Sellas Life Sciences Group (ehemals Galena Biopharma) (Seite 145)
eröffnet am 30.10.12 22:43:19 von
neuester Beitrag 19.01.24 22:21:17 von
neuester Beitrag 19.01.24 22:21:17 von
Beiträge: 2.935
ID: 1.177.530
ID: 1.177.530
Aufrufe heute: 0
Gesamt: 345.742
Gesamt: 345.742
Aktive User: 0
ISIN: US81642T2096 · WKN: A2PU3T · Symbol: SLS
1,3500
USD
+5,47 %
+0,0700 USD
Letzter Kurs 02:00:00 Nasdaq
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,2000 | +471,16 | |
| 13,110 | +38,44 | |
| 1,8250 | +35,19 | |
| 1,2100 | +21,00 | |
| 27,50 | +19,57 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 4,6300 | -11,81 | |
| 40,23 | -12,54 | |
| 4,5001 | -13,46 | |
| 0,9378 | -18,55 | |
| 36,70 | -22,87 |
Beitrag zu dieser Diskussion schreiben
Antwort auf Beitrag Nr.: 50.674.584 von Growth2012 am 21.09.15 20:58:13hatte den artikel am wochenende gelesen und heute mittag bereits gepostet...war dann zunächst doch etwas verwundert über die (abwinkende) reaktion in deiner ersten mail...wenn man hier nämlich genau zwischen den zeilen liest, dann denke ich weiß man auch, warum der kurs heute >5% mit zunehmendem volumen steigt...finde das schon sehr "gehaltvoll"...
The Houston Chronicle Article in full... (Es scheint viel Gehör zu finden stateside
(Es scheint viel Gehör zu finden stateside )
)
Doctors take shot at keeping breast cancer away
Dr. Elizabeth Mittendorf had never met the patient waiting in the exam room, but a quick glance at her chart suggested she was a perfect candidate to test the oncologist's experimental drug.
Sadaf Zaidi had been treated successfully for breast cancer, though worrisome aspects remained: The cancer had been Stage 3C, so malignant cells had spread to the lymph nodes; a surgeon had to clean up a significant tumor not killed by the chemotherapy; and Zaidi is 31, an age when cancers tend to be more aggressive.
Those factors pointed to one conclusion. Zaidi, a mother of two, faces a high risk the cancer will return.
Some of the greatest strides in the war against cancer have been made battling tumors in the breast, but recurrence occurs in 20 percent of patients within five years of treatment, typically with fatal results.
Mittendorf, a surgeon-immunologist at M.D. Anderson Cancer Center, has been working on the problem for nearly 15 years. This year, she finished enrollment of a clinical trial - likely the final one required for regulatory approval - that promises a better outcome.
Related
Nurse Beena Varghese greets Bruce Campbell during his appointment at MD Anderson Cancer Center last month. Campbell, who has Stage 4 lung cancer, has been receiving immunotherapy for the last year and a half. His oncologist, John Heymach, below, proposed the treatment.
Patients clamor to get into trials for immunotherapy, the new
Jill Stuckey places "Jimmy Carter for Cancer Survivor" signs in Plains, Ga., Thursday in advance of the former president's return to his hometown.
Carter getting immunotherapy for cancer in his brain
The approach is the same one that conquered many of the 20th-century's worst scourges: vaccination.
Meeting with Zaidi, Mittendorf laid it all out. The vaccine revs up the immune system to seek out and attack rogue tumor cells in the circulation, when they're most vulnerable, before they coalescence into a mass. It causes some itchiness, perhaps a mild headache, but no chemotherapy-like side effects. In research thus far, it has dramatically reduced recurrence in women at high risk.
In one small trial, a precursor to the one in which Zaidi would be enrolled, Mittendorf noted that the vaccine helped lower the recurrence rate to zero.
"Do I think we've cured breast cancer?" Mittendorf said to Zaidi. "I'd like to think that, but the honest answer is 'not yet.' But this strategy definitely warrants further investigation."
***
Vaccines are the original immunotherapy, the field now generating such excitement in cancer care. They're credited with bringing seven major infectious conditions under some degree of control - smallpox, tetanus, diphtheria, yellow fever, whooping cough, polio and measles - and saving an estimated 9 million lives worldwide each year.
Health
New overactive-bladder medication blamed for dry eyes
Too much visceral fat around your organs and fat under your skin can trigger inflammation and increase your risk of everything from depression to cancer.
Get rid of food 'felons' to avoid vicious cycle
Sadaf Zaidi, left, a recently recovered breast cancer patient accepts an invitation by Dr. Elizabeth Mittendorf of M.D. Anderson Cancer Center to participate on the clinical trial of a vaccine that shows promise for preventing the recurrence of breast cancer.
Doctors take shot at keeping breast cancer away
A Houston girl, age 3, is the youngest child ever diagnosed with adult onset diabetes (Type 2).
Case of Houston child, 3, with diabetes highlights increase among
This product image provided by Boehringer Ingelheim Pharmaceuticals shows bottles of Jardiance, a daily pill for Type 2 diabetes. Jardiance sharply reduced chances of dying in diabetic patients at high risk of heart complications, a study shows, making the medication the first shown to lengthen diabetics' lives. (Boehringer Ingelheim Pharmaceuticals via AP)
Study: Type 2 diabetes pill cuts death risk
In cancer, they've been considered a flop.
While traditional vaccines mostly are aimed at preventing disease, cancer research has focused on therapeutic vaccines, which seek to generate an immune response in existing tumors. That premise was supported by lab work that showed vaccines shrank tumors in cell cultures.
Actual patients, however, proved another story. A 2004 review of 1990s clinical trials employing cancer vaccines found a 3 percent response rate. Promising candidates by GlaxoSmithKline, Amgen, Oncothyreon, Ziopharm and Keryx all failed to hold up in trials. It seemed a watershed when the Food and Drug Administration approved the first therapeutic cancer vaccine in 2010 - Provenge, for prostate cancer - but it remains little used, likely because it only extends patients' lives four months and costs more than $100,000. The company that developed it, Dendreon, filed for bankruptcy last year.
"The field was more religion than science," says Dr. George Peoples, Mittendorf's early mentor and one of the high priests. "You either believed or you didn't. There wasn't a lot of supporting data."
Believers now say vaccines are poised to break through, thanks to an improved understanding of the immune system and lessons learned from failures. Dr. Patrick Hwu, M.D. Anderson's head of the Division of Cancer Medicine and associate director of its Center for Cancer Immunology, says vaccines will start changing cancer care within five years.
Click on the arrows above to read a brief history of cancer treatments.
Peoples credits much of the turnaround to Houston scientist Jim Allison, who won a Lasker Award this month for his 1996 discovery of the first of a network of brakes that rein in the immune system and that cancer cells exploit in escaping detection. The discovery partly explains why vaccines provoked a strong immune response but stopped short of killing tumor cells.
Most of the new hope involves combining vaccines with checkpoint inhibitors, the class of drugs Allison pioneered that removes the brakes and unleashes the immune system to attack cancer. The expectation is that the approaches should prove complementary - vaccines triggering a response in cancers where there's not robust enough immune activity for checkpoint inhibitors to help, and those inhibitors taking off the brakes in cancers where vaccines' positive response was thwarted.
The bigger vaccine breakthrough may turn out to be stopping cancer before it gets started.
***
For Mittendorf, the epiphany came in the months following 9/11. A newly minted surgeon paying back an Air Force-funded medical school scholarship, she had been called to Walter Reed Medical Center in Bethesda, Md., to fill positions vacated by Army doctors deployed to Afghanistan. She took care of not just soldiers returning from the Middle East, but lots of breast cancer patients - active duty personnel, retirees and family members.
Among them was one woman whose breast cancer recurred 15 years after she was told she was cured. The cancer returned in her bone and eventually spread to her liver.
"It didn't make sense, I remember thinking," Mittendorf said. "Clearly, all the treatment she received at the time worked. What failed, ultimately, was her immune system."
Mittendorf, whose medical education was almost curtailed by a propensity to faint at patients' suffering, resolved to learn all she could about the system's workings. At M.D. Anderson following the stint at Walter Reed, she told her adviser she wanted to focus the research component of her surgical oncology fellowship on immunology. "Immunotherapy's never going to work. I won't let you do that," he told her, and put her in a lab studying molecular biology.
Mittendorf learned from the experience, but after the fellowship, she wasted no time getting back on the immunotherapy path. Now a full-time surgeon at M.D. Anderson, she simultaneously pursued a Ph.D. in immunology, naively thinking, "How hard could it be?"
Seven years later, she's one of only a handful of scientists in the world with such dual expertise, which she says provides a nice balance - the surgeon's need to quickly fix problems fits her lack of patience, while the immunologist's appreciation of long-term responses has prepared her for the decades-long battle ahead.
Throughout the fellowship and doctoral program, Mittendorf's mind focused on vaccines for breast cancer. The only trial in patients whose disease had spread from the breast to other organs had failed, no surprise to Mittendorf, who figured vaccines by themselves were no match for cancers in advanced stages.
Under Peoples' influence at Walter Reed, Mittendorf bought into the idea that vaccines would best work to prevent cancer. A West Point graduate who recently retired from Brooke Army Medical Center in San Antonio, Peoples in 2001 launched the first clinical trial testing whether a vaccine could prevent recurrence of breast tumors, known as "secondary" cancer prevention.
In the next five years, he and Mittendorf accumulated data from about 200 patients proving the radical idea worked.
The data showed a recurrence rate of 20 percent in those who got a placebo, 10 percent in those who got the vaccine and 5 percent in those who got the vaccine's optimal dose. In a smaller trial, no breast cancer recurred in 60 patients who got the vaccine and another drug.
Mittendorf shared the statistics with her newest patient, and Zaidi calmly told the doctor, "I hope I don't get the placebo."
***
In the mind of M.D. Anderson's Hwu, vaccines' past hardships are like the Wright Brothers' early efforts to fly - the trial and error that accompanies the development of any new technology. No one considers that first 12-second flight a failure.
One problem was that the old vaccines weren't designed to biologically resemble a virus, a fundamental ingredient to triggering a robust immune response. For another, they used a particular mineral oil that lured T cells to attack it rather than the cancer, according to a recent study.
Though it never used that mineral oil, Mittendorf's vaccine is somewhat old-school and likely to be tweaked in the future. It's a simple blend of an immune system stimulant and a tumor protein compound, HER2, present, to some extent, in 75 percent to 80 percent of breast cancers.
The vaccine, known as NeuVax, also has potential against ovarian, prostate, pancreatic, colon, bladder and gastric cancers because the protein is common to those tumors, too. In Mittendorf's trials, the vaccine reduced recurrence in women who had both high and low levels of HER2.
Mittendorf's vaccine is the furthest along in testing, but numerous institutions are working with vaccines.
The University of Pittsburgh has published promising results from an early-stage trial of a colon cancer vaccine. Roswell Park Cancer Institute in Buffalo, N.Y., recently agreed to study a lung cancer vaccine developed in Cuba. Bristol-Myers Squibb this year struck a licensing deal that will pay almost $1 billion for a prostate cancer vaccine initially thought to have failed but now showing long-term signs of effectiveness.
At the University of Pennsylvania, whose Dr. Robert Vonderheide says it's now "OK to say the V word," researchers are working with a vaccine targeting an enzyme overly active in the vast majority of cancers, a potential "universal" vaccine. They recently launched an early-stage trial trying to prevent recurrence in cancers of the pancreas, lung and breast.
No one has as much going on as Mittendorf. She has eight breast cancer vaccine trials underway or in planning, foremost among them the one expected to culminate in FDA approval, an international trial of more than 700 patients.
They also include combination trials: two with the drug Herceptin, which is used to treat and prevent recurrence in HER2-positive breast cancers, and a planned one with a checkpoint inhibitor in advanced-stage triple negative breast cancer, a particularly lethal form of the disease that resists most treatment.
Mittendorf is also about to launch research that could bring vaccines closer to "primary prevention:" a trial of patients with precancerous lesions in the breast. The condition, known as Stage 0 and involving abnormal cells confined to the milk ducts, is diagnosed in 60,000 American women annually.
"If these preventive vaccine trials work, researchers in every kind of cancer will jump into the field," says Dr. Elizabeth Jaffee, an oncologist and immunologist at Johns Hopkins.

For nearly 15 years, Mittendorf has focused both her clinical and laboratory efforts on the study of breast cancer with a specific interest in breast cancer immunotherapy.
After so many years in the trenches - one colleague refers to her as being a disciple of "immunotherapy before immunotherapy was cool" - Mittendorf can now make out a finish line, even if results of her latest-phase trial won't be available until 2018.
At that point, she can seek FDA approval for NeuVax. The epitome of the dispassionate scientist, she draws back from a question about whether such approval might be emotional for her, but acknowledges it will be a "tremendous advance," given that roughly 40,000 American women annually die of breast cancer.
A college soccer player and marathon runner, Mittendorf said any celebration will probably be short-lived. Approval of NeuVax will mark a starting point, she said, the opportunity to "run fast in a new direction." She calls the overall cancer vaccine effort an ultra-marathon.
It is not a race without challenges. One thing that facilitated breakthroughs in melanoma and lung cancer, the checkpoint inhibitor drugs recently approved, was that there was so little competing treatment. But many therapies are effective for breast cancer, so researchers will have to identify which immunotherapy benefits which population of patients at which stage of disease.
The process is likely to require a dizzying number of clinical trials and result in pharmaceutical companies charging more for the finished product, already a huge issue in cancer treatment.
It also will tax researchers' ability to design vaccines that remain a step ahead of tumors, given cancer's ability to evolve coping strategies. "There's a saying," said Mittendorf, "one dumb tumor is smarter than 10 smart oncologists."
Still, buoyed by immunotherapy's wave of success and newfound interest from the National Cancer Institute, Mittendorf and other researchers are thinking big. They envision a day when cancer vaccines are truly preventive, just like those used in infectious disease.
Zaidi, fully aware her stage 3C breast cancer is "a big deal," needed no persuasion. She seemed at peace at her first vaccination appointment this month, still anxious only about whether she was assigned to the vaccine or the placebo. It'll be 18 months before she finds out.
"I know the vaccine will work," said Zaidi, who completed medical school in Pakistan and currently works in medical research. "It just makes sense."
http://www.houstonchronicle.com/news/health/article/Doctors-…
 (Es scheint viel Gehör zu finden stateside
(Es scheint viel Gehör zu finden stateside )
) Doctors take shot at keeping breast cancer away
Dr. Elizabeth Mittendorf had never met the patient waiting in the exam room, but a quick glance at her chart suggested she was a perfect candidate to test the oncologist's experimental drug.
Sadaf Zaidi had been treated successfully for breast cancer, though worrisome aspects remained: The cancer had been Stage 3C, so malignant cells had spread to the lymph nodes; a surgeon had to clean up a significant tumor not killed by the chemotherapy; and Zaidi is 31, an age when cancers tend to be more aggressive.
Those factors pointed to one conclusion. Zaidi, a mother of two, faces a high risk the cancer will return.
Some of the greatest strides in the war against cancer have been made battling tumors in the breast, but recurrence occurs in 20 percent of patients within five years of treatment, typically with fatal results.
Mittendorf, a surgeon-immunologist at M.D. Anderson Cancer Center, has been working on the problem for nearly 15 years. This year, she finished enrollment of a clinical trial - likely the final one required for regulatory approval - that promises a better outcome.
Related
Nurse Beena Varghese greets Bruce Campbell during his appointment at MD Anderson Cancer Center last month. Campbell, who has Stage 4 lung cancer, has been receiving immunotherapy for the last year and a half. His oncologist, John Heymach, below, proposed the treatment.
Patients clamor to get into trials for immunotherapy, the new
Jill Stuckey places "Jimmy Carter for Cancer Survivor" signs in Plains, Ga., Thursday in advance of the former president's return to his hometown.
Carter getting immunotherapy for cancer in his brain
The approach is the same one that conquered many of the 20th-century's worst scourges: vaccination.
Meeting with Zaidi, Mittendorf laid it all out. The vaccine revs up the immune system to seek out and attack rogue tumor cells in the circulation, when they're most vulnerable, before they coalescence into a mass. It causes some itchiness, perhaps a mild headache, but no chemotherapy-like side effects. In research thus far, it has dramatically reduced recurrence in women at high risk.
In one small trial, a precursor to the one in which Zaidi would be enrolled, Mittendorf noted that the vaccine helped lower the recurrence rate to zero.
"Do I think we've cured breast cancer?" Mittendorf said to Zaidi. "I'd like to think that, but the honest answer is 'not yet.' But this strategy definitely warrants further investigation."
***
Vaccines are the original immunotherapy, the field now generating such excitement in cancer care. They're credited with bringing seven major infectious conditions under some degree of control - smallpox, tetanus, diphtheria, yellow fever, whooping cough, polio and measles - and saving an estimated 9 million lives worldwide each year.
Health
New overactive-bladder medication blamed for dry eyes
Too much visceral fat around your organs and fat under your skin can trigger inflammation and increase your risk of everything from depression to cancer.
Get rid of food 'felons' to avoid vicious cycle
Sadaf Zaidi, left, a recently recovered breast cancer patient accepts an invitation by Dr. Elizabeth Mittendorf of M.D. Anderson Cancer Center to participate on the clinical trial of a vaccine that shows promise for preventing the recurrence of breast cancer.
Doctors take shot at keeping breast cancer away
A Houston girl, age 3, is the youngest child ever diagnosed with adult onset diabetes (Type 2).
Case of Houston child, 3, with diabetes highlights increase among
This product image provided by Boehringer Ingelheim Pharmaceuticals shows bottles of Jardiance, a daily pill for Type 2 diabetes. Jardiance sharply reduced chances of dying in diabetic patients at high risk of heart complications, a study shows, making the medication the first shown to lengthen diabetics' lives. (Boehringer Ingelheim Pharmaceuticals via AP)
Study: Type 2 diabetes pill cuts death risk
In cancer, they've been considered a flop.
While traditional vaccines mostly are aimed at preventing disease, cancer research has focused on therapeutic vaccines, which seek to generate an immune response in existing tumors. That premise was supported by lab work that showed vaccines shrank tumors in cell cultures.
Actual patients, however, proved another story. A 2004 review of 1990s clinical trials employing cancer vaccines found a 3 percent response rate. Promising candidates by GlaxoSmithKline, Amgen, Oncothyreon, Ziopharm and Keryx all failed to hold up in trials. It seemed a watershed when the Food and Drug Administration approved the first therapeutic cancer vaccine in 2010 - Provenge, for prostate cancer - but it remains little used, likely because it only extends patients' lives four months and costs more than $100,000. The company that developed it, Dendreon, filed for bankruptcy last year.
"The field was more religion than science," says Dr. George Peoples, Mittendorf's early mentor and one of the high priests. "You either believed or you didn't. There wasn't a lot of supporting data."
Believers now say vaccines are poised to break through, thanks to an improved understanding of the immune system and lessons learned from failures. Dr. Patrick Hwu, M.D. Anderson's head of the Division of Cancer Medicine and associate director of its Center for Cancer Immunology, says vaccines will start changing cancer care within five years.
Click on the arrows above to read a brief history of cancer treatments.
Peoples credits much of the turnaround to Houston scientist Jim Allison, who won a Lasker Award this month for his 1996 discovery of the first of a network of brakes that rein in the immune system and that cancer cells exploit in escaping detection. The discovery partly explains why vaccines provoked a strong immune response but stopped short of killing tumor cells.
Most of the new hope involves combining vaccines with checkpoint inhibitors, the class of drugs Allison pioneered that removes the brakes and unleashes the immune system to attack cancer. The expectation is that the approaches should prove complementary - vaccines triggering a response in cancers where there's not robust enough immune activity for checkpoint inhibitors to help, and those inhibitors taking off the brakes in cancers where vaccines' positive response was thwarted.
The bigger vaccine breakthrough may turn out to be stopping cancer before it gets started.
***
For Mittendorf, the epiphany came in the months following 9/11. A newly minted surgeon paying back an Air Force-funded medical school scholarship, she had been called to Walter Reed Medical Center in Bethesda, Md., to fill positions vacated by Army doctors deployed to Afghanistan. She took care of not just soldiers returning from the Middle East, but lots of breast cancer patients - active duty personnel, retirees and family members.
Among them was one woman whose breast cancer recurred 15 years after she was told she was cured. The cancer returned in her bone and eventually spread to her liver.
"It didn't make sense, I remember thinking," Mittendorf said. "Clearly, all the treatment she received at the time worked. What failed, ultimately, was her immune system."
Mittendorf, whose medical education was almost curtailed by a propensity to faint at patients' suffering, resolved to learn all she could about the system's workings. At M.D. Anderson following the stint at Walter Reed, she told her adviser she wanted to focus the research component of her surgical oncology fellowship on immunology. "Immunotherapy's never going to work. I won't let you do that," he told her, and put her in a lab studying molecular biology.
Mittendorf learned from the experience, but after the fellowship, she wasted no time getting back on the immunotherapy path. Now a full-time surgeon at M.D. Anderson, she simultaneously pursued a Ph.D. in immunology, naively thinking, "How hard could it be?"
Seven years later, she's one of only a handful of scientists in the world with such dual expertise, which she says provides a nice balance - the surgeon's need to quickly fix problems fits her lack of patience, while the immunologist's appreciation of long-term responses has prepared her for the decades-long battle ahead.
Throughout the fellowship and doctoral program, Mittendorf's mind focused on vaccines for breast cancer. The only trial in patients whose disease had spread from the breast to other organs had failed, no surprise to Mittendorf, who figured vaccines by themselves were no match for cancers in advanced stages.
Under Peoples' influence at Walter Reed, Mittendorf bought into the idea that vaccines would best work to prevent cancer. A West Point graduate who recently retired from Brooke Army Medical Center in San Antonio, Peoples in 2001 launched the first clinical trial testing whether a vaccine could prevent recurrence of breast tumors, known as "secondary" cancer prevention.
In the next five years, he and Mittendorf accumulated data from about 200 patients proving the radical idea worked.
The data showed a recurrence rate of 20 percent in those who got a placebo, 10 percent in those who got the vaccine and 5 percent in those who got the vaccine's optimal dose. In a smaller trial, no breast cancer recurred in 60 patients who got the vaccine and another drug.
Mittendorf shared the statistics with her newest patient, and Zaidi calmly told the doctor, "I hope I don't get the placebo."
***
In the mind of M.D. Anderson's Hwu, vaccines' past hardships are like the Wright Brothers' early efforts to fly - the trial and error that accompanies the development of any new technology. No one considers that first 12-second flight a failure.
One problem was that the old vaccines weren't designed to biologically resemble a virus, a fundamental ingredient to triggering a robust immune response. For another, they used a particular mineral oil that lured T cells to attack it rather than the cancer, according to a recent study.
Though it never used that mineral oil, Mittendorf's vaccine is somewhat old-school and likely to be tweaked in the future. It's a simple blend of an immune system stimulant and a tumor protein compound, HER2, present, to some extent, in 75 percent to 80 percent of breast cancers.
The vaccine, known as NeuVax, also has potential against ovarian, prostate, pancreatic, colon, bladder and gastric cancers because the protein is common to those tumors, too. In Mittendorf's trials, the vaccine reduced recurrence in women who had both high and low levels of HER2.
Mittendorf's vaccine is the furthest along in testing, but numerous institutions are working with vaccines.
The University of Pittsburgh has published promising results from an early-stage trial of a colon cancer vaccine. Roswell Park Cancer Institute in Buffalo, N.Y., recently agreed to study a lung cancer vaccine developed in Cuba. Bristol-Myers Squibb this year struck a licensing deal that will pay almost $1 billion for a prostate cancer vaccine initially thought to have failed but now showing long-term signs of effectiveness.
At the University of Pennsylvania, whose Dr. Robert Vonderheide says it's now "OK to say the V word," researchers are working with a vaccine targeting an enzyme overly active in the vast majority of cancers, a potential "universal" vaccine. They recently launched an early-stage trial trying to prevent recurrence in cancers of the pancreas, lung and breast.
No one has as much going on as Mittendorf. She has eight breast cancer vaccine trials underway or in planning, foremost among them the one expected to culminate in FDA approval, an international trial of more than 700 patients.
They also include combination trials: two with the drug Herceptin, which is used to treat and prevent recurrence in HER2-positive breast cancers, and a planned one with a checkpoint inhibitor in advanced-stage triple negative breast cancer, a particularly lethal form of the disease that resists most treatment.
Mittendorf is also about to launch research that could bring vaccines closer to "primary prevention:" a trial of patients with precancerous lesions in the breast. The condition, known as Stage 0 and involving abnormal cells confined to the milk ducts, is diagnosed in 60,000 American women annually.
"If these preventive vaccine trials work, researchers in every kind of cancer will jump into the field," says Dr. Elizabeth Jaffee, an oncologist and immunologist at Johns Hopkins.

For nearly 15 years, Mittendorf has focused both her clinical and laboratory efforts on the study of breast cancer with a specific interest in breast cancer immunotherapy.
After so many years in the trenches - one colleague refers to her as being a disciple of "immunotherapy before immunotherapy was cool" - Mittendorf can now make out a finish line, even if results of her latest-phase trial won't be available until 2018.
At that point, she can seek FDA approval for NeuVax. The epitome of the dispassionate scientist, she draws back from a question about whether such approval might be emotional for her, but acknowledges it will be a "tremendous advance," given that roughly 40,000 American women annually die of breast cancer.
A college soccer player and marathon runner, Mittendorf said any celebration will probably be short-lived. Approval of NeuVax will mark a starting point, she said, the opportunity to "run fast in a new direction." She calls the overall cancer vaccine effort an ultra-marathon.
It is not a race without challenges. One thing that facilitated breakthroughs in melanoma and lung cancer, the checkpoint inhibitor drugs recently approved, was that there was so little competing treatment. But many therapies are effective for breast cancer, so researchers will have to identify which immunotherapy benefits which population of patients at which stage of disease.
The process is likely to require a dizzying number of clinical trials and result in pharmaceutical companies charging more for the finished product, already a huge issue in cancer treatment.
It also will tax researchers' ability to design vaccines that remain a step ahead of tumors, given cancer's ability to evolve coping strategies. "There's a saying," said Mittendorf, "one dumb tumor is smarter than 10 smart oncologists."
Still, buoyed by immunotherapy's wave of success and newfound interest from the National Cancer Institute, Mittendorf and other researchers are thinking big. They envision a day when cancer vaccines are truly preventive, just like those used in infectious disease.
Zaidi, fully aware her stage 3C breast cancer is "a big deal," needed no persuasion. She seemed at peace at her first vaccination appointment this month, still anxious only about whether she was assigned to the vaccine or the placebo. It'll be 18 months before she finds out.
"I know the vaccine will work," said Zaidi, who completed medical school in Pakistan and currently works in medical research. "It just makes sense."
http://www.houstonchronicle.com/news/health/article/Doctors-…
Naja, wenn nichts anderes gehaltvolles ...
Doctors take shot at keeping breast cancer away
Promise shown in preventing return of tumors

...dann endlich mal ein sympathisches Bild von Beth Mittendorf (sie schaut meist so 'grimmig')

Doctors take shot at keeping breast cancer away
Promise shown in preventing return of tumors

...dann endlich mal ein sympathisches Bild von Beth Mittendorf (sie schaut meist so 'grimmig')

Antwort auf Beitrag Nr.: 50.635.731 von tancho am 16.09.15 12:33:28eigentlich bin ich hier nur dabei...weil du mal wieder wie immer das richtige näschen hast und in die rakete eingestiegen bist, als sie noch am boden stand...
o.k. vielleicht auch nicht ... sondern eher wegen solchen möglichen perspektiven:
http://www.houstonchronicle.com/news/health/article/Doctors-…
grüße ice_05
o.k. vielleicht auch nicht ... sondern eher wegen solchen möglichen perspektiven:
http://www.houstonchronicle.com/news/health/article/Doctors-…
grüße ice_05
abwärts ist relaiv!

Antwort auf Beitrag Nr.: 50.635.731 von tancho am 16.09.15 12:33:28jetzt wo Du das sagst gehts abwärts 

ist meistens so wenn ich dabei bin

If the trend is really your friend ...
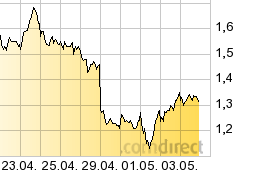
...whats this?

...whats this?


Antwort auf Beitrag Nr.: 50.629.600 von hardliner1A am 15.09.15 15:31:07Hi Hardliner, Augenmerk auf Gale-301 und die ESMO werfen
da bin ich voll & ganz bei dir ...
Vorallem bzgl. ESMO (european footprint & wieder ist man Roche in der Schweiz ganz nah bei , ich frag mich nur ob Schwartz gedenkt (Höchstpersönlich) hier in Erscheinung zu treten
, ich frag mich nur ob Schwartz gedenkt (Höchstpersönlich) hier in Erscheinung zu treten ...wäre -meines Erachtens -ein ganz starken Signalwirkung das positive Zwischendata demnächst im Anmarsch sei
...wäre -meines Erachtens -ein ganz starken Signalwirkung das positive Zwischendata demnächst im Anmarsch sei
da bin ich voll & ganz bei dir ...

Vorallem bzgl. ESMO (european footprint & wieder ist man Roche in der Schweiz ganz nah bei
 , ich frag mich nur ob Schwartz gedenkt (Höchstpersönlich) hier in Erscheinung zu treten
, ich frag mich nur ob Schwartz gedenkt (Höchstpersönlich) hier in Erscheinung zu treten ...wäre -meines Erachtens -ein ganz starken Signalwirkung das positive Zwischendata demnächst im Anmarsch sei
...wäre -meines Erachtens -ein ganz starken Signalwirkung das positive Zwischendata demnächst im Anmarsch sei




















