► BAYER AG ■ Auf neuen Wegen ◄ (Seite 2348)
eröffnet am 29.11.03 14:19:12 von
neuester Beitrag 20.05.24 14:10:48 von
neuester Beitrag 20.05.24 14:10:48 von
Beiträge: 25.000
ID: 800.232
ID: 800.232
Aufrufe heute: 25
Gesamt: 2.272.119
Gesamt: 2.272.119
Aktive User: 0
ISIN: DE000BAY0017 · WKN: BAY001 · Symbol: BAYN
28,77
EUR
+0,65 %
+0,19 EUR
Letzter Kurs 20.05.24 Tradegate
Neuigkeiten
| Bayer: Vorsicht, der Ausbruchsversuch droht zu scheiternAnzeige |
20.05.24 · dpa-AFX Analysen |
19.05.24 · Felix Haupt Anzeige |
19.05.24 · Markus Weingran |
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 6,40 | +184,44 | |
| 0,7019 | +71,15 | |
| 0,8000 | +45,45 | |
| 0,6100 | +25,70 | |
| 2,0600 | +24,85 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 3,5000 | -8,85 | |
| 1,6200 | -8,99 | |
| 20,360 | -11,48 | |
| 4,0400 | -14,95 | |
| 23,700 | -19,52 |
Beitrag zu dieser Diskussion schreiben
Antwort auf Beitrag Nr.: 51.640.153 von Lennypenny am 02.02.16 19:04:56
Für mich bleiben zwei Möglichkeiten:
1. Entweder einige kleinere Zukäufe (bis 5mrd) die Teilbereiche sehr gut abdecken -> z.b. KWS im Zuckerrübenbereich
2. "Große Lösung": Zusammgengehen mit Dowdupont-Sparte oder BASF-Sparte oder Monsanto - entweder als Komplettübernahme oder was mir lieber gefallen würde Bayer-spinoff + Zusammengehen und Anteilseigner werden (mit Monsanto).
Zitat von Lennypenny: welche Firmen würdet Ihr denn im Agro Bereich für Bayer fovorisieren?
Gruß
Lenny
Für mich bleiben zwei Möglichkeiten:
1. Entweder einige kleinere Zukäufe (bis 5mrd) die Teilbereiche sehr gut abdecken -> z.b. KWS im Zuckerrübenbereich
2. "Große Lösung": Zusammgengehen mit Dowdupont-Sparte oder BASF-Sparte oder Monsanto - entweder als Komplettübernahme oder was mir lieber gefallen würde Bayer-spinoff + Zusammengehen und Anteilseigner werden (mit Monsanto).
Antwort auf Beitrag Nr.: 51.637.471 von Low-Risk-Strategie am 02.02.16 15:37:07welche Firmen würdet Ihr denn im Agro Bereich für Bayer fovorisieren?
Gruß
Lenny
Gruß
Lenny
Die Rotation geht weiter:
Bayer will sein Agrochemie-Geschäft weiter ausbauen
http://www.cash.ch/news/alle/rss/bayer_will_sein_agrochemieg…
ChemChina will Syngenta für 44 Milliarden Franken schlucken
http://www.focus.de/finanzen/news/wirtschaftsticker/kreise-c…
Bayer will sein Agrochemie-Geschäft weiter ausbauen
http://www.cash.ch/news/alle/rss/bayer_will_sein_agrochemieg…
ChemChina will Syngenta für 44 Milliarden Franken schlucken
http://www.focus.de/finanzen/news/wirtschaftsticker/kreise-c…
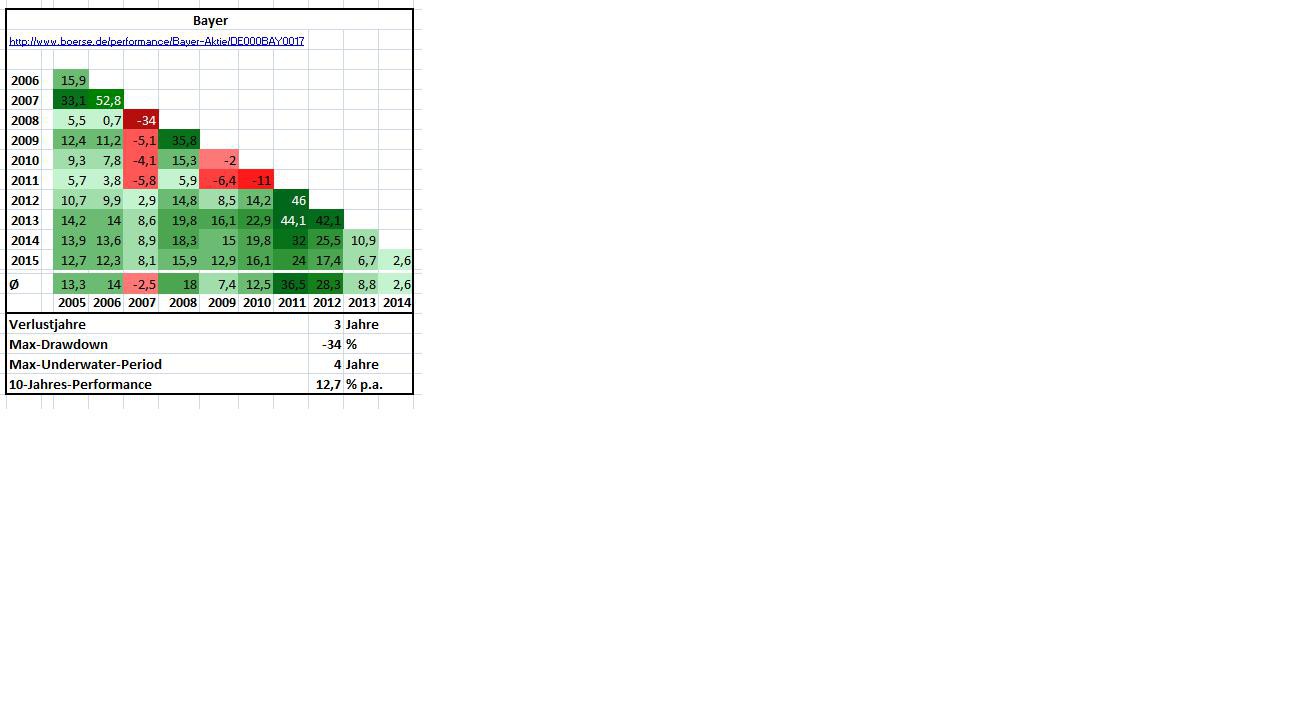
Fazit 2015

"BAYER - Die europäische Arzneizulassungsbehörde EMA nimmt die Studie für Bayers Verkaufsschlager Xarelto unter die Lupe. Es besteht der Verdacht, dass Bayer für die Zulassung ein defektes Testgerät verwendet haben soll. Auch die US-Arzneibehörde FDA horcht auf. (Handelsblatt S. 16)"
Mich würde interesseren ob und wenn ja, welche andere Firmen von dem defekten Testsystem der Alere Inc. betroffen sind.
Mich würde interesseren ob und wenn ja, welche andere Firmen von dem defekten Testsystem der Alere Inc. betroffen sind.
The final decision of the European Commission on the marketing authorization is expected in the coming weeks. Hemophilia affects approximately 400,000 people around the world and is characterized by prolonged or spontaneous bleeding, especially into the muscles, joints or internal organs.
Übersetzt heißt das der Durchbruch für 400,000 Menschen Weltweit.
Die endgültige Entscheidung der Europäischen Kommission über die Genehmigung für das Inverkehrbringen wird in den kommenden Wochen erwartet. Hämophilie betrifft etwa 400.000 Menschen auf der ganzen Welt und ist durch verlängerte und spontane Blutungen charakterisiert, besonders in die Muskeln, Gelenke oder in innere Organe.
Da muss das Zeug aber richtig teuer sein damit da ein Gewinn abfällt.
Übersetzt heißt das der Durchbruch für 400,000 Menschen Weltweit.
Die endgültige Entscheidung der Europäischen Kommission über die Genehmigung für das Inverkehrbringen wird in den kommenden Wochen erwartet. Hämophilie betrifft etwa 400.000 Menschen auf der ganzen Welt und ist durch verlängerte und spontane Blutungen charakterisiert, besonders in die Muskeln, Gelenke oder in innere Organe.
Da muss das Zeug aber richtig teuer sein damit da ein Gewinn abfällt.
Bluterkrankheit
December 18, 2015
Not intended for U.S. and UK Media
Bayer Receives Positive CHMP Opinion for BAY 81-8973 for the Treatment of Hemophilia A in Patients in EU
Berlin, December 18, 2015 - The European Committee for Medicinal Products for Human Use (CHMP) has recommended BAY 81-8973, Bayer's new recombinant factor VIII compound, for approval in the EU for the treatment and prophylaxis of bleeding in patients with hemophilia A for all age groups. BAY 81-8973 is an unmodified full-length recombinant factor VIII compound that, in clinicals trials, has demonstrated control of bleeds and protection from bleeds in hemophilia A patients when used prophylactically two or three times per week.
The final decision of the European Commission on the marketing authorization is expected in the coming weeks. Hemophilia affects approximately 400,000 people around the world and is characterized by prolonged or spontaneous bleeding, especially into the muscles, joints or internal organs.
"Bayer is highly committed to the hemophilia community, exemplified best by our latest development BAY 81-8973, as well as by our long-acting recombinant factor VIII pipeline candidate BAY 94-9027", said Dr. Joerg Moeller, Member of the Bayer HealthCare Executive Committee and Head of Global Development. "In addition, we are also pursuing different early stage treatment approaches in hemophilia including an anti-TFPI-antibody."
The positive CHMP recommendation is based on results from the LEOPOLD (Long-Term Efficacy Open-Label Program in Severe Hemophilia A Disease) clinical trials, which evaluated BAY 81-8973 in children, adolescents and adults using both, prophylaxis dosing regimens and on-demand treatment.
Bayer has submitted marketing applications for BAY 81-8973 in the US and several other countries and is pursuing regulatory approvals worldwide.
Bayer is committed to delivering science for a better life by advancing a portfolio of innovative treatments. Hematology at Bayer includes an approved treatment for hemophilia A and numerous compounds in various stages of development for hemophilia, sickle cell anemia, and other blood and bleeding disorders. Together, these compounds reflect the company's commitment to research and development for these indications, prioritizing specific targets for intervention with the potential to improve the way that rare blood and bleeding disorders are treated.
About LEOPOLD
The LEOPOLD Clinical Development Program consists of three multinational clinical trials designed to evaluate the pharmacokinetics, efficacy, and safety of BAY 81-8973 in subjects with severe hemophilia A (<1% FVIII:C).
LEOPOLD I is an open-label, cross-over Phase III study in males aged 12-65 years. The objectives were to demonstrate the efficacy and safety of BAY 81-8973 when used as prophylaxis, for the treatment of bleeding episodes, and for maintaining hemostasis during surgery. In LEOPOLD I, investigators assigned subjects to either the two- or three-times-weekly dosing regimens based on each patient's phenotype, prior bleeding history and other factors.
LEOPOLD II is a randomized, cross-over, open-label trial also in male subjects aged 12 to 65 years. In this Phase III study, 80 subjects were randomized to receive BAY 81-8973 either as a low-dose prophylaxis regimen (20-30 IU/kg; n=28) twice-per-week, high-dose prophylaxis (30-40 IU/kg; n=31) three-times-per-week, or on-demand (n=21). The primary objective was to demonstrate the superiority of prophylaxis versus on-demand therapy, with the primary endpoint being bleeding frequency at 12 months.
LEOPOLD Kids is an open-label, non-randomized Phase III study. Part A is designed to evaluate the efficacy and safety of BAY 81-8973 for prophylaxis, treatment of bleeds, and surgical management in previously treated children <=12 years of age with twice or three times per week or every other day prophylaxis regimens. Part B of the study, which involves previously untreated patients (PUPs), is ongoing. ? About Bayer HealthCare
The Bayer Group is a global enterprise with core competencies in the fields of health care and agriculture. Bayer HealthCare, a subgroup of Bayer AG with annual sales of around EUR 20.0 billion (2014), is one of the world's leading, innovative companies in the healthcare and medical products industry and is based in Leverkusen, Germany. The company combines the global activities of the Animal Health, Consumer Care, Medical Care and Pharmaceuticals divisions. Bayer HealthCare's aim is to discover, develop, manufacture and market products that will improve human and animal health worldwide. Bayer HealthCare has a global workforce of 60,700 employees (Dec 31, 2014) and is represented in more than 100 countries. More information is available at www.healthcare.bayer.com.
Our online press service is just a click away: press.healthcare.bayer.com Follow us on Facebook: http://www.facebook.com/healthcare.bayer Follow us on Twitter: https://twitter.com/BayerHealthCare
Find more information at www.bayerpharma.com.
Forward-looking statements
This release may contain forward-looking statements based on current assumptions and forecasts made by Bayer Group or subgroup management. Various known and unknown risks, uncertainties and other factors could lead to material differences between the actual future results, financial situation, development or performance of the company and the estimates given here. These factors include those discussed in Bayer's public reports which are available on the Bayer website at www.bayer.com. The company assumes no liability whatsoever to update these forward-looking statements or to conform them to future events or developments.
Stock Price/Index
XETRA: 115.85 down -2.32 %
December 18, 2015 19:50 CEST
DAX: 10,608.19 down -1.21 %
December 18, 2015 19:50 CEST
Download
Investor News
Download ( PDF, 30 KB )
collect
Current Events
February 25, 2016
FY/Q4 2015 Investor Conference Call
February 24, 2016
Announcement of the proposed dividend
Current Links
IR News Archive Overview
Download Acrobat Reader
Email Newsletter
RSS Newsfeed
SMS Newsservice
Search
Enter your search term
Go
Quicklinks
Service
Contact IR Team
Order Publications
Newsletter
RSS Newsfeeds
Podcasts
Mobile Services
Collected Documents
New on this site
FAQs
Investor Factsheet
Last updated: December 04, 2013 Copyright © Bayer AG
December 18, 2015
Not intended for U.S. and UK Media
Bayer Receives Positive CHMP Opinion for BAY 81-8973 for the Treatment of Hemophilia A in Patients in EU
Berlin, December 18, 2015 - The European Committee for Medicinal Products for Human Use (CHMP) has recommended BAY 81-8973, Bayer's new recombinant factor VIII compound, for approval in the EU for the treatment and prophylaxis of bleeding in patients with hemophilia A for all age groups. BAY 81-8973 is an unmodified full-length recombinant factor VIII compound that, in clinicals trials, has demonstrated control of bleeds and protection from bleeds in hemophilia A patients when used prophylactically two or three times per week.
The final decision of the European Commission on the marketing authorization is expected in the coming weeks. Hemophilia affects approximately 400,000 people around the world and is characterized by prolonged or spontaneous bleeding, especially into the muscles, joints or internal organs.
"Bayer is highly committed to the hemophilia community, exemplified best by our latest development BAY 81-8973, as well as by our long-acting recombinant factor VIII pipeline candidate BAY 94-9027", said Dr. Joerg Moeller, Member of the Bayer HealthCare Executive Committee and Head of Global Development. "In addition, we are also pursuing different early stage treatment approaches in hemophilia including an anti-TFPI-antibody."
The positive CHMP recommendation is based on results from the LEOPOLD (Long-Term Efficacy Open-Label Program in Severe Hemophilia A Disease) clinical trials, which evaluated BAY 81-8973 in children, adolescents and adults using both, prophylaxis dosing regimens and on-demand treatment.
Bayer has submitted marketing applications for BAY 81-8973 in the US and several other countries and is pursuing regulatory approvals worldwide.
Bayer is committed to delivering science for a better life by advancing a portfolio of innovative treatments. Hematology at Bayer includes an approved treatment for hemophilia A and numerous compounds in various stages of development for hemophilia, sickle cell anemia, and other blood and bleeding disorders. Together, these compounds reflect the company's commitment to research and development for these indications, prioritizing specific targets for intervention with the potential to improve the way that rare blood and bleeding disorders are treated.
About LEOPOLD
The LEOPOLD Clinical Development Program consists of three multinational clinical trials designed to evaluate the pharmacokinetics, efficacy, and safety of BAY 81-8973 in subjects with severe hemophilia A (<1% FVIII:C).
LEOPOLD I is an open-label, cross-over Phase III study in males aged 12-65 years. The objectives were to demonstrate the efficacy and safety of BAY 81-8973 when used as prophylaxis, for the treatment of bleeding episodes, and for maintaining hemostasis during surgery. In LEOPOLD I, investigators assigned subjects to either the two- or three-times-weekly dosing regimens based on each patient's phenotype, prior bleeding history and other factors.
LEOPOLD II is a randomized, cross-over, open-label trial also in male subjects aged 12 to 65 years. In this Phase III study, 80 subjects were randomized to receive BAY 81-8973 either as a low-dose prophylaxis regimen (20-30 IU/kg; n=28) twice-per-week, high-dose prophylaxis (30-40 IU/kg; n=31) three-times-per-week, or on-demand (n=21). The primary objective was to demonstrate the superiority of prophylaxis versus on-demand therapy, with the primary endpoint being bleeding frequency at 12 months.
LEOPOLD Kids is an open-label, non-randomized Phase III study. Part A is designed to evaluate the efficacy and safety of BAY 81-8973 for prophylaxis, treatment of bleeds, and surgical management in previously treated children <=12 years of age with twice or three times per week or every other day prophylaxis regimens. Part B of the study, which involves previously untreated patients (PUPs), is ongoing. ? About Bayer HealthCare
The Bayer Group is a global enterprise with core competencies in the fields of health care and agriculture. Bayer HealthCare, a subgroup of Bayer AG with annual sales of around EUR 20.0 billion (2014), is one of the world's leading, innovative companies in the healthcare and medical products industry and is based in Leverkusen, Germany. The company combines the global activities of the Animal Health, Consumer Care, Medical Care and Pharmaceuticals divisions. Bayer HealthCare's aim is to discover, develop, manufacture and market products that will improve human and animal health worldwide. Bayer HealthCare has a global workforce of 60,700 employees (Dec 31, 2014) and is represented in more than 100 countries. More information is available at www.healthcare.bayer.com.
Our online press service is just a click away: press.healthcare.bayer.com Follow us on Facebook: http://www.facebook.com/healthcare.bayer Follow us on Twitter: https://twitter.com/BayerHealthCare
Find more information at www.bayerpharma.com.
Forward-looking statements
This release may contain forward-looking statements based on current assumptions and forecasts made by Bayer Group or subgroup management. Various known and unknown risks, uncertainties and other factors could lead to material differences between the actual future results, financial situation, development or performance of the company and the estimates given here. These factors include those discussed in Bayer's public reports which are available on the Bayer website at www.bayer.com. The company assumes no liability whatsoever to update these forward-looking statements or to conform them to future events or developments.
Stock Price/Index
XETRA: 115.85 down -2.32 %
December 18, 2015 19:50 CEST
DAX: 10,608.19 down -1.21 %
December 18, 2015 19:50 CEST
Download
Investor News
Download ( PDF, 30 KB )
collect
Current Events
February 25, 2016
FY/Q4 2015 Investor Conference Call
February 24, 2016
Announcement of the proposed dividend
Current Links
IR News Archive Overview
Download Acrobat Reader
Email Newsletter
RSS Newsfeed
SMS Newsservice
Search
Enter your search term
Go
Quicklinks
Service
Contact IR Team
Order Publications
Newsletter
RSS Newsfeeds
Podcasts
Mobile Services
Collected Documents
New on this site
FAQs
Investor Factsheet
Last updated: December 04, 2013 Copyright © Bayer AG
artikel von sep 2013 ?
Antwort auf Beitrag Nr.: 51.331.842 von SmartCap am 18.12.15 11:08:25
Xarelto steht auch unter Verdacht unerwünschte Nebenwirkungen und Todesfälle zu verursachen!
Quelle: http://www.spiegel.de/wissenschaft/medizin/bayer-blutverduen…
Zitat von SmartCap: bzgl Xarelto: Firma des Testgeräteherstellers: Alere San Diego, Inc. San Diego, California
gibt es andere Firmen die diesbezgl. betroffen sind ?
SC
Xarelto steht auch unter Verdacht unerwünschte Nebenwirkungen und Todesfälle zu verursachen!
Quelle: http://www.spiegel.de/wissenschaft/medizin/bayer-blutverduen…
bzgl Xarelto: Firma des Testgeräteherstellers: Alere San Diego, Inc. San Diego, California
gibt es andere Firmen die diesbezgl. betroffen sind ?
SC
gibt es andere Firmen die diesbezgl. betroffen sind ?
SC
► BAYER AG ■ Auf neuen Wegen ◄





















