investor and notable short-seller Andrew Left.
It also pointed to possible problems with another Vivus patent, with claims related to methods for treating disorders that include obesity "using sulfamates (including topiramate) in combination with psychostimulants (including phentermine)".
Qsymia's second important ingredient is phentermine. The brokerage points to possible copy-cat versions eating up Qsymia's market as the product is made of two key ingredients, which are already available in the market as generics.
Competitor Orexigen Therapeutics Inc's shares also fell 10 percent after the report went public. Orexigen's experimental drug, Contrave, is a combination of slow release versions of naltrexone and a mild psychostimulant, bupropion.
Orexigen is currently conducting a trial to study risks of heart complications from using Contrave.
Shares of Arena Pharmaceuticals Inc -- the first drugmaker to get a diet pill approved in 13 years -- were down 2 percent at $9.75 on the Nasdaq. Arena got the approval late last month.
Vivus shares, which had gained 10 percent in value a day after its diet pill was approved, fell 13 percent to a low of $25.15 in afternoon trading.
(Reporting by Vidya P L Nathan in Bangalore; Editing by Maju Samue
http://www.reuters.com/article/2012/07/19/us-vivus-shares-id…
Vivus falls on concerns of patent protection for diet pill
Thu Jul 19, 2012 4:12pm EDT
(Reuters) - Shares of Vivus Inc fell sharply on Thursday afternoon after well-known short-seller Citron Research cast doubts on the drugmaker's ability to protect its weight-loss pill's patents.
Citron in a report on its website pointed out the possibility that Johnson & Johnson could challenge Vivus in court over patents that cover usage of anti-seizure drug topiramate -- one of the key ingredients in Vivus' freshly approved Qsymia.
The Citron report comes two days after U.S. health regulators approved Qsymia for use in obese and overweight adults who have at least one weight-related condition. The drug was expected to have the potential to bring in more than $1 billion in sales.
Citron is run by California-based investor and notable short-seller Andrew Left.
It also pointed to possible problems with another Vivus patent, with claims related to methods for treating disorders that include obesity "using sulfamates (including topiramate) in combination with psychostimulants (including phentermine)".
Qsymia's second important ingredient is phentermine. The brokerage points to possible copy-cat versions eating up Qsymia's market as the product is made of two key ingredients, which are already available in the market as generics.
Competitor Orexigen Therapeutics Inc's shares also fell 10 percent after the report went public. Orexigen's experimental drug, Contrave, is a combination of slow release versions of naltrexone and a mild psychostimulant, bupropion.
Orexigen is currently conducting a trial to study risks of heart complications from using Contrave.
Shares of Arena Pharmaceuticals Inc -- the first drugmaker to get a diet pill approved in 13 years -- were down 2 percent at $9.75 on the Nasdaq. Arena got the approval late last month.
Vivus shares, which had gained 10 percent in value a day after its diet pill was approved, fell 13 percent to a low of $25.15 in afternoon trading.
(Reporting by Vidya P L Nathan in Bangalore; Editing by Maju Samue
http://www.reuters.com/article/2012/07/19/us-vivus-shares-id…
@AREANICS-Longies......SIERRA WORLD ist mal wieder aktiv  ..
..
...ich gebe euch nun mal ein kleinen SIERRA WORLD ADRENALIN-Stoß fürs Wochenende........
Saturday, July 21, 2012
TOP FOUR REASONS -Arena Pharmaceuticals (ARNA) will become a $100 stock forecasts accurate phenom Sierra World Equity Review
forecasts accurate phenom Sierra World Equity Review
!!
Arena Pharmaceuticals "STOCK OF THE YEAR"-- a $100 Stock in the Making !!
Reason ONE:
-Currently there are only 2 FDA approved obesity drugs, Belviq appears to be the more attractive choice, it's competition Qsymia should NOT be used by pregnant women says the FDA. Nor should it be taken by people who have had a recent stroke or unstable heart disease as it can speed up heart rate or be taken by people with glaucoma or hyperthyroidism. Sierra World Equity Review believes sales of Belviq will dominate those of Qsymia.
Reason TWO:
-Obesity rates are rising around the world especially in China where an emerging middle class has a rising obesity level. To put that into context, by 2015 there will be more obese people in China than the ENTIRE POPULATION of the United States. Revenue from sales of Belviq in China alone would make Arena a $100 stock. Belviq will be available in China, Japan, the European Union, North America and South America.
... this is not a nine or ten or even 20 dollar stock....easily going to $100 and MORE when the sales get going! Even with Eisai the Japanese drug partner reaping a nice profit from Arena Pharmaceuticals Belviq, the revenues are just going to be so massive, nothing can stop this stock!
Reason THREE:
-The BUYOUT, with Pfizer and other major players focusing on Arena Pharmaceuticals, it has become the hottest biotech potential BUYOUT this year! For current shareholders of Arena that translates into a huge windfall.........
Sierra World Equity Review predicts that the day after buyout the stock will open at $60 and race to $100 over the next 4 quarters as those revenues start to pour in!......ich denke, damit könnten wir leben...
One can't help feeling a little envious of the fortunate Arena shareholders who had the foresight and wisdom or luck to invest in Arena when it was dirt cheap.....Sierra World Equity Review is happy for them and applauds their success.
Reason FOUR:
-Belviq will be very inexpensive, costing between perhaps 100-200 dollars per month. In prescription drug terms this is cheap and insurance companies will be cover the cost. The cost benefit for insurance companies is a no brainer....it's far more lucrative to pay for inexpensive weight loss medication than have to cover severe medical conditions that may result from obesity if left untreated. Sierra World Equity Review predicts that insurance companies will see the bottom line and WILL FULLY COVER Belviq as a treatment for obesity.
http://sierraworldequityreview.blogspot.de/2012/07/breakingw…
 ..
.....ich gebe euch nun mal ein kleinen SIERRA WORLD ADRENALIN-Stoß fürs Wochenende........

Saturday, July 21, 2012
TOP FOUR REASONS -Arena Pharmaceuticals (ARNA) will become a $100 stock
 forecasts accurate phenom Sierra World Equity Review
forecasts accurate phenom Sierra World Equity Review!!
Arena Pharmaceuticals "STOCK OF THE YEAR"-- a $100 Stock in the Making !!
Reason ONE:
-Currently there are only 2 FDA approved obesity drugs, Belviq appears to be the more attractive choice, it's competition Qsymia should NOT be used by pregnant women says the FDA. Nor should it be taken by people who have had a recent stroke or unstable heart disease as it can speed up heart rate or be taken by people with glaucoma or hyperthyroidism. Sierra World Equity Review believes sales of Belviq will dominate those of Qsymia.
Reason TWO:
-Obesity rates are rising around the world especially in China where an emerging middle class has a rising obesity level. To put that into context, by 2015 there will be more obese people in China than the ENTIRE POPULATION of the United States. Revenue from sales of Belviq in China alone would make Arena a $100 stock. Belviq will be available in China, Japan, the European Union, North America and South America.
... this is not a nine or ten or even 20 dollar stock....easily going to $100 and MORE when the sales get going! Even with Eisai the Japanese drug partner reaping a nice profit from Arena Pharmaceuticals Belviq, the revenues are just going to be so massive, nothing can stop this stock!
Reason THREE:
-The BUYOUT, with Pfizer and other major players focusing on Arena Pharmaceuticals, it has become the hottest biotech potential BUYOUT this year! For current shareholders of Arena that translates into a huge windfall.........
Sierra World Equity Review predicts that the day after buyout the stock will open at $60 and race to $100 over the next 4 quarters as those revenues start to pour in!......ich denke, damit könnten wir leben...

One can't help feeling a little envious of the fortunate Arena shareholders who had the foresight and wisdom or luck to invest in Arena when it was dirt cheap.....Sierra World Equity Review is happy for them and applauds their success.
Reason FOUR:
-Belviq will be very inexpensive, costing between perhaps 100-200 dollars per month. In prescription drug terms this is cheap and insurance companies will be cover the cost. The cost benefit for insurance companies is a no brainer....it's far more lucrative to pay for inexpensive weight loss medication than have to cover severe medical conditions that may result from obesity if left untreated. Sierra World Equity Review predicts that insurance companies will see the bottom line and WILL FULLY COVER Belviq as a treatment for obesity.
http://sierraworldequityreview.blogspot.de/2012/07/breakingw…
Zitat von bernie55: Sierra World Equity Review predicts that the day after buyout the stock will open at $60 and race to $100 over the next 4 quarters as those revenues start to pour in!
......ich denke, damit könnten wir leben...
http://sierraworldequityreview.blogspot.de/2012/07/breakingw…
......die TOP "Hausnummernprognosen" hat SIERRA wohl doch ein wenig die Birne zugenebelt.......mir übrigens auch...

..also , wenn ein BUYOUT kommt, dann steht der Preis einer Aktie fest und kann deshalb nicht noch bis 100 USD steigen...
Aber vielleicht meinten sie auch, dass "nach einen möglichen beginnenden "BUYOUT-BATTLE" 60 USD das erste Angebot sein wird....


Suzhou Connect Biopharmaceuticals In-Licenses Anti-Inflammatory Compounds from Arena Pharmaceuticals
TAICANG, Suzhou, China, July 24, 2012 /PRNewswire-iReach/ --
Suzhou Connect Biopharmaceuticals, Ltd. today announced it, through its wholly owned subsidiary Connect Biopharm LLC in San Diego, has entered into an exclusive worldwide licensing agreement with Arena Pharmaceuticals, Inc., for the development and commercialization of novel small molecule compounds intended for the treatment of allergic rhinitis and atopic dermatitis.
The allergic inflammation program was initiated and led by Zheng Wei, Ph.D., co-founder and Chief Executive Officer of Suzhou Connect and the former Director of Immunology at Arena. Intellectual property assets under the licensing agreement include two series of anti-inflammatory compounds as well as a new method for treating diseases. The molecular targets and financial terms of the agreement were not disclosed.
"We are extremely excited to acquire the rights to these promising preclinical drug candidates and this method of treatment from Arena, a recognized leader in GPCR drug discovery and development," said Dr. Wei. "Allergic rhinitis and atopic dermatitis together afflict more than one third of the world's population, and current treatments have severe limitations. This agreement provides us with a unique opportunity to address such limitations by advancing drug candidates with novel mechanisms of action. It comes on the heels of our recent financing round, which enables us to fund the program into clinical proof-of-concept studies."
"We look forward to Connect's advancement of the novel anti-inflammatory compounds that comprise this program," said Dominic P. Behan, Ph.D., Arena's Executive Vice President and Chief Scientific Officer.
About Suzhou Connect Biopharmaceuticals
Connect is a startup biopharmaceutical company dedicated to the discovery and development of novel therapeutics targeting G-protein coupled receptors (GPCR) for the treatment of autoimmune diseases, allergy and cancer. The company was founded by Dr. Zheng Wei, formerly Director of Immunology at Arena Pharmaceuticals, Inc., and Dr. William (Wubin) Pan, co-founder and formerly China President and Chief Operation Officer of Crown Biosciences, Inc., with investment from Xiang Tang Group. In addition to developing drug candidates through in-licensing, the company uses its proprietary GMab technology to discover and develop therapeutic antibodies against GPCR drug targets.
About Arena Pharmaceuticals
Arena Pharmaceuticals, Inc., is a biopharmaceutical company focused on discovering, developing and commercializing oral drugs that target G protein-coupled receptors, an important class of validated drug targets, in four major therapeutic areas: cardiovascular, central nervous system, inflammatory and metabolic diseases. For more information about Arena Pharmaceuticals, please visit www.arenapharm.com.
http://www.prnewswire.com/news-releases/suzhou-connect-bioph…" target="_blank" rel="nofollow ugc noopener">
http://www.prnewswire.com/news-releases/suzhou-connect-bioph…
Eisai and Arena Pharmaceuticals Complete Transfer of BELVIQ® (lorcaserin HCl) New Drug Application
WOODCLIFF LAKE, N.J., and SAN DIEGO, July 30, 2012 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) announced today that Arena has transferred the BELVIQ® (lorcaserin hydrochloride) New Drug Application (NDA) to Eisai. The transfer establishes Eisai as the marketing authorization holder responsible for regulatory activities in the United States related to the commercialization of BELVIQ, including pharmacovigilance requirements.
Arena will continue in its oversight role for the global development of BELVIQ while working closely with Eisai for the joint development plan in the United States.
"The NDA transfer furthers our commitment to BELVIQ as we prepare to launch in the United States and submit applications for approval throughout the Americas," said Lonnel Coats, President and Chief Executive Officer, Eisai Inc.
Eisai plans to submit marketing authorization applications based on the NDA to the health authorities throughout the territories in North and South America for which it currently holds commercialization rights, including Mexico, Brazil and Canada.
"We look forward to further exploring the therapeutic potential of BELVIQ and supporting Eisai's efforts to obtain marketing approval in additional North and South American countries," said Jack Lief, Arena's President and Chief Executive Officer.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-co…
WOODCLIFF LAKE, N.J., and SAN DIEGO, July 30, 2012 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) announced today that Arena has transferred the BELVIQ® (lorcaserin hydrochloride) New Drug Application (NDA) to Eisai. The transfer establishes Eisai as the marketing authorization holder responsible for regulatory activities in the United States related to the commercialization of BELVIQ, including pharmacovigilance requirements.
Arena will continue in its oversight role for the global development of BELVIQ while working closely with Eisai for the joint development plan in the United States.
"The NDA transfer furthers our commitment to BELVIQ as we prepare to launch in the United States and submit applications for approval throughout the Americas," said Lonnel Coats, President and Chief Executive Officer, Eisai Inc.
Eisai plans to submit marketing authorization applications based on the NDA to the health authorities throughout the territories in North and South America for which it currently holds commercialization rights, including Mexico, Brazil and Canada.
"We look forward to further exploring the therapeutic potential of BELVIQ and supporting Eisai's efforts to obtain marketing approval in additional North and South American countries," said Jack Lief, Arena's President and Chief Executive Officer.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-co…
Zitat von bernie55: Eisai and Arena Pharmaceuticals Complete Transfer of BELVIQ® (lorcaserin HCl) New Drug Application
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-co…
....hier Auszüge von einem Kommentar aus dem Seeking Alpha zum oben erwähnten Artikel...
Arena Transfers BELVIQ To Eisai - What It Means
July 30, 2012
The most interesting part of the regulation is that the new owner has to agree to take over "agreements, promises, and conditions made by the former owner". This includes all post marketing studies and all pharmacovigilance (reporting of adverse events to FDA) requirements.
What is not explicitly stated in today's announcement is that Eisai, not Arena, will be running all of the pediatric and cardiovascular outcomes trials that are required according to BELVIQ's FDA approval letter (pdf).
Two things strike me about this job posting in light of today's announcement.
First, it is clear that Eisai has been preparing for this transfer of the BELVIQ NDA for at least a few weeks now including addressing staffing needs. Second, the job description above specifically mentions Diabetes. In an earlier article I mentioned that the astounding Hba1c results seen in the BLOOM-DM trials meant that Arena and Eisai would eventually pursue a diabetes indication for BELVIQ. This job description confirms my speculation that exploring a diabetes indication for BELVIQ is probably part of the development plan.
The key takeaway from today's announcement is that Arena and Eisai are further cementing their working relationship through this transfer. The dedicated research team at Arena is being relived of administrative burdens related to marketing BELVIQ so that they can focus on developing the rest of Arena's pipeline.
ARNA longs would do well to tamper their speculation that another large pharmaceutical company will swoop in and buyout Arena. It could happen since both Arena and Eisai have the right to terminate their partnership under certain circumstances and since Arena has not given Eisai the rights to BELVIQ in Europe or the rest of the world, but it is highly unlikely.
A much more likely scenario is that Eisai will eventually acquire Arena once initial sales numbers come in and EU approval is obtained.
http://seekingalpha.com/article/761691-arena-transfers-belvi…
My Conservative Revenue Model For Arena
August 9, 2012
Edited by Kate Boehme
Arena Pharmaceuticals (ARNA) shares have taken a hit recently as investors show concern over the rising competition posed by Vivus (VVUS). In response, Arena has announced an expanded marketing and supply agreement with Eisai. In my previous article, I presented a very simple and straight-forward revenue model for Vivus Inc. Here, a similar effort will be made to analyze the market share and revenue potential of Arena within the domestic market.
The assumptions posed by this model are very straightforward and easy to understand. I have made an effort to refrain from complex methodologies and assumptions. As there is no information available on Arena's pricing strategy, the assumptions concerning revenue are based on the market share and ignore the cost component.
The U.S. obesity market is currently valued at $1.2 billion. In 2011, drug companies achieved revenues of $222 million, and the market is expected to grow at 20 percent annually. Arena has given the domestic marketing rights to Eisai Inc. Arena will therefore get 31.5 percent of the net sales from Eisai as well as an additional 36.5 percent of any sales above $750 million. This deal makes Eisai the primary operator in the domestic obesity market. Therefore, in order to accurately assess Arena's revenues, we need to calculate the market share and sales estimates for Eisai and then filter out the numbers.
This particular model represents a scenario analysis; three scenarios are considered, each with their own probability. The first scenario is the best-case scenario. In the best case, Eisai Inc performs ahead of the competition and, at its peak, captures a market share of 80 percent. As the name suggests, moderate-case scenario gives Eisai Inc a moderate market share, wherein its market share is nearly equal to that of its key competitor. Lastly, the third scenario puts Eisai and Belviq in the worst position in terms of market share and revenues.
Dropout rate for both of my models is quite high at 50 percent. The reason behind keeping the dropout rate high and constant for both companies is that they both face certain challenges. Vivus carries a perhaps less-than-ideal label, which could potentially pose certain side effects for pregnant women. On the other hand, Arena has issues with the efficacy of their drug. It can thus be assumed that this high dropout rates assigned to both companies creates a level playing field.
(Tabelle siehe link)
It must be mentioned that this model only represents the revenue figures for the domestic market. Arena still has the marketing rights for Europe and other territories, and their revenues will surely increase once the company secures approval for these territories. Eisai may also try to market the drug to patients with diabetes as Belviq showed Hba1c reduction of 0.9 percent, promising results during upcoming clinical trials. However, these points are moot with relation to this particular model as, for simplicity's sake, it takes into account only the domestic obesity market.
It is clear from the model that Arena will be able to garner revenues of around $450 million at its peak. Manufacturing costs will comprise 20 percent of net sales. However, results would be better if Arena had exclusive rights for the domestic market. While profits may have been higher if this had been the case, understandably Arena chose a less risky route for the marketing of their first approved drug by partnering with an experienced player. In this manner, Arena was able to shift most of the risks to Eisai Inc.
Arena vs. Vivus
When comparing these two companies directly, it is clear that both parties carry their own unique advantages. While Vivus has a more effective product, Arena has a product with a safer profile. One thing particularly in favor of Vivus is that it has an established pipeline and is not reliant on a single drug for revenue.
In the future, it can be expected that both companies will emerge ahead, as the obesity market is desperate for an effective drug. Though both of these stocks may show some volatility in the short-term, the long run prospects are optimistic. However, it is important to also consider that there always remains the possibility of a takeover; both companies have drugs that could prove to be a vital component of any big player's portfolio.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/793311-my-conservative-reven…
August 9, 2012
Edited by Kate Boehme
Arena Pharmaceuticals (ARNA) shares have taken a hit recently as investors show concern over the rising competition posed by Vivus (VVUS). In response, Arena has announced an expanded marketing and supply agreement with Eisai. In my previous article, I presented a very simple and straight-forward revenue model for Vivus Inc. Here, a similar effort will be made to analyze the market share and revenue potential of Arena within the domestic market.
The assumptions posed by this model are very straightforward and easy to understand. I have made an effort to refrain from complex methodologies and assumptions. As there is no information available on Arena's pricing strategy, the assumptions concerning revenue are based on the market share and ignore the cost component.
The U.S. obesity market is currently valued at $1.2 billion. In 2011, drug companies achieved revenues of $222 million, and the market is expected to grow at 20 percent annually. Arena has given the domestic marketing rights to Eisai Inc. Arena will therefore get 31.5 percent of the net sales from Eisai as well as an additional 36.5 percent of any sales above $750 million. This deal makes Eisai the primary operator in the domestic obesity market. Therefore, in order to accurately assess Arena's revenues, we need to calculate the market share and sales estimates for Eisai and then filter out the numbers.
This particular model represents a scenario analysis; three scenarios are considered, each with their own probability. The first scenario is the best-case scenario. In the best case, Eisai Inc performs ahead of the competition and, at its peak, captures a market share of 80 percent. As the name suggests, moderate-case scenario gives Eisai Inc a moderate market share, wherein its market share is nearly equal to that of its key competitor. Lastly, the third scenario puts Eisai and Belviq in the worst position in terms of market share and revenues.
Dropout rate for both of my models is quite high at 50 percent. The reason behind keeping the dropout rate high and constant for both companies is that they both face certain challenges. Vivus carries a perhaps less-than-ideal label, which could potentially pose certain side effects for pregnant women. On the other hand, Arena has issues with the efficacy of their drug. It can thus be assumed that this high dropout rates assigned to both companies creates a level playing field.
(Tabelle siehe link)
It must be mentioned that this model only represents the revenue figures for the domestic market. Arena still has the marketing rights for Europe and other territories, and their revenues will surely increase once the company secures approval for these territories. Eisai may also try to market the drug to patients with diabetes as Belviq showed Hba1c reduction of 0.9 percent, promising results during upcoming clinical trials. However, these points are moot with relation to this particular model as, for simplicity's sake, it takes into account only the domestic obesity market.
It is clear from the model that Arena will be able to garner revenues of around $450 million at its peak. Manufacturing costs will comprise 20 percent of net sales. However, results would be better if Arena had exclusive rights for the domestic market. While profits may have been higher if this had been the case, understandably Arena chose a less risky route for the marketing of their first approved drug by partnering with an experienced player. In this manner, Arena was able to shift most of the risks to Eisai Inc.
Arena vs. Vivus
When comparing these two companies directly, it is clear that both parties carry their own unique advantages. While Vivus has a more effective product, Arena has a product with a safer profile. One thing particularly in favor of Vivus is that it has an established pipeline and is not reliant on a single drug for revenue.
In the future, it can be expected that both companies will emerge ahead, as the obesity market is desperate for an effective drug. Though both of these stocks may show some volatility in the short-term, the long run prospects are optimistic. However, it is important to also consider that there always remains the possibility of a takeover; both companies have drugs that could prove to be a vital component of any big player's portfolio.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/793311-my-conservative-reven…
Arena Pharmaceuticals Inc. (ARNA): What to Expect from Earnings
By David Woodburn
Published: August 9, 2012 at 10:17 am
Arena Pharmaceuticals, Inc. gets its turn in the earnings parade Thursday when it releases its quarterly report after the closing bell. The company, which has been in the news for the last couple months since receiving FDA approval for its weight-loss drug, Belviq, has come up a bit short of expectations over the past year. However, the stock has made a nice run over the last three months, so it will be interesting to see if the numbers live up to the stock. Here is an inside look at what may be expected from the report.
Over a three-month period from May 9 to August 8 of this year, the stock of Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) rose more than 110 percent, from $3.42 a share to $7.30 a share. From June 7 to June 21, the stock gained 81 percent of its value when the price rose for 11 straight trading days. After that, however, the stock lost 15 percent of its value over six straight losing sessions from July 13 to July 20.
The average estimates among analysts calls for a net EPS loss of 8 cents, which is an improvement from a minus-16 cent EPS loss in the same quarter of 2011. Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) has missed quarterly EPS estimates in each of the last three quarters. On the revenue side, analysts are expecting to see about $7.4 million on the report, which would be a more than two-fold increase from the year-ago quarter and would end two straight quarter of revenue declines. The company reported $2.2 million in revenue in the first quarter of 2012 and $3.9 million in the fourth quarter of 2011. The company lists an asset-to-liability ratio of more than 7.5, so the company’s overall financial health seems solid.
It is possible that Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) will discuss its marketing and sales plans for Belviq at its conference call later Thursday, though the drug is not expected to hit the market until the first quarter of 2013.
http://www.insidermonkey.com/blog/arena-pharmaceuticals-inc-…
By David Woodburn
Published: August 9, 2012 at 10:17 am
Arena Pharmaceuticals, Inc. gets its turn in the earnings parade Thursday when it releases its quarterly report after the closing bell. The company, which has been in the news for the last couple months since receiving FDA approval for its weight-loss drug, Belviq, has come up a bit short of expectations over the past year. However, the stock has made a nice run over the last three months, so it will be interesting to see if the numbers live up to the stock. Here is an inside look at what may be expected from the report.
Over a three-month period from May 9 to August 8 of this year, the stock of Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) rose more than 110 percent, from $3.42 a share to $7.30 a share. From June 7 to June 21, the stock gained 81 percent of its value when the price rose for 11 straight trading days. After that, however, the stock lost 15 percent of its value over six straight losing sessions from July 13 to July 20.
The average estimates among analysts calls for a net EPS loss of 8 cents, which is an improvement from a minus-16 cent EPS loss in the same quarter of 2011. Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) has missed quarterly EPS estimates in each of the last three quarters. On the revenue side, analysts are expecting to see about $7.4 million on the report, which would be a more than two-fold increase from the year-ago quarter and would end two straight quarter of revenue declines. The company reported $2.2 million in revenue in the first quarter of 2012 and $3.9 million in the fourth quarter of 2011. The company lists an asset-to-liability ratio of more than 7.5, so the company’s overall financial health seems solid.
It is possible that Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) will discuss its marketing and sales plans for Belviq at its conference call later Thursday, though the drug is not expected to hit the market until the first quarter of 2013.
http://www.insidermonkey.com/blog/arena-pharmaceuticals-inc-…
Arena Pharmaceuticals Inc Raises FY 2012 Revenue Guidance
Thursday, 9 Aug 2012 04:05pm EDT
Arena Pharmaceuticals Inc announced an update to its fiscal 2012 financial guidance. Arena increased its fiscal 2012 revenue guidance from $66-$72 million to $91-$97 million, which includes the $20.0 million milestone payment received from Eisai for the inclusion in the FDA-approved prescribing information of the BLOOM-DM data and, although the timing remains uncertain, $65.0 million for milestone payments that Arena will receive from Eisai following DEA scheduling designation and delivery of BELVIQ launch supply.
http://www.reuters.com/finance/stocks/ARNA.O/key-development…
Thursday, 9 Aug 2012 04:05pm EDT
Arena Pharmaceuticals Inc announced an update to its fiscal 2012 financial guidance. Arena increased its fiscal 2012 revenue guidance from $66-$72 million to $91-$97 million, which includes the $20.0 million milestone payment received from Eisai for the inclusion in the FDA-approved prescribing information of the BLOOM-DM data and, although the timing remains uncertain, $65.0 million for milestone payments that Arena will receive from Eisai following DEA scheduling designation and delivery of BELVIQ launch supply.
http://www.reuters.com/finance/stocks/ARNA.O/key-development…
Arena Pharmaceuticals-Aktie: "buy"
13.08.12 11:38
Jefferies & Co
New York (www.aktiencheck.de) - Thomas Wei, Thomas Nguyen, Jeffrey Hung und Shaunak Deepak, Analysten von Jefferies & Co stufen die Arena Pharmaceuticals-Aktie mit dem Rating "buy" ein. Das Kursziel werde bei 20 USD belassen. (Analyse vom 10.08.2012) (13.08.2012/ac/a/a)
http://www.aktiencheck.de/analysen/Artikel-Arena_Pharmaceuti…
13.08.12 11:38
Jefferies & Co
New York (www.aktiencheck.de) - Thomas Wei, Thomas Nguyen, Jeffrey Hung und Shaunak Deepak, Analysten von Jefferies & Co stufen die Arena Pharmaceuticals-Aktie mit dem Rating "buy" ein. Das Kursziel werde bei 20 USD belassen. (Analyse vom 10.08.2012) (13.08.2012/ac/a/a)
http://www.aktiencheck.de/analysen/Artikel-Arena_Pharmaceuti…
Arena Cuts Loss, Sales Climb - Analyst Blog
Zacks.com
Arena Pharmaceuticals Inc. ( ARNA ) suffered a loss (excluding special items) of 12 cents per share in the second quarter of 2012 as against a loss of 16 cents incurred in the year-ago quarter. However, second quarter 2012 loss was wider than the Zacks Consensus Estimate of a loss of 8 cents per share. The narrower year-over-year loss was attributable to higher revenues.
Quarterly Results
Arena Pharma recorded second quarter revenues of $22 million compared to revenues of $3.3 million in the prior-year quarter. The jump was mainly attributable to the $20 million milestone payment received from partner, Eisai Inc. related to the agreement between the companies regarding weight loss drug, Belviq. The Zacks Consensus Estimate was much lower at $10 million.
Operating expenses at Arena Pharma declined 13.4% to $20.1 million on account of lower research and development (R&D) and general and administrative (G&A) expenses. R&D expenses recorded a decline of 4.1% to 14.1 million due to decrease in salary and other personnel-related expenses. G&A expenses decreased 14.7% to $5.1 million in the reported quarter due to lower patent-related legal fees.
FDA Approval for Belviq
In June 2012, the US Food and Drug Administration (FDA) approved Belviq (lorcaserin HCl) as an adjunct to a healthy diet (low on calories) and increased physical activity for chronic weight management in obese (Body Mass Index, or BMI, >30) or overweight (BMI >27) adults suffering from at least one weight-related co-morbid condition. The move marks the first FDA approval for a weight-loss drug in 13 years.
While clearing the drug, whose safety and efficacy when coadministered with other weight loss products and the effect on cardiovascular morbidity and mortality are yet to be established, the FDA recommended Belviq to be classified by the US Drug Enforcement Administration (DEA) as a scheduled drug.
Eisai, which will market Belviq in the US, is preparing for the launch of the drug after the DEA gives its verdict on the matter. Eisai is currently expecting Belviq to hit the US market in early 2013.
Both Eisai and Arena Pharma will have to conduct post-marketing studies to evaluate the safety and efficacy of Belviq for weight management in the pediatric population. Post-marketing studies will also be conducted to evaluate the effect of long-term Belviq therapy in overweight and obese patients suffering from cardiovascular disease or who are exposed to cardiovascular risk factors. The companies intend to work with the FDA on the finalization of the post-marketing study designs.
We note that Belviq received a 120 day assessment report from the European Medicines Agency (EMA) for the Marketing Authorization Application (MAA) submitted in the EU. Arena Pharma expects a final decision from the EU in the first half of 2013. Belviq is also under review in Switzerland. Approval in the EU and Switzerland would further boost the sales potential of the drug.
2012 Guidance
For 2012, Arena Pharma increased its revenue guidance to a band of $91 million to $97 million from the earlier range of $66 million to $72 million. This includes a $20 million milestone payment received from Eisai in the first quarter of 2012 and $65 million milestone payments which Arena Pharma will receive following the DEA scheduling designation and launch of Belviq. The company also mentioned that they revenues could be lower by $65 million if the company fails to receive DEA scheduling in 2012.
However Arena Pharma continues to expect R&D expenses in the range of approximately $57 million to $67 million. Arena Pharma also kept its G&A expenses guidance unchanged at $20 million to $24 million.
Arena Pharma expects 2012 cash, cash equivalents and short-term investments to be approximately $215 million instead of the previously mentioned guidance of $84 million to $89 million subject to the DEA scheduling and launch of Belviq.
Our View
We currently have a Neutral recommendation on Arena Pharma which carries a Zacks #2 Rank (short term 'Buy' rating) The FDA approval of Belviq is a huge boost for the company. The drug targets the highly lucrative obesity market. However, we note that competition is fast catching up with Vivus Inc. 's ( VVUS ) obesity drug Qsymia recently gaining approval from the FDA. Another potential competitor could be Orexigen Therapeutics, Inc. 's ( OREX ) Contrave upon approval. We expect investor focus to remain on the DEA scheduling of the drug.
http://community.nasdaq.com/News/2012-08/arena-cuts-loss-sal…
Zacks.com
Arena Pharmaceuticals Inc. ( ARNA ) suffered a loss (excluding special items) of 12 cents per share in the second quarter of 2012 as against a loss of 16 cents incurred in the year-ago quarter. However, second quarter 2012 loss was wider than the Zacks Consensus Estimate of a loss of 8 cents per share. The narrower year-over-year loss was attributable to higher revenues.
Quarterly Results
Arena Pharma recorded second quarter revenues of $22 million compared to revenues of $3.3 million in the prior-year quarter. The jump was mainly attributable to the $20 million milestone payment received from partner, Eisai Inc. related to the agreement between the companies regarding weight loss drug, Belviq. The Zacks Consensus Estimate was much lower at $10 million.
Operating expenses at Arena Pharma declined 13.4% to $20.1 million on account of lower research and development (R&D) and general and administrative (G&A) expenses. R&D expenses recorded a decline of 4.1% to 14.1 million due to decrease in salary and other personnel-related expenses. G&A expenses decreased 14.7% to $5.1 million in the reported quarter due to lower patent-related legal fees.
FDA Approval for Belviq
In June 2012, the US Food and Drug Administration (FDA) approved Belviq (lorcaserin HCl) as an adjunct to a healthy diet (low on calories) and increased physical activity for chronic weight management in obese (Body Mass Index, or BMI, >30) or overweight (BMI >27) adults suffering from at least one weight-related co-morbid condition. The move marks the first FDA approval for a weight-loss drug in 13 years.
While clearing the drug, whose safety and efficacy when coadministered with other weight loss products and the effect on cardiovascular morbidity and mortality are yet to be established, the FDA recommended Belviq to be classified by the US Drug Enforcement Administration (DEA) as a scheduled drug.
Eisai, which will market Belviq in the US, is preparing for the launch of the drug after the DEA gives its verdict on the matter. Eisai is currently expecting Belviq to hit the US market in early 2013.
Both Eisai and Arena Pharma will have to conduct post-marketing studies to evaluate the safety and efficacy of Belviq for weight management in the pediatric population. Post-marketing studies will also be conducted to evaluate the effect of long-term Belviq therapy in overweight and obese patients suffering from cardiovascular disease or who are exposed to cardiovascular risk factors. The companies intend to work with the FDA on the finalization of the post-marketing study designs.
We note that Belviq received a 120 day assessment report from the European Medicines Agency (EMA) for the Marketing Authorization Application (MAA) submitted in the EU. Arena Pharma expects a final decision from the EU in the first half of 2013. Belviq is also under review in Switzerland. Approval in the EU and Switzerland would further boost the sales potential of the drug.
2012 Guidance
For 2012, Arena Pharma increased its revenue guidance to a band of $91 million to $97 million from the earlier range of $66 million to $72 million. This includes a $20 million milestone payment received from Eisai in the first quarter of 2012 and $65 million milestone payments which Arena Pharma will receive following the DEA scheduling designation and launch of Belviq. The company also mentioned that they revenues could be lower by $65 million if the company fails to receive DEA scheduling in 2012.
However Arena Pharma continues to expect R&D expenses in the range of approximately $57 million to $67 million. Arena Pharma also kept its G&A expenses guidance unchanged at $20 million to $24 million.
Arena Pharma expects 2012 cash, cash equivalents and short-term investments to be approximately $215 million instead of the previously mentioned guidance of $84 million to $89 million subject to the DEA scheduling and launch of Belviq.
Our View
We currently have a Neutral recommendation on Arena Pharma which carries a Zacks #2 Rank (short term 'Buy' rating) The FDA approval of Belviq is a huge boost for the company. The drug targets the highly lucrative obesity market. However, we note that competition is fast catching up with Vivus Inc. 's ( VVUS ) obesity drug Qsymia recently gaining approval from the FDA. Another potential competitor could be Orexigen Therapeutics, Inc. 's ( OREX ) Contrave upon approval. We expect investor focus to remain on the DEA scheduling of the drug.
http://community.nasdaq.com/News/2012-08/arena-cuts-loss-sal…
Biotechnology Stocks on the Move; Arena Pharmaceuticals Inc. (ARNA), VIVUS Inc. (VVUS), Orexigen Therapeutics Inc. (OREX)
By Mark Spencer
Aug 14, 2012 12:46:19 PM PDT
ARNA
$8.26 +$1.02 +14.09%
OREX
$4.32 +$0.18 +4.35%
VVUS
$23.24 +$1.52 +7.00%
Arena Pharmaceuticals Inc. (NASDAQ: ARNA) shares are soaring in trading today amid speculation that the biopharmaceutical company could be acquired by a major pharmaceutical company. ARNA’s drug for treatment of obesity, lorcaserin (Belviq), was recently approved by the FDA. In fact, lorcaserin is the first obesity treatment drug approved by the FDA in over a decade.
ARNA also reported its second-quarter financial results recently. The company’s revenue for the quarter totaled $22 million, compared to $3.3 million reported for the same period in the previous year. The increase in revenue was mainly due to a $20 million milestone payment earned under the marketing and supply agreement with Eisai.
ARNA’s net loss for the first half of 2012 was $51.5 million, or $0.29 per share, compared to $65.1 million, or $0.49 per share reported in the first half of 2011.
ARNA expects full-year revenue to come in between $91 million and $97 million. This compares to previous guidance range of $66-$72 million.
ARNA shares rose to an intra-day high of $8.25 in trading today. At last check, the stock was trading 13.81% higher at $8.24 on above average volume of 19.29 million. Year-to-date, ARNA shares gained more than 335%.
Shares of Arena’s rivals, VIVUS Inc. (NASDAQ: VVUS) and Orexigen Therapeutics Inc. (NASDAQ: OREX) have also risen sharply in trading today. Both, VVUS and OREX are also developing drugs for the treatment of obesity.
VVUS shares are currently trading 6.03% higher at $23.03 on volume of 3.44 million. Year-to-date, VVUS shares have gained more than 136%.
OREX shares rose to an intra-day high of $4.43 in trading today. At last check, the stock was trading 4.35% higher at $4.32 on volume of 1.92 million. Year-to-date, the stock gained more than 168%.
http://www.smallcapnetwork.com/Biotechnology-Stocks-on-the-M…
By Mark Spencer
Aug 14, 2012 12:46:19 PM PDT
ARNA
$8.26 +$1.02 +14.09%
OREX
$4.32 +$0.18 +4.35%
VVUS
$23.24 +$1.52 +7.00%
Arena Pharmaceuticals Inc. (NASDAQ: ARNA) shares are soaring in trading today amid speculation that the biopharmaceutical company could be acquired by a major pharmaceutical company. ARNA’s drug for treatment of obesity, lorcaserin (Belviq), was recently approved by the FDA. In fact, lorcaserin is the first obesity treatment drug approved by the FDA in over a decade.
ARNA also reported its second-quarter financial results recently. The company’s revenue for the quarter totaled $22 million, compared to $3.3 million reported for the same period in the previous year. The increase in revenue was mainly due to a $20 million milestone payment earned under the marketing and supply agreement with Eisai.
ARNA’s net loss for the first half of 2012 was $51.5 million, or $0.29 per share, compared to $65.1 million, or $0.49 per share reported in the first half of 2011.
ARNA expects full-year revenue to come in between $91 million and $97 million. This compares to previous guidance range of $66-$72 million.
ARNA shares rose to an intra-day high of $8.25 in trading today. At last check, the stock was trading 13.81% higher at $8.24 on above average volume of 19.29 million. Year-to-date, ARNA shares gained more than 335%.
Shares of Arena’s rivals, VIVUS Inc. (NASDAQ: VVUS) and Orexigen Therapeutics Inc. (NASDAQ: OREX) have also risen sharply in trading today. Both, VVUS and OREX are also developing drugs for the treatment of obesity.
VVUS shares are currently trading 6.03% higher at $23.03 on volume of 3.44 million. Year-to-date, VVUS shares have gained more than 136%.
OREX shares rose to an intra-day high of $4.43 in trading today. At last check, the stock was trading 4.35% higher at $4.32 on volume of 1.92 million. Year-to-date, the stock gained more than 168%.
http://www.smallcapnetwork.com/Biotechnology-Stocks-on-the-M…
5 Upcoming Biotechnology Industry Organization (BIO) Events – (ARNA, SVNT, HEB, GILD)
By Rick Wolfe
On August 27, 2012 At 12:51 pm
Category : Financial News, Today's Hot News
The Biotechnology Industry Organization (BIO) on Friday thanked Gov. Mitt Romney for his declared plan to shore up more market penetration and rivalry among energy sources by maintaining the RFS in “The Romney Plan For A Stronger Middle Class: ENERGY INDEPENDENCE,” released on Thursday.
There is a series of upcoming BIO events starting next month including BIO India International Conference September 12 – 13, 2012 Mumbai, India, Livestock Biotech Summit September 19 – 21, 2012 Kansas City, MO BIO Investor Forum October 9 – 10, 2012 San Francisco, CA, Pacific Rim Summit on Industrial Biotechnology and Bioenergy October 10 – 12, 2012 Vancouver, Canada and The BIO Convention in China October 24 – 25, 2012 Shanghai, China.
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) continues to move up, retaining its top position in the stock market even after posting a loss for the second quarter ended June 30, 2012. For full year 2012, it currently anticipates revenue in a range of $91-$97 million, higher from earlier guidance of $66-$72 million.
Shares of this company traded up 1.77% during trading yesterday, hitting $8.69. The stock has a 52 week low of $1.23 and a 52 week high of $13.50. The company has a market cap of $1.87 billion. The P/S ratio is 63.07 and P/B ratio 17.28. The beta value is 0.21. ARNA’s RSI amounts to 50.50.
Savient Pharmaceuticals, Inc. (NASDAQ:SVNT) has recently surpassed its three-month high on twice the average trading volume, and it seems like it is moving forward.
The company has a market value of $88.93 million. It employs 173 people and over the last 12 months generated revenue of $14.45 million and has a net income of -$108.83 million. The firm’s operating margin is -756.72 percent and net profit margin -753.06 percent. The latest closing price of its shares moved up 76.00% from the 50-day moving average.
Hemispherx BioPharma, Inc (AMEX:HEB) topped its 52-week high on Friday. For this company, a return on equity of -28.92 percent was realized due to its financial situation. Last twelve months earnings per share reached a value of -$0.08. Earnings are projected to move up 25.00 percent for the coming five years. The stock closed at $0.659, up 0.069 points or 11.69% from previous close and at a distance of +61.32% from 20-day simple moving average.
Gilead Sciences, Inc.(NASDAQ:GILD) gapped up and closed higher in the last trading session before a FDA ruling today as many believe the good news could secure its place as the leader of the HIV drug pack for a while.
In the last trading session, the stock’s price moved 19.13% above their 200 day moving average, changing hands as low as $34.45 per share. The stock is currently trading 7.35% up from SMA 50. The worst hit in its 52 week range is $34.45 per share, with $58.84 as the 52 week best price, that compares with a latest closing price of $57.29. The Beta of this stock is 0.44.
Read more: 5 Upcoming Biotechnology Industry Organization (BIO) Events – (ARNA, SVNT, HEB, GILD) | Property Mentor http://www.propertymentorgroup.com/5-upcoming-biotechnology-…
http://www.propertymentorgroup.com/5-upcoming-biotechnology-…
By Rick Wolfe
On August 27, 2012 At 12:51 pm
Category : Financial News, Today's Hot News
The Biotechnology Industry Organization (BIO) on Friday thanked Gov. Mitt Romney for his declared plan to shore up more market penetration and rivalry among energy sources by maintaining the RFS in “The Romney Plan For A Stronger Middle Class: ENERGY INDEPENDENCE,” released on Thursday.
There is a series of upcoming BIO events starting next month including BIO India International Conference September 12 – 13, 2012 Mumbai, India, Livestock Biotech Summit September 19 – 21, 2012 Kansas City, MO BIO Investor Forum October 9 – 10, 2012 San Francisco, CA, Pacific Rim Summit on Industrial Biotechnology and Bioenergy October 10 – 12, 2012 Vancouver, Canada and The BIO Convention in China October 24 – 25, 2012 Shanghai, China.
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) continues to move up, retaining its top position in the stock market even after posting a loss for the second quarter ended June 30, 2012. For full year 2012, it currently anticipates revenue in a range of $91-$97 million, higher from earlier guidance of $66-$72 million.
Shares of this company traded up 1.77% during trading yesterday, hitting $8.69. The stock has a 52 week low of $1.23 and a 52 week high of $13.50. The company has a market cap of $1.87 billion. The P/S ratio is 63.07 and P/B ratio 17.28. The beta value is 0.21. ARNA’s RSI amounts to 50.50.
Savient Pharmaceuticals, Inc. (NASDAQ:SVNT) has recently surpassed its three-month high on twice the average trading volume, and it seems like it is moving forward.
The company has a market value of $88.93 million. It employs 173 people and over the last 12 months generated revenue of $14.45 million and has a net income of -$108.83 million. The firm’s operating margin is -756.72 percent and net profit margin -753.06 percent. The latest closing price of its shares moved up 76.00% from the 50-day moving average.
Hemispherx BioPharma, Inc (AMEX:HEB) topped its 52-week high on Friday. For this company, a return on equity of -28.92 percent was realized due to its financial situation. Last twelve months earnings per share reached a value of -$0.08. Earnings are projected to move up 25.00 percent for the coming five years. The stock closed at $0.659, up 0.069 points or 11.69% from previous close and at a distance of +61.32% from 20-day simple moving average.
Gilead Sciences, Inc.(NASDAQ:GILD) gapped up and closed higher in the last trading session before a FDA ruling today as many believe the good news could secure its place as the leader of the HIV drug pack for a while.
In the last trading session, the stock’s price moved 19.13% above their 200 day moving average, changing hands as low as $34.45 per share. The stock is currently trading 7.35% up from SMA 50. The worst hit in its 52 week range is $34.45 per share, with $58.84 as the 52 week best price, that compares with a latest closing price of $57.29. The Beta of this stock is 0.44.
Read more: 5 Upcoming Biotechnology Industry Organization (BIO) Events – (ARNA, SVNT, HEB, GILD) | Property Mentor http://www.propertymentorgroup.com/5-upcoming-biotechnology-…
http://www.propertymentorgroup.com/5-upcoming-biotechnology-…
Arena Is Poised To Move
August 25, 2012 ,
Spencer Osborne
Investors in Arena Pharmaceuticals, Inc. (ARNA) got great news in late June that the company's obesity drug Belviq had received FDA approval. A combination of excitement and irrational exuberance then took over and sent Arena toward a 52-week high of $13.50. While seeing that range was great, it was based on excitement that was perhaps overstated at least at this early stage. I am not saying that Arena is not worth $13.50. I am simply saying that it moved too fast, and the fact that it did settle down quickly is probably good evidence of that.
So where does that leave us now? It leaves us with a pharmaceutical company that is one of only two that have been successful in getting FDA approval on an obesity drug in the last decade. That is reason enough that Arena has extreme potential. Add to that the company's cash position, and we now have a company than can move forward in a strategic manner. A good sign when you may become the object of potential partnerships or buyouts, as a strong cash position allows you to negotiate from a better position. Simply stated there are reasons why Wall Street is getting more excited about Arena.
What has me more excited are some technical indicators. I like to look at volume, support and resistance, and exponential moving averages as it allows me multiple points to consider. Right now I see something very exciting happening with Arena.
Volume
I use volume to measure the relative strength in a move. Any move that happens on substantial volume has some strength and reasoning behind it. When volume tapers off, it typically means that whatever compelled the above average volume is no longer a catalyst. I typically use the 200 day as my baseline, but monitor the 5 day, 13 day, and 20 day as well. Monitoring all of these allows me to gauge current action against several points to consider the relative meaning.
With Arena, the average volume is 12.9 million shares. Lately, the company has traded below that level - at the same time it has been making a run in price. This is very interesting because to me it demonstrates that the Street is very willing to test higher levels, but not yet doing so with that much conviction. These are the times when a savvy trader can make a play.
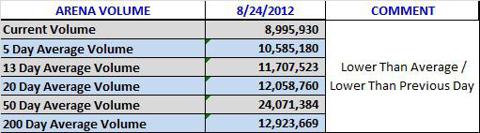
In my opinion, there is not a lot of conviction behind this move, but there is enough action to test higher ground. I see something brewing here.
Support and Resistance
Support and resistance are also very classic technical indicators. I like to refer to these because they are relatively simple to understand, and they give a relative indication as to the potential moves an equity can make. When I look at support and resistance, I gauge the strength of each level as time passes in order to better grasp my risk or reward.
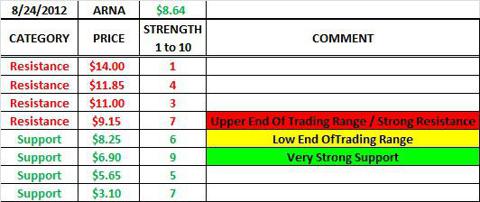
Right now, Arena is in the middle of strong support at $6.90 and strong resistance at $9.15, and testing higher highs often. There seems to be a trend building and much of that is based on the fundamentals of Arena as the launch of Belviq approaches. The beauty of this is that we are seeing a foundation built at current levels that should allow the company to hold strong. Once this equity breaks past $9.00, there is little holding it back. The march upward again is far more healthy that a spike upward.
Exponential Moving Averages
The third category I consider is Exponential Moving Averages. When the successive averages are higher than the next it is a bullish indicator. When they are lower it is bearish. Just two weeks ago, Arena was exhibiting all bearish signals on the EMA. Today, the story is quite different and building in a positive manner.
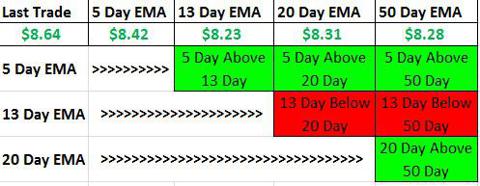
Arena has started a run, and that run has turned several technical indicators bullish. Over the last few trading sessions, the 5 day average has turned all green. The next phase is the 13 day average, and with current action, that will happen sooner rather than later.
So What Does All Of This Mean?
Arena is building on solid fundamentals and an exciting potential. These are core considerations in investing into any equity. It is also building on a technical basis, which makes things even more exciting.
Combing all three of these technical measures gives us some clues of what to watch for both short and long term. It can also give additional confidence in an investment.
I look for moves on good volume. Right now, I see Arena testing the proverbial waters and drifting upward, but not having true conviction in these moves. The key here is bringing up the 13 day averages, while trying to keep above the 5. Believe it or not, this equity can maintain a bullish stance with a close above $8.42. That gives it a little room to work with. This equity is poised to go all green from an EMA standpoint very soon, and it is why, even with this most recent run, there is still upside potential building. On the risk side, we want to see it hold at least $8.25 support.
Things To Consider
News and rumors can fuel or spoil a run. Arena has seen some positive press lately, outside of buyout rumors, and this could help support new support and strength above $8.00. Prior to FDA approval of Belviq, this equity was much more speculative. Once Arena's Belviq gained FDA approval, there were fundamental reasons to invest, as the potential is substantial. The next phase is getting it to market and that is only months away. In my opinion, Arena is worthy of a serious look.
Disclosure: I am long ARNA. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
http://seekingalpha.com/article/827931-arena-is-poised-to-mo…
August 25, 2012 ,
Spencer Osborne
Investors in Arena Pharmaceuticals, Inc. (ARNA) got great news in late June that the company's obesity drug Belviq had received FDA approval. A combination of excitement and irrational exuberance then took over and sent Arena toward a 52-week high of $13.50. While seeing that range was great, it was based on excitement that was perhaps overstated at least at this early stage. I am not saying that Arena is not worth $13.50. I am simply saying that it moved too fast, and the fact that it did settle down quickly is probably good evidence of that.
So where does that leave us now? It leaves us with a pharmaceutical company that is one of only two that have been successful in getting FDA approval on an obesity drug in the last decade. That is reason enough that Arena has extreme potential. Add to that the company's cash position, and we now have a company than can move forward in a strategic manner. A good sign when you may become the object of potential partnerships or buyouts, as a strong cash position allows you to negotiate from a better position. Simply stated there are reasons why Wall Street is getting more excited about Arena.
What has me more excited are some technical indicators. I like to look at volume, support and resistance, and exponential moving averages as it allows me multiple points to consider. Right now I see something very exciting happening with Arena.
Volume
I use volume to measure the relative strength in a move. Any move that happens on substantial volume has some strength and reasoning behind it. When volume tapers off, it typically means that whatever compelled the above average volume is no longer a catalyst. I typically use the 200 day as my baseline, but monitor the 5 day, 13 day, and 20 day as well. Monitoring all of these allows me to gauge current action against several points to consider the relative meaning.
With Arena, the average volume is 12.9 million shares. Lately, the company has traded below that level - at the same time it has been making a run in price. This is very interesting because to me it demonstrates that the Street is very willing to test higher levels, but not yet doing so with that much conviction. These are the times when a savvy trader can make a play.

In my opinion, there is not a lot of conviction behind this move, but there is enough action to test higher ground. I see something brewing here.
Support and Resistance
Support and resistance are also very classic technical indicators. I like to refer to these because they are relatively simple to understand, and they give a relative indication as to the potential moves an equity can make. When I look at support and resistance, I gauge the strength of each level as time passes in order to better grasp my risk or reward.

Right now, Arena is in the middle of strong support at $6.90 and strong resistance at $9.15, and testing higher highs often. There seems to be a trend building and much of that is based on the fundamentals of Arena as the launch of Belviq approaches. The beauty of this is that we are seeing a foundation built at current levels that should allow the company to hold strong. Once this equity breaks past $9.00, there is little holding it back. The march upward again is far more healthy that a spike upward.
Exponential Moving Averages
The third category I consider is Exponential Moving Averages. When the successive averages are higher than the next it is a bullish indicator. When they are lower it is bearish. Just two weeks ago, Arena was exhibiting all bearish signals on the EMA. Today, the story is quite different and building in a positive manner.

Arena has started a run, and that run has turned several technical indicators bullish. Over the last few trading sessions, the 5 day average has turned all green. The next phase is the 13 day average, and with current action, that will happen sooner rather than later.
So What Does All Of This Mean?
Arena is building on solid fundamentals and an exciting potential. These are core considerations in investing into any equity. It is also building on a technical basis, which makes things even more exciting.
Combing all three of these technical measures gives us some clues of what to watch for both short and long term. It can also give additional confidence in an investment.
I look for moves on good volume. Right now, I see Arena testing the proverbial waters and drifting upward, but not having true conviction in these moves. The key here is bringing up the 13 day averages, while trying to keep above the 5. Believe it or not, this equity can maintain a bullish stance with a close above $8.42. That gives it a little room to work with. This equity is poised to go all green from an EMA standpoint very soon, and it is why, even with this most recent run, there is still upside potential building. On the risk side, we want to see it hold at least $8.25 support.
Things To Consider
News and rumors can fuel or spoil a run. Arena has seen some positive press lately, outside of buyout rumors, and this could help support new support and strength above $8.00. Prior to FDA approval of Belviq, this equity was much more speculative. Once Arena's Belviq gained FDA approval, there were fundamental reasons to invest, as the potential is substantial. The next phase is getting it to market and that is only months away. In my opinion, Arena is worthy of a serious look.
Disclosure: I am long ARNA. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
http://seekingalpha.com/article/827931-arena-is-poised-to-mo…
-- Three Corporate Presentations to be Webcast Live --, -- Arena to Also Participate in Panel Presentations --,
By Arena Pharmaceuticals, Inc.
SAN DIEGO, Aug. 30, 2012 — /PRNewswire/ -- Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) announced today that the company is scheduled to present corporate updates at the following three upcoming investor conferences:
Stifel Nicolaus Healthcare Conference Presentation: September 6, 2012, at 9:10 a.m. Eastern Time (6:10 a.m. Pacific Time) at the Four Seasons Hotel in Boston, Massachusetts Presenting: Robert E. Hoffman, Senior Vice President, Finance and Chief Financial Officer
NewsMakers in the Biotech Industry Presentation: September 7, 2012, at 10:30 a.m. Eastern Time (7:30 a.m. Pacific Time) at the Millennium Broadway Hotel in New York, New York Presenting: Craig M. Audet, Senior Vice President, Operations and Head of Global Regulatory Affairs
Bank of America Merrill Lynch Global Healthcare Conference Presentation: September 12, 2012, at 8:55 a.m. British Summer Time (12:55 a.m. Pacific Time) at the Bank of America Merrill Lynch Financial Centre in London, England Presenting: Dominic P. Behan, Ph.D., Executive Vice President and Chief Scientific Officer
Arena's presentations at these conferences will be webcast live. Each of the audio webcasts will be available under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the presentation. Please connect to Arena's website several minutes prior to the start of each webcast to ensure adequate time for any software download that may be necessary.
Arena also announced today that it is scheduled to participate in panel presentations at Citi's 7th Annual Biotech Day on September 5, 2012, at the Mandarin Oriental Hotel in Boston, Massachusetts and at the Rodman and Renshaw Global Investment Conference on September 10, 2012, at The Waldorf Astoria Hotel in New York, New York. These panel presentations will not be webcast.
About Arena Pharmaceuticals Arena Pharmaceuticals is a biopharmaceutical company focused on discovering, developing and commercializing novel drugs for weight management, cardiovascular disease, inflammation and other disorders. Arena's US operations are located in San Diego, California, and its operations outside of the United States, including its commercial manufacturing facility, are located in Zofingen, Switzerland. For more information about Arena, please visit www.arenapharm.com.
Arena Pharmaceuticals® and Arena® are registered service marks of Arena Pharmaceuticals, Inc.
Forward-Looking Statements Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include statements about Arena's efforts, focus, goals, strategy, research and development programs, and ability to discover and develop compounds and commercialize drugs. For such statements, Arena claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Arena's expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, the following: risks related to commercializing drugs, including regulatory, manufacturing and supply issues and the pace of market acceptance; cash and revenues generated from lorcaserin, including the timing and impact of competition; the timing and outcome of regulatory review is uncertain; government and commercial reimbursement and pricing decisions; risks related to relying on collaborative agreements; the timing and receipt of payments and fees, if any, from collaborators; the entry into, modification or termination of collaborative arrangements; unexpected or unfavorable new data; nonclinical and clinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Arena, request additional information, have additional recommendations or change their guidance or requirements before or after approval; data and other information related to any of Arena's research and development programs may not meet safety, efficacy or other regulatory requirements or otherwise be sufficient for regulatory review, approval or continued marketing; Arena's ability to obtain and defend patents; the timing, success and cost of Arena's research and development programs; results of clinical trials and other studies are subject to different interpretations and may not be predictive of future results; clinical trials and other studies may not proceed at the time or in the manner expected or at all; having adequate funds; and satisfactory resolution of litigation or other disagreements with others. Additional factors that could cause actual results to differ materially from those stated or implied by Arena's forward-looking statements are disclosed in Arena's filings with the Securities and Exchange Commission. These forward-looking statements represent Arena's judgment as of the time of this release. Arena disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
Contact: Arena Pharmaceuticals, Inc. Media Contact: Russo Partners
Cindy McGee, Vice President, David Schull, President
Investor Relations & Alliance Management david.schull@russopartnersllc.com
cmcgee@arenapharm.com 858.717.2310
858.453.7200, ext. 1479
www.arenapharm.com
SOURCE Arena Pharmaceuticals, Inc.
Read more here: http://www.heraldonline.com/2012/08/30/4225785/arena-pharmac…
http://www.heraldonline.com/2012/08/30/4225785/arena-pharmac…
By Arena Pharmaceuticals, Inc.
SAN DIEGO, Aug. 30, 2012 — /PRNewswire/ -- Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) announced today that the company is scheduled to present corporate updates at the following three upcoming investor conferences:
Stifel Nicolaus Healthcare Conference Presentation: September 6, 2012, at 9:10 a.m. Eastern Time (6:10 a.m. Pacific Time) at the Four Seasons Hotel in Boston, Massachusetts Presenting: Robert E. Hoffman, Senior Vice President, Finance and Chief Financial Officer
NewsMakers in the Biotech Industry Presentation: September 7, 2012, at 10:30 a.m. Eastern Time (7:30 a.m. Pacific Time) at the Millennium Broadway Hotel in New York, New York Presenting: Craig M. Audet, Senior Vice President, Operations and Head of Global Regulatory Affairs
Bank of America Merrill Lynch Global Healthcare Conference Presentation: September 12, 2012, at 8:55 a.m. British Summer Time (12:55 a.m. Pacific Time) at the Bank of America Merrill Lynch Financial Centre in London, England Presenting: Dominic P. Behan, Ph.D., Executive Vice President and Chief Scientific Officer
Arena's presentations at these conferences will be webcast live. Each of the audio webcasts will be available under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the presentation. Please connect to Arena's website several minutes prior to the start of each webcast to ensure adequate time for any software download that may be necessary.
Arena also announced today that it is scheduled to participate in panel presentations at Citi's 7th Annual Biotech Day on September 5, 2012, at the Mandarin Oriental Hotel in Boston, Massachusetts and at the Rodman and Renshaw Global Investment Conference on September 10, 2012, at The Waldorf Astoria Hotel in New York, New York. These panel presentations will not be webcast.
About Arena Pharmaceuticals Arena Pharmaceuticals is a biopharmaceutical company focused on discovering, developing and commercializing novel drugs for weight management, cardiovascular disease, inflammation and other disorders. Arena's US operations are located in San Diego, California, and its operations outside of the United States, including its commercial manufacturing facility, are located in Zofingen, Switzerland. For more information about Arena, please visit www.arenapharm.com.
Arena Pharmaceuticals® and Arena® are registered service marks of Arena Pharmaceuticals, Inc.
Forward-Looking Statements Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include statements about Arena's efforts, focus, goals, strategy, research and development programs, and ability to discover and develop compounds and commercialize drugs. For such statements, Arena claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Arena's expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, the following: risks related to commercializing drugs, including regulatory, manufacturing and supply issues and the pace of market acceptance; cash and revenues generated from lorcaserin, including the timing and impact of competition; the timing and outcome of regulatory review is uncertain; government and commercial reimbursement and pricing decisions; risks related to relying on collaborative agreements; the timing and receipt of payments and fees, if any, from collaborators; the entry into, modification or termination of collaborative arrangements; unexpected or unfavorable new data; nonclinical and clinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Arena, request additional information, have additional recommendations or change their guidance or requirements before or after approval; data and other information related to any of Arena's research and development programs may not meet safety, efficacy or other regulatory requirements or otherwise be sufficient for regulatory review, approval or continued marketing; Arena's ability to obtain and defend patents; the timing, success and cost of Arena's research and development programs; results of clinical trials and other studies are subject to different interpretations and may not be predictive of future results; clinical trials and other studies may not proceed at the time or in the manner expected or at all; having adequate funds; and satisfactory resolution of litigation or other disagreements with others. Additional factors that could cause actual results to differ materially from those stated or implied by Arena's forward-looking statements are disclosed in Arena's filings with the Securities and Exchange Commission. These forward-looking statements represent Arena's judgment as of the time of this release. Arena disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
Contact: Arena Pharmaceuticals, Inc. Media Contact: Russo Partners
Cindy McGee, Vice President, David Schull, President
Investor Relations & Alliance Management david.schull@russopartnersllc.com
cmcgee@arenapharm.com 858.717.2310
858.453.7200, ext. 1479
www.arenapharm.com
SOURCE Arena Pharmaceuticals, Inc.
Read more here: http://www.heraldonline.com/2012/08/30/4225785/arena-pharmac…
http://www.heraldonline.com/2012/08/30/4225785/arena-pharmac…
 @AREANICS
@AREANICS
Gestern aus dem Urlaub zurückgekommen und volle 4 Wochen " Biotech-börsenfrei" gewesen...

....tja, das nennt man dann wohl geile Ferien......

Der punktuelle Infoservice per SMS zum Urlaubsort hat sehr gut geklappt ( Danke EARTHIE, well done
 ) und wie ich auf den ersten Blick sehe, hat der ARENA-INFO-Thread auch genügend Futter bekommen ( Danke Poppie, auch well done
) und wie ich auf den ersten Blick sehe, hat der ARENA-INFO-Thread auch genügend Futter bekommen ( Danke Poppie, auch well done  )
)..so, dann werde ich in den nächsten Tagen mal meinen Wissensstand ein bisschen auf Vordermann bringen....

Grüße bernie55

3 Things to Watch With Arena Pharmaceuticals
By Sean Williams , The Motley Fool
Posted 11:21AM 09/10/12 Posted under: Investing
------
Arena Pharmaceuticals (NAS: ARNA) is a clinical-stage biopharmaceutical company focused on developing oral drugs that treat cardiovascular, central nervous system, inflammatory, and metabolic diseases. It currently has one FDA-approved drug to treat obesity, Belviq.
Today, let's look at three things that investors should be watching regarding Arena Pharmaceuticals, as they will provide us with better insight into the company.
1. European Medicines Agency decision
With Belviq approved by the FDA, Arena has fought about half of the battle. Next on the docket is the review from the Drug Enforcement Agency, which should give investors a better idea of what protocol will need to be undertaken in order to prescribe Belviq, and the big decision by the EMA as to whether Belviq will get a thumbs-up or down in Europe.
Considering that Belviq passed the hurdle of approval in the U.S., it seems likely that the EMA will also approve it as long as the safety profile meets its high standards. Since receiving its 120-day assessment in June, we know that the EMA will make its decision on, or before, Nov. 12. The question now is: Can Belviq become the first drug approved worldwide to treat chronic weight management problems -- and will its marketing partner, Eisai (OTC: ESALY), step up and purchase the company if Belviq gets the nod of approval in the European Union? Arena has already been moving higher on these rumors, and the idea isn't completely far-fetched if the EMA approves Belviq.
2. Safety concerns
Let's get back to talking about the safety issues soon to be addressed by the DEA review letter. Since the DEA is labeling Belviq as a scheduled drug, it means that there are still safety concerns that will require Arena to complete six additional tests to satisfy the DEA.
First off, it looks like there could be a warning label applied to Belviq saying that it shouldn't be used during pregnancy because of adverse side effects, which could include serotonin syndrome. Don't feel bad, Arena shareholders; VIVUS (NAS: VVUS) -- which just had its obesity drug, Qsymia, approved by the FDA -- will probably also contend with a pregnancy warning as one of its components, topiramate, has been linked to birth defects in the first trimester of pregnancy.
It's also worth noting that the FDA's approval letter didn't mention requiring that an echocardiogram be performed prior to prescribing the drug despite an increase in serotonin-2B levels noted in patients on the drug. This statement will likely result in a warning for those with moderate to severe cardiovascular problems (i.e., heart disease) who are prescribed Belviq. Among Arena's six safety trials, Eisai will be paying to run trials looking at Belviq's effects on heart attacks and strokes. Similarly, Orexigen Therapeutics (NAS: OREX) will be running a cardiovascular safety review on its obesity drug, Contrave.
3. Drug launch
This is the part where Arena shareholders either hold their breath or gasp in horror. We've seen how big of a nightmare drug launches can be on more than one occasion over the past couple of years. Dendreon's (NAS: DNDN) Provenge prostate cancer treatment was meant to revolutionize treatment and life expectancy for late-stage patients. Instead, sales of the drug limped out of the gate because of its enormous $90,000-plus price tag and physicians' unwillingness to prescribe it on fears that they may not get reimbursed. The recently purchased Human Genome Sciences had the first approved lupus treatment in more than 50 years, and it also failed to launch effectively.
On one side, we can clearly see the advantage of having a premier marketing partner that understands how to launch a drug. Eisai, which holds marketing rights for Belviq in most of North and South America, is one of the world's largest pharmaceutical companies and likely won't have any problem generating sales with its experienced marketing team. On the flip side, having a marketing partner also means that Arena will be sharing its total revenue with Eisai. Don't get me wrong, with Wall Street analysts having projected peak sales of $2 billion, that still leaves the potential for huge profits, but considering that VIVUS owns Qsymia outright, it could give the profitability edge to VIVUS, assuming it can launch Qsymia effectively.
Foolish roundup
Now that you know what to watch for, it should be easier to analyze Arena Pharmaceuticals' successes and pitfalls in the future and hopefully give you a leg up on other investors.
If you're still craving even more info on where this company's headed, get your copy of our latest premium research report on Arena Pharmaceuticals. It gives you an in-depth look at opportunities and events that could move Arena's share price and comes with one year of regular updates. Click here to get this report and claim your investing edge.
The article 3 Things to Watch With Arena Pharmaceuticals originally appeared on Fool.com.
http://www.dailyfinance.com/2012/09/10/3-things-to-watch-wit…
By Sean Williams , The Motley Fool
Posted 11:21AM 09/10/12 Posted under: Investing
------
Arena Pharmaceuticals (NAS: ARNA) is a clinical-stage biopharmaceutical company focused on developing oral drugs that treat cardiovascular, central nervous system, inflammatory, and metabolic diseases. It currently has one FDA-approved drug to treat obesity, Belviq.
Today, let's look at three things that investors should be watching regarding Arena Pharmaceuticals, as they will provide us with better insight into the company.
1. European Medicines Agency decision
With Belviq approved by the FDA, Arena has fought about half of the battle. Next on the docket is the review from the Drug Enforcement Agency, which should give investors a better idea of what protocol will need to be undertaken in order to prescribe Belviq, and the big decision by the EMA as to whether Belviq will get a thumbs-up or down in Europe.
Considering that Belviq passed the hurdle of approval in the U.S., it seems likely that the EMA will also approve it as long as the safety profile meets its high standards. Since receiving its 120-day assessment in June, we know that the EMA will make its decision on, or before, Nov. 12. The question now is: Can Belviq become the first drug approved worldwide to treat chronic weight management problems -- and will its marketing partner, Eisai (OTC: ESALY), step up and purchase the company if Belviq gets the nod of approval in the European Union? Arena has already been moving higher on these rumors, and the idea isn't completely far-fetched if the EMA approves Belviq.
2. Safety concerns
Let's get back to talking about the safety issues soon to be addressed by the DEA review letter. Since the DEA is labeling Belviq as a scheduled drug, it means that there are still safety concerns that will require Arena to complete six additional tests to satisfy the DEA.
First off, it looks like there could be a warning label applied to Belviq saying that it shouldn't be used during pregnancy because of adverse side effects, which could include serotonin syndrome. Don't feel bad, Arena shareholders; VIVUS (NAS: VVUS) -- which just had its obesity drug, Qsymia, approved by the FDA -- will probably also contend with a pregnancy warning as one of its components, topiramate, has been linked to birth defects in the first trimester of pregnancy.
It's also worth noting that the FDA's approval letter didn't mention requiring that an echocardiogram be performed prior to prescribing the drug despite an increase in serotonin-2B levels noted in patients on the drug. This statement will likely result in a warning for those with moderate to severe cardiovascular problems (i.e., heart disease) who are prescribed Belviq. Among Arena's six safety trials, Eisai will be paying to run trials looking at Belviq's effects on heart attacks and strokes. Similarly, Orexigen Therapeutics (NAS: OREX) will be running a cardiovascular safety review on its obesity drug, Contrave.
3. Drug launch
This is the part where Arena shareholders either hold their breath or gasp in horror. We've seen how big of a nightmare drug launches can be on more than one occasion over the past couple of years. Dendreon's (NAS: DNDN) Provenge prostate cancer treatment was meant to revolutionize treatment and life expectancy for late-stage patients. Instead, sales of the drug limped out of the gate because of its enormous $90,000-plus price tag and physicians' unwillingness to prescribe it on fears that they may not get reimbursed. The recently purchased Human Genome Sciences had the first approved lupus treatment in more than 50 years, and it also failed to launch effectively.
On one side, we can clearly see the advantage of having a premier marketing partner that understands how to launch a drug. Eisai, which holds marketing rights for Belviq in most of North and South America, is one of the world's largest pharmaceutical companies and likely won't have any problem generating sales with its experienced marketing team. On the flip side, having a marketing partner also means that Arena will be sharing its total revenue with Eisai. Don't get me wrong, with Wall Street analysts having projected peak sales of $2 billion, that still leaves the potential for huge profits, but considering that VIVUS owns Qsymia outright, it could give the profitability edge to VIVUS, assuming it can launch Qsymia effectively.
Foolish roundup
Now that you know what to watch for, it should be easier to analyze Arena Pharmaceuticals' successes and pitfalls in the future and hopefully give you a leg up on other investors.
If you're still craving even more info on where this company's headed, get your copy of our latest premium research report on Arena Pharmaceuticals. It gives you an in-depth look at opportunities and events that could move Arena's share price and comes with one year of regular updates. Click here to get this report and claim your investing edge.
The article 3 Things to Watch With Arena Pharmaceuticals originally appeared on Fool.com.
http://www.dailyfinance.com/2012/09/10/3-things-to-watch-wit…
Arena Takedown - Is It Fair?
September 12, 2012 by Spencer Osborne
Arena Pharmaceuticals (ARNA) has developed a weight loss drug, Belviq, that is one of only a couple that have received FDA approval in the last decade. The company is awaiting DEA approvals, and then it can begin marketing what appears to many a blockbuster drug. Sales of Belviq could begin as early as late 2012 and this has investors and Wall Street excited, sending the stock to over $9.00 per share. Combine this with rumors of a possible buyout and there is little wonder why Arena saw a nice climb over the past month.
Over the past week Arena has been trending downward and now sits just above $8. Credit Suisse initiated coverage on the equity and established a $6 price target that has many passionate investors crying foul. The reasoning of these investors appears to be sound and warranted, but there are a couple of sides to every story.
What I am going to say here may be frustrating to some, but it is what it is. The market has many players in it and understanding the fundamental story of an equity is not always a recipe for investing success. Sometimes it is more advantageous to understand how the market is actually played than a moral philosophy of what is right and wrong. I know this will ire some, but it is reality.
In theory investment banks have a wall between the activities and position of the bank and the analysts opinions. In practice that may seem impossible, so investors should make an effort to understand the possibility and plan a strategy that takes advantage of it.
Arena Pharmaceuticals is on the cusp of releasing what many hope is a monumental drug (Belviq) in a monumental category (obesity). There is plenty of reason to see massive potential. In fact, many do see that potential, and that is exactly what drives the stock upward.
What we need to realize is that betting on potential is great, but we need to comprehend that we are betting on an unproven potential. What creates value is real success and real numbers. I happen to believe that the potential of Arena will develop into impressive numbers as soon as the end of this year (if released by then). I also know that this equity saw a rise upward on FDA approval and rumor of a buyout. The FDA approval is now past and digested, as is the buyout speculation. What we are left with is the potential of Belviq.
To their credit, Credit Suisse stated that Arena may not meet up to lofty expectations and I have seen some pretty lofty expectations. To their detriment, Credit Suisse also assigned lofty expectations to another drug by Orexigen because they have a pending drug in the same category. The issue is that Orexigen is a couple of years away from a realistic market debut.
Had Credit Suisse simply stopped at valuation concerns on Arena the low price target, while perhaps undeserved, would have been more palatable to investors. The combination of a low price target on Arena, a high price target on Orexigen, and the fact that Credit Suisse is buying Arena shares all add up to investors wondering whether or not the market is stacked against them.
While what is happening to Arena may not seem fair, we all have watched the market for quite some time, and the rules of the market, fair or not, need to carry weight in a trading strategy. Some will voice complaints and frustration while others will look for an edge and perhaps add to their positions. If you believe in the long term prospects of Arena, and believe that Belviq is a compelling market changer, and are holding out for that day, then you are likely worrying about something that you shouldn't be.
Analysts have opinions. Some are bullish and some are bearish. Take it all with a grain of salt. Look at the opinions, even if you do not agree with them. Likely there are many out there that will agree, and that will impact the equity in some form or another.
Fair does not really have a place in the market as frustrating as that may be. Savvy thinking does. Support is at $7.10 and is moderately strong. Resistance is at $8.75 and is very strong. Arena sits in the middle. Arena is now below all meaningful moving averages and volume is lighter than normal. Perhaps consolidation is in order now. Watch things closely over the next few days, and remember, word from the DEA could come at any time and that would be positive.
http://seekingalpha.com/article/861891-arena-takedown-is-it-…
September 12, 2012 by Spencer Osborne
Arena Pharmaceuticals (ARNA) has developed a weight loss drug, Belviq, that is one of only a couple that have received FDA approval in the last decade. The company is awaiting DEA approvals, and then it can begin marketing what appears to many a blockbuster drug. Sales of Belviq could begin as early as late 2012 and this has investors and Wall Street excited, sending the stock to over $9.00 per share. Combine this with rumors of a possible buyout and there is little wonder why Arena saw a nice climb over the past month.
Over the past week Arena has been trending downward and now sits just above $8. Credit Suisse initiated coverage on the equity and established a $6 price target that has many passionate investors crying foul. The reasoning of these investors appears to be sound and warranted, but there are a couple of sides to every story.
What I am going to say here may be frustrating to some, but it is what it is. The market has many players in it and understanding the fundamental story of an equity is not always a recipe for investing success. Sometimes it is more advantageous to understand how the market is actually played than a moral philosophy of what is right and wrong. I know this will ire some, but it is reality.
In theory investment banks have a wall between the activities and position of the bank and the analysts opinions. In practice that may seem impossible, so investors should make an effort to understand the possibility and plan a strategy that takes advantage of it.
Arena Pharmaceuticals is on the cusp of releasing what many hope is a monumental drug (Belviq) in a monumental category (obesity). There is plenty of reason to see massive potential. In fact, many do see that potential, and that is exactly what drives the stock upward.
What we need to realize is that betting on potential is great, but we need to comprehend that we are betting on an unproven potential. What creates value is real success and real numbers. I happen to believe that the potential of Arena will develop into impressive numbers as soon as the end of this year (if released by then). I also know that this equity saw a rise upward on FDA approval and rumor of a buyout. The FDA approval is now past and digested, as is the buyout speculation. What we are left with is the potential of Belviq.
To their credit, Credit Suisse stated that Arena may not meet up to lofty expectations and I have seen some pretty lofty expectations. To their detriment, Credit Suisse also assigned lofty expectations to another drug by Orexigen because they have a pending drug in the same category. The issue is that Orexigen is a couple of years away from a realistic market debut.
Had Credit Suisse simply stopped at valuation concerns on Arena the low price target, while perhaps undeserved, would have been more palatable to investors. The combination of a low price target on Arena, a high price target on Orexigen, and the fact that Credit Suisse is buying Arena shares all add up to investors wondering whether or not the market is stacked against them.
While what is happening to Arena may not seem fair, we all have watched the market for quite some time, and the rules of the market, fair or not, need to carry weight in a trading strategy. Some will voice complaints and frustration while others will look for an edge and perhaps add to their positions. If you believe in the long term prospects of Arena, and believe that Belviq is a compelling market changer, and are holding out for that day, then you are likely worrying about something that you shouldn't be.
Analysts have opinions. Some are bullish and some are bearish. Take it all with a grain of salt. Look at the opinions, even if you do not agree with them. Likely there are many out there that will agree, and that will impact the equity in some form or another.
Fair does not really have a place in the market as frustrating as that may be. Savvy thinking does. Support is at $7.10 and is moderately strong. Resistance is at $8.75 and is very strong. Arena sits in the middle. Arena is now below all meaningful moving averages and volume is lighter than normal. Perhaps consolidation is in order now. Watch things closely over the next few days, and remember, word from the DEA could come at any time and that would be positive.
http://seekingalpha.com/article/861891-arena-takedown-is-it-…
Qsymia von Vivus ist nun schon verfügbar > Vertrieb erfolgt durch zertifizierte Apotheken
Qsymia™ is only available by mail order through certified pharmacies in the Qsymia Home Delivery Network.
http://www.qsymia.com/
Qsymia™ is only available by mail order through certified pharmacies in the Qsymia Home Delivery Network.
http://www.qsymia.com/
Arena Pharmaceuticals to Present at the UBS Global Life Sciences Conference
SAN DIEGO, Sept. 18, 2012 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at the UBS Global Life Sciences Conference on Thursday, September 20, 2012, at 7:30 a.m. Eastern Time (4:30 a.m. Pacific Time) at the Grand Hyatt New York in New York City.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
SAN DIEGO, Sept. 18, 2012 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at the UBS Global Life Sciences Conference on Thursday, September 20, 2012, at 7:30 a.m. Eastern Time (4:30 a.m. Pacific Time) at the Grand Hyatt New York in New York City.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
Arena Pharmaceuticals - The Obesity Blockbuster Drug Company and More
By Joseph Dedvukaj - September 18, 2012
Retail investors were right and Wall Street was wrong about the approval of Arena Pharmaceuticals,
and Wall Street was wrong about the approval of Arena Pharmaceuticals, Inc's (NASDAQ: ARNA) lead drug candidate Belviq (Lorcaserin). Here are the four coming catalysts for Arena:
Inc's (NASDAQ: ARNA) lead drug candidate Belviq (Lorcaserin). Here are the four coming catalysts for Arena:
1. The Obesity epidemic gives Arena's Belviq blockbuster drug status.
2. The growing obesity market is enormous and Belviq has a broad label.
3. Physicians control the demand for Belviq not the consumer.
4. ARNA is a buyout, merger, or partner candidate.
I believe anyone of these will drive the price of the stock much higher.
My citizen petition filed with the FDA helped ordinary citizens, the FDA, scientists, retail investors, and physicians understand why the FDA must approve Belviq. In my research we saw retail investors fight a bitter battle with Wall Street, with many analysts preoccupied with spreading fear, and heavily shorting the company's stock, hoping to reap the benefits of a company shorted into oblivion. A whopping 43 million shares of Wall Street shorted stock interest still remain trapped turning over and over almost on a daily basis to keep the lid on the stock while institutional investors take their ever increasing positions. Virtually, millions of shares are being traded almost daily, trying to tire and weaken the long investor (the investor who invests because the future is profitable). Steadfastly, we believe Belviq will be a blockbuster drug generating more than $1 billion of revenue for its owner each year. We attempted to school Wall Street financial "wizards" who didn't have the medical expertise to see the fundamentals of the equation here. They ignored our efforts, choosing instead to focus on a competing drug?
with many analysts preoccupied with spreading fear, and heavily shorting the company's stock, hoping to reap the benefits of a company shorted into oblivion. A whopping 43 million shares of Wall Street shorted stock interest still remain trapped turning over and over almost on a daily basis to keep the lid on the stock while institutional investors take their ever increasing positions. Virtually, millions of shares are being traded almost daily, trying to tire and weaken the long investor (the investor who invests because the future is profitable). Steadfastly, we believe Belviq will be a blockbuster drug generating more than $1 billion of revenue for its owner each year. We attempted to school Wall Street financial "wizards" who didn't have the medical expertise to see the fundamentals of the equation here. They ignored our efforts, choosing instead to focus on a competing drug?
Reiterating some of the attempted education:
First, Belviq is a unique single-agent molecule developed using a very innovative high technology called GPCR CART Technology. The FDA correctly approved more than just a new drug candidate for the worst advancing epidemic in U.S. History - Obesity and all the comorbidities that come along with it.
The FDA gave a seal of approval to Arena's proprietary technology that has yielded hundreds of patents as revealed on page 19 of Arena's 2011 annual report. Yes, a promising future for Arena has just begun. We encourage retail investors to hold their winning stock positions in ARNA, and add to positions if possible, because we believe long investors will be handsomely rewarded for patience in this stock. Reading investor message boards on Yahoo, Google and Microsoft can be misleading and often times confusing. The FDA's approval is a testament that Belviq is a super drug because medicines may only be placed on the US market when they are safe, effective and of good quality. This is verified in the new drug authorization process. On June 27, 2012, the FDA approved Belviq for marketing. However, what few investors realize, including Wall Street, is that Belviq also received the very broad label. This usually is more important than the drug approval itself, since drugs can be approved, but with severe label restrictions which restrict use and marketing and therefore revenues. Additionaly, there is not a single documented FDA drug ever that has achieved blockbuster status being sold through mail order. This is NOT the case with Arena, as it is with the competing drug.
In fact, the very broad label the FDA gave Belviq actually gives physicians, broad discretion in the way a patient's illnesses, diseases, or co-morbidities can be managed with the use of Belviq. The demand for Belviq will come from knowledgable physicians who have their patient's best interests at heart.
1. The Obesity epidemic gives Arena's Belviq blockbuster drug status.
We learned from past blockbuster drug sales history that even non-epidemic treatment drugs' have grown to blockbuster status. For example, past experience shows blockbuster drugs meet annual sales targets from serving a huge population with medical need:
Drug - Condition - Pharma : 2010 US Sales
Lipitor - Cholesterol - Pfizer (NYSE: PFE) : $5,329,000,000
Zyprexa - Antipsychotic - Eli Lily : $2,496,000,000
Levaquin - Antibiotic - Johnson & Johnson : $1,312,000,000
Concerta - ADHD/ADD - Johnson & Johnson : $929,000,000
Protonix - Antacid - Pfizer : $690,000,000
Plavix - Anti-Platelet - Bristol-Myers : $6,154,000,000
Seroquel - Antipsychotic - AstraZeneca (NYSE: AZN) : $3,747,000,000
Singulair - Asthma - Merck (NYSE: MRK) : $3,351,000,000
Actos - Type 2 Diabetes - Amgen : $2,304,000,000
Can there be any doubt Arena's Belviq is a blockbuster drug, given the broad labeling for comorbidities? We think not.
2. The Obesity market is enormous:
The FDA knows the obesity market represents the largest healthcare crisis facing the country today. Retail investors know statistics that Wall Street doesn't seem to grasp. Obesity has a far-ranging negative effect on health. Obesity-related diseases account for nearly 10 percent of all US medical spending, or an estimated $147 Billion Dollars each year and cause an estimated 300,000 premature deaths in the US. Current estimates of the economic cost of obesity in the United States. The recent Gallop Poll found that Obesity costs cities an estimated $80 billion a year in healthcare costs. Health insurance will cover Belviq.
3. Physicians control the Demand Side for Belviq not the consumer:
Belviq's demand side is rather unique. It is characterized by a complex interrelationship amongst patients, doctors, hospitals, insurance providers and reimbursement systems. Usually, prescription medicines, the ultimate consumer (i.e. the patient) systematically differs from the decision maker (generally the prescribing doctor) and very often also from the bearer of the costs (generally the health system). I believe our health insurance system should cover Belviq because of the comorbidities in the labeling. Unlike other markets, the patient is normally not in a position to choose directly which product he/she wishes to use. The relationship between patient and doctor is characterized by an information asymmetry where the patient generally must rely on the doctor's expertise. Doctors are therefore decisive for the choice of obesity pharmaceutical products used (type and volume). This explains why it is historically so important for Arena and Eisai (originator companies) to remain in constant contact with doctors. In addition to detailing (visiting of doctors by pharmaceutical companies), the originator companies confirm that medical journals and seminars are the main source of information for doctors on developments in relation to medicines.
Arena as an active participant in the pharmaceutical sector is R&D driven and regulated. On the supply side, there are two types of companies. So-called "originator" companies are active in research, development, manufacturing, marketing and supply of innovative medicines. The average time taken from patent application filing to product launch is typically 8.6 years. Arena's Belviq is subject to secure patent protection, which is needed to provide a reward for innovation and incentives for future research. Arena's patent protection will not expire for over 14 years and in some cases can be extended. Competition from generic manufacturers cannot enter the market with medicines that are equivalent to Belviq typically at significantly lower prices for the benefit of the consumer.
In general terms, the life cycle of an originator product can be divided into three distinct phases: the pre-launch period, where R&D as well as regulatory (governmental) approval take place; the marketing and sales period, during which the product benefits from exclusivity; and a later period when the patent protection is lost and generic competition is possible.
Arena partnership with Eisai demonstrates management knows the importance of maintaining its freedom to operate, which is essential for an originator company to ensure that its research options remain as open as possible, in particular with regard to further development of its own inventions. Wall Street doesn't understand this aspect of the business as demonstrated by their bet against Belviq approval.
4. ARNA is a buyout, merger, or partner candidate.
According to Ibisworld, 13 blockbuster drugs will lose patent protection through 2013. One example, between 2010 and 2012, drugs that make up 42% of Pfizer pharmaceuticals revenue will lose patent protection. Big pharmaceutical companies need blockbuster drug companies more than ever. I believe Arena will either be bought out or merge with Eisai. It is very common for a pharmaceutical company with a high stock price (P/E Multiple) relative to earnings to use the stock as currency. A merger with Eisai in a stock for stock deal is the most likely event.
On the other hand, in this environment of shrinking blockbuster sales, a Big Pharmaceutical can easily afford to buy Arena in cash or stock and cash combination. Accountants and financial experts will agree that pharmaceutical companies like Pfizer, Abbot Labs, Astrazeneca , Novartis, and Merck & Co.,, just to name a few, have existing complimentary marketing infrastructure capable of making Belviq sales a source of virtually pure profit. The value of Arena on a 7 times multiple of past projected historical sales data (referred to above for drugs serving huge medical needs) alone would put Arena at $7-$10 Billion in a buyout scenario. However, Arena comes with some enticing benefits for a pharmaceutical suitor - 10 year Swiss Tax Haven and R&D Write-offs. How does Arena's proven unique drug discovery technology factor into the equation? Is there any doubt a pharmaceutical company would have to pay a premium for the hundreds of patents this technology has created. We challenge Wall Street either to agree with us or give us concrete analysis to the contrary.
Therefore Arena is a red-hot buy in my opinion. Arena's stock is temporarily beholden to the wave of short interest attacks sitting at all time highs of 43,934,871 as of August 31, 2012. The shorts, according to some experts who teach short selling, appear to have been misled into believing DEA Classification is the equivalent to FDA approval. This is absurd, and the short's are wrong again! The Drug Enforcement Administration is a scheduling classification used for drugs that have a likelihood or potential of being abused. For the record, during all pre-approval testing Belviq did not show any clinical abuse potential. No matter what DEA classification Belviq is given, I think it will be a blockbuster drug! Eisai will announce pricing and when Belviq will be available.
Arena's stock is trading at an extremely low price of $8.37/share. The 52-week high is $13.50/share. Arena is a long-term growth stock and the floodgates are about to open. The first catalyst is receipt of DEA classification. Then, we will have an obesity drug with broad FDA labeling, ready to treat obesity related comorbidities, by physicians who hold the key to demand and will use this drug with broad discretion. Simply put, it is the best single agent for obesity and its associated diseases. The news about the company's fundamentals will get better with the stream of news from the DEA, Eisai Pricing, Belviq Roll-Out, and the Big Pharmaceutical deal which could come at any time.
http://beta.fool.com/mrjosephd/2012/09/18/arena-pharmaceutic…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/mrjosephd/2012/09/18/arena-pharmaceutic…
By Joseph Dedvukaj - September 18, 2012
Retail investors were right
 and Wall Street was wrong about the approval of Arena Pharmaceuticals,
and Wall Street was wrong about the approval of Arena Pharmaceuticals, Inc's (NASDAQ: ARNA) lead drug candidate Belviq (Lorcaserin). Here are the four coming catalysts for Arena:
Inc's (NASDAQ: ARNA) lead drug candidate Belviq (Lorcaserin). Here are the four coming catalysts for Arena:1. The Obesity epidemic gives Arena's Belviq blockbuster drug status.
2. The growing obesity market is enormous and Belviq has a broad label.
3. Physicians control the demand for Belviq not the consumer.
4. ARNA is a buyout, merger, or partner candidate.
I believe anyone of these will drive the price of the stock much higher.
My citizen petition filed with the FDA helped ordinary citizens, the FDA, scientists, retail investors, and physicians understand why the FDA must approve Belviq. In my research we saw retail investors fight a bitter battle with Wall Street,
 with many analysts preoccupied with spreading fear, and heavily shorting the company's stock, hoping to reap the benefits of a company shorted into oblivion. A whopping 43 million shares of Wall Street shorted stock interest still remain trapped turning over and over almost on a daily basis to keep the lid on the stock while institutional investors take their ever increasing positions. Virtually, millions of shares are being traded almost daily, trying to tire and weaken the long investor (the investor who invests because the future is profitable). Steadfastly, we believe Belviq will be a blockbuster drug generating more than $1 billion of revenue for its owner each year. We attempted to school Wall Street financial "wizards" who didn't have the medical expertise to see the fundamentals of the equation here. They ignored our efforts, choosing instead to focus on a competing drug?
with many analysts preoccupied with spreading fear, and heavily shorting the company's stock, hoping to reap the benefits of a company shorted into oblivion. A whopping 43 million shares of Wall Street shorted stock interest still remain trapped turning over and over almost on a daily basis to keep the lid on the stock while institutional investors take their ever increasing positions. Virtually, millions of shares are being traded almost daily, trying to tire and weaken the long investor (the investor who invests because the future is profitable). Steadfastly, we believe Belviq will be a blockbuster drug generating more than $1 billion of revenue for its owner each year. We attempted to school Wall Street financial "wizards" who didn't have the medical expertise to see the fundamentals of the equation here. They ignored our efforts, choosing instead to focus on a competing drug?Reiterating some of the attempted education:
First, Belviq is a unique single-agent molecule developed using a very innovative high technology called GPCR CART Technology. The FDA correctly approved more than just a new drug candidate for the worst advancing epidemic in U.S. History - Obesity and all the comorbidities that come along with it.
The FDA gave a seal of approval to Arena's proprietary technology that has yielded hundreds of patents as revealed on page 19 of Arena's 2011 annual report. Yes, a promising future for Arena has just begun. We encourage retail investors to hold their winning stock positions in ARNA, and add to positions if possible, because we believe long investors will be handsomely rewarded for patience in this stock. Reading investor message boards on Yahoo, Google and Microsoft can be misleading and often times confusing. The FDA's approval is a testament that Belviq is a super drug because medicines may only be placed on the US market when they are safe, effective and of good quality. This is verified in the new drug authorization process. On June 27, 2012, the FDA approved Belviq for marketing. However, what few investors realize, including Wall Street, is that Belviq also received the very broad label. This usually is more important than the drug approval itself, since drugs can be approved, but with severe label restrictions which restrict use and marketing and therefore revenues. Additionaly, there is not a single documented FDA drug ever that has achieved blockbuster status being sold through mail order. This is NOT the case with Arena, as it is with the competing drug.
In fact, the very broad label the FDA gave Belviq actually gives physicians, broad discretion in the way a patient's illnesses, diseases, or co-morbidities can be managed with the use of Belviq. The demand for Belviq will come from knowledgable physicians who have their patient's best interests at heart.
1. The Obesity epidemic gives Arena's Belviq blockbuster drug status.
We learned from past blockbuster drug sales history that even non-epidemic treatment drugs' have grown to blockbuster status. For example, past experience shows blockbuster drugs meet annual sales targets from serving a huge population with medical need:
Drug - Condition - Pharma : 2010 US Sales
Lipitor - Cholesterol - Pfizer (NYSE: PFE) : $5,329,000,000
Zyprexa - Antipsychotic - Eli Lily : $2,496,000,000
Levaquin - Antibiotic - Johnson & Johnson : $1,312,000,000
Concerta - ADHD/ADD - Johnson & Johnson : $929,000,000
Protonix - Antacid - Pfizer : $690,000,000
Plavix - Anti-Platelet - Bristol-Myers : $6,154,000,000
Seroquel - Antipsychotic - AstraZeneca (NYSE: AZN) : $3,747,000,000
Singulair - Asthma - Merck (NYSE: MRK) : $3,351,000,000
Actos - Type 2 Diabetes - Amgen : $2,304,000,000
Can there be any doubt Arena's Belviq is a blockbuster drug, given the broad labeling for comorbidities? We think not.
2. The Obesity market is enormous:
The FDA knows the obesity market represents the largest healthcare crisis facing the country today. Retail investors know statistics that Wall Street doesn't seem to grasp. Obesity has a far-ranging negative effect on health. Obesity-related diseases account for nearly 10 percent of all US medical spending, or an estimated $147 Billion Dollars each year and cause an estimated 300,000 premature deaths in the US. Current estimates of the economic cost of obesity in the United States. The recent Gallop Poll found that Obesity costs cities an estimated $80 billion a year in healthcare costs. Health insurance will cover Belviq.
3. Physicians control the Demand Side for Belviq not the consumer:
Belviq's demand side is rather unique. It is characterized by a complex interrelationship amongst patients, doctors, hospitals, insurance providers and reimbursement systems. Usually, prescription medicines, the ultimate consumer (i.e. the patient) systematically differs from the decision maker (generally the prescribing doctor) and very often also from the bearer of the costs (generally the health system). I believe our health insurance system should cover Belviq because of the comorbidities in the labeling. Unlike other markets, the patient is normally not in a position to choose directly which product he/she wishes to use. The relationship between patient and doctor is characterized by an information asymmetry where the patient generally must rely on the doctor's expertise. Doctors are therefore decisive for the choice of obesity pharmaceutical products used (type and volume). This explains why it is historically so important for Arena and Eisai (originator companies) to remain in constant contact with doctors. In addition to detailing (visiting of doctors by pharmaceutical companies), the originator companies confirm that medical journals and seminars are the main source of information for doctors on developments in relation to medicines.
Arena as an active participant in the pharmaceutical sector is R&D driven and regulated. On the supply side, there are two types of companies. So-called "originator" companies are active in research, development, manufacturing, marketing and supply of innovative medicines. The average time taken from patent application filing to product launch is typically 8.6 years. Arena's Belviq is subject to secure patent protection, which is needed to provide a reward for innovation and incentives for future research. Arena's patent protection will not expire for over 14 years and in some cases can be extended. Competition from generic manufacturers cannot enter the market with medicines that are equivalent to Belviq typically at significantly lower prices for the benefit of the consumer.
In general terms, the life cycle of an originator product can be divided into three distinct phases: the pre-launch period, where R&D as well as regulatory (governmental) approval take place; the marketing and sales period, during which the product benefits from exclusivity; and a later period when the patent protection is lost and generic competition is possible.
Arena partnership with Eisai demonstrates management knows the importance of maintaining its freedom to operate, which is essential for an originator company to ensure that its research options remain as open as possible, in particular with regard to further development of its own inventions. Wall Street doesn't understand this aspect of the business as demonstrated by their bet against Belviq approval.
4. ARNA is a buyout, merger, or partner candidate.
According to Ibisworld, 13 blockbuster drugs will lose patent protection through 2013. One example, between 2010 and 2012, drugs that make up 42% of Pfizer pharmaceuticals revenue will lose patent protection. Big pharmaceutical companies need blockbuster drug companies more than ever. I believe Arena will either be bought out or merge with Eisai. It is very common for a pharmaceutical company with a high stock price (P/E Multiple) relative to earnings to use the stock as currency. A merger with Eisai in a stock for stock deal is the most likely event.
On the other hand, in this environment of shrinking blockbuster sales, a Big Pharmaceutical can easily afford to buy Arena in cash or stock and cash combination. Accountants and financial experts will agree that pharmaceutical companies like Pfizer, Abbot Labs, Astrazeneca , Novartis, and Merck & Co.,, just to name a few, have existing complimentary marketing infrastructure capable of making Belviq sales a source of virtually pure profit. The value of Arena on a 7 times multiple of past projected historical sales data (referred to above for drugs serving huge medical needs) alone would put Arena at $7-$10 Billion in a buyout scenario. However, Arena comes with some enticing benefits for a pharmaceutical suitor - 10 year Swiss Tax Haven and R&D Write-offs. How does Arena's proven unique drug discovery technology factor into the equation? Is there any doubt a pharmaceutical company would have to pay a premium for the hundreds of patents this technology has created. We challenge Wall Street either to agree with us or give us concrete analysis to the contrary.
Therefore Arena is a red-hot buy in my opinion. Arena's stock is temporarily beholden to the wave of short interest attacks sitting at all time highs of 43,934,871 as of August 31, 2012. The shorts, according to some experts who teach short selling, appear to have been misled into believing DEA Classification is the equivalent to FDA approval. This is absurd, and the short's are wrong again! The Drug Enforcement Administration is a scheduling classification used for drugs that have a likelihood or potential of being abused. For the record, during all pre-approval testing Belviq did not show any clinical abuse potential. No matter what DEA classification Belviq is given, I think it will be a blockbuster drug! Eisai will announce pricing and when Belviq will be available.
Arena's stock is trading at an extremely low price of $8.37/share. The 52-week high is $13.50/share. Arena is a long-term growth stock and the floodgates are about to open. The first catalyst is receipt of DEA classification. Then, we will have an obesity drug with broad FDA labeling, ready to treat obesity related comorbidities, by physicians who hold the key to demand and will use this drug with broad discretion. Simply put, it is the best single agent for obesity and its associated diseases. The news about the company's fundamentals will get better with the stream of news from the DEA, Eisai Pricing, Belviq Roll-Out, and the Big Pharmaceutical deal which could come at any time.
http://beta.fool.com/mrjosephd/2012/09/18/arena-pharmaceutic…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/mrjosephd/2012/09/18/arena-pharmaceutic…
2 Biotech Stocks Making Waves
By David Williamson, The Motley Fool
Posted 9:11PM 09/18/12 Posted under: Investing
------
Once again, biotech investors are reminded that a slow day on the markets doesn't guarantee we'll be drama free in the health-care sector. We had several big moves and a few notable headlines grabbing investor attention. Let's dive into two of those stories and highlight key investor takeaways.
Targacept (NAS: TRGT) managed to completely roll back yesterday's 11% decline, which happened when its ADHD drug candidate TC-5619 didn't pass its phase 2 trial. Adam Feuerstein of The Street noted that the support came from institutional buyer and major shareholder Fidelity. TC-5619 might not work for ADHD, but it may for schizophrenia and Alzheimer's, two massive markets.
This morning, shares traded for under cash on hand, although for an unprofitable biotech, cash is certainly a moving target. Before investors jump in with real money, though, remember that just because Targacept has institutional support, that doesn't mean its drug will be successful; Alzheimer's is a particularly tough disease to crack. Just ask Elan (NYS: ELN) investors, who lost 15% when bapineuzumab was shelved, or the fact that Eli Lilly (NYS: LLY) climbed 3% even as its drug, solanezumab, failed both phase 3 trials simply because it had a hint of efficacy.
Arena Pharmaceuticals (NAS: ARNA) added another 7% to yesterday's 3% gain, despite the news that rival obesity drug Qsymia by Vivus (NAS: VVUS) was commercially available. Having a competitor beat you to market is generally a bad thing, but this was widely expected, since Arena's Belviq is tied up in the DEA classification process. Besides, Belviq will be on the market soon enough, and the winner won't be decided in the first few months. Arena's rise this week is probably just momentum behind both obesity-drug makers, but at these lofty levels, a stumble by either one on launch could dampen enthusiasm for the space.
For an in-depth look at the hottest stock in biotech, try The Motley Fool's new premium report on Arena Pharmaceuticals. This report outlines key opportunities and risks facing the company plus the must-watch areas for investors. Click here to receive your copy now!
The article 2 Biotech Stocks Making Waves originally appeared on Fool.com.
http://www.dailyfinance.com/2012/09/18/2-biotech-stocks-maki…
By David Williamson, The Motley Fool
Posted 9:11PM 09/18/12 Posted under: Investing
------
Once again, biotech investors are reminded that a slow day on the markets doesn't guarantee we'll be drama free in the health-care sector. We had several big moves and a few notable headlines grabbing investor attention. Let's dive into two of those stories and highlight key investor takeaways.
Targacept (NAS: TRGT) managed to completely roll back yesterday's 11% decline, which happened when its ADHD drug candidate TC-5619 didn't pass its phase 2 trial. Adam Feuerstein of The Street noted that the support came from institutional buyer and major shareholder Fidelity. TC-5619 might not work for ADHD, but it may for schizophrenia and Alzheimer's, two massive markets.
This morning, shares traded for under cash on hand, although for an unprofitable biotech, cash is certainly a moving target. Before investors jump in with real money, though, remember that just because Targacept has institutional support, that doesn't mean its drug will be successful; Alzheimer's is a particularly tough disease to crack. Just ask Elan (NYS: ELN) investors, who lost 15% when bapineuzumab was shelved, or the fact that Eli Lilly (NYS: LLY) climbed 3% even as its drug, solanezumab, failed both phase 3 trials simply because it had a hint of efficacy.
Arena Pharmaceuticals (NAS: ARNA) added another 7% to yesterday's 3% gain, despite the news that rival obesity drug Qsymia by Vivus (NAS: VVUS) was commercially available. Having a competitor beat you to market is generally a bad thing, but this was widely expected, since Arena's Belviq is tied up in the DEA classification process. Besides, Belviq will be on the market soon enough, and the winner won't be decided in the first few months. Arena's rise this week is probably just momentum behind both obesity-drug makers, but at these lofty levels, a stumble by either one on launch could dampen enthusiasm for the space.
For an in-depth look at the hottest stock in biotech, try The Motley Fool's new premium report on Arena Pharmaceuticals. This report outlines key opportunities and risks facing the company plus the must-watch areas for investors. Click here to receive your copy now!
The article 2 Biotech Stocks Making Waves originally appeared on Fool.com.
http://www.dailyfinance.com/2012/09/18/2-biotech-stocks-maki…
Bloomberg News
Obesity Drugs Gain Attention on FDA Backing for Therapies
By Meg Tirrell on September 19, 2012
Obesity drugs are getting a lift after years of languishing even as populations around the globe get heavier. Rather than pharmaceutical giants, though, it’s small companies with an appetite for risk leading the charge.
Plagued with failures and side effects, drug development in obesity slowed to a near halt in the last decade as most large pharmaceutical companies abandoned efforts, leaving smaller biotechnology companies to forge ahead. This year, after 13 years with no new drug approvals, the U.S. Food and Drug Administration cleared two medicines for obesity, kick starting what many hope will be a rejuvenation for the field.
“A few years ago we were in the darker days of obesity where the regulatory environment was uncertain and pharma was doubtful this would ever be a market segment,” Kevin Starr, a partner at Boston-based venture capital firm Third Rock Ventures LLC, said in a telephone interview. “There’s been a sea change at the FDA in their recent approvals.”
Prospects brightened for obesity drug development this year as the FDA signaled it would be more willing to seriously consider drugs as a viable way to treat some forms of obesity, calling it a “major public health concern.” Now the betting is more approvals may follow.
Almost 36 percent of U.S. adults are obese, a condition that can lead to heart disease, Type 2 diabetes and stroke, according to the Centers for Disease Control and Prevention. Medical costs associated with obesity are estimated to have been $147 billion in 2008.
Early Support
Third Rock Ventures is an early backer of Zafgen Inc., a biotechnology company in mid-stage trials of a therapy to treat obesity. The Food and Drug Administration cleared Belviq, from Arena Pharmaceuticals Inc. (ARNA) and Eisai Co. (4523), in June and Vivus Inc. (VVUS)’s Qsymia in July, the first U.S. approvals for obesity drugs since 1999. They were shown to help obese patients lose about 5 to 10 percent of their body weight in clinical trials.
That’s a start, say many in the field. Still, the need for even more new drugs remains, they said.
“The concern among clinicians is that the overall pipeline for new drugs is very, very small,” said Carey Lumeng, an obesity researcher at the University of Michigan in Ann Arbor. “We aren’t getting enough new ones to market.”
Drug development will be among the topics discussed when the 30th annual meeting of the Obesity Society opens tomorrow in San Antonio, Texas. The program will touch on all areas of obesity research, from behavioral changes and exercise to surgery and therapeutics.
Multiple Therapies
In past years,“the biggest focus of the obesity meeting has been on exercise and behavior modification,” said Simos Simeonidis, an analyst with Cowen & Co., in a telephone interview. “Those things don’t work on their own; sometimes you have to do all of them together with drugs or surgery.”
Tom Hughes, chief executive officer of Cambridge, Massachusetts-based Zafgen, spent two decades at Novartis AG. (NOVN) He’s led Zafgen since 2008, forging a battle against accepted perceptions of obesity as a lifestyle choice -- the result of laziness or a lack of discipline. That view has kept many out of obesity drug development, he said.
“There are forces that keep people obese,” Hughes said in an interview. “People are fighting their physiology.”
An obese person’s body processes food differently from a lean person’s, Hughes said. One way is that the liver overproduces fat molecules, which get transported back to fat cells, or adipose tissue. Zafgen’s drug, beloranib, is designed to reset the way the body processes fat by inhibiting an enzyme tied to the liver’s fat production. It also changes the production of hormones that control trafficking of fat and the use of fat as energy, Hughes said.
Small Companies
Zafgen, with five full-time employees including Hughes, uses contract researchers to run its clinical trials. Its small size is typical of companies still willing to work on obesity drug development, after larger companies concluded in recent years that getting approvals for such drugs would be too long and cumbersome a process.
Arena, based in San Diego, and Vivus, in Mountain View, California, were both small biotechs before their drugs were cleared. Both had market valuations of less than $1 billion at the start of the year; now, Vivus is worth about $2.5 billion and Arena, $2 billion.
“It’s been the small companies that were willing to take the risks,” Mike King, an analyst with Rodman & Renshaw, said in a telephone interview. The approvals this year may draw some interest from larger drugmakers, he said. Obesity “is one of the last kind of monolithic markets that’s out there right now. Given the numbers, you have to expect that these would be blockbuster drugs.”
Safety Roadblocks
Big pharmaceutical companies would have to overcome bad memories of recent efforts in obesity drug development to jump back in. Safety issues tied to medicines’ effects on the heart and brain led to withdrawals and programs being shuttered throughout the 1990s and 2000s.
Wyeth’s Pondimin and Redux, also known as fenfluramine and dexfenfluramine, were pulled from the market in 1997 after they were linked to heart valve problems in patients who took it with phentermine, a combination known as fen-phen. The drugmaker, now owned by Pfizer Inc. (PFE), reserved more than $21 billion to settle lawsuits over the drugs.
Drug Failures
Sanofi (SAN), the Paris-based drugmaker, stopped developing its obesity pill Acomplia in 2008 after it was pulled from the market in Europe for possible links to suicide and depression. Pfizer and Merck & Co. (MRK) also ended obesity programs that year, with Pfizer, the world’s largest drugmaker, citing “likely new regulatory requirements for approval.” All three drugs targeted a brain receptor known to make marijuana smokers hungry. New York-based Pfizer bought Wyeth in 2009 for $68 billion.
Abbott Laboratories (ABT) pulled its diet pill, Meridia, off U.S. shelves in October 2010 after it was tied to heart attacks and strokes.
The two medicines approved this year were the first cleared by the FDA since Roche Holding AG (ROG)’s Xenical in 1999.
It was an uphill battle. The drugs were both rejected by the FDA in 2010, Arena’s for concerns over cancer links and Vivus’s for worries about birth defects and heart risks. They were cleared this year on the condition the companies perform post-marketing studies to monitor safety. A third drug, Contrave, from La Jolla, California-based Orexigen Therapeutics Inc. (OREX), must undergo a cardiovascular outcomes trial before it may be approved.
Zafgen’s Treatment
Zafgen’s therapy aims to avoid those concerns. It’s designed for severely obese patients, ones who may consider weight-loss surgery. The company has completed three early-stage trials, exposing 50 patients to the drug. In the first two, patients had an average body mass index of about 39; obesity is defined as 30 or greater. The newly approved drugs, Belviq and Qsymia, are approved for patients with BMI of at least 30, or of at least 27 with another weight-related condition such as Type 2 diabetes or hypertension.
BMI is calculated using height and weight. A 6-foot man who weighs more than 220 pounds is considered obese using the formula, according to the National Institutes of Health.
Zafgen’s beloranib helped patients lose an average of 4.3 kilograms (9.5 pounds) over 25 days, compared with little change for those taking a placebo. Patients taking the medicine also saw declines in their triglycerides and low-density lipoprotein cholesterol, contributors to heart disease, and had their waist circumference shrink.
‘Starvation Diets’
“The weight loss is three to four times what you get on other therapies,” Hughes said. “It’s roughly similar to starvation diets.”
Zafgen has started a longer study of more patients to look at safety and efficacy on a bigger scale. The mid-stage trial will examine the drug’s use for 12 weeks in 120 patients, with data expected in mid-2013.
Before this year’s approvals, Zafgen planned to seek a sale of the company once it received longer-term data on the drug. Now, the company plans to continue independently, Hughes said.
“The twin approvals reinstated a lot of confidence in our path,” he said. Hughes said he received e-mails of congratulations after the FDA’s announcements, even though he wasn’t working on those programs.
The success of the new-to-market drugs will factor into how much interest obesity therapeutics generate, Simeonidis said.
More Competition
“We will see other companies get back into it,” said Craig Audet, head of global regulatory affairs at Arena, in a telephone interview. “I think they will take a wait-and-see approach.”
Large drugmakers’ efforts have turned to diabetes, with some testing those compounds later for obesity. Novo Nordisk A/S (NOVOB), the world’s largest insulin maker, is in late-stage trials in obesity of its diabetes medicine Victoza. Eli Lilly & Co. (LLY), the Indianapolis-based maker of medicines for diabetes, cancer and depression, views obesity as “an area of interest,” CEO John Lechleiter said in a July interview.
“It’s a very important area of medicine,” Lechleiter said, noting “Lilly doesn’t have any molecules today that I can talk about.”
Merck, based in Whitehouse Station, New Jersey, doesn’t have anything listed in its pipeline for obesity, Caroline Lappetito, a spokeswoman, said. “Obesity research is not deemed a priority area for Merck at this time.”
Arena’s Audet said a “broad development gap” occurred after Sanofi’s Acomplia was pulled from the market, prompting other drugmakers to lose interest. Arena is looking for more obesity compounds to bring to the market, he said.
“There are some smaller companies in Phase 1 and 2, but no one really that far along,” he said.
Third Rock’s Starr sees that changing.
“Drug development had been paralyzed for awhile because of the lack of movement at the FDA and pharma exiting the space,” he said. “It has changed and will continue to change. If you fast forward five to 10 years from now, I would suspect this could be one of the more prolific areas for drug discovery because of the size of the problem.”
To contact the reporter on this story: Meg Tirrell in New York at mtirrell@bloomberg.net
To contact the editor responsible for this story: Reg Gale at rgale5@bloomberg.net
http://www.businessweek.com/news/2012-09-19/obesity-drugs-ga…
Obesity Drugs Gain Attention on FDA Backing for Therapies
By Meg Tirrell on September 19, 2012
Obesity drugs are getting a lift after years of languishing even as populations around the globe get heavier. Rather than pharmaceutical giants, though, it’s small companies with an appetite for risk leading the charge.
Plagued with failures and side effects, drug development in obesity slowed to a near halt in the last decade as most large pharmaceutical companies abandoned efforts, leaving smaller biotechnology companies to forge ahead. This year, after 13 years with no new drug approvals, the U.S. Food and Drug Administration cleared two medicines for obesity, kick starting what many hope will be a rejuvenation for the field.
“A few years ago we were in the darker days of obesity where the regulatory environment was uncertain and pharma was doubtful this would ever be a market segment,” Kevin Starr, a partner at Boston-based venture capital firm Third Rock Ventures LLC, said in a telephone interview. “There’s been a sea change at the FDA in their recent approvals.”
Prospects brightened for obesity drug development this year as the FDA signaled it would be more willing to seriously consider drugs as a viable way to treat some forms of obesity, calling it a “major public health concern.” Now the betting is more approvals may follow.
Almost 36 percent of U.S. adults are obese, a condition that can lead to heart disease, Type 2 diabetes and stroke, according to the Centers for Disease Control and Prevention. Medical costs associated with obesity are estimated to have been $147 billion in 2008.
Early Support
Third Rock Ventures is an early backer of Zafgen Inc., a biotechnology company in mid-stage trials of a therapy to treat obesity. The Food and Drug Administration cleared Belviq, from Arena Pharmaceuticals Inc. (ARNA) and Eisai Co. (4523), in June and Vivus Inc. (VVUS)’s Qsymia in July, the first U.S. approvals for obesity drugs since 1999. They were shown to help obese patients lose about 5 to 10 percent of their body weight in clinical trials.
That’s a start, say many in the field. Still, the need for even more new drugs remains, they said.
“The concern among clinicians is that the overall pipeline for new drugs is very, very small,” said Carey Lumeng, an obesity researcher at the University of Michigan in Ann Arbor. “We aren’t getting enough new ones to market.”
Drug development will be among the topics discussed when the 30th annual meeting of the Obesity Society opens tomorrow in San Antonio, Texas. The program will touch on all areas of obesity research, from behavioral changes and exercise to surgery and therapeutics.
Multiple Therapies
In past years,“the biggest focus of the obesity meeting has been on exercise and behavior modification,” said Simos Simeonidis, an analyst with Cowen & Co., in a telephone interview. “Those things don’t work on their own; sometimes you have to do all of them together with drugs or surgery.”
Tom Hughes, chief executive officer of Cambridge, Massachusetts-based Zafgen, spent two decades at Novartis AG. (NOVN) He’s led Zafgen since 2008, forging a battle against accepted perceptions of obesity as a lifestyle choice -- the result of laziness or a lack of discipline. That view has kept many out of obesity drug development, he said.
“There are forces that keep people obese,” Hughes said in an interview. “People are fighting their physiology.”
An obese person’s body processes food differently from a lean person’s, Hughes said. One way is that the liver overproduces fat molecules, which get transported back to fat cells, or adipose tissue. Zafgen’s drug, beloranib, is designed to reset the way the body processes fat by inhibiting an enzyme tied to the liver’s fat production. It also changes the production of hormones that control trafficking of fat and the use of fat as energy, Hughes said.
Small Companies
Zafgen, with five full-time employees including Hughes, uses contract researchers to run its clinical trials. Its small size is typical of companies still willing to work on obesity drug development, after larger companies concluded in recent years that getting approvals for such drugs would be too long and cumbersome a process.
Arena, based in San Diego, and Vivus, in Mountain View, California, were both small biotechs before their drugs were cleared. Both had market valuations of less than $1 billion at the start of the year; now, Vivus is worth about $2.5 billion and Arena, $2 billion.
“It’s been the small companies that were willing to take the risks,” Mike King, an analyst with Rodman & Renshaw, said in a telephone interview. The approvals this year may draw some interest from larger drugmakers, he said. Obesity “is one of the last kind of monolithic markets that’s out there right now. Given the numbers, you have to expect that these would be blockbuster drugs.”
Safety Roadblocks
Big pharmaceutical companies would have to overcome bad memories of recent efforts in obesity drug development to jump back in. Safety issues tied to medicines’ effects on the heart and brain led to withdrawals and programs being shuttered throughout the 1990s and 2000s.
Wyeth’s Pondimin and Redux, also known as fenfluramine and dexfenfluramine, were pulled from the market in 1997 after they were linked to heart valve problems in patients who took it with phentermine, a combination known as fen-phen. The drugmaker, now owned by Pfizer Inc. (PFE), reserved more than $21 billion to settle lawsuits over the drugs.
Drug Failures
Sanofi (SAN), the Paris-based drugmaker, stopped developing its obesity pill Acomplia in 2008 after it was pulled from the market in Europe for possible links to suicide and depression. Pfizer and Merck & Co. (MRK) also ended obesity programs that year, with Pfizer, the world’s largest drugmaker, citing “likely new regulatory requirements for approval.” All three drugs targeted a brain receptor known to make marijuana smokers hungry. New York-based Pfizer bought Wyeth in 2009 for $68 billion.
Abbott Laboratories (ABT) pulled its diet pill, Meridia, off U.S. shelves in October 2010 after it was tied to heart attacks and strokes.
The two medicines approved this year were the first cleared by the FDA since Roche Holding AG (ROG)’s Xenical in 1999.
It was an uphill battle. The drugs were both rejected by the FDA in 2010, Arena’s for concerns over cancer links and Vivus’s for worries about birth defects and heart risks. They were cleared this year on the condition the companies perform post-marketing studies to monitor safety. A third drug, Contrave, from La Jolla, California-based Orexigen Therapeutics Inc. (OREX), must undergo a cardiovascular outcomes trial before it may be approved.
Zafgen’s Treatment
Zafgen’s therapy aims to avoid those concerns. It’s designed for severely obese patients, ones who may consider weight-loss surgery. The company has completed three early-stage trials, exposing 50 patients to the drug. In the first two, patients had an average body mass index of about 39; obesity is defined as 30 or greater. The newly approved drugs, Belviq and Qsymia, are approved for patients with BMI of at least 30, or of at least 27 with another weight-related condition such as Type 2 diabetes or hypertension.
BMI is calculated using height and weight. A 6-foot man who weighs more than 220 pounds is considered obese using the formula, according to the National Institutes of Health.
Zafgen’s beloranib helped patients lose an average of 4.3 kilograms (9.5 pounds) over 25 days, compared with little change for those taking a placebo. Patients taking the medicine also saw declines in their triglycerides and low-density lipoprotein cholesterol, contributors to heart disease, and had their waist circumference shrink.
‘Starvation Diets’
“The weight loss is three to four times what you get on other therapies,” Hughes said. “It’s roughly similar to starvation diets.”
Zafgen has started a longer study of more patients to look at safety and efficacy on a bigger scale. The mid-stage trial will examine the drug’s use for 12 weeks in 120 patients, with data expected in mid-2013.
Before this year’s approvals, Zafgen planned to seek a sale of the company once it received longer-term data on the drug. Now, the company plans to continue independently, Hughes said.
“The twin approvals reinstated a lot of confidence in our path,” he said. Hughes said he received e-mails of congratulations after the FDA’s announcements, even though he wasn’t working on those programs.
The success of the new-to-market drugs will factor into how much interest obesity therapeutics generate, Simeonidis said.
More Competition
“We will see other companies get back into it,” said Craig Audet, head of global regulatory affairs at Arena, in a telephone interview. “I think they will take a wait-and-see approach.”
Large drugmakers’ efforts have turned to diabetes, with some testing those compounds later for obesity. Novo Nordisk A/S (NOVOB), the world’s largest insulin maker, is in late-stage trials in obesity of its diabetes medicine Victoza. Eli Lilly & Co. (LLY), the Indianapolis-based maker of medicines for diabetes, cancer and depression, views obesity as “an area of interest,” CEO John Lechleiter said in a July interview.
“It’s a very important area of medicine,” Lechleiter said, noting “Lilly doesn’t have any molecules today that I can talk about.”
Merck, based in Whitehouse Station, New Jersey, doesn’t have anything listed in its pipeline for obesity, Caroline Lappetito, a spokeswoman, said. “Obesity research is not deemed a priority area for Merck at this time.”
Arena’s Audet said a “broad development gap” occurred after Sanofi’s Acomplia was pulled from the market, prompting other drugmakers to lose interest. Arena is looking for more obesity compounds to bring to the market, he said.
“There are some smaller companies in Phase 1 and 2, but no one really that far along,” he said.
Third Rock’s Starr sees that changing.
“Drug development had been paralyzed for awhile because of the lack of movement at the FDA and pharma exiting the space,” he said. “It has changed and will continue to change. If you fast forward five to 10 years from now, I would suspect this could be one of the more prolific areas for drug discovery because of the size of the problem.”
To contact the reporter on this story: Meg Tirrell in New York at mtirrell@bloomberg.net
To contact the editor responsible for this story: Reg Gale at rgale5@bloomberg.net
http://www.businessweek.com/news/2012-09-19/obesity-drugs-ga…
Arena Pharmaceuticals - Market Recap
Posted on 09/18/2012 by Peter Mankowitz
NEW YORK (AVAFIN) -- Block trades help investors understand the sentiment of large financial institutions. A total of 164 block trades were executed during the last trading session, typically at least 10,000 shares or more represent a single block unit. Further analysis reveals that 2,274,308 shares were bought and 1,661,992 shares were sold. The bought/sold ratio for shares of Arena Pharmaceuticals is 1.37, representing a positive net cash flow of $5,591,948 into the stock.
On the derivative side, a total of 5,310 put and 29,616 call contracts exchanged hands yesterday leading to a 0.18 put/call ratio.
Arena Pharmaceuticals opened at $8.60 and the stock price rose $0.64 (+7.42%) to $9.26 in the last trading session. ARNA is trading between the range of $8.50 - $9.35. To date, the stock has gained 4.87% within the last week.
Arena Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company committed to discovering and developing innovative therapies offering medical advances and new options for patients.
http://www.avafin.com/articles/1017662.html
Posted on 09/18/2012 by Peter Mankowitz
NEW YORK (AVAFIN) -- Block trades help investors understand the sentiment of large financial institutions. A total of 164 block trades were executed during the last trading session, typically at least 10,000 shares or more represent a single block unit. Further analysis reveals that 2,274,308 shares were bought and 1,661,992 shares were sold. The bought/sold ratio for shares of Arena Pharmaceuticals is 1.37, representing a positive net cash flow of $5,591,948 into the stock.
On the derivative side, a total of 5,310 put and 29,616 call contracts exchanged hands yesterday leading to a 0.18 put/call ratio.
Arena Pharmaceuticals opened at $8.60 and the stock price rose $0.64 (+7.42%) to $9.26 in the last trading session. ARNA is trading between the range of $8.50 - $9.35. To date, the stock has gained 4.87% within the last week.
Arena Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company committed to discovering and developing innovative therapies offering medical advances and new options for patients.
http://www.avafin.com/articles/1017662.html
Sixty-three percent of adults in Alabama may be obese by 2030, report warns
Tuesday, September 18, 2012, 11:21 AM
Alabama health care burdens will soar if rates of obesity continue on their present trajectory, according to a policy report released this morning by two nonprofit organizations focused on improving health.
By 2030, 62.6 percent of adult Alabamians would be obese, nearly double the 32 percent in 2011, according to "F as in Fat: How Obesity Threatens America's Future," produced by the Trust for America's Health and the Robert Wood Johnson Foundation.
That increase would contribute to an estimated 661,673 new cases of type 2 diabetes, 1,458,880 new cases of coronary heart disease and stroke, 1,286,270 new cases of hypertension, 818,339 new cases of arthritis, and 200,226 new cases of obesity-related cancer in Alabama, the report found. Alabama would be one of 16 states -- mostly in the South and Midwest -- with more than 60 percent rates of obesity.
"The rise in health care costs could be staggering," Jeff Levi, executive director of Trust for America's Health, said in a telephone news conference. "That's a huge impact on our society. Overall, our states are on track for even worse health and significantly higher health care costs."
"But," Levi said, "that's not the way it has to be."
If Alabama adults could reduce their body mass indexes -- a measure of obesity that uses height and weight -- by 5 percent, the report says, the state would save 7.1 percent in health care costs for a cumulative savings of $9.4 billion in Alabama by 2030. That 5 percent would be equivalent to a 200-pound, 6-foot-tall person losing 10 pounds.
The report recommends making investments in obesity prevention to match the health and financial toll that the obesity epidemic is making on the nation.
http://blog.al.com/spotnews/2012/09/sixty-three_percent_of_a…" target="_blank" rel="nofollow ugc noopener">
http://blog.al.com/spotnews/2012/09/sixty-three_percent_of_a…
Tuesday, September 18, 2012, 11:21 AM
Alabama health care burdens will soar if rates of obesity continue on their present trajectory, according to a policy report released this morning by two nonprofit organizations focused on improving health.
By 2030, 62.6 percent of adult Alabamians would be obese, nearly double the 32 percent in 2011, according to "F as in Fat: How Obesity Threatens America's Future," produced by the Trust for America's Health and the Robert Wood Johnson Foundation.
That increase would contribute to an estimated 661,673 new cases of type 2 diabetes, 1,458,880 new cases of coronary heart disease and stroke, 1,286,270 new cases of hypertension, 818,339 new cases of arthritis, and 200,226 new cases of obesity-related cancer in Alabama, the report found. Alabama would be one of 16 states -- mostly in the South and Midwest -- with more than 60 percent rates of obesity.
"The rise in health care costs could be staggering," Jeff Levi, executive director of Trust for America's Health, said in a telephone news conference. "That's a huge impact on our society. Overall, our states are on track for even worse health and significantly higher health care costs."
"But," Levi said, "that's not the way it has to be."
If Alabama adults could reduce their body mass indexes -- a measure of obesity that uses height and weight -- by 5 percent, the report says, the state would save 7.1 percent in health care costs for a cumulative savings of $9.4 billion in Alabama by 2030. That 5 percent would be equivalent to a 200-pound, 6-foot-tall person losing 10 pounds.
The report recommends making investments in obesity prevention to match the health and financial toll that the obesity epidemic is making on the nation.
http://blog.al.com/spotnews/2012/09/sixty-three_percent_of_a…" target="_blank" rel="nofollow ugc noopener">
http://blog.al.com/spotnews/2012/09/sixty-three_percent_of_a…
Vivus Shares Feel The Pain: Blockbuster Diet Drug Likely Won't Be Sold In Europe
....was bereits immer wieder in den Foren diskutiert wurde, dass zum einen Phentermine als auch die erheblichen Nebenwirkungen ( siehe REMS)ein Approval in Europa unwahrscheinlich machen würden...
Vivus stock slumped more than 10% this morning after the company said its weight-loss drug will likely be denied approval in Europe.
The European Medicines Agency will make a formal decision on the drug, Qsiva, at a meeting next month. But initial comments from the organization make Qsiva’s future seem clouded. Vivus will have the opportunity to resubmit the medicine or appeal.
“We await the official decision and the formal report which should provide us specifics on any additional requirements leading to the approval of Qsiva in Europe,” said Vivus President Peter Tam. “We will work … to address the Committee’s concerns. VIVUS is committed to making this important medication available to obese patients in Europe.”
Shares of Vivus fell 12.1% to $20.85 in pre-market trading.
The drug is already approved in the United States, where Vivus markets the weight-loss pill under the name Qysmia. Meant to treat obesity in patients with at lease one weight-related condition, like high blood pressure or diabetes, Qysmia is seen as a potential blockbuster for Vivus. Blocked access to Europe—an area where half of the adult population is obese or overweight, greater than the alarming rates seen in America—would be a severe disappointment for the drugmaker.
Wells Fargo analyst Michael Tong says it’s a surprise decision by the European group. “The probable regulatory setback in Europe is an incremental modest negative for Vivus, in our view,” say Tong, who rates the stock as Market Perform. “Although we had always believed the U.S. end-user market represents the larger opportunity for Qsymia, if the approval pathway for Qsiva in Europe ultimately proves too onerous for Vivus to pursue, it does lower the peak sales potential for the Qsymia-Qsiva franchise”
Another diet drug, Arena Pharmaceuticals’ Belviq, was approved earlier this year in the U.S.
Arena and Vivus are small players in the global drug business, compete with such giants as GaxloSmithKline, Eli Lilly and Pfizer.
It’s taken Vivus years to gain approval for the weight-loss medicine in the U.S. It first applied for FDA approval in 2010 only to be met with rejection: The agency feared the drug could cause birth defects in children if taken by pregnant women.
http://www.forbes.com/sites/abrambrown/2012/09/21/vivus-shar…
....was bereits immer wieder in den Foren diskutiert wurde, dass zum einen Phentermine als auch die erheblichen Nebenwirkungen ( siehe REMS)ein Approval in Europa unwahrscheinlich machen würden...
Vivus stock slumped more than 10% this morning after the company said its weight-loss drug will likely be denied approval in Europe.
The European Medicines Agency will make a formal decision on the drug, Qsiva, at a meeting next month. But initial comments from the organization make Qsiva’s future seem clouded. Vivus will have the opportunity to resubmit the medicine or appeal.
“We await the official decision and the formal report which should provide us specifics on any additional requirements leading to the approval of Qsiva in Europe,” said Vivus President Peter Tam. “We will work … to address the Committee’s concerns. VIVUS is committed to making this important medication available to obese patients in Europe.”
Shares of Vivus fell 12.1% to $20.85 in pre-market trading.
The drug is already approved in the United States, where Vivus markets the weight-loss pill under the name Qysmia. Meant to treat obesity in patients with at lease one weight-related condition, like high blood pressure or diabetes, Qysmia is seen as a potential blockbuster for Vivus. Blocked access to Europe—an area where half of the adult population is obese or overweight, greater than the alarming rates seen in America—would be a severe disappointment for the drugmaker.
Wells Fargo analyst Michael Tong says it’s a surprise decision by the European group. “The probable regulatory setback in Europe is an incremental modest negative for Vivus, in our view,” say Tong, who rates the stock as Market Perform. “Although we had always believed the U.S. end-user market represents the larger opportunity for Qsymia, if the approval pathway for Qsiva in Europe ultimately proves too onerous for Vivus to pursue, it does lower the peak sales potential for the Qsymia-Qsiva franchise”
Another diet drug, Arena Pharmaceuticals’ Belviq, was approved earlier this year in the U.S.
Arena and Vivus are small players in the global drug business, compete with such giants as GaxloSmithKline, Eli Lilly and Pfizer.
It’s taken Vivus years to gain approval for the weight-loss medicine in the U.S. It first applied for FDA approval in 2010 only to be met with rejection: The agency feared the drug could cause birth defects in children if taken by pregnant women.
http://www.forbes.com/sites/abrambrown/2012/09/21/vivus-shar…
As Door Closes On Vivus It Swings Wide Open For Arena
By Reza Ganjavi - September 22, 2012
Reza is a member of The Motley Fool Blog Network -- entries represent the personal opinions of our bloggers and are not formally edited.
Vivus (NASDAQ: VVUS) announced today that the company's weight loss drug, Qsymia will likely be rejected by the European Medicines Agency committee. This news couldn't have come at a worse time for Vivus as it is participating in the Obesity Society's 30th Annual Scientific Meeting and word around this rejection could circulate among American doctors like wild fire.
Many believe FDA should not have approved Qsymia due to safety concerns and many believe at the very least FDA should have made pregnancy tests mandatory due to dangers around birth defect which is not the only safety concern with Qsymia.
I have heard of doctors holding a strong stance that they would never prescribe Qsymia due to safety concerns. Apparently European regulators agree with these doctors and are not going to allow Qsymia to be sold in Europe.
Vivus has indicated that it would appeal the decision but it seems like an uphill battle.
Today's news likely hurts Qsymia's chances of approvals in other regions as well.
A European rejection of Qsymia takes out a large chunk of the global obesity market away from Vivus and hands it to Arena Pharmaceuticals (NASDAQ: ARNA) whose chance of getting approval in Europe is very good given the excellent safety profile of Belviq and its strong efficacy.
Completers in the bloom and blossom studies lost an average of 8% of body weight; responders lost between 11% and 12% of body weight in a year. The top 25% of weight losers lost an average of 35 pounds in one year. It's comical to hear some analysts and journalists call this "marginal" efficacy. There's nothing marginal about these amazing numbers.
Arena will file answers to the EMA's 120-day questions next quarter with approval expected sometimes in first half of 2013.
This news also does not bode so well for Orexigen Therapeutics (NASDAQ: OREX) whose drug, Contrave doesn't have as good of a safety profile as Arena's Belviq.
Both VVUS and OREX were down today while ARNA was up 3.14% on above average volume. Vivus was down 11.47% at almost 4.5 times average volume.
SAFETY SAFETY SAFETY
Thumbs down to Qsymia in Europe puts the Eisai sales team at a stronger competitive advantage over Qsymia. Eisai has a top-notch sales team which has experience selling a blockbuster drug. Telling doctors that Qsymia was rejected in Europe due to safety concerns will not bode well to a physician community which is very safety conscious.
Speaking about safety, reading the Qsymia label is like watching a horror movie. It talks about the patient having to immediately stop the drug if a certain condition occurs (e.g. pregnancy) and then it talks about not immediately stopping the drug - that it should be tapered. No wonder some doctors are so adamantly against prescribing Qsymia and much prefer Belviq which has an excellent safety profile with the most common side effect being a transient mild headache.
THE ONLY GAME ON THE CONTINENT
For Arena, the most important aspect of this news is that the company will be able to ink an even better deal with a European partner now that Belviq appears to be the only game on... the continent.
Arena has indicated a strong level of interest from potential partners in Europe and other territories. This is one of the many catalysts which will move the stock which in my opinion is grossly under-valued and flooded with artificial supply (around 43 Million shorted shares) and an institutional interest which I think will want to at least double in the months ahead. This is an explosive combination with two strong streams of demand meeting a shortage of supply as retail investors who are holding 69% of the float don't seem to want to let it go at these prices no matter how many analysts, journalists, investment bankers and brokers tell us to sell by initiating or changing coverage with ridiculous, low-ball price targets.
IRON TIGHT RETAIL HANDS
I know several large retail investors, some holding in excess of a million shares, who are absolutely determined to keep their shares until ARNA reaches fair value which many of them believe is at least triple the current price.
Some sell-side analysts have been consistently wrong about Arena and their bashing of Arena and pumping of Vivus and Orexigen is almost comical to read based upon their apparently clueless yet manipulative commentary.
Imagine if Arena had a news item similar to what was released by Vivus about the anticipated EMA rejection of Qsymia. The same analysts would be all over it.
The best saying on this subject is "doctors write prescriptions not analysts". In this extremely important obesity market retail investors have beat professional high-paid analysts hands-down. Maybe some sell-side analysts should be called "paid shills of hedge funds" instead.
"DO AS I DO, NOT AS I SAY"
Despite all the pumping of Vivus and Orexigen, and all the bashing of Arena, institutions have been quietly accumulating ARNA more than they have either VVUS or OREX. Wall Street’s motto should be “Do As I Do, Not As I Say”. I'd rather do as Jack Lief, President and CEO of Arena has done:
not sell a single share since co-founding the company.
http://beta.fool.com/beatlesforever/2012/09/22/as-door-close…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/beatlesforever/2012/09/22/as-door-close…
By Reza Ganjavi - September 22, 2012
Reza is a member of The Motley Fool Blog Network -- entries represent the personal opinions of our bloggers and are not formally edited.
Vivus (NASDAQ: VVUS) announced today that the company's weight loss drug, Qsymia will likely be rejected by the European Medicines Agency committee. This news couldn't have come at a worse time for Vivus as it is participating in the Obesity Society's 30th Annual Scientific Meeting and word around this rejection could circulate among American doctors like wild fire.
Many believe FDA should not have approved Qsymia due to safety concerns and many believe at the very least FDA should have made pregnancy tests mandatory due to dangers around birth defect which is not the only safety concern with Qsymia.
I have heard of doctors holding a strong stance that they would never prescribe Qsymia due to safety concerns. Apparently European regulators agree with these doctors and are not going to allow Qsymia to be sold in Europe.
Vivus has indicated that it would appeal the decision but it seems like an uphill battle.
Today's news likely hurts Qsymia's chances of approvals in other regions as well.
A European rejection of Qsymia takes out a large chunk of the global obesity market away from Vivus and hands it to Arena Pharmaceuticals (NASDAQ: ARNA) whose chance of getting approval in Europe is very good given the excellent safety profile of Belviq and its strong efficacy.
Completers in the bloom and blossom studies lost an average of 8% of body weight; responders lost between 11% and 12% of body weight in a year. The top 25% of weight losers lost an average of 35 pounds in one year. It's comical to hear some analysts and journalists call this "marginal" efficacy. There's nothing marginal about these amazing numbers.
Arena will file answers to the EMA's 120-day questions next quarter with approval expected sometimes in first half of 2013.
This news also does not bode so well for Orexigen Therapeutics (NASDAQ: OREX) whose drug, Contrave doesn't have as good of a safety profile as Arena's Belviq.
Both VVUS and OREX were down today while ARNA was up 3.14% on above average volume. Vivus was down 11.47% at almost 4.5 times average volume.
SAFETY SAFETY SAFETY
Thumbs down to Qsymia in Europe puts the Eisai sales team at a stronger competitive advantage over Qsymia. Eisai has a top-notch sales team which has experience selling a blockbuster drug. Telling doctors that Qsymia was rejected in Europe due to safety concerns will not bode well to a physician community which is very safety conscious.
Speaking about safety, reading the Qsymia label is like watching a horror movie. It talks about the patient having to immediately stop the drug if a certain condition occurs (e.g. pregnancy) and then it talks about not immediately stopping the drug - that it should be tapered. No wonder some doctors are so adamantly against prescribing Qsymia and much prefer Belviq which has an excellent safety profile with the most common side effect being a transient mild headache.
THE ONLY GAME ON THE CONTINENT
For Arena, the most important aspect of this news is that the company will be able to ink an even better deal with a European partner now that Belviq appears to be the only game on... the continent.
Arena has indicated a strong level of interest from potential partners in Europe and other territories. This is one of the many catalysts which will move the stock which in my opinion is grossly under-valued and flooded with artificial supply (around 43 Million shorted shares) and an institutional interest which I think will want to at least double in the months ahead. This is an explosive combination with two strong streams of demand meeting a shortage of supply as retail investors who are holding 69% of the float don't seem to want to let it go at these prices no matter how many analysts, journalists, investment bankers and brokers tell us to sell by initiating or changing coverage with ridiculous, low-ball price targets.

IRON TIGHT RETAIL HANDS
I know several large retail investors, some holding in excess of a million shares, who are absolutely determined to keep their shares until ARNA reaches fair value which many of them believe is at least triple the current price.

Some sell-side analysts have been consistently wrong about Arena and their bashing of Arena and pumping of Vivus and Orexigen is almost comical to read based upon their apparently clueless yet manipulative commentary.
Imagine if Arena had a news item similar to what was released by Vivus about the anticipated EMA rejection of Qsymia. The same analysts would be all over it.
The best saying on this subject is "doctors write prescriptions not analysts". In this extremely important obesity market retail investors have beat professional high-paid analysts hands-down. Maybe some sell-side analysts should be called "paid shills of hedge funds" instead.
"DO AS I DO, NOT AS I SAY"
Despite all the pumping of Vivus and Orexigen, and all the bashing of Arena, institutions have been quietly accumulating ARNA more than they have either VVUS or OREX. Wall Street’s motto should be “Do As I Do, Not As I Say”. I'd rather do as Jack Lief, President and CEO of Arena has done:
not sell a single share since co-founding the company.

http://beta.fool.com/beatlesforever/2012/09/22/as-door-close…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/beatlesforever/2012/09/22/as-door-close…
Some Good News For Vivus And Arena Regarding Insurance
October 1, 2012; by Spencer Osborne
Disclosure: I am long ARNA. I have no position in VVUS.
I've already seen the message boards chiming in with debate over some recent news released about Vivus (VVUS). According to Bloomberg, recently launched Vivus is seeing that about one-third of the prescriptions written for its product, Qsymia, are being covered by insurance companies. That is great news for Vivus as well as competitor Arena Pharmaceuticals (ARNA). One major issue with anti-obesity drugs was whether or not insurance companies would cover the pills. According to Vivus, the one-third rate is higher than the company was anticipating.
I can tell from my email inbox that some investors are focusing on other aspects of the Bloomberg article, however. If you are serious about your investment in this sector, please try to remove the noise of message board debate. This article has news that bodes well for both Vivus and Arena.
Insurance Vs. Cash
It almost goes without saying that when a consumer perceives that he or she is getting a product cheaply, or for free, he or she is much more likely to participate. Thus, having insurance companies see the cost benefit to getting people down to a healthy weight and deciding to cover the treatment is good news.
There will certainly be some passionate investors in these companies saying things like, "Only one-third of patients getting insurance to cover Qsymia is a huge negative." This is noise. This type of debate accomplishes nothing. What we want to focus on here is that having one-third of the insurance companies pay for a new drug like this out of the gate is a victory for the sector. The number of insurers that cover it will only grow from here.
If you have ever had to take medication, you are likely aware that you sometimes have to jump through hoops to get a particular drug covered. This is particularly true when a drug or treatment is new. In my opinion, I could easily see the coverage rate jumping to 50% in short order and then moving up from there. Simply stated, remove the noise of those few passionate investors who are one-sided and look at what is happening for the sector.
The next piece of news in the Bloomberg article was that in the first week there were 106 prescriptions written for Vivus' Qsymia. This was another favorite of the passionate one-sided investors who filled my email inbox. One message said, "LOL... Only 106 prescriptions written... Vivus is DOOMED!!!" More noise.
Vivus is doomed? Really? Come on. Vivis has conducted what equates to a soft launch of its drug Qsymia. The company created a website and launched the product. It is testing the proverbial waters like any company that is essentially creating a new category does. Vivus has not yet even begun to "pound the pavement" on this. Rather than look at 106 prescriptions as a negative, we should be looking at this information for what it is.
In the first week of launch, the company obviously had doctors prescribe Qsymia 106 times. That is impressive. I am not sure about you, but I cannot typically get an appointment with my doctor with just five days notice. Typically, I have to set appointments weeks in advance. As the word about Vivus spreads, appointments will increase, as will consultations, and the numbers will increase at an impressive rate. Patience is required here, not fly-by-the-seat-of-your-pants rants.
Simos Simeonidis, an analyst with Cowen & Co., did some research that indicates great potential. In his analysis, about 70% to 75% of doctors surveyed stated that they would not be hesitant to prescribe Vivus' Qsymia or Arena's Belviq. Again, a huge positive in the piece. That market will grow as well.
Simply stated, the early indications seem to be that Vivus is off to a good start. There are positives in the early data, and there is a huge need for treatment. Set aside the Vivus vs. Arena noise and look at the positive developments for both of these companies. As I have said in the past, the potential market is massive and there is room for two or three companies to perform well.
http://seekingalpha.com/article/898531-some-good-news-for-vi…
October 1, 2012; by Spencer Osborne
Disclosure: I am long ARNA. I have no position in VVUS.
I've already seen the message boards chiming in with debate over some recent news released about Vivus (VVUS). According to Bloomberg, recently launched Vivus is seeing that about one-third of the prescriptions written for its product, Qsymia, are being covered by insurance companies. That is great news for Vivus as well as competitor Arena Pharmaceuticals (ARNA). One major issue with anti-obesity drugs was whether or not insurance companies would cover the pills. According to Vivus, the one-third rate is higher than the company was anticipating.
I can tell from my email inbox that some investors are focusing on other aspects of the Bloomberg article, however. If you are serious about your investment in this sector, please try to remove the noise of message board debate. This article has news that bodes well for both Vivus and Arena.
Insurance Vs. Cash
It almost goes without saying that when a consumer perceives that he or she is getting a product cheaply, or for free, he or she is much more likely to participate. Thus, having insurance companies see the cost benefit to getting people down to a healthy weight and deciding to cover the treatment is good news.
There will certainly be some passionate investors in these companies saying things like, "Only one-third of patients getting insurance to cover Qsymia is a huge negative." This is noise. This type of debate accomplishes nothing. What we want to focus on here is that having one-third of the insurance companies pay for a new drug like this out of the gate is a victory for the sector. The number of insurers that cover it will only grow from here.
If you have ever had to take medication, you are likely aware that you sometimes have to jump through hoops to get a particular drug covered. This is particularly true when a drug or treatment is new. In my opinion, I could easily see the coverage rate jumping to 50% in short order and then moving up from there. Simply stated, remove the noise of those few passionate investors who are one-sided and look at what is happening for the sector.
The next piece of news in the Bloomberg article was that in the first week there were 106 prescriptions written for Vivus' Qsymia. This was another favorite of the passionate one-sided investors who filled my email inbox. One message said, "LOL... Only 106 prescriptions written... Vivus is DOOMED!!!" More noise.
Vivus is doomed? Really? Come on. Vivis has conducted what equates to a soft launch of its drug Qsymia. The company created a website and launched the product. It is testing the proverbial waters like any company that is essentially creating a new category does. Vivus has not yet even begun to "pound the pavement" on this. Rather than look at 106 prescriptions as a negative, we should be looking at this information for what it is.
In the first week of launch, the company obviously had doctors prescribe Qsymia 106 times. That is impressive. I am not sure about you, but I cannot typically get an appointment with my doctor with just five days notice. Typically, I have to set appointments weeks in advance. As the word about Vivus spreads, appointments will increase, as will consultations, and the numbers will increase at an impressive rate. Patience is required here, not fly-by-the-seat-of-your-pants rants.
Simos Simeonidis, an analyst with Cowen & Co., did some research that indicates great potential. In his analysis, about 70% to 75% of doctors surveyed stated that they would not be hesitant to prescribe Vivus' Qsymia or Arena's Belviq. Again, a huge positive in the piece. That market will grow as well.
Simply stated, the early indications seem to be that Vivus is off to a good start. There are positives in the early data, and there is a huge need for treatment. Set aside the Vivus vs. Arena noise and look at the positive developments for both of these companies. As I have said in the past, the potential market is massive and there is room for two or three companies to perform well.
http://seekingalpha.com/article/898531-some-good-news-for-vi…
Antwort auf Beitrag Nr.: 43.667.274 von Poppholz am 02.10.12 07:42:27die Grundlage für den Kommentar aus Bloomberg:
Vivus’s Weight-Loss Pill Covered by Insurers 30% of the Time
By Drew Armstrong and Ryan Flinn - Oct 1, 2012 4:28 PM GMT+0200
Vivus Inc. (VVUS)’s obesity drug Qsymia was covered by health insurers more often than anticipated in its first week on the market, which may help drive sales.
Vivus had said it didn’t expect health plans to agree to pay for the medicine, according to Andrew Berens, an analyst with Bloomberg Industries. Qsymia was covered about a third of the time in the week starting Sept. 21. Insurers paying for the Mountain View, California-based company’s treatment may boost use of Qsymia, since patients wouldn’t have to pay cash.
“This is definitely a major concern among obesity physicians; there was a lot of talk -- some wishful thinking maybe -- that there will eventually be reimbursement for drug therapy in obesity,” Simos Simeonidis, an analyst with Cowen & Co., said in a Sept. 24 note to clients.
Qsymia is the first new weight loss drug on the market in more than a decade and may generate as much as $1.1 billion a year by 2016, Berens said. It will take time for sales of the drug to solidify, and in the first week, only 106 prescriptions were written, according to the data compiled by Bloomberg.
Vivus climbed 4.3 percent to $18.57 at 10:24 a.m. New York time. The shares had increased 83 percent this year through yesterday.
Arena Pharmaceuticals Inc. (ARNA), based in San Diego, has its obesity own drug, Belviq, that was approved by U.S. regulators in June. Orexigen Therapeutics Inc. (OREX), based in La Jolla, California, is developing a therapy as well, which if approved will be sold under the name Contrave.
Slow Expansion
The obesity drug market probably will expand slowly, Simeonidis said in an interview, as patients and doctors learn more about the medications and who should take them. He attended the annual meeting of the Obesity Society in San Antonio last month, and spoke to physicians to gauge their interest. He found about 70 to 75 percent said they would prescribe Qsymia or Belviq, while the other 25 to 30 percent were more skeptical, he wrote in a note last week.
“The biggest demand I saw was from the obesity specialists,” Simeonidis said in an interview. “From the general practitioner perspective, they’re going to be slower to adopt.” He compared the market for these new obesity drugs to the early development of medication to treat depression.
“It was similar to the obesity space, in that obesity is seen as a lifestyle and motivation issue, and depression was seen like that by some people, and that has become a huge market.”
Only a segment of patients are likely to get the drugs, he said, with those looking to lose 5 percent or less of their body weight being advised to focus on diet and exercise. On the other end of the scale, those who want or need to lose 30 percent or more of their body weight probably would require surgery. Still, Simeonidis said some doctors told him they would put these patients on the medications for months prior to surgery to reduce the amount that needs to be removed.
To contact the reporters on this story: Drew Armstrong in New York at darmstrong17@bloomberg.net; Ryan Flinn in San Francisco at rflinn@bloomberg.net
To contact the editor responsible for this story: Reg Gale at rgale5@bloomberg.net
http://www.bloomberg.com/news/2012-10-01/vivus-s-weight-loss…
Vivus’s Weight-Loss Pill Covered by Insurers 30% of the Time
By Drew Armstrong and Ryan Flinn - Oct 1, 2012 4:28 PM GMT+0200
Vivus Inc. (VVUS)’s obesity drug Qsymia was covered by health insurers more often than anticipated in its first week on the market, which may help drive sales.
Vivus had said it didn’t expect health plans to agree to pay for the medicine, according to Andrew Berens, an analyst with Bloomberg Industries. Qsymia was covered about a third of the time in the week starting Sept. 21. Insurers paying for the Mountain View, California-based company’s treatment may boost use of Qsymia, since patients wouldn’t have to pay cash.
“This is definitely a major concern among obesity physicians; there was a lot of talk -- some wishful thinking maybe -- that there will eventually be reimbursement for drug therapy in obesity,” Simos Simeonidis, an analyst with Cowen & Co., said in a Sept. 24 note to clients.
Qsymia is the first new weight loss drug on the market in more than a decade and may generate as much as $1.1 billion a year by 2016, Berens said. It will take time for sales of the drug to solidify, and in the first week, only 106 prescriptions were written, according to the data compiled by Bloomberg.
Vivus climbed 4.3 percent to $18.57 at 10:24 a.m. New York time. The shares had increased 83 percent this year through yesterday.
Arena Pharmaceuticals Inc. (ARNA), based in San Diego, has its obesity own drug, Belviq, that was approved by U.S. regulators in June. Orexigen Therapeutics Inc. (OREX), based in La Jolla, California, is developing a therapy as well, which if approved will be sold under the name Contrave.
Slow Expansion
The obesity drug market probably will expand slowly, Simeonidis said in an interview, as patients and doctors learn more about the medications and who should take them. He attended the annual meeting of the Obesity Society in San Antonio last month, and spoke to physicians to gauge their interest. He found about 70 to 75 percent said they would prescribe Qsymia or Belviq, while the other 25 to 30 percent were more skeptical, he wrote in a note last week.
“The biggest demand I saw was from the obesity specialists,” Simeonidis said in an interview. “From the general practitioner perspective, they’re going to be slower to adopt.” He compared the market for these new obesity drugs to the early development of medication to treat depression.
“It was similar to the obesity space, in that obesity is seen as a lifestyle and motivation issue, and depression was seen like that by some people, and that has become a huge market.”
Only a segment of patients are likely to get the drugs, he said, with those looking to lose 5 percent or less of their body weight being advised to focus on diet and exercise. On the other end of the scale, those who want or need to lose 30 percent or more of their body weight probably would require surgery. Still, Simeonidis said some doctors told him they would put these patients on the medications for months prior to surgery to reduce the amount that needs to be removed.
To contact the reporters on this story: Drew Armstrong in New York at darmstrong17@bloomberg.net; Ryan Flinn in San Francisco at rflinn@bloomberg.net
To contact the editor responsible for this story: Reg Gale at rgale5@bloomberg.net
http://www.bloomberg.com/news/2012-10-01/vivus-s-weight-loss…
Auszug:
...
2. Arena Pharmaceuticals
Arena is a clinical-stage biopharmaceutical company, engages in discovering, developing, and commercializing oral drugs that target G protein-coupled receptors in the therapeutic areas of cardiovascular, central nervous system, inflammatory, and metabolic diseases. In June 2012, the U.S. Food and Drug Administration (FDA) approved it internally discovered and developed drug, BELVIQ® (lorcaserin HCl).
Pipeline Portfolio
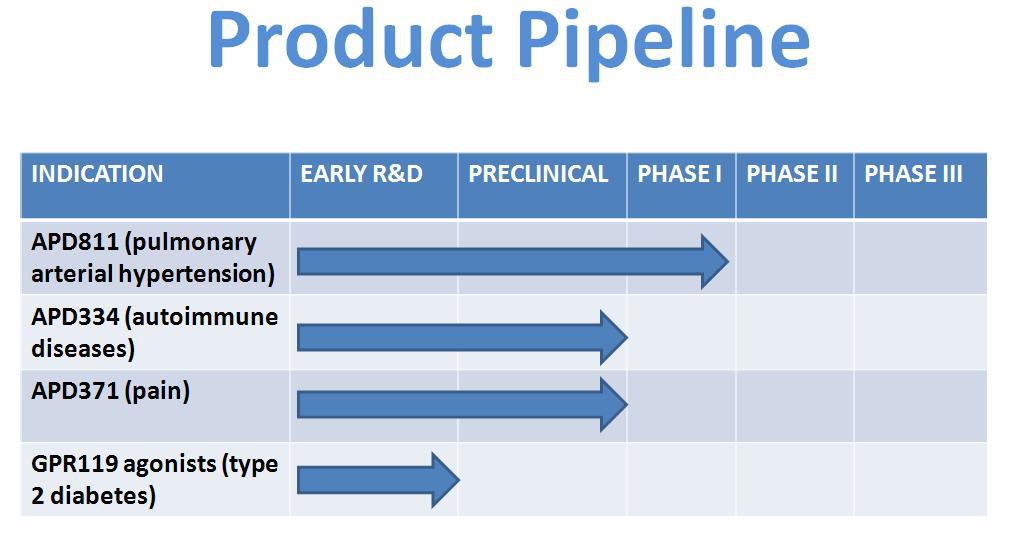
New Catalyst
On August, 2012 The company announced that it had received the 120 day assessment report as part of the European Medicines Agency review of the Marketing Authorization Application for lorcaserin. The questions and requests for additional information in this report will need to be addressed before lorcaserin can be recommended for marketing approval in the European Union.
Next Catalyst
Arena plans to respond to the 120 day assessment in the fourth quarter of 2012.
Fundamentals Analysis
Short interest
Settlement Date Short Interest Avg Daily Share Volume Days To Cover
10/15/2012 47,445,300 8,060,361 5.886250
9/28/2012 47,695,393 10,229,479 4.662544
9/14/2012 45,676,405 10,272,397 4.446519
Source: Nasdaq.com
Analyst Research
Source: Nasdaq.com
Financials
The company reported the second-quarter financial results on August 8, with the following highlights:
Revenue
$21.97MM
Net Income
($22.09MM)
Cash
$143.81MM
My Fundamental and Chart Analysis
Arena Pharmaceuticals has a market cap of $1.83 billion and an enterprise value of $1.77 Million. Its trailing P/E is incalculable, and its forward P/E is just incalculable. Arena's estimated growth rate for this year is 29.52%. It has a total cash position on its balance sheet of just $143.81 Million.
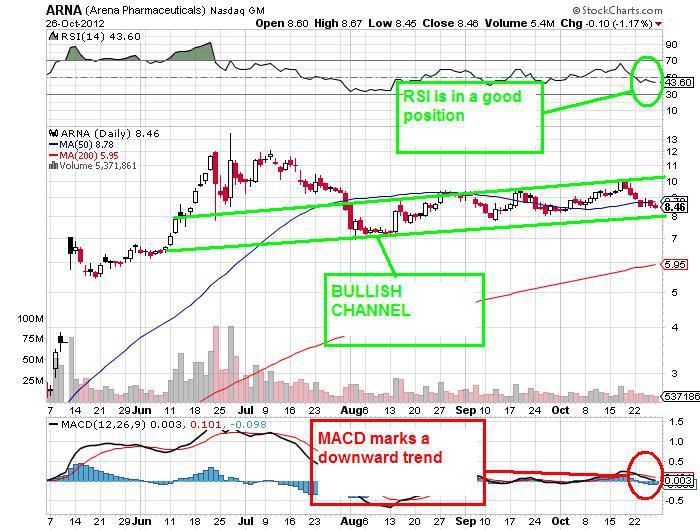
(click to enlarge)
This stock plunged from its June high of $13.50 to a recent low in August of $7.21. If you are bullish on this stock, I would look to be a buyer on the next high-volume move above some near-term overhead resistance at $8.75 a share. Look for volume that's tracking in close to or above its three-month average action of 8,565,650 shares. The RSI is in a good position and MACD marks a downward trend. The stock is in a bullish channel.
http://seekingalpha.com/article/958151-3-best-biotech-stocks…
...
2. Arena Pharmaceuticals
Arena is a clinical-stage biopharmaceutical company, engages in discovering, developing, and commercializing oral drugs that target G protein-coupled receptors in the therapeutic areas of cardiovascular, central nervous system, inflammatory, and metabolic diseases. In June 2012, the U.S. Food and Drug Administration (FDA) approved it internally discovered and developed drug, BELVIQ® (lorcaserin HCl).
Pipeline Portfolio

New Catalyst
On August, 2012 The company announced that it had received the 120 day assessment report as part of the European Medicines Agency review of the Marketing Authorization Application for lorcaserin. The questions and requests for additional information in this report will need to be addressed before lorcaserin can be recommended for marketing approval in the European Union.
Next Catalyst
Arena plans to respond to the 120 day assessment in the fourth quarter of 2012.
Fundamentals Analysis
Short interest
Settlement Date Short Interest Avg Daily Share Volume Days To Cover
10/15/2012 47,445,300 8,060,361 5.886250
9/28/2012 47,695,393 10,229,479 4.662544
9/14/2012 45,676,405 10,272,397 4.446519
Source: Nasdaq.com
Analyst Research

Source: Nasdaq.com
Financials
The company reported the second-quarter financial results on August 8, with the following highlights:
Revenue
$21.97MM
Net Income
($22.09MM)
Cash
$143.81MM
My Fundamental and Chart Analysis
Arena Pharmaceuticals has a market cap of $1.83 billion and an enterprise value of $1.77 Million. Its trailing P/E is incalculable, and its forward P/E is just incalculable. Arena's estimated growth rate for this year is 29.52%. It has a total cash position on its balance sheet of just $143.81 Million.

(click to enlarge)
This stock plunged from its June high of $13.50 to a recent low in August of $7.21. If you are bullish on this stock, I would look to be a buyer on the next high-volume move above some near-term overhead resistance at $8.75 a share. Look for volume that's tracking in close to or above its three-month average action of 8,565,650 shares. The RSI is in a good position and MACD marks a downward trend. The stock is in a bullish channel.
http://seekingalpha.com/article/958151-3-best-biotech-stocks…
Who’s Winning the Weight-Loss Drug Race?
By Meghan Foley | More Articles
October 30, 2012
Even though the market for obesity treatments is a large one, with approximately two-thirds of Americans overweight, no new long-term weight-loss drugs have been developed for the American market in more than a decade. But this year, three companies primed treatments for regulatory approval: Vivus (NASDAQ:VVUS), Arena Pharmaceuticals (NASDAQ:ARNA), and Orexigen Therapeutics (NASDAQ: OREX).
While Vivus launched its drug Qysmia at the end of September, ahead of rivals Arena and Orexigen, the race for the most successful treatment has not yet been won.
Since its release at the end of September, sales of Qysmia have been hampered by problems with distribution. Before the pill became available to consumers, analysts had worried about whether Qsymia would be covered by health insurers; even the company itself said that it did not expect health plans to agree to pay for the medicine. However, during the drug’s first week on the market, Bloomberg analysts reported that the drug was covered about a third of the time it was prescribed. Yet sales were still slow. The drug was only approved under a REMS (Risk Evaluation and Mitigation Strategy) by the FDA, which limited the drug’s availability to mail order purchases. On October 17, Vivus submitted an amendment to the agency to allow Qysmia to be sold at retail pharmacies, although Belviq has already been guaranteed this right.
Further problems have halted Qysmia’s expansion into Europe. The European Medicines Agency rejected Qsymia’s Marketing Authorization Application on October 18, after months of delays. While Vivus intends to resubmit its application, the agency’s decision suggests that the drug’s risk profile was too high. But this pronouncement was not unexpected. The Food and Drug Administration approved the drug in the United States only on the condition that a REMS was put in place to guard against the dangers associated with the drug’s components.
The other contenders, Arena’s Belviq and Orexigen’s Contrave, still have regulatory hurdles to overcome before reaching the market. While Belviq awaits Drug Enforcement Agency scheduling, Orexigen must resubmit Contrave to the FDA. The treatment was initially dismissed by the regulator because further data was needed to prove that the drug was safe enough for patients with heightened risk to cardiovascular events. On October 22, Orexigen announced that the FDA’s Center for Drug Evaluation and Research had issued a formal dispute resolution request that would give the company a faster path to resubmit Contrave. The company subsequently announced plans to enroll 9,000 patients in a new study by the end of 2012.
For much of their history, these three stocks have traded in sync. But as their respective obesity treatments came closer to hitting the market, the stocks began to diverged at the end of last year. Seeking Alpha contributor Brian L. Wilson noted in a recent article that the correlation between the three stocks has begun to wear thin. While all three pharmaceutical companies are targeting the “supposedly giant and undiscovered drug market” of obesity treatments,” Wilson questions whether all three can be successful in the same market.
His thesis is supported by recent declines in the companies’ stock prices. Vivus, although Qysmia reached the market ahead of other treatments, has seen its shares drop 22 percent over the last year. In comparison, Arena dropped only 6 percent and Orexigen just under 4 percent in the same period. It may seem counterintuitive that Vivus shares have fallen by a greater percentage, but its treatment has yet to post significant sales numbers. According to Seeking Alpha, Arena’s drug is in a better position to be a success because “there is a notion that Belviq is superior to Qsymia due to the big discrepancy in safety between the two drugs, although their efficacy in weight loss was never directly compared.”
http://wallstcheatsheet.com/stocks/whos-winning-the-weight-l…
By Meghan Foley | More Articles
October 30, 2012
Even though the market for obesity treatments is a large one, with approximately two-thirds of Americans overweight, no new long-term weight-loss drugs have been developed for the American market in more than a decade. But this year, three companies primed treatments for regulatory approval: Vivus (NASDAQ:VVUS), Arena Pharmaceuticals (NASDAQ:ARNA), and Orexigen Therapeutics (NASDAQ: OREX).
While Vivus launched its drug Qysmia at the end of September, ahead of rivals Arena and Orexigen, the race for the most successful treatment has not yet been won.
Since its release at the end of September, sales of Qysmia have been hampered by problems with distribution. Before the pill became available to consumers, analysts had worried about whether Qsymia would be covered by health insurers; even the company itself said that it did not expect health plans to agree to pay for the medicine. However, during the drug’s first week on the market, Bloomberg analysts reported that the drug was covered about a third of the time it was prescribed. Yet sales were still slow. The drug was only approved under a REMS (Risk Evaluation and Mitigation Strategy) by the FDA, which limited the drug’s availability to mail order purchases. On October 17, Vivus submitted an amendment to the agency to allow Qysmia to be sold at retail pharmacies, although Belviq has already been guaranteed this right.
Further problems have halted Qysmia’s expansion into Europe. The European Medicines Agency rejected Qsymia’s Marketing Authorization Application on October 18, after months of delays. While Vivus intends to resubmit its application, the agency’s decision suggests that the drug’s risk profile was too high. But this pronouncement was not unexpected. The Food and Drug Administration approved the drug in the United States only on the condition that a REMS was put in place to guard against the dangers associated with the drug’s components.
The other contenders, Arena’s Belviq and Orexigen’s Contrave, still have regulatory hurdles to overcome before reaching the market. While Belviq awaits Drug Enforcement Agency scheduling, Orexigen must resubmit Contrave to the FDA. The treatment was initially dismissed by the regulator because further data was needed to prove that the drug was safe enough for patients with heightened risk to cardiovascular events. On October 22, Orexigen announced that the FDA’s Center for Drug Evaluation and Research had issued a formal dispute resolution request that would give the company a faster path to resubmit Contrave. The company subsequently announced plans to enroll 9,000 patients in a new study by the end of 2012.
For much of their history, these three stocks have traded in sync. But as their respective obesity treatments came closer to hitting the market, the stocks began to diverged at the end of last year. Seeking Alpha contributor Brian L. Wilson noted in a recent article that the correlation between the three stocks has begun to wear thin. While all three pharmaceutical companies are targeting the “supposedly giant and undiscovered drug market” of obesity treatments,” Wilson questions whether all three can be successful in the same market.
His thesis is supported by recent declines in the companies’ stock prices. Vivus, although Qysmia reached the market ahead of other treatments, has seen its shares drop 22 percent over the last year. In comparison, Arena dropped only 6 percent and Orexigen just under 4 percent in the same period. It may seem counterintuitive that Vivus shares have fallen by a greater percentage, but its treatment has yet to post significant sales numbers. According to Seeking Alpha, Arena’s drug is in a better position to be a success because “there is a notion that Belviq is superior to Qsymia due to the big discrepancy in safety between the two drugs, although their efficacy in weight loss was never directly compared.”
http://wallstcheatsheet.com/stocks/whos-winning-the-weight-l…
Antwort auf Beitrag Nr.: 43.766.422 von Poppholz am 30.10.12 13:58:11sounds good.
Aber wos isn des:
"In June 2012, the U.S. Food and Drug Administration (FDA) approved it internally discovered and developed drug, BELVIQ® (lorcaserin HCl)."
und im Original genauso.
"its" ????
ich verstehs nich
Aber wos isn des:
"In June 2012, the U.S. Food and Drug Administration (FDA) approved it internally discovered and developed drug, BELVIQ® (lorcaserin HCl)."
und im Original genauso.
"its" ????
ich verstehs nich
Antwort auf Beitrag Nr.: 43.766.939 von me_2 am 30.10.12 15:58:34ist in diesem Zusammenhang als "Füllwort" zu nehmen.
genehmigen es (das Medikament).
genehmigen es (das Medikament).
Arena Pharmaceuticals to Host Corporate Update and Financial Results Conference Call and Webcast on Tuesday, November 6
SAN DIEGO, Oct. 31, 2012 /PRNewswire/ --Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) today announced that it will provide a corporate update and report third quarter 2012 financial results after the NASDAQ Global Select Market closes on Tuesday, November 6, 2012. The company will host a conference call and webcast at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time) that day to discuss the corporate update and financial results.
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' Third Quarter 2012 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
SAN DIEGO, Oct. 31, 2012 /PRNewswire/ --Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) today announced that it will provide a corporate update and report third quarter 2012 financial results after the NASDAQ Global Select Market closes on Tuesday, November 6, 2012. The company will host a conference call and webcast at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time) that day to discuss the corporate update and financial results.
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' Third Quarter 2012 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
Trading Analysis for Arena Pharmaceuticals
Posted on 11/02/2012 by Edward Connelly
NEW YORK (AVAFIN) -- A record number of Arena Pharmaceuticals call contracts were traded during the busy trading session. There were 3.3 call contracts traded for each put contract yielding a 0.30 put/call ratio where 7,132 put and 23,495 call contracts exchanged hands.
Put/Call ratio is often used to measure investment sentiment, the ratio serves as a predictor of investor behavior. Unusual options volume provides reliable clues that the stock is expected to make a move.
Arena Pharmaceuticals is currently trading at $7.79, down $0.04 (0.51%) in today's trading session. The shares of the stock were trading between $7.76 and $8.05. Within the last week, the shares have lost -8.21% of their value. Todays's volume of 6M shares is less than the average volume of 10M shares. ARNA is trading below the 50 day moving average and higher than the 200 day moving average.
http://www.avafin.com/articles/1018608.html
Posted on 11/02/2012 by Edward Connelly
NEW YORK (AVAFIN) -- A record number of Arena Pharmaceuticals call contracts were traded during the busy trading session. There were 3.3 call contracts traded for each put contract yielding a 0.30 put/call ratio where 7,132 put and 23,495 call contracts exchanged hands.
Put/Call ratio is often used to measure investment sentiment, the ratio serves as a predictor of investor behavior. Unusual options volume provides reliable clues that the stock is expected to make a move.
Arena Pharmaceuticals is currently trading at $7.79, down $0.04 (0.51%) in today's trading session. The shares of the stock were trading between $7.76 and $8.05. Within the last week, the shares have lost -8.21% of their value. Todays's volume of 6M shares is less than the average volume of 10M shares. ARNA is trading below the 50 day moving average and higher than the 200 day moving average.
http://www.avafin.com/articles/1018608.html
Beststocksdaily.com Issues Investment Alert On (NASDAQ:GALE), (NASDAQ:VVUS), (NASDAQ:ARNA), (NASDAQ:SIFY), (OTC:SANP)
Beststocksdaily.com MacReport Media
Galena Biopharma Inc(NASDAQ: GALE) stock increased 3.63% to $2.26. The company presented data from the Phase 1/2 clinical trial of NeuVax (nelipepimut-S or E75) at the 27th Annual Meeting of the Society for Immunotherapy of Cancer. The event is being held October 26-28, 2012 at the Bethesda North Marriott Hotel & Conference Center in North Bethesda, Maryland.
Will GALE Move Higher After Todays Sudden Selling Activities? Dont Miss The Free Trend Analysis here http://beststocksdaily.com/p2/index.php?company=GALE
VIVUS, Inc.(NASDAQ: VVUS) shares fell 2.07% to $14.72 in the early hour. The company announced that it will report financial results for the third quarter ended September 30, 2012 before the NASDAQ Market opens on Tuesday, November 6, 2012. The company will conduct a conference call and an audio webcast at 8:45am ET on the same day. The company will conduct a conference call and an audio webcast at 8:45am ET on the same day.
How Should Investors Trade VVUS After The Recent Momentum, Find Out Here http://beststocksdaily.com/p2/index.php?company=VVUS
Arena Pharmaceuticals, Inc.(NASDAQ:ARNA) shares gained 1.02% to $7.91 in the morning hour. The company announced that it will provide a corporate update and report third quarter 2012 financial results after the NASDAQ Global Select Market closes on Tuesday, November 6, 2012. The company will host a conference call and webcast at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time) that day to discuss the corporate update and financial results.
Get Free Special Trend Analysis On ARNA Here http://beststocksdaily.com/p2/index.php?company=ARNA
Sify Technologies Limited (ADR)(NASDAQ: SIFY) stock fell 2.03% to $1.93. The company reported revenues of INR 2059 million for the quarter ended September 30, 2012 against revenues of INR 1815 million for the corresponding quarter of the previous year, a growth of 13%. EBITDA for the quarter increased to INR 161 million, as compared to INR 136 million in the corresponding quarter previous year. Net profit for the quarter was INR 612 million, as against a net loss of INR 89 million in the corresponding quarter previous year.
Get Free Special Trend Analysis On SIFY Here http://beststocksdaily.com/p2/index.php?company=SIFY
Santo Mining Corp(OTC:SANP) stock climbed 2.56% to $0.836 after the company has instructed its counsel to prepare an application to FINRA regarding a 2-1 forward split of the Company's common shares. It is expected that the split will be undertaken as soon as practicable and will be administered on a 'payable upon surrender' basis; requiring shareholders to deliver their certificates to the Company's transfer agent in order to receive their new post-split shares.
Get Free Special Trend Analysis On SANP Here http://beststocksdaily.com/p2/index.php?company=SANP
About Beststocksdaily
Beststocksdailys team is engaged in providing valuable and updated news information on U.S. stocks on a regular basis. Beststocksdailys instant stock news on Major Gainers, Hot Stocks and various other stocks, guides investors in making the wise stock market investments decision. To Get Instant updates in the inbox, readers are advised to sign up for free at Beststocksdaily.com.
Disclaimer:
The assembled information disseminated by beststocksdaily.com is for information purposes only, and is neither a solicitation to buy nor an offer to sell securities. beststocksdaily.com does expect that investors will buy and sell securities based on information assembled and presented in beststocksdaily.com. PLEASE always do your own due diligence, and consult your financial advisor.
Contact:
Brain Burr
Beststocksdaily
1419 Westwood Blvd Los Angeles, CA
90024-4911
staff@beststocksdaily.com
www.beststocksdaily.com
SOURCE Beststocksdaily.com
http://www.equities.com/news/headline-story?dt=2012-11-02&va…
Beststocksdaily.com MacReport Media
Galena Biopharma Inc(NASDAQ: GALE) stock increased 3.63% to $2.26. The company presented data from the Phase 1/2 clinical trial of NeuVax (nelipepimut-S or E75) at the 27th Annual Meeting of the Society for Immunotherapy of Cancer. The event is being held October 26-28, 2012 at the Bethesda North Marriott Hotel & Conference Center in North Bethesda, Maryland.
Will GALE Move Higher After Todays Sudden Selling Activities? Dont Miss The Free Trend Analysis here http://beststocksdaily.com/p2/index.php?company=GALE
VIVUS, Inc.(NASDAQ: VVUS) shares fell 2.07% to $14.72 in the early hour. The company announced that it will report financial results for the third quarter ended September 30, 2012 before the NASDAQ Market opens on Tuesday, November 6, 2012. The company will conduct a conference call and an audio webcast at 8:45am ET on the same day. The company will conduct a conference call and an audio webcast at 8:45am ET on the same day.
How Should Investors Trade VVUS After The Recent Momentum, Find Out Here http://beststocksdaily.com/p2/index.php?company=VVUS
Arena Pharmaceuticals, Inc.(NASDAQ:ARNA) shares gained 1.02% to $7.91 in the morning hour. The company announced that it will provide a corporate update and report third quarter 2012 financial results after the NASDAQ Global Select Market closes on Tuesday, November 6, 2012. The company will host a conference call and webcast at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time) that day to discuss the corporate update and financial results.
Get Free Special Trend Analysis On ARNA Here http://beststocksdaily.com/p2/index.php?company=ARNA
Sify Technologies Limited (ADR)(NASDAQ: SIFY) stock fell 2.03% to $1.93. The company reported revenues of INR 2059 million for the quarter ended September 30, 2012 against revenues of INR 1815 million for the corresponding quarter of the previous year, a growth of 13%. EBITDA for the quarter increased to INR 161 million, as compared to INR 136 million in the corresponding quarter previous year. Net profit for the quarter was INR 612 million, as against a net loss of INR 89 million in the corresponding quarter previous year.
Get Free Special Trend Analysis On SIFY Here http://beststocksdaily.com/p2/index.php?company=SIFY
Santo Mining Corp(OTC:SANP) stock climbed 2.56% to $0.836 after the company has instructed its counsel to prepare an application to FINRA regarding a 2-1 forward split of the Company's common shares. It is expected that the split will be undertaken as soon as practicable and will be administered on a 'payable upon surrender' basis; requiring shareholders to deliver their certificates to the Company's transfer agent in order to receive their new post-split shares.
Get Free Special Trend Analysis On SANP Here http://beststocksdaily.com/p2/index.php?company=SANP
About Beststocksdaily
Beststocksdailys team is engaged in providing valuable and updated news information on U.S. stocks on a regular basis. Beststocksdailys instant stock news on Major Gainers, Hot Stocks and various other stocks, guides investors in making the wise stock market investments decision. To Get Instant updates in the inbox, readers are advised to sign up for free at Beststocksdaily.com.
Disclaimer:
The assembled information disseminated by beststocksdaily.com is for information purposes only, and is neither a solicitation to buy nor an offer to sell securities. beststocksdaily.com does expect that investors will buy and sell securities based on information assembled and presented in beststocksdaily.com. PLEASE always do your own due diligence, and consult your financial advisor.
Contact:
Brain Burr
Beststocksdaily
1419 Westwood Blvd Los Angeles, CA
90024-4911
staff@beststocksdaily.com
www.beststocksdaily.com
SOURCE Beststocksdaily.com
http://www.equities.com/news/headline-story?dt=2012-11-02&va…
Arena Looks To Be The Clear Winner In The Weight Loss Space
November 4, 2012 | 59 comments | about: ARNA, includes: VVUS
Disclosure: I am long ARNA. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Since October 17, shares of Vivus (VVUS) have fallen 35%. In the past, shares have been volatile, but never have they fallen to this degree since the FDA approval of Qsymia. The stock is still trading with a 50% gain in 2012, but in light of all that's occurred, it's becoming obvious that Arena Pharmaceuticals (ARNA) will be the clear winner in the weight loss sweepstakes. As a result, let's take a look at a timeline of events that have led shares of VVUS lower and how it benefits ARNA.
October 15 - VVUS gains momentum as Qsymia sees early success and strong demand. In the third week following its launch, Qsymia prescriptions rose 150% sequentially. This data also showed that 53% of the scripts were paid for by commercial insurers versus 36% in the week prior. This was the last round of positive news that the company has seen; which did appear encouraging as insurers were covering the drug and sales were booming. However, it all went south from here.
October 17 - Vivus asks the FDA to allow wider pharmacy sales of its weight loss drug. The company can not advertise or market its drug to the fullest extent, and the product is only available through certified mail order pharmacies. This has been a daunting concern for investors, as the simple fact is that some consumers don't know that Qnexa exists. Some believed that this may be a sign of desperation, or that VVUS now realizes its demand will be weak after the initial push.
October 19 - Vivus fails to win European Agency backing. This was no real surprise. Earlier this year Vivus prepared investors for the fact that the EU would not support its weight loss drug. However, the concerns of cardiovascular and nervous system effects brought back bad memories, and were the start of a vicious downtrend.
November 2 - Credit Suisse analyst noted that initial sales for Qsymia may be slower than expected due to a more cautious distribution method. This has been the number one concern among investors, and has become a significant problem for the company. The sales guidance of $12 million for Q4 was way below the consensus.
After the company's drug being rejected in the EU and sales projections continuing to fall, the stock has been crushed in anticipation of earnings on November 6. It appears as though no one wants to hold this stock ahead of the announcement, in fear of weak sales and lower guidance.
Arena's Benefit
I should probably begin by saying that it's really too early to tell if Qsymia will be a long-term failure. I have said time-after-time-after-time that the best time to buy a biotechnology stock, following an approval, is right after its launch. Almost always, companies with excessive valuations and high expectations do not live up to high expectations during the initial launch. It takes time to build a network, market, and distribute the product. However, the threat of looming competition and a very strict distribution/marketing policy is making it very difficult for Vivus to succeed.
All of the problems facing Vivus are a non-issue for Arena Pharmaceuticals. Its FDA approved drug Belviq is safe, effective, and will most likely launch sometime early in 2013. In fact, the company's only visible problem has been a slower than expected launch. But the good news is that the launch and all of the marketing will be handled by a third-party partner, and is expected to be one of the largest launches to date.
Arena will only receive up to 35% of the sales from its product, however EU approval is not expected to be a problem. The company is awaiting DEA scheduling, but it will have a much more favorable marketing program compared to Vivus. Belviq will be marketed by reps, it will be advertised, it will be covered by insurers, it will be safe, and it will not in any way be hidden from the public like Qsymia as a result of strict regulations.
Conclusion
Despite Vivus' early problems there is one thing that has not changed, the size of the weight loss market, which is still one of the largest unmet medical needs in healthcare. Aside from Vivus' valuation, there is something else that has changed: The valuation of Arena Pharmaceuticals. The stock has declined 22% since October 18.
The market for weight loss products is huge, there are two approved products, and at least one is going to benefit mightily from the pure size and demand within the industry. In my opinion, the restrictions placed on Vivus and its inability to properly market is going to be a blessing for Arena, a company that is also valued for a worst case scenario.
A few months ago I purchased shares of both companies, a small position. My reason was because I thought both would see an early pop during the first year but then only one would emerge as the clear winner. I thought that Vivus' product was more effective but that Arena's was safer, and I wasn't sure which the consumer would prefer. However, if consumers aren't aware of Qsymia's existence then there isn't much competition, because although the FDA did approve Qsymia, it seems almost evident that it was only to quiet the critics who wanted an effective weight loss product approved. It almost seems as though the FDA set Vivus up to fail, and so far I think they succeeded, meanwhile Arena looks to benefit. As a result, I think Arena's valuation is attractive and that it presents solid upside potential from this point forward.
http://seekingalpha.com/article/976241-arena-looks-to-be-the…
November 4, 2012 | 59 comments | about: ARNA, includes: VVUS
Disclosure: I am long ARNA. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Since October 17, shares of Vivus (VVUS) have fallen 35%. In the past, shares have been volatile, but never have they fallen to this degree since the FDA approval of Qsymia. The stock is still trading with a 50% gain in 2012, but in light of all that's occurred, it's becoming obvious that Arena Pharmaceuticals (ARNA) will be the clear winner in the weight loss sweepstakes. As a result, let's take a look at a timeline of events that have led shares of VVUS lower and how it benefits ARNA.
October 15 - VVUS gains momentum as Qsymia sees early success and strong demand. In the third week following its launch, Qsymia prescriptions rose 150% sequentially. This data also showed that 53% of the scripts were paid for by commercial insurers versus 36% in the week prior. This was the last round of positive news that the company has seen; which did appear encouraging as insurers were covering the drug and sales were booming. However, it all went south from here.
October 17 - Vivus asks the FDA to allow wider pharmacy sales of its weight loss drug. The company can not advertise or market its drug to the fullest extent, and the product is only available through certified mail order pharmacies. This has been a daunting concern for investors, as the simple fact is that some consumers don't know that Qnexa exists. Some believed that this may be a sign of desperation, or that VVUS now realizes its demand will be weak after the initial push.
October 19 - Vivus fails to win European Agency backing. This was no real surprise. Earlier this year Vivus prepared investors for the fact that the EU would not support its weight loss drug. However, the concerns of cardiovascular and nervous system effects brought back bad memories, and were the start of a vicious downtrend.
November 2 - Credit Suisse analyst noted that initial sales for Qsymia may be slower than expected due to a more cautious distribution method. This has been the number one concern among investors, and has become a significant problem for the company. The sales guidance of $12 million for Q4 was way below the consensus.
After the company's drug being rejected in the EU and sales projections continuing to fall, the stock has been crushed in anticipation of earnings on November 6. It appears as though no one wants to hold this stock ahead of the announcement, in fear of weak sales and lower guidance.
Arena's Benefit
I should probably begin by saying that it's really too early to tell if Qsymia will be a long-term failure. I have said time-after-time-after-time that the best time to buy a biotechnology stock, following an approval, is right after its launch. Almost always, companies with excessive valuations and high expectations do not live up to high expectations during the initial launch. It takes time to build a network, market, and distribute the product. However, the threat of looming competition and a very strict distribution/marketing policy is making it very difficult for Vivus to succeed.
All of the problems facing Vivus are a non-issue for Arena Pharmaceuticals. Its FDA approved drug Belviq is safe, effective, and will most likely launch sometime early in 2013. In fact, the company's only visible problem has been a slower than expected launch. But the good news is that the launch and all of the marketing will be handled by a third-party partner, and is expected to be one of the largest launches to date.
Arena will only receive up to 35% of the sales from its product, however EU approval is not expected to be a problem. The company is awaiting DEA scheduling, but it will have a much more favorable marketing program compared to Vivus. Belviq will be marketed by reps, it will be advertised, it will be covered by insurers, it will be safe, and it will not in any way be hidden from the public like Qsymia as a result of strict regulations.
Conclusion
Despite Vivus' early problems there is one thing that has not changed, the size of the weight loss market, which is still one of the largest unmet medical needs in healthcare. Aside from Vivus' valuation, there is something else that has changed: The valuation of Arena Pharmaceuticals. The stock has declined 22% since October 18.
The market for weight loss products is huge, there are two approved products, and at least one is going to benefit mightily from the pure size and demand within the industry. In my opinion, the restrictions placed on Vivus and its inability to properly market is going to be a blessing for Arena, a company that is also valued for a worst case scenario.
A few months ago I purchased shares of both companies, a small position. My reason was because I thought both would see an early pop during the first year but then only one would emerge as the clear winner. I thought that Vivus' product was more effective but that Arena's was safer, and I wasn't sure which the consumer would prefer. However, if consumers aren't aware of Qsymia's existence then there isn't much competition, because although the FDA did approve Qsymia, it seems almost evident that it was only to quiet the critics who wanted an effective weight loss product approved. It almost seems as though the FDA set Vivus up to fail, and so far I think they succeeded, meanwhile Arena looks to benefit. As a result, I think Arena's valuation is attractive and that it presents solid upside potential from this point forward.
http://seekingalpha.com/article/976241-arena-looks-to-be-the…
Theravance: Still A Buy On Weakness
November 4, 2012 | 2 comments | about: THRX, includes: MRK
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. (More...)
The negative trading in biopharmaceutical companies continued to hurt companies that successfully obtained FDA approvals but have yet to release a product to market. For the year-to-date, the S&P 500 is up around 12%, while the SPDR S&P Pharmaceutical (XPH) is up 10%. More recently, over the last month, negativity in the markets was acute: the S&P is down 2.5%, while the pharmaceutical ETF is down even more, declining 7%. Arena Pharmaceuticals (ARNA), VVUS Inc. (VVUS), and Theravance Inc. (THRX) are down even more than the pharmaceutical index:
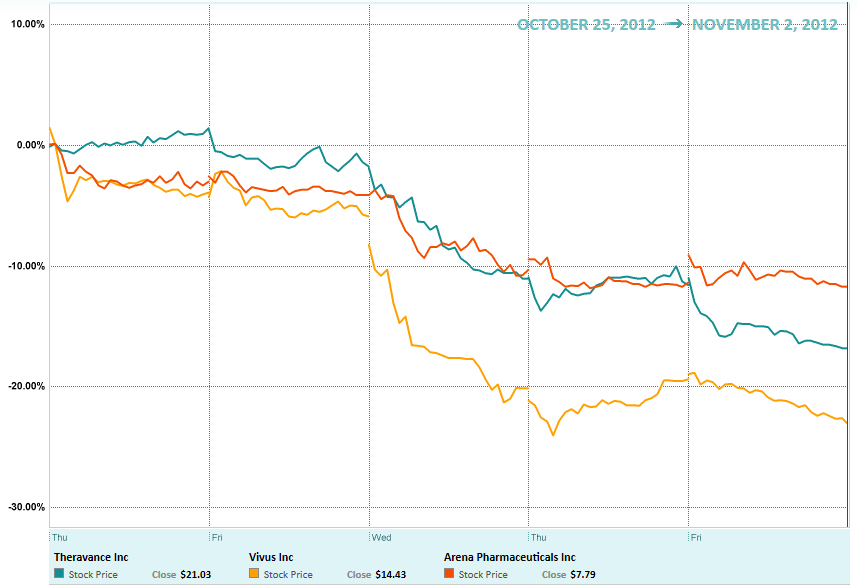
Skittish investors are now pricing in operational risks inherent in bringing a drug product to market. VIVUS shares are having an especially difficult time, as investors renew concerns for Qsymia and its side effects. The European Medicines Agency rejection and operational risks in launching the drug also continue to weigh heavily on shares. Arena, whose Belviq obesity drug is expected to launch next year, will update shareholders on its progress when it reports earnings on November 6. If earnings from Theravance and Valeant Pharmaceuticals (VRX) are any indication, Arena and VIVUS could still fall even if good news is reported.
During the third quarter conference call, Theravance provided a number of positive updates to its studies. The company said that:
Phase 3 data for FF/VI (fluticasone furoate and vilanterol) was presented, and a review of the regulator findings began in the United States and in Europe. Registration is in progress for FF/VI, known as "Relvar" in the European Union and Japan and "Breo" in the United States.
Phase 3 for umeclidinium bromide (UMEC), vilanterol (VI), and Chronic Obstructive Pulmonary Disease ("COPD") was completed and was on track for regulatory filing for COPD from the end of this year.
Results were positive for phase 2B for TD-1211. TD-1211 is used to alleviate constipation in patients receiving opioid therapy. Discussions with regulators to continue to the next study phase could take place before the end of the year.
TD-9855, a norepinephrine serotonin reuptake inhibitor, is undergoing a phase 2 safety and efficacy study for adult ADHD.
Upside in Arena could continue to be limited in the near-term, due mostly to investors being impatient. Even when Theravance signed a collaboration agreement with Merck (MRK) to study treatments for hypertension and heart failure, shares still declined sharply from the $26-level. Theravance closed recently at $21.03. The short-sighted nature of speculators gives investors an entry-point at a lower price. Theravance will receive $5 million from Merck initially, and will be eligible for milestone payments that total $148 million for royalties and first indication on any products coming from the joint efforts.
Theravance also made an agreement with a Russian firm, R-Pharm. Theravance may earn up to $2 million in licensing, $4 million in development milestones, $6 million in sales milestones, and 15% royalty for net sales of TD-1792. TD-1792 is an antibiotic used to treat drug-resistant Gram-positive infections.
Earnings Review
In the third quarter, Theravance lost $0.37 per share, or $34.7 million on revenue of $1.4 million. Revenue declined from $6.4 million last year because the VIBATIV collaboration agreement with Astellas was terminated on January 6 2012. VIBATIV is used to treat methicillin-resistant Staphylococcus aureus (MRSA).
Theravance ended the quarter with cash and cash equivalents totaling $352.4 million.
Theravance reiterated that operating expenses will be at the higher end of between the $120 million and $130 million guidance.
Conclusion
The market sell-off in companies like Theravance, Arena, and Theravance-partner GlaxoSmithKline plc (GSK), created an opportunity for investors with a longer-term timeframe. Investors who want exposure to biopharmaceutical companies and understand the risks associated with drug development should consider Theravance. Theravance has a pipeline of products. Most notably, it is working on treatment for illnesses like COPD. When brought to market, its drug will be able to treat a substantial proportion of the population suffering from COPD.
The company is planning to provide an update on MABA (Bifunctional Muscarinic Antagonist-Beta2 Agonist) by the end of the year, and will give more updates at conferences scheduled for next year. Theravance will present at the European Respiratory Society congress held between September 7 - 11 2013. Investors should also expect Theravance to report updates to LABA (Long-Acting Beta2 Agonist) and VI (Vilanterol Trifenatate) for Asthma treatment in adults at the American Thoracic Society conference held on May 17-22 2013.
http://seekingalpha.com/article/976631-theravance-still-a-bu…
November 4, 2012 | 2 comments | about: THRX, includes: MRK
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. (More...)
The negative trading in biopharmaceutical companies continued to hurt companies that successfully obtained FDA approvals but have yet to release a product to market. For the year-to-date, the S&P 500 is up around 12%, while the SPDR S&P Pharmaceutical (XPH) is up 10%. More recently, over the last month, negativity in the markets was acute: the S&P is down 2.5%, while the pharmaceutical ETF is down even more, declining 7%. Arena Pharmaceuticals (ARNA), VVUS Inc. (VVUS), and Theravance Inc. (THRX) are down even more than the pharmaceutical index:

Skittish investors are now pricing in operational risks inherent in bringing a drug product to market. VIVUS shares are having an especially difficult time, as investors renew concerns for Qsymia and its side effects. The European Medicines Agency rejection and operational risks in launching the drug also continue to weigh heavily on shares. Arena, whose Belviq obesity drug is expected to launch next year, will update shareholders on its progress when it reports earnings on November 6. If earnings from Theravance and Valeant Pharmaceuticals (VRX) are any indication, Arena and VIVUS could still fall even if good news is reported.
During the third quarter conference call, Theravance provided a number of positive updates to its studies. The company said that:
Phase 3 data for FF/VI (fluticasone furoate and vilanterol) was presented, and a review of the regulator findings began in the United States and in Europe. Registration is in progress for FF/VI, known as "Relvar" in the European Union and Japan and "Breo" in the United States.
Phase 3 for umeclidinium bromide (UMEC), vilanterol (VI), and Chronic Obstructive Pulmonary Disease ("COPD") was completed and was on track for regulatory filing for COPD from the end of this year.
Results were positive for phase 2B for TD-1211. TD-1211 is used to alleviate constipation in patients receiving opioid therapy. Discussions with regulators to continue to the next study phase could take place before the end of the year.
TD-9855, a norepinephrine serotonin reuptake inhibitor, is undergoing a phase 2 safety and efficacy study for adult ADHD.
Upside in Arena could continue to be limited in the near-term, due mostly to investors being impatient. Even when Theravance signed a collaboration agreement with Merck (MRK) to study treatments for hypertension and heart failure, shares still declined sharply from the $26-level. Theravance closed recently at $21.03. The short-sighted nature of speculators gives investors an entry-point at a lower price. Theravance will receive $5 million from Merck initially, and will be eligible for milestone payments that total $148 million for royalties and first indication on any products coming from the joint efforts.
Theravance also made an agreement with a Russian firm, R-Pharm. Theravance may earn up to $2 million in licensing, $4 million in development milestones, $6 million in sales milestones, and 15% royalty for net sales of TD-1792. TD-1792 is an antibiotic used to treat drug-resistant Gram-positive infections.
Earnings Review
In the third quarter, Theravance lost $0.37 per share, or $34.7 million on revenue of $1.4 million. Revenue declined from $6.4 million last year because the VIBATIV collaboration agreement with Astellas was terminated on January 6 2012. VIBATIV is used to treat methicillin-resistant Staphylococcus aureus (MRSA).
Theravance ended the quarter with cash and cash equivalents totaling $352.4 million.
Theravance reiterated that operating expenses will be at the higher end of between the $120 million and $130 million guidance.
Conclusion
The market sell-off in companies like Theravance, Arena, and Theravance-partner GlaxoSmithKline plc (GSK), created an opportunity for investors with a longer-term timeframe. Investors who want exposure to biopharmaceutical companies and understand the risks associated with drug development should consider Theravance. Theravance has a pipeline of products. Most notably, it is working on treatment for illnesses like COPD. When brought to market, its drug will be able to treat a substantial proportion of the population suffering from COPD.
The company is planning to provide an update on MABA (Bifunctional Muscarinic Antagonist-Beta2 Agonist) by the end of the year, and will give more updates at conferences scheduled for next year. Theravance will present at the European Respiratory Society congress held between September 7 - 11 2013. Investors should also expect Theravance to report updates to LABA (Long-Acting Beta2 Agonist) and VI (Vilanterol Trifenatate) for Asthma treatment in adults at the American Thoracic Society conference held on May 17-22 2013.
http://seekingalpha.com/article/976631-theravance-still-a-bu…
Arena Pharmaceuticals Third Quarter Earnings Sneak Peek
By Derek Hoffman | More Articles
November 05, 2012
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) will unveil its latest earnings on Tuesday, November 6, 2012. Arena Pharmaceuticals, Inc. is a biotechnology company that has developed a receptor-based screening assay using their own CART technology.
Arena Pharmaceuticals, Inc. Earnings Preview Cheat Sheet
Wall St. Earnings Expectations: The average estimate of analysts is for net loss of 8 cents per share, a narrower loss from the year-earlier quarter net loss of 16 cents. During the past three months, the average estimate has moved up from a loss of 9 cents. Between one and three months ago, the average estimate moved up. It has been unchanged at a loss of 8 cents during the last month.
Past Earnings Performance: Last quarter, the company fell short of estimates by 4 cents, coming in at a loss of 12 cents a share versus the estimate of net loss of 8 cents a share. It was the fourth straight quarter of missing estimates.
Earnings season is back and more important than ever. Get our newest CHEAT SHEET stock picks now
Balance Sheet Analysis: The company’s current ratio of assets to liabilities came in at 8.92 last quarter. Having a ratio above 2:1 is usually considered a good indicator of a company’s liquidity and ability to meet creditor demands. The company improved this liquidity measure from 7.55 in the first quarter to the last quarter driven in part by an increase in current assets. Current assets increased 81.8% to $167.3 million while liabilities rose by 53.9% to $18.8 million.
Stock Price Performance: Between October 4, 2012 and November 1, 2012, the stock price dropped 94 cents (-10.7%), from $8.77 to $7.83. The stock price saw one of its best stretches over the last year between June 7, 2012 and June 21, 2012, when shares rose for 11 straight days, increasing 81.9% (+$5.26) over that span. It saw one of its worst periods between September 4, 2012 and September 11, 2012 when shares fell for six straight days, dropping 14.1% (-$1.31) over that span.
A Look Back: In the second quarter, the company’s loss narrowed to a loss of $22.1 million (12 cents a share) from a loss of $22.9 million (16 cents) a year earlier, but missed analyst expectations. Revenue rose more than sixfold to $22 million from $3.3 million.
Key Stats:
The company is looking to build on last quarter’s top line growth, which snapped a string of revenue declines. Revenue fell 48.3% in the fourth quarter of the last fiscal year and 44.2% in the first quarter before climbing in the second quarter.
Analyst Ratings: There are mostly holds on the stock with six of nine analysts surveyed giving that rating.
Wall St. Revenue Expectations: Analysts predict a rise of 1.4% in revenue from the year-earlier quarter to $3.5 million.
Stocks with improving earnings metrics are worthy of your extra attention. In fact, “E = Earnings Are Increasing Quarter-Over-Quarter” is a core component of our CHEAT SHEET investing framework for this very reason.
http://wallstcheatsheet.com/stocks/arena-pharmaceuticals-thi…
By Derek Hoffman | More Articles
November 05, 2012
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) will unveil its latest earnings on Tuesday, November 6, 2012. Arena Pharmaceuticals, Inc. is a biotechnology company that has developed a receptor-based screening assay using their own CART technology.
Arena Pharmaceuticals, Inc. Earnings Preview Cheat Sheet
Wall St. Earnings Expectations: The average estimate of analysts is for net loss of 8 cents per share, a narrower loss from the year-earlier quarter net loss of 16 cents. During the past three months, the average estimate has moved up from a loss of 9 cents. Between one and three months ago, the average estimate moved up. It has been unchanged at a loss of 8 cents during the last month.
Past Earnings Performance: Last quarter, the company fell short of estimates by 4 cents, coming in at a loss of 12 cents a share versus the estimate of net loss of 8 cents a share. It was the fourth straight quarter of missing estimates.
Earnings season is back and more important than ever. Get our newest CHEAT SHEET stock picks now
Balance Sheet Analysis: The company’s current ratio of assets to liabilities came in at 8.92 last quarter. Having a ratio above 2:1 is usually considered a good indicator of a company’s liquidity and ability to meet creditor demands. The company improved this liquidity measure from 7.55 in the first quarter to the last quarter driven in part by an increase in current assets. Current assets increased 81.8% to $167.3 million while liabilities rose by 53.9% to $18.8 million.
Stock Price Performance: Between October 4, 2012 and November 1, 2012, the stock price dropped 94 cents (-10.7%), from $8.77 to $7.83. The stock price saw one of its best stretches over the last year between June 7, 2012 and June 21, 2012, when shares rose for 11 straight days, increasing 81.9% (+$5.26) over that span. It saw one of its worst periods between September 4, 2012 and September 11, 2012 when shares fell for six straight days, dropping 14.1% (-$1.31) over that span.
A Look Back: In the second quarter, the company’s loss narrowed to a loss of $22.1 million (12 cents a share) from a loss of $22.9 million (16 cents) a year earlier, but missed analyst expectations. Revenue rose more than sixfold to $22 million from $3.3 million.
Key Stats:
The company is looking to build on last quarter’s top line growth, which snapped a string of revenue declines. Revenue fell 48.3% in the fourth quarter of the last fiscal year and 44.2% in the first quarter before climbing in the second quarter.
Analyst Ratings: There are mostly holds on the stock with six of nine analysts surveyed giving that rating.
Wall St. Revenue Expectations: Analysts predict a rise of 1.4% in revenue from the year-earlier quarter to $3.5 million.
Stocks with improving earnings metrics are worthy of your extra attention. In fact, “E = Earnings Are Increasing Quarter-Over-Quarter” is a core component of our CHEAT SHEET investing framework for this very reason.
http://wallstcheatsheet.com/stocks/arena-pharmaceuticals-thi…
Obesity drug maker Arena books smaller 3Q loss
Arena Pharma books smaller 3rd-quarter loss as it prepares for US launch of Belviq
SAN DIEGO (AP) -- Weight loss drugmaker Arena Pharmaceuticals Inc. said Tuesday that its third-quarter loss shrank as its research and development and manufacturing costs declined.
The Food and Drug Administration approved Belviq in June, and Arena's partner Eisai Co. will start selling the drug in the first quarter of 2013. Belviq was the first long-term weight loss drug to get FDA approval in more than a decade.
Regulators in the European Union and Switzerland are also reviewing Belviq for sale there.
Arena reported losses of $15.5 million, or 7 cents per share, for the three months ended Sept. 30. In the third quarter of 2011 Arena lost $22.7 million, or 16 cents per share. Its revenue declined to $1.5 million from $3.5 million, but most costs declined and the company booked a gain on revaluing derivatives and recorded interest income.
Analysts were forecasting a larger loss of 9 cents per share and $2.4 million in revenue, according to FactSet.
On Tuesday Arena also announced a marketing partnership for Belviq in South Korea.
It said Ildong Pharmaceutical Co. will work to win marketing approval for Belviq in South Korea and will market the drug there if it is approved.
Arena will be paid $5 million upfront and $3 million more if the drug is cleared for marketing. San Diego-based Arena will manufacture Belviq and sell it to Ildong.
Arena shares lost 35 cents, or 4.4 percent, to $7.60 on Tuesday.
---------------------------------------------------------------------------
Separately, shares of Arena's competitor Vivus Inc. plunged after Vivus reported early data for its weight loss drug Qsymia, which was approved a few weeks after Belviq and reached the market in September. Vivus said large numbers of Qsymia prescriptions were going unfilled because of limited insurance coverage and high copays for the drug.
Vivus shares fell 21 percent to $11.82.
http://finance.yahoo.com/news/obesity-drug-maker-arena-books…

Arena Pharma books smaller 3rd-quarter loss as it prepares for US launch of Belviq
SAN DIEGO (AP) -- Weight loss drugmaker Arena Pharmaceuticals Inc. said Tuesday that its third-quarter loss shrank as its research and development and manufacturing costs declined.
The Food and Drug Administration approved Belviq in June, and Arena's partner Eisai Co. will start selling the drug in the first quarter of 2013. Belviq was the first long-term weight loss drug to get FDA approval in more than a decade.
Regulators in the European Union and Switzerland are also reviewing Belviq for sale there.
Arena reported losses of $15.5 million, or 7 cents per share, for the three months ended Sept. 30. In the third quarter of 2011 Arena lost $22.7 million, or 16 cents per share. Its revenue declined to $1.5 million from $3.5 million, but most costs declined and the company booked a gain on revaluing derivatives and recorded interest income.
Analysts were forecasting a larger loss of 9 cents per share and $2.4 million in revenue, according to FactSet.
On Tuesday Arena also announced a marketing partnership for Belviq in South Korea.

It said Ildong Pharmaceutical Co. will work to win marketing approval for Belviq in South Korea and will market the drug there if it is approved.
Arena will be paid $5 million upfront and $3 million more if the drug is cleared for marketing. San Diego-based Arena will manufacture Belviq and sell it to Ildong.
Arena shares lost 35 cents, or 4.4 percent, to $7.60 on Tuesday.
---------------------------------------------------------------------------
Separately, shares of Arena's competitor Vivus Inc. plunged after Vivus reported early data for its weight loss drug Qsymia, which was approved a few weeks after Belviq and reached the market in September. Vivus said large numbers of Qsymia prescriptions were going unfilled because of limited insurance coverage and high copays for the drug.
Vivus shares fell 21 percent to $11.82.
http://finance.yahoo.com/news/obesity-drug-maker-arena-books…
Antwort auf Beitrag Nr.: 43.794.751 von bernie55 am 07.11.12 09:21:35Hi Bernie, alles klar?
UPDATE: Jefferies & Company Reiterates Buy Rating, Lowers PT on Arena Pharmaceuticals
Dwight Einhorn, Benzinga Staff Writer November 07, 2012 10:11 AM
+ Follow
Print
Email
Tickers: ARNA
inShare
In a report published Wednesday, Jefferies & Company reiterated its Buy rating on Arena Pharmaceuticals (NASDAQ: ARNA [FREE Stock Trend Analysis]), but lowered its price target from $20.00 to $14.00.
Jefferies noted, “ARNA reiterated its plans for Belviq (1Q13 U.S. launch) and outlined a new South Korean partnership with Ildong. We remain confident in the long-term potential for Belviq based on combination use with phentermine, but we are lowering our PT from $20 to $14, as we moderate our launch expectations for Belviq monotherapy following disappointing sales for competitor Qsymia.”
Arena Pharmaceuticals closed on Tuesday at $7.60.
Read more: http://www.benzinga.com/analyst-ratings/analyst-color/12/11/…
UPDATE: Jefferies & Company Reiterates Buy Rating, Lowers PT on Arena Pharmaceuticals
Dwight Einhorn, Benzinga Staff Writer November 07, 2012 10:11 AM
+ Follow
Tickers: ARNA
inShare
In a report published Wednesday, Jefferies & Company reiterated its Buy rating on Arena Pharmaceuticals (NASDAQ: ARNA [FREE Stock Trend Analysis]), but lowered its price target from $20.00 to $14.00.
Jefferies noted, “ARNA reiterated its plans for Belviq (1Q13 U.S. launch) and outlined a new South Korean partnership with Ildong. We remain confident in the long-term potential for Belviq based on combination use with phentermine, but we are lowering our PT from $20 to $14, as we moderate our launch expectations for Belviq monotherapy following disappointing sales for competitor Qsymia.”
Arena Pharmaceuticals closed on Tuesday at $7.60.
Read more: http://www.benzinga.com/analyst-ratings/analyst-color/12/11/…
Arena Pharmaceuticals (ARNA) - Trading Recap
Posted on 11/08/2012 by Allen Bersch
NEW YORK (AVAFIN) -- Trading of Arena Pharmaceuticals options resulted in establishing a new 90-day call volume record, where 28,110 call contracts exchanged hands between the buyers and the sellers. A total of 9,300 put and 28,110 call contracts was traded raising a 0.33 put/call ratio on shares of ARNA
Put/Call ratio can be regarded as a predictor of investment sentiment, indicating what experienced investors are doing in preparation for a move of an underlying equity. A high put/call ratio suggests that the investor sentiment is bearish and that investors are expecting the underlying stock price to decrease. On the other hand, a low put/call ratio implies that the investor sentiment is bullish and that investors are expecting the underlying stock price to increase. Thus, unusual volume provides reliable clues that the stock is expected to make a move.
Arena Pharmaceuticals is trading at $7.57, up $0.33 (+4.56%) in today's trading session. The daily low is $7.24 and the high is $7.66. The trading volume of 9M is below the average volume of 10M shares. ARNA is trading below the 50 day moving average and higher than the 200 day moving average. The stock's 52 week low is $1.27 and 52 week high is $13.50. To date, the stock has lost -8.47% within the last week.
Arena Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company committed to discovering and developing innovative therapies offering medical advances and new options for patients.
http://www.avafin.com/articles/1018750.html
Arena Pharmaceuticals'Management Hosts Lazard Capital Markets Healthcare Conference (Transcript)
November 14, 2012 |
AUSZÜGE daraus:
Robert Hoffman - Chief Financial Officer
We are so pleased to get our drug approved BELVIQ on June 27th of 2012. The first drug approved for a weight-loss indication in 13 year.
And so we’re just waiting on DEA scheduling right now. BELVIQ is a product of our GPCR platform technology and we have multiple shorts and go behind it. I think we’ll touch on that the pipeline a little bit later but we have a PAH APD811 for PAH which is in a Phase I Multiple Ascending Dose clinical trial right now.
We plan on initiating the Phase I for APD334 which is for autoimmune indication. And then beyond that we have a pain indication as well. So we are thrilled with that. I don’t think that to tell anyone in the audience here that obesity is a large market. One-third of all U.S adults are classified as obese, two-thirds are classified as overweight or obese.
Significant market opportunity, worldwide it’s a 0.5 billion individuals are classified as obese. So it’s a significant market opportunity for us. We actually are very much ready for the launch. We’ve delivered we announced in our last 10-Q launch supply to Eisai. So we delivered a $11.6 million worth. It’s a portion of the launch supply. So we’ll be ready to launch as soon as we get DEA scheduling.
Eisai has been an excellent marketing partner. They had been able to market successfully two block busters in Aricept as well as Aciphex both of that come in our patent. So they’re really focused on the launch of BELVIQ going forward. The economics are significant to us we get we sell them product and from for the very first dollar we get 31.5% of their net sales not a profit share but net sale, very significant.
So in terms of looking at further down the economics at $250 million in net sale. We’ll get 31.5% of that which is about $80 million plus that threshold we achieved some purchase price adjustments as well as some milestones which another $55 million take on top of that when we get DEA scheduling is another $65 million. So if I do my math right that’s an excess of $200 million on their first $250 million of sale.
So it makes significant opportunity for us. In terms of other opportunities we just announced last week a nice collaboration with the South Korean company Ildong but we received a $5 million upfront payment great economic service as well first dollar on net sales is at 35% and that will ramps up to 45% on net sale.
In terms of other areas that we are moving forward with the European strategy. We did file in the EU in March of this year. I can’t believe it’s for this year but and so we founded the 120 day questions and we are hopeful to get a decision on that in first half of 2013 probably behind that is actually Switzerland as well which is where our manufacturing facility is.
So we filed in Switzerland and we expect a decision on that in the first half of 2013 as well. In terms of financials we adjusted our guidance to and 2012 the $165 million in cash that is not include the DEA scheduling milestone of $65 million and it does include the purchase price excuse me the purchase payment as well as the $5 million from Ildong.
Again we are very well financed and we’ve reiterated that we don’t haven’t need to finance at this time. In terms of upcoming milestones I did mention the DEA scheduling. Again we are very much looking forward to launching this drug with our experience partner Eisai
We will announce the results from the APD811 in 2013 and we expect to go into the clinic for APD334 for autoimmune diseases. Again the EU and the first half of 2013 and we look to partner the drug in another opportunities in addition to North and South America with Eisai as well as Ildong with Korea.
With that I am sure Josh you have plenty questions for us and we’re ready to get started.
http://seekingalpha.com/article/1005411-arena-pharmaceutical…
November 14, 2012 |
AUSZÜGE daraus:
Robert Hoffman - Chief Financial Officer
We are so pleased to get our drug approved BELVIQ on June 27th of 2012. The first drug approved for a weight-loss indication in 13 year.
And so we’re just waiting on DEA scheduling right now. BELVIQ is a product of our GPCR platform technology and we have multiple shorts and go behind it. I think we’ll touch on that the pipeline a little bit later but we have a PAH APD811 for PAH which is in a Phase I Multiple Ascending Dose clinical trial right now.
We plan on initiating the Phase I for APD334 which is for autoimmune indication. And then beyond that we have a pain indication as well. So we are thrilled with that. I don’t think that to tell anyone in the audience here that obesity is a large market. One-third of all U.S adults are classified as obese, two-thirds are classified as overweight or obese.
Significant market opportunity, worldwide it’s a 0.5 billion individuals are classified as obese. So it’s a significant market opportunity for us. We actually are very much ready for the launch. We’ve delivered we announced in our last 10-Q launch supply to Eisai. So we delivered a $11.6 million worth. It’s a portion of the launch supply. So we’ll be ready to launch as soon as we get DEA scheduling.
Eisai has been an excellent marketing partner. They had been able to market successfully two block busters in Aricept as well as Aciphex both of that come in our patent. So they’re really focused on the launch of BELVIQ going forward. The economics are significant to us we get we sell them product and from for the very first dollar we get 31.5% of their net sales not a profit share but net sale, very significant.
So in terms of looking at further down the economics at $250 million in net sale. We’ll get 31.5% of that which is about $80 million plus that threshold we achieved some purchase price adjustments as well as some milestones which another $55 million take on top of that when we get DEA scheduling is another $65 million. So if I do my math right that’s an excess of $200 million on their first $250 million of sale.
So it makes significant opportunity for us. In terms of other opportunities we just announced last week a nice collaboration with the South Korean company Ildong but we received a $5 million upfront payment great economic service as well first dollar on net sales is at 35% and that will ramps up to 45% on net sale.
In terms of other areas that we are moving forward with the European strategy. We did file in the EU in March of this year. I can’t believe it’s for this year but and so we founded the 120 day questions and we are hopeful to get a decision on that in first half of 2013 probably behind that is actually Switzerland as well which is where our manufacturing facility is.
So we filed in Switzerland and we expect a decision on that in the first half of 2013 as well. In terms of financials we adjusted our guidance to and 2012 the $165 million in cash that is not include the DEA scheduling milestone of $65 million and it does include the purchase price excuse me the purchase payment as well as the $5 million from Ildong.
Again we are very well financed and we’ve reiterated that we don’t haven’t need to finance at this time. In terms of upcoming milestones I did mention the DEA scheduling. Again we are very much looking forward to launching this drug with our experience partner Eisai
We will announce the results from the APD811 in 2013 and we expect to go into the clinic for APD334 for autoimmune diseases. Again the EU and the first half of 2013 and we look to partner the drug in another opportunities in addition to North and South America with Eisai as well as Ildong with Korea.
With that I am sure Josh you have plenty questions for us and we’re ready to get started.
http://seekingalpha.com/article/1005411-arena-pharmaceutical…
Is Belviq The Next Global Blockbuster?
By Reza Ganjavi - November 13, 2012
In its recent quarterly update, Arena Pharmaceuticals (NASDAQ: ARNA) announced some important new developments. For example, the company announced that it is working on establishing Belviq as a therapy for various indications such as smoking cessation, and doing trials in combination with agents such as phentermine and metformin. Arena will disclose more details after consulting with the FDA on further studies.
Another important announcement was partnership with Ildong Pharmaceuticals to penetrate the South Korean market that has a huge number of obese patients. I believe this is one in a series of strategic partnership that we will hear from Arena, to address the obesity problem in a number of countries.
As an investor, I like the idea of receiving milestone payments and various streams of income from different partners based on their net sales, and partners usually cover the costs of getting local approvals. In the case of Ildong, the payments include a $5 million cash upfront and $3 million once the drug is approved by KFDA in South Korea. I believe having the US FDA approval opens doors to Arena and its partners obtaining approvals around the globe as most other regulatory agencies will use FDA’s due diligence as basis of their assessment.
The idea of Belviq becoming a blockbuster is emerging as a reality before our eyes. I think in the next six months not only we will see a Big Pharma partner with Arena in the EU, but we will see strategic partnerships covering most of the globe including other large markets of Asia and beyond. Arena’s partner already is working on penetrating the North and South American markets.
In my opinion, once these partnerships flourish, Arena will be every investor’s dream: a small research and development joint pounding out global blockbuster molecular agents and combination therapies!
It’s noteworthy that Belviq is not only a great treatment for obesity, it could potentially become the favorite first-line therapy for Type II diabetes and Metabolic Syndrome which provide Belviq an even bigger market than the existing, humongous, under-served, global obesity market.
Arena’s margins on Belviq are high enough to make Belviq very profitable. Arena provides the global market "Swiss Quality" tablets manufactured to highest standards in Zofingen, Switzerland. A negotiated, friendly Swiss tax system adds icing on the cake of Arena’s balance-sheet and profitability.
In its conference call Arena indicated that its US marketing partner Eisai believes:
“Belviq will be one of the most important compounds in the history of their organization, based on the drug’s strong marketing position. Eisai is focused on delivering increasing sales of Belviq each year by expanding its sales force, following the initial market penetration and improved reimbursement.”
Initially, Eisai will target 30,000 physicians in the US. I have a friend who has 300 obese patients! If these 30,000 doctors have half as many patients, we are looking about initial targeting of about 4.5 million patients which is a fraction of the US obesity market – add to that Type II diabetes and Metabolic Syndrome and we’re looking at a huge growth potential for Arena & Eisai in the US alone.
Eisai’s US CEO recently indicated they will eventually look into expanding their sales force through direct hire or contract sales organizations – or I suppose through partnerships with other big pharmas.
Arena’s global market is set to increase dramatically once it obtains European approval in the first half of 2013. I anticipate a positive recommendation from CHMP will be issued 1Q2013. I am confident Belviq will be approved in Europe.
Obesity is a real problem in Europe and EU has recently rejected Qsymia by Vivus (NASDAQ: VVUS) due to safety issues.
Belviq is far safer than Qsymia and Arena indicated EU did not ask any surprise questions, or questions the FDA had not asked. Arena has addressed EU’s 120 day questions so the application is progressing smoothly.
Furthermore, Arena expects a decision from Switzerland’s regulatory body, Swissmedic in the first half of 2013 as well. I expect that to be positive as well.
It's noteworthy that Arena is the only company with a new breakthrough obesity medication that has started to penetrate global markets. Orexigen Therapeutics (NASDAQ: OREX) is at least a year away from obtaining FDA approval, and longer in obtaining EU approval if it doesn't face the same challenges Vivus had in Europe. Both Qsymia and Orexigen's Contrave also face generic competition which Arena doesn't have to worry about.
As Belviq moves towards a global blockbuster status, Arena short sellers have been increasing their exposure, setting themselves up for a painful short squeeze. I’ll go over this in further detail in my next MF article.
http://beta.fool.com/beatlesforever/2012/11/13/arena-pharmas…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/beatlesforever/2012/11/13/arena-pharmas…
By Reza Ganjavi - November 13, 2012
In its recent quarterly update, Arena Pharmaceuticals (NASDAQ: ARNA) announced some important new developments. For example, the company announced that it is working on establishing Belviq as a therapy for various indications such as smoking cessation, and doing trials in combination with agents such as phentermine and metformin. Arena will disclose more details after consulting with the FDA on further studies.
Another important announcement was partnership with Ildong Pharmaceuticals to penetrate the South Korean market that has a huge number of obese patients. I believe this is one in a series of strategic partnership that we will hear from Arena, to address the obesity problem in a number of countries.
As an investor, I like the idea of receiving milestone payments and various streams of income from different partners based on their net sales, and partners usually cover the costs of getting local approvals. In the case of Ildong, the payments include a $5 million cash upfront and $3 million once the drug is approved by KFDA in South Korea. I believe having the US FDA approval opens doors to Arena and its partners obtaining approvals around the globe as most other regulatory agencies will use FDA’s due diligence as basis of their assessment.
The idea of Belviq becoming a blockbuster is emerging as a reality before our eyes. I think in the next six months not only we will see a Big Pharma partner with Arena in the EU, but we will see strategic partnerships covering most of the globe including other large markets of Asia and beyond. Arena’s partner already is working on penetrating the North and South American markets.
In my opinion, once these partnerships flourish, Arena will be every investor’s dream: a small research and development joint pounding out global blockbuster molecular agents and combination therapies!
It’s noteworthy that Belviq is not only a great treatment for obesity, it could potentially become the favorite first-line therapy for Type II diabetes and Metabolic Syndrome which provide Belviq an even bigger market than the existing, humongous, under-served, global obesity market.
Arena’s margins on Belviq are high enough to make Belviq very profitable. Arena provides the global market "Swiss Quality" tablets manufactured to highest standards in Zofingen, Switzerland. A negotiated, friendly Swiss tax system adds icing on the cake of Arena’s balance-sheet and profitability.
In its conference call Arena indicated that its US marketing partner Eisai believes:
“Belviq will be one of the most important compounds in the history of their organization, based on the drug’s strong marketing position. Eisai is focused on delivering increasing sales of Belviq each year by expanding its sales force, following the initial market penetration and improved reimbursement.”
Initially, Eisai will target 30,000 physicians in the US. I have a friend who has 300 obese patients! If these 30,000 doctors have half as many patients, we are looking about initial targeting of about 4.5 million patients which is a fraction of the US obesity market – add to that Type II diabetes and Metabolic Syndrome and we’re looking at a huge growth potential for Arena & Eisai in the US alone.
Eisai’s US CEO recently indicated they will eventually look into expanding their sales force through direct hire or contract sales organizations – or I suppose through partnerships with other big pharmas.
Arena’s global market is set to increase dramatically once it obtains European approval in the first half of 2013. I anticipate a positive recommendation from CHMP will be issued 1Q2013. I am confident Belviq will be approved in Europe.

Obesity is a real problem in Europe and EU has recently rejected Qsymia by Vivus (NASDAQ: VVUS) due to safety issues.
Belviq is far safer than Qsymia and Arena indicated EU did not ask any surprise questions, or questions the FDA had not asked. Arena has addressed EU’s 120 day questions so the application is progressing smoothly.
Furthermore, Arena expects a decision from Switzerland’s regulatory body, Swissmedic in the first half of 2013 as well. I expect that to be positive as well.
It's noteworthy that Arena is the only company with a new breakthrough obesity medication that has started to penetrate global markets. Orexigen Therapeutics (NASDAQ: OREX) is at least a year away from obtaining FDA approval, and longer in obtaining EU approval if it doesn't face the same challenges Vivus had in Europe. Both Qsymia and Orexigen's Contrave also face generic competition which Arena doesn't have to worry about.
As Belviq moves towards a global blockbuster status, Arena short sellers have been increasing their exposure, setting themselves up for a painful short squeeze. I’ll go over this in further detail in my next MF article.
http://beta.fool.com/beatlesforever/2012/11/13/arena-pharmas…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/beatlesforever/2012/11/13/arena-pharmas…
Arena Pharmaceuticals to Webcast Live Presentation at the Piper Jaffray 24th Annual Healthcare Conference
Arena to Also Present at the Leerink Swann 6th Annual POLARxPRESS Bus Tour
SAN DIEGO, Nov. 21, 2012 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present at the Piper Jaffray 24th Annual Healthcare Conference on Wednesday, November 28, 2012, at 8:00 a.m. Eastern Time (5:00 a.m. Pacific Time) at The New York Palace Hotel in New York City.
Jack Lief , Arena's President and Chief Executive Officer, and Craig M. Audet, Arena's Senior Vice President, Operations and Head of Global Regulatory Affairs, are scheduled to provide a corporate overview.
, Arena's President and Chief Executive Officer, and Craig M. Audet, Arena's Senior Vice President, Operations and Head of Global Regulatory Affairs, are scheduled to provide a corporate overview.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event.
Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
Arena also announced today that it is scheduled to present at the Leerink Swann 6th Annual POLARxPRESS Bus Tour
on November 28, 2012, in New York City. This presentation will not be webcast.
http://finance.yahoo.com/news/arena-pharmaceuticals-webcast-…
Arena to Also Present at the Leerink Swann 6th Annual POLARxPRESS Bus Tour
SAN DIEGO, Nov. 21, 2012 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present at the Piper Jaffray 24th Annual Healthcare Conference on Wednesday, November 28, 2012, at 8:00 a.m. Eastern Time (5:00 a.m. Pacific Time) at The New York Palace Hotel in New York City.
Jack Lief
 , Arena's President and Chief Executive Officer, and Craig M. Audet, Arena's Senior Vice President, Operations and Head of Global Regulatory Affairs, are scheduled to provide a corporate overview.
, Arena's President and Chief Executive Officer, and Craig M. Audet, Arena's Senior Vice President, Operations and Head of Global Regulatory Affairs, are scheduled to provide a corporate overview.A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event.
Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
Arena also announced today that it is scheduled to present at the Leerink Swann 6th Annual POLARxPRESS Bus Tour
on November 28, 2012, in New York City. This presentation will not be webcast.
http://finance.yahoo.com/news/arena-pharmaceuticals-webcast-…
Clinical Policy Bulletin:
Weight Reduction Medications and Programs
http://www.aetna.com/cpb/medical/data/1_99/0039.html
Weight Reduction Medications and Programs
http://www.aetna.com/cpb/medical/data/1_99/0039.html
Zitat von bernie55: Clinical Policy Bulletin:
Weight Reduction Medications and Programs
http://www.aetna.com/cpb/medical/data/1_99/0039.html
Vivus Leads a Weight-Loss Rally after Aetna Release
By Avi Salzman
Vivus (VVUS) rose 14% in early trading after Aetna (AET) said it will cover weight-loss drug Qsymia and other weight loss drugs in situations where it is medically necessary.
Aetna outlined its decision in a clinical policy bulletin and listed the drugs it will cover including Didrex from Pfizer (PFE), Xenical from Roche (RHHBY), Belviq from Arena Pharmaceuticals (ARNA) and others.
Orexigen (OREX), which is developing another weight-loss drug also rose 6.9%.
http://blogs.barrons.com/stockstowatchtoday/2012/11/21/vivus…
Arena 2,7 % 

Antwort auf Beitrag Nr.: 43.848.223 von me_2 am 21.11.12 17:41:26Arena ist der "winner", aber alle warten auf das DEA scheduling, d.h. die Einstufung eines neuen Präperates in eine Suchtkategorieklasse!
Arena hat viel weniger Nebenwirkungen als das Vivus Präperat und wird weltweit für Furore sorgen!
Ich halte meine 1000 Stück bis zum Verkauf bei US Dollar 25!
Arena hat viel weniger Nebenwirkungen als das Vivus Präperat und wird weltweit für Furore sorgen!
Ich halte meine 1000 Stück bis zum Verkauf bei US Dollar 25!

Antwort auf Beitrag Nr.: 43.848.283 von Magnetfeldfredy am 21.11.12 17:53:12Meine vollste Zustimmung! DEA classification IV und ab gehts! Ich machs mit meinen 800 St. genauso ...
@AREANICS
Hier noch einmal die anstehenden News, die bis spätestens März /April 2013 rausgekommen sind
> Bekanntgabe der Missbrauchsklassifikation seitens der DEA - Zeitpunkt ????
> Approval Entscheidung in Europa durch die EMA - ca. März 2013
Da werden wir , ich gehe mal von positiven Entscheidungen aus , andere Kurse bei ARENA sehen.
, andere Kurse bei ARENA sehen.
ARENA ist und bleibt ein LONGinvestment !!!!
Hier noch einmal die anstehenden News, die bis spätestens März /April 2013 rausgekommen sind
> Bekanntgabe der Missbrauchsklassifikation seitens der DEA - Zeitpunkt ????
> Approval Entscheidung in Europa durch die EMA - ca. März 2013
Da werden wir , ich gehe mal von positiven Entscheidungen aus
 , andere Kurse bei ARENA sehen.
, andere Kurse bei ARENA sehen.ARENA ist und bleibt ein LONGinvestment !!!!

 UK/Europe: NICE has to appraise the clinical and cost effectiveness of lorcaserin hydrochloride!
UK/Europe: NICE has to appraise the clinical and cost effectiveness of lorcaserin hydrochloride! 
http://guidance.nice.org.uk/TA/Wave21/19
Zitat von bernie55:UK/Europe: NICE has to appraise the clinical and cost effectiveness of lorcaserin hydrochloride!
http://guidance.nice.org.uk/TA/Wave21/19
Auszug aus dem IV Board:
NICE is the National Institute for Health and Clinical Excellence in the United Kingdom.
NICE is busy with an appraisal about Lorcaserin and will issue an economic analysis. In dependence on this analysis it will be decided whether the costs of a drug will be covered by the compulsory health insurance or not.
Costs will be considered from an NHS (National Health Service) and Personal Social Services perspective. Please note that the process had been accelerated by the manufacturer ".. this appraisal has been rescheduled to align with the latest regulatory expectations."
This means an approval could occur earlier as expected
 and no oral presentation will probably be requested
and no oral presentation will probably be requested and the clock within the European approval process wont be stopped again on day180 (maybe just before Christmas or after the holiday season in early January) and the final Commission decision (d277) could be published in late March or early April 2013! wow!
and the clock within the European approval process wont be stopped again on day180 (maybe just before Christmas or after the holiday season in early January) and the final Commission decision (d277) could be published in late March or early April 2013! wow!Approval in Europe in early April 2013 at the latest .....this sounds not too bad....
http://www.investorvillage.com/smbd.asp?mb=633&mn=44137&pt=m…
Zitat von bernie55:Zitat von bernie55:UK/Europe: NICE has to appraise the clinical and cost effectiveness of lorcaserin hydrochloride!
http://guidance.nice.org.uk/TA/Wave21/19
Auszug aus dem IV Board:
NICE is the National Institute for Health and Clinical Excellence in the United Kingdom.
NICE is busy with an appraisal about Lorcaserin and will issue an economic analysis. In dependence on this analysis it will be decided whether the costs of a drug will be covered by the compulsory health insurance or not.
Costs will be considered from an NHS (National Health Service) and Personal Social Services perspective. Please note that the process had been accelerated by the manufacturer ".. this appraisal has been rescheduled to align with the latest regulatory expectations."
This means an approval could occur earlier as expectedand no oral presentation will probably be requested
and the clock within the European approval process wont be stopped again on day180 (maybe just before Christmas or after the holiday season in early January) and the final Commission decision (d277) could be published in late March or early April 2013! wow!
Approval in Europe in early April 2013 at the latest .....this sounds not too bad....
http://www.investorvillage.com/smbd.asp?mb=633&mn=44137&pt=m…
...eine kleine Ergänzung zum o.g. Artikel aus dem IV...
Project history
DATE
23 July 2012
Following on from advice received from the manufacturer, this appraisal has now been rescheduled to align with latest regulatory expectations. Therefore, we now anticipate that the appraisal will begin during early December 2012.
The deadline for submissions is expected in approximately mid February 2013.
Project history
DATE
2 November 2012
Please note that following on from advice received from the manufacturer, this appraisal has been rescheduled to align with latest regulatory expectations. Therefore, we now anticipate that the appraisal will begin during mid October 2012.
The deadline for submissions is expected in approximately mid December 2012.
Increased Insurance Coverage: A Big Step For Anti-Obesity Drug Manufacturers
November 25, 2012
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. (More...)
By: Ahmed Ishtiaq
Companies operating in the obesity market have had a lot of ups and downs in the past few months. Such is the volatile situation of the market that a small piece of news can cause massive swings in the stock price. Vivus (VVUS) share price took a heavy beating after the company reported poor sales figures from its anti-obesity drug, Qsymia. These poor figures weighed heavily on the whole sector as Arena Pharmaceuticals (ARNA) and Orexigen Therapeutics (OREX) also fell. Investors react emotionally to the news in the BioPharma sector, especially with the development stage companies. As a result, wild swings in the prices of these stocks are expected. After the bad news of poor earnings, these companies recently received probably the biggest positive news after the approval of their drugs.
Aetna Inc (AET), the third largest insurer in the country decided to provide coverage to these anti-obesity drugs. As a result, stock prices in the sector went up substantially. The decision by the third largest insurer in the country will have far reaching affect on the fortunes of these companies. Let's see how the decision will affect the companies.
Aetna Policy Bulletin:
In a recent policy bulletin, Aetna announced that it will provide coverage to the anti-obesity drugs manufactured by Vivus Inc and Arena Pharmaceuticals. In my previous articles, I have always maintained that the insurers will be inclined to provide coverage for anti-obesity drugs. There are two reasons why I felt the coverage will be provided- first, the cost from obesity related diseases is much higher than the cost of anti-obesity drugs. At the moment, total expenditure on obesity related drugs is over $140 billion in the country. In fact, these medical expenses were a major factor in the change of FDA's stance over the anti-obesity drugs. Diseases like diabetes and coronary heart disease are far more expensive to cover than the cure for obesity itself.
The second reason is the recognition of obesity as a disease rather than a mere choice of life style. FDA recognizes that obesity is a disease, and serious steps are needed to fight this epidemic. FDA accepted the problem, and their solution was the approval of anti-obesity drugs by Arena and Vivus. However, a solution to this problem will not be complete until the insurance companies back the FDA. In my opinion, most of the insurers would like to become a part of the solution rather than a problem. Aetna is the first to become a part of the solution.
Affect on the Participants:
In its most recent earnings announcement, Vivus announced that only 20% of the prescriptions received insurance. Low insurance coverage was the biggest factor in low sales of Qsymia. Approximately 30% of patients, who were prescribed Qsymia, did not pick up their prescriptions due to the excessive cost of the drug. Insurance coverage is extremely important for anti-obesity drugs as a lot of patients will not be able to afford these drugs. Aetna's decision is a particularly welcome sign for the anti-obesity drug manufacturers. Smaller insurers will now be inclined to provide coverage for Qsymia and Belviq.
The news had an instant impact on the stock prices of these companies. Vivus stock went up by 12.85% to $11.68 after hitting high of $12.59, shares of Arena also rose 3% to $9.19 on Wednesday. Third player in the market is Orexigen Therapeutics ; however, its drug is still in the trial phases. Orexigen is working on presenting its drug for approval in 2013. Shares of Orexigen went up by 9% to $4.73. As a result of increased insurance coverage, fourth quarter prospects are already looking bright for Vivus. I expect Qsymia sales to go up substantially during the current quarter. It can very well prove to be the first quarter when the company reports a profit. Although Aetna's decision is extremely positive news, further support is needed from other insurers.
Summary:
All the pieces of the puzzle are falling into place nicely for anti-obesity drug manufacturers. Insurance coverage for these drugs is a massive event in this sector. We can expect a slight decrease in the volatility now that one of the biggest hurdles in revenues has been removed. I expect these stocks to move up in a steady manner now. Fourth quarter results will be hugely impressive for Vivus due to increased coverage. Arena will also go into the market with a lot more belief than before. All the players in the obesity sector will come out winners, and the patient investors will be rewarded handsomely.
http://seekingalpha.com/article/1026241-increased-insurance-…
November 25, 2012
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. (More...)
By: Ahmed Ishtiaq
Companies operating in the obesity market have had a lot of ups and downs in the past few months. Such is the volatile situation of the market that a small piece of news can cause massive swings in the stock price. Vivus (VVUS) share price took a heavy beating after the company reported poor sales figures from its anti-obesity drug, Qsymia. These poor figures weighed heavily on the whole sector as Arena Pharmaceuticals (ARNA) and Orexigen Therapeutics (OREX) also fell. Investors react emotionally to the news in the BioPharma sector, especially with the development stage companies. As a result, wild swings in the prices of these stocks are expected. After the bad news of poor earnings, these companies recently received probably the biggest positive news after the approval of their drugs.
Aetna Inc (AET), the third largest insurer in the country decided to provide coverage to these anti-obesity drugs. As a result, stock prices in the sector went up substantially. The decision by the third largest insurer in the country will have far reaching affect on the fortunes of these companies. Let's see how the decision will affect the companies.
Aetna Policy Bulletin:
In a recent policy bulletin, Aetna announced that it will provide coverage to the anti-obesity drugs manufactured by Vivus Inc and Arena Pharmaceuticals. In my previous articles, I have always maintained that the insurers will be inclined to provide coverage for anti-obesity drugs. There are two reasons why I felt the coverage will be provided- first, the cost from obesity related diseases is much higher than the cost of anti-obesity drugs. At the moment, total expenditure on obesity related drugs is over $140 billion in the country. In fact, these medical expenses were a major factor in the change of FDA's stance over the anti-obesity drugs. Diseases like diabetes and coronary heart disease are far more expensive to cover than the cure for obesity itself.
The second reason is the recognition of obesity as a disease rather than a mere choice of life style. FDA recognizes that obesity is a disease, and serious steps are needed to fight this epidemic. FDA accepted the problem, and their solution was the approval of anti-obesity drugs by Arena and Vivus. However, a solution to this problem will not be complete until the insurance companies back the FDA. In my opinion, most of the insurers would like to become a part of the solution rather than a problem. Aetna is the first to become a part of the solution.
Affect on the Participants:
In its most recent earnings announcement, Vivus announced that only 20% of the prescriptions received insurance. Low insurance coverage was the biggest factor in low sales of Qsymia. Approximately 30% of patients, who were prescribed Qsymia, did not pick up their prescriptions due to the excessive cost of the drug. Insurance coverage is extremely important for anti-obesity drugs as a lot of patients will not be able to afford these drugs. Aetna's decision is a particularly welcome sign for the anti-obesity drug manufacturers. Smaller insurers will now be inclined to provide coverage for Qsymia and Belviq.
The news had an instant impact on the stock prices of these companies. Vivus stock went up by 12.85% to $11.68 after hitting high of $12.59, shares of Arena also rose 3% to $9.19 on Wednesday. Third player in the market is Orexigen Therapeutics ; however, its drug is still in the trial phases. Orexigen is working on presenting its drug for approval in 2013. Shares of Orexigen went up by 9% to $4.73. As a result of increased insurance coverage, fourth quarter prospects are already looking bright for Vivus. I expect Qsymia sales to go up substantially during the current quarter. It can very well prove to be the first quarter when the company reports a profit. Although Aetna's decision is extremely positive news, further support is needed from other insurers.
Summary:
All the pieces of the puzzle are falling into place nicely for anti-obesity drug manufacturers. Insurance coverage for these drugs is a massive event in this sector. We can expect a slight decrease in the volatility now that one of the biggest hurdles in revenues has been removed. I expect these stocks to move up in a steady manner now. Fourth quarter results will be hugely impressive for Vivus due to increased coverage. Arena will also go into the market with a lot more belief than before. All the players in the obesity sector will come out winners, and the patient investors will be rewarded handsomely.
http://seekingalpha.com/article/1026241-increased-insurance-…
first, the cost from obesity related diseases is much higher than the cost of anti-obesity drugs.
das sind super news, aber immer diese simplifizierenden Statements: wenn alle Dicken pills für 140 Mia. einwerfen, sind alle damit verbundenen Probleme weg. So einfach isses doch nicht! Die drugs sind mäßig wirksam wenn sie mit entspr. Änderungen des Lebensstils Bewegung, Ernährung (McDonalds verbieten?) etc. kombiniert werden. Und das funktioniert in Studien manchmal noch ganz gut, aber in der Realität siehts doch ganz anders aus....
das sind super news, aber immer diese simplifizierenden Statements: wenn alle Dicken pills für 140 Mia. einwerfen, sind alle damit verbundenen Probleme weg. So einfach isses doch nicht! Die drugs sind mäßig wirksam wenn sie mit entspr. Änderungen des Lebensstils Bewegung, Ernährung (McDonalds verbieten?) etc. kombiniert werden. Und das funktioniert in Studien manchmal noch ganz gut, aber in der Realität siehts doch ganz anders aus....
The Future Is Bright For Arena Pharmaceuticals
November 26, 2012
This summer, the FDA took action to make Arena Pharmaceuticals' (ARNA) Belviq the first drug approved for the treatment of obesity in thirteen years. By now, most are aware that Belviq's main competition is Vivus' (VVUS) Qsymia, and this has led to a sharp divide in investor opinion concerning the two drugs.
While many have supported one drug or the other on a basis of safety or efficacy, I contend that the relative marketability of Belviq and its potential uses outside the realm of obesity position Arena Pharmaceuticals for a very exciting future. Indeed, the company's vision for Belviq is promising, and it warrants far more attention than it's been given to this point.
A Closer Look at Belviq's Marketability
When considering whether or not a product will be successful in the marketplace, it is important to investigate the product's marketability. Some determinants of marketability include the size of the target market and how easily the target market can be accessed via advertisement, thereby increasing public awareness of the product.
For both Belviq and Qsymia, the target market is enormous. In the United States alone, 150 million people are considered overweight or obese (BMI≥25). Globally, the numbers are even more staggering: at least 500 million people are considered obese (BMI≥30). While both Belviq and Qsymia have been approved for use in the United States, neither has been approved for use anywhere else in the world.
Currently, Arena is pursuing EMA approval for use of Belviq in the European Union. Since Qsymia was recently rejected by the EMA for use in Europe, a vote of confidence from the EMA would leave Arena in a great position to take advantage of the entire European market. At this point, then, it would seem that Belviq has a small edge in terms of potential target market size.
As far as ability to access the target market, most would agree that Arena has the edge there, as well. The restrictive REMS placed upon Qsymia at the time of FDA approval is very limiting, and it's something that has definitely impacted sales of Qsymia to this point. As part of the REMS, Vivus is not allowed to advertise Qsymia directly to consumers, adversely affecting Vivus' ability to access the target market.
Another element of the Qsymia REMS requires that patients who are prescribed Qsymia use a mail-order pharmacy service. This means that a patient cannot simply fill a Qsymia prescription at the neighborhood pharmacy; rather, upon completing some extra paperwork, the Qsymia prescription is delivered to the patient's door. While this sounds very convenient, the excess paperwork is often considered an unnecessary inconvenience. Additionally, patients using a mail-order pharmacy can't speak with a pharmacist in person, which makes some patients uneasy ("who will answer my questions?").
Development of Combination Therapies Based on Belviq
While Belviq's advantage over Qsymia with respect to marketability is important, I believe that the prospect of using Belviq in combination therapies could have an even greater impact. In the recent Credit Suisse Healthcare Conference, Craig Audet specifically mentioned Arena's plans to pursue both Belviq/Metformin and Belviq/Phenteramine combination therapies.
In the recent Credit Suisse Healthcare Conference, Craig Audet specifically mentioned Arena's plans to pursue both Belviq/Metformin and Belviq/Phenteramine combination therapies.
Data from Arena's BLOOM-DM trial suggest that Belviq is effective in lowering HbA1C in type 2 diabetics, meaning that type 2 diabetics taking Belviq enjoy better long-term glycemic control than those taking placebo. As such, a combination of Belviq and Metformin (the current standard of care for glycemic control in type 2 diabetes) could allow for even better gylcemic control in type 2 diabetes than either drug alone. Indeed, if studies of a Belviq/Metformin combination yield positive results, Arena would have a whole new target market of patients.
In my opinion, a combination therapy of Belviq/Phenteramine is truly exciting. While Fenfluramine/Phenteramine ("FenPhen") was an exquisitely effective anti-obesity drug, it was heavily associated with valvular heart disease and subsequently pulled from the market. It is now known that Fenfluramine induced valvular heart disease via stimulation of the 5HT2B receptor in valvular tissue. Fortunately, Belviq is 100x more selective for the 5HT2C receptor than the 5HT2B receptor, which basically limits Belviq's action to central 5HT2C receptors. While Fenfluramine worked at the same central 5HT2C receptors, its secondary action on peripheral 5HT2B receptors is what caused problems. As such, many have hopes that a Belviq/Phenteramine combination therapy could be as effective as FenPhen, without the associated risk of valvular heart disease.
Time to Invest, Not Trade
Clearly, Belviq gives Arena the chance to become a legitimate pharmaceutical company that deserves consideration as an investment, not just a fleeting trade. With patent protection of Belviq until 2023 in most jurisdictions, Arena has plenty of time to expand Belviq's target market and explore alternative ways to monetize the drug.
Recently, many people have made bold predictions regarding early sales numbers for Belviq. Whether or not these predictions come true, I believe that the Arena's real value lies in its future plans for Belviq.
In other words,
don't make a trade for today...make an investment for tomorrow.
http://seekingalpha.com/article/1029111-the-future-is-bright…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/1029111-the-future-is-bright…
November 26, 2012
This summer, the FDA took action to make Arena Pharmaceuticals' (ARNA) Belviq the first drug approved for the treatment of obesity in thirteen years. By now, most are aware that Belviq's main competition is Vivus' (VVUS) Qsymia, and this has led to a sharp divide in investor opinion concerning the two drugs.
While many have supported one drug or the other on a basis of safety or efficacy, I contend that the relative marketability of Belviq and its potential uses outside the realm of obesity position Arena Pharmaceuticals for a very exciting future. Indeed, the company's vision for Belviq is promising, and it warrants far more attention than it's been given to this point.
A Closer Look at Belviq's Marketability
When considering whether or not a product will be successful in the marketplace, it is important to investigate the product's marketability. Some determinants of marketability include the size of the target market and how easily the target market can be accessed via advertisement, thereby increasing public awareness of the product.
For both Belviq and Qsymia, the target market is enormous. In the United States alone, 150 million people are considered overweight or obese (BMI≥25). Globally, the numbers are even more staggering: at least 500 million people are considered obese (BMI≥30). While both Belviq and Qsymia have been approved for use in the United States, neither has been approved for use anywhere else in the world.
Currently, Arena is pursuing EMA approval for use of Belviq in the European Union. Since Qsymia was recently rejected by the EMA for use in Europe, a vote of confidence from the EMA would leave Arena in a great position to take advantage of the entire European market. At this point, then, it would seem that Belviq has a small edge in terms of potential target market size.
As far as ability to access the target market, most would agree that Arena has the edge there, as well. The restrictive REMS placed upon Qsymia at the time of FDA approval is very limiting, and it's something that has definitely impacted sales of Qsymia to this point. As part of the REMS, Vivus is not allowed to advertise Qsymia directly to consumers, adversely affecting Vivus' ability to access the target market.
Another element of the Qsymia REMS requires that patients who are prescribed Qsymia use a mail-order pharmacy service. This means that a patient cannot simply fill a Qsymia prescription at the neighborhood pharmacy; rather, upon completing some extra paperwork, the Qsymia prescription is delivered to the patient's door. While this sounds very convenient, the excess paperwork is often considered an unnecessary inconvenience. Additionally, patients using a mail-order pharmacy can't speak with a pharmacist in person, which makes some patients uneasy ("who will answer my questions?").
Development of Combination Therapies Based on Belviq

While Belviq's advantage over Qsymia with respect to marketability is important, I believe that the prospect of using Belviq in combination therapies could have an even greater impact.
 In the recent Credit Suisse Healthcare Conference, Craig Audet specifically mentioned Arena's plans to pursue both Belviq/Metformin and Belviq/Phenteramine combination therapies.
In the recent Credit Suisse Healthcare Conference, Craig Audet specifically mentioned Arena's plans to pursue both Belviq/Metformin and Belviq/Phenteramine combination therapies.
Data from Arena's BLOOM-DM trial suggest that Belviq is effective in lowering HbA1C in type 2 diabetics, meaning that type 2 diabetics taking Belviq enjoy better long-term glycemic control than those taking placebo. As such, a combination of Belviq and Metformin (the current standard of care for glycemic control in type 2 diabetes) could allow for even better gylcemic control in type 2 diabetes than either drug alone. Indeed, if studies of a Belviq/Metformin combination yield positive results, Arena would have a whole new target market of patients.
In my opinion, a combination therapy of Belviq/Phenteramine is truly exciting. While Fenfluramine/Phenteramine ("FenPhen") was an exquisitely effective anti-obesity drug, it was heavily associated with valvular heart disease and subsequently pulled from the market. It is now known that Fenfluramine induced valvular heart disease via stimulation of the 5HT2B receptor in valvular tissue. Fortunately, Belviq is 100x more selective for the 5HT2C receptor than the 5HT2B receptor, which basically limits Belviq's action to central 5HT2C receptors. While Fenfluramine worked at the same central 5HT2C receptors, its secondary action on peripheral 5HT2B receptors is what caused problems. As such, many have hopes that a Belviq/Phenteramine combination therapy could be as effective as FenPhen, without the associated risk of valvular heart disease.
Time to Invest, Not Trade
Clearly, Belviq gives Arena the chance to become a legitimate pharmaceutical company that deserves consideration as an investment, not just a fleeting trade. With patent protection of Belviq until 2023 in most jurisdictions, Arena has plenty of time to expand Belviq's target market and explore alternative ways to monetize the drug.
Recently, many people have made bold predictions regarding early sales numbers for Belviq. Whether or not these predictions come true, I believe that the Arena's real value lies in its future plans for Belviq.
In other words,
don't make a trade for today...make an investment for tomorrow.

http://seekingalpha.com/article/1029111-the-future-is-bright…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/1029111-the-future-is-bright…
Arena Pharmaceuticals and Ildong Pharmaceutical Enter Into Co-Development and License Agreement for Temanogrel, a Novel Agent for Thrombotic Diseases
Agreement Enables Clinical Development of Temanogrel to Resume with Ildong
SAN DIEGO, Nov. 28, 2012 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that it has entered into a co-development and license agreement with Ildong Pharmaceutical Co., Ltd., for temanogrel, Arena's internally discovered inverse agonist of the serotonin 2A receptor. The agreement grants Ildong exclusive rights to commercialize temanogrel in South Korea for myocardial infarction, acute coronary syndrome, stroke, peripheral artery disease, and other cardiovascular diseases, subject to further development and regulatory approval of temanogrel. Initially, Ildong will be responsible for funding and conducting, under the direction of a joint steering committee, the next two planned clinical trials in this program: an additional Phase 1 trial in healthy volunteers and a Phase 2a proof-of-concept trial in patients.
"The Phase 1 trials Arena previously conducted support temanogrel's potential to treat a variety of conditions related to thrombosis through a novel mechanism that may provide advantages over currently available therapies," said Jack Lief, Arena's President and Chief Executive Officer. "A successful product in this category has the potential to serve as an important treatment option for many patients, and this collaboration with Ildong enables us to resume clinical development in a cost-effective manner."
Arena will maintain ownership of temanogrel outside of South Korea, and has the rights to use data generated by Ildong for the development and potential commercialization of temanogrel outside of South Korea by Arena or other Arena licensees. In addition, Ildong has agreed to pay Arena a $2 million development milestone if the planned additional Phase 1 and Phase 2a clinical trials conducted by Ildong support continued development and Arena or another Arena licensee initiates a Phase 2b clinical trial of temanogrel.
Arena is also eligible to receive a royalty on net sales of temanogrel in South Korea, while Ildong is eligible to receive a share of future payments received by Arena related to licensing transactions and sales of temanogrel in other territories.
"It is a great pleasure to establish this collaboration with Arena for temanogrel," said Jung-chi Lee, Ildong's Chairman and Chief Executive Officer. "We look forward to supporting the global development of this drug candidate and having the opportunity to bring temanogrel to patients in South Korea as a treatment for thrombotic diseases."
About Thrombosis
Thrombosis is the formation of a clot, or thrombus, inside a blood vessel. Thrombus formation that occurs in the blood vessels of the heart or brain can lead to serious thrombotic diseases including myocardial infarction, acute coronary syndrome and stroke. One of the initial events in thrombus formation is the activation of platelets, which then aggregate and adhere to one another as they release certain factors, including high concentrations of serotonin. Serotonin promotes further platelet aggregation and also causes constriction, or narrowing, of the blood vessels. Elevated serotonin levels have been associated with increased cardiovascular risk. The prothrombotic effects of serotonin on platelets and blood vessels are mediated by the serotonin 2A receptor, and inverse agonists of the serotonin 2A receptor have the potential to inhibit this activity.
About Temanogrel
Temanogrel is an inverse agonist of the serotonin 2A receptor intended for the treatment of thrombotic diseases, and has completed single- and multiple-ascending dose Phase 1 trials in healthy volunteers. Temanogrel has the potential to prevent serotonin-mediated platelet aggregation and reverse serotonin-mediated vasoconstriction. This dual mechanism of temanogrel may be therapeutically useful for the treatment or prevention of thrombotic diseases.
http://finance.yahoo.com/news/arena-pharmaceuticals-ildong-p…
Die Zusammenarbeit für die Entwicklung und Licenzierung des Blutverdünnungsmittels ist sicher eine gute Nachricht. Heißt sie doch, dass wieder was in der Pipeline ist, die Kosten geteilt werden und die Aussichten auch.
Kursbewegend wird die Nachricht eher nicht sein, weil eine Zulassung irgendwann in Jahren käme.
Kursbewegend wird die Nachricht eher nicht sein, weil eine Zulassung irgendwann in Jahren käme.
Arena Pharma Will Dominate US, Europe & Rest of Globe's Obesity and Diabetes Markets
By Reza Ganjavi - December 4, 2012
Many retail investors believe Arena Pharmaceuticals (NASDAQ: ARNA) has an emerging blockbuster in its obesity and diabetes medicine, Belviq. Institutional investors have not caught on fully but are getting there. Arena's institutional ownership has been streadily on the rise since FDA approval of Belviq on June 27, from 25% to 45% of the float. The American market is ripe and wide open, but Arena's prospects are global, another fact most Wall Street analysts have not yet considered.
Most analysts are still on the sidelines regarding the US market, let alone Europe. They were dealt such a fiction by hedge funds and Vivus (NASDAQ: VVUS) proponents that in the thawing of that fiction into nowhereland, the paradigm shift is taking them some time and pain. Main Street has been highly consistent throughout, however. Dr. Steven Vig, who treats many obesity and diabetic patients, has been one of Belviq's proponents for a long time. He's recently put together the a fine video explaining the science and the market thoroughly.
Obesity treatment has been an area in which retail investors have beat Wall Street hands down. Retail still owns majority of Arena shares while Wall Street's apetite for Arena is growing. It's not often that Main Street calls the shots but in this case we do -- we decide when and at what price to sell our shares to Wall Street.
Patience is the name of the game , in my opinon, and investors need to do good, thorough research for themselves. The pros are not providing the needed guidance. Many pros are providing analysis to suit their agenda, be it their customers who are short and need shares or those who missed the boat and need to get in at higher prices.
, in my opinon, and investors need to do good, thorough research for themselves. The pros are not providing the needed guidance. Many pros are providing analysis to suit their agenda, be it their customers who are short and need shares or those who missed the boat and need to get in at higher prices.
A company you want to invest in is often the best source of information. Arena has provided plenty of important information in its recent investor conferences. There is also plenty of material on regulatory agencies' websites, and also blog posts such as these and message boards can be a good source of information as many people participate and share their views, but it's important to be skeptical while reading reading public boards as they may contain inaccurate information.
While Wall Street isn't even debating ROW (rest of the world) potential for Arena, Main Street is ahead of the game again. Check out these statistics:
The size of the obesity market is well known: two-thirds of the population in the United States is overweight and one-third, obese. The statistics in many other countries are similar. In Korea, where Arena recently signed a marketing agreement for Belviq, one-third of adults are obese. In Central and South America, over 30% of women are obese. In Mexico, 30% of adults are obese and 70% are overweight.
In the UK 25% of adults are obese and 57% are overweight. In Kuwait over one-third of men and almost half of all adult women are obese. In other Arabic countries the numbers are similar to Kuwait.
In other regions too obesity rates have skyrocketed and are projected to continue to increase, including child-obesity. That’s why experts call obesity a pandemic. Globally, over 500 million adults are obese.
Arena has a partnership with Eisai for North and South Americas.
Arena also has a partnership with a large Korean pharmaceutical, Ildong, for not only Belviq but funding of research for another novel agent of Arena's, for thrombotic diseases. Arena has indicated it is talking to many interested parties globally. I expect we will hear more partnerships in the near future. Arena's CEO recently stated there's high demand for Belviq in Asia, including China, India, and the Middle East, and Arena will pursue all these opportunities.
However, Europe remains the short-term, low-hanging fruit internationally. Many Wall Street analysts, hedge funds, and journalists have been consistently wrong so far in their predictions for Arena, and now they're skeptical about European approval.
My research indicates that European as well as Swiss approvals of Belviq are slam dunks. ....einen Baseketball kraftvoll ( und sicher !!) im Korb versenken...
Here's why:
From "GUIDELINE ON CLINICAL EVALUATION OF MEDICINAL PRODUCTS USED IN WEIGHT CONTROL -- section 4.2.1": "Proportions of responders in the various treatment arms could be considered as an alternative primary efficacy criterion where response is more than 10% weight loss at the end of a 12-month period."
Arena indeed meets and exceeds the above criteria. Comparing "treatment arms," the number of responders who lost more than 10% of their weight was significantly higher in the Belviq group than in the placebo group.
Separately, a cardiovascular working group in Europe has recommended to CHMP that the efficacy threshold is lowered to 5% based on the cardiovascular and other health benefits observed at 5% weight loss. We know that a modest 5% weight loss can have significant improvements on overall health -- two Phase III trials of Belviq showed completers lost an average of 8% of body weight and responders lost between 11% and 12% of body weight in a year. The top 25% of weight losers lost an average of 35 pounds in one year. This is superior efficacy, which will even improve further though upcoming trials in combination therapies.
I believe EMA will view Belviq as very safe ....yepp...
-- just as FDA did by not requiring a REMS program. Therefore, European approval of Belviq will happen in the first half of 2013 -- most likely by February 2013 we will have the CHMP recommendation for approval and it will get finalized by member states by April. This opens a huge market where Arena will be the only player. Qsymia by Vivus was recently rejected in the EU and despite Vivus management's desire for appeal, Qsymia will never get approved in Europe.
There is a dire need for a safe and effective obesity drug in the EU and Belviq will be it. I expect Jack Lief, Arena's shrewd CEO, to strike yet another deal for the company with a global player for European partnership (click here to vote for Jack to be CEO of the year).
I will not be surprised at all to see a buyout by that time given strong US sales and Eisai's need to increase its sales presence, which it has done in the past in partnerships with Pfizer (NYSE: PFE) and Johnson & Johnson (NYSE: JNJ). Any of those companies or Merck (NYSE: MRK), Abbott Laboratories (NYSE: ABT), or another big pharma could be a potential suiter for Arena, providing a creative deal that covers Arena's deal with Eisai.
With strong US sales and EU approval, the price tag I believe will be north of $30 per share.
 ..time will tell...
..time will tell...
http://beta.fool.com/beatlesforever/2012/12/04/arena-pharma-…
By Reza Ganjavi - December 4, 2012
Many retail investors believe Arena Pharmaceuticals (NASDAQ: ARNA) has an emerging blockbuster in its obesity and diabetes medicine, Belviq. Institutional investors have not caught on fully but are getting there. Arena's institutional ownership has been streadily on the rise since FDA approval of Belviq on June 27, from 25% to 45% of the float. The American market is ripe and wide open, but Arena's prospects are global, another fact most Wall Street analysts have not yet considered.
Most analysts are still on the sidelines regarding the US market, let alone Europe. They were dealt such a fiction by hedge funds and Vivus (NASDAQ: VVUS) proponents that in the thawing of that fiction into nowhereland, the paradigm shift is taking them some time and pain. Main Street has been highly consistent throughout, however. Dr. Steven Vig, who treats many obesity and diabetic patients, has been one of Belviq's proponents for a long time. He's recently put together the a fine video explaining the science and the market thoroughly.
Obesity treatment has been an area in which retail investors have beat Wall Street hands down. Retail still owns majority of Arena shares while Wall Street's apetite for Arena is growing. It's not often that Main Street calls the shots but in this case we do -- we decide when and at what price to sell our shares to Wall Street.

Patience is the name of the game
 , in my opinon, and investors need to do good, thorough research for themselves. The pros are not providing the needed guidance. Many pros are providing analysis to suit their agenda, be it their customers who are short and need shares or those who missed the boat and need to get in at higher prices.
, in my opinon, and investors need to do good, thorough research for themselves. The pros are not providing the needed guidance. Many pros are providing analysis to suit their agenda, be it their customers who are short and need shares or those who missed the boat and need to get in at higher prices.A company you want to invest in is often the best source of information. Arena has provided plenty of important information in its recent investor conferences. There is also plenty of material on regulatory agencies' websites, and also blog posts such as these and message boards can be a good source of information as many people participate and share their views, but it's important to be skeptical while reading reading public boards as they may contain inaccurate information.
While Wall Street isn't even debating ROW (rest of the world) potential for Arena, Main Street is ahead of the game again. Check out these statistics:
The size of the obesity market is well known: two-thirds of the population in the United States is overweight and one-third, obese. The statistics in many other countries are similar. In Korea, where Arena recently signed a marketing agreement for Belviq, one-third of adults are obese. In Central and South America, over 30% of women are obese. In Mexico, 30% of adults are obese and 70% are overweight.
In the UK 25% of adults are obese and 57% are overweight. In Kuwait over one-third of men and almost half of all adult women are obese. In other Arabic countries the numbers are similar to Kuwait.
In other regions too obesity rates have skyrocketed and are projected to continue to increase, including child-obesity. That’s why experts call obesity a pandemic. Globally, over 500 million adults are obese.
Arena has a partnership with Eisai for North and South Americas.
Arena also has a partnership with a large Korean pharmaceutical, Ildong, for not only Belviq but funding of research for another novel agent of Arena's, for thrombotic diseases. Arena has indicated it is talking to many interested parties globally. I expect we will hear more partnerships in the near future. Arena's CEO recently stated there's high demand for Belviq in Asia, including China, India, and the Middle East, and Arena will pursue all these opportunities.

However, Europe remains the short-term, low-hanging fruit internationally. Many Wall Street analysts, hedge funds, and journalists have been consistently wrong so far in their predictions for Arena, and now they're skeptical about European approval.
My research indicates that European as well as Swiss approvals of Belviq are slam dunks. ....einen Baseketball kraftvoll ( und sicher !!) im Korb versenken...

Here's why:
From "GUIDELINE ON CLINICAL EVALUATION OF MEDICINAL PRODUCTS USED IN WEIGHT CONTROL -- section 4.2.1": "Proportions of responders in the various treatment arms could be considered as an alternative primary efficacy criterion where response is more than 10% weight loss at the end of a 12-month period."
Arena indeed meets and exceeds the above criteria. Comparing "treatment arms," the number of responders who lost more than 10% of their weight was significantly higher in the Belviq group than in the placebo group.
Separately, a cardiovascular working group in Europe has recommended to CHMP that the efficacy threshold is lowered to 5% based on the cardiovascular and other health benefits observed at 5% weight loss. We know that a modest 5% weight loss can have significant improvements on overall health -- two Phase III trials of Belviq showed completers lost an average of 8% of body weight and responders lost between 11% and 12% of body weight in a year. The top 25% of weight losers lost an average of 35 pounds in one year. This is superior efficacy, which will even improve further though upcoming trials in combination therapies.
I believe EMA will view Belviq as very safe ....yepp...

-- just as FDA did by not requiring a REMS program. Therefore, European approval of Belviq will happen in the first half of 2013 -- most likely by February 2013 we will have the CHMP recommendation for approval and it will get finalized by member states by April. This opens a huge market where Arena will be the only player. Qsymia by Vivus was recently rejected in the EU and despite Vivus management's desire for appeal, Qsymia will never get approved in Europe.
There is a dire need for a safe and effective obesity drug in the EU and Belviq will be it. I expect Jack Lief, Arena's shrewd CEO, to strike yet another deal for the company with a global player for European partnership (click here to vote for Jack to be CEO of the year).
I will not be surprised at all to see a buyout by that time given strong US sales and Eisai's need to increase its sales presence, which it has done in the past in partnerships with Pfizer (NYSE: PFE) and Johnson & Johnson (NYSE: JNJ). Any of those companies or Merck (NYSE: MRK), Abbott Laboratories (NYSE: ABT), or another big pharma could be a potential suiter for Arena, providing a creative deal that covers Arena's deal with Eisai.
With strong US sales and EU approval, the price tag I believe will be north of $30 per share.
 ..time will tell...
..time will tell...
http://beta.fool.com/beatlesforever/2012/12/04/arena-pharma-…
Arena Pharmaceuticals to Present at the Oppenheimer 23rd Annual Healthcare Conference
PR NewswirePress Release: Arena Pharmaceuticals, Inc. – 35 minutes ago
SAN DIEGO, Dec. 6, 2012 /PRNewswire/
Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at the Oppenheimer 23rd Annual Healthcare Conference on Wednesday, December 12, 2012, at 9:25 a.m. Eastern Time (6:25 a.m. Pacific Time) at The Waldorf Astoria in New York City.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
PR NewswirePress Release: Arena Pharmaceuticals, Inc. – 35 minutes ago
SAN DIEGO, Dec. 6, 2012 /PRNewswire/
Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at the Oppenheimer 23rd Annual Healthcare Conference on Wednesday, December 12, 2012, at 9:25 a.m. Eastern Time (6:25 a.m. Pacific Time) at The Waldorf Astoria in New York City.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
Arena Pressures Short Sellers as Hedge Fund Owner Pressures Vivus
By Reza Ganjavi - December 11, 2012 |
Examining the top institutional holders of Arena Pharmaceuticals (NASDAQ: ARNA) vs. Vivus (NASDAQ: VVUS) reveals a stark difference in the quality of investors in these two obesity drug makers. Vivus’ top holders are two hedge funds: QVT and Passport Capital.
 Arena's top two holders are among the world's largest investment management companies, Wellington Management and Vanguard; they manage a combined $2.3 trillion of assets.
Arena's top two holders are among the world's largest investment management companies, Wellington Management and Vanguard; they manage a combined $2.3 trillion of assets. 
News was out recently that QVT is pressuring Vivus management to sell the company. On Twitter, investors had fun with a less significant point about QVT: their General Counsel & Partner, Fati Sadeghi-Nejad, is a cousin of one of Jim Cramer's writers who's been bashing Arena (and has been consistently wrong). Just before Belviq was approved, in line with a streak of misrepresentations and disparaging articles out of Jim Cramer’s bash machine, cousin Sadeghi-Nejad tweeted: "I just want to be right. I've followed the FDA for 15 yrs. You are walking into a buzz saw. Sell." The FDA proved him wrong.
Following Belviq's approval, an investor wrote: “Adam Feuerstein’s FUD shook me out of riding ARNA through the Advisory Committee meeting.” But many investors didn't listen to Cramer's group, held on to their shares, and saw their investment go through the roof. Cramer's team are still repeating the same weak bashing mantras that were proved false. With over 60 million shares shorted it's hardly surprising that the company is intensely bashed.
Cramer's team are still repeating the same weak bashing mantras that were proved false. With over 60 million shares shorted it's hardly surprising that the company is intensely bashed.
Wall Street had very high hopes for Vivus. As early as about a month ago, analysts were still insisting on price targets of around $50. Vivus insiders sold massive number of shares at much higher prices than today’s $10.54. Shares of Vivus have lost two-thirds of their value in the last few months as reality set in.
I know several investors who follow the obesity space very closely. We do a deep level of due diligence and consult with medical experts in the field. Similar to what Wall Street analysts do, but Main Street research seems to be a lot more focused and free from influences of investment banking, clients, etc. We follow a much smaller number of stocks, so our research has more depth and accuracy than many of pros. Several of us saw through Vivus’ ridiculous valuation and unreasonable analyst estimates when the company was over $30.
At the core of our prediction for Vivus was the weak demand factor based on feedback we received from physicians about Qsymia’s risks and generic competition. Both these predictions seem to have come true. Qsymia’s sales have been weak and insurance coverage is not boding well for Qsymia.
Andy Baron, one of the thought leaders in the obesity investing space, pointed out the problem for Vivus as follows:
"The Blue Cross/Blue Shield, policy requiring doctors to try generic phentermine first, is significant bad news for VVUS. This will only get worse when Supernus Pharmaceuticals (NASDAQ: SUPN) starts selling their extended-release topiramate, Trokendi, in June. It comes in 50 mg and 100 mg doses (among others), which is pretty close to the 46 mg and 92 mg in Qsymia. With that and generic phentermine, doctors will be able to prescribe almost the identical medications in Qsymia with no REMS, no pharmacy restriction, and a much lower cost."
"It's also very interesting that they don't specify the dosage of generic phentermine that must be tried (and fail) before they'll pay for Qsymia. The most common dose is 37.5 mg, which is more than twice the amount in the highest dose of Qsymia. In fact the amount of phentermine in the recommended dose of Qsymia, 7.5 mg, is not widely available. So the initial choice is to try phentermine at a higher dose than is in Qsymia (with a likely increase in heart rate) or to try Belviq, which many doctors would prefer anyway."
Perhaps QVT wants Vivus to be sold because Big Pharmas may not be interested in the proposition Vivus offers. Arena's management, on the other hand, have expressed that there is a high degree of interest globally in establishing relationships with Arena.
Wall Street had it wrong with Arena just as it did about Vivus, but inversely. This mistake resulted in astronomical short interest, which was confronted with the painful fact of a strong FDA approval of Belviq last June.
Since then, Arena short sellers were caught between a rock and a hard place with few options:
1) Cover and incur a potential billion dollar loss;
2) Short more shares in hope of a) increasing sales-basis b) spooking retail holders to sell.
As discussed in some of my previous MF articles, the demand for shares of Arena by the short interest is augmented by institutional interest, which has been on a constant rise -- this second wave of demand does not bode well for shorts. We’ve seen a recurring cycle of ARNA’s shares dropping followed by big gains in a typical consolidation pattern. At the end of one such cycle recently ARNA was naked shorted and on the RegSho list. Selling what you don't have and what you have not borrowed should be criminal.
Arena’s business is developing rapidly.
It recently announced a new partnership with a large South Korean pharma, and Arena has indicated it’s in talks with lots of other companies across the globe for partnerships;
European approval of Belviq is very likely and positive indication could come as early as Christmas if EU/EMA’S CHMP doesn’t ask for an oral hearing.
The DEA should finally complete its review of Belviq any day, and the agency may even give Arena/Eisai an exception to start marketing earlier than expected. Strong initial sales numbers will result in upgrades, and the beginning of combination therapies trials will get Belviq closer to the blockbuster status.
These and other positive developments for Arena are putting pressure on short sellers whose time is running out . I see a very dangerous and explosive situation for Arena short sellers. Their sales basis may have risen by a dollar, but they need more shares to cover the additional sales. As pressure builds they may have no choice but to be forced to cover, which causes competition among different short sellers and a short squeeze.
. I see a very dangerous and explosive situation for Arena short sellers. Their sales basis may have risen by a dollar, but they need more shares to cover the additional sales. As pressure builds they may have no choice but to be forced to cover, which causes competition among different short sellers and a short squeeze.
There doesn’t seem to be a reasonable chance that retail investors would sell 61,748,793 shares at current prices.
Even then, shorts are looking at a loss of several hundred million dollars . A more likely scenario is that shorts will chase the price in order to cover.
. A more likely scenario is that shorts will chase the price in order to cover.
Given the daily volume and price action I don’t believe anybody can buy over 60 million shares without bidding the price up at least $5. That would put the loss at around $600,000,000.....das wäre doch mal ein Schlag gegen die Heuschrecken...
How many hedge fund traders will lose their job when they lose $600 million? Even that number is conservative, and doesn’t take into consideration a bidding war for Arena by Big Pharma, whose patent pipelines are dwindling. A buyout offer could put the loss of short sellers well over a billion dollars. Short of a major market crash, Arena’s short sellers are in for what the band Bananarama would call "A Cruel, Cruel Winter.”
http://beta.fool.com/beatlesforever/2012/12/11/arena-pressur…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/beatlesforever/2012/12/11/arena-pressur…
By Reza Ganjavi - December 11, 2012 |
Examining the top institutional holders of Arena Pharmaceuticals (NASDAQ: ARNA) vs. Vivus (NASDAQ: VVUS) reveals a stark difference in the quality of investors in these two obesity drug makers. Vivus’ top holders are two hedge funds: QVT and Passport Capital.
 Arena's top two holders are among the world's largest investment management companies, Wellington Management and Vanguard; they manage a combined $2.3 trillion of assets.
Arena's top two holders are among the world's largest investment management companies, Wellington Management and Vanguard; they manage a combined $2.3 trillion of assets. 
News was out recently that QVT is pressuring Vivus management to sell the company. On Twitter, investors had fun with a less significant point about QVT: their General Counsel & Partner, Fati Sadeghi-Nejad, is a cousin of one of Jim Cramer's writers who's been bashing Arena (and has been consistently wrong). Just before Belviq was approved, in line with a streak of misrepresentations and disparaging articles out of Jim Cramer’s bash machine, cousin Sadeghi-Nejad tweeted: "I just want to be right. I've followed the FDA for 15 yrs. You are walking into a buzz saw. Sell." The FDA proved him wrong.
Following Belviq's approval, an investor wrote: “Adam Feuerstein’s FUD shook me out of riding ARNA through the Advisory Committee meeting.” But many investors didn't listen to Cramer's group, held on to their shares, and saw their investment go through the roof.
 Cramer's team are still repeating the same weak bashing mantras that were proved false. With over 60 million shares shorted it's hardly surprising that the company is intensely bashed.
Cramer's team are still repeating the same weak bashing mantras that were proved false. With over 60 million shares shorted it's hardly surprising that the company is intensely bashed.Wall Street had very high hopes for Vivus. As early as about a month ago, analysts were still insisting on price targets of around $50. Vivus insiders sold massive number of shares at much higher prices than today’s $10.54. Shares of Vivus have lost two-thirds of their value in the last few months as reality set in.
I know several investors who follow the obesity space very closely. We do a deep level of due diligence and consult with medical experts in the field. Similar to what Wall Street analysts do, but Main Street research seems to be a lot more focused and free from influences of investment banking, clients, etc. We follow a much smaller number of stocks, so our research has more depth and accuracy than many of pros. Several of us saw through Vivus’ ridiculous valuation and unreasonable analyst estimates when the company was over $30.
At the core of our prediction for Vivus was the weak demand factor based on feedback we received from physicians about Qsymia’s risks and generic competition. Both these predictions seem to have come true. Qsymia’s sales have been weak and insurance coverage is not boding well for Qsymia.
Andy Baron, one of the thought leaders in the obesity investing space, pointed out the problem for Vivus as follows:
"The Blue Cross/Blue Shield, policy requiring doctors to try generic phentermine first, is significant bad news for VVUS. This will only get worse when Supernus Pharmaceuticals (NASDAQ: SUPN) starts selling their extended-release topiramate, Trokendi, in June. It comes in 50 mg and 100 mg doses (among others), which is pretty close to the 46 mg and 92 mg in Qsymia. With that and generic phentermine, doctors will be able to prescribe almost the identical medications in Qsymia with no REMS, no pharmacy restriction, and a much lower cost."
"It's also very interesting that they don't specify the dosage of generic phentermine that must be tried (and fail) before they'll pay for Qsymia. The most common dose is 37.5 mg, which is more than twice the amount in the highest dose of Qsymia. In fact the amount of phentermine in the recommended dose of Qsymia, 7.5 mg, is not widely available. So the initial choice is to try phentermine at a higher dose than is in Qsymia (with a likely increase in heart rate) or to try Belviq, which many doctors would prefer anyway."
Perhaps QVT wants Vivus to be sold because Big Pharmas may not be interested in the proposition Vivus offers. Arena's management, on the other hand, have expressed that there is a high degree of interest globally in establishing relationships with Arena.
Wall Street had it wrong with Arena just as it did about Vivus, but inversely. This mistake resulted in astronomical short interest, which was confronted with the painful fact of a strong FDA approval of Belviq last June.
Since then, Arena short sellers were caught between a rock and a hard place with few options:
1) Cover and incur a potential billion dollar loss;
2) Short more shares in hope of a) increasing sales-basis b) spooking retail holders to sell.
As discussed in some of my previous MF articles, the demand for shares of Arena by the short interest is augmented by institutional interest, which has been on a constant rise -- this second wave of demand does not bode well for shorts. We’ve seen a recurring cycle of ARNA’s shares dropping followed by big gains in a typical consolidation pattern. At the end of one such cycle recently ARNA was naked shorted and on the RegSho list. Selling what you don't have and what you have not borrowed should be criminal.
Arena’s business is developing rapidly.
It recently announced a new partnership with a large South Korean pharma, and Arena has indicated it’s in talks with lots of other companies across the globe for partnerships;
European approval of Belviq is very likely and positive indication could come as early as Christmas if EU/EMA’S CHMP doesn’t ask for an oral hearing.
The DEA should finally complete its review of Belviq any day, and the agency may even give Arena/Eisai an exception to start marketing earlier than expected. Strong initial sales numbers will result in upgrades, and the beginning of combination therapies trials will get Belviq closer to the blockbuster status.
These and other positive developments for Arena are putting pressure on short sellers whose time is running out
 . I see a very dangerous and explosive situation for Arena short sellers. Their sales basis may have risen by a dollar, but they need more shares to cover the additional sales. As pressure builds they may have no choice but to be forced to cover, which causes competition among different short sellers and a short squeeze.
. I see a very dangerous and explosive situation for Arena short sellers. Their sales basis may have risen by a dollar, but they need more shares to cover the additional sales. As pressure builds they may have no choice but to be forced to cover, which causes competition among different short sellers and a short squeeze.There doesn’t seem to be a reasonable chance that retail investors would sell 61,748,793 shares at current prices.
Even then, shorts are looking at a loss of several hundred million dollars
 . A more likely scenario is that shorts will chase the price in order to cover.
. A more likely scenario is that shorts will chase the price in order to cover.Given the daily volume and price action I don’t believe anybody can buy over 60 million shares without bidding the price up at least $5. That would put the loss at around $600,000,000.....das wäre doch mal ein Schlag gegen die Heuschrecken...

How many hedge fund traders will lose their job when they lose $600 million? Even that number is conservative, and doesn’t take into consideration a bidding war for Arena by Big Pharma, whose patent pipelines are dwindling. A buyout offer could put the loss of short sellers well over a billion dollars. Short of a major market crash, Arena’s short sellers are in for what the band Bananarama would call "A Cruel, Cruel Winter.”
http://beta.fool.com/beatlesforever/2012/12/11/arena-pressur…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/beatlesforever/2012/12/11/arena-pressur…
Antwort auf Beitrag Nr.: 43.919.210 von bernie55 am 12.12.12 12:14:45..eine sehr schöne Übersicht über die anstehenden Events (kurz - und längerfristig) bei ARENA...
Most investors don't realize that Arena has several strong short-term and long-term catalysts coming:
1. DEA schedule IV announcement any day now
2. $65,000,000 milestone payment
3. December, 2012, EMA preliminary assessment of Belviq's EU application
4. First quarter, 2013, Belviq U.S. sales and marketing
5. EU Belviq approval first half 2013
6. Continued institution and fund ownership growth
7. EU Partnership
8. Additional Rest of World Partnerships
9. Quarterly sales results
10. EU Launch
11. Approval in Switzerland, Canada, Mexico, Brazil and Korea
12. Belviq-Phentermine combination drug
13. Belviq for treating addiction (such as smoking cessation support)
14. Belviq-Metformin combination drug (Diabetes is a huge market by itself)
15. Advancing APD811 towards phase IIb (PAH treatment)
16. Starting clinical trials with at-least one more promising NCE
17. Potential Buy-out by Big Pharmaceutical before Belviq becomes a success
18. Short squeeze coming soon.
entnommen aus http://beta.fool.com/mrjosephd/2012/11/26/what-investors-sho…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/mrjosephd/2012/11/26/what-investors-sho…
Most investors don't realize that Arena has several strong short-term and long-term catalysts coming:
1. DEA schedule IV announcement any day now
2. $65,000,000 milestone payment
3. December, 2012, EMA preliminary assessment of Belviq's EU application
4. First quarter, 2013, Belviq U.S. sales and marketing
5. EU Belviq approval first half 2013
6. Continued institution and fund ownership growth
7. EU Partnership
8. Additional Rest of World Partnerships
9. Quarterly sales results
10. EU Launch
11. Approval in Switzerland, Canada, Mexico, Brazil and Korea
12. Belviq-Phentermine combination drug
13. Belviq for treating addiction (such as smoking cessation support)
14. Belviq-Metformin combination drug (Diabetes is a huge market by itself)
15. Advancing APD811 towards phase IIb (PAH treatment)
16. Starting clinical trials with at-least one more promising NCE
17. Potential Buy-out by Big Pharmaceutical before Belviq becomes a success
18. Short squeeze coming soon.
entnommen aus http://beta.fool.com/mrjosephd/2012/11/26/what-investors-sho…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/mrjosephd/2012/11/26/what-investors-sho…
 .....also, hier eine Vorankündigung, dass die DEA am 19.12.12 Lorcaserin ( Belviq) die Klassifikation IV geben wird...
.....also, hier eine Vorankündigung, dass die DEA am 19.12.12 Lorcaserin ( Belviq) die Klassifikation IV geben wird... 
Schedules of Controlled Substances: Placement of Lorcaserin into Schedule IV
An unpublished Proposed Rule by the Drug Enforcement Administration on 12/19/2012.
This document is unpublished, but on 12/19/2012 it is scheduled to be published and available on this page.
Until then, you can download the pre-publication PDF version.
https://www.federalregister.gov/articles/2012/12/19/2012-305…" target="_blank" rel="nofollow ugc noopener">
https://www.federalregister.gov/articles/2012/12/19/2012-305…
Drug Enforcement Administration
Public Inspection Documents Pending Publication
December 2012 Wednesday 19
Schedules of Controlled Substances: Placement of Lorcaserin into Schedule IV
https://www.federalregister.gov/agencies/drug-enforcement-ad…

Public Inspection Documents Pending Publication
December 2012 Wednesday 19
Schedules of Controlled Substances: Placement of Lorcaserin into Schedule IV
https://www.federalregister.gov/agencies/drug-enforcement-ad…
...irgendwie funzen die Links nicht, deshalb hier nochmals die vollständigen Webadressen zur obengenannten News :
https://www.federalregister.gov/agencies/drug-enforcement-ad…
https://www.federalregister.gov/articles/2012/12/19/2012-305…
https://www.federalregister.gov/agencies/drug-enforcement-ad…
https://www.federalregister.gov/articles/2012/12/19/2012-305…
Antwort auf Beitrag Nr.: 43.940.309 von bernie55 am 18.12.12 16:41:37ist ja super!
Leider reagiert der Kurs gar nicht.
Wundert allerdings schon a weng dass eine unpublished document dann doch auf der FDA-Site einsehbar sein sollte ...
Leider reagiert der Kurs gar nicht.
Wundert allerdings schon a weng dass eine unpublished document dann doch auf der FDA-Site einsehbar sein sollte ...
Antwort auf Beitrag Nr.: 43.940.584 von me_2 am 18.12.12 17:30:52Der Kurs wird nach offizieller Bekanntgabe sehrwohl reagieren, Arena wird durch die Glukosesenkung für Diabetiker und Fettsüchtige der Megablockbuster!
Antwort auf Beitrag Nr.: 43.940.881 von Magnetfeldfredy am 18.12.12 18:24:56morgen wissen wir mehr ...
Antwort auf Beitrag Nr.: 43.940.935 von me_2 am 18.12.12 18:33:14Ich habe heute nachgelegt, aber nicht für morgen oder übermorgen, Arena hat mit Belviq einen Blockbuster an der Hand der in 1-2 Jahren die Aktie in den 20-30 US Dollar Bereich bringen kann!
Wahrscheinlich gibts zuvor eine Übernahme von big pharma da ja die EU-Rechte Arena ganz alleine gehören!
Wahrscheinlich gibts zuvor eine Übernahme von big pharma da ja die EU-Rechte Arena ganz alleine gehören!

Antwort auf Beitrag Nr.: 43.940.309 von bernie55 am 18.12.12 16:41:37Super Recherche Bernie, wie meine 5 Kopfbälle pro Sekunde!

Antwort auf Beitrag Nr.: 43.942.745 von bernie55 am 19.12.12 08:52:01Genau und jetzt heißt Arena go........

Money Managers Love These 6 High-Growth Biotech Stocks
Posted:12/19/12
By:Sabina Bhatia

The Biotech industry is relatively volatile, where share prices are capable of soaring or plummeting from the smallest signal from the FDA, announcement of medical breakthroughs and passage of laws. For that reason many investors say that the time, knowledge and attention required to actively trade the industry makes it not worth their while. But if you’re looking to enter the space anyway, following the investing activities of “smart money” investors provides a good starting point. We ran a screen with this idea in mind.
We began by screening the biotech industry for bullish sentiment from institutional investors, with significant net institutional purchases over the last quarter representing at least 5% of share float. This indicates that institutional investors such as hedge fund managers and mutual fund managers expect these names to outperform into the future.
We then screened for the stocks with high growth projections, with 5-year projected EPS growth rates above 15%.
For an interactive version of this chart, click on the image below.Analyst ratings sourced from Zacks Investment Research.

https://www.kapitall.com/framework/tool/Comparator/embedded.…
Do you think hedge funds are calling it right on these names? Use this list as a starting point for your own analysis.
List sorted by net institutional purchases as a percent of share float.
1. Arena Pharmaceuticals, Inc. (ARNA): Focuses on discovering, developing, and commercializing oral drugs in the therapeutic areas of cardiovascular, central nervous system, inflammatory, and metabolic diseases. Market cap at $2.01B, most recent closing price at $9.25. Net institutional purchases in the current quarter at 29.4M shares, which represents about 13.6% of the company’s float of 216.13M shares. 5-year EPS growth at 25%.
2. Exact Sciences Corporation (EXAS): Focuses on developing a molecular diagnostic screening technology for the early detection and prevention of colorectal pre-cancer and cancer. Market cap at $656.19M, most recent closing price at $10.29. Net institutional purchases in the current quarter at 5.8M shares, which represents about 9.95% of the company’s float of 58.27M shares. 5-year EPS growth at 18%.
3. VIVUS Inc. (VVUS): Engages in the development and commercialization of therapeutic products for underserved markets in the United States. Market cap at $1.32B, most recent closing price at $13.08. Net institutional purchases in the current quarter at 6.1M shares, which represents about 7.38% of the company’s float of 82.66M shares. 5-year EPS growth at 51%.
4. Illumina Inc. (ILMN): Develops, manufactures, and markets integrated systems for the analysis of genetic variation and biological function. Market cap at $6.4B, most recent closing price at $51.86. Net institutional purchases in the current quarter at 8.6M shares, which represents about 7.07% of the company’s float of 121.60M shares. 5-year EPS growth at 17.4%.
5. Onyx Pharmaceuticals, Inc. (ONXX): Engages in developing therapies targeting the molecular mechanisms causing cancer in the United States and internationally. Market cap at $5.27B, most recent closing price at $78.46. Net institutional purchases in the current quarter at 3.3M shares, which represents about 5.23% of the company’s float of 63.14M shares. 5-year EPS growth at 38%.
6. Affymax, Inc. (AFFY): Engages in the development of drugs for the treatment of serious and life-threatening conditions. Market cap at $811.42M, most recent closing price at $21.83. Net institutional purchases in the current quarter at 1.6M shares, which represents about 5.% of the company’s float of 31.97M shares. 5-year EPS growth at 24%.
http://wire.kapitall.com/investment-idea/money-managers-love…
Posted:12/19/12
By:Sabina Bhatia

The Biotech industry is relatively volatile, where share prices are capable of soaring or plummeting from the smallest signal from the FDA, announcement of medical breakthroughs and passage of laws. For that reason many investors say that the time, knowledge and attention required to actively trade the industry makes it not worth their while. But if you’re looking to enter the space anyway, following the investing activities of “smart money” investors provides a good starting point. We ran a screen with this idea in mind.
We began by screening the biotech industry for bullish sentiment from institutional investors, with significant net institutional purchases over the last quarter representing at least 5% of share float. This indicates that institutional investors such as hedge fund managers and mutual fund managers expect these names to outperform into the future.
We then screened for the stocks with high growth projections, with 5-year projected EPS growth rates above 15%.
For an interactive version of this chart, click on the image below.Analyst ratings sourced from Zacks Investment Research.

https://www.kapitall.com/framework/tool/Comparator/embedded.…
Do you think hedge funds are calling it right on these names? Use this list as a starting point for your own analysis.
List sorted by net institutional purchases as a percent of share float.
1. Arena Pharmaceuticals, Inc. (ARNA): Focuses on discovering, developing, and commercializing oral drugs in the therapeutic areas of cardiovascular, central nervous system, inflammatory, and metabolic diseases. Market cap at $2.01B, most recent closing price at $9.25. Net institutional purchases in the current quarter at 29.4M shares, which represents about 13.6% of the company’s float of 216.13M shares. 5-year EPS growth at 25%.
2. Exact Sciences Corporation (EXAS): Focuses on developing a molecular diagnostic screening technology for the early detection and prevention of colorectal pre-cancer and cancer. Market cap at $656.19M, most recent closing price at $10.29. Net institutional purchases in the current quarter at 5.8M shares, which represents about 9.95% of the company’s float of 58.27M shares. 5-year EPS growth at 18%.
3. VIVUS Inc. (VVUS): Engages in the development and commercialization of therapeutic products for underserved markets in the United States. Market cap at $1.32B, most recent closing price at $13.08. Net institutional purchases in the current quarter at 6.1M shares, which represents about 7.38% of the company’s float of 82.66M shares. 5-year EPS growth at 51%.
4. Illumina Inc. (ILMN): Develops, manufactures, and markets integrated systems for the analysis of genetic variation and biological function. Market cap at $6.4B, most recent closing price at $51.86. Net institutional purchases in the current quarter at 8.6M shares, which represents about 7.07% of the company’s float of 121.60M shares. 5-year EPS growth at 17.4%.
5. Onyx Pharmaceuticals, Inc. (ONXX): Engages in developing therapies targeting the molecular mechanisms causing cancer in the United States and internationally. Market cap at $5.27B, most recent closing price at $78.46. Net institutional purchases in the current quarter at 3.3M shares, which represents about 5.23% of the company’s float of 63.14M shares. 5-year EPS growth at 38%.
6. Affymax, Inc. (AFFY): Engages in the development of drugs for the treatment of serious and life-threatening conditions. Market cap at $811.42M, most recent closing price at $21.83. Net institutional purchases in the current quarter at 1.6M shares, which represents about 5.% of the company’s float of 31.97M shares. 5-year EPS growth at 24%.
http://wire.kapitall.com/investment-idea/money-managers-love…
Das müßte uns doch auch was bringen:
Vivus shares climb after PBM accepts Qsymia
Vivus shares climb after nation's largest pharmacy benefits manager accepts diet pill Qsymia
http://finance.yahoo.com/news/vivus-shares-climb-pbm-accepts…
Vivus shares climb after PBM accepts Qsymia
Vivus shares climb after nation's largest pharmacy benefits manager accepts diet pill Qsymia
http://finance.yahoo.com/news/vivus-shares-climb-pbm-accepts…
Antwort auf Beitrag Nr.: 43.958.544 von Magnetfeldfredy am 24.12.12 09:58:03http://beta.fool.com/springer23/2012/12/11/dendreons-roller-…
US: Pharmaceuticals
Pharma Groups Hope for Diet Drugs Binge
Financial Times
Published: Thursday, 27 Dec 2012 | 2:18 AM ET
By: Andrew Jack
As Christmas overeating gives way to under-fulfilled new year diets, the pharmaceutical industry's appetite has been whetted for a fresh surge in business.
After years of caution and setbacks, several drug companies are preparing to capitalize on what they hope will be a surge in sales from prescription medicines linked to the growing global trend of obesity.
"It has been a very challenged category, but there is a feeling we have to do something about obesity with the realization that it is a medical epidemic," says Peter Tam, president of Vivus, a California-based biotechnology company. Last autumn it launched Qsymia, the first weight loss drug to win US regulatory approval in 13 years.
In the coming weeks, Arena, another West Coast biotech, is set to launch its drug Belviq after receiving authorization. A third, Orexigen, is preparing to submit for fresh regulatory approval for its experimental medicine Contrave after previous rejections.
"There has been a very significant and rather sudden shift in the views of the regulator over the past year," says Simos Simeonidis, senior biotech analyst with Cowen. "Companies had been laying off staff and putting products on hold. Now there has been a resurgence and a lot of investor interest."
Weight loss drugs have long been viewed with suspicion by healthcare specialists both because of their limited efficacy and the risks of side effects – factors which continue to haunt the field. Qsymia's weight loss impact is slightly more than 10 percent and Belviq's less than 6 percent – both when tested in ideal conditions with strong medical supervision.
Meanwhile, Wyeth, now part of Pfizer, is still fighting claims of heart valve defects and other problems linked to Fen-Phen, a combination of diet drugs withdrawn in 1997 for which it earmarked reserves of $21 billion to cover settlements. Regulators subsequently pulled Abbott's drug Meridia, and – in Europe – both Servier's Mediator and Sanofi's Acomplia, which was linked to suicidal feelings.
Only one prescription weight loss drug has remained on the market in the US: Roche's Xenical, approved in 1999 and also now sold over-the-counter by GlaxoSmithKline as Alli. It appears relatively safe, but only modestly effective and brings unpleasant side effects including oily stools. That has limited its commercial success and frustrated attempts by GSK to find a buyer.
Mr Tam suggests that fresh interest in diet drugs by US regulators began in 2007 with studies on the significant impact of bariatric surgery in reducing not only obesity but related mortality. In recent months, he says high-level scientific meetings coupled with public discussion led by Michelle Obama, the US first lady, may have proved important in urging a more receptive attitude towards medicines.
For now, larger western pharmaceutical companies remain reluctant, with little in their late-stage pipelines except for Novo Nordisk of Denmark, the specialist diabetes company. It is currently testing expanded use of its injectable treatment Victoza for weight loss.
Japanese companies have proved more active: Eisai partnered with Arena and Takeda with Orexigen. Mr Simeonidis suggests this may reflect that until recently the investments in weight-loss biotech companies were "a free option" given high skepticism and low valuations. "I wouldn't be surprised if we start to see more involvement from big pharma now."
For now, Mr Tam says Vivus is rebuffing alliances. "We have preferred to shake off suitors and do it alone to maintain value for our shareholders." But he foresees the prospect ahead of using direct-to-consumer advertising and a larger scale sales force to market Qsymia to doctors – something likely to require a big pharma partner.
The path will not prove easy. Vivus had to agree a tight monitoring program with US regulators to track each user, notably to avoid the risk of its use by pregnant women, since Qsymia can cause birth defects. The more aggressively any of the new diet pills are marketed, the greater the risks of misuse, failure to ensure the drug is accompanied by diet and exercise and danger of consumer disappointment.
Gary Palmer, chief medical officer in the US for Eisai, which will co-ordinate Belviq's launch for Arena, says: "We are still considering whether to use direct-to-consumer advertising. Our approach will start with specialists. This is about being responsible and educating the physicians."
Concerns linger about safety. Sidney Wolfe, head of the health research group at Public Citizen, a US consumer watchdog that has fought approval of the new drugs, wrote recently: "Obesity is unquestionably a serious public health concern, but that doesn't give the FDA licence to ignore the scientific evidence."
European regulators, stung by their recent bad experiences with Acomplia and Mediator, remain skeptical and have so far not approved the newer drugs. And many health insurers in the US remain cautious about reimbursing a medicine with modest efficacy and only potentially very long-term benefits – leaving consumers to pay out of pocket.
Donny Wong, head of metabolic disorders at Decision Resources, a market research agency, predicts rising obesity will increase demand in the years to come. But he warns: "Expectations are really sky high. Patients are expecting at 55 years old to go back to their ideal weight at 20, with a 15-20 per cent loss that is unachievable. They will very rapidly become disappointed."
http://www.cnbc.com/id/100340682
Pharma Groups Hope for Diet Drugs Binge
Financial Times
Published: Thursday, 27 Dec 2012 | 2:18 AM ET
By: Andrew Jack
As Christmas overeating gives way to under-fulfilled new year diets, the pharmaceutical industry's appetite has been whetted for a fresh surge in business.
After years of caution and setbacks, several drug companies are preparing to capitalize on what they hope will be a surge in sales from prescription medicines linked to the growing global trend of obesity.
"It has been a very challenged category, but there is a feeling we have to do something about obesity with the realization that it is a medical epidemic," says Peter Tam, president of Vivus, a California-based biotechnology company. Last autumn it launched Qsymia, the first weight loss drug to win US regulatory approval in 13 years.
In the coming weeks, Arena, another West Coast biotech, is set to launch its drug Belviq after receiving authorization. A third, Orexigen, is preparing to submit for fresh regulatory approval for its experimental medicine Contrave after previous rejections.
"There has been a very significant and rather sudden shift in the views of the regulator over the past year," says Simos Simeonidis, senior biotech analyst with Cowen. "Companies had been laying off staff and putting products on hold. Now there has been a resurgence and a lot of investor interest."
Weight loss drugs have long been viewed with suspicion by healthcare specialists both because of their limited efficacy and the risks of side effects – factors which continue to haunt the field. Qsymia's weight loss impact is slightly more than 10 percent and Belviq's less than 6 percent – both when tested in ideal conditions with strong medical supervision.
Meanwhile, Wyeth, now part of Pfizer, is still fighting claims of heart valve defects and other problems linked to Fen-Phen, a combination of diet drugs withdrawn in 1997 for which it earmarked reserves of $21 billion to cover settlements. Regulators subsequently pulled Abbott's drug Meridia, and – in Europe – both Servier's Mediator and Sanofi's Acomplia, which was linked to suicidal feelings.
Only one prescription weight loss drug has remained on the market in the US: Roche's Xenical, approved in 1999 and also now sold over-the-counter by GlaxoSmithKline as Alli. It appears relatively safe, but only modestly effective and brings unpleasant side effects including oily stools. That has limited its commercial success and frustrated attempts by GSK to find a buyer.
Mr Tam suggests that fresh interest in diet drugs by US regulators began in 2007 with studies on the significant impact of bariatric surgery in reducing not only obesity but related mortality. In recent months, he says high-level scientific meetings coupled with public discussion led by Michelle Obama, the US first lady, may have proved important in urging a more receptive attitude towards medicines.
For now, larger western pharmaceutical companies remain reluctant, with little in their late-stage pipelines except for Novo Nordisk of Denmark, the specialist diabetes company. It is currently testing expanded use of its injectable treatment Victoza for weight loss.
Japanese companies have proved more active: Eisai partnered with Arena and Takeda with Orexigen. Mr Simeonidis suggests this may reflect that until recently the investments in weight-loss biotech companies were "a free option" given high skepticism and low valuations. "I wouldn't be surprised if we start to see more involvement from big pharma now."
For now, Mr Tam says Vivus is rebuffing alliances. "We have preferred to shake off suitors and do it alone to maintain value for our shareholders." But he foresees the prospect ahead of using direct-to-consumer advertising and a larger scale sales force to market Qsymia to doctors – something likely to require a big pharma partner.
The path will not prove easy. Vivus had to agree a tight monitoring program with US regulators to track each user, notably to avoid the risk of its use by pregnant women, since Qsymia can cause birth defects. The more aggressively any of the new diet pills are marketed, the greater the risks of misuse, failure to ensure the drug is accompanied by diet and exercise and danger of consumer disappointment.
Gary Palmer, chief medical officer in the US for Eisai, which will co-ordinate Belviq's launch for Arena, says: "We are still considering whether to use direct-to-consumer advertising. Our approach will start with specialists. This is about being responsible and educating the physicians."
Concerns linger about safety. Sidney Wolfe, head of the health research group at Public Citizen, a US consumer watchdog that has fought approval of the new drugs, wrote recently: "Obesity is unquestionably a serious public health concern, but that doesn't give the FDA licence to ignore the scientific evidence."
European regulators, stung by their recent bad experiences with Acomplia and Mediator, remain skeptical and have so far not approved the newer drugs. And many health insurers in the US remain cautious about reimbursing a medicine with modest efficacy and only potentially very long-term benefits – leaving consumers to pay out of pocket.
Donny Wong, head of metabolic disorders at Decision Resources, a market research agency, predicts rising obesity will increase demand in the years to come. But he warns: "Expectations are really sky high. Patients are expecting at 55 years old to go back to their ideal weight at 20, with a 15-20 per cent loss that is unachievable. They will very rapidly become disappointed."
http://www.cnbc.com/id/100340682
Zitat von Magnetfeldfredy: http://us.rd.yahoo.com/finance/external/pssa/SIG=14c9ebb0i;_ylt=As7dzE8IQ1RpHAOnzfIpi3iiuYdG;_ylu=X3oDMTIxdmYzYjNrBG1pdANXaWRlIFF1b3RlcyBNb2R1bGUEcG9zAzMzBHNlYwNNZWRpYVJlY2VudFF1b3Rlc1BvcnRmb2xpb3NXaWRl;_ylg=X3oDMTFpNzk0NjhtBGludGwDdXMEbGFuZwNlbi11cwRwc3RhaWQDBHBzdGNhdANob21lBHB0A3NlY3Rpb25z/*http://seekingalpha.com/article/1079721-arena-s-marketing-partner-eisai-pharma-seeks-expedited-final-ruling-from-dea-to-begin-belviq-sales?source=yahoo
..zum besseren " Leseverständnis"
 des o.g. links von Fredy
des o.g. links von Fredy  ...
...Arena's Marketing Partner Eisai Pharma Seeks Expedited Final Ruling From DEA To Begin Belviq Sales
December 24, 2012
Arena's (ARNA) February 2013 commercial launch of Belviq is quickly approaching, its partner Eisai's seems to be ready to hit the ground running. The DEA placed Belviq in a schedule IV classification allowing physicians to liberally prescribe Belviq to patients by fast electronic script through local pharmacies for a three-month supply (90 pills) at a time.
Eisai is evidently chomping at the bit ready to launch. On December 19, 2012, Eisai wrote the DEA citing exceptional circumstances exist related to the obesity epidemic to immediately grant a final order at the end of the notice period (January 18, 2013):
"For the reasons described above, Eisai, Inc., respectfully requests that the DEA upon finalizing the proposed rule, make effective the date the same date as the publication of the final rule. This would enable physicians and their patients to have access to Lorcaserin as soon as possible in this area of growing unmet medical need. The Administrator has included an earlier effective date in the final rule for other drugs. Such precedents include zopiclone (70 Fed Reg 63 (April 4, 2005), pregabalin (70 Fed Reg (July 28, 2005), ezogabine (76 Fed Reg. 241 (December 15, 2011))."
In researching this issue, I discovered the DEA has never turned down this kind of request, especially under these well-grounded exceptional circumstances. I expect the DEA to grant Eisai's request and Belviq will be available in local pharmacy's by February 7, 2012, considering the average time it normally takes for the DEA to grant such a request.
Legally, Eisai can market Belviq already because the FDA approved the drug. Normally, however, the pharmaceutical company waits for a DEA final order to make sure the marketing inserts reflect the proper drug classification. Eisai has an existing sales force in place, already marketing to physicians, as evidenced by some of the physicians I surveyed who have indicated Eisai sales representatives called to inform them Belviq, a new revolutionary weight management product, will be available in February 2013. Internet posts have already experimented with catchy marketing slogans- "Belviq will make you chic/sleek."
Eisai has confirmed that Belviq is being loaded in drug store databases at many major pharmacies, such as CVS, and will soon be ready for distribution. Additionally, major group and individual health plans have already begun to cover Belviq. Aetna and Blue Cross plan to cover Belviq. Arena's CEO Jack Lief hinted that Belviq's price should be approximately $3.80 per day, using the two-pill daily dosing measure..
..ein durchaus akkzeptabler Preis..
 ...
...Personally, I would pay the price of a Starbuck's Latte when one considers the health benefits for weight loss, biomarkers, diabetes and pre-diabetes that Belviq may offers patients. I would imagine most health plans will eventually follow Aetna and Blue Cross the significant improvement in biomarkers (.9 HBA1C).
In addition, Belviq is very close to European Medicine Agency approval. I expect Belviq will receive a positive CHMP..me too..
 review by the first week in January, 2013. In anticipation, investors have debated why Arena has not partnered Belviq. The obvious reason is Belviq is certain to fetch a better price upon EU approval. I confirmed with Arena that they are in active discussions with several potential EU partners and will provide an update upon EU approval.
review by the first week in January, 2013. In anticipation, investors have debated why Arena has not partnered Belviq. The obvious reason is Belviq is certain to fetch a better price upon EU approval. I confirmed with Arena that they are in active discussions with several potential EU partners and will provide an update upon EU approval.Whenever the EU approves Belviq and/or Arena partner's Belviq in Europe, the news will certainly give Arena's share price a boost.
It is becoming evident that the market for Arena's drug and Eisai's ability to deliver on sales is currently being underestimated by analysts and Wall Street alike as Arena closed on December 21, 2012, in regular session trading at $8.65, which is at it's 50 day EMA and nearly 36% below it's 52 week high. I suspect Wall Street's general "show me the money" sentiment towards Arena will change once institutions and hedge funds begin factoring in EU approval and track Belviq's US weekly prescription data.
With plenty of cash on hand, zero debt, no meaningful competition, new indications, milestone payments, Belviq combinations to pursue, and growing partnership deals, Arena appears to be in the driver's seat and in complete control of its destiny. Provided that their sales and marketing strategies are well executed by marketing powerhouse Eisai, there seems to be little to stand in the way keeping them from becoming a successful commercial enterprise and providing shareholder value for many years to come. I rate the stock a strong buy ahead of these catalyst.

Events & Presentations
31st Annual J.P. Morgan Healthcare Conference
January 7 - 10, 2013
San Francisco, California
http://invest.arenapharm.com/eventdetail.cfm?EventID=121537
Posted : Dec 27, 2012
Biotech Stocks Watch List – ARNA, MNKD, YMI, AMGN
Biotech

The Association of Biotechnology-Led Enterprises (ABLE ), the association of biotechnology firms in India, has arranged a list of estimates for the sector from the Budget to be presented in March. For 2011-12, Indian biotechnology industry marked 18.55 per cent growth to hit Rs 20,441 crore landmark. However, the gross domestic expenditure on R&D represents just 2.1 per cent of worldwide R&D, according to ABLE.
In the U.S. on Wednesday, biotech companies followed broader decline in the markets. Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) jumped 0.34% with the closing price of $8.75. The overall volume in the last trading session was 7.74 million shares. Its fifty two week range was $1.51-$13.50. The total market capitalization remained $1.90 billion.
ARNA is ahead its 52 week low with 479.47% and its last month price volatility remained 4.21%. Its beta coefficient was 0.23 with a target price of $9.67. In its share capital ARNA has 217.29million outstanding shares while 215.68million shares have been floated in market. ARNA has insider ownership of 0.02% with its institutional ownership remained 45.76%. Company’s current year earnings per share grew with 29.52% while the five year EPS growth rate was +12.65%.
MannKind Corporation (NASDAQ:MNKD)reported the gain of +5.00% and closed at $2.31 with the overall traded volume of 6.41 million shares more than the average volume of 1.86 million. MNKD has outstanding shares of 245.78 million and its total market cap is 567.75 million. Its beta value stands at 1.06 times. Mann Kind Corporation (Mann Kind) is a development-stage bio pharmaceutical company and focus on the discovery, development and commercialization of therapeutic products for diseases such as diabetes and cancer.
YM BioSciences Inc. (USA) (NYSEAMEX:YMI) declared last week that the Supreme Court of Nova Scotia has endowed a provisional order, setting, among other things, December 31, 2012 as the record date related with the formerly declared plan of arrangement.
In the last trading session, YM BioSciences Inc. (USA) (NYSEAMEX:YMI) dropped 0.70% to close at $2.85 and its total traded volume was 4.01 million shares. It has market cap of 449.01 million.
Amgen, Inc. (NASDAQ:AMGN) plunged -0.58% and closed at $86.89 with the overall traded volume of 2.72 million shares. AMGN has earnings per share of $5.59 with the net profit margin of 26.71% and operating margin of 33.60%. Its price to earnings ratio ended at 15.54. Amgen Inc. (Amgen) is a biotechnology medicines company. The Company discovers, develops, manufactures and markets medicines for illnesses.
http://www.therem.org/biotech-stocks-watch-list-arna-mnkd-ym…
Biotech Stocks Watch List – ARNA, MNKD, YMI, AMGN
Biotech

The Association of Biotechnology-Led Enterprises (ABLE ), the association of biotechnology firms in India, has arranged a list of estimates for the sector from the Budget to be presented in March. For 2011-12, Indian biotechnology industry marked 18.55 per cent growth to hit Rs 20,441 crore landmark. However, the gross domestic expenditure on R&D represents just 2.1 per cent of worldwide R&D, according to ABLE.
In the U.S. on Wednesday, biotech companies followed broader decline in the markets. Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) jumped 0.34% with the closing price of $8.75. The overall volume in the last trading session was 7.74 million shares. Its fifty two week range was $1.51-$13.50. The total market capitalization remained $1.90 billion.
ARNA is ahead its 52 week low with 479.47% and its last month price volatility remained 4.21%. Its beta coefficient was 0.23 with a target price of $9.67. In its share capital ARNA has 217.29million outstanding shares while 215.68million shares have been floated in market. ARNA has insider ownership of 0.02% with its institutional ownership remained 45.76%. Company’s current year earnings per share grew with 29.52% while the five year EPS growth rate was +12.65%.
MannKind Corporation (NASDAQ:MNKD)reported the gain of +5.00% and closed at $2.31 with the overall traded volume of 6.41 million shares more than the average volume of 1.86 million. MNKD has outstanding shares of 245.78 million and its total market cap is 567.75 million. Its beta value stands at 1.06 times. Mann Kind Corporation (Mann Kind) is a development-stage bio pharmaceutical company and focus on the discovery, development and commercialization of therapeutic products for diseases such as diabetes and cancer.
YM BioSciences Inc. (USA) (NYSEAMEX:YMI) declared last week that the Supreme Court of Nova Scotia has endowed a provisional order, setting, among other things, December 31, 2012 as the record date related with the formerly declared plan of arrangement.
In the last trading session, YM BioSciences Inc. (USA) (NYSEAMEX:YMI) dropped 0.70% to close at $2.85 and its total traded volume was 4.01 million shares. It has market cap of 449.01 million.
Amgen, Inc. (NASDAQ:AMGN) plunged -0.58% and closed at $86.89 with the overall traded volume of 2.72 million shares. AMGN has earnings per share of $5.59 with the net profit margin of 26.71% and operating margin of 33.60%. Its price to earnings ratio ended at 15.54. Amgen Inc. (Amgen) is a biotechnology medicines company. The Company discovers, develops, manufactures and markets medicines for illnesses.
http://www.therem.org/biotech-stocks-watch-list-arna-mnkd-ym…
Antwort auf Beitrag Nr.: 43.962.270 von Poppholz am 27.12.12 15:19:32http://us.rd.yahoo.com/finance/external/pssa/SIG=11kh22vgl/*…
Antwort auf Beitrag Nr.: 43.964.507 von Magnetfeldfredy am 28.12.12 10:50:01Arena goes Europe:
http://www.dailymail.co.uk/health/article-2255912/Belviq-pil…
http://www.dailymail.co.uk/health/article-2255912/Belviq-pil…
Zitat von bernie55:![]()
Events & Presentations
31st Annual J.P. Morgan Healthcare Conference
January 7 - 10, 2013
San Francisco, California
http://invest.arenapharm.com/eventdetail.cfm?EventID=121537
SAN DIEGO, Jan. 2, 2013 /PRNewswire/
Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present at the 31st Annual J.P. Morgan Healthcare Conference on Wednesday, January 9, 2013, at 3:30 p.m. Pacific Time (6:30 p.m. Eastern Time) at The Westin St. Francis in San Francisco, California. Jack Lief, Arena's President and Chief Executive Officer, is scheduled to provide a corporate overview.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
Thursday's Small Cap Biotech Catalyst Watch List
January 3, 2013; by Scott Matusow
(...)
Arena Pharmaceuticals (ARNA)
Arena engages in discovering, developing, and commercializing oral drugs that target G protein-coupled receptors in the therapeutic areas of cardiovascular, central nervous system, inflammatory, and metabolic diseases.
Since the approval of its weight loss drug Belviq in June, the stock has fallen from a yearly high of $11.39 to $7.21, a fall of 37%. I feel long term investors have excellent value here at its current price level of around $9 a share.
Recently, the Drug Enforcement Agency placed Belviq in a schedule IV classification which will allow physicians to prescribe Belviq to patients via fast electronic script through local pharmacies for a three-month supply (90 pills) at a time. This is a big plus in factoring in potential sales for the drug.
The catalyst for Arena is in early 2013 when the company along with its marketing partner Eisai Pharma will officially launch Belviq. One well known biotech writer believes Belviq will see failure on its launch while I disagree. A few doctors I have spoken to have told me they receive many requests from patients looking for help to lose weight, and are looking forward to prescribing Belviq to their patients. Belviq can certainly help those who suffer from weight issues as studies have shown (which is why the FDA approved it).
While I do not think Belviq is the "magic pill" in and of itself, many who try it are likely to receive a strong placebo effect from the drug, believing it will help them. Arena has a solid marketing partner with Eisai, a company with a good track record. So I believe Belviq will see a successful launch. I also believe it's important to separate one's personal bias when forecasting business results. Personally, I am not wild about Belviq, as I mentioned, I really do not think it's the magic pill some believe it is to treat obesity. Regardless, business wise it should be a big winner, because many people who are obese are desperate for help. When patients have a strong belief a drug will help them, especially when it comes to losing weight, these same patients might be motivated to seek better eating and exercise habits. Therefore, it comes down to business, and not my personal or moral opinion about the drug. My opinion is that Belviq will be a big winner business wise for Arena. Also, it's my strong opinion that Belviq will gain European Medicine Agency (EMA) approval for the same reason it gained FDA approval, because it works and it's safe.
(...)
http://seekingalpha.com/article/1091991-thursday-s-small-cap…
January 3, 2013; by Scott Matusow
(...)
Arena Pharmaceuticals (ARNA)
Arena engages in discovering, developing, and commercializing oral drugs that target G protein-coupled receptors in the therapeutic areas of cardiovascular, central nervous system, inflammatory, and metabolic diseases.
Since the approval of its weight loss drug Belviq in June, the stock has fallen from a yearly high of $11.39 to $7.21, a fall of 37%. I feel long term investors have excellent value here at its current price level of around $9 a share.
Recently, the Drug Enforcement Agency placed Belviq in a schedule IV classification which will allow physicians to prescribe Belviq to patients via fast electronic script through local pharmacies for a three-month supply (90 pills) at a time. This is a big plus in factoring in potential sales for the drug.
The catalyst for Arena is in early 2013 when the company along with its marketing partner Eisai Pharma will officially launch Belviq. One well known biotech writer believes Belviq will see failure on its launch while I disagree. A few doctors I have spoken to have told me they receive many requests from patients looking for help to lose weight, and are looking forward to prescribing Belviq to their patients. Belviq can certainly help those who suffer from weight issues as studies have shown (which is why the FDA approved it).
While I do not think Belviq is the "magic pill" in and of itself, many who try it are likely to receive a strong placebo effect from the drug, believing it will help them. Arena has a solid marketing partner with Eisai, a company with a good track record. So I believe Belviq will see a successful launch. I also believe it's important to separate one's personal bias when forecasting business results. Personally, I am not wild about Belviq, as I mentioned, I really do not think it's the magic pill some believe it is to treat obesity. Regardless, business wise it should be a big winner, because many people who are obese are desperate for help. When patients have a strong belief a drug will help them, especially when it comes to losing weight, these same patients might be motivated to seek better eating and exercise habits. Therefore, it comes down to business, and not my personal or moral opinion about the drug. My opinion is that Belviq will be a big winner business wise for Arena. Also, it's my strong opinion that Belviq will gain European Medicine Agency (EMA) approval for the same reason it gained FDA approval, because it works and it's safe.
(...)
http://seekingalpha.com/article/1091991-thursday-s-small-cap…
Antwort auf Beitrag Nr.: 43.977.827 von Poppholz am 03.01.13 14:42:38Inhaltlich nix neues. Außer Herrn Matusow's "my strong opinion".
Ansonsten Guads Neis Joor!
Ansonsten Guads Neis Joor!
Antwort auf Beitrag Nr.: 43.978.376 von me_2 am 03.01.13 16:44:27Arena Seeks Partners in Europe, China for Obesity Drug

By Ryan Flinn - Jan 7, 2013 9:40 PM GMT+0100
Facebook Share
LinkedIn
Google +1
COMMENTS
Print
QUEUE
Q
Arena Pharmaceuticals Inc. (ARNA), the maker of weight-loss drug Belviq, is looking for partners to sell the medicine abroad once it clears regulatory hurdles, Jack Lief, Arena’s president and chief executive officer, said.
Belviq, approved in the U.S. in June as the first new obesity drug in 13 years, is being promoted in North America by Tokyo-based Eisai Co. (4523) Lief said San Diego-based Arena is in talks with other companies to promote the therapy in Europe if it gains clearance there, with a decision expected in the first half of this year.
“Europe is not partnered yet -- we’re waiting for approval,” Lief said in an interview at the annual health-care conference sponsored by JPMorgan Chase & Co. (JPM) in San Francisco. “We’re talking to partners presently, even at this meeting.”
Lief said the biggest market outside the U.S. for the drug is China, and the company is looking for partners there as well.
The pill works in a similar way to fenfluramine, part of the fen-phen appetite-suppression drug combination pulled from pharmacies in 1997 when it was linked to heart valve abnormalities.
Belviq is indicated for people who have at least one coexisting condition such as high blood pressure or Type 2 diabetes. The drug will be available in the U.S. after the Drug Enforcement Administration completes a review to classify Belviq based on its potential for abuse, Arena said in an earlier statement.
Craig Audet, Arena’s senior vice president of operations, said the company doesn’t want the drug to be promoted just for cosmetic purposes.
Obesity Management
“Another thing we’re really looking for is someone who looks at Belviq for medical management of obesity, and not just as a weight-loss drug,” Audet said in an interview. “We don’t want someone marketing this as a cosmetic drug.”
Arena turned down a company offering to promote the drug in Korea for this reason, choosing instead a less lucrative partnership with Ildong Pharmaceutical Co., Audet said.
“You can’t talk out of both sides of your mouth,” Audet said.
Arena’s drug, previously known by its chemical name as lorcaserin, was rejected by the FDA in 2010 because the agency had concerns about cancer. Advisers to the agency determined the benefits of the drug outweighed the risks.
The FDA has said Arena’s pill shouldn’t be used by pregnant women. Belviq may cause serious side effects, including a fatal increase in the chemical serotonin produced by the body that can cause muscle rigidity, fever and seizures, especially when used with drugs that treat migraines and depression, the agency said. Belviq may also cause memory or attention disturbances, the FDA said.
The medication’s label also instructs patients to discontinue use if 5 percent of weight loss is not achieved by the 12th week of treatment.
Arena and Eisai agreed to conduct six post-market studies to analyst the safety and efficacy of the medicine.
To contact the reporter on this story: Ryan Flinn in San Francisco at rflinn@bloomberg.net
To contact the editor responsible for this story: Reg Gale at rgale5@bloomberg.net
More News:
Science
Facebook Share
LinkedIn
Google +1
COMMENTS


By Ryan Flinn - Jan 7, 2013 9:40 PM GMT+0100
Facebook Share
Google +1
COMMENTS
QUEUE
Q
Arena Pharmaceuticals Inc. (ARNA), the maker of weight-loss drug Belviq, is looking for partners to sell the medicine abroad once it clears regulatory hurdles, Jack Lief, Arena’s president and chief executive officer, said.
Belviq, approved in the U.S. in June as the first new obesity drug in 13 years, is being promoted in North America by Tokyo-based Eisai Co. (4523) Lief said San Diego-based Arena is in talks with other companies to promote the therapy in Europe if it gains clearance there, with a decision expected in the first half of this year.
“Europe is not partnered yet -- we’re waiting for approval,” Lief said in an interview at the annual health-care conference sponsored by JPMorgan Chase & Co. (JPM) in San Francisco. “We’re talking to partners presently, even at this meeting.”
Lief said the biggest market outside the U.S. for the drug is China, and the company is looking for partners there as well.
The pill works in a similar way to fenfluramine, part of the fen-phen appetite-suppression drug combination pulled from pharmacies in 1997 when it was linked to heart valve abnormalities.
Belviq is indicated for people who have at least one coexisting condition such as high blood pressure or Type 2 diabetes. The drug will be available in the U.S. after the Drug Enforcement Administration completes a review to classify Belviq based on its potential for abuse, Arena said in an earlier statement.
Craig Audet, Arena’s senior vice president of operations, said the company doesn’t want the drug to be promoted just for cosmetic purposes.
Obesity Management
“Another thing we’re really looking for is someone who looks at Belviq for medical management of obesity, and not just as a weight-loss drug,” Audet said in an interview. “We don’t want someone marketing this as a cosmetic drug.”
Arena turned down a company offering to promote the drug in Korea for this reason, choosing instead a less lucrative partnership with Ildong Pharmaceutical Co., Audet said.
“You can’t talk out of both sides of your mouth,” Audet said.
Arena’s drug, previously known by its chemical name as lorcaserin, was rejected by the FDA in 2010 because the agency had concerns about cancer. Advisers to the agency determined the benefits of the drug outweighed the risks.
The FDA has said Arena’s pill shouldn’t be used by pregnant women. Belviq may cause serious side effects, including a fatal increase in the chemical serotonin produced by the body that can cause muscle rigidity, fever and seizures, especially when used with drugs that treat migraines and depression, the agency said. Belviq may also cause memory or attention disturbances, the FDA said.
The medication’s label also instructs patients to discontinue use if 5 percent of weight loss is not achieved by the 12th week of treatment.
Arena and Eisai agreed to conduct six post-market studies to analyst the safety and efficacy of the medicine.
To contact the reporter on this story: Ryan Flinn in San Francisco at rflinn@bloomberg.net
To contact the editor responsible for this story: Reg Gale at rgale5@bloomberg.net
More News:
Science
Facebook Share
Google +1
COMMENTS
Zitat von Magnetfeldfredy: Arena Seeks Partners in Europe, China for Obesity Drug
By Ryan Flinn - Jan 7, 2013 9:40 PM GMT+0100
Arena Pharmaceuticals Inc. (ARNA), the maker of weight-loss drug Belviq, is looking for partners to sell the medicine abroad once it clears regulatory hurdles, Jack Lief, Arena’s president and chief executive officer, said.
Belviq, approved in the U.S. in June as the first new obesity drug in 13 years, is being promoted in North America by Tokyo-based Eisai Co. (4523) Lief said San Diego-based Arena is in talks with other companies to promote the therapy in Europe if it gains clearance there, with a decision expected in the first half of this year.
“Europe is not partnered yet -- we’re waiting for approval,” Lief said in an interview at the annual health-care conference sponsored by JPMorgan Chase & Co. (JPM) in San Francisco. “We’re talking to partners presently, even at this meeting.”
...also, meiner Meinung nach weiß Jack Lief schon mehr.....ich denke, dass das Approval in Europa immer greifbarer wird......zumindest haben wir von der CHMP noch nichts gehört bzgl. eines " oral hearing"..
..und das bewerte ich als sehr gutes Zeichen !!!!
Lief said the biggest market outside the U.S. for the drug is China, and the company is looking for partners there as well.
The pill works in a similar way to fenfluramine, part of the fen-phen appetite-suppression drug combination pulled from pharmacies in 1997 when it was linked to heart valve abnormalities.
Belviq is indicated for people who have at least one coexisting condition such as high blood pressure or Type 2 diabetes. The drug will be available in the U.S. after the Drug Enforcement Administration completes a review to classify Belviq based on its potential for abuse, Arena said in an earlier statement.
Craig Audet, Arena’s senior vice president of operations, said the company doesn’t want the drug to be promoted just for cosmetic purposes.
Obesity Management
“Another thing we’re really looking for is someone who looks at Belviq for medical management of obesity, and not just as a weight-loss drug,” Audet said in an interview. “We don’t want someone marketing this as a cosmetic drug.”
Arena turned down a company offering to promote the drug in Korea for this reason, choosing instead a less lucrative partnership with Ildong Pharmaceutical Co., Audet said.
“You can’t talk out of both sides of your mouth,” Audet said.
Arena’s drug, previously known by its chemical name as lorcaserin, was rejected by the FDA in 2010 because the agency had concerns about cancer. Advisers to the agency determined the benefits of the drug outweighed the risks.
The FDA has said Arena’s pill shouldn’t be used by pregnant women. Belviq may cause serious side effects, including a fatal increase in the chemical serotonin produced by the body that can cause muscle rigidity, fever and seizures, especially when used with drugs that treat migraines and depression, the agency said. Belviq may also cause memory or attention disturbances, the FDA said.
The medication’s label also instructs patients to discontinue use if 5 percent of weight loss is not achieved by the 12th week of treatment.
Arena and Eisai agreed to conduct six post-market studies to analyst the safety and efficacy of the medicine.
To contact the reporter on this story: Ryan Flinn in San Francisco at rflinn@bloomberg.net
To contact the editor responsible for this story: Reg Gale at rgale5@bloomberg.net
http://www.bloomberg.com/news/2013-01-07/arena-seeks-partner…
http://www.bild.de/ratgeber/gesundheit/uebergewicht/neue-met…
Guten Morgen @ll - heute auch in der Bildzeitung davon die Rede -- Auszug:
3. Die neuen Pillen
2012 ließ die US-Gesundheitsbehörde FDA zum ersten Mal seit 1999 wieder zwei Abnehmmedikamente zu. „Belviq“ unterdrückt über die Serotonin-Rezeptoren im Gehirn das Hungergefühl. „Qsymia“ ist eine Kombination zweier bereits eingesetzter Medikamente, eines Appetitzüglers und eines Anti-Epileptikums. Es unterdrückt ebenfalls das Hungergefühl und lässt Essen weniger reizvoll erscheinen.
In klinischen Studien nahmen die Probanden mit „Belviq“ durchschnittlich 13 Kilo im Jahr ab, mit „Qsymia“ durschschnittlich 11 Kilo. Die Nebenwirkungen können Taubheitsgefühle, Herzrasen oder depressive Verstimmungen sein.
Die Medikamente müssen vom Arzt verordnet werden, „Belviq“ und „Qsymia“ sollen sehr wahrscheinlich in diesem Jahr in Europa zugelassen werden.
Klingt doch gut und viele Tausende lesen die Bildzeitung ...
Guten Morgen @ll - heute auch in der Bildzeitung davon die Rede -- Auszug:
3. Die neuen Pillen
2012 ließ die US-Gesundheitsbehörde FDA zum ersten Mal seit 1999 wieder zwei Abnehmmedikamente zu. „Belviq“ unterdrückt über die Serotonin-Rezeptoren im Gehirn das Hungergefühl. „Qsymia“ ist eine Kombination zweier bereits eingesetzter Medikamente, eines Appetitzüglers und eines Anti-Epileptikums. Es unterdrückt ebenfalls das Hungergefühl und lässt Essen weniger reizvoll erscheinen.
In klinischen Studien nahmen die Probanden mit „Belviq“ durchschnittlich 13 Kilo im Jahr ab, mit „Qsymia“ durschschnittlich 11 Kilo. Die Nebenwirkungen können Taubheitsgefühle, Herzrasen oder depressive Verstimmungen sein.
Die Medikamente müssen vom Arzt verordnet werden, „Belviq“ und „Qsymia“ sollen sehr wahrscheinlich in diesem Jahr in Europa zugelassen werden.
Klingt doch gut und viele Tausende lesen die Bildzeitung ...
Zitat von bernie55 >: Arena Seeks Partners in Europe, China for Obesity Drug
By Ryan Flinn - Jan 7, 2013 9:40 PM GMT+0100
Arena Pharmaceuticals Inc. (ARNA), the maker of weight-loss drug Belviq, is looking for partners to sell the medicine abroad once it clears regulatory hurdles, Jack Lief, Arena’s president and chief executive officer, said.
Belviq, approved in the U.S. in June as the first new obesity drug in 13 years, is being promoted in North America by Tokyo-based Eisai Co. (4523) Lief said San Diego-based Arena is in talks with other companies to promote the therapy in Europe if it gains clearance there, with a decision expected in the first half of this year.
“Europe is not partnered yet -- we’re waiting for approval,” Lief said in an interview at the annual health-care conference sponsored by JPMorgan Chase & Co. (JPM) in San Francisco. “We’re talking to partners presently, even at this meeting.”
...also, meiner Meinung nach weiß Jack Lief schon mehr.....ich denke, dass das Approval in Europa immer greifbarer wird......zumindest haben wir von der CHMP noch nichts gehört bzgl. eines " oral hearing"..
..und das bewerte ich als sehr gutes Zeichen!!!!
http://www.bloomberg.com/news/2013-01-07/arena-seeks-partner…
..vielleicht passsend hierzu ein Kommentar aus dem IV Board vom 1/8/2013 1:57:38 AM by Franca_ole
AUTHORISATION OF MEDICINAL PRODUCTS FOR HUMAN USE in Europe
The Agency shall ensure that the opinion of the Committee for Medicinal Products for Human Use (CHMP) is given within 210 days after receipt of a valid application.
Vivus had published the negative outlook 27 days before the official decision has been announced. Regarding Lorcaserin day210 must be in January because the restart of the clock (day121) had been confirmed for October 2012.
The topic will be almost certainly on the agenda for the upcoming January CHMP meeting.
But the fact that a negative outlook has not been given by the company up to now is more than a positive sign
 .
.http://www.investorvillage.com/smbd.asp?mb=633&mn=46945&pt=m…
Ich konnte wirklich nicht vorhersehen, dass die beiden Zitate so eine positive Reaktion hervorrufen werden. 
Arena Pharmaceuticals, Inc. (ARNA)
-Nasdaq-
10.11 Up 0.89 (9.89%)
Volume: 30,453,518
Avg Vol (3m): 8,139,770

Arena Pharmaceuticals, Inc. (ARNA)
-Nasdaq-
10.11 Up 0.89 (9.89%)

Volume: 30,453,518

Avg Vol (3m): 8,139,770
Insight & Intelligence™ : Jan 9, 2013
Getting FDA to Yes
As PDUFA, patent cliff drive 2012 approvals, can industry keep cranking out new molecules?
Alex Philippidis
Years of industry complaints appeared to pay off when the clock struck midnight on New Year’s Eve. FDA rang out 2012 with 39 approvals for new molecular entities—the second-highest number since 1996, when the agency approved Lipitor (atorvastatin) among 53 drug candidates.
“FDA is encouraged by the increase in NME approvals; but it’s too early to tell if it reflects a long-term trend,” FDA spokeswoman Lisa Kubaska, PharmD, told GEN.
Several factors explain the sharp increase from 30 drugs approved in 2011, and 21 in 2010; an average 23 new drugs were approved annually between 2002 and 2011. Those factors include FDA’s expanded options for faster new-drug reviews under the fifth authorization of the Prescription Drug User Fee Act (PDUFA V); more applications for new drug approvals, as big biopharma scrambles to recoup revenue lost as aging blockbusters lose patent protection; and jawboning by industry and investors that blamed the high cost of drug development on what they termed slow and complicated FDA reviews after the agency’s post-Vioxx shift toward more risk-benefit analysis.
While a final budget for the 2013 fiscal year has yet to be adopted, Dr. Kubaska said FDA is reviewing new drugs under PDUFA V, part of the FDA Safety and Innovation Act (FDASIA), enacted in July by President Barack Obama after sailing through Congress with rare bipartisan majorities.
FDASIA commits FDA to review and act on 90% of standard applications within 10–12 months from filing date, and on 90% of priority submissions within six to eight months from filing date. As of Nov. 30, all new drugs met PDUFA deadlines, with 81% approved in their first review cycle. However, FDASIA also pushed back the start of that first cycle to after the 60-day administrative filing review period.
New Breakthrough
FDASIA also created a new “breakthrough therapy” designation to speed up review of drugs intended “to treat a serious or life-threatening disease or condition, [where] preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints.”
FDASIA also expanded fast-track products to include “qualified infectious disease products” such as new antibacterial drugs and drugs for rare diseases, as well as new drugs that, alone or with one or more drugs, are intended to treat serious or life-threatening diseases or conditions.
“FDA cannot expect a continuing upward trend for NME approvals until a sustained increase in the number of applications is also demonstrated,” added Dr. Kubaska, a lieutenant commander in the U.S. Public Health Service Commissioned Corps.
Rick Edmunds, senior partner and global health practice leader with Booz & Co., told GEN that while he is unsure whether drug approvals can continue rising annually, the increases seen until now stem from drug companies rebuilding their portfolios to make up for patent-cliff losses.
“I think it’s the direct result of the shift in portfolio strategy by most pharma over the past five-plus years. Specifically, you’re seeing more focus on specialty-oriented products, where the needs are much more fundamental unmet needs, rather than incremental or even reasonably significant clinical improvement in primary care, broad patient-based products,” Edmunds said.
One example of that focus: Of the 39 new drugs, 12 are for rare diseases. Eleven rare-disease drugs were approved in 2011.
Secondly, Edmunds said, drug developers have been more diligent in how they approach advancing products through FDA reviews: “Anything they thought could get approved, or had a shot at getting approved, they were pushing forward a few years ago because their pipelines were so bare. I think increasingly you’re seeing that having done some earlier weeding out, the products they’re bringing in they have more confidence in, in terms of market.”
18 New Cancer Drugs
Of the 39 new drugs approved in 2012, the largest disease category was cancer, where 18 drugs were approved for 19 indications. Among approved drugs were Novartis’ Afinitor (everolimus) for two indications, hormone receptor-positive, HER2-negative breast cancer and renal angiomyolipoma associated with tuberous sclerosis complex; Pfizer’s Bosulif (bosutinib) for Ph+ chronic myelogenous leukemia and Inlyta (axitinib) for advanced renal cell carcinoma; Roche subsidiary Genentech’s Perjeta (pertuzumab) for first-line HER2+ metastatic breast cancer and Erivedge (vismodegib) for basal cell carcinoma; and Ariad Pharmaceuticals’ Iclusig (ponatinib) for chronic myeloid leukemia and Philadelphia chromosome positive acute lymphoblastic leukemia.
“I think part of that is that the industry is in that area, doing a lot of R&D in oncology. But it’s also that the FDA is doing more to ensure that those products actually reach the marketplace,” Kenneth I. Kaitin, Ph.D., professor and director of the Tufts Center for the Study of Drug Development at Tufts University School of Medicine, told GEN.
As late as about three years ago, Dr. Kaitin recalled, FDA’s cancer division was under fire for what industry deemed were too-onerous requirements for getting a new cancer drug on the market: “They don’t look like the same division any more. There have been lots of oncology products that have been approved relative to the total number of product approvals this year and last year.”
Other significant categories:
Neurology—Eight drugs approved, including Bristol-Myers Squibb’s Eliquis (apixaban) for prevention of stroke and systemic embolism due to nonvalvular atrial fibrillation; GlaxiSmithKline’s Horizant (gabapentin enacarbil) for postherpetic neuralgia; and Pfizer’s Lyrica (pregabalin) for neuropathic pain associated with spinal cord injury.
Pulmonary and respiratory diseases—Eight drugs approved, including the first tuberculosis drug approved in four decades, Sirturo (bedaquiline) by Johnson & Johnson’s Janssen Therapeutics unit; and Vertex Pharmaceuticals’ Kalydeco (ivacaftor) for cystic fibrosis with the G551D mutation in the CFTR gene, the first CFTR potentiator.
Mixed and Slower
“If you’re in cancer or infectious diseases or if you’ve got an orphan drug, the agency is using pretty much all the tools at its disposal to move along those reviews and approvals. But in other areas—diabetes, endocrinology, CMS—the performance is much more mixed and slower,” David L. Gollaher, Ph.D., president and CEO of the California Healthcare Institute (CHI), told GEN.
CHI, which represents California’s biomedical industry, joined the Boston Consulting Group (BCG) in May to issue a report concluding that despite FDA efforts to shorten reviews, the agency took longer to approve drugs for diseases and conditions posing serious public health threats—including cardiovascular disease, obesity, and diabetes—than for cancer, hepatitis C, lupus, pneumonia, and orphan diseases.
During 2012, FDA approved three cardiovascular drugs, and one diabetes drug—Eli Lilly’s Jentadueto (linagliptin plus metformin hydrochloride) for type II diabetes. But the agency also okayed the first two weight loss drugs in more than a decade, Vivus’ Qsymia (phentermine and topiramate extended-release) and Belviq (lorcaserin hydrochloride), made by Arena Pharmaceuticals and distributed by Japan’s Eisai.
“Taken as a whole, the FDA has remained committed to getting important new products to the marketplace,” Dr. Kaitin said.
A key to that commitment, he said, was the series of PDUFA laws under which drug developers are charged “user fees” through which FDA funds new drug reviews. Every five years since 1992, PDUFA laws have sought to balance industry interests with FDA’s traditional interests in ensuring drug safety and efficacy.
“These issues that tended to fester over a long time, they really can’t anymore,” Dr. Kaitin said. “I don’t see a return the way we had in the past, where we went from 1962 to 1992 with an overly cautious, difficult to deal with, often confrontational regulatory agency.”
PDUFA laws alone won’t ensure smoother sailing for drug developers. As FDA likes to say, it can’t approve applications that don’t exist. That puts the burden on industry to continue developing drug candidates capable of withstanding agency scrutiny. But as Barry I. Eisenstein, M.D., senior vp, scientific affairs with Cubist Pharmaceuticals, correctly noted to GEN last month, all therapeutic areas are struggling with a greater degree of difficulty in finding new leads for breakthrough therapeutics.
“The modern genomic era, which 15 years ago we thought was going to allow for breakthroughs in a rapid way, has still not yet evolved to the point where it’s demonstrating that degree of proficiency in getting new leads,” Dr. Eisenstein said.
Until that improves, FDA is unlikely to see the sustained increase in new applications needed so it can approve more new drugs in coming years.
http://www.genengnews.com/keywordsandtools/print/3/29936/
Getting FDA to Yes
As PDUFA, patent cliff drive 2012 approvals, can industry keep cranking out new molecules?
Alex Philippidis
Years of industry complaints appeared to pay off when the clock struck midnight on New Year’s Eve. FDA rang out 2012 with 39 approvals for new molecular entities—the second-highest number since 1996, when the agency approved Lipitor (atorvastatin) among 53 drug candidates.
“FDA is encouraged by the increase in NME approvals; but it’s too early to tell if it reflects a long-term trend,” FDA spokeswoman Lisa Kubaska, PharmD, told GEN.
Several factors explain the sharp increase from 30 drugs approved in 2011, and 21 in 2010; an average 23 new drugs were approved annually between 2002 and 2011. Those factors include FDA’s expanded options for faster new-drug reviews under the fifth authorization of the Prescription Drug User Fee Act (PDUFA V); more applications for new drug approvals, as big biopharma scrambles to recoup revenue lost as aging blockbusters lose patent protection; and jawboning by industry and investors that blamed the high cost of drug development on what they termed slow and complicated FDA reviews after the agency’s post-Vioxx shift toward more risk-benefit analysis.
While a final budget for the 2013 fiscal year has yet to be adopted, Dr. Kubaska said FDA is reviewing new drugs under PDUFA V, part of the FDA Safety and Innovation Act (FDASIA), enacted in July by President Barack Obama after sailing through Congress with rare bipartisan majorities.
FDASIA commits FDA to review and act on 90% of standard applications within 10–12 months from filing date, and on 90% of priority submissions within six to eight months from filing date. As of Nov. 30, all new drugs met PDUFA deadlines, with 81% approved in their first review cycle. However, FDASIA also pushed back the start of that first cycle to after the 60-day administrative filing review period.
New Breakthrough
FDASIA also created a new “breakthrough therapy” designation to speed up review of drugs intended “to treat a serious or life-threatening disease or condition, [where] preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints.”
FDASIA also expanded fast-track products to include “qualified infectious disease products” such as new antibacterial drugs and drugs for rare diseases, as well as new drugs that, alone or with one or more drugs, are intended to treat serious or life-threatening diseases or conditions.
“FDA cannot expect a continuing upward trend for NME approvals until a sustained increase in the number of applications is also demonstrated,” added Dr. Kubaska, a lieutenant commander in the U.S. Public Health Service Commissioned Corps.
Rick Edmunds, senior partner and global health practice leader with Booz & Co., told GEN that while he is unsure whether drug approvals can continue rising annually, the increases seen until now stem from drug companies rebuilding their portfolios to make up for patent-cliff losses.
“I think it’s the direct result of the shift in portfolio strategy by most pharma over the past five-plus years. Specifically, you’re seeing more focus on specialty-oriented products, where the needs are much more fundamental unmet needs, rather than incremental or even reasonably significant clinical improvement in primary care, broad patient-based products,” Edmunds said.
One example of that focus: Of the 39 new drugs, 12 are for rare diseases. Eleven rare-disease drugs were approved in 2011.
Secondly, Edmunds said, drug developers have been more diligent in how they approach advancing products through FDA reviews: “Anything they thought could get approved, or had a shot at getting approved, they were pushing forward a few years ago because their pipelines were so bare. I think increasingly you’re seeing that having done some earlier weeding out, the products they’re bringing in they have more confidence in, in terms of market.”
18 New Cancer Drugs
Of the 39 new drugs approved in 2012, the largest disease category was cancer, where 18 drugs were approved for 19 indications. Among approved drugs were Novartis’ Afinitor (everolimus) for two indications, hormone receptor-positive, HER2-negative breast cancer and renal angiomyolipoma associated with tuberous sclerosis complex; Pfizer’s Bosulif (bosutinib) for Ph+ chronic myelogenous leukemia and Inlyta (axitinib) for advanced renal cell carcinoma; Roche subsidiary Genentech’s Perjeta (pertuzumab) for first-line HER2+ metastatic breast cancer and Erivedge (vismodegib) for basal cell carcinoma; and Ariad Pharmaceuticals’ Iclusig (ponatinib) for chronic myeloid leukemia and Philadelphia chromosome positive acute lymphoblastic leukemia.
“I think part of that is that the industry is in that area, doing a lot of R&D in oncology. But it’s also that the FDA is doing more to ensure that those products actually reach the marketplace,” Kenneth I. Kaitin, Ph.D., professor and director of the Tufts Center for the Study of Drug Development at Tufts University School of Medicine, told GEN.
As late as about three years ago, Dr. Kaitin recalled, FDA’s cancer division was under fire for what industry deemed were too-onerous requirements for getting a new cancer drug on the market: “They don’t look like the same division any more. There have been lots of oncology products that have been approved relative to the total number of product approvals this year and last year.”
Other significant categories:
Neurology—Eight drugs approved, including Bristol-Myers Squibb’s Eliquis (apixaban) for prevention of stroke and systemic embolism due to nonvalvular atrial fibrillation; GlaxiSmithKline’s Horizant (gabapentin enacarbil) for postherpetic neuralgia; and Pfizer’s Lyrica (pregabalin) for neuropathic pain associated with spinal cord injury.
Pulmonary and respiratory diseases—Eight drugs approved, including the first tuberculosis drug approved in four decades, Sirturo (bedaquiline) by Johnson & Johnson’s Janssen Therapeutics unit; and Vertex Pharmaceuticals’ Kalydeco (ivacaftor) for cystic fibrosis with the G551D mutation in the CFTR gene, the first CFTR potentiator.
Mixed and Slower
“If you’re in cancer or infectious diseases or if you’ve got an orphan drug, the agency is using pretty much all the tools at its disposal to move along those reviews and approvals. But in other areas—diabetes, endocrinology, CMS—the performance is much more mixed and slower,” David L. Gollaher, Ph.D., president and CEO of the California Healthcare Institute (CHI), told GEN.
CHI, which represents California’s biomedical industry, joined the Boston Consulting Group (BCG) in May to issue a report concluding that despite FDA efforts to shorten reviews, the agency took longer to approve drugs for diseases and conditions posing serious public health threats—including cardiovascular disease, obesity, and diabetes—than for cancer, hepatitis C, lupus, pneumonia, and orphan diseases.
During 2012, FDA approved three cardiovascular drugs, and one diabetes drug—Eli Lilly’s Jentadueto (linagliptin plus metformin hydrochloride) for type II diabetes. But the agency also okayed the first two weight loss drugs in more than a decade, Vivus’ Qsymia (phentermine and topiramate extended-release) and Belviq (lorcaserin hydrochloride), made by Arena Pharmaceuticals and distributed by Japan’s Eisai.
“Taken as a whole, the FDA has remained committed to getting important new products to the marketplace,” Dr. Kaitin said.
A key to that commitment, he said, was the series of PDUFA laws under which drug developers are charged “user fees” through which FDA funds new drug reviews. Every five years since 1992, PDUFA laws have sought to balance industry interests with FDA’s traditional interests in ensuring drug safety and efficacy.
“These issues that tended to fester over a long time, they really can’t anymore,” Dr. Kaitin said. “I don’t see a return the way we had in the past, where we went from 1962 to 1992 with an overly cautious, difficult to deal with, often confrontational regulatory agency.”
PDUFA laws alone won’t ensure smoother sailing for drug developers. As FDA likes to say, it can’t approve applications that don’t exist. That puts the burden on industry to continue developing drug candidates capable of withstanding agency scrutiny. But as Barry I. Eisenstein, M.D., senior vp, scientific affairs with Cubist Pharmaceuticals, correctly noted to GEN last month, all therapeutic areas are struggling with a greater degree of difficulty in finding new leads for breakthrough therapeutics.
“The modern genomic era, which 15 years ago we thought was going to allow for breakthroughs in a rapid way, has still not yet evolved to the point where it’s demonstrating that degree of proficiency in getting new leads,” Dr. Eisenstein said.
Until that improves, FDA is unlikely to see the sustained increase in new applications needed so it can approve more new drugs in coming years.
http://www.genengnews.com/keywordsandtools/print/3/29936/
ZUR ERINNERUNG - heute auf der 31st Annual J.P. Morgan Healthcare Conference

Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present at the 31st Annual J.P. Morgan Healthcare Conference on Wednesday, January 9, 2013, at 3:30 p.m. Pacific Time (6:30 p.m. Eastern Time) at The Westin St. Francis in San Francisco, California. Jack Lief, Arena's President and Chief Executive Officer, is scheduled to provide a corporate overview.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…

Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present at the 31st Annual J.P. Morgan Healthcare Conference on Wednesday, January 9, 2013, at 3:30 p.m. Pacific Time (6:30 p.m. Eastern Time) at The Westin St. Francis in San Francisco, California. Jack Lief, Arena's President and Chief Executive Officer, is scheduled to provide a corporate overview.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
Antwort auf Beitrag Nr.: 43.998.934 von bernie55 am 09.01.13 11:43:40Was ist das für eine späte Zeit, 23.30 Uhr bei uns?
Nur was für Studenten und Künstler!
Nur was für Studenten und Künstler!

!
Dieser Beitrag wurde von m.klemm moderiert. Grund: verstößt gegen Urheberrechte
29 Biotech stocks on the move after news
Posted by Jessica Lindell on Jan 09, 2013
The BioEnterprise Midwest Healthcare Venture Investment report revealed that healthcare technology companies in Ohio attracted $292 million in 2012, scoring a 65 percent growth from 2011, when $178 million was invested.
By sector, 2012 equity funding in the Midwest was to be paid to biopharmaceutical companies ($487 million), medical device companies ($309 million) and healthcare IT and service companies ($177 million).
...
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) got a boost yesterday after President and CEO Jack Lief said the company is seeking partners to sell its weight-loss drug Belviq abroad once it clears regulatory hurdles.
...
VIVUS, Inc. (NASDAQ:VVUS) was down after unusual trading activity was linked to a possible insider trade.
...
http://www.gaininggreen.com/29-biotech-stocks-on-the-move-af…
Posted by Jessica Lindell on Jan 09, 2013
The BioEnterprise Midwest Healthcare Venture Investment report revealed that healthcare technology companies in Ohio attracted $292 million in 2012, scoring a 65 percent growth from 2011, when $178 million was invested.
By sector, 2012 equity funding in the Midwest was to be paid to biopharmaceutical companies ($487 million), medical device companies ($309 million) and healthcare IT and service companies ($177 million).
...
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) got a boost yesterday after President and CEO Jack Lief said the company is seeking partners to sell its weight-loss drug Belviq abroad once it clears regulatory hurdles.
...
VIVUS, Inc. (NASDAQ:VVUS) was down after unusual trading activity was linked to a possible insider trade.
...
http://www.gaininggreen.com/29-biotech-stocks-on-the-move-af…
Zitat von bernie55: Annual J.P. Morgan Healthcare Conference
![]()
Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present at the 31st Annual J.P. Morgan Healthcare Conference on Wednesday, January 9, 2013, at 3:30 p.m. Pacific Time (6:30 p.m. Eastern Time) at The Westin St. Francis in San Francisco, California. Jack Lief, Arena's President and Chief Executive Officer, is scheduled to provide a corporate overview.
"Inhaltlich" scheint es von ARENA auf der Konferenz nichts Neues gegeben zu haben.
Auf die Frage bzgl. eines "oral-hearing" seitens der CHMP und einer möglichen " PR" antwortete Jack Lief.
" We have - and there were no questions and no request for an oral hearing - so nothing for us to report there.
We will just have to wait and see. The 210 day letter with the CHMP recommendation is due before the end of the month so we will just have to wait and see what the recommendation is. We will update as appropriate."
Also, die guten News sind, dass es keine News gibt.

Antwort auf Beitrag Nr.: 44.003.627 von bernie55 am 10.01.13 09:57:22Hier die JPM - " Powerpoint"-Präsentation von ARENA
https://jpmorgan.metameetings.com/confbook/healthcare13/stas…
https://jpmorgan.metameetings.com/confbook/healthcare13/stas…
Im IV Board gefunden
> die "Vorgehensweise zur Begutachtung von Medikamenten" im Vorfeld der EMA Entscheidung
http://www.investorvillage.com/smbd.asp?mb=633&mn=47330&pt=m…
Risk Management Plan (RMP) will be requested by the European Medicines Agency
--------------------------------------------------------------------------------------------------------------------------------------------------------------------
Pharmacovigilance Risk Assessment Committee (PRAC) draft agenda of meeting 7-10 January 2013
(Item 5 of the PRAC agenda)
The RMP describes what is known and not known about the side effects of a medicine and states how these risks will be prevented or minimised in patients.
It also includes plans for studies and other activities to gain more knowledge about the safety of the medicine and risk factors for developing side effects. RMPs are continually modified and updated throughout the lifetime of the medicine as new information becomes available.
5.1.8. Lorcaserin Hydrochloride
• Evaluation of the RMP in the context of an initial Marketing Authorisation Application procedure
Status: for discussion and agreement of advice to CHMP
http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_e…
--------------------------------------------------------------------------------------------------------------------------------------------------------------
Committee for Advanced Therapies (CAT) meeting 9-11 Januari 2013
The Committee for Advanced Therapies (CAT) is the committee that is responsible for assessing the quality, safety and efficacy of advanced-therapy medicines. It does not currently publish the minutes or agendas of its meetings. It does publish a meeting report after each meeting
http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/l…
--------------------------------------------------------------------------------------------------------------------------------------------------------------
...the Committee for Medicinal Products for Human Use (CHMP) will discuss Lorcaserin in the time 14-17 Januari 2013 :
http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/l…" target="_blank" rel="nofollow ugc noopener">
http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/l…
On Friday at the latest we will be informed about the result of the scientific evaluation!
....also spätestens am 18.01.13
> die "Vorgehensweise zur Begutachtung von Medikamenten" im Vorfeld der EMA Entscheidung
http://www.investorvillage.com/smbd.asp?mb=633&mn=47330&pt=m…
Risk Management Plan (RMP) will be requested by the European Medicines Agency
--------------------------------------------------------------------------------------------------------------------------------------------------------------------
Pharmacovigilance Risk Assessment Committee (PRAC) draft agenda of meeting 7-10 January 2013
(Item 5 of the PRAC agenda)
The RMP describes what is known and not known about the side effects of a medicine and states how these risks will be prevented or minimised in patients.
It also includes plans for studies and other activities to gain more knowledge about the safety of the medicine and risk factors for developing side effects. RMPs are continually modified and updated throughout the lifetime of the medicine as new information becomes available.
5.1.8. Lorcaserin Hydrochloride
• Evaluation of the RMP in the context of an initial Marketing Authorisation Application procedure
Status: for discussion and agreement of advice to CHMP
http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_e…
--------------------------------------------------------------------------------------------------------------------------------------------------------------
Committee for Advanced Therapies (CAT) meeting 9-11 Januari 2013
The Committee for Advanced Therapies (CAT) is the committee that is responsible for assessing the quality, safety and efficacy of advanced-therapy medicines. It does not currently publish the minutes or agendas of its meetings. It does publish a meeting report after each meeting
http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/l…
--------------------------------------------------------------------------------------------------------------------------------------------------------------
...the Committee for Medicinal Products for Human Use (CHMP) will discuss Lorcaserin in the time 14-17 Januari 2013 :
http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/l…" target="_blank" rel="nofollow ugc noopener">
http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/l…
On Friday at the latest we will be informed about the result of the scientific evaluation!
....also spätestens am 18.01.13
Analyst Blog
Update on Orexigen's Contrave
by Zacks Equity Research
January 10, 2013
Orexigen Therapeutics, Inc. (OREX - Snapshot Report) recently provided an update on its obesity candidate, Contrave. The company, which had received a complete response letter (CRL) from the US Food and Drug Administration (FDA) in Jan 2011 for Contrave, said that it could be in a position to resubmit the NDA for Contrave in the second half of 2013.
At the time of issuing the CRL, the FDA had expressed concerns regarding the long-term cardiovascular safety profile of Contrave and had asked Orexigen to conduct an additional study. The FDA requested a randomized, double-blind, placebo-controlled trial of sufficient size and duration which would show that the risk of major adverse cardiovascular events in overweight and obese subjects treated with Contrave does not adversely affect its benefit-risk profile.
In Feb 2012, the company reached an agreement with the FDA on a Special Protocol Assessment (SPA) for the Contrave outcomes trial and the Light Study was initiated in Jun 2012.
The company said that the Division of Metabolism and Endocrinology Products (DMEP) has proposed a resubmission procedure under which an interim analysis report submitted by the independent Data Monitoring Committee of the Light Study can be used for the resubmission of the Contrave NDA.
While the timing of the interim analysis depends on the rate of occurrence of major adverse cardiovascular events in the Light Study, Orexigen is hopeful of gaining approval in early 2014.
Our Recommendation
The company has a collaboration agreement with Takeda for the development and commercialization of Contrave in the US, Canada and Mexico. Takeda has experience in the metabolic disorder market which should prove beneficial. However, we note that Contrave, once launched, will be a late entrant in the obesity market. Last year, two obesity prevention drugs were approved: Arena Pharmaceuticals, Inc.’s (ARNA - Snapshot Report) Belviq and VIVUS Inc.’s (VVUS - Snapshot Report) Qsymia.
We currently have a Neutral recommendation on Orexigen. The stock carries a Zacks Rank #3 (Hold).
http://www.zacks.com/stock/news/90196/update-on-orexigens-co…
Update on Orexigen's Contrave
by Zacks Equity Research
January 10, 2013
Orexigen Therapeutics, Inc. (OREX - Snapshot Report) recently provided an update on its obesity candidate, Contrave. The company, which had received a complete response letter (CRL) from the US Food and Drug Administration (FDA) in Jan 2011 for Contrave, said that it could be in a position to resubmit the NDA for Contrave in the second half of 2013.
At the time of issuing the CRL, the FDA had expressed concerns regarding the long-term cardiovascular safety profile of Contrave and had asked Orexigen to conduct an additional study. The FDA requested a randomized, double-blind, placebo-controlled trial of sufficient size and duration which would show that the risk of major adverse cardiovascular events in overweight and obese subjects treated with Contrave does not adversely affect its benefit-risk profile.
In Feb 2012, the company reached an agreement with the FDA on a Special Protocol Assessment (SPA) for the Contrave outcomes trial and the Light Study was initiated in Jun 2012.
The company said that the Division of Metabolism and Endocrinology Products (DMEP) has proposed a resubmission procedure under which an interim analysis report submitted by the independent Data Monitoring Committee of the Light Study can be used for the resubmission of the Contrave NDA.
While the timing of the interim analysis depends on the rate of occurrence of major adverse cardiovascular events in the Light Study, Orexigen is hopeful of gaining approval in early 2014.
Our Recommendation
The company has a collaboration agreement with Takeda for the development and commercialization of Contrave in the US, Canada and Mexico. Takeda has experience in the metabolic disorder market which should prove beneficial. However, we note that Contrave, once launched, will be a late entrant in the obesity market. Last year, two obesity prevention drugs were approved: Arena Pharmaceuticals, Inc.’s (ARNA - Snapshot Report) Belviq and VIVUS Inc.’s (VVUS - Snapshot Report) Qsymia.
We currently have a Neutral recommendation on Orexigen. The stock carries a Zacks Rank #3 (Hold).
http://www.zacks.com/stock/news/90196/update-on-orexigens-co…
The beauty of Belviq
Jogging around the order of my expectations a bit, Lief noted that a decision is expected on approval in the EU within the first half of the year, and in Switzerland just a few months later. In addition, Eisai is getting its data together to file for a new drug application in Mexico, Canada, and Brazil sometime in 2013, while Ildong -- its newest partner in South Korea -- should also being filing an NDA this year.
In terms of compensation, investors have been wondering for a while if Arena had a shot at making big profits, or if its costs and low royalties would simply get the company by. Yesterday's presentation cleared that up in a big way!
On the low end of the scale, Arena will be getting 31.5% of sales on all U.S. revenue, with the potential to hit as high as 36.5% of the split when Belviq sales expand beyond $750 million.
This doesn't even factor in the countless milestone payments and purchase price adjustments that can boost Arena's bottom line and provide it up to $2.5 billion in one-time payments!
If you're curious about its Ildong partnership, Arena will be netting around 35% to 45% of total sales there in that collaborative partnership.
What's really telling is how well Arena's has orchestrated keeping its costs to a minimum. Unlike VIVUS, which is going it alone, Arena's choice to collaborate with Eisai has removed 90% of the costs associated with a $200 million-$300 million cardiovascular follow-up study from its plate, and places almost the entirety of Belviq's launch costs squarely on Eisai's shoulders.
Arena also received a 10-year tax holiday for its manufacturing facility in Switzerland, further lowering its costs.
We are, GPCR!
In what was a nice change of pace, we also got an intimate look at Arena's pipeline beyond Belviq.
CEO Jack Lief -- who was this year's runner-up for CEO of the year as voted by The Motley Fool community -- noted his company's focus on G protein-coupled receptors, or GPCRs, and highlighted three pipeline compounds in particular: APD811, APD334, and Temanogrel.
APD811, which is an oral treatment being targeted at pulmonary arterial hypertension, is currently in two phase 1 studies, and Lief expects to release certain data on the drug later this quarter. Lief specifically noted that APD811 could be a once-a-day oral solution to those needing constant infusions and works at the prostacyclin receptor -- a key target for the most effective pulmonary arterial hypertension medications currently on the market.
APD334 is being advanced as an autoimmune disease drug, such as for multiple sclerosis, and should advance to phase 1 trials this year along with APD811. Specifically, Lief notes that this compound is expected to target the S1P1 receptor, but he feels confident that its specialization will not result in bradycardia or other adverse pulmonary side effects like competing compounds.
Finally, Temanogrel, an inverse agonist of the serotonin 2A receptor, is being targeted at thrombotic diseases. Arena actually completed phase 1 trials of the drug years ago but is currently undergoing proof-of-concept trials with partner Ildong in South Korea. Temanogrel's focus on preventing serotonin-induced coagulation without creating an increase in bleeding risk could be a major benefit to those suffering from thrombotic diseases.
The makings of a winner
All told, Arena has all the makings of a winner. It ended its most recent quarter with $165 million in cash and stands to receive another $65 million milestone payment when the DEA classifies Belviq in the next month or so. With the majority of its costs covered by its partners, a sea of milestone payments still to come, and about 500 million people worldwide that could benefit from Belviq -- even more potentially if Belviq gains additional indications --
I see no reason why Arena can't be a solid long-term hold for serious biotech investors.
http://www.dailyfinance.com/2013/01/10/jpmorgan-healthcare-c…
Jogging around the order of my expectations a bit, Lief noted that a decision is expected on approval in the EU within the first half of the year, and in Switzerland just a few months later. In addition, Eisai is getting its data together to file for a new drug application in Mexico, Canada, and Brazil sometime in 2013, while Ildong -- its newest partner in South Korea -- should also being filing an NDA this year.
In terms of compensation, investors have been wondering for a while if Arena had a shot at making big profits, or if its costs and low royalties would simply get the company by. Yesterday's presentation cleared that up in a big way!
On the low end of the scale, Arena will be getting 31.5% of sales on all U.S. revenue, with the potential to hit as high as 36.5% of the split when Belviq sales expand beyond $750 million.
This doesn't even factor in the countless milestone payments and purchase price adjustments that can boost Arena's bottom line and provide it up to $2.5 billion in one-time payments!
If you're curious about its Ildong partnership, Arena will be netting around 35% to 45% of total sales there in that collaborative partnership.
What's really telling is how well Arena's has orchestrated keeping its costs to a minimum. Unlike VIVUS, which is going it alone, Arena's choice to collaborate with Eisai has removed 90% of the costs associated with a $200 million-$300 million cardiovascular follow-up study from its plate, and places almost the entirety of Belviq's launch costs squarely on Eisai's shoulders.
Arena also received a 10-year tax holiday for its manufacturing facility in Switzerland, further lowering its costs.
We are, GPCR!
In what was a nice change of pace, we also got an intimate look at Arena's pipeline beyond Belviq.
CEO Jack Lief -- who was this year's runner-up for CEO of the year as voted by The Motley Fool community -- noted his company's focus on G protein-coupled receptors, or GPCRs, and highlighted three pipeline compounds in particular: APD811, APD334, and Temanogrel.
APD811, which is an oral treatment being targeted at pulmonary arterial hypertension, is currently in two phase 1 studies, and Lief expects to release certain data on the drug later this quarter. Lief specifically noted that APD811 could be a once-a-day oral solution to those needing constant infusions and works at the prostacyclin receptor -- a key target for the most effective pulmonary arterial hypertension medications currently on the market.
APD334 is being advanced as an autoimmune disease drug, such as for multiple sclerosis, and should advance to phase 1 trials this year along with APD811. Specifically, Lief notes that this compound is expected to target the S1P1 receptor, but he feels confident that its specialization will not result in bradycardia or other adverse pulmonary side effects like competing compounds.
Finally, Temanogrel, an inverse agonist of the serotonin 2A receptor, is being targeted at thrombotic diseases. Arena actually completed phase 1 trials of the drug years ago but is currently undergoing proof-of-concept trials with partner Ildong in South Korea. Temanogrel's focus on preventing serotonin-induced coagulation without creating an increase in bleeding risk could be a major benefit to those suffering from thrombotic diseases.
The makings of a winner
All told, Arena has all the makings of a winner. It ended its most recent quarter with $165 million in cash and stands to receive another $65 million milestone payment when the DEA classifies Belviq in the next month or so. With the majority of its costs covered by its partners, a sea of milestone payments still to come, and about 500 million people worldwide that could benefit from Belviq -- even more potentially if Belviq gains additional indications --
I see no reason why Arena can't be a solid long-term hold for serious biotech investors.

http://www.dailyfinance.com/2013/01/10/jpmorgan-healthcare-c…
Schaut mal was fürn schöner Kurs da in US für ARNA gerade angezeigt wird:
$32.5 +235.05% - Data as of 1/14/2013
Was ist da los?
http://www.nasdaq.com/symbol/arna/real-time
$32.5 +235.05% - Data as of 1/14/2013
Was ist da los?
http://www.nasdaq.com/symbol/arna/real-time
Antwort auf Beitrag Nr.: 44.017.307 von me_2 am 14.01.13 09:35:43Hab`s schon gesehen, Fehler oder Übernahme?

Zitat von me_2: Schaut mal was fürn schöner Kurs da in US für ARNA gerade angezeigt wird:
$32.5 +235.05% - Data as of 1/14/2013
Was ist da los?
http://www.nasdaq.com/symbol/arna/real-time
Habe ich gestern auch schon gesehen.
Ich würde eindeutig sagen Funktionsstörung, siehe auch AMRN und MSFT.
AMRN > $19.5
+ 10.88
+126.22%
MSFT > $1000
+ 973.17
+ 3627.17%
...aber bei einem Buy-out wäre ich natürlich mit $32.5 durchaus zufrieden...

2012's Top Russell 2000 Gainer Poised for a Repeat Performance in 2013
Ich habe erst ab Absatz >DOCTORS EXPECT SUCCESSFUL LAUNCH > meine "lines" plaziert, da in den vorherigen Absätzen inhaltlich nichts Neues berichtet wird.
By Reza Ganjavi - January 11, 2013 |
The first weight management medication approved by the FDA in over a decade will soon hit the USA market. Belviq, by Arena Pharmaceuticals (NASDAQ: ARNA), is a single-molecule novel agent developed in California using an advanced GPCR technology based on a Nobel Prize winning scientific discovery.
Arena’s stock had a stellar 382% Gain in 2012, placing it as the top gainer in the Russell 2000 index. For the reasons outlined below, many investors believe Arena’s stock is poised to do even better in 2013.
SAFE MECHANISM OF ACTION
Years of extensive clinical trials on over 8000 patients have confirmed Belviq’s safety and efficacy. Unlike other weight-loss medications, which have had severe risks, Belviq’s most common side effect is a mild headache for a couple of days. It does not stimulate metabolism nor does it raise heart rate. Instead, Belviq promotes satiety—the experience of not being hungry. Consistent with Belviq's excellent safety profile, the FDA did not require a REMS (risk management) program.
SIGNIFICANT EFFICACY
Clinical trials revealed that responders lost an average of about 12% of body weight in a year. Scientists have determined that even a modest 5% loss in body weight has significant health benefits.
Being overweight often leads to an array of other illnesses. Belviq has significant benefits for treatment of pre-diabetic symptoms, Type 2 diabetes, as well as other comorbidities associated with being overweight, such as heart disease and cancer. Data indicates Belviq improves values for biomarkers that may be predictive of future cardiovascular events, including lipid levels, insulin resistance, levels of inflammatory markers and high blood pressure, and significant positive impact on type 2 diabetes patients.
WIDE INSURANCE COVERAGE
Two major insurance companies (Aetna and Blue Cross) have indicated they would cover obesity drugs, and others are expected to follow suit. Arena's marketing partner Eisai has a large team focused on insurance coverage.
Employers and insurance companies alike have an incentive to encourage weight loss because it leads to better overall health and significantly decreased healthcare costs. It is anticipated that Belviq will be covered by most insurance plans, and eventually by government programs such as Medicare and Medicaid.
HUGE TARGET MARKET
Belviq’s potential market is huge. In the USA alone, where obesity is a growing epidemic, one-third of adults are clinically obese and two-thirds are overweight. The treatment of obesity and type 2 diabetes costs the USA healthcare system an estimated $1 billion per day.
Obesity continues to expand throughout the world. The WHO estimated that “in 2008, more than 1.4 billion adults, 20 and older, were overweight. Of these, over 200 million men and nearly 300 million women were obese.” Many investors predict that Belviq will be a global blockbuster. Capturing even a fraction of the potential market means huge earnings for Arena.
DOCTORS EXPECT SUCCESSFUL LAUNCH
One of the many doctors who is predicting a successful launch for Belviq is Dr. Steven Vig, M.D., a specialist in internal medicine in Arizona who has thousands of patients including many obese, overweight, and diabetic patients. Dr. Vig correctly predicted Belviq’s FDA approval in 2012. He is now predicting Belviq’s launch will be a colossal success:
“Patients are calling my office every day asking when the new weight loss pill (Belviq) will be available. Based on current information, we are telling them that it will likely be available in pharmacies in the United States in March of 2013. Eisai will be targeting primary care doctors as well as specialists like endocrinology in their initial marketing efforts.”
Dr. Vig, M.D., anticipates European approval in the first half of 2013.
Dr. Vig, M.D., opines Belviq will be a blockbuster drug, and will be used by overweight and obese patients all over the planet, some of which have type 2 diabetes.
Later studies will focus on Belviq plus metformin for faster weight loss, Belviq plus phentermine for more weight loss, Belviq alone (the latter for smoking cessation), and pediatric obesity studies. Doctors will be saying to their patients, "GET SLEEK WITH BELVIQ!"
BELVIQ WILL BE APPROVED IN THE EUROPEAN UNION
The European Union is expected to grant marketing authorization for Belviq in the first half of 2013. Belviq meets the EU’s efficacy requirement, and has an excellent safety profile. Arena will then be well positioned to capture the majority of EU’s obesity market.
Vivus (NASDAQ: VVUS)'s Qsymia was recently rejected in Europe due to safety concerns.
The EU's regulatory process is complex but a close examination of the EU's EMA/CHMP timeline indicates that at the latest by February 21, 2013 we should expect CHMP's recommendation for approval to the EU unless the regulatory clock stops if EMA has further questions.
It is not clear if Belviq is in stage 2 or 3 of the approval process but if in stage 3, CHMP's recommendation could come as early as January 13.
On January 8 which corresponds with a EMA/PRAC meeting, ARNA increased over 10%.
BELVIQ’S OTHER USES
Arena has indicated that it plans to conduct trials for the use of Belviq in a variety of other health problems such as smoking cessation and trials in combination with other agents to address additional addictive behaviors.
BELVIQ HAS DISTRIBUTION PARTNERS
Belviq will be distributed in the USA by Eisai, which has marketing rights to Belviq in North and South America. Arena is in discussions with several international partners for making Belviq available in the rest of the world (ROW) including China which has a huge obesity and diabetic problem. The second of such agreements was inked with a large South Korean pharmaceutical, Ildong, which includes royalties, lucrative milestone payments, and research cooperation. Many more deals are expected if Arena is not acquired soon.
ARENA POSITIONED FOR BIG PHARMA ACQUISITION
Most large pharmaceuticals are faced with ever narrowing pipelines due to expiring patents and generic competition. Arena is a ripe takeover target by a Big Pharma acquirer , which could happen in 2013 following initial USA sales figures, but at a hefty premium — not less than $6B or $26 per share :kiss
, which could happen in 2013 following initial USA sales figures, but at a hefty premium — not less than $6B or $26 per share :kiss vs. the current share price of $9).
vs. the current share price of $9).
 The share price could be further boosted prior to final sale by a combination of short squeeze and competitive bidding.
The share price could be further boosted prior to final sale by a combination of short squeeze and competitive bidding.
The buyout could also result from negotiations for a EU partner with a global player such as Johnson & Johnson (NYSE: JNJ); Pfizer (NYSE: PFE); Roche (PINK: RHHBY); GlaxoSmithKline (NYSE: GSK); Novartis (NYSE: NVS); Sanofi (NYSE: SNY); Abbott Laboratories (NYSE: ABT); Merck (NYSE: MRK); Eli Lilly (NYSE: LLY); Bristol Myers Squibb (NYSE: BMY).
SHORT INTEREST IS STARING AT A BILLION DOLLAR LOSS
Short interest has risen since FDA approval to an astronomical level of 55 million shares. The only rational explanation for this is an attempt to raise their sales basis, believed to be under $5/share. Given the daily volume, behavior of the stock and several strong catalysts on the horizon, we estimate covering the short position could cost the short interest a billion dollar loss.
Any catalyst—like EU approval—could worsen the loss for short sellers. Even if half of their position is hedged by options, the potential loss is still staggering and at a level which has caused some hedge funds to close their doors. Options activity in the next few months should be very interesting as shorts should use their options leverage.
Adding to shorts’ woes is a stream of demand by institutions that sat on the sidelines prior to approval. Strong demand for shares together with limited supply means higher prices. Arena has indicated it has no plans to dilute the shares, given a strong cash position, expected revenues from Belviq and further milestone-payment receivable from existing marketing partner.
INSTITUTIONAL OWNERSHIP
Institutional ownership of Arena has increased significantly post-approval to the current level of about 45% of the float. Analysts expect this will increase to 80% .
.
The transfer has to occur from retail investors to institutions. Many retail investors bought Arena under $3 while Arena was under heavy attack by short sellers and like-minded journalists and analysts who were all proven to be embarrassingly wrong when Belviq was approved by the FDA. (The bashers are still at it, repeatedly attacking Arena with the same mantras that led short interest into hot water in the first place, which is where they still find themselves stuck today.)
Retail investors’ prediction of Belviq’s approval was due to solid scientific evidence and the guidance of several medical doctors who were excited about Belviq and firmly believed it would be approved, given the scale and consequences of the obesity epidemic. The FDA sided with the science, not the fiction promoted by short-sellers.
ADDITIONAL REASONS WHY WE LIKE INVESTING IN ARENA
Arena has a strong pipeline of other potential novel agents based on its proven, leading edge, and GPCR technology.
Arena’s founder and CEO has not sold a single share since founding the company.
A strong balance sheet, and attractive profit margins.
A strong intellectual property position.
Valuation models paint a very positive picture for Arena’s share price (model 1, model 2).
10-year tax holiday in Switzerland where Belviq is manufactured adds a delicious “icing on the cake”.
Finally, in 2013, we expect Arena’s shares to beat their stellar 2012 performance, and reward us patient investors handsomely.
http://beta.fool.com/beatlesforever/2013/01/11/2012s-top-ru…
Ich habe erst ab Absatz >DOCTORS EXPECT SUCCESSFUL LAUNCH > meine "lines" plaziert, da in den vorherigen Absätzen inhaltlich nichts Neues berichtet wird.
By Reza Ganjavi - January 11, 2013 |
The first weight management medication approved by the FDA in over a decade will soon hit the USA market. Belviq, by Arena Pharmaceuticals (NASDAQ: ARNA), is a single-molecule novel agent developed in California using an advanced GPCR technology based on a Nobel Prize winning scientific discovery.
Arena’s stock had a stellar 382% Gain in 2012, placing it as the top gainer in the Russell 2000 index. For the reasons outlined below, many investors believe Arena’s stock is poised to do even better in 2013.
SAFE MECHANISM OF ACTION
Years of extensive clinical trials on over 8000 patients have confirmed Belviq’s safety and efficacy. Unlike other weight-loss medications, which have had severe risks, Belviq’s most common side effect is a mild headache for a couple of days. It does not stimulate metabolism nor does it raise heart rate. Instead, Belviq promotes satiety—the experience of not being hungry. Consistent with Belviq's excellent safety profile, the FDA did not require a REMS (risk management) program.
SIGNIFICANT EFFICACY
Clinical trials revealed that responders lost an average of about 12% of body weight in a year. Scientists have determined that even a modest 5% loss in body weight has significant health benefits.
Being overweight often leads to an array of other illnesses. Belviq has significant benefits for treatment of pre-diabetic symptoms, Type 2 diabetes, as well as other comorbidities associated with being overweight, such as heart disease and cancer. Data indicates Belviq improves values for biomarkers that may be predictive of future cardiovascular events, including lipid levels, insulin resistance, levels of inflammatory markers and high blood pressure, and significant positive impact on type 2 diabetes patients.
WIDE INSURANCE COVERAGE
Two major insurance companies (Aetna and Blue Cross) have indicated they would cover obesity drugs, and others are expected to follow suit. Arena's marketing partner Eisai has a large team focused on insurance coverage.
Employers and insurance companies alike have an incentive to encourage weight loss because it leads to better overall health and significantly decreased healthcare costs. It is anticipated that Belviq will be covered by most insurance plans, and eventually by government programs such as Medicare and Medicaid.
HUGE TARGET MARKET
Belviq’s potential market is huge. In the USA alone, where obesity is a growing epidemic, one-third of adults are clinically obese and two-thirds are overweight. The treatment of obesity and type 2 diabetes costs the USA healthcare system an estimated $1 billion per day.
Obesity continues to expand throughout the world. The WHO estimated that “in 2008, more than 1.4 billion adults, 20 and older, were overweight. Of these, over 200 million men and nearly 300 million women were obese.” Many investors predict that Belviq will be a global blockbuster. Capturing even a fraction of the potential market means huge earnings for Arena.
DOCTORS EXPECT SUCCESSFUL LAUNCH
One of the many doctors who is predicting a successful launch for Belviq is Dr. Steven Vig, M.D., a specialist in internal medicine in Arizona who has thousands of patients including many obese, overweight, and diabetic patients. Dr. Vig correctly predicted Belviq’s FDA approval in 2012. He is now predicting Belviq’s launch will be a colossal success:

“Patients are calling my office every day asking when the new weight loss pill (Belviq) will be available. Based on current information, we are telling them that it will likely be available in pharmacies in the United States in March of 2013. Eisai will be targeting primary care doctors as well as specialists like endocrinology in their initial marketing efforts.”
Dr. Vig, M.D., anticipates European approval in the first half of 2013.

Dr. Vig, M.D., opines Belviq will be a blockbuster drug, and will be used by overweight and obese patients all over the planet, some of which have type 2 diabetes.

Later studies will focus on Belviq plus metformin for faster weight loss, Belviq plus phentermine for more weight loss, Belviq alone (the latter for smoking cessation), and pediatric obesity studies. Doctors will be saying to their patients, "GET SLEEK WITH BELVIQ!"
BELVIQ WILL BE APPROVED IN THE EUROPEAN UNION
The European Union is expected to grant marketing authorization for Belviq in the first half of 2013. Belviq meets the EU’s efficacy requirement, and has an excellent safety profile. Arena will then be well positioned to capture the majority of EU’s obesity market.
Vivus (NASDAQ: VVUS)'s Qsymia was recently rejected in Europe due to safety concerns.
The EU's regulatory process is complex but a close examination of the EU's EMA/CHMP timeline indicates that at the latest by February 21, 2013 we should expect CHMP's recommendation for approval to the EU unless the regulatory clock stops if EMA has further questions.
It is not clear if Belviq is in stage 2 or 3 of the approval process but if in stage 3, CHMP's recommendation could come as early as January 13.
On January 8 which corresponds with a EMA/PRAC meeting, ARNA increased over 10%.
BELVIQ’S OTHER USES
Arena has indicated that it plans to conduct trials for the use of Belviq in a variety of other health problems such as smoking cessation and trials in combination with other agents to address additional addictive behaviors.
BELVIQ HAS DISTRIBUTION PARTNERS
Belviq will be distributed in the USA by Eisai, which has marketing rights to Belviq in North and South America. Arena is in discussions with several international partners for making Belviq available in the rest of the world (ROW) including China which has a huge obesity and diabetic problem. The second of such agreements was inked with a large South Korean pharmaceutical, Ildong, which includes royalties, lucrative milestone payments, and research cooperation. Many more deals are expected if Arena is not acquired soon.
ARENA POSITIONED FOR BIG PHARMA ACQUISITION
Most large pharmaceuticals are faced with ever narrowing pipelines due to expiring patents and generic competition. Arena is a ripe takeover target by a Big Pharma acquirer
 , which could happen in 2013 following initial USA sales figures, but at a hefty premium — not less than $6B or $26 per share :kiss
, which could happen in 2013 following initial USA sales figures, but at a hefty premium — not less than $6B or $26 per share :kiss vs. the current share price of $9).
vs. the current share price of $9).  The share price could be further boosted prior to final sale by a combination of short squeeze and competitive bidding.
The share price could be further boosted prior to final sale by a combination of short squeeze and competitive bidding.
The buyout could also result from negotiations for a EU partner with a global player such as Johnson & Johnson (NYSE: JNJ); Pfizer (NYSE: PFE); Roche (PINK: RHHBY); GlaxoSmithKline (NYSE: GSK); Novartis (NYSE: NVS); Sanofi (NYSE: SNY); Abbott Laboratories (NYSE: ABT); Merck (NYSE: MRK); Eli Lilly (NYSE: LLY); Bristol Myers Squibb (NYSE: BMY).
SHORT INTEREST IS STARING AT A BILLION DOLLAR LOSS
Short interest has risen since FDA approval to an astronomical level of 55 million shares. The only rational explanation for this is an attempt to raise their sales basis, believed to be under $5/share. Given the daily volume, behavior of the stock and several strong catalysts on the horizon, we estimate covering the short position could cost the short interest a billion dollar loss.
Any catalyst—like EU approval—could worsen the loss for short sellers. Even if half of their position is hedged by options, the potential loss is still staggering and at a level which has caused some hedge funds to close their doors. Options activity in the next few months should be very interesting as shorts should use their options leverage.
Adding to shorts’ woes is a stream of demand by institutions that sat on the sidelines prior to approval. Strong demand for shares together with limited supply means higher prices. Arena has indicated it has no plans to dilute the shares, given a strong cash position, expected revenues from Belviq and further milestone-payment receivable from existing marketing partner.
INSTITUTIONAL OWNERSHIP
Institutional ownership of Arena has increased significantly post-approval to the current level of about 45% of the float. Analysts expect this will increase to 80%
 .
. The transfer has to occur from retail investors to institutions. Many retail investors bought Arena under $3 while Arena was under heavy attack by short sellers and like-minded journalists and analysts who were all proven to be embarrassingly wrong when Belviq was approved by the FDA. (The bashers are still at it, repeatedly attacking Arena with the same mantras that led short interest into hot water in the first place, which is where they still find themselves stuck today.)
Retail investors’ prediction of Belviq’s approval was due to solid scientific evidence and the guidance of several medical doctors who were excited about Belviq and firmly believed it would be approved, given the scale and consequences of the obesity epidemic. The FDA sided with the science, not the fiction promoted by short-sellers.
ADDITIONAL REASONS WHY WE LIKE INVESTING IN ARENA
Arena has a strong pipeline of other potential novel agents based on its proven, leading edge, and GPCR technology.
Arena’s founder and CEO has not sold a single share since founding the company.
A strong balance sheet, and attractive profit margins.
A strong intellectual property position.
Valuation models paint a very positive picture for Arena’s share price (model 1, model 2).
10-year tax holiday in Switzerland where Belviq is manufactured adds a delicious “icing on the cake”.
Finally, in 2013, we expect Arena’s shares to beat their stellar 2012 performance, and reward us patient investors handsomely.

http://beta.fool.com/beatlesforever/2013/01/11/2012s-top-ru…
Antwort auf Beitrag Nr.: 44.017.665 von bernie55 am 14.01.13 10:50:43ja, DVAX auch so ähnlich. Wohl ein technischer Bug. SChade 

Antwort auf Beitrag Nr.: 44.017.887 von me_2 am 14.01.13 11:29:34Arena Pharmaceuticals (ARNA_) is a clinical-stage biopharmaceutical company focused on discovering, developing and commercializing oral drugs that target G protein-coupled receptors. This stock is trading up 4.8% at $10.13 in recent trading.
Today's Volume: 12.34 million
Average Volume: 8.61 million
Volume % Change: 135%
From a technical perspective, ARNA is spiking higher here right off some near-term support at $9.50 with monster upside volume. This stock has been uptrending strongly for the last two months, with shares moving higher from its recent low of $7.09 to its intraday high of $10.28. During that move, shares of ARAN have been consistently making higher lows and higher highs, which is bullish technical price action. That move has now pushed ARNA within range of triggering a major breakout trade. That trade will hit if ARNA manages to take out some key overhead resistance levels at $12.07 to $13.50 with high volume.
Traders should now look for long-biased trades in ARNA as long as it's trending above $9.65 to $10.14, and then once it sustains a move or close above those breakout levels with volume that this near or above 8.61 million shares. If that breakout triggers soon, then ARNA will set up to enter new 52-week high territory once it clears $13.50. Some possible upside targets off that breakout are $15 to $17.

Today's Volume: 12.34 million
Average Volume: 8.61 million
Volume % Change: 135%
From a technical perspective, ARNA is spiking higher here right off some near-term support at $9.50 with monster upside volume. This stock has been uptrending strongly for the last two months, with shares moving higher from its recent low of $7.09 to its intraday high of $10.28. During that move, shares of ARAN have been consistently making higher lows and higher highs, which is bullish technical price action. That move has now pushed ARNA within range of triggering a major breakout trade. That trade will hit if ARNA manages to take out some key overhead resistance levels at $12.07 to $13.50 with high volume.
Traders should now look for long-biased trades in ARNA as long as it's trending above $9.65 to $10.14, and then once it sustains a move or close above those breakout levels with volume that this near or above 8.61 million shares. If that breakout triggers soon, then ARNA will set up to enter new 52-week high territory once it clears $13.50. Some possible upside targets off that breakout are $15 to $17.


Zitat von Magnetfeldfredy: From a technical perspective......
......If that breakout triggers soon, then ARNA will set up to enter new 52-week high territory once it clears $13.50. Some possible upside targets off that breakout are $15 to $17.
Hi Fredy und all die anderen " Areanics",
das Avg Vol. (der letzten 3 Monate) liegt bei 8,809,270.
Wegen der anstehende CHMP Empfehlung + der bevorstehende Einführung von Belviq in den USA ist in den letzten 6 Tagen das Vol. aber enorm angestiegen.
.9,400,000
30,600,000
19,950,000
12,750,000
.6,500,000
20,200,000
Ich habe aber aktuell immer noch das Szenario vor Augen, dass einige BIGS bereits auf Tuchfühlung mit dem Management von ARNA gegangen sind und diverse Angebote für ein buy-out vorbereiten..... einfach mal nur so ein Gedanke

Dann sollten die " technischen " Argumente bei ARNA hinfällig werden, da ein endgültiges Übernahmeangebot meiner Meinung höher liegen würde als bei 18$..
....und damit , denke ich, könnten wir alle gut leben.

Grüße
bernie55

Antwort auf Beitrag Nr.: 44.021.371 von Magnetfeldfredy am 14.01.13 23:01:57$15,- bis $17,- halte ich auch für realistisch.
Möglich sind natürlich auch Kurse von $20,- und darüber.
Mal sehen.
Aktuell ist ARNA vom Kurswert zum ersten Mal über ELN.

Möglich sind natürlich auch Kurse von $20,- und darüber.
Mal sehen.
Aktuell ist ARNA vom Kurswert zum ersten Mal über ELN.

ARNA, CLSN, HALO, TEAR Among Charts to Watch
by Harry Boxer, Mon January 14th, 2013
There was some sloppy action and the stock market ended mixed on Monday. Many of the stocks we follow are still doing well. We were going to go over the short side, but decided not just yet. Instead, we’ll take a look at some of the longs we have been following and swing trades that we put out.
Arena Pharmaceuticals, Inc. (ARNA) has a nice base and a long pattern that goes back to July. It ran up May, June, July, which took it from 2.25 to 14, almost seven-fold in just a couple months. Then it resulted in a big consolidation, freebased, and now it’s up against resistance near the 10.35 range, reaching as high as 10.31 on Monday, and closing at 10.20, up 54 cents, or 5.59%, on 20 million shares. That’s the second biggest volume in a couple months. If it does follow through on Tuesday, we could see this stock run quickly to 12, the short-term target, and then to the secondary target at 14.
Celsion Corp. (CLSN) has been acting great. We put another swing trade out on it on Monday. There was a wonderful trade during most of November that went from the 4.00-range up to 8.50 or so. It consolidated in a wedge the last few weeks, and may have popped out of it on 2.9 million shares on Monday. That’s the biggest volume in two or three weeks. It closed at 27.22, up 2.39, or 9.6%, and looks like it may run up to test the 10 3/4 zone, which is the near-term target.
Halozyme Therapeutics, Inc. (HALO) had the declining topsline and lateral price resistance taken out as well as the flag that was in that same area. It popped, pulled back for a couple days, and then exploded on Monday, up 1.12 to 8.34, or nearly 16%, on 3 million shares. That’s the second biggest volume since September. At this point, if it can get across the line where it closed on Monday, then it should be able to get up around 10-10.25.
TearLab Corporation (TEAR) was put out as a swing trade, and it popped through resistance, pulled back to retest that zone, and then it bounced, up 13 cents to 5.44, or 2.45%, on nearly a half million shares. That was the best volume in the last three or four sessions. It looks like it can extend. The long-term chart shows some big upside in the 10 zone, which means it will have doubled if it makes that move up.
Other stocks on Harry’s Charts of the Day are ACADIA Pharmaceuticals Inc. (ACAD), Amyris, Inc. (AMRS), Acme Packet, Inc. (APKT), BIOLASE, Inc. (BIOL), Flowers Foods, Inc. (FLO), Krispy Kreme Doughnuts, Inc. (KKD), Opko Health, Inc. (OPK), Perion Network Ltd (PERI), Research In Motion Limited (RIMM), Sangamo Biosciences Inc. (SGMO), and TrovaGene, Inc. (TROV).
http://www.advicetrade.com/chartsoftheday/ARNA-CLSN-HALO-TEA…
by Harry Boxer, Mon January 14th, 2013
There was some sloppy action and the stock market ended mixed on Monday. Many of the stocks we follow are still doing well. We were going to go over the short side, but decided not just yet. Instead, we’ll take a look at some of the longs we have been following and swing trades that we put out.
Arena Pharmaceuticals, Inc. (ARNA) has a nice base and a long pattern that goes back to July. It ran up May, June, July, which took it from 2.25 to 14, almost seven-fold in just a couple months. Then it resulted in a big consolidation, freebased, and now it’s up against resistance near the 10.35 range, reaching as high as 10.31 on Monday, and closing at 10.20, up 54 cents, or 5.59%, on 20 million shares. That’s the second biggest volume in a couple months. If it does follow through on Tuesday, we could see this stock run quickly to 12, the short-term target, and then to the secondary target at 14.
Celsion Corp. (CLSN) has been acting great. We put another swing trade out on it on Monday. There was a wonderful trade during most of November that went from the 4.00-range up to 8.50 or so. It consolidated in a wedge the last few weeks, and may have popped out of it on 2.9 million shares on Monday. That’s the biggest volume in two or three weeks. It closed at 27.22, up 2.39, or 9.6%, and looks like it may run up to test the 10 3/4 zone, which is the near-term target.
Halozyme Therapeutics, Inc. (HALO) had the declining topsline and lateral price resistance taken out as well as the flag that was in that same area. It popped, pulled back for a couple days, and then exploded on Monday, up 1.12 to 8.34, or nearly 16%, on 3 million shares. That’s the second biggest volume since September. At this point, if it can get across the line where it closed on Monday, then it should be able to get up around 10-10.25.
TearLab Corporation (TEAR) was put out as a swing trade, and it popped through resistance, pulled back to retest that zone, and then it bounced, up 13 cents to 5.44, or 2.45%, on nearly a half million shares. That was the best volume in the last three or four sessions. It looks like it can extend. The long-term chart shows some big upside in the 10 zone, which means it will have doubled if it makes that move up.
Other stocks on Harry’s Charts of the Day are ACADIA Pharmaceuticals Inc. (ACAD), Amyris, Inc. (AMRS), Acme Packet, Inc. (APKT), BIOLASE, Inc. (BIOL), Flowers Foods, Inc. (FLO), Krispy Kreme Doughnuts, Inc. (KKD), Opko Health, Inc. (OPK), Perion Network Ltd (PERI), Research In Motion Limited (RIMM), Sangamo Biosciences Inc. (SGMO), and TrovaGene, Inc. (TROV).
http://www.advicetrade.com/chartsoftheday/ARNA-CLSN-HALO-TEA…
Arena Pharmaceuticals - Trading Review
Posted on 01/14/2013 by Jane Lacave
NEW YORK (AVAFIN) -- A total of 144 block trades were executed today, typically at least 10,000 shares or more represent a single block unit. Block trades help investors understand the sentiment of large financial institutions. Further trading analysis reveals that 1,260,640 shares were bought and 2,078,849 shares were sold. The bought/sold ratio for shares of Arena Pharmaceuticals is 0.61, representing a negative net cash flow of $8,257,435 out of the stock.
Options traders were busy as well, a total of 35,452 call and 10,947 put contracts were traded yesterday yielding a 0.31 put/call ratio.
Arena Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company committed to discovering and developing innovative therapies offering medical advances and new options for patients.
Arena Pharmaceuticals opened at $9.82 and the stock price rose $0.54 (+5.60%) to $10.20 during the market session. ARNA is trading between the range of $9.65 - $10.31. To date, the stock has gained 7.45% within the last quarter. Volume is 20M in relation to the three month average volume of 8M shares. ARNA is trading above the 50 day moving average and higher than the 200 day moving average.
http://www.avafin.com/articles/1020227.html
Posted on 01/14/2013 by Jane Lacave
NEW YORK (AVAFIN) -- A total of 144 block trades were executed today, typically at least 10,000 shares or more represent a single block unit. Block trades help investors understand the sentiment of large financial institutions. Further trading analysis reveals that 1,260,640 shares were bought and 2,078,849 shares were sold. The bought/sold ratio for shares of Arena Pharmaceuticals is 0.61, representing a negative net cash flow of $8,257,435 out of the stock.
Options traders were busy as well, a total of 35,452 call and 10,947 put contracts were traded yesterday yielding a 0.31 put/call ratio.
Arena Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company committed to discovering and developing innovative therapies offering medical advances and new options for patients.
Arena Pharmaceuticals opened at $9.82 and the stock price rose $0.54 (+5.60%) to $10.20 during the market session. ARNA is trading between the range of $9.65 - $10.31. To date, the stock has gained 7.45% within the last quarter. Volume is 20M in relation to the three month average volume of 8M shares. ARNA is trading above the 50 day moving average and higher than the 200 day moving average.
http://www.avafin.com/articles/1020227.html
Gainer Watch List: Dendreon Corp. (NASDAQ NDN), Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) and Comverse Technology Inc. (CMVT) Have Been Added to Growing Stock Report's NASDAQ Watch List
NDN), Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) and Comverse Technology Inc. (CMVT) Have Been Added to Growing Stock Report's NASDAQ Watch List
New York, NY -- (SBWIRE) -- 01/14/2013 -- Growing Stock Report iniates a Percentage Gainer Watch List and Investor Polls for the following stocks: Dendreon Corp. (NASDAQ NDN), Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) and Comverse Technology Inc. (NASDAQ:CMVT).
NDN), Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) and Comverse Technology Inc. (NASDAQ:CMVT).
Dendreon Corp. (NASDAQ NDN) As of This Writing up (+5.02%) on 17,022,879 Shares Traded.
NDN) As of This Writing up (+5.02%) on 17,022,879 Shares Traded.
Dendreon Corp. (NASDAQ NDN) a company that engages in the discovery, development, and commercialization of novel therapeutics to enhance cancer treatment options for patients crossed above their 200 day moving average of $6.30, changing hands as high as $6.69 per share. Dendreon Corp shares are currently trading up about (+5.02%) on the day, which has prompted Growing Stock Report to Add Dendreon Corp. (NASDAQ
NDN) a company that engages in the discovery, development, and commercialization of novel therapeutics to enhance cancer treatment options for patients crossed above their 200 day moving average of $6.30, changing hands as high as $6.69 per share. Dendreon Corp shares are currently trading up about (+5.02%) on the day, which has prompted Growing Stock Report to Add Dendreon Corp. (NASDAQ NDN to it's NASDAQ Gainer Watch List.
NDN to it's NASDAQ Gainer Watch List.
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) As of This Writing up (+5.75%) on 13,326,481 Shares Traded.
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) a clinical-stage biopharmaceutical company, engages in discovering, developing, and commercializing oral drugs that target G protein-coupled receptors in the therapeutic areas of cardiovascular, central nervous system, inflammatory, and metabolic diseases is currently waiting on European approval for it's obesity drug Belviq, shares are currently up (+5.75%) which has prompted Growing Stock Report to Add Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) to it's NASDAQ Gainer Watch List.
Comverse Technology Inc. (NASDAQ:CMVT) As of This Writing up (+6.91%) on 8,711,623 Shares Traded.
Comverse Technology Inc. (NASDAQ:CMVT) a company that through its subsidiaries, provides software-based products, systems, and related services for wireless, wireline, and cable network communication service providers is currently up (+6.91%) on news that Nice Systems Ltd. (NICE) advanced to the highest level in almost six months after Calcalist said the Israeli maker of analytical telecommunications products is in talks to buy competitor Verint Systems Inc. (VRNT) or which Comverse Technology Inc. (Nasdaq:CMVT) owns a 41 percent stake in Verint, which has prompted Growing Stock Report to Add Comverse Technology Inc. (Nasdaq:CMVT) to it's Nasdaq Gainer Watch List.
Get CMVT Poll & Consensus Report Here: http://www.growingstockreport.com/Survey.aspx?stock=CMVT
GrowingStockReport.com monitors and scans the markets for stock related signals as well as any external factors that might bring trading opportunities.
Through a vast network of IR professionals GrowingStockReport.com is often aware of several large investor awareness campaigns being deployed.
Timing is important when trading Small Caps and Penny Stocks.
Simply sign up for free and start receiving exclusive alerts.
Subscribe Here: http://www.GrowingStockReport.com
Disclosure
GrowingStockReport.com is not a registered investment advisor and nothing contained in any materials should be construed as a recommendation to buy or sell securities. Investors should always conduct their own due diligence with any potential investment. Please visit GrowingStockReport.com website, for complete risks and disclosures.
Contact Info:
Growing Stock Report
editor@GrowingStockReport.com
SBWire (http://s.tt/1yr8k)
http://www.sbwire.com/press-releases/gainer-watch-list-dendr…
 NDN), Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) and Comverse Technology Inc. (CMVT) Have Been Added to Growing Stock Report's NASDAQ Watch List
NDN), Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) and Comverse Technology Inc. (CMVT) Have Been Added to Growing Stock Report's NASDAQ Watch ListNew York, NY -- (SBWIRE) -- 01/14/2013 -- Growing Stock Report iniates a Percentage Gainer Watch List and Investor Polls for the following stocks: Dendreon Corp. (NASDAQ
 NDN), Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) and Comverse Technology Inc. (NASDAQ:CMVT).
NDN), Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) and Comverse Technology Inc. (NASDAQ:CMVT).Dendreon Corp. (NASDAQ
 NDN) As of This Writing up (+5.02%) on 17,022,879 Shares Traded.
NDN) As of This Writing up (+5.02%) on 17,022,879 Shares Traded.Dendreon Corp. (NASDAQ
 NDN) a company that engages in the discovery, development, and commercialization of novel therapeutics to enhance cancer treatment options for patients crossed above their 200 day moving average of $6.30, changing hands as high as $6.69 per share. Dendreon Corp shares are currently trading up about (+5.02%) on the day, which has prompted Growing Stock Report to Add Dendreon Corp. (NASDAQ
NDN) a company that engages in the discovery, development, and commercialization of novel therapeutics to enhance cancer treatment options for patients crossed above their 200 day moving average of $6.30, changing hands as high as $6.69 per share. Dendreon Corp shares are currently trading up about (+5.02%) on the day, which has prompted Growing Stock Report to Add Dendreon Corp. (NASDAQ NDN to it's NASDAQ Gainer Watch List.
NDN to it's NASDAQ Gainer Watch List.Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) As of This Writing up (+5.75%) on 13,326,481 Shares Traded.
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) a clinical-stage biopharmaceutical company, engages in discovering, developing, and commercializing oral drugs that target G protein-coupled receptors in the therapeutic areas of cardiovascular, central nervous system, inflammatory, and metabolic diseases is currently waiting on European approval for it's obesity drug Belviq, shares are currently up (+5.75%) which has prompted Growing Stock Report to Add Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) to it's NASDAQ Gainer Watch List.
Comverse Technology Inc. (NASDAQ:CMVT) As of This Writing up (+6.91%) on 8,711,623 Shares Traded.
Comverse Technology Inc. (NASDAQ:CMVT) a company that through its subsidiaries, provides software-based products, systems, and related services for wireless, wireline, and cable network communication service providers is currently up (+6.91%) on news that Nice Systems Ltd. (NICE) advanced to the highest level in almost six months after Calcalist said the Israeli maker of analytical telecommunications products is in talks to buy competitor Verint Systems Inc. (VRNT) or which Comverse Technology Inc. (Nasdaq:CMVT) owns a 41 percent stake in Verint, which has prompted Growing Stock Report to Add Comverse Technology Inc. (Nasdaq:CMVT) to it's Nasdaq Gainer Watch List.
Get CMVT Poll & Consensus Report Here: http://www.growingstockreport.com/Survey.aspx?stock=CMVT
GrowingStockReport.com monitors and scans the markets for stock related signals as well as any external factors that might bring trading opportunities.
Through a vast network of IR professionals GrowingStockReport.com is often aware of several large investor awareness campaigns being deployed.
Timing is important when trading Small Caps and Penny Stocks.
Simply sign up for free and start receiving exclusive alerts.
Subscribe Here: http://www.GrowingStockReport.com
Disclosure
GrowingStockReport.com is not a registered investment advisor and nothing contained in any materials should be construed as a recommendation to buy or sell securities. Investors should always conduct their own due diligence with any potential investment. Please visit GrowingStockReport.com website, for complete risks and disclosures.
Contact Info:
Growing Stock Report
editor@GrowingStockReport.com
SBWire (http://s.tt/1yr8k)
http://www.sbwire.com/press-releases/gainer-watch-list-dendr…
Top Bullish Stocks On Monday: Cisco, HP And Arena Pharmaceuticals With Unusual Call Activities
January 15, 2013 |
...
Arena Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company focused on discovering, developing and commercializing oral drugs that target G protein-coupled receptors, GPCRs. ARNA is currently waiting on European approval for its obesity drug, Belviq. Technically, ARNA is bullish while MACD (12, 26, 9) is showing a bullish trend and RSI (14) is picking up with increasing buying momentum at 67.12. ARNA is currently trading above its 50-day MA of $8.85 and 200-day MA of $7.73. By closing at $10.20 on Monday, ARNA had broken through and closed above its R2 pivot point of $10.13, as seen from the chart below.
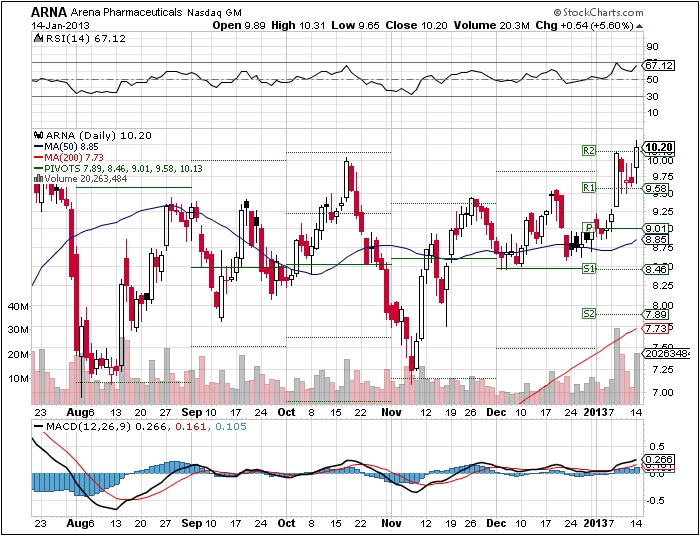
http://seekingalpha.com/article/1111641-top-bullish-stocks-o…
January 15, 2013 |
...
Arena Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company focused on discovering, developing and commercializing oral drugs that target G protein-coupled receptors, GPCRs. ARNA is currently waiting on European approval for its obesity drug, Belviq. Technically, ARNA is bullish while MACD (12, 26, 9) is showing a bullish trend and RSI (14) is picking up with increasing buying momentum at 67.12. ARNA is currently trading above its 50-day MA of $8.85 and 200-day MA of $7.73. By closing at $10.20 on Monday, ARNA had broken through and closed above its R2 pivot point of $10.13, as seen from the chart below.

http://seekingalpha.com/article/1111641-top-bullish-stocks-o…
Major Revenue Losses From Patent Expirations Forces Big Pharma Companies to Look to the Biotech Industry for Replacements
Five Star Equities Provides Stock Research on Arena Pharmaceuticals and VIVUS
NEW YORK, NY--(Marketwire - Jan 15, 2013) - Lost revenues from expiring patents has played a major part in the Biotech Industry's success in recent years. A total of 676 takeovers of biotechnology and pharmaceutical companies have occurred in the past three years, with an average premium of 38 percent, according to data collected by Bloomberg.
Five Star Equities examines the outlook for companies in the Biotech Industry and provides equity research on Arena Pharmaceuticals, Inc. ( NASDAQ : ARNA ) and VIVUS, Inc. ( NASDAQ : VVUS ).
Access to the full company reports can be found at:
www.FiveStarEquities.com/ARNA
www.FiveStarEquities.com/VVUS
At the end of the third quarter five of the biggest drug makers in the U.S. held over $70 billion in cash, near cash and short-term investments. Major revenue losses from patent expirations have forced big pharmaceutical companies to look to biotech companies to help fill the void.
Pfizer's Lipitor and Bristol-Myers' Plavix, which lost exclusivity in late 2011, had combined annuals revenues of $17 billion at their peaks.
"We're through many cost-cutting programs, restructurings and portfolio arrangements," said Henry Gosebruch, Managing Director, Mergers & Acquisitions J.P. Morgan. "When you put that together with record levels of cash available and improving, but still moderate R&D productivity, we think there will be more big pharma M&A activity in 2013."
Arena Pharmaceuticals is focused on discovering, developing and commercializing novel drugs for weight management, cardiovascular disease, inflammation and other disorders. The company is currently seeking partners to help market their weight-loss drug Belviq in Europe and China. "Europe is not partnered yet -- we're waiting for approval,"
Jack Lief, Arena's President and CEO, said at a recent health-care conference. "We're talking to partners presently, even at this meeting."
VIVUS is developing innovative, next-generation therapies to address unmet needs in obesity, diabetes, sleep apnea and sexual health. The company recently reported that prescription shipments for Qsymia, their anti-obesity drug, totaled 12,978 for the four weeks ended Dec. 21, a monthly increase of 67 percent.
Five Star Equities provides Market Research focused on equities that offer growth opportunities, value, and strong potential return. We strive to provide the most up-to-date market activities. We constantly create research reports and newsletters for our members. Five Star Equities has not been compensated by any of the above-mentioned companies. We act as an independent research portal and are aware that all investment entails inherent risks. Please view the full disclaimer at:
www.FiveStarEquities.com/disclaimer
http://finance.yahoo.com/news/major-revenue-losses-patent-ex…
Five Star Equities Provides Stock Research on Arena Pharmaceuticals and VIVUS
NEW YORK, NY--(Marketwire - Jan 15, 2013) - Lost revenues from expiring patents has played a major part in the Biotech Industry's success in recent years. A total of 676 takeovers of biotechnology and pharmaceutical companies have occurred in the past three years, with an average premium of 38 percent, according to data collected by Bloomberg.
Five Star Equities examines the outlook for companies in the Biotech Industry and provides equity research on Arena Pharmaceuticals, Inc. ( NASDAQ : ARNA ) and VIVUS, Inc. ( NASDAQ : VVUS ).
Access to the full company reports can be found at:
www.FiveStarEquities.com/ARNA
www.FiveStarEquities.com/VVUS
At the end of the third quarter five of the biggest drug makers in the U.S. held over $70 billion in cash, near cash and short-term investments. Major revenue losses from patent expirations have forced big pharmaceutical companies to look to biotech companies to help fill the void.
Pfizer's Lipitor and Bristol-Myers' Plavix, which lost exclusivity in late 2011, had combined annuals revenues of $17 billion at their peaks.
"We're through many cost-cutting programs, restructurings and portfolio arrangements," said Henry Gosebruch, Managing Director, Mergers & Acquisitions J.P. Morgan. "When you put that together with record levels of cash available and improving, but still moderate R&D productivity, we think there will be more big pharma M&A activity in 2013."
Arena Pharmaceuticals is focused on discovering, developing and commercializing novel drugs for weight management, cardiovascular disease, inflammation and other disorders. The company is currently seeking partners to help market their weight-loss drug Belviq in Europe and China. "Europe is not partnered yet -- we're waiting for approval,"
Jack Lief, Arena's President and CEO, said at a recent health-care conference. "We're talking to partners presently, even at this meeting."
VIVUS is developing innovative, next-generation therapies to address unmet needs in obesity, diabetes, sleep apnea and sexual health. The company recently reported that prescription shipments for Qsymia, their anti-obesity drug, totaled 12,978 for the four weeks ended Dec. 21, a monthly increase of 67 percent.
Five Star Equities provides Market Research focused on equities that offer growth opportunities, value, and strong potential return. We strive to provide the most up-to-date market activities. We constantly create research reports and newsletters for our members. Five Star Equities has not been compensated by any of the above-mentioned companies. We act as an independent research portal and are aware that all investment entails inherent risks. Please view the full disclaimer at:
www.FiveStarEquities.com/disclaimer
http://finance.yahoo.com/news/major-revenue-losses-patent-ex…
mal was von "Feuerstein":
Drugs
Arena Pharma Investors: DEA, Hedge Funds Conspiring To Destroy Belviq
By Adam Feuerstein 01/15/13 - 11:13 AM EST
SAN DIEGO (TheStreet) -- Some of Arena Pharmaceuticals' (ARNA_) retail-investor shareholders accuse the U.S. Drug Enforcement Agency of conspiring with Wall Street hedge funds to delay the commercial launch of the company's weight-loss pill Belviq.
More on ARNA
The DEA is collecting public comments on its proposed rule making Belviq a Schedule IV controlled substance. The 30-day comment period ends on Jan. 18, after which DEA will finalize its ruling. After another 30-day waiting period, the rule becomes effective and Arena's marketing partner Eisai can start selling Belviq and begin to compete against Vivus' (VVUS_) Qsymia.
But this routine bureaucratic procedure is too long and unnecessary, according to some Arena supporters who believe Belviq should be made commercially available immediately. That the drug isn't yet stocked on pharmacy shelves is another sign that crooked Wall Street is trying to destroy Arena and keep obese Americans from the live-saving therapy they so desperately need.
Read through the eye-opening (and head-scratching) public comments posted on the DEA's proposed rule.
My favorite, from Roger Carter posted yesterday:
If there is one thing I'm sure of, the DEA will act at the last minute of the last day... on Belviq...The FDA and now the DEA, has killed more Americans than any Terrorist could dream about by delaying this safe and effective drug to so many Americans who need them. The problem in the US is these agencies have all been captured by Wall Street. Wall Street don't make widgets, they lie, steal and manipulate everything. They canot make the billions any other way. They have to manipulate. Does it really take 6 months to review a drug? I don't think so. Wall Street hedge funds are behind the delays in order to make millions by manipulating the stock with out fear. There is no other way there could be 56 million shorts on the company unless they have the inside scoop of the delays. So you people at the DEA have to look in the mirror and ask yourself, is it worth to delay a drug that can save lives for a few bucks in a Swiss account?...
Reza Ganjavi, echoing Carter's sentiments:
Dear DEA In reviewing the comments submitted please note that there is an interest by some hedge funds to disparage lorcasersin because they are facing a huge loss due to fighting against science and truth, and fortunately losing that fight when FDA approved the drug...
Mark Davis, who identifies himself as an Arena shareholder:
It has taken over 6 mths for the DEA to review Belviq. In the mean time, thousand have died and are dying who could have been helped with this new novel drug from Arena. I'm sure there are people at the DEA saying well, "Tough Tinsel, it's not my problem we have rules and guidelines we must follow, we are the DEA" while sitting behind there desk sipping on a 16 ounce Coke... Said to see all government agencies have been captured by Wall Street hedge funds at the expensue of Dying Americans. Yesterday was to late already.
David Kistler:
This process took six months -- time the drug could have been available to begin treating the obesity epidemic. I personally know many people who desperately need a solution to their obesity problem and are being adversely impacted by the unnecessary delays in bringing this drug to market. Although Arena Pharmaceuticals, the company that developed lorcaserin, spent over $1 billion bringing it through approval, they have been unable to sell the drug will they waited six months for this classification decision. There is no legitimate reason to classify lorcasering in any higher level of the Schedule and I suggest that any comments urging that position have probably been submitted by competitors or hedge funds either invested in competitors or short shares of Arena Pharmaceuticals....
While waiting for the final DEA scheduling to become effective, Arena and Eisai are also working through the regulatory approval process for Belviq in Europe. The European Medicine Agency's Committee for Medicinal Products for Human Use (CHMP) is meeting this week (Jan. 14-17) and will make its recommendation public on Friday, Jan. 18.
It's not known if Belviq is on this week's CHMP docket. [The committee doesn't make its monthly meeting agendas public in advance.] Arena executives have told investors to expect a European approval decision on Belviq in the first half of the year. Arena's retail investor shareholders fully expect a CHMP positive recommendation to be announced on Friday.
The recent strength in Arena shares -- up 19% since the beginning of the year -- is largely tied to anticipation of a positive European approval recommendation on Friday. Arena shares are up 5% to $10.72 in Tuesday trading.
-- Reported by Adam Feuerstein in Boston.
http://www.thestreet.com/story/11813587/1/arena-pharma-inves…
Drugs
Arena Pharma Investors: DEA, Hedge Funds Conspiring To Destroy Belviq
By Adam Feuerstein 01/15/13 - 11:13 AM EST
SAN DIEGO (TheStreet) -- Some of Arena Pharmaceuticals' (ARNA_) retail-investor shareholders accuse the U.S. Drug Enforcement Agency of conspiring with Wall Street hedge funds to delay the commercial launch of the company's weight-loss pill Belviq.
More on ARNA
The DEA is collecting public comments on its proposed rule making Belviq a Schedule IV controlled substance. The 30-day comment period ends on Jan. 18, after which DEA will finalize its ruling. After another 30-day waiting period, the rule becomes effective and Arena's marketing partner Eisai can start selling Belviq and begin to compete against Vivus' (VVUS_) Qsymia.
But this routine bureaucratic procedure is too long and unnecessary, according to some Arena supporters who believe Belviq should be made commercially available immediately. That the drug isn't yet stocked on pharmacy shelves is another sign that crooked Wall Street is trying to destroy Arena and keep obese Americans from the live-saving therapy they so desperately need.
Read through the eye-opening (and head-scratching) public comments posted on the DEA's proposed rule.
My favorite, from Roger Carter posted yesterday:
If there is one thing I'm sure of, the DEA will act at the last minute of the last day... on Belviq...The FDA and now the DEA, has killed more Americans than any Terrorist could dream about by delaying this safe and effective drug to so many Americans who need them. The problem in the US is these agencies have all been captured by Wall Street. Wall Street don't make widgets, they lie, steal and manipulate everything. They canot make the billions any other way. They have to manipulate. Does it really take 6 months to review a drug? I don't think so. Wall Street hedge funds are behind the delays in order to make millions by manipulating the stock with out fear. There is no other way there could be 56 million shorts on the company unless they have the inside scoop of the delays. So you people at the DEA have to look in the mirror and ask yourself, is it worth to delay a drug that can save lives for a few bucks in a Swiss account?...
Reza Ganjavi, echoing Carter's sentiments:
Dear DEA In reviewing the comments submitted please note that there is an interest by some hedge funds to disparage lorcasersin because they are facing a huge loss due to fighting against science and truth, and fortunately losing that fight when FDA approved the drug...
Mark Davis, who identifies himself as an Arena shareholder:
It has taken over 6 mths for the DEA to review Belviq. In the mean time, thousand have died and are dying who could have been helped with this new novel drug from Arena. I'm sure there are people at the DEA saying well, "Tough Tinsel, it's not my problem we have rules and guidelines we must follow, we are the DEA" while sitting behind there desk sipping on a 16 ounce Coke... Said to see all government agencies have been captured by Wall Street hedge funds at the expensue of Dying Americans. Yesterday was to late already.
David Kistler:
This process took six months -- time the drug could have been available to begin treating the obesity epidemic. I personally know many people who desperately need a solution to their obesity problem and are being adversely impacted by the unnecessary delays in bringing this drug to market. Although Arena Pharmaceuticals, the company that developed lorcaserin, spent over $1 billion bringing it through approval, they have been unable to sell the drug will they waited six months for this classification decision. There is no legitimate reason to classify lorcasering in any higher level of the Schedule and I suggest that any comments urging that position have probably been submitted by competitors or hedge funds either invested in competitors or short shares of Arena Pharmaceuticals....
While waiting for the final DEA scheduling to become effective, Arena and Eisai are also working through the regulatory approval process for Belviq in Europe. The European Medicine Agency's Committee for Medicinal Products for Human Use (CHMP) is meeting this week (Jan. 14-17) and will make its recommendation public on Friday, Jan. 18.
It's not known if Belviq is on this week's CHMP docket. [The committee doesn't make its monthly meeting agendas public in advance.] Arena executives have told investors to expect a European approval decision on Belviq in the first half of the year. Arena's retail investor shareholders fully expect a CHMP positive recommendation to be announced on Friday.
The recent strength in Arena shares -- up 19% since the beginning of the year -- is largely tied to anticipation of a positive European approval recommendation on Friday. Arena shares are up 5% to $10.72 in Tuesday trading.
-- Reported by Adam Feuerstein in Boston.
http://www.thestreet.com/story/11813587/1/arena-pharma-inves…
Auszug aus:
Biotech
Biotech Stock Mailbag: Peregrine, Arena, Dendreon, NPS Pharma
By Adam Feuerstein 01/18/13 - 08:18 AM EST
...
Mark emails: "Adam, I have been following your comments on Arena Pharmaceuticals (ARNA_) and Vivus (VVUS_) for five months now. Don't you believe it's time to throw in the towel and admit you are wrong? Vivus has lost 40 to 50% of its value, Arena is up 25%-30%. Any hedge fund owning Vivus presents overhead resistance, not support. Although both companies are well capitalized, Vivus is much more at risk than Arena. Arena's partner is financing 90% of their expansion. Arena is in the enviable position of deciding whether to bring eventual European acceptance of their drug to market by themselves or use their money to invest in a secondary biotech."
I'm not a Vivus bull. Arena fans thinking otherwise are mistaken. I was out front in predicting a disappointing Qsymia launch and warned Street estimates were too high, before the stock sold off.
Mark's correct about Arena shares rising in value as we get closer to Belviq's projected first-quarter/second-quarter U.S. commercial launch. To me, Arena's $2.4 billion enterprise value makes me more bearish, not less. I agree with the fundamental bear thesis on the Belviq launch laid out by my former contributor Nate Sadeghi. If anything, Sadeghi's call was too early. I'm looking forward to Belviq hitting the market because all the talk will end and we'll finally start seeing disappointing prescriptions and sales.
Arena at $10 plus change is over-valued. Let's look at the numbers:
Using a generous six-times multiple for Belviq, Arena's current $2.4 billion enterprise value implies $400 million in royalty revenue. Arena gets roughly one-third of Belviq's economics, which again, implies $1.3 billion in Belviq sales already baked into Arena's market value.
If Belviq costs $2,500 per year, Arena and Eisai need 520,000 patients on the weight loss drug for a full year in order to generate $1.3 billion in sales. More than half of Belviq patients in the clinical trials failed to lose a minimum 5% of their baseline weight after 12 weeks, so assuming patients will stay on the drug for a year in the real world is a pipe dream. If patients stay on the drug for six months, Arena and Eisai need 1 million patients just to generate the Belviq sales already baked into Arena's valuation.
That's not going to happen.
One more note: The European Medicines Agency did not take any action on Belviq during its January CHMP meeting, which just wrapped yesterday. This is bound to disappoint Arena retail investor supporters convinced European regulators were going to approve Belviq this month. I believe Europe will reject Belviq. The next CHMP meeting takes place in February 18-21.
http://www.thestreet.com/story/11816254/3/biotech-stock-mail…
Biotech
Biotech Stock Mailbag: Peregrine, Arena, Dendreon, NPS Pharma
By Adam Feuerstein 01/18/13 - 08:18 AM EST
...
Mark emails: "Adam, I have been following your comments on Arena Pharmaceuticals (ARNA_) and Vivus (VVUS_) for five months now. Don't you believe it's time to throw in the towel and admit you are wrong? Vivus has lost 40 to 50% of its value, Arena is up 25%-30%. Any hedge fund owning Vivus presents overhead resistance, not support. Although both companies are well capitalized, Vivus is much more at risk than Arena. Arena's partner is financing 90% of their expansion. Arena is in the enviable position of deciding whether to bring eventual European acceptance of their drug to market by themselves or use their money to invest in a secondary biotech."
I'm not a Vivus bull. Arena fans thinking otherwise are mistaken. I was out front in predicting a disappointing Qsymia launch and warned Street estimates were too high, before the stock sold off.
Mark's correct about Arena shares rising in value as we get closer to Belviq's projected first-quarter/second-quarter U.S. commercial launch. To me, Arena's $2.4 billion enterprise value makes me more bearish, not less. I agree with the fundamental bear thesis on the Belviq launch laid out by my former contributor Nate Sadeghi. If anything, Sadeghi's call was too early. I'm looking forward to Belviq hitting the market because all the talk will end and we'll finally start seeing disappointing prescriptions and sales.
Arena at $10 plus change is over-valued. Let's look at the numbers:
Using a generous six-times multiple for Belviq, Arena's current $2.4 billion enterprise value implies $400 million in royalty revenue. Arena gets roughly one-third of Belviq's economics, which again, implies $1.3 billion in Belviq sales already baked into Arena's market value.
If Belviq costs $2,500 per year, Arena and Eisai need 520,000 patients on the weight loss drug for a full year in order to generate $1.3 billion in sales. More than half of Belviq patients in the clinical trials failed to lose a minimum 5% of their baseline weight after 12 weeks, so assuming patients will stay on the drug for a year in the real world is a pipe dream. If patients stay on the drug for six months, Arena and Eisai need 1 million patients just to generate the Belviq sales already baked into Arena's valuation.
That's not going to happen.
One more note: The European Medicines Agency did not take any action on Belviq during its January CHMP meeting, which just wrapped yesterday. This is bound to disappoint Arena retail investor supporters convinced European regulators were going to approve Belviq this month. I believe Europe will reject Belviq. The next CHMP meeting takes place in February 18-21.
http://www.thestreet.com/story/11816254/3/biotech-stock-mail…
Antwort auf Beitrag Nr.: 44.037.700 von Poppholz am 18.01.13 15:01:40Drecks Basher, er glaubte schon bei ca. 1 US Dollar , daß Locaserin, das jetzige Belviq, nicht von der FDA zugelassen wird, wurde aber und wurde ein Tenbagger!
J.Lief der CEO von Arena hat beim letzten CC Anfang Januar von einer Zulassung im ersten Halbjahr 2013 gesprochen und nicht von Anfang 2013, Mensch sind die Leute blöd!
J.Lief der CEO von Arena hat beim letzten CC Anfang Januar von einer Zulassung im ersten Halbjahr 2013 gesprochen und nicht von Anfang 2013, Mensch sind die Leute blöd!

Zitat von Poppholz:
Biotech
Biotech Stock Mailbag: Arena,
"Adam, I have been following your comments on Arena Pharmaceuticals (ARNA_)
Don't you believe it's time to throw in the towel and admit you are wrong?

ADAM FUCKELSTONE
Arena at $10 plus change .....is over-valued.....wie so viele Aktien..
The European Medicines Agency did not take any action on Belviq during its January CHMP meeting, which just wrapped yesterday. ........gut erkannt, Adam Fuckelstone..
This is bound to disappoint Arena retail investor supporters convinced European regulators were going to approve Belviq this month. ......der Termin ist nur verschoben worden, mehr nicht.....
I believe Europe will reject Belviq......YEPP, genauso wie in den USA.....
The next CHMP meeting takes place in February 18-21.....bloody well right, Mr. Fuckelstone...
Hi good old „AREANICS” > be patient !!!
Am Freitag ist Arena wieder down gegangen nachdem am Freitag die erhofften News aus Europa nicht gekommen sind.OK, es ist immer frustierend so einen Knick nach unten mitzubekommen aber da müssen wir durch.
Und wer als „Biotechanleger“ aktiv ist, der kennt solch heftige Kurssprünge, sowohl nach oben als auch nach unten.
Fundamental hat sich aktuell überhaupt nichts geändert, A:F und Co. nutzen solche Situationen immer wieder aus, um negative Stimmungen gegen ARNA zu verbreiten – das hat Methode und das kennen wir nicht nur bei Arena !!!
Es gibt viele Anleger, die schon seit 1,05 $ , seit 2 $ , seit 4 $ etc. dabei sind, also „ longiemäßig“ gesehen auf einem sehr guten Kurs liegen.
Auf noch weitere längere Sicht gesehen ( ein späteres EU-Approval vorausgesetzt) ist es völlig unerheblich ob wir von der CHMP im Februar oder sogar erst im März 2013 hören.
Zur „ Aufmunterung“
„ Aufmunterung“  möchte ich euch nochmals „ kurz- bzw. langfristige BELVIQ-Burner“ auflisten, die ARENA nach oben katapultieren werden.
möchte ich euch nochmals „ kurz- bzw. langfristige BELVIQ-Burner“ auflisten, die ARENA nach oben katapultieren werden. 
- DEA schedule IV announcement any day now
- $65,000,000 milestone payment
- U.S Launch
- First and second quarter , Belviq U.S. sales and marketing
- EU Belviq approval first half 2013
- Continued institution and fund ownership growth
- EU Partnership
- Additional Rest of World Partnerships
- Quarterly sales results
- EU Launch
- Approval in Switzerland, Canada, Mexico, Brazil and Korea
- Belviq-Phentermine combination drug
- Belviq for treating addiction (such as smoking cessation support)
- Belviq-Metformin combination drug (Diabetes is a huge market by itself)
Darüber hinaus möchte ich euch auch nochmals an die möglichen TOP-Burner der klinischen Pipeline erinnern.
- Advancing APD811 towards phase IIb (PAH treatment)
- Starting clinical trials with at-least one more promising NCE
- Potential Buy-out by Big Pharmaceutical before Belviq becomes a success
- APD 811 for the treament of pulmonary arterial hypertension
- APD 334 for the treatment of multiple sclerosis and rheumatoid arthritis
- APD 371 for the treatment of pain
- GPR 119 for the treatment of diabetes Typ 2
- TEMANOGREL for the treatment of arterial thrombobis
Uns allen " Good Luck" -
Grüße
bernie55
Am Freitag ist Arena wieder down gegangen nachdem am Freitag die erhofften News aus Europa nicht gekommen sind.OK, es ist immer frustierend so einen Knick nach unten mitzubekommen aber da müssen wir durch.
Und wer als „Biotechanleger“ aktiv ist, der kennt solch heftige Kurssprünge, sowohl nach oben als auch nach unten.
Fundamental hat sich aktuell überhaupt nichts geändert, A:F und Co. nutzen solche Situationen immer wieder aus, um negative Stimmungen gegen ARNA zu verbreiten – das hat Methode und das kennen wir nicht nur bei Arena !!!
Es gibt viele Anleger, die schon seit 1,05 $ , seit 2 $ , seit 4 $ etc. dabei sind, also „ longiemäßig“ gesehen auf einem sehr guten Kurs liegen.
Auf noch weitere längere Sicht gesehen ( ein späteres EU-Approval vorausgesetzt) ist es völlig unerheblich ob wir von der CHMP im Februar oder sogar erst im März 2013 hören.
Zur
 „ Aufmunterung“
„ Aufmunterung“  möchte ich euch nochmals „ kurz- bzw. langfristige BELVIQ-Burner“ auflisten, die ARENA nach oben katapultieren werden.
möchte ich euch nochmals „ kurz- bzw. langfristige BELVIQ-Burner“ auflisten, die ARENA nach oben katapultieren werden. 
- DEA schedule IV announcement any day now
- $65,000,000 milestone payment
- U.S Launch
- First and second quarter , Belviq U.S. sales and marketing
- EU Belviq approval first half 2013
- Continued institution and fund ownership growth
- EU Partnership
- Additional Rest of World Partnerships
- Quarterly sales results
- EU Launch
- Approval in Switzerland, Canada, Mexico, Brazil and Korea
- Belviq-Phentermine combination drug
- Belviq for treating addiction (such as smoking cessation support)
- Belviq-Metformin combination drug (Diabetes is a huge market by itself)
Darüber hinaus möchte ich euch auch nochmals an die möglichen TOP-Burner der klinischen Pipeline erinnern.

- Advancing APD811 towards phase IIb (PAH treatment)
- Starting clinical trials with at-least one more promising NCE
- Potential Buy-out by Big Pharmaceutical before Belviq becomes a success
- APD 811 for the treament of pulmonary arterial hypertension
- APD 334 for the treatment of multiple sclerosis and rheumatoid arthritis
- APD 371 for the treatment of pain
- GPR 119 for the treatment of diabetes Typ 2
- TEMANOGREL for the treatment of arterial thrombobis
Uns allen " Good Luck" -
Grüße
bernie55

...mal was " Reißerisches " von der PRO-Seite 
Ventures Sierra World Equity Review
HEADLINE:
Be Prepared To Be Shocked Shorts_Arena Pharmaceuticals (ARNA) To Release Very Good News This Week.
Still one of my favorite stocks I have noticed some unusual activity that leads me to believe ARNA shareholders are in for a very big week! Remember when it happens I told you so!
http://sierraworldequityreview.blogspot.de/2013/01/be-prepar…" target="_blank" rel="nofollow ugc noopener">
http://sierraworldequityreview.blogspot.de/2013/01/be-prepar…
..würde mich natürlich freuen wenn diese Woche eine Knaller-News kommt....
...würde im übrigen die kommende "russische Kaltfront" > Russenpeitsche < temperaturmäßig dann voll ausgleichen...

Ventures Sierra World Equity Review
HEADLINE:
Be Prepared To Be Shocked Shorts_Arena Pharmaceuticals (ARNA) To Release Very Good News This Week.
Still one of my favorite stocks I have noticed some unusual activity that leads me to believe ARNA shareholders are in for a very big week! Remember when it happens I told you so!
http://sierraworldequityreview.blogspot.de/2013/01/be-prepar…" target="_blank" rel="nofollow ugc noopener">
http://sierraworldequityreview.blogspot.de/2013/01/be-prepar…
..würde mich natürlich freuen wenn diese Woche eine Knaller-News kommt....

...würde im übrigen die kommende "russische Kaltfront" > Russenpeitsche < temperaturmäßig dann voll ausgleichen...

Warum es grad um 7% abwärts geht (etvl. noch weiter):
Arena Pharma (ARNA) Receives Day 180 from CHMP for Belviq
January 22, 2013 8:04 AM EST
In March 2012, Arena Pharmaceuticals (Nasdaq: ARNA) filed a Marketing Authorization Application, or MAA, through the Centralized Procedure with the European Medicines Agency, or EMA, for the marketing approval of BELVIQ (lorcaserin HCl) in the European Union, or EU. The proposed indication for BELVIQ in the EU is as an adjunct to diet and exercise for weight control in patients over the age of 18 years old who are obese (BMI ³ 30 kg/m2), or are overweight (BMI>27 kg/m2) with associated risk factor(s), such as hypertension, dyslipidemia, cardiovascular disease, type 2 diabetes, or sleep apnea.
Arena commented:
We have received the Day 180 List of Outstanding Issues from the EMA’s Committee for Medicinal Products for Human Use, or CHMP. The issues will need to be addressed before the CHMP can recommend BELVIQ for marketing approval in the EU. The major objections relate to previously identified non-clinical and clinical issues, including tumors in rats, valvulopathy and psychiatric events, and the CHMP requests that we further justify BELVIQ’s overall benefit-risk balance taking these issues into consideration.
In accordance with the CHMP’s process, we have been asked to address the issues in writing. The CHMP also plans to consult with independent experts who will provide recommendations on the outstanding issues. In addition to the written response, we have been invited by the CHMP to provide an oral explanation.
The CHMP is expected to reach its final opinion on the BELVIQ MAA by Day 210, which we continue to expect in the first half of 2013.
http://www.streetinsider.com/Corporate+News/Arena+Pharma+%28…
Arena Pharma (ARNA) Receives Day 180 from CHMP for Belviq
January 22, 2013 8:04 AM EST
In March 2012, Arena Pharmaceuticals (Nasdaq: ARNA) filed a Marketing Authorization Application, or MAA, through the Centralized Procedure with the European Medicines Agency, or EMA, for the marketing approval of BELVIQ (lorcaserin HCl) in the European Union, or EU. The proposed indication for BELVIQ in the EU is as an adjunct to diet and exercise for weight control in patients over the age of 18 years old who are obese (BMI ³ 30 kg/m2), or are overweight (BMI>27 kg/m2) with associated risk factor(s), such as hypertension, dyslipidemia, cardiovascular disease, type 2 diabetes, or sleep apnea.
Arena commented:
We have received the Day 180 List of Outstanding Issues from the EMA’s Committee for Medicinal Products for Human Use, or CHMP. The issues will need to be addressed before the CHMP can recommend BELVIQ for marketing approval in the EU. The major objections relate to previously identified non-clinical and clinical issues, including tumors in rats, valvulopathy and psychiatric events, and the CHMP requests that we further justify BELVIQ’s overall benefit-risk balance taking these issues into consideration.
In accordance with the CHMP’s process, we have been asked to address the issues in writing. The CHMP also plans to consult with independent experts who will provide recommendations on the outstanding issues. In addition to the written response, we have been invited by the CHMP to provide an oral explanation.
The CHMP is expected to reach its final opinion on the BELVIQ MAA by Day 210, which we continue to expect in the first half of 2013.
http://www.streetinsider.com/Corporate+News/Arena+Pharma+%28…
Zitat von me_2: Warum es grad um 7% abwärts geht (etvl. noch weiter):
Arena Pharma (ARNA) Receives Day 180 from CHMP for Belviq
January 22, 2013 8:04 AM EST
Arena commented:
We have received the Day 180 List of Outstanding Issues from the EMA’s Committee for Medicinal Products for Human Use, or CHMP. The issues will need to be addressed before the CHMP can recommend BELVIQ for marketing approval in the EU. The major objections relate to previously identified non-clinical and clinical issues, including tumors in rats, valvulopathy and psychiatric events, and the CHMP requests that we further justify BELVIQ’s overall benefit-risk balance taking these issues into consideration.
In accordance with the CHMP’s process, we have been asked to address the issues in writing. The CHMP also plans to consult with independent experts who will provide recommendations on the outstanding issues. In addition to the written response, we have been invited by the CHMP to provide an oral explanation.
The CHMP is expected to reach its final opinion on the BELVIQ MAA by Day 210, which we continue to expect in the first half of 2013.
http://www.streetinsider.com/Corporate+News/Arena+Pharma+%28…
..also jetzt ist das " DAY 180" leider doch noch gekommen...
.. so sei es drum...
...an dieser Stelle sei gesagt, dass die geforderten Fragen keine Ablehnung bzgl. eines Approvals bedeuten......die Erfolgs-Chancen hinsichtlich eines Approvals sind prozentual gesunken und sind aktuell wohl nicht mehr als ein " Slam dunk" anzusehen..
..so sei`s drum
Die Entscheidung über ein Approval von Belviq wird sich zeitlich somit nach hinten verschieben....in the first half of 2013
...sicherlich kommen durch zusätzliche wissenschaftliche Anliegen ( issues in writing, oral hearing, consulting independant experts) Unsicherheiten in das Spiel, die auf dem Markt "hart" bestraft werden und von Cramer, A. Fuckelstone und Konsorte rigoros ausgenutzt werden.
Ich muss sagen, das nervt mich langsam immer mehr, dass solche Ur-Gestalten ihr Unwesen am Markt treiben dürfen...
Wenn ich mir Kursentwicklung von ARNA seit April/Mai 2012 anschaue , bin ich mit dem Kursverlauf sehr zufrieden. Das was für mich zählt ist , dass ich weiß, dass ARNA als Biotechpharma-Unternehmen langfristig eine gewichtige Rolle spielen wird.
Wenn ich mir die aktuelle " bürokratische" Gurkerei in den USA anschaue, dann kann ich wirklich nur noch mit dem Kopf schütteln - seit Approval durch die FDA im Juni 2012 sind mittlerweile 7 Monate vergangen und BELVIQ ist immer noch nicht auf dem Markt.Und da steckt wohl leider auch Methode drin !!!
Lasst uns nach vorne schauen

Grüße
bernie55

Antwort auf Beitrag Nr.: 44.050.313 von bernie55 am 22.01.13 18:24:38Ja wir dachten das würde einfach von der EMA durchgewunken.
Jetzt bin ich nicht mehr so optimistisch. Die Europäer sind bei Herzklappenerkrankungen wohl kritischer als die FDA (memento Mediator !!), viell. müssen noch Langzeit-Safety -Daten (über 24 Mo. hinaus ) dazu geliefert werden.
Bin erstmal ausgestiegen ...
Jetzt bin ich nicht mehr so optimistisch. Die Europäer sind bei Herzklappenerkrankungen wohl kritischer als die FDA (memento Mediator !!), viell. müssen noch Langzeit-Safety -Daten (über 24 Mo. hinaus ) dazu geliefert werden.
Bin erstmal ausgestiegen ...
Antwort auf Beitrag Nr.: 44.050.313 von bernie55 am 22.01.13 18:24:38Hallo Bernie55
ich bin gestern erst mal raus. Wollte eigentlich schon Freitag raus - aber eben die Hoffnung...
Wer sagt denn dass die beiden sich nicht abgesprochen haben; einer schreibt Positiv, der andere Negativ .. also trauen tu ich schon lange keinem mehr. Da nun alles verlängert wurde, bis zur eventuellen Zulassung, da kann es noch sehr sehr böse werden. So wie es heute aussieht, wird wahrscheinlich auf Tagestief geschlossen. Dann knallt es morgen noch weiter runter, denn die ganzen zusätzlichen Aufgaben kosten wieder eine Menge Geld, um die Auflagen zu erfüllen. Und gegen die Pharma-Mafia ( egal ob in EU oder USA ) keiner sowieso nichts machen und wenn die sich auf ein Produkt negativ eingeschossen haben, dann gehen die auch nicht so schnell zurück davon.
Ich habe zwar nicht viel Ahnung von Charttechnik .. aber meiner Meinung nach geht es hier noch bis ca. 5 USD runter oder weiter, pendelt dann ewig lang auf der Stelle. Denn, Fred Feuerstein und Konsorten wollen das Ding runter haben und wie man sieht, hören viele darauf und schmeissen alles auf den Markt.
Ist nur meine persönliche Meinung. Ich würde mich auch freuen, wenn es anders herum kommt und ihr weiter gute Gewinne macht. Und ich glaube immer noch an eine Absprache der beiden Typen.....
ich bin gestern erst mal raus. Wollte eigentlich schon Freitag raus - aber eben die Hoffnung...
Wer sagt denn dass die beiden sich nicht abgesprochen haben; einer schreibt Positiv, der andere Negativ .. also trauen tu ich schon lange keinem mehr. Da nun alles verlängert wurde, bis zur eventuellen Zulassung, da kann es noch sehr sehr böse werden. So wie es heute aussieht, wird wahrscheinlich auf Tagestief geschlossen. Dann knallt es morgen noch weiter runter, denn die ganzen zusätzlichen Aufgaben kosten wieder eine Menge Geld, um die Auflagen zu erfüllen. Und gegen die Pharma-Mafia ( egal ob in EU oder USA ) keiner sowieso nichts machen und wenn die sich auf ein Produkt negativ eingeschossen haben, dann gehen die auch nicht so schnell zurück davon.
Ich habe zwar nicht viel Ahnung von Charttechnik .. aber meiner Meinung nach geht es hier noch bis ca. 5 USD runter oder weiter, pendelt dann ewig lang auf der Stelle. Denn, Fred Feuerstein und Konsorten wollen das Ding runter haben und wie man sieht, hören viele darauf und schmeissen alles auf den Markt.
Ist nur meine persönliche Meinung. Ich würde mich auch freuen, wenn es anders herum kommt und ihr weiter gute Gewinne macht. Und ich glaube immer noch an eine Absprache der beiden Typen.....
me_2 :Bin erstmal ausgestiegen ...
philipini: ich bin gestern erst mal raus.......
Hi ihr 2,
zum einen weiß ich nicht , mit welchem EK ihr bei ARNA eingestiegen seid ,zum anderen weiß ich nicht mit welcher Grundeinstellung ihr in Biotechaktien investiert seid.( Kurz-Trading" oder "Longie-Investment" ).
Ich habe schon sehr oft dazu Stellung bezogen und denke, dass die eigene Einstellung zu Investments das eigene Handeln bestimmt.
Und wenn ihr das Gefühl habt , auszusteigen, dann tut es.
Ich bin in ARNA in der Zeit vom 20.04 - 27.04 und dann nochmals im Mai richtig dick eingestiegen – ich setze dabei auf " längerfristig" und das hat seinen Grund....
> Ich setzte mein Geld erst nach intensiven Recherchen eines BIOTECH-Unternehmens ein.
> Ich investiere mein Geld in ein BIOTECH-Unternehmen , von dessen Produkt ich überzeugt bin und wovon ich denke, dass ein Approval Erfolg haben könnte.
Dabei liegt der Zeitpunkt des Einstiegs teilweise schon vor dem eigentlichen RUN, wenn alles so kommt wie man es sich erhofft.
Ich sehe mein LONGINVESTMENT auf eine Sicht von 1-4 Jahren. Auch wenn " Störfeuer" seitens Hedgies und CO. wirklich sowas von nerven, so bin ich mittlerweile doch der Auffassung, dass bei einem guten Investment längerfristig gesehen , die dicke Belohnung kommen wird.
Auch wenn " Störfeuer" seitens Hedgies und CO. wirklich sowas von nerven, so bin ich mittlerweile doch der Auffassung, dass bei einem guten Investment längerfristig gesehen , die dicke Belohnung kommen wird.
Bei sehr guter Performance werden natürlich auch schon Zwischenverkäufe getätigt...
Ich versuche mit einem Investment mindestens mehr als 100 % Gewinn zu machen, das ist bei Biotechaktien gar nicht so abwegig, vor allem dann, wenn man bereits vor dem RUN dabei ist.
Hat natürlich nicht bei allen Biotech-Aktien geklappt aber im Großen und Ganzen bin ich mehr als zufrieden.
aber im Großen und Ganzen bin ich mehr als zufrieden.
-------------------------------------------------------------------------------------------------
So, jetzt und stehen als nächstes die Einführung und der Verkauf von Belviq in den USA an.
EISAI steht quasi schon in den Startlöchern, um dann im Februar endlich mit dem Verkauf zu beginnen.
Allen Biotechlern weiterhin " GOOD LUCK"
bernie55
philipini: ich bin gestern erst mal raus.......
Hi ihr 2,

zum einen weiß ich nicht , mit welchem EK ihr bei ARNA eingestiegen seid ,zum anderen weiß ich nicht mit welcher Grundeinstellung ihr in Biotechaktien investiert seid.( Kurz-Trading" oder "Longie-Investment" ).
Ich habe schon sehr oft dazu Stellung bezogen und denke, dass die eigene Einstellung zu Investments das eigene Handeln bestimmt.
Und wenn ihr das Gefühl habt , auszusteigen, dann tut es.

Ich bin in ARNA in der Zeit vom 20.04 - 27.04 und dann nochmals im Mai richtig dick eingestiegen – ich setze dabei auf " längerfristig" und das hat seinen Grund....
> Ich setzte mein Geld erst nach intensiven Recherchen eines BIOTECH-Unternehmens ein.
> Ich investiere mein Geld in ein BIOTECH-Unternehmen , von dessen Produkt ich überzeugt bin und wovon ich denke, dass ein Approval Erfolg haben könnte.
Dabei liegt der Zeitpunkt des Einstiegs teilweise schon vor dem eigentlichen RUN, wenn alles so kommt wie man es sich erhofft.

Ich sehe mein LONGINVESTMENT auf eine Sicht von 1-4 Jahren.
 Auch wenn " Störfeuer" seitens Hedgies und CO. wirklich sowas von nerven, so bin ich mittlerweile doch der Auffassung, dass bei einem guten Investment längerfristig gesehen , die dicke Belohnung kommen wird.
Auch wenn " Störfeuer" seitens Hedgies und CO. wirklich sowas von nerven, so bin ich mittlerweile doch der Auffassung, dass bei einem guten Investment längerfristig gesehen , die dicke Belohnung kommen wird.Bei sehr guter Performance werden natürlich auch schon Zwischenverkäufe getätigt...

Ich versuche mit einem Investment mindestens mehr als 100 % Gewinn zu machen, das ist bei Biotechaktien gar nicht so abwegig, vor allem dann, wenn man bereits vor dem RUN dabei ist.

Hat natürlich nicht bei allen Biotech-Aktien geklappt
 aber im Großen und Ganzen bin ich mehr als zufrieden.
aber im Großen und Ganzen bin ich mehr als zufrieden.
-------------------------------------------------------------------------------------------------
So, jetzt und stehen als nächstes die Einführung und der Verkauf von Belviq in den USA an.
EISAI steht quasi schon in den Startlöchern, um dann im Februar endlich mit dem Verkauf zu beginnen.
Allen Biotechlern weiterhin " GOOD LUCK"
bernie55

Antwort auf Beitrag Nr.: 44.054.825 von bernie55 am 23.01.13 16:57:52ich sehe die aktuellen Kurse auch eher als "Nachkaufmöglichkeit".
Der Kurs ist gestern (Mittwoch) schon sehr stabil seitwärts gelaufen. Mal sehen ob er heute und morgen noch weiter nach unten geht oder ob der Boden schon gefunden ist.
Charttechnisch sind die $ 5,00 nicht drin, da müßte die USA schon die Zulassung entziehen.

Der Kurs ist gestern (Mittwoch) schon sehr stabil seitwärts gelaufen. Mal sehen ob er heute und morgen noch weiter nach unten geht oder ob der Boden schon gefunden ist.
Charttechnisch sind die $ 5,00 nicht drin, da müßte die USA schon die Zulassung entziehen.

Antwort auf Beitrag Nr.: 44.057.238 von Poppholz am 24.01.13 09:09:10Wo der genaue Nachkaufkurs sein wird weiß keiner von uns, daß aber die Adipositas = Fettsucht ein globales Megaproblem ist weiß jeder!
Arena hat die Zulassung im "fettem Amiland", einen super Partner mit Esai der die Kosten für die Trials trägt, eine steuerfreie Herstellung in der Schweiz.... Korea-Partner, Brasilien, Mexico.....
Und ganz nebenbei Belviq wirkt gegen Diabetis, Bluthochdruck, Cholesterin...
Was kann man bei den Fakten falsch machen:
http://us.rd.yahoo.com/finance/external/pssa/SIG=1262mm22l;_…
Arena hat die Zulassung im "fettem Amiland", einen super Partner mit Esai der die Kosten für die Trials trägt, eine steuerfreie Herstellung in der Schweiz.... Korea-Partner, Brasilien, Mexico.....
Und ganz nebenbei Belviq wirkt gegen Diabetis, Bluthochdruck, Cholesterin...
Was kann man bei den Fakten falsch machen:
http://us.rd.yahoo.com/finance/external/pssa/SIG=1262mm22l;_…
Antwort auf Beitrag Nr.: 44.054.825 von bernie55 am 23.01.13 16:57:52Hallo Bernie,
deine Überlegungen kann ich gut nachvollziehen.
Aber jeder agiert doch aufgrund seiner eigenen Einstellung, Philosophie, Cashsituation, Erfahrungen, Information etcetc.
Für mich waren die Einwände des CHMP unerwartet und ich sehe, wie Philipini schon angemerkt hat, drohende Verluste an Zeit und Resourcen. (siehe Mediator-skandal in Frankreich/Spanien ein, beide Substanzen wirken am selben Rezeptor)
Ich persönlich definiere mich nicht in deisen Kategorien "Long" oder "Trader", ich reagiere oft intuitiv (Intuition=kondensierte unbewußte Erfahrung). Manche Invests bleiben 10 Jahre im Depot, manche nur 1 Woche ...
Arena ist für mich langfristig aussichtsreich, aber kurzfristig bin ich vorsichtig. Vielleicht gibt es ja die Möglichkeit, evtl. 15-20% günstiger wieder einzusteigen, wenn es der weitere Newsflow und Kursverlauf hergibt.
So "long"
me_2
deine Überlegungen kann ich gut nachvollziehen.
Aber jeder agiert doch aufgrund seiner eigenen Einstellung, Philosophie, Cashsituation, Erfahrungen, Information etcetc.
Für mich waren die Einwände des CHMP unerwartet und ich sehe, wie Philipini schon angemerkt hat, drohende Verluste an Zeit und Resourcen. (siehe Mediator-skandal in Frankreich/Spanien ein, beide Substanzen wirken am selben Rezeptor)
Ich persönlich definiere mich nicht in deisen Kategorien "Long" oder "Trader", ich reagiere oft intuitiv (Intuition=kondensierte unbewußte Erfahrung). Manche Invests bleiben 10 Jahre im Depot, manche nur 1 Woche ...
Arena ist für mich langfristig aussichtsreich, aber kurzfristig bin ich vorsichtig. Vielleicht gibt es ja die Möglichkeit, evtl. 15-20% günstiger wieder einzusteigen, wenn es der weitere Newsflow und Kursverlauf hergibt.
So "long"
me_2
Zitat von me_2: Hallo Bernie,
deine Überlegungen kann ich gut nachvollziehen.
Aber jeder agiert doch aufgrund seiner eigenen Einstellung, Philosophie, Cashsituation, Erfahrungen, Information etcetc...YEPP
Ich persönlich definiere mich nicht in deisen Kategorien "Long" oder "Trader", ich reagiere oft intuitiv (Intuition=kondensierte unbewußte Erfahrung).....wenn du damit an der Börse Erfolg hast, PRIMA .....
Manche Invests bleiben 10 Jahreim Depot manche nur 1 Woche ...also, dann bist du doch sehr flexibel..
...im übrigen gibt es neben meinen BIOTECH-Longinvestments auch manchmal Aktien , die ich " tradermäßig" betrachte .... .
Arena ist für mich langfristig aussichtsreich.....YEPP , für mich auch......wer hätte das gedacht....
...aber kurzfristig bin ich vorsichtig. Vielleicht gibt es ja die Möglichkeit, evtl. 15-20% günstiger wieder einzusteigen, wenn es der weitere Newsflow und Kursverlauf hergibt..
....nicht,dass ich dir diesen Einstieg nicht gönne, aber das will ich nicht hoffen
So "long"
me_2
Grüße und weiterhin " good hunting"
bernie55
Zitat von bernie55:Zitat von me_2: Hallo Bernie,
deine Überlegungen kann ich gut nachvollziehen.
Aber jeder agiert doch aufgrund seiner eigenen Einstellung, Philosophie, Cashsituation, Erfahrungen, Information etcetc...YEPP
Ich persönlich definiere mich nicht in deisen Kategorien "Long" oder "Trader", ich reagiere oft intuitiv (Intuition=kondensierte unbewußte Erfahrung).....wenn du damit an der Börse Erfolg hast, PRIMA .....
Manche Invests bleiben 10 Jahreim Depot manche nur 1 Woche ...also, dann bist du doch sehr flexibel..
...im übrigen gibt es neben meinen BIOTECH-Longinvestments auch manchmal Aktien , die ich " tradermäßig" betrachte .... .
Arena ist für mich langfristig aussichtsreich.....YEPP , für mich auch......wer hätte das gedacht....
...aber kurzfristig bin ich vorsichtig. Vielleicht gibt es ja die Möglichkeit, evtl. 15-20% günstiger wieder einzusteigen, wenn es der weitere Newsflow und Kursverlauf hergibt..
....nicht,dass ich dir diesen Einstieg nicht gönne, aber das will ich nicht hoffen
So "long"
me_2
Grüße und weiterhin " good hunting"
bernie55
Antwort auf Beitrag Nr.: 44.058.623 von me_2 am 24.01.13 12:51:36Das war ja Blödsinn, wollte ich gar nicht, lapsus technicus
Antwort auf Beitrag Nr.: 44.058.630 von me_2 am 24.01.13 12:52:53http://us.rd.yahoo.com/finance/external/pssa/SIG=12crp0eu8/*…
!
Dieser Beitrag wurde von m.klemm moderiert. Grund: verstößt gegen Urheberrechte
Interessante EMA Statistik von 2012:
Die EMA hat bei insgesamt 27 " drugs" eine "List of Outstanding Issues" ( LOOI)= offenstehende Diskussionspunkte herausgearbeitet.
Von diesen "drugs" haben am Ende 25, somit knapp 93% ,die Zulassung erhalten.
Review of Drugs Rejected by EMA in 2012
Total Number of drugs issued with LOOI in 2012 = 27
Total Number of approved drugs with LOOI in 2012 = 25
Total Number of drugs rejected on 2nd CHMP Review = 2
Conclusion:
- If EMA has serious objection to a drug, it'll likely reject it in the first CHMP.
- Approval Percentage for drugs issued with LOOI is 25/27 = 93%
After seeing this analysis by losnachospicante as well as the one I just posted by tiger-joseph, I must say that this helps my confidence level in Belviq being approved in EU from a statistical point of view.
http://www.investorvillage.com/smbd.asp?mb=633&mn=49094&pt=m…
Die EMA hat bei insgesamt 27 " drugs" eine "List of Outstanding Issues" ( LOOI)= offenstehende Diskussionspunkte herausgearbeitet.
Von diesen "drugs" haben am Ende 25, somit knapp 93% ,die Zulassung erhalten.
Review of Drugs Rejected by EMA in 2012
Total Number of drugs issued with LOOI in 2012 = 27
Total Number of approved drugs with LOOI in 2012 = 25
Total Number of drugs rejected on 2nd CHMP Review = 2
Conclusion:
- If EMA has serious objection to a drug, it'll likely reject it in the first CHMP.
- Approval Percentage for drugs issued with LOOI is 25/27 = 93%
After seeing this analysis by losnachospicante as well as the one I just posted by tiger-joseph, I must say that this helps my confidence level in Belviq being approved in EU from a statistical point of view.
http://www.investorvillage.com/smbd.asp?mb=633&mn=49094&pt=m…
Arena Pharmaceuticals Inc. Stock Sell Recommendation Reiterated (ARNA)
By TheStreet Wire 01/28/13
Dieser Artikel mit o.g. Wortlautwurde von THE STREET WIRE am
17/08/12
06/09/12
18/10/12
26/12/12
18/01/13
28/01/13
bereits immer wieder veröffentlicht
By TheStreet Wire 01/28/13
Dieser Artikel mit o.g. Wortlautwurde von THE STREET WIRE am
17/08/12
06/09/12
18/10/12
26/12/12
18/01/13
28/01/13
bereits immer wieder veröffentlicht
Ja die von Street wire sind echt bärisch.... sind wohl alle short.
Sollte verboten werden dieses öffentliche drücken.
Arena Pharmaceuticals Inc. Stock Sell Recommendation Reiterated (ARNA)
Immerhin ist die Community von ARNA überzeugt. sehen ARNA steigend.
sehen ARNA steigend.
Community Poll Results
percentage of investors predicting on this security.

 75%
75%

 25%
25%
Sollte verboten werden dieses öffentliche drücken.
Arena Pharmaceuticals Inc. Stock Sell Recommendation Reiterated (ARNA)
Immerhin ist die Community von ARNA überzeugt.
 sehen ARNA steigend.
sehen ARNA steigend.Community Poll Results
percentage of investors predicting on this security.

 75%
75%
 25%
25%
In WDAM-TV America short video:
FDA approves new weight loss drugs
http://www.wdam.com/story/20602440/fda-approves-new-weight-l…
Good news for people in the battle against the bulge.
For the first time in more than a dozen years, the FDA has approved new drugs for weight loss.
For many, battling excess weight has been a life-long struggle, but the new drugs, tentatively called Belviq and Qsymia, offer hope.
Cleveland Clinic Bariatric Specialist Dr. Karen Cooper says surgery right now is the most effective tool for obese patients.
But that's not a viable option for everyone. The new drugs show the promise of, in the simplest terms, tricking your brain into making you feel full.
"What that means is that your portion sizes will be less. If portion sizes are smaller, you're taking less calories and that will prevent weight gain and hopefully promote some weight loss," said Dr. Cooper.
These drugs can be used long term, with fewer known side effects.
FDA approves new weight loss drugs
http://www.wdam.com/story/20602440/fda-approves-new-weight-l…
Good news for people in the battle against the bulge.
For the first time in more than a dozen years, the FDA has approved new drugs for weight loss.
For many, battling excess weight has been a life-long struggle, but the new drugs, tentatively called Belviq and Qsymia, offer hope.
Cleveland Clinic Bariatric Specialist Dr. Karen Cooper says surgery right now is the most effective tool for obese patients.
But that's not a viable option for everyone. The new drugs show the promise of, in the simplest terms, tricking your brain into making you feel full.
"What that means is that your portion sizes will be less. If portion sizes are smaller, you're taking less calories and that will prevent weight gain and hopefully promote some weight loss," said Dr. Cooper.
These drugs can be used long term, with fewer known side effects.
@AREANICS
Auch wenn ich bei sehr günstigen Kursen im April/Mai 2012 bei ARENA eingestiegen bin, so finde ich es aktuell schon hart mitanzusehen, wie der Aktienkurs von ARNA von den Hedgies systematisch in den letzten 12 Tagen nach unten geprügelt wurde - jetzt wurde gerade mit 300 Stk. der erste Premarketkurs von 7,90$ taxiert.
Im letzten Jahr haben Jim Cramer, Adam Fuckerstone und Konsorte immer wieder versucht, ARENA "negativ" zu bewerten ,um ARENA nach unten zu prügeln, was aber damals durch das seitens der Hedgie-Mafia nicht erwartete FDA Approval ganz schön in die Hose ging - so und jetzt hat die Hedgie-Mafia zum zweiten großen Angriff geblasen.
Aktuell kann man im Yahoo Board unter den letzten Beiträgen zu 90 % Bash-Attacken lesen, die die Angst bei den LONGIES schüren soll.
Ich hoffe, dass diesem gemeinen Spiel bald ein Ende gesetzt wird und ein BIG-NEWS FLOW endlich eine andere Richtung vorgibt.
For us " a lot of patience" -
patience which will reward us
Auch wenn ich bei sehr günstigen Kursen im April/Mai 2012 bei ARENA eingestiegen bin, so finde ich es aktuell schon hart mitanzusehen, wie der Aktienkurs von ARNA von den Hedgies systematisch in den letzten 12 Tagen nach unten geprügelt wurde - jetzt wurde gerade mit 300 Stk. der erste Premarketkurs von 7,90$ taxiert.
Im letzten Jahr haben Jim Cramer, Adam Fuckerstone und Konsorte immer wieder versucht, ARENA "negativ" zu bewerten ,um ARENA nach unten zu prügeln, was aber damals durch das seitens der Hedgie-Mafia nicht erwartete FDA Approval ganz schön in die Hose ging - so und jetzt hat die Hedgie-Mafia zum zweiten großen Angriff geblasen.
Aktuell kann man im Yahoo Board unter den letzten Beiträgen zu 90 % Bash-Attacken lesen, die die Angst bei den LONGIES schüren soll.
Ich hoffe, dass diesem gemeinen Spiel bald ein Ende gesetzt wird und ein BIG-NEWS FLOW endlich eine andere Richtung vorgibt.
For us " a lot of patience" -
patience which will reward us

Antwort auf Beitrag Nr.: 44.087.490 von bernie55 am 31.01.13 14:24:58Das endgültige DEA Klasse IV scheduling müßte eignetlich täglich kommen, dann wird`s auch wieder bergaufgehen mit Arena!
Zitat von Magnetfeldfredy: Das endgültige DEA Klasse IV scheduling müßte eignetlich täglich kommen, dann wird`s auch wieder bergaufgehen mit Arena!
YEPP......

..erst DEA, dann baldiger Beginn der USA Vermarktung..

...die EISAI Vertreter stehen schon startbereit in den Löchern und scharren mit den Hufen...

EISAI
1 Februar 2013 > Third Quarter Financial Results / Presentations <
...vielleicht ein paar News zu Belviq ?????
http://www.investorvillage.com/smbd.asp?mb=633&mn=49385&pt=m…
1 Februar 2013 > Third Quarter Financial Results / Presentations <
...vielleicht ein paar News zu Belviq ?????
http://www.investorvillage.com/smbd.asp?mb=633&mn=49385&pt=m…
Antwort auf Beitrag Nr.: 44.087.849 von bernie55 am 31.01.13 15:28:59Angesichts dieser schlagenden Argumente gehts auch schon wieder aufwärts in US.
Bin auch wieder dabei, der kurzfristige Ausstieg aus dem fallenden Zug hat nach Abzug der Unkosten nur ein paar Prozente gebracht.
Nichts gegen die zu erwartenden Gewinne, wenns hier mal richtig los geht.
patience and awareness!
Bin auch wieder dabei, der kurzfristige Ausstieg aus dem fallenden Zug hat nach Abzug der Unkosten nur ein paar Prozente gebracht.
Nichts gegen die zu erwartenden Gewinne, wenns hier mal richtig los geht.
patience and awareness!
Antwort auf Beitrag Nr.: 44.089.104 von me_2 am 31.01.13 19:04:21me_2 -- ,ir juckt auch der Finger immer wieder, weil ich denke, JETZT geht es aufwärts ... aber ich warte noch ab, sieht auch heute nicht gut aus .. Positiv zu erklären .. das können nur DIE, die den Kurs bestimmen. Sicher werde ich wieder dabei sein, wenn - ja wenn ich denke, dass es der richtige Zeitpunkt ist.. ob ich den treffe, dass ist eine andere Frage..aber es ist zu früh dazu..
bernie55
ich bin mit 12% Gewinn raus, hätte viel mehr sein können .. aber sollte nicht ... besser Reissleine ziehen und tiefer weiter sehen...
Aber ich hoffe auch, dass es wieder besser wird
Ahh so .. ich meinte natürlich 5€, da ich gerade auf der Seite war
Hoffe es wird alles wie wir es uns wünschen
lg philipini
bernie55
ich bin mit 12% Gewinn raus, hätte viel mehr sein können .. aber sollte nicht ... besser Reissleine ziehen und tiefer weiter sehen...
Aber ich hoffe auch, dass es wieder besser wird
Ahh so .. ich meinte natürlich 5€, da ich gerade auf der Seite war
Hoffe es wird alles wie wir es uns wünschen
lg philipini
Antwort auf Beitrag Nr.: 44.093.976 von philipini am 01.02.13 17:54:33Leerink Swann Resumes Arena Pharma (ARNA) at Outperform
Article
Related Articles (2)
Stock Quotes (1)
Comments (0)
FREE Breaking News Alerts from StreetInsider.com!
E-mail Address
Top News
Most Read
Highlighted
Virgin Media (VMED) Acquired by Liberty Global (LBTYA) in $23.3B Deal
Despite ESPN Headwinds, Walt Disney's (DIS) Q1 Delivers; Has Good News for Star Wars Buffs
Market Wrap: Dell Does Deal; U.S. Services Expand...Slowly; BlackBerry is Back!
Dell (DELL) to Go Private for $13.65/Share in Cash
How Can Apple (AAPL) Regain its Mojo?
February 6, 2013 7:14 AM EST
Get Alerts ARNA Hot Sheet
Price: $8.63 --0%
Rating Summary:
3 Buy, 8 Hold, 5 Sell
Rating Trend: Down
Today's Overall Ratings:
Up: 7 | Down: 18 | New: 15
Leerink Swann initiates coverage on Arena Pharma (NASDAQ: ARNA) with a Outperform rating and $11-$12 valuation range.
The firm comments, "Our due diligence points to a significant transformation occurring in the obesity space, similar in scope to smoking cessation a decade ago. The result should be a robust market expansion story advanced by: 1) novel more potent drugs; 2) increasing reimbursement; 3) significant sales & marketing efforts, and; 4) more approvals of effective therapies with co-morbid disease benefits. While the VVUS (OP) Qsymia launch has been slow, ARNA/Eisai's Belviq upcoming launch should work synergistically to broaden the market."
For an analyst ratings summary and ratings history on Arena Pharma click here. For more ratings news on Arena Pharma click here.
Shares of Arena Pharma closed at $8.63 yesterday, with a 52 week range of $1.70-$13.50.
Article
Related Articles (2)
Stock Quotes (1)
Comments (0)
FREE Breaking News Alerts from StreetInsider.com!
E-mail Address
Top News
Most Read
Highlighted
Virgin Media (VMED) Acquired by Liberty Global (LBTYA) in $23.3B Deal
Despite ESPN Headwinds, Walt Disney's (DIS) Q1 Delivers; Has Good News for Star Wars Buffs
Market Wrap: Dell Does Deal; U.S. Services Expand...Slowly; BlackBerry is Back!
Dell (DELL) to Go Private for $13.65/Share in Cash
How Can Apple (AAPL) Regain its Mojo?
February 6, 2013 7:14 AM EST
Get Alerts ARNA Hot Sheet
Price: $8.63 --0%
Rating Summary:
3 Buy, 8 Hold, 5 Sell
Rating Trend: Down
Today's Overall Ratings:
Up: 7 | Down: 18 | New: 15
Leerink Swann initiates coverage on Arena Pharma (NASDAQ: ARNA) with a Outperform rating and $11-$12 valuation range.
The firm comments, "Our due diligence points to a significant transformation occurring in the obesity space, similar in scope to smoking cessation a decade ago. The result should be a robust market expansion story advanced by: 1) novel more potent drugs; 2) increasing reimbursement; 3) significant sales & marketing efforts, and; 4) more approvals of effective therapies with co-morbid disease benefits. While the VVUS (OP) Qsymia launch has been slow, ARNA/Eisai's Belviq upcoming launch should work synergistically to broaden the market."
For an analyst ratings summary and ratings history on Arena Pharma click here. For more ratings news on Arena Pharma click here.
Shares of Arena Pharma closed at $8.63 yesterday, with a 52 week range of $1.70-$13.50.
Leerink Swann initiates coverage on Arena Pharma (NASDAQ: ARNA) with a Outperform rating and $11-$12 valuation range.
 ...it´s only a beginning...
...it´s only a beginning...
 ...it´s only a beginning...
...it´s only a beginning...
Arena Pharmaceuticals to Present at Two Upcoming Investor Conferences
PR NewswirePress Release: Arena Pharmaceuticals, Inc.
SAN DIEGO, Feb. 6, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at two upcoming investor conferences at The Waldorf Astoria in New York City:
15th Annual BIO CEO & Investor Conference
Presentation: February 12, 2013, at 1:30 p.m. Eastern Time (10:30 a.m. Pacific Time)
Leerink Swann Global Healthcare Conference
Presentation: February 13, 2013, at 2:30 p.m. Eastern Time (11:30 a.m. Pacific Time)
A live audio webcast of each presentation will be available under the investor relations section of Arena's website at www.arenapharm.com
A replay of each presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
PR NewswirePress Release: Arena Pharmaceuticals, Inc.
SAN DIEGO, Feb. 6, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at two upcoming investor conferences at The Waldorf Astoria in New York City:
15th Annual BIO CEO & Investor Conference
Presentation: February 12, 2013, at 1:30 p.m. Eastern Time (10:30 a.m. Pacific Time)
Leerink Swann Global Healthcare Conference
Presentation: February 13, 2013, at 2:30 p.m. Eastern Time (11:30 a.m. Pacific Time)
A live audio webcast of each presentation will be available under the investor relations section of Arena's website at www.arenapharm.com
A replay of each presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
Eine NEWS am Rande
EnteroMedics' ReCharge Trial Demonstrates Statistically Significant Weight Loss in Obesity
But Does Not Meet Predefined Efficacy Endpoints
http://boardvote.com/symbol/ETRM/communique/212064
EnteroMedics' ReCharge Trial Demonstrates Statistically Significant Weight Loss in Obesity
But Does Not Meet Predefined Efficacy Endpoints
http://boardvote.com/symbol/ETRM/communique/212064
Antwort auf Beitrag Nr.: 44.118.429 von bernie55 am 08.02.13 12:10:05"significant"?
Zitat von me_2: "significant"?
Hi me_2,
Enteromedics versuchte wohl, "katastrophale" Ergebnisdaten aus dem Trial III als " überzeugend " zu verkaufen...
...deshalb die knapp 60 % Minus !!!
EnteroMedics Weight-Loss Device Is A Pulsing Placebo
EnteroMedics tried to spin the disastrous results from the RECHARGE trial as a near miss and said it would still seek FDA approval for the VBLOC device.
[A previous phase III study of VBLOC, known as EMPOWER, also failed.]
http://www.thestreet.com/story/11836352/1/enteromedics-weigh…
What Is It With Arena Pharmaceuticals, Inc. (ARNA) and the EMA?
By The Motley Fool in News
Published: February 19, 2013 at 4:20 pm
Regardless of FDA approval, safety issues have been a great concern with regulators and medical practitioners when it comes to obesity drugs. It is no wonder then that it took USFDA thirteen long years to approve another obesity drug after Xenical, Belviq of Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) and Qysmia of VIVUS, Inc. (NASDAQ:VVUS), despite obesity being a priority with researchers.
Belviq was approved by the FDA in June 2012 while Qsymia was approved in July 2012. There was much hype regarding the approval of these diet pills because of prevalence of huge demand. Improved profitability of the two companies was expected to reflect in their stock prices. However, VIVUS stock lost momentum after July 2012 and fell by more than 52% between July 2012 and February 12, 2013. Although Arena's stock gained almost 26% since approval, it has been more or less an up-and-down story and is currently trading at a sizeable discount to the peak it achieved in the month it was approved.

Both Qsymia and Belviq seem to have run into troubles of different sorts.
Qsymia had issues with REMS (Risk Evaluation and Mitigation Strategy), pricing and insurance and the way it was marketed. Belviq, on the other hand was supposedly much safer, although with slower efficacy and Arena Pharmaceuticals was expected not to repeat mistakes committed by Vivus while marketing Qsymia.
The major issue with Belviq however was approval by the European agency for the evaluation of medicinal products, European Medicines Agency (EMA).
EMA has still not approved Belviq (active ingredient: lorcaserin). Although, it was expected that EMA will take its own time to approve Belviq, there has been an inordinate delay and still no signs of approval. Belviq was expected to be launched in the US market early this year after the DEA completed its scheduling. It now appears that even DEA had problems with Belviq (see below).
What is it with the EMA?
Even at the time of approaching EMA (March 2012), there were fears that the agency would have problems with approval as it is not known to follow USFDA. Those fears seem to have come true.
Lorcaserin was once rejected by FDA nearly two years ago because it had reasons to believe that it caused “mammary neoplasms (to) occur near clinical exposure and the tumorigenic MOA remains unresolved." This ground for rejection was resolved by the FDA this time after independent pathologists came to the conclusion that the drug was not likely to cause tumors in humans as its carcinogenicity was species specific.
Belviq is considered to be safe by the FDA and did not require any REMS, which is a strategy that the regulator mandates so that the benefits of a drug outweigh the known and/or potential serious risks associated with it. It was expected that CHMP (Committee for Medicinal Products for Human Use), the committee responsible for elaborating EMA’s opinions on human use of medicinal products, would come up with a favorable recommendation and the agency will approve Belviq a few months after that. That has not happened and it is almost a year since Arena applied for approval.
EMA’s advisory committee is seeking more information and risk/benefit analysis on Belviq. Besides more information on detection of tumors in rats, the agency also wants to know more about other clinical and non-clinical issues identified during the present and previous studies of lorcaserin.
CHMP has sent Arena a 180-day list of questions relating to various issues including those relating to heart valve and psychiatric side effects and some of them are pretty difficult. The current 180-day list follows the earlier 120-day list that comprised some of the same concerns, which indicates that the European regulators were not fully satisfied by Arena’s responses.
Problems with US Launch
The Drug Enforcement Agency (DEA) proposes to place lorcaserin in Schedule IV of the Controlled Substances Act. The proposal is on the recommendation of Assistant Secretary for Health of the Department of Health and Human Services (HHS) and on an evaluation of all other relevant data by DEA. Those adversely affected or aggrieved by the proposal were to file their comments by January 18, 2013. Hopefully, the rule becomes effective after a 30-day wait and Eisai, Arena’s marketing partner, can then start selling Belviq.
It would also be of interest to investors who fancy the obesity drug space that Vanderbilt University, a private research university in Nashville, Tennessee is partnering with GlaxoSmithKline plc (ADR) (NYSE:GSK) to develop a new obesity drug that is safer and would not raise blood pressure in patients. Phase 1 trials will begin in 2016 and we can expect similar hurdles this time also if at all there are any positive results. The target of the GSK research is melanocortin-4 receptor, which plays a role in balancing food intake and energy expenditure.
Conclusion
Bureaucratic delays, necessary or unnecessary cannot and should not be taken lying down. Whether or not there is substance in Arena shareholders’ accusation that DEA’s bureaucratic delay is unnecessary is a debatable issue. If shareholders are to be believed, then hedge funds are conspiring with DEA to destroy Belviq. With additional 4.25 million shares purchased by institutions, institutional holding of ARNA stock has increased by 5.7%.
With the 30-day deadline set by DEA rules about to expire, Arena and its marketing partner are sure to make a strong launch of Belviq and gain advantage over Vivus’ Qsymia. However, analysts have lowered forecast of 2013 sales by 70% – down from $115 million to $35 million. JPMorgan analyst, Cory Kasimov, however, forecasts 2013 sales at $74 million growing to $481 million in 2016.
So far so good in as far as the US market is concerned.
Forget DEA and hedge funds for the time being but EMA’s concerns are for real. Even at the cost of repeating what I said in the beginning, I would like to bring to notice the fact that it took thirteen long years for the second and third diet pills to be approved by the FDA. With obesity assuming epidemic proportions, it is a major concern in the US. If despite that the FDA has been wary of approving diet pills, then there is definitely a reason for that.
While delay by EMA is not the end of the world for Arena, losing the European market will have a major impact on Arena’s revenues. Already, the ARNA stock has suffered a loss of 14.2% after CHMP failed to approve Belviq in its last meeting in January (not assuming a correlation, however). This means another wait of 30 days for a ruling to come.
Safety issues must necessarily be addressed. EMA’s delay in approving Belviq indicates that they think the FDA has overlooked certain safety issues. If Arena’s answers to the EMA’s questions in the 180-day list fail to satisfy the agency, then doubts about FDA approval may have some substance giving Areana investors a reason to worry. I mean, can we trust the Europeans enough to doubt the FDA's ruling?
The article What Is It With ARENA and the EMA? originally appeared on Fool.com and is written by Sujata Dutta.
Copyright © 1995 - 2013 The Motley Fool, LLC. All rights reserved. The Motley Fool has a disclosure policy.
http://www.insidermonkey.com/blog/what-is-it-with-arena-phar…
By The Motley Fool in News
Published: February 19, 2013 at 4:20 pm
Regardless of FDA approval, safety issues have been a great concern with regulators and medical practitioners when it comes to obesity drugs. It is no wonder then that it took USFDA thirteen long years to approve another obesity drug after Xenical, Belviq of Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) and Qysmia of VIVUS, Inc. (NASDAQ:VVUS), despite obesity being a priority with researchers.
Belviq was approved by the FDA in June 2012 while Qsymia was approved in July 2012. There was much hype regarding the approval of these diet pills because of prevalence of huge demand. Improved profitability of the two companies was expected to reflect in their stock prices. However, VIVUS stock lost momentum after July 2012 and fell by more than 52% between July 2012 and February 12, 2013. Although Arena's stock gained almost 26% since approval, it has been more or less an up-and-down story and is currently trading at a sizeable discount to the peak it achieved in the month it was approved.

Both Qsymia and Belviq seem to have run into troubles of different sorts.
Qsymia had issues with REMS (Risk Evaluation and Mitigation Strategy), pricing and insurance and the way it was marketed. Belviq, on the other hand was supposedly much safer, although with slower efficacy and Arena Pharmaceuticals was expected not to repeat mistakes committed by Vivus while marketing Qsymia.
The major issue with Belviq however was approval by the European agency for the evaluation of medicinal products, European Medicines Agency (EMA).
EMA has still not approved Belviq (active ingredient: lorcaserin). Although, it was expected that EMA will take its own time to approve Belviq, there has been an inordinate delay and still no signs of approval. Belviq was expected to be launched in the US market early this year after the DEA completed its scheduling. It now appears that even DEA had problems with Belviq (see below).
What is it with the EMA?
Even at the time of approaching EMA (March 2012), there were fears that the agency would have problems with approval as it is not known to follow USFDA. Those fears seem to have come true.
Lorcaserin was once rejected by FDA nearly two years ago because it had reasons to believe that it caused “mammary neoplasms (to) occur near clinical exposure and the tumorigenic MOA remains unresolved." This ground for rejection was resolved by the FDA this time after independent pathologists came to the conclusion that the drug was not likely to cause tumors in humans as its carcinogenicity was species specific.
Belviq is considered to be safe by the FDA and did not require any REMS, which is a strategy that the regulator mandates so that the benefits of a drug outweigh the known and/or potential serious risks associated with it. It was expected that CHMP (Committee for Medicinal Products for Human Use), the committee responsible for elaborating EMA’s opinions on human use of medicinal products, would come up with a favorable recommendation and the agency will approve Belviq a few months after that. That has not happened and it is almost a year since Arena applied for approval.
EMA’s advisory committee is seeking more information and risk/benefit analysis on Belviq. Besides more information on detection of tumors in rats, the agency also wants to know more about other clinical and non-clinical issues identified during the present and previous studies of lorcaserin.
CHMP has sent Arena a 180-day list of questions relating to various issues including those relating to heart valve and psychiatric side effects and some of them are pretty difficult. The current 180-day list follows the earlier 120-day list that comprised some of the same concerns, which indicates that the European regulators were not fully satisfied by Arena’s responses.
Problems with US Launch
The Drug Enforcement Agency (DEA) proposes to place lorcaserin in Schedule IV of the Controlled Substances Act. The proposal is on the recommendation of Assistant Secretary for Health of the Department of Health and Human Services (HHS) and on an evaluation of all other relevant data by DEA. Those adversely affected or aggrieved by the proposal were to file their comments by January 18, 2013. Hopefully, the rule becomes effective after a 30-day wait and Eisai, Arena’s marketing partner, can then start selling Belviq.
It would also be of interest to investors who fancy the obesity drug space that Vanderbilt University, a private research university in Nashville, Tennessee is partnering with GlaxoSmithKline plc (ADR) (NYSE:GSK) to develop a new obesity drug that is safer and would not raise blood pressure in patients. Phase 1 trials will begin in 2016 and we can expect similar hurdles this time also if at all there are any positive results. The target of the GSK research is melanocortin-4 receptor, which plays a role in balancing food intake and energy expenditure.
Conclusion
Bureaucratic delays, necessary or unnecessary cannot and should not be taken lying down. Whether or not there is substance in Arena shareholders’ accusation that DEA’s bureaucratic delay is unnecessary is a debatable issue. If shareholders are to be believed, then hedge funds are conspiring with DEA to destroy Belviq. With additional 4.25 million shares purchased by institutions, institutional holding of ARNA stock has increased by 5.7%.
With the 30-day deadline set by DEA rules about to expire, Arena and its marketing partner are sure to make a strong launch of Belviq and gain advantage over Vivus’ Qsymia. However, analysts have lowered forecast of 2013 sales by 70% – down from $115 million to $35 million. JPMorgan analyst, Cory Kasimov, however, forecasts 2013 sales at $74 million growing to $481 million in 2016.
So far so good in as far as the US market is concerned.
Forget DEA and hedge funds for the time being but EMA’s concerns are for real. Even at the cost of repeating what I said in the beginning, I would like to bring to notice the fact that it took thirteen long years for the second and third diet pills to be approved by the FDA. With obesity assuming epidemic proportions, it is a major concern in the US. If despite that the FDA has been wary of approving diet pills, then there is definitely a reason for that.
While delay by EMA is not the end of the world for Arena, losing the European market will have a major impact on Arena’s revenues. Already, the ARNA stock has suffered a loss of 14.2% after CHMP failed to approve Belviq in its last meeting in January (not assuming a correlation, however). This means another wait of 30 days for a ruling to come.
Safety issues must necessarily be addressed. EMA’s delay in approving Belviq indicates that they think the FDA has overlooked certain safety issues. If Arena’s answers to the EMA’s questions in the 180-day list fail to satisfy the agency, then doubts about FDA approval may have some substance giving Areana investors a reason to worry. I mean, can we trust the Europeans enough to doubt the FDA's ruling?
The article What Is It With ARENA and the EMA? originally appeared on Fool.com and is written by Sujata Dutta.
Copyright © 1995 - 2013 The Motley Fool, LLC. All rights reserved. The Motley Fool has a disclosure policy.
http://www.insidermonkey.com/blog/what-is-it-with-arena-phar…
Trading Activity Report for Arena Pharmaceuticals (ARNA)
Posted on 02/20/2013 by Tom Kaplan
NEW YORK (AVAFIN) -- Arena Pharmaceuticals options contracts experienced a new 90-day record for call contracts where a total of 12,529 call contracts were traded in the busy trading session. The contract spread yielded a 0.74 put/call ratio where 1.4 call contracts were traded for each put contract.
Investors use options to manage risk and to speculate on price changes. Options can provide significant leverage if the stock moves in the right direction but aslo end up worthless if it doesn't.
Arena Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company committed to discovering and developing innovative therapies offering medical advances and new options for patients.
Arena Pharmaceuticals opened at $8.36 and the stock price declined $0.04 (0.48%) to $8.31 during the market session. ARNA is trading between the range of $8.31 - $8.45. Performance indicators show that the stock has lost -20.10% within the last month. Volume is 3M in relation to the three month average volume of 8M shares. ARNA is trading below the 50 day moving average and lower than the 200 day moving average.
http://www.avafin.com/articles/1021336.html
European regulators reject Vivus diet pill again
...was wohl zu erwarten war...
Thu Feb 21, 2013 5:41pm EST
Feb 21 (Reuters) - European health regulators for a second time rejected a diet pill developed by Vivus Inc and said the obesity treatment would not be approved there unless the company conducts a large lengthy trial to prove its heart safety, Vivus said on Thursday.
The drug, sold in the United States as Qsymia, was approved by U.S. regulators last year. The U.S. Food and Drug Administration also approved another diet pill, Belviq, sold by Arena Pharmaceuticals Inc, making them the first new obesity drugs approved in the United States in more than a decade.
Qsymia, which was to be sold in Europe under the brand name Qsiva, was initially rejected by the European Committee for Medicinal Products for Human Use (CHMP) last October. Vivus requested a re-examination of the decision and was turned down again on Thursday.
"We are disappointed with the CHMP decision regarding Qsiva and the position the Committee adopted with respect to the need for a preapproval cardiovascular outcomes trial," Vivus President Peter Tam said in a statement.
Another diet drug developed by Orexigen Therapeutics Inc faces a similar hurdle in the United States after the FDA said it would not approve its pill, which is called Contrave, without a lengthy, expensive study to prove that it does not increase the risk of heart attacks and strokes.
Vivus shares fell 2.4 percent to $12.57 in extended-hours trading from their Nasdaq close at $12.88.
http://www.reuters.com/article/2013/02/21/vivus-europe-idUSL…
...was wohl zu erwarten war...
Thu Feb 21, 2013 5:41pm EST
Feb 21 (Reuters) - European health regulators for a second time rejected a diet pill developed by Vivus Inc and said the obesity treatment would not be approved there unless the company conducts a large lengthy trial to prove its heart safety, Vivus said on Thursday.
The drug, sold in the United States as Qsymia, was approved by U.S. regulators last year. The U.S. Food and Drug Administration also approved another diet pill, Belviq, sold by Arena Pharmaceuticals Inc, making them the first new obesity drugs approved in the United States in more than a decade.
Qsymia, which was to be sold in Europe under the brand name Qsiva, was initially rejected by the European Committee for Medicinal Products for Human Use (CHMP) last October. Vivus requested a re-examination of the decision and was turned down again on Thursday.
"We are disappointed with the CHMP decision regarding Qsiva and the position the Committee adopted with respect to the need for a preapproval cardiovascular outcomes trial," Vivus President Peter Tam said in a statement.
Another diet drug developed by Orexigen Therapeutics Inc faces a similar hurdle in the United States after the FDA said it would not approve its pill, which is called Contrave, without a lengthy, expensive study to prove that it does not increase the risk of heart attacks and strokes.
Vivus shares fell 2.4 percent to $12.57 in extended-hours trading from their Nasdaq close at $12.88.
http://www.reuters.com/article/2013/02/21/vivus-europe-idUSL…
Hoffen wir, daß es uns mit der EMA nicht ähnlich geht ...
Zitat von me_2: Hoffen wir, daß es uns mit der EMA nicht ähnlich geht ...
...yepp, hoffen ist immer gut..

Die Entscheidung seitens der EMA wird erst mal auf sich warten lassen > Zeitraum innerhalb des ersten Halbjahrs 2013..

Und bedenke: Ein mögliches "Approval" durch die zusätzliche Nachfrage seitens der EMA ist noch lange nicht vom Tisch....

Bis zur Verkündung durch die EMA werden wir hoffentlich schon erste Verkaufszahlen von BELVIQ erfahren und sehen wie der Verkauf von BELVIQ anläuft.
Sollten die ersten Verkaufszahlen schon beeindruckend sein, dann wird uns eine eventuelle Ablehnung seitens der EMA nur ein müdes Lächeln kosten..

Mit der nächste NEWS ( "DEA ") sollten wir bis spätestens Anfang März rechnen...
Danach könnte das "launch" seitens EISAI auch schon recht zügig begonnen werden.

Ich würde deine Zuversicht ja gerne teilen, warte damit aber bis alle regulatorischen Voraussetzungen dieseits und jenseits des Ozeans vorliegen, und ich denke daß wir beide brauchen werden.
Die CHMP Stellungsnahme sollte bis zum 21.2.13 erfolgen, man sieht daß sich EMA und Berater zunehmend schwertun, sich von der Verträglichkeit von neuen Medikamenten bei nicht-lebensbedrohlichen (und das liegt hier nun mal vor) Indikationen überzeugen zu lassen.
Einziger Trost aus meiner Sicht ist, daß das downside potential inzwischen wohl eher gering ist.
Die CHMP Stellungsnahme sollte bis zum 21.2.13 erfolgen, man sieht daß sich EMA und Berater zunehmend schwertun, sich von der Verträglichkeit von neuen Medikamenten bei nicht-lebensbedrohlichen (und das liegt hier nun mal vor) Indikationen überzeugen zu lassen.
Einziger Trost aus meiner Sicht ist, daß das downside potential inzwischen wohl eher gering ist.
Arena Pharmaceuticals to Host Corporate Update and Financial Results Conference Call and Webcast on Monday, March 4
SAN DIEGO, Feb. 25, 2013 /PRNewswire/ --
Arena Pharmaceuticals, Inc. (ARNA) announced today that it will host a conference call and webcast to provide a corporate update and discuss fourth quarter and full year 2012 financial results before the NASDAQ Global Select Market opens on
Monday, March 4, 2013.
The conference call and webcast will begin at 8:30 a.m. Eastern Time (5:30 a.m. Pacific Time).
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' Fourth Quarter and Full Year 2012 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-host-cor…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-host-cor…
SAN DIEGO, Feb. 25, 2013 /PRNewswire/ --
Arena Pharmaceuticals, Inc. (ARNA) announced today that it will host a conference call and webcast to provide a corporate update and discuss fourth quarter and full year 2012 financial results before the NASDAQ Global Select Market opens on
Monday, March 4, 2013.
The conference call and webcast will begin at 8:30 a.m. Eastern Time (5:30 a.m. Pacific Time).
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' Fourth Quarter and Full Year 2012 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-host-cor…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-host-cor…
25-Feb-2013
Regulation FD Disclosure
Item 7.01 Regulation FD Disclosure.
On February 25, 2013, Eisai Inc. disclosed a wholesale acquisition cost of BELVIQ �
(lorcaserin HCl) of $199.50 for a bottle of 60 10 mg tablets.
http://biz.yahoo.com/e/130225/arna8-k.html
Regulation FD Disclosure
Item 7.01 Regulation FD Disclosure.
On February 25, 2013, Eisai Inc. disclosed a wholesale acquisition cost of BELVIQ �
(lorcaserin HCl) of $199.50 for a bottle of 60 10 mg tablets.
http://biz.yahoo.com/e/130225/arna8-k.html
Vivius geht ordentlich in den Keller:
Feb 25, 2013, 1:40pm PST
Vivus Inc. loss grows to $57M due to weight loss drug sales costs
Enlarge
Steven E.F. Brown
Vivus Inc., which sells an FDA-approved weight loss drug called Qsymia, lost $56.7 million in the fourth quarter of 2012, up from a loss of $11.5 million a year earlier.
The higher costs of selling Qsymia, along with associated general and administrative expenses, pushed up the company's loss.
This latest loss pushed Vivus' accumulated deficit -- how much money it has lost or written off since it started -- to $486,146,000, close to half a billion dollars.
Sales, however, which were zero in the fourth quarter a year ago, rose to $1.97 million in the most recent quarter.
For the year ended December, Vivus (NASDAQ: VVUS) lost $139.9 million on sales of $2.01 million. That compares with 2011, when Vivus lost $46.1 million on zero sales.
Mountain View-based Vivus also sells a treatment for erectile dysfunction that was approved by the Food and Drug Administration in April. The company is seeking approval to sell that product in Europe as well.
Leland Wilson is CEO of Vivus. He said that this year the company will be seeking reimbursement coverage for patients using Qsymia and also sales partners for the erectile dysfunction treatment.
Qsymia, which went on sale in the United States in September, was the first new weight loss medication approved by the FDA in 13 years.
http://www.bizjournals.com/sanfrancisco/blog/biotech/2013/02…
Feb 25, 2013, 1:40pm PST
Vivus Inc. loss grows to $57M due to weight loss drug sales costs
Enlarge
Steven E.F. Brown
Vivus Inc., which sells an FDA-approved weight loss drug called Qsymia, lost $56.7 million in the fourth quarter of 2012, up from a loss of $11.5 million a year earlier.
The higher costs of selling Qsymia, along with associated general and administrative expenses, pushed up the company's loss.
This latest loss pushed Vivus' accumulated deficit -- how much money it has lost or written off since it started -- to $486,146,000, close to half a billion dollars.
Sales, however, which were zero in the fourth quarter a year ago, rose to $1.97 million in the most recent quarter.
For the year ended December, Vivus (NASDAQ: VVUS) lost $139.9 million on sales of $2.01 million. That compares with 2011, when Vivus lost $46.1 million on zero sales.
Mountain View-based Vivus also sells a treatment for erectile dysfunction that was approved by the Food and Drug Administration in April. The company is seeking approval to sell that product in Europe as well.
Leland Wilson is CEO of Vivus. He said that this year the company will be seeking reimbursement coverage for patients using Qsymia and also sales partners for the erectile dysfunction treatment.
Qsymia, which went on sale in the United States in September, was the first new weight loss medication approved by the FDA in 13 years.
http://www.bizjournals.com/sanfrancisco/blog/biotech/2013/02…
Antwort auf Beitrag Nr.: 44.188.268 von Poppholz am 26.02.13 16:22:04Das ist das schöne an unserer Arena, die Kosten trägt Esai, wir haben 30 % der Einnahmen, alles Drecksbasher die Ami-Medien!
Schöner Beitrag, besonder durch den Vergleich von ARENA mit VIVIUS:
3 Bio-Pharmaceuticals You Can’t Ignore: Arena Pharmaceuticals, Inc. (ARNA), VIVUS Inc. (VVUS)
By The Motley Fool in News
Published: February 26, 2013 at 4:24 pm
...
Arena Pharmaceuticals
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) is a much safer bet as compared to Vivus due to less strict safety guidance by FDA. Initially the FDA asked for a DEA review into potential abuse of Belviq, but a recent ruling has put all such concerns to rest. According to the DEA, Belviq has been classified as a schedule IV drug, i.e. having a low risk of abuse. Therefore physicians are allowed to prescribe Belviq for a 3 month period through local pharmacies.
Unlike the dramatic fluctuations in the stock price of its rival Vivus, Arena's stock has fluctuated between $8 and $11 during the last six months. The trial results of Belviq show that it can reduce weight in the range of 10% to 11%, making it ideal for patients who do not want to undertake a lot of risk. It will also be easier for doctors to prescribe Belviq due to its less strict safety guidelines; therefore Arena is the safest obesity bet out there.
http://www.insidermonkey.com/blog/3-bio-pharmaceuticals-you-…
3 Bio-Pharmaceuticals You Can’t Ignore: Arena Pharmaceuticals, Inc. (ARNA), VIVUS Inc. (VVUS)
By The Motley Fool in News
Published: February 26, 2013 at 4:24 pm
...
Arena Pharmaceuticals
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) is a much safer bet as compared to Vivus due to less strict safety guidance by FDA. Initially the FDA asked for a DEA review into potential abuse of Belviq, but a recent ruling has put all such concerns to rest. According to the DEA, Belviq has been classified as a schedule IV drug, i.e. having a low risk of abuse. Therefore physicians are allowed to prescribe Belviq for a 3 month period through local pharmacies.
Unlike the dramatic fluctuations in the stock price of its rival Vivus, Arena's stock has fluctuated between $8 and $11 during the last six months. The trial results of Belviq show that it can reduce weight in the range of 10% to 11%, making it ideal for patients who do not want to undertake a lot of risk. It will also be easier for doctors to prescribe Belviq due to its less strict safety guidelines; therefore Arena is the safest obesity bet out there.
http://www.insidermonkey.com/blog/3-bio-pharmaceuticals-you-…
Antwort auf Beitrag Nr.: 44.190.578 von Poppholz am 27.02.13 07:48:13hier auch mal als Grafik dargestellt, wie sich die beiden Kurse verhalten:

Arena Pharmaceuticals to Present at the Cowen and Company 33rd Annual Health Care Conference
SAN DIEGO, Feb. 27, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at the Cowen and Company 33rd Annual Health Care Conference on Wednesday, March 6, 2013, at 10:00 a.m. Eastern Time (7:00 a.m. Pacific Time), at The Boston Marriott Copley Place in Boston, Massachusetts.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
SAN DIEGO, Feb. 27, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at the Cowen and Company 33rd Annual Health Care Conference on Wednesday, March 6, 2013, at 10:00 a.m. Eastern Time (7:00 a.m. Pacific Time), at The Boston Marriott Copley Place in Boston, Massachusetts.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
........zur Erinnerung...
.....heute vor Börsenhandelsbeginn in den USA ....
Arena Pharmaceuticals to Host Corporate Update and Financial Results Conference Call and Webcast on Monday, March 4
SAN DIEGO, Feb. 25, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that it will host a conference call and webcast to provide a corporate update and discuss fourth quarter and full year 2012 financial results before the NASDAQ Global Select Market opens on Monday, March 4, 2013.
The conference call and webcast will begin at 8:30 a.m. Eastern Time (5:30 a.m. Pacific Time).
.....heute vor Börsenhandelsbeginn in den USA ....
Arena Pharmaceuticals to Host Corporate Update and Financial Results Conference Call and Webcast on Monday, March 4
SAN DIEGO, Feb. 25, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that it will host a conference call and webcast to provide a corporate update and discuss fourth quarter and full year 2012 financial results before the NASDAQ Global Select Market opens on Monday, March 4, 2013.
The conference call and webcast will begin at 8:30 a.m. Eastern Time (5:30 a.m. Pacific Time).
Antwort auf Beitrag Nr.: 44.209.879 von bernie55 am 04.03.13 09:53:58ich vermute mal, dass die Zahlen nicht so gut sind.
Die Kosten bei einer "Produkteinführung" sind meist sehr hoch, bzw. erst kommen die Kosten dann der Ertrag.
Vivius hat das gleiche "Problem". Da ist der Kurs dann abgerutscht.
Interessant ist die Zukunftsaussicht bei Arena.
Ich werde keine Aktien verkaufen, um dann wieder in ein paar Tagen einzusteigen, da bei meinem Glück der Kursverlauf genau anders verläuft.

Die Kosten bei einer "Produkteinführung" sind meist sehr hoch, bzw. erst kommen die Kosten dann der Ertrag.
Vivius hat das gleiche "Problem". Da ist der Kurs dann abgerutscht.
Interessant ist die Zukunftsaussicht bei Arena.
Ich werde keine Aktien verkaufen, um dann wieder in ein paar Tagen einzusteigen, da bei meinem Glück der Kursverlauf genau anders verläuft.

Antwort auf Beitrag Nr.: 44.210.117 von Poppholz am 04.03.13 10:44:44Kosten hat Arena fast keine, Esai trägt Hauptlast, Arnea hat 1/3 der Einnahmen!

Antwort auf Beitrag Nr.: 44.210.307 von Magnetfeldfredy am 04.03.13 11:31:35Das ist 13:30 Bavarian Standard Time, hört jemand zu?
Ich glaub wir warten noch immer auf die endgültige Bekanntgabe der DEA-Klassifizierung, oder gibts da news?

Arena Pharmaceuticals Provides Corporate Update and Reviews Fourth Quarter and Full Year 2012 Financial Results
http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=744…" target="_blank" rel="nofollow ugc noopener">
http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=744…
Antwort auf Beitrag Nr.: 44.210.756 von bernie55 am 04.03.13 13:24:36Soweit ich gelesen und gehört habe, nichts wesentlich neues. Insbesondere nur sehr magere Info zum Ausblick 2013. Entsprechend auch die Kursreaktion.
Arena Pharmaceuticals, Inc. (ARNA):
Small High-Risk Biotech Offers Potential Big Returns
March 4th, 2013

Arena Pharmaceuticals
George Leong: The biotechnology sector provides some of the best investment opportunities for significant returns. This is especially true with the emerging smaller biotech companies that are in the early stages of clinical trials and commercialization. Yet the risk is high. Success in clinical trials from phases one to three could reap major price appreciation for shareholders. On the other hand, many drugs in the pre-clinical and clinical trial stages also fail and never reach commercialization. This is the risk; but in our view, success could easily compensate for taking a chance.
As an investor, you can simply invest in Pfizer Inc. (NYSE:PFE) with its proven track record and market-cap of $198 billion; but the upside investment opportunity, while positive longer-term, will not make you rich in the shorter term due to the lack of strong revenue growth. Just take a look at the Thomson Financial estimates: revenues at Pfizer are estimated by Thomson Financial to contract by 2.5% in 2013, followed by another 1.8% in 2014.
Pfizer is an excellent long-term investment opportunity for the conservative investor looking for income and some capital gains; but for us, most of the easy money has already been made. We want to see stellar growth and superlative price appreciation. Given this, companies such as Pfizer are out of the equation for us, unless you’re happy with small capital-gains potential and a 3.5% dividend.
Have you ever wondered how billionaires continue to get RICHER, while the rest of the world is struggling?
"I study billionaires for a living. To be more specific, I study how these investors generate such huge and consistent profits in the stock markets -- year-in and year-out."
When I search for emerging biotech stocks as an investment opportunity, I look for a strong drug pipeline. The ideal investment opportunity would be a company with at least one drug in commercialization or close to it, more drugs in late-stage phase three clinical trials, and even more drugs in development.
Sometimes, all three areas of the drug lifecycle are not available, especially to small biotech companies; but one small bio-tech company, Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) is beginning to reward patient investors after its obesity drug—“BELVIQ”—was approved by the Federal Drug Administration (FDA).
And what makes the approval special is that BELVIQ is the first weight loss pill approved in the last 13 years. (Source: Liston, E., “Which Of These 3 Biotech Stocks Looks Better?” Seeking Alpha web site, January 28, 2013, last accessed March 3, 2013.) Arena will get 12 years of exclusivity with its new drug due to President Obama’s Biologics Price Competition and Innovation (BPCI) Act of 2009.
For Arena, that means over a decade to dominate the market and lock in record profits, making the stock a good investment opportunity.
And what is amazing is that Arena is waiting to hear if BELVIQ will also get approved by the European Medical Association—and if that happens, we have no doubt ....let`s hope and see.... ......it could see another bull run and could provide a good investment opportunity.
......it could see another bull run and could provide a good investment opportunity.
The global market for Arena could be massive. Obesity is a chronic problem in the U.S. and worldwide, including China. The National Institutes of Health (NIH) predict that spending on health care related to obesity will rise to $343 billion in 2018. According to the American Journal of Preventive Medicine, about 42% of Americans could be obese by 2030. (Source: “Arena Pharmaceuticals and VIVUS Look to Benefit as Aetna Provides Coverage for Anti-Obesity Drug,” Yahoo! Finance, November 22, 2012, last accessed March 3, 2013.) So drugs like BELVIQ could have great potential.
Since its upward move, Arena has paused and is looking for direction in a tight sideways channel. The near-term technical indicators point to some near-term weakness, but we would view breakdowns as an investment opportunity.
A move to the 50-day moving average (MA) around $7.73 would be an area in which you could look to buy. And in the unlikely case that Arena falls to its 200-day MA of $4.01, this would be a strong buy, based on my technical analysis.

The chart above shows a bullish formation with a price target of $18.50, representing a potential move of 132% and a good investment opportunity.
The reality is that Arena could easily surge on the chart, especially as BELVIQ gathers worldwide acceptance and sales pick up.
Please note: the information on Pfizer and Arena contained in this article is not to be construed as advice to buy the stock; rather, it is meant to provide an example of a potential good investment opportunity.
Find out what some of the top shale oil plays are in “How to Play the Oil Rush in North Dakota.”
This article is brought to you courtesy of George Leong from Profit Confidential.
http://marketdailynews.com/2013/03/04/arena-pharmaceuticals-…
Small High-Risk Biotech Offers Potential Big Returns
March 4th, 2013

Arena Pharmaceuticals
George Leong: The biotechnology sector provides some of the best investment opportunities for significant returns. This is especially true with the emerging smaller biotech companies that are in the early stages of clinical trials and commercialization. Yet the risk is high. Success in clinical trials from phases one to three could reap major price appreciation for shareholders. On the other hand, many drugs in the pre-clinical and clinical trial stages also fail and never reach commercialization. This is the risk; but in our view, success could easily compensate for taking a chance.
As an investor, you can simply invest in Pfizer Inc. (NYSE:PFE) with its proven track record and market-cap of $198 billion; but the upside investment opportunity, while positive longer-term, will not make you rich in the shorter term due to the lack of strong revenue growth. Just take a look at the Thomson Financial estimates: revenues at Pfizer are estimated by Thomson Financial to contract by 2.5% in 2013, followed by another 1.8% in 2014.
Pfizer is an excellent long-term investment opportunity for the conservative investor looking for income and some capital gains; but for us, most of the easy money has already been made. We want to see stellar growth and superlative price appreciation. Given this, companies such as Pfizer are out of the equation for us, unless you’re happy with small capital-gains potential and a 3.5% dividend.
Have you ever wondered how billionaires continue to get RICHER, while the rest of the world is struggling?
"I study billionaires for a living. To be more specific, I study how these investors generate such huge and consistent profits in the stock markets -- year-in and year-out."
When I search for emerging biotech stocks as an investment opportunity, I look for a strong drug pipeline. The ideal investment opportunity would be a company with at least one drug in commercialization or close to it, more drugs in late-stage phase three clinical trials, and even more drugs in development.
Sometimes, all three areas of the drug lifecycle are not available, especially to small biotech companies; but one small bio-tech company, Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) is beginning to reward patient investors after its obesity drug—“BELVIQ”—was approved by the Federal Drug Administration (FDA).

And what makes the approval special is that BELVIQ is the first weight loss pill approved in the last 13 years. (Source: Liston, E., “Which Of These 3 Biotech Stocks Looks Better?” Seeking Alpha web site, January 28, 2013, last accessed March 3, 2013.) Arena will get 12 years of exclusivity with its new drug due to President Obama’s Biologics Price Competition and Innovation (BPCI) Act of 2009.

For Arena, that means over a decade to dominate the market and lock in record profits, making the stock a good investment opportunity.
And what is amazing is that Arena is waiting to hear if BELVIQ will also get approved by the European Medical Association—and if that happens, we have no doubt ....let`s hope and see....
 ......it could see another bull run and could provide a good investment opportunity.
......it could see another bull run and could provide a good investment opportunity.The global market for Arena could be massive. Obesity is a chronic problem in the U.S. and worldwide, including China. The National Institutes of Health (NIH) predict that spending on health care related to obesity will rise to $343 billion in 2018. According to the American Journal of Preventive Medicine, about 42% of Americans could be obese by 2030. (Source: “Arena Pharmaceuticals and VIVUS Look to Benefit as Aetna Provides Coverage for Anti-Obesity Drug,” Yahoo! Finance, November 22, 2012, last accessed March 3, 2013.) So drugs like BELVIQ could have great potential.
Since its upward move, Arena has paused and is looking for direction in a tight sideways channel. The near-term technical indicators point to some near-term weakness, but we would view breakdowns as an investment opportunity.
A move to the 50-day moving average (MA) around $7.73 would be an area in which you could look to buy. And in the unlikely case that Arena falls to its 200-day MA of $4.01, this would be a strong buy, based on my technical analysis.

The chart above shows a bullish formation with a price target of $18.50, representing a potential move of 132% and a good investment opportunity.
The reality is that Arena could easily surge on the chart, especially as BELVIQ gathers worldwide acceptance and sales pick up.
Please note: the information on Pfizer and Arena contained in this article is not to be construed as advice to buy the stock; rather, it is meant to provide an example of a potential good investment opportunity.
Find out what some of the top shale oil plays are in “How to Play the Oil Rush in North Dakota.”
This article is brought to you courtesy of George Leong from Profit Confidential.
http://marketdailynews.com/2013/03/04/arena-pharmaceuticals-…
Arena Pharmaceuticals' Diet Drug Drama A Nail Biter
Mar 7 2013, 01:40
The wait for regulatory approval of Arena Pharmaceutical's (ARNA) diet drug has left investors in an anxious state. Although the U.S. Federal Drug Administration (FDA) approved Arena's compound lorcaserin in June 2012, the FDA classified it as a Schedule IV controlled drug, making it subject to additional controls by the Drug Enforcement Administration (DEA). Ever since, Arena has been waiting for the DEA to complete a review and provide a final classification for lorcaserin, which Arena plans to market under the brand name Belviq. A process that was originally supposed to require four to six months has now taken eight months and counting.
In early 2012, Arena had also submitted an application to the European Union for approval of Belviq in that market. Investors were nonplussed as Arena responded to the usual set of questions from the European Medicines Agency (EMA). However, a second notice of outstanding issues coming by letter from the EMA in January 2013, has challenged the resolve of even the most patient shareholders. Issues raised by the EMA committee, such as tumors in rats and psychiatric events, sent a chill across trading in ARNA.
Six weeks after the EMA notice, a sizeable "short" position has developed in Arena Pharmaceutical shares. To be clear, 55.3 million shares have been sold short by investors expecting the worst for Belviq. This development is no surprise. It has been accompanied by a series of negative or less than supportive commentary from sell-side analysts.
The question is whether the bear-case view is a bit overdone. The short interest in ARNA represents 26% of the flotation (total shares outstanding less shares held by insiders). That in itself means something. More importantly, consider that as deep and swift as trading has become in ARNA, it would take almost seven days of trading to clear all the short interest in the stock. Called the Days-to-Cover Ratio (Total Shares Sold Short divided by Average Daily Trading Volume), the measure is a good barometer for stocks under a cloud of controversy. Compare the ARNA ratio to the average Days-to-Cover for all stocks sold short on the Nasdaq - 3.22 days. Stocks sold short on the New York Stock Exchange could be mopped up before noon as indicated by an average Days-to-Cover of 0.39 days for NYSE stocks.
With such a build-up in short interest, even the slightest good news for Arena could trigger a so-called "short squeeze."
A shift to bullish sentiment would likely drive the bid high enough to cause short-sellers to "buy-in" and close short-positions. Eye-balling historic trading patterns in ARNA suggests the majority of the short interest in the stock was built up at prices between $6.00 and $12.00. A 25% shift higher in stock price would leave the majority of short interest well in the negative.
What is the likelihood of positive news for Arena's Belviq? Well, pretty high!
While approval in Europe appears to be a long way off or even a non-starter, Belviq is more likely than not to be sold in the U.S. - perhaps the largest market for diet drugs. Granted during the wait for the DEA to finalize a classification for Belviq, a competing diet drug has been approved and launched. Vivus, Inc. (VVUS) is already selling its Qsymia drug in the U.S. for chronic weight control. For many drugs, arriving in second to the market would be a serious letdown. However, both Arena's Belviq and Vivus' Qsymia are intended for the chronically obese as well as those who may be less hefty, but have a risk factor such as diabetes or heart disease. That means as many as two out of three Americans could be candidates for prescription diet therapies. That is arguably one of the largest drug markets in the world. Even second place in such a large market will be lucrative.
The hitch for Arena has been the extra step required for classification as a Schedule IV drug by the DEA.
Vivus' Qsymia is the brand name for an extended release formulation of phentermine and topiaramate. Phentermine is an appetite suppressant and topiramate is a seizure medication. Arena's Belviq is composed of lorcaserin, which decreases activity within the appetite centers of the brain. Apparently the FDA decided Belviq was too vulnerable to abuse to let it go to the market without added oversight. Even with DEA involvement in the process, it is still more a matter of when and not if, Belviq will get a berth on the U.S. pharmacy shelves.
This brings us back to that measure of short interest 55.32 million shares. Is it wise to sit in a short position with such a large crowd when there is impending positive news? > NÖ <
It appears to me that investors have already factored a U.S. launch into Arena's future. However, the value attributed to that opportunity has been muted by news of Vivus' head start. Arena's case has not been helped by the news that Vivus has already resorted to discounting after experiencing a tepid response to its Qsymia price tag. Hence ARNA hovers near the $8.50 price level, about 37% below its 52-week high price.
A final clearance for take-off in the U.S. market for Arena's Belviq would likely be accompanied by renewed discussion of milestone payments and royalties. Arena is expected to receive $5.0 million from its marketing partner upon receiving a DEA designation and another $60 million upon Belviq's launch. . Then royalties begin.
Arena's CFO at one point suggested the first $1.0 billion in total sales could trigger royalties in excess of $300 million.
Chatter about payments and cash flows along with the enthusiastic re-calculation of future revenue and profits are likely to generate a dramatic shift in sentiment, turning up the volume on Arena valuation and stock price.
If that happens, it might be ARNA bears who are nibbling on their digits.
http://seekingalpha.com/article/1254141-arena-pharmaceutical…
Mar 7 2013, 01:40
The wait for regulatory approval of Arena Pharmaceutical's (ARNA) diet drug has left investors in an anxious state. Although the U.S. Federal Drug Administration (FDA) approved Arena's compound lorcaserin in June 2012, the FDA classified it as a Schedule IV controlled drug, making it subject to additional controls by the Drug Enforcement Administration (DEA). Ever since, Arena has been waiting for the DEA to complete a review and provide a final classification for lorcaserin, which Arena plans to market under the brand name Belviq. A process that was originally supposed to require four to six months has now taken eight months and counting.
In early 2012, Arena had also submitted an application to the European Union for approval of Belviq in that market. Investors were nonplussed as Arena responded to the usual set of questions from the European Medicines Agency (EMA). However, a second notice of outstanding issues coming by letter from the EMA in January 2013, has challenged the resolve of even the most patient shareholders. Issues raised by the EMA committee, such as tumors in rats and psychiatric events, sent a chill across trading in ARNA.
Six weeks after the EMA notice, a sizeable "short" position has developed in Arena Pharmaceutical shares. To be clear, 55.3 million shares have been sold short by investors expecting the worst for Belviq. This development is no surprise. It has been accompanied by a series of negative or less than supportive commentary from sell-side analysts.
The question is whether the bear-case view is a bit overdone. The short interest in ARNA represents 26% of the flotation (total shares outstanding less shares held by insiders). That in itself means something. More importantly, consider that as deep and swift as trading has become in ARNA, it would take almost seven days of trading to clear all the short interest in the stock. Called the Days-to-Cover Ratio (Total Shares Sold Short divided by Average Daily Trading Volume), the measure is a good barometer for stocks under a cloud of controversy. Compare the ARNA ratio to the average Days-to-Cover for all stocks sold short on the Nasdaq - 3.22 days. Stocks sold short on the New York Stock Exchange could be mopped up before noon as indicated by an average Days-to-Cover of 0.39 days for NYSE stocks.
With such a build-up in short interest, even the slightest good news for Arena could trigger a so-called "short squeeze."
A shift to bullish sentiment would likely drive the bid high enough to cause short-sellers to "buy-in" and close short-positions. Eye-balling historic trading patterns in ARNA suggests the majority of the short interest in the stock was built up at prices between $6.00 and $12.00. A 25% shift higher in stock price would leave the majority of short interest well in the negative.
What is the likelihood of positive news for Arena's Belviq? Well, pretty high!
While approval in Europe appears to be a long way off or even a non-starter, Belviq is more likely than not to be sold in the U.S. - perhaps the largest market for diet drugs. Granted during the wait for the DEA to finalize a classification for Belviq, a competing diet drug has been approved and launched. Vivus, Inc. (VVUS) is already selling its Qsymia drug in the U.S. for chronic weight control. For many drugs, arriving in second to the market would be a serious letdown. However, both Arena's Belviq and Vivus' Qsymia are intended for the chronically obese as well as those who may be less hefty, but have a risk factor such as diabetes or heart disease. That means as many as two out of three Americans could be candidates for prescription diet therapies. That is arguably one of the largest drug markets in the world. Even second place in such a large market will be lucrative.
The hitch for Arena has been the extra step required for classification as a Schedule IV drug by the DEA.
Vivus' Qsymia is the brand name for an extended release formulation of phentermine and topiaramate. Phentermine is an appetite suppressant and topiramate is a seizure medication. Arena's Belviq is composed of lorcaserin, which decreases activity within the appetite centers of the brain. Apparently the FDA decided Belviq was too vulnerable to abuse to let it go to the market without added oversight. Even with DEA involvement in the process, it is still more a matter of when and not if, Belviq will get a berth on the U.S. pharmacy shelves.
This brings us back to that measure of short interest 55.32 million shares. Is it wise to sit in a short position with such a large crowd when there is impending positive news? > NÖ <

It appears to me that investors have already factored a U.S. launch into Arena's future. However, the value attributed to that opportunity has been muted by news of Vivus' head start. Arena's case has not been helped by the news that Vivus has already resorted to discounting after experiencing a tepid response to its Qsymia price tag. Hence ARNA hovers near the $8.50 price level, about 37% below its 52-week high price.
A final clearance for take-off in the U.S. market for Arena's Belviq would likely be accompanied by renewed discussion of milestone payments and royalties. Arena is expected to receive $5.0 million from its marketing partner upon receiving a DEA designation and another $60 million upon Belviq's launch. . Then royalties begin.
Arena's CFO at one point suggested the first $1.0 billion in total sales could trigger royalties in excess of $300 million.
Chatter about payments and cash flows along with the enthusiastic re-calculation of future revenue and profits are likely to generate a dramatic shift in sentiment, turning up the volume on Arena valuation and stock price.
If that happens, it might be ARNA bears who are nibbling on their digits.
http://seekingalpha.com/article/1254141-arena-pharmaceutical…
Kleiner zeitlicher Lückenfüller
Das "WARTEN AUF DEA" ( von Arena) ist fast schon mit dem "WARTEN AUF GODOT" ( S. Beckett) zu vergleichen.
Nur mit dem Unterschied, dass das "WARTEN AUF DEA" nicht vergeblich sein wird....
Schöne Grüße
bernie55
Das "WARTEN AUF DEA" ( von Arena) ist fast schon mit dem "WARTEN AUF GODOT" ( S. Beckett) zu vergleichen.
Nur mit dem Unterschied, dass das "WARTEN AUF DEA" nicht vergeblich sein wird....

Schöne Grüße
bernie55

Lorcaserin ist nicht auf dem CHMP März-Treffen diskutiert worden.
http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_e…" target="_blank" rel="nofollow ugc noopener">
http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_e…
http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_e…" target="_blank" rel="nofollow ugc noopener">
http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_e…
Antwort auf Beitrag Nr.: 44.287.465 von bernie55 am 22.03.13 16:28:04http://us.rd.yahoo.com/finance/external/pssa/SIG=12qct2vq1/*…
irgendwann muß doch DEA -selbst bei großzügigster Interpretation der vorgesehenen Fristen- mal zu einem Ende des Bewertungsprozesses kommen. Hat noch jemand den Überblick über den Zeitplan und Deadlines? Gefühlt müßte das Verfahren doch schon lange abgehakt sein.
Antwort auf Beitrag Nr.: 44.295.021 von me_2 am 25.03.13 14:08:51Die DEA hat keine Fristen und auch keine Deadline.
Für den derzeitigen "Review of public Comments-Prozess" hat sie alle Zeit der Welt.
Geduld ist hier der Schlüssel zum Erfolg.
Für den derzeitigen "Review of public Comments-Prozess" hat sie alle Zeit der Welt.

Geduld ist hier der Schlüssel zum Erfolg.

Antwort auf Beitrag Nr.: 44.296.157 von NoSelters am 25.03.13 16:08:05Ich erinnere mich etwas gelesen zu haben mit 30 d comment phase und dann 15-45 d effective date ...(?)
Antwort auf Beitrag Nr.: 44.296.325 von me_2 am 25.03.13 16:20:14Die 30-Tage Frist war für die öffentlichen Kommentare bindend,die 15-45 Tage sind nicht bindend,da alle relevanten Kommentare geprüft und abgehandelt werden müssen und dies nicht planbar ist....


Die spinnen,die Amis...aber da müssen wir durch.
Loooong and stroooong!



Die spinnen,die Amis...aber da müssen wir durch.
Loooong and stroooong!
Arena Pharmaceuticals Announces Eisai's Submission of BELVIQ® (lorcaserin HCl) Marketing Authorization Application in Mexico
Application for Approval as a Treatment for Chronic Weight Management Triggers $500,000 Milestone Payment to Arena --
SAN DIEGO, April 1, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that Eisai Laboratorios S. de R.L. de C.V., a subsidiary of Eisai Inc., has submitted a marketing authorization application (MAA) for BELVIQ® (lorcaserin HCl) in Mexico with the Federal Commission for the Protection Against Sanitary Risk (COFEPRIS). The intended indication for BELVIQ is as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index (BMI) of 30 kg/m2 or greater (obese), or 27 kg/m2 or greater (overweight) in the presence of at least one weight-related comorbid condition. Based on the MAA submission, Arena will receive a milestone payment of $500,000 from Eisai.
"We look forward to the opportunity to help address this unmet medical need by bringing BELVIQ to patients in Mexico who can benefit from treatment for chronic weight management," said Jack Lief, Arena's President and Chief Executive Officer. "We plan to commercialize BELVIQ globally through collaborations with organizations such as Eisai that are committed to and have proven capabilities in serving patients and physicians."
Approximately 30 percent of adults in Mexico are obese and 70 percent are overweight or obese. The rapidly increasing prevalence of overweight and obese patients is leading to a large health and economic burden in Mexico.
"Eisai is committed to keeping patients' medical needs at the forefront of all that we do, as part of our human health care corporate mission," said Frank Ciriello, Senior Vice President, Eisai Inc. "We believe that Eisai has an opportunity with BELVIQ to bring a new medical option to help address the needs of patients affected by overweight and obesity in Mexico."
Eisai is responsible for the regulatory approval and, ultimately, marketing and distribution of BELVIQ in Mexico. Subject to approval, Arena will manufacture BELVIQ at its facility in Switzerland and sell finished commercial product to Eisai for distribution in Mexico. Arena is eligible to receive payments based upon Eisai's net sales of BELVIQ and is also eligible to receive regulatory and development milestone payments.
Beyond Mexico, Eisai has marketing and distribution rights in most of North and South America and also plans to submit MAAs for BELVIQ in Canada and Brazil this year. In addition, Arena has granted marketing and distribution rights to Ildong Pharmaceutical Co., Ltd., for South Korea and plans to enter into additional collaborations to commercialize BELVIQ outside of these territories. Arena has composition of matter patents for BELVIQ issued in major jurisdictions globally that, in most cases, are capable of continuing into 2023, and has filed applications for patent extension in the United States, which, if granted, will extend the patent term for BELVIQ into 2026.
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…
Application for Approval as a Treatment for Chronic Weight Management Triggers $500,000 Milestone Payment to Arena --
SAN DIEGO, April 1, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that Eisai Laboratorios S. de R.L. de C.V., a subsidiary of Eisai Inc., has submitted a marketing authorization application (MAA) for BELVIQ® (lorcaserin HCl) in Mexico with the Federal Commission for the Protection Against Sanitary Risk (COFEPRIS). The intended indication for BELVIQ is as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index (BMI) of 30 kg/m2 or greater (obese), or 27 kg/m2 or greater (overweight) in the presence of at least one weight-related comorbid condition. Based on the MAA submission, Arena will receive a milestone payment of $500,000 from Eisai.
"We look forward to the opportunity to help address this unmet medical need by bringing BELVIQ to patients in Mexico who can benefit from treatment for chronic weight management," said Jack Lief, Arena's President and Chief Executive Officer. "We plan to commercialize BELVIQ globally through collaborations with organizations such as Eisai that are committed to and have proven capabilities in serving patients and physicians."
Approximately 30 percent of adults in Mexico are obese and 70 percent are overweight or obese. The rapidly increasing prevalence of overweight and obese patients is leading to a large health and economic burden in Mexico.
"Eisai is committed to keeping patients' medical needs at the forefront of all that we do, as part of our human health care corporate mission," said Frank Ciriello, Senior Vice President, Eisai Inc. "We believe that Eisai has an opportunity with BELVIQ to bring a new medical option to help address the needs of patients affected by overweight and obesity in Mexico."
Eisai is responsible for the regulatory approval and, ultimately, marketing and distribution of BELVIQ in Mexico. Subject to approval, Arena will manufacture BELVIQ at its facility in Switzerland and sell finished commercial product to Eisai for distribution in Mexico. Arena is eligible to receive payments based upon Eisai's net sales of BELVIQ and is also eligible to receive regulatory and development milestone payments.
Beyond Mexico, Eisai has marketing and distribution rights in most of North and South America and also plans to submit MAAs for BELVIQ in Canada and Brazil this year. In addition, Arena has granted marketing and distribution rights to Ildong Pharmaceutical Co., Ltd., for South Korea and plans to enter into additional collaborations to commercialize BELVIQ outside of these territories. Arena has composition of matter patents for BELVIQ issued in major jurisdictions globally that, in most cases, are capable of continuing into 2023, and has filed applications for patent extension in the United States, which, if granted, will extend the patent term for BELVIQ into 2026.
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…
Arena Pharmaceuticals Initiates Phase 1 Clinical Trial of APD334 for Autoimmune Diseases
SAN DIEGO, April 5, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today the initiation of dosing in a Phase 1 clinical trial of APD334, a novel oral drug candidate that targets the sphingosine 1-phosphate subtype 1 (S1P1) receptor for the potential treatment of autoimmune diseases.
This randomized, double-blind and placebo-controlled Phase 1 trial will evaluate the safety, tolerability and pharmacokinetics of single-ascending doses of APD334 in up to 64 healthy adult volunteers.
"We are pleased to expand our clinical-stage pipeline by initiating a Phase 1 trial of APD334, and look forward to advancing this novel compound through our validated development platform," said William R. Shanahan, M.D., Arena's Senior Vice President and Chief Medical Officer. "APD334's selectivity for the S1P1 receptor has the potential to improve upon the adverse event profile of currently available treatments for a spectrum of autoimmune diseases."
About Autoimmune Diseases
Autoimmune diseases are characterized by an inappropriate immune response against substances and tissues that are normally present in the body. In an autoimmune reaction, a person's antibodies and immune cells target healthy tissues, triggering an inflammatory response. Reducing the immune and/or inflammatory response is an important goal in the treatment of autoimmune disease.
About APD334
APD334 is an orally available drug candidate discovered by Arena that targets the S1P1 receptor for the potential treatment of a number of conditions related to autoimmune diseases, including multiple sclerosis, psoriasis and rheumatoid arthritis. S1P1 receptors have been demonstrated to be involved in the modulation of several biological responses, including lymphocyte trafficking from lymph nodes to the peripheral blood. By isolating lymphocytes in lymph nodes, fewer immune cells are available in the circulating blood to effect tissue damage. Arena has optimized APD334 as a potent and selective small molecule S1P1 receptor agonist that reduces the severity of disease in preclinical autoimmune disease models.
About Arena Pharmaceuticals
Arena is a biopharmaceutical company focused on discovering, developing and commercializing novel drugs that target G protein-coupled receptors, or GPCRs, to address unmet medical needs. BELVIQ® (lorcaserin HCl), Arena's internally discovered drug, was approved by the US Food and Drug Administration in June 2012 and is under review for regulatory approval in additional territories. Arena's US operations are located in San Diego, California, and its operations outside of the United States, including its commercial manufacturing facility, are located in Zofingen, Switzerland. For more information, visit Arena's website at www.arenapharm.com.
Arena Pharmaceuticals® and Arena® are registered service marks of Arena Pharmaceuticals, Inc. BELVIQ® is a registered trademark of Arena Pharmaceuticals GmbH.
Forward-Looking Statements
Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include statements about the advancement, therapeutic indication and use, safety, efficacy, tolerability, selectivity and mechanism of action of APD334; the protocol, design, scope, enrollment and other aspects of the Phase 1 clinical trial of APD334; the potential of APD334 and treatment of autoimmune diseases in general; the regulatory review of BELVIQ; and Arena's focus, plans, goals, strategy, expectations, research and development programs, and ability to discover and develop compounds and commercialize drugs. For such statements, Arena claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Arena's expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, the following: APD334 may not have an adequate safety margin or otherwise be sufficient for further development or regulatory review or approval; risks related to commercializing drugs, including regulatory, manufacturing and supply issues and the pace of market acceptance; cash and revenues generated from BELVIQ, including the impact of competition; Arena's revenues will be based in part on management's estimates, judgment and accounting policies, and incorrect estimates or disagreement regarding Arena's estimates or accounting policies may result in changes to Arena's guidance or previously reported results; the timing and outcome of regulatory review is uncertain, and BELVIQ may not be approved for marketing when expected or ever by any other regulatory agency; government and commercial reimbursement and pricing decisions; risks related to relying on collaborative arrangements; the timing and receipt of payments and fees, if any, from collaborators; the entry into or modification or termination of collaborative arrangements; unexpected or unfavorable new data; nonclinical and clinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Arena or others, request additional information, have additional recommendations or change their guidance or requirements before or after approval; data and other information related to any of Arena's research and development may not meet safety, efficacy or other regulatory requirements or otherwise be sufficient for further research and development, regulatory review or approval or continued marketing; Arena's ability to obtain and defend patents; the timing, success and cost of Arena's research and development programs; results of clinical trials and other studies are subject to different interpretations and may not be predictive of future results; clinical trials and other studies may not proceed at the time or in the manner expected or at all; having adequate funds; and satisfactory resolution of litigation or other disagreements with others. Additional factors that could cause actual results to differ materially from those stated or implied by Arena's forward-looking statements are disclosed in Arena's filings with the Securities and Exchange Commission. These forward-looking statements represent Arena's judgment as of the time of this release. Arena disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
http://finance.yahoo.com/news/arena-pharmaceuticals-initiate…
SAN DIEGO, April 5, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today the initiation of dosing in a Phase 1 clinical trial of APD334, a novel oral drug candidate that targets the sphingosine 1-phosphate subtype 1 (S1P1) receptor for the potential treatment of autoimmune diseases.
This randomized, double-blind and placebo-controlled Phase 1 trial will evaluate the safety, tolerability and pharmacokinetics of single-ascending doses of APD334 in up to 64 healthy adult volunteers.
"We are pleased to expand our clinical-stage pipeline by initiating a Phase 1 trial of APD334, and look forward to advancing this novel compound through our validated development platform," said William R. Shanahan, M.D., Arena's Senior Vice President and Chief Medical Officer. "APD334's selectivity for the S1P1 receptor has the potential to improve upon the adverse event profile of currently available treatments for a spectrum of autoimmune diseases."
About Autoimmune Diseases
Autoimmune diseases are characterized by an inappropriate immune response against substances and tissues that are normally present in the body. In an autoimmune reaction, a person's antibodies and immune cells target healthy tissues, triggering an inflammatory response. Reducing the immune and/or inflammatory response is an important goal in the treatment of autoimmune disease.
About APD334
APD334 is an orally available drug candidate discovered by Arena that targets the S1P1 receptor for the potential treatment of a number of conditions related to autoimmune diseases, including multiple sclerosis, psoriasis and rheumatoid arthritis. S1P1 receptors have been demonstrated to be involved in the modulation of several biological responses, including lymphocyte trafficking from lymph nodes to the peripheral blood. By isolating lymphocytes in lymph nodes, fewer immune cells are available in the circulating blood to effect tissue damage. Arena has optimized APD334 as a potent and selective small molecule S1P1 receptor agonist that reduces the severity of disease in preclinical autoimmune disease models.
About Arena Pharmaceuticals
Arena is a biopharmaceutical company focused on discovering, developing and commercializing novel drugs that target G protein-coupled receptors, or GPCRs, to address unmet medical needs. BELVIQ® (lorcaserin HCl), Arena's internally discovered drug, was approved by the US Food and Drug Administration in June 2012 and is under review for regulatory approval in additional territories. Arena's US operations are located in San Diego, California, and its operations outside of the United States, including its commercial manufacturing facility, are located in Zofingen, Switzerland. For more information, visit Arena's website at www.arenapharm.com.
Arena Pharmaceuticals® and Arena® are registered service marks of Arena Pharmaceuticals, Inc. BELVIQ® is a registered trademark of Arena Pharmaceuticals GmbH.
Forward-Looking Statements
Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include statements about the advancement, therapeutic indication and use, safety, efficacy, tolerability, selectivity and mechanism of action of APD334; the protocol, design, scope, enrollment and other aspects of the Phase 1 clinical trial of APD334; the potential of APD334 and treatment of autoimmune diseases in general; the regulatory review of BELVIQ; and Arena's focus, plans, goals, strategy, expectations, research and development programs, and ability to discover and develop compounds and commercialize drugs. For such statements, Arena claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Arena's expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, the following: APD334 may not have an adequate safety margin or otherwise be sufficient for further development or regulatory review or approval; risks related to commercializing drugs, including regulatory, manufacturing and supply issues and the pace of market acceptance; cash and revenues generated from BELVIQ, including the impact of competition; Arena's revenues will be based in part on management's estimates, judgment and accounting policies, and incorrect estimates or disagreement regarding Arena's estimates or accounting policies may result in changes to Arena's guidance or previously reported results; the timing and outcome of regulatory review is uncertain, and BELVIQ may not be approved for marketing when expected or ever by any other regulatory agency; government and commercial reimbursement and pricing decisions; risks related to relying on collaborative arrangements; the timing and receipt of payments and fees, if any, from collaborators; the entry into or modification or termination of collaborative arrangements; unexpected or unfavorable new data; nonclinical and clinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Arena or others, request additional information, have additional recommendations or change their guidance or requirements before or after approval; data and other information related to any of Arena's research and development may not meet safety, efficacy or other regulatory requirements or otherwise be sufficient for further research and development, regulatory review or approval or continued marketing; Arena's ability to obtain and defend patents; the timing, success and cost of Arena's research and development programs; results of clinical trials and other studies are subject to different interpretations and may not be predictive of future results; clinical trials and other studies may not proceed at the time or in the manner expected or at all; having adequate funds; and satisfactory resolution of litigation or other disagreements with others. Additional factors that could cause actual results to differ materially from those stated or implied by Arena's forward-looking statements are disclosed in Arena's filings with the Securities and Exchange Commission. These forward-looking statements represent Arena's judgment as of the time of this release. Arena disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
http://finance.yahoo.com/news/arena-pharmaceuticals-initiate…
...zur Abwechslung mal etwas zum Thema ARENA und DEA....
As Arena Pharma Begins To Address Mexican Obesity, All Eyes Are Now On The DEA
http://seekingalpha.com/article/1326161-as-arena-pharma-begi…
The Arena Conspiracy And DEA Scheduling
http://seekingalpha.com/article/1326431-the-arena-conspiracy…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/1326431-the-arena-conspiracy…

As Arena Pharma Begins To Address Mexican Obesity, All Eyes Are Now On The DEA
http://seekingalpha.com/article/1326161-as-arena-pharma-begi…
The Arena Conspiracy And DEA Scheduling
http://seekingalpha.com/article/1326431-the-arena-conspiracy…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/1326431-the-arena-conspiracy…
Antwort auf Beitrag Nr.: 44.384.955 von bernie55 am 08.04.13 13:55:11Da wollen die Amis mal Kosten sparen im Gesundheitswesen und verzögern den Markteintritt durch die lahme DEA um Monate....
Oder rigged game again?:O
Oder rigged game again?:O
Aus der PRAC-Agenda von heute:
5.1.15. Lorcaserin
• Evaluation of an RMP in the context of an initial Marketing Authorisation Application procedure
Pharmacovigilance Risk Assessment Committee (PRAC)
EMA/218110/2013 Page 15/38
Status: for discussion and agreement of advice to CHMP
5.1.15. Lorcaserin
• Evaluation of an RMP in the context of an initial Marketing Authorisation Application procedure
Pharmacovigilance Risk Assessment Committee (PRAC)
EMA/218110/2013 Page 15/38
Status: for discussion and agreement of advice to CHMP
April 21, 2013 11:42 pm
Mystery objectors delay weight loss drug
By Andrew Jack
The launch of a new weight loss drug is being held up by the US Drug Enforcement Authority, after a surge in anonymous objections that some investors fear is manipulating the process.
Belviq, developed by Arena, the US biotech company, was authorised as safe and effective by the Food and Drug Administration last June, but has yet to be ratified by the DEA under a process designed to ensure controlled use of medicines that come with a risk of abuse.
The FDA recommended that Belviq, known generically as Lorcaserin, be classified as a “schedule IV” drug, a low-risk category, which gives regulators some supervisory powers to oversee prescriptions.
But an unusually high number of 69 comments have been filed on Belviq, creating a greater workload for DEA officials in making an assessment.
The stalling has allowed Qsymia, a weight-loss drug made by Vivus, a rival US biotech, to gain a lead over Belviq, even though the drug was approved after Belviq by the FDA.
While most of the 47 positive remarks on the DEA website are identified by the name of the author, 19 of 22 negative ones are anonymous, sparking debate over whether individuals with a vested interest in delaying Belviq have been posting criticisms.
http://www.ft.com/cms/s/0/ef3ca4ae-aa65-11e2-9a38-00144feabd…
Mystery objectors delay weight loss drug
By Andrew Jack
The launch of a new weight loss drug is being held up by the US Drug Enforcement Authority, after a surge in anonymous objections that some investors fear is manipulating the process.
Belviq, developed by Arena, the US biotech company, was authorised as safe and effective by the Food and Drug Administration last June, but has yet to be ratified by the DEA under a process designed to ensure controlled use of medicines that come with a risk of abuse.
The FDA recommended that Belviq, known generically as Lorcaserin, be classified as a “schedule IV” drug, a low-risk category, which gives regulators some supervisory powers to oversee prescriptions.
But an unusually high number of 69 comments have been filed on Belviq, creating a greater workload for DEA officials in making an assessment.
The stalling has allowed Qsymia, a weight-loss drug made by Vivus, a rival US biotech, to gain a lead over Belviq, even though the drug was approved after Belviq by the FDA.
While most of the 47 positive remarks on the DEA website are identified by the name of the author, 19 of 22 negative ones are anonymous, sparking debate over whether individuals with a vested interest in delaying Belviq have been posting criticisms.
http://www.ft.com/cms/s/0/ef3ca4ae-aa65-11e2-9a38-00144feabd…
Antwort auf Beitrag Nr.: 44.482.583 von bernie55 am 22.04.13 09:29:59Hi Bernie,
bei Arena wird doch genauso beschissen wie bei Dendreon, so macht Börse keinen Spaß mehr, oder?
bei Arena wird doch genauso beschissen wie bei Dendreon, so macht Börse keinen Spaß mehr, oder?
Ich kann mir sehr gut vorstellen das hier das Gap unter 4 erstmal geschlossen wird und es dementsprechend schnell bergab gehen kann.
Antwort auf Beitrag Nr.: 44.506.803 von MACD am 24.04.13 21:16:08GAP bei 4, da müßte die DEA das Scheduling verändern, so ein Schmarrn!

Antwort auf Beitrag Nr.: 44.506.957 von Magnetfeldfredy am 24.04.13 21:43:41Die Behauptung, dass jedes Gap irgendwann einmal geschlossen wird, ist Quatsch. Weil sie aufgrund der nötigen Beobachtungsdauer (=unendlich!!) formal nicht falsifizierbar ist.
((Und wenn doch, mit hoher Wahrscheinlichkeit nicht während meiner Börsenlebenszeit
 ))
))
((Und wenn doch, mit hoher Wahrscheinlichkeit nicht während meiner Börsenlebenszeit

 ))
))
Habt ihr das bei Dendreon nicht auch gesagt? Gibt es hier keine Parallelen zwischen der Zulassungseuphorie dieser Werte (ARNA, VVUS), der eingepreisten Marktkapitalsierungen und den Risiken das diese letztenendes nicht gehalten werden können?
Antwort auf Beitrag Nr.: 44.509.859 von MACD am 25.04.13 10:55:55Mußt ja nicht kaufen, weißt ja eh alles besser!
Fettsucht ist eine globale Epedemie, weltweit!
Arena hat mit Belviq eine gut verträgliche Pille die von der FDA zugelassen wurde und jederzeit mit dem Verkauf in den USA nach der DEA starten kann.
EU-Zulassung gibt hier meiner Meinung nach die Phantasie nach oben bzw. auch nach unten!
Dieselben Betrüger und Manipulatoren wie bei Dendreon sind hier am Werke, time will tell!
Ich halte Arena seit US Dollar 2, Adam Fuckinstein und Konsorten haben immer behauptet Arena bekommt keine Zulassung......., seitdem vervierfacht und das Zeug um globalen Blockbuster!
Fettsucht ist eine globale Epedemie, weltweit!
Arena hat mit Belviq eine gut verträgliche Pille die von der FDA zugelassen wurde und jederzeit mit dem Verkauf in den USA nach der DEA starten kann.
EU-Zulassung gibt hier meiner Meinung nach die Phantasie nach oben bzw. auch nach unten!
Dieselben Betrüger und Manipulatoren wie bei Dendreon sind hier am Werke, time will tell!
Ich halte Arena seit US Dollar 2, Adam Fuckinstein und Konsorten haben immer behauptet Arena bekommt keine Zulassung......., seitdem vervierfacht und das Zeug um globalen Blockbuster!

Ich habe nicht behauptet irgendetwas besser zu wissen und das jedes Gap geschlossen wird, nur die Wahrscheinlichkeiten sind halt gegeben. Aber wie hoch kann das Risiko eingeschätzt werden das diese Pille am Markt nicht nachgefragt wird wie erwartet (und schon eingepreist im Kurs). Es passt eben nicht so gut ins Bild wenn auch mal die enormen Risiken erwähnt werden, aber sowas gehört auch angesprochen.
Das die besagten Schreiberlinge nicht ernstgenommen werden sollten ist auch klar.
Das die besagten Schreiberlinge nicht ernstgenommen werden sollten ist auch klar.
Antwort auf Beitrag Nr.: 44.510.997 von MACD am 25.04.13 12:43:16Ich nehme Dich sehrwohl ernst und schätze Deine Chartaussagen!
Das Risiko nochmals 4 US Dollar zu sehen ist genauso hoch wie das Risiko neue Highs bei US Dollar 13-14 zu sehen, oder?
Deine GAP-Theorie ist für Dendreon gut, dann müßte es ja irgendwann wieder brutal aufwärts gehen?
Das Risiko nochmals 4 US Dollar zu sehen ist genauso hoch wie das Risiko neue Highs bei US Dollar 13-14 zu sehen, oder?

Deine GAP-Theorie ist für Dendreon gut, dann müßte es ja irgendwann wieder brutal aufwärts gehen?
Bei diesen Spezialwerten sind charttechnische Analysen wohl bei weitem weniger aussagekräftig als bei normalen Werten. Es handelt sich ja nicht um Gaps von wenigen Prozent sondern von vergleichsweise riesigen Kurssprüngen. Ein Gap-Closing bei Arena wäre in einem Zusammenwirken mit schlechter werdenden fundamentalen Erwartungen eher wahrscheinlich (Delay im Belviq-Verkauf, schlechte Nachfrage etc.). Die Gefahr ist bei diesen Werten eben die Überschätzung der Verkaufserlöse nach Zulassung (Blockbuster-Euphorie in Foren:-)
@Areanics-Hardcore 
@Areanites-Light
Wir alle wissen, dass wir eigentlich nichts wissen und deshalb haben wir alle recht bzw. unrecht – dass einige „rechter“ haben , wird sich immer erst am Ende zeigen. ( "Siehste,habe ich es nicht gleich gesagt bzw. habe ich doch gewusst")
Deshalb an dieser Stelle nochmals kurz zu den Fakten –
BELVIQ hat die FDA Zulassung und kann in den USA vermarktet werden sobald die DEA ihr grünes Licht für Schedule IV gibt.
Dann werden wir sehen wie erfolgreich der Marketingpartner Eisai das Produkt Belviq „an den Mann bringt“ ( die Umsatzzahlen des ersten Halbjahres werden m.M.n. die ersten Tendenzen aufzeigen und die weitere Reiserichtung von ARNA bestimmen)
("wer hätte das gedacht")
Die CHMP wird im ersten Halbjahr 2013 eine Empfehlung für ein Approval/Non-Approval aussprechen.
Durch ein Approval wird sich ("wer hätte das gedacht") eine zusätzliche Marktchance für die Umsatzgenerierung von ARNA ergeben. In welcher Höhe , das wird sich ebenfalls zeigen.
Durch ein Non-Approval natürlich nicht.
So einfach ist Börse.
Uns allen , good luck
LG
bernie55

@Areanites-Light

Wir alle wissen, dass wir eigentlich nichts wissen und deshalb haben wir alle recht bzw. unrecht – dass einige „rechter“ haben , wird sich immer erst am Ende zeigen. ( "Siehste,habe ich es nicht gleich gesagt bzw. habe ich doch gewusst")

Deshalb an dieser Stelle nochmals kurz zu den Fakten –
BELVIQ hat die FDA Zulassung und kann in den USA vermarktet werden sobald die DEA ihr grünes Licht für Schedule IV gibt.
Dann werden wir sehen wie erfolgreich der Marketingpartner Eisai das Produkt Belviq „an den Mann bringt“ ( die Umsatzzahlen des ersten Halbjahres werden m.M.n. die ersten Tendenzen aufzeigen und die weitere Reiserichtung von ARNA bestimmen)
("wer hätte das gedacht")
Die CHMP wird im ersten Halbjahr 2013 eine Empfehlung für ein Approval/Non-Approval aussprechen.
Durch ein Approval wird sich ("wer hätte das gedacht") eine zusätzliche Marktchance für die Umsatzgenerierung von ARNA ergeben. In welcher Höhe , das wird sich ebenfalls zeigen.
Durch ein Non-Approval natürlich nicht.
So einfach ist Börse.

Uns allen , good luck
LG
bernie55

Antwort auf Beitrag Nr.: 44.516.913 von bernie55 am 26.04.13 08:46:35klasse, fundamental neue erkenntnisse ...
Zitat von me_2: klasse, fundamental neue erkenntnisse .....("wer hätte das gedacht")...
 .......oooohhhhmmmmmmmm......
.......oooohhhhmmmmmmmm......
 .....Sssssssssssorrryyyy, dass ich dich mit meinem Beitrag aus dem Gleichgewicht gebracht habe.....
.....Sssssssssssorrryyyy, dass ich dich mit meinem Beitrag aus dem Gleichgewicht gebracht habe.....
Aber wenn du die Ironie meines Beitrages ("wer hätte das gedacht")
 nicht herausgehört hast, so sei es drum.
nicht herausgehört hast, so sei es drum.Der Beitrag war eigentlich nur eine Reaktion auf die immer wiederkehrenden nie endenden Grundsatz-Diskussionen in Foren
- mehr nicht

Nun ist einfach nur noch gemeinsames Warten auf News angesagt...
...klasse, fundamental neue erkenntnisse ...("wer hätte das gedacht")...

Grüße
bernie55

Arena Pharmaceuticals to Host Corporate Update and Financial Results Conference Call and Webcast on Thursday, May 2, 2013
SAN DIEGO, April 26, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that it will provide a corporate update and discuss first quarter 2013 financial results after the NASDAQ Global Select Market closes on Thursday, May 2, 2013.
The conference call and webcast will begin at 5:15 p.m. Eastern Time (2:15 p.m. Pacific Time).
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' First Quarter 2013 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-host-cor…
SAN DIEGO, April 26, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that it will provide a corporate update and discuss first quarter 2013 financial results after the NASDAQ Global Select Market closes on Thursday, May 2, 2013.
The conference call and webcast will begin at 5:15 p.m. Eastern Time (2:15 p.m. Pacific Time).
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' First Quarter 2013 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-host-cor…
 Arena Withdrawing Obesity Drug Regulatory Application in Europe
Arena Withdrawing Obesity Drug Regulatory Application in Europe 
By Ryan Flinn - May 2, 2013 11:12 PM GMT+0200
Arena Pharmaceuticals Inc. (ARNA), the maker of the weight-loss drug Belviq, said it is withdrawing its application to gain regulatory approval in Europe for the obesity medicine.

Arena will review its application to the European Medicines Union for Belviq, approved in the U.S. in June as the first new obesity drug in 13 years, before deciding when to resubmit it, the San Diego-based company said today in a statement.
The pill, being promoted in North America by Tokyo-based Eisai Co. (4523), works in a similar way to fenfluramine, part of the fen-phen appetite-suppression drug combination pulled from pharmacies in 1997 when it was linked to heart valve abnormalities. It will be available in the U.S. after the Drug Enforcement Administration completes a review to classify Belviq based on its potential for abuse.
Arena didn’t give a timetable for its application in Europe, saying it was “evaluating the best approach for submitting at a later date.”
Shares declined 14 percent to $7.25 in extended trading at 5:01 p.m. New York time after gaining 2.1 percent to close at $8.40.
Arena’s drug, previously known by its chemical name as lorcaserin, was rejected by the FDA in 2010 because the agency had concerns about cancer. Advisers to the agency determined the benefits of the drug outweighed the risks.
http://www.bloomberg.com/news/2013-05-02/arena-withdrawing-o…
...vielleicht einmal gar nicht so dumm mit dem Antrag zu warten....es scheinen wohl noch einige Unsicherheiten bzgl. des Zulas-antrages zu bestehen.......ARNA will wohl bei dem Zula-Antrag auf "volle Nummer" sicher gehen...
....aber trotzalledem kommt diese Ankündigung einfach zu einem sehr sehr sehr ungünstiger Zeitpunkt....
Arena Pharmaceuticals to Host Corporate Update and Financial Results Conference Call and Webcast on Thursday, May 2, 2013
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) had a loss and missed Wall Street’s expectations, AND came up short on beating the revenue expectation. The revenue miss is a negative sign to shareholders seeking high growth out of the company. Shares are down 17.85%.
Arena Pharmaceuticals, Inc. Earnings Cheat Sheet
Results: Adjusted Earnings Per Share decreased to $-0.09 in the quarter versus EPS of $-0.18 in the year-earlier quarter.
Revenue: Rose 8.22% to $2.37 million from the year-earlier quarter.
Actual vs. Wall St. Expectations: Arena Pharmaceuticals, Inc. reported adjusted EPS loss of $0.09 per share. By that measure, the company missed the mean analyst estimate of $0.11. It missed the average revenue estimate of $50.36 million.
Quoting Management: “We are pleased with AACE’s recently published treatment guidelines and the specific reference to BELVIQ, which we believe will increase awareness among physicians and support the expansion of reimbursement,” said Jack Lief, Arena’s President and Chief Executive Officer. “We look forward to the completion of BELVIQ’s scheduling designation in the United States, and we are focused on making this new treatment option available to patients in additional parts of the world.”
Revenue increased 22.16% from $1.94 million in the previous quarter. EPS decreased to $-0.09 in the quarter versus EPS of $-0.10 in the previous quarter.
Looking Forward: Analysts have a more positive outlook for the company’s next-quarter performance. Over the past three months, the average estimate for next quarter’s earnings has risen from a loss of $0.05 to a loss $0.03. For the current year, the average estimate has moved down from a loss of $0.04 to a loss of $0.06 over the last ninety days.
http://wallstcheatsheet.com/stocks/arena-pharmaceuticals-ear…
..so kann man es natürlich auch sehen.....
Msg 57745 of 57784 at 5/2/2013 8:19:43 PM by
Mr. Joseph
The good news is that tomorrow the weak hands will sell, the shorts will cover, and we’ll get rid of both of them at last!
....die Hedgies werden jetzt noch einmal "auf Teufel komm raus" covern...
...vielleicht die letzte Chance ARNA bis auf 6 USD runterzuprügeln ???
Msg 57745 of 57784 at 5/2/2013 8:19:43 PM by
Mr. Joseph
The good news is that tomorrow the weak hands will sell, the shorts will cover, and we’ll get rid of both of them at last!
....die Hedgies werden jetzt noch einmal "auf Teufel komm raus" covern...
...vielleicht die letzte Chance ARNA bis auf 6 USD runterzuprügeln ???
... yeah and I am definitely one of the mentioned "weak Hands"...
mit EU bricht der halbe Markt weg. Die Issues die zum Rückzug von der EMA geführt haben (wird ja wohl irgendwas Safety-relevantes sein) müssen schon gravierend sein. Diese Probleme kriegt der US-Markt auch früher oder später ...
Denke ich
mit EU bricht der halbe Markt weg. Die Issues die zum Rückzug von der EMA geführt haben (wird ja wohl irgendwas Safety-relevantes sein) müssen schon gravierend sein. Diese Probleme kriegt der US-Markt auch früher oder später ...
Denke ich
Zitat von me_2: ... yeah and I am definitely one of the mentioned "weak Hands"...
mit EU bricht der halbe Markt weg. Die Issues die zum Rückzug von der EMA geführt haben (wird ja wohl irgendwas Safety-relevantes sein) müssen schon gravierend sein.
Diese Probleme kriegt der US-Markt auch früher oder später ...
Denke ich
Hallo me_2
Zu meinem Geamt-EK sind es zwar noch einige viele xx Prozente nach unten, jedoch habe ich bereits auch schon Teilverkäufe getätigt.
Viele Anleger ( me too
 ) haben natürlich viel Hoffnung auf ein mögliches Approval in Europa gelegt. Mit der Nachricht hat sicherlich keiner gerechnet.
) haben natürlich viel Hoffnung auf ein mögliches Approval in Europa gelegt. Mit der Nachricht hat sicherlich keiner gerechnet. Bzgl.der "issues" kann ich nichts sagen,ich denke aber auch , dass viele Unsicherheiten bestehen, die ARNA dazu veranlasst haben, diesen Schritt zu gehen.
Jetzt heißt es abwarten, DEA "announcen" lassen und dann sehen, wie Belviq in den USA vermarktet wird.
Grüße
bernie55

Barron's 500: America's Top Companies Leave One Wishing For A Thicker Wallet
..unter anderem was zu ARENA...
There are now two newly FDA-approved anti-obesity drugs on the market, or will be "imminently" according to Arena's (ARNA) CEO on May 2. Arena's Belviq and Vivus's (VVUS) Qsymia are poised to profit from this major change in policy. Both have markets, but a 16-point comparison indicates Belviq will gain greater acceptance by patients and primary care physicians and another analysis indicates Belviq will dominate in the obesity clinic market where most of the anti-obesity drugs are prescribed.
This may also be the best possible time to buy Arena since the worst news just passed and it faces many positive catalysts.
The worst possible news hit Thursday when Arena temporarily forfeited an edge over Vivus by strategically withdrawing its application to the European Medical Association (EMA). That dropped Arena 22.6% to $6.50 (a price last seen in early June 2012) before bouncing a bit to finish down an ugly 9.2%. Arena was already down 23.6% from its $11 peak in January and holding flat on news that the EMA had questions. Arena is now down a whopping 31.6%.
Because Arena and Vivus generally trade in tandem, contrasting their reduction in share price upon bad European news is one measure of knowing if Arena overshot its bottom and if it created a buying opportunity. It appears so because in contrast to Arena, Vivus only dropped 6% when the EMA rejected it (-11.5% on news of likely rejection followed by a 60% rebound before dropping 2% when officially rejected in October 2012).
In addition to looking backwards to determine if the Street overreacted, we should also look forward and we find plenty of compelling reasons to buy into this dip since there are more than ten near and mid-term catalysts that should boost Arena's stock.
While there is always more than meets the eye, Arena's stated strategy is to conduct nonclinical studies that could not be completed fast enough to meet the EMA's strict timeline. Because clinical trials are relatively short, we may yet see Belviq before the EMA in 6-18 months with a much clearer and stronger hand, including a better deal with European marketing partner for some of the reasons stated below.
Additional catalysts for Arena include:
(1) the imminent DEA scheduling of Belviq, probably as a schedule IV per FDA recommendations with a $65 million milestone payment from Eisai (ESALY.PK);
(2) Marketing of Belviq by Eisai, perhaps immediately upon scheduling;
(3) Potential approval of Belviq by the South Korean equivalent of the FDA which will result in a $3 million milestone payment from Ildong;
(4) Potential approval of Eisai's recent application for Belviq in Mexico and Switzerland;
(5) Results from phase I trials that may ultimately pit Arena against Novartis's multiple sclerosis drug Gilenya which generated $500 million in 2012 despite its many side effects;
(6) Applications for Belviq to Canada and other countries;
(7) a potential study of Belviq on smoking cessation;
(8) a new application to Europe;
(9) several more drugs in the pipeline, sales results, et cetera.
Thus, by all measures, this would be the time to buy into Arena, as a hedge or as a pure play.
http://seekingalpha.com/article/1408101-barron-s-500-america…
..unter anderem was zu ARENA...
There are now two newly FDA-approved anti-obesity drugs on the market, or will be "imminently" according to Arena's (ARNA) CEO on May 2. Arena's Belviq and Vivus's (VVUS) Qsymia are poised to profit from this major change in policy. Both have markets, but a 16-point comparison indicates Belviq will gain greater acceptance by patients and primary care physicians and another analysis indicates Belviq will dominate in the obesity clinic market where most of the anti-obesity drugs are prescribed.
This may also be the best possible time to buy Arena since the worst news just passed and it faces many positive catalysts.
The worst possible news hit Thursday when Arena temporarily forfeited an edge over Vivus by strategically withdrawing its application to the European Medical Association (EMA). That dropped Arena 22.6% to $6.50 (a price last seen in early June 2012) before bouncing a bit to finish down an ugly 9.2%. Arena was already down 23.6% from its $11 peak in January and holding flat on news that the EMA had questions. Arena is now down a whopping 31.6%.
Because Arena and Vivus generally trade in tandem, contrasting their reduction in share price upon bad European news is one measure of knowing if Arena overshot its bottom and if it created a buying opportunity. It appears so because in contrast to Arena, Vivus only dropped 6% when the EMA rejected it (-11.5% on news of likely rejection followed by a 60% rebound before dropping 2% when officially rejected in October 2012).
In addition to looking backwards to determine if the Street overreacted, we should also look forward and we find plenty of compelling reasons to buy into this dip since there are more than ten near and mid-term catalysts that should boost Arena's stock.
While there is always more than meets the eye, Arena's stated strategy is to conduct nonclinical studies that could not be completed fast enough to meet the EMA's strict timeline. Because clinical trials are relatively short, we may yet see Belviq before the EMA in 6-18 months with a much clearer and stronger hand, including a better deal with European marketing partner for some of the reasons stated below.
Additional catalysts for Arena include:
(1) the imminent DEA scheduling of Belviq, probably as a schedule IV per FDA recommendations with a $65 million milestone payment from Eisai (ESALY.PK);
(2) Marketing of Belviq by Eisai, perhaps immediately upon scheduling;
(3) Potential approval of Belviq by the South Korean equivalent of the FDA which will result in a $3 million milestone payment from Ildong;
(4) Potential approval of Eisai's recent application for Belviq in Mexico and Switzerland;
(5) Results from phase I trials that may ultimately pit Arena against Novartis's multiple sclerosis drug Gilenya which generated $500 million in 2012 despite its many side effects;
(6) Applications for Belviq to Canada and other countries;
(7) a potential study of Belviq on smoking cessation;
(8) a new application to Europe;
(9) several more drugs in the pipeline, sales results, et cetera.
Thus, by all measures, this would be the time to buy into Arena, as a hedge or as a pure play.
http://seekingalpha.com/article/1408101-barron-s-500-america…
Antwort auf Beitrag Nr.: 44.581.879 von bernie55 am 07.05.13 13:37:18Hi Bernie,
das sind viele schöne Argumente die seit Monaten/Jahren vorgebetet werden. Aber so langsam fange ich an an der Story zu verzweifeln. Die 65M Meilensteine haben wir inzwischen wohl schon während des DEA-Prozesses verfrühstückt. Rückzug einer Marketing Application hab ich noch nie gehört, da müssen schon heftige (Safety) Bedenken vorliegen. Und wer sagt uns daß die anderen Länder CH, Canada, SüdKorea, Mex u.a. da nicht auch Einwände haben? Bei VVUS läufts auch nicht ... Die Leute von Eisai warten schon seit Monaten auf ihren Einsatz, hoffentlich haben die in der Zwischenzeit noch was anderes zu tun, auch hier entstehen zusätzl. Vorhaltungkosten. Die sonstige Pipeline war bisher auch nie im Gespräch, wieso sollte die jetzt plötzlich einen Wert bekommen, wo der Hoffnungsträger Probleme macht?
Die Liste der potential de-catalysts wäre auch ganz schön lang.
Bin zwar noch dabei, weiß aber nicht wie lange.
das sind viele schöne Argumente die seit Monaten/Jahren vorgebetet werden. Aber so langsam fange ich an an der Story zu verzweifeln. Die 65M Meilensteine haben wir inzwischen wohl schon während des DEA-Prozesses verfrühstückt. Rückzug einer Marketing Application hab ich noch nie gehört, da müssen schon heftige (Safety) Bedenken vorliegen. Und wer sagt uns daß die anderen Länder CH, Canada, SüdKorea, Mex u.a. da nicht auch Einwände haben? Bei VVUS läufts auch nicht ... Die Leute von Eisai warten schon seit Monaten auf ihren Einsatz, hoffentlich haben die in der Zwischenzeit noch was anderes zu tun, auch hier entstehen zusätzl. Vorhaltungkosten. Die sonstige Pipeline war bisher auch nie im Gespräch, wieso sollte die jetzt plötzlich einen Wert bekommen, wo der Hoffnungsträger Probleme macht?
Die Liste der potential de-catalysts wäre auch ganz schön lang.
Bin zwar noch dabei, weiß aber nicht wie lange.
So DEA-Scheduling ist erfolgt, Vermarktung in 30 Tagen ab Morgen.
Einen schönen Tag den Standhaften.
Gruß
Oberländler
http://ofr.gov/OFRUpload/OFRData/2013-10895_PI.pdf
Einen schönen Tag den Standhaften.

Gruß
Oberländler
http://ofr.gov/OFRUpload/OFRData/2013-10895_PI.pdf
Antwort auf Beitrag Nr.: 44.583.097 von Oberlaendler am 07.05.13 16:01:14Daß ich das noch erleben darf ... 

Antwort auf Beitrag Nr.: 44.583.135 von me_2 am 07.05.13 16:06:09Kurs reagiert recht verhalten, da hatten wir doch a wenig mehr erwartet, oder??
Antwort auf Beitrag Nr.: 44.583.827 von me_2 am 07.05.13 17:15:40Es gibt wohl noch Zweifel, bezüglich der möglichen Verkaufszahlen bzw. eines schwierigen Startes (natürlich AF).
Siehe auch http://www.thestreet.com/_nasdaq/story/11915869/1/happy-day-…
(Und noch einige Leute, die wohl mit ca. 62 Mio Shares short sind, nach Angabe NASDAQ.)
Oberländler
Siehe auch http://www.thestreet.com/_nasdaq/story/11915869/1/happy-day-…
(Und noch einige Leute, die wohl mit ca. 62 Mio Shares short sind, nach Angabe NASDAQ.)
Oberländler
Arena Pharmaceuticals Provides Update on Upcoming US Launch of BELVIQ® (lorcaserin HCl) for Chronic Weight Management
SAN DIEGO, May 7, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the Office of the Federal Register filed for public inspection the US Drug Enforcement Administration's (DEA) final rule placing BELVIQ (pronounced BEL-VEEK) into Schedule IV of the Controlled Substances Act. The scheduling designation will be effective 30 days after tomorrow's expected publication in the Federal Register. Following the effective date, BELVIQ will be available to patients in the United States by prescription, and Arena will receive $65 million in milestone payments from Eisai Inc. under their marketing and supply agreement.
BELVIQ is approved for use as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index (BMI) of 30 kg/m2 or greater (obese), or 27 kg/m2 or greater (overweight) in the presence of at least one weight-related comorbid condition (e.g., hypertension, dyslipidemia, type 2 diabetes). The indication includes the following limitations of use: The safety and efficacy of coadministration of BELVIQ with other products intended for weight loss and the effect of BELVIQ on cardiovascular morbidity and mortality have not been established.
"We are thrilled that BELVIQ will soon be available in the United States as a new treatment option for the medical management of patients who are overweight with a comorbidity or obese," said Jack Lief, Arena's President and Chief Executive Officer.
that BELVIQ will soon be available in the United States as a new treatment option for the medical management of patients who are overweight with a comorbidity or obese," said Jack Lief, Arena's President and Chief Executive Officer.
Eisai is responsible for the marketing and distribution of BELVIQ in the United States. Arena manufactures BELVIQ at its facility in Switzerland, and sells finished commercial product to Eisai for a purchase price starting at 31.5% of Eisai's annual net product sales. The purchase price increases on a tiered basis up to 36.5% on the portion of annual net product sales exceeding $750 million. Arena is also eligible to receive $1.16 billion in purchase price adjustment payments based on annual net sales levels of BELVIQ.
http://finance.yahoo.com/news/arena-pharmaceuticals-provides…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-provides…
SAN DIEGO, May 7, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the Office of the Federal Register filed for public inspection the US Drug Enforcement Administration's (DEA) final rule placing BELVIQ (pronounced BEL-VEEK) into Schedule IV of the Controlled Substances Act. The scheduling designation will be effective 30 days after tomorrow's expected publication in the Federal Register. Following the effective date, BELVIQ will be available to patients in the United States by prescription, and Arena will receive $65 million in milestone payments from Eisai Inc. under their marketing and supply agreement.
BELVIQ is approved for use as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index (BMI) of 30 kg/m2 or greater (obese), or 27 kg/m2 or greater (overweight) in the presence of at least one weight-related comorbid condition (e.g., hypertension, dyslipidemia, type 2 diabetes). The indication includes the following limitations of use: The safety and efficacy of coadministration of BELVIQ with other products intended for weight loss and the effect of BELVIQ on cardiovascular morbidity and mortality have not been established.
"We are thrilled
 that BELVIQ will soon be available in the United States as a new treatment option for the medical management of patients who are overweight with a comorbidity or obese," said Jack Lief, Arena's President and Chief Executive Officer.
that BELVIQ will soon be available in the United States as a new treatment option for the medical management of patients who are overweight with a comorbidity or obese," said Jack Lief, Arena's President and Chief Executive Officer.Eisai is responsible for the marketing and distribution of BELVIQ in the United States. Arena manufactures BELVIQ at its facility in Switzerland, and sells finished commercial product to Eisai for a purchase price starting at 31.5% of Eisai's annual net product sales. The purchase price increases on a tiered basis up to 36.5% on the portion of annual net product sales exceeding $750 million. Arena is also eligible to receive $1.16 billion in purchase price adjustment payments based on annual net sales levels of BELVIQ.
http://finance.yahoo.com/news/arena-pharmaceuticals-provides…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-provides…
Antwort auf Beitrag Nr.: 44.589.413 von bernie55 am 08.05.13 10:22:38Sobald wir jenseits der 10 notieren bin ich auch thrilled 



Arena Pharmaceuticals to Receive $65M in Milestone Payments from Eisai Inc.
Wednesday, May 8, 2013
San Diego’s Arena Pharmaceuticals Inc. will receive $65 million in milestone payments from Japanese pharma company Eisai Inc. after receiving U.S. Drug Enforcement Administration approval of its weight loss drug, Belviq. This approval will allow the drug to be sold on the U.S. market.
The DEA will officially designate Belviq a Schedule IV controlled substance in about a month’s time, meaning it has a relatively low potential to be addictive in patients.
Shares for the company, whose market cap is $1.81 billion, rose 11 percent, trading at $8.34 at market close.
Belviq was approved in June 2012 by the U.S Food and Drug Administration — the first anti-obesity drug approved by the FDA since 2000.
Eisai is responsible for the marketing and distribution of Belviq in the U.S. It is manufactured by Arena in its Switzerland facility, and sells the finished commercial product to Eisai for a purchase price starting at 31.5 percent of Eisai’s annual net product sales, the company said in a statement.
Arena is eligible to receive $1.16 billion based on annual net sales levels of Belviq, the company said.
— Meghana Keshavan
http://www.sdbj.com/news/2013/may/08/arena-pharmaceuticals-r…
Wednesday, May 8, 2013
San Diego’s Arena Pharmaceuticals Inc. will receive $65 million in milestone payments from Japanese pharma company Eisai Inc. after receiving U.S. Drug Enforcement Administration approval of its weight loss drug, Belviq. This approval will allow the drug to be sold on the U.S. market.
The DEA will officially designate Belviq a Schedule IV controlled substance in about a month’s time, meaning it has a relatively low potential to be addictive in patients.
Shares for the company, whose market cap is $1.81 billion, rose 11 percent, trading at $8.34 at market close.
Belviq was approved in June 2012 by the U.S Food and Drug Administration — the first anti-obesity drug approved by the FDA since 2000.
Eisai is responsible for the marketing and distribution of Belviq in the U.S. It is manufactured by Arena in its Switzerland facility, and sells the finished commercial product to Eisai for a purchase price starting at 31.5 percent of Eisai’s annual net product sales, the company said in a statement.
Arena is eligible to receive $1.16 billion based on annual net sales levels of Belviq, the company said.
— Meghana Keshavan
http://www.sdbj.com/news/2013/may/08/arena-pharmaceuticals-r…
Zitat von me_2: Sobald wir jenseits der 10 notieren bin ich auch thrilled
Dein Zitat vom 03.05.13 hat dich doch bereits " gethrilled"
... yeah and I am definitely one of the mentioned "weak Hands"..
Aber ich gebe zu, dass dein Zitat " Sobald wir jenseits der 10 notieren bin ich auch thrilled" dich emotional sicherlich
anders "thrillen" wird....
In diesem Sinne, good " thrill" to all ARENIACS and AREANITES

Grüße
bernie55

..und noch was zum " Thrillen"...
Vivus reports weaker sales of diet pill Qsymia
Wed May 8, 2013 7:50am EDT
May 8 (Reuters) - Vivus Inc reported weaker-than-expected quarterly sales of its diet drug Qsymia amid difficulties over reimbursement for obesity treatments and a restricted sales channel.
Net product revenue, reflecting sales of Qsymia, rose to $4.1 million in the first quarter from $2 million in the preceding quarter. The drug was launched late last year.
The company's net loss widened to $53.6 million, or 53 cents per share, from $18.8 million, or 20 cents per share, a year earlier.
Analysts had expected a loss of 51 cents per share on revenue of $5.2 million, according to Thomson Reuters I/B/E/S.
Qsymia was the first weight-loss pill to be launched in the United States in 13 years.
http://www.reuters.com/article/2013/05/08/vivus-results-idUS…

Vivus reports weaker sales of diet pill Qsymia
Wed May 8, 2013 7:50am EDT
May 8 (Reuters) - Vivus Inc reported weaker-than-expected quarterly sales of its diet drug Qsymia amid difficulties over reimbursement for obesity treatments and a restricted sales channel.
Net product revenue, reflecting sales of Qsymia, rose to $4.1 million in the first quarter from $2 million in the preceding quarter. The drug was launched late last year.
The company's net loss widened to $53.6 million, or 53 cents per share, from $18.8 million, or 20 cents per share, a year earlier.
Analysts had expected a loss of 51 cents per share on revenue of $5.2 million, according to Thomson Reuters I/B/E/S.
Qsymia was the first weight-loss pill to be launched in the United States in 13 years.
http://www.reuters.com/article/2013/05/08/vivus-results-idUS…
Antwort auf Beitrag Nr.: 44.591.683 von bernie55 am 08.05.13 14:15:51Yeah yeah, VVUS's Gegenwart könnte unsere Zukunft sein. Ain't that thrilling? 



Antwort auf Beitrag Nr.: 44.591.683 von bernie55 am 08.05.13 14:15:51..und noch was zum " Thrillen"...







Antwort auf Beitrag Nr.: 44.592.341 von franzisca am 08.05.13 15:29:27A Gitter mit nix drin, no thrill
Antwort auf Beitrag Nr.: 44.592.361 von me_2 am 08.05.13 15:31:12kommt noch! 

Arena Pharmaceuticals buy
Finanzen.net Up-/Downgr.Von Quelle: Finanzen.net / Aktiencheck.de AG | Finanzen.net Up-/Downgr. – Do., 8. Nov 2012 09:50 MEZ
E-Mail
Freigeben
Drucken
Firmen:
NEW YORK
Arena Pharmaceuticals Inc
AKTUELLER KURS
Symbol Kurs Veränderung
ARNA 8,35 +0,01
New York (Frankfurt: A0DKRK - Nachrichten) (www.aktiencheck.de) - Die Analysten von Jefferies & Co stufen die Arena Pharmaceuticals (NasdaqGM: ARNA - Nachrichten) -Aktie (ISIN US0400471027/ WKN 939027) mit dem Votum "buy" ein. Das Kursziel werde von 20,00 USD auf 14,00 USD gesenkt. (Analyse vom 07.11.2012) (08.11.2012/ac/a/a)
Offenlegung von möglichen Interessenskonflikten: Mögliche Interessenskonflikte können Sie auf der Site des Erstellers/ der Quelle der Analyse einsehen.
Finanzen.net Up-/Downgr.Von Quelle: Finanzen.net / Aktiencheck.de AG | Finanzen.net Up-/Downgr. – Do., 8. Nov 2012 09:50 MEZ
Freigeben
Firmen:
NEW YORK
Arena Pharmaceuticals Inc
AKTUELLER KURS
Symbol Kurs Veränderung
ARNA 8,35 +0,01
New York (Frankfurt: A0DKRK - Nachrichten) (www.aktiencheck.de) - Die Analysten von Jefferies & Co stufen die Arena Pharmaceuticals (NasdaqGM: ARNA - Nachrichten) -Aktie (ISIN US0400471027/ WKN 939027) mit dem Votum "buy" ein. Das Kursziel werde von 20,00 USD auf 14,00 USD gesenkt. (Analyse vom 07.11.2012) (08.11.2012/ac/a/a)
Offenlegung von möglichen Interessenskonflikten: Mögliche Interessenskonflikte können Sie auf der Site des Erstellers/ der Quelle der Analyse einsehen.
Oh sorry.....alter mist....habe ich gerade erst gesehen...Sorry...zuviel Multitasking 

 ..so viel zum " Thrillen"...
..so viel zum " Thrillen"...
Ahead of the Bell: Vivus 1Q loss widens
VVUS: 12.81 Up 0.76(6.06%) 10:09AM EDT -

DEA Scheduling IV für BELVIQ (ARNA)
ARNA: 8.32 Down 0.02(0.24%) 10:09AM EDT -

Arena Pharmaceuticals to Present at the Bank of America Merrill Lynch 2013 Health Care Conference
SAN DIEGO, May 10, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at the Bank of America Merrill Lynch 2013 Health Care Conference on Wednesday, May 15, 2013, at 2:20 p.m. Pacific Time (5:20 p.m. Eastern Time), at the Encore at the Wynn Las Vegas Hotel in Las Vegas, Nevada.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
SAN DIEGO, May 10, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at the Bank of America Merrill Lynch 2013 Health Care Conference on Wednesday, May 15, 2013, at 2:20 p.m. Pacific Time (5:20 p.m. Eastern Time), at the Encore at the Wynn Las Vegas Hotel in Las Vegas, Nevada.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
Antwort auf Beitrag Nr.: 44.606.489 von bernie55 am 10.05.13 15:24:13nice venue, kann man anschließend noch ne Runde Blackjack spielen...
Antwort auf Beitrag Nr.: 44.606.979 von me_2 am 10.05.13 16:11:51Angesichts aller aktuellen positiven Catalysts tut sich am Kurs nix. No thrill !! und die zu erwartende enttäuschenden sales Zahlen kommen erst noch ... ob das noch was wird?
Antwort auf Beitrag Nr.: 44.608.495 von me_2 am 10.05.13 19:19:21Byebye ARNA, hab mich verabschiedet, andere Inc.s haben auch schöne Stocks. Und aussichtsreichere. Wünsche allen Arenauten noch gute Investments.
Wir haben ja lange genug darauf gewartet , aber jetzt ist es soweit.
Am 7 Juni wird in den USA das Medikament Belviq endlich auf den Markt eingeführt werden.
Innerhalb des Monats Juni können dann die ersten Umsätzen eingefahren werden.
Marketing partner Eisa's (ESALF.PK) CEO Lonnel Coats, from the May 11th conference call, states that by June 11, Belviq will be available in 20,000 pharmacies and within the first 30 days on the market, the sales force will have touched 20,000 physicians.
CEO L.C. "We will penetrate this market very quickly."
http://seekingalpha.com/article/1457311-arena-buyout-more-hi…

Am 7 Juni wird in den USA das Medikament Belviq endlich auf den Markt eingeführt werden.
Innerhalb des Monats Juni können dann die ersten Umsätzen eingefahren werden.
Marketing partner Eisa's (ESALF.PK) CEO Lonnel Coats, from the May 11th conference call, states that by June 11, Belviq will be available in 20,000 pharmacies and within the first 30 days on the market, the sales force will have touched 20,000 physicians.
CEO L.C. "We will penetrate this market very quickly."
http://seekingalpha.com/article/1457311-arena-buyout-more-hi…
Arena Pharmaceuticals to Present at Two Upcoming Investor Conferences
SAN DIEGO, May 24, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at the following investor conferences:
Deutsche Bank Securities 38th Annual Health Care Conference
Presentation: May 30, 2013, at 12:00 p.m. Eastern Time (9:00 a.m. Pacific Time) at The Westin Boston Waterfront Hotel in Boston, Massachusetts
Jefferies 2013 Global Healthcare Conference
Presentation: June 4, 2013, at 9:30 a.m. Eastern Time (6:30 a.m. Pacific Time) at the Grand Hyatt New York in New York City
A live audio webcast of each presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of each presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of each webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
SAN DIEGO, May 24, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at the following investor conferences:
Deutsche Bank Securities 38th Annual Health Care Conference
Presentation: May 30, 2013, at 12:00 p.m. Eastern Time (9:00 a.m. Pacific Time) at The Westin Boston Waterfront Hotel in Boston, Massachusetts
Jefferies 2013 Global Healthcare Conference
Presentation: June 4, 2013, at 9:30 a.m. Eastern Time (6:30 a.m. Pacific Time) at the Grand Hyatt New York in New York City
A live audio webcast of each presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of each presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of each webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
fängt die Gurke nun wieder an zu laufen????
Sieht ja sehr schön aus Heute
Sieht ja sehr schön aus Heute
In der Ruhe liegt die Kraft. :-)
Arena's Belviq Set To Launch
Aus o.g. Artikel habe ich mal die Einschätzungen vom Management von EISAI und ARENA bzgl. der möglichen Vermarktung von Belviq herauskopiert.
In an interview on Bloomberg Tuesday, Eisai and Arena management guided that the team was shorting for Belviq sales to hit $150 million this year. In modeling a breakdown, the prescription levels might need to look like the chart below.
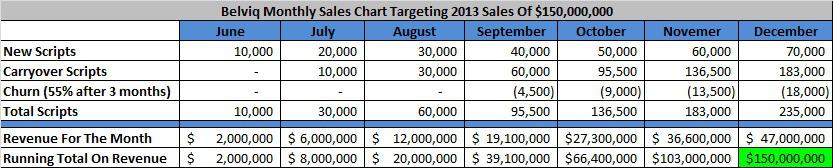
http://seekingalpha.com/article/1481361-arena-s-belviq-set-t…
Aus o.g. Artikel habe ich mal die Einschätzungen vom Management von EISAI und ARENA bzgl. der möglichen Vermarktung von Belviq herauskopiert.
In an interview on Bloomberg Tuesday, Eisai and Arena management guided that the team was shorting for Belviq sales to hit $150 million this year. In modeling a breakdown, the prescription levels might need to look like the chart below.

http://seekingalpha.com/article/1481361-arena-s-belviq-set-t…
Antwort auf Beitrag Nr.: 44.789.399 von bernie55 am 05.06.13 10:52:05nice!!!!
und ARNA ist im grünen Bereich (in USA) obwohl die großen im Minus stehen.

und ARNA ist im grünen Bereich (in USA) obwohl die großen im Minus stehen.

Antwort auf Beitrag Nr.: 44.789.399 von bernie55 am 05.06.13 10:52:05Ich glaub ja nicht dass die wirklichen Zahlen so aussehen werden. Das sind ja erst mal nur Phantasien vom Excelsheet.
 Arena Pharmaceuticals Reports on Launch of BELVIQ® (lorcaserin HCl) CIV for Chronic Weight Management in Adults who are Overweight with a Comorbidity or Obese
Arena Pharmaceuticals Reports on Launch of BELVIQ® (lorcaserin HCl) CIV for Chronic Weight Management in Adults who are Overweight with a Comorbidity or Obese 
> BELVIQ Available in US Pharmacies within One Week --
> Arena Achieves $65 Million in Milestones and is Eligible for Additional Payments Based on Product Sales --
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
Antwort auf Beitrag Nr.: 44.807.805 von bernie55 am 07.06.13 15:33:32Hi Bernie,
hebt unsere Arnea ab?
Arena Pharmaceuticals, Inc. Echtzeit Börsenkurse
$ 10* 1.31 15,07%
* Real-Time - Daten per 2013.06.10
Exchange: NASDAQ
Industrie: Health Care
Gemeinschaft Rating: Bullish
ARNA
AMRN
DNDN
Bearbeiten Symbol Liste
Symbol Listenansichten
FlashQuotes
InfoQuotes
Lager Details
Zusammenfassung Quote
Real-Time finden
After Hours Quote
Pre-Markt finden
Historische Quote
Option Kette
CHARTS
Standard Chart
Chart
COMPANY NEWS
Unternehmen Nachrichten
Pressemitteilungen
Markt-Stream
Aktien-Analyse
Analyst Research
Guru Analysis
Stock Report
Wettbewerber
Auf Consultant
Lager Vergleich
FUNDAMENTALS
Rufen Transkripte
Geschäftsbericht
Gewinn-und Verlustrechnung
Umsatz / EPS
SEC-Hinterlegungen
Short Interest
Dividende Geschichte
HOLDINGS
Ownership Zusammenfassung
Institutionelle Holdings
Insiders
(SEC Form 4)
Sparen Aktien
Wechseln Sie zu dem führenden Online-Forex-Plattform. Genießen Copy Trader und OpenBook Echtzeit-Handel.
ARNA der NASDAQ Last Sale (NLS)
NLS Volume Vortag Heute ist High / Low 52 Wk Hoch / Tief
10 1.31 15.07%

Daten per 2013.06.10 04.35.00
3132926
$ 8.69
10 / 8,50
13,50 $ / 6,36 $
Read more: http://www.nasdaq.com/symbol/arna/real-time#ixzz2Vnh8JUnI
hebt unsere Arnea ab?
Arena Pharmaceuticals, Inc. Echtzeit Börsenkurse
$ 10* 1.31 15,07%
* Real-Time - Daten per 2013.06.10
Exchange: NASDAQ
Industrie: Health Care
Gemeinschaft Rating: Bullish
ARNA
AMRN
DNDN
Bearbeiten Symbol Liste
Symbol Listenansichten
FlashQuotes
InfoQuotes
Lager Details
Zusammenfassung Quote
Real-Time finden
After Hours Quote
Pre-Markt finden
Historische Quote
Option Kette
CHARTS
Standard Chart
Chart
COMPANY NEWS
Unternehmen Nachrichten
Pressemitteilungen
Markt-Stream
Aktien-Analyse
Analyst Research
Guru Analysis
Stock Report
Wettbewerber
Auf Consultant
Lager Vergleich
FUNDAMENTALS
Rufen Transkripte
Geschäftsbericht
Gewinn-und Verlustrechnung
Umsatz / EPS
SEC-Hinterlegungen
Short Interest
Dividende Geschichte
HOLDINGS
Ownership Zusammenfassung
Institutionelle Holdings
Insiders
(SEC Form 4)
Sparen Aktien
Wechseln Sie zu dem führenden Online-Forex-Plattform. Genießen Copy Trader und OpenBook Echtzeit-Handel.
ARNA der NASDAQ Last Sale (NLS)
NLS Volume Vortag Heute ist High / Low 52 Wk Hoch / Tief
10 1.31 15.07%


Daten per 2013.06.10 04.35.00
3132926
$ 8.69
10 / 8,50
13,50 $ / 6,36 $
Read more: http://www.nasdaq.com/symbol/arna/real-time#ixzz2Vnh8JUnI
Antwort auf Beitrag Nr.: 44.817.485 von Magnetfeldfredy am 10.06.13 10:36:26Schaun wir mal ob das echt ist oder ein fake-trade?
Tolle Homepage von Belviq unter: www.belviq.com
Tolle Homepage von Belviq unter: www.belviq.com
Hi Fredy,
heute morgen haben in den USA alle noch von einer Funktionsstörung ( glitch) geredet, jetzt sind aber bereits 1500 Stk.(in 100,300 500 Paketen) gehandelt worden.
Es sind zwar nicht viele , aber in D haben die Makler zumindest auch hochgetaxt.
Somit gehe ich mal davon aus, dass diese Trades kein "fakes" sind.
Grüße
bernie55

heute morgen haben in den USA alle noch von einer Funktionsstörung ( glitch) geredet, jetzt sind aber bereits 1500 Stk.(in 100,300 500 Paketen) gehandelt worden.
Es sind zwar nicht viele , aber in D haben die Makler zumindest auch hochgetaxt.
Somit gehe ich mal davon aus, dass diese Trades kein "fakes" sind.
Grüße
bernie55

Antwort auf Beitrag Nr.: 44.818.565 von bernie55 am 10.06.13 13:06:33http://www.cbsnews.com/video/watch/?id=50148592n
wir stehen vorbörslich bei $9,09


Vorbörse interessiert aber leider keinen bei den paar Kleinigkeiten, die da gehandelt werden /wurden
Kurs derzeit bei USD 7,75. Es sickert wohl durch das es sich um einen Ladenhüter handelt bzw. die ersten Sales-Ergebnisse absolut enttäuschend sind. Vermutlich wieder ein Wert wo es besser gewesen wäre sich direkt nach der Zulassungseuphorie zu verabschieden.
Die harte Realität, wo Unternehmensbewertungen dann auch durch Ergebnisse gerechtfertigt werden müssen sieht halt meist anders aus.
Die harte Realität, wo Unternehmensbewertungen dann auch durch Ergebnisse gerechtfertigt werden müssen sieht halt meist anders aus.
Antwort auf Beitrag Nr.: 44.853.861 von MACD am 14.06.13 21:49:27Entäuschender Verkauf, wo hast Du den diesen Quatsch her,
das ist der Grund:
http://biz.yahoo.com/e/130614/arna8-k.html;_ylt=ApWhGuTIxjHd…
Schön sauber recherchieren und keine stories erfinden!
das ist der Grund:
http://biz.yahoo.com/e/130614/arna8-k.html;_ylt=ApWhGuTIxjHd…
Schön sauber recherchieren und keine stories erfinden!

Zitat von MACD: ......Es sickert wohl durch das es sich um einen Ladenhüter handelt bzw. die ersten Sales-Ergebnisse absolut enttäuschend sind.
Der Beginn des Verkaufs von Belviq erfolgte aufgrund des ein wenig verzögerten
 " DEA schedulings" erst ab dem 07.06/10.06.
" DEA schedulings" erst ab dem 07.06/10.06. Nach einer Periode von ca. 5/6 Tagen schon von "ersten enttäuschenden Verkaufszahlen" zu sprechen bzw. von einem " Ladenhüter" zu sprechen, kann ich gedanklich absolut nicht nachvollziehen.
Ich denke,wir müssen zuerst die Ergebnisse des dritten/vierten Quartals abwarten , um dann von aussagekräftigen Tendenzen zu sprechen.
Wir sind im übrigen alle realistisch genug, um uns nicht mit progostizierten Fantasiezahlen zu "dopen". Ich glaube aber auch, dass über die Kooperation mit Eisai ein Riesen-Potential bzgl. der Umsätze ausgeschöpft werden kann
Obwohl ich kein Charttechniker bin, habe ich mal ein bisschen "gechartert" und festgestellt , dass die Indikatoren (MACD
 und SSt.) ein Verkaufssignal generierten.Vielleicht neben dem "Change in "Directors or Principal Officers" ( siehe Magnetfeldfredy) und der wieder beginnenden "Hedgiezockerei" nach dem DEA Scheduling, auch ein Grund.
und SSt.) ein Verkaufssignal generierten.Vielleicht neben dem "Change in "Directors or Principal Officers" ( siehe Magnetfeldfredy) und der wieder beginnenden "Hedgiezockerei" nach dem DEA Scheduling, auch ein Grund.Arena Pharmaceuticals im After Hours Trading im übrigen wieder ein bisschen " up" Inc.
$7.96 > + 0.18 > + 2.31% > After Hours Volume: 50,202
Ok, dann kommen vielleicht die ersten offiziellen Verkaufszahlen, aber erste Erfolge / Misserfolge und Tendenzen sickern sicher schon vorher durch und werden sich dementsprechend auf den Kurs auswirken.
Aktuell 7,52 bei recht hohem Umsatz.
Aktuell 7,52 bei recht hohem Umsatz.
Congress Presses DEA to Open up on Regulatory 'Black Hole' ???
http://www.raps.org/focus-online/news/news-article-view/arti…
By Alexander Gaffney, RF News Editor
The case study for these delays may well be the case of Arena Pharmaceuticals' Belviq (lorcaserin), a weight loss drug approved by FDA in June 2012 – the first weight loss drug approved by FDA in more than a decade, in fact.
But despite the drug's potential to be first to market, the drug entered a 10-month long quagmire at DEA undergoing the scheduling process, only to emerge as a Class IV controlled substance.
".....The letter goes on the request four pieces of information from DEA:
■The number of FDA-approved substances reviewed each year since 2007 and the time spent to review each product.
■Instances of DEA deviating from FDA's scheduling recommendation
■The average time spent to schedule a drug since 2007, and whether this has increased or decreased over time.
■Common reasons for delaying a final scheduling decision for a drug, and what steps or discussions are taken to address outstanding questions with the sponsor.
DEA has until 26 June 2013 to reply, per the letter's request
http://energycommerce.house.gov/sites/republicans.energycomm…" target="_blank" rel="nofollow ugc noopener">
http://energycommerce.house.gov/sites/republicans.energycomm…
....immerhin....
Nun scheinen sich auch Kongressabgeordnete für die DEA zu interessieren.
Die extremen zeitlichen Verzögerungen für die Erstellung eines " einfachen" "schedulings IV " seitens der DEA werden kritisiert und deshalb ist o.g Brief an die DEA gegangen...
http://www.raps.org/focus-online/news/news-article-view/arti…
By Alexander Gaffney, RF News Editor
The case study for these delays may well be the case of Arena Pharmaceuticals' Belviq (lorcaserin), a weight loss drug approved by FDA in June 2012 – the first weight loss drug approved by FDA in more than a decade, in fact.
But despite the drug's potential to be first to market, the drug entered a 10-month long quagmire at DEA undergoing the scheduling process, only to emerge as a Class IV controlled substance.
".....The letter goes on the request four pieces of information from DEA:
■The number of FDA-approved substances reviewed each year since 2007 and the time spent to review each product.
■Instances of DEA deviating from FDA's scheduling recommendation
■The average time spent to schedule a drug since 2007, and whether this has increased or decreased over time.
■Common reasons for delaying a final scheduling decision for a drug, and what steps or discussions are taken to address outstanding questions with the sponsor.
DEA has until 26 June 2013 to reply, per the letter's request
http://energycommerce.house.gov/sites/republicans.energycomm…" target="_blank" rel="nofollow ugc noopener">
http://energycommerce.house.gov/sites/republicans.energycomm…
....immerhin....
Nun scheinen sich auch Kongressabgeordnete für die DEA zu interessieren.
Die extremen zeitlichen Verzögerungen für die Erstellung eines " einfachen" "schedulings IV " seitens der DEA werden kritisiert und deshalb ist o.g Brief an die DEA gegangen...
Antwort auf Beitrag Nr.: 44.869.111 von bernie55 am 18.06.13 12:45:19die entsprechenden Stellen werden sich schon rausreden können, bzw. Ihr Vorgehen nur im Interesse der Allgemeinheit begründen.
Die Interessen von Aktionären können die Herrschaften natürlich nicht berücksichtigen.

Haben wir in der Vergangenheit doch schon oft genug erlebt. Ob gegebenenfalls Familienangehörige ein großes Aktiendepot bzw. entsprechenden Optionshandel betrieben haben kann wahrscheinlich nicht im Detail geprüft werden.

Die Interessen von Aktionären können die Herrschaften natürlich nicht berücksichtigen.

Haben wir in der Vergangenheit doch schon oft genug erlebt. Ob gegebenenfalls Familienangehörige ein großes Aktiendepot bzw. entsprechenden Optionshandel betrieben haben kann wahrscheinlich nicht im Detail geprüft werden.

Antwort auf Beitrag Nr.: 44.863.909 von MACD am 17.06.13 17:00:27die $7,50 scheinen nun auch der Boden gewesen zu sein. Nachbörslich sind wir noch auf $7,62 gestiegen.
Bin gespannt.
Bin gespannt.
NEWS von der American Medical Association = AMA
 ..in allen Medien TV, Rundfunk, Internet und vielen Tageszeitungen u.a....
..in allen Medien TV, Rundfunk, Internet und vielen Tageszeitungen u.a....
BUSINESS DAY
 A.M.A. Recognizes Obesity as a Disease
A.M.A. Recognizes Obesity as a Disease 
http://www.nytimes.com/2013/06/19/business/ama-recognizes-ob…
UPI COM
 AMA declares obesity a disease needing treatment
AMA declares obesity a disease needing treatment
http://www.upi.com/Health_News/2013/06/19/AMA-declares-obesi…
LOS ANGELES TIMES
 AMA declares obesity a disease
AMA declares obesity a disease
http://www.latimes.com/news/science/la-sci-obesity-disease-2…
 ..in allen Medien TV, Rundfunk, Internet und vielen Tageszeitungen u.a....
..in allen Medien TV, Rundfunk, Internet und vielen Tageszeitungen u.a....
BUSINESS DAY
 A.M.A. Recognizes Obesity as a Disease
A.M.A. Recognizes Obesity as a Disease 
http://www.nytimes.com/2013/06/19/business/ama-recognizes-ob…
UPI COM
 AMA declares obesity a disease needing treatment
AMA declares obesity a disease needing treatment
http://www.upi.com/Health_News/2013/06/19/AMA-declares-obesi…
LOS ANGELES TIMES
 AMA declares obesity a disease
AMA declares obesity a disease
http://www.latimes.com/news/science/la-sci-obesity-disease-2…
..die ersten Zahlen bzgl. der Verschreibung von Belviq lesen sich nicht schlecht..
Das Volumen am Handelstag Freitag 21.06.13 lag mit knapp 14 Millionen erheblich über dem 3 Monats-Durchschnitt von ca. 5,7 Millionen...
..also ein UP mit erhöhtem Volumen
> 8.05 + 0.53(+7.12%)<
Arena Surges On Script Data
IMS Health is reporting that Belviq had 1,087 prescriptions filled during the week ending June 14th.
Belviq launched on June 7th, but was not available in pharmacies until June 11th.
http://seekingalpha.com/article/1514692-arena-surges-on-scri…
Das Volumen am Handelstag Freitag 21.06.13 lag mit knapp 14 Millionen erheblich über dem 3 Monats-Durchschnitt von ca. 5,7 Millionen...
..also ein UP mit erhöhtem Volumen
> 8.05 + 0.53(+7.12%)<
Arena Surges On Script Data
IMS Health is reporting that Belviq had 1,087 prescriptions filled during the week ending June 14th.
Belviq launched on June 7th, but was not available in pharmacies until June 11th.
http://seekingalpha.com/article/1514692-arena-surges-on-scri…
Arena Pharmaceuticals, Inc. : Arena Pharmaceuticals Announces BELVIQ(TM) (lorcaserin HCl) New Drug Submission in Canada
06/26/2013| 08:05am US/Eastern
SAN DIEGO, June 26, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) announced today that Eisai Limited (based in Mississauga, Ontario), a subsidiary of Eisai Inc., has submitted a New Drug Submission (NDS) for BELVIQ(TM) (lorcaserin HCl) with Health Canada. BELVIQ is being evaluated in Canada as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management (including weight loss and weight maintenance) in adult obese patients (initial body mass index, or BMI, >= 30 kg/m(2)), or overweight patients (initial BMI >= 27 kg/m(2)) in the presence of at least one additional cardiovascular risk factor (e.g., hypertension, dyslipidemia, type 2 diabetes, or sleep apnea). In connection with the NDS, Arena will receive a milestone payment of $500,000 from Eisai.
"Approximately 60 percent of Canadians are overweight or obese, but there are limited treatment options available in Canada beyond diet and exercise," said Jack Lief, Arena's President and Chief Executive Officer. "With this New Drug Submission for BELVIQ, we hope to provide physicians with a new tool to help motivated patients manage their weight."
Eisai is responsible for the regulatory approval and, ultimately, marketing and distribution of BELVIQ in Canada. Beyond Canada, Arena has granted Eisai marketing and distribution rights to BELVIQ in most of North and South America. Arena manufactures BELVIQ at its facility in Switzerland, and sells finished commercial product to Eisai for distribution. Arena is eligible to receive payments based upon Eisai's net sales of BELVIQ and is also eligible to receive regulatory and development milestone payments.
...
http://www.4-traders.com/ARENA-PHARMACEUTICALS-IN-8425/news/…
06/26/2013| 08:05am US/Eastern
SAN DIEGO, June 26, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) announced today that Eisai Limited (based in Mississauga, Ontario), a subsidiary of Eisai Inc., has submitted a New Drug Submission (NDS) for BELVIQ(TM) (lorcaserin HCl) with Health Canada. BELVIQ is being evaluated in Canada as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management (including weight loss and weight maintenance) in adult obese patients (initial body mass index, or BMI, >= 30 kg/m(2)), or overweight patients (initial BMI >= 27 kg/m(2)) in the presence of at least one additional cardiovascular risk factor (e.g., hypertension, dyslipidemia, type 2 diabetes, or sleep apnea). In connection with the NDS, Arena will receive a milestone payment of $500,000 from Eisai.
"Approximately 60 percent of Canadians are overweight or obese, but there are limited treatment options available in Canada beyond diet and exercise," said Jack Lief, Arena's President and Chief Executive Officer. "With this New Drug Submission for BELVIQ, we hope to provide physicians with a new tool to help motivated patients manage their weight."
Eisai is responsible for the regulatory approval and, ultimately, marketing and distribution of BELVIQ in Canada. Beyond Canada, Arena has granted Eisai marketing and distribution rights to BELVIQ in most of North and South America. Arena manufactures BELVIQ at its facility in Switzerland, and sells finished commercial product to Eisai for distribution. Arena is eligible to receive payments based upon Eisai's net sales of BELVIQ and is also eligible to receive regulatory and development milestone payments.
...
http://www.4-traders.com/ARENA-PHARMACEUTICALS-IN-8425/news/…
TOTAL SCRIPTS on Belviq
1. Woche> 1087
2. Woche> 1829
...knapp 3000 in 2 Wochen...
...soweit sind die Verkaufszahlen der zweiten Woche meiner Meinung nach ok..
...immerhin ein knappes Plus von 69 % zur Vorwoche...
Meiner Meinung nach wurde im Vorfeld der Ankündigung der Verkaufszahlen wieder eine "konzertierte" Aktion gegen Arena gefahren. Im Yahoo Board wurden teilweise bis zu 6000 "prescriptions" prognostiziert,wohl um die Erwartungen so hoch zu schrauben, dass bei einer Ankündigung unter 6000 sich Enttäuschung breit macht und der Kurs wieder nach unten gedrückt werden kann.
Also, nur eine reine Vermutung
Grüße
bernie
1. Woche> 1087
2. Woche> 1829
...knapp 3000 in 2 Wochen...
...soweit sind die Verkaufszahlen der zweiten Woche meiner Meinung nach ok..
...immerhin ein knappes Plus von 69 % zur Vorwoche...
Meiner Meinung nach wurde im Vorfeld der Ankündigung der Verkaufszahlen wieder eine "konzertierte" Aktion gegen Arena gefahren. Im Yahoo Board wurden teilweise bis zu 6000 "prescriptions" prognostiziert,wohl um die Erwartungen so hoch zu schrauben, dass bei einer Ankündigung unter 6000 sich Enttäuschung breit macht und der Kurs wieder nach unten gedrückt werden kann.
Also, nur eine reine Vermutung
Grüße
bernie

nun ist der Kurs doch unter die $ 7,50 Marke gelaufen und dort geblieben.
Zur Zeit bringen die Investments in Pharma-Aktien keinen großen Spass.

Zur Zeit bringen die Investments in Pharma-Aktien keinen großen Spass.


Arena Pharmaceuticals to Host Corporate Update and Financial Results Conference Call and Webcast on Thursday, August 1
SAN DIEGO, July 24, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that it will provide a corporate update and report second quarter 2013 financial results before the NASDAQ Global Select Market opens on Thursday, August 1, 2013.
That same morning, Arena will host a conference call and webcast at 8:30 a.m. Eastern Time (5:30 a.m. Pacific Time).
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' Second Quarter 2013 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-host-cor…
 Arena Pharmaceuticals and CY Biotech Enter into Marketing and Supply Agreement for BELVIQ® (lorcaserin HCl) in Taiwan
Arena Pharmaceuticals and CY Biotech Enter into Marketing and Supply Agreement for BELVIQ® (lorcaserin HCl) in Taiwan
SAN DIEGO, July 25, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that its wholly owned subsidiary, Arena Pharmaceuticals GmbH, has entered into an exclusive marketing and supply agreement with CY Biotech Company Ltd. (CYB) for BELVIQ® (lorcaserin HCl) in Taiwan
 .
. Under the agreement, Arena granted CYB the rights to market and distribute BELVIQ in Taiwan for weight loss or weight management in obese and overweight patients, subject to regulatory approval of BELVIQ by the Taiwan Food and Drug Administration (TFDA).
Arena will manufacture BELVIQ at its facility in Switzerland, and sell finished product to CYB for a purchase price at 45% of CYB's annual net sales. In addition, Arena received from CYB a net upfront payment of $2 million, and is eligible to receive purchase price adjustment payments based on CYB's annual net sales, as well as a milestone payment upon approval of the first additional indication for BELVIQ by the TFDA.
CYB is responsible for the development, regulatory approval and, ultimately, marketing and distribution of BELVIQ in Taiwan, including related costs and expenses.
"We will continue to expand the geographic footprint for BELVIQ through agreements with pharmaceutical companies
 that demonstrate a combination of local regulatory expertise and proven marketing strength," said Jack Lief, Arena's President and Chief Executive Officer. "CYB was formerly part of TTY Biopharm, a leading Taiwanese pharmaceutical company. While part of TTY, CYB was the operating group that previously co-marketed the weight loss drug Reductil® with Abbott in Taiwan, and we believe CYB is well positioned to bring BELVIQ to a territory where new weight management pharmacotherapies are needed."
that demonstrate a combination of local regulatory expertise and proven marketing strength," said Jack Lief, Arena's President and Chief Executive Officer. "CYB was formerly part of TTY Biopharm, a leading Taiwanese pharmaceutical company. While part of TTY, CYB was the operating group that previously co-marketed the weight loss drug Reductil® with Abbott in Taiwan, and we believe CYB is well positioned to bring BELVIQ to a territory where new weight management pharmacotherapies are needed."
According to published media reports, half of adult men and a third of adult women in Taiwan are overweight or obese, which are defined in Taiwan as a body mass index >24 kg/m2 or >27 kg/m2, respectively. Eight out of the 10 leading causes of death in Taiwan are associated with obesity, and approximately 10% of total healthcare spending goes to treat problems related to obesity.
"Health and a focus on weight management have become a countrywide movement in Taiwan," said Jacky Wang, CYB's Chairman and President. "This addition to our product portfolio reflects our commitment to innovation and our strong belief in the safety and efficacy of BELVIQ. Our previous experience in building the weight management market will be important for the regulatory approval and marketing of BELVIQ. CYB has a comprehensive sales network in place as well as relationships with the academic and physician thought leaders throughout Taiwan."

http://finance.yahoo.com/news/arena-pharmaceuticals-cy-biote…
Arena Pharmaceuticals' BELVIQ® (lorcaserin HCl) CIV Nominated for 2013 Prix Galien USA Award
Pharmaceuticals, Inc. (ARNA) today announced that BELVIQ® (lorcaserin HCl) has been nominated for the 2013 Prix Galien USA Award in the Best Pharmaceutical Agent category. BELVIQ is approved by the US Food and Drug Administration (FDA) for chronic weight management in adults.
The Prix Galien Award is considered one of the biomedical industry's top accolades, as it recognizes outstanding achievements in improving the human condition through the development of innovative therapies. Nominees are selected from among those medicines and devices approved by the FDA in the last five years.
"BELVIQ is a novel single agent internally discovered at Arena, and was the first new prescription weight-loss treatment approved by the FDA in 13 years," said Dominic P. Behan, Ph.D., Arena's Executive Vice President and Chief Scientific Officer.
"Nomination for the Prix Galien is a prestigious honor that is underscored by the caliber of innovative therapies included alongside BELVIQ in this category."
The Prix Galien Award ceremony will be held in New York City on October 22, 2013.
http://finance.yahoo.com/news/arena-pharmaceuticals-belviq-l…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-belviq-l…
Pharmaceuticals, Inc. (ARNA) today announced that BELVIQ® (lorcaserin HCl) has been nominated for the 2013 Prix Galien USA Award in the Best Pharmaceutical Agent category. BELVIQ is approved by the US Food and Drug Administration (FDA) for chronic weight management in adults.
The Prix Galien Award is considered one of the biomedical industry's top accolades, as it recognizes outstanding achievements in improving the human condition through the development of innovative therapies. Nominees are selected from among those medicines and devices approved by the FDA in the last five years.
"BELVIQ is a novel single agent internally discovered at Arena, and was the first new prescription weight-loss treatment approved by the FDA in 13 years," said Dominic P. Behan, Ph.D., Arena's Executive Vice President and Chief Scientific Officer.
"Nomination for the Prix Galien is a prestigious honor that is underscored by the caliber of innovative therapies included alongside BELVIQ in this category."
The Prix Galien Award ceremony will be held in New York City on October 22, 2013.
http://finance.yahoo.com/news/arena-pharmaceuticals-belviq-l…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-belviq-l…
Jeder fünfte Amerikaner stirbt an Fettleibigkeit:
http://www.webmd.com/diet/news/20130815/obesitys-death-toll-…
aktuelle Studie im „American Journal of Public Health“
http://www.webmd.com/diet/news/20130815/obesitys-death-toll-…
aktuelle Studie im „American Journal of Public Health“
Arena Pharmaceuticals Completes Phase 1b Clinical Trial Evaluating APD811 for Pulmonary Arterial Hypertension
-- Preparation for APD811 Phase 2 Clinical Trial Now Underway --
SAN DIEGO, Aug. 28, 2013 /PRNewswire/
Arena Pharmaceuticals, Inc. (ARNA) today announced the completion of a Phase 1b clinical trial for APD811, an investigational oral prostacyclin (IP) receptor agonist intended for the treatment of pulmonary arterial hypertension (PAH). The company plans to initiate a Phase 2 clinical trial for APD811 in the first quarter of 2014.
"Arena's internal GPCR-focused efforts led to the discovery of this novel drug candidate," said William R. Shanahan, M.D., Arena's Senior Vice President and Chief Medical Officer. "We believe APD811 is a promising new chemical entity that could improve current patient care by providing an oral option for IP-targeted therapy. Based on the encouraging Phase 1 results, we look forward to initiating the Phase 2 program early next year."
This randomized, double-blind and placebo-controlled Phase 1b clinical trial evaluated the safety, tolerability and pharmacokinetics of multiple-ascending doses of APD811. Arena previously evaluated single-ascending doses of APD811 in a Phase 1a clinical trial.
In the Phase 1b clinical trial, 40 healthy volunteers received APD811 and 15 received placebo, and the safety profile of APD811 was characteristic of IP receptor agonists. No serious adverse events were observed, and the most frequent treatment-emergent adverse events were headache, nausea and jaw pain. The results of the Phase 1 program led to the decision to proceed with Phase 2 development of APD811, which will include further exploration of dosing regimens in PAH patients.
About Pulmonary Arterial Hypertension (PAH)
PAH is a progressive, life-threatening disorder characterized by increased pressure in the arteries that carry blood from the heart to the lungs. The increased pressure strains the heart, which can limit physical activity, result in heart failure and reduce life expectancy. Based on data from the Registry to EValuate Early And Long-term PAH disease management (REVEAL) of patients in the United States, there is an estimated five-year survival rate of 57% from diagnosis.
About APD811
APD811, an orally available agonist of the prostacyclin (IP) receptor, is an investigational drug candidate internally discovered and developed by Arena and intended for the treatment of PAH. Treatment with IP agonists, which can slow disease progression and improve exercise tolerance in PAH patients, is considered standard of care for advanced PAH. Currently available IP agonists belong to the prostanoid class of molecules, and these products need to be administered frequently or continuously through intravenous, subcutaneous or inhaled delivery methods. Arena believes that an orally available, non-prostanoid IP agonist such as APD811 may have the potential to improve the standard of care for PAH.
http://finance.yahoo.com/news/arena-pharmaceuticals-complete…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-complete…
-- Preparation for APD811 Phase 2 Clinical Trial Now Underway --
SAN DIEGO, Aug. 28, 2013 /PRNewswire/
Arena Pharmaceuticals, Inc. (ARNA) today announced the completion of a Phase 1b clinical trial for APD811, an investigational oral prostacyclin (IP) receptor agonist intended for the treatment of pulmonary arterial hypertension (PAH). The company plans to initiate a Phase 2 clinical trial for APD811 in the first quarter of 2014.
"Arena's internal GPCR-focused efforts led to the discovery of this novel drug candidate," said William R. Shanahan, M.D., Arena's Senior Vice President and Chief Medical Officer. "We believe APD811 is a promising new chemical entity that could improve current patient care by providing an oral option for IP-targeted therapy. Based on the encouraging Phase 1 results, we look forward to initiating the Phase 2 program early next year."
This randomized, double-blind and placebo-controlled Phase 1b clinical trial evaluated the safety, tolerability and pharmacokinetics of multiple-ascending doses of APD811. Arena previously evaluated single-ascending doses of APD811 in a Phase 1a clinical trial.
In the Phase 1b clinical trial, 40 healthy volunteers received APD811 and 15 received placebo, and the safety profile of APD811 was characteristic of IP receptor agonists. No serious adverse events were observed, and the most frequent treatment-emergent adverse events were headache, nausea and jaw pain. The results of the Phase 1 program led to the decision to proceed with Phase 2 development of APD811, which will include further exploration of dosing regimens in PAH patients.
About Pulmonary Arterial Hypertension (PAH)
PAH is a progressive, life-threatening disorder characterized by increased pressure in the arteries that carry blood from the heart to the lungs. The increased pressure strains the heart, which can limit physical activity, result in heart failure and reduce life expectancy. Based on data from the Registry to EValuate Early And Long-term PAH disease management (REVEAL) of patients in the United States, there is an estimated five-year survival rate of 57% from diagnosis.
About APD811
APD811, an orally available agonist of the prostacyclin (IP) receptor, is an investigational drug candidate internally discovered and developed by Arena and intended for the treatment of PAH. Treatment with IP agonists, which can slow disease progression and improve exercise tolerance in PAH patients, is considered standard of care for advanced PAH. Currently available IP agonists belong to the prostanoid class of molecules, and these products need to be administered frequently or continuously through intravenous, subcutaneous or inhaled delivery methods. Arena believes that an orally available, non-prostanoid IP agonist such as APD811 may have the potential to improve the standard of care for PAH.
http://finance.yahoo.com/news/arena-pharmaceuticals-complete…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-complete…
Antwort auf Beitrag Nr.: 45.348.163 von bernie55 am 29.08.13 11:20:23warum dann der Kurs um 3% auf $6,35 einknicken muss ist mir wieder unverständlich, aber wahrscheinlich muss dass so sein:
"Sell on good news."

"Sell on good news."

vielleicht ist die Marktkapitalisierung mit 1,3 Milliarden einfach zu hoch?
http://www.fool.com/investing/general/2013/08/30/arena-pharm…
Vielleicht können solche Statements den Kurs auch wieder stabilisieren.
Vielleicht können solche Statements den Kurs auch wieder stabilisieren.
Antwort auf Beitrag Nr.: 45.434.935 von dottore am 12.09.13 12:12:143 Monate nach der Markt-Einführung von Belviq lesen sich die ersten Verkaufszahlen meiner Meinung nach nicht schlecht.
https://docs.google.com/spreadsheet/ccc?key=0AncYq0a43hMcdHp…
Zudem läuft jetzt jetzt eine Werbekampagne in den Medien an, die die Konsumenten direkt anspricht.
http://seekingalpha.com/article/1695092-advertising-starts-o…
Das DTC ( direct to consumer) wird sich hoffentlich !!! auch auf die Verkaufszahlen positiv auswirken.
We have to be patient !!
https://docs.google.com/spreadsheet/ccc?key=0AncYq0a43hMcdHp…
Zudem läuft jetzt jetzt eine Werbekampagne in den Medien an, die die Konsumenten direkt anspricht.
http://seekingalpha.com/article/1695092-advertising-starts-o…
Das DTC ( direct to consumer) wird sich hoffentlich !!! auch auf die Verkaufszahlen positiv auswirken.
We have to be patient !!

Arena Pharmaceuticals Provides Update as Eisai's Launch of BELVIQ® (lorcaserin HCl) CIV Enters Consumer Phase 
Press Release: Arena Pharmaceuticals, Inc. – 12 minutes ago
SAN DIEGO, Sept. 16, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today provided an update on Eisai Inc.'s marketing activities for BELVIQ® (lorcaserin HCl) as the US launch campaign enters its consumer phase.
Beginning this month, full-page announcements communicating the availability of BELVIQ are being placed in major US magazines, including O, The Oprah Magazine. These announcements are intended to educate consumers about BELVIQ as a treatment option for weight loss and weight maintenance. In addition, Eisai recently launched the BELIEVE EVERYDAY SUPPORTSM program to provide comprehensive support and savings for patients treated with BELVIQ. Patients can enroll in the program at BelieveSupport.com. For Eisai's announcement with additional details about the program, click here.
"Patient awareness and education about treatment options is an important component of the patient/physician relationship," said Jack Lief, Arena's President and Chief Executive Officer. "Eisai's consumer-focused efforts will complement their comprehensive healthcare professional education initiatives already underway."
Eisai is responsible for the marketing and distribution of BELVIQ in the United States under its agreement with Arena.
http://finance.yahoo.com/news/arena-pharmaceuticals-provides…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-provides…

Press Release: Arena Pharmaceuticals, Inc. – 12 minutes ago
SAN DIEGO, Sept. 16, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today provided an update on Eisai Inc.'s marketing activities for BELVIQ® (lorcaserin HCl) as the US launch campaign enters its consumer phase.
Beginning this month, full-page announcements communicating the availability of BELVIQ are being placed in major US magazines, including O, The Oprah Magazine. These announcements are intended to educate consumers about BELVIQ as a treatment option for weight loss and weight maintenance. In addition, Eisai recently launched the BELIEVE EVERYDAY SUPPORTSM program to provide comprehensive support and savings for patients treated with BELVIQ. Patients can enroll in the program at BelieveSupport.com. For Eisai's announcement with additional details about the program, click here.
"Patient awareness and education about treatment options is an important component of the patient/physician relationship," said Jack Lief, Arena's President and Chief Executive Officer. "Eisai's consumer-focused efforts will complement their comprehensive healthcare professional education initiatives already underway."
Eisai is responsible for the marketing and distribution of BELVIQ in the United States under its agreement with Arena.
http://finance.yahoo.com/news/arena-pharmaceuticals-provides…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-provides…
obwohl sich die Meldung interessant anhört, darf nicht vergessen werden: Wer so viel Übergewicht hat, dass er mithilfe von Medikamenten abnehmen will, gibt auch schnell wieder auf, wenn er überhaupt einen Versuch startet.
Die Erfolgsmeldungen und die Umsatzmeldungen in den kommenden Quartalen werden zeigen, welches Potential wirklich besteht. Damit auch, ob die aktuelle Marktkapitalisation gerechtfertigt ist.
Aber da ist ja auch noch mehr in der Pipeline. Und das scheint mir zur Zeit nicht gespielt zu werden.
Charttechnisch sieht es derzeit jedenfalls hoffnungslos aus.
Es wird sich lohnen, trotz Verluste auszusteigen und bei knapp 4 $ (nicht €)wieder einzusteigen, immer mit der Bereitschaft dann auch noch zu verbilligen.
Mit dem dann folgenden Hype kann man die einzelnen Positionen wieder rausschmeißen, natürlich mit Gewinn und hoffentlich schon im kommenden Jahr.
Die Erfolgsmeldungen und die Umsatzmeldungen in den kommenden Quartalen werden zeigen, welches Potential wirklich besteht. Damit auch, ob die aktuelle Marktkapitalisation gerechtfertigt ist.
Aber da ist ja auch noch mehr in der Pipeline. Und das scheint mir zur Zeit nicht gespielt zu werden.
Charttechnisch sieht es derzeit jedenfalls hoffnungslos aus.
Es wird sich lohnen, trotz Verluste auszusteigen und bei knapp 4 $ (nicht €)wieder einzusteigen, immer mit der Bereitschaft dann auch noch zu verbilligen.
Mit dem dann folgenden Hype kann man die einzelnen Positionen wieder rausschmeißen, natürlich mit Gewinn und hoffentlich schon im kommenden Jahr.
Arena Pharmaceuticals to Present Clinical Data for BELVIQ® (lorcaserin HCl) CIV at the 49th Annual Meeting of the European Association for the Study of Diabetes (EASD)
SAN DIEGO, Sept. 18, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc.
(NASDAQ: ARNA) today announced that data from the BELVIQ® (lorcaserin HCl) Phase 3 clinical trial program will be presented at the following scientific meeting:
49th Annual Meeting of the European Association for the Study of Diabetes (EASD)
Barcelona, Spain: September 23-27, 2013
http://boardvote.com/symbol/ARNA/communique/444360
SAN DIEGO, Sept. 18, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc.
(NASDAQ: ARNA) today announced that data from the BELVIQ® (lorcaserin HCl) Phase 3 clinical trial program will be presented at the following scientific meeting:
49th Annual Meeting of the European Association for the Study of Diabetes (EASD)
Barcelona, Spain: September 23-27, 2013
http://boardvote.com/symbol/ARNA/communique/444360
ist nun mal so, dass in Deutschland Lifestylemedikamente keine Kassenleistung darstellen.
Vor einigen Jahren wurde der Versuch unternommen, einen Appetittzügler für Diabetiker mit BMI über 30 einzuführen. Das wurde nicht akzeptiert und nebenbei bemerkt ging das wegen massiver Nebenwirkung auch wieder vom Markt.
Jedenfalls wird zumindest in Deutschland ein Abnehmmedikament nicht Kassenleistung werden.
Damit wird der Umsatz nicht so rosig, wie viele es sich vorstellen. Die gute Verträglichkeit sollte aber Selbstzahler auf den Plan rufen....
Und damit ist wieder die Frage da, ob die doch sehr hohe Marktkapitalisierung jetzt schon gerechtfertigt ist.
Vor einigen Jahren wurde der Versuch unternommen, einen Appetittzügler für Diabetiker mit BMI über 30 einzuführen. Das wurde nicht akzeptiert und nebenbei bemerkt ging das wegen massiver Nebenwirkung auch wieder vom Markt.
Jedenfalls wird zumindest in Deutschland ein Abnehmmedikament nicht Kassenleistung werden.
Damit wird der Umsatz nicht so rosig, wie viele es sich vorstellen. Die gute Verträglichkeit sollte aber Selbstzahler auf den Plan rufen....
Und damit ist wieder die Frage da, ob die doch sehr hohe Marktkapitalisierung jetzt schon gerechtfertigt ist.
Was soll man zur aktuellen Situation von ARENA sagen?
Ungeachtet der vielleicht zu hoch gesteckten Erwartungen bzgl. der Verkaufszahlen von Belviq hat der aktuelle Downtrend von ARENA nichts mehr rationales - es wird bis zum Abwinken an dem Kurs manipuliert, die Hedgefonds schlagen erbarmungslos zu.
Die täglichen Downs von 3-6 % können als nicht mehr normal betrachtet werden. Dieses Szenario ist von langer Hand vorbereitet, sehr zum Leidwesen vieler Anleger, die vielleicht bei erst 7-9 $ eingestiegen sind.
ARENA ist zur Zeit ein Spielball der Hedgefonds und das Schlimmste ist, dass die SEC trotz vieler Mitteilungen von ARENA Aktionären bisher in keinster Weise darauf reagiert hat.
Keep patience
bernie55
Ungeachtet der vielleicht zu hoch gesteckten Erwartungen bzgl. der Verkaufszahlen von Belviq hat der aktuelle Downtrend von ARENA nichts mehr rationales - es wird bis zum Abwinken an dem Kurs manipuliert, die Hedgefonds schlagen erbarmungslos zu.
Die täglichen Downs von 3-6 % können als nicht mehr normal betrachtet werden. Dieses Szenario ist von langer Hand vorbereitet, sehr zum Leidwesen vieler Anleger, die vielleicht bei erst 7-9 $ eingestiegen sind.
ARENA ist zur Zeit ein Spielball der Hedgefonds und das Schlimmste ist, dass die SEC trotz vieler Mitteilungen von ARENA Aktionären bisher in keinster Weise darauf reagiert hat.
Keep patience
bernie55

Zitat von bernie55: ARENA ist zur Zeit ein Spielball der Hedgefonds und das Schlimmste ist, dass die SEC trotz vieler Mitteilungen von ARENA Aktionären bisher in keinster Weise darauf reagiert hat.
Keep patience
bernie55
..oder vielleicht kommt die SEC doch in die "Puschen"..
SEC ANNOUNCED TODAY GOING AFTER ALL MANIPULATORS
WASHINGTON (Reuters)
The top U.S. securities regulator on Wednesday placed everyone from auditors to fund board members on notice, saying her agency plans to look for violations in all corners of the market, from major Wall Street investment firms to boiler room operations.
"Minor violations that are overlooked or ignored can feed bigger ones, and, perhaps more importantly, can foster a culture where laws are increasingly treated as toothless guidelines," Securities and Exchange Commission Chair Mary Jo White said in a speech at a conference about SEC enforcement issues.
"I believe it is important to pursue even the smallest infractions."
White, a former federal prosecutor, has made tough enforcement a cornerstone of her chairmanship at the SEC since she took the helm of the agency in April.
She has already broken from the SEC's long-time practice of allowing defendants to settle cases without admitting or denying charges, saying the SEC will seek to extract admissions if the circumstances are right.
Just last month, she also said the SEC planned to step up efforts to bring charges against individuals and would not shy away from seeking large penalties against executives and companies.
http://finance.yahoo.com/mbview/threadview/;_ylt=Ak33piL__UM…
Zitat von bernie55:Zitat von bernie55: ARENA ist zur Zeit ein Spielball der Hedgefonds und das Schlimmste ist, dass die SEC trotz vieler Mitteilungen von ARENA Aktionären bisher in keinster Weise darauf reagiert hat.
Keep patience
bernie55
..oder vielleicht kommt die SEC doch in die "Puschen"..
SEC ANNOUNCED TODAY GOING AFTER ALL MANIPULATORS
..oder vielleicht auch nur mehr "Schein als Sein" ???
Antwort auf Beitrag Nr.: 45.603.191 von bernie55 am 10.10.13 15:12:33Servus Bernie,
und täglich grüßt das Murmeltier, siehe Dendreon, Amarin....
Drecksamies, Betrüger ohne Scham, Hochfrequenzhandel und Dreckshedjefonds machen alles kaputt!
Das Land ist pleite, keine Einigung in Sicht aber großkotzig die Welt belehren wollen, lachhaft wenn es für uns nicht so übel wäre.....
und täglich grüßt das Murmeltier, siehe Dendreon, Amarin....
Drecksamies, Betrüger ohne Scham, Hochfrequenzhandel und Dreckshedjefonds machen alles kaputt!
Das Land ist pleite, keine Einigung in Sicht aber großkotzig die Welt belehren wollen, lachhaft wenn es für uns nicht so übel wäre.....
Antwort auf Beitrag Nr.: 45.603.261 von Magnetfeldfredy am 10.10.13 15:18:19Hi Fredy,
deinem "scharfen" Kommentar ist von meiner Seite her nichts mehr hinzuzufügen.
YOU ARE RIGHT
deinem "scharfen" Kommentar ist von meiner Seite her nichts mehr hinzuzufügen.
YOU ARE RIGHT

..ein weiteres Patent für ARENA ...

5-HT2C RECEPTOR AGONISTS IN THE TREATMENT OF DISORDERS AMELIORATED BY REDUCTION OF NOREPINEPHRINE LEVEL
Abstract
Uses of 5-HT.sub.2C receptor agonists in the treatment of disorders ameliorated by reduction of an individual's norepinephrine level, wherein said disorders include but are not limited to hypernorepinephrinemia, cardiomyopathy, cardiac hypertrophy, cardiomyocyte hypertrophy in post-myocardial infarction remodeling, elevated heart rate, vasoconstriction, acute pulmonary vasoconstriction, hypertension, heart failure, cardiac dysfunction after stroke, cardiac arrhythmia, metabolic syndrome, abnormal lipid metabolism, hyperthermia, Cushing syndrome, pheochromocytoma, epilepsy, obstructive sleep apnea, insomnia, glaucoma, osteoarthritis, rheumatoid arthritis, and asthma.
http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
5-HT2C RECEPTOR AGONISTS IN THE TREATMENT OF DISORDERS AMELIORATED BY REDUCTION OF NOREPINEPHRINE LEVEL
Abstract
Uses of 5-HT.sub.2C receptor agonists in the treatment of disorders ameliorated by reduction of an individual's norepinephrine level, wherein said disorders include but are not limited to hypernorepinephrinemia, cardiomyopathy, cardiac hypertrophy, cardiomyocyte hypertrophy in post-myocardial infarction remodeling, elevated heart rate, vasoconstriction, acute pulmonary vasoconstriction, hypertension, heart failure, cardiac dysfunction after stroke, cardiac arrhythmia, metabolic syndrome, abnormal lipid metabolism, hyperthermia, Cushing syndrome, pheochromocytoma, epilepsy, obstructive sleep apnea, insomnia, glaucoma, osteoarthritis, rheumatoid arthritis, and asthma.
http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
irgendwann musste doch ein Kursanstieg auch mal kommen. Der wird die shortys aufs Feld zwingen und es folgt ein wenig mehr als ein Strohfeuer.
Und dann? ....
Und dann? ....
Arena Pharmaceuticals Reports That Eisai Will Double BELVIQ® (lorcaserin HCl) CIV Sales Force by December
Expansion to Approximately 400 Sales Specialists Follows Increased Coverage of BELVIQ by Health Plans and Pharmacy Benefit Managers
SAN DIEGO, Oct. 15, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today reported that Eisai Inc. will increase its BELVIQ® sales force to approximately 400 representatives by December 2013, doubling the size of the sales force from when BELVIQ became available in June 2013.
The expansion of the sales force, which has commenced and follows increases in coverage of BELVIQ by health plans and pharmacy benefit managers (PBMs) since the launch, will enable Eisai to reach approximately 65,000 physicians in the United States, including primary care providers, endocrinologists, cardiovascular specialists and gastrointestinal specialists. BELVIQ is now covered by several prominent health plans and PBMs, including, among others, Express Scripts (including its legacy Express Scripts and Medco operations), Tufts, Health Alliance Plan, Excellus BCBS, Highmark BCBS, BCBS of Michigan, BCBS of North Carolina and Healthnet (California), according to BusinessOne Technologies, Inc. While the exact coverage for BELVIQ varies by patient, this improved access means more patients will receive coverage support from their health plan or PBM.
"Eisai launched BELVIQ with a focus on educating an initial group of physicians, expanding reimbursement coverage, introducing patient support programs and increasing patient awareness," said Jack Lief, Arena's President and Chief Executive Officer. "We have seen increasing physician interest in BELVIQ as an important treatment option for chronic weight management, the payor landscape has improved and we are encouraged by the early response to the patient support programs and recently launched patient campaign. Eisai's efforts have led to month over month increases in BELVIQ prescriptions to date, and we expect that the expansion of the BELVIQ sales force will help communicate the safety and efficacy of BELVIQ to an increased number of physicians."
Lonnel Coats, Eisai Inc.'s President and Chief Executive Officer, added, "Eisai is deeply committed to launching innovative therapies with strong support from the payor community. The expansion of our sales force demonstrates the success we have gained in discussing the potential value of BELVIQ in chronic weight management within the payor community, and the subsequent agreements we have reached with many of our payor partners. We are in very active and productive discussions with other major payors and fully expect the reimbursement landscape will further improve as we head into 2014."
Eisai is responsible for the marketing and distribution of BELVIQ in the United States under its agreement with Arena.
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
Expansion to Approximately 400 Sales Specialists Follows Increased Coverage of BELVIQ by Health Plans and Pharmacy Benefit Managers
SAN DIEGO, Oct. 15, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today reported that Eisai Inc. will increase its BELVIQ® sales force to approximately 400 representatives by December 2013, doubling the size of the sales force from when BELVIQ became available in June 2013.
The expansion of the sales force, which has commenced and follows increases in coverage of BELVIQ by health plans and pharmacy benefit managers (PBMs) since the launch, will enable Eisai to reach approximately 65,000 physicians in the United States, including primary care providers, endocrinologists, cardiovascular specialists and gastrointestinal specialists. BELVIQ is now covered by several prominent health plans and PBMs, including, among others, Express Scripts (including its legacy Express Scripts and Medco operations), Tufts, Health Alliance Plan, Excellus BCBS, Highmark BCBS, BCBS of Michigan, BCBS of North Carolina and Healthnet (California), according to BusinessOne Technologies, Inc. While the exact coverage for BELVIQ varies by patient, this improved access means more patients will receive coverage support from their health plan or PBM.
"Eisai launched BELVIQ with a focus on educating an initial group of physicians, expanding reimbursement coverage, introducing patient support programs and increasing patient awareness," said Jack Lief, Arena's President and Chief Executive Officer. "We have seen increasing physician interest in BELVIQ as an important treatment option for chronic weight management, the payor landscape has improved and we are encouraged by the early response to the patient support programs and recently launched patient campaign. Eisai's efforts have led to month over month increases in BELVIQ prescriptions to date, and we expect that the expansion of the BELVIQ sales force will help communicate the safety and efficacy of BELVIQ to an increased number of physicians."
Lonnel Coats, Eisai Inc.'s President and Chief Executive Officer, added, "Eisai is deeply committed to launching innovative therapies with strong support from the payor community. The expansion of our sales force demonstrates the success we have gained in discussing the potential value of BELVIQ in chronic weight management within the payor community, and the subsequent agreements we have reached with many of our payor partners. We are in very active and productive discussions with other major payors and fully expect the reimbursement landscape will further improve as we head into 2014."
Eisai is responsible for the marketing and distribution of BELVIQ in the United States under its agreement with Arena.
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
 Arena Pharmaceuticals and Eisai Expand Marketing and Supply Agreement for BELVIQ® (lorcaserin HCl) to Include Most Countries Worldwide
Arena Pharmaceuticals and Eisai Expand Marketing and Supply Agreement for BELVIQ® (lorcaserin HCl) to Include Most Countries Worldwide 
-- Collaboration to Focus on Global Commercialization and Development for Weight Management and Potential New Indications --
PR Newswire Arena Pharmaceuticals, Inc.
SAN DIEGO, Calif. and WOODCLIFF LAKE, N.J., Nov. 7, 2013 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) and Eisai Inc. announced today the expansion of the BELVIQ® (lorcaserin HCl) marketing and supply agreement between Arena Pharmaceuticals, Inc.'s wholly owned subsidiary, Arena Pharmaceuticals GmbH, and Eisai Inc. along with its parent company Eisai Co., Ltd. The expanded agreement provides Eisai with exclusive commercialization rights for all countries worldwide, except for South Korea, Taiwan, Australia, Israel and New Zealand. This agreement amends and restates the previous agreement that granted Eisai rights to commercialize BELVIQ in most of North and South America. Eisai's commercialization rights are subject to applicable regulatory approval.
"This new agreement not only expands Eisai's territory for commercialization, but it also prioritizes our global development program for BELVIQ," said Jack Lief, Arena's President and Chief Executive Officer. "Expanding our collaboration with Eisai provides the potential to make BELVIQ available to physicians and patients worldwide for weight management and creates a powerful platform to investigate this novel compound for possible new indications by leveraging our combined drug development and regulatory expertise."
Lonnel Coats, Eisai Inc.'s President and Chief Executive Officer, added, "Our expanded commitment to BELVIQ reflects the company's human health care mission to bring new, innovative therapies to patients. Our belief in this product is strengthened by the feedback we have received from patients, physicians and payers since the US launch of BELVIQ. We have a strong foundation on which Eisai and Arena can build a global franchise for BELVIQ."
Under the terms of the agreement, Arena will receive from Eisai an upfront payment of $60 million and is eligible to receive up to a total of $176.5 million in regulatory and development milestone payments. The total for milestone payments represents an increase of $123 million from the amount remaining available under the previous agreement.
Arena will continue to sell BELVIQ to Eisai for commercialization in the US and in the other territories in North and South America for a purchase price starting at 31.5% and 30.75% of Eisai's net sales, respectively. With respect to the new territories added under the amended agreement, the purchase price starts at 27.5% of Eisai's net sales in Europe, China and Japan, and at 30.75% of Eisai's net sales in all other territories. The purchase price will increase on a tiered basis in the US and the other territories, other than Europe, China and Japan, to as high as 36.5% and 35.75%, respectively, on the portion of Eisai's annual net sales exceeding $750 million. The purchase price will increase to 35% in Europe, China and Japan on the portion of Eisai's annual net sales exceeding $500 million.
Arena is also eligible to receive a total of $1.56 billion in one-time purchase price adjustment payments based on sales in the territories covered by the agreement. The total for purchase price adjustment payments represents an increase of $185 million from the amount available under the previous agreement.
In addition to pursuing regulatory approval for weight management in the expanded territory, under the terms of the agreement, Eisai and Arena plan to investigate the potential of BELVIQ in new areas, such as smoking cessation, a once-daily formulation, a fixed-dose combination with phentermine, as well as explore BELVIQ's impact on diabetes and cardiovascular outcomes.
http://finance.yahoo.com/news/arena-pharmaceuticals-eisai-ex…
Antwort auf Beitrag Nr.: 45.789.894 von bernie55 am 08.11.13 09:27:36ZEITPLAN für Vermarktung von Belviq in anderen Ländern:
..da kommt jetzt doch einiges ins Rollen...
entnommen aus Earnings Call Transcript vom 08.11.13
( Craig Audet - Operations and Head of Global Regulatory affairs )
Beyond the US, we are committed to bringing Belviq (B.) to other markets worldwide where as the data show, the growing problems of obesity and overweight impact the lives of many.
Eisai submitted an application for the approval of B. in Mexico in March, for Canada in June and plans to submit an application in Brazil around the end of this year. We look forward to updating you asour registration strategies in the rest of the world evolve based on our newly expanded agreement Regarding our additional collaborations, Ildong has completed the study to evaluate the tolerability and pharmacokinetics of B. in Korea and is planning to submit an application for approval in South Korea around the end of this year. In addition CYB is developing its regulatory submission for BELVIQ in Taiwan.
its regulatory submission for B. in Taiwan and we´re likely also be in a position to submit their application for approval around the end of this year. Our interaction with Swissmedic continue as we pursue regulatory approval of B. in Switzerland. We will keep you updated on this process as appropriate..
Together (with Eisai) we are focused on developing on fixed dose combination with phentermine, a once-daily formulation, a treatment for smoking cessation as well as exploring the drug impact on diabetes and cardiovascular outcome.
http://seekingalpha.com/article/1825442-arena-pharmaceutical…
..da kommt jetzt doch einiges ins Rollen...

entnommen aus Earnings Call Transcript vom 08.11.13
( Craig Audet - Operations and Head of Global Regulatory affairs )
Beyond the US, we are committed to bringing Belviq (B.) to other markets worldwide where as the data show, the growing problems of obesity and overweight impact the lives of many.
Eisai submitted an application for the approval of B. in Mexico in March, for Canada in June and plans to submit an application in Brazil around the end of this year. We look forward to updating you asour registration strategies in the rest of the world evolve based on our newly expanded agreement Regarding our additional collaborations, Ildong has completed the study to evaluate the tolerability and pharmacokinetics of B. in Korea and is planning to submit an application for approval in South Korea around the end of this year. In addition CYB is developing its regulatory submission for BELVIQ in Taiwan.
its regulatory submission for B. in Taiwan and we´re likely also be in a position to submit their application for approval around the end of this year. Our interaction with Swissmedic continue as we pursue regulatory approval of B. in Switzerland. We will keep you updated on this process as appropriate..
Together (with Eisai) we are focused on developing on fixed dose combination with phentermine, a once-daily formulation, a treatment for smoking cessation as well as exploring the drug impact on diabetes and cardiovascular outcome.
http://seekingalpha.com/article/1825442-arena-pharmaceutical…
Antwort auf Beitrag Nr.: 45.801.360 von bernie55 am 10.11.13 12:00:52Hi Bernie
da sitzen wir wieder mal auf dem selben Pferd - es wird doch hoffentlich kein Esel sein - die Aussichten sind wirklich viel versprechend: Lorcaserin ist eine Pipeline für sich, da die Anwendung in verschiedenen Indikationen (zB Nikotinentwöhnung) möglich erscheint. Die Kombipille (LorPhen) sollte als "Turbofettverbrenner"zudem für zusätzliches Potenzial sorgen. Natürlich ist Geduld gefragt, zumal einige Hedge-Fonds noch immer dagegen wetten, aber ich bin mir fast sicher, dass zweistellige Kurse mittelfristig (1 Jahr) möglich sind.
- die Aussichten sind wirklich viel versprechend: Lorcaserin ist eine Pipeline für sich, da die Anwendung in verschiedenen Indikationen (zB Nikotinentwöhnung) möglich erscheint. Die Kombipille (LorPhen) sollte als "Turbofettverbrenner"zudem für zusätzliches Potenzial sorgen. Natürlich ist Geduld gefragt, zumal einige Hedge-Fonds noch immer dagegen wetten, aber ich bin mir fast sicher, dass zweistellige Kurse mittelfristig (1 Jahr) möglich sind.
Grüsse
ch / Fr_ole
da sitzen wir wieder mal auf dem selben Pferd - es wird doch hoffentlich kein Esel sein
 - die Aussichten sind wirklich viel versprechend: Lorcaserin ist eine Pipeline für sich, da die Anwendung in verschiedenen Indikationen (zB Nikotinentwöhnung) möglich erscheint. Die Kombipille (LorPhen) sollte als "Turbofettverbrenner"zudem für zusätzliches Potenzial sorgen. Natürlich ist Geduld gefragt, zumal einige Hedge-Fonds noch immer dagegen wetten, aber ich bin mir fast sicher, dass zweistellige Kurse mittelfristig (1 Jahr) möglich sind.
- die Aussichten sind wirklich viel versprechend: Lorcaserin ist eine Pipeline für sich, da die Anwendung in verschiedenen Indikationen (zB Nikotinentwöhnung) möglich erscheint. Die Kombipille (LorPhen) sollte als "Turbofettverbrenner"zudem für zusätzliches Potenzial sorgen. Natürlich ist Geduld gefragt, zumal einige Hedge-Fonds noch immer dagegen wetten, aber ich bin mir fast sicher, dass zweistellige Kurse mittelfristig (1 Jahr) möglich sind.Grüsse
ch / Fr_ole
Antwort auf Beitrag Nr.: 45.821.844 von Cyberhexe am 13.11.13 10:23:35Assessment report
Belviq
International non-proprietary name: Lorcaserin
Procedure No. EMEA/H/C/002597
http://www.ema.europa.eu/ema/index.jsp?curl=search.jsp&q=lor…
http://www.ema.europa.eu/docs/en_GB/document_library/Applica…
...für alle die des Englischen mächtig sind, sei das nachfolgende Forum bei Investor Village wärmstens empfohlen - es gibt kein Besseres!http://www.investorvillage.com/smbd.asp?mb=633
Belviq
International non-proprietary name: Lorcaserin
Procedure No. EMEA/H/C/002597
http://www.ema.europa.eu/ema/index.jsp?curl=search.jsp&q=lor…
http://www.ema.europa.eu/docs/en_GB/document_library/Applica…
...für alle die des Englischen mächtig sind, sei das nachfolgende Forum bei Investor Village wärmstens empfohlen - es gibt kein Besseres!http://www.investorvillage.com/smbd.asp?mb=633
Nov 13, 2013
WOODCLIFF LAKE, N.J. and SAN DIEGO, Nov. 13, 2013 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc., (NASDAQ:ARNA) announced today that five abstracts highlighting new data analyses from the BELVIQ® (lorcaserin HCl) Phase 3 clinical trial program will be presented during ObesityWeekSM 2013, hosted by The Obesity Society and the American Society for Metabolic and Bariatric Surgery. ObesityWeekSM 2013 is taking place November 11-16 in Atlanta, Georgia.
The following abstracts highlighting BELVIQ data will be presented:
Impact of Lorcaserin on Glycemic Control in Overweight and Obese Patients with Type 2 Diabetes: Analysis of Week 52 Responders and Non-responders
Oral Presentation: T-32-OR
This post-hoc analysis of a previously published Phase 3 trial, BLOOM-DM, evaluated the effects of lorcaserin on glycemic parameters in overweight and obese patients (body mass index, or BMI, 27-45 kg/m2) with type 2 diabetes who achieved greater than or equal to 5% weight loss at week 52. 509 patients in the BLOOM-DM trial were randomized to lorcaserin 10 mg twice daily or placebo with diet and exercise counseling. This analysis assessed fasting plasma glucose, HbA1c, and use of anti-hyperglycemic medications at week 52, stratified by week 52 weight response.
Effects of Lorcaserin on Lean and Fat Mass Loss in Patients with Type 2 Diabetes Mellitus from the BLOOM-DM Study of Obese and Overweight Patients
Poster Presentation: T-738-P
This analysis of patients from the BLOOM-DM trial evaluated the loss of lean and fat body mass and overall weight loss achieved over one year as determined by dual-emission X-ray absorptiometry (DXA) in a subset of 63 patients with type 2 diabetes. Together with diet and exercise counseling, patients were randomized to lorcaserin or placebo, and DXA scans were performed at baseline, six months, and one year. Lean mass and body fat were stratified by baseline HbA1c.
Effects of Lorcaserin on Lean and Fat Mass Loss in the BLOSSOM Study of Obese and Overweight Patients
Poster Presentation: T-739-P
This analysis of patients from the BLOSSOM trial evaluated the loss of lean and fat body mass relative to overall weight loss achieved over one year as determined by DXA in a subset of 189 obese and overweight patients. Together with diet and exercise counseling, patients were randomized to lorcaserin or placebo, and DXA scans were performed at baseline, six months, and one year. Lean mass and body fat were analyzed by magnitude of weight loss.
Number Needed to Treat Analysis of Lorcaserin in Overweight and Obese Patients
Poster Presentation: T-742-P
A Number Needed to Treat (NNT) analysis was conducted for three Phase 3 trials of lorcaserin: BLOOM, BLOSSOM and BLOOM-DM. This NNT analysis evaluated one-year endpoints including weight loss, reduction in waist circumference, and change in HbA1C (BLOOM-DM only) in week 12 responders - defined as patients achieving greater than or equal to 5% weight loss after 12 weeks of treatment - and the overall lorcaserin population, in comparison to the placebo group.
Health-related Quality of Life in Overweight and Obese Patients Treated with Lorcaserin
Poster Presentation: T-753-P
This analysis assessed the impact of lorcaserin on health-related quality of life in overweight and obese patients enrolled in the Phase 3 BLOOM and BLOSSOM trials.
WOODCLIFF LAKE, N.J. and SAN DIEGO, Nov. 13, 2013 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc., (NASDAQ:ARNA) announced today that five abstracts highlighting new data analyses from the BELVIQ® (lorcaserin HCl) Phase 3 clinical trial program will be presented during ObesityWeekSM 2013, hosted by The Obesity Society and the American Society for Metabolic and Bariatric Surgery. ObesityWeekSM 2013 is taking place November 11-16 in Atlanta, Georgia.
The following abstracts highlighting BELVIQ data will be presented:
Impact of Lorcaserin on Glycemic Control in Overweight and Obese Patients with Type 2 Diabetes: Analysis of Week 52 Responders and Non-responders
Oral Presentation: T-32-OR
This post-hoc analysis of a previously published Phase 3 trial, BLOOM-DM, evaluated the effects of lorcaserin on glycemic parameters in overweight and obese patients (body mass index, or BMI, 27-45 kg/m2) with type 2 diabetes who achieved greater than or equal to 5% weight loss at week 52. 509 patients in the BLOOM-DM trial were randomized to lorcaserin 10 mg twice daily or placebo with diet and exercise counseling. This analysis assessed fasting plasma glucose, HbA1c, and use of anti-hyperglycemic medications at week 52, stratified by week 52 weight response.
Effects of Lorcaserin on Lean and Fat Mass Loss in Patients with Type 2 Diabetes Mellitus from the BLOOM-DM Study of Obese and Overweight Patients
Poster Presentation: T-738-P
This analysis of patients from the BLOOM-DM trial evaluated the loss of lean and fat body mass and overall weight loss achieved over one year as determined by dual-emission X-ray absorptiometry (DXA) in a subset of 63 patients with type 2 diabetes. Together with diet and exercise counseling, patients were randomized to lorcaserin or placebo, and DXA scans were performed at baseline, six months, and one year. Lean mass and body fat were stratified by baseline HbA1c.
Effects of Lorcaserin on Lean and Fat Mass Loss in the BLOSSOM Study of Obese and Overweight Patients
Poster Presentation: T-739-P
This analysis of patients from the BLOSSOM trial evaluated the loss of lean and fat body mass relative to overall weight loss achieved over one year as determined by DXA in a subset of 189 obese and overweight patients. Together with diet and exercise counseling, patients were randomized to lorcaserin or placebo, and DXA scans were performed at baseline, six months, and one year. Lean mass and body fat were analyzed by magnitude of weight loss.
Number Needed to Treat Analysis of Lorcaserin in Overweight and Obese Patients
Poster Presentation: T-742-P
A Number Needed to Treat (NNT) analysis was conducted for three Phase 3 trials of lorcaserin: BLOOM, BLOSSOM and BLOOM-DM. This NNT analysis evaluated one-year endpoints including weight loss, reduction in waist circumference, and change in HbA1C (BLOOM-DM only) in week 12 responders - defined as patients achieving greater than or equal to 5% weight loss after 12 weeks of treatment - and the overall lorcaserin population, in comparison to the placebo group.
Health-related Quality of Life in Overweight and Obese Patients Treated with Lorcaserin
Poster Presentation: T-753-P
This analysis assessed the impact of lorcaserin on health-related quality of life in overweight and obese patients enrolled in the Phase 3 BLOOM and BLOSSOM trials.
Antwort auf Beitrag Nr.: 45.821.844 von Cyberhexe am 13.11.13 10:23:35Hallo Hexchen,
schön von Dir zu hören.
Bin auch seit fast genau 2 Jahren "dick" investiert und hatte leider versäumt,nach der Zulassung zu verkaufen....
Aber zuversichtlich bin ich wie noch nie zuvor.
ARENA wird ein Monster !!!
Liebe Grüsse in die Schweiz aus dem "Badnerländle"
NoSelters
schön von Dir zu hören.

Bin auch seit fast genau 2 Jahren "dick" investiert und hatte leider versäumt,nach der Zulassung zu verkaufen....

Aber zuversichtlich bin ich wie noch nie zuvor.
ARENA wird ein Monster !!!
Liebe Grüsse in die Schweiz aus dem "Badnerländle"
NoSelters
Zitat von Cyberhexe: Hi Bernie
da sitzen wir wieder mal auf dem selben Pferd - es wird doch hoffentlich kein Esel sein- die Aussichten sind wirklich viel versprechend: Lorcaserin ist eine Pipeline für sich, da die Anwendung in verschiedenen Indikationen (zB Nikotinentwöhnung) möglich erscheint. Die Kombipille (LorPhen) sollte als "Turbofettverbrenner"zudem für zusätzliches Potenzial sorgen. Natürlich ist Geduld gefragt, zumal einige Hedge-Fonds noch immer dagegen wetten, aber ich bin mir fast sicher, dass zweistellige Kurse mittelfristig (1 Jahr) möglich sind.
Grüsse
ch / Fr_ole
Hi "Cybi",
als "Longie-Investoren"
 wissen wir mittlerweile( oder auch nicht
wissen wir mittlerweile( oder auch nicht  ), auf welches Pferd wir setzen sollen.
), auf welches Pferd wir setzen sollen.Auch wenn der störrische Esel sich in den letzten Monaten manchmal zu oft durchgesetzt hat, weil Hedgies ihr böses Treiber-Block-Spiel bis zum " Geht nicht Mehr" durchgesetzt haben, gehe ich perspektivisch genau von dem Szenario aus wie Du es oben beschreibst.
Wir beide harren mittlerweile schon so lange zusammen aus( ok, du hast einen günstigeren EK als ich und bist mit xxx Prozenten im Plus - ich halt "noch" mit xx Prozenten aber nicht mehr lange), dass eine zusätzliche Wartezeit bestimmt nicht ins Gewicht fällt.
In diesem Sinne auf hoffentlich weitere gemeinsame xxx Prozente PLUS .

BM für Dich
Schöne Grüße
bernie55

Arena Pharmaceuticals initiated with a BUY at WallachBeth Target $9.
http://www.theflyonthewall.com/permalinks/entry.php/ARNAid19…
http://www.theflyonthewall.com/permalinks/entry.php/ARNAid19…
"Prescriptions" von BELVIQ und QYSIMIA für Zeitraum bis zur 23. Woche nach Markteintritt.

http://www.investorvillage.com/smbd.asp?mb=633&mn=85570&pt=m…

http://www.investorvillage.com/smbd.asp?mb=633&mn=85570&pt=m…
BELVIQ® (lorcaserin HCl) CIV Data to be Presented at American Heart Association 2013 Scientific Sessions
PR Newswire Arena Pharmaceuticals, Inc.
WOODCLIFF LAKE, N.J. and SAN DIEGO, Nov. 18, 2013 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) announced today that a new data analysis from the BELVIQ® (lorcaserin HCl) Phase 3 clinical trial program will be presented at the American Heart Association 2013 Scientific Sessions, November 16-20 in Dallas, Texas.
The following abstract highlighting BELVIQ data will be presented:
The Effects of Lorcaserin on High-Sensitivity C-reactive Protein in Overweight and Obese Patients:
Data From Two Phase 3 Trials
Poster number: 2063
This post-hoc analysis of two previously published Phase 3 trials, BLOOM and BLOOM-DM, evaluated the effect of weight loss on high-sensitivity C-reactive protein (hsCRP) in patients without diabetes who were obese (body mass index, or BMI, 30-45 kg/m2) or overweight (BMI 27-45 kg/m2) in the presence of at least one weight-related comorbid condition and patients with type 2 diabetes who were overweight or obese. Patients were randomized to placebo or lorcaserin for 52 weeks, and all patients received diet and exercise counseling. At week 52, hsCRP levels were assessed for the placebo and lorcaserin groups of the modified intent-to-treat last observation carried forward (MITT/LOCF) population of each trial with a baseline hsCRP greater than 3 mg/L. Results were stratified by 5% or greater weight loss at week 52 and baseline hsCRP levels greater than 3 mg/L.
http://finance.yahoo.com/news/belviq-lorcaserin-hcl-civ-data…
PR Newswire Arena Pharmaceuticals, Inc.
WOODCLIFF LAKE, N.J. and SAN DIEGO, Nov. 18, 2013 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) announced today that a new data analysis from the BELVIQ® (lorcaserin HCl) Phase 3 clinical trial program will be presented at the American Heart Association 2013 Scientific Sessions, November 16-20 in Dallas, Texas.
The following abstract highlighting BELVIQ data will be presented:
The Effects of Lorcaserin on High-Sensitivity C-reactive Protein in Overweight and Obese Patients:
Data From Two Phase 3 Trials
Poster number: 2063
This post-hoc analysis of two previously published Phase 3 trials, BLOOM and BLOOM-DM, evaluated the effect of weight loss on high-sensitivity C-reactive protein (hsCRP) in patients without diabetes who were obese (body mass index, or BMI, 30-45 kg/m2) or overweight (BMI 27-45 kg/m2) in the presence of at least one weight-related comorbid condition and patients with type 2 diabetes who were overweight or obese. Patients were randomized to placebo or lorcaserin for 52 weeks, and all patients received diet and exercise counseling. At week 52, hsCRP levels were assessed for the placebo and lorcaserin groups of the modified intent-to-treat last observation carried forward (MITT/LOCF) population of each trial with a baseline hsCRP greater than 3 mg/L. Results were stratified by 5% or greater weight loss at week 52 and baseline hsCRP levels greater than 3 mg/L.
http://finance.yahoo.com/news/belviq-lorcaserin-hcl-civ-data…
Artikel über Lorcaserin in Journal of Clinical Investigation.
Review
Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype–targeted drugs
Herbert Y. Meltzer, Bryan L. Roth
Published December 2, 2013
Serotonin (5-hydroxytryptamine, or 5-HT) receptors mediate a plethora of physiological phenomena in the brain and the periphery. Additionally, serotonergic dysfunction has been implicated in nearly every neuropsychiatric disorder. The effects of serotonin are mediated by fourteen GPCRs. Both the therapeutic actions and side effects of commonly prescribed drugs are frequently due to nonspecific actions on various 5-HT receptor subtypes. For more than 20 years, the search for clinically efficacious drugs that selectively target 5-HT receptor subtypes has been only occasionally successful. This review provides an overview of 5-HT receptor pharmacology and discusses two recent 5-HT receptor subtype–selective drugs, lorcaserin and pimavanserin, which target the 5HT2C and 5HT2A receptors and provide new treatments for obesity and Parkinson’s disease psychosis, respectively.
http://www.jci.org/articles/view/70678
Review
Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype–targeted drugs
Herbert Y. Meltzer, Bryan L. Roth
Published December 2, 2013
Serotonin (5-hydroxytryptamine, or 5-HT) receptors mediate a plethora of physiological phenomena in the brain and the periphery. Additionally, serotonergic dysfunction has been implicated in nearly every neuropsychiatric disorder. The effects of serotonin are mediated by fourteen GPCRs. Both the therapeutic actions and side effects of commonly prescribed drugs are frequently due to nonspecific actions on various 5-HT receptor subtypes. For more than 20 years, the search for clinically efficacious drugs that selectively target 5-HT receptor subtypes has been only occasionally successful. This review provides an overview of 5-HT receptor pharmacology and discusses two recent 5-HT receptor subtype–selective drugs, lorcaserin and pimavanserin, which target the 5HT2C and 5HT2A receptors and provide new treatments for obesity and Parkinson’s disease psychosis, respectively.
http://www.jci.org/articles/view/70678
moin. beschäftigt sich einer von euch mit achillion pharma?
Arena Pharmaceuticals to Present at Two Upcoming Investor Conferences
SAN DIEGO, Feb. 5, 2014 /PRNewswire/ --
Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at two upcoming investor conferences at The Waldorf Astoria in New York City:
16th Annual BIO CEO & Investor Conference
Presentation: February 11, 2014, at 9:30 a.m. Eastern Time (6:30 a.m. Pacific Time)
2014 Leerink Global Healthcare Conference
Presentation: February 12, 2014, at 1:50 p.m. Eastern Time (10:50 a.m. Pacific Time)
A live audio webcast of each presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of each presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
SAN DIEGO, Feb. 5, 2014 /PRNewswire/ --
Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview at two upcoming investor conferences at The Waldorf Astoria in New York City:
16th Annual BIO CEO & Investor Conference
Presentation: February 11, 2014, at 9:30 a.m. Eastern Time (6:30 a.m. Pacific Time)
2014 Leerink Global Healthcare Conference
Presentation: February 12, 2014, at 1:50 p.m. Eastern Time (10:50 a.m. Pacific Time)
A live audio webcast of each presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of each presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
Arena Pharmaceuticals Reports Coverage for BELVIQ® (lorcaserin HCl) CIV Now Greater Than 50% of Insured Commercial Lives
BELVIQ Now Covered by CVS Caremark -
SAN DIEGO, Calif., Feb. 10, 2014 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today reported that Eisai has secured improved patient access of BELVIQ® (lorcaserin HCl) with two leading healthcare benefit companies, and that the estimated number of insured commercial lives in the United States that have coverage for BELVIQ now exceeds 50 percent.
CVS Caremark customers (employers and health plans) have broadened the coverage of BELVIQ, and eligible CVS Caremark members may have access to BELVIQ in either a preferred or non-preferred brand position depending on the design of the employer benefit or health plan. In addition, Aetna recently announced that it would offer BELVIQ as a preferred brand to eligible patients as part of its pilot program to self-insured plan sponsors nationwide. The Aetna program, which offers access to lifestyle management programs and surgical options, will also measure improvements in health outcomes, productivity and medical costs.
"Securing broad reimbursement coverage for patients is an important part of the foundation for the long-term success of BELVIQ in the United States, and Eisai has continued to demonstrate its expertise in this area," said Jack Lief, Arena's President and Chief Executive Officer. "Over 50 percent of insured commercial lives with coverage for BELVIQ represents a significant increase from the approximately 30 percent of insured commercial lives that were estimated to have coverage at launch in June 2013."
Lonnel Coats, President and Chief Executive Officer of Eisai Inc., added, "We are committed to ensuring that patients have access to BELVIQ. Given how many people are struggling with their weight, it's important to provide treatment options to those who have not been able to sustain long-term weight loss with diet and exercise alone."
A patient's individual coverage for BELVIQ will vary, and may depend on the design of the patient's employer benefit or health plan. The estimated number of insured commercial lives is derived from Fingertip Formulary data, which is generally based on enrollment reported by health plans and pharmacy benefit managers.
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
BELVIQ Now Covered by CVS Caremark -
SAN DIEGO, Calif., Feb. 10, 2014 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today reported that Eisai has secured improved patient access of BELVIQ® (lorcaserin HCl) with two leading healthcare benefit companies, and that the estimated number of insured commercial lives in the United States that have coverage for BELVIQ now exceeds 50 percent.
CVS Caremark customers (employers and health plans) have broadened the coverage of BELVIQ, and eligible CVS Caremark members may have access to BELVIQ in either a preferred or non-preferred brand position depending on the design of the employer benefit or health plan. In addition, Aetna recently announced that it would offer BELVIQ as a preferred brand to eligible patients as part of its pilot program to self-insured plan sponsors nationwide. The Aetna program, which offers access to lifestyle management programs and surgical options, will also measure improvements in health outcomes, productivity and medical costs.
"Securing broad reimbursement coverage for patients is an important part of the foundation for the long-term success of BELVIQ in the United States, and Eisai has continued to demonstrate its expertise in this area," said Jack Lief, Arena's President and Chief Executive Officer. "Over 50 percent of insured commercial lives with coverage for BELVIQ represents a significant increase from the approximately 30 percent of insured commercial lives that were estimated to have coverage at launch in June 2013."
Lonnel Coats, President and Chief Executive Officer of Eisai Inc., added, "We are committed to ensuring that patients have access to BELVIQ. Given how many people are struggling with their weight, it's important to provide treatment options to those who have not been able to sustain long-term weight loss with diet and exercise alone."
A patient's individual coverage for BELVIQ will vary, and may depend on the design of the patient's employer benefit or health plan. The estimated number of insured commercial lives is derived from Fingertip Formulary data, which is generally based on enrollment reported by health plans and pharmacy benefit managers.
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
Arena Pharmaceuticals Secures Belviq Deal With CVS Caremark
KATEY TROUTMAN | MORE ARTICLES
FEBRUARY 11, 2014
Arena Pharmaceuticals (NASDAQ:ARNA) and Eisai Inc. announced Monday that their weight loss drug, Belviq, will be available to more people thanks to a new partnership with CVS Caremark (NYSE:CVS), a pharmacy benefits manager.
Pharmacy benefits managers like Caremark act as a kind of intermediary within the health care system; PBMs are third-party administrators of prescription drug programs and are primarily responsible for processing and paying for prescription drug claims.
Employers and health plans that use CVS Caremark as a PBM have agreed to broaden the coverage of Belviq. In addition, the health insurer Aetna, which has been evaluating Belviq along with a competing drug made by Vivus, called Qsymia, announced it will now offer Belviq as a preferred brand to eligible patients through its pilot program to self-insured plan sponsors across the country.
An Arena Pharmaceuticals press release stated that as a result of the agreement with CVS Caremark, more than half of Americans with insurance should now have access to Belviq.....................
http://wallstcheatsheet.com/stocks/arena-pharmaceuticals-sec…
KATEY TROUTMAN | MORE ARTICLES
FEBRUARY 11, 2014
Arena Pharmaceuticals (NASDAQ:ARNA) and Eisai Inc. announced Monday that their weight loss drug, Belviq, will be available to more people thanks to a new partnership with CVS Caremark (NYSE:CVS), a pharmacy benefits manager.
Pharmacy benefits managers like Caremark act as a kind of intermediary within the health care system; PBMs are third-party administrators of prescription drug programs and are primarily responsible for processing and paying for prescription drug claims.
Employers and health plans that use CVS Caremark as a PBM have agreed to broaden the coverage of Belviq. In addition, the health insurer Aetna, which has been evaluating Belviq along with a competing drug made by Vivus, called Qsymia, announced it will now offer Belviq as a preferred brand to eligible patients through its pilot program to self-insured plan sponsors across the country.
An Arena Pharmaceuticals press release stated that as a result of the agreement with CVS Caremark, more than half of Americans with insurance should now have access to Belviq.....................
http://wallstcheatsheet.com/stocks/arena-pharmaceuticals-sec…
 Arena Pharmaceuticals Announces Filing for Marketing Authorization of BELVIQ® (lorcaserin HCl) in Brazil
Arena Pharmaceuticals Announces Filing for Marketing Authorization of BELVIQ® (lorcaserin HCl) in Brazil
Eisai's Filing for Approval of BELVIQ as a Treatment for Chronic Weight Management Triggers $500,000 Milestone Payment to Arena --
SAN DIEGO, Feb. 12, 2014 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that Eisai Laboratorios Ltda., a subsidiary of Eisai Inc., has filed for marketing authorization of BELVIQ® as a treatment for chronic weight management with the Brazilian Health Surveillance Agency (Anvisa). In connection with the filing, Arena will receive a milestone payment of $500,000 from Eisai.
"With over half of adults in Brazil being overweight or obese, there is a significant need for new chronic weight management treatments to help address this medical need," said Jack Lief, Arena's President and Chief Executive Officer.
"The filing of this marketing application further illustrates Eisai's commitment to make BELVIQ available to patients and physicians around the world."
BELVIQ is being submitted for marketing authorization in Brazil as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult obese patients (initial body mass index, or BMI, ≥ 30 kg/m2), or overweight patients (initial BMI ≥ 27 kg/m2) in the presence of at least one weight-related comorbid condition (e.g., hypertension, dyslipidemia, cardiovascular disease, type 2 diabetes managed with oral hypoglycemic agents, or sleep apnea).
Eisai is responsible for seeking the regulatory approval and the subsequent marketing and distribution of BELVIQ in Brazil. Arena has granted Eisai marketing and distribution rights to BELVIQ for all countries worldwide, except South Korea, Taiwan, Australia, New Zealand and Israel.
Arena manufactures BELVIQ at its facility in Switzerland, and sells finished commercial product to Eisai for distribution. Arena is eligible to receive payments based upon Eisai's net sales of BELVIQ, and is also eligible to receive regulatory and development milestone payments.
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…
Arena Pharmaceuticals to Provide Corporate Update and Report Financial Results on Thursday, February 27
PR Newswire Arena Pharmaceuticals, Inc.
SAN DIEGO, Feb. 20, 2014 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that it will provide a corporate update and report fourth quarter and full year 2013 financial results after the NASDAQ Global Select Market closes on Thursday, February 27, 2014.
That same day, Arena will host a conference call and webcast at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time).
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' Fourth Quarter and Full Year 2013 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-corporat…
PR Newswire Arena Pharmaceuticals, Inc.
SAN DIEGO, Feb. 20, 2014 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that it will provide a corporate update and report fourth quarter and full year 2013 financial results after the NASDAQ Global Select Market closes on Thursday, February 27, 2014.
That same day, Arena will host a conference call and webcast at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time).
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' Fourth Quarter and Full Year 2013 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-corporat…
Arena Pharmaceuticals to Host Corporate Update and Financial Results Conference Call and Webcast
SAN DIEGO, May 5, 2014 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that it will provide a corporate update and report first quarter 2014 financial results after the NASDAQ Global Select Market closes on Monday, May 12, 2014.
That same day, Arena will host a conference call and webcast at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time).
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' First Quarter 2014 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-host-cor…
SAN DIEGO, May 5, 2014 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that it will provide a corporate update and report first quarter 2014 financial results after the NASDAQ Global Select Market closes on Monday, May 12, 2014.
That same day, Arena will host a conference call and webcast at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time).
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' First Quarter 2014 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-host-cor…
Arena's Belviq Sales See Nice Rise
by Spencer Osborne
May. 2, 2014 12:23 PM ET
> Sales are up by more than 15% week over week.
> New scripts are up almost 20%, showing awareness traction.
> Television ad campaign will continue to drive new sales.
http://seekingalpha.com/article/2186773-arenas-belviq-sales-…
...die Verkaufszahlen von Belviq sind seit der TV Kampagne gestiegen...
...dann hoffen wir mal auf weitere Steigerungen....
by Spencer Osborne
May. 2, 2014 12:23 PM ET
> Sales are up by more than 15% week over week.
> New scripts are up almost 20%, showing awareness traction.
> Television ad campaign will continue to drive new sales.
http://seekingalpha.com/article/2186773-arenas-belviq-sales-…
...die Verkaufszahlen von Belviq sind seit der TV Kampagne gestiegen...
...dann hoffen wir mal auf weitere Steigerungen....

Arena Pharma Q1 Loss Widens - Quick Facts
Arena Pharma Q1 Loss Widens, Misses View
Arena Pharmaceuticals Q4 Loss Widens - Quick Facts
Trade ARNA now with
Advertisement
5/12/2014 4:11 PM ET
Arena Pharmaceuticals, Inc. (ARNA: Quote) Monday said first-quarter loss widened to $25.26 million or $0.12 per share from $18.88 million or $0.09 per share in the same period last year.
On average, 11 analysts polled by Thomson Reuters expected the company to report a loss of $0.10 per share for the quarter. Analysts' estimates typically exclude special items.
Revenues jumped to $6.81 million from $2.37 million last year. Analysts expected revenues of $7.98 million.
Click here to receive FREE breaking news email alerts for Arena Pharmaceutical Inc. and others in your portfolio
by RTT Staff Writer
For comments and feedback: editorial@rttnews.com
http://www.rttnews.com/2319939/arena-pharma-q1-loss-widens-q…
Arena Pharma Q1 Loss Widens, Misses View
Arena Pharmaceuticals Q4 Loss Widens - Quick Facts
Trade ARNA now with
Advertisement
5/12/2014 4:11 PM ET
Arena Pharmaceuticals, Inc. (ARNA: Quote) Monday said first-quarter loss widened to $25.26 million or $0.12 per share from $18.88 million or $0.09 per share in the same period last year.
On average, 11 analysts polled by Thomson Reuters expected the company to report a loss of $0.10 per share for the quarter. Analysts' estimates typically exclude special items.
Revenues jumped to $6.81 million from $2.37 million last year. Analysts expected revenues of $7.98 million.
Click here to receive FREE breaking news email alerts for Arena Pharmaceutical Inc. and others in your portfolio
by RTT Staff Writer
For comments and feedback: editorial@rttnews.com
http://www.rttnews.com/2319939/arena-pharma-q1-loss-widens-q…
May 15, 2014
Arena Pharmaceuticals Posts First Quarter Results
-Eisai recorded net product sales for Belviq of $8.4 million in the first quarter of 2014.. -Eisai announced plans to add another 200 representatives to its sales force for BELVIQ by July 2014, increasing the number of representatives to approximately 600. Eisai believes this expansion of the sales force will enable them to reach approximately 90,000...
Arena Pharmaceuticals, Inc. provided a corporate update and reported financial results for the first quarter ended March 31.
In a release on May 12, the company reported first quarter and recent developments.
Belviq (lorcaserin HCl) CIV US Commercial Update
-Approximately 77,000 prescriptions for Belviq were filled in the first quarter of 2014, according to IMS Health, representing growth of approximately 31 percent in total prescriptions as compared to the previous quarter.
-Eisai recorded net product sales for Belviq of $8.4 million in the first quarter of 2014.
-Eisai announced plans to add another 200 representatives to its sales force for BELVIQ by July 2014, increasing the number of representatives to approximately 600. Eisai believes this expansion of the sales force will enable them to reach approximately 90,000 physicians in the United States. This increase is in addition to the 200 sales representatives for Belviq that Eisai added around the end of 2013.
-Eisai announced that its continued work to expand reimbursement has resulted in additional insurance coverage for Belviq. According to Fingertip Formulary, the number of insured commercial lives in the United States with access to Belviq is now estimated to exceed 60 percent. While the exact coverage for Belviq varies by each patient's insurance plan, this improved access means more patients will receive coverage support from their health plan or pharmacy benefit manager.
-Eisai launched a national television advertising campaign for Belviq that illustrates the struggles many people face with appetite and body weight. The television advertisement is airing on numerous networks, including Lifetime, Oxygen and AMC, and presents Belviq as a targeted approach to weight loss, that, when combined with diet and increased activity, may help patients lose weight and keep it off.
-Eisai Laboratorios filed for marketing authorization of Belviq as a treatment for chronic weight management in Brazil. In connection with the filing, Arena achieved a $0.5 million milestone payment from Eisai.
Research & Development
-Eisai completed enrollment in a 12-week pilot study of lorcaserin and phentermine when co-administered. The primary endpoint of this study is safety.
-Arena initiated dosing in a 12-week randomized, double-blind and placebo-controlled Phase 2 clinical trial that will enroll approximately 600 active smokers to evaluate lorcaserin as a potential aid to smoking cessation.
-Ildong Pharmaceutical Co., initiated dosing in a Phase 1 multiple-ascending dose clinical trial of temanogrel, an orally available inverse agonist of the serotonin 2A receptor intended for the treatment of thrombotic diseases.
First Quarter 2014 Financial Results
-Revenues totaled $6.8 million, including $2.9 million in net product sales of Belviq, of which $2.7 million represented 31.5 percent of Eisai's net product sales and $0.2 million related to redemptions of the 15-day free voucher.
-Research and development expenses totaled $21.0 million.
-General and administrative expenses totaled $8.0 million.
-Net loss was $25.3 million, or $0.12 per share.
-At March 31, cash, cash equivalents and short-term investments available-for-sale totaled $256.5 million.
-At March 31, approximately 219.5 million shares of common stock were outstanding.
"We are off to a productive start in 2014 with significant new marketing initiatives for Belviq for chronic weight management, the advancement of our lorcaserin life-cycle management programs and the development of our internally discovered pipeline," said Jack Lief, Arena's President and Chief Executive Officer. "Prescriptions for BELVIQ increased 31 percent in the first quarter over the previous quarter, and Eisai continues to boost its investment in key strategic areas."
Upcoming Conference Participation
Arena is planning to participate at upcoming investment and industry conferences, including:
-Jefferies 2014 Global Healthcare Conference, June 2-5, New York, New York
-American Diabetes Association's 74th Scientific Sessions, June 13-17, San Francisco, California
-Wells Fargo Securities Healthcare Conference, June 17-18, Boston, Massachusetts
-Piper Jaffray Heartland Summit, August 6-7, Minneapolis, Minnesota
Foresters SMART universal life insurance.
Arena seeks to bring medicines targeting G protein-coupled receptors to patients.
More information:
www.BELVIQ.com.
www.arenapharm.com.
((Comments on this story may be sent to newsdesk@closeupmedia.com))
Copyright: (c) 2014 ProQuest Information and Learning Company; All Rights Reserved.
http://insurancenewsnet.com/oarticle/2014/05/15/arena-pharma…
Arena Pharmaceuticals Posts First Quarter Results
-Eisai recorded net product sales for Belviq of $8.4 million in the first quarter of 2014.. -Eisai announced plans to add another 200 representatives to its sales force for BELVIQ by July 2014, increasing the number of representatives to approximately 600. Eisai believes this expansion of the sales force will enable them to reach approximately 90,000...
Arena Pharmaceuticals, Inc. provided a corporate update and reported financial results for the first quarter ended March 31.
In a release on May 12, the company reported first quarter and recent developments.
Belviq (lorcaserin HCl) CIV US Commercial Update
-Approximately 77,000 prescriptions for Belviq were filled in the first quarter of 2014, according to IMS Health, representing growth of approximately 31 percent in total prescriptions as compared to the previous quarter.
-Eisai recorded net product sales for Belviq of $8.4 million in the first quarter of 2014.
-Eisai announced plans to add another 200 representatives to its sales force for BELVIQ by July 2014, increasing the number of representatives to approximately 600. Eisai believes this expansion of the sales force will enable them to reach approximately 90,000 physicians in the United States. This increase is in addition to the 200 sales representatives for Belviq that Eisai added around the end of 2013.
-Eisai announced that its continued work to expand reimbursement has resulted in additional insurance coverage for Belviq. According to Fingertip Formulary, the number of insured commercial lives in the United States with access to Belviq is now estimated to exceed 60 percent. While the exact coverage for Belviq varies by each patient's insurance plan, this improved access means more patients will receive coverage support from their health plan or pharmacy benefit manager.
-Eisai launched a national television advertising campaign for Belviq that illustrates the struggles many people face with appetite and body weight. The television advertisement is airing on numerous networks, including Lifetime, Oxygen and AMC, and presents Belviq as a targeted approach to weight loss, that, when combined with diet and increased activity, may help patients lose weight and keep it off.
-Eisai Laboratorios filed for marketing authorization of Belviq as a treatment for chronic weight management in Brazil. In connection with the filing, Arena achieved a $0.5 million milestone payment from Eisai.
Research & Development
-Eisai completed enrollment in a 12-week pilot study of lorcaserin and phentermine when co-administered. The primary endpoint of this study is safety.
-Arena initiated dosing in a 12-week randomized, double-blind and placebo-controlled Phase 2 clinical trial that will enroll approximately 600 active smokers to evaluate lorcaserin as a potential aid to smoking cessation.
-Ildong Pharmaceutical Co., initiated dosing in a Phase 1 multiple-ascending dose clinical trial of temanogrel, an orally available inverse agonist of the serotonin 2A receptor intended for the treatment of thrombotic diseases.
First Quarter 2014 Financial Results
-Revenues totaled $6.8 million, including $2.9 million in net product sales of Belviq, of which $2.7 million represented 31.5 percent of Eisai's net product sales and $0.2 million related to redemptions of the 15-day free voucher.
-Research and development expenses totaled $21.0 million.
-General and administrative expenses totaled $8.0 million.
-Net loss was $25.3 million, or $0.12 per share.
-At March 31, cash, cash equivalents and short-term investments available-for-sale totaled $256.5 million.
-At March 31, approximately 219.5 million shares of common stock were outstanding.
"We are off to a productive start in 2014 with significant new marketing initiatives for Belviq for chronic weight management, the advancement of our lorcaserin life-cycle management programs and the development of our internally discovered pipeline," said Jack Lief, Arena's President and Chief Executive Officer. "Prescriptions for BELVIQ increased 31 percent in the first quarter over the previous quarter, and Eisai continues to boost its investment in key strategic areas."
Upcoming Conference Participation
Arena is planning to participate at upcoming investment and industry conferences, including:
-Jefferies 2014 Global Healthcare Conference, June 2-5, New York, New York
-American Diabetes Association's 74th Scientific Sessions, June 13-17, San Francisco, California
-Wells Fargo Securities Healthcare Conference, June 17-18, Boston, Massachusetts
-Piper Jaffray Heartland Summit, August 6-7, Minneapolis, Minnesota
Foresters SMART universal life insurance.
Arena seeks to bring medicines targeting G protein-coupled receptors to patients.
More information:
www.BELVIQ.com.
www.arenapharm.com.
((Comments on this story may be sent to newsdesk@closeupmedia.com))
Copyright: (c) 2014 ProQuest Information and Learning Company; All Rights Reserved.
http://insurancenewsnet.com/oarticle/2014/05/15/arena-pharma…
...dann hoffen wir mal, dass durch diese Maßnahme die Verkaufszahlen von Belviq weiter nach oben "schnellen"...
......so nach dem Motto >> YES WE CAN <<
Arena Pharmaceuticals Reports that Eisai Completes Planned Increase in Sales Force for BELVIQ® (lorcaserin HCl)
Sales Force Increased to Approximately 600 Sales Specialists
PR Newswire Arena Pharmaceuticals, Inc.
July 10, 2014 8:00 AM
SAN DIEGO, July 10, 2014 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today reported that Eisai Inc. has completed its planned increase of more than 200 new contract sales representatives in its Metabolic Business Unit, increasing the sales force for BELVIQ® by 50% to approximately 600.
Eisai expects this expansion of the sales force will enable them to increase their reach to approximately 92,000 physicians in the United States.
"We are enthusiastic about the commercial ramp-up for BELVIQ with this further increase in the sales force," said Jack Lief, Arena's President and Chief Executive Officer. "This involves a large-scale deployment of additional sales representatives who will increase Eisai's ability to educate physicians. We support the approach and believe that it will help produce greater results."
BELVIQ is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index (BMI) of 30 kg/m2 or greater (obese), or 27 kg/m2 or greater (overweight) in the presence of at least one weight-related, comorbid condition (e.g., hypertension, dyslipidemia, type 2 diabetes). It is not known if BELVIQ is safe and effective when taken with other prescription, over-the-counter, or herbal weight loss products, and the effect of BELVIQ on cardiovascular morbidity and mortality has not been established.
Eisai is responsible for the marketing and distribution of BELVIQ in the United States under its agreement with Arena.
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
......so nach dem Motto >> YES WE CAN <<

Arena Pharmaceuticals Reports that Eisai Completes Planned Increase in Sales Force for BELVIQ® (lorcaserin HCl)
Sales Force Increased to Approximately 600 Sales Specialists

PR Newswire Arena Pharmaceuticals, Inc.
July 10, 2014 8:00 AM
SAN DIEGO, July 10, 2014 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today reported that Eisai Inc. has completed its planned increase of more than 200 new contract sales representatives in its Metabolic Business Unit, increasing the sales force for BELVIQ® by 50% to approximately 600.
Eisai expects this expansion of the sales force will enable them to increase their reach to approximately 92,000 physicians in the United States.
"We are enthusiastic about the commercial ramp-up for BELVIQ with this further increase in the sales force," said Jack Lief, Arena's President and Chief Executive Officer. "This involves a large-scale deployment of additional sales representatives who will increase Eisai's ability to educate physicians. We support the approach and believe that it will help produce greater results."
BELVIQ is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index (BMI) of 30 kg/m2 or greater (obese), or 27 kg/m2 or greater (overweight) in the presence of at least one weight-related, comorbid condition (e.g., hypertension, dyslipidemia, type 2 diabetes). It is not known if BELVIQ is safe and effective when taken with other prescription, over-the-counter, or herbal weight loss products, and the effect of BELVIQ on cardiovascular morbidity and mortality has not been established.
Eisai is responsible for the marketing and distribution of BELVIQ in the United States under its agreement with Arena.
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
Arena Pharmaceuticals Enters into Marketing and Supply Agreement for BELVIQ® (lorcaserin HCl) in Israel
SAN DIEGO, July 21, 2014 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that its wholly owned subsidiary, Arena Pharmaceuticals GmbH, has entered into an exclusive marketing and supply agreement for BELVIQ® (lorcaserin HCl) with Teva Pharmaceutical Industries Limited's local Israeli subsidiary, Abic Marketing Limited. Under the agreement, Arena granted Abic the rights to market and distribute BELVIQ in Israel for weight loss or weight management in obese and overweight patients, subject to regulatory approval by the State of Israel Ministry of Health (MOH).
"Founded in Israel in 1901, Teva is the leading pharmaceutical company in the Israeli market," said Jack Lief, Arena's President and Chief Executive Officer. "Their local presence and proven commercialization expertise are important factors toward making BELVIQ available in Israel as a new treatment option for chronic weight management."
Steadily rising obesity rates is a well-known phenomenon in western countries, and Israel is no exception. The number of overweight and obese Israelis has nearly tripled between 1967 and 2003. In 2011, the MOH estimated that approximately 48% of the population is overweight or obese.
Abic is responsible for regulatory approval and, ultimately, marketing and distribution of BELVIQ in Israel, including related costs and expenses. Arena will manufacture finished drug product at its facility in Switzerland, which it will sell to Abic at a purchase price equal to a percentage of Abic's annual net sales of BELVIQ. In addition, Arena will receive an upfront payment, and is eligible to receive milestone payments upon regulatory submission and regulatory approval of BELVIQ as well as one-time purchase price adjustment payments based on Abic's annual net sales.
http://finance.yahoo.com/news/arena-pharmaceuticals-enters-m…
SAN DIEGO, July 21, 2014 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that its wholly owned subsidiary, Arena Pharmaceuticals GmbH, has entered into an exclusive marketing and supply agreement for BELVIQ® (lorcaserin HCl) with Teva Pharmaceutical Industries Limited's local Israeli subsidiary, Abic Marketing Limited. Under the agreement, Arena granted Abic the rights to market and distribute BELVIQ in Israel for weight loss or weight management in obese and overweight patients, subject to regulatory approval by the State of Israel Ministry of Health (MOH).
"Founded in Israel in 1901, Teva is the leading pharmaceutical company in the Israeli market," said Jack Lief, Arena's President and Chief Executive Officer. "Their local presence and proven commercialization expertise are important factors toward making BELVIQ available in Israel as a new treatment option for chronic weight management."
Steadily rising obesity rates is a well-known phenomenon in western countries, and Israel is no exception. The number of overweight and obese Israelis has nearly tripled between 1967 and 2003. In 2011, the MOH estimated that approximately 48% of the population is overweight or obese.
Abic is responsible for regulatory approval and, ultimately, marketing and distribution of BELVIQ in Israel, including related costs and expenses. Arena will manufacture finished drug product at its facility in Switzerland, which it will sell to Abic at a purchase price equal to a percentage of Abic's annual net sales of BELVIQ. In addition, Arena will receive an upfront payment, and is eligible to receive milestone payments upon regulatory submission and regulatory approval of BELVIQ as well as one-time purchase price adjustment payments based on Abic's annual net sales.
http://finance.yahoo.com/news/arena-pharmaceuticals-enters-m…
 Nach so langer Pause jetzt mal eine HAMMER NEWS
Nach so langer Pause jetzt mal eine HAMMER NEWS
Arena Pharmaceuticals Reports Positive Results from Phase 1b Clinical Trial Evaluating APD334 for the Treatment of Autoimmune Diseases
- Trial results show novel S1P1 modulator produced strong, dose-dependent lymphocyte lowering -
- No clinically significant safety findings relative to heart, liver or pulmonary function -
SAN DIEGO, Jan. 7, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced top-line results from a Phase 1b multiple ascending dose clinical trial for APD334, an oral drug candidate that targets the sphingosine 1-phosphate subtype 1 (S1P1) receptor for the potential treatment of autoimmune diseases.
In the Phase 1b clinical trial, APD334 demonstrated a dose-dependent effect on lymphocyte count lowering in blood, with mean decreases from baseline of up to 69%. Lymphocyte counts, on average, recovered to baseline within one week of conclusion of dosing. There were no clinically significant safety findings with respect to heart rate or rhythm or pulmonary function, and no clinically significant elevations in liver enzyme tests. The most common treatment-emergent adverse events were mild or moderate contact dermatitis, headache, constipation and diarrhea, with none being clearly drug related. There were no discontinuations for adverse events, and no serious adverse events were observed.
"Lymphocyte lowering at the level demonstrated in this trial has been shown to correlate with clinical efficacy in Phase 2 and Phase 3 trials of other S1P1 modulators in multiple sclerosis, psoriasis and ulcerative colitis," said William R. Shanahan, M.D., Arena's Senior Vice President and Chief Medical Officer. "The results of this trial support investigation of the efficacy and safety of APD334 in patients with autoimmune diseases."
The randomized, double-blind, placebo-controlled Phase 1b clinical trial evaluated the safety, tolerability, pharmacodynamics and pharmacokinetics of multiple-ascending doses of APD334. In five different dosing cohorts, a total of 50 healthy volunteers received APD334 and 10 received placebo for 21 days.
"Based on these impressive results, we plan to expedite APD334 into Phase 2 clinical trials for ulcerative colitis and Crohn's disease," said Jack Lief, Arena's President and Chief Executive Officer. "The advancement of this promising drug candidate further demonstrates Arena's focused expertise in discovering and developing innovative drug candidates targeting G protein-coupled receptors that have the potential to improve health."
About Autoimmune Diseases
Autoimmune diseases, such as multiple sclerosis, psoriasis, ulcerative colitis, Crohn's disease and rheumatoid arthritis, are characterized by an inappropriate immune response against substances and tissues that are normally present in the body. In an autoimmune reaction, a person's antibodies and immune cells target healthy tissue, triggering an inflammatory response. Reducing the immune and/or inflammatory response is an important goal in the treatment of autoimmune diseases.
About APD334
APD334 is a potent and selective, orally available investigational drug candidate that targets the S1P1 receptor. Discovered by Arena, APD334 has therapeutic potential in autoimmune diseases. S1P1 receptors have been demonstrated to be involved in the modulation of several biological responses, including lymphocyte trafficking from lymph nodes to the peripheral blood. By isolating lymphocytes in lymph nodes, fewer immune cells are available in the circulating blood to effect tissue damage.
About Arena Pharmaceuticals
Arena is embracing the challenge of improving health by seeking to bring innovative medicines targeting G protein-coupled receptors to patients. Arena's internally discovered drug, BELVIQ® (lorcaserin HCl), is approved in the United States, and Arena is focused on discovering, developing and commercializing additional drugs to address unmet medical needs. Arena's US operations are located in San Diego, California, and its operations outside of the United States, including its commercial manufacturing facility, are located in Zofingen, Switzerland. For more information, visit Arena's website at www.arenapharm.com.
Arena Pharmaceuticals® and Arena® are registered service marks of Arena Pharmaceuticals, Inc. BELVIQ® is a registered trademark of Arena Pharmaceuticals GmbH.
Forward-Looking Statements
Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include statements about the advancement, investigation, therapeutic indication, use, safety, efficacy, mechanism of action, and potential of APD334, including as a potential treatment for one or more autoimmune diseases; the significance of the trial results for APD334, including with respect to lymphocyte lowering and safety; the significance of lymphocyte lowering and reducing the immune and/or inflammatory response; expediting the development of APD334, including future trials and investigated indications; Arena's expertise in discovering and developing drug candidates that have the potential to improve health; embracing the challenge of improving health; seeking to bring innovative medicines to patients; and Arena's focus, plans, goals, strategy, expectations, research and development programs, and ability to discover and develop compounds and commercialize drugs. For such statements, Arena claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Arena's expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, the following: top-line results are not comprehensive and are based on a preliminary analysis of then available data, and findings and conclusions related to the trial are subject to change following a more comprehensive review of the data; APD334 may not be developed, approved for marketing or commercialized for any disease or condition; risks related to commercializing drugs, including regulatory, manufacturing, supply and marketing issues and the availability and use of BELVIQ; cash and revenues generated from BELVIQ, including the impact of competition; Arena's revenues will be based in part on estimates, judgment and accounting policies, and incorrect estimates or disagreement regarding estimates or accounting policies may result in changes to Arena's guidance or previously reported results; the timing and outcome of regulatory review is uncertain, and BELVIQ may not be approved for marketing when expected or ever in combination with another drug, for another indication or using a different formulation or in any other territory for any indication; regulatory decisions in one territory may impact other regulatory decisions and Arena's business prospects; government and commercial reimbursement and pricing decisions; risks related to relying on collaborative arrangements; the timing and receipt of payments and fees, if any, from collaborators; the entry into or modification or termination of collaborative arrangements; unexpected or unfavorable new data; nonclinical and clinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Arena or others, request additional information, have additional recommendations or change their guidance or requirements before or after approval; data and other information related to any of Arena's research and development may not meet regulatory requirements or otherwise be sufficient for (or Arena or a collaborator may not pursue) further research and development, regulatory review or approval or continued marketing; Arena's and third parties' intellectual property rights; the timing, success and cost of Arena's research and development; results of clinical trials and other studies are subject to different interpretations and may not be predictive of future results; clinical trials and other studies may not proceed at the time or in the manner expected or at all; having adequate funds; and satisfactory resolution of litigation or other disagreements with others. Additional factors that could cause actual results to differ materially from those stated or implied by Arena's forward-looking statements are disclosed in Arena's filings with the Securities and Exchange Commission. These forward-looking statements represent Arena's judgment as of the time of this release. Arena disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
....aus dem IV Board...
APD334 Trial results are all about the SAFETY
The significance of these findings today, is that APD 334 is now more likely to replace Gilenya as the first line therapy drug of choice for multiple autoimmune conditions, rather than simply being a second to market competitor with similar efficacy and side effect profile.
This was Arena's plan all along in designing a drug that targets the S1P1 receptor responsible for the reduction in white blood count (lymphocytes) and efficacy, but not the S1P3 receptor which was responsible for the bradycardia in animal studies. Notice that this is exactly what they did with Belviq that selectively targets the 5HT2c receptor (responsible for weight loss), but not the 5HT2b receptor linked to the heart valve damage seen with Fenfluramine.
This unique approach to drug development is something that the market still does not fully understand. It significantly increases the chances that a novel drug will be both efficacious and yet have fewer side effects than an already approved drug. APD334 appears to be our next winner from Arena's unique drug development approach.
http://www.investorvillage.com/smbd.asp?mb=633&mn=146481&pt=…
APD334 Trial results are all about the SAFETY
The significance of these findings today, is that APD 334 is now more likely to replace Gilenya as the first line therapy drug of choice for multiple autoimmune conditions, rather than simply being a second to market competitor with similar efficacy and side effect profile.
This was Arena's plan all along in designing a drug that targets the S1P1 receptor responsible for the reduction in white blood count (lymphocytes) and efficacy, but not the S1P3 receptor which was responsible for the bradycardia in animal studies. Notice that this is exactly what they did with Belviq that selectively targets the 5HT2c receptor (responsible for weight loss), but not the 5HT2b receptor linked to the heart valve damage seen with Fenfluramine.
This unique approach to drug development is something that the market still does not fully understand. It significantly increases the chances that a novel drug will be both efficacious and yet have fewer side effects than an already approved drug. APD334 appears to be our next winner from Arena's unique drug development approach.
http://www.investorvillage.com/smbd.asp?mb=633&mn=146481&pt=…
Antwort auf Beitrag Nr.: 48.719.834 von bernie55 am 07.01.15 19:43:43Hallo Bernie,
anscheinend sind wir die beiden letzten Überbleibsel aus der ARENA-Geschichte.

Gruß
Rainer
anscheinend sind wir die beiden letzten Überbleibsel aus der ARENA-Geschichte.

Gruß
Rainer
Antwort auf Beitrag Nr.: 48.722.045 von Poppholz am 08.01.15 07:40:57
.....also sozusagen > die letzten AREANICS der ersten Stunde < ....
Zitat von Poppholz: Hallo Bernie,
anscheinend sind wir die beiden letzten Überbleibsel aus der ARENA-Geschichte.
Gruß
Rainer
.....also sozusagen > die letzten AREANICS der ersten Stunde < ....

Aber ich weiß auch , dass einige gestern wieder eingestiegen sind. 
Grüße
bernie55

Grüße
bernie55

Antwort auf Beitrag Nr.: 48.724.286 von bernie55 am 08.01.15 11:31:29nicht das wir hier nur unsere Aktien hin und her tauschen.


Arena Pharmaceuticals to Present at the 33rd Annual J.P. Morgan Healthcare Conference
PR Newswire Arena Pharmaceuticals, Inc.
January 6, 2015 8:00 AM
SAN DIEGO, Jan. 6, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview and update at the 33rd Annual J.P. Morgan Healthcare Conference on Wednesday,
January 14, 2015, at 2:30 p.m. Pacific Time (5:30 p.m. Eastern Time), at The Westin St. Francis Hotel in San Francisco, California.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
PR Newswire Arena Pharmaceuticals, Inc.
January 6, 2015 8:00 AM
SAN DIEGO, Jan. 6, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview and update at the 33rd Annual J.P. Morgan Healthcare Conference on Wednesday,
January 14, 2015, at 2:30 p.m. Pacific Time (5:30 p.m. Eastern Time), at The Westin St. Francis Hotel in San Francisco, California.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
Arena Pharmaceuticals Initiates Phase 2 Clinical Trial Evaluating Ralinepag for Pulmonary Arterial Hypertension
SAN DIEGO, Jan. 12, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today the initiation of patient dosing in a Phase 2 clinical trial of ralinepag, an oral, non-prostanoid prostacyclin (IP) receptor agonist intended for the treatment of pulmonary arterial hypertension (PAH).
"An intriguing component of this compound is the smooth peak-to-trough ratio, which may potentiate efficacy and minimize toxicity relative to currently available therapies," said Lewis J. Rubin, M.D., Emeritus Professor of Medicine at University of California, San Diego, School of Medicine. "This clinical trial should help to further elucidate how ralinepag's intrinsic properties translate to its efficacy and safety profile."
This 22-week, randomized, double-blind and placebo-controlled Phase 2 trial will evaluate the hemodynamic and exercise capacity effects, safety and tolerability of ralinepag in up to 60 patients with PAH. During the first nine weeks of the trial, patients will be titrated to their individual tolerance level, and then sustained at this level for the remainder of the trial.
"We believe ralinepag offers promise in the IP receptor class of molecules given its oral availability, long plasma half-life, and high selectivity and potency in vitro," said Jack Lief, Arena's President and Chief Executive Officer. "In conjunction with our other clinical-stage programs, this compound further enhances our optimism about Arena's value drivers."
About Pulmonary Arterial Hypertension
PAH is a progressive, life-threatening disorder characterized by increased pressure in the arteries that carry blood from the heart to the lungs. The increased pressure strains the heart, which can limit physical activity, result in heart failure and reduce life expectancy. Based on data from the Registry to EValuate Early And Long-term PAH disease management (REVEAL) of patients in the United States, there is an estimated five-year survival rate of 57% from diagnosis.
About Ralinepag
Ralinepag, an orally available agonist of the IP receptor, is an investigational drug candidate internally discovered and developed by Arena and intended for the treatment of vascular diseases, including PAH. In Phase 1 trials, ralinepag showed an approximate 25-hour half-life, indicating that the compound could be dosed once or twice daily. Arena believes that an orally available, non-prostanoid IP receptor agonist that provides clinical benefits similar to currently available IP receptor agonists has the potential to improve treatment for patients with PAH. The FDA has granted ralinepag orphan drug status for the treatment of PAH.
http://finance.yahoo.com/news/arena-pharmaceuticals-initiate…
SAN DIEGO, Jan. 12, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today the initiation of patient dosing in a Phase 2 clinical trial of ralinepag, an oral, non-prostanoid prostacyclin (IP) receptor agonist intended for the treatment of pulmonary arterial hypertension (PAH).
"An intriguing component of this compound is the smooth peak-to-trough ratio, which may potentiate efficacy and minimize toxicity relative to currently available therapies," said Lewis J. Rubin, M.D., Emeritus Professor of Medicine at University of California, San Diego, School of Medicine. "This clinical trial should help to further elucidate how ralinepag's intrinsic properties translate to its efficacy and safety profile."
This 22-week, randomized, double-blind and placebo-controlled Phase 2 trial will evaluate the hemodynamic and exercise capacity effects, safety and tolerability of ralinepag in up to 60 patients with PAH. During the first nine weeks of the trial, patients will be titrated to their individual tolerance level, and then sustained at this level for the remainder of the trial.
"We believe ralinepag offers promise in the IP receptor class of molecules given its oral availability, long plasma half-life, and high selectivity and potency in vitro," said Jack Lief, Arena's President and Chief Executive Officer. "In conjunction with our other clinical-stage programs, this compound further enhances our optimism about Arena's value drivers."
About Pulmonary Arterial Hypertension
PAH is a progressive, life-threatening disorder characterized by increased pressure in the arteries that carry blood from the heart to the lungs. The increased pressure strains the heart, which can limit physical activity, result in heart failure and reduce life expectancy. Based on data from the Registry to EValuate Early And Long-term PAH disease management (REVEAL) of patients in the United States, there is an estimated five-year survival rate of 57% from diagnosis.
About Ralinepag
Ralinepag, an orally available agonist of the IP receptor, is an investigational drug candidate internally discovered and developed by Arena and intended for the treatment of vascular diseases, including PAH. In Phase 1 trials, ralinepag showed an approximate 25-hour half-life, indicating that the compound could be dosed once or twice daily. Arena believes that an orally available, non-prostanoid IP receptor agonist that provides clinical benefits similar to currently available IP receptor agonists has the potential to improve treatment for patients with PAH. The FDA has granted ralinepag orphan drug status for the treatment of PAH.
http://finance.yahoo.com/news/arena-pharmaceuticals-initiate…
Arena's Belviq On Track With Big Prescription Gains 


Summary
Belviq prescriptions recover from New Year's weekend, post 26% weekly increase.
Belviq regains a 50% share; rival Qsymia has much lower totals than last year, while Contrave launch complete stalls.
Eisai initiated a new marketing initiative to counter Takeda's Direct Save program; its impact may be felt as soon as next week.
New prescription numbers have been released by Symphony for Belviq (see Table 1), the anti-obesity agent from Arena Pharmaceuticals (NASDAQ:ARNA). These are excellent gains following New Year's week. The growth was even higher than the first full week of 2014 when Belviq was still in launch mode. It also bested the gains of Qsymia, the more established product from Vivus (NASDAQ:VVUS).
New Symphony prescription numbers are also available for Qsymia and Contrave from Orexigen (NASDAQ:OREX). There is continued growth in the sector (Table 2), with the 3 products gaining 16.6% week to week (28,097 vs. 24,099). Belviq has regained a 50% share among the anorexiants despite Contrave's launch; Contrave's Week 12 increase of 1.2% is its slowest thus far. On the other hand, Qsymia's numbers, while growing 13.5%, are much lower than last year at this time (-15.8%).
Table 1. Symphony Reported Prescriptions
Belviq
Belviq
Qsymia
Week Ended
Total
Week Ended
Total
Total
12/26/14*
9,327
12/27/13
3,708
6,834
01/02/15
11,257
01/03/14
4,880
8,606
Weekly Change
20.7%
Weekly Change
31.6%
25.9%
01/09/15
14,154
01/10/14
5,778
10,411
Weekly Change
25.7%
Weekly Change
18.4%
21.0%
*Estimated
Table 2. Market Share by Symphony Reported Totals
Belviq
Belviq
Qsymia
Qsymia
Contrave
Contrave
Week Ended
Total
Share
Total
Share
Total
Share
01/02/15
11257
46.7%
7721
32.0%
5121
21.2%
01/09/15
14154
50.4%
8762
31.2%
5181
18.4%
Belviq should continue to steadily pull away in the first quarter, with Contrave trying to catching up to Qsymia. The reasons for these expected dynamics were discussed in a previous article. In brief, the marketers for Belviq and Contrave, Eisai (OTCPK:ESALY) and Takeda (OTCPK:TKPHF), have more sales reps, 450 and 900, respectively, versus the 150 of Vivus. Eisai is the sole company engaging in direct-to-consumer advertising (DTCA). Vivus is financially unable to hire more reps or launch a DTCA effort. While Takeda offers the most generous pricing among all three, including a $60 maintenance price for cash patients, Eisai countered with a new patient assistance program this week that may start showing effects next week. Belviq (and Qsymia) also enjoys better insurance coverage than Contrave from the largest insurers. Qsymia does have an advantage of being the only weight loss drug on the Performance Drug List of CVS/Caremark (NYSE:CVS), the nation's second largest pharmacy benefits manager. However, savings programs from the others bring maintenance copays for those with commercial insurance down to $45-$50. Vivus reported a -2% decrease in cumulative prescriptions between Q1 2014 and Q4 2013 and is likely to do the same. Predicting a growth for Belviq that approximates its 2014 path appears more and more reasonable; Eisai having a larger sales force than in fall 2013 helps. For the first quarter of 2014, there were three weeks of greater than 10% week-over-week increases, including this second week of January. Applying the same trajectory for Contrave may be conservative, but the model did forecast 4,000 IMS prescriptions during the Christmas week. Pending confirmatory IMS numbers, projections ending in March may still look like Figure 1.
Figure 1. Projected Total Prescriptions by Week
(click to enlarge)
The breakdown of the model in numbers gives the following for each drug:
Estimated cumulative number of prescriptions filled for Quarter 1 2015;
Estimated total prescriptions just for week ending March 27, 2015 (market share);
Year-over-year change; and
Quarter-to-quarter change.
Belviq: 187,000, 17,446 (46.3% share), +228%, +27%
Qsymia: 131,000, 11,162 (29.6% share), +6%, -3%
Contrave: 97,000, 9,073 (24.1% share), N/A, +188%
Combined: 415.000, 36,659, +32%, +130%
Previously, Arena had been getting close to $40 from Eisai per prescription. It will be difficult to know who many take advantage of Eisai's new discount program. How much Arena earns from Eisai and other collaborative revenues as well as manufacturing services varies every quarter, but the smallest amount for these items the past 3 reports totaled $2.44 million for the quarter ended September 30, 2014.
http://seekingalpha.com/article/2827426-arenas-belviq-on-tra…
Artikel ist von gestern abend
Bin seit gestern dann wieder mit an Bord
Grüße und nice WE
EF



Summary
Belviq prescriptions recover from New Year's weekend, post 26% weekly increase.
Belviq regains a 50% share; rival Qsymia has much lower totals than last year, while Contrave launch complete stalls.
Eisai initiated a new marketing initiative to counter Takeda's Direct Save program; its impact may be felt as soon as next week.
New prescription numbers have been released by Symphony for Belviq (see Table 1), the anti-obesity agent from Arena Pharmaceuticals (NASDAQ:ARNA). These are excellent gains following New Year's week. The growth was even higher than the first full week of 2014 when Belviq was still in launch mode. It also bested the gains of Qsymia, the more established product from Vivus (NASDAQ:VVUS).
New Symphony prescription numbers are also available for Qsymia and Contrave from Orexigen (NASDAQ:OREX). There is continued growth in the sector (Table 2), with the 3 products gaining 16.6% week to week (28,097 vs. 24,099). Belviq has regained a 50% share among the anorexiants despite Contrave's launch; Contrave's Week 12 increase of 1.2% is its slowest thus far. On the other hand, Qsymia's numbers, while growing 13.5%, are much lower than last year at this time (-15.8%).
Table 1. Symphony Reported Prescriptions
Belviq
Belviq
Qsymia
Week Ended
Total
Week Ended
Total
Total
12/26/14*
9,327
12/27/13
3,708
6,834
01/02/15
11,257
01/03/14
4,880
8,606
Weekly Change
20.7%
Weekly Change
31.6%
25.9%
01/09/15
14,154
01/10/14
5,778
10,411
Weekly Change
25.7%
Weekly Change
18.4%
21.0%
*Estimated
Table 2. Market Share by Symphony Reported Totals
Belviq
Belviq
Qsymia
Qsymia
Contrave
Contrave
Week Ended
Total
Share
Total
Share
Total
Share
01/02/15
11257
46.7%
7721
32.0%
5121
21.2%
01/09/15
14154
50.4%
8762
31.2%
5181
18.4%
Belviq should continue to steadily pull away in the first quarter, with Contrave trying to catching up to Qsymia. The reasons for these expected dynamics were discussed in a previous article. In brief, the marketers for Belviq and Contrave, Eisai (OTCPK:ESALY) and Takeda (OTCPK:TKPHF), have more sales reps, 450 and 900, respectively, versus the 150 of Vivus. Eisai is the sole company engaging in direct-to-consumer advertising (DTCA). Vivus is financially unable to hire more reps or launch a DTCA effort. While Takeda offers the most generous pricing among all three, including a $60 maintenance price for cash patients, Eisai countered with a new patient assistance program this week that may start showing effects next week. Belviq (and Qsymia) also enjoys better insurance coverage than Contrave from the largest insurers. Qsymia does have an advantage of being the only weight loss drug on the Performance Drug List of CVS/Caremark (NYSE:CVS), the nation's second largest pharmacy benefits manager. However, savings programs from the others bring maintenance copays for those with commercial insurance down to $45-$50. Vivus reported a -2% decrease in cumulative prescriptions between Q1 2014 and Q4 2013 and is likely to do the same. Predicting a growth for Belviq that approximates its 2014 path appears more and more reasonable; Eisai having a larger sales force than in fall 2013 helps. For the first quarter of 2014, there were three weeks of greater than 10% week-over-week increases, including this second week of January. Applying the same trajectory for Contrave may be conservative, but the model did forecast 4,000 IMS prescriptions during the Christmas week. Pending confirmatory IMS numbers, projections ending in March may still look like Figure 1.
Figure 1. Projected Total Prescriptions by Week
(click to enlarge)
The breakdown of the model in numbers gives the following for each drug:
Estimated cumulative number of prescriptions filled for Quarter 1 2015;
Estimated total prescriptions just for week ending March 27, 2015 (market share);
Year-over-year change; and
Quarter-to-quarter change.
Belviq: 187,000, 17,446 (46.3% share), +228%, +27%
Qsymia: 131,000, 11,162 (29.6% share), +6%, -3%
Contrave: 97,000, 9,073 (24.1% share), N/A, +188%
Combined: 415.000, 36,659, +32%, +130%
Previously, Arena had been getting close to $40 from Eisai per prescription. It will be difficult to know who many take advantage of Eisai's new discount program. How much Arena earns from Eisai and other collaborative revenues as well as manufacturing services varies every quarter, but the smallest amount for these items the past 3 reports totaled $2.44 million for the quarter ended September 30, 2014.
http://seekingalpha.com/article/2827426-arenas-belviq-on-tra…
Artikel ist von gestern abend

Bin seit gestern dann wieder mit an Bord
Grüße und nice WE
EF

Arena Pharmaceuticals Announces Agreement to Sell Common Stock
SAN DIEGO, Jan. 21, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that it has sold 21,000,000 shares of common stock to Jefferies LLC and Piper Jaffray & Co. as underwriters for its previously announced public offering. The underwriters offered the shares to purchasers in transactions on The NASDAQ Global Select Market, in the over-the-counter market, through negotiated transactions or otherwise at market prices prevailing at the time of sale, at prices related to prevailing market prices or at negotiated prices. The offering is expected to close on or about January 26, 2015, subject to customary closing conditions. In addition, Arena has granted the underwriters a 30-day option to purchase up to an aggregate of 3,000,000 additional shares of common stock. All of the shares are being offered by Arena. Arena anticipates using the net proceeds from the offering for the clinical and preclinical development of drug candidates, for discovery research for new drug candidates, and for general corporate purposes, including working capital, costs associated with product sales and manufacturing services, capital expenditures and potentially for the commercialization of any new approved drugs.
Jefferies LLC and Piper Jaffray & Co. are acting as joint book-running managers for the offering.
A registration statement relating to the shares described above was previously filed with the Securities and Exchange Commission (SEC) and is effective. A preliminary prospectus supplement related to the offering has been filed with the SEC and is available on the SEC's website located at http://www.sec.gov. Copies of the final prospectus supplement and the accompanying prospectus relating to this offering, when available, may be obtained from Jefferies LLC, Attention: Equity Syndicate Prospectus Department, 520 Madison Avenue, 12th Floor, New York, NY 10022, by telephone at (877) 547-6340, or by e-mail at Prospectus_Department@Jefferies.com; or Piper Jaffray & Co., Attention: Prospectus Department, 800 Nicollet Mall, Suite 800, Minneapolis, MN 55402, by telephone at (800) 747-3924, or by email at prospectus@pjc.com.
This press release shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of any such state or other jurisdiction.
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…
SAN DIEGO, Jan. 21, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that it has sold 21,000,000 shares of common stock to Jefferies LLC and Piper Jaffray & Co. as underwriters for its previously announced public offering. The underwriters offered the shares to purchasers in transactions on The NASDAQ Global Select Market, in the over-the-counter market, through negotiated transactions or otherwise at market prices prevailing at the time of sale, at prices related to prevailing market prices or at negotiated prices. The offering is expected to close on or about January 26, 2015, subject to customary closing conditions. In addition, Arena has granted the underwriters a 30-day option to purchase up to an aggregate of 3,000,000 additional shares of common stock. All of the shares are being offered by Arena. Arena anticipates using the net proceeds from the offering for the clinical and preclinical development of drug candidates, for discovery research for new drug candidates, and for general corporate purposes, including working capital, costs associated with product sales and manufacturing services, capital expenditures and potentially for the commercialization of any new approved drugs.
Jefferies LLC and Piper Jaffray & Co. are acting as joint book-running managers for the offering.
A registration statement relating to the shares described above was previously filed with the Securities and Exchange Commission (SEC) and is effective. A preliminary prospectus supplement related to the offering has been filed with the SEC and is available on the SEC's website located at http://www.sec.gov. Copies of the final prospectus supplement and the accompanying prospectus relating to this offering, when available, may be obtained from Jefferies LLC, Attention: Equity Syndicate Prospectus Department, 520 Madison Avenue, 12th Floor, New York, NY 10022, by telephone at (877) 547-6340, or by e-mail at Prospectus_Department@Jefferies.com; or Piper Jaffray & Co., Attention: Prospectus Department, 800 Nicollet Mall, Suite 800, Minneapolis, MN 55402, by telephone at (800) 747-3924, or by email at prospectus@pjc.com.
This press release shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of any such state or other jurisdiction.
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…
Significant Weight Loss and Drop in A1c with New Combo of Lorcaserin and Phentermine
A new combination of two FDA-approved drugs has shown a remarkable 8.7% average weight loss and a reduction of 1 point for A1c in just 12 weeks...
http://www.diabetesincontrol.com/articles/diabetes-news/1744…
A new combination of two FDA-approved drugs has shown a remarkable 8.7% average weight loss and a reduction of 1 point for A1c in just 12 weeks...
http://www.diabetesincontrol.com/articles/diabetes-news/1744…
Arena Pharmaceuticals Announces Marketing Approval in South Korea of BELVIQ® (lorcaserin HCl) for Weight Management
http://ih.advfn.com/p.php?pid=nmona&article=65333022&symbol=…
http://ih.advfn.com/p.php?pid=nmona&article=65333022&symbol=…
Antwort auf Beitrag Nr.: 48.961.844 von Capret am 03.02.15 11:47:45
Arena Pharmaceuticals Announces Marketing Approval in South Korea of BELVIQ® (lorcaserin HCl) for Weight Management
Today : Wednesday 4 February 2015
SAN DIEGO, Feb. 3, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) today announced that Ildong Pharmaceutical Co., Ltd., has informed Arena that the Ministry of Food and Drug Safety has approved BELVIQ® (lorcaserin HCl) for marketing for weight management in South Korea. Ildong will market and distribute BELVIQ in South Korea under its marketing and supply agreement with Arena's wholly owned subsidiary, Arena Pharmaceuticals GmbH, or Arena GmbH.
In connection with the approval, Arena GmbH will receive a milestone payment of $3.0 million from Ildong.
"We are pleased with this achievement, and continue to work with our collaborators to obtain approval for BELVIQ in additional territories," said Jack Lief, Arena's President and Chief Executive Officer. "As a leading pharmaceutical company in South Korea with significant experience marketing obesity products, we are confident in Ildong's ability to make this novel therapy available to patients in South Korea."
BELVIQ is approved in South Korea as an adjunct to a reduced-calorie diet and increased physical activity for weight management in adult patients with an initial body mass index (BMI) of 30 kg/m2 or greater (obese), or 27 kg/m2 or greater (overweight) in the presence of at least one weight related comorbid condition (e.g., hypertension, dyslipidemia, type 2 diabetes).
Arena GmbH will manufacture BELVIQ at its facility in Switzerland, and sell BELVIQ to Ildong for a purchase price starting at 35% of Ildong's annual net sales. The purchase price will increase on a tiered basis up to 45% on the portion of annual net sales exceeding $15.0 million.
Zitat von Capret: Arena Pharmaceuticals Announces Marketing Approval in South Korea of BELVIQ® (lorcaserin HCl) for Weight Management
http://ih.advfn.com/p.php?pid=nmona&article=65333022&symbol=…
Arena Pharmaceuticals Announces Marketing Approval in South Korea of BELVIQ® (lorcaserin HCl) for Weight Management
Today : Wednesday 4 February 2015
SAN DIEGO, Feb. 3, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) today announced that Ildong Pharmaceutical Co., Ltd., has informed Arena that the Ministry of Food and Drug Safety has approved BELVIQ® (lorcaserin HCl) for marketing for weight management in South Korea. Ildong will market and distribute BELVIQ in South Korea under its marketing and supply agreement with Arena's wholly owned subsidiary, Arena Pharmaceuticals GmbH, or Arena GmbH.
In connection with the approval, Arena GmbH will receive a milestone payment of $3.0 million from Ildong.
"We are pleased with this achievement, and continue to work with our collaborators to obtain approval for BELVIQ in additional territories," said Jack Lief, Arena's President and Chief Executive Officer. "As a leading pharmaceutical company in South Korea with significant experience marketing obesity products, we are confident in Ildong's ability to make this novel therapy available to patients in South Korea."
BELVIQ is approved in South Korea as an adjunct to a reduced-calorie diet and increased physical activity for weight management in adult patients with an initial body mass index (BMI) of 30 kg/m2 or greater (obese), or 27 kg/m2 or greater (overweight) in the presence of at least one weight related comorbid condition (e.g., hypertension, dyslipidemia, type 2 diabetes).
Arena GmbH will manufacture BELVIQ at its facility in Switzerland, and sell BELVIQ to Ildong for a purchase price starting at 35% of Ildong's annual net sales. The purchase price will increase on a tiered basis up to 45% on the portion of annual net sales exceeding $15.0 million.
Antwort auf Beitrag Nr.: 48.972.359 von bernie55 am 04.02.15 09:34:40Arena Pharmaceuticals to Present at the 2015 RBC Capital Markets Healthcare Conference
PR Newswire Arena Pharmaceuticals, IInc
SAN DIEGO, Feb. 18, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview and update at the 2015 RBC Capital Markets Healthcare Conference on
Tuesday, February 24, 2015, at 10:00 a.m. Eastern Time (7:00 a.m. Pacific Time), at The New York Palace Hotel in New York City.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
PR Newswire Arena Pharmaceuticals, IInc
SAN DIEGO, Feb. 18, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview and update at the 2015 RBC Capital Markets Healthcare Conference on
Tuesday, February 24, 2015, at 10:00 a.m. Eastern Time (7:00 a.m. Pacific Time), at The New York Palace Hotel in New York City.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
Arena Pharmaceuticals Announces Fourth Quarter and Full Year 2014 Financial Results Conference Call and Webcast on
Monday, March 2
SAN DIEGO, Feb. 23, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that it will host a conference call and webcast at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time) on Monday, March 2, 2015, to provide a corporate update and discuss fourth quarter and full year 2014 financial results. Arena will release its financial results after the NASDAQ Global Select Market closes that day.
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' Fourth Quarter and Full Year 2014 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…
Monday, March 2
SAN DIEGO, Feb. 23, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that it will host a conference call and webcast at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time) on Monday, March 2, 2015, to provide a corporate update and discuss fourth quarter and full year 2014 financial results. Arena will release its financial results after the NASDAQ Global Select Market closes that day.
The conference call may be accessed by dialing 877.643.7155 for domestic callers and 914.495.8552 for international callers. Please specify to the operator that you would like to join the "Arena Pharmaceuticals' Fourth Quarter and Full Year 2014 Financial Results Call." The conference call will be webcast live under the investor relations section of Arena's website at www.arenapharm.com, and will be archived there for 30 days following the call. Please connect to Arena's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…
Antwort auf Beitrag Nr.: 49.150.070 von bernie55 am 23.02.15 17:04:36Arena Pharmaceuticals Misses Q4 Expectations
Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) reported financial results Monday for the fourth quarter and full year ended December 31, 2014.
Revenues totaled $9.2 million, below expectations of $10.36 million and above the $7.44 million reported in the prior year period.
Revenues included $3.8 million in net product sales of BELVIQ, of which $3.2 million represented 31.5 percent of Eisai's net product sales and $0.6 million related to redemptions of the 15-day free voucher and product samples.
The fourth quarter net loss was $32.1 million, or $(0.15) per share, greater than the expected loss of $(0.12). The company reported a loss of $(0.11) in the prior year period.
Research and development expenses totaled $27.8 million and general and administrative expenses totaled $8.9 million.
View more earnings on ARNA
As of December 31, 2014, cash and cash equivalents totaled $163.2 million.
In the first quarter of 2015, the company raised approximately $100.7 million in net proceeds through the sale of 21.0 million shares of common stock at a price to the underwriters of $4.8139 per share.
CEO Jack Lief said, "2014 was a pivotal year for Arena, marked by the advancement of multiple development programs and the growth of sales of BELVIQ. We reported impressive clinical trial results from our pipeline of internally discovered GPCR drug candidates, including for our lorcaserin lifecycle management programs. In 2015 and beyond, we plan to build on this momentum by aggressively pursuing our promising drug development opportunities both internally and through collaboration, while continuing to support the delivery of patient benefits with BELVIQ."
Arena was unable to predict first quarter sales and did not provide guidance for its overall 2015 revenues.
The company expected full year 2015 research and development expenses of approximately $114.0 million to $122.0 million, including non-cash expenses of approximately $13.0 million and $6.0 million in development expenses reimbursed by Eisai (with such reimbursed expenses included in revenue).
http://finance.yahoo.com/news/arena-pharmaceuticals-misses-q…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-misses-q…
Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) reported financial results Monday for the fourth quarter and full year ended December 31, 2014.
Revenues totaled $9.2 million, below expectations of $10.36 million and above the $7.44 million reported in the prior year period.
Revenues included $3.8 million in net product sales of BELVIQ, of which $3.2 million represented 31.5 percent of Eisai's net product sales and $0.6 million related to redemptions of the 15-day free voucher and product samples.
The fourth quarter net loss was $32.1 million, or $(0.15) per share, greater than the expected loss of $(0.12). The company reported a loss of $(0.11) in the prior year period.
Research and development expenses totaled $27.8 million and general and administrative expenses totaled $8.9 million.
View more earnings on ARNA
As of December 31, 2014, cash and cash equivalents totaled $163.2 million.
In the first quarter of 2015, the company raised approximately $100.7 million in net proceeds through the sale of 21.0 million shares of common stock at a price to the underwriters of $4.8139 per share.
CEO Jack Lief said, "2014 was a pivotal year for Arena, marked by the advancement of multiple development programs and the growth of sales of BELVIQ. We reported impressive clinical trial results from our pipeline of internally discovered GPCR drug candidates, including for our lorcaserin lifecycle management programs. In 2015 and beyond, we plan to build on this momentum by aggressively pursuing our promising drug development opportunities both internally and through collaboration, while continuing to support the delivery of patient benefits with BELVIQ."
Arena was unable to predict first quarter sales and did not provide guidance for its overall 2015 revenues.
The company expected full year 2015 research and development expenses of approximately $114.0 million to $122.0 million, including non-cash expenses of approximately $13.0 million and $6.0 million in development expenses reimbursed by Eisai (with such reimbursed expenses included in revenue).
http://finance.yahoo.com/news/arena-pharmaceuticals-misses-q…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-misses-q…
Antwort auf Beitrag Nr.: 49.224.929 von bernie55 am 03.03.15 11:59:46Top FDA Official Says OREXIGEN Study Result 'Unreliable,' 'Misleading'
..............The study results showing that Orexigen’s obesity drug Contrave reduced the risk of heart attacks and cardiovascular death and sent shares of the tiny La Jolla, Calif., biotechnology company soaring 30% were probably “unreliable,” “misleading,” and “likely false,” according to a top Food and Drug Administration official.
If Orexigen cannot find way to set things right, it could face fines, civil penalties,
 or even the withdrawal of Contrave from the market
or even the withdrawal of Contrave from the market  .
.
John Jenkins is the director of the Office of New Drugs. He had a key role in negotiating the specifics of a big heart safety study of Contrave, as well as the safety of the guidance used to design big heart trials that are required of diabetes and obesity drugs. I interviewed him earlier today about the release of the Contrave data, which Orexigen released on Tuesday via a patent and a filing with the Securities and Exchange Commission over the protests of the FDA, the researchers leading the clinical trial, and its marketing partner, Takeda, and about Orexigen’s earlier failure to keep the data confidential to even its own executives.......
http://www.forbes.com/sites/matthewherper/2015/03/05/top-fda…" target="_blank" rel="nofollow ugc noopener">
http://www.forbes.com/sites/matthewherper/2015/03/05/top-fda…
..............The study results showing that Orexigen’s obesity drug Contrave reduced the risk of heart attacks and cardiovascular death and sent shares of the tiny La Jolla, Calif., biotechnology company soaring 30% were probably “unreliable,” “misleading,” and “likely false,” according to a top Food and Drug Administration official.
If Orexigen cannot find way to set things right, it could face fines, civil penalties,
 or even the withdrawal of Contrave from the market
or even the withdrawal of Contrave from the market  .
.John Jenkins is the director of the Office of New Drugs. He had a key role in negotiating the specifics of a big heart safety study of Contrave, as well as the safety of the guidance used to design big heart trials that are required of diabetes and obesity drugs. I interviewed him earlier today about the release of the Contrave data, which Orexigen released on Tuesday via a patent and a filing with the Securities and Exchange Commission over the protests of the FDA, the researchers leading the clinical trial, and its marketing partner, Takeda, and about Orexigen’s earlier failure to keep the data confidential to even its own executives.......
http://www.forbes.com/sites/matthewherper/2015/03/05/top-fda…" target="_blank" rel="nofollow ugc noopener">
http://www.forbes.com/sites/matthewherper/2015/03/05/top-fda…
Antwort auf Beitrag Nr.: 49.259.687 von bernie55 am 06.03.15 13:12:02Arena Pharmaceuticals Receives Additional Patent for BELVIQ® (lorcaserin HCl) from United States Patent and Trademark Office
PR Newswire Arena Pharmaceuticals, Inc.
SAN DIEGO and WOODCLIFF LAKE, N.J., April 7, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) and Eisai Inc., announced today that the US Patent and Trademark Office granted Arena US Patent No. 8,999,970, which describes a method for selecting appropriate patients based on renal function for BELVIQ® (lorcaserin HCl), a serotonin 2C receptor agonist approved for weight management.
"Seeking strong intellectual property protection has always been a key component of Arena's business model," said Jack Lief, Arena's President and Chief Executive Officer. "We expect this new patent to extend exclusivity for BELVIQ until 2033."
In addition to this method-of-treatment patent, composition of matter patents for BELVIQ are issued in major jurisdictions globally that, in most cases, are capable of continuing into 2023. Arena has filed applications for patent term extension on patents directed to composition of matter in the United States, which, if granted, would extend the composition of matter patent term for BELVIQ into 2026 or potentially 2027.
http://finance.yahoo.com/news/arena-pharmaceuticals-receives…
PR Newswire Arena Pharmaceuticals, Inc.
SAN DIEGO and WOODCLIFF LAKE, N.J., April 7, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) and Eisai Inc., announced today that the US Patent and Trademark Office granted Arena US Patent No. 8,999,970, which describes a method for selecting appropriate patients based on renal function for BELVIQ® (lorcaserin HCl), a serotonin 2C receptor agonist approved for weight management.
"Seeking strong intellectual property protection has always been a key component of Arena's business model," said Jack Lief, Arena's President and Chief Executive Officer. "We expect this new patent to extend exclusivity for BELVIQ until 2033."
In addition to this method-of-treatment patent, composition of matter patents for BELVIQ are issued in major jurisdictions globally that, in most cases, are capable of continuing into 2023. Arena has filed applications for patent term extension on patents directed to composition of matter in the United States, which, if granted, would extend the composition of matter patent term for BELVIQ into 2026 or potentially 2027.
http://finance.yahoo.com/news/arena-pharmaceuticals-receives…
Antwort auf Beitrag Nr.: 49.508.747 von bernie55 am 07.04.15 15:28:41Arena, Eisai Finish Phase I Studies on Obesity Drug Belviq -
Analyst Blog
Zacks By Zacks Equity Research
Arena Pharmaceuticals, Inc. ARNA and Eisai Inc. ESALY announced the completion of two phase I studies on their obesity drug, Belviq. The companies are looking to develop Belviq as a once-daily extended release formulation. Presently, a twice-daily immediate release formulation of Belviq is available in the markets.
The randomized phase I studies evaluated the safety, tolerability, pharmacokinetics and bioavailability of an extended-release formulation of Belviq in 36 healthy adult subjects. Positive results would allow the company to file a new drug application with the FDA later this year.
We are encouraged by Belviq’s progress. Once successfully developed and approved, the extended-release formulation will provide patients with additional treatment and dosing options.
The news comes shortly after the companies announced that the U.S. Patent and Trademark Office issued a new patent (U.S. Patent No. 8,999,970) to Belviq. The patent, which covers the method of selecting appropriate patients based on renal function, is expected to extend Belviq’s exclusivity until 2033.
Belviq is approved for weight management in the U.S. Arena Pharma and Eisai are evaluating Belviq for additional indications. Currently, Belviq is being evaluated for smoking cessation and weight management in combination with phentermine.
We note that Belviq is Arena Pharma’s sole marketed product. Arena Pharma has a collaboration agreement with Eisai in the U.S.
Meanwhile, the obesity market is getting more crowded – Novo Nordisk’s NVO Saxenda is the latest entrant in this market. Several health care companies are also looking to bring obesity treatments to the market.
http://finance.yahoo.com/news/arena-eisai-finish-phase-studi…
Analyst Blog
Zacks By Zacks Equity Research
Arena Pharmaceuticals, Inc. ARNA and Eisai Inc. ESALY announced the completion of two phase I studies on their obesity drug, Belviq. The companies are looking to develop Belviq as a once-daily extended release formulation. Presently, a twice-daily immediate release formulation of Belviq is available in the markets.
The randomized phase I studies evaluated the safety, tolerability, pharmacokinetics and bioavailability of an extended-release formulation of Belviq in 36 healthy adult subjects. Positive results would allow the company to file a new drug application with the FDA later this year.
We are encouraged by Belviq’s progress. Once successfully developed and approved, the extended-release formulation will provide patients with additional treatment and dosing options.
The news comes shortly after the companies announced that the U.S. Patent and Trademark Office issued a new patent (U.S. Patent No. 8,999,970) to Belviq. The patent, which covers the method of selecting appropriate patients based on renal function, is expected to extend Belviq’s exclusivity until 2033.
Belviq is approved for weight management in the U.S. Arena Pharma and Eisai are evaluating Belviq for additional indications. Currently, Belviq is being evaluated for smoking cessation and weight management in combination with phentermine.
We note that Belviq is Arena Pharma’s sole marketed product. Arena Pharma has a collaboration agreement with Eisai in the U.S.
Meanwhile, the obesity market is getting more crowded – Novo Nordisk’s NVO Saxenda is the latest entrant in this market. Several health care companies are also looking to bring obesity treatments to the market.
http://finance.yahoo.com/news/arena-eisai-finish-phase-studi…
Antwort auf Beitrag Nr.: 49.557.518 von bernie55 am 14.04.15 10:10:07Arena Pharmaceuticals to Present at the Annual Needham Healthcare Conference
PR Newswire Arena Pharmaceuticals, Inc.
April 9, 2015 8:00 AM
SAN DIEGO, April 9, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview and update at the 14th Annual Needham Healthcare Conference on Wednesday, April 15, 2015, at 12:50 p.m. Eastern Time (9:50 a.m. Pacific Time), at the Westin Grand Central Hotel in New York City.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
PR Newswire Arena Pharmaceuticals, Inc.
April 9, 2015 8:00 AM
SAN DIEGO, April 9, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) announced today that the company is scheduled to present a corporate overview and update at the 14th Annual Needham Healthcare Conference on Wednesday, April 15, 2015, at 12:50 p.m. Eastern Time (9:50 a.m. Pacific Time), at the Westin Grand Central Hotel in New York City.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
A replay of the presentation will be available for 30 days following the event.
http://files.shareholder.com/downloads/ARNA/49474337x0x82139…
http://files.shareholder.com/downloads/ARNA/49474337x0x82139…
Antwort auf Beitrag Nr.: 49.576.271 von bernie55 am 16.04.15 09:59:28Arena Pharmaceuticals Reports Favorable Results from Phase 1 Single-Ascending Dose Clinical Trial of APD371
-- Results support advancement of the novel, oral drug candidate to the next stage of development
PR Newswire Arena Pharmaceuticals, Inc.
SAN DIEGO, April 29, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced favorable results from a Phase 1 single-ascending dose clinical trial of APD371, a highly selective and potent agonist of the cannabinoid 2 (CB2) receptor currently in development for the treatment of pain and potentially fibrotic diseases.
The randomized, double-blind and placebo-controlled Phase 1 clinical trial enrolled 56 healthy adults to evaluate the safety, tolerability and pharmacokinetics of single-ascending doses of APD371. Dose responsive exposure was observed over the explored dose range of 10-400 mg with good tolerability at all doses administered.
"In this trial, APD371 was well tolerated, as evidenced by the lack of dose-limiting adverse events, at drug levels greatly exceeding those anticipated as needed for activating the CB2 receptor," said William R. Shanahan, Jr., M.D., Arena's Senior Vice President and Chief Medical Officer. "We believe that the intrinsic properties of this compound could offer advantages over currently marketed treatments for pain."
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
-- Results support advancement of the novel, oral drug candidate to the next stage of development
PR Newswire Arena Pharmaceuticals, Inc.
SAN DIEGO, April 29, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced favorable results from a Phase 1 single-ascending dose clinical trial of APD371, a highly selective and potent agonist of the cannabinoid 2 (CB2) receptor currently in development for the treatment of pain and potentially fibrotic diseases.
The randomized, double-blind and placebo-controlled Phase 1 clinical trial enrolled 56 healthy adults to evaluate the safety, tolerability and pharmacokinetics of single-ascending doses of APD371. Dose responsive exposure was observed over the explored dose range of 10-400 mg with good tolerability at all doses administered.
"In this trial, APD371 was well tolerated, as evidenced by the lack of dose-limiting adverse events, at drug levels greatly exceeding those anticipated as needed for activating the CB2 receptor," said William R. Shanahan, Jr., M.D., Arena's Senior Vice President and Chief Medical Officer. "We believe that the intrinsic properties of this compound could offer advantages over currently marketed treatments for pain."
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
Antwort auf Beitrag Nr.: 49.671.336 von bernie55 am 29.04.15 14:18:35Arena Pharmaceuticals reports 1Q loss
SAN DIEGO (AP) _ Arena Pharmaceuticals Inc. (ARNA) on Monday reported a loss of $24.3 million in its first quarter.
The San Diego-based company said it had a loss of 10 cents per share.
The drugmaker posted revenue of $12.3 million in the period.
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
SAN DIEGO (AP) _ Arena Pharmaceuticals Inc. (ARNA) on Monday reported a loss of $24.3 million in its first quarter.
The San Diego-based company said it had a loss of 10 cents per share.
The drugmaker posted revenue of $12.3 million in the period.
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
Antwort auf Beitrag Nr.: 49.761.183 von bernie55 am 12.05.15 10:44:20Arena Pharmaceuticals and Roivant Sciences Enter into Collaboration for Nelotanserin, a Novel Inverse Agonist of the 5-HT2A Receptor
- Roivant to develop nelotanserin for patients with behavioral and neuropsychiatric disturbances across multiple therapeutic indications -
PR Newswire Arena Pharmaceuticals, Inc.
12 hours ago
SAN DIEGO, May 11, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that its subsidiary, Arena Pharmaceuticals GmbH, and Roivant Sciences Ltd., have entered into a Development, Marketing and Supply Agreement for nelotanserin, Arena's internally discovered inverse agonist of the 5-HT2A receptor. The agreement grants Roivant exclusive worldwide rights to develop and commercialize nelotanserin.
Nelotanserin has been studied to date in multiple clinical trials involving over 900 subjects. Roivant intends to initiate additional Phase 2 clinical trials for the treatment of behavioral and neuropsychiatric disturbances, including psychoses, in patients with dementia and other neurological diseases. In addition, Roivant may pursue the development of nelotanserin for other neuropsychiatric disorders. Roivant will be responsible for funding the development and commercialization of nelotanserin.
"Arena's prior clinical trials support nelotanserin's tolerability, activity in the brain and potential to treat a variety of neuropsychiatric conditions," said Jack Lief, Arena's President and Chief Executive Officer. "Our collaboration with Roivant enables clinical development to proceed for this novel compound that has the potential to provide treatment in an area of unmet medical need."
"Roivant is uniquely positioned to maximize the therapeutic and commercial value of nelotanserin," said Vivek Ramaswamy, Founder of Roivant. "The 5-HT2A receptor is one of the most promising targets for the treatment of behavioral disturbances and neuropsychiatric symptoms in patients with dementia and other neurological diseases. We applaud Arena for discovering and advancing a potent, selective and potential best-in-class 5-HT2A inverse agonist, and we look forward to consummating the development and commercialization of nelotanserin."
Under the agreement, Arena will manufacture clinical supply and commercial product to sell to Roivant.
Arena will receive a $4.0 million upfront payment and is eligible to receive $41.5 million in regulatory and development milestone payments. Arena is also eligible to receive 15% of net sales of nelotanserin in exchange for the manufacture and supply of finished commercial drug product, and up to a total of $60.0 million in one-time purchase price adjustment payments tied to certain commercial sales milestones.
http://finance.yahoo.com/news/arena-pharmaceuticals-roivant-…
- Roivant to develop nelotanserin for patients with behavioral and neuropsychiatric disturbances across multiple therapeutic indications -
PR Newswire Arena Pharmaceuticals, Inc.
12 hours ago
SAN DIEGO, May 11, 2015 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that its subsidiary, Arena Pharmaceuticals GmbH, and Roivant Sciences Ltd., have entered into a Development, Marketing and Supply Agreement for nelotanserin, Arena's internally discovered inverse agonist of the 5-HT2A receptor. The agreement grants Roivant exclusive worldwide rights to develop and commercialize nelotanserin.
Nelotanserin has been studied to date in multiple clinical trials involving over 900 subjects. Roivant intends to initiate additional Phase 2 clinical trials for the treatment of behavioral and neuropsychiatric disturbances, including psychoses, in patients with dementia and other neurological diseases. In addition, Roivant may pursue the development of nelotanserin for other neuropsychiatric disorders. Roivant will be responsible for funding the development and commercialization of nelotanserin.
"Arena's prior clinical trials support nelotanserin's tolerability, activity in the brain and potential to treat a variety of neuropsychiatric conditions," said Jack Lief, Arena's President and Chief Executive Officer. "Our collaboration with Roivant enables clinical development to proceed for this novel compound that has the potential to provide treatment in an area of unmet medical need."
"Roivant is uniquely positioned to maximize the therapeutic and commercial value of nelotanserin," said Vivek Ramaswamy, Founder of Roivant. "The 5-HT2A receptor is one of the most promising targets for the treatment of behavioral disturbances and neuropsychiatric symptoms in patients with dementia and other neurological diseases. We applaud Arena for discovering and advancing a potent, selective and potential best-in-class 5-HT2A inverse agonist, and we look forward to consummating the development and commercialization of nelotanserin."
Under the agreement, Arena will manufacture clinical supply and commercial product to sell to Roivant.
Arena will receive a $4.0 million upfront payment and is eligible to receive $41.5 million in regulatory and development milestone payments. Arena is also eligible to receive 15% of net sales of nelotanserin in exchange for the manufacture and supply of finished commercial drug product, and up to a total of $60.0 million in one-time purchase price adjustment payments tied to certain commercial sales milestones.
http://finance.yahoo.com/news/arena-pharmaceuticals-roivant-…
Antwort auf Beitrag Nr.: 49.761.210 von bernie55 am 12.05.15 10:48:34Eisai and Arena Pharmaceuticals Announce Presentation of Lorcaserin HCl Data at Upcoming Medical Meetings
WOODCLIFF LAKE, N.J., and SAN DIEGO, May 14, 2015 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) announced today that lorcaserin HCl data will be presented at the following medical meetings:
American Thoracic Society International Conference taking place in Denver, Colorado, May 15-20, 2015
Lorcaserin for Smoking Cessation Treatment in Cigarette Smokers: A Randomized Phase 2 Trial
Poster Number: 118
This study evaluated the efficacy of lorcaserin 10 mg twice daily for smoking cessation and body weight change in a population of dependent smokers over placebo.
International Society for Pharmacoeconomics and Outcomes Research taking place in Philadelphia, Pennsylvania, May 16-20, 2015
Cost Implication of Using Lorcaserin in Weight Management Prior to Bariatric Surgery
Poster Number: PSY21
This study evaluated the cost implication of using lorcaserin for weight loss prior to bariatric surgery over a two-year horizon from the payer's perspective.
Lorcaserin is an investigational product for smoking cessation. The efficacy and safety of lorcaserin for smoking cessation have not been established.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-an…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-an…
WOODCLIFF LAKE, N.J., and SAN DIEGO, May 14, 2015 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) announced today that lorcaserin HCl data will be presented at the following medical meetings:
American Thoracic Society International Conference taking place in Denver, Colorado, May 15-20, 2015
Lorcaserin for Smoking Cessation Treatment in Cigarette Smokers: A Randomized Phase 2 Trial
Poster Number: 118
This study evaluated the efficacy of lorcaserin 10 mg twice daily for smoking cessation and body weight change in a population of dependent smokers over placebo.
International Society for Pharmacoeconomics and Outcomes Research taking place in Philadelphia, Pennsylvania, May 16-20, 2015
Cost Implication of Using Lorcaserin in Weight Management Prior to Bariatric Surgery
Poster Number: PSY21
This study evaluated the cost implication of using lorcaserin for weight loss prior to bariatric surgery over a two-year horizon from the payer's perspective.
Lorcaserin is an investigational product for smoking cessation. The efficacy and safety of lorcaserin for smoking cessation have not been established.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-an…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-an…
Beim "Konkurrenten" OREXIGEN scheinen die Probleme immer größer zu werden.
Orexigen Therapeutics (OREX) Stock Continues to Sink on Contrave Study Fallout
http://www.thestreet.com/story/13149885/1/orexigen-therapeut…
Orexigen and Takeda Feud Over Cost of a Controversial Diet Drug Trial
http://blogs.wsj.com/pharmalot/2015/05/13/orexigen-and-taked…
Orexigen Therapeutics (OREX) Stock Continues to Sink on Contrave Study Fallout
http://www.thestreet.com/story/13149885/1/orexigen-therapeut…
Orexigen and Takeda Feud Over Cost of a Controversial Diet Drug Trial
http://blogs.wsj.com/pharmalot/2015/05/13/orexigen-and-taked…
Antwort auf Beitrag Nr.: 49.783.632 von bernie55 am 15.05.15 11:23:20..
Piper views Receptos buyout as good for Arena
Piper Jaffray analyst Edward Tenthoff views Celgene's (CELG) acquisition of Receptos (RCPT) as good for Arena Pharmaceuticals (ARNA). Tenthoff views Arena as a "derivative call" on the deal with the company's APD334 already showing dose-dependent decreases in circulating lymphocyte counts. Arena will also initiate Phase II studies in ulcerative colitis and Crohn's disease this year, the analyst points out.
Receptos' ozanimod is being studied in Phase III trials for relapsing multiple sclerosis and ulcerative colitis. Tenthoff reiterates an Overweight rating on Arena with a $7.50 price target. The company has a market capitalization around $1B and closed yesterday up 8c to $4.47
http://www.thefly.com/landingPageNews.php?id=2220966&headlin…
Piper views Receptos buyout as good for Arena
Piper Jaffray analyst Edward Tenthoff views Celgene's (CELG) acquisition of Receptos (RCPT) as good for Arena Pharmaceuticals (ARNA). Tenthoff views Arena as a "derivative call" on the deal with the company's APD334 already showing dose-dependent decreases in circulating lymphocyte counts. Arena will also initiate Phase II studies in ulcerative colitis and Crohn's disease this year, the analyst points out.
Receptos' ozanimod is being studied in Phase III trials for relapsing multiple sclerosis and ulcerative colitis. Tenthoff reiterates an Overweight rating on Arena with a $7.50 price target. The company has a market capitalization around $1B and closed yesterday up 8c to $4.47
http://www.thefly.com/landingPageNews.php?id=2220966&headlin…
11/30 08:03 AM Eisai, Arena Pharma Unveil FDA Acceptance Of New Drug Application For Extended Release Formulation Of Lorcaserin (ARNA)
Antwort auf Beitrag Nr.: 51.204.840 von dottore am 30.11.15 14:18:41
WOODCLIFF LAKE, N.J. and SAN DIEGO, Nov. 30, 2015 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) today announced that the U.S. Food and Drug Administration (FDA) has accepted for filing the New Drug Application (NDA) for an extended release formulation of lorcaserin. If approved, the extended release formulation will offer patients a chronic weight management treatment in a once-daily dosing option.
Lorcaserin (sold under the brand name BELVIQ®) is currently approved as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults who have a body mass index (BMI) of 30 kg/m2 or greater (obese), or BMI of 27 kg/m2 or greater (overweight) with at least one weight-related medical condition, such as high blood pressure, high cholesterol, or type 2 diabetes. It is not known if BELVIQ is safe and effective when taken with other prescription, over-the-counter, or herbal weight loss products, nor is it known if BELVIQ changes the risk of heart problems or stroke, or death due to heart problems or stroke.
"The filing of this application by the FDA means they have made a threshold determination that it is sufficiently complete to permit a substantive review," said Craig M. Audet, Ph.D., Arena's Senior Vice President of Operations & Head of Global Regulatory Affairs. "This extended release formulation has the potential to offer patients once-daily dosing of lorcaserin, which can be an important addition to their chronic weight management plan."
The regulatory filing for the extended release formulation is based on the results of two Phase 1 registrational clinical trials evaluating bioequivalence of a once-daily, 20 mg extended release formulation of lorcaserin, as compared to the currently approved, twice-daily 10 mg immediate release formulation. If approved, the extended release formulation is expected to be marketed as BELVIQ XR®, which is the brand name conditionally approved by the FDA.
"The dramatic rise in obesity has major consequences for public health," said Frank Ciriello, President, Eisai Global Neurology Business Unit and Established Products. "If approved, this once-a-day option will provide another choice for appropriate patients who find it difficult to lose weight through diet and exercise alone."
Lorcaserin is believed to decrease food consumption and promote satiety by selectively activating the serotonin 2C receptors in the brain.
The extended release formulation of lorcaserin is investigational and not approved by any regulatory agency.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-an…
Zitat von dottore: 11/30 08:03 AM Eisai, Arena Pharma Unveil FDA Acceptance Of New Drug Application For Extended Release Formulation Of Lorcaserin (ARNA)
WOODCLIFF LAKE, N.J. and SAN DIEGO, Nov. 30, 2015 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) today announced that the U.S. Food and Drug Administration (FDA) has accepted for filing the New Drug Application (NDA) for an extended release formulation of lorcaserin. If approved, the extended release formulation will offer patients a chronic weight management treatment in a once-daily dosing option.
Lorcaserin (sold under the brand name BELVIQ®) is currently approved as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults who have a body mass index (BMI) of 30 kg/m2 or greater (obese), or BMI of 27 kg/m2 or greater (overweight) with at least one weight-related medical condition, such as high blood pressure, high cholesterol, or type 2 diabetes. It is not known if BELVIQ is safe and effective when taken with other prescription, over-the-counter, or herbal weight loss products, nor is it known if BELVIQ changes the risk of heart problems or stroke, or death due to heart problems or stroke.
"The filing of this application by the FDA means they have made a threshold determination that it is sufficiently complete to permit a substantive review," said Craig M. Audet, Ph.D., Arena's Senior Vice President of Operations & Head of Global Regulatory Affairs. "This extended release formulation has the potential to offer patients once-daily dosing of lorcaserin, which can be an important addition to their chronic weight management plan."
The regulatory filing for the extended release formulation is based on the results of two Phase 1 registrational clinical trials evaluating bioequivalence of a once-daily, 20 mg extended release formulation of lorcaserin, as compared to the currently approved, twice-daily 10 mg immediate release formulation. If approved, the extended release formulation is expected to be marketed as BELVIQ XR®, which is the brand name conditionally approved by the FDA.
"The dramatic rise in obesity has major consequences for public health," said Frank Ciriello, President, Eisai Global Neurology Business Unit and Established Products. "If approved, this once-a-day option will provide another choice for appropriate patients who find it difficult to lose weight through diet and exercise alone."
Lorcaserin is believed to decrease food consumption and promote satiety by selectively activating the serotonin 2C receptors in the brain.
The extended release formulation of lorcaserin is investigational and not approved by any regulatory agency.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-an…
Eisai and Arena Pharmaceuticals Announce Completion of Enrollment in BELVIQ® (lorcaserin HCI) CAMELLIA-TIMI 61 Study
Long-Term, Large-Scale Outcomes Study Assesses Cardiovascular and Metabolic Effects of BELVIQ in Overweight and Obese Patients
WOODCLIFF LAKE, N.J. and SAN DIEGO, Dec. 1, 2015 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) today announced that the CAMELLIA-TIMI 61 study has reached its target enrollment of 12,000 patients at more than 470 sites in eight countries. The CAMELLIA-TIMI 61 outcomes study is designed to evaluate the impact of long-term treatment with BELVIQ® (lorcaserin HCl) CIV on the incidence of major adverse cardiovascular events and conversion to type 2 diabetes mellitus in obese and overweight patients with cardiovascular disease and/or multiple cardiovascular risk factors.
"We are pleased to see full enrollment in this important study, which will advance scientific understanding of the long-term safety and efficacy of BELVIQ in overweight and obese patients," said Andrew Satlin, MD, Executive Vice President, Neuroscience and General Medicine PCU, Eisai Inc. "As a company focused on human health care, we are committed to increasing our knowledge of the effects of BELVIQ on cardiovascular health."
The five-year study, conducted in partnership with the Thrombolysis in Myocardial Infarction (TIMI) Study Group, will address the post-marketing requirement from the U.S. Food and Drug Administration to evaluate the long-term cardiovascular safety of BELVIQ. In addition, the study includes two primary efficacy endpoints: (1) to assess the effect of BELVIQ on cardiovascular event risk reduction and (2) to assess the effect of BELVIQ on the conversion to type 2 diabetes mellitus among patients without diabetes at baseline.
"We are very excited that CAMELLIA-TIMI 61 has completed enrollment in such a timely fashion," said Benjamin M. Scirica, MD, MPH, Senior Investigator, TIMI Study Group and Associate Professor of Medicine, Harvard Medical School and Co-Primary Investigator of the CAMELLIA-TIMI 61 Trial. "The enthusiasm and speed of enrollment highlights the commitment to evaluate the efficacy and safety of pharmacotherapy in obese and overweight patients who have cardiovascular disease or are at risk of cardiovascular complications. As the largest ongoing outcomes trial in obesity, CAMELLIA-TIMI 61 will provide invaluable information about this traditionally difficult to treat population."
BELVIQ is used along with a reduced-calorie diet and increased physical activity for chronic weight management in adults who have a body mass index (BMI) of 30 kg/m2 or greater (obese), or BMI of 27 kg/m2 or greater (overweight) with at least one weight-related medical condition such as high blood pressure, high cholesterol, or type 2 diabetes. It is not known if BELVIQ is safe and effective when taken with other prescription, over-the-counter, or herbal weight loss products, nor is it known if BELVIQ changes the risk of heart problems or stroke, or death due to heart problems or stroke. BELVIQ is believed to promote satiety and decrease food consumption by selectively activating serotonin 2C receptors in the brain. The exact mechanism of action of BELVIQ is not known.
"We commend Eisai on the progress they have made in advancing CAMELLIA and on reaching this significant milestone," said Harry F. Hixson, Jr., Arena's interim Chief Executive Officer. "Data from this cardiovascular outcomes trial will provide a better understanding of the potential of long-term treatment with BELVIQ, and we look forward to the study results."
About the Study
The CAMELLIA (Cardiovascular And Metabolic Effects of Lorcaserin In Overweight And Obese Patients) TIMI 61 study is the largest ongoing double-blind, placebo-controlled, parallel-group Phase IIIB/IV study among weight loss medications. The primary safety objective is to evaluate the incidence of major adverse cardiovascular events (MACE), defined as cardiovascular death, myocardial infarction or stroke. If the primary safety objective is met, the co-primary efficacy objectives are to evaluate the impact of BELVIQ on the incidence of: (1) MACE+, defined as MACE or hospitalization due to unstable angina or heart failure, or any coronary revascularization, and (2) conversion to type 2 diabetes mellitus for subjects without diabetes at baseline. In addition, the study will evaluate the efficacy of BELVIQ with respect to glycemic control in patients with type 2 diabetes mellitus.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-an…
Long-Term, Large-Scale Outcomes Study Assesses Cardiovascular and Metabolic Effects of BELVIQ in Overweight and Obese Patients
WOODCLIFF LAKE, N.J. and SAN DIEGO, Dec. 1, 2015 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) today announced that the CAMELLIA-TIMI 61 study has reached its target enrollment of 12,000 patients at more than 470 sites in eight countries. The CAMELLIA-TIMI 61 outcomes study is designed to evaluate the impact of long-term treatment with BELVIQ® (lorcaserin HCl) CIV on the incidence of major adverse cardiovascular events and conversion to type 2 diabetes mellitus in obese and overweight patients with cardiovascular disease and/or multiple cardiovascular risk factors.
"We are pleased to see full enrollment in this important study, which will advance scientific understanding of the long-term safety and efficacy of BELVIQ in overweight and obese patients," said Andrew Satlin, MD, Executive Vice President, Neuroscience and General Medicine PCU, Eisai Inc. "As a company focused on human health care, we are committed to increasing our knowledge of the effects of BELVIQ on cardiovascular health."
The five-year study, conducted in partnership with the Thrombolysis in Myocardial Infarction (TIMI) Study Group, will address the post-marketing requirement from the U.S. Food and Drug Administration to evaluate the long-term cardiovascular safety of BELVIQ. In addition, the study includes two primary efficacy endpoints: (1) to assess the effect of BELVIQ on cardiovascular event risk reduction and (2) to assess the effect of BELVIQ on the conversion to type 2 diabetes mellitus among patients without diabetes at baseline.
"We are very excited that CAMELLIA-TIMI 61 has completed enrollment in such a timely fashion," said Benjamin M. Scirica, MD, MPH, Senior Investigator, TIMI Study Group and Associate Professor of Medicine, Harvard Medical School and Co-Primary Investigator of the CAMELLIA-TIMI 61 Trial. "The enthusiasm and speed of enrollment highlights the commitment to evaluate the efficacy and safety of pharmacotherapy in obese and overweight patients who have cardiovascular disease or are at risk of cardiovascular complications. As the largest ongoing outcomes trial in obesity, CAMELLIA-TIMI 61 will provide invaluable information about this traditionally difficult to treat population."
BELVIQ is used along with a reduced-calorie diet and increased physical activity for chronic weight management in adults who have a body mass index (BMI) of 30 kg/m2 or greater (obese), or BMI of 27 kg/m2 or greater (overweight) with at least one weight-related medical condition such as high blood pressure, high cholesterol, or type 2 diabetes. It is not known if BELVIQ is safe and effective when taken with other prescription, over-the-counter, or herbal weight loss products, nor is it known if BELVIQ changes the risk of heart problems or stroke, or death due to heart problems or stroke. BELVIQ is believed to promote satiety and decrease food consumption by selectively activating serotonin 2C receptors in the brain. The exact mechanism of action of BELVIQ is not known.
"We commend Eisai on the progress they have made in advancing CAMELLIA and on reaching this significant milestone," said Harry F. Hixson, Jr., Arena's interim Chief Executive Officer. "Data from this cardiovascular outcomes trial will provide a better understanding of the potential of long-term treatment with BELVIQ, and we look forward to the study results."
About the Study
The CAMELLIA (Cardiovascular And Metabolic Effects of Lorcaserin In Overweight And Obese Patients) TIMI 61 study is the largest ongoing double-blind, placebo-controlled, parallel-group Phase IIIB/IV study among weight loss medications. The primary safety objective is to evaluate the incidence of major adverse cardiovascular events (MACE), defined as cardiovascular death, myocardial infarction or stroke. If the primary safety objective is met, the co-primary efficacy objectives are to evaluate the impact of BELVIQ on the incidence of: (1) MACE+, defined as MACE or hospitalization due to unstable angina or heart failure, or any coronary revascularization, and (2) conversion to type 2 diabetes mellitus for subjects without diabetes at baseline. In addition, the study will evaluate the efficacy of BELVIQ with respect to glycemic control in patients with type 2 diabetes mellitus.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-an…
Antwort auf Beitrag Nr.: 51.479.121 von zz74 am 13.01.16 10:30:06
Boehringer Ingelheim and Arena Pharmaceuticals Collaborate to Advance Research in Schizophrenia
- Collaboration partners to strengthen research in psychiatric diseases -
- The new partnership further supports Boehringer Ingelheim's CNS pipeline projects -
- Mental diseases are an area of high unmet medical need and represent a huge economic and social burden globally -
INGELHEIM, Germany, and SAN DIEGO, Jan. 12, 2016 /PRNewswire/ -- Boehringer Ingelheim and Arena Pharmaceuticals, Inc. (ARNA) announced today that they have signed an exclusive agreement to conduct joint research to identify drug candidates targeting an undisclosed G protein-coupled receptor (GPCR), which belongs to the group of orphan CNS receptors. An "orphan receptor" is structurally related to a family of proteins that are known to act as functional cell-surface receptors but whose ligand has not yet been identified.
Arena will provide Boehringer Ingelheim exclusive rights to its internally discovered, novel compounds and intellectual property for an orphan CNS receptor. The companies will jointly conduct research to identify additional drug candidates that are suitable for continued research and development as therapeutic compounds for various disease indications. The agreement grants Boehringer Ingelheim exclusive worldwide rights to develop, manufacture and commercialize products resulting from the collaboration.
According to the World Health Organization, mental illness - together with substance abuse disorders - remains a leading cause of disability worldwide. At least 450 million people suffer from mental health problems. The global economic burden of mental illness is larger than for cancer, cardiovascular disease or diabetes and continues to grow – with significant health, social and economic consequences. As currently available treatment options leave many patients unsatisfactorily treated, more effective medicines for mental diseases are urgently needed.
"This agreement reflects our new corporate focus to enter into collaboration opportunities at various stages of development," said Harry F. Hixson, Jr., Arena's interim Chief Executive Officer. "Collaborations are an essential part of our drug discovery and development efforts, so we are pleased to be part of this shared goal to identify novel drugs targeting an orphan CNS receptor with Boehringer Ingelheim, who possesses demonstrated capabilities in research, development, manufacturing, and marketing of pharmaceutical products."
"Together with our research partners in neuroscience, we are targeting brain circuits underlying major untreated symptom domains in our CNS focus areas of Alzheimer's disease, schizophrenia, and depression. We are delighted to enter into this new collaboration with Arena, who have recognized expertise in orphan CNS GPCR drug discovery and development," said Corporate Senior Vice President Clive R. Wood, Head of Discovery Research of Boehringer Ingelheim. "We believe that this alliance with Arena will allow us to deliver innovative, new medicines for the treatment of patients with psychiatric diseases such as schizophrenia and contribute to our vision of helping the millions of people with mental illness, to live fuller, more independent lives, for longer."
The new partnership combines Arena's expertise in GPCR focused drug discovery and development with Boehringer Ingelheim's unique approach to CNS research and development and its proven capabilities in bringing novel medications of high therapeutic value to the market in areas of high unmet medical need.
The collaboration is part of Boehringer Ingelheim's commitment to researching and developing new and more effective treatments for psychiatric diseases and their symptoms.
Under the terms of the agreement, Arena is eligible to receive payments up to $262 million in success milestones in case of full commercial success of multiple drug products, including an upfront payment and research funding. In addition, Arena is eligible to receive tiered royalties on future sales of products that arise from the collaboration. Further details of the agreement were not disclosed.
Zitat von zz74: http://www.nasdaq.com/press-release/boehringer-ingelheim-and…
Boehringer Ingelheim and Arena Pharmaceuticals Collaborate to Advance Research in Schizophrenia
- Collaboration partners to strengthen research in psychiatric diseases -
- The new partnership further supports Boehringer Ingelheim's CNS pipeline projects -
- Mental diseases are an area of high unmet medical need and represent a huge economic and social burden globally -
INGELHEIM, Germany, and SAN DIEGO, Jan. 12, 2016 /PRNewswire/ -- Boehringer Ingelheim and Arena Pharmaceuticals, Inc. (ARNA) announced today that they have signed an exclusive agreement to conduct joint research to identify drug candidates targeting an undisclosed G protein-coupled receptor (GPCR), which belongs to the group of orphan CNS receptors. An "orphan receptor" is structurally related to a family of proteins that are known to act as functional cell-surface receptors but whose ligand has not yet been identified.
Arena will provide Boehringer Ingelheim exclusive rights to its internally discovered, novel compounds and intellectual property for an orphan CNS receptor. The companies will jointly conduct research to identify additional drug candidates that are suitable for continued research and development as therapeutic compounds for various disease indications. The agreement grants Boehringer Ingelheim exclusive worldwide rights to develop, manufacture and commercialize products resulting from the collaboration.
According to the World Health Organization, mental illness - together with substance abuse disorders - remains a leading cause of disability worldwide. At least 450 million people suffer from mental health problems. The global economic burden of mental illness is larger than for cancer, cardiovascular disease or diabetes and continues to grow – with significant health, social and economic consequences. As currently available treatment options leave many patients unsatisfactorily treated, more effective medicines for mental diseases are urgently needed.
"This agreement reflects our new corporate focus to enter into collaboration opportunities at various stages of development," said Harry F. Hixson, Jr., Arena's interim Chief Executive Officer. "Collaborations are an essential part of our drug discovery and development efforts, so we are pleased to be part of this shared goal to identify novel drugs targeting an orphan CNS receptor with Boehringer Ingelheim, who possesses demonstrated capabilities in research, development, manufacturing, and marketing of pharmaceutical products."
"Together with our research partners in neuroscience, we are targeting brain circuits underlying major untreated symptom domains in our CNS focus areas of Alzheimer's disease, schizophrenia, and depression. We are delighted to enter into this new collaboration with Arena, who have recognized expertise in orphan CNS GPCR drug discovery and development," said Corporate Senior Vice President Clive R. Wood, Head of Discovery Research of Boehringer Ingelheim. "We believe that this alliance with Arena will allow us to deliver innovative, new medicines for the treatment of patients with psychiatric diseases such as schizophrenia and contribute to our vision of helping the millions of people with mental illness, to live fuller, more independent lives, for longer."
The new partnership combines Arena's expertise in GPCR focused drug discovery and development with Boehringer Ingelheim's unique approach to CNS research and development and its proven capabilities in bringing novel medications of high therapeutic value to the market in areas of high unmet medical need.
The collaboration is part of Boehringer Ingelheim's commitment to researching and developing new and more effective treatments for psychiatric diseases and their symptoms.
Under the terms of the agreement, Arena is eligible to receive payments up to $262 million in success milestones in case of full commercial success of multiple drug products, including an upfront payment and research funding. In addition, Arena is eligible to receive tiered royalties on future sales of products that arise from the collaboration. Further details of the agreement were not disclosed.
Antwort auf Beitrag Nr.: 51.479.286 von bernie55 am 13.01.16 10:49:18Arena's (ARNA) Q4 Loss Narrower but Revenues Fall Short
Zacks By Arpita Dutt
ARNA reported a loss of 11 cents per share (adjusted for restructuring expenses and one-time items) in the fourth quarter of 2015, narrower than the year-ago loss of 14 cents per share. The Zacks Consensus Estimate was also a loss of 14 cents.
Total revenues declined 15.7% year over year to $7.8 million, missing the Zacks Consensus Estimate of $9 million.
Full year 2015 revenues increased 3.7% to $38.3 million, while the company reported a loss of 44 cents per share in 2015, narrower than the loss of 52 cents in 2014.
http://finance.yahoo.com/news/arenas-arna-q4-loss-narrower-1…
Zacks By Arpita Dutt
ARNA reported a loss of 11 cents per share (adjusted for restructuring expenses and one-time items) in the fourth quarter of 2015, narrower than the year-ago loss of 14 cents per share. The Zacks Consensus Estimate was also a loss of 14 cents.
Total revenues declined 15.7% year over year to $7.8 million, missing the Zacks Consensus Estimate of $9 million.
Full year 2015 revenues increased 3.7% to $38.3 million, while the company reported a loss of 44 cents per share in 2015, narrower than the loss of 52 cents in 2014.
http://finance.yahoo.com/news/arenas-arna-q4-loss-narrower-1…
Arena Pharmaceuticals Reports Favorable Results from Phase 1b Multiple-Ascending Dose Clinical Trial of APD371
SAN DIEGO, April 12, 2016 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced favorable results from its Phase 1b multiple-ascending dose clinical trial of APD371, a highly selective and potent agonist of the cannabinoid 2 (CB2) receptor with potential utility in the treatment of pain.
This randomized, double-blind, placebo-controlled Phase 1b clinical trial enrolled 36 healthy adults to evaluate the safety, tolerability and pharmacokinetics of multiple-ascending doses of APD371. Cohorts of 12 subjects (9 active, 3 placebo) were administered doses of 50 mg, 100 mg, or 200 mg of APD371 or placebo three times daily for 10 days and, in connection with the pharmacokinetic evaluation, one time on the 11th day. The most common adverse events were headache and nausea. All adverse events were classified as mild, and there were no serious adverse events reported. There was one discontinuation in the high-dose group due to an adverse event of mild thirst and somnolence. Reductions in blood pressure and heart rate were observed, but none were symptomatic or resulted in an adverse event. Drug levels at all doses tested in the trial, including the lowest dose, were well above those believed to be needed to stimulate the CB2 receptor.
"The results of this trial substantiate data from our single-ascending dose trial of APD371. In both trials, we achieved dose-responsive exposure without dose-limiting adverse events across the range studied," said William R. Shanahan, Jr., M.D., Arena's Senior Vice President and Chief Medical Officer. "Our scientists designed APD371 as a highly selective, peripherally restricted, full agonist to provide pain relief without psychotropic effects, loss of efficacy over time, or the dependence, abuse potential or adverse event profile associated with other pain treatments."
About APD371
APD371, an orally available agonist of the CB2 receptor, is Arena's internally discovered investigational drug candidate intended for the treatment of pain and potentially other indications. With its high level of selectivity for the CB2 receptor versus the CB1 receptor and its high peripheral restriction observed in preclinical studies, APD371 is designed to provide pain relief without psychotropic effects. Targeting the CB2 receptor may also avoid or reduce the potential for the dependence or abuse associated with opioid drugs or the adverse event profile associated with NSAIDs. Preclinical efficacy with APD371 has been shown in animal models of osteoarthritic and neuropathic pain.
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
SAN DIEGO, April 12, 2016 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced favorable results from its Phase 1b multiple-ascending dose clinical trial of APD371, a highly selective and potent agonist of the cannabinoid 2 (CB2) receptor with potential utility in the treatment of pain.
This randomized, double-blind, placebo-controlled Phase 1b clinical trial enrolled 36 healthy adults to evaluate the safety, tolerability and pharmacokinetics of multiple-ascending doses of APD371. Cohorts of 12 subjects (9 active, 3 placebo) were administered doses of 50 mg, 100 mg, or 200 mg of APD371 or placebo three times daily for 10 days and, in connection with the pharmacokinetic evaluation, one time on the 11th day. The most common adverse events were headache and nausea. All adverse events were classified as mild, and there were no serious adverse events reported. There was one discontinuation in the high-dose group due to an adverse event of mild thirst and somnolence. Reductions in blood pressure and heart rate were observed, but none were symptomatic or resulted in an adverse event. Drug levels at all doses tested in the trial, including the lowest dose, were well above those believed to be needed to stimulate the CB2 receptor.
"The results of this trial substantiate data from our single-ascending dose trial of APD371. In both trials, we achieved dose-responsive exposure without dose-limiting adverse events across the range studied," said William R. Shanahan, Jr., M.D., Arena's Senior Vice President and Chief Medical Officer. "Our scientists designed APD371 as a highly selective, peripherally restricted, full agonist to provide pain relief without psychotropic effects, loss of efficacy over time, or the dependence, abuse potential or adverse event profile associated with other pain treatments."
About APD371
APD371, an orally available agonist of the CB2 receptor, is Arena's internally discovered investigational drug candidate intended for the treatment of pain and potentially other indications. With its high level of selectivity for the CB2 receptor versus the CB1 receptor and its high peripheral restriction observed in preclinical studies, APD371 is designed to provide pain relief without psychotropic effects. Targeting the CB2 receptor may also avoid or reduce the potential for the dependence or abuse associated with opioid drugs or the adverse event profile associated with NSAIDs. Preclinical efficacy with APD371 has been shown in animal models of osteoarthritic and neuropathic pain.
http://finance.yahoo.com/news/arena-pharmaceuticals-reports-…
Antwort auf Beitrag Nr.: 52.170.640 von bernie55 am 12.04.16 15:01:38Arena's (ARNA) Q1 Loss Narrower but Revenues Fall Short
Zacks By Arpita Dutt
May 10, 2016 8:27 AM
Arena Pharmaceuticals, Inc. ARNA reported a loss of 9 cents per share in the first quarter of 2016, narrower than the year-ago loss and the Zacks Consensus Estimate of a loss of 10 cents. Total revenues, however, declined 19.7% year over year to $9.8 million, missing the Zacks Consensus Estimate of $11 million.
Arena recorded Belviq (weight management) sales of $3.5 million, down 10.3% sequentially. As per IMS Health, total prescriptions for Belviq were about 121,000, down 7.6% sequentially.
Research & development (R&D) expenses declined 15.8% year over year to $18.5 million. General & administrative (G&A) expenses declined 17.9% year over year to $6.9 million. Lower expenses reflected the reduction of workforce.
Focus in 2016 will remain on the advancement of the company’s proprietary pipeline candidates, which include APD334 (phase II for ulcerative colitis), ralinepag (in a phase II study for pulmonary arterial hypertension), and APD371 (pain; completed a phase I multiple-ascending dose study with favorable results). Top-line data on both ralinepag and APD334 are expected in the second quarter of 2017. Arena also intends to pursue strategic collaborations for certain clinical and pre-clinical programs.
The company is also seeking FDA approval for a once-daily formulation of Belviq (Belviq XR) with a response from the agency expected in the third quarter.
In addition to reporting first quarter results, Arena announced the appointment of Amit D. Munshi as President, Chief Executive Officer and interim principal financial officer, effective May 11.
http://finance.yahoo.com/news/arenas-arna-q1-loss-narrower-1…
Zacks By Arpita Dutt
May 10, 2016 8:27 AM
Arena Pharmaceuticals, Inc. ARNA reported a loss of 9 cents per share in the first quarter of 2016, narrower than the year-ago loss and the Zacks Consensus Estimate of a loss of 10 cents. Total revenues, however, declined 19.7% year over year to $9.8 million, missing the Zacks Consensus Estimate of $11 million.
Arena recorded Belviq (weight management) sales of $3.5 million, down 10.3% sequentially. As per IMS Health, total prescriptions for Belviq were about 121,000, down 7.6% sequentially.
Research & development (R&D) expenses declined 15.8% year over year to $18.5 million. General & administrative (G&A) expenses declined 17.9% year over year to $6.9 million. Lower expenses reflected the reduction of workforce.
Focus in 2016 will remain on the advancement of the company’s proprietary pipeline candidates, which include APD334 (phase II for ulcerative colitis), ralinepag (in a phase II study for pulmonary arterial hypertension), and APD371 (pain; completed a phase I multiple-ascending dose study with favorable results). Top-line data on both ralinepag and APD334 are expected in the second quarter of 2017. Arena also intends to pursue strategic collaborations for certain clinical and pre-clinical programs.
The company is also seeking FDA approval for a once-daily formulation of Belviq (Belviq XR) with a response from the agency expected in the third quarter.
In addition to reporting first quarter results, Arena announced the appointment of Amit D. Munshi as President, Chief Executive Officer and interim principal financial officer, effective May 11.
http://finance.yahoo.com/news/arenas-arna-q1-loss-narrower-1…
Diabetes/Herz-Kreislauf
das liest sich doch sehr viel versprechend - bin derzeit vollständig ausgestiegen, bin aber sehr zuversichtlich, dass der präventive Nutzen von Lorcaserin bei Diabetes und Herz-Kreislauf-Erkrankungen spätestens 2018 mit Auswertung von CAMELLIA-TIMI nachgewiesen wird:A Study to Evaluate the Effect of Long-term Treatment With BELVIQ (Lorcaserin HCl) on the Incidence of Major Adverse Cardiovascular Events and Conversion to Type 2 Diabetes Mellitus in Obese and Overweight Subjects With Cardiovascular Disease or Multiple Cardiovascular Risk Factors (CAMELLIA-TIMI)
Die zahlreichen Chancen (Pipeline + Lorcaserin) in Relation zu der aktuellen Marktkapitalisierung machen ein Investment wieder interessant. Werde wohl langsam aber stetig eine neue Position aufbauen!
Zu den bisherigen ph3-Studien wurde wiederum in einem Journal ein Beitrag veröffentlicht:
Postgrad Med. 2016 May;128(4):364-70. doi: 10.1080/00325481.2016.1178590.
Evaluation of lorcaserin on progression of prediabetes to type 2 diabetes and reversion to euglycemia.
Nesto R1, Fain R2, Li Y3, Shanahan W4.
Author information
Abstract
OBJECTIVES:
Lorcaserin is a selective 5-HT2C (5-hydroxytryptamine 2C) receptor agonist indicated for weight management. Here, we assess the impact of lorcaserin on progression from prediabetes to type 2 diabetes (T2D) and on reversion from prediabetes to euglycemia.
METHODS:
This is a post hoc analysis of pooled data from two Phase 3 studies, BLOOM and BLOSSOM (N = 6136), evaluating the impact of lorcaserin on weight and glycemic parameters over 52 weeks in the subpopulation of obese/overweight subjects with prediabetes, alternately defined by fasting plasma glucose (FPG) 100-125 mg/dl or glycated hemoglobin (HbA1c) 5.7-6.4% at baseline.
RESULTS:
At Week 52, in the subpopulation with prediabetes, nearly twice as many lorcaserin-treated subjects achieved ≥5% weight loss versus placebo (HbA1c: 55.6% vs. 27.5%, p < 0.001; FPG: 52.8% vs. 28.8%, p < 0.001), and a significantly lower percentage of lorcaserin-treated subjects progressed to T2D versus placebo based on HbA1c (lorcaserin 3.2%, placebo 5.0%, p = 0.032) but not FPG (lorcaserin 1.6%, placebo 2.6%, p = 0.227). A significantly greater proportion of lorcaserin-treated subjects versus placebo also reverted to euglycemia based on both HbA1c (lorcaserin 40%, placebo 29.5%, p < 0.001) and FPG (lorcaserin 52.4%, placebo 46.5%, p = 0.047).
CONCLUSION:
In subjects with prediabetes, lorcaserin may contribute to weight loss and improve glycemic parameters, and thus may help with preventing progression to T2D and promoting reversion to euglycemia.
Antwort auf Beitrag Nr.: 52.400.304 von cyberhexe123 am 13.05.16 11:01:38Arena Pharmaceuticals Announces USAN Approval of Nonproprietary Name "Etrasimod" for its Drug Candidate APD334
- Etrasimod being studied in a Phase 2 clinical trial for ulcerative colitis -
SAN DIEGO, June 13, 2016 /PRNewswire/ --
Arena Pharmaceuticals, Inc. (ARNA) announced today that the United States Adopted Names (USAN) Council has approved the nonproprietary name etrasimod (pronounced Et-ras'-i-mod) for APD334, a selective oral, investigational, Sphingosine 1-Phosphate Subtype 1 (S1P1) receptor modulator with therapeutic potential in autoimmune diseases. Arena is currently studying etrasimod in a Phase 2 clinical trial for ulcerative colitis.
"Given the limitations of currently available treatments for ulcerative colitis, including challenging effect profiles, we believe etrasimod has the potential to become the standard of care," said Amit Munshi, President and Chief Executive Officer of Arena. "We look forward to the results of our Phase 2 clinical trial of this promising compound."
Etrasimod is a potent and highly selective, orally available investigational drug candidate that targets the S1P1 receptor. S1P1 receptors have been demonstrated to be involved in the modulation of several biological responses, including lymphocyte trafficking from lymph nodes to the peripheral blood. By isolating subpopulations of lymphocytes in lymph nodes, fewer immune cells are available in the circulating blood to effect tissue damage.
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…
- Etrasimod being studied in a Phase 2 clinical trial for ulcerative colitis -
SAN DIEGO, June 13, 2016 /PRNewswire/ --
Arena Pharmaceuticals, Inc. (ARNA) announced today that the United States Adopted Names (USAN) Council has approved the nonproprietary name etrasimod (pronounced Et-ras'-i-mod) for APD334, a selective oral, investigational, Sphingosine 1-Phosphate Subtype 1 (S1P1) receptor modulator with therapeutic potential in autoimmune diseases. Arena is currently studying etrasimod in a Phase 2 clinical trial for ulcerative colitis.
"Given the limitations of currently available treatments for ulcerative colitis, including challenging effect profiles, we believe etrasimod has the potential to become the standard of care," said Amit Munshi, President and Chief Executive Officer of Arena. "We look forward to the results of our Phase 2 clinical trial of this promising compound."
Etrasimod is a potent and highly selective, orally available investigational drug candidate that targets the S1P1 receptor. S1P1 receptors have been demonstrated to be involved in the modulation of several biological responses, including lymphocyte trafficking from lymph nodes to the peripheral blood. By isolating subpopulations of lymphocytes in lymph nodes, fewer immune cells are available in the circulating blood to effect tissue damage.
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…
Antwort auf Beitrag Nr.: 52.610.699 von bernie55 am 14.06.16 10:36:20Arena (ARNA) to Cut 73% Workforce, Shifts Focus to Pipeline
Zacks By Arpita Dutt
July 1, 2016 9:37 AM
Arena Pharmaceuticals, Inc. ARNA announced a 73% cut in its workforce and said that it will be shifting its priorities to its proprietary clinical stage pipeline.
Workforce Reduction to Cut Costs
The U.S. workforce will be cut by approximately 100 employees by Aug 31, 2016, mainly in the areas of research, manufacturing and G&A. The company estimates that this move will reduce annualized cash expenditures for personnel by about $17 million and related other operating expenses of between $6-8 million.
Additional cost reduction measures include reductions at the company’s Swiss manufacturing facility.
Arena expects to incur restructuring charges of about $6.1 million (a majority of which are cash expenditures), mostly in the second quarter of 2016.
Focus Shifts to Pipeline
Arena, which has obesity treatment, Belviq, in its marketed portfolio, intends to focus on its pipeline which comprises etrasimod (APD334; phase II study ongoing for ulcerative colitis, with potential for additional indications including beyond inflammatory bowel disease), APD371 (recently completed a phase I multiple-ascending dose study with favorable results; under evaluation for pain indications) and ralinepag (APD811; phase II study ongoing for pulmonary arterial hypertension).
The company also intends to support its collaborations with companies like Eisai Co., Ltd. ESALY for Belviq, Axovant Sciences Ltd. (nelotanserin for central nervous system disorders; currently in phase II studies), Ildong Pharmaceuticals Co., Ltd. (temanogrel for thrombotic diseases –phase I study ongoing) and Boehringer Ingelheim (preclinical development of candidates targeting a central nervous system receptor for psychiatric diseases).
http://finance.yahoo.com/news/arena-arna-cut-73-workforce-13…
Zacks By Arpita Dutt
July 1, 2016 9:37 AM
Arena Pharmaceuticals, Inc. ARNA announced a 73% cut in its workforce and said that it will be shifting its priorities to its proprietary clinical stage pipeline.
Workforce Reduction to Cut Costs
The U.S. workforce will be cut by approximately 100 employees by Aug 31, 2016, mainly in the areas of research, manufacturing and G&A. The company estimates that this move will reduce annualized cash expenditures for personnel by about $17 million and related other operating expenses of between $6-8 million.
Additional cost reduction measures include reductions at the company’s Swiss manufacturing facility.
Arena expects to incur restructuring charges of about $6.1 million (a majority of which are cash expenditures), mostly in the second quarter of 2016.
Focus Shifts to Pipeline
Arena, which has obesity treatment, Belviq, in its marketed portfolio, intends to focus on its pipeline which comprises etrasimod (APD334; phase II study ongoing for ulcerative colitis, with potential for additional indications including beyond inflammatory bowel disease), APD371 (recently completed a phase I multiple-ascending dose study with favorable results; under evaluation for pain indications) and ralinepag (APD811; phase II study ongoing for pulmonary arterial hypertension).
The company also intends to support its collaborations with companies like Eisai Co., Ltd. ESALY for Belviq, Axovant Sciences Ltd. (nelotanserin for central nervous system disorders; currently in phase II studies), Ildong Pharmaceuticals Co., Ltd. (temanogrel for thrombotic diseases –phase I study ongoing) and Boehringer Ingelheim (preclinical development of candidates targeting a central nervous system receptor for psychiatric diseases).
http://finance.yahoo.com/news/arena-arna-cut-73-workforce-13…
Antwort auf Beitrag Nr.: 52.757.011 von bernie55 am 04.07.16 10:55:58Arena Pharmaceuticals Announces Shift to Focus on Proprietary Clinical Stage Pipeline
- Additional cost reductions implemented to support development program prioritization -
SAN DIEGO, June 30, 2016 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) today announced a strategic shifting of priorities to emphasize its proprietary clinical stage pipeline. The Company also announced the implementation of additional cost reductions to streamline the organization to support its development programs.
"We believe our clinical-stage pipeline has the potential to deliver first or best-in-class compounds for a broad range of indications. Building a streamlined and highly-focused organization supports our primary objective - developing our pipeline in a timely and efficient manner," said Amit Munshi, Arena's President and Chief Executive Officer.
Arena will reduce its US workforce by approximately 100 employees, or 73%, primarily in areas of research, manufacturing and G&A, which Arena estimates will reduce annualized cash expenditures for (i) personnel by approximately $17 million and (ii) related other operating expenses of between $6-8 million. Arena plans to implement additional cost control measures to further reduce its expenditures, including reductions at its Swiss manufacturing facility.
As a result of the US workforce reduction, which is planned to be completed by August 31, 2016, Arena estimates it will incur restructuring charges, primarily in the second quarter of 2016, of approximately $6.1 million (a majority of which are cash expenditures) in connection with one-time employee termination costs, including severance and other benefits.
Arena expects to discuss its strategic focus and organizational streamlining during its upcoming second quarter conference call.
About Arena Pharmaceuticals
We are a biopharmaceutical company focused on discovering and developing novel, small molecule drugs. We are currently directing our activities and resources primarily on the following activities:
1. Advancing our proprietary clinical programs:
a. Etrasimod (APD334) - a next generation, highly specific modulator of the sphingosine 1-phosphate subtype 1, or S1P1, receptor - in an ongoing Phase 2 clinical trial for ulcerative colitis, and potentially exploring additional indications, including beyond inflammatory bowel disease
b. APD371- an agonist of the cannabinoid-2, or CB2, receptor - most recently completed a Phase 1 multiple-ascending dose clinical trial with favorable results. and is under evaluation for pain indications
c. Ralinepag (APD811) - an agonist of the prostacyclin receptor - in an ongoing Phase 2 clinical trial for pulmonary arterial hypertension, or PAH
2. Supporting our collaborations:
a. Eisai Inc. and Eisai Co., Ltd. and others - in their efforts with respect to the approved product BELVIQ for weight management
b. Axovant Sciences Ltd. - in Phase 2 clinical trials for nelotanserin, an inverse agonist of the serotonin 2A receptor for central nervous system disorders
c. Ildong Pharmaceuticals Co., Ltd. - in a Phase 1 clinical trial for temanogrel, an inverse agonist of the serotonin 2A receptor for thrombotic diseases
d. Boehringer Ingelheim International GmbH - in preclinical development of drug candidates targeting a central nervous system, or CNS, receptor for psychiatric diseases
For more information, please visit www.arenapharm.com
http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=977…
- Additional cost reductions implemented to support development program prioritization -
SAN DIEGO, June 30, 2016 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) today announced a strategic shifting of priorities to emphasize its proprietary clinical stage pipeline. The Company also announced the implementation of additional cost reductions to streamline the organization to support its development programs.
"We believe our clinical-stage pipeline has the potential to deliver first or best-in-class compounds for a broad range of indications. Building a streamlined and highly-focused organization supports our primary objective - developing our pipeline in a timely and efficient manner," said Amit Munshi, Arena's President and Chief Executive Officer.
Arena will reduce its US workforce by approximately 100 employees, or 73%, primarily in areas of research, manufacturing and G&A, which Arena estimates will reduce annualized cash expenditures for (i) personnel by approximately $17 million and (ii) related other operating expenses of between $6-8 million. Arena plans to implement additional cost control measures to further reduce its expenditures, including reductions at its Swiss manufacturing facility.
As a result of the US workforce reduction, which is planned to be completed by August 31, 2016, Arena estimates it will incur restructuring charges, primarily in the second quarter of 2016, of approximately $6.1 million (a majority of which are cash expenditures) in connection with one-time employee termination costs, including severance and other benefits.
Arena expects to discuss its strategic focus and organizational streamlining during its upcoming second quarter conference call.
About Arena Pharmaceuticals
We are a biopharmaceutical company focused on discovering and developing novel, small molecule drugs. We are currently directing our activities and resources primarily on the following activities:
1. Advancing our proprietary clinical programs:
a. Etrasimod (APD334) - a next generation, highly specific modulator of the sphingosine 1-phosphate subtype 1, or S1P1, receptor - in an ongoing Phase 2 clinical trial for ulcerative colitis, and potentially exploring additional indications, including beyond inflammatory bowel disease
b. APD371- an agonist of the cannabinoid-2, or CB2, receptor - most recently completed a Phase 1 multiple-ascending dose clinical trial with favorable results. and is under evaluation for pain indications
c. Ralinepag (APD811) - an agonist of the prostacyclin receptor - in an ongoing Phase 2 clinical trial for pulmonary arterial hypertension, or PAH
2. Supporting our collaborations:
a. Eisai Inc. and Eisai Co., Ltd. and others - in their efforts with respect to the approved product BELVIQ for weight management
b. Axovant Sciences Ltd. - in Phase 2 clinical trials for nelotanserin, an inverse agonist of the serotonin 2A receptor for central nervous system disorders
c. Ildong Pharmaceuticals Co., Ltd. - in a Phase 1 clinical trial for temanogrel, an inverse agonist of the serotonin 2A receptor for thrombotic diseases
d. Boehringer Ingelheim International GmbH - in preclinical development of drug candidates targeting a central nervous system, or CNS, receptor for psychiatric diseases
For more information, please visit www.arenapharm.com
http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=977…
Antwort auf Beitrag Nr.: 52.758.367 von bernie55 am 04.07.16 13:53:33BELVIQ - Zulassung in Mexico unter anderem Namen
Eisai, Arena Pharmaceuticals Get Regulatory Approval For VENESPRI In Mexico
Benzinga
R. Chandrasekaran
July 14, 20161 Comment
Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) and Eisai Inc. revealed Thursday that the Federal Commission for the Protection Against Sanitary Risk (COFEPRIS) has given its approval for the chronic weight management agent VENESPRI. It would proceed for commercialization by Eisai Laboratorios, S. de R.L. de C.V. (Eisai Mexico) in Mexico.
Arena and Eisai said that VENESPRI is currently approved in Mexico as an adjunct to a reduced-calorie diet, as well as, increased physical activity for chronic weight management in adult patients with a body mass index (BMI) of 30 kg/m2 or greater (obese). According to them, patients with a BMI of 27 kg/m2 or higher or overweight with minimum one weight-related medical condition like high blood pressure, high cholesterol, or type 2 diabetes, were also eligible to be treated with VENESPRI.
The companies indicated the product would become available later this year. As a result of the latest regulatory approval, Arena would get a $1 million milestone payment.
Arena's SVP of Operations and Head of Global Regulatory Affairs, Craig Audet, said, "We are pleased that the Mexican health authority has approved VENESPRI as an option for patients who find it difficult to lose weight through diet and exercise alone."
Eisai CEO, Shaji Procida, reacted to the approval saying, "Currently more than 70 percent of the Mexican population is either overweight or obese. Without intervention, these numbers are projected to increase. Eisai remains committed to help address the health care needs of this patient population."
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-re…
Eisai, Arena Pharmaceuticals Get Regulatory Approval For VENESPRI In Mexico
Benzinga
R. Chandrasekaran
July 14, 20161 Comment
Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) and Eisai Inc. revealed Thursday that the Federal Commission for the Protection Against Sanitary Risk (COFEPRIS) has given its approval for the chronic weight management agent VENESPRI. It would proceed for commercialization by Eisai Laboratorios, S. de R.L. de C.V. (Eisai Mexico) in Mexico.
Arena and Eisai said that VENESPRI is currently approved in Mexico as an adjunct to a reduced-calorie diet, as well as, increased physical activity for chronic weight management in adult patients with a body mass index (BMI) of 30 kg/m2 or greater (obese). According to them, patients with a BMI of 27 kg/m2 or higher or overweight with minimum one weight-related medical condition like high blood pressure, high cholesterol, or type 2 diabetes, were also eligible to be treated with VENESPRI.
The companies indicated the product would become available later this year. As a result of the latest regulatory approval, Arena would get a $1 million milestone payment.
Arena's SVP of Operations and Head of Global Regulatory Affairs, Craig Audet, said, "We are pleased that the Mexican health authority has approved VENESPRI as an option for patients who find it difficult to lose weight through diet and exercise alone."
Eisai CEO, Shaji Procida, reacted to the approval saying, "Currently more than 70 percent of the Mexican population is either overweight or obese. Without intervention, these numbers are projected to increase. Eisai remains committed to help address the health care needs of this patient population."
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-re…
Eisai And Arena Pharmaceuticals Get FDA Approval For BELVIQ XR Extended-Release Tablets
July 19, 2016
Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) and Eisai Inc. revealed that the Food and Drug Administration has approved its New Drug Application (NDA) for BELVIQ XR (lorcaserin HCl) CIV extended-release 20 mg tablets. According to the company, the new formulation of lorcaserin would provide patients a once-a-day dosing option that might help them achieve, as well as maintain weight loss.
Arena expects BELVIQ XR to be available in the fall season of the current year. The company indicated it would get a $10 million milestone payment in connection with the latest regulatory approval.
The company's president and CEO, Amit Munshi, said, "We are pleased that once-daily BELVIQ XR has been approved by the FDA and will provide patients another option for weight loss. The approval of this new formulation is another example of Arena's success in supporting our collaborators."
Similarly, Eisai EVP for Neurology Business Group, Andrew Satlin, commented, "We're excited to offer this once-a-day option of lorcaserin. This option may provide another choice for patients who are overweight or obese and find it difficult to lose weight through diet and exercise alone. The development of this new formulation further underscores Eisai's ongoing commitment to help address the health care needs of this underserved population."
The companies disclosed that bioequivalence, as well as bioavailability of once-daily BELVIQ XR 20 mg compared with twice-daily BELVIQ 10 mg was based on two first stage registrational clinical studies among healthy adult subjects. The company added that the most common treatment-emergent adverse events were similar to those seen in the final stage of the clinical studies of BELVIQ 10 mg twice-daily.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-fd…
July 19, 2016
Arena Pharmaceuticals, Inc. (NASDAQ: ARNA) and Eisai Inc. revealed that the Food and Drug Administration has approved its New Drug Application (NDA) for BELVIQ XR (lorcaserin HCl) CIV extended-release 20 mg tablets. According to the company, the new formulation of lorcaserin would provide patients a once-a-day dosing option that might help them achieve, as well as maintain weight loss.
Arena expects BELVIQ XR to be available in the fall season of the current year. The company indicated it would get a $10 million milestone payment in connection with the latest regulatory approval.
The company's president and CEO, Amit Munshi, said, "We are pleased that once-daily BELVIQ XR has been approved by the FDA and will provide patients another option for weight loss. The approval of this new formulation is another example of Arena's success in supporting our collaborators."
Similarly, Eisai EVP for Neurology Business Group, Andrew Satlin, commented, "We're excited to offer this once-a-day option of lorcaserin. This option may provide another choice for patients who are overweight or obese and find it difficult to lose weight through diet and exercise alone. The development of this new formulation further underscores Eisai's ongoing commitment to help address the health care needs of this underserved population."
The companies disclosed that bioequivalence, as well as bioavailability of once-daily BELVIQ XR 20 mg compared with twice-daily BELVIQ 10 mg was based on two first stage registrational clinical studies among healthy adult subjects. The company added that the most common treatment-emergent adverse events were similar to those seen in the final stage of the clinical studies of BELVIQ 10 mg twice-daily.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-fd…
Eisai Inc. and Arena Pharmaceuticals Announce Availability of Once-Daily BELVIQ XR® (lorcaserin HCl) Extended-Release Tablets
WOODCLIFF LAKE, N.J. and SAN DIEGO, Oct. 3, 2016 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) today announced the availability of BELVIQ XR® (lorcaserin HCl) CIV extended-release 20 mg tablets, a new once-daily dosing option that may help some patients achieve and maintain weight loss.
"With more than 78 million adults who are obese in this country and obesity rates on the rise, there is an increased need for additional therapeutic options to help patients better manage their weight," said Caroline Apovian, M.D., Professor of Medicine at Boston University School of Medicine. "A once-daily, extended-release tablet provides a treatment regimen that may help patients meet their weight loss goals."
BELVIQ XR is proven to be slowly absorbed in the body and last throughout the day. The 20 mg once-daily extended-release formulation is approved for use with a reduced-calorie diet and increased physical activity for chronic weight management in adults who have a body mass index (BMI) of 30 kg/m2 or greater (obese), or BMI of 27 kg/m2 or greater (overweight) with at least one weight-related medical condition, such as high blood pressure, high cholesterol or type 2 diabetes. It is not known if BELVIQ XR, when taken with other prescription, over-the-counter, or herbal weight-loss products, is safe and effective. It is not known if BELVIQ XR changes your risk of heart problems, stroke or death due to heart problems or stroke.
"We are pleased that BELVIQ XR is now available to patients and may provide them with a new option that can be used as part of their weight management armamentarium," said Amit Munshi, President and Chief Executive Officer, Arena Pharmaceuticals, Inc."The launch of this new formulation is another example of Arena's success in supporting our collaborators."
"Eisai is committed to those living with obesity—a chronic, progressive disease that has serious health consequences," said Paul Hawthorne, Senior Vice President, Neurology Business Group, Eisai Inc. "We are excited to offer patients a once-daily option for chronic weight management that may help them achieve and sustain their weight loss goals."
For those who qualify, including those with Medicare Part D coverage (restrictions apply), Eisai will also offer a savings card, with two ways to save money on prescriptions. For more information about the program, patients can visit www.belviqxr.com or call 1(855) BELVIQ-1 (1-855-235-8471).
http://finance.yahoo.com/news/eisai-inc-arena-pharmaceutical…
WOODCLIFF LAKE, N.J. and SAN DIEGO, Oct. 3, 2016 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) today announced the availability of BELVIQ XR® (lorcaserin HCl) CIV extended-release 20 mg tablets, a new once-daily dosing option that may help some patients achieve and maintain weight loss.
"With more than 78 million adults who are obese in this country and obesity rates on the rise, there is an increased need for additional therapeutic options to help patients better manage their weight," said Caroline Apovian, M.D., Professor of Medicine at Boston University School of Medicine. "A once-daily, extended-release tablet provides a treatment regimen that may help patients meet their weight loss goals."
BELVIQ XR is proven to be slowly absorbed in the body and last throughout the day. The 20 mg once-daily extended-release formulation is approved for use with a reduced-calorie diet and increased physical activity for chronic weight management in adults who have a body mass index (BMI) of 30 kg/m2 or greater (obese), or BMI of 27 kg/m2 or greater (overweight) with at least one weight-related medical condition, such as high blood pressure, high cholesterol or type 2 diabetes. It is not known if BELVIQ XR, when taken with other prescription, over-the-counter, or herbal weight-loss products, is safe and effective. It is not known if BELVIQ XR changes your risk of heart problems, stroke or death due to heart problems or stroke.
"We are pleased that BELVIQ XR is now available to patients and may provide them with a new option that can be used as part of their weight management armamentarium," said Amit Munshi, President and Chief Executive Officer, Arena Pharmaceuticals, Inc."The launch of this new formulation is another example of Arena's success in supporting our collaborators."
"Eisai is committed to those living with obesity—a chronic, progressive disease that has serious health consequences," said Paul Hawthorne, Senior Vice President, Neurology Business Group, Eisai Inc. "We are excited to offer patients a once-daily option for chronic weight management that may help them achieve and sustain their weight loss goals."
For those who qualify, including those with Medicare Part D coverage (restrictions apply), Eisai will also offer a savings card, with two ways to save money on prescriptions. For more information about the program, patients can visit www.belviqxr.com or call 1(855) BELVIQ-1 (1-855-235-8471).
http://finance.yahoo.com/news/eisai-inc-arena-pharmaceutical…
Arena (ARNA) Q3 Loss Lower than Expected, Revenues Rise
Zacks Equity Research
ZacksNovember 8, 2016Comment
Arena Pharmaceuticals, Inc. ARNA reported a loss of 5 cents per share in the third quarter of 2016, narrower than both the year-ago loss of 11 cents and the Zacks Consensus Estimate of a loss of 7 cents.
Total revenue surged 110% year over year to $19.2 million, which includes $11 million in milestone payments earned from partner Eisai for Belviq XR.
Belviq XR is a once-daily, extended-release formulation of Belviq (obesity), the only approved product in Arena’s portfolio, which received FDA approval in July this year and was launched this month.
http://finance.yahoo.com/news/arena-arna-q3-loss-lower-13110…
Zacks Equity Research
ZacksNovember 8, 2016Comment
Arena Pharmaceuticals, Inc. ARNA reported a loss of 5 cents per share in the third quarter of 2016, narrower than both the year-ago loss of 11 cents and the Zacks Consensus Estimate of a loss of 7 cents.
Total revenue surged 110% year over year to $19.2 million, which includes $11 million in milestone payments earned from partner Eisai for Belviq XR.
Belviq XR is a once-daily, extended-release formulation of Belviq (obesity), the only approved product in Arena’s portfolio, which received FDA approval in July this year and was launched this month.
http://finance.yahoo.com/news/arena-arna-q3-loss-lower-13110…
Arena Pharmaceuticals Announces Poster at the American Heart Association (AHA) Scientific Sessions 2016
PR Newswire PR NewswireNovember 9, 2016Comment
SAN DIEGO, Nov. 9, 2016 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that Professor Lucie Clapp will present a poster at the American Heart Association (AHA) Scientific Sessions 2016 on Monday, November 14 at 10:45am CT. The scientific sessions are taking place November 12-16, 2016 in New Orleans, Louisiana at the Ernest N. Morial Convention Center.
The following poster will be presented:
APD811, a Novel and Highly Selective Non-prostanoid IP Receptor Agonist in Smooth Muscle Cells From Patients With Pulmonary Hypertension
Session Type: Abstract Poster Session
Session Number: VA.APS.P189
Poster Session: Novel Mechanisms of Pulmonary Vascular Disease
Date: Monday, November 14, 2016, 10:45 am - 12:00 noon
Location: Ernest N. Morial Convention Center, Science and Technology Hall-Clinical Science Section
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…
PR Newswire PR NewswireNovember 9, 2016Comment
SAN DIEGO, Nov. 9, 2016 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that Professor Lucie Clapp will present a poster at the American Heart Association (AHA) Scientific Sessions 2016 on Monday, November 14 at 10:45am CT. The scientific sessions are taking place November 12-16, 2016 in New Orleans, Louisiana at the Ernest N. Morial Convention Center.
The following poster will be presented:
APD811, a Novel and Highly Selective Non-prostanoid IP Receptor Agonist in Smooth Muscle Cells From Patients With Pulmonary Hypertension
Session Type: Abstract Poster Session
Session Number: VA.APS.P189
Poster Session: Novel Mechanisms of Pulmonary Vascular Disease
Date: Monday, November 14, 2016, 10:45 am - 12:00 noon
Location: Ernest N. Morial Convention Center, Science and Technology Hall-Clinical Science Section
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-announce…
Arena Pharmaceuticals to Present at the 28th Annual Piper Jaffray Healthcare Conference
PR Newswire PR NewswireNovember 9, 20161 Comment
SAN DIEGO, Nov. 9, 2016 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that Amit Munshi, the Company's President and Chief Executive Officer will present a corporate overview at the 28th Annual Piper Jaffray Healthcare Conference on Tuesday, November 29 at 1:30pm ET. The conference will take place November 29-30, 2016 at the Lotte New York Palace in New York, NY.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
PR Newswire PR NewswireNovember 9, 20161 Comment
SAN DIEGO, Nov. 9, 2016 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that Amit Munshi, the Company's President and Chief Executive Officer will present a corporate overview at the 28th Annual Piper Jaffray Healthcare Conference on Tuesday, November 29 at 1:30pm ET. The conference will take place November 29-30, 2016 at the Lotte New York Palace in New York, NY.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
Arena Pharmaceuticals Completes Enrollment in Ralinepag Phase 2 Clinical Trial for Pulmonary Arterial Hypertension (PAH)
PR Newswire PR NewswireDecember 7, 20161 Comment
SAN DIEGO, Dec. 7, 2016 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that it has completed enrollment in the ralinepag phase 2 trial. Ralinepag is an oral, selective IP receptor agonist targeting the prostacyclin pathway for the treatment of pulmonary arterial hypertension (PAH). The study enrolled approximately 60 patients at sites globally.
"This marks an important step in the development of ralinepag and is evidence of our strategic focus on our pipeline," said Amit Munshi, Arena's President and CEO. "We believe ralinepag has the potential to achieve a best-in-class profile for patients suffering from PAH and we look forward to seeing the results mid-year to confirm our hypothesis."
The trial is a 22-week, randomized, double-blind, placebo-controlled Phase 2 trial evaluating the effectiveness in reducing pulmonary vascular resistance, improving exercise capacity, tolerability and safety of ralinepag.
About PAH
Pulmonary arterial hypertension (PAH) is a rare, progressive, life-threatening disorder characterized by increased pressure in the arteries that carry blood from the heart to the lungs. The increased pressure strains the heart, which can limit physical activity, result in heart failure and reduce life expectancy. Based on data from the Registry to EValuate Early And Long-term PAH disease management (REVEAL) of patients in the United States, there is an estimated five-year survival rate of 57% from diagnosis.
About Ralinepag
Ralinepag, an orally available agonist of the IP receptor, is an investigational drug candidate internally discovered and developed by Arena and intended for the treatment of vascular diseases, including PAH. In Phase 1 trials, ralinepag showed an approximate 25-hour half-life, indicating that the compound could be dosed once or twice daily. Arena believes that ralinepag's high intrinsic potency and activity at the human IP receptor has the potential to improve treatment for patients with PAH. The FDA has granted ralinepag orphan drug status for the treatment of PAH.
http://finance.yahoo.com/news/arena-pharmaceuticals-complete…
PR Newswire PR NewswireDecember 7, 20161 Comment
SAN DIEGO, Dec. 7, 2016 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that it has completed enrollment in the ralinepag phase 2 trial. Ralinepag is an oral, selective IP receptor agonist targeting the prostacyclin pathway for the treatment of pulmonary arterial hypertension (PAH). The study enrolled approximately 60 patients at sites globally.
"This marks an important step in the development of ralinepag and is evidence of our strategic focus on our pipeline," said Amit Munshi, Arena's President and CEO. "We believe ralinepag has the potential to achieve a best-in-class profile for patients suffering from PAH and we look forward to seeing the results mid-year to confirm our hypothesis."
The trial is a 22-week, randomized, double-blind, placebo-controlled Phase 2 trial evaluating the effectiveness in reducing pulmonary vascular resistance, improving exercise capacity, tolerability and safety of ralinepag.
About PAH
Pulmonary arterial hypertension (PAH) is a rare, progressive, life-threatening disorder characterized by increased pressure in the arteries that carry blood from the heart to the lungs. The increased pressure strains the heart, which can limit physical activity, result in heart failure and reduce life expectancy. Based on data from the Registry to EValuate Early And Long-term PAH disease management (REVEAL) of patients in the United States, there is an estimated five-year survival rate of 57% from diagnosis.
About Ralinepag
Ralinepag, an orally available agonist of the IP receptor, is an investigational drug candidate internally discovered and developed by Arena and intended for the treatment of vascular diseases, including PAH. In Phase 1 trials, ralinepag showed an approximate 25-hour half-life, indicating that the compound could be dosed once or twice daily. Arena believes that ralinepag's high intrinsic potency and activity at the human IP receptor has the potential to improve treatment for patients with PAH. The FDA has granted ralinepag orphan drug status for the treatment of PAH.
http://finance.yahoo.com/news/arena-pharmaceuticals-complete…
Arena Pharmaceuticals to Present at the 35th Annual J.P. Morgan Healthcare Conference
PR Newswire PR Newswire December 13, 2016
SAN DIEGO, Dec. 13, 2016 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that Amit Munshi, the Company's President and Chief Executive Officer will present a corporate update at the 35th Annual J.P. Morgan Healthcare Conference on Thursday, January 12 at 9:00am PT. The conference will take place January 9-12, 2017, at the Westin St. Francis in San Francisco, CA.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
PR Newswire PR Newswire December 13, 2016
SAN DIEGO, Dec. 13, 2016 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that Amit Munshi, the Company's President and Chief Executive Officer will present a corporate update at the 35th Annual J.P. Morgan Healthcare Conference on Thursday, January 12 at 9:00am PT. The conference will take place January 9-12, 2017, at the Westin St. Francis in San Francisco, CA.
A live audio webcast of the presentation will be available under the investor relations section of Arena's website at www.arenapharm.com. A replay of the presentation will be available for 30 days following the event. Please connect to Arena's website several minutes prior to the start of the webcast to ensure adequate time for any software download that may be necessary.
http://finance.yahoo.com/news/arena-pharmaceuticals-present-…
Eisai and Arena Pharmaceuticals Announce Regulatory Approval of BELVIQ® (lorcaserin HCl) in Brazil
PR Newswire PR Newswire December 19, 20161
WOODCLIFF LAKE, N.J. and SAN DIEGO, Dec. 19, 2016 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) today announced that Eisai Laboratórios Ltda., a subsidiary of Eisai Inc., has received regulatory approval from the Brazilian Health Surveillance Agency (ANVISA) for BELVIQ® (lorcaserin HCl) for chronic weight management.
BELVIQ is approved in Brazil as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with a body mass index (BMI) of 30 kg/m2 or greater (obese), or overweight patients with a BMI greater than or equal to 27 kg/m2 in the presence of at least one weight-related comorbid condition such as high blood pressure, high cholesterol, cardiovascular disease, type 2 diabetes managed with oral hypoglycemic agents or sleep apnea. The product is expected to become available following review by Brazil's Medicines Market Regulation Board (CMED), which will trigger a $1 million milestone payment to Arena.
"Currently, more than half of the Brazilian population is overweight or obese and, without intervention, these numbers are projected to increase," said Shaji Procida, President and Chief Operating Officer, Eisai Inc. "This approval represents a new option for Brazilians who find it difficult to lose weight through diet and exercise alone. At Eisai, we remain committed to help address the needs of this patient population."
In addition to Brazil, BELVIQ is approved for weight management in the United States and South Korea. Lorcaserin is also approved in Mexico under the brand name VENESPRI® (lorcaserin HCl).
About BELVIQ® (lorcaserin HCl) CIV for Chronic Weight Management in the United States
BELVIQ is an FDA-approved prescription weight-loss medication that, when used with diet and exercise, can help some adults (body mass index [BMI] ≥27 kg/m2) living with extra weight, with a weight-related medical problem, or adults living with obesity (BMI ≥30 kg/m2), lose weight and keep it off.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-an…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-an…
PR Newswire PR Newswire December 19, 20161
WOODCLIFF LAKE, N.J. and SAN DIEGO, Dec. 19, 2016 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) today announced that Eisai Laboratórios Ltda., a subsidiary of Eisai Inc., has received regulatory approval from the Brazilian Health Surveillance Agency (ANVISA) for BELVIQ® (lorcaserin HCl) for chronic weight management.
BELVIQ is approved in Brazil as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with a body mass index (BMI) of 30 kg/m2 or greater (obese), or overweight patients with a BMI greater than or equal to 27 kg/m2 in the presence of at least one weight-related comorbid condition such as high blood pressure, high cholesterol, cardiovascular disease, type 2 diabetes managed with oral hypoglycemic agents or sleep apnea. The product is expected to become available following review by Brazil's Medicines Market Regulation Board (CMED), which will trigger a $1 million milestone payment to Arena.
"Currently, more than half of the Brazilian population is overweight or obese and, without intervention, these numbers are projected to increase," said Shaji Procida, President and Chief Operating Officer, Eisai Inc. "This approval represents a new option for Brazilians who find it difficult to lose weight through diet and exercise alone. At Eisai, we remain committed to help address the needs of this patient population."
In addition to Brazil, BELVIQ is approved for weight management in the United States and South Korea. Lorcaserin is also approved in Mexico under the brand name VENESPRI® (lorcaserin HCl).
About BELVIQ® (lorcaserin HCl) CIV for Chronic Weight Management in the United States
BELVIQ is an FDA-approved prescription weight-loss medication that, when used with diet and exercise, can help some adults (body mass index [BMI] ≥27 kg/m2) living with extra weight, with a weight-related medical problem, or adults living with obesity (BMI ≥30 kg/m2), lose weight and keep it off.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-an…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-an…
Arena Pharmaceuticals and Eisai Amend Marketing and Supply Agreement for BELVIQ Globally
PR Newswire PR NewswireJanuary 4, 20171 Comment
SAN DIEGO, Jan. 4, 2017 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that it has amended its BELVIQ® (lorcaserin HCl) marketing and supply agreement with Eisai Co., Ltd. and Eisai Inc. (collectively, "Eisai"). Under the revised agreement, Eisai is acquiring global commercialization rights to BELVIQ, including in the territories retained by Arena under the parties' prior agreement, with control over global development and commercialization decisions, and is responsible for all lorcaserin development expenses going forward.
The financial terms of the revised agreement are expected to provide Arena with $23 million of cash payments and over $80 million of potential cost relief on current lorcaserin development obligations.
Arena will continue to be eligible to receive royalty payments on net sales of BELVIQ and participate in the upside potential of lorcaserin from additional geographies and clinical trials such as the ongoing cardiovascular outcomes trial, CAMELLIA.
"We would like to thank Eisai for its continued efforts on BELVIQ," said Amit Munshi, President and Chief Executive Officer of Arena. "This amended agreement allows Arena to focus more of its financial resources on our clinical stage programs, with a goal of developing first- or best-in-class assets with our three proprietary compounds, from which we expect results from multiple Phase 2 clinical trials later this year."
Under the revised agreement, Arena will:
Transfer to Eisai certain intellectual property, assets and records related to lorcaserin
Assign its rights under the agreements with Ildong Pharmaceutical Co. LTD., Abic Marketing Limited and CY Biotech Company Limited to Eisai
Continue to manufacture lorcaserin at its facility in Switzerland and sell finished product to Eisai for marketing and distribution
Provide for technology transfer to Eisai for the option to manufacture lorcaserin
Eisai will be responsible for all further development, sales and marketing, regulatory and patent expenses.
Under the revised agreement,
Arena is eligible to receive approximately $23 million over a two-year period for inventory and ongoing support for its manufacturing obligations, plus payments comparable to a contract manufacturer for continuing to supply lorcaserin
Arena will no longer incur lorcaserin clinical development expenses, which could have exceeded $80 million over the next several years
Arena is eligible to receive royalty payments of 9.5% on annual global net sales of lorcaserin less than or equal to $175 million, 13.5% on annual global net sales greater than $175 million but less than or equal to $500 million and 18.5% on annual global net sales greater than $500 million
Arena is eligible to receive $26 million in potential sales and regulatory milestones including $25 million upon global net sales reaching $250 million in any 12 month period and $1 million for approval in Brazil.
http://finance.yahoo.com/news/arena-pharmaceuticals-eisai-am…
PR Newswire PR NewswireJanuary 4, 20171 Comment
SAN DIEGO, Jan. 4, 2017 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (ARNA) today announced that it has amended its BELVIQ® (lorcaserin HCl) marketing and supply agreement with Eisai Co., Ltd. and Eisai Inc. (collectively, "Eisai"). Under the revised agreement, Eisai is acquiring global commercialization rights to BELVIQ, including in the territories retained by Arena under the parties' prior agreement, with control over global development and commercialization decisions, and is responsible for all lorcaserin development expenses going forward.
The financial terms of the revised agreement are expected to provide Arena with $23 million of cash payments and over $80 million of potential cost relief on current lorcaserin development obligations.
Arena will continue to be eligible to receive royalty payments on net sales of BELVIQ and participate in the upside potential of lorcaserin from additional geographies and clinical trials such as the ongoing cardiovascular outcomes trial, CAMELLIA.
"We would like to thank Eisai for its continued efforts on BELVIQ," said Amit Munshi, President and Chief Executive Officer of Arena. "This amended agreement allows Arena to focus more of its financial resources on our clinical stage programs, with a goal of developing first- or best-in-class assets with our three proprietary compounds, from which we expect results from multiple Phase 2 clinical trials later this year."
Under the revised agreement, Arena will:
Transfer to Eisai certain intellectual property, assets and records related to lorcaserin
Assign its rights under the agreements with Ildong Pharmaceutical Co. LTD., Abic Marketing Limited and CY Biotech Company Limited to Eisai
Continue to manufacture lorcaserin at its facility in Switzerland and sell finished product to Eisai for marketing and distribution
Provide for technology transfer to Eisai for the option to manufacture lorcaserin
Eisai will be responsible for all further development, sales and marketing, regulatory and patent expenses.
Under the revised agreement,
Arena is eligible to receive approximately $23 million over a two-year period for inventory and ongoing support for its manufacturing obligations, plus payments comparable to a contract manufacturer for continuing to supply lorcaserin
Arena will no longer incur lorcaserin clinical development expenses, which could have exceeded $80 million over the next several years
Arena is eligible to receive royalty payments of 9.5% on annual global net sales of lorcaserin less than or equal to $175 million, 13.5% on annual global net sales greater than $175 million but less than or equal to $500 million and 18.5% on annual global net sales greater than $500 million
Arena is eligible to receive $26 million in potential sales and regulatory milestones including $25 million upon global net sales reaching $250 million in any 12 month period and $1 million for approval in Brazil.
http://finance.yahoo.com/news/arena-pharmaceuticals-eisai-am…
Why Arena Pharmaceuticals Stock Sank in 2016
Belviq's weak sales weighed heavily on Arena's stock last year.
George Budwell (TMFGBudwell) Jan 13, 2017 at 8:07AM
So what
At the start of 2017, Arena decided to admit defeat on Belviq by transferring the remaining commercial rights to Eisai under an amended marketing and licensing agreement. Although the biotech will receive $23 million in cash and over $80 million in cost relief associated with Belviq's development per this modified agreement, this move essentially reverts Arena back into a clinical-stage company.
Now what
From a valuation standpoint, Arena's near-term prospects are now going to be mostly dependent on the fate of its mid-stage drug candidates etrasimod for ulcerative colitis and ralinepag for pulmonary arterial hypertension. Both drugs are expected to produce top-line data this year, which should determine if they progress into a pivotal-stage trial perhaps in early 2018.
While Arena is going after some big game from a commercial standpoint with its clinical pipeline -- etrasimod, for example, is targeting a market forecast to grow to an impressive $7 billion by 2021 -- there are ample reasons to remain cautious with this small-cap biotech this year. Even if both of these experimental drugs hit the mark in their ongoing mid-stage studies, pivotal-stage trials can easily take three to five years to complete.
Unfortunately, Arena's definitely going to need to raise capital well before then, based on its last-stated cash position of around $101 million, implying that more dilutive financing is nearly guaranteed in the years ahead. Even if Arena's shares do "pop" on a positive mid-stage result this year, this stock may have trouble sustaining any upward momentum due to the strong possibility of a sizable secondary offering coming into play.
http://www.fool.com/investing/2017/01/13/why-arena-pharmaceu…
@AREANICS
Meine Restportion an ARENA-Aktien habe ich in dieser Woche verkauft.
Zum einen, weil es 2017 viele Biotech-Aktien gibt, die bzgl. der Produkt-Pipeline und der damit verbundenen möglichen Performance im Kurs ein wesentlich größeres Potential versprechen.
Zum anderen wollte ich die Verluste realisieren, da ich durch die Übernahme von ARIAD durch TAKEDA Riesengewinne erzielen konnte und somit die 25 % Kapitalertragssteuer ein bisschen
erzielen konnte und somit die 25 % Kapitalertragssteuer ein bisschen  nach unten drücken konnte.
nach unten drücken konnte.
Ich wünsche alle Investierten weiterhin good luck und ein gutes Näschen in der manchmal rauen Börsenluft.
Grüße
bernie55
Belviq's weak sales weighed heavily on Arena's stock last year.
George Budwell (TMFGBudwell) Jan 13, 2017 at 8:07AM
So what
At the start of 2017, Arena decided to admit defeat on Belviq by transferring the remaining commercial rights to Eisai under an amended marketing and licensing agreement. Although the biotech will receive $23 million in cash and over $80 million in cost relief associated with Belviq's development per this modified agreement, this move essentially reverts Arena back into a clinical-stage company.
Now what
From a valuation standpoint, Arena's near-term prospects are now going to be mostly dependent on the fate of its mid-stage drug candidates etrasimod for ulcerative colitis and ralinepag for pulmonary arterial hypertension. Both drugs are expected to produce top-line data this year, which should determine if they progress into a pivotal-stage trial perhaps in early 2018.
While Arena is going after some big game from a commercial standpoint with its clinical pipeline -- etrasimod, for example, is targeting a market forecast to grow to an impressive $7 billion by 2021 -- there are ample reasons to remain cautious with this small-cap biotech this year. Even if both of these experimental drugs hit the mark in their ongoing mid-stage studies, pivotal-stage trials can easily take three to five years to complete.
Unfortunately, Arena's definitely going to need to raise capital well before then, based on its last-stated cash position of around $101 million, implying that more dilutive financing is nearly guaranteed in the years ahead. Even if Arena's shares do "pop" on a positive mid-stage result this year, this stock may have trouble sustaining any upward momentum due to the strong possibility of a sizable secondary offering coming into play.
http://www.fool.com/investing/2017/01/13/why-arena-pharmaceu…
@AREANICS
Meine Restportion an ARENA-Aktien habe ich in dieser Woche verkauft.
Zum einen, weil es 2017 viele Biotech-Aktien gibt, die bzgl. der Produkt-Pipeline und der damit verbundenen möglichen Performance im Kurs ein wesentlich größeres Potential versprechen.
Zum anderen wollte ich die Verluste realisieren, da ich durch die Übernahme von ARIAD durch TAKEDA Riesengewinne
 erzielen konnte und somit die 25 % Kapitalertragssteuer ein bisschen
erzielen konnte und somit die 25 % Kapitalertragssteuer ein bisschen  nach unten drücken konnte.
nach unten drücken konnte.Ich wünsche alle Investierten weiterhin good luck und ein gutes Näschen in der manchmal rauen Börsenluft.
Grüße
bernie55

Antwort auf Beitrag Nr.: 54.079.790 von bernie55 am 13.01.17 18:12:45wann könnte es dort zu einer KE kommen?
Antwort auf Beitrag Nr.: 54.087.299 von sharepicker321 am 15.01.17 12:40:16das hätte ich so nicht erwartet: Ralinepag hat in einer Phase-2-Studie (n=61) ein mehr als beachtliches Resultat geliefert, nämlich eine Reduktion des Lungenblutdruck um 30% verglichen mit Placebo, und dies auch noch statistisch signifikant den primären Endpunkt erreichend. Not too bad!
Die Erwartungen im Vorfeld waren weitaus skeptischer, da lediglich eine Senkung um 20 -25% prognostiziert wurde. Allerdings wurde ein sekundärer Endpunkt nicht erreicht, nämlich die Wegstrecke, die ein Patient innerhalb von 6 Minuten zurücklegen kann: Ralinepag-Patienten legten 36m zurück, während Patienten unter Plazebo gerade einmal 30m zurücklegten - allerdings stat. nicht sign. Ausserdem sind einige Ungereimtheiten auszumachen, die man beachten sollte: der Umstand, dass Ralinepag-Patienten jünger waren als diejenigen unter Plazebo, könnte zu dessen Vorteil gewesen sein. Hingegen waren die Verum-Patienten in einem schlechteren Zustand als unter Plazebo.
Ralinepag gehört zur Klasse von bereits etablierten Wirkstoffen zur Behandlung von PAH (pulmonary arterial hypertension), nämlich den IP Prostacyclin-Rezeptor-Agonisten, die in 2016 insgesamt fast 2 Mrd USD Verkaufserlöse erzielt haben. Einer dieser Wirkstoffe am Markt ist Actelions Uptravi (generic name: Selexipag), mit welchem das CH-Unternehmen im vergangegen Jahr (2016) $240m Umsatz erzielt hat.
Das Wachstumspotenzial innerhalb Actelions PAH-Portfolio ist ursächlich dafür verantwortlich, dass JNJ das Unternehmen erst kürzlich für sage und schreibe 30 Milliarden USD übernommen hat.
Ich bin Heute eingestiegen und habe mir vorbörslich 1000 Stück ins Depot gelegt. Je nach Eröffnung werden in den US weitere Positionen hinzugekauft.
Die Erwartungen im Vorfeld waren weitaus skeptischer, da lediglich eine Senkung um 20 -25% prognostiziert wurde. Allerdings wurde ein sekundärer Endpunkt nicht erreicht, nämlich die Wegstrecke, die ein Patient innerhalb von 6 Minuten zurücklegen kann: Ralinepag-Patienten legten 36m zurück, während Patienten unter Plazebo gerade einmal 30m zurücklegten - allerdings stat. nicht sign. Ausserdem sind einige Ungereimtheiten auszumachen, die man beachten sollte: der Umstand, dass Ralinepag-Patienten jünger waren als diejenigen unter Plazebo, könnte zu dessen Vorteil gewesen sein. Hingegen waren die Verum-Patienten in einem schlechteren Zustand als unter Plazebo.
Ralinepag gehört zur Klasse von bereits etablierten Wirkstoffen zur Behandlung von PAH (pulmonary arterial hypertension), nämlich den IP Prostacyclin-Rezeptor-Agonisten, die in 2016 insgesamt fast 2 Mrd USD Verkaufserlöse erzielt haben. Einer dieser Wirkstoffe am Markt ist Actelions Uptravi (generic name: Selexipag), mit welchem das CH-Unternehmen im vergangegen Jahr (2016) $240m Umsatz erzielt hat.
Das Wachstumspotenzial innerhalb Actelions PAH-Portfolio ist ursächlich dafür verantwortlich, dass JNJ das Unternehmen erst kürzlich für sage und schreibe 30 Milliarden USD übernommen hat.
Ich bin Heute eingestiegen und habe mir vorbörslich 1000 Stück ins Depot gelegt. Je nach Eröffnung werden in den US weitere Positionen hinzugekauft.
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +2,53 | |
| 0,00 | |
| -0,80 | |
| 0,00 | |
| -0,66 | |
| -1,43 | |
| 0,00 | |
| +2,27 | |
| +0,88 | |
| -33,33 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 225 | ||
| 119 | ||
| 105 | ||
| 76 | ||
| 57 | ||
| 38 | ||
| 36 | ||
| 36 | ||
| 33 | ||
| 31 |
























