OpGen - Spezialist gegen antibiotikaresistente Infektionen (Seite 23)
eröffnet am 13.08.15 09:48:54 von
neuester Beitrag 10.11.23 14:21:39 von
neuester Beitrag 10.11.23 14:21:39 von
Beiträge: 402
ID: 1.217.076
ID: 1.217.076
Aufrufe heute: 0
Gesamt: 36.933
Gesamt: 36.933
Aktive User: 0
ISIN: US68373L4068 · WKN: A3D38S · Symbol: 650
0,4620
EUR
0,00 %
0,0000 EUR
Letzter Kurs 26.04.24 Stuttgart
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,9000 | +59,66 | |
| 0,5922 | +44,44 | |
| 6,8660 | +44,27 | |
| 11,690 | +40,84 | |
| 0,7000 | +36,69 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,6900 | -14,50 | |
| 2,8150 | -15,21 | |
| 7,6100 | -17,20 | |
| 1,6100 | -18,27 | |
| 2,1200 | -21,77 |
Beitrag zu dieser Diskussion schreiben
OpGen CEO and Chairman of the Board Issue Letters to Stockholders
https://www.globenewswire.com/news-release/2020/05/11/203098…

https://www.globenewswire.com/news-release/2020/05/11/203098…
First Quarter 2020 Financial Results of OpGen, Inc. Stand-alone
Total revenue for the first quarter of 2020 was approximately $617,000 down from $1.0 million in the first quarter of 2019. This decrease can be attributed to Acuitas AMR Gene Panel and Acuitas® Lighthouse revenue, which was approximately $254,000, while revenues from the company’s rapid FISH products decreased to $363,000. Curetis and Ares revenue was approximately $900,000 for the first quarter generating a pro forma combined unaudited revenue of over $1.5 million.
Operating expenses for the first quarter of 2020 were $4.6 million, compared with $4.8 million in the first quarter of 2019.
The net loss for the first quarter of 2020 was $3.9 million or $0.53 per share, compared with $3.9 million or $8.25 per share in the first quarter of 2019.
http://ir.opgen.com/news-releases/news-release-details/opgen…
The Company also provided the following business updates that were achieved during the first quarter of 2020:
Successful closure of the business combination between OpGen and Curetis on April 1, 2020. At the closing, William E. Rhodes III, the former chairman of the Supervisory Board of Curetis N.V., was appointed chairman of the board of OpGen, and Oliver Schacht, Ph.D., the former Chief Executive Officer of Curetis N.V., was appointed the President and Chief Executive Officer of OpGen and to the board of directors;
The newly formed board of directors of OpGen now also includes Evan Jones, former Chairman and CEO of OpGen, Don Elsey, Mario Crovetto, and Prabhavathi Fernandes, PhD;
Announced the start of an investigator initiated collaboration with Karolinska Institutet, Sweden, to identify bacterial co-infections in patients admitted to the ICU for COVID-19 pneumonia using the Unyvero HPN panel;
OpGen significantly improved its working capital position in the first quarter of 2020 through $5.8 million of sales under the company’s ATM program and $8.1 million in proceeds from the exercise of warrants issued in the company’s public offering in October 2019;
Clinical trials were initiated during the first quarter of 2020 at nine participating sites for the Acuitas AMR Gene Panel (Urine) test. Testing and the trial have been suspended due to hospital actions to focus resources on the COVID-19 pandemic;
Acuitas Lighthouse® was utilized in a research study conducted by the Mayo Clinic to predict phenotypic resistance and antimicrobial susceptibility among clinical isolates, with findings published in Diagnostic Microbiology & Infectious Disease; and
Curetis, Ares Genetics, and BGI announced a partnership around BGI’s CoV-2 test kit commercialization in Europe; Curetis has begun selling the BGI CoV-2 product via its distribution network in EMEA during Q1 2020 and to date has sold over 15,000 tests into its distribution network.
Additional key business updates and strategic milestones include:
OpGen’ expects that its submission to the U.S. Food and Drug Administration (“FDA”) for clearance of the Acuitas® AMR Gene Panel (Isolates) for the detection of antimicrobial resistance genes in bacterial isolates is nearing completion. OpGen has responded, and is continuing to respond, to the FDA’s additional information requests and now anticipates approaching a clearance decision for the Acuitas® AMR Gene Panel for isolates. Exact timing is unknown as a result of the COVID-19 pandemic;
OpGen successfully achieved the last of the first year milestones in the groundbreaking collaboration with the New York State Department of Health and ILÚM Health Solutions, LLC, to develop a state-of-the-art research program to detect, track, and manage antimicrobial-resistant infections at healthcare institutions statewide. In response to the COVID-19 emergency in New York State, testing under the program has been put on hold by the Wadsworth Center and participating hospitals; however, the parties are currently engaged in active negotiations and discussions for a second year of the collaboration and expect to resume the project and expand it into its second year once the COVID-19 situation permits;
OpGen’s subsidiary Ares Genetics in April announced that a study on the feasibility and potential of antibiotic susceptibility testing and bacterial pathogen identification using next-generation sequencing (NGS) has been pre-published in the Journal of Clinical Microbiology. The study was performed by Ares Genetics GmbH and Curetis GmbH, and scientists at the Max Perutz Labs (Austria), a joint venture of the University of Vienna and the Medical University of Vienna, and the Mayo Clinic (Rochester, MN, U.S.A.); and
Curetis and Quaphaco entered into an exclusive three-year distribution partnership for the Unyvero product line in Vietnam; the contract includes minimum commitments by Quaphaco totaling approximately $ 2.1 million over the initial three-year term.
Mr. Schacht continued, “We have a strong pipeline heading into the second quarter of 2020 despite the unique market challenges presented at this time. With the anticipated near term FDA clearance decision and subsequent launch of our Acuitas AMR Gene Panel product for isolate and the exciting combination of the Acuitas Lighthouse® database with the ARESdb, our strong, proprietary product pipeline attests to our ability to deliver premier AI-powered bioinformatics solutions and diagnostic products. We are especially excited about an upcoming major milestone in the Ares collaboration with its strategic R&D collaboration partner, a leading global IVD corporation, where completion of the R&D phase is expected for the third quarter this year. The IVD corporate partner has pre-funded an option which upon exercise would then grant it 90 days of exclusive negotiations with Ares regarding a potential future partnering and licensing deal.”
Total revenue for the first quarter of 2020 was approximately $617,000 down from $1.0 million in the first quarter of 2019. This decrease can be attributed to Acuitas AMR Gene Panel and Acuitas® Lighthouse revenue, which was approximately $254,000, while revenues from the company’s rapid FISH products decreased to $363,000. Curetis and Ares revenue was approximately $900,000 for the first quarter generating a pro forma combined unaudited revenue of over $1.5 million.
Operating expenses for the first quarter of 2020 were $4.6 million, compared with $4.8 million in the first quarter of 2019.
The net loss for the first quarter of 2020 was $3.9 million or $0.53 per share, compared with $3.9 million or $8.25 per share in the first quarter of 2019.
http://ir.opgen.com/news-releases/news-release-details/opgen…
The Company also provided the following business updates that were achieved during the first quarter of 2020:
Successful closure of the business combination between OpGen and Curetis on April 1, 2020. At the closing, William E. Rhodes III, the former chairman of the Supervisory Board of Curetis N.V., was appointed chairman of the board of OpGen, and Oliver Schacht, Ph.D., the former Chief Executive Officer of Curetis N.V., was appointed the President and Chief Executive Officer of OpGen and to the board of directors;
The newly formed board of directors of OpGen now also includes Evan Jones, former Chairman and CEO of OpGen, Don Elsey, Mario Crovetto, and Prabhavathi Fernandes, PhD;
Announced the start of an investigator initiated collaboration with Karolinska Institutet, Sweden, to identify bacterial co-infections in patients admitted to the ICU for COVID-19 pneumonia using the Unyvero HPN panel;
OpGen significantly improved its working capital position in the first quarter of 2020 through $5.8 million of sales under the company’s ATM program and $8.1 million in proceeds from the exercise of warrants issued in the company’s public offering in October 2019;
Clinical trials were initiated during the first quarter of 2020 at nine participating sites for the Acuitas AMR Gene Panel (Urine) test. Testing and the trial have been suspended due to hospital actions to focus resources on the COVID-19 pandemic;
Acuitas Lighthouse® was utilized in a research study conducted by the Mayo Clinic to predict phenotypic resistance and antimicrobial susceptibility among clinical isolates, with findings published in Diagnostic Microbiology & Infectious Disease; and
Curetis, Ares Genetics, and BGI announced a partnership around BGI’s CoV-2 test kit commercialization in Europe; Curetis has begun selling the BGI CoV-2 product via its distribution network in EMEA during Q1 2020 and to date has sold over 15,000 tests into its distribution network.
Additional key business updates and strategic milestones include:
OpGen’ expects that its submission to the U.S. Food and Drug Administration (“FDA”) for clearance of the Acuitas® AMR Gene Panel (Isolates) for the detection of antimicrobial resistance genes in bacterial isolates is nearing completion. OpGen has responded, and is continuing to respond, to the FDA’s additional information requests and now anticipates approaching a clearance decision for the Acuitas® AMR Gene Panel for isolates. Exact timing is unknown as a result of the COVID-19 pandemic;
OpGen successfully achieved the last of the first year milestones in the groundbreaking collaboration with the New York State Department of Health and ILÚM Health Solutions, LLC, to develop a state-of-the-art research program to detect, track, and manage antimicrobial-resistant infections at healthcare institutions statewide. In response to the COVID-19 emergency in New York State, testing under the program has been put on hold by the Wadsworth Center and participating hospitals; however, the parties are currently engaged in active negotiations and discussions for a second year of the collaboration and expect to resume the project and expand it into its second year once the COVID-19 situation permits;
OpGen’s subsidiary Ares Genetics in April announced that a study on the feasibility and potential of antibiotic susceptibility testing and bacterial pathogen identification using next-generation sequencing (NGS) has been pre-published in the Journal of Clinical Microbiology. The study was performed by Ares Genetics GmbH and Curetis GmbH, and scientists at the Max Perutz Labs (Austria), a joint venture of the University of Vienna and the Medical University of Vienna, and the Mayo Clinic (Rochester, MN, U.S.A.); and
Curetis and Quaphaco entered into an exclusive three-year distribution partnership for the Unyvero product line in Vietnam; the contract includes minimum commitments by Quaphaco totaling approximately $ 2.1 million over the initial three-year term.
Mr. Schacht continued, “We have a strong pipeline heading into the second quarter of 2020 despite the unique market challenges presented at this time. With the anticipated near term FDA clearance decision and subsequent launch of our Acuitas AMR Gene Panel product for isolate and the exciting combination of the Acuitas Lighthouse® database with the ARESdb, our strong, proprietary product pipeline attests to our ability to deliver premier AI-powered bioinformatics solutions and diagnostic products. We are especially excited about an upcoming major milestone in the Ares collaboration with its strategic R&D collaboration partner, a leading global IVD corporation, where completion of the R&D phase is expected for the third quarter this year. The IVD corporate partner has pre-funded an option which upon exercise would then grant it 90 days of exclusive negotiations with Ares regarding a potential future partnering and licensing deal.”
Antwort auf Beitrag Nr.: 63.595.620 von Plomo_o_plata am 07.05.20 15:40:35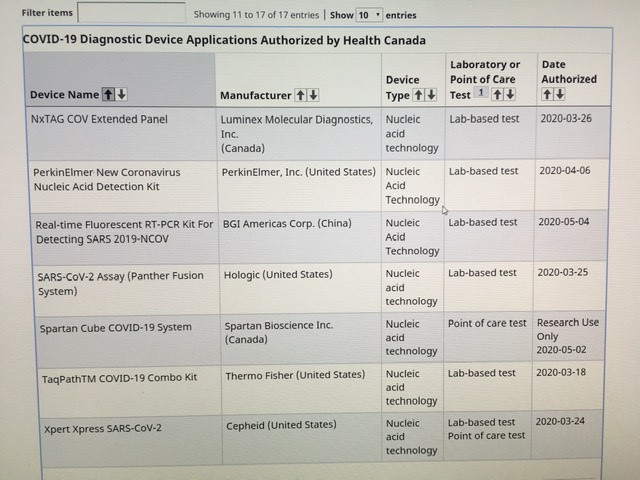

Antwort auf Beitrag Nr.: 63.569.553 von Turnover15 am 05.05.20 16:15:29
Mein fehler
Stimmt Donnerstag, habe das mit datametrex ai verwechselt da gab es heute einen sehr erfolgreichen webinar
Antwort auf Beitrag Nr.: 63.567.294 von Plomo_o_plata am 05.05.20 13:26:27Ich denke Donnerstag AH 🙄
https://finance.yahoo.com/news/opgen-business-financial-resu…
https://finance.yahoo.com/news/opgen-business-financial-resu…
Antwort auf Beitrag Nr.: 63.567.150 von Growth2012 am 05.05.20 13:13:12
Heute Abend
Werden wir mehr wissen, earnings nach der Glocke
Antwort auf Beitrag Nr.: 63.560.874 von Plomo_o_plata am 04.05.20 21:52:12Danke dir 
Tja, sie lebt (offensichtlich) noch.... 💹
💹
Geld 2,20
Brief 2,26

Tja, sie lebt (offensichtlich) noch....
 💹
💹
Geld 2,20
Brief 2,26
Antwort auf Beitrag Nr.: 63.526.754 von Growth2012 am 01.05.20 10:14:19
Danke dir
Hier noch ein Interview von https://www.technologynetworks.com/diagnostics/blog/the-need…
Antwort auf Beitrag Nr.: 63.524.363 von Plomo_o_plata am 30.04.20 22:42:51Moin Plomo_o_plata, wirklich beeindruckende content , Danke fürs posting.
, Danke fürs posting.
Das ganze kommt zudem sehr professionell & durchdacht rüber.
Bin sehr gespannt was alles ab KW 20 "operativ" auf uns zukommt
 , Danke fürs posting.
, Danke fürs posting.Das ganze kommt zudem sehr professionell & durchdacht rüber.
Bin sehr gespannt was alles ab KW 20 "operativ" auf uns zukommt






















