Empower Clinics weltweit erste Cannabis Klinik (Seite 536)
eröffnet am 02.04.19 14:56:27 von
neuester Beitrag 28.03.24 09:05:02 von
neuester Beitrag 28.03.24 09:05:02 von
Beiträge: 6.034
ID: 1.301.202
ID: 1.301.202
Aufrufe heute: 1
Gesamt: 518.021
Gesamt: 518.021
Aktive User: 0
ISIN: CA29246V1040 · WKN: A2JKED · Symbol: EPW
0,0100
CAD
0,00 %
0,0000 CAD
Letzter Kurs 20.06.23 CSE
Neuigkeiten
19.08.23 · Accesswire |
15.08.23 · Accesswire |
09.08.23 · Accesswire |
01.08.23 · Accesswire |
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,2000 | +471,16 | |
| 13,110 | +38,44 | |
| 6,5000 | +27,45 | |
| 1,0580 | +22,03 | |
| 1,2100 | +21,00 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 24,050 | -12,55 | |
| 4,0300 | -12,96 | |
| 6,2600 | -14,25 | |
| 3,8500 | -14,45 | |
| 36,70 | -22,87 |
Beitrag zu dieser Diskussion schreiben
Zitat: "Phase 4 allows Empower to service enterprise level clients, including movie and television studios that require reliable, accurate, fast and mass batch testing capabilities in order to resume production in a safe and compliant manner."
Empower Clinics Signs North American Distribution Agreement with API Pharma USA For FDA EUA COVID-Rapid Antigen & Antibody Tests. Supported Three Months of Clinical Trials
EMPOWER'S KAI Medical Laboratory Also Supporting API Pharma With Health Canada Submission of COVID Rapid Antigen Test and Signs National Distribution Agreement
VANCOUVER, BC / ACCESSWIRE / November 30, 2020 / EMPOWER CLINICS INC. (CBDT:CSE) (8EC:Frankfurt) (EPWCF:OTCQB) ("Empower" or the "Company") an integrated healthcare company serving a database of 165,000 patients through clinics in the Southwest United States, a telemedicine platform and medical diagnostics laboratory is pleased to announce the North American Re-Seller & Distribution Agreement with API Pharma ("API") for its FDA EUA submitted Rapid Antigen Test and Rapid Antibody Test.
Empower's KAI Medical Laboratory ("KAI") provided API significant scientific data through three months of clinical trials. As a result, Empower and API are now able to sell these products with confidence, on an unlimited basis, to its customers and prospective customers nationwide in the United States and in European countries supporting CE mark approval.
Furthermore, Empower and KAI are providing API with support for its Health Canada submission for its Rapid Antigen Detection Test ("RADT") (COVID-19 Application For Authorization If Import Or Sale Of Medical Devices). Empower will have Canadian distribution rights upon Health Canada approval.
Steven McAuley, Chairman and CEO of Empower stated "Our society is experiencing unprecedented changes that are affecting how we go back to work, how we travel and how we might gain permission to interact with others. Testing protocols and testing products are becoming a part of our daily lives, and there are key differences between PCR, antigen and antibody tests. They are all important, irrespective of how potential vaccines come to market. Our company is leading and positioned to provide all of these important tests to the markets we serve."
The global COVID-19 diagnostics market size is valued at USD 19.8 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 3.1% from 2021 to 2027. These tests are critical in the management of the ongoing COVID-19 pandemic for accurate diagnosis as well as for tackling the spread of the infection (https://www.grandviewresearch.com/industry-analysis/covid-19…
WHAT IS A RAPID ANTIGEN DETECTION TEST (RADT)?
An RADT is a rapid diagnostic test suitable for point-of-care testing that directly detects the presence or absence of an antigen. This distinguishes it from other medical tests that detect antibodies or nucleic acid, of either laboratory or point of care types.
WHAT IS AN ANTIBODY TEST?
An antibody test, also known as a serology test, looks for specific antibodies in your blood. This test is useful because it shows if someone has had the infection in the past, even if you had only mild symptoms.
One inherent advantage of an antigen test over an antibody test is that it can take time for the immune system to develop antibodies after infection begins, but the foreign antigen is present within a preliminary incubation period of five days or less.
GOVERNMENT OF CANADA - RADT's WILL HAVE AN IMPORTANT ROLE TO PLAY
The Government of Canada website for the use of RADT stated the following"
"Potential characteristics of these tests (faster turnaround time, lower per-test cost, ability to do the test in a setting by non-professionals on a more frequent basis, amongst others) suggest that they will have an important role to play in the next phase of the response. It is important for the public health, infectious diseases experts and laboratory communities to identify the scenarios where the use of RADTs may further strengthen the public health response by expanding access to testing"
(https://www.canada.ca/en/public-health/services/diseases/201…
API PHARMA - NO CHOICE BUT TO RAPID TEST
Rapid testing is critical to any strategy for containing COVID-19. It helps determine where the virus is spreading, who has immunity and how widely the virus has spread.
The COVID-Rapid Antigen Test determines who has the virus at present and is likely to be contagious. With >99% sensitivity for those who are contagious, the rapid results allow healthcare providers to make quick and accurate medical decisions.
The COVID-Rapid Antibody Test, which screens blood for antibodies, can allow hospitals and workplaces to identify people who were infected and may have already developed immunity. Studies have shown that one-third to one-half of those infected with COVID-19 are asymptomatic. As a result, serology tests help determine who can lead the way back to reopen businesses and work on the front lines of health care.
The COVID-Rapid line of tests are accurate and affordable so it can be used for testing large populations. America has no choice but to rapid test for COVID-19 so that governments, schools, and workplaces can test large groups of individuals, not just those who present with symptoms.
API PHARMA RADT DEMONSTRATED HIGHLY EFFECTIVE ON 3 CONTINENTS
API Pharma's COVID-Rapid Antigen test has been tested and demonstrated to be highly effective on three continents. This is important as it demonstrates all the different strains of the virus will respond equally to the rapid antigen test. Clinical studies were carried out specifically in China, the United States and Poland.
PARTNERSHIP FURTHER SOLIDIFIES KAI MEDICAL FOOTHOLD IN COVID-19 TESTING SPACE
The clinical trials of API products performed a variety of studies including the precision, accuracy, sensitivity, and interfering substances, as well as, the efficacy of operators to perform the test accurately. These studies ensure that API-products meet industry standards for testing and will accurately identify COVID-19 positives as well as COVID-19 negatives.
Because of API's focus on Rapid, Point-of-Care testing for both antibodies and antigens, in addition to Kai Medical's extraordinary capacity to handle industry-standard turnaround times for RT-PCR testing, this partnership further solidifies Kai Medical's foothold in the COVID-19 testing space.
Yoshi Tyler, President of KAI Medical Laboratory stated "We anticipate that, moving forward, there will be a focus on much more rapid and accurate point-of-care testing, in order to return to everyday life. Like the virus, our testing strategies must be adaptable. As we move forward, we continue to prioritize the best and most efficacious testing strategies."
KAI Medical Laboratory operates a high-complexity CLIA and COLA accredited laboratory that provides reliable and accurate testing solutions to hospitals, medical clinics, pharmacies, and employer groups. KAI has taken an active role in COVID-19 testing, battling the pandemic through RT-PCR testing and serology testing with the capacity to process 4,000 RT-PCR test specimens per day. While the RT-PCR test identifies if a patient has an active virus, the serology or antibody test detects if a patient has previously been exposed to the virus. Both of these test results are vital to managing outbreaks and the potential spread of coronavirus.
As a result of this capability, Empower is now able to expand phase four of its COVID-19 testing rollout which was first announced on April 27, 2020 beginning with testing in-clinic testing (Phase 1) and culminating with a nationwide roll-out across the United States (Phase 4). Phase 4 allows Empower to service enterprise level clients, including movie and television studios that require reliable, accurate, fast and mass batch testing capabilities in order to resume production in a safe and compliant manner.
ABOUT API PHARMA
API Pharma (API) is an industry leader in designing and distributing rapid tests for a range of viruses and diseases, including rotovirus, influenza, hepatitis, herpes, HIV, and others.
API's rapid COVID-19 test is nearly as simple as a home pregnancy test, requiring only a few drops of blood from a finger stick and a drop of reagent. Results are available in under 20 minutes and are simple to interpret: one line means negative, two lines means positive. The test has 97% sensitivity and 97% specificity, and API's long experience with rapid tests makes it a reliable provider. API currently has approval to provide test kits to medical facilities in the U.S. and expects to have FDA approval for home use soon. The test is already in use in Europe.
ABOUT EMPOWER
Empower is creating a network of physicians and practitioners who integrate to serve patient needs, in-clinic, through telemedicine, and with decentralized mobile delivery. A simplified, streamlined care model bringing key attributes of the healthcare supply chain together, always focused on patient experience. The Company provides COVID-19 testing services to consumers and businesses as part of a four-phased nationwide testing initiative in the United States. Empower recently acquired Kai Medical Laboratory, LLC as a wholly owned subsidiary with large-scale testing capability.
ABOUT Kai Medical Laboratory
Our mission is to change healthcare through science & innovative quality care by providing value added services, accuracy, and consistency. Our unwavering commitment to quality compliance and scientific innovation elevates Kai Medical Laboratory to a new standard in patient care. Kai Medical Laboratory is located in the Dallas Medical District in close proximity to some of the largest healthcare groups in the U.S. including Parkland Hospital, UT Southwestern, Children's Medical Center, Baylor Scott & White Health (Dallas), Tenet Healthcare (Dallas), CHRISTUS Healthcare (Dallas).
ON BEHALF OF THE BOARD OF DIRECTORS:
Steven McAuley
Chief Executive Officer
CONTACTS:
Investors: Dustin Klein
Director
dustin@svmmjcc.com
720-352-1398
Investors: Steven McAuley
CEO
s.mcauley@empowerclinics.com
604-789-2146
DISCLAIMER FOR FORWARD-LOOKING STATEMENTS
This news release contains certain "forward-looking statements" or "forward-looking information" (collectively "forward looking statements") within the meaning of applicable Canadian securities laws. All statements, other than statements of historical fact, are forward-looking statements and are based on expectations, estimates and projections as at the date of this news release.Forward-looking statements can frequently be identified by words such as "plans", "continues", "expects", "projects", "intends", "believes", "anticipates", "estimates", "may", "will", "potential", "proposed" and other similar words, or information that certain events or conditions "may" or "will" occur. Forward-looking statements in this news release include, but are not limited to, statements regarding: the expected benefits to the Company and its shareholders as a result of the acquisition of Kai Medical Laboratory; the transaction terms; the expected number of clinics and patients following the closing; the future potential success of Kai Medical Laboratory, Sun Valley's franchise model; the anticipated date of closing of the acquisition and the occurrence thereof; and that the Company will be positioned to be a market-leading service provider for complex patient requirements in 2020 and beyond. Such statements are only projections, are based on assumptions known to management at this time, and are subject to risks and uncertainties that may cause actual results, performance or developments to differ materially from those contained in the forward-looking statements, including: that the Kai Medical Laboratory acquisition may not be completed on the terms expected or at all; that the Company's products may not work as expected; that the Company may not be able to expand COVID-19 testing; that legislative changes may have an adverse affect on the Company's business and product development; that the Company may not be able to obtain adequate financing to pursue its business plan; general business, economic, competitive, political and social uncertainties; failure to obtain any necessary approvals in connection with the proposed transaction; and other factors beyond the Company's control. No assurance can be given that any of the events anticipated by the forward-looking statements will occur or, if they do occur, what benefits the Company will obtain from them. Readers are cautioned not to place undue reliance on the forward-looking statements in this release, which are qualified in their entirety by these cautionary statements. The Company is under no obligation, and expressly disclaims any intention or obligation, to update or revise any forward-looking statements in this release, whether as a result of new information, future events or otherwise, except as expressly required by applicable laws.
SOURCE: Empower Clinics Inc.
View source version on accesswire.com:
https://www.accesswire.com/618756/Empower-Clinics-Signs-Nort…
EMPOWER'S KAI Medical Laboratory Also Supporting API Pharma With Health Canada Submission of COVID Rapid Antigen Test and Signs National Distribution Agreement
VANCOUVER, BC / ACCESSWIRE / November 30, 2020 / EMPOWER CLINICS INC. (CBDT:CSE) (8EC:Frankfurt) (EPWCF:OTCQB) ("Empower" or the "Company") an integrated healthcare company serving a database of 165,000 patients through clinics in the Southwest United States, a telemedicine platform and medical diagnostics laboratory is pleased to announce the North American Re-Seller & Distribution Agreement with API Pharma ("API") for its FDA EUA submitted Rapid Antigen Test and Rapid Antibody Test.
Empower's KAI Medical Laboratory ("KAI") provided API significant scientific data through three months of clinical trials. As a result, Empower and API are now able to sell these products with confidence, on an unlimited basis, to its customers and prospective customers nationwide in the United States and in European countries supporting CE mark approval.
Furthermore, Empower and KAI are providing API with support for its Health Canada submission for its Rapid Antigen Detection Test ("RADT") (COVID-19 Application For Authorization If Import Or Sale Of Medical Devices). Empower will have Canadian distribution rights upon Health Canada approval.
Steven McAuley, Chairman and CEO of Empower stated "Our society is experiencing unprecedented changes that are affecting how we go back to work, how we travel and how we might gain permission to interact with others. Testing protocols and testing products are becoming a part of our daily lives, and there are key differences between PCR, antigen and antibody tests. They are all important, irrespective of how potential vaccines come to market. Our company is leading and positioned to provide all of these important tests to the markets we serve."
The global COVID-19 diagnostics market size is valued at USD 19.8 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 3.1% from 2021 to 2027. These tests are critical in the management of the ongoing COVID-19 pandemic for accurate diagnosis as well as for tackling the spread of the infection (https://www.grandviewresearch.com/industry-analysis/covid-19…
WHAT IS A RAPID ANTIGEN DETECTION TEST (RADT)?
An RADT is a rapid diagnostic test suitable for point-of-care testing that directly detects the presence or absence of an antigen. This distinguishes it from other medical tests that detect antibodies or nucleic acid, of either laboratory or point of care types.
WHAT IS AN ANTIBODY TEST?
An antibody test, also known as a serology test, looks for specific antibodies in your blood. This test is useful because it shows if someone has had the infection in the past, even if you had only mild symptoms.
One inherent advantage of an antigen test over an antibody test is that it can take time for the immune system to develop antibodies after infection begins, but the foreign antigen is present within a preliminary incubation period of five days or less.
GOVERNMENT OF CANADA - RADT's WILL HAVE AN IMPORTANT ROLE TO PLAY
The Government of Canada website for the use of RADT stated the following"
"Potential characteristics of these tests (faster turnaround time, lower per-test cost, ability to do the test in a setting by non-professionals on a more frequent basis, amongst others) suggest that they will have an important role to play in the next phase of the response. It is important for the public health, infectious diseases experts and laboratory communities to identify the scenarios where the use of RADTs may further strengthen the public health response by expanding access to testing"
(https://www.canada.ca/en/public-health/services/diseases/201…
API PHARMA - NO CHOICE BUT TO RAPID TEST
Rapid testing is critical to any strategy for containing COVID-19. It helps determine where the virus is spreading, who has immunity and how widely the virus has spread.
The COVID-Rapid Antigen Test determines who has the virus at present and is likely to be contagious. With >99% sensitivity for those who are contagious, the rapid results allow healthcare providers to make quick and accurate medical decisions.
The COVID-Rapid Antibody Test, which screens blood for antibodies, can allow hospitals and workplaces to identify people who were infected and may have already developed immunity. Studies have shown that one-third to one-half of those infected with COVID-19 are asymptomatic. As a result, serology tests help determine who can lead the way back to reopen businesses and work on the front lines of health care.
The COVID-Rapid line of tests are accurate and affordable so it can be used for testing large populations. America has no choice but to rapid test for COVID-19 so that governments, schools, and workplaces can test large groups of individuals, not just those who present with symptoms.
API PHARMA RADT DEMONSTRATED HIGHLY EFFECTIVE ON 3 CONTINENTS
API Pharma's COVID-Rapid Antigen test has been tested and demonstrated to be highly effective on three continents. This is important as it demonstrates all the different strains of the virus will respond equally to the rapid antigen test. Clinical studies were carried out specifically in China, the United States and Poland.
PARTNERSHIP FURTHER SOLIDIFIES KAI MEDICAL FOOTHOLD IN COVID-19 TESTING SPACE
The clinical trials of API products performed a variety of studies including the precision, accuracy, sensitivity, and interfering substances, as well as, the efficacy of operators to perform the test accurately. These studies ensure that API-products meet industry standards for testing and will accurately identify COVID-19 positives as well as COVID-19 negatives.
Because of API's focus on Rapid, Point-of-Care testing for both antibodies and antigens, in addition to Kai Medical's extraordinary capacity to handle industry-standard turnaround times for RT-PCR testing, this partnership further solidifies Kai Medical's foothold in the COVID-19 testing space.
Yoshi Tyler, President of KAI Medical Laboratory stated "We anticipate that, moving forward, there will be a focus on much more rapid and accurate point-of-care testing, in order to return to everyday life. Like the virus, our testing strategies must be adaptable. As we move forward, we continue to prioritize the best and most efficacious testing strategies."
KAI Medical Laboratory operates a high-complexity CLIA and COLA accredited laboratory that provides reliable and accurate testing solutions to hospitals, medical clinics, pharmacies, and employer groups. KAI has taken an active role in COVID-19 testing, battling the pandemic through RT-PCR testing and serology testing with the capacity to process 4,000 RT-PCR test specimens per day. While the RT-PCR test identifies if a patient has an active virus, the serology or antibody test detects if a patient has previously been exposed to the virus. Both of these test results are vital to managing outbreaks and the potential spread of coronavirus.
As a result of this capability, Empower is now able to expand phase four of its COVID-19 testing rollout which was first announced on April 27, 2020 beginning with testing in-clinic testing (Phase 1) and culminating with a nationwide roll-out across the United States (Phase 4). Phase 4 allows Empower to service enterprise level clients, including movie and television studios that require reliable, accurate, fast and mass batch testing capabilities in order to resume production in a safe and compliant manner.
ABOUT API PHARMA
API Pharma (API) is an industry leader in designing and distributing rapid tests for a range of viruses and diseases, including rotovirus, influenza, hepatitis, herpes, HIV, and others.
API's rapid COVID-19 test is nearly as simple as a home pregnancy test, requiring only a few drops of blood from a finger stick and a drop of reagent. Results are available in under 20 minutes and are simple to interpret: one line means negative, two lines means positive. The test has 97% sensitivity and 97% specificity, and API's long experience with rapid tests makes it a reliable provider. API currently has approval to provide test kits to medical facilities in the U.S. and expects to have FDA approval for home use soon. The test is already in use in Europe.
ABOUT EMPOWER
Empower is creating a network of physicians and practitioners who integrate to serve patient needs, in-clinic, through telemedicine, and with decentralized mobile delivery. A simplified, streamlined care model bringing key attributes of the healthcare supply chain together, always focused on patient experience. The Company provides COVID-19 testing services to consumers and businesses as part of a four-phased nationwide testing initiative in the United States. Empower recently acquired Kai Medical Laboratory, LLC as a wholly owned subsidiary with large-scale testing capability.
ABOUT Kai Medical Laboratory
Our mission is to change healthcare through science & innovative quality care by providing value added services, accuracy, and consistency. Our unwavering commitment to quality compliance and scientific innovation elevates Kai Medical Laboratory to a new standard in patient care. Kai Medical Laboratory is located in the Dallas Medical District in close proximity to some of the largest healthcare groups in the U.S. including Parkland Hospital, UT Southwestern, Children's Medical Center, Baylor Scott & White Health (Dallas), Tenet Healthcare (Dallas), CHRISTUS Healthcare (Dallas).
ON BEHALF OF THE BOARD OF DIRECTORS:
Steven McAuley
Chief Executive Officer
CONTACTS:
Investors: Dustin Klein
Director
dustin@svmmjcc.com
720-352-1398
Investors: Steven McAuley
CEO
s.mcauley@empowerclinics.com
604-789-2146
DISCLAIMER FOR FORWARD-LOOKING STATEMENTS
This news release contains certain "forward-looking statements" or "forward-looking information" (collectively "forward looking statements") within the meaning of applicable Canadian securities laws. All statements, other than statements of historical fact, are forward-looking statements and are based on expectations, estimates and projections as at the date of this news release.Forward-looking statements can frequently be identified by words such as "plans", "continues", "expects", "projects", "intends", "believes", "anticipates", "estimates", "may", "will", "potential", "proposed" and other similar words, or information that certain events or conditions "may" or "will" occur. Forward-looking statements in this news release include, but are not limited to, statements regarding: the expected benefits to the Company and its shareholders as a result of the acquisition of Kai Medical Laboratory; the transaction terms; the expected number of clinics and patients following the closing; the future potential success of Kai Medical Laboratory, Sun Valley's franchise model; the anticipated date of closing of the acquisition and the occurrence thereof; and that the Company will be positioned to be a market-leading service provider for complex patient requirements in 2020 and beyond. Such statements are only projections, are based on assumptions known to management at this time, and are subject to risks and uncertainties that may cause actual results, performance or developments to differ materially from those contained in the forward-looking statements, including: that the Kai Medical Laboratory acquisition may not be completed on the terms expected or at all; that the Company's products may not work as expected; that the Company may not be able to expand COVID-19 testing; that legislative changes may have an adverse affect on the Company's business and product development; that the Company may not be able to obtain adequate financing to pursue its business plan; general business, economic, competitive, political and social uncertainties; failure to obtain any necessary approvals in connection with the proposed transaction; and other factors beyond the Company's control. No assurance can be given that any of the events anticipated by the forward-looking statements will occur or, if they do occur, what benefits the Company will obtain from them. Readers are cautioned not to place undue reliance on the forward-looking statements in this release, which are qualified in their entirety by these cautionary statements. The Company is under no obligation, and expressly disclaims any intention or obligation, to update or revise any forward-looking statements in this release, whether as a result of new information, future events or otherwise, except as expressly required by applicable laws.
SOURCE: Empower Clinics Inc.
View source version on accesswire.com:
https://www.accesswire.com/618756/Empower-Clinics-Signs-Nort…
NEWS: Empower Clinics Signs North American Distribution Agreement with API Pharma USA
https://www.stockwatch.com/News/Item?bid=U-by0618756-U:EPWCF…Empower Clinics Signs North American Distribution Agreement with API Pharma USA For FDA EUA COVID-Rapid Antigen & Antibody Tests. Supported Three Months of Clinical Trials
Alle die Nase erst einmal voll, verständlich.
Für mich heißt es abwarten.
Ich hoffe der Docht deutet auf eine Beendigung des Abverkaufs.
Die untere Trendlinie, spätestens die 65er-Unterstützung müssen halten, ansonsten geht es massiv abwärts.
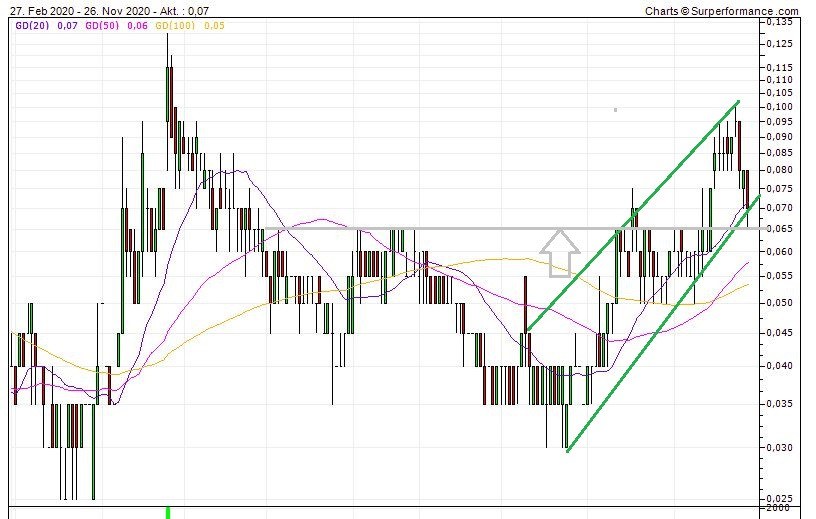
Für mich heißt es abwarten.
Ich hoffe der Docht deutet auf eine Beendigung des Abverkaufs.
Die untere Trendlinie, spätestens die 65er-Unterstützung müssen halten, ansonsten geht es massiv abwärts.

Antwort auf Beitrag Nr.: 65.834.862 von Pappnase81 am 25.11.20 10:01:27
Wenn unter den Aktionären keine Hellseher sind wussten hier definitiv bereits einige vorher etwas.
Q3: Gut, aber mal wieder nicht gut genug?
Mal schauen, ob jetzt eine Seitwärtsbewegung folgt. Werde möglicherweise etwas nachsammeln wenn sich nicht anderswo Möglichkeiten bieten.
Da die letzten Deals erst ab Q4 wirken, sollte dieses dann bedeutend besser ausfallen als Q3.
Zitat von Pappnase81: Würde mich nicht wundern wenn da gestern schon was durchgesickert ist und deshalb erstmal viele Gewinne mitgenommen haben.
Wenn unter den Aktionären keine Hellseher sind wussten hier definitiv bereits einige vorher etwas.
Q3: Gut, aber mal wieder nicht gut genug?
Mal schauen, ob jetzt eine Seitwärtsbewegung folgt. Werde möglicherweise etwas nachsammeln wenn sich nicht anderswo Möglichkeiten bieten.
Da die letzten Deals erst ab Q4 wirken, sollte dieses dann bedeutend besser ausfallen als Q3.
Antwort auf Beitrag Nr.: 65.835.039 von Lubi_1 am 25.11.20 10:13:23Wenn man sich überlegt was im Sommer in den USA wegen Covid-19 los war,muss man sich nicht wundern,dass die Kliniken weniger Besucher/Umsatz hatten.
Antwort auf Beitrag Nr.: 65.835.039 von Lubi_1 am 25.11.20 10:13:23Klar.Aber in Q4.
Antwort auf Beitrag Nr.: 65.834.451 von Othernick am 25.11.20 09:38:22Umsatz +67% in 2020gegenüber 2019.
Der Umsatz im 3.Quartal 2020 ist aber niedriger als der Umsatz in Q3 2019.
Ich habe eigentlich viel bessere Zahlen erwartet, vorallem was den Umsatz in diesem Quartal betrifft.
Dachte eigentlich durch das Covid-19 Testing gehen die Umsätze viel stärker hoch🤔
Der Umsatz im 3.Quartal 2020 ist aber niedriger als der Umsatz in Q3 2019.
Ich habe eigentlich viel bessere Zahlen erwartet, vorallem was den Umsatz in diesem Quartal betrifft.
Dachte eigentlich durch das Covid-19 Testing gehen die Umsätze viel stärker hoch🤔
Ich finde die Q3 zahlen eher enttäuschend! War schlechter als letztes Jahr, obwohl sie so viel testen wollten. Yoy ist es ok, aber q3 war in meinen Augen ein Rückschritt. Würde mich nicht wundern wenn da gestern schon was durchgesickert ist und deshalb erstmal viele Gewinne mitgenommen haben. Naja, der erste Deal mit 1mio c$ war sehr gut. Hoffen wir das weitere in dieser Größenordnung kommen.
Status zur cbd extraktionsanlage wäre auch nicht schlecht.
Status zur cbd extraktionsanlage wäre auch nicht schlecht.
Antwort auf Beitrag Nr.: 65.833.959 von Othernick am 25.11.20 09:12:29Zahlen sind da.Umsatz +67 Prozent! Da kommt heute noch was zu den weiteren Aussichten...





















