Iomai Corporation (IOMI) - 500 Beiträge pro Seite
eröffnet am 03.08.07 20:09:51 von
neuester Beitrag 15.01.08 15:58:33 von
neuester Beitrag 15.01.08 15:58:33 von
Beiträge: 10
ID: 1.131.241
ID: 1.131.241
Aufrufe heute: 0
Gesamt: 1.024
Gesamt: 1.024
Aktive User: 0
ISIN: US46202P1030
Profile:Iomai Corporation, a biopharmaceutical company, engages in the discovery, development, and commercialization of vaccines and immune system stimulants. Its products under development include IS patch for pandemic flu program, a Phase 1 clinical trial IS patch that, when used in conjunction with an injectable flu vaccine, is designed to stimulate an immune response, as well as that allows public health officials to extend vaccine supply in the event of an influenza pandemic; and IS patch for elderly receiving flu vaccines, a Phase 2 clinical trial IS patch to improve the immune response of the elderly to existing injectable influenza vaccines. The company also develops a needle-free flu vaccine patch, a Phase 1 clinical trial product candidate, which combines flu antigens with an adjuvant in a single patch; a needle-free pandemic flu vaccine patch, a product candidate under preclinical development, which combines pandemic flu antigen with an adjuvant in a single patch to solve issues regarding mass vaccination in the event of an outbreak of pandemic flu; and a needle-free travelers' diarrhea vaccine patch, a Phase 2 clinical trial product candidate, which is designed to induce a immune response that diminished the severity and delayed the onset of the common form of travelers' diarrhea. Iomai Corporation's vaccines and immune system stimulants are delivered to the skin via a needle-free technology, transcutaneous immunization, a proprietary technology designed to trigger an immune response by targeting Langerhans cells. The company was incorporated in 1997 and is based in Gaithersburg, Maryland.
http://www.iomai.com/

http://www.iomai.com/

Iomai Vaccine Confers Statistically Significant Protection Against Travelers' Diarrhea in Phase 2 Study
Wednesday August 1, 6:45 am ET
- Interim Data from Field Study Also Shows Patch Vaccine Limits Severity, Shortens Duration of Travelers' Diarrhea -
- Conference Call Scheduled for 8:30 a.m. Eastern Today -
GAITHERSBURG, Md., Aug. 1 /PRNewswire-FirstCall/ -- Iomai Corporation (Nasdaq: IOMI - News) today announced that its patch-based vaccine for enterotoxigenic E. coli (ETEC) bacteria conferred statistically significant protection from travelers' diarrhea as compared to placebo, particularly for more severe cases, according to interim data from a double-blind Phase 2 field study. Vaccinated travelers were 75 percent less likely to suffer moderate or severe diarrhea from any cause (p<0.01) and 84 percent less likely to be afflicted by severe diarrhea (p<0.05). No vaccine-related serious adverse events were reported.
In addition to the protective effects, investigators found that the frequency and duration of diarrhea in vaccinated subjects who did contract disease was significantly lower than in their unvaccinated peers.
"The results from this study strongly suggest that the use of Iomai's needle-free, patch-based vaccine can have a notable impact in reducing the chances of suffering from the ravages of travelers' diarrhea," said Herbert L. DuPont, M.D., professor and director of the Center for Infectious Diseases at The University of Texas School of Public Health and the principal investigator on the trial. "Right now, we are generally limited to administering antibiotics after the illness has begun. An effective, easy-to-use vaccine would be a vast improvement and have an immediate impact on travel medicine."
The Trek Phase 2 field study followed 170 subjects, 111 who received a placebo and 59 who received two doses of Iomai's patch-based vaccine before traveling to Mexico or Guatemala. These efficacy results were particularly notable as the trial was conducted largely to gather information on the logistics of conducting such a study in the field. The study was conducted in collaboration with researchers from the Johns Hopkins Bloomberg School of Public Health and the University of Texas School of Public Health.
Iomai's vaccine uses the company's novel transcutaneous immunization (TCI) technology, which allows the vaccine to be delivered to the immune system via a simple patch affixed to the skin. ETEC causes illness through the toxins it produces, including one known as heat-labile toxin or LT. Iomai's unique patch-based vaccine enables the safe administration of this potent immunogen into the skin to stimulate the immune response.
"These convincing results, demonstrating high levels of efficacy in a real-world setting, serve as validation of our TCI approach," said Dr. Gregory Glenn, M.D., Iomai's founder and chief scientific officer. "The data suggest that our TCI technology can be used to provide protection against other pathogens. We are moving as quickly as possible to complete our Phase 2 work so we can begin pivotal trials and bring our travelers' diarrhea vaccine candidate to market."
The study met its primary endpoints, which were designed to evaluate the safety of the vaccine and the incidence of ETEC. The secondary objectives included evaluation of vaccine-preventable outcomes, immunogenicity and stool frequency. The study also gathered data on the protective efficacy of the vaccine. Iomai plans to submit the full dataset from the trial for publication as soon as possible. The company intends to launch a Phase 3 program for the vaccine in 2008.
About Travelers' Diarrhea
This year, approximately 55 million international travelers will visit countries where bacteria that cause travelers' diarrhea are endemic, particularly Africa, Asia and Latin America, and about 20 million of those travelers will develop travelers' diarrhea. "Though we always caution travelers about the best ways to avoid food and drink that may harbor the bacteria, that advice is often not enough, and an effective means of vaccination could substantially reduce the burden of disease," said Robin McKenzie, M.D., an assistant professor at Johns Hopkins and an investigator on the trial.
ETEC, the most common cause of travelers' diarrhea, represents just under half of all cases of travelers' diarrhea for international travelers to areas where ETEC is common. A recently completed market study suggested that there is a large market for an effective ETEC vaccine, potentially exceeding $500 million annually. If approved, the Iomai vaccine would be the first vaccine for travelers' diarrhea available in the United States.
ETEC's impact goes beyond travelers. The World Health Organization estimates that children in the developing world suffer 210 million episodes of diarrhea caused by ETEC annually, causing 380,000 deaths each year.
Conference Call Details
To access the live conference call today at 8:30 a.m. Eastern Time via phone, please dial 866-510-0705 from the United States and Canada or 617-597- 5363 internationally. The conference ID is 26738905. Please dial in approximately ten minutes prior to the start of the call. A telephone replay will be available beginning approximately one hour after the call through Aug. 8 and may be accessed by dialing 888-286-8010 from the United States and Canada or 617-801-6888 internationally. The replay passcode is 85144095.
To access the live and subsequently archived webcast of the conference call, go to the Investor Relations section of the Company's website at www.iomai.com. Please connect to the web site at least 15 minutes prior to the call to allow for any software download that may be necessary. The webcast is also being distributed through the Thomson StreetEvents Network. Individual investors can listen to the call at www.earnings.com, Thomson's individual investor portal, powered by StreetEvents. Institutional investors can access the call via Thomson StreetEvents (www.streetevents.com), a password-protected event management site.
ABOUT IOMAI CORPORATION
Iomai Corporation discovers and develops vaccines and immune system stimulants, delivered via a novel, needle-free technology called transcutaneous immunization (TCI). TCI, discovered by researchers at the Walter Reed Army Institute of Research, taps into the unique benefits of a major group of antigen-presenting cells found in the outer layers of the skin (Langerhans cells) to generate an enhanced immune response. Iomai is leveraging TCI to enhance the efficacy of existing vaccines, develop new vaccines that are viable only through transcutaneous administration and expand the global vaccine market. Iomai currently has four product candidates in development: three targeting influenza and pandemic flu and one to prevent travelers' diarrhea. For more information on Iomai, please visit www.iomai.com.
Some matters discussed in this press release constitute "forward-looking statements" that involve known and unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements. Such forward-looking statements include statements about the timing and expectation for Iomai completing Phase 2 work on this product candidate, commencing Phase 3 studies, and bringing the product candidate to market; the potential size of the market for an ETEC vaccine; the applicability of TCI technology to other pathogens; and that, if approved, Iomai's vaccine would be the first available in the U.S. Applicable risks and uncertainties include, among others, that Iomai may not be able to enroll sufficient numbers of patients in future clinical trials; that future clinical trials may not replicate results seen in the trial described in this press release; that Iomai may be unable to obtain the regulatory approvals necessary to conduct additional clinical trials or to market any product for travelers' diarrhea; that estimates of market size overstate the number of travelers who would use such a product, if it were approved; that competitors may develop products that are safer, more effective, or more convenient to use; and the risks identified under the heading "Factors That May Impact Future Results" in Management's Discussion and Analysis of Financial Condition and Results of Operations in Iomai's Quarterly Report on Form 10-Q for the three months ended March 31, 2007, and filed with the Securities and Exchange Commission. While Iomai believes that this product candidate is amenable to self-administration, in the Phase 2 study described in this press release, medical professionals administered the vaccine. Whether any approved product would be self-administered would depend on many factors, including the outcome of any studies the Company conducts evaluating self-administration and the views of regulatory agencies. Iomai cautions investors and others not to place undue reliance on the forward-looking statements contained in this press release. Iomai's business is subject to many risks. For a discussion of such risks, you are encouraged to read the documents the Company files with the U.S. Securities and Exchange Commission, available at http://www.sec.gov. The statements in this press release speak only as of the date of this document, and Iomai undertakes no obligation to update or revise the statements.
--------------------------------------------------------------------------------
Source: Iomai Corporation
Wednesday August 1, 6:45 am ET
- Interim Data from Field Study Also Shows Patch Vaccine Limits Severity, Shortens Duration of Travelers' Diarrhea -
- Conference Call Scheduled for 8:30 a.m. Eastern Today -
GAITHERSBURG, Md., Aug. 1 /PRNewswire-FirstCall/ -- Iomai Corporation (Nasdaq: IOMI - News) today announced that its patch-based vaccine for enterotoxigenic E. coli (ETEC) bacteria conferred statistically significant protection from travelers' diarrhea as compared to placebo, particularly for more severe cases, according to interim data from a double-blind Phase 2 field study. Vaccinated travelers were 75 percent less likely to suffer moderate or severe diarrhea from any cause (p<0.01) and 84 percent less likely to be afflicted by severe diarrhea (p<0.05). No vaccine-related serious adverse events were reported.
In addition to the protective effects, investigators found that the frequency and duration of diarrhea in vaccinated subjects who did contract disease was significantly lower than in their unvaccinated peers.
"The results from this study strongly suggest that the use of Iomai's needle-free, patch-based vaccine can have a notable impact in reducing the chances of suffering from the ravages of travelers' diarrhea," said Herbert L. DuPont, M.D., professor and director of the Center for Infectious Diseases at The University of Texas School of Public Health and the principal investigator on the trial. "Right now, we are generally limited to administering antibiotics after the illness has begun. An effective, easy-to-use vaccine would be a vast improvement and have an immediate impact on travel medicine."
The Trek Phase 2 field study followed 170 subjects, 111 who received a placebo and 59 who received two doses of Iomai's patch-based vaccine before traveling to Mexico or Guatemala. These efficacy results were particularly notable as the trial was conducted largely to gather information on the logistics of conducting such a study in the field. The study was conducted in collaboration with researchers from the Johns Hopkins Bloomberg School of Public Health and the University of Texas School of Public Health.
Iomai's vaccine uses the company's novel transcutaneous immunization (TCI) technology, which allows the vaccine to be delivered to the immune system via a simple patch affixed to the skin. ETEC causes illness through the toxins it produces, including one known as heat-labile toxin or LT. Iomai's unique patch-based vaccine enables the safe administration of this potent immunogen into the skin to stimulate the immune response.
"These convincing results, demonstrating high levels of efficacy in a real-world setting, serve as validation of our TCI approach," said Dr. Gregory Glenn, M.D., Iomai's founder and chief scientific officer. "The data suggest that our TCI technology can be used to provide protection against other pathogens. We are moving as quickly as possible to complete our Phase 2 work so we can begin pivotal trials and bring our travelers' diarrhea vaccine candidate to market."
The study met its primary endpoints, which were designed to evaluate the safety of the vaccine and the incidence of ETEC. The secondary objectives included evaluation of vaccine-preventable outcomes, immunogenicity and stool frequency. The study also gathered data on the protective efficacy of the vaccine. Iomai plans to submit the full dataset from the trial for publication as soon as possible. The company intends to launch a Phase 3 program for the vaccine in 2008.
About Travelers' Diarrhea
This year, approximately 55 million international travelers will visit countries where bacteria that cause travelers' diarrhea are endemic, particularly Africa, Asia and Latin America, and about 20 million of those travelers will develop travelers' diarrhea. "Though we always caution travelers about the best ways to avoid food and drink that may harbor the bacteria, that advice is often not enough, and an effective means of vaccination could substantially reduce the burden of disease," said Robin McKenzie, M.D., an assistant professor at Johns Hopkins and an investigator on the trial.
ETEC, the most common cause of travelers' diarrhea, represents just under half of all cases of travelers' diarrhea for international travelers to areas where ETEC is common. A recently completed market study suggested that there is a large market for an effective ETEC vaccine, potentially exceeding $500 million annually. If approved, the Iomai vaccine would be the first vaccine for travelers' diarrhea available in the United States.
ETEC's impact goes beyond travelers. The World Health Organization estimates that children in the developing world suffer 210 million episodes of diarrhea caused by ETEC annually, causing 380,000 deaths each year.
Conference Call Details
To access the live conference call today at 8:30 a.m. Eastern Time via phone, please dial 866-510-0705 from the United States and Canada or 617-597- 5363 internationally. The conference ID is 26738905. Please dial in approximately ten minutes prior to the start of the call. A telephone replay will be available beginning approximately one hour after the call through Aug. 8 and may be accessed by dialing 888-286-8010 from the United States and Canada or 617-801-6888 internationally. The replay passcode is 85144095.
To access the live and subsequently archived webcast of the conference call, go to the Investor Relations section of the Company's website at www.iomai.com. Please connect to the web site at least 15 minutes prior to the call to allow for any software download that may be necessary. The webcast is also being distributed through the Thomson StreetEvents Network. Individual investors can listen to the call at www.earnings.com, Thomson's individual investor portal, powered by StreetEvents. Institutional investors can access the call via Thomson StreetEvents (www.streetevents.com), a password-protected event management site.
ABOUT IOMAI CORPORATION
Iomai Corporation discovers and develops vaccines and immune system stimulants, delivered via a novel, needle-free technology called transcutaneous immunization (TCI). TCI, discovered by researchers at the Walter Reed Army Institute of Research, taps into the unique benefits of a major group of antigen-presenting cells found in the outer layers of the skin (Langerhans cells) to generate an enhanced immune response. Iomai is leveraging TCI to enhance the efficacy of existing vaccines, develop new vaccines that are viable only through transcutaneous administration and expand the global vaccine market. Iomai currently has four product candidates in development: three targeting influenza and pandemic flu and one to prevent travelers' diarrhea. For more information on Iomai, please visit www.iomai.com.
Some matters discussed in this press release constitute "forward-looking statements" that involve known and unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements. Such forward-looking statements include statements about the timing and expectation for Iomai completing Phase 2 work on this product candidate, commencing Phase 3 studies, and bringing the product candidate to market; the potential size of the market for an ETEC vaccine; the applicability of TCI technology to other pathogens; and that, if approved, Iomai's vaccine would be the first available in the U.S. Applicable risks and uncertainties include, among others, that Iomai may not be able to enroll sufficient numbers of patients in future clinical trials; that future clinical trials may not replicate results seen in the trial described in this press release; that Iomai may be unable to obtain the regulatory approvals necessary to conduct additional clinical trials or to market any product for travelers' diarrhea; that estimates of market size overstate the number of travelers who would use such a product, if it were approved; that competitors may develop products that are safer, more effective, or more convenient to use; and the risks identified under the heading "Factors That May Impact Future Results" in Management's Discussion and Analysis of Financial Condition and Results of Operations in Iomai's Quarterly Report on Form 10-Q for the three months ended March 31, 2007, and filed with the Securities and Exchange Commission. While Iomai believes that this product candidate is amenable to self-administration, in the Phase 2 study described in this press release, medical professionals administered the vaccine. Whether any approved product would be self-administered would depend on many factors, including the outcome of any studies the Company conducts evaluating self-administration and the views of regulatory agencies. Iomai cautions investors and others not to place undue reliance on the forward-looking statements contained in this press release. Iomai's business is subject to many risks. For a discussion of such risks, you are encouraged to read the documents the Company files with the U.S. Securities and Exchange Commission, available at http://www.sec.gov. The statements in this press release speak only as of the date of this document, and Iomai undertakes no obligation to update or revise the statements.
--------------------------------------------------------------------------------
Source: Iomai Corporation
IOMI: UBS Securities Ups to Buy from Neutral; Keeps Tgt @ $2.7; Analyst Notes
Wednesday, August 01, 2007 09:12ET
Issuer: Iomai Corporation (NasdaqNM: IOMI)
Analyst Firm: UBS Securities Inc.
Ratings Action: UPGRADE
Current Rating: Buy (from Neutral)
Target Price Action: MAINTAIN
Target Price: $2.70
Analyst Comments: The firm based their recommendation on valuation
Wednesday, August 01, 2007 09:12ET
Issuer: Iomai Corporation (NasdaqNM: IOMI)
Analyst Firm: UBS Securities Inc.
Ratings Action: UPGRADE
Current Rating: Buy (from Neutral)
Target Price Action: MAINTAIN
Target Price: $2.70
Analyst Comments: The firm based their recommendation on valuation
Iomai Trial Shows 100 Percent Immune Response in Elderly Subjects Vaccinated With Novel Travelers' Diarrhea Patch Vaccine
Thursday August 9, 8:30 am ET
GAITHERSBURG, Md., Aug. 9 /PRNewswire-FirstCall/ -- Iomai Corporation (Nasdaq: IOMI - News) today announced that interim results from a Phase 1 safety and immunogenicity trial of its patch-based vaccine for travelers' diarrhea found that all subjects who received the vaccine -- 19 elderly and 17 adult participants -- mounted an immune response, demonstrating that even elderly subjects with weaker immune systems respond to the vaccine.
ADVERTISEMENT
The trial was designed to compare the number of elderly subjects (65 and older) who seroconverted -- developed antibodies to the vaccine's active ingredient -- with the number of adult participants (aged 18 to 40) who seroconverted. Because the immune system is often less powerful in older people, many vaccines do not work as well in the elderly. However, all subjects who received the Iomai patch, regardless of age, seroconverted with IgG antibodies. Moreover, 18 of 19 elderly subjects and 15 of 17 adult subjects also seroconverted with IgA antibodies. The study was conducted in Belfast, Ireland, and subjects received two patches, 21 days apart, containing "heat labile" toxin, or LT, and were followed for 42 days. No treatment- related serious adverse effects were reported.
"The results of this trial further confirm that we can effectively deliver the active ingredient in our patch-based vaccine for travelers' diarrhea to adults of all ages, and based on this data, the Iomai travelers' diarrhea vaccine could be expected to extend coverage to this important at-risk segment of the traveling population," said Gregory M. Glenn, M.D., senior vice president and chief scientific officer of Iomai. "These results, coupled with the remarkable findings from our field study that we announced last week, provide additional evidence of the significant potential for Iomai's transcutaneous immunization (TCI) approach."
Iomai last week announced that a Phase 2 field trial of the vaccine found that healthy adults who traveled to Guatemala and Mexico and were vaccinated with the Iomai patch were 75 percent less likely to suffer moderate or severe travelers' diarrhea. With the high seroconversion rates in the elderly, the interim results from this new study suggest that the protective effect of Iomai's travelers' diarrhea vaccine may apply to all adults, not just young adults. Iomai will look to confirm this in its Phase 3 trials for its travelers' diarrhea vaccine, which the company intends to launch in 2008.
The study was designed to evaluate the safety of the travelers' diarrhea vaccine patch in the elderly and it met this primary endpoint. The secondary objective included evaluation of immunogenicity of the vaccine in the elderly compared with young adults. Iomai plans to submit the study data from the trial for publication as soon as possible.
ABOUT TRAVELERS' DIARRHEA
This year, approximately 55 million international travelers will visit countries where bacteria that cause travelers' diarrhea are endemic, particularly Africa, Asia and Latin America, and about 20 million of those travelers will develop travelers' diarrhea.
ETEC, the most common cause of travelers' diarrhea, represents just under half of all cases of travelers' diarrhea for international travelers to areas where ETEC is common. A recently completed market study suggested that there is a large market for an effective ETEC vaccine, potentially exceeding $500 million annually. If approved, the Iomai vaccine would be the first vaccine for travelers' diarrhea available in the United States.
ETEC's impact goes beyond travelers. The World Health Organization estimates that children in the developing world suffer 210 million episodes of diarrhea caused by ETEC annually, causing 380,000 deaths each year.
ABOUT IOMAI CORPORATION
Iomai Corporation discovers and develops vaccines and immune system stimulants, delivered via a novel, needle-free technology called transcutaneous immunization (TCI). TCI, discovered by researchers at the Walter Reed Army Institute of Research, taps into the unique benefits of a major group of antigen-presenting cells found in the outer layers of the skin (Langerhans cells) to generate an enhanced immune response. Iomai is leveraging TCI to enhance the efficacy of existing vaccines, develop new vaccines that are viable only through transcutaneous administration and expand the global vaccine market. Iomai currently has four product candidates in development: three targeting influenza and pandemic flu and one to prevent travelers' diarrhea. For more information on Iomai, please visit www.iomai.com.
Some matters discussed in this press release constitute "forward-looking statements" that involve known and unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements. Such forward-looking statements include statements about the predictability of the data from this trial in preventing travelers' diarrhea in the elderly, timing and expectation for Iomai commencing Phase 3 studies for this product candidate, and bringing the product candidate to market; the potential size of the market for an ETEC vaccine; the applicability of TCI technology to other pathogens; and that, if approved, Iomai's vaccine would be the first available in the U.S. Applicable risks and uncertainties include, among others, that Iomai may not be able to enroll sufficient numbers of patients in future clinical trials; that future clinical trials may not replicate results seen in the trial described in this press release; that Iomai may be unable to obtain the regulatory approvals necessary to conduct additional clinical trials or to market any product for travelers' diarrhea; that estimates of market size overstate the number of travelers who would use such a product, if it were approved; that competitors may develop products that are safer, more effective, or more convenient to use; and the risks identified under the heading "Factors That May Impact Future Results" in Management's Discussion and Analysis of Financial Condition and Results of Operations in Iomai's Quarterly Report on Form 10-Q for the three months ended March 31, 2007, and filed with the Securities and Exchange Commission. While Iomai believes that this product candidate is amenable to self-administration, in the Phase 2 study described in this press release, medical professionals administered the vaccine. Whether any approved product would be self-administered would depend on many factors, including the outcome of any studies the Company conducts evaluating self-administration and the views of regulatory agencies. Iomai cautions investors and others not to place undue reliance on the forward-looking statements contained in this press release. Iomai's business is subject to many risks. For a discussion of such risks, you are encouraged to read the documents the Company files with the U.S. Securities and Exchange Commission, available at http://www.sec.gov.
The statements in this press release speak only as of the date of this document, and Iomai undertakes no obligation to update or revise the statements.
--------------------------------------------------------------------------------
Source: Iomai Corporation
Thursday August 9, 8:30 am ET
GAITHERSBURG, Md., Aug. 9 /PRNewswire-FirstCall/ -- Iomai Corporation (Nasdaq: IOMI - News) today announced that interim results from a Phase 1 safety and immunogenicity trial of its patch-based vaccine for travelers' diarrhea found that all subjects who received the vaccine -- 19 elderly and 17 adult participants -- mounted an immune response, demonstrating that even elderly subjects with weaker immune systems respond to the vaccine.
ADVERTISEMENT
The trial was designed to compare the number of elderly subjects (65 and older) who seroconverted -- developed antibodies to the vaccine's active ingredient -- with the number of adult participants (aged 18 to 40) who seroconverted. Because the immune system is often less powerful in older people, many vaccines do not work as well in the elderly. However, all subjects who received the Iomai patch, regardless of age, seroconverted with IgG antibodies. Moreover, 18 of 19 elderly subjects and 15 of 17 adult subjects also seroconverted with IgA antibodies. The study was conducted in Belfast, Ireland, and subjects received two patches, 21 days apart, containing "heat labile" toxin, or LT, and were followed for 42 days. No treatment- related serious adverse effects were reported.
"The results of this trial further confirm that we can effectively deliver the active ingredient in our patch-based vaccine for travelers' diarrhea to adults of all ages, and based on this data, the Iomai travelers' diarrhea vaccine could be expected to extend coverage to this important at-risk segment of the traveling population," said Gregory M. Glenn, M.D., senior vice president and chief scientific officer of Iomai. "These results, coupled with the remarkable findings from our field study that we announced last week, provide additional evidence of the significant potential for Iomai's transcutaneous immunization (TCI) approach."
Iomai last week announced that a Phase 2 field trial of the vaccine found that healthy adults who traveled to Guatemala and Mexico and were vaccinated with the Iomai patch were 75 percent less likely to suffer moderate or severe travelers' diarrhea. With the high seroconversion rates in the elderly, the interim results from this new study suggest that the protective effect of Iomai's travelers' diarrhea vaccine may apply to all adults, not just young adults. Iomai will look to confirm this in its Phase 3 trials for its travelers' diarrhea vaccine, which the company intends to launch in 2008.
The study was designed to evaluate the safety of the travelers' diarrhea vaccine patch in the elderly and it met this primary endpoint. The secondary objective included evaluation of immunogenicity of the vaccine in the elderly compared with young adults. Iomai plans to submit the study data from the trial for publication as soon as possible.
ABOUT TRAVELERS' DIARRHEA
This year, approximately 55 million international travelers will visit countries where bacteria that cause travelers' diarrhea are endemic, particularly Africa, Asia and Latin America, and about 20 million of those travelers will develop travelers' diarrhea.
ETEC, the most common cause of travelers' diarrhea, represents just under half of all cases of travelers' diarrhea for international travelers to areas where ETEC is common. A recently completed market study suggested that there is a large market for an effective ETEC vaccine, potentially exceeding $500 million annually. If approved, the Iomai vaccine would be the first vaccine for travelers' diarrhea available in the United States.
ETEC's impact goes beyond travelers. The World Health Organization estimates that children in the developing world suffer 210 million episodes of diarrhea caused by ETEC annually, causing 380,000 deaths each year.
ABOUT IOMAI CORPORATION
Iomai Corporation discovers and develops vaccines and immune system stimulants, delivered via a novel, needle-free technology called transcutaneous immunization (TCI). TCI, discovered by researchers at the Walter Reed Army Institute of Research, taps into the unique benefits of a major group of antigen-presenting cells found in the outer layers of the skin (Langerhans cells) to generate an enhanced immune response. Iomai is leveraging TCI to enhance the efficacy of existing vaccines, develop new vaccines that are viable only through transcutaneous administration and expand the global vaccine market. Iomai currently has four product candidates in development: three targeting influenza and pandemic flu and one to prevent travelers' diarrhea. For more information on Iomai, please visit www.iomai.com.
Some matters discussed in this press release constitute "forward-looking statements" that involve known and unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements. Such forward-looking statements include statements about the predictability of the data from this trial in preventing travelers' diarrhea in the elderly, timing and expectation for Iomai commencing Phase 3 studies for this product candidate, and bringing the product candidate to market; the potential size of the market for an ETEC vaccine; the applicability of TCI technology to other pathogens; and that, if approved, Iomai's vaccine would be the first available in the U.S. Applicable risks and uncertainties include, among others, that Iomai may not be able to enroll sufficient numbers of patients in future clinical trials; that future clinical trials may not replicate results seen in the trial described in this press release; that Iomai may be unable to obtain the regulatory approvals necessary to conduct additional clinical trials or to market any product for travelers' diarrhea; that estimates of market size overstate the number of travelers who would use such a product, if it were approved; that competitors may develop products that are safer, more effective, or more convenient to use; and the risks identified under the heading "Factors That May Impact Future Results" in Management's Discussion and Analysis of Financial Condition and Results of Operations in Iomai's Quarterly Report on Form 10-Q for the three months ended March 31, 2007, and filed with the Securities and Exchange Commission. While Iomai believes that this product candidate is amenable to self-administration, in the Phase 2 study described in this press release, medical professionals administered the vaccine. Whether any approved product would be self-administered would depend on many factors, including the outcome of any studies the Company conducts evaluating self-administration and the views of regulatory agencies. Iomai cautions investors and others not to place undue reliance on the forward-looking statements contained in this press release. Iomai's business is subject to many risks. For a discussion of such risks, you are encouraged to read the documents the Company files with the U.S. Securities and Exchange Commission, available at http://www.sec.gov.
The statements in this press release speak only as of the date of this document, and Iomai undertakes no obligation to update or revise the statements.
--------------------------------------------------------------------------------
Source: Iomai Corporation
insiderkauf!!!
2007-08-15
Purchase 2007-08-15
5:48 pm IOMAI CORP IOMI Glenn Gregory M
(Chief Scientific Officer) 10,000 $1.79 $17,900 81,738
(Direct)
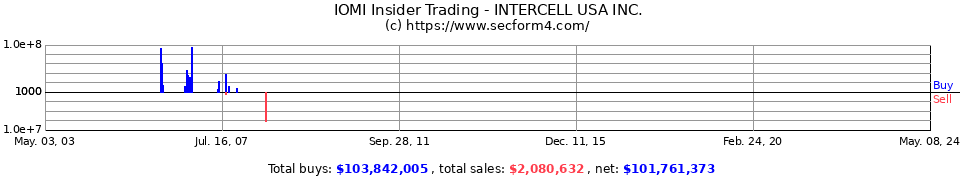
2007-08-15
Purchase 2007-08-15
5:48 pm IOMAI CORP IOMI Glenn Gregory M
(Chief Scientific Officer) 10,000 $1.79 $17,900 81,738
(Direct)

nochmals neue insiderkäufe!
Purchase 2007-08-20
4:39 pm IOMAI CORP IOMI Meyer F Weller
(Director) 36,200 $1.746 $63,189 40,900
2007-08-16
Purchase 2007-08-20
4:38 pm IOMAI CORP IOMI Glenn Gregory M
(Chief Scientific Officer) 2,000 $1.73 $3,460 83,738
(Direct)
2007-08-15
Purchase 2007-08-15
5:48 pm IOMAI CORP IOMI Glenn Gregory M
(Chief Scientific Officer) 10,000 $1.79 $17,900 81,738
Purchase 2007-08-20
4:39 pm IOMAI CORP IOMI Meyer F Weller
(Director) 36,200 $1.746 $63,189 40,900
2007-08-16
Purchase 2007-08-20
4:38 pm IOMAI CORP IOMI Glenn Gregory M
(Chief Scientific Officer) 2,000 $1.73 $3,460 83,738
(Direct)
2007-08-15
Purchase 2007-08-15
5:48 pm IOMAI CORP IOMI Glenn Gregory M
(Chief Scientific Officer) 10,000 $1.79 $17,900 81,738
kursziel- 4$
Gap schließen
Gap schließen
Iomai Announces Data from Phase 2 Field Study of Travelers' Diarrhea Vaccine Accepted for Presentation at ICAAC
Thursday August 23, 8:30 am ET
GAITHERSBURG, Md., Aug. 23 /PRNewswire-FirstCall/ -- Iomai Corporation (Nasdaq: IOMI - News) today announced that the abstract detailing efficacy and safety results from its double-blind Phase 2 field study of a patch-based vaccine for travelers' diarrhea has been accepted as a "late breaker" presentation at Interscience Conference on Antimicrobials and Chemotherapy (ICAAC), to be held in Chicago Sept. 17 to 20.
The abstract, "Transcutaneous Immunization with the Heat Labile Toxin (LT) of Enterotoxigenic Escherichia coli (ETEC) Protects in a Phase 2 Field Trial in Travelers to Guatemala and Mexico," will be presented by Gregory Glenn, M.D., Iomai's chief scientific officer, on Tuesday, Sept. 18, during the session on "Vaccines and Pediatric Infections." The session will be held from 1:30 to 4 p.m.
About Travelers' Diarrhea
This year, approximately 55 million international travelers will visit countries where bacteria that cause travelers' diarrhea are endemic, particularly Africa, Asia and Latin America, and about 20 million of those travelers will develop travelers' diarrhea.
Travelers' diarrhea (TD) is caused by ingestion of pathogens in contaminated food and water. Of the 54 million travelers to endemic areas, an estimated 17 million will contract TD, making it one of the most common illnesses in travelers. Symptoms last for 3 to 5 days, and 55 percent of subjects who become ill have more than six stools daily, with an average of 18 total stools per episode. TD is often accompanied by nausea, vomiting and cramping and can result in severe dehydration, mimicking the symptoms of cholera. Although generally self-limited, TD is often accompanied by prostration and can require hospitalization, often in settings where health care is inadequate. Additionally, 10 to 30 percent of travelers who contract diarrhea also develop a more chronic condition, irritable bowel syndrome.
About Iomai Corporation
Iomai Corporation discovers and develops vaccines and immune system stimulants, delivered via a novel, needle-free technology called transcutaneous immunization (TCI). TCI taps into the unique benefits of a major group of antigen-presenting cells found in the outer layers of the skin (Langerhans cells) to generate an enhanced immune response. Iomai is leveraging TCI to enhance the efficacy of existing vaccines, develop new vaccines that are viable only through transcutaneous administration and expand the global vaccine market. Iomai currently has four product candidates in development: three targeting influenza and pandemic flu and one to prevent travelers' diarrhea. For more information on Iomai, please visit http://www.iomai.com.
--------------------------------------------------------------------------------
Source: Iomai Corporation
Thursday August 23, 8:30 am ET
GAITHERSBURG, Md., Aug. 23 /PRNewswire-FirstCall/ -- Iomai Corporation (Nasdaq: IOMI - News) today announced that the abstract detailing efficacy and safety results from its double-blind Phase 2 field study of a patch-based vaccine for travelers' diarrhea has been accepted as a "late breaker" presentation at Interscience Conference on Antimicrobials and Chemotherapy (ICAAC), to be held in Chicago Sept. 17 to 20.
The abstract, "Transcutaneous Immunization with the Heat Labile Toxin (LT) of Enterotoxigenic Escherichia coli (ETEC) Protects in a Phase 2 Field Trial in Travelers to Guatemala and Mexico," will be presented by Gregory Glenn, M.D., Iomai's chief scientific officer, on Tuesday, Sept. 18, during the session on "Vaccines and Pediatric Infections." The session will be held from 1:30 to 4 p.m.
About Travelers' Diarrhea
This year, approximately 55 million international travelers will visit countries where bacteria that cause travelers' diarrhea are endemic, particularly Africa, Asia and Latin America, and about 20 million of those travelers will develop travelers' diarrhea.
Travelers' diarrhea (TD) is caused by ingestion of pathogens in contaminated food and water. Of the 54 million travelers to endemic areas, an estimated 17 million will contract TD, making it one of the most common illnesses in travelers. Symptoms last for 3 to 5 days, and 55 percent of subjects who become ill have more than six stools daily, with an average of 18 total stools per episode. TD is often accompanied by nausea, vomiting and cramping and can result in severe dehydration, mimicking the symptoms of cholera. Although generally self-limited, TD is often accompanied by prostration and can require hospitalization, often in settings where health care is inadequate. Additionally, 10 to 30 percent of travelers who contract diarrhea also develop a more chronic condition, irritable bowel syndrome.
About Iomai Corporation
Iomai Corporation discovers and develops vaccines and immune system stimulants, delivered via a novel, needle-free technology called transcutaneous immunization (TCI). TCI taps into the unique benefits of a major group of antigen-presenting cells found in the outer layers of the skin (Langerhans cells) to generate an enhanced immune response. Iomai is leveraging TCI to enhance the efficacy of existing vaccines, develop new vaccines that are viable only through transcutaneous administration and expand the global vaccine market. Iomai currently has four product candidates in development: three targeting influenza and pandemic flu and one to prevent travelers' diarrhea. For more information on Iomai, please visit http://www.iomai.com.
--------------------------------------------------------------------------------
Source: Iomai Corporation
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| -99,00 | |
| -0,30 | |
| +2,46 | |
| -0,17 | |
| +0,78 | |
| -0,81 | |
| +0,88 | |
| -3,30 | |
| -0,51 | |
| +0,54 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 237 | ||
| 110 | ||
| 100 | ||
| 65 | ||
| 55 | ||
| 39 | ||
| 34 | ||
| 32 | ||
| 29 | ||
| 25 |





















