Cel-Sci Corp. (CVM; 871006) - 500 Beiträge pro Seite (Seite 6)
eröffnet am 30.10.03 18:31:27 von
neuester Beitrag 11.02.15 00:07:02 von
neuester Beitrag 11.02.15 00:07:02 von
Beiträge: 7.656
ID: 791.230
ID: 791.230
Aufrufe heute: 5
Gesamt: 442.884
Gesamt: 442.884
Aktive User: 0
Top-Diskussionen
| Titel | letzter Beitrag | Aufrufe |
|---|---|---|
| vor 42 Minuten | 4407 | |
| vor 33 Minuten | 4043 | |
| heute 16:01 | 3915 | |
| vor 1 Stunde | 2051 | |
| vor 32 Minuten | 1883 | |
| vor 1 Stunde | 1625 | |
| vor 1 Stunde | 1478 | |
| heute 13:04 | 1416 |
Meistdiskutierte Wertpapiere
| Platz | vorher | Wertpapier | Kurs | Perf. % | Anzahl | ||
|---|---|---|---|---|---|---|---|
| 1. | 1. | 18.726,81 | -0,10 | 99 | |||
| 2. | 3. | 167,24 | +4,11 | 53 | |||
| 3. | 5. | 6,3580 | -1,85 | 52 | |||
| 4. | 10. | 10,240 | -5,01 | 42 | |||
| 5. | 11. | 5,6500 | -0,74 | 29 | |||
| 6. | 7. | 13,372 | +2,48 | 28 | |||
| 7. | 36. | 70.168,90 | +2,84 | 28 | |||
| 8. | Neu! | 97,65 | -11,31 | 26 |
http://www.tradingmarkets.com/.site/news/Stock%20News/179925…
*Revised* Analyst Reports for Elixir Gaming Technologies Inc., CEL-SCI Corporation, 3PAR Inc., and Denison Mines Corp.
Friday, August 01, 2008; Posted: 11:13 AM
*Revised* Analyst Reports for Elixir Gaming Technologies Inc., CEL-SCI Corporation, 3PAR Inc., and Denison Mines Corp.
Friday, August 01, 2008; Posted: 11:13 AM
 hab mich mal angemeldet, finde aber keine Infos über cvm
hab mich mal angemeldet, finde aber keine Infos über cvm 
Hallo Leute,
ich halte mich ja derzeit sehr bedeckt, aber nachdem erfreulichen Kursverlauf heute ( +12% ), dachte ich, ich muss mal folgendes los werden:
Wenn ich mich bei Yahoo nicht verguckt habe, hat zuletzt am 02.10.07 ein CVM Insider verkauft. Anschließend wurde nur noch gekauft. Siehe die folgende Auflistung:
INSIDER TRANSACTIONS REPORTED - LAST TWO YEARS
Date Insider Shares Type Transaction Value*
30-Jul-08 KERSTEN GEERT R
Officer 7,400 Direct Purchase at $0.61 per share. $4,514
30-Jun-08 CIPRIANO JOHN
Officer 3,931 Direct Acquisition (Non Open Market) at $0.65 per share. $2,555
30-Jun-08 KERSTEN GEERT R
Officer 5,192 Direct Acquisition (Non Open Market) at $0.65 per share. $3,374
30-Jun-08 TALOR EYAL
Officer 3,692 Direct Acquisition (Non Open Market) at $0.65 per share. $2,399
30-Jun-08 ZIMMERMAN DANIEL H
Officer 4,045 Direct Acquisition (Non Open Market) at $0.65 per share. $2,629
30-Jun-08 PRICHEP PATRICIA B
Officer 4,270 Direct Acquisition (Non Open Market) at $0.65 per share. $2,775
8-Apr-08 KERSTEN GEERT R
Officer 4,800 Direct Purchase at $0.66 per share. $3,168
31-Mar-08 CIPRIANO JOHN
Officer 3,871 Direct Acquisition (Non Open Market) at $0.66 per share. $2,554
31-Mar-08 KERSTEN GEERT R
Officer 5,113 Direct Acquisition (Non Open Market) at $0.66 per share. $3,374
31-Mar-08 TALOR EYAL
Officer 3,636 Direct Acquisition (Non Open Market) at $0.66 per share. $2,399
31-Mar-08 ZIMMERMAN DANIEL H
Officer 3,983 Direct Acquisition (Non Open Market) at $0.66 per share. $2,628
31-Mar-08 PRICHEP PATRICIA B
Officer 4,205 Direct Acquisition (Non Open Market) at $0.66 per share. $2,775
5-Mar-08 DE CLARA MAXIMILIAN
Officer 200,000 Direct Acquisition (Non Open Market) at $0.62 per share. $124,000
5-Mar-08 ESTERHAZY ALEXANDER G
Director 100,000 Direct Acquisition (Non Open Market) at $0.62 per share. $62,000
5-Mar-08 CIPRIANO JOHN
Officer 50,000 Direct Acquisition (Non Open Market) at $0.62 per share. $31,000
5-Mar-08 YOUNG PETER R
Director 100,000 Direct Acquisition (Non Open Market) at $0.62 per share. $62,000
5-Mar-08 KINSOLVING C RICHARD
Director 100,000 Direct Acquisition (Non Open Market) at $0.62 per share. $62,000
5-Mar-08 KERSTEN GEERT R
Officer 200,000 Direct Acquisition (Non Open Market) at $0.62 per share. $124,000
5-Mar-08 TALOR EYAL
Officer 100,000 Direct Acquisition (Non Open Market) at $0.62 per share. $62,000
5-Mar-08 ZIMMERMAN DANIEL H
Officer 50,000 Direct Acquisition (Non Open Market) at $0.62 per share. $31,000
5-Mar-08 PRICHEP PATRICIA B
Officer 100,000 Direct Acquisition (Non Open Market) at $0.62 per share. $62,000
22-Jan-08 KERSTEN GEERT R
Officer 8,500 Direct Purchase at $0.45 per share. $3,825
15-Jan-08 CIPRIANO JOHN
Officer 5,200 Direct Acquisition (Non Open Market) at $0.52 per share. $2,704
15-Jan-08 KERSTEN GEERT R
Officer 12,847 Direct Acquisition (Non Open Market) at $0.52 per share. $6,680
15-Jan-08 TALOR EYAL
Officer 6,973 Direct Acquisition (Non Open Market) at $0.52 per share. $3,625
15-Jan-08 ZIMMERMAN DANIEL H
Officer 5,351 Direct Acquisition (Non Open Market) at $0.52 per share. $2,782
15-Jan-08 PRICHEP PATRICIA B
Officer 5,649 Direct Acquisition (Non Open Market) at $0.52 per share. $2,937
31-Dec-07 CIPRIANO JOHN
Officer 4,867 Direct Acquisition (Non Open Market) at $0.50 per share. $2,433
31-Dec-07 KERSTEN GEERT R
Officer 6,750 Direct Acquisition (Non Open Market) at $0.50 per share. $3,375
31-Dec-07 TALOR EYAL
Officer 4,800 Direct Acquisition (Non Open Market) at $0.50 per share. $2,400
31-Dec-07 ZIMMERMAN DANIEL H
Officer 5,008 Direct Acquisition (Non Open Market) at $0.50 per share. $2,504
31-Dec-07 PRICHEP PATRICIA B
Officer 5,287 Direct Acquisition (Non Open Market) at $0.50 per share. $2,643
17-Oct-07 CIPRIANO JOHN
Officer 3,938 Direct Acquisition (Non Open Market) at $0.61 per share. $2,402
17-Oct-07 KERSTEN GEERT R
Officer 5,532 Direct Acquisition (Non Open Market) at $0.61 per share. $3,374
17-Oct-07 TALOR EYAL
Officer 3,934 Direct Acquisition (Non Open Market) at $0.61 per share. $2,399
17-Oct-07 ZIMMERMAN DANIEL H
Officer 4,052 Direct Acquisition (Non Open Market) at $0.61 per share. $2,471
17-Oct-07 PRICHEP PATRICIA B
Officer 4,250 Direct Acquisition (Non Open Market) at $0.61 per share. $2,592
17-Oct-07 KERSTEN GEERT R
Officer 10,500 Direct Purchase at $0.63 - $0.63 per share. $6,6152
2-Oct-07 ZIMMERMAN DANIEL H
Officer 18,000 Direct Disposition (Non Open Market) at $0 per share. N/A
Zu den + 12% heute muss ich aber der fairneshalber sagen, dass wir die vieleicht gleich am Montag wieder abgeben. War ja vor einigen Tagen auch so. Oder es ist was im Busch?!
Eine CVM Übernahme auf Grund der derzeitigen Bewertung halte ich nicht mehr ausgeschlossen. Ich erinnere nur an den Kurs von Iomai, die ja von Intercell übernommen wurden. Von ca 0,55 € auf über 4 €.
Auf Grund des Potentials von Multikine halte ich eine vergleichbare Wertentwickling nicht mal für ausgeschlossen. Oder was meint Ihr??
Im übrigen habe ich mich noch weiter im Biotechbereich angagiert. Ganz viel Intercell, Medigene und natürlich ganz viel CVM.
Bis PIII / Jahresende ist es nicht mehr weit. Und, auch wenn es wie eine durchhalteparole klingt, alles wird gut! Hoffe ich zumindestens Schließlich hab ich dank dem Dollarverfall ca 30 % verlust in CVM.
mfg Plaste
ich halte mich ja derzeit sehr bedeckt, aber nachdem erfreulichen Kursverlauf heute ( +12% ), dachte ich, ich muss mal folgendes los werden:
Wenn ich mich bei Yahoo nicht verguckt habe, hat zuletzt am 02.10.07 ein CVM Insider verkauft. Anschließend wurde nur noch gekauft. Siehe die folgende Auflistung:
INSIDER TRANSACTIONS REPORTED - LAST TWO YEARS
Date Insider Shares Type Transaction Value*
30-Jul-08 KERSTEN GEERT R
Officer 7,400 Direct Purchase at $0.61 per share. $4,514
30-Jun-08 CIPRIANO JOHN
Officer 3,931 Direct Acquisition (Non Open Market) at $0.65 per share. $2,555
30-Jun-08 KERSTEN GEERT R
Officer 5,192 Direct Acquisition (Non Open Market) at $0.65 per share. $3,374
30-Jun-08 TALOR EYAL
Officer 3,692 Direct Acquisition (Non Open Market) at $0.65 per share. $2,399
30-Jun-08 ZIMMERMAN DANIEL H
Officer 4,045 Direct Acquisition (Non Open Market) at $0.65 per share. $2,629
30-Jun-08 PRICHEP PATRICIA B
Officer 4,270 Direct Acquisition (Non Open Market) at $0.65 per share. $2,775
8-Apr-08 KERSTEN GEERT R
Officer 4,800 Direct Purchase at $0.66 per share. $3,168
31-Mar-08 CIPRIANO JOHN
Officer 3,871 Direct Acquisition (Non Open Market) at $0.66 per share. $2,554
31-Mar-08 KERSTEN GEERT R
Officer 5,113 Direct Acquisition (Non Open Market) at $0.66 per share. $3,374
31-Mar-08 TALOR EYAL
Officer 3,636 Direct Acquisition (Non Open Market) at $0.66 per share. $2,399
31-Mar-08 ZIMMERMAN DANIEL H
Officer 3,983 Direct Acquisition (Non Open Market) at $0.66 per share. $2,628
31-Mar-08 PRICHEP PATRICIA B
Officer 4,205 Direct Acquisition (Non Open Market) at $0.66 per share. $2,775
5-Mar-08 DE CLARA MAXIMILIAN
Officer 200,000 Direct Acquisition (Non Open Market) at $0.62 per share. $124,000
5-Mar-08 ESTERHAZY ALEXANDER G
Director 100,000 Direct Acquisition (Non Open Market) at $0.62 per share. $62,000
5-Mar-08 CIPRIANO JOHN
Officer 50,000 Direct Acquisition (Non Open Market) at $0.62 per share. $31,000
5-Mar-08 YOUNG PETER R
Director 100,000 Direct Acquisition (Non Open Market) at $0.62 per share. $62,000
5-Mar-08 KINSOLVING C RICHARD
Director 100,000 Direct Acquisition (Non Open Market) at $0.62 per share. $62,000
5-Mar-08 KERSTEN GEERT R
Officer 200,000 Direct Acquisition (Non Open Market) at $0.62 per share. $124,000
5-Mar-08 TALOR EYAL
Officer 100,000 Direct Acquisition (Non Open Market) at $0.62 per share. $62,000
5-Mar-08 ZIMMERMAN DANIEL H
Officer 50,000 Direct Acquisition (Non Open Market) at $0.62 per share. $31,000
5-Mar-08 PRICHEP PATRICIA B
Officer 100,000 Direct Acquisition (Non Open Market) at $0.62 per share. $62,000
22-Jan-08 KERSTEN GEERT R
Officer 8,500 Direct Purchase at $0.45 per share. $3,825
15-Jan-08 CIPRIANO JOHN
Officer 5,200 Direct Acquisition (Non Open Market) at $0.52 per share. $2,704
15-Jan-08 KERSTEN GEERT R
Officer 12,847 Direct Acquisition (Non Open Market) at $0.52 per share. $6,680
15-Jan-08 TALOR EYAL
Officer 6,973 Direct Acquisition (Non Open Market) at $0.52 per share. $3,625
15-Jan-08 ZIMMERMAN DANIEL H
Officer 5,351 Direct Acquisition (Non Open Market) at $0.52 per share. $2,782
15-Jan-08 PRICHEP PATRICIA B
Officer 5,649 Direct Acquisition (Non Open Market) at $0.52 per share. $2,937
31-Dec-07 CIPRIANO JOHN
Officer 4,867 Direct Acquisition (Non Open Market) at $0.50 per share. $2,433
31-Dec-07 KERSTEN GEERT R
Officer 6,750 Direct Acquisition (Non Open Market) at $0.50 per share. $3,375
31-Dec-07 TALOR EYAL
Officer 4,800 Direct Acquisition (Non Open Market) at $0.50 per share. $2,400
31-Dec-07 ZIMMERMAN DANIEL H
Officer 5,008 Direct Acquisition (Non Open Market) at $0.50 per share. $2,504
31-Dec-07 PRICHEP PATRICIA B
Officer 5,287 Direct Acquisition (Non Open Market) at $0.50 per share. $2,643
17-Oct-07 CIPRIANO JOHN
Officer 3,938 Direct Acquisition (Non Open Market) at $0.61 per share. $2,402
17-Oct-07 KERSTEN GEERT R
Officer 5,532 Direct Acquisition (Non Open Market) at $0.61 per share. $3,374
17-Oct-07 TALOR EYAL
Officer 3,934 Direct Acquisition (Non Open Market) at $0.61 per share. $2,399
17-Oct-07 ZIMMERMAN DANIEL H
Officer 4,052 Direct Acquisition (Non Open Market) at $0.61 per share. $2,471
17-Oct-07 PRICHEP PATRICIA B
Officer 4,250 Direct Acquisition (Non Open Market) at $0.61 per share. $2,592
17-Oct-07 KERSTEN GEERT R
Officer 10,500 Direct Purchase at $0.63 - $0.63 per share. $6,6152
2-Oct-07 ZIMMERMAN DANIEL H
Officer 18,000 Direct Disposition (Non Open Market) at $0 per share. N/A
Zu den + 12% heute muss ich aber der fairneshalber sagen, dass wir die vieleicht gleich am Montag wieder abgeben. War ja vor einigen Tagen auch so. Oder es ist was im Busch?!
Eine CVM Übernahme auf Grund der derzeitigen Bewertung halte ich nicht mehr ausgeschlossen. Ich erinnere nur an den Kurs von Iomai, die ja von Intercell übernommen wurden. Von ca 0,55 € auf über 4 €.
Auf Grund des Potentials von Multikine halte ich eine vergleichbare Wertentwickling nicht mal für ausgeschlossen. Oder was meint Ihr??
Im übrigen habe ich mich noch weiter im Biotechbereich angagiert. Ganz viel Intercell, Medigene und natürlich ganz viel CVM.
Bis PIII / Jahresende ist es nicht mehr weit. Und, auch wenn es wie eine durchhalteparole klingt, alles wird gut! Hoffe ich zumindestens Schließlich hab ich dank dem Dollarverfall ca 30 % verlust in CVM.
mfg Plaste
Der Dollar kommt uns jetzt mal zu gute,sollten jetzt langsam mal wieder News´s kommen.Oder,neue Bilder von der Produktion und dann der Beginn der Phase 3.Dann rechne ich mit einem einer Kursexplosion.Potenzial zwischen 100 und 500 Prozent,meine Meinung.Bin schon über 3 Jahre hier dabei.Gruß
Antwort auf Beitrag Nr.: 34.741.326 von VFBLER am 16.08.08 09:27:09 ja, das Volumen stiegt ja die letzten Tage - vielleicht kommt Montag was, wird langsam Zeit. Ich werde von meinen mühsam gesammelten Shares nix abgeben, da ich in dieser Ausgangsposition ca. plus/minus bin und man kann doch auch mal dfür belohnt werden über Jahre durchzuhalten, Solarworld gab es auch mal für einen Euro
ja, das Volumen stiegt ja die letzten Tage - vielleicht kommt Montag was, wird langsam Zeit. Ich werde von meinen mühsam gesammelten Shares nix abgeben, da ich in dieser Ausgangsposition ca. plus/minus bin und man kann doch auch mal dfür belohnt werden über Jahre durchzuhalten, Solarworld gab es auch mal für einen Euro 
 ja, das Volumen stiegt ja die letzten Tage - vielleicht kommt Montag was, wird langsam Zeit. Ich werde von meinen mühsam gesammelten Shares nix abgeben, da ich in dieser Ausgangsposition ca. plus/minus bin und man kann doch auch mal dfür belohnt werden über Jahre durchzuhalten, Solarworld gab es auch mal für einen Euro
ja, das Volumen stiegt ja die letzten Tage - vielleicht kommt Montag was, wird langsam Zeit. Ich werde von meinen mühsam gesammelten Shares nix abgeben, da ich in dieser Ausgangsposition ca. plus/minus bin und man kann doch auch mal dfür belohnt werden über Jahre durchzuhalten, Solarworld gab es auch mal für einen Euro 
war mal wieder ein Nuller
 es ist zum kotzen
es ist zum kotzen
CEL-SCI Announces Exclusive Licensing Agreement with Teva for Cancer Drug Multikine
Tuesday August 19, 11:00 am ET
VIENNA, Va., Aug. 19 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (Amex: CVM - News) announced today that it has entered into an exclusive licensing agreement with Teva Pharmaceutical Industries Ltd. (Teva), a leading global pharmaceutical company, under which CEL-SCI has granted Teva an exclusive license to market and distribute the Company's cancer drug Multikine for Israel and Turkey (the "Territory"). The licensing agreement is initially restricted to the areas of head and neck cancer. Teva has the right, subject to certain conditions, to extend the licensing agreement to include other cancers during the term of this agreement. Multikine is currently thought to be potentially useful in treating many tumor types.
ADVERTISEMENT
Pursuant to the agreement, Teva will participate in CEL-SCI's upcoming global Phase III clinical trial. Teva will fund a portion of the Phase III clinical study and Teva's clinical group will conduct part of the clinical study in Israel under the auspices of CEL-SCI and its Clinical Research Organization. Teva will also be responsible for registering the product in the Territory. Once Multikine has been approved, CEL-SCI will be responsible for manufacturing the product, while Teva will be responsible for sales in the Territory. Revenues will be split 50/50 between CEL-SCI and Teva.
"We believe that Teva's expertise and knowledge in successfully conducting large pivotal clinical trials and in developing markets for large unmet medical needs will prove invaluable in maximizing Multikine's potential. This agreement is consistent with our strategy to share the clinical and regulatory expenses associated with the development of Multikine while retaining rights to market Multikine in North America and Europe," said Geert Kersten, Chief Executive Officer of CEL-SCI.
CEL-SCI is developing Multikine for approval as a first line indication in head and neck cancer. To that end, the Company's upcoming Phase III clinical trial is an 800 patient clinical study designed to demonstrate that administration of its cancer drug Multikine to head and neck cancer patients before they receive any conventional cancer treatment will increase their survival. Head and neck cancer is one of the world's biggest cancers affecting about 600,000 people per annum worldwide.
About CEL-SCI's Phase III Cancer Drug Multikine:
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck in May 2007. In the summer of 2007 CEL-SCI started construction of the manufacturing facility to produce Multikine for the Phase III trial and subsequent sale following approval. This facility is expected to be completed soon.
Multikine, a patented defined mixture of naturally derived cytokines, is the first immunotherapeutic agent in a new class of drugs called "Immune SIMULATORS." Immune SIMULATORS simulate the way our natural immune system acts in defending us against cancer. As opposed to other immunotherapies which are designed to target a single or limited number of specific antigens or molecules, Immune SIMULATORS are multi-targeted; they simultaneously cause a direct and targeted killing of the specific tumor cells and they activate the immune system to produce a stronger anti-tumor attack on multiple fronts.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
CEL-SCI has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense. Most recently CEL-SCI announced that its newly discovered rheumatoid arthritis vaccine showed excellent results in animal tests.
Tuesday August 19, 11:00 am ET
VIENNA, Va., Aug. 19 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (Amex: CVM - News) announced today that it has entered into an exclusive licensing agreement with Teva Pharmaceutical Industries Ltd. (Teva), a leading global pharmaceutical company, under which CEL-SCI has granted Teva an exclusive license to market and distribute the Company's cancer drug Multikine for Israel and Turkey (the "Territory"). The licensing agreement is initially restricted to the areas of head and neck cancer. Teva has the right, subject to certain conditions, to extend the licensing agreement to include other cancers during the term of this agreement. Multikine is currently thought to be potentially useful in treating many tumor types.
ADVERTISEMENT
Pursuant to the agreement, Teva will participate in CEL-SCI's upcoming global Phase III clinical trial. Teva will fund a portion of the Phase III clinical study and Teva's clinical group will conduct part of the clinical study in Israel under the auspices of CEL-SCI and its Clinical Research Organization. Teva will also be responsible for registering the product in the Territory. Once Multikine has been approved, CEL-SCI will be responsible for manufacturing the product, while Teva will be responsible for sales in the Territory. Revenues will be split 50/50 between CEL-SCI and Teva.
"We believe that Teva's expertise and knowledge in successfully conducting large pivotal clinical trials and in developing markets for large unmet medical needs will prove invaluable in maximizing Multikine's potential. This agreement is consistent with our strategy to share the clinical and regulatory expenses associated with the development of Multikine while retaining rights to market Multikine in North America and Europe," said Geert Kersten, Chief Executive Officer of CEL-SCI.
CEL-SCI is developing Multikine for approval as a first line indication in head and neck cancer. To that end, the Company's upcoming Phase III clinical trial is an 800 patient clinical study designed to demonstrate that administration of its cancer drug Multikine to head and neck cancer patients before they receive any conventional cancer treatment will increase their survival. Head and neck cancer is one of the world's biggest cancers affecting about 600,000 people per annum worldwide.
About CEL-SCI's Phase III Cancer Drug Multikine:
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck in May 2007. In the summer of 2007 CEL-SCI started construction of the manufacturing facility to produce Multikine for the Phase III trial and subsequent sale following approval. This facility is expected to be completed soon.
Multikine, a patented defined mixture of naturally derived cytokines, is the first immunotherapeutic agent in a new class of drugs called "Immune SIMULATORS." Immune SIMULATORS simulate the way our natural immune system acts in defending us against cancer. As opposed to other immunotherapies which are designed to target a single or limited number of specific antigens or molecules, Immune SIMULATORS are multi-targeted; they simultaneously cause a direct and targeted killing of the specific tumor cells and they activate the immune system to produce a stronger anti-tumor attack on multiple fronts.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
CEL-SCI has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense. Most recently CEL-SCI announced that its newly discovered rheumatoid arthritis vaccine showed excellent results in animal tests.
Auftakt zu weitern guten News???



 das schwankt ja ganz schön heftig, und ich bin ab morgen im Krankenhaus
das schwankt ja ganz schön heftig, und ich bin ab morgen im Krankenhaus 
Teva Pharmaceutical Industries Ltd. (hebr.: טבע תעשיות פרמצבטיות בע"מ) ist ein Pharmakonzern mit Sitz in Petach Tikwa, Israel. Teva ist Marktführer in Israel, es gehört zu den 25 größten Pharmakonzernen weltweit (nach eigenen Angaben) und ist die größte Firma im weltweiten Generika-Markt. Im Jahr 2004 betrug der Jahresumsatz des Konzerns 4,8 Milliarden US-Dollar, die Zahl der Beschäftigten belief sich auf etwa 14.000.
Firmengeschichte [Bearbeiten]Teva (hebräisch für Natur) wurde 1901 als Drogerie unter dem Namen Salomon, Levin und Elstein Ltd. gegründet; das Unternehmen war zunächst ein reines Vertriebsunternehmen für importierte Medikamente. Ab 1930 kam es in der Folge der Einwanderung hochqualifizierter Wissenschaftler zur Gründung erster Labors, die Medikamente herstellten; viele dieser Labors gingen später in Teva auf. Nach einer starken Expansion der Pharmaindustrie ab dem 2. Weltkrieg ging Teva als eines der ersten Pharmaunternehmen 1951 an die Tel Aviver Börse. Im Verlauf der folgenden Jahrzehnte kam es zu Konzentrations- und Konsolidierungsprozessen; im Jahr 1976 kam es zur formalen Gründung der Teva Pharmaceutical Industries Ltd.. International expandiert die Firma seit der zweiten Hälfte der 1980er Jahre. Sie war zunächst in den USA vertreten, inzwischen bestehen weltweit Niederlassungen. Im Januar 2006 übernahm Teva das US-amerikanische Pharmazieunternehmen Ivax, das zu den zehn größten Generikaherstellern weltweit gehörte.
Firmengeschichte [Bearbeiten]Teva (hebräisch für Natur) wurde 1901 als Drogerie unter dem Namen Salomon, Levin und Elstein Ltd. gegründet; das Unternehmen war zunächst ein reines Vertriebsunternehmen für importierte Medikamente. Ab 1930 kam es in der Folge der Einwanderung hochqualifizierter Wissenschaftler zur Gründung erster Labors, die Medikamente herstellten; viele dieser Labors gingen später in Teva auf. Nach einer starken Expansion der Pharmaindustrie ab dem 2. Weltkrieg ging Teva als eines der ersten Pharmaunternehmen 1951 an die Tel Aviver Börse. Im Verlauf der folgenden Jahrzehnte kam es zu Konzentrations- und Konsolidierungsprozessen; im Jahr 1976 kam es zur formalen Gründung der Teva Pharmaceutical Industries Ltd.. International expandiert die Firma seit der zweiten Hälfte der 1980er Jahre. Sie war zunächst in den USA vertreten, inzwischen bestehen weltweit Niederlassungen. Im Januar 2006 übernahm Teva das US-amerikanische Pharmazieunternehmen Ivax, das zu den zehn größten Generikaherstellern weltweit gehörte.
 babelfish
babelfishCEL-SCI verkündet exklusive Lizenzvereinbarung mit Teva für Krebsmedikament Multikine Dienstag, die 19.,11. August: 00 morgens UND WIEN, VA., 19. August /PRNewswire-FirstCall/ -- CEL-SCI Corporation (Amex: CVM - Nachrichten) heute verkündet, dass sie einen Lizenzvertrag mit Teva Pharmaindustrien Ltd. (Teva), ein führendes globales pharmazeutisches Unternehmen schlossen hat, unter dem CEL-SCI Teva eine ausschliessliche Lizenz bewilligt hat, das Company' zu vermarkten und zu verteilen; s-Krebsmedikament Multikine für Israel und die Türkei (das " Territory"). Die Lizenzvereinbarung wird zuerst auf die Bereiche Haupt- und Ansatzkrebses eingeschränkt. Teva hat das Recht abhängig von bestimmten Bedingungen, die Lizenzvereinbarung zu verlängern, andere Krebse während dieser Vereinbarung einzuschließen. Multikine ist z.Z. wahrscheinlich möglicherweise nützlich, wenn man viele Tumorarten behandelt. REKLAMEANZEIGE Gemäß der Vereinbarung nimmt Teva an CEL-SCI' teil; klinische Studie der s-bevorstehende globale Phase III. Teva finanziert einen Teil der klinischen Studie der Phase III und des Teva' s-klinische Gruppe leitet Teil der klinischen Studie in Israel unter die Auspizien von CEL-SCI und von seiner klinischen Forschungsorganisation. Teva ist auch für das Registrieren des Produktes in der Gegend verantwortlich. Sobald Multikine genehmigt worden ist, ist CEL-SCI für die Herstellung des Produktes verantwortlich, während Teva für Verkäufe in der Gegend verantwortlich ist. Einkommen werden 50/50 zwischen CEL-SCI und Teva aufgespaltet. " Wir glauben dass Teva' s-Sachkenntnis und Wissen, in der großen Angelklinischer Studien erfolgreich leiten und in sich entwickelnden Märkten für große unmet medizinische Notwendigkeiten, prüfen unschätzbares in maximierenMultikine' s-Potenzial. Diese Vereinbarung ist mit unserer Strategie in Einklang, die klinischen und regelnden Unkosten zu teilen, die mit der Entwicklung von Multikine verbunden sind, während das Behalten berichtigt, um Multikine in Nordamerika und in Europa zu vermarkten, " besagter Geert Kersten, Generaldirektor von CEL-SCI. CEL-SCI entwickelt Multikine für Zustimmung wie erstes Zeilenangabe in Haupt- und Ansatzkrebs. Zu diesem Zweck das Company' klinische Studie der s-ist bevorstehende Phase III eine klinische Studie mit 800 Patienten, die entworfen ist, um diese Verwaltung seines Krebsmedikaments Multikine zu Haupt zu demonstrieren und Ansatzkrebspatienten, bevor sie jede herkömmliche Krebsbehandlung empfangen, erhöhen ihr Überleben. Haupt- und Ansatzkrebs ist einer des world' s-größte Krebse, die pro Jahr ungefähr 600.000 Leute weltweit beeinflussen.
 Warum kommt nach dieser News die Aktie zurück, obwohl sich Teva an den Kosten der Studie III beteiligt ???
Warum kommt nach dieser News die Aktie zurück, obwohl sich Teva an den Kosten der Studie III beteiligt ???
wer weiß,
evtl. wird versucht, den Kurs so lange wie möglich unten zu halten, um günstig zuzukaufen.
Ist aber reine spekulation. Vieleicht kommt ja bald auch eine ganz andere Meldung, z.B. in Form von einer Beteiligung an CVM. Wer weiss.
mfg Plaste
PS: zwischendurch +25%, jetzt wieder 0%, ich glaube das war schon mal ein Vorgeschmack auf das was kommen kann...
auf das was kommen kann...
evtl. wird versucht, den Kurs so lange wie möglich unten zu halten, um günstig zuzukaufen.
Ist aber reine spekulation. Vieleicht kommt ja bald auch eine ganz andere Meldung, z.B. in Form von einer Beteiligung an CVM. Wer weiss.
mfg Plaste
PS: zwischendurch +25%, jetzt wieder 0%, ich glaube das war schon mal ein Vorgeschmack
 auf das was kommen kann...
auf das was kommen kann...
Antwort auf Beitrag Nr.: 34.769.496 von Plaste am 19.08.08 20:19:03 Teva ist ein Riesenkonzern mit viel Kohle - die sind doch nicht so blöd und verpulvern Millionen wenn da nicht was rauszuholen ist
Teva ist ein Riesenkonzern mit viel Kohle - die sind doch nicht so blöd und verpulvern Millionen wenn da nicht was rauszuholen ist 
 Teva ist ein Riesenkonzern mit viel Kohle - die sind doch nicht so blöd und verpulvern Millionen wenn da nicht was rauszuholen ist
Teva ist ein Riesenkonzern mit viel Kohle - die sind doch nicht so blöd und verpulvern Millionen wenn da nicht was rauszuholen ist 
und dann werden wir noch durchgereicht bis 0,51 cent,echt toll
 vom Tageshoch 0.78 auf 0.51 nachbörslich, das soll mir mal jemand erklären - bei der News
vom Tageshoch 0.78 auf 0.51 nachbörslich, das soll mir mal jemand erklären - bei der News 


schlechter Quartalsbericht?Kapitalerhöhung?
Teva ist auch nur ein Generikahersteller,eine Pharmafirma würde einfach mehr Fantashie entfachen
20.08.2008 16:40
CEL-SCI CORPORATION Releases Letter to Shareholders
VIENNA, Va., Aug. 20 /PRNewswire-FirstCall/ -- The following letter is being released by CEL-SCI CORPORATION (News) to its shareholders:
Dear Fellow Shareholders,
I am writing to you in response to the many phone calls and e-mails that we received within the last 24 hours. You were universally enthused by the licensing deal for our Phase III cancer drug Multikine with one of the most successful and respected pharmaceutical companies in the world. You were excited that they are putting their reputation on the line by actually running and funding the costs of a part of our Phase III clinical trial. You were enthused with the initial increase in the share price, yet no one could understand why our shares, after trading 30 times the normal volume, took such a steep fall in the afternoon. You asked, "How is it possible that the stock can be down after such a good announcement?" As the Company's largest shareholder, and somebody who has purchased shares in the open market regularly, I am as puzzled and disappointed as you.
While we may never know what happened, I have been told by many that the stock traded as if it could have been under an attack from short sellers. Short sellers play a role in the stock market, correcting imbalances in supply and demand that occur periodically. However, it appears that trading in our shares may have involved more than opportunistic short sellers, but may actually have involved manipulation, which is illegal.
Short sellers make money by betting that a stock will decline in price. They sell shares they do not own (yes that is legal as long as you follow certain rules which we think were not followed in this case) and hope to repurchase them at a lower price. Short sellers may look for companies that have been doing better than the averages and/or their peers on the theory that everyone should be knocked down equally. Our share price has been doing much better than the averages and/or our peers in what has been a very challenging market for micro-cap stocks.
We believe that in all likelihood the short seller has been shorting CEL-SCI stock for a while. The selling of our stock increased in the last month, but more importantly, the seller was not acting like a shareholder, but like someone who trades deceptively to create the impression of a decreasing share price. Remember, the short seller makes money from a declining share price. For example, one day we traded about 85,000 shares throughout the day and in the last minute another 80,000 shares were sold. That may have been done to drive the share price down and is euphemistically known as "painting the tape," an illegal activity which makes the stock look weak and unattractive.
Yesterday's announcement of the licensing deal brought in a large amount of buying to our stock and initially increased the share price substantially on huge volume. Having been taken by surprise by this great news, the short seller may have been faced with taking a large loss (having to buy back at higher prices the shares that he sold earlier) or selling again a very large number of shares in order to drive down the share price and stop the share's upward momentum. It appears that he may have opted for shorting many more shares. And again, in the last few minutes of the day's trading, large amounts of stock were sold depressing our share price.
The goal of the short seller may have been to scare investors out of their stock at lower prices so that the short seller can cover (buy the shares back) at a profit (a lower price). So what can you do about this? Short sellers can benefit from creating panic among the shareholders and getting them to sell their shares cheaply. Therefore CEL-SCI shareholders should be aware of the game that is being played with their Company's shares and not be intimidated.
We accept that short selling is a legal activity, but only when conducted according to the rules. WE BELIEVE THAT SHORT SELLERS IN OUR CASE MAY NOT HAVE FOLLOWED THE RULES AND THAT IS WHY WE NEED YOUR HELP. WE NEED YOU TO CONTACT YOUR CONGRESSPERSON AND/OR SENATOR AS EXPLAINED AT THE END OF THE LETTER.
Short selling CEL-SCI stock is allowed ONLY if you can borrow an equivalent amount of CEL-SCI stock. We have checked with brokers and it is very difficult to borrow CEL-SCI shares. Therefore, it is highly unlikely that the massive short sales that have hurt the CEL-SCI share price are legal short sales which have been transacted with a corresponding number of shares borrowed. Most likely these sales were conducted in violation of the short sale rules. These sales were most likely conducted to keep the share price from gaining upward momentum since an increase in the share price would hurt the short seller. If we are correct, we are the victim of vicious and illegal manipulation.
The only way to prove this malfeasance, or disprove it, is to get the regulators to subpoena the trading records of the last few weeks. Only they can find out whether short sellers may have followed the rules. That is where you can be of great help. Twice in the last month we have contacted the SEC and other regulatory bodies and have asked them to investigate. Now we are asking you to contact your local Congressman and/or Senator and request their help in getting the regulators to focus on our issue. Short sellers must abide by the rules. CEL-SCI was created to help people. Now CEL-SCI needs your help.
In attacking CEL-SCI, they are attacking not just your money but also the hope of cancer patients. This affects almost every family. Our Multikine is a late stage cancer drug that appears to be non-toxic and showed significant increases in survival in the Phase II studies. Hence, by jeopardizing the Company, short sellers may be destroying the dreams of many people seeking a novel and non-toxic cancer treatment. I have dedicated 20 years to the development of this drug. We are about to enter the end stage and we are starting to find acceptance even among the big pharmaceutical companies, and I am not going to let these people push us around. I and the management and scientific team at CEL-SCI remain steadfast in our commitment to bring this promising drug to market.
Thank you for your help, Geert Kersten Chief Executive Officer
CEL-SCI CORPORATION Releases Letter to Shareholders
VIENNA, Va., Aug. 20 /PRNewswire-FirstCall/ -- The following letter is being released by CEL-SCI CORPORATION (News) to its shareholders:
Dear Fellow Shareholders,
I am writing to you in response to the many phone calls and e-mails that we received within the last 24 hours. You were universally enthused by the licensing deal for our Phase III cancer drug Multikine with one of the most successful and respected pharmaceutical companies in the world. You were excited that they are putting their reputation on the line by actually running and funding the costs of a part of our Phase III clinical trial. You were enthused with the initial increase in the share price, yet no one could understand why our shares, after trading 30 times the normal volume, took such a steep fall in the afternoon. You asked, "How is it possible that the stock can be down after such a good announcement?" As the Company's largest shareholder, and somebody who has purchased shares in the open market regularly, I am as puzzled and disappointed as you.
While we may never know what happened, I have been told by many that the stock traded as if it could have been under an attack from short sellers. Short sellers play a role in the stock market, correcting imbalances in supply and demand that occur periodically. However, it appears that trading in our shares may have involved more than opportunistic short sellers, but may actually have involved manipulation, which is illegal.
Short sellers make money by betting that a stock will decline in price. They sell shares they do not own (yes that is legal as long as you follow certain rules which we think were not followed in this case) and hope to repurchase them at a lower price. Short sellers may look for companies that have been doing better than the averages and/or their peers on the theory that everyone should be knocked down equally. Our share price has been doing much better than the averages and/or our peers in what has been a very challenging market for micro-cap stocks.
We believe that in all likelihood the short seller has been shorting CEL-SCI stock for a while. The selling of our stock increased in the last month, but more importantly, the seller was not acting like a shareholder, but like someone who trades deceptively to create the impression of a decreasing share price. Remember, the short seller makes money from a declining share price. For example, one day we traded about 85,000 shares throughout the day and in the last minute another 80,000 shares were sold. That may have been done to drive the share price down and is euphemistically known as "painting the tape," an illegal activity which makes the stock look weak and unattractive.
Yesterday's announcement of the licensing deal brought in a large amount of buying to our stock and initially increased the share price substantially on huge volume. Having been taken by surprise by this great news, the short seller may have been faced with taking a large loss (having to buy back at higher prices the shares that he sold earlier) or selling again a very large number of shares in order to drive down the share price and stop the share's upward momentum. It appears that he may have opted for shorting many more shares. And again, in the last few minutes of the day's trading, large amounts of stock were sold depressing our share price.
The goal of the short seller may have been to scare investors out of their stock at lower prices so that the short seller can cover (buy the shares back) at a profit (a lower price). So what can you do about this? Short sellers can benefit from creating panic among the shareholders and getting them to sell their shares cheaply. Therefore CEL-SCI shareholders should be aware of the game that is being played with their Company's shares and not be intimidated.
We accept that short selling is a legal activity, but only when conducted according to the rules. WE BELIEVE THAT SHORT SELLERS IN OUR CASE MAY NOT HAVE FOLLOWED THE RULES AND THAT IS WHY WE NEED YOUR HELP. WE NEED YOU TO CONTACT YOUR CONGRESSPERSON AND/OR SENATOR AS EXPLAINED AT THE END OF THE LETTER.
Short selling CEL-SCI stock is allowed ONLY if you can borrow an equivalent amount of CEL-SCI stock. We have checked with brokers and it is very difficult to borrow CEL-SCI shares. Therefore, it is highly unlikely that the massive short sales that have hurt the CEL-SCI share price are legal short sales which have been transacted with a corresponding number of shares borrowed. Most likely these sales were conducted in violation of the short sale rules. These sales were most likely conducted to keep the share price from gaining upward momentum since an increase in the share price would hurt the short seller. If we are correct, we are the victim of vicious and illegal manipulation.
The only way to prove this malfeasance, or disprove it, is to get the regulators to subpoena the trading records of the last few weeks. Only they can find out whether short sellers may have followed the rules. That is where you can be of great help. Twice in the last month we have contacted the SEC and other regulatory bodies and have asked them to investigate. Now we are asking you to contact your local Congressman and/or Senator and request their help in getting the regulators to focus on our issue. Short sellers must abide by the rules. CEL-SCI was created to help people. Now CEL-SCI needs your help.
In attacking CEL-SCI, they are attacking not just your money but also the hope of cancer patients. This affects almost every family. Our Multikine is a late stage cancer drug that appears to be non-toxic and showed significant increases in survival in the Phase II studies. Hence, by jeopardizing the Company, short sellers may be destroying the dreams of many people seeking a novel and non-toxic cancer treatment. I have dedicated 20 years to the development of this drug. We are about to enter the end stage and we are starting to find acceptance even among the big pharmaceutical companies, and I am not going to let these people push us around. I and the management and scientific team at CEL-SCI remain steadfast in our commitment to bring this promising drug to market.
Thank you for your help, Geert Kersten Chief Executive Officer
bin nicht besonders begeistert von dem shareholder letter. Mag vielleicht sein, dass der Verantwortliche in Rage war, mag aber auch sein, dass er von anderen Verkäufen ablenken wollte.
Abgesehen davon ist es normal nicht Aufgabe eines Vorstands, die aktuelle Marktlage zu kommentieren.
Abgesehen davon ist es normal nicht Aufgabe eines Vorstands, die aktuelle Marktlage zu kommentieren.
Data Suggest CEL-SCI's CEL-2000 Vaccine Prevents or Retards Permanent Damage of Rheumatoid Arthritis
Friday September 5, 9:45 am ET
VIENNA, Va., Sept. 5 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (Amex: CVM - News) announced today that it presented new rheumatoid arthritis data at the Marcus Evans conference on Immunogenicity in Amsterdam. The data, presented by Dr. Daniel Zimmerman, Senior Vice President of Research, Cellular Immunology of CEL-SCI, indicate that CEL-SCI's rheumatoid arthritis vaccine CEL-2000 prevents or retards the permanent tissue damage caused by rheumatoid arthritis in an animal model of the disease. These new findings further support previous positive results announced for this vaccine in June 2008. The data were derived from a histopathological analysis of tissues samples collected in comparative studies of CEL-2000 and Enbrel® that were conducted in a well established animal model of rheumatoid arthritis. Enbrel is a leading treatment for people with rheumatoid arthritis.
ADVERTISEMENT
In the studies, the mice were injected with collagen on days 0 and 21 to induce the disease. Once the mice reached a significant and uniform disease state, therapy with Enbrel or CEL-2000 was initiated and continued for 28 days. CEL-2000 was administered only twice and Enbrel was administered either every day for the first 14 days (for the histopathology and immunology studies) or every other day over the entire study period of 28 days (for the immunology studies only).
Dr. Zimmerman reported previously that in studies conducted in mice treated similarly, CEL-2000 was equivalent or possibly superior to Enbrel in slowing disease progression and lessening symptoms. The new data presented today indicate that, in mice vaccinated with CEL-2000 after appearance of visible disease, statistically significant less inflammation and permanent damage with regard to 1) bone erosion, 2) cartilage destruction, and 3) pannus formation were observed. These are some of the same parameters that can be seen in rheumatoid arthritis damage in humans. CEL-2000 was as good as, and possibly superior to, Enbrel in slowing further disease progression as evaluated by these histological parameters and by footpad swelling as well as externally visible joint damage.
"It is very exciting to see the reduction of severe rheumatoid arthritis damage in these animals through a simple vaccination," said Dr. Zimmerman. "I hope that CEL-2000 will some day be used to lessen the damage caused by rheumatoid arthritis in patients."
CEL-2000 may offer a number of potential advantages over the existing rheumatoid arthritis treatments. Data collected in the animal studies conducted with CEL-2000 demonstrate it is effective with fewer and smaller doses. It is also potentially less toxic and more disease specific therapy. Finally, CEL-2000 could also be useful for patients who are not able to take or who may be unresponsive to existing products.
Rheumatoid arthritis treatments comprise a $13 billion market. Enbrel, a leading rheumatoid arthritis treatment sold by Amgen and Wyeth, reported US sales in 2007 of about $3.2 billion. Enbrel is a soluble recombinant protein of a human TNF-alpha receptor linked to human IgG Fc. In some cases, human or humanized monoclonal antibodies to TNF-alpha have also been used for therapy in rheumatoid arthritis. These therapies remove or inactivate TNF-alpha, a natural human cytokine required in many immune functions for normal defenses.
CEL-SCI's rheumatoid arthritis vaccine CEL-2000 was discovered as part of work with the Company's ongoing research and development activities with its L.E.A.P.S.(TM) (Ligand Epitope Antigen Presentation System) technology. L.E.A.P.S.(TM) is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S.(TM) technology.
The concept behind the L.E.A.P.S.(TM) technology is to directly mimic cell/cell interactions on the T-cell surface with synthetic peptides. The L.E.A.P.S.(TM) constructs containing the antigenic disease epitope linked to a T-cell binding ligand (TCBL) can be manufactured by peptide synthesis or by covalently linking the two peptides. Depending upon the type of L.E.A.P.S.(TM) construct and TCBL used, CEL-SCI is able to direct the outcome of the immune response towards the development of T-cell function with primarily effector T-cell functions (T Lymphocyte; helper/effector T lymphocyte, type 1 or 2 [Th1 or Th2], cytotoxic [Tc] or suppressor [Ts]). Therefore, it would appear that the L.E.A.P.S.(TM) construct represents a chimeric peptide with bi-functional behavior.
About CEL-SCI:
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine®. In Phase II clinical trials, Multikine was shown to be safe and well tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck in May 2007.
The Company has operations in Vienna, Virginia, and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense and avian flu.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K for the year ended September 30, 2007. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
--------------------------------------------------------------------------------
Source: CEL-SCI Corporation
Friday September 5, 9:45 am ET
VIENNA, Va., Sept. 5 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (Amex: CVM - News) announced today that it presented new rheumatoid arthritis data at the Marcus Evans conference on Immunogenicity in Amsterdam. The data, presented by Dr. Daniel Zimmerman, Senior Vice President of Research, Cellular Immunology of CEL-SCI, indicate that CEL-SCI's rheumatoid arthritis vaccine CEL-2000 prevents or retards the permanent tissue damage caused by rheumatoid arthritis in an animal model of the disease. These new findings further support previous positive results announced for this vaccine in June 2008. The data were derived from a histopathological analysis of tissues samples collected in comparative studies of CEL-2000 and Enbrel® that were conducted in a well established animal model of rheumatoid arthritis. Enbrel is a leading treatment for people with rheumatoid arthritis.
ADVERTISEMENT
In the studies, the mice were injected with collagen on days 0 and 21 to induce the disease. Once the mice reached a significant and uniform disease state, therapy with Enbrel or CEL-2000 was initiated and continued for 28 days. CEL-2000 was administered only twice and Enbrel was administered either every day for the first 14 days (for the histopathology and immunology studies) or every other day over the entire study period of 28 days (for the immunology studies only).
Dr. Zimmerman reported previously that in studies conducted in mice treated similarly, CEL-2000 was equivalent or possibly superior to Enbrel in slowing disease progression and lessening symptoms. The new data presented today indicate that, in mice vaccinated with CEL-2000 after appearance of visible disease, statistically significant less inflammation and permanent damage with regard to 1) bone erosion, 2) cartilage destruction, and 3) pannus formation were observed. These are some of the same parameters that can be seen in rheumatoid arthritis damage in humans. CEL-2000 was as good as, and possibly superior to, Enbrel in slowing further disease progression as evaluated by these histological parameters and by footpad swelling as well as externally visible joint damage.
"It is very exciting to see the reduction of severe rheumatoid arthritis damage in these animals through a simple vaccination," said Dr. Zimmerman. "I hope that CEL-2000 will some day be used to lessen the damage caused by rheumatoid arthritis in patients."
CEL-2000 may offer a number of potential advantages over the existing rheumatoid arthritis treatments. Data collected in the animal studies conducted with CEL-2000 demonstrate it is effective with fewer and smaller doses. It is also potentially less toxic and more disease specific therapy. Finally, CEL-2000 could also be useful for patients who are not able to take or who may be unresponsive to existing products.
Rheumatoid arthritis treatments comprise a $13 billion market. Enbrel, a leading rheumatoid arthritis treatment sold by Amgen and Wyeth, reported US sales in 2007 of about $3.2 billion. Enbrel is a soluble recombinant protein of a human TNF-alpha receptor linked to human IgG Fc. In some cases, human or humanized monoclonal antibodies to TNF-alpha have also been used for therapy in rheumatoid arthritis. These therapies remove or inactivate TNF-alpha, a natural human cytokine required in many immune functions for normal defenses.
CEL-SCI's rheumatoid arthritis vaccine CEL-2000 was discovered as part of work with the Company's ongoing research and development activities with its L.E.A.P.S.(TM) (Ligand Epitope Antigen Presentation System) technology. L.E.A.P.S.(TM) is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S.(TM) technology.
The concept behind the L.E.A.P.S.(TM) technology is to directly mimic cell/cell interactions on the T-cell surface with synthetic peptides. The L.E.A.P.S.(TM) constructs containing the antigenic disease epitope linked to a T-cell binding ligand (TCBL) can be manufactured by peptide synthesis or by covalently linking the two peptides. Depending upon the type of L.E.A.P.S.(TM) construct and TCBL used, CEL-SCI is able to direct the outcome of the immune response towards the development of T-cell function with primarily effector T-cell functions (T Lymphocyte; helper/effector T lymphocyte, type 1 or 2 [Th1 or Th2], cytotoxic [Tc] or suppressor [Ts]). Therefore, it would appear that the L.E.A.P.S.(TM) construct represents a chimeric peptide with bi-functional behavior.
About CEL-SCI:
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine®. In Phase II clinical trials, Multikine was shown to be safe and well tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck in May 2007.
The Company has operations in Vienna, Virginia, and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense and avian flu.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K for the year ended September 30, 2007. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
--------------------------------------------------------------------------------
Source: CEL-SCI Corporation
Daten legen nahe, CEL-SCI's CEL-2000 Impfstoff verhindert oder verzögert dauerhaften Schädigung der rheumatoiden Arthritis
Freitag 5. September, 9:45 Uhr ET
VIENNA, Va., September 5 / PRNewswire-FirstCall / - CEL-SCI Corporation (AMEX CVM - News) gab heute bekannt, dass es neue rheumatoider Arthritis Daten auf der Marcus Evans Konferenz über die Immunogenität in Amsterdam. Die Daten, präsentiert von Dr. Daniel Zimmermann, Senior Vice President of Research, Zelluläre Immunologie der CEL-SCI, deuten darauf hin, dass CEL-SCI's rheumatoider Arthritis Impfstoff CEL-2000 verhindert oder verzögert die permanente Gewebe Schäden, die durch die rheumatoide Arthritis in einem Tiermodell der der Krankheit. Diese neuen Erkenntnisse weitere Unterstützung der bisherigen positiven Ergebnisse der Bekanntgabe für dieses Impfstoffs im Juni 2008. Die Daten stammen aus einer histopathologischen Analyse von Geweben gesammelten Proben in vergleichenden Studien von CEL-2000 und Enbrel ®, die in einem etablierten Tiermodell der rheumatoiden Arthritis. Enbrel ist ein führender Behandlung für Menschen mit rheumatoider Arthritis.
WERBUNG
In den Studien, die Mäusen injiziert wurden mit Kollagen an den Tagen 0 und 21, um die Krankheit. Sobald die Maus erreicht eine signifikante und einheitlichen Staat Krankheit, Therapie mit Enbrel oder CEL-2000 initiiert wurde und weiterhin für 28 Tage. CEL-2000 verabreicht wurde nur zweimal und Enbrel wurde entweder jeden Tag für die ersten 14 Tage (für die Histopathologie Studien und klinische Immunologie) oder jeden zweiten Tag über die gesamte Studiendauer von 28 Tagen (für die Immunologie nur Studien).
Dr. Zimmerman zuvor berichtet, dass in Studien an Mäusen ähnlich behandelt, CEL-2000 entsprach oder eventuell überlegen Enbrel bei Fortschreiten der Krankheit verlangsamt und das Abnehmen Symptome. Die neuen Daten deuten darauf hin, dass heute, im geimpften Mäusen mit CEL-2000 nach Erscheinen des sichtbaren Krankheit, statistisch signifikant weniger Entzündungen und dauerhafte Schäden im Hinblick auf 1) Knochen Erosion, 2) Knorpel-Zerstörung, und 3) Pannus Bildung beobachtet. Dies sind nur einige der gleichen Parameter, kann man bei rheumatoider Arthritis Schäden beim Menschen. CEL-2000 war so gut, wie und eventuell überlegen, Enbrel zu bremsen weitere Fortschreiten der Krankheit bewertet als von diesen Parametern und histologischen von footpad Schwellung sowie nach außen sichtbare gemeinsame Schäden.
"Es ist sehr spannend zu sehen, die Reduzierung von schweren Schäden rheumatoider Arthritis bei diesen Tieren durch eine einfache Impfung", sagt Dr. Zimmermann. "Ich hoffe, dass CEL-2000 wird einige Tage benutzt werden, um die Schäden, die durch die rheumatoide Arthritis bei Patienten."
CEL-2000 kann eine Reihe potenzieller Vorteile gegenüber den bestehenden rheumatoiden Arthritis Behandlungen. Daten, die im Tierexperimentelle Studien, die mit CEL-2000 zeigen, ist es effektiver mit weniger und kleineren Dosen. Es ist auch potenziell weniger toxisch und mehr Krankheit spezifische Therapie. Schließlich CEL-2000 könnte auch nützlich sein für Patienten, nicht in der Lage sind, zu entnehmen oder kann reagiert auf bereits vorhandene Produkte.
Rheumatoide Arthritis Behandlungen umfassen ein $ 13 Milliarden Markt. Enbrel, einem der führenden Behandlung rheumatoider Arthritis Versand durch Amgen und Wyeth, berichteten US-Umsatz in 2007 auf rund USD 3,2 Milliarden. Enbrel ist ein rekombinanter Protein löslich eines menschlichen TNF-alpha-Rezeptor im Zusammenhang mit Human-IgG Fc. In einigen Fällen, von Mensch oder humanisierter monoklonaler Antikörper gegen TNF-alpha wurden auch für die Therapie bei rheumatoider Arthritis. Diese Therapien zu entfernen oder zu inaktivieren TNF-alpha, einem natürlichen menschlichen Zytokin in vielen Immun-Funktionen für die normale Abwehr.
CEL-SCI's rheumatoider Arthritis Impfstoff CEL-2000 entdeckt wurde als Teil der Arbeit mit der Firma der laufenden Forschungs-und Entwicklungsaktivitäten mit ihren LEAPS (TM) (Epitope Ligand Antigen Presentation System)-Technologie. LEAPS (TM) ist ein neuartiges T-Zell-Modulation-Plattform-Technologie ermöglicht es, dass CEL-SCI zu entwerfen und zu synthetisieren proprietäre immunogens. Jede Krankheit, für die eine Antigen-Sequenz identifiziert wurde, wie Infektionskrankheiten, Parasiten, Autoimmunerkrankungen oder maligne Erkrankungen und Allergien, sind die potenziellen therapeutischen oder präventiven Standorte für die Anwendung von LEAPS (TM)-Technologie.
Das Konzept hinter dem LEAPS (TM)-Technologie ist direkt imitieren Zelle / Zell-Interaktionen auf der T-Zell-Oberfläche mit synthetischen Peptiden. Die LEAPS (TM)-Konstrukte mit den Antigen-Epitop-Krankheit in Verbindung mit einem T-Zell-bindenden Liganden (TCBL) werden kann, hergestellt von der Peptid-Synthese oder durch kovalente Verknüpfung der beiden Peptide. Abhängig von der Art der LEAPS (TM) konstruieren und TCBL verwendet, CEL-SCI ist in der Lage, die direkte Ergebnis der Immunantwort auf die Entwicklung von T-Zell-Funktion in erster Linie mit Effektor T-Zell-Funktionen (T-Lymphozyten; Helfer / Effektor T Lymphozyten, Typ 1 oder 2 [Th1 oder Th2], zytotoxische [Tc] oder Suppressor [TS]). Daher hat es den Anschein, dass die LEAPS (TM)-Konstrukt ist ein chimärer Peptid mit bi-funktionale Verhalten.
Freitag 5. September, 9:45 Uhr ET
VIENNA, Va., September 5 / PRNewswire-FirstCall / - CEL-SCI Corporation (AMEX CVM - News) gab heute bekannt, dass es neue rheumatoider Arthritis Daten auf der Marcus Evans Konferenz über die Immunogenität in Amsterdam. Die Daten, präsentiert von Dr. Daniel Zimmermann, Senior Vice President of Research, Zelluläre Immunologie der CEL-SCI, deuten darauf hin, dass CEL-SCI's rheumatoider Arthritis Impfstoff CEL-2000 verhindert oder verzögert die permanente Gewebe Schäden, die durch die rheumatoide Arthritis in einem Tiermodell der der Krankheit. Diese neuen Erkenntnisse weitere Unterstützung der bisherigen positiven Ergebnisse der Bekanntgabe für dieses Impfstoffs im Juni 2008. Die Daten stammen aus einer histopathologischen Analyse von Geweben gesammelten Proben in vergleichenden Studien von CEL-2000 und Enbrel ®, die in einem etablierten Tiermodell der rheumatoiden Arthritis. Enbrel ist ein führender Behandlung für Menschen mit rheumatoider Arthritis.
WERBUNG
In den Studien, die Mäusen injiziert wurden mit Kollagen an den Tagen 0 und 21, um die Krankheit. Sobald die Maus erreicht eine signifikante und einheitlichen Staat Krankheit, Therapie mit Enbrel oder CEL-2000 initiiert wurde und weiterhin für 28 Tage. CEL-2000 verabreicht wurde nur zweimal und Enbrel wurde entweder jeden Tag für die ersten 14 Tage (für die Histopathologie Studien und klinische Immunologie) oder jeden zweiten Tag über die gesamte Studiendauer von 28 Tagen (für die Immunologie nur Studien).
Dr. Zimmerman zuvor berichtet, dass in Studien an Mäusen ähnlich behandelt, CEL-2000 entsprach oder eventuell überlegen Enbrel bei Fortschreiten der Krankheit verlangsamt und das Abnehmen Symptome. Die neuen Daten deuten darauf hin, dass heute, im geimpften Mäusen mit CEL-2000 nach Erscheinen des sichtbaren Krankheit, statistisch signifikant weniger Entzündungen und dauerhafte Schäden im Hinblick auf 1) Knochen Erosion, 2) Knorpel-Zerstörung, und 3) Pannus Bildung beobachtet. Dies sind nur einige der gleichen Parameter, kann man bei rheumatoider Arthritis Schäden beim Menschen. CEL-2000 war so gut, wie und eventuell überlegen, Enbrel zu bremsen weitere Fortschreiten der Krankheit bewertet als von diesen Parametern und histologischen von footpad Schwellung sowie nach außen sichtbare gemeinsame Schäden.
"Es ist sehr spannend zu sehen, die Reduzierung von schweren Schäden rheumatoider Arthritis bei diesen Tieren durch eine einfache Impfung", sagt Dr. Zimmermann. "Ich hoffe, dass CEL-2000 wird einige Tage benutzt werden, um die Schäden, die durch die rheumatoide Arthritis bei Patienten."
CEL-2000 kann eine Reihe potenzieller Vorteile gegenüber den bestehenden rheumatoiden Arthritis Behandlungen. Daten, die im Tierexperimentelle Studien, die mit CEL-2000 zeigen, ist es effektiver mit weniger und kleineren Dosen. Es ist auch potenziell weniger toxisch und mehr Krankheit spezifische Therapie. Schließlich CEL-2000 könnte auch nützlich sein für Patienten, nicht in der Lage sind, zu entnehmen oder kann reagiert auf bereits vorhandene Produkte.
Rheumatoide Arthritis Behandlungen umfassen ein $ 13 Milliarden Markt. Enbrel, einem der führenden Behandlung rheumatoider Arthritis Versand durch Amgen und Wyeth, berichteten US-Umsatz in 2007 auf rund USD 3,2 Milliarden. Enbrel ist ein rekombinanter Protein löslich eines menschlichen TNF-alpha-Rezeptor im Zusammenhang mit Human-IgG Fc. In einigen Fällen, von Mensch oder humanisierter monoklonaler Antikörper gegen TNF-alpha wurden auch für die Therapie bei rheumatoider Arthritis. Diese Therapien zu entfernen oder zu inaktivieren TNF-alpha, einem natürlichen menschlichen Zytokin in vielen Immun-Funktionen für die normale Abwehr.
CEL-SCI's rheumatoider Arthritis Impfstoff CEL-2000 entdeckt wurde als Teil der Arbeit mit der Firma der laufenden Forschungs-und Entwicklungsaktivitäten mit ihren LEAPS (TM) (Epitope Ligand Antigen Presentation System)-Technologie. LEAPS (TM) ist ein neuartiges T-Zell-Modulation-Plattform-Technologie ermöglicht es, dass CEL-SCI zu entwerfen und zu synthetisieren proprietäre immunogens. Jede Krankheit, für die eine Antigen-Sequenz identifiziert wurde, wie Infektionskrankheiten, Parasiten, Autoimmunerkrankungen oder maligne Erkrankungen und Allergien, sind die potenziellen therapeutischen oder präventiven Standorte für die Anwendung von LEAPS (TM)-Technologie.
Das Konzept hinter dem LEAPS (TM)-Technologie ist direkt imitieren Zelle / Zell-Interaktionen auf der T-Zell-Oberfläche mit synthetischen Peptiden. Die LEAPS (TM)-Konstrukte mit den Antigen-Epitop-Krankheit in Verbindung mit einem T-Zell-bindenden Liganden (TCBL) werden kann, hergestellt von der Peptid-Synthese oder durch kovalente Verknüpfung der beiden Peptide. Abhängig von der Art der LEAPS (TM) konstruieren und TCBL verwendet, CEL-SCI ist in der Lage, die direkte Ergebnis der Immunantwort auf die Entwicklung von T-Zell-Funktion in erster Linie mit Effektor T-Zell-Funktionen (T-Lymphozyten; Helfer / Effektor T Lymphozyten, Typ 1 oder 2 [Th1 oder Th2], zytotoxische [Tc] oder Suppressor [TS]). Daher hat es den Anschein, dass die LEAPS (TM)-Konstrukt ist ein chimärer Peptid mit bi-funktionale Verhalten.
das 3 Quartal ist fast rum,immer noch keine Nachrichten zum Start von Phase 3 ????????????????????????????????
Antwort auf Beitrag Nr.: 35.089.610 von VFBLER am 13.09.08 13:30:55 die nächste sinnvolle Nachricht wäre die Fertigstellung der Fabrik ( mit Fotos ), dann brache ich Arbeiter und Fachkräfte die alles bedienen können, und dann kann die P III beginnen.
die nächste sinnvolle Nachricht wäre die Fertigstellung der Fabrik ( mit Fotos ), dann brache ich Arbeiter und Fachkräfte die alles bedienen können, und dann kann die P III beginnen.
--- dafür brauche ich aber erst auch die Leute an denen ich " teste " !!!
 die nächste sinnvolle Nachricht wäre die Fertigstellung der Fabrik ( mit Fotos ), dann brache ich Arbeiter und Fachkräfte die alles bedienen können, und dann kann die P III beginnen.
die nächste sinnvolle Nachricht wäre die Fertigstellung der Fabrik ( mit Fotos ), dann brache ich Arbeiter und Fachkräfte die alles bedienen können, und dann kann die P III beginnen.--- dafür brauche ich aber erst auch die Leute an denen ich " teste " !!!
 Lies mal genau
Lies mal genau 
Wednesday, August 20, 2008
Cel-Sci lets Teva sell cancer drugWashington Business Journal - by Tierney Plumb Staff Reporter
Print Email Reprints RSS Feeds Add to Del.icio.us Digg This CommentsRelated News
Cel-Sci compound gets more play
Duramed parent Barr settles patent dispute
Duramed parent Barr Pharmaceuticals to be acquired by Teva
Generic sales help Barr to 2Q profit hike
Change at the Top
Teva Pharmaceutical Industries Ltd. got the OK to market and distribute Cel-Sci Corp.’s cancer drug Multikine in Israel and Turkey.
Once the drug has been approved, Vienna-based Cel-Sci (AMEX:CVM) will make the product, while the Israel-based generic pharmaceutical giant will be responsible for sales. Revenue will be split evenly between both companies.
The licensing agreement is initially restricted to the areas of head and neck cancer. Teva has the rights, subject to certain conditions, to extend the licensing agreement to include other cancers during the term of this agreement. The drug is currently thought to be useful in treating different tumor types.
Teva will participate in Cel-Sci’s upcoming global Phase 3 clinical trial and fund a portion of the Phase 3 clinical study. Teva’s clinical group will conduct part of the clinical study in Israel.
Last summer, Cel-Sci started constructing a manufacturing facility to produce Multikine for the trial and subsequent sale following approval. The facility is expected to be completed soon.
“We believe that Teva’s expertise and knowledge in successfully conducting large pivotal clinical trials and in developing markets for large unmet medical needs will prove invaluable in maximizing Multikine’s potential,” said Geert Kersten, chief executive of Cel-Sci. “This agreement is consistent with our strategy to share the clinical and regulatory expenses associated with the development of Multikine while retaining rights to market Multikine in North America and Europe.”
Last month, Teva (NASDAQ: TEVA) expanded its U.S. operations when it agreed to acquire Barr Pharmaceuticals Inc., which owns Duramed Pharmaceuticals in Cincinnati, for $7.5 billion.
Reader Comments
CEL-SCI Expects to Take Delivery of New Manufacturing Facility on October 8, 2008
Tuesday September 16, 9:40 am ET
VIENNA, Va., Sept. 16 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (Amex: CVM - News) announced today that it expects to take delivery of the new manufacturing facility for its lead drug Multikine® on October 8, 2008. This dedicated facility will produce the Multikine that will be used for CEL-SCI's pivotal Phase III clinical trial for first-line therapy of previously untreated head and neck cancer patients, and subsequently for sale following approval of the drug. The facility, which cost about $22 million to build, is state of the art and commercial ready.
ADVERTISEMENT
Geert Kersten, CEL-SCI's Chief Executive Officer said, "Multikine started with the idea that activating the immune system to fight cancer could be beneficial and successful, as long as you could activate the immune system before it was weakened by surgery, radiation and chemotherapy. Our clinical studies showed significant benefit to the cancer patients treated with Multikine. We are now in the home stretch. Having our own Multikine dedicated manufacturing facility gives us control and eliminates a great deal of risk from our product development."
CEL-SCI is developing Multikine for approval as a first line indication in head and neck cancer. To that end, the Company's upcoming Phase III clinical trial is an 800 patient clinical study designed to demonstrate that administration of its cancer drug Multikine to head and neck cancer patients before they receive any conventional cancer treatment will increase their survival. Head and neck cancer is one of the world's biggest cancers affecting about 650,000 people per annum worldwide.
About CEL-SCI's Phase III Cancer Drug Multikine:
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck in May 2007. In the summer of 2007 CEL-SCI started construction of the manufacturing facility to produce Multikine for the Phase III trial and subsequent sale following approval. This facility is expected to be completed on October 8, 2008.
Multikine, a patented defined mixture of naturally derived cytokines, is the first immunotherapeutic agent in a new class of drugs called "Immune SIMULATORS". Immune SIMULATORS simulate the way our natural immune system acts in defending us against cancer. As opposed to other immunotherapies which are designed to target a single or limited number of specific antigens or molecules, Immune SIMULATORS are multi-targeted; they simultaneously cause a direct and targeted killing of the specific tumor cells and they activate the immune system to produce a stronger anti-tumor attack on multiple fronts.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
CEL-SCI has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense. Most recently CEL-SCI announced that its newly discovered rheumatoid arthritis vaccine showed excellent results in animal tests.
--------------------------------------------------------------------------------
Source: CEL-SCI Corporation
Tuesday September 16, 9:40 am ET
VIENNA, Va., Sept. 16 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (Amex: CVM - News) announced today that it expects to take delivery of the new manufacturing facility for its lead drug Multikine® on October 8, 2008. This dedicated facility will produce the Multikine that will be used for CEL-SCI's pivotal Phase III clinical trial for first-line therapy of previously untreated head and neck cancer patients, and subsequently for sale following approval of the drug. The facility, which cost about $22 million to build, is state of the art and commercial ready.
ADVERTISEMENT
Geert Kersten, CEL-SCI's Chief Executive Officer said, "Multikine started with the idea that activating the immune system to fight cancer could be beneficial and successful, as long as you could activate the immune system before it was weakened by surgery, radiation and chemotherapy. Our clinical studies showed significant benefit to the cancer patients treated with Multikine. We are now in the home stretch. Having our own Multikine dedicated manufacturing facility gives us control and eliminates a great deal of risk from our product development."
CEL-SCI is developing Multikine for approval as a first line indication in head and neck cancer. To that end, the Company's upcoming Phase III clinical trial is an 800 patient clinical study designed to demonstrate that administration of its cancer drug Multikine to head and neck cancer patients before they receive any conventional cancer treatment will increase their survival. Head and neck cancer is one of the world's biggest cancers affecting about 650,000 people per annum worldwide.
About CEL-SCI's Phase III Cancer Drug Multikine:
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck in May 2007. In the summer of 2007 CEL-SCI started construction of the manufacturing facility to produce Multikine for the Phase III trial and subsequent sale following approval. This facility is expected to be completed on October 8, 2008.
Multikine, a patented defined mixture of naturally derived cytokines, is the first immunotherapeutic agent in a new class of drugs called "Immune SIMULATORS". Immune SIMULATORS simulate the way our natural immune system acts in defending us against cancer. As opposed to other immunotherapies which are designed to target a single or limited number of specific antigens or molecules, Immune SIMULATORS are multi-targeted; they simultaneously cause a direct and targeted killing of the specific tumor cells and they activate the immune system to produce a stronger anti-tumor attack on multiple fronts.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
CEL-SCI has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense. Most recently CEL-SCI announced that its newly discovered rheumatoid arthritis vaccine showed excellent results in animal tests.
--------------------------------------------------------------------------------
Source: CEL-SCI Corporation
 anschnallen, mal sehen wer das registriert
anschnallen, mal sehen wer das registriert 
Antwort auf Beitrag Nr.: 35.145.591 von ALF-FRED am 17.09.08 10:50:05babelfish :
WIEN, VA., Sept. 16 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (Amex: CVM - Nachrichten) heute verkündet worden, dass sie erwartet, Anlieferung der neuen Produktionsanlage für seine Bleidroge Multikine® am 8. Oktober 2008 zu nehmen. Diese engagierte Anlage produziert das Multikine, das für CEL-SCI' verwendet wird; klinische Studie der s-Angelphase III für first-line Therapie der vorher unbehandelten Haupt- und Ansatzkrebspatienten und nachher für Verkauf nach Zustimmung der Droge. Die Anlage, die ungefähr $22 Million kostete, um zu errichten, ist Entwicklungs- und vorbereiten Handels. REKLAMEANZEIGE Geert Kersten, CEL-SCI' s-sagte Generaldirektor, " Multikine begann mit der Idee, dass, das Immunsystem zu aktivieren, um Krebs zu kämpfen vorteilhaft und erfolgreich sein könnte, solange Sie das Immunsystem aktivieren konnten, bevor es durch Chirurgie, Strahlung und Chemotherapie geschwächt wurde. Unsere klinischen Studien zeigten den Krebspatienten bedeutenden Nutzen, die mit Multikine behandelt wurden. Wir sind jetzt in der Ausgangsausdehnung. Haben unserer eigenen Multikine eingeweihten Produktionsanlage gibt uns Steuerung und beseitigt viel Risiko von unserem Produkt development." CEL-SCI entwickelt Multikine für Zustimmung wie erstes Zeilenangabe in Haupt- und Ansatzkrebs. Zu diesem Zweck das Company' klinische Studie der s-ist bevorstehende Phase III eine klinische Studie mit 800 Patienten, die entworfen ist, um diese Verwaltung seines Krebsmedikaments Multikine zu Haupt zu demonstrieren und Ansatzkrebspatienten, bevor sie jede herkömmliche Krebsbehandlung empfangen, erhöhen ihr Überleben. Haupt- und Ansatzkrebs ist einer des world' s-größte Krebse, die pro Jahr ungefähr 650.000 Leute weltweit beeinflussen
WIEN, VA., Sept. 16 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (Amex: CVM - Nachrichten) heute verkündet worden, dass sie erwartet, Anlieferung der neuen Produktionsanlage für seine Bleidroge Multikine® am 8. Oktober 2008 zu nehmen. Diese engagierte Anlage produziert das Multikine, das für CEL-SCI' verwendet wird; klinische Studie der s-Angelphase III für first-line Therapie der vorher unbehandelten Haupt- und Ansatzkrebspatienten und nachher für Verkauf nach Zustimmung der Droge. Die Anlage, die ungefähr $22 Million kostete, um zu errichten, ist Entwicklungs- und vorbereiten Handels. REKLAMEANZEIGE Geert Kersten, CEL-SCI' s-sagte Generaldirektor, " Multikine begann mit der Idee, dass, das Immunsystem zu aktivieren, um Krebs zu kämpfen vorteilhaft und erfolgreich sein könnte, solange Sie das Immunsystem aktivieren konnten, bevor es durch Chirurgie, Strahlung und Chemotherapie geschwächt wurde. Unsere klinischen Studien zeigten den Krebspatienten bedeutenden Nutzen, die mit Multikine behandelt wurden. Wir sind jetzt in der Ausgangsausdehnung. Haben unserer eigenen Multikine eingeweihten Produktionsanlage gibt uns Steuerung und beseitigt viel Risiko von unserem Produkt development." CEL-SCI entwickelt Multikine für Zustimmung wie erstes Zeilenangabe in Haupt- und Ansatzkrebs. Zu diesem Zweck das Company' klinische Studie der s-ist bevorstehende Phase III eine klinische Studie mit 800 Patienten, die entworfen ist, um diese Verwaltung seines Krebsmedikaments Multikine zu Haupt zu demonstrieren und Ansatzkrebspatienten, bevor sie jede herkömmliche Krebsbehandlung empfangen, erhöhen ihr Überleben. Haupt- und Ansatzkrebs ist einer des world' s-größte Krebse, die pro Jahr ungefähr 650.000 Leute weltweit beeinflussen
haben nur wir die New´s kappiert?
Antwort auf Beitrag Nr.: 35.152.447 von VFBLER am 17.09.08 17:47:00 die kennt hier keiner, oder kaum einer - wir werden sehen wenn der Start der P III beginnt was es Wert ist
die kennt hier keiner, oder kaum einer - wir werden sehen wenn der Start der P III beginnt was es Wert ist 

 die kennt hier keiner, oder kaum einer - wir werden sehen wenn der Start der P III beginnt was es Wert ist
die kennt hier keiner, oder kaum einer - wir werden sehen wenn der Start der P III beginnt was es Wert ist 

Antwort auf Beitrag Nr.: 35.145.591 von ALF-FRED am 17.09.08 10:50:05Hi Alfred,
gute News. Die eigene Prosuktionsstaette...dann kann es jetzt endlich losgehen...
Schon naechstes Jahre wird spannend...
Gruss,
Sil
gute News. Die eigene Prosuktionsstaette...dann kann es jetzt endlich losgehen...
Schon naechstes Jahre wird spannend...

Gruss,
Sil
Antwort auf Beitrag Nr.: 35.163.830 von Sillak am 18.09.08 10:05:15 ich hatte schon gedacht dich gibt es nicht mehr, wollen wir hoffen das unsere Ausdauer belohnt wird !!!
ich hatte schon gedacht dich gibt es nicht mehr, wollen wir hoffen das unsere Ausdauer belohnt wird !!!

 ich hatte schon gedacht dich gibt es nicht mehr, wollen wir hoffen das unsere Ausdauer belohnt wird !!!
ich hatte schon gedacht dich gibt es nicht mehr, wollen wir hoffen das unsere Ausdauer belohnt wird !!!
sollte eigentlich schon beim Start der Phase 3 dieses Jahr ein Kurssprung anstehen.Übernahme Phanatasie,siehe Bayer(hat auch eine kleine Klitsche für 210 mio Euro erst jetzt übernommen).Wie lange dauert eine 3 Phase(Jahre)?????
Antwort auf Beitrag Nr.: 35.190.205 von VFBLER am 19.09.08 17:26:02Hi VFBLER!
Ich meine die Studie ist auf zwei bis drei Jahre ausgelegt. Aber man muss jetzt nicht Jahre warten. Ergebnisse werden immer mal wieder praesentiert, so dass man sehr schnell erfahren wird, wie der Status ist.
Gruss,
Sil
Ich meine die Studie ist auf zwei bis drei Jahre ausgelegt. Aber man muss jetzt nicht Jahre warten. Ergebnisse werden immer mal wieder praesentiert, so dass man sehr schnell erfahren wird, wie der Status ist.
Gruss,
Sil


 im Yahoo BoaRD wird gemutmaßt , dass der Rückgang von diesem Artikel kommen könnte
im Yahoo BoaRD wird gemutmaßt , dass der Rückgang von diesem Artikel kommen könnte 


Is this why we are down today?
http://www.biospace.com/news_story.aspx
ImClone Systems Incorporated (NASDAQ: IMCL - News) and Bristol-Myers Squibb Company (NYSE: BMY - News) today announced five-year overall survival data from the pivotal Phase 3 study examining ERBITUX® (cetuximab) combined with radiation in patients with locally or regionally advanced squamous cell carcinoma of the head and neck (SCCHN). These results were presented today at the American Society for Therapeutic Radiology and Oncology (ASTRO) Annual Meeting in Boston.
Analysis of these five-year data demonstrate that the addition of ERBITUX to radiation therapy resulted in a significant increase in median overall survival for patients with SCCHN, when compared to radiation therapy alone [49.0 vs. 29.3 months, respectively; p=0.018, Hazard Ratio (HR)=0.725, 95% CI (0.556-0.946)]. The overall survival rate at five years was 45 percent vs. 36 percent, respectively (p=0.018); survival rate at three years was 55 percent vs. 45 percent (p=0.05), respectively. These data are consistent with results in the current head and neck labeling for ERBITUX, which include the median overall survival rate [49.0 vs. 29.3 months, respectively; p=0.03, HR=0.74, 95% CI (0.57-0.97)].
"ERBITUX in combination with radiation demonstrated a statistically significant improvement in overall survival versus radiation alone at five years,” said James Bonner, M.D., University of Alabama, principal investigator for the study. “These results are important for both physicians and patients because this is a difficult type of cancer to treat."
Antwort auf Beitrag Nr.: 35.248.104 von ALF-FRED am 24.09.08 09:51:53Hi ALF!
Zu finden auf der HP von ImClone...
IMPORTANT SAFETY INFORMATION
Severe allergic reactions, have occurred in 3% of 1485 patients receiving ERBITUX (Cetuximab) during clinical studies. Symptoms can include trouble with breathing, rash, itching, low blood pressure, and/or heart attack. These reactions are due to ERBITUX infusions and have resulted in death on rare occasions. Your doctor or nurse should watch you closely for these symptoms during treatment. Severe allergic reactions require that treatment with ERBITUX be stopped immediately and not started again.
Heart attack and/or sudden death occurred in 2% of 208 patients with head and neck cancer treated with radiation therapy and ERBITUX. Notify your doctor if you have a history of any heart disease.
Die Uberlebensrate sind ohne Srahlungsbehandlung bei MK gleich gross. Entscheidend ist aber, dass MK keine dieser Allergierekationen bisher lieferte.
Falls MK gleich gute Ergebnisse in PIII bringt, dann wird sich der Markt eher fuer MK entscheiden.
Auf jeden Fall ist es gut, dass man ein zweites Produkt am Markt hat (hoffentlich)!
Gruss,
Sil
Zu finden auf der HP von ImClone...
IMPORTANT SAFETY INFORMATION
Severe allergic reactions, have occurred in 3% of 1485 patients receiving ERBITUX (Cetuximab) during clinical studies. Symptoms can include trouble with breathing, rash, itching, low blood pressure, and/or heart attack. These reactions are due to ERBITUX infusions and have resulted in death on rare occasions. Your doctor or nurse should watch you closely for these symptoms during treatment. Severe allergic reactions require that treatment with ERBITUX be stopped immediately and not started again.
Heart attack and/or sudden death occurred in 2% of 208 patients with head and neck cancer treated with radiation therapy and ERBITUX. Notify your doctor if you have a history of any heart disease.
Die Uberlebensrate sind ohne Srahlungsbehandlung bei MK gleich gross. Entscheidend ist aber, dass MK keine dieser Allergierekationen bisher lieferte.
Falls MK gleich gute Ergebnisse in PIII bringt, dann wird sich der Markt eher fuer MK entscheiden.
Auf jeden Fall ist es gut, dass man ein zweites Produkt am Markt hat (hoffentlich)!
Gruss,
Sil
Dann bin ich ja mal auf den 8 Oktober gespannt

Trans Date Filer Ownership Type Price Shares
Sep 30, 2008 ZIMMERMAN DANIEL H
Officer direct Acquisition (Non Open Market) 0.4000 6,573
Sep 30, 2008 CIPRIANO JOHN
Officer direct Acquisition (Non Open Market) 0.4000 6,388
Sep 30, 2008 TALOR EYAL
Officer direct Acquisition (Non Open Market) 0.4000 6,000
Sep 30, 2008 PRICHEP PATRICIA B
Officer direct Acquisition (Non Open Market) 0.4000 6,939
Sep 30, 2008 KERSTEN GEERT R
Officer direct Acquisition (Non Open Market) 0.4000 8,437
die Ruhe vor dem Sturm?
Antwort auf Beitrag Nr.: 35.384.325 von VFBLER am 02.10.08 19:46:29 CEL-SCI Enters Agreement With National Institutes of Health Clinical Center To Determine the Molecular Basis of Multikine Anti-Tumor Effect
CEL-SCI Enters Agreement With National Institutes of Health Clinical Center To Determine the Molecular Basis of Multikine Anti-Tumor Effect
Tuesday October 7, 10:34 am ET
VIENNA, Va., Oct. 7 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE: CVM - News) announced today that it has entered into a Material Transfer Agreement (MTA) with the National Institutes of Health Clinical Center (NIHCC) and the laboratory of Dr. Francesco M. Marincola, M.D., to investigate the molecular basis of changes to the tumor microenvironment caused by CEL-SCI's cancer drug Multikine. Dr. Marincola is the Chief of the Infectious Disease and Immunogentics Section, Department of Transfusion Medicine Clinical Center, National Institutes of Health, a leading national and international medical research laboratory.
ADVERTISEMENT
Pursuant to the agreement, CEL-SCI will provide tumor samples of Multikine treated and untreated matched control patients to the NIHCC, which will then use the latest molecular genomic microarray technology developed by Dr. Marincola's laboratory to look for molecular genomic differences in the tumor microenvironment in patients with squamous cell carcinoma of the head and neck. Should these pre-clinical experiments find significant differences between the Multikine treated and untreated patients, a formal collaboration between CEL-SCI and the NIHCC may be developed in order to expand the study to include patients from CEL-SCI's Pivotal global Phase III clinical trial.
"Multikine's potential to change the tumor microenvironment and, in doing so, break tumor tolerance has been a long-time goal of anti-cancer immunotherapy. We are very excited to be working with Dr. Marincola and the NIH Clinical Center to determine the molecular basis of Multikine's ability to cause tumor microenvironment changes," said Dr. Talor, Senior VP of Research and Manufacturing at CEL-SCI and the developer of Multikine.
About CEL-SCI's Phase III Cancer Drug Multikine:
CEL-SCI is developing Multikine for approval as a first-line indication in head and neck cancer. The company's upcoming Pivotal global Phase III clinical trial is an 800 patient study designed to demonstrate that administration of its anti-cancer drug Multikine to head and neck cancer patients before they receive their first conventional cancer treatment will increase their survival. Head and neck cancer is one of the world's biggest cancers affecting about 650,000 people per annum.
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine for the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck, in May 2007. In the summer of 2007 CEL-SCI started construction of the manufacturing facility to produce Multikine for the global Phase III trial and subsequent sale following approval. This facility is expected to be completed soon.
Multikine, a patented defined mixture of naturally derived cytokines is the first immunotherapeutic agent in a new class of drugs called "Immune SIMULATORS." Immune SIMULATORS simulate the way our natural immune system acts in defending us against cancer. As opposed to other immunotherapies which are designed to target a single or limited number of specific antigens or molecules, Immune SIMULATORS are multi-targeted; they simultaneously cause a direct and targeted killing of the specific tumor cells and they activate the immune system to produce a stronger anti-tumor attack on multiple fronts.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
The Company has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense.
NO ENDORSEMENT OF ANY ORGANIZATION, PRODUCT OR SERVICE MENTIONED IN THIS ARTICLE IS INTENDED OR INFERRED BY THE NATIONAL INSTITUTES OF HEALTH OR ITS EMPLOYEES.
 CEL-SCI Enters Agreement With National Institutes of Health Clinical Center To Determine the Molecular Basis of Multikine Anti-Tumor Effect
CEL-SCI Enters Agreement With National Institutes of Health Clinical Center To Determine the Molecular Basis of Multikine Anti-Tumor EffectTuesday October 7, 10:34 am ET
VIENNA, Va., Oct. 7 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE: CVM - News) announced today that it has entered into a Material Transfer Agreement (MTA) with the National Institutes of Health Clinical Center (NIHCC) and the laboratory of Dr. Francesco M. Marincola, M.D., to investigate the molecular basis of changes to the tumor microenvironment caused by CEL-SCI's cancer drug Multikine. Dr. Marincola is the Chief of the Infectious Disease and Immunogentics Section, Department of Transfusion Medicine Clinical Center, National Institutes of Health, a leading national and international medical research laboratory.
ADVERTISEMENT
Pursuant to the agreement, CEL-SCI will provide tumor samples of Multikine treated and untreated matched control patients to the NIHCC, which will then use the latest molecular genomic microarray technology developed by Dr. Marincola's laboratory to look for molecular genomic differences in the tumor microenvironment in patients with squamous cell carcinoma of the head and neck. Should these pre-clinical experiments find significant differences between the Multikine treated and untreated patients, a formal collaboration between CEL-SCI and the NIHCC may be developed in order to expand the study to include patients from CEL-SCI's Pivotal global Phase III clinical trial.
"Multikine's potential to change the tumor microenvironment and, in doing so, break tumor tolerance has been a long-time goal of anti-cancer immunotherapy. We are very excited to be working with Dr. Marincola and the NIH Clinical Center to determine the molecular basis of Multikine's ability to cause tumor microenvironment changes," said Dr. Talor, Senior VP of Research and Manufacturing at CEL-SCI and the developer of Multikine.
About CEL-SCI's Phase III Cancer Drug Multikine:
CEL-SCI is developing Multikine for approval as a first-line indication in head and neck cancer. The company's upcoming Pivotal global Phase III clinical trial is an 800 patient study designed to demonstrate that administration of its anti-cancer drug Multikine to head and neck cancer patients before they receive their first conventional cancer treatment will increase their survival. Head and neck cancer is one of the world's biggest cancers affecting about 650,000 people per annum.
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine for the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck, in May 2007. In the summer of 2007 CEL-SCI started construction of the manufacturing facility to produce Multikine for the global Phase III trial and subsequent sale following approval. This facility is expected to be completed soon.
Multikine, a patented defined mixture of naturally derived cytokines is the first immunotherapeutic agent in a new class of drugs called "Immune SIMULATORS." Immune SIMULATORS simulate the way our natural immune system acts in defending us against cancer. As opposed to other immunotherapies which are designed to target a single or limited number of specific antigens or molecules, Immune SIMULATORS are multi-targeted; they simultaneously cause a direct and targeted killing of the specific tumor cells and they activate the immune system to produce a stronger anti-tumor attack on multiple fronts.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
The Company has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense.
NO ENDORSEMENT OF ANY ORGANIZATION, PRODUCT OR SERVICE MENTIONED IN THIS ARTICLE IS INTENDED OR INFERRED BY THE NATIONAL INSTITUTES OF HEALTH OR ITS EMPLOYEES.
fallender kurs???
hat heut nicht sollen die neue Fabrik in betrieb kommen sollen?
Antwort auf Beitrag Nr.: 35.483.208 von VFBLER am 08.10.08 19:42:02jepp - mal sehen - ist ja überall land unter, 

CEL-SCI Takes Delivery of New Manufacturing Facility
Thursday October 9, 9:45 am ET
VIENNA, Va., Oct. 9 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE: CVM - News) announced today that it has taken delivery of the new manufacturing facility for its lead drug Multikine®. This dedicated facility, located in the Baltimore area, will produce the Multikine that will be used for CEL-SCI's pivotal Phase III clinical trial for first-line therapy of previously untreated head and neck cancer patients, and subsequently for sale following approval of the drug. The facility, which cost about $22 million to build, is state of the art and will soon be commercial ready. As it stands today, the facility can produce about $600 million worth of drug per year. Within one year it can be built out to product almost $2 billion worth of drug per year.
ADVERTISEMENT
Geert Kersten, CEL-SCI's Chief Executive Officer said, "Multikine started with the idea that activating the immune system to fight cancer could be beneficial and successful, as long as you could activate the immune system before it was weakened by surgery, radiation and chemotherapy. Our clinical studies showed significant benefit to the cancer patients treated with Multikine. We are now in the home stretch. Having our own Multikine dedicated manufacturing facility gives us control and eliminates a great deal of risk from our product development. Our next step is to completely validate the facility and to bring it on line for manufacturing."
CEL-SCI is developing Multikine for approval as a first line indication in head and neck cancer. To that end, the Company's upcoming Phase III clinical trial is an 800 patient clinical study designed to demonstrate that administration of its cancer drug Multikine to head and neck cancer patients before they receive any conventional cancer treatment will increase their survival. Head and neck cancer is one of the world's biggest cancers affecting about 650,000 people per annum worldwide.
About CEL-SCI's Phase III Cancer Drug Multikine:
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck in May 2007. In the summer of 2007 CEL-SCI started construction of the manufacturing facility to produce Multikine for the Phase III trial and subsequent sale following approval.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
CEL-SCI has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense. Most recently CEL-SCI announced that its newly discovered rheumatoid arthritis vaccine showed excellent results in animal tests.
Thursday October 9, 9:45 am ET
VIENNA, Va., Oct. 9 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE: CVM - News) announced today that it has taken delivery of the new manufacturing facility for its lead drug Multikine®. This dedicated facility, located in the Baltimore area, will produce the Multikine that will be used for CEL-SCI's pivotal Phase III clinical trial for first-line therapy of previously untreated head and neck cancer patients, and subsequently for sale following approval of the drug. The facility, which cost about $22 million to build, is state of the art and will soon be commercial ready. As it stands today, the facility can produce about $600 million worth of drug per year. Within one year it can be built out to product almost $2 billion worth of drug per year.
ADVERTISEMENT
Geert Kersten, CEL-SCI's Chief Executive Officer said, "Multikine started with the idea that activating the immune system to fight cancer could be beneficial and successful, as long as you could activate the immune system before it was weakened by surgery, radiation and chemotherapy. Our clinical studies showed significant benefit to the cancer patients treated with Multikine. We are now in the home stretch. Having our own Multikine dedicated manufacturing facility gives us control and eliminates a great deal of risk from our product development. Our next step is to completely validate the facility and to bring it on line for manufacturing."
CEL-SCI is developing Multikine for approval as a first line indication in head and neck cancer. To that end, the Company's upcoming Phase III clinical trial is an 800 patient clinical study designed to demonstrate that administration of its cancer drug Multikine to head and neck cancer patients before they receive any conventional cancer treatment will increase their survival. Head and neck cancer is one of the world's biggest cancers affecting about 650,000 people per annum worldwide.
About CEL-SCI's Phase III Cancer Drug Multikine:
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck in May 2007. In the summer of 2007 CEL-SCI started construction of the manufacturing facility to produce Multikine for the Phase III trial and subsequent sale following approval.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
CEL-SCI has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense. Most recently CEL-SCI announced that its newly discovered rheumatoid arthritis vaccine showed excellent results in animal tests.


 aufbauen und anfangen, ist doch super - warte mal wenn die ersten Fotos veröffentlich werden - etweder ist alles Schmuh ( was ich nicht glaube ) oder wir sind wirklich die wenigen die es über Jahre beobachten und daran glauben und dann sind wir gut dabei,
aufbauen und anfangen, ist doch super - warte mal wenn die ersten Fotos veröffentlich werden - etweder ist alles Schmuh ( was ich nicht glaube ) oder wir sind wirklich die wenigen die es über Jahre beobachten und daran glauben und dann sind wir gut dabei, so schnell kann man garnicht gucken - natürlich müssen die PIII Ergebnisse stimmen !!!
ich tippe auf 50/50 erheblich besser als Lotto, habe das Geld schon abgeschrieben und würde auch nie bei 2 Euro rausgehen, alles oder nix

das war eigentlich mein zielkurs,2 euro,hab zwar nur 16k.aber jetzt könnte man mit dem Geld gute aktien billig kaufen
Antwort auf Beitrag Nr.: 35.500.516 von ALF-FRED am 09.10.08 19:19:04 wenn das Zeug sich durchsetzt und so gut ist, dann ist 30 Dollar minimum !!!
wenn das Zeug sich durchsetzt und so gut ist, dann ist 30 Dollar minimum !!!
wenn es nix taugt - ist die Kohle weg, oder ich bekomme plus minus, hab ja nicht viel für bezahlt,
 wenn das Zeug sich durchsetzt und so gut ist, dann ist 30 Dollar minimum !!!
wenn das Zeug sich durchsetzt und so gut ist, dann ist 30 Dollar minimum !!!wenn es nix taugt - ist die Kohle weg, oder ich bekomme plus minus, hab ja nicht viel für bezahlt,

bin mal gespannt wieviel Geld noch da ist?
Wie lange es noch reicht?
und ob es nicht besser wär eine Pharmafirma ins Boot zu holen?
Die Kreditkrise wird halt vor unserer CelSci auch kein halt machen.
Wie lange es noch reicht?
und ob es nicht besser wär eine Pharmafirma ins Boot zu holen?
Die Kreditkrise wird halt vor unserer CelSci auch kein halt machen.
Antwort auf Beitrag Nr.: 35.533.475 von VFBLER am 11.10.08 19:43:35 da könntest du Recht haben, eventuell Risikokapital od. Geld aus der Schweiz - Max wird bestimmt Beziehungen haben
da könntest du Recht haben, eventuell Risikokapital od. Geld aus der Schweiz - Max wird bestimmt Beziehungen haben 
 da könntest du Recht haben, eventuell Risikokapital od. Geld aus der Schweiz - Max wird bestimmt Beziehungen haben
da könntest du Recht haben, eventuell Risikokapital od. Geld aus der Schweiz - Max wird bestimmt Beziehungen haben 
kursaufschwung an uns vorbei gegangen,scheiße
heutiges Volumen in den USA jetzt schon über 480.000 ???
 mal sehen was morgen oder Montag kommt, wir werden sehen - kann sich nur noch um eine kurze Zeit handeln, ich hoffe das das warten sich gelohnt hat !!!
mal sehen was morgen oder Montag kommt, wir werden sehen - kann sich nur noch um eine kurze Zeit handeln, ich hoffe das das warten sich gelohnt hat !!! 
http://www.marketwire.com/press-release/Industrial-Info-Reso…
Oct 17, 2008 08:00 ET
BE&K Building Group Completes $22 Million Biomanufacturing Plant in Baltimore for CEL-SCI, an Industrial Info News Alert

Oct 17, 2008 08:00 ET
BE&K Building Group Completes $22 Million Biomanufacturing Plant in Baltimore for CEL-SCI, an Industrial Info News Alert

Antwort auf Beitrag Nr.: 35.602.761 von Plaste am 17.10.08 14:43:02 hab es mal nach amiland gestellt - da wissen die ja nix -
hab es mal nach amiland gestellt - da wissen die ja nix -
supi Info, warum springt denn der Kurs nicht an ???
thanks Alf-Fred


 hab es mal nach amiland gestellt - da wissen die ja nix -
hab es mal nach amiland gestellt - da wissen die ja nix -supi Info, warum springt denn der Kurs nicht an ???
thanks Alf-Fred



Antwort auf Beitrag Nr.: 35.605.220 von ALF-FRED am 17.10.08 18:46:23Gute Frage.
Wobei ich darauf keine Antwort weiß.
Aber bei CVM ist ja bekanntlich alles möglich. Egal in welche Richtung.
mfg Plaste
PS: Kann ja nicht mehr lange dauern...
Wobei ich darauf keine Antwort weiß.
Aber bei CVM ist ja bekanntlich alles möglich. Egal in welche Richtung.
mfg Plaste
PS: Kann ja nicht mehr lange dauern...

also fabrik ist nun fertiggestellt?
Antwort auf Beitrag Nr.: 35.611.332 von VFBLER am 18.10.08 10:58:08 so deute ich das, kann doch auch nix englisch gut -
so deute ich das, kann doch auch nix englisch gut -
mal sehn, die werden doch wohl eine news bringen die Tage !!!!
 so deute ich das, kann doch auch nix englisch gut -
so deute ich das, kann doch auch nix englisch gut -mal sehn, die werden doch wohl eine news bringen die Tage !!!!
Antwort auf Beitrag Nr.: 35.615.810 von ALF-FRED am 18.10.08 21:13:37

 die Lieferadresse mal bei google earth eingeben und schwupps die wupps seht ihr das neue Firmengebäude von oben
die Lieferadresse mal bei google earth eingeben und schwupps die wupps seht ihr das neue Firmengebäude von oben 





 die Lieferadresse mal bei google earth eingeben und schwupps die wupps seht ihr das neue Firmengebäude von oben
die Lieferadresse mal bei google earth eingeben und schwupps die wupps seht ihr das neue Firmengebäude von oben 



der euro kommt uns ja mal zu gute
Antwort auf Beitrag Nr.: 35.646.892 von VFBLER am 21.10.08 20:01:48 ich hätte nie gedacht, dass ich bei meinen " günstigenen
ich hätte nie gedacht, dass ich bei meinen " günstigenen  " Durchschnittskursen noch bei - 30 % lande !!!
" Durchschnittskursen noch bei - 30 % lande !!!
würd ja gerne noch nachkaufen, aber irgendwie kommt hier nix mehr, auf News springt der Kurs nicht mehr an, nix mehr neues auf der Homepage, keine Fotos die mal von der Firma aktualisiert werden, etc.,
 Silak ist auch weg
Silak ist auch weg 

hoffentlich ärger ich mich nicht in ein paar Tagen, denn ich hab als Hobbyaktionär einfach keinen Bock mehr auf die ganze Scheiße, jeder betrügt und bescheißt ( siehe Bankenskandal ) und der kleinen Anleger, der sich etwas mehr Sicherheit für sein Alter wünscht schaut dumm in die Röhre.
 ich hätte nie gedacht, dass ich bei meinen " günstigenen
ich hätte nie gedacht, dass ich bei meinen " günstigenen  " Durchschnittskursen noch bei - 30 % lande !!!
" Durchschnittskursen noch bei - 30 % lande !!!würd ja gerne noch nachkaufen, aber irgendwie kommt hier nix mehr, auf News springt der Kurs nicht mehr an, nix mehr neues auf der Homepage, keine Fotos die mal von der Firma aktualisiert werden, etc.,
 Silak ist auch weg
Silak ist auch weg 

hoffentlich ärger ich mich nicht in ein paar Tagen, denn ich hab als Hobbyaktionär einfach keinen Bock mehr auf die ganze Scheiße, jeder betrügt und bescheißt ( siehe Bankenskandal ) und der kleinen Anleger, der sich etwas mehr Sicherheit für sein Alter wünscht schaut dumm in die Röhre.

dachte ich war besoffen,wo ich heut nacht heim gekommen bin und cel sci im plus war.aber sie wären im plus,ich aber auch betrunken
Antwort auf Beitrag Nr.: 35.790.389 von VFBLER am 01.11.08 16:23:28

 es macht keinen Spass mehr, ich verstehe es auch nicht mehr
es macht keinen Spass mehr, ich verstehe es auch nicht mehr 


jemand eine Idee ?
Alfred


 es macht keinen Spass mehr, ich verstehe es auch nicht mehr
es macht keinen Spass mehr, ich verstehe es auch nicht mehr 


jemand eine Idee ?
Alfred
 CEL-SCI Announces Expansion of Exclusive Licensing Agreement for Cancer Drug Multikine
CEL-SCI Announces Expansion of Exclusive Licensing Agreement for Cancer Drug MultikineMonday November 3, 9:45 am ET
Partner to Make Direct Investment in CEL-SCI
VIENNA, Va., Nov. 3 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE: CVM - News) announced today that it has expanded its exclusive licensing agreement for Multikine® with Orient Europharma Co., Ltd. ("Orient Europharma"), a leading pharmaceutical company from Taiwan. The new agreement extends the Multikine collaboration to also cover South Korea, the Philippines, Australia and New Zealand. The licensing agreement initially focuses on the areas of head and neck cancer, nasopharyngeal cancer and potentially cervical cancer.
ADVERTISEMENT
As part of this new agreement, Orient Europharma will invest $500,000 in CEL-SCI and fund a portion of the Company's global Phase III clinical trial for Multikine due to start in 2009. Orient Europharma's clinical group will conduct part of the clinical study in its territory. In addition to making a direct investment in the Company and funding a portion of the clinical trials, Orient Europharma will also be responsible for registering the product in the Territory. Once Multikine has been approved, CEL-SCI will be responsible for manufacturing the product, while Orient Europharma will be responsible for sales in the Territory. Revenues will be split between CEL-SCI and Orient Europharma.
"We are pleased that Orient Europharma has recognized the potential for Multikine and has extended its license into more countries," said Geert Kersten, Chief Executive Officer of CEL-SCI. "We expect enrollment in Taiwan to be very speedy, particularly with Orient Europharma running that part of the Phase III study. Orient Europharma proved that it is capable of running part of an international Phase III cancer trial when it successfully executed its part in the Phase III clinical trial that led to the approval of Navelbine®."
CEL-SCI has also licensed certain rights related to Multikine to Teva Pharmaceuticals in Israel and Turkey.
CEL-SCI is developing Multikine for approval as a first line indication in head and neck cancer. To that end, the Company's upcoming Phase III clinical trial is an 800 patient clinical study designed to demonstrate that administration of its cancer drug Multikine to head and neck cancer patients before they receive any conventional cancer treatment will increase their survival. Head and neck cancer is one of the world's biggest cancers affecting about 600,000 people per annum worldwide.
About CEL-SCI's Phase III Cancer Drug Multikine:
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck. Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
CEL-SCI has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense. Most recently CEL-SCI announced that its newly discovered rheumatoid arthritis vaccine showed excellent results in animal tests.
 ich hab mal nicht die Nachricht - sondern die E Mail Benachrichtigung übersetzt !!! ( Babelfish hat natürlich übersetzt
ich hab mal nicht die Nachricht - sondern die E Mail Benachrichtigung übersetzt !!! ( Babelfish hat natürlich übersetzt  )
)CEL-SCI verkündet Expansion der exklusiven Lizenzvereinbarung für Krebsmedikament Multikine Partner, UM DIREKTINVESTITION IN CEL-SCI ZU BILDEN Wien, VA, 3. November 2008 - CEL-SCI Corporation (NYSE Alternext US: CVM) heute verkündet, dass es seine exklusive Lizenzvereinbarung für Multikine® mit Orient Europharma Co. erweitert hat, Ltd. („Orient Europharma "), ein führendes pharmazeutisches Unternehmen von Taiwan. Die neue Vereinbarung verlängert die Multikine Zusammenarbeit, um Südkorea, die Philippinen, Australien und Neuseeland auch zu bedecken. Die Lizenzvereinbarung konzentriert zuerst sich auf die Bereiche Haupt- und Ansatzkrebses, des Nasenrachenraumkrebses und des möglicherweise Gebärmutterkrebses. Als Teil dieser neuen Vereinbarung investiert Orient Europharma $500.000 in CEL-SCI und finanziert einen Teil der globalen klinischen Studie der Phase III der Firma für Multikine wegen des Anfangs 2009. Orient Europharma `s klinische Gruppe leitet Teil der klinischen Studie in seiner Gegend. Zusätzlich zur Herstellung einer Direktinvestition in der Firma und zur Finanzierung eines Teils der klinischen Studien, ist Orient Europharma auch für das Registrieren des Produktes in der Gegend verantwortlich. Sobald Multikine genehmigt worden ist, ist CEL-SCI für die Herstellung des Produktes verantwortlich, während Orient Europharma für Verkäufe in der Gegend verantwortlich ist. Einkommen werden zwischen CEL-SCI und Orient Europharma aufgespaltet. „Wir sind, dass Orient Europharma hat das Potenzial für Multikine erkannt und hat seine Lizenz in mehr Länder verlängert,“ sagten Geert Kersten, Generaldirektor von CEL-SCI erfreut. „Wir erwarten Einschreibung in Taiwan, um, besonders mit Betrieb Orient-Europharma sehr schnell zu sein, der Teil der Studie der Phase III. Orientieren Sie Europharma prüfte, dass es zum Betrieb des Teils eines internationalen Krebsversuches der Phase III fähig ist, als es erfolgreich sein Teil in der klinischen Studie der Phase III durchführte, die das führte zu die Zustimmung von Navelbine®.“ CEL-SCI hat auch bestimmte Rechte genehmigt, die auf Multikine zu den Teva pharmazeutischen Produkten in Israel und in der Türkei bezogen werden. CEL-SCI entwickelt Multikine für Zustimmung wie erstes Zeilenangabe in Haupt- und Ansatzkrebs. Zu diesem Zweck ist die bevorstehende klinische Studie der Phase III der Firma eine klinische Studie mit 800 Patienten, die entworfen ist, um diese Verwaltung seines Krebsmedikaments Multikine zu Haupt zu demonstrieren und Ansatzkrebspatienten, bevor sie jede herkömmliche Krebsbehandlung empfangen, erhöhen ihr Überleben. Haupt- und Ansatzkrebs ist einer größten Krebse der Welt, die pro Jahr ungefähr 600.000 Leute weltweit beeinflussen. Ungefähr Krebsmedikament Multikine CEL-SCIS der Phasen-III: In den klinischen Studien der Phase II wurde Multikine, um sicher zu sein und gut-zugelassen, gezeigt und das patients' zu verbessern; Gesamtüberleben durch 33% an einem Mittelpunkt von drei und an einer Hälfte Jahr Chirurgie folgend. Das US-Behörde zur Überwachung von Nahrungs- und Arzneimitteln (FDA) gab grünen Licht für eine klinische Studie der Phase III mit Multikine und bewilligte Waisendrogestatus Multikine in der neoadjuvant Therapie des Krebsgeschwürs der squamous Zelle (Krebs) des Kopfes und des Ansatzes. Multikine ist auch das erste immunotherapeutic Mittel, das als first-line Standard der Sorgfaltbehandlung für Krebs sich entwickelt wird. Es wird vor jeder möglicher anderen Krebstherapie ausgeübt, weil der der Zeitraum ist, als die immune Antitumorantwort noch völlig aktiviert werden kann. Sobald der Patient Krankheit vorangebracht oder Chirurgie hatte hat oder Strahlung und/oder Chemotherapie empfangen hat, wird das Immunsystem streng geschwächt und in der Lage ist weniger, eine wirkungsvolle immune Antitumorantwort anzubringen. Andere Immunotherapies werden ausgeübt, nachdem der Patient Chemotherapie- und/oder Strahlentherapie empfangen hat, die ihre Wirksamkeit begrenzen kann. CEL-SCI hat Betriebe in Wien, in Virginia und in Baltimore, Maryland. CEL-SCI' s andere Produkte, die z.Z. im präklinischen Stadium sind, haben Schutz gegen einige Krankheiten in den Tiertests gezeigt und werden gegen die Krankheiten geprüft, die mit Bio-verteidigung verbunden sind. Kürzlich verkündete CEL-SCI, dass sein eben entdeckter Impfstoff der rheumatischen Arthritis ausgezeichnete Resultate in den Tiertests zeigte. Wenn Sie von den zukünftigen eMail-Mitteilungen entfernt werden möchten, antworten Sie bitte auf cel-sci@cel-sci.com und Art ENTFERNEN in der vorbehaltlichen Linie. Wir entschuldigen uns für jede mögliche Unannehmlichkeit.
Fabrik ist fertig,jetzt warten wir auf den start der Phase 3?Die New´s heut mit der drittklassigen Pharmafirma kam man auch vergessen.Da hat man lieber ein richtige Firma an Bord geholt.

Antwort auf Beitrag Nr.: 35.813.248 von VFBLER am 03.11.08 18:38:45 Anfang 2009 ( weltweit )steht in der Übersetzung
Anfang 2009 ( weltweit )steht in der Übersetzung 
 Anfang 2009 ( weltweit )steht in der Übersetzung
Anfang 2009 ( weltweit )steht in der Übersetzung 
Antwort auf Beitrag Nr.: 35.813.248 von VFBLER am 03.11.08 18:38:45Hallo VFBLER,
Anfang 2009 find ich persönlich nicht unbdingt toll. Schließlich wurde bis jetzt immer von Ende 2008 gesprochen. Wobei das wohl schon einige wussten und der Kurs dementsprechend reagiert hat.
Aber wollen wir vorwärts schauen:
http://www.oep.com.tw/history.html
Die "drittklassige Pharmafirma" hat über 600 Mitarbeiter. Weiteres findet Ihr unter dem angegebenen Link. Im übrigen sind auch die internationalen Kooperationspartner sehr interessant ( Celltech USA, Elan - Ireland, Wyeth - ASIA und viele andere)
mfg Plaste
Anfang 2009 find ich persönlich nicht unbdingt toll. Schließlich wurde bis jetzt immer von Ende 2008 gesprochen. Wobei das wohl schon einige wussten und der Kurs dementsprechend reagiert hat.
Aber wollen wir vorwärts schauen:
http://www.oep.com.tw/history.html
Die "drittklassige Pharmafirma" hat über 600 Mitarbeiter. Weiteres findet Ihr unter dem angegebenen Link. Im übrigen sind auch die internationalen Kooperationspartner sehr interessant ( Celltech USA, Elan - Ireland, Wyeth - ASIA und viele andere)
mfg Plaste
Antwort auf Beitrag Nr.: 35.815.841 von Plaste am 03.11.08 21:59:49Hi Plast!
Ist zwar kein Gigant am Pharmahimmel aber kann zumnindest ein solides Wachstum nachweisen. Keien krummen Zahlen, was doch sehr zuversichtlich stimmt...
Nun kann man auch auf 2009 warten...so viel Zeit muss sein
Egal was kommt, es wird spannend!
Gruss,
Sil
Ist zwar kein Gigant am Pharmahimmel aber kann zumnindest ein solides Wachstum nachweisen. Keien krummen Zahlen, was doch sehr zuversichtlich stimmt...
Nun kann man auch auf 2009 warten...so viel Zeit muss sein

Egal was kommt, es wird spannend!
Gruss,
Sil
Antwort auf Beitrag Nr.: 35.815.841 von Plaste am 03.11.08 21:59:49Aber zum Beispiel ist TEVA, die fuer den Vertrieb in Israel und der Tuerkei verantwortlich sind...
http://www.tevapharm.com/pdf/Q2FactSheet290708.pdf
...ein bisschen groesser.
Aber ich denke nicht, dass die Groesse der kooperierenden Pharmafirmen in der Phase III uebr den Erfolg von MK entscheiden werden...
Gruss,
Sil
http://www.tevapharm.com/pdf/Q2FactSheet290708.pdf
...ein bisschen groesser.
Aber ich denke nicht, dass die Groesse der kooperierenden Pharmafirmen in der Phase III uebr den Erfolg von MK entscheiden werden...
Gruss,
Sil
Antwort auf Beitrag Nr.: 35.817.930 von Sillak am 04.11.08 02:06:16Hallo Sillak,
sehe ich auch so. Entscheidend ist das Produkt und dessen Nutzen.
Im Augenblick habe ich nur ein wenig Angst, dass unsere CVM kurzfristig übernommen wird. Bei einer MK von nicht einmal 40 Mio USD
ist meine Sorge wohl sehr berechtigt.
Bei Corixa hatte ich ein ähnliches Spiel durch. Sie fiel und viel über einen längeren Zeitraum um im Anschluss für "n Abl un n Ei" übernommen zu werden.
übernommen zu werden.
mfg
Plaste
PS: Hatte schon gedacht, Du bist nicht mehr dabei.
sehe ich auch so. Entscheidend ist das Produkt und dessen Nutzen.
Im Augenblick habe ich nur ein wenig Angst, dass unsere CVM kurzfristig übernommen wird. Bei einer MK von nicht einmal 40 Mio USD
ist meine Sorge wohl sehr berechtigt.
Bei Corixa hatte ich ein ähnliches Spiel durch. Sie fiel und viel über einen längeren Zeitraum um im Anschluss für "n Abl un n Ei"
 übernommen zu werden.
übernommen zu werden.mfg
Plaste
PS: Hatte schon gedacht, Du bist nicht mehr dabei.

Antwort auf Beitrag Nr.: 35.818.486 von Plaste am 04.11.08 06:13:46Also....gerade jetzt sollte man doch dabei sein.
Auf der HP von CEL-SCI gibt es ja diesen Link mit den Bericht ueber die kommenden 10 Blockbuster.
Einige sind in der Pipeline von bereits grossen Pharma(riesen) andere jedoch noch eher Firmen bescheidener Groesse.
Besonder Nicox scheint hier interessant. Wenn man es richtig deutet, dann ist die Kurshausse auf gute Resultate in der PIII zurueckzufuehren...
Der Kursrueckgang dann wohl eher mit der Tatsache von Gewinnmitnahmen und der Tatsache, dass bs zum ertsen Geld noch ein wenig Zeit vergehen kann. Ich habe jetzt nicht alles bis in das letzte Detail gelesen, deswegen kann ich auch nicht sagen, wann der erste Cashflow eingeplant ist...
Bei einem parallelen Verlauf wird es also schon 2009 sehr interessant.
Gruss,
Sil
Auf der HP von CEL-SCI gibt es ja diesen Link mit den Bericht ueber die kommenden 10 Blockbuster.
Einige sind in der Pipeline von bereits grossen Pharma(riesen) andere jedoch noch eher Firmen bescheidener Groesse.
Besonder Nicox scheint hier interessant. Wenn man es richtig deutet, dann ist die Kurshausse auf gute Resultate in der PIII zurueckzufuehren...
Der Kursrueckgang dann wohl eher mit der Tatsache von Gewinnmitnahmen und der Tatsache, dass bs zum ertsen Geld noch ein wenig Zeit vergehen kann. Ich habe jetzt nicht alles bis in das letzte Detail gelesen, deswegen kann ich auch nicht sagen, wann der erste Cashflow eingeplant ist...
Bei einem parallelen Verlauf wird es also schon 2009 sehr interessant.
Gruss,
Sil
Antwort auf Beitrag Nr.: 35.819.205 von Sillak am 04.11.08 09:32:30denk mal wenn pfizer oder ein anderer großer konzern partner wär,hätten wir schon längst einen anderen kurs.mit finanzierungen müßten wir uns auch nicht rummplagen.zum schluß dann noch dieübernahmefantasie.
hier geht gar nichts mehr?
Antwort auf Beitrag Nr.: 35.875.015 von VFBLER am 07.11.08 19:38:25 unter 20 werd ich nochmal nachfassen , wenn nicht auch nicht schlimm,
unter 20 werd ich nochmal nachfassen , wenn nicht auch nicht schlimm,
ich verstehe das nicht mehr, hab aber auch keine Ahnung - wird Zeit das ich bald mit der Börse aufhöre
 unter 20 werd ich nochmal nachfassen , wenn nicht auch nicht schlimm,
unter 20 werd ich nochmal nachfassen , wenn nicht auch nicht schlimm, ich verstehe das nicht mehr, hab aber auch keine Ahnung - wird Zeit das ich bald mit der Börse aufhöre

New Data Points to Long Lasting Beneficial Effects of CEL-SCI's Rheumatoid Arthritis Vaccine
Friday November 14, 10:00 am ET
VIENNA, Va., Nov. 14 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE: CVM - News) announced today the presentation of new rheumatoid arthritis data at the 6th annual GTCbio Vaccine Conference in Vienna, Va. The data, presented by Dr. Daniel Zimmerman, Senior Vice President of Research, Cellular Immunology of CEL-SCI, indicate that CEL-SCI's rheumatoid arthritis treatment vaccine CEL-2000 prevents or retards the permanent tissue damage caused by rheumatoid arthritis. The long term results obtained with CEL-2000 vaccine, for the rheumatoid arthritis, were in line with those seen with Enbrel®, a leading treatment for people with rheumatoid arthritis.
ADVERTISEMENT
CEL-2000 may also offer a number of potential advantages over existing rheumatoid arthritis treatments, such as Enbrel. Data collected in the animal studies conducted with CEL-2000 demonstrated that CEL-2000 is an effective treatment against arthritis even with the administration of fewer treatments. CEL-2000 is also potentially a more disease-type specific therapy, should be significantly less expensive and finally, CEL-2000 could also be useful for patients who are not able to take or who may be unresponsive to existing anti-arthritis therapies.
In these studies, mice were injected with collagen on days 0 and 21 to induce the disease. Once the mice cohorts reached a significant and uniform and measurable disease (arthritis) state, therapy with Enbrel or CEL-2000 was initiated and continued for 28 days. CEL-2000 was administered only twice and Enbrel was administered every other day for the first 28 days and an arthritic Index score was determined for both groups. The observation period was doubled from previous studies and was continued for another 28 days for a total study period of 56 days.
"It is very exciting to see the reduction of severe rheumatoid arthritis damage in these animals through a simple vaccination," said Dr. Zimmerman. "I am hopeful that CEL-2000 will one day be used to lessen the damage caused by rheumatoid arthritis in patients."
Rheumatoid arthritis treatments comprise a $13 billion market. Enbrel, a leading rheumatoid arthritis treatment sold by Amgen and Wyeth, reported US sales in 2007 of about $3.2 billion. Enbrel is a soluble recombinant protein of a human TNF-alpha receptor linked to human IgG Fc. In some cases, human or humanized monoclonal antibodies specific against TNF-alpha have also been used for therapy in rheumatoid arthritis. These therapies remove or inactivate TNF-alpha, a natural human cytokine required in many immune functions for normal defenses.
CEL-SCI's rheumatoid arthritis vaccine CEL-2000 was discovered as part of work with the Company's ongoing research and development activities with its L.E.A.P.S.(TM) (Ligand Epitope Antigen Presentation System) technology. L.E.A.P.S. is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S. technology.
The concept behind the L.E.A.P.S. technology is to directly mimic cell/cell interactions on the T-cell surface with synthetic peptides. The L.E.A.P.S. constructs containing the antigenic disease epitope linked to a T-cell binding ligand (TCBL) can be manufactured by peptide synthesis or by covalently linking the two peptides. Depending upon the type of L.E.A.P.S. construct and TCBL used, CEL-SCI is able to direct the outcome of the immune response towards the development of T-cell function with primarily effector T-cell functions (T Lymphocyte; helper/effector T lymphocyte, type 1 or 2 [Th1 or Th2], cytotoxic [Tc] or suppressor [Ts]). Therefore, it would appear that the L.E.A.P.S. construct represents a chimeric peptide with bi-functional behavior.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense and avian flu.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K for the year ended September 30, 2007. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
Friday November 14, 10:00 am ET
VIENNA, Va., Nov. 14 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE: CVM - News) announced today the presentation of new rheumatoid arthritis data at the 6th annual GTCbio Vaccine Conference in Vienna, Va. The data, presented by Dr. Daniel Zimmerman, Senior Vice President of Research, Cellular Immunology of CEL-SCI, indicate that CEL-SCI's rheumatoid arthritis treatment vaccine CEL-2000 prevents or retards the permanent tissue damage caused by rheumatoid arthritis. The long term results obtained with CEL-2000 vaccine, for the rheumatoid arthritis, were in line with those seen with Enbrel®, a leading treatment for people with rheumatoid arthritis.
ADVERTISEMENT
CEL-2000 may also offer a number of potential advantages over existing rheumatoid arthritis treatments, such as Enbrel. Data collected in the animal studies conducted with CEL-2000 demonstrated that CEL-2000 is an effective treatment against arthritis even with the administration of fewer treatments. CEL-2000 is also potentially a more disease-type specific therapy, should be significantly less expensive and finally, CEL-2000 could also be useful for patients who are not able to take or who may be unresponsive to existing anti-arthritis therapies.
In these studies, mice were injected with collagen on days 0 and 21 to induce the disease. Once the mice cohorts reached a significant and uniform and measurable disease (arthritis) state, therapy with Enbrel or CEL-2000 was initiated and continued for 28 days. CEL-2000 was administered only twice and Enbrel was administered every other day for the first 28 days and an arthritic Index score was determined for both groups. The observation period was doubled from previous studies and was continued for another 28 days for a total study period of 56 days.
"It is very exciting to see the reduction of severe rheumatoid arthritis damage in these animals through a simple vaccination," said Dr. Zimmerman. "I am hopeful that CEL-2000 will one day be used to lessen the damage caused by rheumatoid arthritis in patients."
Rheumatoid arthritis treatments comprise a $13 billion market. Enbrel, a leading rheumatoid arthritis treatment sold by Amgen and Wyeth, reported US sales in 2007 of about $3.2 billion. Enbrel is a soluble recombinant protein of a human TNF-alpha receptor linked to human IgG Fc. In some cases, human or humanized monoclonal antibodies specific against TNF-alpha have also been used for therapy in rheumatoid arthritis. These therapies remove or inactivate TNF-alpha, a natural human cytokine required in many immune functions for normal defenses.
CEL-SCI's rheumatoid arthritis vaccine CEL-2000 was discovered as part of work with the Company's ongoing research and development activities with its L.E.A.P.S.(TM) (Ligand Epitope Antigen Presentation System) technology. L.E.A.P.S. is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S. technology.
The concept behind the L.E.A.P.S. technology is to directly mimic cell/cell interactions on the T-cell surface with synthetic peptides. The L.E.A.P.S. constructs containing the antigenic disease epitope linked to a T-cell binding ligand (TCBL) can be manufactured by peptide synthesis or by covalently linking the two peptides. Depending upon the type of L.E.A.P.S. construct and TCBL used, CEL-SCI is able to direct the outcome of the immune response towards the development of T-cell function with primarily effector T-cell functions (T Lymphocyte; helper/effector T lymphocyte, type 1 or 2 [Th1 or Th2], cytotoxic [Tc] or suppressor [Ts]). Therefore, it would appear that the L.E.A.P.S. construct represents a chimeric peptide with bi-functional behavior.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense and avian flu.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K for the year ended September 30, 2007. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
nichts mehr los hier
Antwort auf Beitrag Nr.: 36.054.975 von VFBLER am 24.11.08 19:40:14 nee, ich warte auf ein Zeichen !!! PIII oder auch nicht, ich glaub hier an nix mehr -
nee, ich warte auf ein Zeichen !!! PIII oder auch nicht, ich glaub hier an nix mehr -

 nee, ich warte auf ein Zeichen !!! PIII oder auch nicht, ich glaub hier an nix mehr -
nee, ich warte auf ein Zeichen !!! PIII oder auch nicht, ich glaub hier an nix mehr -
50 % in einer woche,so kann´s weiter gehen.daß wir wenigstens zu unserem kaufkursen wieder kommen
 Press Release Source: CEL-SCI Corporation
Press Release Source: CEL-SCI Corporation Updated Pictures of CEL-SCI Manufacturing Facility Available Online
Wednesday December 3, 9:45 am ET
VIENNA, Va., Dec. 3 /PRNewswire-FirstCall/ -- In response to numerous shareholder inquiries, CEL-SCI Corporation (NYSE Alternext US: CVM) announced today that it has added updated pictures of it new manufacturing facility to its website. The pictures can be accessed through CEL-SCI's homepage (http://www.cel-sci.com) by clicking on the link "Updated Pictures of Manufacturing Facility" in the special highlights section.
ADVERTISEMENT
CEL-SCI is developing its cancer product Multikine® for approval as a first line indication in head and neck cancer. The manufacturing facility was built to supply the upcoming and FDA cleared Phase III clinical trial, as well as commercial sale, of Multikine as a first line indication in treatment naive head and neck cancer patients. Head and neck cancer is one of the world's biggest cancers affecting about 650,000 people per annum worldwide.
CEL-SCI already has two partners who will participate in the Phase III trial: Teva Pharmaceuticals and Orient Europharma of Taiwan. Discussions with other potential partners are ongoing.
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, eliminate all of the tumor in 12% of the patients and to improve the patients' overall survival by 33% at a median of three and a half years following surgery.
CEL-SCI has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense. Most recently CEL-SCI announced that its newly discovered rheumatoid arthritis vaccine showed very promising results in animal tests.
 sieht doch aus wie eine richtige Produktionsfirma - mal sehen wann es los geht !!!!
sieht doch aus wie eine richtige Produktionsfirma - mal sehen wann es los geht !!!! 


was heißt das / der Kurs ist etwas angesprungen ???
9-Dec-2008
Change in Directors or Principal Officers
Item 5.02 Departure of Directors or Certain Officers; Election of Directors;
Appointment of Certain Officers; Compensatory Arrangements of Certain Officers.
Effective December 3, 2008, Dr. Daniel Zimmerman, the Company's Senior Vice President of Research, Cellular Immunology, and John Cipriano, the Company's Senior Vice President of Regulatory Affairs, have agreed to become consultants to the Company on an as needed basis thereby ending their full time employment with the Company.
Antwort auf Beitrag Nr.: 36.176.967 von ALF-FRED am 10.12.08 01:35:46Hi Alf!
Die beiden sind ab sofort keine Mitarbeiter mehr von CEL-SCI, sondern Berater, die im Bedarfsfall die Firma unterstuetzen...
Gruss,
Sil
Die beiden sind ab sofort keine Mitarbeiter mehr von CEL-SCI, sondern Berater, die im Bedarfsfall die Firma unterstuetzen...
Gruss,
Sil
Antwort auf Beitrag Nr.: 36.177.247 von Sillak am 10.12.08 08:29:57 danke !
danke !
 danke !
danke !  bringen nix mehr solche newsletter, die sollen endlich mit der P III anfangen, denn sonst nutzt meine pt2N0MX mir nix mehr
bringen nix mehr solche newsletter, die sollen endlich mit der P III anfangen, denn sonst nutzt meine pt2N0MX mir nix mehr 
8229 Boone Boulevard, Suite 802 COMPANY CONTACT:
Vienna, VA 22182. USA Gavin de Windt
Telephone (703) 506-9460 CEL-SCI Corporation
www.cel-sci.com (703) 506-9460
CEL-SCI CORPORATION RELEASES LETTER TO SHAREHOLDERS
VIENNA, VA, December 12, 2008 – The following letter is being released by CEL-SCI CORPORATION (NYSE Alternext US: CVM) to its shareholders:
Dear Fellow Shareholders:
Recently we have had two pieces of important news for CEL-SCI Corporation and its shareholders. First, the U.N. World Health Organization’s Agency for Research on Cancer report, in reference to the “cancer burden” wrote that “the global burden doubled in the last 30 years of the 20th Century, and it is estimated that this will double again between 2000 and 2020 and nearly triple by 2030”. Cancer will soon become the Number One killer in the world, ahead of heart disease. Those increases are much greater than many people had expected and clearly make our non-toxic Multikine cancer product even more important to develop. Second, we need your help with a legislative proposal advocated by the Biotechnology Industry Organization (BIO) which would help CEL-SCI bring Multikine to market faster. Last, what percentage of people do you think will get cancer and how many of them will die from it? The answer may shake you (see below).
In this letter I also want to review the general status of our Multikine cancer drug:
1. In the last few months we have hit major milestones. We signed a Multikine licensing agreement with Teva Pharmaceuticals, a multinational pharmaceutical company, which will participate in and pay for part of our Phase III trial, we took delivery of our new manufacturing facility in Baltimore (see the pictures at www.cel-sci.com and click on Updated Pictures of Manufacturing Facility), and we extended our existing Multikine partnership with Orient Europharma (OEP) of Taiwan to cover a larger territory. OEP, like Teva, will participate in and pay for part of the Phase III clinical trial. We also presented very promising results on a newly discovered rheumatoid arthritis vaccine.
2. Cancer, our main target for Multikine, is quickly becoming the number one killer, surpassing heart disease. Cancer treatment is not a discretionary expenditure and therefore pharmaceutical companies and investors alike are attracted to cancer therapies.
3. Many pharmaceutical companies are making cancer research a priority. This is a change from their prior focus on life style, mass marketed drugs. After the Vioxx debacle and other issues discovered later, it has become significantly more difficult to receive FDA approval for life style drugs. Recently Eli Lilly purchased the cancer company Imclone for $6.5 billion, Pfizer announced that it would focus on cancer and biologics (Multikine
is a biologic for the treatment of cancer) and Roche is trying to purchase the half of the cancer company Genentech that it does not own for about $45 billion.
4. As a late stage Phase III cancer company focusing on a large unmet medical need, we believe that CEL-SCI is well positioned to partner Multikine with other companies and thereby pay for future development costs.
5. The reason we believe that Multikine is an attractive cancer therapy is because we have shown very good clinical data:
a. 12% of patients receiving Multikine have no tumor left before the normal treatment has even started,
b. overall the Multikine treated patients have 50% less tumor cells before the normal treatment has even started, and
c. long-term survival improvement was 33%, all without Multikine induced toxicity.
We believe that Multikine is able to achieve these excellent results for three reasons:
a. Multikine is the first immunotherapy drug to be used before the immune system is destroyed by radiation and chemotherapy. Up to now other immunotherapy drugs have been given after radiation and chemotherapy, a time when the immune system is severely weakened.
b. Multikine is also able to circumvent the tumor’s defense mechanisms by creating a new and different attack through CD-4 cells instead of the usual CD-8 cells which the tumor has learned to shut down.
c. Multikine renders the remaining tumor cells susceptible to the effect of the follow-on radiation and chemotherapy, thereby potentially increasing the effectiveness of these treatments.
6. Only one pivotal Phase III trial is needed to get to approval from the FDA and if approved, Multikine would become the first line standard of care in head and neck cancer, a multi-billion market.
Now let me share with you a staggering statistic. In a June 5, 2007 interview in the Wall Street Journal Genentech’s CEO, Dr. Arthur Levinson, stated that that “42% of everybody out there is going to get cancer. And half of those 42% are going to die of cancer.” That means that almost every 1 in 2 persons will get cancer. I have devoted my life to finding a new and better way of treating cancer, but I had no idea that the number was going to be that big. The effects on patients and their families from cancer are devastating. We must try to do better!
With that in mind I want to highlight a very recent effort by the The Biotechnology Industry Organization (BIO) to help American biotechnology companies get through the current financial crisis. BIO is lobbying Congress to include tax provisions in fiscal stimulus legislation to help small, capital-intensive companies, such as CEL-SCI. The name of the proposal is “One Time Refund of NOLs in Lieu of Other Tax Benefits to Sustain Critical R&D During Financial Distress”. The proposal is not a hand-out. It calls for Congress to allow small companies to “temporarily elect to receive a refund of their accumulated net operating losses (NOLs) at a discounted rate in lieu of claiming qualified research expenses." The plan, which applies only to tax year 2008, requires that refunds be reinvested in U.S. - based research. Refund recipients would permanently forgo the ability to claim all NOLs involved in the computation of the refund. This plan would really help CEL-SCI and might allow us to launch our very important Phase III clinical trial much faster. I ask you to please contact your Congressperson and Senator to express support for this proposal. This is very important to everyone’s life because, unfortunately, I do not know of any family that has escaped the devastating effects of cancer. Congresspersons and Senators listen to you, their constituents. I have already written to my own representatives.
Please write to your representatives right away and make a personal difference in the fight against cancer. Please tell them that you support the “One Time Refund of NOLs in Lieu of Other Tax Benefits to Sustain Critical R&D During Financial Distress” proposed by the Biotechnology Industry Organization (BIO). This is a critical bill and it needs your lobbying to become reality.
The internet address for contacting your Congressperson is:
https://writerep.house.gov/writerep/welcome.shtml
The internet address for contacting your Senators is:
http://www.senate.gov/general/contact_information/senators_c…
We thank you for your support.
Sincerely,
Geert Kersten

By ANDREW POLLACK
Published: December 9, 2008
Move over Big Three. Little Biotech is joining you in seeking federal assistance.
Biotechnology industry executives plan to visit Congress on Wednesday to ask for a temporary change in the tax law that would let money-losing companies get cash from the government now, in exchange for tax credits they would pledge not to take if they eventually become profitable.
The change, if Washington approved of it, could enable the industry to receive potentially hundreds of millions or even billions of dollars, on the condition that the money would be used for research and development.
The effort comes as many smaller biotechnology companies, particularly those trying to develop drugs, are facing a severe cash shortage that is forcing them to dismiss workers, curtail research and even file for bankruptcy protection or liquidation.
Prospects for the proposal are unclear.
But Allyson Y. Schwartz, a Democratic member of the House Ways and Means Committee, which handles tax matters, said she would push to include the proposal in the forthcoming economic stimulus package and expected many of her colleagues to view it favorably.
“Innovation and technology are growth areas for American businesses and American workers and should be part of this package,” said Ms. Schwartz, whose district in the Philadelphia area is home to some pharmaceutical and biotechnology offices.
A spokeswoman for President-elect Barack Obama said his transition team was not commenting on individual provisions of the stimulus package beyond what has been revealed publicly.
The big profitable biotech companies like Amgen and Genentech remain financially sound. But of the 370 publicly traded American biotechnology companies, the number with less than six months cash on hand is approximately 125 — nearly double the total last year, according to the Biotechnology Industry Organization, a trade group. Developing a drug can require hundreds of millions of dollars and 10 years or more, so even in good economic times some small companies struggle to raise money.
But with financial markets in turmoil, investors have become reluctant or unable to provide the infusions of cash, pushing many biotech companies to the brink.
In seeking assistance, the industry is quick to distinguish itself from the automakers, banks and other supplicants in Washington, portraying itself as a model of innovation and American competitiveness.
“This is not a question of our companies operating with what some perceive as a flawed business model,” said Alan F. Eisenberg, an executive vice president of the Biotechnology Industry Organization, which is known as BIO. “This is about our companies taking a decade to get a product on the market, and during that time they need to have investor capital, and that capital is not available.”
The industry’s idea is to let companies turn their very weakness — their huge losses — into an asset. Under current law, net operating losses can eventually be used to offset some taxes once a company is profitable. But that does little good for companies struggling with losses and a lack of cash now.
So the industry’s proposal would let companies receive payments from the government now in exchange for giving up those tax deductions later. The industry would agree to a cap, perhaps $30 million, on the amount any single company could receive.
Mr. Eisenberg of BIO said the proposed change would be in effect only for a year and would apply to companies outside of biotechnology as well. Few other industries, though, require as much time and money to develop a product as the biotechnology industry.
Biotechnology might not seem an obvious candidate for a bailout. The industry employs only about 200,000 people in the United States, according to the accounting firm Ernst & Young; the automobile industry employs millions.
“Research-based companies that employ 30 people don’t necessarily stimulate the economy,” said a Washington lobbyist for a large pharmaceutical company, who was skeptical the proposal would win backing.
This person, who spoke on the condition of anonymity because he was not authorized to talk to the media, said the proposal’s opponents could argue that assistance was not needed because a company with a truly promising product would be able to sell the rights to that product, or the entire company, to a larger drug company.
The biotechnology industry is also notoriously risky, with many companies never achieving profitability despite spending hundreds of millions of dollars. The chief executive of Genentech has estimated the industry as a whole has lost about $100 billion since the field’s inception in 1970s. Even as of last year, the American biotechnology industry as a whole was not profitable, although it was getting close, according to Ernst & Young.
The biotechnology industry will argue that it is responsible for creating a large number of drugs now being tested that will be needed by an aging population in the future. And it will argue that it is a bastion of American competitiveness.
“It’s one of the few places where the U.S. is the undisputed leader of the world,” said Alexis Borisy, the chief executive of CombinatoRx, a biotech company in Cambridge, Mass., that recently cut 80 employees, or two-thirds of its work force, to conserve its cash after two drugs did not perform well in clinical trials. “Do we allow that to be cannibalized?”
The industry has paved the way in developing ways to treat rheumatoid arthritis and multiple sclerosis as well as cancer drugs like Genentech’s Avastin, ImClone’s Erbitux and Celgene’s Revlimid. OSI Pharmaceuticals, which developed the lung cancer drug Tarceva, lost a cumulative $1.3 billion from its inception in 1983 through 2006 before finally breaking into the black.
Since many companies will never reach profitability, the industry acknowledges that an upfront cash payment would have to be significantly less than the value of the deductions.
For instance, Mr. Eisenberg said, a company with $100 million in net operating losses would be entitled to about $35 million in lower federal taxes when it eventually became profitable. So perhaps the company could be paid about $20 million now, he said.
More Articles in Business » A version of this article appeared in print on December 10, 2008, on page B1 of the New York edition. Free trial. Read the complete New York edition of The Times on computer, just as it appears in print.
Entry into a Material Definitive Agreement
Item 1.01 Entry Into a Material Definitive Agreement
Equity Line of Credit
On December 30, 2008, CEL-SCI entered into an equity line of credit agreement with Ascendiant Capital Group, LLC in order to establish a possible source of funding for CEL-SCI. The equity line of credit agreement establishes what is sometimes also referred to as an equity drawdown facility.
Under the equity line of credit agreement, Ascendiant has agreed to provide CEL-SCI with up to $5,000,000 of funding prior to January 6, 2011. During this period, CEL-SCI may request a drawdown under the equity line of credit by selling shares of its common stock to Ascendiant and Ascendiant will be obligated to purchase the shares. CEL-SCI may request a drawdown once every ten trading days, although CEL-SCI is under no obligation to request any drawdowns under the equity line of credit. There must be a minimum of two trading days between each drawdown request.
During the ten trading days following a drawdown request, CEL-SCI will calculate the amount of shares it will sell to Ascendiant and the purchase price per share. The purchase price per share of common stock will be based on the daily volume weighted average price of CEL-SCI's common stock during each of the ten trading days immediately following the drawdown date, less a discount of 9%.
CEL-SCI may request a drawdown by faxing a drawdown notice to Ascendiant, stating the amount of the drawdown and the lowest price, if any, at which CEL-SCI is willing to sell the shares. The lowest price will be set by CEL-SCI's Chief Executive Officer in his sole and absolute discretion.
Calculation of Drawdown Amount, Purchase Price and Number of Shares Sold
There is no minimum amount CEL-SCI can draw down at any one time. The maximum amount CEL-SCI can draw down at any one time is an amount equal to:
o 10% of the total trading volume of CEL-SCI's common stock for the ten trading days prior to the date of the drawdown request, multiplied by
o the weighted average price of CEL-SCI's common stock for the same ten trading days,
or any other amount mutually agreed upon by CEL-SCI and Ascendiant.
On the day following the delivery of the drawdown notice, a valuation period of ten trading days will start:
o On each of the ten trading days during the valuation period, the number of shares to be sold to Ascendiant will be determined by dividing 1/10 of the drawdown amount by the purchase price on each
trading day. The purchase price will be 91% of the volume weighted average price of CEL-SCI's common stock on that day.
o If the purchase price on any trading day during the ten trading day calculation period is below the minimum price specified by CEL-SCI, then Ascendiant will not purchase any shares on that day, and the drawdown amount will be reduced by 1/10.
Using the formula described above, if CEL-SCI had requested a drawdown on December 12, 2008, the maximum amount CEL-SCI could draw down during the subsequent ten trading days would have been approximately $72,125. Based upon the volume weighted average of CEL-SCI's common stock during the ten trading days subsequent to December 12, 2008, CEL-SCI would have sold 258,000 shares of its common stock to Ascendiant and would have received proceeds from the sale of these shares of approximately $72,125.
If CEL-SCI sets a minimum price which is too high and CEL-SCI's stock price does not consistently meet that level during the ten trading days after its drawdown request, the amount CEL-SCI can draw and the number of shares CEL-SCI will sell to Ascendiant will be reduced. On the other hand, if CEL-SCI sets a minimum price which is too low and its stock price falls significantly but stays above the minimum price, CEL-SCI will have to issue a greater number of shares to Ascendiant based on the reduced market price.
Payment for Shares
The shares purchased on the ten trading days following a drawdown request will be issued and paid for on the 13th trading day following the drawdown request.
Termination of the Equity Line of Credit Agreement
The equity line of credit agreement will be terminated if:
o CEL-SCI's common stock is de-listed from the American Stock Exchange unless the de-listing is in connection with CEL-SCI's subsequent listing of its common stock on the NASDAQ National Market, the NASDAQ SmallCap Market, the New York Stock Exchange, the OTC Bulletin Board,
o CEL-SCI files for protection from its creditors under the Federal Bankruptcy laws,
o The shares which are to be sold to Ascendiant are not covered by an effective registration statement,
o Ascendiant fails to honor a drawdown notice, or
o the equity line of credit agreement violates any other agreement to which CEL-SCI is a party.
General
On the trading day immediately prior to the date CEL-SCI sends its first drawdown notice to Ascendiant, and in consideration for providing the equity drawdown facility, CEL-SCI will deliver to Ascendiant shares of its common stock equal in number to the amount determined by:
o dividing $250,000 by
o the greater of the weighted average price of CEL-SCI's common stock or the closing bid price of CEL-SCI's common stock on the second trading day immediately preceding the drawdown notice.
CEL-SCI paid $20,000 to Feldman Weinstein Smith LLP, legal counsel to Ascendiant, for Ascendiant's legal expenses relating to the equity line of credit.
Ascendiant is entitled to customary indemnification from CEL-SCI for any losses or liabilities it suffers as a result of any breach by CEL-SCI of any provisions of the equity line of credit agreement, or as a result of any lawsuit brought by any shareholder of CEL-SCI, provided the shareholder instituting the lawsuit is not an officer, director or principal shareholder of CEL-SCI.
Item 1.01 Entry Into a Material Definitive Agreement
Equity Line of Credit
On December 30, 2008, CEL-SCI entered into an equity line of credit agreement with Ascendiant Capital Group, LLC in order to establish a possible source of funding for CEL-SCI. The equity line of credit agreement establishes what is sometimes also referred to as an equity drawdown facility.
Under the equity line of credit agreement, Ascendiant has agreed to provide CEL-SCI with up to $5,000,000 of funding prior to January 6, 2011. During this period, CEL-SCI may request a drawdown under the equity line of credit by selling shares of its common stock to Ascendiant and Ascendiant will be obligated to purchase the shares. CEL-SCI may request a drawdown once every ten trading days, although CEL-SCI is under no obligation to request any drawdowns under the equity line of credit. There must be a minimum of two trading days between each drawdown request.
During the ten trading days following a drawdown request, CEL-SCI will calculate the amount of shares it will sell to Ascendiant and the purchase price per share. The purchase price per share of common stock will be based on the daily volume weighted average price of CEL-SCI's common stock during each of the ten trading days immediately following the drawdown date, less a discount of 9%.
CEL-SCI may request a drawdown by faxing a drawdown notice to Ascendiant, stating the amount of the drawdown and the lowest price, if any, at which CEL-SCI is willing to sell the shares. The lowest price will be set by CEL-SCI's Chief Executive Officer in his sole and absolute discretion.
Calculation of Drawdown Amount, Purchase Price and Number of Shares Sold
There is no minimum amount CEL-SCI can draw down at any one time. The maximum amount CEL-SCI can draw down at any one time is an amount equal to:
o 10% of the total trading volume of CEL-SCI's common stock for the ten trading days prior to the date of the drawdown request, multiplied by
o the weighted average price of CEL-SCI's common stock for the same ten trading days,
or any other amount mutually agreed upon by CEL-SCI and Ascendiant.
On the day following the delivery of the drawdown notice, a valuation period of ten trading days will start:
o On each of the ten trading days during the valuation period, the number of shares to be sold to Ascendiant will be determined by dividing 1/10 of the drawdown amount by the purchase price on each
trading day. The purchase price will be 91% of the volume weighted average price of CEL-SCI's common stock on that day.
o If the purchase price on any trading day during the ten trading day calculation period is below the minimum price specified by CEL-SCI, then Ascendiant will not purchase any shares on that day, and the drawdown amount will be reduced by 1/10.
Using the formula described above, if CEL-SCI had requested a drawdown on December 12, 2008, the maximum amount CEL-SCI could draw down during the subsequent ten trading days would have been approximately $72,125. Based upon the volume weighted average of CEL-SCI's common stock during the ten trading days subsequent to December 12, 2008, CEL-SCI would have sold 258,000 shares of its common stock to Ascendiant and would have received proceeds from the sale of these shares of approximately $72,125.
If CEL-SCI sets a minimum price which is too high and CEL-SCI's stock price does not consistently meet that level during the ten trading days after its drawdown request, the amount CEL-SCI can draw and the number of shares CEL-SCI will sell to Ascendiant will be reduced. On the other hand, if CEL-SCI sets a minimum price which is too low and its stock price falls significantly but stays above the minimum price, CEL-SCI will have to issue a greater number of shares to Ascendiant based on the reduced market price.
Payment for Shares
The shares purchased on the ten trading days following a drawdown request will be issued and paid for on the 13th trading day following the drawdown request.
Termination of the Equity Line of Credit Agreement
The equity line of credit agreement will be terminated if:
o CEL-SCI's common stock is de-listed from the American Stock Exchange unless the de-listing is in connection with CEL-SCI's subsequent listing of its common stock on the NASDAQ National Market, the NASDAQ SmallCap Market, the New York Stock Exchange, the OTC Bulletin Board,
o CEL-SCI files for protection from its creditors under the Federal Bankruptcy laws,
o The shares which are to be sold to Ascendiant are not covered by an effective registration statement,
o Ascendiant fails to honor a drawdown notice, or
o the equity line of credit agreement violates any other agreement to which CEL-SCI is a party.
General
On the trading day immediately prior to the date CEL-SCI sends its first drawdown notice to Ascendiant, and in consideration for providing the equity drawdown facility, CEL-SCI will deliver to Ascendiant shares of its common stock equal in number to the amount determined by:
o dividing $250,000 by
o the greater of the weighted average price of CEL-SCI's common stock or the closing bid price of CEL-SCI's common stock on the second trading day immediately preceding the drawdown notice.
CEL-SCI paid $20,000 to Feldman Weinstein Smith LLP, legal counsel to Ascendiant, for Ascendiant's legal expenses relating to the equity line of credit.
Ascendiant is entitled to customary indemnification from CEL-SCI for any losses or liabilities it suffers as a result of any breach by CEL-SCI of any provisions of the equity line of credit agreement, or as a result of any lawsuit brought by any shareholder of CEL-SCI, provided the shareholder instituting the lawsuit is not an officer, director or principal shareholder of CEL-SCI.
Eintritt in ein Material endgültige Vereinbarung
Posten 1/01 Eintritt in ein Material endgültigen Vereinbarung
Equity Line of Credit
Am 30. Dezember 2008, CEL-SCI, die ein Eigenkapital Kreditlinie Einvernehmen mit Ascendiant Capital Group, LLC, um eine mögliche Finanzierungsquelle für CEL-SCI. Die Eigenkapitalquote Leitung der Kreditvertrag wird, was manchmal auch als ein Eigenkapital Inanspruchnahme der Einrichtung.
Nach der Equity-Kreditlinie Vereinbarung, Ascendiant hat sich bereit erklärt, die CEL-SCI mit bis zu $ 5000000 der Finanzierung vor dem 6. Januar 2011. Während dieser Zeit, CEL-SCI, können einen Antrag auf Inanspruchnahme im Rahmen der Equity-Kreditlinie durch den Verkauf von Aktien der Stammaktien zu Ascendiant und Ascendiant werden verpflichtet zum Kauf der Aktie. CEL-SCI, können einen Antrag auf Inanspruchnahme einmal alle zehn Börsentagen, obwohl CEL-SCI ist nicht verpflichtet zu verlangen, jede in Anspruch genommene nach der Equity-Kreditlinie. Es muss ein Minimum von zwei Börsentagen zwischen den einzelnen Inanspruchnahme Anfrage.
Während der zehn Börsentagen nach einer Inanspruchnahme Anfrage, CEL-SCI berechnet die Höhe der Aktien wird es zu verkaufen Ascendiant und der Kaufpreis pro Aktie. Der Kaufpreis je Aktie von Stammaktien werden auf der Grundlage der täglichen Volumen gewichteten durchschnittlichen Kurs von CEL-SCI-Aktien in jedem der zehn Börsentage unmittelbar nach der Inanspruchnahme Datum, weniger einen Rabatt in Höhe von 9%.
CEL-SCI, können einen Antrag auf Inanspruchnahme von Faxen ein Drawdown Mitteilung an Ascendiant, in denen die Höhe der Inanspruchnahme und der niedrigste Preis, wenn überhaupt, auf die CEL-SCI ist bereit zum Verkauf der Aktien. Der niedrigste Preis werden von CEL-SCI's Chief Executive Officer in seinem alleinigen und absoluten Ermessen.
Berechnung der Drawdown Betrag, Kaufpreis und Anzahl der Aktien Verkauft
Es gibt keinen Mindestbetrag CEL-SCI ziehen können Sie zu jeder Zeit. Der Höchstbetrag CEL-SCI ziehen können Sie zu jeder Zeit ist ein Betrag in Höhe von:
o 10% der gesamten Handelsvolumen von CEL-SCI-Stammaktien für die zehn Börsentage vor dem Zeitpunkt der Inanspruchnahme Anfrage, multipliziert mit
o dem gewogenen durchschnittlichen Preis von CEL-SCI-Stammaktien für den gleichen zehn Börsentagen,
oder einer anderen Höhe gegenseitigem Einvernehmen zwischen dem CEL-SCI und Ascendiant.
Am Tag nach der Auslieferung der Inanspruchnahme Hinweis, eine Bewertung Zeitraum von zehn Börsentagen beginnt:
o Auf jeder der zehn Börsentagen bei der Bewertung Zeitraum, die Anzahl der Aktien zum Verkauf an Ascendiant wird ermittelt, indem 1 / 10 der Inanspruchnahme Betrag, um den Kaufpreis zu den einzelnen
Handelstag. Der Kaufpreis wird 91% des Volumens gewogenen durchschnittlichen Preis von CEL-SCI-Stammaktien an diesem Tag.
o Wenn der Kaufpreis auf jedem Handelstag während der zehn Handelstag Berechnung Zeitraum ist unter dem Mindestpreis von CEL-SCI, dann wird nicht Ascendiant Kauf von Wertpapieren an diesem Tag, und die Inanspruchnahme Betrag wird um 1 / 10.
Mit der Formel oben beschrieben, wenn CEL-SCI hatte ein Drawdown auf der 12. Dezember 2008, sowie des Höchstbetrags CEL-SCI könnten sich während der anschließenden zehn Börsentage gewesen wäre, etwa 72.125 Dollar. Basierend auf dem gewichteten Durchschnitt von CEL-SCI-Stammaktien während der zehn Börsentage nach dem 12. Dezember 2008, CEL-SCI hätte verkauft 258.000 Aktien der Stammaktien zu Ascendiant und erhalten hätte der Erlös aus dem Verkauf dieser Anteile an der rund 72.125 Dollar.
Wenn CEL-SCI setzt einen Mindestpreis, zu dem ist zu hoch und CEL-SCI Aktie Preis, nicht konsequent erfüllen, dass während der zehn Börsentagen nach ihrer Inanspruchnahme Antrag ist die Höhe CEL-SCI ziehen können und die Anzahl der Aktien CEL-SCI wird verkaufen zu Ascendiant reduziert werden. Auf der anderen Seite, wenn CEL-SCI setzt einen Mindestpreis, zu dem zu niedrig ist und seine Aktienkurs fällt, sondern bleibt deutlich über dem Mindestpreis, CEL-SCI wird sich die Ausstellung eine größere Anzahl von Aktien an Ascendiant auf der Grundlage der Verringerung der Marktstützung Preis .
Die Zahlung für die Anteile der
Die Aktien erworben über die zehn Börsentagen nach einer Inanspruchnahme Anfrage wird ausgestellt und bezahlt am 13. Handelstag nach der Inanspruchnahme Anfrage.
Beendigung der Equity Line of Credit-Abkommen
Die Equity-Kreditlinie Vereinbarung wird gekündigt, wenn:
o CEL-SCI-Stammaktien ist de-gelistet ab der American Stock Exchange, es sei denn, die De-Listing ist im Zusammenhang mit CEL-SCI's spätere Notierung seiner Stammaktien auf dem NASDAQ National Market, der NASDAQ SMALLCAP Markt, der New York Stock Exchange, der OTC Bulletin Board,
o CEL-SCI-Dateien für den Schutz vor seinen Gläubigern im Rahmen des Bundes-Konkursrecht,
o Die Aktien, die verkauft werden sollen, um Ascendiant sind nicht durch eine wirksame Eintragung Erklärung,
o Ascendiant nicht zu Ehren eines Kredit-Auszahlungsbetrags Hinweis, oder
o der Equity-Kreditlinie Vereinbarung verstößt gegen jede andere Vereinbarung, auf die CEL-SCI ist eine Partei.
Allgemeine
Auf dem Handelstag unmittelbar vor dem Tag CEL-SCI schickt seine ersten Inanspruchnahme Mitteilung an Ascendiant, und unter Berücksichtigung der für die Bereitstellung von Eigenkapital Inanspruchnahme der Einrichtung, CEL-SCI wird zu Ascendiant Anteile seiner Stammaktien gleich der Zahl der festgelegten Betrag von :
o Aufteilung von 250.000 Dollar
o desto größer ist der gewogene durchschnittliche Preis von CEL-SCI-Stammaktien oder die Schließung Angebots Preis von CEL-SCI-Stammaktien am zweiten Handelstag unmittelbar vor der Inanspruchnahme gekündigt werden.
CEL-SCI gezahlt 20.000 Dollar zu Feldman Weinstein Smith LLP, Rechtsbeistand zu Ascendiant, für Ascendiant der gesetzliche Kosten im Zusammenhang mit der Equity-Kreditlinie.
Ascendiant ist berechtigt, übliche Entschädigung von CEL-SCI für eventuelle Verluste oder Verbindlichkeiten er leidet als Folge einer Verletzung von CEL-SCI über alle Bestimmungen der Equity-Kreditlinie Vereinbarung, oder als Folge einer Klage von jedem Aktionär von CEL-SCI, sofern der Aktionär zur Einführung der Klage ist nicht ein Offizier, Regisseur oder Hauptaktionär von CEL-SCI.
Posten 1/01 Eintritt in ein Material endgültigen Vereinbarung
Equity Line of Credit
Am 30. Dezember 2008, CEL-SCI, die ein Eigenkapital Kreditlinie Einvernehmen mit Ascendiant Capital Group, LLC, um eine mögliche Finanzierungsquelle für CEL-SCI. Die Eigenkapitalquote Leitung der Kreditvertrag wird, was manchmal auch als ein Eigenkapital Inanspruchnahme der Einrichtung.
Nach der Equity-Kreditlinie Vereinbarung, Ascendiant hat sich bereit erklärt, die CEL-SCI mit bis zu $ 5000000 der Finanzierung vor dem 6. Januar 2011. Während dieser Zeit, CEL-SCI, können einen Antrag auf Inanspruchnahme im Rahmen der Equity-Kreditlinie durch den Verkauf von Aktien der Stammaktien zu Ascendiant und Ascendiant werden verpflichtet zum Kauf der Aktie. CEL-SCI, können einen Antrag auf Inanspruchnahme einmal alle zehn Börsentagen, obwohl CEL-SCI ist nicht verpflichtet zu verlangen, jede in Anspruch genommene nach der Equity-Kreditlinie. Es muss ein Minimum von zwei Börsentagen zwischen den einzelnen Inanspruchnahme Anfrage.
Während der zehn Börsentagen nach einer Inanspruchnahme Anfrage, CEL-SCI berechnet die Höhe der Aktien wird es zu verkaufen Ascendiant und der Kaufpreis pro Aktie. Der Kaufpreis je Aktie von Stammaktien werden auf der Grundlage der täglichen Volumen gewichteten durchschnittlichen Kurs von CEL-SCI-Aktien in jedem der zehn Börsentage unmittelbar nach der Inanspruchnahme Datum, weniger einen Rabatt in Höhe von 9%.
CEL-SCI, können einen Antrag auf Inanspruchnahme von Faxen ein Drawdown Mitteilung an Ascendiant, in denen die Höhe der Inanspruchnahme und der niedrigste Preis, wenn überhaupt, auf die CEL-SCI ist bereit zum Verkauf der Aktien. Der niedrigste Preis werden von CEL-SCI's Chief Executive Officer in seinem alleinigen und absoluten Ermessen.
Berechnung der Drawdown Betrag, Kaufpreis und Anzahl der Aktien Verkauft
Es gibt keinen Mindestbetrag CEL-SCI ziehen können Sie zu jeder Zeit. Der Höchstbetrag CEL-SCI ziehen können Sie zu jeder Zeit ist ein Betrag in Höhe von:
o 10% der gesamten Handelsvolumen von CEL-SCI-Stammaktien für die zehn Börsentage vor dem Zeitpunkt der Inanspruchnahme Anfrage, multipliziert mit
o dem gewogenen durchschnittlichen Preis von CEL-SCI-Stammaktien für den gleichen zehn Börsentagen,
oder einer anderen Höhe gegenseitigem Einvernehmen zwischen dem CEL-SCI und Ascendiant.
Am Tag nach der Auslieferung der Inanspruchnahme Hinweis, eine Bewertung Zeitraum von zehn Börsentagen beginnt:
o Auf jeder der zehn Börsentagen bei der Bewertung Zeitraum, die Anzahl der Aktien zum Verkauf an Ascendiant wird ermittelt, indem 1 / 10 der Inanspruchnahme Betrag, um den Kaufpreis zu den einzelnen
Handelstag. Der Kaufpreis wird 91% des Volumens gewogenen durchschnittlichen Preis von CEL-SCI-Stammaktien an diesem Tag.
o Wenn der Kaufpreis auf jedem Handelstag während der zehn Handelstag Berechnung Zeitraum ist unter dem Mindestpreis von CEL-SCI, dann wird nicht Ascendiant Kauf von Wertpapieren an diesem Tag, und die Inanspruchnahme Betrag wird um 1 / 10.
Mit der Formel oben beschrieben, wenn CEL-SCI hatte ein Drawdown auf der 12. Dezember 2008, sowie des Höchstbetrags CEL-SCI könnten sich während der anschließenden zehn Börsentage gewesen wäre, etwa 72.125 Dollar. Basierend auf dem gewichteten Durchschnitt von CEL-SCI-Stammaktien während der zehn Börsentage nach dem 12. Dezember 2008, CEL-SCI hätte verkauft 258.000 Aktien der Stammaktien zu Ascendiant und erhalten hätte der Erlös aus dem Verkauf dieser Anteile an der rund 72.125 Dollar.
Wenn CEL-SCI setzt einen Mindestpreis, zu dem ist zu hoch und CEL-SCI Aktie Preis, nicht konsequent erfüllen, dass während der zehn Börsentagen nach ihrer Inanspruchnahme Antrag ist die Höhe CEL-SCI ziehen können und die Anzahl der Aktien CEL-SCI wird verkaufen zu Ascendiant reduziert werden. Auf der anderen Seite, wenn CEL-SCI setzt einen Mindestpreis, zu dem zu niedrig ist und seine Aktienkurs fällt, sondern bleibt deutlich über dem Mindestpreis, CEL-SCI wird sich die Ausstellung eine größere Anzahl von Aktien an Ascendiant auf der Grundlage der Verringerung der Marktstützung Preis .
Die Zahlung für die Anteile der
Die Aktien erworben über die zehn Börsentagen nach einer Inanspruchnahme Anfrage wird ausgestellt und bezahlt am 13. Handelstag nach der Inanspruchnahme Anfrage.
Beendigung der Equity Line of Credit-Abkommen
Die Equity-Kreditlinie Vereinbarung wird gekündigt, wenn:
o CEL-SCI-Stammaktien ist de-gelistet ab der American Stock Exchange, es sei denn, die De-Listing ist im Zusammenhang mit CEL-SCI's spätere Notierung seiner Stammaktien auf dem NASDAQ National Market, der NASDAQ SMALLCAP Markt, der New York Stock Exchange, der OTC Bulletin Board,
o CEL-SCI-Dateien für den Schutz vor seinen Gläubigern im Rahmen des Bundes-Konkursrecht,
o Die Aktien, die verkauft werden sollen, um Ascendiant sind nicht durch eine wirksame Eintragung Erklärung,
o Ascendiant nicht zu Ehren eines Kredit-Auszahlungsbetrags Hinweis, oder
o der Equity-Kreditlinie Vereinbarung verstößt gegen jede andere Vereinbarung, auf die CEL-SCI ist eine Partei.
Allgemeine
Auf dem Handelstag unmittelbar vor dem Tag CEL-SCI schickt seine ersten Inanspruchnahme Mitteilung an Ascendiant, und unter Berücksichtigung der für die Bereitstellung von Eigenkapital Inanspruchnahme der Einrichtung, CEL-SCI wird zu Ascendiant Anteile seiner Stammaktien gleich der Zahl der festgelegten Betrag von :
o Aufteilung von 250.000 Dollar
o desto größer ist der gewogene durchschnittliche Preis von CEL-SCI-Stammaktien oder die Schließung Angebots Preis von CEL-SCI-Stammaktien am zweiten Handelstag unmittelbar vor der Inanspruchnahme gekündigt werden.
CEL-SCI gezahlt 20.000 Dollar zu Feldman Weinstein Smith LLP, Rechtsbeistand zu Ascendiant, für Ascendiant der gesetzliche Kosten im Zusammenhang mit der Equity-Kreditlinie.
Ascendiant ist berechtigt, übliche Entschädigung von CEL-SCI für eventuelle Verluste oder Verbindlichkeiten er leidet als Folge einer Verletzung von CEL-SCI über alle Bestimmungen der Equity-Kreditlinie Vereinbarung, oder als Folge einer Klage von jedem Aktionär von CEL-SCI, sofern der Aktionär zur Einführung der Klage ist nicht ein Offizier, Regisseur oder Hauptaktionär von CEL-SCI.
Hi, ganz interessante News. Diesmal fuer CEL-2000
2009-01-07 09:35 ET - News Release
Results expected to Support Licensing Activities
VIENNA, Va., Jan. 7 /PRNewswire-FirstCall/ -- CEL-SCI Corporation announced today the initiation of a definitive study of its new rheumatoid arthritis treatment vaccine (CEL-2000) to determine the long-lasting beneficial effects on animals with the disease. The study is being conducted at the request of a large pharmaceutical company which is evaluating the possibility of acquiring a global license for the vaccine from CEL-SCI. The long-term results, previously presented at the 6th annual GTCbio Vaccine conference, for the CEL-2000 vaccine were better than or comparable to those for Enbrel(R), a leading treatment for people with rheumatoid arthritis.
"We are very excited to see a prominent pharmaceutical company express interest in licensing the vaccine," said Geert Kersten, CEL-SCI's Chief Executive Officer. "As a result of the very good data with this novel approach, we believe that the prospects for licensing the vaccine are promising."
The definitive experiment will confirm the efficacy and durability of the response to the vaccine and will further assess the safety of the CEL-2000 vaccine, in preparation for possible human trials. CEL-2000 may also offer a number of other potential advantages over existing rheumatoid arthritis treatments, such as Enbrel. The data from animal studies of rheumatoid arthritis using CEL-2000 treatment vaccine demonstrated that CEL-2000 is an effective treatment against arthritis with fewer administrations than those required by other anti-rheumatoid arthritis treatments, including Enbrel(R). CEL-2000 is also potentially a more disease type specific therapy is calculated to be significantly less expensive and may be useful in patients unable to tolerate or who may not be responsive to existing anti-arthritis therapies.
Rheumatoid arthritis treatments comprise a $13 billion market. Enbrel, a leading rheumatoid arthritis treatment sold by Amgen and Wyeth, reported US sales in 2007 of about $3.2 billion. Enbrel is a soluble recombinant protein of a human TNF-alpha receptor linked to human IgG Fc. In some cases, human or humanized monoclonal antibodies specific against TNF-alpha have also been used for therapy in rheumatoid arthritis. These therapies remove or inactivate TNF-alpha, a natural human cytokine required in many immune functions for normal defenses.
Gruss,
Sil
2009-01-07 09:35 ET - News Release
Results expected to Support Licensing Activities
VIENNA, Va., Jan. 7 /PRNewswire-FirstCall/ -- CEL-SCI Corporation announced today the initiation of a definitive study of its new rheumatoid arthritis treatment vaccine (CEL-2000) to determine the long-lasting beneficial effects on animals with the disease. The study is being conducted at the request of a large pharmaceutical company which is evaluating the possibility of acquiring a global license for the vaccine from CEL-SCI. The long-term results, previously presented at the 6th annual GTCbio Vaccine conference, for the CEL-2000 vaccine were better than or comparable to those for Enbrel(R), a leading treatment for people with rheumatoid arthritis.
"We are very excited to see a prominent pharmaceutical company express interest in licensing the vaccine," said Geert Kersten, CEL-SCI's Chief Executive Officer. "As a result of the very good data with this novel approach, we believe that the prospects for licensing the vaccine are promising."
The definitive experiment will confirm the efficacy and durability of the response to the vaccine and will further assess the safety of the CEL-2000 vaccine, in preparation for possible human trials. CEL-2000 may also offer a number of other potential advantages over existing rheumatoid arthritis treatments, such as Enbrel. The data from animal studies of rheumatoid arthritis using CEL-2000 treatment vaccine demonstrated that CEL-2000 is an effective treatment against arthritis with fewer administrations than those required by other anti-rheumatoid arthritis treatments, including Enbrel(R). CEL-2000 is also potentially a more disease type specific therapy is calculated to be significantly less expensive and may be useful in patients unable to tolerate or who may not be responsive to existing anti-arthritis therapies.
Rheumatoid arthritis treatments comprise a $13 billion market. Enbrel, a leading rheumatoid arthritis treatment sold by Amgen and Wyeth, reported US sales in 2007 of about $3.2 billion. Enbrel is a soluble recombinant protein of a human TNF-alpha receptor linked to human IgG Fc. In some cases, human or humanized monoclonal antibodies specific against TNF-alpha have also been used for therapy in rheumatoid arthritis. These therapies remove or inactivate TNF-alpha, a natural human cytokine required in many immune functions for normal defenses.
Gruss,
Sil
Antwort auf Beitrag Nr.: 36.326.801 von Sillak am 07.01.09 19:13:48
was sagst du den als alter Hase dazu wie es weiter geht ????
@ VFBler
was machst du im Lukoil Thread ???
mal sehen ob Cel oder Lukoil ( einstieg Anfang Dez. 2008 ) mein Jahressieger 2009 wird !!!
könnte aber auch Argues ( Ende 2008 Einstieg )werden
Frohes Neues Alf - Fred


was sagst du den als alter Hase dazu wie es weiter geht ????
@ VFBler
was machst du im Lukoil Thread ???
mal sehen ob Cel oder Lukoil ( einstieg Anfang Dez. 2008 ) mein Jahressieger 2009 wird !!!
könnte aber auch Argues ( Ende 2008 Einstieg )werden

Frohes Neues Alf - Fred


Antwort auf Beitrag Nr.: 36.352.053 von ALF-FRED am 11.01.09 21:39:11Hi Alf!
Ich finde CEL ist das grosste Schnaeppchen auf dem Markt. Immer mal wieder kaufe ich mir ein paar dazu.
Jetzt wird die Produktion aufgenommen werden muesen. Wenn dann MK zur Verfuegung steht, kann endlich die PIII beginnen. Ich habe leider noch nicht lesen koennen, zu wann man dies plant...
Aber Geduld sollte man bei CEL ja haben...und lange kann es nicht mehr dauern...

Gruss,
Sil
Ich finde CEL ist das grosste Schnaeppchen auf dem Markt. Immer mal wieder kaufe ich mir ein paar dazu.
Jetzt wird die Produktion aufgenommen werden muesen. Wenn dann MK zur Verfuegung steht, kann endlich die PIII beginnen. Ich habe leider noch nicht lesen koennen, zu wann man dies plant...
Aber Geduld sollte man bei CEL ja haben...und lange kann es nicht mehr dauern...


Gruss,
Sil
zu sillak,hab leider schon 4 jahre geduld,könnt sich langsam mal auszahlen
bei lukoil thread bin ich,weil ich ein ölwert suche.
steig aber lieber bei omv wahrscheinlich ein,wenn mein limit greift
bei lukoil thread bin ich,weil ich ein ölwert suche.
steig aber lieber bei omv wahrscheinlich ein,wenn mein limit greift
Antwort auf Beitrag Nr.: 36.354.455 von VFBLER am 12.01.09 12:33:20Hallo VFBLER!
Die kommenden 24 Monate werden mit Sicherheit die spektakulaersten in der Geschichte von CEL-SCI. Ich hoffe fuer alle Investierten und Betroffenen, dass alles positv ausgehen wird!
(Ich kann einen bitteren Beigeschmack des letzten Satzes nicht leugnen, aber es ist wirklich nur gut gemeint)
Oelwert: Sichere Dividende oder Risiko?
Gruss,
Sil
Die kommenden 24 Monate werden mit Sicherheit die spektakulaersten in der Geschichte von CEL-SCI. Ich hoffe fuer alle Investierten und Betroffenen, dass alles positv ausgehen wird!
(Ich kann einen bitteren Beigeschmack des letzten Satzes nicht leugnen, aber es ist wirklich nur gut gemeint)
Oelwert: Sichere Dividende oder Risiko?
Gruss,
Sil
hi,soll hier kein ölwert thread werden,halt aber lukoil,petrobras und omv für ziemlich ausgeboomt.spricht für die dividende und kurspotenzial.wärs gern sicher hat kauft eine exxon,nur meine meinung und keine empfehlung oder gepushe,was man bei so werten auch nicht machen kann.
cel sci,würd ich gern bei 1 oder 2 dollar einfach mal teilgewinne nicht nehmen.hab´s vor 2 jahren vorpaßt,wo wir kurz auf 2 dollar waren.will´s dieses mal besser machen.bei der phase 3 bin ich mal gespannt ob der kurs richtig anspringt????
Fehlt meiner meinung nach immer noch die richtige pharmafirma,wo auch ein langlebiges bestehen der firma sichert.dann war alles in ordnung,oder zumindest wie zuletzt gemeldet,ein arzneimittel mit einem bigplayer herzustellen,bzw.sich daran beteiligt.
da war dann unendlich kursphantasie
cel sci,würd ich gern bei 1 oder 2 dollar einfach mal teilgewinne nicht nehmen.hab´s vor 2 jahren vorpaßt,wo wir kurz auf 2 dollar waren.will´s dieses mal besser machen.bei der phase 3 bin ich mal gespannt ob der kurs richtig anspringt????
Fehlt meiner meinung nach immer noch die richtige pharmafirma,wo auch ein langlebiges bestehen der firma sichert.dann war alles in ordnung,oder zumindest wie zuletzt gemeldet,ein arzneimittel mit einem bigplayer herzustellen,bzw.sich daran beteiligt.
da war dann unendlich kursphantasie
OH, OH - Hack oder Kack sucht euch schnell einen Partner 
CEL-SCI Corporation Announces 2008 Financial Results
Wednesday January 14, 2009, 9:15 am EST
Yahoo! Buzz Print Related:CEL-SCI Corp.
VIENNA, Va., Jan. 14 /PRNewswire-FirstCall/ -- CEL-SCI CORPORATION
Related Quotes
Symbol Price Change
CVM 0.2399 -0.0501
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} (NYSE Alternext US: CVM) reports financial results for the fiscal year ended September 30, 2008.
CEL-SCI reported a net loss for fiscal year 2008 of $(7,703,415) versus a net loss of $(9,629,657) in fiscal year 2007. The loss per share per common share for fiscal year 2008 was $(0.07) compared to a net loss per common share in fiscal year 2007 of $(0.10). Included in the loss in 2008 were non-cash expenditures that added up to approximately $3.3 million, including $1.3 million for employee-stock compensation charges and $1.4 million for various consulting and other related expenses.
The net loss included research and development (R&D) expenses of $4,101,563 in 2008 compared to $2,528,528 in 2007. R&D expenses increased due to higher costs associated with preparing for the Company's upcoming Phase III clinical trial of its cancer drug Multikine.
Geert Kersten, Chief Executive Officer said, "We are pleased by the progress we made in fiscal 2008 in preparing for our pivotal trial of Multikine for head and neck cancer. Expenditures for 2009 are expected to be significantly reduced because of the completion of the manufacturing facility and other material reductions in expenditures. Our focus now is to secure strategic partners to assist us in funding the Phase III study of our cancer drug."
The report by the Company's accountants also contained a "going concern" qualification. This means that based upon only the existing and committed amount of cash in CEL-SCI today, the accountants cannot be sure that CEL-SCI will have enough cash to stay in business until January 2010. As is clearly explained in the Company's 10-K filing, CEL-SCI's management is aware of this, and is currently working on licensing agreements (in addition to the two agreements it currently has with Teva Pharmaceuticals and Orient Europharma), joint ventures and/or financings to start the Phase III clinical trial with its cancer drug Multikine. In addition, in late December 2008 CEL-SCI put in place a $5 million equity line of credit. In the meantime, in light of the challenging capital markets, management has been very successful in reducing its monthly cash expenditures.
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck in May 2007.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
The Company has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense.
CEL-SCI CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
YEARS ENDED SEPTEMBER 30, 2008 and 2007
2008 2007
GRANT REVENUE AND OTHER $5,065 $57,043
OPERATING EXPENSES:
Research and development (excluding
R&D depreciation of $124,705 and
$91,292 respectively,
included below) 4,101,563 2,528,528
Depreciation and amortization 215,060 176,186
General & administrative 5,200,735 6,704,538
Total operating expenses 9,517,358 9,409,252
NET OPERATING LOSS (9,512,293) (9,352,209)
GAIN ON DERIVATIVE INSTRUMENTS 1,799,393 868,182
COSTS ASSOCIATED WITH CONVERTIBLE DEBT - -
INTEREST INCOME 483,252 562,973
INTEREST EXPENSE (473,767) (1,708,603)
NET LOSS $(7,703,415) $(9,629,657)
DIVIDENDS (424,815) -
NET LOSS AVAILABLE TO COMMON
SHAREHOLDERS $(8,128,230) $(9,629,657)
NET LOSS PER COMMON SHARE
BASIC $(0.07) $(0.10)
DILUTED $(0.07) $(0.10)
WEIGHTED AVERAGE COMMON SHARES
OUTSTANDING
BASIC 117,060,866 97,310,488
DILUTED 117,060,866 97,310,488

CEL-SCI Corporation Announces 2008 Financial Results
Wednesday January 14, 2009, 9:15 am EST
Yahoo! Buzz Print Related:CEL-SCI Corp.
VIENNA, Va., Jan. 14 /PRNewswire-FirstCall/ -- CEL-SCI CORPORATION
Related Quotes
Symbol Price Change
CVM 0.2399 -0.0501
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} (NYSE Alternext US: CVM) reports financial results for the fiscal year ended September 30, 2008.
CEL-SCI reported a net loss for fiscal year 2008 of $(7,703,415) versus a net loss of $(9,629,657) in fiscal year 2007. The loss per share per common share for fiscal year 2008 was $(0.07) compared to a net loss per common share in fiscal year 2007 of $(0.10). Included in the loss in 2008 were non-cash expenditures that added up to approximately $3.3 million, including $1.3 million for employee-stock compensation charges and $1.4 million for various consulting and other related expenses.
The net loss included research and development (R&D) expenses of $4,101,563 in 2008 compared to $2,528,528 in 2007. R&D expenses increased due to higher costs associated with preparing for the Company's upcoming Phase III clinical trial of its cancer drug Multikine.
Geert Kersten, Chief Executive Officer said, "We are pleased by the progress we made in fiscal 2008 in preparing for our pivotal trial of Multikine for head and neck cancer. Expenditures for 2009 are expected to be significantly reduced because of the completion of the manufacturing facility and other material reductions in expenditures. Our focus now is to secure strategic partners to assist us in funding the Phase III study of our cancer drug."
The report by the Company's accountants also contained a "going concern" qualification. This means that based upon only the existing and committed amount of cash in CEL-SCI today, the accountants cannot be sure that CEL-SCI will have enough cash to stay in business until January 2010. As is clearly explained in the Company's 10-K filing, CEL-SCI's management is aware of this, and is currently working on licensing agreements (in addition to the two agreements it currently has with Teva Pharmaceuticals and Orient Europharma), joint ventures and/or financings to start the Phase III clinical trial with its cancer drug Multikine. In addition, in late December 2008 CEL-SCI put in place a $5 million equity line of credit. In the meantime, in light of the challenging capital markets, management has been very successful in reducing its monthly cash expenditures.
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck in May 2007.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
The Company has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense.
CEL-SCI CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
YEARS ENDED SEPTEMBER 30, 2008 and 2007
2008 2007
GRANT REVENUE AND OTHER $5,065 $57,043
OPERATING EXPENSES:
Research and development (excluding
R&D depreciation of $124,705 and
$91,292 respectively,
included below) 4,101,563 2,528,528
Depreciation and amortization 215,060 176,186
General & administrative 5,200,735 6,704,538
Total operating expenses 9,517,358 9,409,252
NET OPERATING LOSS (9,512,293) (9,352,209)
GAIN ON DERIVATIVE INSTRUMENTS 1,799,393 868,182
COSTS ASSOCIATED WITH CONVERTIBLE DEBT - -
INTEREST INCOME 483,252 562,973
INTEREST EXPENSE (473,767) (1,708,603)
NET LOSS $(7,703,415) $(9,629,657)
DIVIDENDS (424,815) -
NET LOSS AVAILABLE TO COMMON
SHAREHOLDERS $(8,128,230) $(9,629,657)
NET LOSS PER COMMON SHARE
BASIC $(0.07) $(0.10)
DILUTED $(0.07) $(0.10)
WEIGHTED AVERAGE COMMON SHARES
OUTSTANDING
BASIC 117,060,866 97,310,488
DILUTED 117,060,866 97,310,488
Antwort auf Beitrag Nr.: 36.375.960 von ALF-FRED am 14.01.09 20:35:40Hi Alf,
gestern war der Markt eh ein enig angeschlagen. CEL-SCI sollet keine Probleme haben noch einmal ein wenig Kapital zu beschaffen. Zudem hat es eher gestoert, dass man immer noch nicht alle Partner fuer eine PIII Studie hat...
Dies ist auch aergerlich, da sich die ersten Ergebisse immer mehr nach hinten verschieben.
Der Kurs geht erts hoch, wenn auch die PIII endlich richtig los geht.
Gruss,
Sil
gestern war der Markt eh ein enig angeschlagen. CEL-SCI sollet keine Probleme haben noch einmal ein wenig Kapital zu beschaffen. Zudem hat es eher gestoert, dass man immer noch nicht alle Partner fuer eine PIII Studie hat...
Dies ist auch aergerlich, da sich die ersten Ergebisse immer mehr nach hinten verschieben.
Der Kurs geht erts hoch, wenn auch die PIII endlich richtig los geht.
Gruss,
Sil
Antwort auf Beitrag Nr.: 36.378.569 von Sillak am 15.01.09 10:12:35 ich lass mich überraschen, mal sehen wie es so kommt !
ich lass mich überraschen, mal sehen wie es so kommt !
 ich lass mich überraschen, mal sehen wie es so kommt !
ich lass mich überraschen, mal sehen wie es so kommt !
Antwort auf Beitrag Nr.: 36.380.042 von ALF-FRED am 15.01.09 12:46:02 Was könnte es dem Kursverlauf in Prozenten bringen, wenn der Start der P III definitiv mit Datum bekannt gegeben würde ?
Was könnte es dem Kursverlauf in Prozenten bringen, wenn der Start der P III definitiv mit Datum bekannt gegeben würde ?
Hat da jemand irgendwelche Vorstellungen oder Erfahrungen mit anderen Werten ?
schönes Wochenende
Alf-Fred
 Was könnte es dem Kursverlauf in Prozenten bringen, wenn der Start der P III definitiv mit Datum bekannt gegeben würde ?
Was könnte es dem Kursverlauf in Prozenten bringen, wenn der Start der P III definitiv mit Datum bekannt gegeben würde ?Hat da jemand irgendwelche Vorstellungen oder Erfahrungen mit anderen Werten ?
schönes Wochenende
Alf-Fred
Antwort auf Beitrag Nr.: 36.394.905 von ALF-FRED am 17.01.09 11:47:18 und wir warten weiter !!!
und wir warten weiter !!!
 und wir warten weiter !!!
und wir warten weiter !!!
Antwort auf Beitrag Nr.: 36.462.957 von ALF-FRED am 28.01.09 10:57:00 Und wenn wir nicht gestorben sind , dann warten wir noch immer!
Und wenn wir nicht gestorben sind , dann warten wir noch immer!
 Und wenn wir nicht gestorben sind , dann warten wir noch immer!
Und wenn wir nicht gestorben sind , dann warten wir noch immer!
Friday, January 30, 2009
Biotech
Biotechs face millions of debt due in recessionWashington Business Journal - by Vandana Sinha Staff Reporter
Print Email Reprints RSS Feeds Add to Del.icio.us Digg This CommentsRelated News
Bioheart delinquent on loan, bankruptcy possible
In a coup for Pr. George's, BioServe stays, expands
Research Triangle Park buys back land from Sumitomo
As if tight-fisted investors and unforgiving shareholders weren’t enough, some area biotechs may see more bills come due this year.
A handful of companies have maturing debt at a time when the capital markets are in upheaval. It’s another beat-the-odds scenario for biotechs, which have already suffered losses in personnel and portfolios as a result of the recession. But those saddled this year with potential debt payments — routine financing tactics for biotechs hungry for cash for clinical tests but short on revenue — say they’ll find a way to pay it off.
The dollar amounts range widely, from $2.2 million in convertible debt notes for Cel-Sci Corp. to $250 million in convertible debt notes for United Therapeutics Corp. Some biotechs have pruned their overall loan balances by shipping off monthly checks to their lenders for the past few years. But the maturation date for a loan or debt note acts as the final deadline — and prime time for the bill collectors to come knocking.
Rockville’s Emergent BioSolutions Inc. will see its annual debt payments nearly double this year over last year to $6 million. GenVec Inc. of Gaithersburg is paying off a $5 million Maryland state bond that matures this June. Bethesda’s Northwest Biotherapeutics Inc. is in talks with its lender to put off a $4 million loan due at the end of last year, even as another $3 million could become payable between now and April. And even one of the largest biotechs in the area, Qiagen NV, whose U.S. headquarters is in Germantown, must shell out $25 million of a $500 million loan this year.
Biotech
Biotechs face millions of debt due in recessionWashington Business Journal - by Vandana Sinha Staff Reporter
Print Email Reprints RSS Feeds Add to Del.icio.us Digg This CommentsRelated News
Bioheart delinquent on loan, bankruptcy possible
In a coup for Pr. George's, BioServe stays, expands
Research Triangle Park buys back land from Sumitomo
As if tight-fisted investors and unforgiving shareholders weren’t enough, some area biotechs may see more bills come due this year.
A handful of companies have maturing debt at a time when the capital markets are in upheaval. It’s another beat-the-odds scenario for biotechs, which have already suffered losses in personnel and portfolios as a result of the recession. But those saddled this year with potential debt payments — routine financing tactics for biotechs hungry for cash for clinical tests but short on revenue — say they’ll find a way to pay it off.
The dollar amounts range widely, from $2.2 million in convertible debt notes for Cel-Sci Corp. to $250 million in convertible debt notes for United Therapeutics Corp. Some biotechs have pruned their overall loan balances by shipping off monthly checks to their lenders for the past few years. But the maturation date for a loan or debt note acts as the final deadline — and prime time for the bill collectors to come knocking.
Rockville’s Emergent BioSolutions Inc. will see its annual debt payments nearly double this year over last year to $6 million. GenVec Inc. of Gaithersburg is paying off a $5 million Maryland state bond that matures this June. Bethesda’s Northwest Biotherapeutics Inc. is in talks with its lender to put off a $4 million loan due at the end of last year, even as another $3 million could become payable between now and April. And even one of the largest biotechs in the area, Qiagen NV, whose U.S. headquarters is in Germantown, must shell out $25 million of a $500 million loan this year.
Antwort auf Beitrag Nr.: 36.496.184 von ALF-FRED am 02.02.09 17:16:26Hi Alf,
ich bin erstmal raus. Warte auf den offiziellen Start der PIII...
Gruss,
Sil
ich bin erstmal raus. Warte auf den offiziellen Start der PIII...
Gruss,
Sil
Antwort auf Beitrag Nr.: 36.521.903 von Sillak am 05.02.09 19:15:37 Schade, hoffe du verpasst denn Einstieg nicht ( wenn er den kommt ) ich lasse laufen mit ca. 60 % miese
Schade, hoffe du verpasst denn Einstieg nicht ( wenn er den kommt ) ich lasse laufen mit ca. 60 % miese 


 Schade, hoffe du verpasst denn Einstieg nicht ( wenn er den kommt ) ich lasse laufen mit ca. 60 % miese
Schade, hoffe du verpasst denn Einstieg nicht ( wenn er den kommt ) ich lasse laufen mit ca. 60 % miese 


Antwort auf Beitrag Nr.: 36.521.919 von ALF-FRED am 05.02.09 19:18:02 vielleicht hab ich ja keine Ahnung - aber mein Verstand sagt, dass ich doch nicht einfach so eine Produktionsfirma baue und sie komplett einrichte - wenn es kein Sinn hat !!!
vielleicht hab ich ja keine Ahnung - aber mein Verstand sagt, dass ich doch nicht einfach so eine Produktionsfirma baue und sie komplett einrichte - wenn es kein Sinn hat !!! 
die Bilder stehen auf der Homepage und man kann sie bei gooogle earth vergleichen, die gibt es wirklich !
 vielleicht hab ich ja keine Ahnung - aber mein Verstand sagt, dass ich doch nicht einfach so eine Produktionsfirma baue und sie komplett einrichte - wenn es kein Sinn hat !!!
vielleicht hab ich ja keine Ahnung - aber mein Verstand sagt, dass ich doch nicht einfach so eine Produktionsfirma baue und sie komplett einrichte - wenn es kein Sinn hat !!! 
die Bilder stehen auf der Homepage und man kann sie bei gooogle earth vergleichen, die gibt es wirklich !

Antwort auf Beitrag Nr.: 36.563.188 von ALF-FRED am 12.02.09 09:40:43Hi Alfred!
Das ist nicht der Punkt. Aufgrund der bisherigen Daten ist man so zuversichtlich, dass man die nalge gebaut hat. Aber dazu muss man ertsmal ausreichend Partner finden, die die PIII mit betreuen und dann muss man natuerlich die markerife erlangen, bevor man voll produzieen kann.
Gruss,
Sil
Das ist nicht der Punkt. Aufgrund der bisherigen Daten ist man so zuversichtlich, dass man die nalge gebaut hat. Aber dazu muss man ertsmal ausreichend Partner finden, die die PIII mit betreuen und dann muss man natuerlich die markerife erlangen, bevor man voll produzieen kann.
Gruss,
Sil
 Was kacken die ab
Was kacken die ab 
Press Release Source: CEL-SCI Corporation
CEL-SCI Corporation Releases Letter to Shareholders
Friday February 20, 8:55 am ET
VIENNA, Va., Feb. 20 /PRNewswire-FirstCall/ -- The following letter is being released by CEL-SCI Corporation (NYSE Alternext US: CVM) to its shareholders:
Dear Fellow Shareholders:
I am writing today to provide you with a review of 2008 as well as to update you on our plans for dealing with the current financial downturn. In looking back at the last year, I see a year of meaningful accomplishments which put CEL-SCI in a valuable position, and among a very select group of companies that have a completely novel medicine with blockbuster potential ready to go into a Phase III clinical trial.
Key Company highlights of 2008:
We signed a Multikine® licensing agreement with Teva Pharmaceuticals, one of the top 20 pharmaceutical companies in the world. Teva will participate in and pay for part of the Multikine Phase III trial.
We extended our Multikine licensing agreement with Orient EuroPharma of Taiwan (a leading pharmaceutical company in Taiwan) to cover additional countries in the Far East. Orient EuroPharma will also participate in and pay for part of our Phase III trial.
Multikine was named one of 10 future blockbuster drugs by MedAdNews. "These 10 medicines are expected to eventually garner FDA approval and break the annual billion-dollar sales barrier."
At the end of 2008 we took delivery of the Multikine manufacturing facility in Baltimore. This facility will supply Multikine for the Phase III clinical trial as well as commercial sale following approval. We have also designed the facility to be used as a contract manufacturing facility during those times (e.g., during certain times in the Phase III study) when we will not be producing Multikine. This lucrative business could quickly become a multi-million dollar revenue generator for CEL-SCI.
We presented very promising data on a new rheumatoid arthritis vaccine discovered by CEL-SCI scientists. The data presented was as good as that achieved with Enbrel, a leading treatment for rheumatoid arthritis.
We started planning and pricing our global clinical trial designed to lead to marketing approval for Multikine. The recent strength of the dollar will reduce our Phase III study costs by several million dollars.
While we are pleased by these accomplishments, we also recognize that there are major challenges due to the severe financial downturn. This downturn has affected all of us, but we have taken immediate action. We very quickly reduced our expenditures and expanded our discussions with various pharmaceutical companies for a world-wide Multikine partnership. Things are very difficult all around for most companies, but we are seeing new strength in the bigger biotechnology names. This will hopefully trickle down to the smaller ones like us as well. The huge buyouts in the industry, Pfizer buying Wyeth and Roche trying to buy Genentech, will add over $100 billion in liquidity to the industry, and that too should help us down the road.
Let me share with you how we at CEL-SCI are dealing with and planning for recovery from this financial downturn. In our opinion, during such a difficult time as this you cannot rely on one strategy alone. Therefore we are pursuing the following multi-pronged strategy. To help us with this process we have hired BroadOak Capital Partners, an investment bank with a comprehensive focus on the life sciences industry.
We are working on licensing agreements for Multikine to pay for the Phase III trial because raising the amount of money required for the trial at the current prices would be harmful to our shareholders;
We are working on a joint venturing agreement for the same reason;
We are in discussions to use our manufacturing facility as a contract manufacturing facility while it is not being used to produce Multikine;
We have also signed a $5 million line of credit to be used if necessary. This line is not intended to be our primary funding source but does serve as additional insurance to shareholders;
We have a few other, some of them "out of the box" ideas, ongoing.
We hope to bring at least one of these projects to a conclusion very soon.
We cannot change the economic climate or the damage that the financial crisis has caused to the countless stocks, us included, but we are trying to structure our future steps to create the greatest value for CEL-SCI shareholders. We are being very careful and deliberate, working closely with our various partners, suppliers, etc as we adapt to the current environment.
We believe that as a cancer company ready to start a pivotal Phase III clinical trial with a completely novel and non-toxic cancer therapy, we are well positioned for licensing, joint venture and other business arrangements. In normal times these deals might already have been completed a while ago and we would have already started the Phase III trial. In the last few months almost all investments have been hit hard and everything just seems to take longer. Our valuation is currently very low. However, please do not confuse our share price with the real underlying value of the company. One announcement can change the share price dramatically. We have created a late stage cancer company with very large potential both to help patients and create profit, particularly since Multikine is not toxic. What is the value of such a product? Once proven to work, probably billions of dollars. Therefore I believe that our value should be much higher today than the current share price, particularly since a cancer drug is not really related to the economic conditions. If we are successful in just some of the projects listed above, the additional value should very quickly be recognized and the current times will be quickly forgotten.
In closing, we believe that the immune system holds the key to treating cancer. We believe that Multikine will be a multi-billion dollar drug because it works with the body to destroy those tumor metastases thought to be responsible for recurrence of the tumor. In our Phase II studies, Multikine when given for only 3 weeks prior to the normal treatment was able to eliminate all tumor in 12% of the patients, reduce the number of tumor cells by an average of 50% in the treatment group and increase long-term overall survival by 33%. This is why you are a shareholder in CEL-SCI! Tell a friend about our great results.
We thank you for your continued support.
Sincerely,
Geert Kersten
Chief Executive Officer
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital or finalize necessary agreements and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10-K for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
CEL-SCI Corporation Releases Letter to Shareholders
Friday February 20, 8:55 am ET
VIENNA, Va., Feb. 20 /PRNewswire-FirstCall/ -- The following letter is being released by CEL-SCI Corporation (NYSE Alternext US: CVM) to its shareholders:
Dear Fellow Shareholders:
I am writing today to provide you with a review of 2008 as well as to update you on our plans for dealing with the current financial downturn. In looking back at the last year, I see a year of meaningful accomplishments which put CEL-SCI in a valuable position, and among a very select group of companies that have a completely novel medicine with blockbuster potential ready to go into a Phase III clinical trial.
Key Company highlights of 2008:
We signed a Multikine® licensing agreement with Teva Pharmaceuticals, one of the top 20 pharmaceutical companies in the world. Teva will participate in and pay for part of the Multikine Phase III trial.
We extended our Multikine licensing agreement with Orient EuroPharma of Taiwan (a leading pharmaceutical company in Taiwan) to cover additional countries in the Far East. Orient EuroPharma will also participate in and pay for part of our Phase III trial.
Multikine was named one of 10 future blockbuster drugs by MedAdNews. "These 10 medicines are expected to eventually garner FDA approval and break the annual billion-dollar sales barrier."
At the end of 2008 we took delivery of the Multikine manufacturing facility in Baltimore. This facility will supply Multikine for the Phase III clinical trial as well as commercial sale following approval. We have also designed the facility to be used as a contract manufacturing facility during those times (e.g., during certain times in the Phase III study) when we will not be producing Multikine. This lucrative business could quickly become a multi-million dollar revenue generator for CEL-SCI.
We presented very promising data on a new rheumatoid arthritis vaccine discovered by CEL-SCI scientists. The data presented was as good as that achieved with Enbrel, a leading treatment for rheumatoid arthritis.
We started planning and pricing our global clinical trial designed to lead to marketing approval for Multikine. The recent strength of the dollar will reduce our Phase III study costs by several million dollars.
While we are pleased by these accomplishments, we also recognize that there are major challenges due to the severe financial downturn. This downturn has affected all of us, but we have taken immediate action. We very quickly reduced our expenditures and expanded our discussions with various pharmaceutical companies for a world-wide Multikine partnership. Things are very difficult all around for most companies, but we are seeing new strength in the bigger biotechnology names. This will hopefully trickle down to the smaller ones like us as well. The huge buyouts in the industry, Pfizer buying Wyeth and Roche trying to buy Genentech, will add over $100 billion in liquidity to the industry, and that too should help us down the road.
Let me share with you how we at CEL-SCI are dealing with and planning for recovery from this financial downturn. In our opinion, during such a difficult time as this you cannot rely on one strategy alone. Therefore we are pursuing the following multi-pronged strategy. To help us with this process we have hired BroadOak Capital Partners, an investment bank with a comprehensive focus on the life sciences industry.
We are working on licensing agreements for Multikine to pay for the Phase III trial because raising the amount of money required for the trial at the current prices would be harmful to our shareholders;
We are working on a joint venturing agreement for the same reason;
We are in discussions to use our manufacturing facility as a contract manufacturing facility while it is not being used to produce Multikine;
We have also signed a $5 million line of credit to be used if necessary. This line is not intended to be our primary funding source but does serve as additional insurance to shareholders;
We have a few other, some of them "out of the box" ideas, ongoing.
We hope to bring at least one of these projects to a conclusion very soon.
We cannot change the economic climate or the damage that the financial crisis has caused to the countless stocks, us included, but we are trying to structure our future steps to create the greatest value for CEL-SCI shareholders. We are being very careful and deliberate, working closely with our various partners, suppliers, etc as we adapt to the current environment.
We believe that as a cancer company ready to start a pivotal Phase III clinical trial with a completely novel and non-toxic cancer therapy, we are well positioned for licensing, joint venture and other business arrangements. In normal times these deals might already have been completed a while ago and we would have already started the Phase III trial. In the last few months almost all investments have been hit hard and everything just seems to take longer. Our valuation is currently very low. However, please do not confuse our share price with the real underlying value of the company. One announcement can change the share price dramatically. We have created a late stage cancer company with very large potential both to help patients and create profit, particularly since Multikine is not toxic. What is the value of such a product? Once proven to work, probably billions of dollars. Therefore I believe that our value should be much higher today than the current share price, particularly since a cancer drug is not really related to the economic conditions. If we are successful in just some of the projects listed above, the additional value should very quickly be recognized and the current times will be quickly forgotten.
In closing, we believe that the immune system holds the key to treating cancer. We believe that Multikine will be a multi-billion dollar drug because it works with the body to destroy those tumor metastases thought to be responsible for recurrence of the tumor. In our Phase II studies, Multikine when given for only 3 weeks prior to the normal treatment was able to eliminate all tumor in 12% of the patients, reduce the number of tumor cells by an average of 50% in the treatment group and increase long-term overall survival by 33%. This is why you are a shareholder in CEL-SCI! Tell a friend about our great results.
We thank you for your continued support.
Sincerely,
Geert Kersten
Chief Executive Officer
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital or finalize necessary agreements and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10-K for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
Antwort auf Beitrag Nr.: 36.621.772 von ALF-FRED am 20.02.09 16:29:53Hi Alf! Ein paar interessante Inhalte...zumindest heute fuer dich ein wenig Erholung.
Nur Geduld...
Evtl. doch wieder rein...ich denke drueber nach. Aber erst warte ich, ob der Kurs nicht doch wieder ein wenig nachgibt...
Gruss,
Sil
Nur Geduld...
Evtl. doch wieder rein...ich denke drueber nach. Aber erst warte ich, ob der Kurs nicht doch wieder ein wenig nachgibt...
Gruss,
Sil
Antwort auf Beitrag Nr.: 36.624.814 von Sillak am 20.02.09 22:08:49 Ja, ich setze auf die 50 / 50 Change ! Kauf nix mehr bei und verkauf auch nix - wenn es klappt hab ich genug
Ja, ich setze auf die 50 / 50 Change ! Kauf nix mehr bei und verkauf auch nix - wenn es klappt hab ich genug 
steuerfrei
 Ja, ich setze auf die 50 / 50 Change ! Kauf nix mehr bei und verkauf auch nix - wenn es klappt hab ich genug
Ja, ich setze auf die 50 / 50 Change ! Kauf nix mehr bei und verkauf auch nix - wenn es klappt hab ich genug 
steuerfrei

CEL-SCI Corporation Releases Brief an die Aktionäre
Freitag 20. Februar, 8.55 Uhr ET
VIENNA, Va., Feb. 20 / Update wird - Die folgenden Buchstaben werden, die von CEL-SCI Corporation (NYSE Alternext USA: CVM) an die Aktionäre:
Sehr geehrte Aktionärinnen und Aktionäre:
Ich schreibe Ihnen heute, um Ihnen eine Überprüfung der 2008 sowie um Sie über unsere Pläne für den Umgang mit der derzeitigen finanziellen Abschwung. Im Rückblick auf das letzte Jahr, sehe ich ein Jahr der sinnvolle Leistungen, die die CEL-SCI in einen wertvollen Position, und auf eine sehr kleine Gruppe von Unternehmen, die eine völlig neuartige Medikament mit Blockbuster-Potenzial bereit, in eine klinische Phase-III - Gerichtsverfahren.
Die wichtigsten Unternehmen Highlights des Jahres 2008:
Wir haben einen Multikine ® Lizenz-Vereinbarung mit Teva Pharmaceuticals, einer der 20 führenden pharmazeutischen Unternehmen in der Welt. Teva wird sich an und zahlen für einen Teil der Multikine Phase-III-Studie.
Wir haben unsere Multikine Lizenzvertrag mit Orient EuroPharma von Taiwan (einer der führenden Pharma-Unternehmen in Taiwan) zur Deckung der zusätzlichen Ländern im Fernen Osten. Orient EuroPharma wird auch an und zahlen für einen Teil der Phase-III-Studie.
Multikine wurde zu einer der 10 künftigen Blockbuster-Medikamente von MedAdNews. "Diese 10 Arzneimittel wird erwartet, dass sie schließlich Garner FDA-Zulassung und Bruch der jährlichen Milliarden-Dollar-Umsatz Barriere."
Am Ende des Jahres 2008 haben wir die Lieferung von Multikine Produktionsstätte in Baltimore. Diese Anlage liefert Multikine für die Phase-III-Studie sowie der Verkauf nach Genehmigung. Wir haben auch die Möglichkeit, als Contract-Manufacturing-Anlage während dieser Zeit (z. B. zu bestimmten Zeiten in der Phase-III-Studie), wenn wir nicht in der Herstellung von Multikine. Dieses lukrative Geschäft könnte sich schnell als ein Multi-Millionen-Dollar-Umsatz-Generator für CEL-SCI.
Wir haben sehr viel versprechende Daten über einen neuen Impfstoff entdeckt der rheumatoiden Arthritis durch die CEL-SCI Wissenschaftler. Die Daten wurden so gut wie erreicht, dass die mit Enbrel, einem führenden Behandlung von rheumatoider Arthritis.
Wir haben die Planung und Preise unserer weltweiten klinischen Studie, die zur Marktzulassung für Multikine. Die jüngste Stärke des Dollar wird unsere Phase-III-Studie Kosten von mehreren Millionen Dollar.
Während wir freuen uns über diese Erfolge haben wir auch erkennen, dass es großen Herausforderungen aufgrund der schwierigen finanziellen Rückgang. Dieser Rückgang betrifft alle von uns, aber wir haben ein sofortiges Eingreifen. Wir sehr schnell unsere Ausgaben und unsere Gespräche mit verschiedenen Pharma-Unternehmen für eine weltweite Partnerschaft Multikine. Die Dinge sind sehr schwierig, alle etwa für die meisten Unternehmen, aber wir werden sehen, neue Kraft in den grösseren Biotechnologie Namen. Das wird sich hoffentlich Rinnsal auf den kleineren, wie auch uns. Die große Buy-outs in der Industrie, Pfizer kaufen Wyeth und Roche zu kaufen Genentech, wird mehr als 100 Milliarden Dollar an Liquidität in der Branche, und das sollte uns helfen, auch die Straße.
Lassen Sie mich mit Ihnen teilen, wie wir auf CEL-SCI sind den Umgang mit und die Planung für die Erholung von dieser finanziellen Rückgang. Wir sind der Meinung, während einer so schwierigen Zeit wie dieser kann man sich nicht auf eine Strategie allein. Daher sind wir um die folgenden Multi-Säulen-Strategie. So helfen Sie uns mit diesem Prozess haben wir gemietet BroadOak Capital Partners, einer Investmentbank mit einem umfassenden konzentrieren sich auf die Life-Science-Industrie.
Wir arbeiten auf Lizenzvereinbarungen für Multikine die Kosten für die Phase-III-Studie, weil die Erhöhung der Menge von Geld, die für die Prüfung in den aktuellen Preisen wäre schädlich für unsere Aktionäre;
Wir arbeiten an einem gemeinsamen venturing Vereinbarung aus dem gleichen Grund;
Wir befinden uns in Diskussionen zu verwenden unsere Fertigung als Contract-Manufacturing-Anlage, während es nicht zur Herstellung von Multikine;
Wir haben auch einen $ 5 Mio. Kreditlinie dann verwendet werden, wenn erforderlich. Diese Zeile ist nicht unsere primäre Finanzierungsquelle, aber als zusätzliche Versicherung an die Aktionäre;
Wir haben ein paar andere, einige von ihnen "out-of-the-box"-Ideen, im Gange.
Wir hoffen, dass die mindestens eines dieser Projekte zu einem Abschluss in Kürze.
Wir können nicht die Konjunktur oder der Schaden, dass die Finanzkrise verursacht hat, um die unzähligen Bestände, uns eingeschlossen, aber wir versuchen, unsere Struktur zu schaffen, die künftigen Schritte der größte Wert für CEL-SCI-Aktionäre. Wir sind sehr vorsichtig und beabsichtigten, in enger Zusammenarbeit mit unseren verschiedenen Partnern, Lieferanten, etc., wie wir an die aktuelle Umgebung.
Wir glauben, dass die als Krebs Unternehmen bereit, um eine entscheidende Phase-III-Studie mit einem völlig neuartigen und nicht-toxischen Krebs-Therapie, wir sind gut positioniert für die Lizenzierung, Joint-Venture-Vereinbarungen und anderen Geschäftspartnern. In normalen Zeiten dieser Transaktionen könnte schon eine Weile her und wir haben bereits die Phase-III-Studie. In den letzten Monaten fast alle Investitionen wurden hart getroffen und alles hat den Anschein, etwas länger dauern. Unsere Bewertung ist derzeit sehr gering. Sie aber bitte nicht verwechseln unsere Aktie mit dem realen Wert der zugrunde liegenden Unternehmen. Eine Ankündigung kann der Kurs der Aktie dramatisch. Wir haben einen späten Stadium Krebs mit sehr großes Potenzial sowohl für Patienten und Gewinn, zumal Multikine ist nicht giftig. Was ist der Wert, der ein solches Produkt? Sobald sich der Arbeit, wahrscheinlich Milliarden von Dollar. Deshalb glaube ich, dass unser Wert sollte heute viel höher als der aktuelle Aktienkurs, insbesondere, da ein Krebsmedikament ist nicht wirklich im Zusammenhang mit der wirtschaftlichen Bedingungen. Wenn es uns gelingt, nur einige der Projekte vor, die zusätzlichen Wert sollte sehr schnell erkannt werden und die aktuelle Zeit wird schnell vergessen.
Abschließend sind wir der Meinung, dass das Immunsystem ist der Schlüssel zur Behandlung von Krebs. Wir glauben, dass Multikine wird eine Multi-Milliarden-Dollar-Medikament, weil es mit dem Körper zu zerstören, die Tumor Metastasen gedacht, die für die Wiederholung des Tumors. In unserer Phase-II-Studien, Multikine, wenn sie für nur 3 Wochen vor der normalen Behandlung war in der Lage, alle Tumor in 12% der Patienten, die Senkung der Zahl von Tumorzellen mit einem Durchschnitt von 50% in der Behandlungsgruppe und die lange -term-Überlebensrate von 33%. Dies ist der Grund, warum Sie sich ein Aktionär in CEL-SCI! Informieren Sie einen Freund über unsere große Ergebnisse.
Wir danken Ihnen für Ihre weitere Unterstützung.
nur wann die phase 3 beginnt,steht mal wieder nichts da????
Freitag 20. Februar, 8.55 Uhr ET
VIENNA, Va., Feb. 20 / Update wird - Die folgenden Buchstaben werden, die von CEL-SCI Corporation (NYSE Alternext USA: CVM) an die Aktionäre:
Sehr geehrte Aktionärinnen und Aktionäre:
Ich schreibe Ihnen heute, um Ihnen eine Überprüfung der 2008 sowie um Sie über unsere Pläne für den Umgang mit der derzeitigen finanziellen Abschwung. Im Rückblick auf das letzte Jahr, sehe ich ein Jahr der sinnvolle Leistungen, die die CEL-SCI in einen wertvollen Position, und auf eine sehr kleine Gruppe von Unternehmen, die eine völlig neuartige Medikament mit Blockbuster-Potenzial bereit, in eine klinische Phase-III - Gerichtsverfahren.
Die wichtigsten Unternehmen Highlights des Jahres 2008:
Wir haben einen Multikine ® Lizenz-Vereinbarung mit Teva Pharmaceuticals, einer der 20 führenden pharmazeutischen Unternehmen in der Welt. Teva wird sich an und zahlen für einen Teil der Multikine Phase-III-Studie.
Wir haben unsere Multikine Lizenzvertrag mit Orient EuroPharma von Taiwan (einer der führenden Pharma-Unternehmen in Taiwan) zur Deckung der zusätzlichen Ländern im Fernen Osten. Orient EuroPharma wird auch an und zahlen für einen Teil der Phase-III-Studie.
Multikine wurde zu einer der 10 künftigen Blockbuster-Medikamente von MedAdNews. "Diese 10 Arzneimittel wird erwartet, dass sie schließlich Garner FDA-Zulassung und Bruch der jährlichen Milliarden-Dollar-Umsatz Barriere."
Am Ende des Jahres 2008 haben wir die Lieferung von Multikine Produktionsstätte in Baltimore. Diese Anlage liefert Multikine für die Phase-III-Studie sowie der Verkauf nach Genehmigung. Wir haben auch die Möglichkeit, als Contract-Manufacturing-Anlage während dieser Zeit (z. B. zu bestimmten Zeiten in der Phase-III-Studie), wenn wir nicht in der Herstellung von Multikine. Dieses lukrative Geschäft könnte sich schnell als ein Multi-Millionen-Dollar-Umsatz-Generator für CEL-SCI.
Wir haben sehr viel versprechende Daten über einen neuen Impfstoff entdeckt der rheumatoiden Arthritis durch die CEL-SCI Wissenschaftler. Die Daten wurden so gut wie erreicht, dass die mit Enbrel, einem führenden Behandlung von rheumatoider Arthritis.
Wir haben die Planung und Preise unserer weltweiten klinischen Studie, die zur Marktzulassung für Multikine. Die jüngste Stärke des Dollar wird unsere Phase-III-Studie Kosten von mehreren Millionen Dollar.
Während wir freuen uns über diese Erfolge haben wir auch erkennen, dass es großen Herausforderungen aufgrund der schwierigen finanziellen Rückgang. Dieser Rückgang betrifft alle von uns, aber wir haben ein sofortiges Eingreifen. Wir sehr schnell unsere Ausgaben und unsere Gespräche mit verschiedenen Pharma-Unternehmen für eine weltweite Partnerschaft Multikine. Die Dinge sind sehr schwierig, alle etwa für die meisten Unternehmen, aber wir werden sehen, neue Kraft in den grösseren Biotechnologie Namen. Das wird sich hoffentlich Rinnsal auf den kleineren, wie auch uns. Die große Buy-outs in der Industrie, Pfizer kaufen Wyeth und Roche zu kaufen Genentech, wird mehr als 100 Milliarden Dollar an Liquidität in der Branche, und das sollte uns helfen, auch die Straße.
Lassen Sie mich mit Ihnen teilen, wie wir auf CEL-SCI sind den Umgang mit und die Planung für die Erholung von dieser finanziellen Rückgang. Wir sind der Meinung, während einer so schwierigen Zeit wie dieser kann man sich nicht auf eine Strategie allein. Daher sind wir um die folgenden Multi-Säulen-Strategie. So helfen Sie uns mit diesem Prozess haben wir gemietet BroadOak Capital Partners, einer Investmentbank mit einem umfassenden konzentrieren sich auf die Life-Science-Industrie.
Wir arbeiten auf Lizenzvereinbarungen für Multikine die Kosten für die Phase-III-Studie, weil die Erhöhung der Menge von Geld, die für die Prüfung in den aktuellen Preisen wäre schädlich für unsere Aktionäre;
Wir arbeiten an einem gemeinsamen venturing Vereinbarung aus dem gleichen Grund;
Wir befinden uns in Diskussionen zu verwenden unsere Fertigung als Contract-Manufacturing-Anlage, während es nicht zur Herstellung von Multikine;
Wir haben auch einen $ 5 Mio. Kreditlinie dann verwendet werden, wenn erforderlich. Diese Zeile ist nicht unsere primäre Finanzierungsquelle, aber als zusätzliche Versicherung an die Aktionäre;
Wir haben ein paar andere, einige von ihnen "out-of-the-box"-Ideen, im Gange.
Wir hoffen, dass die mindestens eines dieser Projekte zu einem Abschluss in Kürze.
Wir können nicht die Konjunktur oder der Schaden, dass die Finanzkrise verursacht hat, um die unzähligen Bestände, uns eingeschlossen, aber wir versuchen, unsere Struktur zu schaffen, die künftigen Schritte der größte Wert für CEL-SCI-Aktionäre. Wir sind sehr vorsichtig und beabsichtigten, in enger Zusammenarbeit mit unseren verschiedenen Partnern, Lieferanten, etc., wie wir an die aktuelle Umgebung.
Wir glauben, dass die als Krebs Unternehmen bereit, um eine entscheidende Phase-III-Studie mit einem völlig neuartigen und nicht-toxischen Krebs-Therapie, wir sind gut positioniert für die Lizenzierung, Joint-Venture-Vereinbarungen und anderen Geschäftspartnern. In normalen Zeiten dieser Transaktionen könnte schon eine Weile her und wir haben bereits die Phase-III-Studie. In den letzten Monaten fast alle Investitionen wurden hart getroffen und alles hat den Anschein, etwas länger dauern. Unsere Bewertung ist derzeit sehr gering. Sie aber bitte nicht verwechseln unsere Aktie mit dem realen Wert der zugrunde liegenden Unternehmen. Eine Ankündigung kann der Kurs der Aktie dramatisch. Wir haben einen späten Stadium Krebs mit sehr großes Potenzial sowohl für Patienten und Gewinn, zumal Multikine ist nicht giftig. Was ist der Wert, der ein solches Produkt? Sobald sich der Arbeit, wahrscheinlich Milliarden von Dollar. Deshalb glaube ich, dass unser Wert sollte heute viel höher als der aktuelle Aktienkurs, insbesondere, da ein Krebsmedikament ist nicht wirklich im Zusammenhang mit der wirtschaftlichen Bedingungen. Wenn es uns gelingt, nur einige der Projekte vor, die zusätzlichen Wert sollte sehr schnell erkannt werden und die aktuelle Zeit wird schnell vergessen.
Abschließend sind wir der Meinung, dass das Immunsystem ist der Schlüssel zur Behandlung von Krebs. Wir glauben, dass Multikine wird eine Multi-Milliarden-Dollar-Medikament, weil es mit dem Körper zu zerstören, die Tumor Metastasen gedacht, die für die Wiederholung des Tumors. In unserer Phase-II-Studien, Multikine, wenn sie für nur 3 Wochen vor der normalen Behandlung war in der Lage, alle Tumor in 12% der Patienten, die Senkung der Zahl von Tumorzellen mit einem Durchschnitt von 50% in der Behandlungsgruppe und die lange -term-Überlebensrate von 33%. Dies ist der Grund, warum Sie sich ein Aktionär in CEL-SCI! Informieren Sie einen Freund über unsere große Ergebnisse.
Wir danken Ihnen für Ihre weitere Unterstützung.
nur wann die phase 3 beginnt,steht mal wieder nichts da????
 Geld ist ja genug da !!!
Geld ist ja genug da !!!TEVA PHARMACEUTICAL –
Rekordzahlen für 2008
Im NASDAQ 100 war die Aktie des israelischen
Generikaspezialisten Teva mit
einem Plus von gut 4% einer der besten
Werte. Der weltgrößte Generikahersteller
meldete für 2008 einen Rekordumsatz
von 11,1 Mrd. US-Dollar, was einem Anstieg
von 18% entspricht. Der operative
Gewinn wuchs um 22% auf 6 Mrd. USDollar.
Der Rückgang beim Nettogewinn
von 1,9 Mrd. US-Dollar auf 635 Mio. USDollar
lag an Einmalaufwendungen für
eine Übernahme.
ab wann müssen die meilensteinzahlungen machen?mit start der 3 phase oder erst später,bei welchen weitern zielen?
Antwort auf Beitrag Nr.: 36.628.752 von VFBLER am 22.02.09 13:15:27 das wird wohl ausgehandelt werden und ist Bestand der Verträge, wenn welche zu Stande kommen !
das wird wohl ausgehandelt werden und ist Bestand der Verträge, wenn welche zu Stande kommen !
Diese Verträge werden bestimmt nicht veröffentlicht werden, ich lass mich überraschen, ich warte ja auch schon ein paar Jahre.
 das wird wohl ausgehandelt werden und ist Bestand der Verträge, wenn welche zu Stande kommen !
das wird wohl ausgehandelt werden und ist Bestand der Verträge, wenn welche zu Stande kommen !Diese Verträge werden bestimmt nicht veröffentlicht werden, ich lass mich überraschen, ich warte ja auch schon ein paar Jahre.




Friday, February 20, 2009, 1:13pm EST | Modified: Friday, February 20, 2009, 1:17pm
Cel-Sci unveils plan to raise fundsWashington Business Journal - by Tierney Plumb Staff Reporter
Print Email Reprints RSS Feeds Add to Del.icio.us Digg This CommentsRelated News
Cel-Sci CEO: Investors played ‘game’ with stock
Cel-Sci lets Teva sell cancer drug
Cel-Sci unveils plan to raise funds
Biotechs face millions of debt due in recession
Cel-Sci Corp., which is trying to scrounge up enough money to run a final trial for its best drug prospect, sent out a letter to shareholders outlining several fund raising tactics being attempted.
“This downturn has affected all of us, but we have taken immediate action,” said Geert Kersten, chief executive officer, in the letter. “We very quickly reduced our expenditures and expanded our discussions with various pharmaceutical companies for a world-wide Multikine partnership.”
To conserve cash, the Vienna-based company laid off three Cel-Sci employees in December and kept them on as consultants.
Last August Cel-Sci announced a licensing deal with Teva, an Israeli pharmaceutical company that will run the last trial for the drug Multikine, which uses the body’s immune system to attack cancerous tumors.
Both Teva and Orient EuroPharma, a pharmaceutical company in Taiwan, said they will participate in and pay for part of the trial.
The company is now working on more licensing agreements for the drug to further fund its three-to-four year trial “because raising the amount of money required for the trial at the current prices would be harmful to our shareholders,” said Kersten, in the letter.
Cel-Sci is also working on a joint venture agreement for the same reason.
The company is also in discussions with biotechs to contract out its new manufacturing facility while it is not producing Multikine. The $22 million factory in Howard County delivered last year and can potentially produce almost $2 billion worth of drug per year.
According to its 10-Q, Cel-Sci subleased a chunk of the facility last month to an undisclosed tenant through January 2011 and will get paid $10,000 a month in rent.
The company also said it inked a $5 million line of credit to be used if necessary. “This line is not intended to be our primary funding source but does serve as additional insurance to shareholders,” he said.
It means for a two-year period, the company can sell up to $5 million of its common stock.
The company said it hired investment bank BroadOak Capital Partners, which focuses on the life sciences industry, to help get least one of the initiatives completed soon.
“In the last few months almost all investments have been hit hard and everything just seems to take longer. Our valuation is currently very low,” he added.
Cel-Sci (NYSE Alternext US: CVM) shares, which have continually dropped from 30 cents since the start of 2009, jumped 27 percent in mid-morning trading on Friday to 19 cents per share.
He said the company thinks Multikine will be a multi-billion dollar drug.
“Therefore I believe that our value should be much higher today than the current share price, particularly since a cancer drug is not really related to the economic conditions.”
 CEL-SCI Announces Exclusive Licensing Agreement for Cancer Drug Multikine(R) for Republic of South Africa
CEL-SCI Announces Exclusive Licensing Agreement for Cancer Drug Multikine(R) for Republic of South AfricaMonday March 9, 2009, 9:30 am EDT
Buzz up! Print Related:CEL-SCI Corp.
VIENNA, Va., March 9 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE Alternext US: CVM) announced today that it has entered into an exclusive licensing agreement with Byron Biopharma ("Byron") under which CEL-SCI has granted Byron an exclusive license to market and distribute the Company's cancer drug Multikine® in the Republic of South Africa (the "Territory"). CEL-SCI already has existing licensing agreements for Multikine with Teva Pharmaceuticals and Orient Europharma.
Related Quotes
Symbol Price Change
CVM 0.22 +0.02
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} Pursuant to the agreement, Byron will purchase $750,000 worth of stock from CEL-SCI and will make a payment of $125,000 in 12 months. Byron will also be responsible for registering the product in the Territory. Once Multikine has been approved, CEL-SCI will be responsible for manufacturing the product, while Byron will be responsible for sales in the Territory. Revenues will be split 50/50 between CEL-SCI and Byron.
"This agreement is consistent with our strategy to license Multikine in the emerging markets to share the expenses of bringing Multikine to market," said Geert Kersten, Chief Executive Officer of CEL-SCI. "We are working on additional agreements around the world."
CEL-SCI is developing Multikine for approval as a first line indication in head and neck cancer. To that end, the Company's planned Phase III clinical trial is an 800 patient clinical study designed to demonstrate that administration of its cancer drug Multikine to head and neck cancer patients before they receive any conventional cancer treatment will increase their survival. Head and neck cancer is one of the world's biggest cancers affecting about 600,000 people per annum worldwide.
In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial and recently CEL-SCI took delivery of its new state of the art manufacturing facility.
Multikine, a patented defined mixture of naturally derived cytokines, is the first immunotherapeutic agent in a new class of drugs called "Immune SIMULATORS". Immune SIMULATORS simulate the way our natural immune system acts in defending us against cancer. As opposed to other immunotherapies which are designed to target a single or limited number of specific antigens or molecules, Immune SIMULATORS are multi-targeted; they simultaneously cause a direct and targeted killing of the specific tumor cells and they activate the immune system to produce a stronger anti-tumor attack on multiple fronts.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
CEL-SCI has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense. Most recently CEL-SCI announced that its newly discovered rheumatoid arthritis vaccine showed excellent results in animal tests. www.cel-sci.com.
 langsam müßte der Kurs doch anspringen !!!
langsam müßte der Kurs doch anspringen !!!CEL-SCI Corporation to Launch Aseptic Filling for Stem Cell Produced Therapies and Other Biological Products
Tuesday March 10, 2009, 8:30 am EDT
Buzz up! Print Related:CEL-SCI Corp.
VIENNA, Va., March 10 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE Alternext US: CVM) announced today that it is planning to launch a new manufacturing process that could allow drugs developed using stem cells and other biological products to maintain their potency and thereby potentially also their shelf life. The availability of this new process developed by CEL-SCI may also significantly accelerate the time to market by eliminating complicated and time consuming validation studies and tests required when these products are filled at room temperature.
Related Quotes
Symbol Price Change
CVM 0.22 +0.02
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} CEL-SCI's new state-of-the-art manufacturing facility near Baltimore, Maryland, where it expects to manufacture its lead cancer product Multikine® for a pivotal Phase III trial, will be used for the process known as cold 4 degrees Celsius Aseptic Filling on a contract basis to stem cell and biologic companies, academic institutions and commercial media suppliers. The facility will allow CEL-SCI to perform cold 4 degrees Celsius aseptic filling of small volume parenteral drug products and sterile media products. The use of a cold 4 degrees Celsius fill, as opposed to the normal room temperature fill, significantly increases the probability of maintaining drug activity, potency and thus potentially extending the shelf life of new biological and stem cell produced products. CEL-SCI will be the only company providing this cold 4 degrees Celsius filling service on a contract basis to other companies and academic institutions.
Many stem cell derived products are expected to have similar requirements to biologically derived drug products, such as Multikine, allowing CEL-SCI to utilize many of the same clean rooms and production facilities. Many Biologic products benefit from being maintained at refrigerated (cold) temperatures, usually between 2 and 8 degrees Celsius throughout their manufacturing process and storage. CEL-SCI's unique, cold aseptic filling suite can be operated at temperatures between 2 degrees Celsius and room temperatures, including humidity control. The Aseptic filling suites are maintained at FDA and EU ISO classifications of 5/6. CEL-SCI also has additional capability to formulate, inspect, label and package these products at cold temperatures.
"We developed this unique cold fill technology over the course of many years and built this new facility for our cancer drug Multikine at a cost of about $22 million because we had not been able to find a company that could do a true 4 degrees Celsius cold fill for us. The best they could do was turn the temperature down to 60 degrees Fahrenheit, without the needed humidity control. We have now created something very unique and something that addresses a true need in the market place. Through our new service we will help advance new treatment therapies while also creating substantial shareholder value through the income from the facility," said Geert Kersten, CEL-SCI's Chief Executive Officer.
Background of CEL-SCI's cold 4 degrees Celsius Fill/Finish facility:
The fastest area of growth in the Biopharmaceutical and Pharmaceutical market is the area of biologics, and most recently the area of stem cells. These compounds and therapies are derived from or mimic human cells or proteins and other molecules (e.g., hormones, etc.) Nearly all of the major new billion dollar drugs developed for unmet medical needs (e. g., Avastin, Erbitux, Rituxan, Herceptin, Copaxon, etc.) are biologics. Biologics are usually very sensitive to heat and quickly lose their biological activity if exposed to room or elevated temperature for various periods of time. These elevated temperatures may also affect the shelf-life of a Biologic product, which means that the product cannot be stored for as long as one might find desirable. However, stem cell products and biologics do not generally lose activity when kept at 4 degrees Celsius.
Therefore CEL-SCI has developed a completely novel technology to be able to accomplish the Fill/Finish operation at 4 degrees Celsius (as well as at room temperature if the client so desires). This is of great importance to companies that produce the latest drugs, biologics and stem cell therapies. It is an absolute requirement by FDA and any other regulatory agency that a drug developer must demonstrate the safety, purity and potency of a drug being produced for use in humans. When filling a product at CEL-SCI's new facility at 4 degrees Celsius, minimal to no biological losses will have occurred due to temperature and therefore the potency of the drug is maintained throughout this final critical step of the drug's manufacturing process. When the same temperature sensitive drug is instead aseptically filled at room temperature, very expensive and time consuming validation studies must be conducted, first, to be able to obtain a complete understanding of the product's potency loss during the room temperature fill process, then to create solutions to the drug potency losses, which also will need to be tested and validated. Therefore CEL-SCI's new cold fill facility will provide several critical advantages to companies developing Biologics and stem cell therapies: it will save them a great deal of money (wasted product and extra validations) and it will save them a great deal of time usually spent on the development of expensive validations.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense and rheumatoid arthritis.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital to achieve the planned objectives and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10-K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
wo kann ich den in amerika cel nachkaufen?über die amex gehts net und über die nasdaq auch nicht?frankfurt gibts eher kursprobleme?
Antwort auf Beitrag Nr.: 36.739.379 von VFBLER am 10.03.09 17:58:30 ich mache es grundsätzlich über die Bank, da ich nicht trade !
ich mache es grundsätzlich über die Bank, da ich nicht trade !
irgendwas ist aber im Busch - ich pers. erwarte Bekanntgabe von Start P III !
mal sehen was kommt -
 ich mache es grundsätzlich über die Bank, da ich nicht trade !
ich mache es grundsätzlich über die Bank, da ich nicht trade !irgendwas ist aber im Busch - ich pers. erwarte Bekanntgabe von Start P III !
mal sehen was kommt -

Antwort auf Beitrag Nr.: 36.759.269 von ALF-FRED am 13.03.09 10:48:55 ich hoffe Sil verpasst nicht den Einstieg !!!
ich hoffe Sil verpasst nicht den Einstieg !!!
 ich hoffe Sil verpasst nicht den Einstieg !!!
ich hoffe Sil verpasst nicht den Einstieg !!!
Antwort auf Beitrag Nr.: 36.759.275 von ALF-FRED am 13.03.09 10:49:35Moin moin,
mal schauen ob der Kurs so weiter geht, habe da leider etwas bedenken, meist ist nach kurzen Hochs am nächsten Tag der Rücksetzer gekommen.
Aber wir hoffen ja immer noch
Habe bei 0.16 nochmals 20000 nachgekauft, wenn dann mit Anlauf ins Verderben... lol
Habe zwar immer noch Bedenken wegen der Finanzierung der P3, aber wenn Sie es wirklich schaffen, die Produktionsstätte zu vermarkten, könnte das gute Einnahmen kreieren, die zumindest einen Teil der Ausgaben decken.
gruss Mimm
mal schauen ob der Kurs so weiter geht, habe da leider etwas bedenken, meist ist nach kurzen Hochs am nächsten Tag der Rücksetzer gekommen.
Aber wir hoffen ja immer noch

Habe bei 0.16 nochmals 20000 nachgekauft, wenn dann mit Anlauf ins Verderben... lol
Habe zwar immer noch Bedenken wegen der Finanzierung der P3, aber wenn Sie es wirklich schaffen, die Produktionsstätte zu vermarkten, könnte das gute Einnahmen kreieren, die zumindest einen Teil der Ausgaben decken.
gruss Mimm
hab´s natürlich auch über die bank versucht,kann aber immer kein handel an der nasdaq und der amex?über frankfurt hab ich´s natürlich mit limit versucht,aber da ist der handel auch gleich null,schade,hab mir jetzt solarworld nachgekauft
Antwort auf Beitrag Nr.: 36.760.722 von VFBLER am 13.03.09 13:07:28 was ist das für ein Zock an der Amex
was ist das für ein Zock an der Amex 
Da ist aber jemand raus gegangen
 was ist das für ein Zock an der Amex
was ist das für ein Zock an der Amex 
Da ist aber jemand raus gegangen

hat aer auch wieder jemand gekauft,aber hohe umsätze.bin leider diese woche nicht mehr zu meinem kauf gekommen.habe schon ein paar.vielleicht kommt jetzt langsam die meldung,daß wir die phase 3 beginnen und wir stehen dann vielleicht über einem euro oder dollar

 ich hab bei Yahoo einen after hours Kurs von 0.35 kan das jemand bestätigen ?
ich hab bei Yahoo einen after hours Kurs von 0.35 kan das jemand bestätigen ?hier noch das filling zu Süd Africa
Form 8-K for CEL SCI CORP
--------------------------------------------------------------------------------
12-Mar-2009
Entry into a Material Definitive Agreement
Item 1.01 Entry Into a Material Definitive Agreement
Effective March 6, 2009 CEL-SCI Corporation entered into a licensing agreement with Byron Biopharma LLC ("Byron") under which CEL-SCI granted Byron an exclusive license to market and distribute CEL-SCI's cancer drug Multikine(R) in the Republic of South Africa. CEL-SCI has existing licensing agreements for Multikine with Teva Pharmaceuticals and Orient Europharma.
Pursuant to the agreement Byron will be responsible for registering the product in South Africa. Once Multikine has been approved for sale, CEL-SCI will be responsible for manufacturing the product, while Byron will be responsible for sales in South Africa. Revenues will be divided equally between CEL-SCI and Byron. To maintain the license Byron, among other requirements, must make milestone payments to CEL-SCI totaling $125,000 on or before March 15, 2010. Byron also agreed to purchase shares of common stock and warrants from CEL-SCI for $750,000.
ja,steht dran bei yahoo.ber diese kurse snd nicht aussage kräftig.hat man ja am freitag gesehen,da waren wir auch im hoh bei 0,35 dollar
CEL SCI CP(AMEX: CVM)
After Hours: 0.35 N/A (N/A) 9:28am EThelp
CEL SCI CP(AMEX: CVM)
After Hours: 0.35 N/A (N/A) 9:28am EThelp
Antwort auf Beitrag Nr.: 36.768.799 von VFBLER am 15.03.09 08:51:40 irgendwie will die Aktien ja doch jemand haben, aber ich finde einfach nix zum Partner in Süd Africa beim googeln,
irgendwie will die Aktien ja doch jemand haben, aber ich finde einfach nix zum Partner in Süd Africa beim googeln,
 irgendwie will die Aktien ja doch jemand haben, aber ich finde einfach nix zum Partner in Süd Africa beim googeln,
irgendwie will die Aktien ja doch jemand haben, aber ich finde einfach nix zum Partner in Süd Africa beim googeln,
Ich habe gestern auch vergebens gesucht.
Ist ein wenig seltsam, hoffe aber, dass es seine Richtigkeit hat.
Aufgrund der aktuellen Nachrichtenlage sieht es ja wieder ein wenig besser aus mit der Finanzierung.
Wenn jetzt P3 beginnen würde, könnten wir uns über deutlich höhere Kurse freuen.
Hoffentlich ist das, was wir die letzten Tage gesehen haben, nicht nur ein Strohfeuer.
Sollte es anhaltender sein, müssen wir erst einmal über die 0.38$ kommen.
Gruss Mimm
Ist ein wenig seltsam, hoffe aber, dass es seine Richtigkeit hat.
Aufgrund der aktuellen Nachrichtenlage sieht es ja wieder ein wenig besser aus mit der Finanzierung.
Wenn jetzt P3 beginnen würde, könnten wir uns über deutlich höhere Kurse freuen.
Hoffentlich ist das, was wir die letzten Tage gesehen haben, nicht nur ein Strohfeuer.
Sollte es anhaltender sein, müssen wir erst einmal über die 0.38$ kommen.
Gruss Mimm



CEL SCI CP(RT-ECN: CVM)
Last Trade: N/A
Trade Time: N/A
Change: N/A
Bid: 0.26 x 100,000
Ask: N/A
This is a free Real-Time quote. Each Real-Time quote is from BATS Exchange and does not necessarily represent the top bids or asks in the marketplace.
Order Book
Top of Book
Bid Price Size
0.26 100,000
0.01 2,430
überraschende wende,cel sci meldet den beginn der phase 3 mit einem potenzial 5 billionen.gleichzeitig würde bekannt,daß ein übernahmenangebot zu einem kurs von 155 dollar eingehen ist.
stand 1 april 2009
stand 1 april 2009
Antwort auf Beitrag Nr.: 36.874.156 von VFBLER am 29.03.09 14:33:17 Wau und dafür habe ich nun Jahre gewartet - für 10% +.
Wau und dafür habe ich nun Jahre gewartet - für 10% +.
Die Nachrichten schlagen ja ein wie eine Bombe



 Wau und dafür habe ich nun Jahre gewartet - für 10% +.
Wau und dafür habe ich nun Jahre gewartet - für 10% +.Die Nachrichten schlagen ja ein wie eine Bombe




Antwort auf Beitrag Nr.: 36.875.430 von rk1901 am 29.03.09 19:46:15Na da können wir ja wohl von einem vorgezogenen 1.Apri-Scherz ausgehen 

bin einfach nur enttäuscht hier von cel sci....
klar braucht man bei einem biotechwert geduld,aber dann darf man auch nicht sagen,daß man phase3 im 1quartal start....

klar braucht man bei einem biotechwert geduld,aber dann darf man auch nicht sagen,daß man phase3 im 1quartal start....

Tja das ist sicher im moment sehr schwierig.
Aber ich sehe das wirklich als eine langfristige Investition und mir ist es egal,obs nächstes oder übernächstes Jahr stattfindet.
Mir stellt sich eher langsam die Frage, ob es überhaupt je los geht
Ich habe vor kurzem sogar noch nachgekauft und hoffe das beste.
Denke das Produkt an sich ist gut, nur die Frage der finanzierung ist ungewiss.
Also hoffen und dann wird schon was gehen.
Aber ich gebs zu, die Ankündigungen über die P3 sind schon überfällig bzw. der Start.
Nix für schwache Nerven
Aber ich sehe das wirklich als eine langfristige Investition und mir ist es egal,obs nächstes oder übernächstes Jahr stattfindet.
Mir stellt sich eher langsam die Frage, ob es überhaupt je los geht

Ich habe vor kurzem sogar noch nachgekauft und hoffe das beste.
Denke das Produkt an sich ist gut, nur die Frage der finanzierung ist ungewiss.
Also hoffen und dann wird schon was gehen.
Aber ich gebs zu, die Ankündigungen über die P3 sind schon überfällig bzw. der Start.
Nix für schwache Nerven

ist 4 jahre langfristig ? so lange bin ich schon dabei....
Antwort auf Beitrag Nr.: 36.935.656 von VFBLER am 07.04.09 17:52:254 jahre ist schon sehr lange, wobei ich sogar noch länger dabei bin.
Habe mittlerweile fast 80000 Aktien und hänge schon kräftig drin jetzt mit dem tiefen Kurs.
Ich habe zwischen 0,16 und 0,46 gekauft und hoffe natürlich auf das grosse Los
Nur meine ersten 1000 Aktien hatte ich bei einem Kurs zu 1€ gekauft aber was solls.
Wenn die P3 starten sollte und es wirklich zu einer Zulassung vom MK kommt, können wir uns zur Ruhe setzen
Habe mittlerweile fast 80000 Aktien und hänge schon kräftig drin jetzt mit dem tiefen Kurs.
Ich habe zwischen 0,16 und 0,46 gekauft und hoffe natürlich auf das grosse Los

Nur meine ersten 1000 Aktien hatte ich bei einem Kurs zu 1€ gekauft aber was solls.
Wenn die P3 starten sollte und es wirklich zu einer Zulassung vom MK kommt, können wir uns zur Ruhe setzen

habe nur 16 k,aber möchte eventuell ein paar nachkaufen...habs auch schon über usa probiert.aber wie hier schon beschrieben kann man über nasdaq oder amex keine kaufen...kann mir jemand helfen...
Hmmm, also ich habe über Frankfurt oder München nie Probleme gehabt zum kaufen.
Man sieht ja immer, was die leute wollen unter Bid und Ask.
Bid 0,177 Ask 0,192
10.000 10000
Das ist aktuell Frankfurt.
Denke für 0,18-0,185 wird man sicher erfolgreich sein.
Kannst ja auch ein tieferes Bid abgeben, in der Hoffnung der Kurs fällt und wartest eventuell 1-2 Tage.
Gruss Mimm
Man sieht ja immer, was die leute wollen unter Bid und Ask.
Bid 0,177 Ask 0,192
10.000 10000
Das ist aktuell Frankfurt.
Denke für 0,18-0,185 wird man sicher erfolgreich sein.
Kannst ja auch ein tieferes Bid abgeben, in der Hoffnung der Kurs fällt und wartest eventuell 1-2 Tage.
Gruss Mimm
CEL-SCI Corporation Releases Letter to Shareholders
* Tuesday April 14, 2009, 2:09 pm EDT
*
Buzz up!
* Print
Related:
* CEL-SCI Corp.
VIENNA, Va., April 14 /PRNewswire-FirstCall/ -- The following letter is being released by CEL-SCI Corporation (NYSE AMEX: CVM) to its shareholders:
Related Quotes
Symbol Price Change
CVM 0.2997 +0.0597
Chart for CEL SCI CP
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""}
Dear Fellow Shareholders:
A very important event has just transpired for our company. Dendreon Corporation has announced that its pivotal Phase III trial with advanced prostate cancer met its primary endpoint of improving overall survival compared to a placebo control. The full data from the 512 patient study will be presented on April 28 at the American Urological Association conference.
The significance of Dendreon's success to CEL-SCI is great. For years most investors have shunned the cancer immunotherapy space because of the many failures of various companies involved in the space. While we at CEL-SCI have always tried to differentiate why our immunotherapy approach to treating cancer should be successful where other immunotherapy approaches have failed, the whole cancer immunotherapy space has been so tainted that it was almost impossible to be heard. The successful outcome of the Dendreon study gives the cancer immunotherapy field the needed validation to attract new money to the space. The success of Dendreon proves that it is possible to harness the human immune system against a patient's own cancer.
Geert Kersten, CEO of CEL-SCI said, "We believe that the success of Dendreon validates the cancer immunotherapy area as a promising alternative/adjunct to existing cancer therapies and think that it could lead to enhanced opportunities for CEL-SCI in both the investment and scientific communities."
* Tuesday April 14, 2009, 2:09 pm EDT
*
Buzz up!
Related:
* CEL-SCI Corp.
VIENNA, Va., April 14 /PRNewswire-FirstCall/ -- The following letter is being released by CEL-SCI Corporation (NYSE AMEX: CVM) to its shareholders:
Related Quotes
Symbol Price Change
CVM 0.2997 +0.0597
Chart for CEL SCI CP
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""}
Dear Fellow Shareholders:
A very important event has just transpired for our company. Dendreon Corporation has announced that its pivotal Phase III trial with advanced prostate cancer met its primary endpoint of improving overall survival compared to a placebo control. The full data from the 512 patient study will be presented on April 28 at the American Urological Association conference.
The significance of Dendreon's success to CEL-SCI is great. For years most investors have shunned the cancer immunotherapy space because of the many failures of various companies involved in the space. While we at CEL-SCI have always tried to differentiate why our immunotherapy approach to treating cancer should be successful where other immunotherapy approaches have failed, the whole cancer immunotherapy space has been so tainted that it was almost impossible to be heard. The successful outcome of the Dendreon study gives the cancer immunotherapy field the needed validation to attract new money to the space. The success of Dendreon proves that it is possible to harness the human immune system against a patient's own cancer.
Geert Kersten, CEO of CEL-SCI said, "We believe that the success of Dendreon validates the cancer immunotherapy area as a promising alternative/adjunct to existing cancer therapies and think that it could lead to enhanced opportunities for CEL-SCI in both the investment and scientific communities."
Antwort auf Beitrag Nr.: 36.939.941 von Mimm am 08.04.09 11:07:48

Antwort auf Beitrag Nr.: 36.968.548 von [KERN]Codex am 14.04.09 22:48:43So Leute, jetzt haben wir wenigstens kurzfristig einen Aufwärtstrend mit unserer Aktie, obwohl ich glaube, dass dieser Hype mit der Grippe nur kurzfristig sein wird und es danach wieder einen Abwärtstrend geben wird.
Hoffe nur, dass es nicht zuviel nach unten korrigiert wird.
Denke es wird erst dann langfristig nach oben gehen, wenn die P3 beginnt.
Gruss Mimm
Hoffe nur, dass es nicht zuviel nach unten korrigiert wird.
Denke es wird erst dann langfristig nach oben gehen, wenn die P3 beginnt.
Gruss Mimm
Ja wenn die WHO auf phase 5 erhöht wird es ernst, und dan sollte man auch nur die "leader" wie nvax, bcrx kaufen, weil alles andere was irgendwie mit swine flu verbunden is auch steigt aber genauso schnell wieder fällt, und wir werden sehen worein das geld vom kongress fliesst. immerhin 1,5 mrd dollar.
CEL-SCI's L.E.A.P.S. Technology Shows Potential to Treat Swine Influenza in Preclinical Studies
On Tuesday April 28, 2009, 9:37 am EDT
http://finance.yahoo.com/news/CELSCIs-LEAPS-Technology-prnew…
On Tuesday April 28, 2009, 9:37 am EDT
http://finance.yahoo.com/news/CELSCIs-LEAPS-Technology-prnew…
hab mir gestern ein paar über die amex gekauft,zu 0,33 cent hoffentlich war das nicht zu teuer.jetzt hat´s auch geklappt,glaub sonst war immmer das volumen zu dünn.hoffentlich gehts bald steil aufwärts

it news siehst´s gerade auch nicht gut aus....
bin mal gespannt wenn´s endlich los geht...
bin mal gespannt wenn´s endlich los geht...
Antwort auf Beitrag Nr.: 37.057.584 von [KERN]Codex am 28.04.09 21:25:59Bei einer Pandemie gibt es vor allem ein Problem - zu wenig Antigen zum impfen für alle.
Dazu kommt, dass die originären Impfstoffe oft nur eine schwache Immunantwort erzeugen.
Die Antigene via dendritische Zellen anzubieten, damit Antigene einzusparen und zugleich bessere Immunantworten zu erhalten ist ausgesprochen interessant.
>>>>Title: The L.E.A.P.S. approach to vaccine development.
Author: Zimmerman DH , Rosenthal KS
Source: Front Biosci, 10(): 790-8 2005
Service Fee: $13.00 ; Copyright Royalties: $35.00
Abstract: The Ligand Epitope Antigen Presentation System (L.E.A.P.S.) approach to vaccine development utilizes immune peptides to promote the immunogenicity and influence the type of immune response generated towards epitopes in peptides which may be too small to elicit an immune response. The covalent attachment of these immune peptides to the antigenic peptide promotes the interaction of the epitope with T cells (T cell binding ligand (TCBL)) or antigen presenting cells (immune cell binding ligand (ICBL)) and ultimately promotes binding with the T cell receptor on CD4 or CD8 T cells. The, J, ICBL/TCBL peptide derived from the beta-2-microglobulin chain of MHC I molecules promotes Th1 type responses to the antigenic peptide while the, G, ICBL/TCBL peptide derived from the beta chain of MHC II molecules promotes Th2 types of responses. The efficacy of this approach has been demonstrated by characterization of the immune responses to L.E.A.P.S. vaccines and by elicitation of protection from infectious challenge with herpes simplex virus and other pathogens. The protection studies show that the L.E.A.P.S. approach allows customization of the immune response appropriate for inducing protection from disease. The theory, background, examples and studies of the mechanism of action of the L.E.A.P.S. vaccines will be discussed. <<
Weiss zufällig jemand was es mit der Kooperation mit Teva Pharmaceuticals auf sich hat?
Gruss.
M.
Wie immer sind alle Angaben ohne Gewähr. Die gemachten Angaben stellen keine Handelsaufforderung dar. Aktien können steigen oder fallen. Jeder Investor handelt auf eigenes Risiko.
Dazu kommt, dass die originären Impfstoffe oft nur eine schwache Immunantwort erzeugen.
Die Antigene via dendritische Zellen anzubieten, damit Antigene einzusparen und zugleich bessere Immunantworten zu erhalten ist ausgesprochen interessant.
>>>>Title: The L.E.A.P.S. approach to vaccine development.
Author: Zimmerman DH , Rosenthal KS
Source: Front Biosci, 10(): 790-8 2005
Service Fee: $13.00 ; Copyright Royalties: $35.00
Abstract: The Ligand Epitope Antigen Presentation System (L.E.A.P.S.) approach to vaccine development utilizes immune peptides to promote the immunogenicity and influence the type of immune response generated towards epitopes in peptides which may be too small to elicit an immune response. The covalent attachment of these immune peptides to the antigenic peptide promotes the interaction of the epitope with T cells (T cell binding ligand (TCBL)) or antigen presenting cells (immune cell binding ligand (ICBL)) and ultimately promotes binding with the T cell receptor on CD4 or CD8 T cells. The, J, ICBL/TCBL peptide derived from the beta-2-microglobulin chain of MHC I molecules promotes Th1 type responses to the antigenic peptide while the, G, ICBL/TCBL peptide derived from the beta chain of MHC II molecules promotes Th2 types of responses. The efficacy of this approach has been demonstrated by characterization of the immune responses to L.E.A.P.S. vaccines and by elicitation of protection from infectious challenge with herpes simplex virus and other pathogens. The protection studies show that the L.E.A.P.S. approach allows customization of the immune response appropriate for inducing protection from disease. The theory, background, examples and studies of the mechanism of action of the L.E.A.P.S. vaccines will be discussed. <<
Weiss zufällig jemand was es mit der Kooperation mit Teva Pharmaceuticals auf sich hat?
Gruss.
M.
Wie immer sind alle Angaben ohne Gewähr. Die gemachten Angaben stellen keine Handelsaufforderung dar. Aktien können steigen oder fallen. Jeder Investor handelt auf eigenes Risiko.
die aktie steigt ja langsam...und umsätze sind auch langsam hier,grins,so kanns weiter gehen.der start darf dann auch mal langsam losgehen
Hallo zusammen,
habe in einem amerikanischen blog gelesen, dass CEL eventuell demnächst eine neue Partnerschaft bekannt geben wird und dass mit dem Start der P3 Mitte des Q4 gerechnet wird.
Das muss aber alles erst mal als Spekulation angesehen werden, ich habe schon vieles gelesen
Aber generell wird der Einstieg in die Aktie jetzt als bestenr Zeitpunkt angesehen.
Hoffen wir mal, dass es wirklich soweit kommt.
Gruss Mimm
habe in einem amerikanischen blog gelesen, dass CEL eventuell demnächst eine neue Partnerschaft bekannt geben wird und dass mit dem Start der P3 Mitte des Q4 gerechnet wird.
Das muss aber alles erst mal als Spekulation angesehen werden, ich habe schon vieles gelesen

Aber generell wird der Einstieg in die Aktie jetzt als bestenr Zeitpunkt angesehen.
Hoffen wir mal, dass es wirklich soweit kommt.
Gruss Mimm
wann ist das 4 q. bei cel sci????????????ß
Antwort auf Beitrag Nr.: 37.166.712 von VFBLER am 13.05.09 17:17:10Das sollte ungefähr zwischen Oktober und Dezember liegen 
Warten wirs mal ab.
Gruss Mimm

Warten wirs mal ab.
Gruss Mimm
15-May-2009
Quarterly Report
Item 2. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Liquidity and Capital Resources
The Company has had only limited revenues from operations since its inception in March 1983. The Company has relied upon proceeds realized from the public and private sale of its Common Stock and convertible notes as well as short-term borrowings to meet its funding requirements. Funds raised by the Company have been expended primarily in connection with the acquisition of an exclusive worldwide license to, and later purchase of, certain patented and unpatented proprietary technology and know-how relating to the human immunological defense system, patent applications, the repayment of debt, the continuation of Company sponsored research and development and administrative costs, and the construction of laboratory facilities. Inasmuch as the Company does not anticipate realizing significant revenues until such time as it enters into licensing arrangements regarding its technology and know-how or until such time it receives permission to sell its product (which could take a number of years), the Company has been dependent upon the proceeds from the sale of its securities to meet all of its liquidity and capital resource requirements and will have to continue doing so in the future.
During the six-month periods ended March 31, 2009 and 2008, the Company used cash totaling $57,092 and $10,728,203, respectively. For the six months ended March 31, 2009 and 2008, cash used in operating activities totaled $1,708,634 and $3,251,036. For the six months ended March 31, 2009 and 2008, cash provided by financing activities totaled $1,218,734 and $180,443, respectively. Licensing proceeds of $1,249,982 and receipt of short-term loans of $570,000 provided funds. The repayment of convertible notes ($365,000), financing costs ($36,248) and the repayment of the short-term loan ($200,000) were used in financing activities during the six months ended March 31, 2009. For the six months ended March 31, 2008, cash provided by financing was from the exercise of employee options ($14,403). Repayment of convertible notes of $480,000 used cash in financing activities. Cash provided by investing activities was $432,808 and $7,657,610 was used in investing activities for the six months ended March 31, 2009 and 2008, respectively. For the six months ended March 31, 2009 and 2008, the use of cash in investing activities consisted of purchases of equipment and legal costs incurred in patent applications and, for the six months ended March 31, 2009, the sale of the final $200,000 in ARPs.
The Company has two partners who have agreed to participate in and pay for part of the Phase III clinical trial for Multikine. However in light of the current capital market environment, the Company believes it is prudent not to start the Phase III clinical trial until it has firm commitments in the form of partnerships and/or money raised for a substantial amount of cash to support the Phase III clinical trial. In the meantime, the Company will operate at significantly reduced cash expenditure levels and additional cash may be raised by offering contract manufacturing services to the pharmaceutical industry in its new manufacturing facility. The Company expects that it will need to raise additional capital in fiscal year 2009 in the form of corporate partnerships and/or equity financings to support its operations at its current rate. If the Company does not raise this additional capital during 2009, the Company expects to finance its operations either through its $5 million equity line of credit or additional loans from the Company's President. The Company is currently working
towards a transaction which will finance its Phase III clinical trial of Multikine. The Company believes that it will be able to obtain additional financing since Multikine is a Phase III product designed to treat cancer, an area that pharmaceutical companies are increasingly targeting. It is important to note that the Company's expenditures for fiscal year 2008 included several very large non-recurring expenses that amounted to several million dollars, mostly related to the build out of the manufacturing facility. These expenses will not recur in fiscal year 2009, thereby reducing the Company's expenditures significantly. Beyond those savings the Company has also made other very significant cuts in its expenditures. In addition, the Company has put in place a $5 million Equity Line of Credit (see Note D). With this Equity Line of Credit in place the Company believes it will have the required capital to continue operations through March 2010. However, if necessary the Company can make further reductions in expenditures by a reduction in force or by implementation of a salary reduction program.
The Company has determined that the convertible debt holders of the Series K Notes may require repayment of the entire remaining principal balance at any time after August 4, 2009. This debt can be paid in stock and may not require a cash payment. In addition, in December 2008, CEL-SCI was not in compliance with certain lease requirements (i.e., failure to pay an installment of Base Annual Rent). This resulted in a lease amendment pursuant to which the landlord agreed to defer 3 months (December - February) of rent which will be paid back incrementally from future financings. In return, the Company extended 3,000,000 warrants by one year and repriced these warrants from $1.25 to $0.75 and the landlord was issued an additional 787,000 warrants at $0.75. Both warrants expire on January 26, 2014. The cost of the warrants ($115,721) was accounted for as a debit to deferred rent and a credit to additional paid-in capital. In March 2009, the Company began paying half of the basic monthly rent while it is negotiating for additional capital. As of March 31, 2009, the Company and the landlord were cooperating while the Company is negotiating various financial transactions.
While there can be no assurance that the debt holders will not exercise their put option, and the landlord of the manufacturing facility will not issue a default notice, the Company continues to work on solutions for additional financing and ways to reduce expenses. The Company has shown in the past that they are able to secure financing to continue operations. However, there is no assurance to do so in the future.
It should be noted that substantial funds will be needed for the clinical trial which will be necessary before the Company will be able to apply to the FDA for approval to sell any products which may be developed on a commercial basis throughout the United States. In the absence of revenues, the Company will be required to raise additional funds through the sale of securities, debt financing or other arrangements in order to continue with its research efforts. However, there can be no assurance that such financing will be available or be available on favorable terms. Ultimately, the Company must complete the development of its products, obtain appropriate regulatory approvals and obtain sufficient revenues to support its cost structure.
Since all of the Company's projects are under development the Company cannot predict with any certainty the funds required for future research and clinical trials, the timing of future research and development projects, or when it will be able to generate any revenue from the sale of any of its products.
The Company had invested in ARPs (See Note C). Because of liquidity issues with these ARPs, the Company borrowed $200,000 on a line of credit which was paid off in November of 2008.
Results of Operations and Financial Condition
During the six-month period ended March 31, 2009, research and development expenses increased by $147,433 compared to the six-month period ended March 31, 2008. This increase was due to continuing expenses relating to the preparation for the Phase III clinical trial on Multikine. During the three-month period ended March 31, 2009, research and development expense was relatively unchanged, with a decrease of only $11,827, caused by the layoff of some nonessential personnel in the lab. The Company is preparing for the beginning of the Phase III clinical trial.
During the six-month period ended March 31, 2009, general and administrative expenses decreased by $685,044 compared to the six-month period ended March 31, 2008. This decrease was caused by the Company having extended and repriced options during the six-month period ended March 31, 2008 of $465,008 and the expensing of stock issued to employees in the six-month period ended March 31, 2008 of $378,350 compared to a cost of employee stock issued in prior periods but expensed in the six-month period ended March 31, 2009 of only $81,372, a decrease of $296,978. This decrease from March 31, 2008 to March 31, 2009 was partially offset by higher insurance costs of approximately $16,500. During the three-month period ended March 31, 2009, general and administrative expenses increased slightly, $45,579, primarily because of a writeoff of abandoned patents of approximately $17,000, an increase in insurance of approximately $7,650 and an increase in filing fees of approximately $15,000.
Interest income during the six months ended March 31, 2009 decreased by $196,590 compared to the six-month period ended March 31, 2008. The decrease was due to the decrease in the funds available for investment. Interest income declined by approximately $89,100 during the three months ended March 31, 2009 for the same reason.
The gain on derivative instruments of $656,243 for the six months ended March 31, 2009, and the gain on derivative instruments of $264,554 was the result of the change in fair value of the Series K Notes and Series K Warrants during the period. These gains were caused by fluctuations in the share price of the Company's common stock.
The interest expense of $169,493 for the six months ended March 31, 2009 was composed of four elements: 1) amortization of the Series K discount ($80,551),
2) interest paid and accrued on the Series K debt ($74,650), 3) margin interest ($813) , and 4) interest on the short term loan ($13,479). This is a decline of approximately $96,000 from the six months ended March 31, 2008 because of the lower balance of Series K debt. The corresponding amounts for the three months ended March 31, 2009 are: 1)$36,902, 2) $34,495, 3) $-0- and 4) $13,479.
Research and Development Expenses
During the six-month periods ended March 31, 2009 and 2008, the Company's
research and development efforts involved Multikine and L.E.A.P.S.(TM). The
table below shows the research and development expenses associated with each
project during the six and three-month periods.
Six Months Ended Three Months Ended
March 31, March 31,
2009 2008 2009 2008
---- ---- ---- ----
MULTIKINE $2,158,414 $1,865,345 $ 970,188 $ 956,397
L.E.A.P.S 55,048 200,684 55,048 80,666
---------- ---------- ---------- ----------
TOTAL $2,213,462 $2,066,029 $1,025,236 $1,037,063
========== ========== ========== ==========
In January 2007, the Company received a "no objection" letter from the FDA indicating that it could proceed with the Phase III protocol with Multikine in head & neck cancer patients. The protocol for the Phase III clinical trial was designed to develop conclusive evidence of the safety and efficacy of Multikine in the treatment of advanced primary squamous cell carcinoma of the oral cavity. The Company had previously received a "no objection" letter from the Canadian Biologics and Genetic Therapies Directorate which enabled the Company to begin its Phase III clinical trial in Canada.
As of March 31, 2009, the Company was involved in a number of pre-clinical studies with respect to its L.E.A.P.S. technology. The Company does not know what obstacles it will encounter in future pre-clinical and clinical studies involving its L.E.A.P.S. technology.
Clinical and other studies necessary to obtain regulatory approval of a new drug involve significant costs and require several years to complete. The extent of the Company's clinical trials and research programs are primarily based upon the amount of capital available to the Company and the extent to which the Company has received regulatory approvals for clinical trials. The inability of the Company to conduct clinical trials or research, whether due to a lack of capital or regulatory approval, will prevent the Company from completing the studies and research required to obtain regulatory approval for any products which the Company is developing. Without regulatory approval, the Company will be unable to sell any of its products.
In August 2007, the Company leased a building near Baltimore, Maryland. The building, which consists of approximately 73,000 square feet, will be remodeled in accordance with the Company specifications so that it can be used by the Company to manufacture Multikine for the Company's Phase III clinical trial and sales of the drug if approved by the FDA. The lease is for a term of twenty years and requires annual base rent payments of $1,575,000 during the first year of the lease. The annual base rent escalates each year at 3%. the Company is also required to pay all real and personal property taxes, insurance premiums, maintenance expenses, repair costs and utilities. The lease allows the Company, at its election, to extend the lease for two ten-year periods or to purchase the building at the end of the 20-year lease. The lease required the Company to pay $3,150,000 towards the remodeling costs, which will be recouped by reductions in the annual base rent of $303,228 in years six through twenty of the lease. In January 2008, the Company signed a second amendment to the lease. In accordance
with the lease, on February 8, 2008, the Company paid an additional $1,295,528 toward the remodeling costs and a further $518,790 to pay for lab equipment. In addition, in April 2008, an additional $288,474 was paid for the completion of the facility. In July 2008, CEL-SCI was required to deposit the equivalent of one year's base rent in accordance with the contract. The $1,575,000 was required to be deposited when the amount of cash CEL-SCI had fell below the amount stipulated in the lease. The Company took possession of the manufacturing facility in October 2008.
Regulatory authorities prefer to see biologics such as Multikine manufactured for commercial sale in the same manufacturing facility for Phase III clinical trials and the sale of the product since this arrangement helps to ensure that the drug lots used to conduct the clinical trials will be consistent with those that may be subsequently sold commercially. Although some biotech companies outsource their manufacturing, this can be risky with biologics because they require intense manufacturing and process control. With biologic products a minor change in manufacturing and process control can result in a major change in the final product. Good and consistent manufacturing and process control is critical and is best assured if the product is manufactured and controlled in the manufacturer's own facility by their own specially trained personnel. Since all of the Company's projects are under development, the Company cannot predict when it will be able to generate any revenue from the sale of any of its products.
Critical Accounting Estimates and Policies
Management's discussion and analysis of the Company's financial condition and results of operations is based on its unaudited condensed consolidated financial statements. The preparation of these financial statements is based on the selection of accounting policies and the application of significant accounting estimates, some of which require management to make judgments, estimates and assumptions that affect the amounts reported in the financial statements and notes. The Company believes some of the more critical estimates and policies that affect its financial condition and results of operations are in the areas of revenue recognition, operating leases, asset retirement obligations, stock-based compensation and income taxes. For more information regarding the Company's critical accounting estimates and policies, see Part II, Item 7, MD&A "Critical Accounting Estimates and Policies" of the Company's 2008 10-K. We have discussed the application of these critical accounting policies and estimates with the Audit Committee of the Company's Board of Directors.
Quarterly Report
Item 2. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Liquidity and Capital Resources
The Company has had only limited revenues from operations since its inception in March 1983. The Company has relied upon proceeds realized from the public and private sale of its Common Stock and convertible notes as well as short-term borrowings to meet its funding requirements. Funds raised by the Company have been expended primarily in connection with the acquisition of an exclusive worldwide license to, and later purchase of, certain patented and unpatented proprietary technology and know-how relating to the human immunological defense system, patent applications, the repayment of debt, the continuation of Company sponsored research and development and administrative costs, and the construction of laboratory facilities. Inasmuch as the Company does not anticipate realizing significant revenues until such time as it enters into licensing arrangements regarding its technology and know-how or until such time it receives permission to sell its product (which could take a number of years), the Company has been dependent upon the proceeds from the sale of its securities to meet all of its liquidity and capital resource requirements and will have to continue doing so in the future.
During the six-month periods ended March 31, 2009 and 2008, the Company used cash totaling $57,092 and $10,728,203, respectively. For the six months ended March 31, 2009 and 2008, cash used in operating activities totaled $1,708,634 and $3,251,036. For the six months ended March 31, 2009 and 2008, cash provided by financing activities totaled $1,218,734 and $180,443, respectively. Licensing proceeds of $1,249,982 and receipt of short-term loans of $570,000 provided funds. The repayment of convertible notes ($365,000), financing costs ($36,248) and the repayment of the short-term loan ($200,000) were used in financing activities during the six months ended March 31, 2009. For the six months ended March 31, 2008, cash provided by financing was from the exercise of employee options ($14,403). Repayment of convertible notes of $480,000 used cash in financing activities. Cash provided by investing activities was $432,808 and $7,657,610 was used in investing activities for the six months ended March 31, 2009 and 2008, respectively. For the six months ended March 31, 2009 and 2008, the use of cash in investing activities consisted of purchases of equipment and legal costs incurred in patent applications and, for the six months ended March 31, 2009, the sale of the final $200,000 in ARPs.
The Company has two partners who have agreed to participate in and pay for part of the Phase III clinical trial for Multikine. However in light of the current capital market environment, the Company believes it is prudent not to start the Phase III clinical trial until it has firm commitments in the form of partnerships and/or money raised for a substantial amount of cash to support the Phase III clinical trial. In the meantime, the Company will operate at significantly reduced cash expenditure levels and additional cash may be raised by offering contract manufacturing services to the pharmaceutical industry in its new manufacturing facility. The Company expects that it will need to raise additional capital in fiscal year 2009 in the form of corporate partnerships and/or equity financings to support its operations at its current rate. If the Company does not raise this additional capital during 2009, the Company expects to finance its operations either through its $5 million equity line of credit or additional loans from the Company's President. The Company is currently working
towards a transaction which will finance its Phase III clinical trial of Multikine. The Company believes that it will be able to obtain additional financing since Multikine is a Phase III product designed to treat cancer, an area that pharmaceutical companies are increasingly targeting. It is important to note that the Company's expenditures for fiscal year 2008 included several very large non-recurring expenses that amounted to several million dollars, mostly related to the build out of the manufacturing facility. These expenses will not recur in fiscal year 2009, thereby reducing the Company's expenditures significantly. Beyond those savings the Company has also made other very significant cuts in its expenditures. In addition, the Company has put in place a $5 million Equity Line of Credit (see Note D). With this Equity Line of Credit in place the Company believes it will have the required capital to continue operations through March 2010. However, if necessary the Company can make further reductions in expenditures by a reduction in force or by implementation of a salary reduction program.
The Company has determined that the convertible debt holders of the Series K Notes may require repayment of the entire remaining principal balance at any time after August 4, 2009. This debt can be paid in stock and may not require a cash payment. In addition, in December 2008, CEL-SCI was not in compliance with certain lease requirements (i.e., failure to pay an installment of Base Annual Rent). This resulted in a lease amendment pursuant to which the landlord agreed to defer 3 months (December - February) of rent which will be paid back incrementally from future financings. In return, the Company extended 3,000,000 warrants by one year and repriced these warrants from $1.25 to $0.75 and the landlord was issued an additional 787,000 warrants at $0.75. Both warrants expire on January 26, 2014. The cost of the warrants ($115,721) was accounted for as a debit to deferred rent and a credit to additional paid-in capital. In March 2009, the Company began paying half of the basic monthly rent while it is negotiating for additional capital. As of March 31, 2009, the Company and the landlord were cooperating while the Company is negotiating various financial transactions.
While there can be no assurance that the debt holders will not exercise their put option, and the landlord of the manufacturing facility will not issue a default notice, the Company continues to work on solutions for additional financing and ways to reduce expenses. The Company has shown in the past that they are able to secure financing to continue operations. However, there is no assurance to do so in the future.
It should be noted that substantial funds will be needed for the clinical trial which will be necessary before the Company will be able to apply to the FDA for approval to sell any products which may be developed on a commercial basis throughout the United States. In the absence of revenues, the Company will be required to raise additional funds through the sale of securities, debt financing or other arrangements in order to continue with its research efforts. However, there can be no assurance that such financing will be available or be available on favorable terms. Ultimately, the Company must complete the development of its products, obtain appropriate regulatory approvals and obtain sufficient revenues to support its cost structure.
Since all of the Company's projects are under development the Company cannot predict with any certainty the funds required for future research and clinical trials, the timing of future research and development projects, or when it will be able to generate any revenue from the sale of any of its products.
The Company had invested in ARPs (See Note C). Because of liquidity issues with these ARPs, the Company borrowed $200,000 on a line of credit which was paid off in November of 2008.
Results of Operations and Financial Condition
During the six-month period ended March 31, 2009, research and development expenses increased by $147,433 compared to the six-month period ended March 31, 2008. This increase was due to continuing expenses relating to the preparation for the Phase III clinical trial on Multikine. During the three-month period ended March 31, 2009, research and development expense was relatively unchanged, with a decrease of only $11,827, caused by the layoff of some nonessential personnel in the lab. The Company is preparing for the beginning of the Phase III clinical trial.
During the six-month period ended March 31, 2009, general and administrative expenses decreased by $685,044 compared to the six-month period ended March 31, 2008. This decrease was caused by the Company having extended and repriced options during the six-month period ended March 31, 2008 of $465,008 and the expensing of stock issued to employees in the six-month period ended March 31, 2008 of $378,350 compared to a cost of employee stock issued in prior periods but expensed in the six-month period ended March 31, 2009 of only $81,372, a decrease of $296,978. This decrease from March 31, 2008 to March 31, 2009 was partially offset by higher insurance costs of approximately $16,500. During the three-month period ended March 31, 2009, general and administrative expenses increased slightly, $45,579, primarily because of a writeoff of abandoned patents of approximately $17,000, an increase in insurance of approximately $7,650 and an increase in filing fees of approximately $15,000.
Interest income during the six months ended March 31, 2009 decreased by $196,590 compared to the six-month period ended March 31, 2008. The decrease was due to the decrease in the funds available for investment. Interest income declined by approximately $89,100 during the three months ended March 31, 2009 for the same reason.
The gain on derivative instruments of $656,243 for the six months ended March 31, 2009, and the gain on derivative instruments of $264,554 was the result of the change in fair value of the Series K Notes and Series K Warrants during the period. These gains were caused by fluctuations in the share price of the Company's common stock.
The interest expense of $169,493 for the six months ended March 31, 2009 was composed of four elements: 1) amortization of the Series K discount ($80,551),
2) interest paid and accrued on the Series K debt ($74,650), 3) margin interest ($813) , and 4) interest on the short term loan ($13,479). This is a decline of approximately $96,000 from the six months ended March 31, 2008 because of the lower balance of Series K debt. The corresponding amounts for the three months ended March 31, 2009 are: 1)$36,902, 2) $34,495, 3) $-0- and 4) $13,479.
Research and Development Expenses
During the six-month periods ended March 31, 2009 and 2008, the Company's
research and development efforts involved Multikine and L.E.A.P.S.(TM). The
table below shows the research and development expenses associated with each
project during the six and three-month periods.
Six Months Ended Three Months Ended
March 31, March 31,
2009 2008 2009 2008
---- ---- ---- ----
MULTIKINE $2,158,414 $1,865,345 $ 970,188 $ 956,397
L.E.A.P.S 55,048 200,684 55,048 80,666
---------- ---------- ---------- ----------
TOTAL $2,213,462 $2,066,029 $1,025,236 $1,037,063
========== ========== ========== ==========
In January 2007, the Company received a "no objection" letter from the FDA indicating that it could proceed with the Phase III protocol with Multikine in head & neck cancer patients. The protocol for the Phase III clinical trial was designed to develop conclusive evidence of the safety and efficacy of Multikine in the treatment of advanced primary squamous cell carcinoma of the oral cavity. The Company had previously received a "no objection" letter from the Canadian Biologics and Genetic Therapies Directorate which enabled the Company to begin its Phase III clinical trial in Canada.
As of March 31, 2009, the Company was involved in a number of pre-clinical studies with respect to its L.E.A.P.S. technology. The Company does not know what obstacles it will encounter in future pre-clinical and clinical studies involving its L.E.A.P.S. technology.
Clinical and other studies necessary to obtain regulatory approval of a new drug involve significant costs and require several years to complete. The extent of the Company's clinical trials and research programs are primarily based upon the amount of capital available to the Company and the extent to which the Company has received regulatory approvals for clinical trials. The inability of the Company to conduct clinical trials or research, whether due to a lack of capital or regulatory approval, will prevent the Company from completing the studies and research required to obtain regulatory approval for any products which the Company is developing. Without regulatory approval, the Company will be unable to sell any of its products.
In August 2007, the Company leased a building near Baltimore, Maryland. The building, which consists of approximately 73,000 square feet, will be remodeled in accordance with the Company specifications so that it can be used by the Company to manufacture Multikine for the Company's Phase III clinical trial and sales of the drug if approved by the FDA. The lease is for a term of twenty years and requires annual base rent payments of $1,575,000 during the first year of the lease. The annual base rent escalates each year at 3%. the Company is also required to pay all real and personal property taxes, insurance premiums, maintenance expenses, repair costs and utilities. The lease allows the Company, at its election, to extend the lease for two ten-year periods or to purchase the building at the end of the 20-year lease. The lease required the Company to pay $3,150,000 towards the remodeling costs, which will be recouped by reductions in the annual base rent of $303,228 in years six through twenty of the lease. In January 2008, the Company signed a second amendment to the lease. In accordance
with the lease, on February 8, 2008, the Company paid an additional $1,295,528 toward the remodeling costs and a further $518,790 to pay for lab equipment. In addition, in April 2008, an additional $288,474 was paid for the completion of the facility. In July 2008, CEL-SCI was required to deposit the equivalent of one year's base rent in accordance with the contract. The $1,575,000 was required to be deposited when the amount of cash CEL-SCI had fell below the amount stipulated in the lease. The Company took possession of the manufacturing facility in October 2008.
Regulatory authorities prefer to see biologics such as Multikine manufactured for commercial sale in the same manufacturing facility for Phase III clinical trials and the sale of the product since this arrangement helps to ensure that the drug lots used to conduct the clinical trials will be consistent with those that may be subsequently sold commercially. Although some biotech companies outsource their manufacturing, this can be risky with biologics because they require intense manufacturing and process control. With biologic products a minor change in manufacturing and process control can result in a major change in the final product. Good and consistent manufacturing and process control is critical and is best assured if the product is manufactured and controlled in the manufacturer's own facility by their own specially trained personnel. Since all of the Company's projects are under development, the Company cannot predict when it will be able to generate any revenue from the sale of any of its products.
Critical Accounting Estimates and Policies
Management's discussion and analysis of the Company's financial condition and results of operations is based on its unaudited condensed consolidated financial statements. The preparation of these financial statements is based on the selection of accounting policies and the application of significant accounting estimates, some of which require management to make judgments, estimates and assumptions that affect the amounts reported in the financial statements and notes. The Company believes some of the more critical estimates and policies that affect its financial condition and results of operations are in the areas of revenue recognition, operating leases, asset retirement obligations, stock-based compensation and income taxes. For more information regarding the Company's critical accounting estimates and policies, see Part II, Item 7, MD&A "Critical Accounting Estimates and Policies" of the Company's 2008 10-K. We have discussed the application of these critical accounting policies and estimates with the Audit Committee of the Company's Board of Directors.
CEL-SCI Developing Immune-Based Treatment Against Swine and Other H1N1 Flu Viruses Using Proprietary L.E.A.P.S. Technology
On Tuesday May 26, 2009, 9:00 am EDT
Buzz up! Print Related:CEL-SCI Corp.
VIENNA, Va. May 26 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE AMEX: CVM) announced today that it is developing an immune-based treatment for the "swine flu and related H1N1" flu viruses, utilizing its proprietary L.E.A.P.S.(TM) (Ligand Epitope Antigen Presentation System) vaccine technology. The Company plans to utilize the expertise and knowledge it has gained from developing protective and therapeutic vaccines utilizing L.E.A.P.S. to develop a therapeutic treatment based upon the technology for people infected with the swine and H1N1 flu viruses. CEL-SCI has already commenced pre-clinical testing.
Related Quotes
Symbol Price Change
CVM 0.30 +0.01
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} Previously, CEL-SCI announced that its CEL-1000 peptide, which is derived from the L.E.A.P.S. technology, showed adjuvant activity when used with a peptide based malaria vaccine and a DNA based malaria vaccine in animal challenge studies as part of a Cooperative Research arrangement with the Naval Medical Research Center, Silver Spring, MD. In both cases, the addition of CEL-1000 to the vaccines resulted in significant increases in the protection of the animals. In addition, several different L.E.A.P.S. conjugates induced protection and/or improvement in diseases as diverse as rheumatoid arthritis, malaria, TB and herpes simplex viruses. This shows that the L.E.A.P.S. technology can be successfully applied to many different diseases and these results further suggest that L.E.A.P.S. has the potential to be used either alone as a therapy or as an adjuvant to vaccines under development for the treatment of H1N1 related flu viruses. Adjuvants are designed to improve the effectiveness of vaccines.
"We believe that our L.E.A.P.S. technology platform offers strong potential to address the current swine and H1N1 flu viruses," said Dr. Daniel Zimmerman of CEL-SCI, the inventor of the technology. "With the swine flu currently creating major global health problems despite it occurring outside of the traditional windows for the flu, we are working diligently to provide a solution as it is expected that the disease may become even more virulent later this year during the traditional flu season."
The reason why the L.E.A.P.S. technology lends itself to creating a much more effective immune therapy against the H1N1 flu virus is because the L.E.A.P.S. conjugates induce an effective and powerful immune response without causing excessive amounts of pro-inflammatory cytokines. In the case of prior pandemic influenza, such as the "Spanish Influenza" and more recently the "Avian Flu", patients with stronger immune systems had a greater chance of dying because their immune response was too strong (too many pro-inflammatory cytokines). This is called a cytokine storm. While normally many cytokines play a key role in preventing and treating swine flu, in some cases excessive cytokine amounts may exacerbate the disease as it appears that, unlike the normal flu which affects the very young and very old most severely, swine flu may be more like the avian flu which hits people in their prime more severely.
In addition, LEAPS conjugates may be used to potentially overcome the virus' mutations by focusing on more conserved and common epitopes critical for critical viral function.
The L.E.A.P.S. technology is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S. technology. Each L.E.A.P.S. construct is composed of a T cell binding ligand (TCBL) which has previously demonstrated the ability to induce and elicit protective immunity and antigen specific antibody production in animal models.
The concept behind the L.E.A.P.S. technology is to directly mimic cell/cell interactions on the dendritic and T-cell surface with synthetic peptides. The L.E.A.P.S. constructs containing the antigenic disease epitope linked to an Immune/T-cell binding ligand (I/TCBL) can be manufactured by peptide synthesis or by covalently linking the two peptides. Depending upon the type of L.E.A.P.S. construct and I/TCBL used, CEL-SCI is able to direct the outcome of the immune response towards the development of T-cell function with primarily effector T-cell functions (T Lymphocyte; helper/effector T lymphocyte, type 1 or 2 [Th1 or Th2], cytotoxic [Tc] or suppressor [Ts]). The L.E.A.P.S. vaccine constructs are chimeric peptides which combine antigen specificity with immune response modulation.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine which is being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
On Tuesday May 26, 2009, 9:00 am EDT
Buzz up! Print Related:CEL-SCI Corp.
VIENNA, Va. May 26 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE AMEX: CVM) announced today that it is developing an immune-based treatment for the "swine flu and related H1N1" flu viruses, utilizing its proprietary L.E.A.P.S.(TM) (Ligand Epitope Antigen Presentation System) vaccine technology. The Company plans to utilize the expertise and knowledge it has gained from developing protective and therapeutic vaccines utilizing L.E.A.P.S. to develop a therapeutic treatment based upon the technology for people infected with the swine and H1N1 flu viruses. CEL-SCI has already commenced pre-clinical testing.
Related Quotes
Symbol Price Change
CVM 0.30 +0.01
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} Previously, CEL-SCI announced that its CEL-1000 peptide, which is derived from the L.E.A.P.S. technology, showed adjuvant activity when used with a peptide based malaria vaccine and a DNA based malaria vaccine in animal challenge studies as part of a Cooperative Research arrangement with the Naval Medical Research Center, Silver Spring, MD. In both cases, the addition of CEL-1000 to the vaccines resulted in significant increases in the protection of the animals. In addition, several different L.E.A.P.S. conjugates induced protection and/or improvement in diseases as diverse as rheumatoid arthritis, malaria, TB and herpes simplex viruses. This shows that the L.E.A.P.S. technology can be successfully applied to many different diseases and these results further suggest that L.E.A.P.S. has the potential to be used either alone as a therapy or as an adjuvant to vaccines under development for the treatment of H1N1 related flu viruses. Adjuvants are designed to improve the effectiveness of vaccines.
"We believe that our L.E.A.P.S. technology platform offers strong potential to address the current swine and H1N1 flu viruses," said Dr. Daniel Zimmerman of CEL-SCI, the inventor of the technology. "With the swine flu currently creating major global health problems despite it occurring outside of the traditional windows for the flu, we are working diligently to provide a solution as it is expected that the disease may become even more virulent later this year during the traditional flu season."
The reason why the L.E.A.P.S. technology lends itself to creating a much more effective immune therapy against the H1N1 flu virus is because the L.E.A.P.S. conjugates induce an effective and powerful immune response without causing excessive amounts of pro-inflammatory cytokines. In the case of prior pandemic influenza, such as the "Spanish Influenza" and more recently the "Avian Flu", patients with stronger immune systems had a greater chance of dying because their immune response was too strong (too many pro-inflammatory cytokines). This is called a cytokine storm. While normally many cytokines play a key role in preventing and treating swine flu, in some cases excessive cytokine amounts may exacerbate the disease as it appears that, unlike the normal flu which affects the very young and very old most severely, swine flu may be more like the avian flu which hits people in their prime more severely.
In addition, LEAPS conjugates may be used to potentially overcome the virus' mutations by focusing on more conserved and common epitopes critical for critical viral function.
The L.E.A.P.S. technology is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S. technology. Each L.E.A.P.S. construct is composed of a T cell binding ligand (TCBL) which has previously demonstrated the ability to induce and elicit protective immunity and antigen specific antibody production in animal models.
The concept behind the L.E.A.P.S. technology is to directly mimic cell/cell interactions on the dendritic and T-cell surface with synthetic peptides. The L.E.A.P.S. constructs containing the antigenic disease epitope linked to an Immune/T-cell binding ligand (I/TCBL) can be manufactured by peptide synthesis or by covalently linking the two peptides. Depending upon the type of L.E.A.P.S. construct and I/TCBL used, CEL-SCI is able to direct the outcome of the immune response towards the development of T-cell function with primarily effector T-cell functions (T Lymphocyte; helper/effector T lymphocyte, type 1 or 2 [Th1 or Th2], cytotoxic [Tc] or suppressor [Ts]). The L.E.A.P.S. vaccine constructs are chimeric peptides which combine antigen specificity with immune response modulation.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine which is being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
CEL-SCI Entwicklung von Immun-Behandlung gegen die Schweinepest und andere Flu H1N1-Viren mit proprietären LEAPS Technologie
Am Dienstag, 26. Mai 2009, 9:00 AM EDT
Buzz up! Drucken Related: CEL-SCI Corp
WIEN, 26. Mai Va. Update wird - CEL-SCI Corporation (NYSE AMEX: CVM) gab heute bekannt, dass es die Entwicklung einer immun-basierte Therapie für die "Schweine-Grippe und die damit verbundenen H1N1" Grippe-Viren, die Nutzung seiner proprietären LEAPS (TM) (Ligand Epitop Antigen Präsentation System) Impfstoff-Technologie. Das Unternehmen plant, nutzen das Know-how und Wissen sie hat sich den Schutz und die Entwicklung von therapeutischen Impfstoffen Verwendung LEAPS die Entwicklung eines therapeutischen Behandlung auf der Grundlage der Technologie für die Menschen mit den infizierten Schweinen und H1N1-Grippe-Viren. CEL-SCI hat bereits vor der klinischen Erprobung.
Verwandte Angebote
Symbol Preis ändern
CVM 0,30 +0,01
( "s": "CVM", "K": "C10, L10, P20, T10", "o": "", "J": "") Zuvor, CEL-SCI bekannt gegeben, dass sein CEL-1000-Peptid, der sich aus dem LEAPS Technologie, zeigte Adjuvans-Aktivität, wenn sie mit einem Peptid-basierte Malaria-Impfstoff und eine DNA-basierte Malaria-Impfstoff in tierischen Herausforderung Studien im Rahmen einer kooperativen Forschung Absprache mit dem Naval Medical Research Center, Silver Spring, MD. In beiden Fällen ist der Zusatz von CEL-1000 auf die Impfstoffe zu erheblichen Steigerungen in den Schutz der Tiere. Darüber hinaus verschiedene L.E.A.P.S. Konjugaten induziert und / oder Verbesserungen in so unterschiedlichen Krankheiten wie rheumatoider Arthritis, Malaria, Tuberkulose und Herpes-simplex-Viren. Dies zeigt, dass die L.E.A.P.S. Technologie lassen sich erfolgreich auf vielen verschiedenen Krankheiten, und diese Ergebnisse deuten darauf hin, dass weitere LEAPS hat das Potenzial, die entweder allein als Therapie oder als Adjuvans in der Entwicklung von Impfstoffen, um für die Behandlung von H1N1 im Zusammenhang Grippeviren. Adjuvantien sind zur Verbesserung der Wirksamkeit von Impfstoffen.
"Wir glauben, dass unsere Technologie-Plattform LEAPS bietet erhebliches Potenzial zur Bewältigung der aktuellen Schweinepest und Vogelgrippe H1N1-Viren", sagte Dr. Daniel Zimmerman von CEL-SCI, der Erfinder der Technologie. "Mit der Schweinepest Grippe derzeit Schaffung großen globalen Gesundheitsproblemen, obwohl er außerhalb der traditionellen Fenster für die Grippe, wir arbeiten mit Nachdruck an einer Lösung, wie es ist zu erwarten, dass sich die Krankheit noch virulent später in diesem Jahr während der traditionellen Grippe Saison. "
Der Grund, warum die L.E.A.P.S. Technologie eignet sich für die Schaffung eines wesentlich effektiver Immun-Therapie gegen die Grippe-Virus H1N1 ist, weil die LEAPS Konjugaten induziert eine wirksame und leistungsfähige Immunantwort, ohne dass übermäßige Mengen von pro-inflammatorischen Zytokinen. Im Fall der vor Influenza-Pandemie, wie die "Spanische Grippe" und seit kurzem die "Vogelgrippe", Patienten mit stärkeren Immunsystem hat eine größere Chance, zu sterben, weil ihre Immunabwehr war zu stark (zu viele pro-inflammatorische Zytokine) . Dies wird als Zytokin Sturm. Während normalerweise viele Zytokine spielen eine wichtige Rolle bei der Vorbeugung und Behandlung von Schweinen Grippe, in einigen Fällen übermäßige Mengen Zytokin kann sich die Krankheit, wie es scheint, dass im Gegensatz zu der normalen Grippe, die vor allem die sehr jungen und sehr alten Menschen am stärksten, Schweinen Grippe kann mehr wie die Vogelgrippe, die Menschen in ihrer Hits prime mehr schwer.
Darüber hinaus LEAPS Konjugate können verwendet werden, um möglicherweise das Virus überwinden "Mutationen durch die Konzentration auf mehr erhalten und gemeinsame Epitope, die für die virale kritische Funktion.
Die L.E.A.P.S. Technologie ist ein neuartiger T-Zell-Modulation-Plattform-Technologie ermöglicht es, dass die CEL-SCI zu entwerfen und zu synthetisieren proprietäre immunogens. Jede Krankheit, für die eine Antigen-Sequenz identifiziert wurde, wie Infektionskrankheiten, Parasiten, maligne oder Autoimmun-Erkrankungen und Allergien, die potenzielle therapeutische oder präventive Standorte für die Anwendung der LEAPS Technologie. Jeder L.E.A.P.S. Bau besteht aus einem T-Zell-bindenden Liganden (TCBL), die zuvor gezeigt, die Fähigkeit zur Induktion und entlocken schützenden Immunität und Antigen-spezifische Antikörper in Tiermodellen.
Das Konzept hinter dem L.E.A.P.S. Technologie ist es, direkt imitieren Zelle / Zell-Wechselwirkungen auf der dendritischen und T-Zell-Oberfläche mit synthetischen Peptiden. Die L.E.A.P.S. Konstrukte, die die Antigen-Epitop-Krankheit in Verbindung mit einer Immun-/ T-Zell-bindenden Liganden (I / TCBL) werden können, hergestellt von der Peptid-Synthese oder durch kovalente Verknüpfung der beiden Peptide. Je nach der Art der L.E.A.P.S. Bau-und I / TCBL verwendet, CEL-SCI ist in der Lage, direkt das Ergebnis der Immunantwort auf die Entwicklung der T-Zell-Funktion in erster Linie Effektor T-Zell-Funktionen (T-Lymphozyten, Helfer / Effektor-T-Lymphozyten, Typ 1 oder 2 [ Th1 oder Th2], zytotoxische [Tc] oder Suppressor [V]). Die L.E.A.P.S. Impfstoff-Konstrukte werden chimäre Peptide denen Antigen-spezifischen Immunantwort mit Modulation.
CEL-SCI Corporation ist die Entwicklung von Produkten, die Immunabwehr stärken. Die Führung ist Multikine, das bereit für eine weltweite Phase-III-Studie. Das Unternehmen hat Niederlassungen in Vienna, Virginia, und Baltimore, Maryland.
Wenn in diesem Bericht werden die Worte "beabsichtigen", "glaubt", "erwartet" und "erwartet" und ähnliche Ausdrücke sollen kennzeichnen solche vorausschauenden Aussagen. Solche Aussagen unterliegen Risiken und Unsicherheiten können dazu führen, dass die tatsächlichen Ergebnisse erheblich von den prognostizierten. Faktoren, die verursachen oder dazu beitragen, diese Unterschiede sind, die Unfähigkeit zu duplizieren die klinischen Ergebnisse gezeigt, die in klinischen Studien, rechtzeitige Entwicklung der potenziellen Produkte, die sich gezeigt, dass sichere und wirksame, Empfangen notwendigen regulatorischen Genehmigungen, Schwierigkeiten bei der Herstellung eines der Unternehmen das Potenzial Produkten, die Unfähigkeit, das notwendige Kapital und die Risikofaktoren festgelegten von Zeit zu Zeit in CEL-SCI Corporation's SEC, einschließlich, aber nicht beschränkt auf seinen Bericht auf dem Formular 10 - K / A für das Geschäftsjahr zum 30. September 2008 . Die Gesellschaft übernimmt keinerlei Verpflichtung, öffentlich Release das Ergebnis der Überarbeitung dieser zukunftsgerichteten Aussagen, die gemacht werden, um die Ereignisse oder Umstände nach dem Datum dieses oder um das Eintreten unerwarteter Ereignisse.
Am Dienstag, 26. Mai 2009, 9:00 AM EDT
Buzz up! Drucken Related: CEL-SCI Corp
WIEN, 26. Mai Va. Update wird - CEL-SCI Corporation (NYSE AMEX: CVM) gab heute bekannt, dass es die Entwicklung einer immun-basierte Therapie für die "Schweine-Grippe und die damit verbundenen H1N1" Grippe-Viren, die Nutzung seiner proprietären LEAPS (TM) (Ligand Epitop Antigen Präsentation System) Impfstoff-Technologie. Das Unternehmen plant, nutzen das Know-how und Wissen sie hat sich den Schutz und die Entwicklung von therapeutischen Impfstoffen Verwendung LEAPS die Entwicklung eines therapeutischen Behandlung auf der Grundlage der Technologie für die Menschen mit den infizierten Schweinen und H1N1-Grippe-Viren. CEL-SCI hat bereits vor der klinischen Erprobung.
Verwandte Angebote
Symbol Preis ändern
CVM 0,30 +0,01
( "s": "CVM", "K": "C10, L10, P20, T10", "o": "", "J": "") Zuvor, CEL-SCI bekannt gegeben, dass sein CEL-1000-Peptid, der sich aus dem LEAPS Technologie, zeigte Adjuvans-Aktivität, wenn sie mit einem Peptid-basierte Malaria-Impfstoff und eine DNA-basierte Malaria-Impfstoff in tierischen Herausforderung Studien im Rahmen einer kooperativen Forschung Absprache mit dem Naval Medical Research Center, Silver Spring, MD. In beiden Fällen ist der Zusatz von CEL-1000 auf die Impfstoffe zu erheblichen Steigerungen in den Schutz der Tiere. Darüber hinaus verschiedene L.E.A.P.S. Konjugaten induziert und / oder Verbesserungen in so unterschiedlichen Krankheiten wie rheumatoider Arthritis, Malaria, Tuberkulose und Herpes-simplex-Viren. Dies zeigt, dass die L.E.A.P.S. Technologie lassen sich erfolgreich auf vielen verschiedenen Krankheiten, und diese Ergebnisse deuten darauf hin, dass weitere LEAPS hat das Potenzial, die entweder allein als Therapie oder als Adjuvans in der Entwicklung von Impfstoffen, um für die Behandlung von H1N1 im Zusammenhang Grippeviren. Adjuvantien sind zur Verbesserung der Wirksamkeit von Impfstoffen.
"Wir glauben, dass unsere Technologie-Plattform LEAPS bietet erhebliches Potenzial zur Bewältigung der aktuellen Schweinepest und Vogelgrippe H1N1-Viren", sagte Dr. Daniel Zimmerman von CEL-SCI, der Erfinder der Technologie. "Mit der Schweinepest Grippe derzeit Schaffung großen globalen Gesundheitsproblemen, obwohl er außerhalb der traditionellen Fenster für die Grippe, wir arbeiten mit Nachdruck an einer Lösung, wie es ist zu erwarten, dass sich die Krankheit noch virulent später in diesem Jahr während der traditionellen Grippe Saison. "
Der Grund, warum die L.E.A.P.S. Technologie eignet sich für die Schaffung eines wesentlich effektiver Immun-Therapie gegen die Grippe-Virus H1N1 ist, weil die LEAPS Konjugaten induziert eine wirksame und leistungsfähige Immunantwort, ohne dass übermäßige Mengen von pro-inflammatorischen Zytokinen. Im Fall der vor Influenza-Pandemie, wie die "Spanische Grippe" und seit kurzem die "Vogelgrippe", Patienten mit stärkeren Immunsystem hat eine größere Chance, zu sterben, weil ihre Immunabwehr war zu stark (zu viele pro-inflammatorische Zytokine) . Dies wird als Zytokin Sturm. Während normalerweise viele Zytokine spielen eine wichtige Rolle bei der Vorbeugung und Behandlung von Schweinen Grippe, in einigen Fällen übermäßige Mengen Zytokin kann sich die Krankheit, wie es scheint, dass im Gegensatz zu der normalen Grippe, die vor allem die sehr jungen und sehr alten Menschen am stärksten, Schweinen Grippe kann mehr wie die Vogelgrippe, die Menschen in ihrer Hits prime mehr schwer.
Darüber hinaus LEAPS Konjugate können verwendet werden, um möglicherweise das Virus überwinden "Mutationen durch die Konzentration auf mehr erhalten und gemeinsame Epitope, die für die virale kritische Funktion.
Die L.E.A.P.S. Technologie ist ein neuartiger T-Zell-Modulation-Plattform-Technologie ermöglicht es, dass die CEL-SCI zu entwerfen und zu synthetisieren proprietäre immunogens. Jede Krankheit, für die eine Antigen-Sequenz identifiziert wurde, wie Infektionskrankheiten, Parasiten, maligne oder Autoimmun-Erkrankungen und Allergien, die potenzielle therapeutische oder präventive Standorte für die Anwendung der LEAPS Technologie. Jeder L.E.A.P.S. Bau besteht aus einem T-Zell-bindenden Liganden (TCBL), die zuvor gezeigt, die Fähigkeit zur Induktion und entlocken schützenden Immunität und Antigen-spezifische Antikörper in Tiermodellen.
Das Konzept hinter dem L.E.A.P.S. Technologie ist es, direkt imitieren Zelle / Zell-Wechselwirkungen auf der dendritischen und T-Zell-Oberfläche mit synthetischen Peptiden. Die L.E.A.P.S. Konstrukte, die die Antigen-Epitop-Krankheit in Verbindung mit einer Immun-/ T-Zell-bindenden Liganden (I / TCBL) werden können, hergestellt von der Peptid-Synthese oder durch kovalente Verknüpfung der beiden Peptide. Je nach der Art der L.E.A.P.S. Bau-und I / TCBL verwendet, CEL-SCI ist in der Lage, direkt das Ergebnis der Immunantwort auf die Entwicklung der T-Zell-Funktion in erster Linie Effektor T-Zell-Funktionen (T-Lymphozyten, Helfer / Effektor-T-Lymphozyten, Typ 1 oder 2 [ Th1 oder Th2], zytotoxische [Tc] oder Suppressor [V]). Die L.E.A.P.S. Impfstoff-Konstrukte werden chimäre Peptide denen Antigen-spezifischen Immunantwort mit Modulation.
CEL-SCI Corporation ist die Entwicklung von Produkten, die Immunabwehr stärken. Die Führung ist Multikine, das bereit für eine weltweite Phase-III-Studie. Das Unternehmen hat Niederlassungen in Vienna, Virginia, und Baltimore, Maryland.
Wenn in diesem Bericht werden die Worte "beabsichtigen", "glaubt", "erwartet" und "erwartet" und ähnliche Ausdrücke sollen kennzeichnen solche vorausschauenden Aussagen. Solche Aussagen unterliegen Risiken und Unsicherheiten können dazu führen, dass die tatsächlichen Ergebnisse erheblich von den prognostizierten. Faktoren, die verursachen oder dazu beitragen, diese Unterschiede sind, die Unfähigkeit zu duplizieren die klinischen Ergebnisse gezeigt, die in klinischen Studien, rechtzeitige Entwicklung der potenziellen Produkte, die sich gezeigt, dass sichere und wirksame, Empfangen notwendigen regulatorischen Genehmigungen, Schwierigkeiten bei der Herstellung eines der Unternehmen das Potenzial Produkten, die Unfähigkeit, das notwendige Kapital und die Risikofaktoren festgelegten von Zeit zu Zeit in CEL-SCI Corporation's SEC, einschließlich, aber nicht beschränkt auf seinen Bericht auf dem Formular 10 - K / A für das Geschäftsjahr zum 30. September 2008 . Die Gesellschaft übernimmt keinerlei Verpflichtung, öffentlich Release das Ergebnis der Überarbeitung dieser zukunftsgerichteten Aussagen, die gemacht werden, um die Ereignisse oder Umstände nach dem Datum dieses oder um das Eintreten unerwarteter Ereignisse.

seid ihr ernsthaft der meinung, dass diese aktie potential hat? wenn ja kann mir jemand kurz erläutern warum? (bin wirklich interessiert!)
ist cel sci auch auf der asco?bin mal gespannt,ob wir in den nächsten 10 jahren die phase 3 einläuteten...
mag mir nicht jemand eine kurze infoübersicht bzw. fundierte einschätzung geben?  würde ich wirklich freuen!
würde ich wirklich freuen!
 würde ich wirklich freuen!
würde ich wirklich freuen!
irgend was ist im busch,ganz hohe umsätze heut,aber noch keine nachricht
CEL-SCI-Sci Corp
(AMEX: CVM)
Real-Time: 0.60 Up 0.25 (71.40%) 9:50am ET
(AMEX: CVM)
Real-Time: 0.60 Up 0.25 (71.40%) 9:50am ET
Die Sonne scheint 
Interessant, dass diese Aktie hier auf ein nicht sehr grosses Interesse stoesst!
Zumindest nicht hier bei WO...
Gruesse,
Sil

Interessant, dass diese Aktie hier auf ein nicht sehr grosses Interesse stoesst!
Zumindest nicht hier bei WO...
Gruesse,
Sil

CEL SCI Collaborators Demonstrate Novel L.E.A.P.S. Vaccines Immunize Mice Against Tuberculosis Antigens and Suggest Potential to Treat Swine and Other Influenzas
On Friday June 5, 2009, 8:59 am EDT
Buzz up! Print Related:CEL-SCI Corp.
VIENNA, Va., June 5 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE AMEX: CVM) announced today that its collaborators at the University of Hawaii reported on data at the annual American Society for Microbiology in Philadelphia, PA. This data demonstrates that vaccines utilizing its L.E.A.P.S.(TM) (Ligand Epitope Antigen Presentation System) vaccine technology with specificity for particular Mycobacterium tuberculosis (TB) antigens can elicit immune responses that would be protective against tuberculosis and have the potential to treat swine and other H1N1 influenzas.
Related Quotes
Symbol Price Change
CVM 0.62 +0.27
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} The investigators presented data that showed that blood cells from immunized mice produced gamma interferon in response to the vaccine, while the blood cells from mice in the various control groups did not. Gamma interferon, a cytokine that helps regulate the body's immune response, is considered to be a good indicator of protection against TB and other diseases. The production of too many pro-inflammatory cytokines is thought to be a cause of death in the case of H1N1 influenza. It is for this reason that CEL-SCI believes that the L.E.A.P.S. technology, which induces an effective protective immune response without causing excessive amounts of pro-inflammatory cytokines, may be effective against H1N1 influenza.
In prior tests involving L.E.A.P.S. herpes simplex vaccines, CEL-SCI and other researchers showed that the production of gamma interferon was a good indicator for protection against herpes simplex as well. Dr. Borthakur, of the University of Hawaii, reported at the Microbiology Conference that mouse blood cells taken from L.E.A.P.S. TB immunized mice made detectable amounts of gamma interferon within one day of treatment.
Dr. Daniel Zimmerman, the inventor of the technology believes that the data presented by Dr. Borthakur's group will also be beneficial in developing more improved TB vaccines, perhaps ones including the L.E.A.P.S. conjugates known to elicit protective cytokines such as IL-12, a precursor to producing gamma interferon, and gamma interferon itself.
The L.E.A.P.S. technology combines a small peptide that activates the immune system with a small peptide from a disease-related protein, such as a herpes simplex virus (HSV) glycoprotein to make a vaccine that induces a defined immune response. Last month Dr. Kenneth S. Rosenthal, Professor of Microbiology, Immunology and Biochemistry at Northeastern Ohio Universities Colleges of Medicine and Pharmacy and colleagues showed that CEL-SCI's L.E.A.P.S. vaccines can activate and cause human immature dendritic cells from blood monocyte cells to become dendritic cells that secrete the IL-12 cytokine. The dendritic cells that result initiate a protective cell mediated and antibody immune response. These results were obtained for L.E.A.P.S. vaccines against HSV and HIV. The use of the L.E.A.P.S. vaccine technology may thus open a whole new way of maturing dendritic cell vaccines for infectious diseases such as pandemic flu and cancer. The cytokine IL-12 is the first step in gamma interferon production which is known to be protective with many viruses and pathogens.
"It is very exciting to see the effect of L.E.A.P.S. vaccines on isolated human immature dendritic cells using a simple molecule, in two different instances," said Dr. Zimmerman. "I am hopeful that other L.E.A.P.S. vaccine candidates, such as CEL-2000 being developed as a vaccine for rheumatoid arthritis, can also be used with comparable results in humans. The lack of proinflammatory cytokine production in responses to the L.E.A.P.S. vaccines is especially important for an immunotherapy aimed at rheumatoid arthritis, since these cytokines cause much of the damage seen in rheumatoid arthritis patients, and has important implications for our ability to develop an effective treatment for swine flu and other H1N1 flu viruses."
L.E.A.P.S. technology is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S. technology.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include: an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10-K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
On Friday June 5, 2009, 8:59 am EDT
Buzz up! Print Related:CEL-SCI Corp.
VIENNA, Va., June 5 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE AMEX: CVM) announced today that its collaborators at the University of Hawaii reported on data at the annual American Society for Microbiology in Philadelphia, PA. This data demonstrates that vaccines utilizing its L.E.A.P.S.(TM) (Ligand Epitope Antigen Presentation System) vaccine technology with specificity for particular Mycobacterium tuberculosis (TB) antigens can elicit immune responses that would be protective against tuberculosis and have the potential to treat swine and other H1N1 influenzas.
Related Quotes
Symbol Price Change
CVM 0.62 +0.27
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} The investigators presented data that showed that blood cells from immunized mice produced gamma interferon in response to the vaccine, while the blood cells from mice in the various control groups did not. Gamma interferon, a cytokine that helps regulate the body's immune response, is considered to be a good indicator of protection against TB and other diseases. The production of too many pro-inflammatory cytokines is thought to be a cause of death in the case of H1N1 influenza. It is for this reason that CEL-SCI believes that the L.E.A.P.S. technology, which induces an effective protective immune response without causing excessive amounts of pro-inflammatory cytokines, may be effective against H1N1 influenza.
In prior tests involving L.E.A.P.S. herpes simplex vaccines, CEL-SCI and other researchers showed that the production of gamma interferon was a good indicator for protection against herpes simplex as well. Dr. Borthakur, of the University of Hawaii, reported at the Microbiology Conference that mouse blood cells taken from L.E.A.P.S. TB immunized mice made detectable amounts of gamma interferon within one day of treatment.
Dr. Daniel Zimmerman, the inventor of the technology believes that the data presented by Dr. Borthakur's group will also be beneficial in developing more improved TB vaccines, perhaps ones including the L.E.A.P.S. conjugates known to elicit protective cytokines such as IL-12, a precursor to producing gamma interferon, and gamma interferon itself.
The L.E.A.P.S. technology combines a small peptide that activates the immune system with a small peptide from a disease-related protein, such as a herpes simplex virus (HSV) glycoprotein to make a vaccine that induces a defined immune response. Last month Dr. Kenneth S. Rosenthal, Professor of Microbiology, Immunology and Biochemistry at Northeastern Ohio Universities Colleges of Medicine and Pharmacy and colleagues showed that CEL-SCI's L.E.A.P.S. vaccines can activate and cause human immature dendritic cells from blood monocyte cells to become dendritic cells that secrete the IL-12 cytokine. The dendritic cells that result initiate a protective cell mediated and antibody immune response. These results were obtained for L.E.A.P.S. vaccines against HSV and HIV. The use of the L.E.A.P.S. vaccine technology may thus open a whole new way of maturing dendritic cell vaccines for infectious diseases such as pandemic flu and cancer. The cytokine IL-12 is the first step in gamma interferon production which is known to be protective with many viruses and pathogens.
"It is very exciting to see the effect of L.E.A.P.S. vaccines on isolated human immature dendritic cells using a simple molecule, in two different instances," said Dr. Zimmerman. "I am hopeful that other L.E.A.P.S. vaccine candidates, such as CEL-2000 being developed as a vaccine for rheumatoid arthritis, can also be used with comparable results in humans. The lack of proinflammatory cytokine production in responses to the L.E.A.P.S. vaccines is especially important for an immunotherapy aimed at rheumatoid arthritis, since these cytokines cause much of the damage seen in rheumatoid arthritis patients, and has important implications for our ability to develop an effective treatment for swine flu and other H1N1 flu viruses."
L.E.A.P.S. technology is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S. technology.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include: an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10-K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
CEL SCI Collaborators Demonstrate Novel L.E.A.P.S. Vaccines Immunize Mice Against Tuberculosis Antigens and Suggest Potential to Treat Swine and Other Influenzas
http://finance.yahoo.com/news/CEL-SCI-Collaborators-prnews-1…
http://finance.yahoo.com/news/CEL-SCI-Collaborators-prnews-1…
CEL SCI-Mitarbeiter Demonstrieren Roman LEAPS Impfstoffe gegen Tuberkulose Immunisieren Mäuse Antigene und Vorschläge Mögliche Behandlung Schweinepest und andere Influenza
Am Freitag, der 5. Juni 2009, 8.59 Uhr MESZ
Buzz up! Drucken Related: CEL-SCI Corp
VIENNA, Va., June 5 / Update wird - CEL-SCI Corporation (NYSE AMEX: CVM) gab heute bekannt, dass seine Mitarbeiter an der Universität von Hawaii berichtet über Daten auf der jährlichen Amerikanischen Gesellschaft für Mikrobiologie in Philadelphia, PA. Diese Daten zeigen, dass Impfstoffe unter Verwendung seiner LEAPS (TM) (Ligand Epitop Antigen Präsentation System) Impfstoff mit Spezifität für bestimmte Mycobacterium Tuberkulose (TB)-Antigene können Immunreaktionen auslösen, die als Schutz gegen die Tuberkulose und haben das Potenzial zur Behandlung von Schweinen und anderen H1N1 Influenza.
Verwandte Angebote
Symbol Preis ändern
CVM 0,62 +0,27
( "s": "CVM", "K": "C10, L10, P20, T10", "o": "", "J": "") Die Forscher präsentierten Daten, die zeigten, dass Zellen aus Blut immunisiert Mäusen produziert Gamma-Interferon als Reaktion auf den Impfstoff, während die Blutzellen von Mäusen in den verschiedenen Kontrollgruppen nicht. Gamma-Interferon, ein Zytokin, das regeln die körpereigene Immunantwort, ist ein guter Indikator für den Schutz gegen Tuberkulose und andere Krankheiten. Die Produktion von zu viele pro-inflammatorische Zytokine gilt als Todesursache im Fall von H1N1 Influenza. Es ist aus diesem Grund, dass CEL-SCI ist der Auffassung, dass die LEAPS Technologie, die induziert eine schützende Immunantwort wirksam, ohne dass übermäßige Mengen von pro-inflammatorischen Zytokinen, kann wirksam gegen H1N1 Influenza.
In früheren Tests mit L.E.A.P.S. Herpes-simplex-Impfstoffe, CEL-SCI und andere Forscher zeigten, dass die Produktion von Gamma-Interferon ist ein guter Indikator für den Schutz gegen Herpes-Simplex-als auch. Dr. Borthakur von der Universität von Hawaii, berichtete auf der Konferenz, dass die Mikrobiologie Maus Blutzellen aus LEAPS TB-Mäuse immunisiert, die nachweisbare Mengen von Gamma-Interferon im Rahmen eines Tages der Behandlung.
Dr. Daniel Zimmermann, der Erfinder der Technologie ist der Auffassung, dass die Daten, die von Dr. Borthakur Gruppe wird auch von Vorteil bei der Entwicklung verbesserter Impfstoffe TB, vielleicht sind auch die LEAPS Konjugaten bekannt zu entlocken Schutzmaßnahmen Zytokinen wie IL-12, ein Vorläufer für die Produktion von Interferon gamma, und Gamma-Interferon sich.
Die L.E.A.P.S. Technologie verbindet ein kleines Peptid, aktiviert das Immunsystem mit einem kleinen Peptid an einer Krankheit im Zusammenhang mit Proteinen, wie z. B. eine Herpes-simplex-Virus (HSV) Glycoprotein, um einen Impfstoff induziert eine Immunantwort definiert. Im vergangenen Monat Dr. Kenneth S. Rosenthal, Professor für Mikrobiologie, Immunologie und Biochemie an der Northeastern Ohio Universities College of Medicine und Pharmazie und Kollegen zeigten, dass CEL-SCI's LEAPS Impfstoffe können und zu menschlichen unreifen dendritischen Zellen aus dem Blut Monozyten Zellen zu werden, dass dendritische Zellen sezernieren IL-12 Zytokin. Die dendritischen Zellen, die einen schützenden Zellen und Antikörper-vermittelte Immunantwort. Diese Ergebnisse wurden für L.E.A.P.S. Impfstoffe gegen HIV und HSV. Die Verwendung der L.E.A.P.S. Impfstoff-Technologie können somit eine ganz neue Art und Weise der Reifung dendritischen Zelle Impfstoffe für Infektionskrankheiten wie Grippe-Pandemie und Krebs. Das Zytokin IL-12 ist der erste Schritt in der Gamma-Interferon-Produktion, die bekannt ist als Schutz mit vielen Viren und Krankheitserreger.
"Es ist sehr spannend zu sehen, die Wirkung von Impfstoffen LEAPS am isolierten humanen unreifen dendritischen Zellen mit Hilfe eines einfachen Molekül in zwei verschiedenen Fällen", sagt Dr. Zimmermann. "Ich bin zuversichtlich, dass andere LEAPS Impfstoff-Kandidaten, wie CEL-2000 entwickelt, als Impfstoff gegen rheumatoide Arthritis, kann auch verwendet werden, mit vergleichbaren Ergebnissen beim Menschen. Der Mangel an proinflammatorischen Zytokinproduktion in Antworten auf die LEAPS Impfstoffe ist besonders wichtig für ein Immuntherapie zur rheumatoiden Arthritis, da diese Zytokine verursachen einen Großteil der Schäden in der rheumatoiden Arthritis-Patienten, und hat bedeutende Auswirkungen auf unsere Fähigkeit, eine wirksame Behandlung für Schweine und andere Grippe H1N1-Grippe-Viren. "
L.E.A.P.S. Technologie ist ein neuartiger T-Zell-Modulation-Plattform-Technologie ermöglicht es, dass die CEL-SCI zu entwerfen und zu synthetisieren proprietäre immunogens. Jede Krankheit, für die eine Antigen-Sequenz identifiziert wurde, wie Infektionskrankheiten, Parasiten, maligne oder Autoimmun-Erkrankungen und Allergien, die potenzielle therapeutische oder präventive Standorte für die Anwendung der LEAPS Technologie.
CEL-SCI Corporation ist die Entwicklung von Produkten, die Immunabwehr stärken. Die Führung ist Multikine ®, das bereit für eine weltweite Phase-III-Studie. Das Unternehmen hat Niederlassungen in Vienna, Virginia, und Baltimore, Maryland.
Wenn in diesem Bericht werden die Worte "beabsichtigen", "glaubt", "erwartet" und "erwartet" und ähnliche Ausdrücke sollen kennzeichnen solche vorausschauenden Aussagen. Solche Aussagen unterliegen Risiken und Unsicherheiten können dazu führen, dass die tatsächlichen Ergebnisse erheblich von den prognostizierten. Faktoren, die verursachen oder dazu beitragen, diese Unterschiede sind: die Unfähigkeit zu duplizieren die klinischen Ergebnisse gezeigt, die in klinischen Studien, rechtzeitige Entwicklung der potenziellen Produkte, die sich gezeigt, dass sichere und wirksame, Empfangen notwendigen regulatorischen Genehmigungen, Schwierigkeiten bei der Herstellung eines der Unternehmen das Potenzial Produkten, die Unfähigkeit, das notwendige Kapital und die Risikofaktoren festgelegten von Zeit zu Zeit in CEL-SCI Corporation's SEC, einschließlich, aber nicht beschränkt auf seinen Bericht auf dem Formular 10-K / A für das Geschäftsjahr zum 30. September 2008 . Die Gesellschaft übernimmt keinerlei Verpflichtung, öffentlich Release das Ergebnis der Überarbeitung dieser zukunftsgerichteten Aussagen, die gemacht werden, um die Ereignisse oder Umstände nach dem Datum dieses oder um das Eintreten unerwarteter Ereignisse.
Am Freitag, der 5. Juni 2009, 8.59 Uhr MESZ
Buzz up! Drucken Related: CEL-SCI Corp
VIENNA, Va., June 5 / Update wird - CEL-SCI Corporation (NYSE AMEX: CVM) gab heute bekannt, dass seine Mitarbeiter an der Universität von Hawaii berichtet über Daten auf der jährlichen Amerikanischen Gesellschaft für Mikrobiologie in Philadelphia, PA. Diese Daten zeigen, dass Impfstoffe unter Verwendung seiner LEAPS (TM) (Ligand Epitop Antigen Präsentation System) Impfstoff mit Spezifität für bestimmte Mycobacterium Tuberkulose (TB)-Antigene können Immunreaktionen auslösen, die als Schutz gegen die Tuberkulose und haben das Potenzial zur Behandlung von Schweinen und anderen H1N1 Influenza.
Verwandte Angebote
Symbol Preis ändern
CVM 0,62 +0,27
( "s": "CVM", "K": "C10, L10, P20, T10", "o": "", "J": "") Die Forscher präsentierten Daten, die zeigten, dass Zellen aus Blut immunisiert Mäusen produziert Gamma-Interferon als Reaktion auf den Impfstoff, während die Blutzellen von Mäusen in den verschiedenen Kontrollgruppen nicht. Gamma-Interferon, ein Zytokin, das regeln die körpereigene Immunantwort, ist ein guter Indikator für den Schutz gegen Tuberkulose und andere Krankheiten. Die Produktion von zu viele pro-inflammatorische Zytokine gilt als Todesursache im Fall von H1N1 Influenza. Es ist aus diesem Grund, dass CEL-SCI ist der Auffassung, dass die LEAPS Technologie, die induziert eine schützende Immunantwort wirksam, ohne dass übermäßige Mengen von pro-inflammatorischen Zytokinen, kann wirksam gegen H1N1 Influenza.
In früheren Tests mit L.E.A.P.S. Herpes-simplex-Impfstoffe, CEL-SCI und andere Forscher zeigten, dass die Produktion von Gamma-Interferon ist ein guter Indikator für den Schutz gegen Herpes-Simplex-als auch. Dr. Borthakur von der Universität von Hawaii, berichtete auf der Konferenz, dass die Mikrobiologie Maus Blutzellen aus LEAPS TB-Mäuse immunisiert, die nachweisbare Mengen von Gamma-Interferon im Rahmen eines Tages der Behandlung.
Dr. Daniel Zimmermann, der Erfinder der Technologie ist der Auffassung, dass die Daten, die von Dr. Borthakur Gruppe wird auch von Vorteil bei der Entwicklung verbesserter Impfstoffe TB, vielleicht sind auch die LEAPS Konjugaten bekannt zu entlocken Schutzmaßnahmen Zytokinen wie IL-12, ein Vorläufer für die Produktion von Interferon gamma, und Gamma-Interferon sich.
Die L.E.A.P.S. Technologie verbindet ein kleines Peptid, aktiviert das Immunsystem mit einem kleinen Peptid an einer Krankheit im Zusammenhang mit Proteinen, wie z. B. eine Herpes-simplex-Virus (HSV) Glycoprotein, um einen Impfstoff induziert eine Immunantwort definiert. Im vergangenen Monat Dr. Kenneth S. Rosenthal, Professor für Mikrobiologie, Immunologie und Biochemie an der Northeastern Ohio Universities College of Medicine und Pharmazie und Kollegen zeigten, dass CEL-SCI's LEAPS Impfstoffe können und zu menschlichen unreifen dendritischen Zellen aus dem Blut Monozyten Zellen zu werden, dass dendritische Zellen sezernieren IL-12 Zytokin. Die dendritischen Zellen, die einen schützenden Zellen und Antikörper-vermittelte Immunantwort. Diese Ergebnisse wurden für L.E.A.P.S. Impfstoffe gegen HIV und HSV. Die Verwendung der L.E.A.P.S. Impfstoff-Technologie können somit eine ganz neue Art und Weise der Reifung dendritischen Zelle Impfstoffe für Infektionskrankheiten wie Grippe-Pandemie und Krebs. Das Zytokin IL-12 ist der erste Schritt in der Gamma-Interferon-Produktion, die bekannt ist als Schutz mit vielen Viren und Krankheitserreger.
"Es ist sehr spannend zu sehen, die Wirkung von Impfstoffen LEAPS am isolierten humanen unreifen dendritischen Zellen mit Hilfe eines einfachen Molekül in zwei verschiedenen Fällen", sagt Dr. Zimmermann. "Ich bin zuversichtlich, dass andere LEAPS Impfstoff-Kandidaten, wie CEL-2000 entwickelt, als Impfstoff gegen rheumatoide Arthritis, kann auch verwendet werden, mit vergleichbaren Ergebnissen beim Menschen. Der Mangel an proinflammatorischen Zytokinproduktion in Antworten auf die LEAPS Impfstoffe ist besonders wichtig für ein Immuntherapie zur rheumatoiden Arthritis, da diese Zytokine verursachen einen Großteil der Schäden in der rheumatoiden Arthritis-Patienten, und hat bedeutende Auswirkungen auf unsere Fähigkeit, eine wirksame Behandlung für Schweine und andere Grippe H1N1-Grippe-Viren. "
L.E.A.P.S. Technologie ist ein neuartiger T-Zell-Modulation-Plattform-Technologie ermöglicht es, dass die CEL-SCI zu entwerfen und zu synthetisieren proprietäre immunogens. Jede Krankheit, für die eine Antigen-Sequenz identifiziert wurde, wie Infektionskrankheiten, Parasiten, maligne oder Autoimmun-Erkrankungen und Allergien, die potenzielle therapeutische oder präventive Standorte für die Anwendung der LEAPS Technologie.
CEL-SCI Corporation ist die Entwicklung von Produkten, die Immunabwehr stärken. Die Führung ist Multikine ®, das bereit für eine weltweite Phase-III-Studie. Das Unternehmen hat Niederlassungen in Vienna, Virginia, und Baltimore, Maryland.
Wenn in diesem Bericht werden die Worte "beabsichtigen", "glaubt", "erwartet" und "erwartet" und ähnliche Ausdrücke sollen kennzeichnen solche vorausschauenden Aussagen. Solche Aussagen unterliegen Risiken und Unsicherheiten können dazu führen, dass die tatsächlichen Ergebnisse erheblich von den prognostizierten. Faktoren, die verursachen oder dazu beitragen, diese Unterschiede sind: die Unfähigkeit zu duplizieren die klinischen Ergebnisse gezeigt, die in klinischen Studien, rechtzeitige Entwicklung der potenziellen Produkte, die sich gezeigt, dass sichere und wirksame, Empfangen notwendigen regulatorischen Genehmigungen, Schwierigkeiten bei der Herstellung eines der Unternehmen das Potenzial Produkten, die Unfähigkeit, das notwendige Kapital und die Risikofaktoren festgelegten von Zeit zu Zeit in CEL-SCI Corporation's SEC, einschließlich, aber nicht beschränkt auf seinen Bericht auf dem Formular 10-K / A für das Geschäftsjahr zum 30. September 2008 . Die Gesellschaft übernimmt keinerlei Verpflichtung, öffentlich Release das Ergebnis der Überarbeitung dieser zukunftsgerichteten Aussagen, die gemacht werden, um die Ereignisse oder Umstände nach dem Datum dieses oder um das Eintreten unerwarteter Ereignisse.
was sagt euch die nachricht?wundert mich daß der kurs so anspringt....kommt da nochmal was...
Antwort auf Beitrag Nr.: 37.335.793 von VFBLER am 06.06.09 15:50:22 Cel-Sci management anticipates eventual multibillion-dollar sales from Multikine on an annual basis. Within the oncology market, company executives estimate that revenue from the head and neck cancer market for Multikine could reach about $1.2 billion in the United States and Canada and $3 billion in Europe, according to a report compiled by Cel-Sci and Crystal Research Associates LLC (crystalra.com), with stats from the Mouth Cancer Foundation (mouthcancerfoundation. org). These values are based on the company's internal metrics, assuming a value of about $30,000 for each of the about 40,000 head and neck cancer patients in the United States and Canada and roughly 100,000 patients in Europe.
See CVM website for more info.
http://www.cel-sci.com/
See CVM website for more info.
http://www.cel-sci.com/
Antwort auf Beitrag Nr.: 37.298.849 von Tobberus am 01.06.09 22:30:01Hallo zusammen,
@ Tobberus : kurze Zusammenfassung.
CVM hat ein Produkt (Multikine) das die P2 in Amerika und Canada bestanden hat (FDA approval)und haben eine Fabrik für die Herstellung des Impfstoffes aufgebaut, in der auch die für die P3 benötigten Medikamente hergestellt werden.
CVM hat den Orphan-Drug Status durch die FDA bekommen (ermöglicht beschleunigte Zulassung und etwas finanzielle Unterstützung).
Zusätzlich sind im moment noch weiter Medikamente in der Erprobung, wobei diese (meiner Meinung nach) noch lange nicht in die Testphase gehen(event. Phantasie für die Zukunft).
CVM geht das Geld im Moment aus und Sie sind auf der Suche nach Partnern, wobei 3 schon gefunden wurden (Asien,Israel und Afrika).
Multikine hat (meiner Meinung nach) grosses Potential, auch die P3 zu bestehen, wenn diese angefangen wird.
Allerdings ist im Moment die grösste Befürchtung, dass das Geld nicht ausreicht um diese Phase durchzuziehen.
Sollte ein Partner gefunden werden, der genug Geld mitbringt, ist diese Aktie eine Rakete, wird keiner gefunden ist sie es auch, nur leider dann mit Richtung Erdinnern.
Also hochspekulative Sache, Hop oder Top.
Habe in einem amerikanischen Board heute gelesen, dass es eventuell noch einen Partner geben soll, schauen wir mal.
Meine persönliche Einschätzung zu der aktuellen Preisentwicklung :
Am Montag wird es einen Rücksetzer geben, da die Nachrichten eigentlich nur "alte" News sind.
Sollte die Aktie Montag entgegen meiner Meinung steigen ist es ein positives Zeichen, denn das Volumen war extrem hoch und eventuell gibt es Insiderwissen über News (Partner ?) die erst noch bekannt gegeben werden.
Bin selber seit Jahren investiert und habe vor kurzem nochmals nachgekauft, bin also kein "Basher".
Was interessant ist, bei jeder noch so kleinen Neuigkeit springt die Aktie kurz an, macht dann aber meist wieder einen Rücksetzer.
Ich denke die Aktie wird erst dann nachhaltig steigen, wenn die P3 beginnt.
Alles andere vorher ist nur "Vorspiel"
Gruss Mimm
Ps. ich hoffe natürlich auch, dass die Aktie vorher bzw. Montag steigen wird
@ Tobberus : kurze Zusammenfassung.
CVM hat ein Produkt (Multikine) das die P2 in Amerika und Canada bestanden hat (FDA approval)und haben eine Fabrik für die Herstellung des Impfstoffes aufgebaut, in der auch die für die P3 benötigten Medikamente hergestellt werden.
CVM hat den Orphan-Drug Status durch die FDA bekommen (ermöglicht beschleunigte Zulassung und etwas finanzielle Unterstützung).
Zusätzlich sind im moment noch weiter Medikamente in der Erprobung, wobei diese (meiner Meinung nach) noch lange nicht in die Testphase gehen(event. Phantasie für die Zukunft).
CVM geht das Geld im Moment aus und Sie sind auf der Suche nach Partnern, wobei 3 schon gefunden wurden (Asien,Israel und Afrika).
Multikine hat (meiner Meinung nach) grosses Potential, auch die P3 zu bestehen, wenn diese angefangen wird.
Allerdings ist im Moment die grösste Befürchtung, dass das Geld nicht ausreicht um diese Phase durchzuziehen.
Sollte ein Partner gefunden werden, der genug Geld mitbringt, ist diese Aktie eine Rakete, wird keiner gefunden ist sie es auch, nur leider dann mit Richtung Erdinnern.
Also hochspekulative Sache, Hop oder Top.
Habe in einem amerikanischen Board heute gelesen, dass es eventuell noch einen Partner geben soll, schauen wir mal.
Meine persönliche Einschätzung zu der aktuellen Preisentwicklung :
Am Montag wird es einen Rücksetzer geben, da die Nachrichten eigentlich nur "alte" News sind.
Sollte die Aktie Montag entgegen meiner Meinung steigen ist es ein positives Zeichen, denn das Volumen war extrem hoch und eventuell gibt es Insiderwissen über News (Partner ?) die erst noch bekannt gegeben werden.
Bin selber seit Jahren investiert und habe vor kurzem nochmals nachgekauft, bin also kein "Basher".
Was interessant ist, bei jeder noch so kleinen Neuigkeit springt die Aktie kurz an, macht dann aber meist wieder einen Rücksetzer.
Ich denke die Aktie wird erst dann nachhaltig steigen, wenn die P3 beginnt.
Alles andere vorher ist nur "Vorspiel"

Gruss Mimm
Ps. ich hoffe natürlich auch, dass die Aktie vorher bzw. Montag steigen wird

Antwort auf Beitrag Nr.: 37.336.770 von Mimm am 06.06.09 22:16:43Hi Mimm!
Alte News sind es nun wirklich nicht. Eine Vermutung wurde bewiesen...dies ist schon mal ein grosser Anfang...
Allein eine Frage bleibt. Ist diese News wirklich den groessten Umsatz der letzten zwoelf Monate wert???
Antwort: Nein!
Was man daraus Schlussfolgern kann...Partner fuer PIII scheint die einzige logische Erklaerung...
Man wird es sicher bald erfahren...wenn der Kurs in drei Wochen wieder bei 0,3 USD stehen sollten, war es wohl ein gewaltiger pump and dump.
Gruesse,
Sil
Alte News sind es nun wirklich nicht. Eine Vermutung wurde bewiesen...dies ist schon mal ein grosser Anfang...
Allein eine Frage bleibt. Ist diese News wirklich den groessten Umsatz der letzten zwoelf Monate wert???
Antwort: Nein!
Was man daraus Schlussfolgern kann...Partner fuer PIII scheint die einzige logische Erklaerung...
Man wird es sicher bald erfahren...wenn der Kurs in drei Wochen wieder bei 0,3 USD stehen sollten, war es wohl ein gewaltiger pump and dump.
Gruesse,
Sil
ja,der große umsatz macht mich auch stutzig,sind ja schon am donnerstag fast 700000 stück gehandelt worden(was auch schon viel für cel sci sind).aber 32 mio,wegen der nachricht,denk da kommt noch was und weihnachten vielleicht dieses jahr schon im juni ist





CEL-SCI Expands Testing of Its Vaccine to Determine Efficacy Against More Virulent Strain of H1N1 Swine and Other Influenza Viruses
--VACCINE USING L.E.A.P.S.(TM) TECHNOLOGY PLATFORM IS DESIGNED TO ADDRESS MUTATED FORMS OF SWINE INFLUENZA AND CAN ACT AS BOTH PREVENTATIVE AND THERAPEUTIC VACCINE
VIENNA, Va., June 9, 2009 /PRNewswire-FirstCall via COMTEX/ -- CEL-SCI Corporation (NYSE Amex: CVM) announced today that it is expanding the pre-clinical testing of its flu vaccine, utilizing its proprietary L.E.A.P.S. technology (Ligand Epitope Antigen Presentation System) to determine its efficacy against the more dangerous and virulent virus strains that may arise during the up coming winter flu season. The Company has begun pre-clinical formulation, evaluation and testing of a new application of its L.E.A.P.S vaccine, which will allow the targeting of "mutated" versions of H1N1 swine and other influenza viruses. It is believed that the influenza virus may mutate and evolve between now and the winter flu season. In conjunction with the testing, CEL-SCI has produced several L.E.A.P.S. flu vaccines that focus on the conserved, non changing epitopes of the different strains of Type A Influenza viruses (H1N1, H5N1, H3N1, etc.), including "swine", "avian or bird", and "Spanish Influenza", in order to minimize the chance of viral "escape by mutations" from immune recognition. CEL-SCI's L.E.A.P.S. flu vaccine contains epitopes known to be associated with immune protection against influenza in animal models. The Company had previously announced that it had begun pre-clinical testing of swine and H1N1 flu viruses, which were non-mutated versions of the virus.
The use of L.E.A.P.S. vaccine technology for immunization in animal models has already been shown to provide protection from viral diseases without causing an immune response associated with the deadly "cytokine-storm" seen in many of the victims of influenza.
Dr. Daniel Zimmerman, inventor of the L.E.A.P.S. technology, and currently a consultant to CEL-SCI, said, "Various L.E.A.P.S. technology constructs have already been shown to induce protection in animal challenge models against a variety of diseases such as malaria and herpes simplex virus and as therapeutic vaccines in two different autoimmune conditions. Data showed that L.E.A.P.S. vaccines were able to induce these protective immune responses without the excessive induction of pro-inflammatory cytokines. This is thought to be very important in the swine flu, or the avian flu, since it appears that the excessive production of pro-inflammatory cytokines during the course of the disease is responsible for and may lead to the increased number of deaths from these illnesses."
Recently, collaborators at the University of Hawaii reported on data at the annual American Society for Microbiology in Philadelphia, PA. This data demonstrates that vaccines utilizing its L.E.A.P.S. vaccine technology with specificity for particular Mycobacterium tuberculosis (TB) antigens can elicit immune responses that would be protective against tuberculosis and have the potential to treat swine and other H1N1 influenzas. The investigators presented data that showed that blood and spleen cells from immunized mice produced gamma interferon in response to the vaccine, while the cells from mice in the various control groups did not.
The L.E.A.P.S. technology is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S. technology. Each L.E.A.P.S. construct is composed of a T cell binding ligand (TCBL) which has previously demonstrated the ability to induce and elicit protective immunity and antigen specific antibody production in animal models.
The concept behind the L.E.A.P.S. technology is to directly mimic cell/cell interactions on the dendritic and T-cell surface with synthetic peptides. The L.E.A.P.S. constructs containing the antigenic disease epitope linked to an Immune/T-cell binding ligand (I/TCBL) can be manufactured by peptide synthesis or by covalently linking the two peptides. Depending upon the type of L.E.A.P.S. construct and I/TCBL used, CEL-SCI is able to direct the outcome of the immune response towards the development of T-cell function with primarily effector T-cell functions (T Lymphocyte; helper/effector T lymphocyte, type 1 or 2 [Th1 or Th2], cytotoxic [Tc] or suppressor [Ts]). The L.E.A.P.S. vaccine constructs are chimeric peptides which combine antigen specificity with immune response modulation.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine(R) which is currently being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
SOURCE CEL-SCI Corporation
URL: http://www.cel-sci.com/
www.prnewswire.com
Copyright (C) 2009 PR Newswire. All rights reserved
--VACCINE USING L.E.A.P.S.(TM) TECHNOLOGY PLATFORM IS DESIGNED TO ADDRESS MUTATED FORMS OF SWINE INFLUENZA AND CAN ACT AS BOTH PREVENTATIVE AND THERAPEUTIC VACCINE
VIENNA, Va., June 9, 2009 /PRNewswire-FirstCall via COMTEX/ -- CEL-SCI Corporation (NYSE Amex: CVM) announced today that it is expanding the pre-clinical testing of its flu vaccine, utilizing its proprietary L.E.A.P.S. technology (Ligand Epitope Antigen Presentation System) to determine its efficacy against the more dangerous and virulent virus strains that may arise during the up coming winter flu season. The Company has begun pre-clinical formulation, evaluation and testing of a new application of its L.E.A.P.S vaccine, which will allow the targeting of "mutated" versions of H1N1 swine and other influenza viruses. It is believed that the influenza virus may mutate and evolve between now and the winter flu season. In conjunction with the testing, CEL-SCI has produced several L.E.A.P.S. flu vaccines that focus on the conserved, non changing epitopes of the different strains of Type A Influenza viruses (H1N1, H5N1, H3N1, etc.), including "swine", "avian or bird", and "Spanish Influenza", in order to minimize the chance of viral "escape by mutations" from immune recognition. CEL-SCI's L.E.A.P.S. flu vaccine contains epitopes known to be associated with immune protection against influenza in animal models. The Company had previously announced that it had begun pre-clinical testing of swine and H1N1 flu viruses, which were non-mutated versions of the virus.
The use of L.E.A.P.S. vaccine technology for immunization in animal models has already been shown to provide protection from viral diseases without causing an immune response associated with the deadly "cytokine-storm" seen in many of the victims of influenza.
Dr. Daniel Zimmerman, inventor of the L.E.A.P.S. technology, and currently a consultant to CEL-SCI, said, "Various L.E.A.P.S. technology constructs have already been shown to induce protection in animal challenge models against a variety of diseases such as malaria and herpes simplex virus and as therapeutic vaccines in two different autoimmune conditions. Data showed that L.E.A.P.S. vaccines were able to induce these protective immune responses without the excessive induction of pro-inflammatory cytokines. This is thought to be very important in the swine flu, or the avian flu, since it appears that the excessive production of pro-inflammatory cytokines during the course of the disease is responsible for and may lead to the increased number of deaths from these illnesses."
Recently, collaborators at the University of Hawaii reported on data at the annual American Society for Microbiology in Philadelphia, PA. This data demonstrates that vaccines utilizing its L.E.A.P.S. vaccine technology with specificity for particular Mycobacterium tuberculosis (TB) antigens can elicit immune responses that would be protective against tuberculosis and have the potential to treat swine and other H1N1 influenzas. The investigators presented data that showed that blood and spleen cells from immunized mice produced gamma interferon in response to the vaccine, while the cells from mice in the various control groups did not.
The L.E.A.P.S. technology is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S. technology. Each L.E.A.P.S. construct is composed of a T cell binding ligand (TCBL) which has previously demonstrated the ability to induce and elicit protective immunity and antigen specific antibody production in animal models.
The concept behind the L.E.A.P.S. technology is to directly mimic cell/cell interactions on the dendritic and T-cell surface with synthetic peptides. The L.E.A.P.S. constructs containing the antigenic disease epitope linked to an Immune/T-cell binding ligand (I/TCBL) can be manufactured by peptide synthesis or by covalently linking the two peptides. Depending upon the type of L.E.A.P.S. construct and I/TCBL used, CEL-SCI is able to direct the outcome of the immune response towards the development of T-cell function with primarily effector T-cell functions (T Lymphocyte; helper/effector T lymphocyte, type 1 or 2 [Th1 or Th2], cytotoxic [Tc] or suppressor [Ts]). The L.E.A.P.S. vaccine constructs are chimeric peptides which combine antigen specificity with immune response modulation.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine(R) which is currently being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
SOURCE CEL-SCI Corporation
URL: http://www.cel-sci.com/
www.prnewswire.com
Copyright (C) 2009 PR Newswire. All rights reserved
wär ja schon ein erfolg wenn wir so um 50 cent rum bleiben würden und dann auf zu neuen ufern....1 dann 2 dollar

wir kommen mal wieder nicht voran,nichts neues zu phase 3 und von der vermietung zur fabrik redet auch keiner mehr...
Antwort auf Beitrag Nr.: 37.386.463 von VFBLER am 13.06.09 11:39:37Hallo zusammen,
leider wahr, aber ich sehe es immer noch ositiv, denke es wird in den nächsten Wochen neues kommen, was dann auch den Kurs nach oben treiben wird.
Habe bei OTC gelesen, dass China interesse an der L.E.A.P.S. Technologie hat, bei denen kommt es auf ein paar Dollar mehr oder weniger nicht drauf an
Gruss Mimm
leider wahr, aber ich sehe es immer noch ositiv, denke es wird in den nächsten Wochen neues kommen, was dann auch den Kurs nach oben treiben wird.
Habe bei OTC gelesen, dass China interesse an der L.E.A.P.S. Technologie hat, bei denen kommt es auf ein paar Dollar mehr oder weniger nicht drauf an

Gruss Mimm
CEL-SCI Scientist Invited to Discuss Plans to Launch Unique Manufacturing Process That Saves Cost While Enhancing the Shelf Life of Drugs at 5th Annual Aseptic Processing of Sterile Drug Products Conference
On Tuesday June 16, 2009, 9:00 am EDT
Buzz up! Print Related:CEL-SCI Corp.
VIENNA, Va., June 16 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE Amex: CVM) announced today that Eyal Talor, Ph.D., Senior Vice President of Research and Manufacturing at CEL-SCI, has accepted an invitation to be a featured speaker at the 5th Annual Aseptic Processing of Sterile Drug Products Conference to be held June 17th - 19th. The Conference, sponsored by The Institute of Validation Technology (IVT), takes place at the Sheraton National Hotel in Arlington, VA. At the workshop titled "Aseptic Facility Design Fundamentals - Cold Fill/Finish Design for a Biologic, Liquid Injectable", Dr. Talor will discuss the Company's plans to launch a unique manufacturing process that could allow drugs using stem cell technology and other biological products to maintain their potency through the entire manufacturing process, as well as enhance the shelf life of these drugs.
Related Quotes
Symbol Price Change
CVM 0.47 -0.06
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} The availability of this unique aseptic filling process developed by CEL-SCI may also significantly accelerate the time to market by eliminating complicated, costly and time consuming validation studies and tests required when these products are filled at room temperature. This process is also likely to save money in the production of follow-on biologics and biosimilars (the general equivalent of generics in the biological arena) likely to be approved in the U.S. soon.
CEL-SCI's new state-of-the-art manufacturing facility near Baltimore, Maryland, where CEL-SCI plans to manufacture its lead cancer product Multikine®, will be offering this service to outside companies on a contract basis. The facility will allow CEL-SCI to perform aseptic filling of small volume parenteral drug products.
The Company will employ a process known as "true cold" +4 degrees Celsius Aseptic Filling for products derived from stem cell technology, biotechnology/pharmaceutical companies and academic research institutions. The use of a cold +4 degrees Celsius aseptic fill, as opposed to the room temperature fill usually employed for these and other products, significantly increases the probability of maintaining the biological activity and potency of these drugs, and thus potentially extending the drugs' shelf-life. When launched, CEL-SCI will be the only company providing this "true cold" +4 degrees Celsius filling service on a contractual basis to other companies and academic institutions. CEL-SCI will also be able to offer normal room temperature fills at the same facility.
Dr. Talor said, "Many of the large pharmaceutical and generic companies are getting ready for new biosimilar legislation in the US. They have invested a great deal of effort and money in this area. However, as best we know, none have "true cold" fill capability that will allow them to bring these drugs to market faster, while saving substantial cost. We believe that we will be in an excellent position to work with them to meet their growing needs as they will try to rush new drugs to market."
CEL-SCI's unique, cold aseptic filling suite can be operated at temperatures between 2 degrees Celsius and room temperatures, including humidity control. The Fill/Finish Facility and the aseptic filling suites have all been designed to meet both FDA and EU ISO classifications. CEL-SCI also has additional capability to formulate, inspect, label and package these products at cold temperatures.
Background on CEL-SCI's "true cold" +4 degrees Celsius Fill/Finish facility:
The fastest area of growth in the Biopharmaceutical and Pharmaceutical market is the area of biologics, most recently the area of stem cell derived products, and in the future biosimilars (copies of biologics). These compounds and therapies are derived from or mimic human cells or proteins and other molecules (e.g., hormones, etc.). Nearly all of the major new billion-dollar-drugs developed for unmet medical needs (e.g., Avastin, Erbitux, Rituxan, Herceptin, Copaxon, etc.) are biologics. Biologics are usually very sensitive to heat and quickly lose their biological activity if exposed to room or elevated temperature for various periods of time. These elevated temperatures may also affect the shelf life of a Biologic product, which means that the product cannot be stored for as long as one might find desirable.
Therefore, CEL-SCI has developed a completely novel technology to be able to accomplish the aseptic Fill/Finish operation at "true cold" +4 degrees Celsius. This is of great importance to companies that produce the latest drugs, biologics and stem cell derived therapies. It is an absolute requirement by FDA and any other regulatory agency that a drug developer must demonstrate the safety, purity and potency of a drug being produced for use in humans.
When aseptically filling a product at CEL-SCI's new facility at +4 degrees Celsius, minimal to no biological losses will have occurred due to the maintenance of cold temperature, and therefore the potency of the drug is maintained throughout this final critical step of the drug's manufacturing process. When the same temperature sensitive drug is instead aseptically filled at room temperature, very expensive and time consuming validation studies must be conducted, first, to be able to obtain a complete understanding of the product's potency loss during the room temperature fill process, then to create solutions to the drug potency losses, which then will also need to be tested and validated. Therefore, CEL-SCI's new "true cold" aseptic fill facility will provide two critical advantages to companies developing biologics and stem cell derived therapies: it will save them a great deal of money (wasted product and extra validations) and it will save them a great deal of time usually spent on the development of these expensive validations.
About IVT:
The Institute of Validation Technology (IVT) Conference Division features the most-notable validation and compliance experts in the FDA regulated industry. IVT stands alone in its quest to continually educate pharmaceutical, biopharmaceutical and medical device professionals in validation concepts from A-Z, AND global GXP regulatory initiatives. Through over 15 years of providing timely, cutting-edge conferences, IVT's philosophy remains that: "each of our events provides valuable take-home knowledge that can be immediately implemented at every level."
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is currently being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
Available Topic Expert(s): For information on the listed expert(s), click appropriate link.
Eyal Talor
On Tuesday June 16, 2009, 9:00 am EDT
Buzz up! Print Related:CEL-SCI Corp.
VIENNA, Va., June 16 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE Amex: CVM) announced today that Eyal Talor, Ph.D., Senior Vice President of Research and Manufacturing at CEL-SCI, has accepted an invitation to be a featured speaker at the 5th Annual Aseptic Processing of Sterile Drug Products Conference to be held June 17th - 19th. The Conference, sponsored by The Institute of Validation Technology (IVT), takes place at the Sheraton National Hotel in Arlington, VA. At the workshop titled "Aseptic Facility Design Fundamentals - Cold Fill/Finish Design for a Biologic, Liquid Injectable", Dr. Talor will discuss the Company's plans to launch a unique manufacturing process that could allow drugs using stem cell technology and other biological products to maintain their potency through the entire manufacturing process, as well as enhance the shelf life of these drugs.
Related Quotes
Symbol Price Change
CVM 0.47 -0.06
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} The availability of this unique aseptic filling process developed by CEL-SCI may also significantly accelerate the time to market by eliminating complicated, costly and time consuming validation studies and tests required when these products are filled at room temperature. This process is also likely to save money in the production of follow-on biologics and biosimilars (the general equivalent of generics in the biological arena) likely to be approved in the U.S. soon.
CEL-SCI's new state-of-the-art manufacturing facility near Baltimore, Maryland, where CEL-SCI plans to manufacture its lead cancer product Multikine®, will be offering this service to outside companies on a contract basis. The facility will allow CEL-SCI to perform aseptic filling of small volume parenteral drug products.
The Company will employ a process known as "true cold" +4 degrees Celsius Aseptic Filling for products derived from stem cell technology, biotechnology/pharmaceutical companies and academic research institutions. The use of a cold +4 degrees Celsius aseptic fill, as opposed to the room temperature fill usually employed for these and other products, significantly increases the probability of maintaining the biological activity and potency of these drugs, and thus potentially extending the drugs' shelf-life. When launched, CEL-SCI will be the only company providing this "true cold" +4 degrees Celsius filling service on a contractual basis to other companies and academic institutions. CEL-SCI will also be able to offer normal room temperature fills at the same facility.
Dr. Talor said, "Many of the large pharmaceutical and generic companies are getting ready for new biosimilar legislation in the US. They have invested a great deal of effort and money in this area. However, as best we know, none have "true cold" fill capability that will allow them to bring these drugs to market faster, while saving substantial cost. We believe that we will be in an excellent position to work with them to meet their growing needs as they will try to rush new drugs to market."
CEL-SCI's unique, cold aseptic filling suite can be operated at temperatures between 2 degrees Celsius and room temperatures, including humidity control. The Fill/Finish Facility and the aseptic filling suites have all been designed to meet both FDA and EU ISO classifications. CEL-SCI also has additional capability to formulate, inspect, label and package these products at cold temperatures.
Background on CEL-SCI's "true cold" +4 degrees Celsius Fill/Finish facility:
The fastest area of growth in the Biopharmaceutical and Pharmaceutical market is the area of biologics, most recently the area of stem cell derived products, and in the future biosimilars (copies of biologics). These compounds and therapies are derived from or mimic human cells or proteins and other molecules (e.g., hormones, etc.). Nearly all of the major new billion-dollar-drugs developed for unmet medical needs (e.g., Avastin, Erbitux, Rituxan, Herceptin, Copaxon, etc.) are biologics. Biologics are usually very sensitive to heat and quickly lose their biological activity if exposed to room or elevated temperature for various periods of time. These elevated temperatures may also affect the shelf life of a Biologic product, which means that the product cannot be stored for as long as one might find desirable.
Therefore, CEL-SCI has developed a completely novel technology to be able to accomplish the aseptic Fill/Finish operation at "true cold" +4 degrees Celsius. This is of great importance to companies that produce the latest drugs, biologics and stem cell derived therapies. It is an absolute requirement by FDA and any other regulatory agency that a drug developer must demonstrate the safety, purity and potency of a drug being produced for use in humans.
When aseptically filling a product at CEL-SCI's new facility at +4 degrees Celsius, minimal to no biological losses will have occurred due to the maintenance of cold temperature, and therefore the potency of the drug is maintained throughout this final critical step of the drug's manufacturing process. When the same temperature sensitive drug is instead aseptically filled at room temperature, very expensive and time consuming validation studies must be conducted, first, to be able to obtain a complete understanding of the product's potency loss during the room temperature fill process, then to create solutions to the drug potency losses, which then will also need to be tested and validated. Therefore, CEL-SCI's new "true cold" aseptic fill facility will provide two critical advantages to companies developing biologics and stem cell derived therapies: it will save them a great deal of money (wasted product and extra validations) and it will save them a great deal of time usually spent on the development of these expensive validations.
About IVT:
The Institute of Validation Technology (IVT) Conference Division features the most-notable validation and compliance experts in the FDA regulated industry. IVT stands alone in its quest to continually educate pharmaceutical, biopharmaceutical and medical device professionals in validation concepts from A-Z, AND global GXP regulatory initiatives. Through over 15 years of providing timely, cutting-edge conferences, IVT's philosophy remains that: "each of our events provides valuable take-home knowledge that can be immediately implemented at every level."
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is currently being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
Available Topic Expert(s): For information on the listed expert(s), click appropriate link.
Eyal Talor
CEL-SCI Scientist Invited to Discuss Plans to Launch Unique Manufacturing Process That Saves Cost While Enhancing the Shelf Life of Drugs at 5th Annual Aseptic Processing of Sterile Drug Products Conference
On Tuesday June 16, 2009, 9:00 am EDT
Buzz up! Print Related:CEL-SCI Corp.
VIENNA, Va., June 16 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE Amex: CVM) announced today that Eyal Talor, Ph.D., Senior Vice President of Research and Manufacturing at CEL-SCI, has accepted an invitation to be a featured speaker at the 5th Annual Aseptic Processing of Sterile Drug Products Conference to be held June 17th - 19th. The Conference, sponsored by The Institute of Validation Technology (IVT), takes place at the Sheraton National Hotel in Arlington, VA. At the workshop titled "Aseptic Facility Design Fundamentals - Cold Fill/Finish Design for a Biologic, Liquid Injectable", Dr. Talor will discuss the Company's plans to launch a unique manufacturing process that could allow drugs using stem cell technology and other biological products to maintain their potency through the entire manufacturing process, as well as enhance the shelf life of these drugs.
Related Quotes
Symbol Price Change
CVM 0.47 -0.06
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} The availability of this unique aseptic filling process developed by CEL-SCI may also significantly accelerate the time to market by eliminating complicated, costly and time consuming validation studies and tests required when these products are filled at room temperature. This process is also likely to save money in the production of follow-on biologics and biosimilars (the general equivalent of generics in the biological arena) likely to be approved in the U.S. soon.
CEL-SCI's new state-of-the-art manufacturing facility near Baltimore, Maryland, where CEL-SCI plans to manufacture its lead cancer product Multikine®, will be offering this service to outside companies on a contract basis. The facility will allow CEL-SCI to perform aseptic filling of small volume parenteral drug products.
The Company will employ a process known as "true cold" +4 degrees Celsius Aseptic Filling for products derived from stem cell technology, biotechnology/pharmaceutical companies and academic research institutions. The use of a cold +4 degrees Celsius aseptic fill, as opposed to the room temperature fill usually employed for these and other products, significantly increases the probability of maintaining the biological activity and potency of these drugs, and thus potentially extending the drugs' shelf-life. When launched, CEL-SCI will be the only company providing this "true cold" +4 degrees Celsius filling service on a contractual basis to other companies and academic institutions. CEL-SCI will also be able to offer normal room temperature fills at the same facility.
Dr. Talor said, "Many of the large pharmaceutical and generic companies are getting ready for new biosimilar legislation in the US. They have invested a great deal of effort and money in this area. However, as best we know, none have "true cold" fill capability that will allow them to bring these drugs to market faster, while saving substantial cost. We believe that we will be in an excellent position to work with them to meet their growing needs as they will try to rush new drugs to market."
CEL-SCI's unique, cold aseptic filling suite can be operated at temperatures between 2 degrees Celsius and room temperatures, including humidity control. The Fill/Finish Facility and the aseptic filling suites have all been designed to meet both FDA and EU ISO classifications. CEL-SCI also has additional capability to formulate, inspect, label and package these products at cold temperatures.
Background on CEL-SCI's "true cold" +4 degrees Celsius Fill/Finish facility:
The fastest area of growth in the Biopharmaceutical and Pharmaceutical market is the area of biologics, most recently the area of stem cell derived products, and in the future biosimilars (copies of biologics). These compounds and therapies are derived from or mimic human cells or proteins and other molecules (e.g., hormones, etc.). Nearly all of the major new billion-dollar-drugs developed for unmet medical needs (e.g., Avastin, Erbitux, Rituxan, Herceptin, Copaxon, etc.) are biologics. Biologics are usually very sensitive to heat and quickly lose their biological activity if exposed to room or elevated temperature for various periods of time. These elevated temperatures may also affect the shelf life of a Biologic product, which means that the product cannot be stored for as long as one might find desirable.
Therefore, CEL-SCI has developed a completely novel technology to be able to accomplish the aseptic Fill/Finish operation at "true cold" +4 degrees Celsius. This is of great importance to companies that produce the latest drugs, biologics and stem cell derived therapies. It is an absolute requirement by FDA and any other regulatory agency that a drug developer must demonstrate the safety, purity and potency of a drug being produced for use in humans.
When aseptically filling a product at CEL-SCI's new facility at +4 degrees Celsius, minimal to no biological losses will have occurred due to the maintenance of cold temperature, and therefore the potency of the drug is maintained throughout this final critical step of the drug's manufacturing process. When the same temperature sensitive drug is instead aseptically filled at room temperature, very expensive and time consuming validation studies must be conducted, first, to be able to obtain a complete understanding of the product's potency loss during the room temperature fill process, then to create solutions to the drug potency losses, which then will also need to be tested and validated. Therefore, CEL-SCI's new "true cold" aseptic fill facility will provide two critical advantages to companies developing biologics and stem cell derived therapies: it will save them a great deal of money (wasted product and extra validations) and it will save them a great deal of time usually spent on the development of these expensive validations.
About IVT:
The Institute of Validation Technology (IVT) Conference Division features the most-notable validation and compliance experts in the FDA regulated industry. IVT stands alone in its quest to continually educate pharmaceutical, biopharmaceutical and medical device professionals in validation concepts from A-Z, AND global GXP regulatory initiatives. Through over 15 years of providing timely, cutting-edge conferences, IVT's philosophy remains that: "each of our events provides valuable take-home knowledge that can be immediately implemented at every level."
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is currently being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
Available Topic Expert(s): For information on the listed expert(s), click appropriate link.
Eyal Talor
On Tuesday June 16, 2009, 9:00 am EDT
Buzz up! Print Related:CEL-SCI Corp.
VIENNA, Va., June 16 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE Amex: CVM) announced today that Eyal Talor, Ph.D., Senior Vice President of Research and Manufacturing at CEL-SCI, has accepted an invitation to be a featured speaker at the 5th Annual Aseptic Processing of Sterile Drug Products Conference to be held June 17th - 19th. The Conference, sponsored by The Institute of Validation Technology (IVT), takes place at the Sheraton National Hotel in Arlington, VA. At the workshop titled "Aseptic Facility Design Fundamentals - Cold Fill/Finish Design for a Biologic, Liquid Injectable", Dr. Talor will discuss the Company's plans to launch a unique manufacturing process that could allow drugs using stem cell technology and other biological products to maintain their potency through the entire manufacturing process, as well as enhance the shelf life of these drugs.
Related Quotes
Symbol Price Change
CVM 0.47 -0.06
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} The availability of this unique aseptic filling process developed by CEL-SCI may also significantly accelerate the time to market by eliminating complicated, costly and time consuming validation studies and tests required when these products are filled at room temperature. This process is also likely to save money in the production of follow-on biologics and biosimilars (the general equivalent of generics in the biological arena) likely to be approved in the U.S. soon.
CEL-SCI's new state-of-the-art manufacturing facility near Baltimore, Maryland, where CEL-SCI plans to manufacture its lead cancer product Multikine®, will be offering this service to outside companies on a contract basis. The facility will allow CEL-SCI to perform aseptic filling of small volume parenteral drug products.
The Company will employ a process known as "true cold" +4 degrees Celsius Aseptic Filling for products derived from stem cell technology, biotechnology/pharmaceutical companies and academic research institutions. The use of a cold +4 degrees Celsius aseptic fill, as opposed to the room temperature fill usually employed for these and other products, significantly increases the probability of maintaining the biological activity and potency of these drugs, and thus potentially extending the drugs' shelf-life. When launched, CEL-SCI will be the only company providing this "true cold" +4 degrees Celsius filling service on a contractual basis to other companies and academic institutions. CEL-SCI will also be able to offer normal room temperature fills at the same facility.
Dr. Talor said, "Many of the large pharmaceutical and generic companies are getting ready for new biosimilar legislation in the US. They have invested a great deal of effort and money in this area. However, as best we know, none have "true cold" fill capability that will allow them to bring these drugs to market faster, while saving substantial cost. We believe that we will be in an excellent position to work with them to meet their growing needs as they will try to rush new drugs to market."
CEL-SCI's unique, cold aseptic filling suite can be operated at temperatures between 2 degrees Celsius and room temperatures, including humidity control. The Fill/Finish Facility and the aseptic filling suites have all been designed to meet both FDA and EU ISO classifications. CEL-SCI also has additional capability to formulate, inspect, label and package these products at cold temperatures.
Background on CEL-SCI's "true cold" +4 degrees Celsius Fill/Finish facility:
The fastest area of growth in the Biopharmaceutical and Pharmaceutical market is the area of biologics, most recently the area of stem cell derived products, and in the future biosimilars (copies of biologics). These compounds and therapies are derived from or mimic human cells or proteins and other molecules (e.g., hormones, etc.). Nearly all of the major new billion-dollar-drugs developed for unmet medical needs (e.g., Avastin, Erbitux, Rituxan, Herceptin, Copaxon, etc.) are biologics. Biologics are usually very sensitive to heat and quickly lose their biological activity if exposed to room or elevated temperature for various periods of time. These elevated temperatures may also affect the shelf life of a Biologic product, which means that the product cannot be stored for as long as one might find desirable.
Therefore, CEL-SCI has developed a completely novel technology to be able to accomplish the aseptic Fill/Finish operation at "true cold" +4 degrees Celsius. This is of great importance to companies that produce the latest drugs, biologics and stem cell derived therapies. It is an absolute requirement by FDA and any other regulatory agency that a drug developer must demonstrate the safety, purity and potency of a drug being produced for use in humans.
When aseptically filling a product at CEL-SCI's new facility at +4 degrees Celsius, minimal to no biological losses will have occurred due to the maintenance of cold temperature, and therefore the potency of the drug is maintained throughout this final critical step of the drug's manufacturing process. When the same temperature sensitive drug is instead aseptically filled at room temperature, very expensive and time consuming validation studies must be conducted, first, to be able to obtain a complete understanding of the product's potency loss during the room temperature fill process, then to create solutions to the drug potency losses, which then will also need to be tested and validated. Therefore, CEL-SCI's new "true cold" aseptic fill facility will provide two critical advantages to companies developing biologics and stem cell derived therapies: it will save them a great deal of money (wasted product and extra validations) and it will save them a great deal of time usually spent on the development of these expensive validations.
About IVT:
The Institute of Validation Technology (IVT) Conference Division features the most-notable validation and compliance experts in the FDA regulated industry. IVT stands alone in its quest to continually educate pharmaceutical, biopharmaceutical and medical device professionals in validation concepts from A-Z, AND global GXP regulatory initiatives. Through over 15 years of providing timely, cutting-edge conferences, IVT's philosophy remains that: "each of our events provides valuable take-home knowledge that can be immediately implemented at every level."
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is currently being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
Available Topic Expert(s): For information on the listed expert(s), click appropriate link.
Eyal Talor
Cel-Sci (CVM): While the Cel-Sci stock price has declined back to the fifty cent level after a spike to over a dollar, the company is intent on keeping itself in the news. A recent press release highlighted the company's process that could maintain the shelf life of stem cell drugs.
The real bread and butter for Cel-Sci is Multikine, a head and neck cancer vaccine that is due to begin Phase III trials any time. However, the shelf-life extending manufacturing process and the company's LEAPS technology, although only in early phases of development, may offer additional value to the company, if successful.
CVM is bargain for under a dollar, in my opinion, and I'm still accumulating as long as it remains under that level.
was schreiben die amis noch über Cel Sci?
The real bread and butter for Cel-Sci is Multikine, a head and neck cancer vaccine that is due to begin Phase III trials any time. However, the shelf-life extending manufacturing process and the company's LEAPS technology, although only in early phases of development, may offer additional value to the company, if successful.
CVM is bargain for under a dollar, in my opinion, and I'm still accumulating as long as it remains under that level.
was schreiben die amis noch über Cel Sci?
zwischen 40 und 50 cent kann man gut einsammeln, es wird den zeitpunkt geben in den kommenden jahren, da steht cvm bei 2,50.
wenn ich nochmal nachkaufe,dann irgendwo um 0,30 cent.aber wiee man sieht kann es sehr schnell gehen,bei den kleinen werten...jetzt liegts nur noch am start der phase 3,das nervt.dann sehen wir sicher schnell kurse zwischen 1 und 2 dollar,meine meinung.
schönes wochenende
schönes wochenende
CEL-SCI Signs Definitive Agreement to Raise $5 Million in Registered Direct Offering
Press Release
Source: CEL-SCI Corporation
On Wednesday June 24, 2009, 9:20 am EDT
Buzz up! 0 Print
Companies:Cel-sci corp.
VIENNA, Va., June 24 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE AMEX: CVM), a late stage cancer immunotherapy company, today announced that it has entered into a definitive agreement with one institutional investor to sell 12.5 million units, with each unit consisting of one of the Company's common shares and 0.67 warrants to purchase one share of common stock, for gross proceeds of approximately $5.0 million, before deducting placement agent fees and estimated offering expenses, in a "registered direct" offering. The investor has agreed to purchase the units at a purchase price of $0.40 per unit. The warrants, which represent the right to acquire an aggregate of up to 8.375 million common shares, will be exercisable at any time on or after 181 days from the Closing Date and prior to the 5-year anniversary at an exercise price of $0.50 per share, which was above the closing price of the Company's common shares on the NYSE AMEX Market on June 23, 2009. Chardan Capital Markets, LLC acted as placement agent for the offering.
Related Quotes
Symbol Price Change
CVM 0.40 -0.05
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} The transaction is expected to close on or about June 29, 2009, subject to satisfaction of customary closing conditions.
Geert Kersten, Chief Executive Officer of CEL-SCI, said: "We are planning to use this money to achieve a number of major milestones, key among them are the acceleration of our H1N1 swine flu work and the validation of our manufacturing facility for contract manufacturing services and to produce our cancer drug Multikine for the planned Phase III clinical trial."
The securities described above are being offered by CEL-SCI Corporation pursuant to a registration statement previously filed and declared effective by the Securities and Exchange Commission.
This press release shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state. The securities may be offered only by means of a prospectus. Copies of the final prospectus supplement and accompanying base prospectus relating to this offering may be obtained at the Securities and Exchange Commission's website at www.sec.gov.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is being readied for a global Phase III trial in advancer primary head and neck cancer. CEL-SCI is also developing a vaccine to prevent and treat swine and other influenzas using its L.E.A.P.S. technology platform and expects to soon finish the validation of its state-of-the-art facility in Maryland which it expects to utilize to launch aseptic filling for stem cell produced therapies and other biological products. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
Certain statements contained herein relating to the anticipated closing of the offering or product development, or that otherwise relate to future periods, are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These statements are based on assumptions that may not prove accurate. Actual results could differ materially from those anticipated due to certain risks inherent in the biotechnology industry and for companies engaged in the development of new products in a regulated market. These risks, including those related to whether the offering will close when anticipated or at all, the results of discovery research and preclinical testing; the timing or results of pending and future clinical trials (including the design and progress of clinical trials; safety and efficacy of the products being tested; action, inaction or delay by the FDA, European or other regulators or their advisory bodies; and analysis or interpretation by, or submission to, these entities or others of scientific data); uncertainties regarding the status of biotechnology patents; uncertainties as to the cost of protecting intellectual property; changes in the status of the existing collaborative and licensing relationships; the ability of collaborators, licensees and other third parties to meet their obligations; market demand for products; scale up and marketing capabilities; competition; international operations; share price volatility; and CEL-SCI's financing needs and opportunities are described in more detail in CEL-SCI's most recent annual report on Form 10-K/A and in other SEC filings. Consider such risks carefully in considering CEL-SCI's prospects.
Press Release
Source: CEL-SCI Corporation
On Wednesday June 24, 2009, 9:20 am EDT
Buzz up! 0 Print
Companies:Cel-sci corp.
VIENNA, Va., June 24 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE AMEX: CVM), a late stage cancer immunotherapy company, today announced that it has entered into a definitive agreement with one institutional investor to sell 12.5 million units, with each unit consisting of one of the Company's common shares and 0.67 warrants to purchase one share of common stock, for gross proceeds of approximately $5.0 million, before deducting placement agent fees and estimated offering expenses, in a "registered direct" offering. The investor has agreed to purchase the units at a purchase price of $0.40 per unit. The warrants, which represent the right to acquire an aggregate of up to 8.375 million common shares, will be exercisable at any time on or after 181 days from the Closing Date and prior to the 5-year anniversary at an exercise price of $0.50 per share, which was above the closing price of the Company's common shares on the NYSE AMEX Market on June 23, 2009. Chardan Capital Markets, LLC acted as placement agent for the offering.
Related Quotes
Symbol Price Change
CVM 0.40 -0.05
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} The transaction is expected to close on or about June 29, 2009, subject to satisfaction of customary closing conditions.
Geert Kersten, Chief Executive Officer of CEL-SCI, said: "We are planning to use this money to achieve a number of major milestones, key among them are the acceleration of our H1N1 swine flu work and the validation of our manufacturing facility for contract manufacturing services and to produce our cancer drug Multikine for the planned Phase III clinical trial."
The securities described above are being offered by CEL-SCI Corporation pursuant to a registration statement previously filed and declared effective by the Securities and Exchange Commission.
This press release shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state. The securities may be offered only by means of a prospectus. Copies of the final prospectus supplement and accompanying base prospectus relating to this offering may be obtained at the Securities and Exchange Commission's website at www.sec.gov.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is being readied for a global Phase III trial in advancer primary head and neck cancer. CEL-SCI is also developing a vaccine to prevent and treat swine and other influenzas using its L.E.A.P.S. technology platform and expects to soon finish the validation of its state-of-the-art facility in Maryland which it expects to utilize to launch aseptic filling for stem cell produced therapies and other biological products. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
Certain statements contained herein relating to the anticipated closing of the offering or product development, or that otherwise relate to future periods, are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These statements are based on assumptions that may not prove accurate. Actual results could differ materially from those anticipated due to certain risks inherent in the biotechnology industry and for companies engaged in the development of new products in a regulated market. These risks, including those related to whether the offering will close when anticipated or at all, the results of discovery research and preclinical testing; the timing or results of pending and future clinical trials (including the design and progress of clinical trials; safety and efficacy of the products being tested; action, inaction or delay by the FDA, European or other regulators or their advisory bodies; and analysis or interpretation by, or submission to, these entities or others of scientific data); uncertainties regarding the status of biotechnology patents; uncertainties as to the cost of protecting intellectual property; changes in the status of the existing collaborative and licensing relationships; the ability of collaborators, licensees and other third parties to meet their obligations; market demand for products; scale up and marketing capabilities; competition; international operations; share price volatility; and CEL-SCI's financing needs and opportunities are described in more detail in CEL-SCI's most recent annual report on Form 10-K/A and in other SEC filings. Consider such risks carefully in considering CEL-SCI's prospects.
CEL-SCI Files Patent Application to Support Company's Treatment for More Virulent Strain of H1N1 Swine and Other Influenza Viruses
Press Release
Source: CEL-SCI Corporation
On Wednesday June 24, 2009, 9:25 am EDT
Buzz up! 0 Print
Companies:Cel-sci corp.
VIENNA, Va., June 24 /PRNewswire-FirstCall/ -- CEL-SCI CORPORATION (NYSE AMEX: CVM) announced today that it has filed a provisional U.S. patent application covering its L.E.A.P.S.(TM) immune therapy drugs (vaccines) for the prevention/treatment of H1N1, swine, bird flu, Influenza A and/or evolving mutants or variants of these viruses. Some experts believe that by the next flu season the swine flu virus will have evolved and/or combined with other viruses to create a much more lethal new virus. That is what happened in the case of the Spanish flu pandemic. CEL-SCI's efforts to fight this virus are focused on using conserved epitopes from essential proteins to be found in the A influenza virus for H1N1, H1N5, swine, bird flu and Spanish influenza to create an effective vaccine/treatment that could potentially fight such a mutant virus.
Related Quotes
Symbol Price Change
CVM 0.4050 -0.0450
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} Geert Kersten, Chief Executive Officer of CEL-SCI said, "By filing this provisional patent in the U.S., we are preserving our rights to file patents on these inventions and for their use world-wide either as an injected vaccine before a person is infected or exposed or as a therapeutic vaccine for treatment."
Experimental work has been initiated on these various methods of use and applications for the A influenza vaccines. These L.E.A.P.S. vaccines, when used individually or together, are expected to induce antigen specific immune response(s) which, based on other L.E.A.P.S. animal tests in multiple disease models will hopefully lead to a protective immune response.
The most recent such presentation by an outside university investigator (Dr. Borthakur, University of Hawaii) reported new L.E.A.P.S. Tuberculosis data at the Annual American Society for Microbiology in Philadelphia, PA. This TB data demonstrated that vaccines utilizing the L.E.A.P.S. vaccine technology with specificity for particular Mycobacterium tuberculosis (TB) antigens can elicit immune responses that would be expected to be protective against tuberculosis and have the potential to treat swine and other H1N1 influenzas. The TB investigators presented data that showed that blood and spleen cells from immunized mice produced gamma interferon in response to the vaccine, while the cells from mice in the various control groups did not. Other recent L.E.A.P.S. data, presented by Dr. Kenneth S. Rosenthal, Professor of Microbiology, Immunology and Biochemistry at Northeastern Ohio Universities Colleges of Medicine and Pharmacy and colleagues at the 12th NFID meeting in Baltimore showed that CEL-SCI's L.E.A.P.S. vaccines can activate and cause human blood monocyte cells to become dendritic cells that secrete the IL-12 cytokine. The dendritic cells that result initiate a protective cell mediated and antibody immune response. These results were obtained for L.E.A.P.S. vaccines against herpes simplex and HIV.
The L.E.A.P.S. technology is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S. technology. Each L.E.A.P.S. construct is composed of a T cell binding ligand (TCBL) which has previously demonstrated the ability to induce and elicit protective immunity and antigen specific antibody production in animal models.
The concept behind the L.E.A.P.S. technology is to directly mimic cell/cell interactions on the dendritic and T-cell surface with synthetic peptides. The L.E.A.P.S. constructs containing the antigenic disease epitope linked to an Immune/T-cell binding ligand (I/TCBL) can be manufactured by peptide synthesis or by covalently linking the two peptides. Depending upon the type of L.E.A.P.S. construct and I/TCBL used, CEL-SCI is able to direct the outcome of the immune response towards the development of T-cell function with primarily effector T-cell functions (T Lymphocyte; helper/effector T lymphocyte, type 1 or 2 [Th1 or Th2], cytotoxic [Tc] or suppressor [Ts]). The L.E.A.P.S. vaccine constructs are chimeric peptides which combine antigen specificity with immune response modulation.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is currently being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
Press Release
Source: CEL-SCI Corporation
On Wednesday June 24, 2009, 9:25 am EDT
Buzz up! 0 Print
Companies:Cel-sci corp.
VIENNA, Va., June 24 /PRNewswire-FirstCall/ -- CEL-SCI CORPORATION (NYSE AMEX: CVM) announced today that it has filed a provisional U.S. patent application covering its L.E.A.P.S.(TM) immune therapy drugs (vaccines) for the prevention/treatment of H1N1, swine, bird flu, Influenza A and/or evolving mutants or variants of these viruses. Some experts believe that by the next flu season the swine flu virus will have evolved and/or combined with other viruses to create a much more lethal new virus. That is what happened in the case of the Spanish flu pandemic. CEL-SCI's efforts to fight this virus are focused on using conserved epitopes from essential proteins to be found in the A influenza virus for H1N1, H1N5, swine, bird flu and Spanish influenza to create an effective vaccine/treatment that could potentially fight such a mutant virus.
Related Quotes
Symbol Price Change
CVM 0.4050 -0.0450
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} Geert Kersten, Chief Executive Officer of CEL-SCI said, "By filing this provisional patent in the U.S., we are preserving our rights to file patents on these inventions and for their use world-wide either as an injected vaccine before a person is infected or exposed or as a therapeutic vaccine for treatment."
Experimental work has been initiated on these various methods of use and applications for the A influenza vaccines. These L.E.A.P.S. vaccines, when used individually or together, are expected to induce antigen specific immune response(s) which, based on other L.E.A.P.S. animal tests in multiple disease models will hopefully lead to a protective immune response.
The most recent such presentation by an outside university investigator (Dr. Borthakur, University of Hawaii) reported new L.E.A.P.S. Tuberculosis data at the Annual American Society for Microbiology in Philadelphia, PA. This TB data demonstrated that vaccines utilizing the L.E.A.P.S. vaccine technology with specificity for particular Mycobacterium tuberculosis (TB) antigens can elicit immune responses that would be expected to be protective against tuberculosis and have the potential to treat swine and other H1N1 influenzas. The TB investigators presented data that showed that blood and spleen cells from immunized mice produced gamma interferon in response to the vaccine, while the cells from mice in the various control groups did not. Other recent L.E.A.P.S. data, presented by Dr. Kenneth S. Rosenthal, Professor of Microbiology, Immunology and Biochemistry at Northeastern Ohio Universities Colleges of Medicine and Pharmacy and colleagues at the 12th NFID meeting in Baltimore showed that CEL-SCI's L.E.A.P.S. vaccines can activate and cause human blood monocyte cells to become dendritic cells that secrete the IL-12 cytokine. The dendritic cells that result initiate a protective cell mediated and antibody immune response. These results were obtained for L.E.A.P.S. vaccines against herpes simplex and HIV.
The L.E.A.P.S. technology is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S. technology. Each L.E.A.P.S. construct is composed of a T cell binding ligand (TCBL) which has previously demonstrated the ability to induce and elicit protective immunity and antigen specific antibody production in animal models.
The concept behind the L.E.A.P.S. technology is to directly mimic cell/cell interactions on the dendritic and T-cell surface with synthetic peptides. The L.E.A.P.S. constructs containing the antigenic disease epitope linked to an Immune/T-cell binding ligand (I/TCBL) can be manufactured by peptide synthesis or by covalently linking the two peptides. Depending upon the type of L.E.A.P.S. construct and I/TCBL used, CEL-SCI is able to direct the outcome of the immune response towards the development of T-cell function with primarily effector T-cell functions (T Lymphocyte; helper/effector T lymphocyte, type 1 or 2 [Th1 or Th2], cytotoxic [Tc] or suppressor [Ts]). The L.E.A.P.S. vaccine constructs are chimeric peptides which combine antigen specificity with immune response modulation.
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is currently being readied for a global Phase III trial. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
jetzt gibts wenigstens mal wieder bares...leider bringt uns daß
im kurs nicht weiter,eher belastend
im kurs nicht weiter,eher belastend
aber geld gibts ja auch wieder,zahlt man heut ja auch nicht so ohne wenn und aber...
Antwort auf Beitrag Nr.: 37.481.459 von VFBLER am 28.06.09 10:24:28CEL-SCI to Present Data from Multikine Clinical Trials at National Institutes of Health (NIH), National Cancer Institute (NCI) Seminar Series
CEL-SCI's Presentation will Highlight Multikine's Unique Ability to Utilize The Patient's Own Immune System to Generate a Robust Anti-Tumor Response
Press Release
Source: CEL-SCI Corporation
On Friday July 10, 2009, 8:30 am EDT
Buzz up! 2 Print
Companies:Cel-sci corp.
VIENNA, Va., July 10 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE Amex: CVM), a late stage cancer immunotherapy company, announced today that Dr. Eyal Talor, CEL-SCI's Senior Vice President of Research and Manufacturing, has been invited to present to the NIH, NCI's Clinical Center and the Center for Human Immunology, NERD (New Research and Development) Seminar Series, which will take place on Friday July 10, 2009 at the NIH's Clinical Center located at the main NIH campus, on 9000 Rockville Pike, Bethesda, MD. The title of Dr. Talor's presentation is: "Multikine: Cancer Immunotherapy; how to make immunotherapy for cancer work".
Related Quotes
Symbol Price Change
CVM 0.4012 +0.0201
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} In the presentation, Dr. Talor will discuss Multikine's immunotherapy clinical program for cancer, the unique mechanism of action of Multikine (published in the Journal of Clinical Oncology) and the way Multikine imparts the patient's own immune response with the ability to mount a robust anti-tumor immune response against his/her tumor. Dr. Talor will also discuss the clinical findings from the different Multikine Phase II clinical trials which highlight the advantages that Multikine treatment provides for early disease management in newly diagnosed (treatment naive) patients with Squamous Cell Carcinoma of the Head and Neck. Early intervention in this disease is seen by many clinicians and researchers in the field as a key to improving disease outcome in these patients.
Dr. Talor will also discuss the objectives of the collaborative study between the NIH, NCI under a current Material Transfer Agreement. This work is aimed at elucidating the tumor microenvironment changes, at the molecular level, brought about by Multikine treatment of head and neck cancer patients. The CEL-SCI / NIH collaborative study will use samples collected from patients with advanced primary Head and Neck cancer (Oral Squamous Cell Carcinoma) during the impending Multikine Pivotal Global Phase III trial and these samples will be tested by the NIH, NCI using Genomic Microarray technology, developed at the NIH, NCI laboratories.
About CEL-SCI Corporation
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is being readied for a global Phase III trial in advanced primary head and neck cancer. CEL-SCI is also developing a vaccine to prevent and treat swine and other influenzas using its L.E.A.P.S.(TM) technology platform and expects to soon finish the validation of its state-of-the-art facility in Maryland which it expects to utilize to launch aseptic filling for stem cell produced therapies and other biological products. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
For more information, please visit www.cel-sci.com.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
Available Topic Expert(s): For information on the listed expert(s), click appropriate link.
Eyal Talor
https://profnet.prnewswire.com/Subscriber/ExpertProfile.aspx…
Buzz up! 2
Related Headlines
CEL-SCI to Present Data from Multikine Clinical Trials at National Institutes of Health (NIH), National Cancer Institute (NCI) Seminar Series - PR Newswire
CEL-SCI Corporation Releases Letter to Shareholders - PR Newswire
CEL SCI CORP Files SEC form 8-K, Entry into a Material Definitive Agreement, Financial Statements and Exhibits - EDGAR Online
CEL-SCI Completes $5.85 Million Registered Direct Offering - PR Newswire
CEL SCI CORP Files SEC form 8-K, Entry into a Material Definitive Agreement, Financial Statements and Exhibits - EDGAR Online
Related Blog Headlines
6 More Stock Briefs - at Seeking Alpha
7 More Stock Briefs - at Seeking Alpha
CEL-SCI: A Great Buy Looking for Attention - at Seeking Alpha
Top Stories
GM Chief: Company Will Make Money, Repay Loans - AP
Stocks seesaw as earnings jitters increase - AP
Geithner says derivatives blindsided the gov't - AP
Consumers' mood sours in early July - Reuters
Oil below $60 as traders eye company results - AP
Related Message Boards
Cel-sci corp.
Sponsored Links
Sunnyvale Mom Cures Skin Wrinkles
I wasted more than $1000 before I found Two FREE Products that work.
AskErinTurner.com
Top Finance Jobs 2009 - Medical Billing
Make $90,000 - Free Training Online - Fill Shortage.
StimulusMedicalJobs.comBusiness Finance
$5,000 A Month, Part Time. Take Our Free Tour & Earn Cash Today!
www.Fresh-Off-The-Press.com
Do You Have Financial Worries?
Making Extra Money From Home Is Possible! We Found Out How.
TheUsaDailyNews.comADVERTISEMENT
Tech Ticker Recent Posts
IRS "Turning Over Every Rock" to Raise Revenue: Obama Targeting Overseas Assets - Aaron Task
GM Out Of Bankruptcy - Jay Yarow
USA to UBS: Hand Over Tax Evaders, Or Else! Swiss Bank's American Assets at Risk - Aaron Task
View More »
Subscribe to Topics
Top Stories
Add Alert
cvm Headlines
Add Alert
CEL-SCI's Presentation will Highlight Multikine's Unique Ability to Utilize The Patient's Own Immune System to Generate a Robust Anti-Tumor Response
Press Release
Source: CEL-SCI Corporation
On Friday July 10, 2009, 8:30 am EDT
Buzz up! 2 Print
Companies:Cel-sci corp.
VIENNA, Va., July 10 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE Amex: CVM), a late stage cancer immunotherapy company, announced today that Dr. Eyal Talor, CEL-SCI's Senior Vice President of Research and Manufacturing, has been invited to present to the NIH, NCI's Clinical Center and the Center for Human Immunology, NERD (New Research and Development) Seminar Series, which will take place on Friday July 10, 2009 at the NIH's Clinical Center located at the main NIH campus, on 9000 Rockville Pike, Bethesda, MD. The title of Dr. Talor's presentation is: "Multikine: Cancer Immunotherapy; how to make immunotherapy for cancer work".
Related Quotes
Symbol Price Change
CVM 0.4012 +0.0201
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} In the presentation, Dr. Talor will discuss Multikine's immunotherapy clinical program for cancer, the unique mechanism of action of Multikine (published in the Journal of Clinical Oncology) and the way Multikine imparts the patient's own immune response with the ability to mount a robust anti-tumor immune response against his/her tumor. Dr. Talor will also discuss the clinical findings from the different Multikine Phase II clinical trials which highlight the advantages that Multikine treatment provides for early disease management in newly diagnosed (treatment naive) patients with Squamous Cell Carcinoma of the Head and Neck. Early intervention in this disease is seen by many clinicians and researchers in the field as a key to improving disease outcome in these patients.
Dr. Talor will also discuss the objectives of the collaborative study between the NIH, NCI under a current Material Transfer Agreement. This work is aimed at elucidating the tumor microenvironment changes, at the molecular level, brought about by Multikine treatment of head and neck cancer patients. The CEL-SCI / NIH collaborative study will use samples collected from patients with advanced primary Head and Neck cancer (Oral Squamous Cell Carcinoma) during the impending Multikine Pivotal Global Phase III trial and these samples will be tested by the NIH, NCI using Genomic Microarray technology, developed at the NIH, NCI laboratories.
About CEL-SCI Corporation
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is being readied for a global Phase III trial in advanced primary head and neck cancer. CEL-SCI is also developing a vaccine to prevent and treat swine and other influenzas using its L.E.A.P.S.(TM) technology platform and expects to soon finish the validation of its state-of-the-art facility in Maryland which it expects to utilize to launch aseptic filling for stem cell produced therapies and other biological products. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
For more information, please visit www.cel-sci.com.
When used in this report, the words "intends," "believes," "anticipated" and "expects" and similar expressions are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties which could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include, an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI Corporation's SEC filings, including but not limited to its report on Form 10- K/A for the year ended September 30, 2008. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
Available Topic Expert(s): For information on the listed expert(s), click appropriate link.
Eyal Talor
https://profnet.prnewswire.com/Subscriber/ExpertProfile.aspx…
Buzz up! 2
Related Headlines
CEL-SCI to Present Data from Multikine Clinical Trials at National Institutes of Health (NIH), National Cancer Institute (NCI) Seminar Series - PR Newswire
CEL-SCI Corporation Releases Letter to Shareholders - PR Newswire
CEL SCI CORP Files SEC form 8-K, Entry into a Material Definitive Agreement, Financial Statements and Exhibits - EDGAR Online
CEL-SCI Completes $5.85 Million Registered Direct Offering - PR Newswire
CEL SCI CORP Files SEC form 8-K, Entry into a Material Definitive Agreement, Financial Statements and Exhibits - EDGAR Online
Related Blog Headlines
6 More Stock Briefs - at Seeking Alpha
7 More Stock Briefs - at Seeking Alpha
CEL-SCI: A Great Buy Looking for Attention - at Seeking Alpha
Top Stories
GM Chief: Company Will Make Money, Repay Loans - AP
Stocks seesaw as earnings jitters increase - AP
Geithner says derivatives blindsided the gov't - AP
Consumers' mood sours in early July - Reuters
Oil below $60 as traders eye company results - AP
Related Message Boards
Cel-sci corp.
Sponsored Links
Sunnyvale Mom Cures Skin Wrinkles
I wasted more than $1000 before I found Two FREE Products that work.
AskErinTurner.com
Top Finance Jobs 2009 - Medical Billing
Make $90,000 - Free Training Online - Fill Shortage.
StimulusMedicalJobs.comBusiness Finance
$5,000 A Month, Part Time. Take Our Free Tour & Earn Cash Today!
www.Fresh-Off-The-Press.com
Do You Have Financial Worries?
Making Extra Money From Home Is Possible! We Found Out How.
TheUsaDailyNews.comADVERTISEMENT
Tech Ticker Recent Posts
IRS "Turning Over Every Rock" to Raise Revenue: Obama Targeting Overseas Assets - Aaron Task
GM Out Of Bankruptcy - Jay Yarow
USA to UBS: Hand Over Tax Evaders, Or Else! Swiss Bank's American Assets at Risk - Aaron Task
View More »
Subscribe to Topics
Top Stories
Add Alert
cvm Headlines
Add Alert
ist hier wieder was im busch?heut sehr hohes handelsvolumen...dann kommt meist 1 oder 2 tage später eine meldung.bin mal gespannt...
Der Newsflow ist gut....vielleicht knacken wir die 0,50 US Cent noch. Ein "US Analyst" meinte kürzlich, Biodelivery wäre gut, würde aber stagnieren, er wäre beim Umschichten nach hier. Das Risiko wäre größer, aber überschaubar. Nun,ich bin bei beiden dabei, denn Biodel. hat heute Geld bekommen und Cel hat Super-Aussichten 

heut ist schon wieder dampf drin,über 3 mio gehandelt und erst halbzeit.die meldung kommt bald und cel sc wird explodieren

Hallo Leute,
bin etwas überrascht über den kurs und das Volumen gestern und heute.
Bin normalerweise nicht so euphorisch, denke aber, das da etwas im Busch ist, was eventuell die nächsten Tage bekannt gegeben wird.
Hoffen wir das beste.
Gruss Mimm
bin etwas überrascht über den kurs und das Volumen gestern und heute.
Bin normalerweise nicht so euphorisch, denke aber, das da etwas im Busch ist, was eventuell die nächsten Tage bekannt gegeben wird.
Hoffen wir das beste.
Gruss Mimm
Antwort auf Beitrag Nr.: 37.626.474 von Mimm am 22.07.09 21:19:05 ob Cel. für die HV in München wieder Eintritt haben will ?
ob Cel. für die HV in München wieder Eintritt haben will ?
Kommt jemand aus der Ecke und geht hin ?
Gruß Alfred ( wieder entsperrt )
 ob Cel. für die HV in München wieder Eintritt haben will ?
ob Cel. für die HV in München wieder Eintritt haben will ?Kommt jemand aus der Ecke und geht hin ?
Gruß Alfred ( wieder entsperrt )
Konsolidierung ist okay, aber nicht unter 0,44 bitte
Konso leicht übertrieben, Cel is a great one, don't forget, also bitte 

 jetzt gehts wohl endlich los after hours --- 0,50 ----
jetzt gehts wohl endlich los after hours --- 0,50 ----Who's Afraid of the Big Bad Flu? One company is diligently preparing for the worst case scenario with promising results.
Written by M.E.Garza
Wednesday, 29 July 2009 12:11
“…Off the record, in a deep conversation in a dark bar, a government scientist researching pandemic flu would say that they’re scared to death because they see just how intelligent this virus is…”
Like many of you, over the last few months, I’ve been drawn in by the alarming- often unnerving- headlines about the H1N1 flu pandemic.
“It’s all hype,” some say. “Don’t buy into what the establishment is selling. “
From the start, I couldn’t help myself from suspiciously scoffing at some of the ratings and readership driven headlines.
That all changed for me late last month when I decided to research what all this noise was really about.
Now, frankly, I wish I had been less overcritical and more ostrich-like. Some part of me wishes to rediscover the peace of mind that got lost since my journey to into the darkness of the coming flu pandemic and the brutal truth behind it.
That this is a space about biotech investing reminds me to tell you, my captive audience, that I’m very cognizant of that fact and that this isn’t going to turn into the type of exposé found in a sensational cheap magazine.
Better yet, I can tell you that this company is flying under the same radar screens that point the flock of eager investors into playing the “swine flu stocks.”
As with the rest of story, the truth is, there is much more substance than sizzle here.
As with the rest of the tale, there is also much more terror in the truth than I bargained for.
I realized early on that if I were to get anywhere near the truth about H1N1, my first challenge was to find someone trustworthy and experienced who could help point the way. I needed to clear my already cynical mind from further clouding.
H1N1 Vaccine Shortage Possible
KMSP FOX 9 Minneapolis | Jul. 29, 2009
I began to scour the Centers for Disease Control and Prevention website and found various estimates of flu-related deaths (since apparently there is no true method or count for these deaths) and experts there will tell you that the actual number of deaths vary greatly from year to year because flu seasons often fluctuate in length and severity.
I found alarming press clippings from the U.S. Military stating that they are confident that years of pandemic planning will help it deal with H1N1. Those of us who watched the events surrounding Hurricane Katrina and our national government’s reactions can rest easy now, right?
I dug deeper.
I connected to someone who has spent twenty-five years working on biopharmaceutical development and regulatory issues in the U.S. He also spent the last quarter of a century working on various therapeutic and preventative platforms as well as vaccines, but he would only speak to me on the record under strict conditions of anonymity.
Having taken part in many collaborations with the government at various levels with several agencies, he wanted the public to know the truth, but he was also not thrilled by the idea of being castigated or confused in any way with chicken little.
After a brief pause, I slipped on my best Woodward and Bernstein and told him I’d agree to his conditions.
“The biggest concern is to ask ourselves what this is and how is it being addressed, “ he began.
“ There are some great limitations of existing technologies and approaches that are being used currently and that have been used historically. Where they’ve got the biggest challenge from a public health perspective is that these methods are all backwards looking, and we’re using a strain of this virus that is a strain that was identified at the end of April in California.”
While that’s not unimportant – since this is the strain of the virus that is currently plaguing the United States and pretty much the entire rest of the globe- the CDC and the scientific community have pointed out consistently that, as is always the case with influenza generally, this virus is inherently unstable. It mutates.
“That’s precisely why we’ve got this particular strain- one that, despite it being summer in the northern hemisphere- is still propagating and still causing significant morbidity and a fair amount of mortality, which is highly unusual,” he explained calmly.
“Obviously it’s also circulating in the Southern Hemisphere, but at some point, consistent with the variations that we’ve already seen with this virus in terms of Tamiflu resistance, this virus is going to- for lack of a better word- mutate again and how it will change and how it will present itself going forward is totally unpredictable.”
What is known about influenza viruses is that as they evolve and when they genetically alter through the process of resortment , natural selection principles tell us that the viruses look to alter in ways that continue to maintain their ease of transmissibility and certainly their morbidity potential.
“In an ideal world, the ideal virus doesn’t try to wreak mass mortality because at some point if it’s killing its hosts, its own ability to survive is compromised, but this virus obviously has been smart enough to figure out how to survive in the summer heat when it usually survives in the winter cold. It has figured out how to transmit from human to human on a global basis faster than any influenza virus before. Part of that is a reality of the current global marketplace and its global transportation modes, but it’s also a reflection of the virus.”
This guy may have been shy, but he didn’t mince any words.
“There are obviously other influenza viruses on the planet that are known and some that are probably unknown and as, we look forward, all the approaches right now are focused on that California strain- which in essence is the same strain that originated down in Mexico. As we look forward, as this virus continues to circle the globe, as it interfaces in humans and more importantly in pigs, there is a significant chance that it will evolve and could soon have greater morbidity effects and far worse mortality effects on the global population, comparable to what happened in 1918 – which also involved an H1N1 virus. With the last recirculating H1N1 being in 1957, the global population’s level of natural immunity may be assumed to be low.”
In looking at the approaches on the table today, scientists and pharmaceutical companies are simply trying to give us vaccines that look at building up immunity against only that original strain, and while that is all well and good, a basic problem remains.
“It’s a critically-important step that must be taken but it’s still backwards looking, not forward looking, and those vaccines cannot and do not anticipate future variations that occur through natural selection, especially as a result of resortment. So in essence, we hope to have a vaccine that will protect against this virus as it was discovered in late-April and exists now, but it’s ability to protect against the evolution of this virus is pretty much non-existent- especially if we end up with yet another entirely new strain that has greater virulence.”
That sobering, if not shocking dose of the truth led me to dig further and eventually to the doorsteps of the CEL-SCI Corporation (Amex: CVM) and it’s CEO, Geert Kersten.
Kersten has been with CEL-SCI from the early days of its inception in the late 80’s. And for better or worse, he’s been involved in the pioneering field of cancer immunotherapy for almost two decades, through its many challenging cycles.
“The government needs to continue doing what it’s doing, because that’s their job,” says Kersten in a calm voice layered with his thick, native German accent. “But at the same time, no one is addressing the issue of a more dangerous, yet very likely, mutated virus. What happens when it stops responding to Tamiflu or Relenza ? ”
Good questions, indeed.
Just five years ago, Relenza- the influenza treatment developed in Australia- was headed for the drug scrapheap. Today, as the world scrambles to cope with the flu pandemic, suddenly Relenza is headed for "blockbuster" status and that’s a term which is reserved for drugs that generate over $1 billion a year in sales. Mere days ago there were news headlines that GlaxoSmithKline (NYSE:GSK) is attempting to triple its global capacity in order to produce Relenza. Those efforts are going to justify its blockbuster tag with ease, but that’s only going to work for so long.
“If something knocks out the virus’ ability to survive, it will, by definition, following ‘selfish gene’ theory, continue to evolve and figure out a way to work around that imposition.” I am reminded by my biologic informant.
In other words, it will genetically modify itself and circumvent the vaccines, as well as the Tamiflus and Relenzas.
“Since this is such a doomsday scenario, shouldn’t we be taking out some insurance?” asks CEL-SCI’s Kersten. “
CEL-SCI’s preclinical studies have demonstrated that vaccines utilizing its proprietary L.E.A.P.S. (Ligand Epitope Antigen Presentation System) technology potentially induce protection against illnesses such as the swine influenza. L.E.A.P.S. helps direct the immune response to the vaccine epitope.
What his company’s technology proposes is the ability to attack what comes out of those mutations the moment it shows up, as opposed to what we have now.
“The idea is very simple, if I’m looking for a bad guy and I know that bad guy is going to undergo facial reconstruction or hair color changes, the only way I’m going to be able to capture that bad guy is by focusing on parts of that person that cannot be changed,” explains Kersten.
“While he can wear colored contact lenses on his eyes, he can’t actually change his eyes. So if I have a biometric measurement machine that checks for eyes as he walks past it, then no matter what he’s done, he cannot escape from me. Much like that analogy, there are various parts of the virus that are central to its survival. The virus mutates in order to defend itself and those regions which mutate are actually the easiest target for vaccine development. They ‘stand out.’ It’s almost like a ruse that the virus creates to divert you from parts of itself that are important to survival, so the current vaccine development focuses on the easy targets and you get strong immune responses to them, but then the virus mutates and the vaccine doesn’t work anymore.
“Our work is different. We focus on parts of the virus that may not make for targets that are as easy as the current ones but they cannot change because if they change the virus simply dies. So if you now make a combination of these parts and you take them from the current swine flu, you move forward knowing that the mutated swine flu will have the same parts that will remain unchanged. Then you add to that some parts from, say, the current avian flu. And you take another from the Spanish flu [the 1918 pandemic also was caused by an H1N1]. You end up with several peptides mixed to form one vaccine which should provide at least partial protection and treatment against a mutated virus which itself has taken different pieces from different viruses and put them together.”
The key focus of the L.E.A.P.S. technology approach is that it’s selecting common regions across a range of viruses that through the course of time have shown high virulence, high morbidity, and high mortality and then helps the immune system process these regions in a manner that will lead to clinical benefit, whether that is prevention or treatment. It uses non changing parts of the viruses as a base for constructing a treatment paradigm or a preventative paradigm. The technology could potentially be used in either or both contexts. By having those common foundational elements, there is a much greater likelihood to be able to catch up , stay current with and even at some levels stay ahead of the virus as it goes through both global circulation and a resortment process, especially in the context of H5N1 or any of the other influenzas that we know about, or more importantly that we don’t know about.
That work is not that expensive. It involves peptides and other technologies that, for a little bit of money, could potentially forestall an apocalyptic type of disaster.
“One of the fascinating things that the media and most everyone else pays no attention to is that early on, very early on, the CDC knew that there were problems growing this strain of virus used for the conventional vaccine approach now being ramped up for swine flu,” says my informant. “ The low yields and long time to production should not be news to anyone in the know. The current vaccine manufacturers have come out and said, ‘hey we’re getting low yields and its taking us a long time to produce that low-yielding vaccine,’ but insiders have known this since last April.
“This gets back to just how smart a virus we’re dealing with. To boil it down to simplistic almost cartoonish terms, this thing has figured out how to survive the environmental conditions in which influenza viruses have never survived, namely heat and humidity.
“It is contrary to all of influenza history to see a virus surviving the way this one is. It knows enough that vaccines- ever since we started creating vaccines- are produced in eggs and it’s genetic code knows enough not to allow a vaccine to be produced in eggs quickly. This is not by chance that we’ve got this problem. This all gets back to a virus that has been around for hundreds of years and through hundreds of years of experience has morphed itself into something that is wary of being trapped or deceived.
“It has figured out a way to continue its propagation almost unimpeded and that would suggest that off the record, in a deep conversation in a dark bar, a government scientist researching pandemic flu would say that they’re scared to death because they see just how intelligent this virus is, they see what it’s done already and they know its potential. It’s ability to have done what it’s already done suggests a genetic intelligence- a genetic code internal to the virus- that will allow it to adapt in ways in which we have not seen influenza adapt before. “
There are many more reasons to like CEL-SCI for the work they’re doing in the influenza space and beyond.
In the coming days I’ll continue to explore those reasons with you.
In the meantime, stay tuned and informed, and if you can help it all- stay protected.
Disclosure: No Positions
Antwort auf Beitrag Nr.: 37.675.363 von ALF-FRED am 30.07.09 09:46:11Hoert sich gut an...
Gruesse,
Sil

Gruesse,
Sil
hab mich nachbörslich eingekauft -
der artikel steht auf JEDER seite in den verschiedenen
us-boards...ich glaube, dass gibt nen riesen hype in den
nächsten tagen...
und ich gehöre eher zu den konservativen biotech-investoren
der artikel steht auf JEDER seite in den verschiedenen
us-boards...ich glaube, dass gibt nen riesen hype in den
nächsten tagen...
und ich gehöre eher zu den konservativen biotech-investoren

Antwort auf Beitrag Nr.: 37.675.698 von Gustl24 am 30.07.09 10:27:00

danke für den tipp !
was meinst Du, geht hier ?
LG


danke für den tipp !
was meinst Du, geht hier ?
LG
Also schluss war 44 cent das wären dann umgerechnet
31 cent ca.
und wenn wir die pre Marketkurse ansehen die stehen bei 50 cent
umgerechnet 35,4 cent!! und hier wird 41 cent bezahlt finder ich schon recht heftig!
31 cent ca.
und wenn wir die pre Marketkurse ansehen die stehen bei 50 cent
umgerechnet 35,4 cent!! und hier wird 41 cent bezahlt finder ich schon recht heftig!
Hallo alle,
Servus Gustl...so hab mich eingelesen in die Story und mal was gesetzt...paar K können nicht schaden...hat zwar gut vorgelegt in D...sollte aber kein Problem sein...was ich so gelesen habe...lass mich mal positiv überraschen
Servus Gustl...so hab mich eingelesen in die Story und mal was gesetzt...paar K können nicht schaden...hat zwar gut vorgelegt in D...sollte aber kein Problem sein...was ich so gelesen habe...lass mich mal positiv überraschen
Scheint interessant zu werden...geht ja schon was im Pre Bereich
mal sehen ob ich eine weitere Posi etws tiefer noch ziehen kann
NASDAQ Last Sale
0.50 0.06 ++13.64%
Volume
3,166,152 Previous Close
$ 0.44
Today's High
$ 0.52 Today's Low
$ 0.38
mal sehen ob ich eine weitere Posi etws tiefer noch ziehen kann
NASDAQ Last Sale
0.50 0.06 ++13.64%
Volume
3,166,152 Previous Close
$ 0.44
Today's High
$ 0.52 Today's Low
$ 0.38
Antwort auf Beitrag Nr.: 37.677.116 von Expertchen007 am 30.07.09 12:51:03Musss ich korrigieren...Kurs ist After Hour...nicht PRE Markt
So hier gibts denn 1. Kurs PRE
NASDAQ Last Sale
0.56 0.12 +++27.27%
NASDAQ Last Sale
0.56 0.12 +++27.27%
Kursveraluf...alles drin heute von 0,40 -0,65 eine riesen Range
Pre-Market
Last: $ .49 Pre-Market
High: $ .57
Pre-Market
Volume: 61,450 Pre-Market
Low: $ .49
Pre-Market
Last: $ .49 Pre-Market
High: $ .57
Pre-Market
Volume: 61,450 Pre-Market
Low: $ .49
wenn das Nievau vom Pre bis zur Eröffnung hält,
leiste ich mir auch ne kleine Posi, kann leider
nur zur regulären Zeit traden
leiste ich mir auch ne kleine Posi, kann leider
nur zur regulären Zeit traden
Antwort auf Beitrag Nr.: 37.677.772 von 3ckv am 30.07.09 13:58:20Schwer zu sagen was passiert...kommt drauf an was die Company selbst jetzt bringt...bei der Geschichte...gabs einige Hypes..aber auch gewaltige Tiefschläge...mal abwarten
So jetzt kommt langsam Leben in den Kurs...sollte an der Story was dran sein ist es wohl egal ob ein EK bei 0,30 oder bei 0,45 € liegt...da hatt der eine dann halt XXX % und der andere paar mehr...alles relativ...aber bleibt alles abzuwarten....aber was man so liest ...Hmmmm ...vieles möglich. Gab auch schon mehrere Storys um das Theman...leider gings dort in die Hose....bin mal gespannt
Antwort auf Beitrag Nr.: 37.677.975 von Expertchen007 am 30.07.09 14:23:16bist Du schon drin ?

pre market 0,55
Antwort auf Beitrag Nr.: 37.678.081 von ALF-FRED am 30.07.09 14:33:52http://www.nasdaq.com/aspxcontent/ExtendedTradingTrades.aspx…
jungs drüben pushen ding schon vorbörslich auf 30%

Antwort auf Beitrag Nr.: 37.678.022 von Mato3118 am 30.07.09 14:28:26Ja mit 2 Positonen bereits eingestiegen...aber relativ hoch...sollte aber egal sein wenns den klappt...mal sehen...
Antwort auf Beitrag Nr.: 37.678.130 von Expertchen007 am 30.07.09 14:38:35ok, Du alter Bio-Zocker

Antwort auf Beitrag Nr.: 37.678.120 von split66 am 30.07.09 14:38:02Da pusht niemand...es kommt nur darauf an welche Wahrheit dahintersteckt...somit wäre das kein Push sondern ein billiger Kurs...kann genauso gut in die Hose gehen
Jetzt kommt Volumen rein in die Story
NASDAQ Last Sale
0.58 0.14 +++ 31.82%
Volume
866,375 Previous Close
$ 0.44
Today's High
$ 0.59 Today's Low
$ 0.49
NASDAQ Last Sale
0.58 0.14 +++ 31.82%
Volume
866,375 Previous Close
$ 0.44
Today's High
$ 0.59 Today's Low
$ 0.49
Antwort auf Beitrag Nr.: 37.678.285 von Expertchen007 am 30.07.09 14:52:47ich sehe hier 1,7 mio umsatz
http://data.cnbc.com/quotes/CVM
http://data.cnbc.com/quotes/CVM
Will ja jetzt nicht jeden cent bejubeln...aber setzt meine obere Range im Kurs für heute mal etwas hoch in Richtung 0,77 US$...kann durchaus sein...im Moment ist bei Biotec alles möglich
NASDAQ Last Sale
0.59 0.15 +++34.09%
NASDAQ Last Sale
0.59 0.15 +++34.09%
Antwort auf Beitrag Nr.: 37.678.285 von Expertchen007 am 30.07.09 14:52:47wenn ich nur wüßte, ob das nicht nur wieder vorbörsliches
Hochgezocke ist ???
Hochgezocke ist ???

Antwort auf Beitrag Nr.: 37.678.308 von split66 am 30.07.09 14:55:31Stimmt sorry war nicht aktuell...geht bereits in die 2 Millionen ran
Antwort auf Beitrag Nr.: 37.678.349 von Mato3118 am 30.07.09 14:58:31Das werden wir heute Abend wissen,...nur sei Dir im klaren wenn da eine Bestätigung seitens Cel kommt...dannn gehts im Kurs mehr als rund ...aber auch drauf einstellen das alles nicht so war 

Antwort auf Beitrag Nr.: 37.678.364 von Expertchen007 am 30.07.09 15:00:13

Was mit an der Story gefällt ist das es nur wenige Zocker im Moment wissen was läuft....das wird sich wohl bald ändern...noch ist CEL mehr oder weniger ein kleiner Fisch im Becken
Antwort auf Beitrag Nr.: 37.678.376 von Mato3118 am 30.07.09 15:00:56Eröffnung schätze ich auf die 0,55 US$...danach muss was seitens CEl kommen...werd mal nachschauen
Antwort auf Beitrag Nr.: 37.678.496 von Expertchen007 am 30.07.09 15:09:41 wenn sie was bringen, dann um 16.00 MEZ - Cel.Sci macht das immer eine halbe Std. nach eröffnung der Amibörse
wenn sie was bringen, dann um 16.00 MEZ - Cel.Sci macht das immer eine halbe Std. nach eröffnung der Amibörse 
 wenn sie was bringen, dann um 16.00 MEZ - Cel.Sci macht das immer eine halbe Std. nach eröffnung der Amibörse
wenn sie was bringen, dann um 16.00 MEZ - Cel.Sci macht das immer eine halbe Std. nach eröffnung der Amibörse 
Nix neues ausser der Artikel..war allerdings heute früh bereits drin
http://seekingalpha.com/article/152378-cel-sci-s-flu-work-di…
http://seekingalpha.com/article/152378-cel-sci-s-flu-work-di…
Antwort auf Beitrag Nr.: 37.678.605 von ALF-FRED am 30.07.09 15:18:38Danke für den Hinweis..na dann warten wir mal...kann auch heut noch nix kommen...nun gut ich werds erfahren
Es kann losgehen:


Ziemlich Volumen drin ..aber noch keine Tendenz
Gewinnmitnahmen...na klar hätt ich auch gemacht !!! Muss allerdings wohl bisschen warten...
Antwort auf Beitrag Nr.: 37.678.886 von Expertchen007 am 30.07.09 15:39:41Geht wohl doch etwas gegen süden. ALles nur Gewinnmitnahmen? Glaub nicht ...
Antwort auf Beitrag Nr.: 37.678.937 von daytrader_100 am 30.07.09 15:44:11Sind schon Mitnahmen...aber wurde auch hoch gespielt....da muss was vom Unternehmen kommen...aber ist ne gute Möglichkeit hier...würd mal sagen 70 % Chance....bleibt aber spekultiv...bin zwar 10-15 % im minus...aber das Risiko muss drin sein....lass ich mal 4 Wochen stehen...
Antwort auf Beitrag Nr.: 37.678.982 von Expertchen007 am 30.07.09 15:47:47Na mal abwarten was da noch so kommt ...
Würd mal sagen so bis 16 Uhr waren die Mitnahmen erst mal beendet..alles danach dürften Leute sein die jetzt Einsteigen...und auf die News hoffen ...aif jeden Fall laufen die letzen 30 Minuten mehr Käufe ein
Antwort auf Beitrag Nr.: 37.679.909 von Expertchen007 am 30.07.09 17:03:46Hallo zusammen,
also ich fürchte leider, dass das wieder mal nur ein Strohfeuer sein wird, sind ja eigentlich auch keine wirklichen "News".
Wenn nicht bald P3 gestartet wird oder Geld über die neue Fabrik rein kommt, wird das Geld knapp.
Alles mit der L.E.A.P.S. technologie ist "nice to have" aber wenn man es nüchtern betrachtet, hat da keiner wirkliches Interesse dran im Moment und ich glaube auch nicht, das CVM das Geld und vor allem die Zeit hat etwas in einem sinnvollen Zeitrahmen entwickeln zu können, leider.
Wobei ich natürlich immer dafür bin, wenn es aufwärts geht
@ Expertchen007 auf welcher Seite siehst du die Verkauf/Ankaufszahlen ?
Realtimequotes find ich viele, aber wieviele buyer oder seller am Markt sind weiss ich nicht.
Gruss Mimm
also ich fürchte leider, dass das wieder mal nur ein Strohfeuer sein wird, sind ja eigentlich auch keine wirklichen "News".
Wenn nicht bald P3 gestartet wird oder Geld über die neue Fabrik rein kommt, wird das Geld knapp.
Alles mit der L.E.A.P.S. technologie ist "nice to have" aber wenn man es nüchtern betrachtet, hat da keiner wirkliches Interesse dran im Moment und ich glaube auch nicht, das CVM das Geld und vor allem die Zeit hat etwas in einem sinnvollen Zeitrahmen entwickeln zu können, leider.
Wobei ich natürlich immer dafür bin, wenn es aufwärts geht

@ Expertchen007 auf welcher Seite siehst du die Verkauf/Ankaufszahlen ?
Realtimequotes find ich viele, aber wieviele buyer oder seller am Markt sind weiss ich nicht.
Gruss Mimm
Irgendwie ist mit Biotech heut nix los...alles down..( nun gut cel im plus )
Antwort auf Beitrag Nr.: 37.680.123 von Mimm am 30.07.09 17:26:40Realtimequotes find ich viele, aber wieviele buyer oder seller am Markt sind weiss ich nicht.
Sehe ich über http://de.advfn.com
Bei Amex....40,1 % Käufe zu 30,2 % Verkäufe ...Rest vermutlich Shorts....also gut ausgeglichen....bleibt mal alles abzuwarten was geht oder nicht....klar ich hätte die Teile auch billiger haben können...aber wenn was kommt sollte das egal sein...ansonsten sind halt paar hunderter kaputt..aber gehört dazu
Sehe ich über http://de.advfn.com
Bei Amex....40,1 % Käufe zu 30,2 % Verkäufe ...Rest vermutlich Shorts....also gut ausgeglichen....bleibt mal alles abzuwarten was geht oder nicht....klar ich hätte die Teile auch billiger haben können...aber wenn was kommt sollte das egal sein...ansonsten sind halt paar hunderter kaputt..aber gehört dazu
Antwort auf Beitrag Nr.: 37.680.123 von Mimm am 30.07.09 17:26:40Hier auch das Orderbuch NASDAQ Level II....CVM eingeben
http://www.level2stockquotes.com/level-ii-quotes.html
http://www.level2stockquotes.com/level-ii-quotes.html
125 000 im Bid auf der 0,5050 US$...ne Menge Holz..muss erst mal weg
bin heut zu 0,5 usd rein
Who's Afraid of the Big Bad Flu? One company is diligently preparing for the worst case scenario with promising results
http://biomedreports.com/component/content/article/3544-whos…
Who's Afraid of the Big Bad Flu? One company is diligently preparing for the worst case scenario with promising results
http://biomedreports.com/component/content/article/3544-whos…
Antwort auf Beitrag Nr.: 37.681.504 von Expertchen007 am 30.07.09 20:07:39Danke für die Infos, muss mich erstmal schlau machen, wie die Daten genau zu lesen sind.
Aber leider geht es wieder mal mit CEL bergab, hoffe nur, es bleibt ein kleines Plus heute übrig.
Gruss Mimm
Aber leider geht es wieder mal mit CEL bergab, hoffe nur, es bleibt ein kleines Plus heute übrig.
Gruss Mimm
Antwort auf Beitrag Nr.: 37.681.863 von Mimm am 30.07.09 20:49:21Gap geschlossen - Jetzt gehts wieder aufwärts. 

kann mir ein "insider" kurzgefasst paar infos zu
pIII des krebsmedikaments, der verlauf der studien in der vergangenheit und der weiteren prognose geben...
zieht das CVM allein durch, gibt es partnerschaften etc.,
mögliches potential des medikaments etc.vielen dank gustl
pIII des krebsmedikaments, der verlauf der studien in der vergangenheit und der weiteren prognose geben...
zieht das CVM allein durch, gibt es partnerschaften etc.,
mögliches potential des medikaments etc.vielen dank gustl
 cel. weiß von nix - schreiben sie jedenfalls im Newsletter
cel. weiß von nix - schreiben sie jedenfalls im NewsletterJuly 30, 2009
Dear Fellow Shareholder:
A number of you have called this morning to ask why our CEL-SCI stock (NYSE AMEX: CVM) is showing so much activity. While we cannot be sure, we believe that this new interest in CEL-SCI comes from a story that was published by a leading healthcare website, BioMedReports.com. The article is available at:
http://biomedreports.com/articles/most-popular/3544-whos-afr…
I hope that this communication is helpful to you.
Sincerely,
Geert Kersten
Chief Executive Officer
ja, dann wars wohl ein Sturm im Wasserglas
oder was meint Ihr?
steckt villeicht doch noch was dahinter?
oder was meint Ihr?
steckt villeicht doch noch was dahinter?

Antwort auf Beitrag Nr.: 37.684.692 von 3ckv am 31.07.09 10:27:10Schwer zu sagen. Der Text ist ewig lang und auf arbeit ist schwierig sich das mal reinzuziehen. Hoffen wir das was dran ist und nicht nur nen Sturm im Wasserglas...
der gleiche artikel von biomedreports nochmals
heute bei marketwire erschienen -
nur mit noch optimistischerer headline:
Biotech Drug Company Preparing for Worst Case H1N1 Flu Scenario Using Extremely Promising New Technology (NYSE Amex: CVM)
schau mer mal, obs was bringt in sachen kurs...
steter tropfen...
we will see
heute bei marketwire erschienen -
nur mit noch optimistischerer headline:
Biotech Drug Company Preparing for Worst Case H1N1 Flu Scenario Using Extremely Promising New Technology (NYSE Amex: CVM)
schau mer mal, obs was bringt in sachen kurs...
steter tropfen...
we will see

Biotech Drug Company Preparing for Worst Case H1N1 Flu Scenario Using Extremely Promising New Technology (NYSE Amex: CVM)
"...Off the record, in a deep conversation in a dark bar, a government scientist researching pandemic flu would say that they're scared to death because they see just how intelligent this virus is..."
Press Release
Source: BioMedReports
On Friday July 31, 2009, 7:00 am EDT
Buzz up! 0 Print
Companies:CEL-SCI Corp.
LOS ANGELES, CA--(Marketwire - 07/31/09) - BioMedReports, the news portal covering the biomedical news and financial sector, in a special report about the H1N1 flu pandemic, is reporting that CEL-SCI Corporation (AMEX:CVM - News) is positioned to help combat the virus after diligently preparing for the worst case scenario with promising results.
Related Quotes
Symbol Price Change
CVM 0.46 0.00
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} CEL-SCI's preclinical studies have demonstrated that vaccines utilizing its proprietary L.E.A.P.S. (Ligand Epitope Antigen Presentation System) technology potentially induce protection against illnesses such as the swine influenza.
L.E.A.P.S. helps direct the immune response to the vaccine epitope and company scientists have been working to ensure that it will be available should the virus continue to spread and mutate.
"What this company's technology proposes is the ability to attack what comes out of the Swine Flu's mutations the moment it shows up, as opposed to what we have now," reports M.E. Garza of BioMedReports.
"The idea is very simple, if I'm looking for a bad guy and I know that bad guy is going to undergo facial reconstruction or hair color changes, the only way I'm going to be able to capture that bad guy is by focusing on parts of that person that cannot be changed," explains CEL-SCI's CEO Geert Kersten as he describes the dangerous influenza virus.
While some drug makers like Novartis AG, Sanofi-Aventis, Baxter, Novavax, Inc. and others are stockpiling vaccines and other treatments, government officials feel that may not be enough and it has been reported recently that there may even be a shortage of vaccines.
The alarming new special report titled "Who's Afraid of the Big Bad Flu" appears now at BioMedReports.com:
http://biomedreports.com/component/content/article/3544-whos… scenario.html
Biotech investors interested in accessing the complete database of clinical trials and upcoming FDA decisions can access that information on the same site:
http://biomedreports.com/fda-calendar/fda-calendar.html
About BioMedReports.com
BioMedReports.com is a news portal covering the biomedical news and financial sector. It features stock research reports, news, videos, stock commentaries, and more -- including FDA and Clinical Trial Calendars.
Disclosure: No positions
Contact:
Contact:Mike HavrillaManaging EditorBioMedReports.comEmail Contact323-472-4480 Phone888-210-3556 Fax
"...Off the record, in a deep conversation in a dark bar, a government scientist researching pandemic flu would say that they're scared to death because they see just how intelligent this virus is..."
Press Release
Source: BioMedReports
On Friday July 31, 2009, 7:00 am EDT
Buzz up! 0 Print
Companies:CEL-SCI Corp.
LOS ANGELES, CA--(Marketwire - 07/31/09) - BioMedReports, the news portal covering the biomedical news and financial sector, in a special report about the H1N1 flu pandemic, is reporting that CEL-SCI Corporation (AMEX:CVM - News) is positioned to help combat the virus after diligently preparing for the worst case scenario with promising results.
Related Quotes
Symbol Price Change
CVM 0.46 0.00
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} CEL-SCI's preclinical studies have demonstrated that vaccines utilizing its proprietary L.E.A.P.S. (Ligand Epitope Antigen Presentation System) technology potentially induce protection against illnesses such as the swine influenza.
L.E.A.P.S. helps direct the immune response to the vaccine epitope and company scientists have been working to ensure that it will be available should the virus continue to spread and mutate.
"What this company's technology proposes is the ability to attack what comes out of the Swine Flu's mutations the moment it shows up, as opposed to what we have now," reports M.E. Garza of BioMedReports.
"The idea is very simple, if I'm looking for a bad guy and I know that bad guy is going to undergo facial reconstruction or hair color changes, the only way I'm going to be able to capture that bad guy is by focusing on parts of that person that cannot be changed," explains CEL-SCI's CEO Geert Kersten as he describes the dangerous influenza virus.
While some drug makers like Novartis AG, Sanofi-Aventis, Baxter, Novavax, Inc. and others are stockpiling vaccines and other treatments, government officials feel that may not be enough and it has been reported recently that there may even be a shortage of vaccines.
The alarming new special report titled "Who's Afraid of the Big Bad Flu" appears now at BioMedReports.com:
http://biomedreports.com/component/content/article/3544-whos… scenario.html
Biotech investors interested in accessing the complete database of clinical trials and upcoming FDA decisions can access that information on the same site:
http://biomedreports.com/fda-calendar/fda-calendar.html
About BioMedReports.com
BioMedReports.com is a news portal covering the biomedical news and financial sector. It features stock research reports, news, videos, stock commentaries, and more -- including FDA and Clinical Trial Calendars.
Disclosure: No positions
Contact:
Contact:Mike HavrillaManaging EditorBioMedReports.comEmail Contact323-472-4480 Phone888-210-3556 Fax
Biotech Drug Company Preparing for Worst Case H1N1 Flu Scenario Using Extremely Promising New Technology (NYSE Amex: CVM)
"...Off the record, in a deep conversation in a dark bar, a government scientist researching pandemic flu would say that they're scared to death because they see just how intelligent this virus is..."
Press Release
Source: BioMedReports
On Friday July 31, 2009, 7:00 am EDT
Buzz up! 0 Print
Companies:CEL-SCI Corp.
LOS ANGELES, CA--(Marketwire - 07/31/09) - BioMedReports, the news portal covering the biomedical news and financial sector, in a special report about the H1N1 flu pandemic, is reporting that CEL-SCI Corporation (AMEX:CVM - News) is positioned to help combat the virus after diligently preparing for the worst case scenario with promising results.
Related Quotes
Symbol Price Change
CVM 0.50 +0.04
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} CEL-SCI's preclinical studies have demonstrated that vaccines utilizing its proprietary L.E.A.P.S. (Ligand Epitope Antigen Presentation System) technology potentially induce protection against illnesses such as the swine influenza.
L.E.A.P.S. helps direct the immune response to the vaccine epitope and company scientists have been working to ensure that it will be available should the virus continue to spread and mutate.
"What this company's technology proposes is the ability to attack what comes out of the Swine Flu's mutations the moment it shows up, as opposed to what we have now," reports M.E. Garza of BioMedReports.
"The idea is very simple, if I'm looking for a bad guy and I know that bad guy is going to undergo facial reconstruction or hair color changes, the only way I'm going to be able to capture that bad guy is by focusing on parts of that person that cannot be changed," explains CEL-SCI's CEO Geert Kersten as he describes the dangerous influenza virus.
While some drug makers like Novartis AG, Sanofi-Aventis, Baxter, Novavax, Inc. and others are stockpiling vaccines and other treatments, government officials feel that may not be enough and it has been reported recently that there may even be a shortage of vaccines.
The alarming new special report titled "Who's Afraid of the Big Bad Flu" appears now at BioMedReports.com:
http://biomedreports.com/component/content/article/3544-whos… scenario.html
Biotech investors interested in accessing the complete database of clinical trials and upcoming FDA decisions can access that information on the same site:
http://biomedreports.com/fda-calendar/fda-calendar.html
About BioMedReports.com
BioMedReports.com is a news portal covering the biomedical news and financial sector. It features stock research reports, news, videos, stock commentaries, and more -- including FDA and Clinical Trial Calendars.
Disclosure: No positions
"...Off the record, in a deep conversation in a dark bar, a government scientist researching pandemic flu would say that they're scared to death because they see just how intelligent this virus is..."
Press Release
Source: BioMedReports
On Friday July 31, 2009, 7:00 am EDT
Buzz up! 0 Print
Companies:CEL-SCI Corp.
LOS ANGELES, CA--(Marketwire - 07/31/09) - BioMedReports, the news portal covering the biomedical news and financial sector, in a special report about the H1N1 flu pandemic, is reporting that CEL-SCI Corporation (AMEX:CVM - News) is positioned to help combat the virus after diligently preparing for the worst case scenario with promising results.
Related Quotes
Symbol Price Change
CVM 0.50 +0.04
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} CEL-SCI's preclinical studies have demonstrated that vaccines utilizing its proprietary L.E.A.P.S. (Ligand Epitope Antigen Presentation System) technology potentially induce protection against illnesses such as the swine influenza.
L.E.A.P.S. helps direct the immune response to the vaccine epitope and company scientists have been working to ensure that it will be available should the virus continue to spread and mutate.
"What this company's technology proposes is the ability to attack what comes out of the Swine Flu's mutations the moment it shows up, as opposed to what we have now," reports M.E. Garza of BioMedReports.
"The idea is very simple, if I'm looking for a bad guy and I know that bad guy is going to undergo facial reconstruction or hair color changes, the only way I'm going to be able to capture that bad guy is by focusing on parts of that person that cannot be changed," explains CEL-SCI's CEO Geert Kersten as he describes the dangerous influenza virus.
While some drug makers like Novartis AG, Sanofi-Aventis, Baxter, Novavax, Inc. and others are stockpiling vaccines and other treatments, government officials feel that may not be enough and it has been reported recently that there may even be a shortage of vaccines.
The alarming new special report titled "Who's Afraid of the Big Bad Flu" appears now at BioMedReports.com:
http://biomedreports.com/component/content/article/3544-whos… scenario.html
Biotech investors interested in accessing the complete database of clinical trials and upcoming FDA decisions can access that information on the same site:
http://biomedreports.com/fda-calendar/fda-calendar.html
About BioMedReports.com
BioMedReports.com is a news portal covering the biomedical news and financial sector. It features stock research reports, news, videos, stock commentaries, and more -- including FDA and Clinical Trial Calendars.
Disclosure: No positions
Impfstoff haben die anderen, Cel hat was Besonderes
andere aktien haben sich ver. 5 oder ver.10 facht.also haben wir noch starkes potenzial
 after hours etwas nach oben - mal sehen wie es nachher mit dem pre market geht
after hours etwas nach oben - mal sehen wie es nachher mit dem pre market geht wenn Nachrichten kommen , war es meistens Montag 16.00 Uhr MEZ
CVMCel-Sci Corporation
Jul. 31, 2009 Market Close: $ 0.50 Did you know you can trade some stocks after the closing bell rings on Wall Street? Every trading day between 4:00 and 6:30 p.m. ET, traders take advantage of the After-Hours trading session. Learn more about the After-Hours trading session and how you can benefit from it.
After Hours Trade Reporting
Pre-Market Charts | After Hours Charts After Hours
Last: $ .52 After Hours
High: $ .52
After Hours
Volume: 11,000 After Hours
Low: $ .47
After Hours
Time (ET) After Hours
Price After Hours
Share Volume
19:40 $ .52 500
19:40 $ .52 500
17:46 $ .49 975
17:20 $ .4701 3,000
16:59 $ .47 1,000
16:58 $ .47 1,000
16:52 $ .47 1,000
16:51 $ .47 2,000
16:44 $ .49 425
16:17 $ .49 500
16:17 $ .49 100
 0,55
0,55 
Schon wieder schönes Volumen drin... Schaun wa ma wo die reise hingeht. Auf steigende Kurse...
Antwort auf Beitrag Nr.: 37.699.862 von Fafnir77 am 03.08.09 15:50:56jetzt gehts aber ganz schön nach unten
 aFTER HOURS 0,58
aFTER HOURS 0,58
Hallo zusammen,
muss den Urlaub mal 2 -3 Tage unterbrechen und eine neue Lenkung ins Auto bauen lassen ( thats life ), aber ich sehe es gibt ja die tollsten Gerüchte im US Board - der Kurs passt auch in USA...und es werden wohl spannende Tage folgen - könnten neue Höchstände kommen....
), aber ich sehe es gibt ja die tollsten Gerüchte im US Board - der Kurs passt auch in USA...und es werden wohl spannende Tage folgen - könnten neue Höchstände kommen.... ...lass mich mal überraschen -
...lass mich mal überraschen -
US Board
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
muss den Urlaub mal 2 -3 Tage unterbrechen und eine neue Lenkung ins Auto bauen lassen ( thats life
 ), aber ich sehe es gibt ja die tollsten Gerüchte im US Board - der Kurs passt auch in USA...und es werden wohl spannende Tage folgen - könnten neue Höchstände kommen....
), aber ich sehe es gibt ja die tollsten Gerüchte im US Board - der Kurs passt auch in USA...und es werden wohl spannende Tage folgen - könnten neue Höchstände kommen.... ...lass mich mal überraschen -
...lass mich mal überraschen - US Board
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
IM AH...wars ja schon mal eine gute Tendenz
8/4/2009 6:12:09 AM Market Closed
NASDAQ Last Sale
0.58 0.02 +++3.57%
Volume
4,185,059 Previous Close
$ 0.56
" müsste " heute über die 0,60 US$ gehen ( aber was muss an der Börse schon )
8/4/2009 6:12:09 AM Market Closed
NASDAQ Last Sale
0.58 0.02 +++3.57%
Volume
4,185,059 Previous Close
$ 0.56
" müsste " heute über die 0,60 US$ gehen ( aber was muss an der Börse schon )
heute wird man wahrscheinlich nochmal günstig nachkaufen können 





CEL SCI CP(AMEX: CVM)
Real-Time: 0.5255 0.0345 (6.16%) 9:36AM ET
nachkaufmöglichkeit


Real-Time: 0.5255 0.0345 (6.16%) 9:36AM ET
nachkaufmöglichkeit



 dann aber schnell, siehe level II
dann aber schnell, siehe level II 
Antwort auf Beitrag Nr.: 37.707.859 von ALF-FRED am 04.08.09 15:43:58glaub ich nicht !!!!
bin mir relativ sicher dass die heut noch bis 0,50 zurück kommt
bin mir relativ sicher dass die heut noch bis 0,50 zurück kommt
hi,ein bissi fakten von cel sci war schon ganz gut....
nur immer eine ankündigung zu h1n1 reicht da wohl nicht.
der start zu phase 3 von multikine oder die vermietung der produktionhalle war mal eine gute nachricht.
freu mich ja auch über steigende kurse,bin ja schon lange genug dabei....
nur immer eine ankündigung zu h1n1 reicht da wohl nicht.
der start zu phase 3 von multikine oder die vermietung der produktionhalle war mal eine gute nachricht.
freu mich ja auch über steigende kurse,bin ja schon lange genug dabei....
Antwort auf Beitrag Nr.: 37.712.336 von VFBLER am 05.08.09 08:38:46 ich habe auch über die letzten 5 Jahre gesammelt und liege ganz gut ( steuerfrei )
ich habe auch über die letzten 5 Jahre gesammelt und liege ganz gut ( steuerfrei )
die letzten Tage habe ich nochmals eine 50 % tige Tradingposition gekauft, den wenn Nachrichten kommen möchte ich auch mal einen Ritt wagen und die Longposition bleibt noch mindestens 3 - 4 Jahre im Depot.
 ich habe auch über die letzten 5 Jahre gesammelt und liege ganz gut ( steuerfrei )
ich habe auch über die letzten 5 Jahre gesammelt und liege ganz gut ( steuerfrei )die letzten Tage habe ich nochmals eine 50 % tige Tradingposition gekauft, den wenn Nachrichten kommen möchte ich auch mal einen Ritt wagen und die Longposition bleibt noch mindestens 3 - 4 Jahre im Depot.

 Kann sich noch jemand daran erinnern, das es für alte Aktien ( bis zu einem bestimmten Datum ? ) Zusatzaktien ( eventuell zum Vorzugspreis ) geben sollte wann das ungfähr war und wo das steht ?
Kann sich noch jemand daran erinnern, das es für alte Aktien ( bis zu einem bestimmten Datum ? ) Zusatzaktien ( eventuell zum Vorzugspreis ) geben sollte wann das ungfähr war und wo das steht ? irgendwas mit Serie k oder so, davon müßte ich welche haben war so ca. 2006 rum
schade, hätte gestern, gerne noch welche zu 0,35 kaufen wollen. in usa ging es bis 0,52 aber nicht darunter, bin wohl ein bisschen zu optimistischt gewesen.
hab gott sei dank, heute noch welche in den usa zu 0,51 bekommen 


würde mich nicht wundern, wenn wir heute um 22.00 uhr noch mit einem plus schliessen. unglaublich !!!



würde mich nicht wundern, wenn wir heute um 22.00 uhr noch mit einem plus schliessen. unglaublich !!!
Ich finde nirgendwo schlechte Nachrichten und den Rücksetzer kann ich mir nicht erklären, weiß Jemand mehr?
 Hie4r braucht man Geduld, eine extra Tradingposition zum zocken und eine long Position für Multikine
Hie4r braucht man Geduld, eine extra Tradingposition zum zocken und eine long Position für MultikineDer Rest ist nur PR zum Reiten, diesmal war es die Schweinegrippe, vor 3 Jahren die Vogelgrippe etc.,
Die Firma ( Gebäude der Produktion ) ist fertig ich warte auf den Start der PIII !
Fährt jemand nach München zur HV ?


kann auch schnell gehen
die letzten tage die hier
http://isht.comdirect.de/html/detail/main.html?type=candle&s…
die letzten tage die hier
http://isht.comdirect.de/html/detail/main.html?type=candle&s…

Cel-Sci has a 3000 Percent Increase Potential
Recs
1
Follow
Share
Report
August 07, 2009 – Comments (0) | RELATED TICKERS: CVM
In the next three years Cel-Sci could light up the Biotech world by releasing Phase III results on a new product called Multikine®.
Multikine® is CEL-SCI’s late stage cancer product, which is an immunotherapeutic agent for first-line standard of care treatment in head and neck cancer patients. The drugs approach is different in the way it fights against cancer, by directly affecting both the tumor cells and by activating an anti-tumor immune response by the body.
This multipronged approach has produced fabulous Phase II results. Multikine achieved a 33% improvement in median overall survival at a median of 3.5 years post surgery with no adverse events. Along with these impressive safety and efficacy results, Multikine showed a 50% reduction in tumor cells, 12% complete response, 8% increase in the Local Regional Control.
Folks I’m not usually this gitty, but the Phase II results are just not seen like this! I also like the risk factors ahead. Multikine will only need to demonstrate a 10% improvement in overall survival for the Phase III trials to be successful. We have a good 20% to play with from the Phase II results.
So why is Cel-Sci’s stock at $0.48 for today’s close and its market cap sitting on a measly 62M? Its because Wall Street is wondering why a US partner has not yet been identified if you ask me. So why am I not worried about this pending risk? Because TEVA has already signed for the Israeli market and they are about the most conservative company known to man on the branded side. They see the clinical risk being very low and the potential for a best in class product being high – just like I do!
Before I get too long winded let me tell you a few other reasons why I like the stock; Cel-Sci has enough cash until next March (unusual for Biotech’s in this credit climate), has only 130M shares outstanding, and is finishing up on its manufacturing facility where it plans to make its Phase III clinical trial material.
As with the other Biotech’s I’ve recommended I’m in for a handsome sum!
I’ll keep you posted
Becon800
http://caps.fool.com/Blogs/ViewPost.aspx?bpid=240336&t=01005…
 After hours 0,506 in Amiland - Artikel mit mehren Seiten Teil I und II
After hours 0,506 in Amiland - Artikel mit mehren Seiten Teil I und IIhttp://www.thestreet.com/_yahoo/story/10567479/1/inovio-digs…
Beacon Equity Issues Trading Outlook for CEL-SCI Corp.
Press Release
Source: Beacon Equity
On Monday August 10, 2009, 6:50 am EDT
Buzz up! 0 Print
Companies:CEL-SCI Corp.
DALLAS, Aug. 10, 2009 (GLOBE NEWSWIRE) -- BeaconEquity.com announces an investment report featuring CEL-SCI Corp. (NYSE Amex:CVM). The report includes financial, comparative and investment analyses, and pertinent industry information you need to know to make an educated investment decision.
Related Quotes
Symbol Price Change
CVM 0.4795 0.0000
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} The investment report on CEL-SCI Corp. (NYSE Amex:CVM) should be of particular interest to other biotechnology companies: Introgen Therapeutics Inc. (Pink Sheets:INGNQ - News), Targeted Genetics Corp. (Nasdaq:TGEN - News), Vical Inc. (Nasdaq:VICL - News) and Immunogen Inc. (Nasdaq:IMGN - News).
It is available at: http://www.beaconequity.com/i/CVM/" target="_blank" rel="nofollow ugc noopener">http://www.beaconequity.com/i/CVM/
Get our alerts BEFORE the rest of the market. Follow us on Twitter: http://twitter.com/BeaconEquity
CEL-SCI Corp. (NYSE Amex:CVM) is a developer, manufacturer and marketer of novel therapies through proprietary anti-cancer technologies to fight cancer and heteroconjugate technologies referred to as Ligand Epitope Antigen Presentation System (L.E.A.P.S.) for the treatment of bacterial, viral and parasitic infections. The Company's flagship drug therapy, Multikine, is a new cancer immunotherapy drug developed to stimulate the human body's own immune system to fight and kill cancer cells.
In the report, the analyst notes:
"The Company's flagship drug therapy, Multikine, is a new cancer immunotherapy drug developed to stimulate the human body's own immune system to fight and kill cancer cells. Multikine has cleared phase II drug trial and moved into phase III in the United States and Canada to treat advanced primary head and neck cancer patients. The Company also focuses its efforts on the improvement of immunotherapies to prevent/treatment of avian flu, H1N1, herpes simplex, malaria, viral encephalitis, smallpox, vaccinia and other infections.
"The Company has received recent media and investor attention due to official responses to the rapid spread of the H1N1 virus reported by the World Health Organization (WHO), which recently elevated its warning to world pandemic level six by Dr. Margaret Chan, director-general of WHO, on June 11, 2009. Additional investor interest followed the Company's announcement that the goal of its experimental H1N1 drug candidate Cel-1000 is not only to combat the current strain but future mutations of the strain as well."
To read the entire report visit: http://www.beaconequity.com/i/CVM
See what investors are saying about these stocks at: http://www.stockhideout.com/
BeaconEquity.com is one of the industry's largest small-cap report providers. Beacon strives to provide a balanced view of many promising small-cap companies that would otherwise fall under the radar of the typical Wall Street investor. We provide investors with an excellent first step in their research and due diligence by providing daily trading ideas, and consolidating the public information available on them. For more information on Beacon, please visit http://www.BeaconEquity.com
BeaconEquity Disclosure
DO NOT BASE ANY INVESTMENT DECISION UPON ANY MATERIALS FOUND ON THIS REPORT. We are not registered as a securities broker-dealer or an investment adviser either with the U.S. Securities and Exchange Commission (the "SEC") or with any state securities regulatory authority. We are neither licensed nor qualified to provide investment advice. Beacon nor its affiliates have a beneficial interest in the mentioned company; nor have they received compensation of any kind for any of the companies listed in this communication. The information contained in our report is not an offer to buy or sell securities. We distribute opinions and information free of charge exclusively to individuals who wish to receive them.
Press Release
Source: Beacon Equity
On Monday August 10, 2009, 6:50 am EDT
Buzz up! 0 Print
Companies:CEL-SCI Corp.
DALLAS, Aug. 10, 2009 (GLOBE NEWSWIRE) -- BeaconEquity.com announces an investment report featuring CEL-SCI Corp. (NYSE Amex:CVM). The report includes financial, comparative and investment analyses, and pertinent industry information you need to know to make an educated investment decision.
Related Quotes
Symbol Price Change
CVM 0.4795 0.0000
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} The investment report on CEL-SCI Corp. (NYSE Amex:CVM) should be of particular interest to other biotechnology companies: Introgen Therapeutics Inc. (Pink Sheets:INGNQ - News), Targeted Genetics Corp. (Nasdaq:TGEN - News), Vical Inc. (Nasdaq:VICL - News) and Immunogen Inc. (Nasdaq:IMGN - News).
It is available at: http://www.beaconequity.com/i/CVM/" target="_blank" rel="nofollow ugc noopener">http://www.beaconequity.com/i/CVM/
Get our alerts BEFORE the rest of the market. Follow us on Twitter: http://twitter.com/BeaconEquity
CEL-SCI Corp. (NYSE Amex:CVM) is a developer, manufacturer and marketer of novel therapies through proprietary anti-cancer technologies to fight cancer and heteroconjugate technologies referred to as Ligand Epitope Antigen Presentation System (L.E.A.P.S.) for the treatment of bacterial, viral and parasitic infections. The Company's flagship drug therapy, Multikine, is a new cancer immunotherapy drug developed to stimulate the human body's own immune system to fight and kill cancer cells.
In the report, the analyst notes:
"The Company's flagship drug therapy, Multikine, is a new cancer immunotherapy drug developed to stimulate the human body's own immune system to fight and kill cancer cells. Multikine has cleared phase II drug trial and moved into phase III in the United States and Canada to treat advanced primary head and neck cancer patients. The Company also focuses its efforts on the improvement of immunotherapies to prevent/treatment of avian flu, H1N1, herpes simplex, malaria, viral encephalitis, smallpox, vaccinia and other infections.
"The Company has received recent media and investor attention due to official responses to the rapid spread of the H1N1 virus reported by the World Health Organization (WHO), which recently elevated its warning to world pandemic level six by Dr. Margaret Chan, director-general of WHO, on June 11, 2009. Additional investor interest followed the Company's announcement that the goal of its experimental H1N1 drug candidate Cel-1000 is not only to combat the current strain but future mutations of the strain as well."
To read the entire report visit: http://www.beaconequity.com/i/CVM
See what investors are saying about these stocks at: http://www.stockhideout.com/
BeaconEquity.com is one of the industry's largest small-cap report providers. Beacon strives to provide a balanced view of many promising small-cap companies that would otherwise fall under the radar of the typical Wall Street investor. We provide investors with an excellent first step in their research and due diligence by providing daily trading ideas, and consolidating the public information available on them. For more information on Beacon, please visit http://www.BeaconEquity.com
BeaconEquity Disclosure
DO NOT BASE ANY INVESTMENT DECISION UPON ANY MATERIALS FOUND ON THIS REPORT. We are not registered as a securities broker-dealer or an investment adviser either with the U.S. Securities and Exchange Commission (the "SEC") or with any state securities regulatory authority. We are neither licensed nor qualified to provide investment advice. Beacon nor its affiliates have a beneficial interest in the mentioned company; nor have they received compensation of any kind for any of the companies listed in this communication. The information contained in our report is not an offer to buy or sell securities. We distribute opinions and information free of charge exclusively to individuals who wish to receive them.
Antwort auf Beitrag Nr.: 37.745.097 von ALF-FRED am 10.08.09 14:52:56"The Company's flagship drug therapy, Multikine, is a new cancer immunotherapy drug developed to stimulate the human body's own immune system to fight and kill cancer cells. Multikine has cleared phase II drug trial and moved into phase III in the United States and Canada to treat advanced primary head and neck cancer patients. The Company also focuses its efforts on the improvement of immunotherapies to prevent/treatment of avian flu, H1N1, herpes simplex, malaria, viral encephalitis, smallpox, vaccinia and other infections.
Das kann was werden Ende 2009 / Anfang 2010
Das kann was werden Ende 2009 / Anfang 2010

 Pre-Market Trade Reporting
Pre-Market Trade ReportingPre-Market Charts | After Hours Charts Pre-Market
Last: $ .53 Pre-Market
High: $ .54
Pre-Market
Volume: 64,885 Pre-Market
Low: $ .49
Pre-Market
Time (ET) Pre-Market
Price Pre-Market
Share Volume
09:08 $ .53 5,390
09:08 $ .53 5,000
09:08 $ .53 2,000
09:08 $ .53 110
09:04 $ .50 400
09:04 $ .50 2,600
08:53 $ .51 100
08:25 $ .49 2,100
08:14 $ .49 42,385
08:06 $ .53 300
08:06 $ .54 100
08:05 $ .52 2,000
08:05 $ .53 200
08:03 $ .52 200
08:03 $ .51 2,000
Wird wohl alles dauern bei Cel - aber konnte heute günstig Nachlegen....denk mal so 6- 12 Monate muss gerechnet werden bis eine wirkliche Tendenz da ist....sollte es aber so kommen wie ich hoffe...dann sind diese Kurse bald Geschichte ( im positiven )...müssen ja nicht gleich die 3000 % plus sein...wie der US Kollege meint....paar 100 langen auch 
Noch ist es hier ja schön ruhig....wird sich aber wohl irgendwann dann ändern

Noch ist es hier ja schön ruhig....wird sich aber wohl irgendwann dann ändern
Sk mit 0,52 US$ heute...wäre mal nicht schlecht...sieht recht gut aus heute..auch vom Volumen her
Nachdem ich leider nach langer Zeit bei Freddie Mac raus bin ( leider 1 Woche zu früh )...müssen es halt kleinere Brötchen sein...aber 9 % passt ja auch...mal sehen wie es AH weitergeht
NASDAQ Last Sale
0.52 0.04 +++8.34%
Volume
1,616,201
NASDAQ Last Sale
0.52 0.04 +++8.34%
Volume
1,616,201
Ohne Update nix los bei Cel Kurs Range 0,48 - 0,52 US$.....Phase 3 Results müssen her....oder die 3000 % plus vom US Kollegen
Wieder ein Tag mit Abtastkursen...aber wehe es kommt die wirklich entscheidende NEWS...dann dürfte hier aber ein Tanz beginnen....Biotech ein reines Gedultspiel mit Hintergrundwissen
Antwort auf Beitrag Nr.: 37.765.167 von Expertchen007 am 12.08.09 17:45:32diese ganze RS geschichte bremst den kurs
aus - brauchen mal ne meldung bez.
pIII - am besten finanzierung mit solventem
partner
aus - brauchen mal ne meldung bez.
pIII - am besten finanzierung mit solventem
partner

Antwort auf Beitrag Nr.: 37.774.582 von Gustl24 am 13.08.09 16:48:19Aber das RS ist doch ein Gerücht - oder hab ich was nicht mitbekommen ????
 wenn rs re split heißen soll wird in den Amiboards schon Jahre darüber diskutiert !!!
wenn rs re split heißen soll wird in den Amiboards schon Jahre darüber diskutiert !!!
Antwort auf Beitrag Nr.: 37.775.173 von Expertchen007 am 13.08.09 17:36:12ist kein gerücht...
aus dem letzten von dir reingestellten sec-filing:
NOTICE OF ANNUAL MEETING OF SHAREHOLDERS
TO BE HELD SEPTEMBER 14, 2009
To the Shareholders:
Notice is hereby given that the annual meeting of the shareholders of CEL-SCI Corporation ("CEL-SCI") will be held at the Hilton Munich
Park, Am Tucherplatz 7, 80538 Munich, Germany, on September 14, 2009, at 10:30 a.m., for the following purposes:
(1) to elect the directors who shall constitute CEL-SCI's Board of Directors for the ensuing year;
(2) to approve the adoption of CEL-SCI's 2009 Incentive Stock Option Plan which provides that up to 5,000,000 shares of common stock may
be issued upon the exercise of options granted pursuant to the Incentive Stock Option Plan;
(3) to approve the adoption of CEL-SCI's 2009 Non-Qualified Stock Option Plan which provides that up to 15,000,000 shares of common stock
may be issued upon the exercise of options granted pursuant to the Non-Qualified Stock Option Plan;
(4) to approve the adoption of CEL-SCI's 2009 Stock Bonus Plan which provides that up to 2,000,000 shares of common stock may be issued
to persons granted stock bonuses pursuant to the Stock Bonus Plan;
(5) to approve an amendment to CEL-SCI's Stock Compensation Plan to provide for the issuance of up to 2,000,000 additional restricted shares
of common stock to CEL-SCI's directors, officers, employees and consultants for services provided to the Company;
(6) to amend CEL-SCI's Articles of Incorporation such that CEL-SCI would be authorized to issue 450,000,000 shares of common stock;
(7) subject to the determination of CEL-SCI's directors that a reverse split would be in the best interest of CEL-SCI's shareholders, to approve a
reverse split of CEL-SCI's common stock.(8) to ratify the appointment of BDO Seidman, LLP as CEL-SCI's independent registered public accounting firm for the fiscal year ending
September 30, 2009.
to transact such other business as may properly come before the meeting.
und weiter...unten im text ( seite 26):
APPROVAL OF A REVERSE SPLIT OF CEL-SCI'S COMMON STOCK, SUBJECT TO THE DETERMINATION OF CEL-SCI'S
DIRECTORS THAT A REVERSE SPLIT WOULD BE IN THE BEST INTEREST OF CEL-SCI'S SHAREHOLDERS.
CEL-SCI's Board of Directors is seeking approval to adopt a reverse split of its outstanding common stock in a ratio no less than 1-for-2 and no
greater than 1-for-10 at any time before its next Annual Meeting of Stockholders in 2010. CEL-SCI's Board of Directors has not made any
determination of whether it wants to actually proceed with a reverse split of the Company's common stock; it is only seeking the shareholders'
approval for such a step at this time. CEL-SCI's Directors asked for and received this approval from the shareholders last year, but did not use
it. CEL-SCI's Directors believe that, because it is not possible to predict future market conditions, it would be in the best interests of the
stockholders if the Board was able to determine, at any time prior to the next Annual Meeting of Stockholders in 2010...
klare fakten...
das produkt ist super...aber company braucht geld für pIII
und möchte in gewisse börsensegmente um investoren zu bekommen...
so weit so gut...dann sollen sie mal ein paar meldungen raushauen -
dann klappt das mit einem kurs über 1$...von 20$ möchte ich
hier wie in ami-boards nicht sprechen, aber 1,5$ wären
realistisch

aus dem letzten von dir reingestellten sec-filing:
NOTICE OF ANNUAL MEETING OF SHAREHOLDERS
TO BE HELD SEPTEMBER 14, 2009
To the Shareholders:
Notice is hereby given that the annual meeting of the shareholders of CEL-SCI Corporation ("CEL-SCI") will be held at the Hilton Munich
Park, Am Tucherplatz 7, 80538 Munich, Germany, on September 14, 2009, at 10:30 a.m., for the following purposes:
(1) to elect the directors who shall constitute CEL-SCI's Board of Directors for the ensuing year;
(2) to approve the adoption of CEL-SCI's 2009 Incentive Stock Option Plan which provides that up to 5,000,000 shares of common stock may
be issued upon the exercise of options granted pursuant to the Incentive Stock Option Plan;
(3) to approve the adoption of CEL-SCI's 2009 Non-Qualified Stock Option Plan which provides that up to 15,000,000 shares of common stock
may be issued upon the exercise of options granted pursuant to the Non-Qualified Stock Option Plan;
(4) to approve the adoption of CEL-SCI's 2009 Stock Bonus Plan which provides that up to 2,000,000 shares of common stock may be issued
to persons granted stock bonuses pursuant to the Stock Bonus Plan;
(5) to approve an amendment to CEL-SCI's Stock Compensation Plan to provide for the issuance of up to 2,000,000 additional restricted shares
of common stock to CEL-SCI's directors, officers, employees and consultants for services provided to the Company;
(6) to amend CEL-SCI's Articles of Incorporation such that CEL-SCI would be authorized to issue 450,000,000 shares of common stock;
(7) subject to the determination of CEL-SCI's directors that a reverse split would be in the best interest of CEL-SCI's shareholders, to approve a
reverse split of CEL-SCI's common stock.(8) to ratify the appointment of BDO Seidman, LLP as CEL-SCI's independent registered public accounting firm for the fiscal year ending
September 30, 2009.
to transact such other business as may properly come before the meeting.
und weiter...unten im text ( seite 26):
APPROVAL OF A REVERSE SPLIT OF CEL-SCI'S COMMON STOCK, SUBJECT TO THE DETERMINATION OF CEL-SCI'S
DIRECTORS THAT A REVERSE SPLIT WOULD BE IN THE BEST INTEREST OF CEL-SCI'S SHAREHOLDERS.
CEL-SCI's Board of Directors is seeking approval to adopt a reverse split of its outstanding common stock in a ratio no less than 1-for-2 and no
greater than 1-for-10 at any time before its next Annual Meeting of Stockholders in 2010. CEL-SCI's Board of Directors has not made any
determination of whether it wants to actually proceed with a reverse split of the Company's common stock; it is only seeking the shareholders'
approval for such a step at this time. CEL-SCI's Directors asked for and received this approval from the shareholders last year, but did not use
it. CEL-SCI's Directors believe that, because it is not possible to predict future market conditions, it would be in the best interests of the
stockholders if the Board was able to determine, at any time prior to the next Annual Meeting of Stockholders in 2010...
klare fakten...

das produkt ist super...aber company braucht geld für pIII
und möchte in gewisse börsensegmente um investoren zu bekommen...
so weit so gut...dann sollen sie mal ein paar meldungen raushauen -
dann klappt das mit einem kurs über 1$...von 20$ möchte ich
hier wie in ami-boards nicht sprechen, aber 1,5$ wären
realistisch


Antwort auf Beitrag Nr.: 37.780.604 von Gustl24 am 14.08.09 11:15:29 ja, weiß ich - wird aber regelmäßig angekündigt und nicht durchgeführt
ja, weiß ich - wird aber regelmäßig angekündigt und nicht durchgeführt
1,5 $ bei 1 zu 10 wäre aber nicht so dolle - mal sehen was kommt -
fährt denn keiner nach München und kann berichten ???
 ja, weiß ich - wird aber regelmäßig angekündigt und nicht durchgeführt
ja, weiß ich - wird aber regelmäßig angekündigt und nicht durchgeführt1,5 $ bei 1 zu 10 wäre aber nicht so dolle - mal sehen was kommt -
fährt denn keiner nach München und kann berichten ???

Antwort auf Beitrag Nr.: 37.780.744 von ALF-FRED am 14.08.09 11:28:01ich meinte ja auch 1,5$ VOR annual meeting und
OHNE split...
OHNE split...
wohne im raum münchen, aber sich montags da reinzuhocken
geht nicht...da geht arbeit vor
geht nicht...da geht arbeit vor

Antwort auf Beitrag Nr.: 37.782.050 von Gustl24 am 14.08.09 13:38:58 ach so dann sei es entschuldigt ---- ich wollte mal vor einigen Jahren zum meeting nach Frankfurt, da wollte Cel.SCI ca. 90 Euro Eintritt ( 2 Personen ) für die HV laut Aussagen meiner Bank, da habe ich es gelassen !!!
ach so dann sei es entschuldigt ---- ich wollte mal vor einigen Jahren zum meeting nach Frankfurt, da wollte Cel.SCI ca. 90 Euro Eintritt ( 2 Personen ) für die HV laut Aussagen meiner Bank, da habe ich es gelassen !!!
 ach so dann sei es entschuldigt ---- ich wollte mal vor einigen Jahren zum meeting nach Frankfurt, da wollte Cel.SCI ca. 90 Euro Eintritt ( 2 Personen ) für die HV laut Aussagen meiner Bank, da habe ich es gelassen !!!
ach so dann sei es entschuldigt ---- ich wollte mal vor einigen Jahren zum meeting nach Frankfurt, da wollte Cel.SCI ca. 90 Euro Eintritt ( 2 Personen ) für die HV laut Aussagen meiner Bank, da habe ich es gelassen !!!
Antwort auf Beitrag Nr.: 37.780.604 von Gustl24 am 14.08.09 11:15:29Hatte ich gar nicht gesehen...sowas...na ja denke das kommt nicht...
CEL-SCI Corporation Reports Third Quarter 2009 Financial Results
Press Release
Source: CEL-SCI Corporation
On Friday August 14, 2009, 4:58 pm EDT
Buzz up! 0 Print
Companies:CEL-SCI Corp.
Topics:Health Care Sector
VIENNA, Va., Aug. 14 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE Amex: CVM) reports financial results for the three and nine months ended June 30, 2009.
The Company's loss from operations for the quarter ended June 30, 2009 was $3.7 million versus a loss of $2.2 million during the same quarter in 2008. Included in the fiscal 2009 third quarter loss of $3.7 million was $2.6 million in non-cash expense. The Company's loss from operations for the nine months ended June 30, 2009 was $8.1 million versus a loss of $7.1 million during the same nine months in 2008. Included in the fiscal 2009 nine-month loss of $8.1 million were $4 million in non-cash expense.
The net loss per common share for the quarter ended June 30, 2009 was $0.05 versus a net loss per common share of $0.02 during the same quarter in 2008. The Company's net loss per common share for the nine months ended June 30, 2009 was $0.08 versus a net loss per common share of $0.06 during the same nine month period in 2008.
During the three month period ended June 30, 2009, research and development charges were $1.6 million compared to $1.0 million during the same period in 2008. During the nine month period ended June 30, 2009, research and development expenses were $3.8 million compared to $3.0 million during the same period in 2008.
During the three month period ended June 30, 2009, general and administrative expenses were $1.9 million compared to $1.2 million during the same period in 2008. During the nine month period ended June 30, 2009, general and administrative expenses were $4.0 million compared to $3.9 million during the same period in 2008. A large part of the G&A expenditures was non-cash charges. The Company ended the 2009 third quarter with cash and cash equivalents of $5.6 million.
Geert Kersten, Chief Executive Officer of CEL-SCI said, "Despite a disruption in the capital markets, CEL-SCI has emerged stronger than ever. We are nearing the completion of the validation of our manufacturing facility in order to position the Company to proceed with a pivotal Phase III trial of Multikine as quickly as possible. The Company also expanded its efforts to develop its L.E.A.P.S.(TM) technology platform to combat a potentially more aggressive future form of the H1N1 virus. It also strengthened our balance sheet through the completion of a registered direct offering, allowing us to invest in many of these exciting clinical programs."
CEL-SCI Corporation develops immune-based cancer and infectious disease therapies. Its primary product candidate is Multikine, a next-generation, comprehensive immunotherapy that targets newly diagnosed head and neck cancer. Multikine is cleared by the FDA and the Canadian regulators for a global Phase III clinical trial in newly diagnosed head and neck cancer. In a Phase II long term follow-up trial with Multikine, a 33% improvement in overall survival was observed.
The Company has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being/have been tested against diseases associated with bio-defense, rheumatoid arthritis, and pandemic flu.
For more information, please visit http://www.cel-sci.com/.
Press Release
Source: CEL-SCI Corporation
On Friday August 14, 2009, 4:58 pm EDT
Buzz up! 0 Print
Companies:CEL-SCI Corp.
Topics:Health Care Sector
VIENNA, Va., Aug. 14 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE Amex: CVM) reports financial results for the three and nine months ended June 30, 2009.
The Company's loss from operations for the quarter ended June 30, 2009 was $3.7 million versus a loss of $2.2 million during the same quarter in 2008. Included in the fiscal 2009 third quarter loss of $3.7 million was $2.6 million in non-cash expense. The Company's loss from operations for the nine months ended June 30, 2009 was $8.1 million versus a loss of $7.1 million during the same nine months in 2008. Included in the fiscal 2009 nine-month loss of $8.1 million were $4 million in non-cash expense.
The net loss per common share for the quarter ended June 30, 2009 was $0.05 versus a net loss per common share of $0.02 during the same quarter in 2008. The Company's net loss per common share for the nine months ended June 30, 2009 was $0.08 versus a net loss per common share of $0.06 during the same nine month period in 2008.
During the three month period ended June 30, 2009, research and development charges were $1.6 million compared to $1.0 million during the same period in 2008. During the nine month period ended June 30, 2009, research and development expenses were $3.8 million compared to $3.0 million during the same period in 2008.
During the three month period ended June 30, 2009, general and administrative expenses were $1.9 million compared to $1.2 million during the same period in 2008. During the nine month period ended June 30, 2009, general and administrative expenses were $4.0 million compared to $3.9 million during the same period in 2008. A large part of the G&A expenditures was non-cash charges. The Company ended the 2009 third quarter with cash and cash equivalents of $5.6 million.
Geert Kersten, Chief Executive Officer of CEL-SCI said, "Despite a disruption in the capital markets, CEL-SCI has emerged stronger than ever. We are nearing the completion of the validation of our manufacturing facility in order to position the Company to proceed with a pivotal Phase III trial of Multikine as quickly as possible. The Company also expanded its efforts to develop its L.E.A.P.S.(TM) technology platform to combat a potentially more aggressive future form of the H1N1 virus. It also strengthened our balance sheet through the completion of a registered direct offering, allowing us to invest in many of these exciting clinical programs."
CEL-SCI Corporation develops immune-based cancer and infectious disease therapies. Its primary product candidate is Multikine, a next-generation, comprehensive immunotherapy that targets newly diagnosed head and neck cancer. Multikine is cleared by the FDA and the Canadian regulators for a global Phase III clinical trial in newly diagnosed head and neck cancer. In a Phase II long term follow-up trial with Multikine, a 33% improvement in overall survival was observed.
The Company has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being/have been tested against diseases associated with bio-defense, rheumatoid arthritis, and pandemic flu.
For more information, please visit http://www.cel-sci.com/.
Die Gesellschaft, ausgehend vom Betriebsergebnis für das Quartal zum 30. Juni 2009 $ 3,7 Mio. gegenüber einem Verlust von $ 2.2 Mio. im gleichen Quartal 2008. Inbegriffen im Geschäftsjahr 2009 dritten Quartal Verlust von 3,7 Mio. $ 2,6 Mio. im nicht-zahlungswirksame Aufwendungen. Die Gesellschaft, ausgehend vom Betriebsergebnis für die ersten neun Monate zum 30. Juni 2009 $ 8,1 Mio. gegenüber einem Verlust von $ 7,1 Millionen im gleichen neun Monaten im Jahr 2008. Inbegriffen im Geschäftsjahr 2009 Neun-Monats-Verlust von $ 8,1 Mio. $ 4 Millionen in nicht-zahlungswirksame Aufwendungen.
Der Nettoverlust je Aktie für das Quartal zum 30. Juni 2009 war $ 0,05 im Vergleich zu einem Nettoverlust je Aktie von $ 0.02 im gleichen Quartal 2008. Die Firma Netto-Verlust pro Aktie für die neun Monate zum 30. Juni 2009 0,08 US-Dollar gegenüber einem Nettoverlust je Aktie von $ 0.06 im gleichen Zeitraum neun Monate im Jahr 2008.
Während der drei Monate zum 30. Juni 2009, Forschung und Entwicklung Kosten wurden $ 1.6 Mio. verglichen mit $ 1,0 Mio. für den gleichen Zeitraum in 2008. Während der neun Monate endend am 30. Juni 2009, Aufwendungen für Forschung und Entwicklung wurden $ 3,8 Mio. gegenüber $ 3,0 Mio. für den gleichen Zeitraum in 2008.
Während der drei Monate zum 30. Juni 2009, Verwaltungs-und Gemeinkosten wurden $ 1,9 Millionen im Vergleich zu 1,2 Millionen Dollar für den gleichen Zeitraum in 2008. Während der neun Monate endend am 30. Juni 2009, Verwaltungs-und Gemeinkosten wurden $ 4,0 Mio. verglichen mit $ 3.9 Millionen für den gleichen Zeitraum in 2008. Ein großer Teil der G & A-Ausgaben wurde nicht liquiditätswirksame Aufwendungen. Das Unternehmen beendete das dritte Quartal 2009 mit flüssigen Mitteln von $ 5,6 Millionen.
Geert Kersten, Chief Executive Officer von CEL-SCI sagte: "Trotz einer Störung an den Kapitalmärkten, CEL-SCI hat sich stärker als je zuvor. Wir sind kurz vor dem Abschluss der Validierung unserer Produktionsstätte in Position, um die Gesellschaft zu gehen mit einer Phase-III-Studie von Multikine so schnell wie möglich. Das Unternehmen auch ihre Bemühungen um die Entwicklung ihrer LEAPS (TM) Technologie-Plattform zur Bekämpfung einer potenziell aggressiver zukünftige Gestaltung der H1N1-Virus. Auch unsere Bilanz durch die Vollendung eines eingetragenen direkt anzubieten, die es uns ermöglicht, in vielen dieser spannenden klinischen Programme. CEL-SCI Corporation entwickelt immun-basierte Krebs und Infektionskrankheiten Therapien. Die Primärprodukts Bewerber Multikine, der nächsten Generation und umfassenden Immuntherapie, dass die Ziele neu diagnostizierten Kopf-Hals-Krebs. Multikine ist von der FDA und dem kanadischen Regulierungsbehörden für eine globale klinische Phase-III-Studie bei neu diagnostizierten Kopf-Hals-Krebs. In einer Phase-II-langfristigen Follow-up-Studie mit Multikine, eine 33% Verbesserung der Überlebensrate beobachtet.
Das Unternehmen hat Niederlassungen in Vienna, Virginia und Baltimore, Maryland. CEL-SCI's andere Produkte, die derzeit in der vorklinischen Phase, haben gezeigt, den Schutz gegen eine Reihe von Krankheiten in Tierversuchen und werden / wurden getestet, gegen Krankheiten, die mit Bio-Verteidigung, rheumatoider Arthritis und Grippe-Pandemie.
Der Nettoverlust je Aktie für das Quartal zum 30. Juni 2009 war $ 0,05 im Vergleich zu einem Nettoverlust je Aktie von $ 0.02 im gleichen Quartal 2008. Die Firma Netto-Verlust pro Aktie für die neun Monate zum 30. Juni 2009 0,08 US-Dollar gegenüber einem Nettoverlust je Aktie von $ 0.06 im gleichen Zeitraum neun Monate im Jahr 2008.
Während der drei Monate zum 30. Juni 2009, Forschung und Entwicklung Kosten wurden $ 1.6 Mio. verglichen mit $ 1,0 Mio. für den gleichen Zeitraum in 2008. Während der neun Monate endend am 30. Juni 2009, Aufwendungen für Forschung und Entwicklung wurden $ 3,8 Mio. gegenüber $ 3,0 Mio. für den gleichen Zeitraum in 2008.
Während der drei Monate zum 30. Juni 2009, Verwaltungs-und Gemeinkosten wurden $ 1,9 Millionen im Vergleich zu 1,2 Millionen Dollar für den gleichen Zeitraum in 2008. Während der neun Monate endend am 30. Juni 2009, Verwaltungs-und Gemeinkosten wurden $ 4,0 Mio. verglichen mit $ 3.9 Millionen für den gleichen Zeitraum in 2008. Ein großer Teil der G & A-Ausgaben wurde nicht liquiditätswirksame Aufwendungen. Das Unternehmen beendete das dritte Quartal 2009 mit flüssigen Mitteln von $ 5,6 Millionen.
Geert Kersten, Chief Executive Officer von CEL-SCI sagte: "Trotz einer Störung an den Kapitalmärkten, CEL-SCI hat sich stärker als je zuvor. Wir sind kurz vor dem Abschluss der Validierung unserer Produktionsstätte in Position, um die Gesellschaft zu gehen mit einer Phase-III-Studie von Multikine so schnell wie möglich. Das Unternehmen auch ihre Bemühungen um die Entwicklung ihrer LEAPS (TM) Technologie-Plattform zur Bekämpfung einer potenziell aggressiver zukünftige Gestaltung der H1N1-Virus. Auch unsere Bilanz durch die Vollendung eines eingetragenen direkt anzubieten, die es uns ermöglicht, in vielen dieser spannenden klinischen Programme. CEL-SCI Corporation entwickelt immun-basierte Krebs und Infektionskrankheiten Therapien. Die Primärprodukts Bewerber Multikine, der nächsten Generation und umfassenden Immuntherapie, dass die Ziele neu diagnostizierten Kopf-Hals-Krebs. Multikine ist von der FDA und dem kanadischen Regulierungsbehörden für eine globale klinische Phase-III-Studie bei neu diagnostizierten Kopf-Hals-Krebs. In einer Phase-II-langfristigen Follow-up-Studie mit Multikine, eine 33% Verbesserung der Überlebensrate beobachtet.
Das Unternehmen hat Niederlassungen in Vienna, Virginia und Baltimore, Maryland. CEL-SCI's andere Produkte, die derzeit in der vorklinischen Phase, haben gezeigt, den Schutz gegen eine Reihe von Krankheiten in Tierversuchen und werden / wurden getestet, gegen Krankheiten, die mit Bio-Verteidigung, rheumatoider Arthritis und Grippe-Pandemie.
Tja, die brauchen wieder Geld 

Im grossen und ganzen keine Überraschung bei Cel- Zahlen...aber die Zukunft zählt....
Abschlag schätze ich heute so 8-10 % ...nun gut werd mich mal unten anstellen für eien weitere Position...die 0,40 € im Depot ist noch zu hoch
Antwort auf Beitrag Nr.: 37.795.399 von Expertchen007 am 17.08.09 15:41:53hoffe du hast bei 0,45 $ nochmal zugeschlagen
hätte mir von schweinegrippe-boom hier mehr versprochen -
wobei dieses kapitel bez. schweinegrippe wird nochmal
im herbst ( leider !) nmm. nochmal die welt bewegen -
nach der urlaubszeit werden die ansteckungszahlen nochmal
weit nach oben gehen...
für den nachhaltigen kursverlauf wäre hier aber die p3 massgebend...

hätte mir von schweinegrippe-boom hier mehr versprochen -
wobei dieses kapitel bez. schweinegrippe wird nochmal
im herbst ( leider !) nmm. nochmal die welt bewegen -
nach der urlaubszeit werden die ansteckungszahlen nochmal
weit nach oben gehen...
für den nachhaltigen kursverlauf wäre hier aber die p3 massgebend...
Antwort auf Beitrag Nr.: 37.798.038 von Gustl24 am 17.08.09 21:46:43Auf der 03,X hats gereicht
Was ist heute los....Ausbruch ????
Antwort auf Beitrag Nr.: 37.802.711 von Expertchen007 am 18.08.09 16:00:34 orders auf Level II laufen wie Zäpchen, da muß was kommen !!!
orders auf Level II laufen wie Zäpchen, da muß was kommen !!!
 orders auf Level II laufen wie Zäpchen, da muß was kommen !!!
orders auf Level II laufen wie Zäpchen, da muß was kommen !!!  ich habs, da ist es
ich habs, da ist es 
CEL-SCI Expands H1N1 Flu Virus Work
Press Release
Source: CEL-SCI Corporation
On Tuesday August 18, 2009, 10:09 am EDT
Buzz up! 0 Print
Companies:CEL-SCI Corp.
Topics:Health Care Sector
VIENNA, Va., Aug. 18 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (NYSE Amex: CVM), announces that it is expanding the scope of its work towards creating a novel treatment and vaccination against the current H1N1 virus, as well as a future mutated form in which the virus has acquired greater morbidity and mortality. The new work will add regions of the hemagglutinin molecule which are highly conserved and essential for the virus' survival. This work comes on top of the ongoing work being conducted against other non-changing parts of the virus. CEL-SCI scientists believe that the combination of various non-changing regions on the virus in one treatment or vaccine will allow for a greater ability to treat and protect against the current H1N1 virus and any possible future mutation.
Related Quotes
Symbol Price Change
CVM 0.50 +0.03
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} CEL-SCI has two on-going research programs directed towards the H1N1 virus. The first one is directed against a future mutated form of the virus. It is the fear of many experts that the H1N1 virus will continue to mutate and "swap genes" to become more lethal. CEL-SCI is focused on creating a treatment/vaccine against such a virus by combining non-changing parts of the H1N1 virus, the Avian Flu virus and the Spanish Flu virus. When a new virus surfaces, this treatment/vaccine is expected to provide an important cornerstone for the fight against the virus.
The second program is directed at helping very sick patients infected with the current form of H1N1. CEL-SCI expects to give updated communication on that program within the next month.
CEL-SCI's L.E.A.P.S.(TM) (Ligand Epitope Antigen Presentation System) technology allows the Company to direct an immune response against a specific disease epitope, in this case non-changing regions of H1N1, Avian Flu and the Spanish Flu. This makes it possible to 1) program the intended immune response and 2) avoid the administration of regions of the H1N1, and other viruses, that may exacerbate the problem of cytokine storm. Cytokine storm is very much involved in the death of many H1N1 patients.
The L.E.A.P.S. technology combines a small peptide that activates the immune system with a small peptide from a disease-related protein, such as the H1N1 hemagglutinin molecule, to make a vaccine that induces a defined immune response. Each L.E.A.P.S. construct is composed of a T cell binding ligand (TCBL) which has previously demonstrated the ability to induce and elicit protective immunity and antigen specific antibody production in animal models. In CEL-SCI's L.E.A.P.S. swine flu vaccine the TCBLs will be coupled to three different highly conserved protective epitopes from three different essential proteins common to all influenza A virus strains.
L.E.A.P.S. technology is a novel T-cell modulation platform technology that enables CEL-SCI to design and synthesize proprietary immunogens. Any disease for which an antigenic sequence has been identified, such as infectious, parasitic, malignant or autoimmune diseases and allergies, are potential therapeutic or preventive sites for the application of L.E.A.P.S. technology.
About CEL-SCI Corporation
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is being readied for a global Phase III trial in advanced primary head and neck cancer. CEL-SCI is also developing a vaccine to prevent and treat swine and other influenzas using its L.E.A.P.S. technology platform and expects to soon finish the validation of its state-of-the-art facility in Maryland which it expects to utilize to launch aseptic filling for stem cell produced therapies and other biological products. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
For more information, please visit www.cel-sci.com.
Naja, auch kein Riesenausbruch 

Antwort auf Beitrag Nr.: 37.802.878 von ALF-FRED am 18.08.09 16:14:01Das ist ja mal geil...nun heißt es nur noch warten...

Antwort auf Beitrag Nr.: 37.806.848 von carpe--diem am 19.08.09 08:07:53Wenns dem Kurs hilft...dann auch der !!!!! 
****************************
Der Makler manipuliert den Kurs in D auch schön runter...prima

****************************
Der Makler manipuliert den Kurs in D auch schön runter...prima

Range sehe ich heute 0,51 - 0,56 US$ ...Tendenz steigend....why not 
After Hours
Last: $ .52 After Hours
High: $ .54
After Hours
Volume: 15,975 After Hours
Low: $ .51
AH war schon mal nicht schlecht...nur halt wenig Volumen...alles andere regelt der Markt ....noch sitzen die Shorter am Hebel...aber auch deren Tag des covern wird kommen...und dann Bahn frei

After Hours
Last: $ .52 After Hours
High: $ .54
After Hours
Volume: 15,975 After Hours
Low: $ .51
AH war schon mal nicht schlecht...nur halt wenig Volumen...alles andere regelt der Markt ....noch sitzen die Shorter am Hebel...aber auch deren Tag des covern wird kommen...und dann Bahn frei
Antwort auf Beitrag Nr.: 37.808.554 von Expertchen007 am 19.08.09 11:27:48ich hoffe sie spielen das blatt swine flu nochmals
die nächsten tage von firmenseite agressiv weiter...
würde dem kurs sehr gut tun...
mann expertchen, du alter zocker, dass deine glcc
nochmals golden glänzen, gratuliere...aber vorsicht
garr ist und bleibt ein verbrecher...und dass mona und
glcc gleichzeitig steigen ist wohl kein zufall...
aber gewinne an der börse bleiben gewinne...dir solls recht sein...
die nächsten tage von firmenseite agressiv weiter...
würde dem kurs sehr gut tun...
mann expertchen, du alter zocker, dass deine glcc
nochmals golden glänzen, gratuliere...aber vorsicht
garr ist und bleibt ein verbrecher...und dass mona und
glcc gleichzeitig steigen ist wohl kein zufall...
aber gewinne an der börse bleiben gewinne...dir solls recht sein...

Antwort auf Beitrag Nr.: 37.808.968 von Gustl24 am 19.08.09 12:14:10 ich warte schon ein paar Jahre, nun habe ich das Gefühl es kommt bald was ( Umsätze, Nachrichenflow etc.)
ich warte schon ein paar Jahre, nun habe ich das Gefühl es kommt bald was ( Umsätze, Nachrichenflow etc.)
meinetwegen auch erst die Schweinegrippe - dann Start P III - großer Partner und 2010 die dann zu erwartene neue Fischgrippe ,
Kursziel 2009 2 - 3 Euro
2010 5
2011 10 und mehr bei positiven Ergebnissen
2013 25 und mehr bei der Produktion und Vertrieb des Wundermittels
 ich warte schon ein paar Jahre, nun habe ich das Gefühl es kommt bald was ( Umsätze, Nachrichenflow etc.)
ich warte schon ein paar Jahre, nun habe ich das Gefühl es kommt bald was ( Umsätze, Nachrichenflow etc.)meinetwegen auch erst die Schweinegrippe - dann Start P III - großer Partner und 2010 die dann zu erwartene neue Fischgrippe ,
Kursziel 2009 2 - 3 Euro
2010 5
2011 10 und mehr bei positiven Ergebnissen
2013 25 und mehr bei der Produktion und Vertrieb des Wundermittels
Antwort auf Beitrag Nr.: 37.808.968 von Gustl24 am 19.08.09 12:14:10Ich weiß das ist ein Drecksladen ( GLCC ) ...aber letztendlich hat sichs gelohnt...will keine Zahlen nennen..aber ist schon heftig...Einsatz raus + nochmals 600 %....( und hab noch einiges stehen )stehe bereit in Cel zu investieren . ( Hätt ich selbst nie mehr dran geglaubt - never !!! ....sollte das Game dort weitergehen - wirds im Depot heftig werden - aber ich bin zufrieden und o.k -wenn der Rest verreckt solls so sein 
*****************************************
ASK bereits bei 0,58 US$...glaub aber o,55 wäre o.k für heute

*****************************************
ASK bereits bei 0,58 US$...glaub aber o,55 wäre o.k für heute

Das war heute nix..aber gar nix...der Sack von Makler soll mir mal die Teile geben zu dem Kurs den er stellt...ich nehm reichlich davon
Press Release
Source: CEL-SCI Corporation
On Thursday August 20, 2009, 9:10 am EDT
Buzz up! 0 Print
Companies:CEL-SCI Corp.
VIENNA, Va., Aug. 20 /PRNewswire-FirstCall/ -- CEL-SCI Corporation. (NYSE Amex: CVM), a biotechnology company entering a late stage oncology trial and focused on infectious disease, today announced that it has entered into a definitive agreement with several institutional investors to sell 9.7 million units, with each unit consisting of one of the Company's common shares and 0.50 warrants to purchase one share of common stock, for gross proceeds of approximately $4.4 million, before deducting placement agent fees and estimated offering expenses, in a "registered direct" offering. The investors have agreed to purchase the units at a purchase price of $0.45 per unit. The warrants, which represent the right to acquire 4.85 million common shares, will be exercisable at any time on or after February 20, 2010 and prior to the 5-year anniversary of the closing of the transaction at an exercise price of $0.55 per share, which was above the closing price of the Company's common shares on the NYSE AMEX Market on August 19, 2009. Chardan Capital Markets, LLC acted as placement agent for the offering.
Related Quotes
Symbol Price Change
CVM 0.50 0.00
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} The transaction is expected to close on or about August 25, 2009, subject to satisfaction of customary closing conditions.
The securities described above are being offered by CEL-SCI Corporation pursuant to a registration statement previously filed and declared effective by the Securities and Exchange Commission. This press release shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state. The securities may be offered only by means of a prospectus. Copies of the final prospectus supplement and accompanying base prospectus relating to this offering may be obtained at the Securities and Exchange Commission's website at http://www.sec.gov .
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is being readied for a global Phase III trial in advanced primary head and neck cancer. CEL-SCI is also developing a vaccine to prevent and treat swine and other influenzas using its L.E.A.P.S. technology platform and expects to soon finish the validation of its state-of-the-art facility in Maryland which it expects to utilize to launch aseptic filling for stem cell produced therapies and other biological products. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
Certain statements contained herein relating to the anticipated closing of the offering or product development, or that otherwise relate to future periods, are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These statements are based on assumptions that may not prove accurate. Actual results could differ materially from those anticipated due to certain risks inherent in the biotechnology industry and for companies engaged in the development of new products in a regulated market. These risks, including those related to whether the offering will close when anticipated or at all, the results of discovery research and preclinical testing; the timing or results of pending and future clinical trials (including the design and progress of clinical trials; safety and efficacy of the products being tested; action, inaction or delay by the FDA, European or other regulators or their advisory bodies; and analysis or interpretation by, or submission to, these entities or others of scientific data); uncertainties regarding the status of biotechnology patents; uncertainties as to the cost of protecting intellectual property; changes in the status of the existing collaborative and licensing relationships; the ability of collaborators, licensees and other third parties to meet their obligations; market demand for products; scale up and marketing capabilities; competition; international operations; share price volatility; and CEL-SCI's financing needs and opportunities are described in more detail in CEL-SCI's most recent annual report on Form 10-K/A and in other SEC filings. Consider such risks carefully in considering CEL-SCI's prospects.
Source: CEL-SCI Corporation
On Thursday August 20, 2009, 9:10 am EDT
Buzz up! 0 Print
Companies:CEL-SCI Corp.
VIENNA, Va., Aug. 20 /PRNewswire-FirstCall/ -- CEL-SCI Corporation. (NYSE Amex: CVM), a biotechnology company entering a late stage oncology trial and focused on infectious disease, today announced that it has entered into a definitive agreement with several institutional investors to sell 9.7 million units, with each unit consisting of one of the Company's common shares and 0.50 warrants to purchase one share of common stock, for gross proceeds of approximately $4.4 million, before deducting placement agent fees and estimated offering expenses, in a "registered direct" offering. The investors have agreed to purchase the units at a purchase price of $0.45 per unit. The warrants, which represent the right to acquire 4.85 million common shares, will be exercisable at any time on or after February 20, 2010 and prior to the 5-year anniversary of the closing of the transaction at an exercise price of $0.55 per share, which was above the closing price of the Company's common shares on the NYSE AMEX Market on August 19, 2009. Chardan Capital Markets, LLC acted as placement agent for the offering.
Related Quotes
Symbol Price Change
CVM 0.50 0.00
{"s" : "cvm","k" : "c10,l10,p20,t10","o" : "","j" : ""} The transaction is expected to close on or about August 25, 2009, subject to satisfaction of customary closing conditions.
The securities described above are being offered by CEL-SCI Corporation pursuant to a registration statement previously filed and declared effective by the Securities and Exchange Commission. This press release shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state. The securities may be offered only by means of a prospectus. Copies of the final prospectus supplement and accompanying base prospectus relating to this offering may be obtained at the Securities and Exchange Commission's website at http://www.sec.gov .
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine® which is being readied for a global Phase III trial in advanced primary head and neck cancer. CEL-SCI is also developing a vaccine to prevent and treat swine and other influenzas using its L.E.A.P.S. technology platform and expects to soon finish the validation of its state-of-the-art facility in Maryland which it expects to utilize to launch aseptic filling for stem cell produced therapies and other biological products. The Company has operations in Vienna, Virginia, and Baltimore, Maryland.
Certain statements contained herein relating to the anticipated closing of the offering or product development, or that otherwise relate to future periods, are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These statements are based on assumptions that may not prove accurate. Actual results could differ materially from those anticipated due to certain risks inherent in the biotechnology industry and for companies engaged in the development of new products in a regulated market. These risks, including those related to whether the offering will close when anticipated or at all, the results of discovery research and preclinical testing; the timing or results of pending and future clinical trials (including the design and progress of clinical trials; safety and efficacy of the products being tested; action, inaction or delay by the FDA, European or other regulators or their advisory bodies; and analysis or interpretation by, or submission to, these entities or others of scientific data); uncertainties regarding the status of biotechnology patents; uncertainties as to the cost of protecting intellectual property; changes in the status of the existing collaborative and licensing relationships; the ability of collaborators, licensees and other third parties to meet their obligations; market demand for products; scale up and marketing capabilities; competition; international operations; share price volatility; and CEL-SCI's financing needs and opportunities are described in more detail in CEL-SCI's most recent annual report on Form 10-K/A and in other SEC filings. Consider such risks carefully in considering CEL-SCI's prospects.
 ...nicht schön...allerdings wirds billiger...hilft nix...nun gut bin eh noch nicht fertig mit meinen Positionen
...nicht schön...allerdings wirds billiger...hilft nix...nun gut bin eh noch nicht fertig mit meinen Positionen
So mal einige K heute eingepackt...na ja ist zwar ein S **** Kurs...aber solange ich am Einkaufen bin auch nicht schlecht..konnte meinen Schnitt doch schön senken...aber irgendwann sollte dann mal was passieren
So ne Gurke heute wieder...die hätten auch 0,50 US$ machen können...jetzt krebseln wir da unten rum...aber o.k ich hab Zeit...jede Menge
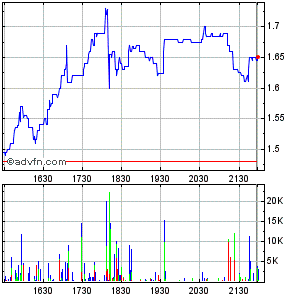
Die brauchst du "Expertchen" auch 

Antwort auf Beitrag Nr.: 37.819.676 von carpe--diem am 20.08.09 17:20:31Kann es sein das Du Dich irgendwie verirrst hast ???
Ist vom 7.7.09
**************************************************************
CVM - Letter To Shareholders (Info) 21-Aug-09 02:26 am On 7/7/09, CEL-SCI Corp. (AMEX:CVM) ($0.50) provided the following updates in a letter to shareholders. The company's three business units include:
Vaccines/treatment: H1N1 (swine) and other influenza viruses, as well as a vaccine for rheumatoid arthritis;
Late-stage non-toxic cancer immunotherapy, Multikine, designed to make the first cancer treatment more successful; and
Unique contract manufacturing services using new manufacturing facility.
CVM recently completed its $22 million manufacturing facility, which is expected to be validated within the next 3 months (i.e. early October 2009) for the manufacture of Multikine for the company's pending Phase III trial and subsequent sale if approved for marketing.
CVM stated that the validation of this facility is a critical step to starting the pivotal Phase 3 trial for Multikine. CVM stated that the Phase 3 study protocol was designed in consultation with the FDA and is expected to enroll about 800 patients to assess overall patient survival as the primary outcome.
The company also stated that the FDA has granted Multikine Orphan Drug status in the USA, and (if the Phase 3 study is successful) Multikine would be on course to become the recommended first-line treatment for head and neck cancer.
**************************************************************
CVM - Letter To Shareholders (Info) 21-Aug-09 02:26 am On 7/7/09, CEL-SCI Corp. (AMEX:CVM) ($0.50) provided the following updates in a letter to shareholders. The company's three business units include:
Vaccines/treatment: H1N1 (swine) and other influenza viruses, as well as a vaccine for rheumatoid arthritis;
Late-stage non-toxic cancer immunotherapy, Multikine, designed to make the first cancer treatment more successful; and
Unique contract manufacturing services using new manufacturing facility.
CVM recently completed its $22 million manufacturing facility, which is expected to be validated within the next 3 months (i.e. early October 2009) for the manufacture of Multikine for the company's pending Phase III trial and subsequent sale if approved for marketing.
CVM stated that the validation of this facility is a critical step to starting the pivotal Phase 3 trial for Multikine. CVM stated that the Phase 3 study protocol was designed in consultation with the FDA and is expected to enroll about 800 patients to assess overall patient survival as the primary outcome.
The company also stated that the FDA has granted Multikine Orphan Drug status in the USA, and (if the Phase 3 study is successful) Multikine would be on course to become the recommended first-line treatment for head and neck cancer.
Antwort auf Beitrag Nr.: 37.825.335 von carpe--diem am 21.08.09 12:45:24Hat sich gerade ...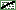 ...erledigt...schönes Restleben noch
...erledigt...schönes Restleben noch
Antwort auf Beitrag Nr.: 37.825.502 von Expertchen007 am 21.08.09 13:05:35Wat bist du nur empfindlich... Keine beleidigende Aussage seitens carpe--diem, nur der Hinweis auf Geduld. Und dein Name ist nun mal Expertchen, wat erwartest du? Das er dich mit Prinz anspricht? Und du darfst mich gern auf ignore stellen wenn dir dieser Kommentar gegen den Strich geht. Vogel Straus Taktik...
Aber gut, jedem das seine... Schönes Wocheneende an alle
Aber gut, jedem das seine... Schönes Wocheneende an alle
 pre markt die ersten käufe bei 0.45 --- schade kann heute nicht zugucken, aber immer wenn ich weg war ist was passiert !!!
pre markt die ersten käufe bei 0.45 --- schade kann heute nicht zugucken, aber immer wenn ich weg war ist was passiert !!! 
Antwort auf Beitrag Nr.: 37.825.571 von Fafnir77 am 21.08.09 13:15:14Ne darum gehts nicht...sondern um dämliche Kommentare...brauch ich nicht...
Antwort auf Beitrag Nr.: 37.826.228 von ALF-FRED am 21.08.09 14:32:12Alf-fred...das sind Spielkurse....wir werden zum Schluß mal abrrechnen..und ich denke da sieht es anders aus. ..der Sack von Makler rückt nix raus zu 0,304...erscheint nicht mal im Bid....so langsam füllt sich das CEL Depot...50 K ist Ende
..der Sack von Makler rückt nix raus zu 0,304...erscheint nicht mal im Bid....so langsam füllt sich das CEL Depot...50 K ist Ende
 ..der Sack von Makler rückt nix raus zu 0,304...erscheint nicht mal im Bid....so langsam füllt sich das CEL Depot...50 K ist Ende
..der Sack von Makler rückt nix raus zu 0,304...erscheint nicht mal im Bid....so langsam füllt sich das CEL Depot...50 K ist Ende 
Wer gibt denn dauernd dämliche Kommentare

 mal ein Auszugaus dem u.g. filling
mal ein Auszugaus dem u.g. fillingCEL-SCI'S PRODUCTS AND "COLD FILL" MANUFACTURING SERVICE
CEL-SCI's business consists of the following:
1) Multikine cancer therapy;
2) New "cold fill" manufacturing service to the pharmaceutical industry; and
3) LEAPS technology, with two products, H1N1 swine flu vaccine/treatment and CEL-2000, a rheumatoid arthritis treatment vaccine.
http://secfilings.com/searchresultswide.aspx?link=1&filingid…
Antwort auf Beitrag Nr.: 37.833.150 von ALF-FRED am 23.08.09 08:59:00Guter Artlikel...sag doch die hätten die 4,4 Mill. nur mit 0,50 ansetzen müssen...aber ne muss ja mit 0,45 gemacht werden....nun gut es zählt was in Phase 3 rauskommt....der Markt ist heiß auf das Produkt...zumal immer mehr Panik mit dem Virus aufkommt....das jenige Unternehmen welches am schnellsten Resultate hat wird Milliarden verdienen
Panik 











So jetzt wirds wieder mal normal....Volumen allerdings kann man vergessen
NASDAQ Last Sale
0.49 0.04 +8.93%
Volume
1,700 Previous Close
$ 0.45
Today's High
$ 0.50 Today's Low
$ 0.49
NASDAQ Last Sale
0.49 0.04 +8.93%
Volume
1,700 Previous Close
$ 0.45
Today's High
$ 0.50 Today's Low
$ 0.49
 da geht noch was minütlich
da geht noch was minütlichPre-Market Trade Reporting
Pre-Market Charts | After Hours Charts Pre-Market
Last: $ .51 Pre-Market
High: $ .51
Pre-Market
Volume: 38,232 Pre-Market
Low: $ .49
Antwort auf Beitrag Nr.: 37.837.955 von ALF-FRED am 24.08.09 14:30:08
43,132 Pre-Market
Low: $ .49
Pre-Market
Time (ET) Pre-Market
Price Pre-Market
Share Volume
08:30 $ .50 400
08:30 $ .50 2,000
08:29 $ .50 2,500
08:28 $ .51 150
08:27 $ .50 100
08:27 $ .50 1,693
08:27 $ .50 479
08:22 $ .50 4,700
08:22 $ .50 2,000
08:22 $ .50 2,300
08:22 $ .50 100
08:22 $ .50 100
08:22 $ .50 193
08:22 $ .50 4,200
08:22 $ .50 100
08:22 $ .50 100
08:22 $ .50 200
08:21 $ .50 5,000
08:21 $ .50 1,000
08:21 $ .50 300
08:20 $ .51 190
08:19 $ .51 220
08:18 $ .50 3,000
08:16 $ .49 1,000
08:14 $ .50 100
08:14 $ .50 1,807
08:14 $ .50 5,000
08:14 $ .49 1,000
08:13 $ .50 1,000
08:13 $ .50 1,500
08:06 $ .50 300
08:05 $ .498 200
08:05 $ .50 200

43,132 Pre-Market
Low: $ .49
Pre-Market
Time (ET) Pre-Market
Price Pre-Market
Share Volume
08:30 $ .50 400
08:30 $ .50 2,000
08:29 $ .50 2,500
08:28 $ .51 150
08:27 $ .50 100
08:27 $ .50 1,693
08:27 $ .50 479
08:22 $ .50 4,700
08:22 $ .50 2,000
08:22 $ .50 2,300
08:22 $ .50 100
08:22 $ .50 100
08:22 $ .50 193
08:22 $ .50 4,200
08:22 $ .50 100
08:22 $ .50 100
08:22 $ .50 200
08:21 $ .50 5,000
08:21 $ .50 1,000
08:21 $ .50 300
08:20 $ .51 190
08:19 $ .51 220
08:18 $ .50 3,000
08:16 $ .49 1,000
08:14 $ .50 100
08:14 $ .50 1,807
08:14 $ .50 5,000
08:14 $ .49 1,000
08:13 $ .50 1,000
08:13 $ .50 1,500
08:06 $ .50 300
08:05 $ .498 200
08:05 $ .50 200
 dicke dinger auf level 2 im orderbuch
dicke dinger auf level 2 im orderbuch 
Pre...
NASDAQ Last Sale
0.50 0.03 + 6.32%
Volume
20,000 Previous Close
$ 0.48
Today's High
$ 0.50 Today's Low
$ 0.50
soll wie immer nicht viel bedeuten...wobei die Amis ja Cel bereits in Richtung 0,80 US$ sehen...bleibt aber erst mal abzuwarten...das regelt dann der Markt nicht die Boards... ...Mittelfristig könnte es in die Richtung gehen
...Mittelfristig könnte es in die Richtung gehen
NASDAQ Last Sale
0.50 0.03 + 6.32%
Volume
20,000 Previous Close
$ 0.48
Today's High
$ 0.50 Today's Low
$ 0.50
soll wie immer nicht viel bedeuten...wobei die Amis ja Cel bereits in Richtung 0,80 US$ sehen...bleibt aber erst mal abzuwarten...das regelt dann der Markt nicht die Boards...
 ...Mittelfristig könnte es in die Richtung gehen
...Mittelfristig könnte es in die Richtung gehen
Cel-Sci Thrives with New Manufacturing Facility
by: M. E. Garza August 25, 2009
M. E. Garza
New details have begun to emerge about Cel-Sci Corporation's (CVM) new, state-of-the-art "Cold-Fill" manufacturing facility which will not only be used to manufacture Multikine® the world's first immunotherapeutic agent (which is being developed as a first-line standard of care treatment for cancer) for both the Phase III trials and commercial sales, but Cel-Sci plans to offer the use of the facility as a service to pharmaceutical companies and others, particularly those that need to "fill and finish" their drugs in a cold environment (4 degrees Celsius, or approximately 39 degrees Fahrenheit).
This is a key process of filling injectable drugs in a sterile manner and is an important part of the manufacturing process for many medicines.
This lab, which many now consider a cornerstone to the future financial success of the company is located near Baltimore, MD. According to newly released documents filed with the SEC, it was designed over several years, and was built out to Cel-Sci's specifications during the past 18 months.
One insider calls it "absolutely state-of-the art" and "extremely impressive down to the smallest detail."
An interview between Cel-Sci's CEO, Geert Kersten, has been scheduled and more details will follow at BioMedReports, but I can tell you that Cel-Sci believes it will be able to charge approximately $150,000 for an eight hour fill and finish "run" at this extremely unique facility whose aseptic filling suites are maintained at FDA and EU ISO classifications of 5/6.
Cel-Sci also has the capability to formulate, inspect, label and package biologic products at cold temperatures. Furthermore, the company discloses that they do not know of any other facility in the United States which is able to provide cold 4 degrees Celsius finish and fill services on a contract basis.
What this will mean for the financial security and future of the company is significant, particularly given the fact that the fastest area of growth in the biopharmaceutical and pharmaceutical markets is biologics, and most recently stem cell products.
These compounds and therapies are derived from or mimic human cells or proteins and other molecules (e.g., hormones, etc.). Nearly all of the major drugs developed for unmet medical needs (e.g., Avastin(R), Erbitux(R), Rituxan(R), Herceptin(R), Copaxon(R), etc.) are biologics. Biologics are usually very sensitive to such things as heat and humidity and are known to quickly lose their biological activity if exposed to room or elevated temperatures (which may also affect the shelf-life of a biologic - with the result being that the product cannot be stored for as long as desired).
These products do not generally lose activity when kept at 4 degrees Celsius.
Shares of Cel-Sci continue to tick upward as signs point to a resurrection of sorts for this biotech play that many had on the ropes and ready for a knock-out punch
by: M. E. Garza August 25, 2009
M. E. Garza
New details have begun to emerge about Cel-Sci Corporation's (CVM) new, state-of-the-art "Cold-Fill" manufacturing facility which will not only be used to manufacture Multikine® the world's first immunotherapeutic agent (which is being developed as a first-line standard of care treatment for cancer) for both the Phase III trials and commercial sales, but Cel-Sci plans to offer the use of the facility as a service to pharmaceutical companies and others, particularly those that need to "fill and finish" their drugs in a cold environment (4 degrees Celsius, or approximately 39 degrees Fahrenheit).
This is a key process of filling injectable drugs in a sterile manner and is an important part of the manufacturing process for many medicines.
This lab, which many now consider a cornerstone to the future financial success of the company is located near Baltimore, MD. According to newly released documents filed with the SEC, it was designed over several years, and was built out to Cel-Sci's specifications during the past 18 months.
One insider calls it "absolutely state-of-the art" and "extremely impressive down to the smallest detail."
An interview between Cel-Sci's CEO, Geert Kersten, has been scheduled and more details will follow at BioMedReports, but I can tell you that Cel-Sci believes it will be able to charge approximately $150,000 for an eight hour fill and finish "run" at this extremely unique facility whose aseptic filling suites are maintained at FDA and EU ISO classifications of 5/6.
Cel-Sci also has the capability to formulate, inspect, label and package biologic products at cold temperatures. Furthermore, the company discloses that they do not know of any other facility in the United States which is able to provide cold 4 degrees Celsius finish and fill services on a contract basis.
What this will mean for the financial security and future of the company is significant, particularly given the fact that the fastest area of growth in the biopharmaceutical and pharmaceutical markets is biologics, and most recently stem cell products.
These compounds and therapies are derived from or mimic human cells or proteins and other molecules (e.g., hormones, etc.). Nearly all of the major drugs developed for unmet medical needs (e.g., Avastin(R), Erbitux(R), Rituxan(R), Herceptin(R), Copaxon(R), etc.) are biologics. Biologics are usually very sensitive to such things as heat and humidity and are known to quickly lose their biological activity if exposed to room or elevated temperatures (which may also affect the shelf-life of a biologic - with the result being that the product cannot be stored for as long as desired).
These products do not generally lose activity when kept at 4 degrees Celsius.
Shares of Cel-Sci continue to tick upward as signs point to a resurrection of sorts for this biotech play that many had on the ropes and ready for a knock-out punch
BioDelivery Sciences, Antigenics, Cel-Sci All Look Like Winners
by: VFC's Stock House August 25, 2009
These three stocks have rebounded nicely on Monday, bouncing off recent lows and an uptrend is in order for each of these stocks.
For BioDelivery Sciences (BDSI), the company is a long term keeper now, in my opinion, and the stock price will eventually reflect that fact - post-Onsolis-approval price action aside.
The 'prime time' for cancer immunotherapy treatments is near, in my opinion, and both Antigenics (AGEN) and Cel-Sci (CVM) should benefit from the growing awareness of the treatment. It's my opinion that Cel Sci, after recently raising capital, is gearing up for a news announcement regarding the much anticipated Multikine Phase III trial. Once that trial date is announced, I believe that the stock could inch towards the one dollar level; therefore I'm buying CVM under fifty cents now, where my previous buy price was for under forty cents.
In my opinion, BDSI, AGEN and CVM are all long term winners.
Disclosure: VFC is long all mentioned stocks.
http://seekingalpha.com/article/158087-biodelivery-sciences-…
by: VFC's Stock House August 25, 2009
These three stocks have rebounded nicely on Monday, bouncing off recent lows and an uptrend is in order for each of these stocks.
For BioDelivery Sciences (BDSI), the company is a long term keeper now, in my opinion, and the stock price will eventually reflect that fact - post-Onsolis-approval price action aside.
The 'prime time' for cancer immunotherapy treatments is near, in my opinion, and both Antigenics (AGEN) and Cel-Sci (CVM) should benefit from the growing awareness of the treatment. It's my opinion that Cel Sci, after recently raising capital, is gearing up for a news announcement regarding the much anticipated Multikine Phase III trial. Once that trial date is announced, I believe that the stock could inch towards the one dollar level; therefore I'm buying CVM under fifty cents now, where my previous buy price was for under forty cents.
In my opinion, BDSI, AGEN and CVM are all long term winners.
Disclosure: VFC is long all mentioned stocks.
http://seekingalpha.com/article/158087-biodelivery-sciences-…
Antwort auf Beitrag Nr.: 37.853.138 von ALF-FRED am 26.08.09 10:22:31Hmmm...war das die 1. Reaktion darauf im AH !!!  ...na ja mal abwarten was nun daraus gemacht wird...aber wird immer interessanter - von Tag zu Tag
...na ja mal abwarten was nun daraus gemacht wird...aber wird immer interessanter - von Tag zu Tag
NASDAQ Last Sale
0.52 0.03 ++ 6.06%
Volume
2,482,311 Previous Close
$ 0.50
 ...na ja mal abwarten was nun daraus gemacht wird...aber wird immer interessanter - von Tag zu Tag
...na ja mal abwarten was nun daraus gemacht wird...aber wird immer interessanter - von Tag zu TagNASDAQ Last Sale
0.52 0.03 ++ 6.06%
Volume
2,482,311 Previous Close
$ 0.50
Hallo Leute,
sind ja doch noch einige der alten Hasen hier.
Schön das sich bei CVM nun langsam was tut. Die Grippe hilft uns wohl oder übel auch noch bei der "Kurspflege". Wobei ich das Gefühl nicht los werde, dass irgend was im Anmarsch ist. Wobei die PIII für M doch bald anlaufen sollte.
bis dann Plaste
sind ja doch noch einige der alten Hasen hier.
Schön das sich bei CVM nun langsam was tut. Die Grippe hilft uns wohl oder übel auch noch bei der "Kurspflege". Wobei ich das Gefühl nicht los werde, dass irgend was im Anmarsch ist. Wobei die PIII für M doch bald anlaufen sollte.
bis dann Plaste
 weiter so
weiter soPre-Market Trade Reporting
Pre-Market Charts | After Hours Charts Pre-Market
Last: $ .58 Pre-Market
High: $ .58
Pre-Market
Volume: 100 Pre-Market
Low: $ .58
Pre-Market
Time (ET) Pre-Market
Price Pre-Market
Share Volume
07:06 $ .58 100
 wir waren scon bis 0.60 - da könnte was kommen noch 1.5 Std. bis zur Eröffnung schon 50 000 im Pre market
wir waren scon bis 0.60 - da könnte was kommen noch 1.5 Std. bis zur Eröffnung schon 50 000 im Pre market8/26/2009 8:07:35 AM Market Closed
NASDAQ Last Sale
0.58 0.08 16.16%
Volume
50,892 Previous Close
$ 0.50
Today's High
$ 0.60 Today's Low
$ 0.53
52 Wk High
$ .80 52 Wk Low
$ .1402
NASDAQ Official Price
Open Price/Date
$ .51
Aug 25, 2009 Close Price/Date
$ .495
Aug 25, 2009
1y Target Est
$ 1
 ich lass mal den mittagsschlaf ausfallen
ich lass mal den mittagsschlaf ausfallen 
8/26/2009 8:10:23 AM Market Closed
NASDAQ Last Sale
0.65 0.16 32.32%
Antwort auf Beitrag Nr.: 37.855.439 von ALF-FRED am 26.08.09 14:11:30Heut brennt der BAum... Da gehen ja schon ordentlich Shares übern Tisch. Bin wirklich gespant wat da kommen mag...
Antwort auf Beitrag Nr.: 37.855.515 von Fafnir77 am 26.08.09 14:21:27http://seekingalpha.com/article/158365-increased-interest-in…
@ Plaste - wir hätten es verdient hab jetzt 2/3 long und 1/3 zum Traden 

Pre-Market
Last: $ .61 Pre-Market
High: $ .65
Pre-Market
Volume: 346,264 Pre-Market
Low: $ .53
Last: $ .61 Pre-Market
High: $ .65
Pre-Market
Volume: 346,264 Pre-Market
Low: $ .53
 noch eine Stunde
noch eine Stunde Pre-Market
Last: $ .6299 Pre-Market
High: $ .65
Pre-Market
Volume: 411,284 Pre-Market
Low: $ .53
Antwort auf Beitrag Nr.: 37.855.536 von Plaste am 26.08.09 14:23:47Danke... Weiß ick bescheid. Aber müsste da nicht mehr hinterstecken bei den Shares die über den Tisch gehen bzw. der Kurs im Pre schon so anzieht? Kann mir nich so vorstellen dass die Aussage seitens der Regierung zu Erkranten und Todesfällen solchen Run auslöst oder wie ist eure MEinung?
 wenn ich das wüsste - habe schon Tagessprünge von über 100 % bei der Aktie gesehen und dann ein paar Tage später alles beim alten - ich warte auf was Nachhaltiges und Fakten P III oder Beteiligung einer großen Pharmafirma - das andere ist Spekulation, aber wenn es dem Kurs hilft
wenn ich das wüsste - habe schon Tagessprünge von über 100 % bei der Aktie gesehen und dann ein paar Tage später alles beim alten - ich warte auf was Nachhaltiges und Fakten P III oder Beteiligung einer großen Pharmafirma - das andere ist Spekulation, aber wenn es dem Kurs hilft 
vom yahoo board - ob es stimmt weiß ich nicht !
ceo coming on cnbc 9;30 baby
by pigeondic [3 minutes ago]
ceo coming on cnbc 9;30 baby
by pigeondic [3 minutes ago]
Pre-Market
Last: $ .5999 Pre-Market
High: $ .65
Pre-Market
Volume: 575,119 Pre-Market
Low: $ .53
Last: $ .5999 Pre-Market
High: $ .65
Pre-Market
Volume: 575,119 Pre-Market
Low: $ .53
Antwort auf Beitrag Nr.: 37.855.744 von ALF-FRED am 26.08.09 14:42:06Ok, es bleibt spannend... Danke der recherchen, auf Arbeit hab ich nich die Zeit dazu. Kann nur ab und an mal reinschauen...
Leck fett...geht ja noch schneller als erwartet...nun gut...immer noch nicht viel...mal abwarten...aber in Depot macht sich das schon mal ganz gut - farblich gesehen
Bin immer noch Buff von dem Volumen im Pre... Besser gehts kaum
Und ich konnte nicht mehr nachlegen 
Na egal

Na egal

die Mille im Pre wirds wohl locker werden -
Pre-Market
Last: $ .60 Pre-Market
High: $ .65
Pre-Market
Volume: 697,457 Pre-Market
Low: $ .53
Pre-Market
Last: $ .60 Pre-Market
High: $ .65
Pre-Market
Volume: 697,457 Pre-Market
Low: $ .53
Gibts heute ein neues Rekorvolumen im Handel..... ...obwohl 30 Millionen ist ne Menge Holz...aber wer weis ...auf jeden Fall Kursmässig bereits die 6 Monate im Pre geschafft !!!!
...obwohl 30 Millionen ist ne Menge Holz...aber wer weis ...auf jeden Fall Kursmässig bereits die 6 Monate im Pre geschafft !!!! 
6 Monatschart

 ...obwohl 30 Millionen ist ne Menge Holz...aber wer weis ...auf jeden Fall Kursmässig bereits die 6 Monate im Pre geschafft !!!!
...obwohl 30 Millionen ist ne Menge Holz...aber wer weis ...auf jeden Fall Kursmässig bereits die 6 Monate im Pre geschafft !!!! 
6 Monatschart
Antwort auf Beitrag Nr.: 37.855.978 von Expertchen007 am 26.08.09 15:06:06Müsste dann gegen 15:30 Uhr unserer Zeit kommen
Pre-Market Trade Reporting
Pre-Market Charts | After Hours Charts Pre-Market
Last: $ .6008 Pre-Market
High: $ .65
Pre-Market
Volume: 902,791 Pre-Market
Low: $ .53
Pre-Market Charts | After Hours Charts Pre-Market
Last: $ .6008 Pre-Market
High: $ .65
Pre-Market
Volume: 902,791 Pre-Market
Low: $ .53
 die mille ist voll , viel spass heute
die mille ist voll , viel spass heute Pre-Market Trade Reporting
Pre-Market Charts | After Hours Charts Pre-Market
Last: $ .57 Pre-Market
High: $ .65
Pre-Market
Volume: 1,000,513 Pre-Market
Low: $ .53
Antwort auf Beitrag Nr.: 37.856.119 von ALF-FRED am 26.08.09 15:18:23Dürfte interessant werden..oder aber clever gemacht seitens USA...wie auch immer...finds interessant...gewaltige Range din
/26/2009 9:21:11 AM Market Closed
NASDAQ Last Sale
0.57 0.07 ++ 14.14%
Volume
550,743 Previous Close
$ 0.50
Today's High
$ 0.65 Today's Low
$ 0.53
/26/2009 9:21:11 AM Market Closed
NASDAQ Last Sale
0.57 0.07 ++ 14.14%
Volume
550,743 Previous Close
$ 0.50
Today's High
$ 0.65 Today's Low
$ 0.53
RT USA

Antwort auf Beitrag Nr.: 37.856.369 von LaPlue am 26.08.09 15:37:41liest sich ja prima!!! aber da müssen nun Bestätigungen des Unternehmens folgen...momentan ein Abtasten des Kurses würd ich mal sagen
Antwort auf Beitrag Nr.: 37.856.486 von Expertchen007 am 26.08.09 15:45:13 wenn dann 16.00 uhr mez wie immer - noch 13 min.
wenn dann 16.00 uhr mez wie immer - noch 13 min.
 wenn dann 16.00 uhr mez wie immer - noch 13 min.
wenn dann 16.00 uhr mez wie immer - noch 13 min.
Antwort auf Beitrag Nr.: 37.856.513 von ALF-FRED am 26.08.09 15:47:44Äh...war doch 9:30 East...also + 6 Stunden..oder wie ?????
So - auf gehts zum fröhlichen Jagen....war zwar noch nicht fertig mit Sammeln...aber bin sehr gut dabei....das TH vom RRE will ich aber wenigstens noch mal sehen...gerne auch etws mehr...
Antwort auf Beitrag Nr.: 37.856.513 von ALF-FRED am 26.08.09 15:47:44 jetzt gehts hoch !!
jetzt gehts hoch !!
 jetzt gehts hoch !!
jetzt gehts hoch !!
Antwort auf Beitrag Nr.: 37.856.677 von ALF-FRED am 26.08.09 15:57:55Ja..viel blau drin...sag ja mal sehen obs ein Rekordvolumen reicht...könnte passieren...sollte dann mal so bei 0,81 -0,84 US$ stehen...falls es die 30 Millionen knackt....bin da relaxt...so nun ab zur Show
 immer mit 100 runtergeprügelt
immer mit 100 runtergeprügelt 
Antwort auf Beitrag Nr.: 37.856.867 von ALF-FRED am 26.08.09 16:11:55Also ich find auf CNBC nix...oder wie ?????
Antwort auf Beitrag Nr.: 37.856.947 von Expertchen007 am 26.08.09 16:17:15ich auch nicht,
Aus dem USA Board...musss ich erst mal anschauen
************************
REASON FOR JUMP IS... 1 minute ago a SENSATIONAL report today on BIOMEDREPORTS.COM--i'd be amazed if this stock isn't pushing $5 very soon.
************************
REASON FOR JUMP IS... 1 minute ago a SENSATIONAL report today on BIOMEDREPORTS.COM--i'd be amazed if this stock isn't pushing $5 very soon.
Antwort auf Beitrag Nr.: 37.856.974 von Expertchen007 am 26.08.09 16:19:10http://biomedreports.com/ ????
Ja dann mal los...
Antwort auf Beitrag Nr.: 37.856.972 von ALF-FRED am 26.08.09 16:19:06Tja Alf-fred...wie ich sagte...vor einiger Zeit " vorbei ists mit der Ruhe " 

Tradegate jetzt auch wieder aktiv dabei mit Bid 0,428 € bereits ...hatten seither kein Volumen gestellt
über 10 Millionen jetzt schon !!! gehandelt...aufällig fast 20 % Shortpositionen....obs zu einem Squezze kommt...??????
Antwort auf Beitrag Nr.: 37.857.138 von Expertchen007 am 26.08.09 16:31:48Artikelauszug...aus dem Link
********************************************
Tuesday, 25 August 2009 02:27
Shares of CEL-SCI continue to tick upward as signs point to a resurection of sorts for this biotech play that many had on the ropes and ready for a knock-out punch.
This was a company struggling to keep their doors open before a recent influx of millions in bullish investment capital and recent debt pay-offs.
Once can see trading volume increasing as speculators have begun to move in now that the most recent filings with the SEC have confirmed that CEL-SCI has also been quietly positioning itself as a player in the H1N1 space with solutions that will get the attention of parties at the CDC, FDA and beyond (if they haven't already). This possibility now ringing more true than ever given the warning bells sounded by the Obama administration's advisory group on Science and Technology, who said in a report released yesterday afternoon that the H1N1 flu virus could cause as many as 30,000 and 90,000 deaths in the United States and that it "poses a serious health threat" to young people and children- unlike past strains of the virus.
The widely published report predicts that at least 1.8 million people will be hospitalized during the epidemic, with up to 300,000 patients requiring intensive care units. These patients could occupy 50-100 percent of all ICU beds in affected regions at the peak of the epidemic and would place "enormous stress" on ICU units.
Keep in mind that these are U.S. numbers alone and that this is one of the few countries with the financial resources to begin "hording" vaccine suuplies. Imagine what the impact might be in coutries with far
********************************************
Tuesday, 25 August 2009 02:27
Shares of CEL-SCI continue to tick upward as signs point to a resurection of sorts for this biotech play that many had on the ropes and ready for a knock-out punch.
This was a company struggling to keep their doors open before a recent influx of millions in bullish investment capital and recent debt pay-offs.
Once can see trading volume increasing as speculators have begun to move in now that the most recent filings with the SEC have confirmed that CEL-SCI has also been quietly positioning itself as a player in the H1N1 space with solutions that will get the attention of parties at the CDC, FDA and beyond (if they haven't already). This possibility now ringing more true than ever given the warning bells sounded by the Obama administration's advisory group on Science and Technology, who said in a report released yesterday afternoon that the H1N1 flu virus could cause as many as 30,000 and 90,000 deaths in the United States and that it "poses a serious health threat" to young people and children- unlike past strains of the virus.
The widely published report predicts that at least 1.8 million people will be hospitalized during the epidemic, with up to 300,000 patients requiring intensive care units. These patients could occupy 50-100 percent of all ICU beds in affected regions at the peak of the epidemic and would place "enormous stress" on ICU units.
Keep in mind that these are U.S. numbers alone and that this is one of the few countries with the financial resources to begin "hording" vaccine suuplies. Imagine what the impact might be in coutries with far
shit, wollte noch zu 0,60 $ rein. hat aber nicht geklappt. 
was sagt ihr zum jetzigen kurs als einstieg. recht hoch, wie mir scheint.
welche ist die news, die diesen kurssprung heute ausgelöst hat?
bye
schlumpftrader

was sagt ihr zum jetzigen kurs als einstieg. recht hoch, wie mir scheint.
welche ist die news, die diesen kurssprung heute ausgelöst hat?
bye
schlumpftrader
Antwort auf Beitrag Nr.: 37.857.176 von schlumpftrader am 26.08.09 16:35:091 Seite zurück blättern....na ja wenn es so ist wie die sagen...das ist wohl dieser Kurs nix als nur ein Anfang...meine persönliche Meinung....aber wait and see
Neues TH ist schon mal da...ab der 0,66 US$ wirds interessant...dann ist das TH PRE gefallen ...aber wird hart
Kann man sich ja nicht beschweren..für einen bunten Nachmittag...mal so zwischendurch
NASDAQ Last Sale
0.64 0.15 +30.30%
NASDAQ Last Sale
0.64 0.15 +30.30%
So jetzt müsste aber dann der Short Squezze einsetzen..wenn ich es richtig gerechnet habe...dann mal anschnallen
Antwort auf Beitrag Nr.: 37.857.276 von Expertchen007 am 26.08.09 16:43:38wie weit sollte das gehen?
lirius
lirius
So muss rüber zu Genta,,,da gibts jetzt auch ne Liveschaltung...bis dann mal
Antwort auf Beitrag Nr.: 37.857.393 von lirius am 26.08.09 16:52:503 Minuten hab ich noch...na ja möglichst weit....der Markt ist gewaltig...und daran mag ich jetzt gar nicht denken...alles von 0,50 - 50 US$ ist möglich.....aber keiner weis was passiert....der wo zuerst das Produkt hat...gewinnt den Jackpot
Antwort auf Beitrag Nr.: 37.857.458 von Expertchen007 am 26.08.09 16:57:57alles von 0,50 - 50 US$ ist möglich.....aber keiner weis was passiert....der wo zuerst das Produkt hat...gewinnt den Jackpot
das klingt sesationell, aber wenn es klappt und cel-sci den impfstoff entwickelt kann das der knaller 2009 werden....sach ich mal leichtsinnig...
das klingt sesationell, aber wenn es klappt und cel-sci den impfstoff entwickelt kann das der knaller 2009 werden....sach ich mal leichtsinnig...

Der Artikelauszug mal auf googledeutsch...
**************************************************
Dienstag, 25 August 2009 02:27
Die Aktien der CEL-SCI weiter nach oben zu kreuzen als Zeichen deuten auf eine resurection für diese Art von Biotech-Spiel, das viele auf die Seile und bereit für eine Knock-out-Schlag.
Dies war ein Unternehmen, das bemüht zu halten ihre Türen öffnen, bevor eine aktuelle Zustrom von Millionen Menschen in bullish Investitionskapital und bisherigen Schulden zu begleichen-offs.
Einmal sehen Handelsvolumen zunimmt, da Spekulanten haben damit begonnen, sich jetzt zu bewegen, dass die neuesten Einreichungen bei der SEC bestätigt haben, dass CEL-SCI hat auch ruhig gewesen positioniert sich als ein Spieler in der H1N1-Raum mit Lösungen, die die Aufmerksamkeit der Parteien erhalten bei der CDC, FDA und darüber hinaus (wenn sie nicht bereits geschehen). Diese Möglichkeit mehr, jetzt klingelt denn je angesichts der Warnung läuteten die Glocken von Beratergruppe der Regierung Obama für Wissenschaft und Technologie, die in einem veröffentlichten Bericht gestern Nachmittag gesagt, dass die Grippe-Virus H1N1 nicht weniger als 30.000 und 90.000 Todesfälle in den Vereinigten Staaten dazu führen könnten, und dass es "stellt eine ernste Gefahr für die Gesundheit" für Jugendliche und Kinder, anders als bei früheren Stämmen des Virus.
Die weithin veröffentlichten Bericht sagt voraus, dass mindestens 1,8 Millionen Menschen während der Epidemie ins Krankenhaus eingeliefert werden, werden mit bis zu 300.000 Patienten, die Intensivstationen. Diese Patienten könnten 50-100 Prozent aller ICU-Betten in den betroffenen Regionen auf dem Höhepunkt der Epidemie zu besetzen und würde statt "einem enormen Druck" auf Intensivstation Einheiten.
Beachten Sie, dass diese US-Zahlen allein sind und dass dies eines der wenigen Länder, mit den finanziellen Mitteln zu beginnen "Horten"-Impfstoff suuplies. Stellen Sie sich vor, was die Auswirkungen auf die Länder einsetzen könnten mit weit

**************************************************
Dienstag, 25 August 2009 02:27
Die Aktien der CEL-SCI weiter nach oben zu kreuzen als Zeichen deuten auf eine resurection für diese Art von Biotech-Spiel, das viele auf die Seile und bereit für eine Knock-out-Schlag.
Dies war ein Unternehmen, das bemüht zu halten ihre Türen öffnen, bevor eine aktuelle Zustrom von Millionen Menschen in bullish Investitionskapital und bisherigen Schulden zu begleichen-offs.
Einmal sehen Handelsvolumen zunimmt, da Spekulanten haben damit begonnen, sich jetzt zu bewegen, dass die neuesten Einreichungen bei der SEC bestätigt haben, dass CEL-SCI hat auch ruhig gewesen positioniert sich als ein Spieler in der H1N1-Raum mit Lösungen, die die Aufmerksamkeit der Parteien erhalten bei der CDC, FDA und darüber hinaus (wenn sie nicht bereits geschehen). Diese Möglichkeit mehr, jetzt klingelt denn je angesichts der Warnung läuteten die Glocken von Beratergruppe der Regierung Obama für Wissenschaft und Technologie, die in einem veröffentlichten Bericht gestern Nachmittag gesagt, dass die Grippe-Virus H1N1 nicht weniger als 30.000 und 90.000 Todesfälle in den Vereinigten Staaten dazu führen könnten, und dass es "stellt eine ernste Gefahr für die Gesundheit" für Jugendliche und Kinder, anders als bei früheren Stämmen des Virus.
Die weithin veröffentlichten Bericht sagt voraus, dass mindestens 1,8 Millionen Menschen während der Epidemie ins Krankenhaus eingeliefert werden, werden mit bis zu 300.000 Patienten, die Intensivstationen. Diese Patienten könnten 50-100 Prozent aller ICU-Betten in den betroffenen Regionen auf dem Höhepunkt der Epidemie zu besetzen und würde statt "einem enormen Druck" auf Intensivstation Einheiten.
Beachten Sie, dass diese US-Zahlen allein sind und dass dies eines der wenigen Länder, mit den finanziellen Mitteln zu beginnen "Horten"-Impfstoff suuplies. Stellen Sie sich vor, was die Auswirkungen auf die Länder einsetzen könnten mit weit
So erst mal Pause beim Kurs in USA...mal überraschen lassen wo es langgeht diese Tage...bzw. nächste Woche
Antwort auf Beitrag Nr.: 37.857.857 von hadesitem am 26.08.09 17:34:37und cel-sci den impfstoff entwickelt kann das der knaller 2009 werden
Tja..wenn !!! dann !!!! geanu darin liegt ja das sein oder nicht sein im Biotech
Tja..wenn !!! dann !!!! geanu darin liegt ja das sein oder nicht sein im Biotech
irre range auf jeden Fall heute...hätte man fast traden können..
NASDAQ Last Sale
0.5848 0.09 ++18.18%
Volume
12,854,925 Previous Close
$ 0.50
Today's High
$ 0.68 Today's Low
$ 0.53
NASDAQ Last Sale
0.5848 0.09 ++18.18%
Volume
12,854,925 Previous Close
$ 0.50
Today's High
$ 0.68 Today's Low
$ 0.53
Antwort auf Beitrag Nr.: 37.857.898 von Expertchen007 am 26.08.09 17:38:31 dann trinken wir mal einen
dann trinken wir mal einen 
 dann trinken wir mal einen
dann trinken wir mal einen 
Antwort auf Beitrag Nr.: 37.858.019 von ALF-FRED am 26.08.09 17:49:59wenns klappt...dürfen es auch 2 sein...aber wie immer ...erst mal langsam...jetzt sind erst mal wieder die Trader an der Reihe...hilft nix...heute ist nur 1 Tag von vielen..die noch kommen...obwohl es sich gelohnt hätte...wären schon einige K gewesen...nun gut, nehm ich irgendwann die große Portion mit ( so hoffe ich mal )
Antwort auf Beitrag Nr.: 37.857.898 von Expertchen007 am 26.08.09 17:38:31wieviel unternehmen arbeiten denn an der entwicklung eines solchen impfstoffes....??
das sind doch weltweit einige sicherlich auch deutsche institute...
das ist ein spannender sektor....wer macht das rennen
das sind doch weltweit einige sicherlich auch deutsche institute...
das ist ein spannender sektor....wer macht das rennen

auf der 0,39 oder 0,397 nehm ich weitere auf...aber es ist wie immer bei Cel...unten gibt der nix raus...ich warte 

Antwort auf Beitrag Nr.: 37.858.075 von hadesitem am 26.08.09 17:55:52sind schon ein paar...was mir bekannt ist 5 wichtige...nur die erzählen alle nicht gerne wie weit sie sind...nicht mal uns....bleibt nur zu hoffen den richtigen zu haben....es würde aber schon das Wort " wahrscheinlich " genügen...dann gehts schon rund...na ja mal sehen wie weit die US Regierung da noch mitspielt.
Details kannste von " Gustl24 " erfahren der ist da sehr gut drin in der Story
Details kannste von " Gustl24 " erfahren der ist da sehr gut drin in der Story
Antwort auf Beitrag Nr.: 37.858.133 von Expertchen007 am 26.08.09 18:01:04
die übersetzungen sind eben immer etwas schlech zu verstehen.....schreibt gustl 24 hier regelmäßig..??

die übersetzungen sind eben immer etwas schlech zu verstehen.....schreibt gustl 24 hier regelmäßig..??
Antwort auf Beitrag Nr.: 37.858.234 von hadesitem am 26.08.09 18:12:22Wie er Zeit hat...triffst ihn aber auch bei X*** hin und wieder...am besten per BM anschreiben...machen wir auch zumeist
Das war wohl ein Ausfall bei W.O...nun gut -we are back...und der Kurs auch...allerdings etwas tiefer
Mal sehen obs eine leichte W Formation gibt...denke allerdings erst zum Schluß hin...ist ja auch ganz ordentlich bereits gestiegen
Volumen lässt doch sehr nacn...aber der Rekord dürfte fallen...Rest hängt auch mit schwachem Dow jetzt zusammen...
DOW RT
DOW RT
Ein Blick auf den...
5 Jahreschart
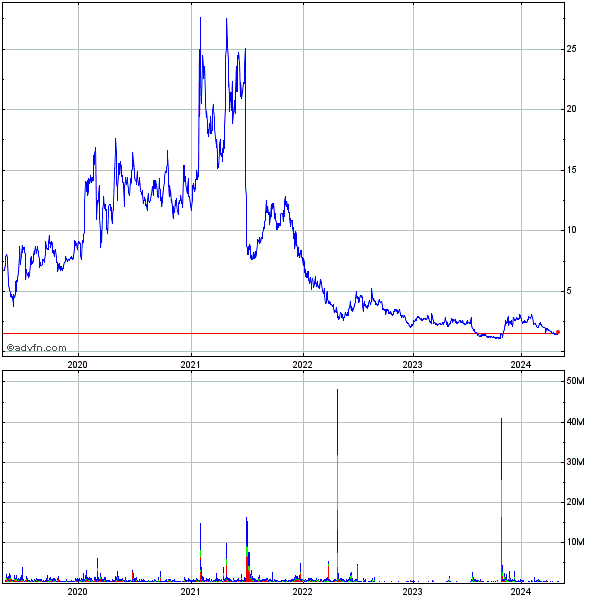
sagt uns das wir fast/ oder auch doch das Rekordvolumen / Allzeit schaffen könnten....allerdings müsste da noch was kommen...im Moment rund 26 Millionen gehandelt...mal sehen was am Finale passiert
5 Jahreschart
sagt uns das wir fast/ oder auch doch das Rekordvolumen / Allzeit schaffen könnten....allerdings müsste da noch was kommen...im Moment rund 26 Millionen gehandelt...mal sehen was am Finale passiert
Sieht gut aus bis jetzt...dürfte hinten stark werden so ab 21:50 Uhr...mal sehen wer das Rennen macht die Bashs--oder die Pushs...
Ein wirklich spannendes Finale ...Cortal bereits wieder bei 0,465 €...dürfte morgen noch interessanter werden
After hours zieht das Teil auch noch an.
Mal sehen ob es wieder einmal nur heiße Luft ist.
Hab die schon so lange und auch solche Reaktionen gab es schon zigmal.
Alleine die Phase III scheint nie enden zu wollen - nur wenn sie wirklich einen positiven FDA Bescheid für Multikine erlangen sehe ich da Licht am Ende des Tunnels.
Von Bioterror mit Milzbrand über AvialFlu und nun Schweinegrippe, hatten wir doch alles schon.......
Vielleicht wieder einmal eine Gelegenheit für Raus und Rein.

Mal sehen ob es wieder einmal nur heiße Luft ist.
Hab die schon so lange und auch solche Reaktionen gab es schon zigmal.
Alleine die Phase III scheint nie enden zu wollen - nur wenn sie wirklich einen positiven FDA Bescheid für Multikine erlangen sehe ich da Licht am Ende des Tunnels.
Von Bioterror mit Milzbrand über AvialFlu und nun Schweinegrippe, hatten wir doch alles schon.......
Vielleicht wieder einmal eine Gelegenheit für Raus und Rein.

Antwort auf Beitrag Nr.: 37.860.545 von franzwerner am 26.08.09 23:46:09NASDAQ Last Sale
0.68 0.19 + 38.38%
Volume
19,219,584 Previous Close
$ 0.50
AH geb ich dir absolut recht...nur hier scheint sich eine neue -Dimension der Entwicklung anzuberaumen...gewalitg !!! aber gut
0.68 0.19 + 38.38%
Volume
19,219,584 Previous Close
$ 0.50
AH geb ich dir absolut recht...nur hier scheint sich eine neue -Dimension der Entwicklung anzuberaumen...gewalitg !!! aber gut

So weit ich weis ist das bereits Fakt ...bin gespannnt auf Deine Antwort ?? !!!
Antwort auf Beitrag Nr.: 37.860.545 von franzwerner am 26.08.09 23:46:09Hi franzwerner!
Bevor etwas enden kann muss es erst einmal beginnen...
Phase III Start ist für Q4 2009 geplant...vorher gibt es aber noch ein paar andere Ergebnisse...
Gruss,
Sil
Bevor etwas enden kann muss es erst einmal beginnen...
Phase III Start ist für Q4 2009 geplant...vorher gibt es aber noch ein paar andere Ergebnisse...
Gruss,
Sil
Wie wahr Sillak, die hat ja noch nicht einmal begonnen (noch schlimmer). Vergaß auch noch HIV anzuführen, das Thema war ja Ende 98 die "virale Bedrohung" schlechthin. Gut dass es Avianflu und Schweinegrippe gibt.
Scherz beseite, eigentlich bin ich seinerzeit in diese Aktie eingestiegen weil mir der Ansatz Immuntherapie in der Onkologie als der einzig plausible erscheint.
Man braucht sich heute nur etwas in der onkologischen Praxis umzusehen und wird unschwer erkennen, dass bereits ein beträchtlicher Teil,insbesondere hämatologischer Neoplasien,mit monoklonalen Antikörpern angegangen wird. Diese sind meist muriner Natur.
Den Körper selbst zur Heilung zu bewegen indem man sein Immunsystem auf Vordermann bringt (welches in der Onkogenese ja offenbar versagt hat) - das ist es was diese Firma für mich ausmacht.
Ich bin da schon so lang drinnen, das sehe ich mir bis zum Ende an, vielleicht löse ich bei einem Lauf wieder einmal einen Teil der Position auf um dann wieder einzusteigen. Dies hat sich schon etliche Male gut machen lassen.
Keine Frage dies ist prinzipiell eine Longposition.
Scherz beseite, eigentlich bin ich seinerzeit in diese Aktie eingestiegen weil mir der Ansatz Immuntherapie in der Onkologie als der einzig plausible erscheint.
Man braucht sich heute nur etwas in der onkologischen Praxis umzusehen und wird unschwer erkennen, dass bereits ein beträchtlicher Teil,insbesondere hämatologischer Neoplasien,mit monoklonalen Antikörpern angegangen wird. Diese sind meist muriner Natur.
Den Körper selbst zur Heilung zu bewegen indem man sein Immunsystem auf Vordermann bringt (welches in der Onkogenese ja offenbar versagt hat) - das ist es was diese Firma für mich ausmacht.
Ich bin da schon so lang drinnen, das sehe ich mir bis zum Ende an, vielleicht löse ich bei einem Lauf wieder einmal einen Teil der Position auf um dann wieder einzusteigen. Dies hat sich schon etliche Male gut machen lassen.
Keine Frage dies ist prinzipiell eine Longposition.
Antwort auf Beitrag Nr.: 37.861.162 von franzwerner am 27.08.09 08:34:39Ob der Kurs ohne H5N1 solchen Hype erlebt hätte...wer weiss...eher nicht.
Es ist gut, dass mit LEAPS CEL etwas hat, was evtl. die Risiken einer Pandemie vermindert, aber letzendlich freue ich mich mehr auf die PIII für MK. Krebs ist immer noch das Geschwür, was der Mensch endlich los werden muss...(das Cashinteresse erwähne ich jetzt mal nicht. So ein Invest in eine Krebsbiotech hat immer ihre zwei Seiten (Ethik/Moral und Börse)
Da die PIII open label ist sollte es dann ausreichend Zündstoff für die kommenden Monate nach dem Start geben...der dann hoffentlich bald kommt.
Gruss,
Sil
Es ist gut, dass mit LEAPS CEL etwas hat, was evtl. die Risiken einer Pandemie vermindert, aber letzendlich freue ich mich mehr auf die PIII für MK. Krebs ist immer noch das Geschwür, was der Mensch endlich los werden muss...(das Cashinteresse erwähne ich jetzt mal nicht. So ein Invest in eine Krebsbiotech hat immer ihre zwei Seiten (Ethik/Moral und Börse)
Da die PIII open label ist sollte es dann ausreichend Zündstoff für die kommenden Monate nach dem Start geben...der dann hoffentlich bald kommt.
Gruss,
Sil
 bid - ask 0.68 - 0.69 $
bid - ask 0.68 - 0.69 $
Antwort auf Beitrag Nr.: 37.863.149 von ALF-FRED am 27.08.09 12:20:50Bin gespannt ob die 0,70 US$ fällt ...dann ist der Weg wirklich frei auf die 0,80 US$...was ich am Montag ja noch sehr bezweifelt habe. Aber Cel überrascht ja sehr gerne....vor allem dann wenn keiner mit rechnet...ganz zu schweigen von den verrückten Ami Prognosen im Moment...
Orderbuch sieht sehr gut aus...bereits jetzt... 0,70 US$ die Untergrenze...bin gespannt was die im Pre Market so treiben...dürfte ordentlich Volumen geben
Pre-Market
Last: $ .76 Pre-Market
High: $ .76
Pre-Market
Volume: 500 Pre-Market
Low: $ .76
Last: $ .76 Pre-Market
High: $ .76
Pre-Market
Volume: 500 Pre-Market
Low: $ .76
Wenn es so bleiben " sollte " ! 0,76 US$ = 0,5331 €
sieht mir sehr nach gap close aus....
NASDAQ Last Sale
0.6588 0.04 ++ 6.45%
Volume
316,565 Previous Close
$ 0.62
NASDAQ Last Sale
0.6588 0.04 ++ 6.45%
Volume
316,565 Previous Close
$ 0.62
heute brauchten wir etwas futter vom ceo - keine infos sondern nur hoffnung, die infos erst wenn der hype abflacht 

Pre-Market Trade Reporting
Pre-Market Charts | After Hours Charts Pre-Market
Last: $ .65 Pre-Market
High: $ .76
Pre-Market
Volume: 504,065 Pre-Market
Low: $ .64
Pre-Market Charts | After Hours Charts Pre-Market
Last: $ .65 Pre-Market
High: $ .76
Pre-Market
Volume: 504,065 Pre-Market
Low: $ .64

Antwort auf Beitrag Nr.: 37.864.698 von Expertchen007 am 27.08.09 15:05:11hoffe im interesse aller investierten, dass Du da mal nicht richtig liegst. 
heutiger trend bis jetzt ist aber nicht so schön.

heutiger trend bis jetzt ist aber nicht so schön.

Antwort auf Beitrag Nr.: 37.864.735 von ALF-FRED am 27.08.09 15:08:54Pflichte deiner Aussage bei. Wir brauchen etwas Phantasie. Hoffe es kommt noch, um in deinem Jargon zu bleiben, etwas Futter heut..
was meint ihr wo die reise hingeht? bei großem volume bleibt der kurs im augenblick relativ stabil. die ruhe vor dem sturm?
Antwort auf Beitrag Nr.: 37.865.484 von schlumpftrader am 27.08.09 16:18:25 ich tipp auf tageshoch zum börsenschluss
ich tipp auf tageshoch zum börsenschluss 
 ich tipp auf tageshoch zum börsenschluss
ich tipp auf tageshoch zum börsenschluss 
 gleich kommt die lange weiße kerze
gleich kommt die lange weiße kerze 
 ...aha war mal ne Stunde nicht da...und nix wars....der Tag ist noch lang...hatte das gap close ja befürchtet..nun gut muss immer mal wieder eine Basis geschaffen werden...
...aha war mal ne Stunde nicht da...und nix wars....der Tag ist noch lang...hatte das gap close ja befürchtet..nun gut muss immer mal wieder eine Basis geschaffen werden...
Der Dow trägt auch was dazu bei...ist halt so
DOW RT

DOW RT
rund 7 Millionen gehandelt...allerdings heute mit 33,3 % Käufe und 48,3 % Verkäufe...Rest dürften Shortpositionen sein..also insgesant schwächer als gestern
Quelle : advfn
Quelle : advfn
Antwort auf Beitrag Nr.: 37.866.267 von Expertchen007 am 27.08.09 17:28:28vielen dank für die aufschlussreichen grafiken erstmals. 
das würde jetzt aber nicht unbedingt für die unmittelbare entwicklung des kurses für steigende kurse sprechen, richtig?

das würde jetzt aber nicht unbedingt für die unmittelbare entwicklung des kurses für steigende kurse sprechen, richtig?
Antwort auf Beitrag Nr.: 37.866.294 von schlumpftrader am 27.08.09 17:31:07Nein das alleine nicht....kommt nun darauf an was an weiteren News folgt....heute ist ein Shortertag..so wie ich das sehe...einfach mal abwarten...ist klar das erst mal eine Tendenz her muss...seitens USA...wir können daran eh nix ändern
So der Dow wird stärker sollte dem Gesamtmarkt Auftrieb geben...und Cel wohl auch
Im ASK läuft gar nix im Moment steht bei 0,65....alles aus dem Bid
Antwort auf Beitrag Nr.: 37.866.789 von Expertchen007 am 27.08.09 18:23:05das bedeutet, dass niemand verkaufen will?
Antwort auf Beitrag Nr.: 37.866.892 von schlumpftrader am 27.08.09 18:36:43ne leider andersherum..jetzt allerdings läuft auch der ASK mit!!....
Antwort auf Beitrag Nr.: 37.866.913 von Expertchen007 am 27.08.09 18:38:52muss ich leider zurücknehmen..bleibt beim bid...die 0,65 muss fallen dann gehts weiter
Antwort auf Beitrag Nr.: 37.866.913 von Expertchen007 am 27.08.09 18:38:52ups. 

 gefällt mir der tageschart, morgen ne news und wir haben den dollar
gefällt mir der tageschart, morgen ne news und wir haben den dollar 
Antwort auf Beitrag Nr.: 37.867.097 von ALF-FRED am 27.08.09 19:05:03von mir aus kann der tageschart schön langsam bis am abend so weiter gehen; hab nix dagegen. 
auch im englisch-forum wird von news gemunkelt. steht da wirklich was an (womöglich von CEL-SCI angekündigt) oder ist das einfach nur ne vermutung von ein paar leuten?

auch im englisch-forum wird von news gemunkelt. steht da wirklich was an (womöglich von CEL-SCI angekündigt) oder ist das einfach nur ne vermutung von ein paar leuten?
Antwort auf Beitrag Nr.: 37.867.097 von ALF-FRED am 27.08.09 19:05:03gefällt mir der tageschart, morgen ne news und wir haben den dollar
Das wird auch in den US Boards vermutet..aber der 1 US$ ist ein Stück hin
Das wird auch in den US Boards vermutet..aber der 1 US$ ist ein Stück hin
Antwort auf Beitrag Nr.: 37.867.186 von schlumpftrader am 27.08.09 19:18:43 news kamen schon oft freitags ( sogar nach Börsenschluss ), wir werden es morgen sehen-
news kamen schon oft freitags ( sogar nach Börsenschluss ), wir werden es morgen sehen-
bis morgen, muß jetzt pizza holen und dann popstars gucken,
morgen früh schaue ich mir dann die 0.70 an ( und wenn es der after eight kurs ist )
 news kamen schon oft freitags ( sogar nach Börsenschluss ), wir werden es morgen sehen-
news kamen schon oft freitags ( sogar nach Börsenschluss ), wir werden es morgen sehen-bis morgen, muß jetzt pizza holen und dann popstars gucken,
morgen früh schaue ich mir dann die 0.70 an ( und wenn es der after eight kurs ist )

Antwort auf Beitrag Nr.: 37.867.239 von ALF-FRED am 27.08.09 19:25:41uns ich geh in Biergarten..in meinem Büro unterm Dach hats so 50 grad...schätz ich mal ...jetzt noch
So bin wieder da...zum Finale...und stark ist das Th ...sauber...da ghets ab im AH Handel....
NASDAQ Last Sale
0.6999 0.08 ++12.90%
0.08 ++12.90%
Volume
8,376,989 Previous Close
$ 0.62
NASDAQ Last Sale
0.6999
 0.08 ++12.90%
0.08 ++12.90% Volume
8,376,989 Previous Close
$ 0.62
Ist ja irre das Teil...wo wills denn hin !!!
NASDAQ Last Sale
0.7098 0.09 ++14.52%
0.09 ++14.52%
NASDAQ Last Sale
0.7098
 0.09 ++14.52%
0.09 ++14.52%
Also hier der SK...nun frag ich mich will die im AH Handel denn schon die 0,80 angreifen...durchaus möglich...Hammer Range von 0,58 bis 0,72 alles dabei heute...gibts auch nicht so oft...na das wird noch was werden
SK
8/27/2009 4:00:41 PM Market Closed
NASDAQ Last Sale
0.7098 0.09 +++ 14.52
SK
8/27/2009 4:00:41 PM Market Closed
NASDAQ Last Sale
0.7098 0.09 +++ 14.52
Antwort auf Beitrag Nr.: 37.868.440 von Expertchen007 am 27.08.09 22:02:40Hallo Leute,
ich mach mich ja seit einiger Zeit ziemlich rahr hier bei WO.
Aber bei dem Kursverlauf...
Zum Thema News. Hatte ich nicht was gelesen, das irgend ein US Börsenpusher ein Interviewe mit CVM machen wollte? Ich glaube auch noch diesen Freitag, also morgen ??
ich mach mich ja seit einiger Zeit ziemlich rahr hier bei WO.
Aber bei dem Kursverlauf...
Zum Thema News. Hatte ich nicht was gelesen, das irgend ein US Börsenpusher ein Interviewe mit CVM machen wollte? Ich glaube auch noch diesen Freitag, also morgen ??
Antwort auf Beitrag Nr.: 37.868.678 von Plaste am 27.08.09 22:32:10Hab da auch was gelesen drüber...aber keine genaue Angabe gefunden.....mal abwarten...auf jeden Fall sehe ich in D morgen Kurse um die 0,53 -0,55 € kommen....ein irres Teil ist das hier
After Hours
Last: $ .73 After Hours
High: $ .7399
After Hours
Volume: 137,990 After Hours
Low: $ .70
After Hours
Last: $ .73 After Hours
High: $ .7399
After Hours
Volume: 137,990 After Hours
Low: $ .70
Antwort auf Beitrag Nr.: 37.868.739 von Expertchen007 am 27.08.09 22:43:59hallo mein zockerfreund.
ich bin heute sehr spät in deutschland eingestiegen,mit bauchweh.



ich bin heute sehr spät in deutschland eingestiegen,mit bauchweh.



Antwort auf Beitrag Nr.: 37.868.779 von fliesenbatscher am 27.08.09 22:51:06Locker bleiben. Mein Durchschnittskurs liegt bei 0,62 €. Wobei ich schon seit Jahren investiert bin.
Wobei ich kein Zocker bin. Und wenn PIII von Multikine wirklich ( ENDLICH !) im nächsten Quartal an den Start gehen sollte, sehe ich das was wir heute sehen als absolute Schnäppchen.
Nebenbei haben wir ja nun auch noch LAPS und die Grippe....
mfg Plaste
Wobei ich kein Zocker bin. Und wenn PIII von Multikine wirklich ( ENDLICH !) im nächsten Quartal an den Start gehen sollte, sehe ich das was wir heute sehen als absolute Schnäppchen.
Nebenbei haben wir ja nun auch noch LAPS und die Grippe....
mfg Plaste
 ich sollte börsenbriefe herausgeben
ich sollte börsenbriefe herausgeben 
Antwort auf Beitrag Nr.: 37.868.779 von fliesenbatscher am 27.08.09 22:51:06Hallo Fliese,
kannst aber nicht sagen ich hätte es Dir nicht schon von langer Zeit gesagt.. ....nicht verzagen......entweder es kommt dick oder halt etwas tiefer wieder .......hoffe Du hast bisschen Zeit mitgebracht. - oder halt die 20 % mal mitnehmen...mach ich ja auch öft bei anderen Werten
....nicht verzagen......entweder es kommt dick oder halt etwas tiefer wieder .......hoffe Du hast bisschen Zeit mitgebracht. - oder halt die 20 % mal mitnehmen...mach ich ja auch öft bei anderen Werten
kannst aber nicht sagen ich hätte es Dir nicht schon von langer Zeit gesagt..
 ....nicht verzagen......entweder es kommt dick oder halt etwas tiefer wieder .......hoffe Du hast bisschen Zeit mitgebracht. - oder halt die 20 % mal mitnehmen...mach ich ja auch öft bei anderen Werten
....nicht verzagen......entweder es kommt dick oder halt etwas tiefer wieder .......hoffe Du hast bisschen Zeit mitgebracht. - oder halt die 20 % mal mitnehmen...mach ich ja auch öft bei anderen Werten
Antwort auf Beitrag Nr.: 37.870.694 von Plaste am 28.08.09 10:20:46siehe posting 2953 

Antwort auf Beitrag Nr.: 37.870.736 von ALF-FRED am 28.08.09 10:23:56Ja da warst Du schon richtig gelegen....die Amis und ihre 5 US$ Theorie bis Oktober wieder mal.....also ich wäre erst mal mit 1 US$ zufrieden...der kommt wohl auch so wie es aussieht ( hoffe ich doch stark ).....wenn dann mal 2 US$ da " wären"...o.k dann ist alles offen....wir wären dann aber bereits beim Thema Multi. / bzw Phase 3 ....danach kanns dann in wirklich andere Region gehen...aber das ist alles Phantasie....im Moment sollten wir erst mal den eK 0,62 € des Kollegen sehen....wäe ja schon mal ein Schritt....nun gut heute ist Freitag.....bin gespannt wie es weitergeht.
Mal sehen ob heute die News kommt....wobei keiner wirklich weis was dran ist oder nicht.....ich werd auf jeden Fall die tieferen Kurse nochmals nutzen- wenn sie kommen ....Vorbörse schätz ich mal im Hoch auf die 0,78
Mal sehen ob heute die News kommt....wobei keiner wirklich weis was dran ist oder nicht.....ich werd auf jeden Fall die tieferen Kurse nochmals nutzen- wenn sie kommen ....Vorbörse schätz ich mal im Hoch auf die 0,78
Antwort auf Beitrag Nr.: 37.870.927 von Expertchen007 am 28.08.09 10:39:40Freitag war schon öffters Newstag - bei dem Volumen die letzten Tage wissen einige Leute bestimmt mehr.
Ich kenn noch die Tage wo in Amiland nur 50 k umgesetzt wurden, und wo von cel. nur 70 Millionen Aktien in Umlauf waren.
Deswegen hatte ich ja auch vor kurzem die Frage gestellt ob sich noch jemand erinnern kann, das bis zu einem gewissen Tag Altaktionäre Bonusaktien bekommen , wenn sie ihre Aktien noch halten und sie nicht veräußert haben, ist schon 3 - 4 Jahre her ,
eventuell kann Silak sich erinnern, ich bin dem englischen nicht so mächtig, das ich das über Babelfish verstehe,
wenn heute ne News kommt laufen wir locker über den Dollar
wir werden sehen
Ich kenn noch die Tage wo in Amiland nur 50 k umgesetzt wurden, und wo von cel. nur 70 Millionen Aktien in Umlauf waren.
Deswegen hatte ich ja auch vor kurzem die Frage gestellt ob sich noch jemand erinnern kann, das bis zu einem gewissen Tag Altaktionäre Bonusaktien bekommen , wenn sie ihre Aktien noch halten und sie nicht veräußert haben, ist schon 3 - 4 Jahre her ,
eventuell kann Silak sich erinnern, ich bin dem englischen nicht so mächtig, das ich das über Babelfish verstehe,
wenn heute ne News kommt laufen wir locker über den Dollar
wir werden sehen
Antwort auf Beitrag Nr.: 37.871.107 von ALF-FRED am 28.08.09 10:56:21Na ja mal sehen was so kommt....was ich bei Cel noch nie vertanden habe ist der riesen Spread in D jedesmal....das sind rund 13 % dazwischen....scheint ein Makler die Hoheit darüber zu haben ....find ich ja unmöglich
oh...ha cortal im ASK bereits auf der 0,532 €...die sind wohl schon vorraus
Antwort auf Beitrag Nr.: 37.871.511 von Expertchen007 am 28.08.09 11:39:44Und Tradegate hinkt hinterher... mit letztem Trade von 0,473
Antwort auf Beitrag Nr.: 37.871.533 von Fafnir77 am 28.08.09 11:42:01Stimmt...na ja auch die werden hoffentlich anpassen müssen....denk heute geht es wieder früh los im PRE - Bereich...frag mich nur wann die Shorts gecovert werden müssen .... ...so langsam sollten die doch mal kalte Füsse bekommen
...so langsam sollten die doch mal kalte Füsse bekommen
 ...so langsam sollten die doch mal kalte Füsse bekommen
...so langsam sollten die doch mal kalte Füsse bekommen
Antwort auf Beitrag Nr.: 37.871.107 von ALF-FRED am 28.08.09 10:56:21Irgend sowas hab ich auch im Kopf, jetzt wo Du es erwähnst.
Leider weiß ich auch nix mehr genaues.
mfg Plaste
Leider weiß ich auch nix mehr genaues.
mfg Plaste
Antwort auf Beitrag Nr.: 37.871.608 von Expertchen007 am 28.08.09 11:49:06Hm ich denke so langsam wirds eng. Der Kurs in US war schon lang nich mehr da, wo er jetzt ist und wir haben 2 Tage in Folge mit Tageshöchstkurs geschlossen... Fehlt noch der Funke
Nicht aufs Volumen schauen...
Pre-Market
Last: $ .75 Pre-Market
High: $ .75
Pre-Market
Volume: 250 Pre-Market
Low: $ .75

Pre-Market
Last: $ .75 Pre-Market
High: $ .75
Pre-Market
Volume: 250 Pre-Market
Low: $ .75
0,56 € im ASK bei Cortal...der Rest keine Reaktion...nun denn
Antwort auf Beitrag Nr.: 37.872.718 von Expertchen007 am 28.08.09 14:03:13 16.00 Uhr wird es spannend - News or not -
16.00 Uhr wird es spannend - News or not -
aber sieht doch wieder grün aus
 16.00 Uhr wird es spannend - News or not -
16.00 Uhr wird es spannend - News or not - aber sieht doch wieder grün aus

Eine Eröffnung auf der 0,74 US$...wäre der Knaller....danach wirds wohl wieder erst mal heftig schwanken...im Pre ist die 0,74 immer wieder mal da...vom Ordebuch her ist Platz bis zur 0,99 US$

 immer diese fillings wo mehr shares im umlauf kommen !
immer diese fillings wo mehr shares im umlauf kommen !
Antwort auf Beitrag Nr.: 37.873.393 von ALF-FRED am 28.08.09 15:24:33 ..meinst du das mit der 1 Million...oder welches denn ????
..meinst du das mit der 1 Million...oder welches denn ????
 ..meinst du das mit der 1 Million...oder welches denn ????
..meinst du das mit der 1 Million...oder welches denn ????
Uppps...geht erst mal steil ...

 für den Kurs
für den Kurs28-Aug-2009
Entry into a Material Definitive Agreement, Financial Statements and Exhibits
Item 1.01 Entry Into a Material Definitive Agreement
On August 20, 2009 CEL-SCI Corporation sold 9,701,000 shares of its common stock to a group of private investors for $4,365,450 or $0.45 per share. The investors also received warrants which entitle the investors to purchase 4,850,500 shares of CEL-SCI's common stock.
On August 26, 2009 CEL-SCI Corporation sold an additional 1,083,435 shares of its common stock to a separate group of private investors for $487,545 or $0.45 per share. The investors also received warrants which entitle the investors to purchase 541,717 shares of CEL-SCI's common stock.
The warrants sold on August 26, and August 20, 2009 may be exercised at any time on or after February 20, 2010 and on or prior to February 20, 2015 at a price of $0.55 per share.
CEL-SCI has filed with the Securities and Exchange Commission a prospectus supplement to its shelf Registration Statement on Form S-3 registering the shares of common stock and warrants sold to the private investors.
Antwort auf Beitrag Nr.: 37.873.469 von ALF-FRED am 28.08.09 15:35:46Diese News war im Kurs bereits drin....keep.. ....immer dasselbe Spiel in der 1. Stunde...beide Seiten kämpfen...
....immer dasselbe Spiel in der 1. Stunde...beide Seiten kämpfen...
 ....immer dasselbe Spiel in der 1. Stunde...beide Seiten kämpfen...
....immer dasselbe Spiel in der 1. Stunde...beide Seiten kämpfen...  gleiche Spiel wie gestern es wird gequetscht -
gleiche Spiel wie gestern es wird gequetscht -
Antwort auf Beitrag Nr.: 37.873.553 von Expertchen007 am 28.08.09 15:43:50 ich hab ja nur so ein Hilfslevel II sehe aber fast doppelt soviele buy Orders ,
ich hab ja nur so ein Hilfslevel II sehe aber fast doppelt soviele buy Orders ,
wenn die News kommt um 16.00 gehts gewalltig hoch !
 ich hab ja nur so ein Hilfslevel II sehe aber fast doppelt soviele buy Orders ,
ich hab ja nur so ein Hilfslevel II sehe aber fast doppelt soviele buy Orders , wenn die News kommt um 16.00 gehts gewalltig hoch !
Antwort auf Beitrag Nr.: 37.873.554 von ALF-FRED am 28.08.09 15:43:57tja und was sagt uns Deine glaskugel heute? 

Wenns mal zu den 0,99 US$ kommen " würde " werd ich wohl etwas rappelig werden...deshalb hoffe ich es geht dann gleich auf 1,02 US$ und gut ist..


Antwort auf Beitrag Nr.: 37.873.581 von schlumpftrader am 28.08.09 15:46:31 ich hab ja keine Ahnung - aber wenn ich den Level II beobachte geht noch was - unter 0.76 wird immer aufgefüllt aber erheblich langsamer als gestern
ich hab ja keine Ahnung - aber wenn ich den Level II beobachte geht noch was - unter 0.76 wird immer aufgefüllt aber erheblich langsamer als gestern

 ich hab ja keine Ahnung - aber wenn ich den Level II beobachte geht noch was - unter 0.76 wird immer aufgefüllt aber erheblich langsamer als gestern
ich hab ja keine Ahnung - aber wenn ich den Level II beobachte geht noch was - unter 0.76 wird immer aufgefüllt aber erheblich langsamer als gestern 
Antwort auf Beitrag Nr.: 37.873.703 von ALF-FRED am 28.08.09 15:59:16Ja ist wieder dasBid Spiel wie gestern auch...die 0,76 US$ ist das Ziel dann kanns erst weiter gehen im ASK
Antwort auf Beitrag Nr.: 37.873.899 von Expertchen007 am 28.08.09 16:16:21
hallo EXpertchen und alle anderen.
sehr ruhig hier.......und das ist gut so.
hallo EXpertchen und alle anderen.

sehr ruhig hier.......und das ist gut so.

Antwort auf Beitrag Nr.: 37.874.180 von fliesenbatscher am 28.08.09 16:41:08Sevus Fliese,
gut Ding braucht Weile...Cel im besonderen...hier ist eh die letzte 1/2 Std. zumeist die entscheidende....wir werde sehen...aber erst muss mal die 0,76 wieder fallen...
gut Ding braucht Weile...Cel im besonderen...hier ist eh die letzte 1/2 Std. zumeist die entscheidende....wir werde sehen...aber erst muss mal die 0,76 wieder fallen...
ich würde sagen; ähnlicher verlauf wie gestern. wollen wir mal hoffen, dass dieser intraday-trend bleibt. wenn es wirklich so bleibt und am ende der krönende abschluss folgt, dann habe ich nichts dagegen. 

pow...ist das langweilig heute...geht ja gar nix vorwärts....Schlagabtausch
 Was haltet ihr denn von meiner Theorie ?
Was haltet ihr denn von meiner Theorie ?Da Cel. ja die Produktionsstätte fertig hat , insbesondere auch auf die Verfahrensweise ( was mit cold produktion oder so ) hingewiesen hat, bestände ja die Möglichkeit, einen Teil des zu erwartenden Impfstoffes gegen H1N1 zu produzieren.
Da sehr viele Dosen schnell gebrauchr werden wird das sicher in Lizenz oder ähnlichem von mehreren Firmen produziert. Da gehts ja darum so schnell wie möglich viel herzustellen.
Das spült Geld in die Kassen und danach kann man sich auf Multikine P III konzentrieren.
Vielleicht kennt sich jemand damit aus, ob das Herstellungsverfahren der Firma dafür geeignet ist diesen Impfstoff mit zu produzieren.
Diskussion erwünscht
Alf-Fred
Antwort auf Beitrag Nr.: 37.878.133 von ALF-FRED am 29.08.09 10:02:59Obama Advisers: Swine Flu Could Infect Nearly Half the U.S. Population
"President Obama's science advisers warned Monday that swine flu could infect nearly half the U.S. population this fall and winter and cause up to 90,000 deaths, mostly in kids and young adults. The estimate is double the deaths normally associated with the seasonal flu.
The report by the President's Council of Advisors on Science and Technology laid out a "plausible scenario" where the H1N1 virus could infect 60 million to 120 million people. As many as 1.8 million people could be admitted to hospitals with up to 300,000 of them requiring treatment in intensive care units, the council found.
The number of people hospitalized could put a strain on the U.S. healthcare system because those patients could completely fill intensive care beds at the peak of the flu season. The report estimates that the epidemic will peak on October 15, the exact date U.S. health officials are expected to deliver a vaccine.
"This is going to be fairly serious," said Harold E. Varmus of Memorial Sloan-Kettering Cancer Center in New York, cochair of the 21-member council. "It's going to stress every aspect of our health system."
Health and Human Services Secretary Kathleen Sebelius called the H1N1 virus "unusual" because it typically infects children and young adults. "This isn't the flu that we're used to," Sebelius said. "The 2009 H1N1 virus will cause a more serious threat this fall."
The council recommended that manufacturers speed up the preparation of the flu vaccine so it can be distributed to high-risk patients such as pregnant women by mid-September. Originally, the government expected 120 million doses to be available on October 15, but U.S. health officials now estimates there will only be 45 million available, with 20 million more each week through December.
The Centers for Disease Control and Prevention recommends that parents get their family vaccinated once the shot is available."
http://www.usnews.com/articles/news/national/2009/08/25/obam…
"President Obama's science advisers warned Monday that swine flu could infect nearly half the U.S. population this fall and winter and cause up to 90,000 deaths, mostly in kids and young adults. The estimate is double the deaths normally associated with the seasonal flu.
The report by the President's Council of Advisors on Science and Technology laid out a "plausible scenario" where the H1N1 virus could infect 60 million to 120 million people. As many as 1.8 million people could be admitted to hospitals with up to 300,000 of them requiring treatment in intensive care units, the council found.
The number of people hospitalized could put a strain on the U.S. healthcare system because those patients could completely fill intensive care beds at the peak of the flu season. The report estimates that the epidemic will peak on October 15, the exact date U.S. health officials are expected to deliver a vaccine.
"This is going to be fairly serious," said Harold E. Varmus of Memorial Sloan-Kettering Cancer Center in New York, cochair of the 21-member council. "It's going to stress every aspect of our health system."
Health and Human Services Secretary Kathleen Sebelius called the H1N1 virus "unusual" because it typically infects children and young adults. "This isn't the flu that we're used to," Sebelius said. "The 2009 H1N1 virus will cause a more serious threat this fall."
The council recommended that manufacturers speed up the preparation of the flu vaccine so it can be distributed to high-risk patients such as pregnant women by mid-September. Originally, the government expected 120 million doses to be available on October 15, but U.S. health officials now estimates there will only be 45 million available, with 20 million more each week through December.
The Centers for Disease Control and Prevention recommends that parents get their family vaccinated once the shot is available."
http://www.usnews.com/articles/news/national/2009/08/25/obam…
Servus,
na ja solange auch nur 0,0001 % Risiko beim Impstoff noch dabei ist was gegen CEl verewendet werden kann...bestimmt nicht...anderseits sollten die Menschen ohne sterben ....dann ist ein "etwas " besser als nix...aber dazu bin ich zuwenig Medizinmensch um da was sagen zu können....GUSTL24 - bitte dazu mal melden .
Vom Kurs her wars ein plus gestern wenn auch nur ein kleines..aber für Feitag ganz o.k....somit den 3. Tag im plus...also was solls...und nächste Woche kommt das Finale....neue Bsais die 0,72 US$...ich könnte wetten der 1 US$ ist in Sichtweite
na ja solange auch nur 0,0001 % Risiko beim Impstoff noch dabei ist was gegen CEl verewendet werden kann...bestimmt nicht...anderseits sollten die Menschen ohne sterben ....dann ist ein "etwas " besser als nix...aber dazu bin ich zuwenig Medizinmensch um da was sagen zu können....GUSTL24 - bitte dazu mal melden .
Vom Kurs her wars ein plus gestern wenn auch nur ein kleines..aber für Feitag ganz o.k....somit den 3. Tag im plus...also was solls...und nächste Woche kommt das Finale....neue Bsais die 0,72 US$...ich könnte wetten der 1 US$ ist in Sichtweite
Hallo Alfred,
dazu habe ich zu wenig Ahnung von der Materie. Aber ich hab was anderes für Dich.
Ich wurde heute morgen ganz früh von Dachdeckern geweckt und hatte dann einiges an Zeit zu viel. Da hab ich mal den Thread durchsucht, ob ich was zum Thema Gratisaktien finde. Das u.a. habe ich dazu gefunden.
Weiterhin hab ich bei der Suche gleich noch einen Beweis für Deine helseherischen Fähigkeiten gefunden.
gefunden.
Die Fertigstellung der Produktionsanlage haben wir ja nun hinter uns und der 1 USD scheint auch nicht mehr weit zu sein. Wollen wir hoffen dass der Rest auch noch stimmt ….
09.11.2007 15:07
CEL-SCI Corporation Announces Adoption of Shareholder Rights Plan
VIENNA, Va., Nov. 9 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (Nachrichten) announced today that its Board of Directors has approved a Stockholder Rights Plan designed to ensure that all of its stockholders receive fair and equal treatment in the event of any proposal to acquire control of CEL-SCI.
Geert Kersten, Chief Executive Officer, said "We have finally gotten to the Phase III clinical trial stage for a cancer indication that has a multi- billion dollar market with no competing product and we have retained our key marketing rights. That is a fairly unique situation. Therefore we need to be vigilant and protect the shareholders during this crucial time period. Distribution of the Rights is not intended to prevent a takeover of CEL-SCI on terms beneficial to its stockholders; however this action by our Board increases its ability to effectively represent the interests of all Stockholders in the event of an unsolicited takeover attempt." Kersten added that the plan was not adopted in response to any specific effort to gain control of CEL-SCI and that he was not aware of any such attempt.
The Stockholder Rights Plan guards against partial or two-tier tender offers, coercive stock accumulation programs, and other tactics that may be used to gain control of CEL-SCI without offering a fair price to all stockholders. Many other publicity held companies have adopted such plans.
Under the Rights Plan, each stockholder will receive a dividend of one Series A Right and one Series B Right for each share of CEL-SCI common stock registered in the stockholders' name. The Right will not become exercisable until either of two events occurs. First, if any person or group (i) acquires 15% or more of CEL-SCI's common stock, or (ii) begin a tender offer or exchange offer that would result in such person owing 15% or more common stock (except pursuant to an offer for all shares deemed fair by CEL-SCI's Board of Directors), each Series A Right would entitle its holder, beginning on the fifteenth business day after the announcement of the acquisition or the commencement of the tender offer, to buy one share of CEL-SCI's common stock at a price equal to 20% of the public market price of the common stock.
Second, if CEL-SCI is acquired in a merger or other business combination or disposes of more than half its assets after a person or group acquires 15% or more of CEL-SCI's common stock (other than a transaction with a person who acquired Common Shares through a tender offer or exchange offer for all outstanding Common Shares approved by the Board of Directors of the Company in accordance with the terms of Rights Agreement), each Series B right would entitle its holder to purchase shares of the acquiring company's common stock at a 50% discount to market value.
CEL-SCI may redeem the Rights at $0.0001 per Right at any time up to fifteen business days after a public announcement that a 15% position has been acquired or a tender offer has begun.
The distribution of the Series A and Series B Rights will be made on November 9, 2007 to stockholders of record on that date. The Right will expire October 30, 2015. The distribution of the Rights is not taxable to stockholders or CEL-SCI.
About CEL-SCI:
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine(R). In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck in May 2007.
Multikine, a patented defined mixture of naturally derived cytokines, is the first immunotherapeutic agent in a new class of drugs called "Immune SIMULATORS". Immune SIMULATORS simulate the way our natural immune system acts in defending us against cancer. As opposed to other immunotherapies which are designed to target a single or limited number of specific antigens or molecules, Immune SIMULATORS are multi-targeted; they simultaneously cause a direct and targeted killing of the specific tumor cells and they activate the immune system to produce a stronger anti-tumor attack on multiple fronts.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
The Company has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense and avian flu.
+++++++++++++
Damals für uns von Mimm übersetzt:
In der Kurzfassung
Alle die Aktien bis zum heutigen Tage gekauft haben, bekommen bis ins Jahr 2015 die Rechte
, im Falle eines übernahme Versuchs durch andere Investoren (feindliche Übernahme) Aktien zu einem Vorzugspreis kaufen zu dürfen.
Bin mir auch nicht so ganz im klaren was die genauen Unterschiede sind.
Auf alle Fälle kann man Aktien zu 20% des aktuellen Aktienkurses kaufen oder zu 50% des Kurses wenn weitere Merkmale erreicht werden ( alles im Falle einer versuchten Übernahme)
Im Prinzip geht es einfach um den Schutz der Kleinanleger.
Wie gesagt, die Rechte verfallen im Jahre 2015, wobei CEL-SCI wohl davon ausgeht, dass der Wert des Unternehmens bis dahin solche Kurse ereicht, dass eine Übernahme unwahrscheinlich oder aber sehr profitabel sein wird .
+++++++++++++++
#2367 von ALF-FRED 21.12.07 21:11:44 Beitrag Nr.: 32.843.923
Dieses Posting: versenden | melden
Folgende Antwort bezieht sich auf Beitrag Nr.: 32.843.844 von Plaste am 21.12.07 21:05:14
im Sommer 2008 geht die Post ab
mal so meine Meinung
Fertigstellung Produktionsfirma ca. 1 Dollar
P III Anfang 3-4 Dollar mit starken Schwankungen
positive PIII 8 - 12 Dollar
Vertrieb 15 Dollar
Ausweitung als Blockbuster in 2 Jahren bis zu 50 Dollar
Felize Navidad
mfg
Plaste
dazu habe ich zu wenig Ahnung von der Materie. Aber ich hab was anderes für Dich.
Ich wurde heute morgen ganz früh von Dachdeckern geweckt und hatte dann einiges an Zeit zu viel. Da hab ich mal den Thread durchsucht, ob ich was zum Thema Gratisaktien finde. Das u.a. habe ich dazu gefunden.
Weiterhin hab ich bei der Suche gleich noch einen Beweis für Deine helseherischen Fähigkeiten
 gefunden.
gefunden. Die Fertigstellung der Produktionsanlage haben wir ja nun hinter uns und der 1 USD scheint auch nicht mehr weit zu sein. Wollen wir hoffen dass der Rest auch noch stimmt ….
09.11.2007 15:07
CEL-SCI Corporation Announces Adoption of Shareholder Rights Plan
VIENNA, Va., Nov. 9 /PRNewswire-FirstCall/ -- CEL-SCI Corporation (Nachrichten) announced today that its Board of Directors has approved a Stockholder Rights Plan designed to ensure that all of its stockholders receive fair and equal treatment in the event of any proposal to acquire control of CEL-SCI.
Geert Kersten, Chief Executive Officer, said "We have finally gotten to the Phase III clinical trial stage for a cancer indication that has a multi- billion dollar market with no competing product and we have retained our key marketing rights. That is a fairly unique situation. Therefore we need to be vigilant and protect the shareholders during this crucial time period. Distribution of the Rights is not intended to prevent a takeover of CEL-SCI on terms beneficial to its stockholders; however this action by our Board increases its ability to effectively represent the interests of all Stockholders in the event of an unsolicited takeover attempt." Kersten added that the plan was not adopted in response to any specific effort to gain control of CEL-SCI and that he was not aware of any such attempt.
The Stockholder Rights Plan guards against partial or two-tier tender offers, coercive stock accumulation programs, and other tactics that may be used to gain control of CEL-SCI without offering a fair price to all stockholders. Many other publicity held companies have adopted such plans.
Under the Rights Plan, each stockholder will receive a dividend of one Series A Right and one Series B Right for each share of CEL-SCI common stock registered in the stockholders' name. The Right will not become exercisable until either of two events occurs. First, if any person or group (i) acquires 15% or more of CEL-SCI's common stock, or (ii) begin a tender offer or exchange offer that would result in such person owing 15% or more common stock (except pursuant to an offer for all shares deemed fair by CEL-SCI's Board of Directors), each Series A Right would entitle its holder, beginning on the fifteenth business day after the announcement of the acquisition or the commencement of the tender offer, to buy one share of CEL-SCI's common stock at a price equal to 20% of the public market price of the common stock.
Second, if CEL-SCI is acquired in a merger or other business combination or disposes of more than half its assets after a person or group acquires 15% or more of CEL-SCI's common stock (other than a transaction with a person who acquired Common Shares through a tender offer or exchange offer for all outstanding Common Shares approved by the Board of Directors of the Company in accordance with the terms of Rights Agreement), each Series B right would entitle its holder to purchase shares of the acquiring company's common stock at a 50% discount to market value.
CEL-SCI may redeem the Rights at $0.0001 per Right at any time up to fifteen business days after a public announcement that a 15% position has been acquired or a tender offer has begun.
The distribution of the Series A and Series B Rights will be made on November 9, 2007 to stockholders of record on that date. The Right will expire October 30, 2015. The distribution of the Rights is not taxable to stockholders or CEL-SCI.
About CEL-SCI:
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product is Multikine(R). In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery. The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine in January 2007 and granted orphan drug status to Multikine in the neoadjuvant therapy of squamous cell carcinoma (cancer) of the head and neck in May 2007.
Multikine, a patented defined mixture of naturally derived cytokines, is the first immunotherapeutic agent in a new class of drugs called "Immune SIMULATORS". Immune SIMULATORS simulate the way our natural immune system acts in defending us against cancer. As opposed to other immunotherapies which are designed to target a single or limited number of specific antigens or molecules, Immune SIMULATORS are multi-targeted; they simultaneously cause a direct and targeted killing of the specific tumor cells and they activate the immune system to produce a stronger anti-tumor attack on multiple fronts.
Multikine is also the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
The Company has operations in Vienna, Virginia and Baltimore, Maryland. CEL-SCI's other products, which are currently in pre-clinical stage, have shown protection against a number of diseases in animal tests and are being tested against diseases associated with bio-defense and avian flu.
+++++++++++++
Damals für uns von Mimm übersetzt:
In der Kurzfassung
Alle die Aktien bis zum heutigen Tage gekauft haben, bekommen bis ins Jahr 2015 die Rechte
, im Falle eines übernahme Versuchs durch andere Investoren (feindliche Übernahme) Aktien zu einem Vorzugspreis kaufen zu dürfen.
Bin mir auch nicht so ganz im klaren was die genauen Unterschiede sind.
Auf alle Fälle kann man Aktien zu 20% des aktuellen Aktienkurses kaufen oder zu 50% des Kurses wenn weitere Merkmale erreicht werden ( alles im Falle einer versuchten Übernahme)
Im Prinzip geht es einfach um den Schutz der Kleinanleger.
Wie gesagt, die Rechte verfallen im Jahre 2015, wobei CEL-SCI wohl davon ausgeht, dass der Wert des Unternehmens bis dahin solche Kurse ereicht, dass eine Übernahme unwahrscheinlich oder aber sehr profitabel sein wird .
+++++++++++++++
#2367 von ALF-FRED 21.12.07 21:11:44 Beitrag Nr.: 32.843.923
Dieses Posting: versenden | melden
Folgende Antwort bezieht sich auf Beitrag Nr.: 32.843.844 von Plaste am 21.12.07 21:05:14
im Sommer 2008 geht die Post ab
mal so meine Meinung
Fertigstellung Produktionsfirma ca. 1 Dollar
P III Anfang 3-4 Dollar mit starken Schwankungen
positive PIII 8 - 12 Dollar
Vertrieb 15 Dollar
Ausweitung als Blockbuster in 2 Jahren bis zu 50 Dollar
Felize Navidad
mfg
Plaste
Antwort auf Beitrag Nr.: 37.878.424 von Plaste am 29.08.09 12:02:45Schade da bekomm ich nix davon  ...na ja mir langt der Aktienkurs...aber erst am Schluß der Story ...
...na ja mir langt der Aktienkurs...aber erst am Schluß der Story ...
ansonsten wr die Prognose aus 2007 ja wirklich na dran
 ...na ja mir langt der Aktienkurs...aber erst am Schluß der Story ...
...na ja mir langt der Aktienkurs...aber erst am Schluß der Story ...ansonsten wr die Prognose aus 2007 ja wirklich na dran
 das meinte ich, danke - da bin ich mit 2/3 meiner shares auf der sicheren seite
das meinte ich, danke - da bin ich mit 2/3 meiner shares auf der sicheren seite 
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 99 | ||
| 53 | ||
| 52 | ||
| 42 | ||
| 29 | ||
| 28 | ||
| 28 | ||
| 26 | ||
| 23 | ||
| 21 |
| Wertpapier | Beiträge | |
|---|---|---|
| 21 | ||
| 20 | ||
| 20 | ||
| 20 | ||
| 19 | ||
| 19 | ||
| 18 | ||
| 17 | ||
| 16 | ||
| 15 |



















