GENTA (GNTA) kommt da was? - 500 Beiträge pro Seite
eröffnet am 01.07.06 07:19:11 von
neuester Beitrag 17.11.06 07:07:17 von
neuester Beitrag 17.11.06 07:07:17 von
Beiträge: 53
ID: 1.068.951
ID: 1.068.951
Aufrufe heute: 0
Gesamt: 14.091
Gesamt: 14.091
Aktive User: 0
Top-Diskussionen
| Titel | letzter Beitrag | Aufrufe |
|---|---|---|
| vor 1 Stunde | 4431 | |
| heute 11:23 | 3299 | |
| vor 49 Minuten | 2500 | |
| heute 11:11 | 2140 | |
| heute 08:50 | 1848 | |
| vor 56 Minuten | 1739 | |
| vor 1 Stunde | 1058 | |
| vor 53 Minuten | 1051 |
Meistdiskutierte Wertpapiere
| Platz | vorher | Wertpapier | Kurs | Perf. % | Anzahl | ||
|---|---|---|---|---|---|---|---|
| 1. | 1. | 18.057,55 | +0,71 | 236 | |||
| 2. | 3. | 0,1905 | +0,79 | 118 | |||
| 3. | 2. | 1,1900 | -13,77 | 100 | |||
| 4. | 5. | 9,3150 | +0,76 | 88 | |||
| 5. | 4. | 160,34 | +1,05 | 59 | |||
| 6. | 12. | 2.346,94 | +0,64 | 39 | |||
| 7. | Neu! | 11,905 | +14,97 | 36 | |||
| 8. | Neu! | 4,7990 | +7,00 | 35 |
Hallo Community,
der Kursverlauf von GENTA war heute wie das Deutschlandspiel gegen Argentinien. Voller Spannung und Emotionen. Zuerst brav im satten Grün und in der letzten Stunde massiv ins Minus gefahren (-7%). Was in den AH aber abgelaufen ist, kann sich kaum einer erklären:
+21.34 (AH)
http://www.nasdaq.com/aspxcontent/ExtendedTradingTrades.aspx…
18:20 $ 1.64 14,057
18:12 $ 1.64 22,838
17:51 $ 1.6211 1,755
17:39 $ 1.64 2,338,739


17:33 $ 1.6378 173,700
17:27 $ 1.625 45,900
17:24 $ 1.64 732
17:13 $ 1.6382 703,400

16:48 $ 1.625 350,600

Zum möglichst allerletztem Zeitpunkt hatten es auf einmal einige seeeehr eilig und es war fast schon egal zu welchem Preis.
Damit sollten einige interessante BUY-Signale generiert worden sein...

Interessant bei GNTA ist auch der Einstieg von SOROS!
http://finance.yahoo.com/q/mh?s=GNTA
GNTA kommt aus ganz anderen Chartbereichen...

GNTA musste den letzten Kurseinbruch verkraften, nachdem das neue Produkt Genasense nicht zugelassen wurde. Inzwischen wurde die Entwicklung vorangetrieben und die Ergebnisse sehen positiv aus. Bei Genasense handelt es sich um ein Ergänzungsmittel mit dem anscheinend die Chemotherapie deutlich wirksamer verlaufen kann.
http://biz.yahoo.com/prnews/060405/nyw056.html?.v=45
http://www.nasdaq.com/aspxcontent/NewsStory.aspx?cpath=20060…
http://aktien.onvista.de/news-filter.html?ID_OSI=88568&ID_OS…
http://aktien.onvista.de/empfehlungen.html?ID_OSI=88568#news
http://www.finanznachrichten.de/nachrichten-2006-02/artikel-…
Insiders scheinen sich bereits eingedeckt zu haben.
http://edgar.brand.edgar-online.com/default.aspx?sym=gnta
und die finanzielle Situation
http://www.advfn.com/p.php?pid=financials&symbol=NASDAQ%3Agn…
Derzeit sind 4,868,793 Stücke short
Es bleibt spannend bei GENTA, aber die Vorzeichen hinterlassen ein eindeutiges Signal, BUY.
nort
der Kursverlauf von GENTA war heute wie das Deutschlandspiel gegen Argentinien. Voller Spannung und Emotionen. Zuerst brav im satten Grün und in der letzten Stunde massiv ins Minus gefahren (-7%). Was in den AH aber abgelaufen ist, kann sich kaum einer erklären:
+21.34 (AH)
http://www.nasdaq.com/aspxcontent/ExtendedTradingTrades.aspx…
18:20 $ 1.64 14,057
18:12 $ 1.64 22,838
17:51 $ 1.6211 1,755
17:39 $ 1.64 2,338,739



17:33 $ 1.6378 173,700
17:27 $ 1.625 45,900
17:24 $ 1.64 732
17:13 $ 1.6382 703,400


16:48 $ 1.625 350,600


Zum möglichst allerletztem Zeitpunkt hatten es auf einmal einige seeeehr eilig und es war fast schon egal zu welchem Preis.
Damit sollten einige interessante BUY-Signale generiert worden sein...
Interessant bei GNTA ist auch der Einstieg von SOROS!
http://finance.yahoo.com/q/mh?s=GNTA
GNTA kommt aus ganz anderen Chartbereichen...
GNTA musste den letzten Kurseinbruch verkraften, nachdem das neue Produkt Genasense nicht zugelassen wurde. Inzwischen wurde die Entwicklung vorangetrieben und die Ergebnisse sehen positiv aus. Bei Genasense handelt es sich um ein Ergänzungsmittel mit dem anscheinend die Chemotherapie deutlich wirksamer verlaufen kann.
http://biz.yahoo.com/prnews/060405/nyw056.html?.v=45
http://www.nasdaq.com/aspxcontent/NewsStory.aspx?cpath=20060…
http://aktien.onvista.de/news-filter.html?ID_OSI=88568&ID_OS…
http://aktien.onvista.de/empfehlungen.html?ID_OSI=88568#news
http://www.finanznachrichten.de/nachrichten-2006-02/artikel-…
Insiders scheinen sich bereits eingedeckt zu haben.
http://edgar.brand.edgar-online.com/default.aspx?sym=gnta
und die finanzielle Situation
http://www.advfn.com/p.php?pid=financials&symbol=NASDAQ%3Agn…
Derzeit sind 4,868,793 Stücke short

Es bleibt spannend bei GENTA, aber die Vorzeichen hinterlassen ein eindeutiges Signal, BUY.
nort
Morge Nort!
das war in der Tat sehr auffällig was gestern Abend bei GNTA in der AH Handel gelaufen ist
18:20 $ 1.64 14,057
18:12 $ 1.64 22,838
17:51 $ 1.6211 1,755
17:39 $ 1.64 2,338,739
17:33 $ 1.6378 173,700
17:27 $ 1.625 45,900
17:24 $ 1.64 732
17:13 $ 1.6382 703,400
16:48 $ 1.625 350,600
Tja, der Pförtner, Taxifahrer & Hausfrau hat diese Kursbewegung sicherlich nicht ausgelöst

Bitte bedenken: das ganze fand an einen späten Freitag Abend statt wo die meisten Amerikaner am kommende Montag bereits frei genommen haben um deren Independece day(4th Juli) ausgiebig feiern zu können...
Es könnte sein das wir hier so'n Art "Initialzündung" gesehen haben

Nächste Woche dürfte es richtig Spannend sein mit GNTA-(bin echt froh nicht short zu sein)
Schönes WE,
Whyso
das war in der Tat sehr auffällig was gestern Abend bei GNTA in der AH Handel gelaufen ist

18:20 $ 1.64 14,057
18:12 $ 1.64 22,838
17:51 $ 1.6211 1,755
17:39 $ 1.64 2,338,739
17:33 $ 1.6378 173,700
17:27 $ 1.625 45,900
17:24 $ 1.64 732
17:13 $ 1.6382 703,400
16:48 $ 1.625 350,600
Tja, der Pförtner, Taxifahrer & Hausfrau hat diese Kursbewegung sicherlich nicht ausgelöst


Bitte bedenken: das ganze fand an einen späten Freitag Abend statt wo die meisten Amerikaner am kommende Montag bereits frei genommen haben um deren Independece day(4th Juli) ausgiebig feiern zu können...
Es könnte sein das wir hier so'n Art "Initialzündung" gesehen haben
Nächste Woche dürfte es richtig Spannend sein mit GNTA-(bin echt froh nicht short zu sein)
Schönes WE,
Whyso

...bitte nicht vergessen daß wir gestern den 30.06. hatten, das zweite Quartal ist somit ausgelaufen. Die gesehenen Bewegungen in AH können auch entsprechende "Buchungen" von MM sein, die da noch getätigte Bewegungen auf das abgelaufene Quartal verbuchen.
Wie gesagt - kann sein muß aber nicht! Zu beobachten sind solche Vorgänge allerdings bei genauerem Hinsehen öfter mal...
Wenn´s tatsächlich eine aufkommende Veränderung wegen positiver News sein sollte freut es mich für euch - seid ja in den letzten Monaten auch furchtbar gerupft worden...
Schönes WE
Wie gesagt - kann sein muß aber nicht! Zu beobachten sind solche Vorgänge allerdings bei genauerem Hinsehen öfter mal...
Wenn´s tatsächlich eine aufkommende Veränderung wegen positiver News sein sollte freut es mich für euch - seid ja in den letzten Monaten auch furchtbar gerupft worden...
Schönes WE

Antwort auf Beitrag Nr.: 22.368.632 von bioperformer am 01.07.06 10:26:06bioperformer, mag sicher sein mit den Rückbuchungen, aber in solch einem Ausmaß? Immerhin sind es an die 25% Kursdifferenz. Es wurden in den AH 3,741,749 Stück umgesetzt. Das 50 day avg volume beträgt dagegen 2,060,193.
Antwort auf Beitrag Nr.: 22.368.048 von nort. am 01.07.06 07:19:11Hi!
Ich habe mir mal die T&S angesehen. Der "Kurssprung" auf Island war ein Kauf von 25 Stück zu 1,99$. Also ein Ausreisser oder versuchte Manipulation ohne jede Relevanz.
18:44:49.684 B 25 1.9900
17:24:59.835 S 732 1.6400
16:42:54.595 B 200 1.6600
16:31:47.862 S 400 1.6100
16:16:19.292 S 500 1.5600
16:16:18.893 S 500 1.5600
16:15:55.524 S 1,000 1.5700
16:15:44.894 S 1,000 1.5800
16:15:44.894 S 100 1.5800
16:15:42.112 S 1,000 1.5900
16:15:35.763 S 1,000 1.6000
16:13:07.229 S 1,000 1.5900
16:12:57.791 S 1,000 1.6000
16:12:38.919 S 1,000 1.6000
16:12:24.438 S 1,000 1.6000
16:09:55.475 S 10,000 1.6000
16:09:39.575 S 1,000 1.6200
16:01:38.183 S 485 1.6800
16:01:37.071 S 1,000 1.6800
16:00:15.504 S 1,000 1.6900
Gruss
s.
Ich habe mir mal die T&S angesehen. Der "Kurssprung" auf Island war ein Kauf von 25 Stück zu 1,99$. Also ein Ausreisser oder versuchte Manipulation ohne jede Relevanz.
18:44:49.684 B 25 1.9900
17:24:59.835 S 732 1.6400
16:42:54.595 B 200 1.6600
16:31:47.862 S 400 1.6100
16:16:19.292 S 500 1.5600
16:16:18.893 S 500 1.5600
16:15:55.524 S 1,000 1.5700
16:15:44.894 S 1,000 1.5800
16:15:44.894 S 100 1.5800
16:15:42.112 S 1,000 1.5900
16:15:35.763 S 1,000 1.6000
16:13:07.229 S 1,000 1.5900
16:12:57.791 S 1,000 1.6000
16:12:38.919 S 1,000 1.6000
16:12:24.438 S 1,000 1.6000
16:09:55.475 S 10,000 1.6000
16:09:39.575 S 1,000 1.6200
16:01:38.183 S 485 1.6800
16:01:37.071 S 1,000 1.6800
16:00:15.504 S 1,000 1.6900
Gruss
s.
Antwort auf Beitrag Nr.: 22.369.879 von stupidgame am 01.07.06 12:54:24Dann guck doch mal bitte hier...
http://www.nasdaq.com/aspxcontent/ExtendedTradingTrades.aspx…
After Hours
Price After Hours
Share Volume
18:20 $ 1.64 14,057
18:12 $ 1.64 22,838
17:51 $ 1.6211 1,755
17:39 $ 1.64 2,338,739
17:33 $ 1.6378 173,700
17:27 $ 1.625 45,900
17:24 $ 1.64 732
17:13 $ 1.6382 703,400
16:48 $ 1.625 350,600
16:42 $ 1.66 200
16:31 $ 1.61 400
16:31 $ 1.58 100
16:31 $ 1.57 500
16:16 $ 1.56 12,500
16:16 $ 1.56 500
16:16 $ 1.56 500
16:16 $ 1.55 7,000
16:15 $ 1.60 5,000
http://www.nasdaq.com/aspxcontent/ExtendedTradingTrades.aspx…
After Hours
Price After Hours
Share Volume
18:20 $ 1.64 14,057
18:12 $ 1.64 22,838
17:51 $ 1.6211 1,755
17:39 $ 1.64 2,338,739
17:33 $ 1.6378 173,700
17:27 $ 1.625 45,900
17:24 $ 1.64 732
17:13 $ 1.6382 703,400
16:48 $ 1.625 350,600
16:42 $ 1.66 200
16:31 $ 1.61 400
16:31 $ 1.58 100
16:31 $ 1.57 500
16:16 $ 1.56 12,500
16:16 $ 1.56 500
16:16 $ 1.56 500
16:16 $ 1.55 7,000
16:15 $ 1.60 5,000
Hat es was mit dem Russel Index zu tun? In diesen wurde doch GNTA aufgenommen...
Aus dem yahoo board:
Here's what happened
by: navy_aircrew_wings86 (M/Deployed)
Long-Term Sentiment: Strong Buy 07/01/06 01:49 am
Msg: 318123 of 318126
It is interesting to watch the discussion here from such knowledgable "investors" like Matt and the rest who are posting as to what happened. I told you all two weeks ago, when GNTA went into the Russell index look for a massive move at the closing bell on 6/30/06. There was a massive sale. Why? Because the price was too high, we are approaching $1.75 at 3:57PM. Remember GNTA qualified for inclusing at $1.71. When you buy massive shares evey fraction of a penny counts. Someone then was told to dump at the bell, lower the price and inclusion went on AH. This is how the big boys play.
Now, Matt, Alchemy and Yachmaster1 (probably the same person) can go back to playing Xbox on the floor.
NAVY
PS: We are going to the moon
Aus dem yahoo board:
Here's what happened
by: navy_aircrew_wings86 (M/Deployed)
Long-Term Sentiment: Strong Buy 07/01/06 01:49 am
Msg: 318123 of 318126
It is interesting to watch the discussion here from such knowledgable "investors" like Matt and the rest who are posting as to what happened. I told you all two weeks ago, when GNTA went into the Russell index look for a massive move at the closing bell on 6/30/06. There was a massive sale. Why? Because the price was too high, we are approaching $1.75 at 3:57PM. Remember GNTA qualified for inclusing at $1.71. When you buy massive shares evey fraction of a penny counts. Someone then was told to dump at the bell, lower the price and inclusion went on AH. This is how the big boys play.
Now, Matt, Alchemy and Yachmaster1 (probably the same person) can go back to playing Xbox on the floor.
NAVY
PS: We are going to the moon

Russell Index:
The Russell 3000 Index measures the performance of the 3,000 largest U.S. companies based on total market capitalization, which represents approximately 98% of the investable U.S. equity market.
http://www.russell.com/US/Indexes/US/Reconstitution/recon_ad…
The Russell 3000 Index measures the performance of the 3,000 largest U.S. companies based on total market capitalization, which represents approximately 98% of the investable U.S. equity market.
http://www.russell.com/US/Indexes/US/Reconstitution/recon_ad…
nvestor's Business Daily
Russell Reshuffle Usually Creates Trading Surge By Heavy Hitters
Thursday June 29, 7:00 pm ET
Ken Hoover
Look out for the final minutes of trading today. The Russell indexes go through their annual rebalancing, and the action can be fast and furious.
Last year, NYSE volume swelled to 3.12 billion shares, 102% above average, on rebalancing day, with 403.8 million shares executed at the close. Nasdaq volume was 73% above average at 3.04 billion, with 428 million traded at the close.
"It's going to be an exciting day," said Adena Friedman, Nasdaq executive vice president. "It's always a big day. We make sure everybody is at their post and ready to go."
There's $3.8 trillion benchmarked to the 24 U.S. Russell indexes. They're revised on the last Friday in June to reflect changes in the companies that make them up. Money managers who track them must buy companies coming into the indexes and sell those leaving.
"We represent the fish ponds that managers use to look for stocks," said Lori Richards, who runs the indexes for Russell Investment Group.
Small companies that have grown move from the small-cap Russell 2000 to the big-cap Russell 1000. Big companies that have stumbled are sent back to the small-cap index. Growth and value indexes are adjusted to reflect changes in the fortunes of the companies in those indexes.
These indexes are important to money managers. Their bonuses and livelihoods depend on their ability to meet or beat their benchmark.
The yearly event hasn't always gone smoothly. In 2001, Nasdaq's computers crashed in the final hour. To compensate, the exchange opened late for an extra hour. Prices jumped wildly.
Since then, the Nasdaq and Tacoma, Wash.-based Russell have strived to ensure there's no repeat.
Chief among the innovations is the Nasdaq Closing Cross, an auction that combines buy and sell interest to create a single closing price.
Professional traders get messages on trade imbalances every 15 seconds in the final 15 minutes of the day. That increases to every five seconds in the final minute. Friedman says that as the close draws nearer, prices tend to be brought back close to an equilibrium point.
IPOs Phased In
Russell has reduced the effect of rebalancing by inserting IPOs into its indexes each quarter rather than all at once. Russell has also released preliminary lists of changes and a parallel index that tracks its revisions.
That lets managers work into new stocks over a period of weeks. But many want to precisely track their index and will wait until today's close. That means they might have to pay up slightly as arbitrageurs try to profit at their expense.
But that trade is too well known, said Steven DeSanctis, head of small-cap research at Prudential Securities.
"It's really hard to make any money on reconstitution," DeSanctis said. "There's too many hedge funds and trading desks involved."
The variations in volume on rebalancing day can vary greatly. In 2004, NYSE trade was up just 23% above average. The Nasdaq was up 59%.
The NYSE has a similar closing auction. Officials there expressed confidence they could handle whatever the market throws at them.
More Reasons For Big Friday
Today is also the end of the quarter, a day when mutual fund trading picks up. And some managers might take care of business they have ahead of an extra long weekend leading up to July Fourth.
DeSanctis also believes many managers were reluctant to trade ahead of Thursday's Fed meeting.
But a factor that might hold volume down is that the indexes haven't changed as much as in past years, possibly because the market has been relatively flat.
Some stocks in the hot sectors, such as basic materials and energy, are moving up to the Russell 1000. Some retailers and home builders that have lagged will go down to the Russell 2000.
Hewlett-Packard will increase its weight in the Russell 1000 Growth Index. Procter & Gamble becomes a bigger piece of the corresponding value index.

http://biz.yahoo.com/ibd/060629/general.html
Russell Reshuffle Usually Creates Trading Surge By Heavy Hitters
Thursday June 29, 7:00 pm ET
Ken Hoover
Look out for the final minutes of trading today. The Russell indexes go through their annual rebalancing, and the action can be fast and furious.
Last year, NYSE volume swelled to 3.12 billion shares, 102% above average, on rebalancing day, with 403.8 million shares executed at the close. Nasdaq volume was 73% above average at 3.04 billion, with 428 million traded at the close.
"It's going to be an exciting day," said Adena Friedman, Nasdaq executive vice president. "It's always a big day. We make sure everybody is at their post and ready to go."
There's $3.8 trillion benchmarked to the 24 U.S. Russell indexes. They're revised on the last Friday in June to reflect changes in the companies that make them up. Money managers who track them must buy companies coming into the indexes and sell those leaving.
"We represent the fish ponds that managers use to look for stocks," said Lori Richards, who runs the indexes for Russell Investment Group.
Small companies that have grown move from the small-cap Russell 2000 to the big-cap Russell 1000. Big companies that have stumbled are sent back to the small-cap index. Growth and value indexes are adjusted to reflect changes in the fortunes of the companies in those indexes.
These indexes are important to money managers. Their bonuses and livelihoods depend on their ability to meet or beat their benchmark.
The yearly event hasn't always gone smoothly. In 2001, Nasdaq's computers crashed in the final hour. To compensate, the exchange opened late for an extra hour. Prices jumped wildly.
Since then, the Nasdaq and Tacoma, Wash.-based Russell have strived to ensure there's no repeat.
Chief among the innovations is the Nasdaq Closing Cross, an auction that combines buy and sell interest to create a single closing price.
Professional traders get messages on trade imbalances every 15 seconds in the final 15 minutes of the day. That increases to every five seconds in the final minute. Friedman says that as the close draws nearer, prices tend to be brought back close to an equilibrium point.
IPOs Phased In
Russell has reduced the effect of rebalancing by inserting IPOs into its indexes each quarter rather than all at once. Russell has also released preliminary lists of changes and a parallel index that tracks its revisions.
That lets managers work into new stocks over a period of weeks. But many want to precisely track their index and will wait until today's close. That means they might have to pay up slightly as arbitrageurs try to profit at their expense.
But that trade is too well known, said Steven DeSanctis, head of small-cap research at Prudential Securities.
"It's really hard to make any money on reconstitution," DeSanctis said. "There's too many hedge funds and trading desks involved."
The variations in volume on rebalancing day can vary greatly. In 2004, NYSE trade was up just 23% above average. The Nasdaq was up 59%.
The NYSE has a similar closing auction. Officials there expressed confidence they could handle whatever the market throws at them.
More Reasons For Big Friday
Today is also the end of the quarter, a day when mutual fund trading picks up. And some managers might take care of business they have ahead of an extra long weekend leading up to July Fourth.
DeSanctis also believes many managers were reluctant to trade ahead of Thursday's Fed meeting.
But a factor that might hold volume down is that the indexes haven't changed as much as in past years, possibly because the market has been relatively flat.
Some stocks in the hot sectors, such as basic materials and energy, are moving up to the Russell 1000. Some retailers and home builders that have lagged will go down to the Russell 2000.
Hewlett-Packard will increase its weight in the Russell 1000 Growth Index. Procter & Gamble becomes a bigger piece of the corresponding value index.

http://biz.yahoo.com/ibd/060629/general.html
hey nort ,..

vor 21:00 uhr habe ich noch gekauft 2.???.??? Mio umsatz dann plötzlich über 10 Mio
aber mit down trend bei 10 Mio 1,64$ TT ist nicht beängstigend sondern ist vielleicht ein Durchbruch!
d.h. jemand wollte billig kaufen nun gut 6 Cents hin oder her wenn etwas stinkt wäre der kurs abgefurtzt
hin oder her wenn etwas stinkt wäre der kurs abgefurtzt
so oder AH 1,99 wie gesagt irgnored aber 10 mio + über 3 mio in den letzten stunden machen mich nervös auf das was da kommt
welke
vor 21:00 uhr habe ich noch gekauft 2.???.??? Mio umsatz dann plötzlich über 10 Mio

aber mit down trend bei 10 Mio 1,64$ TT ist nicht beängstigend sondern ist vielleicht ein Durchbruch!
d.h. jemand wollte billig kaufen nun gut 6 Cents
 hin oder her wenn etwas stinkt wäre der kurs abgefurtzt
hin oder her wenn etwas stinkt wäre der kurs abgefurtzt so oder AH 1,99 wie gesagt irgnored aber 10 mio + über 3 mio in den letzten stunden machen mich nervös auf das was da kommt
welke
Preclinical Results Show GenasenseTM Synergizes with Campath® in Chronic
Lymphocytic Leukemia
Chicago, IL – June 4, 2003 - Genta Incorporated (Nasdaq: GNTA) today announced the publication of preclinical data that showed GenasenseTM(oblimersen sodium), the Company’s lead anticancer compound, enhances the effectiveness of Campath®(alemtuzumab; ILEX) against both chronic lymphocytic leukemia (CLL) and non-Hodgkin’s lymphoma (NHL) cells. The results were reported by a group from St. Bartholomew’s Hospital and the Royal London School of Medicine, London, UK, and were published in the Proceedings of the Annual Meeting of the American Society of Clinical Oncology (ASCO).
Genasense inhibits production of Bcl-2, a protein that is highly expressed in malignant cells from patients with both NHL and CLL. Bcl-2 is widely believed to be a fundamental cause of resistance to anticancer therapy in both illnesses. Previous studies from this research group showed that Genasense amplified the activity of another monoclonal antibody, Rituxan® (rituximab; Genentech/Idec), in CLL and NHL cells. Moreover, the synergy with Rituxan® was independently confirmed in a separate in vivo study of NHL.
In the current study, fresh leukemic cells taken from patients with CLL were evaluated for the amount of cell death induced by Genasense or Campath, a monoclonal antibody that was recently approved for the treatment of patients with CLL. The effectiveness of Campath and Genasense each used alone was first evaluated, followed by the use of both drugs together. Both drugs used alone were shown to trigger cell death; however, pretreatment of these cells with Genasense followed by Campath markedly increased the amount of cell death compared with Campath used alone. The data for the fresh human CLL cells are depicted in the table below. (Similar results were also noted in experiments using human NHL cell lines.) Lastly, the use of Genasense pretreatment enabled a 5- fold reduction in the equivalent effective dose of Campath.
Treatment
Apoptotic Level(mean % of cell death)
Campath alone
56%
Genasense alone
83%
Genasense plus Campath
92%
“Our new findings corroborate prior observations from our laboratory, which show that Genasense increases the apoptotic activity of a number of chemotherapeutic agents,” noted Dr. Finbarr Cotter, senior author on the publication. “Clinical use of Campath can be associated with significant immunosuppression in patients with advanced CLL, so we are particularly encouraged by the marked activity seen when using reduced levels of that agent. These results suggest that Genasense might be useful in circumstances when reduced doses of highly toxic anticancer drugs may be important.”
About Genasense
Genasense works by inhibiting the production of Bcl-2, a protein made by cancer cells that is thought to block chemotherapy-induced cell death. By reducing the amount of Bcl-2 in cancer cells, Genasense may enhance the effectiveness of current anticancer treatments. Genasense is currently in multiple late-stage randomized clinical trials including malignant melanoma, multiple myeloma, chronic lymphocytic leukemia (CLL) and non-small cell lung cancer.
http://www.genta.com/Genta/InvestorRelation/2003/Press_20030…
Lymphocytic Leukemia
Chicago, IL – June 4, 2003 - Genta Incorporated (Nasdaq: GNTA) today announced the publication of preclinical data that showed GenasenseTM(oblimersen sodium), the Company’s lead anticancer compound, enhances the effectiveness of Campath®(alemtuzumab; ILEX) against both chronic lymphocytic leukemia (CLL) and non-Hodgkin’s lymphoma (NHL) cells. The results were reported by a group from St. Bartholomew’s Hospital and the Royal London School of Medicine, London, UK, and were published in the Proceedings of the Annual Meeting of the American Society of Clinical Oncology (ASCO).
Genasense inhibits production of Bcl-2, a protein that is highly expressed in malignant cells from patients with both NHL and CLL. Bcl-2 is widely believed to be a fundamental cause of resistance to anticancer therapy in both illnesses. Previous studies from this research group showed that Genasense amplified the activity of another monoclonal antibody, Rituxan® (rituximab; Genentech/Idec), in CLL and NHL cells. Moreover, the synergy with Rituxan® was independently confirmed in a separate in vivo study of NHL.
In the current study, fresh leukemic cells taken from patients with CLL were evaluated for the amount of cell death induced by Genasense or Campath, a monoclonal antibody that was recently approved for the treatment of patients with CLL. The effectiveness of Campath and Genasense each used alone was first evaluated, followed by the use of both drugs together. Both drugs used alone were shown to trigger cell death; however, pretreatment of these cells with Genasense followed by Campath markedly increased the amount of cell death compared with Campath used alone. The data for the fresh human CLL cells are depicted in the table below. (Similar results were also noted in experiments using human NHL cell lines.) Lastly, the use of Genasense pretreatment enabled a 5- fold reduction in the equivalent effective dose of Campath.
Treatment
Apoptotic Level(mean % of cell death)
Campath alone
56%
Genasense alone
83%
Genasense plus Campath
92%
“Our new findings corroborate prior observations from our laboratory, which show that Genasense increases the apoptotic activity of a number of chemotherapeutic agents,” noted Dr. Finbarr Cotter, senior author on the publication. “Clinical use of Campath can be associated with significant immunosuppression in patients with advanced CLL, so we are particularly encouraged by the marked activity seen when using reduced levels of that agent. These results suggest that Genasense might be useful in circumstances when reduced doses of highly toxic anticancer drugs may be important.”
About Genasense
Genasense works by inhibiting the production of Bcl-2, a protein made by cancer cells that is thought to block chemotherapy-induced cell death. By reducing the amount of Bcl-2 in cancer cells, Genasense may enhance the effectiveness of current anticancer treatments. Genasense is currently in multiple late-stage randomized clinical trials including malignant melanoma, multiple myeloma, chronic lymphocytic leukemia (CLL) and non-small cell lung cancer.
http://www.genta.com/Genta/InvestorRelation/2003/Press_20030…
Antwort auf Beitrag Nr.: 22.374.579 von nort. am 02.07.06 03:06:36Achtung die Meldung ist vom June 4, 2003. Zeigt allerdings das Potential von Genasense.
Hat jemand die Time & Sales der letzten Handelsstunden ?


Kann einfach nicht den Trade zu 1,99$ finden 

Antwort auf Beitrag Nr.: 22.374.721 von WILIMAZEHAZE am 02.07.06 09:11:03@WILIMAZEHAZE
Den Trade zu 1,99 findest Du im Posting #5. Ich habe das extra reinkopiert, da Island diese Daten immer am Folgetag spätestens 12:55 Uhr löscht. Die T&S sind direkt von der Island-Homepage. Ansonsten findest Du T&S, wenn Du rechts auf "GENTA INC." klickst. Danach dann links auf "Times & Sales" und Börsenplatz "NASDAQ" auswählen. Dort wird allerdings der Island-Trade nicht angezeigt.
Gruss
s.
Den Trade zu 1,99 findest Du im Posting #5. Ich habe das extra reinkopiert, da Island diese Daten immer am Folgetag spätestens 12:55 Uhr löscht. Die T&S sind direkt von der Island-Homepage. Ansonsten findest Du T&S, wenn Du rechts auf "GENTA INC." klickst. Danach dann links auf "Times & Sales" und Börsenplatz "NASDAQ" auswählen. Dort wird allerdings der Island-Trade nicht angezeigt.
Gruss
s.
Ab 5th Juli kann der folgende link im sachen GNTA auch sehr nutzlich sein:
http://host.businessweek.com/businessweek/Historical_Quotes.…
Keep the faith,
Whyso
http://host.businessweek.com/businessweek/Historical_Quotes.…
Keep the faith,
Whyso

Antwort auf Beitrag Nr.: 22.368.828 von nort. am 01.07.06 11:00:56...ich will ja auch garnicht unbedingt rechthaben aber:
GNTA ist nicht die einzige Aktie bei der solch hohe nachbörsliche Umsätze stattfanden...
Zudem habe ich das gerade an Quartalsenden - solches wie wir derzeit ja exakt haben - schön öfters beobachten können. Schön fur dich wenn das dann tatsächlich auch kursrelevant wird - sei aber nicht unbedingt maßlos enttäuscht wenn du Montags wieder ein nahtloses Anküpfen an dan Freitags-SK feststellst....
GNTA ist nicht die einzige Aktie bei der solch hohe nachbörsliche Umsätze stattfanden...
Zudem habe ich das gerade an Quartalsenden - solches wie wir derzeit ja exakt haben - schön öfters beobachten können. Schön fur dich wenn das dann tatsächlich auch kursrelevant wird - sei aber nicht unbedingt maßlos enttäuscht wenn du Montags wieder ein nahtloses Anküpfen an dan Freitags-SK feststellst....
Antwort auf Beitrag Nr.: 22.382.294 von bioperformer am 02.07.06 17:35:23Hallo bioperformer,
kein Problem, es geht nicht um Recht haben, sondern um Erklärungen der Situation. Evtl. ist die Erklärung eine Mischung zwischen Quartalsende und dem Russell-Index... Ab Montag wissen wir mit Sicherheit mehr
Ab Montag wissen wir mit Sicherheit mehr 
nort
kein Problem, es geht nicht um Recht haben, sondern um Erklärungen der Situation. Evtl. ist die Erklärung eine Mischung zwischen Quartalsende und dem Russell-Index...
 Ab Montag wissen wir mit Sicherheit mehr
Ab Montag wissen wir mit Sicherheit mehr 
nort
Antwort auf Beitrag Nr.: 22.382.484 von nort. am 02.07.06 17:43:50also von normal kann man nicht sprechen wenn 14 Mill Shares über den Ladentisch gegangen sind und das dazu mit relativ wenig Spielraum begriffen auf diese Aktie.
Denke eher das die Papiere von den zittrigen zu den hartgesottenen gewandert sind. Das mit den 1,99 messe ich ebenfalls kaum Bedeutung zu, das passiert mir als relativ kleinen Trader mehrmals im Jahr ob nachbörslich stark oder schwach ins Plus.
Denke eher das die Papiere von den zittrigen zu den hartgesottenen gewandert sind. Das mit den 1,99 messe ich ebenfalls kaum Bedeutung zu, das passiert mir als relativ kleinen Trader mehrmals im Jahr ob nachbörslich stark oder schwach ins Plus.
Antwort auf Beitrag Nr.: 22.374.579 von nort. am 02.07.06 03:06:36thx nort,
muss gestehen von dieser kombi wusst ich nix.
ist schon erachtlich das der stärkste Widersacher Campath bei Leukämie Genasense bei der Apoptose schon ziemlich unterlegen ist!
Wieder einmal ein Beispiel dafür dass die Welt nicht länger ohne Genasense auskommen kann zum Wohle des Patienten jedenfalls!
welke
muss gestehen von dieser kombi wusst ich nix.
ist schon erachtlich das der stärkste Widersacher Campath bei Leukämie Genasense bei der Apoptose schon ziemlich unterlegen ist!
Wieder einmal ein Beispiel dafür dass die Welt nicht länger ohne Genasense auskommen kann zum Wohle des Patienten jedenfalls!
welke
Genta In. (GNTA): shares of Berkeley heights, NJ based cancer drugs maker rose 6.8% to $1.71 per share. The stock is still weak as the 50 day moving average is below 200 day moving average. However with K line rising pass D line and MACD about to break above 0 there is the chance that the stock can be back to bull market again. [Accumulate]
http://smartstockinvestment.blogspot.com/2006/06/high-return…
http://smartstockinvestment.blogspot.com/2006/06/high-return…
Antwort auf Beitrag Nr.: 22.388.812 von nort. am 03.07.06 06:14:17große leistung nort 

Antwort auf Beitrag Nr.: 22.382.484 von nort. am 02.07.06 17:43:50hallo nort.,
...also geklärt, war wie vermutet ´ne Verbuchungsaktion im Rahmen des Quartalsendes..
...also geklärt, war wie vermutet ´ne Verbuchungsaktion im Rahmen des Quartalsendes..
Pfizer Visits Hundreds of Companies in Shopping Spree (Update3)
June 30 (Bloomberg) -- Pfizer Inc. will scrutinize hundreds of drug and biotechnology companies this year in a hunt for the next $1 billion medicine, company officials said.
Pfizer, the world's largest drugmaker, has earmarked as much as $17 billion to acquire new drugs or biotechnology treatments during the next two years, the New York-based company said on June 26.
John L. LaMattina, Pfizer's global research president, said this week that he is directing a search for deals -- acquisitions or product licenses -- that would cost between $1 billion and $4 billion. Pfizer will have about $29 billion in cash after the $16.6 billion sale of its consumer-products unit to New Brunswick, New Jersey-based Johnson & Johnson, announced this week.


June 30 (Bloomberg) -- Pfizer Inc. will scrutinize hundreds of drug and biotechnology companies this year in a hunt for the next $1 billion medicine, company officials said.
Pfizer, the world's largest drugmaker, has earmarked as much as $17 billion to acquire new drugs or biotechnology treatments during the next two years, the New York-based company said on June 26.
John L. LaMattina, Pfizer's global research president, said this week that he is directing a search for deals -- acquisitions or product licenses -- that would cost between $1 billion and $4 billion. Pfizer will have about $29 billion in cash after the $16.6 billion sale of its consumer-products unit to New Brunswick, New Jersey-based Johnson & Johnson, announced this week.


GENTA sucht ja derzeit Sales-Leute ohne Ende:
http://jobsearch.monster.com/jobsearch.asp?q=oncology+sales&…
http://genta.com/genta/Employment/careers.html
Ist ein gutes Zeichen. Höchste Zeit sein Winterdepötchen vollzumachen
nort
http://jobsearch.monster.com/jobsearch.asp?q=oncology+sales&…
http://genta.com/genta/Employment/careers.html
Ist ein gutes Zeichen. Höchste Zeit sein Winterdepötchen vollzumachen

nort
Aus dem Yahoo-Board ein guter Beitrag von cxhyixgt:
all the cheerleaders ... (6 Ratings) 10-Jul-06 10:29 pm
have now decided they figured out the stock price movement. They were -supposedly- long and now are -supposedly- getting out. Good for them. Lots of advise from those who lost while they held long positions. Why are they still posting per minute? They seemingly are now the biggest players along the negative sentiment. Why? Are they going short? Very doubtful that they would have guts to play it on the short side at these levels. They are heavily bashing so that people may dump and they can buy back at lower prices. They would not have stayed long until the results would have been heard, had the stock appreciate in anticipation.
What are these people claiming?
- GNTA does not pump the stock with PRs: Big deal. This is how uncertain events are handled. If you cannot stocmach it, get out and shut up. There is no reson to speculate.
- Weakness in the stock price indicates that approvals will not happen: Totally wrong. There is no correlation between pre-approval price movement and "approval" likelihood. We have discussed this here. Going second time around, funds are playing it safe. If you were to keep it until after a decision, what does it matter if it goes to $1 or $3 pre-approval?
Summary of Events:
First of all, I resommend that you all do your own due diligence.
- Previous Genasense FDA negative opinion was formed primarily because of GNTA not hitting the primary end-point with statistical significance. Then, the ODAC advisory comiittee, said, in the absence of statistically significant survival benefit, the claimed benefit *could* have been a result of experiemtn design. The new FDA submittal on CLL shows statistically significant benefit and they met the primary end-point. There is no reason to believe that this data is not approvable from the way FDA responded during the last application process.
- As for the new EMEA application, it is true that they still did not meet the primary end point with statistical significance. However, this statistical significance is a measure of likelihood being less than 5%. Hypothesis testing is not a pure science. Generally accepted terms indicate that if probability values is less than 5% (p<0.05) then the results are statistically significant. During the first application, primary end point had 17% probability after 12month follow up. The most recent application shows 7.7% probability (while is not below 5%, it is much closer to being considered statistically significant). Moreover, a subgroup study (based on LDH level) shows survival benefit to be statistically significant (1.7%). The EMEA would not be able to dismiss this data easily either for the whole study (involving 771 patients, or the subgroup results involving 508 patients). Additionally, remember than GNTA has already discussed the results with 4 countries and that EMEA process is different in nature. It can very well be approved conditionally with LDH levels being the pre-screening measure.
- As for partnership, if it were not for the timeliness of Sanofi acquisition, Aventis could have been the partner to date (ok some speculation on my side, and some specualtion on analysts side). There is no clear reason given for the parting of that partnership other than the fact that Sanofi wanted to show cost reduction synergies from the costly acquisition they have gone through (Ave). It is for true that if the results were a sure thing and close in time, they most likley would not have parted ways, but as non-obvious as it is, genasense has a very good chance of approval...
all the cheerleaders ... (6 Ratings) 10-Jul-06 10:29 pm
have now decided they figured out the stock price movement. They were -supposedly- long and now are -supposedly- getting out. Good for them. Lots of advise from those who lost while they held long positions. Why are they still posting per minute? They seemingly are now the biggest players along the negative sentiment. Why? Are they going short? Very doubtful that they would have guts to play it on the short side at these levels. They are heavily bashing so that people may dump and they can buy back at lower prices. They would not have stayed long until the results would have been heard, had the stock appreciate in anticipation.
What are these people claiming?
- GNTA does not pump the stock with PRs: Big deal. This is how uncertain events are handled. If you cannot stocmach it, get out and shut up. There is no reson to speculate.
- Weakness in the stock price indicates that approvals will not happen: Totally wrong. There is no correlation between pre-approval price movement and "approval" likelihood. We have discussed this here. Going second time around, funds are playing it safe. If you were to keep it until after a decision, what does it matter if it goes to $1 or $3 pre-approval?
Summary of Events:
First of all, I resommend that you all do your own due diligence.
- Previous Genasense FDA negative opinion was formed primarily because of GNTA not hitting the primary end-point with statistical significance. Then, the ODAC advisory comiittee, said, in the absence of statistically significant survival benefit, the claimed benefit *could* have been a result of experiemtn design. The new FDA submittal on CLL shows statistically significant benefit and they met the primary end-point. There is no reason to believe that this data is not approvable from the way FDA responded during the last application process.
- As for the new EMEA application, it is true that they still did not meet the primary end point with statistical significance. However, this statistical significance is a measure of likelihood being less than 5%. Hypothesis testing is not a pure science. Generally accepted terms indicate that if probability values is less than 5% (p<0.05) then the results are statistically significant. During the first application, primary end point had 17% probability after 12month follow up. The most recent application shows 7.7% probability (while is not below 5%, it is much closer to being considered statistically significant). Moreover, a subgroup study (based on LDH level) shows survival benefit to be statistically significant (1.7%). The EMEA would not be able to dismiss this data easily either for the whole study (involving 771 patients, or the subgroup results involving 508 patients). Additionally, remember than GNTA has already discussed the results with 4 countries and that EMEA process is different in nature. It can very well be approved conditionally with LDH levels being the pre-screening measure.
- As for partnership, if it were not for the timeliness of Sanofi acquisition, Aventis could have been the partner to date (ok some speculation on my side, and some specualtion on analysts side). There is no clear reason given for the parting of that partnership other than the fact that Sanofi wanted to show cost reduction synergies from the costly acquisition they have gone through (Ave). It is for true that if the results were a sure thing and close in time, they most likley would not have parted ways, but as non-obvious as it is, genasense has a very good chance of approval...
Can Genasense Bounce Back?
New subgroup analysis submitted with melanoma application.
By Malorye Branca
To some people, Genta's Genasense looks like an important step forward in the treatment of some dreadful diseases. To others, it's a drug with questionable efficacy and serious drawbacks. Now Genta is in the tense situation of waiting for two major regulatory agencies -- the U.S. FDA and Europe's EMA -- to show how they feel about the compound.
In the United States, the drug is up for approval in treatment of relapsed or refractory chronic lymphocytic leukemia (CLL). In Europe, Genta is going for approval in advanced melanoma. In both cases, the drug is given in combination with other, standard therapies. Genasense (oblimersen sodium) is an antisense oligonucleotide targeted against BCL-2, which is a major inhibitor of apoptosis (see Hot Targets. The drug is supposed to work by making tumor cells sensitive to chemotherapy.

The FDA has already had one look at Genasense a couple of years ago, when Genta applied to market the drug for treatment of advanced melanoma in the United States. That submission included data from the largest clinical trial ever done in this disease. But the drug had failed to meet its main end point -- a specific gain in survival. Genta scientists argued that it had at least made a significant difference in disease progression. When an Oncologic Drugs Advisory Committee (ODAC) reviewed that data back in May 2004, they were simply not convinced any positive effects outweighed the side effects.
Genta is now resubmitting that melanoma trial data to the EMA, with some important changes and additions. For one thing, they have followed some of the patients out much further. Looking back, says Genta CEO Raymond Warrell, it was "a big weakness to specify an event-driven analysis in a disease that is characterized by high mortality." The first study was designed with an endpoint of 500 patient deaths: Genta researchers expected the survival curves for the responders and nonresponders to start diverging early. That didn't happen. Instead, the study mortality endpoint was reached before any effect could be seen in the long-term survival curves.
This time, with more survivors included in the data, the drug's effect is much clearer, according to Warrell. "We can now show the drug really extended progression-free survival," he says. Even better, the response is even clearer in a subgroup of patients -- those with normal serum lactate dehydrogenase (LDH) levels. Looking just at that group, patients taking only the standard therapy (dacarbazine) lived an average of 9.7 months, while those also on Genasense as adjunctive therapy lived 11.4 months. Those data were statistically very significant.
But according to Brian Rye, an analyst at Janney Montgomery Scott, the melanoma approval is still questionable because that study's primary end point was still not met even with the extended analysis. Approval, he says, will likely depend on how much unmet need there is in the disease. However, Rye believes the CLL data being submitted in the United States sounds promising: "They did hit [that] primary end point the first time out," he says. Overall, then, Rye sees Genasense as "an approvable drug."
Because it is the only antisense product currently near market, Genasense's future will impact the entire field. The first and last antisense-based drug approved so far is Isis' Vitravene, an eye disease treatment that reached the market several years ago. Since that time, there have only been a string of failures.
The stakes are highest for Genta, of course. "Given what happened two years ago [with FDA submission], we think it is better to let the company prove itself than give it the benefit of the doubt," says Rye.
http://www.pharmaceuticaldiscovery.com/archives/May%2016%20%…
New subgroup analysis submitted with melanoma application.
By Malorye Branca
To some people, Genta's Genasense looks like an important step forward in the treatment of some dreadful diseases. To others, it's a drug with questionable efficacy and serious drawbacks. Now Genta is in the tense situation of waiting for two major regulatory agencies -- the U.S. FDA and Europe's EMA -- to show how they feel about the compound.
In the United States, the drug is up for approval in treatment of relapsed or refractory chronic lymphocytic leukemia (CLL). In Europe, Genta is going for approval in advanced melanoma. In both cases, the drug is given in combination with other, standard therapies. Genasense (oblimersen sodium) is an antisense oligonucleotide targeted against BCL-2, which is a major inhibitor of apoptosis (see Hot Targets. The drug is supposed to work by making tumor cells sensitive to chemotherapy.

The FDA has already had one look at Genasense a couple of years ago, when Genta applied to market the drug for treatment of advanced melanoma in the United States. That submission included data from the largest clinical trial ever done in this disease. But the drug had failed to meet its main end point -- a specific gain in survival. Genta scientists argued that it had at least made a significant difference in disease progression. When an Oncologic Drugs Advisory Committee (ODAC) reviewed that data back in May 2004, they were simply not convinced any positive effects outweighed the side effects.
Genta is now resubmitting that melanoma trial data to the EMA, with some important changes and additions. For one thing, they have followed some of the patients out much further. Looking back, says Genta CEO Raymond Warrell, it was "a big weakness to specify an event-driven analysis in a disease that is characterized by high mortality." The first study was designed with an endpoint of 500 patient deaths: Genta researchers expected the survival curves for the responders and nonresponders to start diverging early. That didn't happen. Instead, the study mortality endpoint was reached before any effect could be seen in the long-term survival curves.
This time, with more survivors included in the data, the drug's effect is much clearer, according to Warrell. "We can now show the drug really extended progression-free survival," he says. Even better, the response is even clearer in a subgroup of patients -- those with normal serum lactate dehydrogenase (LDH) levels. Looking just at that group, patients taking only the standard therapy (dacarbazine) lived an average of 9.7 months, while those also on Genasense as adjunctive therapy lived 11.4 months. Those data were statistically very significant.
But according to Brian Rye, an analyst at Janney Montgomery Scott, the melanoma approval is still questionable because that study's primary end point was still not met even with the extended analysis. Approval, he says, will likely depend on how much unmet need there is in the disease. However, Rye believes the CLL data being submitted in the United States sounds promising: "They did hit [that] primary end point the first time out," he says. Overall, then, Rye sees Genasense as "an approvable drug."
Because it is the only antisense product currently near market, Genasense's future will impact the entire field. The first and last antisense-based drug approved so far is Isis' Vitravene, an eye disease treatment that reached the market several years ago. Since that time, there have only been a string of failures.
The stakes are highest for Genta, of course. "Given what happened two years ago [with FDA submission], we think it is better to let the company prove itself than give it the benefit of the doubt," says Rye.
http://www.pharmaceuticaldiscovery.com/archives/May%2016%20%…
If the FDA announcement is favorable, this $2 stock could double or triple in a week. If the announcement is bad, look out—the stock could fall to zero. Those are the stakes.
The company in question is Genta Inc. (GNTA:NASDAQ). And the drug application waiting FDA approval is something called Genasense, a drug given to cancer patients to assist chemotherapy. Genasense is an antisense treatment, which consist of chemically modified strands of DNA that bind to mRNA.
Without getting too technical, this inhibits the production of a protein -- in this case, Bcl-2 -- a protein that is found in many kinds of cancer cells that can "block" chemotherapy form killing them. By getting Bcl-2 out of the picture, Genasense makes chemo and other anticancer therapy more effective at targeting and eliminating these cancer cells.
Genta’s goal is to see Genasense treatment as the preferred aid to chemotherapy for many different kinds of cancer. And based on recent clinical trial results, the company is well on its way.
In the trial, 241 patients underwent cancer treatment with or without Genasense. 17 percent of those on Genasense achieved complete or partial remission, while only 7 percent of patients who were administered chemotherapy only did. Those who were given Genasense also were in remission longer.
Translation: Genasense’s cancer application was twice as effective as normal chemotherapy.
Thanks to these tremendous results, the FDA accepted the company’s application for Genasense on March 1. The basis for the acceptance is that this treatment can help patients with relapsed or refractory chronic lymphocytic leukemia. The FDA is set to make its decision on whether the drug can be marketed on Oct. 28, 2006.
Given the fact that trial results have been fantastic and FDA has "fast-tracked" the approval process, how is this not a sure fire 100% gain in the makings?
When you are dealing with the FDA, nothing is certain.
Back in 2003 and 2004 Genta had a lucrative partnership with pharmaceutical giant Aventis to developand commercialize Genasense. Yes, the same Genasense in advanced FDA trials today. At the time, the company believed Genasense could be used effectively to help treat malignant melanoma.
Then came the bad news. In May 2004, the FDA decided it would not approve the drug to be used with melanoma patients, and the stock lost most of its value, settling in around $2. Then in November 2004, Aventis -- around the same time as its merger with a major European pharmaceutical giant Sanofi -- decided to terminate the agreement with Genta.
From the sanofi-aventis 2004 annual report:"In the wake of the FDA’s rejection of the NDA for Genasense in advanced melanoma, and in the light of unconvincing results in chronic lymphocytic leukemia (CLL), Sanofi-Aventis decided in November 2004 to terminate its agreement with Genta concerning the development of Genasense."
The company in question is Genta Inc. (GNTA:NASDAQ). And the drug application waiting FDA approval is something called Genasense, a drug given to cancer patients to assist chemotherapy. Genasense is an antisense treatment, which consist of chemically modified strands of DNA that bind to mRNA.
Without getting too technical, this inhibits the production of a protein -- in this case, Bcl-2 -- a protein that is found in many kinds of cancer cells that can "block" chemotherapy form killing them. By getting Bcl-2 out of the picture, Genasense makes chemo and other anticancer therapy more effective at targeting and eliminating these cancer cells.
Genta’s goal is to see Genasense treatment as the preferred aid to chemotherapy for many different kinds of cancer. And based on recent clinical trial results, the company is well on its way.
In the trial, 241 patients underwent cancer treatment with or without Genasense. 17 percent of those on Genasense achieved complete or partial remission, while only 7 percent of patients who were administered chemotherapy only did. Those who were given Genasense also were in remission longer.
Translation: Genasense’s cancer application was twice as effective as normal chemotherapy.
Thanks to these tremendous results, the FDA accepted the company’s application for Genasense on March 1. The basis for the acceptance is that this treatment can help patients with relapsed or refractory chronic lymphocytic leukemia. The FDA is set to make its decision on whether the drug can be marketed on Oct. 28, 2006.
Given the fact that trial results have been fantastic and FDA has "fast-tracked" the approval process, how is this not a sure fire 100% gain in the makings?
When you are dealing with the FDA, nothing is certain.
Back in 2003 and 2004 Genta had a lucrative partnership with pharmaceutical giant Aventis to developand commercialize Genasense. Yes, the same Genasense in advanced FDA trials today. At the time, the company believed Genasense could be used effectively to help treat malignant melanoma.
Then came the bad news. In May 2004, the FDA decided it would not approve the drug to be used with melanoma patients, and the stock lost most of its value, settling in around $2. Then in November 2004, Aventis -- around the same time as its merger with a major European pharmaceutical giant Sanofi -- decided to terminate the agreement with Genta.
From the sanofi-aventis 2004 annual report:"In the wake of the FDA’s rejection of the NDA for Genasense in advanced melanoma, and in the light of unconvincing results in chronic lymphocytic leukemia (CLL), Sanofi-Aventis decided in November 2004 to terminate its agreement with Genta concerning the development of Genasense."
wie sehen hier denn die bio profis die chancen einer zulassung??
derzeit:
+17.83%



 OHNE news!!!
OHNE news!!!
+17.83%



 OHNE news!!!
OHNE news!!!
sitze hier vor dem Rechner mit ner Popkorn Tütte und schaue dem Kurs beim steigen zu: +19.38%
Is,n hier los...Strohfeuer?


Antwort auf Beitrag Nr.: 22.770.158 von Nostarowie am 19.07.06 21:40:35Hmm, dafür ist das Volumen zu hoch... 
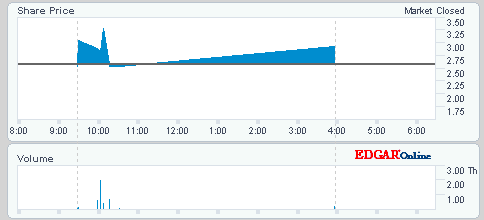

Antwort auf Beitrag Nr.: 22.770.322 von nort. am 19.07.06 21:45:57Ich hatte echt schon den Finger am abzug sprich SL da ich Tags keine Zeit habe..wenn wir unter 1,28 gerodelt wären...aber so is gut. Da wart mer ma.
Sollte es jetzt konstant hochgehen dann ist das doch ein schöner Boden bei $1,30 

und ein W noch dazu...und dann kommt noch Herbst und und und

und ein W noch dazu...und dann kommt noch Herbst und und und

Antwort auf Beitrag Nr.: 22.770.322 von nort. am 19.07.06 21:45:57This should now be fun (for a change) up to Christmas and beyond...
Sonnige Grüße,
Whyso

Sonnige Grüße,
Whyso

hallo, kann mir jemand sagen ob Genta ein fondwert ist, und ob man jetzt noch einsteigen soll ???
Antwort auf Beitrag Nr.: 22.847.064 von noah-allessandro am 21.07.06 20:13:52http://finance.yahoo.com/q/mh?s=GNTA
Major holders
DEr gute alte Soros ist auch mit dabei ...
Major holders
DEr gute alte Soros ist auch mit dabei ...
Gute Frage Nort -Kommt da was????...eindeutig ja




July 26 /PRNewswire-FirstCall/ -- Genta Incorporated (Nasdaq: GNTA - News) announced that the Company's proposal for a confirmatory trial of Genasense® (oblimersen sodium) Injection in patients with chronic lymphocytic leukemia (CLL) has completed Special Protocol Assessment (SPA) by the Food and Drug Administration (FDA). The SPA documents FDA's agreement regarding the trial's design and planned analyses to fulfill commitments that may be required under provisions related to SubPart H regulations regarding accelerated approval. Genta has filed a New Drug Application (NDA) under SubPart H for Genasense plus chemotherapy, which is currently under review by FDA. The NDA has a target action date for a marketing approval decision by October 29, 2006 under the Prescription Drug User Fee Act (PDUFA).
The trial, which will be conducted in symptomatic patients who have not previously received chemotherapy, will randomize patients to receive fludarabine plus rituximab (Rituxan®; Genentech, Inc.) with or without Genasense. The pivotal trial in the pending NDA employed a combination of fludarabine plus cyclophosphamide with or without Genasense in patients with relapsed or refractory CLL.
"Data from our previous randomized trials have suggested that extensive prior treatment may introduce other mechanisms of acquired drug resistance," said Dr. Loretta M. Itri, Genta's Chief Medical Officer and President, Pharmaceutical Development. "This study targets treatment-naïve patients with a regimen that is still considered investigational, but which includes the most widely prescribed drugs for CLL in the U.S. With this trial, we reaffirm our commitment to patients with this illness, and we are very pleased with FDA's review of this next-step proposal."





July 26 /PRNewswire-FirstCall/ -- Genta Incorporated (Nasdaq: GNTA - News) announced that the Company's proposal for a confirmatory trial of Genasense® (oblimersen sodium) Injection in patients with chronic lymphocytic leukemia (CLL) has completed Special Protocol Assessment (SPA) by the Food and Drug Administration (FDA). The SPA documents FDA's agreement regarding the trial's design and planned analyses to fulfill commitments that may be required under provisions related to SubPart H regulations regarding accelerated approval. Genta has filed a New Drug Application (NDA) under SubPart H for Genasense plus chemotherapy, which is currently under review by FDA. The NDA has a target action date for a marketing approval decision by October 29, 2006 under the Prescription Drug User Fee Act (PDUFA).
The trial, which will be conducted in symptomatic patients who have not previously received chemotherapy, will randomize patients to receive fludarabine plus rituximab (Rituxan®; Genentech, Inc.) with or without Genasense. The pivotal trial in the pending NDA employed a combination of fludarabine plus cyclophosphamide with or without Genasense in patients with relapsed or refractory CLL.
"Data from our previous randomized trials have suggested that extensive prior treatment may introduce other mechanisms of acquired drug resistance," said Dr. Loretta M. Itri, Genta's Chief Medical Officer and President, Pharmaceutical Development. "This study targets treatment-naïve patients with a regimen that is still considered investigational, but which includes the most widely prescribed drugs for CLL in the U.S. With this trial, we reaffirm our commitment to patients with this illness, and we are very pleased with FDA's review of this next-step proposal."

Grüße von Tara drüben:
------------------
-----Ursprüngliche Nachricht-----
Von: Investor_Relations [mailto:Investor_Relations@genta.com]
Gesendet: Mittwoch, 26. Juli 2006 14:04
An: undisclosed-recipients:
Betreff: Genta Press Release - Wednesday, July 26, 2006
Attached please find a Genta press release, Genta Receives Special Protocol Assessment from FDA for Confirmatory Trial of Genasense® Plus Chemotherapy in Chronic Lymphocytic Leukemia, which crossed the wire today, Wednesday, July 26, 2006 at 8:00 am ET.
If you have any questions, please contact Investor Relations at 908-286-3980.
Thank you.
<<SPA Phase 4 CLL receipt v2 7-06.pdf>>
Investor Relations
908-286-3980 (T)
info@genta.com
-------------------
1,68 pre market
------------------
-----Ursprüngliche Nachricht-----
Von: Investor_Relations [mailto:Investor_Relations@genta.com]
Gesendet: Mittwoch, 26. Juli 2006 14:04
An: undisclosed-recipients:
Betreff: Genta Press Release - Wednesday, July 26, 2006
Attached please find a Genta press release, Genta Receives Special Protocol Assessment from FDA for Confirmatory Trial of Genasense® Plus Chemotherapy in Chronic Lymphocytic Leukemia, which crossed the wire today, Wednesday, July 26, 2006 at 8:00 am ET.
If you have any questions, please contact Investor Relations at 908-286-3980.
Thank you.
<<SPA Phase 4 CLL receipt v2 7-06.pdf>>
Investor Relations
908-286-3980 (T)
info@genta.com
-------------------
1,68 pre market

also dann, nächster Anlauf auf den Bergstrum...

Man sollte ebenfalls beachten, wer heute so zugelangt hat am Markt
http://thomson.finance.lycos.com/lycos/iwatch/cgi-bin/iw_tic…
Name
GENTA INC
Last Price
1.6100
Net Change
+0.2100
+0.0600 (AH)
Shares Matched
835,084
2,400 (AH)
Market
G
Previous Close
1.4000
Percent Change
+15.00%
+3.87 (AH)
Man sollte ebenfalls beachten, wer heute so zugelangt hat am Markt

http://thomson.finance.lycos.com/lycos/iwatch/cgi-bin/iw_tic…
Name
GENTA INC
Last Price
1.6100
Net Change
+0.2100
+0.0600 (AH)
Shares Matched
835,084
2,400 (AH)
Market
G
Previous Close
1.4000
Percent Change
+15.00%
+3.87 (AH)
Antwort auf Beitrag Nr.: 23.108.276 von nort. am 27.07.06 06:51:50naja, es haben statt fünf Prozent (30-Tages-Durchschnitt) neun Prozent Instis zugelangt, relativ gesehen ist das zwar fast eine Verdopplung des institutionellen Interesses, aber absolut gesehen? Außerdem werden drei Viertel des Volumes in der I-Watch Graphik nicht erfasst, man weiß also nicht, wie repräsentativ diese Zahlen sind...
Gruß, hecht
Gruß, hecht
Es wird apannend!
Genta Announces FDA's Oncology Drug Advisory Committee Will Review Genasense(R) as Treatment for Chronic Lymphocytic Leukemia
http://biz.yahoo.com/prnews/060727/nyth110.html?.v=53
Genta Announces FDA's Oncology Drug Advisory Committee Will Review Genasense(R) as Treatment for Chronic Lymphocytic Leukemia
http://biz.yahoo.com/prnews/060727/nyth110.html?.v=53
schläft noch ...  aber immer auf der Lauer (RSI)
aber immer auf der Lauer (RSI)
http://stockcharts.com/c-sc/sc?s=GNTA&p=D&yr=0&mn=6&dy=15&id…
 aber immer auf der Lauer (RSI)
aber immer auf der Lauer (RSI)http://stockcharts.com/c-sc/sc?s=GNTA&p=D&yr=0&mn=6&dy=15&id…
Wie mit dem Lineal gezogen der Kurs heute !
1.39 -1,42 $ und das über Stunden...da wurde heute Stunden lange 1,7 Mio Aktien geschoben und der Kurs rappelt sich nicht...
Innerhalb von Sekunden/Minuten gingen 500.000 Shares über den Tisch und kein ruckeln am Kurs....kleine, feine Paketevon 500-1000 Stocks.....naja hoffen wir mal das Beste....
1.39 -1,42 $ und das über Stunden...da wurde heute Stunden lange 1,7 Mio Aktien geschoben und der Kurs rappelt sich nicht...
Innerhalb von Sekunden/Minuten gingen 500.000 Shares über den Tisch und kein ruckeln am Kurs....kleine, feine Paketevon 500-1000 Stocks.....naja hoffen wir mal das Beste....
Das Startniveau wurde gefunden. 
Anbei ein Link aus dem Yahoo-Board. Eine gute Zusammenfassung in Form eines Blogs über Genta:
http://equityinvestmentideas.blogspot.com/2006/04/catalysts-…
nort
# This $235 million company has a treatment in the works that could make chemotherapy twice as effective in the quest for killing deadly cancer cells. Its treatment is in stage 3 clinical trials, and the FDA granted the company fast track status to bring this application to market since no other treatment like it exists.
# 60% of all drugs given fast-track status are brought to market successfully. That’s good news for this small-cap company. The odds are in its favor.
# The only thing that stands in its way now from potential million in profits is a few months and the FDA’s approval. Approval will either be granted or denied on Oct. 28, 2006, less six seven months from today.
In the trial, 241 patients underwent cancer treatment with or without Genasense. 17% of those on Genasense achieved complete or partial remission, while only 7% of patients who were administered chemotherapy only did. Those who were given Genasense also were in remission longer. Genasense’s cancer application was twice as effective as normal chemotherapy.

Anbei ein Link aus dem Yahoo-Board. Eine gute Zusammenfassung in Form eines Blogs über Genta:
http://equityinvestmentideas.blogspot.com/2006/04/catalysts-…
nort
# This $235 million company has a treatment in the works that could make chemotherapy twice as effective in the quest for killing deadly cancer cells. Its treatment is in stage 3 clinical trials, and the FDA granted the company fast track status to bring this application to market since no other treatment like it exists.
# 60% of all drugs given fast-track status are brought to market successfully. That’s good news for this small-cap company. The odds are in its favor.
# The only thing that stands in its way now from potential million in profits is a few months and the FDA’s approval. Approval will either be granted or denied on Oct. 28, 2006, less six seven months from today.
In the trial, 241 patients underwent cancer treatment with or without Genasense. 17% of those on Genasense achieved complete or partial remission, while only 7% of patients who were administered chemotherapy only did. Those who were given Genasense also were in remission longer. Genasense’s cancer application was twice as effective as normal chemotherapy.
Antwort auf Beitrag Nr.: 23.705.466 von nort. am 31.08.06 18:31:15na wo rennt sie denn

Its all about this tommorow:
" Overall, the study found that 20 out of 120 patients, or 16.7%, receiving Genasense and other therapy temporarily had their disease improve compared with 8 out of 121, or 6.6%.
The FDA agreed with the study findings. However, the FDA said overall survival among both patient groups was similar, lasting just less than three years. But, both the company and the FDA noted that no cancer drug has so far been shown to improve overall survival among such leukemia patients. "
" Overall, the study found that 20 out of 120 patients, or 16.7%, receiving Genasense and other therapy temporarily had their disease improve compared with 8 out of 121, or 6.6%.
The FDA agreed with the study findings. However, the FDA said overall survival among both patient groups was similar, lasting just less than three years. But, both the company and the FDA noted that no cancer drug has so far been shown to improve overall survival among such leukemia patients. "

Antwort auf Beitrag Nr.: 23.782.209 von whyso am 06.09.06 00:46:11Du postest in allen GENTA Threas deine Skepsiss.
Warum sagst Du nicht das Du raus bist ????
In einem Thread hast Du ja schon angefangen eine neue Aktie zu puschen.
Warum seid Ihr nicht ehrlich??
Ach ja mit Ehrlichkeit läßt sich an der Börse ja kein Geld verdienen.
Ich vergaß.
Warum sagst Du nicht das Du raus bist ????
In einem Thread hast Du ja schon angefangen eine neue Aktie zu puschen.
Warum seid Ihr nicht ehrlich??
Ach ja mit Ehrlichkeit läßt sich an der Börse ja kein Geld verdienen.
Ich vergaß.
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 236 | ||
| 118 | ||
| 100 | ||
| 88 | ||
| 59 | ||
| 39 | ||
| 36 | ||
| 35 | ||
| 32 | ||
| 31 |
| Wertpapier | Beiträge | |
|---|---|---|
| 30 | ||
| 28 | ||
| 26 | ||
| 25 | ||
| 24 | ||
| 20 | ||
| 19 | ||
| 18 | ||
| 18 | ||
| 17 |





















