ANALYSE: Akorn-Aktie möglicherweise vor noch größerem Absturz - Raymond James - 500 Beiträge pro Seite | Diskussion im Forum
eröffnet am 01.03.18 21:25:17 von
neuester Beitrag 07.10.20 22:22:31 von
neuester Beitrag 07.10.20 22:22:31 von
Beiträge: 146
ID: 1.275.357
ID: 1.275.357
Aufrufe heute: 0
Gesamt: 8.853
Gesamt: 8.853
Aktive User: 0
ISIN: US0097281069 · WKN: 888920
0,0270
USD
0,00 %
0,0000 USD
Letzter Kurs 03.10.20 Nasdaq OTC
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,6500 | +25,75 | |
| 6,0000 | +25,00 | |
| 56,69 | +20,00 | |
| 0,6400 | +18,52 | |
| 491,30 | +17,07 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 2,6000 | -12,16 | |
| 8,5000 | -15,00 | |
| 0,7300 | -18,59 | |
| 92,06 | -19,84 | |
| 2,7280 | -29,14 |
Es handelt sich um einen automatisiert angelegten Thread zur Nachricht "ANALYSE: Akorn-Aktie möglicherweise vor noch größerem Absturz - Raymond James" vom Autor dpa-AFX
Das Investmenthaus Raymond James zeichnet angesichts der aktuellen Probleme beim US-Generikahersteller Akorn für dessen Aktie ein düsteres Szenario. Der deutsche Gesundheitskonzern Fresenius SE will Akorn übernehmen, doch mögliche …
Lesen Sie den ganzen Artikel: ANALYSE: Akorn-Aktie möglicherweise vor noch größerem Absturz - Raymond James
Das Investmenthaus Raymond James zeichnet angesichts der aktuellen Probleme beim US-Generikahersteller Akorn für dessen Aktie ein düsteres Szenario. Der deutsche Gesundheitskonzern Fresenius SE will Akorn übernehmen, doch mögliche …
Lesen Sie den ganzen Artikel: ANALYSE: Akorn-Aktie möglicherweise vor noch größerem Absturz - Raymond James
http://investors.akorn.com/phoenix.zhtml?c=78132&p=irol-news…
=>
Akorn Issues Statement on Investigation
LAKE FOREST, Ill., Feb. 26, 2018 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq:AKRX), a leading specialty generic pharmaceutical company, today issued the following statement:
“Akorn and Fresenius Kabi AG, with the assistance of outside consultants, are investigating alleged breaches of FDA data integrity requirements relating to product development at the Company. To date, the Company’s investigation has not found any facts that would result in a material impact on Akorn’s operations and the Company does not believe this investigation should affect the closing of the transaction with Fresenius. The Company does not intend to provide further updates as the investigation proceeds. The Company is continuing to work to obtain regulatory clearance for the transaction.”
=>
Akorn Issues Statement on Investigation
LAKE FOREST, Ill., Feb. 26, 2018 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq:AKRX), a leading specialty generic pharmaceutical company, today issued the following statement:
“Akorn and Fresenius Kabi AG, with the assistance of outside consultants, are investigating alleged breaches of FDA data integrity requirements relating to product development at the Company. To date, the Company’s investigation has not found any facts that would result in a material impact on Akorn’s operations and the Company does not believe this investigation should affect the closing of the transaction with Fresenius. The Company does not intend to provide further updates as the investigation proceeds. The Company is continuing to work to obtain regulatory clearance for the transaction.”
Antwort auf Beitrag Nr.: 57.164.457 von faultcode am 01.03.18 21:25:17=>
* FRE fällt heute deutlich
* AKRX steigt heute deutlich ??
=> ich leg mir hier mal ne kleine Posi zu...
Frei nach dem Motto von George Soros (*):
Invest first and then investigate.
(*) https://www.elitetrader.com/et/threads/george-soros-on-tradi…
* FRE fällt heute deutlich
* AKRX steigt heute deutlich ??
=> ich leg mir hier mal ne kleine Posi zu...
Frei nach dem Motto von George Soros (*):
Invest first and then investigate.
(*) https://www.elitetrader.com/et/threads/george-soros-on-tradi…
Antwort auf Beitrag Nr.: 57.164.628 von faultcode am 01.03.18 21:45:39
da Fresenius SE nun das Kneifen anfängt, indem sie einen Vorwand suchen, aus dem Deal herauszukommen, nachdem sie wohl feststellten, dass sie - in ihrer Anfangseuphorie - zuviel Geld bezahlen müssten
=> ob's wirklich so laufen wird bis Jahresende?
Akorn (AKRX) Issues Statement as Fresenius Seeks to Terminate Merger
https://www.streetinsider.com/Corporate+News/Akorn+%28AKRX%2…
=>
“We categorically disagree with Fresenius’ accusations. The previously disclosed ongoing investigation, which is not a condition to closing, has not found any facts that would result in a material adverse effect on Akorn’s business and therefore there is no basis to terminate the transaction.
We intend to vigorously enforce our rights, and Fresenius’ obligations, under our binding merger agreement.”
=> da hat jemand bei Fresenius (Kabis) nicht richtig aufgepasst
heute mit Expansion Breakdown
(noch unbestätigt)da Fresenius SE nun das Kneifen anfängt, indem sie einen Vorwand suchen, aus dem Deal herauszukommen, nachdem sie wohl feststellten, dass sie - in ihrer Anfangseuphorie - zuviel Geld bezahlen müssten


=> ob's wirklich so laufen wird bis Jahresende?
Akorn (AKRX) Issues Statement as Fresenius Seeks to Terminate Merger
https://www.streetinsider.com/Corporate+News/Akorn+%28AKRX%2…
=>
“We categorically disagree with Fresenius’ accusations. The previously disclosed ongoing investigation, which is not a condition to closing, has not found any facts that would result in a material adverse effect on Akorn’s business and therefore there is no basis to terminate the transaction.
We intend to vigorously enforce our rights, and Fresenius’ obligations, under our binding merger agreement.”
=> da hat jemand bei Fresenius (Kabis) nicht richtig aufgepasst

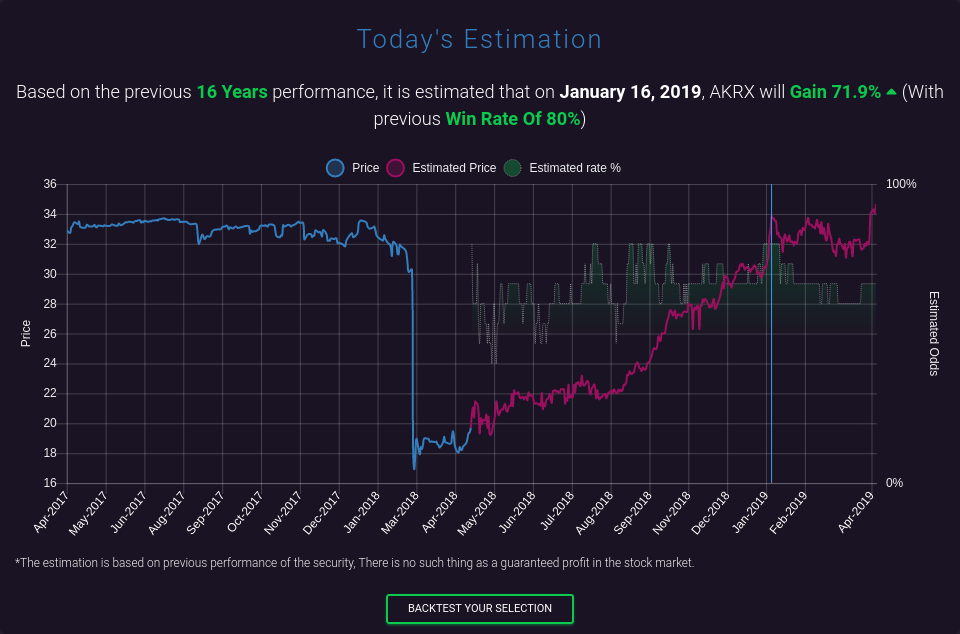
Antwort auf Beitrag Nr.: 57.606.063 von faultcode am 23.04.18 12:45:37pre-market standen mal kurz USD12.99 an
--> den Gefallen wird mir der Markt aber wohl nicht mehr machen heute --> dennoch "3-Tage-Regel" abwarten --> Mittwoch im Laufe des Tages noch mal vorbeischauen..
mein Total Fair Value (konversativ) liegt momentan bei USD8.40 mit:
- mat.book value -USD1.79 (2016 (*)) --> AR2017 noch immer nicht draussen --> wird aber wohl auch zum 31.12.2017 <0 sein
- EPS2018e: USD0.61
__FactSet: USD0.62
__Thomson Reuters: USD0.05 !!
- CAGR EPS 2018e-2028e: 10%
- term.CAGR EPS 2029e-2038e: 2%
- discount rate: 10%
=> durch die vielen M&A-Aktivitäten ist sowohl Umsatz-, Cash Flow- und Ergebnis-Stabilität praktisch nicht gegeben
=> aber unter USD10 pro Aktie kann man mMn nichts falsch machen, ganz unabhängig davon wie die FRE-Geschichte ausgehen wird!
(*) Ende 2016 schleppte AKRX grob USD1b an Goodwill und intangible assets mit sich herum!
--> den Gefallen wird mir der Markt aber wohl nicht mehr machen heute --> dennoch "3-Tage-Regel" abwarten --> Mittwoch im Laufe des Tages noch mal vorbeischauen..
mein Total Fair Value (konversativ) liegt momentan bei USD8.40 mit:
- mat.book value -USD1.79 (2016 (*)) --> AR2017 noch immer nicht draussen --> wird aber wohl auch zum 31.12.2017 <0 sein
- EPS2018e: USD0.61
__FactSet: USD0.62
__Thomson Reuters: USD0.05 !!
- CAGR EPS 2018e-2028e: 10%
- term.CAGR EPS 2029e-2038e: 2%
- discount rate: 10%
=> durch die vielen M&A-Aktivitäten ist sowohl Umsatz-, Cash Flow- und Ergebnis-Stabilität praktisch nicht gegeben
=> aber unter USD10 pro Aktie kann man mMn nichts falsch machen, ganz unabhängig davon wie die FRE-Geschichte ausgehen wird!
(*) Ende 2016 schleppte AKRX grob USD1b an Goodwill und intangible assets mit sich herum!
Antwort auf Beitrag Nr.: 57.606.534 von faultcode am 23.04.18 13:43:45
=>
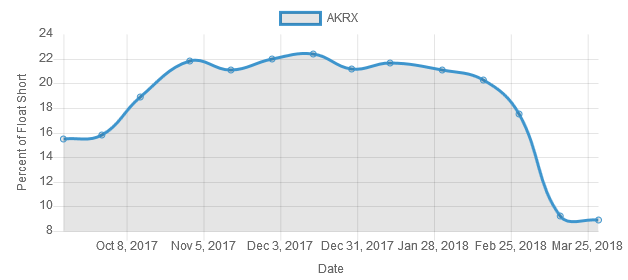
Shortquote noch bei 8...9%
https://www.shortpainbot.com/?s=akrx=>

Antwort auf Beitrag Nr.: 57.606.063 von faultcode am 23.04.18 12:45:37
http://investors.akorn.com/phoenix.zhtml?c=78132&p=irol-news…
=>
Akorn, Inc. (NASDAQ:AKRX), today filed a complaint in Delaware Chancery Court asking that Fresenius Kabi AG be required to fulfill its obligations under the definitive merger agreement, and issued the following statement:
“Fresenius’ attempt to terminate the transaction on the pretext that the findings from the ongoing investigation are a breach of the merger agreement is completely without merit.
The previously disclosed ongoing investigation, of which we have voluntarily notified and are in regular communication with the Food and Drug Administration, has not found any facts that would result in a material adverse effect on Akorn’s business and therefore there is no basis to terminate the transaction.
The investigation is not a condition to closing and the only remaining condition is approval from the Federal Trade Commission. We intend to vigorously enforce our rights, and Fresenius’ obligations, under our binding merger agreement.”...
Klage seitens Akorn schon da
Akorn Asks Delaware Court to Require Fresenius Kabi to Fulfill Its Obligations under Merger Agreementhttp://investors.akorn.com/phoenix.zhtml?c=78132&p=irol-news…
=>
Akorn, Inc. (NASDAQ:AKRX), today filed a complaint in Delaware Chancery Court asking that Fresenius Kabi AG be required to fulfill its obligations under the definitive merger agreement, and issued the following statement:
“Fresenius’ attempt to terminate the transaction on the pretext that the findings from the ongoing investigation are a breach of the merger agreement is completely without merit.
The previously disclosed ongoing investigation, of which we have voluntarily notified and are in regular communication with the Food and Drug Administration, has not found any facts that would result in a material adverse effect on Akorn’s business and therefore there is no basis to terminate the transaction.
The investigation is not a condition to closing and the only remaining condition is approval from the Federal Trade Commission. We intend to vigorously enforce our rights, and Fresenius’ obligations, under our binding merger agreement.”...
ist der Abschlag da übertrieben ?
Antwort auf Beitrag Nr.: 57.607.584 von Hufeisenmagnet am 23.04.18 15:24:28wenn man das wüsste es wird auf jedenfall eine schöne Summe Geld fliessen Vertrag ist Vertrag und die müssen erfüllt werden oder es muss gezahlt werden
Antwort auf Beitrag Nr.: 57.608.055 von dig101 am 23.04.18 16:10:47das mit dem Vertrag ist aber strittig..............
Antwort auf Beitrag Nr.: 57.608.055 von dig101 am 23.04.18 16:10:47Hallo Dig101,
sehr optimistische Einschätzung. Ich nehme an Du hast den Vertrag gelesen, den der Freseniusvorstand behauptet, dass eine Kündigung ohne Ausgleich lt. Vertrag möglich ist.
Okay der Akornvorstand behauptet dass eine Kündigung nicht möglich ist, aber der behauptet ja auch sich mit seinen Produkten an die gesetzlichen Vorgaben gehalten zu haben.
Der Markt tendiert jedenfalls deutlich.
Gruß
Schlummschütze
sehr optimistische Einschätzung. Ich nehme an Du hast den Vertrag gelesen, den der Freseniusvorstand behauptet, dass eine Kündigung ohne Ausgleich lt. Vertrag möglich ist.
Okay der Akornvorstand behauptet dass eine Kündigung nicht möglich ist, aber der behauptet ja auch sich mit seinen Produkten an die gesetzlichen Vorgaben gehalten zu haben.
Der Markt tendiert jedenfalls deutlich.
Gruß
Schlummschütze
Antwort auf Beitrag Nr.: 57.608.154 von Hufeisenmagnet am 23.04.18 16:20:59Sagt Fres. am Ende ist es ein Vertrag der erfüllt werden muss
informieren muss man sich auf jeden Fall
Der Chart sieht für mich gefährlich und zugleich interessant aus.
Eröffnung 14,110EUR
Tageshoch 14,110EUR
Tagestief 8,000EUR
Vortageskurs 16,020EUR
Volumen 27,09 Tsd.Stk.
52-Wochen Hoch 16,100EUR
52-Wochen Tief 13,900EUR
ich riskier erst mal einen Blick
Der Chart sieht für mich gefährlich und zugleich interessant aus.
Einfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
Eröffnung 14,110EUR
Tageshoch 14,110EUR
Tagestief 8,000EUR
Vortageskurs 16,020EUR
Volumen 27,09 Tsd.Stk.
52-Wochen Hoch 16,100EUR
52-Wochen Tief 13,900EUR
ich riskier erst mal einen Blick

Antwort auf Beitrag Nr.: 57.608.205 von Schlummschuetze am 23.04.18 16:25:25Tja wer hat jetzt recht falls fre. aber nicht recht hat sollte der kurs sehr schnell sehr hoch wieder gehen
Die Frage ist wer schliesst so einen Vertrag ab ich glaube niemand die leute müssten sehr dumm sein einen Vertrag abzuschließen den man jederzeit ohne Strafe kündigen kann.
Die Frage ist wer schliesst so einen Vertrag ab ich glaube niemand die leute müssten sehr dumm sein einen Vertrag abzuschließen den man jederzeit ohne Strafe kündigen kann.
Antwort auf Beitrag Nr.: 57.608.268 von Hufeisenmagnet am 23.04.18 16:30:36Falls hier ein paar 100 $ an Abfindung fliessen sollte man schnell wieder sehr hoch steigen vielleicht sogar über 20 $ .
Antwort auf Beitrag Nr.: 57.608.436 von dig101 am 23.04.18 16:45:24So richtig überzeugst du mich nicht.
Da lese ich liebe die News und mach mir mein eigenes Bild.
Du schmeisst hier mit Zahlen um dich, da finde ich nix konkretes und sachliches
Da lese ich liebe die News und mach mir mein eigenes Bild.
Du schmeisst hier mit Zahlen um dich, da finde ich nix konkretes und sachliches
Antwort auf Beitrag Nr.: 57.608.577 von Hufeisenmagnet am 23.04.18 17:00:53Am Ende wird man sich schon einigen aber nix bekommen bei Vertragsbruch kann ich mir schwer vorstellen.
Fres. will einfach nur günstig aus der Sache raus kommen und dafür sind alle Mittel recht.
Der Kurs wird zeigen wer recht hat die erste Panik ist vorbei und vielleicht kauft sich fres wieder günstig ein
Fres. will einfach nur günstig aus der Sache raus kommen und dafür sind alle Mittel recht.
Der Kurs wird zeigen wer recht hat die erste Panik ist vorbei und vielleicht kauft sich fres wieder günstig ein
Antwort auf Beitrag Nr.: 57.608.322 von dig101 am 23.04.18 16:35:20Hi Dig101.
grundsätzlich sind Verträge dazu da um gebrochen zu werden.
Fre beruft sich ja auch darauf, dass Akorn gegen mehrere Punkte verstossen hätte.
Akorn muss seinerseits unabhängig von den Chancen Klagen, da ihnen die
Anwälte der Aktionäre im Nacken sitzen, die Akorn verklagt haben, wegen Verschweigens
von negativen kursrelevanten Aussichten.
Also selbst bei Abfindung wird viel Geld direkt wieder aus Akorn rausgesaugt.
Übernahme würde ich immer noch nicht ausschließen, erwarte dann aber einen großen
Preisnachlass (mindestens 50 %). Wäre natürlich ein deutliches Aufgeld zu aktuellen Kursen
und für beide Seiten vielleicht immer noch die beste Lösung.
Ansonsten wird es eng für Akorn (hohe Umsatzeinbrüche, hohe Verluste, mit Rechtsfällen beschäftigter Vorstand, der sich nicht ausreichend um die eigentlichen Geschäfte kümmern kann.).
Gruß
Schlummschütze
grundsätzlich sind Verträge dazu da um gebrochen zu werden.

Fre beruft sich ja auch darauf, dass Akorn gegen mehrere Punkte verstossen hätte.
Akorn muss seinerseits unabhängig von den Chancen Klagen, da ihnen die
Anwälte der Aktionäre im Nacken sitzen, die Akorn verklagt haben, wegen Verschweigens
von negativen kursrelevanten Aussichten.
Also selbst bei Abfindung wird viel Geld direkt wieder aus Akorn rausgesaugt.
Übernahme würde ich immer noch nicht ausschließen, erwarte dann aber einen großen
Preisnachlass (mindestens 50 %). Wäre natürlich ein deutliches Aufgeld zu aktuellen Kursen
und für beide Seiten vielleicht immer noch die beste Lösung.
Ansonsten wird es eng für Akorn (hohe Umsatzeinbrüche, hohe Verluste, mit Rechtsfällen beschäftigter Vorstand, der sich nicht ausreichend um die eigentlichen Geschäfte kümmern kann.).
Gruß
Schlummschütze
aus einer Sekundärquelle:
"Im Übernahmevertrag hatten die Bad Homburger keine Auflösungsgebühr im Falle eines Scheiterns (Breakup fee) vereinbart. Fresenius bestätigte entsprechend seine Jahresprognose für Konzernergebnis und -umsatz."
https://boerse.ard.de/aktien/fresenius-will-akorn-nicht100.h…
"Im Übernahmevertrag hatten die Bad Homburger keine Auflösungsgebühr im Falle eines Scheiterns (Breakup fee) vereinbart. Fresenius bestätigte entsprechend seine Jahresprognose für Konzernergebnis und -umsatz."
https://boerse.ard.de/aktien/fresenius-will-akorn-nicht100.h…
Akorn zieht nach Übernahme-Absage gegen Fresenius vor Gericht
Der US-Generikahersteller weist die Vorwürfe des Gesundheitskonzerns kategorisch zurück.
Fresenius droht nun ein juristisches Nachspiel.
http://www.handelsblatt.com/unternehmen/industrie/us-generik…
da geht die Schlammschlacht los................
Der US-Generikahersteller weist die Vorwürfe des Gesundheitskonzerns kategorisch zurück.
Fresenius droht nun ein juristisches Nachspiel.
http://www.handelsblatt.com/unternehmen/industrie/us-generik…
da geht die Schlammschlacht los................
Antwort auf Beitrag Nr.: 57.608.994 von Global-Player83 am 23.04.18 17:37:40Würde schreiben dumm gelaufen wenn es wirklich so ist.
Ganz ehrlich, wenn Fresenius Akorn übernehmen will/wollte, dann diktieren die doch die Konditionen, Strafen etc.
Und was soll Akorn seinen Aktionären denn sagen? "Ja, Fresenius hatte recht, es gab Unregelmäßigkeiten mit der FDA". Dann könnten die Chefs aber ihren Hut nehmen, so hat man stillschweigen mit Fresenius vereinbart und zieht eine Pseudo Klage durch, bei der mann sich als Opfer darstellt. Der US-Generika Hersteller steht im harten Konkurrenzkampf und der Preisdruck macht sich negativ auf die Gewinnentwicklung bemerkbar. Wenn es "innenpolitisch" nicht so läuft, muss man halt "außenpolitisch" (für Ablenkung Sorgen = Klage) auf die Pauke hauen.
Und was soll Akorn seinen Aktionären denn sagen? "Ja, Fresenius hatte recht, es gab Unregelmäßigkeiten mit der FDA". Dann könnten die Chefs aber ihren Hut nehmen, so hat man stillschweigen mit Fresenius vereinbart und zieht eine Pseudo Klage durch, bei der mann sich als Opfer darstellt. Der US-Generika Hersteller steht im harten Konkurrenzkampf und der Preisdruck macht sich negativ auf die Gewinnentwicklung bemerkbar. Wenn es "innenpolitisch" nicht so läuft, muss man halt "außenpolitisch" (für Ablenkung Sorgen = Klage) auf die Pauke hauen.
Fresenius alleges 'blatant fraud' at U.S. drugmaker Akorn
heute (auch) <-15% wg.: https://www.reuters.com/article/us-akorn-m-a-fresenius/frese…=>
...German healthcare group Fresenius (FREG.DE) alleged it uncovered “blatant fraud at the very top level” of U.S. generic drugmaker Akorn Inc (AKRX.O) after Fresenius agreed to acquire the company for $4.75 billion, according to a court filing made public late on Tuesday...
=> The court scheduled the trial to begin on July 9.
...
The drugmaker will ask a Delaware judge at a hearing on Tuesday to fast-track its lawsuit and schedule a trial as soon as next month, according to court documents.
Fresenius wants the trial to be held in January.

...
Akorn said in its lawsuit last month that Fresenius uncovered data integrity problems that are common in the generic drug industry and is seizing on them to try to back out of a deal it soured on for financial reasons.
=> ehrlich: ich sehe das auch so.
But Fresenius alleged that an Akorn executive vice president for quality assurance, whose name was redacted from the court filing, knowingly directed the submission of fraudulent testing data to the U.S. Food and Drug Administration.
The fabricated data concerned Akorn’s application to market the antibiotic azithromycin, and Fresenius alleged the fraudulent scheme began in 2012.
Fresenius also alleged that “the same scheme has infected” at least five other Akorn products.
In the court filing, Fresenius said its investigation revealed “blatant fraud at the very top level of Akorn’s executive team, stunning evidence of blatant and pervasive data integrity violations.”
Akorn has said in court documents it investigated the possible submission of falsified data and fired an executive who was involved.
“Critically, azithromycin and the five other drug products in question either have never been marketed or are not currently being marketed and were never forecasted to form a material portion of Akorn’s future earnings,” the company said in one of the documents.
=> als ob Datenschummel in der Pharmaindustrie was ganz Neues wäre!

--> d.h., ich gehe davon aus, dass AKRX so schummelt wie im Prinzip alle anderen auch -- inklusive Fresenius Kabi

--> oder im Umkehrschluss: das ist nicht ungefährlich für Fresenius (im wichtigen US-Markt), wenn sie sich dort wie die grossen Aufklärer und Aufräumer aufführen...
ich gehe von folgenden Annahmen aus:
=> Datenschummel seitens Akorn hat stattgefunden
=> Fresenius nutzt das als "Vorwand" um die geplante Übernahme abzusagen
=> u.a vor allem weil die Kennzahlen von Akorn sich deutlich eingetrübt haben
"It’s easy to see why Fresenius might want out; the deal “math has clearly changed,” Stanicky noted, adding that Akorn is expecting 2018 earnings of $156 million, well below Fresenius’ initial outlook of $380 million ot $420 million. And the FTC may be requiring divestments that Fresenius isn’t keen on, pressuring the equation even further.
But “worsened economics do not impact FRE's obligation to close this deal. Hence the focus on ‘failure to fulfill closing conditions,’” Stanicky wrote."
Quelle: https://www.fiercepharma.com/pharma/fresenius-dumps-4-3b-ako…
Die für mich spannendere Frage ist, wo liegt der faire Wert der Akorn-Aktie ohne Berücksichtigung des Übernahme-Streits?
=> Datenschummel seitens Akorn hat stattgefunden
=> Fresenius nutzt das als "Vorwand" um die geplante Übernahme abzusagen
=> u.a vor allem weil die Kennzahlen von Akorn sich deutlich eingetrübt haben
"It’s easy to see why Fresenius might want out; the deal “math has clearly changed,” Stanicky noted, adding that Akorn is expecting 2018 earnings of $156 million, well below Fresenius’ initial outlook of $380 million ot $420 million. And the FTC may be requiring divestments that Fresenius isn’t keen on, pressuring the equation even further.
But “worsened economics do not impact FRE's obligation to close this deal. Hence the focus on ‘failure to fulfill closing conditions,’” Stanicky wrote."
Quelle: https://www.fiercepharma.com/pharma/fresenius-dumps-4-3b-ako…
Die für mich spannendere Frage ist, wo liegt der faire Wert der Akorn-Aktie ohne Berücksichtigung des Übernahme-Streits?
Antwort auf Beitrag Nr.: 57.685.269 von Global-Player83 am 03.05.18 12:28:14Hi,
ohne die Übernahmephantasie ist Akorn wohl wertlos.
Akorn schreibt rote Zahlen, die Umsätze brechen weg, eine Lawine von Prozessen (mit Fresenius, die Schadenersatzforderungen der sich betrogen fühlenden eigenen Aktionäre, Betrug bei Medikamentenzulassungen und damit auch hier Bußgelder und Schadenersatzprozesse)-
Wenn die Freseniusbehauptungen stimmen bleibt nicht mehr viel.
Gruß
Schlummschütze
ohne die Übernahmephantasie ist Akorn wohl wertlos.
Akorn schreibt rote Zahlen, die Umsätze brechen weg, eine Lawine von Prozessen (mit Fresenius, die Schadenersatzforderungen der sich betrogen fühlenden eigenen Aktionäre, Betrug bei Medikamentenzulassungen und damit auch hier Bußgelder und Schadenersatzprozesse)-
Wenn die Freseniusbehauptungen stimmen bleibt nicht mehr viel.
Gruß
Schlummschütze
Antwort auf Beitrag Nr.: 57.692.418 von Schlummschuetze am 04.05.18 08:57:59
=> das wäre aus Sicht von Fresenius aber unlogisch. Gut, es steht das einschränkende Wort "wohl" oben dabei.
Denn dann stellt sich die Frage: was wollte Fresenius (Kabi) eigentlich von Akorn?
=> vor diesem Hintergrund gibt es ja nur noch zwei Möglichkeiten:
(A) Fresenius (Kabi) ist zu doof Übernahmeziele hinreichend zu prüfen:
...Stephan Sturm, Vorstandsvorsitzender von Fresenius, sagte: „Mit diesen Akquisitionen (FC: gemeint Merck Biosimilar + Akorn) stellen wir bei Fresenius Kabi die Weichen für ein noch breiter angelegtes und dauerhaft kräftiges Wachstum über das laufende Jahrzehnt hinaus. Akorn ergänzt das Produktangebot von Fresenius Kabi im Generika-Geschäft bestens.
...
Die US-amerikanischen Unternehmenszentralen von Akorn und Fresenius Kabi liegen nur wenige Kilometer voneinander entfernt im Norden des US-Bundesstaats Illinois...
aus: https://www.fresenius.de/5770
--> diese ganz Meldung überschlägt sich fast für Freude auf das Geschäft von Akorn
=> dagegen spricht aber der M&A-Trackrekord von Fresenius und von auch von Stephan Sturm als CFO und später CEO (2016) von Fresenius
--> OK, vielleicht war das nun sein erster grosser Fehler bei Fresenius (als CEO)
oder
(B) man ist auf ganz hinterhältige Betrüger reingefallen, was man nicht absehen konnte bei einem Übernahmeziel in der Nachbarschaft:
- ich habe auf Google Maps nachgemessen: es sind vom:
Three Corporate Drive Lake Zurich, IL 60047 (FRE/HQ)
bis
Lake Forest, IL, (AKRX/HQ)
nur 15 miles = 30min Autofahrt!
=> das ist keine Fern-Übernahme. Beide Standorte sind etabliert, auch mit Produktion (Manufacturing Locations), z.B.:
FRE:
* Melrose Park village, Cook County, IL
<-- 180 miles -->
AKRX:
* 1222 W. Grand Ave., Decatur, IL
=> kaum zu glauben, dass nicht mal Info's und Personen hin und her gegangen sind
- in IL alleine arbeiten jeweils mehrere hundert Personen bei Fresenius Kabi, als auch bei Akorn
=> und da soll ich glauben, dass die eine Gruppe über Jahre ruchlose Betrüger sind ("unter anderen schwerwiegende Verstöße gegen FDA-Vorgaben", FRE, 22.4.), die nur noch auf naive Deutsche mit ihrem "stupid German money" gewartet haben?
Einer Gruppe, die auch regelmässig von der FDA (indirekt wenigstens) kontrolliert wird?
Im Heimatland der FDA-Regularien wie 21 CFR Part 11, denen sich auch alle europäischen Pharma-Produktions-Standorte mutmasslich und streng unterwerfen?
__
nebenbei:
- bei glassdoor bekommt Fresenius Kabi USA (2,4) schlechtere Noten als Akorn (3,3 - was aber auch nicht wirklich gut ist), bei fast identischer Anzahl von Bewertungen mit Anzahl >= 60 (auch wenn man das nicht überbewerten sollte - v.a. nicht als Aktionär; gibt gute Gegenbeispiele dazu...)
Zitat von Schlummschuetze: ...ohne die Übernahmephantasie ist Akorn wohl wertlos...
=> das wäre aus Sicht von Fresenius aber unlogisch. Gut, es steht das einschränkende Wort "wohl" oben dabei.
Denn dann stellt sich die Frage: was wollte Fresenius (Kabi) eigentlich von Akorn?
=> vor diesem Hintergrund gibt es ja nur noch zwei Möglichkeiten:
(A) Fresenius (Kabi) ist zu doof Übernahmeziele hinreichend zu prüfen:
...Stephan Sturm, Vorstandsvorsitzender von Fresenius, sagte: „Mit diesen Akquisitionen (FC: gemeint Merck Biosimilar + Akorn) stellen wir bei Fresenius Kabi die Weichen für ein noch breiter angelegtes und dauerhaft kräftiges Wachstum über das laufende Jahrzehnt hinaus. Akorn ergänzt das Produktangebot von Fresenius Kabi im Generika-Geschäft bestens.
...
Die US-amerikanischen Unternehmenszentralen von Akorn und Fresenius Kabi liegen nur wenige Kilometer voneinander entfernt im Norden des US-Bundesstaats Illinois...
aus: https://www.fresenius.de/5770
--> diese ganz Meldung überschlägt sich fast für Freude auf das Geschäft von Akorn
=> dagegen spricht aber der M&A-Trackrekord von Fresenius und von auch von Stephan Sturm als CFO und später CEO (2016) von Fresenius
--> OK, vielleicht war das nun sein erster grosser Fehler bei Fresenius (als CEO)
oder
(B) man ist auf ganz hinterhältige Betrüger reingefallen, was man nicht absehen konnte bei einem Übernahmeziel in der Nachbarschaft:
- ich habe auf Google Maps nachgemessen: es sind vom:
Three Corporate Drive Lake Zurich, IL 60047 (FRE/HQ)
bis
Lake Forest, IL, (AKRX/HQ)
nur 15 miles = 30min Autofahrt!
=> das ist keine Fern-Übernahme. Beide Standorte sind etabliert, auch mit Produktion (Manufacturing Locations), z.B.:
FRE:
* Melrose Park village, Cook County, IL
<-- 180 miles -->
AKRX:
* 1222 W. Grand Ave., Decatur, IL
=> kaum zu glauben, dass nicht mal Info's und Personen hin und her gegangen sind

- in IL alleine arbeiten jeweils mehrere hundert Personen bei Fresenius Kabi, als auch bei Akorn
=> und da soll ich glauben, dass die eine Gruppe über Jahre ruchlose Betrüger sind ("unter anderen schwerwiegende Verstöße gegen FDA-Vorgaben", FRE, 22.4.), die nur noch auf naive Deutsche mit ihrem "stupid German money" gewartet haben?

Einer Gruppe, die auch regelmässig von der FDA (indirekt wenigstens) kontrolliert wird?
Im Heimatland der FDA-Regularien wie 21 CFR Part 11, denen sich auch alle europäischen Pharma-Produktions-Standorte mutmasslich und streng unterwerfen?
__
nebenbei:
- bei glassdoor bekommt Fresenius Kabi USA (2,4) schlechtere Noten als Akorn (3,3 - was aber auch nicht wirklich gut ist), bei fast identischer Anzahl von Bewertungen mit Anzahl >= 60 (auch wenn man das nicht überbewerten sollte - v.a. nicht als Aktionär; gibt gute Gegenbeispiele dazu...)
Antwort auf Beitrag Nr.: 57.685.269 von Global-Player83 am 03.05.18 12:28:14
=> USD17.50 laut Morning Star (angeblich)
Quelle: Post auf StockTwits, 4.5.
=> (17.50 - 11.70)/11.70 = +49.6%
=> allerdings, wenn dem so tatsächlich wäre, kann das Drift dahin (zurück) längere Zeit in Anspruch nehmen, denn Zusatz-Kosten kommen auf Akorn auf jeden Fall zu, neben einem Malus nun (vielleicht so 6m <= t < 18m)
__
Andere Frage: warum wartet Fresenius eigentlich das Ergebnis der (unabh.) Untersuchung bei Akron nicht ab?
--> woher wissen die eigentlich schon nach 2 Monaten, dass es "schwerwiegende Verstöße gegen FDA-Vorgaben" gab?
=> was passiert, wenn es nur "nicht-schwerwiegende Verstöße gegen FDA-Vorgaben" gab?
=> ..und was passiert, wenn im Melrose Park village bei Fresenius Kabi nun auch "Verstösse gegen FDA-Vorgaben" gefunden werden sollten?
--> ich meine, wenn man schon mal in Illinois beim vertieften Prüfen ist...
Zitat von Global-Player83: ...Die für mich spannendere Frage ist, wo liegt der faire Wert der Akorn-Aktie ohne Berücksichtigung des Übernahme-Streits?
=> USD17.50 laut Morning Star (angeblich)
Quelle: Post auf StockTwits, 4.5.
=> (17.50 - 11.70)/11.70 = +49.6%
=> allerdings, wenn dem so tatsächlich wäre, kann das Drift dahin (zurück) längere Zeit in Anspruch nehmen, denn Zusatz-Kosten kommen auf Akorn auf jeden Fall zu, neben einem Malus nun (vielleicht so 6m <= t < 18m)
__
Andere Frage: warum wartet Fresenius eigentlich das Ergebnis der (unabh.) Untersuchung bei Akron nicht ab?

--> woher wissen die eigentlich schon nach 2 Monaten, dass es "schwerwiegende Verstöße gegen FDA-Vorgaben" gab?

=> was passiert, wenn es nur "nicht-schwerwiegende Verstöße gegen FDA-Vorgaben" gab?
=> ..und was passiert, wenn im Melrose Park village bei Fresenius Kabi nun auch "Verstösse gegen FDA-Vorgaben" gefunden werden sollten?

--> ich meine, wenn man schon mal in Illinois beim vertieften Prüfen ist...

Antwort auf Beitrag Nr.: 57.699.597 von faultcode am 05.05.18 02:33:18
Zwei Mängelberichte von 2016 und 2017 sind leicht im Internet zu finden, wenn auch mit teilweise Schwärzungen:
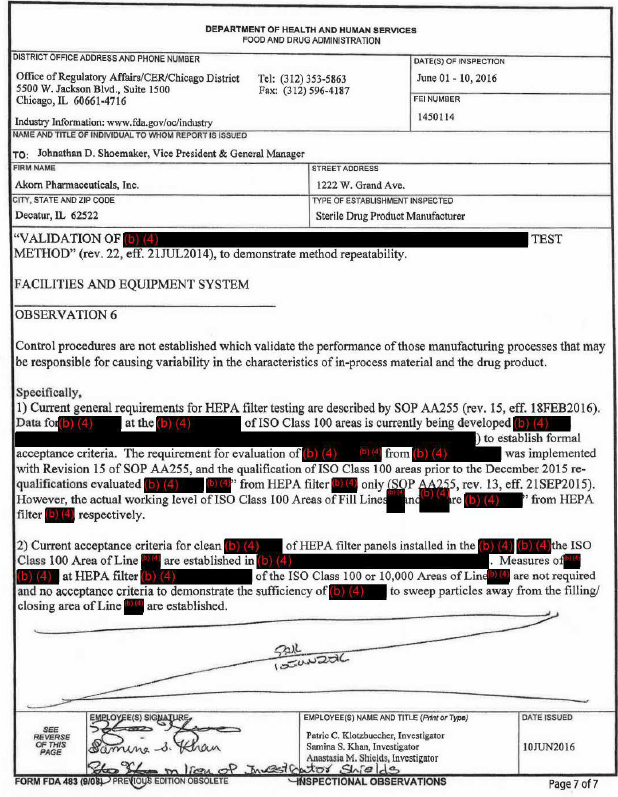
(a) 2016-06-01..10, 7 Seiten, Mängel in:
- production system
- quality system
- lab controls system
- facilities and equipment system
(b) 2017-04-20..26, 4 Seiten - quasi ein Follow-up von (a), da die Fabrik immer noch Defizite aufzeigte
Aus https://www.fiercepharma.com/manufacturing/akorn-sterile-pla…, 2017-10-24:
...
- Despite the observations (FC: gemeint ist auch der Zustand nach April 2017), the FDA has continued to approve new products from that plant. Akorn's last five approved drugs, four of them sterile injectables, are being produced at the facility.
- (Fresenius) got three U.S. plants and one in India in the Akorn deal, which is slated to close in early 2018.
- Sterile injectables accounted for about 35% of Akorn's $1.1 billion in sales last year, but analysts also liked that Fresenius was additionally acquiring some new segments like ophthalmics and topical solutions, which might be less vulnerable to the generics pricing pressure that has made life difficult for generics producers in the last few years.
...
=> also die FDA genehmigte den Weiterbetrieb (mit welchen Einschränkungen, wenn überhaupt?) der Fabrik, aber Fresenius - mit eigener, nicht-unabhängiger Prüfung - will von all dem nichts gewusst haben?
__
(*) Form FDA 483 "Inspectional Observations"
- form used by the FDA to document and communicate concerns discovered during these inspections
- https://en.wikipedia.org/wiki/Form_FDA_483
Form FDA 483 "Inspectional Observations" - Mängelanzeigen gut bekannt
Mindestens ein FDA-Mängelbericht (*) über die Decatur, IL-Fabrik von Akorn muss Fresenius vor der Übernahme bekannt gewesen sein.Zwei Mängelberichte von 2016 und 2017 sind leicht im Internet zu finden, wenn auch mit teilweise Schwärzungen:

(a) 2016-06-01..10, 7 Seiten, Mängel in:
- production system
- quality system
- lab controls system
- facilities and equipment system
(b) 2017-04-20..26, 4 Seiten - quasi ein Follow-up von (a), da die Fabrik immer noch Defizite aufzeigte
Aus https://www.fiercepharma.com/manufacturing/akorn-sterile-pla…, 2017-10-24:
...
- Despite the observations (FC: gemeint ist auch der Zustand nach April 2017), the FDA has continued to approve new products from that plant. Akorn's last five approved drugs, four of them sterile injectables, are being produced at the facility.
- (Fresenius) got three U.S. plants and one in India in the Akorn deal, which is slated to close in early 2018.
- Sterile injectables accounted for about 35% of Akorn's $1.1 billion in sales last year, but analysts also liked that Fresenius was additionally acquiring some new segments like ophthalmics and topical solutions, which might be less vulnerable to the generics pricing pressure that has made life difficult for generics producers in the last few years.
...
=> also die FDA genehmigte den Weiterbetrieb (mit welchen Einschränkungen, wenn überhaupt?) der Fabrik, aber Fresenius - mit eigener, nicht-unabhängiger Prüfung - will von all dem nichts gewusst haben?

__
(*) Form FDA 483 "Inspectional Observations"
- form used by the FDA to document and communicate concerns discovered during these inspections
- https://en.wikipedia.org/wiki/Form_FDA_483
Antwort auf Beitrag Nr.: 57.705.550 von faultcode am 06.05.18 22:27:49
Finanzakrobat wird Fresenius-Chef
http://www.rp-online.de/wirtschaft/stephan-sturm-finanzakrob…
=>
...
Banker finden es beachtlich, dass Fresenius viele Fusionen und Übernahmen (M&A) mit einem kleinen Experten-Team durchzieht, meist geräuschlos und ohne externe Hilfe.
"M&A ist schließlich kein Hexenwerk", sagt Sturm dazu.
"Banker als Berater engagieren wir nur, wenn es die Rahmenbedingungen erfordern."
Als sich Sturm und eine Handvoll Kollegen 2013 mit Managern des Konkurrenten Rhön-Klinikum treffen, um über den Kauf zahlreicher Rhön-Kliniken zu verhandeln, sitzt auf Fresenius-Seite außer Sturm kein Banker mit am Tisch.
...
Keine SWOT-Analyse von Akorn durch Fresenius?
Nur Zahlen geguckt, und keine FDA-Berichte gelesen, bzw. lesen lassen?
Einsame Entscheidung im Elfenbeinturm getroffen?
Stephan Sturm hat rund 3 Jahre (1989 – 1991) als Management Consultant bei McKinsey & Co., Duesseldorf and Frankfurt gearbeitet. Er sollte es eigentlich besser wissen.
=> viel Spass mit dem US-Rechtssystem
"M&A ist schließlich kein Hexenwerk" -- Stephan Sturm
28.6.2016Finanzakrobat wird Fresenius-Chef
http://www.rp-online.de/wirtschaft/stephan-sturm-finanzakrob…
=>
...
Banker finden es beachtlich, dass Fresenius viele Fusionen und Übernahmen (M&A) mit einem kleinen Experten-Team durchzieht, meist geräuschlos und ohne externe Hilfe.
"M&A ist schließlich kein Hexenwerk", sagt Sturm dazu.
"Banker als Berater engagieren wir nur, wenn es die Rahmenbedingungen erfordern."
Als sich Sturm und eine Handvoll Kollegen 2013 mit Managern des Konkurrenten Rhön-Klinikum treffen, um über den Kauf zahlreicher Rhön-Kliniken zu verhandeln, sitzt auf Fresenius-Seite außer Sturm kein Banker mit am Tisch.
...
Keine SWOT-Analyse von Akorn durch Fresenius?

Nur Zahlen geguckt, und keine FDA-Berichte gelesen, bzw. lesen lassen?
Einsame Entscheidung im Elfenbeinturm getroffen?
Stephan Sturm hat rund 3 Jahre (1989 – 1991) als Management Consultant bei McKinsey & Co., Duesseldorf and Frankfurt gearbeitet. Er sollte es eigentlich besser wissen.
=> viel Spass mit dem US-Rechtssystem

Antwort auf Beitrag Nr.: 57.699.597 von faultcode am 05.05.18 02:33:18Hallo Faultcode,
Du fragtest nach dem Akornwert ohne Übernahme.
Fresenius wollte Akorn kaufen. Zu dem Zeitpunkt machte Akorn gute Gewinne und Umsatzsprünge.
Inzwischen ist der Umsatz drastisch gefallen und die Gewinne sind weg. Der Akornwert liegt also heute weit unter dem Wert vor der Vertragsunterzeichnung.
Dazu wenn Fresenius aussteigen kann und Recht mit seinen Behauptungen hat, dann kam es zu Zulassungstricksereien bei Medikamenten. Folglich dann zu Bußgeldern und Schadenersatzforderungen von Patienten und Krankenkassen.
Darüberhinaus klagen ja auch schon Aktionäre gegen die Firma, die sich betrogen fühlen, wegen kursrelevanten Falschinformationen. Also auch hier Schadenersatzforderungen.
Zieh beides vom eh schon deutlich geringeren Akornwert ab. Da bleibt nicht mehr viel.
Hat Akorn Recht wird Geld fließen, wenn es aber Betrügereien gab .....
Gruß
Schlummschütze
Du fragtest nach dem Akornwert ohne Übernahme.
Fresenius wollte Akorn kaufen. Zu dem Zeitpunkt machte Akorn gute Gewinne und Umsatzsprünge.
Inzwischen ist der Umsatz drastisch gefallen und die Gewinne sind weg. Der Akornwert liegt also heute weit unter dem Wert vor der Vertragsunterzeichnung.
Dazu wenn Fresenius aussteigen kann und Recht mit seinen Behauptungen hat, dann kam es zu Zulassungstricksereien bei Medikamenten. Folglich dann zu Bußgeldern und Schadenersatzforderungen von Patienten und Krankenkassen.
Darüberhinaus klagen ja auch schon Aktionäre gegen die Firma, die sich betrogen fühlen, wegen kursrelevanten Falschinformationen. Also auch hier Schadenersatzforderungen.
Zieh beides vom eh schon deutlich geringeren Akornwert ab. Da bleibt nicht mehr viel.
Hat Akorn Recht wird Geld fließen, wenn es aber Betrügereien gab .....
Gruß
Schlummschütze
Antwort auf Beitrag Nr.: 57.705.568 von Schlummschuetze am 06.05.18 22:39:50
ich nicht --> Global-Player83 war es oben #24 --> eine Antwort dazu auf #27
=> das wusste Fresenius (Kabi) bereits vor dem Merger agreement (FTC filing am May 8, 2017):
aus (+) unten: --> Financial Forecasts:
...
The March 2017 Management Case was not made available to Fresenius Kabi.
However, the Company’s management provided information to Fresenius Kabi as to how certain assumptions and estimates underlying the November 2016 Management Case would change to reflect the information about recent developments that was used to prepare the March 2017 Management Case....
(e) aus (+) --> March 2017 Management Case:
Revenues:
- 2014: USD555m
- 2015: USD985m
- 2016: USD1,120m
- 2017: USD841m <--- 2017e (+), 2017-03: USD834m = ~ -0.8% ==> diese Abweichung ist marginal
- 2018e: USD1,157m
- 2019e: USD1,258m
...
- 2026e: USD1,680m
=> CAGR 2016-2026: +4.1%
Operating Income / EBIT after Unusual Expense:
- 2014: USD-32.8m
- 2015: USD-36.1m
- 2016: USD327.6m
- 2017: USD-17.7m <--- 2017e (+), 2017-03: USD268m <-- allerdings ist das ein EBIT ohne "unusual expenses"
=> vorher die hohe Abweichung in 2017?
--> wg. "unusual expenses" von rund USD117m
- 2018e: USD394m
- 2019e: USD453m
...
- 2026e: USD545m
=> CAGR 2016-2026: +5.2%
=> man sieht: Akorn hat oft ein "unusual expenses" Problem --> Fresenius muss das - mit einem Investmentbanker an der Spitze - gewusst haben
--> Fresenius muss das - mit einem Investmentbanker an der Spitze - gewusst haben 
Allerdings:
- der Free Cash Flow (FCF) von 2010 bis 2017 war immer > 0 !!
Mit 2017-FCF/share von USD1.27 > 2016-FCF/share von USD0.80
FCF hier definiert als "Simple" FCF = Operating Cash Flow - CAPEX
Der vielleicht beste Artikel zu AKRX-FRE Kabi:
2.5.2018
Reviewing The Akorn-Fresenius Merger Fallout
https://seekingalpha.com/article/4168444-reviewing-akorn-fre…
--> sehr lang!
(+) SCHEDULE 14A
https://www.sec.gov/Archives/edgar/data/3116/000130817917000…
--> sehr interessantes Dokument mit viel Info's
AKRX-Deal: closing date is set for July 24. 2018 (1)
Zitat von Schlummschuetze: ...Du fragtest nach dem Akornwert ohne Übernahme...
ich nicht --> Global-Player83 war es oben #24 --> eine Antwort dazu auf #27
Zitat von Schlummschuetze: ...Inzwischen ist der Umsatz drastisch gefallen und die Gewinne sind weg...
=> das wusste Fresenius (Kabi) bereits vor dem Merger agreement (FTC filing am May 8, 2017):
aus (+) unten: --> Financial Forecasts:
...
The March 2017 Management Case was not made available to Fresenius Kabi.
However, the Company’s management provided information to Fresenius Kabi as to how certain assumptions and estimates underlying the November 2016 Management Case would change to reflect the information about recent developments that was used to prepare the March 2017 Management Case....
(e) aus (+) --> March 2017 Management Case:
Revenues:
- 2014: USD555m
- 2015: USD985m
- 2016: USD1,120m
- 2017: USD841m <--- 2017e (+), 2017-03: USD834m = ~ -0.8% ==> diese Abweichung ist marginal
- 2018e: USD1,157m
- 2019e: USD1,258m
...
- 2026e: USD1,680m
=> CAGR 2016-2026: +4.1%
Operating Income / EBIT after Unusual Expense:
- 2014: USD-32.8m
- 2015: USD-36.1m
- 2016: USD327.6m
- 2017: USD-17.7m <--- 2017e (+), 2017-03: USD268m <-- allerdings ist das ein EBIT ohne "unusual expenses"
=> vorher die hohe Abweichung in 2017?
--> wg. "unusual expenses" von rund USD117m
- 2018e: USD394m
- 2019e: USD453m
...
- 2026e: USD545m
=> CAGR 2016-2026: +5.2%
=> man sieht: Akorn hat oft ein "unusual expenses" Problem
 --> Fresenius muss das - mit einem Investmentbanker an der Spitze - gewusst haben
--> Fresenius muss das - mit einem Investmentbanker an der Spitze - gewusst haben 
Allerdings:
- der Free Cash Flow (FCF) von 2010 bis 2017 war immer > 0 !!
Mit 2017-FCF/share von USD1.27 > 2016-FCF/share von USD0.80
FCF hier definiert als "Simple" FCF = Operating Cash Flow - CAPEX
Der vielleicht beste Artikel zu AKRX-FRE Kabi:
2.5.2018
Reviewing The Akorn-Fresenius Merger Fallout
https://seekingalpha.com/article/4168444-reviewing-akorn-fre…
--> sehr lang!
(+) SCHEDULE 14A
https://www.sec.gov/Archives/edgar/data/3116/000130817917000…
--> sehr interessantes Dokument mit viel Info's

Antwort auf Beitrag Nr.: 57.705.799 von faultcode am 07.05.18 00:38:20
November 2016 Management Case
vom
March 2017 Management Case ?
Beides sind Eingangsgrössen ins SCHEDULE 14A zur Abstimmung für die AKRX-Aktionäre seinerzeit:
Revenues, 2016-11 <--> 2017-03:
2026e: USD1,821m <--> USD1,680m
EBIT, 2016-11 <--> 2017-03:
2026e: USD600m <--> USD545m
..ohne "unusual expenses"
---
Mal schnell nachgerechnet zu https://www.wallstreet-online.de/diskussion/1275357-21-30/an… oben
=> fairer Preis|Morning Star: USD17.50 (~)
Nach DCF mit den "normalen Jahren" 2015 + 2016 und mit:
* mittig EPS ~USD1.35/share
* CAGR +5.5% beim EPS für 10Y
* term.growth 10Y mit 2%
* disc.rate 10%
* Null(!) mat.Buchwert/share (z.Z. leicht < 0)
=> kommt man so auf ~USD16.60 !
=> also kommt oben ganz gut (~) hin!
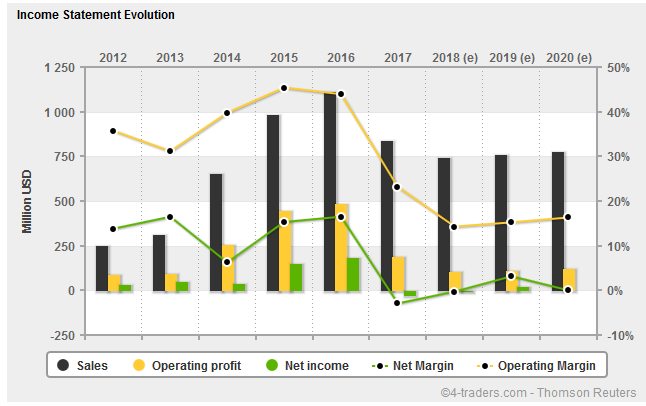
http://www.4-traders.com/AKORN-INC-42504/financials/
=> für mich sieht das nach worst case (2018e ff.) aus
ABER: es gibt Analysten, die das für zu hoch auf Generika-Multiples-Basis halten, und auf ~USD8 kommen (ich glaube auch selbe Quelle wie oben):
=> aber: Akorn ist schon länger nicht mehr als "normaler" US-Generika-Hersteller bewertet worden - auch nicht ab 2015 (das Ende des Wachstums im US-Gesundheitsmarkt)
=> der harmonische Mittelwert beim P/E|year-end in den profitablen Jahren 2010-2016 lag bei 32 !!
=> mit der (konversativen) Schätzung von Thomson Reuters vom 4.5.2018 für 2018e mit EPS = USD0.19 läge man damit bei circa:
USD6 ! für eine Aktie als fairen Wert, den ich für einen worst case halte!
=> nehme ich den Mittelwert aus (~) und den USD6 komme ich auf USD11.75
=> und wo war der letzte Kurs am Freitag? => bei USD11.74
=> so gesehen gibt es momentan sehr wahrscheinlich zwei Lager bei der Akorn-Aktie:
(a) die einen sagen, ab 2018/19 erholen sich die Parameter wieder Richtung 2015 + 2016
(man beachte #31 oben --> der FCF nahm 2017 satt zu, weil der Oper.Cashflow wieder stark zunahm - auch bei erneut erhöhtem CAPEX!)
und:
(b) die anderen sagen, nein - so wie Thomason Reuters - alles wie vor 2015.
Die anhängigen Rechtsfälle sind alle normale Pillepalle. Nicht ein Fall dabei wg. schlechtem Medikament oder IP-Verletzungen. Nur die sind gewöhnlich potenziell gefährlich.
..und man beachte auch:
- Fresenius dachte doch hier nicht kurzfristig mit dem (teilweise) schlechten 2017, sondern langfristig - wie immer eigentlich in der Vergangenheit:
=> nein, an den 2017er-Zahlen von Akorn hat sich Fresenius (Kabi) nicht gestört, sondern mindestens an ihrem eigenen Kaufpreis von USD4.75b = USD34/share
Dieser "Aufsteiger" hat einfach gepennt - und das Merger agreement (auch) unterschrieben:
Philipp Schulte-Noelle
Chief Financial Officer & Chief Compliance Officer, FRESENIUS KABI AG
https://heft.manager-magazin.de/MM/2015/7/135518114/index.ht…
=> klar, wenn der Chef unterschreibt, unterschreibt der CFO halt auch
AKRX-Deal: closing date is set for July 24. 2018 (2)
dazu ein Detail aus (+) oben: wie unterscheiden sich eigentlich der November 2016 Management Case
vom
March 2017 Management Case ?
Beides sind Eingangsgrössen ins SCHEDULE 14A zur Abstimmung für die AKRX-Aktionäre seinerzeit:
Revenues, 2016-11 <--> 2017-03:
2026e: USD1,821m <--> USD1,680m
EBIT, 2016-11 <--> 2017-03:
2026e: USD600m <--> USD545m
..ohne "unusual expenses"

---
Mal schnell nachgerechnet zu https://www.wallstreet-online.de/diskussion/1275357-21-30/an… oben
=> fairer Preis|Morning Star: USD17.50 (~)
Nach DCF mit den "normalen Jahren" 2015 + 2016 und mit:
* mittig EPS ~USD1.35/share
* CAGR +5.5% beim EPS für 10Y
* term.growth 10Y mit 2%
* disc.rate 10%
* Null(!) mat.Buchwert/share (z.Z. leicht < 0)
=> kommt man so auf ~USD16.60 !
=> also kommt oben ganz gut (~) hin!

http://www.4-traders.com/AKORN-INC-42504/financials/
=> für mich sieht das nach worst case (2018e ff.) aus

ABER: es gibt Analysten, die das für zu hoch auf Generika-Multiples-Basis halten, und auf ~USD8 kommen (ich glaube auch selbe Quelle wie oben):
=> aber: Akorn ist schon länger nicht mehr als "normaler" US-Generika-Hersteller bewertet worden - auch nicht ab 2015 (das Ende des Wachstums im US-Gesundheitsmarkt)
=> der harmonische Mittelwert beim P/E|year-end in den profitablen Jahren 2010-2016 lag bei 32 !!
=> mit der (konversativen) Schätzung von Thomson Reuters vom 4.5.2018 für 2018e mit EPS = USD0.19 läge man damit bei circa:
USD6 ! für eine Aktie als fairen Wert, den ich für einen worst case halte!
=> nehme ich den Mittelwert aus (~) und den USD6 komme ich auf USD11.75
=> und wo war der letzte Kurs am Freitag? => bei USD11.74

=> so gesehen gibt es momentan sehr wahrscheinlich zwei Lager bei der Akorn-Aktie:
(a) die einen sagen, ab 2018/19 erholen sich die Parameter wieder Richtung 2015 + 2016
(man beachte #31 oben --> der FCF nahm 2017 satt zu, weil der Oper.Cashflow wieder stark zunahm - auch bei erneut erhöhtem CAPEX!)
und:
(b) die anderen sagen, nein - so wie Thomason Reuters - alles wie vor 2015.
Die anhängigen Rechtsfälle sind alle normale Pillepalle. Nicht ein Fall dabei wg. schlechtem Medikament oder IP-Verletzungen. Nur die sind gewöhnlich potenziell gefährlich.
..und man beachte auch:
- Fresenius dachte doch hier nicht kurzfristig mit dem (teilweise) schlechten 2017, sondern langfristig - wie immer eigentlich in der Vergangenheit:
=> nein, an den 2017er-Zahlen von Akorn hat sich Fresenius (Kabi) nicht gestört, sondern mindestens an ihrem eigenen Kaufpreis von USD4.75b = USD34/share
Dieser "Aufsteiger" hat einfach gepennt - und das Merger agreement (auch) unterschrieben:
Philipp Schulte-Noelle
Chief Financial Officer & Chief Compliance Officer, FRESENIUS KABI AG
https://heft.manager-magazin.de/MM/2015/7/135518114/index.ht…
=> klar, wenn der Chef unterschreibt, unterschreibt der CFO halt auch


Antwort auf Beitrag Nr.: 57.705.799 von faultcode am 07.05.18 00:38:20
aus (+) oben, oder hier die Links zu den SEC Proxy filings:
http://investors.akorn.com/phoenix.zhtml?c=78132&p=irol-sec&…
--> dort gibt es auch das Haupt-Proxy-Dokument mit 235 PDF-Seiten (neben einigen Ergänzungen bis 2017-06) -- besser zum Verarbeiten:
=>
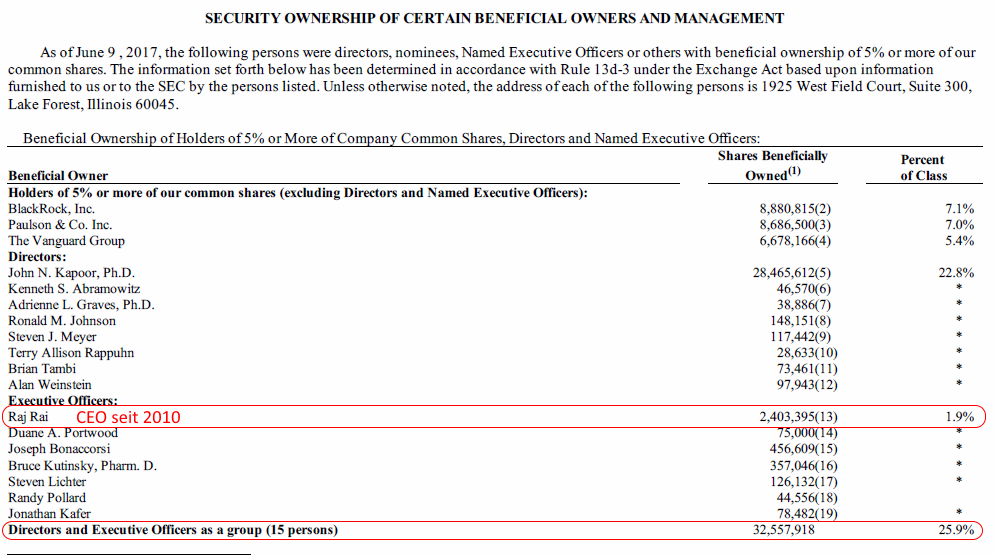
http://investors.akorn.com/phoenix.zhtml?c=78132&p=irol-govM…
--> die Direktoren und Executives haben alle das Voting agreement unterschrieben (neben dem sehr vielen Kleingedruckten...), so dass obige Besitzangaben auch heute noch zutreffen sollten
=> ich sag's mal so: wenn der Fresenius-Deal nicht zustandekommt, kommt irgendwann 2019ff. ein anderer Übernehmer -- aber nicht mehr für USD34
AKRX-Deal: closing date is set for July 24. 2018 (3) -- Insider dick in der Aktie
=> daher auch der Furor wg. der Fresenius-Deal-Absage 
aus (+) oben, oder hier die Links zu den SEC Proxy filings:
http://investors.akorn.com/phoenix.zhtml?c=78132&p=irol-sec&…
--> dort gibt es auch das Haupt-Proxy-Dokument mit 235 PDF-Seiten (neben einigen Ergänzungen bis 2017-06) -- besser zum Verarbeiten:
=>

http://investors.akorn.com/phoenix.zhtml?c=78132&p=irol-govM…
--> die Direktoren und Executives haben alle das Voting agreement unterschrieben (neben dem sehr vielen Kleingedruckten...), so dass obige Besitzangaben auch heute noch zutreffen sollten
=> ich sag's mal so: wenn der Fresenius-Deal nicht zustandekommt, kommt irgendwann 2019ff. ein anderer Übernehmer -- aber nicht mehr für USD34

Erstaunlich was Du hier leistest.
Ich frage mich jedoch, wieso hat Frisenius hier nicht eher reagiert. Schliesslich hatten die schon länger Interesse an AKORN.
Da schau ich mir doch den Partner genauer an ?
Ich frage mich jedoch, wieso hat Frisenius hier nicht eher reagiert. Schliesslich hatten die schon länger Interesse an AKORN.
Da schau ich mir doch den Partner genauer an ?
Vlt sehen die Umsatz-Gewinn Modelle von Fresenius für Akorn in den nächsten Jahren noch düsterer aus, als die Modelle von Akorn selbst...
Was Fresenius mit Akorn wollte sollte eigentlich klar sein.
Akorn hat einige sehr gute Medikamente (gute Pipeline) und vertreibt diese bisher nur in der USA und Indien.
Fresenius ist mit KABI hingegen global aufgestellt und möchte diese global vertreiben. U.a. deswegen wurde auch immer von 2020 ff mit hohen Gewinnen gerechnet. Das sind einfache Skaleneffekte.
warten wir die Quartalszahlen in paar Tagen mal ab.
Akorn hat einige sehr gute Medikamente (gute Pipeline) und vertreibt diese bisher nur in der USA und Indien.
Fresenius ist mit KABI hingegen global aufgestellt und möchte diese global vertreiben. U.a. deswegen wurde auch immer von 2020 ff mit hohen Gewinnen gerechnet. Das sind einfache Skaleneffekte.
warten wir die Quartalszahlen in paar Tagen mal ab.
Akorn - ein paar Fakten zum Geschäftsmodell
alles aus dem Annual Report 2017 (28.2.2018):1/ man hat (2015, 2016, 2017) 3 Hauptkunden, nämlich US-Distributoren (die aber "natürlich" keine Endkunden sind), nach Net Revenue:
* AmerisourceBergen: 19% (2017)
* Cardinal Health: 18% (2017)
* McKesson: 27% (2017)
In diesen Jahren machten diese immer:
- mindestens 77.4% der Gross Sales aus,
- ..und mindestens 63.5% der Net Revenues aus
(Underschied beider Grössen nach AKRX:
- allowances for chargebacks
- rebates
- administrative fees and others
- promotions
- product returns)
2/ die Geschäfts-Saisonalität ist "nicht signifikant" bei den meisten Produkten
(bei ein paar schon wg. warmer + kalter US-Jahreszeiten)
3/ Haupt-Produkte:
• AK-FLUOR (fluorescein injection, USP) --> Fluoreszenzangiographie des Augenhintergrundes
• Atropine Sulfate Ophthalmic Solution --> Augen
• Clobetasol Propionate Cream --> entzündliche Hauterkrankungen
• Dehydrated Alcohol Injection --> degeneration of nerve function (neurolysis) for control of chronic pain
• Ephedrine Sulfate Injection: >= 10% der Total Net Revenues
--> allergische Störungen
• Myorisan (isotretinoin capsules, USP) --> Akne
• Nembutal Sodium Solution (pentobarbital sodium injection, USP) --> beruhigende Hypnotika,
Behandlung oder Verhinderung von epileptischen, z. B. tonisch-klonischen Anfällen
• Phenylephrine Hydrochloride Ophthalmic Solution --> Augen
• TheraTears --> Augen
• Zioptan --> Augen
USP = United States Pharmacopeial Convention
=> alle anderen Produkte jeweils < 10% der Total Net Revenues
3a/
- nur 8% sind "Consumer Health"-Produkte, der Rest verschreibungspflichtig (2017)
4/ F+E: im Bereich alternativer Dosierformen:
- injectable
- ophthalmic (Augen)
- topical (topisch: Gel, Creme, ...)
- oral
- inhaled liquid
- nasal spray
- otic (Ohren)
- ausgewählte oral solid dose formulations
=> die Arzneien, die man selber herstellt, werden auch selber entwickelt;
die anderen verpartnert man (also Arzneien, die man NICHT selber herstellt)
Seit 2017 gibt es ein neues R&D Center in Cranbury, NJ
5/ Auslandsgeschäft (Währungsrisiko):
- sehr gering mit: USD26m von USD841m (2017, net revenue)
6/ Mitarbeiter 2017 (full-time):
- USA: 1706
- Indien: 425 --> sterile injectable facility -- nicht FDA-approved! Wird angestrebt.
- Schweiz: 153 --> ehemals Excelvision AG: Augen-Produkte
7/ man hat auch Tier-Arzneien im Programm
8/ der (aktuelle) Auditor ist BDO, Chicago IL, und das seit 2016
----
noch Ratings von Moody's für Akorn, Inc. :
- Long Term Rtg B1, 23 Apr 2018
--> B1 = LT Speculative grade: Judged as being speculative and a high credit risk
(mit ... Ba3 besser als B1 besser als B2 besser als B3...)
- Long Term Watch Uncertain
- ST Issuer Level Rtg SGL-1, 23 Apr 2018
- ST Issuer Level Watch Not on Watch
- Outlook Ratings Under Review, 26 Apr 2017
LT = Long Term
ST = Short Term
---
aktueller Chart:
"Sturm" - der Name scheint Programm zu sein --> Ende des Verfahrens 2019 mit einem Vergleich?
https://www.finanzen.net/nachricht/aktien/ausblick-fresenius…=>
...Doch die geplante rund 4,8 Milliarden Dollar schwere Übernahme des US-Generikaherstellers Akorn ließ der Konzern kürzlich wegen Betrugsvorwürfen gegen die Amerikaner platzen.
Beide Seiten streiten demnächst vor einem US-Gericht. Sturm rechnet damit, dass das Verfahren spätestens 2019 abgeschlossen sein sollte, sagte er am Rande des Aktionärstreffens der Nachrichtenagentur Bloomberg....
=> der Mann scheint sehr forsch zu sein. Mir soll's recht sein.
Also würde eine Beilegung in 2020 oder sogar noch später nicht so gut für ihn aussehen
=> wie also "spätestens 2019" zu einem Abschluss des Verfahrens kommen?
=> ich meine, mit einem Vergleich
=> d.h., Fresenius gibt Akorn Geld und zieht weiter, nachdem das Delaware Chancery Court dem Vergleich zugestimmt hat.
Antwort auf Beitrag Nr.: 57.790.252 von faultcode am 18.05.18 14:24:30
https://www.finanzen.net/nachricht/aktien/fresenius-verteidi…
=>...
Sturm wies Vorwürfe zurück, der Konzern habe vor dem Angebot bei Akorn womöglich nicht richtig hingeschaut. "Das war die intensivste Prüfung, die ich bei Fresenius erlebt habe. Sie entsprach höchsten Standards." Die Verstöße seien in Bereichen passiert, in die Fresenius keinen Einblick haben durfte. Sturm führte dies auf die Börsennotierung von Akorn zurück und darauf, dass das Unternehmen ein direkter Wettbewerber der Tochter Kabi sei. "Wir sind an den Rand dessen gegangen, was zulässig war. Überall dort, wo wir nicht hinschauen konnten, haben wir uns im Rahmen des Kaufvertrags sehr gute Absicherungen geben lassen."
Für Fresenius sei der Fall Akorn eine neue Situation. "So etwas gab es in der Tat noch nicht", sagte Sturm. Das Unternehmen ist durch mehrere Milliardenzukäufe zu einem globalen Firmenkonglomerat in der Gesundheitsbranche gewachsen.
Immer wieder wird Fresenius auch als "Akquisitionsmaschine" bezeichnet. Auch die Tochter Kabi, deren Nordamerikageschäft durch die Akorn-Übernahme hätte gestärkt werden sollen, wurde wiederholt durch Zukäufe ausgebaut. So beruht Sturm zufolge der Erfolg von Kabi in Nordamerika auf der 3,7 Milliarden Euro schweren Übernahme des Generikaspezialisten APP, mit der Kabi 2008 überhaupt erst in den US-Pharmamarkt eintrat. Nun will Fresenius auch ohne Akorn sein Geschäft mit Nachahmermedikamenten in Nordamerika ausbauen.
...
Anfang Juli werden sich die beiden Unternehmen vor Gericht wiedertreffen. Aktionärsvertreter wollten vom Vorstand vor allem wissen, mit welchen finanziellen Risiken durch den abgesagten Akorn-Kauf zu rechnen ist und wie lange sich das Verfahren hinziehen wird. Bislang seien bei Fresenius transaktionsbezogene Kosten von insgesamt 60 Millionen Euro vor Steuern angefallen, sagte Sturm.
Der Konzern müsse noch prüfen, inwiefern Rückstellungen im Zusammenhang mit dem Prozess gebildet werden müssten. Von Akorn fordert Fresenius Schadenersatz...
Zu:
"Das war die intensivste Prüfung, die ich bei Fresenius erlebt habe. Sie entsprach höchsten Standards."
=> wie kann das sein, wenn obige FDA-Mängelberichte (Beitrag Nr. 28) vom JUNI 2016 sind -- und leicht auffindbar im Internet


Fresenius verteidigt abgeblasene Akorn-Übernahme
noch Ergänzung von der FRE-HV:https://www.finanzen.net/nachricht/aktien/fresenius-verteidi…
=>...
Sturm wies Vorwürfe zurück, der Konzern habe vor dem Angebot bei Akorn womöglich nicht richtig hingeschaut. "Das war die intensivste Prüfung, die ich bei Fresenius erlebt habe. Sie entsprach höchsten Standards." Die Verstöße seien in Bereichen passiert, in die Fresenius keinen Einblick haben durfte. Sturm führte dies auf die Börsennotierung von Akorn zurück und darauf, dass das Unternehmen ein direkter Wettbewerber der Tochter Kabi sei. "Wir sind an den Rand dessen gegangen, was zulässig war. Überall dort, wo wir nicht hinschauen konnten, haben wir uns im Rahmen des Kaufvertrags sehr gute Absicherungen geben lassen."
Für Fresenius sei der Fall Akorn eine neue Situation. "So etwas gab es in der Tat noch nicht", sagte Sturm. Das Unternehmen ist durch mehrere Milliardenzukäufe zu einem globalen Firmenkonglomerat in der Gesundheitsbranche gewachsen.
Immer wieder wird Fresenius auch als "Akquisitionsmaschine" bezeichnet. Auch die Tochter Kabi, deren Nordamerikageschäft durch die Akorn-Übernahme hätte gestärkt werden sollen, wurde wiederholt durch Zukäufe ausgebaut. So beruht Sturm zufolge der Erfolg von Kabi in Nordamerika auf der 3,7 Milliarden Euro schweren Übernahme des Generikaspezialisten APP, mit der Kabi 2008 überhaupt erst in den US-Pharmamarkt eintrat. Nun will Fresenius auch ohne Akorn sein Geschäft mit Nachahmermedikamenten in Nordamerika ausbauen.
...
Anfang Juli werden sich die beiden Unternehmen vor Gericht wiedertreffen. Aktionärsvertreter wollten vom Vorstand vor allem wissen, mit welchen finanziellen Risiken durch den abgesagten Akorn-Kauf zu rechnen ist und wie lange sich das Verfahren hinziehen wird. Bislang seien bei Fresenius transaktionsbezogene Kosten von insgesamt 60 Millionen Euro vor Steuern angefallen, sagte Sturm.
Der Konzern müsse noch prüfen, inwiefern Rückstellungen im Zusammenhang mit dem Prozess gebildet werden müssten. Von Akorn fordert Fresenius Schadenersatz...
Zu:
"Das war die intensivste Prüfung, die ich bei Fresenius erlebt habe. Sie entsprach höchsten Standards."
=> wie kann das sein, wenn obige FDA-Mängelberichte (Beitrag Nr. 28) vom JUNI 2016 sind -- und leicht auffindbar im Internet



Antwort auf Beitrag Nr.: 57.791.860 von faultcode am 18.05.18 17:53:47
• seit er CEO wurde (2016) "beschleunigt (er) das Tempo", gemeint sind die Übernahmen, hier Quirónsalud (Spanien, Kliniken) und eben Akorn (Frühjahr 2017)
• nach dem Übernahmeangebot soll er "(3) anonyme Hinweise (Schreiben) bekommen haben, dass (Akorn) ... Testreihen für die Zulassung bei .... (der) FDA manipuliert haben soll."
• AR-Chef Gerd Krick (langjähriger Chef von Fresenius) wird als unabhängig dargestellt, der sich in der Akorn-Frage hinter Sturm stellte (und stellt)
• MM meint, dass sich der Prozess bis "weit ins kommende Jahr" ziehen wird
--> das klingt anders als oben (#38):
Sturm rechnet damit, dass das Verfahren spätestens 2019 abgeschlossen sein sollte, sagte er am Rande des Aktionärstreffens der Nachrichtenagentur Bloomberg
• dann noch so zwei Sätze: "Fresenius' Nimbus ist akut gefährdet und Sturms erst recht. Sollte sich am Ende herausstellen, dass er bei der Übernahme an den entscheidenden Stellen zu nachlässig war, kann es sehr schnell eng für ihn werden."
• Rest ist Sturm's Bio von ganz unten bis ganz oben: nicht mit dem Silberlöffel im Mund geboren; sehr ehrgezig, aber zweimal an etablierten Institutionen gescheitert:
-- London School of Economics: keine Aufnahme
-- keine Wall Street-Investmentbank wollte ihn nehmen --> und so landet er (kurz) bei McKinsey und optimiert für VW eine Kabelstränge-Fertigung u.a.
• "neigt gelegentlich zu Stimmungsumschwüngen"; Vorgänger Schneider war "berechenbarer"
• Sturm wurde 2016 CEO, als Fresenius:
- vgl.-weise wenig Schulden hat, ..
- ..und eine volle (Kriegs-)Kasse
• die Quirónsalud-Übernahme gelingt sehr gut
• in den USA sanken bereits die Generika-Preise; nur noch 3 Distributoren sind übrig geblieben; siehe oben Beitrag Nr. 37
• Akorn sei da stark, wo Fresenius Kabi Nachholbedarf hat: bei Arznei-Mitteln, "die in kleinen Dosen verabreicht werden"
• Akorn Chairman John N. Kapoor (*1942/43 in India: https://en.wikipedia.org/wiki/John_Kapoor --> founder of Akorn) als Grossaktionär (bei Akorn) "erweist sich als harter Brocken": siehe Beitrag Nr. 33 oben: 22.8%; "initiiert ein regelrechtes Wettbieten mit zeitweise:
- 4 Pharmafirmen
- 1 Staatsfonds
- 5 Finanzinvestoren
=> Fresenius bietet das meiste Geld mit USD34, +40% zum vorherigen Aktienkurs
Jetzt wird's interessant:
• Akorn's Ruf war damals schon administrativ und finanziell belastet:
- 2014 wurde die Bilanz korrigiert
- 2012...2015: EY, KPMG und BDO: Bilanz-Vermerk: "kein adäquates Controlling-System"
• John N. Kapoor wird in 2017-10 verhaftet wg. Bestechungsgelder in einem anderen Zusammenhang; "Opium-Krise" in den USA: https://www.yahoo.com/news/billionaire-insys-founder-charged…
• das MM: "Sturm glaubt, dass die Akorn-Pipeline stark genug ist, um die Prämie zu rechtfertigen."
• indem Sturm öffentlich die Übernahme abbläst, lässt er den Akorn-Aktienkurs abstürzen und:
"Damit hat er Akorn-CEO Rajat Rai in eine Ecke manövriert, aus der der sich kaum herausverhandeln kann." Grund: die Ansammlung von Hedge funds (HF's) in der Akorn-Aktie bei USD30...32 --> Arbitrage zu den USD34
--> damit kann es keinen Rabatt auf den Akorn-Kaufpreis geben, ohne (erfolgreiche/FC) Schadenersatz-Klagen der HF's zu riskieren.
=> liebes MM: der Mann heisst "Raj Rai" nicht "Rajat Rai" --> bitte, z.B. oben, in den SEC-Dokumenten nachlesen (das richtige Schreiben von Namen wird in den USA sehr ernst genommen...)
• Sturm kaufte am 8.5. für ~EUR1Mio Fresenius-Aktien: http://www.dgap.de/dgap/News/directors_dealings/kauf-sturm-s…
__
mir fällt auf: Stephan Sturm hatte in seiner Karriere zuvor keinen "richtigen" USA-Bezug. Das ist mMn zunächst kein Problem.
Aber für forsches Vorgehen in den USA taugt kein "deutsches" forsches Vorgehen. Siehe Klaus Kleinfeld bei Alcoa/Arconic.
=> das rächt sich eben nun.
MM-Artikel
Ich lese gerade den MM-Artikel 6/2018 ab Seite 70 zu Stephan Sturm. Meine eigene Zusammenfassung daraus:• seit er CEO wurde (2016) "beschleunigt (er) das Tempo", gemeint sind die Übernahmen, hier Quirónsalud (Spanien, Kliniken) und eben Akorn (Frühjahr 2017)
• nach dem Übernahmeangebot soll er "(3) anonyme Hinweise (Schreiben) bekommen haben, dass (Akorn) ... Testreihen für die Zulassung bei .... (der) FDA manipuliert haben soll."
• AR-Chef Gerd Krick (langjähriger Chef von Fresenius) wird als unabhängig dargestellt, der sich in der Akorn-Frage hinter Sturm stellte (und stellt)
• MM meint, dass sich der Prozess bis "weit ins kommende Jahr" ziehen wird
--> das klingt anders als oben (#38):
Sturm rechnet damit, dass das Verfahren spätestens 2019 abgeschlossen sein sollte, sagte er am Rande des Aktionärstreffens der Nachrichtenagentur Bloomberg
• dann noch so zwei Sätze: "Fresenius' Nimbus ist akut gefährdet und Sturms erst recht. Sollte sich am Ende herausstellen, dass er bei der Übernahme an den entscheidenden Stellen zu nachlässig war, kann es sehr schnell eng für ihn werden."
• Rest ist Sturm's Bio von ganz unten bis ganz oben: nicht mit dem Silberlöffel im Mund geboren; sehr ehrgezig, aber zweimal an etablierten Institutionen gescheitert:
-- London School of Economics: keine Aufnahme
-- keine Wall Street-Investmentbank wollte ihn nehmen --> und so landet er (kurz) bei McKinsey und optimiert für VW eine Kabelstränge-Fertigung u.a.
• "neigt gelegentlich zu Stimmungsumschwüngen"; Vorgänger Schneider war "berechenbarer"
• Sturm wurde 2016 CEO, als Fresenius:
- vgl.-weise wenig Schulden hat, ..
- ..und eine volle (Kriegs-)Kasse
• die Quirónsalud-Übernahme gelingt sehr gut
• in den USA sanken bereits die Generika-Preise; nur noch 3 Distributoren sind übrig geblieben; siehe oben Beitrag Nr. 37
• Akorn sei da stark, wo Fresenius Kabi Nachholbedarf hat: bei Arznei-Mitteln, "die in kleinen Dosen verabreicht werden"
• Akorn Chairman John N. Kapoor (*1942/43 in India: https://en.wikipedia.org/wiki/John_Kapoor --> founder of Akorn) als Grossaktionär (bei Akorn) "erweist sich als harter Brocken": siehe Beitrag Nr. 33 oben: 22.8%; "initiiert ein regelrechtes Wettbieten mit zeitweise:
- 4 Pharmafirmen
- 1 Staatsfonds
- 5 Finanzinvestoren
=> Fresenius bietet das meiste Geld mit USD34, +40% zum vorherigen Aktienkurs
Jetzt wird's interessant:
• Akorn's Ruf war damals schon administrativ und finanziell belastet:
- 2014 wurde die Bilanz korrigiert
- 2012...2015: EY, KPMG und BDO: Bilanz-Vermerk: "kein adäquates Controlling-System"
• John N. Kapoor wird in 2017-10 verhaftet wg. Bestechungsgelder in einem anderen Zusammenhang; "Opium-Krise" in den USA: https://www.yahoo.com/news/billionaire-insys-founder-charged…
• das MM: "Sturm glaubt, dass die Akorn-Pipeline stark genug ist, um die Prämie zu rechtfertigen."
• indem Sturm öffentlich die Übernahme abbläst, lässt er den Akorn-Aktienkurs abstürzen und:
"Damit hat er Akorn-CEO Rajat Rai in eine Ecke manövriert, aus der der sich kaum herausverhandeln kann." Grund: die Ansammlung von Hedge funds (HF's) in der Akorn-Aktie bei USD30...32 --> Arbitrage zu den USD34
--> damit kann es keinen Rabatt auf den Akorn-Kaufpreis geben, ohne (erfolgreiche/FC) Schadenersatz-Klagen der HF's zu riskieren.
=> liebes MM: der Mann heisst "Raj Rai" nicht "Rajat Rai" --> bitte, z.B. oben, in den SEC-Dokumenten nachlesen (das richtige Schreiben von Namen wird in den USA sehr ernst genommen...)
• Sturm kaufte am 8.5. für ~EUR1Mio Fresenius-Aktien: http://www.dgap.de/dgap/News/directors_dealings/kauf-sturm-s…
__
mir fällt auf: Stephan Sturm hatte in seiner Karriere zuvor keinen "richtigen" USA-Bezug. Das ist mMn zunächst kein Problem.
Aber für forsches Vorgehen in den USA taugt kein "deutsches" forsches Vorgehen. Siehe Klaus Kleinfeld bei Alcoa/Arconic.
=> das rächt sich eben nun.
jetzt geht's los: Fresenius und Ex-Übernahmeziel Akorn treffen sich vor Gericht --> 9.7.
https://www.finanzen.net/nachricht/aktien/nach-geplatzter-ue…=>
...
Nachdem der Gesundheitskonzern die geplante Übernahme des Generikaherstellers Ende April wegen seiner Einschätzung nach schwer wiegender Verstöße des US-Unternehmens gegen Vorgaben der US-Gesundheitsbehörde FDA zur Datenintegrität in der Produktentwicklung hat platzen lassen, wird nun ein Gericht darüber entscheiden müssen, ob der DAX-Konzern Akorn vielleicht doch übernehmen muss.
Denn Akorn pocht auf den Vollzug der Transaktion und will dies auf juristischem Wege durchsetzen. Erster Verhandlungstag vor dem zuständigen Court of Chancery im US-Bundesstaat Delaware ist Montag, der 9. Juli.
...
Vor einem amerikanischen Gericht stehen sich ein deutsches und ein US-Unternehmen gegenüber....!? handelt es sich hierbei um ein unparteiisches Schiedsgericht?
die Anleger sind jedenfalls optimistisch bei AKRX gestimmt: heute auch +2%
dazu aus: https://thefly.com/landingPageNews.php?id=2754829
...RBC SEES 'PATH TO CLOSING': In a note published this Monday, RBC Capital analyst Randall Stanicky said the trial should bring some clarity to the ongoing deal uncertainty.
He said he continues to see a path to closing and notes risk and reward are both high, as Akorn's stock is trading at $16-$17 versus the $34 deal price. The analyst added he has not yet seen persuasive evidence justifying the deal break based on his review of litigation documents and a call with legal experts.
AKORN UPSIDE/DOWNSIDE 'LOOKS INTERESTING': On Tuesday of this week, Deutsche Bank analyst Gregg Gilbert noted that Akorn is seeking a ruling that Fresenius be required to complete its acquisition per the merger agreement.
The analyst believes the upside versus downside profile for Akorn from the current price is worth considering.
He sees three potential scenarios:
(a) Fresenius is required to acquire Akorn for $34 per share, representing 102% upside;
(b) the deal breaks and Akorn trades down to single digits on a standalone basis, or 69% downside;
(c) the companies settle for a price below $34 but well above the current stock price, which could yield more upside than downside.
Gilbert believes Akorn's upside/downside "looks interesting" ahead of the key event, he told investors.
...
The trial is set to begin on Monday and is scheduled to last through Friday July 13 at this point, RBC recently told investors...

dazu aus: https://thefly.com/landingPageNews.php?id=2754829
...RBC SEES 'PATH TO CLOSING': In a note published this Monday, RBC Capital analyst Randall Stanicky said the trial should bring some clarity to the ongoing deal uncertainty.
He said he continues to see a path to closing and notes risk and reward are both high, as Akorn's stock is trading at $16-$17 versus the $34 deal price. The analyst added he has not yet seen persuasive evidence justifying the deal break based on his review of litigation documents and a call with legal experts.
AKORN UPSIDE/DOWNSIDE 'LOOKS INTERESTING': On Tuesday of this week, Deutsche Bank analyst Gregg Gilbert noted that Akorn is seeking a ruling that Fresenius be required to complete its acquisition per the merger agreement.
The analyst believes the upside versus downside profile for Akorn from the current price is worth considering.
He sees three potential scenarios:
(a) Fresenius is required to acquire Akorn for $34 per share, representing 102% upside;
(b) the deal breaks and Akorn trades down to single digits on a standalone basis, or 69% downside;
(c) the companies settle for a price below $34 but well above the current stock price, which could yield more upside than downside.
Gilbert believes Akorn's upside/downside "looks interesting" ahead of the key event, he told investors.
...
The trial is set to begin on Monday and is scheduled to last through Friday July 13 at this point, RBC recently told investors...
Kurzer Zock:
So, morgen soll also bereits ein Urteil in der Causa Fresenius vs. Akorn Fallen. Ich sehe drei mögliche Szenarien:
1. Das Gericht verdonnert Fresenius dazu die Akquisition wie ursprünglich abgemacht durchzuziehen. Heißt für 34$ pro Aktie, was eine Verdoppelung des jetzigen Kurses bedeuten würde (Eintrittswahrscheinlichkeit 25%).
2. Das Gericht gibt Fresenius auf ganzer Linie recht und der Deal wird abgeblasen. Würde bedeuten, dass der Kurs sich durchaus halbieren könnte (Eintrittswahrscheinlichkeit 25%).
3. US-Gerichte neigen in solchen Fällen oft zu Kompromisslösungen. Würde hier bedeuten, dass der Deal mit einem gewissen Preisabschlag über die Bühne gehen muss. Also unter 34$ aber wohl deutlich über dem jetzigen Kursniveau. Könnte mir hier Aufschlag von ca. 50% vorstellen (Eintrittswahrscheinlichkeit 50%)
Bei Eintritt von Szenario 2. müsste man die Papier ggf. doch länger halten, bis sich der Kurs nach dem ersten Schock wieder berappelt, immerhin Stand der Laden vor dem ganzen Drama auch schon deutlich über 20$. Einen Totalverlust kann man hier ausschließen, der Laden macht ja solide Gewinne.
So ein Chance/Risiko Verhältnis bietet einem kein Casino… Achtung nur etwas für Zocker und auf keinen Fall eine „normale“ Kaufempfehlung…
Achtung nur etwas für Zocker und auf keinen Fall eine „normale“ Kaufempfehlung…
So, morgen soll also bereits ein Urteil in der Causa Fresenius vs. Akorn Fallen. Ich sehe drei mögliche Szenarien:
1. Das Gericht verdonnert Fresenius dazu die Akquisition wie ursprünglich abgemacht durchzuziehen. Heißt für 34$ pro Aktie, was eine Verdoppelung des jetzigen Kurses bedeuten würde (Eintrittswahrscheinlichkeit 25%).
2. Das Gericht gibt Fresenius auf ganzer Linie recht und der Deal wird abgeblasen. Würde bedeuten, dass der Kurs sich durchaus halbieren könnte (Eintrittswahrscheinlichkeit 25%).
3. US-Gerichte neigen in solchen Fällen oft zu Kompromisslösungen. Würde hier bedeuten, dass der Deal mit einem gewissen Preisabschlag über die Bühne gehen muss. Also unter 34$ aber wohl deutlich über dem jetzigen Kursniveau. Könnte mir hier Aufschlag von ca. 50% vorstellen (Eintrittswahrscheinlichkeit 50%)
Bei Eintritt von Szenario 2. müsste man die Papier ggf. doch länger halten, bis sich der Kurs nach dem ersten Schock wieder berappelt, immerhin Stand der Laden vor dem ganzen Drama auch schon deutlich über 20$. Einen Totalverlust kann man hier ausschließen, der Laden macht ja solide Gewinne.
So ein Chance/Risiko Verhältnis bietet einem kein Casino…
 Achtung nur etwas für Zocker und auf keinen Fall eine „normale“ Kaufempfehlung…
Achtung nur etwas für Zocker und auf keinen Fall eine „normale“ Kaufempfehlung… gestern +7.8%
Herr Sturm: wie war das mit:Der Entscheidung liegen unter anderen schwerwiegende Verstöße gegen FDA-Vorgaben zur Datenintegrität bei Akorn zugrunde, die während der von Fresenius eingeleiteten, unabhängigen Untersuchung gefunden wurden. (*)
Fresenius Had Testing Woes Similar to Akorn, Unit Head Says
https://www.bloomberg.com/news/articles/2018-07-12/fresenius…
=>
-- FDA officials cited India plant for computer software issues
-- Drugmaker cited similar problems in canceling Akorn buyout
A Fresenius SE executive acknowledged his company has grappled with testing problems similar to those the German health-care company cited as a basis for pulling out of a $4.3 billion deal to buy U.S. generic drugmaker Akorn Inc.
Mats Henriksson, chief executive of a Fresenius unit, testified Thursday that U.S. Food and Drug Administration inspectors in 2013 questioned drug-injection testing and test-result storage at one of the firm’s plants in India. It took the company two years to address those problems, Henriksson told Delaware Chancery Judge Travis Laster.
If Fresenius is forced to go through with the combination, the health-care conglomerate would take steps to recall all Akorn products tied to the questionable test results and spend four years changing operational procedures, Henriksson said. It would cost Fresenius an estimated $254 million, he said.
Fresenius officials have criticized Lake Forest, Illinois-based Akorn for using the exact same tests at some of its facilities and questioned whether the drugmaker’s computer security was too lax to guarantee the reliability of its test results.
Company officials say Akorn’s testing miscues, along with operational problems that drew FDA sanction, justified canceling the $34-per-share deal. Akorn counters Fresenius is using minor mistakes as a pretext for backing out of the deal.
The German company’s shares dropped the most in two months on Friday, trading down as much as 3.7 percent to 66.2 euros in Frankfurt.
Altered Akorn Test Data Cited as a Reason for Canceled Deal
“The big difference is that we were talking about one site for Fresenius,” Henriksson said. “At Akorn, we were talking about problems with research and development and at multiple sites.”
Laster must decide whether Fresenius properly walked away from the Akorn buyout in April or whether the German company must finalize the deal.
Once the FDA flagged testing problems at the Kalyani plant in West Bengal, India, Fresenius officials shut down the facility and stopped distribution of all products made there, Henriksson said. It brought in consultants to investigate how the testing problems occurred, he said.
The FDA found the company’s computer software wasn’t properly overseeing injection tests of drug ingredients and wasn’t allowing quality checks. “Out-of-specification or undesirable results were ignored and not investigated,” regulators noted in a warning letter. “Only passing results were considered valid,” they added.
Fresenius complained about similar shoddy testing methods used at Akorn, noting its former head of quality control altered test results and then submitted them to the FDA. Officials of the Bad Homburg, Germany-based company also uncovered examples of falsified entries in lab notebooks and destroyed testing notes.
Under cross examination, Henriksson conceded Fresenius executives began to weigh pulling out of the merger in September 2017 after Akorn had two quarters of disappointing sales. Fresenius officials should “build a legal case” to justify a withdrawal from the combination, the company’s senior management noted in an email.
“That was one avenue we had to march,” Henriksson said. While scuttling the merger was one option, the executive said he was focused on plans for rejuvenating Akorn rather than destroying the combination’s potential value.
That email came about two weeks before a whistleblower sent a letter to Fresenius executives alerting them to falsified test data Akorn had submitted to the FDA in a new drug application. Fresenius later learned Mark Silverberg, Akorn’s former head of quality control, had fudged test results on an antibiotic on which the company was seeking FDA approval.
Nathan Sheers, a Washington-based lawyer hired by Fresenius to investigate the depth of Akorn’s data-integrity woes, told Laster he found problems “across the board” at the drugmaker. He said the firm’s computer system has such systemic problems Akorn officials couldn’t substantiate their drug-testing data, the attorney said.
Once the FDA gets the full picture of Akorn’s operational snafus, Sheers said regulators are likely to “throw the book” at the generic drugmaker and may recommend criminal prosecution of some executives.
The case is Akorn Inc. v. Fresenius Kabi AG, 2018-0300, Delaware Chancery Court (Wilmington).
(*) 22.04.2018 http://www.dgap.de/dgap/News/adhoc/fresenius-kgaa-fresenius-…
Antwort auf Beitrag Nr.: 58.207.781 von faultcode am 13.07.18 14:24:23
=> gut, man weiss nie so recht wie so eine vermutlich noch länger andauernde juristische Auseinandersetzung gehen wird, aber diese Aussage klingt nach meinem US-Verständnis nicht gut aus Sicht von Fresenius:
--> Grund: denn wenn diese Sicht der Dinge nun stimmen würde, würde hier klar der Fall einer Täuschung vorliegen, und es kann sein, daß ein US-Richter das gar nicht gerne sieht.
Hat Fresenius (in den USA) getäuscht?
der Bloomberg-Artikel oben ist recht lang, daher nochmal dieser Ausschnitt:Zitat von faultcode: ...Under cross examination, Henriksson conceded Fresenius executives began to weigh pulling out of the merger in September 2017 after Akorn had two quarters of disappointing sales. Fresenius officials should “build a legal case” to justify a withdrawal from the combination, the company’s senior management noted in an email.
“That was one avenue we had to march,” Henriksson said. While scuttling the merger was one option, the executive said he was focused on plans for rejuvenating Akorn rather than destroying the combination’s potential value...
=> gut, man weiss nie so recht wie so eine vermutlich noch länger andauernde juristische Auseinandersetzung gehen wird, aber diese Aussage klingt nach meinem US-Verständnis nicht gut aus Sicht von Fresenius:
--> Grund: denn wenn diese Sicht der Dinge nun stimmen würde, würde hier klar der Fall einer Täuschung vorliegen, und es kann sein, daß ein US-Richter das gar nicht gerne sieht.
Warum beharrt den Akorn so darauf übernommen zu werden? haben sie es so nötig?
Wenn ich mir ein Stück Butter in den Einkaufswagen lege, und mich kurz vor der Kasse dazu entscheide es zurück zu legen ins Kühlregel, kann doch der Supermarkt auch nicht von mir verlangen, das Stück Butter zu kaufen.
Und sofern ich weiß gibt es keine bind. Verpflichtungen zum Kauf, angeblich gib es nicht mal vertragliche Strafgelder wenn die Übernahme nicht zustande kommt.
Wenn ich mir ein Stück Butter in den Einkaufswagen lege, und mich kurz vor der Kasse dazu entscheide es zurück zu legen ins Kühlregel, kann doch der Supermarkt auch nicht von mir verlangen, das Stück Butter zu kaufen.
Und sofern ich weiß gibt es keine bind. Verpflichtungen zum Kauf, angeblich gib es nicht mal vertragliche Strafgelder wenn die Übernahme nicht zustande kommt.
Antwort auf Beitrag Nr.: 58.210.307 von Global-Player83 am 13.07.18 19:02:34
=> doch, die Vereinbarung war ein (unterzeichnetes) binding merger agreement
=> das ist nicht der Streitpunkt. Der Knackpunkt dabei ist, daß nun vom Richter (und Jury?) geprüft werden muss, ob die daran geknüpften Bedingungen von Akorn (und auch Fresenius) hinreichend eingehalten wurden.
SCHEDULE 14A --> oben ist der Link (unten!): https://www.wallstreet-online.de/diskussion/1275357-31-40/an…
--> dort ist die Termination Fee vereinbart:
Pursuant to the merger agreement, the Company will be required to pay Fresenius Kabi a termination fee equal to $129 million if the merger agreement is terminated: --> eine ganze Reihe von Bedingungen...
...doch zur wichtigsten Frage:
(a)
=> dem Akorn-Management bleibt keine Wahl bei dem vereinbarten Preis von USD34 pro Aktie:
--> denn es ist für ein US-Management viel einfacher eine Nicht-US-Company auf Einhaltung des Agreements zu verklagen, als sich später von US-Investoren verklagen zu lassen, denen diese USD34 bei Absage durch die Lappen gegangen sind
(b) zu "haben sie es so nötig?"
--> ja!
• Mats Henriksson/CEO Fresenius Kabi AG sagte ja, daß bei Übernahme Fresenius USD254m über vier Jahre in die Hand nehmen würde, um bei Akorn aufzuräumen
=> das wissen nun alle! Auch die Akorn-Aktionäre.
=> ganz einfach:
• Fresenius: tiefe Taschen
• Akorn: nicht so tiefe Taschen
=> d.h. bei Deal-Abschluss könnten die Akorn-Verantwortlichen zur FDA sagen: "Seht her! Nun wird alles deutsch-gründlich gefixt!"
Zitat von Global-Player83: ...Und sofern ich weiß gibt es keine bind. Verpflichtungen zum Kauf,..
=> doch, die Vereinbarung war ein (unterzeichnetes) binding merger agreement
=> das ist nicht der Streitpunkt. Der Knackpunkt dabei ist, daß nun vom Richter (und Jury?) geprüft werden muss, ob die daran geknüpften Bedingungen von Akorn (und auch Fresenius) hinreichend eingehalten wurden.
SCHEDULE 14A --> oben ist der Link (unten!): https://www.wallstreet-online.de/diskussion/1275357-31-40/an…
--> dort ist die Termination Fee vereinbart:
Pursuant to the merger agreement, the Company will be required to pay Fresenius Kabi a termination fee equal to $129 million if the merger agreement is terminated: --> eine ganze Reihe von Bedingungen...
...doch zur wichtigsten Frage:
Zitat von Global-Player83: Warum beharrt den Akorn so darauf übernommen zu werden? haben sie es so nötig?
(a)
=> dem Akorn-Management bleibt keine Wahl bei dem vereinbarten Preis von USD34 pro Aktie:
--> denn es ist für ein US-Management viel einfacher eine Nicht-US-Company auf Einhaltung des Agreements zu verklagen, als sich später von US-Investoren verklagen zu lassen, denen diese USD34 bei Absage durch die Lappen gegangen sind
(b) zu "haben sie es so nötig?"
--> ja!
• Mats Henriksson/CEO Fresenius Kabi AG sagte ja, daß bei Übernahme Fresenius USD254m über vier Jahre in die Hand nehmen würde, um bei Akorn aufzuräumen
=> das wissen nun alle! Auch die Akorn-Aktionäre.
=> ganz einfach:
• Fresenius: tiefe Taschen
• Akorn: nicht so tiefe Taschen
=> d.h. bei Deal-Abschluss könnten die Akorn-Verantwortlichen zur FDA sagen: "Seht her! Nun wird alles deutsch-gründlich gefixt!"
Antwort auf Beitrag Nr.: 58.219.977 von faultcode am 16.07.18 02:41:39Aus der Traum von der Übernahme:
https://www.wallstreet-online.de/nachricht/10727034-freseniu…
https://www.wallstreet-online.de/nachricht/10727034-freseniu…

Antwort auf Beitrag Nr.: 58.223.136 von Carola11 am 16.07.18 12:54:19
=> Managing Director Hussam Masri hat halt nun die undankbare Aufgabe das legalisierte Anlage-Betrugsinstrument Aktienanleihe Plus 08/2019 (WKN DK0RF9) an den Anleger in Deutschland zu bringen
=> man beachte: ausserhalb Deutschlands und Luxemburg darf diese "Aktienanleihe" (offiziell) nicht vertrieben werden
=> allerdings fiel der Akorn-Kurs am Freitag bis Ende um fast -7%
=> nach meinem Kenntnisstand steht noch gar ncht fest, wann eine erste Entscheidung in diesem Rechtsstreit getroffen wird
noch offen
also mal ganz ehrlich: das ist schon am 22.April von Fresenius selber bekannt gegeben worden => http://www.dgap.de/dgap/News/adhoc/fresenius-kgaa-fresenius-…=> Managing Director Hussam Masri hat halt nun die undankbare Aufgabe das legalisierte Anlage-Betrugsinstrument Aktienanleihe Plus 08/2019 (WKN DK0RF9) an den Anleger in Deutschland zu bringen
=> man beachte: ausserhalb Deutschlands und Luxemburg darf diese "Aktienanleihe" (offiziell) nicht vertrieben werden

=> allerdings fiel der Akorn-Kurs am Freitag bis Ende um fast -7%
=> nach meinem Kenntnisstand steht noch gar ncht fest, wann eine erste Entscheidung in diesem Rechtsstreit getroffen wird
Hmmm...oder aber Szenario 2,5: das ganze zieht sich noch etwas hin...*grummel*
könnte aber für Szenario 3 sprechen...also keine schwarz oder weiß Entscheidung sondern ein Abschlag vom Ursprungspreis in Höhe der Kosten einer Überarbeitung der Betriebsprozesse bei Akorn (ca. 200-300 Millionen $)...wäre immer noch ein enormer Aufschlag zum jetzigen Kurs...
könnte aber für Szenario 3 sprechen...also keine schwarz oder weiß Entscheidung sondern ein Abschlag vom Ursprungspreis in Höhe der Kosten einer Überarbeitung der Betriebsprozesse bei Akorn (ca. 200-300 Millionen $)...wäre immer noch ein enormer Aufschlag zum jetzigen Kurs...

So geht es weiter:
The remaining timeline is as follows post the trial:
July 26 - Fresenius Opening Brief
August 6 - AKRX Response
August 13 - Fresenius Counterclaim
August 20 - AKRX Counterclaim
August 23 - Post-Trial Argument
AKRX wanted a much longer post-trial brief period suggesting that ARKX believes the following:
1. Good news from the FDA (maybe an ANDA approval?)
2. No news from the FDA coming soon (no facility shutdown?/warning letter?)
Quelle: Seeking Alpha
Die Royal Bank of Canada sieht die Chancen für eine Gerichtsentscheidung zu Gunsten von Akorn mittlerweile bei 70% und hat das Kursziel auf 27$ angehoben...es bleibt also spannend!
The remaining timeline is as follows post the trial:
July 26 - Fresenius Opening Brief
August 6 - AKRX Response
August 13 - Fresenius Counterclaim
August 20 - AKRX Counterclaim
August 23 - Post-Trial Argument
AKRX wanted a much longer post-trial brief period suggesting that ARKX believes the following:
1. Good news from the FDA (maybe an ANDA approval?)
2. No news from the FDA coming soon (no facility shutdown?/warning letter?)
Quelle: Seeking Alpha
Die Royal Bank of Canada sieht die Chancen für eine Gerichtsentscheidung zu Gunsten von Akorn mittlerweile bei 70% und hat das Kursziel auf 27$ angehoben...es bleibt also spannend!

-16% -- Akorn falls after judge says he has more to do before decision
https://thefly.com/landingPageNews.php?id=2781295&headline=A…Shares of Akorn (AKRX) are falling after Judge Delaware Chancery Court Judge Travis Laster said he has more to do before making a decision on the company's case against Fresenius (FSNUY).
The latter is seeking to cancel its proposed acquisition of Akorn at $34 per share. "I've got a lot to do before I decide this case," Laster said during a hearing today, according to Bloomberg.
The news agency also cited Fresenius lawyer Lewis Clayton as telling Laster that his company has a "powerful case" that Akorn violated the merger agreement. Shares of Akorn are down 10%, or $1.85, to $16.26 in midday trading.
Antwort auf Beitrag Nr.: 58.521.570 von faultcode am 23.08.18 21:31:13
=>
Law360 (August 23, 2018, 8:24 PM EDT) -- A Delaware vice chancellor warned Thursday that he saw no obvious winner in a battle over Fresenius Kabi AG’s bid to walk away from a $4.3 billion deal to buy generic-drug maker Akorn Inc., with both sides accusing the other of fatal breaches during post-trial arguments.
In the case, now submitted for a ruling, an attorney for Akorn argued that “buyer’s remorse” motivated Fresenius to declare a termination of the tie-up on April 22, before the deal had closed...
No Clear Winner In .3B Akorn Deal Row: Vice Chancellor
https://www.law360.com/securities/articles/1076087/no-clear-…=>
Law360 (August 23, 2018, 8:24 PM EDT) -- A Delaware vice chancellor warned Thursday that he saw no obvious winner in a battle over Fresenius Kabi AG’s bid to walk away from a $4.3 billion deal to buy generic-drug maker Akorn Inc., with both sides accusing the other of fatal breaches during post-trial arguments.
In the case, now submitted for a ruling, an attorney for Akorn argued that “buyer’s remorse” motivated Fresenius to declare a termination of the tie-up on April 22, before the deal had closed...
Dem Einbruch der Akorn Aktien zufolge scheint der Richter ein paar kritische Fragen ggüber Akorn aufgeworfen zu haben.
Judge says Fresenius can walk away from $4.8 bln Akorn deal --> -47% premarket
1.10.https://finance.yahoo.com/news/judge-says-fresenius-walk-awa…
=>
A Delaware judge ruled on Monday that German healthcare group Fresenius SE could walk away from its $4.75 billion deal for U.S. drugmaker Akorn Inc and rejected Akorn's claim that the merger agreement had been breached.
Delaware Vice Chancellor Travis Laster said Fresenius validly terminated the merger agreement and he found Akorn's representations regarding its compliance with regulatory requirements were "not true and correct."
Shares of Akorn plunged 47 percent in premarket U.S. trading, while shares of Fresenius jumped 7.5 percent.
Akorn did not immediately respond to a request for comment.
Laster, who oversaw a five-day trial, wrote in a 246-page opinion that Akorn's "magnitude of inaccuracies would reasonably be expected to result in a material adverse effect," allowing Fresenius to walk away.
Laster also said Akorn breached its obligation to continue operating in the ordinary course after signing the deal.
The judge has said he anticipates one or both sides would appeal his opinion to the state's Supreme Court...
Antwort auf Beitrag Nr.: 58.842.870 von faultcode am 01.10.18 15:23:59Aktienkurs nähert sich damit langsam dem Buchwert an bei so USD5.75 per 30.6.2018
Antwort auf Beitrag Nr.: 58.842.924 von faultcode am 01.10.18 15:29:59
9:26 am ET October 1, 2018 (Benzinga) Print
“We are disappointed by the ruling by the Delaware Chancery Court determining not to force Fresenius to close and we continue to believe Fresenius’ attempt to terminate the transaction is in breach of our binding merger agreement.
We intend to appeal, in an effort to vigorously enforce our rights and continue to protect the interests of our Company and our shareholders.”
© 2018 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
AKRX: intention to appeal
Akorn Offers Comment On Delaware Complaint Against Fresenius9:26 am ET October 1, 2018 (Benzinga) Print
“We are disappointed by the ruling by the Delaware Chancery Court determining not to force Fresenius to close and we continue to believe Fresenius’ attempt to terminate the transaction is in breach of our binding merger agreement.
We intend to appeal, in an effort to vigorously enforce our rights and continue to protect the interests of our Company and our shareholders.”
© 2018 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Fresenius muss Akorn nicht übernehmen
https://boerse.ard.de/aktien/Fresenius-muss-akorn-nicht-uebe…
https://boerse.ard.de/aktien/Fresenius-muss-akorn-nicht-uebe…
live 5,47. mal sehen ob es heute wieder hoch geht
sehe bei 5,25 eine bodenbildung
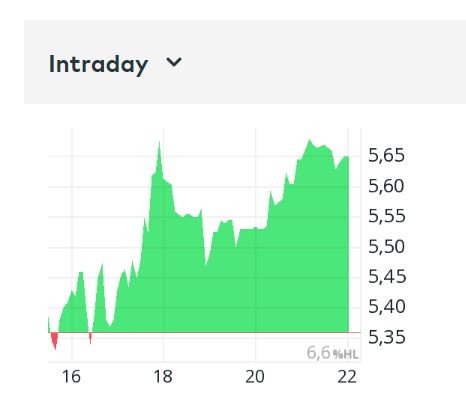




Antwort auf Beitrag Nr.: 58.843.059 von faultcode am 01.10.18 15:42:21Eigentlich kann das in den TSA (Trump States of America) doch nicht sein. Oder ist Akorn zu klein oder Fresenius halten die Amis für eine US-Firma (wie die Telekom :laugh ?
?
Kurs hat sich vom Hoch jetzt in etwas gezehntelt. Aber können die alleine überleben? Deren Bilanz gefällt mir weniger.
 ?
?Kurs hat sich vom Hoch jetzt in etwas gezehntelt. Aber können die alleine überleben? Deren Bilanz gefällt mir weniger.
Antwort auf Beitrag Nr.: 58.868.484 von mindgames1001 am 04.10.18 13:33:07Hallo,
1/ ich habe Schwierigkeiten Deine Charts zuzuordnen - zeitlich, als auch bzgl. des Marktes
2/ "Bodenbildung"?? --> Chart in USD seit dem besagten Montag, 1.10., an der NASDAQ:
--> der "Boden" war ratzfatz durch, wenn er denn hält bezogen auf den vom Trader anvisierten Zeithorizont --> wird mMn so gesehen viel eher eine "V"-Formation mittelfristig betrachtet
__
abgesehen davon:
• Akorn hätte gerne Geld von Fresenius, damit die wieder ihre Ruhe haben können, und Herr Sturm seinen "USA-Schock" endlich überwinden kann
1/ ich habe Schwierigkeiten Deine Charts zuzuordnen - zeitlich, als auch bzgl. des Marktes
2/ "Bodenbildung"?? --> Chart in USD seit dem besagten Montag, 1.10., an der NASDAQ:

--> der "Boden" war ratzfatz durch, wenn er denn hält bezogen auf den vom Trader anvisierten Zeithorizont --> wird mMn so gesehen viel eher eine "V"-Formation mittelfristig betrachtet
__
abgesehen davon:
• Akorn hätte gerne Geld von Fresenius, damit die wieder ihre Ruhe haben können, und Herr Sturm seinen "USA-Schock" endlich überwinden kann

Brief 5,30
Akorn Receives Product Approval
LAKE FOREST, Ill., Oct. 10, 2018 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty generic pharmaceutical company, today announced that it has received a new Abbreviated New Drug Application (ANDA) approval from the U.S. Food and Drug Administration (FDA) for Bimatoprost Ophthalmic Solution, 0.03%. The product is manufactured at Akorn’s Amityville, New York manufacturing facility.
According to IQVIA, sales of bimatoprost ophthalmic solution, 0.03% were approximately $63.5 million for the twelve months ended August 2018.
Bimatoprost ophthalmic solution, 0.03% is indicated to treat hypotrichosis of the eyelashes by increasing their growth including length, thickness and darkness.
LAKE FOREST, Ill., Oct. 10, 2018 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty generic pharmaceutical company, today announced that it has received a new Abbreviated New Drug Application (ANDA) approval from the U.S. Food and Drug Administration (FDA) for Bimatoprost Ophthalmic Solution, 0.03%. The product is manufactured at Akorn’s Amityville, New York manufacturing facility.
According to IQVIA, sales of bimatoprost ophthalmic solution, 0.03% were approximately $63.5 million for the twelve months ended August 2018.
Bimatoprost ophthalmic solution, 0.03% is indicated to treat hypotrichosis of the eyelashes by increasing their growth including length, thickness and darkness.
Antwort auf Beitrag Nr.: 58.882.683 von faultcode am 05.10.18 21:38:36
https://www.finanzen.net/nachricht/aktien/klage-auf-uebernah…
=> das war abzusehen, alleine schon, um (in den USA) die Rechte der eigenen Aktionäre zu weit wie möglich zu wahren
=> dann noch dieses:
Außerdem beantragte Akorn, den Berufungsantrag beschleunigt zu behandeln.
=> mal schauen; bei Gerichtsprognosen bin ich vorsichtig
Akorn legt Berufung gegen Urteil im Prozess gegen Fresenius ein
18.10.https://www.finanzen.net/nachricht/aktien/klage-auf-uebernah…
=> das war abzusehen, alleine schon, um (in den USA) die Rechte der eigenen Aktionäre zu weit wie möglich zu wahren
=> dann noch dieses:
Außerdem beantragte Akorn, den Berufungsantrag beschleunigt zu behandeln.
=> mal schauen; bei Gerichtsprognosen bin ich vorsichtig

Antwort auf Beitrag Nr.: 58.997.742 von faultcode am 18.10.18 21:22:08die werden sich wieder erholen, ich hab gestern investiert glück auf
Antwort auf Beitrag Nr.: 59.056.705 von sir1 am 25.10.18 17:50:16die Umschichtung in Defensiv-Werte in den USA ist nach wie vor am Laufen --> davon profitiert (momentan) auch eine Akorn
--> eine oberlangweilige Procter & Gamble mit ~+8% in einem Monat (keine Reklame - nur anderes Beispiel)
(keine Reklame - nur anderes Beispiel)
--> eine oberlangweilige Procter & Gamble mit ~+8% in einem Monat
 (keine Reklame - nur anderes Beispiel)
(keine Reklame - nur anderes Beispiel)
unter welchen Gesichtspunkten erachtest du Akorn als defensiven Wert....?
Antwort auf Beitrag Nr.: 59.057.281 von Global-Player83 am 25.10.18 18:25:42Pharma-Generika
Q3 --> -6% after hours
Akorn Provides Third Quarter 2018 ResultsLAKE FOREST, Ill., Nov. 06, 2018 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty generic pharmaceutical company, today announced its financial results for the third quarter of 2018.
Business Highlights
• Revenues declined predominantly due to the effect of competition on key products such as Ephedrine Sulfate Injection, Lidocaine Ointment, Methylene Blue Injection and Nembutal
• In the third quarter of 2018, the decrease in revenues due to pricing was approximately 3% compared to the same period in 2017, the lowest drop in the trailing six quarters
• Overall portfolio well-balanced and diversified with no product contributing greater than 10% of total revenues
• Received six new ANDA approvals and launched three new ANDAs in the first 10 months of 2018
• Completed construction of new laboratory facilities at Decatur and Somerset manufacturing sites
• Completed installation and qualification of serialization equipment on all required packaging lines ahead of FDA’s November 2018 enforcement deadline for the Drug Supply Chain Security Act
• Continued to make progress on FDA action items and milestones as a result of recent inspections of our facilities in Decatur and Somerset, as well as data integrity assessments and remediation globally
70 Mio. $ Verlust in Q3, nach 9 Monaten sind es bereits 187 Mio. $ Verlust, Cash und Equitypositionen nehmen ab, Bilanz verschlechtert sich von Q zu Q
Da kann ich gut verstehen, dass Fresenius den Rückzug angetreten hat.
Da kann ich gut verstehen, dass Fresenius den Rückzug angetreten hat.
Antwort auf Beitrag Nr.: 59.157.723 von Global-Player83 am 07.11.18 13:01:01Um so bitterer wäre es, wenn Fresenius die Berufung verlieren und Akorn doch noch kaufen bzw. entschädigen müsste.
Wie hoch schätzt du dieses Risiko ein?
ich denke 10/90 wenn überhaupt, wenn dann nur politisch und protektionistisch motiviert
Die Probleme bei Akorn scheinen ja nicht aus der Luft gegriffen zu sein:
https://www.fiercepharma.com/manufacturing/fda-cited-akorn-n…
Die Probleme bei Akorn scheinen ja nicht aus der Luft gegriffen zu sein:
https://www.fiercepharma.com/manufacturing/fda-cited-akorn-n…
Antwort auf Beitrag Nr.: 59.167.791 von Global-Player83 am 08.11.18 12:44:23Keine Ahnung, 10% wäre schon denkbar.
Akorn-Fresenius Fight Over Canceled Deal Is Argued in Court
5.12.https://www.bloomberg.com/news/articles/2018-12-05/akorn-fre…
=>
• Akorn targets decision allowing German company to walk away
• Delaware court’s ruling may shed light on ‘material change’
Akorn still trying to salvage its multibillion-dollar sale to German drugmaker Fresenius SE, argued that a trial judge improperly rewrote state law and used “guesswork” in allowing Fresenius to scrap the deal.
The case marks the first time the Delaware Supreme Court has weighed the thorny question of when a company can cancel a purchase because the target’s business took a sharp downturn before a deal closed. The five justices said they’d deliver their decision later.
“Since it’s the first, people will compare their cases to this one to see if they have enough to walk away,” said Charles Elson, director of the John L. Weinberg Center for Corporate Governance at the University of Delaware.
The decision is eagerly anticipated by arbitragers who bet on merger-and-acquisition deals, and will shed light on what amounts to a “material adverse change” in a firm’s business under Delaware law. Many high-profile disputes over merger and buyout deals are heard in Delaware, corporate home to more than half the U.S.’s public companies and more than 60 percent of Fortune 500 firms. Its chancery court specializes in quickly hearing big-dollar business cases.
Judge Travis Laster erred in allowing Fresenius to renege on its agreement to pay $4.3 billion for Akorn because it knew the risks of acquiring the maker of generic injectable drugs, Daniel Slifkin, one of the company’s lawyers, told the state’s highest court Wednesday.
Laster ignored evidence showing that Fresenius’s executives “set out to tank the deal” and used his own “personal intuition and guesswork,” rather than solid legal reasoning, to ratify the German drugmaker’s move to scuttle the buyout, Slifkin said.
Fresenius’s lawyer countered that Laster relied on stacks of evidence showing a laundry list of problems at Akorn that went unaddressed as its revenues plummeted, in finding that the company’s business had radically declined prior to the closing deadlines.
“Every time somebody picked up a rock at Akorn, they found more problems, each worse than the last,” Lewis Clayton, the German company’s lawyer, told the court. While Laster found multiple reasons to conclude Akorn’s business suffered a material change, Fresenius needed only one reason to win, he added.
Akorn sued in April after Fresenius pulled out of the deal, citing the U.S. company’s plunging revenues and operational problems. As the deal was being finalized, Fresenius officials discovered Akorn wouldn’t meet profit projections. An anonymous whistleblower also tipped off Fresenius executives about a longstanding pattern of problems in Akorn’s drug-development and manufacturing systems.
Akorn officials claimed Fresenius focused on minor regulatory and manufacturing miscues as a pretext for canceling the buyout after suffering “buyer’s remorse.” Fresenius had suffered some of the same kinds of operational problems, they said.
After a weeklong trial, Laster ruled in October that the deterioration of Akorn’s business was severe enough to create a “material adverse event” allowing Fresenius to walk away. That caused Akorn’s stock to plunge almost 60 percent in New York.
While other chancery judges have found that such double-digit declines didn’t amount to a material change, Laster said Akorn’s nosedive was attributable to “company-specific problems” rather than industrywide trends. Those problems included phony test results presented to the U.S. Food and Drug Administration, a computer system with inadequate security and a history of manufacturing problems that drew a slew of regulatory warnings.
Salvage Deal
In briefs filed with the appellate court, Akorn contends Laster didn’t give it sufficient credit for quickly moving to address the problems uncovered during the dispute in hopes of salvaging the deal. It also said Fresenius Chief Executive Officer Stephan Sturm ordered underlings to find a way to extricate the company from the transaction.
In its own filings, the German company pointed to Laster’s comment that Akorn’s business “fell off a cliff” before the deal was consummated. He also found “overwhelming evidence of widespread regulatory problems and compliance problems.” That was enough to create a material adverse change under Delaware law, Fresenius’s lawyers argue.
Fresenius also rejected Akorn’s claims it quickly addressed data and operational problems, noting the generic drugmaker ignored manufacturing miscues and took “extraordinary steps to conceal its violations in the hope Fresenius would not discover them until after the transaction closed.”
The case is Akorn Inc. v. Fresenius Kabi AG, No. 535-2018, Delaware Supreme Court (Dover)...
Fresenius muss Akorn nicht übernehmen
Antwort auf Beitrag Nr.: 59.391.305 von Global-Player83 am 07.12.18 18:28:59Die Bilanz ist kurzfristig noch o.k., W/C fast so groß wie die MK. Aber sie müssten mal profitabel werden. Irgendwann müssen sie auch die 0,8 Mrd. long terms zurückzahlen.
Kann jemand eine positive Gewinnprognose abgeben? Der alte CEO konnte es offenbar nicht, dann muss er auch weg. Fresenius als Retter ist jedenfalls Geschichte.
Kann jemand eine positive Gewinnprognose abgeben? Der alte CEO konnte es offenbar nicht, dann muss er auch weg. Fresenius als Retter ist jedenfalls Geschichte.
Antwort auf Beitrag Nr.: 59.380.889 von faultcode am 06.12.18 16:44:54
http://investors.akorn.com/news-releases/news-release-detail…
=>
...Akorn, Inc. (NASDAQ: AKRX) today said it will move forward and rebuild shareholder value as an independent company following the disappointing decision from the Supreme Court of the State of Delaware upholding the lower court’s decision to allow Fresenius Kabi AG to terminate the April 2017 merger agreement.
With the litigation process concluded, Akorn’s Board of Directors announced that it is engaged in a formal search for a new chief executive officer to lead the company into its next phase. Current CEO Raj Rai has decided to retire and will assist the board to ensure a smooth transition and remain in his role until the hiring date of the new chief executive.
“We recognize that this has been an extended period of uncertainty for Akorn’s customers, employees and investors and the Board is committed to ensuring the company’s stability and long-term growth,” said Board Chairman Alan Weinstein. “While there is work to do, Akorn’s future remains bright thanks to its manufacturing, quality and generics expertise and is not dependent upon a consummated transaction with Fresenius. We thank Raj for his years of service with Akorn and his success in building the company into a leading organization in a highly competitive industry.”...
Supreme Court of the State of Delaware Rules Against Akorn, New CEO Search Underway
Dec. 07, 2018http://investors.akorn.com/news-releases/news-release-detail…
=>
...Akorn, Inc. (NASDAQ: AKRX) today said it will move forward and rebuild shareholder value as an independent company following the disappointing decision from the Supreme Court of the State of Delaware upholding the lower court’s decision to allow Fresenius Kabi AG to terminate the April 2017 merger agreement.
With the litigation process concluded, Akorn’s Board of Directors announced that it is engaged in a formal search for a new chief executive officer to lead the company into its next phase. Current CEO Raj Rai has decided to retire and will assist the board to ensure a smooth transition and remain in his role until the hiring date of the new chief executive.
“We recognize that this has been an extended period of uncertainty for Akorn’s customers, employees and investors and the Board is committed to ensuring the company’s stability and long-term growth,” said Board Chairman Alan Weinstein. “While there is work to do, Akorn’s future remains bright thanks to its manufacturing, quality and generics expertise and is not dependent upon a consummated transaction with Fresenius. We thank Raj for his years of service with Akorn and his success in building the company into a leading organization in a highly competitive industry.”...
!
Dieser Beitrag wurde von CloudMOD moderiert. Grund: Tatsachenbehauptungen bitte immer mit Quellenangaben
Q3 Verlust 70 mio. $, nach 9 Monaten schon rund 187 Mio. $ verlust!
http://investors.akorn.com/news-releases/news-release-detail…
http://investors.akorn.com/news-releases/news-release-detail…
ja die zahlen sind von DO, dem 6.11.
aus dem grund und der nicht-übernahme hat sich der kurs ja schon halbiert
ps ich hoffe oder denke dass das tief von 3,50 dollar nochmal kurz getestet wird. aber man wird sehen.
aus dem grund und der nicht-übernahme hat sich der kurs ja schon halbiert
ps ich hoffe oder denke dass das tief von 3,50 dollar nochmal kurz getestet wird. aber man wird sehen.
Antwort auf Beitrag Nr.: 59.406.105 von mindgames1001 am 10.12.18 16:15:09Ich habe mir das Ding noch mal angeschaut. Umsätze fallen, Kosten steigen. Der einzige Lichtblick ist, dass die Schulden erst 2021 fällig werden.
Was kann die Firma retten? Wohl nur die neuen Produkte. Ich sehe aber insgesamt wenig Transparenz. Ich denke, die Verluste gehen weiter und der Kurs Richtung 1 USD.
Wenn der neue CEO kein großer Aufräumer ist, geht das Ding pleite. Mir ist völlig rätselhaft, warum Sturm von Fresenius die kaufen wollte. Wirft wohl auch ein Licht auf ihn, bisher fand ich den eigentlich sehr clever.
Was kann die Firma retten? Wohl nur die neuen Produkte. Ich sehe aber insgesamt wenig Transparenz. Ich denke, die Verluste gehen weiter und der Kurs Richtung 1 USD.
Wenn der neue CEO kein großer Aufräumer ist, geht das Ding pleite. Mir ist völlig rätselhaft, warum Sturm von Fresenius die kaufen wollte. Wirft wohl auch ein Licht auf ihn, bisher fand ich den eigentlich sehr clever.
bei ca. 4,60 dollar sollte ein widerstand sein, bin immer noch short
live 4,26 -4,5 %
live 3,69 eur -6%
Akorn Names Douglas S. Boothe as President and CEO
20.12.http://investors.akorn.com/news-releases/news-release-detail…
=>
Akorn, Inc. (NASDAQ: AKRX), today announced that Douglas S. Boothe has been named president and chief executive officer effective January 1, 2019, bringing deep pharmaceuticals expertise and a proven track record as Akorn looks forward to its next phase as an independent company committed to rebuilding shareholder value.
Boothe is an industry veteran with extensive accomplishments in the specialty, generic and over-the-counter pharmaceuticals business. He brings to Akorn a long history of senior level expertise in general management, strategy, sales and marketing, product selection, supply chain and business development in the US and global arenas.
Highlights among his many skills are the abilities to produce strong revenue growth for specialty and generic products and lead companies through challenging environments with key stakeholders. Boothe has overseen many successful new product development projects, regulatory reviews and approvals, paragraph IV litigations and commercial launches. He has also reinvigorated research and development, regulatory, compliance and supply chain platforms in his past roles.
Most recently, Boothe served as the president of the $600 million generics division of publicly held Impax Laboratories, which developed, manufactured and marketed bioequivalent pharmaceuticals and was acquired by Amneal Pharmaceuticals LLC in a reverse merger transaction in May 2018.
Prior to that Boothe was the executive vice president and general manager of Perrigo Company Plc, with responsibility for the approximately $1 billion U.S. pharmaceuticals business, which included generics and specialty pharmaceutical products. He also served as the CEO of Actavis Inc., the U.S. manufacturing and marketing division of Actavis Group, and held senior positions at Alpharma and Pharmacia Corp.
“My fellow board members and I are truly excited to welcome Doug to Akorn at this pivotal time for the company,” said Board of Directors Chairman Alan Weinstein. “After a comprehensive search, Doug quickly rose to the top of the list due to his proven track record in helping build businesses, navigate regulatory complexities and guide companies through competitive market environments.”
Boothe said: “I am excited and humbled with the opportunity to lead Akorn and our dedicated employees as we focus our energies toward expanding our offerings to customers, improving our financial performance and enhancing long-term shareholder value. Akorn and I are committed to manufacturing excellence and quality as we move forward.”
Boothe received his undergraduate degree from Princeton University and his MBA from the Wharton School of Business at the University of Pennsylvania.
=> na dann, frohes Gelingen
Antwort auf Beitrag Nr.: 59.492.781 von faultcode am 21.12.18 20:08:39
https://www.marketwatch.com/story/akorn-receives-warning-let…
=>
...Akorn Inc. said Wednesday that it received a warning letter from the Food and Drug Administration, related to an inspection of the generic drug maker's Decatur, Illinois manufacturing facility.
The stock was halted for news, and was inactive prior to the halt. The FDA had investigated the facility in April and May 2018. The company said it will respond to the FDA letter, dated Jan. 4, within 15 working days.
"Akorn is committed to the highest standards of quality and compliance, and will continue to work collaboratively with the FDA to resolve all issues addressed in the warning letter," Akorn said in a statement...
Akorn receives 'warning letter' from FDA related to a manufacturing facility
9.1.https://www.marketwatch.com/story/akorn-receives-warning-let…
=>
...Akorn Inc. said Wednesday that it received a warning letter from the Food and Drug Administration, related to an inspection of the generic drug maker's Decatur, Illinois manufacturing facility.
The stock was halted for news, and was inactive prior to the halt. The FDA had investigated the facility in April and May 2018. The company said it will respond to the FDA letter, dated Jan. 4, within 15 working days.
"Akorn is committed to the highest standards of quality and compliance, and will continue to work collaboratively with the FDA to resolve all issues addressed in the warning letter," Akorn said in a statement...
Antwort auf Beitrag Nr.: 59.592.743 von faultcode am 09.01.19 14:03:25Au - Fresenius hat nachgelegt ("amendment"):
20.2.
Akorn Comments on Fresenius’ Proposed Amended Claims
http://investors.akorn.com/news-releases/news-release-detail…
=>
...Akorn, Inc. (Nasdaq: AKRX) today announced that it strongly contests Fresenius’ proposed amended claims filed earlier today.
Akorn believes these claims are meritless and overreaching. Akorn denies the allegations and will vigorously defend itself in this litigation, while continuing to focus on advancing its pipeline, strengthening its business and developing a strategic plan for improving financial performance and long-term shareholder value....
=> -9% gestern
20.2.
Akorn Comments on Fresenius’ Proposed Amended Claims
http://investors.akorn.com/news-releases/news-release-detail…
=>
...Akorn, Inc. (Nasdaq: AKRX) today announced that it strongly contests Fresenius’ proposed amended claims filed earlier today.
Akorn believes these claims are meritless and overreaching. Akorn denies the allegations and will vigorously defend itself in this litigation, while continuing to focus on advancing its pipeline, strengthening its business and developing a strategic plan for improving financial performance and long-term shareholder value....
=> -9% gestern
Antwort auf Beitrag Nr.: 59.931.692 von faultcode am 21.02.19 13:43:3628.2.
http://investors.akorn.com/news-releases/news-release-detail…
=>
Fourth Quarter 2018 and Recent Business Highlights
• Net revenue was $153 million, a decline of $33 million, or 17.6%, compared to the fourth quarter of 2017, predominantly due to the effect of competition on key products and shortfalls in supply
• Net loss was $215 million compared to a net loss of $65 million in the fourth quarter of 2017. Adjusted EBITDA was $(20) million compared to $28 million in the fourth quarter of 2017
• Continued progress on completion of FDA action items related to the inspections of our facilities in Decatur and Somerset
• Appointed Douglas Boothe as President and Chief Executive Officer, bringing significant generic and specialty pharmaceutical leadership experience
• Announced new additions to Board of Directors and Executive Management team adding significant expertise to the Company’s leadership
• Focused on moving forward and rebuilding shareholder value as an independent company following the terminated Fresenius Kabi AG merger agreement
• Engaged financial, operational, and legal advisors to help develop and execute long-term growth plan
• Healthy cash position of $225 million as of December 31, 2018
http://investors.akorn.com/news-releases/news-release-detail…
=>
Fourth Quarter 2018 and Recent Business Highlights
• Net revenue was $153 million, a decline of $33 million, or 17.6%, compared to the fourth quarter of 2017, predominantly due to the effect of competition on key products and shortfalls in supply
• Net loss was $215 million compared to a net loss of $65 million in the fourth quarter of 2017. Adjusted EBITDA was $(20) million compared to $28 million in the fourth quarter of 2017
• Continued progress on completion of FDA action items related to the inspections of our facilities in Decatur and Somerset
• Appointed Douglas Boothe as President and Chief Executive Officer, bringing significant generic and specialty pharmaceutical leadership experience
• Announced new additions to Board of Directors and Executive Management team adding significant expertise to the Company’s leadership
• Focused on moving forward and rebuilding shareholder value as an independent company following the terminated Fresenius Kabi AG merger agreement
• Engaged financial, operational, and legal advisors to help develop and execute long-term growth plan
• Healthy cash position of $225 million as of December 31, 2018
Antwort auf Beitrag Nr.: 59.989.915 von faultcode am 28.02.19 20:24:27
=> LAKE FOREST, Ill., March 01, 2019 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq: AKRX) announced today that the Delaware Court of Chancery has denied a request by Fresenius Kabi AG to bring a fraud claim against Akorn. ...
Akorn Comments on Favorable Court Order
http://investors.akorn.com/news-releases/news-release-detail…" target="_blank" rel="nofollow ugc noopener">http://investors.akorn.com/news-releases/news-release-detail…=> LAKE FOREST, Ill., March 01, 2019 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq: AKRX) announced today that the Delaware Court of Chancery has denied a request by Fresenius Kabi AG to bring a fraud claim against Akorn. ...
Akorn Provides Preliminary First Quarter 2019 Results and Full Year Guidance => +18% premarket
7.5http://investors.akorn.com/news-releases/news-release-detail…
=>
...
First Quarter 2019 and Recent Business Highlights
• Net revenue was $166 million, up 8% from the fourth quarter of 2018, down 10% from the prior year quarter
• Net loss was $82 million, compared to $215 million in the fourth quarter of 2018 and $29 million in the prior year quarter
• Adjusted EBITDA was $10 million, compared to ($20) million in the fourth quarter of 2018 and $25 million in the prior year quarter
• Cash position of $184 million as of March 31, 2019
• Full year 2019 guidance anticipates continued sequential improvement throughout the year
• Ongoing progress towards completion of FDA action items related to the inspections of our facilities in Decatur and Somerset
• Further strengthening of leadership team and organizational capabilities
• Significant reduction in backorders and failure to supply penalties
• Two products launched year-to-date: Ropivacaine Hydrochloride Injection, USP (2mg/ml) and TheraTears® SteriLid® Antimicrobial, the 1st FDA Accepted Antimicrobial Eyelid Cleanser
• Two New Product Approvals: Loteprednol Etabonate Ophthalmic Suspension, 0.5% and Fluticasone Propionate Nasal Spray USP, 50 mcg per spray (OTC)
• Extended revolving credit facility and executed a Standstill Agreement with current lending institutions
See "Non-GAAP Financial Measures" below.
Antwort auf Beitrag Nr.: 60.505.692 von faultcode am 07.05.19 14:01:17..und nun +27%
AH +3&
May 20, 2019 4:05 PM EDT
Akorn Receives FDA Approval for Azelastine Hydrochloride Nasal Spray, 0.1%
http://investors.akorn.com/news-releases/news-release-detail…
May 20, 2019 4:05 PM EDT
Akorn Receives FDA Approval for Azelastine Hydrochloride Nasal Spray, 0.1%
http://investors.akorn.com/news-releases/news-release-detail…
Akorn Receives FDA Warning Letter
http://investors.akorn.com/news-releases/news-release-detail…=>
...Akorn, Inc. (Nasdaq: AKRX), a leading specialty generic pharmaceutical company, announced that it received a warning letter from the U.S. Food and Drug Administration (FDA) related to the inspection of its Somerset, New Jersey manufacturing facility in July and August of 2018.
Akorn is committed to the highest standards of quality and compliance, and will continue to work collaboratively with the FDA to resolve all issues addressed in the warning letter. The Company will respond to the FDA letter within the required 15 working days from receipt of the letter....
Antwort auf Beitrag Nr.: 60.886.584 von faultcode am 25.06.19 15:16:23Akorn auf dem Weg zurück zur alten Kampflinie bei ~USD6.6

Antwort auf Beitrag Nr.: 60.914.265 von faultcode am 28.06.19 16:12:15
LAKE FOREST, Ill., Aug. 01, 2019 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty generic pharmaceutical company, today announced its preliminary financial results for the second quarter of 2019.
Second Quarter 2019 and Recent Business Highlights
• Net revenue was $178 million, up 7% from the first quarter of 2019, down 7% from the prior year quarter
• Net loss was $112 million, compared to $82 million in the first quarter of 2019 and $88 million in the prior year quarter
• Adjusted EBITDA was $22 million, compared to $10 million in the first quarter of 2019 and $35 million in the prior year quarter
• Generated positive operating cash flow during the second quarter
• Continued sequential reduction in backorders and failure to supply penalties
• Launched three products: TheraTears® SteriLid® Antimicrobial, the first FDA Accepted Antimicrobial Eyelid Cleanser, Loteprednol Etabonate Ophthalmic Suspension, 0.5%, and Dicyclomine Hydrochloride Injection, USP
• Received three ANDA approvals: Loteprednol Etabonate Ophthalmic Suspension, 0.5%, Fluticasone Propionate Nasal Spray USP, 50 mcg per spray (OTC), and Azelastine Hydrochloride Nasal Spray, 0.1%
• Responded to FDA warning letter related to the 2018 inspection of our Somerset, NJ manufacturing facility
• Reached non-binding agreement in principle with lead plaintiffs in the securities class action litigation
See "Non-GAAP Financial Measures" below.
Douglas Boothe, Akorn’s President and Chief Executive Officer, stated, “We are pleased that our second quarter results showed continued financial and operational improvements across the business, a strong signal that our operational initiatives are creating value and generating momentum. We saw reductions in backorders and failure to supply penalties, which have a direct impact on both financial performance and customer satisfaction.”
Boothe continued, “As we look to the second half of 2019, we feel confident in the fundamentals of our business and believe that our focus on compliance, transparency, and accountability will allow us to execute against our strategic growth objectives.
As such, we are updating our net loss guidance and affirming our net revenue and adjusted EBITDA guidance for the full year of 2019 as we strive to return the Company to a path of long-term profitable growth for our stakeholders.”
....
(FC: Format)
Akorn Provides Preliminary Second Quarter 2019 Results
http://investors.akorn.com/news-releases/news-release-detail…LAKE FOREST, Ill., Aug. 01, 2019 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty generic pharmaceutical company, today announced its preliminary financial results for the second quarter of 2019.
Second Quarter 2019 and Recent Business Highlights
• Net revenue was $178 million, up 7% from the first quarter of 2019, down 7% from the prior year quarter
• Net loss was $112 million, compared to $82 million in the first quarter of 2019 and $88 million in the prior year quarter
• Adjusted EBITDA was $22 million, compared to $10 million in the first quarter of 2019 and $35 million in the prior year quarter
• Generated positive operating cash flow during the second quarter
• Continued sequential reduction in backorders and failure to supply penalties
• Launched three products: TheraTears® SteriLid® Antimicrobial, the first FDA Accepted Antimicrobial Eyelid Cleanser, Loteprednol Etabonate Ophthalmic Suspension, 0.5%, and Dicyclomine Hydrochloride Injection, USP
• Received three ANDA approvals: Loteprednol Etabonate Ophthalmic Suspension, 0.5%, Fluticasone Propionate Nasal Spray USP, 50 mcg per spray (OTC), and Azelastine Hydrochloride Nasal Spray, 0.1%
• Responded to FDA warning letter related to the 2018 inspection of our Somerset, NJ manufacturing facility
• Reached non-binding agreement in principle with lead plaintiffs in the securities class action litigation
See "Non-GAAP Financial Measures" below.
Douglas Boothe, Akorn’s President and Chief Executive Officer, stated, “We are pleased that our second quarter results showed continued financial and operational improvements across the business, a strong signal that our operational initiatives are creating value and generating momentum. We saw reductions in backorders and failure to supply penalties, which have a direct impact on both financial performance and customer satisfaction.”
Boothe continued, “As we look to the second half of 2019, we feel confident in the fundamentals of our business and believe that our focus on compliance, transparency, and accountability will allow us to execute against our strategic growth objectives.
As such, we are updating our net loss guidance and affirming our net revenue and adjusted EBITDA guidance for the full year of 2019 as we strive to return the Company to a path of long-term profitable growth for our stakeholders.”
....
(FC: Format)
Antwort auf Beitrag Nr.: 61.152.113 von faultcode am 01.08.19 13:57:14nächstes Ziel mMn: USD6..7
--> aus der Q2-Präsentation:
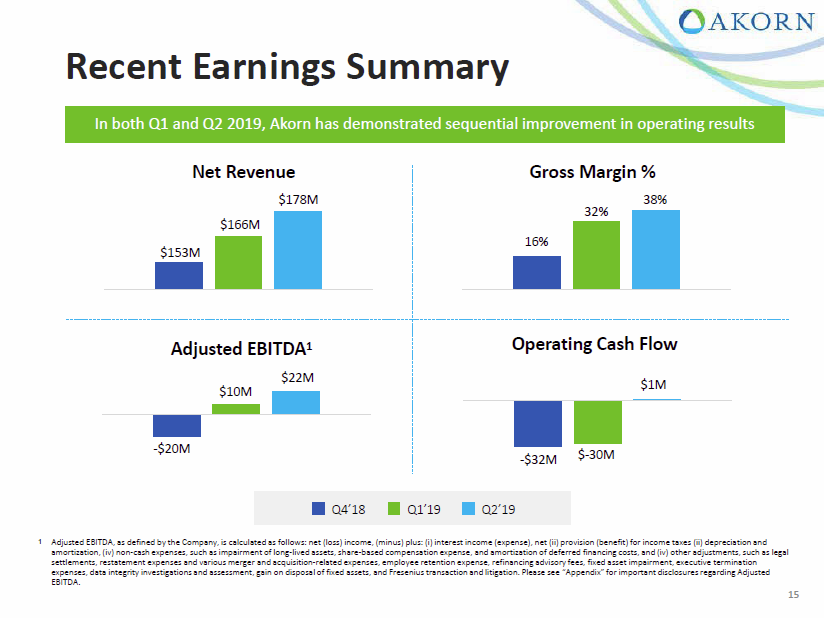
-->
• die großen Hedgies sind alle raus
• die anderen sind gebrannte Kinder
• ...und der Rest traut sich nicht
• die Shorties bauten zuletzt ab, liegen aber immer noch höher als Ende 2018 --> https://www.nasdaq.com/market-activity/stocks/akrx/short-int…
--> https://www.nasdaq.com/market-activity/stocks/akrx/short-int…
=> nur die beinharten sind z.Z. (long) drin
--> ich überlege, vor Q3 nachzukaufen: 31.10.? -- noch unbestätigt
--> aus der Q2-Präsentation:

-->
• die großen Hedgies sind alle raus
• die anderen sind gebrannte Kinder
• ...und der Rest traut sich nicht
• die Shorties bauten zuletzt ab, liegen aber immer noch höher als Ende 2018
 --> https://www.nasdaq.com/market-activity/stocks/akrx/short-int…
--> https://www.nasdaq.com/market-activity/stocks/akrx/short-int…=> nur die beinharten sind z.Z. (long) drin

--> ich überlege, vor Q3 nachzukaufen: 31.10.? -- noch unbestätigt
https://www.akorn2019securitiessettlement.com/documents --> Proof of Claim and Release Form
--> oben kann man mit Hilfe der Form PROOF OF CLAIM AND RELEASE bis zum 24.1.2020 eventuelle Ansprüche an den SETTLEMENT FUND geltend machen, wenn man Akorn-Aktien vom NOVEMBER 3, 2016 bis APRIL 8, 2019 hatte, bzw. erworben hatte - und eine ganze Reihe von Bedingungen erfüllt sind
--> oben kann man mit Hilfe der Form PROOF OF CLAIM AND RELEASE bis zum 24.1.2020 eventuelle Ansprüche an den SETTLEMENT FUND geltend machen, wenn man Akorn-Aktien vom NOVEMBER 3, 2016 bis APRIL 8, 2019 hatte, bzw. erworben hatte - und eine ganze Reihe von Bedingungen erfüllt sind
Antwort auf Beitrag Nr.: 61.700.329 von faultcode am 15.10.19 23:49:04
-->
... it plans to issue a press release before market open on Thursday, October 31, 2019 outlining its third quarter 2019 financial results.
http://investors.akorn.com/news-releases/news-release-detail…
Zitat von faultcode: ...--> ich überlege, vor Q3 nachzukaufen: 31.10.? -- noch unbestätigt
-->
... it plans to issue a press release before market open on Thursday, October 31, 2019 outlining its third quarter 2019 financial results.
http://investors.akorn.com/news-releases/news-release-detail…
Antwort auf Beitrag Nr.: 61.791.985 von faultcode am 29.10.19 18:47:49
http://investors.akorn.com/news-releases/news-release-detail…
Updates net loss and affirms net revenue and adjusted EBITDA guidance
LAKE FOREST, Ill., Oct. 31, 2019 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty pharmaceutical company, today announced its preliminary financial results for the third quarter of 2019.
Third Quarter 2019 and Recent Business Highlights
• Net revenue was $176 million, up $11 million, 6% from the prior year quarter
• Net income was $48 million, compared to $70 million loss in the prior year quarter
• Adjusted EBITDA was $29 million, compared to $10 million in the prior year quarter
• Generated positive operating cash flow during the third quarter
• Launched five new products year-to-date including one in the third quarter: Diclofenac Sodium Topical Gel 1%
• Received five ANDA approvals year-to-date including two in the third quarter: Azelastine Hydrochloride Nasal Spray, 0.15% and Betamethasone Dipropionate Lotion USP (Augmented), 0.05%
• Continued progress on operational initiatives leading to stability in backorders and significant reduction in failure to supply penalties
...
=> zack!
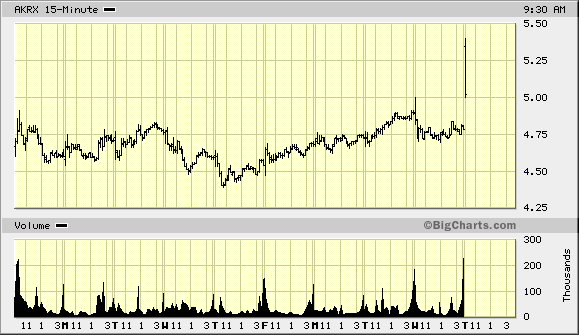
Akorn Provides Preliminary Third Quarter 2019 Results
31.10.http://investors.akorn.com/news-releases/news-release-detail…
Updates net loss and affirms net revenue and adjusted EBITDA guidance
LAKE FOREST, Ill., Oct. 31, 2019 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty pharmaceutical company, today announced its preliminary financial results for the third quarter of 2019.
Third Quarter 2019 and Recent Business Highlights
• Net revenue was $176 million, up $11 million, 6% from the prior year quarter
• Net income was $48 million, compared to $70 million loss in the prior year quarter
• Adjusted EBITDA was $29 million, compared to $10 million in the prior year quarter
• Generated positive operating cash flow during the third quarter
• Launched five new products year-to-date including one in the third quarter: Diclofenac Sodium Topical Gel 1%
• Received five ANDA approvals year-to-date including two in the third quarter: Azelastine Hydrochloride Nasal Spray, 0.15% and Betamethasone Dipropionate Lotion USP (Augmented), 0.05%
• Continued progress on operational initiatives leading to stability in backorders and significant reduction in failure to supply penalties
...
=> zack!

Antwort auf Beitrag Nr.: 61.756.193 von faultcode am 24.10.19 12:38:17ich habe die Claim-Formulare mal in vorgeschriebener eidesstattlicher Form ausgefüllt und abgeschickt.
Ich sollte nun in ~60 Tagen eine Postkarte vom Claims Administrator bekommen, daß mein Claim bei ihm eingegangen ist.
Ich sollte nun in ~60 Tagen eine Postkarte vom Claims Administrator bekommen, daß mein Claim bei ihm eingegangen ist.
16.12.
Akorn Announces Extension of Standstill Agreement
http://investors.akorn.com/news-releases/news-release-detail…
...
Akorn ... today announced that Akorn and certain of its lenders have reached an agreement that extends the standstill period to February 7, 2020.
Doug Boothe, Akorn’s President and Chief Executive Officer, commented, “We are pleased to have reached this agreement, which provides additional time for Akorn, our Board of Directors, and advisors to evaluate strategic alternatives to address the Company’s litigation-related liabilities and position Akorn for long-term success.
Meanwhile, we are maintaining our momentum, investing in our business, and building on the operational improvements we have made over the past year. I am excited about the opportunities ahead, and I look forward to seeing Akorn reach its full potential.”
A copy of the agreement was filed with the SEC on the Company’s Form 8-K earlier today....
Akorn Announces Extension of Standstill Agreement
http://investors.akorn.com/news-releases/news-release-detail…
...
Akorn ... today announced that Akorn and certain of its lenders have reached an agreement that extends the standstill period to February 7, 2020.
Doug Boothe, Akorn’s President and Chief Executive Officer, commented, “We are pleased to have reached this agreement, which provides additional time for Akorn, our Board of Directors, and advisors to evaluate strategic alternatives to address the Company’s litigation-related liabilities and position Akorn for long-term success.
Meanwhile, we are maintaining our momentum, investing in our business, and building on the operational improvements we have made over the past year. I am excited about the opportunities ahead, and I look forward to seeing Akorn reach its full potential.”
A copy of the agreement was filed with the SEC on the Company’s Form 8-K earlier today....
Warnt vor einem möglichen Bankrott!
tatsächlich:
18.12.
Akorn's stock extends plunge toward record low after disclosing it was exploring a bankruptcy
https://www.marketwatch.com/story/akorns-stock-extends-plung…
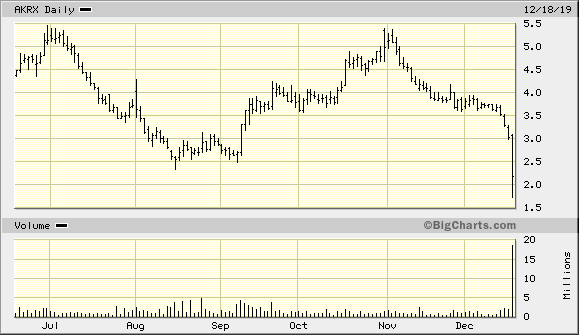
18.12.
Akorn's stock extends plunge toward record low after disclosing it was exploring a bankruptcy
https://www.marketwatch.com/story/akorns-stock-extends-plung…

Antwort auf Beitrag Nr.: 62.193.318 von faultcode am 19.12.19 00:47:00Ich wunderte mich schon etwas, woher Akorn das Geld zur möglichen Entschädigung der Alt-Aktionäre hernehmen wollte/will.
Diese Aktion läuft ja schon eine Weile, und der Claims Administrator sah vielleicht bereits, wie viel bereits eingegangen ist - und eventuell, wer (bislang) nicht mitmacht und eigenständig weiterklagt.
Das derzeitige, öffentliche Nachdenken über Chapter 11 (was mMn unbedingt ein Wink mit dem Zaunpfahl ist) muss also nicht zwangsläufig heißen, daß es bislang in 2019Q4 operativ schlecht gelaufen sein muss.
Diese Aktion läuft ja schon eine Weile, und der Claims Administrator sah vielleicht bereits, wie viel bereits eingegangen ist - und eventuell, wer (bislang) nicht mitmacht und eigenständig weiterklagt.
Das derzeitige, öffentliche Nachdenken über Chapter 11 (was mMn unbedingt ein Wink mit dem Zaunpfahl ist) muss also nicht zwangsläufig heißen, daß es bislang in 2019Q4 operativ schlecht gelaufen sein muss.
Antwort auf Beitrag Nr.: 62.193.318 von faultcode am 19.12.19 00:47:00hier liegt das Problem mMn:
• seit einem 1/2 Jahr schon sind die Schulden in der laufenden Berichtsperiode zu tilgen, wenn die Gläubiger sich eben nicht bewegen wollen bis dahin (und AKRX hat nicht mal eben ~USD841m übrig, sondern nur so ~USD206 an Cash & Short Term Investments|30.9.):
https://www.marketwatch.com/investing/stock/akrx/financials/…
• seit einem 1/2 Jahr schon sind die Schulden in der laufenden Berichtsperiode zu tilgen, wenn die Gläubiger sich eben nicht bewegen wollen bis dahin (und AKRX hat nicht mal eben ~USD841m übrig, sondern nur so ~USD206 an Cash & Short Term Investments|30.9.):

https://www.marketwatch.com/investing/stock/akrx/financials/…
Antwort auf Beitrag Nr.: 62.198.016 von faultcode am 19.12.19 15:17:25ich habe heute beide Tranchen verkauft:
• in rund 1/2 Jahre keine Refinanzierung - bei einem Generika-Hersteller - hinzubekommen, ist mMn kein gutes Zeichen
Man müsste mal die Struktur der Gläubiger ansehen. Ich gehe - ohne Nachweis - von (entsprechend spezialisierten) Hedge Funds oder Distressed assets-Investoren aus, die tatsächlich am Ende Akorn im Rahmen von Chapter 11 übernehmen, um es anschließend wieder eines Tages an die Börse zu bringen
Das ist in den USA ja nicht unüblich.
• in rund 1/2 Jahre keine Refinanzierung - bei einem Generika-Hersteller - hinzubekommen, ist mMn kein gutes Zeichen
Man müsste mal die Struktur der Gläubiger ansehen. Ich gehe - ohne Nachweis - von (entsprechend spezialisierten) Hedge Funds oder Distressed assets-Investoren aus, die tatsächlich am Ende Akorn im Rahmen von Chapter 11 übernehmen, um es anschließend wieder eines Tages an die Börse zu bringen
Das ist in den USA ja nicht unüblich.
kurse unter 1,10euro sollten kurzfristig ganz ok gewesen sein um mal wieder einzusteigen

https://www.finanznachrichten.de/nachrichten-2020-01/4855496…
https://www.finanznachrichten.de/nachrichten-2020-01/4855496…
The Irish giant Perrigo is in "advanced talks" to take over Akorn, a Nasdaq-listed company that manufactures generic products, after Kapoor, one of the largest shareholders, sold before the end of the year the shares he owned in the company.
Akorn, currently in suspension of payments, will thus be absorbed by Perrigo, the Irish manufacturer of private label over-the-counter pharmaceutical products and whose sales come from the US health care system. Doug Boothe, President and Chief Executive Officer of Akorn, said he was pleased to have reached an agreement that provides additional time for Akorn, the board of directors and the advisors to evaluate strategic alternatives to address the responsibilities and position related to litigation in the Company.
Perrigo's financial advisor, Morgan Stanley, would be willing to assume all of Akorn's debt and pay up to $ 380 million for his capital.
Akorn's litigation with Fresenius after his failed operation and winning the right to withdraw from the agreement in October would not affect the operation.
Since Boothe took over late last year, the new CEO said Akorn has made structural and organizational changes to "emphasize compliance, transparency and accountability" at its three manufacturing facilities in the United States. Akorn is also exploring a sale for its site in India. Fruitful conversations are expected to become a reality before the end of the month.
das waren die news wegen was der kurs am 30.1. um 30% angestiegen ist. nun mal sehen was noch so kommt bis zum 7.2.
https://okdiario.com/economia/perrigo-esta-conversaciones-av…

Akorn, currently in suspension of payments, will thus be absorbed by Perrigo, the Irish manufacturer of private label over-the-counter pharmaceutical products and whose sales come from the US health care system. Doug Boothe, President and Chief Executive Officer of Akorn, said he was pleased to have reached an agreement that provides additional time for Akorn, the board of directors and the advisors to evaluate strategic alternatives to address the responsibilities and position related to litigation in the Company.
Perrigo's financial advisor, Morgan Stanley, would be willing to assume all of Akorn's debt and pay up to $ 380 million for his capital.
Akorn's litigation with Fresenius after his failed operation and winning the right to withdraw from the agreement in October would not affect the operation.
Since Boothe took over late last year, the new CEO said Akorn has made structural and organizational changes to "emphasize compliance, transparency and accountability" at its three manufacturing facilities in the United States. Akorn is also exploring a sale for its site in India. Fruitful conversations are expected to become a reality before the end of the month.
das waren die news wegen was der kurs am 30.1. um 30% angestiegen ist. nun mal sehen was noch so kommt bis zum 7.2.
https://okdiario.com/economia/perrigo-esta-conversaciones-av…
soll natürlich 13.1. heissen *
gestern wurde angeblich einer der lawsuits geklärt, sieht man auch etwas am chart. langsam denke ich bei ca. 1,20-1,30 dollar ist der boden erreicht. mal sehen ob heute die 1,50 dollar wiederkommen
aktuell ASK 1.24 €
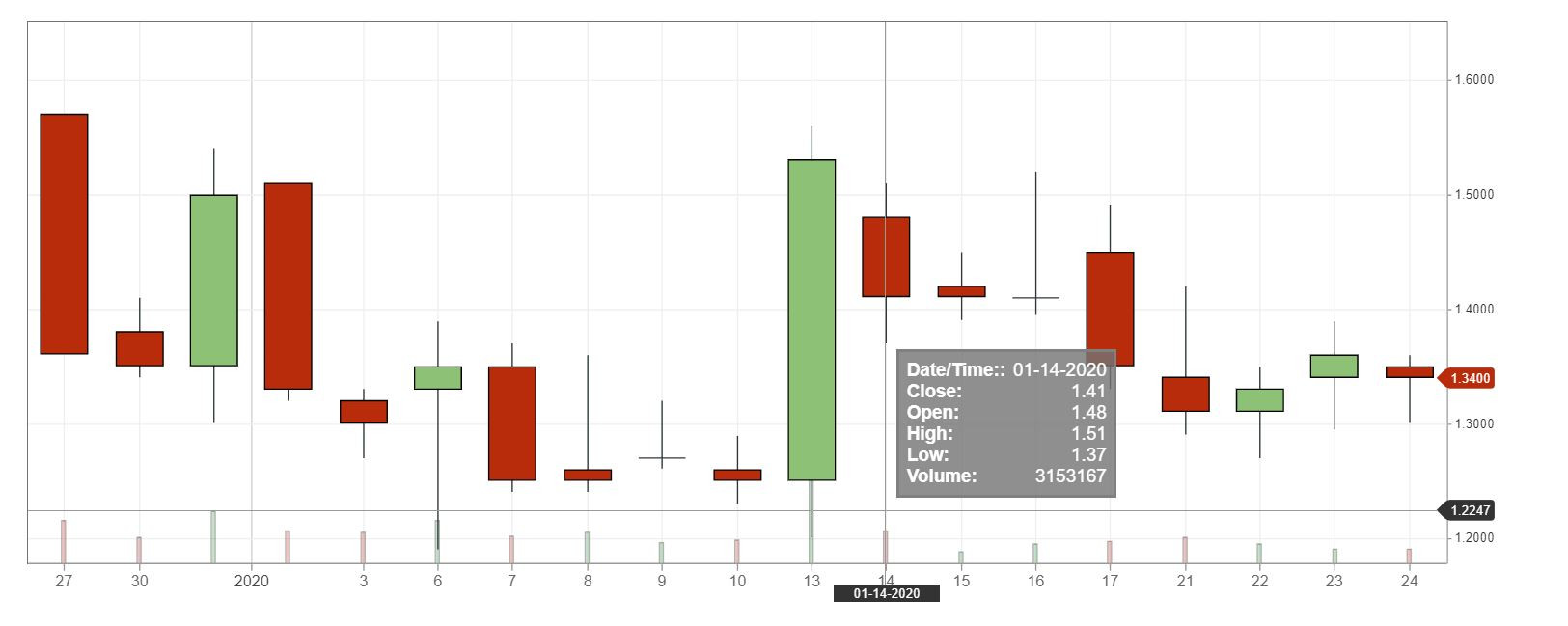
aktuell ASK 1.24 €



premarket teilweise +8%, mal sehen ob man den kurs halten kann



Akorn Continues Negotiations with Lenders
LAKE FOREST, Ill., Feb. 10, 2020 /PRNewswire/ -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty pharmaceutical company, today confirmed that negotiations with certain of its lenders are ongoing following the expiration of its standstill agreement on February 7, 2020. Akorn continues to evaluate strategic alternatives to address the Company's litigation-related liabilities and position Akorn for long-term success.
...
http://investors.akorn.com/news-releases/news-release-detail…
LAKE FOREST, Ill., Feb. 10, 2020 /PRNewswire/ -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty pharmaceutical company, today confirmed that negotiations with certain of its lenders are ongoing following the expiration of its standstill agreement on February 7, 2020. Akorn continues to evaluate strategic alternatives to address the Company's litigation-related liabilities and position Akorn for long-term success.
...
http://investors.akorn.com/news-releases/news-release-detail…
Antwort auf Beitrag Nr.: 62.633.794 von faultcode am 10.02.20 14:01:0012.2.
Akorn Announces New Extension of Standstill with Lenders and Agrees to Pursue a Sale of its Business
http://investors.akorn.com/news-releases/news-release-detail…
...
Akorn, Inc. (Nasdaq: AKRX), a leading specialty pharmaceutical company, today announced it has reached an agreement with certain of its lenders to extend the standstill period. The agreement defines a path forward and outlines milestones to execute a sale of Akorn's business, potentially using Chapter 11 protection in order to address Akorn's litigation-related overhangs and execute a transaction that maximizes value.
Doug Boothe, Akorn's President and Chief Executive Officer, commented, "Akorn is a fundamentally strong business with exciting opportunities ahead. Our efforts to stabilize and strengthen our business have already achieved meaningful results. The decision to pursue a sale of the Company gives Akorn the opportunity to address its capital structure and litigation-related overhangs by seeking a new owner that will continue to invest in our business and future growth. I believe that this decisive action is the right path for our business and will deliver greater financial security in the long term."
...
Akorn Announces New Extension of Standstill with Lenders and Agrees to Pursue a Sale of its Business
http://investors.akorn.com/news-releases/news-release-detail…
...
Akorn, Inc. (Nasdaq: AKRX), a leading specialty pharmaceutical company, today announced it has reached an agreement with certain of its lenders to extend the standstill period. The agreement defines a path forward and outlines milestones to execute a sale of Akorn's business, potentially using Chapter 11 protection in order to address Akorn's litigation-related overhangs and execute a transaction that maximizes value.
Doug Boothe, Akorn's President and Chief Executive Officer, commented, "Akorn is a fundamentally strong business with exciting opportunities ahead. Our efforts to stabilize and strengthen our business have already achieved meaningful results. The decision to pursue a sale of the Company gives Akorn the opportunity to address its capital structure and litigation-related overhangs by seeking a new owner that will continue to invest in our business and future growth. I believe that this decisive action is the right path for our business and will deliver greater financial security in the long term."
...

Antwort auf Beitrag Nr.: 62.676.646 von faultcode am 14.02.20 00:45:45Akorn Provides Fourth Quarter and Full Year 2019 Results
LAKE FOREST, Ill., Feb. 26, 2020 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty pharmaceutical company, today announced its financial results for the quarter and year ended December 31, 2019.
Fourth Quarter 2019 Results and Recent Developments
• Net revenue was $162 million, an increase of $9 million, or 5.8%, compared to the fourth quarter of 2018.
• Net loss was $81 million compared to a net loss of $215 million in the fourth quarter of 2018.
• Adjusted EBITDA was $18 million compared to $(20) million in the fourth quarter of 2018.
• Reached an agreement with our lenders to execute a sale of Akorn’s business, potentially using Chapter 11 protection, as previously disclosed on the Company’s Form 8-K filed on February 12, 2020.
Douglas Boothe, Akorn’s President and Chief Executive Officer, stated, “Our fourth quarter and full year 2019 results reflect the operational progress achieved throughout the course of the year. As we move forward through the sale process, we will continue to fulfill contractual obligations to suppliers and customers and deliver safe and effective pharmaceutical products for patients that depend on them.”
...
http://investors.akorn.com/news-releases/news-release-detail…
LAKE FOREST, Ill., Feb. 26, 2020 (GLOBE NEWSWIRE) -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty pharmaceutical company, today announced its financial results for the quarter and year ended December 31, 2019.
Fourth Quarter 2019 Results and Recent Developments
• Net revenue was $162 million, an increase of $9 million, or 5.8%, compared to the fourth quarter of 2018.
• Net loss was $81 million compared to a net loss of $215 million in the fourth quarter of 2018.
• Adjusted EBITDA was $18 million compared to $(20) million in the fourth quarter of 2018.
• Reached an agreement with our lenders to execute a sale of Akorn’s business, potentially using Chapter 11 protection, as previously disclosed on the Company’s Form 8-K filed on February 12, 2020.
Douglas Boothe, Akorn’s President and Chief Executive Officer, stated, “Our fourth quarter and full year 2019 results reflect the operational progress achieved throughout the course of the year. As we move forward through the sale process, we will continue to fulfill contractual obligations to suppliers and customers and deliver safe and effective pharmaceutical products for patients that depend on them.”
...
http://investors.akorn.com/news-releases/news-release-detail…
Antwort auf Beitrag Nr.: 62.805.725 von faultcode am 27.02.20 12:48:161.4.
Akorn Provides Update on Sale Process
LAKE FOREST, Ill., April 1, 2020 /PRNewswire/ -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty pharmaceutical company, today announced that it no longer has any bids in the Sale Process that are sufficient to pay all obligations under its term loan agreement. Accordingly, the Company now toggles to the alternative milestones that were detailed in the Second Amended Standstill Agreement and are summarized in the 8-K filed earlier today.
Doug Boothe, Akorn's President and Chief Executive Officer, commented, "Unfortunately, our sale process has been negatively impacted by the broader market uncertainties related to the COVID-19 crisis. However, we are working closely with our lenders to determine the best path forward to ensure that the Company is positioned for long-term success."
"We remain confident in the fundamental strength of Akorn's business. Our commitment to patients, customers and communities is unwavering as we work to fulfill our mission which, now more than ever, is critical for those we serve. We continue to work tirelessly to improve patients' lives through the quality, availability and affordability of our products."
The Company plans to continue to operate as usual, including delivering safe and effective pharmaceutical products to customers and fulfilling contractual obligations, including payments to vendors.
--> ich nehme an, daß es für Bestandsaktionäre keinen Unterschied mehr zwischen RSA und Chapter 11 macht:

<8-K von heute>
Akorn Provides Update on Sale Process
LAKE FOREST, Ill., April 1, 2020 /PRNewswire/ -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty pharmaceutical company, today announced that it no longer has any bids in the Sale Process that are sufficient to pay all obligations under its term loan agreement. Accordingly, the Company now toggles to the alternative milestones that were detailed in the Second Amended Standstill Agreement and are summarized in the 8-K filed earlier today.
Doug Boothe, Akorn's President and Chief Executive Officer, commented, "Unfortunately, our sale process has been negatively impacted by the broader market uncertainties related to the COVID-19 crisis. However, we are working closely with our lenders to determine the best path forward to ensure that the Company is positioned for long-term success."
"We remain confident in the fundamental strength of Akorn's business. Our commitment to patients, customers and communities is unwavering as we work to fulfill our mission which, now more than ever, is critical for those we serve. We continue to work tirelessly to improve patients' lives through the quality, availability and affordability of our products."
The Company plans to continue to operate as usual, including delivering safe and effective pharmaceutical products to customers and fulfilling contractual obligations, including payments to vendors.
--> ich nehme an, daß es für Bestandsaktionäre keinen Unterschied mehr zwischen RSA und Chapter 11 macht:

<8-K von heute>
Ging ja gestern wild zu, hat jemand eine Meinung zu denen ?
Einerseits steht Umstrukturierung und/oder Chapter 11 im Raum, anderseits ist es ja nicht so das die Firma nichts hat Ostern oder ist
Einerseits steht Umstrukturierung und/oder Chapter 11 im Raum, anderseits ist es ja nicht so das die Firma nichts hat Ostern oder ist
Heute Tag der Entscheidung hier. Ich kann mir nicht vorstellen, dass so eine Firma Bankrott gehen wird. Durch Corona konnten die Verkaufszahlen nicht erreicht werden und deshalb können die Zahlungsanforderungen nicht beglichen werden. Diese Firma hat rund 900M Schulden und zugleich 145M Cash. Ich bin mir sicher, dass hier eine Lösung gefunden wurde. Sieht aus wie bei Mallinkrodt!
Auffallend sind 5.2M Shares bei 0.21$! Das sind enorme Blöcke welche mich zudem optimistisch stimmen! Deswegen habe ich gestern kurz vor Börsenschluss bei 0.203 zugeschlagen! Nachbörslich auf 0.22$. Schauen wir mal was hier heute passiert!
Auffallend sind 5.2M Shares bei 0.21$! Das sind enorme Blöcke welche mich zudem optimistisch stimmen! Deswegen habe ich gestern kurz vor Börsenschluss bei 0.203 zugeschlagen! Nachbörslich auf 0.22$. Schauen wir mal was hier heute passiert!
Sehr bullish hier, 40% in 1 Tag bin zu frieden 

Pleite wäre blöde, besser Einigung mit Gläubigern und Kurse über 1,00.
Ist ne große Firma, mit vielen Produkten. Gut möglich das Sie überleben. Ich tippe 70:30 das es gut ausgeht.
Ist ne große Firma, mit vielen Produkten. Gut möglich das Sie überleben. Ich tippe 70:30 das es gut ausgeht.
Antwort auf Beitrag Nr.: 63.533.009 von KHL911 am 01.05.20 20:08:50Kann ich mir fast nicht vorstellen! Ich meine diese Bude macht 1 Milliarde Umsatz! Haben weitere top Produkte in der Pipeline! Die werden sicher eine Lösung mit den Gläubigern finden! Diese Kurse sind ein Witz! Wenn hier eine positive finanzierungs PR kommt, sehen wir hier 1-2$ da bin ich sowas von sicher! Darum heute getradet, vorher wieder rein und lasse die nun laufen.
Ja, ich hätte nix dagegen wenn es auf 1 geht. Oder 2. Oder 3😉
sieht sehr spannend aus .....
setzten sie mal auf die WL
setzten sie mal auf die WL
Antwort auf Beitrag Nr.: 63.533.438 von BTRRR am 01.05.20 20:46:18Die sind schlichtweg überschuldet! Machen Verluste und das Eigenkapital ist komplett aufgebraucht...hier geht es nur noch um die Verwertung der Assets, ob da was für die Aktionäre übrig bleibt wage ich zu bezweifeln....
In ein paar Wochen weiß man mehr, vielleicht wird’s jetzt volatiler, sodass man traden kann
heute: Chapter 11
Antwort auf Beitrag Nr.: 62.199.582 von faultcode am 19.12.19 17:48:06
--> man scheint auf dem Weg dahin zu sein:
21.5.
Akorn to Use Voluntary Chapter 11 Process to Position Business for Long-Term Success
Continues Operations as Usual, Delivering Safe and Effective Products to Customers and Patients
LAKE FOREST, Ill., May 20, 2020 /PRNewswire/ -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty pharmaceutical company ("Akorn" or the "Company"), today announced that the Company and its U.S. subsidiaries filed for voluntary protection under Chapter 11 ("Chapter 11") of the U.S. Bankruptcy Code in United States Bankruptcy Court for the District of Delaware (the "Bankruptcy Court") to execute an in‑court sale of its business while addressing litigation-related overhangs and best positioning the business for long-term success under new ownership.
In connection with the filing, the Company has executed a Restructuring Support Agreement with lenders representing more than 75% of its secured debt, who will collectively serve as a "stalking horse" bidder in the Company's sale process and provide additional liquidity to fund the Company's business operations during this process.
...
http://investors.akorn.com/news-releases/news-release-detail…
Zu "um es anschließend wieder eines Tages an die Börse zu bringen": ich gehe dabei davon aus, daß man dann auch einen neuen Firmennamen haben wird
Zitat von faultcode: ...Man müsste mal die Struktur der Gläubiger ansehen. Ich gehe - ohne Nachweis - von (entsprechend spezialisierten) Hedge Funds oder Distressed assets-Investoren aus, die tatsächlich am Ende Akorn im Rahmen von Chapter 11 übernehmen, um es anschließend wieder eines Tages an die Börse zu bringen...
--> man scheint auf dem Weg dahin zu sein:
21.5.
Akorn to Use Voluntary Chapter 11 Process to Position Business for Long-Term Success
Continues Operations as Usual, Delivering Safe and Effective Products to Customers and Patients
LAKE FOREST, Ill., May 20, 2020 /PRNewswire/ -- Akorn, Inc. (Nasdaq: AKRX), a leading specialty pharmaceutical company ("Akorn" or the "Company"), today announced that the Company and its U.S. subsidiaries filed for voluntary protection under Chapter 11 ("Chapter 11") of the U.S. Bankruptcy Code in United States Bankruptcy Court for the District of Delaware (the "Bankruptcy Court") to execute an in‑court sale of its business while addressing litigation-related overhangs and best positioning the business for long-term success under new ownership.
In connection with the filing, the Company has executed a Restructuring Support Agreement with lenders representing more than 75% of its secured debt, who will collectively serve as a "stalking horse" bidder in the Company's sale process and provide additional liquidity to fund the Company's business operations during this process.
...
http://investors.akorn.com/news-releases/news-release-detail…
Zu "um es anschließend wieder eines Tages an die Börse zu bringen": ich gehe dabei davon aus, daß man dann auch einen neuen Firmennamen haben wird

Antwort auf Beitrag Nr.: 63.763.222 von faultcode am 21.05.20 16:19:06In dem Fall gibt’s da dann wohl nicht viel zu traden 🥺
Antwort auf Beitrag Nr.: 63.763.555 von KHL911 am 21.05.20 16:53:16Ich habe hier 200k Shares und das ist absolutes high risk! Nur schaut euch die Pipeline und die Produkte an, die sind wirklich gut. Ich sage es klar, kauft das Teil hier nicht. Nur ist es mir ein Zock wert! Daher um es klar auszudrücken, ich glaube nicht dass die Bankrott gehen werden! Chapter 11 ist nicht Chapter 7!!!! Daher solange das nicht kommt, sehe ich hier nicht schwarz!
Antwort auf Beitrag Nr.: 63.764.749 von BTRRR am 21.05.20 19:31:27Korrektur! Habe bei 0.187 alles verkauft und RMED gekauft! So ein Schnäppchen habe ich schon lange nicht mehr gesehen. Wünsche einen schönen Abend.
Schade, scheint wohl doch on the way to zero zu sein
RMED ..... Die mit dem public offering 🥺
Antwort auf Beitrag Nr.: 63.777.538 von KHL911 am 23.05.20 09:36:34Jap wurde aber gestern abgeschlossen! Nachbörslich auf 0.53$! 32M Cash und Mkab 7M klingelt da etwas?
Morgen ist ja in Amerika zu. Dann mal schauen wie tief es noch runtergeht.
ab Montag, 1.6., OTC-Handel unter AKRXQ
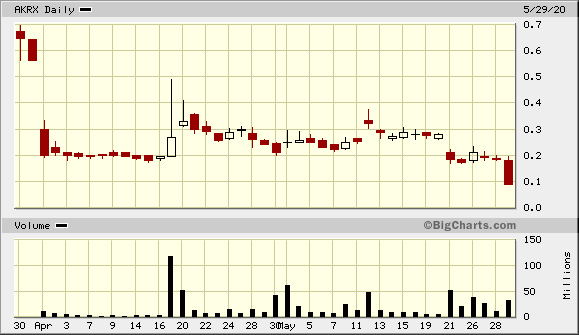



Vielleicht jetzt nochmal einsteigen 🤷♂️
Antwort auf Beitrag Nr.: 63.849.287 von faultcode am 29.05.20 23:29:4412.8.
AKORN $AKRXQ To Release Details Of New Joint Venture With Unnamed Nasdaq Traded Stock Following Talks In Chicago
https://twitter.com/StockWatchHQ/status/1293274211113304064
AKORN $AKRXQ To Release Details Of New Joint Venture With Unnamed Nasdaq Traded Stock Following Talks In Chicago
https://twitter.com/StockWatchHQ/status/1293274211113304064

Das Ende:
2.9.
Akorn Receives Court Approval of Sale to Lenders
http://investors.akorn.com/news-releases/news-release-detail…
...
2.9.
Akorn Receives Court Approval of Sale to Lenders
http://investors.akorn.com/news-releases/news-release-detail…
...

Antwort auf Beitrag Nr.: 64.971.606 von faultcode am 02.09.20 22:53:12Das Ende der alten Akorn - formal:

...
..und bis zum IPO wieder

...
..und bis zum IPO wieder

Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +1,14 | |
| +0,51 | |
| +1,05 | |
| +2,80 | |
| 0,00 | |
| +3,98 | |
| +1,12 | |
| 0,00 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 215 | ||
| 90 | ||
| 76 | ||
| 58 | ||
| 54 | ||
| 36 | ||
| 34 | ||
| 29 | ||
| 27 | ||
| 25 |





















