Seattle Genetics (WKN 602322): Chance zum Einstieg? - 500 Beiträge pro Seite
eröffnet am 13.10.09 08:43:54 von
neuester Beitrag 16.08.17 15:40:27 von
neuester Beitrag 16.08.17 15:40:27 von
Beiträge: 219
ID: 1.153.620
ID: 1.153.620
Aufrufe heute: 0
Gesamt: 14.077
Gesamt: 14.077
Aktive User: 0
ISIN: US81181C1045 · WKN: A2QFAQ · Symbol: SGT
203,20
EUR
-0,39 %
-0,80 EUR
Letzter Kurs 03.11.23 Quotrix Düsseldorf
Neuigkeiten
06.02.24 · dpa-AFX |
05.02.24 · dpa-AFX |
05.02.24 · dpa-AFX |
13.12.23 · dpa-AFX |
13.12.23 · dpa-AFX |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,2500 | +92,01 | |
| 0,8905 | +74,51 | |
| 1,2099 | +34,52 | |
| 0,9500 | +33,67 | |
| 2,6802 | +27,02 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 4,3700 | -14,81 | |
| 1,6100 | -15,26 | |
| 7,9300 | -15,91 | |
| 13,010 | -19,94 | |
| 3,5000 | -26,24 |
Moin,

da ich nur noch kleine, historische Threads finden konnte (z.B. von asics01), hier ein Neuanfang für interessierte:
Seattle Genetics entwickelt in Zusammenarbeit mit diversen "BigPharmas" Medikamente auf Antikörperbasis. Besonders interessant ist dabei eine eigene Konjugattechnik.
Der Kurs konnte sich vom Tief Anfang März (ca. 7$) gut verdoppeln. wobei ein Ausbruch zu den Halbjahreszahlen zusammen mit Daten am 23. Juli erfolgte.
Der Kurs stieg bis auf ca. 15$, stürzte jedoch kürzlich nach Mitteilung der Einstellung einer PIIb-Studie (SGN40 = Dacetuzumab in Kombination mit Rituxan) bis auf unter 10$ ab.
Sehe hier eine Einstiegschance (habe auch eine erste Posi).
Kurs kann freilich auch das Gap vom Juli schliessen und ca. 9$ testen.
Meinungen?
Grüße q.

da ich nur noch kleine, historische Threads finden konnte (z.B. von asics01), hier ein Neuanfang für interessierte:
Seattle Genetics entwickelt in Zusammenarbeit mit diversen "BigPharmas" Medikamente auf Antikörperbasis. Besonders interessant ist dabei eine eigene Konjugattechnik.
Der Kurs konnte sich vom Tief Anfang März (ca. 7$) gut verdoppeln. wobei ein Ausbruch zu den Halbjahreszahlen zusammen mit Daten am 23. Juli erfolgte.
Der Kurs stieg bis auf ca. 15$, stürzte jedoch kürzlich nach Mitteilung der Einstellung einer PIIb-Studie (SGN40 = Dacetuzumab in Kombination mit Rituxan) bis auf unter 10$ ab.
Sehe hier eine Einstiegschance (habe auch eine erste Posi).
Kurs kann freilich auch das Gap vom Juli schliessen und ca. 9$ testen.
Meinungen?
Grüße q.
Hallo,

gleich schon Nachrichten heute (durchaus gute, wie ich meine).
Erfahrener Ex-Genentech-Mann jetzt bei SGEN für geplante Vermarktung von brentuximab (vedotin) 2011.
Hier der Text:
Seattle Genetics Names Head of Commercial and Announces Management Promotion
BOTHELL, Wash.--(BUSINESS WIRE)--Oct. 13, 2009-- Seattle Genetics, Inc. (Nasdaq: SGEN) today announced the appointment of Bruce J. Seeley as Executive Vice President, Commercial. Mr. Seeley brings more than 18 years of experience in oncology drug marketing and sales to the company, most recently from Genentech BioOncology. In addition, the company announced the promotion of Kirk D. Schumacher to Vice President, Legal Affairs and Compliance, and General Counsel.
“Bruce will lead our efforts to build Seattle Genetics’ marketing and sales functions as we position the company for commercial operations, including the planned 2011 product launch of brentuximab vedotin (SGN-35),” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “His depth of experience in the commercialization of oncology drugs, including two of the most important therapies for the treatment of cancer, Herceptin® and Taxotere®, will be a tremendous asset to the company. Kirk has been instrumental at Seattle Genetics, including his contributions to several strategic collaborations and equity financings, and his oversight of our legal affairs. Bruce and Kirk will play key leadership roles as Seattle Genetics continues to execute on its corporate goals.”
Bruce Seeley previously worked at Genentech (a wholly owned member of the Roche Group), most recently as Senior Director for Herceptin (trastuzumab) and T-DM1 marketing. His responsibilities included development and implementation of marketing and launch strategies, including product differentiation, pricing, distribution, medical education and promotion. Before that, Mr. Seeley spent four years at Aventis Pharma in increasing roles of responsibility, including Head of New Product Commercialization and Licensing, Global Marketing, Oncology. While at Aventis, he managed the global marketing activities for Taxotere (docetaxel). Mr. Seeley also held various marketing and sales positions at Rhone-Poulenc Rorer and Bristol-Myers Squibb. He received a Bachelor of Arts in Sociology from the University of California at Los Angeles.
Kirk Schumacher joined Seattle Genetics in October 2003, serving most recently as General Counsel. Since joining the company, he has participated in the negotiation and completion of multiple corporate alliances and equity financings, first as outside legal advisor and then as internal corporate counsel. Prior to joining the company, Mr. Schumacher was with the law firms of Venture Law Group and Riddell Williams. He received his J.D. from Columbia Law School and a B.A. from the University of Wisconsin.

gleich schon Nachrichten heute (durchaus gute, wie ich meine).
Erfahrener Ex-Genentech-Mann jetzt bei SGEN für geplante Vermarktung von brentuximab (vedotin) 2011.
Hier der Text:
Seattle Genetics Names Head of Commercial and Announces Management Promotion
BOTHELL, Wash.--(BUSINESS WIRE)--Oct. 13, 2009-- Seattle Genetics, Inc. (Nasdaq: SGEN) today announced the appointment of Bruce J. Seeley as Executive Vice President, Commercial. Mr. Seeley brings more than 18 years of experience in oncology drug marketing and sales to the company, most recently from Genentech BioOncology. In addition, the company announced the promotion of Kirk D. Schumacher to Vice President, Legal Affairs and Compliance, and General Counsel.
“Bruce will lead our efforts to build Seattle Genetics’ marketing and sales functions as we position the company for commercial operations, including the planned 2011 product launch of brentuximab vedotin (SGN-35),” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “His depth of experience in the commercialization of oncology drugs, including two of the most important therapies for the treatment of cancer, Herceptin® and Taxotere®, will be a tremendous asset to the company. Kirk has been instrumental at Seattle Genetics, including his contributions to several strategic collaborations and equity financings, and his oversight of our legal affairs. Bruce and Kirk will play key leadership roles as Seattle Genetics continues to execute on its corporate goals.”
Bruce Seeley previously worked at Genentech (a wholly owned member of the Roche Group), most recently as Senior Director for Herceptin (trastuzumab) and T-DM1 marketing. His responsibilities included development and implementation of marketing and launch strategies, including product differentiation, pricing, distribution, medical education and promotion. Before that, Mr. Seeley spent four years at Aventis Pharma in increasing roles of responsibility, including Head of New Product Commercialization and Licensing, Global Marketing, Oncology. While at Aventis, he managed the global marketing activities for Taxotere (docetaxel). Mr. Seeley also held various marketing and sales positions at Rhone-Poulenc Rorer and Bristol-Myers Squibb. He received a Bachelor of Arts in Sociology from the University of California at Los Angeles.
Kirk Schumacher joined Seattle Genetics in October 2003, serving most recently as General Counsel. Since joining the company, he has participated in the negotiation and completion of multiple corporate alliances and equity financings, first as outside legal advisor and then as internal corporate counsel. Prior to joining the company, Mr. Schumacher was with the law firms of Venture Law Group and Riddell Williams. He received his J.D. from Columbia Law School and a B.A. from the University of Wisconsin.
Moin,

Analysten (Leerink Swann): "Market Perform":
(leider nur als Textauszug)
Beurteilen SGEN als gute Firma, unklar aber Kaufzeitpunkt. Das wird erst die Zukunft zeigen können.

Gruß q.
Leerink Swann initiates coverage on Seattle Genetics (Nasdaq: SGEN) with a Market Perform rating. Price target $12.
Leerink analyst says, "Seattle Genetics is a development-stage biotechnology company founded on the antibody drug conjugate (ADC) technology that, in our view, is an important and validated platform, and has three ADC- or antibody-based agents in mid- to late-stage development for hematological malignancies...SGN-35 looks to be a highly active agent for relapsed and refractory Hodgkin's lymphoma (HL) and has generated high enthusiasm among MEDACorp consultants we spoke to. Positive considerations in our view include consistent results by independent review, and rapid enrollment of the pivotal Phase II trial, which typically indicates high investigator and patient interest. Recent early termination of a Phase II trial of dacetuzumab (SGN-40) for non-Hodgkin's lymphoma appears to dim its prospects and reduce the potential upside of the stock. There are clear signals of activity in highly durable responses in Phase I, but the rate of response looks to be inadequate. The early data on patient selection based on gene signature are encouraging, but there may need to be a greater degree of enrichment, and we await further data to ascertain whether the selection scheme is reliable and practical...Key to the stock's near-term outlook could be randomized Phase IIb data for lintuzumab (SGN-33) in acute myeloid leukemia in 1H:10...We believe SGEN is a good company with solid prospects of becoming one of the limited number of commercial-stage biotech companies. To us, it is a matter of when is the best time to buy the stock."
www.streetinsider.com/New+Coverage/Leeri...

Analysten (Leerink Swann): "Market Perform":
(leider nur als Textauszug)
Beurteilen SGEN als gute Firma, unklar aber Kaufzeitpunkt. Das wird erst die Zukunft zeigen können.

Gruß q.
Leerink Swann initiates coverage on Seattle Genetics (Nasdaq: SGEN) with a Market Perform rating. Price target $12.
Leerink analyst says, "Seattle Genetics is a development-stage biotechnology company founded on the antibody drug conjugate (ADC) technology that, in our view, is an important and validated platform, and has three ADC- or antibody-based agents in mid- to late-stage development for hematological malignancies...SGN-35 looks to be a highly active agent for relapsed and refractory Hodgkin's lymphoma (HL) and has generated high enthusiasm among MEDACorp consultants we spoke to. Positive considerations in our view include consistent results by independent review, and rapid enrollment of the pivotal Phase II trial, which typically indicates high investigator and patient interest. Recent early termination of a Phase II trial of dacetuzumab (SGN-40) for non-Hodgkin's lymphoma appears to dim its prospects and reduce the potential upside of the stock. There are clear signals of activity in highly durable responses in Phase I, but the rate of response looks to be inadequate. The early data on patient selection based on gene signature are encouraging, but there may need to be a greater degree of enrichment, and we await further data to ascertain whether the selection scheme is reliable and practical...Key to the stock's near-term outlook could be randomized Phase IIb data for lintuzumab (SGN-33) in acute myeloid leukemia in 1H:10...We believe SGEN is a good company with solid prospects of becoming one of the limited number of commercial-stage biotech companies. To us, it is a matter of when is the best time to buy the stock."
www.streetinsider.com/New+Coverage/Leeri...
Und dies hier auch gleich noch:
(Müssen ja irgendwie ein paar Beiträge zusammenkommen)

Nächste Woche Q3-Ergebnisse und conference call. Dabei vielleicht auch ein paar Infos zu den laufenden Projekten...
Seattle Genetics to Host Conference Call and Webcast Discussion of Third Quarter 2009 Financial Results on October 22, 2009
Press Release
Source: Seattle Genetics, Inc.
On 9:00 am EDT, Thursday October 15, 2009
Buzz up! 0 Print
Companies:Seattle Genetics Inc.
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq:SGEN - News) announced today that it will report its third quarter 2009 financial results on Thursday, October 22, 2009, after the close of financial markets. Following the announcement, company management will host a conference call and webcast discussion of the results and provide a general corporate update. Access to the event can be obtained as follows:
Related Quotes
Symbol Price Change
SGEN 10.29 0.00
{"s" : "sgen","k" : "c10,l10,p20,t10","o" : "","j" : ""} LIVE access on Thursday, October 22, 2009, 2:00 p.m. Pacific Time (PT) / 5:00 p.m. Eastern Time (ET)
Telephone 800-762-8908 (domestic) or 480-629-9774 (international); conference ID 4172548
Webcast available at www.seattlegenetics.com in the Investors and News section
REPLAY access
Telephone replay will be available beginning at approximately 4:00 p.m. PT on October 22, 2009 through 4:00 p.m. PT on October 26, 2009 by calling 800-406-7325 (domestic) or 303-590-3030 (international); conference ID 4172548
Webcast replay will be available on the Seattle Genetics website at www.seattlegenetics.com in the Investors and News section
About Seattle Genetics
Seattle Genetics is a clinical stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company’s lead product candidate, brentuximab vedotin (SGN-35), is in a pivotal trial under a special protocol assessment with the FDA. Brentuximab vedotin is empowered by Seattle Genetics’ proprietary antibody-drug conjugate (ADC) technology comprising highly potent synthetic drugs and stable linkers for attaching the drugs to monoclonal antibodies. In addition, Seattle Genetics has three other product candidates in ongoing clinical trials: lintuzumab (SGN-33), dacetuzumab (SGN-40) and SGN-70. Dacetuzumab is being developed under a worldwide collaboration with Genentech (a wholly-owned member of the Roche Group). Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Genentech, Bayer, CuraGen, Progenics, Daiichi Sankyo, MedImmune, a subsidiary of AstraZeneca, and Millennium: The Takeda Oncology Company, as well as an ADC co-development agreement with Agensys, a subsidiary of Astellas Pharma. More information can be found at www.seattlegenetics.com.
Contact:
Seattle Genetics, Inc.Peggy Pinkston, 425-527-4160ppinkston@seagen.com
(Müssen ja irgendwie ein paar Beiträge zusammenkommen)

Nächste Woche Q3-Ergebnisse und conference call. Dabei vielleicht auch ein paar Infos zu den laufenden Projekten...
Seattle Genetics to Host Conference Call and Webcast Discussion of Third Quarter 2009 Financial Results on October 22, 2009
Press Release
Source: Seattle Genetics, Inc.
On 9:00 am EDT, Thursday October 15, 2009
Buzz up! 0 Print
Companies:Seattle Genetics Inc.
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq:SGEN - News) announced today that it will report its third quarter 2009 financial results on Thursday, October 22, 2009, after the close of financial markets. Following the announcement, company management will host a conference call and webcast discussion of the results and provide a general corporate update. Access to the event can be obtained as follows:
Related Quotes
Symbol Price Change
SGEN 10.29 0.00
{"s" : "sgen","k" : "c10,l10,p20,t10","o" : "","j" : ""} LIVE access on Thursday, October 22, 2009, 2:00 p.m. Pacific Time (PT) / 5:00 p.m. Eastern Time (ET)
Telephone 800-762-8908 (domestic) or 480-629-9774 (international); conference ID 4172548
Webcast available at www.seattlegenetics.com in the Investors and News section
REPLAY access
Telephone replay will be available beginning at approximately 4:00 p.m. PT on October 22, 2009 through 4:00 p.m. PT on October 26, 2009 by calling 800-406-7325 (domestic) or 303-590-3030 (international); conference ID 4172548
Webcast replay will be available on the Seattle Genetics website at www.seattlegenetics.com in the Investors and News section
About Seattle Genetics
Seattle Genetics is a clinical stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company’s lead product candidate, brentuximab vedotin (SGN-35), is in a pivotal trial under a special protocol assessment with the FDA. Brentuximab vedotin is empowered by Seattle Genetics’ proprietary antibody-drug conjugate (ADC) technology comprising highly potent synthetic drugs and stable linkers for attaching the drugs to monoclonal antibodies. In addition, Seattle Genetics has three other product candidates in ongoing clinical trials: lintuzumab (SGN-33), dacetuzumab (SGN-40) and SGN-70. Dacetuzumab is being developed under a worldwide collaboration with Genentech (a wholly-owned member of the Roche Group). Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Genentech, Bayer, CuraGen, Progenics, Daiichi Sankyo, MedImmune, a subsidiary of AstraZeneca, and Millennium: The Takeda Oncology Company, as well as an ADC co-development agreement with Agensys, a subsidiary of Astellas Pharma. More information can be found at www.seattlegenetics.com.
Contact:
Seattle Genetics, Inc.Peggy Pinkston, 425-527-4160ppinkston@seagen.com
Hallo schon wieder,

auch Motley Fool bleiben relativ optimistisch.
Auch mein Hauptkaufargument, dass der Kursverfall übertrieben war und daher eine Gegenreaktion erfolgen sollte.
Hier der Artikel (in Gänze im Yahoo-Board erhältlich), Markierung von mir:
3 Stocks in a Tailspin
By Dave Mock
October 15, 2009 | Comments (0)
Recs
ShareThis SGEN
Seattle Genetics
...
Individual stocks can surge 10%, 25%, or even higher in a short period of time. And they can fall just as far, just as quickly. For example, shares of Digital River (Nasdaq: DRIV) lost more than a third of their value when one of its major customers, Symantec, said it won't extend its contract.
Big drops in share price can sometimes signal material defects or new risks. But at other times, they're simply pullbacks along with the larger pessimism facing the market today. Fortunately, we have Motley Fool CAPS, a great resource to help us understand the larger picture behind big price drops.
Is the sky falling?
CAPS contains more than just the crowd's opinions. Its best-performing members' votes count more in shaping each company's rating than do the picks of their poorer-performing peers. That way, investors can intelligently use the collective wisdom of more than 140,000 CAPS members to make better decisions.
We'll use CAPS' handy stock screening tool to quickly zero in on companies that have been slashed by at least 20% in the last four weeks, and which have a market cap greater than $100 million and a beta of less than 3. If you want to run this screen for yourself, please do -- just keep in mind that the results will update with the market.
Company
CAPS Rating
(out of 5)
4-Week
Price Change
KB Home (NYSE: KBH)
*
(22.7%)
Seattle Genetics (Nasdaq: SGEN)
***
(30.7%)
Xerox (NYSE: XRX)
***
(20.1%)
Source: Motley Fool CAPS. Price return Sept. 18 through Oct. 13.
KB Home
In addition to the weak earnings report from homebuilder KB Home that echoed the struggling quarters of peers Hovnanian (NYSE: HOV) and Lennar, the SEC is looking into possible accounting and disclosure issues at the company. KB has been the focal point of investigations in the past, resulting in the departure of several executives, but the company doesn't believe this investigation will have any material effect on operations. Although there have been some signs of optimism in the business and KB expects to generate positive cash flow from operations this year, it doesn't expect any meaningful improvement in U.S. housing in the near future, and expects an uneven recovery. Regardless of how operations are going inside the company, many investors are weary of the housing market in general; as such, nearly 61% of the 1,200 CAPS members rating KB Home expect it to underperform the market.
Seattle Genetics
Clinical-stage biotech Seattle Genetics' shares have been on the retreat since it abruptly ended a phase-two trial for its cancer drug dacetuzumab, being developed with Roche's Genentech, as an interim analysis of that trial determined that the drug would likely fail to meet its goal. The company still has four ongoing trials with the drug and a couple of other trials -- including one of its lead product, SGN-35 -- that are expected to report data next year, giving investors hope that one of its many opportunities will pan out. Today, 92% of the 294 CAPS members rating Seattle Genetics see it beating the broader market.Xerox
Document processing company Xerox hopes to triple its annual revenue from services and looks for annualized cost savings of $300 million to $400 million in the first three years with its recently announced plans to buy Affiliated Computer Services. While large shareholder BlackRock recently disclosed that it's cut its stake in Xerox by a third since the beginning of this year, partially mitigating the hit shares have taken recently, some CAPS members say they like the deal. The combination is similar to recent moves by others like Dell (Nasdaq: DELL) and Hewlett-Packard (NYSE: HPQ) to diversify in the technology services sector, but some investors are concerned with the integration process and resulting share dilution. As such, only a lukewarm 84% of the 401 CAPS members rating Xerox are bullish and believe there's a good chance to score big gains on Xerox.
...

auch Motley Fool bleiben relativ optimistisch.
Auch mein Hauptkaufargument, dass der Kursverfall übertrieben war und daher eine Gegenreaktion erfolgen sollte.
Hier der Artikel (in Gänze im Yahoo-Board erhältlich), Markierung von mir:
3 Stocks in a Tailspin
By Dave Mock
October 15, 2009 | Comments (0)
Recs
ShareThis SGEN
Seattle Genetics
...
Individual stocks can surge 10%, 25%, or even higher in a short period of time. And they can fall just as far, just as quickly. For example, shares of Digital River (Nasdaq: DRIV) lost more than a third of their value when one of its major customers, Symantec, said it won't extend its contract.
Big drops in share price can sometimes signal material defects or new risks. But at other times, they're simply pullbacks along with the larger pessimism facing the market today. Fortunately, we have Motley Fool CAPS, a great resource to help us understand the larger picture behind big price drops.
Is the sky falling?
CAPS contains more than just the crowd's opinions. Its best-performing members' votes count more in shaping each company's rating than do the picks of their poorer-performing peers. That way, investors can intelligently use the collective wisdom of more than 140,000 CAPS members to make better decisions.
We'll use CAPS' handy stock screening tool to quickly zero in on companies that have been slashed by at least 20% in the last four weeks, and which have a market cap greater than $100 million and a beta of less than 3. If you want to run this screen for yourself, please do -- just keep in mind that the results will update with the market.
Company
CAPS Rating
(out of 5)
4-Week
Price Change
KB Home (NYSE: KBH)
*
(22.7%)
Seattle Genetics (Nasdaq: SGEN)
***
(30.7%)
Xerox (NYSE: XRX)
***
(20.1%)
Source: Motley Fool CAPS. Price return Sept. 18 through Oct. 13.
KB Home
In addition to the weak earnings report from homebuilder KB Home that echoed the struggling quarters of peers Hovnanian (NYSE: HOV) and Lennar, the SEC is looking into possible accounting and disclosure issues at the company. KB has been the focal point of investigations in the past, resulting in the departure of several executives, but the company doesn't believe this investigation will have any material effect on operations. Although there have been some signs of optimism in the business and KB expects to generate positive cash flow from operations this year, it doesn't expect any meaningful improvement in U.S. housing in the near future, and expects an uneven recovery. Regardless of how operations are going inside the company, many investors are weary of the housing market in general; as such, nearly 61% of the 1,200 CAPS members rating KB Home expect it to underperform the market.
Seattle Genetics
Clinical-stage biotech Seattle Genetics' shares have been on the retreat since it abruptly ended a phase-two trial for its cancer drug dacetuzumab, being developed with Roche's Genentech, as an interim analysis of that trial determined that the drug would likely fail to meet its goal. The company still has four ongoing trials with the drug and a couple of other trials -- including one of its lead product, SGN-35 -- that are expected to report data next year, giving investors hope that one of its many opportunities will pan out. Today, 92% of the 294 CAPS members rating Seattle Genetics see it beating the broader market.Xerox
Document processing company Xerox hopes to triple its annual revenue from services and looks for annualized cost savings of $300 million to $400 million in the first three years with its recently announced plans to buy Affiliated Computer Services. While large shareholder BlackRock recently disclosed that it's cut its stake in Xerox by a third since the beginning of this year, partially mitigating the hit shares have taken recently, some CAPS members say they like the deal. The combination is similar to recent moves by others like Dell (Nasdaq: DELL) and Hewlett-Packard (NYSE: HPQ) to diversify in the technology services sector, but some investors are concerned with the integration process and resulting share dilution. As such, only a lukewarm 84% of the 401 CAPS members rating Xerox are bullish and believe there's a good chance to score big gains on Xerox.
...
Moin,

in der Tat musste das Gap wohl erst geschlossen werden...
aber...

es geht auch schon wieder in die andere Richtung.
Demnächst findet das ASH-Meeting statt (American Society of Haematology), hier die SGEN betreffenden Beiträge als Abstracts, das sollte interessant werden und könnte auch Kursimpulse liefern!
http://ash.confex.com/ash/2009/webprogra...
http://ash.confex.com/ash/2009/webprogra...
1.
Paper: Dacetuzumab (SGN-40), Lenalidomide, and Weekly Dexamethasone in Relapsed or Refractory Multiple Myeloma: Multiple Responses Observed in a Phase 1b Study
2.
Paper: The Antibody-Drug Conjugate Brentuximab Vedotin (SGN-35) Induced Multiple Objective Responses in Patients with Relapsed or Refractory CD30-Positive Lymphomas in a Phase 1 Weekly Dosing Study
3.
Paper: CD40 Pathway Activation Status Predicts Response to CD40 Targeted Therapy in Diffuse Large b-Cell Lymphoma
... trials. Using baseline gene expression profiling, we found that the in vitro activity of an antibody that stimulates the CD40 pathway, Dacetuzumab (SGN-40), correlated strikingly with a transcriptional profile that was representative of an inactive CD40 pathway. Resistant cells, on the other hand, displayed ...
4.
Paper: A Phase 1b Clinical Trial of Dacetuzumab in Combination with Rituximab and Gemcitabine: Multiple Responses Observed in Patients with Relapsed Diffuse Large B-Cell Lymphoma
... of Colorado, Aurora, CO 7Seattle Genetics, Inc., Bothell, WA 8Memorial Sloan-Kettering Cancer Center, New York, NY Background: Dacetuzumab (SGN-40), a humanized monoclonal antibody that targets CD40, mediates antitumor activity through multiple mechanisms of action, including effector cell functions ...
5.
Paper: Evaluation of Alternative, \"Low-intensity\" Induction Regimens in Elderly Adults with Acute Myeloid Leukemia (AML)
... ; either a standard ara-C-anthracycline regimen (n=38) or a second non-intensive regimen (decitabine +/- gemtuzumab ozogamicin, low-dose ara-c, SGN-33,) (n=34). Overall 25/72 (35%) achieved a CR with salvage therapy; 16/38 CR’s (42%) with standard induction and 9/34 CR’s (26%) with ...
6.
Paper: Novel Targeted Therapy for Relapsed Hodgkin Lymphoma
... ) Anas Younes, MD Department of Lymphoma/Myeloma, The University of Texas M. D. Anderson Cancer Center, Houston, TX Disclosures: Off Label Use: SGN-35, HDAC inhibitors, Xmab2513. See more of: Hodgkin Lymphoma See more of: Education Program << Previous Presentation | Next Presentation
7.
51st ASH Annual Meeting and Exposition (December 5-8, 2009): Lymphoma: Therapy with Biologic Agents, excluding Pre-Clinical Models Poster II
>
8.
51st ASH Annual Meeting and Exposition (December 5-8, 2009): Myeloma - Therapy, excluding Transplantation Poster II
... College Medical School, London, United Kingdom 7Clinical Trials Research Unit, University of Leeds, Leeds, United Kingdom 2870 Dacetuzumab (SGN-40), Lenalidomide, and Weekly Dexamethasone in Relapsed or Refractory Multiple Myeloma: Multiple Responses Observed in a Phase 1b Study Edward Agura, ...

in der Tat musste das Gap wohl erst geschlossen werden...
aber...

es geht auch schon wieder in die andere Richtung.
Demnächst findet das ASH-Meeting statt (American Society of Haematology), hier die SGEN betreffenden Beiträge als Abstracts, das sollte interessant werden und könnte auch Kursimpulse liefern!
http://ash.confex.com/ash/2009/webprogra...
http://ash.confex.com/ash/2009/webprogra...
1.
Paper: Dacetuzumab (SGN-40), Lenalidomide, and Weekly Dexamethasone in Relapsed or Refractory Multiple Myeloma: Multiple Responses Observed in a Phase 1b Study
2.
Paper: The Antibody-Drug Conjugate Brentuximab Vedotin (SGN-35) Induced Multiple Objective Responses in Patients with Relapsed or Refractory CD30-Positive Lymphomas in a Phase 1 Weekly Dosing Study
3.
Paper: CD40 Pathway Activation Status Predicts Response to CD40 Targeted Therapy in Diffuse Large b-Cell Lymphoma
... trials. Using baseline gene expression profiling, we found that the in vitro activity of an antibody that stimulates the CD40 pathway, Dacetuzumab (SGN-40), correlated strikingly with a transcriptional profile that was representative of an inactive CD40 pathway. Resistant cells, on the other hand, displayed ...
4.
Paper: A Phase 1b Clinical Trial of Dacetuzumab in Combination with Rituximab and Gemcitabine: Multiple Responses Observed in Patients with Relapsed Diffuse Large B-Cell Lymphoma
... of Colorado, Aurora, CO 7Seattle Genetics, Inc., Bothell, WA 8Memorial Sloan-Kettering Cancer Center, New York, NY Background: Dacetuzumab (SGN-40), a humanized monoclonal antibody that targets CD40, mediates antitumor activity through multiple mechanisms of action, including effector cell functions ...
5.
Paper: Evaluation of Alternative, \"Low-intensity\" Induction Regimens in Elderly Adults with Acute Myeloid Leukemia (AML)
... ; either a standard ara-C-anthracycline regimen (n=38) or a second non-intensive regimen (decitabine +/- gemtuzumab ozogamicin, low-dose ara-c, SGN-33,) (n=34). Overall 25/72 (35%) achieved a CR with salvage therapy; 16/38 CR’s (42%) with standard induction and 9/34 CR’s (26%) with ...
6.
Paper: Novel Targeted Therapy for Relapsed Hodgkin Lymphoma
... ) Anas Younes, MD Department of Lymphoma/Myeloma, The University of Texas M. D. Anderson Cancer Center, Houston, TX Disclosures: Off Label Use: SGN-35, HDAC inhibitors, Xmab2513. See more of: Hodgkin Lymphoma See more of: Education Program << Previous Presentation | Next Presentation
7.
51st ASH Annual Meeting and Exposition (December 5-8, 2009): Lymphoma: Therapy with Biologic Agents, excluding Pre-Clinical Models Poster II
>
8.
51st ASH Annual Meeting and Exposition (December 5-8, 2009): Myeloma - Therapy, excluding Transplantation Poster II
... College Medical School, London, United Kingdom 7Clinical Trials Research Unit, University of Leeds, Leeds, United Kingdom 2870 Dacetuzumab (SGN-40), Lenalidomide, and Weekly Dexamethasone in Relapsed or Refractory Multiple Myeloma: Multiple Responses Observed in a Phase 1b Study Edward Agura, ...
Moin,

habe nun doch (leider) nicht bei Schließung des Gaps, also ca. 9$ bzw. 6€ zugekauft...
Da war mir das Gap zu klein (bei fallenden Bios weiß man nie).

SGEN heute mit neuer P1 und sogar deutlicher Kursreaktion:
Seattle Genetics Initiates Phase I Clinical Trial of Antibody-Drug Conjugate SGN-75
Press Release
Source: Seattle Genetics, Inc.
On 9:00 am EST, Monday November 16, 2009
Buzz up! 0 Print
Companies:Seattle Genetics Inc.
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq: SGEN - News) today announced that it has initiated a phase I clinical trial of SGN-75 for metastatic renal cell carcinoma and relapsed and refractory non-Hodgkin lymphoma. SGN-75 is an antibody-drug conjugate (ADC) targeting CD70 that utilizes the company’s proprietary technology.
Related Quotes
Symbol Price Change
SGEN 9.76 +0.39
{"s" : "sgen","k" : "c10,l10,p20,t10","o" : "","j" : ""} “Our compelling preclinical data demonstrate that SGN-75 possesses potent antitumor activity in models of both CD70-positive renal cell carcinoma and hematologic malignancies,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “CD70 is expressed on a variety of solid tumors, including renal cell carcinoma, pancreatic, ovarian and lung cancers and glioblastoma as well as multiple myeloma and several types of lymphoma. This broad expression profile provides substantial and diverse therapeutic opportunities to address unmet needs for patients with these malignancies.”
The single-agent phase I study is designed to enroll up to 80 patients at multiple centers in the United States. The trial will evaluate the safety, tolerability, pharmacokinetic profile and antitumor activity of SGN-75 in order to identify a dose and schedule for future clinical trials.
SGN-75 is an ADC comprising an anti-CD70 antibody attached to a potent, synthetic drug payload, monomethyl auristatin F (MMAF), using Seattle Genetics’ proprietary technology. The ADC is designed to be stable in the bloodstream, but to release its payload upon internalization into CD70-expressing tumor cells, resulting in targeted cell-killing.
About Non-Hodgkin Lymphoma
Non-Hodgkin lymphoma represents a diverse group of cancers that develop in the lymphatic system and are characterized by uncontrolled growth and accumulation of abnormal lymphocytes. Lymphocytes are white blood cells that are responsible for defending the body against infection. The most common forms of non-Hodgkin lymphoma are follicular and diffuse large B-cell lymphoma. According to the American Cancer Society, approximately 66,000 cases of non-Hodgkin lymphoma are expected to be diagnosed in the United States during 2009 and approximately 19,500 patients will die from the disease.
About Renal Cell Carcinoma
Renal cell carcinoma (RCC) forms in the kidney, which filters and cleans the blood. Metastatic RCC occurs when the cancer has spread to other parts of the body. RCC is the most common type of kidney cancer in adults, representing approximately 90 percent of cases. The American Cancer Society estimates that there will be more than 57,700 new cases of kidney cancer in the United States during 2009, and about 13,000 people will die from the disease.
About Seattle Genetics
Seattle Genetics is a clinical stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company’s lead product candidate, brentuximab vedotin (SGN-35), is in a pivotal trial under a special protocol assessment with the FDA. Brentuximab vedotin is empowered by Seattle Genetics’ proprietary ADC technology comprising highly potent synthetic drugs and stable linkers for attaching the drugs to monoclonal antibodies. In addition, Seattle Genetics has four other product candidates in ongoing clinical trials: lintuzumab (SGN-33), dacetuzumab (SGN-40), SGN-70 and SGN-75. Dacetuzumab is being developed under a worldwide collaboration with Genentech (a wholly-owned member of the Roche Group). Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Genentech, Bayer, CuraGen, a subsidiary of Celldex Therapeutics, Progenics, Daiichi Sankyo, MedImmune, a subsidiary of AstraZeneca, and Millennium: The Takeda Oncology Company, as well as an ADC co-development agreement with Agensys, a subsidiary of Astellas Pharma. More information can be found at www.seattlegenetics.com.
Certain of the statements made in this press release are forward looking, such as those, among others, relating to the therapeutic potential of SGN-75. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include the inability to show sufficient safety in this phase I clinical trial and the risk of adverse clinical results as SGN-75 moves into and advances in clinical trials. More information about the risks and uncertainties faced by Seattle Genetics is contained in the company’s 10-Q for the quarter ended September 30, 2009 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact:
Seattle Genetics, Inc.Peggy Pinkston, 425-527-4160ppinkston@seagen.com

habe nun doch (leider) nicht bei Schließung des Gaps, also ca. 9$ bzw. 6€ zugekauft...
Da war mir das Gap zu klein (bei fallenden Bios weiß man nie).

SGEN heute mit neuer P1 und sogar deutlicher Kursreaktion:
Seattle Genetics Initiates Phase I Clinical Trial of Antibody-Drug Conjugate SGN-75
Press Release
Source: Seattle Genetics, Inc.
On 9:00 am EST, Monday November 16, 2009
Buzz up! 0 Print
Companies:Seattle Genetics Inc.
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq: SGEN - News) today announced that it has initiated a phase I clinical trial of SGN-75 for metastatic renal cell carcinoma and relapsed and refractory non-Hodgkin lymphoma. SGN-75 is an antibody-drug conjugate (ADC) targeting CD70 that utilizes the company’s proprietary technology.
Related Quotes
Symbol Price Change
SGEN 9.76 +0.39
{"s" : "sgen","k" : "c10,l10,p20,t10","o" : "","j" : ""} “Our compelling preclinical data demonstrate that SGN-75 possesses potent antitumor activity in models of both CD70-positive renal cell carcinoma and hematologic malignancies,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “CD70 is expressed on a variety of solid tumors, including renal cell carcinoma, pancreatic, ovarian and lung cancers and glioblastoma as well as multiple myeloma and several types of lymphoma. This broad expression profile provides substantial and diverse therapeutic opportunities to address unmet needs for patients with these malignancies.”
The single-agent phase I study is designed to enroll up to 80 patients at multiple centers in the United States. The trial will evaluate the safety, tolerability, pharmacokinetic profile and antitumor activity of SGN-75 in order to identify a dose and schedule for future clinical trials.
SGN-75 is an ADC comprising an anti-CD70 antibody attached to a potent, synthetic drug payload, monomethyl auristatin F (MMAF), using Seattle Genetics’ proprietary technology. The ADC is designed to be stable in the bloodstream, but to release its payload upon internalization into CD70-expressing tumor cells, resulting in targeted cell-killing.
About Non-Hodgkin Lymphoma
Non-Hodgkin lymphoma represents a diverse group of cancers that develop in the lymphatic system and are characterized by uncontrolled growth and accumulation of abnormal lymphocytes. Lymphocytes are white blood cells that are responsible for defending the body against infection. The most common forms of non-Hodgkin lymphoma are follicular and diffuse large B-cell lymphoma. According to the American Cancer Society, approximately 66,000 cases of non-Hodgkin lymphoma are expected to be diagnosed in the United States during 2009 and approximately 19,500 patients will die from the disease.
About Renal Cell Carcinoma
Renal cell carcinoma (RCC) forms in the kidney, which filters and cleans the blood. Metastatic RCC occurs when the cancer has spread to other parts of the body. RCC is the most common type of kidney cancer in adults, representing approximately 90 percent of cases. The American Cancer Society estimates that there will be more than 57,700 new cases of kidney cancer in the United States during 2009, and about 13,000 people will die from the disease.
About Seattle Genetics
Seattle Genetics is a clinical stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company’s lead product candidate, brentuximab vedotin (SGN-35), is in a pivotal trial under a special protocol assessment with the FDA. Brentuximab vedotin is empowered by Seattle Genetics’ proprietary ADC technology comprising highly potent synthetic drugs and stable linkers for attaching the drugs to monoclonal antibodies. In addition, Seattle Genetics has four other product candidates in ongoing clinical trials: lintuzumab (SGN-33), dacetuzumab (SGN-40), SGN-70 and SGN-75. Dacetuzumab is being developed under a worldwide collaboration with Genentech (a wholly-owned member of the Roche Group). Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Genentech, Bayer, CuraGen, a subsidiary of Celldex Therapeutics, Progenics, Daiichi Sankyo, MedImmune, a subsidiary of AstraZeneca, and Millennium: The Takeda Oncology Company, as well as an ADC co-development agreement with Agensys, a subsidiary of Astellas Pharma. More information can be found at www.seattlegenetics.com.
Certain of the statements made in this press release are forward looking, such as those, among others, relating to the therapeutic potential of SGN-75. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include the inability to show sufficient safety in this phase I clinical trial and the risk of adverse clinical results as SGN-75 moves into and advances in clinical trials. More information about the risks and uncertainties faced by Seattle Genetics is contained in the company’s 10-Q for the quarter ended September 30, 2009 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact:
Seattle Genetics, Inc.Peggy Pinkston, 425-527-4160ppinkston@seagen.com
Moin,

nun hat auch SGEN seinen "Wunderkrebsmittel"-Bericht, wie neulich auch schon MITI:

Könnte sehr wohl wichtig werden für den Kurs.
Posted on Tuesday, 11.17.09 Recommend (1)share email print comment reprint
UM SYLVESTER COMPREHENSIVE CANCER CENTER
Student meets man who saved his life
A University of Miami student battling a rare disease is in remission thanks to a discovery made by the UM Sylvester Comprehensive Cancer Center.
Buy PhotoCancer patient Steven Guarin, 21, whose cancer is in remission, meets the UM researcher who concocted the experimental drug he is being administered. The cancer is now in remission. Guarin wanted to meet the man who saved his life, Dr. Eckhard Podack, Chairman of Immunology at The Miller School of Medicine at The University of Miami. They are at the Sylvester Comprehensive Cancer Center on November 17, 2009. AL DIAZ / THE MIAMI HERALD STAFF
Photo Similar stories:
•Mystery cure?
Mystery cure?
At first, it didn't sound like the way a modern cancer treatment would be created. As the story went, it was an elixir extracted from the root of a mysterious plant found deep in the Amazon forest in Ecuador, used there for decades against everything from lupus to AIDS.
It was brought to Miami by a Quito businessman who was being treated here for prostate cancer, which eventually killed him.
The Ecuadoran doctor who developed the elixir was trying to patent it, so he was being evasive about the plant and the process by which the extract is made.
•Kennedy waged war on cancer as both lawmaker and patient
Kennedy waged war on cancer as both lawmaker and patient
Like almost no one else, Sen. Edward M. Kennedy embodied the frustrations of America's 40-year war on cancer.
Kennedy strongly supported the idea of a war on cancer, promoting it for months before President Richard M. Nixon announced the battle was to begin in 1971, and advocating for more money than Nixon initially wanted to spend.
And when Kennedy was diagnosed with brain cancer last year, he became one of the millions whose fate was not much changed by the cancer war. Despite billions that have been spent, the death rate from most cancers barely budged.
•Researchers share new findings about cancer
Researchers share new findings about cancer
Hope for a better prostate cancer test, potential new uses for a largely discredited lung cancer drug and a warning for breast cancer patients all emerged from a meeting earlier this month of the American Society of Clinical Oncology in Orlando.
Prostate cancer is the most common form of cancer in American men, with an estimated 186,320 cases diagnosed in 2008. But every year, at least that many men undergo painful and expensive prostate biopsies after routine screening has revealed high levels of prostate-specific antigen, or PSA, and yet the men turn out to not have tumors.
Many critics have charged that the large number of unnecessary biopsies grossly limits the benefits of the PSA test and have called for a halt to its use.
•Acreage residents try to connect dots in cancer cases
Acreage residents try to connect dots in cancer cases
The Acreage, a corner of unincorporated western Palm Beach County, is a rustic patch of dirt roads, canals and horse trails -- and, among some residents, the simmering fear of cancer.
Parents, some of whom had never met, gather in each other's houses in the quiet hours to discuss the cases -- children stricken with cancerous brain tumors or cysts; the trips to Miami Children's Hospital for surgery; the symptoms, sometimes puzzling or misleading, that eventually led to diagnosis. They talk about the sick adults, too, about the toxins they think may be contaminating the community's air, water, soil and peace of mind.
For months, state and county officials have pored over this sprawling community of farms, groves and suburban homes located 15 miles northwest of West Palm Beach to determine whether a cancer cluster -- a greater-than-expected rate of the same cancer -- can be confirmed. The state Department of Health is in the second phase of the study. And, on Thursday, environmental activist Erin Brockovich
•Dr. Howard A. Engle | Pediatrician led anti-tobacco lawsuit
Dr. Howard A. Engle | Pediatrician led anti-tobacco lawsuit
Dr. Howard A. Engle, the veteran Miami Beach pediatrician who lent his name to a landmark class action suit against Big Tobacco, died Wednesday at home, said son David Engle. He was 89 and suffered from smoking-related respiratory disease and lymphoma.
He had been in hospice care since last fall -- when he finally quit smoking.
Decades before he signed on as lead plaintiff in what became known as the ``sick smokers of Florida'' suit, Engle was an institution in Miami Beach, where he treated multiple generations of many families before retiring from private practice in 1997.
BY LUISA YANEZ
lyanez@MiamiHerald.com
This summer, Steven Guarin had accepted his fate: The rare tumors ravaging his 21-year-old body would probably kill him within weeks.
The University of Miami student twice had received aggressive, traditional cancer treatment for a rare lymphoma at the Sylvester Comprehensive Cancer Center at the UM Miller School of Medicine.
Both times he recovered, only to have the killer tumors return with a vengeance, covering most of his body. But today, Guarin is in total remission, thanks to a medical researcher whom Guarin met for the first time Tuesday.
``I'm thankful you dedicated your life to research,'' Guarin said, shaking Eckhard Podack's hand. ``There are many to thank, but you are the first. It feels good to not be sick.''
That wasn't the case five months ago. Guarin, the son of Colombian immigrants who live in Kendall, was too weak for more chemotherapy. His doctors feared he would die within days. His mother and aunt kept vigil by his bedside at Sylvester, praying.
``He's the baby of the family and my only boy,'' his mother, Maria, said Tuesday. ``Imagine the state we were in.''
Joseph Rosenblatt, his oncologist at Sylvester, said the outlook was bleak. ``We had run out of options.''
But Guarin was at an academic medical center, where physicians and researchers work together to develop and test new therapies for patients. Rosenblatt was testing a new drug to seek out and kill cancer cells -- a drug that was developed in a Sylvester lab almost two decades ago.
Podack, now a distinguished Sylvester professor and chair of the department of microbiology and immunology, sold the first incarnation of the drug to Seattle Genetics Inc. in the early 1990s. The company spent years and millions of dollars testing and refining the drug, adding a powerful chemotherapy to create SGN-35.
GUIDED MISSILE
The result: a guided missile that carries chemo directly to the cancer cells, avoiding healthy tissue.
The problem was Guarin was too sick to be considered a candidate for the promising therapy.
``We had to figure out a way to get Steve into the trial, even though he did not meet the criteria. He was just too sick by then. He had a raging fever, his liver was failing and his blood count was horrible,'' Rosenblatt said.
Rosenblatt convinced Seattle Genetics to accept the communications student as the first patient to enroll in the trial.
The gamble, so far, has paid off. Within two days of his first infusion, Guarin's tumors, which were once hot and visibly growing under his skin, had vanished.
``Within a day and a half, I went from fevers and pain and lymph nodes everywhere to walking,'' Guarin said. ``For me, it's a miracle drug.''
An added benefit of SGN-35: no side effects.
Guarin would soon learn that the drug that is still keeping him alive had been developed in a laboratory just a few hundred yards from his hospital bed -- by a researcher who never had met a patient who his research has helped.
`MY HERO'
Guarin sent Podack a heartfelt e-mail expressing his gratitude. ``You're my hero. . .I owe you my life,'' Guarin wrote.
``I am flattered to be called a hero because I was able to help,'' Podack wrote back. On Tuesday, researcher and patient met for the first time at Sylvester, where Guarin is taking the dose he receives every three weeks and gaining strength to continue his treatment. They were introduced by medical school Dean Pascal Goldschmidt, who arranged the meeting because he was so touched by the pair's e-mail exchange.
Podack told Guarin he had begun work on the antibody in the early 1990s ``back when you were barely born.''
``Steve went from no options to new options,'' Rosenblatt said. ``That's the most important message.''

nun hat auch SGEN seinen "Wunderkrebsmittel"-Bericht, wie neulich auch schon MITI:

Könnte sehr wohl wichtig werden für den Kurs.
Posted on Tuesday, 11.17.09 Recommend (1)share email print comment reprint
UM SYLVESTER COMPREHENSIVE CANCER CENTER
Student meets man who saved his life
A University of Miami student battling a rare disease is in remission thanks to a discovery made by the UM Sylvester Comprehensive Cancer Center.
Buy PhotoCancer patient Steven Guarin, 21, whose cancer is in remission, meets the UM researcher who concocted the experimental drug he is being administered. The cancer is now in remission. Guarin wanted to meet the man who saved his life, Dr. Eckhard Podack, Chairman of Immunology at The Miller School of Medicine at The University of Miami. They are at the Sylvester Comprehensive Cancer Center on November 17, 2009. AL DIAZ / THE MIAMI HERALD STAFF
Photo Similar stories:
•Mystery cure?
Mystery cure?
At first, it didn't sound like the way a modern cancer treatment would be created. As the story went, it was an elixir extracted from the root of a mysterious plant found deep in the Amazon forest in Ecuador, used there for decades against everything from lupus to AIDS.
It was brought to Miami by a Quito businessman who was being treated here for prostate cancer, which eventually killed him.
The Ecuadoran doctor who developed the elixir was trying to patent it, so he was being evasive about the plant and the process by which the extract is made.
•Kennedy waged war on cancer as both lawmaker and patient
Kennedy waged war on cancer as both lawmaker and patient
Like almost no one else, Sen. Edward M. Kennedy embodied the frustrations of America's 40-year war on cancer.
Kennedy strongly supported the idea of a war on cancer, promoting it for months before President Richard M. Nixon announced the battle was to begin in 1971, and advocating for more money than Nixon initially wanted to spend.
And when Kennedy was diagnosed with brain cancer last year, he became one of the millions whose fate was not much changed by the cancer war. Despite billions that have been spent, the death rate from most cancers barely budged.
•Researchers share new findings about cancer
Researchers share new findings about cancer
Hope for a better prostate cancer test, potential new uses for a largely discredited lung cancer drug and a warning for breast cancer patients all emerged from a meeting earlier this month of the American Society of Clinical Oncology in Orlando.
Prostate cancer is the most common form of cancer in American men, with an estimated 186,320 cases diagnosed in 2008. But every year, at least that many men undergo painful and expensive prostate biopsies after routine screening has revealed high levels of prostate-specific antigen, or PSA, and yet the men turn out to not have tumors.
Many critics have charged that the large number of unnecessary biopsies grossly limits the benefits of the PSA test and have called for a halt to its use.
•Acreage residents try to connect dots in cancer cases
Acreage residents try to connect dots in cancer cases
The Acreage, a corner of unincorporated western Palm Beach County, is a rustic patch of dirt roads, canals and horse trails -- and, among some residents, the simmering fear of cancer.
Parents, some of whom had never met, gather in each other's houses in the quiet hours to discuss the cases -- children stricken with cancerous brain tumors or cysts; the trips to Miami Children's Hospital for surgery; the symptoms, sometimes puzzling or misleading, that eventually led to diagnosis. They talk about the sick adults, too, about the toxins they think may be contaminating the community's air, water, soil and peace of mind.
For months, state and county officials have pored over this sprawling community of farms, groves and suburban homes located 15 miles northwest of West Palm Beach to determine whether a cancer cluster -- a greater-than-expected rate of the same cancer -- can be confirmed. The state Department of Health is in the second phase of the study. And, on Thursday, environmental activist Erin Brockovich
•Dr. Howard A. Engle | Pediatrician led anti-tobacco lawsuit
Dr. Howard A. Engle | Pediatrician led anti-tobacco lawsuit
Dr. Howard A. Engle, the veteran Miami Beach pediatrician who lent his name to a landmark class action suit against Big Tobacco, died Wednesday at home, said son David Engle. He was 89 and suffered from smoking-related respiratory disease and lymphoma.
He had been in hospice care since last fall -- when he finally quit smoking.
Decades before he signed on as lead plaintiff in what became known as the ``sick smokers of Florida'' suit, Engle was an institution in Miami Beach, where he treated multiple generations of many families before retiring from private practice in 1997.
BY LUISA YANEZ
lyanez@MiamiHerald.com
This summer, Steven Guarin had accepted his fate: The rare tumors ravaging his 21-year-old body would probably kill him within weeks.
The University of Miami student twice had received aggressive, traditional cancer treatment for a rare lymphoma at the Sylvester Comprehensive Cancer Center at the UM Miller School of Medicine.
Both times he recovered, only to have the killer tumors return with a vengeance, covering most of his body. But today, Guarin is in total remission, thanks to a medical researcher whom Guarin met for the first time Tuesday.
``I'm thankful you dedicated your life to research,'' Guarin said, shaking Eckhard Podack's hand. ``There are many to thank, but you are the first. It feels good to not be sick.''
That wasn't the case five months ago. Guarin, the son of Colombian immigrants who live in Kendall, was too weak for more chemotherapy. His doctors feared he would die within days. His mother and aunt kept vigil by his bedside at Sylvester, praying.
``He's the baby of the family and my only boy,'' his mother, Maria, said Tuesday. ``Imagine the state we were in.''
Joseph Rosenblatt, his oncologist at Sylvester, said the outlook was bleak. ``We had run out of options.''
But Guarin was at an academic medical center, where physicians and researchers work together to develop and test new therapies for patients. Rosenblatt was testing a new drug to seek out and kill cancer cells -- a drug that was developed in a Sylvester lab almost two decades ago.
Podack, now a distinguished Sylvester professor and chair of the department of microbiology and immunology, sold the first incarnation of the drug to Seattle Genetics Inc. in the early 1990s. The company spent years and millions of dollars testing and refining the drug, adding a powerful chemotherapy to create SGN-35.
GUIDED MISSILE
The result: a guided missile that carries chemo directly to the cancer cells, avoiding healthy tissue.
The problem was Guarin was too sick to be considered a candidate for the promising therapy.
``We had to figure out a way to get Steve into the trial, even though he did not meet the criteria. He was just too sick by then. He had a raging fever, his liver was failing and his blood count was horrible,'' Rosenblatt said.
Rosenblatt convinced Seattle Genetics to accept the communications student as the first patient to enroll in the trial.
The gamble, so far, has paid off. Within two days of his first infusion, Guarin's tumors, which were once hot and visibly growing under his skin, had vanished.
``Within a day and a half, I went from fevers and pain and lymph nodes everywhere to walking,'' Guarin said. ``For me, it's a miracle drug.''
An added benefit of SGN-35: no side effects.
Guarin would soon learn that the drug that is still keeping him alive had been developed in a laboratory just a few hundred yards from his hospital bed -- by a researcher who never had met a patient who his research has helped.
`MY HERO'
Guarin sent Podack a heartfelt e-mail expressing his gratitude. ``You're my hero. . .I owe you my life,'' Guarin wrote.
``I am flattered to be called a hero because I was able to help,'' Podack wrote back. On Tuesday, researcher and patient met for the first time at Sylvester, where Guarin is taking the dose he receives every three weeks and gaining strength to continue his treatment. They were introduced by medical school Dean Pascal Goldschmidt, who arranged the meeting because he was so touched by the pair's e-mail exchange.
Podack told Guarin he had begun work on the antibody in the early 1990s ``back when you were barely born.''
``Steve went from no options to new options,'' Rosenblatt said. ``That's the most important message.''
Moin,

hier eine Pressemitteilumg von gestern, demnach erhält SGEN 12 Mio $ von der Firma Agensys.
Finde der Kurs könnte mal wieder in die Zweistelligkeit wechseln, nachdem der Verfal anscheinend gestoppt ist.

Gruß q.
-----------------
Monday, November 23, 2009
6:00 a.m. Pacific Time
Seattle Genetics and Agensys, an Affiliate of Astellas,
Expand Antibody-Drug Conjugate Collaboration
Bothell, WA and Santa Monica, CA – November 23, 2009 – Seattle Genetics, Inc. (Nasdaq: SGEN) and Agensys, Inc., an affiliate of Astellas, announced today an expansion of the companies’ antibody-drug conjugate (ADC) collaboration. Under the amended agreement, Agensys will pay a $12 million fee for exclusive rights to ADC licenses against additional antigen targets. Seattle Genetics also receives an option to co-develop another ADC at the time of investigational new drug (IND) submission.
“Combining Agensys’ proprietary antibodies, directed to novel cancer targets, with Seattle Genetics’ industry-leading ADC technology has already led to product candidates for different cancer indications, including ASG-5ME, an ADC for cancers including prostate, pancreatic and gastric that is being co-developed by both companies,” said Aya Jakobovits, Ph.D., Executive Vice President and Head of Research and Development of Agensys. “Expansion of the collaboration will allow Astellas to further enhance its growing pipeline in oncology.”
“Through this broadened collaboration, we continue to leverage the increasing value of our ADC technology to generate revenue as well as to obtain product rights that expand our pipeline of ADCs for the treatment of cancer,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “We look forward to continuing our work with Agensys to bring ADC programs into clinical trials, including ASG-5ME planned for phase I trials in 2010.”
Seattle Genetics and Agensys originally entered into the ADC collaboration in January 2007, under which the companies agreed to co-develop and co-fund an initial ADC program, ASG-5ME, and share equally in any profits upon commercialization. Agensys also received the right to obtain exclusive ADC licenses to three other cancer targets and Seattle Genetics received the right to exercise an option to co-develop and commercialize any one of those additional ADC programs at IND submission in exchange for 50:50 cost and profit-sharing. For ADC programs solely developed and commercialized by Agensys/Astellas, Seattle Genetics is entitled to receive fees, milestones and royalties.
Under the expanded collaboration, Agensys receives the right to obtain exclusive ADC licenses for multiple additional targets in exchange for payment of the upfront fee. Seattle Genetics also receives an option for 50:50 cost and profit-sharing of a third ADC program at IND filing. The remaining ADC programs will be developed and commercialized exclusively by Agensys. Seattle Genetics is entitled to progress-dependent fees, milestone payments and mid-single digit royalties on worldwide net sales of ADC products developed and commercialized solely by Agensys. Under the terms of the amendment, Seattle Genetics is eligible to receive up to $250 million in development milestones and $100 million in sales milestones if all of the additional ADC programs are successful.
Agensys utilizes its portfolio of novel cancer targets to generate high affinity fully human, proprietary antibodies, and combines selected antibodies with the ADC technology to produce new cancer therapies. ADCs utilize the targeting ability of monoclonal antibodies to deliver potent, cell-killing payloads to specific cells. Seattle Genetics’ technology employs synthetic, highly potent drugs attached to antibodies through proprietary linker systems. The linkers are designed to be stable in the bloodstream but to release the drug payload under specific conditions once inside target cells, thereby sparing non-target cells many of the toxic effects of traditional chemotherapy.

hier eine Pressemitteilumg von gestern, demnach erhält SGEN 12 Mio $ von der Firma Agensys.
Finde der Kurs könnte mal wieder in die Zweistelligkeit wechseln, nachdem der Verfal anscheinend gestoppt ist.

Gruß q.
-----------------
Monday, November 23, 2009
6:00 a.m. Pacific Time
Seattle Genetics and Agensys, an Affiliate of Astellas,
Expand Antibody-Drug Conjugate Collaboration
Bothell, WA and Santa Monica, CA – November 23, 2009 – Seattle Genetics, Inc. (Nasdaq: SGEN) and Agensys, Inc., an affiliate of Astellas, announced today an expansion of the companies’ antibody-drug conjugate (ADC) collaboration. Under the amended agreement, Agensys will pay a $12 million fee for exclusive rights to ADC licenses against additional antigen targets. Seattle Genetics also receives an option to co-develop another ADC at the time of investigational new drug (IND) submission.
“Combining Agensys’ proprietary antibodies, directed to novel cancer targets, with Seattle Genetics’ industry-leading ADC technology has already led to product candidates for different cancer indications, including ASG-5ME, an ADC for cancers including prostate, pancreatic and gastric that is being co-developed by both companies,” said Aya Jakobovits, Ph.D., Executive Vice President and Head of Research and Development of Agensys. “Expansion of the collaboration will allow Astellas to further enhance its growing pipeline in oncology.”
“Through this broadened collaboration, we continue to leverage the increasing value of our ADC technology to generate revenue as well as to obtain product rights that expand our pipeline of ADCs for the treatment of cancer,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “We look forward to continuing our work with Agensys to bring ADC programs into clinical trials, including ASG-5ME planned for phase I trials in 2010.”
Seattle Genetics and Agensys originally entered into the ADC collaboration in January 2007, under which the companies agreed to co-develop and co-fund an initial ADC program, ASG-5ME, and share equally in any profits upon commercialization. Agensys also received the right to obtain exclusive ADC licenses to three other cancer targets and Seattle Genetics received the right to exercise an option to co-develop and commercialize any one of those additional ADC programs at IND submission in exchange for 50:50 cost and profit-sharing. For ADC programs solely developed and commercialized by Agensys/Astellas, Seattle Genetics is entitled to receive fees, milestones and royalties.
Under the expanded collaboration, Agensys receives the right to obtain exclusive ADC licenses for multiple additional targets in exchange for payment of the upfront fee. Seattle Genetics also receives an option for 50:50 cost and profit-sharing of a third ADC program at IND filing. The remaining ADC programs will be developed and commercialized exclusively by Agensys. Seattle Genetics is entitled to progress-dependent fees, milestone payments and mid-single digit royalties on worldwide net sales of ADC products developed and commercialized solely by Agensys. Under the terms of the amendment, Seattle Genetics is eligible to receive up to $250 million in development milestones and $100 million in sales milestones if all of the additional ADC programs are successful.
Agensys utilizes its portfolio of novel cancer targets to generate high affinity fully human, proprietary antibodies, and combines selected antibodies with the ADC technology to produce new cancer therapies. ADCs utilize the targeting ability of monoclonal antibodies to deliver potent, cell-killing payloads to specific cells. Seattle Genetics’ technology employs synthetic, highly potent drugs attached to antibodies through proprietary linker systems. The linkers are designed to be stable in the bloodstream but to release the drug payload under specific conditions once inside target cells, thereby sparing non-target cells many of the toxic effects of traditional chemotherapy.
Moin,
letzte Woche schon Takeda (ging ja leider im SGN-35-Ducheinander unter).
Jetzt also GSK.

Heute, Kinder, wird´s was geben...
Seattle Genetics Announces Antibody-Drug Conjugate Collaboration with GlaxoSmithKline
Seattle Genetics to Receive $12 Million Upfront Payment, up to $390 Million in Potential Milestone Payments and Mid-Single Digit Royalties
Buzz up! 0 Print
Companies:Seattle Genetics Inc. Related Quotes
Symbol Price Change
SGEN 9.17 0.00
{"s" : "sgen","k" : "c10,l10,p20,t10","o" : "","j" : ""} Press Release Source: Seattle Genetics, Inc. On Monday December 21, 2009, 7:00 am
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (NASDAQ:SGEN - News), announced today that it has entered into a collaboration agreement with GlaxoSmithKline (GSK) under which GSK will pay an upfront fee of $12 million for rights to utilize Seattle Genetics’ antibody-drug conjugate (ADC) technology with multiple antigens to be named by GSK.
“By collaborating with leading companies such as GSK, we are broadening the reach of our proprietary ADC technology while also generating substantial non-dilutive capital for Seattle Genetics,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “We now have nine ADC licensees and we have generated more than $35 million during 2009 from new and ongoing ADC collaborations.”
GSK is responsible for research, product development, manufacturing and commercialization of all ADC products under the collaboration. Seattle Genetics is eligible to receive from GSK up to $390 million in milestones if all ADCs in the collaboration are commercialized as well as mid-single digit royalties on worldwide net sales of any resulting ADC products. Seattle Genetics also will receive material supply and annual maintenance fees as well as research support payments for assistance provided to GSK under the collaboration.
ADCs are empowered monoclonal antibodies that carry potent, cell-killing drugs. Seattle Genetics has developed proprietary technology employing synthetic, highly potent drugs that can be attached to antibodies through stable linker systems. The linkers are designed to be stable in the bloodstream and release the drugs under specific conditions once inside targeted cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy.
About Seattle Genetics
Seattle Genetics is a clinical-stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company’s lead product candidate, brentuximab vedotin, is in a pivotal trial under a special protocol assessment with the FDA. Brentuximab vedotin is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has four other product candidates in ongoing clinical trials: lintuzumab (SGN-33), dacetuzumab (SGN-40), SGN-70 and SGN-75. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Genentech, Bayer, CuraGen, a subsidiary of Celldex Therapeutics, Progenics, Daiichi Sankyo, MedImmune, a subsidiary of AstraZeneca, and Millennium: The Takeda Oncology Company, as well as an ADC co-development agreement with Agensys, an affiliate of Astellas. More information can be found at http://cts.businesswire.com/ct/CT?id=smartlink&url=http%3A%2…
Certain of the statements made in this press release are forward looking, such as those, among others, relating to the therapeutic potential and future clinical progress, regulatory approval and commercial launch of products utilizing Seattle Genetics’ ADC technology. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include risks related to adverse clinical results as our product candidates or our collaborators’ product candidates move into and advance in clinical trials, risks inherent in early-stage development and failure by Seattle Genetics to secure or maintain relationships with collaborators. More information about the risks and uncertainties faced by Seattle Genetics is contained in the Company’s Form 10-Q for the quarter ended September 30, 2009, filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact:
Seattle Genetics, Inc.Peggy Pinkston, 425-527-4160ppinkston@seagen.com
letzte Woche schon Takeda (ging ja leider im SGN-35-Ducheinander unter).
Jetzt also GSK.

Heute, Kinder, wird´s was geben...
Seattle Genetics Announces Antibody-Drug Conjugate Collaboration with GlaxoSmithKline
Seattle Genetics to Receive $12 Million Upfront Payment, up to $390 Million in Potential Milestone Payments and Mid-Single Digit Royalties
Buzz up! 0 Print
Companies:Seattle Genetics Inc. Related Quotes
Symbol Price Change
SGEN 9.17 0.00
{"s" : "sgen","k" : "c10,l10,p20,t10","o" : "","j" : ""} Press Release Source: Seattle Genetics, Inc. On Monday December 21, 2009, 7:00 am
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (NASDAQ:SGEN - News), announced today that it has entered into a collaboration agreement with GlaxoSmithKline (GSK) under which GSK will pay an upfront fee of $12 million for rights to utilize Seattle Genetics’ antibody-drug conjugate (ADC) technology with multiple antigens to be named by GSK.
“By collaborating with leading companies such as GSK, we are broadening the reach of our proprietary ADC technology while also generating substantial non-dilutive capital for Seattle Genetics,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “We now have nine ADC licensees and we have generated more than $35 million during 2009 from new and ongoing ADC collaborations.”
GSK is responsible for research, product development, manufacturing and commercialization of all ADC products under the collaboration. Seattle Genetics is eligible to receive from GSK up to $390 million in milestones if all ADCs in the collaboration are commercialized as well as mid-single digit royalties on worldwide net sales of any resulting ADC products. Seattle Genetics also will receive material supply and annual maintenance fees as well as research support payments for assistance provided to GSK under the collaboration.
ADCs are empowered monoclonal antibodies that carry potent, cell-killing drugs. Seattle Genetics has developed proprietary technology employing synthetic, highly potent drugs that can be attached to antibodies through stable linker systems. The linkers are designed to be stable in the bloodstream and release the drugs under specific conditions once inside targeted cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy.
About Seattle Genetics
Seattle Genetics is a clinical-stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company’s lead product candidate, brentuximab vedotin, is in a pivotal trial under a special protocol assessment with the FDA. Brentuximab vedotin is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has four other product candidates in ongoing clinical trials: lintuzumab (SGN-33), dacetuzumab (SGN-40), SGN-70 and SGN-75. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Genentech, Bayer, CuraGen, a subsidiary of Celldex Therapeutics, Progenics, Daiichi Sankyo, MedImmune, a subsidiary of AstraZeneca, and Millennium: The Takeda Oncology Company, as well as an ADC co-development agreement with Agensys, an affiliate of Astellas. More information can be found at http://cts.businesswire.com/ct/CT?id=smartlink&url=http%3A%2…
Certain of the statements made in this press release are forward looking, such as those, among others, relating to the therapeutic potential and future clinical progress, regulatory approval and commercial launch of products utilizing Seattle Genetics’ ADC technology. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include risks related to adverse clinical results as our product candidates or our collaborators’ product candidates move into and advance in clinical trials, risks inherent in early-stage development and failure by Seattle Genetics to secure or maintain relationships with collaborators. More information about the risks and uncertainties faced by Seattle Genetics is contained in the Company’s Form 10-Q for the quarter ended September 30, 2009, filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact:
Seattle Genetics, Inc.Peggy Pinkston, 425-527-4160ppinkston@seagen.com
Antwort auf Beitrag Nr.: 38.608.722 von quepos am 21.12.09 13:43:26
Hi quepos,
finde es gut, dass Du einen SGEN-Thread aufgemacht hast. Ich halte seit ca. 6 Monaten eine kleine Position und denke über eine Aufstockung nach. Vielleicht ist die beste Chance dafür aber schon vertan. Im 1. HJ 2010 sollen die Daten für den IIb-Trial für Lintuzumab in AML kommen. Sollten diese Daten überzeugen, wird der Kurs einen deutlichen Sprung machen, der dann wahrscheinlich auch nachhaltig sein wird imho. Ich denke, wenn man sich bei SGEN positionieren will, dann hat man gar nicht mehr so viel Zeit dafür, denn falls die Lintuzumab-Daten gut sind, dann wirds deutlich teurer (und wenn nicht, dann nicht ).
).
Ein ziemlich spannendes Projekt ist übrigens auch dieses, das auf einer SGEN-Lizenz basiert:
NEEDHAM, Mass.--(BUSINESS WIRE)--Dec. 13, 2009-- Celldex Therapeutics, Inc. (NASDAQ: CLDX) today announced the results of a positive Phase 2 study of CDX-011 (formerly CR011-vcMMAE), in patients with heavily pre-treated, locally advanced or metastatic breast cancers. As presented today at the 32nd Annual CTRC-AACR San Antonio Breast Cancer Symposium, the primary efficacy endpoint for the study has been met with significant antitumor activity in patients whose tumors express the target GPNMB. In addition, encouraging results were seen in patients with “triple-negative disease” where treatment options are relatively limited due to lack of hormone receptor or HER2-neu expression.
http://ir.celldextherapeutics.com/phoenix.zhtml?c=93243&p=ir…
Hi quepos,
finde es gut, dass Du einen SGEN-Thread aufgemacht hast. Ich halte seit ca. 6 Monaten eine kleine Position und denke über eine Aufstockung nach. Vielleicht ist die beste Chance dafür aber schon vertan. Im 1. HJ 2010 sollen die Daten für den IIb-Trial für Lintuzumab in AML kommen. Sollten diese Daten überzeugen, wird der Kurs einen deutlichen Sprung machen, der dann wahrscheinlich auch nachhaltig sein wird imho. Ich denke, wenn man sich bei SGEN positionieren will, dann hat man gar nicht mehr so viel Zeit dafür, denn falls die Lintuzumab-Daten gut sind, dann wirds deutlich teurer (und wenn nicht, dann nicht
 ).
).Ein ziemlich spannendes Projekt ist übrigens auch dieses, das auf einer SGEN-Lizenz basiert:
NEEDHAM, Mass.--(BUSINESS WIRE)--Dec. 13, 2009-- Celldex Therapeutics, Inc. (NASDAQ: CLDX) today announced the results of a positive Phase 2 study of CDX-011 (formerly CR011-vcMMAE), in patients with heavily pre-treated, locally advanced or metastatic breast cancers. As presented today at the 32nd Annual CTRC-AACR San Antonio Breast Cancer Symposium, the primary efficacy endpoint for the study has been met with significant antitumor activity in patients whose tumors express the target GPNMB. In addition, encouraging results were seen in patients with “triple-negative disease” where treatment options are relatively limited due to lack of hormone receptor or HER2-neu expression.
http://ir.celldextherapeutics.com/phoenix.zhtml?c=93243&p=ir…
Antwort auf Beitrag Nr.: 38.622.454 von SLGramann am 23.12.09 09:42:55Ja Hallo SLGrammann und natürlich ein Frohes Neues!

Bisher mußte ich ja immer alles selbst posten, dieses Jahr wird alles anders!

SGEN läuft ja ganz gut im Moment und ja, es gibt so einige Lizenznehmervon deren Technologie. Ganz netter Artikel, der die Chancen der Konjugattechnologie etwas herausstellt hier (aus dem Yahoo-Board kopiert):
http://www.xconomy.com/san-diego/2010/01/06/ambrx-saying-tha…
Ach ja und:
Seattle Genetics to Present at the J.P. Morgan Healthcare Conference (am 12. Januar, also nächsten Dienstag).
Solche Investorenkonferenzen sind auch immer ein guter Weg um Interesse zu wecken.

Bei guten News ist evtl. sogar ein schneller Schluss des Abwärts-Gaps ab ca. 11,50 $ möglich, werden sehen.
Erstmal Grüße q.

Bisher mußte ich ja immer alles selbst posten, dieses Jahr wird alles anders!

SGEN läuft ja ganz gut im Moment und ja, es gibt so einige Lizenznehmervon deren Technologie. Ganz netter Artikel, der die Chancen der Konjugattechnologie etwas herausstellt hier (aus dem Yahoo-Board kopiert):
http://www.xconomy.com/san-diego/2010/01/06/ambrx-saying-tha…
Ach ja und:
Seattle Genetics to Present at the J.P. Morgan Healthcare Conference (am 12. Januar, also nächsten Dienstag).
Solche Investorenkonferenzen sind auch immer ein guter Weg um Interesse zu wecken.

Bei guten News ist evtl. sogar ein schneller Schluss des Abwärts-Gaps ab ca. 11,50 $ möglich, werden sehen.
Erstmal Grüße q.
Moin,

noch ein Nachtrag, hatte ich ganz vergessen.
Interessant!
Gruß q.
Biotech, cancer, Antibodies
Seattle Genetics Maps Out a Future With Antibody Drugs That Are “Empowered”
Luke Timmerman 1/6/10
Some of the best-selling drugs in the pharmaceutical industry are what biotechies call “naked” antibodies. These are engineered Y-shaped proteins that zero in on markers of diseased cells, while sparing healthy ones. They generate an estimated $30 billion in annual sales, from big names like Roche’s trastuzumab (Herceptin) for breast cancer.
But to hear one of the nation’s leading developers of antibody drugs talk today, those treatments aren’t really on the cutting edge anymore.
“It’s unlikely in the future of Seattle Genetics that we’ll put another naked antibody into the clinic,” CEO Clay Siegall says. “The future will be with antibodies that are empowered.”
Seattle Genetics (NASDAQ: SGEN) has come to that conclusion after it has spent more than a decade trying to make antibodies more potent. The company, founded in 1998, has never been able to take a naked antibody drug all the way through to FDA approval, and it has shelved a number of candidates over the years—SGN-10, SGN-15, SGN-30. But Seattle Genetics has generated its most promising data yet from a next-generation “empowered” antibody for Hodgkin’s disease. The technology that makes this possible—linking a regular antibody to a toxin that can give it extra-tumor killing punch—has now been licensed to eight other drug companies. More data emerged this year from competitors Roche and ImmunoGen that suggests they, too, have found a way to link an antibody with a toxin to create a souped-up antibody drug that beats the original. And Seattle Genetics has designs on pushing the envelope further in the future with another next-generation technique for making antibodies more potent.
Clay Siegall
Scientists have been dreaming about creating powerful targeted therapies, known as antibody-drug conjugates, for three decades, but the efforts usually failed because the linkers weren’t stable, and the toxins broke off in the bloodstream before they could reach the intended target, causing side effects. Seattle Genetics’ technology is built on the idea that its synthetic linkers remain stable until the antibody reaches the tumor and unleashes its toxic payload.
The lead drug candidate at Seattle Genetics, brentuximab vedotin, opened the eyes of cancer researchers a little more than a year ago with its data. Scientists said that 17 of the 44 patients (38 percent) had their tumors completely disappear or mostly go away after they took the drug. When they looked at patients who got higher doses that are more likely to be tested in late stages, the data look even better. Of the 28 patients who got those doses, about one-third had their tumors completely disappear, while 93 percent had at least some measurable tumor shrinkage. That drug is now in a pivotal clinical trial that could produce results by the end of this year, and may reach the U.S. market by the end of 2011.
While Seattle Genetics still has a couple of naked antibodies moving through clinical trials, Wall Street isn’t counting on them. Last month, Roche pulled the plug on a partnership to develop one of these, dacetuzumab, after it failed in a mid-stage clinical trial of 224 patients with diffuse large B-cell lymphoma. Another naked antibody from Seattle Genetics, SGN-33, is in a mid-stage trial for patients with acute myeloid leukemia. Results from that trial are expected before the end of June.
“Expectations [for SGN-33] are very low given the tough nature of the disease and lack of convincing earlier-stage data,” said JP Morgan analyst Cory Kasimov in a note to clients last month.
Siegall doesn’t want to go quite so far as to declare naked antibodies dead, noting that Seattle Genetics hasn’t decided what to do yet with dacetuzumab, and it doesn’t yet have the data from SGN-33.
“If you are talking about the future, I’d say yes, it’s unlikely that we’ll move another naked antibody into the clinic,” Siegall says.
To that end, Siegall noted he has high hopes for a second antibody-drug conjugate in the clinical pipeline, known as SGN-75. There’s a “high likelihood” the drug will generate clinical trial results in 2010, Siegall says. Plus, Seattle Genetics is looking to introduce a third member of the antibody-drug conjugate class into clinical trials this year.
All told, between Seattle Genetics’ internally developed antibody-drug conjugates and those from its partners, “I would bet that by the end of 2011, there will be close to 10 antibody drug conjugates in clinical trials,” Siegall says.
But Seattle Genetics has more than just antibody-drug conjugates in mind when it talks about making antibodies more potent. The company has also developed another technique, which it calls sugar-engineered antibody technology (SEA).
The basic idea, which the company unveiled back in September, was that Seattle Genetics has found a way to modify naked antibodies with a chemical to block the formation of a certain sugar called fucose that often hangs off the end of a regular antibody. By getting rid of that type of sugar, scientists think they can trigger the immune system to unleash a more powerful assault on the cells that the antibodies bind with, Siegall says.
Since this approach can make antibodies more potent without using a toxin, it’s possible that sugar-engineered antibodies might be better tolerated for long-term usage. That could be useful for diseases in which the antibody needs to be given for a chronic disease, like rheumatoid arthritis, Siegall says.
At least two other companies appear to be further along than Seattle Genetics with this sort of sugar-based approach for empowering antibodies, Siegall says. One is BioWa, a unit of Japan-based Kyowa Hakko Kirin, and the other is GlycArt, now a unit of Roche. The GlycArt technology is being used for a drug candidate called GA-101, an empowered version of Roche and Biogen Idec’s hit rituximab (Rituxan). But Siegall says the Seattle Genetics approach should have an advantage because it only adds one chemical step to the antibody production process, and doesn’t slow things down like the others can.
Like Seattle Genetics’ earlier experience with antibody-drug conjugates, it hopes to use the sugar-engineered antibody approach both for internal programs, and for licensing to other companies, Siegall says. “We are talking to a number of companies about it, and there is interest,” he says.
Since that technology is still relatively new, Siegall tried not to pump up expectations too high about it becoming a moneymaker for the company anytime soon. But it’s a clear indicator that Seattle Genetics is looking at a number of ways to build upon the successes of the plain antibodies from the past.
“These products have been around for a while and they help patients,” Siegall says. “Now we have an opportunity to take antibodies to the next level. The data speaks louder than anything else, and the data are strong.”
Luke Timmerman is the National Biotechnology Editor for Xconomy. You can e-mail him at ltimmerman@xconomy.com, call 206-624-2374, or follow him on Twitter at http://twitter.com/ldtimmerman.

noch ein Nachtrag, hatte ich ganz vergessen.
Interessant!
Gruß q.
Biotech, cancer, Antibodies
Seattle Genetics Maps Out a Future With Antibody Drugs That Are “Empowered”
Luke Timmerman 1/6/10
Some of the best-selling drugs in the pharmaceutical industry are what biotechies call “naked” antibodies. These are engineered Y-shaped proteins that zero in on markers of diseased cells, while sparing healthy ones. They generate an estimated $30 billion in annual sales, from big names like Roche’s trastuzumab (Herceptin) for breast cancer.
But to hear one of the nation’s leading developers of antibody drugs talk today, those treatments aren’t really on the cutting edge anymore.
“It’s unlikely in the future of Seattle Genetics that we’ll put another naked antibody into the clinic,” CEO Clay Siegall says. “The future will be with antibodies that are empowered.”
Seattle Genetics (NASDAQ: SGEN) has come to that conclusion after it has spent more than a decade trying to make antibodies more potent. The company, founded in 1998, has never been able to take a naked antibody drug all the way through to FDA approval, and it has shelved a number of candidates over the years—SGN-10, SGN-15, SGN-30. But Seattle Genetics has generated its most promising data yet from a next-generation “empowered” antibody for Hodgkin’s disease. The technology that makes this possible—linking a regular antibody to a toxin that can give it extra-tumor killing punch—has now been licensed to eight other drug companies. More data emerged this year from competitors Roche and ImmunoGen that suggests they, too, have found a way to link an antibody with a toxin to create a souped-up antibody drug that beats the original. And Seattle Genetics has designs on pushing the envelope further in the future with another next-generation technique for making antibodies more potent.
Clay Siegall
Scientists have been dreaming about creating powerful targeted therapies, known as antibody-drug conjugates, for three decades, but the efforts usually failed because the linkers weren’t stable, and the toxins broke off in the bloodstream before they could reach the intended target, causing side effects. Seattle Genetics’ technology is built on the idea that its synthetic linkers remain stable until the antibody reaches the tumor and unleashes its toxic payload.
The lead drug candidate at Seattle Genetics, brentuximab vedotin, opened the eyes of cancer researchers a little more than a year ago with its data. Scientists said that 17 of the 44 patients (38 percent) had their tumors completely disappear or mostly go away after they took the drug. When they looked at patients who got higher doses that are more likely to be tested in late stages, the data look even better. Of the 28 patients who got those doses, about one-third had their tumors completely disappear, while 93 percent had at least some measurable tumor shrinkage. That drug is now in a pivotal clinical trial that could produce results by the end of this year, and may reach the U.S. market by the end of 2011.
While Seattle Genetics still has a couple of naked antibodies moving through clinical trials, Wall Street isn’t counting on them. Last month, Roche pulled the plug on a partnership to develop one of these, dacetuzumab, after it failed in a mid-stage clinical trial of 224 patients with diffuse large B-cell lymphoma. Another naked antibody from Seattle Genetics, SGN-33, is in a mid-stage trial for patients with acute myeloid leukemia. Results from that trial are expected before the end of June.
“Expectations [for SGN-33] are very low given the tough nature of the disease and lack of convincing earlier-stage data,” said JP Morgan analyst Cory Kasimov in a note to clients last month.
Siegall doesn’t want to go quite so far as to declare naked antibodies dead, noting that Seattle Genetics hasn’t decided what to do yet with dacetuzumab, and it doesn’t yet have the data from SGN-33.
“If you are talking about the future, I’d say yes, it’s unlikely that we’ll move another naked antibody into the clinic,” Siegall says.
To that end, Siegall noted he has high hopes for a second antibody-drug conjugate in the clinical pipeline, known as SGN-75. There’s a “high likelihood” the drug will generate clinical trial results in 2010, Siegall says. Plus, Seattle Genetics is looking to introduce a third member of the antibody-drug conjugate class into clinical trials this year.
All told, between Seattle Genetics’ internally developed antibody-drug conjugates and those from its partners, “I would bet that by the end of 2011, there will be close to 10 antibody drug conjugates in clinical trials,” Siegall says.
But Seattle Genetics has more than just antibody-drug conjugates in mind when it talks about making antibodies more potent. The company has also developed another technique, which it calls sugar-engineered antibody technology (SEA).
The basic idea, which the company unveiled back in September, was that Seattle Genetics has found a way to modify naked antibodies with a chemical to block the formation of a certain sugar called fucose that often hangs off the end of a regular antibody. By getting rid of that type of sugar, scientists think they can trigger the immune system to unleash a more powerful assault on the cells that the antibodies bind with, Siegall says.
Since this approach can make antibodies more potent without using a toxin, it’s possible that sugar-engineered antibodies might be better tolerated for long-term usage. That could be useful for diseases in which the antibody needs to be given for a chronic disease, like rheumatoid arthritis, Siegall says.
At least two other companies appear to be further along than Seattle Genetics with this sort of sugar-based approach for empowering antibodies, Siegall says. One is BioWa, a unit of Japan-based Kyowa Hakko Kirin, and the other is GlycArt, now a unit of Roche. The GlycArt technology is being used for a drug candidate called GA-101, an empowered version of Roche and Biogen Idec’s hit rituximab (Rituxan). But Siegall says the Seattle Genetics approach should have an advantage because it only adds one chemical step to the antibody production process, and doesn’t slow things down like the others can.
Like Seattle Genetics’ earlier experience with antibody-drug conjugates, it hopes to use the sugar-engineered antibody approach both for internal programs, and for licensing to other companies, Siegall says. “We are talking to a number of companies about it, and there is interest,” he says.
Since that technology is still relatively new, Siegall tried not to pump up expectations too high about it becoming a moneymaker for the company anytime soon. But it’s a clear indicator that Seattle Genetics is looking at a number of ways to build upon the successes of the plain antibodies from the past.
“These products have been around for a while and they help patients,” Siegall says. “Now we have an opportunity to take antibodies to the next level. The data speaks louder than anything else, and the data are strong.”
Luke Timmerman is the National Biotechnology Editor for Xconomy. You can e-mail him at ltimmerman@xconomy.com, call 206-624-2374, or follow him on Twitter at http://twitter.com/ldtimmerman.
Hallo again,

hier ein Bericht frisch aus dem Yahoo-Board zum Vortrag des CEO´s (von yahoo-user "redhot47fl")

q.
-------------
Here is a quick summary of today's presentation by SGEN CEO Clay Siegall during the 28th Annual JP Morgan Annual Healthcare Conference in San Francisco:
Top line: No surprises, but a continuing sense of progress and optimism. It sounded as if the session was well attended, and Siegall's presentation earned a nice round of applause.
- SGN-35 remains on track for FDA submission during the first half of 2011, and Siegall's enthusiasm suggested that the company senses no worrisome issues in the ongoing trials.
- The SGN-35 overseas collaboration deal announced late last year with Millennium/Takeda should endure well beyond the three years for which revenues have been estimated. Direct quote from Siegall: "It [SGN-35] will be the first commercial product that we have and it will allow us to build a commercial structure to support the robust list of other exciting products that we have." Note that he is not hedging here. "Will be" is the term he used.
- Patients as young as 12 years old are in the SGN-35 trials. Sure hope this stuff continues to work.
- We might see data from SGN-70 and SGN-75 trials late this year
- ADC collaborations are moving ahead at a steady pace. About $110 million already has been generated from ADC collaborations. Direct quote from Siegall: "I think it is likely you will be seeing future collaborations going forward."
- Still no debt, and the company is sitting on $350 million in cash right now.
That's it. As mentioned, nothing hugely new, but a very encouraging report to begin the year.

hier ein Bericht frisch aus dem Yahoo-Board zum Vortrag des CEO´s (von yahoo-user "redhot47fl")

q.
-------------
Here is a quick summary of today's presentation by SGEN CEO Clay Siegall during the 28th Annual JP Morgan Annual Healthcare Conference in San Francisco:
Top line: No surprises, but a continuing sense of progress and optimism. It sounded as if the session was well attended, and Siegall's presentation earned a nice round of applause.
- SGN-35 remains on track for FDA submission during the first half of 2011, and Siegall's enthusiasm suggested that the company senses no worrisome issues in the ongoing trials.
- The SGN-35 overseas collaboration deal announced late last year with Millennium/Takeda should endure well beyond the three years for which revenues have been estimated. Direct quote from Siegall: "It [SGN-35] will be the first commercial product that we have and it will allow us to build a commercial structure to support the robust list of other exciting products that we have." Note that he is not hedging here. "Will be" is the term he used.
- Patients as young as 12 years old are in the SGN-35 trials. Sure hope this stuff continues to work.
- We might see data from SGN-70 and SGN-75 trials late this year
- ADC collaborations are moving ahead at a steady pace. About $110 million already has been generated from ADC collaborations. Direct quote from Siegall: "I think it is likely you will be seeing future collaborations going forward."
- Still no debt, and the company is sitting on $350 million in cash right now.
That's it. As mentioned, nothing hugely new, but a very encouraging report to begin the year.
Moin,

für die riesige Fangemeine von SGEN ("Leser heute: 0") hier noch mal der Vollständigkeit halber die Quartalszahlen.

Den starken Kusrückgang der jüngsten Zeit kann ich mir nicht erklären, was die Zahlen angeht, so liegen sie sogar weit über den Analystenschätzungen, ebenso wie der Ausblick.

Aber Zahlen sollten bei Bios nicht so sehr im Vordergrund stehen.
Produktfortschritte sind wichtiger.
Gruß q.
--------------------
Seattle Genetics 4th-quarter loss narrows on boost in revenue from partnerships
Buzz up! 0 Print
Companies:GENENTECH INCSeattle Genetics Inc. Topics:Earnings Related Quotes
Symbol Price Change
DNA 80.43 0.00
SGEN 9.72 +0.13
{"s" : "dna,sgen","k" : "c10,l10,p20,t10","o" : "","j" : ""} On Tuesday February 9, 2010, 6:18 pm EST
BOTHELL, Wash. (AP) -- Development-stage biotechnology company Seattle Genetics Inc. said Tuesday its fourth-quarter loss narrowed on higher revenue from partnerships.
The company recorded a loss of $12.1 million, or 12 cents per share, compared with a loss of $30.6 million, or 38 cents per share, during the same period a year before. Revenue more than doubled to $21.8 million from $10.1 million.
Analysts polled by Thomson Reuters expected a loss of 23 cents per share on revenue of $11.7 million.
The company's key partnership is with Genentech Inc., now part of Roche. The companies are developing potential cancer treatments.
For the full year, Seattle Genetics lost $81.7 million, or 90 cents per share, compared with a loss of $85.5 million, or $1.09 per share, in 2008. Revenue rose to $52 million from $35.2 million.
Looking ahead, the company expects revenue between $95 million and $105 million in 2010. Analysts expect $47.3 million in revenue.
Shares of Seattle Genetics rose 25 cents, or 2.7 percent, to close at $9.59.

für die riesige Fangemeine von SGEN ("Leser heute: 0") hier noch mal der Vollständigkeit halber die Quartalszahlen.

Den starken Kusrückgang der jüngsten Zeit kann ich mir nicht erklären, was die Zahlen angeht, so liegen sie sogar weit über den Analystenschätzungen, ebenso wie der Ausblick.

Aber Zahlen sollten bei Bios nicht so sehr im Vordergrund stehen.
Produktfortschritte sind wichtiger.
Gruß q.
--------------------
Seattle Genetics 4th-quarter loss narrows on boost in revenue from partnerships
Buzz up! 0 Print
Companies:GENENTECH INCSeattle Genetics Inc. Topics:Earnings Related Quotes
Symbol Price Change
DNA 80.43 0.00
SGEN 9.72 +0.13
{"s" : "dna,sgen","k" : "c10,l10,p20,t10","o" : "","j" : ""} On Tuesday February 9, 2010, 6:18 pm EST
BOTHELL, Wash. (AP) -- Development-stage biotechnology company Seattle Genetics Inc. said Tuesday its fourth-quarter loss narrowed on higher revenue from partnerships.
The company recorded a loss of $12.1 million, or 12 cents per share, compared with a loss of $30.6 million, or 38 cents per share, during the same period a year before. Revenue more than doubled to $21.8 million from $10.1 million.
Analysts polled by Thomson Reuters expected a loss of 23 cents per share on revenue of $11.7 million.
The company's key partnership is with Genentech Inc., now part of Roche. The companies are developing potential cancer treatments.
For the full year, Seattle Genetics lost $81.7 million, or 90 cents per share, compared with a loss of $85.5 million, or $1.09 per share, in 2008. Revenue rose to $52 million from $35.2 million.
Looking ahead, the company expects revenue between $95 million and $105 million in 2010. Analysts expect $47.3 million in revenue.
Shares of Seattle Genetics rose 25 cents, or 2.7 percent, to close at $9.59.
Antwort auf Beitrag Nr.: 38.921.512 von quepos am 10.02.10 20:46:00
Keine Sorge, zwei oder drei interessierte Miteigentümer gibt es hier noch.
Ausblick auf 2010 war zahlentechnisch wirklich gut. Beruhigend bspw das:
The company expects that its net cash used in operating activities for the year 2010 will be less than $20 million, and that it will end the year with more than $265 million in cash and investments.
SGEN ist, wenn mit SGN 35 alles gut geht, bis zur Zulassung des ersten Produkts durchfinanziert. Das ist doch was.
Lintuzumab-Daten kommen in Q2. Darf man da noch auf etwas hoffen? Ich hab keine Ahnung.
Keine Sorge, zwei oder drei interessierte Miteigentümer gibt es hier noch.

Ausblick auf 2010 war zahlentechnisch wirklich gut. Beruhigend bspw das:
The company expects that its net cash used in operating activities for the year 2010 will be less than $20 million, and that it will end the year with more than $265 million in cash and investments.
SGEN ist, wenn mit SGN 35 alles gut geht, bis zur Zulassung des ersten Produkts durchfinanziert. Das ist doch was.
Lintuzumab-Daten kommen in Q2. Darf man da noch auf etwas hoffen? Ich hab keine Ahnung.

Ich gebe noch nicht auf!

(Leser heute: 0)

SGEN schlägt sich in schwierugem Marktumfeld eigentlich ganz gut. Heute Hinweis auf Investorenkonferenzen, vielleicht bringen die neue Bewegung!
Gruß q.
----------
Seattle Genetics to Present at Upcoming Investor Conferences
Buzz up! 0 Print
Companies:Seattle Genetics Inc. Related Quotes
Symbol Price Change
SGEN 10.43 +0.11
{"s" : "sgen","k" : "c10,l10,p20,t10","o" : "","j" : ""} Press Release Source: Seattle Genetics, Inc. On Thursday February 25, 2010, 9:00 am EST
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq:SGEN - News) announced today that management will present at two upcoming investor conferences. The presentations will be webcast live and available for replay from Seattle Genetics’ website, http://cts.businesswire.com/ct/CT?id=smartlink&url=http%3A%2… under the "Investors and News" section.
RBC Capital Markets Healthcare Conference
Clay B. Siegall, Ph.D., President and Chief Executive Officer, will present on a panel, “Building a Proprietary and Partnered Pipeline of Antibodies,” on Wednesday, March 3, 2010, at 2:05 p.m. Eastern Time. The conference is being held at the New York Palace Hotel in New York, NY.
Cowen and Company 30th Annual Healthcare Conference
Dr. Siegall will present a company overview on Monday, March 8, 2010, at 2:30 p.m. Eastern Time. The conference is being held at the Boston Marriott Copley Place in Boston, MA.
About Seattle Genetics
Seattle Genetics is a clinical stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company’s lead product candidate, brentuximab vedotin, is in a pivotal trial under a special protocol assessment with the FDA. Brentuximab vedotin is being developed in collaboration with Millennium Pharmaceuticals, Inc.: The Takeda Oncology Company. In addition, Seattle Genetics has four other product candidates in ongoing clinical trials: lintuzumab (SGN-33), dacetuzumab (SGN-40), SGN-70 and SGN-75. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, MedImmune, a subsidiary of AstraZeneca, Millennium: The Takeda Oncology Company, and Progenics, as well as an ADC co-development agreement with Agensys, an affiliate of Astellas. More information can be found at http://cts.businesswire.com/ct/CT?id=smartlink&url=http%3A%2…
Contact:
Seattle Genetics, Inc.Peggy Pinkston, 425-527-4160ppinkston@seagen.com

(Leser heute: 0)

SGEN schlägt sich in schwierugem Marktumfeld eigentlich ganz gut. Heute Hinweis auf Investorenkonferenzen, vielleicht bringen die neue Bewegung!
Gruß q.
----------
Seattle Genetics to Present at Upcoming Investor Conferences
Buzz up! 0 Print
Companies:Seattle Genetics Inc. Related Quotes
Symbol Price Change
SGEN 10.43 +0.11
{"s" : "sgen","k" : "c10,l10,p20,t10","o" : "","j" : ""} Press Release Source: Seattle Genetics, Inc. On Thursday February 25, 2010, 9:00 am EST
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq:SGEN - News) announced today that management will present at two upcoming investor conferences. The presentations will be webcast live and available for replay from Seattle Genetics’ website, http://cts.businesswire.com/ct/CT?id=smartlink&url=http%3A%2… under the "Investors and News" section.
RBC Capital Markets Healthcare Conference
Clay B. Siegall, Ph.D., President and Chief Executive Officer, will present on a panel, “Building a Proprietary and Partnered Pipeline of Antibodies,” on Wednesday, March 3, 2010, at 2:05 p.m. Eastern Time. The conference is being held at the New York Palace Hotel in New York, NY.
Cowen and Company 30th Annual Healthcare Conference
Dr. Siegall will present a company overview on Monday, March 8, 2010, at 2:30 p.m. Eastern Time. The conference is being held at the Boston Marriott Copley Place in Boston, MA.
About Seattle Genetics
Seattle Genetics is a clinical stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company’s lead product candidate, brentuximab vedotin, is in a pivotal trial under a special protocol assessment with the FDA. Brentuximab vedotin is being developed in collaboration with Millennium Pharmaceuticals, Inc.: The Takeda Oncology Company. In addition, Seattle Genetics has four other product candidates in ongoing clinical trials: lintuzumab (SGN-33), dacetuzumab (SGN-40), SGN-70 and SGN-75. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, MedImmune, a subsidiary of AstraZeneca, Millennium: The Takeda Oncology Company, and Progenics, as well as an ADC co-development agreement with Agensys, an affiliate of Astellas. More information can be found at http://cts.businesswire.com/ct/CT?id=smartlink&url=http%3A%2…
Contact:
Seattle Genetics, Inc.Peggy Pinkston, 425-527-4160ppinkston@seagen.com
Ein IND von Genentech:
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq: SGEN) announced today that it has achieved a milestone under its antibody-drug conjugate (ADC) collaboration with Genentech, Inc., a wholly owned member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY). The milestone was triggered by Genentech’s submission of an Investigational New Drug (IND) application with the U.S. Food and Drug Administration (FDA) for an ADC utilizing Seattle Genetics’ technology for the treatment of cancer.
http://www.businesswire.com/portal/site/home/permalink/?ndmV…
Ich würde echt gern mal die gesamte Partnerpipe inkl. Vorklinik von SGEN sehen, zumindest die Zahl der Projekte kennen - so wie bei MorphoSys...
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq: SGEN) announced today that it has achieved a milestone under its antibody-drug conjugate (ADC) collaboration with Genentech, Inc., a wholly owned member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY). The milestone was triggered by Genentech’s submission of an Investigational New Drug (IND) application with the U.S. Food and Drug Administration (FDA) for an ADC utilizing Seattle Genetics’ technology for the treatment of cancer.
http://www.businesswire.com/portal/site/home/permalink/?ndmV…
Ich würde echt gern mal die gesamte Partnerpipe inkl. Vorklinik von SGEN sehen, zumindest die Zahl der Projekte kennen - so wie bei MorphoSys...

Seattle Genetics Expands Antibody-Drug Conjugate Patent Portfolio
- Issued U.S. Patent Extends Patent Protection for Brentuximab Vedotin (SGN-35) to at Least 2025 -
BOTHELL, Wash., Mar 11, 2010 (BUSINESS WIRE) -- Seattle Genetics, Inc. /quotes/comstock/15*!sgen/quotes/nls/sgen (SGEN 11.73, -0.03, -0.26%) announced today that the U.S. Patent and Trademark Office has issued a patent related to its antibody-drug conjugate (ADC) technology. U.S. Patent No. 7,659,241 covers cleavable linkers and potent auristatin drug payloads used in certain of Seattle Genetics' ADC programs, most notably brentuximab vedotin (SGN-35), as well as many ADC programs in development by its collaborators. Brentuximab vedotin, an ADC that utilizes the vcMMAE drug-linker unit, is in a pivotal trial for relapsed and refractory Hodgkin lymphoma and a phase II trial for relapsed and refractory systemic anaplastic large cell lymphoma. The company anticipates submitting a New Drug Application for brentuximab vedotin in the first half of 2011.
"This patent is an important addition to the intellectual property portfolio surrounding our ADC technology, and significantly enhances the patent position for multiple internal and collaborator programs that use the vcMMAE drug-linker unit," said Eric Dobmeier, Chief Business Officer of Seattle Genetics. "Issuance of this patent, which extends protection in the United States to at least 2025, also reflects the culmination of significant and novel research conducted at Seattle Genetics to continue advancing our industry-leading ADC technology."
ADCs utilize the targeting ability of monoclonal antibodies to deliver potent, cell-killing payloads to specific cells. Seattle Genetics' proprietary technology employs synthetic, highly potent drugs attached to antibodies through stable linker systems. The linkers are designed to release the drug payload only under specific conditions once inside target cells, thereby sparing non-target cells many of the toxic effects of traditional chemotherapy.
Seattle Genetics is developing brentuximab vedotin in collaboration with Millennium: The Takeda Oncology Company, under which Seattle Genetics has U.S. and Canadian commercialization rights and Millennium has exclusive rights to commercialize the product in the rest of the world. Seattle Genetics and Millennium are jointly funding worldwide development costs for brentuximab vedotin on a 50:50 basis. Additionally, Seattle Genetics has nine ongoing ADC collaborations with leading biotechnology and pharmaceutical companies. To date, Seattle Genetics has generated approximately $110 million through ADC technology license agreements.
SOURCE: Seattle Genetics, Inc.
Seattle Genetics, Inc.
Peggy Pinkston, 425-527-4160
ppinkston@seagen.com
- Issued U.S. Patent Extends Patent Protection for Brentuximab Vedotin (SGN-35) to at Least 2025 -
BOTHELL, Wash., Mar 11, 2010 (BUSINESS WIRE) -- Seattle Genetics, Inc. /quotes/comstock/15*!sgen/quotes/nls/sgen (SGEN 11.73, -0.03, -0.26%) announced today that the U.S. Patent and Trademark Office has issued a patent related to its antibody-drug conjugate (ADC) technology. U.S. Patent No. 7,659,241 covers cleavable linkers and potent auristatin drug payloads used in certain of Seattle Genetics' ADC programs, most notably brentuximab vedotin (SGN-35), as well as many ADC programs in development by its collaborators. Brentuximab vedotin, an ADC that utilizes the vcMMAE drug-linker unit, is in a pivotal trial for relapsed and refractory Hodgkin lymphoma and a phase II trial for relapsed and refractory systemic anaplastic large cell lymphoma. The company anticipates submitting a New Drug Application for brentuximab vedotin in the first half of 2011.
"This patent is an important addition to the intellectual property portfolio surrounding our ADC technology, and significantly enhances the patent position for multiple internal and collaborator programs that use the vcMMAE drug-linker unit," said Eric Dobmeier, Chief Business Officer of Seattle Genetics. "Issuance of this patent, which extends protection in the United States to at least 2025, also reflects the culmination of significant and novel research conducted at Seattle Genetics to continue advancing our industry-leading ADC technology."
ADCs utilize the targeting ability of monoclonal antibodies to deliver potent, cell-killing payloads to specific cells. Seattle Genetics' proprietary technology employs synthetic, highly potent drugs attached to antibodies through stable linker systems. The linkers are designed to release the drug payload only under specific conditions once inside target cells, thereby sparing non-target cells many of the toxic effects of traditional chemotherapy.
Seattle Genetics is developing brentuximab vedotin in collaboration with Millennium: The Takeda Oncology Company, under which Seattle Genetics has U.S. and Canadian commercialization rights and Millennium has exclusive rights to commercialize the product in the rest of the world. Seattle Genetics and Millennium are jointly funding worldwide development costs for brentuximab vedotin on a 50:50 basis. Additionally, Seattle Genetics has nine ongoing ADC collaborations with leading biotechnology and pharmaceutical companies. To date, Seattle Genetics has generated approximately $110 million through ADC technology license agreements.
SOURCE: Seattle Genetics, Inc.
Seattle Genetics, Inc.
Peggy Pinkston, 425-527-4160
ppinkston@seagen.com
Hallo,

wollte ich hier natürlich auch gerade reinstellen...
Schön, dass die Kerntechnologie von SGEN nun noch besser mit Patenten abgesichert ist, denn das ist ja das eigentlich Interessante an der Firma!

Mal seh´n, was der Kurs draus macht, immerhin liegt er ja seit Kurzem inmitten des Gaps bis ca. 13$, vielleicht steigt´s ja nun dahin und schließt die Lücke!

Gruß q.

wollte ich hier natürlich auch gerade reinstellen...
Schön, dass die Kerntechnologie von SGEN nun noch besser mit Patenten abgesichert ist, denn das ist ja das eigentlich Interessante an der Firma!

Mal seh´n, was der Kurs draus macht, immerhin liegt er ja seit Kurzem inmitten des Gaps bis ca. 13$, vielleicht steigt´s ja nun dahin und schließt die Lücke!

Gruß q.
Hallo,

hier mal eine Meldung über Insider-Käufe bei SGEN. Ist zwar nicht die Welt, aber immerhin doch schon ein Sümmchen, was der Herr Berger ier angelegt hat (13.000 Stück zu ca. 154.000 $).
Das Ganze ist erst ein paar Tage her und immerhin zu Kursen von fast 12$, was erstaunlich ist.

Hoffen wir mal, dass der Herr mehr weiß als wir und mit seinem Insiderkauf nicht danebenliegt (was auch oft genug vorkommt).

Gruß q.
SGEN (SEATTLE GENETICS INC /WA)
Show: All Transactions | Grouped by Insider Insider Name Insider Title
(if any) Insider Type Num Shares Price Per Share Amount ($) Transaction Date
BERGER FRANKLIN M D 5,000 11.76 58,794 2010-03-12
BERGER FRANKLIN M D 8,000 11.99 95,920 2010-03-10
AKKARAJU SRINIVAS D 2,700 9.21 24,867 2009-06-25
AKKARAJU SRINIVAS D 600 9.21 5,526 2009-06-25
AKKARAJU SRINIVAS D 2,200 9.20 20,240 2009-06-25

hier mal eine Meldung über Insider-Käufe bei SGEN. Ist zwar nicht die Welt, aber immerhin doch schon ein Sümmchen, was der Herr Berger ier angelegt hat (13.000 Stück zu ca. 154.000 $).
Das Ganze ist erst ein paar Tage her und immerhin zu Kursen von fast 12$, was erstaunlich ist.

Hoffen wir mal, dass der Herr mehr weiß als wir und mit seinem Insiderkauf nicht danebenliegt (was auch oft genug vorkommt).

Gruß q.
SGEN (SEATTLE GENETICS INC /WA)
Show: All Transactions | Grouped by Insider Insider Name Insider Title
(if any) Insider Type Num Shares Price Per Share Amount ($) Transaction Date
BERGER FRANKLIN M D 5,000 11.76 58,794 2010-03-12
BERGER FRANKLIN M D 8,000 11.99 95,920 2010-03-10
AKKARAJU SRINIVAS D 2,700 9.21 24,867 2009-06-25
AKKARAJU SRINIVAS D 600 9.21 5,526 2009-06-25
AKKARAJU SRINIVAS D 2,200 9.20 20,240 2009-06-25
Antwort auf Beitrag Nr.: 39.144.707 von quepos am 15.03.10 21:29:43Eigentlich sollte F.M. Berger aber doch wissen, was er tut. Hier ein kurzer Abriss:
--
Franklin M. Berger, CFA, has served as a Director of Seattle Genetics since June 2004 and is Chairman of the Audit Committee. Mr. Berger is a biotechnology industry analyst with over 25 years of experience in capital markets and financial analysis. He served most recently as Managing Director, Equity Research and Senior Biotechnology Analyst at J.P. Morgan Securities from 1998 to 2003. In this position, he initiated team coverage of 26 biotechnology companies and was responsible for technical, scientific and clinical due diligence as well as company selection. Previously, Mr. Berger served in similar capacities at Salomon Smith Barney from 1997 to 1998 and Josephthal & Co. from 1991 to 1997. Prior to his work as a biotechnology analyst, he managed Pantagruel Partners, a firm that developed early-stage pharmaceutical compounds for sale to drug companies and venture capital firms. He holds an M.B.A. from the Harvard Graduate School of Business Administration and an M.A. in International Economics and a B.A. in International Relations from Johns Hopkins University. In addition to Seattle Genetics, Mr. Berger serves as a director of VaxGen, Inc., Isotechnika Inc. and Thallion Pharmaceuticals, all publicly-traded biopharmaceutical companies, and HealthShares, Inc., an investment company.
--
Franklin M. Berger, CFA, has served as a Director of Seattle Genetics since June 2004 and is Chairman of the Audit Committee. Mr. Berger is a biotechnology industry analyst with over 25 years of experience in capital markets and financial analysis. He served most recently as Managing Director, Equity Research and Senior Biotechnology Analyst at J.P. Morgan Securities from 1998 to 2003. In this position, he initiated team coverage of 26 biotechnology companies and was responsible for technical, scientific and clinical due diligence as well as company selection. Previously, Mr. Berger served in similar capacities at Salomon Smith Barney from 1997 to 1998 and Josephthal & Co. from 1991 to 1997. Prior to his work as a biotechnology analyst, he managed Pantagruel Partners, a firm that developed early-stage pharmaceutical compounds for sale to drug companies and venture capital firms. He holds an M.B.A. from the Harvard Graduate School of Business Administration and an M.A. in International Economics and a B.A. in International Relations from Johns Hopkins University. In addition to Seattle Genetics, Mr. Berger serves as a director of VaxGen, Inc., Isotechnika Inc. and Thallion Pharmaceuticals, all publicly-traded biopharmaceutical companies, and HealthShares, Inc., an investment company.
Antwort auf Beitrag Nr.: 39.144.707 von quepos am 15.03.10 21:29:43
Hi quepos,
zumindest sind das ja wohl zwei Geldleute:
Srinivas Akkaraju, M.D., Ph.D., has served as a Director of Seattle Genetics since July 2003. Dr. Akkaraju is a Managing Director and lead of New Leaf Venture Partners' West Coast biopharmaceuticals practice. Previously, he served as a Managing Director at Panorama Capital. Before that, he was with J.P. Morgan Partners, serving as a Principal starting in April 2001 and becoming a Partner in January 2005. From October 1998 to April 2001, he was in Business and Corporate Development at Genentech, Inc., most recently as Senior Manager. Prior to joining Genentech, Dr. Akkaraju was a graduate student at Stanford University, where he earned his M.D. and a Ph.D. in Immunology. He received his undergraduate degrees in Biochemistry and Computer Science from Rice University. He is also a director of Barrier Therapeutics.
-----
Franklin M. Berger, CFA, has served as a Director of Seattle Genetics since June 2004 and is Chairman of the Audit Committee. Mr. Berger is a biotechnology industry analyst with over 25 years of experience in capital markets and financial analysis. He served most recently as Managing Director, Equity Research and Senior Biotechnology Analyst at J.P. Morgan Securities from 1998 to 2003. In this position, he initiated team coverage of 26 biotechnology companies and was responsible for technical, scientific and clinical due diligence as well as company selection. Previously, Mr. Berger served in similar capacities at Salomon Smith Barney from 1997 to 1998 and Josephthal & Co. from 1991 to 1997. Prior to his work as a biotechnology analyst, he managed Pantagruel Partners, a firm that developed early-stage pharmaceutical compounds for sale to drug companies and venture capital firms. He holds an M.B.A. from the Harvard Graduate School of Business Administration and an M.A. in International Economics and a B.A. in International Relations from Johns Hopkins University. In addition to Seattle Genetics, Mr. Berger serves as a director of VaxGen, Inc., Isotechnika Inc. and Thallion Pharmaceuticals, all publicly-traded biopharmaceutical companies, and HealthShares, Inc., an investment company.
----------------
Das sagt zwar auch noch nicht viel - vor allem nicht, wie Du richtig anmerkst, dass die "mehr" wissen und das ausnutzen - aber immerhin scheinen sie das Chance/Risiko-Verhältnis als positiv zu bewerten.
Irgendwann im zweiten Quartal kommen entscheidende Daten zu Lintuzumab. Das ist zwar kein ADC und mir sind die Brentuximab-Daten zum Jahresende viel wichtiger, aber sollte Lintu nicht floppen und in die P III können, dann bedeutet das auch finanziell eine große Chance für SGEN.
Hammer hat dazu mal folgendes geschrieben:
Seattle Genetics believes that if lintuzumab manages to prolong survival by two months or more, it could file for approval with the FDA. This will surely lead to a very high adoption rate by physicians, who currently prefer putting elderly patients in clinical trials or even not treating them at all, due to lack of safe and effective treatments. More than 13,000 new cases of AML are expected in the U.S each year, two thirds of which will be diagnosed in elderly patients. Assuming a similar rate in Europe, the overall market potential in developed countries could reach 25,000 patients, which could translate into sales in the $700-900 million range, depending on durability of responses. Evidently, if lintuzumab proves active and safe in elderly AML patients, it will quickly find its way to younger patients as well. In addition, lintuzumab is currently being evaluated in MDS, an indication that is expected generate more than $500 million in sales in 2010 for Celgene’s (CELG) Vidaza.
Allerdings ist bei mir das Vertrauen in beide naked-Programme seit dem Dacetuzumab-Flop so ziemlich weg.
Wie auch immer: Die nächsten zwei, drei Monate werden spannend.
Hi quepos,
zumindest sind das ja wohl zwei Geldleute:
Srinivas Akkaraju, M.D., Ph.D., has served as a Director of Seattle Genetics since July 2003. Dr. Akkaraju is a Managing Director and lead of New Leaf Venture Partners' West Coast biopharmaceuticals practice. Previously, he served as a Managing Director at Panorama Capital. Before that, he was with J.P. Morgan Partners, serving as a Principal starting in April 2001 and becoming a Partner in January 2005. From October 1998 to April 2001, he was in Business and Corporate Development at Genentech, Inc., most recently as Senior Manager. Prior to joining Genentech, Dr. Akkaraju was a graduate student at Stanford University, where he earned his M.D. and a Ph.D. in Immunology. He received his undergraduate degrees in Biochemistry and Computer Science from Rice University. He is also a director of Barrier Therapeutics.
-----
Franklin M. Berger, CFA, has served as a Director of Seattle Genetics since June 2004 and is Chairman of the Audit Committee. Mr. Berger is a biotechnology industry analyst with over 25 years of experience in capital markets and financial analysis. He served most recently as Managing Director, Equity Research and Senior Biotechnology Analyst at J.P. Morgan Securities from 1998 to 2003. In this position, he initiated team coverage of 26 biotechnology companies and was responsible for technical, scientific and clinical due diligence as well as company selection. Previously, Mr. Berger served in similar capacities at Salomon Smith Barney from 1997 to 1998 and Josephthal & Co. from 1991 to 1997. Prior to his work as a biotechnology analyst, he managed Pantagruel Partners, a firm that developed early-stage pharmaceutical compounds for sale to drug companies and venture capital firms. He holds an M.B.A. from the Harvard Graduate School of Business Administration and an M.A. in International Economics and a B.A. in International Relations from Johns Hopkins University. In addition to Seattle Genetics, Mr. Berger serves as a director of VaxGen, Inc., Isotechnika Inc. and Thallion Pharmaceuticals, all publicly-traded biopharmaceutical companies, and HealthShares, Inc., an investment company.
----------------
Das sagt zwar auch noch nicht viel - vor allem nicht, wie Du richtig anmerkst, dass die "mehr" wissen und das ausnutzen - aber immerhin scheinen sie das Chance/Risiko-Verhältnis als positiv zu bewerten.
Irgendwann im zweiten Quartal kommen entscheidende Daten zu Lintuzumab. Das ist zwar kein ADC und mir sind die Brentuximab-Daten zum Jahresende viel wichtiger, aber sollte Lintu nicht floppen und in die P III können, dann bedeutet das auch finanziell eine große Chance für SGEN.
Hammer hat dazu mal folgendes geschrieben:
Seattle Genetics believes that if lintuzumab manages to prolong survival by two months or more, it could file for approval with the FDA. This will surely lead to a very high adoption rate by physicians, who currently prefer putting elderly patients in clinical trials or even not treating them at all, due to lack of safe and effective treatments. More than 13,000 new cases of AML are expected in the U.S each year, two thirds of which will be diagnosed in elderly patients. Assuming a similar rate in Europe, the overall market potential in developed countries could reach 25,000 patients, which could translate into sales in the $700-900 million range, depending on durability of responses. Evidently, if lintuzumab proves active and safe in elderly AML patients, it will quickly find its way to younger patients as well. In addition, lintuzumab is currently being evaluated in MDS, an indication that is expected generate more than $500 million in sales in 2010 for Celgene’s (CELG) Vidaza.
Allerdings ist bei mir das Vertrauen in beide naked-Programme seit dem Dacetuzumab-Flop so ziemlich weg.

Wie auch immer: Die nächsten zwei, drei Monate werden spannend.
Antwort auf Beitrag Nr.: 39.145.001 von quepos am 15.03.10 22:06:06
Da hatten wir gerade die selbe Idee...
Da hatten wir gerade die selbe Idee...

Muin,

(mal sehn, ob wir wieder die selbe Idee haben...)
Gibt mal Wieder Futter für den Kurs (hoffentlich):
Gruß q.
-------------
Press Release Source: Seattle Genetics, Inc. On Thursday April 8, 2010, 3:15 am EDT
BOTHELL, Wash. & CAMBRIDGE, Mass. & OSAKA, Japan--(BUSINESS WIRE)--Seattle Genetics, Inc. (NASDAQ:SGEN - News), Takeda Pharmaceutical Company Limited (TOKYO:4502 - News) and its wholly owned subsidiary Millennium: The Takeda Oncology Company today announced the initiation of a phase III clinical trial of brentuximab vedotin (SGN-35) for post-transplant Hodgkin lymphoma patients. Brentuximab vedotin is an antibody-drug conjugate (ADC) targeted to CD30, which is expressed on malignant Hodgkin lymphoma cells. The phase III trial, also known as AETHERA, will evaluate brentuximab vedotin versus placebo for the treatment of patients at high risk of residual Hodgkin lymphoma following autologous stem cell transplant (ASCT).
“Our initial approval strategy for brentuximab vedotin is based on our ongoing pivotal trial in relapsed and refractory Hodgkin lymphoma, from which data are expected in the second half of 2010. The pivotal trial is designed to form the basis for registrational filings with the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) during the first half of 2011 under the accelerated approval and conditional authorization regulations respectively,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer of Seattle Genetics. “The AETHERA trial is part of our broader development strategy and is designed to fulfill regulatory requirements for full approval in the United States and Europe. In addition, this trial will provide data on the use of brentuximab vedotin earlier in Hodgkin lymphoma therapy as part of an integrated second-line regimen with ASCT.”
“Brentuximab vedotin has the potential to address a large unmet medical need in the patient community. By utilizing Seattle Genetics’ ADC technology, the molecule selectively targets CD30, potentially making it the first new drug in more than a decade for patients with relapsed Hodgkin lymphoma,” said Nancy Simonian, M.D., Chief Medical Officer of Millennium. “The initiation of this phase III trial is aimed to support the ongoing pivotal trial of brentuximab vedotin and further enables the Takeda Group to move toward its goal of global oncology leadership.”
The AETHERA trial is a randomized, double-blind, placebo-controlled phase III study comparing progression-free survival in approximately 325 post-ASCT patients receiving brentuximab vedotin to those receiving placebo. Patients must be at high risk for residual Hodgkin lymphoma, defined as those with a history of refractory Hodgkin lymphoma, those who relapse or progress within one year from receiving front-line chemotherapy and/or those who have disease outside of the lymph nodes at the time of pre-ASCT relapse. Secondary endpoints of the trial include overall survival, safety and tolerability. Patients will receive brentuximab vedotin every three weeks for up to approximately one year. This international multi-center trial will be conducted in the United States, Europe and Russia.
Seattle Genetics is developing brentuximab vedotin in collaboration with Millennium, under which Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize brentuximab vedotin in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for brentuximab vedotin on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
Under the collaboration, Seattle Genetics and Millennium are conducting a pivotal trial of brentuximab vedotin for relapsed and refractory Hodgkin lymphoma under a Special Protocol Assessment (SPA) with the FDA, and top-line data are expected in the second half of 2010. The pivotal trial also received EU Centralized Scientific Advice from the EMA. The pivotal trial is designed to form the basis for registrational filings with the FDA and EMA under the accelerated approval and conditional authorization regulations. These regulations provide a mechanism for making promising products for life-threatening diseases commercially available on the basis of preliminary evidence prior to formal demonstration of patient benefit.
In addition, the companies are conducting a phase II trial for relapsed and refractory systemic anaplastic large cell lymphoma, a phase II retreatment trial for relapsed patients who previously responded to brentuximab vedotin therapy, and a phase I combination trial for front-line Hodgkin lymphoma.
About Hodgkin Lymphoma
Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is distinguished pathologically from other types of lymphoma by the presence of one characteristic type of cell, known as the Reed-Sternberg cell. A defining attribute of the Reed-Sternberg cell is its expression of the CD30 antigen. According to the American Cancer Society, approximately 8,500 cases of Hodgkin lymphoma were diagnosed in the United States during 2009.
About Seattle Genetics
Seattle Genetics is a clinical stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company’s lead product candidate, brentuximab vedotin, is in a pivotal trial under an SPA with the FDA. Brentuximab vedotin is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has four other product candidates in ongoing clinical trials: lintuzumab (SGN-33), dacetuzumab (SGN-40), SGN-70 and SGN-75. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, MedImmune, a subsidiary of AstraZeneca, Millennium: The Takeda Oncology Company and Progenics, as well as an ADC co-development agreement with Agensys, an affiliate of Astellas. More information can be found at http://cts.businesswire.com/ct/CT?id=smartlink&url=http%3A%2…
About Takeda Pharmaceutical Company Limited
Located in Osaka, Japan, Takeda is a research-based global company with its main focus on pharmaceuticals. As the largest pharmaceutical company in Japan and one of the global leaders of the industry, Takeda is committed to striving toward better health for individuals and progress in medicine by developing superior pharmaceutical products. Additional information about Takeda is available through its corporate website, www.takeda.com
About Millennium
Millennium: The Takeda Oncology Company, a leading biopharmaceutical company based in Cambridge, Mass., markets a first-in-class proteasome inhibitor, and has a robust clinical development pipeline of product candidates. Millennium Pharmaceuticals, Inc. was acquired by Takeda Pharmaceutical Company Ltd. in May, 2008. The Company’s research, development and commercialization activities are focused in oncology. Additional information about Millennium is available through its website, http://cts.businesswire.com/ct/CT?id=smartlink&url=http%3A%2…
For Seattle Genetics: Certain of the statements made in this press release are forward looking, such as those, among others, relating to the submission for approval of and eventual approval of brentuximab vedotin. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include the inability to show statistically significant benefit in our pivotal trial or the phase III trial, the incidence of adverse events as brentuximab vedotin advances in these clinical trials or delays resulting from requests for additional information or clinical evidence from the FDA or EMA. More information about the risks and uncertainties faced by Seattle Genetics is contained in the company’s annual report on Form 10-K for the year ended December 31, 2009 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6241448&lang=en" target="_blank" rel="nofollow ugc noopener">http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6241448&lang=en
MULTIMEDIA AVAILABLE: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6241448
Contact:
Seattle GeneticsPeggy Pinkston, 425-527-4160ppinkston@seagen.comorMillenniumLauren Musto, 617-551-7848lauren.musto@mpi.comorTakedaSeizo Masuda, +81 33 278 2037Masuda_Seizo@takeda.co.jp

(mal sehn, ob wir wieder die selbe Idee haben...)
Gibt mal Wieder Futter für den Kurs (hoffentlich):
Gruß q.
-------------
Press Release Source: Seattle Genetics, Inc. On Thursday April 8, 2010, 3:15 am EDT
BOTHELL, Wash. & CAMBRIDGE, Mass. & OSAKA, Japan--(BUSINESS WIRE)--Seattle Genetics, Inc. (NASDAQ:SGEN - News), Takeda Pharmaceutical Company Limited (TOKYO:4502 - News) and its wholly owned subsidiary Millennium: The Takeda Oncology Company today announced the initiation of a phase III clinical trial of brentuximab vedotin (SGN-35) for post-transplant Hodgkin lymphoma patients. Brentuximab vedotin is an antibody-drug conjugate (ADC) targeted to CD30, which is expressed on malignant Hodgkin lymphoma cells. The phase III trial, also known as AETHERA, will evaluate brentuximab vedotin versus placebo for the treatment of patients at high risk of residual Hodgkin lymphoma following autologous stem cell transplant (ASCT).
“Our initial approval strategy for brentuximab vedotin is based on our ongoing pivotal trial in relapsed and refractory Hodgkin lymphoma, from which data are expected in the second half of 2010. The pivotal trial is designed to form the basis for registrational filings with the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) during the first half of 2011 under the accelerated approval and conditional authorization regulations respectively,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer of Seattle Genetics. “The AETHERA trial is part of our broader development strategy and is designed to fulfill regulatory requirements for full approval in the United States and Europe. In addition, this trial will provide data on the use of brentuximab vedotin earlier in Hodgkin lymphoma therapy as part of an integrated second-line regimen with ASCT.”
“Brentuximab vedotin has the potential to address a large unmet medical need in the patient community. By utilizing Seattle Genetics’ ADC technology, the molecule selectively targets CD30, potentially making it the first new drug in more than a decade for patients with relapsed Hodgkin lymphoma,” said Nancy Simonian, M.D., Chief Medical Officer of Millennium. “The initiation of this phase III trial is aimed to support the ongoing pivotal trial of brentuximab vedotin and further enables the Takeda Group to move toward its goal of global oncology leadership.”
The AETHERA trial is a randomized, double-blind, placebo-controlled phase III study comparing progression-free survival in approximately 325 post-ASCT patients receiving brentuximab vedotin to those receiving placebo. Patients must be at high risk for residual Hodgkin lymphoma, defined as those with a history of refractory Hodgkin lymphoma, those who relapse or progress within one year from receiving front-line chemotherapy and/or those who have disease outside of the lymph nodes at the time of pre-ASCT relapse. Secondary endpoints of the trial include overall survival, safety and tolerability. Patients will receive brentuximab vedotin every three weeks for up to approximately one year. This international multi-center trial will be conducted in the United States, Europe and Russia.
Seattle Genetics is developing brentuximab vedotin in collaboration with Millennium, under which Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize brentuximab vedotin in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for brentuximab vedotin on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
Under the collaboration, Seattle Genetics and Millennium are conducting a pivotal trial of brentuximab vedotin for relapsed and refractory Hodgkin lymphoma under a Special Protocol Assessment (SPA) with the FDA, and top-line data are expected in the second half of 2010. The pivotal trial also received EU Centralized Scientific Advice from the EMA. The pivotal trial is designed to form the basis for registrational filings with the FDA and EMA under the accelerated approval and conditional authorization regulations. These regulations provide a mechanism for making promising products for life-threatening diseases commercially available on the basis of preliminary evidence prior to formal demonstration of patient benefit.
In addition, the companies are conducting a phase II trial for relapsed and refractory systemic anaplastic large cell lymphoma, a phase II retreatment trial for relapsed patients who previously responded to brentuximab vedotin therapy, and a phase I combination trial for front-line Hodgkin lymphoma.
About Hodgkin Lymphoma
Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is distinguished pathologically from other types of lymphoma by the presence of one characteristic type of cell, known as the Reed-Sternberg cell. A defining attribute of the Reed-Sternberg cell is its expression of the CD30 antigen. According to the American Cancer Society, approximately 8,500 cases of Hodgkin lymphoma were diagnosed in the United States during 2009.
About Seattle Genetics
Seattle Genetics is a clinical stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company’s lead product candidate, brentuximab vedotin, is in a pivotal trial under an SPA with the FDA. Brentuximab vedotin is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has four other product candidates in ongoing clinical trials: lintuzumab (SGN-33), dacetuzumab (SGN-40), SGN-70 and SGN-75. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, MedImmune, a subsidiary of AstraZeneca, Millennium: The Takeda Oncology Company and Progenics, as well as an ADC co-development agreement with Agensys, an affiliate of Astellas. More information can be found at http://cts.businesswire.com/ct/CT?id=smartlink&url=http%3A%2…
About Takeda Pharmaceutical Company Limited
Located in Osaka, Japan, Takeda is a research-based global company with its main focus on pharmaceuticals. As the largest pharmaceutical company in Japan and one of the global leaders of the industry, Takeda is committed to striving toward better health for individuals and progress in medicine by developing superior pharmaceutical products. Additional information about Takeda is available through its corporate website, www.takeda.com
About Millennium
Millennium: The Takeda Oncology Company, a leading biopharmaceutical company based in Cambridge, Mass., markets a first-in-class proteasome inhibitor, and has a robust clinical development pipeline of product candidates. Millennium Pharmaceuticals, Inc. was acquired by Takeda Pharmaceutical Company Ltd. in May, 2008. The Company’s research, development and commercialization activities are focused in oncology. Additional information about Millennium is available through its website, http://cts.businesswire.com/ct/CT?id=smartlink&url=http%3A%2…
For Seattle Genetics: Certain of the statements made in this press release are forward looking, such as those, among others, relating to the submission for approval of and eventual approval of brentuximab vedotin. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include the inability to show statistically significant benefit in our pivotal trial or the phase III trial, the incidence of adverse events as brentuximab vedotin advances in these clinical trials or delays resulting from requests for additional information or clinical evidence from the FDA or EMA. More information about the risks and uncertainties faced by Seattle Genetics is contained in the company’s annual report on Form 10-K for the year ended December 31, 2009 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6241448&lang=en" target="_blank" rel="nofollow ugc noopener">http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6241448&lang=en
MULTIMEDIA AVAILABLE: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6241448
Contact:
Seattle GeneticsPeggy Pinkston, 425-527-4160ppinkston@seagen.comorMillenniumLauren Musto, 617-551-7848lauren.musto@mpi.comorTakedaSeizo Masuda, +81 33 278 2037Masuda_Seizo@takeda.co.jp
Solche Nachrichten sind immer gern gesehen. Es zeigt ein mal mehr, dass SGEN über eine echte Platform-Technik verfügt, auf der stabile Partnerschaften begründet sind:
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq: SGEN) today announced that Genentech, Inc., a wholly owned member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), will pay $9.5 million to renew exclusive licenses to specific targets and extend the research term under the parties’ existing antibody-drug conjugate (ADC) collaboration agreement. Under the terms of the agreement, Genentech has rights to use Seattle Genetics’ ADC technology with antibodies against targets selected by Genentech. Genentech is responsible for research, product development, manufacturing and commercialization. Seattle Genetics is entitled to receive fees, progress-dependent milestone payments and royalties on net sales of any resulting ADC products.
.“We believe this collaboration, coupled with our multiple other ADC collaborations, reinforces the value of our industry-leading technology,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “For Seattle Genetics, these collaborations broaden the reach of our technology into diverse disease settings where empowered antibodies may be able to address significant unmet medical needs. In addition, these deals provide a substantial source of capital as we continue to advance our own pipeline of proprietary antibodies and ADCs for cancer.”
Seattle Genetics has generated approximately $120 million through ADC technology license agreements with leading biotechnology and pharmaceutical companies. ADCs utilize the targeting ability of monoclonal antibodies to deliver potent, cell-killing payloads to specific cells. Seattle Genetics’ proprietary technology employs synthetic, highly potent drugs attached to antibodies through stable linker systems. The linkers are designed to release the drug payload only under specific conditions once inside target cells, thereby sparing non-target cells many of the toxic effects of traditional chemotherapy.
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq: SGEN) today announced that Genentech, Inc., a wholly owned member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), will pay $9.5 million to renew exclusive licenses to specific targets and extend the research term under the parties’ existing antibody-drug conjugate (ADC) collaboration agreement. Under the terms of the agreement, Genentech has rights to use Seattle Genetics’ ADC technology with antibodies against targets selected by Genentech. Genentech is responsible for research, product development, manufacturing and commercialization. Seattle Genetics is entitled to receive fees, progress-dependent milestone payments and royalties on net sales of any resulting ADC products.
.“We believe this collaboration, coupled with our multiple other ADC collaborations, reinforces the value of our industry-leading technology,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “For Seattle Genetics, these collaborations broaden the reach of our technology into diverse disease settings where empowered antibodies may be able to address significant unmet medical needs. In addition, these deals provide a substantial source of capital as we continue to advance our own pipeline of proprietary antibodies and ADCs for cancer.”
Seattle Genetics has generated approximately $120 million through ADC technology license agreements with leading biotechnology and pharmaceutical companies. ADCs utilize the targeting ability of monoclonal antibodies to deliver potent, cell-killing payloads to specific cells. Seattle Genetics’ proprietary technology employs synthetic, highly potent drugs attached to antibodies through stable linker systems. The linkers are designed to release the drug payload only under specific conditions once inside target cells, thereby sparing non-target cells many of the toxic effects of traditional chemotherapy.
Antwort auf Beitrag Nr.: 39.367.119 von SLGramann am 20.04.10 13:35:52Hallo,

gerade erst die Pressemitteilung gelesen, MLNM/Roche sind ja auch ein starker Partner, die 9,5 Mille auch kein ganz kleiner Betrag.
Die erhoffen sich schon was!

Die Kursreaktion ist ja erstmal nicht umwerfend, aber muß auch nicht. Der Weg ist richtig und das Ziel noch weit, auch erfolgte wieder aus technischer Sicht ein Rückgang an die Unterseite der noch offenen Kurslücke bis 13,xx $.

Aber das kann ja jetzt einen neuen Anlauf geben.
Gruß q.

gerade erst die Pressemitteilung gelesen, MLNM/Roche sind ja auch ein starker Partner, die 9,5 Mille auch kein ganz kleiner Betrag.
Die erhoffen sich schon was!

Die Kursreaktion ist ja erstmal nicht umwerfend, aber muß auch nicht. Der Weg ist richtig und das Ziel noch weit, auch erfolgte wieder aus technischer Sicht ein Rückgang an die Unterseite der noch offenen Kurslücke bis 13,xx $.

Aber das kann ja jetzt einen neuen Anlauf geben.
Gruß q.
So Hallo und so,

Zahlen kamen anscheinend gut an.
Trotz der griechischen Grippe extrem starke Entwicklung die letzten Tage.

Gapschluss bis 13,xx$ ante portas.
------------
On Tuesday April 27, 2010, 6:22 pm EDT
BOTHELL, Wash. (AP) -- Biotechnology company Seattle Genetics Inc. on Tuesday reported a first-quarter profit on a boost in revenue from the company's key partner, Genentech.
The company earned $11.5 million, or 11 cents per share, compared with a loss of $27.3 million, or 33 cents per share, during the same period a year prior. Revenue surged to $46.5 million from $9.1 million.
Analysts surveyed by Thomson Reuters expected profit of 4 cents per share on $41 million in revenue.
The company's key partnership is with Genentech, now part of Roche. The companies are developing potential cancer treatments. Seattle Genetics said the profit boost was driven by the earned portion of payments in the company's collaboration with Genentech.
Shares of Seattle Genetics fell 30 cents, or 2.6 percent, to close at $11.32. The stock has traded between $8.12 and $14.94 over the last 52 weeks.
Buzz up! 1 SendSharePrintShare this page
DeliciousTwitterMyspaceDiggStumbleUponFacebook
Related Headlines

Zahlen kamen anscheinend gut an.
Trotz der griechischen Grippe extrem starke Entwicklung die letzten Tage.

Gapschluss bis 13,xx$ ante portas.
------------
On Tuesday April 27, 2010, 6:22 pm EDT
BOTHELL, Wash. (AP) -- Biotechnology company Seattle Genetics Inc. on Tuesday reported a first-quarter profit on a boost in revenue from the company's key partner, Genentech.
The company earned $11.5 million, or 11 cents per share, compared with a loss of $27.3 million, or 33 cents per share, during the same period a year prior. Revenue surged to $46.5 million from $9.1 million.
Analysts surveyed by Thomson Reuters expected profit of 4 cents per share on $41 million in revenue.
The company's key partnership is with Genentech, now part of Roche. The companies are developing potential cancer treatments. Seattle Genetics said the profit boost was driven by the earned portion of payments in the company's collaboration with Genentech.
Shares of Seattle Genetics fell 30 cents, or 2.6 percent, to close at $11.32. The stock has traded between $8.12 and $14.94 over the last 52 weeks.
Buzz up! 1 SendSharePrintShare this page
DeliciousTwitterMyspaceDiggStumbleUponFacebook
Related Headlines
Hallo,

die Frage zu Beginn war ja \"Chance zum Einstieg?\", sie lässt sich nun mit \"ja\" beantworten, obbwohl mein durchschnittlicher Kaufkurs noch 10% über dem Minimum lag.
Aber das ist ja auch Glückssache bei angeschossenen Biotech-Aktien.

Habe jetzt aber auch die \"Chance zum Ausstieg\" genutzt, und eine Teilposition versilbert. Nach Gap-Close und über 40% Gewinn bringt das etwas Pulver für eventuell anstehende andere Schnäppchen im \"Schuldenkrisen-Hype\".

Als wenn nicht die ganze Welt seit Jahren und Jahrzehnten über beide Ohren verschuldet wäre...

Aber das wird halt jetzt so gespielt.
Die €/$-Parität war ja angeblich eh schon beschlossen, das wäre ein Argument für weiteres Halten gewesen, aber mal sehn was kommt.
Bleibe ja weiter investiert!

Gruß q.

die Frage zu Beginn war ja \"Chance zum Einstieg?\", sie lässt sich nun mit \"ja\" beantworten, obbwohl mein durchschnittlicher Kaufkurs noch 10% über dem Minimum lag.
Aber das ist ja auch Glückssache bei angeschossenen Biotech-Aktien.

Habe jetzt aber auch die \"Chance zum Ausstieg\" genutzt, und eine Teilposition versilbert. Nach Gap-Close und über 40% Gewinn bringt das etwas Pulver für eventuell anstehende andere Schnäppchen im \"Schuldenkrisen-Hype\".

Als wenn nicht die ganze Welt seit Jahren und Jahrzehnten über beide Ohren verschuldet wäre...

Aber das wird halt jetzt so gespielt.
Die €/$-Parität war ja angeblich eh schon beschlossen, das wäre ein Argument für weiteres Halten gewesen, aber mal sehn was kommt.
Bleibe ja weiter investiert!

Gruß q.
Moin,

bin nun erstmal ganz raus, Aktie ist ja auch super gelaufen, zumal ich bei dem Dip kürzlich wieder nachgekauft hatte.

Ein paar Gewinne mitnehmen schadet jedoch nicht, werde SGEN aber weiterhin verfolgen, mal sehen, was so auf der ASCO-Konferenz so kommt.
Etwas nervig fand ich die hohen Spreads, durch die man jedesmal ein paar Prozent verliert, das ist ziemlich happig.

Aktie ist hier in D aber auch sehr wenig gehandelt, ich hatte selbst schon einen erheblichen Anteil daran.
Werde daher wohl nächstes Mal direkt an der NASDAQ kaufen, wenn das geht.
Bis denne
Gruß q.

bin nun erstmal ganz raus, Aktie ist ja auch super gelaufen, zumal ich bei dem Dip kürzlich wieder nachgekauft hatte.

Ein paar Gewinne mitnehmen schadet jedoch nicht, werde SGEN aber weiterhin verfolgen, mal sehen, was so auf der ASCO-Konferenz so kommt.
Etwas nervig fand ich die hohen Spreads, durch die man jedesmal ein paar Prozent verliert, das ist ziemlich happig.

Aktie ist hier in D aber auch sehr wenig gehandelt, ich hatte selbst schon einen erheblichen Anteil daran.
Werde daher wohl nächstes Mal direkt an der NASDAQ kaufen, wenn das geht.
Bis denne
Gruß q.
Moin,

hier haben wir auch schon den ASCO-Abstract bezüglich Brentuximab-vedotin, liest sich auf jeden Fall gut...
q.
-------------------------
Meeting: 2010 ASCO Annual Meeting
Citation: J Clin Oncol 28:7s, 2010 (suppl; abstr 8062)
Abstract No: 8062
Attend this session at the ASCO Annual Meeting!
Session: Lymphoma and Plasma Cell Disorders
Type: General Poster Session
Time: Saturday June 5, 8:00 AM to 12:00 PM
Location: S Hall A2
Personalize your Annual Meeting experience with a suggested or customized itinerary!
Author(s): N. Bartlett, L. E. Grove, D. A. Kennedy, E. L. Sievers, A. Forero-Torres; Washington University, St Louis, MO; Seattle Genetics, Inc., Bothell, WA; University of Alabama at Birmingham, Birmingham, AL
Abstract:
Background: Brentuximab vedotin is an anti-CD30 antibody conjugated to the highly potent antitubulin agent, monomethyl auristatin E (MMAE), by a plasma-stable linker. Brentuximab vedotin selectively induces apoptosis in CD30+ cells by binding, internalizing, and releasing MMAE. In phase I clinical studies, good tolerability and antitumor activity were demonstrated in patients with CD30+ hematologic malignancies. This case series describes patients who have experienced relapse and were retreated with brentuximab vedotin. Methods: Heavily pretreated, relapsed or refractory patients with CD30+ hematologic malignancies have been retreated with brentuximab vedotin in 3 multicenter studies. Patients had SD with decreasing tumor volume or better (Cheson 2007) with prior brentuximab vedotin treatment, and subsequently experienced relapse. In their retreatment experiences, patients received brentuximab vedotin IV infusions of 1 mg/kg qweek or 1.8 mg/kg q3week, depending on the study. Results: Across these 3 studies, 7 patients had 8 retreatment experiences. Patients had either ALCL (1) or HL (6); ages ranged from 28-39 years. At baseline prior to retreatment, ECOG performance statuses were 0/1 (7), or 2 (1). Since the prior experience with brentuximab vedotin, the number of chemotherapy regimens was either 0 (4) or 1-3 (4), and 1 patient had an autologous stem cell transplant. Adverse events that occurred in more than 1 patient with retreatment were upper respiratory tract infection (3) and peripheral sensory neuropathy (2); all treatment-related AEs were G1/2 in severity. Among the retreatment experiences, there were 2 CR, 4 PR, and 2 SD; tumor regression was observed in all instances. All objective responses were observed 5-13 weeks after the start of retreatment, and retreatment response durations of 52+ weeks have been demonstrated. Conclusions: Retreatment with brentuximab vedotin was well tolerated. Objective responses were observed (6 of 8) with brentuximab vedotin retreatment in this heavily pretreated population. Enrollment to a phase 2 retreatment study in patients who previously experienced an objective response to brentuximab vedotin is ongoing.

hier haben wir auch schon den ASCO-Abstract bezüglich Brentuximab-vedotin, liest sich auf jeden Fall gut...
q.
-------------------------
Meeting: 2010 ASCO Annual Meeting
Citation: J Clin Oncol 28:7s, 2010 (suppl; abstr 8062)
Abstract No: 8062
Attend this session at the ASCO Annual Meeting!
Session: Lymphoma and Plasma Cell Disorders
Type: General Poster Session
Time: Saturday June 5, 8:00 AM to 12:00 PM
Location: S Hall A2
Personalize your Annual Meeting experience with a suggested or customized itinerary!
Author(s): N. Bartlett, L. E. Grove, D. A. Kennedy, E. L. Sievers, A. Forero-Torres; Washington University, St Louis, MO; Seattle Genetics, Inc., Bothell, WA; University of Alabama at Birmingham, Birmingham, AL
Abstract:
Background: Brentuximab vedotin is an anti-CD30 antibody conjugated to the highly potent antitubulin agent, monomethyl auristatin E (MMAE), by a plasma-stable linker. Brentuximab vedotin selectively induces apoptosis in CD30+ cells by binding, internalizing, and releasing MMAE. In phase I clinical studies, good tolerability and antitumor activity were demonstrated in patients with CD30+ hematologic malignancies. This case series describes patients who have experienced relapse and were retreated with brentuximab vedotin. Methods: Heavily pretreated, relapsed or refractory patients with CD30+ hematologic malignancies have been retreated with brentuximab vedotin in 3 multicenter studies. Patients had SD with decreasing tumor volume or better (Cheson 2007) with prior brentuximab vedotin treatment, and subsequently experienced relapse. In their retreatment experiences, patients received brentuximab vedotin IV infusions of 1 mg/kg qweek or 1.8 mg/kg q3week, depending on the study. Results: Across these 3 studies, 7 patients had 8 retreatment experiences. Patients had either ALCL (1) or HL (6); ages ranged from 28-39 years. At baseline prior to retreatment, ECOG performance statuses were 0/1 (7), or 2 (1). Since the prior experience with brentuximab vedotin, the number of chemotherapy regimens was either 0 (4) or 1-3 (4), and 1 patient had an autologous stem cell transplant. Adverse events that occurred in more than 1 patient with retreatment were upper respiratory tract infection (3) and peripheral sensory neuropathy (2); all treatment-related AEs were G1/2 in severity. Among the retreatment experiences, there were 2 CR, 4 PR, and 2 SD; tumor regression was observed in all instances. All objective responses were observed 5-13 weeks after the start of retreatment, and retreatment response durations of 52+ weeks have been demonstrated. Conclusions: Retreatment with brentuximab vedotin was well tolerated. Objective responses were observed (6 of 8) with brentuximab vedotin retreatment in this heavily pretreated population. Enrollment to a phase 2 retreatment study in patients who previously experienced an objective response to brentuximab vedotin is ongoing.
Seattle Genetics, Growing Up in a Hurry With Millennium, Aims to Make Most of Cancer Drug
Luke Timmerman 5/26/10
Seattle Genetics has spent more than a decade thinking about cutting-edge biology, chemistry, and clinical trials to prove its drug candidates work. Then last week, for the first time in nine years I’ve been reporting on CEO Clay Siegall, he talked with passion about things like manufacturing, inventory, quality assurance, quality control, and insurance reimbursement.
It all might sound awfully boring. But it’s a sure sign that Bothell, WA-based Seattle Genetics (NASDAQ: SGEN), with help from its partner Millennium: The Takeda Oncology Company, is learning fast what it takes to be a mature, commercial biotech company. And while it may be a slow news period for Seattle Genetics (NASDAQ: SGEN), it has to grow up in a hurry, because it is only a few months away from finding out if it has really struck gold with a new therapy for Hodgkin’s disease and related lymphomas.
The big story at Seattle Genetics and Millennium centers on brentuximab vedotin, an “empowered antibody” that specifically seeks out cancer cells and unleashes a potent toxin on them for extra tumor-killing punch. This concept has not lived up to its hype over the past 30 years, but by the second half of 2010, Seattle Genetics and Millennium will learn from a pivotal clinical trial of 100 patients how well this therapy really helps sick patients. If successful, the companies will be able to seek FDA approval in early 2011, and potentially get a faster-than-usual six-month review that the agency sometimes gives to drugs with lifesaving potential. Patients, employees, investors, and an entire field of research is counting on Seattle Genetics and Millennium to deliver the goods. So Siegall & Co. are quietly trying to lay the groundwork now to make sure they are truly ready to make sure this drug is a hit.
Clay Siegall
Clay Siegall
“Our drug has a chance to be a very important drug for patients,” Siegall says.
For those just getting up to speed on this story, here’s a quick refresher. Seattle Genetics, founded in 1998, had its breakout moment in June 2008 at the American Society of Clinical Oncology meeting. That’s when the company released preliminary results showing its experimental treatment was able to completely wipe out or partially shrink tumors for 12 of 38 patients, with mild to moderate fatigue, cough, and nausea as side effects. Results only got better when researchers enrolled a few more patients, and longer-term follow-up data arrived.
A lot of things have fallen into place for Seattle Genetics as a business ever since that appearance at ASCO. It raced to the FDA in early 2009 with a proposal for a pivotal clinical trial, and won the agency’s blessing for the study design. The company got this trial up and running at 27 locations in North America and Europe, and completed enrollment six months ahead of schedule—a lightning pace in oncology, where it’s extremely difficult to enroll patients on time. The company raised more than $200 million from investors in 2009, during a dark period in the overall biotech financial market. In December, Millennium wrote a $60 million upfront check to Seattle Genetics to form a partnership, which left the smaller company with 100 percent of the commercial rights to the experimental drug in the North American market.
Much of what has happened since then has been the sort of behind-the-scenes blocking and tackling that biotech companies need to do, and often fail to do, as they prepare to commercialize a new drug. Part of that effort is in hiring new types of people, with skills in things like quality assurance, regulatory affairs, and quality control that weren’t really necessary in the early years of the company, Siegall says.
The Seattle Genetics payroll, which had just 189 people at the last official count before the June 2008 breakout moment at ASCO, has climbed now to about 310 people. The headcount could go as high as 400 people a year from now if everything goes according to plan, Siegall says.
Much of their effort is going into meetings with Millennium to figure out how to best maximize the potential of brentuximab vedotin, Siegall says. One example was the initiation of a trial called “Aethera.” The main purpose of this study, of more than 320 patients, will be to show the new drug is valuable not just for the sickest of the sick who have run out of other options, but that it also works for larger numbers of patients with earlier-stage forms of Hodgkin’s disease. There’s a trial for patients with another rare disease, anaplastic large cell lymphoma, that carries the same target on malignant cells, called CD30.
Millennium, which has global experience with a $1 billion blockbuster called bortezomib (Velcade), has played a key role in honing the strategy of how to start with one disease, and build from there to make the drug a much bigger seller, Siegall says. Millennium has gone so far as to tell my Boston-based colleague, Ryan McBride, that it believes brentuximab vedotin could be useful for patients with autoimmune diseases, because of its high potency and mild side effect profile for a cancer drug.
“They have been through this kind of planning exercise before,” Siegall says. “They understand what they are doing. They are pros. This is our first product that we plan to launch as a company.”
All that said, Siegall noted that he has been recruiting lots of industry vets for his own talent stable. One person he pointed to, which sort of symbolizes a new breed at Seattle Genetics, is Bruce Seeley, his executive vice president of commercial operations. Seeley comes to this job from Genentech, where he was a senior director for marketing of its breast cancer blockbuster drug trastuzumab (Herceptin), and its “empowered antibody” successor, known as T-DM1. Seeley’s imprint was felt at a recent company meeting in which he started talking about how Seattle Genetics needs to make sure that its drug will get to every patient who wants and needs it, regardless of their ability to pay. This drug will surely cost a small fortune, and any company that blindly ignores critics of drug pricing is setting itself up for some major headaches.
“We’re not naïve,” Siegall says.
Siegall, a great admirer of Genentech, also pointed out one of the things he has learned from that company’s playbook which is being applied to brentuximab vedotin. This is the idea of using the new drug in repeated courses of therapy. This practice, of knocking down a malignancy and then delivering another round to the patient at the time of a relapse, has great appeal to oncologists who like to know they have another arrow left in their quiver just in case a patient turns for the worse. This approach has been one of the important factors in the success of Genentech and Biogen Idec’s hit lymphoma drug, rituximab (Rituxan).
A few anecdotal cases of data on this re-treatment idea will be presented on brentuximab vedotin next month at the ASCO meeting. More rigorous data to answer this question will come later, Siegall says. If this can be proven to be useful, and Seattle Genetics and Millennium can execute their entire vision, brentuximab vedotin could potentially be used in newly-diagnosed patients, and could eliminate the need for radiation, Siegall says. A few leading physicians have “bandied around” the idea that brentuximab vedotin is so powerful that it might be able to eliminate at least a couple components of the four-drug chemo regimen that most patients have to endure today, Siegall says.
It’s a lot to process at once, for a company that simply used to be about R&D. Now it’s about planning ahead to make sure it’s ready to handle success.
“We need to make sure we’re thoughtful about this,” Siegall says. “It’s a balancing act, making sure we have the resources applied right.”
Luke Timmerman is the National Biotechnology Editor for Xconomy.
Luke Timmerman 5/26/10
Seattle Genetics has spent more than a decade thinking about cutting-edge biology, chemistry, and clinical trials to prove its drug candidates work. Then last week, for the first time in nine years I’ve been reporting on CEO Clay Siegall, he talked with passion about things like manufacturing, inventory, quality assurance, quality control, and insurance reimbursement.
It all might sound awfully boring. But it’s a sure sign that Bothell, WA-based Seattle Genetics (NASDAQ: SGEN), with help from its partner Millennium: The Takeda Oncology Company, is learning fast what it takes to be a mature, commercial biotech company. And while it may be a slow news period for Seattle Genetics (NASDAQ: SGEN), it has to grow up in a hurry, because it is only a few months away from finding out if it has really struck gold with a new therapy for Hodgkin’s disease and related lymphomas.
The big story at Seattle Genetics and Millennium centers on brentuximab vedotin, an “empowered antibody” that specifically seeks out cancer cells and unleashes a potent toxin on them for extra tumor-killing punch. This concept has not lived up to its hype over the past 30 years, but by the second half of 2010, Seattle Genetics and Millennium will learn from a pivotal clinical trial of 100 patients how well this therapy really helps sick patients. If successful, the companies will be able to seek FDA approval in early 2011, and potentially get a faster-than-usual six-month review that the agency sometimes gives to drugs with lifesaving potential. Patients, employees, investors, and an entire field of research is counting on Seattle Genetics and Millennium to deliver the goods. So Siegall & Co. are quietly trying to lay the groundwork now to make sure they are truly ready to make sure this drug is a hit.
Clay Siegall
Clay Siegall
“Our drug has a chance to be a very important drug for patients,” Siegall says.
For those just getting up to speed on this story, here’s a quick refresher. Seattle Genetics, founded in 1998, had its breakout moment in June 2008 at the American Society of Clinical Oncology meeting. That’s when the company released preliminary results showing its experimental treatment was able to completely wipe out or partially shrink tumors for 12 of 38 patients, with mild to moderate fatigue, cough, and nausea as side effects. Results only got better when researchers enrolled a few more patients, and longer-term follow-up data arrived.
A lot of things have fallen into place for Seattle Genetics as a business ever since that appearance at ASCO. It raced to the FDA in early 2009 with a proposal for a pivotal clinical trial, and won the agency’s blessing for the study design. The company got this trial up and running at 27 locations in North America and Europe, and completed enrollment six months ahead of schedule—a lightning pace in oncology, where it’s extremely difficult to enroll patients on time. The company raised more than $200 million from investors in 2009, during a dark period in the overall biotech financial market. In December, Millennium wrote a $60 million upfront check to Seattle Genetics to form a partnership, which left the smaller company with 100 percent of the commercial rights to the experimental drug in the North American market.
Much of what has happened since then has been the sort of behind-the-scenes blocking and tackling that biotech companies need to do, and often fail to do, as they prepare to commercialize a new drug. Part of that effort is in hiring new types of people, with skills in things like quality assurance, regulatory affairs, and quality control that weren’t really necessary in the early years of the company, Siegall says.
The Seattle Genetics payroll, which had just 189 people at the last official count before the June 2008 breakout moment at ASCO, has climbed now to about 310 people. The headcount could go as high as 400 people a year from now if everything goes according to plan, Siegall says.
Much of their effort is going into meetings with Millennium to figure out how to best maximize the potential of brentuximab vedotin, Siegall says. One example was the initiation of a trial called “Aethera.” The main purpose of this study, of more than 320 patients, will be to show the new drug is valuable not just for the sickest of the sick who have run out of other options, but that it also works for larger numbers of patients with earlier-stage forms of Hodgkin’s disease. There’s a trial for patients with another rare disease, anaplastic large cell lymphoma, that carries the same target on malignant cells, called CD30.
Millennium, which has global experience with a $1 billion blockbuster called bortezomib (Velcade), has played a key role in honing the strategy of how to start with one disease, and build from there to make the drug a much bigger seller, Siegall says. Millennium has gone so far as to tell my Boston-based colleague, Ryan McBride, that it believes brentuximab vedotin could be useful for patients with autoimmune diseases, because of its high potency and mild side effect profile for a cancer drug.
“They have been through this kind of planning exercise before,” Siegall says. “They understand what they are doing. They are pros. This is our first product that we plan to launch as a company.”
All that said, Siegall noted that he has been recruiting lots of industry vets for his own talent stable. One person he pointed to, which sort of symbolizes a new breed at Seattle Genetics, is Bruce Seeley, his executive vice president of commercial operations. Seeley comes to this job from Genentech, where he was a senior director for marketing of its breast cancer blockbuster drug trastuzumab (Herceptin), and its “empowered antibody” successor, known as T-DM1. Seeley’s imprint was felt at a recent company meeting in which he started talking about how Seattle Genetics needs to make sure that its drug will get to every patient who wants and needs it, regardless of their ability to pay. This drug will surely cost a small fortune, and any company that blindly ignores critics of drug pricing is setting itself up for some major headaches.
“We’re not naïve,” Siegall says.
Siegall, a great admirer of Genentech, also pointed out one of the things he has learned from that company’s playbook which is being applied to brentuximab vedotin. This is the idea of using the new drug in repeated courses of therapy. This practice, of knocking down a malignancy and then delivering another round to the patient at the time of a relapse, has great appeal to oncologists who like to know they have another arrow left in their quiver just in case a patient turns for the worse. This approach has been one of the important factors in the success of Genentech and Biogen Idec’s hit lymphoma drug, rituximab (Rituxan).
A few anecdotal cases of data on this re-treatment idea will be presented on brentuximab vedotin next month at the ASCO meeting. More rigorous data to answer this question will come later, Siegall says. If this can be proven to be useful, and Seattle Genetics and Millennium can execute their entire vision, brentuximab vedotin could potentially be used in newly-diagnosed patients, and could eliminate the need for radiation, Siegall says. A few leading physicians have “bandied around” the idea that brentuximab vedotin is so powerful that it might be able to eliminate at least a couple components of the four-drug chemo regimen that most patients have to endure today, Siegall says.
It’s a lot to process at once, for a company that simply used to be about R&D. Now it’s about planning ahead to make sure it’s ready to handle success.
“We need to make sure we’re thoughtful about this,” Siegall says. “It’s a balancing act, making sure we have the resources applied right.”
Luke Timmerman is the National Biotechnology Editor for Xconomy.
Ein Artikel mit einem Blick auf Lintuzumab. Große Hoffnungen bestehen diesbezüglich ja nicht. Aber vielleicht gibts eine positive Überraschung?
Seattle Genetics’ Dark Horse Drug Candidate Approaches Home Stretch in Leukemia Study
Luke Timmerman 6/22/10
[Correction: 10:05 am Pacific] The buzz around Seattle Genetics (NASDAQ: SGEN) has been about whether it will finally prove, after more than a decade, that an “empowered antibody” can be an effective treatment for cancer. But while fewer people are watching, the company is eagerly awaiting clinical results for a traditional antibody that also has the potential to help cancer patients live longer—and could generate real cash.
The lesser-known drug is called lintuzumab, or SGN-33. This is a so-called “naked” antibody, a Y-shaped protein that’s genetically engineered to zero in on a specific marker on cancer cells that’s seldom found on healthy cells. In this case, the drug is made to hit a marker on tumors of patients with acute myeloid leukemia. Sometime between late this month and August, the company expects to rip off the blind from a study of 210 patients, which will say whether the new drug can help some very sick, elderly patients live a few months longer with acceptable side effects.
Acute myeloid leukemia is a fast-growing, aggressive form of cancer that often strikes people over the age of 60, and makes them too frail to withstand the high-dose chemotherapy they are prescribed. Life expectancy, based on past studies, is usually about 4-6 months. Seattle Genetics decided to swing for the fence in this tough-to-treat group of people after preliminary studies showed that a “mid-teens” percentage of patients saw some degree of partial or complete tumor shrinkage, and there were anecdotal reports of people living longer, CEO Clay Siegall says. If the company can prove its drug helps people live longer with this nasty malignancy, then Seattle Genetics could be in a position to offer this new drug for sale in the not-so-distant future. The disease kills an estimated 8,950 people a year in the U.S., according to the American Cancer Society. [Correction 10:05 am Pacific: An earlier version said lintuzumab could be ready for sale next year, but the company says it hasn't provided guidance yet on timing.]
Wall Street isn’t giving this drug much of a chance. One of the bullish analysts covering the company, Cory Kasimov of JP Morgan, said the consensus among investors is that the lintuzumab trial will fail because of the tough patient population and a “lack of convincing earlier-stage data.” Kasimov gives it a 30 percent shot of winning FDA approval and generating peak annual sales of $350 million by 2016.
This big trial began back in September 2007. The company explained some of the rationale a few months later when it presented early stage results at a meeting of the American Society of Hematology. Of 17 patients who got a variety of doses, five patients had complete tumor remission, while two more had a partial shrinkage of their tumors. The drug was well-tolerated, and patients didn’t develop antibodies to attack the drug itself as a foreign invader, which is known to happen.
Those findings were enough for Seattle Genetics to invest some significant money to get a definitive answer from this ongoing trial, which might be enough to persuade the FDA to clear this drug for sale. The study enrolled 210 patients, and randomly assigned them to get a low-dose chemotherapy drug in addition to the Seattle Genetics treatment, or the low-dose chemotherapy alone. The main goal is to show whether the new drug can help patients live longer—the gold standard measurement in cancer trials. The trial is statistically designed to show a 29 percent lower risk of death among patients on the experimental treatment, and Seattle Genetics is hoping to add a couple extra months of median survival time, without any serious added side effects, Siegall says.
The trial is designed in such a way that researchers, and the company, are blinded to the results to prevent any biases that might skew the study until at least 186 patients who entered the study have died. Then, statisticians will look at which patients got the new drug compared with the standard therapy, to see if there was any benefit. The initial findings will be issued by press release, and then the company will go into more detail at a medical meeting, Siegall says.
It’s possible that if the data for the Seattle Genetics drug is compelling enough from this single study, the company could use it as the basis to whip up an application to the FDA, Siegall says. It’s also possible that it could form part of a body of evidence for the drug, and provide the spark for another rigorous study to prove the drug’s worth, he says.
There are no other drugs on the market for acute myeloid leukemia that hit the same target as the Seattle Genetics drug, since Pfizer announced yesterday that it is pulling gemtuzumab ozogamicin (Mylotarg) off the market.
Strangely enough, the Pfizer drug was a trailblazer in the field of “empowered antibodies” that Seattle Genetics is seeking to reinvent today with a next-generation form of the technology. The scientists at what was then Wyeth created Mylotarg as a way to combine the targeting ability of an antibody with a potent toxin attached to give it more tumor-killing kick. The drug ultimately was a poor seller, partly because the linker wasn’t really stable enough to ensure the toxin got exactly where it needed to go in the tumor.
A decade of research and development later, Seattle Genetics has applied new chemistry techniques for empowered antibodies for a variety of forms of cancer. But with acute myeloid leukemia, among elderly patients who are especially frail, the notion is that a simpler “naked” antibody like lintuzumab, without any extra toxin, might be an effective way to disrupt tumors without causing serious side effects.
The company has spent a lot of time and money on getting this answer, as it completed enrollment in the study in February 2009, and stopped treating the last patient 12 months later. Suspense, naturally, is building at the company on whether this dark horse drug candidate is a winner or not.
“Our goal is to see a meaningful improvement in survival,” Siegall says.
Luke Timmerman is the National Biotechnology Editor for Xconomy, and the Editor of Xconomy Seattle. You can e-mail him at ltimmerman@xconomy.com, or call 206-624-2374.
Seattle Genetics’ Dark Horse Drug Candidate Approaches Home Stretch in Leukemia Study
Luke Timmerman 6/22/10
[Correction: 10:05 am Pacific] The buzz around Seattle Genetics (NASDAQ: SGEN) has been about whether it will finally prove, after more than a decade, that an “empowered antibody” can be an effective treatment for cancer. But while fewer people are watching, the company is eagerly awaiting clinical results for a traditional antibody that also has the potential to help cancer patients live longer—and could generate real cash.
The lesser-known drug is called lintuzumab, or SGN-33. This is a so-called “naked” antibody, a Y-shaped protein that’s genetically engineered to zero in on a specific marker on cancer cells that’s seldom found on healthy cells. In this case, the drug is made to hit a marker on tumors of patients with acute myeloid leukemia. Sometime between late this month and August, the company expects to rip off the blind from a study of 210 patients, which will say whether the new drug can help some very sick, elderly patients live a few months longer with acceptable side effects.
Acute myeloid leukemia is a fast-growing, aggressive form of cancer that often strikes people over the age of 60, and makes them too frail to withstand the high-dose chemotherapy they are prescribed. Life expectancy, based on past studies, is usually about 4-6 months. Seattle Genetics decided to swing for the fence in this tough-to-treat group of people after preliminary studies showed that a “mid-teens” percentage of patients saw some degree of partial or complete tumor shrinkage, and there were anecdotal reports of people living longer, CEO Clay Siegall says. If the company can prove its drug helps people live longer with this nasty malignancy, then Seattle Genetics could be in a position to offer this new drug for sale in the not-so-distant future. The disease kills an estimated 8,950 people a year in the U.S., according to the American Cancer Society. [Correction 10:05 am Pacific: An earlier version said lintuzumab could be ready for sale next year, but the company says it hasn't provided guidance yet on timing.]
Wall Street isn’t giving this drug much of a chance. One of the bullish analysts covering the company, Cory Kasimov of JP Morgan, said the consensus among investors is that the lintuzumab trial will fail because of the tough patient population and a “lack of convincing earlier-stage data.” Kasimov gives it a 30 percent shot of winning FDA approval and generating peak annual sales of $350 million by 2016.
This big trial began back in September 2007. The company explained some of the rationale a few months later when it presented early stage results at a meeting of the American Society of Hematology. Of 17 patients who got a variety of doses, five patients had complete tumor remission, while two more had a partial shrinkage of their tumors. The drug was well-tolerated, and patients didn’t develop antibodies to attack the drug itself as a foreign invader, which is known to happen.
Those findings were enough for Seattle Genetics to invest some significant money to get a definitive answer from this ongoing trial, which might be enough to persuade the FDA to clear this drug for sale. The study enrolled 210 patients, and randomly assigned them to get a low-dose chemotherapy drug in addition to the Seattle Genetics treatment, or the low-dose chemotherapy alone. The main goal is to show whether the new drug can help patients live longer—the gold standard measurement in cancer trials. The trial is statistically designed to show a 29 percent lower risk of death among patients on the experimental treatment, and Seattle Genetics is hoping to add a couple extra months of median survival time, without any serious added side effects, Siegall says.
The trial is designed in such a way that researchers, and the company, are blinded to the results to prevent any biases that might skew the study until at least 186 patients who entered the study have died. Then, statisticians will look at which patients got the new drug compared with the standard therapy, to see if there was any benefit. The initial findings will be issued by press release, and then the company will go into more detail at a medical meeting, Siegall says.
It’s possible that if the data for the Seattle Genetics drug is compelling enough from this single study, the company could use it as the basis to whip up an application to the FDA, Siegall says. It’s also possible that it could form part of a body of evidence for the drug, and provide the spark for another rigorous study to prove the drug’s worth, he says.
There are no other drugs on the market for acute myeloid leukemia that hit the same target as the Seattle Genetics drug, since Pfizer announced yesterday that it is pulling gemtuzumab ozogamicin (Mylotarg) off the market.
Strangely enough, the Pfizer drug was a trailblazer in the field of “empowered antibodies” that Seattle Genetics is seeking to reinvent today with a next-generation form of the technology. The scientists at what was then Wyeth created Mylotarg as a way to combine the targeting ability of an antibody with a potent toxin attached to give it more tumor-killing kick. The drug ultimately was a poor seller, partly because the linker wasn’t really stable enough to ensure the toxin got exactly where it needed to go in the tumor.
A decade of research and development later, Seattle Genetics has applied new chemistry techniques for empowered antibodies for a variety of forms of cancer. But with acute myeloid leukemia, among elderly patients who are especially frail, the notion is that a simpler “naked” antibody like lintuzumab, without any extra toxin, might be an effective way to disrupt tumors without causing serious side effects.
The company has spent a lot of time and money on getting this answer, as it completed enrollment in the study in February 2009, and stopped treating the last patient 12 months later. Suspense, naturally, is building at the company on whether this dark horse drug candidate is a winner or not.
“Our goal is to see a meaningful improvement in survival,” Siegall says.
Luke Timmerman is the National Biotechnology Editor for Xconomy, and the Editor of Xconomy Seattle. You can e-mail him at ltimmerman@xconomy.com, or call 206-624-2374.
Seattle Genetics and Agensys, an Affiliate of Astellas, Announce Initiation of Phase I Clinical Trial of ASG-5ME for Pancreatic Cancer
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq:SGEN) and Agensys, Inc., an affiliate of Tokyo-based Astellas Pharma Inc., today announced that they have initiated a phase I clinical trial of ASG-5ME for the treatment of metastatic pancreatic cancer. ASG-5ME is an antibody-drug conjugate (ADC) that is being co-developed by both companies for the treatment of solid tumors.
.“There is significant need for new pancreatic cancer therapies, demonstrated by the fact that most patients with advanced disease die within one year from diagnosis,” said Jonathan Drachman, M.D., Senior Vice President, Research and Translational Medicine at Seattle Genetics. “We believe ASG-5ME, which is an ADC designed to deliver the potent cytotoxic agent MMAE directly to tumor cells, has the potential to provide a new therapeutic option for this aggressive disease.”
The single-agent, phase I, open-label, dose-escalation study will evaluate the safety and tolerability of ASG-5ME in patients with pancreatic cancer and identify the maximum tolerated dose. Secondary objectives include assessing the pharmacokinetics and antitumor activity of ASG-5ME and identifying a recommended dose and regimen for future clinical trials. The study is designed to enroll up to approximately 50 patients at multiple centers in the United States.
Dr. David Stover, Vice President and Head of Research at Agensys, noted that ASG-5ME is an ADC composed of a fully human monoclonal antibody directed to SLC44A4 (AGS-5), a novel cancer target identified by Agensys to be upregulated in a number of epithelial tumors.
The antibody is attached to a highly potent, synthetic agent, monomethyl auristatin E (MMAE), via an enzyme-cleavable linker using Seattle Genetics’ proprietary technology. The novel linker system is designed to be stable in the bloodstream and release the potent cell-killing agent once inside antigen-expressing cancer cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy while enhancing the antitumor activity. Preclinical data demonstrate that SLC44A4 (AGS-5) is expressed on more than 80 percent of samples derived from patients with pancreatic, prostate and gastric cancers. In addition, ASG-5ME induced long-term regressions in preclinical models of established pancreatic, prostate and colon cancers.
About Pancreatic Cancer
Pancreatic cancer is a fast-growing and difficult to detect form of cancer that affects the pancreas. According to the American Cancer Society, the annual incidence of pancreatic cancer is estimated to be greater than 43,000 cases, and more than 36,000 people are expected to die from the disease in 2010. Pancreatic cancer is the fourth leading cause of cancer-related death for both men and women. The 1- and 5-year survival rates for people diagnosed with any stage of pancreatic cancer are 25 percent and 6 percent, respectively.
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq:SGEN) and Agensys, Inc., an affiliate of Tokyo-based Astellas Pharma Inc., today announced that they have initiated a phase I clinical trial of ASG-5ME for the treatment of metastatic pancreatic cancer. ASG-5ME is an antibody-drug conjugate (ADC) that is being co-developed by both companies for the treatment of solid tumors.
.“There is significant need for new pancreatic cancer therapies, demonstrated by the fact that most patients with advanced disease die within one year from diagnosis,” said Jonathan Drachman, M.D., Senior Vice President, Research and Translational Medicine at Seattle Genetics. “We believe ASG-5ME, which is an ADC designed to deliver the potent cytotoxic agent MMAE directly to tumor cells, has the potential to provide a new therapeutic option for this aggressive disease.”
The single-agent, phase I, open-label, dose-escalation study will evaluate the safety and tolerability of ASG-5ME in patients with pancreatic cancer and identify the maximum tolerated dose. Secondary objectives include assessing the pharmacokinetics and antitumor activity of ASG-5ME and identifying a recommended dose and regimen for future clinical trials. The study is designed to enroll up to approximately 50 patients at multiple centers in the United States.
Dr. David Stover, Vice President and Head of Research at Agensys, noted that ASG-5ME is an ADC composed of a fully human monoclonal antibody directed to SLC44A4 (AGS-5), a novel cancer target identified by Agensys to be upregulated in a number of epithelial tumors.
The antibody is attached to a highly potent, synthetic agent, monomethyl auristatin E (MMAE), via an enzyme-cleavable linker using Seattle Genetics’ proprietary technology. The novel linker system is designed to be stable in the bloodstream and release the potent cell-killing agent once inside antigen-expressing cancer cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy while enhancing the antitumor activity. Preclinical data demonstrate that SLC44A4 (AGS-5) is expressed on more than 80 percent of samples derived from patients with pancreatic, prostate and gastric cancers. In addition, ASG-5ME induced long-term regressions in preclinical models of established pancreatic, prostate and colon cancers.
About Pancreatic Cancer
Pancreatic cancer is a fast-growing and difficult to detect form of cancer that affects the pancreas. According to the American Cancer Society, the annual incidence of pancreatic cancer is estimated to be greater than 43,000 cases, and more than 36,000 people are expected to die from the disease in 2010. Pancreatic cancer is the fourth leading cause of cancer-related death for both men and women. The 1- and 5-year survival rates for people diagnosed with any stage of pancreatic cancer are 25 percent and 6 percent, respectively.
schaut gut aus Zahlen
http://phx.corporate-ir.net/preview/phoenix.zhtml?c=124860&p…
http://phx.corporate-ir.net/preview/phoenix.zhtml?c=124860&p…
Antwort auf Beitrag Nr.: 39.886.190 von schnappi am 27.07.10 22:34:33
Brentuximab Vedotin (SGN-35)
* Expect to report top-line data in the late September to October 2010 timeframe from a pivotal trial in relapsed and refractory Hodgkin lymphoma being conducted under a Special Protocol Assessment (SPA) and to submit a New Drug Application in the first half of 2011 to the U.S. Food and Drug Administration (FDA) under the accelerated approval regulations
* Expect to report interim top-line data in the late September to October 2010 timeframe from a phase II trial in relapsed and refractory systemic anaplastic large cell lymphoma (ALCL)
* Reported preliminary data at the 2010 Annual Meeting of the American Society of Clinical Oncology demonstrating that objective responses were achieved in seven out of 11 retreatment experiences and that brentuximab vedotin was well-tolerated in the retreatment setting
Lintuzumab (SGN-33)
* Continued follow-up in a randomized phase IIb trial of lintuzumab plus low-dose chemotherapy for patients 60 years and older with acute myeloid leukemia to evaluate whether the combination extends overall survival
* Target number of 186 events in the phase IIb clinical trial has not been reached; top-line data are currently expected to be reported in the late August to October 2010 timeframe
Lintuzumab nochmals verschoben...
Brentuximab Vedotin (SGN-35)
* Expect to report top-line data in the late September to October 2010 timeframe from a pivotal trial in relapsed and refractory Hodgkin lymphoma being conducted under a Special Protocol Assessment (SPA) and to submit a New Drug Application in the first half of 2011 to the U.S. Food and Drug Administration (FDA) under the accelerated approval regulations
* Expect to report interim top-line data in the late September to October 2010 timeframe from a phase II trial in relapsed and refractory systemic anaplastic large cell lymphoma (ALCL)
* Reported preliminary data at the 2010 Annual Meeting of the American Society of Clinical Oncology demonstrating that objective responses were achieved in seven out of 11 retreatment experiences and that brentuximab vedotin was well-tolerated in the retreatment setting
Lintuzumab (SGN-33)
* Continued follow-up in a randomized phase IIb trial of lintuzumab plus low-dose chemotherapy for patients 60 years and older with acute myeloid leukemia to evaluate whether the combination extends overall survival
* Target number of 186 events in the phase IIb clinical trial has not been reached; top-line data are currently expected to be reported in the late August to October 2010 timeframe
Lintuzumab nochmals verschoben...
Antwort auf Beitrag Nr.: 39.886.707 von SLGramann am 28.07.10 07:01:54
Adam Feuerstein:
Seattle Genetics(SGEN) tops my list with two, potentially pivotal studies of two blood cancer drugs due to report results in the near future.
A phase II study of SGN-35 in Hodgkin's lymphoma is expected to read out in late September or October, said Seattle Genetics on a conference call Tuesday night. This phase II study is considered registration worthy because it's being conducted under a special protocol assessment (SPA) with the FDA. If the SGN-35 study is positive, Seattle Genetics intends to file for accelerated approval in the first half of 2011.
The phase II single-arm study enrolled about 100 patients with Hodgkin's lymphoma that continued to progress after two or three prior therapies. Look for a response rate from SGN-35 treatment in the range of 25-30% with durability of 4-6 months as an important demarcation of the drug's efficacy, although Seattle Genetics CEO Clay Siegall told me in an interview last week that FDA hasn't placed any specific response rate hurdle on the drug in the SPA.
A single-arm response rate study needs strong data to pass muster with FDA these days. A previous and smaller phase I study of SGN-35 in Hodgkin's patients showed a response rate of about 50%.
The second Seattle Genetics study that investors should be watching is a phase II study of SGN-33 in elderly patients with acute myeloid leukemia (AML). I'd consider this study the more risky of the two, given the need for SGN-33 plus chemotherapy to demonstrate a survival benefit over chemotherapy alone in a very sick patient population that has been difficult to treat effectively in studies of other drugs.
On Tuesday night's conference call, Seattle Genetics said to expect results from the SGN-33 study in late August through October. The data from this study were expected earlier so optimists will say the timeline slip could be due to the fact that SGN-33 treated patients are living longer. Or not. Be careful.
Adam Feuerstein:
Seattle Genetics(SGEN) tops my list with two, potentially pivotal studies of two blood cancer drugs due to report results in the near future.
A phase II study of SGN-35 in Hodgkin's lymphoma is expected to read out in late September or October, said Seattle Genetics on a conference call Tuesday night. This phase II study is considered registration worthy because it's being conducted under a special protocol assessment (SPA) with the FDA. If the SGN-35 study is positive, Seattle Genetics intends to file for accelerated approval in the first half of 2011.
The phase II single-arm study enrolled about 100 patients with Hodgkin's lymphoma that continued to progress after two or three prior therapies. Look for a response rate from SGN-35 treatment in the range of 25-30% with durability of 4-6 months as an important demarcation of the drug's efficacy, although Seattle Genetics CEO Clay Siegall told me in an interview last week that FDA hasn't placed any specific response rate hurdle on the drug in the SPA.
A single-arm response rate study needs strong data to pass muster with FDA these days. A previous and smaller phase I study of SGN-35 in Hodgkin's patients showed a response rate of about 50%.
The second Seattle Genetics study that investors should be watching is a phase II study of SGN-33 in elderly patients with acute myeloid leukemia (AML). I'd consider this study the more risky of the two, given the need for SGN-33 plus chemotherapy to demonstrate a survival benefit over chemotherapy alone in a very sick patient population that has been difficult to treat effectively in studies of other drugs.
On Tuesday night's conference call, Seattle Genetics said to expect results from the SGN-33 study in late August through October. The data from this study were expected earlier so optimists will say the timeline slip could be due to the fact that SGN-33 treated patients are living longer. Or not. Be careful.

August 03, 2010 07:00 AM Eastern Daylight Time
Seattle Genetics Expands Antibody-Drug Conjugate Collaboration with Genentech
Seattle Genetics to Receive $12 Million Upfront Payment, Over $900 Million in Potential Fees and Milestone Payments and Mid-Single Digit Royalties
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (NASDAQ:SGEN) announced today that it has expanded its antibody-drug conjugate (ADC) collaboration agreement with Genentech, Inc., a member of the Roche Group (SWX:RO) (SWX:ROG) (Pink Sheets:RHHBY). Under the expanded agreement, Genentech will pay an upfront fee of $12 million for rights to utilize Seattle Genetics' ADC technology with additional antigens to be named by Genentech.
Genentech is responsible for research, preclinical and clinical development, manufacturing and commercialization of ADCs under the expanded agreement. Pursuant to the terms of the expansion, Seattle Genetics is eligible to receive more than $900 million in fees and milestones if all ADCs in the expanded portion of the collaboration are commercialized, as well as mid-single digit royalties on worldwide net sales of any resulting ADC products. Seattle Genetics is also eligible to receive annual maintenance fees and research support payments for potential assistance if requested by Genentech under the collaboration.
Seattle Genetics and Genentech established an initial ADC collaboration in 2002, under which Genentech has paid more than $30 million in collaboration payments. Under that agreement, Seattle Genetics is eligible to receive more than $500 million in milestone payments if all ADCs in the initial collaboration are commercialized, as well as mid-single digit royalties on worldwide net sales of any resulting ADC products.
“This expansion of our ADC collaboration with Genentech is another indication of the increasing value of our proprietary ADC technology,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “Our ongoing ADC collaboration with Genentech has resulted in multiple preclinical and clinical milestones, and we look forward to their continued progress with product candidates utilizing our ADC technology over the next several years.”
“We are pleased to continue our ADC collaboration with Seattle Genetics. We believe ADCs will play an important role in the future of cancer therapy. Genentech is committed to exploring the therapeutic potential of ADCs in a variety of hematologic malignancies and solid tumors,” said James Sabry, M.D., Ph.D., Vice President, Genentech Partnering.
ADCs are monoclonal antibodies that selectively deliver potent anti-cancer agents to tumor cells. With over a decade of experience and knowledge in ADC innovation, Seattle Genetics has developed proprietary technology employing synthetic, highly potent cell-killing agents called auristatins (such as MMAE and MMAF) and stable linker systems that attach auristatin to the antibody. Seattle Genetics’ novel linker systems are designed to be stable in the bloodstream and release the potent cell-killing agent once inside targeted cancer cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy while enhancing the antitumor activity. Seattle Genetics has generated more than $130 million through its ADC technology license agreements with leading biotechnology and pharmaceutical companies.
Seattle Genetics Expands Antibody-Drug Conjugate Collaboration with Genentech
Seattle Genetics to Receive $12 Million Upfront Payment, Over $900 Million in Potential Fees and Milestone Payments and Mid-Single Digit Royalties
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (NASDAQ:SGEN) announced today that it has expanded its antibody-drug conjugate (ADC) collaboration agreement with Genentech, Inc., a member of the Roche Group (SWX:RO) (SWX:ROG) (Pink Sheets:RHHBY). Under the expanded agreement, Genentech will pay an upfront fee of $12 million for rights to utilize Seattle Genetics' ADC technology with additional antigens to be named by Genentech.
Genentech is responsible for research, preclinical and clinical development, manufacturing and commercialization of ADCs under the expanded agreement. Pursuant to the terms of the expansion, Seattle Genetics is eligible to receive more than $900 million in fees and milestones if all ADCs in the expanded portion of the collaboration are commercialized, as well as mid-single digit royalties on worldwide net sales of any resulting ADC products. Seattle Genetics is also eligible to receive annual maintenance fees and research support payments for potential assistance if requested by Genentech under the collaboration.
Seattle Genetics and Genentech established an initial ADC collaboration in 2002, under which Genentech has paid more than $30 million in collaboration payments. Under that agreement, Seattle Genetics is eligible to receive more than $500 million in milestone payments if all ADCs in the initial collaboration are commercialized, as well as mid-single digit royalties on worldwide net sales of any resulting ADC products.
“This expansion of our ADC collaboration with Genentech is another indication of the increasing value of our proprietary ADC technology,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “Our ongoing ADC collaboration with Genentech has resulted in multiple preclinical and clinical milestones, and we look forward to their continued progress with product candidates utilizing our ADC technology over the next several years.”
“We are pleased to continue our ADC collaboration with Seattle Genetics. We believe ADCs will play an important role in the future of cancer therapy. Genentech is committed to exploring the therapeutic potential of ADCs in a variety of hematologic malignancies and solid tumors,” said James Sabry, M.D., Ph.D., Vice President, Genentech Partnering.
ADCs are monoclonal antibodies that selectively deliver potent anti-cancer agents to tumor cells. With over a decade of experience and knowledge in ADC innovation, Seattle Genetics has developed proprietary technology employing synthetic, highly potent cell-killing agents called auristatins (such as MMAE and MMAF) and stable linker systems that attach auristatin to the antibody. Seattle Genetics’ novel linker systems are designed to be stable in the bloodstream and release the potent cell-killing agent once inside targeted cancer cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy while enhancing the antitumor activity. Seattle Genetics has generated more than $130 million through its ADC technology license agreements with leading biotechnology and pharmaceutical companies.
aus dem Blog von Jason Chew:
Wednesday, August 4, 2010
A Rash of Data Expected From Seattle Genetics
This autumn is turning out to be a busy season for Seattle Genetics. The company is expecting results from up to three clinical trials between September and October- each of which, if successful, may form the basis for the filing of an NDA.
Two trials are nearing completion for flagship compound SGN-35, or brentuximab vedotin. SGN-35 is an antibody-drug-congugate (ADC) targeted to CD30. The CD30 antigen is a marker for Hodgkin’s Lymphoma (HL), Anaplastic Large Cell Lymphoma (ALCL), as well as other T-cell lymphomas. The two pivotal trials are in refractory Hodgkin’s Lymphoma and ALCL.
All eyes are on the pivotal SGN-35 trial in refractory Hodgkin’s Lymphoma. Management has consistently said this is their top priority. Newly detected Hodgkin’s Lymphoma is highly curable; a combination of chemo drugs known as ABVD allows about 85% of patients to attain complete remission. However, over time, about one-third of all patients will become refractory to conventional treatment, and in this setting, there is no standardized care and prognosis becomes progressively worse over time.
In the Phase I dose escalating study, patients with advanced disease who were treated with at least 1.2 mg/kg of drug showed a better than 50% overall response rate and greater than 30% had complete remissions. The dose use in the pivotal trial is 1.8mg/kg. It is a single arm trial conducted under an SPA with the primary endpoint being objective response. Success in this trial is key to the success of the program.
A Phase I trial is underway to provide evidence for SGN-35 as an option for first line therapy in combination with ABVD. Another is testing the compound for use in re-treatment of patients who have already failed initial SGN-35 therapy. Promising interim results from the re-treatment trial were disclosed at ASCO. If confirmed, SGN-35 could become an agent used for long-term care in Hodgkin’s Lymphoma patients. Both studies are important in extending the use of the agent, but data will not be available until 2012. Only about 8000 new cases are diagnosed each year, but over 100,000 people are living with the disease, representing a significant market opportunity.
ALCL is another indication management has often alluded to as a possible route to regulatory approval for SGN-35. This disease is a form of T-cell non-Hodgkin’s Lymphoma and is very rare. It comes in two forms, cutaneous and systemic. While cutaneous ALCL is slow growing and may even disappear on its own, systemic ALCL is aggressive, often occurs in children, and is fatal without treatment. Standard chemotherapy typically results in remission, but few options exist for relapsed disease. SGN-35 has shown good efficacy in this setting. As I had mentioned, this is a rare disease; only about 2000 new cases are diagnosed each year. Sales in this indication will be significantly lower than in HL.
While the focus may be on SGN-35, a surprise may lie in store with its other advanced candidate, the naked antibody SGN-33 (lintuzumab). This is an antibody against CD33, an antigen primarily found on AML and MDS cancer cells. The company has downplayed this compound, maintaining that their focus is on SGN-35. Most analysts don’t give it much chance of succeeding- but there lies the upside. Phase I results for SGN-33 were not spectacular, only about 7% of patients in this single agent trial achieved a complete response, compared to 18% for cytarabine in a recent UK study in patients of a similar age. But remember, the SGN-33 results were for patients across all doses in the Phase I study.
The standard treatment for AML is high-dose daunorubicin plus cytarabine. This treatment regimen can put up to 70% of patients into remission. Unfortunately, patients over age 65 (the median age at diagnosis is 63) receive less benefit from this therapy, and often are not candidates for this type of intensive treatment. For them, cytarabine alone is the standard of care. If SGN-33 is able to provide an additional benefit on top of cytarabine, it would give many patients a much needed treatment option.
If successful in AML and MDS, SGN-33 has the potential to be a highly valuable asset. Celgene’s Vidaza for treatment of AML and MDS had sales of $387 million in 2009 and is estimated to hit $500 million in 2010- and it is still growing fast.
There is also SGN-70, SGN-75 and ASG-5ME in the clinic in Phase I, but I’ll skip over them.
In December 2009, Genentech terminated its SGN-40 (dacetuzumab) collaboration with Seattle Genetics- but don’t expect the compound to disappear. SGN-40’s target, CD40 is very compelling, it is found on malignant haemopoietic cells as well as solid tumors. Genentech had big hopes for SGN-40 and ran a broad range of trials that included Multiple Myeloma and Non-Hodgkin’s Lymphoma. It showed activity in the form of objective responses, but wasn’t quite good enough.
Seattle Genetics has seen this before. The naked antibody portion of SGN-35, SGN-30, had also failed in the clinic in Hodgkin’s Lymphoma for lack of activity; but with the addition of a drug conjugate, the newly empowered antibody is now close to a regulatory filing. I fully expect an ADC empowered version of SGN-40 to appear in the near future.
Seattle Genetics signed an agreement with Genentech August 3, 2010 expanding their ADC collaboration with $12 million upfront and up to $900 million in milestones and mid-single digit worldwide royalties. This only confirms my belief in the company’s ADC technology. Seattle Genetics’ technology provides Genentech the ability to increase the potency of its antibodies without sacrificing safety. It will surely help in the life-cycle management of its large stable of drugs and works out nicely for Seattle Genetics as well, since practically all the research will be carried out by its partner. The deal compares well with that of Regeneron, a highly regarded antibody company, which extended a drug development agreement with Astellas into 2018 for total payments of $295 million and mid-single digit royalties.
In a way, Seattle Genetics story has some resemblance to that of PDLI. Both companies developed disruptive antibody technologies that were broadly licensed while at the same time working on their own internal discovery projects. Multiple marketed drugs using PDLI’s technology provided the company with a steady stream of royalty payments though it struggled with its own pipeline.
If Seattle Genetics’ licensees succeed in the future- and there’s every reason to believe at least some of them will- it will get a boost from its own royalty stream. For now at least, it is looking like Seattle Genetics may succeed in the drug discovery game as well.
Author is Long SGEN, RHHBY.PK
Posted by Jason Chew at 2:30 PM
Wednesday, August 4, 2010
A Rash of Data Expected From Seattle Genetics
This autumn is turning out to be a busy season for Seattle Genetics. The company is expecting results from up to three clinical trials between September and October- each of which, if successful, may form the basis for the filing of an NDA.
Two trials are nearing completion for flagship compound SGN-35, or brentuximab vedotin. SGN-35 is an antibody-drug-congugate (ADC) targeted to CD30. The CD30 antigen is a marker for Hodgkin’s Lymphoma (HL), Anaplastic Large Cell Lymphoma (ALCL), as well as other T-cell lymphomas. The two pivotal trials are in refractory Hodgkin’s Lymphoma and ALCL.
All eyes are on the pivotal SGN-35 trial in refractory Hodgkin’s Lymphoma. Management has consistently said this is their top priority. Newly detected Hodgkin’s Lymphoma is highly curable; a combination of chemo drugs known as ABVD allows about 85% of patients to attain complete remission. However, over time, about one-third of all patients will become refractory to conventional treatment, and in this setting, there is no standardized care and prognosis becomes progressively worse over time.
In the Phase I dose escalating study, patients with advanced disease who were treated with at least 1.2 mg/kg of drug showed a better than 50% overall response rate and greater than 30% had complete remissions. The dose use in the pivotal trial is 1.8mg/kg. It is a single arm trial conducted under an SPA with the primary endpoint being objective response. Success in this trial is key to the success of the program.
A Phase I trial is underway to provide evidence for SGN-35 as an option for first line therapy in combination with ABVD. Another is testing the compound for use in re-treatment of patients who have already failed initial SGN-35 therapy. Promising interim results from the re-treatment trial were disclosed at ASCO. If confirmed, SGN-35 could become an agent used for long-term care in Hodgkin’s Lymphoma patients. Both studies are important in extending the use of the agent, but data will not be available until 2012. Only about 8000 new cases are diagnosed each year, but over 100,000 people are living with the disease, representing a significant market opportunity.
ALCL is another indication management has often alluded to as a possible route to regulatory approval for SGN-35. This disease is a form of T-cell non-Hodgkin’s Lymphoma and is very rare. It comes in two forms, cutaneous and systemic. While cutaneous ALCL is slow growing and may even disappear on its own, systemic ALCL is aggressive, often occurs in children, and is fatal without treatment. Standard chemotherapy typically results in remission, but few options exist for relapsed disease. SGN-35 has shown good efficacy in this setting. As I had mentioned, this is a rare disease; only about 2000 new cases are diagnosed each year. Sales in this indication will be significantly lower than in HL.
While the focus may be on SGN-35, a surprise may lie in store with its other advanced candidate, the naked antibody SGN-33 (lintuzumab). This is an antibody against CD33, an antigen primarily found on AML and MDS cancer cells. The company has downplayed this compound, maintaining that their focus is on SGN-35. Most analysts don’t give it much chance of succeeding- but there lies the upside. Phase I results for SGN-33 were not spectacular, only about 7% of patients in this single agent trial achieved a complete response, compared to 18% for cytarabine in a recent UK study in patients of a similar age. But remember, the SGN-33 results were for patients across all doses in the Phase I study.
The standard treatment for AML is high-dose daunorubicin plus cytarabine. This treatment regimen can put up to 70% of patients into remission. Unfortunately, patients over age 65 (the median age at diagnosis is 63) receive less benefit from this therapy, and often are not candidates for this type of intensive treatment. For them, cytarabine alone is the standard of care. If SGN-33 is able to provide an additional benefit on top of cytarabine, it would give many patients a much needed treatment option.
If successful in AML and MDS, SGN-33 has the potential to be a highly valuable asset. Celgene’s Vidaza for treatment of AML and MDS had sales of $387 million in 2009 and is estimated to hit $500 million in 2010- and it is still growing fast.
There is also SGN-70, SGN-75 and ASG-5ME in the clinic in Phase I, but I’ll skip over them.
In December 2009, Genentech terminated its SGN-40 (dacetuzumab) collaboration with Seattle Genetics- but don’t expect the compound to disappear. SGN-40’s target, CD40 is very compelling, it is found on malignant haemopoietic cells as well as solid tumors. Genentech had big hopes for SGN-40 and ran a broad range of trials that included Multiple Myeloma and Non-Hodgkin’s Lymphoma. It showed activity in the form of objective responses, but wasn’t quite good enough.
Seattle Genetics has seen this before. The naked antibody portion of SGN-35, SGN-30, had also failed in the clinic in Hodgkin’s Lymphoma for lack of activity; but with the addition of a drug conjugate, the newly empowered antibody is now close to a regulatory filing. I fully expect an ADC empowered version of SGN-40 to appear in the near future.
Seattle Genetics signed an agreement with Genentech August 3, 2010 expanding their ADC collaboration with $12 million upfront and up to $900 million in milestones and mid-single digit worldwide royalties. This only confirms my belief in the company’s ADC technology. Seattle Genetics’ technology provides Genentech the ability to increase the potency of its antibodies without sacrificing safety. It will surely help in the life-cycle management of its large stable of drugs and works out nicely for Seattle Genetics as well, since practically all the research will be carried out by its partner. The deal compares well with that of Regeneron, a highly regarded antibody company, which extended a drug development agreement with Astellas into 2018 for total payments of $295 million and mid-single digit royalties.
In a way, Seattle Genetics story has some resemblance to that of PDLI. Both companies developed disruptive antibody technologies that were broadly licensed while at the same time working on their own internal discovery projects. Multiple marketed drugs using PDLI’s technology provided the company with a steady stream of royalty payments though it struggled with its own pipeline.
If Seattle Genetics’ licensees succeed in the future- and there’s every reason to believe at least some of them will- it will get a boost from its own royalty stream. For now at least, it is looking like Seattle Genetics may succeed in the drug discovery game as well.
Author is Long SGEN, RHHBY.PK
Posted by Jason Chew at 2:30 PM
eine neue P I
BOTHELL, Wash., Sep 02, 2010 (BUSINESS WIRE) -- Seattle Genetics, Inc. /quotes/comstock/15*!sgen/quotes/nls/sgen (SGEN 11.55, +0.07, +0.61%) announced today that it has achieved a milestone under its antibody-drug conjugate (ADC) collaboration with Agensys, Inc., an affiliate of Tokyo-based Astellas Pharma Inc. The milestone was triggered by Agensys' initiation of a phase I trial for AGS-16M8F, an ADC for the treatment of cancer utilizing Seattle Genetics' technology.
"This trial initiation increases the total number of ADC programs utilizing our technology currently in clinical trials to eight, further extending the investigation of our technology for the treatment of multiple types of cancer," said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. "Our ADC collaborations generate substantial financial resources for Seattle Genetics, including more than $130 million received to date. We also have the potential to receive approximately $2.7 billion in milestone payments if all collaborator ADCs are commercialized, as well as royalties on net sales of resulting ADC products."
Under the terms of the ADC collaboration agreement, Agensys has rights to use Seattle Genetics' ADC technology with monoclonal antibodies against targets selected by Agensys. Agensys is responsible for research, product development, manufacturing and commercialization activities. Seattle Genetics is entitled to progress-dependent fees, milestone payments and mid-single digit royalties on worldwide net sales of ADC products developed and commercialized solely by Agensys. The companies are currently co-developing ASG-5ME, which is in a phase I clinical trial for metastatic pancreatic cancer and a planned phase I trial for advanced prostate cancer. Seattle Genetics also has options under the collaboration to co-develop two additional ADC programs (excluding AGS-16M8F) at the time of Investigational New Drug submission in exchange for 50:50 cost and profit-sharing.
BOTHELL, Wash., Sep 02, 2010 (BUSINESS WIRE) -- Seattle Genetics, Inc. /quotes/comstock/15*!sgen/quotes/nls/sgen (SGEN 11.55, +0.07, +0.61%) announced today that it has achieved a milestone under its antibody-drug conjugate (ADC) collaboration with Agensys, Inc., an affiliate of Tokyo-based Astellas Pharma Inc. The milestone was triggered by Agensys' initiation of a phase I trial for AGS-16M8F, an ADC for the treatment of cancer utilizing Seattle Genetics' technology.
"This trial initiation increases the total number of ADC programs utilizing our technology currently in clinical trials to eight, further extending the investigation of our technology for the treatment of multiple types of cancer," said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. "Our ADC collaborations generate substantial financial resources for Seattle Genetics, including more than $130 million received to date. We also have the potential to receive approximately $2.7 billion in milestone payments if all collaborator ADCs are commercialized, as well as royalties on net sales of resulting ADC products."
Under the terms of the ADC collaboration agreement, Agensys has rights to use Seattle Genetics' ADC technology with monoclonal antibodies against targets selected by Agensys. Agensys is responsible for research, product development, manufacturing and commercialization activities. Seattle Genetics is entitled to progress-dependent fees, milestone payments and mid-single digit royalties on worldwide net sales of ADC products developed and commercialized solely by Agensys. The companies are currently co-developing ASG-5ME, which is in a phase I clinical trial for metastatic pancreatic cancer and a planned phase I trial for advanced prostate cancer. Seattle Genetics also has options under the collaboration to co-develop two additional ADC programs (excluding AGS-16M8F) at the time of Investigational New Drug submission in exchange for 50:50 cost and profit-sharing.
Lintuzumab ist tot. Jetzt hängt alles an Brentuximab. Binnen 6 Wochen entscheidet sich das Schicksal für den SGEN-Kurs der nächsten paar Jahre.
(Die Erwartungen an Lintuzumab waren übrigens nie sonderlich hoch)
September 13, 2010 06:45 AM Eastern Daylight Time
Seattle Genetics Announces Phase IIb Trial of Lintuzumab (SGN-33) in Patients with Acute Myeloid Leukemia (AML) Did Not Meet Primary Endpoint
Company to Discontinue Lintuzumab Development Program to Focus on Robust Pipeline
Top-line Data to be Reported from Two Brentuximab Vedotin (SGN-35) Trials Within Next Six Weeks
Conference Call to be Held Today at 8:30 a.m. ET
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq:SGEN) today announced that its phase IIb clinical trial of lintuzumab (SGN-33) in older patients with acute myeloid leukemia (AML) did not meet its primary endpoint of extending overall survival. Lintuzumab is a naked monoclonal antibody that targets the CD33 antigen. As a result of the outcome of this trial, the company will discontinue its development program for lintuzumab.
“We are disappointed that lintuzumab did not demonstrate a survival benefit for older AML patients in this study. These patients have limited therapeutic alternatives due to their inability to tolerate the toxicities associated with standard high-dose chemotherapy, representing a substantial unmet medical need,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer of Seattle Genetics. “We want to thank the patients, caregivers and investigators for their participation and commitment to the clinical evaluation of lintuzumab.”
“There is a lot of positive momentum at Seattle Genetics and we continue to focus on advancing our lead product candidate, brentuximab vedotin (SGN-35), and our robust pipeline,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “We are on track to report top-line data from two brentuximab vedotin clinical trials within the next six weeks, positioning us for a regulatory submission in the first half of 2011. In addition, our strong financial position allows us to continue to invest in advancing our multiple other programs, including two antibody-drug conjugates, SGN-75 and ASG-5ME, which are both in ongoing phase I clinical trials.”
Lintuzumab Phase IIb Trial
The phase IIb trial was a randomized, double-blind, placebo-controlled, multi-center clinical trial that enrolled 211 previously untreated AML patients age 60 and older who were ineligible for or declined intensive chemotherapy. The study’s primary endpoint evaluated whether the combination of lintuzumab and low-dose cytarabine chemotherapy extended overall survival compared to low-dose cytarabine plus placebo.
No statistically significant difference in overall survival was achieved between treatment arms. The treatment arms were well balanced for multiple demographic and prognostic factors, and the results were consistent across geographies. Overall survival was generally longer in both arms compared to previously published data with low-dose cytarabine. Lintuzumab was well tolerated in combination with cytarabine chemotherapy.
Conference Call Details
Seattle Genetics' management will host a conference call and webcast to discuss this announcement. The event will be held today at 5:30 a.m. Pacific Time (PT); 8:30 a.m. Eastern Time (ET). The live event will be available from Seattle Genetics' website at http://www.seattlegenetics.com, under the Investors and News section, or by calling (877) 941-8631 (domestic) or (480) 629-9819 (international). The access code is 4364358. A replay of the discussion will be available beginning at approximately 7:00 a.m. PT today from Seattle Genetics' website or by calling (800) 406-7325 (domestic) or (303) 590-3030 (international), using access code 4364358. The telephone replay will be available until 8:00 p.m. PT on Friday, September 17, 2010.
(Die Erwartungen an Lintuzumab waren übrigens nie sonderlich hoch)
September 13, 2010 06:45 AM Eastern Daylight Time
Seattle Genetics Announces Phase IIb Trial of Lintuzumab (SGN-33) in Patients with Acute Myeloid Leukemia (AML) Did Not Meet Primary Endpoint
Company to Discontinue Lintuzumab Development Program to Focus on Robust Pipeline
Top-line Data to be Reported from Two Brentuximab Vedotin (SGN-35) Trials Within Next Six Weeks
Conference Call to be Held Today at 8:30 a.m. ET
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq:SGEN) today announced that its phase IIb clinical trial of lintuzumab (SGN-33) in older patients with acute myeloid leukemia (AML) did not meet its primary endpoint of extending overall survival. Lintuzumab is a naked monoclonal antibody that targets the CD33 antigen. As a result of the outcome of this trial, the company will discontinue its development program for lintuzumab.
“We are disappointed that lintuzumab did not demonstrate a survival benefit for older AML patients in this study. These patients have limited therapeutic alternatives due to their inability to tolerate the toxicities associated with standard high-dose chemotherapy, representing a substantial unmet medical need,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer of Seattle Genetics. “We want to thank the patients, caregivers and investigators for their participation and commitment to the clinical evaluation of lintuzumab.”
“There is a lot of positive momentum at Seattle Genetics and we continue to focus on advancing our lead product candidate, brentuximab vedotin (SGN-35), and our robust pipeline,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “We are on track to report top-line data from two brentuximab vedotin clinical trials within the next six weeks, positioning us for a regulatory submission in the first half of 2011. In addition, our strong financial position allows us to continue to invest in advancing our multiple other programs, including two antibody-drug conjugates, SGN-75 and ASG-5ME, which are both in ongoing phase I clinical trials.”
Lintuzumab Phase IIb Trial
The phase IIb trial was a randomized, double-blind, placebo-controlled, multi-center clinical trial that enrolled 211 previously untreated AML patients age 60 and older who were ineligible for or declined intensive chemotherapy. The study’s primary endpoint evaluated whether the combination of lintuzumab and low-dose cytarabine chemotherapy extended overall survival compared to low-dose cytarabine plus placebo.
No statistically significant difference in overall survival was achieved between treatment arms. The treatment arms were well balanced for multiple demographic and prognostic factors, and the results were consistent across geographies. Overall survival was generally longer in both arms compared to previously published data with low-dose cytarabine. Lintuzumab was well tolerated in combination with cytarabine chemotherapy.
Conference Call Details
Seattle Genetics' management will host a conference call and webcast to discuss this announcement. The event will be held today at 5:30 a.m. Pacific Time (PT); 8:30 a.m. Eastern Time (ET). The live event will be available from Seattle Genetics' website at http://www.seattlegenetics.com, under the Investors and News section, or by calling (877) 941-8631 (domestic) or (480) 629-9819 (international). The access code is 4364358. A replay of the discussion will be available beginning at approximately 7:00 a.m. PT today from Seattle Genetics' website or by calling (800) 406-7325 (domestic) or (303) 590-3030 (international), using access code 4364358. The telephone replay will be available until 8:00 p.m. PT on Friday, September 17, 2010.
Hallo,

in der Tat wurde ein Scheitern fast schon erwartet, so dass ich mich ein bisschen über den Kursanstieg auf fast 13$ gewundert hatte.
Jetzt kommt der Kurs etwas zurück. Hatte eigentlich gehofft, irgendwann im Bereich von 10,xx$ wieder eizusteigen, aber ging immer nur bis 11,xx.
Mal seh´n, wie weit es jetzt sackt, vielleicht auch gar nicht so viel, weil die Brentuximab-Studie als wesentlich aussichtsreicher angesehen wird und dieses nun schon im Kurs eingepreist wird.
Ich warte noch etwas ab mit einem Wiedereinstieg.
Gruß q.

in der Tat wurde ein Scheitern fast schon erwartet, so dass ich mich ein bisschen über den Kursanstieg auf fast 13$ gewundert hatte.
Jetzt kommt der Kurs etwas zurück. Hatte eigentlich gehofft, irgendwann im Bereich von 10,xx$ wieder eizusteigen, aber ging immer nur bis 11,xx.
Mal seh´n, wie weit es jetzt sackt, vielleicht auch gar nicht so viel, weil die Brentuximab-Studie als wesentlich aussichtsreicher angesehen wird und dieses nun schon im Kurs eingepreist wird.
Ich warte noch etwas ab mit einem Wiedereinstieg.
Gruß q.
der Vollständikeit halber:
Genmab A/S And Seattle Genetics Enter Into Antibody-Drug Conjugate Research Collaboration
Tuesday, 14 Sep 2010 03:29am EDT
Genmab A/S and Seattle Genetics announced that the companies have entered into an antibody-drug conjugate (ADC) research collaboration agreement. Under the agreement, Genmab has rights to utilize Seattle Genetics' ADC technology with its HuMax-TF antibody targeting the Tissue Factor antigen, which is expressed on numerous types of solid tumors. Seattle Genetics received an undisclosed upfront payment and has the right to exercise a co-development option for any resulting ADC products at the end of Phase I clinical development. Genmab is responsible for research, manufacturing, preclinical development and Phase I clinical trials of ADCs under this collaboration. Seattle Genetics will receive research support payments for any assistance provided to Genmab. If Seattle Genetics opts into an ADC product at the end of Phase I, the companies would co-develop and share all future costs and profits for the product on a 50:50 basis. If Seattle Genetics does not opt in to an ADC product, Genmab would pay Seattle Genetics fees, milestones and mid-single digit royalties on worldwide net sales of the product.
Genmab A/S And Seattle Genetics Enter Into Antibody-Drug Conjugate Research Collaboration
Tuesday, 14 Sep 2010 03:29am EDT
Genmab A/S and Seattle Genetics announced that the companies have entered into an antibody-drug conjugate (ADC) research collaboration agreement. Under the agreement, Genmab has rights to utilize Seattle Genetics' ADC technology with its HuMax-TF antibody targeting the Tissue Factor antigen, which is expressed on numerous types of solid tumors. Seattle Genetics received an undisclosed upfront payment and has the right to exercise a co-development option for any resulting ADC products at the end of Phase I clinical development. Genmab is responsible for research, manufacturing, preclinical development and Phase I clinical trials of ADCs under this collaboration. Seattle Genetics will receive research support payments for any assistance provided to Genmab. If Seattle Genetics opts into an ADC product at the end of Phase I, the companies would co-develop and share all future costs and profits for the product on a 50:50 basis. If Seattle Genetics does not opt in to an ADC product, Genmab would pay Seattle Genetics fees, milestones and mid-single digit royalties on worldwide net sales of the product.
Antwort auf Beitrag Nr.: 40.143.770 von quepos am 13.09.10 20:28:09
quepos, kann ich verstehen, dass Du jetzt abwartest. Wenn Brentuximab scheitert, geht das nicht mit einer kleinen Delle ab, wie bei Lintuzumab, sondern es wird den Kurs zu Kleinholz machen. SGEN wäre dann mehr oder weniger auf ihre Technologieplattform + etwas Zuschlag für frühe Projekte und Cash zurückgeworfen, was die Bewertung angeht.
Der Oktober wird für mich spannend. Sollte Brentuximab scheitern, werde ich aber zu sehr viel tieferen Kursen meine Position ausbauen. Das ist ein echtes Langfristinvestment. Aber ich hoffe trotzdem, dass ich diese Chance nicht bekomme.
quepos, kann ich verstehen, dass Du jetzt abwartest. Wenn Brentuximab scheitert, geht das nicht mit einer kleinen Delle ab, wie bei Lintuzumab, sondern es wird den Kurs zu Kleinholz machen. SGEN wäre dann mehr oder weniger auf ihre Technologieplattform + etwas Zuschlag für frühe Projekte und Cash zurückgeworfen, was die Bewertung angeht.
Der Oktober wird für mich spannend. Sollte Brentuximab scheitern, werde ich aber zu sehr viel tieferen Kursen meine Position ausbauen. Das ist ein echtes Langfristinvestment. Aber ich hoffe trotzdem, dass ich diese Chance nicht bekomme.

Antwort auf Beitrag Nr.: 40.159.239 von SLGramann am 16.09.10 08:01:49SLGramann,
da Celldex die Linkertechnologie von SeattleGenetics verwendet bin ich natürlich auch sehr gespannt, wie die Daten zu SGN-35 ausfallen werden.
Gibt es einen genauen Termin, wann die Ergebnisse veröffentlicht werden?
Gruß
wachholder
da Celldex die Linkertechnologie von SeattleGenetics verwendet bin ich natürlich auch sehr gespannt, wie die Daten zu SGN-35 ausfallen werden.
Gibt es einen genauen Termin, wann die Ergebnisse veröffentlicht werden?
Gruß
wachholder
Seattle Genetics to release lymphoma data Monday
Fri, Sep 24 2010
* Conference call scheduled for Monday morning
* Shares fall 14 percent in after-hours trade (Adds analyst comment, byline, updates share price)
By Deena Beasley
LOS ANGELES, Sept 24 (Reuters) - Biotechnology company Seattle Genetics (SGEN.O: Quote, Profile, Research, Stock Buzz) will announce on Monday results from a pivotal trial of experimental drug brentuximab vedotin in patients with Hodgkin lymphoma.
The company said on Friday it will hold a conference call at 8.30 a.m. EDT (1230 GMT) on Monday to discuss the data, and its shares fell 14 percent in after-hours trade.
Some investors may have been spooked by the fact that Seattle Genetics announced the conference call, but not the actual trial data.
"It is a very expensive stock," said Elliot Favus, at Favus Institutional Research LLC. "There is no way this company is worth $10 a share if they don't show positive data on Monday."
Shares of Seattle Genetics were trading at $10.50 in extended trading after closing Friday at $12.16 on Nasdaq.
Favus, along with many other Wall Street analysts, expects successful lymphoma trial results for brentuximab, a tumor-targeting antibody linked to a chemotherapy drug called monomethyl auristatin E. But opinions differ as to the drug's ultimate market potential.
About 8,500 Americans are diagnosed each year with Hodgkin lymphoma, and most of them are successfully treated with radiation and chemotherapy.
RBC Capital Markets, in a recent research note, said there is a 75 percent chance that the trial will succeed, which would propel shares of Seattle Genetics to over $13, adding that disappointing results could send the stock to $10. RBC estimated the drug's market potential at $400 million.
"I don't see how you get to $400 million," said Favus, who puts the drug's peak sales at no more than $200 million, which is well below the level needed to support the company's current market cap.
Seattle Genetics is developing brentuximab in partnership with Japan's Takeda Pharmaceutical Co Ltd (4502.T: Quote, Profile, Research, Stock Buzz).
The biotechnology company has said it plans to file for U.S. regulatory approval of the drug in the first half of 2011. (Reporting by Deena Beasley. Editing by Tim Dobbyn)
Fri, Sep 24 2010
* Conference call scheduled for Monday morning
* Shares fall 14 percent in after-hours trade (Adds analyst comment, byline, updates share price)
By Deena Beasley
LOS ANGELES, Sept 24 (Reuters) - Biotechnology company Seattle Genetics (SGEN.O: Quote, Profile, Research, Stock Buzz) will announce on Monday results from a pivotal trial of experimental drug brentuximab vedotin in patients with Hodgkin lymphoma.
The company said on Friday it will hold a conference call at 8.30 a.m. EDT (1230 GMT) on Monday to discuss the data, and its shares fell 14 percent in after-hours trade.
Some investors may have been spooked by the fact that Seattle Genetics announced the conference call, but not the actual trial data.
"It is a very expensive stock," said Elliot Favus, at Favus Institutional Research LLC. "There is no way this company is worth $10 a share if they don't show positive data on Monday."
Shares of Seattle Genetics were trading at $10.50 in extended trading after closing Friday at $12.16 on Nasdaq.
Favus, along with many other Wall Street analysts, expects successful lymphoma trial results for brentuximab, a tumor-targeting antibody linked to a chemotherapy drug called monomethyl auristatin E. But opinions differ as to the drug's ultimate market potential.
About 8,500 Americans are diagnosed each year with Hodgkin lymphoma, and most of them are successfully treated with radiation and chemotherapy.
RBC Capital Markets, in a recent research note, said there is a 75 percent chance that the trial will succeed, which would propel shares of Seattle Genetics to over $13, adding that disappointing results could send the stock to $10. RBC estimated the drug's market potential at $400 million.
"I don't see how you get to $400 million," said Favus, who puts the drug's peak sales at no more than $200 million, which is well below the level needed to support the company's current market cap.
Seattle Genetics is developing brentuximab in partnership with Japan's Takeda Pharmaceutical Co Ltd (4502.T: Quote, Profile, Research, Stock Buzz).
The biotechnology company has said it plans to file for U.S. regulatory approval of the drug in the first half of 2011. (Reporting by Deena Beasley. Editing by Tim Dobbyn)
Antwort auf Beitrag Nr.: 40.210.578 von SLGramann am 25.09.10 15:29:11
Das ist also 14:30 Uhr deutscher Zeit.
Vom "After Hours-Trading" sollte man sich nicht verrückt machen lassen. Niemand von denen, die da gehandelt haben, hat irgendeine Ahnung, was uns morgen wirklich erwartet. Am Ende wurde die Aktie After Hours auch wieder mit 11,80 gehandelt, also sehr in der Nähe des Tagesschlusskurses der Börse.
Auf der anderen Seite wirkt die Ankündigung des CC in der Tat befremdlich. Gute Daten könnte man vor Börsenbeginn auch einfach per Pressemitteilung veröffentlichen, ohne vorher so wichtig zu tun. Nur bei schlechten Daten gibt es eigentlich erhöhten Erklärungsbedarf. Nun ja, in 24 Stunden sind wir schlauer...
Das ist also 14:30 Uhr deutscher Zeit.
Vom "After Hours-Trading" sollte man sich nicht verrückt machen lassen. Niemand von denen, die da gehandelt haben, hat irgendeine Ahnung, was uns morgen wirklich erwartet. Am Ende wurde die Aktie After Hours auch wieder mit 11,80 gehandelt, also sehr in der Nähe des Tagesschlusskurses der Börse.
Auf der anderen Seite wirkt die Ankündigung des CC in der Tat befremdlich. Gute Daten könnte man vor Börsenbeginn auch einfach per Pressemitteilung veröffentlichen, ohne vorher so wichtig zu tun. Nur bei schlechten Daten gibt es eigentlich erhöhten Erklärungsbedarf. Nun ja, in 24 Stunden sind wir schlauer...
Antwort auf Beitrag Nr.: 40.212.419 von SLGramann am 26.09.10 14:39:17ja ich bin auch gespannt wie die Ergebnisse für SGN-35 ausfallen werden.
Schlechte Ergebnisse würden einen Rückschlag für die ADC-Technologie bedeuten.
Das wäre echt bitter, hat man sich doch von den zielgerichteten Therapieansätzen soviel versprochen.
Ich hab mal ein bißchen in einem Forum gelesen in dem Patienten, die mit SGN-35 behandelt wurden, ihre Erfahrungen austauschen.
http://forums.lymphoma.com/showthread.php?t=42341
Ich war doch sehr überrascht über die vielen Nebenwirkungen, die bei einigen Patienten aufgetreten sind.
Ich dachte immer, dass einer der großen Vorteile der ADC-Technologie die im Vergleich zur Chemotherapie geringeren Nebenwirkungen sei.
Kann hier jemand etwas dazu sagen?
wachholder
Schlechte Ergebnisse würden einen Rückschlag für die ADC-Technologie bedeuten.
Das wäre echt bitter, hat man sich doch von den zielgerichteten Therapieansätzen soviel versprochen.
Ich hab mal ein bißchen in einem Forum gelesen in dem Patienten, die mit SGN-35 behandelt wurden, ihre Erfahrungen austauschen.
http://forums.lymphoma.com/showthread.php?t=42341
Ich war doch sehr überrascht über die vielen Nebenwirkungen, die bei einigen Patienten aufgetreten sind.
Ich dachte immer, dass einer der großen Vorteile der ADC-Technologie die im Vergleich zur Chemotherapie geringeren Nebenwirkungen sei.
Kann hier jemand etwas dazu sagen?
wachholder
BOTHELL, Wash. (TheStreet) -- Seattle Genetics(SGEN_) announced Monday results from a pivotal study demonstrating that 75% of patients with advanced Hodgkin lymphoma responded for greater than six months to treatment with the experimental drug brentuximab
The positive results from the study are strong enough to allow Seattle Genetics to seek U.S. regulatory approval for brentuximab in the first half of next year, the company said.
Brentuximab, also known as SGN-35, is designed using a technology proprietary to Seattle Genetics that delivers a lethal dose of chemotherapy directly to cancer cells while sparing healthy cells from toxic effects. Brentuximab consists of an antibody that attaches itself to a certain receptor found on tumor cells. Once inside the tumor, brentuximab releases a toxic chemotherapy payload.
The pivotal phase study was designed under a Special Protocol Assessment (SPA) with the U.S. Food and Drug Administration. Just over 100 patients with Hodgkin lymphoma were enrolled in the study, all of whom had disease that was no longer responsive to currently approved therapies, including autologous stem cell transplants.
After treatment with brentuximab, 75% of the patients demonstrated either a complete or partial response. The duration of response was greater than six months. The drug's safety profile was "consistent" with previous studies, Seattle Genetics said. Additional details from the study are being withheld so they can be presented at a future medical meeting.
"Few drugs ever demonstrate this level of response in the refractory setting, " said Seattle Genetics CEO Clay Siegall, in an interview Sunday. " We believe brentuximab provides a real opportunity and hope for Hodgkin patients in this setting for which there has been no major advance in years."
The FDA did not specify a brentuximab response rate necessary for the drug to be approved as a new treatment for Hodgkin lymphoma, but Seattle Genetics said previously that a 25-30% response rate lasting at least six months would be considered robust.
A previous, smaller study of brentuximab yielded response rates in the range of 50-60%.
About 8,500 patients in the U.S. each year are diagnosed with Hodgkin lymphoma, a cancer that affects white blood cells. Most of these patients are treated successfully with a four-drug chemotherapy cocktail or stem-cell transplants. Seattle Genetics intends to seek approval for brentuximab initially in the approximately 30% of patients who do not respond to current therapies or relapse.
Seattle Genetics is developing brentuximab with Millennium Pharmaceuticals, the U.S.-based cancer drug arm of Japanese pharmaceutical giant Takeda. Millennium will be seeking approval of brentuximab in Europe in 2011.
A second study of brentuximab in patients with relapsed or refractory systemic anaplastic large cell lymphoma (ALCL) will be announced within the next few weeks, Seattle Genetics said.
Seattle Genetics will be seeking accelerated approval of brentuximab in Hodgkin lymphoma based on the 75% response rate demonstrated in Monday's pivotal study. Siegall says the carefully crafted SPA agreement under which the study was conducted should insure that brentuximab does not suffer the same fate of Roche's breast cancer drug TDM-1 -- hit by a refuse-to-file letter from FDA in August.
"We interacted with the FDA in a number of ways to make sure we did the right study in the right patient population," said Siegall.
--Written by Adam Feuerstein in Boston.
Brentuximab, also known as SGN-35, is designed using a technology proprietary to Seattle Genetics that delivers a lethal dose of chemotherapy directly to cancer cells while sparing healthy cells from toxic effects. Brentuximab consists of an antibody that attaches itself to a certain receptor found on tumor cells. Once inside the tumor, brentuximab releases a toxic chemotherapy payload.
The pivotal phase study was designed under a Special Protocol Assessment (SPA) with the U.S. Food and Drug Administration. Just over 100 patients with Hodgkin lymphoma were enrolled in the study, all of whom had disease that was no longer responsive to currently approved therapies, including autologous stem cell transplants.
After treatment with brentuximab, 75% of the patients demonstrated either a complete or partial response. The duration of response was greater than six months. The drug's safety profile was "consistent" with previous studies, Seattle Genetics said. Additional details from the study are being withheld so they can be presented at a future medical meeting.
"Few drugs ever demonstrate this level of response in the refractory setting, " said Seattle Genetics CEO Clay Siegall, in an interview Sunday. " We believe brentuximab provides a real opportunity and hope for Hodgkin patients in this setting for which there has been no major advance in years."
The FDA did not specify a brentuximab response rate necessary for the drug to be approved as a new treatment for Hodgkin lymphoma, but Seattle Genetics said previously that a 25-30% response rate lasting at least six months would be considered robust.
A previous, smaller study of brentuximab yielded response rates in the range of 50-60%.
About 8,500 patients in the U.S. each year are diagnosed with Hodgkin lymphoma, a cancer that affects white blood cells. Most of these patients are treated successfully with a four-drug chemotherapy cocktail or stem-cell transplants. Seattle Genetics intends to seek approval for brentuximab initially in the approximately 30% of patients who do not respond to current therapies or relapse.
Seattle Genetics is developing brentuximab with Millennium Pharmaceuticals, the U.S.-based cancer drug arm of Japanese pharmaceutical giant Takeda. Millennium will be seeking approval of brentuximab in Europe in 2011.
A second study of brentuximab in patients with relapsed or refractory systemic anaplastic large cell lymphoma (ALCL) will be announced within the next few weeks, Seattle Genetics said.
Seattle Genetics will be seeking accelerated approval of brentuximab in Hodgkin lymphoma based on the 75% response rate demonstrated in Monday's pivotal study. Siegall says the carefully crafted SPA agreement under which the study was conducted should insure that brentuximab does not suffer the same fate of Roche's breast cancer drug TDM-1 -- hit by a refuse-to-file letter from FDA in August.
"We interacted with the FDA in a number of ways to make sure we did the right study in the right patient population," said Siegall.
--Written by Adam Feuerstein in Boston.
Die Sorgen waren also unbegründet. Brentuximab ist ein Erfolg. Damit dürfte SGEN auf dem Weg zu einem Produktunternehmen sein, wenn sich die FDA nicht doch noch querstellt, was aber meiner Meinung nach nicht zu erwarten ist.
Dieser Erfolg bedeutet nicht nur, dass man in absehbarer Zukunft Produktumsätze generieren wird (wenn auch nicht Blockbusterpotential besteht), sondern darüber hinaus, dass die ADC-Technologie von SGEN endgültig validiert ist. ADCs sind eine wirklich sehr, sehr vielversprechende Option im Kampf gegen den Krebs und SGEN sitzt im Zentrum des Geschehens. Toll!
Dieser Erfolg bedeutet nicht nur, dass man in absehbarer Zukunft Produktumsätze generieren wird (wenn auch nicht Blockbusterpotential besteht), sondern darüber hinaus, dass die ADC-Technologie von SGEN endgültig validiert ist. ADCs sind eine wirklich sehr, sehr vielversprechende Option im Kampf gegen den Krebs und SGEN sitzt im Zentrum des Geschehens. Toll!
SLGramann,
das sind sehr gute Nachrichten für SeattleGenetics, Immunogen und Celldex.
Bin gespannt wie die Aktien dieser drei Unternehmen heute reagieren werden.
Was Seattle angeht wurden diese Ergebnisse eigentlich erwártet und könnten somit schon im aktuellen Kurs eingepreist sein. Die aktuelle Marktkapitaliserung liegt bereits bei 1,2 Mrd USD und ist meines Erachtens schon ganz schön ambitioniert.
Auf jeden Fall ein Meilenstein der ADC-Technologie und des zielorientierten Therapieansatzes.
GRuß
wachholder
das sind sehr gute Nachrichten für SeattleGenetics, Immunogen und Celldex.
Bin gespannt wie die Aktien dieser drei Unternehmen heute reagieren werden.
Was Seattle angeht wurden diese Ergebnisse eigentlich erwártet und könnten somit schon im aktuellen Kurs eingepreist sein. Die aktuelle Marktkapitaliserung liegt bereits bei 1,2 Mrd USD und ist meines Erachtens schon ganz schön ambitioniert.
Auf jeden Fall ein Meilenstein der ADC-Technologie und des zielorientierten Therapieansatzes.
GRuß
wachholder
Antwort auf Beitrag Nr.: 40.215.650 von wachholder am 27.09.10 13:07:39
@wachholder,
gebe Dir recht, was die Einpreisung angeht. Sehr viel Kurspotential sehe ich kurzfristig auch nicht. SGEN könnte vielleicht bis 15 Dollar hoch laufen (in den nächsten Wochen), aber das wäre schon sehr fett.
Die Story hat jetzt aber eine starke Basis auf der sich in den nächsten Jahren viel entwickeln kann.
Gruß
Hier noch mal die offizielle PM:
Seattle Genetics and Millennium Announce Positive Top-Line Brentuximab Vedotin (SGN-35) Data from Pivotal Trial in Relapsed and Refractory Hodgkin Lymphoma
-Objective Responses Achieved in 75 percent of Patients; Median Duration of Response Greater than Six Months-
-BLA Submission Planned in First Half of 2011-
-Seattle Genetics to Host Conference Call and Webcast Today at 8:30 a.m. ET-
BOTHELL, Wash. & CAMBRIDGE, Mass., Sep 27, 2010 (BUSINESS WIRE) --
Seattle Genetics, Inc. (Nasdaq: SGEN) and Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited (TSE:4502), today announced positive top-line results from the pivotal trial of single-agent brentuximab vedotin (SGN-35), an antibody-drug conjugate (ADC) targeted to CD30. The trial was conducted in 102 relapsed or refractory Hodgkin lymphoma (HL) patients.
Seventy-five percent of patients in the pivotal trial achieved an objective response as assessed by an independent central review, the primary endpoint of the trial. The median duration of response was greater than six months. The safety profile of brentuximab vedotin in this trial was generally consistent with prior clinical trial experience. A more complete data set will be presented at an upcoming scientific meeting.
"We are extremely excited with the top-line results, as they move us one step closer to our goal of bringing brentuximab vedotin to patients with relapsed or refractory Hodgkin lymphoma," said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. "We are positioned for a Biologics License Application (BLA) submission to the U.S. Food and Drug Administration (FDA) in the first half of 2011. In addition, we plan to report top-line data from our phase II trial of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma (ALCL) within the next few weeks."
"The lack of adequate therapies for the treatment of relapsed and refractory Hodgkin lymphoma represents a substantial unmet medical need worldwide, with almost a third of the 30,000 newly diagnosed patients relapsing or becoming refractory to front-line therapy annually," said Nancy Simonian, M.D., Chief Medical Officer of Millennium. "These data have the potential to provide an important advance in therapy for Hodgkin lymphoma. We intend to discuss these results with European regulators to support our goal of submitting a Marketing Authorization Application to the European Medicines Agency (EMA) in 2011."
Pivotal Trial Design
The single-arm pivotal trial assessed efficacy and safety of single-agent brentuximab vedotin in relapsed or refractory, post-autologous stem cell transplant (ASCT) HL patients. Patients received 1.8 milligrams per kilogram of brentuximab vedotin every three weeks for up to 16 total doses. The primary endpoint of the trial was objective response rate as assessed by an independent review facility. Response assessments were based on the rigorous and internationally established Revised Response Criteria for Malignant Lymphoma (Cheson, 2007). Secondary endpoints included complete response rate, duration of response, progression-free survival, overall survival and tolerability. The trial was conducted under a Special Protocol Assessment (SPA) with the FDA and was discussed with the EMA during the process of obtaining EU Centralized Scientific Advice on the brentuximab vedotin development program. Brentuximab vedotin has been granted orphan drug designation by the FDA and EMA for the treatment of HL and ALCL and has been granted fast track designation by the FDA for HL.
About Brentuximab Vedotin
Brentuximab vedotin is an ADC comprising an anti-CD30 monoclonal antibody attached by an enzyme cleavable linker to a potent, synthetic drug payload, monomethyl auristatin E (MMAE) utilizing Seattle Genetics' proprietary technology. The ADC employs a novel linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells. This approach is intended to spare non-targeted cells and thus may help minimize the potential toxic effects of traditional chemotherapy while allowing for the selective targeting of CD30-expressing cancer cells, thus potentially enhancing the antitumor activity.
In addition to the pivotal HL trial, Seattle Genetics and Millennium are conducting a phase II trial for relapsed and refractory systemic ALCL, a phase III clinical trial (the AETHERA trial) for patients at high risk of residual HL following autologous stem cell transplant, a phase II retreatment trial for relapsed patients who previously responded to brentuximab vedotin, and a phase I combination trial for front-line treatment of HL.
About Hodgkin Lymphoma
Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is distinguished from other types of lymphoma by the presence of one characteristic type of cell, known as the Reed-Sternberg cell. A defining attribute of the Reed-Sternberg cell is its expression of the CD30 antigen.
According to the American Cancer Society, approximately 8,500 cases of Hodgkin lymphoma will be diagnosed in the United States during 2010 and more than 1,300 will die from the disease. Globally, there are more than 30,000 cases of Hodgkin lymphoma diagnosed each year. Although front-line combination chemotherapy can result in durable response rates, up to 30 percent of these patients relapse or are refractory to front-line treatment and have few therapeutic options beyond ASCT.
About the Seattle Genetics/Millennium Collaboration
Seattle Genetics and Millennium are jointly developing brentuximab vedotin. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize brentuximab vedotin in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for brentuximab vedotin on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
@wachholder,
gebe Dir recht, was die Einpreisung angeht. Sehr viel Kurspotential sehe ich kurzfristig auch nicht. SGEN könnte vielleicht bis 15 Dollar hoch laufen (in den nächsten Wochen), aber das wäre schon sehr fett.
Die Story hat jetzt aber eine starke Basis auf der sich in den nächsten Jahren viel entwickeln kann.
Gruß
Hier noch mal die offizielle PM:
Seattle Genetics and Millennium Announce Positive Top-Line Brentuximab Vedotin (SGN-35) Data from Pivotal Trial in Relapsed and Refractory Hodgkin Lymphoma
-Objective Responses Achieved in 75 percent of Patients; Median Duration of Response Greater than Six Months-
-BLA Submission Planned in First Half of 2011-
-Seattle Genetics to Host Conference Call and Webcast Today at 8:30 a.m. ET-
BOTHELL, Wash. & CAMBRIDGE, Mass., Sep 27, 2010 (BUSINESS WIRE) --
Seattle Genetics, Inc. (Nasdaq: SGEN) and Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited (TSE:4502), today announced positive top-line results from the pivotal trial of single-agent brentuximab vedotin (SGN-35), an antibody-drug conjugate (ADC) targeted to CD30. The trial was conducted in 102 relapsed or refractory Hodgkin lymphoma (HL) patients.
Seventy-five percent of patients in the pivotal trial achieved an objective response as assessed by an independent central review, the primary endpoint of the trial. The median duration of response was greater than six months. The safety profile of brentuximab vedotin in this trial was generally consistent with prior clinical trial experience. A more complete data set will be presented at an upcoming scientific meeting.
"We are extremely excited with the top-line results, as they move us one step closer to our goal of bringing brentuximab vedotin to patients with relapsed or refractory Hodgkin lymphoma," said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. "We are positioned for a Biologics License Application (BLA) submission to the U.S. Food and Drug Administration (FDA) in the first half of 2011. In addition, we plan to report top-line data from our phase II trial of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma (ALCL) within the next few weeks."
"The lack of adequate therapies for the treatment of relapsed and refractory Hodgkin lymphoma represents a substantial unmet medical need worldwide, with almost a third of the 30,000 newly diagnosed patients relapsing or becoming refractory to front-line therapy annually," said Nancy Simonian, M.D., Chief Medical Officer of Millennium. "These data have the potential to provide an important advance in therapy for Hodgkin lymphoma. We intend to discuss these results with European regulators to support our goal of submitting a Marketing Authorization Application to the European Medicines Agency (EMA) in 2011."
Pivotal Trial Design
The single-arm pivotal trial assessed efficacy and safety of single-agent brentuximab vedotin in relapsed or refractory, post-autologous stem cell transplant (ASCT) HL patients. Patients received 1.8 milligrams per kilogram of brentuximab vedotin every three weeks for up to 16 total doses. The primary endpoint of the trial was objective response rate as assessed by an independent review facility. Response assessments were based on the rigorous and internationally established Revised Response Criteria for Malignant Lymphoma (Cheson, 2007). Secondary endpoints included complete response rate, duration of response, progression-free survival, overall survival and tolerability. The trial was conducted under a Special Protocol Assessment (SPA) with the FDA and was discussed with the EMA during the process of obtaining EU Centralized Scientific Advice on the brentuximab vedotin development program. Brentuximab vedotin has been granted orphan drug designation by the FDA and EMA for the treatment of HL and ALCL and has been granted fast track designation by the FDA for HL.
About Brentuximab Vedotin
Brentuximab vedotin is an ADC comprising an anti-CD30 monoclonal antibody attached by an enzyme cleavable linker to a potent, synthetic drug payload, monomethyl auristatin E (MMAE) utilizing Seattle Genetics' proprietary technology. The ADC employs a novel linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells. This approach is intended to spare non-targeted cells and thus may help minimize the potential toxic effects of traditional chemotherapy while allowing for the selective targeting of CD30-expressing cancer cells, thus potentially enhancing the antitumor activity.
In addition to the pivotal HL trial, Seattle Genetics and Millennium are conducting a phase II trial for relapsed and refractory systemic ALCL, a phase III clinical trial (the AETHERA trial) for patients at high risk of residual HL following autologous stem cell transplant, a phase II retreatment trial for relapsed patients who previously responded to brentuximab vedotin, and a phase I combination trial for front-line treatment of HL.
About Hodgkin Lymphoma
Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is distinguished from other types of lymphoma by the presence of one characteristic type of cell, known as the Reed-Sternberg cell. A defining attribute of the Reed-Sternberg cell is its expression of the CD30 antigen.
According to the American Cancer Society, approximately 8,500 cases of Hodgkin lymphoma will be diagnosed in the United States during 2010 and more than 1,300 will die from the disease. Globally, there are more than 30,000 cases of Hodgkin lymphoma diagnosed each year. Although front-line combination chemotherapy can result in durable response rates, up to 30 percent of these patients relapse or are refractory to front-line treatment and have few therapeutic options beyond ASCT.
About the Seattle Genetics/Millennium Collaboration
Seattle Genetics and Millennium are jointly developing brentuximab vedotin. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize brentuximab vedotin in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for brentuximab vedotin on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
Seattle Genetics, Millennium Generate “Dream” Data With Empowered Antibody Drug for Cancer
Luke Timmerman 9/27/10
Seattle Genetics is making history today, after a dozen years of effort, with groundbreaking clinical trial results in the field of cancer drugs. The results pave the way for the first successful “empowered antibody” drug for cancer, and a new therapy for people who have run out of options for fighting Hodgkin’s disease.
The Bothell, WA-based company (NASDAQ: SGEN) and its Cambridge, MA-based partner, Millennium: The Takeda Oncology Company, are reporting today that Seattle Genetics’ lead drug candidate, brentuximab vedotin, was able to partially or completely shrink tumors for 75 percent of the 102 patients with Hodgkin’s disease who enrolled in a pivotal clinical trial. Most cancer drugs shrink tumors in fewer than 30 percent of patients. That means Seattle Genetics unequivocally surpassed the usual definition of success, and is now in a position to seek FDA approval to market the drug in the U.S.
“I’ve been doing cancer research and making cancer drugs for 25 years, and these are the kind of data you dream of,” says Clay Siegall, the co-founder and CEO of Seattle Genetics. “We are going to have an amazing impact on patients.”
Even though the patients were the sickest of the sick, their tumors were kept in check for at least six months. Doctors will continue to follow the patients to see how long the tumors remain stable, and ultimately how long the drug helps the patients live. Detailed results from the study will be presented at an upcoming medical meeting.
Armed with this new batch of results, Seattle Genetics now plans to seek FDA clearance in the first half of 2011, and to ask for a faster-than-usual six-month regulatory review that is sometimes granted for lifesaving therapies.
The results are important for several reasons. If the FDA gives the green light, brentuximab vedotin (formerly known as SGN-35) will become a transformative asset for Seattle Genetics, as the company will morph from an R&D-only operation into a more fully integrated company that discovers, develops, and sells pharmaceuticals. Scientifically, the drug is potentially the first of a new class of drugs designed to zero in specifically on cancerous cells, with a potent toxin to give them extra tumor-killing punch. Medically, the drug could offer a new option for many of the 8,500 patients in the U.S. diagnosed with Hodgkin’s each year, as well as other patients with lymphomas that overexpress a protein marker called CD30, which brentuximab is designed to hit.
Millennium, which last year paid Seattle Genetics $60 million upfront to obtain rights to the product outside North America, said its next move will be to seek approval for the product in Europe in 2011. “These data have the potential to provide an important advance in therapy for Hodgkin lymphoma,” said Millennium’s chief medical officer, Nancy Simonian, in a statement.
The trial, which started in February 2009, was designed in collaboration with the FDA. All patients got the treatment, rather than being randomly assigned to either the standard of care or the new drug. They were given an intravenous infusion of the Seattle Genetics treatment every three weeks. Side effects were mostly mild, and similar to what researchers saw in a preliminary trial: mild tingling and numbness in the fingers and toes, fatigue, white blood cell depletion, and diarrhea. All patients had relapsed after prior therapy and generally had a life expectancy of two to three years.
Seattle Genetics—partly to give its investigators a chance to show the full details at medical conference to be determined—isn’t providing important details yet about how many of the patients had complete disappearance of their tumors and how many just had partial responses. But when Seattle Genetics’ executive team got a look at the data over the weekend, it confirmed what they thought they’d see, given results from a Phase I trial. Back in a story I wrote two years ago, chief medical officer Tom Reynolds said brentuximab vedotin offered the kind of groundbreaking results for a cancer drug that are rarely seen, likening it to Roche and Biogen Idec’s rituximab (Rituxan) for non-Hodgkin’s lymphoma and Novartis’ imatinib (Gleevec) for chronic myeloid leukemia. Yesterday, Siegall had the same reaction.
“When the data was put up on screen, excitement was palpable,” Siegall says. “It was incredibly exciting for us. We work incredibly hard and are passionate about what we do. I can’t tell you how gratifying it is to see that kind of result. Very few agents demonstrate this type of response rate.”
More news is to come on whether this drug can help patients earlier on or with similar diseases. Seattle Genetics plans to release more clinical trial data within a few weeks on whether brentuximab vedotin can help patients with another rare lymphoma, anaplastic large cell lymphoma, that also has an overabundant number of CD30 cell markers, like Hodgkin’s. And while many patients with Hodgkin’s are essentially cured today by prior rounds of chemotherapy, Seattle Genetics and Millennium envision bringing this new antibody therapy to larger numbers of patients with earlier forms of disease because of the new drug’s combination of strong effectiveness and mild side effects. One such trial, called Aethera, is currently enrolling more than 320 patients.
Seattle Genetics hasn’t set a price for this product, but it has discussed hypothetical prices with analysts in the past as they try to get a handle on the market opportunity. Potential patients number 6,000 to 8,000 in the U.S., plus a similar number in Europe, according to Seattle Genetics market research. If the drug cost $45,000 to $50,000 per year, it could generate $300 million to $400 million in sales, Siegall told me in June 2008.
Over the weekend, Siegall said “it could command a premium price” based on the strength of the clinical trial data, although it’s still too early to set the actual price. This is one of the important questions Seattle Genetics will have to work on in the year ahead, along with other key tasks to complete. The to-do list will include getting the FDA application ready, preparing for an FDA advisory panel meeting, presenting at medical conferences, building up manufacturing inventory, and continuing the momentum with enrollment in early stage trials. Siegall talked with me about this all-out commercial push, and how Seattle Genetics was getting ready to handle all this work, in a feature story about the company’s growth in May.
Today’s results validate the long-term technology strategy of Seattle Genetics: to make targeted antibody drugs. The company was founded in 1998 with technology that Bristol-Myers Squibb gave up on: genetically engineered Y-shaped proteins that can be made to hit certain targets on cancer cells, while mostly sparing healthy ones. That is a big advantage over traditional chemotherapy that can be brutal on healthy cells and cause nasty side effects.
But many antibodies have failed over the years. While they might interfere with tumor growth and proliferation, they sometimes lack the potency to create a true knockout punch. For three decades, scientists have tried to add toxins to the antibodies to essentially create targeted chemotherapy, with very little to show for it. Earlier generations of these so-called “antibody-drug conjugates” failed, often because the toxin would break off in the bloodstream before it could reach the intended target. Wyeth (now part of Pfizer) introduced the first “empowered antibody,” gemtuzumab (Mylotarg), back in 2000, but it was a commercial dud because of its side effects, and Pfizer recently pulled it off the market.
Seattle Genetics has sought to improve on drugs like that through the years with a “synthetic linker” technology that keeps the antibody and toxin stable in the bloodstream, releasing the potent payload only into the tumor. The Seattle Genetics technology has been licensed for development on certain cell targets by a number of major pharma and biotech companies, such as GlaxoSmithKline, AstraZeneca’s MedImmune unit, Daiichi Sankyo, Bayer, and Roche’s Genentech unit. Seattle Genetics has retained exclusive rights to the technology’s use in hitting specific targets, and two other candidates against those targets are now in clinical trials.
Roche’s Genentech unit, the world’s leading maker of antibody drugs, has been in hot pursuit of this same concept for years, and also has produced some strong results with a more potent version of its pioneering breast cancer antibody, trastuzumab (Herceptin). That new potent antibody, called T-DM1, uses a different linking technology provided by Waltham, MA-based ImmunoGen (NASDAQ: IMGN). T-DM1 has been at the forefront of development for such empowered antibodies although the drug hit a bump in the road in August, the FDA said it wouldn’t review a Genentech application until it sees results from a more thorough test.
That setback for Genentech means that Seattle Genetics could be in position during 2011 to establish the new paradigm of empowered antibodies. If this drug is cleared by FDA, it will send a ripple effect through the biotech industry, given that “naked antibodies” that lack the extra degree of potency already make up a market worth an estimated $30 billion a year. Once the first of the new empowered antibodies hits the market, analysts will be busy sizing up the pipeline of more to come in the industry, and projecting all new growth curves for this class of drug.
“We think ADC [antibody-drug conjugate] technology is here to stay, and it could be really important for cancer patients and potentially transformative,” Siegall says. “These data establish us as the leaders in ADC technology. We are certainly not going to be sitting back and watching. We are going to push hard and make sure we provide the most promising opportunities for patients with limited therapeutic options.”
From a business perspective, Siegall is dreaming big. “We are now in position to transition from an R&D company into a commercial company. We’re looking forward to growing this company, and building an important biotech company. What’s an important biotech company; how do you define that? To me, it’s a company that makes products that patients really need.”
Luke Timmerman is the National Biotech Editor of Xconomy, and the Editor of Xconomy Seattle. You can e-mail him at ltimmerman@xconomy.com, or follow him at twitter.com/ldtimmerman.
Luke Timmerman 9/27/10
Seattle Genetics is making history today, after a dozen years of effort, with groundbreaking clinical trial results in the field of cancer drugs. The results pave the way for the first successful “empowered antibody” drug for cancer, and a new therapy for people who have run out of options for fighting Hodgkin’s disease.
The Bothell, WA-based company (NASDAQ: SGEN) and its Cambridge, MA-based partner, Millennium: The Takeda Oncology Company, are reporting today that Seattle Genetics’ lead drug candidate, brentuximab vedotin, was able to partially or completely shrink tumors for 75 percent of the 102 patients with Hodgkin’s disease who enrolled in a pivotal clinical trial. Most cancer drugs shrink tumors in fewer than 30 percent of patients. That means Seattle Genetics unequivocally surpassed the usual definition of success, and is now in a position to seek FDA approval to market the drug in the U.S.
“I’ve been doing cancer research and making cancer drugs for 25 years, and these are the kind of data you dream of,” says Clay Siegall, the co-founder and CEO of Seattle Genetics. “We are going to have an amazing impact on patients.”
Even though the patients were the sickest of the sick, their tumors were kept in check for at least six months. Doctors will continue to follow the patients to see how long the tumors remain stable, and ultimately how long the drug helps the patients live. Detailed results from the study will be presented at an upcoming medical meeting.
Armed with this new batch of results, Seattle Genetics now plans to seek FDA clearance in the first half of 2011, and to ask for a faster-than-usual six-month regulatory review that is sometimes granted for lifesaving therapies.
The results are important for several reasons. If the FDA gives the green light, brentuximab vedotin (formerly known as SGN-35) will become a transformative asset for Seattle Genetics, as the company will morph from an R&D-only operation into a more fully integrated company that discovers, develops, and sells pharmaceuticals. Scientifically, the drug is potentially the first of a new class of drugs designed to zero in specifically on cancerous cells, with a potent toxin to give them extra tumor-killing punch. Medically, the drug could offer a new option for many of the 8,500 patients in the U.S. diagnosed with Hodgkin’s each year, as well as other patients with lymphomas that overexpress a protein marker called CD30, which brentuximab is designed to hit.
Millennium, which last year paid Seattle Genetics $60 million upfront to obtain rights to the product outside North America, said its next move will be to seek approval for the product in Europe in 2011. “These data have the potential to provide an important advance in therapy for Hodgkin lymphoma,” said Millennium’s chief medical officer, Nancy Simonian, in a statement.
The trial, which started in February 2009, was designed in collaboration with the FDA. All patients got the treatment, rather than being randomly assigned to either the standard of care or the new drug. They were given an intravenous infusion of the Seattle Genetics treatment every three weeks. Side effects were mostly mild, and similar to what researchers saw in a preliminary trial: mild tingling and numbness in the fingers and toes, fatigue, white blood cell depletion, and diarrhea. All patients had relapsed after prior therapy and generally had a life expectancy of two to three years.
Seattle Genetics—partly to give its investigators a chance to show the full details at medical conference to be determined—isn’t providing important details yet about how many of the patients had complete disappearance of their tumors and how many just had partial responses. But when Seattle Genetics’ executive team got a look at the data over the weekend, it confirmed what they thought they’d see, given results from a Phase I trial. Back in a story I wrote two years ago, chief medical officer Tom Reynolds said brentuximab vedotin offered the kind of groundbreaking results for a cancer drug that are rarely seen, likening it to Roche and Biogen Idec’s rituximab (Rituxan) for non-Hodgkin’s lymphoma and Novartis’ imatinib (Gleevec) for chronic myeloid leukemia. Yesterday, Siegall had the same reaction.
“When the data was put up on screen, excitement was palpable,” Siegall says. “It was incredibly exciting for us. We work incredibly hard and are passionate about what we do. I can’t tell you how gratifying it is to see that kind of result. Very few agents demonstrate this type of response rate.”
More news is to come on whether this drug can help patients earlier on or with similar diseases. Seattle Genetics plans to release more clinical trial data within a few weeks on whether brentuximab vedotin can help patients with another rare lymphoma, anaplastic large cell lymphoma, that also has an overabundant number of CD30 cell markers, like Hodgkin’s. And while many patients with Hodgkin’s are essentially cured today by prior rounds of chemotherapy, Seattle Genetics and Millennium envision bringing this new antibody therapy to larger numbers of patients with earlier forms of disease because of the new drug’s combination of strong effectiveness and mild side effects. One such trial, called Aethera, is currently enrolling more than 320 patients.
Seattle Genetics hasn’t set a price for this product, but it has discussed hypothetical prices with analysts in the past as they try to get a handle on the market opportunity. Potential patients number 6,000 to 8,000 in the U.S., plus a similar number in Europe, according to Seattle Genetics market research. If the drug cost $45,000 to $50,000 per year, it could generate $300 million to $400 million in sales, Siegall told me in June 2008.
Over the weekend, Siegall said “it could command a premium price” based on the strength of the clinical trial data, although it’s still too early to set the actual price. This is one of the important questions Seattle Genetics will have to work on in the year ahead, along with other key tasks to complete. The to-do list will include getting the FDA application ready, preparing for an FDA advisory panel meeting, presenting at medical conferences, building up manufacturing inventory, and continuing the momentum with enrollment in early stage trials. Siegall talked with me about this all-out commercial push, and how Seattle Genetics was getting ready to handle all this work, in a feature story about the company’s growth in May.
Today’s results validate the long-term technology strategy of Seattle Genetics: to make targeted antibody drugs. The company was founded in 1998 with technology that Bristol-Myers Squibb gave up on: genetically engineered Y-shaped proteins that can be made to hit certain targets on cancer cells, while mostly sparing healthy ones. That is a big advantage over traditional chemotherapy that can be brutal on healthy cells and cause nasty side effects.
But many antibodies have failed over the years. While they might interfere with tumor growth and proliferation, they sometimes lack the potency to create a true knockout punch. For three decades, scientists have tried to add toxins to the antibodies to essentially create targeted chemotherapy, with very little to show for it. Earlier generations of these so-called “antibody-drug conjugates” failed, often because the toxin would break off in the bloodstream before it could reach the intended target. Wyeth (now part of Pfizer) introduced the first “empowered antibody,” gemtuzumab (Mylotarg), back in 2000, but it was a commercial dud because of its side effects, and Pfizer recently pulled it off the market.
Seattle Genetics has sought to improve on drugs like that through the years with a “synthetic linker” technology that keeps the antibody and toxin stable in the bloodstream, releasing the potent payload only into the tumor. The Seattle Genetics technology has been licensed for development on certain cell targets by a number of major pharma and biotech companies, such as GlaxoSmithKline, AstraZeneca’s MedImmune unit, Daiichi Sankyo, Bayer, and Roche’s Genentech unit. Seattle Genetics has retained exclusive rights to the technology’s use in hitting specific targets, and two other candidates against those targets are now in clinical trials.
Roche’s Genentech unit, the world’s leading maker of antibody drugs, has been in hot pursuit of this same concept for years, and also has produced some strong results with a more potent version of its pioneering breast cancer antibody, trastuzumab (Herceptin). That new potent antibody, called T-DM1, uses a different linking technology provided by Waltham, MA-based ImmunoGen (NASDAQ: IMGN). T-DM1 has been at the forefront of development for such empowered antibodies although the drug hit a bump in the road in August, the FDA said it wouldn’t review a Genentech application until it sees results from a more thorough test.
That setback for Genentech means that Seattle Genetics could be in position during 2011 to establish the new paradigm of empowered antibodies. If this drug is cleared by FDA, it will send a ripple effect through the biotech industry, given that “naked antibodies” that lack the extra degree of potency already make up a market worth an estimated $30 billion a year. Once the first of the new empowered antibodies hits the market, analysts will be busy sizing up the pipeline of more to come in the industry, and projecting all new growth curves for this class of drug.
“We think ADC [antibody-drug conjugate] technology is here to stay, and it could be really important for cancer patients and potentially transformative,” Siegall says. “These data establish us as the leaders in ADC technology. We are certainly not going to be sitting back and watching. We are going to push hard and make sure we provide the most promising opportunities for patients with limited therapeutic options.”
From a business perspective, Siegall is dreaming big. “We are now in position to transition from an R&D company into a commercial company. We’re looking forward to growing this company, and building an important biotech company. What’s an important biotech company; how do you define that? To me, it’s a company that makes products that patients really need.”
Luke Timmerman is the National Biotech Editor of Xconomy, and the Editor of Xconomy Seattle. You can e-mail him at ltimmerman@xconomy.com, or follow him at twitter.com/ldtimmerman.
Hinsichtlich des Kurses waren wachholder und ich wohl etwas zu vorsichtig. In der Tat hat SGEN in der letzten Woche bei sehr hohen Umsätzen und ohne wesentlichen Rücksetzer die 15 Dollar Marke genommen:

Der von mir vielzitierte und vielgeschätzte Ohad Hammer hat die Daten von Brentuximab Vedotin als Durchbruch bezeichnet.
Zitat:
"SGN-35 results are spectacular, I am very curious to see duration of response numbers but this is a true breakthrough."
Wir können wohl in der nächsten Woche mit einem kleinen Update zu SGEN durch ihn rechnen.
Der von mir vielzitierte und vielgeschätzte Ohad Hammer hat die Daten von Brentuximab Vedotin als Durchbruch bezeichnet.
Zitat:
"SGN-35 results are spectacular, I am very curious to see duration of response numbers but this is a true breakthrough."
Wir können wohl in der nächsten Woche mit einem kleinen Update zu SGEN durch ihn rechnen.
Antwort auf Beitrag Nr.: 40.255.806 von SLGramann am 03.10.10 15:38:09die Phanasie im Kurs bezieht sich sicher nicht nur auf Hodgkin Lymphom (das sind die o.g. 300-400 mio USD p.a. Umsatz Potential) sondern weitere Indikationen in denen CD30 eine Rolle spielt:
" several T-cell lymphomas including anaplastic large cell lymphoma or ALCL"
, sodass hier viele mit rascher Indikationsausweitung und off label use rechnen;
as of today; 9 laufende oder abgeschlossene Studien mit SGN-35, eine wurde abgebrochen,
http://clinicaltrials.gov/ct2/results?term=sgn-35
das könnte sich rasch ändern, schauen wir mal.
meine persönliche Meinung, und keine Empfehlung zum Kauf
" several T-cell lymphomas including anaplastic large cell lymphoma or ALCL"
, sodass hier viele mit rascher Indikationsausweitung und off label use rechnen;
as of today; 9 laufende oder abgeschlossene Studien mit SGN-35, eine wurde abgebrochen,
http://clinicaltrials.gov/ct2/results?term=sgn-35
das könnte sich rasch ändern, schauen wir mal.
meine persönliche Meinung, und keine Empfehlung zum Kauf
Moin,

hab ich offensichtlich doch zum falschen Zeitpunkt verkauft bzw. versäumt, wieder einzusteigen (10-15% günstiger hätte ich die Aktie immerhin haben können, war aber zu geizig).

Was soll´s, ich glaube das Ende der Fahnenstange wird bald erreicht sein, ein Rücksetzer wird kommen, dann bin ich wieder dabei!
Die Technologie hat sich bewährt, angeheizt noch durch den T-DM1-Erfolg.
Gruß q.

hab ich offensichtlich doch zum falschen Zeitpunkt verkauft bzw. versäumt, wieder einzusteigen (10-15% günstiger hätte ich die Aktie immerhin haben können, war aber zu geizig).

Was soll´s, ich glaube das Ende der Fahnenstange wird bald erreicht sein, ein Rücksetzer wird kommen, dann bin ich wieder dabei!
Die Technologie hat sich bewährt, angeheizt noch durch den T-DM1-Erfolg.
Gruß q.
Brentuximab Vedotin nun auch bei ALCL äußerst erfolgreich!
SEATTLE (TheStreet) -- Clinical trial victory two for Seattle Genetics'(SGEN_) lymphoma drug brentuximab vedotin.
More from Adam Feuerstein
Seattle Genetics Inc.|Seattle Genetics said Monday that 86% of patients with advanced systemic anaplastic large cell lymphoma (ALCL) responded to treatment with brentuximab, also known as SGN-35. The phase II study enrolled 58 ALCL patients, all of whom had a form of the rare cancer that was no longer responding to currently available therapies. This is the second positive clinical trial for brentuximab announced in as many weeks. On Sept. 27, Seattle Genetics announced positive results from a study in patients with advanced Hodgkin disease.
Seattle Genetics intends to submit brentuximab for U.S. approval in the first half of 2011 as a treatment for both forms of lymphoma. Millennium Pharmaceuticals, the U.S.-based subsidiary of the Japanese drug maker Takeda, is Seattle Genetics' ex-U.S. partner for brentuximab and will be in charge of getting the drug approved in Europe.
ALCL is an aggressive type of T-cell non-Hodgkin lymphoma that expresses a protein known as CD30. Between 2,000 and 3,000 patients are diagnosed with ALCL in the U.S. each year, with about half of those treated effectively with first-line chemotherapy.
Brentuximab is designed using a technology proprietary to Seattle Genetics that delivers a lethal dose of chemotherapy directly to cancer cells while sparing healthy cells from toxic effects. Brentuximab consists of an antibody that attaches itself to the CD30 receptor found on tumor cells. Once inside the tumor, brentuximab releases a toxic chemotherapy payload.
Seattle Genetics closed Friday at $16.77. The stock is up 38% since Sept. 27 when the positive brentuximab data in Hodgkin lymphoma was announced.
--Written by Adam Feuerstein in Boston.
SEATTLE (TheStreet) -- Clinical trial victory two for Seattle Genetics'(SGEN_) lymphoma drug brentuximab vedotin.
More from Adam Feuerstein
Seattle Genetics Inc.|Seattle Genetics said Monday that 86% of patients with advanced systemic anaplastic large cell lymphoma (ALCL) responded to treatment with brentuximab, also known as SGN-35. The phase II study enrolled 58 ALCL patients, all of whom had a form of the rare cancer that was no longer responding to currently available therapies. This is the second positive clinical trial for brentuximab announced in as many weeks. On Sept. 27, Seattle Genetics announced positive results from a study in patients with advanced Hodgkin disease.
Seattle Genetics intends to submit brentuximab for U.S. approval in the first half of 2011 as a treatment for both forms of lymphoma. Millennium Pharmaceuticals, the U.S.-based subsidiary of the Japanese drug maker Takeda, is Seattle Genetics' ex-U.S. partner for brentuximab and will be in charge of getting the drug approved in Europe.
ALCL is an aggressive type of T-cell non-Hodgkin lymphoma that expresses a protein known as CD30. Between 2,000 and 3,000 patients are diagnosed with ALCL in the U.S. each year, with about half of those treated effectively with first-line chemotherapy.
Brentuximab is designed using a technology proprietary to Seattle Genetics that delivers a lethal dose of chemotherapy directly to cancer cells while sparing healthy cells from toxic effects. Brentuximab consists of an antibody that attaches itself to the CD30 receptor found on tumor cells. Once inside the tumor, brentuximab releases a toxic chemotherapy payload.
Seattle Genetics closed Friday at $16.77. The stock is up 38% since Sept. 27 when the positive brentuximab data in Hodgkin lymphoma was announced.
--Written by Adam Feuerstein in Boston.
SGN 75 zeigt erste Hinweise auf Wirksamkeit!
SEATTLE, Oct 11, 2010 (BUSINESS WIRE) -- Seattle Genetics, Inc. /quotes/comstock/15*!sgen/quotes/nls/sgen (SGEN 16.77, +0.45, +2.76%) today reported data from a phase I clinical trial of SGN-75 in patients with non-Hodgkin lymphoma or renal cell carcinoma (RCC). Preliminary results demonstrate tolerability and antitumor activity, including two objective responses in the first 16 patients treated. Dose-escalation is continuing. SGN-75 is an antibody-drug conjugate (ADC) targeted to CD70. The data were presented at the 35th European Society for Medical Oncology (ESMO) Congress being held in Milan, Italy.
"These preliminary data are encouraging, and show initial evidence of antitumor activity with SGN-75 in patients with relapsed/refractory non-Hodgkin lymphoma or metastatic RCC," said Jonathan Drachman, M.D., Senior Vice President, Research and Translational Medicine of Seattle Genetics. "We are continuing to dose escalate in this trial to further assess the safety and activity of SGN-75, as well as to establish the optimal dosing regimen for future clinical trials."
Data were reported from 16 patients in the open-label phase I clinical trial, including seven with non-Hodgkin lymphoma and nine with RCC. Cohorts of patients received SGN-75 either every three weeks at doses ranging from 0.3 to 2.0 milligrams per kilogram (mg/kg) or on a weekly basis at a dose of 0.3 mg/kg. Enrolled patients in each indication had received a median of three prior systemic therapies.
Best clinical response among non-Hodgkin lymphoma patients included one patient with a complete remission, four patients with stable disease, one patient with progressive disease and one patient who was not evaluable. Best clinical response among RCC patients included one patient with a partial response, three patients with stable disease and five patients with progressive disease. The most common adverse events were fatigue, nausea and peripheral edema (swelling). A maximum tolerated dose has not been established with either dosing schedule and dose escalation continues. (Abstract #532P)
The single-agent phase I study of SGN-75 was initiated in November 2009 and is designed to enroll up to approximately 80 patients at multiple centers in the United States. The trial is evaluating the safety, tolerability, pharmacokinetic profile and antitumor activity of SGN-75. Patients enrolled in the trial must have received at least one prior therapy and have confirmed CD70 expression.
SGN-75 is an ADC comprising an anti-CD70 antibody attached to a potent, synthetic cell-killing agent, monomethyl auristatin F (MMAF), using Seattle Genetics' proprietary technology. The ADC is designed to be stable in the bloodstream, and to release its cell-killing agent upon internalization into CD70-expressing tumor cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy while enhancing the antitumor activity.
About Non-Hodgkin Lymphoma
Non-Hodgkin lymphoma represents a diverse group of cancers that develop in the lymphatic system and are characterized by uncontrolled growth and accumulation of abnormal lymphocytes. Lymphocytes are white blood cells that are responsible for defending the body against infection. The most common forms of non-Hodgkin lymphoma are follicular and diffuse large B-cell lymphoma. According to the American Cancer Society, more than 65,000 cases of non-Hodgkin lymphoma are expected to be diagnosed in the United States during 2010 and more than 20,000 patients will die from the disease.
About Renal Cell Carcinoma
Renal cell carcinoma (RCC) forms in the kidney, which filters and cleans the blood. Metastatic RCC occurs when the cancer has spread to other parts of the body. RCC is the most common type of kidney cancer in adults, representing approximately 90 percent of cases. The American Cancer Society estimates that there will be more than 58,000 new cases of kidney cancer in the United States during 2010, and approximately 13,000 people will die from the disease.
-----------------
Ich maße mir nicht an, das fachlich zu beurteilen, aber 2 OR und 7 x stable disease aus einem Kollektiv mit 16 Patienten ohne dass auch nur die MTD erreicht wurde klingen zumindest nach Weitermachen!
SEATTLE, Oct 11, 2010 (BUSINESS WIRE) -- Seattle Genetics, Inc. /quotes/comstock/15*!sgen/quotes/nls/sgen (SGEN 16.77, +0.45, +2.76%) today reported data from a phase I clinical trial of SGN-75 in patients with non-Hodgkin lymphoma or renal cell carcinoma (RCC). Preliminary results demonstrate tolerability and antitumor activity, including two objective responses in the first 16 patients treated. Dose-escalation is continuing. SGN-75 is an antibody-drug conjugate (ADC) targeted to CD70. The data were presented at the 35th European Society for Medical Oncology (ESMO) Congress being held in Milan, Italy.
"These preliminary data are encouraging, and show initial evidence of antitumor activity with SGN-75 in patients with relapsed/refractory non-Hodgkin lymphoma or metastatic RCC," said Jonathan Drachman, M.D., Senior Vice President, Research and Translational Medicine of Seattle Genetics. "We are continuing to dose escalate in this trial to further assess the safety and activity of SGN-75, as well as to establish the optimal dosing regimen for future clinical trials."
Data were reported from 16 patients in the open-label phase I clinical trial, including seven with non-Hodgkin lymphoma and nine with RCC. Cohorts of patients received SGN-75 either every three weeks at doses ranging from 0.3 to 2.0 milligrams per kilogram (mg/kg) or on a weekly basis at a dose of 0.3 mg/kg. Enrolled patients in each indication had received a median of three prior systemic therapies.
Best clinical response among non-Hodgkin lymphoma patients included one patient with a complete remission, four patients with stable disease, one patient with progressive disease and one patient who was not evaluable. Best clinical response among RCC patients included one patient with a partial response, three patients with stable disease and five patients with progressive disease. The most common adverse events were fatigue, nausea and peripheral edema (swelling). A maximum tolerated dose has not been established with either dosing schedule and dose escalation continues. (Abstract #532P)
The single-agent phase I study of SGN-75 was initiated in November 2009 and is designed to enroll up to approximately 80 patients at multiple centers in the United States. The trial is evaluating the safety, tolerability, pharmacokinetic profile and antitumor activity of SGN-75. Patients enrolled in the trial must have received at least one prior therapy and have confirmed CD70 expression.
SGN-75 is an ADC comprising an anti-CD70 antibody attached to a potent, synthetic cell-killing agent, monomethyl auristatin F (MMAF), using Seattle Genetics' proprietary technology. The ADC is designed to be stable in the bloodstream, and to release its cell-killing agent upon internalization into CD70-expressing tumor cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy while enhancing the antitumor activity.
About Non-Hodgkin Lymphoma
Non-Hodgkin lymphoma represents a diverse group of cancers that develop in the lymphatic system and are characterized by uncontrolled growth and accumulation of abnormal lymphocytes. Lymphocytes are white blood cells that are responsible for defending the body against infection. The most common forms of non-Hodgkin lymphoma are follicular and diffuse large B-cell lymphoma. According to the American Cancer Society, more than 65,000 cases of non-Hodgkin lymphoma are expected to be diagnosed in the United States during 2010 and more than 20,000 patients will die from the disease.
About Renal Cell Carcinoma
Renal cell carcinoma (RCC) forms in the kidney, which filters and cleans the blood. Metastatic RCC occurs when the cancer has spread to other parts of the body. RCC is the most common type of kidney cancer in adults, representing approximately 90 percent of cases. The American Cancer Society estimates that there will be more than 58,000 new cases of kidney cancer in the United States during 2010, and approximately 13,000 people will die from the disease.
-----------------
Ich maße mir nicht an, das fachlich zu beurteilen, aber 2 OR und 7 x stable disease aus einem Kollektiv mit 16 Patienten ohne dass auch nur die MTD erreicht wurde klingen zumindest nach Weitermachen!
Ohad Hammer hat sich mit der ESMO beschäftigt und in Bezug auf SGEN auch die Daten zu SGN 75 interpretiert.
Natürlich kann man in diesem frühen Stadium der Entwicklung noch keine Prognosen machen, aber es wird aus dem Text doch sehr deutlich, dass der Blick auf dieses Projekt erfreulich "gierig" geworden ist:
Seattle Genetics is about to conclude the best year in its history, since it was incorporated 13 years ago. The company’s lead agent, SGN-35 (aka Brentuximab Vedotin), generated astonishing results in two types of blood cancers earlier this year. Based on the results in Hodkin’s Lymphoma, SGN-35’s approval seems inevitable, even though results are not from large randomized studies. Unlike T-DM1’s case, Seattle Genetics negotiated a special protocol assessment (SPA) with the FDA, implying that the trial design and endpoints are acceptable by the FDA.
Although initial market potential is limited ($250-$300M), this amount is still substantial for a company with a market cap of ~$1.7B, assuming a substantial portion of revenues will come from the US, where Seattle Genetics has exclusive rights. The approval will also serve as a final validation for Seattle Genetics’ technology and will position it as the leading antibody drug conjugate (ADC) company with the only approved ADC in the market (Mylotarg, the first ADC to get approved was recently withdrawn due to failed post-approval studies).
At ESMO, Seattle Genetics gave investors another reason for optimism, this time following positive data for another wholly owned agent, SGN-75. SGN-75 is an ADC for the treatment of NHL and renal cancer, two indications that represent a multi-billion dollar opportunity. Data at ESMO included preliminary findings implying that SGN-75 is active in both indications. The trial included only patients whose tumors express CD70, SGN-75’s target.
Seattle Genetics reported a complete response in one patient with NHL (out of 7 patients) and one partial response in a renal cancer patient (out of ~10 patients). As of data presentation the maximal dose was not reached, so the drug’s real activity could be substantially better. This is particularly relevant for solid cancers such as renal cancer, which typically require higher exposure compared to blood cancers. So far, SGN-75’s exposure was significantly lower than that achieved by other ADCs for solid tumors such as T-DM1, so the main challenge now is increasing the dose without causing too many side effects.
Although NHL is a larger market than Renal cancer, the latter should be viewed as more attractive for SGN-75 since it represents a higher unmet need with less competition, especially for antibodies. Until now, the market for renal cancer has been dominated by kinase inhibitors such as Pfizer’s (PFE) Sutent, Onyx’s (ONXX) Nexavar and Novartis’ (NVS) Afinitor. While these agents disrupt signals, ADCs such as SGN-75 deliver a toxic payload to tumor cells and can therefore be viewed as complimentary.
As with any early stage drug candidate, more patients are needed in order to better assess SGN-75’s potential. It remains to be seen whether higher doses lead to a better response rate. Another important issue is the ability to identify tumors which are more sensitive to SGN-75 based on the amount and pattern of CD70 expression. Preclinical data published by Seattle Genetics show good correlation between expression level s of CD70 and sensitivity to SGN-75. As CD70 is found in many other cancers but with less prevalence than in kidney cancer, there is potential opportunity for label expansion in lung, ovarian and pancreatic cancer, as shown by the elegant paper.
In summary, SGN-75 could become the next big ADC, as it contains all the right ingredients: New target, unmet need, potential utility in several solid tumor indications, good safety profile and clear efficacy. Based on the rapid recruitment, investors should have a sense of where this agent stands by next ASCO in June 2011.
Most importantly, SGN-75 is still unpartnered so positive results next year should have a huge impact on the compamy’s valuation. The antibody moiety in SGN-75 was part of a 2001 licensing deal with a research institute in the Netherlands. This implies that Seattle Genetics will probably pay low royalties on sales of SGN-75, but this should not prevent it from getting a lucrative licensing deal down the road.
Seattle Genetics is not the only one with a CD70 program. Medarex also has a CD70 ADC (MDX-1203) in clinical testing, based on its proprietary ADC technology, which is not as validated as that of Seattle Genetics. The two companies were running neck-to-neck with their CD70 programs during the past several years. Medarex beat Seattle Genetics in starting a phase I, probably due to the fact Seattle Genetics was developing a method for selecting patients based on CD70 expression. In contrast, Medarex is not selecting patients based on molecular markers but simply limit accrual to renal cancer and NHL. It will be intriguing to see first data for MDX-1203, which should give investors more perspective on its ADC technology compared to that of Seattle Genetics.
It is also very likely to see a CD70 ADC powered by Immunogen’s (IMGN) technology. Even if Immunogen or its partners are not working on the target, the recent data for SGN-75 clearly marks CD70 as the next hot target. Extrapolating from published work by Genentech, one can assume that Immunogen can use exactly the same non-cleavable linker currently used with T-DM1, which is much more validated than the rest of its linkers. With the exception of T-DM1, all of Immunogen’s compounds in the clinic are based on cleaveable linkers, which are viewed by some as less effective (even though some targets are not suitable for uncleaveable linker).
http://www.hammerstockblog.com/winners-of-esmo-2010/
Natürlich kann man in diesem frühen Stadium der Entwicklung noch keine Prognosen machen, aber es wird aus dem Text doch sehr deutlich, dass der Blick auf dieses Projekt erfreulich "gierig" geworden ist:
Seattle Genetics is about to conclude the best year in its history, since it was incorporated 13 years ago. The company’s lead agent, SGN-35 (aka Brentuximab Vedotin), generated astonishing results in two types of blood cancers earlier this year. Based on the results in Hodkin’s Lymphoma, SGN-35’s approval seems inevitable, even though results are not from large randomized studies. Unlike T-DM1’s case, Seattle Genetics negotiated a special protocol assessment (SPA) with the FDA, implying that the trial design and endpoints are acceptable by the FDA.
Although initial market potential is limited ($250-$300M), this amount is still substantial for a company with a market cap of ~$1.7B, assuming a substantial portion of revenues will come from the US, where Seattle Genetics has exclusive rights. The approval will also serve as a final validation for Seattle Genetics’ technology and will position it as the leading antibody drug conjugate (ADC) company with the only approved ADC in the market (Mylotarg, the first ADC to get approved was recently withdrawn due to failed post-approval studies).
At ESMO, Seattle Genetics gave investors another reason for optimism, this time following positive data for another wholly owned agent, SGN-75. SGN-75 is an ADC for the treatment of NHL and renal cancer, two indications that represent a multi-billion dollar opportunity. Data at ESMO included preliminary findings implying that SGN-75 is active in both indications. The trial included only patients whose tumors express CD70, SGN-75’s target.
Seattle Genetics reported a complete response in one patient with NHL (out of 7 patients) and one partial response in a renal cancer patient (out of ~10 patients). As of data presentation the maximal dose was not reached, so the drug’s real activity could be substantially better. This is particularly relevant for solid cancers such as renal cancer, which typically require higher exposure compared to blood cancers. So far, SGN-75’s exposure was significantly lower than that achieved by other ADCs for solid tumors such as T-DM1, so the main challenge now is increasing the dose without causing too many side effects.
Although NHL is a larger market than Renal cancer, the latter should be viewed as more attractive for SGN-75 since it represents a higher unmet need with less competition, especially for antibodies. Until now, the market for renal cancer has been dominated by kinase inhibitors such as Pfizer’s (PFE) Sutent, Onyx’s (ONXX) Nexavar and Novartis’ (NVS) Afinitor. While these agents disrupt signals, ADCs such as SGN-75 deliver a toxic payload to tumor cells and can therefore be viewed as complimentary.
As with any early stage drug candidate, more patients are needed in order to better assess SGN-75’s potential. It remains to be seen whether higher doses lead to a better response rate. Another important issue is the ability to identify tumors which are more sensitive to SGN-75 based on the amount and pattern of CD70 expression. Preclinical data published by Seattle Genetics show good correlation between expression level s of CD70 and sensitivity to SGN-75. As CD70 is found in many other cancers but with less prevalence than in kidney cancer, there is potential opportunity for label expansion in lung, ovarian and pancreatic cancer, as shown by the elegant paper.
In summary, SGN-75 could become the next big ADC, as it contains all the right ingredients: New target, unmet need, potential utility in several solid tumor indications, good safety profile and clear efficacy. Based on the rapid recruitment, investors should have a sense of where this agent stands by next ASCO in June 2011.
Most importantly, SGN-75 is still unpartnered so positive results next year should have a huge impact on the compamy’s valuation. The antibody moiety in SGN-75 was part of a 2001 licensing deal with a research institute in the Netherlands. This implies that Seattle Genetics will probably pay low royalties on sales of SGN-75, but this should not prevent it from getting a lucrative licensing deal down the road.
Seattle Genetics is not the only one with a CD70 program. Medarex also has a CD70 ADC (MDX-1203) in clinical testing, based on its proprietary ADC technology, which is not as validated as that of Seattle Genetics. The two companies were running neck-to-neck with their CD70 programs during the past several years. Medarex beat Seattle Genetics in starting a phase I, probably due to the fact Seattle Genetics was developing a method for selecting patients based on CD70 expression. In contrast, Medarex is not selecting patients based on molecular markers but simply limit accrual to renal cancer and NHL. It will be intriguing to see first data for MDX-1203, which should give investors more perspective on its ADC technology compared to that of Seattle Genetics.
It is also very likely to see a CD70 ADC powered by Immunogen’s (IMGN) technology. Even if Immunogen or its partners are not working on the target, the recent data for SGN-75 clearly marks CD70 as the next hot target. Extrapolating from published work by Genentech, one can assume that Immunogen can use exactly the same non-cleavable linker currently used with T-DM1, which is much more validated than the rest of its linkers. With the exception of T-DM1, all of Immunogen’s compounds in the clinic are based on cleaveable linkers, which are viewed by some as less effective (even though some targets are not suitable for uncleaveable linker).
http://www.hammerstockblog.com/winners-of-esmo-2010/
Antwort auf Beitrag Nr.: 40.386.812 von SLGramann am 25.10.10 20:16:07"With the exception of T-DM1, all of Immunogen’s compounds in the clinic are based on cleaveable linkers, which are viewed by some as less effective"
Da irrt sich Ohad Hammer!
Moderne spaltbare Disulfid-Linker (wie z.B. SPDB, verwendet z.B. in Biotest´s BT-062) haben gegenüber dem im Immunkonjugat T-DM1 verwendeten Thioether-Linker (MCC) den Vorteil, dass sie lipophile Metaboliten bilden, die auch sogenannte "Bystander Activity" aufweisen, also das die Tumorzelle umgebene (und für die Tumorzelle wichtige) Gewebsstroma schädigen!
Auch führt SPDB zu einer im Vergleich zu MCC erhöhten primären Toxizität gegenüber den Targetzellen!
So sehr ich Ohad Hammer schätze, hier ist er offensichtlich nicht auf dem neuesten Stand.
Da irrt sich Ohad Hammer!
Moderne spaltbare Disulfid-Linker (wie z.B. SPDB, verwendet z.B. in Biotest´s BT-062) haben gegenüber dem im Immunkonjugat T-DM1 verwendeten Thioether-Linker (MCC) den Vorteil, dass sie lipophile Metaboliten bilden, die auch sogenannte "Bystander Activity" aufweisen, also das die Tumorzelle umgebene (und für die Tumorzelle wichtige) Gewebsstroma schädigen!
Auch führt SPDB zu einer im Vergleich zu MCC erhöhten primären Toxizität gegenüber den Targetzellen!
So sehr ich Ohad Hammer schätze, hier ist er offensichtlich nicht auf dem neuesten Stand.
Antwort auf Beitrag Nr.: 40.637.105 von Joschka Schröder am 02.12.10 23:50:01PS: Richtig ist, dass es in Immunogen´s Repertoire spaltbare Linker (wie z.B. SPP) gibt, die im Hinblick auf ihre primäre Toxizität weniger effektiv sind als z.B. MCC.
Darauf beziehen sich auch Krop et al. in ihrer im Journal of Clinical Oncology publizierten Arbeit ("Phase I Study of Trastuzumab-DM1 ...", erschienen im April 2010). Vielleicht hatte Ohad Hammer diese Studie vor Augen.
Der Nachteile spaltbarer Linker besteht in potentiell etwas höherem freien Toxin in der Blutbahn. Dies wird jedoch beim SPDB durch die in #62 beschriebenen Effekte deutlich überkompensiert, so dass per saldo der moderne Disulfid-Linker die besseren Kenndaten aufweist als der im T-DM1 verwendete Thioether-Linker.
Darauf beziehen sich auch Krop et al. in ihrer im Journal of Clinical Oncology publizierten Arbeit ("Phase I Study of Trastuzumab-DM1 ...", erschienen im April 2010). Vielleicht hatte Ohad Hammer diese Studie vor Augen.
Der Nachteile spaltbarer Linker besteht in potentiell etwas höherem freien Toxin in der Blutbahn. Dies wird jedoch beim SPDB durch die in #62 beschriebenen Effekte deutlich überkompensiert, so dass per saldo der moderne Disulfid-Linker die besseren Kenndaten aufweist als der im T-DM1 verwendete Thioether-Linker.
Antwort auf Beitrag Nr.: 40.637.105 von Joschka Schröder am 02.12.10 23:50:01Hi JS, fachlich kann ich das nicht beurteilen und bin nach wie vor froh, dass Du die IMGN-Technik für zumindest gleichwertig hältst. Wenn er schreibt: "which are viewed by some as less effective", macht er sich diese Einschätztung ja auch nicht wirklich zu eigen.
Wie auch immer: Ich habe meine SGEN-Position an diesem eher schwachen Tag in New York verdoppelt. Ich sehe das Unternehmen klar auf dem Weg zum Markt. Und Brentuximab Vedotin ist sicher erst der Anfang der Geschichte.
(Finanziert wird das Ganze übrigens durch einen Teilverkauf meiner eh zu großen MorphoSys-Position...)
Wie auch immer: Ich habe meine SGEN-Position an diesem eher schwachen Tag in New York verdoppelt. Ich sehe das Unternehmen klar auf dem Weg zum Markt. Und Brentuximab Vedotin ist sicher erst der Anfang der Geschichte.
(Finanziert wird das Ganze übrigens durch einen Teilverkauf meiner eh zu großen MorphoSys-Position...)
Ohad Hammer hat nach der ASH ein Update zu SGEN veröffentlicht:
http://www.hammerstockblog.com/the-winner-of-ash-2010-seattl…
Ich fühle mich dadurch in meiner Entscheidung bestärkt, kürzlich recht aggressiv nachzukaufen.
Das wesentliche Argument ist, dass mit Brentuximab Vedotin ein ADC mit sehr hoher Wahrscheinlichkeit auf dem Weg zum Markt ist, der zwar in Nischenindikationen entwickelt worden ist, der aber dennoch SGEN einen beachtlichen, regelmäßigen Einnahmestrom verschaffen dürfte, der es dem Unternehmen erlaubt, 1.) eine eigene Vertriebsstruktur aufzubauen und sich generell breiter aufzustellen und 2.) viel Geld in die eigene Forschung zu lenken, ohne auf den Kapitalmarkt angewiesen zu sein. Da SGEN über eine besondere Kompetenz verfügt, halte ich die Ausgaben für eine eigene Pipeline auch für gut angelegtes Geld. Die Sache ist ja so, dass SGEN auf sehr viel wissenschaftliche Erkenntnisse der letzten 20 Jahre im Bereich Antikörper und Targets zugreifen kann und mit seiner ADC-Technologie Dinge eher veredelt, als das Rad jedes Mal ganz neu zu erfinden.
Außerdem ist SGEN sehr stark in die ADC-Projekte großer Pharmas eingebunden. Das dürfte insbesondere für Roche/Genentech gelten, wo dutzende Programme mit SGEN-Technologie in der vorklinischen Entwicklung sein sollen. Ich gehe davon aus, dass wir in den kommenden Jahren zahlreiche Partnerklinikgänge sehen werden.
Ich hoffe, das Unternehmen wird nicht übernommen, weil ich glaube, dass hier ein wirklich enormes Potential besteht, das sich über die Jahre entwickeln können sollte!
Ein Erfolg mit SGN-75 ist bei meiner Entscheidung unberücksichtigt, da hier noch keine relevanten Daten verfügbar sind. Ich halte SGEN auch so für einen guten Kauf. Dennoch ist ein Erfolg mit SGN-75 auch nicht ausgeschlossen. Sollte er kommen, dann wäre das so etwas wie ein Jackpot. Insofern darf man auf die nächste ASCO gespannt sein. Wir haben da eine heiße Karte im Spiel. Wenn sie sticht, dann wow!
Hier nun der Text von Hammer:
(Hervorhebungen von mir)
The Winner of ASH 2010 - Seattle Genetics
As expected, earlier this month at the annual American Society of Hematology (ASH) meeting, Seattle Genetics (SGEN) reported positive results that will likely lead to the company’s first ever regulatory approval for Brentuximab vedotin (SGN-35). The data will transform Seattle Genetics into a commercial stage company, with an initial market opportunity of ~$250M in the US alone. In addition, the results further validate the company’s ADC (antibody drug conjugate) technology, which has broad utility and huge commercial potential. In particular, Seattle Genetics could become a market leader in hematology by next year’s meeting, with results for two additional ADCs.
SGN-35 demonstrated overwhelming activity and a good safety profile in two relatively small indications: Hodgkin Lymphoma (HL) and ALCL (anaplastic large cell lymphoma). Both trials demonstrated stellar response rates, 75% for HL and 86% in ALCL. The duration of response in the HL study was 6.7 months, somewhat disappointing given SGN-35’s potency, however, it is above the unofficial approval bar of 6 months. Duration of response in ALCL was still not reached.
Duration of response is also important for product sales, as it affects treatment duration and the overall amount of drug patients receive. Still, the small patient population, the lack of other approved therapeutic options and the strong efficacy will probably enable Seattle Genetics and its partner Takeda to price SGN-35 higher than most marketed antibodies for cancer.
As the HL trial was conducted under SPA (special protocol assessment), chances for approval in this indication are extremely high. Although the ALCL trial is not under SPA, chances for approval are very high as well. In fact, SGN-35 looks even more effective in this indication than in HL. Assuming SGN-35 is approved, the drug is expected to be on the market by late 2011.
Evaluations regarding SGN-35’s market potential vary, ranging from $150M to $300M in the US, where Seattle Genetics has marketing rights for the drug. The company is entitled to double digit royalties on sales outside of the US, which could reach $300M (~$40 million in royalties). These values do not represent potential label expansion into earlier treatment lines within HL and ALCL.
In addition, SGN-35 might have utility in other indications based on data in the scientific literature, however, there is still no clinical experience with SGN-35 in these indications. In addition, unlike HL and ALCL, where the vast majority of patients express CD30 (SGN-35’s target), the incidence in other cancers such as certain types of sarcoma and NHL is unknown but probably substantially lower.
Novartis (NVS) also published results in refractory HL with its HDAC inhibitor, LBH589, but results pale in comparison to those of SGN-35, with a response rate of 27% and a response duration of 6.1 months. Importantly, Novartis’ drug rarely leads to complete responses (4%) whereas a third of patients treated with SGN-35 achieved a CR. Novartis plans to file for regulatory approval in the coming weeks but even if LBH589 receives approval and beat SGN-35 by several months, the superiority of the latter is unmistakable.
Growing presence in hematology
SGN-35 success more than compensates for the failures of two previous Seattle Genetics agents for blood cancer, SGN-40 for NHL and SGN-33 (lintuzumab) for AML. Next year, however, the company should have an update on SGN-75, which is being evaluated in NHL as well as in renal cancer. Preliminary data with suboptimal doses that was presented 2 months ago showed activity in NHL.
NHL is a group of diseases that represent a very large yet highly competitive indication. Still, effective and safe treatment options are needed for refractory patients as well as for augmenting approved regimens. Next year, the company should present updated results with SGN-75 that will shed more light on this agent’s prospects in NHL. A response rate of 30% in heavily pretreated patients will make SGN-75, wholly owned by Seattle Genetics, an important asset.
Seattle Genetics has another shot in NHL with an antibody-drug conjugate in development by Genentech. This ADC (DCDT2980S), which utilizes Seattle Genetics’ technology, targets CD22, a well recognized target for NHL as well as other blood cancers. CD22 is considered a validated target thanks to positive data for Pfizer’s (PFE) anti- CD22 ADC (CMC-544), which is now in phase III testing. This makes the probability of seeing activity with Genentech’s agent rather high. Although DCDT2980S will not be a first in class agent, it may very well be a best in class agent as Seattle Genetics’ technology seems to be superior to that of Pfizer in terms of efficacy and safety.
Genentech’s CD22 conjugate just entered the clinic so it could have preliminary data by next year’s ASH meeting. It is the third antibody drug conjugate Genentech is bringing to the clinic (the other two are T-DM1 using Immunogen’s (IMGN) technology and another ADC using Seattle Genetics’ technology that was discontinued). This further demonstrates Genentech/Roche’s enthusiasm with the ADC field. Many additional pharmaceutical companies are developing ADCs, which are considered the hottest topic in the antibody industry. These include Novartis and Sanofi-Aventis (SNY) (both using Immunogen’s technology), Astellas and GSK (GSK) (both using Seattle Genetics’ technology), and Pfizer (proprietary technology). Genentech is using technologies from both companies as well as proprietary technologies and probably has the largest preclinical pipeline of ADCs in the industry.
Interestingly, Genentech evaluated Immunogen’s technology for CD22 as well and eventually decided to go with Seattle Genetics’ technology. Based on Genentech’s publications, Immunogen’s conjugates appeared to also be very effective in animal models using the same technology used in T-DM1. It will therefore not be surprising if Immunogen or one of its partners bring a CD22 ADC to the clinic in the near future. Sanofi Aventis is developing SAR-3419, an anti CD19 ADC for NHL using Immunogen’s technology, which should enter phase II next year.
Biotech portfolio updates
We are selling Celgene (CELG), Myriad Genetics (MYGN) and one of three positions in Micromet (MITI). We are initiating a position in YM Biosciences (YMI), on which we will elaborate in the upcoming article on additional results from ASH.
http://www.hammerstockblog.com/the-winner-of-ash-2010-seattl…
Ich fühle mich dadurch in meiner Entscheidung bestärkt, kürzlich recht aggressiv nachzukaufen.
Das wesentliche Argument ist, dass mit Brentuximab Vedotin ein ADC mit sehr hoher Wahrscheinlichkeit auf dem Weg zum Markt ist, der zwar in Nischenindikationen entwickelt worden ist, der aber dennoch SGEN einen beachtlichen, regelmäßigen Einnahmestrom verschaffen dürfte, der es dem Unternehmen erlaubt, 1.) eine eigene Vertriebsstruktur aufzubauen und sich generell breiter aufzustellen und 2.) viel Geld in die eigene Forschung zu lenken, ohne auf den Kapitalmarkt angewiesen zu sein. Da SGEN über eine besondere Kompetenz verfügt, halte ich die Ausgaben für eine eigene Pipeline auch für gut angelegtes Geld. Die Sache ist ja so, dass SGEN auf sehr viel wissenschaftliche Erkenntnisse der letzten 20 Jahre im Bereich Antikörper und Targets zugreifen kann und mit seiner ADC-Technologie Dinge eher veredelt, als das Rad jedes Mal ganz neu zu erfinden.
Außerdem ist SGEN sehr stark in die ADC-Projekte großer Pharmas eingebunden. Das dürfte insbesondere für Roche/Genentech gelten, wo dutzende Programme mit SGEN-Technologie in der vorklinischen Entwicklung sein sollen. Ich gehe davon aus, dass wir in den kommenden Jahren zahlreiche Partnerklinikgänge sehen werden.
Ich hoffe, das Unternehmen wird nicht übernommen, weil ich glaube, dass hier ein wirklich enormes Potential besteht, das sich über die Jahre entwickeln können sollte!
Ein Erfolg mit SGN-75 ist bei meiner Entscheidung unberücksichtigt, da hier noch keine relevanten Daten verfügbar sind. Ich halte SGEN auch so für einen guten Kauf. Dennoch ist ein Erfolg mit SGN-75 auch nicht ausgeschlossen. Sollte er kommen, dann wäre das so etwas wie ein Jackpot. Insofern darf man auf die nächste ASCO gespannt sein. Wir haben da eine heiße Karte im Spiel. Wenn sie sticht, dann wow!
Hier nun der Text von Hammer:
(Hervorhebungen von mir)
The Winner of ASH 2010 - Seattle Genetics
As expected, earlier this month at the annual American Society of Hematology (ASH) meeting, Seattle Genetics (SGEN) reported positive results that will likely lead to the company’s first ever regulatory approval for Brentuximab vedotin (SGN-35). The data will transform Seattle Genetics into a commercial stage company, with an initial market opportunity of ~$250M in the US alone. In addition, the results further validate the company’s ADC (antibody drug conjugate) technology, which has broad utility and huge commercial potential. In particular, Seattle Genetics could become a market leader in hematology by next year’s meeting, with results for two additional ADCs.
SGN-35 demonstrated overwhelming activity and a good safety profile in two relatively small indications: Hodgkin Lymphoma (HL) and ALCL (anaplastic large cell lymphoma). Both trials demonstrated stellar response rates, 75% for HL and 86% in ALCL. The duration of response in the HL study was 6.7 months, somewhat disappointing given SGN-35’s potency, however, it is above the unofficial approval bar of 6 months. Duration of response in ALCL was still not reached.
Duration of response is also important for product sales, as it affects treatment duration and the overall amount of drug patients receive. Still, the small patient population, the lack of other approved therapeutic options and the strong efficacy will probably enable Seattle Genetics and its partner Takeda to price SGN-35 higher than most marketed antibodies for cancer.
As the HL trial was conducted under SPA (special protocol assessment), chances for approval in this indication are extremely high. Although the ALCL trial is not under SPA, chances for approval are very high as well. In fact, SGN-35 looks even more effective in this indication than in HL. Assuming SGN-35 is approved, the drug is expected to be on the market by late 2011.
Evaluations regarding SGN-35’s market potential vary, ranging from $150M to $300M in the US, where Seattle Genetics has marketing rights for the drug. The company is entitled to double digit royalties on sales outside of the US, which could reach $300M (~$40 million in royalties). These values do not represent potential label expansion into earlier treatment lines within HL and ALCL.
In addition, SGN-35 might have utility in other indications based on data in the scientific literature, however, there is still no clinical experience with SGN-35 in these indications. In addition, unlike HL and ALCL, where the vast majority of patients express CD30 (SGN-35’s target), the incidence in other cancers such as certain types of sarcoma and NHL is unknown but probably substantially lower.
Novartis (NVS) also published results in refractory HL with its HDAC inhibitor, LBH589, but results pale in comparison to those of SGN-35, with a response rate of 27% and a response duration of 6.1 months. Importantly, Novartis’ drug rarely leads to complete responses (4%) whereas a third of patients treated with SGN-35 achieved a CR. Novartis plans to file for regulatory approval in the coming weeks but even if LBH589 receives approval and beat SGN-35 by several months, the superiority of the latter is unmistakable.
Growing presence in hematology
SGN-35 success more than compensates for the failures of two previous Seattle Genetics agents for blood cancer, SGN-40 for NHL and SGN-33 (lintuzumab) for AML. Next year, however, the company should have an update on SGN-75, which is being evaluated in NHL as well as in renal cancer. Preliminary data with suboptimal doses that was presented 2 months ago showed activity in NHL.
NHL is a group of diseases that represent a very large yet highly competitive indication. Still, effective and safe treatment options are needed for refractory patients as well as for augmenting approved regimens. Next year, the company should present updated results with SGN-75 that will shed more light on this agent’s prospects in NHL. A response rate of 30% in heavily pretreated patients will make SGN-75, wholly owned by Seattle Genetics, an important asset.
Seattle Genetics has another shot in NHL with an antibody-drug conjugate in development by Genentech. This ADC (DCDT2980S), which utilizes Seattle Genetics’ technology, targets CD22, a well recognized target for NHL as well as other blood cancers. CD22 is considered a validated target thanks to positive data for Pfizer’s (PFE) anti- CD22 ADC (CMC-544), which is now in phase III testing. This makes the probability of seeing activity with Genentech’s agent rather high. Although DCDT2980S will not be a first in class agent, it may very well be a best in class agent as Seattle Genetics’ technology seems to be superior to that of Pfizer in terms of efficacy and safety.
Genentech’s CD22 conjugate just entered the clinic so it could have preliminary data by next year’s ASH meeting. It is the third antibody drug conjugate Genentech is bringing to the clinic (the other two are T-DM1 using Immunogen’s (IMGN) technology and another ADC using Seattle Genetics’ technology that was discontinued). This further demonstrates Genentech/Roche’s enthusiasm with the ADC field. Many additional pharmaceutical companies are developing ADCs, which are considered the hottest topic in the antibody industry. These include Novartis and Sanofi-Aventis (SNY) (both using Immunogen’s technology), Astellas and GSK (GSK) (both using Seattle Genetics’ technology), and Pfizer (proprietary technology). Genentech is using technologies from both companies as well as proprietary technologies and probably has the largest preclinical pipeline of ADCs in the industry.
Interestingly, Genentech evaluated Immunogen’s technology for CD22 as well and eventually decided to go with Seattle Genetics’ technology. Based on Genentech’s publications, Immunogen’s conjugates appeared to also be very effective in animal models using the same technology used in T-DM1. It will therefore not be surprising if Immunogen or one of its partners bring a CD22 ADC to the clinic in the near future. Sanofi Aventis is developing SAR-3419, an anti CD19 ADC for NHL using Immunogen’s technology, which should enter phase II next year.
Biotech portfolio updates
We are selling Celgene (CELG), Myriad Genetics (MYGN) and one of three positions in Micromet (MITI). We are initiating a position in YM Biosciences (YMI), on which we will elaborate in the upcoming article on additional results from ASH.
@SLGramann
Was ist aus Deiner Sicht eine faire Bewertung für Seattle Genetics (nach Möglichkeit mit kurzer Begründung)?
Was ist aus Deiner Sicht eine faire Bewertung für Seattle Genetics (nach Möglichkeit mit kurzer Begründung)?
Antwort auf Beitrag Nr.: 40.741.965 von Joschka Schröder am 21.12.10 06:44:33Hi JS,
die Frage liegt bei einer Marktkapitalisierung von ca. 1,5 Milliarden Dollar natürlich auf der Hand.
Ich stimme mit Dir überein, dass komplizierte Modelle zur Unternehmensbewertung fragwürdig und oft sogar gefährlich sind. Ich bevorzuge also immer einen möglichst vereinfachten Ansatz, den ich gern mit so etwas wie einer Grundüberzeugung kombiniere.
Zu SGEN:
Bei meiner Investition setze ich voraus, dass Brentuximab Vedotin zugelassen werden wird und dass es für SGEN Spitzenumsätze von mindestens 300 Mio. Dollar (einschließlich Royalties) erwirtschaften wird.
Weiterhin gehe ich davon aus, dass für diese Umsätze ein KUV von 4 angemessen ist.
Damit sind aus meiner Sicht bereits 1,2 Milliarden Dollar Marktkapitalisierung abgesichert.
SGEN verfügt über ca. 300 Mio. Dollar Cash und wird meiner Erwartung nach spätestens ab 2012 Cash generieren, statt Cash zu verbrennen.
Insofern ist für mich die derzeitige Marktkapitalisierung durch greifbare Assets abgesichert.
Dass das methodisch schon deshalb angreifbar ist, weil ich keinen Risikoabschlag für den Fall einer Nichtzulassung vornehme und den Spitzenumsatz von Brentuximab schon jetzt veranschlage, obwohl er noch Jahre in der Zukunft liegt, ist mir bewusst. Aber wie gesagt: Ich will keine BWL-Bewertung, sondern ein Feeling, ob die Idee an sich stimmt. Eine Nichtzulassung ist für mich keine Option. Kommt es doch negativ, werde ich das natürlich bitter bezahlen.
Alles, was ich bis jetzt eigentlich sagen wollte, ist, dass auf mittlere Sicht gesehen meiner Meinung nach die derzeitige Marktkapitalisierung durch Realwerte abgesichert ist.
Das allein rechtfertigt natürlich noch keine Investition.
Aber mit SGEN bekommt man einen der zentralen Akteure im ADC-Bereich, welcher möglicherweise das heißeste Thema im Bereich Antikörper/Biotech überhaupt ist. Man bekommt eine validierte Technologie, gute Partnerschaften, ein kompetentes Entwicklerteam, ein fähiges Management.
Und wenn man meinen Ansichten weiter oben folgen möchte, dass die derzeitige Bewertung auf einer recht sicheren Basis ruht, wird man der Meinung sein, dass man für all das nicht sehr viel zahlen muss.
Die Hoffnung auf künftige Rendite rührt aus der Erwartung her, dass sich aus den genannten Zutaten Großes entwickeln wird. Man muss „nur“ Geduld haben und die Dinge geschehen lassen.
Soweit meine Gedanken dazu. Zugegeben: Wenig Zahlen, viel „Generallinie“. Das ist nicht jedermanns Sache und geht immer dann schief, wenn man mit seiner Generallinie neben der Spur liegt. Ich hoffe, in Bezug auf ADC und SGEN geht es mir nicht so.
Viele Grüße
die Frage liegt bei einer Marktkapitalisierung von ca. 1,5 Milliarden Dollar natürlich auf der Hand.
Ich stimme mit Dir überein, dass komplizierte Modelle zur Unternehmensbewertung fragwürdig und oft sogar gefährlich sind. Ich bevorzuge also immer einen möglichst vereinfachten Ansatz, den ich gern mit so etwas wie einer Grundüberzeugung kombiniere.
Zu SGEN:
Bei meiner Investition setze ich voraus, dass Brentuximab Vedotin zugelassen werden wird und dass es für SGEN Spitzenumsätze von mindestens 300 Mio. Dollar (einschließlich Royalties) erwirtschaften wird.
Weiterhin gehe ich davon aus, dass für diese Umsätze ein KUV von 4 angemessen ist.
Damit sind aus meiner Sicht bereits 1,2 Milliarden Dollar Marktkapitalisierung abgesichert.
SGEN verfügt über ca. 300 Mio. Dollar Cash und wird meiner Erwartung nach spätestens ab 2012 Cash generieren, statt Cash zu verbrennen.
Insofern ist für mich die derzeitige Marktkapitalisierung durch greifbare Assets abgesichert.
Dass das methodisch schon deshalb angreifbar ist, weil ich keinen Risikoabschlag für den Fall einer Nichtzulassung vornehme und den Spitzenumsatz von Brentuximab schon jetzt veranschlage, obwohl er noch Jahre in der Zukunft liegt, ist mir bewusst. Aber wie gesagt: Ich will keine BWL-Bewertung, sondern ein Feeling, ob die Idee an sich stimmt. Eine Nichtzulassung ist für mich keine Option. Kommt es doch negativ, werde ich das natürlich bitter bezahlen.
Alles, was ich bis jetzt eigentlich sagen wollte, ist, dass auf mittlere Sicht gesehen meiner Meinung nach die derzeitige Marktkapitalisierung durch Realwerte abgesichert ist.
Das allein rechtfertigt natürlich noch keine Investition.
Aber mit SGEN bekommt man einen der zentralen Akteure im ADC-Bereich, welcher möglicherweise das heißeste Thema im Bereich Antikörper/Biotech überhaupt ist. Man bekommt eine validierte Technologie, gute Partnerschaften, ein kompetentes Entwicklerteam, ein fähiges Management.
Und wenn man meinen Ansichten weiter oben folgen möchte, dass die derzeitige Bewertung auf einer recht sicheren Basis ruht, wird man der Meinung sein, dass man für all das nicht sehr viel zahlen muss.
Die Hoffnung auf künftige Rendite rührt aus der Erwartung her, dass sich aus den genannten Zutaten Großes entwickeln wird. Man muss „nur“ Geduld haben und die Dinge geschehen lassen.
Soweit meine Gedanken dazu. Zugegeben: Wenig Zahlen, viel „Generallinie“. Das ist nicht jedermanns Sache und geht immer dann schief, wenn man mit seiner Generallinie neben der Spur liegt. Ich hoffe, in Bezug auf ADC und SGEN geht es mir nicht so.
Viele Grüße
Vom Grundsatz her finde ich Deinen Bewertungsansatz absolut vernünftig. Im Speziellen habe ich für Brentuximab aber noch keine Abschätzung durchgeführt (habe dies aber für die Feiertage geplant).
@SLGramann
Noch zu Deinen Überlegungen:
Ich rechne eigentlich lieber auf Basis von Mittelzuflüssen (s. mein IMGN-Beispiel). Nun kann man natürlich Umsatzbewertungen in Cashflow- oder Ergebnisbewertungen überführen. Da SGEN das Immunkonjugat Brentuximab - falls denn die Zulassung klappt - in den USA und Kanada vermutlich selbst vermarkten wird und im Rest der Welt Tantiemen von Millenium erhalten wird, ist das mit einer gemischten Umsatzbewertung allerdings so eine Sache. Deshalb, damit ich Deine Bewertung besser nachvollziehen kann, meine Frage: Mit welchem Umsatz und welcher Vorsteuermarge rechnest Du in den von SGEN selbst bedienten nordamerikanischen Märkten ... und welche Tantiemen (Umsatz x prozentuale Gewinnbeteiligung) erwartest Du für den Rest der Welt?
Mit anderen Worten: Um IMGN mit SGEN besser vergleichen zu können, wäre es wichtig zu wissen, welche Erwartungen Du hinsichtlich des von SGEN durch Bentuximab generierten Vorsteuerergebnisses hast (analog zu den ca. 100 Mio. USD p.a., die man bei IMGN für T-DM1 ansetzen kann).
Noch zu Deinen Überlegungen:
Ich rechne eigentlich lieber auf Basis von Mittelzuflüssen (s. mein IMGN-Beispiel). Nun kann man natürlich Umsatzbewertungen in Cashflow- oder Ergebnisbewertungen überführen. Da SGEN das Immunkonjugat Brentuximab - falls denn die Zulassung klappt - in den USA und Kanada vermutlich selbst vermarkten wird und im Rest der Welt Tantiemen von Millenium erhalten wird, ist das mit einer gemischten Umsatzbewertung allerdings so eine Sache. Deshalb, damit ich Deine Bewertung besser nachvollziehen kann, meine Frage: Mit welchem Umsatz und welcher Vorsteuermarge rechnest Du in den von SGEN selbst bedienten nordamerikanischen Märkten ... und welche Tantiemen (Umsatz x prozentuale Gewinnbeteiligung) erwartest Du für den Rest der Welt?
Mit anderen Worten: Um IMGN mit SGEN besser vergleichen zu können, wäre es wichtig zu wissen, welche Erwartungen Du hinsichtlich des von SGEN durch Bentuximab generierten Vorsteuerergebnisses hast (analog zu den ca. 100 Mio. USD p.a., die man bei IMGN für T-DM1 ansetzen kann).
Noch eine ganz allgemeine Beobachtung: Recht interessant finde ich, dass gegen Ende einer P2-Phase bei guter Datenlage die Investorengemeinde gewöhnlich davon ausgeht, das entsprechende Medikament werde zugelassen. D.h. ab der späten P2-Phase verschiebt sich oft das Chance-Risiko-Verhältnis in Richtung Risiko, weil der Erfolg "eingepreist" wird, ein etwaiger Misserfolg (und den kann es auch in einer späteren Studienphase noch geben, etwa dann, wenn es um das progressionsfreie Überleben oder das Nebenwirkungsprofil geht) jedoch nicht.
Zitat von SLGramann: Bei meiner Investition setze ich voraus, dass Brentuximab Vedotin zugelassen werden wird und dass es für SGEN Spitzenumsätze von mindestens 300 Mio. Dollar (einschließlich Royalties) erwirtschaften wird.
Weiterhin gehe ich davon aus, dass für diese Umsätze ein KUV von 4 angemessen ist.
Damit sind aus meiner Sicht bereits 1,2 Milliarden Dollar Marktkapitalisierung abgesichert.
Hallo SLG,
kurz eine Überlegung zu Brentuzimab: Die von Dir avisierten Umsätzen könnte es nach dem aktuellen Stand der Dinge nur dann geben, wenn das Immunkonjugat auch in der Erstlinien-Therapie zugelassen würde ... und das erscheint zum jetzigen Zeitpunkt eher ungewiss, da sich einige Nebenwirkungen des Brentuzimab mit denen der üblichen ABVD-Therapie überschneiden und wechselseitig verstärken werden. Insoweit würde ich vermutlich ein konservativeres Szenario bevorzugen.
Offensichtlich ist dieser Punkt auch unter Analysten umstritten: Jefferies z.B. geht schon jetzt recht mutig von einem Positiv-Szenario aus (Zulassung 2011, nachfolgend auch für Erstlinien-Behandlung) und rufen ein Kursziel von 20 USD auf. Brean Murray Carret hingegen ist hinsichtlich der Erstlinien-Therapie deutlich vorsichtiger (bezüglich der Zweitlinien-Therapie ist BMC ebenfalls sehr optimistisch) und kommt so auf ein Kursziel in Höhe von 11 USD. Anhand dieser Gegenüberstellung wird die spekulative Komponente eines Investments in SGEN aus meiner Sicht recht deutlich. Um so genauer sollte man die weiteren klinischen Studienergebnisse verfolgen.
Viele Grüße
JS
Antwort auf Beitrag Nr.: 40.772.908 von Joschka Schröder am 29.12.10 13:13:43Bei den Nebenwirkungen ist u.a. hinsichtlich der peripheren Neuropathie und der Neutropenie eine additive (oder potenzierende) Wirkung des Brentuximab und des ABVD-Schemas zu erwarten, so dass die Möglichkeit einer gleichzeitigen Verabreichung zweifelhaft erscheint. Eine entsprechende Toxizitätsstudie befindet sich, wenn ich mich recht erinnere, in Vorbereitung.
Antwort auf Beitrag Nr.: 40.753.601 von Joschka Schröder am 22.12.10 22:35:12Mit anderen Worten: Um IMGN mit SGEN besser vergleichen zu können, wäre es wichtig zu wissen, welche Erwartungen Du hinsichtlich des von SGEN durch Bentuximab generierten Vorsteuerergebnisses hast
Bei dieser Frage will ich mich nicht gern weit aus dem Fenster lehnen...
Also, das folgende ist eher Szenario, als Prognose:
Wenn ich 300 Mio. Dollar Spitzenumsatz unterstelle, dann sind da sicher 30 Mio. Royalties mit drin, die faktisch voll in die Vorsteuermarge eingehen dürften. Bei den verbleibenden 270 Mio. würde ich 50% für Herstellung, Vertrieb und damit unmittelbar zusammenhängende Verwaltung abziehen wollen, so dass theoretisch 135 Mio. Vorsteuergewinn verbleiben würden. Zusammen mit den Royalties könnte der Vorsteuergewinn dann bis zu 150 Mio. Dollar erreichen.
Natürlich wird SGEN einen solchen Vorsteuergewinn nicht ausweisen können, da eine größer werdende Forschung (auch für andere Projekte) und die allgemeine Verwaltung des Unternehmens zu finanzieren ist. Unterm Strich muss das dann alles nicht viel mehr als break-even bedeuten, aber es ging hier ja um das Potential von B Vedotin, wenn man das mal separiert.
Letztlich wird es natürlich darauf ankommen, welches Umsatzpotential man mit B Vedotin wirklich heben kann. Aufgrund der bisher außerordentlichen Wirksamkeitsdaten erlaube ich mir hier eine Portion Optimismus.
Bei dieser Frage will ich mich nicht gern weit aus dem Fenster lehnen...
Also, das folgende ist eher Szenario, als Prognose:
Wenn ich 300 Mio. Dollar Spitzenumsatz unterstelle, dann sind da sicher 30 Mio. Royalties mit drin, die faktisch voll in die Vorsteuermarge eingehen dürften. Bei den verbleibenden 270 Mio. würde ich 50% für Herstellung, Vertrieb und damit unmittelbar zusammenhängende Verwaltung abziehen wollen, so dass theoretisch 135 Mio. Vorsteuergewinn verbleiben würden. Zusammen mit den Royalties könnte der Vorsteuergewinn dann bis zu 150 Mio. Dollar erreichen.
Natürlich wird SGEN einen solchen Vorsteuergewinn nicht ausweisen können, da eine größer werdende Forschung (auch für andere Projekte) und die allgemeine Verwaltung des Unternehmens zu finanzieren ist. Unterm Strich muss das dann alles nicht viel mehr als break-even bedeuten, aber es ging hier ja um das Potential von B Vedotin, wenn man das mal separiert.
Letztlich wird es natürlich darauf ankommen, welches Umsatzpotential man mit B Vedotin wirklich heben kann. Aufgrund der bisher außerordentlichen Wirksamkeitsdaten erlaube ich mir hier eine Portion Optimismus.
Ein neuer Partnerdeal. Diesmal mit Pfizer.
Der Deal ist klein, insofern er sich nur auf ein Target bezieht. Die Konditionen zeigen aber mal wieder, an welch langem Hebel SGEN und IMGN sitzen!
Bis zu 200 Mio. an Meilensteinen in so einem frühen Stadium aushandeln, das kann nicht jeder.
Außerdem ist es sehr beachtenswert, dass Pfizer wohl über eine eigene ADC-Technologie verfügt. Offenkundig ist die aber nicht sonderlich leistungsstark. Das wieder zeigt für mich aber nicht etwa die Inkompetenz von Pfizer, sondern, dass SGEN und IMGN so schnell nicht kopierbar sind - auch nicht von Unternehmen mit sehr großen Ressourcen. Meine Vermutung ist, dass auch einfach Glück dabei war, dass die ADC-Technologien von SGEN und IMGN gut funktionieren.
Posted January 6, 2011
Seattle Genetics Announces Antibody-Drug Conjugate Collaboration with Pfizer
- Seattle Genetics to receive $8 million upfront payment, over $200 million in potential milestone payments plus royalties under single-target collaboration -
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq:SGEN) announced today that it has entered into a collaboration agreement with Pfizer Inc. (NYSE:PFE) under which Pfizer will pay an upfront fee of $8 million for rights to utilize Seattle Genetics' antibody-drug conjugate (ADC) technology with antibodies to a single oncology target.
"This collaboration reflects the increasing value of our ADC technology and strong interest in its potential among leaders in the drug development community," said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. "We now have ten ongoing ADC collaborations, six collaborator ADCs using our technology are in clinical development, and several additional programs are advancing towards the clinic. We have generated more than $145 million from ADC licensing, and we have the potential to receive significant future milestones and royalties for ADCs developed by our collaborators."
Pfizer is responsible for research, product development, manufacturing and commercialization of any ADC products under the collaboration. Seattle Genetics is eligible to receive from Pfizer over $200 million in progress-dependent milestones as well as royalties on worldwide net sales of any resulting ADC products. Seattle Genetics also will receive material supply and annual maintenance fees as well as research support payments for assistance provided to Pfizer under the collaboration.
ADCs are monoclonal antibodies that selectively deliver potent anti-cancer agents to tumor cells. With over a decade of experience and knowledge in ADC innovation, Seattle Genetics has developed proprietary technology employing synthetic, highly potent cell-killing agents called auristatins (such as MMAE and MMAF) and stable linker systems that attach auristatin to the antibody. Seattle Genetics' novel linker systems are designed to be stable in the bloodstream and release the potent cell-killing agent once inside targeted cancer cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy while enhancing the antitumor activity.
Der Deal ist klein, insofern er sich nur auf ein Target bezieht. Die Konditionen zeigen aber mal wieder, an welch langem Hebel SGEN und IMGN sitzen!
Bis zu 200 Mio. an Meilensteinen in so einem frühen Stadium aushandeln, das kann nicht jeder.
Außerdem ist es sehr beachtenswert, dass Pfizer wohl über eine eigene ADC-Technologie verfügt. Offenkundig ist die aber nicht sonderlich leistungsstark. Das wieder zeigt für mich aber nicht etwa die Inkompetenz von Pfizer, sondern, dass SGEN und IMGN so schnell nicht kopierbar sind - auch nicht von Unternehmen mit sehr großen Ressourcen. Meine Vermutung ist, dass auch einfach Glück dabei war, dass die ADC-Technologien von SGEN und IMGN gut funktionieren.
Posted January 6, 2011
Seattle Genetics Announces Antibody-Drug Conjugate Collaboration with Pfizer
- Seattle Genetics to receive $8 million upfront payment, over $200 million in potential milestone payments plus royalties under single-target collaboration -
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq:SGEN) announced today that it has entered into a collaboration agreement with Pfizer Inc. (NYSE:PFE) under which Pfizer will pay an upfront fee of $8 million for rights to utilize Seattle Genetics' antibody-drug conjugate (ADC) technology with antibodies to a single oncology target.
"This collaboration reflects the increasing value of our ADC technology and strong interest in its potential among leaders in the drug development community," said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. "We now have ten ongoing ADC collaborations, six collaborator ADCs using our technology are in clinical development, and several additional programs are advancing towards the clinic. We have generated more than $145 million from ADC licensing, and we have the potential to receive significant future milestones and royalties for ADCs developed by our collaborators."
Pfizer is responsible for research, product development, manufacturing and commercialization of any ADC products under the collaboration. Seattle Genetics is eligible to receive from Pfizer over $200 million in progress-dependent milestones as well as royalties on worldwide net sales of any resulting ADC products. Seattle Genetics also will receive material supply and annual maintenance fees as well as research support payments for assistance provided to Pfizer under the collaboration.
ADCs are monoclonal antibodies that selectively deliver potent anti-cancer agents to tumor cells. With over a decade of experience and knowledge in ADC innovation, Seattle Genetics has developed proprietary technology employing synthetic, highly potent cell-killing agents called auristatins (such as MMAE and MMAF) and stable linker systems that attach auristatin to the antibody. Seattle Genetics' novel linker systems are designed to be stable in the bloodstream and release the potent cell-killing agent once inside targeted cancer cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy while enhancing the antitumor activity.
Schönes Interview:
(gerade bei der JPMorgan Health Care Conference)
http://finance.yahoo.com/video/cnbc-22844419/jpm-health-care…
(gerade bei der JPMorgan Health Care Conference)
http://finance.yahoo.com/video/cnbc-22844419/jpm-health-care…
SGEN mit einer beachtlichen Kapitalerhöhung in Vorbereitung des Launchs für Brentuximab Vedotin. Das Unternehmen geht konsequent voran. Ich begrüße diese Maßnahme, auch wenn die Verwässerung schmerzt. Vielleicht ist es die letzte gewesen.
Seattle Genetics Reloads Cash Reserves With $155M, On Cusp of Selling First Drug
Luke Timmerman 2/2/11
Seattle Genetics has raised a new load of cash to brace itself for a big year ahead, as it prepares to hire a lot of people and introduce its first drug on the U.S. market.
The Bothell, WA-based company (NASDAQ: SGEN) said today it has raised another $155 million through a stock sale. The company sold 10 million new shares at $15.50 apiece, and has granted its underwriters options to buy another 1.5 million shares over the next 30 days. Jefferies & Co., JP Morgan Securities, Leerink Swann, RBC Capital Markets, Needham & Co., William Blair & Co., Oppenheimer & Co., and ThinkEquity all helped manage the offering.
Seattle Genetics has been on a roll the past year seeing its stock rise more than 60 percent, and, not surprisingly, was able to command good terms. The company’s stock closed yesterday at $16.17, before it announced that it planned to sell more shares, essentially diluting the value of existing ones. Even so, Seattle Genetics was able to sell shares at just a 4 percent discount to yesterday’s closing price, which could mean many of the new investors will be motivated to hold on and wait for the shares to climb so they can see a bigger return.
The company hasn’t reported how much cash it had in the bank heading into 2011, but did say it had $315.6 million in cash as of September 30, it most recent date of record. Much of the money will go toward the commercial push for brentuximab vedotin (SGN-35). That’s the new “empowered antibody” that Seattle Genetics and its partner, Cambridge, MA-based Millennium: The Takeda Oncology Company, are developing for Hodgkin’s disease, anaplastic large cell lymphoma, and other related lymphomas with a common protein target called CD30. The drug showed unprecedented tumor shrinkage rates for patients in two pivotal trials last year, and now Seattle Genetics plans to seek FDA approval to start marketing the drug in the U.S.
Getting its first drug on the U.S. market after 13 years in business requires Seattle Genetics to hire a lot more people, with new skills in things like marketing and manufacturing, as CEO Clay Siegall explained in this feature last May. Siegall, in an interview in December, said the company expects to build a commercial team with 110 people by the end of 2011, which will be part of an overall company staff of about 475 to 500 people.
Luke Timmerman is the National Biotech Editor of Xconomy, and the Editor of Xconomy Seattle. You can e-mail him at ltimmerman@xconomy.com, or follow him at twitter.com/ldtimmerman.
Seattle Genetics Reloads Cash Reserves With $155M, On Cusp of Selling First Drug
Luke Timmerman 2/2/11
Seattle Genetics has raised a new load of cash to brace itself for a big year ahead, as it prepares to hire a lot of people and introduce its first drug on the U.S. market.
The Bothell, WA-based company (NASDAQ: SGEN) said today it has raised another $155 million through a stock sale. The company sold 10 million new shares at $15.50 apiece, and has granted its underwriters options to buy another 1.5 million shares over the next 30 days. Jefferies & Co., JP Morgan Securities, Leerink Swann, RBC Capital Markets, Needham & Co., William Blair & Co., Oppenheimer & Co., and ThinkEquity all helped manage the offering.
Seattle Genetics has been on a roll the past year seeing its stock rise more than 60 percent, and, not surprisingly, was able to command good terms. The company’s stock closed yesterday at $16.17, before it announced that it planned to sell more shares, essentially diluting the value of existing ones. Even so, Seattle Genetics was able to sell shares at just a 4 percent discount to yesterday’s closing price, which could mean many of the new investors will be motivated to hold on and wait for the shares to climb so they can see a bigger return.
The company hasn’t reported how much cash it had in the bank heading into 2011, but did say it had $315.6 million in cash as of September 30, it most recent date of record. Much of the money will go toward the commercial push for brentuximab vedotin (SGN-35). That’s the new “empowered antibody” that Seattle Genetics and its partner, Cambridge, MA-based Millennium: The Takeda Oncology Company, are developing for Hodgkin’s disease, anaplastic large cell lymphoma, and other related lymphomas with a common protein target called CD30. The drug showed unprecedented tumor shrinkage rates for patients in two pivotal trials last year, and now Seattle Genetics plans to seek FDA approval to start marketing the drug in the U.S.
Getting its first drug on the U.S. market after 13 years in business requires Seattle Genetics to hire a lot more people, with new skills in things like marketing and manufacturing, as CEO Clay Siegall explained in this feature last May. Siegall, in an interview in December, said the company expects to build a commercial team with 110 people by the end of 2011, which will be part of an overall company staff of about 475 to 500 people.
Luke Timmerman is the National Biotech Editor of Xconomy, and the Editor of Xconomy Seattle. You can e-mail him at ltimmerman@xconomy.com, or follow him at twitter.com/ldtimmerman.
Ohad Hammer hat einen umfassenden vergleichenden Artikel zu SGEN und IMGN geschrieben, den ich hier vollständig einstelle (Fettungen von mir):
http://www.hammerstockblog.com/seattle-genetics-strengthens-…
Seattle Genetics Strengthens Its Foothold Within Genentech (At The Expense Of Immunogen?)
In its earnings release last week, Seattle Genetics (SGEN) did not surprise anyone with the financial guidance and expected timelines for approval of its lead agent, SGN-35. However, on the business development front, the release did include an intriguing announcement that did not receive the attention it deserved. The company announced that Genentech recently advanced 3 new antibody drug conjugates (ADC) based on Seattle Genetics’ technology to phase I, this is in addition to the CD22 ADC already in clinical testing.
The announcement has several important implications for Seattle Genetics. First, the number of clinical programs in its partnered pipeline instantly jumped 50% from 6 to 9. By definition, this provides Seattle Genetics with more shots on goal and increases chances of substantial milestones and royalties down the road. More importantly, it establishes Seattle Genetics’ technology as Genentech’s preferred ADC platform, an attractive position given Genentech’s dominance in oncology and ADCs in particular.Genentech’s decision is a serious blow to Immunogen, but it does not mean that Genentech will not use its technology at all. Genentech still has licenses for 4 targets from Immunogen based on deals from 2005 and 2008. The fact that until now no clinical program has resulted from these deals is a bad indication but it does not completely rule out this option.
Genentech’s growing ADC pipeline
When Genentech started developing antibody drug conjugates it worked with technologies from both Immunogen (IMGN) and Seattle Genetics. The first ADC it advanced to clinical testing was T-DM1, based on Immunogen’s technology. From the initial phase I, T-DM1 has demonstrated remarkable activity and a surprisingly benign safety profile. It is probably the success of this agent which ignited the excitement around ADCs and led to an industry-wide shift towards this field.
In parallel to T-DM1, Seattle Genetics was developing its own ADC, SGN-35 for Hodgkin’s Lymphoma, where the agent demonstrated strong efficacy with an excellent safety profile. Based on the clinical validation of both technologies and the fact Genentech had access to them, it seemed plausible that it would use both platforms in future projects. If anything, one could expect that T-DM1’s remarkable performance and the aggressive development plan Genentech is pursuing for this agent would make it lean towards Immunogen rather than Seattle Genetics.
But this was not the case. In June 2008, Genentech advanced a second ADC to phase I, which was based on Seattle Genetics’ technology, but the program was discontinued shortly afterwards, probably due to safety issues. This was followed by two follow-on licensing deals with Seattle Genetics for additional targets during 2010. The deals included $21.5M in upfront payments and probably over $1B in potential milestone payments.
Since then, Genentech advanced 4 additional ADCs to phase I, all of which employ Seattle Genetics’ technology. This brings the number of Seattle Genetics-based programs to 5 versus only one using Immunogen’s technology (T-DM1). Genentech disclosed the identity of only one of the 5 candidates, a CD22 ADC for the treatment of blood cancers (discussed here). The rest of the programs (the discontinued plus three new programs) are still undisclosed. The discontinued program was probably a MUC16 ADC whereas two of the active programs could be ADCs targeting CD79b (lymphoma) and TENB2 (prostate cancer).
Not all deals are created equal
In the past 2 years, Seattle Genetics managed to bring more deals, generating more cash and opportunities compared to Immunogen. It is unclear whether this reflects a real preference in the industry or simply Immunogen’s decision to be more selective in the deal it signs. During 2009-2010, Seattle reported rich deals with GSK (GSK), Astellas and Daiichi Sankyo in contrast to Immunogen, who signed a meaningful deal only recently with Novartis (NVS). The recent Novartis deal (discussed here) was an important validation after a 2 year drought and the price paid by Novartis certainly showed that Immunogen still has a lot to offer.
Not all deals are created equal, as some represent higher chances of actually generating clinical stage programs. In general, recent deals that involve a higher price tag indicate that a partner is more committed to a given program and is actively pursuing it. Immunogen’s partnerships with Novartis and Amgen (AMGN) are good examples.
Novartis agreed to very generous terms as part of last year’s deal, so one can assume that it would not do the deal unless it were highly excited about these programs. In contrast, Amgen secured Immunogen’s technology for two targets as part of an old collaboration it inherited from Abgenix. Because the agreement was signed when Immunogen’s technology was still unvalidated, it includes very modest financial terms ($1M upfront per program). Amgen’s 2009 licensing of two targets probably reflected the upcoming expiration of the Abgenix-Immunogen collaboration. This enabled Amgen to secure access to Immunogen’s technology for a fraction of the real market price, so it is still unclear what the driving force behind this deal was.
Fundamental questions regarding target selection
Although it seems that when given a choice, Genentech prefers Seattle Genetics’ technology, there are cases in which Immunogen’s technology is superior to that of Seattle Genetics. In particular, targets that do not get internalized efficiently seem more suitable for Immunogen’s cleavable linkers like those used in all of its programs except T-DM1. Genentech appears to avoid these targets, as ADCs based on cleavable linkers are thought to have a less favorable therapeutic window (efficacy/safety ratio), even though Immunogen and its partners were able to reach clinically active doses in most clinical trials.
Indeed, to date, the only two ADCs which demonstrated sufficient potency as single agents are T-DM1 and SGN-35, both utilize non-cleavable linkers for targets that are good internalizers. The rest of ADCs in Immunogen’s pipeline have not performed as nicely as T-DM1, although it is hard to attribute a given clinical profile to a single element. IMGN901, for example, had activity in multiple indications including lung cancer and multiple myeloma, but it was not potent enough as mono-therapy. This forced Immunogen to evaluate IMGN901 in combination with standard of care in three indications.
Developing a drug in combination is by definition more challenging. It requires comparative trials that are longer and more expensive, and it also has safety implications that may prevent optimal dosing. Most importantly, proof of concept is reached only after a large randomized phase II trial, a formidable challenge on its own (discussed here). This brings up the debate on whether targets that require cleavable linkers should be pursued at all.
There may still be cases where ADCs based on cleavable linkers show sufficient potency as single agents, especially in blood cancers that are considered less challenging than solid tumors. This year at ASCO, Sanofi is expected to present data for SAR3419, a CD19 ADC, given weekly as mono-therapy. Sanofi already announced this program will move into phase II as single agent, so one could expect data to be positive (although there are a lot of other promising agents in clinical development for NHL).
But what about cases where an ADC needs to be given in combination? This is still an open question as there is not enough clinical data out there regarding ADCs as part of combination regimens. One encouraging indication comes from Endocyte (ECYT), who is developing small-molecule drug conjugates (SMDC). Although SMDCs have a totally different clinical profile than ADCs, both rely on a similar approach of a targeting moiety used to deliver a toxic payload into cancer cells. Endocyte’s lead agent, EC145, was not effective enough to be given alone, so the company evaluated it in a randomized phase II in combination with chemotherapy. Results demonstrated a remarkable benefit in a subset of patients who expressed EC145’s target in all of their lesions, so this can be viewed as a conceptual proof of concept. Next year, Immunogen expects to start phase I with IMGN853 which is a direct competitor of Endocyte’s EC145.
Interestingly, Sanofi’s SAR3419 utilizes Immunogen’s cleavable linker, even though CD19 is highly internalized. In one of Genentech’s seminal publications on ADCs for blood cancers, researchers also found that CD19 ADCs using non-cleavable linkers were ineffective. In contrast, Seattle Genetics published a paper in 2008, showing good efficacy with its non-cleavable linker. This demonstrates that the activity profile of a given ADC is a result of an interplay between several factors and can sometimes be unpredictable.
Immunogen’s partnered pipeline in 2011
Immunogen expects three new programs in its partnered pipeline to start phase I this year, and there is still a hypothetical chance that one of these programs will be with Genentech. Another partner that could start phase I with an ADC based on Immunogen’s technology is Bayer who is developing an anti-mesothelin ADC. Bayer had a pre-existing collaboration with Seattle Genetics prior to signing the deal with Immunogen, but licensed Immunogen’s technology for this ADC. One potential explanation is the lower activity observed with non-cleavable linkers for mesothelin targeted ADCs. Another active partner is obviously Novartis, who has been very aggressive in bringing antibodies to the clinic through its broad collaboration with Morphosys (MOR.DE).
Sanofi-Aventis already has two ADCs based on Immunogen’s technology in clinical testing. Last month, Oxford Biotherapeutics out-licensed an antibody program to Sanofi-Aventis, who intends to pursue it as an ADC. As the target for this antibody is proprietary of Oxford Bio, Sanofi will probably have to negotiate a new licensing deal with either Seattle Genetics or Immunogen. Based on the broad collaboration between Sanofi and Immunogen, Immunogen stands a fair chance of getting this project. Based on recent ADC deals, this deal will be much more lucrative than the current deals Sanofi has with Immunogen, with an upfront fee of $5-8M and $200M in milestones.
The two big catalysts for Imuunogen’s partnered pipeline will be data presentation for SAR3419 and T-DM1 in the second and third quarters this year, respectively.
Sanofi will publish the long anticipated phase I data using a once weekly schedule. There are additional CD19 programs in development, including Micromet’s (MITI) MT-103 that continues to generate solid data in NHL. Competition is thinning out following the discontinuation of Medarex’s Fc engineered CD19 antibody, probably due to safety issues. This does not bode well for Morphosys and Medimmune who also have Fc engineered CD19 antibodies in phase I.
Roche will present PFS data from a randomized phase II evaluating T-DM1 vs. Herceptin+ chemo in 1st line HER2+ breast cancer patients. This trial generated a positive signal in terms of response rate in favor of T-DM1 but the PFS data will be crucial as a more reliable endpoint. Since Roche is already enrolling patients in a pivotal study using a similar design in a similar patient population, one can assume that T-DM1 was at least comparable to standard of care.
http://www.hammerstockblog.com/seattle-genetics-strengthens-…
Seattle Genetics Strengthens Its Foothold Within Genentech (At The Expense Of Immunogen?)
In its earnings release last week, Seattle Genetics (SGEN) did not surprise anyone with the financial guidance and expected timelines for approval of its lead agent, SGN-35. However, on the business development front, the release did include an intriguing announcement that did not receive the attention it deserved. The company announced that Genentech recently advanced 3 new antibody drug conjugates (ADC) based on Seattle Genetics’ technology to phase I, this is in addition to the CD22 ADC already in clinical testing.
The announcement has several important implications for Seattle Genetics. First, the number of clinical programs in its partnered pipeline instantly jumped 50% from 6 to 9. By definition, this provides Seattle Genetics with more shots on goal and increases chances of substantial milestones and royalties down the road. More importantly, it establishes Seattle Genetics’ technology as Genentech’s preferred ADC platform, an attractive position given Genentech’s dominance in oncology and ADCs in particular.Genentech’s decision is a serious blow to Immunogen, but it does not mean that Genentech will not use its technology at all. Genentech still has licenses for 4 targets from Immunogen based on deals from 2005 and 2008. The fact that until now no clinical program has resulted from these deals is a bad indication but it does not completely rule out this option.
Genentech’s growing ADC pipeline
When Genentech started developing antibody drug conjugates it worked with technologies from both Immunogen (IMGN) and Seattle Genetics. The first ADC it advanced to clinical testing was T-DM1, based on Immunogen’s technology. From the initial phase I, T-DM1 has demonstrated remarkable activity and a surprisingly benign safety profile. It is probably the success of this agent which ignited the excitement around ADCs and led to an industry-wide shift towards this field.
In parallel to T-DM1, Seattle Genetics was developing its own ADC, SGN-35 for Hodgkin’s Lymphoma, where the agent demonstrated strong efficacy with an excellent safety profile. Based on the clinical validation of both technologies and the fact Genentech had access to them, it seemed plausible that it would use both platforms in future projects. If anything, one could expect that T-DM1’s remarkable performance and the aggressive development plan Genentech is pursuing for this agent would make it lean towards Immunogen rather than Seattle Genetics.
But this was not the case. In June 2008, Genentech advanced a second ADC to phase I, which was based on Seattle Genetics’ technology, but the program was discontinued shortly afterwards, probably due to safety issues. This was followed by two follow-on licensing deals with Seattle Genetics for additional targets during 2010. The deals included $21.5M in upfront payments and probably over $1B in potential milestone payments.
Since then, Genentech advanced 4 additional ADCs to phase I, all of which employ Seattle Genetics’ technology. This brings the number of Seattle Genetics-based programs to 5 versus only one using Immunogen’s technology (T-DM1). Genentech disclosed the identity of only one of the 5 candidates, a CD22 ADC for the treatment of blood cancers (discussed here). The rest of the programs (the discontinued plus three new programs) are still undisclosed. The discontinued program was probably a MUC16 ADC whereas two of the active programs could be ADCs targeting CD79b (lymphoma) and TENB2 (prostate cancer).
Not all deals are created equal
In the past 2 years, Seattle Genetics managed to bring more deals, generating more cash and opportunities compared to Immunogen. It is unclear whether this reflects a real preference in the industry or simply Immunogen’s decision to be more selective in the deal it signs. During 2009-2010, Seattle reported rich deals with GSK (GSK), Astellas and Daiichi Sankyo in contrast to Immunogen, who signed a meaningful deal only recently with Novartis (NVS). The recent Novartis deal (discussed here) was an important validation after a 2 year drought and the price paid by Novartis certainly showed that Immunogen still has a lot to offer.
Not all deals are created equal, as some represent higher chances of actually generating clinical stage programs. In general, recent deals that involve a higher price tag indicate that a partner is more committed to a given program and is actively pursuing it. Immunogen’s partnerships with Novartis and Amgen (AMGN) are good examples.
Novartis agreed to very generous terms as part of last year’s deal, so one can assume that it would not do the deal unless it were highly excited about these programs. In contrast, Amgen secured Immunogen’s technology for two targets as part of an old collaboration it inherited from Abgenix. Because the agreement was signed when Immunogen’s technology was still unvalidated, it includes very modest financial terms ($1M upfront per program). Amgen’s 2009 licensing of two targets probably reflected the upcoming expiration of the Abgenix-Immunogen collaboration. This enabled Amgen to secure access to Immunogen’s technology for a fraction of the real market price, so it is still unclear what the driving force behind this deal was.
Fundamental questions regarding target selection
Although it seems that when given a choice, Genentech prefers Seattle Genetics’ technology, there are cases in which Immunogen’s technology is superior to that of Seattle Genetics. In particular, targets that do not get internalized efficiently seem more suitable for Immunogen’s cleavable linkers like those used in all of its programs except T-DM1. Genentech appears to avoid these targets, as ADCs based on cleavable linkers are thought to have a less favorable therapeutic window (efficacy/safety ratio), even though Immunogen and its partners were able to reach clinically active doses in most clinical trials.
Indeed, to date, the only two ADCs which demonstrated sufficient potency as single agents are T-DM1 and SGN-35, both utilize non-cleavable linkers for targets that are good internalizers. The rest of ADCs in Immunogen’s pipeline have not performed as nicely as T-DM1, although it is hard to attribute a given clinical profile to a single element. IMGN901, for example, had activity in multiple indications including lung cancer and multiple myeloma, but it was not potent enough as mono-therapy. This forced Immunogen to evaluate IMGN901 in combination with standard of care in three indications.
Developing a drug in combination is by definition more challenging. It requires comparative trials that are longer and more expensive, and it also has safety implications that may prevent optimal dosing. Most importantly, proof of concept is reached only after a large randomized phase II trial, a formidable challenge on its own (discussed here). This brings up the debate on whether targets that require cleavable linkers should be pursued at all.
There may still be cases where ADCs based on cleavable linkers show sufficient potency as single agents, especially in blood cancers that are considered less challenging than solid tumors. This year at ASCO, Sanofi is expected to present data for SAR3419, a CD19 ADC, given weekly as mono-therapy. Sanofi already announced this program will move into phase II as single agent, so one could expect data to be positive (although there are a lot of other promising agents in clinical development for NHL).
But what about cases where an ADC needs to be given in combination? This is still an open question as there is not enough clinical data out there regarding ADCs as part of combination regimens. One encouraging indication comes from Endocyte (ECYT), who is developing small-molecule drug conjugates (SMDC). Although SMDCs have a totally different clinical profile than ADCs, both rely on a similar approach of a targeting moiety used to deliver a toxic payload into cancer cells. Endocyte’s lead agent, EC145, was not effective enough to be given alone, so the company evaluated it in a randomized phase II in combination with chemotherapy. Results demonstrated a remarkable benefit in a subset of patients who expressed EC145’s target in all of their lesions, so this can be viewed as a conceptual proof of concept. Next year, Immunogen expects to start phase I with IMGN853 which is a direct competitor of Endocyte’s EC145.
Interestingly, Sanofi’s SAR3419 utilizes Immunogen’s cleavable linker, even though CD19 is highly internalized. In one of Genentech’s seminal publications on ADCs for blood cancers, researchers also found that CD19 ADCs using non-cleavable linkers were ineffective. In contrast, Seattle Genetics published a paper in 2008, showing good efficacy with its non-cleavable linker. This demonstrates that the activity profile of a given ADC is a result of an interplay between several factors and can sometimes be unpredictable.
Immunogen’s partnered pipeline in 2011
Immunogen expects three new programs in its partnered pipeline to start phase I this year, and there is still a hypothetical chance that one of these programs will be with Genentech. Another partner that could start phase I with an ADC based on Immunogen’s technology is Bayer who is developing an anti-mesothelin ADC. Bayer had a pre-existing collaboration with Seattle Genetics prior to signing the deal with Immunogen, but licensed Immunogen’s technology for this ADC. One potential explanation is the lower activity observed with non-cleavable linkers for mesothelin targeted ADCs. Another active partner is obviously Novartis, who has been very aggressive in bringing antibodies to the clinic through its broad collaboration with Morphosys (MOR.DE).
Sanofi-Aventis already has two ADCs based on Immunogen’s technology in clinical testing. Last month, Oxford Biotherapeutics out-licensed an antibody program to Sanofi-Aventis, who intends to pursue it as an ADC. As the target for this antibody is proprietary of Oxford Bio, Sanofi will probably have to negotiate a new licensing deal with either Seattle Genetics or Immunogen. Based on the broad collaboration between Sanofi and Immunogen, Immunogen stands a fair chance of getting this project. Based on recent ADC deals, this deal will be much more lucrative than the current deals Sanofi has with Immunogen, with an upfront fee of $5-8M and $200M in milestones.
The two big catalysts for Imuunogen’s partnered pipeline will be data presentation for SAR3419 and T-DM1 in the second and third quarters this year, respectively.
Sanofi will publish the long anticipated phase I data using a once weekly schedule. There are additional CD19 programs in development, including Micromet’s (MITI) MT-103 that continues to generate solid data in NHL. Competition is thinning out following the discontinuation of Medarex’s Fc engineered CD19 antibody, probably due to safety issues. This does not bode well for Morphosys and Medimmune who also have Fc engineered CD19 antibodies in phase I.
Roche will present PFS data from a randomized phase II evaluating T-DM1 vs. Herceptin+ chemo in 1st line HER2+ breast cancer patients. This trial generated a positive signal in terms of response rate in favor of T-DM1 but the PFS data will be crucial as a more reliable endpoint. Since Roche is already enrolling patients in a pivotal study using a similar design in a similar patient population, one can assume that T-DM1 was at least comparable to standard of care.
Hallo again,

als "Gründer" dieser Diskussion melde ich mich hiermit zurück zur aktiven Anlegerschaft.
Vorhin Teilposi wieder erworben ("Fuß in der Tür") zum exakt gleichen Preis wie mein Verkauf letzten Mai (€/$ machts möglich).
Wenn´s passt, verbillige ich.
SGEN ist immer noch nicht besonders preisgünstig, aber wie soll man das schon beurteilen.
Hatte auch meine EXEL mit gutem Gewinn, aber wie sich jetzt zeigt, viel zu früh verkauft...

ay que ver.

Geuß q.

als "Gründer" dieser Diskussion melde ich mich hiermit zurück zur aktiven Anlegerschaft.
Vorhin Teilposi wieder erworben ("Fuß in der Tür") zum exakt gleichen Preis wie mein Verkauf letzten Mai (€/$ machts möglich).
Wenn´s passt, verbillige ich.
SGEN ist immer noch nicht besonders preisgünstig, aber wie soll man das schon beurteilen.
Hatte auch meine EXEL mit gutem Gewinn, aber wie sich jetzt zeigt, viel zu früh verkauft...

ay que ver.

Geuß q.
Antwort auf Beitrag Nr.: 41.095.657 von quepos am 23.02.11 21:48:07Hi,
habe die derzeitige Kursschwäche auch nochmals genutzt und für etwas mehr als 15 Dollar meine Position weiter ausgebaut. Sie ist jetzt wirklich ziemlich groß...
Ich halte SGEN auch nicht für ein Schnäppchen. Die Frage ist aber, ob man an die Technologie glaubt oder nicht. Ich glaube dran und ich finde, dass das gerade durch die enge Zusammenarbeit mit Genentech recht gut verifiziert ist. Langfristig ist das Potential meiner Meinung nach erheblich.
Und wer weiß, vielleicht passiert selbst kurzfristig was tolles mit SGN 75.
habe die derzeitige Kursschwäche auch nochmals genutzt und für etwas mehr als 15 Dollar meine Position weiter ausgebaut. Sie ist jetzt wirklich ziemlich groß...
Ich halte SGEN auch nicht für ein Schnäppchen. Die Frage ist aber, ob man an die Technologie glaubt oder nicht. Ich glaube dran und ich finde, dass das gerade durch die enge Zusammenarbeit mit Genentech recht gut verifiziert ist. Langfristig ist das Potential meiner Meinung nach erheblich.
Und wer weiß, vielleicht passiert selbst kurzfristig was tolles mit SGN 75.
Der Zulassungsantrag für Brentuximab Vedotin ist gestellt. Jetzt ist die FDA am Zug.
BOTHELL, Wash.--(BUSINESS WIRE)-- Seattle Genetics, Inc. (Nasdaq:SGEN - News) announced today that it has submitted a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for the use of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma and relapsed or refractory systemic anaplastic large cell lymphoma (ALCL). Brentuximab vedotin is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of Hodgkin lymphoma and ALCL. Seattle Genetics has requested a Priority Review from the FDA that, if granted, provides six months from receipt of the submission for the FDA to take action on the application.
Gleichzeitig arbeitet man daran, die Zielgruppe zu erweitern und zu einer first-line Therapie zu kommen:
BOTHELL, Wash.--(BUSINESS WIRE)-- Seattle Genetics, Inc. (Nasdaq:SGEN - News) and Millennium: The Takeda Oncology Company today announced that they have initiated a phase I clinical trial of brentuximab vedotin (SGN-35) in combination with chemotherapy for the treatment of newly diagnosed systemic anaplastic large cell lymphoma (ALCL) patients. Brentuximab vedotin is an antibody-drug conjugate (ADC) directed to CD30, which is highly expressed in ALCL.
“The objective and complete response rates observed with single-agent brentuximab vedotin in relapsed or refractory systemic ALCL, and the manageable side-effect profile, provide encouraging justification for its investigation in earlier lines of treatment for this aggressive type of non-Hodgkin lymphoma,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer of Seattle Genetics. “There have been no significant advances for the treatment of front-line systemic ALCL since the introduction of CHOP chemotherapy for aggressive lymphomas decades ago. New approaches to improve upon the outcomes achieved with standard multi-agent chemotherapy regimens in newly diagnosed ALCL patients are needed.”
“ALCL is an area of significant unmet need, and the initiation of this study is an important milestone in our investigation of brentuximab vedotin in earlier lines of CD30-positive hematologic malignancies,” said Nancy Simonian, M.D., Chief Medical Officer, Millennium.
The phase I dose-escalation trial will evaluate the safety profile, pharmacokinetics and antitumor activity of brentuximab vedotin when administered sequentially or in combination with multi-agent front-line chemotherapy regimens. The study is expected to enroll up to approximately 60 patients at multiple centers in the United States and Europe.
BOTHELL, Wash.--(BUSINESS WIRE)-- Seattle Genetics, Inc. (Nasdaq:SGEN - News) announced today that it has submitted a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for the use of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma and relapsed or refractory systemic anaplastic large cell lymphoma (ALCL). Brentuximab vedotin is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of Hodgkin lymphoma and ALCL. Seattle Genetics has requested a Priority Review from the FDA that, if granted, provides six months from receipt of the submission for the FDA to take action on the application.
Gleichzeitig arbeitet man daran, die Zielgruppe zu erweitern und zu einer first-line Therapie zu kommen:
BOTHELL, Wash.--(BUSINESS WIRE)-- Seattle Genetics, Inc. (Nasdaq:SGEN - News) and Millennium: The Takeda Oncology Company today announced that they have initiated a phase I clinical trial of brentuximab vedotin (SGN-35) in combination with chemotherapy for the treatment of newly diagnosed systemic anaplastic large cell lymphoma (ALCL) patients. Brentuximab vedotin is an antibody-drug conjugate (ADC) directed to CD30, which is highly expressed in ALCL.
“The objective and complete response rates observed with single-agent brentuximab vedotin in relapsed or refractory systemic ALCL, and the manageable side-effect profile, provide encouraging justification for its investigation in earlier lines of treatment for this aggressive type of non-Hodgkin lymphoma,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer of Seattle Genetics. “There have been no significant advances for the treatment of front-line systemic ALCL since the introduction of CHOP chemotherapy for aggressive lymphomas decades ago. New approaches to improve upon the outcomes achieved with standard multi-agent chemotherapy regimens in newly diagnosed ALCL patients are needed.”
“ALCL is an area of significant unmet need, and the initiation of this study is an important milestone in our investigation of brentuximab vedotin in earlier lines of CD30-positive hematologic malignancies,” said Nancy Simonian, M.D., Chief Medical Officer, Millennium.
The phase I dose-escalation trial will evaluate the safety profile, pharmacokinetics and antitumor activity of brentuximab vedotin when administered sequentially or in combination with multi-agent front-line chemotherapy regimens. The study is expected to enroll up to approximately 60 patients at multiple centers in the United States and Europe.
Seattle Genetics, Inc. (Nasdaq: SGEN) announced today that Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited (TSE:4502), has paid an undisclosed fee to exercise an option to designate a second antigen target under the parties’ existing antibody-drug conjugate (ADC) collaboration. Seattle Genetics entered into this collaboration with Millennium in April 2009, at which time Millennium obtained an exclusive ADC license to an initial antigen expressed on solid tumors as well as two options for exclusive licenses to additional targets.
"This expansion of our ADC collaboration demonstrates Millennium's continuing commitment to our ADC technology," stated Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. "We currently have 10 ongoing ADC collaborations and have generated nearly $150 million from ADC licensing deals to date. In addition, there are 11 ADCs in clinical development utilizing Seattle Genetics' technology."
Under the terms of the ADC collaboration, Millennium is responsible for research, product development, manufacturing and commercialization of any ADC products resulting from the collaboration. Seattle Genetics is entitled to receive progress-dependent milestone payments and mid-single digit royalties from Millennium on worldwide net sales of any resulting ADC products. Seattle Genetics also receives supply and annual maintenance fees as well as research support payments for assistance provided to Millennium under the collaboration.
"This expansion of our ADC collaboration demonstrates Millennium's continuing commitment to our ADC technology," stated Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. "We currently have 10 ongoing ADC collaborations and have generated nearly $150 million from ADC licensing deals to date. In addition, there are 11 ADCs in clinical development utilizing Seattle Genetics' technology."
Under the terms of the ADC collaboration, Millennium is responsible for research, product development, manufacturing and commercialization of any ADC products resulting from the collaboration. Seattle Genetics is entitled to receive progress-dependent milestone payments and mid-single digit royalties from Millennium on worldwide net sales of any resulting ADC products. Seattle Genetics also receives supply and annual maintenance fees as well as research support payments for assistance provided to Millennium under the collaboration.
Ein weiterer kleiner ADC-Deal mit guten Konditionen. Das läppert sich zusammen.
Seattle Genetics Announces Antibody-Drug Conjugate Collaboration with Abbott
Seattle Genetics to receive $8 million upfront payment, plus potential milestone payments and royalties under single-target collaboration
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (NASDAQ:SGEN) today announced that it has entered into a collaboration agreement with Abbott (NYSE:ABT) under which Abbott will pay an upfront fee of $8 million for rights to utilize Seattle Genetics’ antibody-drug conjugate (ADC) technology with antibodies to a single oncology target.
We are pleased to collaborate with Abbott on our ADC technology given their position as one of the world’s leading pharmaceutical companies and their demonstrated commitment to both biologic and oncology therapeutics,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “This is the second ADC collaboration with a multinational pharmaceutical company that we have announced this year, further illustrating the important role that our ADC technology is poised to play in the treatment of many types of cancer.”
Abbott is responsible for research, product development, manufacturing and commercialization of any ADC products under the collaboration. Pending achievement of certain development, regulatory and commercial milestones, Seattle Genetics is eligible to receive from Abbott up to approximately $200 million in milestone payments, as well as royalties on worldwide net sales of any resulting ADC products. Seattle Genetics also will receive annual maintenance fees and research support payments for assistance provided to Abbott under the collaboration.
Seattle Genetics Announces Antibody-Drug Conjugate Collaboration with Abbott
Seattle Genetics to receive $8 million upfront payment, plus potential milestone payments and royalties under single-target collaboration
BOTHELL, Wash.--(BUSINESS WIRE)--Seattle Genetics, Inc. (NASDAQ:SGEN) today announced that it has entered into a collaboration agreement with Abbott (NYSE:ABT) under which Abbott will pay an upfront fee of $8 million for rights to utilize Seattle Genetics’ antibody-drug conjugate (ADC) technology with antibodies to a single oncology target.
We are pleased to collaborate with Abbott on our ADC technology given their position as one of the world’s leading pharmaceutical companies and their demonstrated commitment to both biologic and oncology therapeutics,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “This is the second ADC collaboration with a multinational pharmaceutical company that we have announced this year, further illustrating the important role that our ADC technology is poised to play in the treatment of many types of cancer.”
Abbott is responsible for research, product development, manufacturing and commercialization of any ADC products under the collaboration. Pending achievement of certain development, regulatory and commercial milestones, Seattle Genetics is eligible to receive from Abbott up to approximately $200 million in milestone payments, as well as royalties on worldwide net sales of any resulting ADC products. Seattle Genetics also will receive annual maintenance fees and research support payments for assistance provided to Abbott under the collaboration.
Jaso Chew wundert sich, dass SGEN noch nicht übernommen wurde. Ich wundere mich mit ihm.
Seattle Genetics Consolidating Its Strong ADC Position
By Jason Chew | March 22, 2011 | Filed under: Drug Development, Recent Posts
Today, Seattle Genetics announced a partnership with Abbott to develop a drug against a single target using their antibody-drug-conjugate (ADC) technology. Abbott will pay $8 million upfront and up to $200 million in milestones as well as royalties on worldwide sales, if any.
This deal comes quickly after news of the expansion of an ADC development project with Millennium Takeda on March 15 from the original April 2009 collaboration. A collaboration with Pfizer announced in January contains similar terms as this new Abbott deal. Seattle Genetics now has collaborations with some of the leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab.
Eleven ADCs based on Seattle Genetics technology are currently in clinical development, up from nine in November 2010. Seattle Genetics itself has the best chance of being the first to commercialize an ADC with its lead product candidate, brentuximab vedotin, for the treatment of relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. It has already filed a BLA in February and expects FDA approval by the end of this year with a launch in 2012.
It is truly a surprise Seattle Genetics has managed to remain a stand-alone company. Its brentuximab vedotin development partner, Millennium Takeda, has been highly acquisitive and interested in the oncology space; Genentech is paying top dollar to access its ADC technology. Certain mid-tier pharmaceutical companies would do well to revitalize their drug discovery efforts with a dose of innovation. Bristol Myers Squibb has demonstrated the gains possible through the acquisition of game-changing technologies with its purchase of Medarex.
I am not advocating Seattle Genetics put itself up for sale; it is obviously more valuable on its own.
Seattle Genetics Consolidating Its Strong ADC Position
By Jason Chew | March 22, 2011 | Filed under: Drug Development, Recent Posts
Today, Seattle Genetics announced a partnership with Abbott to develop a drug against a single target using their antibody-drug-conjugate (ADC) technology. Abbott will pay $8 million upfront and up to $200 million in milestones as well as royalties on worldwide sales, if any.
This deal comes quickly after news of the expansion of an ADC development project with Millennium Takeda on March 15 from the original April 2009 collaboration. A collaboration with Pfizer announced in January contains similar terms as this new Abbott deal. Seattle Genetics now has collaborations with some of the leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab.
Eleven ADCs based on Seattle Genetics technology are currently in clinical development, up from nine in November 2010. Seattle Genetics itself has the best chance of being the first to commercialize an ADC with its lead product candidate, brentuximab vedotin, for the treatment of relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. It has already filed a BLA in February and expects FDA approval by the end of this year with a launch in 2012.
It is truly a surprise Seattle Genetics has managed to remain a stand-alone company. Its brentuximab vedotin development partner, Millennium Takeda, has been highly acquisitive and interested in the oncology space; Genentech is paying top dollar to access its ADC technology. Certain mid-tier pharmaceutical companies would do well to revitalize their drug discovery efforts with a dose of innovation. Bristol Myers Squibb has demonstrated the gains possible through the acquisition of game-changing technologies with its purchase of Medarex.
I am not advocating Seattle Genetics put itself up for sale; it is obviously more valuable on its own.
Moin,
:;
AACR kam wohl zumindest nicht schlecht an.

Nach meinem "Fuß-in-der-Tür"-Kauf bin ich zu keinen weiteren Nachkäufen gekommen, weil der Kurs nicht mehr viel weiter zurückkam.
Aber das wünscht man sich ja eigentlich auch!
Hier der Text der heutigen Meldung:
----------------------------------
Seattle Genetics Highlights ADC Technology Research at AACR
Companies:Seattle Genetics Inc. Related Quotes
Symbol Price Change
SGEN 16.16 0.44
{"s" : "sgen","k" : "a00,a50,b00,b60,c10,g00,h00,l10,p20,t10,v00","o" : "","j" : ""} Press Release Source: Seattle Genetics, Inc. On Tuesday April 5, 2011, 8:00 am EDT
BOTHELL, Wash.--#BUSINESS WIRE#-- Seattle Genetics, Inc. #Nasdaq:SGEN - News# today announced that research related to its antibody-drug conjugate #ADC# technology was presented at the 102nd Annual Meeting of the American Association for Cancer Research #AACR# being held in Orlando, FL. The presentations highlighted advances being made by the company with its ADC technology and preclinical research on its ADC pipeline, including brentuximab vedotin #SGN-35#, SGN-75 and SGN-19A.
“These presentations demonstrate our continued leadership in the field of ADCs, including using the technology to extend our pipeline of preclinical product candidates, exploring ADCs in combination with standard therapies, and measuring their pharmacodynamic effects,” said Jonathan Drachman, M.D., Senior Vice President, Research and Translational Medicine at Seattle Genetics. “We also illustrate how to refine properties of future drug-linkers and how to evaluate this new class of molecules in clinical trials. This research reflects our enthusiasm and continued belief that ADCs are a significant, emerging class of cancer therapeutics.”
ADCs are monoclonal antibodies that selectively deliver potent anti-cancer agents to tumor cells. With over a decade of experience and knowledge in ADC innovation, Seattle Genetics has developed proprietary technology employing synthetic, potent cell-killing agents called auristatins #such as MMAE and MMAF# and stable linker systems that attach auristatin to the antibody. Seattle Genetics’ novel linker systems are designed to be stable in the bloodstream and release the potent cell-killing agent once inside targeted cancer cells. This approach is intended to spare non-malignant cells and reduce many of the toxic effects of traditional chemotherapy while enhancing antitumor activity.
Seattle Genetics’ presentations at AACR demonstrated the following:
Auristatin-based ADCs, including brentuximab vedotin and SGN-75, have synergistic activity when combined with mTOR inhibitors such as sirolimus and everolimus in several solid tumor and hematologic malignancy tumor models. #Abstract #1789#
Methods have been developed to assess the effect of SGN-75 on CD70-positive cells, including in blood samples from a subset of
:;
AACR kam wohl zumindest nicht schlecht an.

Nach meinem "Fuß-in-der-Tür"-Kauf bin ich zu keinen weiteren Nachkäufen gekommen, weil der Kurs nicht mehr viel weiter zurückkam.
Aber das wünscht man sich ja eigentlich auch!
Hier der Text der heutigen Meldung:
----------------------------------
Seattle Genetics Highlights ADC Technology Research at AACR
Companies:Seattle Genetics Inc. Related Quotes
Symbol Price Change
SGEN 16.16 0.44
{"s" : "sgen","k" : "a00,a50,b00,b60,c10,g00,h00,l10,p20,t10,v00","o" : "","j" : ""} Press Release Source: Seattle Genetics, Inc. On Tuesday April 5, 2011, 8:00 am EDT
BOTHELL, Wash.--#BUSINESS WIRE#-- Seattle Genetics, Inc. #Nasdaq:SGEN - News# today announced that research related to its antibody-drug conjugate #ADC# technology was presented at the 102nd Annual Meeting of the American Association for Cancer Research #AACR# being held in Orlando, FL. The presentations highlighted advances being made by the company with its ADC technology and preclinical research on its ADC pipeline, including brentuximab vedotin #SGN-35#, SGN-75 and SGN-19A.
“These presentations demonstrate our continued leadership in the field of ADCs, including using the technology to extend our pipeline of preclinical product candidates, exploring ADCs in combination with standard therapies, and measuring their pharmacodynamic effects,” said Jonathan Drachman, M.D., Senior Vice President, Research and Translational Medicine at Seattle Genetics. “We also illustrate how to refine properties of future drug-linkers and how to evaluate this new class of molecules in clinical trials. This research reflects our enthusiasm and continued belief that ADCs are a significant, emerging class of cancer therapeutics.”
ADCs are monoclonal antibodies that selectively deliver potent anti-cancer agents to tumor cells. With over a decade of experience and knowledge in ADC innovation, Seattle Genetics has developed proprietary technology employing synthetic, potent cell-killing agents called auristatins #such as MMAE and MMAF# and stable linker systems that attach auristatin to the antibody. Seattle Genetics’ novel linker systems are designed to be stable in the bloodstream and release the potent cell-killing agent once inside targeted cancer cells. This approach is intended to spare non-malignant cells and reduce many of the toxic effects of traditional chemotherapy while enhancing antitumor activity.
Seattle Genetics’ presentations at AACR demonstrated the following:
Auristatin-based ADCs, including brentuximab vedotin and SGN-75, have synergistic activity when combined with mTOR inhibitors such as sirolimus and everolimus in several solid tumor and hematologic malignancy tumor models. #Abstract #1789#
Methods have been developed to assess the effect of SGN-75 on CD70-positive cells, including in blood samples from a subset of
Antwort auf Beitrag Nr.: 41.324.228 von quepos am 05.04.11 22:01:34Hi quepos,
da ist Dir ein Teil der Meldung beim Kopieren abhanden gekommen...
Seattle Genetics’ presentations at AACR demonstrated the following:
* Auristatin-based ADCs, including brentuximab vedotin and SGN-75, have synergistic activity when combined with mTOR inhibitors such as sirolimus and everolimus in several solid tumor and hematologic malignancy tumor models. (Abstract #1789)
* Methods have been developed to assess the effect of SGN-75 on CD70-positive cells, including in blood samples from a subset of patients in an ongoing phase I clinical trial. These methods may be appropriate for measuring pharmacodynamic effects. (Abstract #1284)
* SGN-19A, which comprises an anti-CD19 monoclonal antibody linked to MMAF, effectively targets CD19 and induces antitumor activity in models of non-Hodgkin lymphoma and acute lymphoblastic leukemia. SGN-19A is a future investigational new drug candidate. (Abstract #625)
* LIV-1 is a promising new target antigen, expressed at high levels on breast and prostate cancers. An anti-LIV-1 ADC demonstrates antitumor activity in multiple preclinical models. (Abstract #3620)
* A novel screening method was developed to identify drug-linkers that are metabolized differently in tumors compared with normal tissue. These advances could further enhance the specificity of ADCs on cancer cells. (Abstract #2831)
------------
In Bezug auf SGN 75 könnte es zur ASCO Daten geben. Das würde dann wirklich spannend sein. Und wer weiß, vielleicht haben wir da mehr Glück als Verstand?
(theoretisch könnte hier ein enormes Potential stecken, aber noch ist es viel zu früh...)
da ist Dir ein Teil der Meldung beim Kopieren abhanden gekommen...

Seattle Genetics’ presentations at AACR demonstrated the following:
* Auristatin-based ADCs, including brentuximab vedotin and SGN-75, have synergistic activity when combined with mTOR inhibitors such as sirolimus and everolimus in several solid tumor and hematologic malignancy tumor models. (Abstract #1789)
* Methods have been developed to assess the effect of SGN-75 on CD70-positive cells, including in blood samples from a subset of patients in an ongoing phase I clinical trial. These methods may be appropriate for measuring pharmacodynamic effects. (Abstract #1284)
* SGN-19A, which comprises an anti-CD19 monoclonal antibody linked to MMAF, effectively targets CD19 and induces antitumor activity in models of non-Hodgkin lymphoma and acute lymphoblastic leukemia. SGN-19A is a future investigational new drug candidate. (Abstract #625)
* LIV-1 is a promising new target antigen, expressed at high levels on breast and prostate cancers. An anti-LIV-1 ADC demonstrates antitumor activity in multiple preclinical models. (Abstract #3620)
* A novel screening method was developed to identify drug-linkers that are metabolized differently in tumors compared with normal tissue. These advances could further enhance the specificity of ADCs on cancer cells. (Abstract #2831)
------------
In Bezug auf SGN 75 könnte es zur ASCO Daten geben. Das würde dann wirklich spannend sein. Und wer weiß, vielleicht haben wir da mehr Glück als Verstand?

(theoretisch könnte hier ein enormes Potential stecken, aber noch ist es viel zu früh...)
Antwort auf Beitrag Nr.: 41.324.228 von quepos am 05.04.11 22:01:34Ups..
Fehlt irgenwie was, reiche hiermit den Rest nach:
q.

--------------------
Seattle Genetics’ presentations at AACR demonstrated the following:
Auristatin-based ADCs, including brentuximab vedotin and SGN-75, have synergistic activity when combined with mTOR inhibitors such as sirolimus and everolimus in several solid tumor and hematologic malignancy tumor models. (Abstract #1789)
Methods have been developed to assess the effect of SGN-75 on CD70-positive cells, including in blood samples from a subset of patients in an ongoing phase I clinical trial. These methods may be appropriate for measuring pharmacodynamic effects. (Abstract #1284)
SGN-19A, which comprises an anti-CD19 monoclonal antibody linked to MMAF, effectively targets CD19 and induces antitumor activity in models of non-Hodgkin lymphoma and acute lymphoblastic leukemia. SGN-19A is a future investigational new drug candidate. (Abstract #625)
LIV-1 is a promising new target antigen, expressed at high levels on breast and prostate cancers. An anti-LIV-1 ADC demonstrates antitumor activity in multiple preclinical models. (Abstract #3620)
A novel screening method was developed to identify drug-linkers that are metabolized differently in tumors compared with normal tissue. These advances could further enhance the specificity of ADCs on cancer cells. (Abstract #2831)
About Seattle Genetics
Seattle Genetics is a clinical-stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company submitted a Biologics License Application to the U.S. Food and Drug Administration for its lead product candidate, brentuximab vedotin, for the treatment of relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma in February 2011. Brentuximab vedotin is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has four other clinical-stage programs: SGN-75, ASG-5ME, dacetuzumab (SGN-40) and SGN-70. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
Certain of the statements made in this press release are forward-looking, such as those, among others, relating to the therapeutic potential of Seattle Genetics’ product candidates and its ADC technology. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include risks related to failure of our or our collaborators’ product candidates incorporating ADC technologies to show sufficient safety and efficacy to advance in clinical trials or obtain regulatory approval. More information about the risks and uncertainties faced by Seattle Genetics is contained in the company’s 10-K for the year ended December 31, 2010 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact:
Seattle Genetics, Inc.Peggy Pinkston, 425-527-4160ppinkston@seagen.com
-------------------------
Nachbörslich kam auch noch dieses hier:
--------------------------
Seattle Genetics shares rise in heavy trading
Puget Sound Business Journal
Date: Tuesday, April 5, 2011, 4:16pm PDT - Last Modified: Tuesday, April 5, 2011, 4:49pm PDT
Related:Health Care
Seattle Genetics (NASDAQ: SGEN) saw a high volume of trading on Tuesday, with a share volume of 1,857,730 compared to a 50-day average volume of 1,061,936. The stock closed at $16.15, up 2.74 percent from the previous session.
The Bothell-based clinical-stage biotechnology company announced Tuesday that it had presented research related to antibody-drug conjugate (ADC) technology at the 102nd Annual Meeting of the American Association for Cancer Research (AACR) being held in Orlando, FL.
ADCs are monoclonal antibodies that selectively deliver potent anti-cancer agents to tumor cells in an approach intended to spare non-malignant cells and reduce many of the toxic effects of traditional chemotherapy while fighting tumors.
"These presentations demonstrate our continued leadership in the field of ADCs, including using the technology to extend our pipeline of preclinical product candidates, exploring ADCs in combination with standard therapies, and measuring their pharmacodynamic effects," said Jonathan Drachman, senior vice president, Research and Translational Medicine at Seattle Genetics.
"We also illustrate how to refine properties of future drug-linkers and how to evaluate this new class of molecules in clinical trials. This research reflects our enthusiasm and continued belief that ADCs are a significant, emerging class of cancer therapeutics." ...
Read more: Seattle Genetics shares rise in heavy trading | Puget Sound Business Journal
Fehlt irgenwie was, reiche hiermit den Rest nach:
q.

--------------------
Seattle Genetics’ presentations at AACR demonstrated the following:
Auristatin-based ADCs, including brentuximab vedotin and SGN-75, have synergistic activity when combined with mTOR inhibitors such as sirolimus and everolimus in several solid tumor and hematologic malignancy tumor models. (Abstract #1789)
Methods have been developed to assess the effect of SGN-75 on CD70-positive cells, including in blood samples from a subset of patients in an ongoing phase I clinical trial. These methods may be appropriate for measuring pharmacodynamic effects. (Abstract #1284)
SGN-19A, which comprises an anti-CD19 monoclonal antibody linked to MMAF, effectively targets CD19 and induces antitumor activity in models of non-Hodgkin lymphoma and acute lymphoblastic leukemia. SGN-19A is a future investigational new drug candidate. (Abstract #625)
LIV-1 is a promising new target antigen, expressed at high levels on breast and prostate cancers. An anti-LIV-1 ADC demonstrates antitumor activity in multiple preclinical models. (Abstract #3620)
A novel screening method was developed to identify drug-linkers that are metabolized differently in tumors compared with normal tissue. These advances could further enhance the specificity of ADCs on cancer cells. (Abstract #2831)
About Seattle Genetics
Seattle Genetics is a clinical-stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company submitted a Biologics License Application to the U.S. Food and Drug Administration for its lead product candidate, brentuximab vedotin, for the treatment of relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma in February 2011. Brentuximab vedotin is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has four other clinical-stage programs: SGN-75, ASG-5ME, dacetuzumab (SGN-40) and SGN-70. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
Certain of the statements made in this press release are forward-looking, such as those, among others, relating to the therapeutic potential of Seattle Genetics’ product candidates and its ADC technology. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include risks related to failure of our or our collaborators’ product candidates incorporating ADC technologies to show sufficient safety and efficacy to advance in clinical trials or obtain regulatory approval. More information about the risks and uncertainties faced by Seattle Genetics is contained in the company’s 10-K for the year ended December 31, 2010 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact:
Seattle Genetics, Inc.Peggy Pinkston, 425-527-4160ppinkston@seagen.com
-------------------------
Nachbörslich kam auch noch dieses hier:
--------------------------
Seattle Genetics shares rise in heavy trading
Puget Sound Business Journal
Date: Tuesday, April 5, 2011, 4:16pm PDT - Last Modified: Tuesday, April 5, 2011, 4:49pm PDT
Related:Health Care
Seattle Genetics (NASDAQ: SGEN) saw a high volume of trading on Tuesday, with a share volume of 1,857,730 compared to a 50-day average volume of 1,061,936. The stock closed at $16.15, up 2.74 percent from the previous session.
The Bothell-based clinical-stage biotechnology company announced Tuesday that it had presented research related to antibody-drug conjugate (ADC) technology at the 102nd Annual Meeting of the American Association for Cancer Research (AACR) being held in Orlando, FL.
ADCs are monoclonal antibodies that selectively deliver potent anti-cancer agents to tumor cells in an approach intended to spare non-malignant cells and reduce many of the toxic effects of traditional chemotherapy while fighting tumors.
"These presentations demonstrate our continued leadership in the field of ADCs, including using the technology to extend our pipeline of preclinical product candidates, exploring ADCs in combination with standard therapies, and measuring their pharmacodynamic effects," said Jonathan Drachman, senior vice president, Research and Translational Medicine at Seattle Genetics.
"We also illustrate how to refine properties of future drug-linkers and how to evaluate this new class of molecules in clinical trials. This research reflects our enthusiasm and continued belief that ADCs are a significant, emerging class of cancer therapeutics." ...
Read more: Seattle Genetics shares rise in heavy trading | Puget Sound Business Journal
Hallo,
dies hier auch noch:
EBMT-Meeting in Paris, Text folgt
Gruß q.
-------------
Seattle Genetics Reports Brentuximab Vedotin (SGN-35) Data at EBMT Annual Meeting
50 Percent of Post-Allogeneic Transplant Patients Achieved an Objective Response
Companies:Seattle Genetics Inc. Related Quotes
Symbol Price Change
SGEN 16.15 0.00
{"s" : "sgen","k" : "a00,a50,b00,b60,c10,g00,h00,l10,p20,t10,v00","o" : "","j" : ""} Press Release Source: Seattle Genetics, Inc. On Wednesday April 6, 2011, 8:00 am
BOTHELL, Wash.--(BUSINESS WIRE)-- Seattle Genetics, Inc. (Nasdaq:SGEN - News) today announced that data from a case series of Hodgkin lymphoma patients receiving brentuximab vedotin (SGN-35) following allogeneic stem cell transplant were presented in an oral session at the European Group for Blood and Marrow Transplantation (EBMT) Annual Meeting in Paris, France. Brentuximab vedotin is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of Hodgkin lymphoma.
This is the first report of data from brentuximab vedotin in Hodgkin lymphoma patients who relapsed following allogeneic transplant. Patients in the company’s pivotal Hodgkin lymphoma trial had all relapsed following autologous transplant, but none had received an allogeneic transplant. Patients relapsing following allogeneic transplant represent a particularly difficult therapeutic challenge. Key findings from this case series of 25 post-allogeneic transplant patients include:
50 percent of patients achieved an objective response (12 of 24 evaluable), including 38 percent complete remissions; an additional 42 percent of patients had stable disease
Median progression-free survival (PFS) was 34 weeks; median overall survival had not been reached
The median time to objective response was 8.1 weeks and patients received a median of 8 cycles of therapy; five patients remain on treatment
Brentuximab vedotin administration was associated with manageable adverse events, with the most common being cough, fatigue, fever, nausea and peripheral sensory neuropathy
The most common Grade 3 or higher adverse events were neutropenia, anemia, fatigue and fever
“Although allogeneic stem cell transplantation is the only potentially curative option at present for Hodgkin lymphoma patients who relapse following an autologous transplant, only 20 to 25 percent of these patients achieve long-term benefit,” said Owen A. O’Connor, M.D., Ph.D., Professor, and Director, Division of Hematology and Medical Oncology at NYU Cancer Institute. “There are currently no good treatment options for Hodgkin lymphoma patients who fail allogeneic transplant. These data for brentuximab vedotin are encouraging in this post-transplant setting where there is a significant unmet medical need.”
The case series comprises data from Hodgkin lymphoma patients who relapsed following allogeneic stem cell transplant that were enrolled in one of three multicenter, open label clinical trials of brentuximab vedotin. Patients received 1.2 or 1.8 milligrams per kilogram of brentuximab vedotin every three weeks. The median age of patients was 32 years. Enrolled patients had received a median of five prior therapeutic regimens, including 76 percent who had a prior autologous stem cell transplant.
About Brentuximab Vedotin
Brentuximab vedotin is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a potent, synthetic drug, monomethyl auristatin E (MMAE) utilizing Seattle Genetics' proprietary technology. The ADC employs a novel linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells. This approach is intended to spare non-targeted cells and thus may help minimize the potential toxic effects of traditional chemotherapy while allowing for the selective targeting of CD30-expressing cancer cells, thus potentially enhancing the antitumor activity.
Seattle Genetics is jointly developing brentuximab vedotin with Millennium: The Takeda Oncology Company. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize brentuximab vedotin in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for brentuximab vedotin on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
About Hodgkin Lymphoma
Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is distinguished from other types of lymphoma by the presence of one characteristic type of cell, known as the Reed-Sternberg cell. A defining attribute of the Reed-Sternberg cell is its expression of the CD30 antigen.
According to the American Cancer Society, approximately 8,500 cases of Hodgkin lymphoma were diagnosed in the United States during 2010 and more than 1,300 died from the disease. Although front-line combination chemotherapy can result in durable response rates, up to 30 percent of these patients relapse or are refractory to front-line treatment and have few therapeutic options beyond autologous stem cell transplant.
About Seattle Genetics
Seattle Genetics is a clinical-stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company submitted a Biologics License Application to the U.S. Food and Drug Administration for its lead product candidate, brentuximab vedotin, for the treatment of relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma in February 2011. Brentuximab vedotin is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has four other clinical-stage programs: SGN-75, ASG-5ME, dacetuzumab (SGN-40) and SGN-70. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
Certain of the statements made in this press release are forward-looking, such as those, among others, relating to the potential therapeutic benefit of brentuximab vedotin. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include that the safety and/or efficacy results of the pivotal trial in relapsed or refractory Hodgkin lymphoma and phase II trial in relapsed or refractory systemic ALCL will not be sufficient to gain marketing approval in the United States or any other country, that we will be required to amend our submission for marketing approval or that such submission will be refused. In addition, our regulatory plans may change as a result of consultation with the FDA or EMA. More information about the risks and uncertainties faced by Seattle Genetics is contained in the company’s 10-K for the year ended December 31, 2010 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact:
Seattle Genetics, Inc.Peggy Pinkston, 425-527-4160ppinkston@seagen.com
dies hier auch noch:
EBMT-Meeting in Paris, Text folgt
Gruß q.
-------------
Seattle Genetics Reports Brentuximab Vedotin (SGN-35) Data at EBMT Annual Meeting
50 Percent of Post-Allogeneic Transplant Patients Achieved an Objective Response
Companies:Seattle Genetics Inc. Related Quotes
Symbol Price Change
SGEN 16.15 0.00
{"s" : "sgen","k" : "a00,a50,b00,b60,c10,g00,h00,l10,p20,t10,v00","o" : "","j" : ""} Press Release Source: Seattle Genetics, Inc. On Wednesday April 6, 2011, 8:00 am
BOTHELL, Wash.--(BUSINESS WIRE)-- Seattle Genetics, Inc. (Nasdaq:SGEN - News) today announced that data from a case series of Hodgkin lymphoma patients receiving brentuximab vedotin (SGN-35) following allogeneic stem cell transplant were presented in an oral session at the European Group for Blood and Marrow Transplantation (EBMT) Annual Meeting in Paris, France. Brentuximab vedotin is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of Hodgkin lymphoma.
This is the first report of data from brentuximab vedotin in Hodgkin lymphoma patients who relapsed following allogeneic transplant. Patients in the company’s pivotal Hodgkin lymphoma trial had all relapsed following autologous transplant, but none had received an allogeneic transplant. Patients relapsing following allogeneic transplant represent a particularly difficult therapeutic challenge. Key findings from this case series of 25 post-allogeneic transplant patients include:
50 percent of patients achieved an objective response (12 of 24 evaluable), including 38 percent complete remissions; an additional 42 percent of patients had stable disease
Median progression-free survival (PFS) was 34 weeks; median overall survival had not been reached
The median time to objective response was 8.1 weeks and patients received a median of 8 cycles of therapy; five patients remain on treatment
Brentuximab vedotin administration was associated with manageable adverse events, with the most common being cough, fatigue, fever, nausea and peripheral sensory neuropathy
The most common Grade 3 or higher adverse events were neutropenia, anemia, fatigue and fever
“Although allogeneic stem cell transplantation is the only potentially curative option at present for Hodgkin lymphoma patients who relapse following an autologous transplant, only 20 to 25 percent of these patients achieve long-term benefit,” said Owen A. O’Connor, M.D., Ph.D., Professor, and Director, Division of Hematology and Medical Oncology at NYU Cancer Institute. “There are currently no good treatment options for Hodgkin lymphoma patients who fail allogeneic transplant. These data for brentuximab vedotin are encouraging in this post-transplant setting where there is a significant unmet medical need.”
The case series comprises data from Hodgkin lymphoma patients who relapsed following allogeneic stem cell transplant that were enrolled in one of three multicenter, open label clinical trials of brentuximab vedotin. Patients received 1.2 or 1.8 milligrams per kilogram of brentuximab vedotin every three weeks. The median age of patients was 32 years. Enrolled patients had received a median of five prior therapeutic regimens, including 76 percent who had a prior autologous stem cell transplant.
About Brentuximab Vedotin
Brentuximab vedotin is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a potent, synthetic drug, monomethyl auristatin E (MMAE) utilizing Seattle Genetics' proprietary technology. The ADC employs a novel linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells. This approach is intended to spare non-targeted cells and thus may help minimize the potential toxic effects of traditional chemotherapy while allowing for the selective targeting of CD30-expressing cancer cells, thus potentially enhancing the antitumor activity.
Seattle Genetics is jointly developing brentuximab vedotin with Millennium: The Takeda Oncology Company. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize brentuximab vedotin in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for brentuximab vedotin on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
About Hodgkin Lymphoma
Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is distinguished from other types of lymphoma by the presence of one characteristic type of cell, known as the Reed-Sternberg cell. A defining attribute of the Reed-Sternberg cell is its expression of the CD30 antigen.
According to the American Cancer Society, approximately 8,500 cases of Hodgkin lymphoma were diagnosed in the United States during 2010 and more than 1,300 died from the disease. Although front-line combination chemotherapy can result in durable response rates, up to 30 percent of these patients relapse or are refractory to front-line treatment and have few therapeutic options beyond autologous stem cell transplant.
About Seattle Genetics
Seattle Genetics is a clinical-stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The company submitted a Biologics License Application to the U.S. Food and Drug Administration for its lead product candidate, brentuximab vedotin, for the treatment of relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma in February 2011. Brentuximab vedotin is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has four other clinical-stage programs: SGN-75, ASG-5ME, dacetuzumab (SGN-40) and SGN-70. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
Certain of the statements made in this press release are forward-looking, such as those, among others, relating to the potential therapeutic benefit of brentuximab vedotin. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include that the safety and/or efficacy results of the pivotal trial in relapsed or refractory Hodgkin lymphoma and phase II trial in relapsed or refractory systemic ALCL will not be sufficient to gain marketing approval in the United States or any other country, that we will be required to amend our submission for marketing approval or that such submission will be refused. In addition, our regulatory plans may change as a result of consultation with the FDA or EMA. More information about the risks and uncertainties faced by Seattle Genetics is contained in the company’s 10-K for the year ended December 31, 2010 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact:
Seattle Genetics, Inc.Peggy Pinkston, 425-527-4160ppinkston@seagen.com
SGEN heute mit einer Meldung zu neuen Klinikgängen und einer Zusammenfassung des Sachstandes. Es laufen jetzt also 13 klinische Projekte und eines davon steht wohl kurz vor der Zulassung:
(im Vergleich mit dem letzten Update von Hammer ein Partnerprogramm mehr. Da hier von zwei Genentech-INDs die Rede ist, könnte ein Projekt beendet worden sein. Auf jeden Fall macht Genentech extrem Druck. Und das ist extrem wertvoll!)
Seattle Genetics, Inc. (Nasdaq:SGEN) announced today that ten programs utilizing the company’s proprietary antibody-drug conjugate (ADC) technology are now in clinical development across its 11 current ADC collaborations. This includes two additional ADC programs for which Investigational New Drug applications were recently submitted to the U.S. Food and Drug Administration by Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), triggering milestone payments to Seattle Genetics. In addition to the 10 collaborator ADC programs, Seattle Genetics is conducting clinical trials of three other ADCs, including its lead program, brentuximab vedotin (SGN-35).
“Our collaborators’ continued advancement of new ADCs into the clinic illustrates the strong and growing interest in this approach to address the unmet needs of cancer patients,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “Significant progress with ADCs utilizing Seattle Genetics’ technology in multiple ongoing clinical trials is driving substantial technology value and further demonstrates its potential future role in the way cancer is treated.”
The ten collaborator ADCs in clinical development include six by Genentech, and one each by Agensys, an affiliate of Astellas, Bayer, Celldex and Progenics. Seattle Genetics has received more than $155 million to date from its ADC collaborations, and has the potential to receive greater than $3 billion in potential milestone payments plus royalties on future ADC product sales.
(im Vergleich mit dem letzten Update von Hammer ein Partnerprogramm mehr. Da hier von zwei Genentech-INDs die Rede ist, könnte ein Projekt beendet worden sein. Auf jeden Fall macht Genentech extrem Druck. Und das ist extrem wertvoll!)
Seattle Genetics, Inc. (Nasdaq:SGEN) announced today that ten programs utilizing the company’s proprietary antibody-drug conjugate (ADC) technology are now in clinical development across its 11 current ADC collaborations. This includes two additional ADC programs for which Investigational New Drug applications were recently submitted to the U.S. Food and Drug Administration by Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), triggering milestone payments to Seattle Genetics. In addition to the 10 collaborator ADC programs, Seattle Genetics is conducting clinical trials of three other ADCs, including its lead program, brentuximab vedotin (SGN-35).
“Our collaborators’ continued advancement of new ADCs into the clinic illustrates the strong and growing interest in this approach to address the unmet needs of cancer patients,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “Significant progress with ADCs utilizing Seattle Genetics’ technology in multiple ongoing clinical trials is driving substantial technology value and further demonstrates its potential future role in the way cancer is treated.”
The ten collaborator ADCs in clinical development include six by Genentech, and one each by Agensys, an affiliate of Astellas, Bayer, Celldex and Progenics. Seattle Genetics has received more than $155 million to date from its ADC collaborations, and has the potential to receive greater than $3 billion in potential milestone payments plus royalties on future ADC product sales.
Wenn der Kurs 50% tiefer wäre...ja dann wäre die Aktie ein Kauf.
Antwort auf Beitrag Nr.: 41.364.280 von lunatics am 13.04.11 22:02:22Hallo,

der Meinung bin ich im Prinzip auch, aber nachdem ich fast ein ganzes Jahr auf günstige Einstiegskurse gewartet hatte, habe ich doch mal eine erste Position gekauft, da bei positiven Daten mindestens zunächst mit einer netten Aufwärtsbewegung zu rechnen ist.
Auch ist ein gewisser Aufschlag evtl. durch die Technologie gerechtfertigt, aber das wird letzlich erst die Zukunft zeigen.
Sollte der Kurs stark sinken, bin ich mit Nachkäufen dabei (es sei denn, SGN-35 würde floppen).

Gruß q.

der Meinung bin ich im Prinzip auch, aber nachdem ich fast ein ganzes Jahr auf günstige Einstiegskurse gewartet hatte, habe ich doch mal eine erste Position gekauft, da bei positiven Daten mindestens zunächst mit einer netten Aufwärtsbewegung zu rechnen ist.
Auch ist ein gewisser Aufschlag evtl. durch die Technologie gerechtfertigt, aber das wird letzlich erst die Zukunft zeigen.
Sollte der Kurs stark sinken, bin ich mit Nachkäufen dabei (es sei denn, SGN-35 würde floppen).

Gruß q.
Ein neuer Deal mit Genmab. Wäre schön, wenn SGEN künftig regelmäßig Co-Development-Optionen vereinbaren könnte.
Extrem erfolgreich ist mit dieser Strategie bisher Regeneron. Dass SGEN bereits über 3 Co-Optionen verfügt hat, war mir gar nicht bekannt. Sehr schön!
Copenhagen, Denmark and Bothell, WA; April 19, 2011 - Genmab A/S (OMX: GEN) and
Seattle Genetics, Inc. (Nasdaq: SGEN) announced today that the companies have
entered into a second antibody-drug conjugate (ADC) research collaboration
agreement. Under the new agreement, Genmab has rights to utilize Seattle
Genetics´ ADC technology with HuMax-CD74, an antibody in pre-clinical
development to target CD74, which is expressed on a wide range of hematological
malignancies and solid tumors. Seattle Genetics received an undisclosed upfront
payment and has the right to exercise a co-development and co-commercialization
option for any resulting ADC products at the end of Phase I clinical
development.
´We are very pleased to expand our collaboration with Seattle Genetics, who
have been fantastic partners, and at the same time to add a HuMax-CD74 ADC to
Genmab´s pre-clinical product pipeline, ´ said Jan van de Winkel, Ph.D., Chief
Executive Officer of Genmab.
Genmab is responsible for research, manufacturing, pre-clinical development and
Phase I clinical evaluation of ADCs under this new collaboration. Seattle
Genetics will receive research support payments for any assistance provided to
Genmab. If Seattle Genetics opts into an ADC product at the end of Phase I, a
payment would be due to Genmab and the companies would co-develop and share all
future costs and profits for the product on a 50:50 basis. If Seattle Genetics
does not opt in to an ADC product, Genmab would pay Seattle Genetics fees,
milestones and mid-single digit royalties on worldwide net sales of the
product.
´The expanded collaboration with Genmab provides us with another opportunity to
augment our future ADC product pipeline based on data from a phase I clinical
trial, ´ said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. ´We
now have co-development options for four of our collaborators´ ADC programs,
reflecting our ability to maximize the potential of our technology through
strategic collaborations with organizations that have complementary
capabilities.´
Extrem erfolgreich ist mit dieser Strategie bisher Regeneron. Dass SGEN bereits über 3 Co-Optionen verfügt hat, war mir gar nicht bekannt. Sehr schön!
Copenhagen, Denmark and Bothell, WA; April 19, 2011 - Genmab A/S (OMX: GEN) and
Seattle Genetics, Inc. (Nasdaq: SGEN) announced today that the companies have
entered into a second antibody-drug conjugate (ADC) research collaboration
agreement. Under the new agreement, Genmab has rights to utilize Seattle
Genetics´ ADC technology with HuMax-CD74, an antibody in pre-clinical
development to target CD74, which is expressed on a wide range of hematological
malignancies and solid tumors. Seattle Genetics received an undisclosed upfront
payment and has the right to exercise a co-development and co-commercialization
option for any resulting ADC products at the end of Phase I clinical
development.
´We are very pleased to expand our collaboration with Seattle Genetics, who
have been fantastic partners, and at the same time to add a HuMax-CD74 ADC to
Genmab´s pre-clinical product pipeline, ´ said Jan van de Winkel, Ph.D., Chief
Executive Officer of Genmab.
Genmab is responsible for research, manufacturing, pre-clinical development and
Phase I clinical evaluation of ADCs under this new collaboration. Seattle
Genetics will receive research support payments for any assistance provided to
Genmab. If Seattle Genetics opts into an ADC product at the end of Phase I, a
payment would be due to Genmab and the companies would co-develop and share all
future costs and profits for the product on a 50:50 basis. If Seattle Genetics
does not opt in to an ADC product, Genmab would pay Seattle Genetics fees,
milestones and mid-single digit royalties on worldwide net sales of the
product.
´The expanded collaboration with Genmab provides us with another opportunity to
augment our future ADC product pipeline based on data from a phase I clinical
trial, ´ said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. ´We
now have co-development options for four of our collaborators´ ADC programs,
reflecting our ability to maximize the potential of our technology through
strategic collaborations with organizations that have complementary
capabilities.´
Die große Frage der nächsten 14 Tage ist, ob SGEN für Brentuximab Vedotin den priority review Status durch die FDA bekommt. Wenn ja, dürfte im September die Zulassung erfolgen. Wenn nein, würde das kein so günstiges Licht auf das Projekt werfen. Auch für den Kurs dürften die nächsten 2 Wochen aus diesem Grund ziemlich wichtig sein.
Antwort auf Beitrag Nr.: 41.387.247 von SLGramann am 19.04.11 09:16:17Moin,

schade, dass der Kurs von SGEN so gar keine Reaktion zeigt...
Naja, kann ja noch kommen.
Genmab ist ja ein ehemaliger Medarex-Spinoff und nutzt auch deren Technologie. Die Frau von Medarex-Drakeman war ja denn auch Chefin dort. Medarex´ Ipilimumab wurde ja kürzlich zugelassen, die Technologie hat sich also bewährt. Das zusammen mit SGEN´s ADC-Technologie könnte also vielversprechen sein.
Gruß q.

schade, dass der Kurs von SGEN so gar keine Reaktion zeigt...
Naja, kann ja noch kommen.
Genmab ist ja ein ehemaliger Medarex-Spinoff und nutzt auch deren Technologie. Die Frau von Medarex-Drakeman war ja denn auch Chefin dort. Medarex´ Ipilimumab wurde ja kürzlich zugelassen, die Technologie hat sich also bewährt. Das zusammen mit SGEN´s ADC-Technologie könnte also vielversprechen sein.
Gruß q.
Antwort auf Beitrag Nr.: 41.392.855 von quepos am 20.04.11 09:07:07Ja, solche Minikooperationsmeldungen ignoriert der Markt einfach, dafür ist ein mögliches Ergebnis zu weit weg und die Marktkap zu hoch. Zu wenig Einfluss.
Ich bin sehr davon überzeugt, dass jetzt viel am priority review der FDA hängt. Kommt es, gibts wohl einen kleinen Hüpfer im Kurs. Wenn nicht, dürfte eine längere Zeit der Kursschwäche folgen. Entscheidung nach Ostern?
Ich bin sehr davon überzeugt, dass jetzt viel am priority review der FDA hängt. Kommt es, gibts wohl einen kleinen Hüpfer im Kurs. Wenn nicht, dürfte eine längere Zeit der Kursschwäche folgen. Entscheidung nach Ostern?
Moin,

von vielen schon für letzte Woche erwartet, heute hier die Meldung. Dürfte trotz der Erwartung für weitere Zuversicht und etwas Schwung für die Aktie sorgen:
-------
Seattle Genetics Announces FDA Accepts Brentuximab Vedotin BLAs for Filing and Grants Priority Review for Relapsed or Refractory Hodgkin Lymphoma and Systemic ALCL
-PDUFA date set for August 30, 2011-
tweet0EmailPrintCompanies:Seattle Genetics Inc. Related Quotes
Symbol Price Change
SGEN 16.61 0.00
{"s" : "sgen","k" : "a00,a50,b00,b60,c10,g00,h00,l10,p20,t10,v00","o" : "","j" : ""} Press Release Source: Seattle Genetics, Inc. On Monday May 2, 2011, 6:45 am
BOTHELL, Wash.--#BUSINESS WIRE#-- Seattle Genetics, Inc. #Nasdaq:SGEN - News# announced today that the U.S. Food and Drug Administration #FDA# has accepted for filing two Biologics License Applications #BLAs# for brentuximab vedotin, including one for the treatment of patients with relapsed or refractory Hodgkin lymphoma and one for the treatment of patients with relapsed or refractory systemic anaplastic large cell lymphoma #ALCL#. The FDA administratively separated the original BLA submission and will act individually on the application for each indication. In addition, the FDA has granted a six-month priority review of both applications, and has established an action date of August 30, 2011 under the Prescription Drug User Fee Act #PDUFA#. Priority review designation is assigned to drugs that, if approved, would address an unmet medical need for a serious or life-threatening condition. Brentuximab vedotin is an antibody-drug conjugate #ADC# directed to CD30, a defining marker of Hodgkin lymphoma and ALCL.
“These filings and priority review designations are an important step forward in our effort to bring brentuximab vedotin to the many relapsed and refractory Hodgkin lymphoma and systemic ALCL patients in need,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “We look forward to continued interactions with the FDA as they review our brentuximab vedotin BLAs.”
Seattle Genetics announced on February 28, 2011 that it had submitted a BLA for brentuximab vedotin based on results from both a pivotal trial in relapsed or refractory Hodgkin lymphoma and a phase II trial in relapsed or refractory systemic ALCL that were presented at the American Society of Hematology #ASH# Annual Meeting in December 2010. The pivotal trial in Hodgkin lymphoma was conducted under a Special Protocol Assessment #SPA# with the FDA. Brentuximab vedotin has been granted orphan drug designation by the FDA for the treatment of Hodgkin lymphoma and ALCL.
About Brentuximab Vedotin
Brentuximab vedotin is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a potent, synthetic drug, monomethyl auristatin E #MMAE# utiliz

von vielen schon für letzte Woche erwartet, heute hier die Meldung. Dürfte trotz der Erwartung für weitere Zuversicht und etwas Schwung für die Aktie sorgen:
-------
Seattle Genetics Announces FDA Accepts Brentuximab Vedotin BLAs for Filing and Grants Priority Review for Relapsed or Refractory Hodgkin Lymphoma and Systemic ALCL
-PDUFA date set for August 30, 2011-
tweet0EmailPrintCompanies:Seattle Genetics Inc. Related Quotes
Symbol Price Change
SGEN 16.61 0.00
{"s" : "sgen","k" : "a00,a50,b00,b60,c10,g00,h00,l10,p20,t10,v00","o" : "","j" : ""} Press Release Source: Seattle Genetics, Inc. On Monday May 2, 2011, 6:45 am
BOTHELL, Wash.--#BUSINESS WIRE#-- Seattle Genetics, Inc. #Nasdaq:SGEN - News# announced today that the U.S. Food and Drug Administration #FDA# has accepted for filing two Biologics License Applications #BLAs# for brentuximab vedotin, including one for the treatment of patients with relapsed or refractory Hodgkin lymphoma and one for the treatment of patients with relapsed or refractory systemic anaplastic large cell lymphoma #ALCL#. The FDA administratively separated the original BLA submission and will act individually on the application for each indication. In addition, the FDA has granted a six-month priority review of both applications, and has established an action date of August 30, 2011 under the Prescription Drug User Fee Act #PDUFA#. Priority review designation is assigned to drugs that, if approved, would address an unmet medical need for a serious or life-threatening condition. Brentuximab vedotin is an antibody-drug conjugate #ADC# directed to CD30, a defining marker of Hodgkin lymphoma and ALCL.
“These filings and priority review designations are an important step forward in our effort to bring brentuximab vedotin to the many relapsed and refractory Hodgkin lymphoma and systemic ALCL patients in need,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “We look forward to continued interactions with the FDA as they review our brentuximab vedotin BLAs.”
Seattle Genetics announced on February 28, 2011 that it had submitted a BLA for brentuximab vedotin based on results from both a pivotal trial in relapsed or refractory Hodgkin lymphoma and a phase II trial in relapsed or refractory systemic ALCL that were presented at the American Society of Hematology #ASH# Annual Meeting in December 2010. The pivotal trial in Hodgkin lymphoma was conducted under a Special Protocol Assessment #SPA# with the FDA. Brentuximab vedotin has been granted orphan drug designation by the FDA for the treatment of Hodgkin lymphoma and ALCL.
About Brentuximab Vedotin
Brentuximab vedotin is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a potent, synthetic drug, monomethyl auristatin E #MMAE# utiliz
Antwort auf Beitrag Nr.: 41.438.575 von quepos am 02.05.11 13:28:45Hallo,
da fehlte wieder der Rest...

Hier noch mal der ganze Artikel
Gruß q.
----
Press Release Source: Seattle Genetics, Inc. On Monday May 2, 2011, 6:45 am EDT
BOTHELL, Wash.--(BUSINESS WIRE)-- Seattle Genetics, Inc. (Nasdaq:SGEN - News) announced today that the U.S. Food and Drug Administration (FDA) has accepted for filing two Biologics License Applications (BLAs) for brentuximab vedotin, including one for the treatment of patients with relapsed or refractory Hodgkin lymphoma and one for the treatment of patients with relapsed or refractory systemic anaplastic large cell lymphoma (ALCL). The FDA administratively separated the original BLA submission and will act individually on the application for each indication. In addition, the FDA has granted a six-month priority review of both applications, and has established an action date of August 30, 2011 under the Prescription Drug User Fee Act (PDUFA). Priority review designation is assigned to drugs that, if approved, would address an unmet medical need for a serious or life-threatening condition. Brentuximab vedotin is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of Hodgkin lymphoma and ALCL.
“These filings and priority review designations are an important step forward in our effort to bring brentuximab vedotin to the many relapsed and refractory Hodgkin lymphoma and systemic ALCL patients in need,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “We look forward to continued interactions with the FDA as they review our brentuximab vedotin BLAs.”
Seattle Genetics announced on February 28, 2011 that it had submitted a BLA for brentuximab vedotin based on results from both a pivotal trial in relapsed or refractory Hodgkin lymphoma and a phase II trial in relapsed or refractory systemic ALCL that were presented at the American Society of Hematology (ASH) Annual Meeting in December 2010. The pivotal trial in Hodgkin lymphoma was conducted under a Special Protocol Assessment (SPA) with the FDA. Brentuximab vedotin has been granted orphan drug designation by the FDA for the treatment of Hodgkin lymphoma and ALCL.
About Brentuximab Vedotin
Brentuximab vedotin is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a potent, synthetic drug, monomethyl auristatin E (MMAE) utilizing Seattle Genetics' proprietary technology. The ADC employs a novel linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells. This approach is intended to spare non-targeted cells, which may help minimize the potential toxic effects of traditional chemotherapy while allowing for the selective targeting of CD30-expressing cancer cells, thus potentially enhancing the antitumor activity.
Seattle Genetics is developing brentuximab vedotin in collaboration with Millennium: The Takeda Oncology Company. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize brentuximab vedotin in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for brentuximab vedotin on a 50:50 basis, except in Japan where Takeda will be solely responsible for development costs.
About Hodgkin Lymphoma
Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is distinguished from other types of lymphoma by the presence of one characteristic type of cell, known as the Reed-Sternberg cell. A defining attribute of the Reed-Sternberg cell is its expression of the CD30 antigen.
According to the American Cancer Society, approximately 8,500 cases of Hodgkin lymphoma were diagnosed in the United States during 2010 and more than 1,300 people were expected to die from the disease. Although front-line combination chemotherapy can result in durable response rates, up to 30 percent of these patients relapse or are refractory to front-line treatment and have few therapeutic options beyond autologous stem cell transplant.
About Systemic ALCL
ALCL is an aggressive type of T-cell non-Hodgkin lymphoma that highly expresses CD30. In the United States, approximately 2,000 systemic ALCL patients are diagnosed annually. Although front-line combination chemotherapy can result in durable remissions, approximately 50 percent of ALCL patients relapse or are refractory to front-line treatment and have few therapeutic options.
About Seattle Genetics
Seattle Genetics is a clinical-stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The FDA has granted priority review to Biologics License Applications for its lead product candidate, brentuximab vedotin, for the treatment of relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma, with a PDUFA date of August 30 2011. Brentuximab vedotin is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has four other clinical-stage programs: SGN-75, ASG-5ME, dacetuzumab (SGN-40) and SGN-70. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
Certain of the statements made in this press release are forward looking, such as those, among others, relating to the company’s expectations for regulatory approval and commercial launch of brentuximab vedotin. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include risks that data from our clinical trials, including our pivotal Hodgkin lymphoma trial and phase II ALCL trial of brentuximab vedotin, will not support marketing approval for the submitted indications, or that FDA preapproval inspections of our facilities, our manufacturing facilities or inspections of our clinical study sites raise concerns that delay or prevent approval. In addition, we may have to make major amendments to our marketing application and consequently may be delayed in the planned U.S. commercial launch of brentuximab vedotin. Further, brentuximab vedotin may be approved pursuant to the accelerated approval regulations and we may be subject to completing post-marketing requirements and obtaining preapproval of our marketing and promotional materials. More information about the risks and uncertainties faced by Seattle Genetics is contained in the company’s 10-K for the year ended December 31, 2010 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.

da fehlte wieder der Rest...

Hier noch mal der ganze Artikel
Gruß q.
----
Press Release Source: Seattle Genetics, Inc. On Monday May 2, 2011, 6:45 am EDT
BOTHELL, Wash.--(BUSINESS WIRE)-- Seattle Genetics, Inc. (Nasdaq:SGEN - News) announced today that the U.S. Food and Drug Administration (FDA) has accepted for filing two Biologics License Applications (BLAs) for brentuximab vedotin, including one for the treatment of patients with relapsed or refractory Hodgkin lymphoma and one for the treatment of patients with relapsed or refractory systemic anaplastic large cell lymphoma (ALCL). The FDA administratively separated the original BLA submission and will act individually on the application for each indication. In addition, the FDA has granted a six-month priority review of both applications, and has established an action date of August 30, 2011 under the Prescription Drug User Fee Act (PDUFA). Priority review designation is assigned to drugs that, if approved, would address an unmet medical need for a serious or life-threatening condition. Brentuximab vedotin is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of Hodgkin lymphoma and ALCL.
“These filings and priority review designations are an important step forward in our effort to bring brentuximab vedotin to the many relapsed and refractory Hodgkin lymphoma and systemic ALCL patients in need,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “We look forward to continued interactions with the FDA as they review our brentuximab vedotin BLAs.”
Seattle Genetics announced on February 28, 2011 that it had submitted a BLA for brentuximab vedotin based on results from both a pivotal trial in relapsed or refractory Hodgkin lymphoma and a phase II trial in relapsed or refractory systemic ALCL that were presented at the American Society of Hematology (ASH) Annual Meeting in December 2010. The pivotal trial in Hodgkin lymphoma was conducted under a Special Protocol Assessment (SPA) with the FDA. Brentuximab vedotin has been granted orphan drug designation by the FDA for the treatment of Hodgkin lymphoma and ALCL.
About Brentuximab Vedotin
Brentuximab vedotin is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a potent, synthetic drug, monomethyl auristatin E (MMAE) utilizing Seattle Genetics' proprietary technology. The ADC employs a novel linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells. This approach is intended to spare non-targeted cells, which may help minimize the potential toxic effects of traditional chemotherapy while allowing for the selective targeting of CD30-expressing cancer cells, thus potentially enhancing the antitumor activity.
Seattle Genetics is developing brentuximab vedotin in collaboration with Millennium: The Takeda Oncology Company. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize brentuximab vedotin in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for brentuximab vedotin on a 50:50 basis, except in Japan where Takeda will be solely responsible for development costs.
About Hodgkin Lymphoma
Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is distinguished from other types of lymphoma by the presence of one characteristic type of cell, known as the Reed-Sternberg cell. A defining attribute of the Reed-Sternberg cell is its expression of the CD30 antigen.
According to the American Cancer Society, approximately 8,500 cases of Hodgkin lymphoma were diagnosed in the United States during 2010 and more than 1,300 people were expected to die from the disease. Although front-line combination chemotherapy can result in durable response rates, up to 30 percent of these patients relapse or are refractory to front-line treatment and have few therapeutic options beyond autologous stem cell transplant.
About Systemic ALCL
ALCL is an aggressive type of T-cell non-Hodgkin lymphoma that highly expresses CD30. In the United States, approximately 2,000 systemic ALCL patients are diagnosed annually. Although front-line combination chemotherapy can result in durable remissions, approximately 50 percent of ALCL patients relapse or are refractory to front-line treatment and have few therapeutic options.
About Seattle Genetics
Seattle Genetics is a clinical-stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The FDA has granted priority review to Biologics License Applications for its lead product candidate, brentuximab vedotin, for the treatment of relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma, with a PDUFA date of August 30 2011. Brentuximab vedotin is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has four other clinical-stage programs: SGN-75, ASG-5ME, dacetuzumab (SGN-40) and SGN-70. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
Certain of the statements made in this press release are forward looking, such as those, among others, relating to the company’s expectations for regulatory approval and commercial launch of brentuximab vedotin. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include risks that data from our clinical trials, including our pivotal Hodgkin lymphoma trial and phase II ALCL trial of brentuximab vedotin, will not support marketing approval for the submitted indications, or that FDA preapproval inspections of our facilities, our manufacturing facilities or inspections of our clinical study sites raise concerns that delay or prevent approval. In addition, we may have to make major amendments to our marketing application and consequently may be delayed in the planned U.S. commercial launch of brentuximab vedotin. Further, brentuximab vedotin may be approved pursuant to the accelerated approval regulations and we may be subject to completing post-marketing requirements and obtaining preapproval of our marketing and promotional materials. More information about the risks and uncertainties faced by Seattle Genetics is contained in the company’s 10-K for the year ended December 31, 2010 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Moin,

hier mal ein Link zum Webcast von SGEN von gestern, vorgetragen von CBO Dobmeier:
http://investor.seagen.com/phoenix.zhtml?c=124860&p=irol-Eve…
Hört sich alles sehr gut an, auch dass SGEN sich verstärkt als Co-Developer an einzelnen Projekten beteiligen will, also mehr Eigenpipeline und weniger Dienstleister.
(Was ich genauso wie bei Morphosys und anderen für gut und richtig halte).
Auch weitere Partnerschaften werden vorangekündigt. Die Aussichten für SGN-35 sind anscheinend sehr gut.
Auszüge daraus freundlicherweise im Yahoo-Board durch user redhot... bereitgestellt:
------------
Summary of today's SGEN investor presentation - May 3 3-May-11 09:38 am Here are some highlights of today’s investor presentation by SGEN Chief Business Officer Eric Dobmeier during the Deutsche Bank Securities Inc. Health Care Conference in Boston:
- These were the company’s first public comments since the FDA granted priority review to SGN-35 and everything was upbeat. “This is a really exciting time for the company,” Dobmeier said.
- He reviewed the company’s pipeline, with an emphasis on SGN-35, and its ADC technology, with an emphasis on the dozen or so current deals and the prospects of new deals that could be announced soon.
- In response to a question, he said that ADC royalty deals with other biotechs tend to feature royalties “in the mid-single digits,” but as we’ve seen in recent ADC deal announcements, the company now has enough muscle to negotiate co-ownership rights of therapies developed with its ADC technology. “Our business strategy is not necessarily to out-license our technology,” Dobmeier said. “You will see more deals in which we use our technology to build our pipeline [through co-branding].”
- He seemed to predict much higher revenues from ADC deals. “As these programs move into the clinic, as five or so have in the last six months, that’s when the milestones start to get more significant,” he said. “Not a lot of clinical data has been reported yet, but that’s going to change in the near future. Over the next year, we’ll see more attention given to these deals.
- He repeated recent comments about SGN-35 pricing (they’re still working on it, but they plan to be fairly aggressive) and production. “We have established our manufacturing supply chain,” he said. “Everything it proceeding well as we move toward the FDA date of Aug. 30.”
- Every presentation seems to elevate the attention paid to moving SGN-35 into second-line and front-line therapies, and this one was no different. Trials are underway and patients are clamoring to get into them. “Since we reported our data at ASH, there’s been a lot more interest from doctors about ways to get our drug into front-line therapy,” Dobmeier said. “There’s a lot of physician interest into getting this drug into earlier lines of therapy.
- In response to a question, he seemed to say – though he didn’t quite say – that SGEN expects the SGN-35 application to be reviewed by an ODAC – Oncologic Drugs Advisory Committee – en route to a final FDA ruling. I am not knowledgeable enough to say whether this could delay an FDA ruling, but nothing else he said seemed to suggest that SGEN expects a delay beyond Aug. 30.
- His presentation was followed by a large number of questions from the audience. These seems to happen nearly all the time now during these SGEN presentations, a dramatic shift from just a year or so ago, when these presentations did not seem to spark much interest at all from the audiences.
That's all I have. Nothing really brand new, but nothing whatsoever that seemed troubling. A replay soon should be available here: http://investor.seagen.com/phoenix.zhtml...

hier mal ein Link zum Webcast von SGEN von gestern, vorgetragen von CBO Dobmeier:
http://investor.seagen.com/phoenix.zhtml?c=124860&p=irol-Eve…
Hört sich alles sehr gut an, auch dass SGEN sich verstärkt als Co-Developer an einzelnen Projekten beteiligen will, also mehr Eigenpipeline und weniger Dienstleister.
(Was ich genauso wie bei Morphosys und anderen für gut und richtig halte).
Auch weitere Partnerschaften werden vorangekündigt. Die Aussichten für SGN-35 sind anscheinend sehr gut.
Auszüge daraus freundlicherweise im Yahoo-Board durch user redhot... bereitgestellt:
------------
Summary of today's SGEN investor presentation - May 3 3-May-11 09:38 am Here are some highlights of today’s investor presentation by SGEN Chief Business Officer Eric Dobmeier during the Deutsche Bank Securities Inc. Health Care Conference in Boston:
- These were the company’s first public comments since the FDA granted priority review to SGN-35 and everything was upbeat. “This is a really exciting time for the company,” Dobmeier said.
- He reviewed the company’s pipeline, with an emphasis on SGN-35, and its ADC technology, with an emphasis on the dozen or so current deals and the prospects of new deals that could be announced soon.
- In response to a question, he said that ADC royalty deals with other biotechs tend to feature royalties “in the mid-single digits,” but as we’ve seen in recent ADC deal announcements, the company now has enough muscle to negotiate co-ownership rights of therapies developed with its ADC technology. “Our business strategy is not necessarily to out-license our technology,” Dobmeier said. “You will see more deals in which we use our technology to build our pipeline [through co-branding].”
- He seemed to predict much higher revenues from ADC deals. “As these programs move into the clinic, as five or so have in the last six months, that’s when the milestones start to get more significant,” he said. “Not a lot of clinical data has been reported yet, but that’s going to change in the near future. Over the next year, we’ll see more attention given to these deals.
- He repeated recent comments about SGN-35 pricing (they’re still working on it, but they plan to be fairly aggressive) and production. “We have established our manufacturing supply chain,” he said. “Everything it proceeding well as we move toward the FDA date of Aug. 30.”
- Every presentation seems to elevate the attention paid to moving SGN-35 into second-line and front-line therapies, and this one was no different. Trials are underway and patients are clamoring to get into them. “Since we reported our data at ASH, there’s been a lot more interest from doctors about ways to get our drug into front-line therapy,” Dobmeier said. “There’s a lot of physician interest into getting this drug into earlier lines of therapy.
- In response to a question, he seemed to say – though he didn’t quite say – that SGEN expects the SGN-35 application to be reviewed by an ODAC – Oncologic Drugs Advisory Committee – en route to a final FDA ruling. I am not knowledgeable enough to say whether this could delay an FDA ruling, but nothing else he said seemed to suggest that SGEN expects a delay beyond Aug. 30.
- His presentation was followed by a large number of questions from the audience. These seems to happen nearly all the time now during these SGEN presentations, a dramatic shift from just a year or so ago, when these presentations did not seem to spark much interest at all from the audiences.
That's all I have. Nothing really brand new, but nothing whatsoever that seemed troubling. A replay soon should be available here: http://investor.seagen.com/phoenix.zhtml...
Moin,

die Quartalszahlen sind nun auch da, der Text ist relativ lang, daher hier ein Link auf die Seite von SGEN:
http://investor.seagen.com/phoenix.zhtml?c=124860&p=irol-new…
In ganz knappen Worten mein Resumée:
-nix wirklich überraschendes
-Firma offenbar fest überzeugt von der Zulassung von SGEN-35, Vermarktung wird vorbereitet
-Geld genug in der Kasse (auch durch die KE vom Februar und diverse Milestones
Vielleicht gibt´s ja noch ein paar Analystenkommentare.
Soweit erstmal und Grüße
q.

die Quartalszahlen sind nun auch da, der Text ist relativ lang, daher hier ein Link auf die Seite von SGEN:
http://investor.seagen.com/phoenix.zhtml?c=124860&p=irol-new…
In ganz knappen Worten mein Resumée:
-nix wirklich überraschendes
-Firma offenbar fest überzeugt von der Zulassung von SGEN-35, Vermarktung wird vorbereitet
-Geld genug in der Kasse (auch durch die KE vom Februar und diverse Milestones
Vielleicht gibt´s ja noch ein paar Analystenkommentare.
Soweit erstmal und Grüße
q.
Hi quepos,
schön, was so passiert, wenn man im Urlaub ist.
schön, was so passiert, wenn man im Urlaub ist.
War zuletzt wegen des priority review fast nervös geworden, aber nun ist es doch so gekommen wie erwartet. Ich gehe fest von einer Zulassung Ende August aus.
Aus dem Q-Bericht ist vielleicht dies noch eine Erwähnung wert:
SGN-75
* Plan to report additional data from an ongoing phase I clinical trial in patients with relapsed or refractory non-Hodgkin lymphoma or metastatic renal cell carcinoma at the ASCO annual meeting
Mit diesem Projekt darf man einige vorsichtige Hoffnungen verbinden, die mit der ASCO vielleicht gestärkt werden? Warten wirs ab.
Aus dem Q-Bericht ist vielleicht dies noch eine Erwähnung wert:
SGN-75
* Plan to report additional data from an ongoing phase I clinical trial in patients with relapsed or refractory non-Hodgkin lymphoma or metastatic renal cell carcinoma at the ASCO annual meeting
Mit diesem Projekt darf man einige vorsichtige Hoffnungen verbinden, die mit der ASCO vielleicht gestärkt werden? Warten wirs ab.
Antwort auf Beitrag Nr.: 41.503.863 von SLGramann am 14.05.11 21:44:22Hallo,

mache auch noch oft Urlaub ohne Fernsehen und Internet, manchmal habe ich erst auf dem Heimflug den Wirtschaftsteil irgendeiner Zeitung gelesen, aber auch da findet sich natürlich nichts über Aktien wie SGEN.
...
Ja, ist ganz schön gelaufen die letzte Zeit, leider habe ich nicht sehr viele Stücke, da ich nicht zum Aufstocken meiner "Fuß-in-der-Tür-Position" kam. Hatte noch auf eine Schließung des Up-Gaps vom September gewartet, kam ja aber (bisher) nicht. Aber ich will nicht traurig sein, dass die Aktie nicht fällt!

SGN-75 wäre dann der nächste interessante Kandidat, aber erstmal SGN-35...

Grüße q.

mache auch noch oft Urlaub ohne Fernsehen und Internet, manchmal habe ich erst auf dem Heimflug den Wirtschaftsteil irgendeiner Zeitung gelesen, aber auch da findet sich natürlich nichts über Aktien wie SGEN.
...
Ja, ist ganz schön gelaufen die letzte Zeit, leider habe ich nicht sehr viele Stücke, da ich nicht zum Aufstocken meiner "Fuß-in-der-Tür-Position" kam. Hatte noch auf eine Schließung des Up-Gaps vom September gewartet, kam ja aber (bisher) nicht. Aber ich will nicht traurig sein, dass die Aktie nicht fällt!

SGN-75 wäre dann der nächste interessante Kandidat, aber erstmal SGN-35...

Grüße q.
Hier mal das ASCO-Abstract zu SGN 75:
Background: SGN-75 is an ADC composed of a humanized anti-CD70 mAb conjugated to the antimicrotubule agent MMAF, via a plasma-stable linker. Upon binding to CD70, SGN-75 internalizes and releases cys‑mcMMAF, which binds tubulin and induces G2/M arrest and apoptosis. Methods: A phase I, dose-escalation, multicenter study was initiated to investigate the safety, tolerability, PK, pharmacodynamic effects, and antitumor activity of SGN-75 monotherapy in pts with CD70-positive metastatic RCC or relapsed/refractory NHL. SGN-75 was administered IV in cohort-specific doses using 2 schedules (q3wk or q1wk). Pts were eligible to receive cycles of SGN-75 until progression. Results: A total of 26 pts, 14 RCC and 12 NHL, have been treated. Median age was 60 years (range 30–70); all pts had an ECOG status of 0/1. RCC pts had a median 3.5 prior regimens (range 1–7), and all had prior nephrectomy. NHL pts had a median 3 prior regimens (range 1–6), and 2 had prior autoSCT. Doses up to 3 mg/kg q3wk and 0.6 mg/kg q1wk have been tolerated; MTD has not been reached in either dosing schedule. The most common AEs were fatigue (31%), nausea (23%), dyspnea, peripheral edema, and thrombocytopenia (19% each). AEs ≥ Gr 3 observed in >1 pt were thrombocytopenia, dyspnea, and fatigue (3, 2, and 2 pts). Related serious AEs were Gr 2 iridocyclitis and Gr 4 ITP. Preliminary PK analysis demonstrated dose-proportional exposure; mean SGN-75 terminal half-life ranged from 6–13 days across dose levels. Best responses in RCC were 2 PR, 5 SD, 6 PD, and 1 not evaluated (NE); in NHL, responses were 1 CR, 6 SD, 3 PD, and 2 NE. Objective responses were observed at 1 mg/kg q3wk (CR - mantle cell lymphoma; 31 wk duration), 2 mg/kg q3wk (PR - RCC; 7+ wk duration), and 3 mg/kg q3wk (PR - RCC; 2+ days duration). CD70 expression was variable among all patients; the 3 responding pts had nearly uniform CD70 expression on malignant cells. Conclusions: SGN-75 has generally been well tolerated in pts with RCC or NHL; MTD has not been reached in 2 dosing schedules under investigation, and dose escalation is ongoing. Encouraging antitumor activity has been observed in both heavily pretreated RCC pts and in relapsed/refractory NHL pts.
-------------
Präsentation ist am 06.06.:
SGN-75
The effect of SGN-75, a novel antibody-drug conjugate (ADC), in treatment of patients with renal cell carcinoma (RCC) or non-Hodgkin lymphoma (NHL): A phase I study
Monday, June 6, 8:00 a.m.-12:00 p.m. CT
Abstract #3071
First author: Dr. John A. Thompson, Seattle Cancer Care Alliance, Seattle, WA
------------------
Mit einer Einschätzung muss ich mich zurückhalten. Die Ergebnisse scheinen mir die Daten der ESMO einigermaßen zu stützen und könnten möglicherweise positiv interpretierbar sein. Allerdings ist es wohl etwas enttäuschend, dass - trotz Dosiseskalation? - nur eine weitere OR hinzugekommen ist (3 OR bei 26 Patienten...).
Es liegen jetzt Daten von 26 Patienten vor. Auch hier hatte ich mir etwas mehr vorgestellt, denn eigentlich soll der Trial mit über 100 Patienten Anfang 2012 abgeschlossen sein. Dass die Dosiseskalation weitergeht, ist hingegen ermutigend.
Vergleich ESMO ./. ASCO:
RCC:
1 PR ./. 2 PR
3 SD ./. 5 SD
5 PD ./. 6 PD
- ./. 1 NE
NHL:
1 CR ./. 1 CR
4 SD ./. 6 SD
1 PD ./. 3 PD
1 NE ./. 2 NE
Background: SGN-75 is an ADC composed of a humanized anti-CD70 mAb conjugated to the antimicrotubule agent MMAF, via a plasma-stable linker. Upon binding to CD70, SGN-75 internalizes and releases cys‑mcMMAF, which binds tubulin and induces G2/M arrest and apoptosis. Methods: A phase I, dose-escalation, multicenter study was initiated to investigate the safety, tolerability, PK, pharmacodynamic effects, and antitumor activity of SGN-75 monotherapy in pts with CD70-positive metastatic RCC or relapsed/refractory NHL. SGN-75 was administered IV in cohort-specific doses using 2 schedules (q3wk or q1wk). Pts were eligible to receive cycles of SGN-75 until progression. Results: A total of 26 pts, 14 RCC and 12 NHL, have been treated. Median age was 60 years (range 30–70); all pts had an ECOG status of 0/1. RCC pts had a median 3.5 prior regimens (range 1–7), and all had prior nephrectomy. NHL pts had a median 3 prior regimens (range 1–6), and 2 had prior autoSCT. Doses up to 3 mg/kg q3wk and 0.6 mg/kg q1wk have been tolerated; MTD has not been reached in either dosing schedule. The most common AEs were fatigue (31%), nausea (23%), dyspnea, peripheral edema, and thrombocytopenia (19% each). AEs ≥ Gr 3 observed in >1 pt were thrombocytopenia, dyspnea, and fatigue (3, 2, and 2 pts). Related serious AEs were Gr 2 iridocyclitis and Gr 4 ITP. Preliminary PK analysis demonstrated dose-proportional exposure; mean SGN-75 terminal half-life ranged from 6–13 days across dose levels. Best responses in RCC were 2 PR, 5 SD, 6 PD, and 1 not evaluated (NE); in NHL, responses were 1 CR, 6 SD, 3 PD, and 2 NE. Objective responses were observed at 1 mg/kg q3wk (CR - mantle cell lymphoma; 31 wk duration), 2 mg/kg q3wk (PR - RCC; 7+ wk duration), and 3 mg/kg q3wk (PR - RCC; 2+ days duration). CD70 expression was variable among all patients; the 3 responding pts had nearly uniform CD70 expression on malignant cells. Conclusions: SGN-75 has generally been well tolerated in pts with RCC or NHL; MTD has not been reached in 2 dosing schedules under investigation, and dose escalation is ongoing. Encouraging antitumor activity has been observed in both heavily pretreated RCC pts and in relapsed/refractory NHL pts.
-------------
Präsentation ist am 06.06.:
SGN-75
The effect of SGN-75, a novel antibody-drug conjugate (ADC), in treatment of patients with renal cell carcinoma (RCC) or non-Hodgkin lymphoma (NHL): A phase I study
Monday, June 6, 8:00 a.m.-12:00 p.m. CT
Abstract #3071
First author: Dr. John A. Thompson, Seattle Cancer Care Alliance, Seattle, WA
------------------
Mit einer Einschätzung muss ich mich zurückhalten. Die Ergebnisse scheinen mir die Daten der ESMO einigermaßen zu stützen und könnten möglicherweise positiv interpretierbar sein. Allerdings ist es wohl etwas enttäuschend, dass - trotz Dosiseskalation? - nur eine weitere OR hinzugekommen ist (3 OR bei 26 Patienten...).
Es liegen jetzt Daten von 26 Patienten vor. Auch hier hatte ich mir etwas mehr vorgestellt, denn eigentlich soll der Trial mit über 100 Patienten Anfang 2012 abgeschlossen sein. Dass die Dosiseskalation weitergeht, ist hingegen ermutigend.
Vergleich ESMO ./. ASCO:
RCC:
1 PR ./. 2 PR
3 SD ./. 5 SD
5 PD ./. 6 PD
- ./. 1 NE
NHL:
1 CR ./. 1 CR
4 SD ./. 6 SD
1 PD ./. 3 PD
1 NE ./. 2 NE
Ohad Hammer gibt mit Blick auf die ASCO dazu folgenden Kommentar:
Seattle Genetics (SGEN) will provide an update for its anti-CD70 ADC (SGN-75), which is being evaluated in NHL and renal cancer patients. Based on the abstract, this agent is active but the response rate is modest so far even though clinically relevant doses were reached. The efficacy profile could improve as more patients are enrolled at the higher cohorts. Another interesting observation is a correlation between uniform expression of CD70 and response, which could guide investigators towards better patient selection.
Progenics (PGNX) will report data for its ADC against PSMA in prostate cancer patients. Results with this agent, which utilizes Seattle Genetics’ technology continue to be disappointing, with no meaningful signs of clinical activity. The fact that Bayer is not presenting any data for its CA-IX ADC (also utilizing Seattle Genetics’ technology) does not bode well for this program as well.
Next year, however, Seattle Genetics will have multiple shots on goal thanks Genentech’s accelerating ADC pipeline. In less than a year, Genentech advanced 7 new ADC to phase I, all of which are based on Seattle Genetics’ technology. If only one or two of these demonstrate good activity, they could represent important value creation events for the company.
Nun gut, warten wir aufs nächste Jahr...
Seattle Genetics (SGEN) will provide an update for its anti-CD70 ADC (SGN-75), which is being evaluated in NHL and renal cancer patients. Based on the abstract, this agent is active but the response rate is modest so far even though clinically relevant doses were reached. The efficacy profile could improve as more patients are enrolled at the higher cohorts. Another interesting observation is a correlation between uniform expression of CD70 and response, which could guide investigators towards better patient selection.
Progenics (PGNX) will report data for its ADC against PSMA in prostate cancer patients. Results with this agent, which utilizes Seattle Genetics’ technology continue to be disappointing, with no meaningful signs of clinical activity. The fact that Bayer is not presenting any data for its CA-IX ADC (also utilizing Seattle Genetics’ technology) does not bode well for this program as well.
Next year, however, Seattle Genetics will have multiple shots on goal thanks Genentech’s accelerating ADC pipeline. In less than a year, Genentech advanced 7 new ADC to phase I, all of which are based on Seattle Genetics’ technology. If only one or two of these demonstrate good activity, they could represent important value creation events for the company.
Nun gut, warten wir aufs nächste Jahr...

So ist der Stand bei SGN 75:
2011 ASCO Annual Meeting
CHICAGO--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq: SGEN) today announced the presentation of interim data from an ongoing phase I clinical trial of SGN-75 in renal cell carcinoma (RCC) and non-Hodgkin lymphoma at the American Society of Clinical Oncology (ASCO) 2011 Annual Meeting being held in Chicago, IL. SGN-75 is an antibody-drug conjugate (ADC) targeted to CD70. The interim data suggest that SGN-75 is generally well tolerated and induces objective responses in heavily pretreated RCC and non-Hodgkin lymphoma patients.
Among 25 RCC patients treated at escalating doses of SGN-75, two patients achieved a partial response, eight patients had stable disease, 11 patients had progressive disease and four patients were not evaluable for response. At doses of 2.0 milligrams per kilogram (mg/kg) and higher, two of nine patients with RCC who were evaluated for response achieved partial remissions lasting 23 weeks and 19+ weeks. Among 16 non-Hodgkin lymphoma patients treated with escalating doses of SGN-75, one patient with mantle cell lymphoma achieved a complete remission with duration of 31 weeks.
Two schedules of dose administration were tested in this study, a weekly dosing regimen and an every three week dosing regimen. The most common adverse events associated with SGN-75 treatment in the every three week dosing regimen were fatigue (30 percent), nausea (30 percent), dry eye (23 percent) and thrombocytopenia (23 percent). The maximum tolerated dose was determined to be 3.0 mg/kg every three weeks. Based on the observed ADC half life of six to 10 days, increased patient convenience and better safety profile, the every three week dosing schedule has been selected for further study. Enrollment of additional patients in up to 15-patient disease-specific cohorts is continuing at the 3.0 mg/kg every three week dose to further elucidate the safety and antitumor activity of SGN-75. (Abstract #3071)
“Despite the use of immunotherapy, tyrosine kinase inhibitors and mTOR inhibitors, many patients with kidney cancer ultimately experience progression of their disease,” said John A. Thompson, M.D., Professor, Medical Oncology, University of Washington School of Medicine and Seattle Cancer Care Alliance. “SGN-75 represents a novel approach to targeted therapy. These interim data with SGN-75 provide encouraging evidence of antitumor activity in some patients who had experienced treatment failure on other systemic therapies.”
2011 ASCO Annual Meeting
CHICAGO--(BUSINESS WIRE)--Seattle Genetics, Inc. (Nasdaq: SGEN) today announced the presentation of interim data from an ongoing phase I clinical trial of SGN-75 in renal cell carcinoma (RCC) and non-Hodgkin lymphoma at the American Society of Clinical Oncology (ASCO) 2011 Annual Meeting being held in Chicago, IL. SGN-75 is an antibody-drug conjugate (ADC) targeted to CD70. The interim data suggest that SGN-75 is generally well tolerated and induces objective responses in heavily pretreated RCC and non-Hodgkin lymphoma patients.
Among 25 RCC patients treated at escalating doses of SGN-75, two patients achieved a partial response, eight patients had stable disease, 11 patients had progressive disease and four patients were not evaluable for response. At doses of 2.0 milligrams per kilogram (mg/kg) and higher, two of nine patients with RCC who were evaluated for response achieved partial remissions lasting 23 weeks and 19+ weeks. Among 16 non-Hodgkin lymphoma patients treated with escalating doses of SGN-75, one patient with mantle cell lymphoma achieved a complete remission with duration of 31 weeks.
Two schedules of dose administration were tested in this study, a weekly dosing regimen and an every three week dosing regimen. The most common adverse events associated with SGN-75 treatment in the every three week dosing regimen were fatigue (30 percent), nausea (30 percent), dry eye (23 percent) and thrombocytopenia (23 percent). The maximum tolerated dose was determined to be 3.0 mg/kg every three weeks. Based on the observed ADC half life of six to 10 days, increased patient convenience and better safety profile, the every three week dosing schedule has been selected for further study. Enrollment of additional patients in up to 15-patient disease-specific cohorts is continuing at the 3.0 mg/kg every three week dose to further elucidate the safety and antitumor activity of SGN-75. (Abstract #3071)
“Despite the use of immunotherapy, tyrosine kinase inhibitors and mTOR inhibitors, many patients with kidney cancer ultimately experience progression of their disease,” said John A. Thompson, M.D., Professor, Medical Oncology, University of Washington School of Medicine and Seattle Cancer Care Alliance. “SGN-75 represents a novel approach to targeted therapy. These interim data with SGN-75 provide encouraging evidence of antitumor activity in some patients who had experienced treatment failure on other systemic therapies.”
SGEN will mehr vom Kuchen:
Seattle Genetics, Inc. (Nasdaq:SGEN) and Agensys, Inc., an affiliate of Tokyo-based Astellas Pharma Inc. (Tokyo:4503), today announced that Seattle Genetics has exercised an option to co-develop a second antibody-drug conjugate (ADC) under the companies’ existing ADC collaboration agreement. The ADC, known as ASG-22ME (formerly AGS-22M6E), targets the Nectin-4 antigen, which is expressed on multiple solid tumors. During the first quarter of 2011, Agensys submitted an investigational new drug (IND) application to the U.S. Food and Drug Administration (FDA) for a phase I trial of ASG-22ME. Seattle Genetics and Agensys are also co-developing another ADC known as ASG-5ME, which is currently in phase I clinical trials for pancreatic and prostate cancer.
“ASG-22ME is the second ADC we are co-developing under our collaboration with Agensys/Astellas, and the fifteenth ADC using Seattle Genetics’ technology in clinical development across both our internal pipeline and collaborator programs,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “The progress we and Agensys/Astellas are making demonstrates the synergy of combining Seattle Genetics’ innovative, industry-leading ADC technology with Agensys’ proprietary cancer targets and antibodies to develop potential new treatments for patients with cancer.”
“These two co-development programs with Seattle Genetics, coupled with other internal programs such as our AGS-16M8F ADC that is in a phase I trial for renal cell carcinoma, demonstrate our commitment to ADCs and the strength of our growing oncology pipeline,” said Sef Kurstjens, M.D., Ph.D., President and Chief Executive Officer of Agensys. “We look forward to continuing our productive and strong collaboration with Seattle Genetics.”
The single-agent phase I trial will evaluate the safety, tolerability, pharmacokinetic profile and antitumor activity of escalating doses of ASG-22ME. The study is designed to enroll up to 50 patients at multiple centers in the United States.
ASG-22ME is an ADC composed of a fully human antibody directed to Nectin-4, an antigen expressed in multiple cancers including bladder, breast, lung and pancreatic cancers. Preclinically, ASG-22ME has demonstrated potent antitumor activity, including regressions in models of established breast, bladder and lung cancer. The antibody is attached to a potent, synthetic cytotoxic agent, monomethyl auristatin E (MMAE), via an enzyme-cleavable linker using Seattle Genetics’ proprietary technology. The ADC is designed to be stable in the bloodstream, but to release MMAE upon internalization into Nectin-4-expressing tumor cells, resulting in targeted cell-killing.
Seattle Genetics, Inc. (Nasdaq:SGEN) and Agensys, Inc., an affiliate of Tokyo-based Astellas Pharma Inc. (Tokyo:4503), today announced that Seattle Genetics has exercised an option to co-develop a second antibody-drug conjugate (ADC) under the companies’ existing ADC collaboration agreement. The ADC, known as ASG-22ME (formerly AGS-22M6E), targets the Nectin-4 antigen, which is expressed on multiple solid tumors. During the first quarter of 2011, Agensys submitted an investigational new drug (IND) application to the U.S. Food and Drug Administration (FDA) for a phase I trial of ASG-22ME. Seattle Genetics and Agensys are also co-developing another ADC known as ASG-5ME, which is currently in phase I clinical trials for pancreatic and prostate cancer.
“ASG-22ME is the second ADC we are co-developing under our collaboration with Agensys/Astellas, and the fifteenth ADC using Seattle Genetics’ technology in clinical development across both our internal pipeline and collaborator programs,” said Eric L. Dobmeier, Chief Business Officer of Seattle Genetics. “The progress we and Agensys/Astellas are making demonstrates the synergy of combining Seattle Genetics’ innovative, industry-leading ADC technology with Agensys’ proprietary cancer targets and antibodies to develop potential new treatments for patients with cancer.”
“These two co-development programs with Seattle Genetics, coupled with other internal programs such as our AGS-16M8F ADC that is in a phase I trial for renal cell carcinoma, demonstrate our commitment to ADCs and the strength of our growing oncology pipeline,” said Sef Kurstjens, M.D., Ph.D., President and Chief Executive Officer of Agensys. “We look forward to continuing our productive and strong collaboration with Seattle Genetics.”
The single-agent phase I trial will evaluate the safety, tolerability, pharmacokinetic profile and antitumor activity of escalating doses of ASG-22ME. The study is designed to enroll up to 50 patients at multiple centers in the United States.
ASG-22ME is an ADC composed of a fully human antibody directed to Nectin-4, an antigen expressed in multiple cancers including bladder, breast, lung and pancreatic cancers. Preclinically, ASG-22ME has demonstrated potent antitumor activity, including regressions in models of established breast, bladder and lung cancer. The antibody is attached to a potent, synthetic cytotoxic agent, monomethyl auristatin E (MMAE), via an enzyme-cleavable linker using Seattle Genetics’ proprietary technology. The ADC is designed to be stable in the bloodstream, but to release MMAE upon internalization into Nectin-4-expressing tumor cells, resulting in targeted cell-killing.
Seattle Genetics "buy"
20.06.11 11:42
ThinkEquity Partners
Moin,
:#
erstmals sind die 20$ überwunden worden und auch der Schlusskurs liegt über 20.
Das lässt sich ziemlich gut an!
Auch hier in D gibt es ein paar, die die Aktie beobachten. Heute z.B. dieses hier:
----------------------
Rating-Update:
San Francisco #aktiencheck.de AG# - Marko Kozul, Analyst von ThinkEquity Partners, stuft die Aktie von Seattle Genetics #ISIN US8125781026 / WKN 602322# unverändert mit "buy" ein. Das 12-Monats-Kursziel werde nach wie vor bei 26 USD gesehen. #Analyse vom 17.06.2011# #20.06.2011/ac/a/u#
--------------------
26$ wäre ja immerhin 30% noch obendrauf, halte ich aber auch nicht für unrealistisch. Wenn die Restunsicherheit wegen der Zulassung weg ist, sollte es schon noch höhere Kurse geben. Trotz "sell on good news" wäre ja dann der kommerzielle Erfolg zunächst mal sichtbar #Produktion soll ja dann sofort erfolgen# und auch der Rest der Pipeline erscheint dann in neuem Licht.
Da bleibe ich doch weiter dabei!
Gruß q.
20.06.11 11:42
ThinkEquity Partners
Moin,
:#
erstmals sind die 20$ überwunden worden und auch der Schlusskurs liegt über 20.
Das lässt sich ziemlich gut an!
Auch hier in D gibt es ein paar, die die Aktie beobachten. Heute z.B. dieses hier:
----------------------
Rating-Update:
San Francisco #aktiencheck.de AG# - Marko Kozul, Analyst von ThinkEquity Partners, stuft die Aktie von Seattle Genetics #ISIN US8125781026 / WKN 602322# unverändert mit "buy" ein. Das 12-Monats-Kursziel werde nach wie vor bei 26 USD gesehen. #Analyse vom 17.06.2011# #20.06.2011/ac/a/u#
--------------------
26$ wäre ja immerhin 30% noch obendrauf, halte ich aber auch nicht für unrealistisch. Wenn die Restunsicherheit wegen der Zulassung weg ist, sollte es schon noch höhere Kurse geben. Trotz "sell on good news" wäre ja dann der kommerzielle Erfolg zunächst mal sichtbar #Produktion soll ja dann sofort erfolgen# und auch der Rest der Pipeline erscheint dann in neuem Licht.
Da bleibe ich doch weiter dabei!
Gruß q.
Ein schöner Bericht von Luke Timmerman von Xconomy über die Vorbereitungen bei SGEN für den Launch von Brentuximab:
http://www.xconomy.com/seattle/2011/07/05/seattle-genetics-o…
http://www.xconomy.com/seattle/2011/07/05/seattle-genetics-o…
Droht doch noch Ärger auf dem Weg zur Zulassung?
Möglicherweise erfolgt die Zulassung mit Einschränkungen?
Mal wieder die Größe des Trials... Allerdings hatte man ein SPA.
Nach der letztlich nicht verständlichen (und in der Konsequenz amoralischen) Entscheidung der FDA bei T-DM 1 hält man ja vieles für möglich.
Mal sehen, wie die Empfehlung morgen lauten wird. Am wichtigsten ist die Zulassung an sich. Ansonsten gibts off-label und man kann auch Trials nachschieben.
Trial Size May Limit Use Of Seattle Genetics' Experimental Blood Cancer Drug-Reuters
Tuesday, 12 Jul 2011 04:44pm EDT
Reuters reported that U.S. drug reviewers might limit the use of a Seattle Genetics experimental blood cancer drug due to the narrow scope of its clinical trials. In documents released on July 12, 2011, the Food and Drug Administration asked an advisory panel to consider the drug, under the proposed trade name Adcetris, for use in previously treated patients with Hodgkin's lymphoma and anaplastic large cell lymphoma (ALCL). About 9,000 Americans a year are diagnosed with Hodgkin's lymphoma and 3,000 with ALCL. The reviewers suggested the labeling for Hodgkin's should focus on a smaller patient group than expected, limiting potential sales. According to the FDA documents, the drug should be considered for patients who had already been given a stem cell transplant, rather than on all treated patients as suggested by the Company. The FDA advisory panel votes on the drug on July 14, 2011.
Möglicherweise erfolgt die Zulassung mit Einschränkungen?
Mal wieder die Größe des Trials... Allerdings hatte man ein SPA.
Nach der letztlich nicht verständlichen (und in der Konsequenz amoralischen) Entscheidung der FDA bei T-DM 1 hält man ja vieles für möglich.
Mal sehen, wie die Empfehlung morgen lauten wird. Am wichtigsten ist die Zulassung an sich. Ansonsten gibts off-label und man kann auch Trials nachschieben.
Trial Size May Limit Use Of Seattle Genetics' Experimental Blood Cancer Drug-Reuters
Tuesday, 12 Jul 2011 04:44pm EDT
Reuters reported that U.S. drug reviewers might limit the use of a Seattle Genetics experimental blood cancer drug due to the narrow scope of its clinical trials. In documents released on July 12, 2011, the Food and Drug Administration asked an advisory panel to consider the drug, under the proposed trade name Adcetris, for use in previously treated patients with Hodgkin's lymphoma and anaplastic large cell lymphoma (ALCL). About 9,000 Americans a year are diagnosed with Hodgkin's lymphoma and 3,000 with ALCL. The reviewers suggested the labeling for Hodgkin's should focus on a smaller patient group than expected, limiting potential sales. According to the FDA documents, the drug should be considered for patients who had already been given a stem cell transplant, rather than on all treated patients as suggested by the Company. The FDA advisory panel votes on the drug on July 14, 2011.
Antwort auf Beitrag Nr.: 41.779.708 von SLGramann am 13.07.11 07:53:42Hallo,

gibt evtl. einen kleinen Rücksetzer.
Wenn Adcetris hält, was es verspricht, wird der Kurs dennoch mittelfrisitg mit den Umsätzen des Mittels steigen.
Mal sehen, wie die Empfehlung morgen lauten wird. Am wichtigsten ist die Zulassung an sich. Ansonsten gibts off-label und man kann auch Trials nachschieben.
Sehe ich genauso.
Gruß q.

gibt evtl. einen kleinen Rücksetzer.
Wenn Adcetris hält, was es verspricht, wird der Kurs dennoch mittelfrisitg mit den Umsätzen des Mittels steigen.
Mal sehen, wie die Empfehlung morgen lauten wird. Am wichtigsten ist die Zulassung an sich. Ansonsten gibts off-label und man kann auch Trials nachschieben.
Sehe ich genauso.
Gruß q.
Das war der erste Streich und er war ein voller Erfolg!
Der Handel bleibt mit Sicherheit ausgesetzt, bis die Sache ganz über die Bühne ist.
FDA panel backs Seattle Genetics chemotherapy drug
Associated Press, 07.14.11, 11:48 AM EDT
WASHINGTON -- A panel of federal cancer experts has unanimously voted to grant accelerated approval to Seattle Genetics' innovative chemotherapy drug to treat patients with recurring Hodgkin's disease, a cancer of the white blood cells.
All 10 members of the Food and Drug Administration's oncology drug panel voted in favor of approving the drug based on a single study in about 100 patients. Regular approval normally requires two trials. The FDA is not required to follow the group's advice, though it usually does.
"This drug has extremely exciting activity and is a great example of the kind of drug that should go ahead with accelerated approval," said panel chair Dr. Wyndham Wilson of the National Cancer Institute.
The company has submitted its drug, called Adcetris, as a treatment for patients whose cancer has not responded to other drugs or has returned.
Hodgkin's disease and systemic anaplastic large cell lymphoma are both rare cancers that affect the lymphatic system. The panel will vote separately on the use for lymphoma later this afternoon.
Adcetris uses a targeted antibody designed to deliver chemotherapy directly to cancerous tumor cells, sparing healthy cells.
Der Handel bleibt mit Sicherheit ausgesetzt, bis die Sache ganz über die Bühne ist.
FDA panel backs Seattle Genetics chemotherapy drug
Associated Press, 07.14.11, 11:48 AM EDT
WASHINGTON -- A panel of federal cancer experts has unanimously voted to grant accelerated approval to Seattle Genetics' innovative chemotherapy drug to treat patients with recurring Hodgkin's disease, a cancer of the white blood cells.
All 10 members of the Food and Drug Administration's oncology drug panel voted in favor of approving the drug based on a single study in about 100 patients. Regular approval normally requires two trials. The FDA is not required to follow the group's advice, though it usually does.
"This drug has extremely exciting activity and is a great example of the kind of drug that should go ahead with accelerated approval," said panel chair Dr. Wyndham Wilson of the National Cancer Institute.
The company has submitted its drug, called Adcetris, as a treatment for patients whose cancer has not responded to other drugs or has returned.
Hodgkin's disease and systemic anaplastic large cell lymphoma are both rare cancers that affect the lymphatic system. The panel will vote separately on the use for lymphoma later this afternoon.
Adcetris uses a targeted antibody designed to deliver chemotherapy directly to cancerous tumor cells, sparing healthy cells.
Antwort auf Beitrag Nr.: 41.790.088 von SLGramann am 14.07.11 18:09:25

Antwort auf Beitrag Nr.: 41.790.088 von SLGramann am 14.07.11 18:09:25Hallo,

hier auch des Dramas zweiter Teil.
Dann geht´s tatsächlich wohl noch etwas rauf, danach ist aber evtl. erst mal die Luft raus...
Werden sehen.
Gruß q.
--------------------
Seattle Genetics: Adcetris Gets Second OK
By Adam Feuerstein 07/14/11 - 03:39 PM EDT
1 CommentAdd CommentStock quotes in this article:SGEN inShare.1WASHINGTON D.C. (TheStreet) -- Seattle Genetics(SGEN_) brings its "empowered antibody" cancer drug Adcetris in front of an FDA advisory panel today. I'll be following the action and will provide updates here. (Most recent updates will be posted on top.)
More from Adam Feuerstein
Transcept Pharma: FDA Will Reject Sleeping PillExact Sciences' Colon Cancer Screening TrialMore Hot Biotech Trades for Second Half 2011Market Activity
Seattle Genetics Inc.| SGEN UP3:38 pm ET: The FDA panel voted 10-0 to recommend Adcetris for accelerated approval in ALCL. There was ample discussion and little agreement on proposed designs for a follow-on or confirmatory study.

hier auch des Dramas zweiter Teil.
Dann geht´s tatsächlich wohl noch etwas rauf, danach ist aber evtl. erst mal die Luft raus...
Werden sehen.
Gruß q.
--------------------
Seattle Genetics: Adcetris Gets Second OK
By Adam Feuerstein 07/14/11 - 03:39 PM EDT
1 CommentAdd CommentStock quotes in this article:SGEN inShare.1WASHINGTON D.C. (TheStreet) -- Seattle Genetics(SGEN_) brings its "empowered antibody" cancer drug Adcetris in front of an FDA advisory panel today. I'll be following the action and will provide updates here. (Most recent updates will be posted on top.)
More from Adam Feuerstein
Transcept Pharma: FDA Will Reject Sleeping PillExact Sciences' Colon Cancer Screening TrialMore Hot Biotech Trades for Second Half 2011Market Activity
Seattle Genetics Inc.| SGEN UP3:38 pm ET: The FDA panel voted 10-0 to recommend Adcetris for accelerated approval in ALCL. There was ample discussion and little agreement on proposed designs for a follow-on or confirmatory study.
Antwort auf Beitrag Nr.: 41.791.741 von quepos am 14.07.11 22:35:32Sehr schön. 2 x 10:0. Sehr schwer vorstellbar, dass es am 30.08.2011 nun nicht zur Zulassung kommen könnte.
Denke allerdings auch, dass im Kurs schon sehr viel eingepreist ist.
Andererseits: Wirklich langfristig gesehen steht SGEN ganz am Anfang. Sie haben eine innovative, effektive, voll validierte Technologieplattform, jede Menge Partner und darüber einen Haufen Kandidaten in der Klinik.
Da wird in den nächsten Jahren noch viel passieren.
Denke allerdings auch, dass im Kurs schon sehr viel eingepreist ist.
Andererseits: Wirklich langfristig gesehen steht SGEN ganz am Anfang. Sie haben eine innovative, effektive, voll validierte Technologieplattform, jede Menge Partner und darüber einen Haufen Kandidaten in der Klinik.
Da wird in den nächsten Jahren noch viel passieren.
In Amiland war der Kurs bei cnbc 23 $ + ca.13% in Stuttgart gibts noch für um die 14 Euro Stücke
Antwort auf Beitrag Nr.: 41.792.662 von schnappi am 15.07.11 09:21:17gestern Abend im Amiland
Antwort auf Beitrag Nr.: 41.792.662 von schnappi am 15.07.11 09:21:17schnappi, damit wäre ich vorsichtig. Die +13% kamen aus dem "pre-market" und basierten auf gerade mal 100 Stücken Umsatz.
After hours notiert der Kurs bei einigen tausend Stück Umsatz bei ca. -5% und 19,30 Dollar.
Gut möglich, dass heute Gewinnmitnahmen von wilden Tradern kommen. Es wird aber starke Hände geben, die dankbar einsammeln.
Letztlich ist der Kurs heute nicht vorhersehbar. Ich erwarte aber für die nächten Wochen ein wildes Schwanken um die Marke von 20 Dollar (zwischen 18 und 22). Mal sehen.
After hours notiert der Kurs bei einigen tausend Stück Umsatz bei ca. -5% und 19,30 Dollar.
Gut möglich, dass heute Gewinnmitnahmen von wilden Tradern kommen. Es wird aber starke Hände geben, die dankbar einsammeln.
Letztlich ist der Kurs heute nicht vorhersehbar. Ich erwarte aber für die nächten Wochen ein wildes Schwanken um die Marke von 20 Dollar (zwischen 18 und 22). Mal sehen.
Antwort auf Beitrag Nr.: 41.793.130 von SLGramann am 15.07.11 10:26:26Hallo ja ist schon klar ich gehe aber eher von steigenden Kursen hab mit SGEN zur Zeit 120% plus dat tut mal gut 

July 15 (Reuters) - Seattle Genetics Inc: * Jefferies raises Seattle Genetics Inc price target to $25 from $22; rating buy For a summary of rating and price target changes on S&P 500 companies: Reuters Eikon, double-click Reuters 3000Xtra users, double-click Reuters Station users, click .1568 For a summary of rating and price target changes on non-S&P 500 companies: Reuters Eikon, double-click Reuters 3000Xtra users, double-click Reuters Station users, click .2102 For a summary of rating and price target changes on Canadian companies: Reuters Eikon, double-click Reuters 3000Xtra users, double-click Reuters Station users, click .4899 ((Bangalore Equities Newsroom; +91 80 4135 5800; within U.S. +1 646 223 8780)) (For more news, please click here) COPYRIGHT Copyright Thomson Reuters 2011. All rights reserved.
Hier wirft man meiner Meinung nach Haare in Suppe:
Some investors are getting out of Seattle Genetics (SGEN) a day after the company scored a win toward a cancer drug approval in the US.
Shares of the company fell 8% to $18.75 in midday trading Friday after falling by double digits earlier in the morning. Yesterday, Seattle Genetics won the recommendation of a panel of government medical advisers who said that the company’s drug Adcetris should receive an accelerated approval for two types of blood cancer.
Now a sell-off isn’t unusual for a stock that has a big price run-up prior to a catalyst such as a panel meeting. (See Seattle Genetics Moves Closer to Drug Approval.) But the meeting also raised some concerns about market timing and potential of the drug.
The panel advisers twice voted 10-0 to recommend Food and Drug Administration accelerated approval for Adcetris to treat Hodgkin’s lymphoma and anaplastic large cell lymphoma. The FDA is expected to make a decision on the drug by August 30. It’s not bound by the advisers’ recommendations.
The advisers’ endorsements are obviously good news. The FDA wants to get promising drugs out to market quickly and sometimes accelerated approval is granted so patients can get the medicines they need. But companies are required to do follow-up confirmatory studies to make sure the drugs do indeed work and there’s concern whether Seattle Genetics can meet the FDA demands (the company trials so far have shown Adcetris to be highly effective). FDA officials are making it clear that they want agreement with the company on a confirmatory study before they make their decision by the end of next month. There’s a question of labeling restrictions as well given the limitations of the data so far on Adcetris.
“Unanticipated conditional qualifiers and high likelihood of narrow restrictions support our view of limited commercial success,” Canaccord Genuity analyst George Farmer says. He rates Seattle Genetics a sell.
Seattle Genetics plans to eventually seek approval for Adcetris to treat a broader range of patients. It also plans to get overseas approvals with the help of partner Takeda, which will sell the drug outside the US and Canada.
On a conference call Friday morning, Seattle Genetics CEO Clay Siegall assured analysts and investors that the company would reach an agreement with the FDA on a confirmatory trial.
“We have a strong working relationship with the FDA,” Siegall said.
Siegall said yesterday’s panel votes marked “a rare and exciting day” for the company. He noted that unanimous votes are hard to come by.
But Bank of America/ Merrill Lynch analyst Rachel McMinn challenged Siegall on the call, noting apparent tensions between the company and the agency.
“I don’t think you should be celebrating,” McMinn said, adding that she perceived the company has a “massive disconnect with the FDA.”
Richard Pazdur, who heads the FDA’s cancer drug division, announced at Thursday’s meeting that Seattle Genetics had actually requested a regular approval for its drug, which in the agency’s opinion would require more testing. An accelerated approval allows a drug on the market with the understanding that it will do follow-up study to make sure the drug is safe and effective. The FDA recommended that Seattle Genetics seek the accelerated approval but it also wants to make sure that the company has plans in place to complete such a study.
McMinn also took the company to task for not earlier disclosing to investors its application discussions with investors.
Spokeswoman Peggy Pinkston said in a statement, “Seattle Genetics takes disclosure requirements and investor transparency very seriously."
------------------
Vor irgendwelchen follow-up studys hätte ich mal gar keine Angst. Im Gegenteil! Dass das kostet ist natürlich klar, bleibt aber alles im Rahmen imho.
Der Abverkauf ist wohl eher einem sell on good news und der in der Tat sehr hohen Bewertung geschuldet.
Ich würde ja selbst erst wieder bei 15 Dollar nachkaufen...
Some investors are getting out of Seattle Genetics (SGEN) a day after the company scored a win toward a cancer drug approval in the US.
Shares of the company fell 8% to $18.75 in midday trading Friday after falling by double digits earlier in the morning. Yesterday, Seattle Genetics won the recommendation of a panel of government medical advisers who said that the company’s drug Adcetris should receive an accelerated approval for two types of blood cancer.
Now a sell-off isn’t unusual for a stock that has a big price run-up prior to a catalyst such as a panel meeting. (See Seattle Genetics Moves Closer to Drug Approval.) But the meeting also raised some concerns about market timing and potential of the drug.
The panel advisers twice voted 10-0 to recommend Food and Drug Administration accelerated approval for Adcetris to treat Hodgkin’s lymphoma and anaplastic large cell lymphoma. The FDA is expected to make a decision on the drug by August 30. It’s not bound by the advisers’ recommendations.
The advisers’ endorsements are obviously good news. The FDA wants to get promising drugs out to market quickly and sometimes accelerated approval is granted so patients can get the medicines they need. But companies are required to do follow-up confirmatory studies to make sure the drugs do indeed work and there’s concern whether Seattle Genetics can meet the FDA demands (the company trials so far have shown Adcetris to be highly effective). FDA officials are making it clear that they want agreement with the company on a confirmatory study before they make their decision by the end of next month. There’s a question of labeling restrictions as well given the limitations of the data so far on Adcetris.
“Unanticipated conditional qualifiers and high likelihood of narrow restrictions support our view of limited commercial success,” Canaccord Genuity analyst George Farmer says. He rates Seattle Genetics a sell.
Seattle Genetics plans to eventually seek approval for Adcetris to treat a broader range of patients. It also plans to get overseas approvals with the help of partner Takeda, which will sell the drug outside the US and Canada.
On a conference call Friday morning, Seattle Genetics CEO Clay Siegall assured analysts and investors that the company would reach an agreement with the FDA on a confirmatory trial.
“We have a strong working relationship with the FDA,” Siegall said.
Siegall said yesterday’s panel votes marked “a rare and exciting day” for the company. He noted that unanimous votes are hard to come by.
But Bank of America/ Merrill Lynch analyst Rachel McMinn challenged Siegall on the call, noting apparent tensions between the company and the agency.
“I don’t think you should be celebrating,” McMinn said, adding that she perceived the company has a “massive disconnect with the FDA.”
Richard Pazdur, who heads the FDA’s cancer drug division, announced at Thursday’s meeting that Seattle Genetics had actually requested a regular approval for its drug, which in the agency’s opinion would require more testing. An accelerated approval allows a drug on the market with the understanding that it will do follow-up study to make sure the drug is safe and effective. The FDA recommended that Seattle Genetics seek the accelerated approval but it also wants to make sure that the company has plans in place to complete such a study.
McMinn also took the company to task for not earlier disclosing to investors its application discussions with investors.
Spokeswoman Peggy Pinkston said in a statement, “Seattle Genetics takes disclosure requirements and investor transparency very seriously."
------------------
Vor irgendwelchen follow-up studys hätte ich mal gar keine Angst. Im Gegenteil! Dass das kostet ist natürlich klar, bleibt aber alles im Rahmen imho.
Der Abverkauf ist wohl eher einem sell on good news und der in der Tat sehr hohen Bewertung geschuldet.
Ich würde ja selbst erst wieder bei 15 Dollar nachkaufen...
Seattle Genetics Announces Unanimous Recommendations from FDA Advisory Committee in Favor of Accelerated Approval of ADCETRIS for Post-Transplant Relapsed Hodgkin Lymphoma and Relapsed or Refractory Systemic ALCL
14 Jul 2011
Seattle Genetics announced that the Oncologic Drugs Advisory Committee (ODAC) to the U.S. Food and Drug Administration (FDA) voted 10-0 to recommend that the FDA grant accelerated approval of ADCETRIS™ (brentuximab vedotin) for the treatment of patients with Hodgkin lymphoma who relapse after autologous stem cell transplant (ASCT)
BOTHELL, WA, USA | July 14, 2011 | Seattle Genetics, Inc. (Nasdaq:SGEN - News) today announced that the Oncologic Drugs Advisory Committee (ODAC) to the U.S. Food and Drug Administration (FDA) voted 10-0 to recommend that the FDA grant accelerated approval of ADCETRIS™ (brentuximab vedotin) for the treatment of patients with Hodgkin lymphoma who relapse after autologous stem cell transplant (ASCT). In addition, ODAC voted 10-0 to recommend that the FDA grant accelerated approval of ADCETRIS for the treatment of patients with relapsed or refractory systemic anaplastic large cell lymphoma (ALCL). The FDA is expected to act on the two Biologics License Applications (BLAs) for ADCETRIS by August 30, 2011 under the Prescription Drug User Fee Act (PDUFA). ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of Hodgkin lymphoma and ALCL.
“The recommendations by the ODAC panels today are another significant step forward in our development of ADCETRIS,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “If approved, ADCETRIS would be the first in a new class of ADCs, utilizing stable linkers and potent cytotoxic payloads. We look forward to continuing to interact with the FDA in its evaluation of ADCETRIS towards our goal of bringing this CD30-directed drug to patients in need.”
The ADCETRIS BLAs were based primarily on data from a pivotal trial in relapsed or refractory Hodgkin lymphoma that was conducted under a Special Protocol Assessment with the FDA and from a phase II trial in relapsed or refractory systemic ALCL. The FDA is not obligated to follow the guidance of advisory committee panels but normally takes its advice into consideration before making its final decision on approval.
###
About ADCETRIS™
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a potent, synthetic drug, monomethyl auristatin E (MMAE) utilizing Seattle Genetics' proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells. This approach is intended to spare non-targeted cells and thus may help minimize the potential toxic effects of traditional chemotherapy while allowing for the selective targeting of CD30-expressing cancer cells, thus potentially enhancing the antitumor activity.
Seattle Genetics is developing ADCETRIS in collaboration with Millennium: The Takeda Oncology Company. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. In June 2011, the European Medicines Agency accepted the filing of a Marketing Authorization Application (MAA) for ADCETRIS for the treatment of relapsed or refractory Hodgkin lymphoma (HL) and relapsed or refractory systemic anaplastic large cell lymphoma (sALCL). Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where Takeda will be solely responsible for development costs.
About Hodgkin Lymphoma
Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is distinguished from other types of lymphoma by the presence of one characteristic type of cell, known as the Reed-Sternberg cell. A defining attribute of the Reed-Sternberg cell is its expression of the CD30 antigen.
According to the American Cancer Society, more than 8,800 cases of Hodgkin lymphoma will be diagnosed in the United States during 2011 and approximately 1,300 people are expected to die from the disease. Although front-line combination chemotherapy can result in durable response rates, up to 30 percent of these patients relapse or are refractory to front-line treatment and have few therapeutic options beyond ASCT.
About Systemic ALCL
ALCL is an aggressive type of T-cell non-Hodgkin lymphoma that highly expresses CD30. In the United States, approximately 2,000 systemic ALCL patients are diagnosed annually. Although front-line combination chemotherapy can result in durable remissions, approximately 50 percent of ALCL patients relapse or are refractory to front-line treatment and have few therapeutic options.
About Seattle Genetics
Seattle Genetics is a clinical-stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The U.S. Food and Drug Administration has granted priority review to Biologics License Applications for its lead product candidate, ADCETRIS, for the treatment of relapsed or refractory Hodgkin lymphoma and relapsed or refractory systemic anaplastic large cell lymphoma, with a PDUFA date of August 30, 2011. ADCETRIS is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has five other clinical-stage programs: SGN-75, ASG-5ME, ASG-22ME, dacetuzumab (SGN-40) and SGN-70. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
SOURCE: Seattle Genetics, Inc.
14 Jul 2011
Seattle Genetics announced that the Oncologic Drugs Advisory Committee (ODAC) to the U.S. Food and Drug Administration (FDA) voted 10-0 to recommend that the FDA grant accelerated approval of ADCETRIS™ (brentuximab vedotin) for the treatment of patients with Hodgkin lymphoma who relapse after autologous stem cell transplant (ASCT)
BOTHELL, WA, USA | July 14, 2011 | Seattle Genetics, Inc. (Nasdaq:SGEN - News) today announced that the Oncologic Drugs Advisory Committee (ODAC) to the U.S. Food and Drug Administration (FDA) voted 10-0 to recommend that the FDA grant accelerated approval of ADCETRIS™ (brentuximab vedotin) for the treatment of patients with Hodgkin lymphoma who relapse after autologous stem cell transplant (ASCT). In addition, ODAC voted 10-0 to recommend that the FDA grant accelerated approval of ADCETRIS for the treatment of patients with relapsed or refractory systemic anaplastic large cell lymphoma (ALCL). The FDA is expected to act on the two Biologics License Applications (BLAs) for ADCETRIS by August 30, 2011 under the Prescription Drug User Fee Act (PDUFA). ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of Hodgkin lymphoma and ALCL.
“The recommendations by the ODAC panels today are another significant step forward in our development of ADCETRIS,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “If approved, ADCETRIS would be the first in a new class of ADCs, utilizing stable linkers and potent cytotoxic payloads. We look forward to continuing to interact with the FDA in its evaluation of ADCETRIS towards our goal of bringing this CD30-directed drug to patients in need.”
The ADCETRIS BLAs were based primarily on data from a pivotal trial in relapsed or refractory Hodgkin lymphoma that was conducted under a Special Protocol Assessment with the FDA and from a phase II trial in relapsed or refractory systemic ALCL. The FDA is not obligated to follow the guidance of advisory committee panels but normally takes its advice into consideration before making its final decision on approval.
###
About ADCETRIS™
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a potent, synthetic drug, monomethyl auristatin E (MMAE) utilizing Seattle Genetics' proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells. This approach is intended to spare non-targeted cells and thus may help minimize the potential toxic effects of traditional chemotherapy while allowing for the selective targeting of CD30-expressing cancer cells, thus potentially enhancing the antitumor activity.
Seattle Genetics is developing ADCETRIS in collaboration with Millennium: The Takeda Oncology Company. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. In June 2011, the European Medicines Agency accepted the filing of a Marketing Authorization Application (MAA) for ADCETRIS for the treatment of relapsed or refractory Hodgkin lymphoma (HL) and relapsed or refractory systemic anaplastic large cell lymphoma (sALCL). Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where Takeda will be solely responsible for development costs.
About Hodgkin Lymphoma
Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is distinguished from other types of lymphoma by the presence of one characteristic type of cell, known as the Reed-Sternberg cell. A defining attribute of the Reed-Sternberg cell is its expression of the CD30 antigen.
According to the American Cancer Society, more than 8,800 cases of Hodgkin lymphoma will be diagnosed in the United States during 2011 and approximately 1,300 people are expected to die from the disease. Although front-line combination chemotherapy can result in durable response rates, up to 30 percent of these patients relapse or are refractory to front-line treatment and have few therapeutic options beyond ASCT.
About Systemic ALCL
ALCL is an aggressive type of T-cell non-Hodgkin lymphoma that highly expresses CD30. In the United States, approximately 2,000 systemic ALCL patients are diagnosed annually. Although front-line combination chemotherapy can result in durable remissions, approximately 50 percent of ALCL patients relapse or are refractory to front-line treatment and have few therapeutic options.
About Seattle Genetics
Seattle Genetics is a clinical-stage biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease. The U.S. Food and Drug Administration has granted priority review to Biologics License Applications for its lead product candidate, ADCETRIS, for the treatment of relapsed or refractory Hodgkin lymphoma and relapsed or refractory systemic anaplastic large cell lymphoma, with a PDUFA date of August 30, 2011. ADCETRIS is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has five other clinical-stage programs: SGN-75, ASG-5ME, ASG-22ME, dacetuzumab (SGN-40) and SGN-70. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
SOURCE: Seattle Genetics, Inc.
sorry, wurde ja schon gepostet; naja dann seht es als meinen Einstand hier an 

hier gibts im Moment auch kein halten mehr
noch wer da die News wird eher zum verkaufen genutzt scheint es
BOTHELL, Wash., Aug 19, 2011 (BUSINESS WIRE) -- ---Seattle Genetics to implement comprehensive reimbursement support and patient assistance program- ---Conference call Monday, August 22, 2011, at 8:30 a.m. ET to provide further information on the launch and commercialization of ADCETRIS- Seattle Genetics, Inc. (Nasdaq: SGEN) today announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval of ADCETRIS(TM) (brentuximab vedotin) for two indications: (1) the treatment of patients with Hodgkin lymphoma after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates, and (2) the treatment of patients with systemic anaplastic large cell lymphoma (ALCL) after failure of at least one prior multi-agent chemotherapy regimen. The indications for ADCETRIS are based on response rate. There are no data available demonstrating improvement in patient-reported outcomes or survival with ADCETRIS. ADCETRIS is the first drug approved by the FDA for Hodgkin lymphoma in more than 30 years, and provides a new therapeutic alternative for Hodgkin lymphoma and systemic ALCL in these settings. Seattle Genetics expects to make ADCETRIS available to patients next week. ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30.
"Bringing a new product to the market is a significant milestone for Seattle Genetics in fulfilling its mission to improve the lives of people with cancer," said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. "The approval of ADCETRIS is a result of more than a decade of research and development by talented scientists and physicians. The company has deep appreciation for the hundreds of patients who participated in ADCETRIS trials, and the passion and determination of the clinicians at sites around the world in investigating this first in a new class of targeted anticancer agents.
We are committed to continued clinical investigation of ADCETRIS through a broad development program for CD30-positive malignancies, including confirmatory trials in front-line Hodgkin and T-cell lymphomas that we have planned in consultation with the FDA." "The marked single agent activity seen with ADCETRIS, including a high durable complete remission rate, offers an opportunity to improve the treatment paradigm of patients for whom the treatment is indicated," said Owen A. O'Connor, M.D., Ph.D., Professor, and Director, Division of Hematology and Medical Oncology at NYU Cancer Institute. "This approval represents a major advancement in the care of these patients." Seattle Genetics also announced that it has established a patient assistance program named SeaGen Secure(TM) that offers patients and providers access to ADCETRIS reimbursement support, benefit investigations and patient assistance programs. More information about SeaGen Secure is available at (855)-4SEAGEN (855-473-2436) Monday through Friday from 9:00 a.m. to 8:00 p.m. Eastern Time.
About ADCETRIS ADCETRIS (brentuximab vedotin) is an antibody-drug conjugate (ADC) comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics' proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
The ADCETRIS approvals were based on data from two open-label, single-arm clinical trials: a pivotal trial in Hodgkin lymphoma patients who relapsed after ASCT and a pivotal trial in relapsed systemic ALCL patients. The primary endpoint of both trials was overall response rate as assessed by an independent review facility.
In the pivotal Hodgkin lymphoma clinical trial, 102 patients were enrolled who had relapsed after ASCT. Data demonstrated that 73 percent (95 percent CI 65, 83) of patients achieved an objective response following treatment with ADCETRIS, including 32 percent (95 percent CI 23, 42) with complete remissions and 40 percent with partial remissions (95 percent CI 32, 49). The median duration of objective response was 6.7 months (95 percent CI 4.0, 14.8; range 1.3 to 21.9+ months).
In the pivotal systemic ALCL clinical trial, 58 patients with relapsed disease were enrolled. These data demonstrated that 86 percent (95 percent CI 77, 95) of patients achieved an objective response following treatment with ADCETRIS, including 57 percent with complete remissions (95 percent CI 44, 70) and 29 percent with partial remissions (95 percent CI 18, 41). The median duration of objective response was 12.6 months (95 percent CI 5.7, not estimable; range 0.1 to 15.9+ months).
Please see important safety information below, and the full prescribing information for ADCETRIS at www.seattlegenetics.com or www.ADCETRIS.com.
Indications and Usage ADCETRIS is a CD30-directed antibody-drug conjugate indicated for the treatment of patients with Hodgkin lymphoma after failure of ASCT or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates, and for the treatment of patients with systemic ALCL after failure of at least one prior multi-agent chemotherapy regimen. The indications for ADCETRIS are based on response rate. There are no data available demonstrating improvement in patient-reported outcomes or survival with ADCETRIS.
The recommended dose of ADCETRIS is 1.8 milligrams per kilogram administered only as an intravenous infusion over 30 minutes every three weeks. Treatment should be continued until a maximum of 16 cycles, disease progression or unacceptable toxicity.
Important Safety Information Warnings and Precautions: -- Peripheral neuropathy: ADCETRIS treatment causes a peripheral neuropathy that is predominantly sensory. Cases of peripheral motor neuropathy have also been reported. ADCETRIS-induced peripheral neuropathy is cumulative. Treating physicians should monitor patients for neuropathy and institute dose modifications accordingly.
-- Infusion reactions: Infusion-related reactions, including anaphylaxis, have occurred with ADCETRIS. Monitor patients during infusion. If an infusion reaction occurs, the infusion should be interrupted and appropriate medical management instituted. If anaphylaxis occurs, the infusion should be discontinued immediately and appropriate medical management instituted.
-- Neutropenia: Monitor complete blood counts prior to each dose of ADCETRIS. If Grade 3 or 4 neutropenia develops, manage by dose delays, reductions or discontinuation. Prolonged (greater-than or equal to 1 week) severe neutropenia can occur with ADCETRIS.
-- Tumor Lysis Syndrome: Patients with rapidly proliferating tumor and high tumor burden are at risk of tumor lysis syndrome and these patients should be monitored closely and appropriate measures taken.
-- Stevens-Johnson syndrome: Stevens-Johnson syndrome has been reported with ADCETRIS. If Stevens-Johnson syndrome occurs, discontinue ADCETRIS and administer appropriate medical therapy.
-- Progressive Multifocal Leukoencephalopathy (PML): A fatal case of PML has been reported in a patient who received four chemotherapy regimens prior to receiving ADCETRIS.
-- Use in pregnancy: Fetal harm can occur. Pregnant women should be advised of the potential hazard to the fetus.
Adverse Reactions: ADCETRIS was studied as monotherapy in 160 patients in two phase II trials.
Across both trials, the most common adverse reactions (greater-than or equal to 20%), regardless of causality, were neutropenia, peripheral sensory neuropathy, fatigue, nausea, anemia, upper respiratory tract infection, diarrhea, pyrexia, rash, thrombocytopenia, cough and vomiting.
Drug Interactions: Patients who are receiving strong CYP3A4 inhibitors concomitantly with ADCETRIS should be closely monitored for adverse reactions.
For additional important safety information, please see the full prescribing information for ADCETRIS at www.seattlegenetics.com or www.ADCETRIS.com.
Seattle Genetics is jointly developing ADCETRIS with Millennium: The Takeda Oncology Company. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
Conference Call Details Seattle Genetics' management will host a conference call and webcast to discuss the approval, the price of ADCETRIS, and the company's reimbursement and patient assistance program. The call will be held Monday, August 22, 2011 at 5:30 a.m.
Pacific Time (PT); 8:30 a.m. Eastern Time (ET). The live event will be available from Seattle Genetics' website at www.seattlegenetics.com, under the Investors and News section, or by calling (877) 941-8609 (domestic) or (480) 629-9818 (international). The access code is 4466950. A replay of the discussion will be available beginning at approximately 7:30 a.m. PT on August 22, 2011 from Seattle Genetics' website or by calling (800) 406-7325 (domestic) or (303) 590-3030 (international), using access code 4466950. The telephone replay will be available until 5:00 p.m. PT on August 24, 2011.
More information about ADCETRIS is available at (855)-4SEAGEN (855-473-2436) Monday through Friday from 9:00 a.m. to 8:00 p.m. Eastern Time.
About Hodgkin Lymphoma and Systemic ALCL Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is distinguished from other types of lymphoma by the presence of one characteristic type of cell, known as the Reed-Sternberg cell(1). The Reed-Sternberg cell generally expresses CD30(2).
Systemic ALCL is an aggressive type of T-cell non-Hodgkin lymphoma that also expresses CD30(2).
About Seattle Genetics Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer. Seattle Genetics' first product, ADCETRIS(TM), was approved by the FDA on August 19, 2011 for the treatment of patients with Hodgkin lymphoma after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates, and for the treatment of patients with systemic anaplastic large cell lymphoma (ALCL) after failure of at least one prior multi-agent chemotherapy regimen. In addition to ADCETRIS, Seattle Genetics has three other clinical-stage programs: SGN-75, ASG-5ME and ASG-22ME. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
Certain of the statements made in this press release are forward looking, such as those, among others, relating to the company's statements regarding the potential for ADCETRIS to offer an improved treatment paradigm of patients for whom the treatment is indicated, and other statements that are other than statements of historical facts. Actual results or developments may differ materially from those projected or implied in these forward-looking statements.
Factors that may cause such a difference include risks and uncertainties associated with fulfilling sales, marketing and distribution requirements; the acceptance of ADCETRIS in the marketplace; the status of reimbursement from third-party payors; the company's dependence on third-party manufacturers; and the company's compliance with applicable regulatory requirements, including the healthcare fraud and abuse laws and the company's post-marketing requirements.
More information about the risks and uncertainties faced by Seattle Genetics is contained in the company's 10-Q for the quarter ended June 30, 2011, filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
(1)National Cancer Institute. Hodgkin lymphoma. Available at http://www.cancer.gov/cancertopics/types/hodgkin. Accessed August 15, 2011.
(2)Haluska FG, et al. The Cellular Biology of the Reed-Sternberg Cell. Blood.
1994; 84:1005-1019.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6835336&lang… SOURCE: Seattle Genetics, Inc.
CONTACT: Seattle Genetics, Inc. Peggy Pinkston, 425-527-4160 ppinkston@seagen.com Copyright Business Wire 2011 -0- KEYWORD: United States
North America
Washington INDUSTRY KEYWORD: Stem Cells
Health
Biotechnology
Oncology
Pharmaceutical
FDA SUBJECT CODE: Product/Service
Photo/Multimedia
BOTHELL, Wash., Aug 19, 2011 (BUSINESS WIRE) -- ---Seattle Genetics to implement comprehensive reimbursement support and patient assistance program- ---Conference call Monday, August 22, 2011, at 8:30 a.m. ET to provide further information on the launch and commercialization of ADCETRIS- Seattle Genetics, Inc. (Nasdaq: SGEN) today announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval of ADCETRIS(TM) (brentuximab vedotin) for two indications: (1) the treatment of patients with Hodgkin lymphoma after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates, and (2) the treatment of patients with systemic anaplastic large cell lymphoma (ALCL) after failure of at least one prior multi-agent chemotherapy regimen. The indications for ADCETRIS are based on response rate. There are no data available demonstrating improvement in patient-reported outcomes or survival with ADCETRIS. ADCETRIS is the first drug approved by the FDA for Hodgkin lymphoma in more than 30 years, and provides a new therapeutic alternative for Hodgkin lymphoma and systemic ALCL in these settings. Seattle Genetics expects to make ADCETRIS available to patients next week. ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30.
"Bringing a new product to the market is a significant milestone for Seattle Genetics in fulfilling its mission to improve the lives of people with cancer," said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. "The approval of ADCETRIS is a result of more than a decade of research and development by talented scientists and physicians. The company has deep appreciation for the hundreds of patients who participated in ADCETRIS trials, and the passion and determination of the clinicians at sites around the world in investigating this first in a new class of targeted anticancer agents.
We are committed to continued clinical investigation of ADCETRIS through a broad development program for CD30-positive malignancies, including confirmatory trials in front-line Hodgkin and T-cell lymphomas that we have planned in consultation with the FDA." "The marked single agent activity seen with ADCETRIS, including a high durable complete remission rate, offers an opportunity to improve the treatment paradigm of patients for whom the treatment is indicated," said Owen A. O'Connor, M.D., Ph.D., Professor, and Director, Division of Hematology and Medical Oncology at NYU Cancer Institute. "This approval represents a major advancement in the care of these patients." Seattle Genetics also announced that it has established a patient assistance program named SeaGen Secure(TM) that offers patients and providers access to ADCETRIS reimbursement support, benefit investigations and patient assistance programs. More information about SeaGen Secure is available at (855)-4SEAGEN (855-473-2436) Monday through Friday from 9:00 a.m. to 8:00 p.m. Eastern Time.
About ADCETRIS ADCETRIS (brentuximab vedotin) is an antibody-drug conjugate (ADC) comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics' proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
The ADCETRIS approvals were based on data from two open-label, single-arm clinical trials: a pivotal trial in Hodgkin lymphoma patients who relapsed after ASCT and a pivotal trial in relapsed systemic ALCL patients. The primary endpoint of both trials was overall response rate as assessed by an independent review facility.
In the pivotal Hodgkin lymphoma clinical trial, 102 patients were enrolled who had relapsed after ASCT. Data demonstrated that 73 percent (95 percent CI 65, 83) of patients achieved an objective response following treatment with ADCETRIS, including 32 percent (95 percent CI 23, 42) with complete remissions and 40 percent with partial remissions (95 percent CI 32, 49). The median duration of objective response was 6.7 months (95 percent CI 4.0, 14.8; range 1.3 to 21.9+ months).
In the pivotal systemic ALCL clinical trial, 58 patients with relapsed disease were enrolled. These data demonstrated that 86 percent (95 percent CI 77, 95) of patients achieved an objective response following treatment with ADCETRIS, including 57 percent with complete remissions (95 percent CI 44, 70) and 29 percent with partial remissions (95 percent CI 18, 41). The median duration of objective response was 12.6 months (95 percent CI 5.7, not estimable; range 0.1 to 15.9+ months).
Please see important safety information below, and the full prescribing information for ADCETRIS at www.seattlegenetics.com or www.ADCETRIS.com.
Indications and Usage ADCETRIS is a CD30-directed antibody-drug conjugate indicated for the treatment of patients with Hodgkin lymphoma after failure of ASCT or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates, and for the treatment of patients with systemic ALCL after failure of at least one prior multi-agent chemotherapy regimen. The indications for ADCETRIS are based on response rate. There are no data available demonstrating improvement in patient-reported outcomes or survival with ADCETRIS.
The recommended dose of ADCETRIS is 1.8 milligrams per kilogram administered only as an intravenous infusion over 30 minutes every three weeks. Treatment should be continued until a maximum of 16 cycles, disease progression or unacceptable toxicity.
Important Safety Information Warnings and Precautions: -- Peripheral neuropathy: ADCETRIS treatment causes a peripheral neuropathy that is predominantly sensory. Cases of peripheral motor neuropathy have also been reported. ADCETRIS-induced peripheral neuropathy is cumulative. Treating physicians should monitor patients for neuropathy and institute dose modifications accordingly.
-- Infusion reactions: Infusion-related reactions, including anaphylaxis, have occurred with ADCETRIS. Monitor patients during infusion. If an infusion reaction occurs, the infusion should be interrupted and appropriate medical management instituted. If anaphylaxis occurs, the infusion should be discontinued immediately and appropriate medical management instituted.
-- Neutropenia: Monitor complete blood counts prior to each dose of ADCETRIS. If Grade 3 or 4 neutropenia develops, manage by dose delays, reductions or discontinuation. Prolonged (greater-than or equal to 1 week) severe neutropenia can occur with ADCETRIS.
-- Tumor Lysis Syndrome: Patients with rapidly proliferating tumor and high tumor burden are at risk of tumor lysis syndrome and these patients should be monitored closely and appropriate measures taken.
-- Stevens-Johnson syndrome: Stevens-Johnson syndrome has been reported with ADCETRIS. If Stevens-Johnson syndrome occurs, discontinue ADCETRIS and administer appropriate medical therapy.
-- Progressive Multifocal Leukoencephalopathy (PML): A fatal case of PML has been reported in a patient who received four chemotherapy regimens prior to receiving ADCETRIS.
-- Use in pregnancy: Fetal harm can occur. Pregnant women should be advised of the potential hazard to the fetus.
Adverse Reactions: ADCETRIS was studied as monotherapy in 160 patients in two phase II trials.
Across both trials, the most common adverse reactions (greater-than or equal to 20%), regardless of causality, were neutropenia, peripheral sensory neuropathy, fatigue, nausea, anemia, upper respiratory tract infection, diarrhea, pyrexia, rash, thrombocytopenia, cough and vomiting.
Drug Interactions: Patients who are receiving strong CYP3A4 inhibitors concomitantly with ADCETRIS should be closely monitored for adverse reactions.
For additional important safety information, please see the full prescribing information for ADCETRIS at www.seattlegenetics.com or www.ADCETRIS.com.
Seattle Genetics is jointly developing ADCETRIS with Millennium: The Takeda Oncology Company. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
Conference Call Details Seattle Genetics' management will host a conference call and webcast to discuss the approval, the price of ADCETRIS, and the company's reimbursement and patient assistance program. The call will be held Monday, August 22, 2011 at 5:30 a.m.
Pacific Time (PT); 8:30 a.m. Eastern Time (ET). The live event will be available from Seattle Genetics' website at www.seattlegenetics.com, under the Investors and News section, or by calling (877) 941-8609 (domestic) or (480) 629-9818 (international). The access code is 4466950. A replay of the discussion will be available beginning at approximately 7:30 a.m. PT on August 22, 2011 from Seattle Genetics' website or by calling (800) 406-7325 (domestic) or (303) 590-3030 (international), using access code 4466950. The telephone replay will be available until 5:00 p.m. PT on August 24, 2011.
More information about ADCETRIS is available at (855)-4SEAGEN (855-473-2436) Monday through Friday from 9:00 a.m. to 8:00 p.m. Eastern Time.
About Hodgkin Lymphoma and Systemic ALCL Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is distinguished from other types of lymphoma by the presence of one characteristic type of cell, known as the Reed-Sternberg cell(1). The Reed-Sternberg cell generally expresses CD30(2).
Systemic ALCL is an aggressive type of T-cell non-Hodgkin lymphoma that also expresses CD30(2).
About Seattle Genetics Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer. Seattle Genetics' first product, ADCETRIS(TM), was approved by the FDA on August 19, 2011 for the treatment of patients with Hodgkin lymphoma after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates, and for the treatment of patients with systemic anaplastic large cell lymphoma (ALCL) after failure of at least one prior multi-agent chemotherapy regimen. In addition to ADCETRIS, Seattle Genetics has three other clinical-stage programs: SGN-75, ASG-5ME and ASG-22ME. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
Certain of the statements made in this press release are forward looking, such as those, among others, relating to the company's statements regarding the potential for ADCETRIS to offer an improved treatment paradigm of patients for whom the treatment is indicated, and other statements that are other than statements of historical facts. Actual results or developments may differ materially from those projected or implied in these forward-looking statements.
Factors that may cause such a difference include risks and uncertainties associated with fulfilling sales, marketing and distribution requirements; the acceptance of ADCETRIS in the marketplace; the status of reimbursement from third-party payors; the company's dependence on third-party manufacturers; and the company's compliance with applicable regulatory requirements, including the healthcare fraud and abuse laws and the company's post-marketing requirements.
More information about the risks and uncertainties faced by Seattle Genetics is contained in the company's 10-Q for the quarter ended June 30, 2011, filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
(1)National Cancer Institute. Hodgkin lymphoma. Available at http://www.cancer.gov/cancertopics/types/hodgkin. Accessed August 15, 2011.
(2)Haluska FG, et al. The Cellular Biology of the Reed-Sternberg Cell. Blood.
1994; 84:1005-1019.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6835336&lang… SOURCE: Seattle Genetics, Inc.
CONTACT: Seattle Genetics, Inc. Peggy Pinkston, 425-527-4160 ppinkston@seagen.com Copyright Business Wire 2011 -0- KEYWORD: United States
North America
Washington INDUSTRY KEYWORD: Stem Cells
Health
Biotechnology
Oncology
Pharmaceutical
FDA SUBJECT CODE: Product/Service
Photo/Multimedia
Antwort auf Beitrag Nr.: 41.978.507 von schnappi am 19.08.11 21:16:16Ups, das kam ein paar Tage eher als erwartet. Ich kann jetzt allerdings nicht beurteilen, ob die Zulassung stärker beschränkt wurde, als früher mal gedacht.
Wie auch immer: Seit der Markt die nächste Rezession einpreist und das Dendreon-Desaster den Biotech-Markt erst mal versaut hat, ist die Stimmung extrem negativ.
Wenn ich mir so HGS anschaue... na ja... die spinnen doch, oder?
Was SGEN angeht: Ich sehe das Unternehmen als eine langfristige Kerninvestition im Biotech-Bereich.
Adcetris ist der Anfang und nicht das Ende der Geschichte!
Mal ein Blick nur auf die aktuelle Partner-Pipeline:

Daneben hat SGEN noch zumindest 3 eigene interessante Programme am Laufen.
Wie auch immer: Seit der Markt die nächste Rezession einpreist und das Dendreon-Desaster den Biotech-Markt erst mal versaut hat, ist die Stimmung extrem negativ.
Wenn ich mir so HGS anschaue... na ja... die spinnen doch, oder?
Was SGEN angeht: Ich sehe das Unternehmen als eine langfristige Kerninvestition im Biotech-Bereich.
Adcetris ist der Anfang und nicht das Ende der Geschichte!
Mal ein Blick nur auf die aktuelle Partner-Pipeline:

Daneben hat SGEN noch zumindest 3 eigene interessante Programme am Laufen.
Antwort auf Beitrag Nr.: 41.978.507 von schnappi am 19.08.11 21:16:16Seattle Genetics Wins FDA Approval of First Drug, a New Treatment for Lymphomas
Luke Timmerman 8/19/11
Seattle Genetics’ big day has arrived, as it has won FDA clearance to start selling its first new drug on the U.S. market after 14 years in business.
The company has received FDA clearance to start marketing brentuximab vedotin (Adcetris) as a new treatment for U.S. patients with a pair of rare lymphomas—Hodgkin’s disease and anaplastic large-cell lymphoma. The drug was given approval under the faster-than-usual six-month review cycle the FDA sometimes grants to potentially lifesaving therapies, and the official word came a little bit faster than the agency’s legal review deadline of Aug. 30. The regulatory action was really no surprise, since Seattle Genetics won a 10-0 approval recommendation from an FDA advisory panel last month.
“Early clinical data suggest that patients who received Adcetris for Hodgkin lymphoma and systemic anaplastic lymphoma experienced a significant response to the therapy,” said Richard Pazdur, the director of the FDA’s cancer drug review office, in a statement.
The FDA approval is the biggest moment in the history of Seattle Genetics (NASDAQ: SGEN), a company that was founded in 1997 and has run up a deficit of more than $546 million to get to this point, according to its most recent quarterly report.
The company’s new drug represents a significant step ahead for both the science of antibody drug development, and for patients with these two lymphomas. The drug combines the targeting capability of a genetically engineered antibody with an attached toxin that gives the antibody much more potent tumor-killing punch. This sort of “empowered antibody” approach has been pursued by scientists for more than three decades, but Seattle Genetics is only now in position to create what could be the first commercial hit from this class.
The drug showed startling effectiveness in clinical trials, providing significant tumor shrinkage in 75 percent of patients with relapsed forms of Hodgkin’s disease in a clinical trial, and in about 86 percent of patients with anaplastic large-cell lymphoma. Researchers are still following patients to see how long those responses really do last, and to what extent they may help people live longer. The most common side effects found in clinical trials were a depletion in infection-fighting white blood cells, nerve damage in the fingers and toes, fatigue, nausea, and anemia, among other effects, according to today’s statement from the FDA.
Seattle Genetics issued a brief statement today after the FDA announcement. “Bringing a new product to the market is a significant milestone for Seattle Genetics in fulfilling its mission to improve the lives of people with cancer,” said Clay Siegall, the company’s co-founder and CEO.
The company didn’t discuss one of the key facts investors will want to know immediately about the new product—its price. Most analysts expect that the drug will end up costing more than $100,000 per patient, as it is given to patients in a series of intravenous infusions once every three weeks. Seattle Genetics said it plans to discuss more of its commercial plans on a conference call with investors at 8:30 am ET/5:30 am PT on Aug. 22.
Luke Timmerman 8/19/11
Seattle Genetics’ big day has arrived, as it has won FDA clearance to start selling its first new drug on the U.S. market after 14 years in business.
The company has received FDA clearance to start marketing brentuximab vedotin (Adcetris) as a new treatment for U.S. patients with a pair of rare lymphomas—Hodgkin’s disease and anaplastic large-cell lymphoma. The drug was given approval under the faster-than-usual six-month review cycle the FDA sometimes grants to potentially lifesaving therapies, and the official word came a little bit faster than the agency’s legal review deadline of Aug. 30. The regulatory action was really no surprise, since Seattle Genetics won a 10-0 approval recommendation from an FDA advisory panel last month.
“Early clinical data suggest that patients who received Adcetris for Hodgkin lymphoma and systemic anaplastic lymphoma experienced a significant response to the therapy,” said Richard Pazdur, the director of the FDA’s cancer drug review office, in a statement.
The FDA approval is the biggest moment in the history of Seattle Genetics (NASDAQ: SGEN), a company that was founded in 1997 and has run up a deficit of more than $546 million to get to this point, according to its most recent quarterly report.
The company’s new drug represents a significant step ahead for both the science of antibody drug development, and for patients with these two lymphomas. The drug combines the targeting capability of a genetically engineered antibody with an attached toxin that gives the antibody much more potent tumor-killing punch. This sort of “empowered antibody” approach has been pursued by scientists for more than three decades, but Seattle Genetics is only now in position to create what could be the first commercial hit from this class.
The drug showed startling effectiveness in clinical trials, providing significant tumor shrinkage in 75 percent of patients with relapsed forms of Hodgkin’s disease in a clinical trial, and in about 86 percent of patients with anaplastic large-cell lymphoma. Researchers are still following patients to see how long those responses really do last, and to what extent they may help people live longer. The most common side effects found in clinical trials were a depletion in infection-fighting white blood cells, nerve damage in the fingers and toes, fatigue, nausea, and anemia, among other effects, according to today’s statement from the FDA.
Seattle Genetics issued a brief statement today after the FDA announcement. “Bringing a new product to the market is a significant milestone for Seattle Genetics in fulfilling its mission to improve the lives of people with cancer,” said Clay Siegall, the company’s co-founder and CEO.
The company didn’t discuss one of the key facts investors will want to know immediately about the new product—its price. Most analysts expect that the drug will end up costing more than $100,000 per patient, as it is given to patients in a series of intravenous infusions once every three weeks. Seattle Genetics said it plans to discuss more of its commercial plans on a conference call with investors at 8:30 am ET/5:30 am PT on Aug. 22.
Hallo,

nach dem Kurssturz ("sell on good news") von neulich nun immerhin gegen Ende noch ein Zucken in die richtige Richtung.
In diesen Tagen hääte mich noch nicht mal ein Minus an diesem für die Firma so erfreulichem Tage gewundert.
Vielleicht gibt´s noch ein paar Kaufempfehlungen und auch Shorteindeckungen.

Sind schon extreme Wochen...
Gruß q.

nach dem Kurssturz ("sell on good news") von neulich nun immerhin gegen Ende noch ein Zucken in die richtige Richtung.
In diesen Tagen hääte mich noch nicht mal ein Minus an diesem für die Firma so erfreulichem Tage gewundert.
Vielleicht gibt´s noch ein paar Kaufempfehlungen und auch Shorteindeckungen.

Sind schon extreme Wochen...
Gruß q.
Antwort auf Beitrag Nr.: 41.978.567 von SLGramann am 19.08.11 21:34:01So, die Zulassung ist offensichtlich so breit erfolgt, wie derzeit überhaupt möglich gewesen ist:
Fri Aug 19, 2011 5:37pm EDT
* Label broader than expected after July advisory panel
* Shares up 7.8 percent to $15.04
* To begin selling next week, price to be released Monday (Adds analyst comment, CEO comment, price and sales analyst forecast, broader label, July panel concerns)
WASHINGTON, Aug 19 (Reuters) - U.S. drug regulators gave the go ahead on Friday for a blood cancer medicine by Seattle Genetics Inc (SGEN.O), making it the first drug for Hodgkin's lymphoma approved in more than 30 years.
The U.S. Food and Drug Administration approved Seattle Genetics' Adcetris to treat two types of relatively rare blood cancers -- Hodgkin's lymphoma and anaplastic large cell lymphoma (ALCL) -- with a broader label than expected and ahead of the deadline for the decision on the drug.
Adcetris received approval for use in Hodgkin's patients whose have already received a stem cell transplant or two chemotherapy treatments and in ALCL patients who have gone through one chemotherapy treatment.
This is the first drug approved for Hodgkin's lymphoma since 1977 and the first one ever specifically indicated to treat ALCL. About 9,000 Americans a year are diagnosed with Hodgkin's lymphoma and 3,000 with ALCL. The National Cancer Institute estimates some 1,300 people will die of Hodgkin's lymphoma in the United States this year alone.
In July, a panel of FDA advisers raised questions about the drug's trial size and spooked Wall Street with the possibility the label could be restricted to Hodgkin's lymphoma patients whose illness relapsed after a stem cell transplant.
"There was growing concern that (Seattle Genetics) and FDA would not have time to agree on post marketing trials and negotiate a label in the short time since the (panel) meeting or that (Seattle Genetics) would lose negotiating leverage in its attempt to get an improved label," RBC Capital Markets analyst Jason Kantor said in a note.
"The early approval and broad label clearly remove those overhangs and should restore confidence in (Seattle Genetics)management."
The FDA, which was due to decide on Adcetris by Aug. 30, granted priority review status for the drug, meaning the agency believes the medicine is a potentially significant advance over existing therapies.
The company is now conducting more trials to both satisfy the FDA requirement for full approval after such accelerated, or conditional, approval and to reach its own goal of getting Adcetris to more patients and earlier in their treatment.
"We are focused on broadening out the use of Adcetris to bigger patient populations and bringing it to front line treatment," the company's Chief Executive Clay Siegall told Reuters.
"Overall, we are delighted with the label ... This is a label that is the best outcome for patients that need Adcetris," he said.
Seattle Genetics shares rose 7.8 percent to close at $15.04 on Nasdaq.
Adcetris, known chemically as brentuximab vedotin, links a tumor-targeting antibody to a cancer-killing chemotherapy drug with the goal of limiting side effects.
It is designed to home in on an antigen, or foreign substance, in Hodgkin's lymphoma, several types of T-cell lymphoma and other hematologic malignancies.
Seattle Genetics said it planned to begin selling Adcetris next week. Siegall would not disclose the price of the drug, which is expected to be revealed in a company conference call on Aug. 22. J.P. Morgan analyst Cory Kasimov expected a price range per patient of $100,000 to $150,000.
Howard Liang, an analyst at Leerink Swann, has forecast that U.S. sales of Adcetris could reach more than $400 million for both Hodgkin's and ALCL in 2015.
"At some point in the future, we'll consider providing sales guidance," Siegall said, declining to give any forecasts at this time. (Reporting by Alina Selyukh; editing by Dave Zimmerman and Andre Grenon)
--------------------
SGEN hat nunmehr einen nachhaltigen und wachsenden und nicht dilutativen Einnahmestrom, der genutzt werden wird, das Unternehmen in eine neue Dimension hineinwachsen zu lassen. Insbesondere kann die Eigenpipe jetzt stärker gefördert werden.
Warum bspw. nicht als Partner für den Celldex-ADC CDX-011 einsteigen, falls nächstes Jahr dort die erhofften guten Daten kommen?
Fri Aug 19, 2011 5:37pm EDT
* Label broader than expected after July advisory panel
* Shares up 7.8 percent to $15.04
* To begin selling next week, price to be released Monday (Adds analyst comment, CEO comment, price and sales analyst forecast, broader label, July panel concerns)
WASHINGTON, Aug 19 (Reuters) - U.S. drug regulators gave the go ahead on Friday for a blood cancer medicine by Seattle Genetics Inc (SGEN.O), making it the first drug for Hodgkin's lymphoma approved in more than 30 years.
The U.S. Food and Drug Administration approved Seattle Genetics' Adcetris to treat two types of relatively rare blood cancers -- Hodgkin's lymphoma and anaplastic large cell lymphoma (ALCL) -- with a broader label than expected and ahead of the deadline for the decision on the drug.
Adcetris received approval for use in Hodgkin's patients whose have already received a stem cell transplant or two chemotherapy treatments and in ALCL patients who have gone through one chemotherapy treatment.
This is the first drug approved for Hodgkin's lymphoma since 1977 and the first one ever specifically indicated to treat ALCL. About 9,000 Americans a year are diagnosed with Hodgkin's lymphoma and 3,000 with ALCL. The National Cancer Institute estimates some 1,300 people will die of Hodgkin's lymphoma in the United States this year alone.
In July, a panel of FDA advisers raised questions about the drug's trial size and spooked Wall Street with the possibility the label could be restricted to Hodgkin's lymphoma patients whose illness relapsed after a stem cell transplant.
"There was growing concern that (Seattle Genetics) and FDA would not have time to agree on post marketing trials and negotiate a label in the short time since the (panel) meeting or that (Seattle Genetics) would lose negotiating leverage in its attempt to get an improved label," RBC Capital Markets analyst Jason Kantor said in a note.
"The early approval and broad label clearly remove those overhangs and should restore confidence in (Seattle Genetics)management."
The FDA, which was due to decide on Adcetris by Aug. 30, granted priority review status for the drug, meaning the agency believes the medicine is a potentially significant advance over existing therapies.
The company is now conducting more trials to both satisfy the FDA requirement for full approval after such accelerated, or conditional, approval and to reach its own goal of getting Adcetris to more patients and earlier in their treatment.
"We are focused on broadening out the use of Adcetris to bigger patient populations and bringing it to front line treatment," the company's Chief Executive Clay Siegall told Reuters.
"Overall, we are delighted with the label ... This is a label that is the best outcome for patients that need Adcetris," he said.
Seattle Genetics shares rose 7.8 percent to close at $15.04 on Nasdaq.
Adcetris, known chemically as brentuximab vedotin, links a tumor-targeting antibody to a cancer-killing chemotherapy drug with the goal of limiting side effects.
It is designed to home in on an antigen, or foreign substance, in Hodgkin's lymphoma, several types of T-cell lymphoma and other hematologic malignancies.
Seattle Genetics said it planned to begin selling Adcetris next week. Siegall would not disclose the price of the drug, which is expected to be revealed in a company conference call on Aug. 22. J.P. Morgan analyst Cory Kasimov expected a price range per patient of $100,000 to $150,000.
Howard Liang, an analyst at Leerink Swann, has forecast that U.S. sales of Adcetris could reach more than $400 million for both Hodgkin's and ALCL in 2015.
"At some point in the future, we'll consider providing sales guidance," Siegall said, declining to give any forecasts at this time. (Reporting by Alina Selyukh; editing by Dave Zimmerman and Andre Grenon)
--------------------
SGEN hat nunmehr einen nachhaltigen und wachsenden und nicht dilutativen Einnahmestrom, der genutzt werden wird, das Unternehmen in eine neue Dimension hineinwachsen zu lassen. Insbesondere kann die Eigenpipe jetzt stärker gefördert werden.
Warum bspw. nicht als Partner für den Celldex-ADC CDX-011 einsteigen, falls nächstes Jahr dort die erhofften guten Daten kommen?
Antwort auf Beitrag Nr.: 41.979.378 von SLGramann am 20.08.11 09:15:58Danke für die Info.
Gibt es denn schon Reaktionen/Börsenkommentare auf die news?
Werden neue Kursziele genannt, oder heisst es jetzt eher: sell on good news?

Gibt es denn schon Reaktionen/Börsenkommentare auf die news?
Werden neue Kursziele genannt, oder heisst es jetzt eher: sell on good news?

close, in half hour here. seattle genetics, in business for 14 years, no product. finally winning approval for its very first product, a new treatment for hodgkins lymphoma. it is -- this is a first new treatment for that disease in 30 years. so is the company prepared it launch its first ever product? here is how the stock is trading. open higher, now trading lower. how do you price a drug after 14 years of development and more than a half billion dollars in research and development costs? joining us is seattle genetics ceo an president. thank for joining us an congratulations, finally, huh? thank you very much for having me on your show. we are delighted with the approval. we are excited to help the many patients in need. the big question is how you rice it. i just learned from my producer you set the price at $13,500 per dose. it makes you blanch when you consider what's going on in healthcare and the pricing issues and pressures from the government and so forth. how do you justify that cost there? well, the product has a lot of innovation. first of all, you can select the patients based on something on the surface of their tumor cell called cd 30. second of all, in clinical trial, we showed the vast majority of patients respond to this drug. so that's really important. as most cancer drugs, it is hard to select which patient population it treat. also they work on a much smaller grouping of patients. so we feel we have a great drug that's really a patient-friendly drug, 30-minute infusion once every three weeks with very limited side effects compared to cancer chemotherapy. it is still very expensive. does this make you nervous, back it bill's question, in a time when everyone is cutting back on healthcare cost and coverage? we think that the price of the drug was set in an appropriate fashion. we did a lot of works and analysis comparing it to other drugs. in looking at the price to benefit ratio of this drug, we think we did a good job in pricing it in the right way. and we also set up a function called cgen secure to help patients uninsured or underinsured or help with co-payments. so we set up a plan it make sure we can hit the broadest arave patients in need. is it possible there are other uses for this drug down the road? absolutely. thanks for asking that. at the present time, we are in clinical trials in many different diseases where cd 30, its target, is present. that includes a variety of lymphomas and nonlymphoma drugs alike. we should expect, or our guide is that we will be in maybe five or six different type of diseases as well as earlier lines of therapies to improve the outcome for patients and decrease their toxicity from cancer chemotherapy drugs? as what is unique or different with the way this treats lymphoma versus the drugs in place for lymphoma before this? this is a targeted therapeutic drug. it targets by going after the specific target on the cell with an antibody. then after binding to the cancer cell it delivers a cyto toxic drug that kills the cancer cell. where as most cancer chemotherapy drugs target any dividing cell. while cancer does divide rapidly, so do normal tish youies. especially things like bone-marrow that you need. that's where the side effects from cancer chemotherapy come up. congratulations and best of luck to you. thank for coming on. thank you very much. Scroll upScroll downCost/Benefit Challenge of New Cancer DrugsMon 22 Aug 11 | 11:22 AM ET Insight on how to price a drug after 14 years of development and more than half a billion of R&D, with Clay Siegall, Seattle Genetics president/CEO.
COMMENTS .More Comments Thank you for joining our discussion. Your comment has been posted. ADD COMMENTSPlease Sign In or Register to participate. In order to add a comment you must click here and accept our terms and conditions. Edit Screen Name .Your Comments (Up to 1100 characters):Please sign-in/optin or register to be able to submit comments. Remaining characters1100.Preview Comment .CNBC welcomes your contribution. Please respect our community and the integrity of its participants. CNBC reserves the right to moderate and approve your comment. .Sorry, we are no longer accepting comments. PREVIEW COMMENTYour comments have not been posted yet.
Please review your submission to make sure you are comfortable with your entry. Your Comments: Edit Comment .Submit Comment ..
..
RELATED ARTICLES AND CONTENTMore Related Videos
von CNBC
COMMENTS .More Comments Thank you for joining our discussion. Your comment has been posted. ADD COMMENTSPlease Sign In or Register to participate. In order to add a comment you must click here and accept our terms and conditions. Edit Screen Name .Your Comments (Up to 1100 characters):Please sign-in/optin or register to be able to submit comments. Remaining characters1100.Preview Comment .CNBC welcomes your contribution. Please respect our community and the integrity of its participants. CNBC reserves the right to moderate and approve your comment. .Sorry, we are no longer accepting comments. PREVIEW COMMENTYour comments have not been posted yet.
Please review your submission to make sure you are comfortable with your entry. Your Comments: Edit Comment .Submit Comment ..
..
RELATED ARTICLES AND CONTENTMore Related Videos
von CNBC
Hallo,

danke für das Einstellen des CNBC-Beitrages, hatte den Text noch nicht gelesen.
Eigentlich alles - wie erwartet - recht positiv. Die (nur noch mal wiederholte) Verkaufsempfehlung verbunden mit Schlechtrechnungen der Umsätze und Gewinne führten zu weiteren Shortverkäufen, in der Spitze mit über minus sechs Prozent.

Inzwischen setzt eine Erholung ein. Die Erkenntnis wird sich durchsetzen, dass es eben nicht nur diese zwei speziellen Anwendungen eines Präparats von SGEN bleiben werden, zumal der Ansatz ja innovativ ist (auch wenn die Idee dazu ja nun schon diverse Jahrzehnte alt ist).

Ich denke mal, früher oder später (will heißen dieses oder nächstes Jahr) kommt die Übernahme. Wahrscheinlich zu einem nachträglich so empfundenen Schnäppchenpreis, wie bei Medarex.
We´ll see.

Gruß q.

danke für das Einstellen des CNBC-Beitrages, hatte den Text noch nicht gelesen.
Eigentlich alles - wie erwartet - recht positiv. Die (nur noch mal wiederholte) Verkaufsempfehlung verbunden mit Schlechtrechnungen der Umsätze und Gewinne führten zu weiteren Shortverkäufen, in der Spitze mit über minus sechs Prozent.

Inzwischen setzt eine Erholung ein. Die Erkenntnis wird sich durchsetzen, dass es eben nicht nur diese zwei speziellen Anwendungen eines Präparats von SGEN bleiben werden, zumal der Ansatz ja innovativ ist (auch wenn die Idee dazu ja nun schon diverse Jahrzehnte alt ist).

Ich denke mal, früher oder später (will heißen dieses oder nächstes Jahr) kommt die Übernahme. Wahrscheinlich zu einem nachträglich so empfundenen Schnäppchenpreis, wie bei Medarex.
We´ll see.

Gruß q.
Seattle Genetics starts Phase II Adcetris trial
Seattle Genetics has started a Phase II trial to evaluate its antibody-drug conjugate (ADC) Adcetris (brentuximab vedotin) as a treatment for the patients suffering from relapsed or refractory CD30-positive non-Hodgkin lymphomas.
The trial will also evaluate patients with diffuse large B-cell lymphoma, peripheral T-cell lymphoma and other less common lymphoma subtypes.
The trial will investigate the antitumor activity, duration of response and safety profile of Adcetris in these patients.
The company expects to recruit around 55 patients in various centers across the US.
Seattle Genetics chief medical officer Thomas Reynolds said this clinical trial is part of their comprehensive development plan to evaluate the potential of Adcetris in CD30-positive malignancies, building on the data they have generated in certain patients with Hodgkin lymphoma and systemic ALCL.
"We believe the targeting ability of Adcetris to CD30 provides opportunities in selected lymphoma subtypes," Reynolds said.
Seattle Genetics has started a Phase II trial to evaluate its antibody-drug conjugate (ADC) Adcetris (brentuximab vedotin) as a treatment for the patients suffering from relapsed or refractory CD30-positive non-Hodgkin lymphomas.
The trial will also evaluate patients with diffuse large B-cell lymphoma, peripheral T-cell lymphoma and other less common lymphoma subtypes.
The trial will investigate the antitumor activity, duration of response and safety profile of Adcetris in these patients.
The company expects to recruit around 55 patients in various centers across the US.
Seattle Genetics chief medical officer Thomas Reynolds said this clinical trial is part of their comprehensive development plan to evaluate the potential of Adcetris in CD30-positive malignancies, building on the data they have generated in certain patients with Hodgkin lymphoma and systemic ALCL.
"We believe the targeting ability of Adcetris to CD30 provides opportunities in selected lymphoma subtypes," Reynolds said.
Antwort auf Beitrag Nr.: 41.987.064 von quepos am 22.08.11 21:11:16Hi quepos,
der kurzzeitige nochmalige Abverkauf diese Woche wurde wohl vor allem nach dem Muster der Dendreon-Panik gespielt.
Der Preis von Adcetris ist - erwartungsgemäß - hoch:
Seattle Genetics said the drug will cost between $94,500 and $121,500 per patient depending on the number of doses. The price could run even higher because it’s unknown how many doses each patient will require. (The company estimated the price based on seven to nine infusions per patient.)
Bei Dendreon will man glauben, dass die schlechten (was man so schlecht nennt...!) Verkäufe (auch) an dem hohen Preis von Provenge von knappen 100.000 Dollar liegen.
Meiner Meinung nach wird es sich bei Adcetris zeigen, dass der Preis die Verkäufe nicht negativ beeinflussen wird und wahrscheinlich ist der Preis auch bei Provenge nicht das Pudels Kern...
@Fruehrenter,
sollte sich Adcetris auch bei NHL nicht nur als wirksam, sondern als konkurrenzfähig erweisen - und sei es nur bei irgendeinem Subtyp - dann geht ein großes Fenster in eine andere Umsatzdimension auf. Ich halte meine Erwartungen in Bezug auf NHL aber lieber bei ganz genau Null.
Grüße
der kurzzeitige nochmalige Abverkauf diese Woche wurde wohl vor allem nach dem Muster der Dendreon-Panik gespielt.
Der Preis von Adcetris ist - erwartungsgemäß - hoch:
Seattle Genetics said the drug will cost between $94,500 and $121,500 per patient depending on the number of doses. The price could run even higher because it’s unknown how many doses each patient will require. (The company estimated the price based on seven to nine infusions per patient.)
Bei Dendreon will man glauben, dass die schlechten (was man so schlecht nennt...!) Verkäufe (auch) an dem hohen Preis von Provenge von knappen 100.000 Dollar liegen.
Meiner Meinung nach wird es sich bei Adcetris zeigen, dass der Preis die Verkäufe nicht negativ beeinflussen wird und wahrscheinlich ist der Preis auch bei Provenge nicht das Pudels Kern...
@Fruehrenter,
sollte sich Adcetris auch bei NHL nicht nur als wirksam, sondern als konkurrenzfähig erweisen - und sei es nur bei irgendeinem Subtyp - dann geht ein großes Fenster in eine andere Umsatzdimension auf. Ich halte meine Erwartungen in Bezug auf NHL aber lieber bei ganz genau Null.
Grüße
Ja Hallo,

hatte auch mal ein paar DNDN, aber so richtig hat mich Provenge nie überzeugt. War viel Hype und die Vorgänge im Vorfeld der endgültigen Zulassung mehr als fragwürdig.
SGEN präsentiert sich da in meinen Augen ganz anders. Die ADC-Technologie verbunden mit der hohen response-rate wird m.M. für einen Run auf das Medikament sorgen.
Es wird viele zulassungsfremde Anwendungen geben.
Trotz der immer recht hohen Bewertung sehe ich daher noch einiges Potenzial und bleibe dabei.
Gruß q.

hatte auch mal ein paar DNDN, aber so richtig hat mich Provenge nie überzeugt. War viel Hype und die Vorgänge im Vorfeld der endgültigen Zulassung mehr als fragwürdig.
SGEN präsentiert sich da in meinen Augen ganz anders. Die ADC-Technologie verbunden mit der hohen response-rate wird m.M. für einen Run auf das Medikament sorgen.
Es wird viele zulassungsfremde Anwendungen geben.
Trotz der immer recht hohen Bewertung sehe ich daher noch einiges Potenzial und bleibe dabei.
Gruß q.
Antwort auf Beitrag Nr.: 42.013.344 von quepos am 28.08.11 21:39:48Zum Glück sind wir hier nicht die einzigen, die Adcetris auf der Erfolgsspur sehen:
Aug 30, 2011, 8:29 am EDT | By Barry Cohen, Health Care Writer
Evidently one insider at Seattle Genetics (NASDAQ:SGEN) is confident the biotech company can avoid the dreaded Dendreon (NASDAQ NDN) effect.
NDN) effect.
Dendreon was trading at nearly $36 per share in early August when the company reported sluggish sales of its highly touted prostate cancer compound, Provenge, the first medicine to train the body’s immune system to attack cancer cells like a virus. Shareholders saw their investment plummet by two-thirds in a single day as the company withdrew its 2011 revenue estimate of $350 million to $400 million.
SPONSORED LINKS
Julian Baker, a Seattle Genetics director, is betting big the same fate doesn’t befall the company’s new cancer medication, Adcetris. His firm, New York City-based Baker Brothers Life Sciences Capital, last week bought $18 million worth of the company’s shares.
Im laufenden Quartal wird man faktisch noch keine Produktumsätze haben, aber für das 4. Quartal kann man wohl die ersten paar Millionen erwarten.
Aug 30, 2011, 8:29 am EDT | By Barry Cohen, Health Care Writer
Evidently one insider at Seattle Genetics (NASDAQ:SGEN) is confident the biotech company can avoid the dreaded Dendreon (NASDAQ
 NDN) effect.
NDN) effect.Dendreon was trading at nearly $36 per share in early August when the company reported sluggish sales of its highly touted prostate cancer compound, Provenge, the first medicine to train the body’s immune system to attack cancer cells like a virus. Shareholders saw their investment plummet by two-thirds in a single day as the company withdrew its 2011 revenue estimate of $350 million to $400 million.
SPONSORED LINKS
Julian Baker, a Seattle Genetics director, is betting big the same fate doesn’t befall the company’s new cancer medication, Adcetris. His firm, New York City-based Baker Brothers Life Sciences Capital, last week bought $18 million worth of the company’s shares.
Im laufenden Quartal wird man faktisch noch keine Produktumsätze haben, aber für das 4. Quartal kann man wohl die ersten paar Millionen erwarten.
Moin,
:;
gibt wieder ein paar Investoren-Konferenzen, diesmal natürlich von besonderem Interesse (nach der Adcetris-Zulassung).
Könnte mir vorstellen, dass auch Statements zum Anlaufen des Vertriebs und künftigen Erlösen kommen werden. Bin ganz gespannt auf die Reaktionen. Immerhin sollte man meinen, dass der Vertrieb gerade zu Beginn rasant ist. Jeder betroffene Patient wird sich um das Mittel reissen, wenn die Chancen auf Besserung hoch sind und jeder Tag zählt.
Bei Provenge z.B. ist das bestimmt anders, weil nur ein kleiner Bruchteil der Behandlungen Aussicht auf Erfolg hat, daher die Zurückhaltung beim Umsatz und der Kurseinbruch dort.
Bei SGEN dagegen ist der Kurs nach dem etwas rätselhaften "Zulassungseinbruch" ja ganz gut nach oben zurückgekommen, trotz des Marktumfeldes.

We´ll see.
Gruß q.
Press Release Source: Seattle Genetics, Inc. On Thursday September 8, 2011, 9:00 am EDT
BOTHELL, Wash.--(BUSINESS WIRE)-- Seattle Genetics, Inc. (NASDAQ:SGEN - News) announced today that management will present at the following upcoming investor conferences.
Morgan Stanley Global Healthcare Conference
Tuesday, September 13, 2011 at 11:30 a.m. Eastern time in New York, NY
UBS Global Life Sciences Conference
Tuesday, September 20, 2011 at 2:30 p.m. Eastern time in New York, NY
The presentations will be webcast live and available for replay from Seattle Genetics’ website at http://www.seattlegenetics.com in the Investors and News section.
:;
gibt wieder ein paar Investoren-Konferenzen, diesmal natürlich von besonderem Interesse (nach der Adcetris-Zulassung).
Könnte mir vorstellen, dass auch Statements zum Anlaufen des Vertriebs und künftigen Erlösen kommen werden. Bin ganz gespannt auf die Reaktionen. Immerhin sollte man meinen, dass der Vertrieb gerade zu Beginn rasant ist. Jeder betroffene Patient wird sich um das Mittel reissen, wenn die Chancen auf Besserung hoch sind und jeder Tag zählt.
Bei Provenge z.B. ist das bestimmt anders, weil nur ein kleiner Bruchteil der Behandlungen Aussicht auf Erfolg hat, daher die Zurückhaltung beim Umsatz und der Kurseinbruch dort.
Bei SGEN dagegen ist der Kurs nach dem etwas rätselhaften "Zulassungseinbruch" ja ganz gut nach oben zurückgekommen, trotz des Marktumfeldes.

We´ll see.
Gruß q.
Press Release Source: Seattle Genetics, Inc. On Thursday September 8, 2011, 9:00 am EDT
BOTHELL, Wash.--(BUSINESS WIRE)-- Seattle Genetics, Inc. (NASDAQ:SGEN - News) announced today that management will present at the following upcoming investor conferences.
Morgan Stanley Global Healthcare Conference
Tuesday, September 13, 2011 at 11:30 a.m. Eastern time in New York, NY
UBS Global Life Sciences Conference
Tuesday, September 20, 2011 at 2:30 p.m. Eastern time in New York, NY
The presentations will be webcast live and available for replay from Seattle Genetics’ website at http://www.seattlegenetics.com in the Investors and News section.
BOTHELL, Wash. & OXFORD, England, Sep 13, 2011 (BUSINESS WIRE) -- Seattle Genetics, Inc. and Oxford BioTherapeutics (OBT) today announced that they have formed a strategic collaboration to jointly discover novel antibody-drug conjugates (ADCs) for cancer. Under the collaboration, OBT will generate panels of monoclonal antibodies against novel tumor-specific antigens identified using its proprietary Oxford Genome Anatomy Project (OGAP(R)) database. The antibodies generated by OBT will then be screened for activity using Seattle Genetics' ADC technology. The resulting ADCs may be selected by each company for further development and commercialization.
"This collaboration is directly aligned with our goal of identifying novel ADC targets to expand our early-stage product pipeline," said Jonathan Drachman, M.D., Senior Vice President, Research and Translational Medicine at Seattle Genetics. "Through the agreement, we contribute our know-how and proprietary ADC technology and gain access to novel cancer targets obtained from OBT's expertise in target discovery. We believe that this type of multi-product collaboration maximizes the chances for success and capitalizes on each company's strengths."
"We are delighted to be collaborating with Seattle Genetics in this alliance which we believe will add multiple ADC candidates to OBT's rapidly developing pre-clinical product pipeline. ADCs are beginning to transform the outlook for many cancer patients and OBT believes that with its antibody pipeline, it can make an important contribution to the development of this exciting new class of therapeutics. We are looking forward to working with Seattle Genetics to provide improved therapies for patients suffering from cancer," said Christian Rohlff, Chief Executive Officer of OBT.
Under the terms of the multi-year, multi-product agreement, OBT and Seattle Genetics will each have an equal number of alternating options to select programs from among the preclinical ADCs identified for exclusive, worldwide development and commercialization. Each company will receive undisclosed progress-dependent milestone payments and royalties on net sales of any resulting ADCs developed by the other party.
About OGAP(R)
The Oxford Genome Anatomy Project (OGAP(R)) database represents one of the world's largest proprietary collections of disease-associated proteins. OGAP(R) oncology contains proteomic data on over 7,500 cancer membrane proteins combined with genomic and clinical information derived from human blood and cancer tissue studies. OGAP(R) contains proprietary target information on three-quarters of the entire human proteome. Almost two million human protein fragments have been sequenced in OGAP(R) in 50 different human tissues representing 60 diseases, including 25 forms of cancer. OGAP(R) integrates data covering 17,000 different genes and over eight million genetic variants (SNPs and haplotypes).
------------
Das ist die Rubrik "ferne Zukunft". Man merkt, dass SGEN weg will vom Status des Technologielieferanten mit 5% Royalties oder so...
"This collaboration is directly aligned with our goal of identifying novel ADC targets to expand our early-stage product pipeline," said Jonathan Drachman, M.D., Senior Vice President, Research and Translational Medicine at Seattle Genetics. "Through the agreement, we contribute our know-how and proprietary ADC technology and gain access to novel cancer targets obtained from OBT's expertise in target discovery. We believe that this type of multi-product collaboration maximizes the chances for success and capitalizes on each company's strengths."
"We are delighted to be collaborating with Seattle Genetics in this alliance which we believe will add multiple ADC candidates to OBT's rapidly developing pre-clinical product pipeline. ADCs are beginning to transform the outlook for many cancer patients and OBT believes that with its antibody pipeline, it can make an important contribution to the development of this exciting new class of therapeutics. We are looking forward to working with Seattle Genetics to provide improved therapies for patients suffering from cancer," said Christian Rohlff, Chief Executive Officer of OBT.
Under the terms of the multi-year, multi-product agreement, OBT and Seattle Genetics will each have an equal number of alternating options to select programs from among the preclinical ADCs identified for exclusive, worldwide development and commercialization. Each company will receive undisclosed progress-dependent milestone payments and royalties on net sales of any resulting ADCs developed by the other party.
About OGAP(R)
The Oxford Genome Anatomy Project (OGAP(R)) database represents one of the world's largest proprietary collections of disease-associated proteins. OGAP(R) oncology contains proteomic data on over 7,500 cancer membrane proteins combined with genomic and clinical information derived from human blood and cancer tissue studies. OGAP(R) contains proprietary target information on three-quarters of the entire human proteome. Almost two million human protein fragments have been sequenced in OGAP(R) in 50 different human tissues representing 60 diseases, including 25 forms of cancer. OGAP(R) integrates data covering 17,000 different genes and over eight million genetic variants (SNPs and haplotypes).
------------
Das ist die Rubrik "ferne Zukunft". Man merkt, dass SGEN weg will vom Status des Technologielieferanten mit 5% Royalties oder so...
Die erste konkrete Umsatzschätzung, die ich gelesen habe. Finde sie sehr(!) hoch. Na mal sehen...
Seattle Genetics Adcetris sales forecasts raised at RBC Capital
After surveying doctors, RBC Capital raised its 2011 Adcetris sales forecast to $18M, versus the consensus estimate of $10M. The firm notes that 71% of the doctors it surveyed expect Adcetris to become a first-line treatment, and it maintains an Outperform rating on the stock. (SGEN)
Seattle Genetics Adcetris sales forecasts raised at RBC Capital
After surveying doctors, RBC Capital raised its 2011 Adcetris sales forecast to $18M, versus the consensus estimate of $10M. The firm notes that 71% of the doctors it surveyed expect Adcetris to become a first-line treatment, and it maintains an Outperform rating on the stock. (SGEN)
Hallo,

die Vermutungen/Hoffnungen scheinen sich zu bestätigen, dass die Verkäufe von Anfang an hoch sind. Hoffentlich werden die Erwartungen der Patienten auch erfüllt!
Läuft ja super heute gegen den Markttrend.
Wird nach oben aber sehr eng, erst das Gap bei knapp 20, dann das Hoch bei etwas über 21 in diesem Marktumfeld...
wait and see.
Gruß von quepos

die Vermutungen/Hoffnungen scheinen sich zu bestätigen, dass die Verkäufe von Anfang an hoch sind. Hoffentlich werden die Erwartungen der Patienten auch erfüllt!
Läuft ja super heute gegen den Markttrend.
Wird nach oben aber sehr eng, erst das Gap bei knapp 20, dann das Hoch bei etwas über 21 in diesem Marktumfeld...
wait and see.
Gruß von quepos
Moin,
bin aus meiner "Fuß-in-der-Tür"-Position ausgestiegen (leider, da SGEN immer noch weiter steigt).
Dachte eher an ein Doppel-Top und günstigeren Wiedereinstieg -
hay que ver.
Aktie ist recht hoch bewertet, aber die Technologie auch gut.
Gruß q.
bin aus meiner "Fuß-in-der-Tür"-Position ausgestiegen (leider, da SGEN immer noch weiter steigt).
Dachte eher an ein Doppel-Top und günstigeren Wiedereinstieg -
hay que ver.
Aktie ist recht hoch bewertet, aber die Technologie auch gut.
Gruß q.
Antwort auf Beitrag Nr.: 42.186.094 von quepos am 07.10.11 20:28:23Ich stimme zu, dass die Bewertung hoch ist. Die allgemeine Lage der Wirtschaft ist hingegen erbärmlich. Frühindikatoren sind im Sinkflug - heute bspw. der ECRI-Leading-Index. Kann mir für die nächsten 6 Monate gute Kaufkurse bei allen möglichen Aktien vorstellen. Cash generieren kann derzeit eine kluge Taktik sein!
Seattle Genetics Announces Initiation of a Phase II Clinical Trial of ADCETRIS™ in CD30-Positive Non-Lymphoma Malignancies Print
25 Oct 2011
Seattle Genetics has initiated a phase II clinical trial of ADCETRIS (brentuximab vedotin) for patients with CD30-positive non-lymphoma malignancies
BOTHELL, WA, USA I October 25, 2011 I Seattle Genetics, Inc. (Nasdaq: SGEN - News) today announced that it has initiated a phase II clinical trial of ADCETRIS™ (brentuximab vedotin) for patients with CD30-positive non-lymphoma malignancies, including multiple myeloma, leukemia and solid tumors. The trial is designed to assess the antitumor activity and safety profile of ADCETRIS in these patients. ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30. On August 19, 2011, the U.S. Food and Drug Administration granted accelerated approval of ADCETRIS for two indications. ADCETRIS has not been approved for use in any non-lymphoma malignancies.
“This is the first clinical trial of ADCETRIS that expands our investigation beyond lymphoma,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer of Seattle Genetics. “There are reports in published literature of CD30 expression in multiple myeloma, several types of leukemia and solid tumors. Through both corporate-sponsored and investigator-sponsored trials, our development plan is designed to broadly evaluate the potential of ADCETRIS in many types of CD30-positive malignancies.”
The phase II trial is enrolling patients with CD30-positive non-lymphoma malignancies who have failed, refused or have been deemed ineligible for standard therapy. Assessment of CD30 expression will be performed according to a Seattle Genetics screening protocol. The screening protocol facilitates high-throughput assessment of CD30 expression in patients with a variety of non-lymphoma malignancies to identify those eligible for the clinical trial. The primary endpoint of the phase II trial is characterization of the antitumor activity of ADCETRIS. In addition, the trial will assess safety and establish the relationship of CD30 expression with antitumor activity. The study is expected to enroll approximately 40 patients at multiple centers in the United States.
About ADCETRIS
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics’ proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
In addition to the non-lymphoma trial, ADCETRIS is being evaluated in a phase III clinical trial (the AETHERA trial) for patients at high risk of residual Hodgkin lymphoma following autologous stem cell transplant (ASCT), a phase II trial for relapsed or refractory non-Hodgkin lymphoma patients, a phase II retreatment trial for relapsed patients who previously responded to ADCETRIS, a phase I trial in combination with multi-agent chemotherapy for front-line treatment of Hodgkin lymphoma and a phase I trial in combination with multi-agent chemotherapy for front-line treatment of systemic ALCL. A phase III trial in CD30-positive cutaneous T-cell lymphomas is planned for the first half of 2012.
Seattle Genetics is developing ADCETRIS in collaboration with Millennium: The Takeda Oncology Company. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
About Seattle Genetics
Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer. ADCETRIS™ was approved by the FDA on August 19, 2011 for two indications. ADCETRIS is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has three other clinical-stage ADC programs: SGN-75, ASG-5ME and ASG-22ME. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
SOURCE: Seattle Genetics
25 Oct 2011
Seattle Genetics has initiated a phase II clinical trial of ADCETRIS (brentuximab vedotin) for patients with CD30-positive non-lymphoma malignancies
BOTHELL, WA, USA I October 25, 2011 I Seattle Genetics, Inc. (Nasdaq: SGEN - News) today announced that it has initiated a phase II clinical trial of ADCETRIS™ (brentuximab vedotin) for patients with CD30-positive non-lymphoma malignancies, including multiple myeloma, leukemia and solid tumors. The trial is designed to assess the antitumor activity and safety profile of ADCETRIS in these patients. ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30. On August 19, 2011, the U.S. Food and Drug Administration granted accelerated approval of ADCETRIS for two indications. ADCETRIS has not been approved for use in any non-lymphoma malignancies.
“This is the first clinical trial of ADCETRIS that expands our investigation beyond lymphoma,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer of Seattle Genetics. “There are reports in published literature of CD30 expression in multiple myeloma, several types of leukemia and solid tumors. Through both corporate-sponsored and investigator-sponsored trials, our development plan is designed to broadly evaluate the potential of ADCETRIS in many types of CD30-positive malignancies.”
The phase II trial is enrolling patients with CD30-positive non-lymphoma malignancies who have failed, refused or have been deemed ineligible for standard therapy. Assessment of CD30 expression will be performed according to a Seattle Genetics screening protocol. The screening protocol facilitates high-throughput assessment of CD30 expression in patients with a variety of non-lymphoma malignancies to identify those eligible for the clinical trial. The primary endpoint of the phase II trial is characterization of the antitumor activity of ADCETRIS. In addition, the trial will assess safety and establish the relationship of CD30 expression with antitumor activity. The study is expected to enroll approximately 40 patients at multiple centers in the United States.
About ADCETRIS
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics’ proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
In addition to the non-lymphoma trial, ADCETRIS is being evaluated in a phase III clinical trial (the AETHERA trial) for patients at high risk of residual Hodgkin lymphoma following autologous stem cell transplant (ASCT), a phase II trial for relapsed or refractory non-Hodgkin lymphoma patients, a phase II retreatment trial for relapsed patients who previously responded to ADCETRIS, a phase I trial in combination with multi-agent chemotherapy for front-line treatment of Hodgkin lymphoma and a phase I trial in combination with multi-agent chemotherapy for front-line treatment of systemic ALCL. A phase III trial in CD30-positive cutaneous T-cell lymphomas is planned for the first half of 2012.
Seattle Genetics is developing ADCETRIS in collaboration with Millennium: The Takeda Oncology Company. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
About Seattle Genetics
Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer. ADCETRIS™ was approved by the FDA on August 19, 2011 for two indications. ADCETRIS is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has three other clinical-stage ADC programs: SGN-75, ASG-5ME and ASG-22ME. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
SOURCE: Seattle Genetics
Moin,

das sind gute, wenn auch irgendwie erwartete Nachrichten.
Klar dass SGEN nach den bisherigen Erfolgen schnell Studien zu anderen Anwendungen nachschieben will. Man ist offenbar überzeugt, dass auch kleine Patientenzahlen reichen - schön wär´s. Besonders gut natürlich dabei, dass auch solide Tumore in das Programm aufgenommen werden, das ist natürlich hochgradig interessant.
Muss leider schon wieder aufpassen, dass der Kurs nicht davonläuft, da ich momentan ja nicht investiert bin...

We´ll see.
Gruß q.

das sind gute, wenn auch irgendwie erwartete Nachrichten.
Klar dass SGEN nach den bisherigen Erfolgen schnell Studien zu anderen Anwendungen nachschieben will. Man ist offenbar überzeugt, dass auch kleine Patientenzahlen reichen - schön wär´s. Besonders gut natürlich dabei, dass auch solide Tumore in das Programm aufgenommen werden, das ist natürlich hochgradig interessant.
Muss leider schon wieder aufpassen, dass der Kurs nicht davonläuft, da ich momentan ja nicht investiert bin...

We´ll see.
Gruß q.
Hallo,

hab ich wohl Glück gehabt, momentan nicht investiert zu sein bei dem fetten Kurssturz.
Stellt sich wieder die Frage "Chance zum Einstieg?"
Ich glaube auf längere Sicht auf jeden Fall. Durch die Analystendowngrades und den angeschlagenen Chart kann es natürlich noch ein Stück weiter nach unten gehen, ich verstehe das dicke Minus von 18% allerdings überhaupt nicht. Auf Sicht von ein paar Wochen oder Monaten sollte auch das Riesen-Gap wieder geschlossen werden. Andererseits gibt es ja viele, die nach dem schnellen Anstieg seit Anfang August noch Gewinne mitnehmen wollen, nicht zu vergessen auch die hohe Short-Quote.
Vielleicht sollte ich die Entwicklung noch ein paar Tage abwarten.
Die Zahlen waren ja eigentlich äußerst positiv, da die Adcetris-Umsätze (und nur das zählt im Moment wirklich) ja deutlich über den Erwartungen lagen, wie ja auch von mir erhofft.
Mal sehen, vielleicht gibt es ja bald einige positive Patientenberichte, das würde dem Kurs sicher Auftrieb geben.
Dann will ich natürlich auch wieder investiert sein!

Gruß q.

hab ich wohl Glück gehabt, momentan nicht investiert zu sein bei dem fetten Kurssturz.
Stellt sich wieder die Frage "Chance zum Einstieg?"
Ich glaube auf längere Sicht auf jeden Fall. Durch die Analystendowngrades und den angeschlagenen Chart kann es natürlich noch ein Stück weiter nach unten gehen, ich verstehe das dicke Minus von 18% allerdings überhaupt nicht. Auf Sicht von ein paar Wochen oder Monaten sollte auch das Riesen-Gap wieder geschlossen werden. Andererseits gibt es ja viele, die nach dem schnellen Anstieg seit Anfang August noch Gewinne mitnehmen wollen, nicht zu vergessen auch die hohe Short-Quote.
Vielleicht sollte ich die Entwicklung noch ein paar Tage abwarten.
Die Zahlen waren ja eigentlich äußerst positiv, da die Adcetris-Umsätze (und nur das zählt im Moment wirklich) ja deutlich über den Erwartungen lagen, wie ja auch von mir erhofft.
Mal sehen, vielleicht gibt es ja bald einige positive Patientenberichte, das würde dem Kurs sicher Auftrieb geben.
Dann will ich natürlich auch wieder investiert sein!

Gruß q.
Antwort auf Beitrag Nr.: 42.307.317 von quepos am 05.11.11 20:01:19Hi quepos,
habe nach meinem Post vom 07.10. meine SGEN-Position verkauft, die einen ziemlich großen Depotanteil ausgemacht hat und ganz gut im Plus war.
Der Grund für den jüngsten Kursrückschlag wird wahrscheinlich in diesem Artikel angesprochen. Gut möglich, dass das alles ziemlich irrational war:
Seattle Genetics Frontline Trial Plans Are Not New -- and Not a Problem, Either
DAVID MILLER NOV 04, 2011 8:40 AM
Seattle Genetics (SGEN) released earnings for its recently-launched cancer drug Adcetris yesterday after the bell. Revenues of $10 million for the first six weeks on the market far exceeded consensus estimates for Q3 and even some analyst estimates for all of 2011. By any measure, the results were quite good. With about 450 patients now taking Adcetris every three weeks at an average per-dose cost over $11,000, all analysts are likely to be raising their 2011 revenue estimates.
Adcetris received accelerated approval from the FDA for both Hodgkin’s Lymphoma (or HL) and anaplastic large cell lymphoma (or ALCL). Under FDA rules, Seattle Genetics must run a confirmatory trial. The FDA and Seattle Genetics have an agreement this confirmatory trial will be in front-line (treatment naïve) patients and will have a survival endpoint.
Current front-line therapy for HL is a cocktail of four cancer drugs commonly referred to as “ABVD." ABVD was first developed in the 1970s. Even a brief discussion with leading hematological oncologists will inform you nobody really likes ABVD. It works, but it is toxic. There are dozens, if not hundreds, of clinical trials already in the books seeking ways to improve ABVD’s efficacy or reduce its pulmonary side effects. Of the four combination drugs, the “B’ in ABVD (Bleomycin) is responsible for the pulmonary side effects.
To prepare for the Phase III confirmatory trial required by the FDA, Seattle Genetics is running a Phase I trial testing the safety of Adcetris in combination with ABVD and some of its components. The first step of this trial was to test Adcetris plus ABVD. On yesterday’s call, Seattle Genetics announced the pulmonary toxicity already present with ABVD increased meaningfully with the addition of Adcetris. Seattle Genetics said that because of this, the confirmatory trial would be AVD plus Adcetris – in other words, removing Bleomycin from the combination. Early testing of AVD plus Adcetris (“AVDA”) shows no pulmonary toxicity so far.
Some on Wall Street, particularly the fast-money biotech funds who are short Seattle Genetics, have seized upon this as a “new” issue that will delay Seattle Genetics' confirmatory trial and eventual entry into the much larger front-line indication
This isn’t new and it isn’t a problem.
Even before Adcetris was approved, Seattle Genetics management was talking about the front-line trial for HL being Adcetris plus AVD. My firm published this in our 200+ page Anniversary Issue last August in fact. A confirmatory trial design of Adcetris plus AVD versus ABVD is obviously not a new idea.
It’s also not a problem for key opinion leaders in the Hodgkin’s Disease space. I’ve spoken with to several at scientific conferences over the past couple of years who expressed sincere excitement in obtaining Adcetris for their treatment-naïve patients. They talked openly about how they would create combinations. Removing the ‘B’ from ABVD and adding Adcetris was very often communicated as their preferred approach.
When a biotech releases good news but has a short interest as high as Seattle Genetics’ short interest (25.3 million shares with just 3 million shares traded daily and a 81 million share float), bears have to come up with something negative to talk about. My guess is that “something” will be how Seattle Genetics’ front line program is now “in trouble” or “in disarray” or has some “unexpected complications.” This is something the smart, well-researched investor will recognize as spin.
Don’t get me wrong. Seattle Genetics’ market cap is priced for perfect execution on the sales side. With such a fast start, people might get carried away with revenue projections for the remainder of 2011 and especially 2012. There are risks to the Adcetris launch, but one of those risks is not that the front line trial in HL will be Adcetris plus AVD.
http://www.minyanville.com/businessmarkets/articles/Seattle-…
Sehe das wie Du: Der Adcetris-Umsatz war ein Hammer! Ich hatte für das Q3 noch mit so gut wie gar nichts gerechnet. Jetzt könnte ich mir für Q4 40 bis 50 Mio. vorstellen. Wahnsinn.
Nach wie vor misstraue ich aber der wirtschaftlichen Gesamtsituation. Außerdem werde ich am Montag eine kleine sehr spekulative Position bei Exelixis eingehen.
Bei SGEN bleibe ich erst mal an der Seitenlinie. Spätestens bei 15 Dollar wäre ein Rückkauf allerdings Pflicht.
habe nach meinem Post vom 07.10. meine SGEN-Position verkauft, die einen ziemlich großen Depotanteil ausgemacht hat und ganz gut im Plus war.
Der Grund für den jüngsten Kursrückschlag wird wahrscheinlich in diesem Artikel angesprochen. Gut möglich, dass das alles ziemlich irrational war:
Seattle Genetics Frontline Trial Plans Are Not New -- and Not a Problem, Either
DAVID MILLER NOV 04, 2011 8:40 AM
Seattle Genetics (SGEN) released earnings for its recently-launched cancer drug Adcetris yesterday after the bell. Revenues of $10 million for the first six weeks on the market far exceeded consensus estimates for Q3 and even some analyst estimates for all of 2011. By any measure, the results were quite good. With about 450 patients now taking Adcetris every three weeks at an average per-dose cost over $11,000, all analysts are likely to be raising their 2011 revenue estimates.
Adcetris received accelerated approval from the FDA for both Hodgkin’s Lymphoma (or HL) and anaplastic large cell lymphoma (or ALCL). Under FDA rules, Seattle Genetics must run a confirmatory trial. The FDA and Seattle Genetics have an agreement this confirmatory trial will be in front-line (treatment naïve) patients and will have a survival endpoint.
Current front-line therapy for HL is a cocktail of four cancer drugs commonly referred to as “ABVD." ABVD was first developed in the 1970s. Even a brief discussion with leading hematological oncologists will inform you nobody really likes ABVD. It works, but it is toxic. There are dozens, if not hundreds, of clinical trials already in the books seeking ways to improve ABVD’s efficacy or reduce its pulmonary side effects. Of the four combination drugs, the “B’ in ABVD (Bleomycin) is responsible for the pulmonary side effects.
To prepare for the Phase III confirmatory trial required by the FDA, Seattle Genetics is running a Phase I trial testing the safety of Adcetris in combination with ABVD and some of its components. The first step of this trial was to test Adcetris plus ABVD. On yesterday’s call, Seattle Genetics announced the pulmonary toxicity already present with ABVD increased meaningfully with the addition of Adcetris. Seattle Genetics said that because of this, the confirmatory trial would be AVD plus Adcetris – in other words, removing Bleomycin from the combination. Early testing of AVD plus Adcetris (“AVDA”) shows no pulmonary toxicity so far.
Some on Wall Street, particularly the fast-money biotech funds who are short Seattle Genetics, have seized upon this as a “new” issue that will delay Seattle Genetics' confirmatory trial and eventual entry into the much larger front-line indication
This isn’t new and it isn’t a problem.
Even before Adcetris was approved, Seattle Genetics management was talking about the front-line trial for HL being Adcetris plus AVD. My firm published this in our 200+ page Anniversary Issue last August in fact. A confirmatory trial design of Adcetris plus AVD versus ABVD is obviously not a new idea.
It’s also not a problem for key opinion leaders in the Hodgkin’s Disease space. I’ve spoken with to several at scientific conferences over the past couple of years who expressed sincere excitement in obtaining Adcetris for their treatment-naïve patients. They talked openly about how they would create combinations. Removing the ‘B’ from ABVD and adding Adcetris was very often communicated as their preferred approach.
When a biotech releases good news but has a short interest as high as Seattle Genetics’ short interest (25.3 million shares with just 3 million shares traded daily and a 81 million share float), bears have to come up with something negative to talk about. My guess is that “something” will be how Seattle Genetics’ front line program is now “in trouble” or “in disarray” or has some “unexpected complications.” This is something the smart, well-researched investor will recognize as spin.
Don’t get me wrong. Seattle Genetics’ market cap is priced for perfect execution on the sales side. With such a fast start, people might get carried away with revenue projections for the remainder of 2011 and especially 2012. There are risks to the Adcetris launch, but one of those risks is not that the front line trial in HL will be Adcetris plus AVD.
http://www.minyanville.com/businessmarkets/articles/Seattle-…
Sehe das wie Du: Der Adcetris-Umsatz war ein Hammer! Ich hatte für das Q3 noch mit so gut wie gar nichts gerechnet. Jetzt könnte ich mir für Q4 40 bis 50 Mio. vorstellen. Wahnsinn.
Nach wie vor misstraue ich aber der wirtschaftlichen Gesamtsituation. Außerdem werde ich am Montag eine kleine sehr spekulative Position bei Exelixis eingehen.
Bei SGEN bleibe ich erst mal an der Seitenlinie. Spätestens bei 15 Dollar wäre ein Rückkauf allerdings Pflicht.
Hallo,
mal wieder eine leicht negative Nachricht, SGEN mußte für Adcetris eine weitere Warnung vor Nebenwirkungen aufdrucken.
Kurs war aber schon ganz gut am Erholen in den letzten Wochen, nun wieder etwas zurückgekommen. Durch die Euro-Schwäche finde ich (wieder) keinen wesentlich günstigeren Wiedereinstieg
Vielleicht ist schon wieder Zeit für meine "Fuß-in-der-Tür"-Position.
So richtig negativ ist die Meldung ja nicht.
Text:
-----------------
..Seattle Genetics adds warning to Adcetris label
Seattle Genetics will add warning about brain infection to label of its cancer drug Adcetris
Associated Press – Fri, Jan 13, 2012 6:27 PM EST
....
Share0EmailPrint.....Companies:...Seattle Genetics Inc. . ..RELATED QUOTES.
.Symbol Price Change
SGEN 17.65 -0.79
......
BOTHELL, Wash. (AP) -- Seattle Genetics Inc. said Friday that its cancer drug Adcetris will carry a warning about a deadly type of brain infection that can occur in patients.
The company said Adcetris will carry a "black box" or boxed warning, the most serious type of warning label. The label will say that patients who take Adcetris can develop progressive multifocal leukoencephalopathy, a rare brain infection that is associated with weakened immune systems. Seattle Genetics said two cases of PML have been reported in Adcetris patients, and a third is suspected. The label will also warn that Adcetris should not be used with the cancer drug bleomycin because of a risk of heart damage.
Seattle Genetics and the Food and Drug Administration agreed on the changes.
Adcetris is approved as a treatment to treat Hodgkin's lymphoma and systemic anaplastic large cell lymphoma. Bleomycin, or Blenoxane, is used to treat cancers of the head, neck, and genitals.
Shares of Seattle Genetics fell 4.3 percent to $17.65 Friday, and were unchanged in aftermarket trading.
..
mal wieder eine leicht negative Nachricht, SGEN mußte für Adcetris eine weitere Warnung vor Nebenwirkungen aufdrucken.
Kurs war aber schon ganz gut am Erholen in den letzten Wochen, nun wieder etwas zurückgekommen. Durch die Euro-Schwäche finde ich (wieder) keinen wesentlich günstigeren Wiedereinstieg

Vielleicht ist schon wieder Zeit für meine "Fuß-in-der-Tür"-Position.
So richtig negativ ist die Meldung ja nicht.
Text:
-----------------
..Seattle Genetics adds warning to Adcetris label
Seattle Genetics will add warning about brain infection to label of its cancer drug Adcetris
Associated Press – Fri, Jan 13, 2012 6:27 PM EST
....
Share0EmailPrint.....Companies:...Seattle Genetics Inc. . ..RELATED QUOTES.
.Symbol Price Change
SGEN 17.65 -0.79
......
BOTHELL, Wash. (AP) -- Seattle Genetics Inc. said Friday that its cancer drug Adcetris will carry a warning about a deadly type of brain infection that can occur in patients.
The company said Adcetris will carry a "black box" or boxed warning, the most serious type of warning label. The label will say that patients who take Adcetris can develop progressive multifocal leukoencephalopathy, a rare brain infection that is associated with weakened immune systems. Seattle Genetics said two cases of PML have been reported in Adcetris patients, and a third is suspected. The label will also warn that Adcetris should not be used with the cancer drug bleomycin because of a risk of heart damage.
Seattle Genetics and the Food and Drug Administration agreed on the changes.
Adcetris is approved as a treatment to treat Hodgkin's lymphoma and systemic anaplastic large cell lymphoma. Bleomycin, or Blenoxane, is used to treat cancers of the head, neck, and genitals.
Shares of Seattle Genetics fell 4.3 percent to $17.65 Friday, and were unchanged in aftermarket trading.
..
Hallo,

Kurs stabilisiert sich anscheinend bei ca. 18$, habe nun wieder eine Position erworben. Da es nicht weiter fallen will, steigt´s hoffentlich. Ziel zunächst wieder die 21-22$.
Werde nun die Nachrichten rund um SGEN sicherlich wieder intensiver verfolgen.
Gruß q.

Kurs stabilisiert sich anscheinend bei ca. 18$, habe nun wieder eine Position erworben. Da es nicht weiter fallen will, steigt´s hoffentlich. Ziel zunächst wieder die 21-22$.
Werde nun die Nachrichten rund um SGEN sicherlich wieder intensiver verfolgen.
Gruß q.
Hallo,
in dieses Board muss ichs auch noch mal kopieren....
AMGN kauft MITI
(die ich gerade alle verkauft hatte)
grrrrrrrrrrrrrrrrrrr
um es nochmals zu sagen
grrrrrrrrrrrrrrrrrrrrrrrrrrr
Sollte einige Gelder in SGEN umleiten. In MOR ja evtl. auch, da gab es ja ohnehin schon Übernahmefantasien.
hier die Meldung:
January 26, 2012, 9:21 amMergers & Acquisitions
Amgen to Buy Micromet for $1.16 Billion
By EVELYN M. RUSLI
Amgen has agreed to buy Micromet, a biotechnology company that develops cancer drugs, for $1.16 billion.
The offer of $11 a share represents a premium of 33 percent from Micromet’s closing stock price on Wednesday.
Micromet, which currently has no drugs on the market, designs cancer treatments that target blood and solid tumor cancers, including leukemia and non-Hodgkin’s lymphomas. The company has research facilities in Munich, Germany and offices in Rockville, Maryland.
Among Micromet’s drugs in development is blinatumomab, which treats acute lymphoblastic leukemia, that is now in Phase 2 trials.
Article Tools E-mailPrintRecommendShare
Close
Tumblr
Digg
Linkedin
Reddit
Permalink
.Twitter ..Related Links•The press release
.“The acquisition of Micromet is an opportunity to acquire an innovative oncology asset with global rights and a validated technology platform with broad potential clinical applications,” Kevin Sharer, Amgen’s chief executive, said in a statement on Thursday. “Blinatumomab will serve as an important complement to our oncology pipeline.”
Amgen, which is set to reporting earnings after Thursday’s close, plans to acquire Micromet’s shares in two phases. A subsidiary of Amgen will buy at least a majority of Microment’s outstanding shares at $11 a piece. The parent company will then buy any remaining shares, at the same price. The deal will likely close in the first quarter, the company said in a statement.
Amgen, is one of the world’s largest drug makers, with more than $15 billion in annual sales, however, the company has struggled to fill its pipeline with new blockbuster drugs. Last year, the company slashed 6 percent of its research and development staff and issued a $5 billion share buy back in December.
This would be the biggest acquisition by Amgen since it had agreed to acquire Abgenix for $2.2 billion in cash in 2005, according to Capital IQ data.
Amgen was advised by Moelis & Company and the law firm of Sullivan & Cromwell. Goldman Sachs and the law firm of Cooley advised Micromet.
in dieses Board muss ichs auch noch mal kopieren....
AMGN kauft MITI
(die ich gerade alle verkauft hatte)
grrrrrrrrrrrrrrrrrrr
um es nochmals zu sagen
grrrrrrrrrrrrrrrrrrrrrrrrrrr
Sollte einige Gelder in SGEN umleiten. In MOR ja evtl. auch, da gab es ja ohnehin schon Übernahmefantasien.
hier die Meldung:
January 26, 2012, 9:21 amMergers & Acquisitions
Amgen to Buy Micromet for $1.16 Billion
By EVELYN M. RUSLI
Amgen has agreed to buy Micromet, a biotechnology company that develops cancer drugs, for $1.16 billion.
The offer of $11 a share represents a premium of 33 percent from Micromet’s closing stock price on Wednesday.
Micromet, which currently has no drugs on the market, designs cancer treatments that target blood and solid tumor cancers, including leukemia and non-Hodgkin’s lymphomas. The company has research facilities in Munich, Germany and offices in Rockville, Maryland.
Among Micromet’s drugs in development is blinatumomab, which treats acute lymphoblastic leukemia, that is now in Phase 2 trials.
Article Tools E-mailPrintRecommendShare
Close
Tumblr
Digg
Permalink
.Twitter ..Related Links•The press release
.“The acquisition of Micromet is an opportunity to acquire an innovative oncology asset with global rights and a validated technology platform with broad potential clinical applications,” Kevin Sharer, Amgen’s chief executive, said in a statement on Thursday. “Blinatumomab will serve as an important complement to our oncology pipeline.”
Amgen, which is set to reporting earnings after Thursday’s close, plans to acquire Micromet’s shares in two phases. A subsidiary of Amgen will buy at least a majority of Microment’s outstanding shares at $11 a piece. The parent company will then buy any remaining shares, at the same price. The deal will likely close in the first quarter, the company said in a statement.
Amgen, is one of the world’s largest drug makers, with more than $15 billion in annual sales, however, the company has struggled to fill its pipeline with new blockbuster drugs. Last year, the company slashed 6 percent of its research and development staff and issued a $5 billion share buy back in December.
This would be the biggest acquisition by Amgen since it had agreed to acquire Abgenix for $2.2 billion in cash in 2005, according to Capital IQ data.
Amgen was advised by Moelis & Company and the law firm of Sullivan & Cromwell. Goldman Sachs and the law firm of Cooley advised Micromet.
Moin,

mal sehen, wie der Markt die nachfolgenden detaillierten Resultate von Adcetris aufnimmt. Eine Verbesserung trat "nur" in ca. 50% der Patienten auf, 6x "stable disease", ein Patient starb sogar (daher wohl die Sache mit dem Warnhinweis). Gemischtes Bild.
------------------
..Seattle Genetics Announces Data from ADCETRIS™ in Cutaneous T-Cell Lymphoma and Peripheral T-Cell Lymphoma
-Interim Data Demonstrate 65 Percent Response Rate in Patients with Relapsed CTCL-
-Two Case Studies Provide First Report of Activity and Tolerability in PTCL-NOS Patients-
Press Release: Seattle Genetics, Inc. – 15 hours ago
....
Share0EmailPrint.....Companies:...Seattle Genetics Inc. . ..RELATED QUOTES.
.Symbol Price Change
SGEN 18.50 0.00
......
SAN FRANCISCO--(BUSINESS WIRE)-- Seattle Genetics, Inc. (Nasdaq:SGEN - News) today announced that interim results from an investigator-sponsored phase II clinical trial of ADCETRIS (brentuximab vedotin) in patients with cutaneous T-cell lymphoma (CTCL) were presented at the T-Cell Lymphoma Forum being held January 26-28, 2012 in San Francisco, CA. In addition, case studies were presented on two patients with relapsed peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) who were treated with ADCETRIS. ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30. ADCETRIS is not approved for use in CTCL or PTCL-NOS.
Investigator-Sponsored Phase II Trial in CTCL
At the time of data analysis, 17 of 23 enrolled patients had received at least two doses of ADCETRIS and were evaluable for response. All patients had relapsed CD30-positive cutaneous lymphoproliferative disorders, including lymphomatoid papulosis (LyP), primary cutaneous anaplastic large cell lymphoma (pcALCL) or mycosis fungoides (MF). Patients treated in this trial receive 1.8 milligrams per kilogram (mg/kg) of ADCETRIS every three weeks for up to a maximum of eight doses. Patients who achieve partial remission or stable disease are eligible to receive an additional eight doses. Key findings, which were presented by Dr. Madeleine Duvic from The University of Texas MD Anderson Cancer Center, include:
•Eleven of 17 evaluable patients (65 percent) achieved an objective response, including seven complete remissions (CRs) and four partial remissions (PRs). Six patients had stable disease. These investigator-assessed responses were observed in all three subtypes of CTCL.
•The most common adverse events were Grade 1, including diarrhea, chest tightness, alopecia, nausea, elevated liver enzymes and peripheral neuropathy. ADCETRIS treatment was held in two patients with Grade 2 peripheral sensory neuropathy and in one patient with Grade 4 neutropenia and Grade 3 leukopenia. One patient died of sepsis after one dose of ADCETRIS.
•Variable CD30 expression was observed in baseline lesions.
•The clinical trial is ongoing with planned enrollment of 29 patients.
PTCL Case Studies
Data were also presented from case studies of two patients with relapsed CD30-positive PTCL-NOS who were enrolled in phase I trials of ADCETRIS. PTCL-NOS is associated with a poor prognosis and there is no well-established standard of care. Both patients were heavily pretreated, including prior autologous stem cell transplant. The first patient achieved a PR with progression-free survival of eight months and overall survival of 22 months. Adverse events considered related to ADCETRIS were Grade 2 sensory neuropathy in the fingertips and Grade 3 fatigue. The second patient achieved a CR which is still ongoing with a duration of more than 19 months. The patient had Grade 3 anemia and other Grade 1 adverse events, none of which were considered by the investigator to be related to ADCETRIS.
About ADCETRIS
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics’ proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
Seattle Genetics and Millennium: The Takeda Oncology Company are jointly developing ADCETRIS. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group is solely responsible for development costs.
ADCETRIS was granted accelerated approval by the U.S. Food and Drug Administration (FDA) in August 2011 for two indications: (1) the treatment of patients with Hodgkin lymphoma after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates, and (2) the treatment of patients with sALCL after failure of at least one prior multi-agent chemotherapy regimen. The indications for ADCETRIS are based on response rate. There are no data available demonstrating improvement in patient-reported outcomes or survival with ADCETRIS. ADCETRIS has not been approved for use in CTCL or PTCL-NOS.
ADCETRIS is not approved for use outside the United States. The marketing authorization application for ADCETRIS in relapsed or refractory Hodgkin lymphoma and sALCL, filed by Takeda Global Research & Development Centre (Europe), was accepted by the European Medicines Agency for review in June 2011.
Seattle Genetics and Millennium: The Takeda Oncology Company are planning a phase III clinical trial of ADCETRIS in CD30-positive CTCL patients to begin by mid-2012.
About CTCL
Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Cutaneous lymphomas are a category of non-Hodgkin lymphomas that primarily involve the skin. According to the Cutaneous Lymphoma Foundation, CTCL is the most common type of cutaneous lymphoma that typically presents with red, scaly patches or thickened plaques of skin that often mimic eczema or chronic dermatitis. Progression from limited skin involvement is variable and may be accompanied by tumor formation, ulceration and exfoliation, complicated by itching and infections. Advanced stages are defined by involvement of lymph nodes, peripheral blood and internal organs.
About Seattle Genetics
Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer. The FDA granted accelerated approval of ADCETRIS in August 2011 for two indications. ADCETRIS is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has three other clinical-stage ADC programs: SGN-75, ASG-5ME and ASG-22ME. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
U.S. Important Safety Information
BOXED WARNING
Progressive multifocal leukoencephalopathy (PML): JC virus infection resulting in PML and death can occur in patients receiving ADCETRIS.
Contraindication:
Concomitant use of ADCETRIS and bleomycin is contraindicated due to pulmonary toxicity.
Warnings and Precautions:
•Peripheral neuropathy: ADCETRIS treatment causes a peripheral neuropathy that is predominantly sensory. Cases of peripheral motor neuropathy have also been reported. ADCETRIS-induced peripheral neuropathy is cumulative. Treating physicians should monitor patients for symptoms of neuropathy, such as hypoesthesia, hyperesthesia, paresthesia, discomfort, a burning sensation, neuropathic pain or weakness and institute dose modifications accordingly.
•Infusion reactions: Infusion-related reactions, including anaphylaxis, have occurred with ADCETRIS. Monitor patients during infusion. If an infusion reaction occurs, the infusion should be interrupted and appropriate medical management instituted. If anaphylaxis occurs, the infusion should be immediately and permanently discontinued and appropriate medical management instituted.
•Neutropenia: Monitor complete blood counts prior to each dose of ADCETRIS and consider more frequent monitoring for patients with Grade 3 or 4 neutropenia. If Grade 3 or 4 neutropenia develops, manage by dose delays, reductions or discontinuation. Prolonged (≥1 week) severe neutropenia can occur with ADCETRIS.
•Tumor lysis syndrome: Patients with rapidly proliferating tumor and high tumor burden are at risk of tumor lysis syndrome and these patients should be monitored closely and appropriate measures taken.
•Progressive multifocal leukoencephalopathy (PML): JC virus infection resulting in PML and death has been reported in ADCETRIS-treated patients. In addition to ADCETRIS therapy, other possible contributory factors include prior therapies and underlying disease that may cause immunosuppression. Consider the diagnosis of PML in any patient presenting with new-onset signs and symptoms of central nervous system abnormalities. Evaluation of PML includes, but is not limited to, consultation with a neurologist, brain MRI, and lumbar puncture or brain biopsy. Hold ADCETRIS if PML is suspected and discontinue ADCETRIS if PML is confirmed.
•Stevens-Johnson syndrome: Stevens-Johnson syndrome has been reported with ADCETRIS. If Stevens-Johnson syndrome occurs, discontinue ADCETRIS and administer appropriate medical therapy.
•Use in pregnancy: Fetal harm can occur. Pregnant women should be advised of the potential hazard to the fetus.
Adverse Reactions:
ADCETRIS was studied as monotherapy in 160 patients in two phase 2 trials. Across both trials, the most common adverse reactions (≥20%), regardless of causality, were neutropenia, peripheral sensory neuropathy, fatigue, nausea, anemia, upper respiratory tract infection, diarrhea, pyrexia, rash, thrombocytopenia, cough and vomiting.
Drug Interactions:
Patients who are receiving strong CYP3A4 inhibitors concomitantly with ADCETRIS should be closely monitored for adverse reactions.
For additional important safety information, including Boxed WARNING, please see the full U.S. prescribing information for ADCETRIS at www.seattlegenetics.com or www.ADCETRIS.com.
Certain of the statements made in this press release are forward looking, such as those, among others, relating to the therapeutic potential of ADCETRIS and initiation of future clinical trials. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include the inability to show sufficient activity in clinical trials and the risk of adverse events as ADCETRIS advances in such clinical trials. In addition, data from our clinical trials, including our pivotal trials which were the basis for FDA accelerated approval, may not necessarily be indicative of subsequent clinical trial results. More information about the risks and uncertainties faced by Seattle Genetics is contained in the company’s 10-Q for the quarter ended September 30, 2011 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
..Contact:.
.Seattle Genetics, Inc.
Peggy Pinkston, 425-527-4160
ppinkston@seagen.com.
...

mal sehen, wie der Markt die nachfolgenden detaillierten Resultate von Adcetris aufnimmt. Eine Verbesserung trat "nur" in ca. 50% der Patienten auf, 6x "stable disease", ein Patient starb sogar (daher wohl die Sache mit dem Warnhinweis). Gemischtes Bild.
------------------
..Seattle Genetics Announces Data from ADCETRIS™ in Cutaneous T-Cell Lymphoma and Peripheral T-Cell Lymphoma
-Interim Data Demonstrate 65 Percent Response Rate in Patients with Relapsed CTCL-
-Two Case Studies Provide First Report of Activity and Tolerability in PTCL-NOS Patients-
Press Release: Seattle Genetics, Inc. – 15 hours ago
....
Share0EmailPrint.....Companies:...Seattle Genetics Inc. . ..RELATED QUOTES.
.Symbol Price Change
SGEN 18.50 0.00
......
SAN FRANCISCO--(BUSINESS WIRE)-- Seattle Genetics, Inc. (Nasdaq:SGEN - News) today announced that interim results from an investigator-sponsored phase II clinical trial of ADCETRIS (brentuximab vedotin) in patients with cutaneous T-cell lymphoma (CTCL) were presented at the T-Cell Lymphoma Forum being held January 26-28, 2012 in San Francisco, CA. In addition, case studies were presented on two patients with relapsed peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) who were treated with ADCETRIS. ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30. ADCETRIS is not approved for use in CTCL or PTCL-NOS.
Investigator-Sponsored Phase II Trial in CTCL
At the time of data analysis, 17 of 23 enrolled patients had received at least two doses of ADCETRIS and were evaluable for response. All patients had relapsed CD30-positive cutaneous lymphoproliferative disorders, including lymphomatoid papulosis (LyP), primary cutaneous anaplastic large cell lymphoma (pcALCL) or mycosis fungoides (MF). Patients treated in this trial receive 1.8 milligrams per kilogram (mg/kg) of ADCETRIS every three weeks for up to a maximum of eight doses. Patients who achieve partial remission or stable disease are eligible to receive an additional eight doses. Key findings, which were presented by Dr. Madeleine Duvic from The University of Texas MD Anderson Cancer Center, include:
•Eleven of 17 evaluable patients (65 percent) achieved an objective response, including seven complete remissions (CRs) and four partial remissions (PRs). Six patients had stable disease. These investigator-assessed responses were observed in all three subtypes of CTCL.
•The most common adverse events were Grade 1, including diarrhea, chest tightness, alopecia, nausea, elevated liver enzymes and peripheral neuropathy. ADCETRIS treatment was held in two patients with Grade 2 peripheral sensory neuropathy and in one patient with Grade 4 neutropenia and Grade 3 leukopenia. One patient died of sepsis after one dose of ADCETRIS.
•Variable CD30 expression was observed in baseline lesions.
•The clinical trial is ongoing with planned enrollment of 29 patients.
PTCL Case Studies
Data were also presented from case studies of two patients with relapsed CD30-positive PTCL-NOS who were enrolled in phase I trials of ADCETRIS. PTCL-NOS is associated with a poor prognosis and there is no well-established standard of care. Both patients were heavily pretreated, including prior autologous stem cell transplant. The first patient achieved a PR with progression-free survival of eight months and overall survival of 22 months. Adverse events considered related to ADCETRIS were Grade 2 sensory neuropathy in the fingertips and Grade 3 fatigue. The second patient achieved a CR which is still ongoing with a duration of more than 19 months. The patient had Grade 3 anemia and other Grade 1 adverse events, none of which were considered by the investigator to be related to ADCETRIS.
About ADCETRIS
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics’ proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
Seattle Genetics and Millennium: The Takeda Oncology Company are jointly developing ADCETRIS. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group is solely responsible for development costs.
ADCETRIS was granted accelerated approval by the U.S. Food and Drug Administration (FDA) in August 2011 for two indications: (1) the treatment of patients with Hodgkin lymphoma after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates, and (2) the treatment of patients with sALCL after failure of at least one prior multi-agent chemotherapy regimen. The indications for ADCETRIS are based on response rate. There are no data available demonstrating improvement in patient-reported outcomes or survival with ADCETRIS. ADCETRIS has not been approved for use in CTCL or PTCL-NOS.
ADCETRIS is not approved for use outside the United States. The marketing authorization application for ADCETRIS in relapsed or refractory Hodgkin lymphoma and sALCL, filed by Takeda Global Research & Development Centre (Europe), was accepted by the European Medicines Agency for review in June 2011.
Seattle Genetics and Millennium: The Takeda Oncology Company are planning a phase III clinical trial of ADCETRIS in CD30-positive CTCL patients to begin by mid-2012.
About CTCL
Lymphoma is a general term for a group of cancers that originate in the lymphatic system. There are two major categories of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Cutaneous lymphomas are a category of non-Hodgkin lymphomas that primarily involve the skin. According to the Cutaneous Lymphoma Foundation, CTCL is the most common type of cutaneous lymphoma that typically presents with red, scaly patches or thickened plaques of skin that often mimic eczema or chronic dermatitis. Progression from limited skin involvement is variable and may be accompanied by tumor formation, ulceration and exfoliation, complicated by itching and infections. Advanced stages are defined by involvement of lymph nodes, peripheral blood and internal organs.
About Seattle Genetics
Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer. The FDA granted accelerated approval of ADCETRIS in August 2011 for two indications. ADCETRIS is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has three other clinical-stage ADC programs: SGN-75, ASG-5ME and ASG-22ME. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
U.S. Important Safety Information
BOXED WARNING
Progressive multifocal leukoencephalopathy (PML): JC virus infection resulting in PML and death can occur in patients receiving ADCETRIS.
Contraindication:
Concomitant use of ADCETRIS and bleomycin is contraindicated due to pulmonary toxicity.
Warnings and Precautions:
•Peripheral neuropathy: ADCETRIS treatment causes a peripheral neuropathy that is predominantly sensory. Cases of peripheral motor neuropathy have also been reported. ADCETRIS-induced peripheral neuropathy is cumulative. Treating physicians should monitor patients for symptoms of neuropathy, such as hypoesthesia, hyperesthesia, paresthesia, discomfort, a burning sensation, neuropathic pain or weakness and institute dose modifications accordingly.
•Infusion reactions: Infusion-related reactions, including anaphylaxis, have occurred with ADCETRIS. Monitor patients during infusion. If an infusion reaction occurs, the infusion should be interrupted and appropriate medical management instituted. If anaphylaxis occurs, the infusion should be immediately and permanently discontinued and appropriate medical management instituted.
•Neutropenia: Monitor complete blood counts prior to each dose of ADCETRIS and consider more frequent monitoring for patients with Grade 3 or 4 neutropenia. If Grade 3 or 4 neutropenia develops, manage by dose delays, reductions or discontinuation. Prolonged (≥1 week) severe neutropenia can occur with ADCETRIS.
•Tumor lysis syndrome: Patients with rapidly proliferating tumor and high tumor burden are at risk of tumor lysis syndrome and these patients should be monitored closely and appropriate measures taken.
•Progressive multifocal leukoencephalopathy (PML): JC virus infection resulting in PML and death has been reported in ADCETRIS-treated patients. In addition to ADCETRIS therapy, other possible contributory factors include prior therapies and underlying disease that may cause immunosuppression. Consider the diagnosis of PML in any patient presenting with new-onset signs and symptoms of central nervous system abnormalities. Evaluation of PML includes, but is not limited to, consultation with a neurologist, brain MRI, and lumbar puncture or brain biopsy. Hold ADCETRIS if PML is suspected and discontinue ADCETRIS if PML is confirmed.
•Stevens-Johnson syndrome: Stevens-Johnson syndrome has been reported with ADCETRIS. If Stevens-Johnson syndrome occurs, discontinue ADCETRIS and administer appropriate medical therapy.
•Use in pregnancy: Fetal harm can occur. Pregnant women should be advised of the potential hazard to the fetus.
Adverse Reactions:
ADCETRIS was studied as monotherapy in 160 patients in two phase 2 trials. Across both trials, the most common adverse reactions (≥20%), regardless of causality, were neutropenia, peripheral sensory neuropathy, fatigue, nausea, anemia, upper respiratory tract infection, diarrhea, pyrexia, rash, thrombocytopenia, cough and vomiting.
Drug Interactions:
Patients who are receiving strong CYP3A4 inhibitors concomitantly with ADCETRIS should be closely monitored for adverse reactions.
For additional important safety information, including Boxed WARNING, please see the full U.S. prescribing information for ADCETRIS at www.seattlegenetics.com or www.ADCETRIS.com.
Certain of the statements made in this press release are forward looking, such as those, among others, relating to the therapeutic potential of ADCETRIS and initiation of future clinical trials. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include the inability to show sufficient activity in clinical trials and the risk of adverse events as ADCETRIS advances in such clinical trials. In addition, data from our clinical trials, including our pivotal trials which were the basis for FDA accelerated approval, may not necessarily be indicative of subsequent clinical trial results. More information about the risks and uncertainties faced by Seattle Genetics is contained in the company’s 10-Q for the quarter ended September 30, 2011 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
..Contact:.
.Seattle Genetics, Inc.
Peggy Pinkston, 425-527-4160
ppinkston@seagen.com.
...
Hallo,

mein timing bei MITI mag schlecht gewesen sein (obwohl ich schon auch etwas Gewinn hatte, man soll ja nicht meckern), bei SGEN läuft´s dafür gleich (wieder) gut los. Die Daten werden vom Markt offenbar gut aufgenommen, der Anstieg könnte aber auch durch Übernahmegerüchte durch Merck mit beeinflusst worden sein.
We´ll see
q.
------------------------------------------
Seattle Genetics Rises on Positive Trial Data
QBy Ryan Flinn - Jan 27, 2012 7:38 PM GMT+0100 .
inShare.0
More
Business ExchangeBuzz up!DiggPrint Email ...Seattle Genetics Inc. (SGEN), maker of the Hodgkin lymphoma drug Adcetris, is trading at its highest level in more than two months after reporting positive data from additional studies on the medicine.
The drugmaker gained 6.7 percent to $19.74 at 1:10 p.m. New York time, the highest price for shares of the Bothell, Washington-based company since Nov. 3.
Adcetris was approved in August for patients with Hodgkin lymphoma for whom other therapies have failed, the first new medicine cleared by U.S. regulators for the disease since 1977. The drug had a 65 percent response rate according to interim results of a Phase 2 clinical trial of patients with cutaneous T-cell lymphoma, the company said yesterday at a medical meeting in San Francisco. Three stages of clinical trials are usually required for U.S. regulatory approval.
Adcetris, known chemically as brentuximab, is Seattle Genetics’s first product. Sales of the medicine may surpass $275 million in 2014, according to the average estimate of three analysts surveyed by Bloomberg.

mein timing bei MITI mag schlecht gewesen sein (obwohl ich schon auch etwas Gewinn hatte, man soll ja nicht meckern), bei SGEN läuft´s dafür gleich (wieder) gut los. Die Daten werden vom Markt offenbar gut aufgenommen, der Anstieg könnte aber auch durch Übernahmegerüchte durch Merck mit beeinflusst worden sein.
We´ll see
q.
------------------------------------------
Seattle Genetics Rises on Positive Trial Data
QBy Ryan Flinn - Jan 27, 2012 7:38 PM GMT+0100 .
inShare.0
More
Business ExchangeBuzz up!DiggPrint Email ...Seattle Genetics Inc. (SGEN), maker of the Hodgkin lymphoma drug Adcetris, is trading at its highest level in more than two months after reporting positive data from additional studies on the medicine.
The drugmaker gained 6.7 percent to $19.74 at 1:10 p.m. New York time, the highest price for shares of the Bothell, Washington-based company since Nov. 3.
Adcetris was approved in August for patients with Hodgkin lymphoma for whom other therapies have failed, the first new medicine cleared by U.S. regulators for the disease since 1977. The drug had a 65 percent response rate according to interim results of a Phase 2 clinical trial of patients with cutaneous T-cell lymphoma, the company said yesterday at a medical meeting in San Francisco. Three stages of clinical trials are usually required for U.S. regulatory approval.
Adcetris, known chemically as brentuximab, is Seattle Genetics’s first product. Sales of the medicine may surpass $275 million in 2014, according to the average estimate of three analysts surveyed by Bloomberg.
Hallo,

der seeking-alpha-Artikel vom Freitag macht mich doch auch etwas nachdenklich.
Wenn ziemlich viele Patienten die Behandlung abbrechen (wegen Fortschreitens der Krankheit), werden die Adcetris-Umsätze evtl. enttäuschen.
Werde das Geschehen vor den morgigen Zahlen genau beobachten und ggf. sogar wieder verkaufen.
Die Anzahl der hier mitlesenden User scheint ja ohnehin gering, aber egal. Hier der Artikel, leider etwas lang, aber durchaus lesenswert:
----------------
Seattle Genetics' Adcetris Launch: Second Survey Reveals Additional Insights
2 comments | by: Andrew McDonald February 10, 2012 | about: SGEN, includes: ACOR, AUXL, INCY, NVS, OPTR, SPPI Font Size: PrintEmail Recommend 0 Share this page
inShare0Share0 On Monday, February 13, Seattle Genetics (SGEN) will release its fourth quarter and year end results, and investors will get the first full-quarter sales data for Adcetris, the company's first marketed drug which was approved on August 19, 2011. We have conducted two Adcetris usage surveys of hematologists and report a summary of the second survey's results herein. Full results of the surveys can be downloaded for free here. We have found these physician surveys to be helpful in forecasting sales for several new products, including those of Optimer Pharmaceuticals (OPTR), Spectrum Pharmaceuticals (SPPI), Incyte (INCY)/Novartis (NVS), Acorda Therapeutics (ACOR), and Auxilium Pharmaceuticals (AUXL).
Seattle Genetics reported $10MM in Adcetris sales for 3Q11, which represented 6 weeks of sales for that quarter. This amount of sales beat analyst estimates, and the strength of the initial launch was attributed to both patients switching over from the company's expanded access clinical program and to new patients. The trajectory of Adcetris sales remains a question for investors now that (1) the initial bolus of U.S. patients have been transferred to commercial product and (2) patients are starting to progress after treatment.
Another outstanding question for investors is the safety profile of Adcetris, as this profile relates to potential label expansion. For example, in a Phase I trial as a front-line treatment for patients with newly diagnosed HL in which study participants received Adcetris along with a standard chemotherapy regimen, ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine), approximately 40% of patients experienced pulmonary toxicity, compared to a 10-25% historical incidence for patients taking ABVD alone. As a result of the finding, the study protocol was amended and to remove bleomycin, which is also associated with pulmonary toxicities. According to data presented at the American Society for Hematology (ASH) conference, patients treated with Adcetris plus AVD have not yet had problems with pulmonary toxicity. However, the safety question is still an issue that investors will be following closely. This is especially important for front-line treatment. We believe that in order to succeed as a front-line therapy, Adcetris containing regimens should have an acceptable safety profile. The company expects to start a Phase III in font-line late this year or early in 2013.
In addition to seeking a front line label in HL, the company is running multiple other clinical trials to expand the label. A Phase III study is testing Adcetris for post autologous stem cell transplant HL as a means to prevent relapse; enrollment is expected to complete later this year, with data expected sometime in 2013 or 2014. Phase II trials are being conducted in CD30 positive cancers including r/r non-Hodgkin lymphomas and non-lymphoma malignancies, and for the retreatment of hematological malignancies. A Phase I study is underway testing Adcetris as a front-line therapy for CD30 positive mature T-cell malignancies.
Before we look at the survey results, we note the company's valuation. At a price of $19.01 and with 115MM shares outstanding, Seattle Genetics has a market cap of $2.19 billion and an enterprise value of $1.86 billion. The company ended 3Q11 with $375 million in cash and investments and no debt. Revenues for the third quarter totaled $20.7 million, with $10 million in revenue contributed to net Adcetris sales. Net loss for the quarter was $40.7 million.
Our second survey of hematologists in our proprietary Expert Network regarding Adcetris aimed to gauge the number of patients taking Adcetris and to determine, at least qualitatively, the strength of the launch. We note that the survey has inherent biases that may affect the accuracy of the results. Hence, we recommend interpreting the results with caution.
As a reminder, our November survey yielded responses from 79 hematologists who had treated a total of 76 patients with Adcetris. The current survey yielded responses from 72 hematologists, including 36 physicians who responded to the previous survey and 36 new respondents. Overall, survey respondents have treated a total of 84 patients, an increase of 8 patients over the November number.
The 36 hematologists who responded to both surveys reported having treated 50 patients in the November survey and 44 patients in the January survey. Of these 44 patients, 18 have discontinued therapy (41%). For the 36 new responders to the January survey, 14/40 (35%) of patients have discontinued Adcetris treatment.
A significant portion of patients has discontinued Adcetris use, and the most commonly cited reason was disease progression. A total of 32/84 (38%) of patients treated has discontinued. According to the January survey, 45% (22/49) of patients at academic/major referral centers and 29% (10/35) of patients at community centers discontinued Adcetris use.
The survey results lend credence to the hypothesis that there was pent-up demand for Adcetris and that after the initial rush of patients, the rate of new starts may be slower. Reports of patients discontinuing Adcetris should also be reflected in sales.
We had hypothesized, based on the results of the November survey that peak U.S. sales of Adcetris for the r/r HL and ALCL indications could range be as high as $513MM. Based on the results of the November survey, we hypothesize that U.S. sales could be as high as $438MM. Unknown variables still include label expansion potential and ex-U.S. royalty revenue, which could influence share price.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Seattle Genetics' Adcetris Launch: Second Survey Reveals Additional Insights
2 comments | by: Andrew McDonald February 10, 2012 | about: SGEN, includes: ACOR, AUXL, INCY, NVS, OPTR, SPPI Font Size: PrintEmail Recommend 0 Share this page
inShare0Share0 On Monday, February 13, Seattle Genetics (SGEN) will release its fourth quarter and year end results, and investors will get the first full-quarter sales data for Adcetris, the company's first marketed drug which was approved on August 19, 2011. We have conducted two Adcetris usage surveys of hematologists and report a summary of the second survey's results herein. Full results of the surveys can be downloaded for free here. We have found these physician surveys to be helpful in forecasting sales for several new products, including those of Optimer Pharmaceuticals (OPTR), Spectrum Pharmaceuticals (SPPI), Incyte (INCY)/Novartis (NVS), Acorda Therapeutics (ACOR), and Auxilium Pharmaceuticals (AUXL).
Seattle Genetics reported $10MM in Adcetris sales for 3Q11, which represented 6 weeks of sales for that quarter. This amount of sales beat analyst estimates, and the strength of the initial launch was attributed to both patients switching over from the company's expanded access clinical program and to new patients. The trajectory of Adcetris sales remains a question for investors now that (1) the initial bolus of U.S. patients have been transferred to commercial product and (2) patients are starting to progress after treatment.
Another outstanding question for investors is the safety profile of Adcetris, as this profile relates to potential label expansion. For example, in a Phase I trial as a front-line treatment for patients with newly diagnosed HL in which study participants received Adcetris along with a standard chemotherapy regimen, ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine), approximately 40% of patients experienced pulmonary toxicity, compared to a 10-25% historical incidence for patients taking ABVD alone. As a result of the finding, the study protocol was amended and to remove bleomycin, which is also associated with pulmonary toxicities. According to data presented at the American Society for Hematology (ASH) conference, patients treated with Adcetris plus AVD have not yet had problems with pulmonary toxicity. However, the safety question is still an issue that investors will be following closely. This is especially important for front-line treatment. We believe that in order to succeed as a front-line therapy, Adcetris containing regimens should have an acceptable safety profile. The company expects to start a Phase III in font-line late this year or early in 2013.
In addition to seeking a front line label in HL, the company is running multiple other clinical trials to expand the label. A Phase III study is testing Adcetris for post autologous stem cell transplant HL as a means to prevent relapse; enrollment is expected to complete later this year, with data expected sometime in 2013 or 2014. Phase II trials are being conducted in CD30 positive cancers including r/r non-Hodgkin lymphomas and non-lymphoma malignancies, and for the retreatment of hematological malignancies. A Phase I study is underway testing Adcetris as a front-line therapy for CD30 positive mature T-cell malignancies.
Before we look at the survey results, we note the company's valuation. At a price of $19.01 and with 115MM shares outstanding, Seattle Genetics has a market cap of $2.19 billion and an enterprise value of $1.86 billion. The company ended 3Q11 with $375 million in cash and investments and no debt. Revenues for the third quarter totaled $20.7 million, with $10 million in revenue contributed to net Adcetris sales. Net loss for the quarter was $40.7 million.
Our second survey of hematologists in our proprietary Expert Network regarding Adcetris aimed to gauge the number of patients taking Adcetris and to determine, at least qualitatively, the strength of the launch. We note that the survey has inherent biases that may affect the accuracy of the results. Hence, we recommend interpreting the results with caution.
As a reminder, our November survey yielded responses from 79 hematologists who had treated a total of 76 patients with Adcetris. The current survey yielded responses from 72 hematologists, including 36 physicians who responded to the previous survey and 36 new respondents. Overall, survey respondents have treated a total of 84 patients, an increase of 8 patients over the November number.
The 36 hematologists who responded to both surveys reported having treated 50 patients in the November survey and 44 patients in the January survey. Of these 44 patients, 18 have discontinued therapy (41%). For the 36 new responders to the January survey, 14/40 (35%) of patients have discontinued Adcetris treatment.
A significant portion of patients has discontinued Adcetris use, and the most commonly cited reason was disease progression. A total of 32/84 (38%) of patients treated has discontinued. According to the January survey, 45% (22/49) of patients at academic/major referral centers and 29% (10/35) of patients at community centers discontinued Adcetris use.
The survey results lend credence to the hypothesis that there was pent-up demand for Adcetris and that after the initial rush of patients, the rate of new starts may be slower. Reports of patients discontinuing Adcetris should also be reflected in sales.
We had hypothesized, based on the results of the November survey that peak U.S. sales of Adcetris for the r/r HL and ALCL indications could range be as high as $513MM. Based on the results of the November survey, we hypothesize that U.S. sales could be as high as $438MM. Unknown variables still include label expansion potential and ex-U.S. royalty revenue, which could influence share price.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.

der seeking-alpha-Artikel vom Freitag macht mich doch auch etwas nachdenklich.
Wenn ziemlich viele Patienten die Behandlung abbrechen (wegen Fortschreitens der Krankheit), werden die Adcetris-Umsätze evtl. enttäuschen.
Werde das Geschehen vor den morgigen Zahlen genau beobachten und ggf. sogar wieder verkaufen.
Die Anzahl der hier mitlesenden User scheint ja ohnehin gering, aber egal. Hier der Artikel, leider etwas lang, aber durchaus lesenswert:
----------------
Seattle Genetics' Adcetris Launch: Second Survey Reveals Additional Insights
2 comments | by: Andrew McDonald February 10, 2012 | about: SGEN, includes: ACOR, AUXL, INCY, NVS, OPTR, SPPI Font Size: PrintEmail Recommend 0 Share this page
inShare0Share0 On Monday, February 13, Seattle Genetics (SGEN) will release its fourth quarter and year end results, and investors will get the first full-quarter sales data for Adcetris, the company's first marketed drug which was approved on August 19, 2011. We have conducted two Adcetris usage surveys of hematologists and report a summary of the second survey's results herein. Full results of the surveys can be downloaded for free here. We have found these physician surveys to be helpful in forecasting sales for several new products, including those of Optimer Pharmaceuticals (OPTR), Spectrum Pharmaceuticals (SPPI), Incyte (INCY)/Novartis (NVS), Acorda Therapeutics (ACOR), and Auxilium Pharmaceuticals (AUXL).
Seattle Genetics reported $10MM in Adcetris sales for 3Q11, which represented 6 weeks of sales for that quarter. This amount of sales beat analyst estimates, and the strength of the initial launch was attributed to both patients switching over from the company's expanded access clinical program and to new patients. The trajectory of Adcetris sales remains a question for investors now that (1) the initial bolus of U.S. patients have been transferred to commercial product and (2) patients are starting to progress after treatment.
Another outstanding question for investors is the safety profile of Adcetris, as this profile relates to potential label expansion. For example, in a Phase I trial as a front-line treatment for patients with newly diagnosed HL in which study participants received Adcetris along with a standard chemotherapy regimen, ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine), approximately 40% of patients experienced pulmonary toxicity, compared to a 10-25% historical incidence for patients taking ABVD alone. As a result of the finding, the study protocol was amended and to remove bleomycin, which is also associated with pulmonary toxicities. According to data presented at the American Society for Hematology (ASH) conference, patients treated with Adcetris plus AVD have not yet had problems with pulmonary toxicity. However, the safety question is still an issue that investors will be following closely. This is especially important for front-line treatment. We believe that in order to succeed as a front-line therapy, Adcetris containing regimens should have an acceptable safety profile. The company expects to start a Phase III in font-line late this year or early in 2013.
In addition to seeking a front line label in HL, the company is running multiple other clinical trials to expand the label. A Phase III study is testing Adcetris for post autologous stem cell transplant HL as a means to prevent relapse; enrollment is expected to complete later this year, with data expected sometime in 2013 or 2014. Phase II trials are being conducted in CD30 positive cancers including r/r non-Hodgkin lymphomas and non-lymphoma malignancies, and for the retreatment of hematological malignancies. A Phase I study is underway testing Adcetris as a front-line therapy for CD30 positive mature T-cell malignancies.
Before we look at the survey results, we note the company's valuation. At a price of $19.01 and with 115MM shares outstanding, Seattle Genetics has a market cap of $2.19 billion and an enterprise value of $1.86 billion. The company ended 3Q11 with $375 million in cash and investments and no debt. Revenues for the third quarter totaled $20.7 million, with $10 million in revenue contributed to net Adcetris sales. Net loss for the quarter was $40.7 million.
Our second survey of hematologists in our proprietary Expert Network regarding Adcetris aimed to gauge the number of patients taking Adcetris and to determine, at least qualitatively, the strength of the launch. We note that the survey has inherent biases that may affect the accuracy of the results. Hence, we recommend interpreting the results with caution.
As a reminder, our November survey yielded responses from 79 hematologists who had treated a total of 76 patients with Adcetris. The current survey yielded responses from 72 hematologists, including 36 physicians who responded to the previous survey and 36 new respondents. Overall, survey respondents have treated a total of 84 patients, an increase of 8 patients over the November number.
The 36 hematologists who responded to both surveys reported having treated 50 patients in the November survey and 44 patients in the January survey. Of these 44 patients, 18 have discontinued therapy (41%). For the 36 new responders to the January survey, 14/40 (35%) of patients have discontinued Adcetris treatment.
A significant portion of patients has discontinued Adcetris use, and the most commonly cited reason was disease progression. A total of 32/84 (38%) of patients treated has discontinued. According to the January survey, 45% (22/49) of patients at academic/major referral centers and 29% (10/35) of patients at community centers discontinued Adcetris use.
The survey results lend credence to the hypothesis that there was pent-up demand for Adcetris and that after the initial rush of patients, the rate of new starts may be slower. Reports of patients discontinuing Adcetris should also be reflected in sales.
We had hypothesized, based on the results of the November survey that peak U.S. sales of Adcetris for the r/r HL and ALCL indications could range be as high as $513MM. Based on the results of the November survey, we hypothesize that U.S. sales could be as high as $438MM. Unknown variables still include label expansion potential and ex-U.S. royalty revenue, which could influence share price.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Seattle Genetics' Adcetris Launch: Second Survey Reveals Additional Insights
2 comments | by: Andrew McDonald February 10, 2012 | about: SGEN, includes: ACOR, AUXL, INCY, NVS, OPTR, SPPI Font Size: PrintEmail Recommend 0 Share this page
inShare0Share0 On Monday, February 13, Seattle Genetics (SGEN) will release its fourth quarter and year end results, and investors will get the first full-quarter sales data for Adcetris, the company's first marketed drug which was approved on August 19, 2011. We have conducted two Adcetris usage surveys of hematologists and report a summary of the second survey's results herein. Full results of the surveys can be downloaded for free here. We have found these physician surveys to be helpful in forecasting sales for several new products, including those of Optimer Pharmaceuticals (OPTR), Spectrum Pharmaceuticals (SPPI), Incyte (INCY)/Novartis (NVS), Acorda Therapeutics (ACOR), and Auxilium Pharmaceuticals (AUXL).
Seattle Genetics reported $10MM in Adcetris sales for 3Q11, which represented 6 weeks of sales for that quarter. This amount of sales beat analyst estimates, and the strength of the initial launch was attributed to both patients switching over from the company's expanded access clinical program and to new patients. The trajectory of Adcetris sales remains a question for investors now that (1) the initial bolus of U.S. patients have been transferred to commercial product and (2) patients are starting to progress after treatment.
Another outstanding question for investors is the safety profile of Adcetris, as this profile relates to potential label expansion. For example, in a Phase I trial as a front-line treatment for patients with newly diagnosed HL in which study participants received Adcetris along with a standard chemotherapy regimen, ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine), approximately 40% of patients experienced pulmonary toxicity, compared to a 10-25% historical incidence for patients taking ABVD alone. As a result of the finding, the study protocol was amended and to remove bleomycin, which is also associated with pulmonary toxicities. According to data presented at the American Society for Hematology (ASH) conference, patients treated with Adcetris plus AVD have not yet had problems with pulmonary toxicity. However, the safety question is still an issue that investors will be following closely. This is especially important for front-line treatment. We believe that in order to succeed as a front-line therapy, Adcetris containing regimens should have an acceptable safety profile. The company expects to start a Phase III in font-line late this year or early in 2013.
In addition to seeking a front line label in HL, the company is running multiple other clinical trials to expand the label. A Phase III study is testing Adcetris for post autologous stem cell transplant HL as a means to prevent relapse; enrollment is expected to complete later this year, with data expected sometime in 2013 or 2014. Phase II trials are being conducted in CD30 positive cancers including r/r non-Hodgkin lymphomas and non-lymphoma malignancies, and for the retreatment of hematological malignancies. A Phase I study is underway testing Adcetris as a front-line therapy for CD30 positive mature T-cell malignancies.
Before we look at the survey results, we note the company's valuation. At a price of $19.01 and with 115MM shares outstanding, Seattle Genetics has a market cap of $2.19 billion and an enterprise value of $1.86 billion. The company ended 3Q11 with $375 million in cash and investments and no debt. Revenues for the third quarter totaled $20.7 million, with $10 million in revenue contributed to net Adcetris sales. Net loss for the quarter was $40.7 million.
Our second survey of hematologists in our proprietary Expert Network regarding Adcetris aimed to gauge the number of patients taking Adcetris and to determine, at least qualitatively, the strength of the launch. We note that the survey has inherent biases that may affect the accuracy of the results. Hence, we recommend interpreting the results with caution.
As a reminder, our November survey yielded responses from 79 hematologists who had treated a total of 76 patients with Adcetris. The current survey yielded responses from 72 hematologists, including 36 physicians who responded to the previous survey and 36 new respondents. Overall, survey respondents have treated a total of 84 patients, an increase of 8 patients over the November number.
The 36 hematologists who responded to both surveys reported having treated 50 patients in the November survey and 44 patients in the January survey. Of these 44 patients, 18 have discontinued therapy (41%). For the 36 new responders to the January survey, 14/40 (35%) of patients have discontinued Adcetris treatment.
A significant portion of patients has discontinued Adcetris use, and the most commonly cited reason was disease progression. A total of 32/84 (38%) of patients treated has discontinued. According to the January survey, 45% (22/49) of patients at academic/major referral centers and 29% (10/35) of patients at community centers discontinued Adcetris use.
The survey results lend credence to the hypothesis that there was pent-up demand for Adcetris and that after the initial rush of patients, the rate of new starts may be slower. Reports of patients discontinuing Adcetris should also be reflected in sales.
We had hypothesized, based on the results of the November survey that peak U.S. sales of Adcetris for the r/r HL and ALCL indications could range be as high as $513MM. Based on the results of the November survey, we hypothesize that U.S. sales could be as high as $438MM. Unknown variables still include label expansion potential and ex-U.S. royalty revenue, which could influence share price.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Moin,

die Zahlen sind raus und wie so oft bei Biotechs sehr unterschiedlich interpretierbar. Nachbörslich hat´s noch ein dickes Minus gegeben, der Kurs wird zumindest zur Eröffnung an diesem Valentine´s Day absacken, sich dann nach meiner Einschätzung erholen, da die Adcetris-Verkaufszahlen eigentlich gut sind und durch die bevorstehende Zulassung auch in Europa noch an Fahrt gewinnen sollten.
Aber was weiss man schon, es gibt massenhaft Short-Positionen bei SGEN.
Werde jedenfalls dabeibleiben und im Falle eines stärkeren Rücksetzers innerhalb der nächsten Wochen (sagen wir mal auf 15$ oder so) auch nachkaufen.
Erstmal sehen, was die Analysten draus machen.
Gruß q.
---------------------
..Seattle Genetics Inc. Earnings: Beats Analysts’ Estimates as Loss Narrows
Wall St. Cheat Sheet – 8 hours ago
....
Share0EmailPrint.....Companies:...Seattle Genetics Inc. . ..RELATED QUOTES.
.Symbol Price Change
SGEN 18.96 +0.36
......
Helped by revenue growth, Seattle Genetics, Inc. narrowed its loss in the fourth quarter. Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease.
Investing Insights: Will the iPad 3 Be the Next Catalyst for Apple’s Stock?
Seattle Genetics Earnings Cheat Sheet for the Fourth Quarter
Results: Loss narrowed to $27.2 million (loss of 24 cents per diluted share) from $34.5 million (loss of 34 cents per share) in the same quarter a year earlier.
Revenue: Rose more than sixfold to $48.9 million from the year earlier quarter.
Actual vs. Wall St. Expectations: Seattle Genetics, Inc. beat the mean analyst estimate of a loss of 30 cents per share. It beat the average revenue estimate of $39.1 million.
Quoting Management: “We are pleased with the successful launch of ADCETRIS and our execution in bringing this drug to patients in need,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “Our commercialization initiatives continue to focus on expanding awareness of ADCETRIS among oncologists, particularly in the community setting, and ensuring an efficient reimbursement process.”
Key Stats:
The company has now beaten estimates the last two quarters. In the third quarter, it topped expectations with a loss of -35 cents versus a mean estimate of a loss of 46 cents per share.
The company’s revenue has now risen for two straight quarters. In the third quarter, revenue increased 29.2% to $20.7 million from the year earlier quarter.
Looking Forward: Expectations for the company’s next quarter performance are higher than they were ninety days ago. The average estimate for the first quarter of the next fiscal year is now at a loss of 25 cents per share, up from a loss of 31 cents. For the fiscal year, the average estimate has moved from a loss of $1.45 a share to a loss of $1.40 over the last ninety days.
(Company fundamentals provided by Xignite Financials. Earnings estimates provided by Zacks)

die Zahlen sind raus und wie so oft bei Biotechs sehr unterschiedlich interpretierbar. Nachbörslich hat´s noch ein dickes Minus gegeben, der Kurs wird zumindest zur Eröffnung an diesem Valentine´s Day absacken, sich dann nach meiner Einschätzung erholen, da die Adcetris-Verkaufszahlen eigentlich gut sind und durch die bevorstehende Zulassung auch in Europa noch an Fahrt gewinnen sollten.
Aber was weiss man schon, es gibt massenhaft Short-Positionen bei SGEN.
Werde jedenfalls dabeibleiben und im Falle eines stärkeren Rücksetzers innerhalb der nächsten Wochen (sagen wir mal auf 15$ oder so) auch nachkaufen.
Erstmal sehen, was die Analysten draus machen.
Gruß q.
---------------------
..Seattle Genetics Inc. Earnings: Beats Analysts’ Estimates as Loss Narrows
Wall St. Cheat Sheet – 8 hours ago
....
Share0EmailPrint.....Companies:...Seattle Genetics Inc. . ..RELATED QUOTES.
.Symbol Price Change
SGEN 18.96 +0.36
......
Helped by revenue growth, Seattle Genetics, Inc. narrowed its loss in the fourth quarter. Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer and autoimmune disease.
Investing Insights: Will the iPad 3 Be the Next Catalyst for Apple’s Stock?
Seattle Genetics Earnings Cheat Sheet for the Fourth Quarter
Results: Loss narrowed to $27.2 million (loss of 24 cents per diluted share) from $34.5 million (loss of 34 cents per share) in the same quarter a year earlier.
Revenue: Rose more than sixfold to $48.9 million from the year earlier quarter.
Actual vs. Wall St. Expectations: Seattle Genetics, Inc. beat the mean analyst estimate of a loss of 30 cents per share. It beat the average revenue estimate of $39.1 million.
Quoting Management: “We are pleased with the successful launch of ADCETRIS and our execution in bringing this drug to patients in need,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “Our commercialization initiatives continue to focus on expanding awareness of ADCETRIS among oncologists, particularly in the community setting, and ensuring an efficient reimbursement process.”
Key Stats:
The company has now beaten estimates the last two quarters. In the third quarter, it topped expectations with a loss of -35 cents versus a mean estimate of a loss of 46 cents per share.
The company’s revenue has now risen for two straight quarters. In the third quarter, revenue increased 29.2% to $20.7 million from the year earlier quarter.
Looking Forward: Expectations for the company’s next quarter performance are higher than they were ninety days ago. The average estimate for the first quarter of the next fiscal year is now at a loss of 25 cents per share, up from a loss of 31 cents. For the fiscal year, the average estimate has moved from a loss of $1.45 a share to a loss of $1.40 over the last ninety days.
(Company fundamentals provided by Xignite Financials. Earnings estimates provided by Zacks)
Moin,
Die BofA erhöht Kursziel aufgrund geringerer Ausgaben und weil sie keinen Grund für eine KE dieses Jahr sieht.
-------------
4Q sales slightly below, spend expectations a bright spot SGEN reported 4Q Adcetris sales of $33.2m, slightly below our $35M estimate, but generally consistent with recent investor expectations. Management commentary suggests that shorter dosing duration partially offset by higher patient demand drove the variance from our model. While 4Q11 Adcetris sales did little to dampen the raging investor debate on 2012 Adcetris sales, we opted to leave our annual sales forecasts largely unchanged following the results (our quarterly estimates shifted however). SGEN’s 2012 operating expense guidance ($212- $240M non-GAAP) was significantly lower than our prior estimate ($366M), giving us more confidence that the company does not need to finance this year. Our PO moves from $22 to $23 on eliminating the previously expected dilution.

Die BofA erhöht Kursziel aufgrund geringerer Ausgaben und weil sie keinen Grund für eine KE dieses Jahr sieht.
-------------
4Q sales slightly below, spend expectations a bright spot SGEN reported 4Q Adcetris sales of $33.2m, slightly below our $35M estimate, but generally consistent with recent investor expectations. Management commentary suggests that shorter dosing duration partially offset by higher patient demand drove the variance from our model. While 4Q11 Adcetris sales did little to dampen the raging investor debate on 2012 Adcetris sales, we opted to leave our annual sales forecasts largely unchanged following the results (our quarterly estimates shifted however). SGEN’s 2012 operating expense guidance ($212- $240M non-GAAP) was significantly lower than our prior estimate ($366M), giving us more confidence that the company does not need to finance this year. Our PO moves from $22 to $23 on eliminating the previously expected dilution.
Ach ja, fast vergessen, positiver im Text, aber noch zurückhaltender im Kursziel Needham (sie stellen sogar ein Mini-Jahresplus in Aussicht, also "Breakeven"):
----------------
Needham & Company Reiterates a 'Buy' on Seattle Genetics (SGEN); Impressive First Full Quarter of Adcetris Sales
February 14, 2012 8:31 AM EST
Needham & Company reiterates a 'Buy' on Seattle Genetics (NASDAQ: SGEN) price target of $20.00.
Analyst, Alan Carr, said, "SGEN reported $33.2M in 4Q11 Adcetris sales, above sell-side $25.9M consensus. This was the first full quarter of sales for the drug. Management did not provide 2012 sales guidance, but expects to be able to do so later this year...We expect continued adoption in r/r HL and r/r ALCL in the near term, with increasing commercial interest over time in maintenance, retreatment and possibly front-line settings. Greater than expected penetration of the community hospital setting is a positive, given the opportunity for longer duration therapy. Management is leveraging physician interest in the drug to drive a publication strategy that may accelerate inclusion in guidelines and reimbursement. Five investigator-sponsored trials are under way, with 8-10 more expected in 2012."
Needham raises FY12 EPS estimate from (1.22) to $0.02. Also raises U.S. Adcetris sales estimates from $74M to $217M.
----------------
Needham & Company Reiterates a 'Buy' on Seattle Genetics (SGEN); Impressive First Full Quarter of Adcetris Sales
February 14, 2012 8:31 AM EST
Needham & Company reiterates a 'Buy' on Seattle Genetics (NASDAQ: SGEN) price target of $20.00.
Analyst, Alan Carr, said, "SGEN reported $33.2M in 4Q11 Adcetris sales, above sell-side $25.9M consensus. This was the first full quarter of sales for the drug. Management did not provide 2012 sales guidance, but expects to be able to do so later this year...We expect continued adoption in r/r HL and r/r ALCL in the near term, with increasing commercial interest over time in maintenance, retreatment and possibly front-line settings. Greater than expected penetration of the community hospital setting is a positive, given the opportunity for longer duration therapy. Management is leveraging physician interest in the drug to drive a publication strategy that may accelerate inclusion in guidelines and reimbursement. Five investigator-sponsored trials are under way, with 8-10 more expected in 2012."
Needham raises FY12 EPS estimate from (1.22) to $0.02. Also raises U.S. Adcetris sales estimates from $74M to $217M.
Hallo,

gab gestern eine etwas merkwürdige Pflichtmitteilung (s.u.), offenbar ist einer der führenden Köpfe (Bruce J. Seeley) gegangen (worden) und zwar sehr kurzfristig zum 1. März.
Ist sogar jetzt schon komplett von der Homepage (seagen.com) verschwunden. Merkwürdig.
Hat hoffentlich nichts schlimm firmenschädigendes veranstaltet.
Oder ist so ein Weggang normal? Mal sehen, ob das irgendeine Kursreaktion gibt.
Gruß q.
-----------------------------------------------
Form 8-K for SEATTLE GENETICS INC /WA
--------------------------------------------------------------------------------
23-Feb-2012
Change in Directors or Principal Officers, Amendment or Waiver to Code o
Item 5.02 Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers.
(b),(e) On February 23, 2012, Seattle Genetics, Inc., (the "Company") and Bruce J. Seeley, the Company's Executive Vice President, Commercial, entered into a Severance and Release Agreement effective February 23, 2012 (the "Agreement"), providing for the termination of Mr. Seeley's employment on March 1, 2012 (the "Termination Date"). Pursuant to the Agreement, Mr. Seeley will receive a lump sum payment equal to twelve (12) months of his current base salary plus a cash bonus for 2012 equal to 35% of his current base salary pro-rated for the length of his employment during 2012. In addition, the vesting of Mr. Seeley's equity awards to purchase shares of the Company's common stock will accelerate as if Mr. Seeley's employment had continued for a period of twelve (12) months from the Termination Date. The Company will also pay COBRA benefits through March 1, 2013 for Mr. Seeley. The above description of the terms of the Agreement is a summary and is qualified in its entirety by the terms of the Agreement, which is filed with this report as Exhibit 10.1 and its contents are incorporated by reference into this Item 5.02.
(e) 2012 Senior Executive Annual Bonus Plan. On February 16, 2012, the Board of Directors of the Company (the "Board"), upon the recommendation of the Compensation Committee of the Board, approved the 2012 Senior Executive Annual Bonus Plan (the "Plan"), an incentive compensation program, which is designed to motivate, retain and reward the Company's executive officers based on the achievement of specified Company and individual goals. The Compensation Committee administers the Plan. Participants eligible under the Plan are those executives at the Vice President level or higher (each a "Participant"), including the following "named executive officers" (as defined under applicable securities laws): Clay B. Siegall, Todd E. Simpson, Thomas C. Reynolds and Eric L. Dobmeier. The amount of a Participant's bonus is based on a target percentage of such Participant's annual base pay as of the date of payment of the bonus, which target percentages have been determined by the Compensation Committee. The target percentage for Dr. Siegall is sixty percent (75%), Mr. Dobmeier's is fifty percent (50%), and Mr. Simpson's and Dr. Reynolds' target percentage is forty-five percent (45%). Under the Plan, this target percentage is then adjusted, generally based 50% on the Company's performance and 50% on the individual Participant's performance as determined by the Compensation Committee, except for members of the Company's Executive Committee (which include Mr. Simpson and Dr. Reynolds), in which case the percentage adjustment is based 60% on the Company's performance and 40% on the individual Participant's performance. Additionally, Mr. Dobmeier's percentage adjustment is based 80% on the Company's performance and 20% on individual performance, and Dr. Siegall's final performance percentage will be determined by the Compensation Committee it its sole discretion. The corporate performance measures under the Plan for 2012 are primarily based on the initiation of clinical trials of ADCETRIS, achievement of commercial objectives and submission for marketing approval in Canada of ADCETRIS, as well as development and clinical activities related to our other product candidates. Additional goals include hiring and retention goals, strategic objectives and stock performance. The Company's achieved performance percentage and/or the individual achieved Participant performance percentage may exceed 100% in the event the Company and/or the Participant exceed the predetermined goals (provided that neither percentage may exceed 150%), which could result in the payment of cash bonuses under the Plan at a level above target. The Plan is effective for the Company's 2012 calendar year and expires on December 31, 2012 (with any bonus payments under the Plan to be made by February 15, 2013). The above description of the terms of the Plan is a summary and is qualified in its entirety by the terms of the Plan, which is filed with this report as Exhibit 10.2 and its contents are incorporated by reference into this Item 5.02.
Item 5.05 Amendments to the Registrant's Code of Ethics, or Waiver of a Provision of the Code of Ethics.
On February 16, 2012, the Board amended and restated the Company's Code of Ethics. The Code of Ethics, which applies to all of the Company's directors, officers and employees, was amended and restated to, among other things,
(a) emphasize the applicable laws and our guidelines governing interactions with government officials, health-care professionals, customers, suppliers, consumers and other third parties, (b) clarify our expectations regarding employee and manager compliance with and enforcement of the
--------------------------------------------------------------------------------
Code of Ethics, (c) expand our guidelines relating to potential employee conflicts of interest, and (d) reflect revisions to our Employee Handbook. A copy of the Code of Ethics, as amended and restated by the Board on February 16, 2012, is attached as Exhibit 14.1 hereto. The amended and restated Code of Ethics will also be posted on the Company's website at www.seattlegenetics.com under the section entitled "Investors & News" at "Corporate Governance."
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
10.1 Severance and Release Agreement by and between the Company and Bruce J. Seeley effective February 23, 2012.
10.2 Seattle Genetics, Inc. 2012 Senior Executive Annual Bonus Plan.
14.1 Seattle Genetics, Inc. Code of Ethics, as amended and restated on February 16, 2012.

gab gestern eine etwas merkwürdige Pflichtmitteilung (s.u.), offenbar ist einer der führenden Köpfe (Bruce J. Seeley) gegangen (worden) und zwar sehr kurzfristig zum 1. März.
Ist sogar jetzt schon komplett von der Homepage (seagen.com) verschwunden. Merkwürdig.
Hat hoffentlich nichts schlimm firmenschädigendes veranstaltet.
Oder ist so ein Weggang normal? Mal sehen, ob das irgendeine Kursreaktion gibt.
Gruß q.
-----------------------------------------------
Form 8-K for SEATTLE GENETICS INC /WA
--------------------------------------------------------------------------------
23-Feb-2012
Change in Directors or Principal Officers, Amendment or Waiver to Code o
Item 5.02 Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers.
(b),(e) On February 23, 2012, Seattle Genetics, Inc., (the "Company") and Bruce J. Seeley, the Company's Executive Vice President, Commercial, entered into a Severance and Release Agreement effective February 23, 2012 (the "Agreement"), providing for the termination of Mr. Seeley's employment on March 1, 2012 (the "Termination Date"). Pursuant to the Agreement, Mr. Seeley will receive a lump sum payment equal to twelve (12) months of his current base salary plus a cash bonus for 2012 equal to 35% of his current base salary pro-rated for the length of his employment during 2012. In addition, the vesting of Mr. Seeley's equity awards to purchase shares of the Company's common stock will accelerate as if Mr. Seeley's employment had continued for a period of twelve (12) months from the Termination Date. The Company will also pay COBRA benefits through March 1, 2013 for Mr. Seeley. The above description of the terms of the Agreement is a summary and is qualified in its entirety by the terms of the Agreement, which is filed with this report as Exhibit 10.1 and its contents are incorporated by reference into this Item 5.02.
(e) 2012 Senior Executive Annual Bonus Plan. On February 16, 2012, the Board of Directors of the Company (the "Board"), upon the recommendation of the Compensation Committee of the Board, approved the 2012 Senior Executive Annual Bonus Plan (the "Plan"), an incentive compensation program, which is designed to motivate, retain and reward the Company's executive officers based on the achievement of specified Company and individual goals. The Compensation Committee administers the Plan. Participants eligible under the Plan are those executives at the Vice President level or higher (each a "Participant"), including the following "named executive officers" (as defined under applicable securities laws): Clay B. Siegall, Todd E. Simpson, Thomas C. Reynolds and Eric L. Dobmeier. The amount of a Participant's bonus is based on a target percentage of such Participant's annual base pay as of the date of payment of the bonus, which target percentages have been determined by the Compensation Committee. The target percentage for Dr. Siegall is sixty percent (75%), Mr. Dobmeier's is fifty percent (50%), and Mr. Simpson's and Dr. Reynolds' target percentage is forty-five percent (45%). Under the Plan, this target percentage is then adjusted, generally based 50% on the Company's performance and 50% on the individual Participant's performance as determined by the Compensation Committee, except for members of the Company's Executive Committee (which include Mr. Simpson and Dr. Reynolds), in which case the percentage adjustment is based 60% on the Company's performance and 40% on the individual Participant's performance. Additionally, Mr. Dobmeier's percentage adjustment is based 80% on the Company's performance and 20% on individual performance, and Dr. Siegall's final performance percentage will be determined by the Compensation Committee it its sole discretion. The corporate performance measures under the Plan for 2012 are primarily based on the initiation of clinical trials of ADCETRIS, achievement of commercial objectives and submission for marketing approval in Canada of ADCETRIS, as well as development and clinical activities related to our other product candidates. Additional goals include hiring and retention goals, strategic objectives and stock performance. The Company's achieved performance percentage and/or the individual achieved Participant performance percentage may exceed 100% in the event the Company and/or the Participant exceed the predetermined goals (provided that neither percentage may exceed 150%), which could result in the payment of cash bonuses under the Plan at a level above target. The Plan is effective for the Company's 2012 calendar year and expires on December 31, 2012 (with any bonus payments under the Plan to be made by February 15, 2013). The above description of the terms of the Plan is a summary and is qualified in its entirety by the terms of the Plan, which is filed with this report as Exhibit 10.2 and its contents are incorporated by reference into this Item 5.02.
Item 5.05 Amendments to the Registrant's Code of Ethics, or Waiver of a Provision of the Code of Ethics.
On February 16, 2012, the Board amended and restated the Company's Code of Ethics. The Code of Ethics, which applies to all of the Company's directors, officers and employees, was amended and restated to, among other things,
(a) emphasize the applicable laws and our guidelines governing interactions with government officials, health-care professionals, customers, suppliers, consumers and other third parties, (b) clarify our expectations regarding employee and manager compliance with and enforcement of the
--------------------------------------------------------------------------------
Code of Ethics, (c) expand our guidelines relating to potential employee conflicts of interest, and (d) reflect revisions to our Employee Handbook. A copy of the Code of Ethics, as amended and restated by the Board on February 16, 2012, is attached as Exhibit 14.1 hereto. The amended and restated Code of Ethics will also be posted on the Company's website at www.seattlegenetics.com under the section entitled "Investors & News" at "Corporate Governance."
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
10.1 Severance and Release Agreement by and between the Company and Bruce J. Seeley effective February 23, 2012.
10.2 Seattle Genetics, Inc. 2012 Senior Executive Annual Bonus Plan.
14.1 Seattle Genetics, Inc. Code of Ethics, as amended and restated on February 16, 2012.
Moin,

mal wieder ein Kommentar, der die Lage von SGEN m.M. realistisch einschätzt. Ein Kursziel von 24 (bzw. 24,5) $ wird gegeben, SGEN wird als vergleichsweise risikoarm gesehen. Bleibe weiter dabei.
Gruß q.
----------------
Should Seattle Genetics Start Thinking More Like A Pharma Company?
March 14, 2012 | 4 commentsby: Stephen Simpson | about: SGEN, includes: ARQL, AVEO, CLDX, IMGN Approval and launch hasn't quite meant Easy Street for Seattle Genetics (SGEN). Granted, the company now carries a $2 billion market cap and has doubled over the past two years, but there still seems to be a great deal of controversy about the true potential of its drug Adcetris and the subsequent fair value. Oddly enough, perhaps part of the answer is for Seattle Genetics to think a little more like a pharmaceutical company and a little less like a biotech.
Where Will Adcetris Go?
Arguing about market potential and peak sales is nothing new in biotech -- just review the debates at AEterna Zentaris (AEZS) or Vivus (VVUS) sometime -- but the spread in sell-side estimates for Adcetris still surprises me. I've seen expectations as low as $250 million to as much as $900 million. On the low end, it would assume that Seattle Genetics finds largely unmitigated failure in extending the label and usage; on the high end, it presupposes that almost everything goes right.
Certainly the early results from the launch haven't settled much of anything. Sales were a little light in the fourth quarter, as patient recruitment seemed strong, but treatment duration was a little short. So, enter the next debate - is Seattle Genetics going to quickly burn through the "pent up demand" for the drug and fail to extend treatment durations, leading to stagnant sales before additional indications, or is a physician education/marketing program going to bring treatment times more in line with management expectations?
Ultimately I come down close to the middle on that sales potential. Factoring in a 15% discount rate, the fully diluted sharecount, a revenue multiple (8x) and assorted odds and ends, I see fair value for Adcetris of around $15 per share.
Leverage The Technology
Seattle Genetics is not just a one-drug story, though. For starters, there are three other drugs in the pipeline where the company has a sizable (or sole) ownership stake. Granted, these compounds are quite a way back in development, so won't be contributors for five years or more.
One of the other things going for Seattle Genetics is its antibody drug conjugation technology portfolio. ADC basically allows drug companies to make more effective cancer-fighting drugs that tie toxic payloads to cell-specific antibodies. Unlike a lot of companies that tried for too long to make of a go of it as a technology licensing partner before adopting proprietary compounds, Seattle Genetics has had a two-pronged strategy in place for a while now.
Seattle Genetics boasts a sizable roster of ADC partners, including Roche (RHHBY.PK) and GlaxoSmithKline (GSK). These projects are still overwhelmingly in early-stage studies, but the most advanced program (Celldex's (CLDX) CDX-011 for breast cancer) will be showing Phase 2 data soon at the upcoming ASCO meeting in early June.
Are these partnerships going to make Seattle Genetics and its shareholders rich? Probably not, as the royalties are modest (mid single-digit, typically), but they do represent potential cash flows with high margins that require no additional work from the company. I believe this business could be worth an incremental $4 to $5 to Seattle Genetics. Moreover, looking at ImmunoGen (IMGN) (another company that has been actively out-licensing its own engineered antibody technology), that valuation may well be conservative.
Leverage The Infrastructure
Where I think Seattle Genetics could, and should, really start to think more like a pharmaceutical company is where its business infrastructure is concerned. Management made the decision to retain U.S. market rights and build out its own sales effort.
At this point, though, the addressable market for Adcetris is relatively small. That will change to some extent if and when clinical data supports label extensions, but the early stage of Seattle Genetics' other pipeline drugs suggests some potential under-utilization.
Perhaps, then, Seattle Genetics should look for partnering or acquisition opportunities with the idea of running more product through its infrastructure. There are quite a few small oncology biotechs out there that either have not partnered their late-stage compounds or have reached agreements that allow them to opt for co-promotion.
Just to name a few, there are ArQule (ARQL) and AVEO Pharmaceuticals (AVEO) and Celldex. ArQule and AVEO have late-stage oncology drug candidates with U.S. commercialization opt-in rights with their partners. Celldex has no partner for its Phase 2 breast cancer drug CDX-011, nor its Phase 3 brain cancer drug CDX-110. Certainly Seattle Genetics would have to be judicious and careful with these decisions, but I think it would at least be worth exploring.
The Bottom Line
Summing up the parts, I see $15 per share in value from Adcetris, $5 in ADC technology value, and another $4.50 per share from the pipeline and balance sheet. Although the total fair value of $24 is not all that exciting by the high-risk/high-reward standards of biotech, I do think there is an opportunity for the company to build value through in-licensing opportunities.
What's more, I like the cost-free revenue potential that the technology platform offers (to say nothing of ongoing technology development and licensing opportunities) and I think my numbers on Adcetris may well be conservative. Consequently, although I don't think the appreciation potential is as great here as in other names, I do think it's a relatively safer play by the standards of biotech investing.
Disclosure: I am long RHHBY.PK.

mal wieder ein Kommentar, der die Lage von SGEN m.M. realistisch einschätzt. Ein Kursziel von 24 (bzw. 24,5) $ wird gegeben, SGEN wird als vergleichsweise risikoarm gesehen. Bleibe weiter dabei.
Gruß q.
----------------
Should Seattle Genetics Start Thinking More Like A Pharma Company?
March 14, 2012 | 4 commentsby: Stephen Simpson | about: SGEN, includes: ARQL, AVEO, CLDX, IMGN Approval and launch hasn't quite meant Easy Street for Seattle Genetics (SGEN). Granted, the company now carries a $2 billion market cap and has doubled over the past two years, but there still seems to be a great deal of controversy about the true potential of its drug Adcetris and the subsequent fair value. Oddly enough, perhaps part of the answer is for Seattle Genetics to think a little more like a pharmaceutical company and a little less like a biotech.
Where Will Adcetris Go?
Arguing about market potential and peak sales is nothing new in biotech -- just review the debates at AEterna Zentaris (AEZS) or Vivus (VVUS) sometime -- but the spread in sell-side estimates for Adcetris still surprises me. I've seen expectations as low as $250 million to as much as $900 million. On the low end, it would assume that Seattle Genetics finds largely unmitigated failure in extending the label and usage; on the high end, it presupposes that almost everything goes right.
Certainly the early results from the launch haven't settled much of anything. Sales were a little light in the fourth quarter, as patient recruitment seemed strong, but treatment duration was a little short. So, enter the next debate - is Seattle Genetics going to quickly burn through the "pent up demand" for the drug and fail to extend treatment durations, leading to stagnant sales before additional indications, or is a physician education/marketing program going to bring treatment times more in line with management expectations?
Ultimately I come down close to the middle on that sales potential. Factoring in a 15% discount rate, the fully diluted sharecount, a revenue multiple (8x) and assorted odds and ends, I see fair value for Adcetris of around $15 per share.
Leverage The Technology
Seattle Genetics is not just a one-drug story, though. For starters, there are three other drugs in the pipeline where the company has a sizable (or sole) ownership stake. Granted, these compounds are quite a way back in development, so won't be contributors for five years or more.
One of the other things going for Seattle Genetics is its antibody drug conjugation technology portfolio. ADC basically allows drug companies to make more effective cancer-fighting drugs that tie toxic payloads to cell-specific antibodies. Unlike a lot of companies that tried for too long to make of a go of it as a technology licensing partner before adopting proprietary compounds, Seattle Genetics has had a two-pronged strategy in place for a while now.
Seattle Genetics boasts a sizable roster of ADC partners, including Roche (RHHBY.PK) and GlaxoSmithKline (GSK). These projects are still overwhelmingly in early-stage studies, but the most advanced program (Celldex's (CLDX) CDX-011 for breast cancer) will be showing Phase 2 data soon at the upcoming ASCO meeting in early June.
Are these partnerships going to make Seattle Genetics and its shareholders rich? Probably not, as the royalties are modest (mid single-digit, typically), but they do represent potential cash flows with high margins that require no additional work from the company. I believe this business could be worth an incremental $4 to $5 to Seattle Genetics. Moreover, looking at ImmunoGen (IMGN) (another company that has been actively out-licensing its own engineered antibody technology), that valuation may well be conservative.
Leverage The Infrastructure
Where I think Seattle Genetics could, and should, really start to think more like a pharmaceutical company is where its business infrastructure is concerned. Management made the decision to retain U.S. market rights and build out its own sales effort.
At this point, though, the addressable market for Adcetris is relatively small. That will change to some extent if and when clinical data supports label extensions, but the early stage of Seattle Genetics' other pipeline drugs suggests some potential under-utilization.
Perhaps, then, Seattle Genetics should look for partnering or acquisition opportunities with the idea of running more product through its infrastructure. There are quite a few small oncology biotechs out there that either have not partnered their late-stage compounds or have reached agreements that allow them to opt for co-promotion.
Just to name a few, there are ArQule (ARQL) and AVEO Pharmaceuticals (AVEO) and Celldex. ArQule and AVEO have late-stage oncology drug candidates with U.S. commercialization opt-in rights with their partners. Celldex has no partner for its Phase 2 breast cancer drug CDX-011, nor its Phase 3 brain cancer drug CDX-110. Certainly Seattle Genetics would have to be judicious and careful with these decisions, but I think it would at least be worth exploring.
The Bottom Line
Summing up the parts, I see $15 per share in value from Adcetris, $5 in ADC technology value, and another $4.50 per share from the pipeline and balance sheet. Although the total fair value of $24 is not all that exciting by the high-risk/high-reward standards of biotech, I do think there is an opportunity for the company to build value through in-licensing opportunities.
What's more, I like the cost-free revenue potential that the technology platform offers (to say nothing of ongoing technology development and licensing opportunities) and I think my numbers on Adcetris may well be conservative. Consequently, although I don't think the appreciation potential is as great here as in other names, I do think it's a relatively safer play by the standards of biotech investing.
Disclosure: I am long RHHBY.PK.
Seattle Genetics Announces Pivotal ADCETRIS™ (Brentuximab Vedotin) Hodgkin Lymphoma Study Published in Journal of Clinical Oncology
27 Mar 2012
Data Supported FDA Accelerated Approval in Relapsed Patients-
Broad Ongoing Evaluation of ADCETRIS in Earlier Lines of Hodgkin Lymphoma and in Other CD30-Positive Malignancies-
BOTHELL, WA, USA I March 26, 2012 I Seattle Genetics, Inc. (Nasdaq:SGEN - News) today announced that the Journal of Clinical Oncology (JCO) published results of the company’s pivotal clinical trial of ADCETRIS™ (brentuximab vedotin) in Hodgkin lymphoma (HL) patients with relapsed or refractory disease following an autologous stem cell transplant (ASCT). The findings, published today online, demonstrated that treatment with ADCETRIS as a single agent induced durable objective responses in 75 percent of patients and was associated with a manageable safety profile. ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30, which is expressed in HL and anaplastic large cell lymphoma (ALCL).
Additionally, a separate pivotal clinical trial of ADCETRIS for the treatment of relapsed or refractory systemic ALCL has been accepted for publication and is currently in press for an upcoming issue of JCO.
“Although Hodgkin lymphoma is often viewed as a curable disease, up to 30 percent of patients relapse or are refractory following front-line chemotherapy regimens and subsequent treatments, leaving limited therapeutic options,” said Dr. Anas Younes, Professor of Medicine and Director, Clinical Investigation and Translational Research Department of Lymphoma/Myeloma at The University of Texas MD Anderson Cancer Center. “ADCETRIS represents a new approach that is changing the way we treat relapsed and refractory HL patients. The complete response rate and manageable safety profile we observed with ADCETRIS in the pivotal trial have also generated enthusiasm among the medical community for evaluating ADCETRIS in earlier lines of HL therapy.”
“Data from this pivotal trial served as the basis for the accelerated approval of ADCETRIS in August 2011 for relapsed Hodgkin patients, and is the foundation for our robust clinical development plan to broadly evaluate ADCETRIS in earlier lines of therapy, as well as in other CD30-positive malignancies,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer of Seattle Genetics. “We are evaluating ADCETRIS across a broad array of CD30-positive malignancies, towards our goal of bringing it to additional patients in need.”
The open-label, phase II study evaluated the efficacy and safety of ADCETRIS in 102 patients with relapsed or refractory, CD30-positive HL after ASCT.
Highlights from the study include:
75 percent of patients achieved an objective response, the primary endpoint of the trial, as assessed by an independent central review.
34 percent of patients achieved a complete remission.
The median duration of response was 29 weeks by independent central review, and 47 weeks by investigator assessment. Durable complete remissions approaching two years were observed.
Treatment with ADCETRIS was associated with manageable adverse events, the most common being peripheral sensory neuropathy, nausea, fatigue, neutropenia and diarrhea. The most common Grade 3 or higher adverse events were neutropenia, peripheral sensory neuropathy, thrombocytopenia and anemia.
About ADCETRIS
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics’ proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
ADCETRIS is being evaluated in a phase III clinical trial (the AETHERA trial) for patients at high risk of residual Hodgkin lymphoma following autologous stem cell transplant (ASCT), a phase II trial for relapsed or refractory CD30-positive non-Hodgkin lymphomas, a phase II trial for CD30-positive non-lymphoma malignancies, a phase II retreatment trial for relapsed patients who previously responded to ADCETRIS, a phase I trial in combination with multi-agent chemotherapy for front-line treatment of Hodgkin lymphoma and a phase I trial in combination with multi-agent chemotherapy for front-line treatment of mature T-cell lymphomas. Three additional phase III trials are planned, including a trial in CD30-positive cutaneous T-cell lymphomas to begin in mid-2012, a front-line trial in Hodgkin lymphoma and a front-line trial in mature T-cell lymphomas. The front-line trials are expected to begin by late 2012 or early 2013.
Seattle Genetics and Millennium: The Takeda Oncology Company are jointly developing ADCETRIS. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group is solely responsible for development costs.
27 Mar 2012
Data Supported FDA Accelerated Approval in Relapsed Patients-
Broad Ongoing Evaluation of ADCETRIS in Earlier Lines of Hodgkin Lymphoma and in Other CD30-Positive Malignancies-
BOTHELL, WA, USA I March 26, 2012 I Seattle Genetics, Inc. (Nasdaq:SGEN - News) today announced that the Journal of Clinical Oncology (JCO) published results of the company’s pivotal clinical trial of ADCETRIS™ (brentuximab vedotin) in Hodgkin lymphoma (HL) patients with relapsed or refractory disease following an autologous stem cell transplant (ASCT). The findings, published today online, demonstrated that treatment with ADCETRIS as a single agent induced durable objective responses in 75 percent of patients and was associated with a manageable safety profile. ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30, which is expressed in HL and anaplastic large cell lymphoma (ALCL).
Additionally, a separate pivotal clinical trial of ADCETRIS for the treatment of relapsed or refractory systemic ALCL has been accepted for publication and is currently in press for an upcoming issue of JCO.
“Although Hodgkin lymphoma is often viewed as a curable disease, up to 30 percent of patients relapse or are refractory following front-line chemotherapy regimens and subsequent treatments, leaving limited therapeutic options,” said Dr. Anas Younes, Professor of Medicine and Director, Clinical Investigation and Translational Research Department of Lymphoma/Myeloma at The University of Texas MD Anderson Cancer Center. “ADCETRIS represents a new approach that is changing the way we treat relapsed and refractory HL patients. The complete response rate and manageable safety profile we observed with ADCETRIS in the pivotal trial have also generated enthusiasm among the medical community for evaluating ADCETRIS in earlier lines of HL therapy.”
“Data from this pivotal trial served as the basis for the accelerated approval of ADCETRIS in August 2011 for relapsed Hodgkin patients, and is the foundation for our robust clinical development plan to broadly evaluate ADCETRIS in earlier lines of therapy, as well as in other CD30-positive malignancies,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer of Seattle Genetics. “We are evaluating ADCETRIS across a broad array of CD30-positive malignancies, towards our goal of bringing it to additional patients in need.”
The open-label, phase II study evaluated the efficacy and safety of ADCETRIS in 102 patients with relapsed or refractory, CD30-positive HL after ASCT.
Highlights from the study include:
75 percent of patients achieved an objective response, the primary endpoint of the trial, as assessed by an independent central review.
34 percent of patients achieved a complete remission.
The median duration of response was 29 weeks by independent central review, and 47 weeks by investigator assessment. Durable complete remissions approaching two years were observed.
Treatment with ADCETRIS was associated with manageable adverse events, the most common being peripheral sensory neuropathy, nausea, fatigue, neutropenia and diarrhea. The most common Grade 3 or higher adverse events were neutropenia, peripheral sensory neuropathy, thrombocytopenia and anemia.
About ADCETRIS
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics’ proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
ADCETRIS is being evaluated in a phase III clinical trial (the AETHERA trial) for patients at high risk of residual Hodgkin lymphoma following autologous stem cell transplant (ASCT), a phase II trial for relapsed or refractory CD30-positive non-Hodgkin lymphomas, a phase II trial for CD30-positive non-lymphoma malignancies, a phase II retreatment trial for relapsed patients who previously responded to ADCETRIS, a phase I trial in combination with multi-agent chemotherapy for front-line treatment of Hodgkin lymphoma and a phase I trial in combination with multi-agent chemotherapy for front-line treatment of mature T-cell lymphomas. Three additional phase III trials are planned, including a trial in CD30-positive cutaneous T-cell lymphomas to begin in mid-2012, a front-line trial in Hodgkin lymphoma and a front-line trial in mature T-cell lymphomas. The front-line trials are expected to begin by late 2012 or early 2013.
Seattle Genetics and Millennium: The Takeda Oncology Company are jointly developing ADCETRIS. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group is solely responsible for development costs.
Hallo,
und noch mal News zur weiteren Anwendung von Adcetris.
diesmal mit Millennium/Roche
Gruß q.
---------------------
Seattle Genetics and Millennium to Collaborate with Ventana on Companion Diagnostic for ADCETRIS™ in CD30-Positive Malignancies
-Ventana to develop molecular companion diagnostic test to evaluate CD30 expression levels in tissue specimens-
Press Release: Seattle Genetics, Inc. – 11 minutes agotweetShare0EmailPrintCompanies:Seattle Genetics Inc.RELATED QUOTESSymbol Price Change
SGEN 18.77 0.00
Related Content
View Photowww.millennium.comMultimedia Gallery URL
BOTHELL, Wash.& CAMBRIDGE, Mass. & TUCSON, Ariz.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (Nasdaq:SGEN - News) and Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited (TSE:4502), today announced that they have formed a collaboration with Ventana Medical Systems, Inc. (Ventana), a member of the Roche Group. Under the collaboration agreement, Ventana will seek to develop, manufacture and commercialize a molecular companion diagnostic test with the goal of identifying patients who might respond to treatment with ADCETRIS based on CD30 expression levels in their tissue specimens. As part of the ongoing clinical development of ADCETRIS, Millennium and Seattle Genetics are planning two phase III studies that will use the companion diagnostic, one in CD30-positive cutaneous T-cell lymphoma (CTCL) and the other in CD30-positive mature T-cell lymphomas (MTCL).
ADCETRIS was approved by the U.S. Food and Drug Administration in August 2011 for relapsed Hodgkin lymphoma (HL) and systemic anaplastic large call lymphoma (sALCL). A molecular companion diagnostic is not required for the current FDA-approved indications for ADCETRIS.
“Availability of a CD30 companion diagnostic will bring us a step closer to our vision of a more personalized, target-based approach to the treatment of cancer, and supports our broad ongoing and planned clinical development of ADCETRIS for CD30-positive patients in need,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer of Seattle Genetics. “Although the identification of CD30 expression and its role in the diagnosis of Hodgkin lymphoma and systemic ALCL is well-established, CD30 expression in other malignancies is more heterogeneous. The collaboration with Ventana provides an opportunity for development of a diagnostic tool to identify patients who may benefit from ADCETRIS treatment.”
“Translational medicine research is central to Millennium’s strategic focus of developing innovative, targeted therapies that provide a high benefit to patients,” said Karen Ferrante, M.D., Chief Medical Officer, Millennium. “We look forward to collaborating with Ventana and Seattle Genetics to develop this new diagnostic tool and expanding the ongoing clinical development program for ADCETRIS in patients with CD30-positive malignancies.”
CD30 is a member of the tumor necrosis factor receptor (TNFR) family and is a characteristic cell surface receptor for activated T-cells and B-cells, including the malignant cells of HL and sALCL1,2. Published literature also reports CD30 expression in other cancers. Seattle Genetics is currently exploring the potential of ADCETRIS in two phase II clinical trials to further characterize CD30 expression and evaluate antitumor activity of ADCETRIS. One trial is evaluating patients with non-Hodgkin lymphomas, including diffuse large B-cell lymphoma, peripheral T-cell lymphoma and other less common lymphoma subtypes and the second trial is evaluating patients with non-lymphoma malignancies, including multiple myeloma, leukemia and solid tumors. Data from both trials are expected to be reported at upcoming medical conferences during 2012. ADCETRIS is not approved for treatment of the non-Hodgkin lymphomas and non-lymphoma malignancies studied in these trials.
“We are pleased to work with Seattle Genetics and Millennium to develop a companion diagnostic test for detecting CD30 expression levels that may assist in identifying additional patients who might benefit from ADCETRIS,” said Doug Ward, VP and General Manager, Ventana Translational Diagnostics. “We believe that a Personalized Healthcare approach is particularly relevant for targeted agents such as ADCETRIS, an antibody-drug conjugate, and this collaboration provides an opportunity to add to our growing pipeline of companion diagnostic tests.”
About ADCETRIS
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics’ proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
Seattle Genetics and Millennium are jointly developing ADCETRIS. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
About Seattle Genetics
Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer. The U.S. Food and Drug Administration granted accelerated approval of ADCETRIS in August 2011 for two indications. ADCETRIS is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has three other clinical-stage ADC programs: SGN-75, ASG-5ME and ASG-22ME. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
und noch mal News zur weiteren Anwendung von Adcetris.
diesmal mit Millennium/Roche
Gruß q.
---------------------
Seattle Genetics and Millennium to Collaborate with Ventana on Companion Diagnostic for ADCETRIS™ in CD30-Positive Malignancies
-Ventana to develop molecular companion diagnostic test to evaluate CD30 expression levels in tissue specimens-
Press Release: Seattle Genetics, Inc. – 11 minutes agotweetShare0EmailPrintCompanies:Seattle Genetics Inc.RELATED QUOTESSymbol Price Change
SGEN 18.77 0.00
Related Content
View Photowww.millennium.comMultimedia Gallery URL
BOTHELL, Wash.& CAMBRIDGE, Mass. & TUCSON, Ariz.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (Nasdaq:SGEN - News) and Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited (TSE:4502), today announced that they have formed a collaboration with Ventana Medical Systems, Inc. (Ventana), a member of the Roche Group. Under the collaboration agreement, Ventana will seek to develop, manufacture and commercialize a molecular companion diagnostic test with the goal of identifying patients who might respond to treatment with ADCETRIS based on CD30 expression levels in their tissue specimens. As part of the ongoing clinical development of ADCETRIS, Millennium and Seattle Genetics are planning two phase III studies that will use the companion diagnostic, one in CD30-positive cutaneous T-cell lymphoma (CTCL) and the other in CD30-positive mature T-cell lymphomas (MTCL).
ADCETRIS was approved by the U.S. Food and Drug Administration in August 2011 for relapsed Hodgkin lymphoma (HL) and systemic anaplastic large call lymphoma (sALCL). A molecular companion diagnostic is not required for the current FDA-approved indications for ADCETRIS.
“Availability of a CD30 companion diagnostic will bring us a step closer to our vision of a more personalized, target-based approach to the treatment of cancer, and supports our broad ongoing and planned clinical development of ADCETRIS for CD30-positive patients in need,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer of Seattle Genetics. “Although the identification of CD30 expression and its role in the diagnosis of Hodgkin lymphoma and systemic ALCL is well-established, CD30 expression in other malignancies is more heterogeneous. The collaboration with Ventana provides an opportunity for development of a diagnostic tool to identify patients who may benefit from ADCETRIS treatment.”
“Translational medicine research is central to Millennium’s strategic focus of developing innovative, targeted therapies that provide a high benefit to patients,” said Karen Ferrante, M.D., Chief Medical Officer, Millennium. “We look forward to collaborating with Ventana and Seattle Genetics to develop this new diagnostic tool and expanding the ongoing clinical development program for ADCETRIS in patients with CD30-positive malignancies.”
CD30 is a member of the tumor necrosis factor receptor (TNFR) family and is a characteristic cell surface receptor for activated T-cells and B-cells, including the malignant cells of HL and sALCL1,2. Published literature also reports CD30 expression in other cancers. Seattle Genetics is currently exploring the potential of ADCETRIS in two phase II clinical trials to further characterize CD30 expression and evaluate antitumor activity of ADCETRIS. One trial is evaluating patients with non-Hodgkin lymphomas, including diffuse large B-cell lymphoma, peripheral T-cell lymphoma and other less common lymphoma subtypes and the second trial is evaluating patients with non-lymphoma malignancies, including multiple myeloma, leukemia and solid tumors. Data from both trials are expected to be reported at upcoming medical conferences during 2012. ADCETRIS is not approved for treatment of the non-Hodgkin lymphomas and non-lymphoma malignancies studied in these trials.
“We are pleased to work with Seattle Genetics and Millennium to develop a companion diagnostic test for detecting CD30 expression levels that may assist in identifying additional patients who might benefit from ADCETRIS,” said Doug Ward, VP and General Manager, Ventana Translational Diagnostics. “We believe that a Personalized Healthcare approach is particularly relevant for targeted agents such as ADCETRIS, an antibody-drug conjugate, and this collaboration provides an opportunity to add to our growing pipeline of companion diagnostic tests.”
About ADCETRIS
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics’ proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
Seattle Genetics and Millennium are jointly developing ADCETRIS. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
About Seattle Genetics
Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer. The U.S. Food and Drug Administration granted accelerated approval of ADCETRIS in August 2011 for two indications. ADCETRIS is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has three other clinical-stage ADC programs: SGN-75, ASG-5ME and ASG-22ME. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
nehme die Aktie mal auf meine Watchlist.
Gibt ja doch "immer mal wieder" starke Rücksetzer.
Gibt ja doch "immer mal wieder" starke Rücksetzer.
Moin,

Die Zahlen für das erste Quartal sind raus und fielen leider recht enttäuschend aus. Die Aktie ist nachbörslich auch geradezu eingebrochen um fast 9%. Naja, malö sehen, was die Analysten draus machen. Vielleicht ergibt sich auch eine gute Gelegenheit zum Nachlegen, schließlich wollte ich meine Position ja noch ausbauen. Hätte das aber lieber bei steigenden Kursen und guten News gemacht...
Gruß q.
Hier die Meldung:
---------------------------
Seattle Genetics pares 1Q loss on Adcetris sales
Seattle Genetics takes smaller 1st-quarter loss and reports $34.5 million in Adcetris sales
Associated Press – 8 hours agoShare0EmailPrintCompanies:SEATTLE GENETICSSEATTLE GENETICSSeattle Genetics Inc.RELATED QUOTESSymbol Price Change
SGEN 19.03 -0.17
SGT.BE 11.97 0.00
BOTHELL, Wash. (AP) -- Seattle Genetics Inc. said Tuesday it took a smaller loss in the first quarter after launching its cancer drug Adcetris. Its revenue for the quarter, however, came in below Wall Street forecasts and its shares dropped 7 percent in extended trading.
The company said sales of Adcetris totaled $34.5 million in the first quarter. The drug was approved by the Food and Drug Administration in August, and is used as a treatment for Hodgkin's lymphoma and systemic anaplastic large cell lymphoma.
Seattle Genetics said it lost $12.3 million, or 11 cents per share, in the first three months of 2012. The result matched analysts' expectations. A year ago it took a loss of $32.7 million, or 30 cents per share.
Revenue climbed to $48.2 million from $12.2 million. Analysts expected the company post $51.3 million in revenue, according to FactSet.
Seattle Genetics said it expects $140 million to $150 million in Adcetris sales in 2012. It backed its forecast of $55 million to $65 million in revenue from collaboration and license agreements.
Shares of Seattle Genetics dropped $1.38 to $17.65 in aftermarket trading following the release of the earnings report. They had ended the regular session down 17 cents to $19.03.

Die Zahlen für das erste Quartal sind raus und fielen leider recht enttäuschend aus. Die Aktie ist nachbörslich auch geradezu eingebrochen um fast 9%. Naja, malö sehen, was die Analysten draus machen. Vielleicht ergibt sich auch eine gute Gelegenheit zum Nachlegen, schließlich wollte ich meine Position ja noch ausbauen. Hätte das aber lieber bei steigenden Kursen und guten News gemacht...
Gruß q.
Hier die Meldung:
---------------------------
Seattle Genetics pares 1Q loss on Adcetris sales
Seattle Genetics takes smaller 1st-quarter loss and reports $34.5 million in Adcetris sales
Associated Press – 8 hours agoShare0EmailPrintCompanies:SEATTLE GENETICSSEATTLE GENETICSSeattle Genetics Inc.RELATED QUOTESSymbol Price Change
SGEN 19.03 -0.17
SGT.BE 11.97 0.00
BOTHELL, Wash. (AP) -- Seattle Genetics Inc. said Tuesday it took a smaller loss in the first quarter after launching its cancer drug Adcetris. Its revenue for the quarter, however, came in below Wall Street forecasts and its shares dropped 7 percent in extended trading.
The company said sales of Adcetris totaled $34.5 million in the first quarter. The drug was approved by the Food and Drug Administration in August, and is used as a treatment for Hodgkin's lymphoma and systemic anaplastic large cell lymphoma.
Seattle Genetics said it lost $12.3 million, or 11 cents per share, in the first three months of 2012. The result matched analysts' expectations. A year ago it took a loss of $32.7 million, or 30 cents per share.
Revenue climbed to $48.2 million from $12.2 million. Analysts expected the company post $51.3 million in revenue, according to FactSet.
Seattle Genetics said it expects $140 million to $150 million in Adcetris sales in 2012. It backed its forecast of $55 million to $65 million in revenue from collaboration and license agreements.
Shares of Seattle Genetics dropped $1.38 to $17.65 in aftermarket trading following the release of the earnings report. They had ended the regular session down 17 cents to $19.03.
Millennium and Seattle Genetics Initiate Global Phase 3 Trial of ADCETRIS™ in Patients with CD30-Expressing Relapsed Cutaneous T-cell Lymphoma
08 May 2012
-Trial Conducted Under Special Protocol Assessment with FDA-
-Study Includes Evaluation of Companion Diagnostic Test for CD30 Identification-
CAMBRIDGE, MA & BOTHELL, WA, USA I May 7, 2012 I Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited (TSE:4502) and Seattle Genetics, Inc. (Nasdaq: SGEN), today announced the initiation of an international pivotal phase 3 clinical trial evaluating ADCETRIS (brentuximab vedotin) in patients with CD30-expressing cutaneous T-cell lymphoma (CTCL) who received at least one prior systemic therapy. The global multi-center study with ADCETRIS, an antibody-drug conjugate (ADC) directed to CD30, will be conducted in the United States, Europe, Australia and Brazil. The trial is being conducted under a Special Protocol Assessment (SPA) agreement from the U.S. Food and Drug Administration (FDA) regarding the trial design. The study also received European Medicines Agency (EMA) scientific advice.
“Millennium is pleased to announce the initiation of the pivotal trial of ADCETRIS in patients with relapsed CD30-expressing CTCL. We recognize this as a significant milestone in our efforts to explore the potential of this targeted therapy in other indications,” said Karen Ferrante, MD, Chief Medical Officer, Millennium. “Looking forward, this study may support the potential to supplement therapeutic options for patients, from traditional systemic chemotherapy to ADCETRIS, a targeted therapy.”
“Data from patients with cutaneous lesions observed in our pivotal trial in systemic anaplastic large cell lymphoma (sALCL) and interim data from investigator-sponsored trials in CTCL with ADCETRIS provide a strong rationale for initiating this phase 3 trial,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer, Seattle Genetics. “CTCL is an important part of our development plan to broadly evaluate ADCETRIS in CD30-expressing malignancies. This trial complements many other ongoing and planned trials for patients in need, including two additional phase 3 trials for front-line Hodgkin lymphoma (HL) and front-line mature T-cell lymphomas expected to start by late 2012 or early 2013.”
CD30 is a member of the tumor necrosis factor receptor (TNFR) family and is a characteristic cell surface receptor for activated T-cells and B-cells, including the malignant cells of HL and sALCL. According to published literature, up to 50 percent of CTCL patients’ lesions express CD30(1-3). Under a previously announced collaboration agreement with Ventana Medical Systems, Inc. (Ventana), Millennium and Seattle Genetics, Ventana is developing a molecular companion diagnostic test for use in this CTCL patient population.
Study design
The study is a randomized, open-label, phase 3 trial of ADCETRIS versus investigator’s choice of methotrexate or bexarotene in patients with CD30-positive CTCL, including those with primary cutaneous anaplastic large cell lymphoma (pcALCL) or mycosis fungoides (MF). The primary endpoint of the study is overall response rate (ORR), lasting at least 4 months, with ADCETRIS in patients with CD30-positive MF or pcALCL compared to that achieved with therapy in the control arm. The key secondary endpoints are complete response (CR), progression-free survival (PFS), and burden of symptoms. Approximately 124 patients will be enrolled in the pivotal trial.
For more information about the trial, please visit www.clinicaltrials.gov.
About ADCETRIS
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics’ proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
ADCETRISTM (brentuximab vedotin) received accelerated approval from the US FDA for two indications: (1) the treatment of patients with Hodgkin lymphoma after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates, and (2) the treatment of patients with systemic anaplastic large cell lymphoma (ALCL) after failure of at least one prior multi-agent chemotherapy regimen. The indications for ADCETRIS are based on response rate. There are no data available demonstrating improvement in patient-reported outcomes or survival with ADCETRIS.
ADCETRIS is not approved for the treatment of CTCL, front-line HL and front-line ALCL. ADCETRIS is not approved for use outside the United States. The marketing authorization application for ADCETRIS in relapsed or refractory Hodgkin lymphoma and systemic ALCL, filed by Takeda Global Research & Development Centre (Europe), was accepted for review by the European Medicines Agency for review in June 2011.
Seattle Genetics and Millennium are jointly developing ADCETRIS. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
About Cutaneous T-Cell Lymphoma
CTCLs constitute a group of non-Hodgkin lymphomas (NHLs) and are cancers of the T lymphocytes (a type of white blood cell) that mainly affect the skin but can also involve the blood, lymph nodes and/or internal organs in patients with advanced disease.
About Millennium
Millennium: The Takeda Oncology Company, a leading biopharmaceutical company based in Cambridge, Mass., markets VELCADE, a first-in-class proteasome inhibitor, and has a robust clinical development pipeline of product candidates. Millennium Pharmaceuticals, Inc. was acquired by Takeda Pharmaceutical Company Ltd. in May, 2008. The Company’s research, development and commercialization activities are focused in oncology. Additional information about Millennium is available through its website, www.millennium.com.
About Seattle Genetics
Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer. The U.S. Food and Drug Administration granted accelerated approval of ADCETRIS in August 2011 for two indications. ADCETRIS is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has three other clinical-stage ADC programs: SGN-75, ASG-5ME and ASG-22ME. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
08 May 2012
-Trial Conducted Under Special Protocol Assessment with FDA-
-Study Includes Evaluation of Companion Diagnostic Test for CD30 Identification-
CAMBRIDGE, MA & BOTHELL, WA, USA I May 7, 2012 I Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited (TSE:4502) and Seattle Genetics, Inc. (Nasdaq: SGEN), today announced the initiation of an international pivotal phase 3 clinical trial evaluating ADCETRIS (brentuximab vedotin) in patients with CD30-expressing cutaneous T-cell lymphoma (CTCL) who received at least one prior systemic therapy. The global multi-center study with ADCETRIS, an antibody-drug conjugate (ADC) directed to CD30, will be conducted in the United States, Europe, Australia and Brazil. The trial is being conducted under a Special Protocol Assessment (SPA) agreement from the U.S. Food and Drug Administration (FDA) regarding the trial design. The study also received European Medicines Agency (EMA) scientific advice.
“Millennium is pleased to announce the initiation of the pivotal trial of ADCETRIS in patients with relapsed CD30-expressing CTCL. We recognize this as a significant milestone in our efforts to explore the potential of this targeted therapy in other indications,” said Karen Ferrante, MD, Chief Medical Officer, Millennium. “Looking forward, this study may support the potential to supplement therapeutic options for patients, from traditional systemic chemotherapy to ADCETRIS, a targeted therapy.”
“Data from patients with cutaneous lesions observed in our pivotal trial in systemic anaplastic large cell lymphoma (sALCL) and interim data from investigator-sponsored trials in CTCL with ADCETRIS provide a strong rationale for initiating this phase 3 trial,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer, Seattle Genetics. “CTCL is an important part of our development plan to broadly evaluate ADCETRIS in CD30-expressing malignancies. This trial complements many other ongoing and planned trials for patients in need, including two additional phase 3 trials for front-line Hodgkin lymphoma (HL) and front-line mature T-cell lymphomas expected to start by late 2012 or early 2013.”
CD30 is a member of the tumor necrosis factor receptor (TNFR) family and is a characteristic cell surface receptor for activated T-cells and B-cells, including the malignant cells of HL and sALCL. According to published literature, up to 50 percent of CTCL patients’ lesions express CD30(1-3). Under a previously announced collaboration agreement with Ventana Medical Systems, Inc. (Ventana), Millennium and Seattle Genetics, Ventana is developing a molecular companion diagnostic test for use in this CTCL patient population.
Study design
The study is a randomized, open-label, phase 3 trial of ADCETRIS versus investigator’s choice of methotrexate or bexarotene in patients with CD30-positive CTCL, including those with primary cutaneous anaplastic large cell lymphoma (pcALCL) or mycosis fungoides (MF). The primary endpoint of the study is overall response rate (ORR), lasting at least 4 months, with ADCETRIS in patients with CD30-positive MF or pcALCL compared to that achieved with therapy in the control arm. The key secondary endpoints are complete response (CR), progression-free survival (PFS), and burden of symptoms. Approximately 124 patients will be enrolled in the pivotal trial.
For more information about the trial, please visit www.clinicaltrials.gov.
About ADCETRIS
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics’ proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
ADCETRISTM (brentuximab vedotin) received accelerated approval from the US FDA for two indications: (1) the treatment of patients with Hodgkin lymphoma after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates, and (2) the treatment of patients with systemic anaplastic large cell lymphoma (ALCL) after failure of at least one prior multi-agent chemotherapy regimen. The indications for ADCETRIS are based on response rate. There are no data available demonstrating improvement in patient-reported outcomes or survival with ADCETRIS.
ADCETRIS is not approved for the treatment of CTCL, front-line HL and front-line ALCL. ADCETRIS is not approved for use outside the United States. The marketing authorization application for ADCETRIS in relapsed or refractory Hodgkin lymphoma and systemic ALCL, filed by Takeda Global Research & Development Centre (Europe), was accepted for review by the European Medicines Agency for review in June 2011.
Seattle Genetics and Millennium are jointly developing ADCETRIS. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
About Cutaneous T-Cell Lymphoma
CTCLs constitute a group of non-Hodgkin lymphomas (NHLs) and are cancers of the T lymphocytes (a type of white blood cell) that mainly affect the skin but can also involve the blood, lymph nodes and/or internal organs in patients with advanced disease.
About Millennium
Millennium: The Takeda Oncology Company, a leading biopharmaceutical company based in Cambridge, Mass., markets VELCADE, a first-in-class proteasome inhibitor, and has a robust clinical development pipeline of product candidates. Millennium Pharmaceuticals, Inc. was acquired by Takeda Pharmaceutical Company Ltd. in May, 2008. The Company’s research, development and commercialization activities are focused in oncology. Additional information about Millennium is available through its website, www.millennium.com.
About Seattle Genetics
Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer. The U.S. Food and Drug Administration granted accelerated approval of ADCETRIS in August 2011 for two indications. ADCETRIS is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has three other clinical-stage ADC programs: SGN-75, ASG-5ME and ASG-22ME. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
Hallo,

soschnell kann´s gehen, sind wohl ein paar Shorties auf dem falschen Fuß erwischt worden, Kurs schon wieder im Plus...
Hoffentlich bleibt´s auch so.
Gruß q.

soschnell kann´s gehen, sind wohl ein paar Shorties auf dem falschen Fuß erwischt worden, Kurs schon wieder im Plus...
Hoffentlich bleibt´s auch so.
Gruß q.
Seattle Genetics announces phase II clinical trial results for Adcetris
Published 11 May 2012
Biotechnology firm Seattle Genetics has announced the interim results from an investigator-sponsored phase II clinical trial of Adcetris (brentuximab vedotin) in patients with relapsed cutaneous T-cell lymphoma (CTCL).
Adcetris, which is an antibody-drug conjugate (ADC) directed to CD30, has not been approved for use in CTCL.
The trial enrolled 17 CTCL patients, which include 16 mycosis fungoides (MF) patients and one Sezary syndrome patient.
The primary endpoint of the trial is clinical response rate, while correlation of clinical response with CD30 expression levels, duration of response, progression-free survival and safety were included in the secondary endpoints.
The study showed a partial remission in 12 of 16 evaluable patients (75%), while median CD30 expression on lymphoid cells in biopsies of skin lesions was 15%.
It also revealed that 68% of patients maintained response at week 25, and recorded adverse events mostly of Grade 1 or 2, with the most common related events being peripheral neuropathy, followed by fatigue, decreased appetite and generalized skin.
CTCL is the most common type of cutaneous lymphoma and typically presents with red, scaly patches or thickened plaques of skin that often mimic eczema or chronic dermatitis, according to the Cutaneous Lymphoma Foundation.
Adcetris is not approved for use outside the US, and the marketing authorization application for drug filed by Takeda Global Research & Development Centre (Europe) was accepted by the European Medicines Agency for review in June 2011.
Published 11 May 2012
Biotechnology firm Seattle Genetics has announced the interim results from an investigator-sponsored phase II clinical trial of Adcetris (brentuximab vedotin) in patients with relapsed cutaneous T-cell lymphoma (CTCL).
Adcetris, which is an antibody-drug conjugate (ADC) directed to CD30, has not been approved for use in CTCL.
The trial enrolled 17 CTCL patients, which include 16 mycosis fungoides (MF) patients and one Sezary syndrome patient.
The primary endpoint of the trial is clinical response rate, while correlation of clinical response with CD30 expression levels, duration of response, progression-free survival and safety were included in the secondary endpoints.
The study showed a partial remission in 12 of 16 evaluable patients (75%), while median CD30 expression on lymphoid cells in biopsies of skin lesions was 15%.
It also revealed that 68% of patients maintained response at week 25, and recorded adverse events mostly of Grade 1 or 2, with the most common related events being peripheral neuropathy, followed by fatigue, decreased appetite and generalized skin.
CTCL is the most common type of cutaneous lymphoma and typically presents with red, scaly patches or thickened plaques of skin that often mimic eczema or chronic dermatitis, according to the Cutaneous Lymphoma Foundation.
Adcetris is not approved for use outside the US, and the marketing authorization application for drug filed by Takeda Global Research & Development Centre (Europe) was accepted by the European Medicines Agency for review in June 2011.
Hallo,
hier noch mal eine andere Darstellung des selben Events. Der eine nicht auswertbate Patient ist demnach gestorben, allerdings (hoffentlich) nicht als Nebenwirkung, sondern durch eine Lungenentzündung.
Die Wirksamkeit bei CTCLs scheint demnach ganz gut zu sein, mit einer Fortsetzung der Studien kann man also rechnen.
Gruß q.
Hier der Text:
-----------------------
DOW JONES NEWSWIRES
Biotechnology Seattle Genetics Inc. (SGEN) said interim results in a Phase II clinical trial of its Adcetris drug, used for the treatment of relapsed cutaneous T-cell lymphoma, found that 75% of patients in the study achieved a partial remission.
The disorder, also known as CTCLs, is characterized by an abnormal accumulation of cancerous T-cells in the skin, resulting in an itchy, red rash that can thicken or form a tumor, according to the National Institutes of Health.
Of the 17 patients involved, 12 had partial remission, three had a stable disease and one had progressive disease. One died due to respiratory failure, presumably linked to pneumonia, the company said.
In all, 68% of patients maintained a response at 25 weeks. The most-common side effects included nerve damage, fatigue, decreased appetite and generalized skin eruption.
Adcetris isn't approved to treat CTCL, but was granted accelerated approval in August by the Food and Drug Administration.
Seattle Genetics also has three other clinical-stage programs on which it is working in addition to Adcetris.
Shares were down 3 cents at $18.74 in recent trading. The stock is up 12% so far this year.
-By Ben Fox Rubin, Dow Jones Newswires; 212-416-3108; ben.rubin@dowjones.com
hier noch mal eine andere Darstellung des selben Events. Der eine nicht auswertbate Patient ist demnach gestorben, allerdings (hoffentlich) nicht als Nebenwirkung, sondern durch eine Lungenentzündung.
Die Wirksamkeit bei CTCLs scheint demnach ganz gut zu sein, mit einer Fortsetzung der Studien kann man also rechnen.
Gruß q.
Hier der Text:
-----------------------
DOW JONES NEWSWIRES
Biotechnology Seattle Genetics Inc. (SGEN) said interim results in a Phase II clinical trial of its Adcetris drug, used for the treatment of relapsed cutaneous T-cell lymphoma, found that 75% of patients in the study achieved a partial remission.
The disorder, also known as CTCLs, is characterized by an abnormal accumulation of cancerous T-cells in the skin, resulting in an itchy, red rash that can thicken or form a tumor, according to the National Institutes of Health.
Of the 17 patients involved, 12 had partial remission, three had a stable disease and one had progressive disease. One died due to respiratory failure, presumably linked to pneumonia, the company said.
In all, 68% of patients maintained a response at 25 weeks. The most-common side effects included nerve damage, fatigue, decreased appetite and generalized skin eruption.
Adcetris isn't approved to treat CTCL, but was granted accelerated approval in August by the Food and Drug Administration.
Seattle Genetics also has three other clinical-stage programs on which it is working in addition to Adcetris.
Shares were down 3 cents at $18.74 in recent trading. The stock is up 12% so far this year.
-By Ben Fox Rubin, Dow Jones Newswires; 212-416-3108; ben.rubin@dowjones.com
Hallo,
da fährt man mal übers Wochenende weg, dann so ein Kursverlauf. Das Gap ist nun zu, wollte aber eigentlich zu knapp 22 einen Teil verkauft haben...
Hatte aber keine Order erteilt.
Da rast der Kurs 10% rauf und wieder runter in kürzester Zeit, News gibt´s auch nicht, Short-Quote enorm hoch (wie aber z.B. vorher auch bei MEDX oder MITI.
Könnte mir vorstellen, dass bei solchen Bios die Short-Positionen z.T. tatsächlich dem eigentlichen Zweck dienen, zu dem sie mal erschaffen wurden, nämlich um Aktienpositionen zu hedgen.
(Z.B. bei den Bäcker-Brüdern).
Wenn schon nicht zu 22 verkaufen, dann vielleicht zu 20 kaufen?
Ist schließlich ASCO-Time. Mal sehn...
Gruß q.
da fährt man mal übers Wochenende weg, dann so ein Kursverlauf. Das Gap ist nun zu, wollte aber eigentlich zu knapp 22 einen Teil verkauft haben...
Hatte aber keine Order erteilt.
Da rast der Kurs 10% rauf und wieder runter in kürzester Zeit, News gibt´s auch nicht, Short-Quote enorm hoch (wie aber z.B. vorher auch bei MEDX oder MITI.
Könnte mir vorstellen, dass bei solchen Bios die Short-Positionen z.T. tatsächlich dem eigentlichen Zweck dienen, zu dem sie mal erschaffen wurden, nämlich um Aktienpositionen zu hedgen.
(Z.B. bei den Bäcker-Brüdern).
Wenn schon nicht zu 22 verkaufen, dann vielleicht zu 20 kaufen?
Ist schließlich ASCO-Time. Mal sehn...
Gruß q.
Hallo und Moin,
gleich 2x gute Nachrichten:
1. Adcetris-Zulassung für Kanada (bereits eingepreist)
2. Celldex´ Brustkrebs Medikament offenbar wirksam (noch nicht eingepreist, da Mitteilung nach Börsenschluss)
Das Celldex-Medikament basiert ja auf einem ADC von SGEN, könnte den Kurs antreiben.

Gruß q.
gleich 2x gute Nachrichten:
1. Adcetris-Zulassung für Kanada (bereits eingepreist)
2. Celldex´ Brustkrebs Medikament offenbar wirksam (noch nicht eingepreist, da Mitteilung nach Börsenschluss)
Das Celldex-Medikament basiert ja auf einem ADC von SGEN, könnte den Kurs antreiben.

Gruß q.
Moin,

SGEN ein kleines grünes Licht in der roten Steppe...
zwar nur PI-Daten, aber mit beobachteter Tumor-Wirksamkeit.
Und 50:50 Ertragsbeteiligung. Vieleicht ein weiteres Standbein neben Adcetris?
Gruß q.
-------------------
Agensys and Seattle Genetics Announce Interim Phase I Data from ASG-5ME Clinical Trial for Prostate Cancer
Dose-Escalation Completed and Preliminary Antitumor Activity Observed
Press Release: Seattle Genetics, Inc. – 4 hours agoShare0EmailPrintCompanies:Seattle Genetics Inc.RELATED QUOTESSymbol Price Change
SGEN 19.43 +0.32
Related Content
View Photohttp://www.astellas.com/enGenre Notes
SANTA MONICA, Calif. & BOTHELL, Wash.--(BUSINESS WIRE)--
Agensys, Inc., an affiliate of Tokyo-based Astellas Pharma Inc., and Seattle Genetics, Inc. (SGEN) today announced interim data from a phase I clinical trial evaluating ASG-5ME for the treatment of castration-resistant prostate cancer (CRPC). ASG-5ME is an antibody-drug conjugate (ADC) targeting the SLC44A4 antigen that is being co-developed by both companies for the treatment of solid tumors. The data are being presented at the American Society of Clinical Oncology (ASCO) annual meeting being held June 1-5, 2012 in Chicago, IL.
“SLC44A4 is an attractive target in prostate cancer and is present in the majority of patients with both localized and metastatic disease,” said Leonard Reyno, M.D., Senior Vice President and Chief Medical Officer of Agensys. “The current Phase I data demonstrates the tolerability of this antibody drug conjugate and further evaluation of safety and antitumor activity in patients with castration resistant prostate cancer is ongoing.”
“It is encouraging to observe these preliminary data with ASG-5ME in prostate cancer, a disease for which late-stage patients need additional therapeutic options,” said Jonathan Drachman, M.D., Senior Vice President, Research and Translational Medicine of Seattle Genetics. “In addition to prostate cancer, our two companies are continuing to evaluate the potential use of ASG-5ME in other solid tumor indications. In parallel, we are collaborating with Agensys to co-develop ASG-22ME, an ADC targeting Nectin-4 for solid tumors.”
Phase 1 trial of ASG-5ME in metastatic castration-resistant prostate cancer (CRPC) (Abstract #4568)
ASG-5ME is being evaluated in a single-agent phase I clinical trial to determine the maximum tolerated dose (MTD) and to assess the safety, pharmacokinetic profile and antitumor activity of escalating doses of ASG-5ME. At the time of data analysis, 26 patients were enrolled. The median age of the patients was 69.5 years and the median baseline prostate-specific antigen (PSA) level was 82.25.
Key findings, presented by Dr. Michael Morris from Memorial Sloan Kettering Cancer Center in New York, NY, and clinical investigator on the study include:
ASG-5ME was given to cohorts of patients with CRPC as a single IV infusion every three weeks at doses ranging from 0.3 milligrams per kilogram (mg/kg) to 3.0 mg/kg. The MTD was exceeded at 3.0 mg/kg.
Across all dose cohorts, the most common Grade 1 and 2 adverse events occurring in more than 20 percent of patients included fatigue (50.0 percent), decreased appetite (42.3 percent), peripheral neuropathy (34.6 percent) and nausea (23.0 percent).
PSA reductions were observed in several patients, providing preliminary evidence of antitumor effect with ASG-5ME treatment.
The phase I trial is ongoing, with enrollment to two expansion cohorts in chemotherapy naïve and chemotherapy exposed CRPC patients planned.
Seattle Genetics and Agensys recently completed enrollment in a phase I pancreatic cancer trial of ASG-5ME dosed weekly. The companies plan to evaluate ASG-5ME in patients with gastric cancer based on preclinical expression data.

SGEN ein kleines grünes Licht in der roten Steppe...
zwar nur PI-Daten, aber mit beobachteter Tumor-Wirksamkeit.
Und 50:50 Ertragsbeteiligung. Vieleicht ein weiteres Standbein neben Adcetris?
Gruß q.
-------------------
Agensys and Seattle Genetics Announce Interim Phase I Data from ASG-5ME Clinical Trial for Prostate Cancer
Dose-Escalation Completed and Preliminary Antitumor Activity Observed
Press Release: Seattle Genetics, Inc. – 4 hours agoShare0EmailPrintCompanies:Seattle Genetics Inc.RELATED QUOTESSymbol Price Change
SGEN 19.43 +0.32
Related Content
View Photohttp://www.astellas.com/enGenre Notes
SANTA MONICA, Calif. & BOTHELL, Wash.--(BUSINESS WIRE)--
Agensys, Inc., an affiliate of Tokyo-based Astellas Pharma Inc., and Seattle Genetics, Inc. (SGEN) today announced interim data from a phase I clinical trial evaluating ASG-5ME for the treatment of castration-resistant prostate cancer (CRPC). ASG-5ME is an antibody-drug conjugate (ADC) targeting the SLC44A4 antigen that is being co-developed by both companies for the treatment of solid tumors. The data are being presented at the American Society of Clinical Oncology (ASCO) annual meeting being held June 1-5, 2012 in Chicago, IL.
“SLC44A4 is an attractive target in prostate cancer and is present in the majority of patients with both localized and metastatic disease,” said Leonard Reyno, M.D., Senior Vice President and Chief Medical Officer of Agensys. “The current Phase I data demonstrates the tolerability of this antibody drug conjugate and further evaluation of safety and antitumor activity in patients with castration resistant prostate cancer is ongoing.”
“It is encouraging to observe these preliminary data with ASG-5ME in prostate cancer, a disease for which late-stage patients need additional therapeutic options,” said Jonathan Drachman, M.D., Senior Vice President, Research and Translational Medicine of Seattle Genetics. “In addition to prostate cancer, our two companies are continuing to evaluate the potential use of ASG-5ME in other solid tumor indications. In parallel, we are collaborating with Agensys to co-develop ASG-22ME, an ADC targeting Nectin-4 for solid tumors.”
Phase 1 trial of ASG-5ME in metastatic castration-resistant prostate cancer (CRPC) (Abstract #4568)
ASG-5ME is being evaluated in a single-agent phase I clinical trial to determine the maximum tolerated dose (MTD) and to assess the safety, pharmacokinetic profile and antitumor activity of escalating doses of ASG-5ME. At the time of data analysis, 26 patients were enrolled. The median age of the patients was 69.5 years and the median baseline prostate-specific antigen (PSA) level was 82.25.
Key findings, presented by Dr. Michael Morris from Memorial Sloan Kettering Cancer Center in New York, NY, and clinical investigator on the study include:
ASG-5ME was given to cohorts of patients with CRPC as a single IV infusion every three weeks at doses ranging from 0.3 milligrams per kilogram (mg/kg) to 3.0 mg/kg. The MTD was exceeded at 3.0 mg/kg.
Across all dose cohorts, the most common Grade 1 and 2 adverse events occurring in more than 20 percent of patients included fatigue (50.0 percent), decreased appetite (42.3 percent), peripheral neuropathy (34.6 percent) and nausea (23.0 percent).
PSA reductions were observed in several patients, providing preliminary evidence of antitumor effect with ASG-5ME treatment.
The phase I trial is ongoing, with enrollment to two expansion cohorts in chemotherapy naïve and chemotherapy exposed CRPC patients planned.
Seattle Genetics and Agensys recently completed enrollment in a phase I pancreatic cancer trial of ASG-5ME dosed weekly. The companies plan to evaluate ASG-5ME in patients with gastric cancer based on preclinical expression data.
Moin,

nun auch das noch:
(P.S.: halte nichts von Cramer, m.M. nach unseriös. Aber vielleicht hilft´s dem Kurs, zumindest kurzfristig).
-------------
Cramer Interviews Seattle Genetics CEO
Published: Tuesday, 5 Jun 2012 | 6:50 PM ET Text Size By: Kirsten Chang
Special to CNBC
While economies everywhere continue to face a global slowdown, investors should turn to the safety of stocks in a recession-proof sector, Jim Cramer said Tuesday on CNBC's "Mad Money." He cited Seattle Genetics as the perfect example of one such stock.
With a market capitalization of $2.4 billion, Seattle Genetics [SGEN Loading... () ] is a small company, but Cramer thinks it’s worth speculating on.
The Bothell, Wash.-based firm champions the use of innovative biotechnology — like engineered antibodies to target and kill cancer cells while sparing healthy cells. And though the stock isn't profitable just yet, it does have one drug — Adcetris — already on the market and is expected to start raking in material revenues in 2014.
Cramer also noted that at the American Society of Clinical Oncology conference, Seattle Genetics released "bullish phase two data" about the use of Adcetris to treat other types of lymphoma, in addition to the Hodgkin's and large cell strains of lymphoma the drug has already successfully treated.
To find out more about the company's prospects, Cramer sat down to chat with Clay Siegall, president and CEO of Seattle Genetics on Tuesday's show. Watch the video to see the full interview.

nun auch das noch:
(P.S.: halte nichts von Cramer, m.M. nach unseriös. Aber vielleicht hilft´s dem Kurs, zumindest kurzfristig).
-------------
Cramer Interviews Seattle Genetics CEO
Published: Tuesday, 5 Jun 2012 | 6:50 PM ET Text Size By: Kirsten Chang
Special to CNBC
While economies everywhere continue to face a global slowdown, investors should turn to the safety of stocks in a recession-proof sector, Jim Cramer said Tuesday on CNBC's "Mad Money." He cited Seattle Genetics as the perfect example of one such stock.
With a market capitalization of $2.4 billion, Seattle Genetics [SGEN Loading... () ] is a small company, but Cramer thinks it’s worth speculating on.
The Bothell, Wash.-based firm champions the use of innovative biotechnology — like engineered antibodies to target and kill cancer cells while sparing healthy cells. And though the stock isn't profitable just yet, it does have one drug — Adcetris — already on the market and is expected to start raking in material revenues in 2014.
Cramer also noted that at the American Society of Clinical Oncology conference, Seattle Genetics released "bullish phase two data" about the use of Adcetris to treat other types of lymphoma, in addition to the Hodgkin's and large cell strains of lymphoma the drug has already successfully treated.
To find out more about the company's prospects, Cramer sat down to chat with Clay Siegall, president and CEO of Seattle Genetics on Tuesday's show. Watch the video to see the full interview.
Moin nochmal,
oder buenas noches
sehr guter Tag, da All-Time-High bei hohen Umsätzen generiert wurde. Verstehe immer nicht, warum es so wenig Interesse für SGEN gibt...
Seid Thread-Eröffnung immerhin ca. Verdreifachung.
Ist mir allerdings fast schon zuviel im Moment, Cramer´s Jünger dürften über kurz oder lang abgezockt werden (danach Verkaufswelle).
Ich bleibe investiert, werde aber bei Erreichen der 26 einen Teil verkaufen (falls nichts grundlegend Neues kommt).
War zwar nur die "Fu0-in-der-Tür" Position, aber der Fuß war schon breit.
Vielleicht kommt auch noch was (bei dem Volumen)
q.
oder buenas noches
sehr guter Tag, da All-Time-High bei hohen Umsätzen generiert wurde. Verstehe immer nicht, warum es so wenig Interesse für SGEN gibt...
Seid Thread-Eröffnung immerhin ca. Verdreifachung.
Ist mir allerdings fast schon zuviel im Moment, Cramer´s Jünger dürften über kurz oder lang abgezockt werden (danach Verkaufswelle).
Ich bleibe investiert, werde aber bei Erreichen der 26 einen Teil verkaufen (falls nichts grundlegend Neues kommt).
War zwar nur die "Fu0-in-der-Tür" Position, aber der Fuß war schon breit.
Vielleicht kommt auch noch was (bei dem Volumen)
q.
Millennium meldet aktualisierte Überlebensdaten aus zulassungsrelevanter Studie zu ADCETRIS® (Brentuximab Vedotin) mit Patienten mit rezidiviertem oder refraktärem Hodgkin-Lymphom
– Mittlere Gesamtüberlebensdauer nach 26,5 Monaten Folgezeit noch nicht erreicht -
– Daten werden auf der 17. Jahrestagung des Europäischen Hämatologieverbandes vorgelegt -
Millennium: The Takeda Oncology Company, eine hundertprozentige Tochtergesellschaft der Takeda Pharmaceutical Company Limited (TSE:4502), veröffentlichte heute aktualisierte Überlebensdaten aus einer zulassungsrelevanten klinischen Phase-II-Studie zu dem Einzelwirkstoff Brentuximab Vedotin bei Patienten mit rezidiviertem oder refraktärem Hodgkin-Lymphom (HL) nach einer autologen Stammzellentransplantation (ASZT). Aus diesen Daten ging hervor, dass die mittlere Überlebenszeit nach einer Folgezeitspanne von 26,5 Monaten noch nicht erreicht war. Die Daten werden in einem mündlichen Vortrag auf der 17. Jahrestagung des Europäischen Hämatologieverbandes (EHA) vorgelegt, die vom 14. bis 17. Juni 2012 in Amsterdam (Niederlande) stattfindet. Brentuximab Vedotin ist ein Antikörperwirkstoffkonjugat (ADC), das auf CD30, einen definierenden Marker der Mehrzahl von HL-Typen, abzielt.
„Patienten mit stark vorbehandeltem Hodgkin-Lymphom, die nach einer autologen Stammzellentransplantation einen Rückfall erleiden, haben oft sehr schlechte Aussichten. Darum besteht hier eine erhebliche medizinische Versorgungslücke hinsichtlich wirksamer Behandlungsoptionen“, erklärte Dr. Scott Smith, Ph.D., Loyola University Medical Center. „Diese aktualisierten Gesamtüberlebensdaten aus der zulassungsrelevanten Studie sind ermutigend und legen den Schluss nahe, dass Brentuximab Vedotin bei der Therapierung von Patienten mit einer rezidivierten oder refraktären Erkrankung eine wichtige Rolle spielen könnte.“
Langfristige Folgeergebnisse einer laufenden zulassungsrelevanten Studie zu Brentuximab Vedotin bei Patienten mit rezidiviertem oder refraktärem Hodgkin-Lymphom
Die zulassungsrelevante Studie wurde mit 102 Patienten mit rezidiviertem oder refraktärem HL nach ASZT durchgeführt. Der primäre Endpunkt bestand in der Ermittlung der objektiven Ansprechrate (ORR) nach unabhängiger Prüfung. Die sekundären Endpunkte waren komplette Remission (CR), Dauer des Ansprechens, progressionsfreies Überleben (PFS), Gesamtüberlebenszeit (OS) sowie Sicherheit und Verträglichkeit. Zum Zeitpunkt der langfristigen Folgeanalyse betrug die mittlere Beobachtungszeit seit der ersten Dosis 26,5 Monate. Die von Dr. Scott Smith vorgelegten Daten beinhalten folgende Punkte:
Wie bereits berichtet, lag die ORR bei 75 Prozent (76 von 102 Patienten), wobei 33 Prozent der Patienten (n=34) eine komplette Remission erzielten.
Die mittlere Gesamtüberlebensrate war nach einer mittleren Beobachtungszeit von 26,5 Monaten noch nicht erreicht.
Die mittlere progressionsfreie Überlebenszeit für alle Patienten lag bei 5,6 Monaten.
Wie bereits berichtet, waren die häufigsten (≥20 Prozent) nachteiligen Ereignisse (AE) jeglichen Grades periphere sensorische Neuropathie (47 Prozent), Müdigkeit (46 Prozent), Übelkeit (42 Prozent), Infektionen der oberen Atemwege (37 Prozent), Durchfall (36 Prozent), Pyrexie (29 Prozent), Neutropenie (22 Prozent), Erbrechen (22 Prozent) und Husten (21 Prozent).
Unter den nachteiligen Ereignissen, die bei ≥20 der Probanden auftraten, gehörten zu den Ereignissen 3. Grades oder höher Neutropenie (20 Prozent), periphere sensorische Neuropathie (9 Prozent), Müdigkeit (2 Prozent), Pyrexie (2 Prozent) und Durchfall (1 Prozent).
Die Patienten erhielten alle drei Wochen 1,8 Milligramm Brentuximab Vedotin je Kilogramm als 30-minütige, ambulant verabreichte, intravenöse Infusion, und zwar bis zu 16 Zyklen. Im Rahmen der Studie erhielten die Probanden einen Mittelwert von 9 Zyklen Brentuximab Vedotin. Das mittlere Alter der Patienten in der zulassungsrelevanten Studie lag bei 31 Jahren. Die Teilnehmer hatten einen Mittelwert von 3,5 (Bereich 1-13) frühere krebsbezogene systemische Therapien erhalten, darunter auch ASZT. 71 Prozent der Patienten litten an einer primären refraktären Erkrankung. Diese sind im Studienprotokoll als jene Patienten definiert, die innerhalb von drei Monaten nach Erreichen einer CR rezidivierten oder keine CR erreichten. 42 Prozent hatten nicht auf ihre zuletzt erhaltene Therapie angesprochen.
Angaben zu dem mündlichen Vortrag:
Sonntag, 17. Juni, 08.30 Uhr – 08.45 Uhr MESZ
Abstract #1109
Mündlicher Vortrag in Halle 2
Hauptverfasser: Dr. Scott Smith, Ph.D., Loyola University Medical Center
Über Brentuximab Vedotin
Brentuximab Vedotin ist ein Antikörperwirkstoffkonjugat (ADC), das einen monoklonalen Anti-CD30-Antikörper beinhaltet, der unter Nutzung der proprietären Technologie von Seattle Genetics mithilfe eines durch eine Protease abspaltbaren Linkers an einen Wirkstoff namens Monomethyl-Auristatin E (MMAE) gebunden ist, welcher in die Mikrotubulusbildung eingreift. Das Konjugat verwendet ein Linkersystem, das in der Blutbahn stabil bleiben, aber nach Einschluss in CD30-exprimierende Tumorzellen MMAE freisetzen soll.
Brentuximab Vedotin ist nicht für die Verwendung außerhalb der USA zugelassen. Der von Takeda Global Research & Development Centre (Europe) gestellte Zulassungsantrag auf das Inverkehrbringen (MAA) von Brentuximab Vedotin für rezidiviertes oder refraktäres Hodgkin-Lymphom und systemisches ALCL wurde im Juni 2011 von der Europäischen Arzneimittelagentur zur Prüfung angenommen.
Millennium und Seattle Genetics (Nasdaq:SGEN) entwickeln Brentuximab Vedotin gemeinsam. Unter den Bedingungen der Kooperationsvereinbarung verfügt Seattle Genetics über die US-amerikanischen und kanadischen Vermarktungsrechte und die Takeda-Gruppe über die Rechte zur Kommerzialisierung von Brentuximab Vedotin im Rest der Welt. Seattle Genetics und die Takeda-Gruppe finanzieren die Entwicklungskosten für Brentuximab Vedotin gemeinsam auf einer 50-prozentigen Basis, außer in Japan, wo die Takeda-Gruppe die Entwicklungskosten allein übernimmt.
Über das Hodgkin-Lymphom
Lymphom ist ein Sammelbegriff für verschiedene Krebsarten, die ihren Ursprung im lymphatischen System haben. Es gibt zwei Hauptkategorien von Lymphomen: Hodgkin-Lymphom und Non-Hodgkin-Lymphom. Das Hodgkin-Lymphom unterscheidet sich von anderen Lymphomtypen durch das Vorhandensein eines charakteristischen Zelltyps, der Sternberg-Reed-Zelle. Die Sternberg-Reed-Zelle exprimiert im Allgemeinen CD30.
Weltweit werden jedes Jahr mehr als 30.000 Neudiagnosen für Hodgkin-Lymphom gestellt. Obwohl die in der Regel zuerst angewandte Kombinationschemotherapie ein dauerhaftes Ansprechen bewirken kann, rezidivieren bis zu 30 Prozent der Erkrankungen oder sind gegen die Haupttherapie refraktär. In diesem Fall stehen außer ASZT nur wenige Therapieoptionen bereit.
Über Millennium
Millennium: The Takeda Oncology Company, ein führendes biopharmazeutisches Unternehmen mit Sitz in Cambridge im US-Bundesstaat Massachusetts, vermarktet VELCADE, einen Proteasom-Hemmer und ersten Vertreter dieser Arzneimittelklasse, und verfügt über eine robuste klinische Entwicklungspipeline für weitere Produktkandidaten. Millennium Pharmaceuticals Inc. wurde im Mai 2008 von der Takeda Pharmaceutical Company Ltd. übernommen. Der Schwerpunkt der Forschungs-, Entwicklungs- und Vermarktungstätigkeit des Unternehmens liegt auf dem Gebiet der Onkologie. Weitere Informationen zu Millennium erhalten Sie auf der Unternehmenswebsite unter: www.millennium.com.
Über die Takeda Pharmaceuticals International GmbH
Die Takeda Pharmaceuticals International GmbH hat ihren Firmensitz in Zürich und ist ein hundertprozentiges Tochterunternehmen der Takeda Pharmaceutical Company Limited. Als größtes Pharmaunternehmen Japans und eines der führenden in der globalen Pharmaindustrie setzt Takeda sich dafür ein, die Gesundheit von Patienten weltweit durch innovative Führungsarbeit in der Medizin zu verbessern. Takeda ist in mehr als 70 Ländern vertreten, mit besonderen Stärken in Asien, Nordamerika, Europa und den schnell wachsenden Märkten wie Lateinamerika, Russland CIS und China. Im globalen Ranking nach Rx-Umsatz steht Takeda auf Platz 12, in den BRIC-Ländern an 14. Stelle sowie auf der 18. Position in Europa. Die geschäftliche Präsenz des Unternehmens erstreckt sich hauptsächlich auf die therapeutischen Gebiete Stoffwechselerkrankungen, Gastroenterologie, Onkologie, Herz-Kreislauf, Erkrankungen des ZNS, Entzündungs- und Immunerkrankungen, Atemwegserkrankungen und Schmerztherapie.
Weitere Informationen über Takeda erhalten Sie auf der Website des Unternehmens unter: http://www.takeda.com.
Hinweis der Redaktion: Diese Pressemitteilung ist auch in der Rubrik Medien auf der Website des Unternehmens verfügbar unter: www.millennium.com/InTheNews.aspx.
Fotos/Multimedia-Galerie verfügbar unter: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=50309738&lan…
Die Ausgangssprache, in der der Originaltext veröffentlicht wird, ist die offizielle und autorisierte Version. Übersetzungen werden zur besseren Verständigung mitgeliefert. Nur die Sprachversion, die im Original veröffentlicht wurde, ist rechtsgültig. Gleichen Sie deshalb Übersetzungen mit der originalen Sprachversion der Veröffentlichung ab.
Millennium Lindsay Treadway, +1-617-444-3383 lindsay.treadway@mpi.com oder Takeda Pharmaceuticals Europe Ltd. Danny Stepto, +44-20-3116-8000 Corporate Communications danny.stepto@takeda.com
Thumbnail
Quelle: BUSINESS WIRE
– Mittlere Gesamtüberlebensdauer nach 26,5 Monaten Folgezeit noch nicht erreicht -
– Daten werden auf der 17. Jahrestagung des Europäischen Hämatologieverbandes vorgelegt -
Millennium: The Takeda Oncology Company, eine hundertprozentige Tochtergesellschaft der Takeda Pharmaceutical Company Limited (TSE:4502), veröffentlichte heute aktualisierte Überlebensdaten aus einer zulassungsrelevanten klinischen Phase-II-Studie zu dem Einzelwirkstoff Brentuximab Vedotin bei Patienten mit rezidiviertem oder refraktärem Hodgkin-Lymphom (HL) nach einer autologen Stammzellentransplantation (ASZT). Aus diesen Daten ging hervor, dass die mittlere Überlebenszeit nach einer Folgezeitspanne von 26,5 Monaten noch nicht erreicht war. Die Daten werden in einem mündlichen Vortrag auf der 17. Jahrestagung des Europäischen Hämatologieverbandes (EHA) vorgelegt, die vom 14. bis 17. Juni 2012 in Amsterdam (Niederlande) stattfindet. Brentuximab Vedotin ist ein Antikörperwirkstoffkonjugat (ADC), das auf CD30, einen definierenden Marker der Mehrzahl von HL-Typen, abzielt.
„Patienten mit stark vorbehandeltem Hodgkin-Lymphom, die nach einer autologen Stammzellentransplantation einen Rückfall erleiden, haben oft sehr schlechte Aussichten. Darum besteht hier eine erhebliche medizinische Versorgungslücke hinsichtlich wirksamer Behandlungsoptionen“, erklärte Dr. Scott Smith, Ph.D., Loyola University Medical Center. „Diese aktualisierten Gesamtüberlebensdaten aus der zulassungsrelevanten Studie sind ermutigend und legen den Schluss nahe, dass Brentuximab Vedotin bei der Therapierung von Patienten mit einer rezidivierten oder refraktären Erkrankung eine wichtige Rolle spielen könnte.“
Langfristige Folgeergebnisse einer laufenden zulassungsrelevanten Studie zu Brentuximab Vedotin bei Patienten mit rezidiviertem oder refraktärem Hodgkin-Lymphom
Die zulassungsrelevante Studie wurde mit 102 Patienten mit rezidiviertem oder refraktärem HL nach ASZT durchgeführt. Der primäre Endpunkt bestand in der Ermittlung der objektiven Ansprechrate (ORR) nach unabhängiger Prüfung. Die sekundären Endpunkte waren komplette Remission (CR), Dauer des Ansprechens, progressionsfreies Überleben (PFS), Gesamtüberlebenszeit (OS) sowie Sicherheit und Verträglichkeit. Zum Zeitpunkt der langfristigen Folgeanalyse betrug die mittlere Beobachtungszeit seit der ersten Dosis 26,5 Monate. Die von Dr. Scott Smith vorgelegten Daten beinhalten folgende Punkte:
Wie bereits berichtet, lag die ORR bei 75 Prozent (76 von 102 Patienten), wobei 33 Prozent der Patienten (n=34) eine komplette Remission erzielten.
Die mittlere Gesamtüberlebensrate war nach einer mittleren Beobachtungszeit von 26,5 Monaten noch nicht erreicht.
Die mittlere progressionsfreie Überlebenszeit für alle Patienten lag bei 5,6 Monaten.
Wie bereits berichtet, waren die häufigsten (≥20 Prozent) nachteiligen Ereignisse (AE) jeglichen Grades periphere sensorische Neuropathie (47 Prozent), Müdigkeit (46 Prozent), Übelkeit (42 Prozent), Infektionen der oberen Atemwege (37 Prozent), Durchfall (36 Prozent), Pyrexie (29 Prozent), Neutropenie (22 Prozent), Erbrechen (22 Prozent) und Husten (21 Prozent).
Unter den nachteiligen Ereignissen, die bei ≥20 der Probanden auftraten, gehörten zu den Ereignissen 3. Grades oder höher Neutropenie (20 Prozent), periphere sensorische Neuropathie (9 Prozent), Müdigkeit (2 Prozent), Pyrexie (2 Prozent) und Durchfall (1 Prozent).
Die Patienten erhielten alle drei Wochen 1,8 Milligramm Brentuximab Vedotin je Kilogramm als 30-minütige, ambulant verabreichte, intravenöse Infusion, und zwar bis zu 16 Zyklen. Im Rahmen der Studie erhielten die Probanden einen Mittelwert von 9 Zyklen Brentuximab Vedotin. Das mittlere Alter der Patienten in der zulassungsrelevanten Studie lag bei 31 Jahren. Die Teilnehmer hatten einen Mittelwert von 3,5 (Bereich 1-13) frühere krebsbezogene systemische Therapien erhalten, darunter auch ASZT. 71 Prozent der Patienten litten an einer primären refraktären Erkrankung. Diese sind im Studienprotokoll als jene Patienten definiert, die innerhalb von drei Monaten nach Erreichen einer CR rezidivierten oder keine CR erreichten. 42 Prozent hatten nicht auf ihre zuletzt erhaltene Therapie angesprochen.
Angaben zu dem mündlichen Vortrag:
Sonntag, 17. Juni, 08.30 Uhr – 08.45 Uhr MESZ
Abstract #1109
Mündlicher Vortrag in Halle 2
Hauptverfasser: Dr. Scott Smith, Ph.D., Loyola University Medical Center
Über Brentuximab Vedotin
Brentuximab Vedotin ist ein Antikörperwirkstoffkonjugat (ADC), das einen monoklonalen Anti-CD30-Antikörper beinhaltet, der unter Nutzung der proprietären Technologie von Seattle Genetics mithilfe eines durch eine Protease abspaltbaren Linkers an einen Wirkstoff namens Monomethyl-Auristatin E (MMAE) gebunden ist, welcher in die Mikrotubulusbildung eingreift. Das Konjugat verwendet ein Linkersystem, das in der Blutbahn stabil bleiben, aber nach Einschluss in CD30-exprimierende Tumorzellen MMAE freisetzen soll.
Brentuximab Vedotin ist nicht für die Verwendung außerhalb der USA zugelassen. Der von Takeda Global Research & Development Centre (Europe) gestellte Zulassungsantrag auf das Inverkehrbringen (MAA) von Brentuximab Vedotin für rezidiviertes oder refraktäres Hodgkin-Lymphom und systemisches ALCL wurde im Juni 2011 von der Europäischen Arzneimittelagentur zur Prüfung angenommen.
Millennium und Seattle Genetics (Nasdaq:SGEN) entwickeln Brentuximab Vedotin gemeinsam. Unter den Bedingungen der Kooperationsvereinbarung verfügt Seattle Genetics über die US-amerikanischen und kanadischen Vermarktungsrechte und die Takeda-Gruppe über die Rechte zur Kommerzialisierung von Brentuximab Vedotin im Rest der Welt. Seattle Genetics und die Takeda-Gruppe finanzieren die Entwicklungskosten für Brentuximab Vedotin gemeinsam auf einer 50-prozentigen Basis, außer in Japan, wo die Takeda-Gruppe die Entwicklungskosten allein übernimmt.
Über das Hodgkin-Lymphom
Lymphom ist ein Sammelbegriff für verschiedene Krebsarten, die ihren Ursprung im lymphatischen System haben. Es gibt zwei Hauptkategorien von Lymphomen: Hodgkin-Lymphom und Non-Hodgkin-Lymphom. Das Hodgkin-Lymphom unterscheidet sich von anderen Lymphomtypen durch das Vorhandensein eines charakteristischen Zelltyps, der Sternberg-Reed-Zelle. Die Sternberg-Reed-Zelle exprimiert im Allgemeinen CD30.
Weltweit werden jedes Jahr mehr als 30.000 Neudiagnosen für Hodgkin-Lymphom gestellt. Obwohl die in der Regel zuerst angewandte Kombinationschemotherapie ein dauerhaftes Ansprechen bewirken kann, rezidivieren bis zu 30 Prozent der Erkrankungen oder sind gegen die Haupttherapie refraktär. In diesem Fall stehen außer ASZT nur wenige Therapieoptionen bereit.
Über Millennium
Millennium: The Takeda Oncology Company, ein führendes biopharmazeutisches Unternehmen mit Sitz in Cambridge im US-Bundesstaat Massachusetts, vermarktet VELCADE, einen Proteasom-Hemmer und ersten Vertreter dieser Arzneimittelklasse, und verfügt über eine robuste klinische Entwicklungspipeline für weitere Produktkandidaten. Millennium Pharmaceuticals Inc. wurde im Mai 2008 von der Takeda Pharmaceutical Company Ltd. übernommen. Der Schwerpunkt der Forschungs-, Entwicklungs- und Vermarktungstätigkeit des Unternehmens liegt auf dem Gebiet der Onkologie. Weitere Informationen zu Millennium erhalten Sie auf der Unternehmenswebsite unter: www.millennium.com.
Über die Takeda Pharmaceuticals International GmbH
Die Takeda Pharmaceuticals International GmbH hat ihren Firmensitz in Zürich und ist ein hundertprozentiges Tochterunternehmen der Takeda Pharmaceutical Company Limited. Als größtes Pharmaunternehmen Japans und eines der führenden in der globalen Pharmaindustrie setzt Takeda sich dafür ein, die Gesundheit von Patienten weltweit durch innovative Führungsarbeit in der Medizin zu verbessern. Takeda ist in mehr als 70 Ländern vertreten, mit besonderen Stärken in Asien, Nordamerika, Europa und den schnell wachsenden Märkten wie Lateinamerika, Russland CIS und China. Im globalen Ranking nach Rx-Umsatz steht Takeda auf Platz 12, in den BRIC-Ländern an 14. Stelle sowie auf der 18. Position in Europa. Die geschäftliche Präsenz des Unternehmens erstreckt sich hauptsächlich auf die therapeutischen Gebiete Stoffwechselerkrankungen, Gastroenterologie, Onkologie, Herz-Kreislauf, Erkrankungen des ZNS, Entzündungs- und Immunerkrankungen, Atemwegserkrankungen und Schmerztherapie.
Weitere Informationen über Takeda erhalten Sie auf der Website des Unternehmens unter: http://www.takeda.com.
Hinweis der Redaktion: Diese Pressemitteilung ist auch in der Rubrik Medien auf der Website des Unternehmens verfügbar unter: www.millennium.com/InTheNews.aspx.
Fotos/Multimedia-Galerie verfügbar unter: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=50309738&lan…
Die Ausgangssprache, in der der Originaltext veröffentlicht wird, ist die offizielle und autorisierte Version. Übersetzungen werden zur besseren Verständigung mitgeliefert. Nur die Sprachversion, die im Original veröffentlicht wurde, ist rechtsgültig. Gleichen Sie deshalb Übersetzungen mit der originalen Sprachversion der Veröffentlichung ab.
Millennium Lindsay Treadway, +1-617-444-3383 lindsay.treadway@mpi.com oder Takeda Pharmaceuticals Europe Ltd. Danny Stepto, +44-20-3116-8000 Corporate Communications danny.stepto@takeda.com
Thumbnail
Quelle: BUSINESS WIRE
Hallo Laura6,
habe die Meldung auch schon wohlwollend überflogen, das Ansehen von Adcetris wächst ständig und der Kurs auch!

Ist ja fast schon mein (Zwischen-) Ziel von 26$ erreicht (wobei ich mir im Moment nicht mehr so sicher bin, ob ich dann wirklich einen Teil verkaufe).
Schließlich soll man ja auch mal den Kurs laufen lassen.
Was mich interessiert ist deine deutsche Version der Meldung. Ist gut übersetzt, du hast als Quelle businesswire.com genannt. Die lese ich auch öfters, aber wie hast du die deutsche Version bekommen oder muss man sich anmelden?
Habe auf die Schnelle nichts gefunden.

Gruß q.
habe die Meldung auch schon wohlwollend überflogen, das Ansehen von Adcetris wächst ständig und der Kurs auch!

Ist ja fast schon mein (Zwischen-) Ziel von 26$ erreicht (wobei ich mir im Moment nicht mehr so sicher bin, ob ich dann wirklich einen Teil verkaufe).
Schließlich soll man ja auch mal den Kurs laufen lassen.
Was mich interessiert ist deine deutsche Version der Meldung. Ist gut übersetzt, du hast als Quelle businesswire.com genannt. Die lese ich auch öfters, aber wie hast du die deutsche Version bekommen oder muss man sich anmelden?
Habe auf die Schnelle nichts gefunden.

Gruß q.
Hallo quepos,
Hier ist der Link.
http://www.businesswire.com/news/home/20120614005437/de
Der Link ist für jeden zugänglich.
Gruß Laura6
Hier ist der Link.
http://www.businesswire.com/news/home/20120614005437/de
Der Link ist für jeden zugänglich.
Gruß Laura6
Hallo,
vielen Dank, war mein Fehler, hatte nur nicht nach der Suche bei businesswire runter gescrollt, da stehen ja alle Übersetzungen, sogar holländisch und italienisch.

Gruß q.
vielen Dank, war mein Fehler, hatte nur nicht nach der Suche bei businesswire runter gescrollt, da stehen ja alle Übersetzungen, sogar holländisch und italienisch.

Gruß q.
Hallo,

habe mich nun doch aufgerafft, die Hälfte meiner Position zu versilbern für knapp 26$. Kauf war ja zu 18$, so dass sich ein nettes Plus ergibt.
Falls es doch noch weiter steigt, habe ich ja immer noch einige. Der Anstieg war mir im Grunde zu schnell in der letzten Zeit.
Gruß q.

habe mich nun doch aufgerafft, die Hälfte meiner Position zu versilbern für knapp 26$. Kauf war ja zu 18$, so dass sich ein nettes Plus ergibt.
Falls es doch noch weiter steigt, habe ich ja immer noch einige. Der Anstieg war mir im Grunde zu schnell in der letzten Zeit.
Gruß q.
Hier mal die Meinung von jemanden der ein bißchen näher an Seattle Gen. drann ist.
Seattle Genetics Shares Continue To Impress - And It's Not Too Late To Buy
June 11, 2012 |Steven Breazzano
Background
Seattle Genetics (SGEN), based outside of Seattle, WA, focuses on the development and commercialization of antibody-drug-conjugates, or ADCs, for the treatment of cancer. These ADC therapies are designed to combine the strength of monoclonal antibodies (selective targeting) with the toxic, cell killing strength of a small molecule. By attaching the toxic compound monomethyl auristatin E (well, approximately 4 of these toxic molecules!) to an anti-CD30 antibody, SGEN brought Adcetris to market in the US for Hodgkin's Lymphoma (HL) and systemic anaplastic large cell lymphoma (sALCL), in the refractory setting. After some large price swings in 2011, trading has been relatively tame this year with the shares steadily rising from approximately $16/share (when I first wrote about the company) to $24/share, seemingly immune to the troubles plaguing the macroeconomic landscape. Seattle Genetics has been in the news recently with data releases and ASCO presentations, as well as routine quarterly conference calls.
Adcetris Sales, Balance Sheet, and Financials
SGEN received accelerated approval for Adcetris last year based on tremendous efficacy: 73% objective response rate (ORR) in HL and an 86% ORR in sALCL. The drug was partnered in late 2009 with Millennium, a division of Takeda Pharmaceuticals, and they will continue to pay for half of all development costs. Sales of the drug reached $10 MM in the third quarter of 2011; impressive for only 6 weeks on the market. In the fourth quarter of 2011, net sales of Adcetris reached $33.2 MM, and in the first quarter of 2012, net sales reached $34.5 MM. Also during the earnings release in Q1 2012, management felt comfortable giving guidance on Adcetris sales, expecting a total of $140-150 MM in sales during 2012, with an additional $55 to $65 MM in collaboration revenues.
Seattle Genetics had approximately $309 MM in cash and cash equivalents at the end of Q1 2012, a decrease of ~$21 MM from Q4 2012. The balance sheet is clean with no debt, but a significant amount of deferred revenue is listed under liabilities. As SGEN achieves milestones under its collaborations, this will be recognized as revenue in future periods and is no real cause for concern, as this is common for biotechs with partnership agreements. Management expects a total of $245 MM to $270 MM in expenses for 2012 ($30-33MM of non cash expenses, mostly share-based compensation), and 35% of this is expected to be SG&A. SGEN will not be cash-flow positive this year, but with a strong cash balance and increasing sales of Adcetris, they are well positioned to advance the partnered and wholly-owned therapeutics without raising additional capital.
One frequent criticism of Seattle Genetics is, "they spend too much." SGEN does indeed spend a lot. But theoretically speaking, shouldn't effective R&D expenditures be capitalized and not expensed? I understand that in practice, capitalizing most pharmaceutical R&D would lead to messy, and probably inflated and/or fraudulent balance sheets and income statements since most projects are not successful. But in SGEN's case, the programs are doing well and there is significant progress, so why not give them the benefit of the doubt?
Market Potential of Adcetris
SGEN's pipeline is extremely promising with new ADC's in clinical trials, but most of the attention is centered on Adcetris and its future sales. Adcetris is approved in the refractory setting, and SGEN is attempting to move up the ladder to front-line therapy for HL and sALCL. To achieve this goal, SGEN has initiated and supported approximately a dozen new clinical trials. Here some uncertainty arises. Adcetris is fantastically active in the refractory setting, but there is no guarantee it works in other CD30+ malignancies. So far though, the drug appears synergistic with chemotherapy, and hopefully becomes part of the new standard of care. SGEN is also screening non-lymphoma malignancies to see if other CD30+ malignancies may respond (ASCO abstract can be found here). SGEN's management believes that Adcetris may eventually become a blockbuster drug (traditionally defined as sales over $1 BB), and so far preliminary results demonstrate Adcetris' efficacy in these new indications, with manageable side effects. The fact that sales may reach $140-150 MM in its first 12 months combined with the numerous clinical trial initiations is indicative of both physician and payer acceptance for Adcetris, not to mention the truly beneficial effect Adcetris provides for patients.
Validation of the Platform
Although the accelerated approval last year and impressive early sales provide significant validation of the ADC platform, Celldex's (CLDX) recent results are also positive for SGEN. As a licensee of SGEN's technology, Celldex has been developing CDX-011 for breast cancer. In a subset of patients with triple negative breast cancer, a traditionally tough indication, who also express the antigen CDX-011 targets, results were highly statistically significant and impressive. Although the data is based on a small number of patients, I would imagine Celldex will be pursuing a larger trial in the near future for this particular niche indication. Don't expect much of this to flow to SGEN's bottom line though, as SGEN only receives "royalty payments in the mid-single digits on any net product sales," per CLDX's 10k.
Conclusions and Future Directions
While some uncertainty remains with the upcoming trials with Adcetris and treatments targeting solid tumors, let's not forget SGEN already has a treatment on the market that is selling well. SGEN has a large pipeline, and although valuation is a bit tricky with the sheer volume of partnerships (12 active ADC collaborations!), the depth of the clinical and preclinical pipeline is impressive.
Seattle Genetics is at the vanguard of the hot new field of ADCs, and with clinical validation and impressive sales, Seattle Genetics is a speculative stock that investors may wish to consider in their portfolios.
Disclosure: I am long SGEN.
Das klingt doch vielversprechend...
Laura6
Seattle Genetics Shares Continue To Impress - And It's Not Too Late To Buy
June 11, 2012 |Steven Breazzano
Background
Seattle Genetics (SGEN), based outside of Seattle, WA, focuses on the development and commercialization of antibody-drug-conjugates, or ADCs, for the treatment of cancer. These ADC therapies are designed to combine the strength of monoclonal antibodies (selective targeting) with the toxic, cell killing strength of a small molecule. By attaching the toxic compound monomethyl auristatin E (well, approximately 4 of these toxic molecules!) to an anti-CD30 antibody, SGEN brought Adcetris to market in the US for Hodgkin's Lymphoma (HL) and systemic anaplastic large cell lymphoma (sALCL), in the refractory setting. After some large price swings in 2011, trading has been relatively tame this year with the shares steadily rising from approximately $16/share (when I first wrote about the company) to $24/share, seemingly immune to the troubles plaguing the macroeconomic landscape. Seattle Genetics has been in the news recently with data releases and ASCO presentations, as well as routine quarterly conference calls.
Adcetris Sales, Balance Sheet, and Financials
SGEN received accelerated approval for Adcetris last year based on tremendous efficacy: 73% objective response rate (ORR) in HL and an 86% ORR in sALCL. The drug was partnered in late 2009 with Millennium, a division of Takeda Pharmaceuticals, and they will continue to pay for half of all development costs. Sales of the drug reached $10 MM in the third quarter of 2011; impressive for only 6 weeks on the market. In the fourth quarter of 2011, net sales of Adcetris reached $33.2 MM, and in the first quarter of 2012, net sales reached $34.5 MM. Also during the earnings release in Q1 2012, management felt comfortable giving guidance on Adcetris sales, expecting a total of $140-150 MM in sales during 2012, with an additional $55 to $65 MM in collaboration revenues.
Seattle Genetics had approximately $309 MM in cash and cash equivalents at the end of Q1 2012, a decrease of ~$21 MM from Q4 2012. The balance sheet is clean with no debt, but a significant amount of deferred revenue is listed under liabilities. As SGEN achieves milestones under its collaborations, this will be recognized as revenue in future periods and is no real cause for concern, as this is common for biotechs with partnership agreements. Management expects a total of $245 MM to $270 MM in expenses for 2012 ($30-33MM of non cash expenses, mostly share-based compensation), and 35% of this is expected to be SG&A. SGEN will not be cash-flow positive this year, but with a strong cash balance and increasing sales of Adcetris, they are well positioned to advance the partnered and wholly-owned therapeutics without raising additional capital.
One frequent criticism of Seattle Genetics is, "they spend too much." SGEN does indeed spend a lot. But theoretically speaking, shouldn't effective R&D expenditures be capitalized and not expensed? I understand that in practice, capitalizing most pharmaceutical R&D would lead to messy, and probably inflated and/or fraudulent balance sheets and income statements since most projects are not successful. But in SGEN's case, the programs are doing well and there is significant progress, so why not give them the benefit of the doubt?
Market Potential of Adcetris
SGEN's pipeline is extremely promising with new ADC's in clinical trials, but most of the attention is centered on Adcetris and its future sales. Adcetris is approved in the refractory setting, and SGEN is attempting to move up the ladder to front-line therapy for HL and sALCL. To achieve this goal, SGEN has initiated and supported approximately a dozen new clinical trials. Here some uncertainty arises. Adcetris is fantastically active in the refractory setting, but there is no guarantee it works in other CD30+ malignancies. So far though, the drug appears synergistic with chemotherapy, and hopefully becomes part of the new standard of care. SGEN is also screening non-lymphoma malignancies to see if other CD30+ malignancies may respond (ASCO abstract can be found here). SGEN's management believes that Adcetris may eventually become a blockbuster drug (traditionally defined as sales over $1 BB), and so far preliminary results demonstrate Adcetris' efficacy in these new indications, with manageable side effects. The fact that sales may reach $140-150 MM in its first 12 months combined with the numerous clinical trial initiations is indicative of both physician and payer acceptance for Adcetris, not to mention the truly beneficial effect Adcetris provides for patients.
Validation of the Platform
Although the accelerated approval last year and impressive early sales provide significant validation of the ADC platform, Celldex's (CLDX) recent results are also positive for SGEN. As a licensee of SGEN's technology, Celldex has been developing CDX-011 for breast cancer. In a subset of patients with triple negative breast cancer, a traditionally tough indication, who also express the antigen CDX-011 targets, results were highly statistically significant and impressive. Although the data is based on a small number of patients, I would imagine Celldex will be pursuing a larger trial in the near future for this particular niche indication. Don't expect much of this to flow to SGEN's bottom line though, as SGEN only receives "royalty payments in the mid-single digits on any net product sales," per CLDX's 10k.
Conclusions and Future Directions
While some uncertainty remains with the upcoming trials with Adcetris and treatments targeting solid tumors, let's not forget SGEN already has a treatment on the market that is selling well. SGEN has a large pipeline, and although valuation is a bit tricky with the sheer volume of partnerships (12 active ADC collaborations!), the depth of the clinical and preclinical pipeline is impressive.
Seattle Genetics is at the vanguard of the hot new field of ADCs, and with clinical validation and impressive sales, Seattle Genetics is a speculative stock that investors may wish to consider in their portfolios.
Disclosure: I am long SGEN.
Das klingt doch vielversprechend...
Laura6
Ganz aktuell schon wieder gute Nachrichten....
Seattle Genetics Announces ADCETRIS® Receives Positive CHMP Opinion for Conditional Approval in European Union
Fri July 20, 2012 10:45 AM | about: SGEN
NEWS PROVIDED BY:
Business Wire
Recommended as Treatment for Relapsed or Refractory Hodgkin Lymphoma and Systemic ALCL
BOTHELL, Wash.--(BUSINESS WIRE)-- Seattle Genetics, Inc. (Nasdaq:SGEN) today announced that its collaborator, Millennium: The Takeda Oncology Company , a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, has received a positive recommendation from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for the conditional marketing authorization of ADCETRIS (brentuximab vedotin) for two indications: (1) the treatment of adult patients with relapsed or refractory CD30-positive Hodgkin lymphoma (HL) following autologous stem cell transplant (ASCT) or following at least two prior therapies when ASCT or multi-agent chemotherapy is not a treatment option, and (2) for the treatment of adult patients with relapsed or refractory systemic anaplastic large cell lymphoma (sALCL). ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30.
The positive opinion from CHMP and broad label recommendation is a key step in the European regulatory process for ADCETRIS and brings us closer to our goal of making this important new therapy globally available to patients with relapsed Hodgkin lymphoma or systemic ALCL, said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics (SGEN). If approved in the European Union, ADCETRIS will represent the first new therapeutic advance for relapsed Hodgkin lymphoma patients in several decades and further validates the potential of ADCs in the treatment of cancer.
The European Commission, which has the authority to approve medicines for use in the European Union, generally follows the recommendations of the CHMP and typically renders a final decision within three months of the CHMP opinion. If the CHMP recommendation is formally adopted by the European Commission, ADCETRIS would be approved for marketing in all 27 member states of the European Union.
European Commission approval will trigger two milestone payments, one for each indication, totaling $25 million to Seattle Genetics under the collaboration agreement between Seattle Genetics and Millennium: The Takeda Oncology Company. Seattle Genetics is also entitled to tiered double-digit royalties with percentages starting in the mid-teens and escalating to the mid-twenties based on net sales of ADCETRIS within Millenniums territories, subject to offsets for royalties paid by Millennium to third parties.
About ADCETRIS
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
ADCETRIS received accelerated approval from the U.S. Food and Drug Administration (FDA) in August 2011 for relapsed HL and sALCL.
Seattle Genetics and Millennium are jointly developing ADCETRIS. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
About Seattle Genetics
Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer. The FDA granted accelerated approval of ADCETRIS in August 2011 for two indications. ADCETRIS is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has three other clinical-stage ADC programs: SGN-75, ASG-5ME and ASG-22ME. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
Certain of the statements made in this press release are forward looking, such as those, among others, relating to the potential for approval in the European Union and the milestone payment triggered upon approval by the European Commission. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include risks that data from the pivotal clinical trials will not support marketing approval for the submitted indications by the European Commission despite the positive opinion of CHMP. More information about the risks and uncertainties faced by Seattle Genetics is contained in the companys 10-Q for the quarter ended March 31, 2012 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Seattle Genetics, Inc.
Peggy Pinkston, 425-527-4160
ppinkston@seagen.com
Source: Seattle Genetics, Inc.
Copyright Business Wire 2012
Grüße an alle SGEN Fans.
Seattle Genetics Announces ADCETRIS® Receives Positive CHMP Opinion for Conditional Approval in European Union
Fri July 20, 2012 10:45 AM | about: SGEN
NEWS PROVIDED BY:
Business Wire
Recommended as Treatment for Relapsed or Refractory Hodgkin Lymphoma and Systemic ALCL
BOTHELL, Wash.--(BUSINESS WIRE)-- Seattle Genetics, Inc. (Nasdaq:SGEN) today announced that its collaborator, Millennium: The Takeda Oncology Company , a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, has received a positive recommendation from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for the conditional marketing authorization of ADCETRIS (brentuximab vedotin) for two indications: (1) the treatment of adult patients with relapsed or refractory CD30-positive Hodgkin lymphoma (HL) following autologous stem cell transplant (ASCT) or following at least two prior therapies when ASCT or multi-agent chemotherapy is not a treatment option, and (2) for the treatment of adult patients with relapsed or refractory systemic anaplastic large cell lymphoma (sALCL). ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30.
The positive opinion from CHMP and broad label recommendation is a key step in the European regulatory process for ADCETRIS and brings us closer to our goal of making this important new therapy globally available to patients with relapsed Hodgkin lymphoma or systemic ALCL, said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics (SGEN). If approved in the European Union, ADCETRIS will represent the first new therapeutic advance for relapsed Hodgkin lymphoma patients in several decades and further validates the potential of ADCs in the treatment of cancer.
The European Commission, which has the authority to approve medicines for use in the European Union, generally follows the recommendations of the CHMP and typically renders a final decision within three months of the CHMP opinion. If the CHMP recommendation is formally adopted by the European Commission, ADCETRIS would be approved for marketing in all 27 member states of the European Union.
European Commission approval will trigger two milestone payments, one for each indication, totaling $25 million to Seattle Genetics under the collaboration agreement between Seattle Genetics and Millennium: The Takeda Oncology Company. Seattle Genetics is also entitled to tiered double-digit royalties with percentages starting in the mid-teens and escalating to the mid-twenties based on net sales of ADCETRIS within Millenniums territories, subject to offsets for royalties paid by Millennium to third parties.
About ADCETRIS
ADCETRIS (brentuximab vedotin) is an ADC comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seattle Genetics proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-expressing tumor cells.
ADCETRIS received accelerated approval from the U.S. Food and Drug Administration (FDA) in August 2011 for relapsed HL and sALCL.
Seattle Genetics and Millennium are jointly developing ADCETRIS. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize ADCETRIS in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for ADCETRIS on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
About Seattle Genetics
Seattle Genetics is a biotechnology company focused on the development and commercialization of monoclonal antibody-based therapies for the treatment of cancer. The FDA granted accelerated approval of ADCETRIS in August 2011 for two indications. ADCETRIS is being developed in collaboration with Millennium: The Takeda Oncology Company. In addition, Seattle Genetics has three other clinical-stage ADC programs: SGN-75, ASG-5ME and ASG-22ME. Seattle Genetics has collaborations for its ADC technology with a number of leading biotechnology and pharmaceutical companies, including Abbott, Bayer, Celldex Therapeutics, Daiichi Sankyo, Genentech, GlaxoSmithKline, Millennium, Pfizer and Progenics, as well as ADC co-development agreements with Agensys, an affiliate of Astellas, and Genmab. More information can be found at www.seattlegenetics.com.
Certain of the statements made in this press release are forward looking, such as those, among others, relating to the potential for approval in the European Union and the milestone payment triggered upon approval by the European Commission. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include risks that data from the pivotal clinical trials will not support marketing approval for the submitted indications by the European Commission despite the positive opinion of CHMP. More information about the risks and uncertainties faced by Seattle Genetics is contained in the companys 10-Q for the quarter ended March 31, 2012 filed with the Securities and Exchange Commission. Seattle Genetics disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Seattle Genetics, Inc.
Peggy Pinkston, 425-527-4160
ppinkston@seagen.com
Source: Seattle Genetics, Inc.
Copyright Business Wire 2012
Grüße an alle SGEN Fans.
Hier mal ein aktueller Kommentar für alle die mit der Firma und den Produkten von Seattle Genetics noch nicht so vertraut sind ....
Armed Antibodies: The Latest Weapon In The War Against Cancer
July 23, 2012 | von Peter Geschek
ADC-s, otherwise known as Antibody-drug Conjugates or armed antibodies, are a new technology in the fight against cancer. After many years of trial and error, the technology now seems to work. And the companies making it work, like Seattle Genetics, Inc. (SGEN) or ImmunoGen, Inc. (IMGN), offer promising investment opportunities.
ADC-s consist of three parts: the cancer antibody, a small but super-strong chemotherapy charge, and the linker that attaches the chemo to the antibody.
It all sounds easy, but it took decades and several failed attempts before one drug was finally approved and the other is getting ready for approval.
ADC-s are like floating sea mines. They drift harmlessly in the blood until they bump into a cancer cell, invade it, and from inside they kill it. Less then 1% of the drug is estimated to reach the tumor, but that is sufficient to produce better results than ever before.
The basic part of the ADC is a monoclonal antibody, like Herceptin, Rituxan or Erbitux, which are the current staples of targeted cancer therapy, all financially and clinically highly successful.
These laboratory-produced molecules mimic the antibodies made by a person's immune system to fight infection. But instead of attacking pathogens, the antibodies attach to specific proteins on the surface of cancer cells.
The antibodies by themselves have a limited ability to kill tumors. So along with the antibodies, conventional cell-killing chemotherapy drugs are usually given to the patient. While these kill cancer cells, they also attack healthy cells and cause adverse side effects.
What is revolutionary about ADC technology is that the toxins attach to the antibody, giving it killing power. At the same time, the effect is limited to the cancer cells only.
Seattle Genetics' auristatins, as their chemo agents are called, are 100- to 1,000-fold more potent than traditional chemotherapy drugs. They have to be that strong, because of the very small volume used.
Normally chemos that strong could not be given to patients. The linker is the key to this technology.
The linkers have to be stable in the bloodstream and release the potent cytotoxic agent once inside the targeted cancer cells. This approach is meant to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy.
This is a tall order, and making the linker stable has taken considerable time and effort. In 2010, Pfizer Inc. (PFE) pulled an approved ADC called Mylotarg from the market, because it had difficulty staying intact before hitting cancer cells.
Adcetris: In August 2011, the FDA approved Seattle Genetics' ADC drug Adcetris for Hodgkin's lymphoma. Adcetris is intended for Hodgkin's lymphoma patients who cannot receive an autologous stem cell transplant or for those whose disease has progressed even after a transplant or two rounds of chemotherapy treatment.
The antibody used in Adcetris binds to a protein on malignant cells called CD30. It showed little clinical effect when tested alone. But when linked to a toxin, it shrank tumors in 73 percent of those with Hodgkin's lymphoma. The chemo used here is called MMAE and the linker is Valine-Citruline.
Both the linker and cell-killing agents are synthetic, therefore the ADC technology is readily scalable. This represents an improvement over natural product drug systems that are typically more challenging and expensive to produce.
T-DM1: Genentech, the South San Francisco-based biotech company, a unit of Roche Holding Ltd (RHHBY.PK), has run a trial called EMILIA to test its new drug T-DM1.
T-DM1 consists of Herceptin, Roche's wonder drug for many years for breast cancer, plus a chemo called DM1 and a linker called Thioether. The chemo and the linker are supplied by Immunogen. The results were reported in May 2012 at the ASCO meeting in Chicago.
In a trial of 991 patients, the new Genentech drug caused tumors to shrink in more patients, kept them in remission longer, and caused fewer significant side effects than the combo treatment of GlaxoSmithKline's (GSK) Tykerb and Xeloda chemotherapy.
65.4 percent of the T-DM1 patients were still alive after two years of follow-up, compared with 47.5 percent of patients on the combo. The findings were encouraging enough for Genentech to submit an application for FDA approval.
Dietmar Berger, Genentech's vice president of clinical oncology, stated: "All of it validates what we've seen before. The clear surprise was the extent of the 2-year survival difference, which I believe is outstanding."
Genentech had previously sought FDA approval for T-DM1 on the basis of smaller studies and was turned down because regulators wanted an opportunity to review a data package that included results from the Emilia study.
Like any drug, though, T-DM1 comes with side effects. About 12.9 percent of patients on the new Genentech treatment had moderate-to-severe depletion of their clot-forming platelet blood cells, compared with 0.2 percent in the control group.
On the other hand, the new drug caused fewer cases of diarrhea, hand/foot rashes, and vomiting than the other treatments.
Genentech is also conducting a pivotal study called Marianne, which is investigating whether a combo of T-DM1 and another new Genentech antibody called Perjeta could be even more effective in HER2-positive breast cancer patients receiving their first round of treatment.
Collaborations
Both Seattle Genetics and Immunogen have numerous collaborating agreements with major pharmas.
Immunogen has agreements with Eli Lilly and Company (LLY), Roche, Amgen Inc. (AMGN), Novartis AG (NVS), Bayer AG (BAYRY.PK), sanofi-aventis (SNY), and others.
Seattle Genetics has deals with Pfizer, Abbott Laboratories (ABT), Celldex Therapeutics, Inc. (CLDX), Genentech, Bayer, Astellas Pharma Inc. (ALPMY.PK), Daiichi Sankyo (DSKYF.PK), and Millenium Hldg Grp (MNHG).
This idea of antibody-drug conjugates is being pursued by a number of companies from small start-ups to pharma giants. Celtic Therapeutics, an investment firm, has recently committed $50 million to creating a new company, ADC Therapeutics, to develop antibody-drug conjugates. About 25 such drugs from a variety of companies are in clinical trials. Genentech alone has eight drugs besides T-DM1 in clinical trials, and another 17 in earlier stages of development.
Investors' Take
In this story, everybody is a winner. Thanks to the success of the trastuzumab emtansine ((T-DM1)) trials, and assuming FDA approval (which is very likely now), Roche/Genentech will solidify their position as a leading force in the breast cancer field.
The two companies providing ADC technology, Seattle Genetics and Immunogen, are clearly promising. Seattle Genetics's drug Adcetris has been approved for Hodgkin's lymphoma patients who failed on other therapies. For now, this market is narrow.
Hodgkin's lymphoma will be diagnosed in an estimated 9,060 U.S. patients in 2012 and about 1,190 people will die from it, according to the National Cancer Institute. But the company is running trials to extend the drug's indication to other lymphomas and first line treatments. When that happens, hopefully in a few years, Adcetris could become a billion-dollar megaseller, like Roche's Rituxan. Analysts project Adcetris to reach $437 million in sales in 2015, according to a Bloomberg survey.
Immunogen does not have an approved drug and consequently no income from drug sales. But its income from the various collaborative deals will grow 150 percent in the year ending June 2013, to $41.4 million, according to Thomson Financial Network. The company is also running trials of its own proprietary drugs, the ovarian cancer drug IMGN853 and a drug for non-Hodgkin's lymphoma, IMGN529. The company is also in the process of raising $100 million from a secondary stock offering.
Armed Antibodies: The Latest Weapon In The War Against Cancer
July 23, 2012 | von Peter Geschek
ADC-s, otherwise known as Antibody-drug Conjugates or armed antibodies, are a new technology in the fight against cancer. After many years of trial and error, the technology now seems to work. And the companies making it work, like Seattle Genetics, Inc. (SGEN) or ImmunoGen, Inc. (IMGN), offer promising investment opportunities.
ADC-s consist of three parts: the cancer antibody, a small but super-strong chemotherapy charge, and the linker that attaches the chemo to the antibody.
It all sounds easy, but it took decades and several failed attempts before one drug was finally approved and the other is getting ready for approval.
ADC-s are like floating sea mines. They drift harmlessly in the blood until they bump into a cancer cell, invade it, and from inside they kill it. Less then 1% of the drug is estimated to reach the tumor, but that is sufficient to produce better results than ever before.
The basic part of the ADC is a monoclonal antibody, like Herceptin, Rituxan or Erbitux, which are the current staples of targeted cancer therapy, all financially and clinically highly successful.
These laboratory-produced molecules mimic the antibodies made by a person's immune system to fight infection. But instead of attacking pathogens, the antibodies attach to specific proteins on the surface of cancer cells.
The antibodies by themselves have a limited ability to kill tumors. So along with the antibodies, conventional cell-killing chemotherapy drugs are usually given to the patient. While these kill cancer cells, they also attack healthy cells and cause adverse side effects.
What is revolutionary about ADC technology is that the toxins attach to the antibody, giving it killing power. At the same time, the effect is limited to the cancer cells only.
Seattle Genetics' auristatins, as their chemo agents are called, are 100- to 1,000-fold more potent than traditional chemotherapy drugs. They have to be that strong, because of the very small volume used.
Normally chemos that strong could not be given to patients. The linker is the key to this technology.
The linkers have to be stable in the bloodstream and release the potent cytotoxic agent once inside the targeted cancer cells. This approach is meant to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy.
This is a tall order, and making the linker stable has taken considerable time and effort. In 2010, Pfizer Inc. (PFE) pulled an approved ADC called Mylotarg from the market, because it had difficulty staying intact before hitting cancer cells.
Adcetris: In August 2011, the FDA approved Seattle Genetics' ADC drug Adcetris for Hodgkin's lymphoma. Adcetris is intended for Hodgkin's lymphoma patients who cannot receive an autologous stem cell transplant or for those whose disease has progressed even after a transplant or two rounds of chemotherapy treatment.
The antibody used in Adcetris binds to a protein on malignant cells called CD30. It showed little clinical effect when tested alone. But when linked to a toxin, it shrank tumors in 73 percent of those with Hodgkin's lymphoma. The chemo used here is called MMAE and the linker is Valine-Citruline.
Both the linker and cell-killing agents are synthetic, therefore the ADC technology is readily scalable. This represents an improvement over natural product drug systems that are typically more challenging and expensive to produce.
T-DM1: Genentech, the South San Francisco-based biotech company, a unit of Roche Holding Ltd (RHHBY.PK), has run a trial called EMILIA to test its new drug T-DM1.
T-DM1 consists of Herceptin, Roche's wonder drug for many years for breast cancer, plus a chemo called DM1 and a linker called Thioether. The chemo and the linker are supplied by Immunogen. The results were reported in May 2012 at the ASCO meeting in Chicago.
In a trial of 991 patients, the new Genentech drug caused tumors to shrink in more patients, kept them in remission longer, and caused fewer significant side effects than the combo treatment of GlaxoSmithKline's (GSK) Tykerb and Xeloda chemotherapy.
65.4 percent of the T-DM1 patients were still alive after two years of follow-up, compared with 47.5 percent of patients on the combo. The findings were encouraging enough for Genentech to submit an application for FDA approval.
Dietmar Berger, Genentech's vice president of clinical oncology, stated: "All of it validates what we've seen before. The clear surprise was the extent of the 2-year survival difference, which I believe is outstanding."
Genentech had previously sought FDA approval for T-DM1 on the basis of smaller studies and was turned down because regulators wanted an opportunity to review a data package that included results from the Emilia study.
Like any drug, though, T-DM1 comes with side effects. About 12.9 percent of patients on the new Genentech treatment had moderate-to-severe depletion of their clot-forming platelet blood cells, compared with 0.2 percent in the control group.
On the other hand, the new drug caused fewer cases of diarrhea, hand/foot rashes, and vomiting than the other treatments.
Genentech is also conducting a pivotal study called Marianne, which is investigating whether a combo of T-DM1 and another new Genentech antibody called Perjeta could be even more effective in HER2-positive breast cancer patients receiving their first round of treatment.
Collaborations
Both Seattle Genetics and Immunogen have numerous collaborating agreements with major pharmas.
Immunogen has agreements with Eli Lilly and Company (LLY), Roche, Amgen Inc. (AMGN), Novartis AG (NVS), Bayer AG (BAYRY.PK), sanofi-aventis (SNY), and others.
Seattle Genetics has deals with Pfizer, Abbott Laboratories (ABT), Celldex Therapeutics, Inc. (CLDX), Genentech, Bayer, Astellas Pharma Inc. (ALPMY.PK), Daiichi Sankyo (DSKYF.PK), and Millenium Hldg Grp (MNHG).
This idea of antibody-drug conjugates is being pursued by a number of companies from small start-ups to pharma giants. Celtic Therapeutics, an investment firm, has recently committed $50 million to creating a new company, ADC Therapeutics, to develop antibody-drug conjugates. About 25 such drugs from a variety of companies are in clinical trials. Genentech alone has eight drugs besides T-DM1 in clinical trials, and another 17 in earlier stages of development.
Investors' Take
In this story, everybody is a winner. Thanks to the success of the trastuzumab emtansine ((T-DM1)) trials, and assuming FDA approval (which is very likely now), Roche/Genentech will solidify their position as a leading force in the breast cancer field.
The two companies providing ADC technology, Seattle Genetics and Immunogen, are clearly promising. Seattle Genetics's drug Adcetris has been approved for Hodgkin's lymphoma patients who failed on other therapies. For now, this market is narrow.
Hodgkin's lymphoma will be diagnosed in an estimated 9,060 U.S. patients in 2012 and about 1,190 people will die from it, according to the National Cancer Institute. But the company is running trials to extend the drug's indication to other lymphomas and first line treatments. When that happens, hopefully in a few years, Adcetris could become a billion-dollar megaseller, like Roche's Rituxan. Analysts project Adcetris to reach $437 million in sales in 2015, according to a Bloomberg survey.
Immunogen does not have an approved drug and consequently no income from drug sales. But its income from the various collaborative deals will grow 150 percent in the year ending June 2013, to $41.4 million, according to Thomson Financial Network. The company is also running trials of its own proprietary drugs, the ovarian cancer drug IMGN853 and a drug for non-Hodgkin's lymphoma, IMGN529. The company is also in the process of raising $100 million from a secondary stock offering.
Antwort auf Beitrag Nr.: 43.417.574 von Laura6 am 24.07.12 12:30:22der Kurs ist nun schon seit mehreren Tagen rückläufig.
Mal schauen wo die Reise noch hingeht.

Mal schauen wo die Reise noch hingeht.

Hallo,

die Quartalszahlen sind nicht umwerfend, nachbörslich stand sogar ein dickes Minus vor dem Kurs, die Überschriften der Kommentare waren durchweg negativ.
Wohin die Reise geht?
Gestern offenbar erstmal kräftig nach oben!
Hier einmal die Zahlen in Kürze (ansonsten auf der Homepage www.seagen.com)
Bleibe weiter investiert.
Gruß q.
----------------------
..Seattle Genetics 2Q revenue misses estimates
Seattle Genetics says cancer drug sales held steady, revenue misses analysts' estimates
Associated Press – Wed, Aug 8, 2012 5:29 PM EDT....Email
Share0Print.....Companies:...Seattle Genetics Inc. . ..RELATED QUOTES.
.Symbol Price Change
SGEN 24.54
..........
BOTHELL, Wash. (AP) -- Seattle Genetics Inc. said Wednesday that sales of its lymphoma drug Adcetris totaled $34.7 million in the second quarter, up slightly from the first quarter. The results missed analysts' estimates.
Adcetris, Seattle Genetics' only marketed product, is used as a treatment for Hodgkin's lymphoma and systemic anaplastic large cell lymphoma. The Food and Drug Administration approved the drug in August 2011, and sales in the first quarter of 2012 totaled $34.5 million. The company is conducting additional clinical trials to broaden its marketing approval.
Seattle Genetics said it lost $17.2 million, or 15 cents per share, in the second quarter, an improvement from a loss of $51.5 million, or 45 cents per share, a year earlier. Revenue rose to $48.8 million from $13.1 million. In addition to Adcetris sales, Seattle Genetics reported $12.9 million from collaborations and $1.2 million in royalty revenue.
Analysts forecast a loss of 15 cents per share on $50.5 million in revenue, according to FactSet.
Shares of Seattle Genetics fell 46 cents to $23.87 Wednesday.
..

die Quartalszahlen sind nicht umwerfend, nachbörslich stand sogar ein dickes Minus vor dem Kurs, die Überschriften der Kommentare waren durchweg negativ.
Wohin die Reise geht?
Gestern offenbar erstmal kräftig nach oben!
Hier einmal die Zahlen in Kürze (ansonsten auf der Homepage www.seagen.com)
Bleibe weiter investiert.
Gruß q.
----------------------
..Seattle Genetics 2Q revenue misses estimates
Seattle Genetics says cancer drug sales held steady, revenue misses analysts' estimates
Associated Press – Wed, Aug 8, 2012 5:29 PM EDT....Email
Share0Print.....Companies:...Seattle Genetics Inc. . ..RELATED QUOTES.
.Symbol Price Change
SGEN 24.54
..........
BOTHELL, Wash. (AP) -- Seattle Genetics Inc. said Wednesday that sales of its lymphoma drug Adcetris totaled $34.7 million in the second quarter, up slightly from the first quarter. The results missed analysts' estimates.
Adcetris, Seattle Genetics' only marketed product, is used as a treatment for Hodgkin's lymphoma and systemic anaplastic large cell lymphoma. The Food and Drug Administration approved the drug in August 2011, and sales in the first quarter of 2012 totaled $34.5 million. The company is conducting additional clinical trials to broaden its marketing approval.
Seattle Genetics said it lost $17.2 million, or 15 cents per share, in the second quarter, an improvement from a loss of $51.5 million, or 45 cents per share, a year earlier. Revenue rose to $48.8 million from $13.1 million. In addition to Adcetris sales, Seattle Genetics reported $12.9 million from collaborations and $1.2 million in royalty revenue.
Analysts forecast a loss of 15 cents per share on $50.5 million in revenue, according to FactSet.
Shares of Seattle Genetics fell 46 cents to $23.87 Wednesday.
..
Hallo,
:;
mal wieder eine neue klinische Studie mit SGN-75, immerhin eine Ib, sollen 40 Teilnehmer werden. Immer gut solche News!
Gruß q.
----------------------------
..Seattle Genetics Announces Initiation of a Phase Ib Trial of SGN-75 in Combination with Everolimus for Patients with Renal Cell Carcinoma
Press Release: Seattle Genetics – 15 minutes ago....Email
Share0Print.....Companies:...Seattle Genetics Inc. . ..RELATED QUOTES.
.Symbol Price Change
SGEN 26.77
......
BOTHELL, Wash.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (SGEN) today announced the initiation of a phase Ib clinical trial evaluating SGN-75 in combination with everolimus (Afinitor®) for patients with advanced metastatic renal cell carcinoma (RCC). The trial is designed to assess the safety and antitumor activity of SGN-75 in combination with everolimus. Seattle Genetics is a leader in the field of antibody-drug conjugates (ADCs) and SGN-75 is an ADC targeted to CD70.
“ADCs have the potential to change the way many types of cancer are treated, and we are excited to evaluate our ADC product candidate, SGN-75, in this phase Ib trial for patients with CD70-expressing RCC,” said Jonathan Drachman , M.D., Senior Vice President, Research and Translational Medicine at Seattle Genetics. “We are encouraged by the preliminary single-agent activity and tolerability demonstrated by SGN-75 in RCC patients and by our preclinical data suggesting synergy between auristatin-containing ADCs and mTOR inhibitors, including everolimus. We look forward to investigating whether this combination can provide therapeutic benefit to patients who currently have limited treatment options.”
The study is a phase Ib, open-label, dose-escalation clinical trial to evaluate the safety and antitumor activity of SGN-75 in combination with everolimus, an mTOR inhibitor, in patients with CD70-positive metastatic RCC. Everolimus is an oral prescription medication used to treat advanced RCC when certain other medicines, such as sunitinib or sorafenib, have not worked. The trial is enrolling patients who have previously been treated with one or two tyrosine kinase inhibitors (TKIs). The primary endpoint of the trial is safety, with key secondary endpoints of best clinical response, progression-free survival (PFS) and overall survival (OS). The study is expected to enroll up to 40 patients at multiple centers in the United States.
“Despite the use of immunotherapy, tyrosine kinase inhibitors and mTOR inhibitors, many patients with kidney cancer ultimately experience progression of their disease,” said Elisabeth Heath, M.D., Associate Professor of Oncology at Barbara Ann Karmanos Cancer Institute and investigator for this phase Ib clinical trial. “Kidney cancer tends to resist treatments after it stops responding to initial therapy, clearly demonstrating a need to identify new treatment approaches, such as targeted therapies directed to novel targets and combination therapy.”
For more information about the trial, including enrolling centers, please visit www.clinicaltrials.gov.
:;
mal wieder eine neue klinische Studie mit SGN-75, immerhin eine Ib, sollen 40 Teilnehmer werden. Immer gut solche News!
Gruß q.
----------------------------
..Seattle Genetics Announces Initiation of a Phase Ib Trial of SGN-75 in Combination with Everolimus for Patients with Renal Cell Carcinoma
Press Release: Seattle Genetics – 15 minutes ago....Email
Share0Print.....Companies:...Seattle Genetics Inc. . ..RELATED QUOTES.
.Symbol Price Change
SGEN 26.77
......
BOTHELL, Wash.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (SGEN) today announced the initiation of a phase Ib clinical trial evaluating SGN-75 in combination with everolimus (Afinitor®) for patients with advanced metastatic renal cell carcinoma (RCC). The trial is designed to assess the safety and antitumor activity of SGN-75 in combination with everolimus. Seattle Genetics is a leader in the field of antibody-drug conjugates (ADCs) and SGN-75 is an ADC targeted to CD70.
“ADCs have the potential to change the way many types of cancer are treated, and we are excited to evaluate our ADC product candidate, SGN-75, in this phase Ib trial for patients with CD70-expressing RCC,” said Jonathan Drachman , M.D., Senior Vice President, Research and Translational Medicine at Seattle Genetics. “We are encouraged by the preliminary single-agent activity and tolerability demonstrated by SGN-75 in RCC patients and by our preclinical data suggesting synergy between auristatin-containing ADCs and mTOR inhibitors, including everolimus. We look forward to investigating whether this combination can provide therapeutic benefit to patients who currently have limited treatment options.”
The study is a phase Ib, open-label, dose-escalation clinical trial to evaluate the safety and antitumor activity of SGN-75 in combination with everolimus, an mTOR inhibitor, in patients with CD70-positive metastatic RCC. Everolimus is an oral prescription medication used to treat advanced RCC when certain other medicines, such as sunitinib or sorafenib, have not worked. The trial is enrolling patients who have previously been treated with one or two tyrosine kinase inhibitors (TKIs). The primary endpoint of the trial is safety, with key secondary endpoints of best clinical response, progression-free survival (PFS) and overall survival (OS). The study is expected to enroll up to 40 patients at multiple centers in the United States.
“Despite the use of immunotherapy, tyrosine kinase inhibitors and mTOR inhibitors, many patients with kidney cancer ultimately experience progression of their disease,” said Elisabeth Heath, M.D., Associate Professor of Oncology at Barbara Ann Karmanos Cancer Institute and investigator for this phase Ib clinical trial. “Kidney cancer tends to resist treatments after it stops responding to initial therapy, clearly demonstrating a need to identify new treatment approaches, such as targeted therapies directed to novel targets and combination therapy.”
For more information about the trial, including enrolling centers, please visit www.clinicaltrials.gov.
Hier etwas über den Antikörper und die Krankheit gegen die er nun erstmals eingesetzt wird um Wirksamkeit und Verträglichkeit zu testen.
About SGN-75
SGN-75 is an ADC composed of an anti-CD70 antibody attached to a synthetic cytoxic cell-killing agent, monomethyl auristatin F (MMAF), using Seattle Genetics proprietary technology. The ADC is designed to be stable in the bloodstream, and to release its cytoxic agent upon internalization into CD70-expressing tumor cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy while enhancing the antitumor activity.
About Renal Cell Carcinoma
Renal cell carcinoma (RCC) forms in the kidney, which filters and cleans the blood. Metastatic RCC occurs when the cancer has spread to other parts of the body. RCC is the most common type of kidney cancer in adults, representing approximately 90 percent of cases. The American Cancer Society estimates that nearly 65,000 new cases of kidney cancer will be diagnosed in the United States during 2012, and approximately 13,600 people will die from the disease.
Grüße an alle Leser
Laura6
About SGN-75
SGN-75 is an ADC composed of an anti-CD70 antibody attached to a synthetic cytoxic cell-killing agent, monomethyl auristatin F (MMAF), using Seattle Genetics proprietary technology. The ADC is designed to be stable in the bloodstream, and to release its cytoxic agent upon internalization into CD70-expressing tumor cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy while enhancing the antitumor activity.
About Renal Cell Carcinoma
Renal cell carcinoma (RCC) forms in the kidney, which filters and cleans the blood. Metastatic RCC occurs when the cancer has spread to other parts of the body. RCC is the most common type of kidney cancer in adults, representing approximately 90 percent of cases. The American Cancer Society estimates that nearly 65,000 new cases of kidney cancer will be diagnosed in the United States during 2012, and approximately 13,600 people will die from the disease.
Grüße an alle Leser
Laura6
Auch wenn die Wahrscheinlichkeit nicht groß ist, eine Antwort zu bekommen: Weiß zufällig jemand, welches Target das Immunkonjugat RG7598 von Roche/Genentech/Seattle Genetics ansteuert?
Hallo,
habe die Frage schon im MOR-Thread gelesen. Kann dazu leider auch nichts finden.
Die Wahrscheinlichkeit einer Antwort war hoch, aber für dich nicht die gewünschte Antwort.
Gegenfrage: Weiß jemand, warum SGEN diese Tage so stark steigt?
Shorteindeckungen finde ich als Antwort zu einfach.
Gruß q.
habe die Frage schon im MOR-Thread gelesen. Kann dazu leider auch nichts finden.
Die Wahrscheinlichkeit einer Antwort war hoch, aber für dich nicht die gewünschte Antwort.
Gegenfrage: Weiß jemand, warum SGEN diese Tage so stark steigt?
Shorteindeckungen finde ich als Antwort zu einfach.
Gruß q.
Hallo,
ja, das war vielleicht wiklich mal der Grund für den Anstieg. Wäre ja auch schön, wenn wirklich mal die wissenschaftlichen Details gewürdigt werden und nicht irgendwelche dusseligen Analystenkommentare.
Roche hat zwar T-DM1 in Phase III, aber danach kommen eine Reihe von SGEN-ADC´s.
Roche sieht wohl dort die Zukunft.
Gruß q.
Für Interessierte dazu ein Link:
http://seekingalpha.com/article/856431-seattle-genetics-posi…
ja, das war vielleicht wiklich mal der Grund für den Anstieg. Wäre ja auch schön, wenn wirklich mal die wissenschaftlichen Details gewürdigt werden und nicht irgendwelche dusseligen Analystenkommentare.
Roche hat zwar T-DM1 in Phase III, aber danach kommen eine Reihe von SGEN-ADC´s.
Roche sieht wohl dort die Zukunft.
Gruß q.
Für Interessierte dazu ein Link:
http://seekingalpha.com/article/856431-seattle-genetics-posi…
Hallo,

es gibt wieder eine PII mit Adcetris, diesmal mit Ziel Front-line Therapie für HL. War zu erwarten, dass sowas kommt, um die Anwendungsbreite zu erhöhen.
Erklärt wieder mal nicht den plötzlichen Anstieg gestern, aber soll mir ja recht sein!!

Gruß q.
---------------------
..Seattle Genetics Announces Initiation of Phase II Trial of ADCETRIS® as Front-line Therapy for Hodgkin Lymphoma Patients Age 60 and Over
Press Release: Seattle Genetics, Inc. – 5 hours ago....Email
Share0Print.....Companies:...Seattle Genetics Inc. . ..RELATED QUOTES.
.Symbol Price Change
SGEN 27.21 +0.27
......
BOTHELL, Wash.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (SGEN) today announced the initiation of a phase II clinical trial evaluating ADCETRIS (brentuximab vedotin) as a front-line therapy for patients age 60 or older with newly diagnosed Hodgkin lymphoma (HL). The trial is designed to assess the efficacy and tolerability of ADCETRIS as a monotherapy for older HL patients who have received no prior treatment. Seattle Genetics is the leader in the field of antibody-drug conjugates (ADCs) and ADCETRIS is an ADC directed to CD30 for relapsed HL and systemic anaplastic large cell lymphoma (sALCL).
“The current standard of care for the treatment of front-line HL is a combination of multiple chemotherapeutic agents and has not changed in more than three decades. Some older HL patients are not able to tolerate the significant side effects associated with these regimens, and there is a significant need to identify effective and tolerable treatment options for these patients,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer at Seattle Genetics. “We believe the response rate associated with single-agent use of ADCETRIS in the relapsed HL setting supports the evaluation of single-agent ADCETRIS in older patients who have received no prior therapy.”
The phase II single-arm, open-label clinical trial will evaluate the efficacy and tolerability of ADCETRIS as front-line monotherapy in patients age 60 or older with HL. The trial is enrolling patients who are newly diagnosed and have received no prior HL treatment. The primary endpoint of the trial is to assess the objective response rate (ORR), with key secondary endpoints of safety and tolerability, duration of response, complete remission (CR) rate and progression-free survival (PFS). The study is expected to enroll up to 20 patients at multiple centers in the United States.
More information about the trial, including enrolling centers, will be available by visiting www.clinicaltrials.gov.

es gibt wieder eine PII mit Adcetris, diesmal mit Ziel Front-line Therapie für HL. War zu erwarten, dass sowas kommt, um die Anwendungsbreite zu erhöhen.
Erklärt wieder mal nicht den plötzlichen Anstieg gestern, aber soll mir ja recht sein!!

Gruß q.
---------------------
..Seattle Genetics Announces Initiation of Phase II Trial of ADCETRIS® as Front-line Therapy for Hodgkin Lymphoma Patients Age 60 and Over
Press Release: Seattle Genetics, Inc. – 5 hours ago....Email
Share0Print.....Companies:...Seattle Genetics Inc. . ..RELATED QUOTES.
.Symbol Price Change
SGEN 27.21 +0.27
......
BOTHELL, Wash.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (SGEN) today announced the initiation of a phase II clinical trial evaluating ADCETRIS (brentuximab vedotin) as a front-line therapy for patients age 60 or older with newly diagnosed Hodgkin lymphoma (HL). The trial is designed to assess the efficacy and tolerability of ADCETRIS as a monotherapy for older HL patients who have received no prior treatment. Seattle Genetics is the leader in the field of antibody-drug conjugates (ADCs) and ADCETRIS is an ADC directed to CD30 for relapsed HL and systemic anaplastic large cell lymphoma (sALCL).
“The current standard of care for the treatment of front-line HL is a combination of multiple chemotherapeutic agents and has not changed in more than three decades. Some older HL patients are not able to tolerate the significant side effects associated with these regimens, and there is a significant need to identify effective and tolerable treatment options for these patients,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer at Seattle Genetics. “We believe the response rate associated with single-agent use of ADCETRIS in the relapsed HL setting supports the evaluation of single-agent ADCETRIS in older patients who have received no prior therapy.”
The phase II single-arm, open-label clinical trial will evaluate the efficacy and tolerability of ADCETRIS as front-line monotherapy in patients age 60 or older with HL. The trial is enrolling patients who are newly diagnosed and have received no prior HL treatment. The primary endpoint of the trial is to assess the objective response rate (ORR), with key secondary endpoints of safety and tolerability, duration of response, complete remission (CR) rate and progression-free survival (PFS). The study is expected to enroll up to 20 patients at multiple centers in the United States.
More information about the trial, including enrolling centers, will be available by visiting www.clinicaltrials.gov.
Zitat von quepos: Hallo,
es gibt wieder eine PII mit Adcetris, diesmal mit Ziel Front-line Therapie für HL. War zu erwarten, dass sowas kommt, um die Anwendungsbreite zu erhöhen.
Erklärt wieder mal nicht den plötzlichen Anstieg gestern, aber soll mir ja recht sein!!
Gestern war Roche´s Q3-Konferenz! Die Analystengemeinde wacht doch bekanntlich zu den Konferenzen auf (wobei die Wachheit unterschiedlich lange andauert). Im Grunde genommen ein alberner Vorgang, der nicht gerade für vorausschauendes Denken der Beteiligten spricht.
Hallo,

erneuter Deal mit Abbott:
25 Mio. $ Upfront, im Falle des Falles bis 200 Mio. Milestones...
hört sich nach $$ an!
Gruß q.
----------------------------
..Seattle Genetics Expands Antibody-Drug Conjugate Collaboration with Abbott
Press Release: Seattle Genetics, Inc. – 22 hours ago....Email
Share0Print.....RELATED QUOTES.
.Symbol Price Change
SGEN 25.70
......
BOTHELL, Wash.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (SGEN) today announced that it has expanded its antibody-drug conjugate (ADC) collaboration with Abbott (ABT). Under the expanded deal, Abbott will pay an upfront fee of $25 million for rights to utilize Seattle Genetics’ auristatin-based ADC technology with antibodies to additional oncology targets. In addition, Seattle Genetics may receive up to $220 million in potential milestone payments per additional target upon achieving predetermined development and commercial objectives, as well as mid-to-high single-digit royalties on worldwide net sales of any resulting products under the multi-target collaboration.
“ADCs have emerged as an important therapeutic approach to cancer, driven by the FDA approval of ADCETRIS®, and encouraging data from numerous clinical and preclinical ADC programs in development by Seattle Genetics and our collaborators,” said Natasha Hernday, Vice President, Corporate Development at Seattle Genetics. “We are leading the field in ADC development, and this expanded collaboration with Abbott further validates our technology and approach in targeting and treating cancer.”
Seattle Genetics and Abbott originally entered into an ADC collaboration in March 2011 under which Abbott paid an upfront fee of $8 million for rights to utilize Seattle Genetics’ ADC technology with antibodies to a single oncology target. Abbott is responsible for research, product development, manufacturing and commercialization of any ADC products under the expanded collaboration. In addition to the upfront payment and potential milestone payments and royalties, Seattle Genetics will receive annual maintenance fees and research support payments for assistance provided to Abbott under the collaboration.
ADCs are monoclonal antibodies that are designed to selectively deliver cytotoxic agents to tumor cells. With over a decade of experience and knowledge in ADC innovation, Seattle Genetics has developed proprietary technology employing synthetic cytotoxic agents, such as monomethyl auristatin E (MMAE) and monomethyl auristatin F (MMAF), and stable linker systems that attach these cytotoxic agents to the antibody. Seattle Genetics’ linker systems are designed to be stable in the bloodstream and release the potent cell-killing agent once inside targeted cancer cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy while enhancing antitumor activity. ADCETRIS (brentuximab vedotin) is the first drug approved utilizing Seattle Genetics’ ADC technology.

erneuter Deal mit Abbott:
25 Mio. $ Upfront, im Falle des Falles bis 200 Mio. Milestones...
hört sich nach $$ an!
Gruß q.
----------------------------
..Seattle Genetics Expands Antibody-Drug Conjugate Collaboration with Abbott
Press Release: Seattle Genetics, Inc. – 22 hours ago....Email
Share0Print.....RELATED QUOTES.
.Symbol Price Change
SGEN 25.70
......
BOTHELL, Wash.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (SGEN) today announced that it has expanded its antibody-drug conjugate (ADC) collaboration with Abbott (ABT). Under the expanded deal, Abbott will pay an upfront fee of $25 million for rights to utilize Seattle Genetics’ auristatin-based ADC technology with antibodies to additional oncology targets. In addition, Seattle Genetics may receive up to $220 million in potential milestone payments per additional target upon achieving predetermined development and commercial objectives, as well as mid-to-high single-digit royalties on worldwide net sales of any resulting products under the multi-target collaboration.
“ADCs have emerged as an important therapeutic approach to cancer, driven by the FDA approval of ADCETRIS®, and encouraging data from numerous clinical and preclinical ADC programs in development by Seattle Genetics and our collaborators,” said Natasha Hernday, Vice President, Corporate Development at Seattle Genetics. “We are leading the field in ADC development, and this expanded collaboration with Abbott further validates our technology and approach in targeting and treating cancer.”
Seattle Genetics and Abbott originally entered into an ADC collaboration in March 2011 under which Abbott paid an upfront fee of $8 million for rights to utilize Seattle Genetics’ ADC technology with antibodies to a single oncology target. Abbott is responsible for research, product development, manufacturing and commercialization of any ADC products under the expanded collaboration. In addition to the upfront payment and potential milestone payments and royalties, Seattle Genetics will receive annual maintenance fees and research support payments for assistance provided to Abbott under the collaboration.
ADCs are monoclonal antibodies that are designed to selectively deliver cytotoxic agents to tumor cells. With over a decade of experience and knowledge in ADC innovation, Seattle Genetics has developed proprietary technology employing synthetic cytotoxic agents, such as monomethyl auristatin E (MMAE) and monomethyl auristatin F (MMAF), and stable linker systems that attach these cytotoxic agents to the antibody. Seattle Genetics’ linker systems are designed to be stable in the bloodstream and release the potent cell-killing agent once inside targeted cancer cells. This approach is intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy while enhancing antitumor activity. ADCETRIS (brentuximab vedotin) is the first drug approved utilizing Seattle Genetics’ ADC technology.
Hallo,

schon länger erwartet ist es nun endlich soweit, dass Adcetris die EU-Zulassung erhält (inkl. 25 Mio Milestone). Sollte kursfördernd sein, auch wenn´s wohl zum Teil schon eingepreist ist.
Gruß q.
---------------------------------
..Seattle Genetics Announces ADCETRIS® Receives European Commission Conditional Marketing Authorization
Conditional Marketing Authorization Triggers Milestone Payments to Seattle Genetics Totaling $25 Million
Press Release: Seattle Genetics, Inc. – 24 minutes ago....Email
Share0Print.....RELATED QUOTES.
.Symbol Price Change
SGEN 25.39
......
BOTHELL, Wash.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (SGEN) today announced that its collaborator, Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, announced that the European Commission has granted conditional marketing authorization for ADCETRIS (brentuximab vedotin). ADCETRIS was approved for two indications: (1) the treatment of adult patients with relapsed or refractory CD30-positive Hodgkin lymphoma (HL) following autologous stem cell transplant (ASCT) or following at least two prior therapies when ASCT or multi-agent chemotherapy is not a treatment option, and (2) for the treatment of adult patients with relapsed or refractory systemic anaplastic large cell lymphoma (sALCL). As a result, under the collaboration Seattle Genetics will receive two milestone payments from Millennium, one for each indication, totaling $25 million. ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30.
“The approval of ADCETRIS by the European Commission marks a significant milestone for the product and for the many relapsed or refractory HL and systemic ALCL patients in need of effective new treatment options in Europe,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “In addition to the U.S. and EU approvals of ADCETRIS, we are making regulatory progress for approval in Canada while Millennium and Takeda are pursuing regulatory approvals in other countries. Complementing these regulatory activities is a robust ADCETRIS clinical development program to support our goal of establishing it as the foundation of therapy for CD30-positive malignancies.”
The conditional marketing authorization for ADCETRIS is valid in the 27 member states of the European Union (EU) as well as Norway, Liechtenstein and Iceland. Similar to accelerated approval regulations in the United States, conditional marketing authorizations are granted in the EU to medicinal products that fulfill an unmet medical need with a positive benefit/risk assessment and whose immediate availability would result in a significant public health benefit. Conditional marketing authorization by the European Commission includes obligations to provide additional clinical data at a later stage to confirm the positive benefit-risk assessment. The ADCETRIS Marketing Authorization Application was filed by Takeda Global Research & Development Centre (Europe) to the European Medicines Agency.

schon länger erwartet ist es nun endlich soweit, dass Adcetris die EU-Zulassung erhält (inkl. 25 Mio Milestone). Sollte kursfördernd sein, auch wenn´s wohl zum Teil schon eingepreist ist.
Gruß q.
---------------------------------
..Seattle Genetics Announces ADCETRIS® Receives European Commission Conditional Marketing Authorization
Conditional Marketing Authorization Triggers Milestone Payments to Seattle Genetics Totaling $25 Million
Press Release: Seattle Genetics, Inc. – 24 minutes ago....Email
Share0Print.....RELATED QUOTES.
.Symbol Price Change
SGEN 25.39
......
BOTHELL, Wash.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (SGEN) today announced that its collaborator, Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, announced that the European Commission has granted conditional marketing authorization for ADCETRIS (brentuximab vedotin). ADCETRIS was approved for two indications: (1) the treatment of adult patients with relapsed or refractory CD30-positive Hodgkin lymphoma (HL) following autologous stem cell transplant (ASCT) or following at least two prior therapies when ASCT or multi-agent chemotherapy is not a treatment option, and (2) for the treatment of adult patients with relapsed or refractory systemic anaplastic large cell lymphoma (sALCL). As a result, under the collaboration Seattle Genetics will receive two milestone payments from Millennium, one for each indication, totaling $25 million. ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30.
“The approval of ADCETRIS by the European Commission marks a significant milestone for the product and for the many relapsed or refractory HL and systemic ALCL patients in need of effective new treatment options in Europe,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “In addition to the U.S. and EU approvals of ADCETRIS, we are making regulatory progress for approval in Canada while Millennium and Takeda are pursuing regulatory approvals in other countries. Complementing these regulatory activities is a robust ADCETRIS clinical development program to support our goal of establishing it as the foundation of therapy for CD30-positive malignancies.”
The conditional marketing authorization for ADCETRIS is valid in the 27 member states of the European Union (EU) as well as Norway, Liechtenstein and Iceland. Similar to accelerated approval regulations in the United States, conditional marketing authorizations are granted in the EU to medicinal products that fulfill an unmet medical need with a positive benefit/risk assessment and whose immediate availability would result in a significant public health benefit. Conditional marketing authorization by the European Commission includes obligations to provide additional clinical data at a later stage to confirm the positive benefit-risk assessment. The ADCETRIS Marketing Authorization Application was filed by Takeda Global Research & Development Centre (Europe) to the European Medicines Agency.
Hallo,

nun geht´s Schlag auf Schlag:
Frontline-PIII mit Takeda für Adcetris wird angeschoben.
Gruß q.
-----------------
..Millennium and Seattle Genetics Initiate Global Phase 3 Clinical Trial of ADCETRIS® in Previously Untreated Advanced Hodgkin Lymphoma
− First randomized clinical trial designed to examine the use of ADCETRIS as part of a frontline treatment regimen for patients with previously untreated classical Hodgkin lymphoma −
Press Release: Millennium: The Takeda Oncology Company and Seattle Genetics, Inc. – 1 hour 24 minutes ago....Email
Share0Print.....Related Content.
.
.
View Photo.Millennium and Seattle Genetics Initiate Global Phase 3 Clinical Trial of ADCETRIS® in Previously Untreated Advanced Hodgkin Lymphoma
....RELATED QUOTES.
.Symbol Price Change
SGEN 25.16
......
CAMBRIDGE, Mass. & BOTHELL, Wash.--(BUSINESS WIRE)--
Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited (TSE:4502), and Seattle Genetics, Inc. (SGEN) today announced the initiation of an international phase 3 clinical trial evaluating ADCETRIS (brentuximab vedotin) as part of a frontline combination chemotherapy regimen in patients with previously untreated advanced Hodgkin lymphoma (HL). The trial is being conducted under a Special Protocol Assessment (SPA) agreement from the U.S. Food and Drug Administration (FDA) and the trial also received scientific advice from the European Medicines Agency (EMA). ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of classical HL.
“Millennium is pleased to announce the initiation of the phase 3 trial of ADCETRIS in patients with previously untreated advanced Hodgkin lymphoma. This is a key step in our efforts to explore the potential of this targeted therapy as part of a frontline treatment regimen,” said Karen Ferrante, MD, Chief Medical Officer, Millennium. “The trial is part of our ongoing development program to explore patient populations that may benefit from treatment with ADCETRIS in earlier lines of therapy and in other CD30-expressing malignancies.”
“There have been no new therapies approved for patients with newly diagnosed HL in many decades, representing a significant need to identify additional treatment options in this setting,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer, Seattle Genetics. “We believe through this novel ADCETRIS-containing regimen we have the potential to redefine the treatment of frontline HL. This trial is also an important part of our development plan for ADCETRIS, and may serve as confirmatory to our U.S. accelerated approval in relapsed HL and systemic anaplastic large cell lymphoma.”
Study Design
The randomized, open-label, phase 3 trial will investigate ADCETRIS+AVD1 versus ABVD2 as frontline therapy in patients with advanced classical HL. The primary endpoint is modified progression free survival (mPFS) per independent review facility assessment using the Revised Response Criteria for malignant lymphoma. Secondary endpoints include overall survival (OS), complete remission (CR) and safety. The multi-center trial will be conducted in North America, Europe, Latin America and Asia. The study will enroll approximately 1,040 eligible patients (approximately 520 patients per treatment arm) who have histologically-confirmed diagnosis of Stage III or IV classical HL who have not been previously treated with systemic chemotherapy or radiotherapy.
For more information, please visit www.clinicaltrials.gov.

nun geht´s Schlag auf Schlag:
Frontline-PIII mit Takeda für Adcetris wird angeschoben.
Gruß q.
-----------------
..Millennium and Seattle Genetics Initiate Global Phase 3 Clinical Trial of ADCETRIS® in Previously Untreated Advanced Hodgkin Lymphoma
− First randomized clinical trial designed to examine the use of ADCETRIS as part of a frontline treatment regimen for patients with previously untreated classical Hodgkin lymphoma −
Press Release: Millennium: The Takeda Oncology Company and Seattle Genetics, Inc. – 1 hour 24 minutes ago....Email
Share0Print.....Related Content.
.
.
View Photo.Millennium and Seattle Genetics Initiate Global Phase 3 Clinical Trial of ADCETRIS® in Previously Untreated Advanced Hodgkin Lymphoma
....RELATED QUOTES.
.Symbol Price Change
SGEN 25.16
......
CAMBRIDGE, Mass. & BOTHELL, Wash.--(BUSINESS WIRE)--
Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited (TSE:4502), and Seattle Genetics, Inc. (SGEN) today announced the initiation of an international phase 3 clinical trial evaluating ADCETRIS (brentuximab vedotin) as part of a frontline combination chemotherapy regimen in patients with previously untreated advanced Hodgkin lymphoma (HL). The trial is being conducted under a Special Protocol Assessment (SPA) agreement from the U.S. Food and Drug Administration (FDA) and the trial also received scientific advice from the European Medicines Agency (EMA). ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of classical HL.
“Millennium is pleased to announce the initiation of the phase 3 trial of ADCETRIS in patients with previously untreated advanced Hodgkin lymphoma. This is a key step in our efforts to explore the potential of this targeted therapy as part of a frontline treatment regimen,” said Karen Ferrante, MD, Chief Medical Officer, Millennium. “The trial is part of our ongoing development program to explore patient populations that may benefit from treatment with ADCETRIS in earlier lines of therapy and in other CD30-expressing malignancies.”
“There have been no new therapies approved for patients with newly diagnosed HL in many decades, representing a significant need to identify additional treatment options in this setting,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer, Seattle Genetics. “We believe through this novel ADCETRIS-containing regimen we have the potential to redefine the treatment of frontline HL. This trial is also an important part of our development plan for ADCETRIS, and may serve as confirmatory to our U.S. accelerated approval in relapsed HL and systemic anaplastic large cell lymphoma.”
Study Design
The randomized, open-label, phase 3 trial will investigate ADCETRIS+AVD1 versus ABVD2 as frontline therapy in patients with advanced classical HL. The primary endpoint is modified progression free survival (mPFS) per independent review facility assessment using the Revised Response Criteria for malignant lymphoma. Secondary endpoints include overall survival (OS), complete remission (CR) and safety. The multi-center trial will be conducted in North America, Europe, Latin America and Asia. The study will enroll approximately 1,040 eligible patients (approximately 520 patients per treatment arm) who have histologically-confirmed diagnosis of Stage III or IV classical HL who have not been previously treated with systemic chemotherapy or radiotherapy.
For more information, please visit www.clinicaltrials.gov.
Hallo,
nun nochmal (hoffentlich nicht doppelt):
Geht jetzt Schlag auf Schlag:
Front-Line-PIII mit Takeda für Adcetris ist auf dem Weg.
Gruß q.
--------------
..Millennium and Seattle Genetics Initiate Global Phase 3 Clinical Trial of ADCETRIS® in Previously Untreated Advanced Hodgkin Lymphoma
− First randomized clinical trial designed to examine the use of ADCETRIS as part of a frontline treatment regimen for patients with previously untreated classical Hodgkin lymphoma −
Press Release: Millennium: The Takeda Oncology Company and Seattle Genetics, Inc. – 1 hour 24 minutes ago....Email
Share0Print.....Related Content.
.
.
View Photo.Millennium and Seattle Genetics Initiate Global Phase 3 Clinical Trial of ADCETRIS® in Previously Untreated Advanced Hodgkin Lymphoma
....RELATED QUOTES.
.Symbol Price Change
SGEN 25.16
......
CAMBRIDGE, Mass. & BOTHELL, Wash.--(BUSINESS WIRE)--
Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited (TSE:4502), and Seattle Genetics, Inc. (SGEN) today announced the initiation of an international phase 3 clinical trial evaluating ADCETRIS (brentuximab vedotin) as part of a frontline combination chemotherapy regimen in patients with previously untreated advanced Hodgkin lymphoma (HL). The trial is being conducted under a Special Protocol Assessment (SPA) agreement from the U.S. Food and Drug Administration (FDA) and the trial also received scientific advice from the European Medicines Agency (EMA). ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of classical HL.
“Millennium is pleased to announce the initiation of the phase 3 trial of ADCETRIS in patients with previously untreated advanced Hodgkin lymphoma. This is a key step in our efforts to explore the potential of this targeted therapy as part of a frontline treatment regimen,” said Karen Ferrante, MD, Chief Medical Officer, Millennium. “The trial is part of our ongoing development program to explore patient populations that may benefit from treatment with ADCETRIS in earlier lines of therapy and in other CD30-expressing malignancies.”
“There have been no new therapies approved for patients with newly diagnosed HL in many decades, representing a significant need to identify additional treatment options in this setting,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer, Seattle Genetics. “We believe through this novel ADCETRIS-containing regimen we have the potential to redefine the treatment of frontline HL. This trial is also an important part of our development plan for ADCETRIS, and may serve as confirmatory to our U.S. accelerated approval in relapsed HL and systemic anaplastic large cell lymphoma.”
Study Design
The randomized, open-label, phase 3 trial will investigate ADCETRIS+AVD1 versus ABVD2 as frontline therapy in patients with advanced classical HL. The primary endpoint is modified progression free survival (mPFS) per independent review facility assessment using the Revised Response Criteria for malignant lymphoma. Secondary endpoints include overall survival (OS), complete remission (CR) and safety. The multi-center trial will be conducted in North America, Europe, Latin America and Asia. The study will enroll approximately 1,040 eligible patients (approximately 520 patients per treatment arm) who have histologically-confirmed diagnosis of Stage III or IV classical HL who have not been previously treated with systemic chemotherapy or radiotherapy.
For more information, please visit www.clinicaltrials.gov.
nun nochmal (hoffentlich nicht doppelt):
Geht jetzt Schlag auf Schlag:
Front-Line-PIII mit Takeda für Adcetris ist auf dem Weg.
Gruß q.
--------------
..Millennium and Seattle Genetics Initiate Global Phase 3 Clinical Trial of ADCETRIS® in Previously Untreated Advanced Hodgkin Lymphoma
− First randomized clinical trial designed to examine the use of ADCETRIS as part of a frontline treatment regimen for patients with previously untreated classical Hodgkin lymphoma −
Press Release: Millennium: The Takeda Oncology Company and Seattle Genetics, Inc. – 1 hour 24 minutes ago....Email
Share0Print.....Related Content.
.
.
View Photo.Millennium and Seattle Genetics Initiate Global Phase 3 Clinical Trial of ADCETRIS® in Previously Untreated Advanced Hodgkin Lymphoma
....RELATED QUOTES.
.Symbol Price Change
SGEN 25.16
......
CAMBRIDGE, Mass. & BOTHELL, Wash.--(BUSINESS WIRE)--
Millennium: The Takeda Oncology Company, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited (TSE:4502), and Seattle Genetics, Inc. (SGEN) today announced the initiation of an international phase 3 clinical trial evaluating ADCETRIS (brentuximab vedotin) as part of a frontline combination chemotherapy regimen in patients with previously untreated advanced Hodgkin lymphoma (HL). The trial is being conducted under a Special Protocol Assessment (SPA) agreement from the U.S. Food and Drug Administration (FDA) and the trial also received scientific advice from the European Medicines Agency (EMA). ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of classical HL.
“Millennium is pleased to announce the initiation of the phase 3 trial of ADCETRIS in patients with previously untreated advanced Hodgkin lymphoma. This is a key step in our efforts to explore the potential of this targeted therapy as part of a frontline treatment regimen,” said Karen Ferrante, MD, Chief Medical Officer, Millennium. “The trial is part of our ongoing development program to explore patient populations that may benefit from treatment with ADCETRIS in earlier lines of therapy and in other CD30-expressing malignancies.”
“There have been no new therapies approved for patients with newly diagnosed HL in many decades, representing a significant need to identify additional treatment options in this setting,” said Thomas C. Reynolds, M.D., Ph.D., Chief Medical Officer, Seattle Genetics. “We believe through this novel ADCETRIS-containing regimen we have the potential to redefine the treatment of frontline HL. This trial is also an important part of our development plan for ADCETRIS, and may serve as confirmatory to our U.S. accelerated approval in relapsed HL and systemic anaplastic large cell lymphoma.”
Study Design
The randomized, open-label, phase 3 trial will investigate ADCETRIS+AVD1 versus ABVD2 as frontline therapy in patients with advanced classical HL. The primary endpoint is modified progression free survival (mPFS) per independent review facility assessment using the Revised Response Criteria for malignant lymphoma. Secondary endpoints include overall survival (OS), complete remission (CR) and safety. The multi-center trial will be conducted in North America, Europe, Latin America and Asia. The study will enroll approximately 1,040 eligible patients (approximately 520 patients per treatment arm) who have histologically-confirmed diagnosis of Stage III or IV classical HL who have not been previously treated with systemic chemotherapy or radiotherapy.
For more information, please visit www.clinicaltrials.gov.
hallo,
hier die neue ASH-Präsentation zu Adcetris

Gruß q.
-----------------
..Seattle Genetics’ ASH 2012 Presentations Highlight ADCETRIS® and Demonstrate Leadership in the Development of Antibody-Drug Conjugates (ADCs)
-Data Support Plans to Establish ADCETRIS as the Foundation of Therapy for a Broad Array of CD30-Positive Malignancies-
-Company Announces Novel ADC Candidate SGN-CD33A and Encouraging ADC Collaborator Data Presentations-
Press Release: Seattle Genetics, Inc. – 38 minutes ago....Email
Share0Print.....RELATED QUOTES.
.Symbol Price Change
SGEN 24.95 +0.62
......
BOTHELL, Wash.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (SGEN) today announced that more than a dozen abstracts, in addition to several collaborator abstracts, for both ADCETRIS (brentuximab vedotin) and investigational antibody-drug conjugates (ADCs) will be presented at the American Society of Hematology (ASH) Annual Meeting taking place in Atlanta, Georgia, December 8 – 11, 2012. ADCETRIS is an ADC directed to CD30, which is known to be expressed in Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (sALCL), as well as in some types of cutaneous T-cell lymphoma (CTCL), B-cell lymphomas and mature T-cell lymphomas (MTCL). Seattle Genetics is broadly evaluating CD30 expression in many other cancer types. ADCETRIS is currently not approved for use in CTCL, B-cell lymphomas, and front-line treatment of HL or MTCL.
"The comprehensive data presented at ASH 2012 support our goal to establish ADCETRIS as the foundation of therapy for a broad array of CD30-positive malignancies and redefine therapy in the front-line setting of HL and MTCL,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. "As a pioneer in developing ADC therapies, we continue to innovate by expanding the ADCETRIS program into CD30-positive malignancies, advancing additional ADC pipeline candidates, and supporting the progress of our collaborator ADC programs. These important advances represent our continued innovation and ADC leadership position."
Seattle Genetics is the leader in developing ADCs, a technology designed to harness the targeting ability of antibodies to deliver cell-killing agents directly to cancer cells. Of the approximately 30 ADC candidates currently in development, more than half utilize Seattle Genetics’ proprietary ADC technology. Multiple company, investigator and collaborator presentations will be presented at ASH, including data on ADCETRIS in many types of CD30-positive malignancies and preclinical data from a new ADC product candidate called SGN-CD33A. Abstract presentations planned at ASH can be found at www.hematology.org and include the following:
ADCETRIS Data in the Front-line Setting
•Results from a phase I trial evaluating front-line therapy with ADCETRIS combined with multi-agent chemotherapy in newly diagnosed advanced stage HL (Abstract number 798, oral presentation, Monday, December 10, 2012)
•Results from a phase I trial evaluating ADCETRIS in combination with multi-agent chemotherapy as front-line treatment of systemic ALCL and other types of CD30-positive MTCL (Abstract number 60, oral presentation, Sunday, December 9, 2012)
ADCETRIS Data in CTCL
•Results from a phase II trial evaluating ADCETRIS in the treatment of relapsed or refractory mycosis fungoides – the most common type of CTCL (Abstract number 797, oral presentation, Monday, December 10, 2012)
•Results from a phase II study of ADCETRIS in CD30-positive CTCL and lymphoproliferative disorders (Abstract number 3688, poster presentation, Monday, December 10, 2012)
ADCETRIS Data in Hodgkin and Non-Hodgkin Lymphomas
•Multiple data presentations on ADCETRIS in HL (Abstract numbers 3687,3689, 3699, 3701)
•Interim results from a phase II trial of ADCETRIS in relapsed or refractory CD30-positive non-Hodgkin lymphoma (NHL) (Abstract number 2746, poster presentation, Sunday, December 9, 2012)
•ADCETRIS data presentations in other CD30-positive malignancies and long-term follow-up from a pivotal trial in relapsed sALCL (Abstract numbers 1558, 2857, 2745)
Data on Other ADC Candidates and Collaborator Programs
•Preclinical antitumor activity from a novel CD33-directed ADC called SGN-CD33A in acute myeloid leukemia (AML) (Abstract number 3589, poster presentation, Monday, December 10, 2012)
•Results from phase I clinical trials of ADCs targeting CD22 and CD79b being developed by ADC collaborator Genentech (Abstract numbers 59, 56, poster presentations, Sunday, December 9, 2012)
hier die neue ASH-Präsentation zu Adcetris

Gruß q.
-----------------
..Seattle Genetics’ ASH 2012 Presentations Highlight ADCETRIS® and Demonstrate Leadership in the Development of Antibody-Drug Conjugates (ADCs)
-Data Support Plans to Establish ADCETRIS as the Foundation of Therapy for a Broad Array of CD30-Positive Malignancies-
-Company Announces Novel ADC Candidate SGN-CD33A and Encouraging ADC Collaborator Data Presentations-
Press Release: Seattle Genetics, Inc. – 38 minutes ago....Email
Share0Print.....RELATED QUOTES.
.Symbol Price Change
SGEN 24.95 +0.62
......
BOTHELL, Wash.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (SGEN) today announced that more than a dozen abstracts, in addition to several collaborator abstracts, for both ADCETRIS (brentuximab vedotin) and investigational antibody-drug conjugates (ADCs) will be presented at the American Society of Hematology (ASH) Annual Meeting taking place in Atlanta, Georgia, December 8 – 11, 2012. ADCETRIS is an ADC directed to CD30, which is known to be expressed in Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (sALCL), as well as in some types of cutaneous T-cell lymphoma (CTCL), B-cell lymphomas and mature T-cell lymphomas (MTCL). Seattle Genetics is broadly evaluating CD30 expression in many other cancer types. ADCETRIS is currently not approved for use in CTCL, B-cell lymphomas, and front-line treatment of HL or MTCL.
"The comprehensive data presented at ASH 2012 support our goal to establish ADCETRIS as the foundation of therapy for a broad array of CD30-positive malignancies and redefine therapy in the front-line setting of HL and MTCL,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. "As a pioneer in developing ADC therapies, we continue to innovate by expanding the ADCETRIS program into CD30-positive malignancies, advancing additional ADC pipeline candidates, and supporting the progress of our collaborator ADC programs. These important advances represent our continued innovation and ADC leadership position."
Seattle Genetics is the leader in developing ADCs, a technology designed to harness the targeting ability of antibodies to deliver cell-killing agents directly to cancer cells. Of the approximately 30 ADC candidates currently in development, more than half utilize Seattle Genetics’ proprietary ADC technology. Multiple company, investigator and collaborator presentations will be presented at ASH, including data on ADCETRIS in many types of CD30-positive malignancies and preclinical data from a new ADC product candidate called SGN-CD33A. Abstract presentations planned at ASH can be found at www.hematology.org and include the following:
ADCETRIS Data in the Front-line Setting
•Results from a phase I trial evaluating front-line therapy with ADCETRIS combined with multi-agent chemotherapy in newly diagnosed advanced stage HL (Abstract number 798, oral presentation, Monday, December 10, 2012)
•Results from a phase I trial evaluating ADCETRIS in combination with multi-agent chemotherapy as front-line treatment of systemic ALCL and other types of CD30-positive MTCL (Abstract number 60, oral presentation, Sunday, December 9, 2012)
ADCETRIS Data in CTCL
•Results from a phase II trial evaluating ADCETRIS in the treatment of relapsed or refractory mycosis fungoides – the most common type of CTCL (Abstract number 797, oral presentation, Monday, December 10, 2012)
•Results from a phase II study of ADCETRIS in CD30-positive CTCL and lymphoproliferative disorders (Abstract number 3688, poster presentation, Monday, December 10, 2012)
ADCETRIS Data in Hodgkin and Non-Hodgkin Lymphomas
•Multiple data presentations on ADCETRIS in HL (Abstract numbers 3687,3689, 3699, 3701)
•Interim results from a phase II trial of ADCETRIS in relapsed or refractory CD30-positive non-Hodgkin lymphoma (NHL) (Abstract number 2746, poster presentation, Sunday, December 9, 2012)
•ADCETRIS data presentations in other CD30-positive malignancies and long-term follow-up from a pivotal trial in relapsed sALCL (Abstract numbers 1558, 2857, 2745)
Data on Other ADC Candidates and Collaborator Programs
•Preclinical antitumor activity from a novel CD33-directed ADC called SGN-CD33A in acute myeloid leukemia (AML) (Abstract number 3589, poster presentation, Monday, December 10, 2012)
•Results from phase I clinical trials of ADCs targeting CD22 and CD79b being developed by ADC collaborator Genentech (Abstract numbers 59, 56, poster presentations, Sunday, December 9, 2012)
Da bin ich ja mal gespannt, was da kommen wird Anfang Dezember. Hier schon mal eine Abstract-Passage:
Adcetris + AVD achieves 92% complete remission rate in front-line Hodgkin and strong response in non-Hodgkin...
q.
Adcetris + AVD achieves 92% complete remission rate in front-line Hodgkin and strong response in non-Hodgkin...
q.
Anti-CD79b Antibody-Drug Conjugate (ADC) von Roche(Genentech)/SGEN mit erstem schönen Wirkungsnachweis, aber auch ausgeprägten Nebenwirkungen.
Maria Corinna Palanca-Wessels, M.D., Ph.D.1*, Ian W. Flinn, MD, PhD2, Laurie H. Sehn, MD, MPH3, Manish Patel, MD4*, Randeep Sangha, MD5*, Myron S. Czuczman, MD6, Gilles Andre Salles, MD, PhD7, Franck Morschhauser, MD8*, Ranjana Advani, MD9, Oliver W. Press, MD, PhD1, William Ho, MD, PhD10, Robert Kahn10*, Dan Lu10*, Zheng Su10*, Yu-Waye Chu10* and Sarit E. Assouline, MD11
1Fred Hutchinson Cancer Research Center, Seattle, WA
2Sarah Cannon Cancer Institute, Nashville, TN
3Medical Oncology, British Columbia Cancer Agency, Vancouver, BC, Canada
4Sarah Cannon Research Institute and Florida Cancer Specialists, Sarasota, FL
5Cross Cancer Institute, alberta, AB, Canada
6Roswell Park Cancer Institute, Buffalo, NY
7Hematologie, Centre Hospitalier Lyon-Sud, Pierre-Benite, France
8CHU Claude Huriez, Lille Cedex, France
9Stanford University, Stanford, CA
10Genentech, Inc., South San Francisco, CA
11Jewish General Hospital, Montreal, QC, Canada
Introduction: CD79b, a component of the B-cell receptor (BCR), is expressed by nearly all B-cell malignancies including NHL. DCDS4501A is an ADC consisting of an anti-CD79b monoclonal antibody conjugated to monomethyl auristatin E (MMAE), a microtubule disrupting agent via a protease-cleavable peptide linker. DCDS4501A exhibits potent anti-tumor activity in murine xenograft models of B-cell lymphoma.
Methods: A Phase I study of DCDS4501A is being conducted to assess the safety, tolerability, pharmacokinetics (PK), and biologic activity of escalating doses of DCDS4501A in pts with relapsed/refractory B-cell NHL. Pts receive DCDS4501A intravenously every 21 days until disease progression or unacceptable toxicity. Intrapatient dose escalation based on tolerability at higher doses is permitted. Following determination of the recommended Phase II dose (RP2D) based on protocol-defined dose-limiting toxicities (DLTs) occurring within 21 days of dosing, additional pts with indolent and aggressive B-cell NHL will be enrolled to further evaluate safety and efficacy based on Cheson response criteria. Here we report the RP2D and preliminary safety and efficacy results.
Results: To date, 33 pts have been enrolled (64% male), median age of 65 years (range 20-85): follicular lymphoma (FL, n=14), diffuse large B-cell lymphoma (DLBCL, n=11), MCL (n=4), MZL (n=2), transformed FL (n=1), and small lymphocytic lymphoma (SLL, n=1). Enrolled patients were heavily pre-treated: 29 patients had ≥ 3 prior regimens, all pts had received prior rituximab, and 9 pts received prior high-dose therapy followed by stem cell transplantation. Pts received a median of 3 doses (range 1-12) of DCDS4501A in 6 dose-escalation cohorts with doses ranging from 0.1-2.4 mg/kg. The protocol-specified MTD was not formally reached, however, 2.4 mg/kg DCDS4501A was determined to be the RP2D based on the overall safety and tolerability profile at that dose, which included 1 pt with a DLT of Grade 4 febrile neutropenia and pneumonia. The most common treatment-emergent adverse events (AE) occurring in ≥ 20% of pts were neutropenia (59%), diarrhea (38%), nausea (34%), hyperglycemia (31%), fatigue (31%), constipation (28%), peripheral neuropathy (28%), pyrexia (28%), leukopenia (25%), chills (22%), and cough (22%). Neutropenia (39%) and leukopenia (12%) were the only treatment-emergent Grade ≥ 3 AEs in ≥ 10% of pts. Seven of 12 pts treated at the RP2D of 2.4 mg/kg experienced a Grade 3-4 AE: Grade 3-4 neutropenia in 5 pts, and anemia, leukopenia and fatigue each in 2 pts. Eight (24%) pts across all dose levels experienced a serious AE (SAE). Four SAEs were reported in 2 pts treated at the RP2D of 2.4 mg/kg: 1 pt with atrial fibrillation, neutropenia, and pneumonia and 1 pt with cardiac failure; in both cases the SAE resolved and study treatment resumed. Treatment discontinuation due to AE occurred in 1 pt each for Grade 3 neutropenia and Grade 3 hyponatremia. One pt had a dose reduction for Grade 4 febrile neutropenia and pneumonia. No deaths were reported within 30 days of the last dose of DCDS4501A. Most cases of neutropenia were observed in the absence of growth factor support. Assessment of Cycle 1 PK after the first dose of DCDS4501A indicated that exposure of antibody-conjugated MMAE (acMMAE), total antibody, and free MMAE, increased with dose. The clearance estimates of acMMAE and total antibody were similar across doses from 0.1-2.4 mg/kg; volume of distribution estimates of acMMAE and total antibody approximated plasma volume, which also did not change with dose. These results suggested dose proportional increase of acMMAE and total antibody exposures. Early evidence of anti-tumor activity was observed, including 5 pts with > 50% reduction in target lesion burden at the first on-treatment tumor assessment after 3-4 cycles of treatment: 2 with FL, and 1 each with DLBCL, transformed FL, and MCL; 4 pts continue on treatment with DCDS4501A (range 5-12 cycles). Two of four pts treated at 2.4 mg/kg evaluable for anti-tumor activity to date had > 80% reduction in target lesion burden at the first on-treatment tumor assessment.
Conclusions: DCDS4501A, a novel ADC targeting CD79b, has demonstrated an acceptable toxicity profile and encouraging anti-tumor activity in heavily pretreated pts with relapsed/refractory B-cell NHL. Updated clinical data will be presented. These results support additional clinical evaluation of DCDS4501A in B-cell malignancies.
Maria Corinna Palanca-Wessels, M.D., Ph.D.1*, Ian W. Flinn, MD, PhD2, Laurie H. Sehn, MD, MPH3, Manish Patel, MD4*, Randeep Sangha, MD5*, Myron S. Czuczman, MD6, Gilles Andre Salles, MD, PhD7, Franck Morschhauser, MD8*, Ranjana Advani, MD9, Oliver W. Press, MD, PhD1, William Ho, MD, PhD10, Robert Kahn10*, Dan Lu10*, Zheng Su10*, Yu-Waye Chu10* and Sarit E. Assouline, MD11
1Fred Hutchinson Cancer Research Center, Seattle, WA
2Sarah Cannon Cancer Institute, Nashville, TN
3Medical Oncology, British Columbia Cancer Agency, Vancouver, BC, Canada
4Sarah Cannon Research Institute and Florida Cancer Specialists, Sarasota, FL
5Cross Cancer Institute, alberta, AB, Canada
6Roswell Park Cancer Institute, Buffalo, NY
7Hematologie, Centre Hospitalier Lyon-Sud, Pierre-Benite, France
8CHU Claude Huriez, Lille Cedex, France
9Stanford University, Stanford, CA
10Genentech, Inc., South San Francisco, CA
11Jewish General Hospital, Montreal, QC, Canada
Introduction: CD79b, a component of the B-cell receptor (BCR), is expressed by nearly all B-cell malignancies including NHL. DCDS4501A is an ADC consisting of an anti-CD79b monoclonal antibody conjugated to monomethyl auristatin E (MMAE), a microtubule disrupting agent via a protease-cleavable peptide linker. DCDS4501A exhibits potent anti-tumor activity in murine xenograft models of B-cell lymphoma.
Methods: A Phase I study of DCDS4501A is being conducted to assess the safety, tolerability, pharmacokinetics (PK), and biologic activity of escalating doses of DCDS4501A in pts with relapsed/refractory B-cell NHL. Pts receive DCDS4501A intravenously every 21 days until disease progression or unacceptable toxicity. Intrapatient dose escalation based on tolerability at higher doses is permitted. Following determination of the recommended Phase II dose (RP2D) based on protocol-defined dose-limiting toxicities (DLTs) occurring within 21 days of dosing, additional pts with indolent and aggressive B-cell NHL will be enrolled to further evaluate safety and efficacy based on Cheson response criteria. Here we report the RP2D and preliminary safety and efficacy results.
Results: To date, 33 pts have been enrolled (64% male), median age of 65 years (range 20-85): follicular lymphoma (FL, n=14), diffuse large B-cell lymphoma (DLBCL, n=11), MCL (n=4), MZL (n=2), transformed FL (n=1), and small lymphocytic lymphoma (SLL, n=1). Enrolled patients were heavily pre-treated: 29 patients had ≥ 3 prior regimens, all pts had received prior rituximab, and 9 pts received prior high-dose therapy followed by stem cell transplantation. Pts received a median of 3 doses (range 1-12) of DCDS4501A in 6 dose-escalation cohorts with doses ranging from 0.1-2.4 mg/kg. The protocol-specified MTD was not formally reached, however, 2.4 mg/kg DCDS4501A was determined to be the RP2D based on the overall safety and tolerability profile at that dose, which included 1 pt with a DLT of Grade 4 febrile neutropenia and pneumonia. The most common treatment-emergent adverse events (AE) occurring in ≥ 20% of pts were neutropenia (59%), diarrhea (38%), nausea (34%), hyperglycemia (31%), fatigue (31%), constipation (28%), peripheral neuropathy (28%), pyrexia (28%), leukopenia (25%), chills (22%), and cough (22%). Neutropenia (39%) and leukopenia (12%) were the only treatment-emergent Grade ≥ 3 AEs in ≥ 10% of pts. Seven of 12 pts treated at the RP2D of 2.4 mg/kg experienced a Grade 3-4 AE: Grade 3-4 neutropenia in 5 pts, and anemia, leukopenia and fatigue each in 2 pts. Eight (24%) pts across all dose levels experienced a serious AE (SAE). Four SAEs were reported in 2 pts treated at the RP2D of 2.4 mg/kg: 1 pt with atrial fibrillation, neutropenia, and pneumonia and 1 pt with cardiac failure; in both cases the SAE resolved and study treatment resumed. Treatment discontinuation due to AE occurred in 1 pt each for Grade 3 neutropenia and Grade 3 hyponatremia. One pt had a dose reduction for Grade 4 febrile neutropenia and pneumonia. No deaths were reported within 30 days of the last dose of DCDS4501A. Most cases of neutropenia were observed in the absence of growth factor support. Assessment of Cycle 1 PK after the first dose of DCDS4501A indicated that exposure of antibody-conjugated MMAE (acMMAE), total antibody, and free MMAE, increased with dose. The clearance estimates of acMMAE and total antibody were similar across doses from 0.1-2.4 mg/kg; volume of distribution estimates of acMMAE and total antibody approximated plasma volume, which also did not change with dose. These results suggested dose proportional increase of acMMAE and total antibody exposures. Early evidence of anti-tumor activity was observed, including 5 pts with > 50% reduction in target lesion burden at the first on-treatment tumor assessment after 3-4 cycles of treatment: 2 with FL, and 1 each with DLBCL, transformed FL, and MCL; 4 pts continue on treatment with DCDS4501A (range 5-12 cycles). Two of four pts treated at 2.4 mg/kg evaluable for anti-tumor activity to date had > 80% reduction in target lesion burden at the first on-treatment tumor assessment.
Conclusions: DCDS4501A, a novel ADC targeting CD79b, has demonstrated an acceptable toxicity profile and encouraging anti-tumor activity in heavily pretreated pts with relapsed/refractory B-cell NHL. Updated clinical data will be presented. These results support additional clinical evaluation of DCDS4501A in B-cell malignancies.
Hallo,

der Quartalsbericht ist raus - leider schlechte Nachrichten.
Zahlen sehen gar nicht mal schlecht aus, aber die Aussicht auf die Adcetris-Umsätze 2013 und damit auf den Gesamtumsatz 2013 wurde zurückgenommen.
Nachbörslich auch dickes Minus, sowas ist natürlich katastrophal für den Aktienkurs. Werde mir noch überlegen ob ich investiert bleibe.
Langfristig bleibt SGEN und auch Adcetris hochinteressant, aber kurzfristig könnte es noch weiter runtergehen.

Gruß q.

der Quartalsbericht ist raus - leider schlechte Nachrichten.
Zahlen sehen gar nicht mal schlecht aus, aber die Aussicht auf die Adcetris-Umsätze 2013 und damit auf den Gesamtumsatz 2013 wurde zurückgenommen.
Nachbörslich auch dickes Minus, sowas ist natürlich katastrophal für den Aktienkurs. Werde mir noch überlegen ob ich investiert bleibe.
Langfristig bleibt SGEN und auch Adcetris hochinteressant, aber kurzfristig könnte es noch weiter runtergehen.

Gruß q.
Hallo,

hält sich ja doch ganz wacker, es gibt sogar mal wieder eine Empfehlung (von der Credit Suisse), ASH ist ja auch schon bald, so bleibe ich bei meinem ursprünglichen Ausstiegsszenario (Vachkauf bei ca. 22, Verkauf bei ca. 30, aber je nach Nachrichtenlage).
Text:
........................
Adcetris and First Indications Are Tip of an Iceberg; INITIATING Coverage with an OUTPERFORM Rating & Target Price of $28

hält sich ja doch ganz wacker, es gibt sogar mal wieder eine Empfehlung (von der Credit Suisse), ASH ist ja auch schon bald, so bleibe ich bei meinem ursprünglichen Ausstiegsszenario (Vachkauf bei ca. 22, Verkauf bei ca. 30, aber je nach Nachrichtenlage).
Text:
........................
Adcetris and First Indications Are Tip of an Iceberg; INITIATING Coverage with an OUTPERFORM Rating & Target Price of $28
Heute JPM Conference, kam offenbar gut an bei den Investoren. Es laüft anscheinend sehr gut in Q4 und generell.
Hier die Anmerkungen des von mir geschätzten Yahoo-Board-Users und SGEN-Aktionärs "redhot...".
Gruß q.
O-Ton:
-------------------
Here are some highlights of CEO Clay Siegall's presentation today during the 31st Annual J.P. Morgan Healthcare Conference in San Francisco:
I heard nothing brand new (as expected), but the presentation was extremely solid and utterly upbeat. At the conclusion, the audience responded with notable applause.
If anything (and this is an exception to the general rule regarding SGEN presentations), the financial news was a primary highlight.
Siegall noted that the company ended the third quarter with $313.9 million in cash and investments – and no debt, but the situation is even better now. “We ended the year with significantly more than that,” he said. “Our fourth quarter was very strong with milestone payments, licensing deals and Adcetris sales.”
The company plans to release its fourth-quarter and full-year 2012 results on Tuesday, Feb. 12. “We are in a solid financial position…and we will keep our pipeline filled for many years to come,” he said.
Other highlights:
- SGEN has more than 1,000 accounts already for Adcetris, with a potential of 2,000 accounts. Physician awareness of Adcetris is extremely high, at 97 percent.
- More than 20 trials are currently underway or designed to build additional use of Adcetris. “We are building data sets in many additional kinds of lymphomas…and there are many opportunities in solid tumors and other applications,” he said.
- Adcetris now has a permanent J code, making it easier for physician billing offices.
- He strongly emphasized the already-released positive trial results in Hodgkin lymphoma (96 percent complete remission with no pulmonary toxicity). He said SGEN is “redefining” the standard of care.
- He made similarly strong comments about outstanding trial results in CTCL and MTCL
- In addition to the many SGEN and partnered trials of Adcetris now underway, nearly 15 investigator-sponsored trials are underway or planned for 2013.
- Regarding the rest of the pipeline, six trials are underway or planned for 2013. Some targets: renal cell cancers, prostate cancer, breast cancer, bladder and lung cancers.
- He predicted additional ADC-related partnering deals this year.
The session then adjourned to another room for Q&A.
As always, I encourage you to listen to the replay.
Hier die Anmerkungen des von mir geschätzten Yahoo-Board-Users und SGEN-Aktionärs "redhot...".
Gruß q.
O-Ton:
-------------------
Here are some highlights of CEO Clay Siegall's presentation today during the 31st Annual J.P. Morgan Healthcare Conference in San Francisco:
I heard nothing brand new (as expected), but the presentation was extremely solid and utterly upbeat. At the conclusion, the audience responded with notable applause.
If anything (and this is an exception to the general rule regarding SGEN presentations), the financial news was a primary highlight.
Siegall noted that the company ended the third quarter with $313.9 million in cash and investments – and no debt, but the situation is even better now. “We ended the year with significantly more than that,” he said. “Our fourth quarter was very strong with milestone payments, licensing deals and Adcetris sales.”
The company plans to release its fourth-quarter and full-year 2012 results on Tuesday, Feb. 12. “We are in a solid financial position…and we will keep our pipeline filled for many years to come,” he said.
Other highlights:
- SGEN has more than 1,000 accounts already for Adcetris, with a potential of 2,000 accounts. Physician awareness of Adcetris is extremely high, at 97 percent.
- More than 20 trials are currently underway or designed to build additional use of Adcetris. “We are building data sets in many additional kinds of lymphomas…and there are many opportunities in solid tumors and other applications,” he said.
- Adcetris now has a permanent J code, making it easier for physician billing offices.
- He strongly emphasized the already-released positive trial results in Hodgkin lymphoma (96 percent complete remission with no pulmonary toxicity). He said SGEN is “redefining” the standard of care.
- He made similarly strong comments about outstanding trial results in CTCL and MTCL
- In addition to the many SGEN and partnered trials of Adcetris now underway, nearly 15 investigator-sponsored trials are underway or planned for 2013.
- Regarding the rest of the pipeline, six trials are underway or planned for 2013. Some targets: renal cell cancers, prostate cancer, breast cancer, bladder and lung cancers.
- He predicted additional ADC-related partnering deals this year.
The session then adjourned to another room for Q&A.
As always, I encourage you to listen to the replay.
Moin,
hier die Zahlen für das Q4 und das Gesamtjahr. Ausblick für 2013-Umsätze von Adcetris ist flat - dürfte aber (hoffentlich) tief gestapelt sein. Mal sehen, was für Kommentare kommen.
--------------------------
Total revenues in the fourth quarter of 2012 were $63.9 million,
compared to $48.9 million in the fourth quarter of 2011. Total revenues for the year ended December 31, 2012 were $210.8 million, compared to $94.8 million in 2011. Revenues in 2012 include ADCETRIS net product sales of $35.4 million in the fourth quarter and $138.2 million for the year. Revenues in 2012 reflect $2.6 million in ADCETRIS net sales to patients in Canada under a Special Access Program that were recognized in the fourth quarter. Revenues also reflect amounts earned under the company’s ADCETRIS and ADC collaborations totaling $26.4 million in the fourth quarter of 2012 and $67.5 million for the year.
Research and development expenses for the fourth quarter of 2012 were $47.7 million, compared to $40.2 million for the fourth quarter of 2011. For 2012, total research and development expenses were $170.3 million, compared to $163.4 million in 2011. Selling, general and administrative expenses for the fourth quarter of 2012 were $23.4 million, compared to $25.0 million for the fourth quarter of 2011. For 2012, total selling, general and administrative expenses were $84.3 million, compared to $72.7 million in 2011. The planned increases in 2012 expenses were primarily driven by ADCETRIS commercialization activities and research and development of the company’s ADC pipeline programs.
Under the ADCETRIS collaboration with Millennium, development costs incurred by Seattle Genetics are included in research and development expense. Joint development costs are co-funded by Millennium on a 50:50 basis. Net reimbursement funding received from Millennium is recognized as revenue over the development period of the collaboration along with other development payments received, including the upfront payment and milestone payments. Seattle Genetics co-funds development activities performed by Millennium under the collaboration, which reduces the amount of reimbursement funding received from Millennium.
Non-cash, share-based compensation expense for the year in 2012 was $25.3 million, compared to $20.0 million for the year in 2011.
Net loss for the fourth quarter of 2012 was $10.6 million, or $0.09 per share, compared to a net loss of $27.2 million, or $0.24 per share, for the fourth quarter of 2011. For the year ended December 31, 2012, net loss was $53.8 million, or $0.46 per share, compared to a net loss of $152.0 million, or $1.34 per share, for the year ended December 31, 2011.
As of December 31, 2012, Seattle Genetics had $364.3 million in cash, cash equivalents and investments, compared to $330.7 million as of December 31, 2011.
2013 Financial Outlook
Seattle Genetics anticipates that revenues from ADCETRIS net product sales will be in the range of $130 million to $140 million in 2013 and that revenues from collaboration and license agreements in 2013 will be in the range of $65 million to $75 million. These revenues will be generated from fees, milestones and reimbursements earned through the company’s ADCETRIS and ADC collaborations.
Research and development expenses are expected to be in the range of $210 million to $230 million. Selling, general and administration expenses are expected to be in the range of $85 million to $95 million. Operating expenses will be directed primarily towards commercialization and clinical trials of ADCETRIS, development and clinical activities for SGN-75, ASG-5ME, ASG-22ME and SGN-CD19A, and IND-enabling activities for SGN-CD33A and SGN-LIV1A. Cost of sales is expected to be approximately $15 million for the year in 2013, representing a range of 10 percent to 12 percent of net sales. Non-cash expenses are expected to be approximately $40 million in 2013, primarily attributable to share-based compensation expense.
hier die Zahlen für das Q4 und das Gesamtjahr. Ausblick für 2013-Umsätze von Adcetris ist flat - dürfte aber (hoffentlich) tief gestapelt sein. Mal sehen, was für Kommentare kommen.
--------------------------
Total revenues in the fourth quarter of 2012 were $63.9 million,
compared to $48.9 million in the fourth quarter of 2011. Total revenues for the year ended December 31, 2012 were $210.8 million, compared to $94.8 million in 2011. Revenues in 2012 include ADCETRIS net product sales of $35.4 million in the fourth quarter and $138.2 million for the year. Revenues in 2012 reflect $2.6 million in ADCETRIS net sales to patients in Canada under a Special Access Program that were recognized in the fourth quarter. Revenues also reflect amounts earned under the company’s ADCETRIS and ADC collaborations totaling $26.4 million in the fourth quarter of 2012 and $67.5 million for the year.
Research and development expenses for the fourth quarter of 2012 were $47.7 million, compared to $40.2 million for the fourth quarter of 2011. For 2012, total research and development expenses were $170.3 million, compared to $163.4 million in 2011. Selling, general and administrative expenses for the fourth quarter of 2012 were $23.4 million, compared to $25.0 million for the fourth quarter of 2011. For 2012, total selling, general and administrative expenses were $84.3 million, compared to $72.7 million in 2011. The planned increases in 2012 expenses were primarily driven by ADCETRIS commercialization activities and research and development of the company’s ADC pipeline programs.
Under the ADCETRIS collaboration with Millennium, development costs incurred by Seattle Genetics are included in research and development expense. Joint development costs are co-funded by Millennium on a 50:50 basis. Net reimbursement funding received from Millennium is recognized as revenue over the development period of the collaboration along with other development payments received, including the upfront payment and milestone payments. Seattle Genetics co-funds development activities performed by Millennium under the collaboration, which reduces the amount of reimbursement funding received from Millennium.
Non-cash, share-based compensation expense for the year in 2012 was $25.3 million, compared to $20.0 million for the year in 2011.
Net loss for the fourth quarter of 2012 was $10.6 million, or $0.09 per share, compared to a net loss of $27.2 million, or $0.24 per share, for the fourth quarter of 2011. For the year ended December 31, 2012, net loss was $53.8 million, or $0.46 per share, compared to a net loss of $152.0 million, or $1.34 per share, for the year ended December 31, 2011.
As of December 31, 2012, Seattle Genetics had $364.3 million in cash, cash equivalents and investments, compared to $330.7 million as of December 31, 2011.
2013 Financial Outlook
Seattle Genetics anticipates that revenues from ADCETRIS net product sales will be in the range of $130 million to $140 million in 2013 and that revenues from collaboration and license agreements in 2013 will be in the range of $65 million to $75 million. These revenues will be generated from fees, milestones and reimbursements earned through the company’s ADCETRIS and ADC collaborations.
Research and development expenses are expected to be in the range of $210 million to $230 million. Selling, general and administration expenses are expected to be in the range of $85 million to $95 million. Operating expenses will be directed primarily towards commercialization and clinical trials of ADCETRIS, development and clinical activities for SGN-75, ASG-5ME, ASG-22ME and SGN-CD19A, and IND-enabling activities for SGN-CD33A and SGN-LIV1A. Cost of sales is expected to be approximately $15 million for the year in 2013, representing a range of 10 percent to 12 percent of net sales. Non-cash expenses are expected to be approximately $40 million in 2013, primarily attributable to share-based compensation expense.
Antwort auf Beitrag Nr.: 44.134.061 von quepos am 13.02.13 08:13:37Die Adcetris-Zahlen sind enttäuschend, erstaunlich, dass die Börse so gefaßt reagiert.
Moin,

bin mal meinem Gefühl gefolgt und habe den 2. Teil meiner Posi für ca. 31.70 $ abgegeben. Im Langfristchart ist der Kurs in der Nähe des mehrjährigen Aufwärtstrends (Oberkante) und auch fundamental finde ich im Momment SGEN mit fast 4 Mrd. $ gut bewertet.
Mal seh´n ob ich in den Zwanzigern wieder reinkomme!
Gruß q.

bin mal meinem Gefühl gefolgt und habe den 2. Teil meiner Posi für ca. 31.70 $ abgegeben. Im Langfristchart ist der Kurs in der Nähe des mehrjährigen Aufwärtstrends (Oberkante) und auch fundamental finde ich im Momment SGEN mit fast 4 Mrd. $ gut bewertet.
Mal seh´n ob ich in den Zwanzigern wieder reinkomme!
Gruß q.
Moin,
die Anwendungsbreite von Adcetris soll weiter erhöht werden werden (neue BLA).
Text siehe unten.
Übrigens hier noch mal nachgereicht:
Der Titel des Threads "Chance zum Einstieg?" kann rüchblickend bestätigt und leicht verändert werden, es wäre lediglich das Fragezeichen durch ein Ausrufezeichen zu ersetzen. Seit Auflegung des Threads ist der Kurs nun um ca. 270 Prozent gestiegen, ein ziemlich guter Wert für weniger als 3 1/2 Jahre!
Werde mich weiterhin hier melden und evtl. wieder einsteigen, wenn es mir lohnend erscheint, nach wie vor halte ich die Technologie der Firma für eine der Besten im Bereich der Medikamentenentwicklung. Ist im Moment aber mit 4 Mrd. Dollar auch gut bezahlt, finde ich.
Gruß und so long
q.
--------------------------------
Press Release: Seattle Genetics, Inc. – Mon, Mar 18, 2013 9:00 AM EDT....Email0
Share0Print.....RELATED QUOTES..Symbol Price Change
SGEN 32.82 -0.59
......
BOTHELL, Wash.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (SGEN) announced today that it has submitted a supplemental Biologics License Application (sBLA) to the U.S. Food and Drug Administration (FDA) supporting the use of ADCETRIS (brentuximab vedotin) for retreatment and extended duration beyond 16 cycles of therapy in relapsed Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (sALCL). ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of HL and sALCL, that was granted accelerated approval by the FDA in August 2011 for relapsed HL and relapsed sALCL.
“The sBLA submission includes data demonstrating ADCETRIS activity in managing HL and sALCL when used in the retreatment setting, as well as beyond the 16 cycles described in our current label, while retaining a manageable safety profile,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “Our goal is to broaden the ADCETRIS U.S. labeling claims to provide both patients and physicians the opportunity to incorporate ADCETRIS into additional HL and sALCL treatment settings. The sBLA submission includes data that support these uses and we look forward to the regulatory outcome.”
The sBLA is based on results from a phase II clinical trial with two treatment arms. One arm evaluated retreatment with ADCETRIS in patients who previously responded to treatment with ADCETRIS, then discontinued treatment and subsequently had disease progression or relapse. The other arm allowed treatment extension and evaluated prolonged treatment with ADCETRIS beyond 16 cycles of therapy. The sBLA submission includes updated data sets from this phase II trial. Preliminary data from this trial were previously reported at the 2011 American Society of Hematology (ASH) Annual Meeting and at the 2012 American Society of Clinical Oncology (ASCO) Annual meeting.
At the 2012 ASCO Annual Meeting, retreatment data from the phase II trial were reported from 23 patients, including one patient who was treated twice. Patients had received a median of four prior systemic therapies, including ADCETRIS. Of 23 evaluable patients, 70 percent (16 of 23) achieved an objective response after retreatment with ADCETRIS, including nine complete remissions and seven partial remissions. Median duration of retreatment objective response was 8.8 months. Among retreated HL patients, nine of 16 (56 percent) achieved an objective response. Among retreated sALCL patients, seven of eight (88 percent) achieved an objective response. The most common adverse events were peripheral neuropathy (46 percent), nausea (42 percent), fatigue (38 percent), diarrhea (33 percent) and fever (29 percent).
At the 2011 ASH Annual Meeting, prolonged treatment data were reported from 17 patients with a median duration of treatment of 17.3 months (approximately 24 cycles of every three week dosing). The overall objective response rate with extended treatment was 88 percent, including 76 percent complete remissions and 12 percent partial remissions. ADCETRIS was generally well-tolerated, with the most common adverse events being peripheral neuropathy (71 percent), upper respiratory infection (53 percent) and fatigue (47 percent). Prolonged treatment with ADCETRIS was associated with clinically meaningful durations of response without worsening of toxicity over time.
ADCETRIS is currently not approved for retreatment and extended duration beyond 16 cycles of therapy in relapsed HL and sALCL.
die Anwendungsbreite von Adcetris soll weiter erhöht werden werden (neue BLA).
Text siehe unten.
Übrigens hier noch mal nachgereicht:
Der Titel des Threads "Chance zum Einstieg?" kann rüchblickend bestätigt und leicht verändert werden, es wäre lediglich das Fragezeichen durch ein Ausrufezeichen zu ersetzen. Seit Auflegung des Threads ist der Kurs nun um ca. 270 Prozent gestiegen, ein ziemlich guter Wert für weniger als 3 1/2 Jahre!
Werde mich weiterhin hier melden und evtl. wieder einsteigen, wenn es mir lohnend erscheint, nach wie vor halte ich die Technologie der Firma für eine der Besten im Bereich der Medikamentenentwicklung. Ist im Moment aber mit 4 Mrd. Dollar auch gut bezahlt, finde ich.
Gruß und so long
q.
--------------------------------
Press Release: Seattle Genetics, Inc. – Mon, Mar 18, 2013 9:00 AM EDT....Email0
Share0Print.....RELATED QUOTES..Symbol Price Change
SGEN 32.82 -0.59
......
BOTHELL, Wash.--(BUSINESS WIRE)--
Seattle Genetics, Inc. (SGEN) announced today that it has submitted a supplemental Biologics License Application (sBLA) to the U.S. Food and Drug Administration (FDA) supporting the use of ADCETRIS (brentuximab vedotin) for retreatment and extended duration beyond 16 cycles of therapy in relapsed Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (sALCL). ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of HL and sALCL, that was granted accelerated approval by the FDA in August 2011 for relapsed HL and relapsed sALCL.
“The sBLA submission includes data demonstrating ADCETRIS activity in managing HL and sALCL when used in the retreatment setting, as well as beyond the 16 cycles described in our current label, while retaining a manageable safety profile,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “Our goal is to broaden the ADCETRIS U.S. labeling claims to provide both patients and physicians the opportunity to incorporate ADCETRIS into additional HL and sALCL treatment settings. The sBLA submission includes data that support these uses and we look forward to the regulatory outcome.”
The sBLA is based on results from a phase II clinical trial with two treatment arms. One arm evaluated retreatment with ADCETRIS in patients who previously responded to treatment with ADCETRIS, then discontinued treatment and subsequently had disease progression or relapse. The other arm allowed treatment extension and evaluated prolonged treatment with ADCETRIS beyond 16 cycles of therapy. The sBLA submission includes updated data sets from this phase II trial. Preliminary data from this trial were previously reported at the 2011 American Society of Hematology (ASH) Annual Meeting and at the 2012 American Society of Clinical Oncology (ASCO) Annual meeting.
At the 2012 ASCO Annual Meeting, retreatment data from the phase II trial were reported from 23 patients, including one patient who was treated twice. Patients had received a median of four prior systemic therapies, including ADCETRIS. Of 23 evaluable patients, 70 percent (16 of 23) achieved an objective response after retreatment with ADCETRIS, including nine complete remissions and seven partial remissions. Median duration of retreatment objective response was 8.8 months. Among retreated HL patients, nine of 16 (56 percent) achieved an objective response. Among retreated sALCL patients, seven of eight (88 percent) achieved an objective response. The most common adverse events were peripheral neuropathy (46 percent), nausea (42 percent), fatigue (38 percent), diarrhea (33 percent) and fever (29 percent).
At the 2011 ASH Annual Meeting, prolonged treatment data were reported from 17 patients with a median duration of treatment of 17.3 months (approximately 24 cycles of every three week dosing). The overall objective response rate with extended treatment was 88 percent, including 76 percent complete remissions and 12 percent partial remissions. ADCETRIS was generally well-tolerated, with the most common adverse events being peripheral neuropathy (71 percent), upper respiratory infection (53 percent) and fatigue (47 percent). Prolonged treatment with ADCETRIS was associated with clinically meaningful durations of response without worsening of toxicity over time.
ADCETRIS is currently not approved for retreatment and extended duration beyond 16 cycles of therapy in relapsed HL and sALCL.
Antwort auf Beitrag Nr.: 44.273.385 von quepos am 19.03.13 20:25:45Die Technologie ist sicherlich eine der besten. So viele im Bereich antibody conjugat gibt es ja nicht. Interessante Übericht:

Ich kenne zwar die Konditionen nicht, zu denen die ihre Lizenzen vergeben, jedoch scheinen sie in dem Bereich Marktführer und sehr breit aufgestellt.
Interessant auch:
http://www.orf-blog.com/major-trends-in-cancer-drug-developm…

Ich kenne zwar die Konditionen nicht, zu denen die ihre Lizenzen vergeben, jedoch scheinen sie in dem Bereich Marktführer und sehr breit aufgestellt.
Interessant auch:
http://www.orf-blog.com/major-trends-in-cancer-drug-developm…
Ich schau mir gerade den Q1-Bericht an.
Die Umsätze mit Adcetris entwickeln sich wirklich enttäuschend. In Q1 2013 wurden gerade einmal 33,9 Mio. USD umgesetzt, in Q1 2012 waren es noch 34,5 Mio. USD.
Die Umsätze mit Adcetris entwickeln sich wirklich enttäuschend. In Q1 2013 wurden gerade einmal 33,9 Mio. USD umgesetzt, in Q1 2012 waren es noch 34,5 Mio. USD.
Ernszthafte Konkurrenz für Adcetris durch den PD-1-Inhobitor nivolumab -> http://in.reuters.com/article/2014/12/06/us-bristol-myers-ly…
Antwort auf Beitrag Nr.: 48.508.694 von Joschka Schröder am 07.12.14 11:17:24Hier noch ein aktuelles Paper aus dem NEJM zum Thema -> http://www.nejm.org/doi/full/10.1056/NEJMoa1411087#t=article…
Auch Merck mit eigenem PD-1-Inhibitor pembrolizumab in der Indikation Hodgkin-Lymphom aktiv -> http://in.reuters.com/article/2014/12/06/us-merck-lymphoma-i…
Ggf. könnte man die PD-1-Inhibitoren mit SGEN´s Adcetris kombinieren. Ein Investment in SGEN scheint derzeit allerdings recht risikobehaftet, weil doch sehr unklar ist, in welchem Umfang das Adcetris-Marktpotential durch die PD-1-Inhibitoren beeinträchtigt wird. Insbesondere die Nivolumab-Ergebnisse sind überragend.
Ggf. könnte man die PD-1-Inhibitoren mit SGEN´s Adcetris kombinieren. Ein Investment in SGEN scheint derzeit allerdings recht risikobehaftet, weil doch sehr unklar ist, in welchem Umfang das Adcetris-Marktpotential durch die PD-1-Inhibitoren beeinträchtigt wird. Insbesondere die Nivolumab-Ergebnisse sind überragend.
Antwort auf Beitrag Nr.: 48.508.832 von Joschka Schröder am 07.12.14 11:47:52"Ein Investment in SGEN scheint derzeit allerdings recht risikobehaftet, weil doch sehr unklar ist, in welchem Umfang das Adcetris-Marktpotential durch die PD-1-Inhibitoren beeinträchtigt wird. Insbesondere die Nivolumab-Ergebnisse sind überragend."
Man muss die Ergebnisse aber auch relativ betrachten... die Kommentare, die ich dazu gelesen habe, sehen SGEN eher positiv. Z.B. ist die CR-Rate in Patienten, die bereits einmal Adcetris erhalten und dann rückfällig geworden sind, unter einer erneuten Behandlung mit Adcetris deutlich höher als mit den PD-1 AKs:
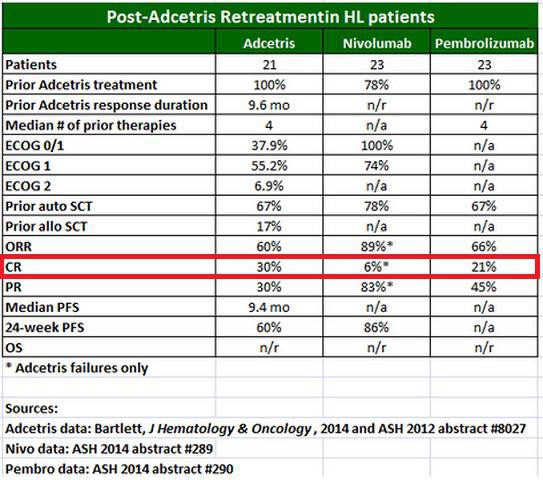
Folgende Kommentare sind auch positiv... Adcetris dürfte SOC werden in HL:
$SGEN @CMoskowitzMD on AETHERA: predict brentuximab vedotin will soon become standard for HL pts undergoing auto SCT
***
Adcetris+RCHOP data amazing. 100% ORR, 80% CR. Normally expect 30-40% ORR with RCHOP in these pts. #ASH14 $SGEN
****
Wenn ich mich richtig erinnere, gibt es auch einen Zusammenhang zwischen Adcetris und den Checkpoint-Hemmern... Adcetris eleminiert in HL über CD30 Tumorzellen, die auch PD-1 expressionieren, was zu langanhaltenden Wirkungen führen kann. Vielleicht verwechsel ich hier auch etwas, im Moment kann ich keine Quelle dafür finden.
Jedenfalls sehen die Langzeit-Daten auch positiv aus...
$SGEN Estimated Four-Year Survival Rate of 64 Percent-Half of Patients who Achieved Complete Remission Remain Disease-free
Mit 9p24.1 könnte es einen Biomarker für PD-1 AKs und JAK2-Hemmer geben... http://www.epvantage.com/Universal/View.aspx?type=Story&id=5… Diese Mutation tritt im wesentlichen in HL auf.
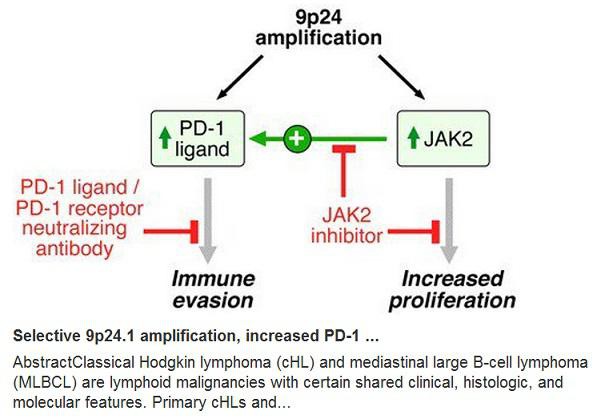
Adcetris in HL ist aber wahrscheinlich schon weitgehend eingepreist in den Kursen.
Gruß
ipollit
Man muss die Ergebnisse aber auch relativ betrachten... die Kommentare, die ich dazu gelesen habe, sehen SGEN eher positiv. Z.B. ist die CR-Rate in Patienten, die bereits einmal Adcetris erhalten und dann rückfällig geworden sind, unter einer erneuten Behandlung mit Adcetris deutlich höher als mit den PD-1 AKs:

Folgende Kommentare sind auch positiv... Adcetris dürfte SOC werden in HL:
$SGEN @CMoskowitzMD on AETHERA: predict brentuximab vedotin will soon become standard for HL pts undergoing auto SCT
***
Adcetris+RCHOP data amazing. 100% ORR, 80% CR. Normally expect 30-40% ORR with RCHOP in these pts. #ASH14 $SGEN
****
Wenn ich mich richtig erinnere, gibt es auch einen Zusammenhang zwischen Adcetris und den Checkpoint-Hemmern... Adcetris eleminiert in HL über CD30 Tumorzellen, die auch PD-1 expressionieren, was zu langanhaltenden Wirkungen führen kann. Vielleicht verwechsel ich hier auch etwas, im Moment kann ich keine Quelle dafür finden.
Jedenfalls sehen die Langzeit-Daten auch positiv aus...
$SGEN Estimated Four-Year Survival Rate of 64 Percent-Half of Patients who Achieved Complete Remission Remain Disease-free
Mit 9p24.1 könnte es einen Biomarker für PD-1 AKs und JAK2-Hemmer geben... http://www.epvantage.com/Universal/View.aspx?type=Story&id=5… Diese Mutation tritt im wesentlichen in HL auf.

Adcetris in HL ist aber wahrscheinlich schon weitgehend eingepreist in den Kursen.
Gruß
ipollit
Antwort auf Beitrag Nr.: 48.511.043 von ipollit am 07.12.14 22:02:50Vielen Dank für das informative Posting. Kannst Du mir bitte noch verraten, woher die interessante Vergleichstabelle stammt?
Antwort auf Beitrag Nr.: 48.511.043 von ipollit am 07.12.14 22:02:50Die EOCG-Daten in der Tabelle scheinen nicht ganz konsistent. Wie interpretierst Du sie? Beim Nivolumab-Kollektiv umfasst die ECOG 0/1-Gruppe auch die ECOG 1-Fälle, bei Adcetris hingegen sind die Kollektive getrennt? Ist für die Interpretation der Daten (-> Schwere der Krankheitsfälle) nicht ganz irrelevant.
Antwort auf Beitrag Nr.: 48.511.361 von Joschka Schröder am 08.12.14 01:17:22die Tabelle wurde über Twitter gepostet...
https://twitter.com/AlpineBV_Miller/status/54197853483591270…
https://twitter.com/search?q=%24sgen&src=typd
von David Miller... https://twitter.com/AlpineBV_Miller/with_replies
Gruß
ipollit
https://twitter.com/AlpineBV_Miller/status/54197853483591270…
https://twitter.com/search?q=%24sgen&src=typd
von David Miller... https://twitter.com/AlpineBV_Miller/with_replies
Gruß
ipollit
Antwort auf Beitrag Nr.: 48.519.833 von ipollit am 08.12.14 22:07:55Vielen Dank, ich hatte die Quelle zwischenzeitlich gefunden.
Antwort auf Beitrag Nr.: 48.508.832 von Joschka Schröder am 07.12.14 11:47:52Die in der vergangenen Nacht publizierten Daten zu SGN-CD33A sind hervorragend. Nach Mylotarg hatte damit wohl kaum noch jemand gerechnet. Eigentlich müßte der SGEN-Kurs heute während der US-Börsensitzung deutlich anziehen.
SAN FRANCISCO--(BUSINESS WIRE)--Dec. 8, 2014-- Seattle Genetics, Inc. (Nasdaq:SGEN) today presented data from an ongoing phase 1 clinical trial evaluating SGN-CD33A, an antibody-drug conjugate (ADC) in development for the treatment of acute myeloid leukemia (AML), at the 56th American Society of Hematology (ASH) Annual Meeting and Exposition taking place in San Francisco, CA December 6-9, 2014. SGN-CD33A is a novel ADC targeted to CD33 utilizing Seattle Genetics’ newest technology. CD33 is expressed on most AML cells regardless of subtype, cytogenetic abnormality or underlying mutational heterogeneity. SGN-CD33A is the first ADC utilizing an engineered cysteine antibody (EC-mAb) and pyrrolobenzodiazepine (PBD) dimer to be advanced into the clinic.
“There is a significant need for improved treatment options for acute myeloid leukemia where patient outcomes and overall survival have not meaningfully changed in the past three decades, particularly for patients unable to undergo intensive chemotherapy,” said Jonathan Drachman, M.D., Chief Medical Officer and Executive Vice President, Research and Development at Seattle Genetics. “The data presented at ASH on SGN-CD33A demonstrate encouraging anti-leukemic activity with a manageable safety profile in primarily elderly, relapsed patients who were not candidates for other therapies. We are pleased to see a high percentage of patients with marked and rapid reduction in bone marrow blasts and low rates of off-target toxicity and 30-day mortality, in addition to observing complete remissions or complete remissions with incomplete recovery across multiple dose levels. We continue to evaluate SGN-CD33A in this trial, and plan to broaden the clinical program to include a phase 1b trial in combination with standard frontline induction and consolidation agents for AML in the near future.”
“From my experience in treating acute myeloid leukemia, it is notable to see a single agent treatment resulting in bone marrows with no detectable blasts, even by highly sensitive flow cytometry,” said Eytan Stein, M.D., Memorial Sloan Kettering Cancer Center. “The SGN-CD33A data presented today are encouraging and show single agent anti-leukemic activity evidenced by a bone marrow blast clearance in 44 percent of patients. This appears to be a highly active agent with a manageable safety profile, and I look forward to evaluating it further as a potential treatment for acute myeloid leukemia.”
Interim Analysis of a Phase 1 Trial of SGN-CD33A in Patients with CD33-Positive Acute Myeloid Leukemia (AML) (Abstract #623, oral presentation at 5:30 p.m. PT on Monday, December 8, 2014 at the Moscone Center South Building, Gateway Ballroom 103)
Data were reported from 56 evaluable AML patients with a median age of 75 years and predominantly intermediate or adverse cytogenetic risk. Of the 56 patients, 43 percent had received intensive therapy and 57 percent had declined intensive therapy. More than 50 percent of patients had evidence of underlying myelodysplasia.
The primary endpoints of the trial are the estimation of the maximum tolerated dose and evaluation of the safety of SGN-CD33A. In addition, the trial is evaluating anti-leukemic activity, pharmacokinetics, progression-free survival and overall survival in patients with CD33-positive AML.
Key findings presented by Dr. Stein include:
Of the 52 evaluable patients treated across all dose levels, the best clinical response by investigator included 11 patients (21 percent) with a complete remission or complete remission with incomplete recovery (CR/CRi). An additional 12 patients (23 percent) achieved a morphologic leukemia free state.
Data suggest an emerging dose-response relationship with rapid and marked decreases in bone marrow blasts. Of the 17 response-evaluable patients treated at 40 micrograms per kilogram (mcg/kg), five patients (29 percent) achieved a CR/CRi. At last follow-up, 12 of 18 patients (67 percent) treated at this dose level remained alive. Ten patients treated at this dose level were elderly and had declined intensive therapy, of which four patients (40 percent) achieved CR/CRi.
Among patients treated at the 40 mcg/kg dose level or higher, 77 percent (17 of 22) had a 50 percent or more reduction in bone marrow blasts.
For CR/CRi patients, median time to neutrophil count recovery was 6.7 weeks and median time to platelet count recovery was 12 weeks.
The most common treatment-related adverse events of any grade occurring in 15 percent or more of patients were febrile neutropenia (32 percent), fatigue (20 percent) and low blood platelet count (16 percent); the most common post-baseline Grade 3 or 4 laboratory abnormalities were low blood neutrophil count (98 percent), low blood leukocyte count (97 percent) and low blood platelet count (87 percent).
The 30-day mortality rate was two percent, with no treatment-related deaths occurring during that time.
The maximum tolerated dose (MTD) has not been reached and dose exploration is continuing, including combination cohorts with hypomethylating agents.
The SGN-CD33A phase 1 clinical trial is ongoing to define an optimal monotherapy dose and schedule for future trials. More information, including enrolling centers, is available by visiting www.clinicaltrials.gov.
SGN-CD33A in Combination with Cytarabine or Hypomethylating Agents Demonstrate Enhanced Anti-leukemic Activity in Preclinical Models of AML (Abstract #3739, poster presentation at 6:00 p.m. PT on Monday, December 8, 2014 at the Moscone Center North Building, Hall E)
A preclinical analysis evaluated the activity of SGN-CD33A in combination with therapies commonly used to treat AML, including cytarabine and hypomethylating agents, in multi-drug resistant AML models. Data presented in a poster presentation by May Sutherland, Ph.D., Seattle Genetics, demonstrated enhanced anti-leukemic activity observed with the combination of SGN-CD33A and cytarabine or hypomethylating agents as well as synergistic mechanisms of action and anti-tumor activity. In addition, use of hypomethylating agents increased CD33 levels on AML cells.
About SGN-CD33A
SGN-CD33A is comprised of three parts: A cysteine-engineered anti-CD33 antibody enabling uniform site-specific conjugation, a cleavable dipeptide linker that is highly stable in circulation, and a PBD dimer that binds DNA with high intrinsic affinity. PBD dimers are a class of DNA-crosslinking agents significantly more potent than systemic chemotherapeutic drugs. Seattle Genetics has been working with PBDs since 2009 under an exclusive research and licensing arrangement with Spirogen Ltd. Over the past four years, Seattle Genetics has selected and optimized specific PBD molecules for its proprietary use in ADCs. In addition, SGN-CD33A employs a novel linker system and proprietary, site-specific conjugation technology (EC-mAb) that allows uniform drug-loading of the cell-killing PBD agent to the anti-CD33 antibody. The ADC is designed to be stable in the bloodstream and to release its cytotoxic agent upon internalization into CD33-expressing cells.
About Acute Myeloid Leukemia
Acute myeloid leukemia, also called acute myelocytic leukemia or AML, is an aggressive type of cancer of the bone marrow and blood that progresses rapidly without treatment. AML is a cancer that starts in the cells that are supposed to mature into different types of blood cells. AML starts in the bone marrow (the soft inner part of the bones, where new blood cells are made) and quickly moves into the blood. According to the American Cancer Society, in 2014 more than 18,500 new cases of AML (mostly in adults) will be diagnosed and nearly 10,500 deaths will occur from AML (almost all will be in adults).
SAN FRANCISCO--(BUSINESS WIRE)--Dec. 8, 2014-- Seattle Genetics, Inc. (Nasdaq:SGEN) today presented data from an ongoing phase 1 clinical trial evaluating SGN-CD33A, an antibody-drug conjugate (ADC) in development for the treatment of acute myeloid leukemia (AML), at the 56th American Society of Hematology (ASH) Annual Meeting and Exposition taking place in San Francisco, CA December 6-9, 2014. SGN-CD33A is a novel ADC targeted to CD33 utilizing Seattle Genetics’ newest technology. CD33 is expressed on most AML cells regardless of subtype, cytogenetic abnormality or underlying mutational heterogeneity. SGN-CD33A is the first ADC utilizing an engineered cysteine antibody (EC-mAb) and pyrrolobenzodiazepine (PBD) dimer to be advanced into the clinic.
“There is a significant need for improved treatment options for acute myeloid leukemia where patient outcomes and overall survival have not meaningfully changed in the past three decades, particularly for patients unable to undergo intensive chemotherapy,” said Jonathan Drachman, M.D., Chief Medical Officer and Executive Vice President, Research and Development at Seattle Genetics. “The data presented at ASH on SGN-CD33A demonstrate encouraging anti-leukemic activity with a manageable safety profile in primarily elderly, relapsed patients who were not candidates for other therapies. We are pleased to see a high percentage of patients with marked and rapid reduction in bone marrow blasts and low rates of off-target toxicity and 30-day mortality, in addition to observing complete remissions or complete remissions with incomplete recovery across multiple dose levels. We continue to evaluate SGN-CD33A in this trial, and plan to broaden the clinical program to include a phase 1b trial in combination with standard frontline induction and consolidation agents for AML in the near future.”
“From my experience in treating acute myeloid leukemia, it is notable to see a single agent treatment resulting in bone marrows with no detectable blasts, even by highly sensitive flow cytometry,” said Eytan Stein, M.D., Memorial Sloan Kettering Cancer Center. “The SGN-CD33A data presented today are encouraging and show single agent anti-leukemic activity evidenced by a bone marrow blast clearance in 44 percent of patients. This appears to be a highly active agent with a manageable safety profile, and I look forward to evaluating it further as a potential treatment for acute myeloid leukemia.”
Interim Analysis of a Phase 1 Trial of SGN-CD33A in Patients with CD33-Positive Acute Myeloid Leukemia (AML) (Abstract #623, oral presentation at 5:30 p.m. PT on Monday, December 8, 2014 at the Moscone Center South Building, Gateway Ballroom 103)
Data were reported from 56 evaluable AML patients with a median age of 75 years and predominantly intermediate or adverse cytogenetic risk. Of the 56 patients, 43 percent had received intensive therapy and 57 percent had declined intensive therapy. More than 50 percent of patients had evidence of underlying myelodysplasia.
The primary endpoints of the trial are the estimation of the maximum tolerated dose and evaluation of the safety of SGN-CD33A. In addition, the trial is evaluating anti-leukemic activity, pharmacokinetics, progression-free survival and overall survival in patients with CD33-positive AML.
Key findings presented by Dr. Stein include:
Of the 52 evaluable patients treated across all dose levels, the best clinical response by investigator included 11 patients (21 percent) with a complete remission or complete remission with incomplete recovery (CR/CRi). An additional 12 patients (23 percent) achieved a morphologic leukemia free state.
Data suggest an emerging dose-response relationship with rapid and marked decreases in bone marrow blasts. Of the 17 response-evaluable patients treated at 40 micrograms per kilogram (mcg/kg), five patients (29 percent) achieved a CR/CRi. At last follow-up, 12 of 18 patients (67 percent) treated at this dose level remained alive. Ten patients treated at this dose level were elderly and had declined intensive therapy, of which four patients (40 percent) achieved CR/CRi.
Among patients treated at the 40 mcg/kg dose level or higher, 77 percent (17 of 22) had a 50 percent or more reduction in bone marrow blasts.
For CR/CRi patients, median time to neutrophil count recovery was 6.7 weeks and median time to platelet count recovery was 12 weeks.
The most common treatment-related adverse events of any grade occurring in 15 percent or more of patients were febrile neutropenia (32 percent), fatigue (20 percent) and low blood platelet count (16 percent); the most common post-baseline Grade 3 or 4 laboratory abnormalities were low blood neutrophil count (98 percent), low blood leukocyte count (97 percent) and low blood platelet count (87 percent).
The 30-day mortality rate was two percent, with no treatment-related deaths occurring during that time.
The maximum tolerated dose (MTD) has not been reached and dose exploration is continuing, including combination cohorts with hypomethylating agents.
The SGN-CD33A phase 1 clinical trial is ongoing to define an optimal monotherapy dose and schedule for future trials. More information, including enrolling centers, is available by visiting www.clinicaltrials.gov.
SGN-CD33A in Combination with Cytarabine or Hypomethylating Agents Demonstrate Enhanced Anti-leukemic Activity in Preclinical Models of AML (Abstract #3739, poster presentation at 6:00 p.m. PT on Monday, December 8, 2014 at the Moscone Center North Building, Hall E)
A preclinical analysis evaluated the activity of SGN-CD33A in combination with therapies commonly used to treat AML, including cytarabine and hypomethylating agents, in multi-drug resistant AML models. Data presented in a poster presentation by May Sutherland, Ph.D., Seattle Genetics, demonstrated enhanced anti-leukemic activity observed with the combination of SGN-CD33A and cytarabine or hypomethylating agents as well as synergistic mechanisms of action and anti-tumor activity. In addition, use of hypomethylating agents increased CD33 levels on AML cells.
About SGN-CD33A
SGN-CD33A is comprised of three parts: A cysteine-engineered anti-CD33 antibody enabling uniform site-specific conjugation, a cleavable dipeptide linker that is highly stable in circulation, and a PBD dimer that binds DNA with high intrinsic affinity. PBD dimers are a class of DNA-crosslinking agents significantly more potent than systemic chemotherapeutic drugs. Seattle Genetics has been working with PBDs since 2009 under an exclusive research and licensing arrangement with Spirogen Ltd. Over the past four years, Seattle Genetics has selected and optimized specific PBD molecules for its proprietary use in ADCs. In addition, SGN-CD33A employs a novel linker system and proprietary, site-specific conjugation technology (EC-mAb) that allows uniform drug-loading of the cell-killing PBD agent to the anti-CD33 antibody. The ADC is designed to be stable in the bloodstream and to release its cytotoxic agent upon internalization into CD33-expressing cells.
About Acute Myeloid Leukemia
Acute myeloid leukemia, also called acute myelocytic leukemia or AML, is an aggressive type of cancer of the bone marrow and blood that progresses rapidly without treatment. AML is a cancer that starts in the cells that are supposed to mature into different types of blood cells. AML starts in the bone marrow (the soft inner part of the bones, where new blood cells are made) and quickly moves into the blood. According to the American Cancer Society, in 2014 more than 18,500 new cases of AML (mostly in adults) will be diagnosed and nearly 10,500 deaths will occur from AML (almost all will be in adults).
Antwort auf Beitrag Nr.: 38.163.840 von quepos am 13.10.09 08:43:54Gratulation für den tollen Riecher, quepos!
Das Interesse an SGEN scheint allerdings inzwischen recht mau zu sein. Zu Unrecht wie ich finde...!
Das Interesse an SGEN scheint allerdings inzwischen recht mau zu sein. Zu Unrecht wie ich finde...!
Wieder über USD 50... sehr schöne Kursentwicklung!
Antwort auf Beitrag Nr.: 53.237.688 von Ville7 am 08.09.16 17:20:24Noch jemand investiert?
Wird dieses Jahr spannend, insbesondere die bladder cancer programme mit Stellas werden hoffentlich erfolgsvorsprechend sein.
Wird dieses Jahr spannend, insbesondere die bladder cancer programme mit Stellas werden hoffentlich erfolgsvorsprechend sein.
Antwort auf Beitrag Nr.: 55.136.951 von biopadawan am 14.06.17 11:14:38Takeda and Seattle Genetics Announce Positive Results from Phase 3 ECHELON-1 Clinical Trial Evaluating ADCETRIS® (brentuximab vedotin) in Frontline Advanced Hodgkin Lymphoma
-Randomized Phase 3 Clinical Trial with ADCETRIS Met Primary Endpoint, Demonstrating a Statistically Significant Improvement in Modified Progression-Free Survival-
-Abstract to be Submitted for Presentation at the 2017 ASH Annual Meeting; Regulatory Submissions Planned-
-Seattle Genetics to Host Conference Call and Webcast Today at 8:30 a.m. ET-
CAMBRIDGE, Mass., OSAKA, Japan & BOTHELL, Wash.--(BUSINESS WIRE)--Jun. 26, 2017-- Takeda Pharmaceutical Company Limited (TSE:4502) and Seattle Genetics, Inc. (NASDAQ: SGEN) today announced that the Phase 3 ECHELON-1 clinical trial met its primary endpoint of a statistically significant improvement in modified progression-free survival (PFS) versus the control arm. ECHELON-1 is a randomized, multicenter trial evaluating ADCETRIS (brentuximab vedotin) as part of a frontline combination chemotherapy regimen in 1,334 patients with previously untreated advanced classical Hodgkin lymphoma. ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of classical Hodgkin lymphoma. ADCETRIS is currently not approved as a frontline therapy for Hodgkin lymphoma.
This Smart News Release features multimedia. View the full release here: http://www.businesswire.com/news/home/20170626005341/en/
Patients in ECHELON-1 were randomized to receive either a combination of ADCETRIS+AVD (Adriamycin, vinblastine, dacarbazine) or ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine), a recognized standard of care for frontline Hodgkin lymphoma. The results of the ECHELON-1 trial demonstrated that combination treatment with ADCETRIS resulted in a statistically significant improvement in modified PFS versus the control arm as assessed by an Independent Review Facility (hazard ratio=0.770; p-value=0.035). The two-year modified PFS rate for patients in the ADCETRIS arm was 82.1 percent compared to 77.2 percent in the control arm. Interim analysis of overall survival (OS), the key secondary endpoint, also trended in favor of the ADCETRIS+AVD arm. An abstract will be submitted for data presentation at the American Society of Hematology (ASH) annual meeting, December 9-12, 2017, in Atlanta, Ga.
The safety profile of ADCETRIS+AVD in the ECHELON-1 trial was consistent with that known for the single-agent components of the regimen. There was an increased incidence of febrile neutropenia and peripheral neuropathy in the ADCETRIS+AVD arm. Febrile neutropenia was reduced through the use of prophylactic growth factors in a subset of patients, and peripheral neuropathy was managed through dose modifications. The control arm had an increased rate and severity of pulmonary toxicity.
“We are excited about the positive result which shows a statistically significant improvement in the primary endpoint of modified PFS,” said Dirk Huebner, M.D., Executive Medical Director, Oncology Therapeutic Area Unit, Takeda Pharmaceutical Company. “The results of this trial signify an important step forward in the development of ADCETRIS and have the potential to change the treatment approach of frontline advanced Hodgkin lymphoma.”
“The outcome of the Phase 3 ECHELON-1 trial represents a significant milestone for the Hodgkin lymphoma community,” said Clay Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “Seattle Genetics’ goal is to establish ADCETRIS as the foundation of care for CD30-expressing lymphomas, including Hodgkin lymphoma. Notably, this is the first clinical trial in frontline advanced Hodgkin lymphoma to show superior efficacy of a regimen that eliminates bleomycin.”
Takeda and Seattle Genetics plan to submit these results to regulatory authorities for approval in their respective territories.
-Randomized Phase 3 Clinical Trial with ADCETRIS Met Primary Endpoint, Demonstrating a Statistically Significant Improvement in Modified Progression-Free Survival-
-Abstract to be Submitted for Presentation at the 2017 ASH Annual Meeting; Regulatory Submissions Planned-
-Seattle Genetics to Host Conference Call and Webcast Today at 8:30 a.m. ET-
CAMBRIDGE, Mass., OSAKA, Japan & BOTHELL, Wash.--(BUSINESS WIRE)--Jun. 26, 2017-- Takeda Pharmaceutical Company Limited (TSE:4502) and Seattle Genetics, Inc. (NASDAQ: SGEN) today announced that the Phase 3 ECHELON-1 clinical trial met its primary endpoint of a statistically significant improvement in modified progression-free survival (PFS) versus the control arm. ECHELON-1 is a randomized, multicenter trial evaluating ADCETRIS (brentuximab vedotin) as part of a frontline combination chemotherapy regimen in 1,334 patients with previously untreated advanced classical Hodgkin lymphoma. ADCETRIS is an antibody-drug conjugate (ADC) directed to CD30, a defining marker of classical Hodgkin lymphoma. ADCETRIS is currently not approved as a frontline therapy for Hodgkin lymphoma.
This Smart News Release features multimedia. View the full release here: http://www.businesswire.com/news/home/20170626005341/en/
Patients in ECHELON-1 were randomized to receive either a combination of ADCETRIS+AVD (Adriamycin, vinblastine, dacarbazine) or ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine), a recognized standard of care for frontline Hodgkin lymphoma. The results of the ECHELON-1 trial demonstrated that combination treatment with ADCETRIS resulted in a statistically significant improvement in modified PFS versus the control arm as assessed by an Independent Review Facility (hazard ratio=0.770; p-value=0.035). The two-year modified PFS rate for patients in the ADCETRIS arm was 82.1 percent compared to 77.2 percent in the control arm. Interim analysis of overall survival (OS), the key secondary endpoint, also trended in favor of the ADCETRIS+AVD arm. An abstract will be submitted for data presentation at the American Society of Hematology (ASH) annual meeting, December 9-12, 2017, in Atlanta, Ga.
The safety profile of ADCETRIS+AVD in the ECHELON-1 trial was consistent with that known for the single-agent components of the regimen. There was an increased incidence of febrile neutropenia and peripheral neuropathy in the ADCETRIS+AVD arm. Febrile neutropenia was reduced through the use of prophylactic growth factors in a subset of patients, and peripheral neuropathy was managed through dose modifications. The control arm had an increased rate and severity of pulmonary toxicity.
“We are excited about the positive result which shows a statistically significant improvement in the primary endpoint of modified PFS,” said Dirk Huebner, M.D., Executive Medical Director, Oncology Therapeutic Area Unit, Takeda Pharmaceutical Company. “The results of this trial signify an important step forward in the development of ADCETRIS and have the potential to change the treatment approach of frontline advanced Hodgkin lymphoma.”
“The outcome of the Phase 3 ECHELON-1 trial represents a significant milestone for the Hodgkin lymphoma community,” said Clay Siegall, Ph.D., President and Chief Executive Officer of Seattle Genetics. “Seattle Genetics’ goal is to establish ADCETRIS as the foundation of care for CD30-expressing lymphomas, including Hodgkin lymphoma. Notably, this is the first clinical trial in frontline advanced Hodgkin lymphoma to show superior efficacy of a regimen that eliminates bleomycin.”
Takeda and Seattle Genetics plan to submit these results to regulatory authorities for approval in their respective territories.
Antwort auf Beitrag Nr.: 55.222.919 von biopadawan am 28.06.17 16:51:19Obige Ergebnisse haben zu einem Bewertungsabschlag in Höhe von ca. 15-20% geführt.
Fraglich ist, ob dies nicht zu viel des Guten war.
Ich für meinen Fall habe nachgekauft. Ich rechne fest mit einer Zulassung sowie guten Marktakzeptanz auch wenn die Ergebnisse nicht wie gewünscht "bahnbrechend" waren.
Schade zudem, dass das Unternehmen in diesem Forum keine Beachtung findet. Ist sonst keiner mehr investiert?
Viele Grüße
Fraglich ist, ob dies nicht zu viel des Guten war.
Ich für meinen Fall habe nachgekauft. Ich rechne fest mit einer Zulassung sowie guten Marktakzeptanz auch wenn die Ergebnisse nicht wie gewünscht "bahnbrechend" waren.
Schade zudem, dass das Unternehmen in diesem Forum keine Beachtung findet. Ist sonst keiner mehr investiert?
Viele Grüße
Antwort auf Beitrag Nr.: 55.363.799 von biopadawan am 20.07.17 16:22:03Bin da auch weiterhin drin...
Antwort auf Beitrag Nr.: 55.364.060 von Ville7 am 20.07.17 16:45:17FDA Accepts Supplemental Biologics License Application and Grants Priority Review for ADCETRIS® (Brentuximab Vedotin) in Cutaneous T-Cell Lymphoma
-Supplemental BLA Based on Clinical Trial Results from the Phase 3 ALCANZA and Phase 2 Investigator-Sponsored Studies in CTCL-
-FDA Previously Granted ADCETRIS Breakthrough Therapy Designation in CTCL-
-PDUFA Action Date is December 16, 2017-
BOTHELL, Wash.--(BUSINESS WIRE)--Aug. 16, 2017-- Seattle Genetics, Inc. (Nasdaq: SGEN) announced today that the U.S. Food and Drug Administration (FDA) has accepted for filing a supplemental Biologics License Application (BLA) based on data from the phase 3 ALCANZA trial and two phase 2 investigator-sponsored trials of ADCETRIS (brentuximab vedotin) in patients with cutaneous T-cell lymphoma (CTCL). In November 2016, the FDA granted ADCETRIS Breakthrough Therapy Designation (BTD) for the treatment of patients with CD30-expressing mycosis fungoides and primary cutaneous anaplastic large cell lymphoma who require systemic therapy and have received one prior systemic therapy. These represent the most common subtypes of CTCL. The FDA granted Priority Review for the application and the Prescription Drug User Fee Act (PDUFA) target action date is December 16, 2017. ADCETRIS is currently not approved for the treatment of CTCL.
“The FDA’s filing of our supplemental BLA with Priority Review status represents a significant milestone towards our goal of making ADCETRIS available to CTCL patients who require systemic therapy,” said Jonathan Drachman, M.D., Chief Medical Officer and Executive Vice President, Research and Development of Seattle Genetics. “Results from our positive phase 3 ALCANZA trial demonstrated that using ADCETRIS in this setting significantly improved the rate of objective responses lasting at least four months with a manageable safety profile. Data from two investigator-sponsored trials in a broader patient population were included in the submission to further support ADCETRIS use in this disease setting. We look forward to working with the FDA during the review of our application for ADCETRIS in CTCL, which, if approved, would be the fourth indication for this product.”
The supplemental BLA is primarily based on positive results from a phase 3 trial called ALCANZA that were presented at the 58th American Society of Hematology (ASH) annual meeting in December 2016 and published online in the Lancet in June 2017. Results from the ALCANZA trial in 128 CTCL patients requiring systemic therapy included:
The trial achieved its primary endpoint with the ADCETRIS treatment arm demonstrating a highly statistically significant improvement in the rate of objective response lasting at least four months (ORR4) versus the control arm as assessed by an independent review facility. ORR4, as assessed by Global Response Score, was 56.3 percent in the ADCETRIS arm compared to 12.5 percent in the control arm (p-value <0.0001).
Key secondary endpoints specified in the protocol, including complete response rate, progression-free survival and reduction in the burden of symptoms during treatment (Skindex-29), were all highly statistically significant in favor of the ADCETRIS arm.
The safety profile associated with ADCETRIS from the ALCANZA trial was generally consistent with the existing prescribing information. The most common adverse events of any grade include: anemia, peripheral sensory neuropathy, nausea, diarrhea, fatigue and neutropenia.
Based on discussions with the FDA, additional data from two investigator-sponsored phase 2 trials have also been incorporated into the supplemental BLA to support the potential for a broader label in CTCL.
-Supplemental BLA Based on Clinical Trial Results from the Phase 3 ALCANZA and Phase 2 Investigator-Sponsored Studies in CTCL-
-FDA Previously Granted ADCETRIS Breakthrough Therapy Designation in CTCL-
-PDUFA Action Date is December 16, 2017-
BOTHELL, Wash.--(BUSINESS WIRE)--Aug. 16, 2017-- Seattle Genetics, Inc. (Nasdaq: SGEN) announced today that the U.S. Food and Drug Administration (FDA) has accepted for filing a supplemental Biologics License Application (BLA) based on data from the phase 3 ALCANZA trial and two phase 2 investigator-sponsored trials of ADCETRIS (brentuximab vedotin) in patients with cutaneous T-cell lymphoma (CTCL). In November 2016, the FDA granted ADCETRIS Breakthrough Therapy Designation (BTD) for the treatment of patients with CD30-expressing mycosis fungoides and primary cutaneous anaplastic large cell lymphoma who require systemic therapy and have received one prior systemic therapy. These represent the most common subtypes of CTCL. The FDA granted Priority Review for the application and the Prescription Drug User Fee Act (PDUFA) target action date is December 16, 2017. ADCETRIS is currently not approved for the treatment of CTCL.
“The FDA’s filing of our supplemental BLA with Priority Review status represents a significant milestone towards our goal of making ADCETRIS available to CTCL patients who require systemic therapy,” said Jonathan Drachman, M.D., Chief Medical Officer and Executive Vice President, Research and Development of Seattle Genetics. “Results from our positive phase 3 ALCANZA trial demonstrated that using ADCETRIS in this setting significantly improved the rate of objective responses lasting at least four months with a manageable safety profile. Data from two investigator-sponsored trials in a broader patient population were included in the submission to further support ADCETRIS use in this disease setting. We look forward to working with the FDA during the review of our application for ADCETRIS in CTCL, which, if approved, would be the fourth indication for this product.”
The supplemental BLA is primarily based on positive results from a phase 3 trial called ALCANZA that were presented at the 58th American Society of Hematology (ASH) annual meeting in December 2016 and published online in the Lancet in June 2017. Results from the ALCANZA trial in 128 CTCL patients requiring systemic therapy included:
The trial achieved its primary endpoint with the ADCETRIS treatment arm demonstrating a highly statistically significant improvement in the rate of objective response lasting at least four months (ORR4) versus the control arm as assessed by an independent review facility. ORR4, as assessed by Global Response Score, was 56.3 percent in the ADCETRIS arm compared to 12.5 percent in the control arm (p-value <0.0001).
Key secondary endpoints specified in the protocol, including complete response rate, progression-free survival and reduction in the burden of symptoms during treatment (Skindex-29), were all highly statistically significant in favor of the ADCETRIS arm.
The safety profile associated with ADCETRIS from the ALCANZA trial was generally consistent with the existing prescribing information. The most common adverse events of any grade include: anemia, peripheral sensory neuropathy, nausea, diarrhea, fatigue and neutropenia.
Based on discussions with the FDA, additional data from two investigator-sponsored phase 2 trials have also been incorporated into the supplemental BLA to support the potential for a broader label in CTCL.
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +0,71 | |
| -0,26 | |
| +1,11 | |
| +0,41 | |
| +1,71 | |
| +0,61 | |
| +3,99 | |
| +6,09 | |
| +2,38 | |
| +4,24 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 216 | ||
| 75 | ||
| 71 | ||
| 62 | ||
| 57 | ||
| 49 | ||
| 37 | ||
| 37 | ||
| 32 | ||
| 24 |
Seattle Genetics (WKN 602322): Chance zum Einstieg?





















