CytomX - ein bahnbrechender Ansatz in der Tumortherapie (Seite 42)
eröffnet am 01.07.16 16:03:16 von
neuester Beitrag 27.01.24 11:32:06 von
neuester Beitrag 27.01.24 11:32:06 von
Beiträge: 1.459
ID: 1.234.534
ID: 1.234.534
Aufrufe heute: 0
Gesamt: 163.656
Gesamt: 163.656
Aktive User: 0
ISIN: US23284F1057 · WKN: A14158 · Symbol: 6C1
1,5320
EUR
+1,06 %
+0,0160 EUR
Letzter Kurs 30.04.24 Tradegate
Neuigkeiten
08.04.24 · globenewswire |
03.04.24 · globenewswire |
21.03.24 · globenewswire |
18.03.24 · globenewswire |
12.03.24 · wO Chartvergleich |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,5888 | +476,69 | |
| 1,8650 | +40,23 | |
| 1,7900 | +35,61 | |
| 3,9000 | +21,12 | |
| 7,9000 | +20,61 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,9600 | -19,67 | |
| 25,81 | -22,12 | |
| 5,1700 | -23,97 | |
| 3,5000 | -26,24 | |
| 0,5103 | -40,66 |
Beitrag zu dieser Diskussion schreiben
By the way...weiterhin sehr informative und herausragende Beiträge hier insb. von Nase_weis_nix und BReal, danke schön👍
Weiter akkumuliert zu 11,50$...MK knapp über Cashbasis, die Korrekturen an den US-Märkten sind dermaßen oversold z.Zt.
Ich habe mal eine Kauforder bei $9.50 gelegt.
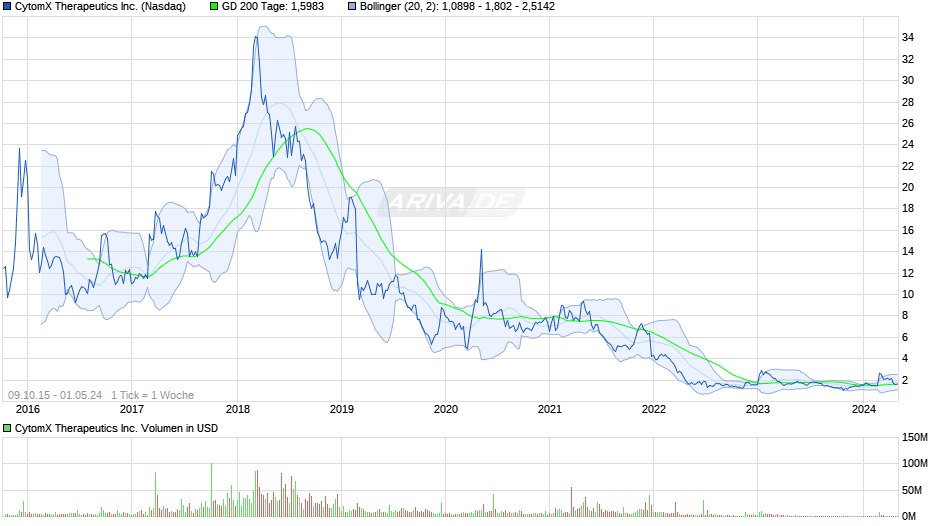

Habe nach längere Recherche und auch den sehr informativen Beiträgen hier ein weiteres Aktienpacket gekauft um mein Bestand zu verbilligen. Ich spekuliere mittelfristig auf eine Erholung bzw. zunächst eine Bodenbildung auf aktuellem Niveau, welches etwa dem 52W Tief entspricht.
Unterm Strich hat sich langfristig doch nichts geändert. Die Einstellung der CX-188 pipeline finde ich strategisch richtig. Der Focus sollte auf der Beantragung Freigabe CX-72 und weitere Entwicklung CX2009 und CX2029 liegen. Der Cashbestand mit ist weiter hervorragend und entspricht etwa 56 % der MK. Das finde ich in Ordnung für eine Unternehmung wie CTX.
Gruß
JM
Unterm Strich hat sich langfristig doch nichts geändert. Die Einstellung der CX-188 pipeline finde ich strategisch richtig. Der Focus sollte auf der Beantragung Freigabe CX-72 und weitere Entwicklung CX2009 und CX2029 liegen. Der Cashbestand mit ist weiter hervorragend und entspricht etwa 56 % der MK. Das finde ich in Ordnung für eine Unternehmung wie CTX.
Gruß
JM
Antwort auf Beitrag Nr.: 59.984.860 von schnappi am 28.02.19 11:30:35Die Technologie bzw die Forschung an sich ist ja nicht schlecht ! Denke da Is noch Potential vorhanden ! Eig geht es ja nur um den Ausstieg des Investors! In geraumer Zeit wird sich auch da wieder was tun !
Antwort auf Beitrag Nr.: 59.973.643 von schnappi am 27.02.19 09:10:49Nachdem ich nun mal in Ruhe alles durchgelesen habe bleibe ich doch investiert ...
In der gestrigen Telko zu den Jahreszahlen wurden einige der Themen, zu denen ich vorher schrieb, in der Q&A-Session angesprochen. Mit den Antworten kann ich im Grunde gut leben.
Zum Thema 2009-Dosiseskalation ohne Prophylaxe:
“Going into this dose escalation as we began to realize that we could dose escalate beyond six mgs per kg and as we kept going, we made a very affirmative decision in collaboration with our Safety Review Committee to dose escalate initially without ocular prophylaxis so that we could get a clear sense of the overall profile of the drug from a safety and efficacy standpoint.
That was a very affirmative decision that we took early on in the program and we took that decision in the full expectation that once we learned about the safety and efficacy of the drug at these upper doses, we would then implement like most likely implement ocular prophylaxis, if needed depending upon what dose we got to which is exactly what we've done. And so the toxicity that we've certainly paid the most attention to and that we have a really good, I believe a really good handle on moving forward is ocular toxicity.
The others are do you really think about it. You look at the safety table, it's still really early days in understanding what their incidence is, what their severity is because the patient numbers are still small, we need to learn more. So we're really quite focused on the ocular tox at this point. We think we've got a handle on it. We're going to need to learn more about some of the other -- about the integrated safety profile as we move forward and there's much still to be learned for sure.”
Zum Thema BMS-Kooperation (Celgene wird nicht erwähnt):
“In conjunction with our broad R&D update yesterday, we announced that BMS has de-prioritized three collaboration targets. This reflects the natural ebb and flow of a long-term multi-target discovery stage biotech pharma partnership. The collaboration remains in full force in effect and we look forward to continuing to make progress on existing collaboration programs and future targets that BMS may select.”
Zum Thema 188:
I mean as I said it's a funny thing actually because we've had several discussions with investors over the last year where we've been asked, why you're doing both and it looks like CX-072 is working fine. We don't quite understand why you're doing CX-188. And so I have to say we were rather surprised by some of the feedback yesterday and really a little taken aback by some of the comments in the biotech press which I think got the wrong end of the stick by a long margin. So yes, I could see if I could see a situation develop as I said yesterday, where in a potential partnership, a partner may have interest in that molecule as well.
But for now, we're really thrilled with where we are with CX-072, it's a couple of years ahead in terms of the clinical program, it's performing just as we designed it to and from a capital allocation standpoint, it just makes all the sense in the world to put PDL-1 on the shelf for the time being.
Zum Thema “Bewusstsein, dem Markt demnächst Entwicklungspfade für die Projekte aufzeigen zu müssen”:
“And as I said in my introductory remarks really for both of the lead programs, we're at a point in time right now where as I said we're making that transition from the platform, proof-of-concept last year to 2019 where the development program supporting the product profiles is going to take more shape. But we'll have more to say about that as the year goes on. We're just not quite ready to do that just yet. So hang in there, it's all coming.”
Zum Thema 2009-Dosiseskalation ohne Prophylaxe:
“Going into this dose escalation as we began to realize that we could dose escalate beyond six mgs per kg and as we kept going, we made a very affirmative decision in collaboration with our Safety Review Committee to dose escalate initially without ocular prophylaxis so that we could get a clear sense of the overall profile of the drug from a safety and efficacy standpoint.
That was a very affirmative decision that we took early on in the program and we took that decision in the full expectation that once we learned about the safety and efficacy of the drug at these upper doses, we would then implement like most likely implement ocular prophylaxis, if needed depending upon what dose we got to which is exactly what we've done. And so the toxicity that we've certainly paid the most attention to and that we have a really good, I believe a really good handle on moving forward is ocular toxicity.
The others are do you really think about it. You look at the safety table, it's still really early days in understanding what their incidence is, what their severity is because the patient numbers are still small, we need to learn more. So we're really quite focused on the ocular tox at this point. We think we've got a handle on it. We're going to need to learn more about some of the other -- about the integrated safety profile as we move forward and there's much still to be learned for sure.”
Zum Thema BMS-Kooperation (Celgene wird nicht erwähnt):
“In conjunction with our broad R&D update yesterday, we announced that BMS has de-prioritized three collaboration targets. This reflects the natural ebb and flow of a long-term multi-target discovery stage biotech pharma partnership. The collaboration remains in full force in effect and we look forward to continuing to make progress on existing collaboration programs and future targets that BMS may select.”
Zum Thema 188:
I mean as I said it's a funny thing actually because we've had several discussions with investors over the last year where we've been asked, why you're doing both and it looks like CX-072 is working fine. We don't quite understand why you're doing CX-188. And so I have to say we were rather surprised by some of the feedback yesterday and really a little taken aback by some of the comments in the biotech press which I think got the wrong end of the stick by a long margin. So yes, I could see if I could see a situation develop as I said yesterday, where in a potential partnership, a partner may have interest in that molecule as well.
But for now, we're really thrilled with where we are with CX-072, it's a couple of years ahead in terms of the clinical program, it's performing just as we designed it to and from a capital allocation standpoint, it just makes all the sense in the world to put PDL-1 on the shelf for the time being.
Zum Thema “Bewusstsein, dem Markt demnächst Entwicklungspfade für die Projekte aufzeigen zu müssen”:
“And as I said in my introductory remarks really for both of the lead programs, we're at a point in time right now where as I said we're making that transition from the platform, proof-of-concept last year to 2019 where the development program supporting the product profiles is going to take more shape. But we'll have more to say about that as the year goes on. We're just not quite ready to do that just yet. So hang in there, it's all coming.”
Hier der Abstract vom Inmmunogen auf der AACR
Abstract
Epithelial Cell Adhesion Molecule (EpCAM) is a glycosylated, 40-kDa type I transmembrane protein that plays a role in cell adhesion and cell signaling. EpCAM is an attractive target for antibody drug conjugate (ADC) development due to its overexpression on a variety of tumors of epithelial origin, including lung, colon, breast, ovarian, prostate and pancreatic cancers. In addition, EpCAM is enriched on tumor-initiating cells (TICs), which are often resistant to conventional cancer therapies. As such, EpCAM-targeted therapies may lead to more durable responses. However, EpCAM is also expressed on a variety of normal epithelia, thus limiting its utility as an ADC target due to potential toxicity. We aim to overcome this limitation by developing an EpCAM-targeting Probody™ drug conjugate (PDC). A Probody therapeutic is an antibody engineered with a mask that blocks the antigen binding site. Probody therapeutics can be selectively activated by tumor associated proteases releasing an active antibody with restored antigen binding activity. Therefore, an EpCAM-targeting PDC could have the anti-tumor potency of an ADC, while limiting binding to healthy tissues and minimizing toxicities.
Here, we describe the development of EpCAM-targeted PDCs, based on a novel human/cynomolgus cross-reactive anti-EpCAM antibody. Probody molecules were successfully conjugated to either the maytansine-derived microtubule disruptor, DM4, linked via a hindered disulfide hydrophilic linker (sulfo-SPDB) or the ultra-potent DNA alkylating payload, DGN549. The antigen binding and in vitro cytotoxicity of intact PDCs were dramatically reduced compared to the corresponding ADC, but could be restored following in vitro proteolytic activation. Furthermore, EpCAM-targeting PDCs displayed compelling and specific anti-tumor activity in xenograft mouse models. In addition, the tolerability and pharmacokinetics (PK) of a selection of the EpCAM PDCs were compared to the EpCAM-targeting ADC in cynomolgus monkeys. EpCAM-targeting PDCs were better tolerated than the corresponding EpCAM-targeting ADC even at higher dose levels and displayed longer half-lives and greater exposure. Therefore, EpCAM PDCs showed greatly improved tolerability and PK profiles compared to the EpCAM ADC.
The studies presented herein support an anti-EpCAM PDC as a promising novel therapeutic to target a wide range of EpCAM-expressing cancers with the potential to overcome the associated on-target toxicities. PROBODY is a trademark of CytomX Therapeutics, Inc.
https://www.abstractsonline.com/pp8/#!/6812/presentation/292…
Klingt vielversprechend. Mal sehen, ob Immunogen die Phase 1 dieses Jahr noch startet.
Abstract
Epithelial Cell Adhesion Molecule (EpCAM) is a glycosylated, 40-kDa type I transmembrane protein that plays a role in cell adhesion and cell signaling. EpCAM is an attractive target for antibody drug conjugate (ADC) development due to its overexpression on a variety of tumors of epithelial origin, including lung, colon, breast, ovarian, prostate and pancreatic cancers. In addition, EpCAM is enriched on tumor-initiating cells (TICs), which are often resistant to conventional cancer therapies. As such, EpCAM-targeted therapies may lead to more durable responses. However, EpCAM is also expressed on a variety of normal epithelia, thus limiting its utility as an ADC target due to potential toxicity. We aim to overcome this limitation by developing an EpCAM-targeting Probody™ drug conjugate (PDC). A Probody therapeutic is an antibody engineered with a mask that blocks the antigen binding site. Probody therapeutics can be selectively activated by tumor associated proteases releasing an active antibody with restored antigen binding activity. Therefore, an EpCAM-targeting PDC could have the anti-tumor potency of an ADC, while limiting binding to healthy tissues and minimizing toxicities.
Here, we describe the development of EpCAM-targeted PDCs, based on a novel human/cynomolgus cross-reactive anti-EpCAM antibody. Probody molecules were successfully conjugated to either the maytansine-derived microtubule disruptor, DM4, linked via a hindered disulfide hydrophilic linker (sulfo-SPDB) or the ultra-potent DNA alkylating payload, DGN549. The antigen binding and in vitro cytotoxicity of intact PDCs were dramatically reduced compared to the corresponding ADC, but could be restored following in vitro proteolytic activation. Furthermore, EpCAM-targeting PDCs displayed compelling and specific anti-tumor activity in xenograft mouse models. In addition, the tolerability and pharmacokinetics (PK) of a selection of the EpCAM PDCs were compared to the EpCAM-targeting ADC in cynomolgus monkeys. EpCAM-targeting PDCs were better tolerated than the corresponding EpCAM-targeting ADC even at higher dose levels and displayed longer half-lives and greater exposure. Therefore, EpCAM PDCs showed greatly improved tolerability and PK profiles compared to the EpCAM ADC.
The studies presented herein support an anti-EpCAM PDC as a promising novel therapeutic to target a wide range of EpCAM-expressing cancers with the potential to overcome the associated on-target toxicities. PROBODY is a trademark of CytomX Therapeutics, Inc.
https://www.abstractsonline.com/pp8/#!/6812/presentation/292…
Klingt vielversprechend. Mal sehen, ob Immunogen die Phase 1 dieses Jahr noch startet.
Antwort auf Beitrag Nr.: 59.979.556 von hinz12 am 27.02.19 19:13:39Dazu findet sich keine Ankündigung bei CytomX.
Gruß
JM
Gruß
JM
Immunogen wird präklinische Daten auf der AACR präsentieren. Heute abend werden die Abstracts veröffentlicht. Da bin ich sehr gespannt.
Wollte Cytomx dort nicht auch noch neuere Daten präsentieren?
Wollte Cytomx dort nicht auch noch neuere Daten präsentieren?
CytomX - ein bahnbrechender Ansatz in der Tumortherapie





















