Noxxon Pharma N.V. (Seite 5)
eröffnet am 30.09.16 09:44:55 von
neuester Beitrag 03.04.24 12:10:35 von
neuester Beitrag 03.04.24 12:10:35 von
Beiträge: 73
ID: 1.239.093
ID: 1.239.093
Aufrufe heute: 0
Gesamt: 11.152
Gesamt: 11.152
Aktive User: 0
ISIN: NL0015000YE1 · WKN: A3DMC3
0,2440
EUR
-4,50 %
-0,0115 EUR
Letzter Kurs 26.04.24 Lang & Schwarz
Neuigkeiten
25.04.24 · Business Wire (engl.) |
23.04.24 · Business Wire (engl.) |
02.04.24 · Business Wire (engl.) |
28.03.24 · Business Wire (engl.) |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,9000 | +59,66 | |
| 0,5922 | +44,44 | |
| 7,3500 | +43,84 | |
| 11,690 | +40,84 | |
| 0,7000 | +36,69 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 10,110 | -13,44 | |
| 8,2200 | -13,47 | |
| 7,6100 | -17,20 | |
| 1,6100 | -18,27 | |
| 2,1200 | -21,77 |
Beitrag zu dieser Diskussion schreiben
Antwort auf Beitrag Nr.: 59.436.435 von R-BgO am 14.12.18 06:26:06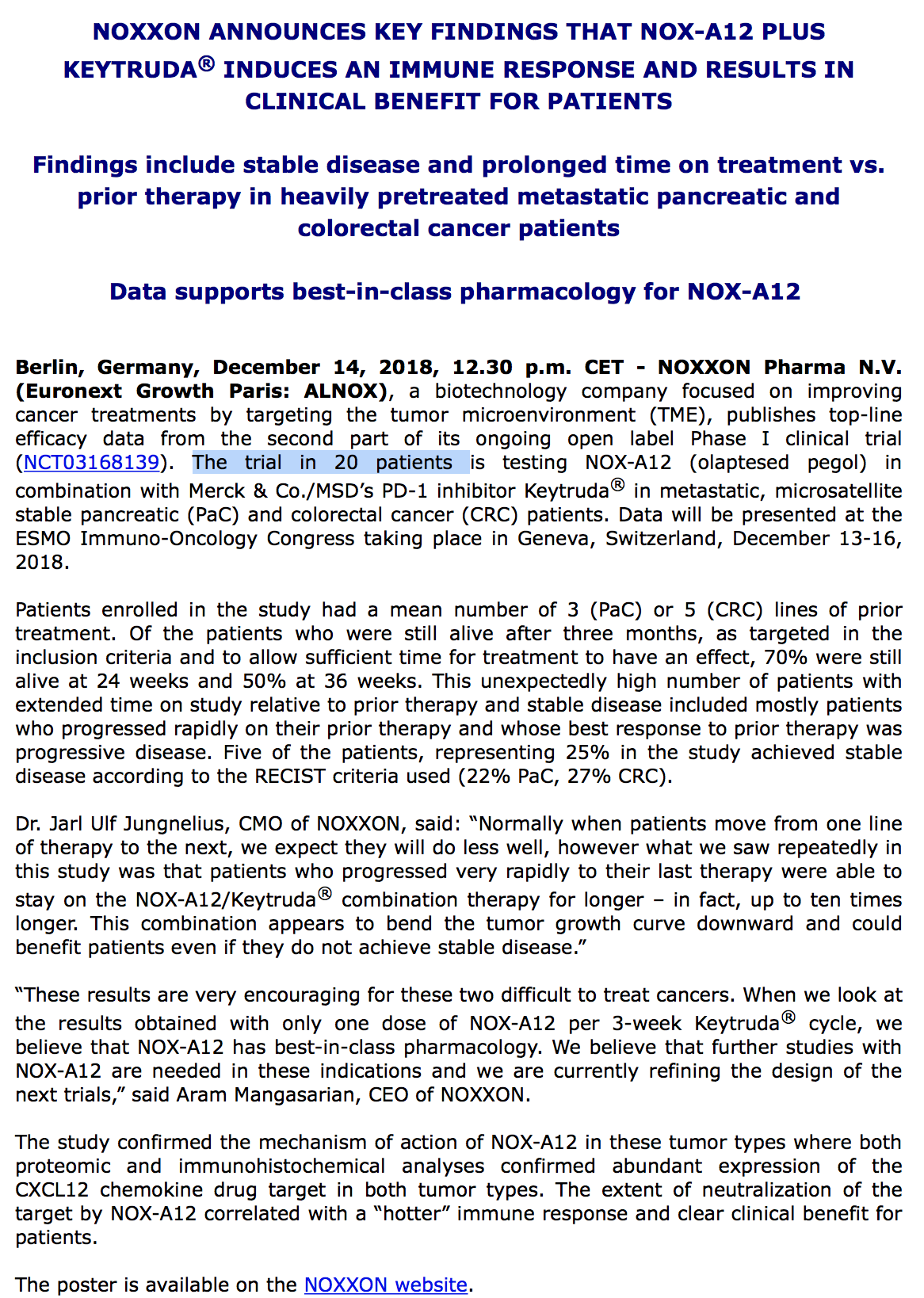

ein Novum:

Zuckt das Ding doch noch mal?

Antwort auf Beitrag Nr.: 59.230.090 von R-BgO am 16.11.18 10:13:47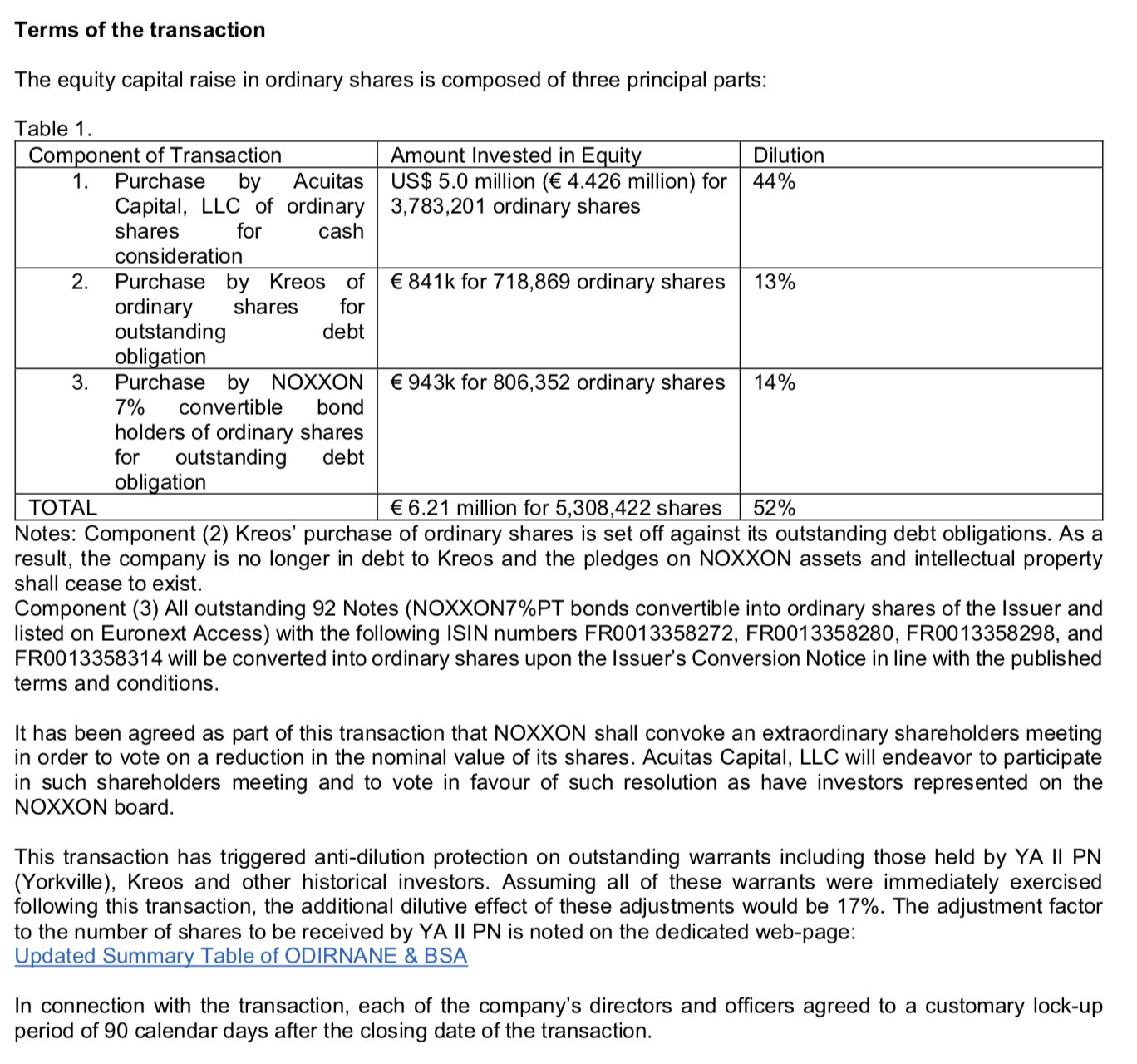

Lebenszeichen:

Performance pur
Lesezeichen...
Antwort auf Beitrag Nr.: 57.992.118 von R-BgO am 15.06.18 09:59:44Berlin, Germany, August 1st, 2018, 08.00 a.m. CEST -
NOXXON Pharma N.V. (Euronext Growth Paris: ALNOX), a biotechnology company focused on improving cancer treatments by targeting the tumor microenvironment (TME), announced today that it has secured additional financing from new investors in the form of convertible bonds which will convert to NOXXON shares at the terms of a future equity financing round, or starting on October 1st, 2018 at the investors option at the market price, which will be reset quarterly to the 10-day volume-weighted average price. The convertible bonds carry an interest rate of 7%, payable in shares upon conversion. A financing of €200,000 was secured on these terms in addition to the €380,000 secured under this same investment vehicle in June 2018 and €400,000 from the ODIRNANE financing vehicle in June and July 2018.
“We appreciate the support of new investors who were attracted by the terms and conditions of the convertible loan. We continue to look actively for additional funds since the company needs to raise additional capital in the near-term, as noted in our Annual Report 2017. We are in active discussions with a number of investors and our financing priorities are first to secure near term clinical data by raising the maximum amount of €1 million authorized under this convertible loan vehicle, and then to finance a NOX-A12 clinical trial in first-line glioblastoma patients,” commented Aram Mangasarian, CEO of NOXXON Pharma.
NOXXON Pharma N.V. (Euronext Growth Paris: ALNOX), a biotechnology company focused on improving cancer treatments by targeting the tumor microenvironment (TME), announced today that it has secured additional financing from new investors in the form of convertible bonds which will convert to NOXXON shares at the terms of a future equity financing round, or starting on October 1st, 2018 at the investors option at the market price, which will be reset quarterly to the 10-day volume-weighted average price. The convertible bonds carry an interest rate of 7%, payable in shares upon conversion. A financing of €200,000 was secured on these terms in addition to the €380,000 secured under this same investment vehicle in June 2018 and €400,000 from the ODIRNANE financing vehicle in June and July 2018.
“We appreciate the support of new investors who were attracted by the terms and conditions of the convertible loan. We continue to look actively for additional funds since the company needs to raise additional capital in the near-term, as noted in our Annual Report 2017. We are in active discussions with a number of investors and our financing priorities are first to secure near term clinical data by raising the maximum amount of €1 million authorized under this convertible loan vehicle, and then to finance a NOX-A12 clinical trial in first-line glioblastoma patients,” commented Aram Mangasarian, CEO of NOXXON Pharma.
Antwort auf Beitrag Nr.: 57.374.795 von erich007 am 23.03.18 23:42:52NOXXON ANNOUNCES THAT IT HAS SECURED ADDITIONAL FINANCING
Berlin, Germany, June 15, 2018, 08.00 a.m. CEST -
NOXXON Pharma N.V. (Euronext Growth Paris: ALNOX), a biotechnology company focused on improving cancer treatments by targeting the tumor microenvironment (TME), announced today that it has secured additional financing from existing investors in the form of a convertible bond which will convert to ALNOX shares at the terms of a future equity financing round, or starting on October 1st, 2018 at the investors option at the market price, which will be reset quarterly to the 10-day volume-weighted average price. The convertible bonds carry an interest rate of 7%, payable in shares upon conversion. €380,000 Euros were secured on these terms in addition to the recently secured €200,000 from the ODIRNANE financing vehicle.
“We are pleased by the continued support of existing investors. We remain committed to completing our clinical trial of NOX-A12 combined with Merck & Co./MSD’s Keytruda® in metastatic colorectal and pancreatic cancer patients and expect to be able to report completion of the recruitment in June with top-line data following shortly thereafter,” commented Aram Mangasarian, CEO of NOXXON Pharma.
Berlin, Germany, June 15, 2018, 08.00 a.m. CEST -
NOXXON Pharma N.V. (Euronext Growth Paris: ALNOX), a biotechnology company focused on improving cancer treatments by targeting the tumor microenvironment (TME), announced today that it has secured additional financing from existing investors in the form of a convertible bond which will convert to ALNOX shares at the terms of a future equity financing round, or starting on October 1st, 2018 at the investors option at the market price, which will be reset quarterly to the 10-day volume-weighted average price. The convertible bonds carry an interest rate of 7%, payable in shares upon conversion. €380,000 Euros were secured on these terms in addition to the recently secured €200,000 from the ODIRNANE financing vehicle.
“We are pleased by the continued support of existing investors. We remain committed to completing our clinical trial of NOX-A12 combined with Merck & Co./MSD’s Keytruda® in metastatic colorectal and pancreatic cancer patients and expect to be able to report completion of the recruitment in June with top-line data following shortly thereafter,” commented Aram Mangasarian, CEO of NOXXON Pharma.
NOXXON PROVIDES CLINICAL UPDATE PRIOR TO AMERICAN SOCIETY OF CLINICAL ONCOLOGY (ASCO) ANNUAL CONFERENCE
Update highlights effects of NOX-A12 monotherapy on tumor microenvironment in all recruited patients with evaluable tumor samples
Berlin, Germany, May 29, 2018, 8.00 a.m. CEST -
NOXXON Pharma N.V. (Euronext Growth Paris: ALNOX), a biotechnology company focused on improving cancer treatments by targeting the tumor microenvironment (TME), announced disclosure of additional data from its ongoing clinical trial (NCT03168139) testing NOX‑A12 alone (part 1) and subsequently in combination with Keytruda® (part 2) in metastatic, microsatellite stable pancreatic and colorectal cancer patients.
The additional data includes the comparison of baseline tumor biopsies to those taken after two weeks of NOX‑A12 monotherapy for all patients for whom matched tumor biopsies were available and analyzed at the time of publication. These data show that markers consistent with a Th1 like immune responses were seen in multiple patients in response to NOX-A12 therapy, in addition to changed levels of CXCL12 in tumors confirming penetration of NOX-A12. Data on changes in cell infiltration looking at a pan-T-cell (CD3) and a Myeloid cell (CD11b) marker will also be provided. The Company confirms that the safety profile of NOX-A12 continued to be consistent with prior experience where its administration was generally safe and well tolerated as monotherapy and in combination with approved anti-cancer agents considering the patient population.
Update highlights effects of NOX-A12 monotherapy on tumor microenvironment in all recruited patients with evaluable tumor samples
Berlin, Germany, May 29, 2018, 8.00 a.m. CEST -
NOXXON Pharma N.V. (Euronext Growth Paris: ALNOX), a biotechnology company focused on improving cancer treatments by targeting the tumor microenvironment (TME), announced disclosure of additional data from its ongoing clinical trial (NCT03168139) testing NOX‑A12 alone (part 1) and subsequently in combination with Keytruda® (part 2) in metastatic, microsatellite stable pancreatic and colorectal cancer patients.
The additional data includes the comparison of baseline tumor biopsies to those taken after two weeks of NOX‑A12 monotherapy for all patients for whom matched tumor biopsies were available and analyzed at the time of publication. These data show that markers consistent with a Th1 like immune responses were seen in multiple patients in response to NOX-A12 therapy, in addition to changed levels of CXCL12 in tumors confirming penetration of NOX-A12. Data on changes in cell infiltration looking at a pan-T-cell (CD3) and a Myeloid cell (CD11b) marker will also be provided. The Company confirms that the safety profile of NOX-A12 continued to be consistent with prior experience where its administration was generally safe and well tolerated as monotherapy and in combination with approved anti-cancer agents considering the patient population.





















