Lexicon Pharmaceuticals Aktie WKN: 936717 ISIN: US5288721047 Symbol: LXRX Typ: Aktie (Seite 10)
eröffnet am 13.03.15 09:18:58 von
neuester Beitrag 13.03.24 08:55:25 von
neuester Beitrag 13.03.24 08:55:25 von
Beiträge: 151
ID: 1.209.260
ID: 1.209.260
Aufrufe heute: 1
Gesamt: 16.675
Gesamt: 16.675
Aktive User: 0
ISIN: US5288723027 · WKN: A14SSK · Symbol: LX31
1,8140
EUR
+4,25 %
+0,0740 EUR
Letzter Kurs 12:41:57 Tradegate
Neuigkeiten
09.05.24 · globenewswire |
02.05.24 · globenewswire |
29.04.24 · globenewswire |
29.04.24 · globenewswire |
18.04.24 · globenewswire |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 9,0770 | +116,20 | |
| 3,2000 | +93,94 | |
| 1,3300 | +30,51 | |
| 1,9800 | +26,11 | |
| 1,4700 | +23,01 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 7,9100 | -16,03 | |
| 7,5000 | -20,97 | |
| 2,4820 | -21,46 | |
| 0,6900 | -21,59 | |
| 4,4320 | -67,44 |
Beitrag zu dieser Diskussion schreiben
Antwort auf Beitrag Nr.: 65.529.997 von Malecon am 28.10.20 23:18:52
Und jetzt geht die Post ab.
🎵
Zitat von Malecon: Der Kurs dieser Aktie wird immer attraktiver
Zitat von Malecon: "Attraktiver für einen Einstieg" meinte ich
Und jetzt geht die Post ab.
🎵
Antwort auf Beitrag Nr.: 65.735.148 von Charly_2 am 17.11.20 11:08:33
Zeigt wohl Wirkung, $4 als Ziel :-)
Zitat von Charly_2: Up we go :-)
https://finance.yahoo.com/news/results-soloist-scored-outcom…
Zeigt wohl Wirkung, $4 als Ziel :-)
"Attraktiver für einen Einstieg" meinte ich.
Das mit dem Boden werden wir sehen, ich warte auch noch eine Weile ab bis sich dieser bildet. Erstmal muss er erreicht werden.
Das mit dem Boden werden wir sehen, ich warte auch noch eine Weile ab bis sich dieser bildet. Erstmal muss er erreicht werden.
Antwort auf Beitrag Nr.: 65.529.997 von Malecon am 28.10.20 23:18:52"attraktiver" ist auch eine Aussage.
spanned ist dass anscheinend bei 1.16 der Boden ist 😜
spanned ist dass anscheinend bei 1.16 der Boden ist 😜
Der Kurs dieser Aktie wird immer attraktiver:
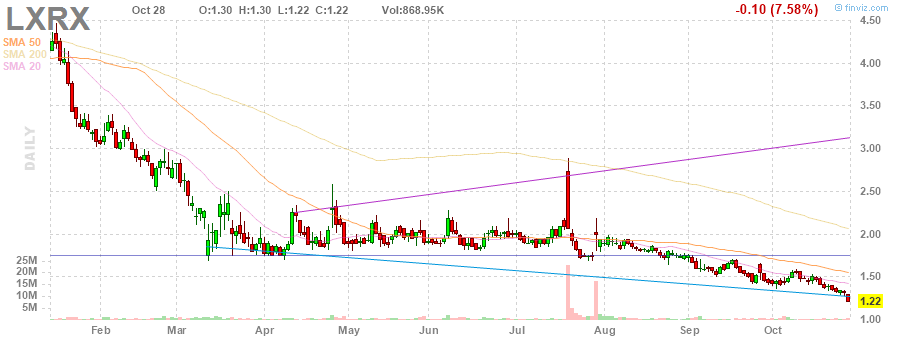

💡

💡
Schlusskurs $2,94

Aktuell +10% auf $2.58
Kursziel $6





















