PCI Biotech - spin-off von Photocure - 500 Beiträge pro Seite
eröffnet am 17.09.10 11:18:25 von
neuester Beitrag 24.03.21 10:38:39 von
neuester Beitrag 24.03.21 10:38:39 von
Beiträge: 18
ID: 1.159.973
ID: 1.159.973
Aufrufe heute: 0
Gesamt: 2.731
Gesamt: 2.731
Aktive User: 0
ISIN: NO0010405640 · WKN: A0Q2FS · Symbol: 4QG
0,1124
EUR
+1,26 %
+0,0014 EUR
Letzter Kurs 03.05.24 Frankfurt
Neuigkeiten
03.05.24 · globenewswire |
26.04.24 · globenewswire |
14.02.24 · globenewswire |
12.02.24 · globenewswire |
13.10.23 · globenewswire |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 38,27 | +28,16 | |
| 8,0780 | +27,90 | |
| 10,155 | +21,04 | |
| 8,1400 | +20,41 | |
| 3,6100 | +17,51 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,6700 | -26,58 | |
| 2,1300 | -34,41 | |
| 3,1600 | -38,64 | |
| 1,7000 | -49,40 | |
| 125,00 | -95,83 |
Following highly promising head and neck results, light mediated therapy development pipeline to be extended to other cancer indications
The Oslo-Axess-listed Norwegian biopharmaceutical company PCI Biotech Holding ASA ("PCI Biotech") today announced that it has raised NOK90 million through a new rights issue. The company received subscriptions for a total of approximately 3.36 million new shares. The initial offering was and remains 2,250,000 new shares, and the rights issue was thus oversubscribed by approximately 50 percent.
CEO Per Walday commented: "We are very satisfied with the share issue being oversubscribed. The capital increase of NOK 90 millions will enable us to complete the planned clinical development for 3 to 4 selected cancer indications. When you take into account this strengthened cash position and the very promising results achieved so far in the ongoing Phase I/II study at University College Hospital in London, we are now well positioned for the further development of both our pipeline and PCI Biotech as a company",
The final allocation of the shares offered in the rights issue is expected to be resolved by the Board of Directors of PCI Biotech on or around June 15, 2010 in accordance with the allocation criteria set out in the prospectus dated May 25, 2010. The final result of the rights issue is expected to be published on or around June 16, 2010
The Oslo-Axess-listed Norwegian biopharmaceutical company PCI Biotech Holding ASA ("PCI Biotech") today announced that it has raised NOK90 million through a new rights issue. The company received subscriptions for a total of approximately 3.36 million new shares. The initial offering was and remains 2,250,000 new shares, and the rights issue was thus oversubscribed by approximately 50 percent.
CEO Per Walday commented: "We are very satisfied with the share issue being oversubscribed. The capital increase of NOK 90 millions will enable us to complete the planned clinical development for 3 to 4 selected cancer indications. When you take into account this strengthened cash position and the very promising results achieved so far in the ongoing Phase I/II study at University College Hospital in London, we are now well positioned for the further development of both our pipeline and PCI Biotech as a company",
The final allocation of the shares offered in the rights issue is expected to be resolved by the Board of Directors of PCI Biotech on or around June 15, 2010 in accordance with the allocation criteria set out in the prospectus dated May 25, 2010. The final result of the rights issue is expected to be published on or around June 16, 2010
Oct 26 (Reuters) - 3 months to Sept. 30 2010:
PCI Biotech ASA
(Millions of Norwegian crowns unless otherwise stated)
Latest Year
Ago
Net Revenue 5.6 2.8
EBIT 1.0 -4.1
Pretax profit 1.9 -3.3
Net profit 1.9 -3.3
PCI Biotech ASA
(Millions of Norwegian crowns unless otherwise stated)
Latest Year
Ago
Net Revenue 5.6 2.8
EBIT 1.0 -4.1
Pretax profit 1.9 -3.3
Net profit 1.9 -3.3
Gesamtverlust 2010: 14 Mio. NOK
Kursentwicklung unfaßbar gut.
Kursentwicklung unfaßbar gut.
Last patient included in the Phase I/II study of PC-A11
Oslo, 23 February 2011 - PCI Biotech (PCIB) the Norwegian biopharmaceutical company, reported today that it has completed the inclusion of patients in its phase I/II study of its lead candidate PC-A11 in cancer patients.
The last patient has been treated with the company’s proprietary photosensitiser Amphinex® used in combination with the cytotoxic agent bleomycin at University College Hospital (UCH) in London. Principal Investigator, Colin Hopper said: “We at UCH are proud of being the first in the world to use the PCI technology in the treatment of cancer patients. The results have been very positive, with strong tumour response in all the treated patients, and we look forward to take part in the further development of PC-A11 in the treatment of Head and Neck cancer patients.”
Per Walday, CEO of PCI Biotech said: “We are very happy to have completed the inclusion of patients in the PC-A11 phase I/II study. This study has proved that we can transfer strong preclinical results to equally strong clinical results, and this is promising for the further development of PC-A11 and other combination products based on the PCI technology platform.”
A total of 19 cancer patients have been treated in the phase I/II study of PC-A11. Reference is made to PCI Biotech’s Q4 2010 report for a summary of the results from the first 14 patients. Complete results from the last 5 patients will be published as soon as all these patients have finished the 3 months follow up.
Oslo, 23 February 2011 - PCI Biotech (PCIB) the Norwegian biopharmaceutical company, reported today that it has completed the inclusion of patients in its phase I/II study of its lead candidate PC-A11 in cancer patients.
The last patient has been treated with the company’s proprietary photosensitiser Amphinex® used in combination with the cytotoxic agent bleomycin at University College Hospital (UCH) in London. Principal Investigator, Colin Hopper said: “We at UCH are proud of being the first in the world to use the PCI technology in the treatment of cancer patients. The results have been very positive, with strong tumour response in all the treated patients, and we look forward to take part in the further development of PC-A11 in the treatment of Head and Neck cancer patients.”
Per Walday, CEO of PCI Biotech said: “We are very happy to have completed the inclusion of patients in the PC-A11 phase I/II study. This study has proved that we can transfer strong preclinical results to equally strong clinical results, and this is promising for the further development of PC-A11 and other combination products based on the PCI technology platform.”
A total of 19 cancer patients have been treated in the phase I/II study of PC-A11. Reference is made to PCI Biotech’s Q4 2010 report for a summary of the results from the first 14 patients. Complete results from the last 5 patients will be published as soon as all these patients have finished the 3 months follow up.
Antwort auf Beitrag Nr.: 41.388.930 von R-BgO am 19.04.11 13:57:43nun wieder gut halbiert...
aus dem Q2-Bericht:
At current cost levels the company is financed into the second quarter 2015. The board of directors
have therefore decided to initiate work on evaluating the company’s capital need and financing
alternatives. DNB Markets and Fondsfinans have been retained as financial advisors in connection
with this evaluation.
At current cost levels the company is financed into the second quarter 2015. The board of directors
have therefore decided to initiate work on evaluating the company’s capital need and financing
alternatives. DNB Markets and Fondsfinans have been retained as financial advisors in connection
with this evaluation.
Antwort auf Beitrag Nr.: 48.197.404 von R-BgO am 02.11.14 12:53:37
soll jetzt wieder für 2 Jahre reichen
in Q1 gab's ne KE
...die zur Verdoppelung der Aktienzahl geführt hat;soll jetzt wieder für 2 Jahre reichen
Antwort auf Beitrag Nr.: 50.393.166 von R-BgO am 13.08.15 12:27:52Oslo, 22 November 2016 – Please find enclosed the financial report for third quarter 2016 for PCI Biotech Holding ASA.
Highlights
fimaCHEM
* Completed Phase I study in bile duct cancer, with early promising results confirmed at central expert review
* Oral presentation of the Phase I results as late-breaking news at United European Gastro Week 2016
* Granted Orphan Drug Designation of fimaporfin for treatment of bile duct cancer in EU
* Lancet Oncology publication of the fimaporfin (Amphinex) first-in-man Phase I study, with independent expert commentary
fimaVACC
* Initiated clinical validation of the vaccination technology – a major development milestone
fimaNAc
* Research collaboration agreement with BioNTech signed
Financials
The Company proposes to carry out a fully underwritten rights issue of NOK 70 million at a subscription price of NOK 7 per share
CEO, Per Walday, comments:
“We have reached several important milestones for the fimaChem program in 2016. On the back of this progress, we were very pleased to recently announce a fully underwritten rights issue of NOK 70 million. The funding will enable the company to complete the initiated interactions with regulatory authorities to determine the best approach to marketing approval.
In addition, the rights issue will fully fund translation of our fimaVacc technology into man. Improving immunogenicity of vaccine candidates is a main priority in the immunotherapy industry and we believe that the fimaVacc technology may play an important part in solving this challenge. We are also facing increased interest in our fimaNAc technology and we look forward to explore synergies with BioNTech’s pioneering disruptive technologies.“
Highlights
fimaCHEM
* Completed Phase I study in bile duct cancer, with early promising results confirmed at central expert review
* Oral presentation of the Phase I results as late-breaking news at United European Gastro Week 2016
* Granted Orphan Drug Designation of fimaporfin for treatment of bile duct cancer in EU
* Lancet Oncology publication of the fimaporfin (Amphinex) first-in-man Phase I study, with independent expert commentary
fimaVACC
* Initiated clinical validation of the vaccination technology – a major development milestone
fimaNAc
* Research collaboration agreement with BioNTech signed
Financials
The Company proposes to carry out a fully underwritten rights issue of NOK 70 million at a subscription price of NOK 7 per share
CEO, Per Walday, comments:
“We have reached several important milestones for the fimaChem program in 2016. On the back of this progress, we were very pleased to recently announce a fully underwritten rights issue of NOK 70 million. The funding will enable the company to complete the initiated interactions with regulatory authorities to determine the best approach to marketing approval.
In addition, the rights issue will fully fund translation of our fimaVacc technology into man. Improving immunogenicity of vaccine candidates is a main priority in the immunotherapy industry and we believe that the fimaVacc technology may play an important part in solving this challenge. We are also facing increased interest in our fimaNAc technology and we look forward to explore synergies with BioNTech’s pioneering disruptive technologies.“
Antwort auf Beitrag Nr.: 53.931.737 von R-BgO am 21.12.16 13:03:31
aktuelle in-depth-Präsentation:
http://pcibiotech.no/wp-content/uploads/2014/01/PCI-Biotech-…
Antwort auf Beitrag Nr.: 53.931.803 von R-BgO am 21.12.16 13:12:53PCI BIOTECH AWARDED NOK 13.8 MILLION FROM THE RESEARCH COUNCIL OF NORWAY TO FURTHER DEVELOPMENT OF PCI FOR USE IN VACCINATION
Oslo, Norway, January 27, 2017
PCI Biotech (OSE:PCIB), a clinical-stage company developing innovative therapeutics that address significant unmet medical needs, today announced that it has been awarded NOK 13.8 million in a BIA grant from The Research Council of Norway to the project "Photochemical vaccination - novel immunotherapy concept for treatment of cancer and infectious diseases".
The main goal of the project is to document in a proof-of-principle clinical study in cancer patients that PCI Biotech's photochemical internalization (PCI) technology can be used to improve the efficacy of a therapeutic cancer vaccine. Other important aspects of the project is to develop the PCI technology for use in vaccination against certain types of viral and bacterial infections, and to explore the technology for use with mRNA-based vaccination.
'This grant supports further development of the promising fimaVacc technology, as well as the important vaccination application of the fimaNAc technology. Both of these applications are well suited for the development of new types of immunotherapy against cancer, and also for the prevention and treatment of some types of infectious diseases, including certain types of chronic virus infections. We are very pleased to see that the expert evaluators and the Research Council share our view on the potential of these technologies.' says CEO in PCI Biotech, Per Walday.
The project will be initiated in Q3 2017 and run for three and a half years. The grant will cover up to 35% of the project costs and the project will be implemented in the company's current plans. The grant is subject to final contract negotiations.
Established in 2006, the BIA programme is the largest industry-oriented programme at the Research council of Norway (Forskningsrådet). This broad-based programme supports high-quality R&D projects with good business and socio-economic potential.
Oslo, Norway, January 27, 2017
PCI Biotech (OSE:PCIB), a clinical-stage company developing innovative therapeutics that address significant unmet medical needs, today announced that it has been awarded NOK 13.8 million in a BIA grant from The Research Council of Norway to the project "Photochemical vaccination - novel immunotherapy concept for treatment of cancer and infectious diseases".
The main goal of the project is to document in a proof-of-principle clinical study in cancer patients that PCI Biotech's photochemical internalization (PCI) technology can be used to improve the efficacy of a therapeutic cancer vaccine. Other important aspects of the project is to develop the PCI technology for use in vaccination against certain types of viral and bacterial infections, and to explore the technology for use with mRNA-based vaccination.
'This grant supports further development of the promising fimaVacc technology, as well as the important vaccination application of the fimaNAc technology. Both of these applications are well suited for the development of new types of immunotherapy against cancer, and also for the prevention and treatment of some types of infectious diseases, including certain types of chronic virus infections. We are very pleased to see that the expert evaluators and the Research Council share our view on the potential of these technologies.' says CEO in PCI Biotech, Per Walday.
The project will be initiated in Q3 2017 and run for three and a half years. The grant will cover up to 35% of the project costs and the project will be implemented in the company's current plans. The grant is subject to final contract negotiations.
Established in 2006, the BIA programme is the largest industry-oriented programme at the Research council of Norway (Forskningsrådet). This broad-based programme supports high-quality R&D projects with good business and socio-economic potential.
Antwort auf Beitrag Nr.: 53.931.737 von R-BgO am 21.12.16 13:03:31
WOW!
von 7 auf 37 NOK in 3 Monaten...
Niveau gehalten und ausgebaut; irgendwas weiß der Kurs, was ich nicht sehe...
Antwort auf Beitrag Nr.: 57.179.417 von R-BgO am 03.03.18 23:39:09
inzwischen auch Handel in Dtld.
und zurück, marsch, marsch...
KE in Q4;inzwischen auch Handel in Dtld.
bei 28 NOK stabilisiert;
fürs Erste haben sie genug cash, um in Ruhe weiterzumachen
fürs Erste haben sie genug cash, um in Ruhe weiterzumachen
Ein bisschen Leben in den Thread zurückbringen ?
PhotoCure Aktie....WKN: 931150..... ISIN: NO0010000045
Die ersten einhundert Prozent sind eingefahren.
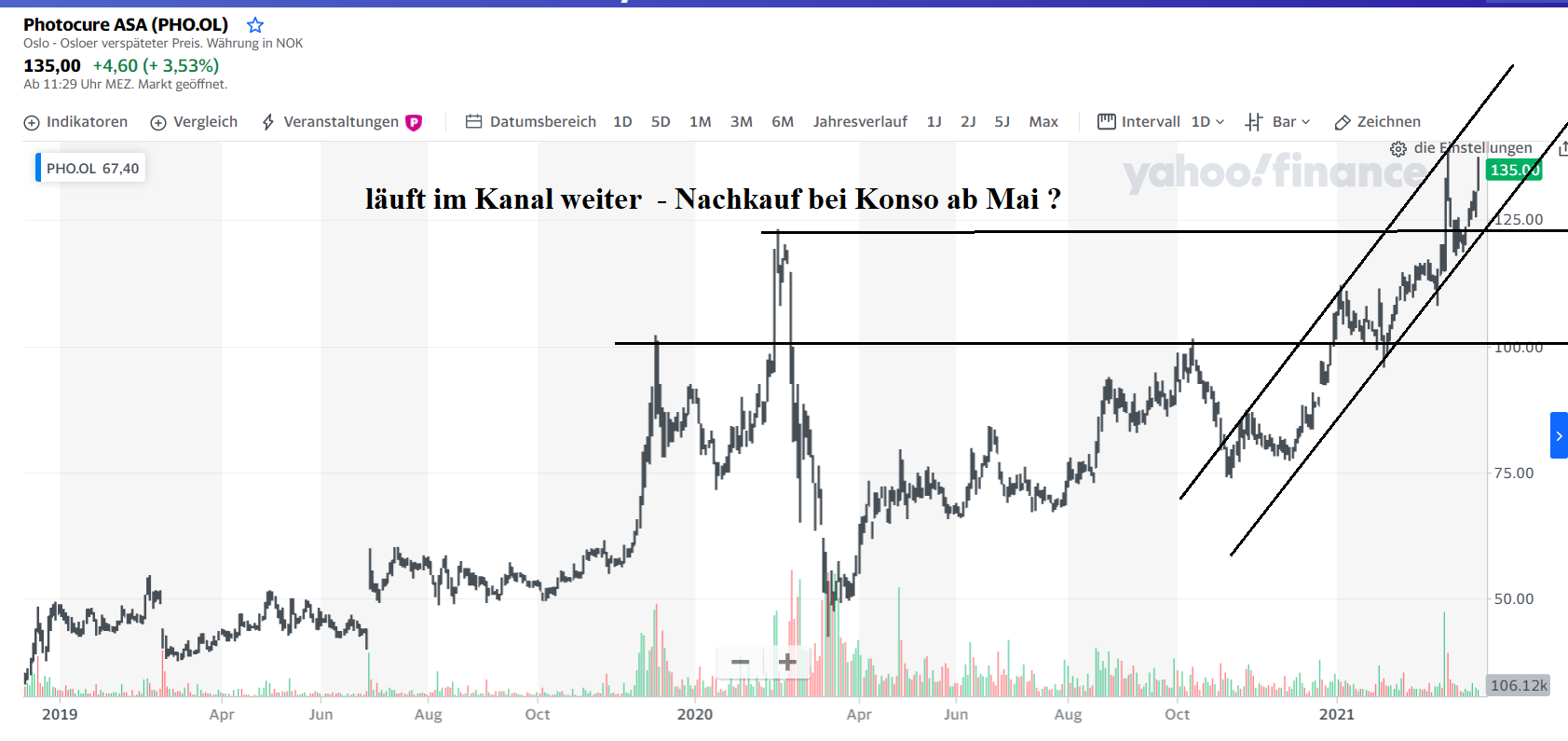
Ergebnisse für das vierte Quartal 2020
Oslo, Norwegen, 3. März 2021: Photocure ASA (OSE: PHO) meldet einen Hexvix ® / Cysview ® -Umsatz von 97,7 Mio. NOK im vierten Quartal 2020 (4. Quartal 2019: 58,8 NOK). Die Produktumsätze stiegen im Geschäftsjahr 2020 um 20%, was einer starken Ausführung in einem beispiellosen Jahr entspricht. Aufgrund der anhaltenden Pandemie setzt das Unternehmen die Prognose für seine Umsatzziele für 2023 im Bereich von 1 Mrd. NOK aus. Photocure plant, die Finanzrichtlinien erneut herauszugeben, sobald die Auswirkungen von Covid-19 erheblich geringer sind und sich die Arztpraxen und der Patientenfluss sowohl in den USA als auch in Europa normalisiert haben.
PhotoCure Aktie....WKN: 931150..... ISIN: NO0010000045
Die ersten einhundert Prozent sind eingefahren.

Ergebnisse für das vierte Quartal 2020
Oslo, Norwegen, 3. März 2021: Photocure ASA (OSE: PHO) meldet einen Hexvix ® / Cysview ® -Umsatz von 97,7 Mio. NOK im vierten Quartal 2020 (4. Quartal 2019: 58,8 NOK). Die Produktumsätze stiegen im Geschäftsjahr 2020 um 20%, was einer starken Ausführung in einem beispiellosen Jahr entspricht. Aufgrund der anhaltenden Pandemie setzt das Unternehmen die Prognose für seine Umsatzziele für 2023 im Bereich von 1 Mrd. NOK aus. Photocure plant, die Finanzrichtlinien erneut herauszugeben, sobald die Auswirkungen von Covid-19 erheblich geringer sind und sich die Arztpraxen und der Patientenfluss sowohl in den USA als auch in Europa normalisiert haben.
Antwort auf Beitrag Nr.: 67.566.345 von Rastelly am 23.03.21 13:43:59Nachtrag:
https://photocure.com/news/photocure-asa-results-for-the-fou…
Die Zukunft schreitet fort: https://photocure.com/news/a-new-study-shows-the-impact-on-r…
Gruss RS
https://photocure.com/news/photocure-asa-results-for-the-fou…
Die Zukunft schreitet fort: https://photocure.com/news/a-new-study-shows-the-impact-on-r…
Gruss RS
Gibt es einen unmittelbaren Zusammenhang zwischen PCI und Photocure?
Sorry sehe gerade die Überschrift des Forums, keine weiteren Fragen
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| -1,86 | |
| +1,66 | |
| +0,60 | |
| +1,69 | |
| +10,22 | |
| -2,81 | |
| 0,00 | |
| 0,00 | |
| -2,81 | |
| -3,03 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 108 | ||
| 77 | ||
| 74 | ||
| 65 | ||
| 45 | ||
| 34 | ||
| 30 | ||
| 26 | ||
| 23 | ||
| 22 |





















