RHYTHM Pharmaceuticals Inc - Entwickler und Vermarkter von Peptidtherapeutika
eröffnet am 14.03.21 16:47:31 von
neuester Beitrag 14.09.22 20:38:31 von
neuester Beitrag 14.09.22 20:38:31 von
Beiträge: 9
ID: 1.344.677
ID: 1.344.677
Aufrufe heute: 0
Gesamt: 887
Gesamt: 887
Aktive User: 0
ISIN: US76243J1051 · WKN: A2H5A0 · Symbol: RYTM
38,28
USD
+0,34 %
+0,13 USD
Letzter Kurs 27.04.24 Nasdaq
Neuigkeiten
24.04.24 · globenewswire |
16.04.24 · globenewswire |
01.04.24 · globenewswire |
25.03.24 · globenewswire |
11.03.24 · globenewswire |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,9000 | +59,66 | |
| 0,5922 | +44,44 | |
| 7,3500 | +43,84 | |
| 11,690 | +40,84 | |
| 0,7000 | +36,69 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 10,110 | -13,44 | |
| 8,2200 | -13,47 | |
| 7,6100 | -17,20 | |
| 1,6100 | -18,27 | |
| 2,1200 | -21,77 |
Beitrag zu dieser Diskussion schreiben
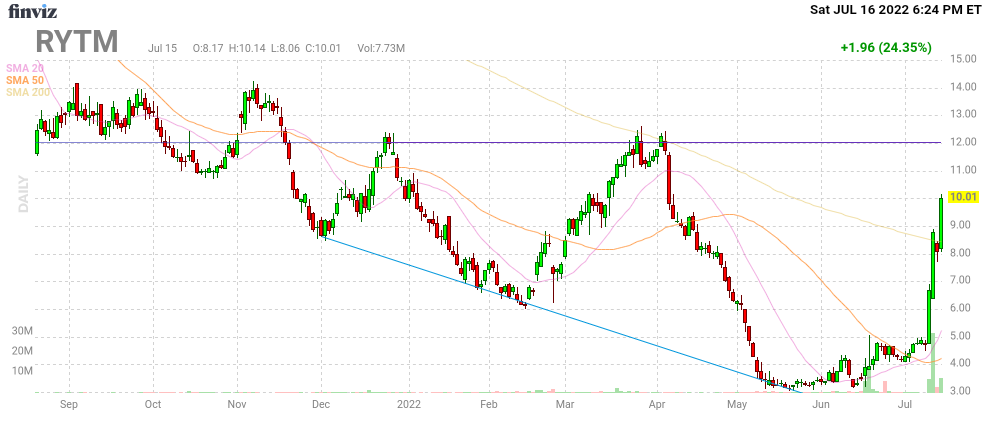
Das Ding steigt ja sogar noch weiter.
Wow.
Zeit, Gewinne mitzunehmen.
🎵

🎵
Ein Paradies für Trader:
🎵

🎵
Rhythm Pharmaceuticals Presents New Data from Phase 2 Basket Study Showing Continued Weight Loss at Up to Nine Months in Patients with HET Obesity on Setmelanotide at ENDO 2021
Rhythm Pharmaceuticals, Inc.
Sat, March 20, 2021, 4:30 PM·10 min read
-- Responders with HET obesity achieved mean weight loss of greater than 12 percent at nine months on setmelanotide therapy --
-- Additional poster presentations include Phase 3 data in Bardet-Biedl and Alström syndromes and analyses of adverse events in Phase 2 and Phase 3 studies in POMC, PCSK1, or LEPR deficiency showing consistent safety results for setmelanotide --
BOSTON, March 20, 2021 (GLOBE NEWSWIRE) -- Rhythm Pharmaceuticals, Inc. (Nasdaq: RYTM), a biopharmaceutical company aimed at developing and commercializing therapies for the treatment of rare genetic diseases of obesity, today announced that three late-breaking data presentations from Phase 2 and Phase 3 studies of setmelanotide were presented during the 103rd Annual Meeting and Expo of the Endocrine Society (ENDO 2021) held virtually March 20-23.
Sadaf Farooqi, M.D., Ph.D., University of Cambridge, UK, presented proof-of-concept data from Rhythm’s Phase 2 study evaluating setmelanotide in individuals living with heterozygous (HET) obesity due to genetic variants in one of two alleles of the POMC, PCSK1 or LEPR gene. The oral presentation included new weight loss data that showed patients with HET obesity who were classified as setmelanotide-responsive at three months continued to lose weight as they remained on treatment, with a mean weight loss of 12.3 percent at nine months on therapy.
In addition, two on-demand posters on setmelanotide were presented. Topline data from Rhythm’s Phase 3 trial evaluating setmelanotide in patients with Bardet-Biedl syndrome (BBS) or Alström syndrome were presented by Robert Haws, M.D., of the Marshfield Clinic Research Institute. Also, Karine Clément, M.D., Ph.D., of Pitié-Salpêtrière Hospital in Paris, presented safety data from three trials evaluating setmelanotide in a total of 35 patients with severe obesity due to leptin receptor (LEPR), proopiomelanocortin (POMC), or proprotein convertase subtilisin/kexin type 1 (PCSK1) deficiency.
“We are pleased to have world-renown physicians and scientists present these data that we believe support setmelanotide’s potential as a targeted therapy to treat genetically defined patients with early-onset, severe obesity and hyperphagia. In particular, these new data in HET obesity patients demonstrate the potential for deeper, durable responses with continued therapy,” said Murray Stewart, M.D., Chief Medical Officer of Rhythm. “These data give us further confidence as we advance setmelanotide through a multi-faceted clinical development program designed to address a range of rare genetic diseases of obesity caused by defects within the melanocortin-4 receptor (MC4R) pathway.”
Effects of setmelanotide observed in patients with HET obesity
Dr. Farooqi, professor at the Wellcome-MRC Institute of Metabolic Science and NIHR Cambridge Biomedical Research Centre delivered an oral presentation entitled, “Effects of Setmelanotide in in Patients with POMC, PCSK1 or LEPR Heterozygous Deficiency Obesity in a Phase 2 Study.”
“Patients with heterozygous mutations in POMC, PCSK1 or LEPR genes that impair the MC4R pathway can suffer from severe obesity and hyperphagia, which cannot be controlled by existing therapeutic interventions, diet or exercise,” Dr. Farooqi said. “These data, showing that treatment with setmelanotide achieved clinically meaningful weight loss at three months that was sustained and deepened at nine months, are quite encouraging, particularly in this refractory patient population. I look forward to learning more about the genetic causes of obesities and about setmelanotide as it is advanced into additional pivotal Phase 3 studies.”
The open-label, single-arm Phase 2 study included patients 6 years old or older with HET obesity. Participants received once daily setmelanotide at the therapeutic dose for 12 weeks. A total of 35 patients, whose mean baseline BMI was 50.3 kg/m2, were included in the analysis, which was previously reported by Rhythm in January 2021. The primary endpoint was mean percent change from baseline in body weight, and 34.3 percent of all patients in the study (12/35) responded with 5 percent or greater weight loss at three months. Mean weight loss among responders was -10.1 percent at three months.
New data presented at ENDO 2021 included an analysis that showed that, among people who responded to treatment with setmelanotide at three months, response was maintained for up to nine months (n=9) with a mean percent change in body weight from baseline of -12.3 percent (90% CI, -16.3% to -8.4%), as of Feb. 23, 2021.
The adverse event (AE) profile for setmelanotide continued to be consistent with what has been previously reported including skin hyperpigmentation, nausea, and injection site pruritis.
Topline data from Phase 3 trial in Bardet-Biedl and Alström syndromes
Dr. Haws, director of the Clinical Research Center at the Marshfield Clinic Research Institute in Marshfield, Wisc., presented a poster entitled, “A Phase 3 Trial in Participants with Obesity Due to Bardet-Biedl Syndrome or Alström Syndrome: Efficacy and Safety of the Melanocortin 4 Receptor Agonist Setmelanotide.”
As previously disclosed, data from this Phase 3 trial showed that treatment with setmelanotide was associated with significant body weight and hunger reduction in obesity due to BBS or Alström syndrome. The study met its primary and all key secondary endpoints, demonstrating statistically significant and clinically meaningful reductions in weight and hunger scores, with patients with BBS comprising all primary endpoint responders. No patients with Alström syndrome met the primary endpoint. The study included 31 patients 12 years old or older who were evaluable for the primary endpoint, including 28 patients with BBS and three patients with Alström syndrome.
This presentation included new weight-loss data specific to adults that showed that patients 18 years old and older with BBS (n=15) had a mean weight loss from baseline of 9.4 percent. Additionally, the presentation included previously disclosed data specific to adolescents and children, which showed that patients younger than 18 years of age with BBS (n=16) had a mean reduction in BMI-Z scores of 0.8. The BMI-Z score, or BMI standard deviation score, represents the number of standard deviations from median BMI by child age and sex.
Setmelanotide safety results from Phase 2 and Phase 3 studies in obesity due to POMC, PCSK1, or LEPR deficiency
Dr. Clément, professor of nutrition at Pitié-Salpêtrière Hospital and Sorbonne Université in Paris presented a poster entitled, “Timing of Onset of Adverse Events with Setmelanotide, an MC4R Agonist, in Patients with Severe Obesity Due to LEPR or POMC Deficiency.”
This presentation provided data from analyses of three studies that evaluated setmelanotide in obesity due to POMC, PCSK1 or LEPR deficiency. The analysis included 35 patients (15 POMC, 2 PCSK1, 18 LEPR) across three trials. Consistent with prior clinical experience, AEs of special interest included hyperpigmentation disorders, disturbances in sexual arousal, nausea, vomiting, and injection site reactions. The onset of AEs of special interest was generally highest during the first month of treatment, with fewer events occurring during subsequent months. Apart from hyperpigmentation, all AEs resolved quickly after onset.
The poster presentations will be available for on-demand viewing on the ENDO 2021 website, https://www.endocrine.org/endo2021, beginning at 11 a.m. on Saturday, March 20. The posters and slides from the oral presentation will be made available on Rhythm’s website, https://www.rhythmtx.com/publications/, following the presentations at ENDO 2021.
Rhythm Pharmaceuticals, Inc.
Sat, March 20, 2021, 4:30 PM·10 min read
-- Responders with HET obesity achieved mean weight loss of greater than 12 percent at nine months on setmelanotide therapy --
-- Additional poster presentations include Phase 3 data in Bardet-Biedl and Alström syndromes and analyses of adverse events in Phase 2 and Phase 3 studies in POMC, PCSK1, or LEPR deficiency showing consistent safety results for setmelanotide --
BOSTON, March 20, 2021 (GLOBE NEWSWIRE) -- Rhythm Pharmaceuticals, Inc. (Nasdaq: RYTM), a biopharmaceutical company aimed at developing and commercializing therapies for the treatment of rare genetic diseases of obesity, today announced that three late-breaking data presentations from Phase 2 and Phase 3 studies of setmelanotide were presented during the 103rd Annual Meeting and Expo of the Endocrine Society (ENDO 2021) held virtually March 20-23.
Sadaf Farooqi, M.D., Ph.D., University of Cambridge, UK, presented proof-of-concept data from Rhythm’s Phase 2 study evaluating setmelanotide in individuals living with heterozygous (HET) obesity due to genetic variants in one of two alleles of the POMC, PCSK1 or LEPR gene. The oral presentation included new weight loss data that showed patients with HET obesity who were classified as setmelanotide-responsive at three months continued to lose weight as they remained on treatment, with a mean weight loss of 12.3 percent at nine months on therapy.
In addition, two on-demand posters on setmelanotide were presented. Topline data from Rhythm’s Phase 3 trial evaluating setmelanotide in patients with Bardet-Biedl syndrome (BBS) or Alström syndrome were presented by Robert Haws, M.D., of the Marshfield Clinic Research Institute. Also, Karine Clément, M.D., Ph.D., of Pitié-Salpêtrière Hospital in Paris, presented safety data from three trials evaluating setmelanotide in a total of 35 patients with severe obesity due to leptin receptor (LEPR), proopiomelanocortin (POMC), or proprotein convertase subtilisin/kexin type 1 (PCSK1) deficiency.
“We are pleased to have world-renown physicians and scientists present these data that we believe support setmelanotide’s potential as a targeted therapy to treat genetically defined patients with early-onset, severe obesity and hyperphagia. In particular, these new data in HET obesity patients demonstrate the potential for deeper, durable responses with continued therapy,” said Murray Stewart, M.D., Chief Medical Officer of Rhythm. “These data give us further confidence as we advance setmelanotide through a multi-faceted clinical development program designed to address a range of rare genetic diseases of obesity caused by defects within the melanocortin-4 receptor (MC4R) pathway.”
Effects of setmelanotide observed in patients with HET obesity
Dr. Farooqi, professor at the Wellcome-MRC Institute of Metabolic Science and NIHR Cambridge Biomedical Research Centre delivered an oral presentation entitled, “Effects of Setmelanotide in in Patients with POMC, PCSK1 or LEPR Heterozygous Deficiency Obesity in a Phase 2 Study.”
“Patients with heterozygous mutations in POMC, PCSK1 or LEPR genes that impair the MC4R pathway can suffer from severe obesity and hyperphagia, which cannot be controlled by existing therapeutic interventions, diet or exercise,” Dr. Farooqi said. “These data, showing that treatment with setmelanotide achieved clinically meaningful weight loss at three months that was sustained and deepened at nine months, are quite encouraging, particularly in this refractory patient population. I look forward to learning more about the genetic causes of obesities and about setmelanotide as it is advanced into additional pivotal Phase 3 studies.”
The open-label, single-arm Phase 2 study included patients 6 years old or older with HET obesity. Participants received once daily setmelanotide at the therapeutic dose for 12 weeks. A total of 35 patients, whose mean baseline BMI was 50.3 kg/m2, were included in the analysis, which was previously reported by Rhythm in January 2021. The primary endpoint was mean percent change from baseline in body weight, and 34.3 percent of all patients in the study (12/35) responded with 5 percent or greater weight loss at three months. Mean weight loss among responders was -10.1 percent at three months.
New data presented at ENDO 2021 included an analysis that showed that, among people who responded to treatment with setmelanotide at three months, response was maintained for up to nine months (n=9) with a mean percent change in body weight from baseline of -12.3 percent (90% CI, -16.3% to -8.4%), as of Feb. 23, 2021.
The adverse event (AE) profile for setmelanotide continued to be consistent with what has been previously reported including skin hyperpigmentation, nausea, and injection site pruritis.
Topline data from Phase 3 trial in Bardet-Biedl and Alström syndromes
Dr. Haws, director of the Clinical Research Center at the Marshfield Clinic Research Institute in Marshfield, Wisc., presented a poster entitled, “A Phase 3 Trial in Participants with Obesity Due to Bardet-Biedl Syndrome or Alström Syndrome: Efficacy and Safety of the Melanocortin 4 Receptor Agonist Setmelanotide.”
As previously disclosed, data from this Phase 3 trial showed that treatment with setmelanotide was associated with significant body weight and hunger reduction in obesity due to BBS or Alström syndrome. The study met its primary and all key secondary endpoints, demonstrating statistically significant and clinically meaningful reductions in weight and hunger scores, with patients with BBS comprising all primary endpoint responders. No patients with Alström syndrome met the primary endpoint. The study included 31 patients 12 years old or older who were evaluable for the primary endpoint, including 28 patients with BBS and three patients with Alström syndrome.
This presentation included new weight-loss data specific to adults that showed that patients 18 years old and older with BBS (n=15) had a mean weight loss from baseline of 9.4 percent. Additionally, the presentation included previously disclosed data specific to adolescents and children, which showed that patients younger than 18 years of age with BBS (n=16) had a mean reduction in BMI-Z scores of 0.8. The BMI-Z score, or BMI standard deviation score, represents the number of standard deviations from median BMI by child age and sex.
Setmelanotide safety results from Phase 2 and Phase 3 studies in obesity due to POMC, PCSK1, or LEPR deficiency
Dr. Clément, professor of nutrition at Pitié-Salpêtrière Hospital and Sorbonne Université in Paris presented a poster entitled, “Timing of Onset of Adverse Events with Setmelanotide, an MC4R Agonist, in Patients with Severe Obesity Due to LEPR or POMC Deficiency.”
This presentation provided data from analyses of three studies that evaluated setmelanotide in obesity due to POMC, PCSK1 or LEPR deficiency. The analysis included 35 patients (15 POMC, 2 PCSK1, 18 LEPR) across three trials. Consistent with prior clinical experience, AEs of special interest included hyperpigmentation disorders, disturbances in sexual arousal, nausea, vomiting, and injection site reactions. The onset of AEs of special interest was generally highest during the first month of treatment, with fewer events occurring during subsequent months. Apart from hyperpigmentation, all AEs resolved quickly after onset.
The poster presentations will be available for on-demand viewing on the ENDO 2021 website, https://www.endocrine.org/endo2021, beginning at 11 a.m. on Saturday, March 20. The posters and slides from the oral presentation will be made available on Rhythm’s website, https://www.rhythmtx.com/publications/, following the presentations at ENDO 2021.
Antwort auf Beitrag Nr.: 67.453.049 von Malecon am 14.03.21 16:59:13Die Daten der Studien sind da... Sieht gut aus oder?
Insider Transaktion:
Todd Foley (BoD) hat am 15.03. satte 1.000.000 Aktien verkauft.
https://insider-analysis.com/search_transactions.php?ticker=…
Todd Foley (BoD) hat am 15.03. satte 1.000.000 Aktien verkauft.
https://insider-analysis.com/search_transactions.php?ticker=…
Kommende Woche wird das Unternehmen wichtige Studien präsentieren:
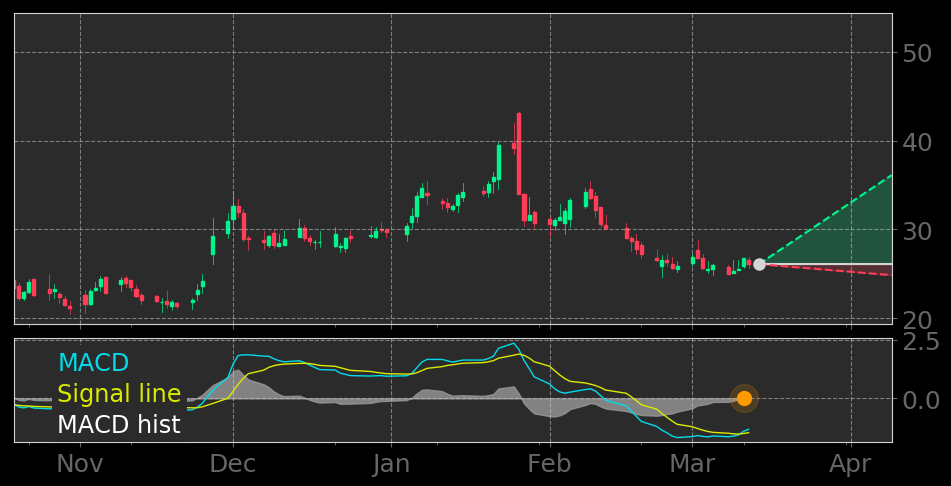
Rein charttechnisch ist eigentlich alles angerichtet.
Oben auf dem Chart sieht man, dass der Kurs die blaue Unterkante erreicht hat und bei positiven Studienergebnissen da wieder nach oben abprallen könnte. Bei negativen Studienergebnissen wird's natürlich krachen und die Unterkante nicht halten.
Es kommt also eine richtungsweisende Woche auf den Aktienkurs zu.
Der MACD hat im Daily die Signallinie nach oben gekreuzt:
🤔
ENDO Meeting Presentations
Rhythm Pharmaceuticals, Inc. (NASDAQ: RYTM): data from the Phase 2 study of setmelanotide in obesity patients and Phase 3 study data for the same investigational therapy in treating obesity due to Bardet-Biedl syndrome or Alstrom syndrome
Quelle: https://m.benzinga.com/article/20155210?utm_referrer=https%3…
Rein charttechnisch ist eigentlich alles angerichtet.
Oben auf dem Chart sieht man, dass der Kurs die blaue Unterkante erreicht hat und bei positiven Studienergebnissen da wieder nach oben abprallen könnte. Bei negativen Studienergebnissen wird's natürlich krachen und die Unterkante nicht halten.
Es kommt also eine richtungsweisende Woche auf den Aktienkurs zu.
Der MACD hat im Daily die Signallinie nach oben gekreuzt:

🤔
Kurzporträt:
Rhythm Pharmaceuticals, Inc. ist ein biopharmazeutisches Unternehmen. Das Unternehmen konzentriert sich auf die Entwicklung und Vermarktung von Peptidtherapeutika zur Behandlung seltener genetischer Mängel, die zu lebensbedrohlichen Stoffwechselstörungen führen. Der führende Peptidproduktkandidat des Unternehmens ist Setmelanotid, ein potenter, erstklassiger Melanocortin-4-Rezeptor oder MC4R, ein Agonist zur Behandlung seltener genetischer Störungen von Fettleibigkeit. Setmelanotid dient als Ersatztherapie zur Behandlung von Melanocortin-4, oder MC4, Wegdefiziten. MC4-Pfaddefizite führen zur Störung von Sättigungssignalen und Energiehomöostase im Körper, was wiederum zu intensiven Hungergefühlen und zu Fettleibigkeit führt. Das Unternehmen konzentrierte sich auch auf Fettleibigkeit im Zusammenhang mit sechs einzelnen genbedingten oder monogenen MC4-Pfaddefiziten Pro-Optiomelanocortin oder POMC, Leptin-Rezeptor oder LepR, Bardet-Biedl-Syndrom, Alstrom-Syndrom, POMC heterozygot und POMC epigenetische Störungen.
Mitarbeiteranzahl : 90 Personen.
Quelle: https://ch.marketscreener.com/kurs/aktie/RHYTHM-PHARMACEUTIC…
Pipeline:
https://www.rhythmtx.com/our-pipeline/
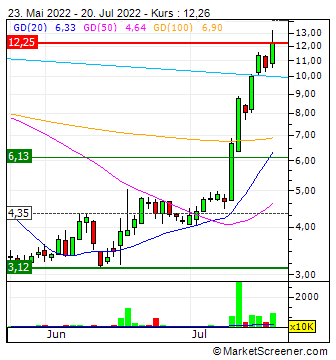
Chart:

Logo:

Rhythm Pharmaceuticals, Inc. ist ein biopharmazeutisches Unternehmen. Das Unternehmen konzentriert sich auf die Entwicklung und Vermarktung von Peptidtherapeutika zur Behandlung seltener genetischer Mängel, die zu lebensbedrohlichen Stoffwechselstörungen führen. Der führende Peptidproduktkandidat des Unternehmens ist Setmelanotid, ein potenter, erstklassiger Melanocortin-4-Rezeptor oder MC4R, ein Agonist zur Behandlung seltener genetischer Störungen von Fettleibigkeit. Setmelanotid dient als Ersatztherapie zur Behandlung von Melanocortin-4, oder MC4, Wegdefiziten. MC4-Pfaddefizite führen zur Störung von Sättigungssignalen und Energiehomöostase im Körper, was wiederum zu intensiven Hungergefühlen und zu Fettleibigkeit führt. Das Unternehmen konzentrierte sich auch auf Fettleibigkeit im Zusammenhang mit sechs einzelnen genbedingten oder monogenen MC4-Pfaddefiziten Pro-Optiomelanocortin oder POMC, Leptin-Rezeptor oder LepR, Bardet-Biedl-Syndrom, Alstrom-Syndrom, POMC heterozygot und POMC epigenetische Störungen.
Mitarbeiteranzahl : 90 Personen.
Quelle: https://ch.marketscreener.com/kurs/aktie/RHYTHM-PHARMACEUTIC…
Pipeline:
https://www.rhythmtx.com/our-pipeline/
Chart:

Logo:

















