Introgen -- weitere positive Nachrichten auf ASGT (4.-8. Juni) - 500 Beiträge pro Seite
eröffnet am 04.06.03 18:14:30 von
neuester Beitrag 28.06.03 20:14:04 von
neuester Beitrag 28.06.03 20:14:04 von
Beiträge: 16
ID: 739.563
ID: 739.563
Aufrufe heute: 0
Gesamt: 905
Gesamt: 905
Aktive User: 0
ISIN: US46119F1075 · WKN: 936231
Neuigkeiten
| TitelBeiträge |
|---|
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,8000 | +2.566,67 | |
| 3,3000 | +44,74 | |
| 1,2200 | +31,76 | |
| 1,2450 | +29,69 | |
| 7,7600 | +29,33 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 2,7900 | -24,80 | |
| 1,0100 | -26,81 | |
| 9,5300 | -34,00 | |
| 1,2400 | -34,39 | |
| 1,0950 | -34,43 |
Ab heute beginnt der Kongress der American Societx of Gene Therapy (ASGT). Abstracts sind schon vorab einzusehen. Abstracts #20,41,60,325,694,795,945,1081,1088,1092,1157 beziehen sich auf Adp53 (ADVEXIN) und vor allem auch auf mda-7/IL-24 dem zweiten vielversprechenden Produkt von INGN.
Phase III Ergebnisse von ADVEXIN werden im Herbst diesen Jahres erwartet, also in wenigen Monaten. Von einer Zulassung ist bei bisher sehr guter Datenlage und kaum vorhandenen Nebenwirkungen zu rechnen. Orphan drug Status hat ADVEXIN bereits erhalten, ich gehe zudem von einer "fast track" Zulassung durch die FDA aus.
Produkt-Pipeline:
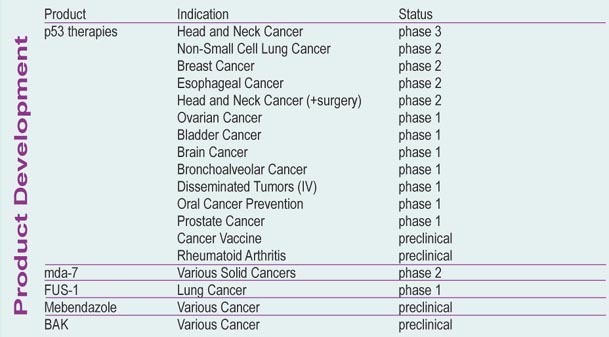
Phase III Ergebnisse von ADVEXIN werden im Herbst diesen Jahres erwartet, also in wenigen Monaten. Von einer Zulassung ist bei bisher sehr guter Datenlage und kaum vorhandenen Nebenwirkungen zu rechnen. Orphan drug Status hat ADVEXIN bereits erhalten, ich gehe zudem von einer "fast track" Zulassung durch die FDA aus.
Produkt-Pipeline:

Und wo siehst du das kursziel in etwa ?
Zwischenzeitlich wurden neue Ergebnisse zu INGN 241 (mda-7/IL24) bei "schwarzem Hautkreb" (Melanom) veröffentlicht, die ebenfalls sehr beeindruckend sind. Ich gehe zum Jahresende von einem Kursziel um 12$ aus. Prognosen von mehreren 100% oder gar 1000% halte ich generell für unrealistisch, wenngleich mehr als 12$ drin sind (meiner Meinung nach).
Danke! Kennst Du noch einen interessanten Wert ?
Hab außer introgen noch cv therapeut,id biomedical und vivus
Hab außer introgen noch cv therapeut,id biomedical und vivus
Wollte mich bedanken für den tollen wert.Gott segne Dich hoffe das Du dafür reich belohnt wirst.
Gruss
Boy
Gruss
Boy

Eine kleine Wunderdroge die ingn da am testen ist
Introgen Reports Safety and Clinical Activity for INGN 241 Therapy; Phase 1/2 Study Shows Potency of INGN 241 as Cancer Cell Killer
Friday June 6, 1:00 pm ET
WASHINGTON, June 6 /PRNewswire-FirstCall/ -- A report of final phase 1 and initial phase 2 evaluation of Introgen Therapeutics` (Nasdaq: INGN - News) anti- cancer drug INGN 241 was presented during a scheduled press conference at the annual meeting of the American Society of Gene Therapy. The trial results in patients with solid tumors demonstrate that INGN 241 is well tolerated, that the agent is biologically active, and importantly, minimal toxicities are associated with the treatment. Introgen`s chief medical officer, Dr. James Merritt, was invited to present these data at the press conference.
ADVERTISEMENT
"Introgen`s belief that INGN 241 is one of the most promising anti-cancer gene drugs in clinical development today has been validated repeatedly in our studies, and we are gratified that our research has been highlighted as newsworthy by the ASGT review committee," said Dr. Merritt.
Previous studies show that INGN 241 causes cancer cells to die and the MDA-7 protein released from the tumor cells may stimulate the immune system to attack additional metastatic tumor cells. A phase 1 study confirmed that INGN 241 is safe and that the MDA-7 protein is active, and importantly, has a wide area of biological effect within injected tumors. In the phase 1 dose- escalating study, tumors from patients treated with the higher dose of the therapy were observed to have the MDA-7 protein in up to 80 percent of cells. The cancer therapeutic has the potential to trigger the human immune system to attack cancer cells. Increases in killer T cells were observed following treatment. To date, in the phase 2 study, although no tumor has responded after a single dose, tumors in two patients regressed after as few as two doses. Complete regression was noted in a melanoma. Regression was also noted in a case of squamous cell cancer.
INGN 241 consists of the human mda-7 gene in Introgen`s proprietary adenovirus vector. The mda-7 gene was discovered by the laboratory of Dr. Paul B. Fisher, professor of clinical pathology and the Michael and Stella Chernow Urological Cancer Research Scientist in the Departments of Neurological Surgery, Pathology and Urology at Columbia University. Introgen holds an exclusive worldwide license from the Corixa Corporation.
Introgen is a leading developer of biopharmaceutical products designed to induce therapeutic protein expression using non-integrating gene agents for the treatment of cancer and other diseases. Introgen maintains integrated research, development, manufacturing, clinical and regulatory departments and operates a commercial-scale, CGMP manufacturing facility.
Certain statements in this press release that are not strictly historical may be "forward-looking" statements, which are based on current expectations and entail various risks and uncertainties. Such forward-looking statements include, but are not limited to, those relating to Introgen`s future success with its clinical development program with INGN 241 for solid tumors. There can be no assurance that Introgen will be able to commercially develop gene- based drugs, that necessary regulatory approvals will be obtained or that any clinical trials or studies undertaken will be successful or that the proposed treatments will prove to be safe and/or effective. The actual results may differ from those described in this press release due to risks and uncertainties that exist in Introgen`s operations and business environment, including, but without limitation, Introgen`s stage of product development and the limited experience in the development of gene-based drugs in general, Introgen`s dependence upon proprietary technology and current competition, history of operating losses and accumulated deficits, reliance on collaborative relationships, and uncertainties related to clinical trials, the safety and efficacy of Introgen`s product candidates, the ability to obtain the appropriate regulatory approvals, patent protection and market acceptance, as well as other risks detailed from time to time in Introgen`s filings with the Securities and Exchange Commission, including its annual report on Form 10-K filed with the SEC on March 31, 2003 and its quarterly report on Form 10- Q filed with the SEC on May 15, 2003. Introgen undertakes no obligation to publicly release the results of any revisions to any forward-looking statements that reflect events or circumstances arising after the date hereof.
gruss meislo
Introgen Reports Safety and Clinical Activity for INGN 241 Therapy; Phase 1/2 Study Shows Potency of INGN 241 as Cancer Cell Killer
Friday June 6, 1:00 pm ET
WASHINGTON, June 6 /PRNewswire-FirstCall/ -- A report of final phase 1 and initial phase 2 evaluation of Introgen Therapeutics` (Nasdaq: INGN - News) anti- cancer drug INGN 241 was presented during a scheduled press conference at the annual meeting of the American Society of Gene Therapy. The trial results in patients with solid tumors demonstrate that INGN 241 is well tolerated, that the agent is biologically active, and importantly, minimal toxicities are associated with the treatment. Introgen`s chief medical officer, Dr. James Merritt, was invited to present these data at the press conference.
ADVERTISEMENT
"Introgen`s belief that INGN 241 is one of the most promising anti-cancer gene drugs in clinical development today has been validated repeatedly in our studies, and we are gratified that our research has been highlighted as newsworthy by the ASGT review committee," said Dr. Merritt.
Previous studies show that INGN 241 causes cancer cells to die and the MDA-7 protein released from the tumor cells may stimulate the immune system to attack additional metastatic tumor cells. A phase 1 study confirmed that INGN 241 is safe and that the MDA-7 protein is active, and importantly, has a wide area of biological effect within injected tumors. In the phase 1 dose- escalating study, tumors from patients treated with the higher dose of the therapy were observed to have the MDA-7 protein in up to 80 percent of cells. The cancer therapeutic has the potential to trigger the human immune system to attack cancer cells. Increases in killer T cells were observed following treatment. To date, in the phase 2 study, although no tumor has responded after a single dose, tumors in two patients regressed after as few as two doses. Complete regression was noted in a melanoma. Regression was also noted in a case of squamous cell cancer.
INGN 241 consists of the human mda-7 gene in Introgen`s proprietary adenovirus vector. The mda-7 gene was discovered by the laboratory of Dr. Paul B. Fisher, professor of clinical pathology and the Michael and Stella Chernow Urological Cancer Research Scientist in the Departments of Neurological Surgery, Pathology and Urology at Columbia University. Introgen holds an exclusive worldwide license from the Corixa Corporation.
Introgen is a leading developer of biopharmaceutical products designed to induce therapeutic protein expression using non-integrating gene agents for the treatment of cancer and other diseases. Introgen maintains integrated research, development, manufacturing, clinical and regulatory departments and operates a commercial-scale, CGMP manufacturing facility.
Certain statements in this press release that are not strictly historical may be "forward-looking" statements, which are based on current expectations and entail various risks and uncertainties. Such forward-looking statements include, but are not limited to, those relating to Introgen`s future success with its clinical development program with INGN 241 for solid tumors. There can be no assurance that Introgen will be able to commercially develop gene- based drugs, that necessary regulatory approvals will be obtained or that any clinical trials or studies undertaken will be successful or that the proposed treatments will prove to be safe and/or effective. The actual results may differ from those described in this press release due to risks and uncertainties that exist in Introgen`s operations and business environment, including, but without limitation, Introgen`s stage of product development and the limited experience in the development of gene-based drugs in general, Introgen`s dependence upon proprietary technology and current competition, history of operating losses and accumulated deficits, reliance on collaborative relationships, and uncertainties related to clinical trials, the safety and efficacy of Introgen`s product candidates, the ability to obtain the appropriate regulatory approvals, patent protection and market acceptance, as well as other risks detailed from time to time in Introgen`s filings with the Securities and Exchange Commission, including its annual report on Form 10-K filed with the SEC on March 31, 2003 and its quarterly report on Form 10- Q filed with the SEC on May 15, 2003. Introgen undertakes no obligation to publicly release the results of any revisions to any forward-looking statements that reflect events or circumstances arising after the date hereof.
gruss meislo
LICENCE TO KILL CANCER CELLS - INGN 007
Introgen Therapeutics, Inc. (ticker: ingn, exchange: NASDAQ) News Release - 6/9/03
--------------------------------------------------------------------------------
New Introgen Product Candidate INGN 007 Presented at American Society of Gene Therapy Meeting
Potent Anti-Cancer Activity Demonstrated in Preclinical Studies
AUSTIN, Texas, Jun 9, 2003 /PRNewswire-FirstCall via COMTEX/ -- New adenoviral technology exclusively licensed by Introgen Therapeutics, Inc. (Nasdaq: INGN) from VirRx, Inc. has yielded a promising anti-cancer product candidate, according to research presented at the annual meeting of the American Society of Gene Therapy. The new product, INGN 007 (VRX-007), over-expresses a gene that allows the vector to saturate the entire tumor and to eradicate cancer in animal models. Introgen`s collaborator, Dr. William S.M. Wold, founder and CEO of VirRx and chairman of the Department of Molecular Microbiology and Immunology at St. Louis University School of Medicine presented the data during the recently concluded meeting.
Dr. Wold said, "The dramatic activity of these oncolytic viruses in accepted animal tumor models is very encouraging and we look forward to moving this approach as rapidly as possible into the clinic."
VirRx has developed a series of replication competent adenovirus vectors that over-express an adenoviral gene (ADP gene) that causes rapid disruption (oncolysis) of tumor cells in which it replicates. The ability to overexpress the ADP gene sets this technology apart from other existing oncolytic viruses and is shown in these studies to provide a powerful antitumor effect. Introgen holds an exclusive license to this technology from VirRx. Most of these vectors have been genetically engineered to incorporate certain genetic features that restrict their replication competency to selected types of cells, such as tumor cells. Additionally, some of these vectors have been further modified with transgenes which specifically kill cancer cells. These vectors have been extensively tested in cell and animal models and shown to be very highly active in killing tumor cells.
"We and others have repeatedly demonstrated that adenovirus based agents can be safely administered in the clinic and have therapeutic activity," said James A. Merritt, M.D., Introgen`s chief medical officer. "Dr. Wold`s new adenovirus concepts are very exciting and have the potential for significantly enhanced activity."
Introgen is a leading developer of biopharmaceutical products designed to induce therapeutic protein expression using non-integrating gene agents for the treatment of cancer and other diseases. Introgen maintains integrated research, development, manufacturing, clinical and regulatory departments and operates a commercial-scale, CGMP manufacturing facility.
Habe bei 7,50 nochmal nachgekauft. Rechne zwar demnächst mit einer Korrektur aber mittel- und langfristig bleibt INGN ein absoluter Kauf. Zudem erwarte ich bald die Bekanntgabe einer Kooperation mit Aventis. Das führende Produkt (INGN201=ADVEXIN-> www.Advexin.de oder .net,.org. usw.) wird bereits über einen Link mit der homepage von Aventis verbunden. Außerdem hat INGN gestern das höchste Handelsvolumen (bei weiter steigenden Kursen) in seiner Geschichte erreicht. In den USA erhält INGN auch in der Presse immer mehr Aufmerksamkeit, sodass ich überdies mit einem coverage verschiedener Brokerhäuser (z.B Prudential Sec.) rechne.
Freue mich für diejenigen, die auf den Zug aufgesprungen sind. Ich denke, es ist immer noch nicht zu spät.
Gruß chirint.
Introgen Therapeutics, Inc. (ticker: ingn, exchange: NASDAQ) News Release - 6/9/03
--------------------------------------------------------------------------------
New Introgen Product Candidate INGN 007 Presented at American Society of Gene Therapy Meeting
Potent Anti-Cancer Activity Demonstrated in Preclinical Studies
AUSTIN, Texas, Jun 9, 2003 /PRNewswire-FirstCall via COMTEX/ -- New adenoviral technology exclusively licensed by Introgen Therapeutics, Inc. (Nasdaq: INGN) from VirRx, Inc. has yielded a promising anti-cancer product candidate, according to research presented at the annual meeting of the American Society of Gene Therapy. The new product, INGN 007 (VRX-007), over-expresses a gene that allows the vector to saturate the entire tumor and to eradicate cancer in animal models. Introgen`s collaborator, Dr. William S.M. Wold, founder and CEO of VirRx and chairman of the Department of Molecular Microbiology and Immunology at St. Louis University School of Medicine presented the data during the recently concluded meeting.
Dr. Wold said, "The dramatic activity of these oncolytic viruses in accepted animal tumor models is very encouraging and we look forward to moving this approach as rapidly as possible into the clinic."
VirRx has developed a series of replication competent adenovirus vectors that over-express an adenoviral gene (ADP gene) that causes rapid disruption (oncolysis) of tumor cells in which it replicates. The ability to overexpress the ADP gene sets this technology apart from other existing oncolytic viruses and is shown in these studies to provide a powerful antitumor effect. Introgen holds an exclusive license to this technology from VirRx. Most of these vectors have been genetically engineered to incorporate certain genetic features that restrict their replication competency to selected types of cells, such as tumor cells. Additionally, some of these vectors have been further modified with transgenes which specifically kill cancer cells. These vectors have been extensively tested in cell and animal models and shown to be very highly active in killing tumor cells.
"We and others have repeatedly demonstrated that adenovirus based agents can be safely administered in the clinic and have therapeutic activity," said James A. Merritt, M.D., Introgen`s chief medical officer. "Dr. Wold`s new adenovirus concepts are very exciting and have the potential for significantly enhanced activity."
Introgen is a leading developer of biopharmaceutical products designed to induce therapeutic protein expression using non-integrating gene agents for the treatment of cancer and other diseases. Introgen maintains integrated research, development, manufacturing, clinical and regulatory departments and operates a commercial-scale, CGMP manufacturing facility.
Habe bei 7,50 nochmal nachgekauft. Rechne zwar demnächst mit einer Korrektur aber mittel- und langfristig bleibt INGN ein absoluter Kauf. Zudem erwarte ich bald die Bekanntgabe einer Kooperation mit Aventis. Das führende Produkt (INGN201=ADVEXIN-> www.Advexin.de oder .net,.org. usw.) wird bereits über einen Link mit der homepage von Aventis verbunden. Außerdem hat INGN gestern das höchste Handelsvolumen (bei weiter steigenden Kursen) in seiner Geschichte erreicht. In den USA erhält INGN auch in der Presse immer mehr Aufmerksamkeit, sodass ich überdies mit einem coverage verschiedener Brokerhäuser (z.B Prudential Sec.) rechne.
Freue mich für diejenigen, die auf den Zug aufgesprungen sind. Ich denke, es ist immer noch nicht zu spät.
Gruß chirint.
Kannst Du mir sagen wie hoch momentan die Marktcap. und Cashbestand ist
Danke
Mfg
Danke
Mfg
Introgen INGN SG Cowen from Outperform
to Mkt Perform vom 11.6.2003
to Mkt Perform vom 11.6.2003
Ich vermute SG Cowen will für einige größere Investoren nochmal billig einkaufen. Wenn man die bid/ask orders intraday verfolgt, kann man da zumindest daraus schliessen. Gezielt wurde der Preis mit Ordergößen von je 100 Stck. nach unten gedrückt (meine Interpretation). Außerdem bleibt zu bedenken, dass INGN immer noch 200-300% höher notiert als noch vor wenigen Wochen. Trotzdem glaube ich, dass SG Cowen (wenn sie genug Leute im Boot haben) INGN wieder "upgraden" wird. Spätestens wenn Ende des Jahres die Phase III Sudien zu Advexin vorliegen werden.
Im übrigen schaffte INGN am Freitag, bei allgemein nach unten tendierenden Märkten (insbesondere Biotechs) ein intraday reversal. Bestätigt meine Theorie, dass der Wert für einige Investoren zu schnell Anstieg und jetzt nach der notwendigen Korrektur nachgekauft wird. Habe selbst auch nochmal nachgekauft (bei 7,45 US$ (leider etwas zu früh) und nochmal bei 6,70 $). Mal sehen, nächstes Wochenende steht die BIO 2003 an, bei der INGN teilnehmen wird.
Gruß und viel Glück, chirint.
Im übrigen schaffte INGN am Freitag, bei allgemein nach unten tendierenden Märkten (insbesondere Biotechs) ein intraday reversal. Bestätigt meine Theorie, dass der Wert für einige Investoren zu schnell Anstieg und jetzt nach der notwendigen Korrektur nachgekauft wird. Habe selbst auch nochmal nachgekauft (bei 7,45 US$ (leider etwas zu früh) und nochmal bei 6,70 $). Mal sehen, nächstes Wochenende steht die BIO 2003 an, bei der INGN teilnehmen wird.
Gruß und viel Glück, chirint.
market cap. ca. 150 Mio $
Chashbestand ca. 21 Mio $, burnrate ca. 4,5 Mio $.
Chash Bestand ist relativ knapp, eas ein gewisses Risiko bei diesem Wert darstellt. Allerdings geht aus der letzten PK hervor, dass die meistens klinischen Studien gesponsort werden. Zudem besteht ein Interesse seitens Aventis, dass das lead product Advexin (Phase III Ende des Jahres abgeschlossen) möglichst rasch in den Markt eingeführt werden kann.
Chashbestand ca. 21 Mio $, burnrate ca. 4,5 Mio $.
Chash Bestand ist relativ knapp, eas ein gewisses Risiko bei diesem Wert darstellt. Allerdings geht aus der letzten PK hervor, dass die meistens klinischen Studien gesponsort werden. Zudem besteht ein Interesse seitens Aventis, dass das lead product Advexin (Phase III Ende des Jahres abgeschlossen) möglichst rasch in den Markt eingeführt werden kann.
Gude Chirint !
Wollte Dich mal fragen was Du von diesem Wert(Aphton) hälst.
Gruss
M.B.
Wollte Dich mal fragen was Du von diesem Wert(Aphton) hälst.
Gruss
M.B.
Hallo chirint,
warst mit dem Nachkaufen etwas früh dran. ich habe mir zunächst für 1000 Euro 150 Stück ins Depot gelegt (7,89/ Stück). Ich werde jetzt dann Nachkaufen. Das mit der Finanzierung war fast klar und ermutigt mich jetzt zum Nachkaufen. Cash war knapp und der Einstieg zu 5,75 mit 11 Mio. ist ein deutlcihes Signal, dass dieses Jahr noch einiges an Kurspotential vorhanden ist. Heute ist ein schönes intraday reversal zu sehen und der Kursabfall der letzten Tage deutet darauf hin, dass grössere Investoren den Kurs bewusst gedrückt haben (nach dem tollen Anstieg sicher auch nicht schwer gewesen)um Einzusteigen. Ich bin sehr zuversichtlich besonders was mda-7 angeht, p53 sehe ich nicht ganz so euphorisch.
warst mit dem Nachkaufen etwas früh dran. ich habe mir zunächst für 1000 Euro 150 Stück ins Depot gelegt (7,89/ Stück). Ich werde jetzt dann Nachkaufen. Das mit der Finanzierung war fast klar und ermutigt mich jetzt zum Nachkaufen. Cash war knapp und der Einstieg zu 5,75 mit 11 Mio. ist ein deutlcihes Signal, dass dieses Jahr noch einiges an Kurspotential vorhanden ist. Heute ist ein schönes intraday reversal zu sehen und der Kursabfall der letzten Tage deutet darauf hin, dass grössere Investoren den Kurs bewusst gedrückt haben (nach dem tollen Anstieg sicher auch nicht schwer gewesen)um Einzusteigen. Ich bin sehr zuversichtlich besonders was mda-7 angeht, p53 sehe ich nicht ganz so euphorisch.
Bekommt INGN vielleicht noch ein Fasttrack
The National Cancer Institute and Introgen Enter Clinical Trials Agreement For Development of Advexin
Tuesday June 24, 1:00 pm ET
Initial Study Includes New Use of Advexin as Mouthwash for Oral Premalignancies
WASHINGTON, June 24 /PRNewswire-FirstCall/ -- Introgen Therapeutics, Inc. (Nasdaq: INGN - News) announced today a Clinical Trials Agreement (CTA) with the Division of Cancer Treatment and Diagnosis (DCTD) of the National Cancer Institute (NCI) to co-develop Introgen`s p53 based cancer agent Advexin. The initial study under this Agreement will be a phase 1/2 study. Advexin will be administered in the form of an oral rinse, or mouthwash for oral premalignancies. Positive data from more than 20 clinical studies support the utility of Advexin in the treatment of established malignant tumors, and this trial will be the first to investigate the effect of Advexin on oral lesions that are at high risk for developing into full blown cancers. The clinical trial, conducted by multiple investigators, will be sponsored by DCTD-NCI. Introgen has supplied Advexin to NCI for distribution to clinical investigators.
"Introgen has developed considerable skill in the clinical use of the p53 gene to treat established cancers," said Dr. James Merritt, Introgen`s chief medical officer. "Cancer prevention is the ultimate goal, and the identification of critical genetic alterations in cancer, such as p53, represents in our view the most promising path to successful early disease intervention and prevention."
The goal of the study is to harness the body`s own cancer control mechanisms in order to destroy those cells that may become malignant. To achieve this, patients with premalignant lesions will be treated for up to six months with Advexin delivered as an oral rinse. Once inside precancerous cells, Advexin leads to the expression of high levels of p53, a protein that plays a central role in the normal pathways that cause damaged cells to undergo apoptosis (also known as cell suicide).
Through a previous Cooperative Research and Development Agreement established in 1999, Introgen and the NCI initiated evaluation of Advexin as an anti-cancer agent for a variety of cancers including breast, ovarian, bladder, liver, lung and brain cancers. The CTA expands and extends development of Advexin for the treatment and prevention of cancer.
Tobacco smoking and alcohol use are contributing factors to the development of oral cancer and the American Cancer Society estimates that in 2003 more than 180,000 cancer deaths are expected to be caused by tobacco use. It is estimated that 30,000 new cases of oral cavity cancer occur annually. Cancers of the oropharynx occur in approximately 4,000 patients annually in the United States. Frequently, cancers in this region of the body recur after surgery, chemotherapy, and radiotherapy; consequently new approaches to preventing recurrence are needed.
Advexin supplies p53 protein in very high concentrations in cancer tissue which selectively kills cancer cells while not harming the surrounding normal cells. P53 is a normal constituent of cells and is known as a tumor suppressor because it provides a powerful halt signal on cell growth. One of the major roles of this protein is to recognize when the cell has been damaged by mutation and to stop cell growth until the damage is repaired. If the cell is heavily damaged and beyond repair, p53 initiates the cell death pathway to prevent the cell from growing out of control. Scientists refer to this process as apoptosis. Apoptosis, or cell death, is a normal process that the body uses to eliminate damaged cells, precancerous cells and cells that are no longer necessary
gruss meislo
The National Cancer Institute and Introgen Enter Clinical Trials Agreement For Development of Advexin
Tuesday June 24, 1:00 pm ET
Initial Study Includes New Use of Advexin as Mouthwash for Oral Premalignancies
WASHINGTON, June 24 /PRNewswire-FirstCall/ -- Introgen Therapeutics, Inc. (Nasdaq: INGN - News) announced today a Clinical Trials Agreement (CTA) with the Division of Cancer Treatment and Diagnosis (DCTD) of the National Cancer Institute (NCI) to co-develop Introgen`s p53 based cancer agent Advexin. The initial study under this Agreement will be a phase 1/2 study. Advexin will be administered in the form of an oral rinse, or mouthwash for oral premalignancies. Positive data from more than 20 clinical studies support the utility of Advexin in the treatment of established malignant tumors, and this trial will be the first to investigate the effect of Advexin on oral lesions that are at high risk for developing into full blown cancers. The clinical trial, conducted by multiple investigators, will be sponsored by DCTD-NCI. Introgen has supplied Advexin to NCI for distribution to clinical investigators.
"Introgen has developed considerable skill in the clinical use of the p53 gene to treat established cancers," said Dr. James Merritt, Introgen`s chief medical officer. "Cancer prevention is the ultimate goal, and the identification of critical genetic alterations in cancer, such as p53, represents in our view the most promising path to successful early disease intervention and prevention."
The goal of the study is to harness the body`s own cancer control mechanisms in order to destroy those cells that may become malignant. To achieve this, patients with premalignant lesions will be treated for up to six months with Advexin delivered as an oral rinse. Once inside precancerous cells, Advexin leads to the expression of high levels of p53, a protein that plays a central role in the normal pathways that cause damaged cells to undergo apoptosis (also known as cell suicide).
Through a previous Cooperative Research and Development Agreement established in 1999, Introgen and the NCI initiated evaluation of Advexin as an anti-cancer agent for a variety of cancers including breast, ovarian, bladder, liver, lung and brain cancers. The CTA expands and extends development of Advexin for the treatment and prevention of cancer.
Tobacco smoking and alcohol use are contributing factors to the development of oral cancer and the American Cancer Society estimates that in 2003 more than 180,000 cancer deaths are expected to be caused by tobacco use. It is estimated that 30,000 new cases of oral cavity cancer occur annually. Cancers of the oropharynx occur in approximately 4,000 patients annually in the United States. Frequently, cancers in this region of the body recur after surgery, chemotherapy, and radiotherapy; consequently new approaches to preventing recurrence are needed.
Advexin supplies p53 protein in very high concentrations in cancer tissue which selectively kills cancer cells while not harming the surrounding normal cells. P53 is a normal constituent of cells and is known as a tumor suppressor because it provides a powerful halt signal on cell growth. One of the major roles of this protein is to recognize when the cell has been damaged by mutation and to stop cell growth until the damage is repaired. If the cell is heavily damaged and beyond repair, p53 initiates the cell death pathway to prevent the cell from growing out of control. Scientists refer to this process as apoptosis. Apoptosis, or cell death, is a normal process that the body uses to eliminate damaged cells, precancerous cells and cells that are no longer necessary
gruss meislo
Cancer Vaccine Trial Expands Introgen`s Application of Advexin; Advexin-Treated Dendritic Cells Orchestrate Anti-Tumor Immune Response
Thursday June 26, 10:38 am ET
AUSTIN, Texas, June 26 /PRNewswire-FirstCall/ -- Introgen Therapeutics, Inc. (Nasdaq: INGN - News) announced that treatments have been initiated in patients using its novel anti-cancer vaccine known as INGN 225. INGN 225 uses Introgen`s Advexin drug in a new way to create a highly specific therapeutic cancer vaccine. Previously published pre-clinical data demonstrated that 60 percent of animals treated with INGN 225 were protected against tumor development. When dendritic cells were activated, 100 percent protection against tumors was achieved.
ADVERTISEMENT
"This is an exciting progression of our effort to find viable vaccines against cancer," said Dmitry Gabrilovich, M.D., Associate Professor of Oncology at the H. Lee Moffitt Cancer Center. "This clinical study will allow us to test the principles we validated in preclinical studies that INGN 225, which uses the p53 gene, has utility in inducing the immune system to attack cancer cells."
The phase 1/2 study is being conducted at the H. Lee Moffitt Cancer Center, in Tampa, Fla., by principal investigators Dr. Scott Antonia, in the department of thoracic oncology and Dr. Dimitry Gabrilovich in the department of immunology. The study is enrolling patients with small-cell lung cancer, who first receive a standard treatment of chemotherapy, followed by INGN 225. The goal of the study is to demonstrate the safety of the treatment and the anticancer potency of the vaccine. The study is also designed to monitor the length of time that the patient`s cancer is kept from progressing.
The INGN 225 vaccine was developed based on work by Dr. Gabrilovich and by Dr. David Carbone, professor of oncology at The Vanderbilt-Ingram Cancer Center. Extensive preclinical testing from Introgen, its collaborators, and many independent scientists has shown that the immune system can recognize and respond to tumor associated p53. Introgen and its collaborators have demonstrated that induction of p53 immunity is safe in animal models.
"p53 mutations and alterations are a hallmark of cancer cells, and this strategy has successfully killed cancer cells throughout the body in preclinical models," said Dr. James Merritt, Introgen`s chief medical officer. "Thus, we believe a vaccine which employs p53 will likely have broad utility in the clinic."
To create the vaccine, Advexin is used to stimulate the patient`s dendritic cells, which are then used as a therapeutic vaccine to repeatedly immunize a patient against their cancer. This study represents the first time that a natural tumor suppressor molecule such as p53 has been used in patients as a vaccine. Dendritic cells are a key orchestrator of the immune response and are specifically responsible for activating the human immune system against specific "invaders," such as cancer cells. The actual killing of these targets is performed by lymphocytes known as CTLs or cytotoxic T lymphocytes. Simply put, dendritic cells present images of the cancer cell to T-lymphocytes and educate them to recognize tumor cells. These T-cells seek out cancer cells and kill them, functioning as a `smart bomb` to selectively eliminate its cancer cell target throughout the body. Efficiently and specifically harnessing the power of CTLs has been a long sought goal of cancer immunology
Thursday June 26, 10:38 am ET
AUSTIN, Texas, June 26 /PRNewswire-FirstCall/ -- Introgen Therapeutics, Inc. (Nasdaq: INGN - News) announced that treatments have been initiated in patients using its novel anti-cancer vaccine known as INGN 225. INGN 225 uses Introgen`s Advexin drug in a new way to create a highly specific therapeutic cancer vaccine. Previously published pre-clinical data demonstrated that 60 percent of animals treated with INGN 225 were protected against tumor development. When dendritic cells were activated, 100 percent protection against tumors was achieved.
ADVERTISEMENT
"This is an exciting progression of our effort to find viable vaccines against cancer," said Dmitry Gabrilovich, M.D., Associate Professor of Oncology at the H. Lee Moffitt Cancer Center. "This clinical study will allow us to test the principles we validated in preclinical studies that INGN 225, which uses the p53 gene, has utility in inducing the immune system to attack cancer cells."
The phase 1/2 study is being conducted at the H. Lee Moffitt Cancer Center, in Tampa, Fla., by principal investigators Dr. Scott Antonia, in the department of thoracic oncology and Dr. Dimitry Gabrilovich in the department of immunology. The study is enrolling patients with small-cell lung cancer, who first receive a standard treatment of chemotherapy, followed by INGN 225. The goal of the study is to demonstrate the safety of the treatment and the anticancer potency of the vaccine. The study is also designed to monitor the length of time that the patient`s cancer is kept from progressing.
The INGN 225 vaccine was developed based on work by Dr. Gabrilovich and by Dr. David Carbone, professor of oncology at The Vanderbilt-Ingram Cancer Center. Extensive preclinical testing from Introgen, its collaborators, and many independent scientists has shown that the immune system can recognize and respond to tumor associated p53. Introgen and its collaborators have demonstrated that induction of p53 immunity is safe in animal models.
"p53 mutations and alterations are a hallmark of cancer cells, and this strategy has successfully killed cancer cells throughout the body in preclinical models," said Dr. James Merritt, Introgen`s chief medical officer. "Thus, we believe a vaccine which employs p53 will likely have broad utility in the clinic."
To create the vaccine, Advexin is used to stimulate the patient`s dendritic cells, which are then used as a therapeutic vaccine to repeatedly immunize a patient against their cancer. This study represents the first time that a natural tumor suppressor molecule such as p53 has been used in patients as a vaccine. Dendritic cells are a key orchestrator of the immune response and are specifically responsible for activating the human immune system against specific "invaders," such as cancer cells. The actual killing of these targets is performed by lymphocytes known as CTLs or cytotoxic T lymphocytes. Simply put, dendritic cells present images of the cancer cell to T-lymphocytes and educate them to recognize tumor cells. These T-cells seek out cancer cells and kill them, functioning as a `smart bomb` to selectively eliminate its cancer cell target throughout the body. Efficiently and specifically harnessing the power of CTLs has been a long sought goal of cancer immunology
J Thorac Cardiovasc Surg. 2003 Jun;125(6):1328-35.
Adenoviral melanoma differentiation-associated gene 7 induces apoptosis in lung cancer cells through mitochondrial permeability transition-independent cytochrome c release.
Pataer A, Chada S, Hunt KK, Roth JA, Swisher SG.
Section of Thoracic Molecular Oncology, Departments of Thoracic and Cardiovascular Surgery and Surgical Oncology, The University of Texas M.D. Anderson Cancer Center, and Introgen Therapeutics Inc, Houston, Tex.
OBJECTIVE: Melanoma differentiation-associated gene 7 is a novel tumor suppressor gene that induces apoptosis in lung cancer cells when delivered by adenoviral gene transfer as Ad-mda7. The mechanisms of action are not well defined but may involve release of cytochrome c from the mitochondria with subsequent caspase activation. METHODS: The lung cancer cell lines A549 and H1299 were transduced with Ad-mda7, adenovirus containing the gene for p53 (Ad-p53), and control adenoviral luciferase vectors. Staurosporine was used as a positive control to induce cytochrome c release through mitochondrial permeability transition-dependent pores, whereas cyclosporine (INN: ciclosporin) was used to specifically inhibit these mitochondrial permeability transition-dependent pores. Apoptosis was evaluated with fluorescence-activated cell sorting analysis of subdiploid populations and mitochondrial membrane potential changes with tetramethylrhodamine ethylester perchlorate. RESULTS: Melanoma differentiation-associated gene 7, transduced by Ad-mda7 into H1299 and A549 lung cancer cells, resulted in sharp increases in cytosolic cytochrome c levels followed by induction of apoptosis and cellular death. The release of cytochrome c from the mitochondria occurred without changes in the mitochondrial membrane potential. Unlike staurosporine treatment, transduction with Ad-p53 and Ad-mda7 caused releases of cytochrome c and apoptosis that were not blocked by cyclosporine, suggesting a mitochondrial permeability transition pore-independent pathway. CONCLUSIONS: Ad-mda7 induces apoptosis in lung cancer cells through mitochondrial cytochrome c release in a process that is not dependent on mitochondrial membrane potential changes and occurs through mitochondrial permeability transition-independent pores. This unique mechanism of action may allow treatment of patients with lung cancer resistant to mitochondrial permeability transition-dependent cell death processes.
Just an in vitro study, but Ad mda-7/ Ad p53 clearly demonstrate biologic activity compared to Ad "placebo"(luciferase). I am convinced adenoviral gene therapy also shows clinical efficiency when compared to control groups. We´ll see in a few months. Nevertheless, I don´t regard Advexin as the CURE for cancer, since vector technology has to be improved. But Advexin will become an very important form of cancer therapy especially in combination with other anticancer drugs and/or radiation.
Adenoviral melanoma differentiation-associated gene 7 induces apoptosis in lung cancer cells through mitochondrial permeability transition-independent cytochrome c release.
Pataer A, Chada S, Hunt KK, Roth JA, Swisher SG.
Section of Thoracic Molecular Oncology, Departments of Thoracic and Cardiovascular Surgery and Surgical Oncology, The University of Texas M.D. Anderson Cancer Center, and Introgen Therapeutics Inc, Houston, Tex.
OBJECTIVE: Melanoma differentiation-associated gene 7 is a novel tumor suppressor gene that induces apoptosis in lung cancer cells when delivered by adenoviral gene transfer as Ad-mda7. The mechanisms of action are not well defined but may involve release of cytochrome c from the mitochondria with subsequent caspase activation. METHODS: The lung cancer cell lines A549 and H1299 were transduced with Ad-mda7, adenovirus containing the gene for p53 (Ad-p53), and control adenoviral luciferase vectors. Staurosporine was used as a positive control to induce cytochrome c release through mitochondrial permeability transition-dependent pores, whereas cyclosporine (INN: ciclosporin) was used to specifically inhibit these mitochondrial permeability transition-dependent pores. Apoptosis was evaluated with fluorescence-activated cell sorting analysis of subdiploid populations and mitochondrial membrane potential changes with tetramethylrhodamine ethylester perchlorate. RESULTS: Melanoma differentiation-associated gene 7, transduced by Ad-mda7 into H1299 and A549 lung cancer cells, resulted in sharp increases in cytosolic cytochrome c levels followed by induction of apoptosis and cellular death. The release of cytochrome c from the mitochondria occurred without changes in the mitochondrial membrane potential. Unlike staurosporine treatment, transduction with Ad-p53 and Ad-mda7 caused releases of cytochrome c and apoptosis that were not blocked by cyclosporine, suggesting a mitochondrial permeability transition pore-independent pathway. CONCLUSIONS: Ad-mda7 induces apoptosis in lung cancer cells through mitochondrial cytochrome c release in a process that is not dependent on mitochondrial membrane potential changes and occurs through mitochondrial permeability transition-independent pores. This unique mechanism of action may allow treatment of patients with lung cancer resistant to mitochondrial permeability transition-dependent cell death processes.
Just an in vitro study, but Ad mda-7/ Ad p53 clearly demonstrate biologic activity compared to Ad "placebo"(luciferase). I am convinced adenoviral gene therapy also shows clinical efficiency when compared to control groups. We´ll see in a few months. Nevertheless, I don´t regard Advexin as the CURE for cancer, since vector technology has to be improved. But Advexin will become an very important form of cancer therapy especially in combination with other anticancer drugs and/or radiation.
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +0,04 | |
| +2,36 | |
| -0,20 | |
| -0,93 | |
| -0,92 | |
| -1,86 | |
| -0,39 | |
| +0,35 | |
| 0,00 | |
| -1,92 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 60 | ||
| 37 | ||
| 19 | ||
| 18 | ||
| 17 | ||
| 17 | ||
| 17 | ||
| 15 | ||
| 14 | ||
| 14 |





















