Zalicus - A low-risk development-stage biotech - 500 Beiträge pro Seite
eröffnet am 10.01.11 20:23:37 von
neuester Beitrag 18.06.14 15:40:49 von
neuester Beitrag 18.06.14 15:40:49 von
Beiträge: 443
ID: 1.162.665
ID: 1.162.665
Aufrufe heute: 0
Gesamt: 32.157
Gesamt: 32.157
Aktive User: 0
ISIN: US29428P1075 · WKN: A117U3 · Symbol: EPRSQ
0,0001
USD
+9.900,00 %
+0,0001 USD
Letzter Kurs 22.04.24 Nasdaq OTC
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,9000 | +59,66 | |
| 0,5922 | +44,44 | |
| 7,3500 | +43,84 | |
| 11,690 | +40,84 | |
| 0,7000 | +36,69 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 10,110 | -13,44 | |
| 8,2200 | -13,47 | |
| 7,6100 | -17,20 | |
| 1,6100 | -18,27 | |
| 2,1200 | -21,77 |
Unbeobachtet und aus dem Winterschlaf erwacht, klettert ZLCS schrittweise in Richtung Norden.
Die frühere CombinatoRx (CRXX ) hat nach der Vereinigung mit Neuromed neuen Schwung genommen.
Zalicus (ZLCS) is my pick from New World Investor for this 2011 list. It is a development-stage, yet low-risk, virtually unknown biotech stock. Zalicus was formed by the merger of CombinatoRx (formerly CRXX) and Neuromed, a private company. Most of the current management is from Neuromed, and they have refocused the company on drugs for pain and immuno-inflammatory disease.
But there were many drugs in preclinical and clinical trials at CombinatoRx before the merger, and several of them are showing more potential than Neuromed may have expected when they agreed to the merger. At the least, these drugs provide ongoing partnering opportunities. Some have world-class potential for eventual royalties.
The core development programs include Synavive, developed by CombinatoRx as CRx-102, to treat immuno-inflammatory disorders. It has completed Phase II clinical trials in subjects with knee osteoarthritis. The other core area are calcium channel blockers for chronic pain, developed by Neuromed
Partnered development programs include Prednisporin (FOV1101), which is being developed by Fovea, now owned by Sanofi-Aventis (SNY). Prednisporin is a topical ophthalmic drug combining low doses of prednisolone acetate and cyclosporine A, an immunosuppressant. A second major partnership with Novartis (NVS) is working on cancer drugs. Phase 2 results are imminent from a collaboration agreement with PgxHealth, a subsidiary of Clinical Data, to develop ATL313, an adenosine A2A receptor agonist, as a combination therapy against multiple myeloma and certain other B-cell malignancies.
At the time of the merger, Neuromed had Exalgo, a once-a-day, extended release version of Dilaudid, in the FDA approval process for pain. The company received approval on March 1, 2010, and its U. S. marketing partner Mallinkrodt, owned by Covidien, introduced the drug in April. June quarter inventory stocking resulted in $1.1 million royalties to Zalicus. Covidien expects Exalgo eventually to get $250 million to $300 million of the $6 billion long-acting opioid market.
However, as frequently happens with a new drug that is widely introduced, once the distributors have their initial stock, sales fall off for a quarter or two as the sell-through builds. That is what happened in the September quarter, when Zalicus reported only $0.15 million in Exalgo royalties. It will happen again in the December quarter, when I am expecting only $0.4 million in royalties. Investors who do not understand how stocking and distribution work gave the stock a drubbing, and gave us an amazing opportunity. I expect a steady, rapid increase in quarterly royalties during 2011.
At about $1.32 per share, Zalicus has a total market capitalization of only $117.5 million. But it has $46.8 million in cash, no debt, a $242.7 million net operating loss carryforward, and a drug on the market that will show accelerating royalties in every quarter of 2011. Exalgo alone is worth $1.50 a share.
The company also has numerous press releases coming as its various company and partnered clinical programs progress. Assuming someone doesn’t buy it for its cash, tax loss and pipeline (Covidien (COV)? Novartis?), I think ZLCS will end 2011 between $4 and $7 a share, and be one of the top-performing stocks of the year. You may have to wait for the second half of the year for most of the gains, but this is what some call an oxymoron: A low-risk development-stage biotech.
http://seekingalpha.com/article/244395-5-stocks-for-2011?sou…
Die frühere CombinatoRx (CRXX ) hat nach der Vereinigung mit Neuromed neuen Schwung genommen.
Zalicus (ZLCS) is my pick from New World Investor for this 2011 list. It is a development-stage, yet low-risk, virtually unknown biotech stock. Zalicus was formed by the merger of CombinatoRx (formerly CRXX) and Neuromed, a private company. Most of the current management is from Neuromed, and they have refocused the company on drugs for pain and immuno-inflammatory disease.
But there were many drugs in preclinical and clinical trials at CombinatoRx before the merger, and several of them are showing more potential than Neuromed may have expected when they agreed to the merger. At the least, these drugs provide ongoing partnering opportunities. Some have world-class potential for eventual royalties.
The core development programs include Synavive, developed by CombinatoRx as CRx-102, to treat immuno-inflammatory disorders. It has completed Phase II clinical trials in subjects with knee osteoarthritis. The other core area are calcium channel blockers for chronic pain, developed by Neuromed
Partnered development programs include Prednisporin (FOV1101), which is being developed by Fovea, now owned by Sanofi-Aventis (SNY). Prednisporin is a topical ophthalmic drug combining low doses of prednisolone acetate and cyclosporine A, an immunosuppressant. A second major partnership with Novartis (NVS) is working on cancer drugs. Phase 2 results are imminent from a collaboration agreement with PgxHealth, a subsidiary of Clinical Data, to develop ATL313, an adenosine A2A receptor agonist, as a combination therapy against multiple myeloma and certain other B-cell malignancies.
At the time of the merger, Neuromed had Exalgo, a once-a-day, extended release version of Dilaudid, in the FDA approval process for pain. The company received approval on March 1, 2010, and its U. S. marketing partner Mallinkrodt, owned by Covidien, introduced the drug in April. June quarter inventory stocking resulted in $1.1 million royalties to Zalicus. Covidien expects Exalgo eventually to get $250 million to $300 million of the $6 billion long-acting opioid market.
However, as frequently happens with a new drug that is widely introduced, once the distributors have their initial stock, sales fall off for a quarter or two as the sell-through builds. That is what happened in the September quarter, when Zalicus reported only $0.15 million in Exalgo royalties. It will happen again in the December quarter, when I am expecting only $0.4 million in royalties. Investors who do not understand how stocking and distribution work gave the stock a drubbing, and gave us an amazing opportunity. I expect a steady, rapid increase in quarterly royalties during 2011.
At about $1.32 per share, Zalicus has a total market capitalization of only $117.5 million. But it has $46.8 million in cash, no debt, a $242.7 million net operating loss carryforward, and a drug on the market that will show accelerating royalties in every quarter of 2011. Exalgo alone is worth $1.50 a share.
The company also has numerous press releases coming as its various company and partnered clinical programs progress. Assuming someone doesn’t buy it for its cash, tax loss and pipeline (Covidien (COV)? Novartis?), I think ZLCS will end 2011 between $4 and $7 a share, and be one of the top-performing stocks of the year. You may have to wait for the second half of the year for most of the gains, but this is what some call an oxymoron: A low-risk development-stage biotech.

http://seekingalpha.com/article/244395-5-stocks-for-2011?sou…
Antwort auf Beitrag Nr.: 40.835.248 von HeinzBork am 10.01.11 20:25:11* Contract for development in oncology extended by a year
* Zalicus to start mid-stage study on arthritis drug in Q2 * Zalicus shares rise 11 pct
Jan 10 (Reuters) - Zalicus Inc (ZLCS.O) said its oncology research collaborator, Novartis (NOVN.VX), was extending their contract by a year to May 2012, sending its shares up 11 percent.
The companies had signed an initial two-year agreement in May 2009 to develop novel anti-cancer compounds using Zalicus' proprietary screening technology.
As per the original agreement, Zalicus got a $4 million upfront payment and was eligible to receive up to $58 million in milestones.
Zalicus also said it plans to advance its arthritis drug Synavive into mid-stage trials in the second quarter.
The Cambridge, Massachusetts-based company's shares, which have gained half their value in the past six months, rose 11 percent to $2.39, their highest in nearly 18 months, on Monday on Nasdaq.
http://www.reuters.com/article/idUSSGE7090C020110110?feedTyp…
* Zalicus to start mid-stage study on arthritis drug in Q2 * Zalicus shares rise 11 pct
Jan 10 (Reuters) - Zalicus Inc (ZLCS.O) said its oncology research collaborator, Novartis (NOVN.VX), was extending their contract by a year to May 2012, sending its shares up 11 percent.
The companies had signed an initial two-year agreement in May 2009 to develop novel anti-cancer compounds using Zalicus' proprietary screening technology.
As per the original agreement, Zalicus got a $4 million upfront payment and was eligible to receive up to $58 million in milestones.
Zalicus also said it plans to advance its arthritis drug Synavive into mid-stage trials in the second quarter.
The Cambridge, Massachusetts-based company's shares, which have gained half their value in the past six months, rose 11 percent to $2.39, their highest in nearly 18 months, on Monday on Nasdaq.
http://www.reuters.com/article/idUSSGE7090C020110110?feedTyp…
endlich mal wieder jemand da.
Nach dem fulminanten Kursanstieg ist der Abschlag gestern in USA von 15 % wohl nur eine chartechnische Reaktion. Meldungen habe ich keine gefunden.
Ob das nun auch schon Einkaufkurse sind? Ich befürchte, es geht noch bis unter 1,75 $
Nach dem fulminanten Kursanstieg ist der Abschlag gestern in USA von 15 % wohl nur eine chartechnische Reaktion. Meldungen habe ich keine gefunden.
Ob das nun auch schon Einkaufkurse sind? Ich befürchte, es geht noch bis unter 1,75 $
Antwort auf Beitrag Nr.: 40.844.622 von dottore am 12.01.11 09:08:53jo.
Mir scheint, da hat jemand Kasse gemacht, die Umsätze mit dem Rückgang waren schon erheblich.
Zumindestens hat der Kurs nicht weiter nach gegeben, das stimmt hoffnungsvoll.
Mir scheint, da hat jemand Kasse gemacht, die Umsätze mit dem Rückgang waren schon erheblich.
Zumindestens hat der Kurs nicht weiter nach gegeben, das stimmt hoffnungsvoll.
heute ging der Kurs schon bis 1,82 runter. Unter 1,75, vielleicht auch erst bei 1,60 greif ich noch einmal zu.
Bin auf jeden Fall positiv gestimmt.
Bin auf jeden Fall positiv gestimmt.
http://online.barrons.com/article/SB500014240529702043316045…
Interessant der Kommentar (ist zwar nur eine Privatmeinung, aber wer weiss ..)
..)
Interessant der Kommentar (ist zwar nur eine Privatmeinung, aber wer weiss
 ..)
..)
hier mal der ganze Artikel
Barron's: The New Doctor in the House: Consolidation
Barron's Cover
| SATURDAY, JANUARY 29, 2011
The New Doctor in the House: Consolidation
By ANDREW BARY | MORE ARTICLES BY AUTHOR
As big drug firms buy up smaller, speciality outfits and their most innovative products, better pipelines and sales-force efficiency will boost profits.
Most major drug makers have enormous cash flows, meager new-medicine pipelines and huge, underutilized sales forces. Lots of small and mid-sized specialty-pharmaceutical and biotech outfits have promising drugs that they find difficult to produce and sell because of strict government manufacturing rules, high marketing costs, and the reluctance of insurers and the government to pay for new and often high-priced treatments.
Could there be a better scenario for more corporate marriages? And for the buyers to pay a nice premium?
Merck & Co. (ticker: MRK) is the most recent casualty of pipeline fatigue: Disappointing trial results for its heralded anticlotting agent, vorapaxar, prompted Wall Street to write off the drug and punish Merck shares.
The big companies are willing to pay up for their smaller brethren because they can use their own sales forces to sell the drugs and slash marketing and overhead at the target company. Pfizer (PFE), may cut about 40% of the overhead at King Pharmaceuticals after its purchase closes in February. Pfizer is paying about 20 times earnings for King, but sees the deal boosting its own profits. Why? The stand-alone profits of a specialty-drug maker understate its earnings potential after it becomes part of a larger company and its costs drop. Ultralow borrowing costs are another plus for buyers.
We've identified 13 promising specialty-drug companies—nearly all of which have at least one product already approved by the Food and Drug Administration—that could prove enticing to the majors.
The 13 each have a stock-market value below $10 billion, making them digestible for buyers. The list includes Alexion Pharmaceuticals (ALXN), Dendreon (DNDN), Human Genome Sciences (HGSI), Cephalon (CEPH) and United Therapeutics (UTHR).
"We foresee the healthy pace of merger activity continuing in 2011, despite the torrid activity of the past five years," wrote Deutsche Bank analyst David Steinberg in a recent research note. He counted 29 deals in 2010 for $75 billion, involving public and private specialty-drug companies. The average premium to the existing stock price paid for public companies last year was about 40%.
"We'll continue to see takeover activity," says Howard Liang, an analyst with Leerink Swann. "Most of these companies have a single drug on the market because it's so hard to develop any product. From an economic perspective, it makes sense to have a sales force sell more than one product."
He sees particular interest among buyers in companies with approved or promising cancer products. In recent years, MGI Pharmaceuticals, OSI Pharmaceuticals and Millennium Pharmaceuticals, all cancer-drug makers, have been snapped up.
Notable specialty-pharma deals last year included Celgene's (CELG) $3.6 billion purchase of Abraxis Bioscience, Astellas Pharmaceuticals' (ALPMF) $3.8 billion deal for OSI, and Pfizer's $3.9 billion purchase of King. Amgen (AMGN) just agreed to pay up to $1 billion for BioVex, a privately held company with a promising cancer product in the final phase of clinical trials.
Some investors take a basket approach to the specialty-pharma group. There are no exchange-traded funds focused on smaller to mid-sized drug companies. The iShares Nasdaq Biotechnology Index Fund (IBB) is dominated by larger companies like Amgen, Teva Pharmaceuticals (TEVA) and Celgene. The $250 million H&Q Life Science Investors Fund (HQL), a closed-end, has some exposure to specialty-drug companies and trades around 11, a 10% discount to its net-asset value.
THE SECTOR IS RISKY for several reasons: Its members' price/earnings ratios generally are high or nonexistent because they're losing money. Takeovers probably will involve only a fraction of sectors. Investor optimism often jumps when a drug gets FDA approval–only to decline if initial sales are disappointing. There also can be controversy about whether an approved drug is particularly effective, or worth its price.
New drugs often target rare conditions and are very expensive, prompting resistance from insurers and other payers. Alexion's Soliris, aimed at a rare blood condition called PNH (paroxysmal nocturnal hemoglobinuria), can cost more than $350,000 a year, making it among the most expensive drugs on the market. Cancer treatments like Dendreon's Provenge can run nearly $100,000 per patient.
Liang says that insurers and the government generally will pay for approved cancer drugs that serve "high unmet needs" in patients with advanced cancer that has spread from the original location, even if treatments extend survival by only months. Large pharmaceutical companies used to be wary of buying companies with super-high-priced drugs because of potentially adverse publicity about gouging the sick. But that reluctance is gone. Sanofi-Aventis (SNY) wants to buy Genzyme (GENZ), whose leading drug, for the rare Gaucher disease, in which a missing enzyme threatens organ function, can cost $500,000 a year.
Another problem: Short sellers can bedevil managements of small-drug producers by trying to publicize or bring to regulatory attention any adverse reactions to new drugs. "With one-product companies, this is a real danger," says a long-time drug investor. "The shorts can destroy these companies. They can't do that to Pfizer, because a single drug doesn't matter much to Pfizer." This can make managements more willing to sell.
Still, it's less dangerous playing smaller outfits with approved drugs than those with treatments still awaiting an okay from an increasingly demanding FDA. That's why investors attracted to the sector probably should own a mix of large and smaller companies. The giants–Pfizer, Merck, Eli Lilly (LLY) Amgen, GlaxoSmithKline (GSK)– are out of favor, and trade cheaply, for an average of just 10 times 2011 profits.
JPMORGAN BIOTECH analysts like companies that haven't partnered with larger drug makers—and thus haven't given away much of the profits of their new products—and those with significant room to boost sales via global distribution or through more effective marketing programs. These include Acorda Therapeutics (ACOR), AMAG Pharmaceuticals (AMAG), Dendreon, Human Genome Sciences and United Therapeutics.
Worldwide annual sales of Dendreon's prostate-cancer drug, Provenge, and Alexion's Soliris could top $1 billion in a few years. Those of Acorda's multiple-sclerosis product, Ampyra, could hit $1 billion.
Analyst Liang says that Provenge amounts to personalized medicine. It's designed to boost a patient's immune system to battle advanced prostate cancer. Immune cells are collected from the blood, treated with Provenge, then put back into the patient. It doesn't have the toxic effects of chemotherapy, but extends the survival of patients by four to 25 months, versus those that get standard treatments. Liang says this "incremental improvement" is the nature of most treatments for advanced cancer.
Dendreon shares, at 35, are down from an May 2010 high of 55–reached around the time the drug was approved. Provenge sales last year were under $50 million, and the Street wonders whether they can ultimately top $1 billion annually. A bullish Liang carries a 55 target for Dendreon, and sees Provenge sales potentially hitting $1.5 billion in 2014.
Acorda also has fallen since its MS drug, Ampyra, was approved last year. It now trades around 23, well below its peak of 40. There's debate about Ampyra's sales potential, with bears pointing to adverse side effects and a potential 2017 patent expiration. JPMorgan analysts are bullish on Ampyra. They have a 48 price target on Acorda, and note that Ampyra sales topped $50 million in the third quarter.
History Lesson
These deals, done at varying multiples of the target's annual revenue, hint at what buyers will now pay for drug outfits.
Alexion has been one of the sector's big winners over the past year, rising 86%, to 85, on hopes for Soliris, whose sales rose 40%, to $384 million, in 2010's first nine months. Soliris has been approved for treatment of PNH, a rare and serious blood disease, and investors are hoping that Alexion gets approval to use the drug for another potentially fatal condition known as aHUS, in which small blood clots form in the body and often destroy kidney function.
The stock is richly priced at 36 times projected 2011 profits, but Liang is still bullish on it, arguing that Alexion has high operating leverage, strong profit growth and acquisition appeal.
Cephalon is a value stock, trading at 61, just seven times projected 2011 earnings. The big concern is the 2012 patent expiration of Provigil, a treatment for narcolepsy, or involuntary sleep during the day, as well as among night-shift workers. Provigil generates $1 billion in annual sales, or a third of its parent's revenue. The bull case on Cephalon is that it will get past the patent problem without a huge hit to profits, and that its valuation anticipates a sharp profit drop.
AMAG IS A VALUE STOCK, too. As of Sept. 30, its cash came to $274 million, or 13 a share. That's just four bucks above the 17 it recently was fetching, down from a peak of 51. The stock has been crushed by disappointing sales of Feraheme, an injectable iron supplement, primarily given to people with chronic kidney disease.
AMAG Pharmaceutical's 2011 sales guidance for Feraheme of $55 million to $60 million was way below the consensus estimate. The company is burning cash because it has a modest revenue base but high expenses. It's expected to lose more than $4 a share in 2011. It aims to broaden the patient population for Feraheme. JPMorgan analyst Geoffrey Meacham is bullish, arguing in a client note that expectations now are very low.
Cadence is a play on Ofirmev, an injectable form of acetaminophen, the active ingredient in Tylenol. It's designed for use by hospitals to supplement or replace morphine for patients in acute pain, often after surgery.
Cadence shares, at around 7, are down 23% in the past year–giving it a stock-market value of just $474 million—as investors wonder about Ofirmev's commercial prospects. The product has advantages over morphine. It isn't addictive, doesn't have narcotics' side-effects and costs only about $10 a dose.
Deutsche Bank's Steinberg is upbeat on Ofirmev, writing in a client note that annual U.S. sales could hit more than $400 million, although it will take time to win over hospitals. He carries a 12 price target, and writes that Cadence could attract a takeover, given the strong interest in companies with pain therapies.
United Therapeutics has two related drugs–Remodulin and Tyvaso—designed to treat pulmonary arterial hypertension, or PAH, a serious condition involving high blood pressure in the pulmonary arteries, which connect the heart to the lungs. Sales and profits of these expensive pharmaceuticals, which can cost more than $100,000 per patient, have risen sharply. The stock, however, is up only a modest 18% in the past year, to 68. Sales of Remodulin and Tyvaso may reach $715 million this year, up from an estimated $575 million in 2010. Profits could hit $3.50 a share, versus 2010's estimated $2.25.
JPMorgan's Meacham carries a 75 price target on United Therapeutics, arguing that it could get a big lift if it can get approval for an oral form of Remodulin—it currently is taken intravenously, via injection or in an inhaled form—because patients prefer pills.
The whole specialty-pharmaceutical area offers plenty of companies like United Therapeutics that boast novel and effective drugs with sharply rising sales. That's just what big drug companies need, and are increasingly willing to buy.
Barron's: The New Doctor in the House: Consolidation
Barron's Cover
| SATURDAY, JANUARY 29, 2011
The New Doctor in the House: Consolidation
By ANDREW BARY | MORE ARTICLES BY AUTHOR
As big drug firms buy up smaller, speciality outfits and their most innovative products, better pipelines and sales-force efficiency will boost profits.
Most major drug makers have enormous cash flows, meager new-medicine pipelines and huge, underutilized sales forces. Lots of small and mid-sized specialty-pharmaceutical and biotech outfits have promising drugs that they find difficult to produce and sell because of strict government manufacturing rules, high marketing costs, and the reluctance of insurers and the government to pay for new and often high-priced treatments.
Could there be a better scenario for more corporate marriages? And for the buyers to pay a nice premium?
Merck & Co. (ticker: MRK) is the most recent casualty of pipeline fatigue: Disappointing trial results for its heralded anticlotting agent, vorapaxar, prompted Wall Street to write off the drug and punish Merck shares.
The big companies are willing to pay up for their smaller brethren because they can use their own sales forces to sell the drugs and slash marketing and overhead at the target company. Pfizer (PFE), may cut about 40% of the overhead at King Pharmaceuticals after its purchase closes in February. Pfizer is paying about 20 times earnings for King, but sees the deal boosting its own profits. Why? The stand-alone profits of a specialty-drug maker understate its earnings potential after it becomes part of a larger company and its costs drop. Ultralow borrowing costs are another plus for buyers.
We've identified 13 promising specialty-drug companies—nearly all of which have at least one product already approved by the Food and Drug Administration—that could prove enticing to the majors.
The 13 each have a stock-market value below $10 billion, making them digestible for buyers. The list includes Alexion Pharmaceuticals (ALXN), Dendreon (DNDN), Human Genome Sciences (HGSI), Cephalon (CEPH) and United Therapeutics (UTHR).
"We foresee the healthy pace of merger activity continuing in 2011, despite the torrid activity of the past five years," wrote Deutsche Bank analyst David Steinberg in a recent research note. He counted 29 deals in 2010 for $75 billion, involving public and private specialty-drug companies. The average premium to the existing stock price paid for public companies last year was about 40%.
"We'll continue to see takeover activity," says Howard Liang, an analyst with Leerink Swann. "Most of these companies have a single drug on the market because it's so hard to develop any product. From an economic perspective, it makes sense to have a sales force sell more than one product."
He sees particular interest among buyers in companies with approved or promising cancer products. In recent years, MGI Pharmaceuticals, OSI Pharmaceuticals and Millennium Pharmaceuticals, all cancer-drug makers, have been snapped up.
Notable specialty-pharma deals last year included Celgene's (CELG) $3.6 billion purchase of Abraxis Bioscience, Astellas Pharmaceuticals' (ALPMF) $3.8 billion deal for OSI, and Pfizer's $3.9 billion purchase of King. Amgen (AMGN) just agreed to pay up to $1 billion for BioVex, a privately held company with a promising cancer product in the final phase of clinical trials.
Some investors take a basket approach to the specialty-pharma group. There are no exchange-traded funds focused on smaller to mid-sized drug companies. The iShares Nasdaq Biotechnology Index Fund (IBB) is dominated by larger companies like Amgen, Teva Pharmaceuticals (TEVA) and Celgene. The $250 million H&Q Life Science Investors Fund (HQL), a closed-end, has some exposure to specialty-drug companies and trades around 11, a 10% discount to its net-asset value.
THE SECTOR IS RISKY for several reasons: Its members' price/earnings ratios generally are high or nonexistent because they're losing money. Takeovers probably will involve only a fraction of sectors. Investor optimism often jumps when a drug gets FDA approval–only to decline if initial sales are disappointing. There also can be controversy about whether an approved drug is particularly effective, or worth its price.
New drugs often target rare conditions and are very expensive, prompting resistance from insurers and other payers. Alexion's Soliris, aimed at a rare blood condition called PNH (paroxysmal nocturnal hemoglobinuria), can cost more than $350,000 a year, making it among the most expensive drugs on the market. Cancer treatments like Dendreon's Provenge can run nearly $100,000 per patient.
Liang says that insurers and the government generally will pay for approved cancer drugs that serve "high unmet needs" in patients with advanced cancer that has spread from the original location, even if treatments extend survival by only months. Large pharmaceutical companies used to be wary of buying companies with super-high-priced drugs because of potentially adverse publicity about gouging the sick. But that reluctance is gone. Sanofi-Aventis (SNY) wants to buy Genzyme (GENZ), whose leading drug, for the rare Gaucher disease, in which a missing enzyme threatens organ function, can cost $500,000 a year.
Another problem: Short sellers can bedevil managements of small-drug producers by trying to publicize or bring to regulatory attention any adverse reactions to new drugs. "With one-product companies, this is a real danger," says a long-time drug investor. "The shorts can destroy these companies. They can't do that to Pfizer, because a single drug doesn't matter much to Pfizer." This can make managements more willing to sell.
Still, it's less dangerous playing smaller outfits with approved drugs than those with treatments still awaiting an okay from an increasingly demanding FDA. That's why investors attracted to the sector probably should own a mix of large and smaller companies. The giants–Pfizer, Merck, Eli Lilly (LLY) Amgen, GlaxoSmithKline (GSK)– are out of favor, and trade cheaply, for an average of just 10 times 2011 profits.
JPMORGAN BIOTECH analysts like companies that haven't partnered with larger drug makers—and thus haven't given away much of the profits of their new products—and those with significant room to boost sales via global distribution or through more effective marketing programs. These include Acorda Therapeutics (ACOR), AMAG Pharmaceuticals (AMAG), Dendreon, Human Genome Sciences and United Therapeutics.
Worldwide annual sales of Dendreon's prostate-cancer drug, Provenge, and Alexion's Soliris could top $1 billion in a few years. Those of Acorda's multiple-sclerosis product, Ampyra, could hit $1 billion.
Analyst Liang says that Provenge amounts to personalized medicine. It's designed to boost a patient's immune system to battle advanced prostate cancer. Immune cells are collected from the blood, treated with Provenge, then put back into the patient. It doesn't have the toxic effects of chemotherapy, but extends the survival of patients by four to 25 months, versus those that get standard treatments. Liang says this "incremental improvement" is the nature of most treatments for advanced cancer.
Dendreon shares, at 35, are down from an May 2010 high of 55–reached around the time the drug was approved. Provenge sales last year were under $50 million, and the Street wonders whether they can ultimately top $1 billion annually. A bullish Liang carries a 55 target for Dendreon, and sees Provenge sales potentially hitting $1.5 billion in 2014.
Acorda also has fallen since its MS drug, Ampyra, was approved last year. It now trades around 23, well below its peak of 40. There's debate about Ampyra's sales potential, with bears pointing to adverse side effects and a potential 2017 patent expiration. JPMorgan analysts are bullish on Ampyra. They have a 48 price target on Acorda, and note that Ampyra sales topped $50 million in the third quarter.
History Lesson
These deals, done at varying multiples of the target's annual revenue, hint at what buyers will now pay for drug outfits.
Alexion has been one of the sector's big winners over the past year, rising 86%, to 85, on hopes for Soliris, whose sales rose 40%, to $384 million, in 2010's first nine months. Soliris has been approved for treatment of PNH, a rare and serious blood disease, and investors are hoping that Alexion gets approval to use the drug for another potentially fatal condition known as aHUS, in which small blood clots form in the body and often destroy kidney function.
The stock is richly priced at 36 times projected 2011 profits, but Liang is still bullish on it, arguing that Alexion has high operating leverage, strong profit growth and acquisition appeal.
Cephalon is a value stock, trading at 61, just seven times projected 2011 earnings. The big concern is the 2012 patent expiration of Provigil, a treatment for narcolepsy, or involuntary sleep during the day, as well as among night-shift workers. Provigil generates $1 billion in annual sales, or a third of its parent's revenue. The bull case on Cephalon is that it will get past the patent problem without a huge hit to profits, and that its valuation anticipates a sharp profit drop.
AMAG IS A VALUE STOCK, too. As of Sept. 30, its cash came to $274 million, or 13 a share. That's just four bucks above the 17 it recently was fetching, down from a peak of 51. The stock has been crushed by disappointing sales of Feraheme, an injectable iron supplement, primarily given to people with chronic kidney disease.
AMAG Pharmaceutical's 2011 sales guidance for Feraheme of $55 million to $60 million was way below the consensus estimate. The company is burning cash because it has a modest revenue base but high expenses. It's expected to lose more than $4 a share in 2011. It aims to broaden the patient population for Feraheme. JPMorgan analyst Geoffrey Meacham is bullish, arguing in a client note that expectations now are very low.
Cadence is a play on Ofirmev, an injectable form of acetaminophen, the active ingredient in Tylenol. It's designed for use by hospitals to supplement or replace morphine for patients in acute pain, often after surgery.
Cadence shares, at around 7, are down 23% in the past year–giving it a stock-market value of just $474 million—as investors wonder about Ofirmev's commercial prospects. The product has advantages over morphine. It isn't addictive, doesn't have narcotics' side-effects and costs only about $10 a dose.
Deutsche Bank's Steinberg is upbeat on Ofirmev, writing in a client note that annual U.S. sales could hit more than $400 million, although it will take time to win over hospitals. He carries a 12 price target, and writes that Cadence could attract a takeover, given the strong interest in companies with pain therapies.
United Therapeutics has two related drugs–Remodulin and Tyvaso—designed to treat pulmonary arterial hypertension, or PAH, a serious condition involving high blood pressure in the pulmonary arteries, which connect the heart to the lungs. Sales and profits of these expensive pharmaceuticals, which can cost more than $100,000 per patient, have risen sharply. The stock, however, is up only a modest 18% in the past year, to 68. Sales of Remodulin and Tyvaso may reach $715 million this year, up from an estimated $575 million in 2010. Profits could hit $3.50 a share, versus 2010's estimated $2.25.
JPMorgan's Meacham carries a 75 price target on United Therapeutics, arguing that it could get a big lift if it can get approval for an oral form of Remodulin—it currently is taken intravenously, via injection or in an inhaled form—because patients prefer pills.
The whole specialty-pharmaceutical area offers plenty of companies like United Therapeutics that boast novel and effective drugs with sharply rising sales. That's just what big drug companies need, and are increasingly willing to buy.
guter tag heute!
erneuter ausbruch, volumen kommt anscheinend wieder, ohne news...
interesse scheint also nach wie vor vorhanden zu sein.
bin gespannt wie weit es diesmal läuft!
ps: danke heinz, freut mich, dass es wieder nen thread gibt!
lg
http://www.nasdaq.com/aspx/chartingbasics.aspx?intraday=off&…
erneuter ausbruch, volumen kommt anscheinend wieder, ohne news...
interesse scheint also nach wie vor vorhanden zu sein.
bin gespannt wie weit es diesmal läuft!
ps: danke heinz, freut mich, dass es wieder nen thread gibt!
lg
http://www.nasdaq.com/aspx/chartingbasics.aspx?intraday=off&…
Wird erneut an Höhe gewinnen.
Erfolgversprechendes Programm und breite Unterstützung durch Big pharma Novartis, die ZLCS einmal übernehmen wird.
Erfolgversprechendes Programm und breite Unterstützung durch Big pharma Novartis, die ZLCS einmal übernehmen wird.
Lesen und staunen...(Heute 1.59€, in 18 Monate auf 10 glatt) http://img.wallstreet-online.de/smilies/eek.gif
Zalicus Climbs on New Price Target, Market Waking Up to Company's Value?
Early Friday morning, JMP Securities issued a "Market Outperform" rating for Zalicus Inc. (ZLCS) and a "target" of $5 per share. Shares jumped 5%-6% on the JMP Securities news following the earlier week's news that Sanofi-Aventis (SNY) featured Zalicus's lead ophthalmology product candidate Prednisporin (FOV1101).
This is a very significant announcement given Zacks' last year target was issued at $3 per share by keen analyst Jason Napodano. In November 2010, Mr. Napodano stated: "Adding in the existing cash on hand totaling $48.6 million as of September 30, 2010, and our sum of parts / NPV analysis for Zalicus yields a total firm value of roughly $260 million, or $3 per share. At the current value of only $105 million we believe the company is far under-valued." It was also observed that in the same quarter of 2010, Jeremy Richards, Manager/Director of Private Wealth Fund, stated: "I believe investors buying the stock under $2 is an exceptional bargain." For those who follow Zalicus, between Q4 2010 and Q1 2011, there was a noticeable jump in daily volume from approximately 500-700,000 traded to 1,879,820 average volume (3M) as of Friday.
It is well-known that Zalicus has been raising an additional $20 million (versus common shares) via Wedbush. The company reported $46 million as of December 2010. On the company's conference call (9 March 2011), Zalicus's CFO Justin Renz reported that the company was over half-way to its goal. I have been told by those close to the firm that the company is aiming to complete all fund raising by the end of this month. If you add together Exalgo's growing royalty figures ($400,000 in Q4 2010) - my projection is $800,000 in Q1 2011, plus the $3,000,000 that Novartis paid to extend its cHTS contract, plus general cHTS contracts (estimate $1,000,000), plus the $20,000,000 fund-raising via Wedbush, Zalicus is going to report a very healthy financial upside (60-70 million dollars) going forward.
Shares traded on the day to a high close of $2.29 per share. Significantly, the company's market cap has exceeded $200 million, and JMP Securities’ new target of $5 per share puts the company in the $450-$500 million corporate value range. However, in my own analysis of Zalicus’ corporate assets, I arrive at a much higher estimate: $1.2 billion, or $13 per share. I base this on two keys points: (1) The internal assets for Zalicus' proprietary cHTS (Cambridge MA location) and electrophysiology screening platform (Vancouver B.C. location) are worth at least $500 million if you study their asset class within other pharmaceutical firms (due diligence I have done). Remember, cHTS is a revenue-generating technology, and (2) the pipeline assets for Zalicus are just beginning to be understood by market analysts. For example, only one ion channel drug candidate (NMED-160) was already worth $450 million to Merck (MRK) just a few years ago. Today, Zalicus has rolled out three ion channel programs with a virtual "army" (a word CEO Corrigan used) of drug candidates in each ion channel program. Furthermore, Zalicus has the Prednisporin license with Sanofi-Aventis, the Exalgo license with Covidien (COV), and Synavive is wholly owned by the company.
Zalicus Climbs on New Price Target, Market Waking Up to Company's Value?
Early Friday morning, JMP Securities issued a "Market Outperform" rating for Zalicus Inc. (ZLCS) and a "target" of $5 per share. Shares jumped 5%-6% on the JMP Securities news following the earlier week's news that Sanofi-Aventis (SNY) featured Zalicus's lead ophthalmology product candidate Prednisporin (FOV1101).
This is a very significant announcement given Zacks' last year target was issued at $3 per share by keen analyst Jason Napodano. In November 2010, Mr. Napodano stated: "Adding in the existing cash on hand totaling $48.6 million as of September 30, 2010, and our sum of parts / NPV analysis for Zalicus yields a total firm value of roughly $260 million, or $3 per share. At the current value of only $105 million we believe the company is far under-valued." It was also observed that in the same quarter of 2010, Jeremy Richards, Manager/Director of Private Wealth Fund, stated: "I believe investors buying the stock under $2 is an exceptional bargain." For those who follow Zalicus, between Q4 2010 and Q1 2011, there was a noticeable jump in daily volume from approximately 500-700,000 traded to 1,879,820 average volume (3M) as of Friday.
It is well-known that Zalicus has been raising an additional $20 million (versus common shares) via Wedbush. The company reported $46 million as of December 2010. On the company's conference call (9 March 2011), Zalicus's CFO Justin Renz reported that the company was over half-way to its goal. I have been told by those close to the firm that the company is aiming to complete all fund raising by the end of this month. If you add together Exalgo's growing royalty figures ($400,000 in Q4 2010) - my projection is $800,000 in Q1 2011, plus the $3,000,000 that Novartis paid to extend its cHTS contract, plus general cHTS contracts (estimate $1,000,000), plus the $20,000,000 fund-raising via Wedbush, Zalicus is going to report a very healthy financial upside (60-70 million dollars) going forward.
Shares traded on the day to a high close of $2.29 per share. Significantly, the company's market cap has exceeded $200 million, and JMP Securities’ new target of $5 per share puts the company in the $450-$500 million corporate value range. However, in my own analysis of Zalicus’ corporate assets, I arrive at a much higher estimate: $1.2 billion, or $13 per share. I base this on two keys points: (1) The internal assets for Zalicus' proprietary cHTS (Cambridge MA location) and electrophysiology screening platform (Vancouver B.C. location) are worth at least $500 million if you study their asset class within other pharmaceutical firms (due diligence I have done). Remember, cHTS is a revenue-generating technology, and (2) the pipeline assets for Zalicus are just beginning to be understood by market analysts. For example, only one ion channel drug candidate (NMED-160) was already worth $450 million to Merck (MRK) just a few years ago. Today, Zalicus has rolled out three ion channel programs with a virtual "army" (a word CEO Corrigan used) of drug candidates in each ion channel program. Furthermore, Zalicus has the Prednisporin license with Sanofi-Aventis, the Exalgo license with Covidien (COV), and Synavive is wholly owned by the company.
Antwort auf Beitrag Nr.: 41.283.740 von Growth2012 am 29.03.11 16:48:18auch sehr gut rechechiert und lesenswert
"Zalicus: Expanding Drug Pipeline Helps Make It a Strong Buy "
http://seekingalpha.com/article/258498-zalicus-expanding-dru…
Beste Smallcap Biotech 2011-2012?
"Zalicus: Expanding Drug Pipeline Helps Make It a Strong Buy "
http://seekingalpha.com/article/258498-zalicus-expanding-dru…
Beste Smallcap Biotech 2011-2012?

Antwort auf Beitrag Nr.: 41.283.866 von Growth2012 am 29.03.11 17:04:38Es wird immer besser
Zalicus Granted Composition-of-Matter Patent for Synavive
http://finance.yahoo.com/news/Zalicus-Granted-bw-2901826003.…
"These two key patents provide the foundation of a solid and enforceable intellectual property estate for Synavive, with issued claims through 2025, and pending applications around our proprietary formulations providing coverage into 2028” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus.
Der Zug rollt nun so gaaaannnzzz langsam aus dem Bahnhof raus, errinert mich sehr an einem frühen DNDN


Zalicus Granted Composition-of-Matter Patent for Synavive
http://finance.yahoo.com/news/Zalicus-Granted-bw-2901826003.…
"These two key patents provide the foundation of a solid and enforceable intellectual property estate for Synavive, with issued claims through 2025, and pending applications around our proprietary formulations providing coverage into 2028” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus.
Der Zug rollt nun so gaaaannnzzz langsam aus dem Bahnhof raus, errinert mich sehr an einem frühen DNDN


Antwort auf Beitrag Nr.: 41.288.780 von Growth2012 am 30.03.11 12:49:16Ein relativ bekannte "Pusherbrief" hat folgendes zu Zalicus geschrieben vor kürzem...
Ein Biotech-Unternehmen ist für uns einer der Gewinner für das Jahr 2011.
Hervorgegangen aus der Verschmelzung von CombuinatoRx, ehemals CRXX, und der Privatfirma Neuromed, hat man sich auf die Forschung von Schmerzmitteln und Medikamenten gegen Autoimmunerkrankungen spezialisiert.
Lesen Sie den Newsletter ganz bis zum Ende, dort haben wir noch eine wichtige Nachricht für Sie!
Bereits vor dem Firmenzusammenschluss gab es eine Reihe von Wirkstoffen, die sich schon in der Phase II oder III befanden. Daneben hat man sich zur Aufgabe gemacht, an Wirkstoffen speziell für Entzündungen der Kniegelenke zu arbeiten, die sogar bis in den Bereich von Knorpelersatzlösungen hineinreichen.
Aufgrund der immer älter werdenden Bevölkerung ist das ein sehr zukunftsträchtiger Geschäftszweig.
Mit dem Pharma-Riesen Sanofi-Aventis ist man diverse Forschungspartnerschaften eingegangen, aus denen zum Beispiel der Wirkstoff Prednisporin hervorgegangen ist. Ein Medikament, welches vornehmlich bei schweren Bindegewebsverletzungen des Auges seine Anwendung findet.
Dieser Wirkstoff bringt Blockbuster-Charakter mit sich.
Eine weitere wichtige Kooperation ist man mit Novartis eingegangen, in der man gemeinsam an Wirkstoffen gegen Krebserkrankungen forscht. Zusätzlich hat man vier andere völlig unterschiedliche Wirkstoffe in der Pipeline, die schon bald anstehen.
Im März letzten Jahres hat man mit Mallinkrodt, das vollständig Covidien gehört, ein erfahrenes Marketing-Unternehmen für sich gewinnen können. Unser Hauptaugenmerk liegt jedoch speziell auf den Wirkstoff Exalgo gerichtet, der bereits während langfristigen Schmerztherapien bei Krebspatienten seine praktische Anwendung findet.
Wenn Sie nun berücksichtigen, dass alleine Exalgo einen Gewinn von rund 1,50 US-Dollar je Aktie ausmacht, wird schnell klar, welches Kurspotenzial in der Aktie unseres Biotech-Favoriten schlummert.
Gänzlich schuldenfrei und Quartal für Quartal mit immer wiederkehrendem Umsatz und Gewinn alleine aus diesem einen Wirkstoff, sehen wir für den Favoriten noch in diesem Jahr Potenzial bis in den Bereich bis zu 7 US-Dollar.
Sie erwartet also, bei einem aktuellen Kurs knapp oberhalb von 2 US-Dollar, ein Kursanstieg von 100 bis 250%.
Übrigens dieses wurde gepostet ohne jegliche weitere Wertung meinerseits


Ein Biotech-Unternehmen ist für uns einer der Gewinner für das Jahr 2011.
Hervorgegangen aus der Verschmelzung von CombuinatoRx, ehemals CRXX, und der Privatfirma Neuromed, hat man sich auf die Forschung von Schmerzmitteln und Medikamenten gegen Autoimmunerkrankungen spezialisiert.
Lesen Sie den Newsletter ganz bis zum Ende, dort haben wir noch eine wichtige Nachricht für Sie!
Bereits vor dem Firmenzusammenschluss gab es eine Reihe von Wirkstoffen, die sich schon in der Phase II oder III befanden. Daneben hat man sich zur Aufgabe gemacht, an Wirkstoffen speziell für Entzündungen der Kniegelenke zu arbeiten, die sogar bis in den Bereich von Knorpelersatzlösungen hineinreichen.
Aufgrund der immer älter werdenden Bevölkerung ist das ein sehr zukunftsträchtiger Geschäftszweig.
Mit dem Pharma-Riesen Sanofi-Aventis ist man diverse Forschungspartnerschaften eingegangen, aus denen zum Beispiel der Wirkstoff Prednisporin hervorgegangen ist. Ein Medikament, welches vornehmlich bei schweren Bindegewebsverletzungen des Auges seine Anwendung findet.
Dieser Wirkstoff bringt Blockbuster-Charakter mit sich.
Eine weitere wichtige Kooperation ist man mit Novartis eingegangen, in der man gemeinsam an Wirkstoffen gegen Krebserkrankungen forscht. Zusätzlich hat man vier andere völlig unterschiedliche Wirkstoffe in der Pipeline, die schon bald anstehen.
Im März letzten Jahres hat man mit Mallinkrodt, das vollständig Covidien gehört, ein erfahrenes Marketing-Unternehmen für sich gewinnen können. Unser Hauptaugenmerk liegt jedoch speziell auf den Wirkstoff Exalgo gerichtet, der bereits während langfristigen Schmerztherapien bei Krebspatienten seine praktische Anwendung findet.
Wenn Sie nun berücksichtigen, dass alleine Exalgo einen Gewinn von rund 1,50 US-Dollar je Aktie ausmacht, wird schnell klar, welches Kurspotenzial in der Aktie unseres Biotech-Favoriten schlummert.
Gänzlich schuldenfrei und Quartal für Quartal mit immer wiederkehrendem Umsatz und Gewinn alleine aus diesem einen Wirkstoff, sehen wir für den Favoriten noch in diesem Jahr Potenzial bis in den Bereich bis zu 7 US-Dollar.
Sie erwartet also, bei einem aktuellen Kurs knapp oberhalb von 2 US-Dollar, ein Kursanstieg von 100 bis 250%.
Übrigens dieses wurde gepostet ohne jegliche weitere Wertung meinerseits


Antwort auf Beitrag Nr.: 41.310.048 von Growth2012 am 03.04.11 15:41:24Michael Murphy spricht für ZLCS (Sein Wort trägt 'Gewicht' Stateside)
#1 Best Stock – Zalicus
Biotech small cap Zalicus (NASDAQ: ZLCS) is our runaway winner, with a whopping 54% return year-to-date. Michael Murphy, an independent technology investment adviser and InvestorPlace.com contributor, initially described this stock as a “low-risk, development-phase biotech,” and the profits have been astounding. The only question is whether the pick can keep this momentum up. According to Murphy, the answer is yes. Read why he thinks Zalicus stock is a buy in his most recent update.
Naja, mal sehen sagt der Blinde

#1 Best Stock – Zalicus
Biotech small cap Zalicus (NASDAQ: ZLCS) is our runaway winner, with a whopping 54% return year-to-date. Michael Murphy, an independent technology investment adviser and InvestorPlace.com contributor, initially described this stock as a “low-risk, development-phase biotech,” and the profits have been astounding. The only question is whether the pick can keep this momentum up. According to Murphy, the answer is yes. Read why he thinks Zalicus stock is a buy in his most recent update.
Naja, mal sehen sagt der Blinde


Antwort auf Beitrag Nr.: 41.314.370 von Growth2012 am 04.04.11 15:22:44>>>Michael Murphy said Synavive was patent protect "until 2028" and Dr. Corrigan is on record stating that Synavive could potentially bring in $300 to $400 million per year once marketed. The indication and market sector bear this estimate out.<<<
Dedicated to pain and immuno-inflammatory drug development, Zalicus' new drugs are identified as Z160, Z212, and Z944. In the world of pain research, Zalicus is arguably among the leaders in what is called ion channel research. In simple terms, Zalicus is seeking to develop oral medications that target pain signaling in the human body. If successful, Zalicus's "Z" drugs would compete in a 20-30 billion dollar market, and particularly, against lucrative opioid drugs that are known to have serious side effects, including addiction.
20-30 billion dollar market, and particularly, against lucrative opioid drugs that are known to have serious side effects, including addiction.
I. Z160
Z160 is identified by Zalicus CEO Mark H. N. Corrigan as a reformulated version of prior failed version NMED-160. Without getting lost in the science, NMED-160 reached phase II clinical studies which led to a highly publicized collaboration with Merck (MRK) in 2006 for approximately 475 million dollars (milestones inclusive). But NMED-160 hit a show-stopper in clinical development because its bioavailability (i.e. the percentage of how much of the drug gets into the body's circulation system) failed to achieve an acceptable target. As a result, in 2009, Merck cancelled its collaboration with then Neuromed Pharmaceuticals (today Zalicus Pharmaceuticals Canada, a subsidiary of Zalicus Inc. USA). However, according to the company's CEO, the bioavailability problem appears resolved, so Z160 is being targeted to start phase I clinical studies assuming it passes all toxicology studies.
II. Z212
Recently, Zalicus announced Z212, including the publication of scholarly research. The Z212 introduction came as a surprise because it is known as a sodium channel modulator while up until recently on the Zalicus website, it was thought that Zalicus was primarily focused on calcium channel modulators. The intrigue here is that Z212 is compared with an already marketed drug called gabapentin. In early preclinical research, the data suggests that Z212 may have similar pain-reducing characteristics as gabapentin. Shareholders also learned from the report that Zalicus scientists have discovered a way to modify their drug compounds to avoid unacceptable cardiac side affects (hERG). Z212 data appears to avoid this challenge that has long plagued ion channel research.
III. Z944
This brings us to Z944, which is known as a calcium T-type ion channel modulator/blocker. According to Zalicus, Z944 should be the first drug candidate entering phase I clinical studies. The value to investors is that Z944 is representative of the company's advance in pain research. In fact, Zalicus claims to have at least 50 other drug candidates in the discovery - preclinical pipeline.
....


Dedicated to pain and immuno-inflammatory drug development, Zalicus' new drugs are identified as Z160, Z212, and Z944. In the world of pain research, Zalicus is arguably among the leaders in what is called ion channel research. In simple terms, Zalicus is seeking to develop oral medications that target pain signaling in the human body. If successful, Zalicus's "Z" drugs would compete in a
 20-30 billion dollar market, and particularly, against lucrative opioid drugs that are known to have serious side effects, including addiction.
20-30 billion dollar market, and particularly, against lucrative opioid drugs that are known to have serious side effects, including addiction.I. Z160
Z160 is identified by Zalicus CEO Mark H. N. Corrigan as a reformulated version of prior failed version NMED-160. Without getting lost in the science, NMED-160 reached phase II clinical studies which led to a highly publicized collaboration with Merck (MRK) in 2006 for approximately 475 million dollars (milestones inclusive). But NMED-160 hit a show-stopper in clinical development because its bioavailability (i.e. the percentage of how much of the drug gets into the body's circulation system) failed to achieve an acceptable target. As a result, in 2009, Merck cancelled its collaboration with then Neuromed Pharmaceuticals (today Zalicus Pharmaceuticals Canada, a subsidiary of Zalicus Inc. USA). However, according to the company's CEO, the bioavailability problem appears resolved, so Z160 is being targeted to start phase I clinical studies assuming it passes all toxicology studies.
II. Z212
Recently, Zalicus announced Z212, including the publication of scholarly research. The Z212 introduction came as a surprise because it is known as a sodium channel modulator while up until recently on the Zalicus website, it was thought that Zalicus was primarily focused on calcium channel modulators. The intrigue here is that Z212 is compared with an already marketed drug called gabapentin. In early preclinical research, the data suggests that Z212 may have similar pain-reducing characteristics as gabapentin. Shareholders also learned from the report that Zalicus scientists have discovered a way to modify their drug compounds to avoid unacceptable cardiac side affects (hERG). Z212 data appears to avoid this challenge that has long plagued ion channel research.
III. Z944
This brings us to Z944, which is known as a calcium T-type ion channel modulator/blocker. According to Zalicus, Z944 should be the first drug candidate entering phase I clinical studies. The value to investors is that Z944 is representative of the company's advance in pain research. In fact, Zalicus claims to have at least 50 other drug candidates in the discovery - preclinical pipeline.
....



Antwort auf Beitrag Nr.: 41.345.210 von Growth2012 am 10.04.11 10:12:34Lesenswert von seeking Alpha Stateside...


Major Events for Zalicus Loom Large in Q2
Zalicus in Q1
In Q1 2011, Zalicus (ZLCS) announced a number of remarkable achievements:
* It rolled out its three-prong ion channel program(s) including: a substantial scientific report on sodium channel 1.7 Z212 (click here), a surprise re-introduction of calcium N-type channel revised NMED-160, now Z160, and its lead advancement for IND filing, a calcium T-type channel designated as Z944 (click here).
* The firm also achieved a key patent (United States Patent 7915265) for Synavive (click here).
* JMP Securities announced coverage of Zalicus on 25 March 2011, setting a target of $5/share compared to Zack's studied analyst Jason Napodano's target of $3/share published in 2010.
Now looking deeply into Q2 2011, the following information should be of interest to Zalicus investors.
Q2 Scheduled Events
21 April 2011: Covidien (COV) reports. Zalicus investors will listen for hints of Exalgo sales in Q1. Most likely, Covidien will not release actual figures, but the firm could comment in general terms on the performance of Exalgo sales.
2-9 May 2011: My understanding is Zalicus will report within this date specific window its Q1 results. Investors will be specifically looking for:
* Exalgo royalty figure - Investors are eager to know . has Exalgo gained exponential sales traction? I expect no less than a 100% gain ($800K) over Q4 2010, which Michael Murphy correctly called at $400K.
* Collaboration news - This is a huge issue within the investor community. In Q1, no names were given, but it was clearly indicated by Zalicus CEO Mark Corrigan that progress of some nature has been/is being made. This includes potential ion channel program partnerships and revenue-generating cHTS collaborations - both were mentioned.
* Amgen (AMGN) - Amgen moved into a second phase of its initial pilot program that was set to end in September 2010. So the question is: Has Zalicus signed Amgen to a longer term collaboration?
* Expect Zalicus to report $3M for the Novartis (NVS) contract extension - Expect Zalicus to report $60-70M in cash reserves following the completion of raising $20M facilitated by Wedbush Securities, adding 8,884,800 common shares (click here). Expect Zalicus to report revenue generated from other cHTS contracts (wild card estimate: $1M). And don't forget to add in Exalgo royalties.
24 May 2011: Annual stockholders' meeting.
8-9 June 2011: World Pharma Congress - Dr. Glenn Short is scheduled to present "Using Combination Ultra High-Throughput Screening (cuHTS) to Address Efficacious Drug Combinations to Responsive Tumor Genotypes." This is the first time for Zalicus, I have read "cuHTS" versus "cHTS" in print. Has Zalicus expanded its high-through-put technology? Additionally, Zalicus Chief Scientific Officer Dr. Terrance Snutch is scheduled to present "Design and Pre-Clinical Development of Novel T-Type and N-Type Calcium Channel Blockers for Pain Intervention." Will Dr. Snutch add sodium channels to that discussion?
Imminent Events
Zalicus investors continue to sit tight for:
* Synavive 2B Clinical Trial Launch - As reported by Zalicus, the firm recently received a "Composition-of-Matter" patent for Synavive (click here) and is scheduled to start the SYNERGY trial in Q2 for rheumatoid arthritis. This is one time I hope CEO Corrigan honors his own timeline. I - like other investors - am growing tired of waiting.
* News on Prednisporin - Since 10 November 2010, per the ClinicalTrials.gov website, the phase 2B has been listed as "completed." Meanwhile, Sanofi-Aventis (SNY) which bought Fovea (which originally licensed Prednisporin) from CombinatoRx (now Zalicus Inc.) announced in Q1 the launch of an ophthalmology division with FOV1101 (i.e. Prednisporin) among those drug candidates assets. In addition, Genzyme (Sanofi's recent acquisition) is known to have an ophthalmology focus (click here). Furthermore, Sanofi announced a "privileged partnership" with The Vision Institute in Paris, France, for ophthalmology, announced on 23 March 2011 (click here). While all of this implies good news, it does have investors sitting on pins and needles. However, it would appear that Sanofi is getting close to making some type of announcement. I have read all of the details of Sanofi's upcoming conferences (including those in French), and Prednisporin is not mentioned; another researcher I trust has stated the same thing.
Imminent and Potentially Lucrative: Ion Channel Programs
* Three-Prongs: In bite-size chunks, CEO Corrigan not only rolled out the lead soldiers of his acclaimed "ion channel army" (terminology he and Dr. Snutch coined) of at least 50 composition candidates, Corrigan made a very defined statement. This is not just an ion channel program (i.e. singular); these are ion channel programs (i.e. plural). Terminology like this should not be lost on investors. Why? Because Zalicus has three separate programs that they can partner (if they choose) separately. Corrigan even hinted that one of those programs, even at its early stage, may already have collaborative interest. Keep in mind, one indication from each program could be worth $500M, so multiply that by 3 ... that's a whopping $1.5B.
* Z160 (Former NMED-160): Like Synavive, which was reformulated into a once/day time-released tablet, I know many investors who have been here since pre-merger days of CombinatoRx and Neuromed were taken aback by the announcement that Z160 was back in the game. After all, Merck (MRK) was ready to put down $450M for NMED-160, so when Corrigan stated that they had resolved the bio-availability problem (i.e. drug absorption) at the molecular level, I sat up and quite frankly cheered. An oral N-type calcium channel pain blocker, you have to think that Zalicus is counting on Z160 to score. The question is, Has Merck come back? Or is someone like Novartis or Amgen, or for that matter Sunovion (Dainippon Sumitomo Pharmaceuticals), looking to step in. All I know is NMED-160 made it deep into phase 2 studies before the bio-availability became insurmountable - but now that problem is resolved.
* Scientific Report and Corporate Presentations: Zalicus is submitting that its ion channel candidates are competitive: Z212 versus Gabapentin (Fanatrex, Gabarone, Gralise, Neurontin) and Z944 versus Naproxen for pain, and Z212 versus Lacosamide or Vimpat (an epilepsy drug). Regarding Lacosamide and Z212 we read: "When tested in the spinal cord slice preparation, lacosamide inhibited lamina I/II neuron AP firing approximately 300 times less potently (IC50 = 150 lM) than Z123212 (Fig. 7C)" (Hildebrand ME et al. A novel slow-inaction-specific ion channel modulator attenuates neuropathic pain. PAIN - 2011 - doi: 10.1016/j.pain.2010.12.035). You don't have to be a scientist to understand what this means: at lower dosages, Z212 was 300 times more potent than Lacosamide. That should make big pharmas interested in epilepsy research to sit up and take notice.
* The Range of Ion Channel Applications: While Zalicus claims to be targeting pain and inflammation, a year ago a report came out that ion channel NP078585 (supplied by Neuromed) may be used to treat alcoholism (also click here). This highlights the diverse business opportunities Zalicus is going to have as it moves forward with its three-prong ion channel programs. As an example, Z212 could be developed as private Canadian firm Xenon Pharmaceuticals is pursuing as a topical cream (XEN402) (click here) for post herpetic neuralgia (PHN) or shingles. Or Z212, if it proved to block one's smell sensory, could be developed into a nasal spray for applicable situations in industry or hospital settings or possibly as an appetite suppressant. These examples may seem extreme, but the ion channel world has a literal myriad of potential applications. Is it possible that Zalicus could decide to sell individual licenses for specific medical indications? If so, it would prove to be extremely lucrative.
* The Competition: Bottom line, Zalicus is deep into the game and is definitely a leader in the pack. Months ago I wrote an unpublished article that detailed Zalicus's key competition. I reference it here. The purpose of that report uncovered that Zalicus is definitely among the world leaders of ion channel research. Keep in mind that is among a pack of Glaxo-Smith-Kline (GSK), Vertex (VRTX), Merck, Hydra BioSciences, Pfizer (PFE), Xention, Grunenthal, Scottish Biomedical, Aurora Biomed, Convergence Pharmaceuticals, Chromocell, Selcia, and ICAGen (ICGN). At that time, I particularly noted the work of Convergence. To that report, I now add Afferent Pharmaceuticals, a private firm in Canada brought to my attention by another investor. I will continue to scan for competition. In any case, Zalicus remains a front-runner.
Conclusion
In a previous article, I made the rationalized claim that I thought Zalicus was worth $13/share. In my opinion, the CEO gets high marks for executing on his originally stated strategic plan in March 2010. My valuation-analysis has not changed; if anything, it has been strengthened. Keep in mind, this present article doesn't even begin to touch on the future of cHTS or cuHTS at Zalicus or its present/future collaborations. We are yet to explore the dissociative steroid market and the low-steroid chemistry platform behind Synavive. What I conclude is that Zalicus is definitely well-funded and major Zalicus events loom large in Q2.
I maintain Zalicus is a Strong Buy.
http://seekingalpha.com/article/263309-major-events-for-zali…



Major Events for Zalicus Loom Large in Q2
Zalicus in Q1
In Q1 2011, Zalicus (ZLCS) announced a number of remarkable achievements:
* It rolled out its three-prong ion channel program(s) including: a substantial scientific report on sodium channel 1.7 Z212 (click here), a surprise re-introduction of calcium N-type channel revised NMED-160, now Z160, and its lead advancement for IND filing, a calcium T-type channel designated as Z944 (click here).
* The firm also achieved a key patent (United States Patent 7915265) for Synavive (click here).
* JMP Securities announced coverage of Zalicus on 25 March 2011, setting a target of $5/share compared to Zack's studied analyst Jason Napodano's target of $3/share published in 2010.
Now looking deeply into Q2 2011, the following information should be of interest to Zalicus investors.
Q2 Scheduled Events
21 April 2011: Covidien (COV) reports. Zalicus investors will listen for hints of Exalgo sales in Q1. Most likely, Covidien will not release actual figures, but the firm could comment in general terms on the performance of Exalgo sales.
2-9 May 2011: My understanding is Zalicus will report within this date specific window its Q1 results. Investors will be specifically looking for:
* Exalgo royalty figure - Investors are eager to know . has Exalgo gained exponential sales traction? I expect no less than a 100% gain ($800K) over Q4 2010, which Michael Murphy correctly called at $400K.
* Collaboration news - This is a huge issue within the investor community. In Q1, no names were given, but it was clearly indicated by Zalicus CEO Mark Corrigan that progress of some nature has been/is being made. This includes potential ion channel program partnerships and revenue-generating cHTS collaborations - both were mentioned.
* Amgen (AMGN) - Amgen moved into a second phase of its initial pilot program that was set to end in September 2010. So the question is: Has Zalicus signed Amgen to a longer term collaboration?
* Expect Zalicus to report $3M for the Novartis (NVS) contract extension - Expect Zalicus to report $60-70M in cash reserves following the completion of raising $20M facilitated by Wedbush Securities, adding 8,884,800 common shares (click here). Expect Zalicus to report revenue generated from other cHTS contracts (wild card estimate: $1M). And don't forget to add in Exalgo royalties.
24 May 2011: Annual stockholders' meeting.
8-9 June 2011: World Pharma Congress - Dr. Glenn Short is scheduled to present "Using Combination Ultra High-Throughput Screening (cuHTS) to Address Efficacious Drug Combinations to Responsive Tumor Genotypes." This is the first time for Zalicus, I have read "cuHTS" versus "cHTS" in print. Has Zalicus expanded its high-through-put technology? Additionally, Zalicus Chief Scientific Officer Dr. Terrance Snutch is scheduled to present "Design and Pre-Clinical Development of Novel T-Type and N-Type Calcium Channel Blockers for Pain Intervention." Will Dr. Snutch add sodium channels to that discussion?
Imminent Events
Zalicus investors continue to sit tight for:
* Synavive 2B Clinical Trial Launch - As reported by Zalicus, the firm recently received a "Composition-of-Matter" patent for Synavive (click here) and is scheduled to start the SYNERGY trial in Q2 for rheumatoid arthritis. This is one time I hope CEO Corrigan honors his own timeline. I - like other investors - am growing tired of waiting.
* News on Prednisporin - Since 10 November 2010, per the ClinicalTrials.gov website, the phase 2B has been listed as "completed." Meanwhile, Sanofi-Aventis (SNY) which bought Fovea (which originally licensed Prednisporin) from CombinatoRx (now Zalicus Inc.) announced in Q1 the launch of an ophthalmology division with FOV1101 (i.e. Prednisporin) among those drug candidates assets. In addition, Genzyme (Sanofi's recent acquisition) is known to have an ophthalmology focus (click here). Furthermore, Sanofi announced a "privileged partnership" with The Vision Institute in Paris, France, for ophthalmology, announced on 23 March 2011 (click here). While all of this implies good news, it does have investors sitting on pins and needles. However, it would appear that Sanofi is getting close to making some type of announcement. I have read all of the details of Sanofi's upcoming conferences (including those in French), and Prednisporin is not mentioned; another researcher I trust has stated the same thing.
Imminent and Potentially Lucrative: Ion Channel Programs
* Three-Prongs: In bite-size chunks, CEO Corrigan not only rolled out the lead soldiers of his acclaimed "ion channel army" (terminology he and Dr. Snutch coined) of at least 50 composition candidates, Corrigan made a very defined statement. This is not just an ion channel program (i.e. singular); these are ion channel programs (i.e. plural). Terminology like this should not be lost on investors. Why? Because Zalicus has three separate programs that they can partner (if they choose) separately. Corrigan even hinted that one of those programs, even at its early stage, may already have collaborative interest. Keep in mind, one indication from each program could be worth $500M, so multiply that by 3 ... that's a whopping $1.5B.
* Z160 (Former NMED-160): Like Synavive, which was reformulated into a once/day time-released tablet, I know many investors who have been here since pre-merger days of CombinatoRx and Neuromed were taken aback by the announcement that Z160 was back in the game. After all, Merck (MRK) was ready to put down $450M for NMED-160, so when Corrigan stated that they had resolved the bio-availability problem (i.e. drug absorption) at the molecular level, I sat up and quite frankly cheered. An oral N-type calcium channel pain blocker, you have to think that Zalicus is counting on Z160 to score. The question is, Has Merck come back? Or is someone like Novartis or Amgen, or for that matter Sunovion (Dainippon Sumitomo Pharmaceuticals), looking to step in. All I know is NMED-160 made it deep into phase 2 studies before the bio-availability became insurmountable - but now that problem is resolved.
* Scientific Report and Corporate Presentations: Zalicus is submitting that its ion channel candidates are competitive: Z212 versus Gabapentin (Fanatrex, Gabarone, Gralise, Neurontin) and Z944 versus Naproxen for pain, and Z212 versus Lacosamide or Vimpat (an epilepsy drug). Regarding Lacosamide and Z212 we read: "When tested in the spinal cord slice preparation, lacosamide inhibited lamina I/II neuron AP firing approximately 300 times less potently (IC50 = 150 lM) than Z123212 (Fig. 7C)" (Hildebrand ME et al. A novel slow-inaction-specific ion channel modulator attenuates neuropathic pain. PAIN - 2011 - doi: 10.1016/j.pain.2010.12.035). You don't have to be a scientist to understand what this means: at lower dosages, Z212 was 300 times more potent than Lacosamide. That should make big pharmas interested in epilepsy research to sit up and take notice.
* The Range of Ion Channel Applications: While Zalicus claims to be targeting pain and inflammation, a year ago a report came out that ion channel NP078585 (supplied by Neuromed) may be used to treat alcoholism (also click here). This highlights the diverse business opportunities Zalicus is going to have as it moves forward with its three-prong ion channel programs. As an example, Z212 could be developed as private Canadian firm Xenon Pharmaceuticals is pursuing as a topical cream (XEN402) (click here) for post herpetic neuralgia (PHN) or shingles. Or Z212, if it proved to block one's smell sensory, could be developed into a nasal spray for applicable situations in industry or hospital settings or possibly as an appetite suppressant. These examples may seem extreme, but the ion channel world has a literal myriad of potential applications. Is it possible that Zalicus could decide to sell individual licenses for specific medical indications? If so, it would prove to be extremely lucrative.
* The Competition: Bottom line, Zalicus is deep into the game and is definitely a leader in the pack. Months ago I wrote an unpublished article that detailed Zalicus's key competition. I reference it here. The purpose of that report uncovered that Zalicus is definitely among the world leaders of ion channel research. Keep in mind that is among a pack of Glaxo-Smith-Kline (GSK), Vertex (VRTX), Merck, Hydra BioSciences, Pfizer (PFE), Xention, Grunenthal, Scottish Biomedical, Aurora Biomed, Convergence Pharmaceuticals, Chromocell, Selcia, and ICAGen (ICGN). At that time, I particularly noted the work of Convergence. To that report, I now add Afferent Pharmaceuticals, a private firm in Canada brought to my attention by another investor. I will continue to scan for competition. In any case, Zalicus remains a front-runner.
Conclusion
In a previous article, I made the rationalized claim that I thought Zalicus was worth $13/share. In my opinion, the CEO gets high marks for executing on his originally stated strategic plan in March 2010. My valuation-analysis has not changed; if anything, it has been strengthened. Keep in mind, this present article doesn't even begin to touch on the future of cHTS or cuHTS at Zalicus or its present/future collaborations. We are yet to explore the dissociative steroid market and the low-steroid chemistry platform behind Synavive. What I conclude is that Zalicus is definitely well-funded and major Zalicus events loom large in Q2.
I maintain Zalicus is a Strong Buy.
http://seekingalpha.com/article/263309-major-events-for-zali…
Ein Zusammenschnitt eines Bloggers von seeking alpha. Was ist daran lesenswert?
Antwort auf Beitrag Nr.: 41.364.384 von lunatics am 13.04.11 22:20:46Pipeline, Aussichten, Co-op Partnern, Finanzierung, für mein dafürhalten ein exzellenter Zussamenfassung die vielen ereignisse im Hause ZLCS seit Combimatrix

Antwort auf Beitrag Nr.: 41.364.535 von Growth2012 am 13.04.11 23:05:21Chapeau, feine Arbeit 
Schaun wir mal, daß es tatsächlich ein gtes Investment wird.

Schaun wir mal, daß es tatsächlich ein gtes Investment wird.
Antwort auf Beitrag Nr.: 41.364.593 von HeinzBork am 13.04.11 23:21:50Tach' Heinz!
Jetzt wird ZLCS erfasst vom "The Bedford Report" stateside, das Geheimtip Platform für small cap Biotech Niechenanbietern...(Falls man wert darauf legt )
)
XenoPort and Zalicus Poised for Growth as Earnings Trends Improve...
http://finance.yahoo.com/news/XenoPort-and-Zalicus-Poised-iw…
Nun ja, mal sehen sagt der ZLCS Blinde

Ach ja, heute hätte man anteile für schlappe 1,68€ bzw. (2,51$) haben können, nur mal so zum Festhalten
Jetzt wird ZLCS erfasst vom "The Bedford Report" stateside, das Geheimtip Platform für small cap Biotech Niechenanbietern...(Falls man wert darauf legt
 )
)XenoPort and Zalicus Poised for Growth as Earnings Trends Improve...
http://finance.yahoo.com/news/XenoPort-and-Zalicus-Poised-iw…
Nun ja, mal sehen sagt der ZLCS Blinde


Ach ja, heute hätte man anteile für schlappe 1,68€ bzw. (2,51$) haben können, nur mal so zum Festhalten

Habe heute meine Anteile bei 2.66 verkauft.
Antwort auf Beitrag Nr.: 41.386.228 von lunatics am 18.04.11 22:23:38...und der Wert ist "trotzdem" nicht sofort eingebrochen-seltsam



Antwort auf Beitrag Nr.: 41.384.805 von Growth2012 am 18.04.11 18:05:47Der Zacks report besagt folgendes:-
Zalicus is in an enviable position within the biotechnology industry. The company has an approved product on the market in Exalgo, and is collecting royalties from a strong partner in Covidien. The company s pipeline contains Synavive, a mid-stage candidate with proof-of-concept data in a significant market opportunity with rheumatoid
arthritis (RA) and osteoarthritis (OA). Zalicus also possess one of the market s leading research and discovery programs in calcium channel programs. Management is focusing the company s early-stage discovery efforts into the development of a new class of analgesics for the treatment of both acute and chronic pain. Finally, the cash
position is strong, with $46.5 million on hand at the end of 2010, with the potential to raise additional funds in 2011 through the sale or out-license of non-core candidates for psoriasis (CRx-191) and diabetes (CRx-401), or exercising Term-B or Term-C on its loan with Oxford. Accordingly, we believe the best valuation methodology for Zalicus is a sum-of-parts analysis that takes into account the strong cash flows from Exalgo and the upside in future development from Synavive, the ion channel programs, and potential out-licensing opportunities.
Our sum of parts / NPV analysis for Zalicus yields a total firm value of roughly $260 million, or $3 per share. At the current value of only $185 million we believe the company is under-valued. A detailed analysis of our sum of parts
valuation can be found in our initiation report from September 2010, and our follow-up reports published in November 2010 and January 2011. We see upside beyond the $3 level based on positive news from Sanofi on prednisporin, or partnering of the early-stage ion channel program. We rate the shares Outperform.
Genau diese Phase knapp unter die 3$ Marke erleben wir zurzeit-alles im lot wurde ich sagen
Zalicus is in an enviable position within the biotechnology industry. The company has an approved product on the market in Exalgo, and is collecting royalties from a strong partner in Covidien. The company s pipeline contains Synavive, a mid-stage candidate with proof-of-concept data in a significant market opportunity with rheumatoid
arthritis (RA) and osteoarthritis (OA). Zalicus also possess one of the market s leading research and discovery programs in calcium channel programs. Management is focusing the company s early-stage discovery efforts into the development of a new class of analgesics for the treatment of both acute and chronic pain. Finally, the cash
position is strong, with $46.5 million on hand at the end of 2010, with the potential to raise additional funds in 2011 through the sale or out-license of non-core candidates for psoriasis (CRx-191) and diabetes (CRx-401), or exercising Term-B or Term-C on its loan with Oxford. Accordingly, we believe the best valuation methodology for Zalicus is a sum-of-parts analysis that takes into account the strong cash flows from Exalgo and the upside in future development from Synavive, the ion channel programs, and potential out-licensing opportunities.
Our sum of parts / NPV analysis for Zalicus yields a total firm value of roughly $260 million, or $3 per share. At the current value of only $185 million we believe the company is under-valued. A detailed analysis of our sum of parts
valuation can be found in our initiation report from September 2010, and our follow-up reports published in November 2010 and January 2011. We see upside beyond the $3 level based on positive news from Sanofi on prednisporin, or partnering of the early-stage ion channel program. We rate the shares Outperform.
Genau diese Phase knapp unter die 3$ Marke erleben wir zurzeit-alles im lot wurde ich sagen

Antwort auf Beitrag Nr.: 41.387.013 von Growth2012 am 19.04.11 08:30:08Moin G2012
Sehr schöner Fund
Ich musste mir noch ein paar Teile letztens für 2,40 reinziehen, mir deucht in 3 Monaten haben wir den Verdoppler, noch ein paar nette news und dann sollte es losgehen.
Sehr schöner Fund

Ich musste mir noch ein paar Teile letztens für 2,40 reinziehen, mir deucht in 3 Monaten haben wir den Verdoppler, noch ein paar nette news und dann sollte es losgehen.
Antwort auf Beitrag Nr.: 41.386.999 von Growth2012 am 19.04.11 08:26:57Keine Angst... Den Verdoppler habe ich bei ZLCS bereits hinter mir.



Antwort auf Beitrag Nr.: 41.388.552 von lunatics am 19.04.11 12:47:15Ich freue mich für jeden Anleger der seine Gewinne absichern kann...und Angst habe ich ganz bestimmt keine!
Hier geht es (meiner bescheidener Meinung nach) nicht um die Gewinne den man evtl. zu früh mal mitnimmt, sondern um die kommenden Steigerungen den mann dann hoffnungslos auf der Strecke lässt....
Allmählich steuern wir den 2€ Brief mal an
Geld Realtime-Kurs1,899
Brief Realtime-Kurs 1,978
An der Börse, sowie im Leben gilt weiterhin ...jedem das seine
Hier geht es (meiner bescheidener Meinung nach) nicht um die Gewinne den man evtl. zu früh mal mitnimmt, sondern um die kommenden Steigerungen den mann dann hoffnungslos auf der Strecke lässt....

Allmählich steuern wir den 2€ Brief mal an
Geld Realtime-Kurs1,899
Brief Realtime-Kurs 1,978
An der Börse, sowie im Leben gilt weiterhin ...jedem das seine

Antwort auf Beitrag Nr.: 41.390.982 von Growth2012 am 19.04.11 19:46:50Mir geht es genau so... Nur sind die Meinung halt verschieden.
Bin aus ZLCS raus weil ich denke dass ich in einem anderen ausgesuchten Investment in der gleichen Zeit mit weniger Risiko in den nächsten Tagen mehr Performance hole.
ZLCS ein gutes Investment keine Frage. Mir persönlich auf dem Top aber jetzt zu heiss.
Bin aus ZLCS raus weil ich denke dass ich in einem anderen ausgesuchten Investment in der gleichen Zeit mit weniger Risiko in den nächsten Tagen mehr Performance hole.
ZLCS ein gutes Investment keine Frage. Mir persönlich auf dem Top aber jetzt zu heiss.
Antwort auf Beitrag Nr.: 41.390.982 von Growth2012 am 19.04.11 19:46:50Moin! ....anbei ein 'one page overview' von Fools bzgl. ZLCS (mit beliebige Presse coverage/links)
http://caps.fool.com/Ticker/ZLCS.aspx
Gestern Abend gabs 6.498 Handlungen stateside (ohne after hours) das ganze von ein stock der unter 100 Million Anteile draußen(98,76) hat.
Tja, es wird Langsam "eng" mit die übrig gebliebene anteile

http://caps.fool.com/Ticker/ZLCS.aspx
Gestern Abend gabs 6.498 Handlungen stateside (ohne after hours) das ganze von ein stock der unter 100 Million Anteile draußen(98,76) hat.
Tja, es wird Langsam "eng" mit die übrig gebliebene anteile


Enter Stock Symbol:
Zalicus Inc. (ZLCS)
2011-04-19 4:47:43 PM
Overall
Our rating system posted a BUY today, same as yesterday. The market seems to continue bullish move. If you bought, continue to hold stock until SELL signal. You are relatively safe to buy now, upward move is expected.
Target
Six months: 3.28 One year: 3.83
Support
Support1: 2.22 Support2: 1.85
Resistance
Resistance1: 2.81 Resistance2: 3.28
Pivot
2.39
Moving Averages
MA(5): 2.51 MA(20): 2.36
MA(100): 1.97 MA(250): 1.62
MACD
MACD(12,26): 0.11 Signal(12,26,9): 0.07
Stochastic Oscillator
%K(14,3): 85.91 %D(3): 72.14
RSI
RSI(14): 74.70
52-Week
High: 2.81 Low: 1.01 Change(%): 105.2
Average Volume(K)
3-Month: 1652 10-Days 1628
Zalicus Inc. (ZLCS)
2011-04-19 4:47:43 PM
Overall
Our rating system posted a BUY today, same as yesterday. The market seems to continue bullish move. If you bought, continue to hold stock until SELL signal. You are relatively safe to buy now, upward move is expected.
Target
Six months: 3.28 One year: 3.83
Support
Support1: 2.22 Support2: 1.85
Resistance
Resistance1: 2.81 Resistance2: 3.28
Pivot
2.39
Moving Averages
MA(5): 2.51 MA(20): 2.36
MA(100): 1.97 MA(250): 1.62
MACD
MACD(12,26): 0.11 Signal(12,26,9): 0.07
Stochastic Oscillator
%K(14,3): 85.91 %D(3): 72.14
RSI
RSI(14): 74.70
52-Week
High: 2.81 Low: 1.01 Change(%): 105.2
Average Volume(K)
3-Month: 1652 10-Days 1628
Antwort auf Beitrag Nr.: 41.396.298 von Growth2012 am 20.04.11 16:28:25Sorry... hier der Link zu Stoxline:
hier der Link zu Stoxline:
http://www.stoxline.com/quote.php?symbol=zlcs
 hier der Link zu Stoxline:
hier der Link zu Stoxline:http://www.stoxline.com/quote.php?symbol=zlcs
Antwort auf Beitrag Nr.: 41.396.319 von Growth2012 am 20.04.11 16:30:34Pipeline Übersicht 2011:-
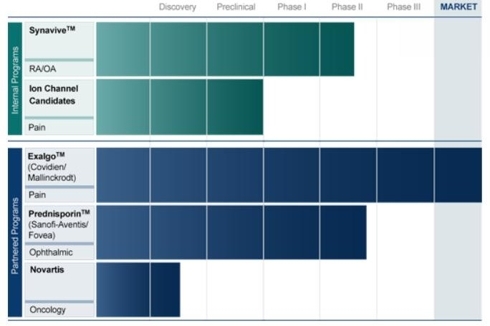
Chart-Tech Entwicklung 2011:- (Bis dato )
)
Time (and regulatory filings) will tell. Though the stock has already quietly risen more than 74%, year-to-date, many believe it still has a long way to go before the year is up. On 04/20/11 it broke resistance and a new 52-week high was forged at $2.99:
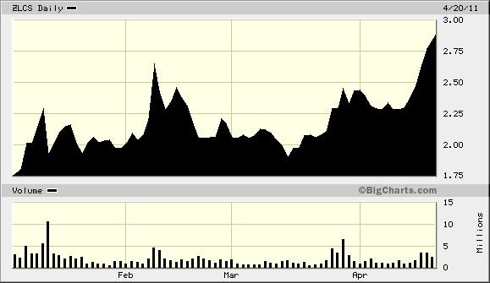
Das ganze Bert Wilkinson Artikel von Seeking Alpha anbei: (20.04.11)
http://seekingalpha.com/article/264523-zalicus-is-wall-stree…
Frohe Ostertage ihr Lieben!

Chart-Tech Entwicklung 2011:- (Bis dato
 )
)Time (and regulatory filings) will tell. Though the stock has already quietly risen more than 74%, year-to-date, many believe it still has a long way to go before the year is up. On 04/20/11 it broke resistance and a new 52-week high was forged at $2.99:

Das ganze Bert Wilkinson Artikel von Seeking Alpha anbei: (20.04.11)
http://seekingalpha.com/article/264523-zalicus-is-wall-stree…
Frohe Ostertage ihr Lieben!
Grober Absturz nachbörslich
Antwort auf Beitrag Nr.: 41.409.672 von lunatics am 26.04.11 00:28:34Ich sehe 300 sh die zu 2,42 gehandelt wurden, das ist doch vernachlässigbar
After Hours Last:
Net / % Change $ 2.81
.005 (.18%) After Hours High: $ 2.81
After Hours Volume: 44,311 After Hours Low: $ 2.42
Learn more about the After-Hours trading session.
Trade Detail
After Hours
Time (ET) After Hours
Price After Hours
Share Volume
19:31 $ 2.81 300
19:01 $ 2.80 1,000
16:42 $ 2.64 2,000
16:42 $ 2.60 1,000
16:42 $ 2.55 2,000
16:42 $ 2.50 300
16:42 $ 2.42 300
16:41 $ 2.70 600
16:41 $ 2.68 400
16:41 $ 2.68 600
16:41 $ 2.66 2,000
16:41 $ 2.65 1,000
16:41 $ 2.65 1,000
16:41 $ 2.65 1,000
16:40 $ 2.77 400
16:40 $ 2.75 500
16:40 $ 2.75 500
16:39 $ 2.78 2,600
16:38 $ 2.79 400
16:15 $ 2.8044 2,411
16:02 $ 2.8085 23,700
16:00 $ 2.79 100
16:00 $ 2.79 100
16:00 $ 2.79 100
Read more: http://www.nasdaq.com/aspxcontent/ExtendedTradingTrades.aspx…
After Hours Last:
Net / % Change $ 2.81
.005 (.18%) After Hours High: $ 2.81
After Hours Volume: 44,311 After Hours Low: $ 2.42
Learn more about the After-Hours trading session.
Trade Detail
After Hours
Time (ET) After Hours
Price After Hours
Share Volume
19:31 $ 2.81 300
19:01 $ 2.80 1,000
16:42 $ 2.64 2,000
16:42 $ 2.60 1,000
16:42 $ 2.55 2,000
16:42 $ 2.50 300
16:42 $ 2.42 300
16:41 $ 2.70 600
16:41 $ 2.68 400
16:41 $ 2.68 600
16:41 $ 2.66 2,000
16:41 $ 2.65 1,000
16:41 $ 2.65 1,000
16:41 $ 2.65 1,000
16:40 $ 2.77 400
16:40 $ 2.75 500
16:40 $ 2.75 500
16:39 $ 2.78 2,600
16:38 $ 2.79 400
16:15 $ 2.8044 2,411
16:02 $ 2.8085 23,700
16:00 $ 2.79 100
16:00 $ 2.79 100
16:00 $ 2.79 100
Read more: http://www.nasdaq.com/aspxcontent/ExtendedTradingTrades.aspx…
Antwort auf Beitrag Nr.: 41.409.672 von lunatics am 26.04.11 00:28:34
"Grober Absturz nachbörslich"
Naja, es darf von mir aus jeden Tag nachbörslich "abstürzen"(sorry, aber vollkommene blödsinn bei der dünne Voluinadecke) und insbesonders im züge der heutigen 'Erholungs- Chartverlauf'

"Grober Absturz nachbörslich"
Naja, es darf von mir aus jeden Tag nachbörslich "abstürzen"(sorry, aber vollkommene blödsinn bei der dünne Voluinadecke) und insbesonders im züge der heutigen 'Erholungs- Chartverlauf'


Antwort auf Beitrag Nr.: 41.410.244 von HeinzBork am 26.04.11 09:52:52Covidien's Report on Exalgo Validates Zalicus' Upward Price
In September 2010, Jason Napodano of Zacks initiated coverage of Zalicus. He later wrote in November: "Our NPV calculations show that Exalgo alone is worth $135 million, or $1.50 per share. This is based on an estimate low double-digit royalty rate on Exalgo peaking at $250 million in 2018." Now we are learning from Covidien that Exalgo "may exceed" their original estimates. I interpret that as great news.
http://seekingalpha.com/article/265269-covidien-s-report-on-…
....
In September 2010, Jason Napodano of Zacks initiated coverage of Zalicus. He later wrote in November: "Our NPV calculations show that Exalgo alone is worth $135 million, or $1.50 per share. This is based on an estimate low double-digit royalty rate on Exalgo peaking at $250 million in 2018." Now we are learning from Covidien that Exalgo "may exceed" their original estimates. I interpret that as great news.
http://seekingalpha.com/article/265269-covidien-s-report-on-…
....

Antwort auf Beitrag Nr.: 41.413.731 von Growth2012 am 26.04.11 21:02:28Neue Resistance levels seit 15:00 Heute Nachmittag
Resistance 1: 3.00 Resistance 2: 3.50

Resistance 1: 3.00 Resistance 2: 3.50
Antwort auf Beitrag Nr.: 41.410.244 von HeinzBork am 26.04.11 09:52:52Wunderbar, die erste Bids jenseits die 2€ Marke 
Geld Realtime-Kurs 2,039
Brief Realtime-Kurs2,118
...und trotzdem stehen wir hier ganz am Anfang der Entwicklung mit ZLCS!
Good trades and steady hands weiterhin

Geld Realtime-Kurs 2,039
Brief Realtime-Kurs2,118
...und trotzdem stehen wir hier ganz am Anfang der Entwicklung mit ZLCS!
Good trades and steady hands weiterhin

$3.15 Pre-Market...
Antwort auf Beitrag Nr.: 41.416.762 von Growth2012 am 27.04.11 14:26:24
Bin froh gestern etwas dazugekauft zu haben....(muss allerdings zugestehen mir ging den Arsch auf Grundeis als ich den Kaufbutton getätigt habe, insbesondere weil ich mitten im stark fallende Kurs befindlich dazugekauft habe
Naja, heute sieht das ganze etwas stabiler aus (und zudem mit weniger Herzkasper involviert
(und zudem mit weniger Herzkasper involviert )
)
Schönes Wö ihr lieben!
Bin froh gestern etwas dazugekauft zu haben....(muss allerdings zugestehen mir ging den Arsch auf Grundeis als ich den Kaufbutton getätigt habe, insbesondere weil ich mitten im stark fallende Kurs befindlich dazugekauft habe

Naja, heute sieht das ganze etwas stabiler aus
 (und zudem mit weniger Herzkasper involviert
(und zudem mit weniger Herzkasper involviert )
)Schönes Wö ihr lieben!
Antwort auf Beitrag Nr.: 41.466.752 von Growth2012 am 06.05.11 17:57:35Not bad at all....
7.496 Handlungen für ZLCS an der Nasdaq heute(Corrigans Bio Presentation Day)
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
Mit knapp 15.18% im Plus geschlossen 'before the bell', nachbörsich sogar etwas mehr (short panic & takeover Angst kehrt zurück)...


7.496 Handlungen für ZLCS an der Nasdaq heute(Corrigans Bio Presentation Day)
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
Mit knapp 15.18% im Plus geschlossen 'before the bell', nachbörsich sogar etwas mehr (short panic & takeover Angst kehrt zurück)...


Antwort auf Beitrag Nr.: 41.466.752 von Growth2012 am 06.05.11 17:57:35Zalicus Selected to Join the NASDAQ Biotechnology Index
http://finance.yahoo.com/news/Zalicus-Selected-to-Join-the-b…
Sollte für etwas zusätzliche Volumina sorgen
(vor allem US Fonds kaufen sehr Index gesteurt)
http://finance.yahoo.com/news/Zalicus-Selected-to-Join-the-b…
Sollte für etwas zusätzliche Volumina sorgen

(vor allem US Fonds kaufen sehr Index gesteurt)

Antwort auf Beitrag Nr.: 41.514.365 von Growth2012 am 17.05.11 14:52:54Ja, das könnte uns ein wenig Schub geben, nachdem wir so abgebürstet wurden.
Antwort auf Beitrag Nr.: 41.514.465 von HeinzBork am 17.05.11 15:04:18Moin Heinz! echt schöner kursverlauf gestern Nachmittag!
Was soll man sagen;- es steckt in jedem Gerücht ein Fünkchen Wahrheit


"Biotech Acquisition Speculation Heats Up"
http://finance.yahoo.com/news/Biotech-Acquisition-iw-3368791…
Dabei wird Bedford als sehr respektierte 'Quelle'(fundiert rechechiert) stateside eingeschätzt...
Aber wie immer gilt -(im Leben und an der Börse)- we will know more in time!

Was soll man sagen;- es steckt in jedem Gerücht ein Fünkchen Wahrheit



"Biotech Acquisition Speculation Heats Up"
http://finance.yahoo.com/news/Biotech-Acquisition-iw-3368791…
Dabei wird Bedford als sehr respektierte 'Quelle'(fundiert rechechiert) stateside eingeschätzt...
Aber wie immer gilt -(im Leben und an der Börse)- we will know more in time!
Antwort auf Beitrag Nr.: 41.514.465 von HeinzBork am 17.05.11 15:04:18Ach ja, ich vergaß vorhin -(auch wenn manche user gar nichts von Seeking Alpha bzw. Michael Murphy halten mögen)-anbei ein (meineserachtens) sehr fundierte Übersicht von MM im sachen ZLCS (May 2011)- wir stehen zurzeit um die 2,60$ und er erwartet 7$ vor Jahresende 2011...Tja, Mut zum festen Prognose hat er als 'Biotech Ikon' allemal bewiesen in meinem Augen...

-------------------------------------------------
Zalicus (ZLCS) is my pick for the InvestorPlace contest for the best stock of 2011, and I wrote it up here and updated it here. The stock was doing well, running from $2.30 in mid-April to over $3 by the end of the month. As so often happens in small-cap and biotech investing, life threw them a curve in the March quarter, and the stock is back to $2.30. I don't think it will stay there for long.
Although Zalicus is a development-stage biotech company focused on alleviating pain, they do have an approved drug, Exalgo, which is marketed by Mallinkrodt, a subsidiary of Covidien (COV). Exalgo is a timed-release version of Dilaudid (hydromorphone), a well-understood pain drug, and is the only long-acting form of hydromorphone approved in the U.S. It is not a hard sale to convince a doctor to prescribe a once-a-day version of this popular drug, as it increases patient compliance and, in an institutional setting, reduces costs.
Exalgo was approved on March 1, 2010, and following the usual practice, Covidien shipped a large amount of the drug to build up pharmacy inventories in the June quarter, generating a $1.1 million royalty payment to Zalicus. Covidien then began marketing the drug, and as prescriptions grew, pharmacies filled them from inventory. This also is typical practice – prescriptions grow much faster than shipments of the drug at first, and the royalty payment to Zalicus fell to $100,000 in the September quarter and increased only slightly to $400,000 in the December quarter.
In the March quarter I was expecting $1 million in royalties and sales of the drug probably were strong enough to justify that. In Covidien's conference call Richard Meelia, Covidien's CEO, said: "Exalgo's right on plan, doing very well, and there's a lot of expectation that it may exceed our original forecast, so that's good." They were very pleased with the uptake of the drug.
But in the Zalicus conference call nine trading days later, we learned that Covidien had made numerous deals with their customers promising rebates and inventory adjustments, and that when these were deducted from the actual sales, the resulting royalty to Zalicus was only $350,000. The stock sank back, giving you an excellent second chance to start or add to positions.
Exalgo is selling more and more each quarter, on a trajectory to reach Covidien's target of $200 million to $300 million in sales. I think the royalty payment to ZLCS will hit $1 million or more this quarter if the Covidien rebate programs are over, and climb from there. That is the first reason to buy or add to ZLCS now.
Zalicus has a second important project that they partnered with Fovea, which was bought by Sanofi-Aventis (SNY) primary for this program. It is Prednisporin for ophthalmic indications, beginning with allergic conjunctivitis. Sanofi has completed a large Phase II study, and we are waiting for them to announce the results. I expect the data to be very positive, and then Sanofi will spell out its plans to advance to Phase III trials. This is the second reason to buy ZLCS now.
Their third and fourth partnered programs are drug discovery collaborations with Novartis (NVS), which exercised an option to continue for a second year, and an early program with Amgen. Zalicus owns a high-throughput drug discovery technology focused on testing combinations of existing approved drugs, and any new combination drugs that come out of these relationships will earn royalties for Zalicus. The Novartis relationship alone calls for $4 million upfront, up to three years of research funding, and up to $58 million in milestone payments per combination drug.
On their own nickel, Zalicus has developed Synavive for immuno-inflammatory diseases, where they have completed multiple Phase II studies. It is essentially a patented, low-dose steroid that avoids the usual side effects, but with an amplified anti-inflammatory effect. The first application is rheumatoid arthritis, where patients eventually advance from oral drugs to expensive injected biologics. Synavive can delay the need to put a patient on a $2,000 a month injectable, with a once-a-day oral drug (much preferred by patients) that has lower toxicity that common steroids.
Synavive has been through many Phase II studies and shown high and sustained efficacy without a single adverse event. The company has planned a Phase IIb study of 250 patients, each dosed for 12 weeks at one of 60 treatment centers around the world. It won't take long to get the efficacy data, and the company will follow with the one-year safety study that eventually will be required for approval. Zalicus is waiting for FDA feedback on their Phase IIb study design any day, and expects to begin enrolling patients in June. This is a third reason to buy ZLCS today.
The focus of the company today is on ion channel blockers, both calcium and sodium channels, to control pain, a $14 billion market. Z160 is an N-type ion channel blocker for chronic pain that Merck developed under a 2006 license, including Phase I and Phase II trials. Merck discontinued the program and gave the drug back to Zalicus. Zalicus scientists reformulated the drug to dramatically increase bioavailability, and it will be in human clinical trials again by year-end.
Z944 is a T-Type ion channel blocker for chronic and acute pain now in animal toxicology studies, which should be in a Phase I human trial by year-end. The company has numerous other T-type and sodium channel blocker candidates for chronic pain in development.
Zalicus has a very experienced management team. The CEO and EVP-R&D have their names on 14 approved drugs between them. Most biotech managements are lucky to be able to name one drug they developed that made it to market. The company raised $19.2 million in March and April, and had $52.8 million in cash at the end of March, plus $4.1 million raised in April, plus a $20.0 million secured credit facility. They burn about $9 million in cash each quarter, but they think they can fund their operations from cash, royalties and milestone payments into 2014.
Even though ZLCS is up about 45% year-to-date, it's still cheap enough to be called a low-risk, development-stage biotech. I am still confident that between the quarterly announcements of the Exalgo royalty payments, the Sanofi data and Phase III plans for Prednisporin, the Synavive Phase IIb trial commencement, the early clinical trials for their ion channel pain medications, and additional partner announcements for their drug discovery platform that ZLCS will be at $7 a share by the end of 2011.
http://seekingalpha.com/article/271496-zalicus-up-in-2011-ex…


-------------------------------------------------
Zalicus (ZLCS) is my pick for the InvestorPlace contest for the best stock of 2011, and I wrote it up here and updated it here. The stock was doing well, running from $2.30 in mid-April to over $3 by the end of the month. As so often happens in small-cap and biotech investing, life threw them a curve in the March quarter, and the stock is back to $2.30. I don't think it will stay there for long.
Although Zalicus is a development-stage biotech company focused on alleviating pain, they do have an approved drug, Exalgo, which is marketed by Mallinkrodt, a subsidiary of Covidien (COV). Exalgo is a timed-release version of Dilaudid (hydromorphone), a well-understood pain drug, and is the only long-acting form of hydromorphone approved in the U.S. It is not a hard sale to convince a doctor to prescribe a once-a-day version of this popular drug, as it increases patient compliance and, in an institutional setting, reduces costs.
Exalgo was approved on March 1, 2010, and following the usual practice, Covidien shipped a large amount of the drug to build up pharmacy inventories in the June quarter, generating a $1.1 million royalty payment to Zalicus. Covidien then began marketing the drug, and as prescriptions grew, pharmacies filled them from inventory. This also is typical practice – prescriptions grow much faster than shipments of the drug at first, and the royalty payment to Zalicus fell to $100,000 in the September quarter and increased only slightly to $400,000 in the December quarter.
In the March quarter I was expecting $1 million in royalties and sales of the drug probably were strong enough to justify that. In Covidien's conference call Richard Meelia, Covidien's CEO, said: "Exalgo's right on plan, doing very well, and there's a lot of expectation that it may exceed our original forecast, so that's good." They were very pleased with the uptake of the drug.
But in the Zalicus conference call nine trading days later, we learned that Covidien had made numerous deals with their customers promising rebates and inventory adjustments, and that when these were deducted from the actual sales, the resulting royalty to Zalicus was only $350,000. The stock sank back, giving you an excellent second chance to start or add to positions.
Exalgo is selling more and more each quarter, on a trajectory to reach Covidien's target of $200 million to $300 million in sales. I think the royalty payment to ZLCS will hit $1 million or more this quarter if the Covidien rebate programs are over, and climb from there. That is the first reason to buy or add to ZLCS now.
Zalicus has a second important project that they partnered with Fovea, which was bought by Sanofi-Aventis (SNY) primary for this program. It is Prednisporin for ophthalmic indications, beginning with allergic conjunctivitis. Sanofi has completed a large Phase II study, and we are waiting for them to announce the results. I expect the data to be very positive, and then Sanofi will spell out its plans to advance to Phase III trials. This is the second reason to buy ZLCS now.
Their third and fourth partnered programs are drug discovery collaborations with Novartis (NVS), which exercised an option to continue for a second year, and an early program with Amgen. Zalicus owns a high-throughput drug discovery technology focused on testing combinations of existing approved drugs, and any new combination drugs that come out of these relationships will earn royalties for Zalicus. The Novartis relationship alone calls for $4 million upfront, up to three years of research funding, and up to $58 million in milestone payments per combination drug.
On their own nickel, Zalicus has developed Synavive for immuno-inflammatory diseases, where they have completed multiple Phase II studies. It is essentially a patented, low-dose steroid that avoids the usual side effects, but with an amplified anti-inflammatory effect. The first application is rheumatoid arthritis, where patients eventually advance from oral drugs to expensive injected biologics. Synavive can delay the need to put a patient on a $2,000 a month injectable, with a once-a-day oral drug (much preferred by patients) that has lower toxicity that common steroids.
Synavive has been through many Phase II studies and shown high and sustained efficacy without a single adverse event. The company has planned a Phase IIb study of 250 patients, each dosed for 12 weeks at one of 60 treatment centers around the world. It won't take long to get the efficacy data, and the company will follow with the one-year safety study that eventually will be required for approval. Zalicus is waiting for FDA feedback on their Phase IIb study design any day, and expects to begin enrolling patients in June. This is a third reason to buy ZLCS today.
The focus of the company today is on ion channel blockers, both calcium and sodium channels, to control pain, a $14 billion market. Z160 is an N-type ion channel blocker for chronic pain that Merck developed under a 2006 license, including Phase I and Phase II trials. Merck discontinued the program and gave the drug back to Zalicus. Zalicus scientists reformulated the drug to dramatically increase bioavailability, and it will be in human clinical trials again by year-end.
Z944 is a T-Type ion channel blocker for chronic and acute pain now in animal toxicology studies, which should be in a Phase I human trial by year-end. The company has numerous other T-type and sodium channel blocker candidates for chronic pain in development.
Zalicus has a very experienced management team. The CEO and EVP-R&D have their names on 14 approved drugs between them. Most biotech managements are lucky to be able to name one drug they developed that made it to market. The company raised $19.2 million in March and April, and had $52.8 million in cash at the end of March, plus $4.1 million raised in April, plus a $20.0 million secured credit facility. They burn about $9 million in cash each quarter, but they think they can fund their operations from cash, royalties and milestone payments into 2014.
Even though ZLCS is up about 45% year-to-date, it's still cheap enough to be called a low-risk, development-stage biotech. I am still confident that between the quarterly announcements of the Exalgo royalty payments, the Sanofi data and Phase III plans for Prednisporin, the Synavive Phase IIb trial commencement, the early clinical trials for their ion channel pain medications, and additional partner announcements for their drug discovery platform that ZLCS will be at $7 a share by the end of 2011.
http://seekingalpha.com/article/271496-zalicus-up-in-2011-ex…
Antwort auf Beitrag Nr.: 41.565.663 von Growth2012 am 27.05.11 10:35:03Moin G2012,
bin im Moment nicht so am Ball,wg. tausend anderer Sachen.
Aber wieder mal schöner Fund von Dir.
Wir scheinen die einzigen zu sein, die die Aktie haben
Aber 7$ am Ende des Jahres halte ich zwar für ambitioniert, aber nicht unmöglich, vorausgesetzt wir bekommen QE3 ohne Finanzkrise 2.
Ich hatte nochmal ein wenig aufgestockt, jetzt heisst es: "auffi gehts, buam"
Schönes Wochenende
HB
bin im Moment nicht so am Ball,wg. tausend anderer Sachen.
Aber wieder mal schöner Fund von Dir.
Wir scheinen die einzigen zu sein, die die Aktie haben

Aber 7$ am Ende des Jahres halte ich zwar für ambitioniert, aber nicht unmöglich, vorausgesetzt wir bekommen QE3 ohne Finanzkrise 2.
Ich hatte nochmal ein wenig aufgestockt, jetzt heisst es: "auffi gehts, buam"
Schönes Wochenende
HB
Antwort auf Beitrag Nr.: 41.571.230 von HeinzBork am 28.05.11 15:56:13Bin auch dabei.....
Antwort auf Beitrag Nr.: 41.514.465 von HeinzBork am 17.05.11 15:04:18Sehr Schöne Platform
Zalicus to Present at the 10th Annual World Pharma Conference
- Novel Calcium Channel Blockers Highlighted at Session on Targeting Pain with Novel Therapeutics -
- Advantages of Combination High-Throughput Screening Reviewed in Session on Cutting Edge Cell-Based Screening Technologies -
http://finance.yahoo.com/news/Zalicus-to-Present-at-the-bw-2…
....
und hier die neusten Novartis Gerüchte (for what they are worth? )
)
Zalicus in Novartis's Sweet Spot Signals More Upside
http://seekingalpha.com/article/272826-zalicus-in-novartis-s…
Schönes Wochenende ihr lieben!

Zalicus to Present at the 10th Annual World Pharma Conference
- Novel Calcium Channel Blockers Highlighted at Session on Targeting Pain with Novel Therapeutics -
- Advantages of Combination High-Throughput Screening Reviewed in Session on Cutting Edge Cell-Based Screening Technologies -
http://finance.yahoo.com/news/Zalicus-to-Present-at-the-bw-2…
....

und hier die neusten Novartis Gerüchte (for what they are worth?
 )
)Zalicus in Novartis's Sweet Spot Signals More Upside
http://seekingalpha.com/article/272826-zalicus-in-novartis-s…
Schönes Wochenende ihr lieben!
3 Stocks Approaching Greatness
By Rich Duprey , June 23, 2011
For every stock out there screaming "buy me," others simply give us a nudge and a nod. While all the attention might be focused on their five-star peers, we can sift through Motley Fool CAPS to find four-star stocks giving us the "high sign" they're approaching greatness.
These opportunities – including familiar names and beaten-down companies -- rank higher than most of the other 5,400 starred companies, and it pays to investigate their potential. For consideration today I've got this trio of stocks on their way to fame.
Fibria Celulose (NYSE: FBR )
Intrepid Potash (NYSE: IPI )
Zalicus (Nasdaq: ZLCS )
................... ........................
ZALICUS:
Not feeling the pain
Pain and immuno-inflammatory disease drug developer Zalicus remains in the good graces of investors despite being subject to the vagaries of Food and Drug Administration approvals. Midstage clinical trials of Synavive for rheumatoid arthritis are poised to begin in the second quarter, and analysts are expecting royalties from Exalgo, which it markets with Covidien, to hit $36 million in two years' time.
Zalicus did suffer a setback when it reported weaker-than-expected quarterly results because Covidien has a rebate program that sapped some of the sales strength.
CAPS member wiaggie says the drug developer not only has a top-notch management team, but with a strong pipeline to support Exalgo, it is a worthy investment:
Well run organization with a revenue-producing drug already in the marketplace and several new ones showing promise in the near future. Unlike many R&D firms, they also have cash-on-hand. Undervalued based on sheer assets, without even mentioning future earning potential.
Let us know your views on its potential for success on the Zalicus CAPS page and add it to your watchlist and keep track of all the news and analysis about this developing drug developer.
http://www.fool.com/investing/general/2011/06/23/3-stocks-ap…
By Rich Duprey , June 23, 2011
For every stock out there screaming "buy me," others simply give us a nudge and a nod. While all the attention might be focused on their five-star peers, we can sift through Motley Fool CAPS to find four-star stocks giving us the "high sign" they're approaching greatness.
These opportunities – including familiar names and beaten-down companies -- rank higher than most of the other 5,400 starred companies, and it pays to investigate their potential. For consideration today I've got this trio of stocks on their way to fame.
Fibria Celulose (NYSE: FBR )
Intrepid Potash (NYSE: IPI )
Zalicus (Nasdaq: ZLCS )
................... ........................
ZALICUS:
Not feeling the pain
Pain and immuno-inflammatory disease drug developer Zalicus remains in the good graces of investors despite being subject to the vagaries of Food and Drug Administration approvals. Midstage clinical trials of Synavive for rheumatoid arthritis are poised to begin in the second quarter, and analysts are expecting royalties from Exalgo, which it markets with Covidien, to hit $36 million in two years' time.
Zalicus did suffer a setback when it reported weaker-than-expected quarterly results because Covidien has a rebate program that sapped some of the sales strength.
CAPS member wiaggie says the drug developer not only has a top-notch management team, but with a strong pipeline to support Exalgo, it is a worthy investment:
Well run organization with a revenue-producing drug already in the marketplace and several new ones showing promise in the near future. Unlike many R&D firms, they also have cash-on-hand. Undervalued based on sheer assets, without even mentioning future earning potential.
Let us know your views on its potential for success on the Zalicus CAPS page and add it to your watchlist and keep track of all the news and analysis about this developing drug developer.
http://www.fool.com/investing/general/2011/06/23/3-stocks-ap…
Antwort auf Beitrag Nr.: 41.694.693 von bernie55 am 24.06.11 12:15:45Bin froh das ich es nicht reinstellen müsste

Danke für deiner Mühe & schönes Wö


Danke für deiner Mühe & schönes Wö

Antwort auf Beitrag Nr.: 41.697.956 von Growth2012 am 24.06.11 21:19:07Hi Groth2012,
und ich bin froh darüber, dass ich Folgendes reinposten darf.
Last Trade: 2.42
Trade Time: Jun 24
Change: Up 0.24 (11.01%)
Volume: 5,377,398
After Hours
Volume: 2,017,713
After Hours
Last: $ 2.4206
After Hours
High: $ 2.47
After Hours
Low: $ 2.3127
und ich bin froh darüber, dass ich Folgendes reinposten darf.

Last Trade: 2.42
Trade Time: Jun 24
Change: Up 0.24 (11.01%)
Volume: 5,377,398

After Hours
Volume: 2,017,713

After Hours
Last: $ 2.4206
After Hours
High: $ 2.47
After Hours
Low: $ 2.3127
Antwort auf Beitrag Nr.: 41.697.956 von Growth2012 am 24.06.11 21:19:07...natürlich Dir auch ein schönes Wochenende...

Antwort auf Beitrag Nr.: 41.698.886 von bernie55 am 25.06.11 08:52:55Happy Weekend for Zalicus Investors
Jun 24, 2011 7:25 PM
After getting hammered since early May, Zalicus LONGS are about to be richly rewarded on plenty of second half upside news, kicked off by the last week of June.
Word out this week revealed further evidence that Prednisporin's future is bright. Two Fovea patent applications indicate that Sanofi patent attorneys may be in the process of securing Prednisporin's patent estate before launching the next stage trial. And it was discovered that Prednisporin's future may go well beyond allergic conjunctivitis and may include age-related macular degeneration. A patent estate should protect Zalicus royalties out to 2028, if not later. With Synavive's patent estate announced by Zalicus in late March, Sanofi lawyers have a reference for making their case with the European patent office that Prednisporin is a novel combination displaying a verifiable synergistic effect. According to Zacks Napodano, Sanofi is working on next stage plans.
Meanwhile, Synavive is about to come out racing. Zalicus has a conference on Tuesday, so it's very likely the CEO would like to use his 15 minutes to brag about Synavive's phase 2B SYNERGY trial. Kudos to Team-Zalicus for putting together one of the best clinical trials I've seen in a long time. Brilliant stuff. 5 arms tightly designed to compliment market DMARDs. I am told enrollment begins in the USA, follows in Europe in July, and turns to Latin America in late summer, early fall.
Likewise, Zalicus Pharmaceuticals, the Vancouver subsidiary of Zalicus Inc, is firing away on all cylinders. A source informs me that Zalicus is targeting
-September as the start-date for Z160's short phase 1 trial;
- couched under old NMED-160, Zalicus plans to jump start it through phase 1 and
into phase 2a in early 2012.
- Meanwhile, Z944 remains on target for phase 1 in Q4.
- Sit down when you read this, but by year end, Zalicus will potentially have 3 internal drug candidates in clinical trials.
Growing rumors from the East (okay in this case Ireland) suggest Covidien is burning the midnight oil ramping up Exalgo sales. The market was disappointed by Q1, but I'm hearing more and more people say that they believe Q2-Q3 will be positively encour…, MA. Maybe this time Covidien will cut Zalicus a break and not bury its progress beneath its own failing drug line and dud Pennsaid. Meanwhile jobs at Mallinckrodt are up for grabs and Exalgo looks to be the prized pony.
Finally, the CEO slipped in his last speech and mentioned Eisai was one of the companies engaged in a pilot program at Zalicus. The slip may have been intentional, but a source confirms that Zalicus has a handful of potential collaborators, among them big pharma. With Zalicus gaining analyst coverage along with a new year in the Russell 2000 and now the NASDAQ Biotech Index, things are looking peachy.
http://seekingalpha.com/instablog/759138-james-stocklasar-th…
Jun 24, 2011 7:25 PM
After getting hammered since early May, Zalicus LONGS are about to be richly rewarded on plenty of second half upside news, kicked off by the last week of June.
Word out this week revealed further evidence that Prednisporin's future is bright. Two Fovea patent applications indicate that Sanofi patent attorneys may be in the process of securing Prednisporin's patent estate before launching the next stage trial. And it was discovered that Prednisporin's future may go well beyond allergic conjunctivitis and may include age-related macular degeneration. A patent estate should protect Zalicus royalties out to 2028, if not later. With Synavive's patent estate announced by Zalicus in late March, Sanofi lawyers have a reference for making their case with the European patent office that Prednisporin is a novel combination displaying a verifiable synergistic effect. According to Zacks Napodano, Sanofi is working on next stage plans.
Meanwhile, Synavive is about to come out racing. Zalicus has a conference on Tuesday, so it's very likely the CEO would like to use his 15 minutes to brag about Synavive's phase 2B SYNERGY trial. Kudos to Team-Zalicus for putting together one of the best clinical trials I've seen in a long time. Brilliant stuff. 5 arms tightly designed to compliment market DMARDs. I am told enrollment begins in the USA, follows in Europe in July, and turns to Latin America in late summer, early fall.
Likewise, Zalicus Pharmaceuticals, the Vancouver subsidiary of Zalicus Inc, is firing away on all cylinders. A source informs me that Zalicus is targeting
-September as the start-date for Z160's short phase 1 trial;
- couched under old NMED-160, Zalicus plans to jump start it through phase 1 and
into phase 2a in early 2012.
- Meanwhile, Z944 remains on target for phase 1 in Q4.
- Sit down when you read this, but by year end, Zalicus will potentially have 3 internal drug candidates in clinical trials.
Growing rumors from the East (okay in this case Ireland) suggest Covidien is burning the midnight oil ramping up Exalgo sales. The market was disappointed by Q1, but I'm hearing more and more people say that they believe Q2-Q3 will be positively encour…, MA. Maybe this time Covidien will cut Zalicus a break and not bury its progress beneath its own failing drug line and dud Pennsaid. Meanwhile jobs at Mallinckrodt are up for grabs and Exalgo looks to be the prized pony.
Finally, the CEO slipped in his last speech and mentioned Eisai was one of the companies engaged in a pilot program at Zalicus. The slip may have been intentional, but a source confirms that Zalicus has a handful of potential collaborators, among them big pharma. With Zalicus gaining analyst coverage along with a new year in the Russell 2000 and now the NASDAQ Biotech Index, things are looking peachy.

http://seekingalpha.com/instablog/759138-james-stocklasar-th…
Antwort auf Beitrag Nr.: 41.698.889 von bernie55 am 25.06.11 08:54:51Guten Morgen bernie!
habe auf die schnelle weitere 1500 stk soeben mal 'gesichert' in Berlin, man weiss ja nie wenn es richtig los geht

Schöner Artikel von James Laasar von Freitag & sehr schöne 'spike' im late trading:

http://seekingalpha.com/instablog/759138-james-stocklasar-th…
We will see...

habe auf die schnelle weitere 1500 stk soeben mal 'gesichert' in Berlin, man weiss ja nie wenn es richtig los geht


Schöner Artikel von James Laasar von Freitag & sehr schöne 'spike' im late trading:
http://seekingalpha.com/instablog/759138-james-stocklasar-th…
We will see...


Antwort auf Beitrag Nr.: 41.703.663 von bernie55 am 27.06.11 11:09:25Fast Zeitgleich reingestellt, sorry Bernie, aber wie heisst es so schön...
"great minds think alike, but unfortunately fools also seldom differ"
Only time will tell
"great minds think alike, but unfortunately fools also seldom differ"

Only time will tell

Reissued: Novartis Files Cancer Patent Citing Zalicus
Jun 27, 2011 9:25 AM > ZLCS
Further research has uncovered Zalicus has its own patents filed in the same chemistry area as Novartis. What will this mean for Zalicus? A partnership? A buy-out? Irrespective, all analysts do not account for this event that has just happened. No one has baked in an actual collaboration having produced a drug indication.
BIG ZALICUS ALERT!!! NOVARTIS FILES PATENT USING cHTS!!! It's official!!!
Novartis on 6/23/11 filed its first Zalicus cHTS patent application for multiple myeloma. This is WHOOPING HUGE NEWS!!!
Proof: Read the patent all the way to the bottom line and your eyes will feast of these words: ""Raw CellTiter GLO relative fluorescent unit (RFU) values were acquired using a microplate reader (PerkinElmer Precisely, Perkin Elmer Life and Analytical Sciences). Data analysis was performed using the Chalice software developed by CombinatoRx (Zalicus Inc., Cambridge, MA, USA). Specifically, the Loewe Additivity (ADD) model was used as the combination reference in the EXAMPLES. "
METHOD FOR TREATING HAEMATOLOGICAL CANCERS
http://www.freepatentsonline.com/WO2011075620A1.html
This could move Zalicus stock pps UP UP UP!!!
The milestones will be worth up to $58M!!!
http://seekingalpha.com/instablog/759138-james-stocklasar-th…
Jun 27, 2011 9:25 AM > ZLCS
Further research has uncovered Zalicus has its own patents filed in the same chemistry area as Novartis. What will this mean for Zalicus? A partnership? A buy-out? Irrespective, all analysts do not account for this event that has just happened. No one has baked in an actual collaboration having produced a drug indication.
BIG ZALICUS ALERT!!! NOVARTIS FILES PATENT USING cHTS!!! It's official!!!
Novartis on 6/23/11 filed its first Zalicus cHTS patent application for multiple myeloma. This is WHOOPING HUGE NEWS!!!
Proof: Read the patent all the way to the bottom line and your eyes will feast of these words: ""Raw CellTiter GLO relative fluorescent unit (RFU) values were acquired using a microplate reader (PerkinElmer Precisely, Perkin Elmer Life and Analytical Sciences). Data analysis was performed using the Chalice software developed by CombinatoRx (Zalicus Inc., Cambridge, MA, USA). Specifically, the Loewe Additivity (ADD) model was used as the combination reference in the EXAMPLES. "
METHOD FOR TREATING HAEMATOLOGICAL CANCERS
http://www.freepatentsonline.com/WO2011075620A1.html
This could move Zalicus stock pps UP UP UP!!!

The milestones will be worth up to $58M!!!
http://seekingalpha.com/instablog/759138-james-stocklasar-th…
Zalicus Initiates Phase 2b Clinical Trial of Synavive in Rheumatoid Arthritis
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ:ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases today announced the initiation of the SYNERGY trial, a Phase 2b clinical trial evaluating Synavive™, a low-dose glucocorticoid with the potential for amplified immuno-inflammatory benefits, in patients with rheumatoid arthritis (RA). Top-line results of the clinical trial are expected to be available in the second half of 2012. In addition, Zalicus has drawn an additional $8.5 million from its $20.0 million secured credit facility with Oxford Finance to fund Synavive advancement.
“RA is an attractive initial indication for Synavive as there is unmet need for a safer glucocorticoid; one that provides the amplified anti-inflammatory activity of a higher dose but without the associated dose-related side effects. There is already a high prevalence of glucocorticoid use in RA for chronic maintenance therapy, as well as significant demand for less expensive, easier to access options to biologic therapy,” said Mark H.N. Corrigan, MD, President and CEO of Zalicus. “Data from a prior Phase 2a clinical trial with Synavive demonstrated encouraging preliminary signs of activity in RA patients and we look forward to further exploring its potential in this indication and reporting top-line SYNERGY results next summer.”
Study Design and Objectives
The Phase 2b clinical trial titled SYNERGY (SYNavivE for Reducing signs and sYmptoms of rheumatoid arthritis trial), is a 12-week, five-arm, global, double-blind, placebo-controlled study to evaluate the safety and efficacy of Synavive as a treatment for the signs and symptoms of RA in approximately 250 subjects with moderate to severe disease. The trial will be conducted in up to 60 centers throughout the United States, Europe and Latin America. The primary objective of the trial is to evaluate Synavive efficacy compared to placebo, while key additional secondary objectives include evaluating the efficacy of Synavive compared to its individual components (2.7mg of Prednisolone and 360mg of Dipyramidamole) as well as how Synavive performs in comparison to 5mg of Prednisone. Subjects who complete the core SYNERGY trial will be eligible to participate in a one-year extension study designed to investigate the long-term safety and durability of response for Synavive. To learn more about the SYNERGY trial and enrollment criteria please visit www.clinicaltrials.gov.
“We will consider the SYNERGY trial a success if Synavive demonstrates a statistically significant benefit compared to placebo and a clinically meaningful, but not necessarily statistically significant, benefit compared to its individual components alone,” said Jonathan Krant, MD, Vice President, Clinical Research of Zalicus. “In addition, we will seek to determine if the efficacy of Synavive is comparable to a commonly prescribed 5 mg dose of the glucocorticoid Prednisone.”
http://finance.yahoo.com/news/Zalicus-Initiates-Phase-2b-bw-…
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ:ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases today announced the initiation of the SYNERGY trial, a Phase 2b clinical trial evaluating Synavive™, a low-dose glucocorticoid with the potential for amplified immuno-inflammatory benefits, in patients with rheumatoid arthritis (RA). Top-line results of the clinical trial are expected to be available in the second half of 2012. In addition, Zalicus has drawn an additional $8.5 million from its $20.0 million secured credit facility with Oxford Finance to fund Synavive advancement.
“RA is an attractive initial indication for Synavive as there is unmet need for a safer glucocorticoid; one that provides the amplified anti-inflammatory activity of a higher dose but without the associated dose-related side effects. There is already a high prevalence of glucocorticoid use in RA for chronic maintenance therapy, as well as significant demand for less expensive, easier to access options to biologic therapy,” said Mark H.N. Corrigan, MD, President and CEO of Zalicus. “Data from a prior Phase 2a clinical trial with Synavive demonstrated encouraging preliminary signs of activity in RA patients and we look forward to further exploring its potential in this indication and reporting top-line SYNERGY results next summer.”
Study Design and Objectives
The Phase 2b clinical trial titled SYNERGY (SYNavivE for Reducing signs and sYmptoms of rheumatoid arthritis trial), is a 12-week, five-arm, global, double-blind, placebo-controlled study to evaluate the safety and efficacy of Synavive as a treatment for the signs and symptoms of RA in approximately 250 subjects with moderate to severe disease. The trial will be conducted in up to 60 centers throughout the United States, Europe and Latin America. The primary objective of the trial is to evaluate Synavive efficacy compared to placebo, while key additional secondary objectives include evaluating the efficacy of Synavive compared to its individual components (2.7mg of Prednisolone and 360mg of Dipyramidamole) as well as how Synavive performs in comparison to 5mg of Prednisone. Subjects who complete the core SYNERGY trial will be eligible to participate in a one-year extension study designed to investigate the long-term safety and durability of response for Synavive. To learn more about the SYNERGY trial and enrollment criteria please visit www.clinicaltrials.gov.
“We will consider the SYNERGY trial a success if Synavive demonstrates a statistically significant benefit compared to placebo and a clinically meaningful, but not necessarily statistically significant, benefit compared to its individual components alone,” said Jonathan Krant, MD, Vice President, Clinical Research of Zalicus. “In addition, we will seek to determine if the efficacy of Synavive is comparable to a commonly prescribed 5 mg dose of the glucocorticoid Prednisone.”
http://finance.yahoo.com/news/Zalicus-Initiates-Phase-2b-bw-…
Antwort auf Beitrag Nr.: 41.710.299 von bernie55 am 28.06.11 12:59:10Spotted at seeking Alpha
Zalicus Hits Grand Slam With Novartis
http://seekingalpha.com/article/277692-zalicus-hits-grand-sl…
Der 'Titel' gefällt schonmal...


Zalicus Hits Grand Slam With Novartis
http://seekingalpha.com/article/277692-zalicus-hits-grand-sl…
Der 'Titel' gefällt schonmal...


Antwort auf Beitrag Nr.: 41.740.225 von Growth2012 am 04.07.11 20:40:59Novartis and Zalicus haben dem Artikel nach dann u.a. wohl ein Abkommen? oder eine " Übereinkunft"? geschlossen bzgl. der Forschung von Multiple Myeloma. Das bedeutet vielleicht für die Zukunft viel Positives .
TIME WILL TRELL
Zalicus Hits Grand Slam With Novartis
July 1, 2011
Congratulations to the Zalicus (ZLCS) team for doing what few biotechs do. What am I thinking of? Delivering on a promise. CEO Corrigan promised investors that Synavive's Phase 2B trial would start by Q2 end. He delivered on that promise (click here). That deserves praise.
Which is why I am strongly encouraging investors to read a stellar instablog post by Zacks analyst, Jason Napodano (click here). Mr. Napodano has provided straight-forward analysis on Zalicus including a $4 price target. But I will add my own comment: Synavive's Phase 2B clinical trial design is among the best I've seen. Having studied the full range of prior Synavive clinical studies, Corrigan's team has created a trial design that if successful will qualify once/day Synavive for Phase 3. I am that confident. Furthermore, the funding for the trial was secured at the end of last year, so it is full speed ahead.
Yes, Synavive's Phase 2B clinical trial is under-way, but equally if not more importantly to market movers, there are four very serious points of information that explain how under-valued Zalicus share price is. That's why I call it a grand slam.
The first issue concerns publicized comments written by Zacks analyst Jason Napodano in various Seeking Alpha articles. He has repeatedly asserted that he is knowledgeable of Sanofi (SNY) moving Prednisporin forward. I for one have privately (e.g. email) and publicly questioned Mr. Napodano about his assertion (click here) and (click here). His source his own, Mr. Napodano repeatedly expresses a very favorable view regarding Prednisporin going forward. Meanwhile, Zalicus officials refuse, perhaps for legal reasons, to answer. Frankly, it would go a long way if Zalicus answered this key question for its shareholders, but maybe they can't and for good reason. Nevertheless, this appears to be great news and beyond speculation given Zacks' analyst Jason Napodano continues to make this ascertain. And I for one, trust Mr. Napodano. Therefore, in my opinion, Zalicus shareholders have very exciting news on the horizon.
The second issue regards the Q2 revelation that Zalicus, per the CEO's comments, has been doing what it can to get Covidien (COV) to file the necessary paperwork to deliver a 32mg version of Exalgo into the market. Apparently the safety data is complete. The question is, has Covidien done this? I'd like to know and so would fellow investors. Covidien, as we all know, may be selling its pharmaceutical division and Exalgo is clearly its number one trophy. Furthermore, according to CEO Corrigan, the 32mg version will add tremendous value to Exalgo sales, which in turn will benefit Zalicus. This story needs to be watched.
The third issue is the carefully guarded ion channel program. I have word that Zalicus plans to announce Z160 will enter Phase 1 clinical studies in September, and Z944 in Q4. Let's just say Zalicus is firing on all cyclinders and an array of patents that have been filed reflect the guarded approach Zalicus is taking to protect its proprietary knowledge under CSO Terrance Preston Snutch's leadership. My single disappointment was not having a front-row seat to hear Dr. Snutch address an audience of key pharmaceticals leaders in Philadelphia in June, and I could add Dr. Short's presentation on Zalicus's cuHTS technology. What does the "u" or "ultra" mean for Zalicus's future? I know investors are asking. Finally, move aside Z212 (the early sodium channel candidate) because Zalicus has already discovered a handful of improved sodium channel candidates. This must be the "army" Drs. Corrigan and Snutch alluded to in Q1.
The fourth issue is the kind of news that makes investors and market movers sit up and take notice. The market is yet to digest this information. It was discovered in June that Novartis (NVS) filed a patent for multiple myeloma (click here). Given Zalicus's Drs. Lee and Rickles work on multiple myeloma which for a time included a brief collaboration with Clinical Data Inc [acquired by Forest Laboratories (FRX)], the Novartis patent immediately pointed to Zalicus's work with dexamethasone in combination with other variables. But this aside, the bottom of the patent reads: "Data analysis was performed using the Chalice software developed by CombinatoRx (Zalicus Inc., Cambridge, MA, USA)" (Ibid). According to my read of the Novartis-Zalicus contract, Zalicus could earn a milestone up to $58M for each (Note: "each") indication, and if Zalicus shares proprietary space with Novartis--and I submit older Zalicus/CombinatoRx patents strongly suggest this (click here) and (here) and (here) and (here) and (here) and (here) and (here) and (here). Wow! That is what we call a very robust patent estate, so whatever Novartis is up to, expect Zalicus and its shareholders to profit in a very big way. Furthermore, shareholders will be eager to learn that along with Amgen (AMGN), apparently Eisai Company is also engaged in a pilot cHTS or cuHTS trial run, and I conclude there are others Zalicus is not disclosing.
Now what is the share price doing other than giving Zalicus LONGS a momentary headache? I have read that up to 5 million shares may be short, but I also agree with those who have noticed how closely Zalicus has been tracking the Russell 2000 Index. As of this report, Zalicus is just below the 50 moving day average, and it recently broke North after heading South following a very defined head and shoulders pattern. This means Zalicus is poised to retest $3.20. In my estimation, if the Novartis situation comes to light, the pop in the share price will be reminiscent of what recently happened with Icagen (ICGN) and Pfizer (PFE) -- a story I think Zalicus and Novartis could closely parallel.
Therefore, I am issuing a STRONG BUY on the information I have very carefully researched including all referenced documentation.
Disclosure: I am long ZLCS.
http://seekingalpha.com/article/277692-zalicus-hits-grand-sl…
TIME WILL TRELL

Zalicus Hits Grand Slam With Novartis
July 1, 2011
Congratulations to the Zalicus (ZLCS) team for doing what few biotechs do. What am I thinking of? Delivering on a promise. CEO Corrigan promised investors that Synavive's Phase 2B trial would start by Q2 end. He delivered on that promise (click here). That deserves praise.
Which is why I am strongly encouraging investors to read a stellar instablog post by Zacks analyst, Jason Napodano (click here). Mr. Napodano has provided straight-forward analysis on Zalicus including a $4 price target. But I will add my own comment: Synavive's Phase 2B clinical trial design is among the best I've seen. Having studied the full range of prior Synavive clinical studies, Corrigan's team has created a trial design that if successful will qualify once/day Synavive for Phase 3. I am that confident. Furthermore, the funding for the trial was secured at the end of last year, so it is full speed ahead.
Yes, Synavive's Phase 2B clinical trial is under-way, but equally if not more importantly to market movers, there are four very serious points of information that explain how under-valued Zalicus share price is. That's why I call it a grand slam.
The first issue concerns publicized comments written by Zacks analyst Jason Napodano in various Seeking Alpha articles. He has repeatedly asserted that he is knowledgeable of Sanofi (SNY) moving Prednisporin forward. I for one have privately (e.g. email) and publicly questioned Mr. Napodano about his assertion (click here) and (click here). His source his own, Mr. Napodano repeatedly expresses a very favorable view regarding Prednisporin going forward. Meanwhile, Zalicus officials refuse, perhaps for legal reasons, to answer. Frankly, it would go a long way if Zalicus answered this key question for its shareholders, but maybe they can't and for good reason. Nevertheless, this appears to be great news and beyond speculation given Zacks' analyst Jason Napodano continues to make this ascertain. And I for one, trust Mr. Napodano. Therefore, in my opinion, Zalicus shareholders have very exciting news on the horizon.
The second issue regards the Q2 revelation that Zalicus, per the CEO's comments, has been doing what it can to get Covidien (COV) to file the necessary paperwork to deliver a 32mg version of Exalgo into the market. Apparently the safety data is complete. The question is, has Covidien done this? I'd like to know and so would fellow investors. Covidien, as we all know, may be selling its pharmaceutical division and Exalgo is clearly its number one trophy. Furthermore, according to CEO Corrigan, the 32mg version will add tremendous value to Exalgo sales, which in turn will benefit Zalicus. This story needs to be watched.
The third issue is the carefully guarded ion channel program. I have word that Zalicus plans to announce Z160 will enter Phase 1 clinical studies in September, and Z944 in Q4. Let's just say Zalicus is firing on all cyclinders and an array of patents that have been filed reflect the guarded approach Zalicus is taking to protect its proprietary knowledge under CSO Terrance Preston Snutch's leadership. My single disappointment was not having a front-row seat to hear Dr. Snutch address an audience of key pharmaceticals leaders in Philadelphia in June, and I could add Dr. Short's presentation on Zalicus's cuHTS technology. What does the "u" or "ultra" mean for Zalicus's future? I know investors are asking. Finally, move aside Z212 (the early sodium channel candidate) because Zalicus has already discovered a handful of improved sodium channel candidates. This must be the "army" Drs. Corrigan and Snutch alluded to in Q1.
The fourth issue is the kind of news that makes investors and market movers sit up and take notice. The market is yet to digest this information. It was discovered in June that Novartis (NVS) filed a patent for multiple myeloma (click here). Given Zalicus's Drs. Lee and Rickles work on multiple myeloma which for a time included a brief collaboration with Clinical Data Inc [acquired by Forest Laboratories (FRX)], the Novartis patent immediately pointed to Zalicus's work with dexamethasone in combination with other variables. But this aside, the bottom of the patent reads: "Data analysis was performed using the Chalice software developed by CombinatoRx (Zalicus Inc., Cambridge, MA, USA)" (Ibid). According to my read of the Novartis-Zalicus contract, Zalicus could earn a milestone up to $58M for each (Note: "each") indication, and if Zalicus shares proprietary space with Novartis--and I submit older Zalicus/CombinatoRx patents strongly suggest this (click here) and (here) and (here) and (here) and (here) and (here) and (here) and (here). Wow! That is what we call a very robust patent estate, so whatever Novartis is up to, expect Zalicus and its shareholders to profit in a very big way. Furthermore, shareholders will be eager to learn that along with Amgen (AMGN), apparently Eisai Company is also engaged in a pilot cHTS or cuHTS trial run, and I conclude there are others Zalicus is not disclosing.
Now what is the share price doing other than giving Zalicus LONGS a momentary headache? I have read that up to 5 million shares may be short, but I also agree with those who have noticed how closely Zalicus has been tracking the Russell 2000 Index. As of this report, Zalicus is just below the 50 moving day average, and it recently broke North after heading South following a very defined head and shoulders pattern. This means Zalicus is poised to retest $3.20. In my estimation, if the Novartis situation comes to light, the pop in the share price will be reminiscent of what recently happened with Icagen (ICGN) and Pfizer (PFE) -- a story I think Zalicus and Novartis could closely parallel.
Therefore, I am issuing a STRONG BUY on the information I have very carefully researched including all referenced documentation.
Disclosure: I am long ZLCS.
http://seekingalpha.com/article/277692-zalicus-hits-grand-sl…
Antwort auf Beitrag Nr.: 41.742.676 von bernie55 am 05.07.11 11:58:19July 5, 2011
06:18 EDT
ZLCS
Zalicus initiated with an Outperform at Oppenheimer
Target $4.
http://www.theflyonthewall.com/permalinks/entry.php/ZLCSid14…
06:18 EDT
ZLCS
Zalicus initiated with an Outperform at Oppenheimer
Target $4.
http://www.theflyonthewall.com/permalinks/entry.php/ZLCSid14…
Zitat von bernie55:
Zalicus initiated with an Outperform at Oppenheimer
Target $4.
http://www.theflyonthewall.com/permalinks/entry.php/ZLCSid14…
Was so ein target gleich mit dem Kurs macht:
Antwort auf Beitrag Nr.: 41.742.676 von bernie55 am 05.07.11 11:58:19
...
Gute 'Chart & Info Quelle'
http://investorshub.advfn.com/boards/board.aspx?board_id=100…
http://ih.advfn.com/p.php?pid=squote&symbol=ZLCS
...

Gute 'Chart & Info Quelle'
http://investorshub.advfn.com/boards/board.aspx?board_id=100…
http://ih.advfn.com/p.php?pid=squote&symbol=ZLCS
Antwort auf Beitrag Nr.: 41.744.119 von HeinzBork am 05.07.11 15:51:20Tach Heinz, gut zu wissen das es dich noch gibt
Endlich mal ein gute Platform/bzw lobende Quelle stateside "MARKETWATCH"
Steigende Grüße

Endlich mal ein gute Platform/bzw lobende Quelle stateside "MARKETWATCH"
Steigende Grüße

Antwort auf Beitrag Nr.: 41.744.966 von Growth2012 am 05.07.11 17:46:09http://www.marketwatch.com/story/drug-stocks-sluggish-zalicu…
....

....


Antwort auf Beitrag Nr.: 41.744.966 von Growth2012 am 05.07.11 17:46:09Hello Growth,
ja klar bin ich noch dabei, hatte letzte Woche nochmal Nachschlag genommen, jetzt darf die Rakete schon mal Fahrt aufnehmen.
Mal sehen wie der Newsflow so ist, die 4$ sind ja hoffentlich nur ein Etappenziel
Vielleicht gibts ja einen Dendreon Effekt, obwohl ZLCS ja noch hier so gut wie unbekannt ist, was das Forum natürlich ganz angenehm macht.
Gruß zurück und hoffentlich bald volle Taschen
ja klar bin ich noch dabei, hatte letzte Woche nochmal Nachschlag genommen, jetzt darf die Rakete schon mal Fahrt aufnehmen.
Mal sehen wie der Newsflow so ist, die 4$ sind ja hoffentlich nur ein Etappenziel

Vielleicht gibts ja einen Dendreon Effekt, obwohl ZLCS ja noch hier so gut wie unbekannt ist, was das Forum natürlich ganz angenehm macht.
Gruß zurück und hoffentlich bald volle Taschen
Hier mal eine fiese Gegenstimme
http://seekingalpha.com/article/278121-the-short-case-for-za…
The Short Case for Zalicus: Not Worth Its Market Cap
The fair value of Zalicus (ZLCS) shares is about $0.50 to $1.00. Our fund is short Zalicus.
Calculating the fair value of Zalicus is relatively straightforward. There are two main assets, Exalgo and Synavive.
Exalgo is a pain drug sold by Covidien (COV). The royalties due to Zalicus are run-rating at approximately $1.5 million annually. The drug seems to have peaked in sales. Even assuming it doubles and then peaks, the Exalgo royalties ($3m X 8x sales for a cost-free royalty) are worth $24 million, or $0.25 per share. Exalgo isn't quite the flop a Nuedexta or Bidil is, but for it to be a meaningful drug for Zalicus' meager royalty share, it will have to do a lot more in revenue to move the needle for this minority owner.
Synavive is a maligned drug. In 2008, the Synavive "COMET-1" study for osteoarthritis failed. How soon the market forgets. This combination of two generic drugs did not do any better (amazingly, it did worse!) than its component drugs. Why should we expect a different outcome from the SYNERGY study? The biological rationale for this drug (formerly known as CRX-102) is flawed, and that was proven in COMET-1. I think Zalicus management is taking this study on because they have nothing better to do. With "SYNERGY", management gets to continue the farce that the company's market cap of $250 million resembles those of other "promising phase 2 companies".
Synavive is the combination of prednisolone and dipyridamole. The company claims they have a composition of matter patent on the combination of two extremely old drugs. Someone should inform management that to win a composition of matter patent, they should probably invent the compound in question first. Synavive won't work in SYNERGY. This asset is worthless. It's worse than worthless--it will eat up $10 to $20 million in cash as the SYNERGY study reads out in the next year.
I value the rest of the pipeline, including the interesting Nav ion channel drugs, with a modest $0.25 value until they enter the clinic.
With 90% downside, ZLCS is a great short. I think the average investor forgets there is no difference between a $2 stock and a $20 stock and a $200 stock and your expected return. A smaller stock price is actually inversely correlated with performance.
Yesterday's stock move in Zalicus is noteworthy. The 3 research analysts covering the stock, JMP Securities, Wedbush and Oppenheimer, have no interest in helping you make money. They exist to support their "business model" of writing new secondary deals for overvalued stocks like Zalicus. Zalicus' CEO made $2.5 million in 2010 according to Bloomberg ($446,000 in salary). Zalicus claims no serious institutional shareholders. Don't subsidize this CEO and this forgettable asset for the illusion of a "cheap" stock. Think in market cap - not stock price. Is this company worth $250 million? Is it worth $500 million or $100 million? If you can't follow this exercise, the market isn't for you. Consider a job selling research at an investment bank.
Disclosure: My hedge fund and I are short ZLCS. We may change our positions at anytime without updating Seeking Alpha.

http://seekingalpha.com/article/278121-the-short-case-for-za…
The Short Case for Zalicus: Not Worth Its Market Cap
The fair value of Zalicus (ZLCS) shares is about $0.50 to $1.00. Our fund is short Zalicus.
Calculating the fair value of Zalicus is relatively straightforward. There are two main assets, Exalgo and Synavive.
Exalgo is a pain drug sold by Covidien (COV). The royalties due to Zalicus are run-rating at approximately $1.5 million annually. The drug seems to have peaked in sales. Even assuming it doubles and then peaks, the Exalgo royalties ($3m X 8x sales for a cost-free royalty) are worth $24 million, or $0.25 per share. Exalgo isn't quite the flop a Nuedexta or Bidil is, but for it to be a meaningful drug for Zalicus' meager royalty share, it will have to do a lot more in revenue to move the needle for this minority owner.
Synavive is a maligned drug. In 2008, the Synavive "COMET-1" study for osteoarthritis failed. How soon the market forgets. This combination of two generic drugs did not do any better (amazingly, it did worse!) than its component drugs. Why should we expect a different outcome from the SYNERGY study? The biological rationale for this drug (formerly known as CRX-102) is flawed, and that was proven in COMET-1. I think Zalicus management is taking this study on because they have nothing better to do. With "SYNERGY", management gets to continue the farce that the company's market cap of $250 million resembles those of other "promising phase 2 companies".
Synavive is the combination of prednisolone and dipyridamole. The company claims they have a composition of matter patent on the combination of two extremely old drugs. Someone should inform management that to win a composition of matter patent, they should probably invent the compound in question first. Synavive won't work in SYNERGY. This asset is worthless. It's worse than worthless--it will eat up $10 to $20 million in cash as the SYNERGY study reads out in the next year.
I value the rest of the pipeline, including the interesting Nav ion channel drugs, with a modest $0.25 value until they enter the clinic.
With 90% downside, ZLCS is a great short. I think the average investor forgets there is no difference between a $2 stock and a $20 stock and a $200 stock and your expected return. A smaller stock price is actually inversely correlated with performance.
Yesterday's stock move in Zalicus is noteworthy. The 3 research analysts covering the stock, JMP Securities, Wedbush and Oppenheimer, have no interest in helping you make money. They exist to support their "business model" of writing new secondary deals for overvalued stocks like Zalicus. Zalicus' CEO made $2.5 million in 2010 according to Bloomberg ($446,000 in salary). Zalicus claims no serious institutional shareholders. Don't subsidize this CEO and this forgettable asset for the illusion of a "cheap" stock. Think in market cap - not stock price. Is this company worth $250 million? Is it worth $500 million or $100 million? If you can't follow this exercise, the market isn't for you. Consider a job selling research at an investment bank.
Disclosure: My hedge fund and I are short ZLCS. We may change our positions at anytime without updating Seeking Alpha.
Antwort auf Beitrag Nr.: 41.749.667 von HeinzBork am 06.07.11 13:48:05The Short Case for Zalicus: Not Worth Its Market Cap
The fair value of Zalicus (ZLCS) shares is about $0.50 to $1.00. Our fund is short Zalicus.
...tja, wenn man sich jetzt aktuell den Kurs anschaut,
(Nasdaq: ZLCS )
Real Time: 2.60 Down 0.03 (1.14%) 11:46AM EDT,
dann hat dieser Artikel den SHORTIES wohl nicht in die Hände gespielt....
The fair value of Zalicus (ZLCS) shares is about $0.50 to $1.00. Our fund is short Zalicus.
...tja, wenn man sich jetzt aktuell den Kurs anschaut,
(Nasdaq: ZLCS )
Real Time: 2.60 Down 0.03 (1.14%) 11:46AM EDT,
dann hat dieser Artikel den SHORTIES wohl nicht in die Hände gespielt....
Antwort auf Beitrag Nr.: 41.751.245 von bernie55 am 06.07.11 17:50:39Mit so ner schwachen Nummer kann man ja auch nicht alles shorten.
Er sollte mal lieber anfangen gegen den Dollar oder Euro zu wetten, da kann er langfristig nur gewinnen.
Er sollte mal lieber anfangen gegen den Dollar oder Euro zu wetten, da kann er langfristig nur gewinnen.
Antwort auf Beitrag Nr.: 41.742.676 von bernie55 am 05.07.11 11:58:19Rebutting "The Short Case for Zalicus"
A short hedge fund published an article today, The Short Case for Zalicus: Not Worth Its Market Cap.
I contend the article is fallacious. For example, the author:
Completely ignored cHTS revenue sources, cash on hand, collaborations. For example the company reported: "As of March 31, 2011, we had cash, cash equivalents and short-term investments of approximately $52.8 million, which includes $1.9 million of restricted cash" (5/5/11 Quarterly report that can be found here). Furthermore the company raised an additional ~$20M via Wedbush the end of March/early April.
Completely misrepresented Synavive. Zalicus is on record that reformulated Synavive has entered a Phase 2-B trial on promising data in rheumatoid and osteo-arthritis; the reformulated version mitigates headaches and throughout all clinical trials including a one-year extension provided an excellent safety profile. Here's the factual truth presented by Zacks analyst Jason Napodano.
Completely contradicts Covidien's (COV) CEO, who has stated on conference calls that Exalgo's sales have not peaked, but have exceeded their expectations. Frankly, the author's assertion is incredulous given Exalgo is just concluding its first year of sales and is still ramping up, with a 32mg version on the horizon. Furthermore, the sales projections from Covidien have ranged from $200-300M/year.
It strikes me as odd that the author provided no sources, documentation, or references for his assertions.
Furthermore, four reputable firms have set targets: Zacks and Oppenheimer: $4/share. JMP and Wedbush: $5/share. In addition, Zalicus has a cancer collaboration with Novartis, and a drug in Phase 2-B completed development with Sanofi (SNY).
I perceive a short squeeze may be in the offing.
Disclosure: I am long ZLCS.
http://seekingalpha.com/article/278231-rebutting-the-short-c…
A short hedge fund published an article today, The Short Case for Zalicus: Not Worth Its Market Cap.
I contend the article is fallacious. For example, the author:
Completely ignored cHTS revenue sources, cash on hand, collaborations. For example the company reported: "As of March 31, 2011, we had cash, cash equivalents and short-term investments of approximately $52.8 million, which includes $1.9 million of restricted cash" (5/5/11 Quarterly report that can be found here). Furthermore the company raised an additional ~$20M via Wedbush the end of March/early April.
Completely misrepresented Synavive. Zalicus is on record that reformulated Synavive has entered a Phase 2-B trial on promising data in rheumatoid and osteo-arthritis; the reformulated version mitigates headaches and throughout all clinical trials including a one-year extension provided an excellent safety profile. Here's the factual truth presented by Zacks analyst Jason Napodano.
Completely contradicts Covidien's (COV) CEO, who has stated on conference calls that Exalgo's sales have not peaked, but have exceeded their expectations. Frankly, the author's assertion is incredulous given Exalgo is just concluding its first year of sales and is still ramping up, with a 32mg version on the horizon. Furthermore, the sales projections from Covidien have ranged from $200-300M/year.
It strikes me as odd that the author provided no sources, documentation, or references for his assertions.

Furthermore, four reputable firms have set targets: Zacks and Oppenheimer: $4/share. JMP and Wedbush: $5/share. In addition, Zalicus has a cancer collaboration with Novartis, and a drug in Phase 2-B completed development with Sanofi (SNY).
I perceive a short squeeze may be in the offing.

Disclosure: I am long ZLCS.
http://seekingalpha.com/article/278231-rebutting-the-short-c…
Zitat von bernie55: Rebutting "The Short Case for Zalicus"
A short hedge fund published an article today, The Short Case for Zalicus: Not Worth Its Market Cap.
I contend the article is fallacious. For example, the author:
Completely ignored cHTS revenue sources, cash on hand, collaborations. For example the company reported: "As of March 31, 2011, we had cash, cash equivalents and short-term investments of approximately $52.8 million, which includes $1.9 million of restricted cash" (5/5/11 Quarterly report that can be found here). Furthermore the company raised an additional ~$20M via Wedbush the end of March/early April.
Completely misrepresented Synavive. Zalicus is on record that reformulated Synavive has entered a Phase 2-B trial on promising data in rheumatoid and osteo-arthritis; the reformulated version mitigates headaches and throughout all clinical trials including a one-year extension provided an excellent safety profile. Here's the factual truth presented by Zacks analyst Jason Napodano.
Completely contradicts Covidien's (COV) CEO, who has stated on conference calls that Exalgo's sales have not peaked, but have exceeded their expectations. Frankly, the author's assertion is incredulous given Exalgo is just concluding its first year of sales and is still ramping up, with a 32mg version on the horizon. Furthermore, the sales projections from Covidien have ranged from $200-300M/year.
It strikes me as odd that the author provided no sources, documentation, or references for his assertions.
Furthermore, four reputable firms have set targets: Zacks and Oppenheimer: $4/share. JMP and Wedbush: $5/share. In addition, Zalicus has a cancer collaboration with Novartis, and a drug in Phase 2-B completed development with Sanofi (SNY).
I perceive a short squeeze may be in the offing.
Disclosure: I am long ZLCS.
http://seekingalpha.com/article/278231-rebutting-the-short-c…
Das war auf jeden Fall ausführlicher als mein Gesabbel

und wenn man sich so ein Chartbild anschaut kommt unweigerlich der Gedanke an High Frequency Trading auf.
So an der Schnur gezogen sieht sehr merkwürdig aus.
Antwort auf Beitrag Nr.: 41.749.667 von HeinzBork am 06.07.11 13:48:05Guten Morgen allerseits!
Heinz, ich finde es wirklich prima das man-(bereits jetzt schon)-versucht neagtive Stimmung gegen Zalicus zu erzeugen...wieso?....dort wo sich kein Potential befindet, hält sich kein shortie lange auf
Wie schneidet Zalicus ab in Amerika im Bezug auf MK/Staus etc?- ein echte fliegenschiß- bei ein gesamten MK von 245,87 Mio. USD mit 94,57 Mio frei verhandelbare Aktienanteile ist die Firma nicht mal erwähenswert (aber irgendwie hat ZLCS begierden geweckt).
Tja, solche kleine mid caps (insbesonderes unter 5$ notierend, sind völlig uninteressant für Fund Managers & ungeduldige Aktionäre sowieso, weil sie halt nicht auf die 'schnelle Welle' mit nach oben schwimmen können...aber manche Stateside haben (im Fall ZLCS) anscheint jetzt schon Zukunfts-Lunte gerochen
Heinz, ich finde es wirklich prima das man-(bereits jetzt schon)-versucht neagtive Stimmung gegen Zalicus zu erzeugen...wieso?....dort wo sich kein Potential befindet, hält sich kein shortie lange auf
Wie schneidet Zalicus ab in Amerika im Bezug auf MK/Staus etc?- ein echte fliegenschiß- bei ein gesamten MK von 245,87 Mio. USD mit 94,57 Mio frei verhandelbare Aktienanteile ist die Firma nicht mal erwähenswert (aber irgendwie hat ZLCS begierden geweckt).
Tja, solche kleine mid caps (insbesonderes unter 5$ notierend, sind völlig uninteressant für Fund Managers & ungeduldige Aktionäre sowieso, weil sie halt nicht auf die 'schnelle Welle' mit nach oben schwimmen können...aber manche Stateside haben (im Fall ZLCS) anscheint jetzt schon Zukunfts-Lunte gerochen

Antwort auf Beitrag Nr.: 41.753.567 von Growth2012 am 07.07.11 08:34:56Ach Ja, dann kamm dieses Gesternnachmittag 
These Cold Stocks Are Heating Up... (nur Drei an der Zahl )
)
http://www.fool.com/investing/general/2011/07/06/these-cold-…

These Cold Stocks Are Heating Up... (nur Drei an der Zahl
 )
)http://www.fool.com/investing/general/2011/07/06/these-cold-…
 ..so dann versuchen wir mal ZALICUS ein bisschen bekannter zu machen...
..so dann versuchen wir mal ZALICUS ein bisschen bekannter zu machen...
Zaliclus: Undervalued, With Strong Pipeline Potential
Zalicus announced it has initiated a phase 2b clinical trial evaluating Synavive for the treatment of rheumatoid arthritis (RA). The trial, SYNERGY (SYNavivE for Reducing signs and sYmptoms of rheumatoid arthritis trial), is a 12-week, 5-arm, double-blind, placebo-controlled study to evaluate the safety and efficacy of Synavive as a treatment for the signs and symptoms of rheumatoid arthritis in approximately 250 subjects with moderate to severe disease. The trial will be conducted in up to 60 centers throughout the U.S., Europe, and Latin America.
The primary objective of the trial is to evaluate Synavive efficacy compared to placebo. The primary endpoint will be efficacy measured by the Disease Activity Score in 28 Joints (DAS28). DAS28 is a common outcome tool used in therapeutic trials to assess disease improvement or response. Key secondary objectives include evaluating the efficacy of Synavive compared to its individual components (2.7mg of prednisolone and
360mg of dipyramidamole) as well as how Synavive performs in comparison to 5mg of prednisolone. Subjects who complete the core SYNERGY trial will be eligible to participate in a one-year extension study designed to investigate the long-term safety and durability of response for Synavive. We estimate the program will take approximately 12 months to complete, thus we are expecting top-line data in the middle of 2012.
Previous Data Supports Optimism
We are optimistic on the outcome of the trial based on results from the previous phase 2 studies with Synavive in RA. As a reminder, in November 2006, management presented positive data from a phase 2a program studying Synavive in patients with RA. The trial compared Synavive plus a disease-modifying antirheumatic drug (DMARD) to placebo plus DMARD (control) in subjects with RA over a 6-week treatment period. The primary endpoint was reduction in C-reactive protein (CRP) levels comparing drug plus DMARD to placebo plus DMARD from baseline at day 42. Secondary endpoints of the trial included ACR-20 responses and DAS-28 scores.
During the trial, Synavive was generally well-tolerated with no serious adverse events (SAE). The most common side effect was headache, with gastro-intestinal symptoms and dizziness also reported. These are all common side effects of the dipyridamole.
We note that Zalicus has also conducted a phase 2b study testing Synavive in patients with knee osteoarthritis (OA). COMET was a large-scale study in 279 patients with knee OA, with a standard flare design where subjects with active disease needed to demonstrate an increase in knee pain as determined by the WOMAC question #1 (related to pain while walking on a flat surface) upon withdrawal of their NSAID/COXIB therapy to be eligible. Subjects were dosed for a total of 14 weeks (98 days) including an initial two-week dipyridamole titration phase. The data shows a dose-related improvement in baseline change, however, the results were not significant for any cohort tested. We believe this was due to the high discontinuation rate (32%), mainly the result of adverse events and subject requests. In follow-up analysis, management identified a number of protocol violators, including use of restricted or prohibited medications during the trial. It is important to note, over half of the protocol violators were in the placebo or prednisolone alone cohort. This dramatically impacted the statistical analysis when compared to the studied doses of Synavive.
Management conducted a modified ITT analysis using the last observation carried forward for these non-compliant patients. Under the mITT analysis, the highest dose of Synavive (2.7/360mg) did demonstrate clinically meaningful benefits in WOMAC Pain, Stiffness, Physical Function, and Hand Pain VAS. The data also showed an improved dose response curve for the low and middle-dose Synavive cohorts. The high dose of Synavive (2.7/360mg) demonstrated an 18.1mm improvement over placebo and a 6.2mm improvement over prednisolone alone on the primary WOMAN Pain endpoint.
Subjects who completed the 14-week core study were eligible to participate in a 12-month, open-label extension study designed to investigate the long-term safety and durability of response for Synavive. This data was presented in October 2009 at the American College of Rheumatology 2009 annual meeting. The data demonstrated that Synavive subjects maintained efficacy levels throughout the 12 month extension trial. These findings further support the mITT data that demonstrated efficacy for the highest dose of Synavive. In addition, subjects on placebo were able to cross-over to Synavive for the 12 month extension study. These patients also experienced statistically significant improvements in all WOMAC measurement subscales upon crossing over.
Importantly, no treatment-related increase in glucocorticoid associated adverse events was observed in any Synavive treated subjects. This includes no worsening of vision, adrenal glucose, infection rate, hypotension, hyperglycemia, or osteoporosis (as measured by bone mineral density).
New Formulation Should Reduce Discontinuations
COMET failed due to the high discontinuation rate of 32%. A significant number of patients in the trial reported headaches. This is most likely the result of the dipyridamole component, which has been known to cause significant headache due to its vasodilating properties. Management has re-formulated the dipyridamole in Synavive from an immediate release to a modified release. The new formulation of Synavive provides time-release co-exposure to both prednisolone and dipyridamole. It also offers a significantly improved tolerability and side-effect profile, as demonstrated in a small (n=24) patient study in which 0% of the subjects experienced headache vs. 41% for conventional dipyridamole. The modified release formulation also allows for a once daily dosing format. We note this new formulation is what Zalicus is studying in the phase 2b SYNERGY trial discussed above.
A Big Opportunity
Rheumatoid arthritis is among the most common degenerative joint diseases and a frequent cause of physical disability among older adults. In the U.S. more than 100 million people suffer from RA and OA, combined. The disease affects the hands, lower back, neck, and weight-bearing joints such as the knees, hips, and feet. Symptoms range from stiffness and intermittent mild pain to severe joint pain and impaired biomechanical function. Over the past year, management has been conducting a series of interviews with key opinion leaders, practicing rheumatologists, primary care doctors and managed care personnel to fully explore the potential for Synavive in RA. Based on their feedback, management believes that RA is attractive on a number of fronts, including current medical practice in which there is already a high prevalence of glucocorticoid use in RA to combat inflammation and restore function, as well as the unmet need for less expensive, easier to access options to biologic therapy.
The SYNERGY trial is set up to demonstrate that Synavive superiority to placebo and the individual components, and offer non-inferiority to the higher dose of 5mg prednisolone. The ultimate goal of Synavive would be to replicate a 7.5mg dose of prednisolone, albeit without the side-effects commonly associated with high doses of corticosteroids. This would allow RA patients to take Synavive daily as a maintenance therapy to prevent flare-ups. The current treatment paradigm is for patients to manage pain by using non-steroidal anti-inflammatory drugs ((NSAIDS)) and Cox-II inhibitors, local analgesics, opioids, oral steroids, disease modifying antirheumatic drugs, intra-articular corticosteroid injection and surgery. The withdrawal of two high-profile Cox-II drugs, Merck s Vioxx and Pfizer s Bextra, has created a significant void the prescription NSAID market. These two drugs were annualizing at sales over $6 billion worldwide before they were pulled from the market for cardiovascular safety concerns. As such, the market has dramatically shifted toward over-the-counter NSAIDs such as naproxen and ibuprofen to management pain.
Rheumatologists typically start patients with RA out on steroids or DARMSs such as cyclosporine and methotrexate. Rheumatologists do not like to prescribe high doses of prednisolone as a maintenance therapy to control flare-ups due to the potential for serious side-effects such as high blood sugar, dyslipidemia, weight gain, insomnia, and weakening immune system. Failure of these pain and steroid medications leads to the use of stronger disease modifying anti-rheumatic drugs such as TNF-alpha molecules and biologics. These drugs carry potential serious metabolic, gastrointestinal, renal, and liver risks. The biologics are also injectable drugs, offering less convenience than a once-daily oral medication. Accordingly, a safer alternative such as Synavive could be a blockbuster product in the hands of a major pharmaceutical company.
The treatment paradigm for osteoarthritis plays out in a similar fashion. In the U.S. more than 100 million people suffer from RA and OA combined. We encourage investors to view our research report from November 2010 that discusses the previous phase 2 data with Synavive in the phase 2a RA program and the phase 2b COMET-1 OA program. Beyond RA and OA, we believe Synavive has potential in other steroid-responsive diseases such as polymyalgia rheumatica, lupus and ulcerative colitis (Crohn's).
IP Position Solid
In April 2011, Zalicus announced that it has been granted a composition-of-matter patent by the U.S. Patent and Trademark office covering Synavive. U.S. patent number 7,915,265 entitled "Combinations for the Treatment of Immunoinflammatory Disorders" provides broad coverage for Synavive through August 2025. This key patent, along with the existing issued method-of-use patent (US patent 7,253,155), fortifies the Synavive patent estate, which includes several patents and patent applications, granted and pending worldwide which collectively provide coverage of Synavive through 2028.
Cash Position Solid
Zalicus exited the first quarter ended March 31, 2011 with $52.8 million in cash and investments. We view this to be sufficient cash to fund operations well into 2013.
We note that on December 22, 2010, Zalicus entered into a loan and security agreement with Oxford Finance Corporation for up to $20 million. The loan included three terms: Term-A: $3.0 million upfront, Term-B: $8.5 million on or before July 15, 2011, and Term-C: $8.5 million on or before January 15, 2012. Management has taken down the initial $3.0 million upfront payment (Term-A) and the second $8.5 million tranche (Term-B) upon the initiation of the SYNERGY trial in late June 2011.
During the first quarter 2011, Zalicus raised approximately $15.1 million through an equity distribution agreement with Wedbush Securities relating to the sale of 7.14 million shares of common stock. We note that the offering was completed in April 2011 with an additional 1.75 million shares, for an additional $4.1 million in cash.
Recommendation
Our sum of parts / NPV analysis for Zalicus yields a total firm value of roughly $350 million, or $4 per share. We see Exalgo alone worth $1.50 per share. We note the current cash balance is over $0.50 per share. At the current value of only $250 million we believe the company is under-valued. We see upside beyond the $4 level based on positive news from Sanofi on prednisporin, or partnering of the early-stage ion channel program. We rate the shares 'Outperform'.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/278359-zaliclus-undervalued-…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/278359-zaliclus-undervalued-…
Heute wurde sie mal wieder von der Leine gelassen, schon 10% im Plus mit respektablen Umsätzen.
http://investing.money.msn.com/investments/stock-price?Symbo…
http://investing.money.msn.com/investments/stock-price?Symbo…
Market Report -- Short Stories (ZLCS)
July 7, 2011 9:16 AM ET
Zalicus initiated with a Mkt Outperform at Rodman & Renshaw; tgt $5 . Rodman & Renshaw initiates (ZLCS) with a Mkt Outperform and a $5 tgt saying they believe that the co's drug combination approach and its innovative ion channel modulator discovery platform provide a renewable source of drug candidates throughout the development spectrum. They believe that the co could be valued at par with the average of a comparable group of drug developers with Phase 2/3 assets.
Briefing.com is the leading Internet provider of live market analysis for U.S. Stock, U.S. Bond, and world FX market participants.
July 7, 2011 9:16 AM ET
Zalicus initiated with a Mkt Outperform at Rodman & Renshaw; tgt $5 . Rodman & Renshaw initiates (ZLCS) with a Mkt Outperform and a $5 tgt saying they believe that the co's drug combination approach and its innovative ion channel modulator discovery platform provide a renewable source of drug candidates throughout the development spectrum. They believe that the co could be valued at par with the average of a comparable group of drug developers with Phase 2/3 assets.
Briefing.com is the leading Internet provider of live market analysis for U.S. Stock, U.S. Bond, and world FX market participants.
Analysts Initiated New Ratings on These 6 Stocks Today
..darunter auch ZALICUS...
On Thursday July 7, 2011, 12:01 pm EDT
Zalicus (NASDAQ:ZLCS): Rodman & Renshaw rates this company a Market Outperform , with a price target of $5.
About the company: Las Vegas Sands Corp. owns and operates casino resorts and convention centers. The Company operates in United States, Macau and Singapore. Las Vegas Sand Corp’s casino’s offer a wide range of gaming activities and entertainment as well as overnight accomodations, while its expo centers host a wide range of entertainment shows, expositions, and other activities.
http://finance.yahoo.com/news/Analysts-Initiated-New-wscheat…
..darunter auch ZALICUS...
On Thursday July 7, 2011, 12:01 pm EDT
Zalicus (NASDAQ:ZLCS): Rodman & Renshaw rates this company a Market Outperform , with a price target of $5.
About the company: Las Vegas Sands Corp. owns and operates casino resorts and convention centers. The Company operates in United States, Macau and Singapore. Las Vegas Sand Corp’s casino’s offer a wide range of gaming activities and entertainment as well as overnight accomodations, while its expo centers host a wide range of entertainment shows, expositions, and other activities.
http://finance.yahoo.com/news/Analysts-Initiated-New-wscheat…
Will Zalicus CEO Surprise Shareholders Today?
Recently, Zalicus (ZLCS) shares touched a new high, but then began to rapidly decline going into early May- in anticipation of the first quarter report. The pre-market release of the quarterly report went down like swallowing an unchewed potato chip as longs like myself braced for the market's profit-taking plunge. I admit, this dip has tested my patience with Covidien (COV). For example, I am sick and tired of Covidien lumping Exalgo results in with Pennsaid. It is obvious that in Q1 2011, Pennsaid, not Exalgo, had miserable results. Exalgo, it appears, had stellar results, but it got lost in the rebate checks. But why isn't that story being told-- that Exalgo is doing so well, Covidien may have to upward-revise its sales expectations? That is, after all, what Covidien's CEO said.
For Zalicus, the angst appears to be centered on the return of Exalgo royalties ($350,000). After all, weeks earlier, Covidien's CEO spoke in glowing terms, but investors were told by Zalicus that Covidien had shaved that royalty check: "[sales] were impacted by multiple Covidien rebate programs associated with initial inventory shipments."
Hier gehts weiter: http://seekingalpha.com/article/268963-will-zalicus-ceo-surp…
Recently, Zalicus (ZLCS) shares touched a new high, but then began to rapidly decline going into early May- in anticipation of the first quarter report. The pre-market release of the quarterly report went down like swallowing an unchewed potato chip as longs like myself braced for the market's profit-taking plunge. I admit, this dip has tested my patience with Covidien (COV). For example, I am sick and tired of Covidien lumping Exalgo results in with Pennsaid. It is obvious that in Q1 2011, Pennsaid, not Exalgo, had miserable results. Exalgo, it appears, had stellar results, but it got lost in the rebate checks. But why isn't that story being told-- that Exalgo is doing so well, Covidien may have to upward-revise its sales expectations? That is, after all, what Covidien's CEO said.
For Zalicus, the angst appears to be centered on the return of Exalgo royalties ($350,000). After all, weeks earlier, Covidien's CEO spoke in glowing terms, but investors were told by Zalicus that Covidien had shaved that royalty check: "[sales] were impacted by multiple Covidien rebate programs associated with initial inventory shipments."
Hier gehts weiter: http://seekingalpha.com/article/268963-will-zalicus-ceo-surp…
Antwort auf Beitrag Nr.: 41.770.860 von HeinzBork am 11.07.11 16:13:43Die nächsten gerüchte kreisen....tja, wir sind mittlerweile sogar bei Pfizer angekommen

http://seekingalpha.com/article/278891-zalicus-a-potential-t…
Gute "Top 15 Liste", und so bleibt ZLCS in alle Munde

http://www.fool.com/investing/high-growth/2011/07/11/the-15-…
Seeking Alpha ist allerding "fast immer" mit Vorsicht zu geniessen... (Je nach Autor
(Je nach Autor )
)


http://seekingalpha.com/article/278891-zalicus-a-potential-t…
Gute "Top 15 Liste", und so bleibt ZLCS in alle Munde


http://www.fool.com/investing/high-growth/2011/07/11/the-15-…
Seeking Alpha ist allerding "fast immer" mit Vorsicht zu geniessen...
 (Je nach Autor
(Je nach Autor )
)
Zalicus Reports Financial Results for the Second Quarter 2011
Press Release Source: Zalicus Inc. On Wednesday August 3, 2011, 7:00 am EDT
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ:ZLCS - News) today reported financial results for the second quarter ended June 30, 2011.
“During the first half of 2011 we diligently executed on our product development plans with the initiation of the Synavive Phase 2b clinical trial for rheumatoid arthritis and the advancement of our Ion channel product candidates, which remain on track to enter the clinic by the end of this year,” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “The initiation of these clinical trials will mark an evolution of the Zalicus pipeline and the progress of our R&D efforts from discovery research toward clinical development.”
http://finance.yahoo.com/news/Zalicus-Reports-Financial-bw-4…
Keiner mehr da...?
Press Release Source: Zalicus Inc. On Wednesday August 3, 2011, 7:00 am EDT
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ:ZLCS - News) today reported financial results for the second quarter ended June 30, 2011.
“During the first half of 2011 we diligently executed on our product development plans with the initiation of the Synavive Phase 2b clinical trial for rheumatoid arthritis and the advancement of our Ion channel product candidates, which remain on track to enter the clinic by the end of this year,” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “The initiation of these clinical trials will mark an evolution of the Zalicus pipeline and the progress of our R&D efforts from discovery research toward clinical development.”
http://finance.yahoo.com/news/Zalicus-Reports-Financial-bw-4…
Keiner mehr da...?
Nach der lange Leideneszeit...meldet sich auch JPM Securities auch noch zu wort(5 Sterne)
This is a list of top research analysts based on the accuracy of earnings estimates on ZLCS, according to StarMine. Analysts that appear here are limited to those covering ZLCS for a significant period of time. Learn More.
Total Ranked Analysts: 5
EPS Accuracy for ZLCS - Trailing Two Fiscal Years and Four Quarters
Top-Ranked Analysts ZLCS Overall Research Reports
Duncan, Charles
JMP Securities
Top Analysts in the Biotechnology Industry:
EPS Accuracy - Industry Excess Return
http://finance.yahoo.com/q/sa?s=zlcs
This is a list of top research analysts based on the accuracy of earnings estimates on ZLCS, according to StarMine. Analysts that appear here are limited to those covering ZLCS for a significant period of time. Learn More.
Total Ranked Analysts: 5
EPS Accuracy for ZLCS - Trailing Two Fiscal Years and Four Quarters
Top-Ranked Analysts ZLCS Overall Research Reports
Duncan, Charles
JMP Securities
Top Analysts in the Biotechnology Industry:
EPS Accuracy - Industry Excess Return
http://finance.yahoo.com/q/sa?s=zlcs
rein @ 1.44
jetzt flieeeeg!
jetzt flieeeeg!

Jaa, sie lebt noch...
Antwort auf Beitrag Nr.: 42.274.312 von HeinzBork am 28.10.11 21:03:12daß ich das noch erleben darf....
Zalicus Reports Financial Results for the Third Quarter 2011
businesswire
Press Release Source: Zalicus Inc. On Wednesday November 2, 2011, 7:50 am EDT
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ:ZLCS - News) today reported financial results for the third quarter ended September 30, 2011.
“We continue to execute on our product development goals. Patient enrollment in the SYNERGY Phase 2b clinical trial of Synavive™ for rheumatoid arthritis is on track with clinical sites actively recruiting subjects across the United States, Europe and Latin America, and we are finalizing preparations to initiate clinical development of Z160, with the goal of moving quickly into Phase 2 trials with a new formulation in 2012,” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “We plan to report top-line clinical results for both Synavive and our clinical-stage Ion channel programs in 2012.”
Third Quarter 2011 and Recent Accomplishments:
Published research on the role of sodium channels in acute and chronic pain processing. The paper, entitled “Identification of Sodium Channel Isoforms that Mediate Action Potential Firing in Lamina I/II Spinal Cord Neurons,” Hildebrand et al., was pre-published on-line in the journal Molecular Pain (2011) and can be found on the Zalicus website. This research provides important new insights into the mediation of spinal cord neuron firing to determine the functional properties of sodium channel expression creating potential new ion channel targets for future pain therapeutics and further validating the pioneering scientific work of the Zalicus Ion channel research team.
Received a notice of allowance from the U.S. patent and trademark office with allowed claims covering methods of use for Synavive as a treatment for osteoarthritis. Once this patent issues, the Synavive portfolio will include patents covering the Synavive composition of matter, methods of treating rheumatoid arthritis, osteoarthritis and other diseases, as well as pending patent applications directed to various Synavive formulations, providing comprehensive protection for Synavive, with patent terms that extend until at least 2028.
Third Quarter 2011 Financial Results (Unaudited):
As of September 30, 2011, Zalicus had cash, cash equivalents, restricted cash and short-term investments of $49.0 million compared to $56.0 million on June 30, 2011.
For the quarter ended September 30, 2011, revenue was $2.4 million compared to $1.2 million for the quarter ended September 30, 2010. Revenue is derived from research and development collaborations with Novartis and Amgen and other pilot research parties, a grant from the United States Army Medical Research Institute for Infectious Diseases and $0.8 million in royalty revenue received from Covidien based on Exalgo sales. To date, Zalicus has recognized $3.3 million in revenue related to Exalgo royalties since its market launch in April 2010. The increase in revenue in the three months ended September 30, 2011 was primarily due to Exalgo royalties from Covidien and an increase in the recognition of collaboration revenue from Novartis.
For the quarter ended September 30, 2011, net loss was $9.3 million, or $0.09 per share, compared to a net loss of $11.4 million, or $0.13 per share, in the quarter ended September 30, 2010.
Research and development expenses were $8.9 million in the quarter ended September 30, 2011 compared to $5.1 million in the quarter ended September 30, 2010. The $3.8 million increase was due primarily to an increase in expenses related to the clinical development of Synavive and preclinical development expenses related to our Ion channel product candidates, including Z160 and Z944. We expect research and development expenses to increase for the year ended December 31, 2011 compared to 2010 due to the Phase 2b SYNERGY trial and the advancement of our Ion channel product candidates such as Z160 and Z944 into Phase 1 clinical development.
General and administrative expenses were $2.6 million in the quarter ended September 30, 2011 compared to $2.8 million in the quarter ended September 30, 2010. We expect our general and administrative expenses for the year ended December 31, 2011 to be consistent with such expenses for 2010.
http://finance.yahoo.com/news/Zalicus-Reports-Financial-bw-3…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/Zalicus-Reports-Financial-bw-3…
businesswire
Press Release Source: Zalicus Inc. On Wednesday November 2, 2011, 7:50 am EDT
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ:ZLCS - News) today reported financial results for the third quarter ended September 30, 2011.
“We continue to execute on our product development goals. Patient enrollment in the SYNERGY Phase 2b clinical trial of Synavive™ for rheumatoid arthritis is on track with clinical sites actively recruiting subjects across the United States, Europe and Latin America, and we are finalizing preparations to initiate clinical development of Z160, with the goal of moving quickly into Phase 2 trials with a new formulation in 2012,” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “We plan to report top-line clinical results for both Synavive and our clinical-stage Ion channel programs in 2012.”
Third Quarter 2011 and Recent Accomplishments:
Published research on the role of sodium channels in acute and chronic pain processing. The paper, entitled “Identification of Sodium Channel Isoforms that Mediate Action Potential Firing in Lamina I/II Spinal Cord Neurons,” Hildebrand et al., was pre-published on-line in the journal Molecular Pain (2011) and can be found on the Zalicus website. This research provides important new insights into the mediation of spinal cord neuron firing to determine the functional properties of sodium channel expression creating potential new ion channel targets for future pain therapeutics and further validating the pioneering scientific work of the Zalicus Ion channel research team.
Received a notice of allowance from the U.S. patent and trademark office with allowed claims covering methods of use for Synavive as a treatment for osteoarthritis. Once this patent issues, the Synavive portfolio will include patents covering the Synavive composition of matter, methods of treating rheumatoid arthritis, osteoarthritis and other diseases, as well as pending patent applications directed to various Synavive formulations, providing comprehensive protection for Synavive, with patent terms that extend until at least 2028.
Third Quarter 2011 Financial Results (Unaudited):
As of September 30, 2011, Zalicus had cash, cash equivalents, restricted cash and short-term investments of $49.0 million compared to $56.0 million on June 30, 2011.
For the quarter ended September 30, 2011, revenue was $2.4 million compared to $1.2 million for the quarter ended September 30, 2010. Revenue is derived from research and development collaborations with Novartis and Amgen and other pilot research parties, a grant from the United States Army Medical Research Institute for Infectious Diseases and $0.8 million in royalty revenue received from Covidien based on Exalgo sales. To date, Zalicus has recognized $3.3 million in revenue related to Exalgo royalties since its market launch in April 2010. The increase in revenue in the three months ended September 30, 2011 was primarily due to Exalgo royalties from Covidien and an increase in the recognition of collaboration revenue from Novartis.
For the quarter ended September 30, 2011, net loss was $9.3 million, or $0.09 per share, compared to a net loss of $11.4 million, or $0.13 per share, in the quarter ended September 30, 2010.
Research and development expenses were $8.9 million in the quarter ended September 30, 2011 compared to $5.1 million in the quarter ended September 30, 2010. The $3.8 million increase was due primarily to an increase in expenses related to the clinical development of Synavive and preclinical development expenses related to our Ion channel product candidates, including Z160 and Z944. We expect research and development expenses to increase for the year ended December 31, 2011 compared to 2010 due to the Phase 2b SYNERGY trial and the advancement of our Ion channel product candidates such as Z160 and Z944 into Phase 1 clinical development.
General and administrative expenses were $2.6 million in the quarter ended September 30, 2011 compared to $2.8 million in the quarter ended September 30, 2010. We expect our general and administrative expenses for the year ended December 31, 2011 to be consistent with such expenses for 2010.
http://finance.yahoo.com/news/Zalicus-Reports-Financial-bw-3…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/Zalicus-Reports-Financial-bw-3…
...interessant daß an den deutschen Börsen im Moment ein gewaltiger Spread von gut 170% zu verzeichnen ist....
Nachbörslich gings an der Nasdaq von den 1,29$ zum Börsenschluß noch auf 1,35$ rauf - und wie es scheint wurden im Pre-market auch schon die ersten zu 1,35$ vertickt (Info yahoo-Board).
Allerdings wird das derzeitige bid/ask von 9600 zu 1,35$/ 100 zu 1,95$ im US-Pre-market von den Börsen hierzulande etwas anders interpretiert...
....eigenartig...?? ...wie kommts?
Nachbörslich gings an der Nasdaq von den 1,29$ zum Börsenschluß noch auf 1,35$ rauf - und wie es scheint wurden im Pre-market auch schon die ersten zu 1,35$ vertickt (Info yahoo-Board).
Allerdings wird das derzeitige bid/ask von 9600 zu 1,35$/ 100 zu 1,95$ im US-Pre-market von den Börsen hierzulande etwas anders interpretiert...
....eigenartig...?? ...wie kommts?
Zalicus Initiates Phase 1 Clinical Trial of Z160
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ: ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases today announced the initiation of a Phase 1 clinical trial evaluating the pharmacokinetics and safety of a new formulation of Z160 (formerly NMED-160), a novel oral N-type calcium channel blocker. Z160 has been reformulated with multiple state-of-the-art delivery techniques to address previous solubility and bioavailability issues. Zalicus plans to run Phase 1 human pharmacokinetic and safety studies with these formulations during the remainder of 2011 and into early 2012. Based on this pharmacokinetic and safety data, Zalicus plans to select the best formulation to advance into Phase 2 clinical development in 2012. A previous formulation of Z160 has been studied in clinical trials of over 200 subjects and was well tolerated.
“Zalicus is a leader in the field of ion channel research and development and we look forward to completing these Phase 1 studies evaluating our new Z160 formulations, and should they prove successful, moving quickly into Phase 2 development for pain in 2012,” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus.
http://finance.yahoo.com/news/Zalicus-Initiates-Phase-1-bw-2…
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ: ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases today announced the initiation of a Phase 1 clinical trial evaluating the pharmacokinetics and safety of a new formulation of Z160 (formerly NMED-160), a novel oral N-type calcium channel blocker. Z160 has been reformulated with multiple state-of-the-art delivery techniques to address previous solubility and bioavailability issues. Zalicus plans to run Phase 1 human pharmacokinetic and safety studies with these formulations during the remainder of 2011 and into early 2012. Based on this pharmacokinetic and safety data, Zalicus plans to select the best formulation to advance into Phase 2 clinical development in 2012. A previous formulation of Z160 has been studied in clinical trials of over 200 subjects and was well tolerated.
“Zalicus is a leader in the field of ion channel research and development and we look forward to completing these Phase 1 studies evaluating our new Z160 formulations, and should they prove successful, moving quickly into Phase 2 development for pain in 2012,” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus.
http://finance.yahoo.com/news/Zalicus-Initiates-Phase-1-bw-2…
Upcoming Events
Zalicus Inc at Oppenheimer & Co. Healthcare Conference
Tuesday, December 13, 2011 1:35 p.m. ET
Location New York, NY
http://phx.corporate-ir.net/phoenix.zhtml?c=148036&p=irol-ca…
Zalicus Inc at Oppenheimer & Co. Healthcare Conference
Tuesday, December 13, 2011 1:35 p.m. ET
Location New York, NY
http://phx.corporate-ir.net/phoenix.zhtml?c=148036&p=irol-ca…
Guten Morgen Bernie, alles gut & fein mit der Phase 1 vorhaben (von daher auch der spike in amiland vor zweitagen)
Das Problem bei Phase1 ist immer die länge der Zulassungsweg und dazugehörigen KOSTEN...Bio hats schwer zurzeit, die besten Ideen/Technologien kriegen alle leider kein billiges Geld zum forschen, auß3er das man selber aus der big pharma Ecke kommt und hat selbst solche tiefe Taschen von vornehinein
Bin trotzdem noch immer 'gut dabei' im sachen ZLCS...
Das Problem bei Phase1 ist immer die länge der Zulassungsweg und dazugehörigen KOSTEN...Bio hats schwer zurzeit, die besten Ideen/Technologien kriegen alle leider kein billiges Geld zum forschen, auß3er das man selber aus der big pharma Ecke kommt und hat selbst solche tiefe Taschen von vornehinein

Bin trotzdem noch immer 'gut dabei' im sachen ZLCS...

Zalicus Initiates Phase 1 Clinical Trial of Z944
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ: ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases, today announced the initiation of a Phase 1 clinical trial evaluating the pharmacokinetics and safety of Z944, a novel oral T-type calcium channel blocker with demonstrated preclinical potential for the treatment of acute and inflammatory pain. In addition, Zalicus has drawn the remaining $8.5 million from its $20.0 million secured credit facility with Oxford Finance to fund ion channel clinical development.
"I am encouraged by the progress we are making in moving our ion channel candidates into clinical development. Z944, a T-type calcium channel blocker, is the second clinical study emanating from our ion channel discovery platform this month, following closely on the heels of Z160, an N-type calcium channel blocker, which initiated Phase 1 studies earlier in December” said Mark H.N. Corrigan, MD, CEO of Zalicus. “We look forward to reporting data from these Phase 1 studies in 2012" commented Mark H.N. Corrigan, MD, President and CEO of Zalicus.
http://finance.yahoo.com/news/Zalicus-Initiates-Phase-1-bw-2…
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ: ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases, today announced the initiation of a Phase 1 clinical trial evaluating the pharmacokinetics and safety of Z944, a novel oral T-type calcium channel blocker with demonstrated preclinical potential for the treatment of acute and inflammatory pain. In addition, Zalicus has drawn the remaining $8.5 million from its $20.0 million secured credit facility with Oxford Finance to fund ion channel clinical development.
"I am encouraged by the progress we are making in moving our ion channel candidates into clinical development. Z944, a T-type calcium channel blocker, is the second clinical study emanating from our ion channel discovery platform this month, following closely on the heels of Z160, an N-type calcium channel blocker, which initiated Phase 1 studies earlier in December” said Mark H.N. Corrigan, MD, CEO of Zalicus. “We look forward to reporting data from these Phase 1 studies in 2012" commented Mark H.N. Corrigan, MD, President and CEO of Zalicus.
http://finance.yahoo.com/news/Zalicus-Initiates-Phase-1-bw-2…
Upgrades & Downgrades History
Date - Research Firm - Action - From - To
21-Dec-11 MLV & Co Initiated Buy
25-Mar-11 JMP Securities Initiated Mkt Outperform
http://finance.yahoo.com/q/ud?s=ZLCS
Date - Research Firm - Action - From - To
21-Dec-11 MLV & Co Initiated Buy
25-Mar-11 JMP Securities Initiated Mkt Outperform
http://finance.yahoo.com/q/ud?s=ZLCS
...ergänzend hinzugefügt daß sie ihr erstes Kursziel bei 4 US$ ansetzen...da ist also noch ordentlich Luft nach oben... :-))
Zalicus to Present at Biotech Showcase 2012 Conference
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ: ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases today announced that Mark H.N. Corrigan, MD, President and CEO is scheduled to present at the Biotech Showcase 2012 Conference at 2:00 p.m. PT on Tuesday, January 10, 2012, at the Parc 55 Wyndham, San Francisco. Interested parties may access a live webcast of the presentation by visiting the Zalicus website at www.zalicus.com. The webcast will be archived on the Zalicus website following the event.
About Zalicus
Zalicus Inc. (Nasdaq: ZLCS - News) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. Zalicus applies its selective ion-channel modulation platform and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease. To learn more about Zalicus, please visit www.zalicus.com.
Forward-Looking Statement:
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus, its product candidates, their potential and the plans for their clinical development, the Zalicus selective ion channel modulation technology, and related preclinical product candidates, Zalicus’s combination drug discovery technology, cHTS, and its other business plans. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus may be identified by words like "believe," "expect," "may," "will," "should," "seek," “plan” or “could” and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the sale and marketing of Exalgo by Covidien, risks related to the development and regulatory approval of Zalicus’s product candidates, including risks relating to formulation and clinical development of Synavive and Prednisporin, the unproven nature of the Zalicus drug discovery technologies, the ability of Covidien and Fovea/Sanofi Aventis to perform their obligations under their agreements with Zalicus, the ability of the Company or its collaboration partners to initiate and successfully complete clinical trials of its product candidates, the Company's ability to obtain additional financing or funding for its research and development and those other risks that can be found in the "Risk Factors" section of Zalicus's annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission. Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management’s current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
(c) 2012 Zalicus Inc. All rights reserved.
http://finance.yahoo.com/news/Zalicus-Present-Biotech-bw-176…
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ: ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases today announced that Mark H.N. Corrigan, MD, President and CEO is scheduled to present at the Biotech Showcase 2012 Conference at 2:00 p.m. PT on Tuesday, January 10, 2012, at the Parc 55 Wyndham, San Francisco. Interested parties may access a live webcast of the presentation by visiting the Zalicus website at www.zalicus.com. The webcast will be archived on the Zalicus website following the event.
About Zalicus
Zalicus Inc. (Nasdaq: ZLCS - News) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. Zalicus applies its selective ion-channel modulation platform and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease. To learn more about Zalicus, please visit www.zalicus.com.
Forward-Looking Statement:
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus, its product candidates, their potential and the plans for their clinical development, the Zalicus selective ion channel modulation technology, and related preclinical product candidates, Zalicus’s combination drug discovery technology, cHTS, and its other business plans. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus may be identified by words like "believe," "expect," "may," "will," "should," "seek," “plan” or “could” and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the sale and marketing of Exalgo by Covidien, risks related to the development and regulatory approval of Zalicus’s product candidates, including risks relating to formulation and clinical development of Synavive and Prednisporin, the unproven nature of the Zalicus drug discovery technologies, the ability of Covidien and Fovea/Sanofi Aventis to perform their obligations under their agreements with Zalicus, the ability of the Company or its collaboration partners to initiate and successfully complete clinical trials of its product candidates, the Company's ability to obtain additional financing or funding for its research and development and those other risks that can be found in the "Risk Factors" section of Zalicus's annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission. Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management’s current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
(c) 2012 Zalicus Inc. All rights reserved.
http://finance.yahoo.com/news/Zalicus-Present-Biotech-bw-176…
Zitat von bernie55: Zalicus to Present at Biotech Showcase 2012 Conference
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ: ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases today announced that Mark H.N. Corrigan, MD, President and CEO is scheduled to present at the Biotech Showcase 2012 Conference at 2:00 p.m. PT on Tuesday, January 10, 2012, at the Parc 55 Wyndham, San Francisco. Interested parties may access a live webcast of the presentation by visiting the Zalicus website at www.zalicus.com. The webcast will be archived on the Zalicus website following the event.
About Zalicus
Zalicus Inc. (Nasdaq: ZLCS - News) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. Zalicus applies its selective ion-channel modulation platform and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease. To learn more about Zalicus, please visit www.zalicus.com.
Forward-Looking Statement:
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus, its product candidates, their potential and the plans for their clinical development, the Zalicus selective ion channel modulation technology, and related preclinical product candidates, Zalicus’s combination drug discovery technology, cHTS, and its other business plans. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus may be identified by words like "believe," "expect," "may," "will," "should," "seek," “plan” or “could” and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the sale and marketing of Exalgo by Covidien, risks related to the development and regulatory approval of Zalicus’s product candidates, including risks relating to formulation and clinical development of Synavive and Prednisporin, the unproven nature of the Zalicus drug discovery technologies, the ability of Covidien and Fovea/Sanofi Aventis to perform their obligations under their agreements with Zalicus, the ability of the Company or its collaboration partners to initiate and successfully complete clinical trials of its product candidates, the Company's ability to obtain additional financing or funding for its research and development and those other risks that can be found in the "Risk Factors" section of Zalicus's annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission. Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management’s current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
(c) 2012 Zalicus Inc. All rights reserved.
http://finance.yahoo.com/news/Zalicus-Present-Biotech-bw-176…
 ..sorry....sorry....sorry....sorry.....sorry.....sorry...
..sorry....sorry....sorry....sorry.....sorry.....sorry...
 ..hier der korrekte Link....
..hier der korrekte Link.... .
. Zalicus to Present at BIO CEO & Investor Conference
Zalicus Inc. (NASDAQ: ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases today announced that Mark H.N. Corrigan, MD, President and CEO is scheduled to present at the 14th Annual BIO CEO & Investor Conference at 8:30 a.m. ET on Monday, February 13, 2012, at the Waldorf Astoria, New York. Interested parties may access a live webcast of the presentation by visiting the Zalicus website at www.zalicus.com. The webcast will be archived on the Zalicus website following the event.
http://finance.yahoo.com/news/Zalicus-Present-BIO-CEO-bw-143…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/Zalicus-Present-BIO-CEO-bw-143…
Antwort auf Beitrag Nr.: 42.709.096 von bernie55 am 06.02.12 22:44:21Zalicus Publishes Data on Novel Ion Channel Compounds
Business WirePress Release: Zalicus – Wed, Feb 15, 2012 4:01 PM EST
- New Paper Published in Science Translational Medicine Reports Anti-Epileptic Activity of Z944, a Novel Oral, T-type Calcium Channel Blocker in Clinical Development for the Treatment of Inflammatory Pain -
- Neuronal Disorders, such as Epilepsy and Pain, Linked Through T-type Calcium Channel Hyper-excitability Mechanisms -
http://finance.yahoo.com/news/Zalicus-Publishes-Data-Novel-b…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/Zalicus-Publishes-Data-Novel-b…
Business WirePress Release: Zalicus – Wed, Feb 15, 2012 4:01 PM EST
- New Paper Published in Science Translational Medicine Reports Anti-Epileptic Activity of Z944, a Novel Oral, T-type Calcium Channel Blocker in Clinical Development for the Treatment of Inflammatory Pain -
- Neuronal Disorders, such as Epilepsy and Pain, Linked Through T-type Calcium Channel Hyper-excitability Mechanisms -
http://finance.yahoo.com/news/Zalicus-Publishes-Data-Novel-b…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/Zalicus-Publishes-Data-Novel-b…
Antwort auf Beitrag Nr.: 42.824.051 von bernie55 am 29.02.12 10:34:00Zalicus and Hydra Collaborate to Advance Novel Ion Channel Product Candidates for the Treatment of Pain
Business WirePress Release: Zalicus Inc. – Thu, Feb 9, 2012 4:30 PM EST
http://finance.yahoo.com/news/Zalicus-Hydra-Collaborate-bw-2…
Business WirePress Release: Zalicus Inc. – Thu, Feb 9, 2012 4:30 PM EST
http://finance.yahoo.com/news/Zalicus-Hydra-Collaborate-bw-2…
Zalicus Successfully Completes Phase 1 Clinical Study with Reformulated Z160
Advancing Into Phase 2 for Neuropathic Pain with Improved Formulation in Second Half of 2012
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Zalicus Inc. (NASDAQ: ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases today announced the successful completion of a Phase 1 clinical trial evaluating the pharmacokinetics and safety of a new formulation of Z160, a novel oral N-type calcium channel blocker. In the study, Z160 demonstrated substantial bioavailability and solubility improvements using a novel, proprietary formulation technology. Based on the data from this study, Zalicus plans to advance Z160 into Phase 2 clinical development for the treatment of neuropathic pain in the second half of 2012. A previous formulation of Z160 was studied in clinical trials of over 200 subjects and was well tolerated.
"We are encouraged by the substantial bioavailability and solubility improvements achieved with the new formulation of Z160 tested in this study,” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “These data provides us with the confidence that we can achieve appropriate and consistent exposure levels to evaluate Z160’s efficacy in pain and we look forward to beginning a Phase 2 clinical study in neuropathic pain in the second half of 2012."
http://finance.yahoo.com/news/zalicus-successfully-completes…
Advancing Into Phase 2 for Neuropathic Pain with Improved Formulation in Second Half of 2012
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Zalicus Inc. (NASDAQ: ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases today announced the successful completion of a Phase 1 clinical trial evaluating the pharmacokinetics and safety of a new formulation of Z160, a novel oral N-type calcium channel blocker. In the study, Z160 demonstrated substantial bioavailability and solubility improvements using a novel, proprietary formulation technology. Based on the data from this study, Zalicus plans to advance Z160 into Phase 2 clinical development for the treatment of neuropathic pain in the second half of 2012. A previous formulation of Z160 was studied in clinical trials of over 200 subjects and was well tolerated.
"We are encouraged by the substantial bioavailability and solubility improvements achieved with the new formulation of Z160 tested in this study,” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “These data provides us with the confidence that we can achieve appropriate and consistent exposure levels to evaluate Z160’s efficacy in pain and we look forward to beginning a Phase 2 clinical study in neuropathic pain in the second half of 2012."
http://finance.yahoo.com/news/zalicus-successfully-completes…
Zalicus Bolsters Cash Position
On March 28, 2012, Zalicus, Inc. (ZLCS) announced via an 8k filing that the company's equity distribution agreement with Wedbush Securities Inc. has been completed at the proposed $15 million in aggregate gross proceeds. The equity distribution agreement, put into place on January 10, 2012 (see 8k filing), allowed for the sale of Zalicus common stock, from time to time, at the market price. Through the "ATM" (at-the-market), Zalicus sold a total of 14.058 million shares into the public market.
...Significant Effort Over The Past Two Weeks...
Inside Zalicus' annual 10k filing, dated March 9, 2012, on page 65, management noted that from January 10, 2012 to March 7, 2012, the company had sold only 5.367 million shares for gross proceeds of $5.5 million.
Over the past two weeks, management sold the remaining 8.691 million shares to raise the remaining $8.8 million allowable under the agreement. This explains the significant increase in volume seen in Zalicus common stock over the past few days.
...Low Visibility / Low Fees...
ATMs are sometimes difficult for shareholders to stomach because management is not obligated to provide an update on the status of the offerings, except in quarterly filings. ATMs are like pulling a bandage off slowly instead of ripping it off in one swipe.
However, the fee that Wedbush charged Zalicus for the ATM was only 2.5%. That's a total cost of $375,000 on a $15.0 million raise. A secondary public offering of 14.1 million shares at $1.05 per share to raise $15.0 million would have likely cost Zalicus 6% fees ($900,000) or more, and the stock would have likely dropped below $1.05 per share on the news. So it's a give / take. In the end, we think this ATM was rather successful for the company.
We remind investors that during the first and second quarter 2011, Zalicus completed a similar equity distribution agreement with Wedbush that raised $20 million in gross proceeds ($19.2 million net after fees).
...Solid Cash Position...
Zalicus exited 2011 with $49.7 million in cash and investments. Subtracting out the estimated operating burn for the first quarter 2012 and then adding back in the net $14.6 million from the ATM, and we forecast that Zalicus will holds just over $50 million in cash today.
With the ATM now complete, downward pressure on the stock has been relieved and we believe that investors can begin to focus on upcoming catalysts for the shares that could drive the stock higher.
These include:
Reporting phase 1 data on Z944 in the second quarter 2012.
Initiating a phase 2 program with Z160 in the third quarter 2012.
Reporting phase 2b data on SYNERGY during the third quarter 2012.
Initiating a phase 2 program with Z944 in the fourth quarter 2012.
Forming additional cHTS or ion channel collaborations during the second half of 2012.
Sanofi initiating a phase 3 trial on Prednisporin during the second half of 2012.
Covidien receiving approval for the 32mg Exalgo dose during the second half of 2012.
Partnering Synavive for phase 3 trials in 2013.
Moving additional ion channel candidates into the clinic in 2013.
http://seekingalpha.com/article/464261-zalicus-bolsters-cash…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/464261-zalicus-bolsters-cash…
On March 28, 2012, Zalicus, Inc. (ZLCS) announced via an 8k filing that the company's equity distribution agreement with Wedbush Securities Inc. has been completed at the proposed $15 million in aggregate gross proceeds. The equity distribution agreement, put into place on January 10, 2012 (see 8k filing), allowed for the sale of Zalicus common stock, from time to time, at the market price. Through the "ATM" (at-the-market), Zalicus sold a total of 14.058 million shares into the public market.
...Significant Effort Over The Past Two Weeks...
Inside Zalicus' annual 10k filing, dated March 9, 2012, on page 65, management noted that from January 10, 2012 to March 7, 2012, the company had sold only 5.367 million shares for gross proceeds of $5.5 million.
Over the past two weeks, management sold the remaining 8.691 million shares to raise the remaining $8.8 million allowable under the agreement. This explains the significant increase in volume seen in Zalicus common stock over the past few days.
...Low Visibility / Low Fees...
ATMs are sometimes difficult for shareholders to stomach because management is not obligated to provide an update on the status of the offerings, except in quarterly filings. ATMs are like pulling a bandage off slowly instead of ripping it off in one swipe.
However, the fee that Wedbush charged Zalicus for the ATM was only 2.5%. That's a total cost of $375,000 on a $15.0 million raise. A secondary public offering of 14.1 million shares at $1.05 per share to raise $15.0 million would have likely cost Zalicus 6% fees ($900,000) or more, and the stock would have likely dropped below $1.05 per share on the news. So it's a give / take. In the end, we think this ATM was rather successful for the company.
We remind investors that during the first and second quarter 2011, Zalicus completed a similar equity distribution agreement with Wedbush that raised $20 million in gross proceeds ($19.2 million net after fees).
...Solid Cash Position...
Zalicus exited 2011 with $49.7 million in cash and investments. Subtracting out the estimated operating burn for the first quarter 2012 and then adding back in the net $14.6 million from the ATM, and we forecast that Zalicus will holds just over $50 million in cash today.
With the ATM now complete, downward pressure on the stock has been relieved and we believe that investors can begin to focus on upcoming catalysts for the shares that could drive the stock higher.
These include:
Reporting phase 1 data on Z944 in the second quarter 2012.
Initiating a phase 2 program with Z160 in the third quarter 2012.
Reporting phase 2b data on SYNERGY during the third quarter 2012.
Initiating a phase 2 program with Z944 in the fourth quarter 2012.
Forming additional cHTS or ion channel collaborations during the second half of 2012.
Sanofi initiating a phase 3 trial on Prednisporin during the second half of 2012.
Covidien receiving approval for the 32mg Exalgo dose during the second half of 2012.
Partnering Synavive for phase 3 trials in 2013.
Moving additional ion channel candidates into the clinic in 2013.
http://seekingalpha.com/article/464261-zalicus-bolsters-cash…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/464261-zalicus-bolsters-cash…
5.04.2012 | 14:07
(41 Leser)
Schrift ändern:
(0 Bewertungen)
Business Wire · Mehr Nachrichten von Business Wire
Zalicus Discovers Novel Multi-Target Mechanism for the Treatment of Multiple Myeloma
New Findings Published in Molecular Cancer Therapeutics Demonstrate Novel and Selective Synergy for Adenosine A2A and Beta-2 Adrenergic Receptor Agonists in Combination with Standard of Care Drugs
Synergy Discovered Through Systematic Combination High Throughput Screening (cHTS™)Platform
Zalicus Inc. (NASDAQ: ZLCS) today announced the publication of new preclinical data in Molecular Cancer Therapeutics, a Journal published by the American Association for Cancer Research. In the article entitled, "Adenosine A2A and Beta-2 Adrenergic Receptor Agonists: Novel Selective and Synergistic Multiple Myeloma Targets Discovered through Systematic Combination Screening," by Rickles, et.al, pre-published online April 4, 2012, Zalicus researchers, in collaboration with the Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute, Harvard Medical School, have discovered that adenosine A2A and beta-2 adrenergic receptor agonists are highly synergistic and selective novel agents that enhance glucocorticoid activity in B-cell malignancies such as multiple myeloma and, importantly, can synergize in combination with current multiple myeloma treatment regimens such as melphalan, lenalidomide, bortezomib and doxorubicin.
"These data demonstrate the power of Zalicus' combination High Throughput Screening (cHTS™) platform to systematically identify synergistic drug combinations for the treatment of cancer," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. "Combination drug regimens are quickly becoming the standard of care in the treatment of cancer and this is especially evident in multiple myeloma where combination therapy has been shown to improve clinical outcomes for patients."
Study highlights:
A2A and beta-2 adrenergic receptor agonists were shown to be highly synergistic and selective agents providing enhanced glucocorticoid activity in B-cell malignancies.
Analysis of agonists in combination with dexamethasone or melphalan in 83 cell lines revealed substantial activity in MM and diffuse large B cell lymphoma cell lines.
In some of the multiple myeloma (MM) cell line models, A2A and beta-2 adrenergic receptor agonists synergized with melphalan, lenalidomide, bortezomib and doxorubicin.
Combination effects were also observed with dexamethasone as well as bortezomib, using MM patient samples and mouse MM xenograft assays.
Results support development of A2A and beta-2 adrenergic receptor agonists for use in multi-drug combination therapy for MM.
Systematic combination screening for the discovery and evaluation of new targets and combination therapies has the potential to improve cancer treatment paradigms and patient outcomes.
About Zalicus
Zalicus Inc. (Nasdaq: ZLCS) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. Zalicus has a portfolio of proprietary clinical-stage product candidates targeting pain and immuno-inflammatory diseases and have entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. Zalicus applies its expertise in the discovery and development of selective ion channel modulators and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease.
To learn more about Zalicus, please visit www.zalicus.com
(41 Leser)
Schrift ändern:
(0 Bewertungen)
Business Wire · Mehr Nachrichten von Business Wire
Zalicus Discovers Novel Multi-Target Mechanism for the Treatment of Multiple Myeloma
New Findings Published in Molecular Cancer Therapeutics Demonstrate Novel and Selective Synergy for Adenosine A2A and Beta-2 Adrenergic Receptor Agonists in Combination with Standard of Care Drugs
Synergy Discovered Through Systematic Combination High Throughput Screening (cHTS™)Platform
Zalicus Inc. (NASDAQ: ZLCS) today announced the publication of new preclinical data in Molecular Cancer Therapeutics, a Journal published by the American Association for Cancer Research. In the article entitled, "Adenosine A2A and Beta-2 Adrenergic Receptor Agonists: Novel Selective and Synergistic Multiple Myeloma Targets Discovered through Systematic Combination Screening," by Rickles, et.al, pre-published online April 4, 2012, Zalicus researchers, in collaboration with the Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute, Harvard Medical School, have discovered that adenosine A2A and beta-2 adrenergic receptor agonists are highly synergistic and selective novel agents that enhance glucocorticoid activity in B-cell malignancies such as multiple myeloma and, importantly, can synergize in combination with current multiple myeloma treatment regimens such as melphalan, lenalidomide, bortezomib and doxorubicin.
"These data demonstrate the power of Zalicus' combination High Throughput Screening (cHTS™) platform to systematically identify synergistic drug combinations for the treatment of cancer," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. "Combination drug regimens are quickly becoming the standard of care in the treatment of cancer and this is especially evident in multiple myeloma where combination therapy has been shown to improve clinical outcomes for patients."
Study highlights:
A2A and beta-2 adrenergic receptor agonists were shown to be highly synergistic and selective agents providing enhanced glucocorticoid activity in B-cell malignancies.
Analysis of agonists in combination with dexamethasone or melphalan in 83 cell lines revealed substantial activity in MM and diffuse large B cell lymphoma cell lines.
In some of the multiple myeloma (MM) cell line models, A2A and beta-2 adrenergic receptor agonists synergized with melphalan, lenalidomide, bortezomib and doxorubicin.
Combination effects were also observed with dexamethasone as well as bortezomib, using MM patient samples and mouse MM xenograft assays.
Results support development of A2A and beta-2 adrenergic receptor agonists for use in multi-drug combination therapy for MM.
Systematic combination screening for the discovery and evaluation of new targets and combination therapies has the potential to improve cancer treatment paradigms and patient outcomes.
About Zalicus
Zalicus Inc. (Nasdaq: ZLCS) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. Zalicus has a portfolio of proprietary clinical-stage product candidates targeting pain and immuno-inflammatory diseases and have entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. Zalicus applies its expertise in the discovery and development of selective ion channel modulators and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease.
To learn more about Zalicus, please visit www.zalicus.com
Antwort auf Beitrag Nr.: 43.041.944 von Growth2012 am 16.04.12 00:27:04Sehr gute nachrichten aus dem Hause ZLCS heute in der früh....
![]()
http://finance.yahoo.com/news/zalicus-novartis-extend-oncolo…
Bahnt sich da evtl. eine übernahme an?!...



http://finance.yahoo.com/news/zalicus-novartis-extend-oncolo…
Bahnt sich da evtl. eine übernahme an?!...



Antwort auf Beitrag Nr.: 43.091.662 von Growth2012 am 26.04.12 14:41:16Ask schon bei 0,93€ in Stuttgart...
(Tendenz stark steigend)

(Tendenz stark steigend)
Zitat von Growth2012: Sehr gute nachrichten aus dem Hause ZLCS heute in der früh....
Bahnt sich da evtl. eine übernahme an?!...
Zalicus and Novartis extend oncology research collaboration
Zalicus provided an update today on its research collaboration with Novartis, based on its combination High Throughput Screening platform. Based on the success of the collaboration up to this point, Novartis has exercised its second option to extend its oncology discovery research collaboration with Zalicus for an additional contract year, through April 2013.
Antwort auf Beitrag Nr.: 43.092.883 von bernie55 am 26.04.12 17:43:21From seeking Alphas website re ZLCS...
![]()
http://seekingalpha.com/article/535341-bullish-sign-zalicus-…
Schöner Auszug (wie ich finde)
"Meanwhile, the bullied stock seems to be getting kicked around for no reason. With Synavive marching through phase 2B clinical trials and Prednisporin being the champion ocular medicament in Sanofi's (SNY) pipeline, I have long championed the potential value of the oncology research highlighted by the Zalicus-Novartis partnership. I alone have often noted the Novartis patents that hint to the long-term value for Zalicus."
Bin Jung...habe Zeit & Hoffnung in/für ZLCS



http://seekingalpha.com/article/535341-bullish-sign-zalicus-…
Schöner Auszug (wie ich finde)
"Meanwhile, the bullied stock seems to be getting kicked around for no reason. With Synavive marching through phase 2B clinical trials and Prednisporin being the champion ocular medicament in Sanofi's (SNY) pipeline, I have long championed the potential value of the oncology research highlighted by the Zalicus-Novartis partnership. I alone have often noted the Novartis patents that hint to the long-term value for Zalicus."
Bin Jung...habe Zeit & Hoffnung in/für ZLCS



Zalicus completes patient enrollment in Phase 2B trial of Synavive in RA
Zalicus announced it has completed enrollment with 292 patients enrolled in the SYNERGY trial, a Phase 2b clinical trial designed to evaluate the safety and efficacy of Synavive®, a low-dose glucocorticoid with the potential for amplified immuno-inflammatory benefits, in patients with rheumatoid arthritis. Top-line results of the clinical trial are expected to be available in the third quarter of 2012. The primary objective of the trial is to evaluate Synavive efficacy compared to placebo, while key additional secondary objectives include evaluating the efficacy of Synavive compared to its individual components (2.7mg of Prednisolone and 360mg of Dipyramidamole) as well as how Synavive performs in comparison to 5mg of Prednisone.
http://www.theflyonthewall.com/permalinks/entry.php/ZLCSid16…
Zalicus announced it has completed enrollment with 292 patients enrolled in the SYNERGY trial, a Phase 2b clinical trial designed to evaluate the safety and efficacy of Synavive®, a low-dose glucocorticoid with the potential for amplified immuno-inflammatory benefits, in patients with rheumatoid arthritis. Top-line results of the clinical trial are expected to be available in the third quarter of 2012. The primary objective of the trial is to evaluate Synavive efficacy compared to placebo, while key additional secondary objectives include evaluating the efficacy of Synavive compared to its individual components (2.7mg of Prednisolone and 360mg of Dipyramidamole) as well as how Synavive performs in comparison to 5mg of Prednisone.
http://www.theflyonthewall.com/permalinks/entry.php/ZLCSid16…
Antwort auf Beitrag Nr.: 43.104.781 von bernie55 am 30.04.12 16:13:02Zalicus (ZLCS) Advances Z160 into Phase 2a Trials
ZLCS Hot Sheet
Overall Analyst Rating: BUY
Zalicus Inc. (Nasdaq: ZLCS), reports that it is advancing Z160, its novel, first in class, oral, N-type calcium channel blocker into multiple Phase 2a clinical trials for chronic pain indications. Zalicus has selected the most promising novel, proprietary formulation for clinical use and is planning to initiate two Phase 2a clinical trials with Z160 for the treatment of neuropathic pain; the first of which is expected to begin enrolling patients in the third quarter of 2012.
Zalicus completed Phase 1 pharmacokinetic and safety studies with a number of formulations and has selected the optimal formulation to move into further clinical development. The selected formulation was well tolerated and demonstrated a 6-fold improvement in Z160’s bioavailability compared to the previously studied formulation of Z160.
Hier handelt es sich um zwei magische medizinische Worte "rheumatoid arthritis "
"rheumatoid arthritis "
(292 test personen für Phase 2B bereits angemeldet-siehe link anbei)
http://clinicaltrials.pharmaceutical-business-review.com/new…
...nun ja, wenn die tests Positiv ausgehen wird Novartis vermutlich "ganz schnell" erheblich mehr als nur 'research partner' sein wollen
wird Novartis vermutlich "ganz schnell" erheblich mehr als nur 'research partner' sein wollen

ZLCS Hot Sheet
Overall Analyst Rating: BUY
Zalicus Inc. (Nasdaq: ZLCS), reports that it is advancing Z160, its novel, first in class, oral, N-type calcium channel blocker into multiple Phase 2a clinical trials for chronic pain indications. Zalicus has selected the most promising novel, proprietary formulation for clinical use and is planning to initiate two Phase 2a clinical trials with Z160 for the treatment of neuropathic pain; the first of which is expected to begin enrolling patients in the third quarter of 2012.
Zalicus completed Phase 1 pharmacokinetic and safety studies with a number of formulations and has selected the optimal formulation to move into further clinical development. The selected formulation was well tolerated and demonstrated a 6-fold improvement in Z160’s bioavailability compared to the previously studied formulation of Z160.
Hier handelt es sich um zwei magische medizinische Worte
 "rheumatoid arthritis "
"rheumatoid arthritis "(292 test personen für Phase 2B bereits angemeldet-siehe link anbei)
http://clinicaltrials.pharmaceutical-business-review.com/new…
...nun ja, wenn die tests Positiv ausgehen
 wird Novartis vermutlich "ganz schnell" erheblich mehr als nur 'research partner' sein wollen
wird Novartis vermutlich "ganz schnell" erheblich mehr als nur 'research partner' sein wollen

Habe soeben weitere 4K in Stuttgart (veremeintlich) "billig" abgeholt(0,695)...das kann doch nicht so ewig weitergehen gen Norden. Der gegenreaktion wird hoffentlich "gewaltig" ausfallen
(Die Zocker brüder von JP Morgan in London lassen wieder Gesamtmarkttechnisch von sich hören/grüßen:O)

(Die Zocker brüder von JP Morgan in London lassen wieder Gesamtmarkttechnisch von sich hören/grüßen:O)
Shit, n' weitere 3K in FRA soeben ins Depot gelegt.
Mal sehen wozu es gut ist...
Mal sehen wozu es gut ist...
Antwort auf Beitrag Nr.: 43.183.645 von Growth2012 am 18.05.12 17:27:53
Lebt der noch??? (war bei minus 13,7% mal heute nachmittag)
Lebt der noch??? (war bei minus 13,7% mal heute nachmittag)
Antwort auf Beitrag Nr.: 43.183.710 von Growth2012 am 18.05.12 17:34:11Nun ja, 10% zurückgekommen nachdem gestrigen zukauf...
Maybe next week? gehts Reibungslos weiter!?!


Maybe next week? gehts Reibungslos weiter!?!



Zalicus to Present at Two Upcoming Investor Conferences
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Zalicus Inc. (NASDAQ: ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases announced that Zalicus management will provide an overview of the Company at two upcoming investor conferences:
Wednesday, May 15, 2012: JMP Securities Research Conference, 11:00 a.m. PT, Ritz Carlton, San Francisco, CA
Monday, June 4, 2012: Jefferies 2012 Global Healthcare Conference, 10:00 a.m. ET, Grand Hyatt, New York, NY
Interested parties may access a live webcast of the presentation by visiting the Zalicus website at www.zalicus.com. The webcast will be archived on the Zalicus website following the event.
http://finance.yahoo.com/news/zalicus-present-two-upcoming-i…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/zalicus-present-two-upcoming-i…
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Zalicus Inc. (NASDAQ: ZLCS - News) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases announced that Zalicus management will provide an overview of the Company at two upcoming investor conferences:
Wednesday, May 15, 2012: JMP Securities Research Conference, 11:00 a.m. PT, Ritz Carlton, San Francisco, CA
Monday, June 4, 2012: Jefferies 2012 Global Healthcare Conference, 10:00 a.m. ET, Grand Hyatt, New York, NY
Interested parties may access a live webcast of the presentation by visiting the Zalicus website at www.zalicus.com. The webcast will be archived on the Zalicus website following the event.
http://finance.yahoo.com/news/zalicus-present-two-upcoming-i…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/zalicus-present-two-upcoming-i…
Antwort auf Beitrag Nr.: 43.195.671 von bernie55 am 22.05.12 11:22:52Neuste Zacks report-(ausgiebig & fundiert rechechiert)- first Taget 3$

http://scr.zacks.com/Theme/Zacks/files/May%203,%202012_ZLCS_…


http://scr.zacks.com/Theme/Zacks/files/May%203,%202012_ZLCS_…
Breakout?
(nördlich der 0,85 marke könnte es noch richtig lustig werden)
Schönes Pfingstwochenende (falls noch jemanden anwesend sein sollte)
(nördlich der 0,85 marke könnte es noch richtig lustig werden)

Schönes Pfingstwochenende (falls noch jemanden anwesend sein sollte)

Antwort auf Beitrag Nr.: 43.198.981 von Growth2012 am 22.05.12 22:31:29Looking good...breakout start gelungen!!!! 
Zalicus Is Locked And Loaded: What You Need To Know
A brief overview of the company, at the end of the 1st quarter of this year they had 52 million in the bank. There are 113 million shares outstanding with their projected cash burn for the next year of roughly 10 million per quarter from the multiple ongoing trials and advancements in their pipeline.
http://seekingalpha.com/article/614891-zalicus-is-locked-and…
über die 2$ Marke bis mitte Juni


Zalicus Is Locked And Loaded: What You Need To Know
A brief overview of the company, at the end of the 1st quarter of this year they had 52 million in the bank. There are 113 million shares outstanding with their projected cash burn for the next year of roughly 10 million per quarter from the multiple ongoing trials and advancements in their pipeline.
http://seekingalpha.com/article/614891-zalicus-is-locked-and…
über die 2$ Marke bis mitte Juni


Antwort auf Beitrag Nr.: 43.213.845 von Growth2012 am 26.05.12 08:30:24Übrigens Wochenabshluß Germany gestern 0,72€ (+13,8%) und nicht 0,62 wie hier im Forumübersicht angezeigt...



Antwort auf Beitrag Nr.: 43.213.850 von Growth2012 am 26.05.12 08:33:10Ab 15:30 heute könnte es lustig werden stateside....



1$ wir kommen (wieder)...
29.05.12 16:00 Uhr
0,9899 USD
+8,64% [0,0787]

29.05.12 16:00 Uhr
0,9899 USD
+8,64% [0,0787]
Ich denke Novartis wird die Firma trotz Partnerschaft bald kaufen. Der Kurs wird dann über 1.50 liegen.

Antwort auf Beitrag Nr.: 43.228.778 von lunatics am 30.05.12 19:47:42Hier einen Link der deine Novartis These etwas Unterstützung verleiht...
Bulls Buy Zalicus As Novartis Collaboration Gains Traction
http://seekingalpha.com/article/623841-bulls-buy-zalicus-as-…

Bulls Buy Zalicus As Novartis Collaboration Gains Traction
http://seekingalpha.com/article/623841-bulls-buy-zalicus-as-…
Antwort auf Beitrag Nr.: 43.228.778 von lunatics am 30.05.12 19:47:42Up, on a down day
...und PING die magische 1$ Marke ist wieder erreicht

04.06.12 17:36 Uhr
1,00 USD
8,86% [0,0814]

...und PING die magische 1$ Marke ist wieder erreicht
04.06.12 17:36 Uhr
1,00 USD
8,86% [0,0814]
Why Comments By Novartis VP Will Send Zalicus' Share Price Up
June 4, 2012 |
Zalicus (ZLCS) shareholders will sit up once they read what a chat board poster floated over the weekend. Who that person is may never be known, but the link that individual shared will have an immediate impact on driving Zalicus's share price to a much higher level.
It was surprising to learn over the weekend of a recent presentation given at the AACR Annual Meeting 2012 held in Chicago, IL, by Novartis (NVS) Vice President and Global Head of Oncology Dr. William R. Sellers wherein he not only cites the ongoing collaborative work at Zalicus, but after viewing the presentation, Dr. Sellers makes it as "plain as day" that Zalicus is playing an integral role in the Novartis global oncology program.
Verification in Spades
Zalicus investors should consider who Dr. Sellers is. He is one of the "oncology top dogs" at Novartis. Meaning, this isn't a presentation given by a management low-level scientist, as Dr. Sellers sits at the top of the Novartis management team. Furthermore, this is definitive proof of the in-depth collaborative relationship Zalicus has with Novartis and the details of Dr. Sellers' presentation are breath-taking--no exaggeration intended. Why is because, to date, Zalicus investors have not heard a peep out of Novartis and next-to-nothing out of Zalicus about this top secret partnership. Truthfully, I think Dr. Sellers' presentation "blows the lid off" of what Zalicus's investors have been guessing about since the collaboration began in May 2009. In an article I recently published I tried to make that case -- now it is verified in spades. Zalicus is sitting dead-center in a major oncology initiative at Novartis.
Don't Let The Science Stop You
Though the presentation is very scientific, it is a must-view for Zalicus shareholders. In a nutshell, investors will learn of the virtual oncology encyclopedia (as Novartis is calling it) of drug-combinations and genetic factors, and how Zalicus's cHTS Chalice technology has been playing a significant role in that process. While you can jump ahead to about the 20 minute mark to hear Dr. Sellers' speak about the research at Zalicus, I highly recommend viewing the entire 30 minute presentation.
Value to Investors
Dr. Sellers' presentation is a window into how Zalicus is operating like an ad-hoc research unit for the global oncology program at Novartis. Given the gross under-valuation of Zalicus's share price, this information should have an exponential impact because the Market rewards companies where value like Dr. Sellers' presentation so explicitly advertises such a high-value program. After all, once everyone sees with their own eyes that Zalicus has been at the center hub of the oncology program at Novartis, it would be shocking not to see the market turn extremely bullish.
CEO Corrigan's Presentation
Meanwhile, here on Monday at the Jefferies Global Healthcare Conference, Zalicus CEO Dr. Corrigan reminded investors of our "long standing program with Novartis" ... "our on-going alliance oncology partnership with Novartis" remaining as always very guarded about what he says. Nevertheless, Dr. Sellers' presentation says it all. Zalicus is in a very enviable position.
Buy Now Because ...
While biotech investing is always risky, with the lid pulled off on the Novartis-Zalicus collaboration, investors have a remarkable opportunity to buy current shares at a bargain-basement price. However, I do anticipate a much bigger move up in the share price, so that window of opportunity may be short-lived. Here's why...
> Novartis may announce at any time an oncology drug combination citing Zalicus's efforts.
> Prednisporin at Sanofi (SNY) must be within months of entering a
phase 3 clinical trial.
>Z160 sailed through phase 1 clinical trials and its two-prong phase 2A
clinical trials will begin this year and Zalicus has consistently met
all of its targets (rare these days among biotechs).
>Z944's phase 1 single dosing results are expected very soon and from the presentation it sounds as though the Sodium channel work for a 3rd drug candidate at Hydra is moving along.
>The odds of Synavive's success in phase 2B look very promising especially if you understand the science and how the clinical trial was designed (e.g. Synavive's primary end-point is matched against placebo, so the likelihood of success 80% or better).
Finally at today's conference where Dr. Corrigan spoke, the normally reserved leader was in rare form ending his talk noting just how bullish he is about his own company. The upside for Zalicus has begun and savvy investors, if not already invested here, should get in very soon because I foresee a major move up in the share price going into summertime.
Disclosure: I am long ZLCS. Investors buy and/or sell at their own risk. I declare that I may day-trade Zalicus shares at any time. "Long" for me means until I sell. I do not "Short" stocks.
http://seekingalpha.com/article/635311-why-comments-by-novar…
June 4, 2012 |
Zalicus (ZLCS) shareholders will sit up once they read what a chat board poster floated over the weekend. Who that person is may never be known, but the link that individual shared will have an immediate impact on driving Zalicus's share price to a much higher level.
It was surprising to learn over the weekend of a recent presentation given at the AACR Annual Meeting 2012 held in Chicago, IL, by Novartis (NVS) Vice President and Global Head of Oncology Dr. William R. Sellers wherein he not only cites the ongoing collaborative work at Zalicus, but after viewing the presentation, Dr. Sellers makes it as "plain as day" that Zalicus is playing an integral role in the Novartis global oncology program.
Verification in Spades
Zalicus investors should consider who Dr. Sellers is. He is one of the "oncology top dogs" at Novartis. Meaning, this isn't a presentation given by a management low-level scientist, as Dr. Sellers sits at the top of the Novartis management team. Furthermore, this is definitive proof of the in-depth collaborative relationship Zalicus has with Novartis and the details of Dr. Sellers' presentation are breath-taking--no exaggeration intended. Why is because, to date, Zalicus investors have not heard a peep out of Novartis and next-to-nothing out of Zalicus about this top secret partnership. Truthfully, I think Dr. Sellers' presentation "blows the lid off" of what Zalicus's investors have been guessing about since the collaboration began in May 2009. In an article I recently published I tried to make that case -- now it is verified in spades. Zalicus is sitting dead-center in a major oncology initiative at Novartis.
Don't Let The Science Stop You
Though the presentation is very scientific, it is a must-view for Zalicus shareholders. In a nutshell, investors will learn of the virtual oncology encyclopedia (as Novartis is calling it) of drug-combinations and genetic factors, and how Zalicus's cHTS Chalice technology has been playing a significant role in that process. While you can jump ahead to about the 20 minute mark to hear Dr. Sellers' speak about the research at Zalicus, I highly recommend viewing the entire 30 minute presentation.
Value to Investors
Dr. Sellers' presentation is a window into how Zalicus is operating like an ad-hoc research unit for the global oncology program at Novartis. Given the gross under-valuation of Zalicus's share price, this information should have an exponential impact because the Market rewards companies where value like Dr. Sellers' presentation so explicitly advertises such a high-value program. After all, once everyone sees with their own eyes that Zalicus has been at the center hub of the oncology program at Novartis, it would be shocking not to see the market turn extremely bullish.
CEO Corrigan's Presentation
Meanwhile, here on Monday at the Jefferies Global Healthcare Conference, Zalicus CEO Dr. Corrigan reminded investors of our "long standing program with Novartis" ... "our on-going alliance oncology partnership with Novartis" remaining as always very guarded about what he says. Nevertheless, Dr. Sellers' presentation says it all. Zalicus is in a very enviable position.
Buy Now Because ...
While biotech investing is always risky, with the lid pulled off on the Novartis-Zalicus collaboration, investors have a remarkable opportunity to buy current shares at a bargain-basement price. However, I do anticipate a much bigger move up in the share price, so that window of opportunity may be short-lived. Here's why...
> Novartis may announce at any time an oncology drug combination citing Zalicus's efforts.
> Prednisporin at Sanofi (SNY) must be within months of entering a
phase 3 clinical trial.
>Z160 sailed through phase 1 clinical trials and its two-prong phase 2A
clinical trials will begin this year and Zalicus has consistently met
all of its targets (rare these days among biotechs).
>Z944's phase 1 single dosing results are expected very soon and from the presentation it sounds as though the Sodium channel work for a 3rd drug candidate at Hydra is moving along.
>The odds of Synavive's success in phase 2B look very promising especially if you understand the science and how the clinical trial was designed (e.g. Synavive's primary end-point is matched against placebo, so the likelihood of success 80% or better).
Finally at today's conference where Dr. Corrigan spoke, the normally reserved leader was in rare form ending his talk noting just how bullish he is about his own company. The upside for Zalicus has begun and savvy investors, if not already invested here, should get in very soon because I foresee a major move up in the share price going into summertime.
Disclosure: I am long ZLCS. Investors buy and/or sell at their own risk. I declare that I may day-trade Zalicus shares at any time. "Long" for me means until I sell. I do not "Short" stocks.
http://seekingalpha.com/article/635311-why-comments-by-novar…
Antwort auf Beitrag Nr.: 43.248.419 von bernie55 am 04.06.12 20:53:59BILLIG-ZLCS 'wiegt' noch immer nur 112Mio USD gesamt MK...
Let the Novartis party begin!

Let the Novartis party begin!


Na Prima!!!...


Jetzt die 1,35$ Schallmauer durchlaufen, dann heisst es endgültig-anschnallen für take off!


Jetzt die 1,35$ Schallmauer durchlaufen, dann heisst es endgültig-anschnallen für take off!

Antwort auf Beitrag Nr.: 43.258.004 von Growth2012 am 06.06.12 21:56:28Chatterbox Stocks (von heute stateside)
http://chatterboxstocks.com/report/ZLCS

http://chatterboxstocks.com/report/ZLCS

As I discussed yesterday, the up and above direction on the shares of Zalicus Inc(ZLCS) continued unabated. The stock is trading well above all its key DMAs, indicating that the up trend is still intact. It now reached overbought conditions so at these levels we should have some cautions. The stochastics are also deep in the overbought zone, and a mild correction cannot be ruled out at this stage. However, I will wait for a reversal signal to actually happen before I take that view. The Parabolic SAR continues with its buy signal. Above 1.36, I will open fresh long positions with an initial target of 1.54.
....



Antwort auf Beitrag Nr.: 43.264.969 von Growth2012 am 08.06.12 16:58:36Nachlademöglichkeit bis 16Uhr Morgennachmittag

Zalicus Inc. (ZLCS)
1.26 + - 0.00(0.00%)
Volume: 4,894,194
Avg Vol (3m): 1,786,770
kommt gut.
kleiner Verschnaufer, abkühlen und dann weiterlaufen lassen.
kleiner Verschnaufer, abkühlen und dann weiterlaufen lassen.
Hier ein super pipeline & catalyst summary von ein Yahoo user:
Sanofi (SNY) announcing Prednisporin will enter Phase III
Novartis (NVS) announcing a collaboration product
Z944 update on its single dosing study
Covidien (COV) announcing the 32 mg version of Exalgo
Synavive's Phase 2B results (September)
Also, from the last presentation by CEO Corrigan, he mentioned that they are still working on the development of new products using their cHTS Chalice technology. Does that mean a new product may show up on the Zalicus pipeline? It's worth thinking about.
Investors should also take note that Dr. Corrigan continues to compare Z160 to morphine and gabapentin, two of the most powerful pain medicaments on the market. This point of information (Z160 = morphine) is one of my main reasons for investing now. Eventually the market is going to digest this news and those who got in early will be handsomely rewarded.
With over $50M in cash reserves and being a highly liquid stock, Dr. Corrigan is right to state that the value is in the firm. If the charts are at all an indicator of where the share price is going, this remains a VERY STRONG BUY.
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
Er sprach allerdings nur von 2$ (kurzfrisitg), ich sehe eher 5$+ als absolut machbar/erreichbar an
Wir sitzen hier auf einen Pulverfaß! (im positiven, versteht sich wohl von selbst :cool
:cool
Sanofi (SNY) announcing Prednisporin will enter Phase III
Novartis (NVS) announcing a collaboration product
Z944 update on its single dosing study
Covidien (COV) announcing the 32 mg version of Exalgo
Synavive's Phase 2B results (September)
Also, from the last presentation by CEO Corrigan, he mentioned that they are still working on the development of new products using their cHTS Chalice technology. Does that mean a new product may show up on the Zalicus pipeline? It's worth thinking about.
Investors should also take note that Dr. Corrigan continues to compare Z160 to morphine and gabapentin, two of the most powerful pain medicaments on the market. This point of information (Z160 = morphine) is one of my main reasons for investing now. Eventually the market is going to digest this news and those who got in early will be handsomely rewarded.
With over $50M in cash reserves and being a highly liquid stock, Dr. Corrigan is right to state that the value is in the firm. If the charts are at all an indicator of where the share price is going, this remains a VERY STRONG BUY.
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
Er sprach allerdings nur von 2$ (kurzfrisitg), ich sehe eher 5$+ als absolut machbar/erreichbar an

Wir sitzen hier auf einen Pulverfaß! (im positiven, versteht sich wohl von selbst
 :cool
:cool
Real time ZLCS (technical chart overview) 
http://barchart.com/chart.php?sym=ZLCS&style=technical&templ…
Ab circa 15:45 erst relevant...

http://barchart.com/chart.php?sym=ZLCS&style=technical&templ…
Ab circa 15:45 erst relevant...

Hallo Growth,
hier auch nochmals ein guter Artikel u.a. mit der zukünftigen Pipeline von ZLCS
hier auch nochmals ein guter Artikel u.a. mit der zukünftigen Pipeline von ZLCS
Zitat von bernie55: Zalicus Bolsters Cash Position
On March 28, 2012, Zalicus, Inc. (ZLCS) announced via an 8k filing that the company's equity distribution agreement with Wedbush Securities Inc. has been completed at the proposed $15 million in aggregate gross proceeds. The equity distribution agreement, put into place on January 10, 2012 (see 8k filing), allowed for the sale of Zalicus common stock, from time to time, at the market price. Through the "ATM" (at-the-market), Zalicus sold a total of 14.058 million shares into the public market.
...Significant Effort Over The Past Two Weeks...
Inside Zalicus' annual 10k filing, dated March 9, 2012, on page 65, management noted that from January 10, 2012 to March 7, 2012, the company had sold only 5.367 million shares for gross proceeds of $5.5 million.
Over the past two weeks, management sold the remaining 8.691 million shares to raise the remaining $8.8 million allowable under the agreement. This explains the significant increase in volume seen in Zalicus common stock over the past few days.
...Low Visibility / Low Fees...
ATMs are sometimes difficult for shareholders to stomach because management is not obligated to provide an update on the status of the offerings, except in quarterly filings. ATMs are like pulling a bandage off slowly instead of ripping it off in one swipe.
However, the fee that Wedbush charged Zalicus for the ATM was only 2.5%. That's a total cost of $375,000 on a $15.0 million raise. A secondary public offering of 14.1 million shares at $1.05 per share to raise $15.0 million would have likely cost Zalicus 6% fees ($900,000) or more, and the stock would have likely dropped below $1.05 per share on the news. So it's a give / take. In the end, we think this ATM was rather successful for the company.
We remind investors that during the first and second quarter 2011, Zalicus completed a similar equity distribution agreement with Wedbush that raised $20 million in gross proceeds ($19.2 million net after fees).
...Solid Cash Position...
Zalicus exited 2011 with $49.7 million in cash and investments. Subtracting out the estimated operating burn for the first quarter 2012 and then adding back in the net $14.6 million from the ATM, and we forecast that Zalicus will holds just over $50 million in cash today.
With the ATM now complete, downward pressure on the stock has been relieved and we believe that investors can begin to focus on upcoming catalysts for the shares that could drive the stock higher.
These include:
Reporting phase 1 data on Z944 in the second quarter 2012.
Initiating a phase 2 program with Z160 in the third quarter 2012.
Reporting phase 2b data on SYNERGY during the third quarter 2012.
Initiating a phase 2 program with Z944 in the fourth quarter 2012.
Forming additional cHTS or ion channel collaborations during the second half of 2012.
Sanofi initiating a phase 3 trial on Prednisporin during the second half of 2012.
Covidien receiving approval for the 32mg Exalgo dose during the second half of 2012.
Partnering Synavive for phase 3 trials in 2013.
Moving additional ion channel candidates into the clinic in 2013.
http://seekingalpha.com/article/464261-zalicus-bolsters-cash…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/464261-zalicus-bolsters-cash…
Antwort auf Beitrag Nr.: 43.282.384 von bernie55 am 14.06.12 10:10:59Klasse Übersicht Bernie, Danke defür...
(bis ende des Jahres wissen wir alle erheblich mehr )
)

(bis ende des Jahres wissen wir alle erheblich mehr
 )
)
Zalicus' Z160 Could Change The World's Pain Market

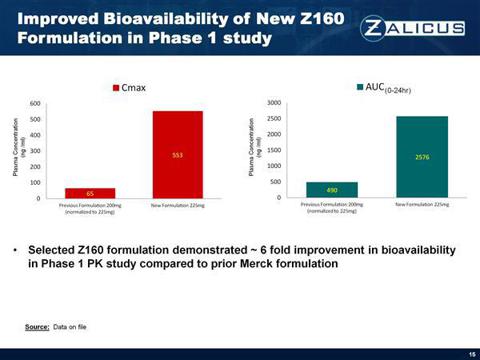
http://seekingalpha.com/article/662721-zalicus-z160-could-ch…
Der 1,30$ close (der späte, aber dafür extrem starke rally zum closing bell hin) lässt richtig positiv hoffen für den weiteren Handelsverlauf nächste Woche...



http://seekingalpha.com/article/662721-zalicus-z160-could-ch…
Der 1,30$ close (der späte, aber dafür extrem starke rally zum closing bell hin) lässt richtig positiv hoffen für den weiteren Handelsverlauf nächste Woche...

Sometimes a picture says more than a thousand words...


Gute Performance auch heute. Spricht für die wahre Substanz dieses Wertes und das enorme zukünftige Potential.
3 Rising Biotech Stars All Under $2/Share 
Resurging Pick: Zalicus (ZLCS)
On late Friday afternoon, fellow SA author Mr. Schrum released a report on Zalicus ($1.30/share) drug candidate Z160 entitled: "Zalicus' Z160 Could Change The World's Pain Market". Within the same hour, volume surged as did the stock price. Whether the two events were connected, the chart requires me to re-highlight to SA readers that my VERY BULLISH CALL on Zalicus is no-bull. I called Zalicus when it dipped into the $0.70-0.80 range and my call remains intact. The upside here is knowing a cadre of pipeline catalysts are near-term and coming in rapid succession. Is there risk here? Of course, but the reward is equally as great, if not greater.
This company has a sound balance sheet and multiple drug catalysts on the near-term horizon (e.g. Z944, Prednisporin, 32 mg Exalgo, and Synavive). With that many catalysts, a $1.30 share price, my analysis of the chart after Friday's close compels me to conclude that this stock is about to break-out. The bullish signs are all over this chart.
(click to enlarge)
Bullish signs:
The 12 June bear raid held the 200 MA.
13-15 June quickly retraced through the dip, volume is once again increasing, and Friday's close at $1.30/share set a new high in recent months. There was quick spike to $1.36.
The 50MA is on track to cross the 200MA, the MACD remains strong, and there's plenty of room left on the RSI upside.
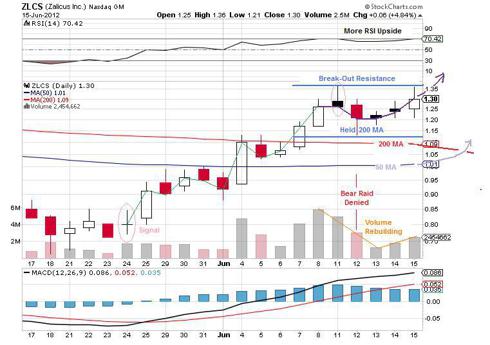
If the share price breaks through $1.36 resistance, it should spike towards to $1.60/share. It could also sideways churn below $1.36, but the bigger risk here may be not holding a position as that Friday afternoon volume spike suggested to me, some very deep-pockets are closing watching this stock.
http://seekingalpha.com/article/665951-3-rising-biotech-star…
Die 3$ Marke ist "wiedermal" (kurzfristig)sehr gut vorstellbar geworden....ZLCS long

Resurging Pick: Zalicus (ZLCS)
On late Friday afternoon, fellow SA author Mr. Schrum released a report on Zalicus ($1.30/share) drug candidate Z160 entitled: "Zalicus' Z160 Could Change The World's Pain Market". Within the same hour, volume surged as did the stock price. Whether the two events were connected, the chart requires me to re-highlight to SA readers that my VERY BULLISH CALL on Zalicus is no-bull. I called Zalicus when it dipped into the $0.70-0.80 range and my call remains intact. The upside here is knowing a cadre of pipeline catalysts are near-term and coming in rapid succession. Is there risk here? Of course, but the reward is equally as great, if not greater.
This company has a sound balance sheet and multiple drug catalysts on the near-term horizon (e.g. Z944, Prednisporin, 32 mg Exalgo, and Synavive). With that many catalysts, a $1.30 share price, my analysis of the chart after Friday's close compels me to conclude that this stock is about to break-out. The bullish signs are all over this chart.
(click to enlarge)
Bullish signs:
The 12 June bear raid held the 200 MA.
13-15 June quickly retraced through the dip, volume is once again increasing, and Friday's close at $1.30/share set a new high in recent months. There was quick spike to $1.36.
The 50MA is on track to cross the 200MA, the MACD remains strong, and there's plenty of room left on the RSI upside.

If the share price breaks through $1.36 resistance, it should spike towards to $1.60/share. It could also sideways churn below $1.36, but the bigger risk here may be not holding a position as that Friday afternoon volume spike suggested to me, some very deep-pockets are closing watching this stock.
http://seekingalpha.com/article/665951-3-rising-biotech-star…
Die 3$ Marke ist "wiedermal" (kurzfristig)sehr gut vorstellbar geworden....ZLCS long

Zalicus Successfully Completes Phase 1 Single Ascending Dose Study with Z944 , a Novel, Oral T-Type Calcium Channel Blocker
Phase 1 Multiple Ascending Dose Study with Z944 to Begin Third Quarter 2012
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Zalicus Inc. (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases, today announced that it is has successfully completed a Phase 1 single ascending dose (SAD) clinical study with Z944, its novel, oral, T-type calcium channel blocker. The study results indicate that Z944 was generally well-tolerated and a maximum tolerated dose was achieved. Z944 will advance into a Phase 1 multiple ascending dose (MAD) study in the third quarter of 2012. Assuming Z944 successfully completes the MAD study, Z944 could enter Phase 2 clinical development in the first quarter of 2013.
“Based on their unique mechanism of action, T-type calcium channel blockers such as Z944 have the potential to provide relief of pain and other symptoms in patients suffering from acute and chronic inflammatory pain, such as post-operative pain, fibromyalgia and inflammatory bowel syndrome, which are significant disease areas in which novel agents with improved tolerability, efficacy, and long-term safety profiles are needed," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “Zalicus is a leader in the discovery and development of Ion channel blockers, with unique expertise in N and T-type calcium channel and sodium channel blockers, and an emerging pipeline of Ion channel blocker drug candidates, including Z160 our oral, N-type calcium channel blocker which is poised to enter Phase 2a clinical trials in neuropathic pain indications in the third quarter of 2012.”
About Z944 and T-type Calcium Channel Blockers
Z944, is a novel, oral, T-type calcium channel blocker that has demonstrated preclinical efficacy in multiple inflammatory pain models. A Phase 1 clinical trial evaluating the safety and tolerability of Z944 is ongoing. If Z944 successfully completes this Phase 1 clinical trial, Zalicus is planning to advance Z944 into Phase 2 clinical development.
The wide distribution of T-type calcium channels found in brain, heart, endocrine cells and other tissues provides the possibility of developing therapeutics for multiple indications, including treatment of pain.
http://finance.yahoo.com/news/zalicus-successfully-completes…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/zalicus-successfully-completes…
Phase 1 Multiple Ascending Dose Study with Z944 to Begin Third Quarter 2012
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Zalicus Inc. (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases, today announced that it is has successfully completed a Phase 1 single ascending dose (SAD) clinical study with Z944, its novel, oral, T-type calcium channel blocker. The study results indicate that Z944 was generally well-tolerated and a maximum tolerated dose was achieved. Z944 will advance into a Phase 1 multiple ascending dose (MAD) study in the third quarter of 2012. Assuming Z944 successfully completes the MAD study, Z944 could enter Phase 2 clinical development in the first quarter of 2013.
“Based on their unique mechanism of action, T-type calcium channel blockers such as Z944 have the potential to provide relief of pain and other symptoms in patients suffering from acute and chronic inflammatory pain, such as post-operative pain, fibromyalgia and inflammatory bowel syndrome, which are significant disease areas in which novel agents with improved tolerability, efficacy, and long-term safety profiles are needed," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “Zalicus is a leader in the discovery and development of Ion channel blockers, with unique expertise in N and T-type calcium channel and sodium channel blockers, and an emerging pipeline of Ion channel blocker drug candidates, including Z160 our oral, N-type calcium channel blocker which is poised to enter Phase 2a clinical trials in neuropathic pain indications in the third quarter of 2012.”
About Z944 and T-type Calcium Channel Blockers
Z944, is a novel, oral, T-type calcium channel blocker that has demonstrated preclinical efficacy in multiple inflammatory pain models. A Phase 1 clinical trial evaluating the safety and tolerability of Z944 is ongoing. If Z944 successfully completes this Phase 1 clinical trial, Zalicus is planning to advance Z944 into Phase 2 clinical development.
The wide distribution of T-type calcium channels found in brain, heart, endocrine cells and other tissues provides the possibility of developing therapeutics for multiple indications, including treatment of pain.
http://finance.yahoo.com/news/zalicus-successfully-completes…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/zalicus-successfully-completes…
Analysten-Bewertung - 19.06.12
Zalicus-Aktie: Imposantes Comeback
Endingen (www.aktiencheck.de) - Die Experten von "Global Biotech Investing" halten die Aktie von Zalicus (<US98887C1053>/ WKN A1C4WQ) auch auf dem aktuellen Niveau für ein Schnäppchen. Das Timing hätte nicht besser sein können. Unmittelbar nach der Nachkaufempfehlung der Experten bei einem Kursniveau von 0,80 USD sei die Aktie bis auf 1,40 USD geklettert, was mutigen Anlegern einen Gewinn von bis zu 75% ins Depot gespült habe.
Analystenkreise würden aktuell einen 2015er Net Profit von 0,50 USD je Aktie prognostizieren. Dann läge das KGV bei unter 3. Researchhäuser würden daher Kursziele von bis zu 5 USD ausflaggen. Vor diesem Hintergrund haltend die Experten von "Global Biotech Investing" die Zalicus-Aktie selbst auf dem aktuellen Niveau für ein Schnäppchen. (Ausgabe 12 vom 18.06.2012) (19.06.2012/ac/a/a)
Quelle: Global Biotech Investing
Zalicus-Aktie: Imposantes Comeback
Endingen (www.aktiencheck.de) - Die Experten von "Global Biotech Investing" halten die Aktie von Zalicus (<US98887C1053>/ WKN A1C4WQ) auch auf dem aktuellen Niveau für ein Schnäppchen. Das Timing hätte nicht besser sein können. Unmittelbar nach der Nachkaufempfehlung der Experten bei einem Kursniveau von 0,80 USD sei die Aktie bis auf 1,40 USD geklettert, was mutigen Anlegern einen Gewinn von bis zu 75% ins Depot gespült habe.
Analystenkreise würden aktuell einen 2015er Net Profit von 0,50 USD je Aktie prognostizieren. Dann läge das KGV bei unter 3. Researchhäuser würden daher Kursziele von bis zu 5 USD ausflaggen. Vor diesem Hintergrund haltend die Experten von "Global Biotech Investing" die Zalicus-Aktie selbst auf dem aktuellen Niveau für ein Schnäppchen. (Ausgabe 12 vom 18.06.2012) (19.06.2012/ac/a/a)
Quelle: Global Biotech Investing
Zalicus to Present at the 2012 JMP Securities Healthcare Conference
Zalicus Inc. (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases, today announced that Zalicus management will present at the 2012 JMP Securities Healthcare Conference at 10:00 a.m. ET on Thursday, July 12, 2012 at the Peninsula Hotel in New York. Interested parties may access a live webcast of the presentation by visiting the Zalicus website at www.zalicus.com. The webcast will be archived on the Zalicus website following the event.
http://finance.yahoo.com/news/zalicus-present-2012-jmp-secur…
Zalicus Inc. (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases, today announced that Zalicus management will present at the 2012 JMP Securities Healthcare Conference at 10:00 a.m. ET on Thursday, July 12, 2012 at the Peninsula Hotel in New York. Interested parties may access a live webcast of the presentation by visiting the Zalicus website at www.zalicus.com. The webcast will be archived on the Zalicus website following the event.
http://finance.yahoo.com/news/zalicus-present-2012-jmp-secur…
Zalicus Is Trading At Bargain Levels
July 9, 2012
Based upon my estimates, Zalicus (ZLCS) has impressive upside potential of 300% based upon two unique drug discovery platforms: Ion Channel Modulation and High Throughput Screening (cHTS) technology. ZLCS is the clear leader in ion channel research wherein ZLCS is developing oral medications that target pain signaling. If successful in trials, ZLCS's "Z" drugs would be a strong competitor in a mega-billion dollar market against lucrative opioid drugs with unpleasant side effects, including addiction.
On June 19, 2012, the company announced that it successfully completed its single ascending dose study of Z944 and that it could move into phase 2 by first quarter of 2013. As commented by Mark H.N. Corrigan, MD, President and CEO of Zalicus:
Based on their unique mechanism of action, T-type calcium channel blockers such as Z944 have the potential to provide relief of pain and other symptoms in patients suffering from acute and chronic inflammatory pain, such as post-operative pain, fibromyalgia and inflammatory bowel syndrome, which are significant disease areas in which novel agents with improved tolerability, efficacy, and long-term safety profiles are needed.
Stock followers may ask why the stock dropped over 25% in the weeks following this announcement. The answer likely lies within news released the same day as this Z944 announcement, that Zalicus Inc. entered into an equity distribution agreement with Wedbush Securities Inc. under which they may sell common stock having aggregate sales proceeds of up to $15,000,000. According to the filing:
Sales of our common stock through Wedbush, if any, will be made by means of ordinary brokers' transactions on the NASDAQ Global Market or otherwise at market prices prevailing at the time of sale, in block transactions, or as otherwise agreed upon by us and Wedbush.
Such At-The-Market sales (ATM sales) are often difficult for shareholders to digest, since management is not obligated to provide status updates on the offerings - except at quarterly filings. I believe the ATM sales are already or nearly completed, as shortly thereafter we saw the highest ZLCS trading volume within the past 12 months.
Aside from the progress made on Z944, there are two drugs in pipeline that are particularly notable. First, Z160 could be a revolutionary pain medication as it would be the first of its kind to be orally administered to block calcium pain receptors without an opioidal side effect. Secondly, ZLCS's most advanced candidate, Synavive, is a treatment for immuno-inflammatory disorders, a $68 billion market, and recently completed the enrollment of patients in a phase 2b clinical trial the results of which are expected later this year.
There are several stock catalysts that may propel the stock to higher levels:
Partnership Announcement and/or Buy-Out Offer:
I would not be surprised if Novartis (NVS) or other large pharmaceuticals show increasing interest in ZLCS for Synavive alone, let alone the aforementioned "Z" drugs. ZLCS is still in partnerships with and, in some cases has, revenue generating collaborations with Amgen (AMGN), NVS, Sanofi (SNY), and Covidien (COV). Those partnerships attest to the perceived value of ZLCS, its management and technologies. In fact, Merck made a $475 million offer for a prior form of Z160 before multiple formulaic improvements were made as demonstrated in Phase 1 PK studies. Even with the dilution created by the ATM and assuming that 12 million shares are sold therein ($15,000,000 / $1.25 per share), the success of Z160 would be worth over $4 per share, well over 3 times the current value.
Zalicus Inc's Presentation at the JMP Securities Healthcare Conference on Thursday, July 12, 2012, 10:00 a.m. EST
Prednisporin: Zalicus has partnered with a division of Sanofy on this drug used to treat inflammatory ocular diseases such as allergic conjunctivitis and positive results of the phase 2b trial could propel the stock to much higher levels.
Exalgo 32 mg: Exalgo was approved as a generic in lower doses, but Covidien reserved the rights to higher doses. According to Zack's analyst, Jason Napodano, "We expect that Covidien will vigorously work to protect the exclusivity of the 32mg dose. In Fact, we believe Covidien's willingness to settle with Watson over the 8, 12 and 16mg doses stems from the fact that once the 32mg dose is approved, Covidien will focus its entire promotional efforts around this dose….our price targets remain unchanged at $3 per share." The drug is currently under FDA review and Napodano expects it to be on the market late in the third quarter of this year.
Synavive: The drug is wholly owned by the company, and as mentioned previously, the market potential is tremendous. Phase 2b study results are due late this quarter.
In addition to strong fundamentals, I believe ZLCS is well-positioned from a technical perspective. It is trading over 140% below its 52-week high and appears to have bottomed. It has pulled back over 25% just in the past 3 weeks which has prompted me to start an aggressive position in the stock. Considering the catalysts noted above, I would continue to buy the stock up into the $1.50 range. Analysts maintain a $3 to $5 target on the stock and I strongly agree with them that this stock should be trading much higher than current levels.
http://seekingalpha.com/article/710101-zalicus-is-trading-at…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/710101-zalicus-is-trading-at…
July 9, 2012
Based upon my estimates, Zalicus (ZLCS) has impressive upside potential of 300% based upon two unique drug discovery platforms: Ion Channel Modulation and High Throughput Screening (cHTS) technology. ZLCS is the clear leader in ion channel research wherein ZLCS is developing oral medications that target pain signaling. If successful in trials, ZLCS's "Z" drugs would be a strong competitor in a mega-billion dollar market against lucrative opioid drugs with unpleasant side effects, including addiction.
On June 19, 2012, the company announced that it successfully completed its single ascending dose study of Z944 and that it could move into phase 2 by first quarter of 2013. As commented by Mark H.N. Corrigan, MD, President and CEO of Zalicus:
Based on their unique mechanism of action, T-type calcium channel blockers such as Z944 have the potential to provide relief of pain and other symptoms in patients suffering from acute and chronic inflammatory pain, such as post-operative pain, fibromyalgia and inflammatory bowel syndrome, which are significant disease areas in which novel agents with improved tolerability, efficacy, and long-term safety profiles are needed.
Stock followers may ask why the stock dropped over 25% in the weeks following this announcement. The answer likely lies within news released the same day as this Z944 announcement, that Zalicus Inc. entered into an equity distribution agreement with Wedbush Securities Inc. under which they may sell common stock having aggregate sales proceeds of up to $15,000,000. According to the filing:
Sales of our common stock through Wedbush, if any, will be made by means of ordinary brokers' transactions on the NASDAQ Global Market or otherwise at market prices prevailing at the time of sale, in block transactions, or as otherwise agreed upon by us and Wedbush.
Such At-The-Market sales (ATM sales) are often difficult for shareholders to digest, since management is not obligated to provide status updates on the offerings - except at quarterly filings. I believe the ATM sales are already or nearly completed, as shortly thereafter we saw the highest ZLCS trading volume within the past 12 months.
Aside from the progress made on Z944, there are two drugs in pipeline that are particularly notable. First, Z160 could be a revolutionary pain medication as it would be the first of its kind to be orally administered to block calcium pain receptors without an opioidal side effect. Secondly, ZLCS's most advanced candidate, Synavive, is a treatment for immuno-inflammatory disorders, a $68 billion market, and recently completed the enrollment of patients in a phase 2b clinical trial the results of which are expected later this year.
There are several stock catalysts that may propel the stock to higher levels:
Partnership Announcement and/or Buy-Out Offer:
I would not be surprised if Novartis (NVS) or other large pharmaceuticals show increasing interest in ZLCS for Synavive alone, let alone the aforementioned "Z" drugs. ZLCS is still in partnerships with and, in some cases has, revenue generating collaborations with Amgen (AMGN), NVS, Sanofi (SNY), and Covidien (COV). Those partnerships attest to the perceived value of ZLCS, its management and technologies. In fact, Merck made a $475 million offer for a prior form of Z160 before multiple formulaic improvements were made as demonstrated in Phase 1 PK studies. Even with the dilution created by the ATM and assuming that 12 million shares are sold therein ($15,000,000 / $1.25 per share), the success of Z160 would be worth over $4 per share, well over 3 times the current value.
Zalicus Inc's Presentation at the JMP Securities Healthcare Conference on Thursday, July 12, 2012, 10:00 a.m. EST
Prednisporin: Zalicus has partnered with a division of Sanofy on this drug used to treat inflammatory ocular diseases such as allergic conjunctivitis and positive results of the phase 2b trial could propel the stock to much higher levels.
Exalgo 32 mg: Exalgo was approved as a generic in lower doses, but Covidien reserved the rights to higher doses. According to Zack's analyst, Jason Napodano, "We expect that Covidien will vigorously work to protect the exclusivity of the 32mg dose. In Fact, we believe Covidien's willingness to settle with Watson over the 8, 12 and 16mg doses stems from the fact that once the 32mg dose is approved, Covidien will focus its entire promotional efforts around this dose….our price targets remain unchanged at $3 per share." The drug is currently under FDA review and Napodano expects it to be on the market late in the third quarter of this year.
Synavive: The drug is wholly owned by the company, and as mentioned previously, the market potential is tremendous. Phase 2b study results are due late this quarter.
In addition to strong fundamentals, I believe ZLCS is well-positioned from a technical perspective. It is trading over 140% below its 52-week high and appears to have bottomed. It has pulled back over 25% just in the past 3 weeks which has prompted me to start an aggressive position in the stock. Considering the catalysts noted above, I would continue to buy the stock up into the $1.50 range. Analysts maintain a $3 to $5 target on the stock and I strongly agree with them that this stock should be trading much higher than current levels.
http://seekingalpha.com/article/710101-zalicus-is-trading-at…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/710101-zalicus-is-trading-at…
Zalicus Initiates Phase 1 Multiple Ascending Dose Study with Z944, a Novel, Oral T-Type Calcium Channel Blocker
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Press Release: Zalicus Inc. – 1 hour 1 minute ago
Zalicus Inc. (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases, today announced that it is has initiated a Phase 1 multiple ascending dose (MAD) clinical study with Z944, its novel, oral, T-type calcium channel blocker. The initiation of this MAD study follows the successfully completed Phase 1 single ascending dose study in which Z944 was determined to be generally well-tolerated and a maximum tolerated dose was achieved. Assuming Z944 successfully completes the MAD study, Z944 could enter Phase 2 clinical development in the first quarter of 2013.
“I am encouraged by the continued progress the Zalicus team is making in advancing the development of our calcium channel blocker programs, including Z944 our T-type calcium channel blocker and Z160, our N-type calcium channel blocker which is poised to enter Phase 2a clinical trials in neuropathic pain later in the third quarter of 2012," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus.
http://finance.yahoo.com/news/zalicus-initiates-phase-1-mult…
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Press Release: Zalicus Inc. – 1 hour 1 minute ago
Zalicus Inc. (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases, today announced that it is has initiated a Phase 1 multiple ascending dose (MAD) clinical study with Z944, its novel, oral, T-type calcium channel blocker. The initiation of this MAD study follows the successfully completed Phase 1 single ascending dose study in which Z944 was determined to be generally well-tolerated and a maximum tolerated dose was achieved. Assuming Z944 successfully completes the MAD study, Z944 could enter Phase 2 clinical development in the first quarter of 2013.
“I am encouraged by the continued progress the Zalicus team is making in advancing the development of our calcium channel blocker programs, including Z944 our T-type calcium channel blocker and Z160, our N-type calcium channel blocker which is poised to enter Phase 2a clinical trials in neuropathic pain later in the third quarter of 2012," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus.
http://finance.yahoo.com/news/zalicus-initiates-phase-1-mult…
Antwort auf Beitrag Nr.: 43.369.002 von bernie55 am 10.07.12 11:11:52und überall die gleichen Leute.


Zalicus: History Tells Us It's Time To Buy
http://seekingalpha.com/article/752221-zalicus-history-tells…
http://seekingalpha.com/article/752221-zalicus-history-tells…

Zitat von Growth2012: Zalicus: History Tells Us It's Time To Buy
http://seekingalpha.com/article/752221-zalicus-history-tells…
....hier mal ein paar kleine statements aus diesem Artikel...

Zalicus: History Tells Us It's Time To Buy
July 26, 2012
- - - - - - - - - - - - - - - - - - - - - - - -
Personally I welcome the fact that Zalicus is raising cash. I see this as confidence in their pipeline. The management team of seasoned executives knows there drugs are worth 100's of millions and want to make sure they are able to advance them to the right stages to get top dollar when they are ready to partner. If Zalicus did not have the cash that it does, it would most certainly have partnered already with a big pharma company and more than likely gotten the short end of the stick.
I confirmed with the CFO Just Rentz a few days ago that there will be an update on the Wedbush offering no later than August 9th along with their 2nd Quarter results. If history shows us correctly, it will be on Friday August 3rd like the year before.
I'm viewing this as a strategic play here that will roll into the run-up for the top line data for Synavive. This is all part of the master plan that Zalicus' executives have put in to motion months ago.
The other big opportunity that people have forgotten about is Sanofi (SNY) with Prednisporin. They, too, will be announcing 2nd quarter results on the 26th of this month. We hope there will be an update with the Phase III initiation for FOV1101. This will trigger a 3 million dollar milestone payment as well as another 38 million dollars in advancement royalties to market.
Covidien (COV) has been tight lipped about the 32 mg Exalgo filing that should be announced in the 2nd half of this year to continue to take the lion's share of the market and keep Watson Pharmaceuticals (WPI) from cutting into the Exalgo market area.
All in all I see this as a lay-up gain. I've have been accumulating at the 1.00 range and am looking for a big run over the next month starting any day.
Disclosure: I am long ZLCS.
Watch this space....

Pain avoidance therapy
It's the long-term that analysts are eyeing when they look at biotech sensation YM BioSciences, which got a much-needed boost when it reported positive data in ongoing early- and mid-stage trials for its treatment of myelofibrosis. I've previously noted my bearishness towards outperformance, because the market for the drug doesn't appear to be particularly large, as once thought, and a rival's drug is already on the market. A once-daily dosage versus twice daily doesn't seem like enough of a change to displace the leader here. No doubt it will get a boost from further positive testing results, but I think there's a long road to go before the real results are in.
It's a different story at another biotech, Zalicus, which actually has a product on the market, and another potential big winner under development. While investors have been watching the ups and downs of its pain-management therapy, Exalgo, because it's the one product it's actually been selling, they also need to keep an eye on Synavive, a treatment for immuno-inflammatory disorders. Top-line results are expected next month that could cause its stock to rise in anticipation.
Although its shares have been cut in half over the past year, it's recovered more than 40% from its low point, after partner Covidien agreed to let Watson Pharmaceuticals produce an Exalgo knock-off. But as I pointed out at the time, it reserved the 32 mg dosage for itself, because it's counting on that version to be a big seller. Because Zalicus has a possible winner in Synavive coming up for review, and a better seller of its successful pain management treatment on the horizon, I've marked it to outperform the broad indexes on CAPS. Let me know in the comments box below, or on the Zalicus CAPS page, if you agree that the biotech has lots of ground it can and will make up.

....


Pain avoidance therapy
It's the long-term that analysts are eyeing when they look at biotech sensation YM BioSciences, which got a much-needed boost when it reported positive data in ongoing early- and mid-stage trials for its treatment of myelofibrosis. I've previously noted my bearishness towards outperformance, because the market for the drug doesn't appear to be particularly large, as once thought, and a rival's drug is already on the market. A once-daily dosage versus twice daily doesn't seem like enough of a change to displace the leader here. No doubt it will get a boost from further positive testing results, but I think there's a long road to go before the real results are in.
It's a different story at another biotech, Zalicus, which actually has a product on the market, and another potential big winner under development. While investors have been watching the ups and downs of its pain-management therapy, Exalgo, because it's the one product it's actually been selling, they also need to keep an eye on Synavive, a treatment for immuno-inflammatory disorders. Top-line results are expected next month that could cause its stock to rise in anticipation.
Although its shares have been cut in half over the past year, it's recovered more than 40% from its low point, after partner Covidien agreed to let Watson Pharmaceuticals produce an Exalgo knock-off. But as I pointed out at the time, it reserved the 32 mg dosage for itself, because it's counting on that version to be a big seller. Because Zalicus has a possible winner in Synavive coming up for review, and a better seller of its successful pain management treatment on the horizon, I've marked it to outperform the broad indexes on CAPS. Let me know in the comments box below, or on the Zalicus CAPS page, if you agree that the biotech has lots of ground it can and will make up.
....


Mir bleibt nur eine offene Frage im sachen ZLCS...
...Ob die 5$ bis zum jahresende immer noch machbar sei

LONG!
...Ob die 5$ bis zum jahresende immer noch machbar sei


LONG!
Zalicus' Potential Might Be Worthy Of A Nobel Prize
The bold statement found in my title is not just for show; Zalicus (ZLCS) is opening a new door that will improve the lives of millions of people around the world.
I listened to the recent conference call in New York at the JMP Securities Healthcare Conference and you can hear the excitement in management's voices. They touched on a topic that is very important to me and I feel that it needs to be loudly voiced.
Z160 and Z944 N type and T type calcium channel blockers have the possibility to create the first pain management drug that is orally administered without the abuse and addictive side effects that come with the use of opioids. Millions are effected every year by opiods and the addictive side effects they cause. Many patients have to deal with withdrawal, overdoses, and the potential these drugs have when in the wrong hands outside a doctor's written consent.
The potential here has Nobel Prize written all over it. Doctors, the FDA, and the effected opioid patients have wanted this advancement for decades and hopefully Zalicus will be there with Z160 & Z944. These two drugs are just the tip of the iceberg to the drugs that will follow from their advancements.
As stated in the Q&A they have already had multiple partnership meetings with big pharmaceutical companies. This is the first time that information has been shared. We all know the potential that these drugs have and that was shown by Merck's (MRK) involvement a few years ago with an earlier formulation of Z160 that Merck ponied up over 475 million dollars for. With the new formulation of Z160 having solved the bioavailability issues, CFO Justin Rentz stated that Zalicus does not want to show its hand too soon. Zalicus is looking out for shareholder value and wants to take it further into studies before letting the cat out of the bag so it can fetch top dollar.
It looks like to me big pharmaceutical companies are clawing to get their hands on Zalicus's pipeline.
Zalicus as of last quarter has 52 million in cash with a current quarterly cash burn rate of 11 million. The cash burn is primarily due to late stage study advancements like Synavive in Phase IIb, as well as the ion channel programs. With the current PPS floating around the $1.00 range it has a market cap of roughly 106 million.
Other things in the meantime to look forward to is top line data for Phase IIb studies on Synavive coming in September.
Sanofi (SNY) announcing a Phase III start on FOV1101 with their 2nd quarter results.
Covidien (COV) is currently working on the new 32mg dosage with Exalgo, as well as possible oncology pre clinical candidates from the Novartis (NVS) collaboration all make for an exciting summer.
All in all very exhilarating news with a once in a lifetime opportunity.
http://seekingalpha.com/article/764171-zalicus-potential-mig…
The bold statement found in my title is not just for show; Zalicus (ZLCS) is opening a new door that will improve the lives of millions of people around the world.
I listened to the recent conference call in New York at the JMP Securities Healthcare Conference and you can hear the excitement in management's voices. They touched on a topic that is very important to me and I feel that it needs to be loudly voiced.
Z160 and Z944 N type and T type calcium channel blockers have the possibility to create the first pain management drug that is orally administered without the abuse and addictive side effects that come with the use of opioids. Millions are effected every year by opiods and the addictive side effects they cause. Many patients have to deal with withdrawal, overdoses, and the potential these drugs have when in the wrong hands outside a doctor's written consent.
The potential here has Nobel Prize written all over it. Doctors, the FDA, and the effected opioid patients have wanted this advancement for decades and hopefully Zalicus will be there with Z160 & Z944. These two drugs are just the tip of the iceberg to the drugs that will follow from their advancements.
As stated in the Q&A they have already had multiple partnership meetings with big pharmaceutical companies. This is the first time that information has been shared. We all know the potential that these drugs have and that was shown by Merck's (MRK) involvement a few years ago with an earlier formulation of Z160 that Merck ponied up over 475 million dollars for. With the new formulation of Z160 having solved the bioavailability issues, CFO Justin Rentz stated that Zalicus does not want to show its hand too soon. Zalicus is looking out for shareholder value and wants to take it further into studies before letting the cat out of the bag so it can fetch top dollar.
It looks like to me big pharmaceutical companies are clawing to get their hands on Zalicus's pipeline.

Zalicus as of last quarter has 52 million in cash with a current quarterly cash burn rate of 11 million. The cash burn is primarily due to late stage study advancements like Synavive in Phase IIb, as well as the ion channel programs. With the current PPS floating around the $1.00 range it has a market cap of roughly 106 million.
Other things in the meantime to look forward to is top line data for Phase IIb studies on Synavive coming in September.
Sanofi (SNY) announcing a Phase III start on FOV1101 with their 2nd quarter results.
Covidien (COV) is currently working on the new 32mg dosage with Exalgo, as well as possible oncology pre clinical candidates from the Novartis (NVS) collaboration all make for an exciting summer.
All in all very exhilarating news with a once in a lifetime opportunity.
http://seekingalpha.com/article/764171-zalicus-potential-mig…
Hmmmm, alleine gestern würden 7,038,207 sharesgetradet

...vermütlich gehts bald 'richtig' los gen norden

Zitat von Growth2012:![]()
Hmmmm, alleine gestern würden 7,038,207 sharesgetradet
...vermütlich gehts bald 'richtig' los gen norden
Ich bin am Montag hier eingestiegen....den Tipp hatte ich von Bernie 55 (danke nochmal an dieser Stelle
 )
)Gestern unter hohem Volumen auf TH geschlossen......

Man konnt gestern schön sehen, das die Shorts immer wieder versucht haben zu drücken...mit mäßigem Erfolg.
Mmn steht Zalicus kurz vor dem Ausbruch...
Halte eine große Position hier......Long

Good Luck @all
Earthfire
Antwort auf Beitrag Nr.: 43.448.246 von Earthfire am 02.08.12 09:36:55"Halte eine große Position hier"
...naja, "groß" ist immer relativ (je nach Anlegertypus & Taschentiefe)
Bin für meine bescheidenen Verhältnisse auch recht 'ambitioniert' investiert.
investiert.

Wünsche jedenfalls viel Glück, gutes timing & starke Nerven bis ans Ziel.
...naja, "groß" ist immer relativ (je nach Anlegertypus & Taschentiefe)
Bin für meine bescheidenen Verhältnisse auch recht 'ambitioniert'
 investiert.
investiert.Wünsche jedenfalls viel Glück, gutes timing & starke Nerven bis ans Ziel.
Zitat von Growth2012: "Halte eine große Position hier"
...naja, "groß" ist immer relativ (je nach Anlegertypus & Taschentiefe)
Bin für meine bescheidenen Verhältnisse auch recht 'ambitioniert'investiert.
![]()
Wünsche jedenfalls viel Glück, gutes timing & starke Nerven bis ans Ziel.
Natürlich ist eine große posi. relativ.......bei mir liegt der Focus eher im Chance-Risikoverhälniss......und weniger in der Taschentiefe d.h. je überzeigter ich von Richung Norden bin...desto größer die Position. !

Man könnte auch sagen....für meine bescheidenen Verhältnisse bin ich hier größer wie üblich in einem Biotec investiert...

Mal sehen was die nächsten Tage und Wochen so bringen.....
Ich wünsche dir auch viel Erfolg....und eine gute Rendite !

Hier noch der Link zu einem Artikel von gestern :
sehr lesenswert :
http://seekingalpha.com/article/768931-4-reasons-to-buy-zali…
sehr lesenswert :
http://seekingalpha.com/article/768931-4-reasons-to-buy-zali…
Ok, take a look at this (absolut kommentarlos & ohne pushen zu wollen...es geht mir nur um das verborgene "potential")
Possible Bullish Inside Day Candle Pattern Detected for Zalicus (NASDAQ:ZLCS)
SmarTrend's candlestick scanner has spotted a possible bullish inside day candle pattern in Zalicus (NASDAQ:ZLCS) based on the price action in the company's shares. Today's price range of $0.96 and $1.01 is within yesterday's high and low of the day. This trading action often signifies indecision by bulls and bears to drive prices higher or lower and often implies a possible change in trend. Traders and investors interested in shares of Zalicus may want to consider an appropriate entry level in the event a reversal to the upside transpires. It is important to look for confirmation.
>>>Zalicus (NASDAQ:ZLCS) has potential upside of 320.8%<<<<... based on a current price of $1.01 and analysts' consensus price target of $4.25. The stock should run into initial resistance at its 200-day moving average (MA) of $1.09 and subsequent resistance at its 50-day MA of $1.10.
Zalicus Inc is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. The Company's drug pipeline includes an arthritis formula in phase 2 development.
In the past 52 weeks, Zalicus share prices have been bracketed by a low of $0.71 and a high of $3.21 and are now at $1.01, 41% above that low price. In the last five trading sessions, the 50-day moving average (MA) has climbed 0.6% while the 200-day MA has remained constant.
SmarTrend is monitoring the recent change of momentum in Zalicus. Please refer to our Company Overview for the results of our proprietary technical indicators that have been scanning shares of Zalicus in search of a potential trend change.
http://www.mysmartrend.com/news-briefs/candlestick/possible-…
we will see....


Possible Bullish Inside Day Candle Pattern Detected for Zalicus (NASDAQ:ZLCS)
SmarTrend's candlestick scanner has spotted a possible bullish inside day candle pattern in Zalicus (NASDAQ:ZLCS) based on the price action in the company's shares. Today's price range of $0.96 and $1.01 is within yesterday's high and low of the day. This trading action often signifies indecision by bulls and bears to drive prices higher or lower and often implies a possible change in trend. Traders and investors interested in shares of Zalicus may want to consider an appropriate entry level in the event a reversal to the upside transpires. It is important to look for confirmation.
>>>Zalicus (NASDAQ:ZLCS) has potential upside of 320.8%<<<<... based on a current price of $1.01 and analysts' consensus price target of $4.25. The stock should run into initial resistance at its 200-day moving average (MA) of $1.09 and subsequent resistance at its 50-day MA of $1.10.
Zalicus Inc is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. The Company's drug pipeline includes an arthritis formula in phase 2 development.
In the past 52 weeks, Zalicus share prices have been bracketed by a low of $0.71 and a high of $3.21 and are now at $1.01, 41% above that low price. In the last five trading sessions, the 50-day moving average (MA) has climbed 0.6% while the 200-day MA has remained constant.
SmarTrend is monitoring the recent change of momentum in Zalicus. Please refer to our Company Overview for the results of our proprietary technical indicators that have been scanning shares of Zalicus in search of a potential trend change.
http://www.mysmartrend.com/news-briefs/candlestick/possible-…
we will see....



After hours gestern (allerdings bei denkbar geringe Volumina)
http://www.nasdaq.com/symbol/zlcs/after-hours

http://www.nasdaq.com/symbol/zlcs/after-hours
N'abend zusammen!
habt ihr das Bid an der Nasdaq soeben wahrgenommen??(bei nur1 cent spread)
Geld 1,12
Brief 1,13
Zeit 06.08.1219:46
Spread 0,88%
Geld Stk. 257.500
Brief Stk. 4.500
Irgend jemand meint es verdammt ernst ...nicht wahr?
...nicht wahr?

..ich denke es wirdt allerdings erst in September so richtig losgehen!
Glück auf
habt ihr das Bid an der Nasdaq soeben wahrgenommen??(bei nur1 cent spread)
Geld 1,12
Brief 1,13
Zeit 06.08.1219:46
Spread 0,88%
Geld Stk. 257.500
Brief Stk. 4.500
Irgend jemand meint es verdammt ernst
 ...nicht wahr?
...nicht wahr?

..ich denke es wirdt allerdings erst in September so richtig losgehen!
Glück auf

Antwort auf Beitrag Nr.: 43.462.701 von Growth2012 am 06.08.12 20:06:33Nabend Growth....da ich ja hier bereits investiert bin...beobachte ich den jeweiligen Intradayhandel sehr genau.
Könntest recht haben mit Sep......tippe aber persönlich auf August...bedingt durch Insider die sich vorher bereits in der Regel vor News positionieren.
Was mir aufgefallen ist, das mit rel. kleinen Stüczahlen gefrückt wird...um im Anschluss einen größeren Kauf zu plazieren.
Desweiteren sieht man kurz vor Handelsschluss, das hier je nach Minus auch noch schnell zugeschlagen wird, und der Kurs entsprechend zulegt.
In meinen Augen alles gute Zeichen, die auf einen baldigen Ausbruch Richtung 2 $ hoffen lassen.
Gruß Earthfire
Könntest recht haben mit Sep......tippe aber persönlich auf August...bedingt durch Insider die sich vorher bereits in der Regel vor News positionieren.
Was mir aufgefallen ist, das mit rel. kleinen Stüczahlen gefrückt wird...um im Anschluss einen größeren Kauf zu plazieren.
Desweiteren sieht man kurz vor Handelsschluss, das hier je nach Minus auch noch schnell zugeschlagen wird, und der Kurs entsprechend zulegt.
In meinen Augen alles gute Zeichen, die auf einen baldigen Ausbruch Richtung 2 $ hoffen lassen.
Gruß Earthfire
News zum schönen Plus heute :
http://www.nasdaq.com/article/zalicus-reports-financial-resu…
auf gehts ...Richtung 2 $

http://www.nasdaq.com/article/zalicus-reports-financial-resu…
auf gehts ...Richtung 2 $


Antwort auf Beitrag Nr.: 43.467.284 von Earthfire am 07.08.12 20:00:59Zalicus Inc (ZLCS) shares climbed 10.71% to $1.24 after the company reported second quarter revenue of $2.9 million, as compared to $1.8 million in the year ago period. For the quarter ended June 30, 2012, net loss was $10.3 million, or $0.09 per share, compared to a net loss of $11.3 million, or $0.11 per share, in the quarter ended June 30, 2011.

in dem Fall liege ich gerne im unrecht earthfire, sicherlich 'more to come' schon im August wie es nun aussieht....

in dem Fall liege ich gerne im unrecht earthfire, sicherlich 'more to come' schon im August wie es nun aussieht....


Zalicus Up 9.8%, Shares Break Through Resistance (ZLCS)
Zalicus (NASDAQ:ZLCS) is one of today's best performing low-priced stocks, up 9.8% to $1.23 on 1.1x average daily volume. Zalicus has traded 3.5 million shares thus far today, vs. average volume of 3.0 million shares per day. The stock has outperformed the Dow (9.8% to the Dow's 0.7%) and outperformed the S&P 500 (9.8% to the S&P's 0.9%) during today's trading.
Zalicus Inc is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. The Company's drug pipeline includes an arthritis formula in phase 2 development.
Over the past year, Zalicus has traded in a range of $0.71 to $3.21 and is now at $1.23, 72% above that low. The 200-day and 50-day moving averages have moved 0.18% higher and 1.78% higher over the past week, respectively.
Zalicus (NASDAQ:ZLCS) has potential upside of 245.5% based on a current price of $1.23 and analysts' consensus price target of $4.25. The stock should find initial support at its 50-day moving average (MA) of $1.14 and further support at its 200-day MA of $1.09.
SmarTrend is tracking the current trend status for Zalicus and will alert subscribers who have ZLCS in their portfolio or watchlist when shares have changed trend direction.
http://www.mysmartrend.com/news-briefs/technical-analysis/za…
Watch this space (insbesondere nach dem gestrigen "schlußpanik" @der Nasdaq ...

Zalicus (NASDAQ:ZLCS) is one of today's best performing low-priced stocks, up 9.8% to $1.23 on 1.1x average daily volume. Zalicus has traded 3.5 million shares thus far today, vs. average volume of 3.0 million shares per day. The stock has outperformed the Dow (9.8% to the Dow's 0.7%) and outperformed the S&P 500 (9.8% to the S&P's 0.9%) during today's trading.
Zalicus Inc is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. The Company's drug pipeline includes an arthritis formula in phase 2 development.
Over the past year, Zalicus has traded in a range of $0.71 to $3.21 and is now at $1.23, 72% above that low. The 200-day and 50-day moving averages have moved 0.18% higher and 1.78% higher over the past week, respectively.
Zalicus (NASDAQ:ZLCS) has potential upside of 245.5% based on a current price of $1.23 and analysts' consensus price target of $4.25. The stock should find initial support at its 50-day moving average (MA) of $1.14 and further support at its 200-day MA of $1.09.
SmarTrend is tracking the current trend status for Zalicus and will alert subscribers who have ZLCS in their portfolio or watchlist when shares have changed trend direction.
http://www.mysmartrend.com/news-briefs/technical-analysis/za…
Watch this space (insbesondere nach dem gestrigen "schlußpanik" @der Nasdaq ...


Antwort auf Beitrag Nr.: 43.468.617 von Growth2012 am 08.08.12 08:35:48morgen Growth, mit den 4,25 $ zb. als Weihnachtsgeschenk könnte ich gut Leben ! 


Spaß beseite.....ich denke das wir hier noch einen mächtigen Nord-Schub erleben werden...
Die Roadmap für Sep. lässt sehr auf Fundamentel`s hoffen....
Mmn.gestern fast auf TH am Nasdaq geschlossen unter rel hohem Vol.....dh. heute wird die Fahrt wohl weiter gehen.
ich Recherchiere heute mal den aktuellen Shortanteil derzeit....
Schönes Ding hier...



Spaß beseite.....ich denke das wir hier noch einen mächtigen Nord-Schub erleben werden...
Die Roadmap für Sep. lässt sehr auf Fundamentel`s hoffen....
Mmn.gestern fast auf TH am Nasdaq geschlossen unter rel hohem Vol.....dh. heute wird die Fahrt wohl weiter gehen.
ich Recherchiere heute mal den aktuellen Shortanteil derzeit....
Schönes Ding hier...

Mahlzeit Aktionäre... ,
,
hier die Shortanalyse von gestern :
http://www.shortanalytics.com/getshortchart.php?tsymbol=zlcs
Long 60 %
Short 40 %
Hier was von gestern (Seeking Alpha) aus der Werberommel....das sollte uns dann Kurstechnisch auch etwas beflügeln :
Why Zalicus Looks Ready For A Bullish Move

Zalicus (ZLCS) has proven to be a great stock to trade, if you know when to time your entry and exit. Still, the day may come when going long-long-and-longer may be the best option; I see that day quickly approaching. By and large due to the volatile nature of biotech trading, stocks like Zalicus, iBio Inc. (IBIO), Arena Pharmaceuticals (ARNA), and now recently Amarin (AMRN) swing up and down. These unnerving stock moves can be unsettling often defying what an investor wants to think about a favorite biotech. Even great companies like Antares Pharma (ATRS) -- a stellar firm in every way -- has been reloading for the fortunate ready to load up on more shares.
In this article, my attention returns to long-time favorite Zalicus that has a number of very exciting catalysts in the 2H 2012. While biotech investing is always risky and events may not turn out in favor of the investor, I have been studying the firm and the technical chart for some time and I think the stock is setting up for a significant pop. That however must be backed up by due diligence, otherwise that is just pumping a stock, but I will explain why I have initiated a position in Zalicus.
Mammoth Buy-Side Bidding
First, there is mammoth buy-side bidding (> 250K shares) that has extended well beyond the recent $15M Wedbush fund-raise. As they say, 'Follow the money!'
Unknown entities, as they often do, appear to be reassuming a significant position in the company. I interpret this as a very bullish sign. Keep in mind, Zalicus has encountered no problems raising additional capital and value-for-value has succeeded at remaining cash-infused above ~$40M. I expect the next quarterly report will confirm this as Zalicus is a research and development firm with a very attractive pipeline. Will the company report a loss? Of course, but if you understand the business plan and the progressive advancements at Zalicus, you'll applaud the firm.
Having followed CEO Corrigan from the beginning of his administration, I am of the opinion that he is very bullish about Z160, the reformulated pain medicament that has already equaled high-powered opioid morphine and/or another popular chemical entity called gabapentin; in my estimation, Z160 is destined for clinical phase 3, and it will be interesting to follow its T-type companion Z944.
Clinical Trial Ramp-Up
Second, counter-intuitive to the frequent angst that Zalicus' fund-raising activities have diluted shareholders or its executives earn outlandish salaries, the opposite view is understanding that the relative success of Zalicus' research pipeline, particularly its ion channel program (e.g. Z160 and Z944) has caused the firm to ramp up its clinical programs--all recently successful, by the way.
Stated another way, one could argue that CEO Corrigan's regime are building a strong firm witnessed by Z160, poised to launch into a phase 2 'proof of concept' clinical trial (August), and Z944 having recently entered a second phase 1 multiple dose clinical trial. In addition, I am expecting the research going on at Hydra Biosciences will yield a new sodium channel candidate within the next year. All told, Zalicus could have three interesting pain drugs in clinical development by 2013--they already have two and that is in a research environment that hasn't seen a powerful new pain drug in decades. Given the addictive nature of opioids, Zalicus is sitting in an enviable sweet spot including the company's unique relationship with Cubist Pharmaceuticals (CBST) where CEO Corrigan is a board member.
A Knowledgeable Outlook on Synavive
It fascinates me to read the outlook of others on Zalicus' drug candidate Synavive because I see so much misinformation. Currently, Synavive is completing a phase 2B clinical trial with a second on-going phase 2B extended safety trial.
While no one can predict the clinical trial outcome (to be reported sometime in September according to the company), Synavive, if it's successful will be a boon for Zalicus. Here's why:
Synavive will offer a low-cost advantage over steroids and most expensive DMARDS (Disease Modifying Antirheumatic Drugs). For cost reasons it would become the first line of treatment beyond NSAIDS (Non-Steroidal Anti-Inflammatory Drugs), postponing the need for rheumatologists to move an arthritis patient directly to a steroid such as prednisone or prednisolone.
The concept behind Synavive is that it will match or out-better the high side effect steroids such as prednisone or prednisolone. The phase 2B trial will either prove this or not, but the latest version of Synavive was reformulated to address its own minor side effect which is a common headache. There is good reason to expect that the slow-release formulation will help here, but that too will need to be proved.
The current treatment regimen for people with rheumatoid arthritis is to move them from NSAIDS to steroids to DMARDS. Not only could Synavive postpone the need for steroids and/or DMARDS, there is some thought at Zalicus that Synavive could postpone the need for much stronger, much higher side effect, and much more costly drugs for a medical indication that affects millions around the globe. However, the synergistic chemistry must be proven.
If the phase 2B proves that Synavive offers a synergistic mechanism that out-betters the common steroids, that will be a grand slam for Zalicus' cHTS Chalice technology -- the same technology that has Novartis (NVS) filing multiple patents of synergistic combinations for the treatment of cancers, among them multiple myeloma.
Investors are warned that no one knows how Synavive's phase 2B trial will turn out and articles that claim omniscience into this matter should be promptly aimed at the nearest trashcan, but so should articles that appear to have no grasp as to how Synavive could be a very marketable drug for millions of rheumatoid arthritis sufferers.
Share-Price Moving Catalysts
While success cannot be guaranteed, savvy investors may benefit from knowing that Zalicus has multiple shots on goal:
Synavive
Z160
Z944
Sodium candidate at Hydra
Novartis oncology program
Prednisporin at Sanofi (SNY) or FOV1101
While I cannot confirm this yet, Zalicus may also have another drug candidate that will soon emerge from its own cHTS-Chalice research; if so, that will deepen the company's already flush pipeline. One thing though is certain: 2H 2012 is going to pop and/or drop the share price.
Chart Looks Bullish
I know some Zalicus long-termers dislike reading coverage on the technical indicators since day-traders have often under-cut visions of long-term greatness, but from what I'm seeing this may be the time when Zalicus is about to make a significant move to the upside going into September hedging on Synavive's outcome.
(click to enlarge)
Notice these indicators:
RSI = bullish room
Sitting at the middle of Bollinger Band = bullish
MACD = recent bullish move
Retest $1.60/share = bullish
Reload complete and bullish move up
200 MA moving to the upside
Touching 50 MA turning bullish
It's a Wrap!
Buying Zalicus may not be for everyone and risk is assumed, but there may be good reason to assume a position. I rate Zalicus a STRONG BUY based on the above discussion. However, late Monday evening I pulled this article to inform Zalicus investors of SIGNIFICANT NEWS--
The Phase 2 trial for Z160 is out! Ahead of a Zalicus news release, shareholders will be excited to learn that the 'proof of concept' clinical trial will: "...compare Z160 and placebo in patients with Lumbosacral Radiculopathy for safety and efficacy for a period of 6 weeks." What is Lumbosacral Radiculopathy? The Laser Spine Institute explains:
Lumbosacral radiculopathy is a broad term referring to a range of symptoms associated with the nerves of the lumbosacral plexus in the lower back. The lumbosacral plexus encompasses the nerves that exit the spinal cord at the lumbar, sacral and coccygeal regions of the spine. Radiculopathy occurs when an anatomical abnormality has caused one or more of these nerves to become irritated, pinched, or impinged.
Zalicus plans to enroll 140 patients and to complete the study within one year (August-September 2013). If you don't think Zalicus is about to make a bullish move, think again. SA readers, depending on when this article is released, will be ahead of the upcoming news release. It is no wonder that Pfizer recently honored Dr. Snutch with a neuropathic pain research award. Yes, the same Pfizer that bought Icagen for its ion channel program. Things are definitely looking bullish at Zalicus.
Happy investing.
Disclosure: I am long ZLCS.
Additional disclosure: Investors buy and/or sell at their own risk. I declare that I may day-trade any stock at any time mentioned in this article. For me 'long' is until I sell. I do not 'short' stocks. I receive one penny per view from SA, and other than my private stock account and publication by SA, I have zero connection to Wall Street. Investors are strongly encouraged to seek the advice of a professional.
Quelle :
http://seekingalpha.com/article/785861-why-zalicus-looks-rea…
Na dann heisst es in den nächsten Monaten...fest die Daumen drücken !!!
Schaun wir mal was heute das bullische Chart macht
 ,
,hier die Shortanalyse von gestern :
http://www.shortanalytics.com/getshortchart.php?tsymbol=zlcs
Long 60 %
Short 40 %
Hier was von gestern (Seeking Alpha) aus der Werberommel....das sollte uns dann Kurstechnisch auch etwas beflügeln :
Why Zalicus Looks Ready For A Bullish Move


Zalicus (ZLCS) has proven to be a great stock to trade, if you know when to time your entry and exit. Still, the day may come when going long-long-and-longer may be the best option; I see that day quickly approaching. By and large due to the volatile nature of biotech trading, stocks like Zalicus, iBio Inc. (IBIO), Arena Pharmaceuticals (ARNA), and now recently Amarin (AMRN) swing up and down. These unnerving stock moves can be unsettling often defying what an investor wants to think about a favorite biotech. Even great companies like Antares Pharma (ATRS) -- a stellar firm in every way -- has been reloading for the fortunate ready to load up on more shares.
In this article, my attention returns to long-time favorite Zalicus that has a number of very exciting catalysts in the 2H 2012. While biotech investing is always risky and events may not turn out in favor of the investor, I have been studying the firm and the technical chart for some time and I think the stock is setting up for a significant pop. That however must be backed up by due diligence, otherwise that is just pumping a stock, but I will explain why I have initiated a position in Zalicus.
Mammoth Buy-Side Bidding
First, there is mammoth buy-side bidding (> 250K shares) that has extended well beyond the recent $15M Wedbush fund-raise. As they say, 'Follow the money!'
Unknown entities, as they often do, appear to be reassuming a significant position in the company. I interpret this as a very bullish sign. Keep in mind, Zalicus has encountered no problems raising additional capital and value-for-value has succeeded at remaining cash-infused above ~$40M. I expect the next quarterly report will confirm this as Zalicus is a research and development firm with a very attractive pipeline. Will the company report a loss? Of course, but if you understand the business plan and the progressive advancements at Zalicus, you'll applaud the firm.
Having followed CEO Corrigan from the beginning of his administration, I am of the opinion that he is very bullish about Z160, the reformulated pain medicament that has already equaled high-powered opioid morphine and/or another popular chemical entity called gabapentin; in my estimation, Z160 is destined for clinical phase 3, and it will be interesting to follow its T-type companion Z944.
Clinical Trial Ramp-Up
Second, counter-intuitive to the frequent angst that Zalicus' fund-raising activities have diluted shareholders or its executives earn outlandish salaries, the opposite view is understanding that the relative success of Zalicus' research pipeline, particularly its ion channel program (e.g. Z160 and Z944) has caused the firm to ramp up its clinical programs--all recently successful, by the way.
Stated another way, one could argue that CEO Corrigan's regime are building a strong firm witnessed by Z160, poised to launch into a phase 2 'proof of concept' clinical trial (August), and Z944 having recently entered a second phase 1 multiple dose clinical trial. In addition, I am expecting the research going on at Hydra Biosciences will yield a new sodium channel candidate within the next year. All told, Zalicus could have three interesting pain drugs in clinical development by 2013--they already have two and that is in a research environment that hasn't seen a powerful new pain drug in decades. Given the addictive nature of opioids, Zalicus is sitting in an enviable sweet spot including the company's unique relationship with Cubist Pharmaceuticals (CBST) where CEO Corrigan is a board member.
A Knowledgeable Outlook on Synavive
It fascinates me to read the outlook of others on Zalicus' drug candidate Synavive because I see so much misinformation. Currently, Synavive is completing a phase 2B clinical trial with a second on-going phase 2B extended safety trial.
While no one can predict the clinical trial outcome (to be reported sometime in September according to the company), Synavive, if it's successful will be a boon for Zalicus. Here's why:
Synavive will offer a low-cost advantage over steroids and most expensive DMARDS (Disease Modifying Antirheumatic Drugs). For cost reasons it would become the first line of treatment beyond NSAIDS (Non-Steroidal Anti-Inflammatory Drugs), postponing the need for rheumatologists to move an arthritis patient directly to a steroid such as prednisone or prednisolone.
The concept behind Synavive is that it will match or out-better the high side effect steroids such as prednisone or prednisolone. The phase 2B trial will either prove this or not, but the latest version of Synavive was reformulated to address its own minor side effect which is a common headache. There is good reason to expect that the slow-release formulation will help here, but that too will need to be proved.
The current treatment regimen for people with rheumatoid arthritis is to move them from NSAIDS to steroids to DMARDS. Not only could Synavive postpone the need for steroids and/or DMARDS, there is some thought at Zalicus that Synavive could postpone the need for much stronger, much higher side effect, and much more costly drugs for a medical indication that affects millions around the globe. However, the synergistic chemistry must be proven.
If the phase 2B proves that Synavive offers a synergistic mechanism that out-betters the common steroids, that will be a grand slam for Zalicus' cHTS Chalice technology -- the same technology that has Novartis (NVS) filing multiple patents of synergistic combinations for the treatment of cancers, among them multiple myeloma.
Investors are warned that no one knows how Synavive's phase 2B trial will turn out and articles that claim omniscience into this matter should be promptly aimed at the nearest trashcan, but so should articles that appear to have no grasp as to how Synavive could be a very marketable drug for millions of rheumatoid arthritis sufferers.
Share-Price Moving Catalysts
While success cannot be guaranteed, savvy investors may benefit from knowing that Zalicus has multiple shots on goal:
Synavive
Z160
Z944
Sodium candidate at Hydra
Novartis oncology program
Prednisporin at Sanofi (SNY) or FOV1101
While I cannot confirm this yet, Zalicus may also have another drug candidate that will soon emerge from its own cHTS-Chalice research; if so, that will deepen the company's already flush pipeline. One thing though is certain: 2H 2012 is going to pop and/or drop the share price.
Chart Looks Bullish
I know some Zalicus long-termers dislike reading coverage on the technical indicators since day-traders have often under-cut visions of long-term greatness, but from what I'm seeing this may be the time when Zalicus is about to make a significant move to the upside going into September hedging on Synavive's outcome.
(click to enlarge)
Notice these indicators:
RSI = bullish room
Sitting at the middle of Bollinger Band = bullish
MACD = recent bullish move
Retest $1.60/share = bullish
Reload complete and bullish move up
200 MA moving to the upside
Touching 50 MA turning bullish
It's a Wrap!
Buying Zalicus may not be for everyone and risk is assumed, but there may be good reason to assume a position. I rate Zalicus a STRONG BUY based on the above discussion. However, late Monday evening I pulled this article to inform Zalicus investors of SIGNIFICANT NEWS--
The Phase 2 trial for Z160 is out! Ahead of a Zalicus news release, shareholders will be excited to learn that the 'proof of concept' clinical trial will: "...compare Z160 and placebo in patients with Lumbosacral Radiculopathy for safety and efficacy for a period of 6 weeks." What is Lumbosacral Radiculopathy? The Laser Spine Institute explains:
Lumbosacral radiculopathy is a broad term referring to a range of symptoms associated with the nerves of the lumbosacral plexus in the lower back. The lumbosacral plexus encompasses the nerves that exit the spinal cord at the lumbar, sacral and coccygeal regions of the spine. Radiculopathy occurs when an anatomical abnormality has caused one or more of these nerves to become irritated, pinched, or impinged.
Zalicus plans to enroll 140 patients and to complete the study within one year (August-September 2013). If you don't think Zalicus is about to make a bullish move, think again. SA readers, depending on when this article is released, will be ahead of the upcoming news release. It is no wonder that Pfizer recently honored Dr. Snutch with a neuropathic pain research award. Yes, the same Pfizer that bought Icagen for its ion channel program. Things are definitely looking bullish at Zalicus.
Happy investing.

Disclosure: I am long ZLCS.
Additional disclosure: Investors buy and/or sell at their own risk. I declare that I may day-trade any stock at any time mentioned in this article. For me 'long' is until I sell. I do not 'short' stocks. I receive one penny per view from SA, and other than my private stock account and publication by SA, I have zero connection to Wall Street. Investors are strongly encouraged to seek the advice of a professional.
Quelle :
http://seekingalpha.com/article/785861-why-zalicus-looks-rea…
Na dann heisst es in den nächsten Monaten...fest die Daumen drücken !!!

Schaun wir mal was heute das bullische Chart macht
Zitat von Earthfire: Orderbuch USA :
http://datasvr.tradearca.com/arcadataserver/ArcaBookData.php…
Na da drüben geht aber was ab :
BID ARCA 1.27 597000 08:19:53
ASK ARCA 1.30 2000 08:00:00
Kann natürlich auch eine Fakeorder sein...die dann plötzlich spurlos verschwindet !
Kann natürlich auch eine Fakeorder sein...die dann plötzlich spurlos verschwindet !
Oder es ist doch kein fake, der dann kurz nach Handeleröffnung ausgeführt wird...
Da muss ich Euch wohl nicht erklären was danach los wäre stateside?!?

Glück auf & Danke @earthfire fürs reinstellen!
Oder es ist doch kein fake, der dann kurz nach Handeleröffnung ausgeführt wird...
Da muss ich Euch wohl nicht erklären was danach los wäre stateside?!?


Glück auf & Danke @earthfire fürs reinstellen!
Zitat von Growth2012: Kann natürlich auch eine Fakeorder sein...die dann plötzlich spurlos verschwindet !
Oder es ist doch kein fake, der dann kurz nach Handeleröffnung ausgeführt wird...
Da muss ich Euch wohl nicht erklären was danach los wäre stateside?!?
Glück auf & Danke @earthfire fürs reinstellen!
Nein scheint keine zu sein !
hier die nächste :
BID ARCA 1.29 400000 08:44:52
ASK ARCA 1.30 22000 08:49:36
Bitte gerne geschehen !

Alter Schwede...könnte sein, das wir noch einen Shortsqueeze erleben...
Noch was aus meiner kleinen Privatanalyse :
Aktienanzahl 113,49 Mio.
Dies ist nach meiner Auffassung rel. wenig...d.h. je mehr feste Hände ( Instis)kaufen desto geringer der Freefloat...
Bei dieser Aktienzahl im Bezug zm derzeitigen ( noch rel. spottbilligem Kurs ) kann es hier schnell zu einem Starken Nachfrageüberhang kommen.....wenn dann die passende pos. News aufgelegt wird, kann sich jeder ausmalen was dies bedeuteten könnte.
Von den Shorts die dann noch drin sind, mal ganz abgesehen !

Was geht da ab ?
BID
ARCA 1.29 500000 09:09:54
ARCA 1.28 110000 09:05:14
ARCA 1.27 200700 08:58:24
ASK
ARCA 1.34 5000 09:08:44
ARCA 1.35 4000 08:56:50
ARCA 1.36 3429 08:36:44
theoretisch müsste der Kurs heute regelrecht explodieren...bei der RATIO
BID
ARCA 1.29 500000 09:09:54
ARCA 1.28 110000 09:05:14
ARCA 1.27 200700 08:58:24
ASK
ARCA 1.34 5000 09:08:44
ARCA 1.35 4000 08:56:50
ARCA 1.36 3429 08:36:44
theoretisch müsste der Kurs heute regelrecht explodieren...bei der RATIO

Antwort auf Beitrag Nr.: 43.470.651 von Earthfire am 08.08.12 15:01:25Ja zweifelsohne earthfire, hier baut sich ein gewisse Druck auf im ZLCS's Kessel... (bin seit knapp zwei jahren jetzt hier geduldig beigeblieben)
(bin seit knapp zwei jahren jetzt hier geduldig beigeblieben)
Off topic
Aber evtl. trotzdem vom Interesse:- schau die Bankia Aktie(WKN: A1JCY3)
mal kurz an, atemberaubend was die Aktie gemacht seit Goldman Sachs als berater ernannt wurde vor knapp zehn handelstagen...

10 short trading days & 30Mrd später

 (bin seit knapp zwei jahren jetzt hier geduldig beigeblieben)
(bin seit knapp zwei jahren jetzt hier geduldig beigeblieben)Off topic
Aber evtl. trotzdem vom Interesse:- schau die Bankia Aktie(WKN: A1JCY3)
mal kurz an, atemberaubend was die Aktie gemacht seit Goldman Sachs als berater ernannt wurde vor knapp zehn handelstagen...

10 short trading days & 30Mrd später


Zitat von Growth2012: Ja zweifelsohne earthfire, hier baut sich ein gewisse Druck auf im ZLCS's Kessel...(bin seit knapp zwei jahren jetzt hier geduldig beigeblieben)
Off topic
Aber evtl. trotzdem vom Interesse:- schau die Bankia Aktie(WKN: A1JCY3)
mal kurz an, atemberaubend was die Aktie gemacht seit Goldman Sachs als berater ernannt wurde vor knapp zehn handelstagen...
![]()
10 short trading days & 30Mrd später
Growth....keine schlechte Rendite für die paar Tage !

2 Jahre ist schon eine Zeit...aber jetzt gehts wohl dann mal los....
Ich steige oft erst rel spät ein.....bei Arena habe ich vor ein paar Monaten so grade nochden Zug erwischt..

Habe eine etwas andere Strategie...d.h. versuche mehr die Hausse zu erwischen....anstatt Jahre mein Geld zu parken.
Bin aber eine längere Zeit als Daytrader aktiv gewesen...daher hat der Begriff Long eine andere Bedeutung für mich....wie für manch anderen.
Ich investiere zur Zeit ausschließlich in Biotecs und Oilexplorer...
Da könntest du dir Pancontinental OIL mal anschauen bei Interesse...der DRill in Kenia startet in ein paar Tagen ...Potenzial bei einem Treffer..vom derzeitigen Kurs aus...ca. 500 %
In dem WO Thread findest du fundierte und rel. emotionslose Infos dazu..
Vermute das Ende nächster Woche die 2 $ Marke bei ZLCS gefallen sein wird.....wenn nicht vorher die Welt untergeht !


Good Luck uns allen !!
Muss weg..........................

@Growth.........Shortsquezze steht vor der Tür....! dh. die Bedingungen dafür sind gut !
Könnte ein richtig schöner 2stelliger Tag werden
und eine News :
Zalicus to Present at the 2012 Wedbush PacGrow Lifesciences Management Access Conference
Published: Wednesday, 8 Aug 2012 | 8:00 AM ET Text Size
CAMBRIDGE, Mass., Aug 08, 2012 (BUSINESS WIRE) -- Zalicus Inc. (NASDAQ: ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases, today announced that Zalicus management will present at the 2012 Wedbush PacGrow Lifesciences Management Access Conference at 9:10 a.m. ET on Tuesday August 14, 2012 at the Le Parker Meridien Hotel in New York. Interested parties may access a live webcast of the presentation by visiting the Zalicus website at
.
The webcast will be archived on the Zalicus website following the event.
About Zalicus Zalicus Inc. (Nasdaq: ZLCS) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. Zalicus has a portfolio of proprietary clinical-stage product candidates targeting pain and immuno-inflammatory diseases and has entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. Zalicus applies its expertise in the discovery and development of selective ion channel modulators and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease.
To learn more about Zalicus, please visit
.
Forward-Looking Statement: This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus, its product candidates, their potential and the plans for their clinical development, the Zalicus selective ion channel modulation technology, and related preclinical product candidates, Zalicus's combination drug discovery technology, cHTS, and its other business plans. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus may be identified by words like "believe," "expect," "may," "will," "should," "seek," "plan" or "could" and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the sale and marketing of Exalgo by Covidien, risks related to the development and regulatory approval of Zalicus's product candidates, including risks relating to formulation and clinical development of Synavive and Prednisporin, the unproven nature of the Zalicus drug discovery technologies, the ability of Covidien and Fovea/Sanofi Aventis to perform their obligations under their agreements with Zalicus, the ability of the Company or its collaboration partners to initiate and successfully complete clinical trials of its product candidates, the Company's ability to obtain additional financing or funding for its research and development and those other risks that can be found in the "Risk Factors" section of Zalicus's annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission. Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management's current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
(c) 2012 Zalicus Inc. All rights reserved.
SOURCE: Zalicus Inc.
CONTACT: Zalicus Inc. Justin Renz, 617-301-7575 CFO JRenz@zalicus.com or Gina Nugent, 617-460-3579 gnugent@zalicus.com Copyright Business Wire 2012 -0- KEYWORD: United States
North America
Massachusetts INDUSTRY KEYWORD: Health
Biotechnology
Quelle :
http://www.cnbc.com/id/48566332
jetzt muss ich aber weg.....gar nicht so einfach, sich on so einem Chart loszureissen !


Könnte ein richtig schöner 2stelliger Tag werden
und eine News :
Zalicus to Present at the 2012 Wedbush PacGrow Lifesciences Management Access Conference
Published: Wednesday, 8 Aug 2012 | 8:00 AM ET Text Size
CAMBRIDGE, Mass., Aug 08, 2012 (BUSINESS WIRE) -- Zalicus Inc. (NASDAQ: ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases, today announced that Zalicus management will present at the 2012 Wedbush PacGrow Lifesciences Management Access Conference at 9:10 a.m. ET on Tuesday August 14, 2012 at the Le Parker Meridien Hotel in New York. Interested parties may access a live webcast of the presentation by visiting the Zalicus website at
.
The webcast will be archived on the Zalicus website following the event.
About Zalicus Zalicus Inc. (Nasdaq: ZLCS) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. Zalicus has a portfolio of proprietary clinical-stage product candidates targeting pain and immuno-inflammatory diseases and has entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. Zalicus applies its expertise in the discovery and development of selective ion channel modulators and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease.
To learn more about Zalicus, please visit
.
Forward-Looking Statement: This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus, its product candidates, their potential and the plans for their clinical development, the Zalicus selective ion channel modulation technology, and related preclinical product candidates, Zalicus's combination drug discovery technology, cHTS, and its other business plans. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus may be identified by words like "believe," "expect," "may," "will," "should," "seek," "plan" or "could" and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the sale and marketing of Exalgo by Covidien, risks related to the development and regulatory approval of Zalicus's product candidates, including risks relating to formulation and clinical development of Synavive and Prednisporin, the unproven nature of the Zalicus drug discovery technologies, the ability of Covidien and Fovea/Sanofi Aventis to perform their obligations under their agreements with Zalicus, the ability of the Company or its collaboration partners to initiate and successfully complete clinical trials of its product candidates, the Company's ability to obtain additional financing or funding for its research and development and those other risks that can be found in the "Risk Factors" section of Zalicus's annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission. Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management's current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
(c) 2012 Zalicus Inc. All rights reserved.
SOURCE: Zalicus Inc.
CONTACT: Zalicus Inc. Justin Renz, 617-301-7575 CFO JRenz@zalicus.com or Gina Nugent, 617-460-3579 gnugent@zalicus.com Copyright Business Wire 2012 -0- KEYWORD: United States
North America
Massachusetts INDUSTRY KEYWORD: Health
Biotechnology
Quelle :
http://www.cnbc.com/id/48566332
jetzt muss ich aber weg.....gar nicht so einfach, sich on so einem Chart loszureissen !



Antwort auf Beitrag Nr.: 43.471.120 von Earthfire am 08.08.12 16:28:59PING...

Hast'd recht gehabt, jetzt wird kräftigst gecovered
P.S. Übrigens,danke für den Tipp, schau ich mir in alle ruhe an heute Abend (Parallel zum Leichtathletik Fest aus London)

Hast'd recht gehabt, jetzt wird kräftigst gecovered

P.S. Übrigens,danke für den Tipp, schau ich mir in alle ruhe an heute Abend (Parallel zum Leichtathletik Fest aus London)

Moin..
hier die Shortsatistik von gestern :
http://www.shortanalytics.com/getshortchart.php?tsymbol=zlcs
lt. dem chart wurden gestern lediglich 3 % gecovert...dh. die Jungs behalten die Nerven !
Wir sukzessive täglich ei wenig glattgestellt werden...
Im After mit 20k sind wir noch knapp 3 % getiegen
So...jetzt erst mal die Marke von 1,8 $ (08.2011) plätten danach she ich bei dem täglichen Volumina keine größeren Wiederstände...eher Kaufsignale...
Die Dienstag (14.08.) wird zeigen, ob es neuigkeiten gibt, die uns fundamental weiterhin stützen werden.
Persönlich bin ich sehr zuversichtlich das es weiter Up gehen wird -
hier die Shortsatistik von gestern :
http://www.shortanalytics.com/getshortchart.php?tsymbol=zlcs
lt. dem chart wurden gestern lediglich 3 % gecovert...dh. die Jungs behalten die Nerven !
Wir sukzessive täglich ei wenig glattgestellt werden...
Im After mit 20k sind wir noch knapp 3 % getiegen

So...jetzt erst mal die Marke von 1,8 $ (08.2011) plätten danach she ich bei dem täglichen Volumina keine größeren Wiederstände...eher Kaufsignale...

Die Dienstag (14.08.) wird zeigen, ob es neuigkeiten gibt, die uns fundamental weiterhin stützen werden.
Persönlich bin ich sehr zuversichtlich das es weiter Up gehen wird -
Hier wieder mal die Shortstatistik von gestern.....nur noch 22 % Shorts am Freitag..
Ich vermute, das am Dienstag News auf den Tish kommen, die uns wieder ein Stück nach Norden schieben werden..
http://www.shortanalytics.com/getshortchart.php?tsymbol=zlcs
hier die Shortinterest von der Nasdaqseite :
http://www.nasdaq.com/symbol/zlcs/short-interest
Settlement Date Short Interest Avg Daily Share Volume Days To Cover 7/31/2012 1,485,884 2,164,805 1.000000
Read more: http://www.nasdaq.com/symbol/zlcs/short-interest#ixzz23ELkwRbq
mit anderen Worten...wir haben derzeit den "tiefsten" Stand im Shortinterest seit 12 Monaten

Das interepretiere ich mal als sehr gutes Zeichen !für die nächsten Wochen.
Ich vermute, das am Dienstag News auf den Tish kommen, die uns wieder ein Stück nach Norden schieben werden..
http://www.shortanalytics.com/getshortchart.php?tsymbol=zlcs
hier die Shortinterest von der Nasdaqseite :
http://www.nasdaq.com/symbol/zlcs/short-interest
Settlement Date Short Interest Avg Daily Share Volume Days To Cover 7/31/2012 1,485,884 2,164,805 1.000000
Read more: http://www.nasdaq.com/symbol/zlcs/short-interest#ixzz23ELkwRbq
mit anderen Worten...wir haben derzeit den "tiefsten" Stand im Shortinterest seit 12 Monaten


Das interepretiere ich mal als sehr gutes Zeichen !für die nächsten Wochen.
Ich halte mich weiter daran:-
" sell in may and go away, but please remeember to come back in September"
Strong close am Freitagnachmittag...und das Zahlenwerk wurde dann am Samstag(gestern, ohne überraschungen)klamm heimlich veröffentlicht:
http://finance.yahoo.com/q/is?s=zlcs
Alles weiterhin im lot...

" sell in may and go away, but please remeember to come back in September"
Strong close am Freitagnachmittag...und das Zahlenwerk wurde dann am Samstag(gestern, ohne überraschungen)klamm heimlich veröffentlicht:
http://finance.yahoo.com/q/is?s=zlcs
Alles weiterhin im lot...


Antwort auf Beitrag Nr.: 43.484.182 von Growth2012 am 12.08.12 09:44:28kurzes Addendum...
Zalicus is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. The company reported that for the second quarter 2012 revenue was $2.9 million compared to $1.8 million for the second quarter 2011. Shares of the company surged nearly 12 percent Tuesday.
http://www.finanznachrichten.de/nachrichten-2012-08/24272566…
Schönes Restwochenende ihr lieben

Zalicus is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. The company reported that for the second quarter 2012 revenue was $2.9 million compared to $1.8 million for the second quarter 2011. Shares of the company surged nearly 12 percent Tuesday.
http://www.finanznachrichten.de/nachrichten-2012-08/24272566…
Schönes Restwochenende ihr lieben

Zalicus: Preparing For A Q3 Catalyst
August 17, 2012 by Longwood Bulls
Typically, the biotech stocks dominating news headlines and Twitter feeds are those approaching FDA approval decisions. Unfortunately, as this summer's most heavily hyped stocks like Arena (ARNA) and Amarin (AMRN) have shown, approval has often already been priced-in by the time the attention comes. More dramatic shifts in share-price tend to occur earlier in the process, with the release of promising clinical results (recent examples include VRTX and ALNY).
With this in mind, investors looking for a speculative play with potential high-upside should be keeping an eye on Zalicus (ZLCS) over the next few weeks for the results of the company's SYNERGY Phase II trial. Zalicus is a company with a rich history of partnerships with Big Pharma, and an intriguing potential entrant into the $14B rheumatoid arthritis market. Despite this, ZLCS' enterprise value is well below $100M. Whether it stays that low for much longer will depend significantly on soon-to-be released clinical trial data. Zalicus's lead drug candidate, Synavive, has demonstrated anti-inflammatory effects, rapid onset of action and excellent safety profiles in clinical studies to-date. The results of SYNERGY, a Phase II clinical trial of Synavive in patients with moderate-to-severe rheumatoid arthritis, are expected to be available in Q3 2012, potentially as soon as mid-August. This analysis will focus on this trial and the potential market implications of positive data.
Synavive: Version 2.0 of a leading anti-inflammatory
Zalicus's leading therapeutic candidate Synavive is a combination therapy comprised of the cardiovascular agent dipyridamole and a low dose of the corticosteroid prednisolone. Prednisolone is in a class of drugs called glucocorticoids, which are a mainstay of anti-inflammatory therapy. Prednisolone prevents the release of substances in the body that cause inflammation and is prescribed to treat a wide range of conditions including rheumatoid arthritis, allergic and respiratory disorders, and psoriasis. Though it is a popular and potent anti-inflammatory therapy, significant adverse effects have limited its therapeutic potential. Prednisolone use is associated with mild to severe side effects including psychiatric issues, insomnia, sodium retention, weight gain, increased appetite, stomach ulcers, glaucoma, cataract, elevated blood sugar, and high blood pressure. These side effects occur more frequently with high-dose or prolonged use of prednisolone.
Novel Combination Therapy.
In order to increase the therapeutic potential of prednisolone, Zalicus pursued a novel multi-component therapeutic approach. In this approach, an enhancing agent (here dipyridamole) was used to sensitize immune cells specifically to the effects of low-dose prednisolone, by modulating relevant signaling pathways. This amplification of the anti-inflammatory effect does not extend to prednisolone's adverse side effects. The synergistic combination therapy of Synavive selectively amplifies the anti-inflammatory activity of low dose prednisolone in immune cells without modulating non-immune signaling pathways mediating glucocorticoid-associated toxicity. Thus, Synavive is designed to enhance or amplify the efficacy of prednisolone without incurring the adverse side effects that plague high-dose prednisolone therapy, a potentially game-changing development in the field of arthritis treatment.
Market competitive landscape
In the SYNERGY clinical trial, Synavive was tested for the indication of Rheumatoid arthritis (RA), which is a chronic, inflammatory disease that causes pain, swelling, and loss of functions primarily in the joints. It affects 1% of the world's population across all ages, although onset occurs most often between 40 and 50. In the US, it is estimated that 1.3 million people, or roughly 0.6% of the US population, suffer from RA. In 2010, the total worldwide pharmaceutical market for RA drugs was in excess of $14B. In addition, previous Synavive clinical trials also targeted hand and knee osteoarthritis (OA), which affects an even larger population of nearly 27 million Americans. With an estimated 28 million Americans affected with either OA or RA, the potential market size for a broadly efficacious Synavive would be substantial.
Two Types of RA Treatment.
The treatment of RA is built on two categories of drugs: (i) disease-modifying antirheumatic drugs (DMARDs) which reduce bone/cartilage damage, and (ii) anti-inflammatory/analgesic agents which treat the symptoms of RA. Corticosteroids including Synavive belong to the latter category. While DMARDs remain a necessity for the long-term treatment of moderate-to-severe arthritis patients, corticosteroids used in low doses complement DMARDs by reducing inflammation as well as acting as "bridge therapy" when starting DMARDs until those DMARDs become effective. Although corticosteroid cannot substitute for DMARDs, effective corticosteroid treatment can postpone the usage of DMARDs, which cost $2,000 to $25,000 a year. In the SYNERGY trial, all patients are receiving Synavive in addition to simultaneous DMARD therapy. However, if the trial is successful, we anticipate Synavive will be an attractive candidate for treatment designed to postpone DMARD-therapy initiation given Synavive's excellent side-effect profile. The significant costs of DMARDs may prove an important incentive for insurance providers to support Synavive prescription.
Corticosteroid Competition.
The major potential direct competitors of Synavive are generic corticosteroids and Horizon Pharma's recently approved time-release corticosteroid, Rayos. With Rayos not currently available, the systemic corticosteroid market has been limited by significant side-effects and price competition from cheap generics. Despite this, systemic corticosteroid sales exceed $500M/year in the United States. Rayos and, if-approved, Synavive, appear poised to increase these revenues substantially for two reasons. First, Synavive and Rayos differentiate themselves from generic corticosteroids with decreased incidence of adverse side-effects, a major concern for the frequent and long-term users of corticosteroids. The negative side effects of corticosteroids increase with duration of exposure and dose, prompting physicians to delay or shorten corticosteroid prescription when feasible. We anticipate prednisolone-containing compounds like Rayos and Synavive will increase the number of patients receiving corticosteroids with increased duration of use. Secondly, we expect that Rayos and Synavive, which do not face competition from identical generics, will likely be able to charge more than the off-patent drugs that currently dominate the market segment. Given the adverse affects experienced by many corticosteroid users, we anticipate that many patients will pay a significant price premium to switch to safer alternatives.
While Horizon Pharma is likely to be a direct competitor, Rayos's recent FDA decision is a positive sign for the approval chances of the similarly prednisolone-modulating Synavive. Rayos sales over the next year will be an indication of physician interest in safer alternatives to generic prednisolone. Investors will be watching these sales numbers as a guide to Synavive's potential market penetration. Additionally, these investors will be analyzing the results of the SYNERGY trial for indications of Synavive/Rayos advantages. Zalicus has reported in preclinical studies that Synavive's combination therapy results in greater efficacy than currently available corticosteroids with diminished side-effects. If confirmed, this efficacy boost would offer Synavive a competitive edge over Rayos (Rayos was compared only to placebo and was not shown to outperform other corticosteroid therapies in its Phase III trial). Furthermore, although SYNERGY covers only RA, the previous clinical trial application of Synavive on OA implies a possible application in another market (30x larger) not occupied by Rayos. Of course, this assumes the new Synavive formulation will prove more efficacious than the one that failed its initial Phase II trial (see below for further discussion of this study).
SYNERGY trial results coming soon: What to look for
The most important indicator of Synavive's potential to reach the market will be the SYNERGY (SYNavivE for Reducing signs and sYmptoms of rheumatoid arthritis) Phase IIb trial, the results of which are expected in the 3Q 2012. The SYNERGY trial is a double-blind study of 292 patients with moderate-to-severe rheumatoid arthritis (arthritis severity is determined by number of joints affected) comparing Synavive to placebo/alternate therapies with regard to both efficacy and safety.
Efficacy.
The Synergy trial is particularly useful in evaluating the market potential of Synavive because it compares the drug not only to a placebo, but also to 3 active competitors. Two of those competitors are the individual components of Synavive taken alone (prednisolone 2.7mg and dipyridamole 360mg); the third is a higher dose of prednisolone (5mg). Well-established clinical research has previously shown that prednisolone is a powerful anti-inflammatory agent; consequently, we consider it highly likely that the prednisolone-containing Synavive will outperform placebo and the dipyridamole-alone conditions. The more interesting data will be in the comparison of Synavive with the two concentrations of prednisolone-alone (dipyridamole is an adjuvant that has shown only modest anti-inflammatory potential when taken alone). Zalicus has significant preclinical data demonstrating an increase in the efficacy of low-dose prednisolone when combined with dipyridamole, but the clinical data has been more mixed. In smaller studies, Synavive has outperformed the placebo (there was no comparison to prednisolone-only treatment) in both RA and osteoarthritis trials.
However, a 279-person Phase II trial (COMET-1) in 2008, which compared Synavive to 2.7mg prednisolone and placebo in osteoarthritis patients, failed to meet statistical significance in its primary outcome measurement- reduction in pain while walking following 12 weeks of treatment. Synavive did show encouraging, though not statistically significant, trends of dose-dependent improvement with regard to joint pain, stiffness, and function. Furthermore high-dose Synavive outperformed both placebo and prednisolone in all of these measurements. The failure of these Synavive-dependent effects to achieve statistical significance was due in large part to unexpectedly positive responses in placebo and prednisolone-only patients. Subsequent analysis revealed that ~10% of patients were simultaneously taking additional unapproved anti-inflammatory drugs (such as an NSAID or COX-2 inhibitor) in violation of the experimental protocol. These protocol violations were disproportionately represented in the placebo and prednisolone-only treatment arms contributing to the unexpectedly high response rate in these groups.
Interpretation of trial data was hampered too by an unexpectedly high percentage of patients who did not complete the trial (~10% not including those later found to have committed protocol violations). This failure to complete was no more common in the Synavive-arm than the placebo, but the lower patient number reduced the statistical power of the study. Re-analysis of the trial data modified to exclude all patients taking unapproved drugs did show a statistically significant improvement in high-dose Synavive over placebo in all efficacy measurements. Also, in this re-analysis, the high-dose Synavive showed improvement over prednisolone-alone in these same measurements (though the difference was not statistically significant in all cases).
Since this trial, Zalicus has reformulated Synavive to result in extended-release of the active components, a change they claim increases anti-inflammatory efficacy and decreases headaches, the most common adverse effect and one contributor to the higher-than-expected trial drop-out rates. If the new formulation of Synavive can support Zalicus's preclinical data and outperform prednisolone-alone (particularly the 5mg prednisolone dose), the market potential for the drug would be far greater. Prednisolone at 5 - 60 mg/day is a standard treatment for moderate rheumatoid arthritis and is available as a generic at a very low price. With a prednisolone level of <5mg, Synavive has demonstrated a safety profile without many of the severe adverse effects that plague current, higher-dose prednisolone therapy regimens (see below for further discussion).
Unfortunately, this improved safety profile will mean little without significant, complementary efficacy data. However positive its safety data, we consider it unlikely that physicians will make significant use of Synavive without clear evidence that the new drug will deliver anti-inflammatory efficacy at least equal to the lower dose end of standard therapy, i.e. 5mg prednisolone. In short, though eventual FDA approval may require only that Synavive outperforms the placebo, market viability will be dependent on clinical data indicating Synavive's superiority to its inexpensive, generic competitor.
Efficacy Outcome Measurements: Digging Deeper.
The primary outcome measurement of the trial will be DAS28 CRP, a score which incorporates both a subjective level of joint tenderness and an objective measurement of C-reactive protein (a factor which increases in blood as a result of inflammation). To justify a Phase 3 trial, Synavive will need to outperform placebo in this scoring system, and DAS28 scores are likely to lead any announcements of top-line results. Savvy investors, however, will also be looking at one of the trial's secondary outcome measurements, ACR20, to judge future market potential. ACR20, like DAS28, integrates both subjective and objective measures of joint discomfort and inflammation. An ACR20 score indicates the number of patients who experience a 20% reduction in a given number of ACR component tests.
Importantly, ACR20 is also the only outcome measurement shared by both the SYNERGY study and the recent Phase III study that led to the approval of likely Synavive-competitor, Rayos. Horizon Pharma's Rayos outperformed placebo 49% to 29% by ACR20 score in a patient population similar to that of the SYNERGY trial in its Phase 3 trial. By comparison the previous formulation of Synavive outperformed placebo 60% to 30% in a 2007 Phase 2a trial. Comparison by ARC20 of the new formulation of Synavive's performance in the SYNERGY trial to Rayos' historical data in its Phase III (CAPRA-2 trial) will be the only opportunity investors and physicians are likely to have to compare the efficacy of these two competing therapies for a long time.
As such, it is an important data point in assessing the competitive landscape of corticosteroids, should Synavive be approved. If Synavive's new formulation continues to outperform placebo to a greater degree than Rayos by ARC20 measurement, the results will be reflected in valuations of both Zalicus and Horizon Pharma.
Safety.
While previous clinical trials have left the efficacy of Synavive in doubt, the superior safety profile of the drug in these trials has been consistently encouraging. In the previous Phase II trial for osteoarthritis, patients taking the 2.7mg prednisolone alone or the prednisolone-containing Synavive reported no incidents of serious adverse effects. Given that high-dose prednisolone is associated with a host of severe adverse side effects including weight gain, psychiatric issues, insomnia, ulcers, glaucoma, and cataract, this is a promising finding. In addition to this, there were even indications that Synavive's combination therapy actually decreased less severe side-effects relative to prednisolone-only therapy. Patients receiving prednisolone alone suffered increases to both systolic blood pressure and serum triglyceride levels (both have been previously reported prednisolone side-effects); those receiving Synavive, like those on the placebo, did not experience increases in either of those factors.
Synavive's most common side-effect was a headache; ~50% of patients reported experiencing them and 4% dropped out of the trial citing this as a reason. Zalicus has reported that the reformulated Synavive with time-delayed release of prednisolone and dipyridamole (which has been associated with headaches in other studies) decreases the incidence of headache. Confirming a decrease in headache frequency as well as maintaining an edge over prednisolone-only in blood-pressure and triglyceride measurements may be important to Synavive's eventual sales strategy. Zalicus has good IP protection for the combination of the drugs prednisolone and dipyridamole but not the individual components which are available as generics.
As the company discussed in its financial filings, there exists the possibility that physicians might prescribe the two components separately to decrease treatment costs, potentially limiting the price Zalicus can charge while maintaining adequate market share. Any evidence that the combination of prednisolone with dipyridamole, particularly the extended-release dipyridamole that Synavive uses, leads to fewer adverse events is particularly crucial in this regard. We consider it very unlikely that physicians will regularly prescribe the drugs separately if there is clinical evidence that separating the two drugs not only decreases efficacy but could lead to additional safety concerns (e.g. elevated blood pressure and triglycerides).
One element of the study that bears additional scrutiny is the comparison of Synavive and the higher dose 5mg prednisolone. Prednisolone-associated side-effects are dose-dependent. If the new formulation maintains the excellent safety profile demonstrated in the previous Phase II trial, the contrast to prednisolone-alone is likely to be even greater with 5mg prednisolone than 2.7mg. The side-effects associated with 2.7mg prednisolone are minor, so the safety benefit offered by the combination therapy is similarly slight. If Synavive efficacy meets or exceeds that of 5mg prednisolone, Synavive may be a replacement for prednisolone regimens associated with far more serious adverse effects (like those listed above). In these cases, Synavive's decreased side-effects, if confirmed, would provide ample reason for physicians to prescribe the Synavive over generic prednisolone, counterbalancing what is likely to be a significant increase in cost.
Zalicus: Prepared for Success:
Should the SYNERGY trial prove successful, there is good evidence that Zalicus is well-prepared to take full advantage of that development. First, Zalicus appears to have a strong IP position with regard to Synavive. Though both components - prednisolone and dipyridamole - are available as generics, Zalicus has both method-of-use (US patent 7,253,155) and composition-of-matter patents (US patent 7,915,265) covering their combination. With those patents granted in 2007 and 2011 respectively, the window of IP protection is substantial.
Additionally, Zalicus has experience partnering profitably with Big Pharma players to develop its technologies and drug candidates. In 2006, Neuromed (which later merged with CombinatoRX to create Zalicus) partnered with Merck (MRK) to develop a drug candidate in a deal estimated at the time to be worth >$450M. Currently, Zalicus and Novartis (NOVN) have partnered to further develop Zalicus' combination high-throughput screening (cHTS) technology that helped design the composition of Synavive. Due in part to that cHTS technology, Zalicus has an intriguing pipeline of drug candidates, though none are as clinically advanced as Synavive (Zalicus's pain-relief pipeline is the subject of in-depth analysis elsewhere on Seeking Alpha).
Finally, despite its low market cap, Zalicus is in a strong position financially with the capital on hand to take a new product into the next stage of development without having to resort to stock dilution or borrowing. The company's financial reports show that Zalicus had approximately $44.1 million in cash and short term investments in Q2 2012; operations are expected to be funded sufficiently into the middle of 2013. The current cash burn rate is higher than ideal at ~40M/year ( ~30% market cap). It is worth noting that this burn rate reflects the cost of continuing the SYNERGY trial which is now complete; we anticipate the burn rate will decrease closer to a pre-SYNERGY level of $4.5M/year (~13% market cap).
Even if the burn rate does not appreciably decrease, the current cash reserves do appear sufficient to last Zalicus well past the release of SYNERGY data and a post-Phase II meeting with the FDA. Should SYNERGY succeed, the positive Phase II data will be reflected in increased share price before any additional financing is necessary.
Recommendation:
In all cases, predicting the results of clinical trials is extremely difficult and comes with a very high level of risk. That an earlier formulation of Synavive has previously failed in another Phase II trial of osteoarthritis patients makes Synavive an even riskier call than most. We believe that the SYNERGY trial has a significant chance of failure. However, we also believe that the science behind Synavive is promising and that, if successful, Synavive has the capability to substantially grow within a Rheumatoid arthritis market that is worth $14B/year.
Given this large market size and Synavive's potential, we believe Zalicus could realize tremendous gains in share-price with positive Phase II data. This massive upside is not reflected in the stock's current pricing. With an enterprise value of ~$100M, ZLCS is significantly undervalued in our opinion. As a potentially high-upside, speculative investment, we believe ZLCS is an appealing opportunity to watch for in 3Q 2012.
Disclosure:
I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/813612-zalicus-preparing-for…
August 17, 2012 by Longwood Bulls
Typically, the biotech stocks dominating news headlines and Twitter feeds are those approaching FDA approval decisions. Unfortunately, as this summer's most heavily hyped stocks like Arena (ARNA) and Amarin (AMRN) have shown, approval has often already been priced-in by the time the attention comes. More dramatic shifts in share-price tend to occur earlier in the process, with the release of promising clinical results (recent examples include VRTX and ALNY).
With this in mind, investors looking for a speculative play with potential high-upside should be keeping an eye on Zalicus (ZLCS) over the next few weeks for the results of the company's SYNERGY Phase II trial. Zalicus is a company with a rich history of partnerships with Big Pharma, and an intriguing potential entrant into the $14B rheumatoid arthritis market. Despite this, ZLCS' enterprise value is well below $100M. Whether it stays that low for much longer will depend significantly on soon-to-be released clinical trial data. Zalicus's lead drug candidate, Synavive, has demonstrated anti-inflammatory effects, rapid onset of action and excellent safety profiles in clinical studies to-date. The results of SYNERGY, a Phase II clinical trial of Synavive in patients with moderate-to-severe rheumatoid arthritis, are expected to be available in Q3 2012, potentially as soon as mid-August. This analysis will focus on this trial and the potential market implications of positive data.
Synavive: Version 2.0 of a leading anti-inflammatory
Zalicus's leading therapeutic candidate Synavive is a combination therapy comprised of the cardiovascular agent dipyridamole and a low dose of the corticosteroid prednisolone. Prednisolone is in a class of drugs called glucocorticoids, which are a mainstay of anti-inflammatory therapy. Prednisolone prevents the release of substances in the body that cause inflammation and is prescribed to treat a wide range of conditions including rheumatoid arthritis, allergic and respiratory disorders, and psoriasis. Though it is a popular and potent anti-inflammatory therapy, significant adverse effects have limited its therapeutic potential. Prednisolone use is associated with mild to severe side effects including psychiatric issues, insomnia, sodium retention, weight gain, increased appetite, stomach ulcers, glaucoma, cataract, elevated blood sugar, and high blood pressure. These side effects occur more frequently with high-dose or prolonged use of prednisolone.
Novel Combination Therapy.
In order to increase the therapeutic potential of prednisolone, Zalicus pursued a novel multi-component therapeutic approach. In this approach, an enhancing agent (here dipyridamole) was used to sensitize immune cells specifically to the effects of low-dose prednisolone, by modulating relevant signaling pathways. This amplification of the anti-inflammatory effect does not extend to prednisolone's adverse side effects. The synergistic combination therapy of Synavive selectively amplifies the anti-inflammatory activity of low dose prednisolone in immune cells without modulating non-immune signaling pathways mediating glucocorticoid-associated toxicity. Thus, Synavive is designed to enhance or amplify the efficacy of prednisolone without incurring the adverse side effects that plague high-dose prednisolone therapy, a potentially game-changing development in the field of arthritis treatment.
Market competitive landscape
In the SYNERGY clinical trial, Synavive was tested for the indication of Rheumatoid arthritis (RA), which is a chronic, inflammatory disease that causes pain, swelling, and loss of functions primarily in the joints. It affects 1% of the world's population across all ages, although onset occurs most often between 40 and 50. In the US, it is estimated that 1.3 million people, or roughly 0.6% of the US population, suffer from RA. In 2010, the total worldwide pharmaceutical market for RA drugs was in excess of $14B. In addition, previous Synavive clinical trials also targeted hand and knee osteoarthritis (OA), which affects an even larger population of nearly 27 million Americans. With an estimated 28 million Americans affected with either OA or RA, the potential market size for a broadly efficacious Synavive would be substantial.
Two Types of RA Treatment.
The treatment of RA is built on two categories of drugs: (i) disease-modifying antirheumatic drugs (DMARDs) which reduce bone/cartilage damage, and (ii) anti-inflammatory/analgesic agents which treat the symptoms of RA. Corticosteroids including Synavive belong to the latter category. While DMARDs remain a necessity for the long-term treatment of moderate-to-severe arthritis patients, corticosteroids used in low doses complement DMARDs by reducing inflammation as well as acting as "bridge therapy" when starting DMARDs until those DMARDs become effective. Although corticosteroid cannot substitute for DMARDs, effective corticosteroid treatment can postpone the usage of DMARDs, which cost $2,000 to $25,000 a year. In the SYNERGY trial, all patients are receiving Synavive in addition to simultaneous DMARD therapy. However, if the trial is successful, we anticipate Synavive will be an attractive candidate for treatment designed to postpone DMARD-therapy initiation given Synavive's excellent side-effect profile. The significant costs of DMARDs may prove an important incentive for insurance providers to support Synavive prescription.
Corticosteroid Competition.
The major potential direct competitors of Synavive are generic corticosteroids and Horizon Pharma's recently approved time-release corticosteroid, Rayos. With Rayos not currently available, the systemic corticosteroid market has been limited by significant side-effects and price competition from cheap generics. Despite this, systemic corticosteroid sales exceed $500M/year in the United States. Rayos and, if-approved, Synavive, appear poised to increase these revenues substantially for two reasons. First, Synavive and Rayos differentiate themselves from generic corticosteroids with decreased incidence of adverse side-effects, a major concern for the frequent and long-term users of corticosteroids. The negative side effects of corticosteroids increase with duration of exposure and dose, prompting physicians to delay or shorten corticosteroid prescription when feasible. We anticipate prednisolone-containing compounds like Rayos and Synavive will increase the number of patients receiving corticosteroids with increased duration of use. Secondly, we expect that Rayos and Synavive, which do not face competition from identical generics, will likely be able to charge more than the off-patent drugs that currently dominate the market segment. Given the adverse affects experienced by many corticosteroid users, we anticipate that many patients will pay a significant price premium to switch to safer alternatives.
While Horizon Pharma is likely to be a direct competitor, Rayos's recent FDA decision is a positive sign for the approval chances of the similarly prednisolone-modulating Synavive. Rayos sales over the next year will be an indication of physician interest in safer alternatives to generic prednisolone. Investors will be watching these sales numbers as a guide to Synavive's potential market penetration. Additionally, these investors will be analyzing the results of the SYNERGY trial for indications of Synavive/Rayos advantages. Zalicus has reported in preclinical studies that Synavive's combination therapy results in greater efficacy than currently available corticosteroids with diminished side-effects. If confirmed, this efficacy boost would offer Synavive a competitive edge over Rayos (Rayos was compared only to placebo and was not shown to outperform other corticosteroid therapies in its Phase III trial). Furthermore, although SYNERGY covers only RA, the previous clinical trial application of Synavive on OA implies a possible application in another market (30x larger) not occupied by Rayos. Of course, this assumes the new Synavive formulation will prove more efficacious than the one that failed its initial Phase II trial (see below for further discussion of this study).
SYNERGY trial results coming soon: What to look for
The most important indicator of Synavive's potential to reach the market will be the SYNERGY (SYNavivE for Reducing signs and sYmptoms of rheumatoid arthritis) Phase IIb trial, the results of which are expected in the 3Q 2012. The SYNERGY trial is a double-blind study of 292 patients with moderate-to-severe rheumatoid arthritis (arthritis severity is determined by number of joints affected) comparing Synavive to placebo/alternate therapies with regard to both efficacy and safety.
Efficacy.
The Synergy trial is particularly useful in evaluating the market potential of Synavive because it compares the drug not only to a placebo, but also to 3 active competitors. Two of those competitors are the individual components of Synavive taken alone (prednisolone 2.7mg and dipyridamole 360mg); the third is a higher dose of prednisolone (5mg). Well-established clinical research has previously shown that prednisolone is a powerful anti-inflammatory agent; consequently, we consider it highly likely that the prednisolone-containing Synavive will outperform placebo and the dipyridamole-alone conditions. The more interesting data will be in the comparison of Synavive with the two concentrations of prednisolone-alone (dipyridamole is an adjuvant that has shown only modest anti-inflammatory potential when taken alone). Zalicus has significant preclinical data demonstrating an increase in the efficacy of low-dose prednisolone when combined with dipyridamole, but the clinical data has been more mixed. In smaller studies, Synavive has outperformed the placebo (there was no comparison to prednisolone-only treatment) in both RA and osteoarthritis trials.
However, a 279-person Phase II trial (COMET-1) in 2008, which compared Synavive to 2.7mg prednisolone and placebo in osteoarthritis patients, failed to meet statistical significance in its primary outcome measurement- reduction in pain while walking following 12 weeks of treatment. Synavive did show encouraging, though not statistically significant, trends of dose-dependent improvement with regard to joint pain, stiffness, and function. Furthermore high-dose Synavive outperformed both placebo and prednisolone in all of these measurements. The failure of these Synavive-dependent effects to achieve statistical significance was due in large part to unexpectedly positive responses in placebo and prednisolone-only patients. Subsequent analysis revealed that ~10% of patients were simultaneously taking additional unapproved anti-inflammatory drugs (such as an NSAID or COX-2 inhibitor) in violation of the experimental protocol. These protocol violations were disproportionately represented in the placebo and prednisolone-only treatment arms contributing to the unexpectedly high response rate in these groups.
Interpretation of trial data was hampered too by an unexpectedly high percentage of patients who did not complete the trial (~10% not including those later found to have committed protocol violations). This failure to complete was no more common in the Synavive-arm than the placebo, but the lower patient number reduced the statistical power of the study. Re-analysis of the trial data modified to exclude all patients taking unapproved drugs did show a statistically significant improvement in high-dose Synavive over placebo in all efficacy measurements. Also, in this re-analysis, the high-dose Synavive showed improvement over prednisolone-alone in these same measurements (though the difference was not statistically significant in all cases).
Since this trial, Zalicus has reformulated Synavive to result in extended-release of the active components, a change they claim increases anti-inflammatory efficacy and decreases headaches, the most common adverse effect and one contributor to the higher-than-expected trial drop-out rates. If the new formulation of Synavive can support Zalicus's preclinical data and outperform prednisolone-alone (particularly the 5mg prednisolone dose), the market potential for the drug would be far greater. Prednisolone at 5 - 60 mg/day is a standard treatment for moderate rheumatoid arthritis and is available as a generic at a very low price. With a prednisolone level of <5mg, Synavive has demonstrated a safety profile without many of the severe adverse effects that plague current, higher-dose prednisolone therapy regimens (see below for further discussion).
Unfortunately, this improved safety profile will mean little without significant, complementary efficacy data. However positive its safety data, we consider it unlikely that physicians will make significant use of Synavive without clear evidence that the new drug will deliver anti-inflammatory efficacy at least equal to the lower dose end of standard therapy, i.e. 5mg prednisolone. In short, though eventual FDA approval may require only that Synavive outperforms the placebo, market viability will be dependent on clinical data indicating Synavive's superiority to its inexpensive, generic competitor.
Efficacy Outcome Measurements: Digging Deeper.
The primary outcome measurement of the trial will be DAS28 CRP, a score which incorporates both a subjective level of joint tenderness and an objective measurement of C-reactive protein (a factor which increases in blood as a result of inflammation). To justify a Phase 3 trial, Synavive will need to outperform placebo in this scoring system, and DAS28 scores are likely to lead any announcements of top-line results. Savvy investors, however, will also be looking at one of the trial's secondary outcome measurements, ACR20, to judge future market potential. ACR20, like DAS28, integrates both subjective and objective measures of joint discomfort and inflammation. An ACR20 score indicates the number of patients who experience a 20% reduction in a given number of ACR component tests.
Importantly, ACR20 is also the only outcome measurement shared by both the SYNERGY study and the recent Phase III study that led to the approval of likely Synavive-competitor, Rayos. Horizon Pharma's Rayos outperformed placebo 49% to 29% by ACR20 score in a patient population similar to that of the SYNERGY trial in its Phase 3 trial. By comparison the previous formulation of Synavive outperformed placebo 60% to 30% in a 2007 Phase 2a trial. Comparison by ARC20 of the new formulation of Synavive's performance in the SYNERGY trial to Rayos' historical data in its Phase III (CAPRA-2 trial) will be the only opportunity investors and physicians are likely to have to compare the efficacy of these two competing therapies for a long time.
As such, it is an important data point in assessing the competitive landscape of corticosteroids, should Synavive be approved. If Synavive's new formulation continues to outperform placebo to a greater degree than Rayos by ARC20 measurement, the results will be reflected in valuations of both Zalicus and Horizon Pharma.
Safety.
While previous clinical trials have left the efficacy of Synavive in doubt, the superior safety profile of the drug in these trials has been consistently encouraging. In the previous Phase II trial for osteoarthritis, patients taking the 2.7mg prednisolone alone or the prednisolone-containing Synavive reported no incidents of serious adverse effects. Given that high-dose prednisolone is associated with a host of severe adverse side effects including weight gain, psychiatric issues, insomnia, ulcers, glaucoma, and cataract, this is a promising finding. In addition to this, there were even indications that Synavive's combination therapy actually decreased less severe side-effects relative to prednisolone-only therapy. Patients receiving prednisolone alone suffered increases to both systolic blood pressure and serum triglyceride levels (both have been previously reported prednisolone side-effects); those receiving Synavive, like those on the placebo, did not experience increases in either of those factors.
Synavive's most common side-effect was a headache; ~50% of patients reported experiencing them and 4% dropped out of the trial citing this as a reason. Zalicus has reported that the reformulated Synavive with time-delayed release of prednisolone and dipyridamole (which has been associated with headaches in other studies) decreases the incidence of headache. Confirming a decrease in headache frequency as well as maintaining an edge over prednisolone-only in blood-pressure and triglyceride measurements may be important to Synavive's eventual sales strategy. Zalicus has good IP protection for the combination of the drugs prednisolone and dipyridamole but not the individual components which are available as generics.
As the company discussed in its financial filings, there exists the possibility that physicians might prescribe the two components separately to decrease treatment costs, potentially limiting the price Zalicus can charge while maintaining adequate market share. Any evidence that the combination of prednisolone with dipyridamole, particularly the extended-release dipyridamole that Synavive uses, leads to fewer adverse events is particularly crucial in this regard. We consider it very unlikely that physicians will regularly prescribe the drugs separately if there is clinical evidence that separating the two drugs not only decreases efficacy but could lead to additional safety concerns (e.g. elevated blood pressure and triglycerides).
One element of the study that bears additional scrutiny is the comparison of Synavive and the higher dose 5mg prednisolone. Prednisolone-associated side-effects are dose-dependent. If the new formulation maintains the excellent safety profile demonstrated in the previous Phase II trial, the contrast to prednisolone-alone is likely to be even greater with 5mg prednisolone than 2.7mg. The side-effects associated with 2.7mg prednisolone are minor, so the safety benefit offered by the combination therapy is similarly slight. If Synavive efficacy meets or exceeds that of 5mg prednisolone, Synavive may be a replacement for prednisolone regimens associated with far more serious adverse effects (like those listed above). In these cases, Synavive's decreased side-effects, if confirmed, would provide ample reason for physicians to prescribe the Synavive over generic prednisolone, counterbalancing what is likely to be a significant increase in cost.
Zalicus: Prepared for Success:
Should the SYNERGY trial prove successful, there is good evidence that Zalicus is well-prepared to take full advantage of that development. First, Zalicus appears to have a strong IP position with regard to Synavive. Though both components - prednisolone and dipyridamole - are available as generics, Zalicus has both method-of-use (US patent 7,253,155) and composition-of-matter patents (US patent 7,915,265) covering their combination. With those patents granted in 2007 and 2011 respectively, the window of IP protection is substantial.
Additionally, Zalicus has experience partnering profitably with Big Pharma players to develop its technologies and drug candidates. In 2006, Neuromed (which later merged with CombinatoRX to create Zalicus) partnered with Merck (MRK) to develop a drug candidate in a deal estimated at the time to be worth >$450M. Currently, Zalicus and Novartis (NOVN) have partnered to further develop Zalicus' combination high-throughput screening (cHTS) technology that helped design the composition of Synavive. Due in part to that cHTS technology, Zalicus has an intriguing pipeline of drug candidates, though none are as clinically advanced as Synavive (Zalicus's pain-relief pipeline is the subject of in-depth analysis elsewhere on Seeking Alpha).
Finally, despite its low market cap, Zalicus is in a strong position financially with the capital on hand to take a new product into the next stage of development without having to resort to stock dilution or borrowing. The company's financial reports show that Zalicus had approximately $44.1 million in cash and short term investments in Q2 2012; operations are expected to be funded sufficiently into the middle of 2013. The current cash burn rate is higher than ideal at ~40M/year ( ~30% market cap). It is worth noting that this burn rate reflects the cost of continuing the SYNERGY trial which is now complete; we anticipate the burn rate will decrease closer to a pre-SYNERGY level of $4.5M/year (~13% market cap).
Even if the burn rate does not appreciably decrease, the current cash reserves do appear sufficient to last Zalicus well past the release of SYNERGY data and a post-Phase II meeting with the FDA. Should SYNERGY succeed, the positive Phase II data will be reflected in increased share price before any additional financing is necessary.
Recommendation:
In all cases, predicting the results of clinical trials is extremely difficult and comes with a very high level of risk. That an earlier formulation of Synavive has previously failed in another Phase II trial of osteoarthritis patients makes Synavive an even riskier call than most. We believe that the SYNERGY trial has a significant chance of failure. However, we also believe that the science behind Synavive is promising and that, if successful, Synavive has the capability to substantially grow within a Rheumatoid arthritis market that is worth $14B/year.
Given this large market size and Synavive's potential, we believe Zalicus could realize tremendous gains in share-price with positive Phase II data. This massive upside is not reflected in the stock's current pricing. With an enterprise value of ~$100M, ZLCS is significantly undervalued in our opinion. As a potentially high-upside, speculative investment, we believe ZLCS is an appealing opportunity to watch for in 3Q 2012.
Disclosure:
I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/813612-zalicus-preparing-for…
Achte auf das "Zahlenwerk", die 44,1 Mio$ warchest ist mächtig beeindrückend für ein aufstrebende US Biotech Firma...
Finally, despite its low market cap, Zalicus is in a strong position financially with the capital on hand to take a new product into the next stage of development without having to resort to stock dilution or borrowing. The company's financial reports show that Zalicus had approximately $44.1 million in cash and short term investments in Q2 2012; operations are expected to be funded sufficiently into the middle of 2013. The current cash burn rate is higher than ideal at ~40M/year ( ~30% market cap). It is worth noting that this burn rate reflects the cost of continuing the SYNERGY trial which is now complete; we anticipate the burn rate will decrease closer to a pre-SYNERGY level of $4.5M/year (~13% market cap).
http://seekingalpha.com/article/813612-zalicus-preparing-for…
....methinks something special is cooking in ZLCS's kitchen!?!


Finally, despite its low market cap, Zalicus is in a strong position financially with the capital on hand to take a new product into the next stage of development without having to resort to stock dilution or borrowing. The company's financial reports show that Zalicus had approximately $44.1 million in cash and short term investments in Q2 2012; operations are expected to be funded sufficiently into the middle of 2013. The current cash burn rate is higher than ideal at ~40M/year ( ~30% market cap). It is worth noting that this burn rate reflects the cost of continuing the SYNERGY trial which is now complete; we anticipate the burn rate will decrease closer to a pre-SYNERGY level of $4.5M/year (~13% market cap).
http://seekingalpha.com/article/813612-zalicus-preparing-for…
....methinks something special is cooking in ZLCS's kitchen!?!



Antwort auf Beitrag Nr.: 43.509.151 von Growth2012 am 19.08.12 09:15:09...kurze addendum:- zudem, wurde ich das Technische Chartbild von Fleck weg kaufen wollen

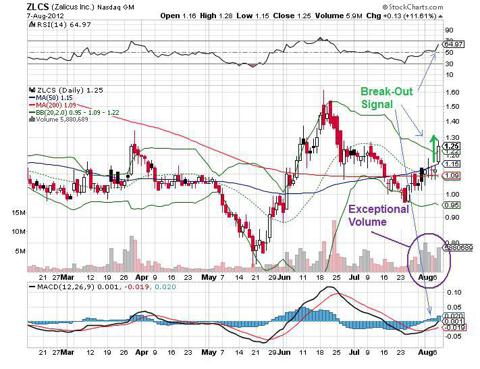
But as always, only time will tell...
Glück auf allerseits & einen schönen Sonntag noch!



But as always, only time will tell...
Glück auf allerseits & einen schönen Sonntag noch!
Na, irgendjemanden auf dem vermeintliche Ausverkauf reingefallen???

und dann plötzlich kommt der "jäger & sammler" Truppe wieder ins spiel
Geld 1,25
Brief 1,26
Zeit 21.08.1217:41
Spread 0,79%
Geld Stk. 252.000
Brief Stk. 3.100
Tja, ich hätte ja eigentlich zuschlagen müssen bei -10%...war leider am arbeiten sodass ich kein "leichtes Geld "verdienen könnte

und dann plötzlich kommt der "jäger & sammler" Truppe wieder ins spiel

Geld 1,25
Brief 1,26
Zeit 21.08.1217:41
Spread 0,79%
Geld Stk. 252.000
Brief Stk. 3.100
Tja, ich hätte ja eigentlich zuschlagen müssen bei -10%...war leider am arbeiten sodass ich kein "leichtes Geld "verdienen könnte

BIG NEWS von ZLCS...

Zalicus files $75M mixed securities shelf
http://finance.yahoo.com/news/zalicus-files-75m-mixed-securi…
Watch this space KW35


Zalicus files $75M mixed securities shelf
http://finance.yahoo.com/news/zalicus-files-75m-mixed-securi…
Watch this space KW35

Antwort auf Beitrag Nr.: 43.532.151 von Growth2012 am 24.08.12 23:09:05OK, big, big news....


8:30AM Zalicus receives FDA approval of 32mg dose strength of EXALGO (ZLCS) 1.26 : Co announces the FDA has approved the supplemental new drug application filed by Mallinckrodt, a subsidiary of Covidien (COV), for the 32 mg dose strength of EXALGO Extended-Release Tablets, for the management of moderate to severe pain in opioid-tolerant patients requiring continuous, around-the-clock opioid analgesia for an extended period of time. The rights to EXALGO were acquired by Mallinckrodt in June 2009 for $15 mln in upfront payments, additional development funding of up to $16 mln and a $40 mln FDA approval milestone payment. Co receives tiered royalties on net sales of EXALGO by Mallinckrodt.
Der Approval bringt zwei dinge mit sich, entweder 5$ bis ende des Jahres, oder ein übernahme Anebot bis ende September...
So, oder so, weiterhin LONG!




8:30AM Zalicus receives FDA approval of 32mg dose strength of EXALGO (ZLCS) 1.26 : Co announces the FDA has approved the supplemental new drug application filed by Mallinckrodt, a subsidiary of Covidien (COV), for the 32 mg dose strength of EXALGO Extended-Release Tablets, for the management of moderate to severe pain in opioid-tolerant patients requiring continuous, around-the-clock opioid analgesia for an extended period of time. The rights to EXALGO were acquired by Mallinckrodt in June 2009 for $15 mln in upfront payments, additional development funding of up to $16 mln and a $40 mln FDA approval milestone payment. Co receives tiered royalties on net sales of EXALGO by Mallinckrodt.
Der Approval bringt zwei dinge mit sich, entweder 5$ bis ende des Jahres, oder ein übernahme Anebot bis ende September...

So, oder so, weiterhin LONG!


Moin zusammen...(noch jemanden da? )
)
Covidien says FDA approves stronger pain drug
Covidien said the FDA approved a 32-milligram dose of Exalgo, which is meant to treat moderate to severe chronic pain. The company said it will launch the new version of Exalgo in the next few weeks.
The FDA approved three lower doses in March 2010.
Covidien shares slipped 9 cents to $55.53 in afternoon trading, while Zalicus Inc. shares rose 12 cents, or 9.5 percent, to $1.38. Exalgo is Zalicus' only approved product, and it gets royalties from the drug's sales.
http://finance.yahoo.com/news/covidien-says-fda-approves-str…
direkter Vergleich zwischen Covidien's vs ZLCS's MK...
Covidien (COV)
Marktkapital. 26,67 Mrd. USD
Stücke 480,10 Mio.
Symbol 6COP
ISIN IE00B68SQD29
WKN A1H7XB
----------------------------
Zalicus (ZLCS)
Marktkapital.174,84 Mio. USD
Stücke 126,70 Mio.
Symbol HGB
ISIN US98887C1053
WKN A1C4WQ
Hmmmmmm,a big player und Amgen & Novartis hören auch mit
und Amgen & Novartis hören auch mit
 )
)Covidien says FDA approves stronger pain drug
Covidien said the FDA approved a 32-milligram dose of Exalgo, which is meant to treat moderate to severe chronic pain. The company said it will launch the new version of Exalgo in the next few weeks.
The FDA approved three lower doses in March 2010.
Covidien shares slipped 9 cents to $55.53 in afternoon trading, while Zalicus Inc. shares rose 12 cents, or 9.5 percent, to $1.38. Exalgo is Zalicus' only approved product, and it gets royalties from the drug's sales.
http://finance.yahoo.com/news/covidien-says-fda-approves-str…
direkter Vergleich zwischen Covidien's vs ZLCS's MK...

Covidien (COV)
Marktkapital. 26,67 Mrd. USD
Stücke 480,10 Mio.
Symbol 6COP
ISIN IE00B68SQD29
WKN A1H7XB
----------------------------
Zalicus (ZLCS)
Marktkapital.174,84 Mio. USD
Stücke 126,70 Mio.
Symbol HGB
ISIN US98887C1053
WKN A1C4WQ
Hmmmmmm,a big player
 und Amgen & Novartis hören auch mit
und Amgen & Novartis hören auch mit
Guten morgen zusammen...
Smartrend hat sich im sachen ZLCS gemeldet, sehr geschätzte Quelle Stateside)
Traders Get Bullish on Shares of Zalicus, Shares Up 6.3% (ZLCS)
Written on Fri, 08/31/2012 - 1:52pm
By James Quinn
Zalicus (NASDAQ:ZLCS) is one of today's best performing low-priced stocks, up 6.3% to $1.52 on 1.4x average daily volume. Thus far today, Zalicus has traded 4.4 million shares, vs. average volume of 3.2 million shares per day. The stock has outperformed the Dow (6.3% to the Dow's 0.5%) and outperformed the S&P 500 (6.3% to the S&P's 0.3%) during today's trading.
Zalicus Inc is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. The Company's drug pipeline includes an arthritis formula in phase 2 development.
Zalicus (NASDAQ:ZLCS) has potential upside of 179.6% based on a current price of $1.52 and analysts' consensus price target of $4.25. The stock should discover initial support at its 50-day moving average (MA) of $1.20 and subsequent support at its 200-day MA of $1.11.
In the past 52 weeks, Zalicus share prices have been bracketed by a low of $0.71 and a high of $3.21 and are now at $1.52, 113% above that low price. Over the past week, the 200-day moving average (MA) has gone up 0.6% while the 50-day MA has remained constant.
SmarTrend recommended that subscribers consider buying shares of Zalicus on August 8th, 2012 as our proprietary SmarTrend analytics indicated a new Uptrend was in progress when shares hit $1.36. Since that recommendation, shares of Zalicus have risen 5.1%. We continue to monitor ZLCS for any potential shift so investors can protect gains and will alert SmarTrend subscribers immediately.
http://www.mysmartrend.com/news-briefs/technical-analysis/tr…
Watch this space bis ende September.... (1,16€ and counting
(1,16€ and counting )
)

Smartrend hat sich im sachen ZLCS gemeldet, sehr geschätzte Quelle Stateside)

Traders Get Bullish on Shares of Zalicus, Shares Up 6.3% (ZLCS)
Written on Fri, 08/31/2012 - 1:52pm
By James Quinn
Zalicus (NASDAQ:ZLCS) is one of today's best performing low-priced stocks, up 6.3% to $1.52 on 1.4x average daily volume. Thus far today, Zalicus has traded 4.4 million shares, vs. average volume of 3.2 million shares per day. The stock has outperformed the Dow (6.3% to the Dow's 0.5%) and outperformed the S&P 500 (6.3% to the S&P's 0.3%) during today's trading.
Zalicus Inc is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. The Company's drug pipeline includes an arthritis formula in phase 2 development.
Zalicus (NASDAQ:ZLCS) has potential upside of 179.6% based on a current price of $1.52 and analysts' consensus price target of $4.25. The stock should discover initial support at its 50-day moving average (MA) of $1.20 and subsequent support at its 200-day MA of $1.11.
In the past 52 weeks, Zalicus share prices have been bracketed by a low of $0.71 and a high of $3.21 and are now at $1.52, 113% above that low price. Over the past week, the 200-day moving average (MA) has gone up 0.6% while the 50-day MA has remained constant.
SmarTrend recommended that subscribers consider buying shares of Zalicus on August 8th, 2012 as our proprietary SmarTrend analytics indicated a new Uptrend was in progress when shares hit $1.36. Since that recommendation, shares of Zalicus have risen 5.1%. We continue to monitor ZLCS for any potential shift so investors can protect gains and will alert SmarTrend subscribers immediately.
http://www.mysmartrend.com/news-briefs/technical-analysis/tr…
Watch this space bis ende September....
 (1,16€ and counting
(1,16€ and counting )
)
Kleine nachtrag  jüngste Seeking Alpha Atikel (sehr fakten basiert, wenn nicht n'bissl zu "pushy" von Freund Lasar)
jüngste Seeking Alpha Atikel (sehr fakten basiert, wenn nicht n'bissl zu "pushy" von Freund Lasar)
Zalicus: When Push Comes To Shove
Well, it's Friday and I'll either be a hero or "toxic" as one chat board poster describes me. As a SA author, I put my confidence in Zalicus (ZLCS) on the line. At a recent Wedbush PAC conference, CFO Renz stated that the firm hoped to launch Z160 in August. In the same presentation, slide #25 also stated that. So what did I do? I wrote articles representing that view. I told investors that come today everything would come to a head. Not a bad thought considering my first entry into Zalicus was below $0.80/share and the stock has since risen to Thursday's close at $1.43/share.
I suppose my optimism and belief in management has some influence on my hunch about Synavive results. I'll admit that I'm nervous, but I'm still holding and considering my options. I've studied the chemistry of Synavive since 2010. I'm no novice to investing and I accept my fate. I take responsibility for my investments.
Hour after hour, I'm studying the situation at Zalicus checking and rechecking the facts. Here's what I've found:
First, Synavive's results are closer than even I calculated. On May 4, 2012, Zalicus released news that the SYNERGY trial was fully enrolled: 292 patients in a study that was targeting 250 patients. Over-enrolled. I find that most curious, but I have no answer. Given that the SYNERGY trial was a 12-week study, that would mean that the study may have completed before the end of July.
Or should I take the date of the clinicaltrials.gov website? The June 1, 2012, update would mean the study was completed by August 24, 2012. Of course, there's lag time, but don't forget an 8-K must be filed within four days of a material event. Yes, I checked. I'm holding at the time of this article and I'll protect every dime I've earned (without the government's help) because I invest to make money, not bemoan I'm too lazy to look up the latest link to three patent applications published by Zalicus on Thursday.
Second, making money requires you to weigh the company's fundamentals, catalysts and stock charts. Here's what I see: the stock price is $1.43/share on the day of anticipated news. This to me is the spirt of SA -- fundamental articles that bring investors cutting-edge news in advance of others. The RSI, MACD, and Bollinger band have more than enough space to run to a new high. But don't take my word. Take the word of an article (that advertises CNBC's Jim Cramer's team) that was published on Thursday that with enough volume, Zalicus could break $2/share. I'd say, "Lock and load! Get ready for the run! Forces much bigger than me are afoot!"
Gee, you'd think that they were reading my articles and studying the same charts. The chart is very bullish. Thursday ended with a 3.62% gain. You see, fundamentals and charts do count and the best investors learn how to use both.
Third, once again I informed Zalicus shareholders that Zalicus had published three critical ion channel patent applications tied to the current program.
I've studied Zalicus over three years, enough so that one analyst, Jason Napodano of Zacks, jokes about my frequent articles on Zalicus. Okay, he's right, but I invest to make money not win a Pulitzer prize or to earn a measly penny per view. I ran with Antares (ATRS), an awesome firm and won. Now my plan is to win over and over with Zalicus, a small biotech firm that has an ion channel portfolio that's about to change the way pain is treated.
Finally, the publication Thursday of those three ion channel patent applications shook me. They shook me because I've never seen Zalicus publish more than one patent on any given day. Zalicus is working overtime to protect its intellectual property and from people like me who actually read their patents.
But the truth is out there. Zalicus has published three patent applications all tied to T-type and sodium-type ion channels and this on the day before the last day of August when Z160 is promised to launch. Now some might say that's a coincidence, but I've never known the stoic CEO Corrigan to operate that way. He's about as strategic as they come including his board seat on Cubist (CBST) or his bullish promise now fulfilled that the 32 mg version of Exalgo with Covidien (COV) would be approved.
Mark H. N. Corrigan doesn't work to lose. CEO Corrigan wouldn't put his name behind Synavive unless he thought it would succeed in a Phase IIB clinical trial or at least have great odds of succeeding. Neither would his former VP (once temporary CEO) Christopher Gallen, who separated from Zalicus, but rather than take a cash payment preferred to keep his shares in Zalicus. Drs. Corrigan and Gallen are not losers, not by any stretch. They play to win and so do I as I sit here fully loaded up to the chin with Zalicus shares.
Therefore, when push comes to shove, I know the risk that Synavive could fail, but I'm going to take responsibility for my choices rather than resort to name-calling. But a final word to all the critics out there: I positively do not believe that the recent S-3 filing implies imminent dilution. I have unwavering confidence in the integrity of this company's CFO and CEO who have stated that the firm has more than enough funds to go into 2013 even 2014.
I expect that come today and next Tuesday, Zalicus is going to soar. I wish everyone the very best in investing. Remember, never invest more than you're willing to lose and do everyone else a favor: take responsibility for your investment choices. "Sticks and stones break my bones, but names never ..." Well, sometimes, being called names does hurt, but if you invest to make money, counting how much you've earned seems to make the pain go away.
http://seekingalpha.com/article/840121-zalicus-when-push-com…
Tja, der Septemberhandel darf kommen ...
 jüngste Seeking Alpha Atikel (sehr fakten basiert, wenn nicht n'bissl zu "pushy" von Freund Lasar)
jüngste Seeking Alpha Atikel (sehr fakten basiert, wenn nicht n'bissl zu "pushy" von Freund Lasar)Zalicus: When Push Comes To Shove
Well, it's Friday and I'll either be a hero or "toxic" as one chat board poster describes me. As a SA author, I put my confidence in Zalicus (ZLCS) on the line. At a recent Wedbush PAC conference, CFO Renz stated that the firm hoped to launch Z160 in August. In the same presentation, slide #25 also stated that. So what did I do? I wrote articles representing that view. I told investors that come today everything would come to a head. Not a bad thought considering my first entry into Zalicus was below $0.80/share and the stock has since risen to Thursday's close at $1.43/share.
I suppose my optimism and belief in management has some influence on my hunch about Synavive results. I'll admit that I'm nervous, but I'm still holding and considering my options. I've studied the chemistry of Synavive since 2010. I'm no novice to investing and I accept my fate. I take responsibility for my investments.
Hour after hour, I'm studying the situation at Zalicus checking and rechecking the facts. Here's what I've found:
First, Synavive's results are closer than even I calculated. On May 4, 2012, Zalicus released news that the SYNERGY trial was fully enrolled: 292 patients in a study that was targeting 250 patients. Over-enrolled. I find that most curious, but I have no answer. Given that the SYNERGY trial was a 12-week study, that would mean that the study may have completed before the end of July.
Or should I take the date of the clinicaltrials.gov website? The June 1, 2012, update would mean the study was completed by August 24, 2012. Of course, there's lag time, but don't forget an 8-K must be filed within four days of a material event. Yes, I checked. I'm holding at the time of this article and I'll protect every dime I've earned (without the government's help) because I invest to make money, not bemoan I'm too lazy to look up the latest link to three patent applications published by Zalicus on Thursday.
Second, making money requires you to weigh the company's fundamentals, catalysts and stock charts. Here's what I see: the stock price is $1.43/share on the day of anticipated news. This to me is the spirt of SA -- fundamental articles that bring investors cutting-edge news in advance of others. The RSI, MACD, and Bollinger band have more than enough space to run to a new high. But don't take my word. Take the word of an article (that advertises CNBC's Jim Cramer's team) that was published on Thursday that with enough volume, Zalicus could break $2/share. I'd say, "Lock and load! Get ready for the run! Forces much bigger than me are afoot!"
Gee, you'd think that they were reading my articles and studying the same charts. The chart is very bullish. Thursday ended with a 3.62% gain. You see, fundamentals and charts do count and the best investors learn how to use both.
Third, once again I informed Zalicus shareholders that Zalicus had published three critical ion channel patent applications tied to the current program.
I've studied Zalicus over three years, enough so that one analyst, Jason Napodano of Zacks, jokes about my frequent articles on Zalicus. Okay, he's right, but I invest to make money not win a Pulitzer prize or to earn a measly penny per view. I ran with Antares (ATRS), an awesome firm and won. Now my plan is to win over and over with Zalicus, a small biotech firm that has an ion channel portfolio that's about to change the way pain is treated.
Finally, the publication Thursday of those three ion channel patent applications shook me. They shook me because I've never seen Zalicus publish more than one patent on any given day. Zalicus is working overtime to protect its intellectual property and from people like me who actually read their patents.
But the truth is out there. Zalicus has published three patent applications all tied to T-type and sodium-type ion channels and this on the day before the last day of August when Z160 is promised to launch. Now some might say that's a coincidence, but I've never known the stoic CEO Corrigan to operate that way. He's about as strategic as they come including his board seat on Cubist (CBST) or his bullish promise now fulfilled that the 32 mg version of Exalgo with Covidien (COV) would be approved.
Mark H. N. Corrigan doesn't work to lose. CEO Corrigan wouldn't put his name behind Synavive unless he thought it would succeed in a Phase IIB clinical trial or at least have great odds of succeeding. Neither would his former VP (once temporary CEO) Christopher Gallen, who separated from Zalicus, but rather than take a cash payment preferred to keep his shares in Zalicus. Drs. Corrigan and Gallen are not losers, not by any stretch. They play to win and so do I as I sit here fully loaded up to the chin with Zalicus shares.
Therefore, when push comes to shove, I know the risk that Synavive could fail, but I'm going to take responsibility for my choices rather than resort to name-calling. But a final word to all the critics out there: I positively do not believe that the recent S-3 filing implies imminent dilution. I have unwavering confidence in the integrity of this company's CFO and CEO who have stated that the firm has more than enough funds to go into 2013 even 2014.
I expect that come today and next Tuesday, Zalicus is going to soar. I wish everyone the very best in investing. Remember, never invest more than you're willing to lose and do everyone else a favor: take responsibility for your investment choices. "Sticks and stones break my bones, but names never ..." Well, sometimes, being called names does hurt, but if you invest to make money, counting how much you've earned seems to make the pain go away.
http://seekingalpha.com/article/840121-zalicus-when-push-com…
Tja, der Septemberhandel darf kommen ...

Zalicus Initiates the First of Two Phase 2a Studies with Z160, a First-in-Class, Oral, State-Dependent, Selective N-type Calcium Channel Blocker, for the Treatment of Chronic Neuropathic Pain
Zalicus Inc. (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases, today announced that it has initiated the first of two Phase 2a clinical studies with Z160, its first-in-class, oral, state-dependent, selective N-type calcium channel blocker for the treatment of chronic neuropathic pain indications. This first Phase 2a study will enroll subjects with chronic neuropathic pain associated with Lumbosacral Radiculopathy (LSR). LSR is a common neuropathic pain condition resulting from the compression or irritation of the nerve roots exiting the lumbar region of the spine. Common symptoms include pain radiating from the lower back and down the legs, as well as numbness and tingling in the lower extremities. The prevalence of LSR is high, affecting 3-5% of the global population1. Currently there are no drug treatments specifically approved to treat this common condition, indicating that LSR is a condition with very high unmet medical need.
Z160 is a state-dependent modulator of the N-type (Cav2.2) calcium channel designed to selectively modulate only those neurons transmitting pain, specifically neurons that are undergoing high-frequency firing. Z160 demonstrated efficacy in several animal models of neuropathic pain. Additionally, phase 1 clinical trials have established Z160 as a very safe and tolerable drug candidate.
A second Phase 2a neuropathic pain clinical study, which is planned to initiate in the fourth quarter of 2012, will evaluate Z160 for the treatment of Postherpetic Neuralgia (PHN), a painful neuropathic condition resulting from an outbreak of the herpes zoster virus, otherwise known as shingles.
Top line data for both studies is expected to be available late in the second half of 2013.
"We are excited to move Z160, with its novel mechanism of action, forward into Phase 2a human clinical testing this year," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “While there are a few treatments currently approved for chronic neuropathic pain, there still exists a significant unmet medical need for a more targeted and efficacious therapy with an improved safety and tolerability profile."
Study Design and Objectives
The Z160 Phase 2a Lumbosacral Radiculopathy (LSR) clinical trial is a 6-week, double-blind, multi-center, randomized, placebo-controlled study designed to evaluate the safety and efficacy of Z160 as a treatment in reducing the neuropathic pain of subjects with the condition. The trial is expected to enroll up to approximately 140 subjects and will be conducted in approximately 20 centers throughout the United States. The primary objective of the trial is to evaluate Z160 efficacy compared to placebo in reducing pain in subjects with LSR as measured by change in average daily pain score from baseline to week 6 of treatment based on an 11-point Pain Intensity Numeral Rating Scale (PI-NRS). To learn more about this trial please visit www.clinicaltrials.gov.
About Z160 and N-type Calcium Channel Blockers
Z160 is a first in class, oral, state dependent, selective N-type calcium channel (Cav 2.2) blocker. Z160 has demonstrated efficacy in multiple animal models of neuropathic and inflammatory pain, suggesting that Z160 has the potential to treat a broad range of chronic pain conditions. In addition, Z160 was well tolerated in previously conducted clinical trials involving over 200 subjects. N-type calcium channels have been recognized as key targets in controlling pain because of their key role in transmitting pain through the spinal nerves to the brain. Zalicus has utilized its expertise in this field to successfully discover high affinity, selective and orally available compounds, such as Z160, that show promise for further development as therapies for pain.
http://finance.yahoo.com/news/zalicus-initiates-first-two-ph…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/zalicus-initiates-first-two-ph…
Zalicus Inc. (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases, today announced that it has initiated the first of two Phase 2a clinical studies with Z160, its first-in-class, oral, state-dependent, selective N-type calcium channel blocker for the treatment of chronic neuropathic pain indications. This first Phase 2a study will enroll subjects with chronic neuropathic pain associated with Lumbosacral Radiculopathy (LSR). LSR is a common neuropathic pain condition resulting from the compression or irritation of the nerve roots exiting the lumbar region of the spine. Common symptoms include pain radiating from the lower back and down the legs, as well as numbness and tingling in the lower extremities. The prevalence of LSR is high, affecting 3-5% of the global population1. Currently there are no drug treatments specifically approved to treat this common condition, indicating that LSR is a condition with very high unmet medical need.
Z160 is a state-dependent modulator of the N-type (Cav2.2) calcium channel designed to selectively modulate only those neurons transmitting pain, specifically neurons that are undergoing high-frequency firing. Z160 demonstrated efficacy in several animal models of neuropathic pain. Additionally, phase 1 clinical trials have established Z160 as a very safe and tolerable drug candidate.
A second Phase 2a neuropathic pain clinical study, which is planned to initiate in the fourth quarter of 2012, will evaluate Z160 for the treatment of Postherpetic Neuralgia (PHN), a painful neuropathic condition resulting from an outbreak of the herpes zoster virus, otherwise known as shingles.
Top line data for both studies is expected to be available late in the second half of 2013.
"We are excited to move Z160, with its novel mechanism of action, forward into Phase 2a human clinical testing this year," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “While there are a few treatments currently approved for chronic neuropathic pain, there still exists a significant unmet medical need for a more targeted and efficacious therapy with an improved safety and tolerability profile."
Study Design and Objectives
The Z160 Phase 2a Lumbosacral Radiculopathy (LSR) clinical trial is a 6-week, double-blind, multi-center, randomized, placebo-controlled study designed to evaluate the safety and efficacy of Z160 as a treatment in reducing the neuropathic pain of subjects with the condition. The trial is expected to enroll up to approximately 140 subjects and will be conducted in approximately 20 centers throughout the United States. The primary objective of the trial is to evaluate Z160 efficacy compared to placebo in reducing pain in subjects with LSR as measured by change in average daily pain score from baseline to week 6 of treatment based on an 11-point Pain Intensity Numeral Rating Scale (PI-NRS). To learn more about this trial please visit www.clinicaltrials.gov.
About Z160 and N-type Calcium Channel Blockers
Z160 is a first in class, oral, state dependent, selective N-type calcium channel (Cav 2.2) blocker. Z160 has demonstrated efficacy in multiple animal models of neuropathic and inflammatory pain, suggesting that Z160 has the potential to treat a broad range of chronic pain conditions. In addition, Z160 was well tolerated in previously conducted clinical trials involving over 200 subjects. N-type calcium channels have been recognized as key targets in controlling pain because of their key role in transmitting pain through the spinal nerves to the brain. Zalicus has utilized its expertise in this field to successfully discover high affinity, selective and orally available compounds, such as Z160, that show promise for further development as therapies for pain.
http://finance.yahoo.com/news/zalicus-initiates-first-two-ph…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/zalicus-initiates-first-two-ph…
Hallo zusammen,
finde die Aktie auch interessant.
Aber bei einer positiven Nachricht -10%???
finde die Aktie auch interessant.
Aber bei einer positiven Nachricht -10%???
Buy Zalicus For A 120% Upside
http://seekingalpha.com/article/847081-buy-zalicus-for-a-120…
Yesterday Zalicus (ZLCS) had a short attack with only 600k shares. The range of the day was between 1.28 low and 1.52 max. During the day more than 6MM of shares were negotiated. So the daily average volume of 3.51MM shares was almost doubled. The stock showed an upside of 90,14% in the last four months. According to analyst opinions and pipeline studies as you will see in the table included in this article, the expected target price will reach $3 in 12 months, with a considerable upside of 120% . In my previous article, I presented four reasons for buying Zalicus. In this article, I am going to expose the new changes as well as the news carried out during that time.
Zalicus Inc. discovers and develops treatments for pain and immuno-inflammatory diseases. An important component of the company's business strategy is collaboration. Zalicus forms collaborations with pharmaceutical and biotechnology companies, as well as U.S. government agencies, to support the development and marketing of select product candidates generated by their discovery technologies.
This small pharmaceutical company has agreements with major companies in the sector, such as Covidien (COV), Novartis (NVS), Sanofi (SNY), Hydra biosciences and the U.S. Army Medical Research Institute for Infectious Diseases.
Pipeline portfolio:
(Click to enlarge)
Synavive
Synavive is a product candidate with a novel mechanism designed to enhance the anti-inflammatory benefits of glucocorticoids without the associated dose-dependent side effects. Developed in a uniquely engineered formulation, Synavive is comprised of the cardiovascular agent dipyridamole, and very low dose of the glucocorticoid prednisolone. Synavive is thought to act through a novel multi-target mechanism of action in which dipyridamole selectively amplifies prednisolone's anti-inflammatory activities without increases in adverse effects typically associated with higher doses of glucocorticoids.
Synavive is in Phase II clinical development and has demonstrated anti-inflammatory effects, rapid onset of action and tolerable safety profiles in clinical studies to-date including rheumatoid arthritis.
About 20 million people in the United States are believed to have rheumatoid arthritis. The market potential is significant and Synavive won't have any problems when it is compared with other similar drugs.
Synavive's estimated market could be between to $14B/year.
The estimated study completion date is in September 2012.
Clinical trial: A Phase II Trial Comparing Z-102 With Placebo In Patients With Moderate To Severe Rheumatoid Arthritis (Synergy)
(Source Clinical Trial)
Z160
Z160 is a first in class, oral, state dependent, selective N-type calcium channel (Cav 2.2) blocker. Z160 has demonstrated efficacy in multiple animal models of neuropathic and inflammatory pain, suggesting that Z160 has the potential to treat a broad range of chronic pain conditions. In addition, Z160 was well tolerated in previously conducted clinical trials involving over 200 subjects. N-type calcium channels have been recognized as key targets in controlling pain because of their key role in transmitting pain through the spinal nerves to the brain. Zalicus has utilized its expertise in this field to successfully discover high affinity, selective and orally available compounds, such as Z160, that show promise for further development as therapies for pain.
Yesterday, Zalicus initiates the first of two Phase IIa studies with Z160, a First-in-Class, Oral, State-Dependent, Selective N-type Calcium Channel Blocker, for the Treatment of Chronic Neuropathic Pain.
Z160's estimated market could be between to $30B/year.
The estimated study completion date is in September 2013.
Clinical trial: Phase II Efficacy Trial of Z160 in Lumbosacral Radiculopathy
(Source clinical trial)
Z944
Z944, is a novel, oral, T-type calcium channel blocker that has demonstrated preclinical efficacy in multiple inflammatory pain models. A Phase I clinical trial evaluating the safety and tolerability of Z944 is ongoing. If Z944 successfully completes this Phase I clinical trial, Zalicus is planning to advance Z944 into Phase II clinical development.
The wide distribution of T-type calcium channels found in, heart, endocrine cells and other tissues provides the possibility of developing therapeutics for multiple indications, including treatment of pain.
Z944 will advance into a Phase I multiple ascending dose study in the third quarter of 2012.
Partnered Programs
Exalgo (Covidien/Mallinckrodt)
The FDA approved the three existing doses of EXALGO (8, 12 and 16 mg) in March 2010. Mallinckrodt subsequently submitted the sNDA in January 2012 with post-marketing data to support the original application's compendium of clinical trials demonstrating safety, efficacy and tolerability. Using OROS technology, EXALGO provides a steady release of hydromorphone throughout the day once steady-state is achieved after three to four days. Additionally, EXALGO has physical properties that may make it difficult to extract the active ingredient using common forms of physical and chemical tampering, including chewing, crushing and dissolving.
The rights to EXALGO were acquired by Mallinckrodt LLC, the pharmaceuticals business of Covidien plc, in June 2009 for $15 million in upfront payments, additional development funding of up to $16 million and a $40 million FDA approval milestone payment. Zalicus receives tiered royalties on net sales of EXALGO by Mallinckrodt.
On 27 August 2012, Zalicus announced that the U.S. Food and Drug Administration has approved the supplemental new drug application filed by Mallinckrodt Inc., a subsidiary of Covidien plc, for the 32 mg dose strength of EXALGO (hydromorphone HCl) Extended-Release Tablets, for the management of moderate to severe pain in opioid-tolerant patients requiring continuous, around-the-clock opioid analgesia for an extended period of time.
Prednisporin (Sanofi)
Prednisporin is a topical ocular drug candidate that Zalicus has exclusively licensed to Fovea Pharmaceuticals SA, a division of Sanofi Aventis (SNY), containing low doses of the glucocorticoid, prednisolone acetate and the immunosuppressant cyclosporine A. Fovea has developed a proprietary co-formulation of Prednisporin and is seeking to develop Prednisporin to treat inflammatory ocular diseases such as allergic conjunctivitis. Fovea has advanced Prednisporin into Phase IIb clinical development in subjects with persistent allergic conjunctivitis.
During 2009, Fovea investigated Prednisporin in a Phase IIa proof-of-concept clinical trial. Fovea has reported that on the primary endpoint of ocular itching, the Prednisporin combination with the higher dose of cyclosporine A is superior to placebo (p=0.048) and non-inferior to the higher dose of prednisolone acetate alone. The Prednisporin combinations were generally well tolerated and there were no serious adverse events reported.
This week Zalicus has been guaranteed the patent of Prednisporin.
News patents of Zalicus
Selective Calcium Channel Modulators. Patent: 20120220564
Oxopiperazine derivatives for the treatment of pain and Epilepsy. Patent: 20120220605
Substituted Heterocyclic derivatives for the treatment of pain and Epilepsy. Patent: 20120220603
In the following table, we will analyze the estimated annual sales of all the ongoing studies in Zalicus.
PIPELINE ESTIMATE ANNUAL SALES
Synavive $14.000.000.000
Z160 $30.000.000.000
Z944 $15.000.000.000
Partnered Programs (royalties) $100.000.000
Ion (cHTP research programs) $5.000.000.000
Total $64.100.000.000 estimate annual sales
The capitalization of Zalicus is only 170MM and the estimated annual sales are around $64B/year. If one of its ongoing studies is approved, the capitalization and its value could be multiplied by 10x or more. Synavive, Z160 and Z944 can make billions of dollars for Zalicus. Any company may be interested in buying Zalicus only by their studies. In the past it was rumored that Merck (MRK) had offered $500MM for Z160. At current prices, Merck could buy Zalicus completely.
Chart Analysis and Comparative
(Click to enlarge)
The Chart marks that Zalicus remains bullish. The red line indicates a possible change of trend if you exceed in the closing daily. The next key resistance is at $1.62.
Yesterday, Zalicus marked a similar candle to that carried out in companies such as Arena Pharmaceuticals (ARNA), Threshold Pharmaceuticals (THLD) and Orexigen Therapeutics (OREX) before communicating positive results in their studies.
Comparative charts
Arena Pharmaceuticals
(Click to enlarge)
Threshold Pharmaceuticals
(Click to enlarge)
Orexigen Therapeutics
(Click to enlarge)
Analyst Ratings
On 28 August 2012, analyst Canaccord Genuity Reiterates a "Buy" on Zalicus with price target of $3.00.
Zalicus: Analyst stock recommendations
(SOURCE)
Conclusion
Extremely important news such as Exalgo approval for dose of 32mg and the start of Phase IIa of Z160, have been very positive for all investors in Zalicus. The target price of Zalicus continues to be $3 for short term. In my opinion, Zalicus could be above two digits in a long-term.
Zalicus is a Strong Buy.
*Pipeline overview data sourced from Zalicus and Chart data sourced from stockcharts, all other data sourced from Nasdaq.com as well as the web of the previously mentioned company.
*Translator: Rut Aznar.
Disclosure: I am long ZLCS. I wrote this article myself, and it
http://seekingalpha.com/article/847081-buy-zalicus-for-a-120…
Yesterday Zalicus (ZLCS) had a short attack with only 600k shares. The range of the day was between 1.28 low and 1.52 max. During the day more than 6MM of shares were negotiated. So the daily average volume of 3.51MM shares was almost doubled. The stock showed an upside of 90,14% in the last four months. According to analyst opinions and pipeline studies as you will see in the table included in this article, the expected target price will reach $3 in 12 months, with a considerable upside of 120% . In my previous article, I presented four reasons for buying Zalicus. In this article, I am going to expose the new changes as well as the news carried out during that time.
Zalicus Inc. discovers and develops treatments for pain and immuno-inflammatory diseases. An important component of the company's business strategy is collaboration. Zalicus forms collaborations with pharmaceutical and biotechnology companies, as well as U.S. government agencies, to support the development and marketing of select product candidates generated by their discovery technologies.
This small pharmaceutical company has agreements with major companies in the sector, such as Covidien (COV), Novartis (NVS), Sanofi (SNY), Hydra biosciences and the U.S. Army Medical Research Institute for Infectious Diseases.
Pipeline portfolio:
(Click to enlarge)
Synavive
Synavive is a product candidate with a novel mechanism designed to enhance the anti-inflammatory benefits of glucocorticoids without the associated dose-dependent side effects. Developed in a uniquely engineered formulation, Synavive is comprised of the cardiovascular agent dipyridamole, and very low dose of the glucocorticoid prednisolone. Synavive is thought to act through a novel multi-target mechanism of action in which dipyridamole selectively amplifies prednisolone's anti-inflammatory activities without increases in adverse effects typically associated with higher doses of glucocorticoids.
Synavive is in Phase II clinical development and has demonstrated anti-inflammatory effects, rapid onset of action and tolerable safety profiles in clinical studies to-date including rheumatoid arthritis.
About 20 million people in the United States are believed to have rheumatoid arthritis. The market potential is significant and Synavive won't have any problems when it is compared with other similar drugs.
Synavive's estimated market could be between to $14B/year.
The estimated study completion date is in September 2012.
Clinical trial: A Phase II Trial Comparing Z-102 With Placebo In Patients With Moderate To Severe Rheumatoid Arthritis (Synergy)
(Source Clinical Trial)
Z160
Z160 is a first in class, oral, state dependent, selective N-type calcium channel (Cav 2.2) blocker. Z160 has demonstrated efficacy in multiple animal models of neuropathic and inflammatory pain, suggesting that Z160 has the potential to treat a broad range of chronic pain conditions. In addition, Z160 was well tolerated in previously conducted clinical trials involving over 200 subjects. N-type calcium channels have been recognized as key targets in controlling pain because of their key role in transmitting pain through the spinal nerves to the brain. Zalicus has utilized its expertise in this field to successfully discover high affinity, selective and orally available compounds, such as Z160, that show promise for further development as therapies for pain.
Yesterday, Zalicus initiates the first of two Phase IIa studies with Z160, a First-in-Class, Oral, State-Dependent, Selective N-type Calcium Channel Blocker, for the Treatment of Chronic Neuropathic Pain.
Z160's estimated market could be between to $30B/year.
The estimated study completion date is in September 2013.
Clinical trial: Phase II Efficacy Trial of Z160 in Lumbosacral Radiculopathy
(Source clinical trial)
Z944
Z944, is a novel, oral, T-type calcium channel blocker that has demonstrated preclinical efficacy in multiple inflammatory pain models. A Phase I clinical trial evaluating the safety and tolerability of Z944 is ongoing. If Z944 successfully completes this Phase I clinical trial, Zalicus is planning to advance Z944 into Phase II clinical development.
The wide distribution of T-type calcium channels found in, heart, endocrine cells and other tissues provides the possibility of developing therapeutics for multiple indications, including treatment of pain.
Z944 will advance into a Phase I multiple ascending dose study in the third quarter of 2012.
Partnered Programs
Exalgo (Covidien/Mallinckrodt)
The FDA approved the three existing doses of EXALGO (8, 12 and 16 mg) in March 2010. Mallinckrodt subsequently submitted the sNDA in January 2012 with post-marketing data to support the original application's compendium of clinical trials demonstrating safety, efficacy and tolerability. Using OROS technology, EXALGO provides a steady release of hydromorphone throughout the day once steady-state is achieved after three to four days. Additionally, EXALGO has physical properties that may make it difficult to extract the active ingredient using common forms of physical and chemical tampering, including chewing, crushing and dissolving.
The rights to EXALGO were acquired by Mallinckrodt LLC, the pharmaceuticals business of Covidien plc, in June 2009 for $15 million in upfront payments, additional development funding of up to $16 million and a $40 million FDA approval milestone payment. Zalicus receives tiered royalties on net sales of EXALGO by Mallinckrodt.
On 27 August 2012, Zalicus announced that the U.S. Food and Drug Administration has approved the supplemental new drug application filed by Mallinckrodt Inc., a subsidiary of Covidien plc, for the 32 mg dose strength of EXALGO (hydromorphone HCl) Extended-Release Tablets, for the management of moderate to severe pain in opioid-tolerant patients requiring continuous, around-the-clock opioid analgesia for an extended period of time.
Prednisporin (Sanofi)
Prednisporin is a topical ocular drug candidate that Zalicus has exclusively licensed to Fovea Pharmaceuticals SA, a division of Sanofi Aventis (SNY), containing low doses of the glucocorticoid, prednisolone acetate and the immunosuppressant cyclosporine A. Fovea has developed a proprietary co-formulation of Prednisporin and is seeking to develop Prednisporin to treat inflammatory ocular diseases such as allergic conjunctivitis. Fovea has advanced Prednisporin into Phase IIb clinical development in subjects with persistent allergic conjunctivitis.
During 2009, Fovea investigated Prednisporin in a Phase IIa proof-of-concept clinical trial. Fovea has reported that on the primary endpoint of ocular itching, the Prednisporin combination with the higher dose of cyclosporine A is superior to placebo (p=0.048) and non-inferior to the higher dose of prednisolone acetate alone. The Prednisporin combinations were generally well tolerated and there were no serious adverse events reported.
This week Zalicus has been guaranteed the patent of Prednisporin.
News patents of Zalicus
Selective Calcium Channel Modulators. Patent: 20120220564
Oxopiperazine derivatives for the treatment of pain and Epilepsy. Patent: 20120220605
Substituted Heterocyclic derivatives for the treatment of pain and Epilepsy. Patent: 20120220603
In the following table, we will analyze the estimated annual sales of all the ongoing studies in Zalicus.
PIPELINE ESTIMATE ANNUAL SALES
Synavive $14.000.000.000
Z160 $30.000.000.000
Z944 $15.000.000.000
Partnered Programs (royalties) $100.000.000
Ion (cHTP research programs) $5.000.000.000
Total $64.100.000.000 estimate annual sales
The capitalization of Zalicus is only 170MM and the estimated annual sales are around $64B/year. If one of its ongoing studies is approved, the capitalization and its value could be multiplied by 10x or more. Synavive, Z160 and Z944 can make billions of dollars for Zalicus. Any company may be interested in buying Zalicus only by their studies. In the past it was rumored that Merck (MRK) had offered $500MM for Z160. At current prices, Merck could buy Zalicus completely.
Chart Analysis and Comparative
(Click to enlarge)
The Chart marks that Zalicus remains bullish. The red line indicates a possible change of trend if you exceed in the closing daily. The next key resistance is at $1.62.
Yesterday, Zalicus marked a similar candle to that carried out in companies such as Arena Pharmaceuticals (ARNA), Threshold Pharmaceuticals (THLD) and Orexigen Therapeutics (OREX) before communicating positive results in their studies.
Comparative charts
Arena Pharmaceuticals
(Click to enlarge)
Threshold Pharmaceuticals
(Click to enlarge)
Orexigen Therapeutics
(Click to enlarge)
Analyst Ratings
On 28 August 2012, analyst Canaccord Genuity Reiterates a "Buy" on Zalicus with price target of $3.00.
Zalicus: Analyst stock recommendations
(SOURCE)
Conclusion
Extremely important news such as Exalgo approval for dose of 32mg and the start of Phase IIa of Z160, have been very positive for all investors in Zalicus. The target price of Zalicus continues to be $3 for short term. In my opinion, Zalicus could be above two digits in a long-term.
Zalicus is a Strong Buy.
*Pipeline overview data sourced from Zalicus and Chart data sourced from stockcharts, all other data sourced from Nasdaq.com as well as the web of the previously mentioned company.
*Translator: Rut Aznar.
Disclosure: I am long ZLCS. I wrote this article myself, and it
Antwort auf Beitrag Nr.: 43.567.346 von BlackOasis am 04.09.12 16:19:43Antwort:- only good stocks ever get bashed bzw. shorted
Mein Rat, buy the dips

Mein Rat, buy the dips

Zalicus: Pain Free Investing
http://seekingalpha.com/article/854201-zalicus-pain-free-inv…
In one of my favorite movie scenes of all time, before his fight with Rocky Balboa in Rocky III, a reporter asks Clubber Lang his prediction for the fight. Clubber stares straight into the camera and utters "Pain." I believe an investment in Zalicus ("ZLCS") can help investors steer clear of pain.
Unfortunately, pain is the reality for many Americans in their daily lives. Millions of Americans suffer from acute or chronic pain every year and the pain exacts a tremendous cost on the country. According to a recent Institute of Medicine Report: Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research, pain is a significant public health problem that costs society at least $560-$635 billion annually, an amount equal to about $2,000.00 for everyone living in the United States. The healthcare component of that total ranges from $261 to $300 billion annually.
Where do American's hurt the most? Good question. When asked about four common types of pain, respondents of a National Institute of Health Statistics survey indicated that lower back pain was the most common form of pain with 27% of respondents citing lower back pain is their source of pain. Similarly according to the same survey, back pain is the leading cause of disability in Americans under 45 years old and more than 26 million Americans between the ages of 20-64 experience frequent back pain. Obviously the current treatments for back pain are not getting the job done.
All of this leads me to Zalicus (ZLCS). Zalicus is a biopharmaceutical company that discovers and develops treatments for patients suffering from pain and immuno-inflammatory diseases. On September 4, 2012, Zalicus announced the initiation of the first of two Phase 2a studies with Z160, which is a first-in-class, oral, state-dependent, selective N-type calcium channel blocker, for the treatment of chronic neuropathic pain. The study is focused on lumbosacral radiculopathy, chronic neuropathic pain originating from the spine.
What does that mean? It means that Zalicus just launched a Phase 2a study of a first-in-class treatment for certain types of lower back pain. In its press release announcing the study, Zalicus estimates the prevalence of the market at 3%-5% of the global population, indicating that there are currently no drugs approved to treat lumbosacral radiculopathy. The market into which Z160 would launch is therefore enormous. While top line results for this study and a second upcoming study are not expected until late in second half of 2013, which I read conservatively as early 2014, the speculative success of Z160 alone makes Zalicus a worthwhile buy at its current price of $1.40 depending on your investing strategy.
What makes ZLCS an even better investment than many other biotech companies that are currently conducting studies of promising drug candidates is that Zalicus is not a one trick pony. Zalicus just announced FDA approval of a 32mg dose strength of Exalgo for the management of moderate to severe pain and Zalicus receives tiered royalties on net sales of Exalgo. Exalgo competes in the opioid pain market with heavyweights such as oxycontin, Embeda (Pfizer ("PFE")), Opana, (Endo Pharmaceuticals ("ENDP")), and Avinza (King Pharmaceuticals which is now part of Pfizer). The market is enormous with sales of $8.5 billion in 2010 and the newly approved 32mg dose should significantly increase Zalicus' revenues going forward as it was the median effective dose upon which patients were stabilized during the pivotal trial. The increase in revenue from Exalgo will help stave off or at least blunt future dilution as Zalicus funds its pipeline.
In addition, sometime this month Zalicus should announce top-line results from its Synergy Phase 2(b) clinical trial of Synavive in rheumatoid arthritis. Positive Synavive results should provide a short term boost to the share price.
I imagine these are the reasons that all analysts covering the company currently maintain buy ratings with a combined price target of $4. If the stock were to reach this consensus price target, an investment today would pocket investors almost 200% in profit.
There are many reasons to buy Zalicus now, but I am having trouble coming up with reasons to sell.
http://seekingalpha.com/article/854201-zalicus-pain-free-inv…
In one of my favorite movie scenes of all time, before his fight with Rocky Balboa in Rocky III, a reporter asks Clubber Lang his prediction for the fight. Clubber stares straight into the camera and utters "Pain." I believe an investment in Zalicus ("ZLCS") can help investors steer clear of pain.
Unfortunately, pain is the reality for many Americans in their daily lives. Millions of Americans suffer from acute or chronic pain every year and the pain exacts a tremendous cost on the country. According to a recent Institute of Medicine Report: Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research, pain is a significant public health problem that costs society at least $560-$635 billion annually, an amount equal to about $2,000.00 for everyone living in the United States. The healthcare component of that total ranges from $261 to $300 billion annually.
Where do American's hurt the most? Good question. When asked about four common types of pain, respondents of a National Institute of Health Statistics survey indicated that lower back pain was the most common form of pain with 27% of respondents citing lower back pain is their source of pain. Similarly according to the same survey, back pain is the leading cause of disability in Americans under 45 years old and more than 26 million Americans between the ages of 20-64 experience frequent back pain. Obviously the current treatments for back pain are not getting the job done.
All of this leads me to Zalicus (ZLCS). Zalicus is a biopharmaceutical company that discovers and develops treatments for patients suffering from pain and immuno-inflammatory diseases. On September 4, 2012, Zalicus announced the initiation of the first of two Phase 2a studies with Z160, which is a first-in-class, oral, state-dependent, selective N-type calcium channel blocker, for the treatment of chronic neuropathic pain. The study is focused on lumbosacral radiculopathy, chronic neuropathic pain originating from the spine.
What does that mean? It means that Zalicus just launched a Phase 2a study of a first-in-class treatment for certain types of lower back pain. In its press release announcing the study, Zalicus estimates the prevalence of the market at 3%-5% of the global population, indicating that there are currently no drugs approved to treat lumbosacral radiculopathy. The market into which Z160 would launch is therefore enormous. While top line results for this study and a second upcoming study are not expected until late in second half of 2013, which I read conservatively as early 2014, the speculative success of Z160 alone makes Zalicus a worthwhile buy at its current price of $1.40 depending on your investing strategy.
What makes ZLCS an even better investment than many other biotech companies that are currently conducting studies of promising drug candidates is that Zalicus is not a one trick pony. Zalicus just announced FDA approval of a 32mg dose strength of Exalgo for the management of moderate to severe pain and Zalicus receives tiered royalties on net sales of Exalgo. Exalgo competes in the opioid pain market with heavyweights such as oxycontin, Embeda (Pfizer ("PFE")), Opana, (Endo Pharmaceuticals ("ENDP")), and Avinza (King Pharmaceuticals which is now part of Pfizer). The market is enormous with sales of $8.5 billion in 2010 and the newly approved 32mg dose should significantly increase Zalicus' revenues going forward as it was the median effective dose upon which patients were stabilized during the pivotal trial. The increase in revenue from Exalgo will help stave off or at least blunt future dilution as Zalicus funds its pipeline.
In addition, sometime this month Zalicus should announce top-line results from its Synergy Phase 2(b) clinical trial of Synavive in rheumatoid arthritis. Positive Synavive results should provide a short term boost to the share price.
I imagine these are the reasons that all analysts covering the company currently maintain buy ratings with a combined price target of $4. If the stock were to reach this consensus price target, an investment today would pocket investors almost 200% in profit.
There are many reasons to buy Zalicus now, but I am having trouble coming up with reasons to sell.
runter auf $0,84
Ist das schon der Boden und kommt jetzt die Gegenreaktion oder ziehen jetzt noch die Investierten ohne STOP LOSS nach.
Ich warte mal ab.
Ist das schon der Boden und kommt jetzt die Gegenreaktion oder ziehen jetzt noch die Investierten ohne STOP LOSS nach.
Ich warte mal ab.
hi Poppholz,
hast du herausgefunden warum die ZLS so krass abgestürtzt ist?
hast du herausgefunden warum die ZLS so krass abgestürtzt ist?
Antwort auf Beitrag Nr.: 43.586.932 von noa9090 am 10.09.12 16:36:26synavive durchgefallen...

http://www.nasdaq.com/article/zalicus-to-halt-further-clinic…

http://www.nasdaq.com/article/zalicus-to-halt-further-clinic…
mal schauen was der Kurs heute so macht.
Ist eigentlich keiner mehr investiert?
Zitat von Poppholz:![]()
mal schauen was der Kurs heute so macht.
Ist eigentlich keiner mehr investiert?
..aber hallo , Poppie....
...immer noch investiert, klaro...

...und heute sogar noch 5000 Stk. nachgekauft bei 0,627 €...
..hoffe, dass der Zeitpunkt des Einstiegs günstig war....
...time will tell....

ZLCS - Moving To Neutral on Zalicus
On September 10, 2012, Zalicus, Inc. (ZLCS) announced top-line results from the phase 2b SYNERGY trial. SYNERGY (SYNavivE for Reducing signs and sYmptoms of rheumatoid arthritis trial) was a 12-week, 5-arm, double-blind, placebo-controlled study to evaluate the safety and efficacy of Synavive as a treatment for the signs and symptoms of rheumatoid arthritis (RA) in approximately 259 subjects with moderate to severe disease conducted at over 50 centers in the U.S., Europe, and Latin America (ClinicalTrials.gov identifier: NCT01369745). Enrollment in SYNERGY completed on April 30, 2012.
The primary endpoint was efficacy measured by the Disease Activity Score in 28 Joints (DAS28) at 12 weeks. DAS28 is a common, patient specific, outcome tool used in therapeutic trials to assess disease improvement or response. The trial was designed to show the efficacy of Synavive compared to its individual components (2.7 mg of prednisolone and 360mg of dipyramidamole) as well as how Synavive performs in comparison to 5 mg of prednisolone alone.
Results showed that patients on Synavive achieved a -0.9 change from baseline on the DAS28 endpoint for Synavive (approximately 17% improvement) compared to a -0.5 change from baseline for placebo (approximately 10% improvement). However, Synavive was not statistically superior to 2.7 mg or 5 mg prednisolone. As a result, management has discontinued development of Synavive. Zalicus will also halt the ongoing one-year extension study which was designed to investigate the long-term safety and durability of response for Synavive.
We believe discontinuing Synavive is the right thing to do, and applaud management for showing the discipline necessary to make the call. Zalicus spent $9.9 million on R&D in the second quarter 2012. Synavive accounted for $2.8 million of this figure. The company spent a total of $6.1 million on Synavive development during the first six months of 2012. In 2011, Zalicus spent $11.8 million on Synavive research and development. We expect a savings of around $3 million in the third and fourth quarter of 2012 now that Synavive has been discontinued. We are lowering our 2013 R&D expense by $5 million as well.
Moving To Neutral
We are disappointed in the failure of SYNERGY and the discontinuation of Synavive. We had previously believed the drug had $250 million potential as a treatment of moderate to severe RA in patients failing high doses of the glucocorticoid prednisolone due to poor tolerability and/or side effects. Our previous price target was $3.00 per share.
We are moving our rating to ‘Neutral’ while we wait for additional data on the two phase 2a ion channel candidates, Z944 and Z160 (discussed below), and lowering our target to $1.50 per share.
We see significant value in these two ion channel candidates, each with novel mechanisms of action for the treatment of acute and chronic pain, respectively. We remind investors that Zalicus also received a royalty on sales of Exalgo at Covidien. Covidien recently received approval for a 32 mg dose of Exalgo, which we believe makes the product far more competitive in the crowded long-acting opioid space. Zalicus also has early-stage oncology and ion channel collaborations, and is eligible to receive both milestones and royalties on the advancement and commercialize
http://finance.yahoo.com/news/zlcs-moving-neutral-zalicus-14…
On September 10, 2012, Zalicus, Inc. (ZLCS) announced top-line results from the phase 2b SYNERGY trial. SYNERGY (SYNavivE for Reducing signs and sYmptoms of rheumatoid arthritis trial) was a 12-week, 5-arm, double-blind, placebo-controlled study to evaluate the safety and efficacy of Synavive as a treatment for the signs and symptoms of rheumatoid arthritis (RA) in approximately 259 subjects with moderate to severe disease conducted at over 50 centers in the U.S., Europe, and Latin America (ClinicalTrials.gov identifier: NCT01369745). Enrollment in SYNERGY completed on April 30, 2012.
The primary endpoint was efficacy measured by the Disease Activity Score in 28 Joints (DAS28) at 12 weeks. DAS28 is a common, patient specific, outcome tool used in therapeutic trials to assess disease improvement or response. The trial was designed to show the efficacy of Synavive compared to its individual components (2.7 mg of prednisolone and 360mg of dipyramidamole) as well as how Synavive performs in comparison to 5 mg of prednisolone alone.
Results showed that patients on Synavive achieved a -0.9 change from baseline on the DAS28 endpoint for Synavive (approximately 17% improvement) compared to a -0.5 change from baseline for placebo (approximately 10% improvement). However, Synavive was not statistically superior to 2.7 mg or 5 mg prednisolone. As a result, management has discontinued development of Synavive. Zalicus will also halt the ongoing one-year extension study which was designed to investigate the long-term safety and durability of response for Synavive.
We believe discontinuing Synavive is the right thing to do, and applaud management for showing the discipline necessary to make the call. Zalicus spent $9.9 million on R&D in the second quarter 2012. Synavive accounted for $2.8 million of this figure. The company spent a total of $6.1 million on Synavive development during the first six months of 2012. In 2011, Zalicus spent $11.8 million on Synavive research and development. We expect a savings of around $3 million in the third and fourth quarter of 2012 now that Synavive has been discontinued. We are lowering our 2013 R&D expense by $5 million as well.
Moving To Neutral
We are disappointed in the failure of SYNERGY and the discontinuation of Synavive. We had previously believed the drug had $250 million potential as a treatment of moderate to severe RA in patients failing high doses of the glucocorticoid prednisolone due to poor tolerability and/or side effects. Our previous price target was $3.00 per share.
We are moving our rating to ‘Neutral’ while we wait for additional data on the two phase 2a ion channel candidates, Z944 and Z160 (discussed below), and lowering our target to $1.50 per share.
We see significant value in these two ion channel candidates, each with novel mechanisms of action for the treatment of acute and chronic pain, respectively. We remind investors that Zalicus also received a royalty on sales of Exalgo at Covidien. Covidien recently received approval for a 32 mg dose of Exalgo, which we believe makes the product far more competitive in the crowded long-acting opioid space. Zalicus also has early-stage oncology and ion channel collaborations, and is eligible to receive both milestones and royalties on the advancement and commercialize
http://finance.yahoo.com/news/zlcs-moving-neutral-zalicus-14…
Antwort auf Beitrag Nr.: 43.596.065 von bernie55 am 12.09.12 16:30:00Tach bernie, ich auch, 7K verbilligt zu 0,61 nachgeladen
Trotzalldem, ich war wirklich extrem enttäuscht von die Synavive Ergbnisse, klar, Dr. Corrigan hat das ganze zwar "geerbt" von seinen vorgänger, aber nun hat seine bisherige weisse weste (exzellenter Ruf in der Branche) doch ein paar flecken abbekommen...
Ich hoffe er wird sich hierdurch umso intensiver um dass ION Programm entsprechend kümmern, dort liegt das wahre Potential, und die einzigste möghlichkeit sein guten Ruf wieder herzustellen...
Naja, aufstehen, abbürsten, aufrichten, und wieder ab nach vorne
Ein schönes wochenende an 'die verbliebenen' in die Runde!

Trotzalldem, ich war wirklich extrem enttäuscht von die Synavive Ergbnisse, klar, Dr. Corrigan hat das ganze zwar "geerbt" von seinen vorgänger, aber nun hat seine bisherige weisse weste (exzellenter Ruf in der Branche) doch ein paar flecken abbekommen...

Ich hoffe er wird sich hierdurch umso intensiver um dass ION Programm entsprechend kümmern, dort liegt das wahre Potential, und die einzigste möghlichkeit sein guten Ruf wieder herzustellen...
Naja, aufstehen, abbürsten, aufrichten, und wieder ab nach vorne
Ein schönes wochenende an 'die verbliebenen' in die Runde!
Zitat von Growth2012: Tach bernie, ich auch, 7K verbilligt zu 0,61 nachgeladen
Trotzalldem, ich war wirklich extrem enttäuscht von die Synavive Ergbnisse,
klar, Dr. Corrigan hat das ganze zwar "geerbt" von seinen vorgänger, aber nun hat seine bisherige weisse weste (exzellenter Ruf in der Branche) doch ein paar flecken abbekommen...
Ich hoffe er wird sich hierdurch umso intensiver um dass ION Programm entsprechend kümmern, dort liegt das wahre Potential, und die einzigste möghlichkeit sein guten Ruf wieder herzustellen...
Naja, aufstehen, abbürsten, aufrichten, und wieder ab nach vorne
Ein schönes wochenende an 'die verbliebenen' in die Runde!
....ganz deiner Meinung, growthie..
... ich denke, der positive Aspekt dieses " Reinfalls" mit Synavive ist der, dass finanziell erhebliche Einsparungen erfolgen, die den cashflow von ZLCS nicht mehr " strapazieren" - siehe meinen Beitrag vom 12.09.12 20:25:18
Grüße
bernie55

22-Oct-2012
Notice of Listing or Failure to Satisfy a Continued Listing Rule or Standard; Transfer of Listing.
On October 22, 2012, Zalicus Inc. (the "Company") received a notification from the NASDAQ Listing Qualifications Department providing notification that, for the last 30 consecutive business days, the bid price of the Company's common stock has closed below the minimum $1.00 per share requirement for continued inclusion under NASDAQ Listing Rule 5450(a)(1) (the "Rule"). There is no change in the trading of the Company's common stock on the NASDAQ Global Market at this time and the Company, in accordance with NASDAQ Listing Rule 5810(c)(3)(A), has been provided 180 calendar days, or until April 22, 2013, to regain compliance with the Rule. To regain compliance with the Rule, the bid price of the Company's common stock must close at $1.00 per share or more for a minimum of ten consecutive business days at any time before April 22, 2013.
If the Company does not regain compliance with the Rule by April 22, 2013, the Company will be notified that its securities are subject to delisting. At that time, the Company may appeal NASDAQ's determination to delist its securities to a NASDAQ "Hearings Panel" in accordance with the NASDAQ Listing Rules. Alternatively, the Company also may consider applying to transfer the listing of its common stock to the Nasdaq Capital Market if it satisfies the requirements for initial inclusion set forth in NASDAQ Listing Rule 5505, with the exception of bid price. If its application is approved, the Company's common stock will continue to trade on the Nasdaq Capital Market and the Company will be afforded the remainder of the Nasdaq Capital Market's second 180 calendar day grace period in order to regain compliance with the bid price requirement while maintaining its listing on the Nasdaq Capital Market.
The Company has not yet determined what action, if any, it will take in response to this notice, although the Company intends to monitor the closing bid price of its common stock and to consider available options if its common stock does not trade at a level likely to result in the Company regaining compliance with the NASDAQ minimum closing bid price requirement. There can be no assurance, however, that the Company will be able to regain compliance with the minimum bid price requirement or will otherwise satisfy the other NASDAQ listing criteria. The Company disclaims any intention or obligation to update this report for purposes of disclosing any action or response that the Company decides to take after the date hereof.
http://biz.yahoo.com/e/121022/zlcs8-k.html
Notice of Listing or Failure to Satisfy a Continued Listing Rule or Standard; Transfer of Listing.
On October 22, 2012, Zalicus Inc. (the "Company") received a notification from the NASDAQ Listing Qualifications Department providing notification that, for the last 30 consecutive business days, the bid price of the Company's common stock has closed below the minimum $1.00 per share requirement for continued inclusion under NASDAQ Listing Rule 5450(a)(1) (the "Rule"). There is no change in the trading of the Company's common stock on the NASDAQ Global Market at this time and the Company, in accordance with NASDAQ Listing Rule 5810(c)(3)(A), has been provided 180 calendar days, or until April 22, 2013, to regain compliance with the Rule. To regain compliance with the Rule, the bid price of the Company's common stock must close at $1.00 per share or more for a minimum of ten consecutive business days at any time before April 22, 2013.
If the Company does not regain compliance with the Rule by April 22, 2013, the Company will be notified that its securities are subject to delisting. At that time, the Company may appeal NASDAQ's determination to delist its securities to a NASDAQ "Hearings Panel" in accordance with the NASDAQ Listing Rules. Alternatively, the Company also may consider applying to transfer the listing of its common stock to the Nasdaq Capital Market if it satisfies the requirements for initial inclusion set forth in NASDAQ Listing Rule 5505, with the exception of bid price. If its application is approved, the Company's common stock will continue to trade on the Nasdaq Capital Market and the Company will be afforded the remainder of the Nasdaq Capital Market's second 180 calendar day grace period in order to regain compliance with the bid price requirement while maintaining its listing on the Nasdaq Capital Market.
The Company has not yet determined what action, if any, it will take in response to this notice, although the Company intends to monitor the closing bid price of its common stock and to consider available options if its common stock does not trade at a level likely to result in the Company regaining compliance with the NASDAQ minimum closing bid price requirement. There can be no assurance, however, that the Company will be able to regain compliance with the minimum bid price requirement or will otherwise satisfy the other NASDAQ listing criteria. The Company disclaims any intention or obligation to update this report for purposes of disclosing any action or response that the Company decides to take after the date hereof.
http://biz.yahoo.com/e/121022/zlcs8-k.html
Antwort auf Beitrag Nr.: 43.744.931 von bernie55 am 24.10.12 08:54:50Hallo bernie, das mit dem Dollar hatte ich ja bereits nach dem Kursrutsch angeführt.....hier gibt es aus meiner Erfahrung 3 Szenarien :
1.) die Nachfrage steigt und hebt den Kurs nachhaltig auf über einen Dollar
2.)Seitens des Unternehmens wird über Broker der Kurs gestützt bzw. manipuliert.....
3.) Es kommt zu einem R/S ( evtl. 2:1)....mit der Ausgabe neuer Shares
Fakt ist, das ich nicht glaube das Zalicus es zu einem Delisting kommen lässt......sollte die Käuferseite nicht in den nächsten Monaten zulegen bzw. pos. News eintrudeln......tippe ich persönlich auf einen R/S.....leider !
Grüße Earthfire
1.) die Nachfrage steigt und hebt den Kurs nachhaltig auf über einen Dollar
2.)Seitens des Unternehmens wird über Broker der Kurs gestützt bzw. manipuliert.....
3.) Es kommt zu einem R/S ( evtl. 2:1)....mit der Ausgabe neuer Shares
Fakt ist, das ich nicht glaube das Zalicus es zu einem Delisting kommen lässt......sollte die Käuferseite nicht in den nächsten Monaten zulegen bzw. pos. News eintrudeln......tippe ich persönlich auf einen R/S.....leider !
Grüße Earthfire

Zalicus Licensee, Sanofi, Provides Status Update on Prednisporin (FOV1101)
Sanofi to Continue Product Development Under a Third-Party Sublicense
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Zalicus Inc. (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain, today announced that Sanofi provided an update on the development status of Prednisporin™ (FOV1101) during the course of Sanofi’s quarterly financial and R&D pipeline update on October 25, 2012. Prednisporin is a fixed dose combination of prednisolone acetate and cyclosporine A being developed for certain ophthalmologic indications including persistent allergic conjunctivitis. Prednisporin was licensed by Zalicus to Fovea Pharmaceuticals SA (now a division of Sanofi) in January 2006.
Based on a recent review of prior Phase 2b results for Prednisporin, Sanofi has reassessed the commercial profile for Prednisporin and has made the decision to continue the development of Prednisporin under a sublicense agreement to be entered into with a third party to be identified by Sanofi. The terms of the Second Amended and Restated Research and License Agreement between Zalicus and Sanofi allows Sanofi to sublicense its rights to develop and commercialize Prednisporin on a global basis, and the milestones and royalties due to Zalicus for successful development and commercialization of Prednisporin would continue to apply in the event Sanofi sublicenses the rights to Prednisporin.
"We are pleased that Prednisporin remains in the Sanofi clinical development pipeline and anticipate that the proposed sublicense by Sanofi will allow for the continued clinical development and potential commercialization of Prednisporin," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus.
About Prednisporin (FOV1101)
Zalicus entered into a research and license agreement with Fovea Pharmaceuticals SA, now a division of Sanofi, in January 2006. Under the agreement, Sanofi received an exclusive worldwide license to Prednisporin (FOV1101) and agreed to fund the development of Prednisporin for certain ophthalmic diseases. Sanofi has advanced Prednisporin into Phase 2b clinical development for persistent allergic conjunctivitis. For Prednisporin, Zalicus has received payments totaling $1.5 million, and is eligible to receive up to $39 million in development and regulatory milestone payments. Zalicus is also eligible to receive royalties on net sales of Prednisporin by Sanofi or any future sublicensee.
About Zalicus
Zalicus Inc. (ZLCS) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain. Zalicus has a portfolio of proprietary clinical-stage product candidates targeting pain such as Z160 and Z944, and has entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. Zalicus applies its expertise in the discovery and development of selective ion channel modulators and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease. To learn more about Zalicus, please visit www.zalicus.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus, the product candidate Prednisporin (FOV1101), its potential, and Sanofi’s plans to sublicense it for further clinical development and commercialization. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus and Prednisporin (FOV1101) may be identified by words like "believe," "expect," "may," "will," "should," "seek," "plan" or "could" and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the clinical development of Prednisporin (FOV1101), the ability of Sanofi to successfully sublicense Prednisporin to a third party, and those other risks that can be found in the "Risk Factors" section of Zalicus' annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission. Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management’s current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
http://finance.yahoo.com/news/zalicus-licensee-sanofi-provid…
Sanofi to Continue Product Development Under a Third-Party Sublicense

CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Zalicus Inc. (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain, today announced that Sanofi provided an update on the development status of Prednisporin™ (FOV1101) during the course of Sanofi’s quarterly financial and R&D pipeline update on October 25, 2012. Prednisporin is a fixed dose combination of prednisolone acetate and cyclosporine A being developed for certain ophthalmologic indications including persistent allergic conjunctivitis. Prednisporin was licensed by Zalicus to Fovea Pharmaceuticals SA (now a division of Sanofi) in January 2006.
Based on a recent review of prior Phase 2b results for Prednisporin, Sanofi has reassessed the commercial profile for Prednisporin and has made the decision to continue the development of Prednisporin under a sublicense agreement to be entered into with a third party to be identified by Sanofi. The terms of the Second Amended and Restated Research and License Agreement between Zalicus and Sanofi allows Sanofi to sublicense its rights to develop and commercialize Prednisporin on a global basis, and the milestones and royalties due to Zalicus for successful development and commercialization of Prednisporin would continue to apply in the event Sanofi sublicenses the rights to Prednisporin.
"We are pleased that Prednisporin remains in the Sanofi clinical development pipeline and anticipate that the proposed sublicense by Sanofi will allow for the continued clinical development and potential commercialization of Prednisporin," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus.
About Prednisporin (FOV1101)
Zalicus entered into a research and license agreement with Fovea Pharmaceuticals SA, now a division of Sanofi, in January 2006. Under the agreement, Sanofi received an exclusive worldwide license to Prednisporin (FOV1101) and agreed to fund the development of Prednisporin for certain ophthalmic diseases. Sanofi has advanced Prednisporin into Phase 2b clinical development for persistent allergic conjunctivitis. For Prednisporin, Zalicus has received payments totaling $1.5 million, and is eligible to receive up to $39 million in development and regulatory milestone payments. Zalicus is also eligible to receive royalties on net sales of Prednisporin by Sanofi or any future sublicensee.
About Zalicus
Zalicus Inc. (ZLCS) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain. Zalicus has a portfolio of proprietary clinical-stage product candidates targeting pain such as Z160 and Z944, and has entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. Zalicus applies its expertise in the discovery and development of selective ion channel modulators and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease. To learn more about Zalicus, please visit www.zalicus.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus, the product candidate Prednisporin (FOV1101), its potential, and Sanofi’s plans to sublicense it for further clinical development and commercialization. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus and Prednisporin (FOV1101) may be identified by words like "believe," "expect," "may," "will," "should," "seek," "plan" or "could" and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the clinical development of Prednisporin (FOV1101), the ability of Sanofi to successfully sublicense Prednisporin to a third party, and those other risks that can be found in the "Risk Factors" section of Zalicus' annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission. Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management’s current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
http://finance.yahoo.com/news/zalicus-licensee-sanofi-provid…
Antwort auf Beitrag Nr.: 43.751.003 von bernie55 am 25.10.12 14:38:28der Kurs fällt immer weiter.
Habe den Wert nach wie vor auf der Watchlist und so langsam sollte der Boden erreicht sein.
Charttechnisch ist dieses noch nicht zu erkennen, schaue aber weiter gespannt auf die Entwicklung.

Habe den Wert nach wie vor auf der Watchlist und so langsam sollte der Boden erreicht sein.
Charttechnisch ist dieses noch nicht zu erkennen, schaue aber weiter gespannt auf die Entwicklung.

Antwort auf Beitrag Nr.: 43.801.683 von Earthfire am 08.11.12 14:03:32Unglaublich was Corrigan (der sogenannte Biotech Experte schlechthin) aus dieser Firma gemacht hat, bin völlig Fassungslos:O
Zalicus Inc.
Condensed Consolidated Statements of Comprehensive Loss
(in thousands, except share and per share amounts)
(Unaudited)
Three months ended September 30, Nine months ended September 30,
2012 2011 2012 2011
Revenue:
Collaborations and other $ 3,381 $ 2,300
$ 8,423 $ 5,042
Government contracts and grants 137 128
339 505
Total revenue 3,518 2,428 8,762 5,547
Operating expenses:
Research and development 12,050 8,871 32,493 25,678
General and administrative 2,201 2,563 7,172 8,030
Amortization of intangible 974 1,285 2,920 3,855
Restructuring (17 ) — 1,112 —
Total operating expenses 15,208 12,719 43,697 37,563
Loss from operations (11,690 ) (10,291 ) (34,935 ) (32,016 )
Interest income 35 33 120 100
Interest expense (532 ) (358 ) (1,686 ) (579 )
Other income 13 148 20 2
Net loss before benefit for income taxes (12,174 ) (10,468 ) (36,481 ) (32,493 )
Income tax benefit — 1,217 441 1,217
Net loss $ (12,174 ) $ (9,251 ) $ (36,040 ) $ (31,276 )
Net loss per share— basic and diluted
$ (0.10 ) $ (0.09 ) $ (0.32 ) $ (0.32 )
Weighted average number of common shares used in net loss per share calculation— basic and diluted
124,562,900 99,214,522 114,069,137 96,712,208
Comprehensive loss $ (12,171 ) $ (9,286 ) $ (36,022 ) $ (31,286 )
Zalicus Inc.
Condensed Consolidated Balance Sheets
(in thousands except per share data)
(Unaudited)
September 30,
2012 December 31,
2011
Assets
Current assets:
Cash and cash equivalents $ 8,971 $ 2,750
Restricted cash — 50
Short-term investments 34,265 45,124
Accounts receivable 3,098 1,886
Prepaid expenses and other current assets 1,039 1,397
Total current assets 47,373 51,207
Property and equipment, net 3,869 5,258
Intangible asset, net 18,627 21,546
Restricted cash and other assets 1,816 1,872
Total assets $ 71,685 $ 79,883
Liabilities and stockholders' equity
Current liabilities:
Accounts payable $ 2,191 $ 1,743
Accrued expenses and other current liabilities 6,090 6,133
Accrued restructuring 189 —
Deferred revenue 4,928 3,349
Current portion of term loan payable 6,137 4,035
Current portion of lease incentive obligation 284 284
Total current liabilities 19,819 15,544
Term loan payable, net of current portion 10,426 15,099
Deferred revenue, net of current portion 1,035 3,000
Deferred rent, net of current portion 494 605
Lease incentive obligation, net of current portion 945 1,159
Other long-term liabilities 28 563
Stockholders' equity:
Preferred stock, $0.001 par value; 5,000 shares authorized; no shares issued and outstanding — —
Common stock, $0.001 par value; 200,000 shares authorized; 126,932 and 99,239 shares issued and outstanding at September 30, 2012 and December 31, 2011, respectively 127 99
Additional paid-in capital 371,537 340,518
Accumulated other comprehensive income (loss) 10 (8 )
Accumulated deficit (332,736 ) (296,696 )
Stockholders' equity 38,938 43,913
Total liabilities and stockholders' equity $ 71,685 $ 79,883
Zalicus Inc.
Condensed Consolidated Statements of Comprehensive Loss
(in thousands, except share and per share amounts)
(Unaudited)
Three months ended September 30, Nine months ended September 30,
2012 2011 2012 2011
Revenue:
Collaborations and other $ 3,381 $ 2,300
$ 8,423 $ 5,042
Government contracts and grants 137 128
339 505
Total revenue 3,518 2,428 8,762 5,547
Operating expenses:
Research and development 12,050 8,871 32,493 25,678
General and administrative 2,201 2,563 7,172 8,030
Amortization of intangible 974 1,285 2,920 3,855
Restructuring (17 ) — 1,112 —
Total operating expenses 15,208 12,719 43,697 37,563
Loss from operations (11,690 ) (10,291 ) (34,935 ) (32,016 )
Interest income 35 33 120 100
Interest expense (532 ) (358 ) (1,686 ) (579 )
Other income 13 148 20 2
Net loss before benefit for income taxes (12,174 ) (10,468 ) (36,481 ) (32,493 )
Income tax benefit — 1,217 441 1,217
Net loss $ (12,174 ) $ (9,251 ) $ (36,040 ) $ (31,276 )
Net loss per share— basic and diluted
$ (0.10 ) $ (0.09 ) $ (0.32 ) $ (0.32 )
Weighted average number of common shares used in net loss per share calculation— basic and diluted
124,562,900 99,214,522 114,069,137 96,712,208
Comprehensive loss $ (12,171 ) $ (9,286 ) $ (36,022 ) $ (31,286 )
Zalicus Inc.
Condensed Consolidated Balance Sheets
(in thousands except per share data)
(Unaudited)
September 30,
2012 December 31,
2011
Assets
Current assets:
Cash and cash equivalents $ 8,971 $ 2,750
Restricted cash — 50
Short-term investments 34,265 45,124
Accounts receivable 3,098 1,886
Prepaid expenses and other current assets 1,039 1,397
Total current assets 47,373 51,207
Property and equipment, net 3,869 5,258
Intangible asset, net 18,627 21,546
Restricted cash and other assets 1,816 1,872
Total assets $ 71,685 $ 79,883
Liabilities and stockholders' equity
Current liabilities:
Accounts payable $ 2,191 $ 1,743
Accrued expenses and other current liabilities 6,090 6,133
Accrued restructuring 189 —
Deferred revenue 4,928 3,349
Current portion of term loan payable 6,137 4,035
Current portion of lease incentive obligation 284 284
Total current liabilities 19,819 15,544
Term loan payable, net of current portion 10,426 15,099
Deferred revenue, net of current portion 1,035 3,000
Deferred rent, net of current portion 494 605
Lease incentive obligation, net of current portion 945 1,159
Other long-term liabilities 28 563
Stockholders' equity:
Preferred stock, $0.001 par value; 5,000 shares authorized; no shares issued and outstanding — —
Common stock, $0.001 par value; 200,000 shares authorized; 126,932 and 99,239 shares issued and outstanding at September 30, 2012 and December 31, 2011, respectively 127 99
Additional paid-in capital 371,537 340,518
Accumulated other comprehensive income (loss) 10 (8 )
Accumulated deficit (332,736 ) (296,696 )
Stockholders' equity 38,938 43,913
Total liabilities and stockholders' equity $ 71,685 $ 79,883
Antwort auf Beitrag Nr.: 43.810.226 von Growth2012 am 10.11.12 17:25:42Third Quarter 2012 and Recent Accomplishments:
Pipeline Progress:
Advanced Z160, a first-in-class, oral, state dependent, selective N-type calcium channel (Cav 2.2) blocker into the first of two planned Phase 2a clinical trials for neuropathic pain. Patient enrollment in the first of these Phase 2a studies, in lumbosacral radiculopathy (LSR), a form of chronic lower back pain, began in the third quarter of 2012. The second Phase 2a clinical trial with Z160, in postherpetic neuralgia, is expected to begin enrolling patients in the fourth quarter of 2012.
Initiated a Phase 1 multiple ascending dose (MAD) clinical study with Z944, its novel, oral, T-type calcium channel blocker which has demonstrated efficacy in a number of preclinical inflammatory pain models and other disease models. The initiation of this MAD study follows the successfully completed Phase 1 single ascending dose study in which Z944 was determined to be generally well-tolerated and a maximum tolerated dose was achieved. T-type calcium channels have been recognized as key targets for therapeutic intervention in a broad range of cell functions and have been implicated in pain signaling. Assuming Z944 successfully completes the MAD study, Z944 could enter Phase 2 clinical development in the first quarter of 2013.
Third Quarter 2012 Financial Results (Unaudited):
As of September 30, 2012, we had cash, cash equivalents, restricted cash and short-term investments of approximately $45.0 million compared to $44.1 million on June 30, 2012.
For the quarter ended September 30, 2012, revenue was $3.5 million, compared to $2.4 million for the quarter ended September 30, 2011. The increase in revenue from the 2011 to the 2012 period was primarily due to increased Exalgo royalties from Covidien and increased collaborative revenue from Novartis. Zalicus recognized $1.4 million in royalty revenue from Covidien based on Exalgo sales for the quarter ended September 30, 2012. This represents an 11% increase in revenue from Exalgo compared to the quarter ended June 30, 2012.
For the quarter ended September 30, 2012, net loss was $12.2 million, or $0.10 per share, compared to a net loss of $9.3 million, or $0.09 per share, in the quarter ended September 30, 2011.
Research and development expenses were $12.1 million in the quarter ended September 30, 2012, compared to $8.9 million in the quarter ended September 30, 2011. The increase in R&D expense from the 2011 period to the 2012 period was primarily due to increased development expenses related to Z160, Synavive and Z944. Zalicus terminated all development activities related to Synavive in the quarter ended September 30, 2012 and as a result, we expect research and development expenses to decrease for the quarter ending December 31, 2012 and for the year ending December 31, 2013.
General and administrative expenses were $2.2 million in the quarter ended September 30, 2012, compared to $2.6 million for the quarter ended September 30, 2011. We expect general and administrative expenses for the quarter ending December 31, 2012 to be consistent with such expenses during the quarter ended September 30, 2012.
Pipeline Progress:
Advanced Z160, a first-in-class, oral, state dependent, selective N-type calcium channel (Cav 2.2) blocker into the first of two planned Phase 2a clinical trials for neuropathic pain. Patient enrollment in the first of these Phase 2a studies, in lumbosacral radiculopathy (LSR), a form of chronic lower back pain, began in the third quarter of 2012. The second Phase 2a clinical trial with Z160, in postherpetic neuralgia, is expected to begin enrolling patients in the fourth quarter of 2012.
Initiated a Phase 1 multiple ascending dose (MAD) clinical study with Z944, its novel, oral, T-type calcium channel blocker which has demonstrated efficacy in a number of preclinical inflammatory pain models and other disease models. The initiation of this MAD study follows the successfully completed Phase 1 single ascending dose study in which Z944 was determined to be generally well-tolerated and a maximum tolerated dose was achieved. T-type calcium channels have been recognized as key targets for therapeutic intervention in a broad range of cell functions and have been implicated in pain signaling. Assuming Z944 successfully completes the MAD study, Z944 could enter Phase 2 clinical development in the first quarter of 2013.
Third Quarter 2012 Financial Results (Unaudited):
As of September 30, 2012, we had cash, cash equivalents, restricted cash and short-term investments of approximately $45.0 million compared to $44.1 million on June 30, 2012.
For the quarter ended September 30, 2012, revenue was $3.5 million, compared to $2.4 million for the quarter ended September 30, 2011. The increase in revenue from the 2011 to the 2012 period was primarily due to increased Exalgo royalties from Covidien and increased collaborative revenue from Novartis. Zalicus recognized $1.4 million in royalty revenue from Covidien based on Exalgo sales for the quarter ended September 30, 2012. This represents an 11% increase in revenue from Exalgo compared to the quarter ended June 30, 2012.
For the quarter ended September 30, 2012, net loss was $12.2 million, or $0.10 per share, compared to a net loss of $9.3 million, or $0.09 per share, in the quarter ended September 30, 2011.
Research and development expenses were $12.1 million in the quarter ended September 30, 2012, compared to $8.9 million in the quarter ended September 30, 2011. The increase in R&D expense from the 2011 period to the 2012 period was primarily due to increased development expenses related to Z160, Synavive and Z944. Zalicus terminated all development activities related to Synavive in the quarter ended September 30, 2012 and as a result, we expect research and development expenses to decrease for the quarter ending December 31, 2012 and for the year ending December 31, 2013.
General and administrative expenses were $2.2 million in the quarter ended September 30, 2012, compared to $2.6 million for the quarter ended September 30, 2011. We expect general and administrative expenses for the quarter ending December 31, 2012 to be consistent with such expenses during the quarter ended September 30, 2012.
Tag zusammen!
habe soeben weitere 5K in Stuttgart zugelegt zu "unglaublichen 0,4050€", mal sehen ob der (so häufig erwartete) rebound dieser Woche endlich stattfinden mag
Zum Festhalten:-ZLCS heute (12.11.12)für 0,4050€ bzw. 0,48$ zu haben..
habe soeben weitere 5K in Stuttgart zugelegt zu "unglaublichen 0,4050€", mal sehen ob der (so häufig erwartete) rebound dieser Woche endlich stattfinden mag

Zum Festhalten:-ZLCS heute (12.11.12)für 0,4050€ bzw. 0,48$ zu haben..

Tages, sowie sechsmonatschart stateside.... 
 ein unfassbare verlauf seit mitte Sept!!!
ein unfassbare verlauf seit mitte Sept!!!


Mal sehen sagt der blinde

 ein unfassbare verlauf seit mitte Sept!!!
ein unfassbare verlauf seit mitte Sept!!!Mal sehen sagt der blinde

Antwort auf Beitrag Nr.: 43.813.620 von Growth2012 am 12.11.12 13:06:06die Charttechniker haben in dieser Aktie ein Paradebeispiel.
Der Kurs befindet sich in einem "sauberen" festen Abwärtstrend, ohne die kleinste Gegenreaktion.
Ein solcher Verfall wird durch die stark automatisierten Verkaufsorder leider noch verstärkt.
Sobald der Boden erreicht ist, sollte es aber auch schnell wieder nach oben gehen, es sei denn es kommen noch News, von denen die "breite Masse" bisher nichts gewusst hat.
(sollte es diese geben, dann macht ein stätiger abverkauf natürlic auch ohne automatisierte Verkaufsorder Sinn)

Der Kurs befindet sich in einem "sauberen" festen Abwärtstrend, ohne die kleinste Gegenreaktion.
Ein solcher Verfall wird durch die stark automatisierten Verkaufsorder leider noch verstärkt.
Sobald der Boden erreicht ist, sollte es aber auch schnell wieder nach oben gehen, es sei denn es kommen noch News, von denen die "breite Masse" bisher nichts gewusst hat.
(sollte es diese geben, dann macht ein stätiger abverkauf natürlic auch ohne automatisierte Verkaufsorder Sinn)

Antwort auf Beitrag Nr.: 43.816.525 von Poppholz am 13.11.12 08:06:45Stuttgart steht aktuell bei €0,33 zu €0,357.
Ich werde aber erst bei Bodenbildung einsteigen und warte erst einmal ab.
Ich werde aber erst bei Bodenbildung einsteigen und warte erst einmal ab.
Antwort auf Beitrag Nr.: 43.816.525 von Poppholz am 13.11.12 08:06:45Alles richtig erkannt-Respekt!...
Ein erste "leichte gegenwehr" ist heute @der Nasdaq zusehen gewesen
13.11.2012 19:06:57 0,4700 21.900
13.11.2012 19:06:57 0,4700 16.600
13.11.2012 19:06:57 0,4700 4.600
13.11.2012 19:06:57 0,4697 1.000
13.11.2012 19:06:57 0,4694 1.800
3.11.2012 19:06:57 0,4697 1.000
13.11.2012 19:03:40 0,4698 10.000
13.11.2012 19:01:06 0,4613 5.000
...aber leider noch nichts weltbewegendes bisher, mal sehenn wo wir stehen am späten Freiatgnachmittag
(no one wants to hold a loser over the weekend, and by the same token nobody wants to miss a re-bounder on its way back to normal trading )
)

Ein erste "leichte gegenwehr" ist heute @der Nasdaq zusehen gewesen
13.11.2012 19:06:57 0,4700 21.900
13.11.2012 19:06:57 0,4700 16.600
13.11.2012 19:06:57 0,4700 4.600
13.11.2012 19:06:57 0,4697 1.000
13.11.2012 19:06:57 0,4694 1.800
3.11.2012 19:06:57 0,4697 1.000
13.11.2012 19:03:40 0,4698 10.000
13.11.2012 19:01:06 0,4613 5.000
...aber leider noch nichts weltbewegendes bisher, mal sehenn wo wir stehen am späten Freiatgnachmittag

(no one wants to hold a loser over the weekend, and by the same token nobody wants to miss a re-bounder on its way back to normal trading
 )
)
Antwort auf Beitrag Nr.: 43.816.527 von Poppholz am 13.11.12 08:08:00weitere 3K in Stuttgart zu 0,381 eben geholt.
(tja, ich kann es einfach nicht lassen momentan, wegen die 'vermeintlich' verbilligte Einstiegskurse natürlich )
)
Werden sehen

(tja, ich kann es einfach nicht lassen momentan, wegen die 'vermeintlich' verbilligte Einstiegskurse natürlich
 )
)Werden sehen


Antwort auf Beitrag Nr.: 43.819.868 von Growth2012 am 13.11.12 19:41:48wünsche Dir viel Erfolg, bleibe aber erst einmal noch an der Seitenlinie.


16.11.12 18:33 Uhr
0,5226 USD
13,61% [0,0626]
Ein kleine trost zum Wochenende hin ...evtl. der Anfang 'from a long climb back up'?!?
...evtl. der Anfang 'from a long climb back up'?!?
Ein schönes we @der Runde
0,5226 USD
13,61% [0,0626]
Ein kleine trost zum Wochenende hin
 ...evtl. der Anfang 'from a long climb back up'?!?
...evtl. der Anfang 'from a long climb back up'?!?Ein schönes we @der Runde

Antwort auf Beitrag Nr.: 43.833.991 von Growth2012 am 16.11.12 18:54:51Ein close um die 0,55USD wäre extrem bullish für den Verlauf ab nächste Woche...

Zurzeit 0.5277+0.0677(14.72%) 1:56pmEST (and counting)

Zurzeit 0.5277+0.0677(14.72%) 1:56pmEST (and counting)
Moin zusammen!
ein sehr stabile Handel gewesen gestern Stateside, die Gewinne vom Freitag würden sehr gut behauptet, sogar mit leichten 6% plus beim 'final bell' abgeschlossen, natürlich rebound bedingt, aber auch deswegen u.a.

"Biotech Industry Experiencing More FDA New Drug Approvals in 2012 / Five Star Equities Provides Stock Research on ImmunoCellular Therapeutics and Zalicus"
http://www.finanznachrichten.de/nachrichten-2012-11/25220029…
More to come...
ein sehr stabile Handel gewesen gestern Stateside, die Gewinne vom Freitag würden sehr gut behauptet, sogar mit leichten 6% plus beim 'final bell' abgeschlossen, natürlich rebound bedingt, aber auch deswegen u.a.


"Biotech Industry Experiencing More FDA New Drug Approvals in 2012 / Five Star Equities Provides Stock Research on ImmunoCellular Therapeutics and Zalicus"
http://www.finanznachrichten.de/nachrichten-2012-11/25220029…
More to come...

Antwort auf Beitrag Nr.: 43.841.513 von Growth2012 am 20.11.12 07:33:29Moin Growth 2012, ja sieht zur Zeit sehr gut aus und ich bin rel. zuversichtlich das wir die Dollarmarke vor dem Stichtag am Nasdaq locker erreichen koennen.
Ich habe auch noch ein paar mal im Downtrend aufgestockt und bin nun für diesen Wert satt mit Shares
Die Frage ist, halten sich die Manipulatoren zurück dh. meiner Einschätzung und Beobachtung des täglichen Handels im Downtrend nach, wurde der Kurs nach unten manipuliert !
Da hat jemand wieder mal schön die Verunsicherung im Wert genutzt um billig zu sammeln.........der elektronische Handel hat dann seinen Rest dazu getan.
Für mich sich das rel. klar so aus, als wenn sie nun normal laufen koennte und wieder steigen darf !
Die nächsten Wochen werden es zeigen............mal sehen wo wir am Jahresande stehen ?
Gruß Earthfire
Ich habe auch noch ein paar mal im Downtrend aufgestockt und bin nun für diesen Wert satt mit Shares

Die Frage ist, halten sich die Manipulatoren zurück dh. meiner Einschätzung und Beobachtung des täglichen Handels im Downtrend nach, wurde der Kurs nach unten manipuliert !
Da hat jemand wieder mal schön die Verunsicherung im Wert genutzt um billig zu sammeln.........der elektronische Handel hat dann seinen Rest dazu getan.
Für mich sich das rel. klar so aus, als wenn sie nun normal laufen koennte und wieder steigen darf !
Die nächsten Wochen werden es zeigen............mal sehen wo wir am Jahresande stehen ?
Gruß Earthfire
Antwort auf Beitrag Nr.: 43.841.869 von Earthfire am 20.11.12 09:31:00N'abend Earthfire , tja, entwickelt sich prächtig wie ich finde (tradet recht beständig, ohne rückartige sprünge- die sowieso langfristig gesehen ungesund wären)
, tja, entwickelt sich prächtig wie ich finde (tradet recht beständig, ohne rückartige sprünge- die sowieso langfristig gesehen ungesund wären)
Ist der Gerücht um evtl. Pfizer Interesse hier schon angekommen
Na denn, schaut mal her...


As you can see, Lyrica provides a high percentage of income to Pfizer. I believe that the company will need to look for a substitute in the short term to ensure the market of Lyrica. One of my favorite candidates for Pfizer is Z160 to N-type calcium channel blocker for chronic pain. This novel compound is being developed by Zalicus (NASDAQ: ZLCS).
Z160 has demonstrated broad preclinical efficacy and clinical safety. This compound is effective in multiple neuropathic and inflammatory pain models. Zalicus showed that Z160 is well tolerated in prior Phase I and Phase IIa trials in approximately 200 subjects. Currently, Z160 is located in two Phase IIa clinical trials in neuropathic pain indications. Z160 has the potential for an Orphan Drug Designation with a clear regulatory path. This market is dominated by Lyrica (pregabalin), Neurontin (gabapetin), Cymbalta (duloxetine), and Lidoderm ( 5% lidocaine patch). These drugs are commonly used to treat all forms of neuropathy. The market of neuropathic pain is a $2 billion market and growing in the US only. For these reasons I think that Z160 will be a future candidate, which may be interesting for Pfizer.
http://beta.fool.com/traderusa/2012/11/20/pfizer-does-it-nee…
Also, Daumen drücken das es stimmen mag, Pfizer wurde ZLCS für 5$+ glatt aus der Kaffekasse bezahlen können... möge es so kommen!
möge es so kommen!
 , tja, entwickelt sich prächtig wie ich finde (tradet recht beständig, ohne rückartige sprünge- die sowieso langfristig gesehen ungesund wären)
, tja, entwickelt sich prächtig wie ich finde (tradet recht beständig, ohne rückartige sprünge- die sowieso langfristig gesehen ungesund wären) Ist der Gerücht um evtl. Pfizer Interesse hier schon angekommen

Na denn, schaut mal her...


As you can see, Lyrica provides a high percentage of income to Pfizer. I believe that the company will need to look for a substitute in the short term to ensure the market of Lyrica. One of my favorite candidates for Pfizer is Z160 to N-type calcium channel blocker for chronic pain. This novel compound is being developed by Zalicus (NASDAQ: ZLCS).
Z160 has demonstrated broad preclinical efficacy and clinical safety. This compound is effective in multiple neuropathic and inflammatory pain models. Zalicus showed that Z160 is well tolerated in prior Phase I and Phase IIa trials in approximately 200 subjects. Currently, Z160 is located in two Phase IIa clinical trials in neuropathic pain indications. Z160 has the potential for an Orphan Drug Designation with a clear regulatory path. This market is dominated by Lyrica (pregabalin), Neurontin (gabapetin), Cymbalta (duloxetine), and Lidoderm ( 5% lidocaine patch). These drugs are commonly used to treat all forms of neuropathy. The market of neuropathic pain is a $2 billion market and growing in the US only. For these reasons I think that Z160 will be a future candidate, which may be interesting for Pfizer.
http://beta.fool.com/traderusa/2012/11/20/pfizer-does-it-nee…
Also, Daumen drücken das es stimmen mag, Pfizer wurde ZLCS für 5$+ glatt aus der Kaffekasse bezahlen können...
 möge es so kommen!
möge es so kommen!
Starke schlussphase in ein eher schwache Gesamtmarktumgebung...
0.58+0.04(7.41%)3:59 PMEST
Kann ich sehr gut mit leben:- nordlich der 0,60$ Marke haben wir jeder menge Platz gen Norden

0.58+0.04(7.41%)3:59 PMEST
Kann ich sehr gut mit leben:- nordlich der 0,60$ Marke haben wir jeder menge Platz gen Norden


Fast zwei kompletten Handeltagen fehlen von der vergangene trading woche wegen 'Thanksgiving'.
Ich denke der neue Trend bei ZLCS sollte mann gut ablesen können ab kommende Mittwochnachmittag, insbesondere falls der Pfizer Gerücht sich bewahrheiten soll (langsam, aber dafür bestängier/stetige zukäufe unter die 1$ Marke )
)
Watch this space
Ich denke der neue Trend bei ZLCS sollte mann gut ablesen können ab kommende Mittwochnachmittag, insbesondere falls der Pfizer Gerücht sich bewahrheiten soll (langsam, aber dafür bestängier/stetige zukäufe unter die 1$ Marke
 )
) Watch this space

Aktuelle standing (Bulls vs Bears) >>>als kopierte Seeking Alpha Beitrag<<<<
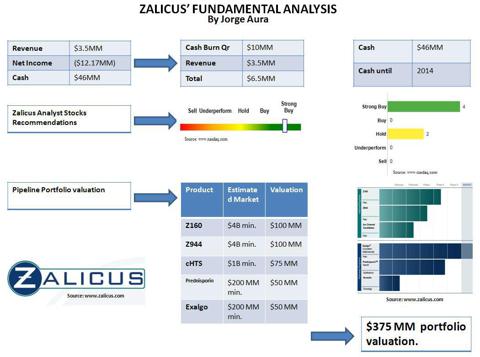
September: Bears
But until this latest financial quarter report, the bears were winning the battle in Zalicus. Its shares were sunk on September 10, 2012. The company announced that it would discontinue the development of Synavive, the promising development to relief the pain of those suffering from Rheumatoid Arthritis "RA". Zalicus' shareholders were disappointed because the company did not explain the reason why it did not continue with this development. The bears took advantage of this circumstance to create fear among the long-term shareholders and remind them the need to trade above $1 per share so as to prevent a future reverse split.
November: Bulls
However, something changed last Friday.(FRi 23.11.12) The shareholders were seeing an opportunity in Zalicus. If readers look at the company's files, they will see that a similar circumstance occurred in the year 2008.
Finanzen:
On November 8, 2012, Zalicus reported financial results for the third quarter ended on September 30, 2012. We saw that the income increased by 45.83 % to $3.5 million compared to $2.4 million of the same financial quarter in 2011. This increase in revenue from 2011 to 2012 was primarily due to increased Exalgo royalties from Covidien and increased collaborative revenue from Novartis. Zalicus recognized $1.4 million in royalty revenue from Covidien based on Exalgo sales for the quarter ended on September 30, 2012. This represents an 11% increase in revenue from Exalgo compared to the quarter ended on June 30, 2012. Furthermore, the collaboration with Novartis achieved revenues close to $2 million.
Risiken vs Vorteile:
On October 22, 2012, Zalicus received a notification from the Nasdaq listing qualifications department in which it is granted a 180 day period to reach the minimum of $1.00 per share requirement before being delisted from Nasdaq. Zalicus' failure in previous studies is another significant risk we must take into account. But we have to think that one of the two Phase II studies of the Z160 will be carried out in only 6 weeks and the results could be completed during the first quarter of 2013. Currently, Zalicus is carrying out the studies that obtained after the merger with Neuromed. Therefore, we cannot compare the previous failed studies about Synavive (contributed by Combinatorx) with current studies about Z160 and Z944.
Conclusion
Studies in progress, strong cash position, oncologic development collaboration with Novartis (platform cHTS), and new opportunities related to obtaining partners as well as the Z-160 results that will come at the end of the year or first quarter of 2013, make me clearly recommend Zalicus.
http://seekingalpha.com/article/1028631-zalicus-bears-and-bu…

September: Bears
But until this latest financial quarter report, the bears were winning the battle in Zalicus. Its shares were sunk on September 10, 2012. The company announced that it would discontinue the development of Synavive, the promising development to relief the pain of those suffering from Rheumatoid Arthritis "RA". Zalicus' shareholders were disappointed because the company did not explain the reason why it did not continue with this development. The bears took advantage of this circumstance to create fear among the long-term shareholders and remind them the need to trade above $1 per share so as to prevent a future reverse split.
November: Bulls
However, something changed last Friday.(FRi 23.11.12) The shareholders were seeing an opportunity in Zalicus. If readers look at the company's files, they will see that a similar circumstance occurred in the year 2008.
Finanzen:
On November 8, 2012, Zalicus reported financial results for the third quarter ended on September 30, 2012. We saw that the income increased by 45.83 % to $3.5 million compared to $2.4 million of the same financial quarter in 2011. This increase in revenue from 2011 to 2012 was primarily due to increased Exalgo royalties from Covidien and increased collaborative revenue from Novartis. Zalicus recognized $1.4 million in royalty revenue from Covidien based on Exalgo sales for the quarter ended on September 30, 2012. This represents an 11% increase in revenue from Exalgo compared to the quarter ended on June 30, 2012. Furthermore, the collaboration with Novartis achieved revenues close to $2 million.
Risiken vs Vorteile:
On October 22, 2012, Zalicus received a notification from the Nasdaq listing qualifications department in which it is granted a 180 day period to reach the minimum of $1.00 per share requirement before being delisted from Nasdaq. Zalicus' failure in previous studies is another significant risk we must take into account. But we have to think that one of the two Phase II studies of the Z160 will be carried out in only 6 weeks and the results could be completed during the first quarter of 2013. Currently, Zalicus is carrying out the studies that obtained after the merger with Neuromed. Therefore, we cannot compare the previous failed studies about Synavive (contributed by Combinatorx) with current studies about Z160 and Z944.
Conclusion
Studies in progress, strong cash position, oncologic development collaboration with Novartis (platform cHTS), and new opportunities related to obtaining partners as well as the Z-160 results that will come at the end of the year or first quarter of 2013, make me clearly recommend Zalicus.
http://seekingalpha.com/article/1028631-zalicus-bears-and-bu…
....


Zalicus’ Z160 for Chronic Neuropathic Pain Selected as a Top 10 Project to Watch
http://finance.yahoo.com/news/zalicus-z160-chronic-neuropath…
Schon um die 0,63 ( 0.6331 Up 0.0401(6.76%) 9 ) vor offizieler Eröffung stateside...



Zalicus’ Z160 for Chronic Neuropathic Pain Selected as a Top 10 Project to Watch
http://finance.yahoo.com/news/zalicus-z160-chronic-neuropath…
Schon um die 0,63 ( 0.6331 Up 0.0401(6.76%) 9 ) vor offizieler Eröffung stateside...

Zitat von Growth2012: ....
Zalicus’ Z160 for Chronic Neuropathic Pain Selected as a Top 10 Project to Watch
http://finance.yahoo.com/news/zalicus-z160-chronic-neuropath…
I believe that the company >Pfizer< will need to look for a substitute in the short term to ensure the market of Lyrica. One of my favorite candidates for Pfizer is Z160 to N-type calcium channel blocker for chronic pain. This novel compound is being developed by Zalicus
...also ich denke,ZLCS könnte jetzt so langsam durchstarten....

Nabend Bernie, Nabend Growth,
ich konnte es nicht lassen und habe mir vor dem Durchstarten noch ein paar k Shares geordert
Nun stehe ich vor meinem Break of even
Ich habe ganz schön geschwitzt mit den Nachkäufen ins fallende Messer.
Wenn ich überlege das meine ersten Positionen um 0,9€ ct lagen, scheint die Strategie aufzugehen.
Nun ist der Dollar wieder in greifbare Nähe gerrückt .
Hoffe weiterhin auf einen Erfolg bzw. eine schöne Übernahme !

Grüße Earthfire
ich konnte es nicht lassen und habe mir vor dem Durchstarten noch ein paar k Shares geordert

Nun stehe ich vor meinem Break of even
Ich habe ganz schön geschwitzt mit den Nachkäufen ins fallende Messer.
Wenn ich überlege das meine ersten Positionen um 0,9€ ct lagen, scheint die Strategie aufzugehen.
Nun ist der Dollar wieder in greifbare Nähe gerrückt .
Hoffe weiterhin auf einen Erfolg bzw. eine schöne Übernahme !


Grüße Earthfire
Antwort auf Beitrag Nr.: 43.867.749 von Earthfire am 27.11.12 19:55:34Hallo Earthfire,
deiner Aussage kann ich sehr gut nachvollziehen da gehört ein größere Portion 'kalkulierte Mut' dazu verbilligen zu wollen, mit ein stock der bereits enttäuscht hat, tja, wie heisst es so schön, den Mutigen gehört die Welt
da gehört ein größere Portion 'kalkulierte Mut' dazu verbilligen zu wollen, mit ein stock der bereits enttäuscht hat, tja, wie heisst es so schön, den Mutigen gehört die Welt
Realistisch betrachtet;- wenn wir nur von eine Wiederherstellung des alten handelsstandes ausgehen, steht uns ein anstieg von ca. 230% zuvor...(3$)
Mit die alten 3$ Platform könnte ich zurzeit wirklich sehr gut & berühight leben.
We will see, good trades & steady hands
deiner Aussage kann ich sehr gut nachvollziehen
 da gehört ein größere Portion 'kalkulierte Mut' dazu verbilligen zu wollen, mit ein stock der bereits enttäuscht hat, tja, wie heisst es so schön, den Mutigen gehört die Welt
da gehört ein größere Portion 'kalkulierte Mut' dazu verbilligen zu wollen, mit ein stock der bereits enttäuscht hat, tja, wie heisst es so schön, den Mutigen gehört die Welt
Realistisch betrachtet;- wenn wir nur von eine Wiederherstellung des alten handelsstandes ausgehen, steht uns ein anstieg von ca. 230% zuvor...(3$)
Mit die alten 3$ Platform könnte ich zurzeit wirklich sehr gut & berühight leben.
We will see, good trades & steady hands

Vertseht ihr dieses evtl.? (zwei renommierte links mit völlig verschiedene basis Angaben zu ZLCS)
Marketwatch:
http://www.marketwatch.com/investing/stock/zlcs/profile
CNBC (mit real time Quotes):
http://data.cnbc.com/quotes/zlcs
z.Bsp Marketwatch sagt aus im Bereich Overview das ZLCS ein MK von
>>>Market cap $75.31M<<< vorzuweisen hat, CNBC sagt das der MK bei >>>Market Cap 89.9M<<< liegen wurde.
Muss ich das verstehen?
P.S Comdirect sagt MK 89,91 Mio. USD, Stücke 126,99 Mio.
Marketwatch:
http://www.marketwatch.com/investing/stock/zlcs/profile
CNBC (mit real time Quotes):
http://data.cnbc.com/quotes/zlcs
z.Bsp Marketwatch sagt aus im Bereich Overview das ZLCS ein MK von
>>>Market cap $75.31M<<< vorzuweisen hat, CNBC sagt das der MK bei >>>Market Cap 89.9M<<< liegen wurde.
Muss ich das verstehen?
P.S Comdirect sagt MK 89,91 Mio. USD, Stücke 126,99 Mio.
Ist zwar von gestern, behält aber heute noch seine aktualität...(wohl geschrieben worden um die 0,67 Marke von gestern) 
Hot Stock: Zalicus, Shares Gain 12.6% (ZLCS)
Zalicus (NASDAQ:ZLCS) is one of today's best performing penny stocks, up 12.6% to $0.67 on 1.2x average daily volume. Thus far today, Zalicus has traded 2.1 million shares, vs. average volume of 1.8 million shares per day. The stock has outperformed the Dow (13.2% to the Dow's -0.1%) and outperformed the S&P 500 (13.2% to the S&P's 0.1%) during today's trading.
Potential upside of 330.5% exists for Zalicus, based on a current level of $0.67 and analysts' average consensus price target of $2.88. The stock should find initial resistance at its 50-day moving average (MA) of $0.85 and further resistance at its 200-day MA of $1.03.
Zalicus Inc is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. The Company's drug pipeline includes an arthritis formula in phase 2 development.
Zalicus share prices have moved between a 52-week high of $3.21 and a 52-week low of $0.38 and are now trading 77% above that low price at $0.67 per share. In the last five trading sessions, the 50-day moving average (MA) has fallen 4.6% while the 200-day MA has slid 0.8%.

Hot Stock: Zalicus, Shares Gain 12.6% (ZLCS)
Zalicus (NASDAQ:ZLCS) is one of today's best performing penny stocks, up 12.6% to $0.67 on 1.2x average daily volume. Thus far today, Zalicus has traded 2.1 million shares, vs. average volume of 1.8 million shares per day. The stock has outperformed the Dow (13.2% to the Dow's -0.1%) and outperformed the S&P 500 (13.2% to the S&P's 0.1%) during today's trading.
Potential upside of 330.5% exists for Zalicus, based on a current level of $0.67 and analysts' average consensus price target of $2.88. The stock should find initial resistance at its 50-day moving average (MA) of $0.85 and further resistance at its 200-day MA of $1.03.
Zalicus Inc is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. The Company's drug pipeline includes an arthritis formula in phase 2 development.
Zalicus share prices have moved between a 52-week high of $3.21 and a 52-week low of $0.38 and are now trading 77% above that low price at $0.67 per share. In the last five trading sessions, the 50-day moving average (MA) has fallen 4.6% while the 200-day MA has slid 0.8%.
...besser spät als nie....
...und eine netten Gruß in die Runde
Ich bin dann seit heute mit einem bescheidenem ersten Teil an Eurer Seite, also geht es morgen richtig up....oder noch 2 Wochen so lala bis down
Danke für Eure Beiträge und Links !!!
Alles Grüne denn für uns !!!
...und eine netten Gruß in die Runde

Ich bin dann seit heute mit einem bescheidenem ersten Teil an Eurer Seite, also geht es morgen richtig up....oder noch 2 Wochen so lala bis down

Danke für Eure Beiträge und Links !!!
Alles Grüne denn für uns !!!
wann kommt da eine entscheidung ?
danke für eine antwort !!!!!
danke für eine antwort !!!!!
Antwort auf Beitrag Nr.: 43.888.831 von Fortunato69 am 04.12.12 13:05:45Grüß Gott,
die Ergebnisse zur Z-160 Phase 2 "können" zum Ende des Jahres oder zum 1. Quartal 13 abgeschlossen sein...las ich eine Seite zuvor.
http://seekingalpha.com/article/1028631-zalicus-bears-and-bu…
+ was da noch so kommen könnte...
toitoitoi
die Ergebnisse zur Z-160 Phase 2 "können" zum Ende des Jahres oder zum 1. Quartal 13 abgeschlossen sein...las ich eine Seite zuvor.
http://seekingalpha.com/article/1028631-zalicus-bears-and-bu…
+ was da noch so kommen könnte...
toitoitoi
Hmmmm...die zweite anstehende Konferenzankündigung binnen wenigen tagen...
http://finance.yahoo.com/news/zalicus-present-oppenheimer-23…
...naja, Zalicus (ZLCS) hat wahrscheinlich irgendetwas "Berichtungs-würdiges" auf Lager demnächst


http://finance.yahoo.com/news/zalicus-present-oppenheimer-23…
...naja, Zalicus (ZLCS) hat wahrscheinlich irgendetwas "Berichtungs-würdiges" auf Lager demnächst



Antwort auf Beitrag Nr.: 43.902.468 von Growth2012 am 07.12.12 13:23:20...aber der Waldorf Astoria (muss das wirklich sein???)
Werde intensiv auf die Kosten/Nützungs effekte entsperechend achten

Werde intensiv auf die Kosten/Nützungs effekte entsperechend achten


ZALICUS "Dashboard" view
http://ycharts.com/companies/ZLCS/dashboard
Ein jahresend Landung zwischen 0,80-0,92$ wäre die perfekte Ausgangslage/Platform für eine möglich rasanter Anstieg im ersten Quartal 2013.
(Das ganze übrigens ohne weitere kooperations Meldungen, Pipeline forstschritte, sowie weitere Übernahmengerüchten die eventuell noch zerstreut werden vor Anfang 2013 )
)
Zwei Konferenzen stehen an, ich rechne stark mit mindestens "one big message" im Bezug auf die ION Entwicklung, watch this space @alle freunde der US Biostock-market

(Zum Festhalten:-gestern für knapp unter die 0,70$ zu haben gewesen)
http://ycharts.com/companies/ZLCS/dashboard
Ein jahresend Landung zwischen 0,80-0,92$ wäre die perfekte Ausgangslage/Platform für eine möglich rasanter Anstieg im ersten Quartal 2013.
(Das ganze übrigens ohne weitere kooperations Meldungen, Pipeline forstschritte, sowie weitere Übernahmengerüchten die eventuell noch zerstreut werden vor Anfang 2013
 )
)Zwei Konferenzen stehen an, ich rechne stark mit mindestens "one big message" im Bezug auf die ION Entwicklung, watch this space @alle freunde der US Biostock-market


(Zum Festhalten:-gestern für knapp unter die 0,70$ zu haben gewesen)
Nett, ganz starke verlauf heute...natürlich alles nur meine bescheidene Meinung und die von meiner Schwiegermutter

Antwort auf Beitrag Nr.: 43.920.580 von Growth2012 am 12.12.12 16:48:12
Yahoos chart is somehow "sexier"...

Yahoos chart is somehow "sexier"...


...Ich wollte schon die Tage schreiben ( obwohl ich gar nicht nervös bin hier  ) ob denn jemand etwas von der Konferenz gehört/gelesen hat...
) ob denn jemand etwas von der Konferenz gehört/gelesen hat...
Man liest nix, man hört nix, kein Webcast auf der Zalicus Seite...selbst auf ihub bescheidenes Schweigen...
...bis gestern....after hours in US 13% auf 0,68...man spekuliert auf News die nächste Woche
Wünsche uns das Beste und in jedem Fall erstmal einen schönen 3. Advent !!!
 ) ob denn jemand etwas von der Konferenz gehört/gelesen hat...
) ob denn jemand etwas von der Konferenz gehört/gelesen hat...Man liest nix, man hört nix, kein Webcast auf der Zalicus Seite...selbst auf ihub bescheidenes Schweigen...
...bis gestern....after hours in US 13% auf 0,68...man spekuliert auf News die nächste Woche

Wünsche uns das Beste und in jedem Fall erstmal einen schönen 3. Advent !!!
Antwort auf Beitrag Nr.: 43.932.376 von Nordenstam am 16.12.12 11:23:45http://www.marketwatch.com/investing/stock/zlcs
Zitat von Nordenstam: ...Ich wollte schon die Tage schreiben ( obwohl ich gar nicht nervös bin hier) ob denn jemand etwas von der Konferenz gehört/gelesen hat...
Man liest nix, man hört nix, kein Webcast auf der Zalicus Seite...selbst auf ihub bescheidenes Schweigen...
...bis gestern....after hours in US 13% auf 0,68...man spekuliert auf News die nächste Woche
Wünsche uns das Beste und in jedem Fall erstmal einen schönen 3. Advent !!!
Hi Nordenstam,
bei dem Volumen sagt der Afterhour nichts aus 1,450 St
DH den kann man fast ignorieren.
Aber wer sich die Trades der vergangen Tage genau anschaut, der sieht das der Kurs mit kleinen Positionen ins Minus getrieben wird, und die Käufe weisen zum Teil eine 10-20 fach höhere Stückzahl auf....
Sagt mir da wird weiter verunsichert und geschickt gesammelt.....nach wie vor.
Ich schließe mich Growth Meinung hier an.....dh. das geringste positive wird hier einen mächtigen Nordschub bewirken.....da sind auch 100% Plus in 1-3 Handelstagen durchaus realistisch.
Und von der Conference habe ich bis dato auch nichts gefunden...
Evtl. droht eine Übernahme.....wer weiß.
Schönen Sonntag noch
Gruß Earthfire
Hallo ihr alle!
Ich bin mehrere Monate in Zalicus. Ich denke, es ist eine einmalige Gelegenheit, auf dem Markt. Zalicus muss mehr wert sein als $ 4 im Jahr 2013.
Über die Konferenz fand ich dies:
http://seekingalpha.com/instablog/1342291-jorge-aura/1365231…
Ich werde auch weiterhin zu akkumulieren. Das Unternehmen wird über $ 1 innerhalb von zwei Wochen.
Grüße an Alle.
Matrixhauer69
Ich bin mehrere Monate in Zalicus. Ich denke, es ist eine einmalige Gelegenheit, auf dem Markt. Zalicus muss mehr wert sein als $ 4 im Jahr 2013.
Über die Konferenz fand ich dies:
http://seekingalpha.com/instablog/1342291-jorge-aura/1365231…
Ich werde auch weiterhin zu akkumulieren. Das Unternehmen wird über $ 1 innerhalb von zwei Wochen.
Grüße an Alle.
Matrixhauer69
Hallo alle:
Dieser Beitrag zeigt, dass Zalicus viele wichtige Katalysatoren hat für das Jahr 2013. Diese Firma ist sehr unterbewertet und sollte mehr wert sein als $ 4 pro Aktie. Zalicus verfügt über 90 laufenden Studien mit der Schweizer Novartis.
Ich mag einige Kommentare aus dem folgenden Beitrag extrahiert hinzu:
http://finance.yahoo.com/mbview/threadview/;_ylt=Ao4oi6iRbmq…
Nur ein paar, die haben vor kurzem aktualisiert und sind ziemlich neu, da die Zusammenarbeit begonnen. Sieht aus wie der Novartis Partnerschaft ist lebendig und gut. Zu viele, um sie hier aufzulisten. Die Liste geht weiter und weiter ......
Gerade in klinischen Studien (dot) Regie schauen und du wirst ein Boot Last der Kombination Arzneimittelstudien für Krebs und es Genotypen zu finden. Dies sicherlich Leitungen bis zu dem, was Mr. Corrigan genannten auf der ASCO-NCI-EORTC Molekulare Marker Treffen.
Noch nicht der Eintragung öffnen
6/2012- A Phase IIIB, Multi-Center, Open-Label-Studie für postmenopausale Frauen mit Östrogenrezeptor-positivem lokal fortgeschrittenem oder metastasiertem Brustkrebs mit Everolimus (RAD001) in Kombination mit Exemestan behandelt: 4EVER
Derzeit Recruiting-
4/2011- einer Phase Ib, Open-Label, Multi-Center, Dosis-Eskalation und Expansion Study einer oral verabreichten Kombination von BEZ235 Plus-MEK162 bei erwachsenen Patienten mit ausgewählten fortgeschrittenen soliden Tumoren
10/2010- A Phase IA, multizentrische, Open-label Dose Escalation Study of Oral BYL719, bei erwachsenen Patienten mit fortgeschrittenen soliden Tumoren, deren Tumor eine Änderung des PIK3CA Gene
5/2012- einer Phase I Studie des LJM716 bei Patienten mit Plattenepithelkarzinom des Kopf-Hals-oder HER2 überexprimierenden metastasierendem Brustkrebs oder Magenkrebs
9/2011-An Open-Label, Multi-Center Phase I-Dosisfindungsstudie der RAD001 (Everolimus, Afinitor ®) in Kombination mit BEZ235 bei Patienten mit fortgeschrittenen soliden Tumoren
6/2010-A Phase Ib, Open-Label, Multi-Center, Dosis-Eskalations-Studie der Oral BKM120 in Kombination mit oralen GSK1120212 bei erwachsenen Patienten mit ausgewählten fortgeschrittenen soliden Tumoren.
5/2012-A Multicenter, Open-Label-, Dosis Escalation, Phase I des LJM716 intravenös in Kombination mit Trastuzumab bei Patientinnen mit HER2 überexprimierenden metastasierendem Brustkrebs verabreicht Studie
Man, was eine Pipeline zur Kombination Arzneimittelstudien, die Liste geht auf eine auf .....
ganz wenige haben Ergebnisse in der ersten Hälfte des Jahres 2013 -
10/2012-A Phase lb / II Multi-center, Open-label, Dosis-Eskalations-Studie der LGX818 und Cetuximab oder LGX818, BYL719 und Cetuximab bei Patienten mit BRAF Mutant metastasiertem Darmkrebs
10/2012-An Open-label, Phase II, Single-Arm-Studie von Everolimus in Kombination mit Letrozol in der Behandlung von postmenopausalen Frauen mit Östrogenrezeptor-positivem metastasierendem Brustkrebs Cancer4/2012- Phase Ib, Dose Escalation Study of Oral LDE225 in Kombination mit BKM120 bei Patienten mit fortgeschrittenen soliden Tumoren
5/2011- einer Phase Ib, Open-Label, Multi-Center, Dosis-Eskalation und Expansion Study einer oral verabreichten Kombination von BKM120 Plus-MEK162 bei erwachsenen Patienten mit ausgewählten fortgeschrittenen soliden Tumoren
1/2012- eine multizentrische, randomisierte, doppelblinde, Placebo-kontrollierte, Phase II Studie zur Evaluierung der Sicherheit und Wirksamkeit von Dovitinib Mit Fulvestrant Kombiniert bei postmenopausalen Patientinnen mit HER2-und HR + Brustkrebs, Evidence of Disease Progression haben, die am oder nach vorheriger Endocrine Therapie
4/2012- A Randomized, Double-blind, Placebo-kontrollierte, Phase II-Studie von BKM120 plus Paclitaxel bei Patienten mit HER2 Negative inoperablem lokal fortgeschrittenem oder metastasiertem Brustkrebs, mit oder ohne PI3K

Dieser Beitrag zeigt, dass Zalicus viele wichtige Katalysatoren hat für das Jahr 2013. Diese Firma ist sehr unterbewertet und sollte mehr wert sein als $ 4 pro Aktie. Zalicus verfügt über 90 laufenden Studien mit der Schweizer Novartis.
Ich mag einige Kommentare aus dem folgenden Beitrag extrahiert hinzu:
http://finance.yahoo.com/mbview/threadview/;_ylt=Ao4oi6iRbmq…
Nur ein paar, die haben vor kurzem aktualisiert und sind ziemlich neu, da die Zusammenarbeit begonnen. Sieht aus wie der Novartis Partnerschaft ist lebendig und gut. Zu viele, um sie hier aufzulisten. Die Liste geht weiter und weiter ......
Gerade in klinischen Studien (dot) Regie schauen und du wirst ein Boot Last der Kombination Arzneimittelstudien für Krebs und es Genotypen zu finden. Dies sicherlich Leitungen bis zu dem, was Mr. Corrigan genannten auf der ASCO-NCI-EORTC Molekulare Marker Treffen.
Noch nicht der Eintragung öffnen
6/2012- A Phase IIIB, Multi-Center, Open-Label-Studie für postmenopausale Frauen mit Östrogenrezeptor-positivem lokal fortgeschrittenem oder metastasiertem Brustkrebs mit Everolimus (RAD001) in Kombination mit Exemestan behandelt: 4EVER
Derzeit Recruiting-
4/2011- einer Phase Ib, Open-Label, Multi-Center, Dosis-Eskalation und Expansion Study einer oral verabreichten Kombination von BEZ235 Plus-MEK162 bei erwachsenen Patienten mit ausgewählten fortgeschrittenen soliden Tumoren
10/2010- A Phase IA, multizentrische, Open-label Dose Escalation Study of Oral BYL719, bei erwachsenen Patienten mit fortgeschrittenen soliden Tumoren, deren Tumor eine Änderung des PIK3CA Gene
5/2012- einer Phase I Studie des LJM716 bei Patienten mit Plattenepithelkarzinom des Kopf-Hals-oder HER2 überexprimierenden metastasierendem Brustkrebs oder Magenkrebs
9/2011-An Open-Label, Multi-Center Phase I-Dosisfindungsstudie der RAD001 (Everolimus, Afinitor ®) in Kombination mit BEZ235 bei Patienten mit fortgeschrittenen soliden Tumoren
6/2010-A Phase Ib, Open-Label, Multi-Center, Dosis-Eskalations-Studie der Oral BKM120 in Kombination mit oralen GSK1120212 bei erwachsenen Patienten mit ausgewählten fortgeschrittenen soliden Tumoren.
5/2012-A Multicenter, Open-Label-, Dosis Escalation, Phase I des LJM716 intravenös in Kombination mit Trastuzumab bei Patientinnen mit HER2 überexprimierenden metastasierendem Brustkrebs verabreicht Studie
Man, was eine Pipeline zur Kombination Arzneimittelstudien, die Liste geht auf eine auf .....
ganz wenige haben Ergebnisse in der ersten Hälfte des Jahres 2013 -
10/2012-A Phase lb / II Multi-center, Open-label, Dosis-Eskalations-Studie der LGX818 und Cetuximab oder LGX818, BYL719 und Cetuximab bei Patienten mit BRAF Mutant metastasiertem Darmkrebs
10/2012-An Open-label, Phase II, Single-Arm-Studie von Everolimus in Kombination mit Letrozol in der Behandlung von postmenopausalen Frauen mit Östrogenrezeptor-positivem metastasierendem Brustkrebs Cancer4/2012- Phase Ib, Dose Escalation Study of Oral LDE225 in Kombination mit BKM120 bei Patienten mit fortgeschrittenen soliden Tumoren
5/2011- einer Phase Ib, Open-Label, Multi-Center, Dosis-Eskalation und Expansion Study einer oral verabreichten Kombination von BKM120 Plus-MEK162 bei erwachsenen Patienten mit ausgewählten fortgeschrittenen soliden Tumoren
1/2012- eine multizentrische, randomisierte, doppelblinde, Placebo-kontrollierte, Phase II Studie zur Evaluierung der Sicherheit und Wirksamkeit von Dovitinib Mit Fulvestrant Kombiniert bei postmenopausalen Patientinnen mit HER2-und HR + Brustkrebs, Evidence of Disease Progression haben, die am oder nach vorheriger Endocrine Therapie
4/2012- A Randomized, Double-blind, Placebo-kontrollierte, Phase II-Studie von BKM120 plus Paclitaxel bei Patienten mit HER2 Negative inoperablem lokal fortgeschrittenem oder metastasiertem Brustkrebs, mit oder ohne PI3K

Stock to Watch: Zalicus Up 7.7% (ZLCS)
Written on Fri, 12/21/2012 - 1:50pm
By David Diaz
Zalicus (NASDAQ:ZLCS) is one of today's best performing penny stocks, up 7.7% to $0.70 on 1.3x average daily volume. Thus far today, Zalicus has traded 1.6 million shares, vs. average volume of 1.2 million shares per day. The stock has outperformed the Dow (7.8% to the Dow's -1.3%) and outperformed the S&P 500 (7.8% to the S&P's -1.4%) during today's trading.
There is potential upside of 149.9% for shares of Zalicus based on a current price of $0.70 and an average consensus analyst price target of $1.75. Zalicus shares should encounter resistance at the 200-day moving average (MA) of $0.96 and support at the 50-day MA of $0.60.
In the past 52 weeks, shares of Zalicus have traded between a low of $0.38 and a high of $3.21 and are now at $0.70, which is 86% above that low price. In the last five trading sessions, the 50-day moving average (MA) has fallen 1.8% while the 200-day MA has slid 1%.
Zalicus Inc is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. The Company's drug pipeline includes an arthritis formula in phase 2 development.
SmarTrend is tracking the current trend status for Zalicus and will alert subscribers who have ZLCS in their portfolio or watchlist when shares have changed trend direction.
http://www.mysmartrend.com/news-briefs/technical-analysis/st…
Meine persönliche Prognose sieht folgendermaßen aus, ende Q1 2013 liegen d wir nördlich die 2$ Marke, zu mitte des Jahres (ende Q2) um die 3$ rum, und zum Jahresende 13 irgendwo zwischen 5$-7$
(Das ganze wohl bemerkt ohne feindliche Übernahme, ansonsten muss ich mit neu geschärften Bleistift nochmal an der jetzige Prognose beherzt wieder rangehen

 )
)
Frohes Fest ihr lieben & nicht vergessen:- steady hands, good trades & bessere nerven zahlen sich FAST IMMER langfrisitg aus...
Written on Fri, 12/21/2012 - 1:50pm
By David Diaz
Zalicus (NASDAQ:ZLCS) is one of today's best performing penny stocks, up 7.7% to $0.70 on 1.3x average daily volume. Thus far today, Zalicus has traded 1.6 million shares, vs. average volume of 1.2 million shares per day. The stock has outperformed the Dow (7.8% to the Dow's -1.3%) and outperformed the S&P 500 (7.8% to the S&P's -1.4%) during today's trading.
There is potential upside of 149.9% for shares of Zalicus based on a current price of $0.70 and an average consensus analyst price target of $1.75. Zalicus shares should encounter resistance at the 200-day moving average (MA) of $0.96 and support at the 50-day MA of $0.60.
In the past 52 weeks, shares of Zalicus have traded between a low of $0.38 and a high of $3.21 and are now at $0.70, which is 86% above that low price. In the last five trading sessions, the 50-day moving average (MA) has fallen 1.8% while the 200-day MA has slid 1%.
Zalicus Inc is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. The Company's drug pipeline includes an arthritis formula in phase 2 development.
SmarTrend is tracking the current trend status for Zalicus and will alert subscribers who have ZLCS in their portfolio or watchlist when shares have changed trend direction.
http://www.mysmartrend.com/news-briefs/technical-analysis/st…
Meine persönliche Prognose sieht folgendermaßen aus, ende Q1 2013 liegen d wir nördlich die 2$ Marke, zu mitte des Jahres (ende Q2) um die 3$ rum, und zum Jahresende 13 irgendwo zwischen 5$-7$
(Das ganze wohl bemerkt ohne feindliche Übernahme, ansonsten muss ich mit neu geschärften Bleistift nochmal an der jetzige Prognose beherzt wieder rangehen


 )
)Frohes Fest ihr lieben & nicht vergessen:- steady hands, good trades & bessere nerven zahlen sich FAST IMMER langfrisitg aus...

Hallo Zalicus Aktionäre  ,
,
Finally, Zalicus Inc. has been working in it for weeks, but put the finishing touches on it today. What's that? The reversal that's been underway since mid-November shortly after the stock made a hard landing. ZLCS has been rallying since then, though hit a wall a couple of weeks ago. Thanks to today's move though, it's back above the 20-day moving average line, and appears to have renewed life.
That being said, there's one more thing ZLCS needs to do to seal the deal...it needs to move above the ceiling at $0.73, which was the contention point in late November or early December. If Zalicus can make it past that hurdle, this bowl-shaped rebound effort will be complete and will have built a technical base in front of it (which was a missing ingredient until now).
Quelle mit Chart :
http://www.smallcapnetwork.com/Two-Out-of-Three-Isnt-Bad-CRD…
Die 0,73$ hatte ich bereits selber endeckt
Der gestrige Handelsverlauf stimmt mich positiv auf einen weiteren bullischen Verlauf in den nächsten Wochen......das Volumen zieht auch langsam aber sicher an......und das unter Plus.
Somit wäre die Basis geschaffen den Sprung über den Dollar bald zu vollziehen !!!
Ich schaue mir täglich im Intradayhandel die Einzeltrades an.....auch gestern war wieder ein regelmäßiges sammeln nach drücken des Kurses mit kleinen Positionen zu beobachten.
Nur was ich auch nicht weiss ist, ob es sich bei den größeren Positionen um eine Sammelorder des MM handelt.
Sei es drum...................ich gehe von einem guten Jahr 2013 bei Zalicus aus
Ich wünsche allen schöne , entspannte und börsenfreie Feiertage
Grüße Earthfire
 ,
,Finally, Zalicus Inc. has been working in it for weeks, but put the finishing touches on it today. What's that? The reversal that's been underway since mid-November shortly after the stock made a hard landing. ZLCS has been rallying since then, though hit a wall a couple of weeks ago. Thanks to today's move though, it's back above the 20-day moving average line, and appears to have renewed life.
That being said, there's one more thing ZLCS needs to do to seal the deal...it needs to move above the ceiling at $0.73, which was the contention point in late November or early December. If Zalicus can make it past that hurdle, this bowl-shaped rebound effort will be complete and will have built a technical base in front of it (which was a missing ingredient until now).
Quelle mit Chart :
http://www.smallcapnetwork.com/Two-Out-of-Three-Isnt-Bad-CRD…
Die 0,73$ hatte ich bereits selber endeckt

Der gestrige Handelsverlauf stimmt mich positiv auf einen weiteren bullischen Verlauf in den nächsten Wochen......das Volumen zieht auch langsam aber sicher an......und das unter Plus.
Somit wäre die Basis geschaffen den Sprung über den Dollar bald zu vollziehen !!!
Ich schaue mir täglich im Intradayhandel die Einzeltrades an.....auch gestern war wieder ein regelmäßiges sammeln nach drücken des Kurses mit kleinen Positionen zu beobachten.
Nur was ich auch nicht weiss ist, ob es sich bei den größeren Positionen um eine Sammelorder des MM handelt.
Sei es drum...................ich gehe von einem guten Jahr 2013 bei Zalicus aus

Ich wünsche allen schöne , entspannte und börsenfreie Feiertage

Grüße Earthfire
Posting auf Yahoo im Bezug auf's Technische Chartentwicklung zum Jahresende...
"Near the breakout zone, look for break above 0.74 to enter longs. Looking at the chart technicals are improving. The MACD gave a buy signal on Friday, daily stochastics and RSI have turned up."
...das tifft die sache wohl ins Mark


"Near the breakout zone, look for break above 0.74 to enter longs. Looking at the chart technicals are improving. The MACD gave a buy signal on Friday, daily stochastics and RSI have turned up."
...das tifft die sache wohl ins Mark


"Innovation – Opportunity – Collaboration
Biotech Showcase™ is an investor and partnering conference devoted to providing private and public biotechnology and life sciences companies with an opportunity to present to, and meet with, investors and pharmaceutical executives in one place during the course of one of the industry's largest annual healthcare investor conferences. Investors and biopharmaceutical executives from around the world gather in San Francisco during this critical week which is widely viewed as setting the tone for the coming year."
Event Details
Title Zalicus presents at Biotech Showcase™ 2013
Date and Time Monday, January 7, 2013 9:30 a.m. PT
Location Parc 55 Wyndham
San Francisco, CA
...stay tuned

Biotech Showcase™ is an investor and partnering conference devoted to providing private and public biotechnology and life sciences companies with an opportunity to present to, and meet with, investors and pharmaceutical executives in one place during the course of one of the industry's largest annual healthcare investor conferences. Investors and biopharmaceutical executives from around the world gather in San Francisco during this critical week which is widely viewed as setting the tone for the coming year."
Event Details
Title Zalicus presents at Biotech Showcase™ 2013
Date and Time Monday, January 7, 2013 9:30 a.m. PT
Location Parc 55 Wyndham
San Francisco, CA
...stay tuned


@die US smallcap Biotech Branche:-ab heute hätten wir Europäern gerne (täglich) Kurssteigerungen um die 10% gen norden wenn's irgendwie geht...

0,51
+27,50 %
+0,110
Tja, der heutige 27,5% Tagesanstieg gibt den Ton an(allerdings verfälscht durch gefrorene Preisfestellungen seitens der Deutsche Börse-sowas gehört eigentlich verboten wie ich finde

(habe trotzalldem wieder 3k in Stuttgart geholt um "letztmalig abzurunden" bevor es hier endlich mal losgeht )
)


0,51
+27,50 %
+0,110
Tja, der heutige 27,5% Tagesanstieg gibt den Ton an(allerdings verfälscht durch gefrorene Preisfestellungen seitens der Deutsche Börse-sowas gehört eigentlich verboten wie ich finde


(habe trotzalldem wieder 3k in Stuttgart geholt um "letztmalig abzurunden" bevor es hier endlich mal losgeht
 )
)  Zalicus Initiates the Second of Two Phase 2a Studies with Z160
Zalicus Initiates the Second of Two Phase 2a Studies with Z160 
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Zalicus Inc. (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain, today announced that it has initiated the second of two Phase 2a clinical studies with Z160, its first-in-class, oral, state-dependent, selective N-type (Cav2.2) calcium channel blocker for the potential treatment of chronic neuropathic pain. The Company also provided an overview of 2012 accomplishments.
Z160 is designed to selectively target neuronal pain signaling by modulating neurons that are undergoing high-frequency firing. Z160 has demonstrated efficacy in several animal models of neuropathic pain, and clinical trials in over 200 subjects have established Z160 as a safe and well tolerated drug candidate.
The second Phase 2a study with Z160 is enrolling subjects with Postherpetic Neuralgia (PHN), a chronic neuropathic pain state resulting from an outbreak of the herpes zoster virus, otherwise known as shingles. Due to this prolonged neuropathic pain, PHN is an industry-accepted standard condition for establishing clinical proof-of-concept for pharmaceutical product candidates seeking to address neuropathic pain. The 6-week, double-blind, multi-center, randomized, placebo-controlled study is expected to enroll approximately 140 subjects and will be conducted in approximately 35 centers throughout the United States. The primary objective of the trial is to evaluate the efficacy of Z160 compared to placebo in reducing pain in subjects with PHN as measured by the change in average weekly pain score from baseline to week 6 of treatment based on a daily 11-point Pain Intensity Numeral Rating Scale (PI-NRS).
"Postherpetic Neuralgia is an important medical condition for evaluating the activity of Z160 for three important reasons. First, it is a well-recognized standard for establishing clinical proof of concept in neuropathic pain; second, with a prevalence of less than 200,000 patients in the U.S., it has the potential for orphan drug status and could be a feasible first indication to pursue from a commercial perspective; and third, significant unmet medical need exists for novel, targeted and more efficacious chronic neuropathic pain therapies with improved safety and tolerability profiles such as Z160,” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus.
The first Phase 2a clinical study with Z160, which began enrolling patients in August of 2012, is evaluating the activity of Z160 in subjects with pain associated with Lumbosacral Radiculopathy, a chronic neuropathic pain condition resulting from the compression or irritation of the nerve roots exiting the lumbar region of the spine.
2012 Accomplishments:
Z160. Advanced Z160, a first-in-class, oral, state dependent, selective N-type calcium channel (Cav 2.2) blocker into two Phase 2a clinical trials for neuropathic pain including lumbosacral radiculopathy (LSR) which began in the third quarter of 2012 and postherpetic neuralgia which began in the fourth quarter of 2012. Top line data from both studies are expected to be available late in the second half of 2013.
Z944. Completed Phase 1 single and multiple ascending dose clinical studies with Z944 and are consulting with regulatory authorities on the clinical path forward. Z944 is a novel, oral, T-type calcium channel blocker which has demonstrated efficacy in a number of preclinical inflammatory pain models and other disease models. T-type calcium channels have been recognized as key targets for therapeutic intervention in a broad range of cell functions and have been implicated in pain signaling.
Sodium Channel Blockers. Working to discover novel, oral, selective, state-dependent sodium channel blockers. Sodium channel blockers are a promising target linked to chronic pain.
Exalgo. A 32mg dosage strength of Exalgo was approved by the FDA in August 2012. We have received over $7.9 million in royalty revenue on Exalgo sales through the quarter ended September 30, 2012.
Prednisporin. Sanofi announced its intention to continue Prednisporin development under a third party sublicense. Future potential milestone payments and royalties to Zalicus will remain in place.
cHTS. Our combination drug discovery research services business on track to generate approximately $7.0 million of revenue in 2012.
“During 2012, we made a number of advances with our novel ion channel programs, including advancing our novel formulation of Z160 into Phase 2 clinical development and advancing Z944 into the clinic,” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “We plan to build on this success in 2013 by generating proof-of-concept data for Z160 in multiple indications and seeking to advance the development of our other ion channel programs.”
http://finance.yahoo.com/news/zalicus-initiates-second-two-p…
Sehr schön...


Aber lass uns nicht vergessen, nördlich die 0,074$ Marke sollte es eigentlich richtig losgehen (short Eindeckungen bis Freitags börsenschluß sind auch nicht ganz auszuschließen
 )
)Bis jetzt 1.735 Handlungen stateside, mit vier weitere Handelsstunden vor uns, langsam aber sicher werden sie drüßen hellhörig, hier als Bsp aus dem Yahoo Forum:
"Regarding Z160, my opinion is that a non-opiate pain solution that is truly successful can exceed all wild expectations on revenue. It could be upto a billion dollars. However, I do not envision Zalicus to manufacture and bring the drug to market. I believe that a partnership will occur when the drug is in Phase 3 to bring it to market. The revenues obviously will not be in the billions to Zalicus, but the royalties will far exceed what anyone can forecast (in the hundreds of millions)"
Steigende Grüße,
Growth12

lets not forget:- der Biotech conference steht für Montag an...

http://finance.yahoo.com/mbview/threadview/?&bn=bfeab61d-25b…
Jetzt, die 0,76USD wurden soeben flott genommen & still counting (short Eindeckungen am morgigen Freitag werden sicherlich folgen )
)


http://finance.yahoo.com/mbview/threadview/?&bn=bfeab61d-25b…
Jetzt, die 0,76USD wurden soeben flott genommen & still counting (short Eindeckungen am morgigen Freitag werden sicherlich folgen
 )
)
Guten morgen zusammen
prima, gesternabend die 0,74$ wieder hinter uns gelassen haben-dieses deutet auf einen wirklich fundierten rebound hin, nicht vergessen, vor knapp fünf monate stand ZLCS um die 1,48$
...der rebound läuft erst jetzt richtig an, die 1$ marke stellt die nächste pschologisiche grenze dar denn es zu überschreiten gilt, die 0,96$ allerdings aus dem Charttechnischensicht.
Stay tuned:- es sollte ein langen, wilden Ritt gen norden werden


prima, gesternabend die 0,74$ wieder hinter uns gelassen haben-dieses deutet auf einen wirklich fundierten rebound hin, nicht vergessen, vor knapp fünf monate stand ZLCS um die 1,48$
...der rebound läuft erst jetzt richtig an, die 1$ marke stellt die nächste pschologisiche grenze dar denn es zu überschreiten gilt, die 0,96$ allerdings aus dem Charttechnischensicht.
Stay tuned:- es sollte ein langen, wilden Ritt gen norden werden


Aus aktuellem anlaß via Seeking Alpha stateside...
"2 Biotech Companies Making Considerable Strides When It Comes To Pain Management"
Zalicus (ZLCS): According to its website, "Zalicus is a biopharmaceutical company which engages in the discovery and development of drug candidates focusing on the treatment of pain and inflammation". Zalicus is primarily focused on the development of the following drugs in its pipeline: Synavive (a Phase IIb candidate being developed for the treatment of rheumatoid arthritis), Z-160 (a Phase I candidate being developed as an N-type calcium channel blocker) and Z-944 (a Phase I candidate focused on the treatment of various chronic pain conditions).
From a fundamental perspective, shares of ZLCS currently carry a market cap of $96.30 million, have traded down 37.50% since July 1, and are also trading at a 19.89% premium to their 50-DMA and at a 16.79% discount to their 200-DMA.
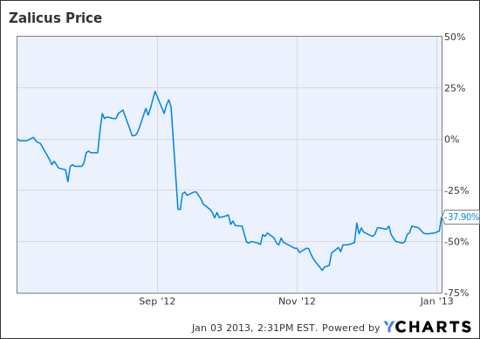
On January 3rd it was announced that Zalicus had initiated the second of two Phase IIa clinical studies with Z160, a selective N-type calcium channel blocker designed to potentially treat chronic neuropathic pain. The second Phase IIa study with Z160 is currently enrolling subjects with Postherpetic Neuralgia (PHN), a chronic neuropathic pain state resulting from an outbreak of the herpes zoster virus, otherwise known as shingles.
According to Dr. Mark H.N. Corrigan, President and CEO of Zalicus, "Postherpetic Neuralgia is an important medical condition for evaluating the activity of Z160 for three important reasons. First, it is a well-recognized standard for establishing clinical proof of concept in neuropathic pain; second, with a prevalence of less than 200,000 patients in the U.S., it has the potential for orphan drug status and could be a feasible first indication to pursue from a commercial perspective; and third, significant unmet medical need exists for novel, targeted and more efficacious chronic neuropathic pain therapies with improved safety and tolerability profiles such as Z160".
http://seekingalpha.com/article/1093581-2-biotech-companies-…

"2 Biotech Companies Making Considerable Strides When It Comes To Pain Management"
Zalicus (ZLCS): According to its website, "Zalicus is a biopharmaceutical company which engages in the discovery and development of drug candidates focusing on the treatment of pain and inflammation". Zalicus is primarily focused on the development of the following drugs in its pipeline: Synavive (a Phase IIb candidate being developed for the treatment of rheumatoid arthritis), Z-160 (a Phase I candidate being developed as an N-type calcium channel blocker) and Z-944 (a Phase I candidate focused on the treatment of various chronic pain conditions).
From a fundamental perspective, shares of ZLCS currently carry a market cap of $96.30 million, have traded down 37.50% since July 1, and are also trading at a 19.89% premium to their 50-DMA and at a 16.79% discount to their 200-DMA.

On January 3rd it was announced that Zalicus had initiated the second of two Phase IIa clinical studies with Z160, a selective N-type calcium channel blocker designed to potentially treat chronic neuropathic pain. The second Phase IIa study with Z160 is currently enrolling subjects with Postherpetic Neuralgia (PHN), a chronic neuropathic pain state resulting from an outbreak of the herpes zoster virus, otherwise known as shingles.
According to Dr. Mark H.N. Corrigan, President and CEO of Zalicus, "Postherpetic Neuralgia is an important medical condition for evaluating the activity of Z160 for three important reasons. First, it is a well-recognized standard for establishing clinical proof of concept in neuropathic pain; second, with a prevalence of less than 200,000 patients in the U.S., it has the potential for orphan drug status and could be a feasible first indication to pursue from a commercial perspective; and third, significant unmet medical need exists for novel, targeted and more efficacious chronic neuropathic pain therapies with improved safety and tolerability profiles such as Z160".
http://seekingalpha.com/article/1093581-2-biotech-companies-…
Antwort auf Beitrag Nr.: 43.980.386 von Growth2012 am 04.01.13 08:53:58 Piper Jaffray initiates coverage on Zalicus (NASDAQ: ZLCS) with a Neutral. PT $1.00.
For an analyst ratings summary and ratings history on Zalicus click here.
For more ratings news on Zalicus click here.
Shares of Zalicus closed at $0.76 yesterday, with a 52 week range of $0.43-$1.62.
http://www.streetinsider.com/New+Coverage/Piper+Jaffray+Star…
For an analyst ratings summary and ratings history on Zalicus click here.
For more ratings news on Zalicus click here.
Shares of Zalicus closed at $0.76 yesterday, with a 52 week range of $0.43-$1.62.
http://www.streetinsider.com/New+Coverage/Piper+Jaffray+Star…
Antwort auf Beitrag Nr.: 43.981.372 von bernie55 am 04.01.13 12:20:24Yeo bernie, langsam aber sicher werden sie alle wieder wach...
Good trades & steady hands für 2013 wünscht dir,
Growth12

Good trades & steady hands für 2013 wünscht dir,
Growth12

Hallo zusammen,
erst mal ein gutes.gesundes und erfolgreiches Neues Jahr !
Der Premarket ist auch schon munter
http://www.nasdaq.com/symbol/zlcs/premarket
Nachdem ich mutig in Zeiten des Downtrends fleissig gesammelt habe, habe ich heute meinen "Break of even" erreicht !!
Ja das hat Nerven gekostet ...........allerdings war mir klar das es im Hinblick auf die Pipeline bei Zalicus nur eine Frage der Zeit für den Rebound ist.
...........allerdings war mir klar das es im Hinblick auf die Pipeline bei Zalicus nur eine Frage der Zeit für den Rebound ist.
Ansonsten wurde ja bereits alles wichtige hier gesagt
Nun lehnen wir uns mit unserem Invest erstmal zurück und schauen wann wir den Dollar reissen werden.....
Auf ein gutes 2013...........auch im Hinblick auf Zalicus
Grüße Earthfire
erst mal ein gutes.gesundes und erfolgreiches Neues Jahr !

Der Premarket ist auch schon munter

http://www.nasdaq.com/symbol/zlcs/premarket
Nachdem ich mutig in Zeiten des Downtrends fleissig gesammelt habe, habe ich heute meinen "Break of even" erreicht !!
Ja das hat Nerven gekostet
 ...........allerdings war mir klar das es im Hinblick auf die Pipeline bei Zalicus nur eine Frage der Zeit für den Rebound ist.
...........allerdings war mir klar das es im Hinblick auf die Pipeline bei Zalicus nur eine Frage der Zeit für den Rebound ist.Ansonsten wurde ja bereits alles wichtige hier gesagt

Nun lehnen wir uns mit unserem Invest erstmal zurück und schauen wann wir den Dollar reissen werden.....

Auf ein gutes 2013...........auch im Hinblick auf Zalicus
Grüße Earthfire

Nun gibt es nichts Neues zu sagen...(schönes Ende gestern)...
...'bin aus dem Urlaub zurück und wünsche allen Investierten hier auch nochmal ein ...
...Frohes und gesundes und möglichst auch erfolgreiches Neues Jahr
Bye...
...'bin aus dem Urlaub zurück und wünsche allen Investierten hier auch nochmal ein ...
...Frohes und gesundes und möglichst auch erfolgreiches Neues Jahr

Bye...
Zitat von Earthfire: Hallo zusammen,
erst mal ein gutes.gesundes und erfolgreiches Neues Jahr !
...dito...
Der Premarket ist auch schon munter
http://www.nasdaq.com/symbol/zlcs/premarket" target="_blank" rel="nofollow ugc noopener">http://www.nasdaq.com/symbol/zlcs/premarket
Nachdem ich mutig in Zeiten des Downtrends fleissig gesammelt habe, habe ich heute meinen "Break of even" erreicht !!
...well done, Earthie, bei 1,50 $ ist dann mal wieder ein Steak fällig..
..dann bezahle ich aber in xxxx die nächste Rechnung..
Ja das hat Nerven gekostet.....
...darfst aber jetzt auch bis ca. April 2013 " rasten "....
..allerdings war mir klar das es im Hinblick auf die Pipeline bei Zalicus nur eine Frage der Zeit für den Rebound ist.
...und da können wir noch einiges mehr als einen Rebound erwarten...
Ansonsten wurde ja bereits alles wichtige hier gesagt
..YEPP..
Nun lehnen wir uns mit unserem Invest erstmal zurück und schauen wann wir den Dollar reissen werden.....
..ich finde, dein LONGIE-Feeling kommt jetzt immer zu Tage...
Auf ein gutes 2013...
..YEPP..
.....auch im Hinblick auf Zalicus
..und ARNA + DNDN + IMGN...
Grüße Earthfire
Grüße
bernie55

Zitat von Growth2012: Yeo bernie, langsam aber sicher werden sie alle wieder wach...![]()
Good trades & steady hands für 2013 wünscht dir,
Growth12
...dasselbe wünsche ich Dir auch, growthie...

...und dass dein Spruch: Stay tuned:- es sollte ein langen, wilden Ritt gen norden werden


...weiterhin Bestand haben möge..

Gestriger Schlusskurs:
0.8176 $
Up 0.0576(7.58%)
Grüße
bernie55

Moin zusammen! ...kennt ihr dieses schon?
Zalicus gains on news of second Phase 2a trial against neuropathic pain
http://www.bizjournals.com/boston/blog/bioflash/2013/01/zali…

Ich weiss nur eins gaz sicher, falls ich "short wäre"(und es gibt noch solche)...wäre mir Angst & Bange beim Chartanblick zurzeit


Zalicus gains on news of second Phase 2a trial against neuropathic pain
http://www.bizjournals.com/boston/blog/bioflash/2013/01/zali…
Ich weiss nur eins gaz sicher, falls ich "short wäre"(und es gibt noch solche)...wäre mir Angst & Bange beim Chartanblick zurzeit



Zitat von Earthfire:Zitat von bernie55: ...
Für uns "Biotech-Longies" heißt das:
Don't make a trade for today.....make an investment for tomorrow.
Grüße
bernie55
Hi Bernie......oder.....make an investment for the day after tomorrow
Grüße Earthfire
tja, damit bist du in die Liga der Biotech-Superlongies aufgestiegen...

Grüße
bernie55

...eine inhaltlich wirklich Super-Longie-Zusammenfassung von ZALICUS...
Will 2013 Be A Better Year For Zalicus?
http://seekingalpha.com/article/1105701-will-2013-be-a-bette…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/1105701-will-2013-be-a-bette…
Will 2013 Be A Better Year For Zalicus?
http://seekingalpha.com/article/1105701-will-2013-be-a-bette…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/1105701-will-2013-be-a-bette…
Warum die 1$ Marke bis spätestens April wieder drin ist...
Hier ein sehr fundierte Yahoo Posting von Heute:
April 22, 2013 is the deadline for NASDAQ 180 day compliance rule. It must be above $1.00 for ten consecutive days or it will be delisted.
Zalicus doesn't want this at all.
#1. they will put out as much positive information as they can to get this back up
#2. they will buy themselves if price is close to a buck
#3. Reverse Split
This company has diluted over 27 Million shares this year sold to Wedbush. This ceased on August 1st, 2012.
100 Million O/S now.
Soon to be 126,000
Current A/S=200,000
Cash or Cash Equivalents: $43.2 Million as of end of September 2012.
Cash burn: @ $10 Million Quarter Expect a decline for Synavive expenses and increases for Z160 and Z994.
Zalicus States they have enough cash for first half of 2014. *Mark my Words: "They Don't!!" Maybe until June or July of 2013, but NO Way mid 2014. At $10 Million a Quarter they will need cash by at least next October 2013.
Nov. 15, 2013 Watson Pharmaceutical can introduce Generic Exalgo and with the Agreement with Covidian, this Reduces Royalty Fees by 50% from Covidian.
Total income from Exalgo: @ $50 million and don't expect much more to come; note that $40 million was just getting it approved.
Zalicus must make sure that they have much better patents on Z160 and 994.
Synavive trial failed because it was not as effective as prednisolone, either 2.7mg or 5mg.
Now off to Z160. Zalicus states that they have "several new formulations of Z160", and these new formulas have demonstrated bioavailability improvements. Zalicus carefully analyzed these new formulas and have selected "the most promising formulation" for continuing Z160 into Phase II-a trials. They also seek to advance Z994 into Phase II clinical development.
So this is basically where we are right now, kind of in Limbo Land, hoping that Z160 can effectively treat shingles and LSR or LumboSacral Radiculopathy..
Z160 is an N Calcium Channel Blocker.
Z994 is a T type Calcium Channel Blocker.
Both are innovative treatments for pain, so it will be a watch, wait and see. Synavive was innovative but didn't provide effective treatment vs pednisolone.
In my opinion Zalicus has made some HUGE mistakes with Exalgo and Synavive. They need to get their act together with: 1. Patents, 2. if they make a partnership, make sure they make it in their favor and think Longer Term, 3. Trial Design...I think Synavive could be a treatment, but I believe their clinical trial could have been designed in a much favorable manner.
All in all, I am neutral on Zalicus, but still remain hopeful. It will be interesting to follow if 160 can effectively treat LSR, if so, then this is a big market. It's all about how they choose to go with clinical trials.
BUT, they have made so many mistakes that you could hand them Gold and they would find a way to convert it to $#%^
http://finance.yahoo.com/mbview/threadview/?&bn=bfeab61d-25b…
Dat wird, dat wird...
Hier ein sehr fundierte Yahoo Posting von Heute:
April 22, 2013 is the deadline for NASDAQ 180 day compliance rule. It must be above $1.00 for ten consecutive days or it will be delisted.
Zalicus doesn't want this at all.
#1. they will put out as much positive information as they can to get this back up
#2. they will buy themselves if price is close to a buck
#3. Reverse Split
This company has diluted over 27 Million shares this year sold to Wedbush. This ceased on August 1st, 2012.
100 Million O/S now.
Soon to be 126,000
Current A/S=200,000
Cash or Cash Equivalents: $43.2 Million as of end of September 2012.
Cash burn: @ $10 Million Quarter Expect a decline for Synavive expenses and increases for Z160 and Z994.
Zalicus States they have enough cash for first half of 2014. *Mark my Words: "They Don't!!" Maybe until June or July of 2013, but NO Way mid 2014. At $10 Million a Quarter they will need cash by at least next October 2013.
Nov. 15, 2013 Watson Pharmaceutical can introduce Generic Exalgo and with the Agreement with Covidian, this Reduces Royalty Fees by 50% from Covidian.
Total income from Exalgo: @ $50 million and don't expect much more to come; note that $40 million was just getting it approved.
Zalicus must make sure that they have much better patents on Z160 and 994.
Synavive trial failed because it was not as effective as prednisolone, either 2.7mg or 5mg.
Now off to Z160. Zalicus states that they have "several new formulations of Z160", and these new formulas have demonstrated bioavailability improvements. Zalicus carefully analyzed these new formulas and have selected "the most promising formulation" for continuing Z160 into Phase II-a trials. They also seek to advance Z994 into Phase II clinical development.
So this is basically where we are right now, kind of in Limbo Land, hoping that Z160 can effectively treat shingles and LSR or LumboSacral Radiculopathy..
Z160 is an N Calcium Channel Blocker.
Z994 is a T type Calcium Channel Blocker.
Both are innovative treatments for pain, so it will be a watch, wait and see. Synavive was innovative but didn't provide effective treatment vs pednisolone.
In my opinion Zalicus has made some HUGE mistakes with Exalgo and Synavive. They need to get their act together with: 1. Patents, 2. if they make a partnership, make sure they make it in their favor and think Longer Term, 3. Trial Design...I think Synavive could be a treatment, but I believe their clinical trial could have been designed in a much favorable manner.
All in all, I am neutral on Zalicus, but still remain hopeful. It will be interesting to follow if 160 can effectively treat LSR, if so, then this is a big market. It's all about how they choose to go with clinical trials.
BUT, they have made so many mistakes that you could hand them Gold and they would find a way to convert it to $#%^
http://finance.yahoo.com/mbview/threadview/?&bn=bfeab61d-25b…
Dat wird, dat wird...

Antwort auf Beitrag Nr.: 44.020.940 von Growth2012 am 14.01.13 21:20:54Hi Growth,damit hatte ich mich bereits im Oktober beschäftigt, hier noch mal mein Beitrag vom 24/10/12
Hallo bernie, das mit dem Dollar hatte ich ja bereits nach dem Kursrutsch angeführt.....hier gibt es aus meiner Erfahrung 3 Szenarien :
1.) die Nachfrage steigt und hebt den Kurs nachhaltig auf über einen Dollar
2.)Seitens des Unternehmens wird über Broker der Kurs gestützt bzw. manipuliert.....
3.) Es kommt zu einem R/S ( evtl. 2:1)....mit der Ausgabe neuer Shares
Fakt ist, das ich nicht glaube das Zalicus es zu einem Delisting kommen lässt......sollte die Käuferseite nicht in den nächsten Monaten zulegen bzw. pos. News eintrudeln......tippe ich persönlich auf einen R/S.....leider !
Grüße Earthfire
aber ich denke das wir den Dollar in den nächsten Wochen im Sack haben werden...............
Time will tell
Grüße Eathfire
Hallo bernie, das mit dem Dollar hatte ich ja bereits nach dem Kursrutsch angeführt.....hier gibt es aus meiner Erfahrung 3 Szenarien :
1.) die Nachfrage steigt und hebt den Kurs nachhaltig auf über einen Dollar
2.)Seitens des Unternehmens wird über Broker der Kurs gestützt bzw. manipuliert.....
3.) Es kommt zu einem R/S ( evtl. 2:1)....mit der Ausgabe neuer Shares
Fakt ist, das ich nicht glaube das Zalicus es zu einem Delisting kommen lässt......sollte die Käuferseite nicht in den nächsten Monaten zulegen bzw. pos. News eintrudeln......tippe ich persönlich auf einen R/S.....leider !
Grüße Earthfire

aber ich denke das wir den Dollar in den nächsten Wochen im Sack haben werden...............

Time will tell
Grüße Eathfire
Die Shorts haben den Kurs in den letzten beiden Tagen gedrückt.....im Gegenzug wurde long massiv gekauft !!
Konnte man gestern dies wieder schön bei der ersten Stunde beobachten...in der Tradinglist....dh es wird wieder fleissig am Kurs manipuliert
http://www.shortanalytics.com/getshortchart.php?tsymbol=zlcs
Mal sehen ob wir heute in die andere Richtung drehen werden.....
Konnte man gestern dies wieder schön bei der ersten Stunde beobachten...in der Tradinglist....dh es wird wieder fleissig am Kurs manipuliert
http://www.shortanalytics.com/getshortchart.php?tsymbol=zlcs
Mal sehen ob wir heute in die andere Richtung drehen werden.....
Antwort auf Beitrag Nr.: 44.032.670 von Earthfire am 17.01.13 13:41:01...heute geht es in die andere Richtung.....

Zitat von Earthfire: Die Shorts haben den Kurs in den letzten beiden Tagen gedrückt.....im Gegenzug wurde long massiv gekauft !!
Konnte man gestern dies wieder schön bei der ersten Stunde beobachten...in der Tradinglist...
...dh es wird wieder fleissig am Kurs manipuliert
http://www.shortanalytics.com/getshortchart.php?tsymbol=zlcs
Mal sehen ob wir heute in die andere Richtung drehen werden.....
Der Autor des Artikels The Future Of Zalicus vom 15 Januar 2013 geht mit dem Vorstand von ZLCS bzgl. der aktuellen Informationspolitik " hart" ins Gericht...
..... wohl deshalb diese "Panik-provozierte" Reaktion bei ZLCS....
http://seekingalpha.com/article/1113571-the-future-of-zalicu…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/1113571-the-future-of-zalicu…
Antwort auf Beitrag Nr.: 44.033.284 von bernie55 am 17.01.13 15:27:11Hi Bernie, habe deinen Bordmail leider übersehen, sorry dafür.
Naja, kein Staatsgeheimnis, mickrige 50K bis dato, also es gilt weiterhin die Devise, day by day, hour by hour, bis hin zur Übernahme (aber dann bitte jenseits der 8$ Marke wenn ich bitte darf)...

Dieser Handelswoche könnte sich als prägend erweisen, nicht zu vergessen, nach seinen verkauf von 114K shares im sachen Steuerdeckung von letzter Woche, hält der CEO noch immer knappe 800K "lebendige ZLCS shares" im Portfolio, er sollte weiterhin relativ motiviert zu seine Arbeitsstätte täglich hinfahren, oder sehe ich das falsch?!?
Good trades & weiterhin steady hands ihr lieben!
Naja, kein Staatsgeheimnis, mickrige 50K bis dato, also es gilt weiterhin die Devise, day by day, hour by hour, bis hin zur Übernahme (aber dann bitte jenseits der 8$ Marke wenn ich bitte darf)...


Dieser Handelswoche könnte sich als prägend erweisen, nicht zu vergessen, nach seinen verkauf von 114K shares im sachen Steuerdeckung von letzter Woche, hält der CEO noch immer knappe 800K "lebendige ZLCS shares" im Portfolio, er sollte weiterhin relativ motiviert zu seine Arbeitsstätte täglich hinfahren, oder sehe ich das falsch?!?

Good trades & weiterhin steady hands ihr lieben!
Heute ein beruhigender Bericht - und schon gehts bergauf...
http://propthink.com/meeting-with-zalicus-management-confirm…
http://propthink.com/meeting-with-zalicus-management-confirm…
Zitat von kmastra: Heute ein beruhigender Bericht - und schon gehts bergauf...
http://propthink.com/meeting-with-zalicus-management-confirm…
Meeting with Zalicus Management Confirms Z160 Potential, Big Pharma Awaiting Trial Data
Jason Napodano, CFA
January 22, 2013
Zitat von Growth2012: Hi Bernie, habe deinen Bordmail leider übersehen, sorry dafür.
Naja, kein Staatsgeheimnis, mickrige 50K bis dato, also es gilt weiterhin die Devise, day by day, hour by hour, bis hin zur Übernahme (aber dann bitte jenseits der 8$ Marke wenn ich bitte darf)...
Dieser Handelswoche könnte sich als prägend erweisen, nicht zu vergessen, nach seinen verkauf von 114K shares im sachen Steuerdeckung von letzter Woche, hält der CEO noch immer knappe 800K "lebendige ZLCS shares" im Portfolio, er sollte weiterhin relativ motiviert zu seine Arbeitsstätte täglich hinfahren, oder sehe ich das falsch?!?
Good trades & weiterhin steady hands ihr lieben!
Hi Growthie,
die Sache mit den 113K shares von CEO Mark Corrigan bzgl. der Steuerdeckung sehe ich ebenfalls als nicht problematisch an - aber solche News sind leider immer wieder " eine steile Vorlage" für diejenigen, die den Kurs nach unten prügeln wollen.
Bzgl. der Eigenmotivation kann ich nur sagen, dass Mark Corrigan - genauso wie wir " German Zalianics"
 - mit dem Ausblick auf zukünftig höhere Kurse bestimmt weiterhin gerne zur Arbeit fahren wird.
- mit dem Ausblick auf zukünftig höhere Kurse bestimmt weiterhin gerne zur Arbeit fahren wird.Grüße
bernie55

Yahoo posting re mögliche Exalgo royalties 2013...
If this rep is speaking the truth, then Exalgo has hit the $100 million benchmark. I really think we will see some big time royalties coming in. Please bear in mind that this thing will keep increasing quarter per quarter untill a plateau or sales flatten out. We could start seeing royalties like $10 million per quarter before patent protection expires. After that, there can/will be residual smaller royalties that come in for a few more years. We might still even see 500k to $1Million per quarter in 2015?
Might see $3 to $5 million this quarter. Would be huge if we do
... "FALLS" diese Prognose einigermaßen eintreffen sollte, dann halt bloß die erworbenen Aktien recht gut fest, es gibt schliesslich nur insgesamt 126,99 Mio davon im Umlauf
Grüße, Growth12/13

If this rep is speaking the truth, then Exalgo has hit the $100 million benchmark. I really think we will see some big time royalties coming in. Please bear in mind that this thing will keep increasing quarter per quarter untill a plateau or sales flatten out. We could start seeing royalties like $10 million per quarter before patent protection expires. After that, there can/will be residual smaller royalties that come in for a few more years. We might still even see 500k to $1Million per quarter in 2015?
Might see $3 to $5 million this quarter. Would be huge if we do
... "FALLS" diese Prognose einigermaßen eintreffen sollte, dann halt bloß die erworbenen Aktien recht gut fest, es gibt schliesslich nur insgesamt 126,99 Mio davon im Umlauf

Grüße, Growth12/13

Seeking Alpha von 22 Jan, 2013...(Das ganze ohne Wertung, weil SA muss halt als Urquelle der recherche für die richtigkeit alleine herhalten) 
Meeting With Zalicus Management Confirms Z160 Potential; Big Pharma Awaiting Trial Data
http://seekingalpha.com/article/1124541-meeting-with-zalicus…
...


Meeting With Zalicus Management Confirms Z160 Potential; Big Pharma Awaiting Trial Data
http://seekingalpha.com/article/1124541-meeting-with-zalicus…
...


Zitat von Growth2012: Seeking Alpha von 22 Jan, 2013...(Das ganze ohne Wertung, weil SA muss halt als Urquelle der recherche für die richtigkeit alleine herhalten)
Meeting With Zalicus Management Confirms Z160 Potential; Big Pharma Awaiting Trial Data
http://seekingalpha.com/article/1124541-meeting-with-zalicus…
...
..ich habe mal etwas aus diesem Artikel herauskopiert....
Market Opportunity
We suspect that Zalicus will look to partner Z160 in a deal similar to the previous collaboration with Merck following results of the two ongoing phase 2a trials. Management told us that partners are already knocking on the door for Z160.

We think with two positive Phase 2a trials in hand, Zalicus will be able to secure a very lucrative deal. For the sake of argument, let's assume Z160 is a $1 billion drug, with a 20% chance to make it to the market in 2018. That could be worth $50 million upfront to a big pharmaceutical company in early 2014, along with $250 million in reasonable back-end potential plus a mid-teen to low-twenty percent royalty on sales.

With a current market capitalization of only $80 million,we think it is fair to say that this sort of transaction is certainly not priced into the shares today.

CEO Selling Shares
This article was ready to publish last week until we noticed a Form-4 filing with the SEC by CEO Mark Corrigan on January 17, 2013. Dr. Corrigan sold 112,850 shares of stock at $0.77 on January 15, 2013. This immediately spooked investors. We even saw an erroneous article noting that Dr. Corrigan sold almost his entire holding in the company - the article has since been retracted.
Dr. Corrigan continues to hold 844,090 shares of Zalicus stock.
We are unconcerned with this selling......growthie, earthie and me too
 ... and believe this misinformation has created a meaningful buying opportunity in the stock (Zalicus traded at $0.80 on January 14, 2012).
... and believe this misinformation has created a meaningful buying opportunity in the stock (Zalicus traded at $0.80 on January 14, 2012).
Antwort auf Beitrag Nr.: 44.086.266 von bernie55 am 31.01.13 11:04:59Hi Bernie  , schöner Upmove gestern in USA
, schöner Upmove gestern in USA  .....
.....
Geschlossen auf TH !!!!
Theoretisch müsste es nach CT....heute weiter steigen !?
Gestern wurde das Downgap vom 16/1 ...punktgenau geschlossen (Range 0,73-0,70ct)
Das nächste liegt über dem Dollar
Ich halte meine shares weiter, wäre doch gelacht wenn wir den Dollar nicht noch vor dem drohenden Delisting sehen würden...
Hier mal eine Stockanalyse zu Zalicus :
http://www.stockta.com/cgi-bin/analysis.pl?symb=ZLCS&cobrand…
 , schöner Upmove gestern in USA
, schöner Upmove gestern in USA  .....
.....Geschlossen auf TH !!!!
Theoretisch müsste es nach CT....heute weiter steigen !?
Gestern wurde das Downgap vom 16/1 ...punktgenau geschlossen (Range 0,73-0,70ct)
Das nächste liegt über dem Dollar

Ich halte meine shares weiter, wäre doch gelacht wenn wir den Dollar nicht noch vor dem drohenden Delisting sehen würden...
Hier mal eine Stockanalyse zu Zalicus :
http://www.stockta.com/cgi-bin/analysis.pl?symb=ZLCS&cobrand…
Antwort auf Beitrag Nr.: 44.086.266 von bernie55 am 31.01.13 11:04:59Tach Bernie, witzig dass du dieses ansprichst...
"and believe this misinformation has created a meaningful buying opportunity in the stock (Zalicus traded at $0.80 on January 14, 2012)."
...ich habe die Vermutung (allerdings reine Vermuntung bzw. gut feeling), das Corrigans teilverkauf eher eine strategische hintergrund hatte:- "weak hands würden dadurch rausgekegelt, und bevorzügt Fonds/Freunde des hauses/ kommen so ein letztes mal noch recht günstig zu pötte, kurz vorm rebound auf die lange Reise Richtung 1$ trading marke.
Der jute Corrigan kann selbst hierbei auch fett profitieren, 800K anteile hat er noch (amtlich)im Portfolio, jetzt kurz ein guten news zu Z160 co-op und das teil steht jenseits der 2$ Marke (ja, ich habe mich verschrieben, die 1$ Marke passieren wir wie ein schnitt durch weiche Butter)
wer will Ihn dann Insider Wissen vorwerfen ...er hat schließlich über 100K shares nachweisslich verkauft um sein Steuerlast bedienen zu können.
...er hat schließlich über 100K shares nachweisslich verkauft um sein Steuerlast bedienen zu können.
Ja, ja, gefickt eingeschädelt lieber Herr Doktor

"and believe this misinformation has created a meaningful buying opportunity in the stock (Zalicus traded at $0.80 on January 14, 2012)."
...ich habe die Vermutung (allerdings reine Vermuntung bzw. gut feeling), das Corrigans teilverkauf eher eine strategische hintergrund hatte:- "weak hands würden dadurch rausgekegelt, und bevorzügt Fonds/Freunde des hauses/ kommen so ein letztes mal noch recht günstig zu pötte, kurz vorm rebound auf die lange Reise Richtung 1$ trading marke.
Der jute Corrigan kann selbst hierbei auch fett profitieren, 800K anteile hat er noch (amtlich)im Portfolio, jetzt kurz ein guten news zu Z160 co-op und das teil steht jenseits der 2$ Marke (ja, ich habe mich verschrieben, die 1$ Marke passieren wir wie ein schnitt durch weiche Butter)
wer will Ihn dann Insider Wissen vorwerfen
 ...er hat schließlich über 100K shares nachweisslich verkauft um sein Steuerlast bedienen zu können.
...er hat schließlich über 100K shares nachweisslich verkauft um sein Steuerlast bedienen zu können.Ja, ja, gefickt eingeschädelt lieber Herr Doktor

Antwort auf Beitrag Nr.: 44.086.450 von Earthfire am 31.01.13 11:31:25Hi Earthfire,
Gestern wurde das Downgap vom 16/1 ...punktgenau geschlossen (Range 0,73-0,70ct) Das nächste liegt über dem Dollar
Man merkt es schon, du läufts auch mit offenen Augen durch deine Börsenumwelt...

So wirds kommen, und für "manche markteilnehmern" wirds (wie immer) einfach zu schnell gehen um effektiv & tief sich dazugesellen zu können

Gestern wurde das Downgap vom 16/1 ...punktgenau geschlossen (Range 0,73-0,70ct) Das nächste liegt über dem Dollar
Man merkt es schon, du läufts auch mit offenen Augen durch deine Börsenumwelt...


So wirds kommen, und für "manche markteilnehmern" wirds (wie immer) einfach zu schnell gehen um effektiv & tief sich dazugesellen zu können


Fein Fein : ZLCS's NASDAQ Last Sale
0.7675[/b] 0.0375 5.13%
nahe TH geschlossen.....ohne News.
Sieht aus als wenn unser Ding hier..langsam aber sicher, richtig Bullish würde !!

0.7675[/b] 0.0375 5.13%
nahe TH geschlossen.....ohne News.
Sieht aus als wenn unser Ding hier..langsam aber sicher, richtig Bullish würde !!


Moin zusammen
ganz kurz (muss weg)...jetzt ist "The Street" sprich Jim Cramer auf Zalicus Aufmerksam geworden, ich sag nur folgendes dazu, 80% von amerikas Anlegerschaft hört auf sein Wort (ich persönlich halte Ihn für ein "Spacko" aber egal, alles Brandbeschleuniger gerne Wilkommen )
)
Hier der Link:- "5 Stocks Under $10 Set to Soar Higher - views"
http://www.stockpickr.com/5-stocks-under-10-set-soar-higher.…
Zalicus
Another under-$10 name that’s trading close to triggering a near-term breakout trade is Zalicus (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. This stock is off to a hot start in 2013, with shares up a whopping 16.5%.
If you take a look at the chart for Zalicus, you’ll notice that this stock has been trending sideways for the last two months and change, with shares moving between 57 cents on the downside and 83 cents on the upside. Shares of ZLCS have just started to bounce off its 50-day moving average of 67 cents and its quickly moving within range of triggering a near-term breakout trade above the upper-end of its recent chart pattern.
Market players should now look for long-biased trades in ZLCS if it manages to break out above some near-term overhead resistance levels at 80 to 83 cents per share and then once it takes out some past overhead resistance levels at 91 cents to $1.10 a share with high volume. Look for a sustained move or close above those levels with volume that hits near or above its three-month average action of 1,141,380 shares. If that breakout triggers soon, then ZLCS will set up to re-fill some of its previous gap down zone above $1.10 a share. That gap started at around $1.40 a share, so any move above 91 cents to $1.10 will put that gap into play for shares of ZLCS.
Traders can look to buy ZLCS off any weakness to anticipate that breakout and then simply use a stop that sits just below its 50-day moving average of 67 cents per share. One could also buy ZLCS off strength once it takes out those breakout levels with volume and then simply use a stop that sits just below 80 cents per share. I would add to either position once ZLCS clears 91 cents to $1.10 a share with volume.
Schönes Wochenende & bloß nicht vergessen- SCHNALLT EUCH AN!!!



ganz kurz (muss weg)...jetzt ist "The Street" sprich Jim Cramer auf Zalicus Aufmerksam geworden, ich sag nur folgendes dazu, 80% von amerikas Anlegerschaft hört auf sein Wort (ich persönlich halte Ihn für ein "Spacko" aber egal, alles Brandbeschleuniger gerne Wilkommen
 )
)Hier der Link:- "5 Stocks Under $10 Set to Soar Higher - views"
http://www.stockpickr.com/5-stocks-under-10-set-soar-higher.…
Zalicus
Another under-$10 name that’s trading close to triggering a near-term breakout trade is Zalicus (ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain and immuno-inflammatory diseases. This stock is off to a hot start in 2013, with shares up a whopping 16.5%.
If you take a look at the chart for Zalicus, you’ll notice that this stock has been trending sideways for the last two months and change, with shares moving between 57 cents on the downside and 83 cents on the upside. Shares of ZLCS have just started to bounce off its 50-day moving average of 67 cents and its quickly moving within range of triggering a near-term breakout trade above the upper-end of its recent chart pattern.
Market players should now look for long-biased trades in ZLCS if it manages to break out above some near-term overhead resistance levels at 80 to 83 cents per share and then once it takes out some past overhead resistance levels at 91 cents to $1.10 a share with high volume. Look for a sustained move or close above those levels with volume that hits near or above its three-month average action of 1,141,380 shares. If that breakout triggers soon, then ZLCS will set up to re-fill some of its previous gap down zone above $1.10 a share. That gap started at around $1.40 a share, so any move above 91 cents to $1.10 will put that gap into play for shares of ZLCS.
Traders can look to buy ZLCS off any weakness to anticipate that breakout and then simply use a stop that sits just below its 50-day moving average of 67 cents per share. One could also buy ZLCS off strength once it takes out those breakout levels with volume and then simply use a stop that sits just below 80 cents per share. I would add to either position once ZLCS clears 91 cents to $1.10 a share with volume.
Schönes Wochenende & bloß nicht vergessen- SCHNALLT EUCH AN!!!



Antwort auf Beitrag Nr.: 44.090.611 von Growth2012 am 01.02.13 08:21:31Hi Growth,Hi Bernie  , ich hoffe wir bleiben in dem Trendkanal....und es geht weiter Up....wir haben schließlich den Dollar zu knacken
, ich hoffe wir bleiben in dem Trendkanal....und es geht weiter Up....wir haben schließlich den Dollar zu knacken 
Hier mal das Chart zu ZlCS
http://stockcharts.com/h-sc/ui
sieht sehr gut aus für den weiteren Verlauf !!
Wenn jetzt noch die Pusherartikel kommen....soll mir auch Recht sein !
Meine persönliche Einschätzung ist, das es über dem Dollar erst richtig los gehen wird DH....für mich ist dies die wichtigste Marke die es zu knacken gilt.
1. psychologisch wichtige Marke
2. drohender R/S ist dann vom Tisch
dies wird auch zu einem größeren Vertrauen der breiten Masse führen...
Wenn dann noch positive Ergebnisse zu Z 160 eintrudeln....zieht Zalicus richtig ab....und eine Übernahme ist dann auch möglich !
Ich drücke und Hardcore-longies die Daumen.....das unsere Nervenstärke und die mutigen Nachkäufe im Downtrend dann fürstlich belohnt werden !!!
 , ich hoffe wir bleiben in dem Trendkanal....und es geht weiter Up....wir haben schließlich den Dollar zu knacken
, ich hoffe wir bleiben in dem Trendkanal....und es geht weiter Up....wir haben schließlich den Dollar zu knacken 
Hier mal das Chart zu ZlCS
http://stockcharts.com/h-sc/ui
sieht sehr gut aus für den weiteren Verlauf !!
Wenn jetzt noch die Pusherartikel kommen....soll mir auch Recht sein !

Meine persönliche Einschätzung ist, das es über dem Dollar erst richtig los gehen wird DH....für mich ist dies die wichtigste Marke die es zu knacken gilt.
1. psychologisch wichtige Marke
2. drohender R/S ist dann vom Tisch
dies wird auch zu einem größeren Vertrauen der breiten Masse führen...
Wenn dann noch positive Ergebnisse zu Z 160 eintrudeln....zieht Zalicus richtig ab....und eine Übernahme ist dann auch möglich !

Ich drücke und Hardcore-longies die Daumen.....das unsere Nervenstärke und die mutigen Nachkäufe im Downtrend dann fürstlich belohnt werden !!!
Antwort auf Beitrag Nr.: 44.091.839 von Earthfire am 01.02.13 12:01:15Ach ja, hier liegt das nächste Downgap :
down Sept-10-2012 1.36 to 1.07 $
down Sept-10-2012 1.36 to 1.07 $

Antwort auf Beitrag Nr.: 44.091.839 von Earthfire am 01.02.13 12:01:15Hi Earthfire, alles richtig, bin echt gespannt auf die nächste Handelswoche(n)...
Hier braut sich (still & leise)ein gewisse Kaufkraft zusammen, unauffällig, aber dafür stimmt die Gesamtrichtung, habe dieses öfters malim Markt erlebt mit US Aktien, bei dünnen Nachrichtenlage wird "trotzdem" kontrolliert & stetig nach oben gefahren im vorfeld des eigentliche Ereignisses
ZLCS ist offentsichtlich im 'Lauerstellungsmodus' übergegangen, ich rechne mit n' richtig fetten news vor ende März/Mitte April, bis dahin werde ich (wie gewöhnt ), weiterhin jeder menge Tee wohl trinken müssen...

Hier braut sich (still & leise)ein gewisse Kaufkraft zusammen, unauffällig, aber dafür stimmt die Gesamtrichtung, habe dieses öfters malim Markt erlebt mit US Aktien, bei dünnen Nachrichtenlage wird "trotzdem" kontrolliert & stetig nach oben gefahren im vorfeld des eigentliche Ereignisses

ZLCS ist offentsichtlich im 'Lauerstellungsmodus' übergegangen, ich rechne mit n' richtig fetten news vor ende März/Mitte April, bis dahin werde ich (wie gewöhnt ), weiterhin jeder menge Tee wohl trinken müssen...

Zalicus to Present at the 2013 BIO CEO & Investor Conference
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ:ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain, today announced that Zalicus management will present at the 2013 BIO CEO & Investor Conference at 9:00 p.m. ET on Tuesday, February 12, 2013 at the Waldorf Astoria in New York. Interested parties may access a live webcast of the presentation by visiting the Zalicus website at www.zalicus.com. The webcast will be archived on the Zalicus website following the event.
About Zalicus
Zalicus Inc. (Nasdaq:ZLCS) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain. Zalicus has a portfolio of proprietary clinical-stage product candidates targeting pain such as Z160 and Z944 and has entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. Zalicus applies its expertise in the discovery and development of selective ion channel modulators and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease. To learn more about Zalicus, please visit www.zalicus.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus, its product candidates, their potential, and its plans for clinical development. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus and its product candidates may be identified by words like "believe," "expect," "may," "will," "should," "seek," "plan" or "could" and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the clinical development of its product candidates, the unproven nature of the Zalicus Ion channel drug discovery technology, and those other risks that can be found in the "Risk Factors" section of Zalicus' annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission. Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management's current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
(c) 2012 Zalicus Inc. All rights reserved.
Wieder im Waldorf Astoria.........unsere schöne Kohle
Aber man gönnt sich ja sonst nichts.................

CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (NASDAQ:ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain, today announced that Zalicus management will present at the 2013 BIO CEO & Investor Conference at 9:00 p.m. ET on Tuesday, February 12, 2013 at the Waldorf Astoria in New York. Interested parties may access a live webcast of the presentation by visiting the Zalicus website at www.zalicus.com. The webcast will be archived on the Zalicus website following the event.
About Zalicus
Zalicus Inc. (Nasdaq:ZLCS) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain. Zalicus has a portfolio of proprietary clinical-stage product candidates targeting pain such as Z160 and Z944 and has entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. Zalicus applies its expertise in the discovery and development of selective ion channel modulators and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease. To learn more about Zalicus, please visit www.zalicus.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus, its product candidates, their potential, and its plans for clinical development. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus and its product candidates may be identified by words like "believe," "expect," "may," "will," "should," "seek," "plan" or "could" and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the clinical development of its product candidates, the unproven nature of the Zalicus Ion channel drug discovery technology, and those other risks that can be found in the "Risk Factors" section of Zalicus' annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission. Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management's current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
(c) 2012 Zalicus Inc. All rights reserved.
Wieder im Waldorf Astoria.........unsere schöne Kohle

Aber man gönnt sich ja sonst nichts.................

Hallo zusammen,auch in Anbetracht das heute Freitag ist  .......bin ich mal auf den heutigen Kursverlauf gespannt.
.......bin ich mal auf den heutigen Kursverlauf gespannt.
Seit langer Zeit haben wir bei Zalicus mal wieder ein Kaufsignal und bewegen uns in einem bullischen Kanal....
Recent CandleStick Analysis
Very Bullish
Date Candle
Feb-07-2013 Bullish Harami
Read more at http://www.stockta.com/cgi-bin/analysis.pl?symb=ZLCS&cobrand…
Mal sehen ob die CT heute greift und die Amis zum Kaufen animiert
Die Burschen hier raten auch zum Kauf :
http://www.americanbulls.com/StockPage.asp?CompanyTicker=ZLC…
Aus meiner Sicht hat sich der Kursverlauf schon positiv verändert dh. wir konnten uns im Bereich des letzten Gaps bis jetzt halten....und sind nicht mehr...wie so oft zuvor, unter die 0,7 ct gefallen.
Bin gespannt ob am 12/2 evtl Neuigkeiten verkündet werden, die für einen weiteren Verlauf des Kurses ...Richtung Norden sorgen.
Den so langsam aber sicher sollten wir uns mal Richtung Dollar bewegen bzw. bis ca. Mitte - Ende März sollte der Dollar stabil anstehen.....da uns ansonsten ein R/S erwischen könnte.
Und damit ist dann Zalicus....aber sicher nicht uns geholfen !!
Schönes WE
 .......bin ich mal auf den heutigen Kursverlauf gespannt.
.......bin ich mal auf den heutigen Kursverlauf gespannt.Seit langer Zeit haben wir bei Zalicus mal wieder ein Kaufsignal und bewegen uns in einem bullischen Kanal....
Recent CandleStick Analysis
Very Bullish
Date Candle
Feb-07-2013 Bullish Harami
Read more at http://www.stockta.com/cgi-bin/analysis.pl?symb=ZLCS&cobrand…
Mal sehen ob die CT heute greift und die Amis zum Kaufen animiert

Die Burschen hier raten auch zum Kauf :
http://www.americanbulls.com/StockPage.asp?CompanyTicker=ZLC…
Aus meiner Sicht hat sich der Kursverlauf schon positiv verändert dh. wir konnten uns im Bereich des letzten Gaps bis jetzt halten....und sind nicht mehr...wie so oft zuvor, unter die 0,7 ct gefallen.
Bin gespannt ob am 12/2 evtl Neuigkeiten verkündet werden, die für einen weiteren Verlauf des Kurses ...Richtung Norden sorgen.
Den so langsam aber sicher sollten wir uns mal Richtung Dollar bewegen bzw. bis ca. Mitte - Ende März sollte der Dollar stabil anstehen.....da uns ansonsten ein R/S erwischen könnte.
Und damit ist dann Zalicus....aber sicher nicht uns geholfen !!

Schönes WE
Antwort auf Beitrag Nr.: 44.118.540 von Earthfire am 08.02.13 12:34:32Hi earthfire, my comment today over on Yahoo re ZlCS...
Spot on comment carlcczc...
That comment will save a lot of people from wading through a lot of rubbish in this forum-I for one personally believe that this -EXTREMELY LONG WAIT- will finally pay off at the end of the day.
Synopsis:-Great product in a strong marketplace, bad leadership team & even weaker communication "experts" within ZLCS.
Will remain long up to 5$+....
http://finance.yahoo.com/mbview/threadview/?&bn=bfeab61d-25b…
Schönes WE...
Spot on comment carlcczc...
That comment will save a lot of people from wading through a lot of rubbish in this forum-I for one personally believe that this -EXTREMELY LONG WAIT- will finally pay off at the end of the day.
Synopsis:-Great product in a strong marketplace, bad leadership team & even weaker communication "experts" within ZLCS.
Will remain long up to 5$+....
http://finance.yahoo.com/mbview/threadview/?&bn=bfeab61d-25b…
Schönes WE...

Moin zusammen,
ich hoffe das wir bald pos. News zu Z160 bekommen !!
Solange keine " fundamentals " da sind, ist der Kurs in der Hand der Manipulatoren................wie so oft bei solchen Werten, insbesondere in USA.
Der Handel wird durch computergestützte Algorithmen in Richtung Süden gedrückt sobald das BID schwächer wird.
Somit wird wie so oft.....mit kleinen "billigen" Verkäufen ins Bid....der Kauf eines größeren Blocktrades ermöglicht....
Ich schaue mir an solchen Tagen immer die Einzeltrades an......gestern zb. mit 3000 Stck runter....um 80 000 Stck 1,5 ct günstiger zu kaufen !
Und da waren noch einige ähnliche Käufe dabei...........schade das man die Käufer nicht sieht
Nun, ich vermute das einige immer wieder versuchen.....sich billig einzudecken.....dies hatten wir in der Vergangenheit schon oft bei Zalicus.
Das einzige was mir hier nach wie vor Bauchschmerzen bereitet, ist der drohende R/S bis ende April.
Nun ist es ja noch einige Zeit bis dahin, aber mich hat bereits 3x in meiner Traderzeit ein R/S erwischt....jedensmal mit Tradinghalt.
Es könnte sein, das eine entsprechende News ( R/S) bereits Anfang April vorliegen könnte.
Deis würde sich aber sicher vorher berreits durch Verkäufe der Instis ankündigen.....die werden mit Sicherheit vorher versuchen gut rauszukommen.
Wie gesagt.......ich hoffe wir haben hier bald positive Fundamentals vorliegen
ich hoffe das wir bald pos. News zu Z160 bekommen !!
Solange keine " fundamentals " da sind, ist der Kurs in der Hand der Manipulatoren................wie so oft bei solchen Werten, insbesondere in USA.
Der Handel wird durch computergestützte Algorithmen in Richtung Süden gedrückt sobald das BID schwächer wird.
Somit wird wie so oft.....mit kleinen "billigen" Verkäufen ins Bid....der Kauf eines größeren Blocktrades ermöglicht....
Ich schaue mir an solchen Tagen immer die Einzeltrades an......gestern zb. mit 3000 Stck runter....um 80 000 Stck 1,5 ct günstiger zu kaufen !
Und da waren noch einige ähnliche Käufe dabei...........schade das man die Käufer nicht sieht

Nun, ich vermute das einige immer wieder versuchen.....sich billig einzudecken.....dies hatten wir in der Vergangenheit schon oft bei Zalicus.
Das einzige was mir hier nach wie vor Bauchschmerzen bereitet, ist der drohende R/S bis ende April.
Nun ist es ja noch einige Zeit bis dahin, aber mich hat bereits 3x in meiner Traderzeit ein R/S erwischt....jedensmal mit Tradinghalt.
Es könnte sein, das eine entsprechende News ( R/S) bereits Anfang April vorliegen könnte.
Deis würde sich aber sicher vorher berreits durch Verkäufe der Instis ankündigen.....die werden mit Sicherheit vorher versuchen gut rauszukommen.
Wie gesagt.......ich hoffe wir haben hier bald positive Fundamentals vorliegen

8 K Report von gestern :
http://www.sec.gov/Archives/edgar/data/1135906/0001181431130…
Hier der Tenor :
At an investor conference on February 12, 2013, Zalicus Inc. (the "Company") will disclose that its unaudited cash, cash equivalents and marketable securities balance as of December 31, 2012 was approximately $36.4 million, that its royalties from net sales of the product Exalgo® by Covidien plc for the Company's fiscal quarter ended December 31, 2012 were approximately $1.5 million and that its revenue from its cHTS™ collaborations for the fiscal year ended December 31, 2012 was approximately $7.3 million.
http://www.sec.gov/Archives/edgar/data/1135906/0001181431130…
Hier der Tenor :
At an investor conference on February 12, 2013, Zalicus Inc. (the "Company") will disclose that its unaudited cash, cash equivalents and marketable securities balance as of December 31, 2012 was approximately $36.4 million, that its royalties from net sales of the product Exalgo® by Covidien plc for the Company's fiscal quarter ended December 31, 2012 were approximately $1.5 million and that its revenue from its cHTS™ collaborations for the fiscal year ended December 31, 2012 was approximately $7.3 million.
Hi,
ist noch jemand da....oder habt ihr alle schon geschmissen ?
Noch mal kurz zu unserem Damoklesschwert :
Fristende für den 1 $ ist der 22 April (180 Tage)
bis dahin muss der Kurs an 10 aufeinanderfolgenden Handelstagen über 1 $ notieren !!
So gesehen haben wir noch 2 Monate um dies zu erfüllen...
Nach meinen Recherchen hat Zalicus auch die Möglichkeit eine Fristverlängerung um weitere 180 Tage zu beantragen.....
Ein R/S würde ja nicht nur das Management...uns kleine ...sondern die Instis hart treffen.
...sondern die Instis hart treffen.
Den zb :
Owner Name Date Shared Held Change (Shares) Change(%) Value(in 1,000s)
MPM ASSET MANAGEMENT LLC 12/31/2012 5,566,980 0 0.00 3,658
VANGUARD GROUP INC 12/31/2012 2,124,194
Read more: http://www.nasdaq.com/symbol/zlcs/institutional-holdings#ixz…
Nun ja....ich hoffe wir haben bald eine positive News...die dem Spuk ein schnelles Ende bereiten wird !!
ist noch jemand da....oder habt ihr alle schon geschmissen ?

Noch mal kurz zu unserem Damoklesschwert :
Fristende für den 1 $ ist der 22 April (180 Tage)
bis dahin muss der Kurs an 10 aufeinanderfolgenden Handelstagen über 1 $ notieren !!

So gesehen haben wir noch 2 Monate um dies zu erfüllen...
Nach meinen Recherchen hat Zalicus auch die Möglichkeit eine Fristverlängerung um weitere 180 Tage zu beantragen.....
Ein R/S würde ja nicht nur das Management...uns kleine
 ...sondern die Instis hart treffen.
...sondern die Instis hart treffen.Den zb :
Owner Name Date Shared Held Change (Shares) Change(%) Value(in 1,000s)
MPM ASSET MANAGEMENT LLC 12/31/2012 5,566,980 0 0.00 3,658
VANGUARD GROUP INC 12/31/2012 2,124,194
Read more: http://www.nasdaq.com/symbol/zlcs/institutional-holdings#ixz…
Nun ja....ich hoffe wir haben bald eine positive News...die dem Spuk ein schnelles Ende bereiten wird !!
Na dann mach ich mal den Alleinunterhalter 
Wenn man sich das Chart ansieht.....sieht aus wie beim letzten mal...im Berreich der 0,64-0,65 ct $...ist der Support dh. hier müsste die Talfahrt wohl scheinbar wieder ihr Ende finden
Mal sehen was heute abend auf dem Volumenzähler steht..

Wenn man sich das Chart ansieht.....sieht aus wie beim letzten mal...im Berreich der 0,64-0,65 ct $...ist der Support dh. hier müsste die Talfahrt wohl scheinbar wieder ihr Ende finden

Mal sehen was heute abend auf dem Volumenzähler steht..

Hola....was ist das den heute im Premarket ?
Pre-Market
Time (ET) Pre-Market
Price Pre-Market
Share Volume
09:14 $ .666 High 103,610
Read more: http://www.nasdaq.com/symbol/zlcs/premarket#ixzz2LM3FNRzD
K
Pre-Market
Time (ET) Pre-Market
Price Pre-Market
Share Volume
09:14 $ .666 High 103,610

Read more: http://www.nasdaq.com/symbol/zlcs/premarket#ixzz2LM3FNRzD
K
Zalicus Granted Composition-of-Matter Patent for Z944 
By Business Wire, February 20, 2013, 08:00:00 AM EDT
Vote up
Key Patent Provides Broad Coverage for Z944 through April 2029
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (Nasdaq Global Market:ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain, today announced that it has been granted a patent by the U.S. Patent and Trademark office (USPTO) covering its product candidate Z944. United States patent number 8,377,968 entitled "N-Piperidinyl Acetamide Derivatives as Calcium Channel Blockers" provides broad coverage for Z944 including compositions of matter and certain therapeutic methods of use through April 2029. Z944 is a novel, oral, T-type calcium channel blocker which has demonstrated efficacy in a number of preclinical inflammatory pain models and other disease models. Z944 completed Phase 1 single and multiple ascending dose clinical studies in late 2012 and the Company plans to continue further clinical development with Z944 during 2013.
"This key patent provides the foundation of a solid and enforceable intellectual property estate for Z944, with issued claims through April 2029," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. "Based on our preclinical and early-stage clinical work, we are excited to further explore the potential of Z944 as a novel, oral, non-opioid pain treatment."
Quelle :
http://www.nasdaq.com/article/zalicus-granted-composition-of…

By Business Wire, February 20, 2013, 08:00:00 AM EDT
Vote up
Key Patent Provides Broad Coverage for Z944 through April 2029
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Zalicus Inc. (Nasdaq Global Market:ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain, today announced that it has been granted a patent by the U.S. Patent and Trademark office (USPTO) covering its product candidate Z944. United States patent number 8,377,968 entitled "N-Piperidinyl Acetamide Derivatives as Calcium Channel Blockers" provides broad coverage for Z944 including compositions of matter and certain therapeutic methods of use through April 2029. Z944 is a novel, oral, T-type calcium channel blocker which has demonstrated efficacy in a number of preclinical inflammatory pain models and other disease models. Z944 completed Phase 1 single and multiple ascending dose clinical studies in late 2012 and the Company plans to continue further clinical development with Z944 during 2013.
"This key patent provides the foundation of a solid and enforceable intellectual property estate for Z944, with issued claims through April 2029," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. "Based on our preclinical and early-stage clinical work, we are excited to further explore the potential of Z944 as a novel, oral, non-opioid pain treatment."
Quelle :
http://www.nasdaq.com/article/zalicus-granted-composition-of…
Zitat von Earthfire: Zalicus Granted Composition-of-Matter Patent for Z944
By Business Wire, February 20, 2013, 08:00:00 AM EDT
Vote up
Key Patent Provides Broad Coverage for Z944 through April 2029
Hi "EARTHIE"

..gute Nachricht

..aber aktuell scheint sich für ZLCS keiner zu interessieren...

..anscheinend nur die BIGs im Hintergrund

...und die kleinen ZALICIANICS u.a. hier im WO Board...

Grüße
bernie55

Speculative Biotech Stocks That Could Bounce 50% On Upcoming Catalysts 
Und die 50% UP brauchen wir auch als ersten Step...damit wir nicht aus dem Nasdaq fliegen bzw. uns ein drohender R/S erspart bleibt.
Zalicus Inc. (ZLCS)
This stock has attracted equal numbers of haters and lovers over the last few years, and especially over the few months after bad news came on its rheumatoid arthritis drug Synavive and the trial was stopped. With partnership deals with Novartis (NVS), Covidien (COV), Sanofi (SNY) and Amgen (AMGN) over the years that investor interest has been warranted. The stock price action over the last year has left many investors disenchanted. This, however may bring opportunity for investors looking for some speculative plays for their biotech stock baskets.
The company discovers and develops drug candidates focusing on the treatment of pain and inflammation. The companys clinical and preclinical product candidates include Synavive, a glucocorticoid prednisolone product candidate, which is in Phase IIb clinical trial for the treatment of rheumatoid arthritis; Z160, an N-type calcium channel blocker that has completed Phase I clinical trial to treat neuropathic and inflammatory pain; and Z944, an oral T-type calcium channel blocker, which is in Phase I clinical trial for the treatment of various chronic pain conditions.
Zalicus' sole FDA approved drug is Exalgo® (hydromorphone HCl) extended-release tablets (8, 12 and 16 mg), for the management of moderate to severe pain. The U.S. commercial rights to Exalgo were acquired in June 2009 by Mallinckrodt, Inc., a subsidiary of Covidien, plc with Zalicus receiving tiered royalties on net sales. At $1.4m last quarter these sales are minimal. In January Zalicus underwent a bear attack when an article surfaced stating that Mark Corrigan, the CEO, sold all his shares. The article was later retracted but the damage was already done and the stock had shed 12% of its value. The CEO had only sold 113,000 shares to cover the tax lax liability of exercising 500,000 shares of stock at 76c.
This brings us to the current pipeline for Zalicus and it looks very attractive, especially at this low valuation. Z160 a first in class, oral, state dependent, selective N-type calcium channel blocker. N-type calcium channels are recognized as important targets in controlling pain since they appear to play a key role in transmitting pain through the spinal nerves to the brain. In September 2012, Zalicus initiated the first Phase 2a study (Clinicaltrials.gov Identifier: NCT01655849) with Z160 in 140 patients with chronic neuropathic pain associated with lumbosacral radiculopathy (LSR). In January the second Phase 2a trial was started. This news sent shares of the stock up 20%. Z944 a novel, oral, T-type calcium channel blocker that has demonstrated preclinical efficacy in multiple inflammatory and acute pain models. The company was just awarded a patent for this drug. On July 10, 2012, Zalicus announced that the company initiated a Phase I multiple ascending dose (MAD) clinical study with Z944. We should be seeing trial result news anytime on Z944 and if positive the drug could enter Phase II trials in the coming weeks. The company stated that it expected this to occur in Q1 in their last earnings report. News could come in the next four weeks. The initiation of Z160 Phase II trials caused a nice 20% pop in Zalicus earlier this year and similar news on Z944 could see the stock move again. Zalicus has also proven that it can attract large pharmaceutical companies to partner with them on drugs so positive news on the Phase I trials could see another deal unfold.
A quick look at the Zalicus chart shows a breakout from the recent consolidation at 69c leading to a move to the 200-day moving average at 88c. Above this level a gap fill to $1.20 would quickly follow.
Quelle :
http://seekingalpha.com/article/1211421-speculative-biotech-…

Und die 50% UP brauchen wir auch als ersten Step...damit wir nicht aus dem Nasdaq fliegen bzw. uns ein drohender R/S erspart bleibt.

Zalicus Inc. (ZLCS)
This stock has attracted equal numbers of haters and lovers over the last few years, and especially over the few months after bad news came on its rheumatoid arthritis drug Synavive and the trial was stopped. With partnership deals with Novartis (NVS), Covidien (COV), Sanofi (SNY) and Amgen (AMGN) over the years that investor interest has been warranted. The stock price action over the last year has left many investors disenchanted. This, however may bring opportunity for investors looking for some speculative plays for their biotech stock baskets.
The company discovers and develops drug candidates focusing on the treatment of pain and inflammation. The companys clinical and preclinical product candidates include Synavive, a glucocorticoid prednisolone product candidate, which is in Phase IIb clinical trial for the treatment of rheumatoid arthritis; Z160, an N-type calcium channel blocker that has completed Phase I clinical trial to treat neuropathic and inflammatory pain; and Z944, an oral T-type calcium channel blocker, which is in Phase I clinical trial for the treatment of various chronic pain conditions.
Zalicus' sole FDA approved drug is Exalgo® (hydromorphone HCl) extended-release tablets (8, 12 and 16 mg), for the management of moderate to severe pain. The U.S. commercial rights to Exalgo were acquired in June 2009 by Mallinckrodt, Inc., a subsidiary of Covidien, plc with Zalicus receiving tiered royalties on net sales. At $1.4m last quarter these sales are minimal. In January Zalicus underwent a bear attack when an article surfaced stating that Mark Corrigan, the CEO, sold all his shares. The article was later retracted but the damage was already done and the stock had shed 12% of its value. The CEO had only sold 113,000 shares to cover the tax lax liability of exercising 500,000 shares of stock at 76c.
This brings us to the current pipeline for Zalicus and it looks very attractive, especially at this low valuation. Z160 a first in class, oral, state dependent, selective N-type calcium channel blocker. N-type calcium channels are recognized as important targets in controlling pain since they appear to play a key role in transmitting pain through the spinal nerves to the brain. In September 2012, Zalicus initiated the first Phase 2a study (Clinicaltrials.gov Identifier: NCT01655849) with Z160 in 140 patients with chronic neuropathic pain associated with lumbosacral radiculopathy (LSR). In January the second Phase 2a trial was started. This news sent shares of the stock up 20%. Z944 a novel, oral, T-type calcium channel blocker that has demonstrated preclinical efficacy in multiple inflammatory and acute pain models. The company was just awarded a patent for this drug. On July 10, 2012, Zalicus announced that the company initiated a Phase I multiple ascending dose (MAD) clinical study with Z944. We should be seeing trial result news anytime on Z944 and if positive the drug could enter Phase II trials in the coming weeks. The company stated that it expected this to occur in Q1 in their last earnings report. News could come in the next four weeks. The initiation of Z160 Phase II trials caused a nice 20% pop in Zalicus earlier this year and similar news on Z944 could see the stock move again. Zalicus has also proven that it can attract large pharmaceutical companies to partner with them on drugs so positive news on the Phase I trials could see another deal unfold.
A quick look at the Zalicus chart shows a breakout from the recent consolidation at 69c leading to a move to the 200-day moving average at 88c. Above this level a gap fill to $1.20 would quickly follow.
Quelle :
http://seekingalpha.com/article/1211421-speculative-biotech-…
Zitat von Earthfire: Speculative Biotech Stocks That Could Bounce 50% On Upcoming Catalysts
Und die 50% UP brauchen wir auch als ersten Step...damit wir nicht aus dem Nasdaq fliegen bzw. uns ein drohender R/S erspart bleibt.
...und dann hoffen wir mal, dass ZLCS diesen Weg gehen wird.....

....ZLCS und natürlich uns allen " good luck"..

Antwort auf Beitrag Nr.: 44.173.178 von bernie55 am 22.02.13 11:05:23Hi Bernie  , na das hoffe ich für alle Beteiligten auch !
, na das hoffe ich für alle Beteiligten auch !
Schon komisch wie unser Papierchen so läuft dh. Gesamtmärkte...blutrot...die meisten Biotecs in USA .....blutrot....und was macht Zalicus...läuft gegen Markt und schließt auch noch grün !
Nun ja...schauen wir mal heute...ob die Richtung und das Volumen anhält
Gemäß der CT müsste sie heute steigen.....
Aber an die CT hat sich Zalicus leider schon oft nicht gehalten...
 , na das hoffe ich für alle Beteiligten auch !
, na das hoffe ich für alle Beteiligten auch !Schon komisch wie unser Papierchen so läuft dh. Gesamtmärkte...blutrot...die meisten Biotecs in USA .....blutrot....und was macht Zalicus...läuft gegen Markt und schließt auch noch grün !
Nun ja...schauen wir mal heute...ob die Richtung und das Volumen anhält

Gemäß der CT müsste sie heute steigen.....

Aber an die CT hat sich Zalicus leider schon oft nicht gehalten...

Na ja, der gestrige Handelsverlauf sah etwas unentschlossen ais....was die Richtung betrifft.
Aber für einen Freitag....können wir uns nicht beklagen
Schönes verschneites WE @all....schau mer mal....was die kommende Woche bringt.
Aber für einen Freitag....können wir uns nicht beklagen

Schönes verschneites WE @all....schau mer mal....was die kommende Woche bringt.

Antwort auf Beitrag Nr.: 44.177.458 von Earthfire am 23.02.13 11:36:24Hi earthfire, mein Gott, ein extrem zähe & langweilig Handelsspanne bie ZLCS zurzeit, es ist wirklich nur zum einpennen geeignet-ABER- ich denke der folgender Satz könnte es in sich haben (und ein schnellen rebound über die 1$ Marke mit sich bringen)
“This key patent provides the foundation of a solid and enforceable intellectual property estate for Z944, with issued claims through April 2029,” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “Based on our preclinical and early-stage clinical work, we are excited to further explore the potential of Z944 as a novel, oral, non-opioid pain treatment.”
Die nächste "Bekanntmachnung" bzgl. Z944 wird nich lange auf sich warten lassen (meiner Meinung nach)...und wenn es richtig bullish rüberkommt, wird hier ganz schnell ein fette Handelsleben in die Aktie wieder eingehaucht
Ich erwarte big turnaround news im laufe der März
“This key patent provides the foundation of a solid and enforceable intellectual property estate for Z944, with issued claims through April 2029,” commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “Based on our preclinical and early-stage clinical work, we are excited to further explore the potential of Z944 as a novel, oral, non-opioid pain treatment.”
Die nächste "Bekanntmachnung" bzgl. Z944 wird nich lange auf sich warten lassen (meiner Meinung nach)...und wenn es richtig bullish rüberkommt, wird hier ganz schnell ein fette Handelsleben in die Aktie wieder eingehaucht

Ich erwarte big turnaround news im laufe der März

Antwort auf Beitrag Nr.: 44.177.458 von Earthfire am 23.02.13 11:36:24Hattet ihr dieses schon gesehen? (Sorry, war/bin viel unterwegs zurzeit)
Possible Bullish Inside Day Candle Pattern Detected for Zalicus
http://www.mysmartrend.com/news-briefs/candlestick/possible-…
Possible Bullish Inside Day Candle Pattern Detected for Zalicus
http://www.mysmartrend.com/news-briefs/candlestick/possible-…
gähnende langeweile, als ob der trading woche gar nicht stattgefunden hätte
 ...ich lobe Besserung!
...ich lobe Besserung!
Hi Growth, kann sein das wir hier du Ruhe vor dem Sturm gerade erleben !
In den US Boards wundern sich die Boys drüben auch über das Volumen der letzten Woche.
Nun ja, man kann auch sagen....solange die großen ihre Shares halten...ist soweit alles in Butter.
Im BID waren immer wieder größere Orders zum billig abgreifen....die wurden aber nicht bedient....dh. keiner will billig verkaufen....was wiederum als positiv interpretiert werden kann !!
Sei es drum...wir brauchen mal ne gute News
Schönes Rest WE @all
In den US Boards wundern sich die Boys drüben auch über das Volumen der letzten Woche.
Nun ja, man kann auch sagen....solange die großen ihre Shares halten...ist soweit alles in Butter.
Im BID waren immer wieder größere Orders zum billig abgreifen....die wurden aber nicht bedient....dh. keiner will billig verkaufen....was wiederum als positiv interpretiert werden kann !!
Sei es drum...wir brauchen mal ne gute News

Schönes Rest WE @all
Zitat von bernie55:Zitat von Earthfire: ..wir brauchen mal ne gute News
Vielleicht schon morgen ?????
ZLCS Earnings announcement/Next Earnings Date: 5-Mar-13
Hi Bernie
 , ja das hatte ich in den US Boards auch gesehen.
, ja das hatte ich in den US Boards auch gesehen.Ich hoffe auch auf was gutes und keine Ankündigung des R/S

Bin mal gespannt ob sich am heutigen Handel etwas ablesen lässt.
Ein R/S wird nicht bereits Wochen vorher angekündigt und muss auch auf einer HV abgesegnet werden !
Grüße

Antwort auf Beitrag Nr.: 44.210.673 von Earthfire am 04.03.13 13:02:30Yeo...etwas news aus dem Hause ZLCS ist ittlerweile wirklich längst überfällig:O
Das heutige Posting auf Yahoo sagt m.E. vieles aus
This is a game of chicken..
--Everyone's watching ZLCS, but nobody is flinching. Few buyers, few sellers.
--The potential buyers are all waiting for proof--either partnership, of trial results. It doesn't matter what management says.They know that this company's drugs could be worth billions, so whether they buy now, or later-- when they know for sure-- at, say, $1.50-$2.00, they would still make a killing...
--They also know that others working on ICB's are either partnered (Convergence) or were bought out (Icagen), so they know there is a risk of NOT being vested at all times...
Tja, etwas 'kenntnis' tut mancmal so richtig weh ...


Das heutige Posting auf Yahoo sagt m.E. vieles aus
This is a game of chicken..
--Everyone's watching ZLCS, but nobody is flinching. Few buyers, few sellers.
--The potential buyers are all waiting for proof--either partnership, of trial results. It doesn't matter what management says.They know that this company's drugs could be worth billions, so whether they buy now, or later-- when they know for sure-- at, say, $1.50-$2.00, they would still make a killing...
--They also know that others working on ICB's are either partnered (Convergence) or were bought out (Icagen), so they know there is a risk of NOT being vested at all times...
Tja, etwas 'kenntnis' tut mancmal so richtig weh ...



Antwort auf Beitrag Nr.: 44.222.526 von Growth2012 am 06.03.13 20:11:38Hi Growth...sieht doch gut...Volumen steigt..Kurs auch 

Zalicus Given Outperform Rating at Oppenheimer 
Zalicus Given Outperform Rating at Oppenheimer (ZLCS)
Source: ABMN Staff | Publish date: Wed, 6 Mar 14:50 | >> Read article in News website
ZLCS
Zalicus (NASDAQ: ZLCS)‘s stock had its “outperform” rating reaffirmed by Oppenheimer in a research note issued on Wednesday. They currently have a $2.00 price target on the stock.
The analysts wrote, “Investors remain focused on the two ongoing Z160 Phase IIa proof-of-concept studies in chronic neuropathic pain. Zalicus now expects to report top-line data from these studies by late-2H13. As a reminder, the two Phase IIa studies include Lumbosacral Radiculopathy (LSR) and Postherpetic Neuralgia (PHN). Zalicus stated that it will continue the clinical development of Z944 in 2013. A key US patent was issued in February 2013 that provides coverage through April 2029. We believe that, with total cash of $36.4M at YE12, Zalicus is capitalized past data readout of its two Z160 Phase IIa studies and into 2014.”
Separately, analysts at Piper Jaffray initiated coverage on shares of Zalicus in a research note to investors on Thursday, January 3rd. They set a “neutral” rating on the stock.
Zalicus traded up 0.45% on Wednesday, hitting $0.6429. Zalicus has a 1-year low of $0.43 and a 1-year high of $1.62. The stock’s 50-day moving average is currently $0.68. The company’s market cap is $81.4 million.
Zalicus last issued its quarterly earnings data on Wednesday, March 6th. The company reported ($0.06) EPS for the quarter, beating the Thomson Reuters consensus estimate of ($0.08) by $0.02. The company had revenue of $3.67 million for the quarter, compared to the consensus estimate of $3.12 million. Analysts expect that Zalicus will post $-0.31 EPS for the current fiscal year.
Zalicus Inc., formerly CombinatoRx, Incorporated, is a biopharmaceutical company engaged in developing drug candidates with a focus on the treatment of pain and inflammation.

Zalicus Given Outperform Rating at Oppenheimer (ZLCS)
Source: ABMN Staff | Publish date: Wed, 6 Mar 14:50 | >> Read article in News website
ZLCS
Zalicus (NASDAQ: ZLCS)‘s stock had its “outperform” rating reaffirmed by Oppenheimer in a research note issued on Wednesday. They currently have a $2.00 price target on the stock.
The analysts wrote, “Investors remain focused on the two ongoing Z160 Phase IIa proof-of-concept studies in chronic neuropathic pain. Zalicus now expects to report top-line data from these studies by late-2H13. As a reminder, the two Phase IIa studies include Lumbosacral Radiculopathy (LSR) and Postherpetic Neuralgia (PHN). Zalicus stated that it will continue the clinical development of Z944 in 2013. A key US patent was issued in February 2013 that provides coverage through April 2029. We believe that, with total cash of $36.4M at YE12, Zalicus is capitalized past data readout of its two Z160 Phase IIa studies and into 2014.”
Separately, analysts at Piper Jaffray initiated coverage on shares of Zalicus in a research note to investors on Thursday, January 3rd. They set a “neutral” rating on the stock.
Zalicus traded up 0.45% on Wednesday, hitting $0.6429. Zalicus has a 1-year low of $0.43 and a 1-year high of $1.62. The stock’s 50-day moving average is currently $0.68. The company’s market cap is $81.4 million.
Zalicus last issued its quarterly earnings data on Wednesday, March 6th. The company reported ($0.06) EPS for the quarter, beating the Thomson Reuters consensus estimate of ($0.08) by $0.02. The company had revenue of $3.67 million for the quarter, compared to the consensus estimate of $3.12 million. Analysts expect that Zalicus will post $-0.31 EPS for the current fiscal year.
Zalicus Inc., formerly CombinatoRx, Incorporated, is a biopharmaceutical company engaged in developing drug candidates with a focus on the treatment of pain and inflammation.
They currently have a $2.00 price target on the stock.
...ich hoffe aber nicht nach dem R/S...
Zalicus Given Outperform Rating at Oppenheimer (ZLCS)
http://www.benzinga.com/analyst-ratings/analyst-color/13/03/…
http://www.i3investor.com/servlets/fdnews/131181.jsp
...ich hoffe aber nicht nach dem R/S...

Zalicus Given Outperform Rating at Oppenheimer (ZLCS)
http://www.benzinga.com/analyst-ratings/analyst-color/13/03/…
http://www.i3investor.com/servlets/fdnews/131181.jsp
Antwort auf Beitrag Nr.: 44.224.418 von bernie55 am 07.03.13 11:04:04morgen Bernie  , nein nicht nach dem R/S
, nein nicht nach dem R/S 
noch ist alles offen...wir haben von heute an...noch ca. 5 Wochen Zeit für den Dollar.
Mit einem R/S würden die investierten Instis sehr geärgert...und das MM hätte da wohl auch nicht viel von !
Bis dato wurde der Kurs sehr manipuliert...damit man schön sammeln konnte !
Jetzt haben wir in den letzten beiden Tagen erst mal wieder Momentum im Chart....ob das so bleibt ist fraglich.
Rein vom Gefühl her...würde ich fast sagen....könnte sein, das sie nun laufen darf.
Hoffe das MM plaziert vor dem drohenden Delisting bzw R/S die passende News um den Kurs zu feuern
Ich bleibe auf jeden Fall erstmal drin....
Grüße Earthfire
 , nein nicht nach dem R/S
, nein nicht nach dem R/S 
noch ist alles offen...wir haben von heute an...noch ca. 5 Wochen Zeit für den Dollar.
Mit einem R/S würden die investierten Instis sehr geärgert...und das MM hätte da wohl auch nicht viel von !

Bis dato wurde der Kurs sehr manipuliert...damit man schön sammeln konnte !
Jetzt haben wir in den letzten beiden Tagen erst mal wieder Momentum im Chart....ob das so bleibt ist fraglich.
Rein vom Gefühl her...würde ich fast sagen....könnte sein, das sie nun laufen darf.
Hoffe das MM plaziert vor dem drohenden Delisting bzw R/S die passende News um den Kurs zu feuern

Ich bleibe auf jeden Fall erstmal drin....
Grüße Earthfire

Tach jungs!...hier ein neuen Beitrag von "Seeking Alpha" im sachen Zalicus...(for what its worth)
On March 6, Zalicus (ZLCS) reported financial results for the fourth quarter and full year 2012. Total revenues in the quarter were $3.8 million. Revenues consisted of $2.4 million in collaborative payments and $1.4 million in royalties on sales of Exalgo at partner Mallinckrodt. Revenues from Exalgo were in-line with my model, whereas collaborative payments exceeded my expectations for the second quarter in a row.
For the full year 2012, total revenues were $12.6 million, comprised of $5.2 million in royalties from Mallinckrodt on Exalgo and $7.4 million in collaborative payments from partner Novartis in oncology and early-stage work with partners utilizing the company's combination High Throughput Screening (cHTS) platform. Revenues were up 53% from 2011. As noted above, collaborative payments have been exceeding my expectations of late. In fact, Zalicus grew collaborative revenues every quarter in 2012.
(click to enlarge)
For 2013, management is guiding for further increases, with a goal of signing one major partnership for early-stage discovery work in 2013. I am pleased to see the company taking advantage of its technology by forming research and discovery collaborations.
For 2013, I model total revenues of approximately $17.0 million, comprised of $7.0 million in royalties on Exalgo and $10.0 million in collaborative payments that utilize the company's vast cHTS platform and ion channel discover engine. I think the potential for Zalicus to strike additional alliances and collaborations is highly discounted by investors.
Here's an interesting way to view Zalicus' valuation - the current market capitalization of $82 million is less than 5X my projected 2013 revenues of $17.0 million, and the company is sitting on $36.5 million in cash and investments.
Two - Shrinking Costs
Net loss for the fourth quarter 2012 was $8.3 million, or $0.07 per share. This was essentially in-line with my estimate for net loss of $0.09 per share on the higher revenues noted above but also higher than expected R&D. Net loss was driven by $1.8 million in G&A, $8.9 million R&D, and $1.0 million in amortization and restructuring. For the full year 2012, net loss totaled $44.8 million, or $0.38 per share. Total expenses in 2012 were comprised of $9.0 million G&A, $41.4 million R&D, and 5.0 million amortization and restructuring.
http://seekingalpha.com/article/1255921-why-zalicus-stock-in…
nun ja, nördlich der 1$ Marke werde ich mich jedenfalls erheblich wohler fühlen...
Good trades & steady hands bis dahin,
Growth12
On March 6, Zalicus (ZLCS) reported financial results for the fourth quarter and full year 2012. Total revenues in the quarter were $3.8 million. Revenues consisted of $2.4 million in collaborative payments and $1.4 million in royalties on sales of Exalgo at partner Mallinckrodt. Revenues from Exalgo were in-line with my model, whereas collaborative payments exceeded my expectations for the second quarter in a row.
For the full year 2012, total revenues were $12.6 million, comprised of $5.2 million in royalties from Mallinckrodt on Exalgo and $7.4 million in collaborative payments from partner Novartis in oncology and early-stage work with partners utilizing the company's combination High Throughput Screening (cHTS) platform. Revenues were up 53% from 2011. As noted above, collaborative payments have been exceeding my expectations of late. In fact, Zalicus grew collaborative revenues every quarter in 2012.
(click to enlarge)
For 2013, management is guiding for further increases, with a goal of signing one major partnership for early-stage discovery work in 2013. I am pleased to see the company taking advantage of its technology by forming research and discovery collaborations.
For 2013, I model total revenues of approximately $17.0 million, comprised of $7.0 million in royalties on Exalgo and $10.0 million in collaborative payments that utilize the company's vast cHTS platform and ion channel discover engine. I think the potential for Zalicus to strike additional alliances and collaborations is highly discounted by investors.
Here's an interesting way to view Zalicus' valuation - the current market capitalization of $82 million is less than 5X my projected 2013 revenues of $17.0 million, and the company is sitting on $36.5 million in cash and investments.
Two - Shrinking Costs
Net loss for the fourth quarter 2012 was $8.3 million, or $0.07 per share. This was essentially in-line with my estimate for net loss of $0.09 per share on the higher revenues noted above but also higher than expected R&D. Net loss was driven by $1.8 million in G&A, $8.9 million R&D, and $1.0 million in amortization and restructuring. For the full year 2012, net loss totaled $44.8 million, or $0.38 per share. Total expenses in 2012 were comprised of $9.0 million G&A, $41.4 million R&D, and 5.0 million amortization and restructuring.
http://seekingalpha.com/article/1255921-why-zalicus-stock-in…
nun ja, nördlich der 1$ Marke werde ich mich jedenfalls erheblich wohler fühlen...

Good trades & steady hands bis dahin,
Growth12

Antwort auf Beitrag Nr.: 44.234.605 von Growth2012 am 10.03.13 11:52:15Hi Growth  @all,
@all,
nun ja, nördlich der 1$ Marke werde ich mich jedenfalls erheblich wohler fühlen...
ab einem Dollar lehnen sich alle Investoren zurück
Vom Charttechnik her...bewegen wir uns wieder in den Bereich der Kaufsignale bzw. einem bullischen Trendkanal.
Aber !!...daran hat sich ZLCS noch nie groß gehalten...leider.
Evtl. darf sie ja jetzt laufen..wie ich vor ein paar Tagen bereits schrieb.
Das Handelsvolumen lässt hoffen.....
Wenn wir über die 0,83 US ct laufen...und uns dort halten können, ist der Dollar nur noch Formsache
Schön wäre in dem UP-Trend ein passende News ( neuer Partner oder dergleichen )....
Ich hoffe nicht, das wir wieder nächste woche das alte Spiel erleben müssen.....2 Schritte vor....und drei Schritte zurück..was den Kurs betrifft !!!
Nochmal zur Erinnerung der 1 $ Regel am Nasdaq.....Stichtag wäre der 22/4/13....bis dahin muss Sie an 10 Handelstagen infolge...über einem Dollar notieren !
wie ich bereits hier erwähnte, besteht die möglichkeit für Zalicus eine Verlängerung der Frist für weitere 180 Tage zu beantragen.
Hoffen wir mal das beste bzw. die Zeit für die paar ct bis zum Dollar, sollte reichen...es sei den die Manipulatoren betreten wieder Bühne und es besteht kein entsprechender Druck auf der Käuferseite !?
Ich denke die nächste Handelswoche wird den UP-Trend bestätigen...oder das alte Spiel beginnt und wir können uns Gedanken machen...ob wir das hier aussitzen oder Handeln !
Grüße Earthfire
 @all,
@all,nun ja, nördlich der 1$ Marke werde ich mich jedenfalls erheblich wohler fühlen...

ab einem Dollar lehnen sich alle Investoren zurück

Vom Charttechnik her...bewegen wir uns wieder in den Bereich der Kaufsignale bzw. einem bullischen Trendkanal.
Aber !!...daran hat sich ZLCS noch nie groß gehalten...leider.
Evtl. darf sie ja jetzt laufen..wie ich vor ein paar Tagen bereits schrieb.
Das Handelsvolumen lässt hoffen.....
Wenn wir über die 0,83 US ct laufen...und uns dort halten können, ist der Dollar nur noch Formsache

Schön wäre in dem UP-Trend ein passende News ( neuer Partner oder dergleichen )....
Ich hoffe nicht, das wir wieder nächste woche das alte Spiel erleben müssen.....2 Schritte vor....und drei Schritte zurück..was den Kurs betrifft !!!
Nochmal zur Erinnerung der 1 $ Regel am Nasdaq.....Stichtag wäre der 22/4/13....bis dahin muss Sie an 10 Handelstagen infolge...über einem Dollar notieren !

wie ich bereits hier erwähnte, besteht die möglichkeit für Zalicus eine Verlängerung der Frist für weitere 180 Tage zu beantragen.
Hoffen wir mal das beste bzw. die Zeit für die paar ct bis zum Dollar, sollte reichen...es sei den die Manipulatoren betreten wieder Bühne und es besteht kein entsprechender Druck auf der Käuferseite !?
Ich denke die nächste Handelswoche wird den UP-Trend bestätigen...oder das alte Spiel beginnt und wir können uns Gedanken machen...ob wir das hier aussitzen oder Handeln !
Grüße Earthfire
News !
Zalicus Presents Data Demonstrating Advantages of State-Dependent Ion Channel Screening
Read more: http://www.nasdaq.com/article/zalicus-presents-data-demonstr…
Zalicus Presents Data Demonstrating Advantages of State-Dependent Ion Channel Screening
Read more: http://www.nasdaq.com/article/zalicus-presents-data-demonstr…
Antwort auf Beitrag Nr.: 44.271.157 von Earthfire am 19.03.13 13:40:44"Rapid neuronal firing which occurs during chronic pathological pain results in accumulation of voltage gated calcium channels in an inactivated state.
By specifically targeting this inactivated state of these channels, it may be possible to broaden the therapeutic window, minimize adverse effects and increase efficacy for these promising pain therapies," said Dr. Lee. "Comparative screening in our cell based assays for state dependent modulation of ion channel function has resulted in the discovery of Z160 and Z944, Zalicus's two most advanced novel, first-in-class calcium channel blockers.
Both Z160 and Z944 have effectively demonstrated enhanced potency for their respective targets in the inactivated state."
http://finance.yahoo.com/marketupdate/inplay#zlcs" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/marketupdate/inplay#zlcs
By specifically targeting this inactivated state of these channels, it may be possible to broaden the therapeutic window, minimize adverse effects and increase efficacy for these promising pain therapies," said Dr. Lee. "Comparative screening in our cell based assays for state dependent modulation of ion channel function has resulted in the discovery of Z160 and Z944, Zalicus's two most advanced novel, first-in-class calcium channel blockers.
Both Z160 and Z944 have effectively demonstrated enhanced potency for their respective targets in the inactivated state."
http://finance.yahoo.com/marketupdate/inplay#zlcs" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/marketupdate/inplay#zlcs
Moin jungs... (echt zäh die Wartezeit hier bei ZLCS )
(echt zäh die Wartezeit hier bei ZLCS )
Zalicus (ZLCS) to Present Positive Data on Calcium Channel Blockers for Pain Treatment
http://www.streetinsider.com/Corporate+News/Zalicus+%28ZLCS%…
...tja, kommt mir vor wie das 'warten auf Godot'

 (echt zäh die Wartezeit hier bei ZLCS )
(echt zäh die Wartezeit hier bei ZLCS )Zalicus (ZLCS) to Present Positive Data on Calcium Channel Blockers for Pain Treatment
http://www.streetinsider.com/Corporate+News/Zalicus+%28ZLCS%…
...tja, kommt mir vor wie das 'warten auf Godot'


Zitat von Growth2012: Moin jungs...(echt zäh die Wartezeit hier bei ZLCS )
...tja, kommt mir vor wie das 'warten auf Godot'
Das Warten auf News bei ZALICUS ist fast genauso " zäh" wie bei ARENA PHARMACEUTICALS
bernie55 schrieb am 11.03.13 13:12:44
Beitrag Nr.200
(44.237.111)
Kleiner zeitlicher Lückenfüller
Das "WARTEN AUF DEA" ( von Arena) ist fast schon mit dem "WARTEN AUF GODOT" ( S. Beckett) zu vergleichen.
Nur mit dem Unterschied, dass das "WARTEN AUF DEA" nicht vergeblich sein wird....

Schöne Grüße
bernie55

@growthie
Boardmail for you...
 Zalicus grantes patent covering formulations of Z160
Zalicus grantes patent covering formulations of Z160
Zalicus announced that it has been granted a patent by the U.S. Patent and Trademark office, or USPTO, covering novel formulations of its product candidate Z160. U.S. patent number 8,409,560, entitled "Solid Dispersion Formulations and Methods of Use Thereof," provides broad coverage for a range of novel Z160 pharmaceutical compositions and methods of using these compositions in the treatment of pain.
http://finance.yahoo.com/news/zalicus-grantes-patent-coverin…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/zalicus-grantes-patent-coverin…
Antwort auf Beitrag Nr.: 44.339.905 von bernie55 am 01.04.13 16:01:52Danke dir Bernie, retourkutsche bzw. Antwortmail bereits auf dem weg...
Hier mein ZLCS investment motto bis dato:


Hier mein ZLCS investment motto bis dato:

4 Healthcare Stock Stories for Tuesday Investment Wellness
http://www.wallstreet-online.de/diskussion/1162665-321-330/z…
http://www.wallstreet-online.de/diskussion/1162665-321-330/z…
Antwort auf Beitrag Nr.: 44.379.689 von Uptick08 am 07.04.13 12:29:44wurde dieses hier evtl. gemeint?
Zalicus (NASDAQ: ZLCS): Current price $0.67
The firm has been granted a patent by the United States Patent and Trademark office covering novel formulations of its product candidate Z160. United States patent number 8,409,560, entitled Solid Dispersion Formulations and Methods of Use Thereof, allows broad coverage for a range of novel Z160 pharmaceutical compositions and methods of using these compositions in the treatment of pain. Zalicus is a biopharmaceutical firm that discovers and develops novel treatments for patients suffering from pain.

http://wallstcheatsheet.com/stocks/4-healthcare-stock-storie…
Zalicus (NASDAQ: ZLCS): Current price $0.67
The firm has been granted a patent by the United States Patent and Trademark office covering novel formulations of its product candidate Z160. United States patent number 8,409,560, entitled Solid Dispersion Formulations and Methods of Use Thereof, allows broad coverage for a range of novel Z160 pharmaceutical compositions and methods of using these compositions in the treatment of pain. Zalicus is a biopharmaceutical firm that discovers and develops novel treatments for patients suffering from pain.

http://wallstcheatsheet.com/stocks/4-healthcare-stock-storie…
Aktueller Stand:
ZLCS hat ein gute und zukunftsträchtige Produktpipeline, jedoch versteht das Management leider nicht,diese in der Öffentlichkeit entsprechend zu "verkaufen".
Der Stichtag , an dem ZLCS 10 Tage in Folge über 1 Dollar stehen muss , ist der 22/4/13
Das "Zeitfenster" ist meiner Meinung nach nicht mehr zu realiseren,ein R/S rückt quasi immer näher - und dieser wird bestimmt nicht kursfördernd für ZLCS sein.
Der aktuelle Aktien-Handel ist unterirdisch lasch und wird sogar durch gute News nicht mehr " aktiver".
Die allgemeine Unsicherheit und Enttäuschung ist aktuell bei vielen Anlegern zu spüren.Deshalb ist es nicht verwunderlich, dass viele Aktionäre ( auch WO User!) einen Großteil bzw. den Gesamtbestand ihrer Aktien verkauft haben.
Good luck
bernie55
ZLCS hat ein gute und zukunftsträchtige Produktpipeline, jedoch versteht das Management leider nicht,diese in der Öffentlichkeit entsprechend zu "verkaufen".
Der Stichtag , an dem ZLCS 10 Tage in Folge über 1 Dollar stehen muss , ist der 22/4/13
Das "Zeitfenster" ist meiner Meinung nach nicht mehr zu realiseren,ein R/S rückt quasi immer näher - und dieser wird bestimmt nicht kursfördernd für ZLCS sein.
Der aktuelle Aktien-Handel ist unterirdisch lasch und wird sogar durch gute News nicht mehr " aktiver".
Die allgemeine Unsicherheit und Enttäuschung ist aktuell bei vielen Anlegern zu spüren.Deshalb ist es nicht verwunderlich, dass viele Aktionäre ( auch WO User!) einen Großteil bzw. den Gesamtbestand ihrer Aktien verkauft haben.
Good luck
bernie55

Hallo, bin jetzt schon seit vier Wochen raus....mit geringem Verlust.
Hatte ich mir gedacht, das die Amis bis zum Delistingdate rausgehen bzw. kein Großer mehr vorher einsteigen wird.
Wenn es zum R/S kommt....werden wahrscheinlich nochmal min. 10-20 % Abschlag kommen.
Nach einem R/S kann man wieder überlegen einzusteigen....
aber zur Zeit sieht es düster für Zalicus aus....den Kursverlauf betreffend !
Hatte ich mir gedacht, das die Amis bis zum Delistingdate rausgehen bzw. kein Großer mehr vorher einsteigen wird.
Wenn es zum R/S kommt....werden wahrscheinlich nochmal min. 10-20 % Abschlag kommen.
Nach einem R/S kann man wieder überlegen einzusteigen....

aber zur Zeit sieht es düster für Zalicus aus....den Kursverlauf betreffend !
Antwort auf Beitrag Nr.: 44.435.919 von Earthfire am 15.04.13 19:27:31Hi earthfire, bin auch komplett ZLCS frei seit heutnachmittag (die letzten 5K in Stuttgart zu 0,43 losgeworden)
Es ist wirklich unglaublich mitanzusehen was schwache managern aus eigentlich gute Firmen & vielversprechend Aktien machen können...
Mach et jut ihr lieben
Es ist wirklich unglaublich mitanzusehen was schwache managern aus eigentlich gute Firmen & vielversprechend Aktien machen können...

Mach et jut ihr lieben

Unglaublich
 ZLCS im freien Fall befindlich, wir wissen alle wohl warum! ...bin heil froh die Rettungsleine mehrmals gezogen zu haben (obwohl es mir ziemlich schwer gefallen ist beim ursprünglichen drücken der 'sell button')
ZLCS im freien Fall befindlich, wir wissen alle wohl warum! ...bin heil froh die Rettungsleine mehrmals gezogen zu haben (obwohl es mir ziemlich schwer gefallen ist beim ursprünglichen drücken der 'sell button') Zitat von Growth2012: UnglaublichZLCS im freien Fall befindlich, wir wissen alle wohl warum!...
...bin heil froh die Rettungsleine mehrmals gezogen zu haben (obwohl es mir ziemlich schwer gefallen ist beim ursprünglichen drücken der 'sell button')
@growth2012
@earthfire ( MateCola- schmecktübrigens prima)

Somit sind "alle Hauptaktionäre des WO Baoards" aus ZLCS ausgestiegen

Jungs, das Leben geht weiter - sowohl mit steigenden und als auch mit fallenden Kursen.....

Grüße
bernie55

noch wer hier dabei? ;-)
ich bin gespannt wann es nun news zur Phase II gibt .... Lange kann es nicht mehr sein!
Z160 ...
Dann ist ja schon schön im plus... Bin erst rein, aber könnte dieses Jahr was nettes werden!
Z160 ...
Dann ist ja schon schön im plus... Bin erst rein, aber könnte dieses Jahr was nettes werden!
Zitat von takeProfit: ich bin gespannt wann es nun news zur Phase II gibt .... Lange kann es nicht mehr sein!
Z160 ...
Dann ist ja schon schön im plus... Bin erst rein, aber könnte dieses Jahr was nettes werden!
News kommen aller vorrausicht nach im Oktober !
musst mal in den US Boards lesen...
Wir müssen über die 0,84usct.....dann bricht sie wahrscheinlich richtig aus

und was ist mit dem RS ?
Zitat von takeProfit: und was ist mit dem RS ?
Der R/S hätte laut den Nasdaqstatuten bereits im Mai spätestens stattfinden sollen bzw. oder ein Delisting...und ab an die OTC
eine offizielle Meldung von Zalicus hierzu habe ich nicht gelesen....
Bei den Regeln am Nasdaq findet sich ein Passus der besagt das das Unternehmen eine Verlängerung von einem Jahr beantragen kann...wenn es schlüssige Gründe für eine Erholung darlegen kann.
Es finden sich nur Spekulationen...R/S kommt bzw. kommt nicht in den US boards
Es gibt aber auch genug Beispiele wo Unternehmen gelistet blieben trotz notierung unter der Dollar Marke und der Kurs irgendwann über einem Dollar wieder notierte...nach längerer Zeit....ohne das es ein Filling hierzu gab.
Aber da blicke ich auch nicht durch...
Vom Datum aus gesehen...ist die offizielle Frist für Zalicus bereits im April diesen Jahres abgelaufen....dh. es hätte eigendlich schon längts etwas passieren müssen !
Persönlich sehe ich bei dem derzeitigen Handelsvolumen bzw. einem anhaltenden Momentum...das der Kurs in ein paar Tagen über der dollarmarke notieren könnte...ohne große Problem.....aber könnte..das ist die Krux.
Im Prinzip könnte man auch sagen....wenn man sich den Verlauf bei Zalicus im chart genau anschaut in den letzten monaten...ist es schon sehr verwunderlich das das volumen und der kurs vor einem eigendlich täglich stattfindenden R/S so anzieht !

Hier ein Text aus einem engl board :
They don't need to conduct an R/S. On Sept 9 their NASDQ status expires, but NASDAQ gives an additional 6 month extension so you are looking at March/April 2014. HOWEVER, with the mere news of Z-160 arriving in Oct., and the PPS highly highly likely to stay above a dollar for at least 30 days, I highly doubt they will entertain an R/S. An R/S would be good though in this instance to allow more Investor firms above $5.00 to make a great investment. If Z-160 is as good as they say it is the revenue potential is incredible.
Also die frist wurde verlängert bis März/April 2014 sollte nun eine positive Meldung im Oktober kommen... Sollte eh klar sein das der kurs länger als 30 Tage über 1 $ sein sollte ...
Also die Daumen drücken oktober ist bald :-) (Hoffentlich knallt das ding nach oben ....)
They don't need to conduct an R/S. On Sept 9 their NASDQ status expires, but NASDAQ gives an additional 6 month extension so you are looking at March/April 2014. HOWEVER, with the mere news of Z-160 arriving in Oct., and the PPS highly highly likely to stay above a dollar for at least 30 days, I highly doubt they will entertain an R/S. An R/S would be good though in this instance to allow more Investor firms above $5.00 to make a great investment. If Z-160 is as good as they say it is the revenue potential is incredible.
Also die frist wurde verlängert bis März/April 2014 sollte nun eine positive Meldung im Oktober kommen... Sollte eh klar sein das der kurs länger als 30 Tage über 1 $ sein sollte ...
Also die Daumen drücken oktober ist bald :-) (Hoffentlich knallt das ding nach oben ....)
Antwort auf Beitrag Nr.: 45.375.899 von takeProfit am 03.09.13 14:43:21Na..in ein paar Wochen kannst du hier aller wahrscheinlichkeit nach..ein paar viel Prozente mitnehmen....dh. Take your Profit 
Spass beiseite...sieht sehr gut aus...Chartechnisch müssen wir die 84 US ct..nachhaltig reissen...dann wird es vermutlich sehr schnell über einen dollar laufen !
Ich hatte heute mal richtig Glück...hatte noch Cash übrig...und mir vor lauter Langeweile...ein paar K zalicus vor Eröffnung gekauft...und dann der Newsflow später...das war doch mal ein schönes timing


Spass beiseite...sieht sehr gut aus...Chartechnisch müssen wir die 84 US ct..nachhaltig reissen...dann wird es vermutlich sehr schnell über einen dollar laufen !
Ich hatte heute mal richtig Glück...hatte noch Cash übrig...und mir vor lauter Langeweile...ein paar K zalicus vor Eröffnung gekauft...und dann der Newsflow später...das war doch mal ein schönes timing


zweimal an der 0,84 ct abgeprallt....aber die kommen noch 





Antwort auf Beitrag Nr.: 45.379.579 von Earthfire am 03.09.13 21:31:24power hour is noch nich um....

Zitat von ThumsUp: power hour is noch nich um....
Zalicus Inc. Real Time Stock Quotes
$0.835
*
0.125
negative
17.61%
*Real-Time - data as of 9/3/2013
Schön geschlossen....und feines Volumen...

Im Pre ist sie über die 0,84ct...aber mit 100 Shares

Wenn der Anstieg morgen hält bzw. weiter ausgebaut werden kann...sehe ich diese woche noch die 1 vorm Komma stehen !

so kann es weiter gehen...
Aber bitte schön regelmäßig positive Meldungen dazu...
Bin gespannt was es am 9. für Infos gibt!
Kenne mich mit Pharma Aktien nicht so aus, außer das wenn es positive Meldungen gibt oft extrem aufwärts geht...
Nur wer jetzt dabei ist dann auch lang genug Mut hat dabei zu bleiben...
Potenzial ist da...
Der Dollar kann kommen und positive News bitte dann auch...
Aber bitte schön regelmäßig positive Meldungen dazu...
Bin gespannt was es am 9. für Infos gibt!
Kenne mich mit Pharma Aktien nicht so aus, außer das wenn es positive Meldungen gibt oft extrem aufwärts geht...
Nur wer jetzt dabei ist dann auch lang genug Mut hat dabei zu bleiben...
Potenzial ist da...
Der Dollar kann kommen und positive News bitte dann auch...
Antwort auf Beitrag Nr.: 45.380.399 von takeProfit am 04.09.13 01:52:11Morgen  , ja bei den Biotecs geht es bei einer positiven Prognose..oft steil auf...aber auch steil ab
, ja bei den Biotecs geht es bei einer positiven Prognose..oft steil auf...aber auch steil ab  ...für den Fall das es nicht passt !
...für den Fall das es nicht passt !
Aussserdem sieht Biotecs sehr Volatil im Tagesgeschäft...dh. kleine 10% Schwankungen muss man schon mal gelassen aussitzen.
Ich kenne und beobachte Zalicus nun bereits seit 1,5 Jahren und alleine anhand des gehandelten Volumen kann ich dir sagen das ich anhand der großen Nachfrage von positiven Ergebnissen ausgehe.
Ich bin damals raus um nicht in den Resplit zu geraten und hatte hier kleine Verluste eingefahren.
Wir müssen nun mal die Fillings nach Insidertrades bzw. Instikäufen durchforsten...ich denke da müsste bald was kommen.
Z160 und Z 944 haben sog. Blockbusterpotenzial dh. hier ist alles möglich....bis in den Bereich von 3-5$ in den nächsten Monaten...
Da du dich nicht so auskennst bei Biotecs....schau dir mal Gale an...auch viel Potenzial...da bin ich auch drin.
So ...und hier der Aftermmarket .
Zalicus Inc. After Hours Trading
$.8899
*
0.0549
negative
6.57%
Read more: http://www.nasdaq.com/symbol/zlcs/after-hours#ixzz2dtmxAL2B
Da bin ich mal gespannt was heute geht....sollte die Nachfrage auf diesem Level bleiben...sehen wir evtl. heute bereits den dollar !

Leider wurde gestern ein Up-Gap gerissen :
Open Gaps
Direction Date range
up Sept-03-2013 0.73 to 0.75
Ich hoffe mal nicht das es unbedingt geschlossen werden muss
 , ja bei den Biotecs geht es bei einer positiven Prognose..oft steil auf...aber auch steil ab
, ja bei den Biotecs geht es bei einer positiven Prognose..oft steil auf...aber auch steil ab  ...für den Fall das es nicht passt !
...für den Fall das es nicht passt !Aussserdem sieht Biotecs sehr Volatil im Tagesgeschäft...dh. kleine 10% Schwankungen muss man schon mal gelassen aussitzen.
Ich kenne und beobachte Zalicus nun bereits seit 1,5 Jahren und alleine anhand des gehandelten Volumen kann ich dir sagen das ich anhand der großen Nachfrage von positiven Ergebnissen ausgehe.
Ich bin damals raus um nicht in den Resplit zu geraten und hatte hier kleine Verluste eingefahren.
Wir müssen nun mal die Fillings nach Insidertrades bzw. Instikäufen durchforsten...ich denke da müsste bald was kommen.
Z160 und Z 944 haben sog. Blockbusterpotenzial dh. hier ist alles möglich....bis in den Bereich von 3-5$ in den nächsten Monaten...

Da du dich nicht so auskennst bei Biotecs....schau dir mal Gale an...auch viel Potenzial...da bin ich auch drin.
So ...und hier der Aftermmarket .
Zalicus Inc. After Hours Trading
$.8899
*
0.0549
negative
6.57%
Read more: http://www.nasdaq.com/symbol/zlcs/after-hours#ixzz2dtmxAL2B
Da bin ich mal gespannt was heute geht....sollte die Nachfrage auf diesem Level bleiben...sehen wir evtl. heute bereits den dollar !


Leider wurde gestern ein Up-Gap gerissen :
Open Gaps
Direction Date range
up Sept-03-2013 0.73 to 0.75
Ich hoffe mal nicht das es unbedingt geschlossen werden muss

fing so gut an...
Eine bessere lange als zuvor und das ding geht trotzdem runter...
Eine bessere lange als zuvor und das ding geht trotzdem runter...
was erwartet ihr von Montag?
Hat sich jetzt gut bei 0,80 USD gehalten, bei positiver Meldung dürfte endlich 1 $ geknackt werden...
Hat sich jetzt gut bei 0,80 USD gehalten, bei positiver Meldung dürfte endlich 1 $ geknackt werden...
Zitat von takeProfit: was erwartet ihr von Montag?
Hat sich jetzt gut bei 0,80 USD gehalten, bei positiver Meldung dürfte endlich 1 $ geknackt werden...
Bei dem derzeitigen Handelsvolumen und dem Momentum habe ich keine großen Zweifel das der Dollar bald falen wird....somit dann auch eine psychologisch wichtige Marke für den weiteren Verlauf des Kurses.
Auf die Vola bei Biotecs hatte ich ja bereits in meinem letzten Beitrag hingewiesen...

Da sind...10 % Minus Intraday...um dann doch noch im Plus zu schliessen keine Seltenheit !
die frage ist: pokern viele auf news heute? Sollte nichts kommen, geht es vielleicht schnell richtig runter...
Oktober ist für Aktien eine lange Zeit...
Oktober ist für Aktien eine lange Zeit...
$ZLCS Zalicus Inc has added a news release to its Investor Relations website.
Title: Zalicus Initiates Phase 1b Clinical Study of Z944, Its Novel, Oral, T-type Calcium Channel Blocker, for the Treatment of Pain
Date(s): 9-Sep-2013 7:30 AM
For a complete listing of our news releases, please click here
Experimental Clinical Study Expected to Complete During the Fourth Quarter 2013
CAMBRIDGE, Mass. --(BUSINESS WIRE)--Sep. 9, 2013-- Zalicus Inc. (Nasdaq Capital Market:ZLCS) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain, today announced that it has initiated a Phase 1b clinical study with Z944, its novel oral T-type calcium channel blocker, in an experimental clinical model of neuropathic pain using Laser-Evoked Potentials (LEP). Results from the LEP study are expected in the fourth quarter 2013.
"Measuring Laser Evoked Potentials is an experimental clinical model that offers both subjective and objective measures to assess the potential of Z944 to modulate pain signaling," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus . "T-type calcium channels are known to be widely implicated in the frequency and intensity of pain signals and the results of this study will further inform Zalicus' development plans for Z944 in a variety of potential indications as we work to optimize a modified release formulation of Z944 for future clinical trials."
The LEP clinical model is a type of experimental medicine study designed to provide both objective and subjective data on a drug's ability to modulate pain signaling. In this model electrical voltage fluctuations evoked by laser thermal stimulation are quantified with vertex-Electroencephalography (EEG) in addition to a subject-reported pain score. Many currently approved and emerging pain drugs have been evaluated using the LEP model, providing the ability to benchmark the efficacy of Z944 against other therapies.
About Z944 and T-type Calcium Channel Blockers
Z944 is a novel, oral, state-dependent, selective T-type calcium channel blocker that has demonstrated preclinical efficacy in multiple inflammatory pain models. T-type calcium channels have been recognized as key targets for therapeutic intervention in a broad range of cell functions and have been implicated in pain signaling. Zalicus has completed Phase 1 single and multiple ascending dose clinical studies evaluating the safety and tolerability of Z944 and is continuing the clinical development of Z944 during 2013.
About Zalicus
Zalicus Inc. (Nasdaq Capital Market:ZLCS) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain. Zalicus has a portfolio of proprietary clinical-stage product candidates targeting pain such as Z160 and Z944 and has entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. Zalicus applies its expertise in the discovery and development of selective ion channel modulators and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease. To learn more about Zalicus , please visit www.zalicus.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus , its product candidate Z944, its potential and the plans for its further clinical preclinical development, the Zalicus selective Ion channel modulation technology and its other business plans. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus and its product candidates may be identified by words like "believe," "expect," "may," "will," "should," "seek," "plan" or "could" and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the development and regulatory approval of Zalicus' product candidates, including risks relating to the clinical development of Z944, the unproven nature of the Zalicus drug discovery technologies, the Company's ability to obtain additional financing or funding for its research and development, and those other risks that can be found in the "Risk Factors" section of Zalicus' annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission . Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management's current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
(c) 2013 Zalicus Inc. All rights reserved.
Title: Zalicus Initiates Phase 1b Clinical Study of Z944, Its Novel, Oral, T-type Calcium Channel Blocker, for the Treatment of Pain
Date(s): 9-Sep-2013 7:30 AM
For a complete listing of our news releases, please click here
Experimental Clinical Study Expected to Complete During the Fourth Quarter 2013
CAMBRIDGE, Mass. --(BUSINESS WIRE)--Sep. 9, 2013-- Zalicus Inc. (Nasdaq Capital Market:ZLCS) a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain, today announced that it has initiated a Phase 1b clinical study with Z944, its novel oral T-type calcium channel blocker, in an experimental clinical model of neuropathic pain using Laser-Evoked Potentials (LEP). Results from the LEP study are expected in the fourth quarter 2013.
"Measuring Laser Evoked Potentials is an experimental clinical model that offers both subjective and objective measures to assess the potential of Z944 to modulate pain signaling," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus . "T-type calcium channels are known to be widely implicated in the frequency and intensity of pain signals and the results of this study will further inform Zalicus' development plans for Z944 in a variety of potential indications as we work to optimize a modified release formulation of Z944 for future clinical trials."
The LEP clinical model is a type of experimental medicine study designed to provide both objective and subjective data on a drug's ability to modulate pain signaling. In this model electrical voltage fluctuations evoked by laser thermal stimulation are quantified with vertex-Electroencephalography (EEG) in addition to a subject-reported pain score. Many currently approved and emerging pain drugs have been evaluated using the LEP model, providing the ability to benchmark the efficacy of Z944 against other therapies.
About Z944 and T-type Calcium Channel Blockers
Z944 is a novel, oral, state-dependent, selective T-type calcium channel blocker that has demonstrated preclinical efficacy in multiple inflammatory pain models. T-type calcium channels have been recognized as key targets for therapeutic intervention in a broad range of cell functions and have been implicated in pain signaling. Zalicus has completed Phase 1 single and multiple ascending dose clinical studies evaluating the safety and tolerability of Z944 and is continuing the clinical development of Z944 during 2013.
About Zalicus
Zalicus Inc. (Nasdaq Capital Market:ZLCS) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain. Zalicus has a portfolio of proprietary clinical-stage product candidates targeting pain such as Z160 and Z944 and has entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. Zalicus applies its expertise in the discovery and development of selective ion channel modulators and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease. To learn more about Zalicus , please visit www.zalicus.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus , its product candidate Z944, its potential and the plans for its further clinical preclinical development, the Zalicus selective Ion channel modulation technology and its other business plans. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus and its product candidates may be identified by words like "believe," "expect," "may," "will," "should," "seek," "plan" or "could" and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the development and regulatory approval of Zalicus' product candidates, including risks relating to the clinical development of Z944, the unproven nature of the Zalicus drug discovery technologies, the Company's ability to obtain additional financing or funding for its research and development, and those other risks that can be found in the "Risk Factors" section of Zalicus' annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission . Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management's current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
(c) 2013 Zalicus Inc. All rights reserved.
A Form 8-K regarding Zalicus Inc has been filed with the United States Securities and Exchange Commission.
http://phx.corporate-ir.net/phoenix.zhtml?c=148036&p=irol-se…
http://phx.corporate-ir.net/phoenix.zhtml?c=148036&p=irol-se…
Antwort auf Beitrag Nr.: 45.410.733 von takeProfit am 09.09.13 14:20:33Pre-Market Volume: Pre-Market High: Pre-Market Low:
8,460 $ .895
(08:09:06 AM) $ .87
(08:52:49 AM)
Read more: http://www.nasdaq.com/symbol/zlcs/premarket#ixzz2eOqZqgcR
Das sieht doch schon mal gut aus; volumen könnte höher sein
8,460 $ .895
(08:09:06 AM) $ .87
(08:52:49 AM)
Read more: http://www.nasdaq.com/symbol/zlcs/premarket#ixzz2eOqZqgcR
Das sieht doch schon mal gut aus; volumen könnte höher sein

also können dieses Jahr noch nette NEWS folgen...
Jetzt heisst es zurücklegen und Daumen drücken!
Gut aufgestellt weit voraus!
Jetzt müsste nur der Dollar nachhaltig sein, das würde beruhigen... wegen dem R/S
Jetzt heisst es zurücklegen und Daumen drücken!
Gut aufgestellt weit voraus!
Jetzt müsste nur der Dollar nachhaltig sein, das würde beruhigen... wegen dem R/S
:-(
naja, fette news sind auch was anderes ...
naja, fette news sind auch was anderes ...
bin noch leicht im Plus ... Die Tage ging es ja NUR runter! :-(
es scheinen doch viele Angst wegen einen eventuellen R/S zu haben...
Wird Zeit das es NEWS gibt... :-) Das ding muss langsam über nem 1$
Wird Zeit das es NEWS gibt... :-) Das ding muss langsam über nem 1$
Antwort auf Beitrag Nr.: 45.499.601 von takeProfit am 23.09.13 10:16:19Ja?
Chart sieht doch o.k. aus, Unterkante getestet und abgeprallt-sieht bullsisch aus mMn
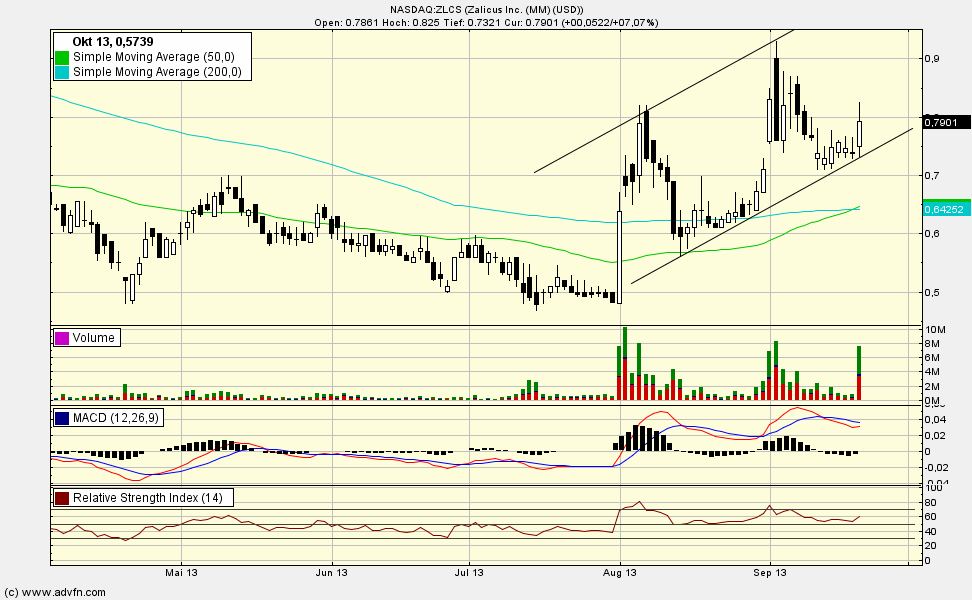
Chart sieht doch o.k. aus, Unterkante getestet und abgeprallt-sieht bullsisch aus mMn
hält sich gut... Jetzt positive NEWS langsam bitte...
Antwort auf Beitrag Nr.: 45.527.985 von takeProfit am 27.09.13 13:13:31News sind drausen ,
,
http://www.accessdata.fda.gov/scripts/opdlisting/oopd/OOPD_R…
Mal schauen was heute geht.
 ,
,http://www.accessdata.fda.gov/scripts/opdlisting/oopd/OOPD_R…
Mal schauen was heute geht.
ZLCS News Orphan Drug Designations 06:37 #55
Search Orphan Drug Designations and ApprovalsPrint Share1 E-mail2 FDA Home3 Developing Products for Rare Diseases & Conditions4 -
Start of page content Return to search Page to restart searchReturn to Orphan Designation Search PageReturn to Search Page
Generic Name: 1-(4-benzhydrylpiperazin-1-yl)-3,3-diphenylpropan-1-one
Trade Name: n/a
Date Designated: 09-26-2013
Orphan Designation: Management of postherpetic neuralgia
Orphan Designation Status: Designated
FDA Orphan Approval Status: Not FDA Approved for Orphan Indication
Sponsor: Zalicus Pharmaceuticals Ltd.
245 First Street
Cambridge, MA 02142
The sponsor address listed is the last reported by the sponsor to OOPD.
Search Orphan Drug Designations and ApprovalsPrint Share1 E-mail2 FDA Home3 Developing Products for Rare Diseases & Conditions4 -
Start of page content Return to search Page to restart searchReturn to Orphan Designation Search PageReturn to Search Page
Generic Name: 1-(4-benzhydrylpiperazin-1-yl)-3,3-diphenylpropan-1-one
Trade Name: n/a
Date Designated: 09-26-2013
Orphan Designation: Management of postherpetic neuralgia
Orphan Designation Status: Designated
FDA Orphan Approval Status: Not FDA Approved for Orphan Indication
Sponsor: Zalicus Pharmaceuticals Ltd.
245 First Street
Cambridge, MA 02142
The sponsor address listed is the last reported by the sponsor to OOPD.
Zalicus’ Z160 Receives Orphan Drug Designation for the Management of Postherpetic Neuralgia
Business WirePress Release: Zalicus Inc. – 6 minutes ago
Email
Print
RELATED CONTENT
RELATED QUOTES
Symbol Price Change
ZLCS 0.82 +0.06
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Zalicus Inc. (Nasdaq Capital Market: ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain, today announced that Z160, its first-in-class, oral, state-dependent, selective N-type calcium channel (Cav 2.2) modulator in development for chronic neuropathic pain has received Orphan Drug designation from the U.S. Food and Drug Administration (FDA) for the management of postherpetic neuralgia. Postherpetic neuralgia (PHN) is a painful neuropathic condition resulting from an outbreak of the herpes zoster virus, otherwise known as shingles.
Zalicus announced it had completed patient enrollment in two Phase 2 clinical studies of Z160 for chronic neuropathic pain indications including postherpetic neuralgia and lumbosacral radiculopathy on September 3, 2013. Top-line results of both studies are expected in the fourth quarter of 2013.
"The FDA’s designation of Z160 as having orphan drug status is an important milestone for Zalicus as we continue the clinical development work required for potential FDA approval of Z160," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “We look forward to evaluating and reporting the activity of Z160 in chronic neuropathic pain indications later this year, including postherpetic neuralgia and lumbosacral radiculopathy.”
Orphan drug designation is granted by the FDA Office of Orphan Drug Products to novel drugs or biologics that treat a rare disease or condition affecting fewer than 200,000 patients in the U.S. The designation provides the drug developer with a seven-year period of U.S. marketing exclusivity if the drug is the first of its type approved for the specified indication or if it demonstrates superior safety, efficacy, or a major contribution to patient care versus another drug of its type previously granted the designation for the same indication, as well as with potential tax credits for clinical research costs, the potential to apply for annual grant funding, clinical research trial design assistance and waiver of Prescription Drug User Fee Act (PDUFA) filing fees.
About Z160
Zalicus is currently advancing Z160, a first-in-class, oral, state-dependent, selective N-type calcium channel (Cav 2.2) modulator, through Phase 2 clinical development in chronic neuropathic pain. Z160 is designed to selectively target neuronal pain signaling by modulating neurons that are undergoing high-frequency firing. Z160 has demonstrated efficacy in multiple animal models of neuropathic and inflammatory pain, suggesting that it has the potential to treat a broad range of chronic pain conditions. Additionally, clinical trials in over 200 subjects have established Z160 as a safe and well tolerated drug candidate. N-type calcium channels have been recognized as key targets in controlling pain because of their key role in transmitting pain through the spinal nerves to the brain.
About Zalicus
Zalicus Inc. (Nasdaq Capital Market: ZLCS) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain. Zalicus has a portfolio of proprietary clinical-stage product candidates targeting pain such as Z160 and Z944 and has entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. Zalicus applies its expertise in the discovery and development of selective ion channel modulators and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease. To learn more about Zalicus, please visit www.zalicus.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus, its product candidate Z160, its potential and the plans for its clinical development, its status as a designated orphan drug, the Zalicus selective Ion channel modulation technology and related preclinical product candidates and Zalicus’ other business plans. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus and its product candidates may be identified by words like "believe," "expect," "may," "will," "should," "seek," “plan” or “could” and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the development and regulatory approval of Zalicus’ product candidates, including risks relating to formulation and clinical development of Z160, the ability of Zalicus to initiate and successfully complete clinical trials of its product candidates, the unproven nature of the Zalicus drug discovery technologies, the Company's ability to obtain additional financing or funding for its research and development, and those other risks that can be found in the "Risk Factors" section of Zalicus' annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission. Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management’s current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
(c) 2013 Zalicus Inc. All rights reserved.
Contact:
Zalicus Inc.
Justin Renz, CFO, 617-301-7575
JRenz@zalicus.com
or
Gina Nugent, 617-460-3579
gnugent@zalicus.com
Business WirePress Release: Zalicus Inc. – 6 minutes ago
RELATED CONTENT
RELATED QUOTES
Symbol Price Change
ZLCS 0.82 +0.06
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Zalicus Inc. (Nasdaq Capital Market: ZLCS), a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain, today announced that Z160, its first-in-class, oral, state-dependent, selective N-type calcium channel (Cav 2.2) modulator in development for chronic neuropathic pain has received Orphan Drug designation from the U.S. Food and Drug Administration (FDA) for the management of postherpetic neuralgia. Postherpetic neuralgia (PHN) is a painful neuropathic condition resulting from an outbreak of the herpes zoster virus, otherwise known as shingles.
Zalicus announced it had completed patient enrollment in two Phase 2 clinical studies of Z160 for chronic neuropathic pain indications including postherpetic neuralgia and lumbosacral radiculopathy on September 3, 2013. Top-line results of both studies are expected in the fourth quarter of 2013.
"The FDA’s designation of Z160 as having orphan drug status is an important milestone for Zalicus as we continue the clinical development work required for potential FDA approval of Z160," commented Mark H.N. Corrigan, MD, President and CEO of Zalicus. “We look forward to evaluating and reporting the activity of Z160 in chronic neuropathic pain indications later this year, including postherpetic neuralgia and lumbosacral radiculopathy.”
Orphan drug designation is granted by the FDA Office of Orphan Drug Products to novel drugs or biologics that treat a rare disease or condition affecting fewer than 200,000 patients in the U.S. The designation provides the drug developer with a seven-year period of U.S. marketing exclusivity if the drug is the first of its type approved for the specified indication or if it demonstrates superior safety, efficacy, or a major contribution to patient care versus another drug of its type previously granted the designation for the same indication, as well as with potential tax credits for clinical research costs, the potential to apply for annual grant funding, clinical research trial design assistance and waiver of Prescription Drug User Fee Act (PDUFA) filing fees.
About Z160
Zalicus is currently advancing Z160, a first-in-class, oral, state-dependent, selective N-type calcium channel (Cav 2.2) modulator, through Phase 2 clinical development in chronic neuropathic pain. Z160 is designed to selectively target neuronal pain signaling by modulating neurons that are undergoing high-frequency firing. Z160 has demonstrated efficacy in multiple animal models of neuropathic and inflammatory pain, suggesting that it has the potential to treat a broad range of chronic pain conditions. Additionally, clinical trials in over 200 subjects have established Z160 as a safe and well tolerated drug candidate. N-type calcium channels have been recognized as key targets in controlling pain because of their key role in transmitting pain through the spinal nerves to the brain.
About Zalicus
Zalicus Inc. (Nasdaq Capital Market: ZLCS) is a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain. Zalicus has a portfolio of proprietary clinical-stage product candidates targeting pain such as Z160 and Z944 and has entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. Zalicus applies its expertise in the discovery and development of selective ion channel modulators and its combination high throughput screening capabilities to discover innovative therapeutics for itself and its collaborators in the areas of pain, inflammation, oncology and infectious disease. To learn more about Zalicus, please visit www.zalicus.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 concerning Zalicus, its product candidate Z160, its potential and the plans for its clinical development, its status as a designated orphan drug, the Zalicus selective Ion channel modulation technology and related preclinical product candidates and Zalicus’ other business plans. These forward-looking statements about future expectations, plans, objectives and prospects of Zalicus and its product candidates may be identified by words like "believe," "expect," "may," "will," "should," "seek," “plan” or “could” and similar expressions and involve significant risks, uncertainties and assumptions, including risks related to the development and regulatory approval of Zalicus’ product candidates, including risks relating to formulation and clinical development of Z160, the ability of Zalicus to initiate and successfully complete clinical trials of its product candidates, the unproven nature of the Zalicus drug discovery technologies, the Company's ability to obtain additional financing or funding for its research and development, and those other risks that can be found in the "Risk Factors" section of Zalicus' annual report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Zalicus periodically files with the Securities and Exchange Commission. Actual results may differ materially from those Zalicus contemplated by these forward-looking statements. These forward-looking statements reflect management’s current views and Zalicus does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
(c) 2013 Zalicus Inc. All rights reserved.
Contact:
Zalicus Inc.
Justin Renz, CFO, 617-301-7575
JRenz@zalicus.com
or
Gina Nugent, 617-460-3579
gnugent@zalicus.com
aber Ergebnisse gab es auch nicht... Meint Ihr jetzt geht es erstmal wieder runter bis die Ergebnisse kommen?
ist es korrekt das vermutet wird das positive NEWS (Studien Ergebnisse) vor dem 7. oktober kommen MÜSSEN ? Um einen R/S zu entkommen?
Der kurs muss 10 Tage hintereinander über 1USD sein ?
Wie seht Ihr die Lage?
Der kurs muss 10 Tage hintereinander über 1USD sein ?
Wie seht Ihr die Lage?
Zitat von takeProfit: ist es korrekt das vermutet wird das positive NEWS (Studien Ergebnisse) vor dem 7. oktober kommen MÜSSEN ? Um einen R/S zu entkommen?
Der kurs muss 10 Tage hintereinander über 1USD sein ?
Wie seht Ihr die Lage?
Hi , die AW auf deine Frage...hast du dir vor Wochen selbst gegeben :
" Also die frist wurde verlängert bis März/April 2014 sollte nun eine positive Meldung im Oktober kommen... Sollte eh klar sein das der kurs länger als 30 Tage über 1 $ sein sollte ...
Also die Daumen drücken oktober ist bald :-) (Hoffentlich knallt das ding nach oben ....) "
Eventuell ist ein R/S auch positiv. Schaut euch mal den Chart von Neostem (Symbol: NBS). Die hatten nen 10/1 R/S gemacht. Seit dem ist der Kurs um ca 30% gestiegen, weil manche Institutionelle Anleger erst ab einem bestimmten Kurswert in eine Aktie invetieren dürfen.
Zitat von Bibbes21: Eventuell ist ein R/S auch positiv. Schaut euch mal den Chart von Neostem (Symbol: NBS). Die hatten nen 10/1 R/S gemacht. Seit dem ist der Kurs um ca 30% gestiegen, weil manche Institutionelle Anleger erst ab einem bestimmten Kurswert in eine Aktie invetieren dürfen.
Dem stimme ich zu....ist auch immer von der "Sache" abhängig wie es nach einem R/S weitergeht....wenn der R/S nur dem kein Delisting dient, und die Aussichten bei dem Unternehmen eher mau sind....geht es meist steil Bergab.
Aber bei Zalicus bedeutet gute Ergebisse....ein Blockbusterpotenzial evtl. sogar einen Buyout....noch vor einem Approval

Ich vermute aber stark, das es in der nächsten Woche sehr grün werden wird hier....und die dauerhaft Notierung über der Dollarmarke zeitnah da sein wird.
Antwort auf Beitrag Nr.: 45.533.267 von Earthfire am 28.09.13 13:42:50Gestern snd 23Mio Aktien, 1/6 aller Aktien gehandelt worden. So hoch wie nie zuvor das Volumen. Und das waren keine Daytrader bei dem Vol-da steckt noch einiges drin denke ich.
JUHHUUU weiter :-)
Wo bleiben alle denn hier ?????
Wo bleiben alle denn hier ?????
huiii hält sich gut... Alles über nen € ist super... Heute ist schon der 2. hoffe auf baldige POSITIVE PII Ergebnisse...
was sagt der Chart? Was meint Ihr ?
Kann ja auch erstmal runter gehen ... Oder ist klar das diesen Monat die Ergebnisse kommen sollen?
Kann ja auch erstmal runter gehen ... Oder ist klar das diesen Monat die Ergebnisse kommen sollen?
Boahhh was ist denn da los?!!!
von +16% jetzt so runter!!!!!!!!!!! warum das?!!!!
von +16% jetzt so runter!!!!!!!!!!! warum das?!!!!
Solange es über einem Dollar bleibt bin ich erstmal zufrieden...
Weiteres kommt bei positiven PII News...
Weiteres kommt bei positiven PII News...
Antwort auf Beitrag Nr.: 45.558.417 von takeProfit am 02.10.13 21:35:51ok, jetzt ist es also raus. wird morgen viele panikvekäufe geben. könnte sich lohnen auf die lauer zu legen. bei den vielen käufern über 1 usd die letzten tage kann ich mir einen krassen absacker auf bis 4 usd vorstellen... wer weiss
lg, thums
lg, thums
Zitat von ThumsUp: ok, jetzt ist es also raus. wird morgen viele panikvekäufe geben. könnte sich lohnen auf die lauer zu legen. bei den vielen käufern über 1 usd die letzten tage kann ich mir einen krassen absacker auf bis 4 usd vorstellen... wer weiss
lg, thums
die Panik wird gerade im Aftermarket bereits abghandelt...denke nicht das es hier noch weiter down gehen wird....die Story und die Aussichten haben sich nicht im geringsten verändert...nur die Sharezahl im Depot..sieht ewas anders aus

Schaun wir mal.........
Antwort auf Beitrag Nr.: 45.558.985 von Earthfire am 02.10.13 22:59:35recht haste; aber psyche tickt anders. morgen wird verkauft was das zeug hält; und diejenigen die von der story überzeugt sind können morgen auf schnäppchenjagd gehen. ich gehe von einem ordentlich dip die ersten 90min aus. dann kann die wundersame wiederauferstehung beginnen.. schließlich geht das gerücht um, viele institutionelle könnten erst oberhalb von 3usd kaufen - ob da was dran ist weiß ich nicht. ich lege mich auf die lauer morgen...
lg, thums
lg, thums
Ist der gewinn jetzt wechh ? Zählt nur die Aktien Anzahl????
Antwort auf Beitrag Nr.: 45.559.051 von takeProfit am 02.10.13 23:10:06dividiere die aktienzahl durch 6 und du kennst deine neue scheinzahl; und der preis: vereinfacht gesagt:mulitpliziere ihn (den heutigen) mit 6. so wird´s wahrscheinlich aber nicht werden; vom th bis in die ah über 30% verlust - das löst emotionen aus. angst und panik werden den kurs (mgl) tüchtig drück, anschließend wird (mgl) die gier zuschlagen... keine ahnung aber möglich. habe einige r/s erlebt die zu üblen einbrüchen geführt habe - zumindest kruzfristig. panikverkäufe einzukalkulieren kann sich hier vorteilhaft auswirken - mmn
ja aber mein gewinn von 60% den ich in USD nun hatte wäre damit nun weg ?
Kann ja nicht mehr verkaufen...
Kann ja nicht mehr verkaufen...
Antwort auf Beitrag Nr.: 45.559.189 von takeProfit am 02.10.13 23:34:18sk heute war 1,20 ergo wären dies morgen 7,20usd; theoretisch stünden dein 60% noch unverändert da - wenn denn 7,20usd erreicht werden. kann auch anders laufen.genau das spiegelt der ah-verlauf wieder - zwischen 1,20 und 0,90 liegen imerhin 25%
Antwort auf Beitrag Nr.: 45.559.301 von Bibbes21 am 02.10.13 23:57:37nee muss auch nicht. kann aber. viele habe weit über 1usd gekauft - von denen werden wahrscheinlich einige panisch verkaufen. und ich bin long hier - für mich wären das schnäppchen. kurzfristig ist mir egal ob ich 50 oder 20% im plus bin. bis dezember sehe ich höhere kurse.
Danke für Deine Erklärung...
Habe gerade mal in mein depot gesehen:
Habe gerade nachgesehen bin mit 11.000 Stück dabei : aktuell +63,17% im Plus...
Dachte immer bei einem Reverse Split zählt die Anzahl der Aktien nur :-/
Sprich der aktuelle Preis wird nicht eingerechnet...
Warum sehen viele ein R/S dann so negativ?
Kann doch Vorteile haben...
Aber ohne R/S wäre mir auch lieber....
Habe gerade mal in mein depot gesehen:
Habe gerade nachgesehen bin mit 11.000 Stück dabei : aktuell +63,17% im Plus...
Dachte immer bei einem Reverse Split zählt die Anzahl der Aktien nur :-/
Sprich der aktuelle Preis wird nicht eingerechnet...
Warum sehen viele ein R/S dann so negativ?
Kann doch Vorteile haben...
Aber ohne R/S wäre mir auch lieber....
Also wer an die Story glaubt bleibt dabei!
Bei einem höheren kurs werden andere die Aktie eher wagen zu kaufen...
Ich lasse alles so.... und warte ab!
Sollte es bald positive Ergebnisse der PII Studie geben sind die Gewinner die Aktien haben....
Bei einem höheren kurs werden andere die Aktie eher wagen zu kaufen...
Ich lasse alles so.... und warte ab!
Sollte es bald positive Ergebnisse der PII Studie geben sind die Gewinner die Aktien haben....
Antwort auf Beitrag Nr.: 45.559.315 von ThumsUp am 03.10.13 00:03:31Hier wird es auch erst mal runter gehen. Nach diesem Anstieg kein Wunder. Da sind zu viele zittrige Hände und Daytrader drin. Die wird sich jetzt erst mal abkühlen und dann gehts weiter.

Aber die frage warum jetzt ein R/S ?
Kritiker oder ängstliche meinen : damit bei keinen guten Studien der Kurs wenigstens noch bei einem Dollar sein wird...
Kritiker oder ängstliche meinen : damit bei keinen guten Studien der Kurs wenigstens noch bei einem Dollar sein wird...
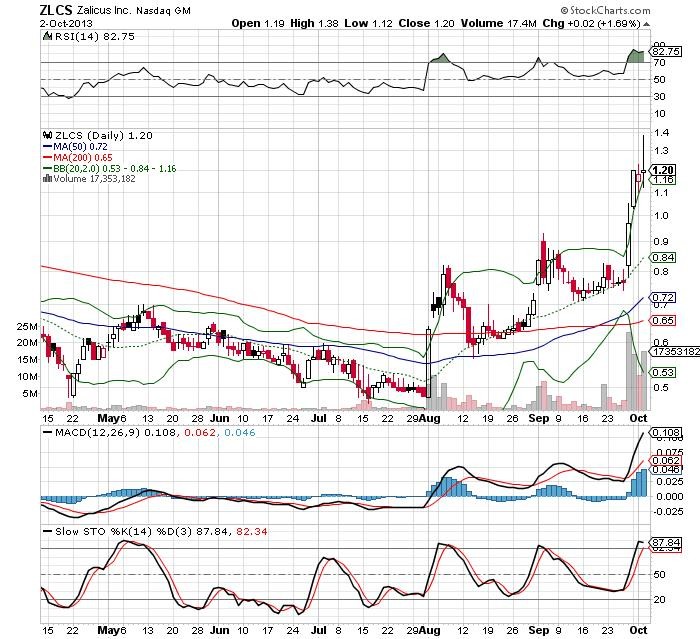
Wie man sieht ist da alles am Anschlag.
Antwort auf Beitrag Nr.: 45.559.385 von takeProfit am 03.10.13 00:25:01Jupp, genau das hab ich mir vorhin auch gedacht. Könnte so rüber kommen. Das Timing ist schon ziemlich blöde. Ich hätte noch zwei, drei Wochen gewartet bis sich der Kurs über 1 Dollar stabilisiert hat und ein erzwungener R/S vom Tisch wäre.
aber wie aus der Erklärung lesen kann ist dies schon länger beschlossen...
Da frage ich mich ob wirklich die Ergebnisse positiv sind...
Werden die bald wirklich veröffentlicht?
Wenn dem so sei wäre doch klar das der kurs in die Decke geht...
Oder Zwischenergebnisse gebracht...
Dann wäre der kurs über ein dollar und der r/S vom Tisch...
Tja, kann wirklich in alle Richtungen gehen...
Was ist am Anschlag? sehe die Grafik nicht auf dem Handy...
Da frage ich mich ob wirklich die Ergebnisse positiv sind...
Werden die bald wirklich veröffentlicht?
Wenn dem so sei wäre doch klar das der kurs in die Decke geht...
Oder Zwischenergebnisse gebracht...
Dann wäre der kurs über ein dollar und der r/S vom Tisch...
Tja, kann wirklich in alle Richtungen gehen...
Was ist am Anschlag? sehe die Grafik nicht auf dem Handy...
Antwort auf Beitrag Nr.: 45.559.425 von takeProfit am 03.10.13 00:38:55Die Technischen Indikatoren in der Cbharttechnik. Schau dir den Chart einfach mal daheim am PC an.
RSI ist auf 82, die Slow Stochastik ist auch an der Decke und der Kurs befindet sich über dem oberen Bollinger Band.
RSI ist auf 82, die Slow Stochastik ist auch an der Decke und der Kurs befindet sich über dem oberen Bollinger Band.
LOL kenne mich mit Indikatoren nicht aus...
Das heisst?
Das heisst?
Gut zu lesen:
Would all of the BLITHERING IDIOTS who sold because of the reverse splits
Please grab your ankles and bend overr while my friend BUTCH THE JANITOR gives you an "examination". HE LOVES PEOPLE LIKE YOU STUPID DOLTS! Do any of you even know what a reverse split is? A split, whether forward or reverse NEVER has a material effect upon shareholder value unless new shares were created. Do any of you realize the absolutely POSITIVE BENEFITS OF TODAYS ANNOUNCEMENT? In an unusual moment of sympathy for the stupid, I have decided to list them below. (you can thank me later)
1. The reverse split eliminated the imminent threat of being delisted from the Nasdaq Capital markets. Had that happened, I can assure you all, it would have had a most detrimental effect upon the market cap of the company's shares.
2. The reverse split CONDENSED the shares outstanding and the float by 83.4%. Thats right, we will have gone from 130 Million Shares outstanding to just under 22 million shares out. (This means the exact same leverage will be applied to your portfolio as the good news rolls in).
3. By using a 1 for 6 ratio, the shares are priced at 6.80 using todays close. This is particularly significant in that the shares are now within access of hedge and mutual funds that were previously prohibited from taking possession of shares trading below 5.00 ( a common threshold)
4. By adjusting the price per share above $5.00, the stock is now marginable in virtually all financial venues, in effect doubling our exposure
5. At prices above 5.00 and at the companies discretion, a market can now be offered for the sale of call and put options. This adds additional liquidity and leverage. This can compound the effect of any news dramatically.
6. ONE MORE TIME: THE VALUE OF THE SHARES IN YOUR ACCOUNT REMAIN EXACTLY THE SAME (Unless a legion of morons dumps their stock at losses because they "just dont get it".
7, WHAT HAPPENED TODAY WAS A GOOD THING!
Would all of the BLITHERING IDIOTS who sold because of the reverse splits
Please grab your ankles and bend overr while my friend BUTCH THE JANITOR gives you an "examination". HE LOVES PEOPLE LIKE YOU STUPID DOLTS! Do any of you even know what a reverse split is? A split, whether forward or reverse NEVER has a material effect upon shareholder value unless new shares were created. Do any of you realize the absolutely POSITIVE BENEFITS OF TODAYS ANNOUNCEMENT? In an unusual moment of sympathy for the stupid, I have decided to list them below. (you can thank me later)
1. The reverse split eliminated the imminent threat of being delisted from the Nasdaq Capital markets. Had that happened, I can assure you all, it would have had a most detrimental effect upon the market cap of the company's shares.
2. The reverse split CONDENSED the shares outstanding and the float by 83.4%. Thats right, we will have gone from 130 Million Shares outstanding to just under 22 million shares out. (This means the exact same leverage will be applied to your portfolio as the good news rolls in).
3. By using a 1 for 6 ratio, the shares are priced at 6.80 using todays close. This is particularly significant in that the shares are now within access of hedge and mutual funds that were previously prohibited from taking possession of shares trading below 5.00 ( a common threshold)
4. By adjusting the price per share above $5.00, the stock is now marginable in virtually all financial venues, in effect doubling our exposure
5. At prices above 5.00 and at the companies discretion, a market can now be offered for the sale of call and put options. This adds additional liquidity and leverage. This can compound the effect of any news dramatically.
6. ONE MORE TIME: THE VALUE OF THE SHARES IN YOUR ACCOUNT REMAIN EXACTLY THE SAME (Unless a legion of morons dumps their stock at losses because they "just dont get it".
7, WHAT HAPPENED TODAY WAS A GOOD THING!
Das heisst die Aktie ist im Moment massiv überkauft. Im normal Fall wird bei jedem gesunden Anstieg immer wieder Druck abgebaut. Hier wird das in den nächsten Tagen denke ich passieren.
Das ist aber noch kein Grund zur Panik. Erstens ist das eine Biotech Aktie, da sind größere Schwankungen normal und zweitens hatten wir im Hoch einen Anstieg von ca. 80%. Da kanns dan schon mal 20-30% runter gehen.
Es sind jetzt halt auch viele kurzorientierte Anleger in der Aktie. Diese werden die nächsten Tage wahrscheinlich ihre Gewinne einstreichen und deswegen wird der Kurs wohl etwas fallen.
Aber les dich mal ein bisschen in die Charttechnik ein. Machte den Aktienhandel um einiges einfacher. Das ist Quasi in Kurven gebrachte Massenpsychologie .
.
Das ist aber noch kein Grund zur Panik. Erstens ist das eine Biotech Aktie, da sind größere Schwankungen normal und zweitens hatten wir im Hoch einen Anstieg von ca. 80%. Da kanns dan schon mal 20-30% runter gehen.
Es sind jetzt halt auch viele kurzorientierte Anleger in der Aktie. Diese werden die nächsten Tage wahrscheinlich ihre Gewinne einstreichen und deswegen wird der Kurs wohl etwas fallen.
Aber les dich mal ein bisschen in die Charttechnik ein. Machte den Aktienhandel um einiges einfacher. Das ist Quasi in Kurven gebrachte Massenpsychologie
 .
.
Antwort auf Beitrag Nr.: 45.559.445 von takeProfit am 03.10.13 00:48:50ich empfehle da eine gewisse auseinandersetzung mit charttechnik. so lassen sich ein- und ausstiegspunkte besser identifzieren. rsi(rel.stärke)>80=überkauft(kurs fällt mgl); rsi<20=überkauft(kurs steigt mgl); bollis: kurs hält sich nicht gerne außerhalb davon auf, nach ca.3 tagen müßte er wieder hineinkorrigieren;hilfreich sind auch trendlinien. guckst du.
Zitat von ThumsUp:Zitat von ThumsUp: rsi<20=überkauft
kaufe "ver"
alles richtig was du schreibst....aber :
Bei Biotecs gelten andere Gesetze dh. in der Charttechnik...und im Bereich der Gesamtmärkte dh. sie sind auch oft Gesamtmarktunabhängig !
Hier können täglich die Phase 2 Ergebnisse kommen....

Und darauf wird spekuliert....oft zeigen sich positive Ergebnisse im Vorfeld
ich denke das wir nicht so viel % abgeben werden...abgesehen von einem DIP nach unten...den es durchaus geben wird.
aber ich ein paar Tagen wird der Kurs das Niveau vor dem Split bzw. darüber erreichen......vermute ich stark.
Aus meiner Erfahrung dauert die Umstellung der Depots bei den deutschen Brokern eh...bis zu 3 Wochen....solange sind die..die drin sind...Handlungsunfähig und zum "Zuschauen" verdammt !

Antwort auf Beitrag Nr.: 45.560.863 von Earthfire am 03.10.13 11:01:44das volumen war extrem; ich gehe auch am ehesten davon aus dass von den ergebnissen was durchgesickert ist...
Das wäre?
sprich diese sind positiv? !!
Oder meint Ihr den abverkauf vor dem R/S ? Also negativ?
Oder war der abverkauf "nur" wegen wissende vom R/S
Ich habe gelesen Zalicus hat Ihren Sitz neben großen anderen bekannten Namen hat...
Auch dort wird viel von der Firma gehalten.
Ich meine klar hätte ich lieber ein großen zock gehabt... von 1$ auf 5$ oder so...
Sah sich Zalicus nicht alleine dort mit News?
Aber für Zalicus ist der Wert wichtig, und ein höherer Preis und dann eine positive Meldung wirkt sich auf das Gesamtbild eher positiv aus.
Aber wenn der kurs künstlich hoch "gehalten" wird, um bei negativen News wenigsten bei nem Dollar zu sein, wäre fatal für das vertrauen... und Investoren...
sprich diese sind positiv? !!
Oder meint Ihr den abverkauf vor dem R/S ? Also negativ?
Oder war der abverkauf "nur" wegen wissende vom R/S
Ich habe gelesen Zalicus hat Ihren Sitz neben großen anderen bekannten Namen hat...
Auch dort wird viel von der Firma gehalten.
Ich meine klar hätte ich lieber ein großen zock gehabt... von 1$ auf 5$ oder so...
Sah sich Zalicus nicht alleine dort mit News?
Aber für Zalicus ist der Wert wichtig, und ein höherer Preis und dann eine positive Meldung wirkt sich auf das Gesamtbild eher positiv aus.
Aber wenn der kurs künstlich hoch "gehalten" wird, um bei negativen News wenigsten bei nem Dollar zu sein, wäre fatal für das vertrauen... und Investoren...
Zitat von ThumsUp: das volumen war extrem; ich gehe auch am ehesten davon aus dass von den ergebnissen was durchgesickert ist...
Noch was...wer sich die Trades im Aftermarket gestern mal genau anschaut...der sieht auch wie die Mmn panisch geschmissenen Shares der kleinen Trader dankbar auch zu Kursen über 1$ aufgesaugt wurden.
In den US Boards wird auch verusucht von einigen Schreibern dort...Panik zu erzeugen.
An der Sache hat sich nichts geändert...das ist das wichtigste hier...
Und die MK bei positiven Ergebnissen von Z160 ...spiegelt sich immer wieder...egal ob ich nun vor dem R/S 12000 Shares habe...oder nun 2000 Shares...die einen entsprechend höheren Kurswert haben.
Locker bleiben....in ein paar Tagen ist hier wieder alles im grünen Bereich !!!

Zitat von takeProfit: Das wäre?
sprich diese sind positiv? !!
Oder meint Ihr den abverkauf vor dem R/S ? Also negativ?
Oder war der abverkauf "nur" wegen wissende vom R/S
Ich habe gelesen Zalicus hat Ihren Sitz neben großen anderen bekannten Namen hat...
Auch dort wird viel von der Firma gehalten.
Ich meine klar hätte ich lieber ein großen zock gehabt... von 1$ auf 5$ oder so...
Sah sich Zalicus nicht alleine dort mit News?
Aber für Zalicus ist der Wert wichtig, und ein höherer Preis und dann eine positive Meldung wirkt sich auf das Gesamtbild eher positiv aus.
Aber wenn der kurs künstlich hoch "gehalten" wird, um bei negativen News wenigsten bei nem Dollar zu sein, wäre fatal für das vertrauen... und Investoren...
Von einem Abverkauf würde ich nach dem im Vorfeld der letzten Handelstage..gestern nicht sprechen...waren doch nur 1, MIO die dort geschmissen haben...wenn es 15 MIO gewesen wären....da würde ich es auch einen Abverkauf nennen.
Das waren Angsthasen..die Angst um ihre Gewinne hatten....
Wenn Z160 gut fluppt...sind selbst 100 % Plus...als lächerlich hier zu betrachten !!
Zitat von takeProfit: Das wäre?
sprich diese sind positiv? !!
Oder meint Ihr den abverkauf vor dem R/S ? Also negativ?
Oder war der abverkauf "nur" wegen wissende vom R/S
Ich habe gelesen Zalicus hat Ihren Sitz neben großen anderen bekannten Namen hat...
Auch dort wird viel von der Firma gehalten.
Ich meine klar hätte ich lieber ein großen zock gehabt... von 1$ auf 5$ oder so...
Sah sich Zalicus nicht alleine dort mit News?
Aber für Zalicus ist der Wert wichtig, und ein höherer Preis und dann eine positive Meldung wirkt sich auf das Gesamtbild eher positiv aus.
Aber wenn der kurs künstlich hoch "gehalten" wird, um bei negativen News wenigsten bei nem Dollar zu sein, wäre fatal für das vertrauen... und Investoren...
Der Kurs spiegelt die MK bzw. die zukünftige MK hier wieder.....zumindest sollte er dies tun !

lol, gerade in mein Depot geschehen:
Zalicius : 58.186,09 +876,71% bei 11.000 Stück :-)
So ein R/S hätte ich dann gerne öfters :-)
Zalicius : 58.186,09 +876,71% bei 11.000 Stück :-)
So ein R/S hätte ich dann gerne öfters :-)
Zitat von Earthfire: In den US Boards wird auch verusucht von einigen Schreibern dort...Panik zu erzeugen.
ich schau da auch viel rein - da ist noch wesentlich mehr (professionelle) meinungsmache unterwegs wie in w:o boards. es macht viel sinn sich die fundamentals in erinnerung zu halten; p160 hat bislang gute ergebnisse gezeigt; die orphan-zulassung ist kein blockbuster - aber damit hat p160 den fuß in der tür und das ist wesentlich. da kein abhängigkeitspotential vorliegt gibt es klare vorteile gg opioiden. wenn also keine sae vorliegen und der effekt gut genug ist ist hier riesenpotential vorhanden. buy the rumors - sell the news
Zitat von ThumsUp:Zitat von Earthfire: In den US Boards wird auch verusucht von einigen Schreibern dort...Panik zu erzeugen.
ich schau da auch viel rein - da ist noch wesentlich mehr (professionelle) meinungsmache unterwegs wie in w:o boards. es macht viel sinn sich die fundamentals in erinnerung zu halten; p160 hat bislang gute ergebnisse gezeigt; die orphan-zulassung ist kein blockbuster - aber damit hat p160 den fuß in der tür und das ist wesentlich. da kein abhängigkeitspotential vorliegt gibt es klare vorteile gg opioiden. wenn also keine sae vorliegen und der effekt gut genug ist ist hier riesenpotential vorhanden. buy the rumors - sell the news
Du sagst es.....

Antwort auf Beitrag Nr.: 45.561.301 von ThumsUp am 03.10.13 11:55:26Das hier...wie bei vielen Werten..Insider am Werk sind, sah man schön daran, das die Meldung des R/S pünktlich zum Handelsschluss veröffendlicht wird....der Kurs aber in der letzten Stunde vorher komplett abverkauft....vor Handelsende drehte er durch die Käufe derer die billig rein wollten sogar wieder ins Plus gedreht ! 
Und dann die News des R/S um 16 Uhr US Ortszeit....Zufall..wer es glaubt


Und dann die News des R/S um 16 Uhr US Ortszeit....Zufall..wer es glaubt


Pre geht los...aktuell 6,1$.....mit 300st
Schaun wir mal...wo wir um 22Uhr stehen...wie gesagt..ich denke spätestens mitte nächster Woche..ist bei Zalicus normalität im Kurs und der R/S vergessen...die User die gestern im Plus waren...werden dann wieder im Plus sein...meine Person eingeschlossen !
Schaun wir mal...wo wir um 22Uhr stehen...wie gesagt..ich denke spätestens mitte nächster Woche..ist bei Zalicus normalität im Kurs und der R/S vergessen...die User die gestern im Plus waren...werden dann wieder im Plus sein...meine Person eingeschlossen !

Du sagst es... Ich hatte den kurs beobachtet und hatte auch das Gefühl das etwas ist...
Konnte wegen einen Termin aber leider nicht verkaufen...
Dann war es schon zu spät.
Fliege Sonntag im Urlaub ... Hoffe das wenn ich wieder komme, vielleicht positiv überrascht werde!
Aber ich habe schon ein mulmiges Gefühl ...
Aber glaube an zalicus und dessen Pipeline
Konnte wegen einen Termin aber leider nicht verkaufen...
Dann war es schon zu spät.
Fliege Sonntag im Urlaub ... Hoffe das wenn ich wieder komme, vielleicht positiv überrascht werde!
Aber ich habe schon ein mulmiges Gefühl ...
Aber glaube an zalicus und dessen Pipeline
Zitat von takeProfit: Du sagst es... Ich hatte den kurs beobachtet und hatte auch das Gefühl das etwas ist...
Konnte wegen einen Termin aber leider nicht verkaufen...
Dann war es schon zu spät.
Fliege Sonntag im Urlaub ... Hoffe das wenn ich wieder komme, vielleicht positiv überrascht werde!
Aber ich habe schon ein mulmiges Gefühl ...
Aber glaube an zalicus und dessen Pipeline
Premarket aktuell 6,4$ mit knapp 10 K Vol....es wird gekauft....
Rein vom gefühl bzw. Interpretation der ersten Käufe...denke ich das wir kein großes Down sehen werden ....und der Kurs rel schnell wieder die 7,2$ erreicht....
wie gesagt...Groß verschenkt...hat sie vorher auch keiner....
Meist stürzen die Buden nach einem R/S heftig ab...die im Anschluss an den R/S dann eine KE machen und eine masse neue shares ausgeben.
Das sehe ich hier nicht...cashmässig ist Zalicus noch gut ausgestattet

Wir müssen halt schauen.....
was hat denn Zalicus an Cash $2.9 million ?
Das ist ja nicht viel!
Könnte das nicht doch ein Grund sein eine KE zu machen?
Eine Verwässerung ist doch nicht vom Tisch.
Zumal warum das alles?
Glaubte das managment nicht an eine Erholung?
Sind die Ergebnisse doch nicht so gut?
Oder sind sie gut und es soll ein ordentlicher kurs sein... ?
Das ist ja nicht viel!
Könnte das nicht doch ein Grund sein eine KE zu machen?
Eine Verwässerung ist doch nicht vom Tisch.
Zumal warum das alles?
Glaubte das managment nicht an eine Erholung?
Sind die Ergebnisse doch nicht so gut?
Oder sind sie gut und es soll ein ordentlicher kurs sein... ?
hat sich gut gehalten .. oder ? Was sagt Ihr ?
Hier nochmals eine gute Zusammenstellung der Fakten. Der RS hat nichts mit den Ergebnissen der Studien zu tun, sondern dient allein dem Verbleib an der Nasdaq.
"The Reverse Split Facts, no conjecture. The facts and nothing but the facts!!!
On Oct 22, 2012, ZLCS received notice of noncompliance from the NASDAQ Global Markets as its stock had a closing price of less than $1 for 30 consecutive days. ZLCS had 180 days to regain compliance. As the share price did not reach the standards of the Nasdaq Global Markets during this initial 180 day period, ZLCS asked for and received from NASDAQ an additional 180 day extension in return for moving its listing to the NASDAQ Capital Market. The final compliance date was extended by NASDAQ to Oct. 21, 2013. As one of the conditions of maintaining its Nasdaq Capital Market listing, ZLCS agreed to ask for shareholder approval of a R/S split to achieve the $1.00 minimum closing bid price should the share price not rise on its own. On June 6, 2013, ZLCS shareholders approved the ability of the ZLCS BOD to implement a reverse stock split between 1 for 5 and 1 for 10 shares.
To administratively affect a R/S split, ZLCS is required to give both NASDAQ and the Depository Trust Corporation a 15 calendar day advance notice of the split and the ratio. In order to accommodate this advance notice requirement, on Sept. 18, 2013, the ZLCS BOD approved a 1 for 6 R/S. ZLCS common share price on that day was $0.74.
With a compliance deadline of Oct. 21, 2013, ZLCS needed to first close above $1.00 per share no later than Oct. 4, 2013, which is ten (10) business days prior to Oct. 21, 2013. If ZLCS did not close above $1.00 each and every business day starting Oct. 4, 2013, NASDAQ would have sent a final delisting notice. The latest day that ZLCS could have initiated the R/S split proceedings to maintain its listing was Sept. 19, 2013, which was one day after when Zalicus began the R/S proceedings. ZLCS closed at $0.75 on Sept. 19, 2013. ZLCS essentially waited as long as possible to initiate the R/S proceedings, as the Company values its NASDAQ listing and did not want to jeopardize its listing qualifications for the Company and its shareholders.
ZLCS did what they had to do.
"The Reverse Split Facts, no conjecture. The facts and nothing but the facts!!!
On Oct 22, 2012, ZLCS received notice of noncompliance from the NASDAQ Global Markets as its stock had a closing price of less than $1 for 30 consecutive days. ZLCS had 180 days to regain compliance. As the share price did not reach the standards of the Nasdaq Global Markets during this initial 180 day period, ZLCS asked for and received from NASDAQ an additional 180 day extension in return for moving its listing to the NASDAQ Capital Market. The final compliance date was extended by NASDAQ to Oct. 21, 2013. As one of the conditions of maintaining its Nasdaq Capital Market listing, ZLCS agreed to ask for shareholder approval of a R/S split to achieve the $1.00 minimum closing bid price should the share price not rise on its own. On June 6, 2013, ZLCS shareholders approved the ability of the ZLCS BOD to implement a reverse stock split between 1 for 5 and 1 for 10 shares.
To administratively affect a R/S split, ZLCS is required to give both NASDAQ and the Depository Trust Corporation a 15 calendar day advance notice of the split and the ratio. In order to accommodate this advance notice requirement, on Sept. 18, 2013, the ZLCS BOD approved a 1 for 6 R/S. ZLCS common share price on that day was $0.74.
With a compliance deadline of Oct. 21, 2013, ZLCS needed to first close above $1.00 per share no later than Oct. 4, 2013, which is ten (10) business days prior to Oct. 21, 2013. If ZLCS did not close above $1.00 each and every business day starting Oct. 4, 2013, NASDAQ would have sent a final delisting notice. The latest day that ZLCS could have initiated the R/S split proceedings to maintain its listing was Sept. 19, 2013, which was one day after when Zalicus began the R/S proceedings. ZLCS closed at $0.75 on Sept. 19, 2013. ZLCS essentially waited as long as possible to initiate the R/S proceedings, as the Company values its NASDAQ listing and did not want to jeopardize its listing qualifications for the Company and its shareholders.
ZLCS did what they had to do.
Antwort auf Beitrag Nr.: 45.567.715 von Druon am 04.10.13 12:54:20Könnt ihr schon handeln? Bin bei Consors und habe noch den alten Bestand.
Antwort auf Beitrag Nr.: 45.567.905 von fortuna924 am 04.10.13 13:14:44nö. diba. willste handeln?
Antwort auf Beitrag Nr.: 45.567.923 von ThumsUp am 04.10.13 13:17:33Nein. Geht doch erst los.
Wird sich alles weiter beruhigen und bis zu den Ergebnissen weiter hochgehen.
Wird sich alles weiter beruhigen und bis zu den Ergebnissen weiter hochgehen.
Antwort auf Beitrag Nr.: 45.567.983 von fortuna924 am 04.10.13 13:22:59bewahrt einen vor panischem auf-den-button-drücken sollte der kurs nochmal auf tauchfahrt gehen. durch den r/s kann ich zwar keine brauchbaren indikator-charts mehr erstellen, gehe aber davon aus dass für die rel.stärke ganz gut ist wenn die bissl abkühlt.
was es gestern ruhig war , dreht es heute ziemlich runter...
Die frage wäre bis wo es nun runter geht.
Dann wenn es dauert bis ZLCS sich meldet kann es noch leider einiges runter gehen...
Die frage wäre bis wo es nun runter geht.
Dann wenn es dauert bis ZLCS sich meldet kann es noch leider einiges runter gehen...
Antwort auf Beitrag Nr.: 45.569.703 von takeProfit am 04.10.13 16:49:40ordentlicher schüttler, so hatte ich mir das gedacht. bei 5,40 habe ich kurz überlegt - und es gelassen. jetzt muss sie sich erstmal behaupten; dabei denke ich nicht dass es größere short-positionen geben wird; ist doch ziemlich riskant. passiert zwar nicht oft, aber letztlich werden auch blockbuster irgendwann mal geboren. schönen urlaub wünscht
thums
thums
Zalicus Inc. (ZLCS) is a developmental-stage biopharma with two pending catalysts for its leading clinical candidate Z160. Specifically, the company has announced that they expect to complete data collection for two Phase II trials for chronic neuropathic pain indications lumbrosacral radiculopathy and postherpetic neuralgia respectively, with top-line results to be announced in late 2013. Z160 is a first in class, oral, state dependent, selective N-type calcium channel blocker that has the potential to be used for a wide variety of chronic pain conditions. Last month, Zalicus also received Orphan Drug Status from the FDA for Z160 as a potential treatment for postherpetic neuralgia.
The potential market size for Z160 as a treatment for neuropathic pain should easily top a billion a year. To back up this assertion, the current market leader in the neuropathic pain market, Pfizer's (PFE) Lyrica, is on track to break the $2B mark this year alone, and has shown steady growth since its initial launch in 2005. Moreover, sales of Eli Lilly's (LLY) Cymbalta for neuropathic pain are also expected to exceed $1B in 2013, showing the strength of this rapidly growing niche-market. Compared to Zalicus's small market cap of approximately $156 M, Z160 thus offers investors a potentially outstanding reward going forward.
Mr. Market has yet to notice Z160's value proposition
While Zalicus is up more than 80% year-to-date, speculative investors, by and large, haven't jumped into this stock with both feet. Namely, the average volume for ZLCS is well shy of 2 M shares per day, despite the fact that company was recently priced around $1 a share prior to its 1 for 6 Reverse Split yesterday. Moreover, the company's market cap still remains demonstrably south of its peers currently performing advanced Phase II trials, e.g., Novavax (NVAX), Inovio (INO)-just to name a few. Essentially, biopharmas with pending top-line Phase II results tend to hover closer to $300 M instead of $100 M in terms of market cap. Based on these comparisons, Zalicus would appear to be undervalued by at least 40% prior to the announcement of its pending Phase II results.
With such a dramatically undervalued market cap, the question potential investors must answer is, why has Zalicus failed to attract the attention of the broader market? I believe the answer to this question is relatively straight-forward but it is critically important for investors to understand.
The answer is that Zalicus is essentially broke, and has a deficit topping $350 M due in large part to the failed development of the company's previous leading drug candidate Synavive. By my estimates, Zalicus only has about $14.5 M remaining in cash and cash equivalents (see hyperlink above), yet is burning nearly $2.5M a month. As the company states in their latest 10-Q, Zalicus only has enough money in the till to keep operating to about February 2014. The message is clear: Zalicus will have to rely heavily on its $25 M purchase agreement with Lincoln Park Capital to keep the doors open, and even this amount won't give the company nearly enough cash to perform a pivotal Phase III trial for Z160-assuming one or both of its current Phase II trials are successful. Even more problematically, a failure of both Phase II trials will undoubtedly be the end of Zalicus; a double failure at this junction will sink the stock and make it nearly impossible to finance the remainder of its drug pipeline. Overall, the serious problems with Zalicus's finances have kept the lid on investor enthusiasm thus far.
Value creation scenarios based on Z160
The risks associated with Zalicus are not exactly a mystery. I believe the company has chosen to perform two Phase II trials simultaneously in order to give themselves the best shot at keeping the business alive, and this strategy gives investors a potentially huge payday waiting in the wings. While the rumor mill about a potential partnership post-Phase II for Z160 has been going strong of late, I believe there are two potential scenarios that could play out if Z160 indeed reports top-line results in the 4th Quarter.
Frankly, Zalicus cannot advance Z160 on its own without wiping out current shareholders. Although the 1-for-6 reverse split has given the company some breathing room to stay listed on the Nasdaq, the size of the offering necessary for a "go it alone" strategy would crater share price; to do so would essentially put a black mark on the company in terms of shareholder trust. Remember, management is supposed to create value for shareholders.
websitePfizer is interested in partnering with innovative collaborators to develop novel and differentiated medicines to address the needs of patients suffering from pain.Phase II stagepremiumpatent protection
What's the potential pay-off if I buy now?
As I stated above, Zalicus is already undervalued by as much as 50% based on prevailing market trends in the biotech sector. Even so, I expect the stock to move closer to its peers in the weeks ahead of any data announcement in the 4th Quarter, giving shares of ZLCS a potential upside of nearly 100% solely as a run-up trade. The potential future valuation will subsequently depend on the strength of the Phase II results. Both trials have decent sample sizes (n = 140), are double-blinded and placebo-controlled, meaning that safety and efficacy results should be decent indicators of larger Phase III trials, and the drug's potential for commercialization.
Based on the market size for pain meds (see hyperlinks above), I believe clear-cut Phase II trial results showing that Z160 is safe and efficacious easily earns the company a minimum market cap of $600 M through either a partnering deal or a buy-out. From current levels, Zalicus would thus appear to have a minimum 300% + upside if Z160 reports the expected top-line results. By contrast, if Z160 turns out to be a dud, Zalicus is probably a goner due to its troubling financial situation. Zalicus is thus an unequivocally high risk-high reward biotech.
Artikel ist vom Freitag den 4/10/13 :
http://seekingalpha.com/article/1728522-a-high-risk-high-rew…
Schönes WE...und auf eine kommende grüne Woche
The potential market size for Z160 as a treatment for neuropathic pain should easily top a billion a year. To back up this assertion, the current market leader in the neuropathic pain market, Pfizer's (PFE) Lyrica, is on track to break the $2B mark this year alone, and has shown steady growth since its initial launch in 2005. Moreover, sales of Eli Lilly's (LLY) Cymbalta for neuropathic pain are also expected to exceed $1B in 2013, showing the strength of this rapidly growing niche-market. Compared to Zalicus's small market cap of approximately $156 M, Z160 thus offers investors a potentially outstanding reward going forward.
Mr. Market has yet to notice Z160's value proposition
While Zalicus is up more than 80% year-to-date, speculative investors, by and large, haven't jumped into this stock with both feet. Namely, the average volume for ZLCS is well shy of 2 M shares per day, despite the fact that company was recently priced around $1 a share prior to its 1 for 6 Reverse Split yesterday. Moreover, the company's market cap still remains demonstrably south of its peers currently performing advanced Phase II trials, e.g., Novavax (NVAX), Inovio (INO)-just to name a few. Essentially, biopharmas with pending top-line Phase II results tend to hover closer to $300 M instead of $100 M in terms of market cap. Based on these comparisons, Zalicus would appear to be undervalued by at least 40% prior to the announcement of its pending Phase II results.
With such a dramatically undervalued market cap, the question potential investors must answer is, why has Zalicus failed to attract the attention of the broader market? I believe the answer to this question is relatively straight-forward but it is critically important for investors to understand.
The answer is that Zalicus is essentially broke, and has a deficit topping $350 M due in large part to the failed development of the company's previous leading drug candidate Synavive. By my estimates, Zalicus only has about $14.5 M remaining in cash and cash equivalents (see hyperlink above), yet is burning nearly $2.5M a month. As the company states in their latest 10-Q, Zalicus only has enough money in the till to keep operating to about February 2014. The message is clear: Zalicus will have to rely heavily on its $25 M purchase agreement with Lincoln Park Capital to keep the doors open, and even this amount won't give the company nearly enough cash to perform a pivotal Phase III trial for Z160-assuming one or both of its current Phase II trials are successful. Even more problematically, a failure of both Phase II trials will undoubtedly be the end of Zalicus; a double failure at this junction will sink the stock and make it nearly impossible to finance the remainder of its drug pipeline. Overall, the serious problems with Zalicus's finances have kept the lid on investor enthusiasm thus far.
Value creation scenarios based on Z160
The risks associated with Zalicus are not exactly a mystery. I believe the company has chosen to perform two Phase II trials simultaneously in order to give themselves the best shot at keeping the business alive, and this strategy gives investors a potentially huge payday waiting in the wings. While the rumor mill about a potential partnership post-Phase II for Z160 has been going strong of late, I believe there are two potential scenarios that could play out if Z160 indeed reports top-line results in the 4th Quarter.
Frankly, Zalicus cannot advance Z160 on its own without wiping out current shareholders. Although the 1-for-6 reverse split has given the company some breathing room to stay listed on the Nasdaq, the size of the offering necessary for a "go it alone" strategy would crater share price; to do so would essentially put a black mark on the company in terms of shareholder trust. Remember, management is supposed to create value for shareholders.
websitePfizer is interested in partnering with innovative collaborators to develop novel and differentiated medicines to address the needs of patients suffering from pain.Phase II stagepremiumpatent protection
What's the potential pay-off if I buy now?
As I stated above, Zalicus is already undervalued by as much as 50% based on prevailing market trends in the biotech sector. Even so, I expect the stock to move closer to its peers in the weeks ahead of any data announcement in the 4th Quarter, giving shares of ZLCS a potential upside of nearly 100% solely as a run-up trade. The potential future valuation will subsequently depend on the strength of the Phase II results. Both trials have decent sample sizes (n = 140), are double-blinded and placebo-controlled, meaning that safety and efficacy results should be decent indicators of larger Phase III trials, and the drug's potential for commercialization.
Based on the market size for pain meds (see hyperlinks above), I believe clear-cut Phase II trial results showing that Z160 is safe and efficacious easily earns the company a minimum market cap of $600 M through either a partnering deal or a buy-out. From current levels, Zalicus would thus appear to have a minimum 300% + upside if Z160 reports the expected top-line results. By contrast, if Z160 turns out to be a dud, Zalicus is probably a goner due to its troubling financial situation. Zalicus is thus an unequivocally high risk-high reward biotech.
Artikel ist vom Freitag den 4/10/13 :
http://seekingalpha.com/article/1728522-a-high-risk-high-rew…
Schönes WE...und auf eine kommende grüne Woche

Antwort auf Beitrag Nr.: 45.574.815 von Earthfire am 05.10.13 19:33:06danke für´s einstellen. hast du schon neue scheine eingebucht? GRÜN ist jetzt am zug...
Zitat von ThumsUp: danke für´s einstellen. hast du schon neue scheine eingebucht? GRÜN ist jetzt am zug...
Gerne
 .....Nein noch nicht umgestellt !
.....Nein noch nicht umgestellt !Hab die Zalicus bei der DIBA liegen....
Das kann auch wenn wir viel Pech haben bis zu 4 Wochen dauern...
Hatte schon 2 R/S ....der erste (Canadischer ÖLexplorer)....hat vier Wochen gedauert.
Der zweite aus den USA, hat 3 Wochen gedauert.
Ein Kauf (Zalicus) war auch nicht möglich...ausser du käufst direkt über einen US Broker
Lt der DIBA ist dies immer davon abhängig, wann das jeweilige Unternehmen die Umbuchung der Shares meldet bzw. die notwendigen Papiere bei der DIBA eintrudeln

Denke aber, bis die Phase 2 Ergebnisse vorliegen....ist alles wieder handelbar.
Habe einen interessanten Vergleich gelesen...Vical..da ist P3 gefloppt...aber vom Kurs aus gesehen...kann man sich an Zalicus bei pos. P2 Ergebnissen annähern...dies würde dann einem Kurs nach dem R/S..von ca. 25-35$ bedeuten dh. so wie der Autor im Seeking Alpha Artikel auch vermutet...ca. 300-400% Up.
Hoffen wir das beste für Zalicus !

Gruß Earthfire
Zitat von ThumsUp: danke für´s einstellen. hast du schon neue scheine eingebucht? GRÜN ist jetzt am zug...
Hier schon mal die neue WKN = A1W6KG
Lt. dieser Bekanntmachung müsste der Handel am Montag in D wieder aufgenommen werden und auch die Umbuchung der Shares zeitnah erfolgen.
EDV-Nummer 6170
Name der Aktiengesellschaft Land WKN ISIN Bemerkung
Zalicus Inc. USA A1C4WQ US98887C1053
ISIN Tausch
Neue WKN: A1W6KG
-
Bekanntmachung: Zeitlich begrenzte Aussetzung der P
reisermittlung ausländischer Aktien
Antragsteller: Baader Bank AG
Marktsegment: Freiverkehr
Elektronischer Handel
EDV-Nummer 6170
Zalicus Inc. USA A1C4WQ
03.10.2013, 08:00 Uhr-
04.10.2013, 22:00 Uhr
ISIN Tausch
(Neue WKN: A1W6KG
Nachzulesen hier :
https://www.boerse-stuttgart.de/media/announcement_archives/…
Morgen..DIBA ..Shares umgestellt !
Auf eine grüne Woche !!!!

Auf eine grüne Woche !!!!

Antwort auf Beitrag Nr.: 45.578.495 von Earthfire am 07.10.13 08:40:01hab´s gesehen. so, boden scheint drin zu sein

hab mir just vor der zacke noch ein paar gezogen. ich glaube so billig wird sie nicht nochmal sein. (spekulier...)

hab mir just vor der zacke noch ein paar gezogen. ich glaube so billig wird sie nicht nochmal sein. (spekulier...)

schöne käufe zum schluss - bald news?
http://finance.yahoo.com/echarts?s=ZLCS+Interactive#symbol=Z…
Hi earthfire, ab 1.38$, ZLCS & ich parted company once again. (ich war damals so long eingstellt,aber heutezutage bin ich nur 48Std geblieben)
Bei Ariad allerdings kein erworbene stk. verkauft bisher wieder verkauft, mal sehen wie es sich dort längerfristig entwickelt (ich rechne fest mit
8$- 10$ vor Februar 2014)
Hi earthfire, ab 1.38$, ZLCS & ich parted company once again. (ich war damals so long eingstellt,aber heutezutage bin ich nur 48Std geblieben)
Bei Ariad allerdings kein erworbene stk. verkauft bisher wieder verkauft, mal sehen wie es sich dort längerfristig entwickelt (ich rechne fest mit
8$- 10$ vor Februar 2014)

noch wer oder wieder dabei???
Zu 1.24 USD dabei
Gestern war es ja sehr volatil. Grund dafür ist, dass Zalincus mit Epirus voraussichtlich im Sommer fusionieren. Hier der Artikel dazu (sogar auf deutsch):
Epirus und Zalicus melden Fusionsvertrag
Fusion schafft börsennotiertes, weltweit tätiges, auf Biosimilars ausgerichtetes Unternehmen mit Schwerpunkt auf der Verbesserung des Zugangs von Patienten zu wichtigen Arzneimitteln
Epirus schließt Serie-B-Finanzierungsrunde in Höhe von 36 Millionen US-Dollar ab
Management hält eine Telefonkonferenz um 8.30 Uhr EDT ab
BOSTON--(BUSINESS WIRE)--Epirus Biopharmaceuticals, Inc. (Epirus), ein Biopharma-Unternehmen mit Sitz in Boston, das sich auf die weltweite Entwicklung und Vermarktung von biosimilaren monoklonalen Antikörpern konzentriert, und Zalicus Inc. (NASDAQ:ZLCS) (Zalicus) gaben heute den Abschluss einer endgültigen Vereinbarung bekannt, die vorsieht, dass Epirus mit einer hundertprozentigen Tochtergesellschaft von Zalicus im Rahmen einer alle Geschäftsanteile einbeziehenden Transaktion fusioniert. Nach erfolgter Schließung von Zalicus wird das Unternehmen zu Epirus Biopharmaceuticals, Inc. umbenannt und unter der Führung des Management-Teams von Epirus tätig sein, dessen President und CEO Amit Munshi ist. Des Weiteren wird Dr. Mark H.N. Corrigan als Chairman des Board of Directors des Unternehmens fungieren, dem Vertreter der bestehenden Vorstände von Epirus und Zalicus angehören.
„Unter der neuen Unternehmensstruktur werden wir das aggressive Voranbringen unserer Pipeline an Biosimilars fortsetzen und uns über lokale Partnerschaften auf unterschiedlichen internationalen Biosimilar-Märkten Präsenz verschaffen“
Im Rahmen der Fusion entsteht ein an der US-Börse Nasdaq notiertes Unternehmen, das sich auf den Aufbau eines weltweiten Biosimilars-Geschäfts konzentriert, damit Patienten besseren Zugang zu wichtigen Arzneimitteln erhalten. Epirus entwickelt zurzeit eine Pipeline von biosimilaren Arzneimitteln, darunter BOW015, ein Biosimilar zu Remicade®, BOW050, ein Biosimilar zu Humira® und BOW030, ein Biosimilar zu Avastin®.
Epirus gab heute außerdem den Abschluss einer von Livzon Mabpharm, Inc. geführten Serie B-Finanzierungsrunde in Höhe von 36 Millionen US-Dollar bekannt. Als weitere Anleger beteiligten sich an dieser Finanzerungsrunde Adage Capital, Greenwoods Investment, Gibralt US, Inc., Monashee Capital Partners LP und ein Investment-Arm von Mousse Partners sowie die bestehenden Investoren TPG Biotech®, Montreux Equity Partners und 5AM Ventures.
„Unter der neuen Unternehmensstruktur werden wir das aggressive Voranbringen unserer Pipeline an Biosimilars fortsetzen und uns über lokale Partnerschaften auf unterschiedlichen internationalen Biosimilar-Märkten Präsenz verschaffen“, so Amit Munshi, President und CEO von Epirus. „Mit dieser Transaktion erschließen wir uns zusätzliche finanzielle Mittel und auch Erfahrungen mit klinischen Studien bei rheumatoider Arthritis. Unser vereintes Team setzt sich für einen erweiterten Zugang von Patienten zu wichtigen Arzneimitteln ein.”
Mark H.N. Corrigan, MD, President und CEO von Zalicus, kommentierte: „Wir sind von den Vorzügen des neuen zusammengeschlossenen Unternehmens und des Segments der biosimilaren Arzneimittel überzeugt. Mit unserem strategischen Modell und erfahrenen Management-Team kombiniert mit der Kompetenz von Zalicus im Bereich der rheumatoiden Arthritis und weltweiten klinischen Entwicklung sind wir gut für einen effektiven Einsatz unserer starken Beziehungen zu führenden Meinungsbildnern und unserer Erfahrung mit der Durchführung klinischer Studien zur rheumatoiden Arthritis in Europa positioniert. Aus dieser Fusion geht ein dynamische und spannendes neues Unternehmen hervor, das sich der erfolgreichen Entwicklung und Vermarktung von Biosimilars für den Weltmarkt widmet. Zalicus identifizierte bei seiner gründlichen Prüfung möglicher Kandidaten für einen Zusammenschluss und ihrer Produkt-Pipelines Epirus als Unternehmen, das beträchtliche Werte für unsere Aktionäre schaffen kann.”
Die Größe des Anteils an dem zusammengeschlossenen Unternehmen, der bei Abschluss der Fusion auf die Zalicus-Aktionäre entfällt, unterliegt einer Anpassung zum Zeitpunkt des Abschlusses, die auf Grundlage des Standes der Netto-Barmittel von Zalicus beim Abschluss erfolgt. Auf Pro-forma-Basis ergibt sich auf Grundlage der Anzahl der Zalicus-Stammaktien, die im Rahmen der Fusion emittiert werden sollen, dass (i) die gegenwärtigen Zalicus-Aktionäre etwa 19 % des zusammengeschlossenen Unternehmens und die gegenwärtigen Epirus-Aktionäre etwa 81 % des Unternehmens besitzen werden, falls die Netto-Barmittel von Zalicus zum Zeitpunkt des Abschlusses 12 Millionen US-Dollar oder mehr betragen, (ii) die gegenwärtigen Zalicus-Aktionäre etwa 17 % des zusammengeschlossenen Unternehmens und die gegenwärtigen Epirus-Aktionäre etwa 83 % des Unternehmens besitzen werden, falls die Netto-Barmittel von Zalicus zum Zeitpunkt des Abschlusses mehr als 9 Millionen aber weniger als 12 Millionen US-Dollar betragen, und (iii) die gegenwärtigen Zalicus-Aktionäre etwa 14 % des zusammengeschlossenen Unternehmens und die gegenwärtigen Epirus-Aktionäre etwa 86 % des Unternehmens besitzen werden, falls die Netto-Barmittel von Zalicus zum Zeitpunkt des Abschlusses 9 Millionen US-Dollar oder weniger betragen. Zalicus prüft zurzeit verschiedene alternative Möglichkeiten zur Erhöhung seiner Netto-Barmittel. Legt man jedoch den aktuellen Stand der Netto-Barmittel von Zalicus zugrunde und bezieht die von Zalicus in Zusammenhang mit der geplanten Transaktion veranschlagten Aufwendungen mit ein, würden die Zalicus-Aktionär bei Abschluss der Fusion am heutigen Tag etwa 14 % des zusammengeschlossenen Unternehmens und die gegenwärtigen Epirus-Aktionäre etwa 86 % des zusammengeschlossenen Unternehmens besitzen. Es kann in keiner Weise gewährleistet werden, dass Maßnahmen, die Zalicus vom jetzigen Zeitpunkt bis zum Abschluss der Fusion im Rahmen seiner Bemühungen um eine Erhöhung seiner Netto-Barmittel ergreifen könnte, Erfolg bringen, oder dass solche Alternativen für Zalicus verfügbar sind.
Die Fusion unterliegt den üblichen Abschlussbedingungen, darunter die Zustimmung der Anteilseigner von Zalicus und Epirus. Die Boards of Directors von Zalicus und Epirus haben einstimmig die Transaktion genehmigt und den Anteilseignern empfohlen, für die Transaktion zu stimmen. Zurzeit wird mit einem Abschluss der Fusion im Laufe des Sommers 2014 gerechnet.
Leerink Partners LLC und Latham & Watkins fungieren als Finanz- bzw. Rechtsberater von Epirus. Wedbush PacGrow Life Sciences und Goodwin Procter sind die Finanz- bzw. Rechtsberater von Zalicus.
Informationen über die Telekonferenz
Epirus und Zalicus halten um 8.30 Uhr EDT eine Telefonkonferenz mit Audio-Webcast zum Fusionsvertrag ab. Um an der Konferenzschaltung teilzunehmen, rufen Sie bitte mindestens fünf Minuten vor Beginn die Nummer 1-877-870-4263 (USA) oder 1-412-317-0790 (international) an und beziehen Sie sich auf die Telefonkonferenz “Epirus and Zalicus Merger Agreement“.
Zugang zu dem Material, das von Epirus und Zalicus im Rahmen der Konferenzschaltung bereitgestellt wird, finden Sie unter www.epirusbiopharma.com bzw. www.zalicus.com.
Außerdem wird ein Audio-Webcast der Telefonkonferenz unter der Rubrik Investor Relations auf der Website von Zalicus unter www.zalicus.com bereitgestellt. Etwa zwei Stunden nach der Veranstaltung wird eine Aufzeichnung des Webcasts auf der Unternehmenswebsite verfügbar sein.
Über EPIRUS
Epirus ist im Begriff, ein weltweites Unternehmen für biosimilare Arzneimittel (Biosimilars) aufzubauen, damit Patienten besseren Zugang zu wichtigen Arzneimitteln erhalten. Dabei stützt sich die kommerzielle Erfolgsstrategie des Unternehmens auf gezielte Ansätze für unterschiedliche internationale Märkte.
In Schwellenländern mit zugänglichen regulatorischen Regelwerken für Biosimilars baut Epirus Partnerschaften mit Unternehmen vor Ort auf, um eine schnellere Genehmigung durch die Zulassungsbehörden erhalten und früher mit der Vermarktung seiner Produkte beginnen zu können.
In schnellwachsenden internationalen Märkten, in denen die Herstellung vor Ort strategische und operationale Vorteile bringt, beabsichtigt Epirus mithilfe der SCALE™-Plattform eine “In Market, For Market™”-Fertigungslösung unter Einbeziehung lokaler Partner bereitzustellen.
Für große Märkte mit etablierten regulatorischen Regelwerken für Biosimilars, wie Europa, plant Epirus als Vermarktungsstrategie für seiner Produkte einer Kombination von Direktvertrieb und lokalen Distributoren.
Weitere Informationen über Epirus finden Sie unter www.epirusbiopharma.com
Über Zalicus
Zalicus Inc. (NASDAQ:ZLCS) ist ein Biopharma-Unternehmen, das sich der Entdeckung und Entwicklung neuartiger Arzneimittel für Patienten mit Schmerzen und Entzündungen verschrieben hat. Zalicus verfügt über ein Portfolio von eigenen Wirkstoffkandidaten im Stadium der klinischen Entwicklung, die auf Schmerzbehandlung abzielen. Das Unternehmen ist mehrere einkommenswirksame Kooperationen mit großen Pharmaunternehmen hinsichtlich weiterer Produkte, Produktkandidaten und Wirkstofffindungstechnologien eingegangen. Zalicus setzt seine Kompetenz in der Entdeckung und Entwicklung von selektiven Ionenkanalmodulatoren und seine Kombination von Screening-Technologien mit hohem Durchsatz für die Findung innovativer Arzneimittel für das eigene Unternehmen und seine Kooperationspartner in den Bereichen Schmerz und Onkologie ein. Weitere Informationen über Zalicus finden Sie unter www.zalicus.com.
Weitere Informationen werden bei der US-Börsenaufsicht SEC eingereicht
Diese Mitteilung stellt weder ein Verkaufsangebot noch die Einholung eines Kaufangebots für Wertpapiere von Epirus oder Zalicus noch eine Aufforderung zur Stimmabgabe oder Zustimmung dar. In Zusammenhang mit der geplanten Transaktion wird Zalicus einen Antrag auf eine Börsenzulassung (Registration Statement) auf Formblatt F-4 einreichen, zu dem auch das gemeinsames Proxy Statement/Prospekt gehört. Das gemeinsames Proxy Statement/Prospekt enthält wichtige Informationen über Epirus, Zalicus, die Transaktion und damit zusammenhängende Fragen. Epirus und Zalicus werden, sobald das gemeinsame Proxy Statement/Prospekt vorliegt, dieses ihren Aktionären auf dem Postweg oder anderweitig zukommen lassen. Anlegern und Wertpapierinhabern von Epirus und Zalicus wird dringend angeraten, das gemeinsame Proxy Statement/Prospekt zu der Fusion (einschließlich aller Änderungen oder Ergänzungen) ganz zu lesen, sobald es zur Verfügung steht, da es wichtige Informationen über die geplante Transaktion enthält.
Anleger und Wertpapierinhaber von Zalicus können kostenlose Exemplare des gemeinsamen Proxy Statement/Prospekts für die geplante Fusion (bei Verfügbarkeit) und weitere bei der SEC von Zalicus eingereichte Unterlagen über die Website der SEC unter www.sec.gov beziehen. Darüber hinaus können Anleger und Wertpapierinhaber von Zalicus kostenlose Exemplare des gemeinsamen Proxy Statement/Prospekts zur geplanten Fusion (bei Verfügbarkeit) beziehen, indem sie sich an Zalicus, z. Hd.: Justin Renz, jrenz@zalicus.com wenden. Anleger und Wertpapierinhaber von Epirus können kostenlose Exemplare des gemeinsamen Proxy Statement/Prospekts zur geplanten Fusion beziehen, indem sie sich an Epirus, z. Hd.: Edward Scott, escott@epirusbiopharma.com wenden.
Epirus und Zalicus sowie deren Directors und gewisse Executive Officers können im Hinblick auf die in der Vereinbarung zwischen Epirus und Zalicus ins Auge gefassten Transaktionen an der Einholung von Stimmrechtsvollmachten beteiligt sein. Information über die Directors und Executive Officers von Zalicus finden sich Jahresbericht des Unternehmens auf Formblatt 10-K für das am 31. Dezember 2013 beendete Geschäftsjahr, der am 14. März 2014 bei der SEC eingereicht wurde. Darüber hinaus werden diese auch in dem gemeinsamen Proxy Statement/Prospekt aufgeführt sein, das von Zalicus bei der SEC in Zusammenhang mit der geplanten Transaktion eingereicht werden wird. Information über die Directors und Officers von Epirus und eine ausführlichere Beschreibung der Interessen der Directors und Officers von Zalicus sowie der geplanten Transaktion finden sich in dem gemeinsamen Proxy Statement/Prospekt, das von Zalicus bei der SEC in Zusammenhang mit der geplanten Transaktion eingereicht werden wird.
Zukunftsgerichtete Aussagen
Alle hierin enthaltenen Aussagen zu künftigen Finanzergebnissen und Geschäftsverläufen, Bedingungen, Plänen, Aussichten, Trends oder Strategien sowie zu weiteren Finanz- und Geschäftsangelegenheiten, unter anderem zu dem möglichen Abschlussdatum der Transaktion, dem Betrag der Netto-Barmittel von Zalicus bei Transaktionsabschluss und den Aussichten für die Vermarktung oder den Vertrieb von Arzneimitteln oder weiteren Produkten sind zukunftsgerichtete Aussagen im Sinne des Private Securities Litigation Reform Act von 1995. Des Weiteren können, wenn in dieser Pressemitteilung verwendetet, die Wörter "möglicherweise", "könnte", "sollte", "davon ausgehen", "meint", "schätzt", "erwartet", "beabsichtigt", "plant", "sieht vorher" und ähnliche Ausdrücke oder Abwandlungen derselben, die sich auf Epirus, Zalicus oder das Management des jeweiligen Unternehmens vor oder nach der oben genannten Fusion beziehen, zukunftsgerichtete Aussagen kenntlich machen. Epirus und Zalicus weisen darauf hin, dass diese zukunftsgerichteten Aussagen zahlreichen Annahmen, Risiken und Unwägbarkeiten unterliegen, die sich Laufe der Zeit ändern können. Wichtige Faktoren, die dazu führen können, dass die tatsächlichen Ergebnisse erheblich von den in den zukunftsgerichteten Aussagen oder Darstellungen früherer Erfahrungen dargelegten Ergebnissen abweichen, beinhalten Risiken und Ungewissheiten, darunter, dass es Epirus oder Zalicus nicht gelingt, die Beziehungen mit Kooperationspartnern zu sichern und zu unterhalten, sowie Risiken in Zusammenhang mit klinischen Studien, Risiken hinsichtlich einer eventuellen Vermarkung der vorgeschlagenen Produktkandidaten von Epirus oder Zalicus (wie Vermarktungs-, zulassungsbehördliche, Produkthaftungs-, Versorgungs-, Wettbewerbs- und andere Risiken), die Abhängigkeit von den Anstrengungen Dritter, die Abhängigkeit von geistigem Eigentum sowie das Risiko, dass Epirus oder Zalicus nicht über die Finanzmittel und den Zugang zu Kapital zur Finanzierung der geplanten Tätigkeiten verfügen. Weitere Informationen zu Faktoren mit möglichen Auswirkungen auf das Geschäft, die Finanzlage und die Betriebsergebnisse von Zalicus finden sich in den Einreichungen des Unternehmens bei der US-Börsenaufsicht SEC, die unter www.sec.gov. verfügbar sind. Die zukunftsgerichteten Aussagen geben ausschließlich die Schätzungen von Epirus und Zalicus zum Datum des Erscheinens dieser Pressemitteilung wieder, und Epirus und Zalicus lehnen ausdrücklich jegliche Verpflichtung zu deren Aktualisierung ab.
Die Ausgangssprache, in der der Originaltext veröffentlicht wird, ist die offizielle und autorisierte Version. Übersetzungen werden zur besseren Verständigung mitgeliefert. Nur die Sprachversion, die im Original veröffentlicht wurde, ist rechtsgültig. Gleichen Sie deshalb Übersetzungen mit der originalen Sprachversion der Veröffentlichung ab.
Kontakte
Russo Partners LLC
Tony Russo, 212-845-4251
tony.russo@russopartnersllc.com
oder
Andreas Marathovouniotis, 212-845-4235
andreas.marathis@russopartnersllc.com
http://www.businesswire.de/news/de/20140416006599/de
Epirus und Zalicus melden Fusionsvertrag
Fusion schafft börsennotiertes, weltweit tätiges, auf Biosimilars ausgerichtetes Unternehmen mit Schwerpunkt auf der Verbesserung des Zugangs von Patienten zu wichtigen Arzneimitteln
Epirus schließt Serie-B-Finanzierungsrunde in Höhe von 36 Millionen US-Dollar ab
Management hält eine Telefonkonferenz um 8.30 Uhr EDT ab
BOSTON--(BUSINESS WIRE)--Epirus Biopharmaceuticals, Inc. (Epirus), ein Biopharma-Unternehmen mit Sitz in Boston, das sich auf die weltweite Entwicklung und Vermarktung von biosimilaren monoklonalen Antikörpern konzentriert, und Zalicus Inc. (NASDAQ:ZLCS) (Zalicus) gaben heute den Abschluss einer endgültigen Vereinbarung bekannt, die vorsieht, dass Epirus mit einer hundertprozentigen Tochtergesellschaft von Zalicus im Rahmen einer alle Geschäftsanteile einbeziehenden Transaktion fusioniert. Nach erfolgter Schließung von Zalicus wird das Unternehmen zu Epirus Biopharmaceuticals, Inc. umbenannt und unter der Führung des Management-Teams von Epirus tätig sein, dessen President und CEO Amit Munshi ist. Des Weiteren wird Dr. Mark H.N. Corrigan als Chairman des Board of Directors des Unternehmens fungieren, dem Vertreter der bestehenden Vorstände von Epirus und Zalicus angehören.
„Unter der neuen Unternehmensstruktur werden wir das aggressive Voranbringen unserer Pipeline an Biosimilars fortsetzen und uns über lokale Partnerschaften auf unterschiedlichen internationalen Biosimilar-Märkten Präsenz verschaffen“
Im Rahmen der Fusion entsteht ein an der US-Börse Nasdaq notiertes Unternehmen, das sich auf den Aufbau eines weltweiten Biosimilars-Geschäfts konzentriert, damit Patienten besseren Zugang zu wichtigen Arzneimitteln erhalten. Epirus entwickelt zurzeit eine Pipeline von biosimilaren Arzneimitteln, darunter BOW015, ein Biosimilar zu Remicade®, BOW050, ein Biosimilar zu Humira® und BOW030, ein Biosimilar zu Avastin®.
Epirus gab heute außerdem den Abschluss einer von Livzon Mabpharm, Inc. geführten Serie B-Finanzierungsrunde in Höhe von 36 Millionen US-Dollar bekannt. Als weitere Anleger beteiligten sich an dieser Finanzerungsrunde Adage Capital, Greenwoods Investment, Gibralt US, Inc., Monashee Capital Partners LP und ein Investment-Arm von Mousse Partners sowie die bestehenden Investoren TPG Biotech®, Montreux Equity Partners und 5AM Ventures.
„Unter der neuen Unternehmensstruktur werden wir das aggressive Voranbringen unserer Pipeline an Biosimilars fortsetzen und uns über lokale Partnerschaften auf unterschiedlichen internationalen Biosimilar-Märkten Präsenz verschaffen“, so Amit Munshi, President und CEO von Epirus. „Mit dieser Transaktion erschließen wir uns zusätzliche finanzielle Mittel und auch Erfahrungen mit klinischen Studien bei rheumatoider Arthritis. Unser vereintes Team setzt sich für einen erweiterten Zugang von Patienten zu wichtigen Arzneimitteln ein.”
Mark H.N. Corrigan, MD, President und CEO von Zalicus, kommentierte: „Wir sind von den Vorzügen des neuen zusammengeschlossenen Unternehmens und des Segments der biosimilaren Arzneimittel überzeugt. Mit unserem strategischen Modell und erfahrenen Management-Team kombiniert mit der Kompetenz von Zalicus im Bereich der rheumatoiden Arthritis und weltweiten klinischen Entwicklung sind wir gut für einen effektiven Einsatz unserer starken Beziehungen zu führenden Meinungsbildnern und unserer Erfahrung mit der Durchführung klinischer Studien zur rheumatoiden Arthritis in Europa positioniert. Aus dieser Fusion geht ein dynamische und spannendes neues Unternehmen hervor, das sich der erfolgreichen Entwicklung und Vermarktung von Biosimilars für den Weltmarkt widmet. Zalicus identifizierte bei seiner gründlichen Prüfung möglicher Kandidaten für einen Zusammenschluss und ihrer Produkt-Pipelines Epirus als Unternehmen, das beträchtliche Werte für unsere Aktionäre schaffen kann.”
Die Größe des Anteils an dem zusammengeschlossenen Unternehmen, der bei Abschluss der Fusion auf die Zalicus-Aktionäre entfällt, unterliegt einer Anpassung zum Zeitpunkt des Abschlusses, die auf Grundlage des Standes der Netto-Barmittel von Zalicus beim Abschluss erfolgt. Auf Pro-forma-Basis ergibt sich auf Grundlage der Anzahl der Zalicus-Stammaktien, die im Rahmen der Fusion emittiert werden sollen, dass (i) die gegenwärtigen Zalicus-Aktionäre etwa 19 % des zusammengeschlossenen Unternehmens und die gegenwärtigen Epirus-Aktionäre etwa 81 % des Unternehmens besitzen werden, falls die Netto-Barmittel von Zalicus zum Zeitpunkt des Abschlusses 12 Millionen US-Dollar oder mehr betragen, (ii) die gegenwärtigen Zalicus-Aktionäre etwa 17 % des zusammengeschlossenen Unternehmens und die gegenwärtigen Epirus-Aktionäre etwa 83 % des Unternehmens besitzen werden, falls die Netto-Barmittel von Zalicus zum Zeitpunkt des Abschlusses mehr als 9 Millionen aber weniger als 12 Millionen US-Dollar betragen, und (iii) die gegenwärtigen Zalicus-Aktionäre etwa 14 % des zusammengeschlossenen Unternehmens und die gegenwärtigen Epirus-Aktionäre etwa 86 % des Unternehmens besitzen werden, falls die Netto-Barmittel von Zalicus zum Zeitpunkt des Abschlusses 9 Millionen US-Dollar oder weniger betragen. Zalicus prüft zurzeit verschiedene alternative Möglichkeiten zur Erhöhung seiner Netto-Barmittel. Legt man jedoch den aktuellen Stand der Netto-Barmittel von Zalicus zugrunde und bezieht die von Zalicus in Zusammenhang mit der geplanten Transaktion veranschlagten Aufwendungen mit ein, würden die Zalicus-Aktionär bei Abschluss der Fusion am heutigen Tag etwa 14 % des zusammengeschlossenen Unternehmens und die gegenwärtigen Epirus-Aktionäre etwa 86 % des zusammengeschlossenen Unternehmens besitzen. Es kann in keiner Weise gewährleistet werden, dass Maßnahmen, die Zalicus vom jetzigen Zeitpunkt bis zum Abschluss der Fusion im Rahmen seiner Bemühungen um eine Erhöhung seiner Netto-Barmittel ergreifen könnte, Erfolg bringen, oder dass solche Alternativen für Zalicus verfügbar sind.
Die Fusion unterliegt den üblichen Abschlussbedingungen, darunter die Zustimmung der Anteilseigner von Zalicus und Epirus. Die Boards of Directors von Zalicus und Epirus haben einstimmig die Transaktion genehmigt und den Anteilseignern empfohlen, für die Transaktion zu stimmen. Zurzeit wird mit einem Abschluss der Fusion im Laufe des Sommers 2014 gerechnet.
Leerink Partners LLC und Latham & Watkins fungieren als Finanz- bzw. Rechtsberater von Epirus. Wedbush PacGrow Life Sciences und Goodwin Procter sind die Finanz- bzw. Rechtsberater von Zalicus.
Informationen über die Telekonferenz
Epirus und Zalicus halten um 8.30 Uhr EDT eine Telefonkonferenz mit Audio-Webcast zum Fusionsvertrag ab. Um an der Konferenzschaltung teilzunehmen, rufen Sie bitte mindestens fünf Minuten vor Beginn die Nummer 1-877-870-4263 (USA) oder 1-412-317-0790 (international) an und beziehen Sie sich auf die Telefonkonferenz “Epirus and Zalicus Merger Agreement“.
Zugang zu dem Material, das von Epirus und Zalicus im Rahmen der Konferenzschaltung bereitgestellt wird, finden Sie unter www.epirusbiopharma.com bzw. www.zalicus.com.
Außerdem wird ein Audio-Webcast der Telefonkonferenz unter der Rubrik Investor Relations auf der Website von Zalicus unter www.zalicus.com bereitgestellt. Etwa zwei Stunden nach der Veranstaltung wird eine Aufzeichnung des Webcasts auf der Unternehmenswebsite verfügbar sein.
Über EPIRUS
Epirus ist im Begriff, ein weltweites Unternehmen für biosimilare Arzneimittel (Biosimilars) aufzubauen, damit Patienten besseren Zugang zu wichtigen Arzneimitteln erhalten. Dabei stützt sich die kommerzielle Erfolgsstrategie des Unternehmens auf gezielte Ansätze für unterschiedliche internationale Märkte.
In Schwellenländern mit zugänglichen regulatorischen Regelwerken für Biosimilars baut Epirus Partnerschaften mit Unternehmen vor Ort auf, um eine schnellere Genehmigung durch die Zulassungsbehörden erhalten und früher mit der Vermarktung seiner Produkte beginnen zu können.
In schnellwachsenden internationalen Märkten, in denen die Herstellung vor Ort strategische und operationale Vorteile bringt, beabsichtigt Epirus mithilfe der SCALE™-Plattform eine “In Market, For Market™”-Fertigungslösung unter Einbeziehung lokaler Partner bereitzustellen.
Für große Märkte mit etablierten regulatorischen Regelwerken für Biosimilars, wie Europa, plant Epirus als Vermarktungsstrategie für seiner Produkte einer Kombination von Direktvertrieb und lokalen Distributoren.
Weitere Informationen über Epirus finden Sie unter www.epirusbiopharma.com
Über Zalicus
Zalicus Inc. (NASDAQ:ZLCS) ist ein Biopharma-Unternehmen, das sich der Entdeckung und Entwicklung neuartiger Arzneimittel für Patienten mit Schmerzen und Entzündungen verschrieben hat. Zalicus verfügt über ein Portfolio von eigenen Wirkstoffkandidaten im Stadium der klinischen Entwicklung, die auf Schmerzbehandlung abzielen. Das Unternehmen ist mehrere einkommenswirksame Kooperationen mit großen Pharmaunternehmen hinsichtlich weiterer Produkte, Produktkandidaten und Wirkstofffindungstechnologien eingegangen. Zalicus setzt seine Kompetenz in der Entdeckung und Entwicklung von selektiven Ionenkanalmodulatoren und seine Kombination von Screening-Technologien mit hohem Durchsatz für die Findung innovativer Arzneimittel für das eigene Unternehmen und seine Kooperationspartner in den Bereichen Schmerz und Onkologie ein. Weitere Informationen über Zalicus finden Sie unter www.zalicus.com.
Weitere Informationen werden bei der US-Börsenaufsicht SEC eingereicht
Diese Mitteilung stellt weder ein Verkaufsangebot noch die Einholung eines Kaufangebots für Wertpapiere von Epirus oder Zalicus noch eine Aufforderung zur Stimmabgabe oder Zustimmung dar. In Zusammenhang mit der geplanten Transaktion wird Zalicus einen Antrag auf eine Börsenzulassung (Registration Statement) auf Formblatt F-4 einreichen, zu dem auch das gemeinsames Proxy Statement/Prospekt gehört. Das gemeinsames Proxy Statement/Prospekt enthält wichtige Informationen über Epirus, Zalicus, die Transaktion und damit zusammenhängende Fragen. Epirus und Zalicus werden, sobald das gemeinsame Proxy Statement/Prospekt vorliegt, dieses ihren Aktionären auf dem Postweg oder anderweitig zukommen lassen. Anlegern und Wertpapierinhabern von Epirus und Zalicus wird dringend angeraten, das gemeinsame Proxy Statement/Prospekt zu der Fusion (einschließlich aller Änderungen oder Ergänzungen) ganz zu lesen, sobald es zur Verfügung steht, da es wichtige Informationen über die geplante Transaktion enthält.
Anleger und Wertpapierinhaber von Zalicus können kostenlose Exemplare des gemeinsamen Proxy Statement/Prospekts für die geplante Fusion (bei Verfügbarkeit) und weitere bei der SEC von Zalicus eingereichte Unterlagen über die Website der SEC unter www.sec.gov beziehen. Darüber hinaus können Anleger und Wertpapierinhaber von Zalicus kostenlose Exemplare des gemeinsamen Proxy Statement/Prospekts zur geplanten Fusion (bei Verfügbarkeit) beziehen, indem sie sich an Zalicus, z. Hd.: Justin Renz, jrenz@zalicus.com wenden. Anleger und Wertpapierinhaber von Epirus können kostenlose Exemplare des gemeinsamen Proxy Statement/Prospekts zur geplanten Fusion beziehen, indem sie sich an Epirus, z. Hd.: Edward Scott, escott@epirusbiopharma.com wenden.
Epirus und Zalicus sowie deren Directors und gewisse Executive Officers können im Hinblick auf die in der Vereinbarung zwischen Epirus und Zalicus ins Auge gefassten Transaktionen an der Einholung von Stimmrechtsvollmachten beteiligt sein. Information über die Directors und Executive Officers von Zalicus finden sich Jahresbericht des Unternehmens auf Formblatt 10-K für das am 31. Dezember 2013 beendete Geschäftsjahr, der am 14. März 2014 bei der SEC eingereicht wurde. Darüber hinaus werden diese auch in dem gemeinsamen Proxy Statement/Prospekt aufgeführt sein, das von Zalicus bei der SEC in Zusammenhang mit der geplanten Transaktion eingereicht werden wird. Information über die Directors und Officers von Epirus und eine ausführlichere Beschreibung der Interessen der Directors und Officers von Zalicus sowie der geplanten Transaktion finden sich in dem gemeinsamen Proxy Statement/Prospekt, das von Zalicus bei der SEC in Zusammenhang mit der geplanten Transaktion eingereicht werden wird.
Zukunftsgerichtete Aussagen
Alle hierin enthaltenen Aussagen zu künftigen Finanzergebnissen und Geschäftsverläufen, Bedingungen, Plänen, Aussichten, Trends oder Strategien sowie zu weiteren Finanz- und Geschäftsangelegenheiten, unter anderem zu dem möglichen Abschlussdatum der Transaktion, dem Betrag der Netto-Barmittel von Zalicus bei Transaktionsabschluss und den Aussichten für die Vermarktung oder den Vertrieb von Arzneimitteln oder weiteren Produkten sind zukunftsgerichtete Aussagen im Sinne des Private Securities Litigation Reform Act von 1995. Des Weiteren können, wenn in dieser Pressemitteilung verwendetet, die Wörter "möglicherweise", "könnte", "sollte", "davon ausgehen", "meint", "schätzt", "erwartet", "beabsichtigt", "plant", "sieht vorher" und ähnliche Ausdrücke oder Abwandlungen derselben, die sich auf Epirus, Zalicus oder das Management des jeweiligen Unternehmens vor oder nach der oben genannten Fusion beziehen, zukunftsgerichtete Aussagen kenntlich machen. Epirus und Zalicus weisen darauf hin, dass diese zukunftsgerichteten Aussagen zahlreichen Annahmen, Risiken und Unwägbarkeiten unterliegen, die sich Laufe der Zeit ändern können. Wichtige Faktoren, die dazu führen können, dass die tatsächlichen Ergebnisse erheblich von den in den zukunftsgerichteten Aussagen oder Darstellungen früherer Erfahrungen dargelegten Ergebnissen abweichen, beinhalten Risiken und Ungewissheiten, darunter, dass es Epirus oder Zalicus nicht gelingt, die Beziehungen mit Kooperationspartnern zu sichern und zu unterhalten, sowie Risiken in Zusammenhang mit klinischen Studien, Risiken hinsichtlich einer eventuellen Vermarkung der vorgeschlagenen Produktkandidaten von Epirus oder Zalicus (wie Vermarktungs-, zulassungsbehördliche, Produkthaftungs-, Versorgungs-, Wettbewerbs- und andere Risiken), die Abhängigkeit von den Anstrengungen Dritter, die Abhängigkeit von geistigem Eigentum sowie das Risiko, dass Epirus oder Zalicus nicht über die Finanzmittel und den Zugang zu Kapital zur Finanzierung der geplanten Tätigkeiten verfügen. Weitere Informationen zu Faktoren mit möglichen Auswirkungen auf das Geschäft, die Finanzlage und die Betriebsergebnisse von Zalicus finden sich in den Einreichungen des Unternehmens bei der US-Börsenaufsicht SEC, die unter www.sec.gov. verfügbar sind. Die zukunftsgerichteten Aussagen geben ausschließlich die Schätzungen von Epirus und Zalicus zum Datum des Erscheinens dieser Pressemitteilung wieder, und Epirus und Zalicus lehnen ausdrücklich jegliche Verpflichtung zu deren Aktualisierung ab.
Die Ausgangssprache, in der der Originaltext veröffentlicht wird, ist die offizielle und autorisierte Version. Übersetzungen werden zur besseren Verständigung mitgeliefert. Nur die Sprachversion, die im Original veröffentlicht wurde, ist rechtsgültig. Gleichen Sie deshalb Übersetzungen mit der originalen Sprachversion der Veröffentlichung ab.
Kontakte
Russo Partners LLC
Tony Russo, 212-845-4251
tony.russo@russopartnersllc.com
oder
Andreas Marathovouniotis, 212-845-4235
andreas.marathis@russopartnersllc.com
http://www.businesswire.de/news/de/20140416006599/de
Gleichzeitig hat eine namenhafte Kanzlei schon erwogen, Klage gegen die Fusion zu erheben, da sie Aktionäre von Zalicus bei der Fusion benachteiligt sieht.
Hier der Artikelm dazu. Allerdings auf englisch:
SHAREHOLDER ALERT: Brodsky & Smith, LLC Announces Investigation of Zalicus, Inc. - ZLCS
Print
Zalicus Inc. (MM) (NASDAQ:ZLCS)
Intraday Stock Chart
Today : Thursday 17 April 2014
Click Here for more Zalicus Inc. (MM) Charts.
BALA CYNWYD, Pa., April 16, 2014 /PRNewswire/ -- Law office of Brodsky & Smith, LLC announces that it is investigating potential claims against the Board of Directors of Zalicus, Inc. ("Zalicus" or the "Company") (Nasdaq: ZLCS) relating to the proposed merger with Epirus Biopharmaceuticals, Inc. ("Epirus").
Click here to learn more about the investigation http://brodsky-smith.com/747-zlcs-zalicus-inc.html, or call 877-534-2590. There is no cost or obligation to you.
Under the terms of the transaction, the percentage of the combined company that Zalicus shareholders will own as of the closing of the merger is subject to an adjustment at closing based on the level of Zalicus net cash at closing. However, if the merger were to close today, the stockholders of Zalicus would own only 14% of the combined company.
The investigation concerns possible breaches of fiduciary duty and other violations of state law by the Board of Directors of Zalicus for not acting in the Company's shareholders' best interests in connection with the sale process. The transaction may undervalue Zalicus and may result in a substantial loss for many long term holders of Zalicus stock. For example, Zalicus stock traded at $7.08 per share as recently as October 1, 2013 and traded at $8.88 per share on August 31, 2012.
If you own shares of Zalicus common stock and wish to discuss the legal ramifications of the investigation, or have any questions, you may e-mail or call the law office of Brodsky & Smith, LLC who will, without obligation or cost to you, attempt to answer your questions. You may contact Jason L. Brodsky, Esquire or Evan J. Smith, Esquire at Brodsky & Smith, LLC, Two Bala Plaza, Suite 602, Bala Cynwyd, PA 19004, by e-mail at investorrelations@brodsky-smith.com, by visiting http://brodsky-smith.com/747-zlcs-zalicus-inc.html, or calling toll free 877-LEGAL-90.
Brodsky & Smith, LLC is a litigation law firm with extensive expertise representing shareholders throughout the nation in securities and case action lawsuits. The attorneys at Brodsky & Smith have been appointed by numerous courts throughout the country to serve as lead counsel in class actions and successfully recovered millions of dollars for our clients and shareholders. Attorney advertising. Prior results do not guarantee a similar outcome.
SOURCE Brodsky & Smith, LLC
Copyright 2014 PR Newswire
http://ih.advfn.com/p.php?pid=nmona&article=61871133
SHAREHOLDER ALERT: Brodsky & Smith, LLC Announces Investigation of Zalicus, Inc. - ZLCS
Zalicus Inc. (MM) (NASDAQ:ZLCS)
Intraday Stock Chart
Today : Thursday 17 April 2014
Click Here for more Zalicus Inc. (MM) Charts.
BALA CYNWYD, Pa., April 16, 2014 /PRNewswire/ -- Law office of Brodsky & Smith, LLC announces that it is investigating potential claims against the Board of Directors of Zalicus, Inc. ("Zalicus" or the "Company") (Nasdaq: ZLCS) relating to the proposed merger with Epirus Biopharmaceuticals, Inc. ("Epirus").
Click here to learn more about the investigation http://brodsky-smith.com/747-zlcs-zalicus-inc.html, or call 877-534-2590. There is no cost or obligation to you.
Under the terms of the transaction, the percentage of the combined company that Zalicus shareholders will own as of the closing of the merger is subject to an adjustment at closing based on the level of Zalicus net cash at closing. However, if the merger were to close today, the stockholders of Zalicus would own only 14% of the combined company.
The investigation concerns possible breaches of fiduciary duty and other violations of state law by the Board of Directors of Zalicus for not acting in the Company's shareholders' best interests in connection with the sale process. The transaction may undervalue Zalicus and may result in a substantial loss for many long term holders of Zalicus stock. For example, Zalicus stock traded at $7.08 per share as recently as October 1, 2013 and traded at $8.88 per share on August 31, 2012.
If you own shares of Zalicus common stock and wish to discuss the legal ramifications of the investigation, or have any questions, you may e-mail or call the law office of Brodsky & Smith, LLC who will, without obligation or cost to you, attempt to answer your questions. You may contact Jason L. Brodsky, Esquire or Evan J. Smith, Esquire at Brodsky & Smith, LLC, Two Bala Plaza, Suite 602, Bala Cynwyd, PA 19004, by e-mail at investorrelations@brodsky-smith.com, by visiting http://brodsky-smith.com/747-zlcs-zalicus-inc.html, or calling toll free 877-LEGAL-90.
Brodsky & Smith, LLC is a litigation law firm with extensive expertise representing shareholders throughout the nation in securities and case action lawsuits. The attorneys at Brodsky & Smith have been appointed by numerous courts throughout the country to serve as lead counsel in class actions and successfully recovered millions of dollars for our clients and shareholders. Attorney advertising. Prior results do not guarantee a similar outcome.
SOURCE Brodsky & Smith, LLC
Copyright 2014 PR Newswire
http://ih.advfn.com/p.php?pid=nmona&article=61871133
Zalicus - A low-risk development-stage biotech 

wo geht der Kurs hin
wenn es eine bestätigte NEWS gibt
.
http://investorshub.advfn.com/boards/read_msg.aspx?message_i…
wenn es eine bestätigte NEWS gibt

.
http://investorshub.advfn.com/boards/read_msg.aspx?message_i…
heuer wird es spannend

Antwort auf Beitrag Nr.: 47.147.744 von gerdass am 13.06.14 10:16:05Na, so spannend wars dann ja doch nicht. letzter Kurd in Stutt. 0,89 €. Fast ganz runter wieder. WIe sind wohl die weiteren Aussichten?
Hier gute NEWS über ZALICUS...endlich!!!!!!!!!!
http://phx.corporate-ir.net/phoenix.zhtml?c=148036&p=irol-ne…
http://phx.corporate-ir.net/phoenix.zhtml?c=148036&p=irol-ne…
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +0,63 | |
| -1,95 | |
| +5,82 | |
| +2,41 | |
| 0,00 | |
| 0,00 | |
| +0,71 | |
| +0,34 | |
| +0,68 | |
| +0,25 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 75 | ||
| 55 | ||
| 54 | ||
| 51 | ||
| 27 | ||
| 23 | ||
| 19 | ||
| 17 | ||
| 15 | ||
| 14 |



















