Vitrolife (Seite 2)
eröffnet am 26.08.16 12:33:38 von
neuester Beitrag 21.04.23 12:23:57 von
neuester Beitrag 21.04.23 12:23:57 von
Beiträge: 31
ID: 1.237.443
ID: 1.237.443
Aufrufe heute: 0
Gesamt: 4.493
Gesamt: 4.493
Aktive User: 0
ISIN: SE0011205202 · WKN: A2JLT3 · Symbol: VTFN
13,720
EUR
+1,40 %
+0,190 EUR
Letzter Kurs 09:58:09 Tradegate
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,9000 | +59,66 | |
| 4,7450 | +35,57 | |
| 25,06 | +30,18 | |
| 10,360 | +24,82 | |
| 0,6650 | +20,91 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 3,4000 | -11,69 | |
| 8,0100 | -12,85 | |
| 0,9800 | -13,27 | |
| 1,6052 | -30,21 | |
| 0,5121 | -30,80 |
Beitrag zu dieser Diskussion schreiben
aber krass teuer: KGV 64
Antwort auf Beitrag Nr.: 59.148.699 von linkshaender am 06.11.18 14:11:51Weiter auf Erfolgskurs.
https://www.vitrolife.com/en/investors/financial-reports/201…
Wieder gutes Quartal.
https://www.vitrolife.com/en/investors/financial-reports/201…
Wieder gutes Quartal.
Antwort auf Beitrag Nr.: 58.205.438 von linkshaender am 13.07.18 10:49:56
3. Quartal sieht wieder gut aus; ggü. VJ und neun-Monats-Schnitt.
Neue Zahlen
https://www.vitrolife.com/en/investors/financial-reports/201…3. Quartal sieht wieder gut aus; ggü. VJ und neun-Monats-Schnitt.
Vitrolife partners with Illumina on preimplantation genetic testing
Vitrolife AB (publ) has entered into a License and Commercialisation Agreement with Illumina, Inc., which provides Vitrolife with exclusive distribution, development and commercialisation rights to Illumina’s preimplantation genetic testing business for IVF in EMEA and Americas.
Beginning in early 2019, Vitrolife will be the exclusive distributor of Illumina’s preimplantation genetic testing for aneuploidy (PGT-A) kit VeriSeq™ PGS and preimplantation genetic testing for monogenic and single gene defects (PGT-M) kit HumanKaryomap-12 in EMEA (Europe, Middle East and Africa) and Americas. In addition, Vitrolife will develop and commercialise new fully kitted products for the in vitro fertilisation (IVF) market using Illumina sequencing. Illumina is today a market leader in technologies used for PGT. Vitrolife estimates that PGT is used for less than ten percent of the world´s IVF cycles. PGT-A, also known as preimplantation genetic screening (PGS), is a test for chromosome copy number that can be used during IVF to help determine the chromosomal status of an embryo from a biopsy of one or more cells. The results of PGS aid in the selection of an embryo likely to have a normal number of chromosomes (euploid) for transfer and help avoid those with abnormal copy number (aneuploid) that may result in IVF failure or miscarriage.
Under the agreement, Vitrolife will make a one-time payment to Illumina of USD 13 million. As Vitrolife develops new kitted sequencing solutions for IVF, Vitrolife will have the exclusive right to commercialise these new products world-wide excluding mainland China for an additional payment of USD 3 million, subject to certain conditions. Vitrolife has agreed to minimum purchase commitments from Illumina through 2023. Illumina will provide transition and support services to Vitrolife. The upfront payment will be financed by available cash balances.
“Through this partnership Vitrolife will become the market leader in PGT technologies, which in combination with the present market leading position in Time-Lapse create a unique opportunity to offer the IVF clinics access to the most effective products to assist their patients’ desire to have children,” says Thomas Axelsson, CEO of Vitrolife.
The transaction is expected to impact EBITDA per share marginally negatively during 2018 and positively as from 2019 and onwards. During 2019, it is expected that the transaction will be accretive to revenue by approximately 10 percent and EBITDA by 3-5 percent. More information about the business will be presented at a Capital Market´s day in November 21 (see www.vitrolife.com for more information).
Vitrolife AB (publ) has entered into a License and Commercialisation Agreement with Illumina, Inc., which provides Vitrolife with exclusive distribution, development and commercialisation rights to Illumina’s preimplantation genetic testing business for IVF in EMEA and Americas.
Beginning in early 2019, Vitrolife will be the exclusive distributor of Illumina’s preimplantation genetic testing for aneuploidy (PGT-A) kit VeriSeq™ PGS and preimplantation genetic testing for monogenic and single gene defects (PGT-M) kit HumanKaryomap-12 in EMEA (Europe, Middle East and Africa) and Americas. In addition, Vitrolife will develop and commercialise new fully kitted products for the in vitro fertilisation (IVF) market using Illumina sequencing. Illumina is today a market leader in technologies used for PGT. Vitrolife estimates that PGT is used for less than ten percent of the world´s IVF cycles. PGT-A, also known as preimplantation genetic screening (PGS), is a test for chromosome copy number that can be used during IVF to help determine the chromosomal status of an embryo from a biopsy of one or more cells. The results of PGS aid in the selection of an embryo likely to have a normal number of chromosomes (euploid) for transfer and help avoid those with abnormal copy number (aneuploid) that may result in IVF failure or miscarriage.
Under the agreement, Vitrolife will make a one-time payment to Illumina of USD 13 million. As Vitrolife develops new kitted sequencing solutions for IVF, Vitrolife will have the exclusive right to commercialise these new products world-wide excluding mainland China for an additional payment of USD 3 million, subject to certain conditions. Vitrolife has agreed to minimum purchase commitments from Illumina through 2023. Illumina will provide transition and support services to Vitrolife. The upfront payment will be financed by available cash balances.
“Through this partnership Vitrolife will become the market leader in PGT technologies, which in combination with the present market leading position in Time-Lapse create a unique opportunity to offer the IVF clinics access to the most effective products to assist their patients’ desire to have children,” says Thomas Axelsson, CEO of Vitrolife.
The transaction is expected to impact EBITDA per share marginally negatively during 2018 and positively as from 2019 and onwards. During 2019, it is expected that the transaction will be accretive to revenue by approximately 10 percent and EBITDA by 3-5 percent. More information about the business will be presented at a Capital Market´s day in November 21 (see www.vitrolife.com for more information).
Antwort auf Beitrag Nr.: 56.056.589 von Fel216 am 30.10.17 09:57:29
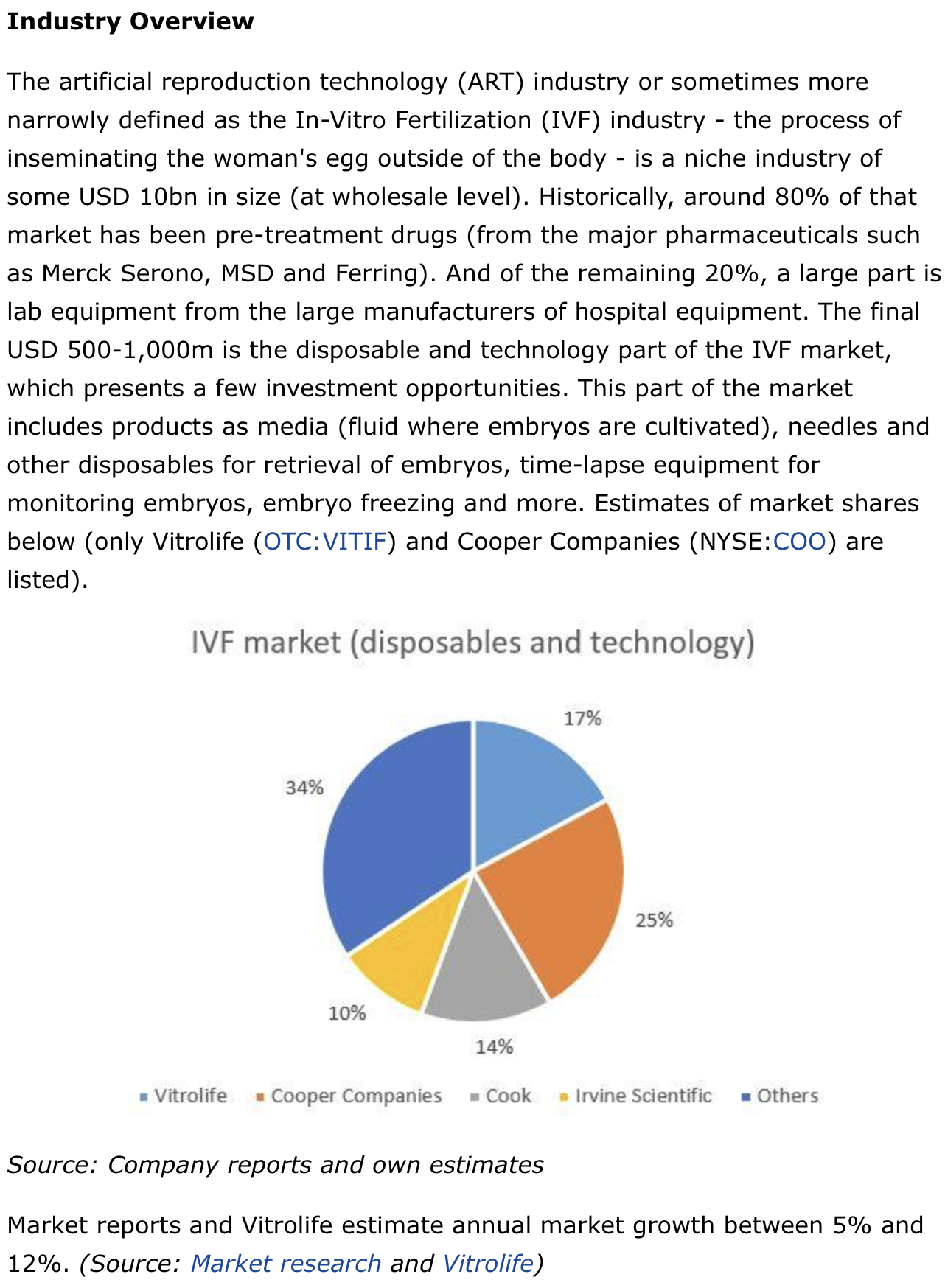
habe eben noch einen gut 2 Jahre alten SA-Artikel gefunden,
der überraschenderweise noch offen ist: https://seekingalpha.com/article/3958386-infertility-treatme…
Zahlen von heute
https://www.vitrolife.com/en/investors/financial-reports/201…Umsatzrückgang in Q2; Verbesserung der Profitabilität. Deutlicher Kursrückgang > 10%.
Vitrolife and GE Healthcare partner to improve assisted reproductive technology offerings
In the lead up to the European Society of Human Reproduction and Embryology (ESHRE) annual conference, GE Healthcare and Vitrolife are partnering to improve patient outcomes in assisted reproductive medicine.
More than 20 years ago, Vitrolife was a key player in introducing the technique and needles for ultrasound-guided oocyte retrieval - now a standard practice in IVF. Vitrolife’s needles feature new echomarking to maximize ultrasound visibility, and an even sharper tip to enable a smooth penetration of the tissue. GE Healthcare’s Voluson™ ultrasound systems enable physicians to monitor stimulated follicles, determine the optimal time for oocyte retrieval, and follow these needles in great detail throughout retrieval.
“With the rise of IVF treatments, we’re committed to providing the highest-quality technology to clinicians, and improving the patient’s experience at every step,” said Roland Rott, General Manager of Women’s Health Ultrasound, GE Healthcare. “We are thrilled to partner with Vitrolife to connect the patient journey in and out of the IVF clinic to ensure the best possible result for the patient.”
From the early diagnosis of potential challenges and evaluating the causes of infertility to monitoring the transfer and ensuring a healthy pregnancy, GE Healthcare’s ultrasound technologies play a key role in reproductive medicine. Similarly, Vitrolife’s assisted reproductive technology offerings incorporate the complete IVF process from the oocyte retrieval through fertilization, embryo culturing, and transfer.
“This partnership supports Vitrolife’s ambition to offer IVF clinics the most effective products in its efforts to assist patients’ desire to have children” said Thomas Axelsson, CEO of Vitrolife AB. “We look forward to partnering with GE Healthcare on joint educational activities for IVF clinics around key procedures and the potential to collaborate on future development projects.”
In the lead up to the European Society of Human Reproduction and Embryology (ESHRE) annual conference, GE Healthcare and Vitrolife are partnering to improve patient outcomes in assisted reproductive medicine.
More than 20 years ago, Vitrolife was a key player in introducing the technique and needles for ultrasound-guided oocyte retrieval - now a standard practice in IVF. Vitrolife’s needles feature new echomarking to maximize ultrasound visibility, and an even sharper tip to enable a smooth penetration of the tissue. GE Healthcare’s Voluson™ ultrasound systems enable physicians to monitor stimulated follicles, determine the optimal time for oocyte retrieval, and follow these needles in great detail throughout retrieval.
“With the rise of IVF treatments, we’re committed to providing the highest-quality technology to clinicians, and improving the patient’s experience at every step,” said Roland Rott, General Manager of Women’s Health Ultrasound, GE Healthcare. “We are thrilled to partner with Vitrolife to connect the patient journey in and out of the IVF clinic to ensure the best possible result for the patient.”
From the early diagnosis of potential challenges and evaluating the causes of infertility to monitoring the transfer and ensuring a healthy pregnancy, GE Healthcare’s ultrasound technologies play a key role in reproductive medicine. Similarly, Vitrolife’s assisted reproductive technology offerings incorporate the complete IVF process from the oocyte retrieval through fertilization, embryo culturing, and transfer.
“This partnership supports Vitrolife’s ambition to offer IVF clinics the most effective products in its efforts to assist patients’ desire to have children” said Thomas Axelsson, CEO of Vitrolife AB. “We look forward to partnering with GE Healthcare on joint educational activities for IVF clinics around key procedures and the potential to collaborate on future development projects.”
Antwort auf Beitrag Nr.: 57.706.744 von R-BgO am 07.05.18 09:23:27neue Stücke sind eingebucht
EmbryoScope+ approved for sales in the USA
Vitrolife AB (publ) has today received market approval for the time-lapse incubator EmbryoScope+ in the USA, the world's third largest market measured in terms of number of IVF treatments.
EmbryoScope+ is a time-lapse system with an integrated incubator with a capacity for fifteen patients. Vitrolife is the market leader of time-lapse systems for use in assisted reproduction for undisturbed culture and improved selection of embryos. “We are delighted to now being able to offer also EmbryoScope+ to the clinics in the USA”, says Thomas Axelsson, CEO of Vitrolife.
Vitrolife AB (publ) has today received market approval for the time-lapse incubator EmbryoScope+ in the USA, the world's third largest market measured in terms of number of IVF treatments.
EmbryoScope+ is a time-lapse system with an integrated incubator with a capacity for fifteen patients. Vitrolife is the market leader of time-lapse systems for use in assisted reproduction for undisturbed culture and improved selection of embryos. “We are delighted to now being able to offer also EmbryoScope+ to the clinics in the USA”, says Thomas Axelsson, CEO of Vitrolife.
Record date for stock split determined
At Vitrolife AB’s Annual General Meeting on April 26, 2018 it was resolved to increase the number of shares in the company by dividing each share into five shares (stock split 5:1). The Board of Directors was authorized to determine the record date for the stock split.
The Board of Directors has now determined that the record date for the stock split will be Friday May 18, 2018, which means that the last day for trading in the share prior to the split is Wednesday May 16, 2018 and the first day for trading with the split shares is Thursday May 17, 2018.
As a result of the split, the shares in Vitrolife AB will as from and including May 17, 2018 change ISIN-number. The new ISIN number is SE0011205202. The shares obtained through the split are expected to be registered on VP-accounts on Monday May 21, 2018.
The split will be carried out automatically by Euroclear and shareholders do not need take any actions. After the split, the number of shares in the company will amount to 108 550 575.
Gothenburg, May 7, 2018
At Vitrolife AB’s Annual General Meeting on April 26, 2018 it was resolved to increase the number of shares in the company by dividing each share into five shares (stock split 5:1). The Board of Directors was authorized to determine the record date for the stock split.
The Board of Directors has now determined that the record date for the stock split will be Friday May 18, 2018, which means that the last day for trading in the share prior to the split is Wednesday May 16, 2018 and the first day for trading with the split shares is Thursday May 17, 2018.
As a result of the split, the shares in Vitrolife AB will as from and including May 17, 2018 change ISIN-number. The new ISIN number is SE0011205202. The shares obtained through the split are expected to be registered on VP-accounts on Monday May 21, 2018.
The split will be carried out automatically by Euroclear and shareholders do not need take any actions. After the split, the number of shares in the company will amount to 108 550 575.
Gothenburg, May 7, 2018





















