Arena Pharmaceuticals - 500 Beiträge pro Seite (Seite 5)
eröffnet am 06.01.11 15:01:01 von
neuester Beitrag 17.01.13 17:43:17 von
neuester Beitrag 17.01.13 17:43:17 von
Beiträge: 2.498
ID: 1.162.543
ID: 1.162.543
Aufrufe heute: 0
Gesamt: 80.367
Gesamt: 80.367
Aktive User: 0
ISIN: US0400476075 · WKN: A2DR4A
99,98
USD
+0,06 %
+0,06 USD
Letzter Kurs 11.03.22 NYSE
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 10,230 | +447,06 | |
| 0,5700 | +55,23 | |
| 0,7200 | +47,03 | |
| 5,4500 | +41,56 | |
| 1,0000 | +33,33 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 11,590 | -14,15 | |
| 4,1900 | -14,49 | |
| 0,9923 | -16,61 | |
| 5,2500 | -19,23 | |
| 0,7500 | -48,28 |
Antwort auf Beitrag Nr.: 43.350.768 von Poppholz am 04.07.12 10:06:46
danke
dann kann ich mich ja heute um meine gurken kümmern
danke
dann kann ich mich ja heute um meine gurken kümmern
Zitat von gauner1: alle zusammen
Ein Vogel wollte Hochzeit halten
In dem grünen Walde.
|: Fi di ra la la :| Fi di ra la la la la
 ....bei diesem Song hast nun wirklich den " VOGEL" abgeschossen..
....bei diesem Song hast nun wirklich den " VOGEL" abgeschossen.. .
...und dafür bekommst du sogar noch eine " positive" Bewertung hinsichtlich " geistiger" Kreativität...

..ich glaubs einfach nicht...







test test test test
Im Juni 12 sind 486250 VIVUS-Aktien von den CEOs verkauft worden.
http://www.secform4.com/insider-trading/881524.htm
hierzu mal ein Artikel der möglichen Beweggründe...
Insider Sales at Vivus: Sign of Things to Come?
By Reza Ganjavi - July 4,
With two weeks to go before the PDUFA date for Qnexa, the heavy selling of shares by the CEO of Vivus (NASDAQ: VVUS) has raised a few eyebrows among some investors. One would normally assume that if Vivus gets FDA approval for Qnexa the company should be worth more than today given Qnexa is the company's most important drug. So why have institutions sold more shares than they bought, and why is the CEO selling before PDUFA? There are a number of reasons discussed among investors, (for example, in this thread), besides the classic explanation that insider sales are automatic/pre-planned sales:
1) Vivus has been in discussions with the FDA over a REMS program for some time. Many investors believe the REMS program and the label could put heavy restrictions on access to Qnexa (e.g., limitation on women in child-bearing age due to risks of birth defects, which is a major concern).
2) The company might not be able to do any marketing and promotion, or at least a highly restrictive one, for an extended period of time.
3) The company wants to set up its own sales organization and "go it alone" in the US. Shorting these types of launches is a favorite of some short sellers because many self-launches fail.
4) Competitive pressure from Arena Pharmaceutical (NASDAQ: ARNA) is huge. Arena is in the game with a boutique drug, Belviq, which is safer than Qnexa; a strong partner, Eisai, which already has hundreds of sales people in the US, ready to hit the ground running; and an FDA label that is better than what most folks could dream about.
5) Quiet period: perhaps after approval insiders enter a quiet period, which would prohibit sales.
6) Vivus' current valuation seems to be over-hyped and many retail investors are predicting a bubble burst. The reasons behind this inflated valuation could be related to several analysts and hedge funds who were proven wrong in their predictions on Arena's success, and were basically caught on the wrong side of the equation. There was a consensus among some analysts that FDA's AdComm would turn Arena down, then they were giving FDA approval a small chance, then they were predicting REMS. Despite being wrong on all these counts, some analysts continued their version of reality against Arena, perhaps to save face, by further promoting Vivus. A good synopsis on this idea can be found here.
Some speculate that the CEO may be selling because Vivus may be overhyped and overpriced, for no other reason than to contain Arena's momentum because there are around 40,000,000 shares of Arena shorted.
Whatever the reason for the insider sales, PDUFA date of July 17 will bring a lot of clarity to this so-called obesity drug race.
My predictions are:
a) I give Qnexa's approval a 50/50 chance. Some argue that due to safety issues it should not be approved. On the positive side, it may limit use of unsafe generics.
b) If approved, a very strict label and REMS program will be demanded by the FDA.
c) If approved, Qnexa will have a slow launch.
d) Most doctors will prefer Belviq due to its better safety profile.
e) If approved, Qnexa's launch will be "shorted" and the valuation will drop to a much more realistic level (perhaps half of what it is today).
f) As sales numbers roll in, analysts will have no choice but to acknowledge the facts vs. the "fiction making," to paraphrase Jim Cramer, which has dominated the obesity drug race so far.
g) Arena will get a boost from a restrictive approval of Qnexa and claim the biggest piece of this huge pie.
http://beta.fool.com/beatlesforever/2012/07/04/insider-sales…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/beatlesforever/2012/07/04/insider-sales…
http://www.secform4.com/insider-trading/881524.htm
hierzu mal ein Artikel der möglichen Beweggründe...
Insider Sales at Vivus: Sign of Things to Come?
By Reza Ganjavi - July 4,
With two weeks to go before the PDUFA date for Qnexa, the heavy selling of shares by the CEO of Vivus (NASDAQ: VVUS) has raised a few eyebrows among some investors. One would normally assume that if Vivus gets FDA approval for Qnexa the company should be worth more than today given Qnexa is the company's most important drug. So why have institutions sold more shares than they bought, and why is the CEO selling before PDUFA? There are a number of reasons discussed among investors, (for example, in this thread), besides the classic explanation that insider sales are automatic/pre-planned sales:
1) Vivus has been in discussions with the FDA over a REMS program for some time. Many investors believe the REMS program and the label could put heavy restrictions on access to Qnexa (e.g., limitation on women in child-bearing age due to risks of birth defects, which is a major concern).
2) The company might not be able to do any marketing and promotion, or at least a highly restrictive one, for an extended period of time.
3) The company wants to set up its own sales organization and "go it alone" in the US. Shorting these types of launches is a favorite of some short sellers because many self-launches fail.
4) Competitive pressure from Arena Pharmaceutical (NASDAQ: ARNA) is huge. Arena is in the game with a boutique drug, Belviq, which is safer than Qnexa; a strong partner, Eisai, which already has hundreds of sales people in the US, ready to hit the ground running; and an FDA label that is better than what most folks could dream about.
5) Quiet period: perhaps after approval insiders enter a quiet period, which would prohibit sales.
6) Vivus' current valuation seems to be over-hyped and many retail investors are predicting a bubble burst. The reasons behind this inflated valuation could be related to several analysts and hedge funds who were proven wrong in their predictions on Arena's success, and were basically caught on the wrong side of the equation. There was a consensus among some analysts that FDA's AdComm would turn Arena down, then they were giving FDA approval a small chance, then they were predicting REMS. Despite being wrong on all these counts, some analysts continued their version of reality against Arena, perhaps to save face, by further promoting Vivus. A good synopsis on this idea can be found here.
Some speculate that the CEO may be selling because Vivus may be overhyped and overpriced, for no other reason than to contain Arena's momentum because there are around 40,000,000 shares of Arena shorted.
Whatever the reason for the insider sales, PDUFA date of July 17 will bring a lot of clarity to this so-called obesity drug race.
My predictions are:
a) I give Qnexa's approval a 50/50 chance. Some argue that due to safety issues it should not be approved. On the positive side, it may limit use of unsafe generics.
b) If approved, a very strict label and REMS program will be demanded by the FDA.
c) If approved, Qnexa will have a slow launch.
d) Most doctors will prefer Belviq due to its better safety profile.
e) If approved, Qnexa's launch will be "shorted" and the valuation will drop to a much more realistic level (perhaps half of what it is today).
f) As sales numbers roll in, analysts will have no choice but to acknowledge the facts vs. the "fiction making," to paraphrase Jim Cramer, which has dominated the obesity drug race so far.
g) Arena will get a boost from a restrictive approval of Qnexa and claim the biggest piece of this huge pie.
http://beta.fool.com/beatlesforever/2012/07/04/insider-sales…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/beatlesforever/2012/07/04/insider-sales…
Antwort auf Beitrag Nr.: 43.351.932 von bernie55 am 04.07.12 14:37:08Im Juni 12 sind 486250 VIVUS-Aktien von den CEOs verkauft worden.
Ds nenne ich doch mal ein positives Signal...würde mal sagen...die haben sich gedacht sicher ist sicher...und ich bringe mal einen Teil meiner Schäfchen ins trocken....hoffe das Vivus die Zulassung verwehrt wird....
Es gibt doch bereits ein gutes Medikament was bald auf den Markt kommt : Belviq !!


Ds nenne ich doch mal ein positives Signal...würde mal sagen...die haben sich gedacht sicher ist sicher...und ich bringe mal einen Teil meiner Schäfchen ins trocken....hoffe das Vivus die Zulassung verwehrt wird....

Es gibt doch bereits ein gutes Medikament was bald auf den Markt kommt : Belviq !!



Antwort auf Beitrag Nr.: 43.350.768 von Poppholz am 04.07.12 10:06:46
der kurs hat sich heute ganz gut gehalten.
der kurs hat sich heute ganz gut gehalten.
Antwort auf Beitrag Nr.: 43.353.191 von Earthfire am 04.07.12 20:31:57
mein moto ist immer gewesen
das leben ist viel zu kurz, um langes gesicht zu machen.
daher kann ich nichts anders
mein moto ist immer gewesen
das leben ist viel zu kurz, um langes gesicht zu machen.
daher kann ich nichts anders
 Hier wird nicht gelacht !!!
Hier wird nicht gelacht !!!
..und schon wieder Sierra World Equity Review ....
..wenn die mit ihren " Sprüchen, Mutmaßungen und Prognose" zeitlich recht behalten sollten, dann fresse ich den berühmt-berüchtigten Besen.....
Big News next week for Arena Pharmaceuticals (ARNA), will Pfizer (PFE) Board of Directors vote for BUYOUT?
Will Pfizer's (PFE) Board of Directors vote YES to buyout Arena Pharmaceuticals (ARNA) at their upcoming meeting next week in Manhattan.? Sierra World Equity Review ventures that this topic will be at the top of the agenda, with other major players in the industry like Johnson and Johnson (JNJ) and Bristol Meyers Squibb (BMY) lurking in the wings time is of the essence! Sierra World Equity Review will stay on top of this story, possibly expect BIG NEWS next week!
Sierra World Equity Review is very confident and will be the first to call this, in our opinion, Pfizer and Arena Pharmaceuticals are already talking! We will continue to highlight Arena Pharmaceuticals and Pfizer going forward.
Sierra World Equity Review currently hold no positions in any of the companies mentioned in this report.
http://sierraworldequityreview.blogspot.de/
..wenn die mit ihren " Sprüchen, Mutmaßungen und Prognose" zeitlich recht behalten sollten, dann fresse ich den berühmt-berüchtigten Besen.....

Big News next week for Arena Pharmaceuticals (ARNA), will Pfizer (PFE) Board of Directors vote for BUYOUT?
Will Pfizer's (PFE) Board of Directors vote YES to buyout Arena Pharmaceuticals (ARNA) at their upcoming meeting next week in Manhattan.? Sierra World Equity Review ventures that this topic will be at the top of the agenda, with other major players in the industry like Johnson and Johnson (JNJ) and Bristol Meyers Squibb (BMY) lurking in the wings time is of the essence! Sierra World Equity Review will stay on top of this story, possibly expect BIG NEWS next week!
Sierra World Equity Review is very confident and will be the first to call this, in our opinion, Pfizer and Arena Pharmaceuticals are already talking! We will continue to highlight Arena Pharmaceuticals and Pfizer going forward.
Sierra World Equity Review currently hold no positions in any of the companies mentioned in this report.
http://sierraworldequityreview.blogspot.de/
Antwort auf Beitrag Nr.: 43.355.114 von bernie55 am 05.07.12 11:35:26dafür dass die so überzeugt sind wiedersprechen die sich aber damit, dass sie keine Aktien der beiden Firmen haben.
Wenn ich solche Informationen habe, dann kaufe ich doch Aktien der betroffenen Firmen bzw. von der kleinen, die übernommen werden soll.

Wenn ich solche Informationen habe, dann kaufe ich doch Aktien der betroffenen Firmen bzw. von der kleinen, die übernommen werden soll.

Zitat von Poppholz: dafür dass die so überzeugt sind wiedersprechen die sich aber damit, dass sie keine Aktien der beiden Firmen haben.
Wenn ich solche Informationen habe, dann kaufe ich doch Aktien der betroffenen Firmen bzw. von der kleinen, die übernommen werden soll.
YEPP.....aber vielleicht wollen sie dadurch ihre Seriosität und Neutralität aufzeigen..

Gong  ..der PRE geht los im Plus :
..der PRE geht los im Plus :
Pre-Market Last:
Net / % Change $ 10.13
.11 (1.10%) Pre-Market High: $ 10.13
(07:38:04 AM)
Pre-Market Volume: 770 Pre-Market Low: $ 10.05
(07:35:01 AM)
Read more: http://www.nasdaq.com/symbol/arna/premarket#ixzz1zkP0Fl2P
Wurde auch Zeit das die Amis wieder anfangen zu handeln...die sind bestimmt ganz heiss heute

 ..der PRE geht los im Plus :
..der PRE geht los im Plus :Pre-Market Last:
Net / % Change $ 10.13
.11 (1.10%) Pre-Market High: $ 10.13
(07:38:04 AM)
Pre-Market Volume: 770 Pre-Market Low: $ 10.05
(07:35:01 AM)
Read more: http://www.nasdaq.com/symbol/arna/premarket#ixzz1zkP0Fl2P
Wurde auch Zeit das die Amis wieder anfangen zu handeln...die sind bestimmt ganz heiss heute


Antwort auf Beitrag Nr.: 43.355.234 von bernie55 am 05.07.12 12:04:28Für eine bevorstehende Übernahme würde deutlich die Tatsache sprechen, dass sich das gesamte ARENA BOD ( Board of directors) Optionen zu hervorragenden Konditionen gesichert hat.
Zuletzt waren es ca. 260.000 shares zu gemittelten 1.60$ wenn ich es richtig in Erinnerung habe.
Und das war nur eine meldepflichtige Nachricht eines BOD members of Arena.
Der Rest von denen bedient sich ebenfall GROSSZÜGIG !
Alles auf UNSEREN Kosten...
Quelle:
http://www.otcmarkets.com/stock/ARNA/filings
Zuletzt waren es ca. 260.000 shares zu gemittelten 1.60$ wenn ich es richtig in Erinnerung habe.
Und das war nur eine meldepflichtige Nachricht eines BOD members of Arena.
Der Rest von denen bedient sich ebenfall GROSSZÜGIG !
Alles auf UNSEREN Kosten...

Quelle:
http://www.otcmarkets.com/stock/ARNA/filings
Antwort auf Beitrag Nr.: 43.355.741 von Earthfire am 05.07.12 13:41:55alles Verbrecher:
Kurs steht schon wieder im roten Bereich.
Kurs steht schon wieder im roten Bereich.
Zitat von Poppholz: alles Verbrecher:
Kurs steht schon wieder im roten Bereich.
Drecksäcke.....hoffe die fallen noch richtig auf die Schnauze...für den Fall das adhoc ein Übernahmeangebot oder sonst eine sehr positive News kommt !!
Hier was neues von Arena :
http://www.nasdaq.com/article/arena-pharmaceuticals-promotes…
Antwort auf Beitrag Nr.: 43.356.205 von Poppholz am 05.07.12 15:09:25über 2.000 Stück zu $9,90 ins BID gestellt.
Da möchte wohl jemand ganz dringend raus.

Da möchte wohl jemand ganz dringend raus.

Antwort auf Beitrag Nr.: 43.356.270 von Poppholz am 05.07.12 15:20:57ich meine 22.000 Stück.
Akutell aber im grünen Bereich.
Hoffentlich hat die Kursdrückerei bald ein Ende.
Akutell aber im grünen Bereich.
Hoffentlich hat die Kursdrückerei bald ein Ende.
Arena Pharmaceuticals Promotes Craig M. Audet to Executive Officer as Senior Vice President, Operations and Head of Global Regulatory Affairs
http://finance.yahoo.com/news/arena-pharmaceuticals-promotes…
http://finance.yahoo.com/news/arena-pharmaceuticals-promotes…
Antwort auf Beitrag Nr.: 43.356.364 von Poppholz am 05.07.12 15:37:19
seit freitag sind unsere freunde verschwunden. lars hat alle gesteinigt
seit freitag sind unsere freunde verschwunden. lars hat alle gesteinigt
war auch ein hartes Stück Arbeit 

beppino, du kleine maus, du ärgerst mich so sehr
such dir einen anderen platz und komm nie wieder her
und jetzt alle zusammen
vieli halla la, villi halla la villi halla la
nach paar tagen pause läuft unsere gurke läuft wieder
such dir einen anderen platz und komm nie wieder her
und jetzt alle zusammen
vieli halla la, villi halla la villi halla la
nach paar tagen pause läuft unsere gurke läuft wieder
arena läuft wieder gegen den trend. dax in minus, arena gut in plus
habe noch einmal 670 Stück nachgekauft.
Mal sehen ob der Kurs jetzt steigt (oder wieder fällt)
Mal sehen ob der Kurs jetzt steigt (oder wieder fällt)
Antwort auf Beitrag Nr.: 43.356.596 von gauner1 am 05.07.12 16:16:14..also hier mal die nächsten Widerstände, die es zu überwinden gilt 
> 11,39 USD
> 11,68 USD
..und ARENA , bitte, bitte, bitte..... schööönnn langsam in Richtung Norden ziehen...
...nicht wieder so viele + Prozente an einem Tag.... .
.
.....stay calm....stay cool....

> 11,39 USD
> 11,68 USD
..und ARENA , bitte, bitte, bitte..... schööönnn langsam in Richtung Norden ziehen...
...nicht wieder so viele + Prozente an einem Tag....
 .
......stay calm....stay cool....

Antwort auf Beitrag Nr.: 43.356.623 von Poppholz am 05.07.12 16:20:37
poppholz oder poppnae, egal
wollte ich bei 8,15 auch 800 kaufen. habe nicht bekommen umd der kurs ist mir abgehauen.
die schlechten shorties sind gesteinigt. die guten, die hochkaufen, sind da und sollen auch da bleiben und jeden tag hochkaufen
poppholz oder poppnae, egal
wollte ich bei 8,15 auch 800 kaufen. habe nicht bekommen umd der kurs ist mir abgehauen.
die schlechten shorties sind gesteinigt. die guten, die hochkaufen, sind da und sollen auch da bleiben und jeden tag hochkaufen
Antwort auf Beitrag Nr.: 43.356.623 von Poppholz am 05.07.12 16:20:3742k bei 10,40 weggekauft
Zitat von vincent-long: Für eine bevorstehende Übernahme würde deutlich die Tatsache sprechen, dass sich das gesamte ARENA BOD ( Board of directors) Optionen zu hervorragenden Konditionen gesichert hat.
Zuletzt waren es ca. 260.000 shares zu gemittelten 1.60$ wenn ich es richtig in Erinnerung habe.
Und das war nur eine meldepflichtige Nachricht eines BOD members of Arena.
Der Rest von denen bedient sich ebenfall GROSSZÜGIG !
Alles auf UNSEREN Kosten...
Quelle:
http://www.otcmarkets.com/stock/ARNA/filings
ich habe eben 500 ELN verkauft, um mir 580 ARNA zu kaufen.
(ich hoffe ich komme nicht in die Hölle)
(ich hoffe ich komme nicht in die Hölle)
Antwort auf Beitrag Nr.: 43.356.623 von Poppholz am 05.07.12 16:20:37wenn ein übernahmeangebot von 35 usd kommt, müssen wir uns gemeinsam überlegen, ob wir annehmen sollen oder erst in verhandlungen treten
ich bitte um ernstgemeinte meinungen



ich bitte um ernstgemeinte meinungen




habe alle meine DNDN verkauft und ARNA nachgekauft.
Vielleicht bekomme ich die Shorties ja noch zum handeln.

Vielleicht bekomme ich die Shorties ja noch zum handeln.

wenn der Kurs weiter so steigt, dann war die Entscheidung richtig:
Antwort auf Beitrag Nr.: 43.356.623 von Poppholz am 05.07.12 16:20:37
dafür habe ich 4 k die griechische hellenic zu 2,20 geholt.
dafür habe ich 4 k die griechische hellenic zu 2,20 geholt.
Arena Pharma AWARDS This Exec and 4 BUZZING Stocks to Know This Morning
By Saul Griffith
July 05 2012
Unfavorable foreign exchange volatility led Costco Wholesale Corp (NASDAQ:COST) to miss estimates of a 3.7 percent gain in same-store sales, reporting only a 3 percent increase; however net sales at the largest warehouse club operator rose 6 percent to $9.18 billion.
Don’t Miss: Wall Street Brief: Apple Bites the Bullet, But Best Buy is Still a Fan.
Led by better sales at its Victoria’s Secret lingerie chain, Limited Brands Inc (NYSE:LTD) posted a commendable 7 percent growth in same-store sales, surpassing the 2.7 percent estimated on the street.
In line with its strategy to exit from the gas station business in markets where fuel demand is declining or flat, ExxonMobil (NYSE:XOM) is said to be considering the sale of its German Esso gas stations; numbering more than 1,100, the filling stations could get Exxon over 1 billion euros.
Arena’s (NASDAQ:ARNA) VP of regulatory affairs, Craig Audet, is promoted to Senior VP, Operations and Head of Global Regulatory Affairs in appreciation for his efforts for securing FDA approval of the company’s weight-loss drug Belviq; Audet is also made responsible for investor relations and alliance management.
Barrick Gold’s (NYSE:ABX) huge mining project located on the border between Argentina and Chile is under a threat as Argentina’s Supreme Court strikes down injunctions that have blocked key parts of a law to protect glaciers, a legal setback for Barrick. At the end of Q1 2012, the company had committed about 70% of the previously announced mine construction capital of $4.7-$5.0 billion with first production is anticipated in mid-2013.
http://wallstcheatsheet.com/stocks/arena-pharmaceuticals-awa…
By Saul Griffith
July 05 2012
Unfavorable foreign exchange volatility led Costco Wholesale Corp (NASDAQ:COST) to miss estimates of a 3.7 percent gain in same-store sales, reporting only a 3 percent increase; however net sales at the largest warehouse club operator rose 6 percent to $9.18 billion.
Don’t Miss: Wall Street Brief: Apple Bites the Bullet, But Best Buy is Still a Fan.
Led by better sales at its Victoria’s Secret lingerie chain, Limited Brands Inc (NYSE:LTD) posted a commendable 7 percent growth in same-store sales, surpassing the 2.7 percent estimated on the street.
In line with its strategy to exit from the gas station business in markets where fuel demand is declining or flat, ExxonMobil (NYSE:XOM) is said to be considering the sale of its German Esso gas stations; numbering more than 1,100, the filling stations could get Exxon over 1 billion euros.
Arena’s (NASDAQ:ARNA) VP of regulatory affairs, Craig Audet, is promoted to Senior VP, Operations and Head of Global Regulatory Affairs in appreciation for his efforts for securing FDA approval of the company’s weight-loss drug Belviq; Audet is also made responsible for investor relations and alliance management.
Barrick Gold’s (NYSE:ABX) huge mining project located on the border between Argentina and Chile is under a threat as Argentina’s Supreme Court strikes down injunctions that have blocked key parts of a law to protect glaciers, a legal setback for Barrick. At the end of Q1 2012, the company had committed about 70% of the previously announced mine construction capital of $4.7-$5.0 billion with first production is anticipated in mid-2013.
http://wallstcheatsheet.com/stocks/arena-pharmaceuticals-awa…
Antwort auf Beitrag Nr.: 43.356.741 von gauner1 am 05.07.12 16:46:06von grichischen Aktien habe ich keine Ahnung. Momentan habe ich mit meinen BIOs genug am Hals.


das sind die freunde von erth, die hochkaufen
Antwort auf Beitrag Nr.: 43.356.828 von gauner1 am 05.07.12 17:06:06aktuell steht der nächste große Brocken erst bei $10,75


lars
wieso heißen alle schwulen Erich ? wenn du nicht weißt, vielleicht weiß jemand anderer
wieso heißen alle schwulen Erich ? wenn du nicht weißt, vielleicht weiß jemand anderer
weil vorne Er und hinten Du






mein letzter kauf am tage der zulassung war bei 11,21
heute zu 98% kein kleinanleger, sondern nur große shorties
Antwort auf Beitrag Nr.: 43.356.868 von LarsKol am 05.07.12 17:15:58
richtig. vorne er, hinten ich
du hast gutes allgemeinwissen



richtig. vorne er, hinten ich
du hast gutes allgemeinwissen




und das, obwohl ich schon in der Grundschule geschwänz(el)t habe






Antwort auf Beitrag Nr.: 43.356.778 von Poppholz am 05.07.12 16:54:45
du brauchst von aktien keine ahnung haben. du brauchst nur zum tiefspunkt und zum höchst verkaufen. so einfach ist es
du brauchst von aktien keine ahnung haben. du brauchst nur zum tiefspunkt und zum höchst verkaufen. so einfach ist es
Antwort auf Beitrag Nr.: 43.356.778 von Poppholz am 05.07.12 16:54:45
fanzisca
lass die kasse klingeln
fanzisca
lass die kasse klingeln
Franzi muss nix klingeln sondern fliegen lassen
Antwort auf Beitrag Nr.: 43.356.778 von Poppholz am 05.07.12 16:54:45
wo bleibt der orphus. der soll mal kommen und schreiben, dass er gewußt hat, dass heute der kurs bis 15% plus geht
wo bleibt der orphus. der soll mal kommen und schreiben, dass er gewußt hat, dass heute der kurs bis 15% plus geht
Gauner,
schreib nicht immer so einen Müll, sonst kommt der lange wieder und weisst dich zurecht!!!
Geh lieber Gurken giessen



schreib nicht immer so einen Müll, sonst kommt der lange wieder und weisst dich zurecht!!!
Geh lieber Gurken giessen



Antwort auf Beitrag Nr.: 43.356.981 von franzisca am 05.07.12 17:40:00der sieht aus, als wenn er Startschwierigkeiten hätte.
Genau wie ARNA.
Aber wenn der Motor erst einmal läuft, ...
Genau wie ARNA.
Aber wenn der Motor erst einmal läuft, ...
Antwort auf Beitrag Nr.: 43.356.936 von gauner1 am 05.07.12 17:31:58da gebe ich Dir recht.
Allerdings sehe ich bei Hellenic (Telecom) nicht das große Potential.
Ich wünsche Dir aber viel Erfolg bei dem Deal.

Allerdings sehe ich bei Hellenic (Telecom) nicht das große Potential.
Ich wünsche Dir aber viel Erfolg bei dem Deal.

Ich bin gerade bei einem SQUEZEE dabei! 

Antwort auf Beitrag Nr.: 43.357.006 von Poppholz am 05.07.12 17:45:22
das potential ist in der kaputten griechischen wirtschaft. bei denen wird langsam die wirtschaft wachsen und entsprechend aktien, die tiefst gefallen sind und alle langsam aufwachen. meine favoritten sind hellenic und die 2 systemrelevante banken. alpha bank und nationalbank of greece
das potential ist in der kaputten griechischen wirtschaft. bei denen wird langsam die wirtschaft wachsen und entsprechend aktien, die tiefst gefallen sind und alle langsam aufwachen. meine favoritten sind hellenic und die 2 systemrelevante banken. alpha bank und nationalbank of greece
Zitat von gauner1: wo bleibt der orphus. der soll mal kommen und schreiben, dass er gewußt hat, dass heute der kurs bis 15% plus geht
gauner ...Ohrfuss kommt nicht bei stegeigenden Kurs zu uns rein..aber kucken tut er bstimmt hier...

Kur steigt..weil meine Freunde mir gasgt haben..die kaufen lieber und gehen raus spazieren...wegen der Angst vom Übernahmeangebot....was evtl. bald kommen könnte !!



Wie schön das ich Montag noch Arenas gekauft habe...


Antwort auf Beitrag Nr.: 43.357.781 von Earthfire am 05.07.12 21:03:59Ich muss am Wochenende immer arbeiten!  .....dafür habe ich aber unter der Woche mehr Zeit für die Börse!
.....dafür habe ich aber unter der Woche mehr Zeit für die Börse! 
 .....dafür habe ich aber unter der Woche mehr Zeit für die Börse!
.....dafür habe ich aber unter der Woche mehr Zeit für die Börse! 
verdammt. die gurke haut mir ab.
die drache muss fliegen
Jetzt bin ich selber baff  ..dachte ich kaufe wieder weil ich Morgen nicht kann aber damit habe ich nicht gerechnet!....Arna halt!
..dachte ich kaufe wieder weil ich Morgen nicht kann aber damit habe ich nicht gerechnet!....Arna halt!
 ..dachte ich kaufe wieder weil ich Morgen nicht kann aber damit habe ich nicht gerechnet!....Arna halt!
..dachte ich kaufe wieder weil ich Morgen nicht kann aber damit habe ich nicht gerechnet!....Arna halt!
Zitat von OrpheusAusDerAsche:Zitat von gauner1: wo bleibt der orphus. der soll mal kommen und schreiben, dass er gewußt hat, dass heute der kurs bis 15% plus geht
Das muß ich gar nicht Wissen, denn mein Programm weiss das.....
Sagen Dir trailing stop orders was !?
Was du alles für tolle Sachen hast !

Ich bin einfach mit der ersten Posi hier bei 2,7 € eingestiegen....klappt auch.....sozusagen Deutsche Wert-Arbeit


Zitat von Earthfire:Zitat von OrpheusAusDerAsche: ...
Das muß ich gar nicht Wissen, denn mein Programm weiss das.....
Sagen Dir trailing stop orders was !?
Was du alles für tolle Sachen hast !
Ich bin einfach mit der ersten Posi hier bei 2,7 € eingestiegen....klappt auch.....sozusagen Deutsche Wert-Arbeit
....ich bei 1,69 € rein.....klappt auch....sozusagen SUPER-Deutsche Wert-Arbeit...


Zitat von bernie55:Zitat von Earthfire: ...
Was du alles für tolle Sachen hast !
Ich bin einfach mit der ersten Posi hier bei 2,7 € eingestiegen....klappt auch.....sozusagen Deutsche Wert-Arbeit
....ich bei 1,69 € rein.....klappt auch....sozusagen SUPER-Deutsche Wert-Arbeit...
och..männo du machst mir auch alles Kaputt !!!!




Dafür habe ich hier meine Longlektion gelernt.....ich bin sooooo entspannt seit dem !


heute war nur coveringtag. siehe auch tageschart. aber egal, hauptsache der kurs steigt. es muss also seinen guten grund haben, dass unsere freunde heute massiv gecovert haben. ist klar. so billig können sie nicht glattstellen, sonst machen die noch mehr verluste.
Antwort auf Beitrag Nr.: 43.357.952 von bernie55 am 05.07.12 21:49:46und morgen dann den anderen...schönes Wochenende gibt das ! 
Es gibt noch eine After Hours!

Es gibt noch eine After Hours!

Zitat von Earthfire:Zitat von bernie55: ...
...den 11,39 USD Widerstand könnten wir heute überspringen...
und morgen dann den anderen...schönes Wochenende gibt das !
..wichtig ist, dass wir in Richtung Norden " langsam steigen"....

..und so wie es aussieht, Earthie, werden wir uns nicht in der DJH zum Kaffeetrinken treffen




Antwort auf Beitrag Nr.: 43.357.852 von franzisca am 05.07.12 21:22:54
nein, bin nicht draußen. wollte 1000 st. nachkaufen. habe zu 8,20 nicht bekommen. dann habe ich hellenic gekauft. hatte gehofft, dass wie immer runterkommt, aber die gurke ist nur gestiegen
nein, bin nicht draußen. wollte 1000 st. nachkaufen. habe zu 8,20 nicht bekommen. dann habe ich hellenic gekauft. hatte gehofft, dass wie immer runterkommt, aber die gurke ist nur gestiegen
Antwort auf Beitrag Nr.: 43.357.852 von franzisca am 05.07.12 21:22:54
übrigens. die drache ist hier zur tradition geworden. warum lässt du sie nicht fliegen ?
übrigens. die drache ist hier zur tradition geworden. warum lässt du sie nicht fliegen ?
Arena Pharmaceuticals, Inc. (ARNA)
11.35 Up 1.33(13.27%) 4:00PM EDT -
..leider nicht > 11,39 USD...
..aber was noch nicht ist, wird noch werden...
11.35 Up 1.33(13.27%) 4:00PM EDT -
..leider nicht > 11,39 USD...
..aber was noch nicht ist, wird noch werden...

Antwort auf Beitrag Nr.: 43.357.852 von franzisca am 05.07.12 21:22:5411,34 schluß. mikrige 13,2% beschämend







Antwort auf Beitrag Nr.: 43.358.025 von franzisca am 05.07.12 22:05:42
die andere drache war schöner
die andere drache war schöner
ich bin ein man und jetzt zufrieden
Zitat von bernie55:Zitat von Earthfire: ...
und morgen dann den anderen...schönes Wochenende gibt das !
..wichtig ist, dass wir in Richtung Norden " langsam steigen"....
..und so wie es aussieht, Earthie, werden wir uns nicht in der DJH zum Kaffeetrinken treffen
Sind noch 14 Tage Bernie...das ist an der Börse eine kleine Ewigkeit...
 ....wer weiß ob wir da noch Arena im Depot haben..evtl. wurden die dann schon in Pfizer...zum entsprechenden Übernahmekurs umgewandelt
....wer weiß ob wir da noch Arena im Depot haben..evtl. wurden die dann schon in Pfizer...zum entsprechenden Übernahmekurs umgewandelt 



Dann leiste ich mir aber einen frisch gepressten O-Saft..statt Kaffee....davon trinke ich eh zuviel

Morgen Urlaub ....geil


Zitat von Earthfire:Zitat von bernie55: ...
..wichtig ist, dass wir in Richtung Norden " langsam steigen"....
..und so wie es aussieht, Earthie, werden wir uns nicht in der DJH zum Kaffeetrinken treffen
Sind noch "14 Tage" Bernie...das ist an der Börse eine kleine Ewigkeit.......wer weiß ob wir da noch Arena im Depot haben..evtl. wurden die dann schon in Pfizer...zum entsprechenden Übernahmekurs umgewandelt
Dann leiste ich mir aber einen frisch gepressten O-Saft..statt Kaffee....davon trinke ich eh zuviel
Morgen Urlaub ....geil
Morgen Earthie,
so, das Wochenende kann nun eingeläutet werden....
ARENA hat sich " einigermaßen"
 geschlagen , aber meinen Ratschlag, ein bisschen langsamer in Richtung Norden zu laufen,nat ARENA nicht beachtet....
geschlagen , aber meinen Ratschlag, ein bisschen langsamer in Richtung Norden zu laufen,nat ARENA nicht beachtet......immer dasselbe mit den Aktien....die tun wirklich nicht das, was man ihnen sagt...

-------------------------------------------------------------------------
..dann wünsche ich dir auf jeden Fall schöne Tage mit deinen Freunden in Rxxxxxxxxxxxxx....
......dort kannst du dich ja schon einmal für deine weiteren "Börsenaktionstreffen" mit O-Saft warmtrinken...

Grüße
bernie55

....also, so wie ich untenstehenden Artikel verstehe, hat ARENA einen " klugen" Kopf an die Spitze gesetzt , der u.a. sehr viel Erfahrungen bei der Vermarktung von Produkten und damit zusammenhängenden gesetzlichen Regularien in diesem Bereich hat.
...inwieweit dadurch eine mögliche Übernahme von "Big Pharmas" beeinflusst bzw. ausgeschlossen werden kann, vermag ich an dieser Stelle nicht sagen....
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) has announced Craig M. Audet’s promotion to Senior VP, Operations and Head of Global Regulatory Affairs. This new role means Audet will serve as and executive officer for the company. He has over 25 years experience in the industry. Before joining Arena, he served as VP and Head of the US Regulatory Affairs Marketed Products Group for Sanofi-Aventis from 2003 to 2008.
http://wallstcheatsheet.com/stocks/jp-morgan-sued-by-ferc-an…" target="_blank" rel="nofollow ugc noopener">
http://wallstcheatsheet.com/stocks/jp-morgan-sued-by-ferc-an…
Zitat von bernie55:
....also, so wie ich untenstehenden Artikel verstehe, hat ARENA einen " klugen" Kopf an die Spitze gesetzt , der u.a. sehr viel Erfahrungen bei der Vermarktung von Produkten und damit zusammenhängenden gesetzlichen Regularien in diesem Bereich hat.
...inwieweit dadurch eine mögliche Übernahme von "Big Pharmas" beeinflusst bzw. ausgeschlossen werden kann, vermag ich an dieser Stelle nicht sagen....
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) has announced Craig M. Audet’s promotion to Senior VP, Operations and Head of Global Regulatory Affairs. This new role means Audet will serve as and executive officer for the company. He has over 25 years experience in the industry. Before joining Arena, he served as VP and Head of the US Regulatory Affairs Marketed Products Group for Sanofi-Aventis from 2003 to 2008.
http://wallstcheatsheet.com/stocks/jp-morgan-sued-by-ferc-an…" target="_blank" rel="nofollow ugc noopener">
http://wallstcheatsheet.com/stocks/jp-morgan-sued-by-ferc-an…
Morgen Bernie, auf jedenfall sehe ich einen zeitlichen Zusammenhang zwischen dieser Personalie und der Zulassung von Belviq

wird wohl eine Strategie hinterstecken
 ....d.h. an einen Zufall glaube ich hier nicht !
....d.h. an einen Zufall glaube ich hier nicht !

 ....eher das Arena ....Gas gibt um sich Marktanteile zu sichern.....und dies schnellstmöglich.
....eher das Arena ....Gas gibt um sich Marktanteile zu sichern.....und dies schnellstmöglich. 
Long and Strong Grüße von
Earthfire


wieso seid ihr so früh wach ?
Zitat von Earthfire:Zitat von bernie55:
....also, so wie ich untenstehenden Artikel verstehe, hat ARENA einen " klugen" Kopf an die Spitze gesetzt , der u.a. sehr viel Erfahrungen bei der Vermarktung von Produkten und damit zusammenhängenden gesetzlichen Regularien in diesem Bereich hat.
...inwieweit dadurch eine mögliche Übernahme von "Big Pharmas" beeinflusst bzw. ausgeschlossen werden kann, vermag ich an dieser Stelle nicht sagen....
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) has announced Craig M. Audet’s promotion to Senior VP, Operations and Head of Global Regulatory Affairs. This new role means Audet will serve as and executive officer for the company. He has over 25 years experience in the industry. Before joining Arena, he served as VP and Head of the US Regulatory Affairs Marketed Products Group for Sanofi-Aventis from 2003 to 2008.
http://wallstcheatsheet.com/stocks/jp-morgan-sued-by-ferc-an…" target="_blank" rel="nofollow ugc noopener">
http://wallstcheatsheet.com/stocks/jp-morgan-sued-by-ferc-an…
Morgen Bernie, auf jedenfall sehe ich einen zeitlichen Zusammenhang zwischen dieser Personalie und der Zulassung von Belviq
wird wohl eine Strategie hinterstecken....d.h. an einen Zufall glaube ich hier nicht !
....eher das Arena ....Gas gibt um sich Marktanteile zu sichern.....und dies schnellstmöglich.
Long and Strong Grüße von
Earthfire
-----------------------------------------------------------------------------
Craig Audet, Vice President of Arena’s (NASDAQ:ARNA) regulatory affairs, has been promoted to Senior VP, Operations and Head of Global Regulatory Affairs, as a reward for the firm obtaining FDA approval for weight-loss drug Belviq.
Investor relations and alliance management will be added to Audet’s new position.
http://wallstcheatsheet.com/stocks/healthcare-business-revie…
...in o.g. Artikel ist die Ernennung als " Belohnung " für besondere Leistungen hinsichtlich des Approvals für Belviq zu verstehen.....sozusagen eine " Belviq-Bonbon-Gratifikation "....

Antwort auf Beitrag Nr.: 43.359.339 von gauner1 am 06.07.12 11:06:23So gauner...
....dann hier mal etwas zum wachwerden...
Die Shortanalyse vom 5.7.12.
http://www.shortanalytics.com/getshortchart.php?tsymbol=arna
....dann hier mal etwas zum wachwerden...

Die Shortanalyse vom 5.7.12.
http://www.shortanalytics.com/getshortchart.php?tsymbol=arna
Antwort auf Beitrag Nr.: 43.359.601 von bernie55 am 06.07.12 12:04:14
mach die selbe analyse auch heute, damit wir auf 13 kommen
mach die selbe analyse auch heute, damit wir auf 13 kommen



 Auf gehts, 13 Euronen heute!
Auf gehts, 13 Euronen heute!
warum so kleinlich?
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA) has announced Craig M. Audet’s promotion to Senior VP, Operations and Head of Global Regulatory Affairs. This new role means Audet will serve as and executive officer for the company. He has over 25 years experience in the industry. Before joining Arena, he served as VP and Head of the US Regulatory Affairs Marketed Products Group for Sanofi-Aventis from 2003 to 2008.
http://wallstcheatsheet.com/stocks/jp-morgan-sued-by-ferc-an…
bernie55 schrieb.also, so wie ich obenstehenden Artikel verstehe, hat ARENA einen " klugen" Kopf an die Spitze gesetzt , der u.a. sehr viel Erfahrungen bei der Vermarktung von Produkten und damit zusammenhängenden gesetzlichen Regularien in diesem Bereich hat.
...inwieweit dadurch eine mögliche Übernahme von "Big Pharmas" beeinflusst bzw. ausgeschlossen werden kann, vermag ich an dieser Stelle nicht sagen....
EARTHIE schrieb , .dass eine Strategie dahinterstecke....
--------------------------------------------------------------------------------------------------------------------------------------------------------

..ok, dann spinne ich unsere " Gedanken" einfach mal weiter..
....am Ende des Jahres steht die Zulassung durch die EMA in Europa an, und ich schätze die Chancen auf ein Approval als sehr gut ein.
Soweit ich richtig informiert bin, besitzt ARENA die alleinigen Vertriebsrechte für Europa und China…
Ausgehend von einem Approval in Europa wird sich ARENA jetzt schon im Vorfeld positionieren und sich wohl auch nach europäischen Partnern umhören - die Ernennung von Craig M. Audet hat wohl den Zweck, diese Aufgabe für Europa zu übernehmen – zum einen um Partner zu finden, zum anderen Kooperationen abzuschließen, die es ARENA ermöglicht,die „ neuen“ Märkte zu erschließen….
So, dann schauen wir mal..
..TIME WILL TELL..
http://wallstcheatsheet.com/stocks/jp-morgan-sued-by-ferc-an…
bernie55 schrieb.also, so wie ich obenstehenden Artikel verstehe, hat ARENA einen " klugen" Kopf an die Spitze gesetzt , der u.a. sehr viel Erfahrungen bei der Vermarktung von Produkten und damit zusammenhängenden gesetzlichen Regularien in diesem Bereich hat.
...inwieweit dadurch eine mögliche Übernahme von "Big Pharmas" beeinflusst bzw. ausgeschlossen werden kann, vermag ich an dieser Stelle nicht sagen....
EARTHIE schrieb , .dass eine Strategie dahinterstecke....
--------------------------------------------------------------------------------------------------------------------------------------------------------

..ok, dann spinne ich unsere " Gedanken" einfach mal weiter..

....am Ende des Jahres steht die Zulassung durch die EMA in Europa an, und ich schätze die Chancen auf ein Approval als sehr gut ein.
Soweit ich richtig informiert bin, besitzt ARENA die alleinigen Vertriebsrechte für Europa und China…

Ausgehend von einem Approval in Europa wird sich ARENA jetzt schon im Vorfeld positionieren und sich wohl auch nach europäischen Partnern umhören - die Ernennung von Craig M. Audet hat wohl den Zweck, diese Aufgabe für Europa zu übernehmen – zum einen um Partner zu finden, zum anderen Kooperationen abzuschließen, die es ARENA ermöglicht,die „ neuen“ Märkte zu erschließen….
So, dann schauen wir mal..
..TIME WILL TELL..

Antwort auf Beitrag Nr.: 43.359.601 von bernie55 am 06.07.12 12:04:14eine SHORT-Quote von über 50%.
Das ist gewaltig, zumal der Kurs gestern so starkt gestiegen ist.
Vielleicht verkaufe ich noch ein paar ELN, dann räumen wir die letzten freien Aktien ab und die Shorties verbrennen sich die Finger.
Dann haben wir die gleiche Kursentwicklung wir bei VW als sich die Herrschaften dort verspekuliert hatten.

Das ist gewaltig, zumal der Kurs gestern so starkt gestiegen ist.
Vielleicht verkaufe ich noch ein paar ELN, dann räumen wir die letzten freien Aktien ab und die Shorties verbrennen sich die Finger.
Dann haben wir die gleiche Kursentwicklung wir bei VW als sich die Herrschaften dort verspekuliert hatten.

Zitat von Poppholz: eine SHORT-Quote von über 50%.
Das ist gewaltig, zumal der Kurs gestern so starkt gestiegen ist.
Vielleicht verkaufe ich noch ein paar ELN, dann räumen wir die letzten freien Aktien ab und die Shorties verbrennen sich die Finger.
Dann haben wir die gleiche Kursentwicklung wir bei VW als sich die Herrschaften dort verspekuliert hatten.
Du glaubst gar nicht wie sehr ich mir dies wünsche !!!!!


Der Tag wird kommen....wo die Shorts drinhängen .........und die Newslage so gut bei ARENA ist....das es kracht....


Zitat von bernie55: bernie55 schrieb.also, so wie ich obenstehenden Artikel verstehe, hat ARENA einen " klugen" Kopf an die Spitze gesetzt , der u.a. sehr viel Erfahrungen bei der Vermarktung von Produkten und damit zusammenhängenden gesetzlichen Regularien in diesem Bereich hat.
...inwieweit dadurch eine mögliche Übernahme von "Big Pharmas" beeinflusst bzw. ausgeschlossen werden kann, vermag ich an dieser Stelle nicht sagen....
EARTHIE schrieb , .dass eine Strategie dahinterstecke....
--------------------------------------------------------------------------------------------------------------------------------------------------------
![]()
..ok, dann spinne ich unsere " Gedanken" einfach mal weiter..
....am Ende des Jahres steht die Zulassung durch die EMA in Europa an, und ich schätze die Chancen auf ein Approval als sehr gut ein.
Soweit ich richtig informiert bin, besitzt ARENA die alleinigen Vertriebsrechte für Europa und China…
Ausgehend von einem Approval in Europa wird sich ARENA jetzt schon im Vorfeld positionieren und sich wohl auch nach europäischen Partnern umhören - die Ernennung von Craig M. Audet hat wohl den Zweck, diese Aufgabe für Europa zu übernehmen – zum einen um Partner zu finden, zum anderen Kooperationen abzuschließen, die es ARENA ermöglicht,die „ neuen“ Märkte zu erschließen….
So, dann schauen wir mal..
..TIME WILL TELL..
Was heißt das für ein mögliches BUY-OUT ??
Wenn ein BUY-OUT durch einen BIG PHARMA wirklich erfolgen sollte, dann ist der Zeitpunkt für eine Übernahme vor einem möglichen Approval in Europa mehr als günstig anzusehen…...
..und wenn man nun das mögliche Potential an „ Obesity“ Menschen in Europa und China dazunimmt, dann relativieren sich auch die Beträge von 30 USD - 40 USD bei einer möglichen Übernahme.

Antwort auf Beitrag Nr.: 43.360.272 von bernie55 am 06.07.12 14:49:0440 $ 


 ist mir zu wenig...da stimme ich nicht zu....un Gauner auch nicht !
ist mir zu wenig...da stimme ich nicht zu....un Gauner auch nicht ! 






 ist mir zu wenig...da stimme ich nicht zu....un Gauner auch nicht !
ist mir zu wenig...da stimme ich nicht zu....un Gauner auch nicht ! 



Antwort auf Beitrag Nr.: 43.360.326 von Earthfire am 06.07.12 14:59:08also bei $40,- würde ich wohl schwach werden.
Aber ich bin ja auch nicht gierig.

Aber ich bin ja auch nicht gierig.

Antwort auf Beitrag Nr.: 43.359.601 von bernie55 am 06.07.12 12:04:14
lard
meine schöne hellena 11 %
deine gurke 2%






lard
meine schöne hellena 11 %
deine gurke 2%






Meine Gurke gerade eben sogar kurz im Minus gewesen






Antwort auf Beitrag Nr.: 43.360.650 von LarsKol am 06.07.12 16:02:35
wenn die gurke in mimus ist, das ist schlimm für einen mam, falls du verstehst, was ich meine









wenn die gurke in mimus ist, das ist schlimm für einen mam, falls du verstehst, was ich meine









Keine Sorge, meine Gurke hat die schöne Hellena auch schon von innen gesehen












Antwort auf Beitrag Nr.: 43.360.650 von LarsKol am 06.07.12 16:02:35
lars
bei 8,50 usd tue ich verbilligen
lars
bei 8,50 usd tue ich verbilligen
also gar nicht mehr



Antwort auf Beitrag Nr.: 43.360.946 von gauner1 am 06.07.12 16:58:31Ich glaub Du bist ein Orfus und willst nur Angst machen














Antwort auf Beitrag Nr.: 43.360.912 von LarsKol am 06.07.12 16:51:32
meinst du nicht, dass der kurs wieder auf 8 zurückkommt ? der lange vincent wartet sogar auf 4 usd. ich biete sogar das doppelte
meinst du nicht, dass der kurs wieder auf 8 zurückkommt ? der lange vincent wartet sogar auf 4 usd. ich biete sogar das doppelte
ja doch bestimmt, aber erst nach dem split






NSPH..hat sich eine W-Formation gebildet....bricht evtl. gleich aus ?
oder auch nicht.....

oder auch nicht.....


@Bernie...kuckst du was Xoma macht.....



Volltreffer !





Volltreffer !


Ich habe meine paar X-Oma`s Gestern verkauft, weil ich süchtig nach ARENA war
unsere freunde sind wieder da. 100 oder 200
Zitat von LarsKol: Ich habe meine paar X-Oma`s Gestern verkauft, weil ich süchtig nach ARENA war
ich habe Montag dienstag und Mittwoch aufgestockt...richtig fett drin jetzt bei XOMA

Chart sagt...Fahnenstange mit sich bildender Wimpelformation..d.h. hier könnte noch ein zusätzlicher Schub kommen ..

Arenas bleiben im Depot...habe genug davon..

heute ist freitag. wer weiß, was heute noch kommt. für heute bin ich sogar mit unch zufrieden.
alle indizes wieder tief in minus
ich verstehe euch nicht. wie kann man in ein unternehmen investieren, bei dem man überhaupt keine ahnung hat. nur weil shorties gibt ? nur weil die aktie ab und zu bischen steigt ? was weoßt ihr von xoma ? wie sind die finanziellen verhältnisse ? usw.usw.usw.
nur so kann mam geld verlieren. im casino hat man bessere chancen. da weiß man zumindestens auf was man setzt
nur so kann mam geld verlieren. im casino hat man bessere chancen. da weiß man zumindestens auf was man setzt
Antwort auf Beitrag Nr.: 43.361.590 von gauner1 am 06.07.12 19:39:32wichtig ist, dass man eine strategie verfolgt.
und nicht bei jeder strategie muss man die finanziellen verhältnisse kennen.
so richtig hundert prozentig kennt sich doch sowieso niemand aus, was bringt also halbwissen bei einer aktie, bei der täglich fast 5 millionen usd umgesetzt werden und die nahezu effizient gehandelt wird?
ich sage, es bringt nur ein gutes gefühl und ein trügerisches sicherheitsempfinden, mehr nicht. ausnahmen sind speziell bei biotechs vielleicht noch anstehende fda-zulassungen, die auf grund der gap problematik mit einkalkuliert werden müssen.
und nicht bei jeder strategie muss man die finanziellen verhältnisse kennen.
so richtig hundert prozentig kennt sich doch sowieso niemand aus, was bringt also halbwissen bei einer aktie, bei der täglich fast 5 millionen usd umgesetzt werden und die nahezu effizient gehandelt wird?
ich sage, es bringt nur ein gutes gefühl und ein trügerisches sicherheitsempfinden, mehr nicht. ausnahmen sind speziell bei biotechs vielleicht noch anstehende fda-zulassungen, die auf grund der gap problematik mit einkalkuliert werden müssen.
wir haben gestern 13% plus gemacht, ziehen davon den heutigen minus von 2 % ab, dann sind wir immer noch mit 11% gegenüber donnerstag glücklich
ein ganz kleiner rücksetzer und das an einem freitag. ist das nicht normal nach den vergangennen gewinnen ?
ein ganz kleiner rücksetzer und das an einem freitag. ist das nicht normal nach den vergangennen gewinnen ?
lars
die sau lief heute durch das dorf medigene, also meine libliengsaktie. war aber nicht dabei, weil ich meine arena und das hellenic nicht verkaufen wollte. und nächste woche geht es dort weiter, denn medigene war tief gefallen und heute kamen beste meldungen. na ja, man kann nicht überall sein
die sau lief heute durch das dorf medigene, also meine libliengsaktie. war aber nicht dabei, weil ich meine arena und das hellenic nicht verkaufen wollte. und nächste woche geht es dort weiter, denn medigene war tief gefallen und heute kamen beste meldungen. na ja, man kann nicht überall sein
Zitat von gauner1: ich verstehe euch nicht. wie kann man in ein unternehmen investieren, bei dem man überhaupt keine ahnung hat. nur weil shorties gibt ? nur weil die aktie ab und zu bischen steigt ? was weoßt ihr von xoma ? wie sind die finanziellen verhältnisse ? usw.usw.usw.
nur so kann mam geld verlieren. im casino hat man bessere chancen. da weiß man zumindestens auf was man setzt
Hallo Gauner...was schreibst du da.....du solltest doch schon längts Wissen wie ich trade..

Steven-Trader..bringt es hiermit auf den Punkt :
Zitat : ["b]wichtig ist, dass man eine strategie verfolgt.[/b]"
und wieder ein Buchtip von mir :
Jeder Trader braucht einen Plan - Detlef Wormstall
Ohne Plan kein Trade
Der Erfolg als Trader wird durch das Handeln und Tun bestimmt, die mentale Stärke und Disziplin ist entscheidend.
Ich hatte ja schon vor ca. 10 Tagen hier mal auf XOMA hingewiesen.....die habe ich permanent auf der WL gehabt...und da kam eine Bewegung mit einer News hinterlegt...die mein Einstiegspunkt markierte...dann noch paar mal nachgekauft .....und fertig ist die Kiste.

Hintergundwissen habe ich hier so viel wie ich benötige um mir ein Bild zu machen....Partner Servier ( Mit Meilensteinzahlungen in Höhe von 450 MIO $ :eek
 ...dann noch Norvatis als Partner...
...dann noch Norvatis als Partner...Mehrere Standbeine...Phase 3 bei einem zukünftigen Blockbuster...und und und..
NSPH wurde hier von Franzica ( Danke :kiss
 erwähnt...bin ich auch an diesem Tag eingestiegen..nach einer Kurzrecherche..KZ 5 $...die ist gestern auch wieder schön gelaufen...und wird Mmn nach bis ende nächster Woche die 4$ klarmachen
erwähnt...bin ich auch an diesem Tag eingestiegen..nach einer Kurzrecherche..KZ 5 $...die ist gestern auch wieder schön gelaufen...und wird Mmn nach bis ende nächster Woche die 4$ klarmachen 
Shortquote ist nur ein Aspekt den ich vor einem Einstieg betrachte....
Aber alles sollte man auch nicht verraten....

Bei Xoma habe ich eine rel. große Posi....dort reichen mir 70- 100 % Plus und ich bin bis auf einen kleinen Rest dort dann raus.
Ich bin ja rel. neu im Biotec Geschäft.....sozusagen Lehrling
 ...aber ch habe das Gefühl als wenn die zur Zeit im Allgemeinen gut laufen.
...aber ch habe das Gefühl als wenn die zur Zeit im Allgemeinen gut laufen.Dies ist mir zb. von Rohstoffexplorern gut bekannt..da gab es auch Zeiten,da konnte man fast "Blind" alles kaufen.....und konnte nur Plus machen..es sei den die Bude stand vor der Pleite !

Schönes WE

Euch allen noch ein entspanntes Wochenende und nächste Woche steigen die Kurse wieder.


hallo berni
wie groß siehst die chance bei vivus für die zulassung ?
ist bei 24 usd die zulassung nicht eingepreist ?
gruß
wie groß siehst die chance bei vivus für die zulassung ?
ist bei 24 usd die zulassung nicht eingepreist ?
gruß
Antwort auf Beitrag Nr.: 43.364.293 von gauner1 am 08.07.12 18:11:46Merk Dir mal, dass an der Börse unvorhersehbare Ereignisse NIE eingepreisst sind.
Und jammer nicht immer so rum!
Und jammer nicht immer so rum!

 BLOOMBERG > positive Presse über Perspektiven von ARENA und über mögliche Übernahme durch Big Pharmas...
BLOOMBERG > positive Presse über Perspektiven von ARENA und über mögliche Übernahme durch Big Pharmas...@ AREANICS....it`s only a beginning ...

First Diet Pill in Decade Turns Arena Into Deal Bait: Real M&A
The first medication in more than a decade to help 78 million obese Americans slim down is turning into takeover bait.
Arena Pharmaceuticals Inc. (ARNA) won Food and Drug Administration approval last month for Belviq, a treatment affecting an area of the brain that helps a person feel full after consuming less food. With the first weight-loss drug to be cleared for sale in the U.S. in 13 years, Arena is projected by analysts to increase revenue 18-fold in the next four years, the fastest growth in the world among specialty pharmaceutical companies greater than $1 billion, according to data compiled by Bloomberg.
Enlarge image First Diet Pill in Decade Turns Arena Into Deal Bait
About 42 percent of the U.S. population may be obese by 2030, up from about a third currently. Photographer: David Paul Morris/Bloomberg
While Arena’s 494 percent stock gain this year makes it the most expensive U.S. specialty drugmaker relative to revenue, the San Diego-based company offers potential buyers a medication that Piper Jaffray Cos. estimates will reach $2 billion in annual sales. Arena, with a $2.2 billion market value, may draw takeover interest from GlaxoSmithKline Plc (GSK), which is divesting its diet pill Alli, said WBB Securities LLC. Tokyo-based Eisai Co. (4523), which has licensed the rights to sell Belviq in the U.S., could also be an acquirer, said Lazard Capital Markets LLC.
“The large pharmaceutical companies are all on the edge of their seats looking at Arena,” Stephen Brozak, president of WBB Securities in Clark, New Jersey, said in a telephone interview. Obesity “is a global pandemic. There are people that absolutely are in need of these types of products. Large pharma are wonderful at marketing drugs and this is a product that lends itself to marketing.”
Appetite Suppression
“Arena is focused on bringing Belviq to the market in the U.S. with Eisai and in obtaining approval of the drug in markets outside of the U.S.,” David Schull, a spokesman for Arena, said in an e-mailed statement when asked whether the company has been approached by potential acquirers about a deal.
The FDA approved Belviq, previously called lorcaserin, on June 27. The pill works in a similar way to fenfluramine, part of the fen-phen appetite-suppression drug combination pulled from pharmacies 15 years ago when it was linked to heart valve abnormalities.
Arena and Eisai agreed to conduct six post-market studies to assess the safety and efficacy of the drug, including determining the potential for major cardiac risks such as heart attack and stroke, the FDA said.
Belviq will be available after the Drug Enforcement Administration completes a review to classify the drug based on its potential for abuse, which Arena has said may take four to six months.
Obesity Rate
“It’s the single most important thing that’s happened to Arena in its entire history,” WBB Securities’ Brozak said. “This is the first approval they’ve ever gotten.”
Analysts project that by 2015 Arena’s sales will have risen faster than every other specialty drugmaker in the world with a market capitalization greater than $1 billion, data compiled by Bloomberg show. The company’s revenue may total $232 million that year, versus $13 million in 2011, according to analysts’ estimates compiled by Bloomberg.
About 42 percent of the U.S. population may be obese by 2030, up from about a third currently, according to a report presented at the Centers for Disease Control and Prevention’s obesity conference in May. Globally, about 500 million people are obese, according to a World Health Organization report. Obesity is defined as having a body mass index of more than 30.
Glaxo, the U.K.’s largest drugmaker, may be interested in purchasing Arena to bolster its presence in the market for weight-loss remedies after sales declined for Alli, its over- the-counter diet pill, according to WBB Securities’ Brozak.
‘Dress Rehearsal’
London-based Glaxo has said it plans to divest Alli, which contains orlistat, a chemical that blocks the intestines from absorbing fat when taken as many as three times a day with meals. Orlistat has been linked to reports of liver injury, prompting consumer advocacy groups to demand its removal from the market. The FDA announced new warnings on the pill’s label in mid-2010, and Glaxo has said that Alli is safe and effective when used as directed.
Glaxo has “done the dress rehearsal,” so it would now know how to best market Arena’s weight-loss drug, Brozak said.
Kevin Colgan, a spokesman for Glaxo, said the company doesn’t comment on speculation, when asked whether it wants to acquire Arena.
Other companies are also seeking approval for weight-loss drugs. Qnexa from Vivus Inc. (VVUS) is slated for an FDA decision by July 17. Orexigen Therapeutics Inc. (OREX), which is developing the pill Contrave with Takeda Pharmaceutical Co., agreed in September to conduct a two-year study of the drug’s heart risks.
Drug Partnership
Belviq is the first prescription obesity medicine to be approved for sale in the U.S. since Roche Holding AG (ROG)’s Xenical in 1999. Glaxo’s Alli is a half-dose version of Xenical’s active ingredient and received FDA clearance in 2007 as the first diet drug available without a prescription.
Once available, lorcaserin will be marketed in the U.S. by Eisai under the name Belviq and the company will pay Arena a portion of the drug’s revenue. The partnership may deter potential acquirers that don’t want to share Belviq’s sales, said Alan Carr, a New York-based analyst for Needham & Co.
“Arena is much more attractive from an M&A perspective if you’re getting worldwide rights,” he said in a phone interview.
Eisai may decide to buy Arena so that it collects all of the revenue from Belviq, said Bill Tanner, an analyst for Lazard in New York. The two companies have a so-called standstill agreement that prevents the Japanese drugmaker from purchasing Arena unless someone else tries to first. It’s intended to protect Arena from a hostile takeover and wouldn’t prevent the companies from negotiating a deal, Tanner said.
Eisai’s Interest
“If this is a big drug, it could be a fairly sizeable payment that they have to make to Arena,” he said in a phone interview. “It probably makes sense that they’d want to take a look at acquiring it.”
Marcia Diljak, a spokeswoman for Eisai, declined to comment on whether the company would be interested in buying Arena.
Eisai will probably want to first gauge Belviq’s commercial success so that it doesn’t risk overpaying for Arena in case sales are weaker than analysts expect, Tanner said.
Arena, which has posted an almost six-fold stock price increase this year, is valued at 52 times analysts’ estimates for fiscal 2012 sales. That’s higher than all 14 other specialty pharmaceutical companies in the U.S. that have a market capitalization greater than $1 billion, data compiled by Bloomberg show. Even based on revenue estimates for fiscal 2015, Arena trades at the second-richest multiple in the group.
“If you knew for a fact that there wasn’t a buyer for the company, I don’t think this stock would be where it is,” Lazard’s Tanner said. “It’s not overly expensive if you think it’s going to be a decent drug.”
Edward Tenthoff, a New York-based analyst for Piper Jaffray, estimates Belviq will reach annual sales of $2 billion just in the U.S. by 2020.
“The approval of a drug with the market potential of Belviq certainly makes Arena” an acquisition candidate, Tenthoff said in a phone interview.
“This could be a true blockbuster drug in an age when there are fewer and fewer blockbusters.”
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…" target="_blank" rel="nofollow ugc noopener">
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…
Trading Covered Calls In The Pharmaceutical Sector
July 9, 2012
As of the close of the market on Friday, three lucrative covered call opportunities existed in three Pharmaceutical companies, Amarin Corporation plc (AMRN), VIVUS Inc. (VVUS), and Arena Pharmaceuticals, Inc. (ARNA).
Do your own due diligence before investing, but here I will outline each trade using a simple online covered call calculator provided by the Options Industry Council.
Amarin Corporation plc
Amarin stock has a $2 billion market capitalization, trades at a large forward P/E ratio, and has negative book value per share. This is not what most people would consider a blue chip stock. There is a potential to make large profits or large losses trading in a company like this. Most of the value is derived from intellectual property.
Still, the stock is trading above the 50-Day moving average, and a covered call could reduce downside risk, if there is reason to sell.

The covered call trade for this stock involves a simple sale of an in-the-money call with a $15 strike price, producing a premium of $1.25. The sale of the call is made against shares purchased at the current market price of $15.12. Assuming a modest level of commissions, your profit will be reduced to 6.6% return, as long as the shares trade above $15 at expiration in 14 days (July 21, 2012).
The break-even price on the trade is $14.07, which means the stock would need to fall below $14.07 before you record a loss.
VIVUS Inc.
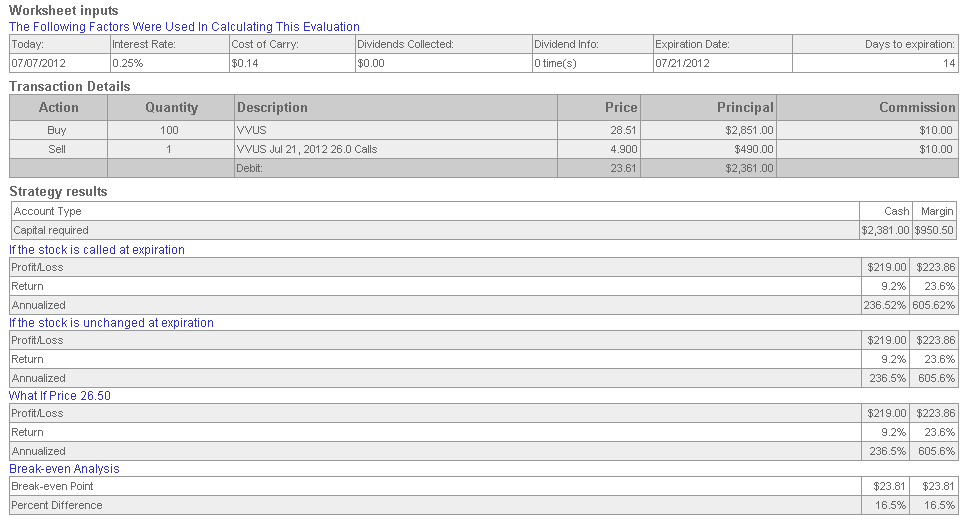
VVUS stock has a $2.8 billion market capitalization, trades at a large forward P/E ratio, and has about $3 book value per share. The company has a blockbuster weight loss drug called Qnexa that is seeking FDA approval on or before July 17th. The tremendous option premium is likely a result of speculation that the drug will be a game-changer for the company.
The simple covered call trade involves buying the stock at the current market price of $28.51 and selling in-the-money call options with a strike price of $26 for a premium of $4.90. The expiration date is July 21, 2012. Factoring in moderate commissions, the return potential over 14 days is 9.2%. Annualized, this is a 236% return.
The break-even price for the trade is $23.81, meaning that the stock would need to fall below this price before any loss is recorded. This is 16.5% below the current market price.
In this type of situation, the risk is that the FDA will disappoint investors by blocking the drug. There is also the risk of a sell-off after good news is announced. Still, the stock chart shows an upward trend and the expiration date for the covered call trade is 14 days away. If the stock falls precipitously, an investor may be able to sell the stock shares before reaching the break-even price for the trade.
Arena Pharmaceuticals, Inc.
Arena also has about a $2 billion market capitalization and shares many of the same characteristics as the other two companies. Unlike VVUS, Arena's weight loss drug, Belviq, has already been approved by the FDA.
The 1-Year chart tells a strong story over the past year, with the stock trading above the 50-day moving average and trending up.
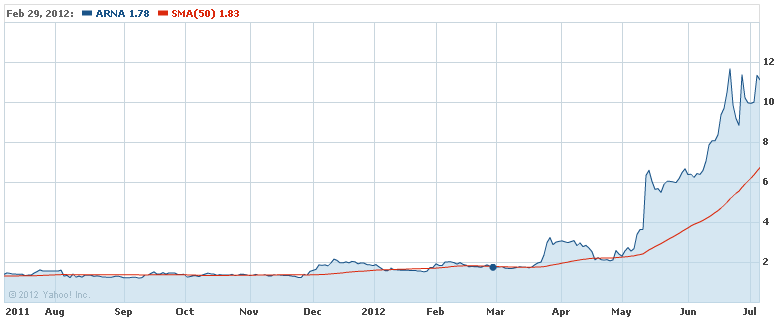
The covered call trade for this stock involves buying shares at the price of $11.11 and selling an in-the-money call option with an $11 strike price, for a premium of $0.93. The option expiration is July 21, 2012. Factoring in modest commissions, the return potential for the trade is limited to about 6%, but over 14 days, that's an annualized return of 153.6%.
The break-even price for the trade is $10.38. Below this stock price, the trade begins to lose money.
(click to enlarge)
The covered call trade on Arena may have lower risk than the trade for VVUS or Amarin, as they just received FDA approval. This is also reflected in the lower return potential for the covered call transaction.
Covered calls have risks similar to owning stocks outright. If a catastrophic event occurs, the stock price could fall precipitously. You still own shares in the company, and will see those shares fall, just like owning shares outright. Selling call options against your shares actually reduces your investment risk, as it returns a portion of your money at risk. However, by selling the call option, you also limit the potential return on the investment.
The benefit to a stock investor is that discipline is added to trading the stock. By selling the call, you are locking in a sales price and creating an exit strategy that should generate a profit.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/707741-trading-covered-calls…
July 9, 2012
As of the close of the market on Friday, three lucrative covered call opportunities existed in three Pharmaceutical companies, Amarin Corporation plc (AMRN), VIVUS Inc. (VVUS), and Arena Pharmaceuticals, Inc. (ARNA).
Do your own due diligence before investing, but here I will outline each trade using a simple online covered call calculator provided by the Options Industry Council.
Amarin Corporation plc
Amarin stock has a $2 billion market capitalization, trades at a large forward P/E ratio, and has negative book value per share. This is not what most people would consider a blue chip stock. There is a potential to make large profits or large losses trading in a company like this. Most of the value is derived from intellectual property.
Still, the stock is trading above the 50-Day moving average, and a covered call could reduce downside risk, if there is reason to sell.

The covered call trade for this stock involves a simple sale of an in-the-money call with a $15 strike price, producing a premium of $1.25. The sale of the call is made against shares purchased at the current market price of $15.12. Assuming a modest level of commissions, your profit will be reduced to 6.6% return, as long as the shares trade above $15 at expiration in 14 days (July 21, 2012).
The break-even price on the trade is $14.07, which means the stock would need to fall below $14.07 before you record a loss.
VIVUS Inc.
VVUS stock has a $2.8 billion market capitalization, trades at a large forward P/E ratio, and has about $3 book value per share. The company has a blockbuster weight loss drug called Qnexa that is seeking FDA approval on or before July 17th. The tremendous option premium is likely a result of speculation that the drug will be a game-changer for the company.
The simple covered call trade involves buying the stock at the current market price of $28.51 and selling in-the-money call options with a strike price of $26 for a premium of $4.90. The expiration date is July 21, 2012. Factoring in moderate commissions, the return potential over 14 days is 9.2%. Annualized, this is a 236% return.
The break-even price for the trade is $23.81, meaning that the stock would need to fall below this price before any loss is recorded. This is 16.5% below the current market price.
In this type of situation, the risk is that the FDA will disappoint investors by blocking the drug. There is also the risk of a sell-off after good news is announced. Still, the stock chart shows an upward trend and the expiration date for the covered call trade is 14 days away. If the stock falls precipitously, an investor may be able to sell the stock shares before reaching the break-even price for the trade.
Arena Pharmaceuticals, Inc.
Arena also has about a $2 billion market capitalization and shares many of the same characteristics as the other two companies. Unlike VVUS, Arena's weight loss drug, Belviq, has already been approved by the FDA.
The 1-Year chart tells a strong story over the past year, with the stock trading above the 50-day moving average and trending up.

The covered call trade for this stock involves buying shares at the price of $11.11 and selling an in-the-money call option with an $11 strike price, for a premium of $0.93. The option expiration is July 21, 2012. Factoring in modest commissions, the return potential for the trade is limited to about 6%, but over 14 days, that's an annualized return of 153.6%.
The break-even price for the trade is $10.38. Below this stock price, the trade begins to lose money.
(click to enlarge)
The covered call trade on Arena may have lower risk than the trade for VVUS or Amarin, as they just received FDA approval. This is also reflected in the lower return potential for the covered call transaction.
Covered calls have risks similar to owning stocks outright. If a catastrophic event occurs, the stock price could fall precipitously. You still own shares in the company, and will see those shares fall, just like owning shares outright. Selling call options against your shares actually reduces your investment risk, as it returns a portion of your money at risk. However, by selling the call option, you also limit the potential return on the investment.
The benefit to a stock investor is that discipline is added to trading the stock. By selling the call, you are locking in a sales price and creating an exit strategy that should generate a profit.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/707741-trading-covered-calls…
Antwort auf Beitrag Nr.: 43.364.558 von vincent-long am 08.07.12 21:28:53
hallo du der lange vincent

wer seit 3,30 usd dabei ist und auch noch getradet hat und fast 400% in plus ist, hat nicht zu jammern.
ich jammere nur dann, wenn die verbrecher kommen. außerdem ist mein recht, von morgens bis abends zu jammern


hallo du der lange vincent


wer seit 3,30 usd dabei ist und auch noch getradet hat und fast 400% in plus ist, hat nicht zu jammern.
ich jammere nur dann, wenn die verbrecher kommen. außerdem ist mein recht, von morgens bis abends zu jammern



Zitat von gauner1: hallo berni
wie groß siehst die chance bei vivus für die zulassung ?
ist bei 24 usd die zulassung nicht eingepreist ?
gruß
Man weiß nie ob und wie ein Medikament zugelassen wird…
...es ist immer ein Spiel mit dem Feuer ( FDA und Approval)……..
Auch wenn der AC sich mit 20 zu 2 für ein Approval ausgesprochen hat, ist das Ding noch nicht gelaufen.
Durch die Zulassung von BELVIQ hat sich meiner Meinung nach die Ausgangssituation bzgl. Approvals bei " Obesity-drugs" grundlegend verändert und die FDA hat einfach nicht mehr den Druck , "alles und nichts" zuzulassen.......
Zudem sprechen die Insider - Verkäufe der CEOs im Juni 2012 ja auch nicht unbedingt von großem Vertrauen und als Anleger von VIVUS Aktien würde ich auf aktuellem Niveau schon "einiges vieles alles "
 verkaufen.
verkaufen. Die Aktie von VIVUS ist bereits sehr gut gelaufen und ich könnte dir aktuell wirklich nicht sagen, wieviel Potential sie überhaupt noch nach oben hätte.
Wenn eine Zulassung für Qnexa ausgesprochen werden würde, dann denke, dass es ein Approval mit sehr strengen Einschränkungen geben wird > z.B. aufgrund nicht auszuschließender Geburten-Missbildungen ist es Frauen im gebärfähigen Alter strengstens untersagt , das Medikament Qnexa einzunehmen.
In meinen Augen wird sich das Rad immer mehr in Richtung ARENA und BELVIQ drehen....es werden immer mehr Artikel zu BELVIQ veröffentlicht..( siehe u.a.Bloomberg Artikel )....und auch immer mehr Investoren anlocken.....
BELVIQ wird bald in aller Munde sein.....äähhmm nicht in aller Munde, aber doch in vielen Körpern...

TIME WILL TELL
Grüße
bernie55

..ein Artikel von Sierra und Co..
..ich denke mal, etwas zum schmunzeln....
Monday, July 9, 2012
"Underground Stock Reporter" opinion on Arena Pharmaceuticals
Sierra World Equity Review is on track...just look at this from the Underground Stock Reporter as of July 9th 2012. Sierra World Equity Review currently hold no positions in any of the companies mentioned in this report.
Underground Stock Reporter
Pfizer will purchase Arena Pharmaceuticals
Share price to be $108
http://sierraworldequityreview.blogspot.de/2012/07/undergrou…
..ich denke mal, etwas zum schmunzeln....

Monday, July 9, 2012
"Underground Stock Reporter" opinion on Arena Pharmaceuticals
Sierra World Equity Review is on track...just look at this from the Underground Stock Reporter as of July 9th 2012. Sierra World Equity Review currently hold no positions in any of the companies mentioned in this report.
Underground Stock Reporter
Pfizer will purchase Arena Pharmaceuticals
Share price to be $108

http://sierraworldequityreview.blogspot.de/2012/07/undergrou…
Sunday, July 8, 2012
Is Eisai Board of Directors to vote on purchasing Arena Pharmaceuticals leveraging their "standstill agreement"?
Sierra World Equity Review forecasts that Japanese drug maker giant Eisai board of directors will be voting shortly if they should leverage the "standstill" agreement they have with Arena Pharmaceuticals. In essence what the agreement that was signed back in July 2010 does, is that it prohibits Eisai from buying out Arena Pharmaceuticals UNLESS someone else attempts to FIRST! Now Sierra World Equity Review has little doubt that Arena Pharmaceuticals will be purchased, with sales of their newly FDA approved obesity weight loss drug, Belviq, projected to hit 2 BILLION DOLLARS by 2020, Arena Pharmaceuticals are THE hottest potential buyout in the biotech sector. Once the first offer is made form Pfizer (PFE), Merck (MRK) or anyone else it will trigger the "standstill" agreement and only add fuel to the bidding war that will develop.
Sierra World Equity Review believes that Eisai is sure to throw their name in the hat for Arena Pharmaceuticals. Please check back for continuing updates on Arena Pharmaceuticals, Eisai, Merck and Pfizer! We will continue to highlight these companies going forward and also examine any additional large pharmaceutical companies that may have an interest in buying out Arena Pharmaceuticals.
Sierra World Equity Review currently hold no positions in any of the companies mentioned in this report.
http://sierraworldequityreview.blogspot.de/2012/07/is-eisai-…
Is Eisai Board of Directors to vote on purchasing Arena Pharmaceuticals leveraging their "standstill agreement"?
Sierra World Equity Review forecasts that Japanese drug maker giant Eisai board of directors will be voting shortly if they should leverage the "standstill" agreement they have with Arena Pharmaceuticals. In essence what the agreement that was signed back in July 2010 does, is that it prohibits Eisai from buying out Arena Pharmaceuticals UNLESS someone else attempts to FIRST! Now Sierra World Equity Review has little doubt that Arena Pharmaceuticals will be purchased, with sales of their newly FDA approved obesity weight loss drug, Belviq, projected to hit 2 BILLION DOLLARS by 2020, Arena Pharmaceuticals are THE hottest potential buyout in the biotech sector. Once the first offer is made form Pfizer (PFE), Merck (MRK) or anyone else it will trigger the "standstill" agreement and only add fuel to the bidding war that will develop.
Sierra World Equity Review believes that Eisai is sure to throw their name in the hat for Arena Pharmaceuticals. Please check back for continuing updates on Arena Pharmaceuticals, Eisai, Merck and Pfizer! We will continue to highlight these companies going forward and also examine any additional large pharmaceutical companies that may have an interest in buying out Arena Pharmaceuticals.
Sierra World Equity Review currently hold no positions in any of the companies mentioned in this report.
http://sierraworldequityreview.blogspot.de/2012/07/is-eisai-…
Zitat von bernie55:BLOOMBERG > positive Presse über Perspektiven von ARENA und über mögliche Übernahme durch Big Pharmas...
@ AREANICS....it`s only a beginning ...
First Diet Pill in Decade Turns Arena Into Deal Bait: Real M&A
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…" target="_blank" rel="nofollow ugc noopener">
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…
Pre-Market
$ 11.44 > + (2.92%)
...und wir freuen uns auf die nächsten Tage und Wochen.
Straight up.
Straight up.
Zitat von dominator1000: ...und wir freuen uns auf die nächsten Tage und Wochen.
Straight up.
...zumal eine mögliche " Übernahmeschlacht" diverser BIG PLAYER durch den Artikel von Bloomberg meiner Meinung nach noch mehr "angeheizt" wurde und wir vielleicht schon kurzfristig das erste Übernahme-Angebot bekommen werden...

Antwort auf Beitrag Nr.: 43.365.957 von bernie55 am 09.07.12 13:38:29bei ARNA halte ich die Theorie einer möglichen Übernahme diesmal wirklich gegeben. Bei den meisten BIOs die ein Medikament an den Start bringen bin ich da nicht so optimistisch, auch wenn in den Foren dieses Thema direkt mit in den Topf geworfen wird.


Wenn ich nichts übersehe dann herrschte vor dem Übernahmeangebot bei HGSI z. B .auch Ruhe und es ging keine Übernahmephantasie durch die Medien zbw. Diskussionsboards...die Anhänger von Dendreon warten auch schon seit Wochen auf die Übernahme zu Phantasiekursen.
Als Insider mit entsprechendem Einfluß (hedge-Fonds-manager usw.) würde ich auch gezielt solche Sachen in Umaluf bringen um meine Bestände zu hohen Kursen zu veräußern.
Als Insider mit entsprechendem Einfluß (hedge-Fonds-manager usw.) würde ich auch gezielt solche Sachen in Umaluf bringen um meine Bestände zu hohen Kursen zu veräußern.

bei 8 usd kaufe ich nach
First Diet Pill in Decade Turns Arena Into Deal Bait: Real M&A
By Tara Lachapelle - 2012-07-09T14:08:12Z
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…
By Tara Lachapelle - 2012-07-09T14:08:12Z
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…
Jammer nicht !

wenn der schäuble heute abend alles richtig macht, dann steigen morgen die spanischen banken
geeignet für tagezocker
vincent der lange
larskohl
erthfire
fanziska
und auch andere gurkenhändler
geeignet für tagezocker
vincent der lange
larskohl
erthfire
fanziska
und auch andere gurkenhändler
Antwort auf Beitrag Nr.: 43.366.610 von gauner1 am 09.07.12 17:04:04dann hast Du wohl Deinen "maximalen Bestand" an ARENA bereits im Depot.


Antwort auf Beitrag Nr.: 43.366.777 von Poppholz am 09.07.12 17:47:41
ja stimmt. 35 st.
ja stimmt. 35 st.
Antwort auf Beitrag Nr.: 43.366.777 von Poppholz am 09.07.12 17:47:41jetzt im ernst
wer weiterhin für biotechaktien intressiert ist, dann soll er sich mal paion anschauen. wegen einer guten meldung bezüglich eines medikamentes ist der kurs heute 9% gestiegen. der kurs ist bis gestern an seinem tiefsten punkt angekommen.
die investierten warten auf die zulassung von remiazolam. wenn die zulassung kommt, dann gibt es ein verXXXXXfacher. zur zeit ist aber die aktie langweilig und braucht viel geduld. ist also was für das depot. ich habe heute 10k gleich nach der meldung ins depot gelegt
wer weiterhin für biotechaktien intressiert ist, dann soll er sich mal paion anschauen. wegen einer guten meldung bezüglich eines medikamentes ist der kurs heute 9% gestiegen. der kurs ist bis gestern an seinem tiefsten punkt angekommen.
die investierten warten auf die zulassung von remiazolam. wenn die zulassung kommt, dann gibt es ein verXXXXXfacher. zur zeit ist aber die aktie langweilig und braucht viel geduld. ist also was für das depot. ich habe heute 10k gleich nach der meldung ins depot gelegt
Antwort auf Beitrag Nr.: 43.366.935 von LarsKol am 09.07.12 18:31:49
wo kommst du gewesen ? wieder in hamburg ?
wo kommst du gewesen ? wieder in hamburg ?
Antwort auf Beitrag Nr.: 43.366.935 von LarsKol am 09.07.12 18:31:49
mit der pille wird heute nix, es sei, kurz vor dem schluß unsere gute freunde kommen und hochkaufen
mit der pille wird heute nix, es sei, kurz vor dem schluß unsere gute freunde kommen und hochkaufen
Richtig, am WE mal wieder in HH, schließlich war dort Schlagermove 2012



Alles nur fröhliche Menschen und niemand hat gejammert



http://www.youtube.com/watch?v=KFkZeVkzwqo



Alles nur fröhliche Menschen und niemand hat gejammert



http://www.youtube.com/watch?v=KFkZeVkzwqo
Antwort auf Beitrag Nr.: 43.366.935 von LarsKol am 09.07.12 18:31:49
hast du heute die schöne hellena gegucken ?
hast du heute die schöne hellena gegucken ?
ja, selbstverständlich, die Hellena wird auch ohne mich immer schöner - vielleicht auch gerade deswegen 

Antwort auf Beitrag Nr.: 43.367.023 von LarsKol am 09.07.12 19:05:54
wenn bei uns alles in ordnung ist bzw. wenn die sache beendet ist - du weiß, was ich meine, will ich auch mit meiner frau bischen reisen. als erstes denke ich ein mittelmeereise, mal sehen
wenn bei uns alles in ordnung ist bzw. wenn die sache beendet ist - du weiß, was ich meine, will ich auch mit meiner frau bischen reisen. als erstes denke ich ein mittelmeereise, mal sehen
Antwort auf Beitrag Nr.: 43.367.023 von LarsKol am 09.07.12 19:05:54die hellena, paion und arena lasse ich einfach laufen, ist mir völlig egal, auch wenn alle 3 100% stegen







Gauner, BM
und nenn mich nicht nochmal ko(H)l .... Du Sack!!!!
Mein Bluumenkohl geht keinen was an

Mein Bluumenkohl geht keinen was an

..hier mal wieder ein Kommentar zu den INSIDER Verkäufen der CEOs von VIVUS - so kurz vor der FDA Entscheidung....
Are Traders WORRIED About This Drug Stock?
By Eric McWhinnie
Vivus Inc. (NASDAQ:VVUS), a California-based bio-pharmaceutical company, has been on a tear this year. In just the past three months, shares have surged more than 30 percent. Recent drug approval developments in the industry have investors’ hearts pumping faster than ever, but insider-selling actions are casting doubt on the company’s upcoming approval decision.
In April, the Food and Drug Administration awarded regulatory approval for Vivus’ Stendra. The drug, known chemically as avanafil, is suppose to provide erections within 15 minutes, nearly half the time of Pfizer Inc.’s (NYSE:PFE) Viagra drug. “I do think it’s a differentiated product. Unlike competitors such as Eli Lilly & Co.’s (NYSE:LLY) Cialis, Vivus’ drug features rapid onset; rapid off,” explained Rodman & Renshaw LLC analyst Michael King, according to Bloomberg. Stendra is the only product on the market for Vivus and may bring in $68 million in sales next year. However, another FDA decision could soon add to those gains.
On July 17, Vivus awaits word from the FDA on its diet pill Qnexa. The approval decision was originally expected in April, but was delayed until next week. It was rejected by the agency in 2010, but received backing earlier this year by a panel of government advisers. If approved, it would become the second weight-loss pill to be approved by the FDA this year.
Last month, Arena Pharmaceutical (NASDAQ:ARNA) won approval for its Lorcaserin drug that affects an area of the brain that helps people eat less and feel full after eating only small amounts of food. It was the first obesity medication cleared for sale in the United States in 13 years.
that affects an area of the brain that helps people eat less and feel full after eating only small amounts of food. It was the first obesity medication cleared for sale in the United States in 13 years.
Although the FDA decision on Stendra is drawing near, Vivus shares closed slightly in the red on Monday. Concerns appear to be growing about insider transactions by three Vivus executives. TheStreet explains, “Insider sales ahead of major FDA drug approvals decisions should always trigger alarm bells. For Vivus, the worry is that insiders — CEO Leland Wilson, President Peter Tam and Chief Commercial Officer Michael Miller — are selling stock now because they know or suspect Qnexa isn’t going to be approved on July 17, as most investors expect it to be. This is the doomsday scenario being spread by shareholders of Arena, Vivus’ rival in the ever-expanding weight-loss pill stock bubble. What’s bad for Vivus is good for Arena, naturally.”
However, as TheStreet points out, these transactions have been occurring under trading plans that are suppose to provide legal cover from insider trading allegations. The insider selling has been going on for years now and is likely a poor indicator of how the FDA will rule next week. Furthermore, you would expect executives to book profits at some point.
Shares of Vivus have surged more than 300 percent over the past three years.
http://wallstcheatsheet.com/stocks/should-traders-be-worried…
Are Traders WORRIED About This Drug Stock?
By Eric McWhinnie
Vivus Inc. (NASDAQ:VVUS), a California-based bio-pharmaceutical company, has been on a tear this year. In just the past three months, shares have surged more than 30 percent. Recent drug approval developments in the industry have investors’ hearts pumping faster than ever, but insider-selling actions are casting doubt on the company’s upcoming approval decision.
In April, the Food and Drug Administration awarded regulatory approval for Vivus’ Stendra. The drug, known chemically as avanafil, is suppose to provide erections within 15 minutes, nearly half the time of Pfizer Inc.’s (NYSE:PFE) Viagra drug. “I do think it’s a differentiated product. Unlike competitors such as Eli Lilly & Co.’s (NYSE:LLY) Cialis, Vivus’ drug features rapid onset; rapid off,” explained Rodman & Renshaw LLC analyst Michael King, according to Bloomberg. Stendra is the only product on the market for Vivus and may bring in $68 million in sales next year. However, another FDA decision could soon add to those gains.
On July 17, Vivus awaits word from the FDA on its diet pill Qnexa. The approval decision was originally expected in April, but was delayed until next week. It was rejected by the agency in 2010, but received backing earlier this year by a panel of government advisers. If approved, it would become the second weight-loss pill to be approved by the FDA this year.
Last month, Arena Pharmaceutical (NASDAQ:ARNA) won approval for its Lorcaserin drug
 that affects an area of the brain that helps people eat less and feel full after eating only small amounts of food. It was the first obesity medication cleared for sale in the United States in 13 years.
that affects an area of the brain that helps people eat less and feel full after eating only small amounts of food. It was the first obesity medication cleared for sale in the United States in 13 years.Although the FDA decision on Stendra is drawing near, Vivus shares closed slightly in the red on Monday. Concerns appear to be growing about insider transactions by three Vivus executives. TheStreet explains, “Insider sales ahead of major FDA drug approvals decisions should always trigger alarm bells. For Vivus, the worry is that insiders — CEO Leland Wilson, President Peter Tam and Chief Commercial Officer Michael Miller — are selling stock now because they know or suspect Qnexa isn’t going to be approved on July 17, as most investors expect it to be. This is the doomsday scenario being spread by shareholders of Arena, Vivus’ rival in the ever-expanding weight-loss pill stock bubble. What’s bad for Vivus is good for Arena, naturally.”
However, as TheStreet points out, these transactions have been occurring under trading plans that are suppose to provide legal cover from insider trading allegations. The insider selling has been going on for years now and is likely a poor indicator of how the FDA will rule next week. Furthermore, you would expect executives to book profits at some point.
Shares of Vivus have surged more than 300 percent over the past three years.
http://wallstcheatsheet.com/stocks/should-traders-be-worried…
Zitat von franzisc am 09.07.12 - 17:17:00 : First Diet Pill in Decade Turns Arena Into Deal Bait: Real M&A
By Tara Lachapelle - 2012-07-09T14:08:12Z
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…
Zitat von bernie55 am 09.07.12 - 08:26:44:...BLOOMBERG > positive Presse über Perspektiven von ARENA und über mögliche Übernahme durch Big Pharmas...
@ AREANICS....it`s only a beginning ...
First Diet Pill in Decade Turns Arena Into Deal Bait: Real M&A
The first medication in more than a decade to help 78 million obese Americans slim down is turning into takeover bait.
Arena Pharmaceuticals Inc. (ARNA) won Food and Drug Administration approval last month for Belviq, a treatment affecting an area of the brain that helps a person feel full after consuming less food. With the first weight-loss drug to be cleared for sale in the U.S. in 13 years, Arena is projected by analysts to increase revenue 18-fold in the next four years, the fastest growth in the world among specialty pharmaceutical companies greater than $1 billion, according to data compiled by Bloomberg.
Enlarge image First Diet Pill in Decade Turns Arena Into Deal Bait
About 42 percent of the U.S. population may be obese by 2030, up from about a third currently. Photographer: David Paul Morris/Bloomberg
While Arena’s 494 percent stock gain this year makes it the most expensive U.S. specialty drugmaker relative to revenue, the San Diego-based company offers potential buyers a medication that Piper Jaffray Cos. estimates will reach $2 billion in annual sales. Arena, with a $2.2 billion market value, may draw takeover interest from GlaxoSmithKline Plc (GSK), which is divesting its diet pill Alli, said WBB Securities LLC. Tokyo-based Eisai Co. (4523), which has licensed the rights to sell Belviq in the U.S., could also be an acquirer, said Lazard Capital Markets LLC.
“The large pharmaceutical companies are all on the edge of their seats looking at Arena,” Stephen Brozak, president of WBB Securities in Clark, New Jersey, said in a telephone interview. Obesity “is a global pandemic. There are people that absolutely are in need of these types of products. Large pharma are wonderful at marketing drugs and this is a product that lends itself to marketing.”
Appetite Suppression
“Arena is focused on bringing Belviq to the market in the U.S. with Eisai and in obtaining approval of the drug in markets outside of the U.S.,” David Schull, a spokesman for Arena, said in an e-mailed statement when asked whether the company has been approached by potential acquirers about a deal.
The FDA approved Belviq, previously called lorcaserin, on June 27. The pill works in a similar way to fenfluramine, part of the fen-phen appetite-suppression drug combination pulled from pharmacies 15 years ago when it was linked to heart valve abnormalities.
Arena and Eisai agreed to conduct six post-market studies to assess the safety and efficacy of the drug, including determining the potential for major cardiac risks such as heart attack and stroke, the FDA said.
Belviq will be available after the Drug Enforcement Administration completes a review to classify the drug based on its potential for abuse, which Arena has said may take four to six months.
Obesity Rate
“It’s the single most important thing that’s happened to Arena in its entire history,” WBB Securities’ Brozak said. “This is the first approval they’ve ever gotten.”
Analysts project that by 2015 Arena’s sales will have risen faster than every other specialty drugmaker in the world with a market capitalization greater than $1 billion, data compiled by Bloomberg show. The company’s revenue may total $232 million that year, versus $13 million in 2011, according to analysts’ estimates compiled by Bloomberg.
About 42 percent of the U.S. population may be obese by 2030, up from about a third currently, according to a report presented at the Centers for Disease Control and Prevention’s obesity conference in May. Globally, about 500 million people are obese, according to a World Health Organization report. Obesity is defined as having a body mass index of more than 30.
Glaxo, the U.K.’s largest drugmaker, may be interested in purchasing Arena to bolster its presence in the market for weight-loss remedies after sales declined for Alli, its over- the-counter diet pill, according to WBB Securities’ Brozak.
‘Dress Rehearsal’
London-based Glaxo has said it plans to divest Alli, which contains orlistat, a chemical that blocks the intestines from absorbing fat when taken as many as three times a day with meals. Orlistat has been linked to reports of liver injury, prompting consumer advocacy groups to demand its removal from the market. The FDA announced new warnings on the pill’s label in mid-2010, and Glaxo has said that Alli is safe and effective when used as directed.
Glaxo has “done the dress rehearsal,” so it would now know how to best market Arena’s weight-loss drug, Brozak said.
Kevin Colgan, a spokesman for Glaxo, said the company doesn’t comment on speculation, when asked whether it wants to acquire Arena.
Other companies are also seeking approval for weight-loss drugs. Qnexa from Vivus Inc. (VVUS) is slated for an FDA decision by July 17. Orexigen Therapeutics Inc. (OREX), which is developing the pill Contrave with Takeda Pharmaceutical Co., agreed in September to conduct a two-year study of the drug’s heart risks.
Drug Partnership
Belviq is the first prescription obesity medicine to be approved for sale in the U.S. since Roche Holding AG (ROG)’s Xenical in 1999. Glaxo’s Alli is a half-dose version of Xenical’s active ingredient and received FDA clearance in 2007 as the first diet drug available without a prescription.
Once available, lorcaserin will be marketed in the U.S. by Eisai under the name Belviq and the company will pay Arena a portion of the drug’s revenue. The partnership may deter potential acquirers that don’t want to share Belviq’s sales, said Alan Carr, a New York-based analyst for Needham & Co.
“Arena is much more attractive from an M&A perspective if you’re getting worldwide rights,” he said in a phone interview.
Eisai may decide to buy Arena so that it collects all of the revenue from Belviq, said Bill Tanner, an analyst for Lazard in New York. The two companies have a so-called standstill agreement that prevents the Japanese drugmaker from purchasing Arena unless someone else tries to first. It’s intended to protect Arena from a hostile takeover and wouldn’t prevent the companies from negotiating a deal, Tanner said.
Eisai’s Interest
“If this is a big drug, it could be a fairly sizeable payment that they have to make to Arena,” he said in a phone interview. “It probably makes sense that they’d want to take a look at acquiring it.”
Marcia Diljak, a spokeswoman for Eisai, declined to comment on whether the company would be interested in buying Arena.
Eisai will probably want to first gauge Belviq’s commercial success so that it doesn’t risk overpaying for Arena in case sales are weaker than analysts expect, Tanner said.
Arena, which has posted an almost six-fold stock price increase this year, is valued at 52 times analysts’ estimates for fiscal 2012 sales. That’s higher than all 14 other specialty pharmaceutical companies in the U.S. that have a market capitalization greater than $1 billion, data compiled by Bloomberg show. Even based on revenue estimates for fiscal 2015, Arena trades at the second-richest multiple in the group.
“If you knew for a fact that there wasn’t a buyer for the company, I don’t think this stock would be where it is,” Lazard’s Tanner said. “It’s not overly expensive if you think it’s going to be a decent drug.”
Edward Tenthoff, a New York-based analyst for Piper Jaffray, estimates Belviq will reach annual sales of $2 billion just in the U.S. by 2020.
“The approval of a drug with the market potential of Belviq certainly makes Arena” an acquisition candidate, Tenthoff said in a phone interview.
“This could be a true blockbuster drug in an age when there are fewer and fewer blockbusters.”
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…" target="_blank" rel="nofollow ugc noopener">
http://www.bloomberg.com/news/2012-07-08/first-diet-pill-in-…
Antwort auf Beitrag Nr.: 43.368.259 von bernie55 am 10.07.12 08:13:24den Verkauf durch den CEO finde ich auch "unüberlegt".
Wenn die Zulassung kommt verschenkt er richtig Geld.
Wenn die Zulassung nicht kommt, hat er eigentlich erst richtig Probleme, da Ihm dann Insiderwissen vorgeworfen wird.

Wenn die Zulassung kommt verschenkt er richtig Geld.
Wenn die Zulassung nicht kommt, hat er eigentlich erst richtig Probleme, da Ihm dann Insiderwissen vorgeworfen wird.

Zitat von Poppholz: den Verkauf durch den CEO finde ich auch "unüberlegt".
Wenn die Zulassung kommt verschenkt er richtig Geld.
Wenn die Zulassung nicht kommt, hat er eigentlich erst richtig Probleme, da Ihm dann Insiderwissen vorgeworfen wird.
Ich weiß gar nicht mehr was ich aktuell darüber denken soll...
...sogar ein ADAM FUCKELSTONE hat sich diesbezüglich schon zu Wort gemeldet...
Alles, was zur Zeit läuft, ist an Spannung nicht mehr zu überbieten...( na ja mit ELAN und DENDREON haben wir ja da auch unsere Erfahrungen gemacht
 )
)Die FDA muss nun eine Entscheidung treffen...
..falls ein Approval für Qnexa ausgesprochen wird,dann werden mit diesem Approval " harte" Einschränkungen verbunden sein...
...ich bin jetzt zwar immer von solch einer Approval-Entscheidung ausgegangen...... aber durch die Zula von BELVIQ hat sich meiner Meinung nach die Ausgangssituation bei Approval-Voraussetzungen bei " Obesity-Drugs" radikal verändert hat...
....die "Safety-Kriterien" von BELVIQ sind jetzt das Maß aller Dinge und werden in weiteren Approval-Entscheidungsprozessen bei " Obesity-Drugs" als " Safety-Standard" miteinbezogen werden....
....und das macht die aktuelle Situation bzgl. Approvals so " unkalkulierbar"....
Ich bin auf jeden Fall froh, dass ARENA das Approval geschafft hat und wir als Aktionäre an der Zukunft dieses Unternehmnens teilhaben werden.
Grüße
bernie55

fanzisca
ich vermisse dich bei meiner alten gurke evotec. lies die heutige nachricht
ich vermisse dich bei meiner alten gurke evotec. lies die heutige nachricht
Antwort auf Beitrag Nr.: 43.369.229 von gauner1 am 10.07.12 12:19:54Muss mich um ein anderes Gemüse kümmern!.....bin Gestern schön auf die Schnauze gefallen!

Antwort auf Beitrag Nr.: 43.369.257 von franzisca am 10.07.12 12:27:28
tut mir leid. was war das. gib bitte per bm
tut mir leid. was war das. gib bitte per bm
Antwort auf Beitrag Nr.: 43.369.388 von gauner1 am 10.07.12 12:56:10Ist kein Geheimniss......PCX! 

Antwort auf Beitrag Nr.: 43.369.434 von franzisca am 10.07.12 13:05:19
verstehe nicht, der kurs ist doch gestern fast nicht gefallen. heute nur 5 cent
kurs 25,47 stutt
verstehe nicht, der kurs ist doch gestern fast nicht gefallen. heute nur 5 cent
kurs 25,47 stutt
Antwort auf Beitrag Nr.: 43.369.527 von gauner1 am 10.07.12 13:29:05?
die haben Insolvenz angemeldet
Kurs FFM €0,34

die haben Insolvenz angemeldet
Kurs FFM €0,34

ich habe gefunden. das it patriot coal
es gibt noch ein pcx
tut mit leid franziska. sicher holst du wo anders den verlust raus
es gibt noch ein pcx
tut mit leid franziska. sicher holst du wo anders den verlust raus
Arena: Why You Should Wait For A Pullback
July 10, 2012 |
Located in San Diego, CA, Arena Pharmaceuticals (ARNA) is a biopharmaceutical company which discovers, develops and commercializes oral drugs that target G protein-coupled receptors. The company mainly focuses on developing drugs for obesity and cardiovascular diseases. The firm's lead product, Lorcaserin, was approved by FDA on June 27th. The drug was approved as an accessory to a low-calorie regime and increased physical activity for chronic weight management. This is the first weight-loss drug that has been approved in the last 13 years. It selectively targets receptors involved in appetite control. Lorcaserin will be marketed under the name Belviq. A research showed that after completing the trial and taking the now-licensed pills regularly, nearly two-thirds of patients lost 5% of their body weight at least compared to one-third for those taking the placebo. Moreover, more than one-third lost 10% of their body weight at least, compared to 13.8% for those on the placebo.
Arena is working in partnership with the Japanese pharmaceutical giant, Eisai Inc. (ESALF.PK) for Lorcaserin.
Arena Pharmaceuticals announced the promotion of Craig Audet to the position of Senior Vice President, Operations and Head of Global Regulatory Affairs on July 5th. Audet will serve as an executive officer of the company in his new role. Audet has been working with the company since 2011 as vice president, regulatory affairs. It is believed that Audet played a crucial role in getting Lorcaserin approved by FDA. Therefore, this promotion should be an admission of Audet's key role in getting Lorcaserin approved. Audet has the experience and track record of good regulatory strategies and submission. Arena still has to get Lorcaserin approved for Europe and other territories. European approval is not expected before the mid of 2013.
Stock Performance
Arena has a market cap of a little over $2 billion. As stated above, the company got approval for Lorcaserin on June, 27. With the approval stock price soared and it went from $8.73 to above $13, in the end settling at $11.35 at the closing. This was a huge gain for the stock. 52 week low-high limits for Arena are $1.23-13.50. Stocks were not alone in this attention from investors. Option contracts for Arena also created a lot of interest. There was an increase of 35% in options traded in the drug market, totaling to 332.000 options. Arena was able to topple Apple (AAPL) and claim the top equity option traded for the day, the first company to do so in the last three months. The stock closed at $11.35 on Thursday, after gaining 13.27%. The volume of stocks was 42.21 million for the day, well over 40% more than the usual volume. Overall, the stock has increased dramatically within the last twelve months. Year-to date, Arena is up by roughly 700%. With a Beta value of 0.23, Arena is one of the least volatile among its peers. However, this sudden skyrocketing has dragged the stock near to the overbought territory. Arena is up by more than 25% in just eleven days. With a Relative Strength Index of 65%, you really need to give the stock a breather.
ARNA Chart
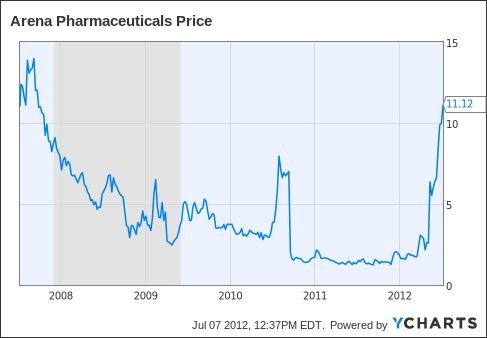
Financial Performance
Arena had total revenues of $2.1 million last year. According to analyst estimates, the revenues for Arena are expected to reach $550 million in U.S. by 2017, while worldwide sales are expected to reach $1.6 billion by 2020. Eisai Inc. has mercantile rights in both South and North America, which annihilates a considerable amount of potential revenue for Arena. Under the agreement with Eisai, Arena will produce the drug in its own plants and sell to Eisai. This enables a part of sales revenues from the Americas to go into Arena's pouch. For Europe and Asia, Arena will hold exclusive rights to market the product which will contribute towards improved revenue numbers. The obesity market is largely unexplored, which leaves Arena's future widely up to speculation.
ARNA Revenues Chart
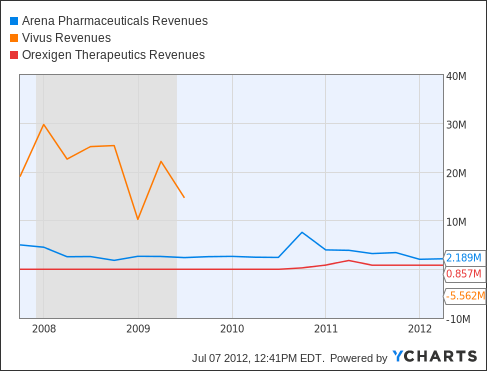
Competitor Analysis
Arena's direct competitors are Vivus (VVUS) and Orexigen (OREX). Vivus has also presented its own weight reduction drug Qnexa, in front of FDA for approval. A decision on the said drug is expected on July 17th. Lorcaserin and Qnexa both received positive votes by the FDA's Endocrinologic and Metabolic Drugs Advisory Committee in May 2012. If Qnexa is also approved then, Arena will have a direct competition to Lorcaserin.
Along with its benefits, Qnexa has some side effects as well. Especially with pregnant women, as a result, the company will put a plan in place to try and make sure that pregnant women do not take the drug. Vivus also plan on adding a label advising people to stop using the drug, should they lose less than 3% of their body weight in three months. There has been a negative sign at Vivus; Insider trading volumes were very high in the last month. Sale of the shares by insiders as compared to the exercise of option to buy was significantly higher.
Summary
The approval of Lorcaserin has opened the obesity market again, which was hugely untapped before. Lorcaserin is the focus of Arena and will contribute a great deal towards the future of the company. Arena is expected to receive approval of its MAA (marketing authorization application) by the European Medicines Agency and grow its entity in Europe. It is also expected that Arena will soon try to get the drug approved for other territories. According to my view, the stock may experience some volatility in the short term, but it should gain in the medium and long term. I think the Arena stock should be bought after a pullback, as all the indications point towards an increase in the stock price in the long term.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/710811-arena-why-you-should-…
July 10, 2012 |
Located in San Diego, CA, Arena Pharmaceuticals (ARNA) is a biopharmaceutical company which discovers, develops and commercializes oral drugs that target G protein-coupled receptors. The company mainly focuses on developing drugs for obesity and cardiovascular diseases. The firm's lead product, Lorcaserin, was approved by FDA on June 27th. The drug was approved as an accessory to a low-calorie regime and increased physical activity for chronic weight management. This is the first weight-loss drug that has been approved in the last 13 years. It selectively targets receptors involved in appetite control. Lorcaserin will be marketed under the name Belviq. A research showed that after completing the trial and taking the now-licensed pills regularly, nearly two-thirds of patients lost 5% of their body weight at least compared to one-third for those taking the placebo. Moreover, more than one-third lost 10% of their body weight at least, compared to 13.8% for those on the placebo.
Arena is working in partnership with the Japanese pharmaceutical giant, Eisai Inc. (ESALF.PK) for Lorcaserin.
Arena Pharmaceuticals announced the promotion of Craig Audet to the position of Senior Vice President, Operations and Head of Global Regulatory Affairs on July 5th. Audet will serve as an executive officer of the company in his new role. Audet has been working with the company since 2011 as vice president, regulatory affairs. It is believed that Audet played a crucial role in getting Lorcaserin approved by FDA. Therefore, this promotion should be an admission of Audet's key role in getting Lorcaserin approved. Audet has the experience and track record of good regulatory strategies and submission. Arena still has to get Lorcaserin approved for Europe and other territories. European approval is not expected before the mid of 2013.
Stock Performance
Arena has a market cap of a little over $2 billion. As stated above, the company got approval for Lorcaserin on June, 27. With the approval stock price soared and it went from $8.73 to above $13, in the end settling at $11.35 at the closing. This was a huge gain for the stock. 52 week low-high limits for Arena are $1.23-13.50. Stocks were not alone in this attention from investors. Option contracts for Arena also created a lot of interest. There was an increase of 35% in options traded in the drug market, totaling to 332.000 options. Arena was able to topple Apple (AAPL) and claim the top equity option traded for the day, the first company to do so in the last three months. The stock closed at $11.35 on Thursday, after gaining 13.27%. The volume of stocks was 42.21 million for the day, well over 40% more than the usual volume. Overall, the stock has increased dramatically within the last twelve months. Year-to date, Arena is up by roughly 700%. With a Beta value of 0.23, Arena is one of the least volatile among its peers. However, this sudden skyrocketing has dragged the stock near to the overbought territory. Arena is up by more than 25% in just eleven days. With a Relative Strength Index of 65%, you really need to give the stock a breather.
ARNA Chart

Financial Performance
Arena had total revenues of $2.1 million last year. According to analyst estimates, the revenues for Arena are expected to reach $550 million in U.S. by 2017, while worldwide sales are expected to reach $1.6 billion by 2020. Eisai Inc. has mercantile rights in both South and North America, which annihilates a considerable amount of potential revenue for Arena. Under the agreement with Eisai, Arena will produce the drug in its own plants and sell to Eisai. This enables a part of sales revenues from the Americas to go into Arena's pouch. For Europe and Asia, Arena will hold exclusive rights to market the product which will contribute towards improved revenue numbers. The obesity market is largely unexplored, which leaves Arena's future widely up to speculation.
ARNA Revenues Chart

Competitor Analysis
Arena's direct competitors are Vivus (VVUS) and Orexigen (OREX). Vivus has also presented its own weight reduction drug Qnexa, in front of FDA for approval. A decision on the said drug is expected on July 17th. Lorcaserin and Qnexa both received positive votes by the FDA's Endocrinologic and Metabolic Drugs Advisory Committee in May 2012. If Qnexa is also approved then, Arena will have a direct competition to Lorcaserin.
Along with its benefits, Qnexa has some side effects as well. Especially with pregnant women, as a result, the company will put a plan in place to try and make sure that pregnant women do not take the drug. Vivus also plan on adding a label advising people to stop using the drug, should they lose less than 3% of their body weight in three months. There has been a negative sign at Vivus; Insider trading volumes were very high in the last month. Sale of the shares by insiders as compared to the exercise of option to buy was significantly higher.
Summary
The approval of Lorcaserin has opened the obesity market again, which was hugely untapped before. Lorcaserin is the focus of Arena and will contribute a great deal towards the future of the company. Arena is expected to receive approval of its MAA (marketing authorization application) by the European Medicines Agency and grow its entity in Europe. It is also expected that Arena will soon try to get the drug approved for other territories. According to my view, the stock may experience some volatility in the short term, but it should gain in the medium and long term. I think the Arena stock should be bought after a pullback, as all the indications point towards an increase in the stock price in the long term.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/710811-arena-why-you-should-…
Insiders Sold These Stocks on Jul-9
Arena Pharmaceuticals (NASDAQ:ARNA): Hoffman Robert (SVP, Finance and CFO) sold 10,000 shares at a price of $11.00.
Arena Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company committed to discovering and developing innovative therapies offering medical advances and new options for patients.
http://fyxnews.com/smw/30437/Insiders-Sold-These-Stocks-on-J…
Arena Pharmaceuticals (NASDAQ:ARNA): Hoffman Robert (SVP, Finance and CFO) sold 10,000 shares at a price of $11.00.
Arena Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company committed to discovering and developing innovative therapies offering medical advances and new options for patients.
http://fyxnews.com/smw/30437/Insiders-Sold-These-Stocks-on-J…
Notable Mid-Cap Insider Sells On July 9
July 10, 2012
Arena Pharmaceuticals, Inc (ARNA): Arena Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company, engages in discovering, developing, and commercializing oral drugs that target G protein-coupled receptors in the therapeutic areas of cardiovascular, central nervous system, inflammatory, and metabolic diseases. Arena Pharmaceuticals has a market cap of $2.09 billion and is currently trading around $11.46 with a 52-week range of $1.23 to $13.50.The stock has gained 512.83% year to date.
According to SEC filings on July 09, 2012, Hoffman Robert (SVP, Finance and CFO) sold a total of 10,000 shares at a total value of $110,000. Over the past three months, there have been a total of 3 insider transactions with 3 Sell transactions. On June 27, 2012, Arena Pharmaceuticals and Eisai Inc. announced that the US FDA has approved BELVIQ (weight loss drug). Arena Pharmaceuticals has still more potential to grow based on EMA approval and potential revenue generation from BELVIQ. For the anti-obesity drugs, Arena is competing with Orexigen Therapeutics' (OREX) Contrave drug and Vivus Inc.'s (VVUS) Qnexa drug. The key financial metrics for Arena Pharmaceuticals are given below:
The following chart provides the insider trading summary for three-month and 12-month periods.
http://seekingalpha.com/article/710601-notable-mid-cap-insid…
July 10, 2012
Arena Pharmaceuticals, Inc (ARNA): Arena Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company, engages in discovering, developing, and commercializing oral drugs that target G protein-coupled receptors in the therapeutic areas of cardiovascular, central nervous system, inflammatory, and metabolic diseases. Arena Pharmaceuticals has a market cap of $2.09 billion and is currently trading around $11.46 with a 52-week range of $1.23 to $13.50.The stock has gained 512.83% year to date.
According to SEC filings on July 09, 2012, Hoffman Robert (SVP, Finance and CFO) sold a total of 10,000 shares at a total value of $110,000. Over the past three months, there have been a total of 3 insider transactions with 3 Sell transactions. On June 27, 2012, Arena Pharmaceuticals and Eisai Inc. announced that the US FDA has approved BELVIQ (weight loss drug). Arena Pharmaceuticals has still more potential to grow based on EMA approval and potential revenue generation from BELVIQ. For the anti-obesity drugs, Arena is competing with Orexigen Therapeutics' (OREX) Contrave drug and Vivus Inc.'s (VVUS) Qnexa drug. The key financial metrics for Arena Pharmaceuticals are given below:

The following chart provides the insider trading summary for three-month and 12-month periods.

http://seekingalpha.com/article/710601-notable-mid-cap-insid…
Antwort auf Beitrag Nr.: 43.369.956 von Poppholz am 10.07.12 15:16:11ich finde ja klasse, wie die "Marktbeobachter" uns davor warnen wollen, diese Aktie zu halten.
Wenn der CFO schon (gewaltige) 10.000 Aktien verkauft, dann muss die Aktie ja demnächst fallen.

Wenn der CFO schon (gewaltige) 10.000 Aktien verkauft, dann muss die Aktie ja demnächst fallen.

Antwort auf Beitrag Nr.: 43.369.973 von Poppholz am 10.07.12 15:19:53ich habe mir noch welche gekauft.
Bei so vielen Bashern kann der Kurs nur steigen.

Bei so vielen Bashern kann der Kurs nur steigen.

Zitat von Poppholz: Bei so vielen Bashern kann der Kurs nur steigen.
AUS DEM YAHOO-BOARD
< 2 minutes ago >
ANALYST EXPECTS OFFERING PRICE OF $9

sell and run




http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
wir begrüssen unsere guten und lieben freunde
die sollen hochkaufen, bis sie verrecken
die sollen hochkaufen, bis sie verrecken
gleich kommt der lars mit seinem affen
los lars
los lars
auch wenn wir gleich bei 12 sind, ist mir egal. für mich ist jetzt gurkenzeit. die sind wichtiger als euere arena
bis nachher
bis nachher

BMTI auch interessant!
Antwort auf Beitrag Nr.: 43.370.520 von franzisca am 10.07.12 17:04:05
habe ich februar und märz damit gearbeitet. hatte kein besonderer erfolg, seit april geht es wieder
habe ich februar und märz damit gearbeitet. hatte kein besonderer erfolg, seit april geht es wieder
morgen
die gewinne von gestern sind nachbörslich verschwunden. der kurs ist sogar etwas in minus
hat man die zulassung entzogen ?

die gewinne von gestern sind nachbörslich verschwunden. der kurs ist sogar etwas in minus
hat man die zulassung entzogen ?


Why Hedge Funds Prefer Vivus Over Arena
July 10, 2012
By Jeffrey Shek
With all the over-the-counter drugs (OTC) for fat loss, it comes as a surprise (for those not in the industry) that it's been 13 long years since the last weight loss drug was approved by FDA (orlistat in 1999, marketed as Alli/Xenical by GlaxoSmithKline Plc (GSK)). America has a weight issue, and the statistics are scary. We have one of the highest obesity ratings in the world, and it isn't getting any better.
Most fat-loss pills don't work. The ones that work often have side-effects. Some of them might work for you, but fail to work for your friends. Doctors are generally unwilling to recommend an OTC pill for weight-loss, instead advocating a nutritional diet and exercise (which is probably the better option of the two). This leaves most of us jaded of the weight-loss industry as a set of fad diets and fake placebo pills.
Enter the recent FDA approval of lorcaserin, a weight loss pill manufactured by Arena Pharmaceuticals (ARNA). Belviq (lorcaserin) was FDA approved on June 27th and hit a 52W high shortly after. The New Drug Application (a process for all new drugs submitted to the FDA) was started in 2009, hit some hurdles in 2010 (where the FDA rejected the application on concerns of safety), and was resubmitted in 2012 with additional restrictions such as use only for certain BMIs (30+) or discretionary health issues. ARNA's stock began a notable uptick back in March, which became more pronounced in May (rising from a mid 2 dollar range to a price level of 6 dollars).
Vivus Inc. (VVUS), a competitor of Arena with a market cap of 2.84bn, is currently seeking FDA approval for Qnexa, a weight-loss pill combined with phentermine and topiramate. It's important to note that both phentermine and topiramate have been approved independently, yet VIVUS is combining the usage of the two in an extended-release capsule for a specific weight-loss treatment. In the past couple of days at Vivus, we've seen a lot of insider activity.
Many of Vivus' insiders have recently exercised their call options and have proceeded to sell the exercised shares. However, don't take this as negative news, as FDA approvals are never a "sure-thing". Instead, insiders are probably looking to lock-in the rise in Vivus' stock price (it's surged 287% since January). Mark your calendars as the FDA's decision to approve or reject Qnexa comes out on July 17th.
One of our observations has been that hedge funds were more open to purchasing VVUS over ARNA during the first quarter in 2012 (see the list of hedge funds here).
Here are some of likely reasons:
1) The FDA decision on Qnexa was supposed to be April 17th; they later pushed the decision to July 17th. Funds such as QVT Financial had probably loaded up on some of these positions expecting the decision in April. In contrast, FDA's decision on Arena's Belviq was expected to be on June 27th.
2) Qnexa was a combination and smaller dosages of two already independently approved drugs (phentermine and topiramate), in contrast to Arena's Belviq (lorcaserin) which was completely new. New drugs can be harder to approve as the potential side effects are less known.
3) FDA's Advisory Committee had given support to Qnexa on February 22nd (Note: This is not a binding decision, only an advisory recommendation to the FDA). FDA's advisory committee on Arena's Belviq did not vote to recommend until May 10th.
Arena's Belviq did get the FDA approval first, has the head-start on market share, and has a strong distributor and marketing relationship with Eisai. Yet, this isn't as significant (as it may appear) if Vivus receives FDA approval.
The weight loss market is largely an untapped growing market. Doctors are not used to prescribing weight-loss pills, nor are patients aware of Belviq and Qnexa. Even if Belviq does have a head-start, assuming Qnexa receives FDA approval, these two blockbuster drugs could very well drive the bottom line for years as doctors and patients become familiar with Belviq and Qnexa. It's a huge untapped industry that can support multiple players. While Vivus is still risky, there's a lot of investor sentiment that Qnexa will obtain FDA approval (some of this is already priced in its current stock price). If Vivus doesn't get the FDA approval, expect Vivus to tank and Arena to surge.
Orexigen Therapeutics Inc. (OREX) is another pharmaceutical to keep interested in the next couple of months. While approval for Orexigen's Contrave, another weight loss drug, has about another year before a final FDA decision to approve or reject based upon the Light Study (Contrave was rejected back in 2011, this study aims to refute some of FDA's concerns), expect to see investor sentiment trying to make some plays leading up to the decision. A review of our hedge fund holdings show that there were only a few funds purchasing Orexigen during Q1 2012. This will probably change over the next couple of months. Observations of Arena and Vivus's respective stocks showed a surge in investor interest and stock price a few months before the FDA Decision was announced.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/713171-why-hedge-funds-prefe…
July 10, 2012
By Jeffrey Shek
With all the over-the-counter drugs (OTC) for fat loss, it comes as a surprise (for those not in the industry) that it's been 13 long years since the last weight loss drug was approved by FDA (orlistat in 1999, marketed as Alli/Xenical by GlaxoSmithKline Plc (GSK)). America has a weight issue, and the statistics are scary. We have one of the highest obesity ratings in the world, and it isn't getting any better.
Most fat-loss pills don't work. The ones that work often have side-effects. Some of them might work for you, but fail to work for your friends. Doctors are generally unwilling to recommend an OTC pill for weight-loss, instead advocating a nutritional diet and exercise (which is probably the better option of the two). This leaves most of us jaded of the weight-loss industry as a set of fad diets and fake placebo pills.
Enter the recent FDA approval of lorcaserin, a weight loss pill manufactured by Arena Pharmaceuticals (ARNA). Belviq (lorcaserin) was FDA approved on June 27th and hit a 52W high shortly after. The New Drug Application (a process for all new drugs submitted to the FDA) was started in 2009, hit some hurdles in 2010 (where the FDA rejected the application on concerns of safety), and was resubmitted in 2012 with additional restrictions such as use only for certain BMIs (30+) or discretionary health issues. ARNA's stock began a notable uptick back in March, which became more pronounced in May (rising from a mid 2 dollar range to a price level of 6 dollars).
Vivus Inc. (VVUS), a competitor of Arena with a market cap of 2.84bn, is currently seeking FDA approval for Qnexa, a weight-loss pill combined with phentermine and topiramate. It's important to note that both phentermine and topiramate have been approved independently, yet VIVUS is combining the usage of the two in an extended-release capsule for a specific weight-loss treatment. In the past couple of days at Vivus, we've seen a lot of insider activity.
Many of Vivus' insiders have recently exercised their call options and have proceeded to sell the exercised shares. However, don't take this as negative news, as FDA approvals are never a "sure-thing". Instead, insiders are probably looking to lock-in the rise in Vivus' stock price (it's surged 287% since January). Mark your calendars as the FDA's decision to approve or reject Qnexa comes out on July 17th.
One of our observations has been that hedge funds were more open to purchasing VVUS over ARNA during the first quarter in 2012 (see the list of hedge funds here).
Here are some of likely reasons:
1) The FDA decision on Qnexa was supposed to be April 17th; they later pushed the decision to July 17th. Funds such as QVT Financial had probably loaded up on some of these positions expecting the decision in April. In contrast, FDA's decision on Arena's Belviq was expected to be on June 27th.
2) Qnexa was a combination and smaller dosages of two already independently approved drugs (phentermine and topiramate), in contrast to Arena's Belviq (lorcaserin) which was completely new. New drugs can be harder to approve as the potential side effects are less known.
3) FDA's Advisory Committee had given support to Qnexa on February 22nd (Note: This is not a binding decision, only an advisory recommendation to the FDA). FDA's advisory committee on Arena's Belviq did not vote to recommend until May 10th.
Arena's Belviq did get the FDA approval first, has the head-start on market share, and has a strong distributor and marketing relationship with Eisai. Yet, this isn't as significant (as it may appear) if Vivus receives FDA approval.
The weight loss market is largely an untapped growing market. Doctors are not used to prescribing weight-loss pills, nor are patients aware of Belviq and Qnexa. Even if Belviq does have a head-start, assuming Qnexa receives FDA approval, these two blockbuster drugs could very well drive the bottom line for years as doctors and patients become familiar with Belviq and Qnexa. It's a huge untapped industry that can support multiple players. While Vivus is still risky, there's a lot of investor sentiment that Qnexa will obtain FDA approval (some of this is already priced in its current stock price). If Vivus doesn't get the FDA approval, expect Vivus to tank and Arena to surge.
Orexigen Therapeutics Inc. (OREX) is another pharmaceutical to keep interested in the next couple of months. While approval for Orexigen's Contrave, another weight loss drug, has about another year before a final FDA decision to approve or reject based upon the Light Study (Contrave was rejected back in 2011, this study aims to refute some of FDA's concerns), expect to see investor sentiment trying to make some plays leading up to the decision. A review of our hedge fund holdings show that there were only a few funds purchasing Orexigen during Q1 2012. This will probably change over the next couple of months. Observations of Arena and Vivus's respective stocks showed a surge in investor interest and stock price a few months before the FDA Decision was announced.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/713171-why-hedge-funds-prefe…
so gut ist mein deutsch nun auch wieder nicht, das ich das verstehen könnte
lars
einige investierte sind engländer, vielleicht auch schottem. die können alle kein deutsch
einige investierte sind engländer, vielleicht auch schottem. die können alle kein deutsch
und vor 200 jahren ist ein teil der schotten nach schwabenland ausgewandert und zuletzt sind sie schwaben geworden
und nimm rücksicht auf den langen vincent und schreib nicht so viel müll
kann jemand ein kurzer kommentar geben ? nicht alle können wirtschaftsenglisch
Heute gehts richtig down. Scheiß Presse.
Überlege mal rauszugehen und später wieder rein, ist mir zu risikobehaftet. Kann man vielleicht ein Schnäppchen machen, wenns heute 10% purzelt.
Überlege mal rauszugehen und später wieder rein, ist mir zu risikobehaftet. Kann man vielleicht ein Schnäppchen machen, wenns heute 10% purzelt.
Antwort auf Beitrag Nr.: 43.373.968 von dominator1000 am 11.07.12 13:48:13Drecks Basher Adam Fuckingstein!
Ich bleib drin, die wollen doch nur schwache Hände rausschütteln, nicht mit mir!
Adam Feuerstein glaubte auch nie, daß Arena die Zulassung erhält, ist eine kleine "Drecksau" und nicht mehr!

Ich bleib drin, die wollen doch nur schwache Hände rausschütteln, nicht mit mir!
Adam Feuerstein glaubte auch nie, daß Arena die Zulassung erhält, ist eine kleine "Drecksau" und nicht mehr!

habe ich mir gedacht, dass es wieder feuerstein gewesen sein muss
Zitat von Magnetfeldfredy: Drecks Basher Adam Fuckingstein!
Ich bleib drin, die wollen doch nur schwache Hände rausschütteln, nicht mit mir! .......ist eine kleine "Drecksau" und nicht mehr!
Jim Cramer: "What's important when you're in that hedge-fund mode is to not do anything that's remotely truthful.
Because the truth is so against your view that it's important to create a new truth to develop a fiction."
http://www.youtube.com/watch?v=NSeTKuNyPr4" target="_blank" rel="nofollow ugc noopener">
http://www.youtube.com/watch?v=NSeTKuNyPr4
Antwort auf Beitrag Nr.: 43.373.968 von dominator1000 am 11.07.12 13:48:13ich lass das.
Feuerstein findet zwar gehör, aber wird am Kursverlauf mittelfristig keinen Einfluss haben.

Feuerstein findet zwar gehör, aber wird am Kursverlauf mittelfristig keinen Einfluss haben.

Zitat von bernie55:Zitat von Magnetfeldfredy: Drecks Basher Adam Fuckingstein!
Ich bleib drin, die wollen doch nur schwache Hände rausschütteln, nicht mit mir! ...
....ist eine kleine "Drecksau" und nicht mehr!
...die BASHER- AKTIONEN werden im Gesamtchart ( LONG-Perspektive
) nicht groß auffallen...
Antwort auf Beitrag Nr.: 43.373.968 von dominator1000 am 11.07.12 13:48:13
press release
July 11, 2012, 2:00 a.m. EDT
Elan-UCD endowment honours Irish business leaders and boosts Ireland's capacity in the 'Business of Biotechnology'
DUBLIN, Jul 11, 2012 (BUSINESS WIRE) -- University College Dublin (UCD) and Elan Corporation plc, ELN +0.29% today announced two scholarships and an annual lecture series at University College Dublin (UCD) in the 'Business of Biotechnology' named in honour of Kieran McGowan, former head of the Industrial Development Authority of Ireland (IDA), Kyran McLaughlin, deputy chairman of Davy Stockbrokers and Laurence Crowley, the well-known accountant and board member of many Irish companies and organisations.
The UCD McGowan and McLaughlin post-doctoral scholarships and the Laurence Crowley 'Business of Biotechnology' lecture series are part of Elan's EUR3.17 million donation to the university to build capacity at the interface between business and science disciplines.
The donation will also establish a new Elan Chair in the 'Business of Biotechnology' to provide academic leadership in this strategically important area. The new professor will be a joint appointment between UCD Business School and UCD College of Science. The appointee will be charged with bolstering the science/technology content of the Business School curriculum and enhancing the business and innovation content of the science curriculum. They will also develop specialised masters programmes and other graduate courses in the business of biotechnology.
"As an Irish-based neuroscience biotechnology company that operates on a global scale, we understand the opportunities and challenges of this business more than most and are delighted in this instance to be in a position to share both financial support and experience with UCD to help create the next generation of biotech leaders out of Ireland," said Kelly Martin, chief executive officer, Elan. "We chose UCD as our academic partner in the business of biotechnology because of the university's track record in driving an innovation culture among its scientists."
"In assisting to create the next generation of Irish biotech leaders with UCD it is apt to acknowledge some of those who have helped to create the environment for this to succeed. I am delighted that we can honour the contribution of Kieran McGowan, Kyran McLaughlin and Laurence Crowley as part of this effort for their contribution to both Elan and the broader business context in Ireland," continued Mr Martin.
Under the direction of Dr Hugh Brady, president of UCD, the university has embedded innovation into the education curriculum, "The overarching goal of the sponsorship is to further strengthen the quality of the Irish graduate pipeline by creating a generation of business graduates who are technology savvy and of science graduates who can realise their innovation and entrepreneurial potential."
According to Kelly Martin the potential for biotechnology is enormous and the current focus of the sector is on the expansion of research and discovery activities to complement Ireland's established strength in manufacturing. Consequently, the Elan/UCD business of biotechnology initiative will open opportunities for scientists in Ireland who wish to commercialise their work.
"The Elan-UCD partnership initiative is an example of how industry and university can support the national economic agenda - getting our brightest minds to provide vision and leadership for future wealth generation," Mr Martin said. "Promoting innovation in core science like this has an eye on creating high value jobs and enterprises that will enable graduates to contribute from Ireland in a meaningful way to the global biotech sector."
The Elan EUR3.17 million to UCD comes after the company's announcement of a strategic alliance with Cambridge University for research into Alzheimer's and Parkinson's disease. "Normally donations of this size are for bricks and mortar but Elan's investment in brainpower demonstrates the company's commitment to Ireland at this juncture. It will have a profound impact on UCD's future and on the expansion of our unique science and business offering," Dr. Brady said.
"Ireland needs to produce the best business and technology graduates if it is to continue to attract major multinational companies and grow its own technology sector. It also needs to take every step possible to increase the commercialisation of its research outputs and to enhance the innovation capacity of Irish-based industry. The creation of a business of biotechnology hub supports the Irish and indeed the European agenda to support emerging enterprise in this area," concluded Dr. Brady.
In recent years, UCD has fostered over 250 industry partnerships and is the national leader in technology transfer and university spinouts through NovaUCD. Over 35 client companies are currently located at NovaUCD covering ICT, biotech, medical devices, wireless and renewable energy start-ups.
Ends/
About UCD
UCD is Ireland's largest university with 25,000 students, including 30% of all international students in the country. UCD educates the largest number of science and engineering graduates in Ireland, including over 31% of the science and engineering PhDs in the country, making it the national leader in fourth-level education. UCD has been ranked number one in research funding in Ireland for the last two years. Its research portfolio is particularly strong in biotechnology and includes the emerging area of systems biology. UCD is currently constructing an iconic 67,000 m(2) Science Centre for teaching and research - the largest science capital development project in the history of Irish third-level education.
In recent years, UCD has fostered over 250 industry partnerships and is the national leader in technology transfer and university spinouts. Supported by the expertise of NovaUCD - the University's hub for knowledge transfer and commercialisation activities - many of these start-ups have gone on to develop new and game-changing products and services.
About Elan
Elan is a neuroscience focused biotechnology company committed to making a difference in the lives of patients and their families by dedicating itself to bringing innovations in science to fill significant unmet medical needs that continue to exist around the world. For additional information about Elan, please visit www.elan.com
Notes to Editor:
Kieran McGowan
A director of Elan, and a director of Charles Schwab Worldwide Funds plc, McGowan was, until May 2012, Chairman of CRH, plc. From 1990 until his retirement in December 1998, he was Chief Executive of the Industrial Development Authority of Ireland. He is a former Chair of the UCD Governing Authority.
Kyran McLaughlin
Deputy chair at Davy, Ireland's largest stockbroker firm, he is a director of Elan and served as chairman from 2005 to 2011. He is also a director of Ryanair Holdings plc and is a director of a number of private companies.
Laurence Crowley
Former partner in KPMG Stokes Kennedy Crowley Chartered Accountants, Laurence has served on many boards in Ireland. He is currently chair of PJ Carroll and Co Ltd, and of Realex Payments. He is also a director of Aer Lingus and of Bord Gais Eireann. He served as a director of Elan Corporation and as executive chairman of UCD Michael Smurfit Graduate School of Business. He is former Governor of Bank of Ireland.
He is a co-chairman of the North South Roundtable Group; chairman of 'Gaisce - The President's Award', the Gate Theatre and The Middletown Centre for Autism.
He is a Director of Economic and Social Research Institute and serves on the Advisory Board of the US-Ireland Alliance.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=50337096&lan…
SOURCE: Elan Corporation plc
UCD
Media:
Eilis O'Brien, +353-1-716-1491
M: +353-87-2057125
or
Elan
Media:
Niamh Lyons, +353-1-709-4176
or
Investor Relations:
Chris Burns, +800-252-3526
David Marshall, +353-1-709-4444
Copyright Business Wire 2012
http://www.marketwatch.com/story/elan-ucd-endowment-honours-…
press release
July 11, 2012, 2:00 a.m. EDT
Elan-UCD endowment honours Irish business leaders and boosts Ireland's capacity in the 'Business of Biotechnology'
DUBLIN, Jul 11, 2012 (BUSINESS WIRE) -- University College Dublin (UCD) and Elan Corporation plc, ELN +0.29% today announced two scholarships and an annual lecture series at University College Dublin (UCD) in the 'Business of Biotechnology' named in honour of Kieran McGowan, former head of the Industrial Development Authority of Ireland (IDA), Kyran McLaughlin, deputy chairman of Davy Stockbrokers and Laurence Crowley, the well-known accountant and board member of many Irish companies and organisations.
The UCD McGowan and McLaughlin post-doctoral scholarships and the Laurence Crowley 'Business of Biotechnology' lecture series are part of Elan's EUR3.17 million donation to the university to build capacity at the interface between business and science disciplines.
The donation will also establish a new Elan Chair in the 'Business of Biotechnology' to provide academic leadership in this strategically important area. The new professor will be a joint appointment between UCD Business School and UCD College of Science. The appointee will be charged with bolstering the science/technology content of the Business School curriculum and enhancing the business and innovation content of the science curriculum. They will also develop specialised masters programmes and other graduate courses in the business of biotechnology.
"As an Irish-based neuroscience biotechnology company that operates on a global scale, we understand the opportunities and challenges of this business more than most and are delighted in this instance to be in a position to share both financial support and experience with UCD to help create the next generation of biotech leaders out of Ireland," said Kelly Martin, chief executive officer, Elan. "We chose UCD as our academic partner in the business of biotechnology because of the university's track record in driving an innovation culture among its scientists."
"In assisting to create the next generation of Irish biotech leaders with UCD it is apt to acknowledge some of those who have helped to create the environment for this to succeed. I am delighted that we can honour the contribution of Kieran McGowan, Kyran McLaughlin and Laurence Crowley as part of this effort for their contribution to both Elan and the broader business context in Ireland," continued Mr Martin.
Under the direction of Dr Hugh Brady, president of UCD, the university has embedded innovation into the education curriculum, "The overarching goal of the sponsorship is to further strengthen the quality of the Irish graduate pipeline by creating a generation of business graduates who are technology savvy and of science graduates who can realise their innovation and entrepreneurial potential."
According to Kelly Martin the potential for biotechnology is enormous and the current focus of the sector is on the expansion of research and discovery activities to complement Ireland's established strength in manufacturing. Consequently, the Elan/UCD business of biotechnology initiative will open opportunities for scientists in Ireland who wish to commercialise their work.
"The Elan-UCD partnership initiative is an example of how industry and university can support the national economic agenda - getting our brightest minds to provide vision and leadership for future wealth generation," Mr Martin said. "Promoting innovation in core science like this has an eye on creating high value jobs and enterprises that will enable graduates to contribute from Ireland in a meaningful way to the global biotech sector."
The Elan EUR3.17 million to UCD comes after the company's announcement of a strategic alliance with Cambridge University for research into Alzheimer's and Parkinson's disease. "Normally donations of this size are for bricks and mortar but Elan's investment in brainpower demonstrates the company's commitment to Ireland at this juncture. It will have a profound impact on UCD's future and on the expansion of our unique science and business offering," Dr. Brady said.
"Ireland needs to produce the best business and technology graduates if it is to continue to attract major multinational companies and grow its own technology sector. It also needs to take every step possible to increase the commercialisation of its research outputs and to enhance the innovation capacity of Irish-based industry. The creation of a business of biotechnology hub supports the Irish and indeed the European agenda to support emerging enterprise in this area," concluded Dr. Brady.
In recent years, UCD has fostered over 250 industry partnerships and is the national leader in technology transfer and university spinouts through NovaUCD. Over 35 client companies are currently located at NovaUCD covering ICT, biotech, medical devices, wireless and renewable energy start-ups.
Ends/
About UCD
UCD is Ireland's largest university with 25,000 students, including 30% of all international students in the country. UCD educates the largest number of science and engineering graduates in Ireland, including over 31% of the science and engineering PhDs in the country, making it the national leader in fourth-level education. UCD has been ranked number one in research funding in Ireland for the last two years. Its research portfolio is particularly strong in biotechnology and includes the emerging area of systems biology. UCD is currently constructing an iconic 67,000 m(2) Science Centre for teaching and research - the largest science capital development project in the history of Irish third-level education.
In recent years, UCD has fostered over 250 industry partnerships and is the national leader in technology transfer and university spinouts. Supported by the expertise of NovaUCD - the University's hub for knowledge transfer and commercialisation activities - many of these start-ups have gone on to develop new and game-changing products and services.
About Elan
Elan is a neuroscience focused biotechnology company committed to making a difference in the lives of patients and their families by dedicating itself to bringing innovations in science to fill significant unmet medical needs that continue to exist around the world. For additional information about Elan, please visit www.elan.com
Notes to Editor:
Kieran McGowan
A director of Elan, and a director of Charles Schwab Worldwide Funds plc, McGowan was, until May 2012, Chairman of CRH, plc. From 1990 until his retirement in December 1998, he was Chief Executive of the Industrial Development Authority of Ireland. He is a former Chair of the UCD Governing Authority.
Kyran McLaughlin
Deputy chair at Davy, Ireland's largest stockbroker firm, he is a director of Elan and served as chairman from 2005 to 2011. He is also a director of Ryanair Holdings plc and is a director of a number of private companies.
Laurence Crowley
Former partner in KPMG Stokes Kennedy Crowley Chartered Accountants, Laurence has served on many boards in Ireland. He is currently chair of PJ Carroll and Co Ltd, and of Realex Payments. He is also a director of Aer Lingus and of Bord Gais Eireann. He served as a director of Elan Corporation and as executive chairman of UCD Michael Smurfit Graduate School of Business. He is former Governor of Bank of Ireland.
He is a co-chairman of the North South Roundtable Group; chairman of 'Gaisce - The President's Award', the Gate Theatre and The Middletown Centre for Autism.
He is a Director of Economic and Social Research Institute and serves on the Advisory Board of the US-Ireland Alliance.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=50337096&lan…
SOURCE: Elan Corporation plc
UCD
Media:
Eilis O'Brien, +353-1-716-1491
M: +353-87-2057125
or
Elan
Media:
Niamh Lyons, +353-1-709-4176
or
Investor Relations:
Chris Burns, +800-252-3526
David Marshall, +353-1-709-4444
Copyright Business Wire 2012
http://www.marketwatch.com/story/elan-ucd-endowment-honours-…
Hab meine Portionen rausgehauen.
Warte jetzt mal ab. Könnte über -10% gehen.
Warte jetzt mal ab. Könnte über -10% gehen.
Ist irgendwo noch ein gap`?
AMRS..der etwas andere Biotechwert!

Antwort auf Beitrag Nr.: 43.375.015 von franzisca am 11.07.12 17:09:56
wird nicht gehandelt, nur glattgestellt. wie bmti gestern
wird nicht gehandelt, nur glattgestellt. wie bmti gestern
Zitat von bernie55:Zitat von Magnetfeldfredy: Drecks Basher Adam Fuckingstein!
Ich bleib drin, die wollen doch nur schwache Hände rausschütteln, nicht mit mir! .......ist eine kleine "Drecksau" und nicht mehr!
Jim Cramer: "What's important when you're in that hedge-fund mode is to not do anything that's remotely truthful.
Because the truth is so against your view that it's important to create a new truth to develop a fiction."
http://www.youtube.com/watch?v=NSeTKuNyPr4" target="_blank" rel="nofollow ugc noopener">
http://www.youtube.com/watch?v=NSeTKuNyPr4
...hier der Stein des Anstosses.....
Arena Pharmaceuticals (ARNA), Vivus Pharmaceuticals (VVUS), Onyx Pharmaceutical (ONXX)
Arena Pharmaceuticals (ARNA) was chosen among followers of Jim Cramer on Twitter as the champion stock for the second half of 2012, but Cramer disagrees with this pick. The stock has rallied $225 on the potential approval of its obesity drug by the FDA; this will be the first major obesity drug for 10 years. The drug could generate $2 billion in revenue, equal to Arena's present market cap of $2 billion. In addition to the fact that Arena has already risen substantially, the story is not perfect. The obesity drug is not as effective as initially thought, and there are substantial cases of side effects, including hallucinations.
Vivus Pharmaceutical (VVUS) also has an obesity drug awaiting approval, and this drug has shown to be more effective than Arena's treatment.
In addition, Vivus owns all of its drug, while Arena just owns 40% of its obesity drug.
Cramer's pick among biotechs is Onyx (ONXX), which has a blood cancer drug which is expected to earn FDA approval, and already has treatments for blood and liver cancer.
http://seekingalpha.com/article/713341-cramer-s-mad-money-fi…
JIM CRAMER versucht mit allen nur erdenklichen Mitteln das Hedgefondsklientel zu bedienen..
...Leute, keep cool....keine SL setzen...
...was sind im übrigen aktuell Minus 5,84 % im Vergleich zu den knapp 500 %, die wir bis jetzt gemacht haben ??
..die 11 USD hält !!!
....vielleicht hat sich der Marktschreier mehr Bewegung nach unten versprochen...
 ...
.......auf jeden Fall steigen jetzt wieder viele bei diesen Kursen ein.....
Die amis laufen wohl noch weiter runter schätze ich.
Antwort auf Beitrag Nr.: 43.375.135 von franzisca am 11.07.12 17:34:52
bei 3 hatte ich nicht den mut. und jetzt nach 18% sowieso nicht
bei 3 hatte ich nicht den mut. und jetzt nach 18% sowieso nicht
würde mich nicht wundern, wenn wir noch bei 11,80 schliessen








ich glaube, dss wir ab etwa 9 usd keinen rücksetzer gehabt haben. wenn morgen auch kleiner minus geben sollte - was ich nicht hoffe - wäre immer noch normal
press release
July 11, 2012, 4:05 p.m. EDT
Arena Pharmaceuticals Files Marketing Authorization Application in Switzerland for Lorcaserin as a Treatment for Weight Control
SAN DIEGO, July 11, 2012 /PRNewswire via COMTEX/ -- Arena Pharmaceuticals, Inc. announced today the filing with the Swiss health authority, Swissmedic, of a Marketing Authorization Application (MAA) for lorcaserin hydrochloride, an investigational drug candidate in Switzerland. The intended indication is as an adjunct to diet and exercise for weight control in patients with an initial body mass index (BMI) of 30 kg/m(2) or greater (obese), or 27 kg/m(2) or greater (overweight) in the presence of at least one weight related comorbid condition. Arena expects that Swissmedic will accept the filing later this month and confirm the filing is sufficiently complete to permit a substantive review process.
"This submission reflects our continued efforts to make lorcaserin available to physicians and patients beyond the United States," said Jack Lief, Arena's President and Chief Executive Officer. "We look forward to Swissmedic's review of our application and to the potential approval of lorcaserin in Switzerland for patients who are obese or overweight with comorbidities."
As part of the Swiss MAA, Arena submitted data from its three double-blind, randomized, placebo-controlled clinical trials, which demonstrated that lorcaserin along with diet and exercise was more effective than diet and exercise alone at helping patients lose 5% or more of their body weight after one year and managing the weight loss for up to two years. In these trials, the most common adverse reactions for patients without diabetes treated with lorcaserin were headache, dizziness, fatigue, nausea, dry mouth, and constipation. In patients with diabetes, the most common adverse reactions were hypoglycemia, headache, back pain, cough, and fatigue.
The MAA filing in Switzerland is the third marketing application submitted to date for lorcaserin. Lorcaserin was approved by the US Food and Drug Administration (FDA) under the trade name BELVIQ in June 2012, and is currently under review with the European Medicines Agency (EMA). Switzerland is not part of the EMA and requires an independent application and approval from Swissmedic.
About Lorcaserin Lorcaserin is believed to decrease food consumption and promote satiety by selectively activating serotonin 2C receptors in the brain. Activation of these receptors may help a person eat less and feel full after eating smaller amounts of food. Arena has patents or patent applications that cover lorcaserin in the United States, Europe, Canada, Mexico, Brazil and many other jurisdictions that, if issued, in most cases would be capable of continuing into 2023 without taking into account any patent term extensions or other exclusivity Arena might obtain.
About Arena Pharmaceuticals Arena Pharmaceuticals, Inc., is a biopharmaceutical company focused on discovering, developing and commercializing oral drugs that target G protein-coupled receptors, an important class of validated drug targets, in four major therapeutic areas: cardiovascular, central nervous system, inflammatory and metabolic diseases. For more information about Arena, please visit www.arenapharm.com .
Arena Pharmaceuticals® and Arena® are registered service marks of Arena Pharmaceuticals, Inc. BELVIQ® is a registered trademark of Arena Pharmaceuticals GmbH.
Forward-Looking Statements Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include statements about lorcaserin's (or BELVIQ's) safety, efficacy, mechanism of action and intended indication; Swissmedic's acceptance and review of the lorcaserin MAA; the potential approval of lorcaserin; lorcaserin's patents; and Arena's efforts, focus, goals, strategy, research and development programs, and ability to develop compounds and commercialize drugs. For such statements, Arena claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Arena's expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, the following: the timing and outcome of DEA, EMA, Swissmedic and other regulatory review is uncertain; limitations on the indicated uses, distribution, marketing and other limitations on BELVIQ or, if approved, any of Arena's other drug candidates; risks related to commercializing drugs, including regulatory, manufacturing and supply issues and the pace of market acceptance; cash and revenues generated from BELVIQ, including timing and impact of competition; government and commercial reimbursement and pricing decisions; risks related to relying on collaborative agreements; the timing and receipt of payments and fees, if any, from collaborators; unexpected or unfavorable new data; nonclinical and clinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Arena or others, request additional information, have additional recommendations or change their guidance or requirements before or after approval; data and other information related to any of Arena's research and development programs may not meet safety, efficacy or other regulatory requirements or otherwise be sufficient for regulatory review, approval or continued marketing; Arena's ability to obtain and defend patents; the timing, success and cost of Arena's research and development programs; results of clinical trials and other studies are subject to different interpretations and may not be predictive of future results; clinical trials and other studies may not proceed at the time or in the manner expected or at all; having adequate funds; and satisfactory resolution of litigation or other disagreements with others. Additional factors that could cause actual results to differ materially from those stated or implied by Arena's forward-looking statements are disclosed in Arena's filings with the Securities and Exchange Commission. These forward-looking statements represent Arena's judgment as of the time of this release. Arena disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
Contacts: Arena Pharmaceuticals, Inc.
858.453.7200
Investor Inquiries: Russo Partners Media Inquiries: Russo Partners
Cindy McGee David Schull
cindy.mcgee@russopartnersllc.com david.schull@russopartnersllc.com
619.213.6995 858.717.2310
www.arenapharm.com
SOURCE Arena Pharmaceuticals, Inc.
Copyright (C) 2012 PR Newswire. All rights reserved
http://www.marketwatch.com/story/arena-pharmaceuticals-files…
July 11, 2012, 4:05 p.m. EDT
Arena Pharmaceuticals Files Marketing Authorization Application in Switzerland for Lorcaserin as a Treatment for Weight Control
SAN DIEGO, July 11, 2012 /PRNewswire via COMTEX/ -- Arena Pharmaceuticals, Inc. announced today the filing with the Swiss health authority, Swissmedic, of a Marketing Authorization Application (MAA) for lorcaserin hydrochloride, an investigational drug candidate in Switzerland. The intended indication is as an adjunct to diet and exercise for weight control in patients with an initial body mass index (BMI) of 30 kg/m(2) or greater (obese), or 27 kg/m(2) or greater (overweight) in the presence of at least one weight related comorbid condition. Arena expects that Swissmedic will accept the filing later this month and confirm the filing is sufficiently complete to permit a substantive review process.
"This submission reflects our continued efforts to make lorcaserin available to physicians and patients beyond the United States," said Jack Lief, Arena's President and Chief Executive Officer. "We look forward to Swissmedic's review of our application and to the potential approval of lorcaserin in Switzerland for patients who are obese or overweight with comorbidities."
As part of the Swiss MAA, Arena submitted data from its three double-blind, randomized, placebo-controlled clinical trials, which demonstrated that lorcaserin along with diet and exercise was more effective than diet and exercise alone at helping patients lose 5% or more of their body weight after one year and managing the weight loss for up to two years. In these trials, the most common adverse reactions for patients without diabetes treated with lorcaserin were headache, dizziness, fatigue, nausea, dry mouth, and constipation. In patients with diabetes, the most common adverse reactions were hypoglycemia, headache, back pain, cough, and fatigue.
The MAA filing in Switzerland is the third marketing application submitted to date for lorcaserin. Lorcaserin was approved by the US Food and Drug Administration (FDA) under the trade name BELVIQ in June 2012, and is currently under review with the European Medicines Agency (EMA). Switzerland is not part of the EMA and requires an independent application and approval from Swissmedic.
About Lorcaserin Lorcaserin is believed to decrease food consumption and promote satiety by selectively activating serotonin 2C receptors in the brain. Activation of these receptors may help a person eat less and feel full after eating smaller amounts of food. Arena has patents or patent applications that cover lorcaserin in the United States, Europe, Canada, Mexico, Brazil and many other jurisdictions that, if issued, in most cases would be capable of continuing into 2023 without taking into account any patent term extensions or other exclusivity Arena might obtain.
About Arena Pharmaceuticals Arena Pharmaceuticals, Inc., is a biopharmaceutical company focused on discovering, developing and commercializing oral drugs that target G protein-coupled receptors, an important class of validated drug targets, in four major therapeutic areas: cardiovascular, central nervous system, inflammatory and metabolic diseases. For more information about Arena, please visit www.arenapharm.com .
Arena Pharmaceuticals® and Arena® are registered service marks of Arena Pharmaceuticals, Inc. BELVIQ® is a registered trademark of Arena Pharmaceuticals GmbH.
Forward-Looking Statements Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include statements about lorcaserin's (or BELVIQ's) safety, efficacy, mechanism of action and intended indication; Swissmedic's acceptance and review of the lorcaserin MAA; the potential approval of lorcaserin; lorcaserin's patents; and Arena's efforts, focus, goals, strategy, research and development programs, and ability to develop compounds and commercialize drugs. For such statements, Arena claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Arena's expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, the following: the timing and outcome of DEA, EMA, Swissmedic and other regulatory review is uncertain; limitations on the indicated uses, distribution, marketing and other limitations on BELVIQ or, if approved, any of Arena's other drug candidates; risks related to commercializing drugs, including regulatory, manufacturing and supply issues and the pace of market acceptance; cash and revenues generated from BELVIQ, including timing and impact of competition; government and commercial reimbursement and pricing decisions; risks related to relying on collaborative agreements; the timing and receipt of payments and fees, if any, from collaborators; unexpected or unfavorable new data; nonclinical and clinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Arena or others, request additional information, have additional recommendations or change their guidance or requirements before or after approval; data and other information related to any of Arena's research and development programs may not meet safety, efficacy or other regulatory requirements or otherwise be sufficient for regulatory review, approval or continued marketing; Arena's ability to obtain and defend patents; the timing, success and cost of Arena's research and development programs; results of clinical trials and other studies are subject to different interpretations and may not be predictive of future results; clinical trials and other studies may not proceed at the time or in the manner expected or at all; having adequate funds; and satisfactory resolution of litigation or other disagreements with others. Additional factors that could cause actual results to differ materially from those stated or implied by Arena's forward-looking statements are disclosed in Arena's filings with the Securities and Exchange Commission. These forward-looking statements represent Arena's judgment as of the time of this release. Arena disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
Contacts: Arena Pharmaceuticals, Inc.
858.453.7200
Investor Inquiries: Russo Partners Media Inquiries: Russo Partners
Cindy McGee David Schull
cindy.mcgee@russopartnersllc.com david.schull@russopartnersllc.com
619.213.6995 858.717.2310
www.arenapharm.com
SOURCE Arena Pharmaceuticals, Inc.
Copyright (C) 2012 PR Newswire. All rights reserved
http://www.marketwatch.com/story/arena-pharmaceuticals-files…
@AREANICS
ich habe zur " Entlastung" dieses Threads noch ein neues Diskussionsforum eröffnet
> ARENA PHARMACEUTICALS + BELVIQ - Wie geht es weiter ???
Das geschieht mit der Absicht, einfach mehr Übersicht in die Bereiche von Fakten, News und möglichen Sachdiskussionen zu bringen.
In diesem Thread werde ich natürlich auch noch " rumstochern" ( also auch noch News,Fakten und Disskussionsstoff reinstellen) und natürlich auch weiterhin an den " regen" Gesprächen von TRADIES und CO. teilhaben
ich habe zur " Entlastung" dieses Threads noch ein neues Diskussionsforum eröffnet
> ARENA PHARMACEUTICALS + BELVIQ - Wie geht es weiter ???
Das geschieht mit der Absicht, einfach mehr Übersicht in die Bereiche von Fakten, News und möglichen Sachdiskussionen zu bringen.
In diesem Thread werde ich natürlich auch noch " rumstochern" ( also auch noch News,Fakten und Disskussionsstoff reinstellen) und natürlich auch weiterhin an den " regen" Gesprächen von TRADIES und CO. teilhaben

Antwort auf Beitrag Nr.: 43.377.840 von bernie55 am 12.07.12 11:40:29
eröffne noch ein threade, indem man nur müll schreiben darf.
überschrift
gurkenthread
eröffne noch ein threade, indem man nur müll schreiben darf.
überschrift
gurkenthread
Zitat von gauner1: eröffne noch ein threade, indem man nur müll schreiben darf.
überschrift
gurkenthread
Er möchte auch einen eigenen Thread!
 ....nein mal im Ernst, man könnte doch im anderem Thread News und Fakten reinstellen und hier so weitermachen wie bisher!
....nein mal im Ernst, man könnte doch im anderem Thread News und Fakten reinstellen und hier so weitermachen wie bisher!Schaut Euch Gnom an.....sehr interessant...Vorbörse schon ca. 6-8% im Plus!

Liebe Grüsse franzisca

Cramer! 
Mad Money Recap
Cramer's 'Mad Money" Recap: Curb Your Enthusiasm
By Scott Rutt 07/11/12 - 07:44 PM EDT
http://www.thestreet.com/_nasdaq/story/11613925/1/cramers-ma…

Mad Money Recap
Cramer's 'Mad Money" Recap: Curb Your Enthusiasm
By Scott Rutt 07/11/12 - 07:44 PM EDT
http://www.thestreet.com/_nasdaq/story/11613925/1/cramers-ma…
Antwort auf Beitrag Nr.: 43.378.273 von franzisca am 12.07.12 13:25:06
ich beobachte seit einer woche. gestern 42 prozent gestiegen und heute ist freitag
ich beobachte seit einer woche. gestern 42 prozent gestiegen und heute ist freitag
Antwort auf Beitrag Nr.: 43.378.454 von gauner1 am 12.07.12 14:15:59Du bist doch schon seit dem 26.05 drin, oder nicht?
und wo ist heute schon Freitag?

und wo ist heute schon Freitag?

nein, bin nicht drinn. und heute ist kein freitag sorry
Zitat von franzisca: Er möchte auch einen eigenen Thread!....nein mal im Ernst, man könnte doch im anderem Thread News und Fakten reinstellen und hier so weitermachen wie bisher!
Liebe Grüsse franzisca
YEPP...Franziska..
....du hast es verstanden...

.....der andere Thread ist nicht als Konkurrenzprodukt zu diesem hier zu verstehen, sondern als "gesunde Beimischung".....

Seventh Annual JMP Securities Healthcare Conference
Jul 12, 2012
9:00 AM ET
http://invest.arenapharm.com/eventdetail.cfm?EventID=116308
Jul 12, 2012
9:00 AM ET
http://invest.arenapharm.com/eventdetail.cfm?EventID=116308
ich bin nicht der orphus, aber für mich war es sonnenklar, dass gestern und heute der kurs fällt und wahrscheinlich weiterfallen wird, was ich für euch nicht hoffe.
grund: anscheind haben einige von euch nicht gesehen, dass vorgestern der kurs nach 5,8% gewinn nachbörslich mit 530k umsatz die ganze gewinne verloren und sogar in minus geraten ist. es hätte euch ja ein licht aufgehen müssen, dass irgendjemand muss was negatives gesprochen haben bzw. ein mensch, der sein wort gilt, mächtig gebasht haben.
man muss nicht an der börse nicht nur auf das fundamentale achten und lediglich in unserem fall über das medikament und konkurenz usw. diskutieren und großartige englische texte reinstellen und groß fachzimpeln usw.usw. sondern zum beispiel den kursverlauf usw. betrachten und seine entscheidungen treffen. einfach da sitzen und gucken, dass der kurs steigt, bringt nichts. arbeiten muss man liebe leute.
dominator und ich haben gestern richtig gemacht. viele von euch nicht.
tut mir leid, dass ich wieder müll schreiben mußte
kurs 10,66
grund: anscheind haben einige von euch nicht gesehen, dass vorgestern der kurs nach 5,8% gewinn nachbörslich mit 530k umsatz die ganze gewinne verloren und sogar in minus geraten ist. es hätte euch ja ein licht aufgehen müssen, dass irgendjemand muss was negatives gesprochen haben bzw. ein mensch, der sein wort gilt, mächtig gebasht haben.
man muss nicht an der börse nicht nur auf das fundamentale achten und lediglich in unserem fall über das medikament und konkurenz usw. diskutieren und großartige englische texte reinstellen und groß fachzimpeln usw.usw. sondern zum beispiel den kursverlauf usw. betrachten und seine entscheidungen treffen. einfach da sitzen und gucken, dass der kurs steigt, bringt nichts. arbeiten muss man liebe leute.
dominator und ich haben gestern richtig gemacht. viele von euch nicht.
tut mir leid, dass ich wieder müll schreiben mußte
kurs 10,66
Zitat von gauner1: ich bin nicht der orphus, aber für mich war es sonnenklar, dass gestern und heute der kurs fällt und wahrscheinlich weiterfallen wird, was ich für euch nicht hoffe.
grund: anscheind haben einige von euch nicht gesehen, dass vorgestern der kurs nach 5,8% gewinn nachbörslich mit 530k umsatz die ganze gewinne verloren und sogar in minus geraten ist. es hätte euch ja ein licht aufgehen müssen, dass irgendjemand muss was negatives gesprochen haben bzw. ein mensch, der sein wort gilt, mächtig gebasht haben.
man muss nicht an der börse nicht nur auf das fundamentale achten und lediglich in unserem fall über das medikament und konkurenz usw. diskutieren und großartige englische texte reinstellen und groß fachzimpeln usw.usw. sondern zum beispiel den kursverlauf usw. betrachten und seine entscheidungen treffen.
einfach da sitzen und gucken, dass der kurs steigt, bringt nichts.
arbeiten muss man liebe leute.....
dominator und ich haben gestern richtig gemacht.....
viele von euch nicht.
tut mir leid, dass ich wieder müll schreiben mußte.......
kurs 10,66
Was ist denn jetzt schon wieder los, gauner1 ????
Rundumschläge gegen wen , warum so aggressiv ???
Es hat dir keiner etwas getan !!!
Unbegreiflich, was du jetzt hier abziehst.....
..hier mal wieder ein großartiger englischer Text...
Ha Ha its official guys...we have a buyer!!!
I am so glad I held onto my shares. I knew that this pps drop activity was probably being orchestrated to swindle the average joe out of his shares. You all should have know this. Obviously cramer was in on the hoax too. If you sold I feel sorry for you but you got what you deserved for not having the confidence that you should have had in your investment.
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…" target="_blank" rel="nofollow ugc noopener">
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
Ha Ha its official guys...we have a buyer!!!
I am so glad I held onto my shares. I knew that this pps drop activity was probably being orchestrated to swindle the average joe out of his shares. You all should have know this. Obviously cramer was in on the hoax too. If you sold I feel sorry for you but you got what you deserved for not having the confidence that you should have had in your investment.
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…" target="_blank" rel="nofollow ugc noopener">
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
Antwort auf Beitrag Nr.: 43.379.209 von bernie55 am 12.07.12 16:44:46
berni
ich ziehe nicht etwas ab. wir sitzen alle in einem boot. wenn einem ein bericht oder irgendetwas auffällt, sollte man in interesse alle hier posten.
ich ziehe nicht was ab, sondern bedauere, dass einige in minus geraten sind bzw. einen teil ihre gewinne gestern und heute verloren haben.
berni
ich ziehe nicht etwas ab. wir sitzen alle in einem boot. wenn einem ein bericht oder irgendetwas auffällt, sollte man in interesse alle hier posten.
ich ziehe nicht was ab, sondern bedauere, dass einige in minus geraten sind bzw. einen teil ihre gewinne gestern und heute verloren haben.
Antwort auf Beitrag Nr.: 43.379.209 von bernie55 am 12.07.12 16:44:46
hast du nicht gemerkt, dass ich der messias bin und die ganze welt retten will ?
hast du nicht gemerkt, dass ich der messias bin und die ganze welt retten will ?
Zitat von gauner1: berni
ich ziehe nicht etwas ab. wir sitzen alle in einem boot. wenn einem ein bericht oder irgendetwas auffällt, sollte man in interesse alle hier posten.
ich ziehe nicht was ab, sondern bedauere, dass einige in minus geraten sind bzw. einen teil ihre gewinne gestern und heute verloren haben
..das ist TRADERmäßig sozial gedacht - rein und raus, um Geld zu verdienen bzw. aber auch zu verlieren...
..EARTHIE hat mir dazu schon einiges erzählt....... und mir wird immer klarer, dass ihr natürlich ganz anders "positioniert" bzw eingestellt zum Aktienkauf und Verkauf..
Viele LONGIES sind übrigens bei ARNA teilweise schon seit ca. 1,70 - 2,90 € EK hier investiert ....
..und da sieht man diese manipulierten Schwankungen natürlich etwas gelassener....
Antwort auf Beitrag Nr.: 43.378.932 von gauner1 am 12.07.12 15:57:16
ich habe übrigens schon am 10. Juli zu $11,80 verkauft (das hat dann den Kurssturz nachbörslich verursacht).
Gekauft hatte ich bereits im August 2011 zu Kursen im Durchschnitt zu $1,30.
Grob hatte ich 2,5 mio. Aktien.





(tut mir Leid für Dich)

ich habe übrigens schon am 10. Juli zu $11,80 verkauft (das hat dann den Kurssturz nachbörslich verursacht).
Gekauft hatte ich bereits im August 2011 zu Kursen im Durchschnitt zu $1,30.
Grob hatte ich 2,5 mio. Aktien.





(tut mir Leid für Dich)
lieber berni
ich bin kein trader, aber wenn ich was negatives sehe, gehe ich raus und wenn die sache vorbei ist, gehe ich wieder rein.
schau bitte, wir wollen alle geld verdienen. gestern raus und heute rein ( bin aber noch nicht ) macht bei mir fast 2800 dollar aus. ist das nicht ein guter gewinn an 2 tagen, wo viele ein ganzer monat durch harte lediglich 2000 euro verdienen ? wozu handeln wir denn eigentlich mit aktien ? daher verstehe ich deinen angriff nicht, wenn ich gut meine
ich bin kein trader, aber wenn ich was negatives sehe, gehe ich raus und wenn die sache vorbei ist, gehe ich wieder rein.
schau bitte, wir wollen alle geld verdienen. gestern raus und heute rein ( bin aber noch nicht ) macht bei mir fast 2800 dollar aus. ist das nicht ein guter gewinn an 2 tagen, wo viele ein ganzer monat durch harte lediglich 2000 euro verdienen ? wozu handeln wir denn eigentlich mit aktien ? daher verstehe ich deinen angriff nicht, wenn ich gut meine
berni
bevor ich mich um meine gurken kümmere, nur eine frage
meinst du, dass man sitzen und nur gucken muss, wie man geld verliert und nichts tut ?
daher verstehe ich dich nicht
bis morgen allerseits
bevor ich mich um meine gurken kümmere, nur eine frage
meinst du, dass man sitzen und nur gucken muss, wie man geld verliert und nichts tut ?
daher verstehe ich dich nicht
bis morgen allerseits
um mal bei den Fakten zu bleiben:
Arena Pharmaceuticals - Daily Trading Update
Posted on 07/12/2012 by Edward Connelly
NEW YORK (AVAFIN) -- Block trading activities for shares of Arena Pharmaceuticals reveal that 121 block trades were executed during the market session. Trade flow analysis shows that $12,344,259 worth of shares were bought and $8,230,650 worth of shares were sold by institutional investors. The positive cash flow $4,113,609 into the stock shows that investors have bullish sentiment.
Institutional investors also took positions in options where a total of 57,093 contracts was traded. Specifically, 45,243 call and 11,850 put contracts were traded during the last session yielding a 0.26 put/call ratio.
Arena Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company committed to discovering and developing innovative therapies offering medical advances and new options for patients.
Shares of Arena Pharmaceuticals edged down $0.52 (4.67%) to $10.61. The price of the stock ranged between a low of $10.08 and $10.92 respectively. The trading volume of 23M is below the 90 day average volume of 24M shares. ARNA is trading above the 50 day moving average. The stock's 52 week low is $1.23 and 52 week high is $13.50. To date, the stock has gained 271.00% within the last quarter.
http://www.avafin.com/articles/1015844.html
Arena Pharmaceuticals - Daily Trading Update
Posted on 07/12/2012 by Edward Connelly
NEW YORK (AVAFIN) -- Block trading activities for shares of Arena Pharmaceuticals reveal that 121 block trades were executed during the market session. Trade flow analysis shows that $12,344,259 worth of shares were bought and $8,230,650 worth of shares were sold by institutional investors. The positive cash flow $4,113,609 into the stock shows that investors have bullish sentiment.
Institutional investors also took positions in options where a total of 57,093 contracts was traded. Specifically, 45,243 call and 11,850 put contracts were traded during the last session yielding a 0.26 put/call ratio.
Arena Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company committed to discovering and developing innovative therapies offering medical advances and new options for patients.
Shares of Arena Pharmaceuticals edged down $0.52 (4.67%) to $10.61. The price of the stock ranged between a low of $10.08 and $10.92 respectively. The trading volume of 23M is below the 90 day average volume of 24M shares. ARNA is trading above the 50 day moving average. The stock's 52 week low is $1.23 and 52 week high is $13.50. To date, the stock has gained 271.00% within the last quarter.
http://www.avafin.com/articles/1015844.html
hier werden mal ARNA und VVUS nebeneinander betrachtet:
Vivus: Getting Ready For FDA Approval
July 12, 2012 |
Vivus (VVUS) is a biopharmaceutical company, which mainly develops medicines for treatment of sleep apnea, diabetes, obesity and male sexual health. While its portfolio is relatively diversified, there is a great deal of interest in company's obesity treatment products. Vivus is currently awaiting final approval from FDA, for Qnexa, their most recent drug. Qnexa is a drug meant to help reduce weight. A few years ago, Qnexa was initially rejected by FDA at the first attempt. At the second presentation in this year, Qnexa got a vote of confidence by a whopping 20-2 margin by the committee.
Vivus will get a final decision about Qnexa on July, 17, 2012. If approved, Qnexa will be the second drug to be approved by FDA in weight loss category in two months. The company plans on marketing the drug by the end of year 2012. European authorities have delayed a decision on the approval of the drug. Vivus is entirely focusing on getting Qnexa approved by FDA at the moment. Currently, Lorcaserin is the only weight reduction drug approved by FDA. Arena Pharmaceuticals (ARNA) is the developer of Lorcaserin.
Clinical Background
Qnexa has already created a strong buzz in the market. Clinical studies have shown a significant weight loss in the participants. According to the results published by the company, average weight loss for top dose participants was 14.4%. Average weight loss for low dose Qnexa was 6.7% and 2.1% for placebo. Mean weight loss after week 56 was 10.9% for top dose and 5.1% for low dose. Participants who completed the top dose treatment, 83.5% lost ≥5%; 67.7% lost ≥10% and 48.1% lost ≥15% of their baseline weight. There are some adverse effect fears, due to which Vivus has to follow a labeling protocol. Qnexa will not be prescribed to the pregnant woman. Vivus plans on advising the patients to stop taking the medicine, if weight loss is less than 3% after three months.
Stock Performance
VVUS Chart
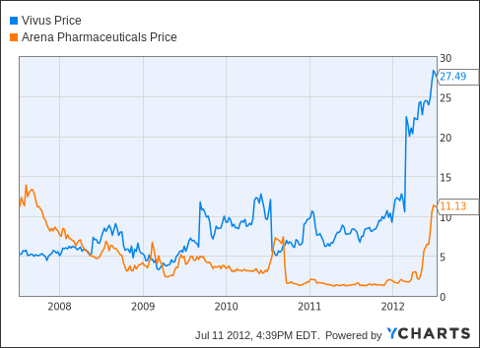
Both Vivus and Arena stocks are currently trading near its 52 week highs. Vivus stock closed at $28.47 on July, 09, 2010. The 52-week trading range for Vivus is $6.13 - $29.99. Even after the recent retreat, the stock is trading near its 52-week highs. Market cap of Vivus Inc. is $2.83 billion, slightly higher than the $2.15 billion market cap of Arena. Vivus stock has a relatively high Beta of 1.42. Trailing twelve month EPS is -0.63. One year analyst target for Vivus is $31.20.
Ratio Analysis
Estimates for the current year EPS are 40% more than the past 12 months EPS. One year forward estimate for EPS is to grow by 166%. Forward P/E is unusually high at 72.90. Current P/B ratio stands at 8.72. Firm is in an exceptionally strong liquidity position. Quick and current ratios are 25.70 and 25.92 respectively. Unlike Arena Pharmaceuticals, Vivus has no debt in its capital structure.
Pipeline Products
Vivus had Avanafil approved by FDA in April, 2012, a drug for men with erectile dysfunction. Vivus plans to sell Avanafil under the brand name Stendra. Stendra promises to work in fifteen minutes, half the time it takes the pills from rivals companies to work. That is a very short period by industry standards.
By getting Stendra approved, Vivus now stand to compete directly with giants of the industry like, Pfizer (PFE), GlaxoSmithKline (GSK) and Bayer. Analysts believe that the sales from Stendra can go up to $68 million in the next year and to $459 million by the year 2017. Erectile dysfunction market itself is currently worth $4.3 billion. Viagra, Pfizer's well-known super-charger, holds almost half of the market share. Vivus is working on getting a partnership to market Stendra as well.
Besides the existing products, Vivus wants to focus on the marketing of Qnexa, if approved. Vivus wants to preserve the entire budget to spend on the marketing of Qnexa. Obesity market is largely unexplored, and it is a large market. There will be less competition in this segment. Vivus has a good marketing network. Vivus has more than one product in the market. As such, the revenues will not be depending on only one drug in a hugely unexplored market.
If approved, Vivus plans to launch Qnexa in the later part of year 2012. On the other hand, Arena Pharmaceutical expects to market the drug in 2013. This will give Vivus Inc a head start on its main competitor, and the firm can capture a large chunk of the market. Vivus is planning to directly market the drug in the U.S. itself and seek partnerships outside the U.S. USA is an immense obesity market. Arena does not have the pure marketing rights for its own obesity drug. Only a certain portion of revenues from sales will go to Arena, whereas the rest will be taken by Aisai Pharmaceuticals, the Japanese partner. This I believe is again an advantage for Vivus over Arena.
Insider Sales
The biggest bearish argument made against Vivus is the sale of shares by three executives in the past two months. Insiders selling stock before the approval are sure to raise concerns among the investors. The main reason, which most people have been ignoring, is something less critical. Vivus executives have been selling stocks under the 10b5-1 trading plan to cover themselves legally against allegations of insider trading. CEO of Vivus recently sold 100,000 shares, but he still owns 4.3 million shares.
Summary
I think the FDA decision is almost certain, and Vivus is extremely hopeful on the approval of Qnexa. This drug will prove to be a catalyst for the success of the company. Analysts believe, after the approval, Vivus stock should be priced at $40. Vivus, currently trading below $30 presents an exciting opportunity to make tidy profits. Insider sales are worrisome, but it is hard to say that insiders are getting rid of their shares, as they hold substantial stake in the company. This still raises a red flag for interested investors. Therefore, as the stock has already made substantial gains, I would suggest a hold and buy at pullback strategy for Vivus.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/718321-vivus-getting-ready-f…
Vivus: Getting Ready For FDA Approval
July 12, 2012 |
Vivus (VVUS) is a biopharmaceutical company, which mainly develops medicines for treatment of sleep apnea, diabetes, obesity and male sexual health. While its portfolio is relatively diversified, there is a great deal of interest in company's obesity treatment products. Vivus is currently awaiting final approval from FDA, for Qnexa, their most recent drug. Qnexa is a drug meant to help reduce weight. A few years ago, Qnexa was initially rejected by FDA at the first attempt. At the second presentation in this year, Qnexa got a vote of confidence by a whopping 20-2 margin by the committee.
Vivus will get a final decision about Qnexa on July, 17, 2012. If approved, Qnexa will be the second drug to be approved by FDA in weight loss category in two months. The company plans on marketing the drug by the end of year 2012. European authorities have delayed a decision on the approval of the drug. Vivus is entirely focusing on getting Qnexa approved by FDA at the moment. Currently, Lorcaserin is the only weight reduction drug approved by FDA. Arena Pharmaceuticals (ARNA) is the developer of Lorcaserin.
Clinical Background
Qnexa has already created a strong buzz in the market. Clinical studies have shown a significant weight loss in the participants. According to the results published by the company, average weight loss for top dose participants was 14.4%. Average weight loss for low dose Qnexa was 6.7% and 2.1% for placebo. Mean weight loss after week 56 was 10.9% for top dose and 5.1% for low dose. Participants who completed the top dose treatment, 83.5% lost ≥5%; 67.7% lost ≥10% and 48.1% lost ≥15% of their baseline weight. There are some adverse effect fears, due to which Vivus has to follow a labeling protocol. Qnexa will not be prescribed to the pregnant woman. Vivus plans on advising the patients to stop taking the medicine, if weight loss is less than 3% after three months.
Stock Performance
VVUS Chart

Both Vivus and Arena stocks are currently trading near its 52 week highs. Vivus stock closed at $28.47 on July, 09, 2010. The 52-week trading range for Vivus is $6.13 - $29.99. Even after the recent retreat, the stock is trading near its 52-week highs. Market cap of Vivus Inc. is $2.83 billion, slightly higher than the $2.15 billion market cap of Arena. Vivus stock has a relatively high Beta of 1.42. Trailing twelve month EPS is -0.63. One year analyst target for Vivus is $31.20.
Ratio Analysis
Estimates for the current year EPS are 40% more than the past 12 months EPS. One year forward estimate for EPS is to grow by 166%. Forward P/E is unusually high at 72.90. Current P/B ratio stands at 8.72. Firm is in an exceptionally strong liquidity position. Quick and current ratios are 25.70 and 25.92 respectively. Unlike Arena Pharmaceuticals, Vivus has no debt in its capital structure.
Pipeline Products
Vivus had Avanafil approved by FDA in April, 2012, a drug for men with erectile dysfunction. Vivus plans to sell Avanafil under the brand name Stendra. Stendra promises to work in fifteen minutes, half the time it takes the pills from rivals companies to work. That is a very short period by industry standards.
By getting Stendra approved, Vivus now stand to compete directly with giants of the industry like, Pfizer (PFE), GlaxoSmithKline (GSK) and Bayer. Analysts believe that the sales from Stendra can go up to $68 million in the next year and to $459 million by the year 2017. Erectile dysfunction market itself is currently worth $4.3 billion. Viagra, Pfizer's well-known super-charger, holds almost half of the market share. Vivus is working on getting a partnership to market Stendra as well.
Besides the existing products, Vivus wants to focus on the marketing of Qnexa, if approved. Vivus wants to preserve the entire budget to spend on the marketing of Qnexa. Obesity market is largely unexplored, and it is a large market. There will be less competition in this segment. Vivus has a good marketing network. Vivus has more than one product in the market. As such, the revenues will not be depending on only one drug in a hugely unexplored market.
If approved, Vivus plans to launch Qnexa in the later part of year 2012. On the other hand, Arena Pharmaceutical expects to market the drug in 2013. This will give Vivus Inc a head start on its main competitor, and the firm can capture a large chunk of the market. Vivus is planning to directly market the drug in the U.S. itself and seek partnerships outside the U.S. USA is an immense obesity market. Arena does not have the pure marketing rights for its own obesity drug. Only a certain portion of revenues from sales will go to Arena, whereas the rest will be taken by Aisai Pharmaceuticals, the Japanese partner. This I believe is again an advantage for Vivus over Arena.
Insider Sales
The biggest bearish argument made against Vivus is the sale of shares by three executives in the past two months. Insiders selling stock before the approval are sure to raise concerns among the investors. The main reason, which most people have been ignoring, is something less critical. Vivus executives have been selling stocks under the 10b5-1 trading plan to cover themselves legally against allegations of insider trading. CEO of Vivus recently sold 100,000 shares, but he still owns 4.3 million shares.
Summary
I think the FDA decision is almost certain, and Vivus is extremely hopeful on the approval of Qnexa. This drug will prove to be a catalyst for the success of the company. Analysts believe, after the approval, Vivus stock should be priced at $40. Vivus, currently trading below $30 presents an exciting opportunity to make tidy profits. Insider sales are worrisome, but it is hard to say that insiders are getting rid of their shares, as they hold substantial stake in the company. This still raises a red flag for interested investors. Therefore, as the stock has already made substantial gains, I would suggest a hold and buy at pullback strategy for Vivus.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
http://seekingalpha.com/article/718321-vivus-getting-ready-f…
Antwort auf Beitrag Nr.: 43.379.444 von Poppholz am 12.07.12 17:29:10Grob hatte ich 2,5 mio. Aktien. tut mir leid für Dich
wieso tut dir leid ? ich habe ja nicht alles verkauft. 1,3 millionen aktien ist noch im depot
wieso tut dir leid ? ich habe ja nicht alles verkauft. 1,3 millionen aktien ist noch im depot
Zitat von gauner1: lieber berni[/b]
ich bin kein trader......doch sind wir alle
Nach WICKIPEDIA sind wir alle Trader...-
Mit dem englischen Wort Trader wird eine Person bezeichnet, die Handel auf Finanzmärkten betreibt,
aber wenn ich was negatives sehe, gehe ich raus ....in Bezug auf ARENA also zu dem Zeitpunkt, als der Kurs von
Hedgies ( Cramer und Co) durch bewusste " Falschmeldungen "nach unten gedrückt wurde ???
und wenn die sache vorbei ist, gehe ich wieder rein....OK
schau bitte, wir wollen alle geld verdienen. YEPP
gestern raus und heute rein ( bin aber noch nicht ) macht bei mir fast 2800 dollar aus. .....SUPER !!!!
ist das nicht ein guter gewinn an 2 tagen, wo viele ein ganzer monat durch harte lediglich 2000 euro verdienen ?
....doch ein sehr guter Gewinn
wozu handeln wir denn eigentlich mit aktien ?
...um als erstes unser eingesetztes Kapital zu schützen und um eine gute Gesamtrendite zu erzielen....und da gibt es verschiedene Sichtweisen bzw Vorgehensweise
> die einen reagieren schneller und öfters auf Kursschwankungen und handeln " ad hoc", so wie DU , Earthie, Dominator etc...und das finde ich OK !!!.
. >ich setzte mein Geld nach intensiven Recherchen eines BIOTECHunternehmens ein ...ich investiere dann mein Geld in ein BIOTECH-Unternehmen , von dessen Produkt ich überzeugt bin....und der Zeitpunkt des Einstiegs liegt teilweise schon vor dem eigentlichen RUN !!!!
.. und dann sehe ich mein LONGINVESTMENT auf eine Sicht von 1-4 Jahren....und dann versuche ich damit mindestens mehr als 100 % Gewinn zu machen, was aber nicht immer klappt
daher verstehe ich deinen angriff nicht, wenn ich gut meine
...ich greife dich nicht an,wenn ich dich gefragt habe , was du hier abziehst....
...ich bin halt immer wieder überrascht darüber , wie stark emotional du dich hier so präsentierst, gerade dann ,wenn die Kurse nach unten manipuliert werden....
Ich hoffe, das Thema ist nun ausdiskutiert .....und du beziehst einfach mal die verschiedenen Wege zur möglichen Gewinnmaximierung
( "Tradies"+"Longies") mit ein.......
dann hoffe ich, dass Du die Sichtweise auch von LONGinvestierten( wie tebi, Poppie, magnetfeldfredy, cyberhexe etc.) nun besser verstehst..
..die einen verdienen ihr Geld mit " schnellem" Agieren ,die anderen mit "long" Agieren.....
Grüße
bernie55
In diesem Schreiben wird noch einmal auf die bewusste " Manipulation" von JIM CRAMER eingegangen und seine Aussagen bzgl. Belviq kritisch beleuchtet und widerlegt...
REAL PHYSICIAN: CRAMER REBUTTAL
11-Jul-12 09:14 am
A very important (but never discussed) point about anti-obesity treatment is what real physicians will prescribe for their patients in coming years. I have discussed with many of my fellow M.D.s, and almost all of us are going with BELVIQ instead of QNEXA, regardless of a CRL delay with the latter.
1) We physicians all well remember the recurrent valvulopathies and pulmonary hypertension that occurred with Fen/Fen. QNEXA will contain phentermine (the first FEN). We have a right and sacred responsibility to assess risk vs. potential benefits for every patient for which we write prescriptions. Belviq causes none of these problems (less than placebo controls) in FDA data.
2) The media (and Jim Cramer) have it all wrong as far as Belviq efficacy in weight loss and he misrepresented Belviq last night.
*****The AVERAGE WEIGHT LOSS WITH BELVIQ IS 8% OF BODY WEIGHT AMONG RESPONDERS. About 50% of patients will lose MORE than 5% of BW in 12 weeks. If not, they need to stop it. Among those that do reach this threshold, 35% lost > 10% of BW, and the top 25% of responders lost > 16.7% of BW!
3) Cramer is mistaken regarding his interpretation of FDA data. Regarding weight loss efficacy, he related BELVIQ was not placebo-controlled, and QNEXA’s data was. Wrong! He has it just backwards. BELVIQ’s data was placebo-controlled, and QNEXA was not. This makes a huge difference in accuracy of drug efficacy.
4) Cramer stated that around 60-70% of patients will not respond to BLEVIQ. Wrong again. The real answer is 47.5% will not lose more than 5%, which makes them classified as non-responders. Nevertheless, do not ever underestimate the power of 5%-8% of weight loss. It can have dramatic effects on overall health, with significant reductions in incidence of Type II diabetes mellitus, coronary vascular disease, hyperlipidemias, sleep apnea, stroke , and even malignancy reduction. Our patients will feel much better about their overall health, and can use the psychological improvement to exercise more, with reduction in pain from osteoarthritis in weight bearing joints.
5) Cramer made another blunder in attempting to use scare tactics about “hallucinations” with BELVIQ. The incidence of hallucinations is ridiculously miniscule as to the absurd. We will not go into the significantly elevated risk of cleft lip incidence in babies born of QNEXA mothers, and the mind-numbing “dopey” side effect of topiramate (called “DOPAMAX”). Who needs that? None of this was discussed by Cramer.
6) Cramer misrepresented BELVIQ’s earnings potential because of “limited numbers of Easai drug reps in America”. How hard would it be to train any drug rep about a medication that will change America’s heath?
7) Cramer misrepresented BELVIQ ‘s earnings potential because of its marketing company taking about 40% of its revenues in the United States. He neglected to mention that outside the U.S (Europe, Latin America, and Asia), ARNA will get significantly more, if not all, of the earnings.
8) Cramer said QNEXA will not need to be a scheduled prescription, and BELVIQ will. He got this just backwards as well . It is highly likely QNEXA will be scheduled. BELVIQ has yet to be determined, but far less likely as highly classified as QNEXA, meaning more restrictions on it. QNEXA will have to be mail ordered, whereas most all pharmacies will actually stock BELVIQ.
9) Finally, the ultimate blunder is that Cramer blatantly said that NO INSURANCE COMPANY WILL EVER COVER AND ANTI-OBESITY MED? Why? Because he had asked one! This is preposterous, because as we all know, covered formulary changes take time. When insurance companies can actually SAVE $, by eliminating other costly meds by addressing the root health problem (obesity), instead of its sequellae, we will have stumbled on a major solution to the cost of healthcare in America!
Sentiment : Strong Buy
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…" target="_blank" rel="nofollow ugc noopener">
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
REAL PHYSICIAN: CRAMER REBUTTAL
11-Jul-12 09:14 am
A very important (but never discussed) point about anti-obesity treatment is what real physicians will prescribe for their patients in coming years. I have discussed with many of my fellow M.D.s, and almost all of us are going with BELVIQ instead of QNEXA, regardless of a CRL delay with the latter.
1) We physicians all well remember the recurrent valvulopathies and pulmonary hypertension that occurred with Fen/Fen. QNEXA will contain phentermine (the first FEN). We have a right and sacred responsibility to assess risk vs. potential benefits for every patient for which we write prescriptions. Belviq causes none of these problems (less than placebo controls) in FDA data.
2) The media (and Jim Cramer) have it all wrong as far as Belviq efficacy in weight loss and he misrepresented Belviq last night.
*****The AVERAGE WEIGHT LOSS WITH BELVIQ IS 8% OF BODY WEIGHT AMONG RESPONDERS. About 50% of patients will lose MORE than 5% of BW in 12 weeks. If not, they need to stop it. Among those that do reach this threshold, 35% lost > 10% of BW, and the top 25% of responders lost > 16.7% of BW!
3) Cramer is mistaken regarding his interpretation of FDA data. Regarding weight loss efficacy, he related BELVIQ was not placebo-controlled, and QNEXA’s data was. Wrong! He has it just backwards. BELVIQ’s data was placebo-controlled, and QNEXA was not. This makes a huge difference in accuracy of drug efficacy.
4) Cramer stated that around 60-70% of patients will not respond to BLEVIQ. Wrong again. The real answer is 47.5% will not lose more than 5%, which makes them classified as non-responders. Nevertheless, do not ever underestimate the power of 5%-8% of weight loss. It can have dramatic effects on overall health, with significant reductions in incidence of Type II diabetes mellitus, coronary vascular disease, hyperlipidemias, sleep apnea, stroke , and even malignancy reduction. Our patients will feel much better about their overall health, and can use the psychological improvement to exercise more, with reduction in pain from osteoarthritis in weight bearing joints.
5) Cramer made another blunder in attempting to use scare tactics about “hallucinations” with BELVIQ. The incidence of hallucinations is ridiculously miniscule as to the absurd. We will not go into the significantly elevated risk of cleft lip incidence in babies born of QNEXA mothers, and the mind-numbing “dopey” side effect of topiramate (called “DOPAMAX”). Who needs that? None of this was discussed by Cramer.
6) Cramer misrepresented BELVIQ’s earnings potential because of “limited numbers of Easai drug reps in America”. How hard would it be to train any drug rep about a medication that will change America’s heath?
7) Cramer misrepresented BELVIQ ‘s earnings potential because of its marketing company taking about 40% of its revenues in the United States. He neglected to mention that outside the U.S (Europe, Latin America, and Asia), ARNA will get significantly more, if not all, of the earnings.
8) Cramer said QNEXA will not need to be a scheduled prescription, and BELVIQ will. He got this just backwards as well . It is highly likely QNEXA will be scheduled. BELVIQ has yet to be determined, but far less likely as highly classified as QNEXA, meaning more restrictions on it. QNEXA will have to be mail ordered, whereas most all pharmacies will actually stock BELVIQ.
9) Finally, the ultimate blunder is that Cramer blatantly said that NO INSURANCE COMPANY WILL EVER COVER AND ANTI-OBESITY MED? Why? Because he had asked one! This is preposterous, because as we all know, covered formulary changes take time. When insurance companies can actually SAVE $, by eliminating other costly meds by addressing the root health problem (obesity), instead of its sequellae, we will have stumbled on a major solution to the cost of healthcare in America!
Sentiment : Strong Buy
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…" target="_blank" rel="nofollow ugc noopener">
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
..die einen verdienen ihr Geld mit " schnellem" Agieren ,die anderen mit "long" Agieren.....
richtig
und ich agiere dazwischen. Seit jahren habe ich den Prinzip, dass ich aus jeder Aktie aussteige, wenn ich merke bzw. sehe, dass der kurs manipuliert wird. diesen Prinzip kann niemand verurteilen.
weiterhin ist nach meiner meinung - wenn man durch längeres beobachten des kursverlaufes- die aktie kennt, bekommt man ein gefühl für den kursverlauf. aus diesem gefühl heraus entscheidet man. auch wenn man negative zeichen sieht, steigt man halt kurzfristig aus und beim günstigeren preis steigt man wieder ein. diese arbeitsmethode - die dir nicht gefällt, was dein recht ist, ist eine strategie, die von mindestens 95% der hobbybörsianer und von börsianer durchgeführt wird- also auch von mir. und wie du siehst, kann man damit gutes geld verdienen.
richtig
und ich agiere dazwischen. Seit jahren habe ich den Prinzip, dass ich aus jeder Aktie aussteige, wenn ich merke bzw. sehe, dass der kurs manipuliert wird. diesen Prinzip kann niemand verurteilen.
weiterhin ist nach meiner meinung - wenn man durch längeres beobachten des kursverlaufes- die aktie kennt, bekommt man ein gefühl für den kursverlauf. aus diesem gefühl heraus entscheidet man. auch wenn man negative zeichen sieht, steigt man halt kurzfristig aus und beim günstigeren preis steigt man wieder ein. diese arbeitsmethode - die dir nicht gefällt, was dein recht ist, ist eine strategie, die von mindestens 95% der hobbybörsianer und von börsianer durchgeführt wird- also auch von mir. und wie du siehst, kann man damit gutes geld verdienen.
übrigens
ich habe ein aktienrückkaufprogram gestertet und habe einen teil der verkauften 2,8k bei 10,60 - fast am neusten tiefsten punkt geholt.
wichtig ist folgendes: seitdem ich hier nicht mehr gepostet habe, ist der thread hier gabz tot. kein wunder, dass auch der kurs nicht mehr so steigt wie früher.

bist du auch bei vivus dabei ? ich seit gestern 0,5k. am dienstag ist der große tag bei vivus. ich denke, dass die zulassung sicher ist, denn zumindestens bis gestern abend hat man von fda nichts gehört
ich habe ein aktienrückkaufprogram gestertet und habe einen teil der verkauften 2,8k bei 10,60 - fast am neusten tiefsten punkt geholt.
wichtig ist folgendes: seitdem ich hier nicht mehr gepostet habe, ist der thread hier gabz tot. kein wunder, dass auch der kurs nicht mehr so steigt wie früher.


bist du auch bei vivus dabei ? ich seit gestern 0,5k. am dienstag ist der große tag bei vivus. ich denke, dass die zulassung sicher ist, denn zumindestens bis gestern abend hat man von fda nichts gehört
ARNA und VVUS Vergleich:
Has Arena Pharmaceuticals Inc. (NASDAQ: ARNA) Peaked or will Biopharmaceutical Company See Greater Gains in the Near Future?
Posted on July 13, 2012 by Editor
To think that less than three months ago shares of Arena Pharmaceuticals Inc. (NASDAQ: ARNA) were trading as low as 2.00 is mind-numbing but with an FDA approval comes plenty of rewards and shareholders of the biopharmaceutical company experienced that first hand when the agency made lorcaserin, to be marked under the commercial name Belviq, the first obesity medication to win favor in 13 years.
That FDA approval came back in late June and shares climbed as high as 13.50 following the news but since that time ARNA has seen a noticeable pullback with shares falling as low as 10.08 on Thursday. While shares have managed to climb to the 11.20 mark on Friday there are concerns about where the price will go in the near future as the company could be facing some competition.
Just how likely it is that the FDA issues yet another approval for an obesity medication is yet to be seen but Vivus Inc. and their shareholders will find out next week with their Prescription Drug User Fee Act (PDUFA), or FDA `Action` date, for Qnexa, scheduled for July 17th. The overwhelming belief is that Vivus will win approval and that could spell trouble for ARNA and their shareholders.
Of course ARNA’s success isn’t dependent upon what happens on July 17th, the facts seem to support the belief that Belviq has fewer side effects than Qnexa and will be accessible to a larger market. That market is massive considering that the FDA approved lorcaserin for those who are obese or who have a body mass index (BMI) of 27 and have another risk factor like high blood pressure, high cholesterol, or diabetes. What that equates to is nearly two-thirds of the population in the United States.
As it stands there are still some concerns over what risks are presented by lorcaserin, namely how it could play a role in heart disease. While those concerns may have some merit the reality is if you are obese then you are already carrying risks of heart disease. That’s not to say that the risks of lorcaserin should be overlooked but common sense should prevail.
Whether or not Vivus and their Qnexa obesity medication can compete with ARNA’s Belviq remains to be seen but one thing is for certain, Qnexa doesn’t have the same market to work with. Even with an FDA approval Qnexa is facing tighter restrictions as IPQ Analytics, an independent research firm, has warned of “risks that exist which are not currently being factored into expectations.” This could lead to a risk management program that would accompany an approval for Qnexa, something that Belviq was able to avoid.
That could be of substantial benefit to ARNA as the risk management program for Qnexa could place restrictions on prescriptions for women of childbearing age and/or those with cardiovascular risk given the fact that the drug had been sent back for additional studies due to concerns about heart valve damage, a permanent and life-threatening side effect.
Not lost on investors is the fact that a number of insider transactions among executives at Vivus have taken place, giving some skeptics reason to believe that the company has its doubts about Qnexa and its impact on the market. While these transactions are well within the rights of executives and may simply be seen as an opportunity to grab profits the timing does appear odd.
Aside from the potential competition in the way of Qnexa, ARNA is also facing doubt from those who believe the company’s diet drug won’t earn as much as they expect. Jim Cramer has been among the most vocal on this front, predicting Arena will gain just below 40% of its revenue from treatment, substantially lower than what the company has predicted.
Without question there are legitimate concerns investors should be aware of when it comes to ARNA, namely that the company’s shares have already jumped more than 500% for the year. That leaves many to question whether or not ARNA has already peaked. With shares as high as they are right now it may make sense to wait for a pullback and if Vivus gets that approval next week there will almost certainly be a downturn for ARNA shares which could then provide an excellent opportunity for investors to build a position.
http://www.otcequity.com/?p=2331
Has Arena Pharmaceuticals Inc. (NASDAQ: ARNA) Peaked or will Biopharmaceutical Company See Greater Gains in the Near Future?
Posted on July 13, 2012 by Editor
To think that less than three months ago shares of Arena Pharmaceuticals Inc. (NASDAQ: ARNA) were trading as low as 2.00 is mind-numbing but with an FDA approval comes plenty of rewards and shareholders of the biopharmaceutical company experienced that first hand when the agency made lorcaserin, to be marked under the commercial name Belviq, the first obesity medication to win favor in 13 years.
That FDA approval came back in late June and shares climbed as high as 13.50 following the news but since that time ARNA has seen a noticeable pullback with shares falling as low as 10.08 on Thursday. While shares have managed to climb to the 11.20 mark on Friday there are concerns about where the price will go in the near future as the company could be facing some competition.
Just how likely it is that the FDA issues yet another approval for an obesity medication is yet to be seen but Vivus Inc. and their shareholders will find out next week with their Prescription Drug User Fee Act (PDUFA), or FDA `Action` date, for Qnexa, scheduled for July 17th. The overwhelming belief is that Vivus will win approval and that could spell trouble for ARNA and their shareholders.
Of course ARNA’s success isn’t dependent upon what happens on July 17th, the facts seem to support the belief that Belviq has fewer side effects than Qnexa and will be accessible to a larger market. That market is massive considering that the FDA approved lorcaserin for those who are obese or who have a body mass index (BMI) of 27 and have another risk factor like high blood pressure, high cholesterol, or diabetes. What that equates to is nearly two-thirds of the population in the United States.
As it stands there are still some concerns over what risks are presented by lorcaserin, namely how it could play a role in heart disease. While those concerns may have some merit the reality is if you are obese then you are already carrying risks of heart disease. That’s not to say that the risks of lorcaserin should be overlooked but common sense should prevail.
Whether or not Vivus and their Qnexa obesity medication can compete with ARNA’s Belviq remains to be seen but one thing is for certain, Qnexa doesn’t have the same market to work with. Even with an FDA approval Qnexa is facing tighter restrictions as IPQ Analytics, an independent research firm, has warned of “risks that exist which are not currently being factored into expectations.” This could lead to a risk management program that would accompany an approval for Qnexa, something that Belviq was able to avoid.
That could be of substantial benefit to ARNA as the risk management program for Qnexa could place restrictions on prescriptions for women of childbearing age and/or those with cardiovascular risk given the fact that the drug had been sent back for additional studies due to concerns about heart valve damage, a permanent and life-threatening side effect.
Not lost on investors is the fact that a number of insider transactions among executives at Vivus have taken place, giving some skeptics reason to believe that the company has its doubts about Qnexa and its impact on the market. While these transactions are well within the rights of executives and may simply be seen as an opportunity to grab profits the timing does appear odd.
Aside from the potential competition in the way of Qnexa, ARNA is also facing doubt from those who believe the company’s diet drug won’t earn as much as they expect. Jim Cramer has been among the most vocal on this front, predicting Arena will gain just below 40% of its revenue from treatment, substantially lower than what the company has predicted.
Without question there are legitimate concerns investors should be aware of when it comes to ARNA, namely that the company’s shares have already jumped more than 500% for the year. That leaves many to question whether or not ARNA has already peaked. With shares as high as they are right now it may make sense to wait for a pullback and if Vivus gets that approval next week there will almost certainly be a downturn for ARNA shares which could then provide an excellent opportunity for investors to build a position.
http://www.otcequity.com/?p=2331
wie hoch schätzt ihr die chance der zulassung für vvus ? bis jetzt hat man ja von fda bezüglich nachfragen, die zu einer verzögerung der zulassung führen, nichts gehört
Zitat von gauner1: ..die einen verdienen ihr Geld mit " schnellem" Agieren ,die anderen mit "long" Agieren.....
.... auch wenn man negative zeichen sieht, steigt man halt kurzfristig aus und beim günstigeren preis steigt man wieder ein. diese arbeitsmethode - die dir nicht gefällt, was dein recht ist, ist eine strategie, die von mindestens 95% der hobbybörsianer und von börsianer durchgeführt wird- also auch von mir. und wie du siehst, kann man damit gutes geld verdienen.
..also, zum Schluss kurz und knackig..
..ich verurteile diese " Arbeitsmethode" nicht !!!!!
Ich schrieb zu " dieser Arbeitsmethode"...
> .....die einen reagieren schneller und öfters auf Kursschwankungen und handeln " ad hoc", so wie DU , Earthie,
Dominator etc...und das finde ich OK !!!.
Habe ich dieser Arbeitsmethode " Gewinnmaximierung " abgesprochen ???? NEIN !!!!
Ich habe dich lediglich auf deine EMOTIONALITÄT hingewiesen,insbesondere bei DOWNs........

...mehr nicht....
und nun ist gut,gauner1...

....zurück zu den börsenalltäglichen Dingen
Grüße
bernie55

Antwort auf Beitrag Nr.: 43.387.937 von bernie55 am 15.07.12 16:31:59Ich habe dich lediglich auf deine EMOTIONALITÄT hingewiesen,insbesondere bei DOWNs........
...mehr nicht....
das stimmt, aber nur wenn ich manipulation sehe. was glaubst du, wie ich zuhause mich aufrege. du kannst mir glauben, dass während der arenazeit bis jetzt 3 tastaturen schon ihr leben verloren haben
der normaler down ist völlig normal. börse läuft nicht nur gradlinig nach oben.
wie schätzt du die chance für vivuszulassung ? wie stark könnte nach deiner einschätzung der kurs fallen, wenn vivus keine bekommt ?
im yahoo-board liest man von 50 zu 50. von den chefs wurde voriger monat über 400k aktien verkauft, warum ? daher bin ich sehr zappelig.
andererseits habe ich gelesen, dass votum des gremiums 20 zu 2 gewesen sein muss. und bis jetzt sind von fda keine nachfragen gekommen. dies gibt wieder hoffnung.
jedenfalls weiß ich nicht, was ich machen soll ? wie ist deine meinung ?
danke

...mehr nicht....
das stimmt, aber nur wenn ich manipulation sehe. was glaubst du, wie ich zuhause mich aufrege. du kannst mir glauben, dass während der arenazeit bis jetzt 3 tastaturen schon ihr leben verloren haben
der normaler down ist völlig normal. börse läuft nicht nur gradlinig nach oben.
wie schätzt du die chance für vivuszulassung ? wie stark könnte nach deiner einschätzung der kurs fallen, wenn vivus keine bekommt ?
im yahoo-board liest man von 50 zu 50. von den chefs wurde voriger monat über 400k aktien verkauft, warum ? daher bin ich sehr zappelig.
andererseits habe ich gelesen, dass votum des gremiums 20 zu 2 gewesen sein muss. und bis jetzt sind von fda keine nachfragen gekommen. dies gibt wieder hoffnung.
jedenfalls weiß ich nicht, was ich machen soll ? wie ist deine meinung ?
danke
Zitat von gauner1: Ich habe dich lediglich auf deine EMOTIONALITÄT hingewiesen,insbesondere bei DOWNs........
...mehr nicht....
das stimmt, aber nur wenn ich manipulation sehe....und die war so was von vorauszusehen...
was glaubst du, wie ich zuhause mich aufrege. du kannst mir glauben, dass während der arenazeit bis jetzt 3 tastaturen schon ihr leben verloren haben ..........uuupppssss.....
der normaler down ist völlig normal. börse läuft nicht nur gradlinig nach oben.......
Zitat von gauner1: wie schätzt du die chance für vivuszulassung ?
ich hatte dazu bereits einiges u.a auch im VVUS Board > 01.07.12 18:21:44 geschrieben...
Merrill Lynch on Lorcaserin approval hurdles
More work to be done before lorcaserin gets approved
ARNA management held an investor call this morning following yesterday’s positive advisory committee vote for lorcaserin in obesity treatment. Management indicated that it has had only preliminary discussions with FDA for any post
approval requirements for the drug. Despite ARNA’s belief that its drug has no safety signals, we expect at a minimum that the PDUFA decision date will be delayed beyond June 27th in order to develop a valvulopathy monitoring program,
a possible post approval cardiovascular outcomes trial, and potentially a cancer monitoring registry, all collectively suggested by panel members. We also expect post approval discussions regarding DEA assignment to further delay the launch. We maintain our Underperform rating with a $5 PO.
..tja, das ist das grundsätzliche Problem dieser " Biotechexperten" , medizinische statements abzugeben und daraus " nur" vage Vermutungen abzuleiten.....im übrigen traf im o.g. Fall nur eine Vorhersage ein (post marketing studies über Auswirkungen auf Her-Kreislauferkrankungen), die aber m.M. nach klar war....
....wenn man die vorherigen Prognosen vieler "ANALysten" ( Adam Feuerstein und CO.) vor dem AC und auch vor der FDA Entscheidung zu BELVIQ( Lorcaserin) gelesen hat, dann hätte man BELVIQ null Chancen einräumen müssen.......bei genaueren eigenen Recherchen ist man aber zu einem völlig anderen Meinungsbild gekommen und hat eine sehr realistische Chance zu einem Approval gesehen.....
Deshalb sehe ich Meinungen von sogenannten Biotechexperten aus der Wall-Street -Mafia immer als sehr kritisch an egal welche Aktien analysiert werden....
....es ist natürlich immer ein Spiel mit dem Feuer ( FDA und Approval), man weiß nie ob und wie ein Medikamentzugelassen wird...... aber wie schon einige hier geschrieben haben, ist der Druck auf die FDA bzgl einer Zulassung für " Obesity-Drugs" von Seiten des Kongresses sehr groß gewesen....
...ich gehe davon aus, dass QNEXA ein Approval mit Einschränkungen ( mit sehr strikten Auflagen gerade im Hinblick auf die möglichen Missbildungen bei Neugeborenen ) schaffen könnte.....
...ich denke aber auch , dass sich durch die Zulassung von BELVIQ die Ausgangssituation bzgl. Approvals bei " Obesity-drugs" grundlegend verändert hat und die FDA nicht mehr den Druck hat, "alles und nichts" zuzulassen....
Die Aktie von VIVUS ist bereits gut gelaufen und ich wüsste aktuell auch gar nicht wieviel Potential sie überhaupt noch nach oben hätte.....
TIME WILL TELL
wie stark könnte nach deiner einschätzung der kurs fallen, wenn vivus keine bekommt ?
...runter auf mindestens 10 - 15 USD.....wer bietet weniger oder mehr ???
andererseits habe ich gelesen, dass votum des gremiums 20 zu 2 gewesen sein muss. und bis jetzt sind von fda keine nachfragen gekommen.
..das hat nichts zu sagen !!! Die FDA muss sich nicht an die Abstimmungsergebnisse des ACs halten...
...die aktuelle Ausgangssituation für VIVUS ist nach der Zulassung von BELVIQ sehr schwer einzuschätzen....
dies gibt wieder hoffnung.
jedenfalls weiß ich nicht, was ich machen soll ?
..das kann ich dir nicht sagen...die Lage ist sehr sehr sehr schwer einzuschätzen...
...also wie gesagt, du kannst auf ein Approval mit sehr strikten Auflagen spekulieren....dann wirst du einen kleinen Hype mitmachen können....
...aber bedenke ,die FDA hat BELVIQ bereits zugelassen....
danke
Grüße
bernie55

Antwort auf Beitrag Nr.: 43.387.067 von Poppholz am 14.07.12 23:53:33
das ist richtig, was du in vivus-thread schreibst, aber fda kann nicht sagen, da ein ähnliches medikament zugelassen ist, wird onexa nicht zugelassen. zumindestens nicht nach dem recht
das ist richtig, was du in vivus-thread schreibst, aber fda kann nicht sagen, da ein ähnliches medikament zugelassen ist, wird onexa nicht zugelassen. zumindestens nicht nach dem recht
Antwort auf Beitrag Nr.: 43.387.067 von Poppholz am 14.07.12 23:53:33
die quote 20 zu 2 und dadurch, dass wir von fda nichts gehört haben, muss ich annehmen, dass vvus die zulassung bekomt. hoffentlich geht arena aber nicht runter, weil jetzt ein konkurenz da ist.
die quote 20 zu 2 und dadurch, dass wir von fda nichts gehört haben, muss ich annehmen, dass vvus die zulassung bekomt. hoffentlich geht arena aber nicht runter, weil jetzt ein konkurenz da ist.
die shorties sind jetzt bei vivusgurke und kaufen hoch
Zitat von gauner1: das ist richtig, was du in vivus-thread schreibst, aber fda kann nicht sagen, da ein ähnliches medikament zugelassen ist, wird onexa nicht zugelassen. zumindestens nicht nach dem recht
..das habe ich nie behauptet
 ...und das wird auch nicht die FDA behaupten...
...und das wird auch nicht die FDA behaupten...
...ich habe sinngemäß geschrieben, dass durch die Zulassung von BELVIQ ein SAFETY-Standard vorgegeben ist, der von anderen " Obesity-Medikamenten" erst mal erreicht werden muss.......und da hat Qnexa mit den Nebenwirkungen bzgl. möglicher Missbildungen bei Neugeborenen nicht gerade die besten Karten.....
Ich denke auch, dass dieser Aspekt bei der Vermarktung der Produkte eine sehr gewichtige Rolle spielen wird......
..also nochmals ...
...wenn ein APPROVAL ausgesprochen wird, dann wird dieses meiner Meinung nach mit sehr strengen Auflagen bei der Anwendung des Medikaments ( REMS )verbunden sein....
TIME WILL TELL
Antwort auf Beitrag Nr.: 43.391.102 von bernie55 am 16.07.12 17:33:05also:
meine Antwort (im VVUS Thread) war nur eine Vermutung, ein Gedanke an die Möglichkeiten aus diesem Bereich.
Nie würde ich behaupten, dass die FDA so vorgeht.
(nur um sicher zu gehen, dass kein Anwalt aus Übersee bei mir klingelt)

meine Antwort (im VVUS Thread) war nur eine Vermutung, ein Gedanke an die Möglichkeiten aus diesem Bereich.
Nie würde ich behaupten, dass die FDA so vorgeht.
(nur um sicher zu gehen, dass kein Anwalt aus Übersee bei mir klingelt)

Antwort auf Beitrag Nr.: 43.391.038 von bernie55 am 16.07.12 17:18:50ja, dann habe ich nicht richtig verstanden gehabt. ich glaube, ich müsste besser lesen.
übrigens, jetzt habe ich einen richtigen

bestehend aus vivus,arna,orex
übrigens, jetzt habe ich einen richtigen
bestehend aus vivus,arna,orex
Antwort auf Beitrag Nr.: 43.391.214 von Poppholz am 16.07.12 17:59:04
du entschuldigt dich also




alles klar

du entschuldigt dich also





alles klar


guen morgen
gestern nachbörslich ins minus geraten. 11 usd
gestern nachbörslich ins minus geraten. 11 usd
schlußkurs 11 usd
heute vorbörslich 10,49 zu 10,89
für heute sieht nicht gut aus. briefseite jetzt schon unter dem schlußkurs
heute vorbörslich 10,49 zu 10,89
für heute sieht nicht gut aus. briefseite jetzt schon unter dem schlußkurs
lars
im moment sieht besser aus
im moment sieht besser aus
 ....so,alle Augen schauen auf VIVUS....
....so,alle Augen schauen auf VIVUS....
...dann bin ich mal gespannt, ob VIVUS ohne die noch nicht beendete FORTRESS Studie ( diese soll erst im 3ten Quartal nachgeliefert werden) ein Approval seitens der FDA erhält......
TIME WILL TELL
vivus ist abgestürzt. wieso ?
Zitat von gauner1: vivus ist abgestürzt. wieso ?
...auf jeden Fall ein DOWN bis 20,91 !!!!..jetzt aktuell 26,80 USD....die HEDGIES manipulieren mal wieder......
...aber vielleicht ist schon irgendetwas durchgesickert und die noch nicht beendeten FORTRESS Studien werden ein Problem für die Zulassung von Qnexa...

>Complete Respond Letter (CRL)??
>Ablehnung oder Verzögerung ??
Zitat von bernie55:Zitat von gauner1: vivus ist abgestürzt. wieso ?
...auf jeden Fall ein DOWN bis 20,91 !!!!..jetzt aktuell 26,80 USD....die HEDGIES manipulieren mal wieder......
...aber vielleicht ist schon irgendetwas durchgesickert und die noch nicht beendeten FORTRESS Studien werden ein Problem für die Zulassung von Qnexa...
>Complete Respond Letter (CRL)??
>Ablehnung oder Verzögerung ??
..sollte sich die noch nicht beendete FORTRESS Studie als Problem für die Zulassung herausstellen, dann dürfte ARENA kursmäßig davon sicherlich profitieren....

ich versuche mit weniger verlust rauszugehen.
auch orex ist abgestürzt
bei beides pech gehabt
auch orex ist abgestürzt
bei beides pech gehabt
11% hat man runtergedrückt gehabt
schau dir den tageschart von vvus und orex an. beide eine fürchterliche shortattake
Auf der Vivus Seite ist bisher noch keine neue Nachricht veröffentlicht
Hallo. Ich denke das vivus heute scheitern koennte......ob da heute die shorts oder ein paar longs die notbremse gezogen haben ist schwer zu sagen.....denke die longs. Wenn vivus scheitert....kann der kurs bei einem th leicht bis 15dollar fallen....der rutsch eben hat gezeigt wie schnell das gehen kann. Hauptsache arena ist gruen...:-). Hallo bernie.....schoene gruesse aus xxxx :-) earthfire
Zitat von Earthfire: Hallo. Ich denke das vivus heute scheitern koennte......ob da heute die shorts oder ein paar longs die notbremse gezogen haben ist schwer zu sagen.....denke die longs. Wenn vivus scheitert....kann der kurs bei einem th leicht bis 15dollar fallen....der rutsch eben hat gezeigt wie schnell das gehen kann. Hauptsache arena ist gruen...:-).
Hallo bernie.....schoene gruesse aus xxxx :-) earthfire
Hauptsache arena ist gruen...:-). YEPP
Hi Earthie......doch noch ein bisschen Zeit fürs WO-ARENA gefunden ??

Ich hoffe, dir geht es gut und du bist fit wie ein Turnschuh...

Grüße
bernie55

Hi bernie.......habe sogar schon getradet hier vom phone.:-) aber ab 15uhr 30..bin ich werktags.....immer mit dem phone live dabei......hoffe das vivus scheitert......das waere fuer unsere arena......suuuuuuper! !! :-) gruesse und freue mich auf samstag :-)
Antwort auf Beitrag Nr.: 43.395.252 von bernie55 am 17.07.12 17:25:20
ich habe mich auf das votum von 20 zu 2 verlassen
erth
willkommen daheim
warst du mit franzisca wieder tauchen ?
ich habe mich auf das votum von 20 zu 2 verlassen
erth
willkommen daheim
warst du mit franzisca wieder tauchen ?
Zitat von Earthfire: Hi bernie.......habe sogar schon getradet hier vom phone.:-) aber ab 15uhr 30..bin ich werktags.....immer mit dem phone live dabei....
..hoffe das vivus scheitert......das waere fuer unsere arena......suuuuuuper! !! :-)
gruesse und freue mich auf samstag :-)
 ..er kanns nicht lassen...
..er kanns nicht lassen...
...."dito"...wir hören bzw. lesen uns...

Zitat von gauner1: ich habe mich auf das votum von 20 zu 2 verlassen
erth
willkommen daheim
warst du mit franzisca wieder tauchen ?
Hi gauner1,
das AC kann zwar eine Richtung vorgeben, aber die FDA muss sich nicht an diese Vorgaben halten....
....es wird auf jeden Fall spannend werden....
...ich bin froh, aktuell bei ARENA auf dem Boot zu sitzen...
...wir haben bereits das Approval !!!

...je länger sich die Ankündigung der FDA Entscheidung seitens VIVUS hinauszögert, desto mehr sehe ich die Chance auf ein mögliches Approval schwinden......einfach mal nur ein Gefühl....

...dann schauen wir mal....

Antwort auf Beitrag Nr.: 43.395.303 von gauner1 am 17.07.12 17:35:17momentan ist noch nichts entschieden, bzw. bekannt gegeben.
Aktuell wird der Kurs ausgetestet. Shorties stellen glatt bzw. versuchen durch eine solche Attake den Kurs nach unten zu jagen, da wahrscheinlich viele mit STOP LOSS gearbeitet haben. Allerdings werden die jetzt wohl draußen sein.
Wenn die Zulassung nicht kommt wird es einen ordentlichen Rutsch geben, da dann viele raus gehen, um gegebenenfalls unten wieder einzusteigen.
Dann bin ich vielleicht auch dabei.

Aktuell wird der Kurs ausgetestet. Shorties stellen glatt bzw. versuchen durch eine solche Attake den Kurs nach unten zu jagen, da wahrscheinlich viele mit STOP LOSS gearbeitet haben. Allerdings werden die jetzt wohl draußen sein.
Wenn die Zulassung nicht kommt wird es einen ordentlichen Rutsch geben, da dann viele raus gehen, um gegebenenfalls unten wieder einzusteigen.
Dann bin ich vielleicht auch dabei.

Antwort auf Beitrag Nr.: 43.395.441 von Poppholz am 17.07.12 18:03:32mit ARNA sind wir auf der "sicheren" Seite:
Bei einer Nichtzulassung bei VVUS wird der Kurs bei uns steigen.
Bei einer Zulassung gegebenenfalls um 10% fallen, die aber in den nächsten Tagen wieder aufgeholt werden wird.
(meine Meinung)

Bei einer Nichtzulassung bei VVUS wird der Kurs bei uns steigen.
Bei einer Zulassung gegebenenfalls um 10% fallen, die aber in den nächsten Tagen wieder aufgeholt werden wird.
(meine Meinung)

Antwort auf Beitrag Nr.: 43.395.252 von bernie55 am 17.07.12 17:25:20
ich verstehe nicht, warum der kurs nicht ausgesetzt wird. jedenfalls wollte ich die nervenbelastung wie bei arena nicht diesmal mitmachen. bin mir geringer verlust raus, ich meine aus vivus
ich verstehe nicht, warum der kurs nicht ausgesetzt wird. jedenfalls wollte ich die nervenbelastung wie bei arena nicht diesmal mitmachen. bin mir geringer verlust raus, ich meine aus vivus
Antwort auf Beitrag Nr.: 43.395.491 von gauner1 am 17.07.12 18:13:04die Entscheidung der FDA soll (glaube ich) erst heute Abend kommen.
Vielleicht erst nachbörslich, dann ist keine Aussetzung nötig.
Vielleicht erst nachbörslich, dann ist keine Aussetzung nötig.
Antwort auf Beitrag Nr.: 43.395.409 von bernie55 am 17.07.12 17:56:21je länger sich die Ankündigung der FDA Entscheidung seitens VIVUS hinauszögert, desto mehr sehe ich die Chance auf ein mögliches Approval schwinden......einfach mal nur ein Gefühl....
gerade aus diesem grund ist mir murmellig geworden und habe vivus verkauft. es muss nicht sein, dass man seine nerven so blastet

gerade aus diesem grund ist mir murmellig geworden und habe vivus verkauft. es muss nicht sein, dass man seine nerven so blastet
halbe stunde nach dem verkauf ist die gurke auf 28 usd gesteigen. ich wäre jetzt sogar im guten gewinn gewesen. so kann einem gehen. meine tastatur hat glück gehabt
die zulassung könnte erteilt sein. wieso steigt der so plötzlich ?
Antwort auf Beitrag Nr.: 43.395.579 von gauner1 am 17.07.12 18:31:01wenn die Zulassung da ist, dann geht der Kurs min. 20% hoch.
Jetzt stellen alle wieder glatt, die vorhin den Abverkauf gestartet haben.
Wahrscheinich sind wir gleich sogar GRÜN.
Damit sollen wieder Käufer in die Aktie gelockt werden.
Zulassung oder nicht, das entscheidet sich alles noch.

Jetzt stellen alle wieder glatt, die vorhin den Abverkauf gestartet haben.
Wahrscheinich sind wir gleich sogar GRÜN.
Damit sollen wieder Käufer in die Aktie gelockt werden.
Zulassung oder nicht, das entscheidet sich alles noch.

ich werde jetzt mit sb arbeiten 30 usd
und wieso steigt der kurs so enorm. was durchgesickert ? könnte sein
ganz proportional wie vivus gestiegen ist, ist arena gefallen
..so gegen 18.30 Uhr haben wir von dem APRROVAL von BELVIQ erfahren....
...aktuell ist von Vivus noch nichts zu hören....
...aktuell ist von Vivus noch nichts zu hören....
Hier mal ein Bild vom Ausschuß, daher zur Zeit auch noch alles schwebend








wahrscheinlich ist für vivius die zulassung so sicher, dass man den kurs nicht ausgesetzt hat
Antwort auf Beitrag Nr.: 43.396.090 von LarsKol am 17.07.12 20:40:02
mach keine scherze mit mir, heute bin ich schlecht drauf

mach keine scherze mit mir, heute bin ich schlecht drauf


Antwort auf Beitrag Nr.: 43.396.056 von Wandenfels2 am 17.07.12 20:29:00....also, falls wirklich die Vorankündigung von dem Artikel der US TODAY und anschließend dieser Bericht von ADAM FUCKELSTONE sich bewahrheiten sollte, dann denke ich "hat die FDA fertig"....
WIE KÖNNEN VORAB SCHON ERGEBNISSE BZGL. DES APPROVALS VERÖFFENTLCIHT WERDEN, OBWOHL SOWOHL DIE FDA ALS AUCH VIVUS NOCHT NICHTS GEMELDET HABEN ???
Vivus Weight-Loss Pill Approved -- Maybe?
By Adam Feuerstein 07/17/12 - 02:11 PM EDT
MOUNTAIN VIEW, Calif. (TheStreet) -- U.S. regulators have apparently approved Vivus'(VVUS_) weight-loss pill Qsymia on Tuesday, which means obese Americans now have a choice in prescription diet pills.
A story touting Qsymia's approval was published prematurely by USA Today earlier today. The story, since removed from the newspaper's web site, quotes Vivus executives and doctors about Qsymia and its approval. Clearly, the reporter wrote the story under embargo with Vivus' cooperation but was not supposed to publish until after an official announcement from FDA that the weight-loss pill had been approved.
The story, since removed from the newspaper's web site, quotes Vivus executives and doctors about Qsymia and its approval. Clearly, the reporter wrote the story under embargo with Vivus' cooperation but was not supposed to publish until after an official announcement from FDA that the weight-loss pill had been approved.
Neither Vivus nor officials from the U.S. Food and Drug Administration have made made any official comment.
Vivus shares are down 1% to $28.30 in recent trading, which has been volatile throughout the day.
So, FDA has now reportedly approved two new medicines to treat obesity in the past three weeks , ending a 13-year drought. The first pill, Arena Pharmaceuticals'(ARNA_) Belviq, was approved on June 27.
, ending a 13-year drought. The first pill, Arena Pharmaceuticals'(ARNA_) Belviq, was approved on June 27.
Approximately one-third of adult Americans are obese today, according to the Centers for Disease Control. Obesity is growing public health problem in the U.S., in part because people who carry unhealthy weight are at greater risk for heart disease, diabetes and other diseases.
Qsymia, formerly known as Qnexa, combines two currently approved drugs -- phentermine, a generic weight loss drug, and topiramate, used to treat epilepsy and migraines.
FDA reportedly approved Qsymia based on data from clinical trials showing the drug helps patients lose approximately 10% of their baseline weight after one year. Similarly, 60-75% of Qsymia-treated patients lost at least 5% of their body weight after one year.
based on data from clinical trials showing the drug helps patients lose approximately 10% of their baseline weight after one year. Similarly, 60-75% of Qsymia-treated patients lost at least 5% of their body weight after one year.
Women who take Qsymia while pregnant are at risk for giving birth to children with birth defects. For this reason, FDA has reportedly placed warnings on Qsymia's label urging women to use birth control while taking the weight-loss drug.
Vivus will conduct a post-approval cardiovascular outcomes study of Qnexa in order to further demonstrate that long-term use of the drug doesn't increase the risk of heart-related side effects.
in order to further demonstrate that long-term use of the drug doesn't increase the risk of heart-related side effects.
Qysmia reportedly received U.S. approval on its second attempt. FDA rejected the drug in 2010 due to safety concerns. A subsequent FDA advisory panel held in February voted 20-2 to recommend the drug's approval.
Arena shares were down 3% to $10.80 in recent trading. Shares of Orexigen Therapeutics(OREX_), developer of a third weight-loss pill still in a late-stage clinical trial, were down 3% to $7.17.
--Written by Adam Feuerstein in Boston.
http://www.thestreet.com/story/11620859/1/vivus-weight-loss-…" target="_blank" rel="nofollow ugc noopener">
http://www.thestreet.com/story/11620859/1/vivus-weight-loss-…
WIE KÖNNEN VORAB SCHON ERGEBNISSE BZGL. DES APPROVALS VERÖFFENTLCIHT WERDEN, OBWOHL SOWOHL DIE FDA ALS AUCH VIVUS NOCHT NICHTS GEMELDET HABEN ???
Vivus Weight-Loss Pill Approved -- Maybe?
By Adam Feuerstein 07/17/12 - 02:11 PM EDT
MOUNTAIN VIEW, Calif. (TheStreet) -- U.S. regulators have apparently approved Vivus'(VVUS_) weight-loss pill Qsymia on Tuesday, which means obese Americans now have a choice in prescription diet pills.
A story touting Qsymia's approval was published prematurely by USA Today earlier today.
 The story, since removed from the newspaper's web site, quotes Vivus executives and doctors about Qsymia and its approval. Clearly, the reporter wrote the story under embargo with Vivus' cooperation but was not supposed to publish until after an official announcement from FDA that the weight-loss pill had been approved.
The story, since removed from the newspaper's web site, quotes Vivus executives and doctors about Qsymia and its approval. Clearly, the reporter wrote the story under embargo with Vivus' cooperation but was not supposed to publish until after an official announcement from FDA that the weight-loss pill had been approved.
Neither Vivus nor officials from the U.S. Food and Drug Administration have made made any official comment.
Vivus shares are down 1% to $28.30 in recent trading, which has been volatile throughout the day.
So, FDA has now reportedly approved two new medicines to treat obesity in the past three weeks
 , ending a 13-year drought. The first pill, Arena Pharmaceuticals'(ARNA_) Belviq, was approved on June 27.
, ending a 13-year drought. The first pill, Arena Pharmaceuticals'(ARNA_) Belviq, was approved on June 27.Approximately one-third of adult Americans are obese today, according to the Centers for Disease Control. Obesity is growing public health problem in the U.S., in part because people who carry unhealthy weight are at greater risk for heart disease, diabetes and other diseases.
Qsymia, formerly known as Qnexa, combines two currently approved drugs -- phentermine, a generic weight loss drug, and topiramate, used to treat epilepsy and migraines.
FDA reportedly approved Qsymia
 based on data from clinical trials showing the drug helps patients lose approximately 10% of their baseline weight after one year. Similarly, 60-75% of Qsymia-treated patients lost at least 5% of their body weight after one year.
based on data from clinical trials showing the drug helps patients lose approximately 10% of their baseline weight after one year. Similarly, 60-75% of Qsymia-treated patients lost at least 5% of their body weight after one year.Women who take Qsymia while pregnant are at risk for giving birth to children with birth defects. For this reason, FDA has reportedly placed warnings on Qsymia's label urging women to use birth control while taking the weight-loss drug.
Vivus will conduct a post-approval cardiovascular outcomes study of Qnexa
 in order to further demonstrate that long-term use of the drug doesn't increase the risk of heart-related side effects.
in order to further demonstrate that long-term use of the drug doesn't increase the risk of heart-related side effects.Qysmia reportedly received U.S. approval on its second attempt. FDA rejected the drug in 2010 due to safety concerns. A subsequent FDA advisory panel held in February voted 20-2 to recommend the drug's approval.
Arena shares were down 3% to $10.80 in recent trading. Shares of Orexigen Therapeutics(OREX_), developer of a third weight-loss pill still in a late-stage clinical trial, were down 3% to $7.17.
--Written by Adam Feuerstein in Boston.
http://www.thestreet.com/story/11620859/1/vivus-weight-loss-…" target="_blank" rel="nofollow ugc noopener">
http://www.thestreet.com/story/11620859/1/vivus-weight-loss-…
Antwort auf Beitrag Nr.: 43.396.134 von bernie55 am 17.07.12 20:54:49
wenn die zulassung erteilt ist, dann ist der kurs entäuschend
wenn die zulassung erteilt ist, dann ist der kurs entäuschend
Ich würd sagen, wenn dem allem so ist, brennt da gerade ganz kräftig die Luft
ich bin der Meinung, dass ich das in den letzten Tagen gelesen habe.
Kann Dir jetzt aber auch keine Quelle nennen.

Kann Dir jetzt aber auch keine Quelle nennen.

Antwort auf Beitrag Nr.: 43.395.805 von gauner1 am 17.07.12 19:26:55das stimmt.
Passt mir meiner Einschätzung was die Zulassung angeht.
Schlecht für VVUS ist gut für ARNA und umgekehrt.

Passt mir meiner Einschätzung was die Zulassung angeht.
Schlecht für VVUS ist gut für ARNA und umgekehrt.

Antwort auf Beitrag Nr.: 43.396.134 von bernie55 am 17.07.12 20:54:49Feuerstein hat doch schon in der Überschrift "MAYBE" geschrieben.
Ist echt erschreckend.
Noch ist gar nichts bekannt.

Ist echt erschreckend.
Noch ist gar nichts bekannt.

Antwort auf Beitrag Nr.: 43.395.612 von Poppholz am 17.07.12 18:38:22der Kursverlauf ist so gekommen wie ich vermutet habe:
Kurz in den grünen Bereich und dann wieder runter.
Mal sehen wann wir Daten bekommen.

Einfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
Kurz in den grünen Bereich und dann wieder runter.
Mal sehen wann wir Daten bekommen.

wer hätte gedacht, dass vivus nach der zulassung um 7 % fällt, sicher niemand, außer orphus
26,51
minus 7,64%

minus 7,64%


und nachbörslich fällt weiter
eine echte gurke
eine echte gurke
Antwort auf Beitrag Nr.: 43.396.414 von gauner1 am 17.07.12 22:00:45kann mir nicht vorstellen, dass die Zulassung da ist.
Dem Artikel von Feuerstein glaube ich nicht.
Zu viel "Maybe".
Dafür ist er (für meinen Geschmack) in der Vergangenheit nicht gerade seriös rüber gekommen.
ARNA ist fast auf Vortagesniveau gestiegen.

Dem Artikel von Feuerstein glaube ich nicht.
Zu viel "Maybe".
Dafür ist er (für meinen Geschmack) in der Vergangenheit nicht gerade seriös rüber gekommen.
ARNA ist fast auf Vortagesniveau gestiegen.

Antwort auf Beitrag Nr.: 43.396.432 von gauner1 am 17.07.12 22:03:37schau Dir mal die Ordergröße im nachbörslichen Bereich an:
http://www.nasdaq.com/symbol/vvus/after-hours
After Hours
Time (ET) After Hours
Price After Hours
Share Volume
16:15 $ 26 100
16:15 $ 27.593 High 181,767
16:15 $ 26 200
16:14 $ 26 100
16:14 $ 26 200
16:14 $ 25.99 100
16:14 $ 25.96 100
16:14 $ 27.55 181,767
16:12 $ 26.47 104
16:12 $ 25.98 714
16:12 $ 26 100
http://www.nasdaq.com/symbol/vvus/after-hours
After Hours
Time (ET) After Hours
Price After Hours
Share Volume
16:15 $ 26 100
16:15 $ 27.593 High 181,767
16:15 $ 26 200
16:14 $ 26 100
16:14 $ 26 200
16:14 $ 25.99 100
16:14 $ 25.96 100
16:14 $ 27.55 181,767
16:12 $ 26.47 104
16:12 $ 25.98 714
16:12 $ 26 100
Antwort auf Beitrag Nr.: 43.396.496 von Poppholz am 17.07.12 22:18:05das ist schon ein echtes Haifischbecken in dem die VVUS-Aktionäre spielen.


Antwort auf Beitrag Nr.: 43.396.439 von Poppholz am 17.07.12 22:04:50
eins ist aber sicher. wenn die beiden pillen vermarktet werden, werden die kurse auch enorm steigen. die beiden haben also viel potential. also eine longi-sache, jedoch nicht für mich
eins ist aber sicher. wenn die beiden pillen vermarktet werden, werden die kurse auch enorm steigen. die beiden haben also viel potential. also eine longi-sache, jedoch nicht für mich
vielleicht ist der kurs von vvus deswegen so stark gefallen, weil einfach nichts gekommen ist und dann hat man auf negative entscheidung der fda spekuliert, jedenfalls ich bin froh, dass ich zwar mit verlust, jedoch raus bin
Zitat von gauner1: vielleicht ist der kurs von vvus deswegen so stark gefallen, weil einfach nichts gekommen ist und dann hat man auf negative entscheidung der fda spekuliert, jedenfalls ich bin froh, dass ich zwar mit verlust, jedoch raus bin
...heute, am 17.07.12, sollte die FDA über die ZULA von Qnexa entschieden haben......
Für Vivus Aktionäre stellt sich nun die Frage :
Wird die noch nicht beendete FORTRESS Studie (diese soll erst im 3ten Quartal nachgeliefert werden) sich als Hindernis für ein mögliches Approval herausstellen ???
N
FDA NEWS RELEASE
For Immediate Release: July 17, 2012
Media Inquiries: Morgan Liscinsky, 301-796-0397; morgan.liscinsky@fda.hhs.gov
Consumer Inquiries: 888-INFO-FDA
FDA approves weight-management drug Qsymia
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/uc…
FDA NEWS RELEASE
For Immediate Release: July 17, 2012
Media Inquiries: Morgan Liscinsky, 301-796-0397; morgan.liscinsky@fda.hhs.gov
Consumer Inquiries: 888-INFO-FDA
FDA approves weight-management drug Qsymia
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/uc…
Antwort auf Beitrag Nr.: 43.396.812 von franzisca am 18.07.12 01:47:34Zulassung für Vivus nur mit hohen Auflagen:
..
Vivus Announces FDA Approval of Once Daily Qsymia™ (Phentermine and Topiramate Extended-release) Capsules CIV
THE FIRST ONCE DAILY COMBINATION TREATMENT FOR CHRONIC WEIGHT MANAGEMENT IN ADULTS WHO ARE OBESE OR OVERWEIGHT WITH A WEIGHT-RELATED COMORBIDITY
Press Release: VIVUS, Inc. – 3 hours ago.. .
.
Email
1
Print
... .
.
Companies:.
.
.VIVUS Inc.
.
.
.
RELATED QUOTES.
.
Symbol
Price
Change
VVUS
26.46
-2.24
.
.
..
.
.
..
.
.
MOUNTAIN VIEW, Calif., July 17, 2012 /PRNewswire/ -- VIVUS, Inc. (VVUS) today announced that the U.S. Food and Drug Administration (FDA) has approved Qsymia™ (pronounced Kyoo sim ee' uh) as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index (BMI) of 30 or greater (obese), or 27 or greater (overweight) in the presence of at least one weight-related comorbidity, such as hypertension, type 2 diabetes mellitus or high cholesterol (dyslipidemia).
"Qsymia is the first FDA-approved once daily combination treatment for patients struggling with obesity," said Peter Tam, President of VIVUS. "The degree and severity of obesity and the lack of effective pharmacological interventions that we face as a society were two primary reasons for the development of Qsymia. We are pleased with FDA's decision today because patients and physicians now have another treatment option available to them. It is expected that Qsymia will be available in the fourth quarter of 2012."
The safety and efficacy of Qsymia were evaluated in two multicenter, phase 3 trials that included severely obese patients (the EQUIP study), and overweight or obese patients with at least two weight-related comorbidities, such as hypertension, hypertriglyceridemia, type 2 diabetes, or central adiposity (the CONQUER study). The average weight loss in EQUIP was 10.9% on Qsymia 15 mg/92 mg and 1.6% for placebo (ITT-LOCF, p<0.0001). The average weight loss in CONQUER was 9.8% on Qsymia 15 mg/92 mg, 7.8% on Qsymia 7.5 mg/46 mg and 1.2% for placebo (ITT-LOCF, p<0.0001).
The most common adverse reactions for patients treated with Qsymia included tingling sensation of hands and feet, dizziness, altered taste, insomnia, constipation and dry mouth.
Qsymia was approved with a Risk Evaluation and Mitigation Strategy (REMS) with a goal of informing prescribers and female patients of reproductive potential about an increased risk of orofacial clefts in infants exposed to Qsymia during the first trimester of pregnancy, the importance of pregnancy prevention for females of reproductive potential receiving Qsymia and the need to discontinue Qsymia immediately if pregnancy occurs. The Qsymia REMS program includes a Medication Guide, Healthcare Provider training, distribution through certified pharmacies, implementation system and a time table for assessments.
As part of the approval of Qsymia, VIVUS is committed to conduct post-marketing studies. The company will conduct a study to assess the long-term treatment effect of Qsymia on the incidence of major adverse cardiovascular events in overweight and obese subjects with confirmed cardiovascular disease, studies to assess the safety and efficacy of Qsymia for weight management in obese pediatric and adolescent subjects, studies to assess drug utilization and pregnancy exposure, a study to assess renal function, as well as animal and in vitro studies.
For more information about Qsymia, go to www.Qsymia.com or for full prescribing information go to http://vivus.com/docs/QsymiaPI.pdf.
Note to Investors
VIVUS will hold a conference call to discuss this update tomorrow, July 18, 2012, beginning at 5:30 a.m. Pacific Time / 8:30 a.m. Eastern Time. You can listen to this call by dialing toll free 877-359-2916 or outside the U.S. at 224-357-2386. A 30-day archive of the call can be accessed at http://ir.vivus.com/.
..
Vivus Announces FDA Approval of Once Daily Qsymia™ (Phentermine and Topiramate Extended-release) Capsules CIV
THE FIRST ONCE DAILY COMBINATION TREATMENT FOR CHRONIC WEIGHT MANAGEMENT IN ADULTS WHO ARE OBESE OR OVERWEIGHT WITH A WEIGHT-RELATED COMORBIDITY
Press Release: VIVUS, Inc. – 3 hours ago.. .
.
1
... .
.
Companies:.
.
.VIVUS Inc.
.
.
.
RELATED QUOTES.
.
Symbol
Price
Change
VVUS
26.46
-2.24
.
.
..
.
.
..
.
.
MOUNTAIN VIEW, Calif., July 17, 2012 /PRNewswire/ -- VIVUS, Inc. (VVUS) today announced that the U.S. Food and Drug Administration (FDA) has approved Qsymia™ (pronounced Kyoo sim ee' uh) as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index (BMI) of 30 or greater (obese), or 27 or greater (overweight) in the presence of at least one weight-related comorbidity, such as hypertension, type 2 diabetes mellitus or high cholesterol (dyslipidemia).
"Qsymia is the first FDA-approved once daily combination treatment for patients struggling with obesity," said Peter Tam, President of VIVUS. "The degree and severity of obesity and the lack of effective pharmacological interventions that we face as a society were two primary reasons for the development of Qsymia. We are pleased with FDA's decision today because patients and physicians now have another treatment option available to them. It is expected that Qsymia will be available in the fourth quarter of 2012."
The safety and efficacy of Qsymia were evaluated in two multicenter, phase 3 trials that included severely obese patients (the EQUIP study), and overweight or obese patients with at least two weight-related comorbidities, such as hypertension, hypertriglyceridemia, type 2 diabetes, or central adiposity (the CONQUER study). The average weight loss in EQUIP was 10.9% on Qsymia 15 mg/92 mg and 1.6% for placebo (ITT-LOCF, p<0.0001). The average weight loss in CONQUER was 9.8% on Qsymia 15 mg/92 mg, 7.8% on Qsymia 7.5 mg/46 mg and 1.2% for placebo (ITT-LOCF, p<0.0001).
The most common adverse reactions for patients treated with Qsymia included tingling sensation of hands and feet, dizziness, altered taste, insomnia, constipation and dry mouth.
Qsymia was approved with a Risk Evaluation and Mitigation Strategy (REMS) with a goal of informing prescribers and female patients of reproductive potential about an increased risk of orofacial clefts in infants exposed to Qsymia during the first trimester of pregnancy, the importance of pregnancy prevention for females of reproductive potential receiving Qsymia and the need to discontinue Qsymia immediately if pregnancy occurs. The Qsymia REMS program includes a Medication Guide, Healthcare Provider training, distribution through certified pharmacies, implementation system and a time table for assessments.
As part of the approval of Qsymia, VIVUS is committed to conduct post-marketing studies. The company will conduct a study to assess the long-term treatment effect of Qsymia on the incidence of major adverse cardiovascular events in overweight and obese subjects with confirmed cardiovascular disease, studies to assess the safety and efficacy of Qsymia for weight management in obese pediatric and adolescent subjects, studies to assess drug utilization and pregnancy exposure, a study to assess renal function, as well as animal and in vitro studies.
For more information about Qsymia, go to www.Qsymia.com or for full prescribing information go to http://vivus.com/docs/QsymiaPI.pdf.
Note to Investors
VIVUS will hold a conference call to discuss this update tomorrow, July 18, 2012, beginning at 5:30 a.m. Pacific Time / 8:30 a.m. Eastern Time. You can listen to this call by dialing toll free 877-359-2916 or outside the U.S. at 224-357-2386. A 30-day archive of the call can be accessed at http://ir.vivus.com/.
morgen
vivus und arena nachbörslich weit gefallen
vvus 24,64 minus 14,12%
arna 10,25 minus 7,74
orex nachbörslich stark gestiegen von 4% minus ins 2,7 %
vivus und arena nachbörslich weit gefallen
vvus 24,64 minus 14,12%
arna 10,25 minus 7,74
orex nachbörslich stark gestiegen von 4% minus ins 2,7 %
Antwort auf Beitrag Nr.: 43.396.920 von gauner1 am 18.07.12 07:19:44die after Hour Kurse werden mit kleinen Order rauf oder runter geschoben.
Entscheidend ist der Kurs um 15:30 Uhr (bei uns).
Wir werden sehen.

Entscheidend ist der Kurs um 15:30 Uhr (bei uns).
Wir werden sehen.

Antwort auf Beitrag Nr.: 43.397.088 von Poppholz am 18.07.12 08:43:40
lieber popholz
der umsatz bei arena war nachbörslich über 1,4 millionen. sonst immer zwischen 30-100k. der nachbörslicher umsatz ist ungemein viel. hat bis jetzt so einen umsatz nie gegeben gehabt. also wir dürfen nicht in die eigene tasche lügen
das selbe gilt auch bei vvus. nachbörslicher umsatz ca. 20-30k. gestern 770K
lieber popholz
der umsatz bei arena war nachbörslich über 1,4 millionen. sonst immer zwischen 30-100k. der nachbörslicher umsatz ist ungemein viel. hat bis jetzt so einen umsatz nie gegeben gehabt. also wir dürfen nicht in die eigene tasche lügen
das selbe gilt auch bei vvus. nachbörslicher umsatz ca. 20-30k. gestern 770K
der kurs von vvus ist ausgesetzt worden. wer weiß, was kommt 15 usd oder 35 usd. letztere glaube ich viel weniger
vorbörsliche kurse von vvus sind da
28 zu 40
sieht nicht schlecht aus
28 zu 40
sieht nicht schlecht aus
Zitat von Magnetfeldfredy: Zulassung für Vivus nur mit hohen Auflagen:
Vivus Announces FDA Approval of Once Daily Qsymia™ (Phentermine and Topiramate Extended-release) Capsules CIV
THE FIRST ONCE DAILY COMBINATION TREATMENT FOR CHRONIC WEIGHT MANAGEMENT IN ADULTS WHO ARE OBESE OR OVERWEIGHT WITH A WEIGHT-RELATED COMORBIDITY
It is expected that Qsymia will be available in the fourth quarter of 2012."
The most common adverse reactions for patients treated with Qsymia included tingling sensation of hands and feet, dizziness, altered taste, insomnia, constipation and dry mouth.
Qsymia was approved with a Risk Evaluation and Mitigation Strategy (REMS) with a goal of informing prescribers and female patients of reproductive potential about an increased risk of orofacial clefts in infants exposed to Qsymia during the first trimester of pregnancy, the importance of pregnancy prevention for females of reproductive potential receiving Qsymia and the need to discontinue Qsymia immediately if pregnancy occurs.
The Qsymia REMS program includes
>a Medication Guide,
>Healthcare Provider training,
>distribution through certified pharmacies,
>implementation system and
>a time table for assessments.
As part of the approval of Qsymia, VIVUS is committed to conduct post-marketing studies.
> The company will conduct a study to assess the long-term treatment effect of Qsymia on the incidence of major adverse cardiovascular events in overweight and obese subjects with confirmed cardiovascular disease,
> studies to assess the safety and efficacy of Qsymia for weight management in obese pediatric and adolescent subjects,
> studies to assess drug utilization and pregnancy exposure,
> a study to assess renal function, as well as animal and in vitro studies.
..das APPROVAL mit post approval studies und mit REMS ist nun doch da.....
....wegen des Risikos möglicher Missbildungen bei Neugeborenen wird die Einnahme von Qsympia bei der " Risikogruppe" prophylaktisch sehr stark zu kontrollieren sein...
..wie " legitimierte" Ärzte mit dieser Ausgangssituation umgehen werden, wird sich zeigen.....
...ich kann mir einfach nicht vorstellen, dass erfahrene " Obesity-Docs" die damit verbundenen " Aufwendungen" durchführen zumal mit BELVIQ bzgl. Safety-Standards andere Möglichkeiten gegeben sind..
TIME WILL TELL
Antwort auf Beitrag Nr.: 43.397.765 von bernie55 am 18.07.12 10:48:11...ich kann mir einfach nicht vorstellen, dass erfahrene " Obesity-Docs" die damit verbundenen " Aufwendungen" durchführen zumal mit BELVIQ bzgl. Safety-Standards andere Möglichkeiten gegeben sind..
ganz richtig
ganz richtig
Antwort auf Beitrag Nr.: 43.397.765 von bernie55 am 18.07.12 10:48:11
können hohe bid/ask bei vvus fakeorder sein ? denn wieso ist der kurs um 14% gefallen ? denn diese zulassung mit auflagen war ja - sagen wir ab 20 uhr den amies bekannt. und trotzdem nachbörslich mit hohem umsatz weiter stark gefallen.
zur zeit 28,01 zu 28,02
können hohe bid/ask bei vvus fakeorder sein ? denn wieso ist der kurs um 14% gefallen ? denn diese zulassung mit auflagen war ja - sagen wir ab 20 uhr den amies bekannt. und trotzdem nachbörslich mit hohem umsatz weiter stark gefallen.
zur zeit 28,01 zu 28,02
Antwort auf Beitrag Nr.: 43.397.227 von gauner1 am 18.07.12 09:17:34wir hatten aber auch schon 4 Order die jeweils über 180.000 Stück lagen.
wir werden sehen.
ARNA wird um die $10,00 eröffnen.
wir werden sehen.
ARNA wird um die $10,00 eröffnen.
Antwort auf Beitrag Nr.: 43.397.684 von gauner1 am 18.07.12 10:34:08aktuell $28,01 zu $28,02
Antwort auf Beitrag Nr.: 43.397.864 von gauner1 am 18.07.12 11:05:15der Handel in den USA ist aktuell ausgesetzt, vor Handelseröffnung wird eine Pressekonferenz gehalten. Pre-market quotes gibt es somit nicht, abwarten ist angesagt. Belastbare BID-ASK quotes gibt es aktuell nicht.
Die Kurs kam gestern nachbörslich stark unter Druck, weil bis 7 pm ET (!) kein offizielles Statement der FDA vorlag und Anleger stark verunsichert waren.
Zwischenzeitlich treffen die ersten Analysten-Upgrades ein, T. Wei von Jefferies - der größte Kritiker - hat seine Vorbehalte aufgegeben und ist von underperform 9 USD zu hold 31 umgeschwenkt, JMP sieht das Kursziel bei 56 USD, Rodman bei 52.
Die Kurs kam gestern nachbörslich stark unter Druck, weil bis 7 pm ET (!) kein offizielles Statement der FDA vorlag und Anleger stark verunsichert waren.
Zwischenzeitlich treffen die ersten Analysten-Upgrades ein, T. Wei von Jefferies - der größte Kritiker - hat seine Vorbehalte aufgegeben und ist von underperform 9 USD zu hold 31 umgeschwenkt, JMP sieht das Kursziel bei 56 USD, Rodman bei 52.
Antwort auf Beitrag Nr.: 43.397.939 von leutzsch2001 am 18.07.12 11:20:28Der Handel ist sowieso "gefakt" und heute und einige Tage total manipuliert!
Arena ohne Stop/Loss ist wichtig auf Wochen/Monate wird sich das Potential mit EU/ROW-Partner herauskristallisieren!
Arena ohne Stop/Loss ist wichtig auf Wochen/Monate wird sich das Potential mit EU/ROW-Partner herauskristallisieren!

Antwort auf Beitrag Nr.: 43.397.939 von leutzsch2001 am 18.07.12 11:20:28
von welcher aktie redest du ? von vvus ? dann gehe hier darauf. da siehst du vorbörsliche kurs
http://datasvr.tradearca.com/arcadataserver/ArcaBookData.php…
von welcher aktie redest du ? von vvus ? dann gehe hier darauf. da siehst du vorbörsliche kurs
http://datasvr.tradearca.com/arcadataserver/ArcaBookData.php…
Antwort auf Beitrag Nr.: 43.398.080 von Magnetfeldfredy am 18.07.12 11:50:05
glaube ich auch, dass die vorbörsliche kurse bei vvus gefakt sind. wahrscheinlich gibt daher ffm keine kurse an.
langfristig sind arna und vvus beide seh sehr gut. kurzfristig schon vorbei.
kurzfristig ist jetzt orex
glaube ich auch, dass die vorbörsliche kurse bei vvus gefakt sind. wahrscheinlich gibt daher ffm keine kurse an.
langfristig sind arna und vvus beide seh sehr gut. kurzfristig schon vorbei.
kurzfristig ist jetzt orex
Zitat von Magnetfeldfredy: Der Handel ist sowieso "gefakt" und heute und einige Tage total manipuliert!
Arena ohne Stop/Loss ist wichtig auf Wochen/Monate wird sich das Potential mit EU/ROW-Partner herauskristallisieren!
Right, Fredy...

...jetzt werden bald die ersten neuen Kursziele reinschneien ( im Yaho-Board bereits geschehen!!) und dann werden Jim Cramer, Fuckelstone und Co. die Posaune auspacken um dann gemeinsam VIVUS nach oben zu " trompeten"......
das es seit 2 tagen die beiden kurse voll in der hand der archlöcher ist und manipuliert wird, ist keine frage. trotzdem sind alle meine 3 tastaturen heil geblieben, was bei mir nicht normal ist
die taxen bei vvus scheinen keine fakeorder zu sein. es wird vorbörslich gehandelt. kurs 30 usd
über 32usd.
aus welchem grunde gabe ich gestern kalte füße bekommen und bei knapp 27 verkauft ?
nachträglich kann man leicht sagen
aus welchem grunde gabe ich gestern kalte füße bekommen und bei knapp 27 verkauft ?
nachträglich kann man leicht sagen
in einem artikel oder bei vivus hat gestanden, dass wenn vivus die zulassung bekommt, wird arena fallen. und das ist auch tatsächlich eingetreten
Antwort auf Beitrag Nr.: 43.399.549 von gauner1 am 18.07.12 16:50:09das ist doch klar gewesen.
Wurde hier auch mehrfach besprochen. Mein Tipp war -10%. Dieses wird aber in der nächsten Zeit wieder ausgeglichen.
alles gut.

Wurde hier auch mehrfach besprochen. Mein Tipp war -10%. Dieses wird aber in der nächsten Zeit wieder ausgeglichen.
alles gut.

Antwort auf Beitrag Nr.: 43.399.707 von Poppholz am 18.07.12 17:16:04
ich bin nicht mehr investiert, jedoch hpffe ich für euch und für meinen freund lars.
wenn der kurs sich völlig konsolidiert hat, komme ich vielleicht wieder rein. ich wäre nicht ausgestiegen, wenn die manipulatoren meine nerven nicht getötet hätten.
ich bin nicht mehr investiert, jedoch hpffe ich für euch und für meinen freund lars.
wenn der kurs sich völlig konsolidiert hat, komme ich vielleicht wieder rein. ich wäre nicht ausgestiegen, wenn die manipulatoren meine nerven nicht getötet hätten.
es ist zwar richtig, dass arena jetzt einen neuen konkurenten hat, aber verstehe nicht, dass der kurs so tief fällt. ich denke der tiefpunkt ist erreicht und sich jetzt alles beruhigt hat, wird es wieder aufwärts gehen, besonders wenn positive news kommen
..der erste Artikel, der die Zulassung von QSYMIA wegen der großen Nebenwirkungen verurteilt....
Drugs
FDA approves weight loss drug Qsymia, but we say skip it
Jul 18, 2012 5:25 PM
The Food and Drug Administration Tuesday approved Qsymia, a combination of the stimulant phentermine and the anti-seizure drug topiramate extended-release, to help obese and overweight people lose weight. But our medical advisers say the pill should be avoided because it can cause several serious side effects.
According to the evidence submitted to the FDA, Qsymia appears to help people drop a few pounds. In studies, obese and overweight people who took Qsymia for one year lost 3.5 to 9.3 more pounds than those who took a placebo. But that small benefit is probably not worth the risks of birth defects, heart attacks, and strokes. In fact, two years ago the FDA rejected the drug, then called Qnexa, due to these concerns, and it is not clear why the FDA reversed course this time, since those side effects are still an issue.
The drug also carries a warning that it can increase heart rate and should not be used by people who have heart disease or have suffered a stroke. Due to the heart concern, Vivus, the manufacturer of Qsymia, is required to conduct a study to determine whether the drug poses a risk of major cardiovascular problems, including heart attack and stroke. Also too, pregnant women should not take Qsymia because it increases the risk of their children being born with a cleft lip or palate.
Qsymia will only be available through specially certified pharmacies under a Risk Evaluation and Mitigation Strategy, or REMS, which is intended to inform doctors and patients about the possibility of birth defects.
"The very idea that a post marketing risk evaluation strategy was a condition required by the FDA for approval of this combination drug product seems like putting the cart ahead of the horse," says Marvin Lipman, M.D., chief medical adviser for Consumer Reports. "Such a study may very well result in preventable mortality and morbidity, a high price to pay in exchange for a few pounds of flesh."
Qsymia contains several additional warnings, including that it can increase the risk of glaucoma, kidney stones, mood problems such as anxiety and depression, and suicidal behavior or thinking about suicide (ideation).
"This drug should only be used in the confines of a research trial in which consumers have been fully informed of its risks and benefits and, someone is responsible for the complications that occur. All the rest of us should wait until we know a lot more about its safety," says John Santa, M.D., director of the Consumer Reports Health Ratings Center.
Bottom Line: We recommend avoiding all weight-loss drugs and supplements. Their benefits are usually marginal. And even if they do help you to shed a few pounds, the side effects can be troublesome and even dangerous.
If you need to lose weight, increasing exercise and limiting portion size when it comes to food are better options.
Additional links:
How to control your weight [Consumer Reports]
Weight-loss drugs: Alli and Xenical (Orlistat):Slim benefits and embarrassing side effects [Consumer Reports]
Lose weight, stay active: Six small changes can help keep off pounds [Consumer Reports]
Sources:
FDA approves weight-management drug Qsymia [FDA]
Panel recommends FDA approve weight loss drug Qnexa [Consumer Reports]
—Steve Mitchell
http://news.consumerreports.org/health/2012/07/fda-approves-…" target="_blank" rel="nofollow ugc noopener">
http://news.consumerreports.org/health/2012/07/fda-approves-…
Drugs
FDA approves weight loss drug Qsymia, but we say skip it
Jul 18, 2012 5:25 PM
The Food and Drug Administration Tuesday approved Qsymia, a combination of the stimulant phentermine and the anti-seizure drug topiramate extended-release, to help obese and overweight people lose weight. But our medical advisers say the pill should be avoided because it can cause several serious side effects.
According to the evidence submitted to the FDA, Qsymia appears to help people drop a few pounds. In studies, obese and overweight people who took Qsymia for one year lost 3.5 to 9.3 more pounds than those who took a placebo. But that small benefit is probably not worth the risks of birth defects, heart attacks, and strokes. In fact, two years ago the FDA rejected the drug, then called Qnexa, due to these concerns, and it is not clear why the FDA reversed course this time, since those side effects are still an issue.
The drug also carries a warning that it can increase heart rate and should not be used by people who have heart disease or have suffered a stroke. Due to the heart concern, Vivus, the manufacturer of Qsymia, is required to conduct a study to determine whether the drug poses a risk of major cardiovascular problems, including heart attack and stroke. Also too, pregnant women should not take Qsymia because it increases the risk of their children being born with a cleft lip or palate.
Qsymia will only be available through specially certified pharmacies under a Risk Evaluation and Mitigation Strategy, or REMS, which is intended to inform doctors and patients about the possibility of birth defects.
"The very idea that a post marketing risk evaluation strategy was a condition required by the FDA for approval of this combination drug product seems like putting the cart ahead of the horse," says Marvin Lipman, M.D., chief medical adviser for Consumer Reports. "Such a study may very well result in preventable mortality and morbidity, a high price to pay in exchange for a few pounds of flesh."
Qsymia contains several additional warnings, including that it can increase the risk of glaucoma, kidney stones, mood problems such as anxiety and depression, and suicidal behavior or thinking about suicide (ideation).
"This drug should only be used in the confines of a research trial in which consumers have been fully informed of its risks and benefits and, someone is responsible for the complications that occur. All the rest of us should wait until we know a lot more about its safety," says John Santa, M.D., director of the Consumer Reports Health Ratings Center.
Bottom Line: We recommend avoiding all weight-loss drugs and supplements. Their benefits are usually marginal. And even if they do help you to shed a few pounds, the side effects can be troublesome and even dangerous.
If you need to lose weight, increasing exercise and limiting portion size when it comes to food are better options.
Additional links:
How to control your weight [Consumer Reports]
Weight-loss drugs: Alli and Xenical (Orlistat):Slim benefits and embarrassing side effects [Consumer Reports]
Lose weight, stay active: Six small changes can help keep off pounds [Consumer Reports]
Sources:
FDA approves weight-management drug Qsymia [FDA]
Panel recommends FDA approve weight loss drug Qnexa [Consumer Reports]
—Steve Mitchell
http://news.consumerreports.org/health/2012/07/fda-approves-…" target="_blank" rel="nofollow ugc noopener">
http://news.consumerreports.org/health/2012/07/fda-approves-…
Zitat von bernie55: ..der erste Artikel, der die Zulassung von QSYMIA wegen der großen Nebenwirkungen verurteilt....
Ich verstehe nicht das man sowas zulassen kann, es gibt genug Frauen die schwanger sind und es nicht merken sogar Ihre Periode weiterhin bekommen........ von den anderen nebenwirkungen ganz zu schweigen!.....vielleicht war der Druck und die Aufmerksamkeikeit seitens der Medien auch zu Gross und so haben sie sich auf einen Kompromiss entschieden und es mit Auflagen zugelassen.
Zitat von franzisca:Zitat von bernie55: ..der erste Artikel, der die Zulassung von QSYMIA wegen der großen Nebenwirkungen verurteilt....
Ich verstehe nicht das man sowas zulassen kann, es gibt genug Frauen die schwanger sind und es nicht merken sogar Ihre Periode weiterhin bekommen........ von den anderen nebenwirkungen ganz zu schweigen!.....vielleicht war der Druck und die Aufmerksamkeikeit seitens der Medien auch zu Gross und so haben sie sich auf einen Kompromiss entschieden und es mit Auflagen zugelassen.
....damit hast du den Nagel auf den Kopf getroffen, Francisca...
..und wenn du noch das AdiPositas der Frauen miteinbeziehst, dann wirst du auch im 6/7 Monat ?? nicht sehen, dass der Bauchumfang aufgrund der Schwangerschaft größer geworden ist....
Ich gehe davon aus, dass die meisten Ärzte, und hoffentlich mehr als mehr, diese Problematik erkennen und bei Bedarf von " Obesity-Drugs" auf "sichere" Medikamente zurückgreifen werden....sprich auf BELVIQ..
....und ganz abgesehen davon,was Ärzte nebenher beachten bzw. an " Diagnostikum" durchführen müssen um dann das Medikament Qsympia zu verabreichen...HAMMER !!!!
...und das kann es nicht wirklich nicht sein.....!!!!
Aber vielleicht wird sich dadurch das " Gesundheitsgefährdende inkl. der vielen Nebenwirkungen" von Qsympia von alleine lösen.......
Antwort auf Beitrag Nr.: 43.402.770 von franzisca am 19.07.12 12:19:45Hallo bernie....ich mache mir keine sorgen bzg. Der konkurenz dh. Bei diesen auflagen wird der marktanteil vermutlich entprecheng gering ausfallen. Wenn ich in vivus investiert gewesen waere.....waere ich ueber diese kuemmerliche steigerung gestern ensprechend entaescht gewesen. Arena wird wieder steigen und uns freude bereiten......da bin ich mir sicher :-). Ich bin seit 2 tagen in amyris investiert.......die laeft auch schoen.....beste gruesse earthfire
..und wenn du noch das AdiPositas der Frauen miteinbeziehst, dann wirst du auch im 6/7 Monat ?? nicht sehen, dass der Bauchumfang aufgrund der Schwangerschaft größer geworden ist....
Genau!...meine ehemalige Schwägerin hat im 7 Monat Wehen bekommen und hatte bis Dato nicht gewusst das sie schwanger war....ich muss dazu sagen das sie sehr kräftig gebaut ist und circa 90-100 kilo auf die Wage bringt!...soviel zum Thema, ich glaube nicht dass Frauen präventiv jeden Monat einen Schwangerschafttest durchführen nur weil der Arzt es empfiehlt!
Genau!...meine ehemalige Schwägerin hat im 7 Monat Wehen bekommen und hatte bis Dato nicht gewusst das sie schwanger war....ich muss dazu sagen das sie sehr kräftig gebaut ist und circa 90-100 kilo auf die Wage bringt!...soviel zum Thema, ich glaube nicht dass Frauen präventiv jeden Monat einen Schwangerschafttest durchführen nur weil der Arzt es empfiehlt!
Zitat von franzisca: ..und wenn du noch das AdiPositas der Frauen miteinbeziehst, dann wirst du auch im 6/7 Monat ?? nicht sehen, dass der Bauchumfang aufgrund der Schwangerschaft größer geworden ist....
Genau!...meine ehemalige Schwägerin hat im 7 Monat Wehen bekommen und hatte bis Dato nicht gewusst das sie schwanger war....ich muss dazu sagen das sie sehr kräftig gebaut ist und circa 90-100 kilo auf die Wage bringt!...soviel zum Thema,
ich glaube nicht dass Frauen präventiv jeden Monat einen Schwangerschafttest durchführen nur weil der Arzt es empfiehlt!
...und dass diese Frauen den auch noch selber bezahlen werden....
Ein Video über VVUS.....Mein Englich ist echt miess aber ich denke das es Negativ ist!
http://video.foxnews.com/v/1741469181001
http://video.foxnews.com/v/1741469181001
...wieder mal ein paar Gerüchte von SIERRA...
Wednesday, July 18, 2012
Pfizer (PFE) to tend offer for Arena Pharmaceuticals (ARNA) after NY Board Meeting ventures Sierra World Equity Review
Ok, so we told you that we believed that their was some exciting things coming Arena Pharmaceuticals way....so here it is....but before we get to it we just want to remind you that you heard it HERE FIRST! We are VERY ACCURATE in our predictions (just look at the links below for proof!!).
links to FDA predictions:
published June 26 2012 - Arena and Lorcaserin
published July 1 2012 - Vivus and Qnexa
Alright let's get right to it, this is going to make ARNA shareholder very happy and also probably a lot of money!
Sierra World Equity Review anticipates that Pfizer has decided on a number, the very maximum they can afford and are willing to pay, a valuation of Arena Pharmaceuticals. At the next Board of Directors meeting in mid-town Manhattan this number will be evaluated and put to a vote. Thereafter, Sierra World Equity Review projects that Pfizer will officially make an offer to purchase Arena Pharmaceuticals offering it a low ball dollar amount. At that point Sierra World Equity Review feels that an all out BIDDING WAR will begin as Eisai, the Japanese Drug giant will then be able to leverage their current "standstill" agreement they have with Arena Pharmaceuticals and attempt to block Pfizer by making a higher offer to Arena Pharmaceuticals. Sierra World Equity Review then believes Pfizer will counter offer and will be prepared to continuing to counter offer until they reach that very maximum figure that they are willing to pay to purchase Arena Pharmaceuticals.
Sierra World Equity Review believes some very interesting and exciting things are going to happen soon, not to mention the VERY REAL possibility that one or more OTHER major pharmaceutical players may also try to get in on the action.
Who can blame them, as of today their are now only 2 FDA approved obseity drugs, Belviq from Arena Pharmaceuticals appears to be far the safer and will be less expensive than Qsymia from Vivus. The lucrative obesity drug market is massive, with sales of Belviq, projected to hit 2 BILLION DOLLARS by 2020, moreover patients often try more than one type of treatment, in other words expect both Arena Pharmaceuticals and Vivus to flourish as will their respective stock price and shareholders!
Remember you heard it hear FIRST! Please check back for continuing updates on Arena Pharmaceuticals and Eisai and Pfizer. We will continue to highlight these companies going forward and also examine any additional large pharmaceutical companies that may have an interest in buying out Arena Pharmaceuticals.Sierra World Equity Review currently hold no positions in any of the companies mentioned in this report......be the first to read our new posts follow us on Twitter at
https://twitter.com/#!/SierraEquity" target="_blank" rel="nofollow ugc noopener">
https://twitter.com/#!/SierraEquity
--------------------------------------------------------------------------------------------------------------
http://sierraworldequityreview.blogspot.de/2012_07_01_archiv…
Wednesday, July 18, 2012
Pfizer (PFE) to tend offer for Arena Pharmaceuticals (ARNA) after NY Board Meeting ventures Sierra World Equity Review
Ok, so we told you that we believed that their was some exciting things coming Arena Pharmaceuticals way....so here it is....but before we get to it we just want to remind you that you heard it HERE FIRST! We are VERY ACCURATE in our predictions (just look at the links below for proof!!).
links to FDA predictions:
published June 26 2012 - Arena and Lorcaserin
published July 1 2012 - Vivus and Qnexa
Alright let's get right to it, this is going to make ARNA shareholder very happy and also probably a lot of money!
Sierra World Equity Review anticipates that Pfizer has decided on a number, the very maximum they can afford and are willing to pay, a valuation of Arena Pharmaceuticals. At the next Board of Directors meeting in mid-town Manhattan this number will be evaluated and put to a vote. Thereafter, Sierra World Equity Review projects that Pfizer will officially make an offer to purchase Arena Pharmaceuticals offering it a low ball dollar amount. At that point Sierra World Equity Review feels that an all out BIDDING WAR will begin as Eisai, the Japanese Drug giant will then be able to leverage their current "standstill" agreement they have with Arena Pharmaceuticals and attempt to block Pfizer by making a higher offer to Arena Pharmaceuticals. Sierra World Equity Review then believes Pfizer will counter offer and will be prepared to continuing to counter offer until they reach that very maximum figure that they are willing to pay to purchase Arena Pharmaceuticals.
Sierra World Equity Review believes some very interesting and exciting things are going to happen soon, not to mention the VERY REAL possibility that one or more OTHER major pharmaceutical players may also try to get in on the action.
Who can blame them, as of today their are now only 2 FDA approved obseity drugs, Belviq from Arena Pharmaceuticals appears to be far the safer and will be less expensive than Qsymia from Vivus. The lucrative obesity drug market is massive, with sales of Belviq, projected to hit 2 BILLION DOLLARS by 2020, moreover patients often try more than one type of treatment, in other words expect both Arena Pharmaceuticals and Vivus to flourish as will their respective stock price and shareholders!
Remember you heard it hear FIRST! Please check back for continuing updates on Arena Pharmaceuticals and Eisai and Pfizer. We will continue to highlight these companies going forward and also examine any additional large pharmaceutical companies that may have an interest in buying out Arena Pharmaceuticals.Sierra World Equity Review currently hold no positions in any of the companies mentioned in this report......be the first to read our new posts follow us on Twitter at
https://twitter.com/#!/SierraEquity" target="_blank" rel="nofollow ugc noopener">
https://twitter.com/#!/SierraEquity
--------------------------------------------------------------------------------------------------------------
http://sierraworldequityreview.blogspot.de/2012_07_01_archiv…
@bernie.....habe heute nochmal nachgekauft bei arena..:-).....auf dem tief erwischt :-)......binnun offen fuer eine uebernahme durch pfizer.....
zur zeit
arena minus 4,4
orex minus 7,5
vvus minus 3,9
mit anderen worten mißt. nix mehr drachen fliegen
arena minus 4,4
orex minus 7,5
vvus minus 3,9
mit anderen worten mißt. nix mehr drachen fliegen
Antwort auf Beitrag Nr.: 43.404.223 von gauner1 am 19.07.12 17:14:16Die sind Alle auf Diät!

...hier ein detailierter Bericht über VIVUS...
....da geht es u.a auch über Patentfragen und möglicherweise Verstöße gegen Patentrechte.....
Vivus (NASDAQ:VVUS): Why FDA Approval Is Not the Prescription
While the company has been busy furthering their FDA submission and selling stock, Vivus has simply avoided confronting the reality that they might not even be able to go to market.
The foundation stone of Vivus' IP protection is a patent licensing deal signed a decade ago with a Dr. Najarian, an obesity doctor and researcher who filed as sole inventor for Qsymia's two-drug combo. One question that has been hanging over the company for years is the possibility of patent infringement on one of Qsymia's two active ingredients, topiramate, a patent held by Johnson & Johnson, who used to sell the drug as an obesity treatment before it went generic. It affords patent protection until 2017.
It originally was filed by a researcher named Shank on June 29, 1996 (US PTO No. 6,071,537)[/i]
There is no evidence of the validity of this patent ever being challenged, either at the USPTO or the corresponding EP patent (EP0915697) filed here. There are no mentions in Vivus' filings of the Shank patent, and therefore no clues as to how Vivus plans to deal with it.
How exactly does Vivus overcome the Shank patent to sell topiramate for weight loss? Citron rates this unresolved question a substantial risk factor.
Vivus has had not just the last 5 months, but 5 years since questions about the Najarian, Shank and McElroy patents were posed by analysts, to create enough clarity to go to market.
Yet to this day, all we hear from the company is assurances that they have legal opinions in hand stating "they are OK". No joint ventures, no counterparty agreements, no releases.
Quite a cloud of uncertainty for a "multi-billion dollar opportunity" resting on the combination of two common generic drugs, don't you think?
If this were a solid pharma with high management credibility, maybe investors could give them the benefit of the doubt. But this management?
Conclusion
Despite years of lead time, Vivus' executives have failed to provide strong patent protection for Qsymia. Taken together, these points provide the best explanation yet why Vivus hasn't been able to recruit a high-profile deep-pocketed pharma partner to leverage its expertise while taking some of the risk of marketing its newly approved diet combo drug.
Instead of selling a stake to a credible pharma marketer, they sold stock – over $230 million to …. you guessed it. The hope that a big pharma company is going to ride in pay $3 billion for Vivus is ludicrous – the strategy of trying to pierce its patent protection for a few million in legal fees is a far lower risk.
The more marketing hype about the FDA approval of this combination of two well-known and inexpensive legacy generic drugs, the more Vivus sets up its own ambush – by legal challenges to its patent, and generic drug competition starting before they can even get to market.
http://www.citronresearch.com/index.php/2012/07/19/vivus-why…
....da geht es u.a auch über Patentfragen und möglicherweise Verstöße gegen Patentrechte.....
Vivus (NASDAQ:VVUS): Why FDA Approval Is Not the Prescription
While the company has been busy furthering their FDA submission and selling stock, Vivus has simply avoided confronting the reality that they might not even be able to go to market.
The foundation stone of Vivus' IP protection is a patent licensing deal signed a decade ago with a Dr. Najarian, an obesity doctor and researcher who filed as sole inventor for Qsymia's two-drug combo. One question that has been hanging over the company for years is the possibility of patent infringement on one of Qsymia's two active ingredients, topiramate, a patent held by Johnson & Johnson, who used to sell the drug as an obesity treatment before it went generic. It affords patent protection until 2017.
It originally was filed by a researcher named Shank on June 29, 1996 (US PTO No. 6,071,537)[/i]
There is no evidence of the validity of this patent ever being challenged, either at the USPTO or the corresponding EP patent (EP0915697) filed here. There are no mentions in Vivus' filings of the Shank patent, and therefore no clues as to how Vivus plans to deal with it.
How exactly does Vivus overcome the Shank patent to sell topiramate for weight loss? Citron rates this unresolved question a substantial risk factor.
Vivus has had not just the last 5 months, but 5 years since questions about the Najarian, Shank and McElroy patents were posed by analysts, to create enough clarity to go to market.
Yet to this day, all we hear from the company is assurances that they have legal opinions in hand stating "they are OK". No joint ventures, no counterparty agreements, no releases.
Quite a cloud of uncertainty for a "multi-billion dollar opportunity" resting on the combination of two common generic drugs, don't you think?
If this were a solid pharma with high management credibility, maybe investors could give them the benefit of the doubt. But this management?
Conclusion
Despite years of lead time, Vivus' executives have failed to provide strong patent protection for Qsymia. Taken together, these points provide the best explanation yet why Vivus hasn't been able to recruit a high-profile deep-pocketed pharma partner to leverage its expertise while taking some of the risk of marketing its newly approved diet combo drug.
Instead of selling a stake to a credible pharma marketer, they sold stock – over $230 million to …. you guessed it. The hope that a big pharma company is going to ride in pay $3 billion for Vivus is ludicrous – the strategy of trying to pierce its patent protection for a few million in legal fees is a far lower risk.
The more marketing hype about the FDA approval of this combination of two well-known and inexpensive legacy generic drugs, the more Vivus sets up its own ambush – by legal challenges to its patent, and generic drug competition starting before they can even get to market.
http://www.citronresearch.com/index.php/2012/07/19/vivus-why…
auf was darf man die kursverläufe alle 3 gurken beziehen ?
waren sie überbewertet ?
haben die shorties auf fallende kurse gesetzt bzw. finden leerverkäufe statt ?
bin bei arna bei 11 raus. möchte wieder rein, bin aber völlig unsicher. einfach angst vor den shorties
waren sie überbewertet ?
haben die shorties auf fallende kurse gesetzt bzw. finden leerverkäufe statt ?
bin bei arna bei 11 raus. möchte wieder rein, bin aber völlig unsicher. einfach angst vor den shorties
Antwort auf Beitrag Nr.: 43.405.355 von gauner1 am 19.07.12 22:19:35Hab Geduld mit Arena, ein Tenbagger kann auch mal zurückkommen um dann wieder durchzustarten.
Vivus hat Probleme mit REM und Patenten, Arena nicht!
Vivus hat Probleme mit REM und Patenten, Arena nicht!

...und noch eine NEWS zu den möglichen Patentproblematiken sowohl bei VIVUS als auch bei OREXIGEN....
Vivus falls on concerns of patent protection for diet pill
Thu Jul 19, 2012 4:12pm EDT
(Reuters) - Shares of Vivus Inc fell sharply on Thursday afternoon after well-known short-seller Citron Research cast doubts on the drugmaker's ability to protect its weight-loss pill's patents.
Citron in a report on its website pointed out the possibility that Johnson & Johnson could challenge Vivus in court over patents that cover usage of anti-seizure drug topiramate -- one of the key ingredients in Vivus' freshly approved Qsymia.
The Citron report comes two days after U.S. health regulators approved Qsymia for use in obese and overweight adults who have at least one weight-related condition. The drug was expected to have the potential to bring in more than $1 billion in sales.
Citron is run by California-based investor and notable short-seller Andrew Left.
It also pointed to possible problems with another Vivus patent, with claims related to methods for treating disorders that include obesity "using sulfamates (including topiramate) in combination with psychostimulants (including phentermine)".
Qsymia's second important ingredient is phentermine. The brokerage points to possible copy-cat versions eating up Qsymia's market as the product is made of two key ingredients, which are already available in the market as generics.
Competitor Orexigen Therapeutics Inc's shares also fell 10 percent after the report went public. Orexigen's experimental drug, Contrave, is a combination of slow release versions of naltrexone and a mild psychostimulant, bupropion.
Orexigen is currently conducting a trial to study risks of heart complications from using Contrave.
Shares of Arena Pharmaceuticals Inc -- the first drugmaker to get a diet pill approved in 13 years -- were down 2 percent at $9.75 on the Nasdaq. Arena got the approval late last month.
Vivus shares, which had gained 10 percent in value a day after its diet pill was approved, fell 13 percent to a low of $25.15 in afternoon trading.
(Reporting by Vidya P L Nathan in Bangalore; Editing by Maju Samue
http://www.reuters.com/article/2012/07/19/us-vivus-shares-id…
Vivus falls on concerns of patent protection for diet pill
Thu Jul 19, 2012 4:12pm EDT
(Reuters) - Shares of Vivus Inc fell sharply on Thursday afternoon after well-known short-seller Citron Research cast doubts on the drugmaker's ability to protect its weight-loss pill's patents.
Citron in a report on its website pointed out the possibility that Johnson & Johnson could challenge Vivus in court over patents that cover usage of anti-seizure drug topiramate -- one of the key ingredients in Vivus' freshly approved Qsymia.
The Citron report comes two days after U.S. health regulators approved Qsymia for use in obese and overweight adults who have at least one weight-related condition. The drug was expected to have the potential to bring in more than $1 billion in sales.
Citron is run by California-based investor and notable short-seller Andrew Left.
It also pointed to possible problems with another Vivus patent, with claims related to methods for treating disorders that include obesity "using sulfamates (including topiramate) in combination with psychostimulants (including phentermine)".
Qsymia's second important ingredient is phentermine. The brokerage points to possible copy-cat versions eating up Qsymia's market as the product is made of two key ingredients, which are already available in the market as generics.
Competitor Orexigen Therapeutics Inc's shares also fell 10 percent after the report went public. Orexigen's experimental drug, Contrave, is a combination of slow release versions of naltrexone and a mild psychostimulant, bupropion.
Orexigen is currently conducting a trial to study risks of heart complications from using Contrave.
Shares of Arena Pharmaceuticals Inc -- the first drugmaker to get a diet pill approved in 13 years -- were down 2 percent at $9.75 on the Nasdaq. Arena got the approval late last month.
Vivus shares, which had gained 10 percent in value a day after its diet pill was approved, fell 13 percent to a low of $25.15 in afternoon trading.
(Reporting by Vidya P L Nathan in Bangalore; Editing by Maju Samue
http://www.reuters.com/article/2012/07/19/us-vivus-shares-id…
Antwort auf Beitrag Nr.: 43.406.006 von bernie55 am 20.07.12 08:32:04meine NEWS und alles was ich im Netz zu unserem Baby finde trage ich in den "Info-Thread von ARNA" ein.
Somit auch immer dort nachschauen, was es an "akutellen" Infos gibt.

Somit auch immer dort nachschauen, was es an "akutellen" Infos gibt.

Antwort auf Beitrag Nr.: 43.405.355 von gauner1 am 19.07.12 22:19:35das ist ja das schöne an unseren BIOs.
Wir wissen nie, welche Adressen gerade dabei sind, unsere Aktienkurse zu beeinflussen und aus welchem Grunde Sie dieses tun, bzw. wann Sie Ihr Ziel erreicht haben und die Aktie wieder in Ruhe lassen.
Es wird halt nie langweilig.

Wir wissen nie, welche Adressen gerade dabei sind, unsere Aktienkurse zu beeinflussen und aus welchem Grunde Sie dieses tun, bzw. wann Sie Ihr Ziel erreicht haben und die Aktie wieder in Ruhe lassen.
Es wird halt nie langweilig.

vivus minus 14 %
Antwort auf Beitrag Nr.: 43.408.501 von bernie55 am 20.07.12 18:01:53
ja, wo sind denn die anderen longies ? z. b. vincent der longe
achso, der wartet immer noch auf 4 euro

ja, wo sind denn die anderen longies ? z. b. vincent der longe
achso, der wartet immer noch auf 4 euro


@AREANICS-Longies......SIERRA WORLD ist mal wieder aktiv  ..
..
...ich gebe euch nun mal ein kleinen SIERRA WORLD ADRENALIN-Stoß fürs Wochenende........
Saturday, July 21, 2012
TOP FOUR REASONS -Arena Pharmaceuticals (ARNA) will become a $100 stock forecasts accurate phenom Sierra World Equity Review
forecasts accurate phenom Sierra World Equity Review
!!
Arena Pharmaceuticals "STOCK OF THE YEAR"-- a $100 Stock in the Making !!
Reason ONE:
-Currently there are only 2 FDA approved obesity drugs, Belviq appears to be the more attractive choice, it's competition Qsymia should NOT be used by pregnant women says the FDA. Nor should it be taken by people who have had a recent stroke or unstable heart disease as it can speed up heart rate or be taken by people with glaucoma or hyperthyroidism. Sierra World Equity Review believes sales of Belviq will dominate those of Qsymia.
Reason TWO:
-Obesity rates are rising around the world especially in China where an emerging middle class has a rising obesity level. To put that into context, by 2015 there will be more obese people in China than the ENTIRE POPULATION of the United States. Revenue from sales of Belviq in China alone would make Arena a $100 stock. Belviq will be available in China, Japan, the European Union, North America and South America.
... this is not a nine or ten or even 20 dollar stock....easily going to $100 and MORE when the sales get going! Even with Eisai the Japanese drug partner reaping a nice profit from Arena Pharmaceuticals Belviq, the revenues are just going to be so massive, nothing can stop this stock!
Reason THREE:
-The BUYOUT, with Pfizer and other major players focusing on Arena Pharmaceuticals, it has become the hottest biotech potential BUYOUT this year! For current shareholders of Arena that translates into a huge windfall.........
Sierra World Equity Review predicts that the day after buyout the stock will open at $60 and race to $100 over the next 4 quarters as those revenues start to pour in!......ich denke, damit könnten wir leben...
One can't help feeling a little envious of the fortunate Arena shareholders who had the foresight and wisdom or luck to invest in Arena when it was dirt cheap.....Sierra World Equity Review is happy for them and applauds their success.
Reason FOUR:
-Belviq will be very inexpensive, costing between perhaps 100-200 dollars per month. In prescription drug terms this is cheap and insurance companies will be cover the cost. The cost benefit for insurance companies is a no brainer....it's far more lucrative to pay for inexpensive weight loss medication than have to cover severe medical conditions that may result from obesity if left untreated. Sierra World Equity Review predicts that insurance companies will see the bottom line and WILL FULLY COVER Belviq as a treatment for obesity.
http://sierraworldequityreview.blogspot.de/2012/07/breakingw…
 ..
.....ich gebe euch nun mal ein kleinen SIERRA WORLD ADRENALIN-Stoß fürs Wochenende........

Saturday, July 21, 2012
TOP FOUR REASONS -Arena Pharmaceuticals (ARNA) will become a $100 stock
 forecasts accurate phenom Sierra World Equity Review
forecasts accurate phenom Sierra World Equity Review!!
Arena Pharmaceuticals "STOCK OF THE YEAR"-- a $100 Stock in the Making !!
Reason ONE:
-Currently there are only 2 FDA approved obesity drugs, Belviq appears to be the more attractive choice, it's competition Qsymia should NOT be used by pregnant women says the FDA. Nor should it be taken by people who have had a recent stroke or unstable heart disease as it can speed up heart rate or be taken by people with glaucoma or hyperthyroidism. Sierra World Equity Review believes sales of Belviq will dominate those of Qsymia.
Reason TWO:
-Obesity rates are rising around the world especially in China where an emerging middle class has a rising obesity level. To put that into context, by 2015 there will be more obese people in China than the ENTIRE POPULATION of the United States. Revenue from sales of Belviq in China alone would make Arena a $100 stock. Belviq will be available in China, Japan, the European Union, North America and South America.
... this is not a nine or ten or even 20 dollar stock....easily going to $100 and MORE when the sales get going! Even with Eisai the Japanese drug partner reaping a nice profit from Arena Pharmaceuticals Belviq, the revenues are just going to be so massive, nothing can stop this stock!
Reason THREE:
-The BUYOUT, with Pfizer and other major players focusing on Arena Pharmaceuticals, it has become the hottest biotech potential BUYOUT this year! For current shareholders of Arena that translates into a huge windfall.........
Sierra World Equity Review predicts that the day after buyout the stock will open at $60 and race to $100 over the next 4 quarters as those revenues start to pour in!......ich denke, damit könnten wir leben...

One can't help feeling a little envious of the fortunate Arena shareholders who had the foresight and wisdom or luck to invest in Arena when it was dirt cheap.....Sierra World Equity Review is happy for them and applauds their success.
Reason FOUR:
-Belviq will be very inexpensive, costing between perhaps 100-200 dollars per month. In prescription drug terms this is cheap and insurance companies will be cover the cost. The cost benefit for insurance companies is a no brainer....it's far more lucrative to pay for inexpensive weight loss medication than have to cover severe medical conditions that may result from obesity if left untreated. Sierra World Equity Review predicts that insurance companies will see the bottom line and WILL FULLY COVER Belviq as a treatment for obesity.
http://sierraworldequityreview.blogspot.de/2012/07/breakingw…
Antwort auf Beitrag Nr.: 43.411.332 von bernie55 am 22.07.12 11:38:37...tja, EARTHIE, da scheinen die Jungs von SIERRA unsere gestrigen Analysegespräche belauscht zu haben....
Grüße
bernie55

Grüße
bernie55

Zitat von bernie55: Sierra World Equity Review predicts that the day after buyout the stock will open at $60 and race to $100 over the next 4 quarters as those revenues start to pour in!......ich denke, damit könnten wir leben...
http://sierraworldequityreview.blogspot.de/2012/07/breakingw…
......die TOP "Hausnummernprognosen" hat SIERRA wohl doch ein wenig die Birne zugenebelt.......mir übrigens auch...

..also , wenn ein BUYOUT kommt, dann steht der Preis einer Aktie fest und kann deshalb nicht noch bis 100 USD steigen...
Aber vielleicht meinten sie auch, dass "nach einen möglichen beginnenden "BUYOUT-BATTLE" 60 USD das erste Angebot sein wird....

Antwort auf Beitrag Nr.: 43.411.393 von bernie55 am 22.07.12 12:25:00
seit wann tust du hier bashen hier ? der faire wert ist 200 usd und das ist immer noch unter dem cashbestand
seit wann tust du hier bashen hier ? der faire wert ist 200 usd und das ist immer noch unter dem cashbestand
Zitat von gauner1: seit wann tust du hier bashen hier ?
der faire wert ist 200 usd und das ist immer noch unter dem cashbestand
Hi gauner1, jetzt hast du doch tatsächlich meine "wahre Funktion" in diesem Thread entlarvt....

...die einen werden von ARENA "bezahlt", um ihre eigenen Gurken im Garten zu gießen

...die anderen werden von ARENA "bezahlt", um hier im Board zu bashen

Grüße
bernie55

Super Performance am crash-day:
These Stocks Couldn't Beat the Blue Chips
By Rich Duprey | More Articles
July 22, 2012 | Comments (3)
Earnings of blue chip stocks drove the Dow higher earlier last week, as did a surprising housing report that showed homebuilders began shoveling more new homes than any time in the past few years. While I don't necessarily find that encouraging, some companies had their own problems to contend with and managed to go in the other direction, some even plunging by double-digit percentages.
So let's see whether they had good reason to drop, as sometimes panic-fueled declines can lead to excellent buying opportunities.
Company
Wednesday's % Change
Price
CAPS Rating (out of 5)
Rovi (Nasdaq: ROVI )
(43.3%)
$10.01
**
New Oriental Education (NYSE: EDU )
(35%)
$9.50
*
Arena Pharmaceuticals (Nasdaq: ARNA )
(9.7%)
$9.98
**
Earnings, fraud allegations, and competition were the reasons behind the midweek grinding these stocks took.
Scrambled signals
Interactive-channel-guide software provider Rovi got its programming mixed up after it said demand in the consumer electronics market is harder to find than water in the desert. Revenues, which it previously pegged at $785 million for the full year, were slashed by a third to $680 million in its new guidance as it hasn't been able to sign up new licensees for its technology that allows viewers to browse TV program listings. Current customers were also unwilling to sign on again, at least not on terms acceptable to Rovi. Losses are also expected to widen for the second quarter, growing from $0.06 per share to $0.18.
Rovi's been fighting Mitsubishi, LG Electronics, and Vizio over their alleged patent infringement of on-screen guides and its V-chip technology that restricts access to certain shows or networks deemed inappropriate. It's also battling Netflix and Roku before the U.S. International Trade Commission.
Some investors have paired the news of Rovi's problems with those at sound specialist DTS (Nasdaq: DTSI ) , which also found demand wanting in consumer electronics, to predict a general malaise in the sector. Considering the faltering economy, no doubt there is extreme weakness, but Rovi and DTS are facing very company-specific issues: a patent challenge and slack Blu-ray player sales. That might not necessarily translate beyond those narrow confines.
A real dunce
For New Oriental Education, it seems like the good ol' days of last year when dozens of Chinese reverse merger stocks were accused of fraud. Muddy Waters, which was one of the prime short sellers leading the charge against the pack, came out with a report questioning its business practices. Coming as it does the day after the provider of English-language training and standardized test prep for Chinese youth revealed the SEC was investigating it -- leading to a nearly identical drop in its stock -- it amounted to a one-two punch for investors.
The Muddy Waters report charges New Oriental with lying about not having franchisees. The say it actually has scads of them, which allows it to improperly "inflate its cash balances in order to receive unqualified audit opinions from its auditor."
Investors might have thought that by this point the dreck of Chinese stocks had been cleared out, but if the allegations and investigations pan out, it's obvious caution is still needed -- though due diligence is always required before you sink your money in. Let me know in the comments section below whether the tag-team duo of SEC investigations and charges of fraud by Muddy Waters has shaken your confidence in Chinese stocks again.
Phat chance
I'll admit to some surprise that Arena Pharmaceuticals dropped as it did on news of the FDA's approval of rival anti-obesity drug Qsymia (formerly known as Qnexa). This is news? The market didn't anticipate that VIVUS (Nasdaq: VVUS ) would be first-to-market? Maybe it was just the reality of having the news settle in.
While Qsymia will be ahead of Arena's Belviq (who comes up with these names?), the few months' lead time might not really give VIVUS an advantage, since it does come with some labeling limitations (although so does Belviq). Yet as the Fool's Brian Orelli notes, it's going to be a while anyway before one can be declared a winner over the other:
"The question becomes whether Belviq's cleaner side-effect profile and potentially stronger marketing muscle can overcome Qsymia's better weight loss. Assuming most patients aren't covered by insurance, the cost of the drugs could be the deciding factor."
While I figured some of the euphoria would wear off on Arena's own approval (we still need to wait for drug scheduling, which is the reason for its delay to market), I think there's still monster potential here, and I'll be trading my underperform rating of the pharma upstart to an outperform. There's a big enough opportunity in obesity for several companies to succeed.
Tell me on the Arena Pharmaceuticals CAPS page or in the comments box below whether you think the stock has pulled back enough to warrant an entry into it at this time.
Ready for a resurrection
Health-care investors are always looking for the next big drug breakthrough like Belviq and Qsymia, and Motley Fool co-founder David Gardner recently identified one small-cap health-care company he believes is poised for monster returns. To uncover this top pick today, enjoy the special free report: "Discover the Next Rule-Breaking Multibagger." Don't miss out on this limited-time offer and your opportunity to discover this game-changing company before the market does. Access your report -- it's totally free.
These Stocks Couldn't Beat the Blue Chips
By Rich Duprey | More Articles
July 22, 2012 | Comments (3)
Earnings of blue chip stocks drove the Dow higher earlier last week, as did a surprising housing report that showed homebuilders began shoveling more new homes than any time in the past few years. While I don't necessarily find that encouraging, some companies had their own problems to contend with and managed to go in the other direction, some even plunging by double-digit percentages.
So let's see whether they had good reason to drop, as sometimes panic-fueled declines can lead to excellent buying opportunities.
Company
Wednesday's % Change
Price
CAPS Rating (out of 5)
Rovi (Nasdaq: ROVI )
(43.3%)
$10.01
**
New Oriental Education (NYSE: EDU )
(35%)
$9.50
*
Arena Pharmaceuticals (Nasdaq: ARNA )
(9.7%)
$9.98
**
Earnings, fraud allegations, and competition were the reasons behind the midweek grinding these stocks took.
Scrambled signals
Interactive-channel-guide software provider Rovi got its programming mixed up after it said demand in the consumer electronics market is harder to find than water in the desert. Revenues, which it previously pegged at $785 million for the full year, were slashed by a third to $680 million in its new guidance as it hasn't been able to sign up new licensees for its technology that allows viewers to browse TV program listings. Current customers were also unwilling to sign on again, at least not on terms acceptable to Rovi. Losses are also expected to widen for the second quarter, growing from $0.06 per share to $0.18.
Rovi's been fighting Mitsubishi, LG Electronics, and Vizio over their alleged patent infringement of on-screen guides and its V-chip technology that restricts access to certain shows or networks deemed inappropriate. It's also battling Netflix and Roku before the U.S. International Trade Commission.
Some investors have paired the news of Rovi's problems with those at sound specialist DTS (Nasdaq: DTSI ) , which also found demand wanting in consumer electronics, to predict a general malaise in the sector. Considering the faltering economy, no doubt there is extreme weakness, but Rovi and DTS are facing very company-specific issues: a patent challenge and slack Blu-ray player sales. That might not necessarily translate beyond those narrow confines.
A real dunce
For New Oriental Education, it seems like the good ol' days of last year when dozens of Chinese reverse merger stocks were accused of fraud. Muddy Waters, which was one of the prime short sellers leading the charge against the pack, came out with a report questioning its business practices. Coming as it does the day after the provider of English-language training and standardized test prep for Chinese youth revealed the SEC was investigating it -- leading to a nearly identical drop in its stock -- it amounted to a one-two punch for investors.
The Muddy Waters report charges New Oriental with lying about not having franchisees. The say it actually has scads of them, which allows it to improperly "inflate its cash balances in order to receive unqualified audit opinions from its auditor."
Investors might have thought that by this point the dreck of Chinese stocks had been cleared out, but if the allegations and investigations pan out, it's obvious caution is still needed -- though due diligence is always required before you sink your money in. Let me know in the comments section below whether the tag-team duo of SEC investigations and charges of fraud by Muddy Waters has shaken your confidence in Chinese stocks again.
Phat chance
I'll admit to some surprise that Arena Pharmaceuticals dropped as it did on news of the FDA's approval of rival anti-obesity drug Qsymia (formerly known as Qnexa). This is news? The market didn't anticipate that VIVUS (Nasdaq: VVUS ) would be first-to-market? Maybe it was just the reality of having the news settle in.
While Qsymia will be ahead of Arena's Belviq (who comes up with these names?), the few months' lead time might not really give VIVUS an advantage, since it does come with some labeling limitations (although so does Belviq). Yet as the Fool's Brian Orelli notes, it's going to be a while anyway before one can be declared a winner over the other:
"The question becomes whether Belviq's cleaner side-effect profile and potentially stronger marketing muscle can overcome Qsymia's better weight loss. Assuming most patients aren't covered by insurance, the cost of the drugs could be the deciding factor."
While I figured some of the euphoria would wear off on Arena's own approval (we still need to wait for drug scheduling, which is the reason for its delay to market), I think there's still monster potential here, and I'll be trading my underperform rating of the pharma upstart to an outperform. There's a big enough opportunity in obesity for several companies to succeed.
Tell me on the Arena Pharmaceuticals CAPS page or in the comments box below whether you think the stock has pulled back enough to warrant an entry into it at this time.
Ready for a resurrection
Health-care investors are always looking for the next big drug breakthrough like Belviq and Qsymia, and Motley Fool co-founder David Gardner recently identified one small-cap health-care company he believes is poised for monster returns. To uncover this top pick today, enjoy the special free report: "Discover the Next Rule-Breaking Multibagger." Don't miss out on this limited-time offer and your opportunity to discover this game-changing company before the market does. Access your report -- it's totally free.
....aktuell läuft ARENA gegen den Trend...
DAX -3,19%
Dow Jones -1,22%
TecDAX -3,11%
Nasdaq -1,96%
ARNA 10.33 ( + 8.57%)
DAX -3,19%
Dow Jones -1,22%
TecDAX -3,11%
Nasdaq -1,96%
ARNA 10.33 ( + 8.57%)

Gut das ich chance zum billigshopping letzte woche genutzt habe :-)....ceo von vivus hat letzte woche shares verkauft.........ratten verlassen das sinkende schiff !! Belviq wird wohl das maß der dinge werden
Zitat von Earthfire: ..ceo von vivus hat letzte woche shares verkauft.........ratten verlassen das sinkende schiff...
YEPP !!!!!!!!!!
VIVUS Inc. (VVUS) > 21.98 Down 2.17(- 8.97%) 11:43AM EDT
7/23/2012 @ 9:50AM
Top CEO Sells: GTXI, VVUS, TWX
According to GuruFocus Insider Data, these are the largest CEO sales during the past week:
GTx, Inc, VIVUS, Inc, Time Warner Inc.
CEO of VIVUS, Inc. (VVUS) Leland F Wilson sold 100,000 shares on 07/18/2012 at an average price of $30.36. VIVUS is a biopharmaceutical company developing innovative, next-generation therapies to address unmet needs in obesity, diabetes and sexual health.
Vivus, Inc. has a market cap of $2.41 billion; its shares were traded at around $24.26 with and P/S ratio of 48.11.
VIVUS Inc reported their first quarter 2012 quarter results. The Company reported net loss of $18.8 million.
CEO Leland F Wilson sold 400,000 shares of VVUS stock in February, June, and July. SVP, Finance and CFO
Timothy E Morris sold 354,358 shares of VVUS stock in February and July.
Chief Commercial Officer Michael Patrick Miller, VP, Operations and General Mgr Guy P Marsh, VP, Clinical Research Wesley Day, President Peter Y Tam, and VP, General Counsel John L Slebir sold 112,991 shares of VVUS stock in July.
http://www.forbes.com/sites/gurufocus/2012/07/23/top-ceo-sel…" target="_blank" rel="nofollow ugc noopener">
http://www.forbes.com/sites/gurufocus/2012/07/23/top-ceo-sel…
heute muss wieder die drache fliegen, damit der thread wieder lebendig wird



dow, nasdaq tief rot

Suzhou Connect Biopharmaceuticals In-Licenses Anti-Inflammatory Compounds from Arena Pharmaceuticals
TAICANG, Suzhou, China, July 24, 2012 /PRNewswire-iReach/ --
Suzhou Connect Biopharmaceuticals, Ltd. today announced it, through its wholly owned subsidiary Connect Biopharm LLC in San Diego, has entered into an exclusive worldwide licensing agreement with Arena Pharmaceuticals, Inc., for the development and commercialization of novel small molecule compounds intended for the treatment of allergic rhinitis and atopic dermatitis.
http://www.prnewswire.com/news-releases/suzhou-connect-bioph…" target="_blank" rel="nofollow ugc noopener">
http://www.prnewswire.com/news-releases/suzhou-connect-bioph…
Antwort auf Beitrag Nr.: 43.421.147 von bernie55 am 25.07.12 10:55:51
dem kurs hat die meldung überhaupt nicht beeinflusst
dem kurs hat die meldung überhaupt nicht beeinflusst
Zitat von gauner1: dem kurs hat die meldung überhaupt nicht beeinflusst
....da dachte ich ,ich könnte wieder " bashen"..und ??..nix war`s mit dem UP

.....Spaß beiseite....

.....nicht jede Message muss unweigerlich zu einem UP führen , doch wird an dieser News deutlich , dass ARENA neben BELVIQ auch mit seinem " medizinischen Know-How " die Entwicklungsprozesse von Medikamenten in China unterstützen kann...
..und das ist für das Image von ARENA im Asiatischen Raum bestimmt nicht schlecht...
Antwort auf Beitrag Nr.: 43.424.853 von bernie55 am 26.07.12 08:31:30....so, jetzt kommt hoffentlich endlich mal etwas mehr " Ruhe " in die Aktie - quasi eine kleine Verschnaufpause...
..und das tut sicherlich nicht nur uns gut...
..hier die gestrige Trading-Range vom 26.07.12...
Hoch 9,69 USD
Tief 9,36 USD
22:00 Uhr > 9,555 USD + 0,68%
..das sieht so richtig " entspannend" aus...
..und das tut sicherlich nicht nur uns gut...

..hier die gestrige Trading-Range vom 26.07.12...
Hoch 9,69 USD
Tief 9,36 USD
22:00 Uhr > 9,555 USD + 0,68%
..das sieht so richtig " entspannend" aus...

Antwort auf Beitrag Nr.: 43.430.382 von bernie55 am 27.07.12 11:00:47morgen.
es ist zwar egal, aber dein schlußkurs von 9,55 ist völlig falsch. schlußkurs 9,42, minus 14%
du hast deinen schlußkurs vom tageschart entnommen und das ist nicht in ordnung. siehe die gesterige kurshiszorie
hier die kurse
16:00:13 $ 9.42 733
16:00:13 $ 9.42 4,388
16:00:13 $ 9.42 3,926
16:00:13 $ 9.42 217
16:00:09 $ 9.4189 500
16:00:08 $ 9.4185 100
16:00:08 $ 9.4185 500
16:00:08 $ 9.4185 400
16:00:07 $ 9.41 100
16:00:06 $ 9.41 1,000
16:00:04 $ 9.41 500
16:00:03 $ 9.41 100
16:00:01 $ 9.42 92,437
16:00:01 $ 9.42 92,437
15:59:59 $ 9.41 100
15:59:59 $ 9.41 100
15:59:59 $ 9.42 200
15:59:59 $ 9.42 615
15:59:59 $ 9.415 100
15:59:59 $ 9.42 100
15:59:59 $ 9.41 100
15:59:59 $ 9.41 100
15:59:59 $ 9.41 100
15:59:58 $ 9.42 100
15:59:57 $ 9.425 200
15:59:57 $ 9.42 100
15:59:57 $ 9.42 1,000
15:59:57 $ 9.42 900
15:59:57 $ 9.42 500
15:59:57 $ 9.42 200
15:59:57 $ 9.42 100
15:59:57 $ 9.42 100
15:59:57 $ 9.42 100
15:59:56 $ 9.4299 500
15:59:56 $ 9.42 100
15:59:56 $ 9.42 100
15:59:56 $ 9.42 100
15:59:56 $ 9.41 100
15:59:54 $ 9.42 100
15:59:54 $ 9.422 100
15:59:54 $ 9.42 100
15:59:52 $ 9.41 300
15:59:52 $ 9.41 864
15:59:52 $ 9.41 107
15:59:52 $ 9.41 100
15:59:52 $ 9.41 2,385
15:59:52 $ 9.41 330
15:59:52 $ 9.41 822
15:59:52 $ 9.41 100
15:59:52 $ 9.41 136
Read more: http://www.nasdaq.com/symbol/arna/time-sales#ixzz21tmf1D7v
es ist zwar egal, aber dein schlußkurs von 9,55 ist völlig falsch. schlußkurs 9,42, minus 14%
du hast deinen schlußkurs vom tageschart entnommen und das ist nicht in ordnung. siehe die gesterige kurshiszorie
hier die kurse
16:00:13 $ 9.42 733
16:00:13 $ 9.42 4,388
16:00:13 $ 9.42 3,926
16:00:13 $ 9.42 217
16:00:09 $ 9.4189 500
16:00:08 $ 9.4185 100
16:00:08 $ 9.4185 500
16:00:08 $ 9.4185 400
16:00:07 $ 9.41 100
16:00:06 $ 9.41 1,000
16:00:04 $ 9.41 500
16:00:03 $ 9.41 100
16:00:01 $ 9.42 92,437
16:00:01 $ 9.42 92,437
15:59:59 $ 9.41 100
15:59:59 $ 9.41 100
15:59:59 $ 9.42 200
15:59:59 $ 9.42 615
15:59:59 $ 9.415 100
15:59:59 $ 9.42 100
15:59:59 $ 9.41 100
15:59:59 $ 9.41 100
15:59:59 $ 9.41 100
15:59:58 $ 9.42 100
15:59:57 $ 9.425 200
15:59:57 $ 9.42 100
15:59:57 $ 9.42 1,000
15:59:57 $ 9.42 900
15:59:57 $ 9.42 500
15:59:57 $ 9.42 200
15:59:57 $ 9.42 100
15:59:57 $ 9.42 100
15:59:57 $ 9.42 100
15:59:56 $ 9.4299 500
15:59:56 $ 9.42 100
15:59:56 $ 9.42 100
15:59:56 $ 9.42 100
15:59:56 $ 9.41 100
15:59:54 $ 9.42 100
15:59:54 $ 9.422 100
15:59:54 $ 9.42 100
15:59:52 $ 9.41 300
15:59:52 $ 9.41 864
15:59:52 $ 9.41 107
15:59:52 $ 9.41 100
15:59:52 $ 9.41 2,385
15:59:52 $ 9.41 330
15:59:52 $ 9.41 822
15:59:52 $ 9.41 100
15:59:52 $ 9.41 136
Read more: http://www.nasdaq.com/symbol/arna/time-sales#ixzz21tmf1D7v
berichtigung. tippfehler. minus 1,4%
Zitat von gauner1: morgen.
es ist zwar egal, aber dein schlußkurs von 9,55 ist völlig falsch. schlußkurs 9,42, minus 14%
Hallo gauner1,
ich schrieb:
..hier die gestrige Trading-Range vom 26.07.12......also nicht vom Freitag,d.27.07.12 !!!
Hoch 9,69 USD
Tief 9,36 USD
22:00 Uhr > 9,555 USD + 0,68%
...das war der Abschlusskurs vom Donnerstag!!!!
...somit dürfte dein Hinweis auf " völlig falsch" hinfällig sein...

..und wenn du jetzt die Days-Range vom 27.07.12 betrachtest, dann liegt die Trading-Range bei
Day's Range: 9.37 - 9.60
Abschlusskurs ist 9,42 USD, korrekt gauner1...

...aber dies entspricht einem Minus von 1,41% und nicht Minus 14 %...

Ich gehe jetzt einfach mal davon aus, dass du heute morgen um 9.17 Uhr noch nicht richtig ausgeschlafen hast und du aufgrund deiner " Altlast-Erfahrungen" mit Arena noch im morgendlichen Tiefschlaf von Minus 14 geträumt hast...

....und siehe da, jetzt gerade gesehen.....
Um 9.18 Uhr bist du etwas wacher geworden und bist dann doch noch auf die 1,4 Minus gekommen bist....


So long, gauner1

Antwort auf Beitrag Nr.: 43.430.382 von bernie55 am 27.07.12 11:00:47
achso, du hast mit 2,555 den sk von donnerstag gemeint. das ist auch ebenfals falsch. der sk von donnerstag war 2,554



mit 14% minus wollte ich dir angst machen, aber anscheind hat das nicht geklappt.


übrigens bei uns in teneriffa haben wir tolles wetter. wie ist es bei euch in alemania ? immer noch scheisswetter ?
achso, du hast mit 2,555 den sk von donnerstag gemeint. das ist auch ebenfals falsch. der sk von donnerstag war 2,554




mit 14% minus wollte ich dir angst machen, aber anscheind hat das nicht geklappt.



übrigens bei uns in teneriffa haben wir tolles wetter. wie ist es bei euch in alemania ? immer noch scheisswetter ?
Antwort auf Beitrag Nr.: 43.430.382 von bernie55 am 27.07.12 11:00:47
eine afrikanische gruppe aus sudan macht im juni,juli,august also 3 monate in deutschland urlaub. nach dem rückkehr wird gefragt, wie war es in deutschland und wie war das wetter ?
ganz toll, alles herlich und das wetter ganz ganz toll. es hat 3 monate täglich nur geregnet.
eine afrikanische gruppe aus sudan macht im juni,juli,august also 3 monate in deutschland urlaub. nach dem rückkehr wird gefragt, wie war es in deutschland und wie war das wetter ?
ganz toll, alles herlich und das wetter ganz ganz toll. es hat 3 monate täglich nur geregnet.
Zitat von gauner1: achso, du hast mit 2,555 den sk von donnerstag gemeint. das ist auch ebenfals falsch. der sk von donnerstag war 2,554
mit 14% minus wollte ich dir angst machen, aber anscheind hat das nicht geklappt.
übrigens bei uns in teneriffa haben wir tolles wetter. wie ist es bei euch in alemania ? immer noch scheisswetter ?
..dann hoffen wir mal, dass deine Gurken bei diesem tollen Wetter nicht vertrocknen...

..
Eisai and Arena Pharmaceuticals Complete Transfer of BELVIQ® (lorcaserin HCl) New Drug Application
-- Eisai Plans to Submit Applications for Marketing Approval throughout the Americas --
Press Release: Arena Pharmaceuticals, Inc. – 1 hour 12 minutes ago.. .
.
Email
Print
... .
.
Companies:.
.
.Arena Pharmaceuticals, Inc.
.
.
.
RELATED QUOTES.
.
Symbol
Price
Change
ARNA
9.42
.
.
..
.
.
..
.
.
WOODCLIFF LAKE, N.J., and SAN DIEGO, July 30, 2012 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) announced today that Arena has transferred the BELVIQ® (lorcaserin hydrochloride) New Drug Application (NDA) to Eisai. The transfer establishes Eisai as the marketing authorization holder responsible for regulatory activities in the United States related to the commercialization of BELVIQ, including pharmacovigilance requirements. Arena will continue in its oversight role for the global development of BELVIQ while working closely with Eisai for the joint development plan in the United States.
"The NDA transfer furthers our commitment to BELVIQ as we prepare to launch in the United States and submit applications for approval throughout the Americas," said Lonnel Coats, President and Chief Executive Officer, Eisai Inc.
Eisai plans to submit marketing authorization applications based on the NDA to the health authorities throughout the territories in North and South America for which it currently holds commercialization rights, including Mexico, Brazil and Canada.
"We look forward to further exploring the therapeutic potential of BELVIQ and supporting Eisai's efforts to obtain marketing approval in additional North and South American countries," said Jack Lief, Arena's President and Chief Executive Officer.
About Arena Pharmaceuticals
Arena Pharmaceuticals is a biopharmaceutical company focused on discovering, developing and commercializing innovative medicines that selectively target G protein-coupled receptors. Arena is committed to delivering novel treatment options to patients for weight management, cardiovascular disease, inflammation and other disorders. Arena's US operations are in San Diego, California, and its operations outside of the United States, including its commercial manufacturing facility, are located in Zofingen, Switzerland. For more information about Arena, please visit www.arenapharm.com.
Arena Pharmaceuticals® and Arena® are registered service marks of Arena Pharmaceuticals, Inc.
BELVIQ® is a registered trademark of Arena Pharmaceuticals GmbH.
About Eisai Inc.
Eisai Inc. was established in 1995 and began marketing its first product in the United States in 1997. Since that time, Eisai Inc. has rapidly grown to become a fully integrated pharmaceutical business. The company serves as the U.S. pharmaceutical operation of Eisai Co., Ltd., a research-based human health care (hhc) company that discovers, develops and markets products throughout the world.
Eisai has a global product creation organization that includes US-based R&D facilities in Massachusetts, New Jersey, North Carolina and Pennsylvania, as well as manufacturing facilities in Maryland and North Carolina. The company's areas of R&D focus include neuroscience; oncology; vascular, inflammatory and immunological reaction; and antibody-based programs. For more information about Eisai, please visit www.eisai.com/US.
Forward-Looking Statements
Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include statements about Eisai's plans to submit marketing authorizations; Arena's and Eisai's roles in continuing development; the potential launch of BELVIQ; the therapeutic potential of BELVIQ; and Arena's efforts, focus, goals, strategy, research and development programs, and ability to develop compounds and commercialize drugs. For such statements, Arena claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Arena's expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, the following: the timing and outcome of regulatory review is uncertain; risks related to commercializing drugs, including regulatory, manufacturing and supply issues and the pace of market acceptance; cash and revenues generated from BELVIQ, including timing and impact of competition; government and commercial reimbursement and pricing decisions; risks related to relying on collaborative agreements; the timing and receipt of payments and fees, if any, from collaborators; unexpected or unfavorable new data; nonclinical and clinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Arena or Eisai, request additional information, have additional recommendations or change their guidance or requirements before or after approval; data and other information related to any of Arena's research and development programs may not meet safety, efficacy or other regulatory requirements or otherwise be sufficient for regulatory review, approval or continued marketing; Arena's ability to obtain and defend patents; the timing, success and cost of Arena's research and development programs; results of clinical trials and other studies are subject to different interpretations and may not be predictive of future results; clinical trials and other studies may not proceed at the time or in the manner expected or at all; having adequate funds; and satisfactory resolution of litigation or other disagreements with others. Additional factors that could cause actual results to differ materially from those stated or implied by Arena's forward-looking statements are disclosed in Arena's filings with the Securities and Exchange Commission. These forward-looking statements represent Arena's judgment as of the time of this release. Arena disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
Contacts: Arena Pharmaceuticals, Inc.
Contacts: Eisai Inc.
858.453.7200
Eisai and Arena Pharmaceuticals Complete Transfer of BELVIQ® (lorcaserin HCl) New Drug Application
-- Eisai Plans to Submit Applications for Marketing Approval throughout the Americas --
Press Release: Arena Pharmaceuticals, Inc. – 1 hour 12 minutes ago.. .
.
... .
.
Companies:.
.
.Arena Pharmaceuticals, Inc.
.
.
.
RELATED QUOTES.
.
Symbol
Price
Change
ARNA
9.42
.
.
..
.
.
..
.
.
WOODCLIFF LAKE, N.J., and SAN DIEGO, July 30, 2012 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) announced today that Arena has transferred the BELVIQ® (lorcaserin hydrochloride) New Drug Application (NDA) to Eisai. The transfer establishes Eisai as the marketing authorization holder responsible for regulatory activities in the United States related to the commercialization of BELVIQ, including pharmacovigilance requirements. Arena will continue in its oversight role for the global development of BELVIQ while working closely with Eisai for the joint development plan in the United States.
"The NDA transfer furthers our commitment to BELVIQ as we prepare to launch in the United States and submit applications for approval throughout the Americas," said Lonnel Coats, President and Chief Executive Officer, Eisai Inc.
Eisai plans to submit marketing authorization applications based on the NDA to the health authorities throughout the territories in North and South America for which it currently holds commercialization rights, including Mexico, Brazil and Canada.
"We look forward to further exploring the therapeutic potential of BELVIQ and supporting Eisai's efforts to obtain marketing approval in additional North and South American countries," said Jack Lief, Arena's President and Chief Executive Officer.
About Arena Pharmaceuticals
Arena Pharmaceuticals is a biopharmaceutical company focused on discovering, developing and commercializing innovative medicines that selectively target G protein-coupled receptors. Arena is committed to delivering novel treatment options to patients for weight management, cardiovascular disease, inflammation and other disorders. Arena's US operations are in San Diego, California, and its operations outside of the United States, including its commercial manufacturing facility, are located in Zofingen, Switzerland. For more information about Arena, please visit www.arenapharm.com.
Arena Pharmaceuticals® and Arena® are registered service marks of Arena Pharmaceuticals, Inc.
BELVIQ® is a registered trademark of Arena Pharmaceuticals GmbH.
About Eisai Inc.
Eisai Inc. was established in 1995 and began marketing its first product in the United States in 1997. Since that time, Eisai Inc. has rapidly grown to become a fully integrated pharmaceutical business. The company serves as the U.S. pharmaceutical operation of Eisai Co., Ltd., a research-based human health care (hhc) company that discovers, develops and markets products throughout the world.
Eisai has a global product creation organization that includes US-based R&D facilities in Massachusetts, New Jersey, North Carolina and Pennsylvania, as well as manufacturing facilities in Maryland and North Carolina. The company's areas of R&D focus include neuroscience; oncology; vascular, inflammatory and immunological reaction; and antibody-based programs. For more information about Eisai, please visit www.eisai.com/US.
Forward-Looking Statements
Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include statements about Eisai's plans to submit marketing authorizations; Arena's and Eisai's roles in continuing development; the potential launch of BELVIQ; the therapeutic potential of BELVIQ; and Arena's efforts, focus, goals, strategy, research and development programs, and ability to develop compounds and commercialize drugs. For such statements, Arena claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Arena's expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, the following: the timing and outcome of regulatory review is uncertain; risks related to commercializing drugs, including regulatory, manufacturing and supply issues and the pace of market acceptance; cash and revenues generated from BELVIQ, including timing and impact of competition; government and commercial reimbursement and pricing decisions; risks related to relying on collaborative agreements; the timing and receipt of payments and fees, if any, from collaborators; unexpected or unfavorable new data; nonclinical and clinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Arena or Eisai, request additional information, have additional recommendations or change their guidance or requirements before or after approval; data and other information related to any of Arena's research and development programs may not meet safety, efficacy or other regulatory requirements or otherwise be sufficient for regulatory review, approval or continued marketing; Arena's ability to obtain and defend patents; the timing, success and cost of Arena's research and development programs; results of clinical trials and other studies are subject to different interpretations and may not be predictive of future results; clinical trials and other studies may not proceed at the time or in the manner expected or at all; having adequate funds; and satisfactory resolution of litigation or other disagreements with others. Additional factors that could cause actual results to differ materially from those stated or implied by Arena's forward-looking statements are disclosed in Arena's filings with the Securities and Exchange Commission. These forward-looking statements represent Arena's judgment as of the time of this release. Arena disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
Contacts: Arena Pharmaceuticals, Inc.
Contacts: Eisai Inc.
858.453.7200
Zitat von Magnetfeldfredy: ..
Eisai and Arena Pharmaceuticals Complete Transfer of BELVIQ® (lorcaserin HCl) New Drug Application
Eisai and Arena Pharmaceuticals Complete Transfer of BELVIQ® (lorcaserin HCl) New Drug Application
WOODCLIFF LAKE, N.J., and SAN DIEGO, July 30, 2012 /PRNewswire/ -- Eisai Inc. and Arena Pharmaceuticals, Inc. (ARNA) announced today that Arena has transferred the BELVIQ® (lorcaserin hydrochloride) New Drug Application (NDA) to Eisai. The transfer establishes Eisai as the marketing authorization holder responsible for regulatory activities in the United States related to the commercialization of BELVIQ, including pharmacovigilance requirements.
Arena will continue in its oversight role for the global development of BELVIQ while working closely with Eisai for the joint development plan in the United States.
"The NDA transfer furthers our commitment to BELVIQ as we prepare to launch in the United States and submit applications for approval throughout the Americas," said Lonnel Coats, President and Chief Executive Officer, Eisai Inc.
Eisai plans to submit marketing authorization applications based on the NDA to the health authorities throughout the territories in North and South America for which it currently holds commercialization rights, including Mexico, Brazil and Canada.
"We look forward to further exploring the therapeutic potential of BELVIQ and supporting Eisai's efforts to obtain marketing approval in additional North and South American countries," said Jack Lief, Arena's President and Chief Executive Officer.
http://finance.yahoo.com/news/eisai-arena-pharmaceuticals-co…
kann jamand mir sagen, was los ist ?
8,511


8,511



Antwort auf Beitrag Nr.: 43.442.757 von gauner1 am 31.07.12 19:16:12Alle Biotechs bluten, kann noch dauern!

komischer tag heute, fast alle biotech sind heute ganz schwer runter.
bei arna hätte heute mindestens 10-20% plus geben müssen
bei arna hätte heute mindestens 10-20% plus geben müssen
Zitat von gauner1: komischer tag heute, fast alle biotech sind heute ganz schwer runter.
bei arna hätte heute mindestens 10-20% plus geben müssen
nabend zusammen....fast alle trifft es !

ich bin noch in Amrs-NSPH- und ZLCS bin ich seit heute investiert....die haben grün geschlossen....und das Volumen Intrady lässt auch dort auf einen weiteren Anstieg hoffen.
Arena habe ich heute verkauft..komplett..dh. die deutsche und die US-posi.
Man könnte sagen Schwein gehabt...war aber nicht so..
Ich bin kein Chartexperte...bin aber mit einem befreundet (Bänker)....dieser hat mir heute vormittag geraten zu verkaufen....nach seiner Analyse würde das CT nicht gut aussehen, und könnte noch mehr down gehen.
Keine Ahnung ob es zutrifft...zumindest hatte er heute Recht...
Fundamental lässt sich das zur Zeit....meiner ansicht nach nicht begünden.
Denke das zur Zeit einfach die Käuferseite zu schwach ist.die Shorties leichtes Spiel haben..und die Longs ihre Gewinne eintüten.
Steige wieder ein, wenn Arena stabil ins Plus läuft.....
Antwort auf Beitrag Nr.: 43.443.521 von Earthfire am 31.07.12 22:56:57
und warum sind fast alle runter ? DNDN sogar 23%
und warum sind fast alle runter ? DNDN sogar 23%
aber diese gurke will heute steigen. dndn nicht
ich nehme an, dass man auf fallende kurse gesetzt hat. wir haben keinen fundamentalen grund, dass der kurs von 11,50 bis jetzt ohne rebound nur fällt.
im gegenteil, gestern haben wir sogar gute nachricht gehabt
im gegenteil, gestern haben wir sogar gute nachricht gehabt
korrigiere
seit 11,80 permanent gefallen und das war am 9.6.12
seit 11,80 permanent gefallen und das war am 9.6.12
Nunja die Zulassungsphantasien bez. ARNA und VVUS sind vorbei und das Kapital sucht sich jetzt wieder andere Spielwiesen. Jetzt geht es darum ob man mit dem Verkauf in den nächsten Monaten profitabel wird bzw. ob die Umsatzerwartungen erfüllt werden.
Bei DNDN sind sicherlich keine Shortspekulanten schuld sondern schlicht und ergreifend die Quartalszahlen und der Ausblick, des weiteren werden 41% der Mitarbeiter entlassen und man schließt wohl das größte Produktionswerk für Provenge weil man gezwungen ist Kosten zu sparen...
Wenn das keine Hiobsbotschaften sind.
Bei DNDN sind sicherlich keine Shortspekulanten schuld sondern schlicht und ergreifend die Quartalszahlen und der Ausblick, des weiteren werden 41% der Mitarbeiter entlassen und man schließt wohl das größte Produktionswerk für Provenge weil man gezwungen ist Kosten zu sparen...
Wenn das keine Hiobsbotschaften sind.
Antwort auf Beitrag Nr.: 43.446.654 von MACD am 01.08.12 18:27:47
ja, schon, aber was hat mit arna zu tun ? der kurs fällt permanent seit 3 wochen.
entweder sind die shorties, oder es ist etwas faul. ich denke jedoch die shorties.
ja, schon, aber was hat mit arna zu tun ? der kurs fällt permanent seit 3 wochen.
entweder sind die shorties, oder es ist etwas faul. ich denke jedoch die shorties.
Naja dann passt die Schwäche doch ins Bild die nach der Zulassung eingesetzt hat. Mir fällt immer auf das in den Boards betr. hochspekulativer Aktien generell Shortys oder Hedge-Fonds usw. für starke Kursverluste verantwortlich gemacht werden und diese sich dann wieder eindecken müssten, aber so einfach kann doch die Rechnung nicht sein.
Jeder der hier ungehedgt shortet hat doch volles Risiko nach oben wenn doch was positives wie ein Buyout etc. durchkommt. Hier werden eben Gewinne mitgenommen und vermutlich auch ein kommendes Cash-burning eingepreist, aber das dauert eben.
Chartmäßig schreit der Kurs ja ebenfalls nach einer Korrektur. Ich glaube nicht das da sobald Momentum nach oben reinkommt. Vielleicht die nächsten Wochen abwarten bis Ende September und dann vor den starken Wintermonaten und US-Wahlen kaufen.
Jeder der hier ungehedgt shortet hat doch volles Risiko nach oben wenn doch was positives wie ein Buyout etc. durchkommt. Hier werden eben Gewinne mitgenommen und vermutlich auch ein kommendes Cash-burning eingepreist, aber das dauert eben.
Chartmäßig schreit der Kurs ja ebenfalls nach einer Korrektur. Ich glaube nicht das da sobald Momentum nach oben reinkommt. Vielleicht die nächsten Wochen abwarten bis Ende September und dann vor den starken Wintermonaten und US-Wahlen kaufen.
Antwort auf Beitrag Nr.: 43.446.821 von MACD am 01.08.12 19:25:16
alles ganz richtig
was mir auffällt, sind die shorties, die glattstellen müssen. wo sind sie seit genau 3 wochen ?
alles ganz richtig
was mir auffällt, sind die shorties, die glattstellen müssen. wo sind sie seit genau 3 wochen ?
Antwort auf Beitrag Nr.: 43.446.821 von MACD am 01.08.12 19:25:16
ja natürlich. das ist das risiko der shorties. wenn was positives kommt, kommen sie durch das hochkaufen in enorme verluste, was ich denen gönne und zwar bis zum verrecken
ja natürlich. das ist das risiko der shorties. wenn was positives kommt, kommen sie durch das hochkaufen in enorme verluste, was ich denen gönne und zwar bis zum verrecken
Also ich bin mit einer Posi zu 11,56 Dollar Short !
Darf nur kein Buyout kommen, dann zerreißt es mich.
Würde bei knapp unter 7 Dollar wieder glattstellen und Long gehen !
Darf nur kein Buyout kommen, dann zerreißt es mich.
Würde bei knapp unter 7 Dollar wieder glattstellen und Long gehen !
vor einigen wochen wurde eine ke mit 12 mil. aktien zu 5,50 usd gemacht. ich hoffe, dass der kurs nicht wieder dahin kommt.
für dich jimmy wäre bestens
für dich jimmy wäre bestens
Antwort auf Beitrag Nr.: 43.446.966 von gauner1 am 01.08.12 20:04:58Nein, kann mir nicht vorstellen, dass es soweit runter läüft !
Werde sicherlich über 6 Dollar wieder einsteigen, wenn auch nur mit 50 %
der gewünschten Positionsgröße !
Werde sicherlich über 6 Dollar wieder einsteigen, wenn auch nur mit 50 %
der gewünschten Positionsgröße !
Zitat von gauner1: ich nehme an, dass man auf fallende kurse gesetzt hat. wir haben keinen fundamentalen grund, dass der kurs von 11,50 bis jetzt ohne rebound nur fällt.
im gegenteil, gestern haben wir sogar gute nachricht gehabt
wenn der kurs bei positiven neuigkeiten fällt, dann ist dass ein sehr schlechtes zeichen.
Zitat von steven_trader:Zitat von gauner1: ich nehme an, dass man auf fallende kurse gesetzt hat. wir haben keinen fundamentalen grund, dass der kurs von 11,50 bis jetzt ohne rebound nur fällt.
im gegenteil, gestern haben wir sogar gute nachricht gehabt
wenn der kurs bei positiven neuigkeiten fällt, dann ist dass ein sehr schlechtes zeichen.
Solange keine Zahlen bezüglich der zukünftigen Kosten...Absatzprognosen von Belviq...zukünftige Ertragsprognosen auf dem Tisch liegen, bleibt Arena ein Spielball der Zocker d.h. weiterhin extrem Volatil !
Theoretisch wäre auch noch eine KE möglich....je nachdem was an Cash für die Produktion und die Vermarktung von Belviq benötigt wird.
Nach dem getstrigen Verlauf halte ich auch einen Kurs von unter 7 $ in dieser Woche für möglich...
Ein buyout ist natürlich auch jederzeit möglich...

hoffen wir das beste !!!
Long zu 5,89 über tradegate !
Aber nur kleine Posi a 600 St.
Aber nur kleine Posi a 600 St.
Antwort auf Beitrag Nr.: 43.451.051 von JimmySpoon am 02.08.12 19:10:06
bist du auch jetzt gurkenhändler geworden ? also schön giessen und düngen und geduldig sein, bis die gurke wächst
bist du auch jetzt gurkenhändler geworden ? also schön giessen und düngen und geduldig sein, bis die gurke wächst
Antwort auf Beitrag Nr.: 43.451.051 von JimmySpoon am 02.08.12 19:10:06
berni
weiß du, ob eisai auch produziert ? oder macht nur die vermarktung
berni
weiß du, ob eisai auch produziert ? oder macht nur die vermarktung
lars
heute war wieder shortitag
heute war wieder shortitag
Quatsch, Heute ist Sonntag
warum geht der kurs täglich nur runter ?
gestern war der kurs so stabil und heute ?
!
Dieser Beitrag wurde von MODernist moderiert. Grund: Spam, WerbungEinfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
ich schätze das der Boden erreicht ist und in den nächsten Tagen der Kurs wieder steigen wird.
Mal sehen wie lange wir noch warten müssen.

Antwort auf Beitrag Nr.: 43.476.187 von Poppholz am 09.08.12 17:07:40Momentan läuft der Kurs seitwärts bei um die $7,20. Mal sehen ob es bald wieder hoch geht:
Arena Pharmaceuticals-Aktie: "buy"
13.08.12 11:38
Jefferies & Co
New York (www.aktiencheck.de) - Thomas Wei, Thomas Nguyen, Jeffrey Hung und Shaunak Deepak, Analysten von Jefferies & Co stufen die Arena Pharmaceuticals-Aktie mit dem Rating "buy" ein. Das Kursziel werde bei 20 USD belassen. (Analyse vom 10.08.2012) (13.08.2012/ac/a/a)
http://www.aktiencheck.de/analysen/Artikel-Arena_Pharmaceuti…
Arena Pharmaceuticals-Aktie: "buy"
13.08.12 11:38
Jefferies & Co
New York (www.aktiencheck.de) - Thomas Wei, Thomas Nguyen, Jeffrey Hung und Shaunak Deepak, Analysten von Jefferies & Co stufen die Arena Pharmaceuticals-Aktie mit dem Rating "buy" ein. Das Kursziel werde bei 20 USD belassen. (Analyse vom 10.08.2012) (13.08.2012/ac/a/a)
http://www.aktiencheck.de/analysen/Artikel-Arena_Pharmaceuti…
Antwort auf Beitrag Nr.: 43.489.661 von Poppholz am 14.08.12 07:56:01den regen Gesprächen hier im Thread gehe ich mal davon aus, dass ich (auch) hier der einzige Aktionär bin.

Nun gut, der Knoten scheint geplatzt und der Kurs steigt.
Dann bin ich mal gespannt, ob wir den Boden jetzt auch langfristig hinter uns lassen.


Nun gut, der Knoten scheint geplatzt und der Kurs steigt.
Dann bin ich mal gespannt, ob wir den Boden jetzt auch langfristig hinter uns lassen.

Antwort auf Beitrag Nr.: 43.494.236 von Poppholz am 15.08.12 07:43:00Nö bist nicht der einzige...
Gestern zweite Charge 1k über Tradegate zu 6,22 gekauft !
Schnitt somit 1,7k zu 6,11, könnte jetzt die nächste Welle geben !
Gestern zweite Charge 1k über Tradegate zu 6,22 gekauft !
Schnitt somit 1,7k zu 6,11, könnte jetzt die nächste Welle geben !
Ich bin auch noch dabei


im Chart schön zu erkennen, wie der Kurs den Abwärtstrend nach oben durchbrochen hat.
Einfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
Antwort auf Beitrag Nr.: 43.495.057 von Poppholz am 15.08.12 10:44:55
Einfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
nach 15 minuten schon 6% minus
Antwort auf Beitrag Nr.: 43.501.181 von gauner1 am 16.08.12 15:47:43nur eine Korrektur.
Steigt doch schon wieder.

Steigt doch schon wieder.

Antwort auf Beitrag Nr.: 43.501.396 von Poppholz am 16.08.12 16:25:40aber über $8,00 soll der Kurs anscheinend auch nicht gehen.
Da wird dann immer ordentlich ins Orderbuch geschmissen:

Da wird dann immer ordentlich ins Orderbuch geschmissen:
Vivius wird abgewertet:
Jefferies Group Lowers VIVUS to Underperform (VVUS)
August 17th, 2012 by Jeff Wilder
VIVUS, Inc. logoVIVUS (NASDAQ: VVUS) was downgraded by equities researchers at Jefferies Group from a “hold” rating to an “underperform” rating in a report issued on Friday. They currently have a $16.00 target price on the stock, down from their previous target price of $31.00.
Jefferies Group Lowers VIVUS to Underperform (VVUS)
August 17th, 2012 by Jeff Wilder
VIVUS, Inc. logoVIVUS (NASDAQ: VVUS) was downgraded by equities researchers at Jefferies Group from a “hold” rating to an “underperform” rating in a report issued on Friday. They currently have a $16.00 target price on the stock, down from their previous target price of $31.00.
Antwort auf Beitrag Nr.: 43.506.079 von Poppholz am 17.08.12 16:09:38das ist sehr gut für uns.
Je mehr am Markt die beiden Produkte verglichen werden,desto besser steht Arena da.

Je mehr am Markt die beiden Produkte verglichen werden,desto besser steht Arena da.

Die Großen kaufen zu!!!!
Da sollte bald noch mehr schwung rein kommen.
http://beta.fool.com/beatlesforever/2012/08/20/institutional…
Da sollte bald noch mehr schwung rein kommen.
http://beta.fool.com/beatlesforever/2012/08/20/institutional…
Antwort auf Beitrag Nr.: 43.518.141 von Biotechspezialx am 21.08.12 21:25:53Wait and enjoy!
der Chartverlauf gefällt.

Antwort auf Beitrag Nr.: 43.538.259 von Poppholz am 27.08.12 17:24:28Bei Arena nach der Zulassung einfach nach Warren Buffett handeln, hinlegen und schlafen in ein paar Monaten haben wir fette Beute!

Antwort auf Beitrag Nr.: 43.538.765 von Magnetfeldfredy am 27.08.12 19:16:10denke auch, dass der Chartverlauf in den letzten Wochen (nach der Zulassung) eine Korrektur des steilen Anstieges gewesen ist.
Diese Phase haben wir aber nun abgeschlossen und der Kurs kann weiter steigen.

Diese Phase haben wir aber nun abgeschlossen und der Kurs kann weiter steigen.

Antwort auf Beitrag Nr.: 43.540.379 von Poppholz am 28.08.12 09:28:13Arena ist momentan gut in der Presse vertreten. Die "interessanten" Artikel trage ich nach Möglichkeit in den "Info-Thread" ein. Es gibt aber auch viele Artikel, in denen Arena nebenbei erwähnt wird und das ist für unsere Aktie sehr gut.
Wir werden sehen wo der Kurs uns noch hinbringen wird.

Wir werden sehen wo der Kurs uns noch hinbringen wird.

eine kleine Analyse:
http://navelliergrowth.investorplace.com/portfolio-grader/st…
sieht ganz gut aus.

http://navelliergrowth.investorplace.com/portfolio-grader/st…
sieht ganz gut aus.

gestern 4 mal an 9,35 gescheitert. heute bis jetzt 2 mal. aber bei diem ganz miesen börsenwetter darf man nicht mehr erwarten. es ist sogar erstaunlich, dass der kurs sich überhaupt hälltund sogar tendenz nach oben zeigt
@AREANICS
Gestern aus dem Urlaub zurückgekommen und volle 4 Wochen " Biotech-börsenfrei" gewesen...
....tja, das nennt man dann wohl geile Ferien......
Der punktuelle Infoservice per SMS zum Urlaubsort hat sehr gut geklappt ( Danke EARTHIE, well done ) und wie ich auf den ersten Blick sehe, hat der ARENA-INFO-Thread auch genügend Futter bekommen ( Danke Poppie, auch well done
) und wie ich auf den ersten Blick sehe, hat der ARENA-INFO-Thread auch genügend Futter bekommen ( Danke Poppie, auch well done  )
)
..so, dann werde ich in den nächsten Tagen mal meinen Wissensstand ein bisschen auf Vordermann bringen....
Grüße bernie55

Gestern aus dem Urlaub zurückgekommen und volle 4 Wochen " Biotech-börsenfrei" gewesen...

....tja, das nennt man dann wohl geile Ferien......

Der punktuelle Infoservice per SMS zum Urlaubsort hat sehr gut geklappt ( Danke EARTHIE, well done
 ) und wie ich auf den ersten Blick sehe, hat der ARENA-INFO-Thread auch genügend Futter bekommen ( Danke Poppie, auch well done
) und wie ich auf den ersten Blick sehe, hat der ARENA-INFO-Thread auch genügend Futter bekommen ( Danke Poppie, auch well done  )
)..so, dann werde ich in den nächsten Tagen mal meinen Wissensstand ein bisschen auf Vordermann bringen....

Grüße bernie55

Antwort auf Beitrag Nr.: 43.563.459 von bernie55 am 03.09.12 15:52:39....unglaublich : VIER Wochen...ist ja schon dekadent....





Antwort auf Beitrag Nr.: 43.564.860 von Tebi am 03.09.12 22:43:54ich hatte schon gedacht, dass Ihr beide verschollen seit.


Hi Ihr Lieben! 
Schaut Euch das an, könnte interessant werden!
http://www.wallstreet-online.de/diskussion/1162130-21-30/pph…
Grüsse franzisca

Schaut Euch das an, könnte interessant werden!
http://www.wallstreet-online.de/diskussion/1162130-21-30/pph…
Grüsse franzisca
heute ging die gurke rückwärts. 5,34% minus
Guten Morgen all - das Interesse scheint ja komplett nachgeslassen zu haben.
Das Teil ist nun schon über 35% im Minus seit dem letztem Höchststand im Juli- ich finde keine neuen Nachrichten und das Kursziel 20 US Dollar scheint in weite Ferne zu rücken. Ich überlege nachzukaufen oder erst mal raus aus der Aktie. Hat keiner eine Meinung mehr? Oder sind alle raus und ich der Einzige, der noch investiert ist? Es wäre doch schön, wenn man vielleicht mal die derzeitige Situation der Aktie kommentieren würde. Was denkt ihr, wie es weiter geht? Ich dachte schon gestern, dass der Abwärtstrend gestopptwird, aber wie es aussieht, geht es immer weiter runter - weiss da einer mehr? Vielen Dank für eure Meinungen und einen schönen Tag
Das Teil ist nun schon über 35% im Minus seit dem letztem Höchststand im Juli- ich finde keine neuen Nachrichten und das Kursziel 20 US Dollar scheint in weite Ferne zu rücken. Ich überlege nachzukaufen oder erst mal raus aus der Aktie. Hat keiner eine Meinung mehr? Oder sind alle raus und ich der Einzige, der noch investiert ist? Es wäre doch schön, wenn man vielleicht mal die derzeitige Situation der Aktie kommentieren würde. Was denkt ihr, wie es weiter geht? Ich dachte schon gestern, dass der Abwärtstrend gestopptwird, aber wie es aussieht, geht es immer weiter runter - weiss da einer mehr? Vielen Dank für eure Meinungen und einen schönen Tag
Arena Takedown - Is It Fair?
September 12, 2012 by Spencer Osborne
Arena Pharmaceuticals (ARNA) has developed a weight loss drug, Belviq, that is one of only a couple that have received FDA approval in the last decade. The company is awaiting DEA approvals, and then it can begin marketing what appears to many a blockbuster drug. Sales of Belviq could begin as early as late 2012 and this has investors and Wall Street excited, sending the stock to over $9.00 per share. Combine this with rumors of a possible buyout and there is little wonder why Arena saw a nice climb over the past month.
Over the past week Arena has been trending downward and now sits just above $8. Credit Suisse initiated coverage on the equity and established a $6 price target that has many passionate investors crying foul. The reasoning of these investors appears to be sound and warranted, but there are a couple of sides to every story.
What I am going to say here may be frustrating to some, but it is what it is. The market has many players in it and understanding the fundamental story of an equity is not always a recipe for investing success. Sometimes it is more advantageous to understand how the market is actually played than a moral philosophy of what is right and wrong. I know this will ire some, but it is reality.
In theory investment banks have a wall between the activities and position of the bank and the analysts opinions. In practice that may seem impossible, so investors should make an effort to understand the possibility and plan a strategy that takes advantage of it.
Arena Pharmaceuticals is on the cusp of releasing what many hope is a monumental drug (Belviq) in a monumental category (obesity). There is plenty of reason to see massive potential. In fact, many do see that potential, and that is exactly what drives the stock upward.
What we need to realize is that betting on potential is great, but we need to comprehend that we are betting on an unproven potential. What creates value is real success and real numbers. I happen to believe that the potential of Arena will develop into impressive numbers as soon as the end of this year (if released by then). I also know that this equity saw a rise upward on FDA approval and rumor of a buyout. The FDA approval is now past and digested, as is the buyout speculation. What we are left with is the potential of Belviq.
To their credit, Credit Suisse stated that Arena may not meet up to lofty expectations and I have seen some pretty lofty expectations. To their detriment, Credit Suisse also assigned lofty expectations to another drug by Orexigen because they have a pending drug in the same category. The issue is that Orexigen is a couple of years away from a realistic market debut.
Had Credit Suisse simply stopped at valuation concerns on Arena the low price target, while perhaps undeserved, would have been more palatable to investors. The combination of a low price target on Arena, a high price target on Orexigen, and the fact that Credit Suisse is buying Arena shares all add up to investors wondering whether or not the market is stacked against them.
While what is happening to Arena may not seem fair, we all have watched the market for quite some time, and the rules of the market, fair or not, need to carry weight in a trading strategy. Some will voice complaints and frustration while others will look for an edge and perhaps add to their positions. If you believe in the long term prospects of Arena, and believe that Belviq is a compelling market changer, and are holding out for that day, then you are likely worrying about something that you shouldn't be.
Analysts have opinions. Some are bullish and some are bearish. Take it all with a grain of salt. Look at the opinions, even if you do not agree with them. Likely there are many out there that will agree, and that will impact the equity in some form or another.
Fair does not really have a place in the market as frustrating as that may be. Savvy thinking does. Support is at $7.10 and is moderately strong. Resistance is at $8.75 and is very strong. Arena sits in the middle. Arena is now below all meaningful moving averages and volume is lighter than normal. Perhaps consolidation is in order now. Watch things closely over the next few days, and remember, word from the DEA could come at any time and that would be positive.
http://seekingalpha.com/article/861891-arena-takedown-is-it-…
September 12, 2012 by Spencer Osborne
Arena Pharmaceuticals (ARNA) has developed a weight loss drug, Belviq, that is one of only a couple that have received FDA approval in the last decade. The company is awaiting DEA approvals, and then it can begin marketing what appears to many a blockbuster drug. Sales of Belviq could begin as early as late 2012 and this has investors and Wall Street excited, sending the stock to over $9.00 per share. Combine this with rumors of a possible buyout and there is little wonder why Arena saw a nice climb over the past month.
Over the past week Arena has been trending downward and now sits just above $8. Credit Suisse initiated coverage on the equity and established a $6 price target that has many passionate investors crying foul. The reasoning of these investors appears to be sound and warranted, but there are a couple of sides to every story.
What I am going to say here may be frustrating to some, but it is what it is. The market has many players in it and understanding the fundamental story of an equity is not always a recipe for investing success. Sometimes it is more advantageous to understand how the market is actually played than a moral philosophy of what is right and wrong. I know this will ire some, but it is reality.
In theory investment banks have a wall between the activities and position of the bank and the analysts opinions. In practice that may seem impossible, so investors should make an effort to understand the possibility and plan a strategy that takes advantage of it.
Arena Pharmaceuticals is on the cusp of releasing what many hope is a monumental drug (Belviq) in a monumental category (obesity). There is plenty of reason to see massive potential. In fact, many do see that potential, and that is exactly what drives the stock upward.
What we need to realize is that betting on potential is great, but we need to comprehend that we are betting on an unproven potential. What creates value is real success and real numbers. I happen to believe that the potential of Arena will develop into impressive numbers as soon as the end of this year (if released by then). I also know that this equity saw a rise upward on FDA approval and rumor of a buyout. The FDA approval is now past and digested, as is the buyout speculation. What we are left with is the potential of Belviq.
To their credit, Credit Suisse stated that Arena may not meet up to lofty expectations and I have seen some pretty lofty expectations. To their detriment, Credit Suisse also assigned lofty expectations to another drug by Orexigen because they have a pending drug in the same category. The issue is that Orexigen is a couple of years away from a realistic market debut.
Had Credit Suisse simply stopped at valuation concerns on Arena the low price target, while perhaps undeserved, would have been more palatable to investors. The combination of a low price target on Arena, a high price target on Orexigen, and the fact that Credit Suisse is buying Arena shares all add up to investors wondering whether or not the market is stacked against them.
While what is happening to Arena may not seem fair, we all have watched the market for quite some time, and the rules of the market, fair or not, need to carry weight in a trading strategy. Some will voice complaints and frustration while others will look for an edge and perhaps add to their positions. If you believe in the long term prospects of Arena, and believe that Belviq is a compelling market changer, and are holding out for that day, then you are likely worrying about something that you shouldn't be.
Analysts have opinions. Some are bullish and some are bearish. Take it all with a grain of salt. Look at the opinions, even if you do not agree with them. Likely there are many out there that will agree, and that will impact the equity in some form or another.
Fair does not really have a place in the market as frustrating as that may be. Savvy thinking does. Support is at $7.10 and is moderately strong. Resistance is at $8.75 and is very strong. Arena sits in the middle. Arena is now below all meaningful moving averages and volume is lighter than normal. Perhaps consolidation is in order now. Watch things closely over the next few days, and remember, word from the DEA could come at any time and that would be positive.
http://seekingalpha.com/article/861891-arena-takedown-is-it-…
Antwort auf Beitrag Nr.: 43.593.924 von philipini am 12.09.12 10:21:01Infos und Stellungnahmen zur Aktie gibt es schon noch, aber diese sind nicht in der Lage eine Bewegung nach oben zu erzeugen.
Ich bin noch investiert und auch noch "gut" im plus.
Langfristig gehe ich von höheren Kursen aus und da ich momentan keine alternative Aktie habe, in die ich investieren möchte, bleibt der Anteil der in Arena geparkt ist auch stehen. Mein STOP LOSS liegt knapp über dem Kaufkurs und somit kann hier eigentlich nichts passieren.

Ich bin noch investiert und auch noch "gut" im plus.
Langfristig gehe ich von höheren Kursen aus und da ich momentan keine alternative Aktie habe, in die ich investieren möchte, bleibt der Anteil der in Arena geparkt ist auch stehen. Mein STOP LOSS liegt knapp über dem Kaufkurs und somit kann hier eigentlich nichts passieren.

Antwort auf Beitrag Nr.: 43.594.917 von Poppholz am 12.09.12 12:57:40einen schönen Chart würde ein fallender Kurs bis $7,40 erzeugen.
Danach könnte der Kurs zu einer schönen W-Formation steigen.
Betrachtungszeitraum von Anfan Juli 2012 bis heute:
Danach könnte der Kurs zu einer schönen W-Formation steigen.
Betrachtungszeitraum von Anfan Juli 2012 bis heute:
Einfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
Cramer: SELL Arena Pharma but BUY .......
By Alex Capel
September 12, 2012
Jim Cramer made the following calls on September 11th, 2012. What do you think about his picks?
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA):
Jim Cramer ranked this stock a Sell
 ..aktuell plus 2,63 %..
..aktuell plus 2,63 %..
The stock closed at $7.99, its 52-week high is $13.50, and its 52-week low is $1.23.
http://wallstcheatsheet.com/investing/cramer-sell-arena-phar…" target="_blank" rel="nofollow ugc noopener">
http://wallstcheatsheet.com/investing/cramer-sell-arena-phar…
By Alex Capel
September 12, 2012
Jim Cramer made the following calls on September 11th, 2012. What do you think about his picks?
Arena Pharmaceuticals, Inc. (NASDAQ:ARNA):
Jim Cramer ranked this stock a Sell

 ..aktuell plus 2,63 %..
..aktuell plus 2,63 %..
The stock closed at $7.99, its 52-week high is $13.50, and its 52-week low is $1.23.
http://wallstcheatsheet.com/investing/cramer-sell-arena-phar…" target="_blank" rel="nofollow ugc noopener">
http://wallstcheatsheet.com/investing/cramer-sell-arena-phar…
Antwort auf Beitrag Nr.: 43.596.188 von bernie55 am 12.09.12 16:46:39Cramer say sell, means to me buy!
Drecksbasher bezahlt von den Hedjies!

Drecksbasher bezahlt von den Hedjies!

Antwort auf Beitrag Nr.: 43.594.949 von Poppholz am 12.09.12 13:03:03das nenne ich doch mal einen Kursverlauf nach meinem Geschmack:

Cramer ist wirklich ein Kontra-Indikator geworden.

Cramer ist wirklich ein Kontra-Indikator geworden.

Qsymia von Vivus nun schon verfügbar > Vertrieb erfolgt durch zertifizierte Apotheken
Qsymia™ is only available by mail order through certified pharmacies in the Qsymia Home Delivery Network.
http://www.qsymia.com/
Qsymia™ is only available by mail order through certified pharmacies in the Qsymia Home Delivery Network.
http://www.qsymia.com/
der nächste Große Widerstand liegt bei $9,30 - $9,40.
Wenn wir den überwunden haben kann es schön weiter nach oben gehen.
Die Korrektur sollte meiner Meinung nach jetzt vorbei sein.
Wir werden sehen, ...

Wenn wir den überwunden haben kann es schön weiter nach oben gehen.
Die Korrektur sollte meiner Meinung nach jetzt vorbei sein.
Wir werden sehen, ...

Arena Pharmaceuticals - The Obesity Blockbuster Drug Company and More
By Joseph Dedvukaj - September 18,
http://us.rd.yahoo.com/finance/external/mfool/SIG=135fhtv3p/…
By Joseph Dedvukaj - September 18,
http://us.rd.yahoo.com/finance/external/mfool/SIG=135fhtv3p/…
Antwort auf Beitrag Nr.: 43.617.439 von Poppholz am 18.09.12 17:20:41da habe ich ja fast eine Punktlandung hingelegt.
After Hour stehen wir nun bei $9,33

Mal schauen ob ich einen schönen Call für ARNA finde der nur noch heute läuft und dann sage ich den entsprechenden "nächsten Widerstand" voraus.

After Hour stehen wir nun bei $9,33

Mal schauen ob ich einen schönen Call für ARNA finde der nur noch heute läuft und dann sage ich den entsprechenden "nächsten Widerstand" voraus.

Antwort auf Beitrag Nr.: 43.619.513 von bernie55 am 19.09.12 08:20:08das stimmt.
Nun gilt es zu beobachten, wie weit ARNA die Seitwärtsbewegung halten kann bzw. wann diese überwunden ist, damit der Kurs weiter steigen kann.
Mit einen fallenden Kurs unter die $9,- Marke rechne ich nicht mehr.
Wir werden sehen welche "Interessengruppen" sich mit unserer Aktie beschäftigen werden.

Nun gilt es zu beobachten, wie weit ARNA die Seitwärtsbewegung halten kann bzw. wann diese überwunden ist, damit der Kurs weiter steigen kann.
Mit einen fallenden Kurs unter die $9,- Marke rechne ich nicht mehr.
Wir werden sehen welche "Interessengruppen" sich mit unserer Aktie beschäftigen werden.

Antwort auf Beitrag Nr.: 43.624.011 von Poppholz am 20.09.12 07:32:22schön den Kurs auf §8,94 gedrückt nur damit Poppholz kein Recht behält, ...


















Vivus drops on worries of rejection overseas
European Rejection Expected for Qsiva


































Vivus disclosed that it expects European officials to recommend against approving its Qsiva obesity drug.
That's sending VVUS lower by 11 percent before the bell on my tradeMONSTER platform.
http://finance.yahoo.com/news/vivus-drops-worries-rejection-…" target="_blank" rel="nofollow ugc noopener">
http://finance.yahoo.com/news/vivus-drops-worries-rejection-…
Vivus Shares Feel The Pain: Blockbuster Diet Drug Likely Won't Be Sold In Europe
....was immer wieder in den Foren von mir bzw. uns diskutiert wurde, war, dass sowohl das Phentermine als auch die erheblichen Nebenwirkungen von Qsymia ( siehe REMS) ein Approval in Europa unwahrscheinlich machen würden.....
Vivus stock slumped more than 10% this morning after the company said its weight-loss drug will likely be denied approval in Europe.
The European Medicines Agency will make a formal decision on the drug, Qsiva, at a meeting next month. But initial comments from the organization make Qsiva’s future seem clouded. Vivus will have the opportunity to resubmit the medicine or appeal.
“We await the official decision and the formal report which should provide us specifics on any additional requirements leading to the approval of Qsiva in Europe,” said Vivus President Peter Tam. “We will work … to address the Committee’s concerns. VIVUS is committed to making this important medication available to obese patients in Europe.”
Shares of Vivus fell 12.1% to $20.85 in pre-market trading.
The drug is already approved in the United States, where Vivus markets the weight-loss pill under the name Qysmia. Meant to treat obesity in patients with at lease one weight-related condition, like high blood pressure or diabetes, Qysmia is seen as a potential blockbuster for Vivus. Blocked access to Europe—an area where half of the adult population is obese or overweight, greater than the alarming rates seen in America—would be a severe disappointment for the drugmaker.
Wells Fargo analyst Michael Tong says it’s a surprise decision by the European group. “The probable regulatory setback in Europe is an incremental modest negative for Vivus, in our view,” say Tong, who rates the stock as Market Perform. “Although we had always believed the U.S. end-user market represents the larger opportunity for Qsymia, if the approval pathway for Qsiva in Europe ultimately proves too onerous for Vivus to pursue, it does lower the peak sales potential for the Qsymia-Qsiva franchise”
Another diet drug, Arena Pharmaceuticals’ Belviq, was approved earlier this year in the U.S.
Arena and Vivus are small players in the global drug business, compete with such giants as GaxloSmithKline, Eli Lilly and Pfizer.
It’s taken Vivus years to gain approval for the weight-loss medicine in the U.S. It first applied for FDA approval in 2010 only to be met with rejection: The agency feared the drug could cause birth defects in children if taken by pregnant women.
http://www.forbes.com/sites/abrambrown/2012/09/21/vivus-shar…
....was immer wieder in den Foren von mir bzw. uns diskutiert wurde, war, dass sowohl das Phentermine als auch die erheblichen Nebenwirkungen von Qsymia ( siehe REMS) ein Approval in Europa unwahrscheinlich machen würden.....
Vivus stock slumped more than 10% this morning after the company said its weight-loss drug will likely be denied approval in Europe.
The European Medicines Agency will make a formal decision on the drug, Qsiva, at a meeting next month. But initial comments from the organization make Qsiva’s future seem clouded. Vivus will have the opportunity to resubmit the medicine or appeal.
“We await the official decision and the formal report which should provide us specifics on any additional requirements leading to the approval of Qsiva in Europe,” said Vivus President Peter Tam. “We will work … to address the Committee’s concerns. VIVUS is committed to making this important medication available to obese patients in Europe.”
Shares of Vivus fell 12.1% to $20.85 in pre-market trading.
The drug is already approved in the United States, where Vivus markets the weight-loss pill under the name Qysmia. Meant to treat obesity in patients with at lease one weight-related condition, like high blood pressure or diabetes, Qysmia is seen as a potential blockbuster for Vivus. Blocked access to Europe—an area where half of the adult population is obese or overweight, greater than the alarming rates seen in America—would be a severe disappointment for the drugmaker.
Wells Fargo analyst Michael Tong says it’s a surprise decision by the European group. “The probable regulatory setback in Europe is an incremental modest negative for Vivus, in our view,” say Tong, who rates the stock as Market Perform. “Although we had always believed the U.S. end-user market represents the larger opportunity for Qsymia, if the approval pathway for Qsiva in Europe ultimately proves too onerous for Vivus to pursue, it does lower the peak sales potential for the Qsymia-Qsiva franchise”
Another diet drug, Arena Pharmaceuticals’ Belviq, was approved earlier this year in the U.S.
Arena and Vivus are small players in the global drug business, compete with such giants as GaxloSmithKline, Eli Lilly and Pfizer.
It’s taken Vivus years to gain approval for the weight-loss medicine in the U.S. It first applied for FDA approval in 2010 only to be met with rejection: The agency feared the drug could cause birth defects in children if taken by pregnant women.
http://www.forbes.com/sites/abrambrown/2012/09/21/vivus-shar…
Antwort auf Beitrag Nr.: 43.631.096 von bernie55 am 21.09.12 14:47:52da bin ich ja mal auf die Kursentwicklung (bei beiden Aktien) gespannt.
Eine Ablehnung in Europa wird auch in den USA zu einer Entscheidung bei "Patienten" führen, die bisher 50 : 50 in Ihrer Erwägung standen.
Eine Ablehnung in Europa wird auch in den USA zu einer Entscheidung bei "Patienten" führen, die bisher 50 : 50 in Ihrer Erwägung standen.
..vor der News, dass VIVUS mit Qsymia in Europa gescheitert ist, hier der Marktschreier JIM CRAMER
Arena Pharmaceuticals Inc. (ARNA): Today's Featured Drugs Laggard
TheStreet Ratings rates Arena Pharmaceuticals as a sell.
Among the areas we feel are negative, one of the most important has been poor profit margins.
http://www.thestreet.com/story/11712926/1/arena-pharmaceutic…
Arena Pharmaceuticals Inc. (ARNA): Today's Featured Drugs Laggard
TheStreet Ratings rates Arena Pharmaceuticals as a sell.
Among the areas we feel are negative, one of the most important has been poor profit margins.
http://www.thestreet.com/story/11712926/1/arena-pharmaceutic…
Kurzer FAKTENCHECK
- VIVUS darf Qsymia in den USA wegen der stark eingeschränkten Zulassung nur über zertifizierte Apotheken verkaufen
- In Europa hat Qsymia keine Genehmigung seitens der EMA erhalten.
- VIVUS darf Qsymia in den USA wegen der stark eingeschränkten Zulassung nur über zertifizierte Apotheken verkaufen
- In Europa hat Qsymia keine Genehmigung seitens der EMA erhalten.
mal schauen was heute läuft.
Antwort auf Beitrag Nr.: 43.631.458 von Poppholz am 21.09.12 15:48:05Das schaut gut aus:
" target="_blank" rel="nofollow ugc noopener">http://beta.fool.com/beatlesforever/2012/09/22/as-door-closes-on-vivus-it-swings-wide-open-for-ar/12686
" target="_blank" rel="nofollow ugc noopener">http://beta.fool.com/beatlesforever/2012/09/22/as-door-closes-on-vivus-it-swings-wide-open-for-ar/12686

Zitat von Magnetfeldfredy: Das schaut gut aus:
YEPP, Fredy
As Door Closes On Vivus It Swings Wide Open For Arena
By Reza Ganjavi - September 22, 2012
Reza is a member of The Motley Fool Blog Network -- entries represent the personal opinions of our bloggers and are not formally edited.
Vivus (NASDAQ: VVUS) announced today that the company's weight loss drug, Qsymia will likely be rejected by the European Medicines Agency committee. This news couldn't have come at a worse time for Vivus as it is participating in the Obesity Society's 30th Annual Scientific Meeting and word around this rejection could circulate among American doctors like wild fire.
Many believe FDA should not have approved Qsymia due to safety concerns and many believe at the very least FDA should have made pregnancy tests mandatory due to dangers around birth defect which is not the only safety concern with Qsymia.
I have heard of doctors holding a strong stance that they would never prescribe Qsymia due to safety concerns. Apparently European regulators agree with these doctors and are not going to allow Qsymia to be sold in Europe.
Vivus has indicated that it would appeal the decision but it seems like an uphill battle.
Today's news likely hurts Qsymia's chances of approvals in other regions as well.
A European rejection of Qsymia takes out a large chunk of the global obesity market away from Vivus and hands it to Arena Pharmaceuticals (NASDAQ: ARNA) whose chance of getting approval in Europe is very good given the excellent safety profile of Belviq and its strong efficacy.
Completers in the bloom and blossom studies lost an average of 8% of body weight; responders lost between 11% and 12% of body weight in a year. The top 25% of weight losers lost an average of 35 pounds in one year. It's comical to hear some analysts and journalists call this "marginal" efficacy. There's nothing marginal about these amazing numbers.
Arena will file answers to the EMA's 120-day questions next quarter with approval expected sometimes in first half of 2013.
This news also does not bode so well for Orexigen Therapeutics (NASDAQ: OREX) whose drug, Contrave doesn't have as good of a safety profile as Arena's Belviq.
Both VVUS and OREX were down today while ARNA was up 3.14% on above average volume. Vivus was down 11.47% at almost 4.5 times average volume.
SAFETY SAFETY SAFETY
Thumbs down to Qsymia in Europe puts the Eisai sales team at a stronger competitive advantage over Qsymia. Eisai has a top-notch sales team which has experience selling a blockbuster drug. Telling doctors that Qsymia was rejected in Europe due to safety concerns will not bode well to a physician community which is very safety conscious.
Speaking about safety, reading the Qsymia label is like watching a horror movie. It talks about the patient having to immediately stop the drug if a certain condition occurs (e.g. pregnancy) and then it talks about not immediately stopping the drug - that it should be tapered. No wonder some doctors are so adamantly against prescribing Qsymia and much prefer Belviq which has an excellent safety profile with the most common side effect being a transient mild headache.
THE ONLY GAME ON THE CONTINENT
For Arena, the most important aspect of this news is that the company will be able to ink an even better deal with a European partner now that Belviq appears to be the only game on... the continent.
Arena has indicated a strong level of interest from potential partners in Europe and other territories. This is one of the many catalysts which will move the stock which in my opinion is grossly under-valued and flooded with artificial supply (around 43 Million shorted shares) and an institutional interest which I think will want to at least double in the months ahead. This is an explosive combination with two strong streams of demand meeting a shortage of supply as retail investors who are holding 69% of the float don't seem to want to let it go at these prices no matter how many analysts, journalists, investment bankers and brokers tell us to sell by initiating or changing coverage with ridiculous, low-ball price targets.

IRON TIGHT RETAIL HANDS
I know several large retail investors, some holding in excess of a million shares, who are absolutely determined to keep their shares until ARNA reaches fair value which many of them believe is at least triple the current price.

Some sell-side analysts have been consistently wrong about Arena and their bashing of Arena and pumping of Vivus and Orexigen is almost comical to read based upon their apparently clueless yet manipulative commentary.
Imagine if Arena had a news item similar to what was released by Vivus about the anticipated EMA rejection of Qsymia. The same analysts would be all over it.
The best saying on this subject is "doctors write prescriptions not analysts". In this extremely important obesity market retail investors have beat professional high-paid analysts hands-down. Maybe some sell-side analysts should be called "paid shills of hedge funds" instead.
"DO AS I DO, NOT AS I SAY"
Despite all the pumping of Vivus and Orexigen, and all the bashing of Arena, institutions have been quietly accumulating ARNA more than they have either VVUS or OREX. Wall Street’s motto should be “Do As I Do, Not As I Say”. I'd rather do as Jack Lief, President and CEO of Arena has done:
not sell a single share since co-founding the company.

http://beta.fool.com/beatlesforever/2012/09/22/as-door-close…" target="_blank" rel="nofollow ugc noopener">
http://beta.fool.com/beatlesforever/2012/09/22/as-door-close…
Hei Bernie,
yes we hope!
yes we hope!
FAKTENCHECK:
> Es muss erst einmal abgewartet werden , was die EMA zu der Ablehnung Qsymia verlauten lässt.
Sollte die „Reject-Argumentation“ in Richtung „des erhöhten Risiko bzgl. Geburtenschädigungen“ gehen, muss VVUS die CVOT in den USA erst mal vervollständigen.
> Das würde dann für eine eventuelle Zulassung von Qsymia in Europa eine Verzögerung von mindestens 2-3 Jahre bedeuten.
-----------------------------------------------------------------------------------------------------------------------------------------------------------
> ARNA hat das Approval für Belviq in Europa wegen der Ablehnung von Qsymia natürlich noch nicht sicher in der Tasche.
> Es muss erst einmal abgewartet werden , was die EMA zu der Ablehnung Qsymia verlauten lässt.
Sollte die „Reject-Argumentation“ in Richtung „des erhöhten Risiko bzgl. Geburtenschädigungen“ gehen, muss VVUS die CVOT in den USA erst mal vervollständigen.
> Das würde dann für eine eventuelle Zulassung von Qsymia in Europa eine Verzögerung von mindestens 2-3 Jahre bedeuten.
-----------------------------------------------------------------------------------------------------------------------------------------------------------
> ARNA hat das Approval für Belviq in Europa wegen der Ablehnung von Qsymia natürlich noch nicht sicher in der Tasche.
ja, darauf habe ich schon lange gewartet..
...der Marktktschreier tritt wieder in Aktion...
...so langsam nervt dieser " Fuzzie" wirklich...
Wenn man die Mundbewegung genaustens betrachtet, so schreit er auf diesem Bild nicht " Sell " sondern eindeutig " Buy"


Arena Pharmaceuticals Inc. Stock SELL Recommendation Reiterated (ARNA)
http://www.thestreet.com/story/11720408/1/arena-pharmaceutic…
...der Marktktschreier tritt wieder in Aktion...
...so langsam nervt dieser " Fuzzie" wirklich...
Wenn man die Mundbewegung genaustens betrachtet, so schreit er auf diesem Bild nicht " Sell " sondern eindeutig " Buy"

Arena Pharmaceuticals Inc. Stock SELL Recommendation Reiterated (ARNA)
http://www.thestreet.com/story/11720408/1/arena-pharmaceutic…
Antwort auf Beitrag Nr.: 43.653.206 von bernie55 am 27.09.12 16:24:16Das ist der größte "Gratler" an den Börsen und mit den Hedgies im Bett, oft ein guter Kontraindikator!

 Anstehende NEWS :
Anstehende NEWS :
Die Veröffentlichung der "Missbrauchklassifikation" seitens der amerikanischen Drogenaufsichtsbehörde (DEA)
So langsam müssten die Jungs mal in die "Pötte" kommen.....
die Versicherungen übernehmen (auf jeden Fall in einigen Fällen) die Kosten für die Medikamente. Es gibt Bestätigung (laut Bloomberg) das bereits 106 Rezepte für Qsymia vergeben wurden (in der ersten Woche).
Dies sollte auch für den Kursverlauf von ARNA positiv sein.
http://seekingalpha.com/article/898531-some-good-news-for-vi…
Dies sollte auch für den Kursverlauf von ARNA positiv sein.
http://seekingalpha.com/article/898531-some-good-news-for-vi…
!
Dieser Beitrag wurde von CloudMOD moderiert. Grund: auf eigenen Wunsch des Users
Antwort auf Beitrag Nr.: 43.667.278 von Poppholz am 02.10.12 07:45:56anbei der Artikel, auf den sich der Kommentar bezieht:
http://www.bloomberg.com/news/2012-10-01/vivus-s-weight-loss…
http://www.bloomberg.com/news/2012-10-01/vivus-s-weight-loss…
The Arena Trading Range Continues
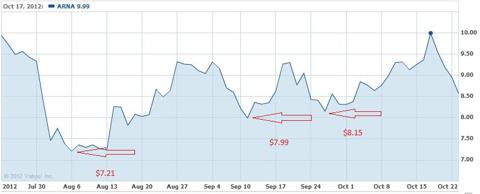
http://seekingalpha.com/article/943681-the-arena-trading-ran…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/943681-the-arena-trading-ran…

http://seekingalpha.com/article/943681-the-arena-trading-ran…" target="_blank" rel="nofollow ugc noopener">
http://seekingalpha.com/article/943681-the-arena-trading-ran…
an diesem Bild haben Chart-Techniker Ihre wahre Freude.
Momentan kann es "nur" eine Richtung geben.
Mal sehen wie lange diese anhält.

Einfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
Momentan kann es "nur" eine Richtung geben.
Mal sehen wie lange diese anhält.

Antwort auf Beitrag Nr.: 43.998.307 von Poppholz am 09.01.13 09:47:52hier noch einmal der US-Chart, ist ja irgendwie aussagekräftiger:
Wenn wir die Hürde von $10,- nun auch heute halten können, von der wir im Oktober noch abgeprallt sind, ist der Weg an die $11,00 frei.
Die nächste Hürde wird dann die $12,- Marke werden, für die wir wohl ein wenig brauchen werden, aber dann ...

Einfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
Wenn wir die Hürde von $10,- nun auch heute halten können, von der wir im Oktober noch abgeprallt sind, ist der Weg an die $11,00 frei.
Die nächste Hürde wird dann die $12,- Marke werden, für die wir wohl ein wenig brauchen werden, aber dann ...

irgendwie ist der Chart von W : O nicht zu sehen. Auf jeden Fall nicht bei mir im Thread.
Somit hier noch einmal eine andere Quelle:
Wenn wir die Hürde von $10,- nun auch heute halten können, von der wir im Oktober noch abgeprallt sind, ist der Weg an die $11,00 frei.
Die nächste Hürde wird dann die $12,- Marke werden, für die wir wohl ein wenig brauchen werden, aber dann ...
Somit hier noch einmal eine andere Quelle:
Wenn wir die Hürde von $10,- nun auch heute halten können, von der wir im Oktober noch abgeprallt sind, ist der Weg an die $11,00 frei.
Die nächste Hürde wird dann die $12,- Marke werden, für die wir wohl ein wenig brauchen werden, aber dann ...
Zitat von Poppholz: hier noch einmal der US-Chart, ist ja irgendwie aussagekräftiger:
Einfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
Wenn wir die Hürde von $10,- nun auch heute halten können, von der wir im Oktober noch abgeprallt sind, ist der Weg an die $11,00 frei.
Die nächste Hürde wird dann die $12,- Marke werden, für die wir wohl ein wenig brauchen werden, aber dann ...
Hi Poppie,

vielen Dank für deine Erläuterungen bzgl der Charttechnik -
Die nächste Hürde wird dann die $12,- Marke werden, für die wir wohl ein wenig brauchen werden, aber dann ...
Wenn heute auf der Konferenz bzw. nächste Woche ( CHMP) schon positive " Hammernews " durchsickern, dann werden wir nicht mehr lange brauchen...

...time will tell..
LG
bernie55

erst einmal wurde der Kurs "erfolgreich" gedrückt.
anscheinend möchten einige nicht, das die automatisierten Käufe durch Orderprogramme aufgrund der Charttechnik greifen.

anscheinend möchten einige nicht, das die automatisierten Käufe durch Orderprogramme aufgrund der Charttechnik greifen.

Antwort auf Beitrag Nr.: 44.000.238 von Poppholz am 09.01.13 15:38:09aktuell $9,83
Mal sehen wie der Schlusskurs aussieht.
Wenn der über $10,- steht wäre das sehr gut für uns.

Mal sehen wie der Schlusskurs aussieht.
Wenn der über $10,- steht wäre das sehr gut für uns.

Antwort auf Beitrag Nr.: 44.000.251 von Poppholz am 09.01.13 15:39:16aktuell $9,63

Da will jetzt aber jemand ganz sicher gehen, dass keine großen Käufe ausgelöst werden.

Da will jetzt aber jemand ganz sicher gehen, dass keine großen Käufe ausgelöst werden.

Antwort auf Beitrag Nr.: 44.000.462 von Poppholz am 09.01.13 16:09:50schön zu sehen, wie sehr darauf geachtet wurde, dass der Kurs die $10,- Marke nicht übersteigt.

Mal sehen wie lange das so geht, bzw. wann die Computerprogramme auf "automatisch" verkaufen gehen.

Mal sehen wie lange das so geht, bzw. wann die Computerprogramme auf "automatisch" verkaufen gehen.

Antwort auf Beitrag Nr.: 44.000.462 von Poppholz am 09.01.13 16:09:50heute wieder gleiches Bild.
Kurs steigt steil an, bis auf $9,94 und wird dann wieder runter geprügelt.
Gestern war es bei $9,98.
Spannende Zeit.

Kurs steigt steil an, bis auf $9,94 und wird dann wieder runter geprügelt.
Gestern war es bei $9,98.
Spannende Zeit.

Antwort auf Beitrag Nr.: 44.006.114 von Poppholz am 10.01.13 16:29:33da wir gestern erfolgreich die $10,- Marke gehalten haben, dürfte heute der Kurs bis $11,- laufen.
(wahrscheinlich nur bis $10,99 nur damit ich kein Recht bekomme)

(wahrscheinlich nur bis $10,99 nur damit ich kein Recht bekomme)

Einfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
.....heute über 11$.....

Antwort auf Beitrag Nr.: 44.025.818 von bernie55 am 15.01.13 21:04:44Glückwunsch Ihr Lieben!

Zitat von Tebi: Glückwunsch Ihr Lieben!
Hi " Tebi"....noch ist nichts passiert....
Wir warten auf das EURO Approval und darauf, dass Eisai bald Belviq veräußern kann ( ca. Mitte Februar)
Ansonsten kannst du uns auch im Fakten-Thread > ARENA PHARMACEUTICALS + BELVIQ - Wie geht es weiter ??? < lesen.....
...ich werde dich in den nächsten Tagen mal " phonen".
LG
bernie55

Antwort auf Beitrag Nr.: 44.024.936 von Poppholz am 15.01.13 18:04:53nun ja, bis $10,95 haben wir es in der Spitze geschafft.
Mal sehen wo wir Freitag Abend stehen.

Einfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
Mal sehen wo wir Freitag Abend stehen.

Antwort auf Beitrag Nr.: 44.026.602 von Poppholz am 16.01.13 07:41:20
die Charts von W O sind irgendwie nicht so optimal zum einpflegen.

die Charts von W O sind irgendwie nicht so optimal zum einpflegen.

Antwort auf Beitrag Nr.: 44.025.902 von Tebi am 15.01.13 21:23:27vielen Dank.
ARNA entwickelt sich zu meinem neuen "Baby", ELN befindet sich momentan in der Pupertät.

ARNA entwickelt sich zu meinem neuen "Baby", ELN befindet sich momentan in der Pupertät.

Antwort auf Beitrag Nr.: 44.026.605 von Poppholz am 16.01.13 07:43:30schön ist auch der Vergleich mit Vivius.
Anbei der Kursverlauf der beiden in den letzten Tagen.

Besonders heute (17.01.2013)
ARNA +3%
VVUS -5%
Bald hat ARNA die VVUS eingeholt.

Anbei der Kursverlauf der beiden in den letzten Tagen.
Besonders heute (17.01.2013)
ARNA +3%
VVUS -5%
Bald hat ARNA die VVUS eingeholt.

Zitat von Poppholz: ........schön ist auch der Vergleich mit Vivius.
Besonders heute (17.01.2013)
ARNA +3%
VVUS -5%
Bald hat ARNA die VVUS eingeholt.
VIVUS downgraded to Sell from Hold at Brean Capital
Theflyonthewall.comTheflyonthewall.com – 5 hours ago
Brean Capital downgraded VIVUS citing rich valuation and lowered Qsymia estimates.
http://finance.yahoo.com/news/vivus-downgraded-sell-hold-bre…
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| -1,55 | |
| -0,07 | |
| +2,09 | |
| +40,00 | |
| +0,12 | |
| -7,46 | |
| -4,19 | |
| 0,00 | |
| -1,65 | |
| 0,00 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 294 | ||
| 108 | ||
| 107 | ||
| 77 | ||
| 70 | ||
| 64 | ||
| 60 | ||
| 47 | ||
| 46 | ||
| 45 |