Oramed Pharmaceuticals (Seite 1450)
eröffnet am 28.03.07 10:26:47 von
neuester Beitrag 19.03.24 16:27:21 von
neuester Beitrag 19.03.24 16:27:21 von
Beiträge: 19.664
ID: 1.121.564
ID: 1.121.564
Aufrufe heute: 4
Gesamt: 1.542.152
Gesamt: 1.542.152
Aktive User: 0
ISIN: US68403P2039 · WKN: A1CTNU · Symbol: OJU1
2,1930
EUR
+0,27 %
+0,0060 EUR
Letzter Kurs 03.05.24 Tradegate
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,2000 | +471,16 | |
| 13,110 | +38,44 | |
| 6,5000 | +27,45 | |
| 1,2100 | +21,00 | |
| 48,25 | +19,94 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 24,050 | -12,55 | |
| 4,0300 | -12,96 | |
| 6,2600 | -14,25 | |
| 3,8500 | -14,45 | |
| 36,70 | -22,87 |
Beitrag zu dieser Diskussion schreiben
Antwort auf Beitrag Nr.: 45.817.150 von kwhistler am 12.11.13 17:54:46hier gleich noch ne Hammer-News hinterher,
Phase 2 steht kurz vorm Abschluß!
Oramed Announces Last Patient Out in Phase 2a FDA Trial of its ORMD-0801 Oral Insulin Capsule

Share with Twitter Share with LinkedIn
Share with Repost.us
JERUSALEM, November 12, 2013 /PRNewswire/ --
Oramed Pharmaceuticals Inc. (NASDAQCM: ORMP) (http://www.oramed.com), a clinical-stage pharmaceutical company focused on the development of oral drug delivery systems, announced today that the last patient has left the clinic from its U.S. FDA Phase 2a safety study for ORMD-0801, an oral insulin capsule based on the Company's platform Protein Oral Delivery (POD™) technology.
The Phase 2a trial, which enlisted 30 type 2 diabetes patients in an in-clinic setting for a seven-day period, was implemented in response to FDA feedback requesting a safety study on ORMD-0801 prior to initiating a larger multi-center trial in the US.
"We are thrilled to announce the last patient out of our Phase 2a clinical trial under the FDA. We look forward to announcing the results," said Oramed CEO Nadav Kidron.
About ORMD-0801 Oral Insulin
Oramed's ORMD-0801 is an orally ingestible insulin capsule indicated for the early stages of type 2 diabetes, when it can still slow the rate of degeneration of the disease by providing additional insulin to the body and allowing pancreatic respite. Moreover, orally administered insulin has the potential benefit of enhanced patient compliance at this crucial stage as well as the advantage of mimicking insulin's natural location and gradients in the body by first passing through the liver before entering the bloodstream. For more information on ORMD-0801, the content of which is not part of this press release, please visit http://oramed.com/index.php?page=14
About Oramed Pharmaceuticals
Oramed Pharmaceuticals is a technology pioneer in the field of oral delivery solutions for drugs and vaccines currently delivered via injection. Established in 2006, Oramed's Protein Oral Delivery (PODTM) technology is based on over 30 years of research by top research scientists at Jerusalem's Hadassah Medical Center. Oramed is seeking to revolutionize the treatment of diabetes through its proprietary flagship product, an orally ingestible insulin capsule (ORMD-0801) currently in Phase 2 clinical trials on patients with type 2 diabetes (T2DM) under an Investigational New Drug application with the U.S. Food and Drug Administration, and with its oral exenatide capsule (ORMD-0901; a GLP-1 analog), with trials on healthy volunteers (Phase 1b) and T2DM patients (Phase 2a) underway. Oramed is also moving forward with clinical trials of ORMD-0801 for the treatment of type 1 diabetes. The company's corporate and R&D headquarters are based in Jerusalem.
For more information, the content of which is not part of this press release, please visit http://www.oramed.com
Forward-looking statements: This press release contains forward-looking statements. For example, we are using forward-looking statements when we discuss our clinical trials or revolutionizing the treatment of diabetes with our products. These forward-looking statements and their implications are based on the current expectations of the management of Oramed only, and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements, including the risks and uncertainties related to the progress, timing, cost, and results of clinical trials and product development programs; difficulties or delays in obtaining regulatory approval or patent protection for our product candidates; competition from other pharmaceutical or biotechnology companies; and our ability to obtain additional funding required to conduct our research, development and commercialization activities. In addition, the following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: changes in technology and market requirements; delays or obstacles in launching our clinical trials; changes in legislation; inability to timely develop and introduce new technologies, products and applications; lack of validation of our technology as we progress further and lack of acceptance of our methods by the scientific community; inability to retain or attract key employees whose knowledge is essential to the development of our products; unforeseen scientific difficulties that may develop with our process; greater cost of final product than anticipated; loss of market share and pressure on pricing resulting from competition; laboratory results that do not translate to equally good results in real settings; our patents may not be sufficient; and final that products may harm recipients, all of which could cause the actual results or performance of Oramed to differ materially from those contemplated in such forward-looking statements. Except as otherwise required by law, Oramed undertakes no obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. For a more detailed description of the risks and uncertainties affecting Oramed, reference is made to Oramed's reports filed from time to time with the Securities and Exchange Commission.
Company Contact:
Oramed Pharmaceuticals
Aviva Sherman
Office: +972-2-566-0001
Int'l: 1-718-831-2512
Mobile: +972-54-792-4438
Email: aviva@oramed.com
SOURCE Oramed Pharmaceuticals Inc.
Phase 2 steht kurz vorm Abschluß!
Oramed Announces Last Patient Out in Phase 2a FDA Trial of its ORMD-0801 Oral Insulin Capsule

Share with Twitter Share with LinkedIn
Share with Repost.us
JERUSALEM, November 12, 2013 /PRNewswire/ --
Oramed Pharmaceuticals Inc. (NASDAQCM: ORMP) (http://www.oramed.com), a clinical-stage pharmaceutical company focused on the development of oral drug delivery systems, announced today that the last patient has left the clinic from its U.S. FDA Phase 2a safety study for ORMD-0801, an oral insulin capsule based on the Company's platform Protein Oral Delivery (POD™) technology.
The Phase 2a trial, which enlisted 30 type 2 diabetes patients in an in-clinic setting for a seven-day period, was implemented in response to FDA feedback requesting a safety study on ORMD-0801 prior to initiating a larger multi-center trial in the US.
"We are thrilled to announce the last patient out of our Phase 2a clinical trial under the FDA. We look forward to announcing the results," said Oramed CEO Nadav Kidron.
About ORMD-0801 Oral Insulin
Oramed's ORMD-0801 is an orally ingestible insulin capsule indicated for the early stages of type 2 diabetes, when it can still slow the rate of degeneration of the disease by providing additional insulin to the body and allowing pancreatic respite. Moreover, orally administered insulin has the potential benefit of enhanced patient compliance at this crucial stage as well as the advantage of mimicking insulin's natural location and gradients in the body by first passing through the liver before entering the bloodstream. For more information on ORMD-0801, the content of which is not part of this press release, please visit http://oramed.com/index.php?page=14
About Oramed Pharmaceuticals
Oramed Pharmaceuticals is a technology pioneer in the field of oral delivery solutions for drugs and vaccines currently delivered via injection. Established in 2006, Oramed's Protein Oral Delivery (PODTM) technology is based on over 30 years of research by top research scientists at Jerusalem's Hadassah Medical Center. Oramed is seeking to revolutionize the treatment of diabetes through its proprietary flagship product, an orally ingestible insulin capsule (ORMD-0801) currently in Phase 2 clinical trials on patients with type 2 diabetes (T2DM) under an Investigational New Drug application with the U.S. Food and Drug Administration, and with its oral exenatide capsule (ORMD-0901; a GLP-1 analog), with trials on healthy volunteers (Phase 1b) and T2DM patients (Phase 2a) underway. Oramed is also moving forward with clinical trials of ORMD-0801 for the treatment of type 1 diabetes. The company's corporate and R&D headquarters are based in Jerusalem.
For more information, the content of which is not part of this press release, please visit http://www.oramed.com
Forward-looking statements: This press release contains forward-looking statements. For example, we are using forward-looking statements when we discuss our clinical trials or revolutionizing the treatment of diabetes with our products. These forward-looking statements and their implications are based on the current expectations of the management of Oramed only, and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements, including the risks and uncertainties related to the progress, timing, cost, and results of clinical trials and product development programs; difficulties or delays in obtaining regulatory approval or patent protection for our product candidates; competition from other pharmaceutical or biotechnology companies; and our ability to obtain additional funding required to conduct our research, development and commercialization activities. In addition, the following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: changes in technology and market requirements; delays or obstacles in launching our clinical trials; changes in legislation; inability to timely develop and introduce new technologies, products and applications; lack of validation of our technology as we progress further and lack of acceptance of our methods by the scientific community; inability to retain or attract key employees whose knowledge is essential to the development of our products; unforeseen scientific difficulties that may develop with our process; greater cost of final product than anticipated; loss of market share and pressure on pricing resulting from competition; laboratory results that do not translate to equally good results in real settings; our patents may not be sufficient; and final that products may harm recipients, all of which could cause the actual results or performance of Oramed to differ materially from those contemplated in such forward-looking statements. Except as otherwise required by law, Oramed undertakes no obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. For a more detailed description of the risks and uncertainties affecting Oramed, reference is made to Oramed's reports filed from time to time with the Securities and Exchange Commission.
Company Contact:
Oramed Pharmaceuticals
Aviva Sherman
Office: +972-2-566-0001
Int'l: 1-718-831-2512
Mobile: +972-54-792-4438
Email: aviva@oramed.com
SOURCE Oramed Pharmaceuticals Inc.
Antwort auf Beitrag Nr.: 45.817.150 von kwhistler am 12.11.13 17:54:46Moin kwhistly
das ist ja ein toller Beitrag
10 Milliarden Dollar Potenzial Minimum
und dann noch von diesem Contributor

ich stelle mich freiwillig als Sklave zu Verfügung

Gruß r
das ist ja ein toller Beitrag

10 Milliarden Dollar Potenzial Minimum
und dann noch von diesem Contributor


ich stelle mich freiwillig als Sklave zu Verfügung


Gruß r

News
http://seekingalpha.com/article/1828722-oramedss-success-in-…
Orameds's Success In Type 1 Diabetes Could Solve Novo Nordisk's Problems
Nov 11 2013, 10:24 | about: ORMP, includes: BMY, GOOG, MNKD, NVO, PFE BOOKMARK / READ LATER
Disclosure: I have no positions in any stocks mentioned, but may initiate a long position in ORMP over the next 72 hours. (More...)
Clinical interest in oral insulin, estimated to have market potential upwards of $10 billion, is escalating as more diabetics are diagnosed each year and patients' petulant demands for an alternative to needles and pens use up more of a doctor's limited practice time. Not just the domain of the smaller biotech, now larger pharmaceuticals are taking up the search. This puts front-runner Oramed Pharmaceuticals (ORMP), furthest along in studies with its ORMD-0801 oral insulin for Type 1 and Type 2 diabetes, in the spotlight.
Last November, Bristol-Myers Squibb Company (BMY) entered into an agreement with India's Biocon to aid in development and commercialization of Biocon's prandial oral insulin product should it pass Phase II. Biocon needs a pharmaceutical advocate after setbacks in early testing with its drug due to placebo effect. However, demonstration of efficacy is years away. Bristol-Myers would gain an innovative new product in a new field of medicine where competition is on the rise.
Novo Nordisk A/S (NVO), a long-time devotee of oral insulin but widely unsuccessful so far, hangs on to its partnership with Ireland-based Merrion Pharmaceuticals and recently finished a single-dose Phase I trial of the jointly-developed insulin tablet NN1954 for Type 2 diabetes. A multiple dose Phase I will follow although the company cannot predict a timeline for Phase II but believes that final approval is a decade away.
Even Google Inc. (GOOG) with its $341 billion market cap knows oral insulin is hot. In September, Google Ventures, the technology giant's alternative investment subsidiary, plowed more than $10 million in start-up Rani Therapeutics of San Jose, California, to further the development of its platform for oral delivery, including insulin. Studies are only at the preclinical stage, however, meaning testing in rodents.
Ahead of the crowd, Oramed has been enrolling patients in early-to-mid stage clinicals for ORMD-0801 since the summer. In July, the first patient had been placed in a Phase IIa for Type 2 diabetes to evaluate safety. Quickly following that, patient recruitment was begun for a Phase I, first-in-human trial using ORMD-0801 for Type 1.
http://seekingalpha.com/article/1828722-oramedss-success-in-…
Orameds's Success In Type 1 Diabetes Could Solve Novo Nordisk's Problems
Nov 11 2013, 10:24 | about: ORMP, includes: BMY, GOOG, MNKD, NVO, PFE BOOKMARK / READ LATER
Disclosure: I have no positions in any stocks mentioned, but may initiate a long position in ORMP over the next 72 hours. (More...)
Clinical interest in oral insulin, estimated to have market potential upwards of $10 billion, is escalating as more diabetics are diagnosed each year and patients' petulant demands for an alternative to needles and pens use up more of a doctor's limited practice time. Not just the domain of the smaller biotech, now larger pharmaceuticals are taking up the search. This puts front-runner Oramed Pharmaceuticals (ORMP), furthest along in studies with its ORMD-0801 oral insulin for Type 1 and Type 2 diabetes, in the spotlight.
Last November, Bristol-Myers Squibb Company (BMY) entered into an agreement with India's Biocon to aid in development and commercialization of Biocon's prandial oral insulin product should it pass Phase II. Biocon needs a pharmaceutical advocate after setbacks in early testing with its drug due to placebo effect. However, demonstration of efficacy is years away. Bristol-Myers would gain an innovative new product in a new field of medicine where competition is on the rise.
Novo Nordisk A/S (NVO), a long-time devotee of oral insulin but widely unsuccessful so far, hangs on to its partnership with Ireland-based Merrion Pharmaceuticals and recently finished a single-dose Phase I trial of the jointly-developed insulin tablet NN1954 for Type 2 diabetes. A multiple dose Phase I will follow although the company cannot predict a timeline for Phase II but believes that final approval is a decade away.
Even Google Inc. (GOOG) with its $341 billion market cap knows oral insulin is hot. In September, Google Ventures, the technology giant's alternative investment subsidiary, plowed more than $10 million in start-up Rani Therapeutics of San Jose, California, to further the development of its platform for oral delivery, including insulin. Studies are only at the preclinical stage, however, meaning testing in rodents.
Ahead of the crowd, Oramed has been enrolling patients in early-to-mid stage clinicals for ORMD-0801 since the summer. In July, the first patient had been placed in a Phase IIa for Type 2 diabetes to evaluate safety. Quickly following that, patient recruitment was begun for a Phase I, first-in-human trial using ORMD-0801 for Type 1.
Antwort auf Beitrag Nr.: 45.787.998 von occitania am 07.11.13 22:11:07Habe 1 Kauforder angeben
Moin Occi,
das hast du gut gemacht
hier die Chart-Oralyse!
ORMP ORAMED PHARMACEUTICALS
LONG BEIBEHALTEN
Letztes Muster:BÄRISCHES VERSCHLINGEN*
Letzter Schlusskurs:7,0100 Veränderung:+0,0300 Prozentsatzänderung:+0,43% 6 Mo.Rating$100⇨100,4512 Mo.Rating1★$100⇨125,8624 Mo.Rating$100⇨125,01Signalaktualisierung
Unsere heutige Systemempfehlung lautet LONG BEIBEHALTEN.
Die vorherige Empfehlung KAUFEN wurde am 06.11.2013, 2 Tage zuvor ausgegeben, als der Aktienkurs 6,6999 betrug.
Seit dem ist ORMP um +4,63% gestiegen.Marktausblick Die Bullen sind in der vollen Kontrolle.
Die negative Stimmung, die zu dem letzten bärischen Muster geführt hat, ist verdampft.
Außerdem deutet das Signal was LONG BEIBEHALTEN hin.
Es ist am besten, dem Signal zu folgen und weiterhin diese Wertpapiere zu halten.
Quelle:
http://www.americanbulls.com/SignalPage.aspx?lang=de&Ticker=…
Gruß r
Moin Occi,
das hast du gut gemacht
hier die Chart-Oralyse!
ORMP ORAMED PHARMACEUTICALS
LONG BEIBEHALTEN
Letztes Muster:BÄRISCHES VERSCHLINGEN*
Letzter Schlusskurs:7,0100 Veränderung:+0,0300 Prozentsatzänderung:+0,43% 6 Mo.Rating$100⇨100,4512 Mo.Rating1★$100⇨125,8624 Mo.Rating$100⇨125,01Signalaktualisierung
Unsere heutige Systemempfehlung lautet LONG BEIBEHALTEN.
Die vorherige Empfehlung KAUFEN wurde am 06.11.2013, 2 Tage zuvor ausgegeben, als der Aktienkurs 6,6999 betrug.
Seit dem ist ORMP um +4,63% gestiegen.Marktausblick Die Bullen sind in der vollen Kontrolle.
Die negative Stimmung, die zu dem letzten bärischen Muster geführt hat, ist verdampft.

Außerdem deutet das Signal was LONG BEIBEHALTEN hin.
Es ist am besten, dem Signal zu folgen und weiterhin diese Wertpapiere zu halten.
Quelle:
http://www.americanbulls.com/SignalPage.aspx?lang=de&Ticker=…
Gruß r

Antwort auf Beitrag Nr.: 45.786.266 von reaaalist am 07.11.13 18:56:38Salut reaaalist
Habe 1 Kauforder angeben
8 Schares zu 8 $...
à bientôt
OC
Habe 1 Kauforder angeben
8 Schares zu 8 $...

à bientôt
OC
Antwort auf Beitrag Nr.: 45.785.946 von occitania am 07.11.13 18:23:37Occi,
da steht drin, daß es nur 5581835 Oramed Aktien gibt.
davon sind 5581827 in sicheren Händen
8 Aktien befinden sich also nur noch auf dem freien Markt
Gruß r
da steht drin, daß es nur 5581835 Oramed Aktien gibt.
davon sind 5581827 in sicheren Händen

8 Aktien befinden sich also nur noch auf dem freien Markt

Gruß r

Antwort auf Beitrag Nr.: 45.785.504 von reaaalist am 07.11.13 17:46:53Salut
Das meine Name nicht dabei ist verstehe
Aber ...Steht auch nicht reaaalist...
Ick kappiere nicht wat dat ist ?
Verteilen die das "Propektus" in die Fußgängerzone von Jerusalem...
à+
OC
Habe endlich wieder Zigarette ...wie lang ist mir das leisten kann ?
...wie lang ist mir das leisten kann ?
Das meine Name nicht dabei ist verstehe
Aber ...Steht auch nicht reaaalist...

Ick kappiere nicht wat dat ist ?
Verteilen die das "Propektus" in die Fußgängerzone von Jerusalem...
à+
OC
Habe endlich wieder Zigarette
 ...wie lang ist mir das leisten kann ?
...wie lang ist mir das leisten kann ?
Antwort auf Beitrag Nr.: 45.776.912 von occitania am 06.11.13 20:22:15Ah, Danke Herr Kollege für die Erinnerung
so ein Darm ist schon recht kompliziert aufgebaut
erst dachte ich es handelt sich um einen Phallus bzw. drei
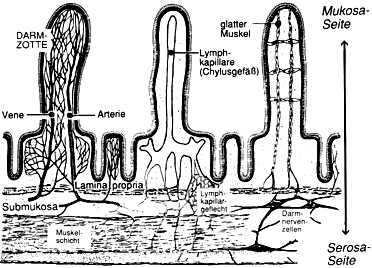
Gruß r
so ein Darm ist schon recht kompliziert aufgebaut
erst dachte ich es handelt sich um einen Phallus bzw. drei


Gruß r

Antwort auf Beitrag Nr.: 45.776.824 von reaaalist am 06.11.13 20:13:34Salut reaaalist
die Resorption von Peptiden und Proteinen durch die Darmwand funktioniert.
Wenn nicht so wäre ...Hätten wir ständig Dünnpfiff ...


à propos Hr Prof.Dr.Reaaalist ,koüpie ihre Vortrag vor 1 paar Monate in XXXXX(D).
à bientôt
OC
die Resorption von Peptiden und Proteinen durch die Darmwand funktioniert.
Wenn nicht so wäre ...Hätten wir ständig Dünnpfiff ...



à propos Hr Prof.Dr.Reaaalist ,koüpie ihre Vortrag vor 1 paar Monate in XXXXX(D).
à bientôt
OC
Oramed Pharmaceuticals






















