NEUROCRINE goes like ImClone - 500 Beiträge pro Seite (Seite 2)
eröffnet am 14.10.06 18:09:23 von
neuester Beitrag 16.09.09 18:17:58 von
neuester Beitrag 16.09.09 18:17:58 von
Beiträge: 754
ID: 1.087.744
ID: 1.087.744
Aufrufe heute: 0
Gesamt: 29.433
Gesamt: 29.433
Aktive User: 0
ISIN: US64125C1099 · WKN: 900964 · Symbol: UOT
143,03
USD
+3,99 %
+5,49 USD
Letzter Kurs 02:00:00 Nasdaq
Neuigkeiten
13.02.24 · wallstreetONLINE Redaktion |
04.02.24 · DR. KALLIWODA RESEARCH |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 5,1300 | +214,72 | |
| 1,2500 | +92,01 | |
| 0,8905 | +74,51 | |
| 3,3700 | +49,45 | |
| 1,2099 | +34,52 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 8,2200 | -13,47 | |
| 1,2029 | -14,15 | |
| 1,6100 | -15,26 | |
| 7,9300 | -15,91 | |
| 3,5000 | -26,24 |
Antwort auf Beitrag Nr.: 28.050.776 von Poppholz am 01.03.07 16:51:33sieht echt nicht gut aus! 
Aber unser ELAN hat sich durchgeschlagen!
Bist Du überhaupt hier investiert?

Aber unser ELAN hat sich durchgeschlagen!
Bist Du überhaupt hier investiert?

auch hier gilt -
wer net hören will muss fühlen...
gelle zen...

Antwort auf Beitrag Nr.: 28.085.876 von GerdKill am 03.03.07 11:43:48man lernt nie aus 

bislang nur 187k vol 
SO gehts net uppi

SO gehts net uppi

für wann erhofft man sich die FDA-Zulassung der 15 mg Variante von Indiplon?
danke+gruss
danke+gruss
meines wissens nach gehts hier um die 5 - 10 mg variante
zulassung bis jahresende denk ich

zulassung bis jahresende denk ich

Antwort auf Beitrag Nr.: 28.362.924 von zenman am 18.03.07 22:19:38ist also zu früh um hier einzusteigen?
im high stand sie vor einigen wochen auf 14,80
hat konsolidiert jetz bis unter 11
vielleicht
je nach markt
gehts sogar noch bis 10
musst du selber entscheiden
wann du für richtig hälst

hat konsolidiert jetz bis unter 11
vielleicht
je nach markt
gehts sogar noch bis 10
musst du selber entscheiden
wann du für richtig hälst

Antwort auf Beitrag Nr.: 28.363.482 von zenman am 18.03.07 23:04:46mir geht es um die Zulassungen und den ggf. gefolgten Rebound. Allerdings habe ich jetzt im Forum Encysive eher bedenkliche Sachen bezüglich der FDA gelesen und dessen Folgen der Unternehmen bzw. dessen Kurswerte...
akt 11,78
wurde aber auch langsam zeit
wurde aber auch langsam zeit

Antwort auf Beitrag Nr.: 28.363.588 von mortem am 18.03.07 23:11:56die qualität und wirksamkeit von indiplon steht bei der FDA ausserhalb der diskussion
NBIX hatte "vergessen" eine verträglichkeitsstudie bzgl der einnahme mit verschiedenen mahlzeiten vorzulegen
aber keine sorge
hat keiner kotzen müssen
man nimmts auch eher abends
und in seltenen fällen
schon kurz nach dem abendessen

NBIX hatte "vergessen" eine verträglichkeitsstudie bzgl der einnahme mit verschiedenen mahlzeiten vorzulegen

aber keine sorge
hat keiner kotzen müssen

man nimmts auch eher abends
und in seltenen fällen
schon kurz nach dem abendessen

leck mich
die 12 sind durch
und ich wollt doch noch zu 11,30 rum zukaufen

die 12 sind durch
und ich wollt doch noch zu 11,30 rum zukaufen

12,09
jetz rennt se mir weg
man is ja nie zufrieden

jetz rennt se mir weg

man is ja nie zufrieden


Antwort auf Beitrag Nr.: 28.454.260 von zenman am 23.03.07 15:39:23gratuliere.
Heute ist generell ein guter Tag für Biotech und Medi-Aktien.
ELN und DNDN machen sich auch gut.
Wäre doch schön, wenn sich die drei gemeinsam nach oben begeben würden.

Heute ist generell ein guter Tag für Biotech und Medi-Aktien.
ELN und DNDN machen sich auch gut.
Wäre doch schön, wenn sich die drei gemeinsam nach oben begeben würden.

Antwort auf Beitrag Nr.: 28.454.608 von Poppholz am 23.03.07 15:51:46holen alle drei auch gerade wieder ein wenig Luft.
(NBIX RT $11,95)
(NBIX RT $11,95)
Antwort auf Beitrag Nr.: 28.454.024 von zenman am 23.03.07 15:30:54Zen, ich habe einige Stücke zu 11,03 bekommen. 
Einbischen verbilligen, so zusagen.

Einbischen verbilligen, so zusagen.
Antwort auf Beitrag Nr.: 28.458.492 von surga am 23.03.07 18:44:08mehr hatte ich gestern leider keine mäuse mehr
akt 12,47 


wassn los hier ?



wassn los hier ?

grad 12,59 
stehn NEWS an, oder was ?
das kann doch nich nur charttechnischer natur sein ?

stehn NEWS an, oder was ?

das kann doch nich nur charttechnischer natur sein ?

By: pinvestment2
Posted as a reply to msg 6448 by Prudentgambler
Re: Stock Movement ,,,,,,,,Fy08 & FY09 CALL OPTION BUYING
we should not forget that NBIX was above 13 and comfortable there before the market fell apart - 13 is really not a big leap especially since NBIX is moving back into a potential news area
last time it appeared there was a leak before the early IR filing PR came out
so
1. is this NBIX getting properly re-valued in a rising market
2. is positive news leaking again and leading to the large amount of call buying
3. is there news coming?
4. short covering
5. buyout rumors again
who knows - but last time once it broke free it just kept rising for weeks - and this is only day two of the rise and 13+ was a stable price for a long time before the market crapped out
i think the IR filing and the new indiplon partner are coming sooner than expected and if NBIX is revaluing with the market and then gets that news I think NBIX is going back above the previous high of 14.90+ - depending on what the market does and when the news comes maybe it is going to happen quickly - maybe it takes until july - we also don't know how much short covering still needs to be done - one thing that today suggests is that there is not a lot of shares available to buy
Posted as a reply to msg 6448 by Prudentgambler
Re: Stock Movement ,,,,,,,,Fy08 & FY09 CALL OPTION BUYING
we should not forget that NBIX was above 13 and comfortable there before the market fell apart - 13 is really not a big leap especially since NBIX is moving back into a potential news area
last time it appeared there was a leak before the early IR filing PR came out
so
1. is this NBIX getting properly re-valued in a rising market
2. is positive news leaking again and leading to the large amount of call buying
3. is there news coming?
4. short covering
5. buyout rumors again
who knows - but last time once it broke free it just kept rising for weeks - and this is only day two of the rise and 13+ was a stable price for a long time before the market crapped out
i think the IR filing and the new indiplon partner are coming sooner than expected and if NBIX is revaluing with the market and then gets that news I think NBIX is going back above the previous high of 14.90+ - depending on what the market does and when the news comes maybe it is going to happen quickly - maybe it takes until july - we also don't know how much short covering still needs to be done - one thing that today suggests is that there is not a lot of shares available to buy
12,70 durchbrochen 
ich rechne mit AM NEWS

ich rechne mit AM NEWS
12,80 durchbrochen 





















13 durchbrochen 

SK 12,58
wahnsinn das teil
wochenlang scheisse gelaufen
und dann an einem tach + 1,58
ohne NEWS
wahnsinn das teil

wochenlang scheisse gelaufen
und dann an einem tach + 1,58
ohne NEWS

!
Dieser Beitrag wurde vom System automatisch gesperrt. Bei Fragen wenden Sie sich bitte an feedback@wallstreet-online.de!
Dieser Beitrag wurde vom System automatisch gesperrt. Bei Fragen wenden Sie sich bitte an feedback@wallstreet-online.de
noch ne meinung 
Partnership is most likely at this time
There might be something going on at NBIX.
A buyout is unlikely at this time. With a 50% or 100% premium, NBIX would be sold at 730/970 million which is too low for the management to accept. Look at how much Tularik got sold with no product and practicely no pipeline, 1.7 billion.
The refiling news is expected in April/May, so it is not really a news anymore. But it might still trigger a mild rally.
Positive clinical trial? I don't know.
The most likely major news is a partnership for Indiplon. NBIX needs it. The time is right for the partner, who is interested in NBIX and has financial and marketing resource, to get in. The deal will be a similar one as Pfizer's and the partner will purchase 5 million share of NBIX with $16-18/share. That will be a major positive news and set NBIX at around 18-20, where it was before Pfizer's divorce. If that is the news, it will leak anyway as so many people involve in making the deal from two parties and investment house.
Another possibility is the stock return to the fair value. In that case, we will in 14-15 in the next few weeks. But with Friday's volume, it is more like a news on its way.
Anyway, it is a fun time for NBIX investor and lets all enjor the Spring.
ich persönlich könnt mir vorstellen
dass in den letzten 2-3 wochen
ALLE, aber auch wirklich alle,
die unter 11 $ gekauft hatten
nun raus sind
sodass
tausende von tradern nur auf ein kaufsignal gewartet haben
der run gestern war so stark
dass ich denke es läuft locker bis an die 14
die nächsten 2 tage
um anschliessend wieder zwischen 13-13,50 zu konsolidieren
zen

Partnership is most likely at this time
There might be something going on at NBIX.
A buyout is unlikely at this time. With a 50% or 100% premium, NBIX would be sold at 730/970 million which is too low for the management to accept. Look at how much Tularik got sold with no product and practicely no pipeline, 1.7 billion.
The refiling news is expected in April/May, so it is not really a news anymore. But it might still trigger a mild rally.
Positive clinical trial? I don't know.
The most likely major news is a partnership for Indiplon. NBIX needs it. The time is right for the partner, who is interested in NBIX and has financial and marketing resource, to get in. The deal will be a similar one as Pfizer's and the partner will purchase 5 million share of NBIX with $16-18/share. That will be a major positive news and set NBIX at around 18-20, where it was before Pfizer's divorce. If that is the news, it will leak anyway as so many people involve in making the deal from two parties and investment house.
Another possibility is the stock return to the fair value. In that case, we will in 14-15 in the next few weeks. But with Friday's volume, it is more like a news on its way.
Anyway, it is a fun time for NBIX investor and lets all enjor the Spring.
ich persönlich könnt mir vorstellen
dass in den letzten 2-3 wochen
ALLE, aber auch wirklich alle,
die unter 11 $ gekauft hatten
nun raus sind
sodass
tausende von tradern nur auf ein kaufsignal gewartet haben
der run gestern war so stark
dass ich denke es läuft locker bis an die 14
die nächsten 2 tage
um anschliessend wieder zwischen 13-13,50 zu konsolidieren
zen

PM
BID 13,00
BID 13,00
PM akt 13,23 
hier is was im busch
bei NBIX leckts immer

hier is was im busch

bei NBIX leckts immer

BID geht auf 13,20 

akt 12,52
NEWS are coming


akt 12,65 

Hi,
achte bitte darauf, das es kein -M- im Chart wird. Ansonsten besteht die Gefahr, das es bis 11,00 Dollar runter geht. Bei einem -W-, kann es schlagartig bis 19,90 laufen. Bei 20,00 ist ein Widerstand, der bei dem zweiten bis drittem Anlauf gemeistert werden sollte. News abhängig!
Gruß W

achte bitte darauf, das es kein -M- im Chart wird. Ansonsten besteht die Gefahr, das es bis 11,00 Dollar runter geht. Bei einem -W-, kann es schlagartig bis 19,90 laufen. Bei 20,00 ist ein Widerstand, der bei dem zweiten bis drittem Anlauf gemeistert werden sollte. News abhängig!
Gruß W
yep
das mach ich mal

das mach ich mal

Sollte es ein -W- werden, geht es Morgen nochmals runter
bis ca. 12,20$ Am Folgetag, sollte dann der Hammer kommen. Wäre dann auch der Sonnerstag. Also Newsstag in dne USA. Passt Doch!
Gruß
Post im E-Mail-Fach
bis ca. 12,20$ Am Folgetag, sollte dann der Hammer kommen. Wäre dann auch der Sonnerstag. Also Newsstag in dne USA. Passt Doch!
Gruß
Post im E-Mail-Fach
grad 12,70 neues TH 

By: pinvestment2
Re: Q2 here we come (pinvestment2)
1. NBIX has a small market cap, no debt, and a sizeable cash position
2. soon it will refile the NDA for indiplon IR - an immediate release sleep medication initially targeted for sleep initiation and middle of the night dosing - it is far more potent, far more clean (very specific for the a1 gaba receptor) than anything else out there - it has a short half life and thus should not pose as large a health risk for the elderly - analysts model the size of the short acting drugs as small because of the niche carved out by sonata - a weak and dirty short acting sleep medication - NBIX lost 2 billion dollars of market cap when its MR version (a long acting version) got delayed and analysts thought they had lost the biggest potential part of the market. Primary care docs I know do not want to treat older people with ambien CR or lunesta because of the long time of being medicated and the myriad side effects associated with them - especially hangovers and loss of cognition that they drugs cause and that can be amplified in hte elderly and lead to accidents - thus they believe that an EFFECTIVE short acting drug will take market share and open up additional markets where docs don't want to tread with the knockout pills - once IR is refiled it should lead to a new partner deal and approval before the end of the year - the partner deal should bring cash, and reduce cash burn
the rest of the NBIX pipeline is impressive
an oral gnrh antagonist for endometriosis without the side effects of current treatments and without hte bone loss of those treatments - and it is a pill and not an injectable drug - in the future can also be used for prostate cancer, breast cancerm and BPH (is in late phase II tecting)
a drug called urocortin2 which seems unblieveably good for congestive heart failure (in early phase II)
and a partnership with GSK for three drugs to treat depression , anxiety, and IBS via a new method - these are in phase II and target very large markets - NBIX can buy in to these to get a co-promotion deal
additionally early drugs in the pipeline
but the pipeline consists of 6 drugs for very large markets and many have already shown impressive testing through phase II and phase III
in a year NBIX can be selling its sleep drug and growing rapidly while adding a sleep maintenance claim to the sleep drug the will further expand its use and then have 4 drugs in phase 3 each targeting very large markets - and most importantly is that the earlier efficacy studies show that these drugs work well and have distinct advantges over current treatments
last week MRK dropped development of its sleep drug and thus there is nothing else marketed by a large pharma in phase III that will be approved for a long period of time. there are plenty of phase II sleeps drugs out there but they are largely rehashing of old drugs from other uses
Re: Q2 here we come (pinvestment2)
1. NBIX has a small market cap, no debt, and a sizeable cash position
2. soon it will refile the NDA for indiplon IR - an immediate release sleep medication initially targeted for sleep initiation and middle of the night dosing - it is far more potent, far more clean (very specific for the a1 gaba receptor) than anything else out there - it has a short half life and thus should not pose as large a health risk for the elderly - analysts model the size of the short acting drugs as small because of the niche carved out by sonata - a weak and dirty short acting sleep medication - NBIX lost 2 billion dollars of market cap when its MR version (a long acting version) got delayed and analysts thought they had lost the biggest potential part of the market. Primary care docs I know do not want to treat older people with ambien CR or lunesta because of the long time of being medicated and the myriad side effects associated with them - especially hangovers and loss of cognition that they drugs cause and that can be amplified in hte elderly and lead to accidents - thus they believe that an EFFECTIVE short acting drug will take market share and open up additional markets where docs don't want to tread with the knockout pills - once IR is refiled it should lead to a new partner deal and approval before the end of the year - the partner deal should bring cash, and reduce cash burn
the rest of the NBIX pipeline is impressive
an oral gnrh antagonist for endometriosis without the side effects of current treatments and without hte bone loss of those treatments - and it is a pill and not an injectable drug - in the future can also be used for prostate cancer, breast cancerm and BPH (is in late phase II tecting)
a drug called urocortin2 which seems unblieveably good for congestive heart failure (in early phase II)
and a partnership with GSK for three drugs to treat depression , anxiety, and IBS via a new method - these are in phase II and target very large markets - NBIX can buy in to these to get a co-promotion deal
additionally early drugs in the pipeline
but the pipeline consists of 6 drugs for very large markets and many have already shown impressive testing through phase II and phase III
in a year NBIX can be selling its sleep drug and growing rapidly while adding a sleep maintenance claim to the sleep drug the will further expand its use and then have 4 drugs in phase 3 each targeting very large markets - and most importantly is that the earlier efficacy studies show that these drugs work well and have distinct advantges over current treatments
last week MRK dropped development of its sleep drug and thus there is nothing else marketed by a large pharma in phase III that will be approved for a long period of time. there are plenty of phase II sleeps drugs out there but they are largely rehashing of old drugs from other uses
NBIX - Neurocrine Biosciences, Inc. - Common Stock
Month ShortInterest PercentChange AverageDailyShareVolume DaystoCover
March 2007 6,492,261 0.67 580,154 11.19
February 2007 6,449,091 12.57 1,368,309 4.71
January 2007 5,729,179 15.67 1,194,230 4.80
December 2006 4,953,188 (17.22) 908,414 5.45
November 2006 5,983,855 (9.88) 1,671,606 3.58
October 2006 6,639,598 8.24 907,021 7.32
September 2006 6,134,299 0.67 1,221,307 5.02
August 2006 6,093,353 2.69 1,069,304 5.70
July 2006 5,933,592 27.03 4,146,861 1.43
June 2006 4,670,846 15.81 4,820,627 1.00
May 2006 4,033,278 (6.53) 1,007,282 4.00
April 2006 4,315,145 13.34 716,489 6.02
knapp 18% short per mitte märz
Month ShortInterest PercentChange AverageDailyShareVolume DaystoCover
March 2007 6,492,261 0.67 580,154 11.19
February 2007 6,449,091 12.57 1,368,309 4.71
January 2007 5,729,179 15.67 1,194,230 4.80
December 2006 4,953,188 (17.22) 908,414 5.45
November 2006 5,983,855 (9.88) 1,671,606 3.58
October 2006 6,639,598 8.24 907,021 7.32
September 2006 6,134,299 0.67 1,221,307 5.02
August 2006 6,093,353 2.69 1,069,304 5.70
July 2006 5,933,592 27.03 4,146,861 1.43
June 2006 4,670,846 15.81 4,820,627 1.00
May 2006 4,033,278 (6.53) 1,007,282 4.00
April 2006 4,315,145 13.34 716,489 6.02
knapp 18% short per mitte märz
By: pinvestment2
accoring to CIBC - NBIX will present on thurs 11:30 - and it will be lyons
it is listed on the CIBC site - now lets see if NBIX confirms
it is a half hour presentation - almost every time lyons has spoke at an investment conference he has given rather interesting insight that you don't hear on the quarterly CC - thus I expect he may give an update on the indiplon refiling (shortly, weeks, end of Q2) and I wouldn't expect him to say end of Q2 if it is going in during april - and of this time he does have a reason to want to see more interest in NBIX and a higher stock price (not that he wouldn't have before) but with the biotech market improving and NBIXs fortunes looking good he should deliver the news that allows people to develop a better understanding of NBIX's value. It will also be interesting to see what he says about an indiplon IR partner (they can't wait long after refiling to start coordinating a launch plan - gnrh enrollment rate would be interesting to hear as well as whether urocortin2 will be moving to longer infusion trials - last presentation NBIX started to stress the progress in the GSK trials and I wonder if this is something that NBIX thinks people should start recognizing.
I did a little bit of reading and saw that the poison pill looks like it expires on april 10 - does anyone think that someone might want to move an investment past 15% as that poison pill expires? (there has been a run up in volume) - I would think that someone would rather get a large stake in NBIX to hold that actually try to buy them
should be an interesting week
accoring to CIBC - NBIX will present on thurs 11:30 - and it will be lyons
it is listed on the CIBC site - now lets see if NBIX confirms
it is a half hour presentation - almost every time lyons has spoke at an investment conference he has given rather interesting insight that you don't hear on the quarterly CC - thus I expect he may give an update on the indiplon refiling (shortly, weeks, end of Q2) and I wouldn't expect him to say end of Q2 if it is going in during april - and of this time he does have a reason to want to see more interest in NBIX and a higher stock price (not that he wouldn't have before) but with the biotech market improving and NBIXs fortunes looking good he should deliver the news that allows people to develop a better understanding of NBIX's value. It will also be interesting to see what he says about an indiplon IR partner (they can't wait long after refiling to start coordinating a launch plan - gnrh enrollment rate would be interesting to hear as well as whether urocortin2 will be moving to longer infusion trials - last presentation NBIX started to stress the progress in the GSK trials and I wonder if this is something that NBIX thinks people should start recognizing.
I did a little bit of reading and saw that the poison pill looks like it expires on april 10 - does anyone think that someone might want to move an investment past 15% as that poison pill expires? (there has been a run up in volume) - I would think that someone would rather get a large stake in NBIX to hold that actually try to buy them
should be an interesting week
die 14 is durchbrochen 

sauber 


Antwort auf Beitrag Nr.: 28.744.088 von zenman am 10.04.07 18:22:31heut könnts endlich ausbruch geben 

natürlich kanns auch ein scheiss - M werden
aber eigentlich
favourisier ich einen kurzfristigen uppi


aber eigentlich
favourisier ich einen kurzfristigen uppi

Neurocrine Biosciences, Inc. Earnings Conference Call (Q1 2007)
Scheduled to start
Thu, May 3, 2007, 5:00 pm Eastern
http://biz.yahoo.com/cc/2/80522.html
Scheduled to start
Thu, May 3, 2007, 5:00 pm Eastern
http://biz.yahoo.com/cc/2/80522.html
Es wird schon wieder. Neuer Anlauf. So ist es halt, wenn die großen Marken überwudnen werden!
gruß W

gruß W

waren die Zahlen so schlecht?
nö
gar nich
aber
es wurde nichts bedeutendes angekündigt für die nächsten wochen
sodass
viele aus angst vor dem downmove erstmal verkaufen
NBIX ist dermassen traderverseucht
die wellen sind schon verdammt hart
fürn nen longie

gar nich
aber
es wurde nichts bedeutendes angekündigt für die nächsten wochen
sodass
viele aus angst vor dem downmove erstmal verkaufen
NBIX ist dermassen traderverseucht

die wellen sind schon verdammt hart
fürn nen longie

is das jetz noch scheiss-bullisch, oder was ? 

also hier
könnten mich höchstens noch 2 LANGE, WEISSE kerzen entschuldigen
für das emotionale ungemach

also hier
könnten mich höchstens noch 2 LANGE, WEISSE kerzen entschuldigen
für das emotionale ungemach

Antwort auf Beitrag Nr.: 29.347.710 von zenman am 16.05.07 18:06:32nur geduld, Zeni 

11,17 
das drecksteil macht mich krank

das drecksteil macht mich krank

Antwort auf Beitrag Nr.: 29.375.084 von zenman am 18.05.07 17:00:10Welcome back Zeni 

heut tut sich was
grad sin 14k auf 12,58 ASK aufgetaucht
sec später wech
un zack kaäufe bis über 12,70
bei grad mal 120k umsatz
käufer sind im markt
grad sin 14k auf 12,58 ASK aufgetaucht
sec später wech
un zack kaäufe bis über 12,70
bei grad mal 120k umsatz
käufer sind im markt

nusere neuro is ungewöhnlich standhaft
im derzeitigen mini-marktcrash
sie war wohl total überverkauft

oder
man steckt in hinblick aus indiplon-filling einfach mehr weg
im derzeitigen mini-marktcrash
sie war wohl total überverkauft
oder
man steckt in hinblick aus indiplon-filling einfach mehr weg
Antwort auf Beitrag Nr.: 29.693.593 von zenman am 07.06.07 20:58:45mir gefällt der Kursverlauf momentan ganz gut.
Wenn der Kurs morgen (Freitag) in FFM unter die €9,- Marke fallen sollte, könnte ich mir vorstellen, meine erste Postition NEUROCINE aufzubauen.

Wenn der Kurs morgen (Freitag) in FFM unter die €9,- Marke fallen sollte, könnte ich mir vorstellen, meine erste Postition NEUROCINE aufzubauen.

DESWEGEN uppi
Neurocrine Biosciences Announces New Research Studies Presented at APSS Reveal Significant Under-Diagnosis of Insomnia
Data Highlights the Impact of Insomnia on Healthcare System and Worker Productivity
SAN DIEGO, June 12 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that results from several research studies on the impact of chronic primary and transient insomnia were presented this week at the Associated Professional Sleep Societies (APSS) Annual Meeting. APSS highlights show that insomnia is significantly under-diagnosed and puts a significant strain on the Healthcare System and Labor Force. New data presented at APSS demonstrates the significant impact insomnia has on daytime functioning including workplace productivity, health, mood and quality of life, regardless of the type of sleep difficulty experienced.
'This research demonstrates that the impact of sleep difficulties on sufferers and the healthcare system is significant. Mounting research confirms that insomnia is a condition to be taken seriously and suggests that treating insomnia properly from the outset could reduce its subsequent impact, cost and safety consequences,' said Christopher O'Brien M.D., Senior Vice President of Clinical Development and Chief Medical Officer of Neurocrine Biosciences.
APSS Presentation Highlights:
Study Demonstrates that Insomnia is Significantly Under-Diagnosed
-- The purpose of this study was to examine the prevalence and clinical
characteristics of subtypes of insomnia in the general population.
-- According to a survey of 14,997 U.S. adults, 43.3% of respondents
self-reported sleeping difficulties related to insomnia; compared to
only 8.5% who met the more stringent Diagnostic and Statistic Manual
of Mental Disorders IV (DSM-IV) criteria for primary insomnia by
self-reported symptoms.
-- Results found that DSM-IV insomnia appears to be under-diagnosed and
under-treated. Additionally, there are a significant number of
individuals with insomnia, who do not meet the DSM-IV criteria, and
experience considerable daytime impairment and symptom frequency.
Study Estimates Insomnia Costs at $13.5 Billion Annually in the U.S. Labor
Force
-- The objective of this study was to estimate the annual indirect costs
in the U.S. Labor Force of DSM-IV diagnosed chronic primary insomnia.
-- Results showed that the annual indirect cost of chronic primary
insomnia in the U.S. labor force, as diagnosed by the DSM-IV, is
estimated to be $13.5 billion which reflects absenteeism from work
directly attributable to an insomnia diagnosis, and productivity
losses attributable to the insomnia-related incidence of accidents and
chronic illnesses (depression, alcohol and/or substance
abuse/dependence)
-- The authors concluded that a DSM-IV diagnosis of insomnia leads to
considerable productivity losses in the U.S. labor force.
Interventions to reduce the severity of chronic primary insomnia among
U.S. workers could substantially reduce its indirect cost burden.
Pharmaceutical Treatment of Insomnia Reduces Overall Economic Costs of Insomnia
-- The objective of this study was to estimate the direct and indirect
costs of treated and untreated insomnia in an employed population.
-- Results showed that insomnia patients who were initially treated with
a non-benzodiazepine hypnotic within two weeks of diagnosis had a much
lower cost burden that those who were not treated for their insomnia.
Demonstrating that treating insomnia was cost-effective relative to
non-treatment, or delayed treatment.
Study Shows that Existing Next Day Impairment Measures May Not Capture True Impact of Insomnia
-- The goal of this study was to evaluate the commonly used tests of
'next day functioning' in insomnia patients to determine if these
tests were successful in measuring key impairment characteristics.
-- The research identified impairment in several areas that were not
identified by traditional tests of functioning. Examples include
abstraction, organization/planning, time management, cognitive
flexibility, judgment and problem solving.
-- Researchers concluded that current measures of daytime functional
impairment may not accurately assess key aspects of daytime functional
impairment.
Increase in Medical Errors As a Result of Insomnia
Results of a new survey that found that insomnia was common in the nursing community, previously announced by Alertness Solutions, were presented at the APSS meeting. The authors concluded that more than one quarter of nurses surveyed suffered from insomnia. The survey conducted by Dr. Mark Rosekind, founder, president and chief scientist of Alertness Solutions, previous director of the Fatigue Countermeasures Program at the NASA Ames Research Center and sleep researcher at Stanford University also identified potential daytime consequences of insomnia that were common among nurses that may affect patient care including a significant increase in medication dispensing errors, charting deviations, and falling asleep unintentionally at work.
Neurocrine is developing indiplon for the treatment of insomnia. Indiplon was licensed from DOV Pharmaceutical in 1998. Neurocrine Biosciences, Inc. is a product-based biopharmaceutical company focused on neurological and endocrine diseases and disorders. The product candidates address some of the largest pharmaceutical markets in the world including insomnia, anxiety, depression, endometriosis, irritable bowel syndrome, and pain. Neurocrine Biosciences, Inc. news releases are available through the Company's website via the Internet at http://www.neurocrine.com.

Neurocrine Biosciences Announces New Research Studies Presented at APSS Reveal Significant Under-Diagnosis of Insomnia
Data Highlights the Impact of Insomnia on Healthcare System and Worker Productivity
SAN DIEGO, June 12 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that results from several research studies on the impact of chronic primary and transient insomnia were presented this week at the Associated Professional Sleep Societies (APSS) Annual Meeting. APSS highlights show that insomnia is significantly under-diagnosed and puts a significant strain on the Healthcare System and Labor Force. New data presented at APSS demonstrates the significant impact insomnia has on daytime functioning including workplace productivity, health, mood and quality of life, regardless of the type of sleep difficulty experienced.
'This research demonstrates that the impact of sleep difficulties on sufferers and the healthcare system is significant. Mounting research confirms that insomnia is a condition to be taken seriously and suggests that treating insomnia properly from the outset could reduce its subsequent impact, cost and safety consequences,' said Christopher O'Brien M.D., Senior Vice President of Clinical Development and Chief Medical Officer of Neurocrine Biosciences.
APSS Presentation Highlights:
Study Demonstrates that Insomnia is Significantly Under-Diagnosed
-- The purpose of this study was to examine the prevalence and clinical
characteristics of subtypes of insomnia in the general population.
-- According to a survey of 14,997 U.S. adults, 43.3% of respondents
self-reported sleeping difficulties related to insomnia; compared to
only 8.5% who met the more stringent Diagnostic and Statistic Manual
of Mental Disorders IV (DSM-IV) criteria for primary insomnia by
self-reported symptoms.
-- Results found that DSM-IV insomnia appears to be under-diagnosed and
under-treated. Additionally, there are a significant number of
individuals with insomnia, who do not meet the DSM-IV criteria, and
experience considerable daytime impairment and symptom frequency.
Study Estimates Insomnia Costs at $13.5 Billion Annually in the U.S. Labor
Force
-- The objective of this study was to estimate the annual indirect costs
in the U.S. Labor Force of DSM-IV diagnosed chronic primary insomnia.
-- Results showed that the annual indirect cost of chronic primary
insomnia in the U.S. labor force, as diagnosed by the DSM-IV, is
estimated to be $13.5 billion which reflects absenteeism from work
directly attributable to an insomnia diagnosis, and productivity
losses attributable to the insomnia-related incidence of accidents and
chronic illnesses (depression, alcohol and/or substance
abuse/dependence)
-- The authors concluded that a DSM-IV diagnosis of insomnia leads to
considerable productivity losses in the U.S. labor force.
Interventions to reduce the severity of chronic primary insomnia among
U.S. workers could substantially reduce its indirect cost burden.
Pharmaceutical Treatment of Insomnia Reduces Overall Economic Costs of Insomnia
-- The objective of this study was to estimate the direct and indirect
costs of treated and untreated insomnia in an employed population.
-- Results showed that insomnia patients who were initially treated with
a non-benzodiazepine hypnotic within two weeks of diagnosis had a much
lower cost burden that those who were not treated for their insomnia.
Demonstrating that treating insomnia was cost-effective relative to
non-treatment, or delayed treatment.
Study Shows that Existing Next Day Impairment Measures May Not Capture True Impact of Insomnia
-- The goal of this study was to evaluate the commonly used tests of
'next day functioning' in insomnia patients to determine if these
tests were successful in measuring key impairment characteristics.
-- The research identified impairment in several areas that were not
identified by traditional tests of functioning. Examples include
abstraction, organization/planning, time management, cognitive
flexibility, judgment and problem solving.
-- Researchers concluded that current measures of daytime functional
impairment may not accurately assess key aspects of daytime functional
impairment.
Increase in Medical Errors As a Result of Insomnia
Results of a new survey that found that insomnia was common in the nursing community, previously announced by Alertness Solutions, were presented at the APSS meeting. The authors concluded that more than one quarter of nurses surveyed suffered from insomnia. The survey conducted by Dr. Mark Rosekind, founder, president and chief scientist of Alertness Solutions, previous director of the Fatigue Countermeasures Program at the NASA Ames Research Center and sleep researcher at Stanford University also identified potential daytime consequences of insomnia that were common among nurses that may affect patient care including a significant increase in medication dispensing errors, charting deviations, and falling asleep unintentionally at work.
Neurocrine is developing indiplon for the treatment of insomnia. Indiplon was licensed from DOV Pharmaceutical in 1998. Neurocrine Biosciences, Inc. is a product-based biopharmaceutical company focused on neurological and endocrine diseases and disorders. The product candidates address some of the largest pharmaceutical markets in the world including insomnia, anxiety, depression, endometriosis, irritable bowel syndrome, and pain. Neurocrine Biosciences, Inc. news releases are available through the Company's website via the Internet at http://www.neurocrine.com.
AH indiplon filling 
June 12, 2007 - 4:05 PM EDT
close Email this News Article
Your Name
Your Email
Friend's Name
Friend's Email
Receive Copy: yes
NBIX 12.62 0.00
Today 5d 1m 3m 1y 5y 10y
Neurocrine Announces Resubmission of New Drug Application for Indiplon Capsules
SAN DIEGO, June 12 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that they have resubmitted its New Drug Application (NDA) for indiplon 5 mg and 10 mg capsules for the treatment of insomnia in both adult and elderly patients to the U.S. Food and Drug Administration (FDA). The resubmission is a complete response to the FDA's May 15, 2006 approvable letter. The indiplon NDA resubmission is based on analyses discussed with statistical, clinical and regulatory consultants. In addition the Company has had interactions with the FDA regarding additional analyses of data previously submitted on indiplon capsules.
'We are pleased to announce the resubmission of the NDA for indiplon capsules and we look forward to working with the FDA in the review process of our resubmission,' said Gary A. Lyons, President and CEO of Neurocrine Biosciences. 'We believe that indiplon's unique profile can offer an effective solution for those patients who need help getting to sleep or returning to sleep after a nighttime awakening.'

June 12, 2007 - 4:05 PM EDT
close Email this News Article
Your Name
Your Email
Friend's Name
Friend's Email
Receive Copy: yes
NBIX 12.62 0.00
Today 5d 1m 3m 1y 5y 10y
Neurocrine Announces Resubmission of New Drug Application for Indiplon Capsules
SAN DIEGO, June 12 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that they have resubmitted its New Drug Application (NDA) for indiplon 5 mg and 10 mg capsules for the treatment of insomnia in both adult and elderly patients to the U.S. Food and Drug Administration (FDA). The resubmission is a complete response to the FDA's May 15, 2006 approvable letter. The indiplon NDA resubmission is based on analyses discussed with statistical, clinical and regulatory consultants. In addition the Company has had interactions with the FDA regarding additional analyses of data previously submitted on indiplon capsules.
'We are pleased to announce the resubmission of the NDA for indiplon capsules and we look forward to working with the FDA in the review process of our resubmission,' said Gary A. Lyons, President and CEO of Neurocrine Biosciences. 'We believe that indiplon's unique profile can offer an effective solution for those patients who need help getting to sleep or returning to sleep after a nighttime awakening.'
PM wird heut zeigen wos langgeht 

Antwort auf Beitrag Nr.: 29.868.804 von zenman am 13.06.07 10:25:04...da hoffe ich mal, dass wir wieder mit Schwung in Richtung Norden gehen....
ALLGEMEINE INFOS zur Zulassung neuer Arzneien
22.06.2007 - 09:28 Uhr
FTD: Das kann ja heiter werden
Die Zulassung neuer Arzneien ist für die Pharmabranche längst keine Kleinigkeit mehr. Regelmäßig lassen die Behörden Hoffnungsträger durchfallen und Börsenträume platzen. Künftig kommt es noch dicker.
Sanofi-Aventis ist fassungslos über die Ablehnung seiner Diätpille Acomplia bei der US-Behörde FDA. Begründung: psychische Nebenwirkungen.
GlaxoSmithKline fürchtet das Aus für das umsatzstarke Diabetesmittel Avandia. Risiko: Herzinfarkt.
Das neue Krebsmittel Vectibix vom weltgrößten Biotechkonzern Amgen findet nicht den Zuspruch der Kontrolleure bei der EU-Behörde Emea. Erklärung: Wirksamkeit zweifelhaft.
Drei Beispiele aus der jüngsten Vergangenheit - eine gemeinsame Wirkung: Aufruhr an der Börse. Die Aktienkurse der betreffenden Konzerne sackten nacheinander auf ein Zweijahrestief. Allen Beteiligten, den Managern, Analysten und Kontrolleuren steckt noch der Skandal um das Schmerzmittel Vioxx in den Knochen. Die Folge für den Hersteller Merck & Co. sowie die Branche waren Tausende Schadensersatzklagen, Imagekrisen und Milliardenverluste. Das mahnte zur Vorsicht. Im Jahr drei nach Vioxx zieht die Politik auf beiden Seiten des Atlantiks die Zügel nun noch fester an.
So feilten am Donnerstag in Washington US-Senatoren an letzten Details für ein Gesetz, das der FDA mehr Einfluss und Geld für zusätzliche Sicherheitskontrollen von Medikamenten gibt. Dazu zahlt die Industrie an die FDA Gebühren von rund 400 Mio. $ jährlich, weitere 225 Mio. $ bringt sie in den kommenden fünf Jahren für die FDA-Observierung von Neueinführungen auf. "Die Nation hat aus den Sicherheitsproblemen mit dem Diabetesmedikament Avandia gelernt", sagte der Vorsitzende des Kongressausschusses, John Dingell, vor wenigen Tagen. Zudem können Verstöße gegen Marketing- und Sicherheitsauflagen mit bis zu 100 Mio. $
Strafe geahndet werden.
Und auch die Emea kann nun härter durchgreifen. Die EU-Kommission hat am 15. Juni eine Verordnung in Kraft gesetzt die Verstöße gegen Emea-Regeln mit hohen Geldbußen ahndet - etwa, wenn Firmen Vorgaben ihrer Arzneimittelzulassungen nicht einhalten, Informationen zur Risikobewertung ihrer Produkte zurückhalten oder deren Nebenwirkungen gar nicht oder erst sehr spät melden.
"Sehr harte Sanktionen"
Die Höchstgrenze der Geldbußen liegt bei fünf Prozent des Jahresumsatzes des betroffenen Zulassungsinhabers. Zulassungsinhaber kann auch eine Konzerntochter sein. "Das dürfte zu ungerechten Bestrafungen führen", sagte Unternehmensanwalt Uwe Fröhlich vom Pharmakonzern Baxter. "Zufälligerweise oder sogar absichtlich kann ein besonders umsatzstarker oder umsatzschwacher Teil eines Konzerns Inhaber der Zulassung sein. Ein Schlupfloch könnten auch Vermarktungspartnerschaften unabhängiger Unternehmen bieten, von denen nur eines die Zulassung hält." Gerechter sei es, die Buße am EU-weiten Umsatz der Arznei festzumachen. "Das sind alles in allem sehr harte Sanktionen", sagt Anwalt Jörg Schickert von der Kanzlei Lovells in München. Er findet manches an der Verordnung unausgereift. "Es gibt noch Schwachstellen, darunter die Frage, wie die Abgrenzung zwischen einzelstaatlichen Strafmaßnahmen und EU-weiten Sanktionen geregelt werden soll."
Das für Zulassungen zuständige Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) habe in der jüngeren Vergangenheit einige Straf- und Bußgeldverfahren eingeleitet, mit unterschiedlichem Ausgang entsprechend der Beweislage, heißt es auf Anfrage. "Ob es in Zukunft wichtiger sein wird, derartige Instrumentarien zur Verfügung zu haben, lässt sich nur schwer einschätzen", so das BfArM. Die im Arzneimittelgesetz für Ordnungswidrigkeiten vorgesehenen Bußgelder von maximal 25.000 Euro seien vergleichsweise gering.
Die Verordnung (EG) 658/2007 zur Auferlegung von Geldbußen durch die Kommission ist ein neuartiges Sanktionsmittel, das es für von der Emea zugelassene Arzneimittel bislang noch nicht gab. "Die Industrie sollte schnellstmöglich alle Schwachstellen abklopfen und Verfahren aufsetzen, die zukünftig Verstöße vermeiden. Dies sollte auch dokumentiert werden", sagte der Anwalt Schickert.
Emea-Chef Thomas Lönngren zumindest will nie mehr einen Tag wie den 30. September 2004 erleben: Weder der Vioxx-Hersteller Merck & Co. noch die FDA-Kollegen hatten ihn frühzeitig über den geplanten Rückruf der Schmerzpille informiert. Die Börse wusste früher Bescheid als er. "Wir wollen von Konzernen so schnell wie möglich informiert werden", sagte er.
Autor/Autoren: Peter Kuchenbuch
http://www.finanztreff.de/ftreff/news.htm?sektion=topthemen&…
22.06.2007 - 09:28 Uhr
FTD: Das kann ja heiter werden
Die Zulassung neuer Arzneien ist für die Pharmabranche längst keine Kleinigkeit mehr. Regelmäßig lassen die Behörden Hoffnungsträger durchfallen und Börsenträume platzen. Künftig kommt es noch dicker.
Sanofi-Aventis ist fassungslos über die Ablehnung seiner Diätpille Acomplia bei der US-Behörde FDA. Begründung: psychische Nebenwirkungen.
GlaxoSmithKline fürchtet das Aus für das umsatzstarke Diabetesmittel Avandia. Risiko: Herzinfarkt.
Das neue Krebsmittel Vectibix vom weltgrößten Biotechkonzern Amgen findet nicht den Zuspruch der Kontrolleure bei der EU-Behörde Emea. Erklärung: Wirksamkeit zweifelhaft.
Drei Beispiele aus der jüngsten Vergangenheit - eine gemeinsame Wirkung: Aufruhr an der Börse. Die Aktienkurse der betreffenden Konzerne sackten nacheinander auf ein Zweijahrestief. Allen Beteiligten, den Managern, Analysten und Kontrolleuren steckt noch der Skandal um das Schmerzmittel Vioxx in den Knochen. Die Folge für den Hersteller Merck & Co. sowie die Branche waren Tausende Schadensersatzklagen, Imagekrisen und Milliardenverluste. Das mahnte zur Vorsicht. Im Jahr drei nach Vioxx zieht die Politik auf beiden Seiten des Atlantiks die Zügel nun noch fester an.
So feilten am Donnerstag in Washington US-Senatoren an letzten Details für ein Gesetz, das der FDA mehr Einfluss und Geld für zusätzliche Sicherheitskontrollen von Medikamenten gibt. Dazu zahlt die Industrie an die FDA Gebühren von rund 400 Mio. $ jährlich, weitere 225 Mio. $ bringt sie in den kommenden fünf Jahren für die FDA-Observierung von Neueinführungen auf. "Die Nation hat aus den Sicherheitsproblemen mit dem Diabetesmedikament Avandia gelernt", sagte der Vorsitzende des Kongressausschusses, John Dingell, vor wenigen Tagen. Zudem können Verstöße gegen Marketing- und Sicherheitsauflagen mit bis zu 100 Mio. $
Strafe geahndet werden.
Und auch die Emea kann nun härter durchgreifen. Die EU-Kommission hat am 15. Juni eine Verordnung in Kraft gesetzt die Verstöße gegen Emea-Regeln mit hohen Geldbußen ahndet - etwa, wenn Firmen Vorgaben ihrer Arzneimittelzulassungen nicht einhalten, Informationen zur Risikobewertung ihrer Produkte zurückhalten oder deren Nebenwirkungen gar nicht oder erst sehr spät melden.
"Sehr harte Sanktionen"
Die Höchstgrenze der Geldbußen liegt bei fünf Prozent des Jahresumsatzes des betroffenen Zulassungsinhabers. Zulassungsinhaber kann auch eine Konzerntochter sein. "Das dürfte zu ungerechten Bestrafungen führen", sagte Unternehmensanwalt Uwe Fröhlich vom Pharmakonzern Baxter. "Zufälligerweise oder sogar absichtlich kann ein besonders umsatzstarker oder umsatzschwacher Teil eines Konzerns Inhaber der Zulassung sein. Ein Schlupfloch könnten auch Vermarktungspartnerschaften unabhängiger Unternehmen bieten, von denen nur eines die Zulassung hält." Gerechter sei es, die Buße am EU-weiten Umsatz der Arznei festzumachen. "Das sind alles in allem sehr harte Sanktionen", sagt Anwalt Jörg Schickert von der Kanzlei Lovells in München. Er findet manches an der Verordnung unausgereift. "Es gibt noch Schwachstellen, darunter die Frage, wie die Abgrenzung zwischen einzelstaatlichen Strafmaßnahmen und EU-weiten Sanktionen geregelt werden soll."
Das für Zulassungen zuständige Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) habe in der jüngeren Vergangenheit einige Straf- und Bußgeldverfahren eingeleitet, mit unterschiedlichem Ausgang entsprechend der Beweislage, heißt es auf Anfrage. "Ob es in Zukunft wichtiger sein wird, derartige Instrumentarien zur Verfügung zu haben, lässt sich nur schwer einschätzen", so das BfArM. Die im Arzneimittelgesetz für Ordnungswidrigkeiten vorgesehenen Bußgelder von maximal 25.000 Euro seien vergleichsweise gering.
Die Verordnung (EG) 658/2007 zur Auferlegung von Geldbußen durch die Kommission ist ein neuartiges Sanktionsmittel, das es für von der Emea zugelassene Arzneimittel bislang noch nicht gab. "Die Industrie sollte schnellstmöglich alle Schwachstellen abklopfen und Verfahren aufsetzen, die zukünftig Verstöße vermeiden. Dies sollte auch dokumentiert werden", sagte der Anwalt Schickert.
Emea-Chef Thomas Lönngren zumindest will nie mehr einen Tag wie den 30. September 2004 erleben: Weder der Vioxx-Hersteller Merck & Co. noch die FDA-Kollegen hatten ihn frühzeitig über den geplanten Rückruf der Schmerzpille informiert. Die Börse wusste früher Bescheid als er. "Wir wollen von Konzernen so schnell wie möglich informiert werden", sagte er.
Autor/Autoren: Peter Kuchenbuch
http://www.finanztreff.de/ftreff/news.htm?sektion=topthemen&…
Antwort auf Beitrag Nr.: 29.868.804 von zenman am 13.06.07 10:25:04By: pinvestment2
Re: Can NBIX hold above $10?
if NBIX ever went below 10$ again then I think I would start selling other prized things in order to get more NBIX - it has so many valuable drugs in the pipeline - and only one of them would make NBIX trade at a multiple of its current price
you can be skeptical - and you can be negative - but when you get to buy significantly undervalued and misunderstood assets - it is not because everyone else can see it - when everyone else can see it then it isn\'t such a good value anymore - and when everyone loves it too much then you know you half to unload some
I still have my NBIX position and have added little bits over time - but overall I try to just stay focused on what I have not try to overload a position - but if NBIX gets much cheaper I will be trying to add more because out of shear relative valuation
Re: Can NBIX hold above $10?
if NBIX ever went below 10$ again then I think I would start selling other prized things in order to get more NBIX - it has so many valuable drugs in the pipeline - and only one of them would make NBIX trade at a multiple of its current price
you can be skeptical - and you can be negative - but when you get to buy significantly undervalued and misunderstood assets - it is not because everyone else can see it - when everyone else can see it then it isn\'t such a good value anymore - and when everyone loves it too much then you know you half to unload some
I still have my NBIX position and have added little bits over time - but overall I try to just stay focused on what I have not try to overload a position - but if NBIX gets much cheaper I will be trying to add more because out of shear relative valuation
so langsam kommt se wieder in schwung 

lyons hat noch net den acceptance letter der FDA bzgl der resubmission of indiplon bekanntgegeben
ergo
müsste DAS die nächsten tage anstehen
sodass
ein ausbruch über die 14 möglich wäre
wenn
das umfeld weiterhin die euphorie-rally fährt

ergo
müsste DAS die nächsten tage anstehen
sodass
ein ausbruch über die 14 möglich wäre
wenn
das umfeld weiterhin die euphorie-rally fährt
Antwort auf Beitrag Nr.: 30.599.832 von zenman am 09.07.07 21:36:25So ein Scheissteil 
Bin gespannt, was am 31. July läuft, wahrscheinlich nicht gutes


Bin gespannt, was am 31. July läuft, wahrscheinlich nicht gutes

Antwort auf Beitrag Nr.: 30.879.990 von surga am 27.07.07 17:50:21ich mach mir auch sorgen wg. resubmission von indi
Antwort auf Beitrag Nr.: 30.883.135 von zenman am 27.07.07 22:10:34Neurocrine Biosciences Announces Conference Call and Webcast to Present Second Quarter 2007 Financial Results
Thursday July 26, 5:03 pm ET
SAN DIEGO, July 26 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that the Company will report its second 2007 financial results after the Nasdaq market closes on Tuesday, July 31, 2007. A live conference call and webcast will be held at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time).
.....
http://biz.yahoo.com/prnews/070726/lath181.html?.v=1
Ich hoffe der Widerstand bei 10,66 hält (sehr eng), sonst sehe ich schwarz.
Kleine Hoffnungschema:
NEUROCRINE BIOSCI (NasdaqGS:NBIX)
After Hours: 10.87 Up 0.17 (1.58%) as of Jul 27 on 07/27/07
Thursday July 26, 5:03 pm ET
SAN DIEGO, July 26 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that the Company will report its second 2007 financial results after the Nasdaq market closes on Tuesday, July 31, 2007. A live conference call and webcast will be held at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time).
.....
http://biz.yahoo.com/prnews/070726/lath181.html?.v=1
Ich hoffe der Widerstand bei 10,66 hält (sehr eng), sonst sehe ich schwarz.

Kleine Hoffnungschema:
NEUROCRINE BIOSCI (NasdaqGS:NBIX)
After Hours: 10.87 Up 0.17 (1.58%) as of Jul 27 on 07/27/07
Antwort auf Beitrag Nr.: 30.889.396 von surga am 28.07.07 09:06:15Q2-ergebnis erwart ich nich so schlimm
die ausstehende antwort der FDA auf die resubmission ist eigentlich längst überfällig
genau deshalb fällt auch der kurs
der markt befürchtet zunehmends eine ablehnung mit nachfolgender zeitverschiebung
eine verzögerung der antwort-veröffentlichung durch das mgt kann eigentlich nicht sein wg. adhoc-pflicht
die ausstehende antwort der FDA auf die resubmission ist eigentlich längst überfällig
genau deshalb fällt auch der kurs
der markt befürchtet zunehmends eine ablehnung mit nachfolgender zeitverschiebung
eine verzögerung der antwort-veröffentlichung durch das mgt kann eigentlich nicht sein wg. adhoc-pflicht
Antwort auf Beitrag Nr.: 30.903.039 von zenman am 28.07.07 22:47:52dann hoffen wir das Beste!
Schön Urlaub noch
Schön Urlaub noch

Antwort auf Beitrag Nr.: 30.926.553 von surga am 29.07.07 21:02:52die scheiss hoffnung is teures börsen-opium
häng selber noch oft genug an dieser hooka
auf IV meinte einer
die Q-zahlen wären diesmal ca 2 wochen früher als üblichh bei NBIX
ob diese so gut sind ?
häng selber noch oft genug an dieser hooka
auf IV meinte einer
die Q-zahlen wären diesmal ca 2 wochen früher als üblichh bei NBIX
ob diese so gut sind ?
Antwort auf Beitrag Nr.: 30.929.874 von zenman am 29.07.07 22:45:38sehr richtig!
manchmal benehmen wir uns wie ein Anfänger und idioten!
Hätte von Anfang an Stop setzen müssen
manchmal benehmen wir uns wie ein Anfänger und idioten!

Hätte von Anfang an Stop setzen müssen

Antwort auf Beitrag Nr.: 30.931.325 von surga am 30.07.07 08:36:59
das habe ich mir gedacht
bald haben wir 10$
das habe ich mir gedacht

bald haben wir 10$

Antwort auf Beitrag Nr.: 30.938.667 von surga am 30.07.07 16:39:0602.08.2007 - 12:19 Uhr
Neurocrine Biosciences overweight
Rating-Update:
Werbung
New York (aktiencheck.de AG) - Die Analysten von Lehman Brothers stufen die Aktie von Neurocrine Biosciences (ISIN US64125C1099/ WKN 900964) unverändert mit "overweight" ein. Das Kursziel werde bei 19 USD gesehen. (02.08.2007/ac/a/u) Analyse-Datum: 02.08.2007
Quelle: Finanzen.net
Nun? Der Kurs ist immer noch im Keller
http://www.finanzen.net/analysen/analysen_detail.asp?Analyse…
Neurocrine Biosciences overweight
Rating-Update:
Werbung
New York (aktiencheck.de AG) - Die Analysten von Lehman Brothers stufen die Aktie von Neurocrine Biosciences (ISIN US64125C1099/ WKN 900964) unverändert mit "overweight" ein. Das Kursziel werde bei 19 USD gesehen. (02.08.2007/ac/a/u) Analyse-Datum: 02.08.2007
Quelle: Finanzen.net
Nun? Der Kurs ist immer noch im Keller

http://www.finanzen.net/analysen/analysen_detail.asp?Analyse…
Antwort auf Beitrag Nr.: 30.986.913 von surga am 02.08.07 16:55:16die cc zu den Q2-zahlen muss lt. den IV-postern die beste seit jahren gewesen sein
die fda-annahme der resub. indi ist lt. mgt bereits telefonisch erfolgt
schriftlich folgt wohl in kürze
für indi wird wohl in bälde kräftig die werbetrommel betätigt werden
bei ärzten und in fachjournalen etc
lest mal im IV-board nach
ist hier nur die kurzform
P1 ergebnisse zu SRN1 (oder so ähnlich)
stehen wohl noch im sommer an
die fda-annahme der resub. indi ist lt. mgt bereits telefonisch erfolgt
schriftlich folgt wohl in kürze
für indi wird wohl in bälde kräftig die werbetrommel betätigt werden
bei ärzten und in fachjournalen etc
lest mal im IV-board nach
ist hier nur die kurzform
P1 ergebnisse zu SRN1 (oder so ähnlich)
stehen wohl noch im sommer an
Antwort auf Beitrag Nr.: 30.994.181 von zenman am 02.08.07 22:05:15
na dann, wenn die Leute fertig eingesammelt haben kann ja wieder steigen
na dann, wenn die Leute fertig eingesammelt haben kann ja wieder steigen

AH am freitag
17:27 $ 9.846 11,100
17:02 $ 9.8448 100
16:44 $ 9.8402 54,600
16:41 $ 9.8402 2,500
16:14 $ 9.8448 200
16:11 $ 9.8402 13,000
16:09 $ 9.8448 100
16:04 $ 9.69 134
angeblich 86% insti-anteil
Company Details
Total Shares Out Standing (millions): 38
Market Capitalization ($ millions): $368
Institutional Ownership: 85.8%
17:27 $ 9.846 11,100
17:02 $ 9.8448 100
16:44 $ 9.8402 54,600
16:41 $ 9.8402 2,500
16:14 $ 9.8448 200
16:11 $ 9.8402 13,000
16:09 $ 9.8448 100
16:04 $ 9.69 134
angeblich 86% insti-anteil

Company Details
Total Shares Out Standing (millions): 38
Market Capitalization ($ millions): $368
Institutional Ownership: 85.8%
eben sind 300k nachbörslich übern tisch 

irgendeiner weiss was 
da müssen infos nach aussen gesickert sein
obwohl der kurs heute 1% gefallen ist
sind die CALL optionen jan08 und jan09
DRASTISCH gestiegen
z.b. der 22,5er jan09 um 100% !!!!

da müssen infos nach aussen gesickert sein
obwohl der kurs heute 1% gefallen ist
sind die CALL optionen jan08 und jan09
DRASTISCH gestiegen
z.b. der 22,5er jan09 um 100% !!!!
Antwort auf Beitrag Nr.: 31.057.267 von zenman am 06.08.07 22:09:37After Hours
Time (ET) After Hours
Price After Hours
Share Volume
16:25 $ 9.5859 11,900
16:18 $ 9.58 1,900
16:18 $ 9.58 2,300
16:16 $ 9.5875 12,333
16:14 $ 9.5859 4,100
16:12 $ 9.5864 36,600
16:02 $ 9.70 300,000


16:02 $ 9.62 48,750
Time (ET) After Hours
Price After Hours
Share Volume
16:25 $ 9.5859 11,900
16:18 $ 9.58 1,900
16:18 $ 9.58 2,300
16:16 $ 9.5875 12,333
16:14 $ 9.5859 4,100
16:12 $ 9.5864 36,600
16:02 $ 9.70 300,000



16:02 $ 9.62 48,750
fettester optionsbrocken
jan08 17,5er CALLs
1344000 st vol
bei kursanstieg von 0,85 auf 1,20
das sind rd. 40% PLUS

jan08 17,5er CALLs
1344000 st vol
bei kursanstieg von 0,85 auf 1,20
das sind rd. 40% PLUS


auch noch AH
17:18 $ 9.62 56,250
16:54 $ 9.5857 1,700
16:52 $ 9.5859 9,900
16:39 $ 9.59 680
17:18 $ 9.62 56,250
16:54 $ 9.5857 1,700
16:52 $ 9.5859 9,900
16:39 $ 9.59 680
der Kursverlauf in den letzten Wochen ist ja leider nicht so erfreulich gewesen.
Ich schaue immer mal wieder rein, bin aber noch nicht investiert.
Wünsche Euch steigende Kurse.

Ich schaue immer mal wieder rein, bin aber noch nicht investiert.
Wünsche Euch steigende Kurse.

Antwort auf Beitrag Nr.: 31.058.689 von zenman am 07.08.07 01:02:10bin dabei zu überlegen meine Stücke zu verbilligen 

akt 10,63
also hatten die hohen AH vol der letzten tage
sowie die CALL-wert-explosionen
ihre bedeutung
vermutlich steht FDA annahme des antrages kurz bevor
d.h. mind. noch eine weitere lange , weisse kerze
also hatten die hohen AH vol der letzten tage
sowie die CALL-wert-explosionen
ihre bedeutung
vermutlich steht FDA annahme des antrages kurz bevor
d.h. mind. noch eine weitere lange , weisse kerze
AH vol
16:35 $ 10.1451 7,304
16:31 $ 10.1451 13,900
16:13 $ 10.1451 1,000
16:13 $ 10.1451 900
16:12 $ 10.1442 200
16:11 $ 10.1442 200
16:11 $ 10.1442 600
16:05 $ 9.94 740
16:01 $ 9.96 1,100
16:00 $ 9.96 500
16:00 $ 9.96 500
16:00 $ 9.95 100
16:35 $ 10.1451 7,304
16:31 $ 10.1451 13,900
16:13 $ 10.1451 1,000
16:13 $ 10.1451 900
16:12 $ 10.1442 200
16:11 $ 10.1442 200
16:11 $ 10.1442 600
16:05 $ 9.94 740
16:01 $ 9.96 1,100
16:00 $ 9.96 500
16:00 $ 9.96 500
16:00 $ 9.95 100
freut mich, dass meine Wünsche für Euch so schnell "Früchte getragen" haben.


AH
After Hours
Time (ET) After Hours
Price After Hours
Share Volume
17:30 $ 9.96 134,900
17:02 $ 10.63 272
16:28 $ 10.3273 900
16:24 $ 10.438 13,900
16:17 $ 10.2773 7,300
16:00 $ 9.8971 100
16:00 $ 9.8971 400
After Hours
Time (ET) After Hours
Price After Hours
Share Volume
17:30 $ 9.96 134,900
17:02 $ 10.63 272
16:28 $ 10.3273 900
16:24 $ 10.438 13,900
16:17 $ 10.2773 7,300
16:00 $ 9.8971 100
16:00 $ 9.8971 400
FDA antrags-annahme ist da
Neurocrine Announces PDUFA Action Date of December 12, 2007 for Indiplon Capsules
SAN DIEGO, Aug. 21 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that the Company has received notification today that the U.S. Food and Drug Administration (FDA) has accepted the Company's resubmission of its New Drug Application (NDA) for indiplon 5 mg and 10 mg capsules for the treatment of insomnia and has set a PDUFA action date of December 12, 2007.
AH nur 14k vol
18:15 $ 10.60 2,000
18:11 $ 10.67 100
18:11 $ 10.61 500
18:11 $ 10.60 400
18:11 $ 10.60 600
18:11 $ 10.60 600
18:11 $ 10.60 600
17:56 $ 10.75 1,000
17:53 $ 11 700
17:52 $ 11 500
17:52 $ 10.95 300
17:46 $ 10.93 400
17:44 $ 11.02 100
17:44 $ 11 100
17:44 $ 11 100
17:44 $ 10.93 200
17:42 $ 11 100
17:40 $ 10.99 100
17:40 $ 11 100
17:40 $ 10.99 100
17:40 $ 10.99 100
17:40 $ 11 100
17:39 $ 10.60 100
17:38 $ 11 100
17:21 $ 10.34 100
17:21 $ 10.34 300
17:19 $ 10.14 300
16:28 $ 9.9202 1,700
16:22 $ 9.87 2,370
da wird dann wohl auch morgen nicht viel passieren
ich warte noch mit wiedereinstieg
Neurocrine Announces PDUFA Action Date of December 12, 2007 for Indiplon Capsules
SAN DIEGO, Aug. 21 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that the Company has received notification today that the U.S. Food and Drug Administration (FDA) has accepted the Company's resubmission of its New Drug Application (NDA) for indiplon 5 mg and 10 mg capsules for the treatment of insomnia and has set a PDUFA action date of December 12, 2007.
AH nur 14k vol
18:15 $ 10.60 2,000
18:11 $ 10.67 100
18:11 $ 10.61 500
18:11 $ 10.60 400
18:11 $ 10.60 600
18:11 $ 10.60 600
18:11 $ 10.60 600
17:56 $ 10.75 1,000
17:53 $ 11 700
17:52 $ 11 500
17:52 $ 10.95 300
17:46 $ 10.93 400
17:44 $ 11.02 100
17:44 $ 11 100
17:44 $ 11 100
17:44 $ 10.93 200
17:42 $ 11 100
17:40 $ 10.99 100
17:40 $ 11 100
17:40 $ 10.99 100
17:40 $ 10.99 100
17:40 $ 11 100
17:39 $ 10.60 100
17:38 $ 11 100
17:21 $ 10.34 100
17:21 $ 10.34 300
17:19 $ 10.14 300
16:28 $ 9.9202 1,700
16:22 $ 9.87 2,370
da wird dann wohl auch morgen nicht viel passieren
ich warte noch mit wiedereinstieg
Antwort auf Beitrag Nr.: 31.248.647 von zenman am 22.08.07 00:23:28das glaube ich auch.
Wenn Du noch warten willst, dann lieber bis Freitagabend, wo die Amies meisten schmeissen (Wochenschluss), vielleicht kriegst Du noch günstiger

Wenn Du noch warten willst, dann lieber bis Freitagabend, wo die Amies meisten schmeissen (Wochenschluss), vielleicht kriegst Du noch günstiger

Antwort auf Beitrag Nr.: 31.251.504 von surga am 22.08.07 10:55:16Feuerstein\'s Biotech-Stock Mailbag
http://www.thestreet.com/_yahoo/newsanalysis/biotech/1037631…
Robert D. writes:
\"Neurocrine BioSciences has a new [approval] date on Dec. 12 for indiplon 5-mg and 10-mg capsules for the treatment of insomnia. Before the original rejection, over a year and a half ago, the stock was at $60. Now it is at $10.30. If the drug gets approval, what do you think the market potential is for the drug? Sales? I believe the stock should double at least from current levels. It was six times as much in the past. Or do you think the insomnia market is saturated now? "
Everyone loves a good comeback or underdog story, right? Neurocrine certainly has the potential to fit in that category. As Robert mentioned, the stock was slammed last May when the Food and Drug Administration failed to approve its insomnia drug indiplon. To make matters worse, Neurocrine\'s big marketing partner, Pfizer (PFE - Cramer\'s Take - Stockpickr - Rating), dropped indiplon like a bad habit.
Now, the drug is back in front of the FDA with an approval decision date of Dec. 12. If Neurocrine can get indiplon approved and persuade the FDA to give the drug a decent label and line up a partner willing to shell out big bucks for direct-to-consumer advertising, the company just might make a dent in the ultra-competitive insomnia market, which is also seeing the entry of cheap generic drugs.
If Neurocrine can pull all that off, the stock might climb out of its 15-month hole.
In other words, I wouldn\'t start cueing up the theme song to Rocky just yet.
Seriously, though, at this point, it\'s hard to see Neurocrine\'s situation getting any worse, unless the FDA rejects indiplon a second time.
But let\'s assume that doesn\'t happen. If indiplon is approved, the black storm clouds hanging over Neurocrine should brighten to a mottled grey, which could be enough to get the stock moving in a positive direction, if only just slightly. So maybe Robert\'s thesis isn\'t so far-fetched after all.
But Neurocrine and indiplon face some tough challenges ahead. Can the company secure a marketing partner willing to spend the big money it will take to market a new insomnia drug?
Neurocrine says discussions are moving forward with several interested parties and that a deal should be done by the time indiplon is ready to launch in the first quarter of next year.
Will indiplon\'s therapeutic profile as a fast-acting sleep-inducing drug be competitive against other sleep drugs that help people both fall asleep and maintain sleep through the night?
Neurocrine cites its own market research to claim that so-called nighttime awakenings are the biggest problem for insomniacs. The company is requesting a label from the FDA that would allow for middle-of-the-night indiplon dosing, as long as people have more than four hours of possible sleep time remaining.
http://www.thestreet.com/_yahoo/newsanalysis/biotech/1037631…
Robert D. writes:
\"Neurocrine BioSciences has a new [approval] date on Dec. 12 for indiplon 5-mg and 10-mg capsules for the treatment of insomnia. Before the original rejection, over a year and a half ago, the stock was at $60. Now it is at $10.30. If the drug gets approval, what do you think the market potential is for the drug? Sales? I believe the stock should double at least from current levels. It was six times as much in the past. Or do you think the insomnia market is saturated now? "
Everyone loves a good comeback or underdog story, right? Neurocrine certainly has the potential to fit in that category. As Robert mentioned, the stock was slammed last May when the Food and Drug Administration failed to approve its insomnia drug indiplon. To make matters worse, Neurocrine\'s big marketing partner, Pfizer (PFE - Cramer\'s Take - Stockpickr - Rating), dropped indiplon like a bad habit.
Now, the drug is back in front of the FDA with an approval decision date of Dec. 12. If Neurocrine can get indiplon approved and persuade the FDA to give the drug a decent label and line up a partner willing to shell out big bucks for direct-to-consumer advertising, the company just might make a dent in the ultra-competitive insomnia market, which is also seeing the entry of cheap generic drugs.
If Neurocrine can pull all that off, the stock might climb out of its 15-month hole.
In other words, I wouldn\'t start cueing up the theme song to Rocky just yet.
Seriously, though, at this point, it\'s hard to see Neurocrine\'s situation getting any worse, unless the FDA rejects indiplon a second time.
But let\'s assume that doesn\'t happen. If indiplon is approved, the black storm clouds hanging over Neurocrine should brighten to a mottled grey, which could be enough to get the stock moving in a positive direction, if only just slightly. So maybe Robert\'s thesis isn\'t so far-fetched after all.
But Neurocrine and indiplon face some tough challenges ahead. Can the company secure a marketing partner willing to spend the big money it will take to market a new insomnia drug?
Neurocrine says discussions are moving forward with several interested parties and that a deal should be done by the time indiplon is ready to launch in the first quarter of next year.
Will indiplon\'s therapeutic profile as a fast-acting sleep-inducing drug be competitive against other sleep drugs that help people both fall asleep and maintain sleep through the night?
Neurocrine cites its own market research to claim that so-called nighttime awakenings are the biggest problem for insomniacs. The company is requesting a label from the FDA that would allow for middle-of-the-night indiplon dosing, as long as people have more than four hours of possible sleep time remaining.
Antwort auf Beitrag Nr.: 31.306.246 von surga am 27.08.07 18:32:43
Neurocrine Biosciences to Present at the BioCentury: Newsmakers in the Biotech Industry Conference
Thursday August 30, 5:30 pm ET
Live audio webcast Thursday, September 6, 2007
SAN DIEGO, Aug. 30 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that Neurocrine Biosciences will present at the BioCentury Newsmakers in the Biotech Industry Conference. The live presentation takes place Thursday, September 6, 2007 at 2:30 p.m. Eastern Time (ET) /11:30 a.m. Pacific Time (PT). The presentation will be simultaneously webcast and may be accessed on the Company's website at http://www.neurocrine.com
Thursday August 30, 5:30 pm ET
Live audio webcast Thursday, September 6, 2007
SAN DIEGO, Aug. 30 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that Neurocrine Biosciences will present at the BioCentury Newsmakers in the Biotech Industry Conference. The live presentation takes place Thursday, September 6, 2007 at 2:30 p.m. Eastern Time (ET) /11:30 a.m. Pacific Time (PT). The presentation will be simultaneously webcast and may be accessed on the Company's website at http://www.neurocrine.com
wollte heute in ffm ordern
der makler hat aber keine
taxe 7,25 zu 7,36
ich biete 7,30
der makler stellt neue taxe 7,30 zu 7,36
heut nachmittag hab ich auf 7,36 erhöht
daraufhin
neue taxe 7,42 zu 7,51

der makler hat keine
und will auch nicht leerverkaufen
obwohl er
sich morgen in amiland eindecken könnte
der makler hat aber keine
taxe 7,25 zu 7,36
ich biete 7,30
der makler stellt neue taxe 7,30 zu 7,36
heut nachmittag hab ich auf 7,36 erhöht
daraufhin
neue taxe 7,42 zu 7,51

der makler hat keine
und will auch nicht leerverkaufen
obwohl er
sich morgen in amiland eindecken könnte
Antwort auf Beitrag Nr.: 31.376.576 von zenman am 03.09.07 18:42:33hat wahrscheinlich Angst, dass er morgen keine für so wenig Geld bekommen wird.


Antwort auf Beitrag Nr.: 31.376.593 von Poppholz am 03.09.07 18:44:50Der Händler in Berlin ist noch besser.
Dort wird €8,75 zu €8,80 getaxt.

Dort wird €8,75 zu €8,80 getaxt.

Antwort auf Beitrag Nr.: 31.376.576 von zenman am 03.09.07 18:42:33Ich dachte, das passierte nur bei mir 

Ich glaube, der MM in FFM hat gar keine Aktien

Und will keine Risiko eingehen


Ich glaube, der MM in FFM hat gar keine Aktien


Und will keine Risiko eingehen

hab fett gekauft
müsste ausbruch aus dem abwärtstrend anstehn
umfeld stimmt
bis ende sep müssten daten P1 für nen wirkstoff kommen
hat lyons in der letzten CC zu den Q2 angekündigt

müsste ausbruch aus dem abwärtstrend anstehn
umfeld stimmt
bis ende sep müssten daten P1 für nen wirkstoff kommen
hat lyons in der letzten CC zu den Q2 angekündigt
das scheissteil kommt nich aussn puschen 
um 10.13-10.14 ami-zeit war ne 100k schiebe-order
d.h. geber und empfänger haben sich abgesprochen
weil
der kurs hat sich dabei keinen cent verändert

um 10.13-10.14 ami-zeit war ne 100k schiebe-order
d.h. geber und empfänger haben sich abgesprochen
weil
der kurs hat sich dabei keinen cent verändert
Antwort auf Beitrag Nr.: 31.390.608 von zenman am 04.09.07 20:54:03sorry
war 14.13-14.14 uhr
war 14.13-14.14 uhr
da mauert einer mit 40k ASK zu 10,00 

bin noch komplett rausgekommen zu 9,82 $ 
irgendwas ist faul im markt

irgendwas ist faul im markt

seh ich das richtig ?
ein dreieck welches sich nach unten auflöst

abgefuckte scheisse
ein dreieck welches sich nach unten auflöst
abgefuckte scheisse

Antwort auf Beitrag Nr.: 31.409.363 von zenman am 06.09.07 00:30:01wenn der chart die NEWS vorweg nimmt ? 
da wird doch bei den phase 1 daten nicht mist rausgekommen sein ?
fakt is
egal welches zukunftspotential in neuro steckt
im moment isses ein heisses eisen
und bei der FDA entscheidung am 12. dez
muss man sich auch MASSIV mit puts absichern
wenn man die aktie während der entscheidung halten will

da wird doch bei den phase 1 daten nicht mist rausgekommen sein ?

fakt is
egal welches zukunftspotential in neuro steckt
im moment isses ein heisses eisen
und bei der FDA entscheidung am 12. dez
muss man sich auch MASSIV mit puts absichern
wenn man die aktie während der entscheidung halten will

Neurocrine Biosciences to Present at the Thomas Weisel Partners Healthcare Conference
Wednesday September 5, 4:00 pm ET
Live Audio Webcast Friday, September 7, 2007

http://biz.yahoo.com/prnews/070905/law148.html?.v=10

sieht bullisch aus

bin über 10 nich mehr rein
war mir zu heiss
grad wo auch die ami-indizes geschwächelt haben
im nachhinein betrachtet hätt man drin bleiben können
sah halt so scheisse aus
die 10,40 war auch ein ziemlicher widerstand
viel glück, surga
dass es weiter gut geht
lg zeni
war mir zu heiss
grad wo auch die ami-indizes geschwächelt haben
im nachhinein betrachtet hätt man drin bleiben können
sah halt so scheisse aus
die 10,40 war auch ein ziemlicher widerstand
viel glück, surga
dass es weiter gut geht
lg zeni

Neurocrine Biosciences to Present at the Bear Stearns Healthcare Conference
Live Audio Webcast Tuesday, September 11, 2007
SAN DIEGO, Sept. 10 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that Neurocrine Biosciences will present at the Bear Stearns Healthcare Conference. The live presentation takes place Tuesday, September 11, 2007 at 10:30 a.m. Eastern Time (ET) /7:30 a.m. Pacific Time (PT). The presentation will be simultaneously webcast and may be accessed on the Company\'s website at http://www.neurocrine.com
-------............
Live Audio Webcast Tuesday, September 11, 2007
SAN DIEGO, Sept. 10 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that Neurocrine Biosciences will present at the Bear Stearns Healthcare Conference. The live presentation takes place Tuesday, September 11, 2007 at 10:30 a.m. Eastern Time (ET) /7:30 a.m. Pacific Time (PT). The presentation will be simultaneously webcast and may be accessed on the Company\'s website at http://www.neurocrine.com
-------............
Antwort auf Beitrag Nr.: 31.917.417 von surga am 09.10.07 20:58:43glückwunsch zu deinem super einstieg 
ich vermute da kommt ne meldung

ich vermute da kommt ne meldung

Antwort auf Beitrag Nr.: 31.928.128 von zenman am 10.10.07 17:36:56Danke Zen,
ich hoffe es, sonst geht es sehr schnell wieder runter!
Wenn wir kurz vor 17:00 Uhr sehen, da steigt jemand mit ca. 1Mio zu 10,45$(?).
Bitte Dein BM sehen!
LG Surga
ich hoffe es, sonst geht es sehr schnell wieder runter!
Wenn wir kurz vor 17:00 Uhr sehen, da steigt jemand mit ca. 1Mio zu 10,45$(?).
Bitte Dein BM sehen!
LG Surga
Antwort auf Beitrag Nr.: 31.928.327 von surga am 10.10.07 17:48:20look mal BM du schlafmütze 

so, wieder raus, immerhin 1$ Gewinn / Stück für paar Tage 

so
da ham wer nun en scheiss-abwärtstrend
die letzte wochenkerze lässt neue tiefs erahnen

da ham wer nun en scheiss-abwärtstrend

die letzte wochenkerze lässt neue tiefs erahnen

Antwort auf Beitrag Nr.: 32.175.251 von zenman am 27.10.07 19:26:26bin seit gestern wieder drin zu 9,1$. Mal sehen wie der Kurs sich entwickelt 

Neurocrine Biosciences and Dainippon Sumitomo Pharma (DSP) Announce Agreement to Develop and Commercialize Indiplon in Japan
Thursday November 1, 7:00 am ET
http://biz.yahoo.com/prnews/071101/lath114.html?.v=70
SAN DIEGO, Nov. 1 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) today announced that it has entered into an exclusive licensing agreement for the development and commercialization of indiplon in Japan with Dainippon Sumitomo Pharma Co, Ltd. (DSP). Neurocrine has submitted its New Drug Application (NDA) for indiplon in the US for the treatment of insomnia. The NDA is currently under review by the FDA with a PUDFA action date of December 12, 2007.
--------------...............
Thursday November 1, 7:00 am ET
http://biz.yahoo.com/prnews/071101/lath114.html?.v=70
SAN DIEGO, Nov. 1 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) today announced that it has entered into an exclusive licensing agreement for the development and commercialization of indiplon in Japan with Dainippon Sumitomo Pharma Co, Ltd. (DSP). Neurocrine has submitted its New Drug Application (NDA) for indiplon in the US for the treatment of insomnia. The NDA is currently under review by the FDA with a PUDFA action date of December 12, 2007.
--------------...............
Antwort auf Beitrag Nr.: 32.232.834 von surga am 01.11.07 14:31:33...bin seit gestern wieder drin zu 9,1$. Mal sehen wie der Kurs sich entwickelt
..gutes " timing " , surga...
..ich bin leider nicht so günstig eingestiegen, denke aber , dass wir bis Ende des Jahres einen " Big Move " machen werden, der uns alle glücklich machen wird...
..time will tell....
..gutes " timing " , surga...

..ich bin leider nicht so günstig eingestiegen, denke aber , dass wir bis Ende des Jahres einen " Big Move " machen werden, der uns alle glücklich machen wird...
..time will tell....

Antwort auf Beitrag Nr.: 32.234.463 von bernie55 am 01.11.07 15:53:57Danke Bernie,
bei NBIX bin ich meistens SHORT, ich denke in kurze geht wieder auf 11 Dollar hoch. Wenn ich sehe, dass es wieder stagniert, dann steige ich wieder aus
bei NBIX bin ich meistens SHORT, ich denke in kurze geht wieder auf 11 Dollar hoch. Wenn ich sehe, dass es wieder stagniert, dann steige ich wieder aus

Antwort auf Beitrag Nr.: 32.238.460 von surga am 01.11.07 18:36:12
Would Wyeth possibly buy NBIX???
Wyeth is looking for acquisitions......part of the story
http://www1.investorvillage.com/smbd.asp?mb=3440&mn=10311&pt…
There remain three big challenges that Mr Poussot must tackle to build on recent success. "I don't think we need massive changes but we need to constantly evolve," Mr Poussot says.
The overarching priority must be to build on Wyeth's biotech strength, getting the most out of a pipeline of new drugs by looking at potential products through the more enterprising eyes of a biotech group, not a "big pharma" company.
He may also be able to use Wyeth's improved financial position more aggressively for targeted acquisitions. The "first priority [for acquisitions] is on science," says Mr Poussot. "The cash situation is very strong now."
As for Wyeth becoming an acquisition target as a result of its sales and earnings growth, Mr Poussot says the only defence is to make the company stronger.
Would Wyeth possibly buy NBIX???
Wyeth is looking for acquisitions......part of the story
http://www1.investorvillage.com/smbd.asp?mb=3440&mn=10311&pt…
There remain three big challenges that Mr Poussot must tackle to build on recent success. "I don't think we need massive changes but we need to constantly evolve," Mr Poussot says.
The overarching priority must be to build on Wyeth's biotech strength, getting the most out of a pipeline of new drugs by looking at potential products through the more enterprising eyes of a biotech group, not a "big pharma" company.
He may also be able to use Wyeth's improved financial position more aggressively for targeted acquisitions. The "first priority [for acquisitions] is on science," says Mr Poussot. "The cash situation is very strong now."
As for Wyeth becoming an acquisition target as a result of its sales and earnings growth, Mr Poussot says the only defence is to make the company stronger.
Neurocrine Biosciences to Present at the CIBC World Markets 18th Annual Healthcare Conference
Monday November 5, 1:40 pm ET
Live Audio Webcast Tuesday, November 6, 2007
SAN DIEGO, Nov. 5 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that Neurocrine Biosciences will present at the CIBC World Markets 18th Annual Healthcare Conference.
The live presentation will take place Tuesday, November 6, 2007 at 8:00 a.m. Eastern Time (ET)/5:00 a.m. Pacific Time (PT).
The presentation will be simultaneously webcast and may be accessed on the Company's website at http://www.neurocrine.com
If you are unable to attend the webcast and would like further information on this announcement please contact the Investor Relations Department at Neurocrine Biosciences at (858) 617-7600. Viewers are encouraged to visit the website approximately 5 minutes prior to the presentation to download or install any necessary software. A replay of the presentation will be archived on the website for two weeks.
Monday November 5, 1:40 pm ET
Live Audio Webcast Tuesday, November 6, 2007
SAN DIEGO, Nov. 5 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that Neurocrine Biosciences will present at the CIBC World Markets 18th Annual Healthcare Conference.
The live presentation will take place Tuesday, November 6, 2007 at 8:00 a.m. Eastern Time (ET)/5:00 a.m. Pacific Time (PT).
The presentation will be simultaneously webcast and may be accessed on the Company's website at http://www.neurocrine.com
If you are unable to attend the webcast and would like further information on this announcement please contact the Investor Relations Department at Neurocrine Biosciences at (858) 617-7600. Viewers are encouraged to visit the website approximately 5 minutes prior to the presentation to download or install any necessary software. A replay of the presentation will be archived on the website for two weeks.
Antwort auf Beitrag Nr.: 32.298.746 von surga am 05.11.07 20:32:31Would Wyeth possibly buy NBIX???
Wyeth is looking for acquisitions......part of the story
NBIX und/oder ELAN ????
Wyeth is looking for acquisitions......part of the story
NBIX und/oder ELAN ????
After Hours
Last: $ 9.9541
After Hours
High: $ 9.9541
After Hours
Volume: 304,000
After Hours
Low: $ 9.92
After Hours
Time (ET)
After Hours
Price After Hours
Share Volume
16:18 $ 9.9541 14,600
16:16 $ 9.95 - 270,000
16:15 $ 9.9541 18,400
16:13 $ 9.9305 900
16:03 $ 9.92 100
Last: $ 9.9541
After Hours
High: $ 9.9541
After Hours
Volume: 304,000
After Hours
Low: $ 9.92
After Hours
Time (ET)
After Hours
Price After Hours
Share Volume
16:18 $ 9.9541 14,600
16:16 $ 9.95 - 270,000

16:15 $ 9.9541 18,400
16:13 $ 9.9305 900
16:03 $ 9.92 100
SAN DIEGO,November 26, 2007 - 8:01 AM EST
Neurocrine Biosciences to Present at the 19th Annual Piper Jaffray Health Care Conference
Live Audio Webcast Tuesday, November 27, 2007 at 1:00 p.m. Eastern Time (ET) / 10:00 a.m. Pacific Time (PT).
The presentation will be simultaneously webcast and may be accessed on the Company's website at http://www.neurocrine.com
If you are unable to attend the webcast and would like further information on this announcement please contact the Investor Relations Department at Neurocrine Biosciences at (858) 617-7600. Viewers are encouraged to visit the website approximately 5 minutes prior to the presentation to download or install any necessary software. A replay of the presentation will be archived on the website for two weeks.
About Neurocrine
Neurocrine Biosciences, Inc. is a product-based biopharmaceutical Company focused on neurological and endocrine diseases and disorders. Our product candidates address some of the largest pharmaceutical markets in the world including insomnia, anxiety, depression, endometriosis, pain, irritable bowel syndrome, and autoimmunity. Neurocrine Biosciences, Inc. news releases are available through the Company's website via the Internet at http://www.neurocrine.com.
SOURCE Neurocrine Biosciences, Inc.
Source: PR Newswire (November 26, 2007 - 8:01 AM EST)
http://www.investorvillage.com/smbd.asp?mb=3440&pt=qn
Neurocrine Biosciences to Present at the 19th Annual Piper Jaffray Health Care Conference
Live Audio Webcast Tuesday, November 27, 2007 at 1:00 p.m. Eastern Time (ET) / 10:00 a.m. Pacific Time (PT).
The presentation will be simultaneously webcast and may be accessed on the Company's website at http://www.neurocrine.com
If you are unable to attend the webcast and would like further information on this announcement please contact the Investor Relations Department at Neurocrine Biosciences at (858) 617-7600. Viewers are encouraged to visit the website approximately 5 minutes prior to the presentation to download or install any necessary software. A replay of the presentation will be archived on the website for two weeks.
About Neurocrine
Neurocrine Biosciences, Inc. is a product-based biopharmaceutical Company focused on neurological and endocrine diseases and disorders. Our product candidates address some of the largest pharmaceutical markets in the world including insomnia, anxiety, depression, endometriosis, pain, irritable bowel syndrome, and autoimmunity. Neurocrine Biosciences, Inc. news releases are available through the Company's website via the Internet at http://www.neurocrine.com.
SOURCE Neurocrine Biosciences, Inc.
Source: PR Newswire (November 26, 2007 - 8:01 AM EST)
http://www.investorvillage.com/smbd.asp?mb=3440&pt=qn
wurde heute viel gekauft 


mal sehen, wie es heute sich entwickelt, der Anfang war schön grün 

Antwort auf Beitrag Nr.: 32.598.953 von surga am 28.11.07 15:34:16aktuell 10,25 - 10,26......" big in plus" ...
...wollen wir mal hoffen, dass am bzw. vor dem Stichtag 12.12.07 das erhoffte Plus von xx Prozenten kommen wird.....

...wollen wir mal hoffen, dass am bzw. vor dem Stichtag 12.12.07 das erhoffte Plus von xx Prozenten kommen wird.....

Antwort auf Beitrag Nr.: 32.599.573 von bernie55 am 28.11.07 16:06:53Das wollen wir doch mal hoffen 
Ist ja nur noch 2 Wochen

Ist ja nur noch 2 Wochen

New sleeping pill?
http://www.revolutionhealth.com/blogs/stevepocetamd/new-slee…
A new report published in the professional medical journal Sleep Medicineindicates that Indipon is an effective hypnotic-sedative. When it might get to the market and be prescribed for people with insomnia is not clear. It has been a long story for this medication. It was granted some type of FDA preliminary approval well over a year ago in 2006, but the approval process hit a snag and it is still not on the market. In the meantime the company that developed it, Neurocrine, was bought by Pfizer. I'm not up on the business side of the drug, but it appears that the companies are still trying to address FDA concerns and ultimately bring it to market. I have been hearing about Indiplon for over 6 years, in part because Neurocrine is based in San Diego, but I have not been involved with its research. It's no wonder that new medications cost so much—this development process might end up taking a decade. The drug is a very short acting non-benzodiazepine. That means it is not in the drug class of the "Valium-like" sleeping pills like Halcion. I don't know its chemical structure, but it is not related to the Ambien and Lunesta chemical class. It does bind, like all these drugs to the GABA-receptor complex. It is novel in that it seems to bind to the alpha subunit of the GABA-A receptor. This difference in chemical binding might make it different from current sleeping pills. It is a very short acting drug, and an extended release version has been developed. In general, the goal for these type of drugs is to have a 6 to 8 hour effect in the body. There are several studies to show effectiveness. The recent study in Sleep Medicine tested the 5 and 10 mg tablets compared to placebo. The study was conducted in 2002 and 2003, so I am not sure if the drug is still on track for approval. Both doses decreased time to fall asleep by about 30 minutes, and also reduced the number of awakenings and increased the total sleep time in the night. Side effects were depression, sleepiness, and decreased appetite in the 10 mg doses. The possibility of depression from all sleeping pills is getting increased attention.
For now, the only way to try this medicine is to participate in a research clinical trial. If it makes it to market, I'll let you know.
J. Steven Poceta MD is a licensed practitioner of neurology and sleep disorders who has been engaged by Revolution Health. No information in this blog is intended to diagnose or treat any condition. The opinions expressed here are Dr. Poceta's own and do not necessarily reflect those of Revolution Health.
http://www.revolutionhealth.com/blogs/stevepocetamd/new-slee…
A new report published in the professional medical journal Sleep Medicineindicates that Indipon is an effective hypnotic-sedative. When it might get to the market and be prescribed for people with insomnia is not clear. It has been a long story for this medication. It was granted some type of FDA preliminary approval well over a year ago in 2006, but the approval process hit a snag and it is still not on the market. In the meantime the company that developed it, Neurocrine, was bought by Pfizer. I'm not up on the business side of the drug, but it appears that the companies are still trying to address FDA concerns and ultimately bring it to market. I have been hearing about Indiplon for over 6 years, in part because Neurocrine is based in San Diego, but I have not been involved with its research. It's no wonder that new medications cost so much—this development process might end up taking a decade. The drug is a very short acting non-benzodiazepine. That means it is not in the drug class of the "Valium-like" sleeping pills like Halcion. I don't know its chemical structure, but it is not related to the Ambien and Lunesta chemical class. It does bind, like all these drugs to the GABA-receptor complex. It is novel in that it seems to bind to the alpha subunit of the GABA-A receptor. This difference in chemical binding might make it different from current sleeping pills. It is a very short acting drug, and an extended release version has been developed. In general, the goal for these type of drugs is to have a 6 to 8 hour effect in the body. There are several studies to show effectiveness. The recent study in Sleep Medicine tested the 5 and 10 mg tablets compared to placebo. The study was conducted in 2002 and 2003, so I am not sure if the drug is still on track for approval. Both doses decreased time to fall asleep by about 30 minutes, and also reduced the number of awakenings and increased the total sleep time in the night. Side effects were depression, sleepiness, and decreased appetite in the 10 mg doses. The possibility of depression from all sleeping pills is getting increased attention.
For now, the only way to try this medicine is to participate in a research clinical trial. If it makes it to market, I'll let you know.
J. Steven Poceta MD is a licensed practitioner of neurology and sleep disorders who has been engaged by Revolution Health. No information in this blog is intended to diagnose or treat any condition. The opinions expressed here are Dr. Poceta's own and do not necessarily reflect those of Revolution Health.
Lyons says NBIX is "a soon to be commercial company"
Ich hoffe aber baldddddddddd
Ich hoffe aber baldddddddddd

wieder grün heute 

I smell a leak
At $12.11, somebody know something.
We have be quite for so long, why the sudden move now - two weeks before the announcement.
Good luck everybody
http://www.investorvillage.com/smbd.asp?mb=3440&mn=10878&pt=…
..da könnte was dran sein....
At $12.11, somebody know something.
We have be quite for so long, why the sudden move now - two weeks before the announcement.
Good luck everybody
http://www.investorvillage.com/smbd.asp?mb=3440&mn=10878&pt=…
..da könnte was dran sein....

Antwort auf Beitrag Nr.: 32.616.224 von bernie55 am 29.11.07 17:23:01Geld
8,18
Brief
8,29
Zeit
29.11.07 17:22:05
Geld Stk.
2.400
Brief Stk.
2.400
..nicht schlecht, Herr Specht...
8,18
Brief
8,29
Zeit
29.11.07 17:22:05
Geld Stk.
2.400
Brief Stk.
2.400
..nicht schlecht, Herr Specht...
Antwort auf Beitrag Nr.: 32.616.237 von bernie55 am 29.11.07 17:24:04Hi Bernie,
sieht so aus, dass Du recht hast!!!!!!!!!!!!!

sieht so aus, dass Du recht hast!!!!!!!!!!!!!


Antwort auf Beitrag Nr.: 32.616.237 von bernie55 am 29.11.07 17:24:04Bernie,
sind wir die letzen Indianer? Wo ist Zeni etc
sind wir die letzen Indianer? Wo ist Zeni etc

starkes MACD-Kaufzeichen heute

Herzlichen Glückwunsch Euch----Bernie + Surga 

Antwort auf Beitrag Nr.: 32.617.567 von Birgit.Tersteegen am 29.11.07 19:04:57danke Bigi 

Antwort auf Beitrag Nr.: 32.617.567 von Birgit.Tersteegen am 29.11.07 19:04:57es entschädigt einbischen, was ich bei VERSATAEL falsch gemacht habe 

Antwort auf Beitrag Nr.: 32.617.603 von surga am 29.11.07 19:07:17...das freut mich! (Börse ist ziemlich heftig im Moment....)
(Börse ist ziemlich heftig im Moment....)
 (Börse ist ziemlich heftig im Moment....)
(Börse ist ziemlich heftig im Moment....)
Antwort auf Beitrag Nr.: 32.617.633 von Birgit.Tersteegen am 29.11.07 19:10:26so ist es! In letzten Wochen habe ich viel falsch gemacht (nicht bei ELAN), zu spät reagiert so dass icg viel Buchverluste habe (Air Berlin und DLG Dialog Semiconductor). So ist halt bei der Börse, alles kann passieren 

ich freu mich auch für euch 

Antwort auf Beitrag Nr.: 32.617.733 von zenman am 29.11.07 19:19:47Danke Zen!!! 

@surga
..knapp 2,8 Millionen Aktien in den USA gehandelt.....
...also, es kann so weiter gehen.....
...vielleicht treffen meine " imaginären " Kursziele ja ein...
.....das wäre natürlich der Oberbringer......und wenn man schaut,wo NBIX im März 2006 stand, dann könnten wir noch viel Spaß mit NBIX bekommen .....
..time will tell....
PS: DANKE für die FREUDE-bekundungen der ELAN Fraktion.....
..knapp 2,8 Millionen Aktien in den USA gehandelt.....
...also, es kann so weiter gehen.....
...vielleicht treffen meine " imaginären " Kursziele ja ein...

.....das wäre natürlich der Oberbringer......und wenn man schaut,wo NBIX im März 2006 stand, dann könnten wir noch viel Spaß mit NBIX bekommen .....
..time will tell....
PS: DANKE für die FREUDE-bekundungen der ELAN Fraktion.....

Nov. 29, 2007 Market
After Hours
Last: $ 12.05
After Hours
High: $ 12.105
After Hours
Volume: 45,260
After Hours
Low: $ 11.94
18:44 $ 12.05 100
18:42 $ 12.02 160
17:09 $ 11.99 1,200
16:40 $ 11.94 300
16:22 $ 12.105 - 21.400
16:13 $ 12.1048 - 14.900
16:12 $ 12.105 100
16:12 $ 12.105 100
16:07 $ 12.098 6.800
16:00 $ 12.01 200
After Hours
Last: $ 12.05
After Hours
High: $ 12.105
After Hours
Volume: 45,260
After Hours
Low: $ 11.94
18:44 $ 12.05 100
18:42 $ 12.02 160
17:09 $ 11.99 1,200
16:40 $ 11.94 300
16:22 $ 12.105 - 21.400

16:13 $ 12.1048 - 14.900

16:12 $ 12.105 100
16:12 $ 12.105 100
16:07 $ 12.098 6.800
16:00 $ 12.01 200
...aber " Hallo " 



Antwort auf Beitrag Nr.: 32.624.190 von bernie55 am 30.11.07 10:06:06Wollen wir doch hoffen 
......time will tell....

......time will tell....

..aktuell 12,34 - 12,44...
..also, geht doch....
..also, geht doch....

Antwort auf Beitrag Nr.: 32.628.650 von bernie55 am 30.11.07 15:44:24da fehlt nur noch 10$ zu Deinem Hochstand 

Author: pinvestment2
send pm · add member to favs · ignore · recommend
http://www.investorvillage.com/smbd.asp?mb=3440&mn=10949&pt=…
next few days should be a little more telling
if this was a short squeeze because too much of the non-institutionally owned float is short and some shares became unavailable then NBIX volume should tail off and it should fall back once the squeeze is over
if some institutions just decided to make their shares unavailable and others follow suit it could be tumultuous and continue for some time further - maybe the institutions pull the rug to run the price up and get a chance to hedge against their positions more cheaply
or if something positive is leaking then I think you should see consistent if intermittent buying pressure because NBIX is so cheap and such a tightly coiled spring - folks should know positive knew could set in motion several positive events in the next 2 months that could lead NBIX substantially higher than its previous range
time will tell - the long term story remains the same - plenty of cash - tons of potential valued at almost nothing
send pm · add member to favs · ignore · recommend
http://www.investorvillage.com/smbd.asp?mb=3440&mn=10949&pt=…
next few days should be a little more telling
if this was a short squeeze because too much of the non-institutionally owned float is short and some shares became unavailable then NBIX volume should tail off and it should fall back once the squeeze is over
if some institutions just decided to make their shares unavailable and others follow suit it could be tumultuous and continue for some time further - maybe the institutions pull the rug to run the price up and get a chance to hedge against their positions more cheaply
or if something positive is leaking then I think you should see consistent if intermittent buying pressure because NBIX is so cheap and such a tightly coiled spring - folks should know positive knew could set in motion several positive events in the next 2 months that could lead NBIX substantially higher than its previous range
time will tell - the long term story remains the same - plenty of cash - tons of potential valued at almost nothing
aktuell in den USA:
12,64 - 12,66
..also wirklich schön grün...
..ich hoffe, wir halten die Range von ca. 12,60 USD...
12,64 - 12,66
..also wirklich schön grün...
..ich hoffe, wir halten die Range von ca. 12,60 USD...
Antwort auf Beitrag Nr.: 32.631.225 von bernie55 am 30.11.07 18:42:17ob wir heute 13$ gelangen? Sieht nach News aus am Montag 

Antwort auf Beitrag Nr.: 32.631.933 von surga am 30.11.07 19:55:09...aber hallo.......nach sssssooooo was von News...

Antwort auf Beitrag Nr.: 32.631.933 von surga am 30.11.07 19:55:09..wwwoohhh....aktuell in den USA 12,89 - 12,92.....
...surga , das könnte was werden mit den 13 USD..
..und wenn nicht heute , dann spätestens nach " Approval "....
....aber dann sehen wir die 13 nur noch von hinten ....
...surga , das könnte was werden mit den 13 USD..
..und wenn nicht heute , dann spätestens nach " Approval "....
....aber dann sehen wir die 13 nur noch von hinten ....

Antwort auf Beitrag Nr.: 32.632.000 von bernie55 am 30.11.07 20:01:07Bernie, Du hast BM
Ein schönes Wochenende wünsche ich Dir

Ein schönes Wochenende wünsche ich Dir

läuft super für euch 
trotzdem
seid vorsichtig

ich mag keine ungesicherten potentiellen halbierer mehr in meinem depot
der vorteil an den futures ist
die laufen IMMER marktkonform
d.h.
es gibt keine beschissenen einzelschicksale
wie bei den aktien
wer ami-aktien kaufen kann
sollte sich freddy mac (FRE) anschauen
nächste woche erwarte ich marktkorrektur
schaut euch mal an
wo FRE herkommt
und vor allem
was die aktie die letzten 2 tage gemacht hat
ausserdem ist ein GAP offen bei 38 $ rum

trotzdem
seid vorsichtig

ich mag keine ungesicherten potentiellen halbierer mehr in meinem depot
der vorteil an den futures ist
die laufen IMMER marktkonform
d.h.
es gibt keine beschissenen einzelschicksale
wie bei den aktien
wer ami-aktien kaufen kann
sollte sich freddy mac (FRE) anschauen
nächste woche erwarte ich marktkorrektur
schaut euch mal an
wo FRE herkommt
und vor allem
was die aktie die letzten 2 tage gemacht hat
ausserdem ist ein GAP offen bei 38 $ rum
sehr schöne Entwicklung.
Freue mich für Euch.

Freue mich für Euch.

wie weit geht es noch heute runter? 
 :O
:O

 :O
:O richtiger Ausverkauf heute

.......der Ausblick nach " approval " ????
..wwwoohh, das wärs...

..wwwoohh, das wärs...


Sleep drug awaits FDA wake-up call
FDA is set to decide whether to approve Neurocrine's sleep aide Indiplon, but analysts question whether the short-acting drug will be a blockbuster.
By Aaron Smith, CNNMoney.com staff writer
December 12 2007: 2:55 PM EST
http://money.cnn.com/2007/12/12/news/companies/indiplon/inde…
NEW YORK (CNNMoney.com) -- Neurocrine Biosciences was set to learn on Wednesday whether federal regulators have approved Indiplon, an experimental short-acting sleeping pill analysts think will have a tough time competing.
Indiplon, if approved by the Food and Drug Administration, would be the newest entrant to the $4 billion U.S. market for sleeping pills. But Indiplon's status as a niche drug, and Neurocrine's lack of a Big Pharma marketing partner, means that the drug faces formidable hurdles in a competitive market.
In fact, analysts believe the drug's annual sales would not rise above $200 million.
Indiplon is a short-acting drug for insomniacs that only works for a short time, rather than the whole night. It would be used to put patients to sleep at the beginning of the night, or back to sleep in the middle of the night if they wake up, without its sedative effects lasting through the morning.
"Some people like the idea of taking a medication only when they need it," said Dr. Thomas Roth, consultant for Neurocrine and director of the Indiplon study that was submitted to the FDA, as well as director of sleep disorders research at the Henry Ford Hospital in Detroit. "This study shows you can take the medicine and not be hung-over the next morning."
Gary Lyons, chief executive of San Diego-based Neurocrine Biosciences (Charts), said in an interview earlier this year that Indiplon takes effect within 15 to 20 minutes and it only lasts for one hour. Lyons said that with longer-acting sleeping pills "you have a woman driving her kids to kindergarten with half the drug still in her system. You don't have that problem with Indiplon."
Merck to seek OK for 2 new drugs
If approved, Indiplon would enter a sleeping pill industry that is already dominated by Sanofi-Aventis' (Charts) Ambien CR, Sepracor's (Charts) Lunesta, and King Pharmaceuticals' (Charts) Sonata, as well as Takeda's Rozerem, the only one of those drugs that does not use a sedative to put patients to sleep. There is also a generic low-cost version of Ambien.
Of all these drugs, Indiplon would compete directly against Sonata, which is also a short-acting sleeping pill, said Aileen Salares, analyst for Leerink Swann.
HIV drug market mutates
But the short-acting niche element to Indiplon is unlikely to make it a big seller, analysts say. Salares and another analyst, Jon LeCroy of Natixis Bleichroeder, believe that annual sales will peak at $150 million.
But the FDA decision could have a big impact on Neurocrine Biosciences, which has no other products on the market.
"The stock should move five bucks either way, depending on the outcome [of the FDA decision,]" said LeCroy. Neurocrine shares were trading at $10.11 a share in midday trade on Wednesday.
Neurocrine had tried to get Indiplon approved for the longer-acting dose, but the FDA shot down that effort in May of 2006. This caused the Neurocrine's stock to plunge, and prompted its Big Pharma partner Pfizer Inc. (Charts, Fortune 500) to sever its partnership to promote the drug.
As for the shorter-acting version of Indiplon, the FDA asked that Neurocrine provide more data about the drug. The FDA decision on Indiplon will be based on this additional data.
Neurocrine's stock activity has been wildly volatile in 2007. Indiplon's rocky road with the FDA has created consternation among investors, says Salares of Leerink Swann.
"We're cautious on this because of the checkered history of Indiplon," said Salares. "The upside is muted, because everyone is concerned about the prospects about the drug and that seems to be the overriding concern. They need a strong partner in order to develop the market."
There don't seem to be any takers from Big Pharma at the moment, said Salares.
The analysts interviewed for this story do not own stock in the companies mentioned here, but Leerink Swann makes a market in Neurocrine. To top of page
FDA is set to decide whether to approve Neurocrine's sleep aide Indiplon, but analysts question whether the short-acting drug will be a blockbuster.
By Aaron Smith, CNNMoney.com staff writer
December 12 2007: 2:55 PM EST
http://money.cnn.com/2007/12/12/news/companies/indiplon/inde…
NEW YORK (CNNMoney.com) -- Neurocrine Biosciences was set to learn on Wednesday whether federal regulators have approved Indiplon, an experimental short-acting sleeping pill analysts think will have a tough time competing.
Indiplon, if approved by the Food and Drug Administration, would be the newest entrant to the $4 billion U.S. market for sleeping pills. But Indiplon's status as a niche drug, and Neurocrine's lack of a Big Pharma marketing partner, means that the drug faces formidable hurdles in a competitive market.
In fact, analysts believe the drug's annual sales would not rise above $200 million.
Indiplon is a short-acting drug for insomniacs that only works for a short time, rather than the whole night. It would be used to put patients to sleep at the beginning of the night, or back to sleep in the middle of the night if they wake up, without its sedative effects lasting through the morning.
"Some people like the idea of taking a medication only when they need it," said Dr. Thomas Roth, consultant for Neurocrine and director of the Indiplon study that was submitted to the FDA, as well as director of sleep disorders research at the Henry Ford Hospital in Detroit. "This study shows you can take the medicine and not be hung-over the next morning."
Gary Lyons, chief executive of San Diego-based Neurocrine Biosciences (Charts), said in an interview earlier this year that Indiplon takes effect within 15 to 20 minutes and it only lasts for one hour. Lyons said that with longer-acting sleeping pills "you have a woman driving her kids to kindergarten with half the drug still in her system. You don't have that problem with Indiplon."
Merck to seek OK for 2 new drugs
If approved, Indiplon would enter a sleeping pill industry that is already dominated by Sanofi-Aventis' (Charts) Ambien CR, Sepracor's (Charts) Lunesta, and King Pharmaceuticals' (Charts) Sonata, as well as Takeda's Rozerem, the only one of those drugs that does not use a sedative to put patients to sleep. There is also a generic low-cost version of Ambien.
Of all these drugs, Indiplon would compete directly against Sonata, which is also a short-acting sleeping pill, said Aileen Salares, analyst for Leerink Swann.
HIV drug market mutates
But the short-acting niche element to Indiplon is unlikely to make it a big seller, analysts say. Salares and another analyst, Jon LeCroy of Natixis Bleichroeder, believe that annual sales will peak at $150 million.
But the FDA decision could have a big impact on Neurocrine Biosciences, which has no other products on the market.
"The stock should move five bucks either way, depending on the outcome [of the FDA decision,]" said LeCroy. Neurocrine shares were trading at $10.11 a share in midday trade on Wednesday.
Neurocrine had tried to get Indiplon approved for the longer-acting dose, but the FDA shot down that effort in May of 2006. This caused the Neurocrine's stock to plunge, and prompted its Big Pharma partner Pfizer Inc. (Charts, Fortune 500) to sever its partnership to promote the drug.
As for the shorter-acting version of Indiplon, the FDA asked that Neurocrine provide more data about the drug. The FDA decision on Indiplon will be based on this additional data.
Neurocrine's stock activity has been wildly volatile in 2007. Indiplon's rocky road with the FDA has created consternation among investors, says Salares of Leerink Swann.
"We're cautious on this because of the checkered history of Indiplon," said Salares. "The upside is muted, because everyone is concerned about the prospects about the drug and that seems to be the overriding concern. They need a strong partner in order to develop the market."
There don't seem to be any takers from Big Pharma at the moment, said Salares.
The analysts interviewed for this story do not own stock in the companies mentioned here, but Leerink Swann makes a market in Neurocrine. To top of page
Antwort auf Beitrag Nr.: 32.745.907 von surga am 12.12.07 21:31:52WANN ist denn jetzt endlich FDA-entscheidung ? 

Antwort auf Beitrag Nr.: 32.747.127 von zenman am 12.12.07 22:56:02...eigentlich am 12.12.07....
Sleep drug awaits FDA wake-up call
FDA is set to decide whether to approve Neurocrine's sleep aide Indiplon, but analysts question whether the short-acting drug will be a blockbuster.
December 12 2007: 2:55 PM EST
NEW YORK (CNNMoney.com) -- Neurocrine Biosciences was set to learn on Wednesday whether federal regulators have approved Indiplon, an experimental short-acting sleeping pill analysts think will have a tough time competing.
Indiplon, if approved by the Food and Drug Administration, would be the newest entrant to the $4 billion U.S. market for sleeping pills. But Indiplon's status as a niche drug, and Neurocrine's lack of a Big Pharma marketing partner, means that the drug faces formidable hurdles in a competitive market.
In fact, analysts believe the drug's annual sales would not rise above $200 million.
Indiplon is a short-acting drug for insomniacs that only works for a short time, rather than the whole night. It would be used to put patients to sleep at the beginning of the night, or back to sleep in the middle of the night if they wake up, without its sedative effects lasting through the morning.
"Some people like the idea of taking a medication only when they need it," said Dr. Thomas Roth, consultant for Neurocrine and director of the Indiplon study that was submitted to the FDA, as well as director of sleep disorders research at the Henry Ford Hospital in Detroit. "This study shows you can take the medicine and not be hung-over the next morning."
Gary Lyons, chief executive of San Diego-based Neurocrine Biosciences (Charts), said in an interview earlier this year that Indiplon takes effect within 15 to 20 minutes and it only lasts for one hour. Lyons said that with longer-acting sleeping pills "you have a woman driving her kids to kindergarten with half the drug still in her system. You don't have that problem with Indiplon."
Merck to seek OK for 2 new drugs
If approved, Indiplon would enter a sleeping pill industry that is already dominated by Sanofi-Aventis' (Charts) Ambien CR, Sepracor's (Charts) Lunesta, and King Pharmaceuticals' (Charts) Sonata, as well as Takeda's Rozerem, the only one of those drugs that does not use a sedative to put patients to sleep. There is also a generic low-cost version of Ambien.
Of all these drugs, Indiplon would compete directly against Sonata, which is also a short-acting sleeping pill, said Aileen Salares, analyst for Leerink Swann.
HIV drug market mutates
But the short-acting niche element to Indiplon is unlikely to make it a big seller, analysts say. Salares and another analyst, Jon LeCroy of Natixis Bleichroeder, believe that annual sales will peak at $150 million.
But the FDA decision could have a big impact on Neurocrine Biosciences, which has no other products on the market.
"The stock should move five bucks either way, depending on the outcome [of the FDA decision,]" said LeCroy. Neurocrine shares were trading at $10.11 a share in midday trade on Wednesday.
Neurocrine had tried to get Indiplon approved for the longer-acting dose, but the FDA shot down that effort in May of 2006. This caused the Neurocrine's stock to plunge, and prompted its Big Pharma partner Pfizer Inc. (Charts, Fortune 500) to sever its partnership to promote the drug.
As for the shorter-acting version of Indiplon, the FDA asked that Neurocrine provide more data about the drug. The FDA decision on Indiplon will be based on this additional data.
Neurocrine's stock activity has been wildly volatile in 2007. Indiplon's rocky road with the FDA has created consternation among investors, says Salares of Leerink Swann.
"We're cautious on this because of the checkered history of Indiplon," said Salares. "The upside is muted, because everyone is concerned about the prospects about the drug and that seems to be the overriding concern. They need a strong partner in order to develop the market."
There don't seem to be any takers from Big Pharma at the moment, said Salares.
http://money.cnn.com/2007/12/12/news/companies/indiplon/inde…
FDA is set to decide whether to approve Neurocrine's sleep aide Indiplon, but analysts question whether the short-acting drug will be a blockbuster.
December 12 2007: 2:55 PM EST
NEW YORK (CNNMoney.com) -- Neurocrine Biosciences was set to learn on Wednesday whether federal regulators have approved Indiplon, an experimental short-acting sleeping pill analysts think will have a tough time competing.
Indiplon, if approved by the Food and Drug Administration, would be the newest entrant to the $4 billion U.S. market for sleeping pills. But Indiplon's status as a niche drug, and Neurocrine's lack of a Big Pharma marketing partner, means that the drug faces formidable hurdles in a competitive market.
In fact, analysts believe the drug's annual sales would not rise above $200 million.
Indiplon is a short-acting drug for insomniacs that only works for a short time, rather than the whole night. It would be used to put patients to sleep at the beginning of the night, or back to sleep in the middle of the night if they wake up, without its sedative effects lasting through the morning.
"Some people like the idea of taking a medication only when they need it," said Dr. Thomas Roth, consultant for Neurocrine and director of the Indiplon study that was submitted to the FDA, as well as director of sleep disorders research at the Henry Ford Hospital in Detroit. "This study shows you can take the medicine and not be hung-over the next morning."
Gary Lyons, chief executive of San Diego-based Neurocrine Biosciences (Charts), said in an interview earlier this year that Indiplon takes effect within 15 to 20 minutes and it only lasts for one hour. Lyons said that with longer-acting sleeping pills "you have a woman driving her kids to kindergarten with half the drug still in her system. You don't have that problem with Indiplon."
Merck to seek OK for 2 new drugs
If approved, Indiplon would enter a sleeping pill industry that is already dominated by Sanofi-Aventis' (Charts) Ambien CR, Sepracor's (Charts) Lunesta, and King Pharmaceuticals' (Charts) Sonata, as well as Takeda's Rozerem, the only one of those drugs that does not use a sedative to put patients to sleep. There is also a generic low-cost version of Ambien.
Of all these drugs, Indiplon would compete directly against Sonata, which is also a short-acting sleeping pill, said Aileen Salares, analyst for Leerink Swann.
HIV drug market mutates
But the short-acting niche element to Indiplon is unlikely to make it a big seller, analysts say. Salares and another analyst, Jon LeCroy of Natixis Bleichroeder, believe that annual sales will peak at $150 million.
But the FDA decision could have a big impact on Neurocrine Biosciences, which has no other products on the market.
"The stock should move five bucks either way, depending on the outcome [of the FDA decision,]" said LeCroy. Neurocrine shares were trading at $10.11 a share in midday trade on Wednesday.
Neurocrine had tried to get Indiplon approved for the longer-acting dose, but the FDA shot down that effort in May of 2006. This caused the Neurocrine's stock to plunge, and prompted its Big Pharma partner Pfizer Inc. (Charts, Fortune 500) to sever its partnership to promote the drug.
As for the shorter-acting version of Indiplon, the FDA asked that Neurocrine provide more data about the drug. The FDA decision on Indiplon will be based on this additional data.
Neurocrine's stock activity has been wildly volatile in 2007. Indiplon's rocky road with the FDA has created consternation among investors, says Salares of Leerink Swann.
"We're cautious on this because of the checkered history of Indiplon," said Salares. "The upside is muted, because everyone is concerned about the prospects about the drug and that seems to be the overriding concern. They need a strong partner in order to develop the market."
There don't seem to be any takers from Big Pharma at the moment, said Salares.
http://money.cnn.com/2007/12/12/news/companies/indiplon/inde…
Antwort auf Beitrag Nr.: 32.747.127 von zenman am 12.12.07 22:56:02ja, eigentlich am Mittwoch 12.12.07!
Heute ist CC, um 9:00 in USA. Ich bin gespannt, was heute berichtet wird!
Neurocrine Biosciences Announces Conference Call and Webcast to Provide Indiplon Update
Wednesday December 12, 10:18 pm ET
http://biz.yahoo.com/prnews/071212/law159.html?.v=11
SAN DIEGO, Dec. 12 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that the Company will host a live conference call and webcast to provide an update on indiplon Thursday morning, December 13, 2007. The call will take place at 9:00 a.m. Eastern Time (6:00 a.m. Pacific Time).
After Hours Last: $ 11
After Hours High: $ 11.25
After Hours Volume: 25,459
After Hours Low: $ 9.60
Heute ist CC, um 9:00 in USA. Ich bin gespannt, was heute berichtet wird!
Neurocrine Biosciences Announces Conference Call and Webcast to Provide Indiplon Update
Wednesday December 12, 10:18 pm ET
http://biz.yahoo.com/prnews/071212/law159.html?.v=11
SAN DIEGO, Dec. 12 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that the Company will host a live conference call and webcast to provide an update on indiplon Thursday morning, December 13, 2007. The call will take place at 9:00 a.m. Eastern Time (6:00 a.m. Pacific Time).
After Hours Last: $ 11
After Hours High: $ 11.25
After Hours Volume: 25,459
After Hours Low: $ 9.60
Antwort auf Beitrag Nr.: 32.750.245 von surga am 13.12.07 07:56:59...ist ja echt der Wahnsinn....
..entweder eine BIG News ( Approval und Bekanntgabe einer Kooperation mit Pfizer ??? )
.....oder eine BAD News ( Approval Letter )
..ok , kurzes Resumee.....
....Zeitpunkt der Ankündigung 12.12.07 - 10.18pm ET
...letzter " Afterhours Kurs" 12.12.07 - 19.48pm ET = 11.00 USD
...zumindest ein steigender Kurs von 6.98 % nach " Announcement des CC "....
..so, dann lasst uns mal hoffen....
..entweder eine BIG News ( Approval und Bekanntgabe einer Kooperation mit Pfizer ??? )
.....oder eine BAD News ( Approval Letter )
..ok , kurzes Resumee.....
....Zeitpunkt der Ankündigung 12.12.07 - 10.18pm ET
...letzter " Afterhours Kurs" 12.12.07 - 19.48pm ET = 11.00 USD
...zumindest ein steigender Kurs von 6.98 % nach " Announcement des CC "....
..so, dann lasst uns mal hoffen....
DAS gefällt mir nicht :O
NBIX weiss die entscheidung seit gestern
und will sie heute auf CC bekanntgeben ?
d.h. da gibts erklärungsbedarf
bei ner zulassung gibt es NIX zu erklären
NBIX weiss die entscheidung seit gestern
und will sie heute auf CC bekanntgeben ?
d.h. da gibts erklärungsbedarf

bei ner zulassung gibt es NIX zu erklären
Antwort auf Beitrag Nr.: 32.750.391 von bernie55 am 13.12.07 08:29:53ist ja echt der Wahnsinn....
..entweder eine BIG News ( Approval und Bekanntgabe einer Kooperation mit Pfizer ??? )
.....oder eine BAD News ( Approvable Letter )
December 13, 2007 - 3:00 AM EST
Neurocrine Receives Approvable Letter for Indiplon Capsules with Additional Safety and Efficacy Data Required by FDA
with Additional Safety and Efficacy Data Required by FDA
SAN DIEGO, Dec. 13 /PRNewswire-FirstCall/
-- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that the Company has received communication from the U.S. Food and Drug Administration (FDA) indicating that the New Drug Application (NDA) for indiplon 5 mg and 10 mg capsules for the treatment of insomnia is approvable pending additional clinical and preclinical data.
On May 15, 2006, the Company received an action letter from the FDA stating that indiplon 5 mg and 10 mg capsules were approvable (2006 Approvable Letter). The 2006 Approvable Letter requested that the company reanalyze data from certain preclinical and clinical studies to support approval of indiplon 5 mg and 10 mg capsules for sleep initiation and middle of the night dosing. The 2006 Approvable Letter also requested reexamination of the safety analyses. At the August 2006 end-of-review meeting where the 2006 Approvable Letter was discussed, the FDA requested that the resubmission include further analyses and modifications of analyses previously submitted to address questions raised by the FDA in the initial review. This reanalysis was completed and was resubmitted on June 12, 2007.
On December 12, 2007, we received an action letter from the FDA stating that indiplon 5mg and 10mg capsules are Approvable (2007 Approvable Letter). The 2007 Approvable Letter did not raise any of the issues previously raised by FDA in the 2006 Approvable Letter.
The requirements as spelled out in the 2007 Approvable Letter raised requirements as follows:
-- An objective/subjective clinical trial in the elderly.
-- A safety study assessing the rates of adverse events occurring with
indiplon when compared to a marketed product.
-- A preclinical study to evaluate indiplon administration during the
third trimester of pregnancy.
'While we are disappointed in the FDA action, we will accept the FDA's offer to discuss the applications via a meeting or telephone conference in order to clarify and determine the next steps required,' said Gary A. Lyons, President and CEO of Neurocrine.
About Neurocrine
Neurocrine Biosciences, Inc. is a product-based biopharmaceutical company focused on neurological and endocrine diseases and disorders. The product candidates address some of the largest pharmaceutical markets in the world including insomnia, anxiety, depression, endometriosis, irritable bowel syndrome, pain, and diabetes. Indiplon was licensed from DOV Pharmaceutical in 1998. Neurocrine Biosciences, Inc. news releases are available through the Company's website via the Internet at http://www.neurocrine.com
In addition to historical facts, this press release may contain forward-looking statements that involve a number of risks and uncertainties relating to Neurocrine's indiplon program that could cause actual results to differ materially from those indicated in the forward-looking statements. Specifically, the risks and uncertainties the Company faces with respect to its indiplon program include, but are not limited to; risk that the Company will not be able to address issues and or requests set forth in the action letter from the FDA in a timely manner if at all; risk that the Company will not be able to address issues and or requests set forth in the action letters from the FDA in a manner acceptable to the FDA if at all; the risk that FDA may reject any future indiplon regulatory filings or find them incomplete or insufficient; risk that indiplon approval and subsequent commercialization may be significantly delayed; and the other risks described in Neurocrine's annual report on Form 10-K for the year ended December 31, 2006 and quarterly report on Form 10-Q for the quarter ended September 30, 2007. Neurocrine undertakes no obligation to update the statements contained in this press release after the date hereof.
SOURCE Neurocrine Biosciences, Inc.
Source: PR Newswire (December 13, 2007 - 3:00 AM EST)
http://www.investorvillage.com/smbd.asp?mb=3440&pt=qn
 ....Wahnsinn...
....Wahnsinn...
.....ich verstehe die Entscheidungen der FDA überhaupt nicht mehr , wenn ich sie überhaupt jemals verstanden habe......
..entweder eine BIG News ( Approval und Bekanntgabe einer Kooperation mit Pfizer ??? )
.....oder eine BAD News ( Approvable Letter )
December 13, 2007 - 3:00 AM EST
Neurocrine Receives Approvable Letter for Indiplon Capsules
 with Additional Safety and Efficacy Data Required by FDA
with Additional Safety and Efficacy Data Required by FDASAN DIEGO, Dec. 13 /PRNewswire-FirstCall/
-- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that the Company has received communication from the U.S. Food and Drug Administration (FDA) indicating that the New Drug Application (NDA) for indiplon 5 mg and 10 mg capsules for the treatment of insomnia is approvable pending additional clinical and preclinical data.
On May 15, 2006, the Company received an action letter from the FDA stating that indiplon 5 mg and 10 mg capsules were approvable (2006 Approvable Letter). The 2006 Approvable Letter requested that the company reanalyze data from certain preclinical and clinical studies to support approval of indiplon 5 mg and 10 mg capsules for sleep initiation and middle of the night dosing. The 2006 Approvable Letter also requested reexamination of the safety analyses. At the August 2006 end-of-review meeting where the 2006 Approvable Letter was discussed, the FDA requested that the resubmission include further analyses and modifications of analyses previously submitted to address questions raised by the FDA in the initial review. This reanalysis was completed and was resubmitted on June 12, 2007.
On December 12, 2007, we received an action letter from the FDA stating that indiplon 5mg and 10mg capsules are Approvable (2007 Approvable Letter). The 2007 Approvable Letter did not raise any of the issues previously raised by FDA in the 2006 Approvable Letter.
The requirements as spelled out in the 2007 Approvable Letter raised requirements as follows:
-- An objective/subjective clinical trial in the elderly.
-- A safety study assessing the rates of adverse events occurring with
indiplon when compared to a marketed product.
-- A preclinical study to evaluate indiplon administration during the
third trimester of pregnancy.
'While we are disappointed in the FDA action, we will accept the FDA's offer to discuss the applications via a meeting or telephone conference in order to clarify and determine the next steps required,' said Gary A. Lyons, President and CEO of Neurocrine.
About Neurocrine
Neurocrine Biosciences, Inc. is a product-based biopharmaceutical company focused on neurological and endocrine diseases and disorders. The product candidates address some of the largest pharmaceutical markets in the world including insomnia, anxiety, depression, endometriosis, irritable bowel syndrome, pain, and diabetes. Indiplon was licensed from DOV Pharmaceutical in 1998. Neurocrine Biosciences, Inc. news releases are available through the Company's website via the Internet at http://www.neurocrine.com
In addition to historical facts, this press release may contain forward-looking statements that involve a number of risks and uncertainties relating to Neurocrine's indiplon program that could cause actual results to differ materially from those indicated in the forward-looking statements. Specifically, the risks and uncertainties the Company faces with respect to its indiplon program include, but are not limited to; risk that the Company will not be able to address issues and or requests set forth in the action letter from the FDA in a timely manner if at all; risk that the Company will not be able to address issues and or requests set forth in the action letters from the FDA in a manner acceptable to the FDA if at all; the risk that FDA may reject any future indiplon regulatory filings or find them incomplete or insufficient; risk that indiplon approval and subsequent commercialization may be significantly delayed; and the other risks described in Neurocrine's annual report on Form 10-K for the year ended December 31, 2006 and quarterly report on Form 10-Q for the quarter ended September 30, 2007. Neurocrine undertakes no obligation to update the statements contained in this press release after the date hereof.
SOURCE Neurocrine Biosciences, Inc.
Source: PR Newswire (December 13, 2007 - 3:00 AM EST)
http://www.investorvillage.com/smbd.asp?mb=3440&pt=qn
 ....Wahnsinn...
....Wahnsinn...
.....ich verstehe die Entscheidungen der FDA überhaupt nicht mehr , wenn ich sie überhaupt jemals verstanden habe......

sorry für euch 
haut den scheiss vorbörslich raus
das kann bis - 50% die nächsten tage gehn

haut den scheiss vorbörslich raus
das kann bis - 50% die nächsten tage gehn
wie geht es jetzt weiter?
Schaue noch immer von der Seitenlinie.
Hatte bisher nicht den Mut (das Geld) hier einzusteigen.

Schaue noch immer von der Seitenlinie.
Hatte bisher nicht den Mut (das Geld) hier einzusteigen.

Antwort auf Beitrag Nr.: 32.755.335 von Poppholz am 13.12.07 15:46:17Lass lieber.....!!Gehe lieber in die Banken----oder stocke unser Elan-Schätzchen auf...



Antwort auf Beitrag Nr.: 32.751.516 von zenman am 13.12.07 10:33:49sieht so aus, dass es bei Dendreon eine Untersuchung bei einigen FDA Mitarbeitern gibt. Ob auch bei NBIX auch der Fall wird? 

Hi surga, Hi Poppie, Hi Zeni, HI alle
..ich weile zur Zeit in NRW, bin noch völlig desinformiert....
WIE ist der aktuellen Stand ??????
..... ich selbst muss erst mal Infos sammeln, was überhaupt Sache ist...........
Grüße, auf die Schnelle
bernie55
..ich weile zur Zeit in NRW, bin noch völlig desinformiert....
WIE ist der aktuellen Stand ??????
..... ich selbst muss erst mal Infos sammeln, was überhaupt Sache ist...........

Grüße, auf die Schnelle
bernie55
Antwort auf Beitrag Nr.: 32.764.107 von bernie55 am 14.12.07 12:15:43
eigentlich gibt es nichts Neues, nur daß NBIX heute mit FDA sprechen will und in Januar Partner für GNRH bekannt geben will
eigentlich gibt es nichts Neues, nur daß NBIX heute mit FDA sprechen will und in Januar Partner für GNRH bekannt geben will

Es ist absolut mir unverständlich was für Manager in den Führungsetagen von Biotech Unternehmen sitzen!
Wie kann man man eine 15 mg Dosis für Indiplon durchdrücken wollen wenn von der FDA 2006 schon drauf hin gewiesen worden ist, dass die Test Daten für so eine hohe Dosierung nicht ausreichen!
Wie so hat man sich nicht auf nur die einreichen der niedrigen Dosierung beschränkt?????
Tja mit der niedrigen Dosis läst sich nicht so viel verdienen .....Statt dessen setzt man alles auf eine Karte....
Ist absolut nicht nachvollziehbar...
Überrascht hat mich allerdings die Extrem schnelle Streichung von 130 Jobs am gleichen Tag!
Das war ein extrem schneller Schritt....an einem Tag 2 solche Meldungen da über den Ticker zu jagen“!
Wird wohl eine neue Studie geben müssen wenn Sie die Dosis bei behalten wollen!
Zudem steht im Januar die Bekannt gabe eines neuen Partner zu GnRH an.
Werde mal das 8 Jahres Tief für den Aufbau einer Position Nutzen!
Interessant ist das gestern die Institutionellen ihre Positionen gehalten haben / der Abgabe Druck lag nur bei niedrigen Stückzahlen bei Privat Verkäufern!
Hoch Interessant das 22.00h Gestern wohl noch ein größer Sharholder die Gunst der Stunde genutzt hat und eine Position von 26.813 aufgebaut hat.
Wie kann man man eine 15 mg Dosis für Indiplon durchdrücken wollen wenn von der FDA 2006 schon drauf hin gewiesen worden ist, dass die Test Daten für so eine hohe Dosierung nicht ausreichen!
Wie so hat man sich nicht auf nur die einreichen der niedrigen Dosierung beschränkt?????
Tja mit der niedrigen Dosis läst sich nicht so viel verdienen .....Statt dessen setzt man alles auf eine Karte....
Ist absolut nicht nachvollziehbar...
Überrascht hat mich allerdings die Extrem schnelle Streichung von 130 Jobs am gleichen Tag!
Das war ein extrem schneller Schritt....an einem Tag 2 solche Meldungen da über den Ticker zu jagen“!
Wird wohl eine neue Studie geben müssen wenn Sie die Dosis bei behalten wollen!
Zudem steht im Januar die Bekannt gabe eines neuen Partner zu GnRH an.
Werde mal das 8 Jahres Tief für den Aufbau einer Position Nutzen!
Interessant ist das gestern die Institutionellen ihre Positionen gehalten haben / der Abgabe Druck lag nur bei niedrigen Stückzahlen bei Privat Verkäufern!
Hoch Interessant das 22.00h Gestern wohl noch ein größer Sharholder die Gunst der Stunde genutzt hat und eine Position von 26.813 aufgebaut hat.

CNN money story on NBIX
althought GL said gnrh deal before the end of this year or early next year this is what the story says
But Neurocrine could have a potential winner in its pipeline, said Woodburn. The company is experimenting with a drug that could be used to treat endometriosis, a problem with uterine tissue that can cause pain, heavy period and infertility. Some five million women in North America suffer from the condition, said Woodburn, who projects $500 million to $1 billion in annual sales for the drug.
Big Pharma's job cuts
That's assuming that the drug, known as a GnRH antagonist, makes it past the FDA. The potential drug is in phase 2 testing, and CEO Lyons said that phase 3 - the final stage before submission to the FDA - will begin in 2008.
This means that it is years away from potentially entering the market.
Lyons said Neurocrine will announce a new partner for the GnRH antagonist in January.
http://money.cnn.com/2007/12/13/news/companies/neurocrine/?p…
http://www.investorvillage.com/smbd.asp?mb=3440&mn=12232&pt=…
althought GL said gnrh deal before the end of this year or early next year this is what the story says
But Neurocrine could have a potential winner in its pipeline, said Woodburn. The company is experimenting with a drug that could be used to treat endometriosis, a problem with uterine tissue that can cause pain, heavy period and infertility. Some five million women in North America suffer from the condition, said Woodburn, who projects $500 million to $1 billion in annual sales for the drug.
Big Pharma's job cuts
That's assuming that the drug, known as a GnRH antagonist, makes it past the FDA. The potential drug is in phase 2 testing, and CEO Lyons said that phase 3 - the final stage before submission to the FDA - will begin in 2008.
This means that it is years away from potentially entering the market.
Lyons said Neurocrine will announce a new partner for the GnRH antagonist in January.
http://money.cnn.com/2007/12/13/news/companies/neurocrine/?p…
http://www.investorvillage.com/smbd.asp?mb=3440&mn=12232&pt=…
Da sollte doch ein Rebound bis 5 Euro möglich sein, oder?
Antwort auf Beitrag Nr.: 32.788.666 von Richy am 17.12.07 10:37:58..ach, Richy, wenn wir das wüssten, würden wir alle so sein, wie du heißt....
..ich habe mich jetzt mal ein bisschen informiert...
...also,so wie ich es verstanden habe, müssen noch zusätzliche Daten zur Sicherheit eingereicht werden und darüber hinaus noch zusätzliche Daten von älteren Menschen und schwangeren Frauen ab 6. Monat ???
Das wird Geld kosten !!!!
Ein potentieller Kooperationspartner bzgl. Indiplon ist auch schon abgesprungen.
Am Freitag will GL sich noch mit der FDA treffen , bevor die nächsten weiteren Schritte bzgl. Indiplon gemacht werden.
..also, irgendwie verstehe ich es nicht mehr....ältere Personen.....Schwangere......da hätte doch einfach eine Aufschrift gereicht...
..die FDA wird mir immer suspekter... ..korrupter..
..korrupter.. ..scheinheiliger....
..scheinheiliger....
, was wir vor Monaten ja bei DNDN und Provenge mitbekommen haben.

..ich habe mich jetzt mal ein bisschen informiert...
...also,so wie ich es verstanden habe, müssen noch zusätzliche Daten zur Sicherheit eingereicht werden und darüber hinaus noch zusätzliche Daten von älteren Menschen und schwangeren Frauen ab 6. Monat ???
Das wird Geld kosten !!!!
Ein potentieller Kooperationspartner bzgl. Indiplon ist auch schon abgesprungen.
Am Freitag will GL sich noch mit der FDA treffen , bevor die nächsten weiteren Schritte bzgl. Indiplon gemacht werden.
..also, irgendwie verstehe ich es nicht mehr....ältere Personen.....Schwangere......da hätte doch einfach eine Aufschrift gereicht...
..die FDA wird mir immer suspekter...
 ..korrupter..
..korrupter.. ..scheinheiliger....
..scheinheiliger....
, was wir vor Monaten ja bei DNDN und Provenge mitbekommen haben.
Neurocrine Biosciences neues Kursziel (Lehman Brothers Inc.)
New York Die Analysten von Lehman Brothers stufen die Aktie von Neurocrine Biosciences (ISIN US64125C1099/ WKN 900964) unverändert mit "overweight" ein. Das Kursziel werde von 19 auf 9,50 USD reduziert.
Analyse-Datum: 18.12.2007
Analyst: Lehman Brothers Inc.
Rating des Analysten: overweight
Quelle:aktienchcheck.de
New York Die Analysten von Lehman Brothers stufen die Aktie von Neurocrine Biosciences (ISIN US64125C1099/ WKN 900964) unverändert mit "overweight" ein. Das Kursziel werde von 19 auf 9,50 USD reduziert.
Analyse-Datum: 18.12.2007
Analyst: Lehman Brothers Inc.
Rating des Analysten: overweight
Quelle:aktienchcheck.de
...goes like imclone 
nu passts erst richtig
imclone war ja auch bei 5 $



nu passts erst richtig
imclone war ja auch bei 5 $


sehr ruhig geworden.
Antwort auf Beitrag Nr.: 32.967.323 von Poppholz am 07.01.08 15:20:23ja, sehr ruhig geworden 
ob schon Boden war?

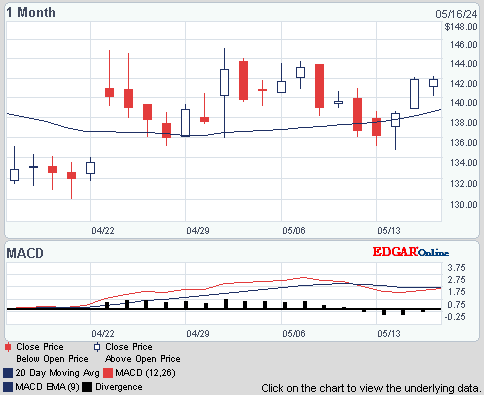

ob schon Boden war?

Neurocrine CEO Gary Lyons Resigns
Monday January 14, 5:16 pm ET
Neurocrine Biosciences CEO Gary Lyons Resigns, Replaced by COO Kevin Gorman
http://biz.yahoo.com/ap/080114/neurocrine_biosciences_ceo.ht…
http://www.investorvillage.com/smbd.asp?mb=3440&mn=13042&pt=…
Re: Deal or no deal?
Author: pinvestment2
for those that were here last january coming off the tax loss selling - NBIX went up 8 days in a row and 11 out of 13 and went up 40% before news was released
since the price is so dirt cheap this could be bargain hunting or maybe someone is betting the deal coming through in january
if GLs final words were correct then there are 9 trading days left in january and something has to give
if a gnrh deal is announced (and has 3$ in upfront cash or easy milestones (like completing the phase IIb or starting the phase III) and we actually even got a measly 2-3$ for the pipeline then we should be back at 10$
and that is still pretty damn cheap since if someone puts up a package deal of cash and milestones of 500 million then if NBIX keeps 30% then the pipeline valuation should be higher
2-3$ for one drug in the pipeline that has 5-6 indications and very large market capability is dirt cheap
if any part of the GSK data is good then NBIX would be seeing more cash from GSK and would have another potential blockbuster in the pipeline
I think there are few shares for sale and this price in retrospect is going to be a great "get"
I still like to think of gnrh ant finishing phase III in 2009 with a couple of GSK partner drugs in phase III and urocortin2 being partnered off and all the promise that NBIX has so that the gnrh deal is the first step to being back in the black and having a rosy future even without indiplon
I wonder what the market will do if NBIX meets with the FDA - outlines a relatively small amount of work to be done and then partners indiplon away for some cash and a royalty with the partner taking over all the development payments
time will tell
which one does everyone think will happen first - gnrh ant or GSK data?
Re: Deal or no deal?
Author: pinvestment2
for those that were here last january coming off the tax loss selling - NBIX went up 8 days in a row and 11 out of 13 and went up 40% before news was released
since the price is so dirt cheap this could be bargain hunting or maybe someone is betting the deal coming through in january
if GLs final words were correct then there are 9 trading days left in january and something has to give
if a gnrh deal is announced (and has 3$ in upfront cash or easy milestones (like completing the phase IIb or starting the phase III) and we actually even got a measly 2-3$ for the pipeline then we should be back at 10$
and that is still pretty damn cheap since if someone puts up a package deal of cash and milestones of 500 million then if NBIX keeps 30% then the pipeline valuation should be higher
2-3$ for one drug in the pipeline that has 5-6 indications and very large market capability is dirt cheap
if any part of the GSK data is good then NBIX would be seeing more cash from GSK and would have another potential blockbuster in the pipeline
I think there are few shares for sale and this price in retrospect is going to be a great "get"
I still like to think of gnrh ant finishing phase III in 2009 with a couple of GSK partner drugs in phase III and urocortin2 being partnered off and all the promise that NBIX has so that the gnrh deal is the first step to being back in the black and having a rosy future even without indiplon
I wonder what the market will do if NBIX meets with the FDA - outlines a relatively small amount of work to be done and then partners indiplon away for some cash and a royalty with the partner taking over all the development payments
time will tell
which one does everyone think will happen first - gnrh ant or GSK data?
Antwort auf Beitrag Nr.: 33.091.128 von surga am 18.01.08 08:59:54@surga
.....interessanter Artikel von pini2....
...habe mich auch gewundert, dass in den letzten Tagen NBIX viel " positiven Dampf " ablässt....
..vielleicht kommen die ereignisreichen Aktivitäten doch schneller auf uns zu als gedacht....mir soll es nur recht sein....
....und etwas Tolles, so ganz nebenbei..
.. wir nähern uns wieder unseren EKs,.....
Grüße bernie55
.....interessanter Artikel von pini2....
...habe mich auch gewundert, dass in den letzten Tagen NBIX viel " positiven Dampf " ablässt....

..vielleicht kommen die ereignisreichen Aktivitäten doch schneller auf uns zu als gedacht....mir soll es nur recht sein....

....und etwas Tolles, so ganz nebenbei..
.. wir nähern uns wieder unseren EKs,.....

Grüße bernie55

Antwort auf Beitrag Nr.: 33.092.612 von bernie55 am 18.01.08 10:49:17Jawohl Bernie, je schneller desto besser 



wann kommt endlich der Partner?







Neurocrine Biosciences Announces Conference Call and Webcast to Present Fourth Quarter and Year End 2007 Financial Results
Thursday January 24, 4:14 pm ET
Conference Call and Webcast Scheduled for Tuesday, February 5
SAN DIEGO, Jan. 24 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that the Company will report its fourth quarter and year-end 2007 financial results after the Nasdaq market closes on Tuesday, February 5, 2008. Neurocrine will then host a live conference call and webcast to discuss its financial results and provide a Company update Tuesday afternoon, February 5, 2008 at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time).
http://biz.yahoo.com/prnews/080124/lath141.html?.v=16
Thursday January 24, 4:14 pm ET
Conference Call and Webcast Scheduled for Tuesday, February 5
SAN DIEGO, Jan. 24 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that the Company will report its fourth quarter and year-end 2007 financial results after the Nasdaq market closes on Tuesday, February 5, 2008. Neurocrine will then host a live conference call and webcast to discuss its financial results and provide a Company update Tuesday afternoon, February 5, 2008 at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time).
http://biz.yahoo.com/prnews/080124/lath141.html?.v=16
Antwort auf Beitrag Nr.: 33.145.098 von surga am 23.01.08 16:53:24wann kommt endlich der Partner?
...the answer , my friend , is blowing in the wind....
...the answer , my friend , is blowing in the wind....
Antwort auf Beitrag Nr.: 33.165.730 von bernie55 am 25.01.08 10:30:33

ziemlich hoher Umsatz am Freitag

was fürn kackteil 
hätt ich nich im traum erwartet
sorry
wenn ich jemand drauf angspitzt hatte


hätt ich nich im traum erwartet
sorry
wenn ich jemand drauf angspitzt hatte

Antwort auf Beitrag Nr.: 33.442.232 von zenman am 21.02.08 22:39:53so is es Zen 





Bist Du hier wieder investiert


Ich hoffe nicht, dass hier wie bei GENTA passiert








Bist Du hier wieder investiert



Ich hoffe nicht, dass hier wie bei GENTA passiert



Antwort auf Beitrag Nr.: 33.450.662 von surga am 22.02.08 18:17:43ich hatte meiner frau 400 st ins depot gelegt 
die haben wir noch

die haben wir noch
Antwort auf Beitrag Nr.: 33.450.918 von zenman am 22.02.08 18:38:03so wie jetzt aussieht, bewegen wir seitwärts um 5$ rum. Sobald einbisschen steigt, kommen Verkaufsorder mit größeren Stückzahl 
Geduld müssen wir haben, siehe Beispiel ENCY

Geduld müssen wir haben, siehe Beispiel ENCY

Antwort auf Beitrag Nr.: 33.474.136 von surga am 26.02.08 08:09:59...wir können aktuell nichts anderes machen , als auf News zu warten, surga...

interesting info - insider trading
in my schwab account, NBIX insider trading activity was updated. it shows a "bought" of 106,000 shares, with the insider title of "Partners LP B/O". They now own 3,340,300. This is a rather large position, but i don't understand who this is. is this their investment banking relationship? is this somehow related to an outside law firm? the rest of the insider list is the usually list of executives, directors, etc..
Does anyone know who this "insider" party is that owns such a HUGE position in NBIX????
http://www.investorvillage.com/smbd.asp?mb=3440&mn=14404&pt=…
..vielleicht kommt jetzt doch mal ein bisschen Bewegung rein...
in my schwab account, NBIX insider trading activity was updated. it shows a "bought" of 106,000 shares, with the insider title of "Partners LP B/O". They now own 3,340,300. This is a rather large position, but i don't understand who this is. is this their investment banking relationship? is this somehow related to an outside law firm? the rest of the insider list is the usually list of executives, directors, etc..
Does anyone know who this "insider" party is that owns such a HUGE position in NBIX????
http://www.investorvillage.com/smbd.asp?mb=3440&mn=14404&pt=…
..vielleicht kommt jetzt doch mal ein bisschen Bewegung rein...
Antwort auf Beitrag Nr.: 33.487.437 von bernie55 am 27.02.08 09:32:00http://www.investorvillage.com/smbd.asp?mb=3440&mn=14406&pt=…
BVF Partners. They are a fund that buys undervalued biotech companies. They are considered an insider as they are buying over 10% of the outstanding shares.
..es scheint also ein Biotechfonds zu sein....
BVF Partners. They are a fund that buys undervalued biotech companies. They are considered an insider as they are buying over 10% of the outstanding shares.
..es scheint also ein Biotechfonds zu sein....
was meint ihr, lohnt sich NEUROCRINE weiter zu halten?
Antwort auf Beitrag Nr.: 33.719.716 von mortem am 25.03.08 14:49:27watum denn gerade jetzt abgeben?
Ich würde warten, bis der Deal mit dem Partner abgeschlossen ist (die Frage nur wann????)


Ich würde warten, bis der Deal mit dem Partner abgeschlossen ist (die Frage nur wann????)



Antwort auf Beitrag Nr.: 33.867.775 von surga am 11.04.08 16:19:44....vielleicht kommt jetzt bald der ersehnte " Partnerdeal ".... 

SAN DIEGO, April 21 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX - News) announced today that the Company will report its first quarter 2008 financial results after the Nasdaq market closes on Wednesday,
April 30, 2008.
Neurocrine will host a live conference call and webcast to discuss its financial results and provide a Company update Wednesday afternoon, April 30, 2008 at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time).
Participants can access the live conference call by dialing 1-800-895-1715 (US) or 785-424-1059 (International) using the conference ID: 7NBIX1. The call can also be accessed via the webcast through the Company's website at http://www.neurocrine.com
http://www.investorvillage.com/mbnews.asp?mb=3440&pt=qn&xid=…
 ...so, dann hoffen wir mal, dass wir nur so mit positiven News zugeschüttet werden.....
...so, dann hoffen wir mal, dass wir nur so mit positiven News zugeschüttet werden.....
April 30, 2008.
Neurocrine will host a live conference call and webcast to discuss its financial results and provide a Company update Wednesday afternoon, April 30, 2008 at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time).
Participants can access the live conference call by dialing 1-800-895-1715 (US) or 785-424-1059 (International) using the conference ID: 7NBIX1. The call can also be accessed via the webcast through the Company's website at http://www.neurocrine.com
http://www.investorvillage.com/mbnews.asp?mb=3440&pt=qn&xid=…
 ...so, dann hoffen wir mal, dass wir nur so mit positiven News zugeschüttet werden.....
...so, dann hoffen wir mal, dass wir nur so mit positiven News zugeschüttet werden.....
Antwort auf Beitrag Nr.: 33.991.281 von bernie55 am 29.04.08 11:01:54 THE DEAL
Author: pinvestment2
think sometimes the points get a bit muddled
NBIX has three phase II programs that address very large potential markets
a. CRF ant - previous versions worked in ssri resistant depression and the CRF pathway seems to be tightly intertwined with anxiety also. IBS appears to be tied in with stress responses also. SocAD is a muddied but large market but efficacy has not been established with these agents. IBS is interesting but also needs to be validated. Depression is the key of these and is a massive market partnered with GSK with 200 million or so in milestones (next significant would be for starting phase III - not expected until late 2009) - addiction also seems to be tied to CRF pathway and good data also to support in animal studies - CRF is going to be a large program with many potential markets - just needs time as three distinct entities to be tested
b. gnrh ant - clear advantages to oral agent as well as side effect profile and bone loss should be much better than current agents - should be a big partner deal - we wait *** the problem here is that gorman sent mixed messages and hasn't delivered yet - multiple potential partners for a drug that could address many markets means big money
c. urocortin2 - early clinical data suggests it is the best potential CHF drug out there - JNJ bought scios for 2.2 billion to get natrecor but natrecor was a bust - but CHF is a big business opportunity - NBIX expect to partner this earlier because of their lack of expertise in cardiac arena - expected late this year or early next year if the tox studies go OK.
d. indiplon is considered dead in US by markets - DSP program continues - if the FDA backs off on the comparator study maybe the previous indiplon partner steps in and takes over the program - would be great for pps but if the FDA meeting was delayed then I don't think we will get an update - last CC gorman said they wouldn't comment until they got FDA written comments after the meeting and that could take 60 days after the meeting - thus I expected that the meeting was scheduled in late feb/early march at the latest - never saw any volume during that time - but also heard that meeting could have been delayed a couple of weeks - to me that means that the meeting probably occurred but NBIX won't have written responses by this week
- each partnership would also reduce cash burn and bring in cash and milestones and thus reduces the cash burn risk of NBIX
- the good of NBIX is that they have the above pipeline and even more in earlier stages
- another good of NBIX is that its price doesn't seem to show much value for the pipeline at all - let alone the cash that will be brought in by partnering
the not so good is that NBIX just seems to be slower than other biopharma companies and just never seems to deliver on time
the frustrating part of NBIX is that management never seems to do what they say they will when they say they will do it or they say one thing and then do another - thus we are saddler with a management penalty because of a lack of confidence in management's ability to deliver
the recent track record of NBIX is that corporate news is released on the CC date and is always later than expected - thus i think it is reasonable to expect that we will hear some news and updated timelines on all of the phase II programs - so yes I expect the deal to be announced on wednesday - if not then gorman will have to give a pretty damn good explanation why it isn't done - an acceptable excuse - we have had multiple partners in an intense competition for the drug - an unacceptable excuse - this is a complex deal and it is a lot of work and we haven't gotten it done yet and it is a little delayed (admission that they are incompetent)
I just don't think that gorman and the board want to have this call before the proxy is voted on and to come out and say no deal yet and no FDA info to comment on
http://www.investorvillage.com/smbd.asp?mb=3440&mn=16320&pt=…
Author: pinvestment2
think sometimes the points get a bit muddled
NBIX has three phase II programs that address very large potential markets
a. CRF ant - previous versions worked in ssri resistant depression and the CRF pathway seems to be tightly intertwined with anxiety also. IBS appears to be tied in with stress responses also. SocAD is a muddied but large market but efficacy has not been established with these agents. IBS is interesting but also needs to be validated. Depression is the key of these and is a massive market partnered with GSK with 200 million or so in milestones (next significant would be for starting phase III - not expected until late 2009) - addiction also seems to be tied to CRF pathway and good data also to support in animal studies - CRF is going to be a large program with many potential markets - just needs time as three distinct entities to be tested
b. gnrh ant - clear advantages to oral agent as well as side effect profile and bone loss should be much better than current agents - should be a big partner deal - we wait *** the problem here is that gorman sent mixed messages and hasn't delivered yet - multiple potential partners for a drug that could address many markets means big money
c. urocortin2 - early clinical data suggests it is the best potential CHF drug out there - JNJ bought scios for 2.2 billion to get natrecor but natrecor was a bust - but CHF is a big business opportunity - NBIX expect to partner this earlier because of their lack of expertise in cardiac arena - expected late this year or early next year if the tox studies go OK.
d. indiplon is considered dead in US by markets - DSP program continues - if the FDA backs off on the comparator study maybe the previous indiplon partner steps in and takes over the program - would be great for pps but if the FDA meeting was delayed then I don't think we will get an update - last CC gorman said they wouldn't comment until they got FDA written comments after the meeting and that could take 60 days after the meeting - thus I expected that the meeting was scheduled in late feb/early march at the latest - never saw any volume during that time - but also heard that meeting could have been delayed a couple of weeks - to me that means that the meeting probably occurred but NBIX won't have written responses by this week
- each partnership would also reduce cash burn and bring in cash and milestones and thus reduces the cash burn risk of NBIX
- the good of NBIX is that they have the above pipeline and even more in earlier stages
- another good of NBIX is that its price doesn't seem to show much value for the pipeline at all - let alone the cash that will be brought in by partnering
the not so good is that NBIX just seems to be slower than other biopharma companies and just never seems to deliver on time
the frustrating part of NBIX is that management never seems to do what they say they will when they say they will do it or they say one thing and then do another - thus we are saddler with a management penalty because of a lack of confidence in management's ability to deliver
the recent track record of NBIX is that corporate news is released on the CC date and is always later than expected - thus i think it is reasonable to expect that we will hear some news and updated timelines on all of the phase II programs - so yes I expect the deal to be announced on wednesday - if not then gorman will have to give a pretty damn good explanation why it isn't done - an acceptable excuse - we have had multiple partners in an intense competition for the drug - an unacceptable excuse - this is a complex deal and it is a lot of work and we haven't gotten it done yet and it is a little delayed (admission that they are incompetent)
I just don't think that gorman and the board want to have this call before the proxy is voted on and to come out and say no deal yet and no FDA info to comment on
http://www.investorvillage.com/smbd.asp?mb=3440&mn=16320&pt=…
http://www.investorvillage.com/smbd.asp?mb=3440&mn=17746&pt=…
Author: daveyjones547
Again, huge volume
Something is going on. I don't know what it is, I can't tell whether it is good or bad, and it could be something as simple as institutions rearranging their positions before the end of the quarter a week from today. But something is up. Maybe the FDA meeting was this week (although I don't know why people would be trading much on the FDA meeting news unless it is positive, because NBIX has already completely 100% written off Indiplon in the U.S.). Maybe the market has heard a whisper of a partner waiting in the wings even if Indiplon doesn't get a break at the FDA meeting, to take it to the rest of the world.
All I know, is that the three biggest volume trading days, by far, for more than a month, have occurred in the last week, and the two biggest trading days in over a month occurred yesterday and today. Monday 440,000 shares traded and we ended even for the day; yesterday 441,000 shares traded and we ended up 10% for the day; and today 442,000 shares traded and we ended down 3% for the day (not bad actually considering the market conditions today).
Something is going on, and I hope we find out soon. And I sure hope it is positive.
Author: daveyjones547
Again, huge volume
Something is going on. I don't know what it is, I can't tell whether it is good or bad, and it could be something as simple as institutions rearranging their positions before the end of the quarter a week from today. But something is up. Maybe the FDA meeting was this week (although I don't know why people would be trading much on the FDA meeting news unless it is positive, because NBIX has already completely 100% written off Indiplon in the U.S.). Maybe the market has heard a whisper of a partner waiting in the wings even if Indiplon doesn't get a break at the FDA meeting, to take it to the rest of the world.
All I know, is that the three biggest volume trading days, by far, for more than a month, have occurred in the last week, and the two biggest trading days in over a month occurred yesterday and today. Monday 440,000 shares traded and we ended even for the day; yesterday 441,000 shares traded and we ended up 10% for the day; and today 442,000 shares traded and we ended down 3% for the day (not bad actually considering the market conditions today).
Something is going on, and I hope we find out soon. And I sure hope it is positive.





5$ ist gedeckelt

tendenz steigend, noch 

Der Widerstand bei 5$ muss überwunden werden, dann freie Bahn bis 5,7$
Mal sehen, was die nächsten Tage bringen


Ich habe bei 4,51 vor paar Tagen zugelegt




Der Widerstand bei 5$ muss überwunden werden, dann freie Bahn bis 5,7$

Mal sehen, was die nächsten Tage bringen



Ich habe bei 4,51 vor paar Tagen zugelegt



ist echt nicht los mit NBIX zurzeit. 
Ich bin erstmal raus, kein Geduld mehr

Ich bin erstmal raus, kein Geduld mehr

SB bei 5,08 eingestellt
Mal sehen, wie lange diese Kursdruckerei noch dauert

Mal sehen, wie lange diese Kursdruckerei noch dauert

Antwort auf Beitrag Nr.: 34.552.890 von surga am 21.07.08 17:02:14...wie gut,dass Du auch Elan hast...!

Antwort auf Beitrag Nr.: 34.552.952 von Birgit.Tersteegen am 21.07.08 17:07:09wie recht Du hast Biggi!
Ich hoffe, es knallt hier bald

Ich hoffe, es knallt hier bald

Charttechnisch sieht eigentlich OK aus

http://www.investorvillage.com/smbd.asp?mb=3440&mn=18101&pt=…
Msg: 18101 of 18105 7/21/2008 2:09:37 PM
Author: daveyjones547
Maybe we are responsible for capping this at $5
It appears that many here do not believe that this stock is going to head over $5 without some news, and news seems to be a month or two away. If people on this board believe this, then I would imagine they are selling shares ~$5 and trying to buy them back in the 4.80s.
I am holding on to my shares, and it is very possible that I will regret not trading this stock off of $5. However, the trading over the last few days has been very different from how it has been for months before. Either someone new is in the game, or one of the old-timers is getting hungry again.
Institutions (who actually have access to management, unlike shareholders) have bought up almost every share in this company, and if they see positive news on the horizon they will buy up every one of your shares while jerking the PPS around in the meantime. I think we are temporarily capped at $5, but I think we will blow through it at some point in the near future.
Msg: 18101 of 18105 7/21/2008 2:09:37 PM
Author: daveyjones547
Maybe we are responsible for capping this at $5
It appears that many here do not believe that this stock is going to head over $5 without some news, and news seems to be a month or two away. If people on this board believe this, then I would imagine they are selling shares ~$5 and trying to buy them back in the 4.80s.
I am holding on to my shares, and it is very possible that I will regret not trading this stock off of $5. However, the trading over the last few days has been very different from how it has been for months before. Either someone new is in the game, or one of the old-timers is getting hungry again.
Institutions (who actually have access to management, unlike shareholders) have bought up almost every share in this company, and if they see positive news on the horizon they will buy up every one of your shares while jerking the PPS around in the meantime. I think we are temporarily capped at $5, but I think we will blow through it at some point in the near future.
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…
Neurocrine Biosciences Announces Conference Call and
Webcast To Present Second Quarter 2008 Financial Results
Conference Call and Webcast Scheduled for Wednesday, July 30
SAN DIEGO, July 23 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that the Company will report its second quarter 2008 financial results after the Nasdaq market closes on Wednesday, July 30, 2008. Neurocrine will host a live conference call and webcast to discuss its financial results and provide a Company update Wednesday afternoon, July 30, 2008 at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time).
Neurocrine Biosciences Announces Conference Call and
Webcast To Present Second Quarter 2008 Financial Results
Conference Call and Webcast Scheduled for Wednesday, July 30
SAN DIEGO, July 23 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that the Company will report its second quarter 2008 financial results after the Nasdaq market closes on Wednesday, July 30, 2008. Neurocrine will host a live conference call and webcast to discuss its financial results and provide a Company update Wednesday afternoon, July 30, 2008 at 5:00 p.m. Eastern Time (2:00 p.m. Pacific Time).
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…
Neurocrine Biosciences Reports Second Quarter 2008 Results
SAN DIEGO, July 30 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) today announced its financial results for the quarter ended June 30, 2008. For the second quarter of 2008, the Company reported a net loss of $21.0 million, or $(0.55) per share compared with a net loss of $26.4 million, or $(0.69) per share, for the same period in 2007. For the six months, the Company reported a net loss of $42.0 million, or $(1.10) per share, as compared to $52.1 million, or $(1.37) per share, for the same period last year.
Revenues for the second quarter of 2008 were $0.7 million compared with $48,000 for the same period last year. Revenues for the six months ended June 30, 2008 were $2.5 million, compared with $0.2 million for the same period in 2007. The increase in revenues is primarily due to revenues recognized in 2008 under collaboration agreements with GlaxoSmithKline (GSK) and Dainippon Sumitomo Pharma Co., Ltd. (DSP).
Research and development expenses decreased to $16.2 million during the second quarter of 2008 compared with $18.8 million for the same period in 2007. For the six months ended June 30, 2008, research and development expenses were $30.4 million, compared to $37.9 million for the same period last year. The decrease in research and development expenses is primarily due to cost savings related to our restructuring in the fourth quarter of 2007.
General and administrative expenses were $4.7 million for the second quarter of 2008 and $8.8 million during the same period last year. For the six months ended June 30, 2008, general and administrative expenses were $13.0 million, compared to $17.1 million for the first half of 2007. The reduction in general and administrative expenses is primarily due to cost savings related to recent restructurings.
The Company's balance sheet on June 30, 2008 reflected total assets of $225.7 million, including cash and investments of $133.5 million compared with balances at December 31, 2007 of $276.7 million and $179.4 million, respectively. The Company expects to end 2008 with in excess of $100 million in cash and investments.
"We have made significant progress during the first half of 2008, moving our GnRH program forward in a large and comprehensive Phase II program, and advancing urocortin 2 through its final preclinical studies to allow for long-term Phase II clinical studies. In addition, we are moving a number of our research programs forward to meet our goal of advancing a novel compound into the clinic each year. All of this is taking place while carefully managing our cash burn," said Kevin Gorman, Chief Executive Officer and President of Neurocrine Biosciences. "We are fortunate to have two Phase II programs that have generated substantial partnership interest and to have an outstanding partner in GSK who is dedicated to our CRF collaboration with three compounds in clinical development."
R & D Pipeline Update
Neurocrine's clinical development group and corporate partners have five programs in clinical development and will report on R & D progress throughout 2008. Neurocrine scientists continue to supply Neurocrine's pipeline to meet the Company-wide goal of bringing one new compound into development each year.
GnRH Antagonists for Endometriosis
Elagolix in Three Phase II Clinical Trials for Endometriosis
Below is a summary of the current ongoing randomized placebo-controlled Phase II trials for elagolix:
Topline
Trial Study Design Endpoints n Status Results
0603 Six-month treatment 1. Impact of 252 Treatment Q3 08
period with elagolix elagolix on bone phase
and DMPA (positive mineral density complete
control) plus using DXA scan
additional long-term 2. Dysmenorrhea
safety assessments and pelvic pain
post treatment
0702 Placebo-controlled Dysmenorrhea 150 Screening 1H 09
trial with 2 doses of and pelvic pain complete
optimized formulation assessed with
tablet modified endpoints
0703 Placebo-controlled Dysmenorrhea 180 Initiated 1H 09
trial with 2 doses of and pelvic pain
elagolix and assessed with
leuprolide depot modified endpoints
comparator
.....................................
Neurocrine Biosciences Reports Second Quarter 2008 Results
SAN DIEGO, July 30 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) today announced its financial results for the quarter ended June 30, 2008. For the second quarter of 2008, the Company reported a net loss of $21.0 million, or $(0.55) per share compared with a net loss of $26.4 million, or $(0.69) per share, for the same period in 2007. For the six months, the Company reported a net loss of $42.0 million, or $(1.10) per share, as compared to $52.1 million, or $(1.37) per share, for the same period last year.
Revenues for the second quarter of 2008 were $0.7 million compared with $48,000 for the same period last year. Revenues for the six months ended June 30, 2008 were $2.5 million, compared with $0.2 million for the same period in 2007. The increase in revenues is primarily due to revenues recognized in 2008 under collaboration agreements with GlaxoSmithKline (GSK) and Dainippon Sumitomo Pharma Co., Ltd. (DSP).
Research and development expenses decreased to $16.2 million during the second quarter of 2008 compared with $18.8 million for the same period in 2007. For the six months ended June 30, 2008, research and development expenses were $30.4 million, compared to $37.9 million for the same period last year. The decrease in research and development expenses is primarily due to cost savings related to our restructuring in the fourth quarter of 2007.
General and administrative expenses were $4.7 million for the second quarter of 2008 and $8.8 million during the same period last year. For the six months ended June 30, 2008, general and administrative expenses were $13.0 million, compared to $17.1 million for the first half of 2007. The reduction in general and administrative expenses is primarily due to cost savings related to recent restructurings.
The Company's balance sheet on June 30, 2008 reflected total assets of $225.7 million, including cash and investments of $133.5 million compared with balances at December 31, 2007 of $276.7 million and $179.4 million, respectively. The Company expects to end 2008 with in excess of $100 million in cash and investments.
"We have made significant progress during the first half of 2008, moving our GnRH program forward in a large and comprehensive Phase II program, and advancing urocortin 2 through its final preclinical studies to allow for long-term Phase II clinical studies. In addition, we are moving a number of our research programs forward to meet our goal of advancing a novel compound into the clinic each year. All of this is taking place while carefully managing our cash burn," said Kevin Gorman, Chief Executive Officer and President of Neurocrine Biosciences. "We are fortunate to have two Phase II programs that have generated substantial partnership interest and to have an outstanding partner in GSK who is dedicated to our CRF collaboration with three compounds in clinical development."
R & D Pipeline Update
Neurocrine's clinical development group and corporate partners have five programs in clinical development and will report on R & D progress throughout 2008. Neurocrine scientists continue to supply Neurocrine's pipeline to meet the Company-wide goal of bringing one new compound into development each year.
GnRH Antagonists for Endometriosis
Elagolix in Three Phase II Clinical Trials for Endometriosis
Below is a summary of the current ongoing randomized placebo-controlled Phase II trials for elagolix:
Topline
Trial Study Design Endpoints n Status Results
0603 Six-month treatment 1. Impact of 252 Treatment Q3 08
period with elagolix elagolix on bone phase
and DMPA (positive mineral density complete
control) plus using DXA scan
additional long-term 2. Dysmenorrhea
safety assessments and pelvic pain
post treatment
0702 Placebo-controlled Dysmenorrhea 150 Screening 1H 09
trial with 2 doses of and pelvic pain complete
optimized formulation assessed with
tablet modified endpoints
0703 Placebo-controlled Dysmenorrhea 180 Initiated 1H 09
trial with 2 doses of and pelvic pain
elagolix and assessed with
leuprolide depot modified endpoints
comparator
.....................................
Indiplon Update
The Company met with the FDA in July for an end of review meeting related to the December 12, 2007 approvable letter for indiplon capsules. The FDA meeting focused on the three additional requirements outlined in the December 12, 2007 approvable letter. At present, the Company is awaiting the final minutes of this meeting to determine the next course of action related to indiplon capsules.
...........................
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…
The Company met with the FDA in July for an end of review meeting related to the December 12, 2007 approvable letter for indiplon capsules. The FDA meeting focused on the three additional requirements outlined in the December 12, 2007 approvable letter. At present, the Company is awaiting the final minutes of this meeting to determine the next course of action related to indiplon capsules.
...........................
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…
http://www1.investorvillage.com/smbd.asp?mb=3440&mn=18516&pt…
Response from IR re DSP/Indiplon
As previously mentioned here, after the last CC I sent an email to IR and asked how they recognized revenue from the DSP upfront payment of 100 Million. There response was boilerplate and I asked a more specific question. I finally got an answer.
Here is my email and their response.
08/05/08
"I appreciate the response, but still have questions. Does the DSP agreement have a specific contract length over which the upfront payments are amortized? Having read the agreement over a year ago, I don't think so. It seems to me that revenue is being recognized as Neurocrine provides technical data and support to facilitate DSP's effort to get Indiplon approved in Japan. If that is the case, it implies that the company does not feel that Indiplon is dead in Japan or in the rest of the world, despite the FDA's concerns as expressed last December. That would be encouraging. I look forward to seeing the FDA Minutes of the July meeting and the PII results for Elagolix. September could be a good month for all of us. If Mr. Coughlin or someone in the accounting department could briefly explain how they arrive at a revenue number to recognize for the DSP contract, that would help. It is an estimate, but using what parameters."
Response 08/19/08
"It is over the expected development period." That is certainly brief and probably came from accounting. Clearly Elagolix is the focus for Neurocrine now, but IMO Indiplon is just on the back burner.
Ochs
Response from IR re DSP/Indiplon
As previously mentioned here, after the last CC I sent an email to IR and asked how they recognized revenue from the DSP upfront payment of 100 Million. There response was boilerplate and I asked a more specific question. I finally got an answer.
Here is my email and their response.
08/05/08
"I appreciate the response, but still have questions. Does the DSP agreement have a specific contract length over which the upfront payments are amortized? Having read the agreement over a year ago, I don't think so. It seems to me that revenue is being recognized as Neurocrine provides technical data and support to facilitate DSP's effort to get Indiplon approved in Japan. If that is the case, it implies that the company does not feel that Indiplon is dead in Japan or in the rest of the world, despite the FDA's concerns as expressed last December. That would be encouraging. I look forward to seeing the FDA Minutes of the July meeting and the PII results for Elagolix. September could be a good month for all of us. If Mr. Coughlin or someone in the accounting department could briefly explain how they arrive at a revenue number to recognize for the DSP contract, that would help. It is an estimate, but using what parameters."
Response 08/19/08
"It is over the expected development period." That is certainly brief and probably came from accounting. Clearly Elagolix is the focus for Neurocrine now, but IMO Indiplon is just on the back burner.
Ochs
so, bin wieder drin

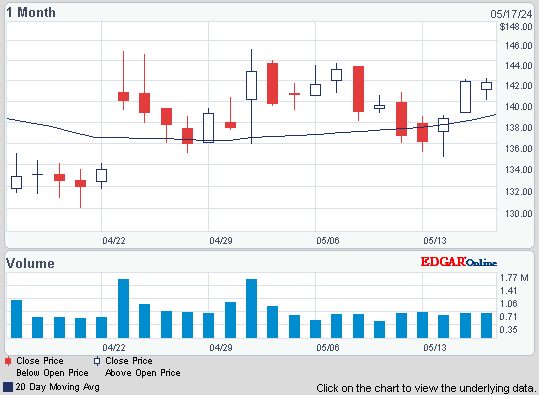


Tageschart

Neurocrine Biosciences, Inc. at BioCentury Publications Inc.
NewsMakers in the Biotech Industry
Thursday, September 4, 2008 10:00 a.m. ET
http://www.biocentury.com/BCApp/BioCenturyCommon/Conferences…
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-cal…
NewsMakers in the Biotech Industry
Thursday, September 4, 2008 10:00 a.m. ET
http://www.biocentury.com/BCApp/BioCenturyCommon/Conferences…
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-cal…
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…
Neurocrine Biosciences to Present at Thomas Weisel Healthcare Conference and the NewsMakers in the Biotech Industry Conference
COMPANY PRESENTS AT THOMAS WEISEL WEDNESDAY, SEPTEMBER 3RD AND NEWSMAKERS IN THE BIOTECH INDUSTRY CONFERENCE THURSDAY, SEPTEMBER 4TH
Neurocrine Biosciences to Present at Thomas Weisel Healthcare Conference and the NewsMakers in the Biotech Industry Conference
COMPANY PRESENTS AT THOMAS WEISEL WEDNESDAY, SEPTEMBER 3RD AND NEWSMAKERS IN THE BIOTECH INDUSTRY CONFERENCE THURSDAY, SEPTEMBER 4TH
sorry, dass ich planlos in die Runde frage, ob es zurzeit Gründe gäbe hier einzusteigen bzw. Neurocrine auf die Watchlist zu setzen?
Antwort auf Beitrag Nr.: 34.938.121 von mortem am 01.09.08 23:28:23Es gäbe Gründe, jedoch spekulativ (siehe Beitrag #715 von bernie55 vom 29.04.08 11:08:44 ).
NBIX Management (Gorman) läßt es nicht durchschauen, was. zz läuft.
Auf dem IV Board schrieben viele, dass im September "was" kommen könnte (News vom FDA wegen INDIPLON, Partnerdeal etc.????), aber wie gesagt, spekulativ halt.
Lese doch mal ab und zu auf dem IV Board:
http://www1.investorvillage.com/smbd.asp?mb=3440&pt=m
Alle sind gespannt, was NBIX auf den beiden Konferenzen am 3. und 4. September bringen wird
(keine Kauf- oder Verkaufsempfehlung)
NBIX Management (Gorman) läßt es nicht durchschauen, was. zz läuft.
Auf dem IV Board schrieben viele, dass im September "was" kommen könnte (News vom FDA wegen INDIPLON, Partnerdeal etc.????), aber wie gesagt, spekulativ halt.

Lese doch mal ab und zu auf dem IV Board:
http://www1.investorvillage.com/smbd.asp?mb=3440&pt=m
Alle sind gespannt, was NBIX auf den beiden Konferenzen am 3. und 4. September bringen wird

(keine Kauf- oder Verkaufsempfehlung)
Neurocrine Biosciences Announces Successful Elagolix PETAL Study in Endometriosis
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…" target="_blank" rel="nofollow ugc noopener">http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…
Phase II Study Meets Primary Bone Mineral Density and Secondary Efficacy Endpoints Company to Host Conference Call and Webcast Wednesday, September 3rd at 8:30 am ET / 5:30 am PT
SAN DIEGO, Sept 02, 2008 /PRNewswire-FirstCall via COMTEX News Network/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) today announced positive safety and efficacy results from its third Phase II clinical trial using its proprietary, orally-active nonpeptide Gonadotropin-Releasing Hormone (GnRH) receptor antagonist, elagolix, in patients with endometriosis. The PETAL study enrolled 252 patients, with a confirmed diagnosis of endometriosis, into three treatment groups; elagolix 150 mg once daily, elagolix 75 mg twice daily, or depo-subQ provera 104(TM) (DMPA) for six months of treatment.
The primary endpoint for the PETAL study was the percent change from baseline in mean bone mineral density (BMD) at Month 6 measured via dual energy X-ray absorptiometry (DXA). Pursuant to discussion with the FDA, the pre-specified statistical analysis plan sought to demonstrate that at Month 6, the lower bound of the 95% confidence interval did not exceed a -2.2% change in BMD from baseline. In women randomized to elagolix 150 mg once daily, the mean percent change from baseline at Month 6 was -0.11% for the spine (lower bound -0.70%) and -0.47% for the femur (lower bound -0.96%). The mean percent change from baseline at Month 6 for the elagolix 75 mg twice daily dosing arm was -1.30% for the spine (lower bound -1.86%) and -0.99% for the femur (lower bound -1.46%).
"The PETAL study demonstrates that elagolix did not induce significant bone loss over a six month treatment of patients with endometriosis, while providing both rapid and significant pain reduction in endometriosis symptoms," said Chris O'Brien, MD, Chief Medical Officer at Neurocrine. "Additionally, this study confirms our decision to move forward with once daily dosing."
Secondary endpoints for the PETAL study were evaluated to assess the improvement of endometriosis symptoms following treatment with elagolix. Improvement in endometriosis symptoms was documented using several different scales for endometriosis pain. The following were assessed before, during and after the six months of treatment:
-- Total Composite Pelvic Sign and Symptoms Score (CPSSS), a validated 0-15 scale that assesses five components of endometriosis pain severity, each on a 0-3 scale.
-- Dysmenorrhea (pelvic pain during menstruation), a component of the CPSSS; 0-3 scale (0=absence of pain, 1=mild pain, 2=moderate pain, 3=severe pain)
-- 98% of patients at baseline had moderate or severe dysmenorrhea.
-- Non-menstrual pelvic pain (pelvic pain outside of menstruation), a component of the CPSSS; 0-3 scale (0=absence of pain, 1=mild pain, 2=moderate pain, 3=severe pain)
-- 97% of patients at baseline had moderate or severe non-menstrual pelvic pain.
-- Responder Rate, percentage of patients who had a one point or greater decrease in pain score.
-- Visual Analog Scale (VAS) to assess pelvic pain levels using daily electronic diary.
Elagolix provided a clinically meaningful and statistically significant reduction in endometriosis pain from baseline as shown below. The magnitude of improvement is comparable to that demonstrated with the currently approved agents, leuprolide and DMPA.
Screening Elagolix
Baseline 150mg qd 75mg bid DMPA
CPSSS 9.1
Week 4 mean -3.9* -3.7* -3.8*
Week 24 mean -5.5* -5.2* -5.3*
Dysmenorrhea 2.4
Week 24 mean -1.5* -1.4* -1.7*
Responder Rate 86% 74% 86%
Non-Menstrual Pelvic
Pain 2.2
Week 24 mean -1.2* -1.2* -1.1*
Responder Rate 86% 77% 77%
VAS 78.7
Week 24 mean -31.8* -33.4* -35.5*
*ITT Analysis, p<0.0001
"We have treated over 600 subjects with elagolix in our extensive endometriosis clinical program, and have consistently seen a robust reduction in endometriosis pain using multiple outcome measures. In our previous Phase II studies we have shown rapid onset of action and sustained efficacy over three months. This study extends those findings, demonstrating significant and sustained pain reduction for six months," said Dr. O'Brien. "There is much more data to come from this study once patients complete the six month follow-up and the study is unblinded at an individual patient level. At that time we will be able to make correlations between pain scores, BMD, pharmacokinetic values, and hormonal levels on an individual patient basis, and we look forward to also sharing those results, as soon as they are available."
Treatment with elagolix was also safe and generally well tolerated. Discontinuation from the clinical trial due to adverse events (AE) was more common among women randomized to DMPA (16%) than those receiving elagolix 150 mg once daily (5%) or 75 mg twice daily (7%). This increased discontinuation in women randomized to DMPA was primarily attributable to irregular vaginal bleeding. The overall rate of AE reporting was comparable across all groups. The most common AE was headache; this was reported 35 times by 19 women (23%) randomized to elagolix 150 mg once daily, 52 times by 22 women (26%) randomized to elagolix 75 mg twice daily, and 43 times by 12 (14%) women randomized to DMPA. There were two serious AEs that led to discontinuation from the trial, both in the DMPA group. The mean frequency of hot flashes was comparable in both the screening period and the treatment period, approximately 0.5 per day across all treatment groups, consistent with our prior Phase II studies.
"We couldn't be more pleased with the results of this study," said Kevin C. Gorman, President and Chief Executive Officer, Neurocrine Biosciences. "We began this program nine years ago with the hope of selectively modulating the GnRH system with an oral antagonist. This trial and our previous Phase II studies have now shown that this is possible. Elagolix is a first in class drug for endometriosis sufferers who desperately need a safe and effective therapy. This is apparent from the 8,000 inquiries to the call center for just this one study. Additionally, to date we had more than 6,000 inquiries regarding the ongoing Lilac PETAL study which is now fully enrolled and on track to report results in 2009."
"These study results are very encouraging," said Shayne M. Plosker, MD, Associate Professor and Division Director, of USF IVF and Reproductive Endocrinology at the University of South Florida College of Medicine in Tampa. "Elagolix has the potential to be an ideal advanced medical therapy for endometriosis-associated pelvic pain and dysmenorrhea. Intermediate suppression of estradiol by a well-tolerated drug appears attainable affording endometriosis symptom reduction without bone loss."
Neurocrine Biosciences would like to thank the patients and the investigators for participating in this important clinical trial.
Conference Call and Webcast Information
The Company will host a live conference call and webcast to provide additional details of this study tomorrow, Wednesday, September 3, 2008 at 8:30 a.m. Eastern Time (5:30 a.m. Pacific Time). Participants can access the live conference call by dialing 1-800-894-5910 (US) or 785-424-1052 (International) using the conference ID: 7GNRH. The call can also be accessed via the webcast through the Company's website at http://www.neurocrine.com.
If you are unable to attend the Webcast and would like further information on this announcement please contact the Investor Relations Department at Neurocrine Biosciences at (858) 617-7600. A replay of the Conference Call will be available approximately one hour after the conclusion of the call by dialing 1-800-374-0934 (US) or 402-220-0680 (International) using the conference ID: 7GNRH. The call will be archived for two weeks.
Neurocrine Biosciences, Inc. is a biopharmaceutical company focused on neurological and endocrine diseases and disorders. Our product candidates address some of the largest pharmaceutical markets in the world including endometriosis, irritable bowel syndrome (IBS), anxiety, depression, pain, diabetes, benign prostatic hyperplasia (BPH) and other neurological and endocrine related diseases and disorders. Neurocrine Biosciences, Inc. news releases are available through the Company's website via the internet at http://www.neurocrine.com.
In addition to historical facts, this press release may contain forward-looking statements that involve a number of risks and uncertainties. Among the factors that could cause actual results to differ materially from those indicated in the forward-looking statements are risks and uncertainties associated with Neurocrine's business and finances in general, as well as risks and uncertainties associated with the Company's GnRH program and Company overall. Specifically, the risks and uncertainties the Company faces with respect to the Company's GnRH program include, but are not limited to, risk that the Company's elagolix Phase II clinical trials will fail to demonstrate that elagolix is safe and effective; risk that elagolix will not proceed to later stage clinical trials; risk associated with the Company's dependence on corporate collaborators for development, commercial manufacturing and marketing and sales activities. With respect to its pipeline overall, the Company faces risk that it will be unable to raise additional funding required to complete development of all of its product candidates; risk relating to the Company's dependence on contract manufacturers for clinical drug supply; risks associated with the Company's dependence on corporate collaborators for commercial manufacturing and marketing and sales activities; uncertainties relating to patent protection and intellectual property rights of third parties; risks and uncertainties relating to competitive products and technological changes that may limit demand for the Company's products; and the other risks described in the Company's report on Form 10-K for the year ended December 31, 2007 and reports on Form 10-Q for the quarters ended March 31, 2008 and June 30, 2008. Neurocrine undertakes no obligation to update the statements contained in this press release after the date hereof.
SOURCE Neurocrine Biosciences, Inc.
http://www.neurocrine.com
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…" target="_blank" rel="nofollow ugc noopener">http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…
Phase II Study Meets Primary Bone Mineral Density and Secondary Efficacy Endpoints Company to Host Conference Call and Webcast Wednesday, September 3rd at 8:30 am ET / 5:30 am PT
SAN DIEGO, Sept 02, 2008 /PRNewswire-FirstCall via COMTEX News Network/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) today announced positive safety and efficacy results from its third Phase II clinical trial using its proprietary, orally-active nonpeptide Gonadotropin-Releasing Hormone (GnRH) receptor antagonist, elagolix, in patients with endometriosis. The PETAL study enrolled 252 patients, with a confirmed diagnosis of endometriosis, into three treatment groups; elagolix 150 mg once daily, elagolix 75 mg twice daily, or depo-subQ provera 104(TM) (DMPA) for six months of treatment.
The primary endpoint for the PETAL study was the percent change from baseline in mean bone mineral density (BMD) at Month 6 measured via dual energy X-ray absorptiometry (DXA). Pursuant to discussion with the FDA, the pre-specified statistical analysis plan sought to demonstrate that at Month 6, the lower bound of the 95% confidence interval did not exceed a -2.2% change in BMD from baseline. In women randomized to elagolix 150 mg once daily, the mean percent change from baseline at Month 6 was -0.11% for the spine (lower bound -0.70%) and -0.47% for the femur (lower bound -0.96%). The mean percent change from baseline at Month 6 for the elagolix 75 mg twice daily dosing arm was -1.30% for the spine (lower bound -1.86%) and -0.99% for the femur (lower bound -1.46%).
"The PETAL study demonstrates that elagolix did not induce significant bone loss over a six month treatment of patients with endometriosis, while providing both rapid and significant pain reduction in endometriosis symptoms," said Chris O'Brien, MD, Chief Medical Officer at Neurocrine. "Additionally, this study confirms our decision to move forward with once daily dosing."
Secondary endpoints for the PETAL study were evaluated to assess the improvement of endometriosis symptoms following treatment with elagolix. Improvement in endometriosis symptoms was documented using several different scales for endometriosis pain. The following were assessed before, during and after the six months of treatment:
-- Total Composite Pelvic Sign and Symptoms Score (CPSSS), a validated 0-15 scale that assesses five components of endometriosis pain severity, each on a 0-3 scale.
-- Dysmenorrhea (pelvic pain during menstruation), a component of the CPSSS; 0-3 scale (0=absence of pain, 1=mild pain, 2=moderate pain, 3=severe pain)
-- 98% of patients at baseline had moderate or severe dysmenorrhea.
-- Non-menstrual pelvic pain (pelvic pain outside of menstruation), a component of the CPSSS; 0-3 scale (0=absence of pain, 1=mild pain, 2=moderate pain, 3=severe pain)
-- 97% of patients at baseline had moderate or severe non-menstrual pelvic pain.
-- Responder Rate, percentage of patients who had a one point or greater decrease in pain score.
-- Visual Analog Scale (VAS) to assess pelvic pain levels using daily electronic diary.
Elagolix provided a clinically meaningful and statistically significant reduction in endometriosis pain from baseline as shown below. The magnitude of improvement is comparable to that demonstrated with the currently approved agents, leuprolide and DMPA.
Screening Elagolix
Baseline 150mg qd 75mg bid DMPA
CPSSS 9.1
Week 4 mean -3.9* -3.7* -3.8*
Week 24 mean -5.5* -5.2* -5.3*
Dysmenorrhea 2.4
Week 24 mean -1.5* -1.4* -1.7*
Responder Rate 86% 74% 86%
Non-Menstrual Pelvic
Pain 2.2
Week 24 mean -1.2* -1.2* -1.1*
Responder Rate 86% 77% 77%
VAS 78.7
Week 24 mean -31.8* -33.4* -35.5*
*ITT Analysis, p<0.0001
"We have treated over 600 subjects with elagolix in our extensive endometriosis clinical program, and have consistently seen a robust reduction in endometriosis pain using multiple outcome measures. In our previous Phase II studies we have shown rapid onset of action and sustained efficacy over three months. This study extends those findings, demonstrating significant and sustained pain reduction for six months," said Dr. O'Brien. "There is much more data to come from this study once patients complete the six month follow-up and the study is unblinded at an individual patient level. At that time we will be able to make correlations between pain scores, BMD, pharmacokinetic values, and hormonal levels on an individual patient basis, and we look forward to also sharing those results, as soon as they are available."
Treatment with elagolix was also safe and generally well tolerated. Discontinuation from the clinical trial due to adverse events (AE) was more common among women randomized to DMPA (16%) than those receiving elagolix 150 mg once daily (5%) or 75 mg twice daily (7%). This increased discontinuation in women randomized to DMPA was primarily attributable to irregular vaginal bleeding. The overall rate of AE reporting was comparable across all groups. The most common AE was headache; this was reported 35 times by 19 women (23%) randomized to elagolix 150 mg once daily, 52 times by 22 women (26%) randomized to elagolix 75 mg twice daily, and 43 times by 12 (14%) women randomized to DMPA. There were two serious AEs that led to discontinuation from the trial, both in the DMPA group. The mean frequency of hot flashes was comparable in both the screening period and the treatment period, approximately 0.5 per day across all treatment groups, consistent with our prior Phase II studies.
"We couldn't be more pleased with the results of this study," said Kevin C. Gorman, President and Chief Executive Officer, Neurocrine Biosciences. "We began this program nine years ago with the hope of selectively modulating the GnRH system with an oral antagonist. This trial and our previous Phase II studies have now shown that this is possible. Elagolix is a first in class drug for endometriosis sufferers who desperately need a safe and effective therapy. This is apparent from the 8,000 inquiries to the call center for just this one study. Additionally, to date we had more than 6,000 inquiries regarding the ongoing Lilac PETAL study which is now fully enrolled and on track to report results in 2009."
"These study results are very encouraging," said Shayne M. Plosker, MD, Associate Professor and Division Director, of USF IVF and Reproductive Endocrinology at the University of South Florida College of Medicine in Tampa. "Elagolix has the potential to be an ideal advanced medical therapy for endometriosis-associated pelvic pain and dysmenorrhea. Intermediate suppression of estradiol by a well-tolerated drug appears attainable affording endometriosis symptom reduction without bone loss."
Neurocrine Biosciences would like to thank the patients and the investigators for participating in this important clinical trial.
Conference Call and Webcast Information
The Company will host a live conference call and webcast to provide additional details of this study tomorrow, Wednesday, September 3, 2008 at 8:30 a.m. Eastern Time (5:30 a.m. Pacific Time). Participants can access the live conference call by dialing 1-800-894-5910 (US) or 785-424-1052 (International) using the conference ID: 7GNRH. The call can also be accessed via the webcast through the Company's website at http://www.neurocrine.com.
If you are unable to attend the Webcast and would like further information on this announcement please contact the Investor Relations Department at Neurocrine Biosciences at (858) 617-7600. A replay of the Conference Call will be available approximately one hour after the conclusion of the call by dialing 1-800-374-0934 (US) or 402-220-0680 (International) using the conference ID: 7GNRH. The call will be archived for two weeks.
Neurocrine Biosciences, Inc. is a biopharmaceutical company focused on neurological and endocrine diseases and disorders. Our product candidates address some of the largest pharmaceutical markets in the world including endometriosis, irritable bowel syndrome (IBS), anxiety, depression, pain, diabetes, benign prostatic hyperplasia (BPH) and other neurological and endocrine related diseases and disorders. Neurocrine Biosciences, Inc. news releases are available through the Company's website via the internet at http://www.neurocrine.com.
In addition to historical facts, this press release may contain forward-looking statements that involve a number of risks and uncertainties. Among the factors that could cause actual results to differ materially from those indicated in the forward-looking statements are risks and uncertainties associated with Neurocrine's business and finances in general, as well as risks and uncertainties associated with the Company's GnRH program and Company overall. Specifically, the risks and uncertainties the Company faces with respect to the Company's GnRH program include, but are not limited to, risk that the Company's elagolix Phase II clinical trials will fail to demonstrate that elagolix is safe and effective; risk that elagolix will not proceed to later stage clinical trials; risk associated with the Company's dependence on corporate collaborators for development, commercial manufacturing and marketing and sales activities. With respect to its pipeline overall, the Company faces risk that it will be unable to raise additional funding required to complete development of all of its product candidates; risk relating to the Company's dependence on contract manufacturers for clinical drug supply; risks associated with the Company's dependence on corporate collaborators for commercial manufacturing and marketing and sales activities; uncertainties relating to patent protection and intellectual property rights of third parties; risks and uncertainties relating to competitive products and technological changes that may limit demand for the Company's products; and the other risks described in the Company's report on Form 10-K for the year ended December 31, 2007 and reports on Form 10-Q for the quarters ended March 31, 2008 and June 30, 2008. Neurocrine undertakes no obligation to update the statements contained in this press release after the date hereof.
SOURCE Neurocrine Biosciences, Inc.
http://www.neurocrine.com



Pipeline
Neurocrine’s research and development efforts are focused on neurological and endocrine diseases and disorders.

Neurocrine’s research and development efforts are focused on neurological and endocrine diseases and disorders.

Re: Venting- Don't read!!! Ray
In response to msg 19078 by goirish1776
http://www1.investorvillage.com/smbd.asp?mb=3440&mn=19085&pt…
You have nailed the reason perfectly. Several weeks ago someone purchased close to 9000 Sept. 5 call options. At that time, the MM had to write those calls collecting the premium and hedge the position by going long NBIX common. Probably close to 900,000 shares. When the stock got into the mid to high 5's the option buyer sold approximately 5000 of the calls (probably back to the MM). The MM probably unhedged those calls by selling almost 500,000 shares which was a source of our weakness. Now with a day to go, the MM probably sold the last of the long position that was hedging the 4000 remaining 5 calls, driving the price well below 5 which would mean those options would expire worthless. With a poor overall market, the option speculator got screwed and the share price of NBIX along with it. The MM will work hard to keep the price below 5 until tomorrows close. Once we are past expiration day, there will be a chance for NBIX to recover above 5 if the market straightens itself out.
I had worried about the potential for this happening last week and sold 15% of my NBIX position. (I should have sold a lot more.) I bought the shares back today around 4.80 (a little too early.) We live in a world where the tail wags the dog with derivatives such as options etc being the tail.
In response to msg 19078 by goirish1776
http://www1.investorvillage.com/smbd.asp?mb=3440&mn=19085&pt…
You have nailed the reason perfectly. Several weeks ago someone purchased close to 9000 Sept. 5 call options. At that time, the MM had to write those calls collecting the premium and hedge the position by going long NBIX common. Probably close to 900,000 shares. When the stock got into the mid to high 5's the option buyer sold approximately 5000 of the calls (probably back to the MM). The MM probably unhedged those calls by selling almost 500,000 shares which was a source of our weakness. Now with a day to go, the MM probably sold the last of the long position that was hedging the 4000 remaining 5 calls, driving the price well below 5 which would mean those options would expire worthless. With a poor overall market, the option speculator got screwed and the share price of NBIX along with it. The MM will work hard to keep the price below 5 until tomorrows close. Once we are past expiration day, there will be a chance for NBIX to recover above 5 if the market straightens itself out.
I had worried about the potential for this happening last week and sold 15% of my NBIX position. (I should have sold a lot more.) I bought the shares back today around 4.80 (a little too early.) We live in a world where the tail wags the dog with derivatives such as options etc being the tail.
Neurocrine Biosciences to Present at the UBS Global Life Sciences Conference
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…" target="_blank" rel="nofollow ugc noopener">
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…
Live Audio Webcast Thursday, September 25, 2008
SAN DIEGO, Sept. 22 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that Kevin Gorman, President and Chief Executive Officer of Neurocrine Biosciences will be presenting at the UBS Global Life Sciences Conference at the Grand Hyatt in New York.
The live presentation takes place Thursday, September 25, 2008 at 10:00 a.m. Eastern Time (ET) /7:00 a.m. Pacific Time (PT). The presentation will be simultaneously webcast and may be accessed on the Company's website at http://www.neurocrine.com which will link you to the UBS Conference site. Once you have been linked to their site, you have the option to search by company name, or by time and date to access the webcast.
If you are unable to attend the webcast and would like further information on this announcement please contact the Investor Relations Department at Neurocrine Biosciences at (858) 617-7600. Viewers are encouraged to visit the website approximately 5 minutes prior to the presentation to download or install any necessary software. A replay of the presentation will be available approximately three hours after the conclusion of the live event and will be archived on the website for two weeks.
About Neurocrine
Neurocrine Biosciences, Inc. is a biopharmaceutical company focused on neurological and endocrine diseases and disorders. Our product candidates address some of the largest pharmaceutical markets in the world including endometriosis, irritable bowel syndrome (IBS), anxiety, depression, pain, diabetes, benign prostatic hyperplasia (BPH) and other neurological and endocrine related diseases and disorders. Neurocrine Biosciences, Inc. news releases are available through the Company's website via the internet at http://www.neurocrine.com
SOURCE Neurocrine Biosciences, Inc.
-0- 09/22/2008
/CONTACT: Claudia Woodworth of Neurocrine Biosciences, Inc.,
+1-858-617-7600/
/Web site: http://www.neurocrine.com /
(NBIX)
CO: Neurocrine Biosciences, Inc.
ST: California, New York
IN: HEA MTC BIO
SU: TDS CCA
SH-JR
-- LAM519 --
3200 09/22/2008 14:40 EDT http://www.prnewswire.com
was ist heute los?




mensch, die lassen sich ja Zeit :O
:O
Indiplon Update
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-sec
The Company met with the FDA in July for an end of review meeting
related to the December 12, 2007 approvable letter for indiplon capsules.
The FDA meeting focused on the three additional requirements outlined in
the approvable letter. After exchange of correspondence regarding meeting
minutes, the Company is awaiting the FDA's final version of these minutes
to determine the next course of action related to indiplon capsules.
 :O
:OIndiplon Update
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-sec
The Company met with the FDA in July for an end of review meeting
related to the December 12, 2007 approvable letter for indiplon capsules.
The FDA meeting focused on the three additional requirements outlined in
the approvable letter. After exchange of correspondence regarding meeting
minutes, the Company is awaiting the FDA's final version of these minutes
to determine the next course of action related to indiplon capsules.
Allen NBIX-ler/in, alles Gute zum neuen Jahr 2009





Antwort auf Beitrag Nr.: 36.294.427 von surga am 02.01.09 19:17:00das wünsch ich ebenso 

Antwort auf Beitrag Nr.: 36.294.580 von zenman am 02.01.09 19:34:20Danke Zen

Neurocrine Biosciences Announces Conference Call and Webcast to Present First Quarter 2009 Financial Results
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…
--Conference Call and Webcast Scheduled for Wednesday, May 6, 2009--
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…
--Conference Call and Webcast Scheduled for Wednesday, May 6, 2009--

http://www.reuters.com/article/marketsNews/idCNBNG5258752009…" target="_blank" rel="nofollow ugc noopener">http://www.reuters.com/article/marketsNews/idCNBNG5258752009…
UPDATE 1-Neurocrine Biosciences gets funding of up to $75 mln
Tue Sep 15, 2009 5:35pm EDT
* Says Kingsbridge Capital to provide up to $75 mln
* Shares rise 12 pct
Sept 15 (Reuters) - Neurocrine Biosciences Inc (NBIX.O) said private investment group Kingsbridge Capital Ltd will provide up to $75 million of capital during the next three years through the purchase of newly issued shares of the biopharmaceutical company.
Shares of Neurocrine rose 12 percent to $3.50 in trading after the bell. They closed at $3.13 Tuesday on Nasdaq.
Neurocrine said it has access to capital at its discretion, and the deal does not impose any restrictions on its operating activities or capital-raising alternatives, other than similar committed equity financing facility (CEFF).
The company is not obligated to utilize any of the $75 million available under the CEFF, and there are no minimum commitments or minimum use penalties, Neurocrine said. For the alerts double-click
[ID:nWNAB9893]
(Reporting by Anand Basu in Bangalore; Editing by Deepak Kannan)
UPDATE 1-Neurocrine Biosciences gets funding of up to $75 mln
Tue Sep 15, 2009 5:35pm EDT
* Says Kingsbridge Capital to provide up to $75 mln
* Shares rise 12 pct
Sept 15 (Reuters) - Neurocrine Biosciences Inc (NBIX.O) said private investment group Kingsbridge Capital Ltd will provide up to $75 million of capital during the next three years through the purchase of newly issued shares of the biopharmaceutical company.
Shares of Neurocrine rose 12 percent to $3.50 in trading after the bell. They closed at $3.13 Tuesday on Nasdaq.
Neurocrine said it has access to capital at its discretion, and the deal does not impose any restrictions on its operating activities or capital-raising alternatives, other than similar committed equity financing facility (CEFF).
The company is not obligated to utilize any of the $75 million available under the CEFF, and there are no minimum commitments or minimum use penalties, Neurocrine said. For the alerts double-click
[ID:nWNAB9893]
(Reporting by Anand Basu in Bangalore; Editing by Deepak Kannan)
Neurocrine Biosciences to Present at the UBS Global Life Sciences Conference
Live Audio Webcast Wednesday, September 23, 2009
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…
SAN DIEGO, Sept. 16 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that Kevin Gorman, President and Chief Executive Officer of Neurocrine Biosciences, will be presenting at the UBS Global Life Sciences Conference in New York.
The live presentation takes place Wednesday, September 23, 2009 at 1:00 p.m. Eastern Time (ET)/10:00 a.m. Pacific Time (PT). The presentation will be simultaneously webcast and may be accessed on the Company's website at http://www.neurocrine.com.
.....
Live Audio Webcast Wednesday, September 23, 2009
http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-new…
SAN DIEGO, Sept. 16 /PRNewswire-FirstCall/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) announced today that Kevin Gorman, President and Chief Executive Officer of Neurocrine Biosciences, will be presenting at the UBS Global Life Sciences Conference in New York.
The live presentation takes place Wednesday, September 23, 2009 at 1:00 p.m. Eastern Time (ET)/10:00 a.m. Pacific Time (PT). The presentation will be simultaneously webcast and may be accessed on the Company's website at http://www.neurocrine.com.
.....
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +1,25 | |
| -0,31 | |
| +0,48 | |
| +1,71 | |
| +0,61 | |
| +1,32 | |
| -0,51 | |
| +2,38 | |
| +0,96 | |
| +0,95 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 120 | ||
| 82 | ||
| 59 | ||
| 57 | ||
| 41 | ||
| 30 | ||
| 26 | ||
| 25 | ||
| 24 | ||
| 23 |
13.02.24 · wallstreetONLINE Redaktion · Amazon |
04.02.24 · DR. KALLIWODA RESEARCH · Neurocrine Biosciences |






















