Ähnlich Biolitec - Photodynamische Therapie -Vertrieb durch GE (Seite 6)
eröffnet am 30.05.07 12:01:47 von
neuester Beitrag 04.11.22 21:21:03 von
neuester Beitrag 04.11.22 21:21:03 von
Beiträge: 133
ID: 1.128.038
ID: 1.128.038
Aufrufe heute: 0
Gesamt: 18.994
Gesamt: 18.994
Aktive User: 0
ISIN: NO0010000045 · WKN: 931150 · Symbol: PHS
4,6950
EUR
+1,08 %
+0,0500 EUR
Letzter Kurs 26.04.24 Tradegate
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,3254 | +82,86 | |
| 0,6500 | +25,75 | |
| 6,0000 | +25,00 | |
| 56,69 | +20,00 | |
| 0,6400 | +18,52 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 8,5000 | -15,00 | |
| 0,7300 | -18,59 | |
| 92,06 | -19,84 | |
| 2,7280 | -29,14 | |
| 1,4500 | -64,37 |
Beitrag zu dieser Diskussion schreiben
Antwort auf Beitrag Nr.: 62.683.114 von boersenwolferl am 14.02.20 14:25:29Die Firma wird nun wohl dank des USA Geschäfts profitabel. 🤘 Denke sie wird steil gehen. 🙋♂️
Hallo AWSX.
Das schaut ja ganz gut aus. Als ich gesehen habe was dieses Unternehmen verkauft hatte ich ein heftiges Déjà-vu. Bei mir wurde 2009 auch der nicht Muskel invasive Blasenkrebs diagnostiziert und ich glaube bei der zweiten oder dritten TUR-B wurde damals auch mit Blaulicht die Blase untersucht. Diese Verfahren gibt es schon länger und ob dieses Produkt oder Verfahren nun neu ist kann ich nicht wirklich beurteilen. Der Blasenkrebs gehört jedenfalls zu den häufigsten Krebsarten. Ich hatte leider danach noch mehrer Rezidive hab dann aber 2014 auf mentale Selbstheilung umgestellt und das mit Erfolg, bin seit 6 Jahren ohne Rezidiv
Ich bin jedenfalls schon wegen meiner Vorgeschichte hier investiert und ich Haffoffe das sich die Technik und die Verfahren noch weiter verbessern um frühzeitige alle Tumore entfernen zu können.
Schönes Wochenende!
boersenwolferl
Das schaut ja ganz gut aus. Als ich gesehen habe was dieses Unternehmen verkauft hatte ich ein heftiges Déjà-vu. Bei mir wurde 2009 auch der nicht Muskel invasive Blasenkrebs diagnostiziert und ich glaube bei der zweiten oder dritten TUR-B wurde damals auch mit Blaulicht die Blase untersucht. Diese Verfahren gibt es schon länger und ob dieses Produkt oder Verfahren nun neu ist kann ich nicht wirklich beurteilen. Der Blasenkrebs gehört jedenfalls zu den häufigsten Krebsarten. Ich hatte leider danach noch mehrer Rezidive hab dann aber 2014 auf mentale Selbstheilung umgestellt und das mit Erfolg, bin seit 6 Jahren ohne Rezidiv

Ich bin jedenfalls schon wegen meiner Vorgeschichte hier investiert und ich Haffoffe das sich die Technik und die Verfahren noch weiter verbessern um frühzeitige alle Tumore entfernen zu können.
Schönes Wochenende!
boersenwolferl
27.02.2020 Results 4th quarter 2019
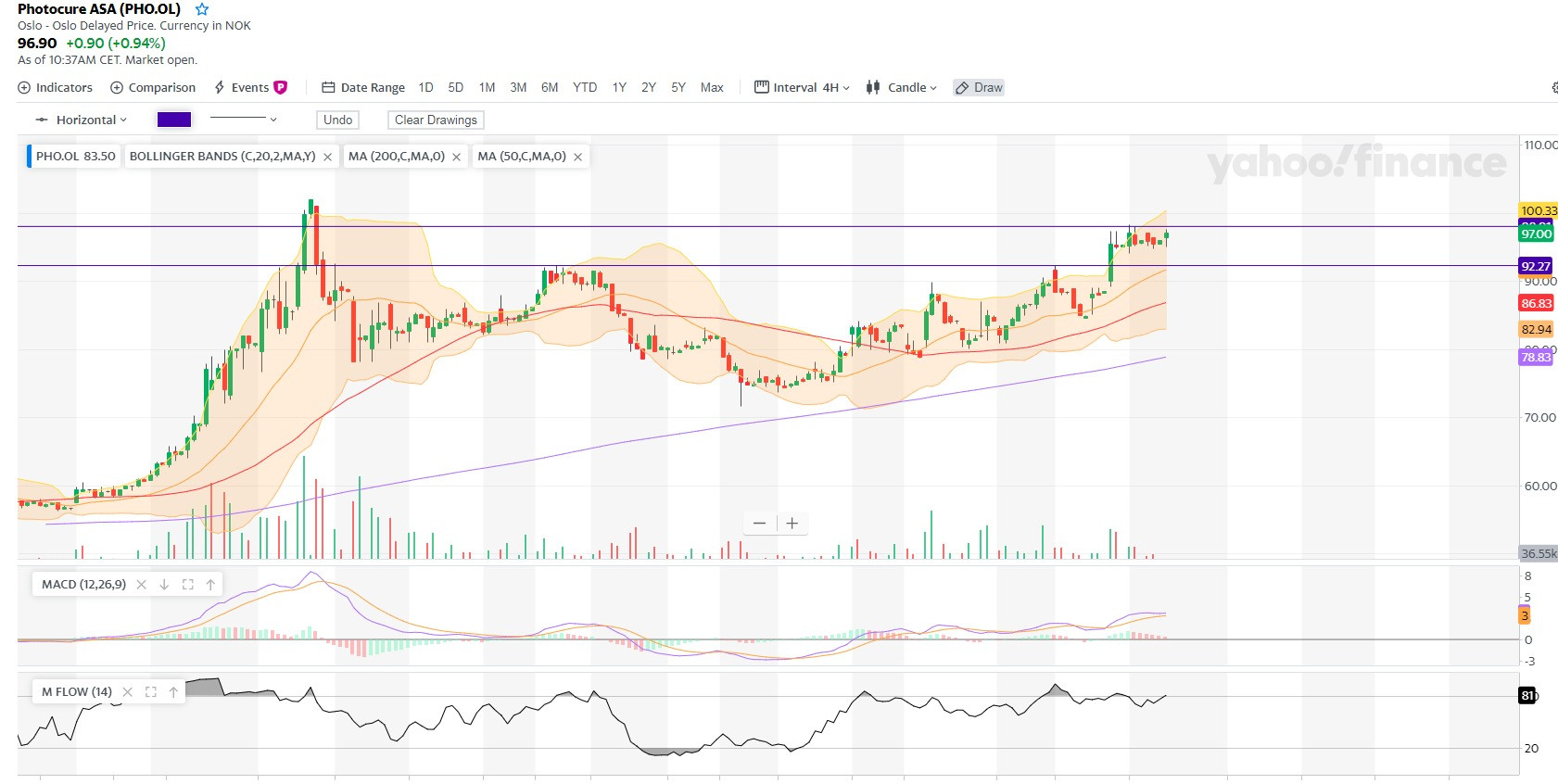
Vielleicht die aufregendste Aktie an der Osloer Börse im Jahr 2020
https://translate.google.com/translate?hl=de&sl=auto&tl=de&u…
Antwort auf Beitrag Nr.: 57.895.032 von R-BgO am 02.06.18 23:35:08
Oslo, Norway July 1, 2019:
Photocure ASA (Photocure, PHO: OSE), today announced that it has entered into a License Agreement providing Asieris Meditech Co., Ltd (Asieris) with a world-wide license to develop and commercialize Cevira® for the treatment of HPV induced cervical precancerous lesions.
Under the agreement, Photocure will receive signing fees, development- and approval milestones, in addition to sales royalties.
“We are proud to announce this agreement with Asieris, providing a global roadmap for the development and commercialization of Cevira. Cevira has the potential to be developed into the standard of care for the treatment of HPV infections and precancerous lesions, as a large population of women could benefit from a non-invasive treatment option for this condition.
This agreement is in line with our vision of becoming a global bladder cancer company by divesting products that do not fit our therapeutic focus.
This agreement provides Photocure with revenue potential from the development and global commercialization of Cevira in the range of USD 250 million including all payments and potential milestones, exclusive royalties of 10 to 20 per cent. We look forward to further cooperation with Asieris into bringing Cevira to the market”, says Daniel Schneider, President and CEO of Photocure.
Cevira® is in development as a treatment for high grade cervical dysplasia. It consists of a convenient, fully integrated drug delivery and light device to be applied intravaginally by the gynecologist. The patient can leave the physician office immediately and go back to daily activities, easily removing the device when the treatment is completed.
Asieris plans to launch a global clinical development program with an initial focus on the China market based on Photocure’s Phase 2b data and the Phase 3 study design elements agreed with the US FDA. The development for the US and EU markets will follow when clinical data from the China focused Phase 3 study confirms the safety and efficacy, estimated to be finished in 2022. Asieris will assume responsibility for the manufacture of the Cevira® product while Photocure retains responsibility for the manufacture of the active pharmaceutical ingredient.
“Cevira is a strategic fit for Asieris’ therapeutic focus on genitourinary (GU) diseases, particularly the oncological ones,” says Kevin Pan, CEO of Asieris. “Asieris has built strong development capabilities in the GU area in China and is rapidly expanding its global capability. Through the partnership with Photocure, we will endeavour to bring this innovative, non-surgical product to global markets and fulfil a substantial unmet medical need in Women’s Health.”
Under the License Agreement, Photocure will receive:
* a total signing fee of USD 5 million within 6 months after signing. In addition, the company
* may receive a total of USD 18 million based upon achievement of certain clinical and regulatory milestones in China and up to USD 36 million for certain clinical and regulatory milestones in USA and EU.
* Approval of a second indication in China, the US and the EU would result in payments of up to USD 14 million.
Additionally, sales royalties will apply in all markets.
Photocure and Asieris enter into a global licensing deal
for Cevira® with up to USD 250 million in milestones and double-digit royaltiesOslo, Norway July 1, 2019:
Photocure ASA (Photocure, PHO: OSE), today announced that it has entered into a License Agreement providing Asieris Meditech Co., Ltd (Asieris) with a world-wide license to develop and commercialize Cevira® for the treatment of HPV induced cervical precancerous lesions.
Under the agreement, Photocure will receive signing fees, development- and approval milestones, in addition to sales royalties.
“We are proud to announce this agreement with Asieris, providing a global roadmap for the development and commercialization of Cevira. Cevira has the potential to be developed into the standard of care for the treatment of HPV infections and precancerous lesions, as a large population of women could benefit from a non-invasive treatment option for this condition.
This agreement is in line with our vision of becoming a global bladder cancer company by divesting products that do not fit our therapeutic focus.
This agreement provides Photocure with revenue potential from the development and global commercialization of Cevira in the range of USD 250 million including all payments and potential milestones, exclusive royalties of 10 to 20 per cent. We look forward to further cooperation with Asieris into bringing Cevira to the market”, says Daniel Schneider, President and CEO of Photocure.
Cevira® is in development as a treatment for high grade cervical dysplasia. It consists of a convenient, fully integrated drug delivery and light device to be applied intravaginally by the gynecologist. The patient can leave the physician office immediately and go back to daily activities, easily removing the device when the treatment is completed.
Asieris plans to launch a global clinical development program with an initial focus on the China market based on Photocure’s Phase 2b data and the Phase 3 study design elements agreed with the US FDA. The development for the US and EU markets will follow when clinical data from the China focused Phase 3 study confirms the safety and efficacy, estimated to be finished in 2022. Asieris will assume responsibility for the manufacture of the Cevira® product while Photocure retains responsibility for the manufacture of the active pharmaceutical ingredient.
“Cevira is a strategic fit for Asieris’ therapeutic focus on genitourinary (GU) diseases, particularly the oncological ones,” says Kevin Pan, CEO of Asieris. “Asieris has built strong development capabilities in the GU area in China and is rapidly expanding its global capability. Through the partnership with Photocure, we will endeavour to bring this innovative, non-surgical product to global markets and fulfil a substantial unmet medical need in Women’s Health.”
Under the License Agreement, Photocure will receive:
* a total signing fee of USD 5 million within 6 months after signing. In addition, the company
* may receive a total of USD 18 million based upon achievement of certain clinical and regulatory milestones in China and up to USD 36 million for certain clinical and regulatory milestones in USA and EU.
* Approval of a second indication in China, the US and the EU would result in payments of up to USD 14 million.
Additionally, sales royalties will apply in all markets.
sie machen zwar immer noch Jahr für Jahr Verluste,
aber die schrumpfen und der Umsatz stiegt;
kaum zu glauben, wie langen Atem man für so was braucht
aber die schrumpfen und der Umsatz stiegt;
kaum zu glauben, wie langen Atem man für so was braucht
FDA GRANTS PRIORITY REVIEW FOR CYSVIEW® SUPPLEMENTAL NEW DRUG APPLICATION (SNDA)
Oslo, Norway, October 18,
Photocure ASA (OSE: PHO) announced today that the U.S. Food and Drug Administration (FDA) has accepted the supplemental New Drug Application (sNDA) for Cysview® on a priority review basis. With the FDA granting a priority review, a decision is expected in the first half of 2018.
The company is looking to expand the label of Cysview to include its use in the outpatient setting to detect the recurrence of bladder cancer using a flexible cystoscope, the detection of carcinoma in situ (CIS) and the repeat administration of Cysview. The filing is a combination drug-device application, with the KARL STORZ D-LIGHT C PDD Flexible Videoscope System.
“We are delighted to see the FDA expedite the review for this sNDA as it will offer patients improved surveillance of their Non-Muscle Invasive Bladder Cancer (NMIBC),” commented Andrea Maddox-Smith CEO, Bladder Cancer Advocacy Network (BCAN). BCAN is the only national advocacy organization devoted to advancing bladder cancer research and supporting those impacted by the disease.
“We look forward to hearing a decision from the FDA early next year on the US Cysview® label expansion to include patients undergoing surveillance cystoscopy using a flexible scope. The sNDA also includes detection of CIS and to allow for repeated use in patients in the operating room and the outpatient settings”, said Kjetil Hestdal, President & CEO, Photocure ASA. “Photocure is dedicated to improving the lives of patients with bladder cancer and we are committed to working with the FDA to bring this important clinical tool to the US market as soon as possible.”
Oslo, Norway, October 18,
Photocure ASA (OSE: PHO) announced today that the U.S. Food and Drug Administration (FDA) has accepted the supplemental New Drug Application (sNDA) for Cysview® on a priority review basis. With the FDA granting a priority review, a decision is expected in the first half of 2018.
The company is looking to expand the label of Cysview to include its use in the outpatient setting to detect the recurrence of bladder cancer using a flexible cystoscope, the detection of carcinoma in situ (CIS) and the repeat administration of Cysview. The filing is a combination drug-device application, with the KARL STORZ D-LIGHT C PDD Flexible Videoscope System.
“We are delighted to see the FDA expedite the review for this sNDA as it will offer patients improved surveillance of their Non-Muscle Invasive Bladder Cancer (NMIBC),” commented Andrea Maddox-Smith CEO, Bladder Cancer Advocacy Network (BCAN). BCAN is the only national advocacy organization devoted to advancing bladder cancer research and supporting those impacted by the disease.
“We look forward to hearing a decision from the FDA early next year on the US Cysview® label expansion to include patients undergoing surveillance cystoscopy using a flexible scope. The sNDA also includes detection of CIS and to allow for repeated use in patients in the operating room and the outpatient settings”, said Kjetil Hestdal, President & CEO, Photocure ASA. “Photocure is dedicated to improving the lives of patients with bladder cancer and we are committed to working with the FDA to bring this important clinical tool to the US market as soon as possible.”
BLUE LIGHT CYSTOSCOPY WITH HEXVIX® REDUCES RECURRENCE RATE AT 3 YEARS IN REAL-LIFE EXPERIENCE STUDY
Oslo, Norway, August 18, 2017:
Photocure ASA (OSE: PHO) today announced that a prospective controlled study investigating the introduction of Blue Light Cystoscopy (BLC™) with Hexvix® at first presentation in patients with non-muscle invasive bladder cancer (NMIBC) in routine clinical practice has been published in the World Journal of Urology. http://bit.ly/RealLifeBLCC
The overall recurrence rates at 3 years were significantly less in patients who received BLC™ with Hexvix® (39.0%) compared to an optimized White Light Cystoscopy (WLC) resection (53.3%; p=0.02). The benefit on the recurrence rate was most pronounced in patients with high-risk disease of (52.1% recurrence at year 3 with BLC™ with Hexvix® versus 80% with WLC; p=0.01).
In this investigator initiated and independent real-life experience study, conducted at a single center in the UK data were prospectively collected on all new patients over 4 years on all new tumour resections. Of 345 patients with resection meeting strict "good quality criteria", 135/153 who underwent resection with white light (WLC) and 146/192 patients who underwent resection guided by BLC™ with Hexvix® were assessed and compared for recurrence at 3 years.
“These long-term recurrence rate results from this high quality prospective real-life experience study are extremely reassuring. They reinforce the results from randomized clinical trials and show that BLC™ with Hexvix®/Cysview® provides long-term benefits in real-life clinical settings for NMIBC patients. The results also strengthen the recommendation to use BLC™ with Hexvix®/Cysview® for all first bladder cancer resections,” says Kjetil Hestdal, M.D., Ph.D., President and CEO, Photocure ASA.
Oslo, Norway, August 18, 2017:
Photocure ASA (OSE: PHO) today announced that a prospective controlled study investigating the introduction of Blue Light Cystoscopy (BLC™) with Hexvix® at first presentation in patients with non-muscle invasive bladder cancer (NMIBC) in routine clinical practice has been published in the World Journal of Urology. http://bit.ly/RealLifeBLCC
The overall recurrence rates at 3 years were significantly less in patients who received BLC™ with Hexvix® (39.0%) compared to an optimized White Light Cystoscopy (WLC) resection (53.3%; p=0.02). The benefit on the recurrence rate was most pronounced in patients with high-risk disease of (52.1% recurrence at year 3 with BLC™ with Hexvix® versus 80% with WLC; p=0.01).
In this investigator initiated and independent real-life experience study, conducted at a single center in the UK data were prospectively collected on all new patients over 4 years on all new tumour resections. Of 345 patients with resection meeting strict "good quality criteria", 135/153 who underwent resection with white light (WLC) and 146/192 patients who underwent resection guided by BLC™ with Hexvix® were assessed and compared for recurrence at 3 years.
“These long-term recurrence rate results from this high quality prospective real-life experience study are extremely reassuring. They reinforce the results from randomized clinical trials and show that BLC™ with Hexvix®/Cysview® provides long-term benefits in real-life clinical settings for NMIBC patients. The results also strengthen the recommendation to use BLC™ with Hexvix®/Cysview® for all first bladder cancer resections,” says Kjetil Hestdal, M.D., Ph.D., President and CEO, Photocure ASA.





















