Auferstanden aus Ruinen? – Welche Zukunft hat Bristol Meyers Squibb? - 500 Beiträge pro Seite
eröffnet am 24.08.12 16:26:47 von
neuester Beitrag 28.03.22 12:14:33 von
neuester Beitrag 28.03.22 12:14:33 von
Beiträge: 97
ID: 1.176.353
ID: 1.176.353
Aufrufe heute: 0
Gesamt: 18.665
Gesamt: 18.665
Aktive User: 0
ISIN: US1101221083 · WKN: 850501
41,24
EUR
0,00 %
0,00 EUR
Letzter Kurs 18:58:03 Lang & Schwarz
Neuigkeiten
10.05.24 · Business Wire (engl.) |
07.05.24 · Business Wire (engl.) |
06.05.24 · Business Wire (engl.) |
05.05.24 · wallstreetONLINE Redaktion |
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,5700 | +55,23 | |
| 5,4500 | +41,56 | |
| 141,00 | +41,00 | |
| 1,1500 | +34,98 | |
| 1,0000 | +33,33 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,8700 | -12,62 | |
| 12,150 | -12,72 | |
| 35,69 | -13,71 | |
| 5,2500 | -19,23 | |
| 0,7300 | -19,34 |
Aus meiner Sicht lohnt es sich, BMS (Ticker: BMY, ISIN: US1101221083) zu beobachten und sogar dort zu investieren. Ende Juli stand die Aktie noch bei 36 Dollar und ist seit dem deutlich gefallen, weil ein Programm gegen Hepatitis-C in der klinischen Prüfung gescheitert ist. Ich habe darin die Chance zum Einstieg gesehen. Deshalb eröffne ich diesen Thread, um hier Nachrichten und Analysen zum Unternehmen zu sammeln. Tradingtips und „charttechnische“ Aussagen wird man hier von mir hingegen nicht zu lesen bekommen.
Bewertung
Am 24.08.2012 beträgt der Aktienkurs 32,15 Dollar, was eine Marktkapitalisierung von ca. 54 Milliarden Dollar bedeutet.
Es wird für 2012 ein Gewinn von 1,92 Dollar / Aktie erwartet, der 2013 auf 1,88 Dollar zurückgehen soll.
Das KGV liegt demnach bei ungefähr 17 für das laufende und das kommende Jahr.
Der Umsatz soll 2012 knappe 18 Milliarden Dollar betragen und dürfte 2013 auf etwas über 17 Milliarden Dollar fallen.
Bis 2015 dürften die Umsätze dann in etwa stagnieren, während der Gewinn ab 2014 wieder moderat steigen und im Jahr 2015 eventuell bei ca. 2,40 Dollar liegen könnte.
Die Jahresdividende liegt bei 1,36 Dollar, was eine Dividendenrendite von ca. 4% vor Steuern ergibt.
Bei BMS handelt es sich also derzeit um ein stagnierendes Pharmaunternehmen mittlerer Größe, das sich mehr und mehr zum Biotech wandelt.
Für den Beobachter stellen sich hier vermutlich zwei Fragen.
1.) Warum stagniert BMS?
2.) Was soll an diesem Unternehmen eigentlich spannend sein?
Stagnation bis 2015/2016 – das „Patentcliff“ bei BMS
BMS hat im letzten Jahr ca. 21 Milliarden Dollar Umsatz generiert.
Im Zeitraum 2012 bis 2015 verlieren indes 4 Medikamente ihren Patentschutz, die in 2011 zusammen für 12,3 Milliarden Dollar bzw. 58%(!) des Gesamtumsatzes standen. Allein Plavix (hemmt die Eigenschaft der Blutplättchen, verklumpen zu können und ist indiziert bspw. für Herzinfarkt- und Schlaganfallpatienten), kam im letzten Jahr auf einen Umsatz von 7 Milliarden Dollar.
Der Patentschutz ist in den USA mittlerweile ausgelaufen. In Europa wird dieses im Februar 2013 geschehen. Der Plavix-Umsatz wird damit bereits 2013 von 7 Milliarden Dollar noch in 2011 auf vielleicht 0,2 Milliarden Dollar einbrechen.
Die ehemals 12,3 Milliarden Dollar Umsatz der Medikamente, die bis 2015 den Patentschutz verlieren (Plavix, Avapro, Abilify und Sustiva) werden in 2016 fast vollständig ausradiert sein.
Stagnation als Erfolg
Wer sich dieses enorme Wegbrechen von Umsätzen vor Augen führt, muss zu dem Urteil kommen, dass es ein beachtlicher Erfolg des BMS-Managements ist, dass das Unternehmen dennoch in etwa seinen Umsatz halten können wird. Der Markt geht davon aus, dass das Umsatztief des Unternehmens schon 2013 mit etwas über 17 Milliarden Dollar erreicht sein wird und die Umsätze dann wieder wachsen. Erst langsam und dann, nach der Bereinigung um die Patentabläufe ab 2016 wieder schneller. Bereits 2015 wird man voraussichtlich wieder in etwa auf dem Niveau von 2011 sein. Dies hängt aber auch von einer Zulassung des Wirkstoffes Eliquis ab. Dies wird weiter unten näher ausgeführt.
Neue Produkte
BMS hat es verstanden, neue Produkte an den Markt zu bringen, deren Umsätze schnell wachsen und die dabei sind, Blockbuster zu werden.
Die beständig wachsenden Umsätze aus diesen neuen Produkten werden bis 2015 die wegfallenden Umsätze der Altprodukte kompensiert haben.
Zu diesen neuen Produkten gehören insbesondere:
- Onglyza (Diabetes), geschätzter Umsatz in 2012: 704 Millionen
- Orencia (Rheumatoide Arthritis), geschätzter Umsatz in 2012: 1 Milliarde
- Yervoy (Melanom), geschätzter Umsatz in 2012: 1 Milliarde
- Sprycel (Leukämie), geschätzter Umsatz in 2012: 650 Millionen
Besonders Yervoy und Sprycel wachsen schnell und könnten bis 2015 jeweils einen Umsatz von 2 Milliarden Dollar erzielen.
Ein Hoffnungsträger
Durch die eben erst erfolgte Übernahme von Amylin hat BMS nun deren Hoffnungsträger Bydureon (Diabetes) im Portfolio. Analysten halten für das erst in diesem Jahr zugelassene Produkt mittelfristig Milliardenumsätze für möglich. Es wird interessant und kursrelevant sein, wie gut die Vermarktung des Produktes in der Zusammenarbeit zwischen BMA und AstraZeneca funktionieren wird. Ende 2013 wird man hier ein erstes Fazit ziehen können.
Der neue König?
Die bisher beschriebenen Neuentwicklungen reichen wohl lediglich aus, um einen regelrechten Absturz bei BMS zu verhindern.
Für eine wirklich positive Entwicklung des Unternehmens und des Aktienkurses in den nächsten Jahren ist aber eine weitere Neuentwicklung entscheidend, nämlich Eliquis (apixaban), ein Ko-Entwicklung mit Pfizer.
Apixaban ist ein Wirkstoff aus der Gruppe der direkten Faktor-Xa-Inhibitoren. Es hemmt selektiv den Faktor Xa, ein Enzym, das in der Blutgerinnung eine zentrale Rolle spielt. Apixaban beugt der Entstehung von Thromben vor und wird zur Thromboseprophylaxe nach Knie- oder Hüftgelenksoperationen eingesetzt. Hierfür gibt es für den europäischen Markt bereits eine Zulassung.
Entscheidend ist aber, dass das Medikament bei Patienten mit Herzrhythmusstörungen (Vorhofflimmern) zum Einsatz kommen soll, um das erhöhte Schlaganfallrisiko der Patienten zu mindern.
Leider hat die amerikanische Zulassungsbehörde FDA im Juni den Zulassungsantrag für diesen Wirkstoff mit einem Complete Response Letter (CRL) zunächst auf das Abstellgleis geschoben.
Ein CRL ist zwar keine Ablehnung, kann aber im Einzelfall die Zulassung um Jahre verzögern oder letztlich zum Aus des Programms führen.
So schlimm dürfte es hier nicht kommen, denn die FDA hat wohl nicht verlangt, dass neue klinische Studien durchgeführt werden. Vielmehr wurde BMS und Pfizer „nur“ aufgegeben, weitere Angaben zum Datenmanagement des für die Zulassung entscheidenden „Aristoteles-Trials“ zu liefern. „Aristoteles“ umfasste 24.000 Patienten und hat Millionen von Datenpunkten generiert. Dass bei einem so komplexen Vorgang Klärungsbedarf zwischen Zulassungsbehörde und Antragsteller entstehen kann, sollte nicht zu kritisch gesehen werden.
Allerdings ist eines auch ganz klar: Die vollständige Zulassung von Eliquis ist essentiell für BMS, denn dem Wirkstoff wird langfristig ein Umsatzpotential wie Plavix zugetraut. Ein potentieller Mega-Blockbuster also.
Eine – wenn auch eher unwahrscheinliche – Ablehnung der Zulassung oder auch „nur“ die Notwendigkeit, zusätzliche Studien durchzuführen, wäre ein mittleres Desaster. In diesem Negativszenario wären Kurse über 25 Dollar für die nächsten 2 Jahre wohl nur schwer zu rechtfertigen.
Die Entscheidung über Eliquis wird aller Voraussicht nach bis spätestens Mitte 2013 gefallen sein. Mit sehr viel Glück könnte die Zulassung bereits Ende 2012 erfolgen. Wahrscheinlicher ist aber eine FDA-Entscheidung im Zeitraum Februar bis Mai 2013.
Persönlich gehe ich davon aus, dass das Mittel in 2013 die Zulassung erhalten wird und BMS dann sehr, sehr stark dastehen wird.
Sollte ich an diesem Punkt irren, werden zumindest die nächsten 2 oder 3 Jahre allerdings wirklich unschön.
Der kommende König der Könige? – Oder, was an BMS so spannend ist.
Während Eliquis auf die Indikation Herz-Kreislauferkrankungen zielt und damit die eine große Geisel der Menschheit adressiert, ist bei BMS noch ein weit weniger fortgeschrittenes Programm in der Entwicklung, das noch wenig bekannt ist, Fachleute aber elektrisiert hat. Es handelt sich um einen Antikörper gegen das Protein PD-1 (Programmed cell death protein 1). Das Programm trägt derzeit noch den Entwicklungsnamen „BMS-936558”.
Die – mittlerweile durch erste klinische Tests gestützte – Erwartung ist, dass durch die Blockade von PD-1 das Immunsystem in die Lage versetzt werden kann, selbst gegen Krebszellen vorzugehen (Immuntherapie).
In diesem Jahr tritt der Wirkstoff in die Phase III der klinischen Entwicklung ein, die um 2015 zu einer ersten Zulassung, möglicherweise bei Lungenkrebs, führen könnte. Tests in weiteren Krebsarten sind auf dem Weg oder werden demnächst beginnen.
Die bisher generierten Phase II-Daten waren überragend gut.
Ich will an dieser Stelle den Biotech-Analysten Ohad Hammer zitieren:
“The biggest news at this year’s ASCO came from BMS’ (BMY) PD-1 antibody, BMS-936558. This antibody belongs to a new class of antibodies that stimulate patients’ immune system to attack cancer. This approach has been recently validated with another BMS antibody, Yervoy, which was approved last year for melanoma.
Based on results presented at the meeting, BMS-936558 is superior to Yervoy by any measure. In fact, it is probably one of the most promising oncology drugs ever to be tested in humans. It induces tumor shrinkage in a substantial portion of patients, creates an immune response that keeps the disease under control for long periods and it does so with limited side effects. To make things even better, there might be a way to pre-select patients who are more likely to respond to this agent.”
Der Wirkstoff würde im Erfolgsfall mit Sicherheit ein Blockbuster, sehr wahrscheinlich ein Mega-Blockbuster, werden. Das Umsatzpotential liegt langfristig wohl deutlich jenseits der 5 Milliarden Dollar-Marke.
Ein Erfolg von BMS-936558 wäre für das Unternehmen transformativ. BMS hätte in diesem Falle viele Jahre eines schnellen Umsatz- und Gewinnwachstums vor sich, die das Unternehmen (und den Aktienkurs) in eine andere Liga führen würde.
Fazit
BMS macht derzeit aufgrund wichtiger Patentabläufe eine schwierige Phase der Unternehmensentwicklung durch.
Das Management hat das Unternehmen aber gut auf diese Zeit eingestellt und eine attraktive Pipeline aus neuen Produkten aufgebaut, die Stück für Stück die wegfallenden Umsätze ersetzen können.
Meine Kaufentscheidung beruhte im Wesentlichen aber auf meiner Erwartung, dass im nächsten Jahr Eliquis eine umfassende Zulassung erhalten und sich in der Folge zum Marktführer im Bereich der Antikoagulantien entwickeln und dass ab 2015/2016 die Zulassung von BMS-936558 das Unternehmen in eine neue Dimension führen wird.
Mit Eliquis und BMS-936558 hat BMS zwei potentielle Mega-Blockbuster in der Pipeline, die, falls sie beide zugelassen werden, ein Investment zu einem großen Erfolg werden lassen sollten. Ich schätze die Zulassungswahrscheinlichkeit für beide Wirkstoffe als überdurchschnittlich hoch ein, würde mir aber um Eliquis sogar etwas mehr Sorgen machen, als um BMS-936558.
Sollte nur einer dieser beiden Kandidaten die Zulassung erhalten, sollte sich ein Investment noch immer gut bezahlt machen, auch wenn, im Falle einer Nichtzulassung von Eliquis, die Jahre 2013/2014 recht bitter werden dürften.
Bewertung
Am 24.08.2012 beträgt der Aktienkurs 32,15 Dollar, was eine Marktkapitalisierung von ca. 54 Milliarden Dollar bedeutet.
Es wird für 2012 ein Gewinn von 1,92 Dollar / Aktie erwartet, der 2013 auf 1,88 Dollar zurückgehen soll.
Das KGV liegt demnach bei ungefähr 17 für das laufende und das kommende Jahr.
Der Umsatz soll 2012 knappe 18 Milliarden Dollar betragen und dürfte 2013 auf etwas über 17 Milliarden Dollar fallen.
Bis 2015 dürften die Umsätze dann in etwa stagnieren, während der Gewinn ab 2014 wieder moderat steigen und im Jahr 2015 eventuell bei ca. 2,40 Dollar liegen könnte.
Die Jahresdividende liegt bei 1,36 Dollar, was eine Dividendenrendite von ca. 4% vor Steuern ergibt.
Bei BMS handelt es sich also derzeit um ein stagnierendes Pharmaunternehmen mittlerer Größe, das sich mehr und mehr zum Biotech wandelt.
Für den Beobachter stellen sich hier vermutlich zwei Fragen.
1.) Warum stagniert BMS?
2.) Was soll an diesem Unternehmen eigentlich spannend sein?
Stagnation bis 2015/2016 – das „Patentcliff“ bei BMS
BMS hat im letzten Jahr ca. 21 Milliarden Dollar Umsatz generiert.
Im Zeitraum 2012 bis 2015 verlieren indes 4 Medikamente ihren Patentschutz, die in 2011 zusammen für 12,3 Milliarden Dollar bzw. 58%(!) des Gesamtumsatzes standen. Allein Plavix (hemmt die Eigenschaft der Blutplättchen, verklumpen zu können und ist indiziert bspw. für Herzinfarkt- und Schlaganfallpatienten), kam im letzten Jahr auf einen Umsatz von 7 Milliarden Dollar.
Der Patentschutz ist in den USA mittlerweile ausgelaufen. In Europa wird dieses im Februar 2013 geschehen. Der Plavix-Umsatz wird damit bereits 2013 von 7 Milliarden Dollar noch in 2011 auf vielleicht 0,2 Milliarden Dollar einbrechen.
Die ehemals 12,3 Milliarden Dollar Umsatz der Medikamente, die bis 2015 den Patentschutz verlieren (Plavix, Avapro, Abilify und Sustiva) werden in 2016 fast vollständig ausradiert sein.
Stagnation als Erfolg
Wer sich dieses enorme Wegbrechen von Umsätzen vor Augen führt, muss zu dem Urteil kommen, dass es ein beachtlicher Erfolg des BMS-Managements ist, dass das Unternehmen dennoch in etwa seinen Umsatz halten können wird. Der Markt geht davon aus, dass das Umsatztief des Unternehmens schon 2013 mit etwas über 17 Milliarden Dollar erreicht sein wird und die Umsätze dann wieder wachsen. Erst langsam und dann, nach der Bereinigung um die Patentabläufe ab 2016 wieder schneller. Bereits 2015 wird man voraussichtlich wieder in etwa auf dem Niveau von 2011 sein. Dies hängt aber auch von einer Zulassung des Wirkstoffes Eliquis ab. Dies wird weiter unten näher ausgeführt.
Neue Produkte
BMS hat es verstanden, neue Produkte an den Markt zu bringen, deren Umsätze schnell wachsen und die dabei sind, Blockbuster zu werden.
Die beständig wachsenden Umsätze aus diesen neuen Produkten werden bis 2015 die wegfallenden Umsätze der Altprodukte kompensiert haben.
Zu diesen neuen Produkten gehören insbesondere:
- Onglyza (Diabetes), geschätzter Umsatz in 2012: 704 Millionen
- Orencia (Rheumatoide Arthritis), geschätzter Umsatz in 2012: 1 Milliarde
- Yervoy (Melanom), geschätzter Umsatz in 2012: 1 Milliarde
- Sprycel (Leukämie), geschätzter Umsatz in 2012: 650 Millionen
Besonders Yervoy und Sprycel wachsen schnell und könnten bis 2015 jeweils einen Umsatz von 2 Milliarden Dollar erzielen.
Ein Hoffnungsträger
Durch die eben erst erfolgte Übernahme von Amylin hat BMS nun deren Hoffnungsträger Bydureon (Diabetes) im Portfolio. Analysten halten für das erst in diesem Jahr zugelassene Produkt mittelfristig Milliardenumsätze für möglich. Es wird interessant und kursrelevant sein, wie gut die Vermarktung des Produktes in der Zusammenarbeit zwischen BMA und AstraZeneca funktionieren wird. Ende 2013 wird man hier ein erstes Fazit ziehen können.
Der neue König?
Die bisher beschriebenen Neuentwicklungen reichen wohl lediglich aus, um einen regelrechten Absturz bei BMS zu verhindern.
Für eine wirklich positive Entwicklung des Unternehmens und des Aktienkurses in den nächsten Jahren ist aber eine weitere Neuentwicklung entscheidend, nämlich Eliquis (apixaban), ein Ko-Entwicklung mit Pfizer.
Apixaban ist ein Wirkstoff aus der Gruppe der direkten Faktor-Xa-Inhibitoren. Es hemmt selektiv den Faktor Xa, ein Enzym, das in der Blutgerinnung eine zentrale Rolle spielt. Apixaban beugt der Entstehung von Thromben vor und wird zur Thromboseprophylaxe nach Knie- oder Hüftgelenksoperationen eingesetzt. Hierfür gibt es für den europäischen Markt bereits eine Zulassung.
Entscheidend ist aber, dass das Medikament bei Patienten mit Herzrhythmusstörungen (Vorhofflimmern) zum Einsatz kommen soll, um das erhöhte Schlaganfallrisiko der Patienten zu mindern.
Leider hat die amerikanische Zulassungsbehörde FDA im Juni den Zulassungsantrag für diesen Wirkstoff mit einem Complete Response Letter (CRL) zunächst auf das Abstellgleis geschoben.
Ein CRL ist zwar keine Ablehnung, kann aber im Einzelfall die Zulassung um Jahre verzögern oder letztlich zum Aus des Programms führen.
So schlimm dürfte es hier nicht kommen, denn die FDA hat wohl nicht verlangt, dass neue klinische Studien durchgeführt werden. Vielmehr wurde BMS und Pfizer „nur“ aufgegeben, weitere Angaben zum Datenmanagement des für die Zulassung entscheidenden „Aristoteles-Trials“ zu liefern. „Aristoteles“ umfasste 24.000 Patienten und hat Millionen von Datenpunkten generiert. Dass bei einem so komplexen Vorgang Klärungsbedarf zwischen Zulassungsbehörde und Antragsteller entstehen kann, sollte nicht zu kritisch gesehen werden.
Allerdings ist eines auch ganz klar: Die vollständige Zulassung von Eliquis ist essentiell für BMS, denn dem Wirkstoff wird langfristig ein Umsatzpotential wie Plavix zugetraut. Ein potentieller Mega-Blockbuster also.
Eine – wenn auch eher unwahrscheinliche – Ablehnung der Zulassung oder auch „nur“ die Notwendigkeit, zusätzliche Studien durchzuführen, wäre ein mittleres Desaster. In diesem Negativszenario wären Kurse über 25 Dollar für die nächsten 2 Jahre wohl nur schwer zu rechtfertigen.
Die Entscheidung über Eliquis wird aller Voraussicht nach bis spätestens Mitte 2013 gefallen sein. Mit sehr viel Glück könnte die Zulassung bereits Ende 2012 erfolgen. Wahrscheinlicher ist aber eine FDA-Entscheidung im Zeitraum Februar bis Mai 2013.
Persönlich gehe ich davon aus, dass das Mittel in 2013 die Zulassung erhalten wird und BMS dann sehr, sehr stark dastehen wird.
Sollte ich an diesem Punkt irren, werden zumindest die nächsten 2 oder 3 Jahre allerdings wirklich unschön.
Der kommende König der Könige? – Oder, was an BMS so spannend ist.
Während Eliquis auf die Indikation Herz-Kreislauferkrankungen zielt und damit die eine große Geisel der Menschheit adressiert, ist bei BMS noch ein weit weniger fortgeschrittenes Programm in der Entwicklung, das noch wenig bekannt ist, Fachleute aber elektrisiert hat. Es handelt sich um einen Antikörper gegen das Protein PD-1 (Programmed cell death protein 1). Das Programm trägt derzeit noch den Entwicklungsnamen „BMS-936558”.
Die – mittlerweile durch erste klinische Tests gestützte – Erwartung ist, dass durch die Blockade von PD-1 das Immunsystem in die Lage versetzt werden kann, selbst gegen Krebszellen vorzugehen (Immuntherapie).
In diesem Jahr tritt der Wirkstoff in die Phase III der klinischen Entwicklung ein, die um 2015 zu einer ersten Zulassung, möglicherweise bei Lungenkrebs, führen könnte. Tests in weiteren Krebsarten sind auf dem Weg oder werden demnächst beginnen.
Die bisher generierten Phase II-Daten waren überragend gut.
Ich will an dieser Stelle den Biotech-Analysten Ohad Hammer zitieren:
“The biggest news at this year’s ASCO came from BMS’ (BMY) PD-1 antibody, BMS-936558. This antibody belongs to a new class of antibodies that stimulate patients’ immune system to attack cancer. This approach has been recently validated with another BMS antibody, Yervoy, which was approved last year for melanoma.
Based on results presented at the meeting, BMS-936558 is superior to Yervoy by any measure. In fact, it is probably one of the most promising oncology drugs ever to be tested in humans. It induces tumor shrinkage in a substantial portion of patients, creates an immune response that keeps the disease under control for long periods and it does so with limited side effects. To make things even better, there might be a way to pre-select patients who are more likely to respond to this agent.”
Der Wirkstoff würde im Erfolgsfall mit Sicherheit ein Blockbuster, sehr wahrscheinlich ein Mega-Blockbuster, werden. Das Umsatzpotential liegt langfristig wohl deutlich jenseits der 5 Milliarden Dollar-Marke.
Ein Erfolg von BMS-936558 wäre für das Unternehmen transformativ. BMS hätte in diesem Falle viele Jahre eines schnellen Umsatz- und Gewinnwachstums vor sich, die das Unternehmen (und den Aktienkurs) in eine andere Liga führen würde.
Fazit
BMS macht derzeit aufgrund wichtiger Patentabläufe eine schwierige Phase der Unternehmensentwicklung durch.
Das Management hat das Unternehmen aber gut auf diese Zeit eingestellt und eine attraktive Pipeline aus neuen Produkten aufgebaut, die Stück für Stück die wegfallenden Umsätze ersetzen können.
Meine Kaufentscheidung beruhte im Wesentlichen aber auf meiner Erwartung, dass im nächsten Jahr Eliquis eine umfassende Zulassung erhalten und sich in der Folge zum Marktführer im Bereich der Antikoagulantien entwickeln und dass ab 2015/2016 die Zulassung von BMS-936558 das Unternehmen in eine neue Dimension führen wird.
Mit Eliquis und BMS-936558 hat BMS zwei potentielle Mega-Blockbuster in der Pipeline, die, falls sie beide zugelassen werden, ein Investment zu einem großen Erfolg werden lassen sollten. Ich schätze die Zulassungswahrscheinlichkeit für beide Wirkstoffe als überdurchschnittlich hoch ein, würde mir aber um Eliquis sogar etwas mehr Sorgen machen, als um BMS-936558.
Sollte nur einer dieser beiden Kandidaten die Zulassung erhalten, sollte sich ein Investment noch immer gut bezahlt machen, auch wenn, im Falle einer Nichtzulassung von Eliquis, die Jahre 2013/2014 recht bitter werden dürften.
da gibt es nur eine lösung bei guten biotechs gute produkte einzukaufen! 

vielleicht finden die auch berlin auf der landkarte


vielleicht finden die auch berlin auf der landkarte

BMS ist hoch spekulativ. In den USA und Europa verlieren sie die Blockbuster die aus dem Patent laufen, in Emerging Markets sind sie nicht stark aufgestellt.
Die Pipeline zeigt wenig hoffnungsvolle Kandidaten. Wie man hier von Koenig der Koenige sprechen kann ist schwer nachzuvollziehen.
Eliquis wird hoechstens im Premiumbereich Fuss fassen, Plavix und seine Nachahmer haben sich zum Golden Standard entwickelt. Bisher sehen die Daten auch noch nicht so gut aus.
Sollte sich BMS 936558 entwickeln wie geplant ergibt sich ein mittelfristiges Potential mit attraktiven Dimensionen.
Allerdings ist das wohl auch schon eingepreist (KGV).
Bei einem KGV ueber 17 wuerde ich jederzeit Roche, Sanofi und Novartis vorziehen. Alle drei stehen besser da, haben bessere Pipelines und eine gute Emerging Market Praesenz. Roche hat ebenso ein praesentes und potentielles Onkologieportfolio mit grossem Potential.
Ebenso sind alle drei dividendenstark.
Nur meine Meinung, keine Empfehlung.
Die Pipeline zeigt wenig hoffnungsvolle Kandidaten. Wie man hier von Koenig der Koenige sprechen kann ist schwer nachzuvollziehen.
Eliquis wird hoechstens im Premiumbereich Fuss fassen, Plavix und seine Nachahmer haben sich zum Golden Standard entwickelt. Bisher sehen die Daten auch noch nicht so gut aus.
Sollte sich BMS 936558 entwickeln wie geplant ergibt sich ein mittelfristiges Potential mit attraktiven Dimensionen.
Allerdings ist das wohl auch schon eingepreist (KGV).
Bei einem KGV ueber 17 wuerde ich jederzeit Roche, Sanofi und Novartis vorziehen. Alle drei stehen besser da, haben bessere Pipelines und eine gute Emerging Market Praesenz. Roche hat ebenso ein praesentes und potentielles Onkologieportfolio mit grossem Potential.
Ebenso sind alle drei dividendenstark.
Nur meine Meinung, keine Empfehlung.
Das Geld, das mir BMS für meine Medarex-Aktien gegeben hat, habe ich dann doch besser in den BMS-spin-off Mead Johnson investiert.
Offensichtlich wurde heute die Entwicklung von BMS986094 (Hepatitis C) eingestellt. Ein weiterer Megaverlust, das produkt war einlizensiert.
Ich werde diese Aktie nicht anfassen...
Ich werde diese Aktie nicht anfassen...
Antwort auf Beitrag Nr.: 43.532.366 von Norbi2 am 25.08.12 03:50:42Norbi, das ist richtig, das Hep-C-Programm ist jetzt offiziell tot und der goodwill aus der Inhibitex-Übernahme wird größtenteils abgeschrieben:
Biopharmaceutical company Bristol-Myers Squibb Co. (BMY:Quote) will take a charge of $1.8 billion in the third quarter related to the voluntarily discontinued development of an experimental hepatitis C drug. The New York-based drugmaker also "does not expect that the impairment charge will result in future cash expenditures."
The move comes just eight months after Bristol-Myers acquired the BMS-986094 drug through February's $2.5 billion all-cash acquisition of hepatitis C drug developer Inhibitex Inc.
Das kann man natürlich nur als Desaster bezeichnen. Klar war das schon seit dem 01.08.2012 als der Trial gestoppt wurde und einige Leute haben das schon lange vorher, nämlich bereits Anfang Mai, kommen sehen.
Siehe hier:
http://www.thestreet.com/story/11523381/1/bristol-myers-miss…
Das war sicher eine der schlechtesten Akquisitionen aller Zeiten. Eigentlich war da schon nach 3 oder 4 Monaten klar, dass das Geld in den Sand gesetzt worden war.
In der Folge hat es dann Anfang August den Kursrutsch von 36 Dollar auf 31,50 gegeben, den ich bei ungefähr 32 zum Einstieg genutzt habe.
Im Hep-C-Bereich ist Gilead nun der große Sieger.
Ich denke aber nicht, dass die Pipeline dadurch signifikant geschwächt ist und ich misstraue dem Hep-C-Hype generell etwas.
Ansonsten:
Zu Eliquis haben wir verschiedene Erwartungen, aber als eventuell kommenden "König der Könige" habe ich BMS 936558 bezeichnet.
Was das KGV angeht: Ich denke nicht, dass BMS 936558 eingepreist ist. Die bereits am Markt befindlichen Produkte + Eliquis haben jede Menge Wachstumspotential auch nach 2015.
Aus meiner Sicht hat BMS eine sehr gute Chance, nach 2015 dynamischer zu wachsen, als bspw. Pfizer oder J&J.
Kommt BMS 936558 noch hinzu, wird sich das natürlich noch viel klarer zeigen.
PS: Danke für die kritischen Kommentare. Ich ahnte schon, dass BMS ziemlich unbeliebt ist.
Mal sehen, ob ich hier recht behalte, mich gegen die allgemeine Meinung zu stellen.
Biopharmaceutical company Bristol-Myers Squibb Co. (BMY:Quote) will take a charge of $1.8 billion in the third quarter related to the voluntarily discontinued development of an experimental hepatitis C drug. The New York-based drugmaker also "does not expect that the impairment charge will result in future cash expenditures."
The move comes just eight months after Bristol-Myers acquired the BMS-986094 drug through February's $2.5 billion all-cash acquisition of hepatitis C drug developer Inhibitex Inc.
Das kann man natürlich nur als Desaster bezeichnen. Klar war das schon seit dem 01.08.2012 als der Trial gestoppt wurde und einige Leute haben das schon lange vorher, nämlich bereits Anfang Mai, kommen sehen.
Siehe hier:
http://www.thestreet.com/story/11523381/1/bristol-myers-miss…
Das war sicher eine der schlechtesten Akquisitionen aller Zeiten. Eigentlich war da schon nach 3 oder 4 Monaten klar, dass das Geld in den Sand gesetzt worden war.
In der Folge hat es dann Anfang August den Kursrutsch von 36 Dollar auf 31,50 gegeben, den ich bei ungefähr 32 zum Einstieg genutzt habe.
Im Hep-C-Bereich ist Gilead nun der große Sieger.
Ich denke aber nicht, dass die Pipeline dadurch signifikant geschwächt ist und ich misstraue dem Hep-C-Hype generell etwas.
Ansonsten:
Zu Eliquis haben wir verschiedene Erwartungen, aber als eventuell kommenden "König der Könige" habe ich BMS 936558 bezeichnet.
Was das KGV angeht: Ich denke nicht, dass BMS 936558 eingepreist ist. Die bereits am Markt befindlichen Produkte + Eliquis haben jede Menge Wachstumspotential auch nach 2015.
Aus meiner Sicht hat BMS eine sehr gute Chance, nach 2015 dynamischer zu wachsen, als bspw. Pfizer oder J&J.
Kommt BMS 936558 noch hinzu, wird sich das natürlich noch viel klarer zeigen.
PS: Danke für die kritischen Kommentare. Ich ahnte schon, dass BMS ziemlich unbeliebt ist.
Mal sehen, ob ich hier recht behalte, mich gegen die allgemeine Meinung zu stellen.

Antwort auf Beitrag Nr.: 43.530.408 von SLGramann am 24.08.12 16:26:47wieviel kaufkraft für biotech firmen sind da verfügbar? 
gab es da einen großen lizensabschluss mit biotest?

gab es da einen großen lizensabschluss mit biotest?
SL,
BMS ist nicht unbeliebt. Darum geht es hier nicht. es ist einfach zur Zeit kein interessantes Investment. Ich habe Deinen Thread anfangs so verstanden dass Du relevante Informationen zur Aktie und zum Unternehmen zusammentragen wolltest und wollte Dir dabei helfen.
Nach Deinem PS (Danke für die kritischen Kommentare. Ich ahnte schon, dass BMS ziemlich unbeliebt ist.
Mal sehen, ob ich hier recht behalte, mich gegen die allgemeine Meinung zu stellen.) verstehe ich dass Du versuchen moechtestStimmen zu sammeln die Deine positive Einstellung wiederspiegeln.
Da kann ich Dir nun leider nicht helfen.
Viel Erfolg,
Norbi
BMS ist nicht unbeliebt. Darum geht es hier nicht. es ist einfach zur Zeit kein interessantes Investment. Ich habe Deinen Thread anfangs so verstanden dass Du relevante Informationen zur Aktie und zum Unternehmen zusammentragen wolltest und wollte Dir dabei helfen.
Nach Deinem PS (Danke für die kritischen Kommentare. Ich ahnte schon, dass BMS ziemlich unbeliebt ist.
Mal sehen, ob ich hier recht behalte, mich gegen die allgemeine Meinung zu stellen.) verstehe ich dass Du versuchen moechtestStimmen zu sammeln die Deine positive Einstellung wiederspiegeln.
Da kann ich Dir nun leider nicht helfen.
Viel Erfolg,
Norbi
hier gibt es noch ein paar (meiner Meinung nach brauchbare) Kommentare zu BMY von www.crosscurrentllc.com/Pharma_Research_JO05.html im Verlauf dieses Jahres...
www.crosscurrentllc.com/uploads/BMY_1-9-12_Must_Have_It.pdf
www.crosscurrentllc.com/uploads/BMY_1-26-12_HCV_Pipeline_Wil…
www.crosscurrentllc.com/uploads/BAYN_2-29-12_What_s_Real_and…
www.crosscurrentllc.com/uploads/BEP_1Q_2012_Part_1_Pharma.pd…
www.crosscurrentllc.com/uploads/BMY_4-26-12_HCV_Doesn_t_Seem…
www.crosscurrentllc.com/uploads/6-4-12_ASCO_Part_2_PD-1_T-DM…
www.crosscurrentllc.com/uploads/BMY_6-25-12_Eliquis.pdf
www.crosscurrentllc.com/uploads/BEP_7-13-12.pdf
www.crosscurrentllc.com/uploads/BMY_7-26-12_What_s_between_h…
www.crosscurrentllc.com/uploads/BMY_8-2-12_Oops.pdf
mfg ipollit
www.crosscurrentllc.com/uploads/BMY_1-9-12_Must_Have_It.pdf
www.crosscurrentllc.com/uploads/BMY_1-26-12_HCV_Pipeline_Wil…
www.crosscurrentllc.com/uploads/BAYN_2-29-12_What_s_Real_and…
www.crosscurrentllc.com/uploads/BEP_1Q_2012_Part_1_Pharma.pd…
www.crosscurrentllc.com/uploads/BMY_4-26-12_HCV_Doesn_t_Seem…
www.crosscurrentllc.com/uploads/6-4-12_ASCO_Part_2_PD-1_T-DM…
www.crosscurrentllc.com/uploads/BMY_6-25-12_Eliquis.pdf
www.crosscurrentllc.com/uploads/BEP_7-13-12.pdf
www.crosscurrentllc.com/uploads/BMY_7-26-12_What_s_between_h…
www.crosscurrentllc.com/uploads/BMY_8-2-12_Oops.pdf
mfg ipollit
Antwort auf Beitrag Nr.: 43.533.139 von Norbi2 am 25.08.12 15:12:28Norbi,
kritische Beiträge sind erwünscht.
Ich habe selbst versucht, auch die negativen Seiten darzustellen. Das allein durch Plavix dieses und nächstes Jahr 7 Milliarden Umsatz wegfällt, habe ich geschrieben.
Und dass dieses Jahr mit dem CRL bei Eliquis und dem Inhibitex-Desaster zwei echte Niederlagen kassiert worden, sehe ich ganz genauso.
Dennoch werde ich hier auch weiter die Chancen sehen.
Hallo ipollit,
danke für die Links.
Der Hinweis auf Elotuzumab war wichtig. Ein weiterer potentieller Blockbuster für den Zeitraum beginnend ab 2014/2015.
kritische Beiträge sind erwünscht.
Ich habe selbst versucht, auch die negativen Seiten darzustellen. Das allein durch Plavix dieses und nächstes Jahr 7 Milliarden Umsatz wegfällt, habe ich geschrieben.
Und dass dieses Jahr mit dem CRL bei Eliquis und dem Inhibitex-Desaster zwei echte Niederlagen kassiert worden, sehe ich ganz genauso.
Dennoch werde ich hier auch weiter die Chancen sehen.
Hallo ipollit,
danke für die Links.
Der Hinweis auf Elotuzumab war wichtig. Ein weiterer potentieller Blockbuster für den Zeitraum beginnend ab 2014/2015.
Weil hier bezweifelt wurde, dass Eliquis überhaupt ein wichtiges Produkt werden könnte, habe ich einige Überlegungen zu möglichen künftigen Umsätzen zusammengesucht:
Bernstein's Tim Anderson estimated that the treatment could grab peak sales of $3.7 billion by 2020 while Leerink Swann's Seamus Fernandez has estimated peak Eliquis sales at $4.2 billion in 2017.
The drug, aimed at patients with heart arrhythmia, would have $2.5 billion a year in sales by 2015 if approved, according to Tim Anderson, a Sanford C. Bernstein & Co. analyst in New York.
he drug: Eliquis (apixaban)
The disease: Cardiovascular
The developers: Pfizer and Bristol-Myers Squibb
Peak projections: $4 billion-$4.5 billion
Pfizer entered 2011 as the symbol for Big Pharma's often dysfunctional R&D efforts. However, it is beginning to look as if Pfizer won't end the year in the dog house. Eliquis--better known in research circles as apixaban--is one of the big reasons for the turnaround.
In August, investigators reported that the blood thinner performed extremely well in Phase III for patients suffering from atrial fibrillation. Not only were the outcomes significantly better than the warfarin arm, but the drug also reduced the risk of stroke or systemic embolism 21%, major bleeding 31% and mortality 11%.
One of the reasons why Pfizer looks so good here is that analysts have been forecasting a $9 billion market for a new wave of blood thinners, and a drug that fit apixaban's efficacy and safety profile could snatch half of that.
--------
Man darf natürlich trotzdem skeptisch sein, dass hier ein potentieller Mega-Blockbuster in der Warteschleife hängt, aber dann vertritt man eine Minderheitenposition und wäre eigentlich etwas stärker in der Begründungspflicht, würde ich meinen.
Fakt ist natürlich auch, dass die Zulassung durch die FDA eben noch fehlt und wahrscheinlich erst nächstes Jahr erfolgen kann.
Erfolgt sie nicht, haben wir den Kurs meines Erachtens bei 25 Dollar.
Hier besteht also durchaus ein echtes Risiko, aber eben gerade weil Eliquis so wichtig ist und eigentlich viel Potential hätte.
Eliquis gilt allgemein noch immer als best-in-class Produkt unter den neuen Antikoagulantien (Pradaxa, Xarelto, Eliquis), insbesondere was safety angeht.
Bernstein's Tim Anderson estimated that the treatment could grab peak sales of $3.7 billion by 2020 while Leerink Swann's Seamus Fernandez has estimated peak Eliquis sales at $4.2 billion in 2017.
The drug, aimed at patients with heart arrhythmia, would have $2.5 billion a year in sales by 2015 if approved, according to Tim Anderson, a Sanford C. Bernstein & Co. analyst in New York.
he drug: Eliquis (apixaban)
The disease: Cardiovascular
The developers: Pfizer and Bristol-Myers Squibb
Peak projections: $4 billion-$4.5 billion
Pfizer entered 2011 as the symbol for Big Pharma's often dysfunctional R&D efforts. However, it is beginning to look as if Pfizer won't end the year in the dog house. Eliquis--better known in research circles as apixaban--is one of the big reasons for the turnaround.
In August, investigators reported that the blood thinner performed extremely well in Phase III for patients suffering from atrial fibrillation. Not only were the outcomes significantly better than the warfarin arm, but the drug also reduced the risk of stroke or systemic embolism 21%, major bleeding 31% and mortality 11%.
One of the reasons why Pfizer looks so good here is that analysts have been forecasting a $9 billion market for a new wave of blood thinners, and a drug that fit apixaban's efficacy and safety profile could snatch half of that.
--------
Man darf natürlich trotzdem skeptisch sein, dass hier ein potentieller Mega-Blockbuster in der Warteschleife hängt, aber dann vertritt man eine Minderheitenposition und wäre eigentlich etwas stärker in der Begründungspflicht, würde ich meinen.
Fakt ist natürlich auch, dass die Zulassung durch die FDA eben noch fehlt und wahrscheinlich erst nächstes Jahr erfolgen kann.
Erfolgt sie nicht, haben wir den Kurs meines Erachtens bei 25 Dollar.
Hier besteht also durchaus ein echtes Risiko, aber eben gerade weil Eliquis so wichtig ist und eigentlich viel Potential hätte.
Eliquis gilt allgemein noch immer als best-in-class Produkt unter den neuen Antikoagulantien (Pradaxa, Xarelto, Eliquis), insbesondere was safety angeht.
Begruendungspflicht? Minderheitenmeinung?
Bist Du noch ganz dicht? Ich wollte Dir beim Argumentesammeln helfen und Du kommst mir mit Begruendungspflicht?
Der Markt bewertet die Aktie, nicht WO. DAS ist die Begruendung!
Bist Du noch ganz dicht? Ich wollte Dir beim Argumentesammeln helfen und Du kommst mir mit Begruendungspflicht?
Der Markt bewertet die Aktie, nicht WO. DAS ist die Begruendung!
Mal etwas Positives zu Eliquis. Das CHMP hat der EMA die Zulassung für die Vorsorge gegen Schlaganfall empfohlen.
Damit ist es sehr wahrscheinlich geworden, dass Eliquis Ende des Jahres in der EU die Zulassung für seine eigentliche Indikation erhalten wird und dass das Produkt in 2013 erstmals nennenswerte Umsätze generieren kann.
Weiter fraglich - und wichtiger - ist indes der Status in den USA. Ich hoffe, dass BMS/Pfizer noch in diesem Jahr den neu formulierten Antrag bei der FDA stellen werden.
Friday, September 21, 2012 7:46 am EDT
PRINCETON, N.J. & NEW YORK--(BUSINESS WIRE)--Bristol-Myers Squibb (NYSE: BMY) and Pfizer Inc. (NYSE: PFE) today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion recommending that ELIQUIS® (apixaban) be granted approval for the prevention of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation (NVAF) and one or more risk factors for stroke. The CHMP's positive opinion will now be reviewed by the European Commission, which has the authority to approve medicines for the European Union. The final decision will be applicable to all 27 European Union member states plus Iceland and Norway.
The positive opinion was based on the pivotal ARISTOTLE and AVERROES studies. These clinical studies evaluated apixaban in approximately 24,000 patients with NVAF in the largest clinical trial program conducted to date in this patient population. The landmark ARISTOTLE trial compared apixaban to warfarin, the standard of care, in more than 18,000 NVAF patients, while AVERROES compared apixaban to aspirin in 5,598 NVAF patients who were unsuitable for vitamin K antagonist (VKA) therapy.
Damit ist es sehr wahrscheinlich geworden, dass Eliquis Ende des Jahres in der EU die Zulassung für seine eigentliche Indikation erhalten wird und dass das Produkt in 2013 erstmals nennenswerte Umsätze generieren kann.
Weiter fraglich - und wichtiger - ist indes der Status in den USA. Ich hoffe, dass BMS/Pfizer noch in diesem Jahr den neu formulierten Antrag bei der FDA stellen werden.
Friday, September 21, 2012 7:46 am EDT
PRINCETON, N.J. & NEW YORK--(BUSINESS WIRE)--Bristol-Myers Squibb (NYSE: BMY) and Pfizer Inc. (NYSE: PFE) today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion recommending that ELIQUIS® (apixaban) be granted approval for the prevention of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation (NVAF) and one or more risk factors for stroke. The CHMP's positive opinion will now be reviewed by the European Commission, which has the authority to approve medicines for the European Union. The final decision will be applicable to all 27 European Union member states plus Iceland and Norway.
The positive opinion was based on the pivotal ARISTOTLE and AVERROES studies. These clinical studies evaluated apixaban in approximately 24,000 patients with NVAF in the largest clinical trial program conducted to date in this patient population. The landmark ARISTOTLE trial compared apixaban to warfarin, the standard of care, in more than 18,000 NVAF patients, while AVERROES compared apixaban to aspirin in 5,598 NVAF patients who were unsuitable for vitamin K antagonist (VKA) therapy.
Antwort auf Beitrag Nr.: 43.635.380 von SLGramann am 23.09.12 09:34:15So, der Status von Eliquis in den USA ist nun auch klar. Die NDA wurde neu eingereicht. Am 17.03.2013 wird die Entscheidung fallen.
Die FDA unterzieht den Antrag offensichtlich einem "class II"-Review. Ich kann nicht beurteilen, ob hier überhaupt ein class I review denkbar gewesen wäre. Dass es class II ist, würde ich dennoch als leicht negativ beurteilen.
If new analysis of existing data is required, it could be a class II review and take six months to review. In the case of a Class I response the NDA could be approved as early as October or November of 2012 and a Class II response would extend approval until March or April of 2013.
Wednesday, September 26, 2012 5:01 pm EDT
PRINCETON, N.J. & NEW YORK--(BUSINESS WIRE)--Bristol-Myers Squibb Company(NYSE: BMY) and Pfizer Inc. (NYSE: PFE) today announced that the U.S. Food and Drug Administration (FDA) has acknowledged receipt of the New Drug Application (NDA) resubmission for ELIQUIS® (apixaban) to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (NVAF). The FDA assigned a new Prescription Drug User Fee Act (PDUFA) goal date of March 17, 2013. The FDA has deemed the resubmission a complete response to its June 22, 2012 Complete Response Letter that requested additional information on data management and verification from the ARISTOTLE trial.
Die FDA unterzieht den Antrag offensichtlich einem "class II"-Review. Ich kann nicht beurteilen, ob hier überhaupt ein class I review denkbar gewesen wäre. Dass es class II ist, würde ich dennoch als leicht negativ beurteilen.
If new analysis of existing data is required, it could be a class II review and take six months to review. In the case of a Class I response the NDA could be approved as early as October or November of 2012 and a Class II response would extend approval until March or April of 2013.
Wednesday, September 26, 2012 5:01 pm EDT
PRINCETON, N.J. & NEW YORK--(BUSINESS WIRE)--Bristol-Myers Squibb Company(NYSE: BMY) and Pfizer Inc. (NYSE: PFE) today announced that the U.S. Food and Drug Administration (FDA) has acknowledged receipt of the New Drug Application (NDA) resubmission for ELIQUIS® (apixaban) to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (NVAF). The FDA assigned a new Prescription Drug User Fee Act (PDUFA) goal date of March 17, 2013. The FDA has deemed the resubmission a complete response to its June 22, 2012 Complete Response Letter that requested additional information on data management and verification from the ARISTOTLE trial.
Antwort auf Beitrag Nr.: 43.534.231 von SLGramann am 26.08.12 10:14:51von Bloomberg:
Pfizer Inc. (PFE), the world’s largest drugmaker, and Bristol-Myers Squibb Co. (BMY) may have to wait until March for word on whether their experimental blood thinner Eliquis passes U.S. regulatory scrutiny.
The pill was rejected by the Food and Drug Administration in June after the agency said it needed more information from the companies’ existing trials. The two New York-based companies said in a statement today they had given the FDA all the data requested and were assigned a target decision date of March 17.
Revenue from Eliquis has the potential to peak at $4.7 billion if approved, Catherine Arnold, an analyst with Credit Suisse Group AG in New York, estimated in a note this month. The pill, which could replace the drug Warfarin as the standard of care, would also help make up for sales that the companies have lost from their own top-selling blockbusters.
Pfizer Inc. (PFE), the world’s largest drugmaker, and Bristol-Myers Squibb Co. (BMY) may have to wait until March for word on whether their experimental blood thinner Eliquis passes U.S. regulatory scrutiny.
The pill was rejected by the Food and Drug Administration in June after the agency said it needed more information from the companies’ existing trials. The two New York-based companies said in a statement today they had given the FDA all the data requested and were assigned a target decision date of March 17.
Revenue from Eliquis has the potential to peak at $4.7 billion if approved, Catherine Arnold, an analyst with Credit Suisse Group AG in New York, estimated in a note this month. The pill, which could replace the drug Warfarin as the standard of care, would also help make up for sales that the companies have lost from their own top-selling blockbusters.
Gestern hat BMS die Zahlen zum dritten Quartal gemeldet. Mit 41 Cent pro Aktien (non-GAAP) blieben sie leicht unter den Erwartungen des Marktes.
Insgesamt war das Quartal natürlich verheerend. Zum einen mussten 1,8 Milliarden wegen der gescheiterten Inhibitex-Übernahme abgeschrieben werden. Zum anderen ist Plavix nun endgültig Geschichte.
Nachdem der Hauptumsatzbringer von BMS faktisch ausradiert ist, macht es für mich Sinn zu schauen, ob und wie BMS den weggefallenen Umsatz langsam ersetzen kann.
Dazu vergleiche ich die beiden Altprodukte Avapro und Plavix, die in diesem Jahr ihren Patentschutz verloren haben, mit den aus meiner Sicht wichtigsten neuen Produkten auf Quartalsbasis.
Zunächst ist zu konstatieren, dass durch Avapro und Plavix seit dem Q1/2012 wahnsinnige 1,741 Milliarden Dollar Umsatz pro Quartal weggefallen sind.
Im Einzelnen:
Im Saldo sind damit nach dieser Rechnung von Q1 bis Q3/2012 auf Quartalsbasis immer noch 1,579 Milliarden Dollar Umsatzausfall zu beklagen.
Wahr ist aber eben auch, dass diese Zahl mit jedem weiteren Quartal kleiner werden wird – je nachdem, wie sich die „Hoffnungsträger“ tatsächlich entwickeln werden.
Der Tiefpunkt der Entwicklung ist jedenfalls mit dem dritten Quartal 2012 bereits durchschritten worden.
Aus der Übernahme von Amylin habe ich den Umsatz von Bydureon mit aufgenommen, den Umsatz mit Byetta (55 Mio.) hingegen unterschlagen. Dies deshalb, weil ich davon ausgehe, dass Bydureon in der Zukunft ohnehin zunächst auf Kosten von Byetta wachsen und die dortigen Umsätze kanibalisieren wird. Daher gehört Byetta für mich definitiv nicht zu den Hoffnungswerten, deren Beobachtung lohnt. Daher nehme ich Byetta von vornherein nicht in die Tabelle auf.
Wie schon mehrfach geschrieben, wäre die vollständige Zulassung von Eliquis extrem wichtig für ein dynamisches Wachstum durch die Hoffnungsträger. Die nächsten Monate werden insofern über die Dynamik der Entwicklung in 2013 und 2014 entscheiden.
Ich werde diese Tabelle künftig nach jedem Quartalsbericht aktualisieren.
Insgesamt war das Quartal natürlich verheerend. Zum einen mussten 1,8 Milliarden wegen der gescheiterten Inhibitex-Übernahme abgeschrieben werden. Zum anderen ist Plavix nun endgültig Geschichte.
Nachdem der Hauptumsatzbringer von BMS faktisch ausradiert ist, macht es für mich Sinn zu schauen, ob und wie BMS den weggefallenen Umsatz langsam ersetzen kann.
Dazu vergleiche ich die beiden Altprodukte Avapro und Plavix, die in diesem Jahr ihren Patentschutz verloren haben, mit den aus meiner Sicht wichtigsten neuen Produkten auf Quartalsbasis.
Zunächst ist zu konstatieren, dass durch Avapro und Plavix seit dem Q1/2012 wahnsinnige 1,741 Milliarden Dollar Umsatz pro Quartal weggefallen sind.
Im Einzelnen:
Q1/2012 Q2/2012 Q3/2012
Avapro 207 117 95
Plavix 1.693 741 64
Gesamt 1.900 858 159
Umsatzverlust durch Patentabläufe bis Q3/12: -1.741
Was konnte davon bisher durch „Hoffnungsträger“ kompensiert werden?
Q1/2012 Q2/2012 Q3/2012
Baraclude 325 357 346
Erbitux 179 179 173
Sprycel 231 244 263
Yveroy 154 162 179
Orencia 254 290 307
Onglyza 161 172 178
Eliquis 0 1 0
Bydureon 0 0 20
Gesamt 1.305 1.405 1.466
Umsatzgewinne durch “Hoffnungsträger” bis Q3/12: +162
Im Saldo sind damit nach dieser Rechnung von Q1 bis Q3/2012 auf Quartalsbasis immer noch 1,579 Milliarden Dollar Umsatzausfall zu beklagen.
Wahr ist aber eben auch, dass diese Zahl mit jedem weiteren Quartal kleiner werden wird – je nachdem, wie sich die „Hoffnungsträger“ tatsächlich entwickeln werden.
Der Tiefpunkt der Entwicklung ist jedenfalls mit dem dritten Quartal 2012 bereits durchschritten worden.
Aus der Übernahme von Amylin habe ich den Umsatz von Bydureon mit aufgenommen, den Umsatz mit Byetta (55 Mio.) hingegen unterschlagen. Dies deshalb, weil ich davon ausgehe, dass Bydureon in der Zukunft ohnehin zunächst auf Kosten von Byetta wachsen und die dortigen Umsätze kanibalisieren wird. Daher gehört Byetta für mich definitiv nicht zu den Hoffnungswerten, deren Beobachtung lohnt. Daher nehme ich Byetta von vornherein nicht in die Tabelle auf.
Wie schon mehrfach geschrieben, wäre die vollständige Zulassung von Eliquis extrem wichtig für ein dynamisches Wachstum durch die Hoffnungsträger. Die nächsten Monate werden insofern über die Dynamik der Entwicklung in 2013 und 2014 entscheiden.
Ich werde diese Tabelle künftig nach jedem Quartalsbericht aktualisieren.
Im CC, dessen Transcript bei seeking alpha zu finden ist, wurden einige interessante Dinge zur Pipeline gesagt.
Vielleicht am überraschendsten sind die sehr positiven Aussagen zu Forxiga. Von diesem Projekt halte ich eigentlich wenig und bin bisher nicht von einer Zulassung ausgegangen. Das Nebenwirkungsprofil erscheint nämlich sehr problematisch.
Sollten die Behörden aber dennoch eine Zulassung beschließen (EMA Ende 2012?, FDA irgendwann 2014?), muss das Programm natürlich in die Liste „Hoffnungswerte“ aufgenommen werden. Abwarten.
Das Jahr 2013 dürfte durch die erhoffte Zulassung von Eliquis geprägt werden. Hier bleibe ich nervös, auch wenn ich die Zulassungswahrscheinlichkeit für Europa bei 90% und für die USA bei 70% ansiedeln würde.
Ende 2014 dürfte dann der Zulassungsantrag für Elotuzumab folgen. Hier mache ich mir wenig Sorgen. Ich gehe von einem großen Erfolg für dieses Programm aus.
Spätestens ab 2015 wird BMS dann wohl durch die Daten des Anti-PD1 geprägt werden. Hier feuert man aus allen Rohren:
- Phase III Study of BMS-936558 Compared to Docetaxel in Previously Treated Advanced or Metastatic Non-squamous NSCLC
http://www.clinicaltrials.gov/ct2/show?term=BMS-936558&rank=…
Bisher 118 Studienstandorte angemeldet. 574 Patienten sind zu rekrutieren.
- Phase III Study of BMS-936558 Compared to Docetaxel in Previously Treated Advanced or Metastatic Squamous Cell Non-small Cell Lung Cancer (NSCLC)
http://www.clinicaltrials.gov/ct2/show?term=BMS-936558&rank=…
Bisher 118 Studienstandorte angemeldet. 264 Patienten sind zu rekrutieren.
- Phase III Study of BMS-936558 vs. Everolimus in Pre-Treated Advanced Or Metastatic Clear-cell RCC
http://www.clinicaltrials.gov/ct2/show?term=BMS-936558&rank=…
Bisher 147 Studienstandorte angemeldet. 844 Patienten sind zu rekrutieren.
Die Anzahl der Studien mit BMS-936558 (bisher 18) wird in den nächsten Jahren deutlich ansteigen, davon bin ich überzeugt.
Hier jetzt die Auszüge aus dem CC, die ich am wichtigsten fand:
Forxiga (dapagliflozin)
I'll take the dapagliflozin FORXIGA question. First, we, as Lamberto indicated, have had extensive discussions with the FDA in the last several months. Based on evolving data, clinical data of patients who've been treated following extensions, the FDA's benefit-risk profile assessment of dapagliflozin may have shifted in favor of the drug. Our discussions have, therefore, resulted in a well-defined path forward. We don't require starting any new clinical data, but we are planning to resubmit in the middle of 2013 with more clinical data from our extension of some studies, plus the results of some dedicated blood pressure studies with dapagliflozin. This medicine, as you know, offers the triad of potential benefits of glucose control, weight reduction and blood pressure reduction. And we do expect to have an advisory committee and a 6-month review. So resubmission of ongoing clinical studies, another look at the benefit-risk, including probably an advisory committee and a 6-month review. Meanwhile, in Europe, we are encouraged by the progress and do feel that we will have this drug approved in Europe by the end of the year.
Eliquis
Regarding ELIQUIS, we received a positive opinion from the CHMP in Europe, and we are expecting a decision by the end of the year. In Japan, our application is progressing and could lead to a decision by the end of the year. And in the U.S., we now have a new PDUFA date on March 17 from the FDA.
Tim, this is Elliott. On ELIQUIS, we do not expect nor have any indication there will be an advisory committee. As I mentioned on our last investor call at the end of July, we had a set of questions. We felt we could submit answers too. By mid-September, we did that. The official receipt by the FDA of those answers were -- was September 26. I am confident our answers addressed all the questions in front of the FDA. That submission date triggers a potential 6-month review. Therefore, we had announced the PDUFA date of March 17 of '13. I am personally hopeful that the decision can come earlier.
Elotuzumab
Yes, Tony, this is Elliott. I'll take the question on elotuzumab, which is our essentially another immuno-oncology approach, this time, focused on a specific antigen that's relevant to multiple myeloma. We are anxiously awaiting these trials. We're going to have to wait a little longer for the final Phase III, which should come in early 2014 in one study, maybe the second quarter of 2014 in the third study.
Anti PD1 (BMS-936558)
With regard to PD-1, we have a very significant, dedicated, intense team on this in development and with our commercial colleagues for advice and involvement in this. It's a very exciting area in specific and in general. We have just recently launched 3 Phase III studies, 2 are in lung studies. Those -- so patients have started to be dosed in second line squamous cells and second line non-squamous cell. A renal cell carcinoma study has also been started and soon, there will be a comprehensive Phase III program, covering all lines of therapy in melanoma probably to start before the end of the year.
Vielleicht am überraschendsten sind die sehr positiven Aussagen zu Forxiga. Von diesem Projekt halte ich eigentlich wenig und bin bisher nicht von einer Zulassung ausgegangen. Das Nebenwirkungsprofil erscheint nämlich sehr problematisch.
Sollten die Behörden aber dennoch eine Zulassung beschließen (EMA Ende 2012?, FDA irgendwann 2014?), muss das Programm natürlich in die Liste „Hoffnungswerte“ aufgenommen werden. Abwarten.
Das Jahr 2013 dürfte durch die erhoffte Zulassung von Eliquis geprägt werden. Hier bleibe ich nervös, auch wenn ich die Zulassungswahrscheinlichkeit für Europa bei 90% und für die USA bei 70% ansiedeln würde.
Ende 2014 dürfte dann der Zulassungsantrag für Elotuzumab folgen. Hier mache ich mir wenig Sorgen. Ich gehe von einem großen Erfolg für dieses Programm aus.
Spätestens ab 2015 wird BMS dann wohl durch die Daten des Anti-PD1 geprägt werden. Hier feuert man aus allen Rohren:
- Phase III Study of BMS-936558 Compared to Docetaxel in Previously Treated Advanced or Metastatic Non-squamous NSCLC
http://www.clinicaltrials.gov/ct2/show?term=BMS-936558&rank=…
Bisher 118 Studienstandorte angemeldet. 574 Patienten sind zu rekrutieren.
- Phase III Study of BMS-936558 Compared to Docetaxel in Previously Treated Advanced or Metastatic Squamous Cell Non-small Cell Lung Cancer (NSCLC)
http://www.clinicaltrials.gov/ct2/show?term=BMS-936558&rank=…
Bisher 118 Studienstandorte angemeldet. 264 Patienten sind zu rekrutieren.
- Phase III Study of BMS-936558 vs. Everolimus in Pre-Treated Advanced Or Metastatic Clear-cell RCC
http://www.clinicaltrials.gov/ct2/show?term=BMS-936558&rank=…
Bisher 147 Studienstandorte angemeldet. 844 Patienten sind zu rekrutieren.
Die Anzahl der Studien mit BMS-936558 (bisher 18) wird in den nächsten Jahren deutlich ansteigen, davon bin ich überzeugt.
Hier jetzt die Auszüge aus dem CC, die ich am wichtigsten fand:
Forxiga (dapagliflozin)
I'll take the dapagliflozin FORXIGA question. First, we, as Lamberto indicated, have had extensive discussions with the FDA in the last several months. Based on evolving data, clinical data of patients who've been treated following extensions, the FDA's benefit-risk profile assessment of dapagliflozin may have shifted in favor of the drug. Our discussions have, therefore, resulted in a well-defined path forward. We don't require starting any new clinical data, but we are planning to resubmit in the middle of 2013 with more clinical data from our extension of some studies, plus the results of some dedicated blood pressure studies with dapagliflozin. This medicine, as you know, offers the triad of potential benefits of glucose control, weight reduction and blood pressure reduction. And we do expect to have an advisory committee and a 6-month review. So resubmission of ongoing clinical studies, another look at the benefit-risk, including probably an advisory committee and a 6-month review. Meanwhile, in Europe, we are encouraged by the progress and do feel that we will have this drug approved in Europe by the end of the year.
Eliquis
Regarding ELIQUIS, we received a positive opinion from the CHMP in Europe, and we are expecting a decision by the end of the year. In Japan, our application is progressing and could lead to a decision by the end of the year. And in the U.S., we now have a new PDUFA date on March 17 from the FDA.
Tim, this is Elliott. On ELIQUIS, we do not expect nor have any indication there will be an advisory committee. As I mentioned on our last investor call at the end of July, we had a set of questions. We felt we could submit answers too. By mid-September, we did that. The official receipt by the FDA of those answers were -- was September 26. I am confident our answers addressed all the questions in front of the FDA. That submission date triggers a potential 6-month review. Therefore, we had announced the PDUFA date of March 17 of '13. I am personally hopeful that the decision can come earlier.
Elotuzumab
Yes, Tony, this is Elliott. I'll take the question on elotuzumab, which is our essentially another immuno-oncology approach, this time, focused on a specific antigen that's relevant to multiple myeloma. We are anxiously awaiting these trials. We're going to have to wait a little longer for the final Phase III, which should come in early 2014 in one study, maybe the second quarter of 2014 in the third study.
Anti PD1 (BMS-936558)
With regard to PD-1, we have a very significant, dedicated, intense team on this in development and with our commercial colleagues for advice and involvement in this. It's a very exciting area in specific and in general. We have just recently launched 3 Phase III studies, 2 are in lung studies. Those -- so patients have started to be dosed in second line squamous cells and second line non-squamous cell. A renal cell carcinoma study has also been started and soon, there will be a comprehensive Phase III program, covering all lines of therapy in melanoma probably to start before the end of the year.
Zulassung von Forxiga in der EU. Wie gesagt, ich mag den Wirkstoff nicht, aber er hat nun mal in Europa die Zulassung bekommen...
Wednesday, November 14, 2012 11:54 am EST
PRINCETON, N.J. and LONDON--(BUSINESS WIRE)--Bristol-Myers Squibb Company (NYSE: BMY) and AstraZeneca (NYSE: AZN) today announced that the European Commission has approved Forxiga™ (dapagliflozin) tablets for the treatment of type 2 diabetes in the European Union (EU). Forxiga is a selective and reversible inhibitor of sodium-glucose cotransporter 2 (SGLT2) that works independently of insulin to help remove excess glucose from the body, a unique mode of action not seen in any other currently available treatments for type 2 diabetes. This is the first medicine in the new SGLT2 class to gain regulatory approval for the treatment of type 2 diabetes, a disease in which high unmet medical need exists.
http://news.bms.com/press-release/rd-news/forxiga-dapagliflo…
Wednesday, November 14, 2012 11:54 am EST
PRINCETON, N.J. and LONDON--(BUSINESS WIRE)--Bristol-Myers Squibb Company (NYSE: BMY) and AstraZeneca (NYSE: AZN) today announced that the European Commission has approved Forxiga™ (dapagliflozin) tablets for the treatment of type 2 diabetes in the European Union (EU). Forxiga is a selective and reversible inhibitor of sodium-glucose cotransporter 2 (SGLT2) that works independently of insulin to help remove excess glucose from the body, a unique mode of action not seen in any other currently available treatments for type 2 diabetes. This is the first medicine in the new SGLT2 class to gain regulatory approval for the treatment of type 2 diabetes, a disease in which high unmet medical need exists.
http://news.bms.com/press-release/rd-news/forxiga-dapagliflo…
Antwort auf Beitrag Nr.: 43.825.656 von SLGramann am 14.11.12 21:47:55Eine Zusammenfassung von Fierce:
After FDA rejection, Bristol-AstraZeneca team wins EC OK for diabetes drug dapagliflozin
November 15, 2012 | By John Carroll
AstraZeneca ($AZN) and Bristol-Myers Squibb ($BMY) were stiff-armed by the FDA on their application for the SGLT2 diabetes drug dapagliflozin, but European regulators believe the benefits outweighed the risks that pushed the agency to reject the innovative therapy early this year.
The regulatory approval for dapagliflozin by the European Commission--which will soon hit the European market as Forxiga--clears the way for the first of several new sodium-glucose cotransporter 2 inhibitors, which spur the body to flush out glucose in urine, working independently of insulin. In the U.S., regulators and independent experts at the FDA turned thumbs down on dapagliflozin, demanding that the developers produce more data to prove that the cancer cases seen in drug studies didn't portend similar troubles if the drug is allowed to hit the huge and growing diabetes market.
This is good news for AstraZeneca, which has been struggling to overcome its reputation for one of the worst late-stage pipelines in the business. Bristol-Myers Squibb, which has had its own setbacks in the wake of a series of new drug approvals, has its own reasons to cheer the decision. The approval is also likely to raise the spirits of the development teams at Johnson & Johnson ($JNJ, developing canagliflozin), Pfizer ($PFE), a Lilly and Boehringer partnership, Lexicon and others which have their own SGLT2 inhibitors in development.
Analysts had projected peak sales of up to $700 million for dapagliflozin before the FDA handed out its complete response letter. But even with an approval from the European Commission, which came after regulators assessed and accepted the companies' risk management plans, a dark cloud is likely to remain over the drug's commercial prospects without a green light in the U.S.
The plan now is to head back to the FDA in mid-2013 with more data drawn from their drug studies.
"We are looking forward to getting approvals everywhere, and that includes with the FDA," Fred Fiedorek, cardiovascular head at Bristol-Myers, told Reuters.
"Diabetes is a progressive disease that requires a combination of treatment approaches over time," said Lamberto Andreotti, the CEO at Bristol-Myers Squibb. "Forxiga is the first of a new class of type 2 diabetes medication that works independently of insulin and represents a new treatment option for patients and physicians across Europe."
After FDA rejection, Bristol-AstraZeneca team wins EC OK for diabetes drug dapagliflozin
November 15, 2012 | By John Carroll
AstraZeneca ($AZN) and Bristol-Myers Squibb ($BMY) were stiff-armed by the FDA on their application for the SGLT2 diabetes drug dapagliflozin, but European regulators believe the benefits outweighed the risks that pushed the agency to reject the innovative therapy early this year.
The regulatory approval for dapagliflozin by the European Commission--which will soon hit the European market as Forxiga--clears the way for the first of several new sodium-glucose cotransporter 2 inhibitors, which spur the body to flush out glucose in urine, working independently of insulin. In the U.S., regulators and independent experts at the FDA turned thumbs down on dapagliflozin, demanding that the developers produce more data to prove that the cancer cases seen in drug studies didn't portend similar troubles if the drug is allowed to hit the huge and growing diabetes market.
This is good news for AstraZeneca, which has been struggling to overcome its reputation for one of the worst late-stage pipelines in the business. Bristol-Myers Squibb, which has had its own setbacks in the wake of a series of new drug approvals, has its own reasons to cheer the decision. The approval is also likely to raise the spirits of the development teams at Johnson & Johnson ($JNJ, developing canagliflozin), Pfizer ($PFE), a Lilly and Boehringer partnership, Lexicon and others which have their own SGLT2 inhibitors in development.
Analysts had projected peak sales of up to $700 million for dapagliflozin before the FDA handed out its complete response letter. But even with an approval from the European Commission, which came after regulators assessed and accepted the companies' risk management plans, a dark cloud is likely to remain over the drug's commercial prospects without a green light in the U.S.
The plan now is to head back to the FDA in mid-2013 with more data drawn from their drug studies.
"We are looking forward to getting approvals everywhere, and that includes with the FDA," Fred Fiedorek, cardiovascular head at Bristol-Myers, told Reuters.
"Diabetes is a progressive disease that requires a combination of treatment approaches over time," said Lamberto Andreotti, the CEO at Bristol-Myers Squibb. "Forxiga is the first of a new class of type 2 diabetes medication that works independently of insulin and represents a new treatment option for patients and physicians across Europe."
So, die Zulassung für Eliquis in der EU ist nun da.
Nächstes Jahr gehts dann natürlich noch um die wichtigere US-Zulassung.
Pfizer, Bristol get EU nod for blood clot preventer
Tue, Nov 20 2012
By Bill Berkrot
(Reuters) - European health regulators on Tuesday approved an eagerly anticipated blood thinner developed by Bristol-Myers Squibb Co and Pfizer Inc for preventing strokes and blood clots in patients with an irregular heartbeat known as atrial fibrillation, the companies said.
The drug Eliquis, also known as apixaban, is widely considered one of the most important new products for the two U.S. drugmakers, with multibillion-dollar annual sales potential.
The European approval was expected after an advisory panel this year recommended it for atrial fibrillation.
"It's not unexpected, but it's positive to finally get an afib approval under the belt for Eliquis," said MKM Partners analyst Jon Lecroy, who sees annual sales reaching $2 billion by about 2017. "We're looking for a March approval or earlier in the U.S. for the same indication," he added.
The U.S. Food and Drug Administration is expected to decide on the proposed use of the drug in the world's biggest market by March 17, after delaying a decision in June to review more information from clinical trials.
The European Commission approval marks the first regulatory approval for Eliquis for stroke prevention in atrial fibrillation patients in any market, the companies said.
Eliquis belongs to a new class of medicines designed to replace decades old warfarin for preventing blood clots in heart patients and after hip or knee replacement surgery.
It already was approved in 27 European Union countries for prevention of certain blood clots called venous thromboembelisms following elective hip or knee replacement surgery.
But atrial fibrillation, which greatly raises the risk of strokes, is considered by far the largest and most important use for the new drugs, that include Xarelto from Bayer and Johnson & Johnson, and Pradaxa from privately held Boehringer Ingelheim.
Eliquis, like Xarelto, works by inhibiting a protein called Factor Xa that plays a critical role in blood clotting. Pradaxa has a slightly different mechanism of action.
About 6 million people in Europe and another 5.8 million in the United States suffer from atrial fibrillation, the most common form of heart arrhythmia, or irregular heartbeat.
In clinical trials, Eliquis demonstrated superiority over warfarin in reducing the risk of strokes, major bleeding and death.
Warfarin, widely used for more than half a century, is inexpensive and works well, but requires close and regular patient monitoring as well as lifestyle and dietary changes that are not necessary with the newer medicines.
"Patients with atrial fibrillation have a five times greater risk of stroke and there remains a critical public health need for improved treatment options to reduce this risk," Lars Wallentin, director of cardiology at Uppsala Clinical Research Centre and University Hospital in Sweden, said in a statement.
He called Eliquis "an important new treatment option for health care professionals, who now have an oral anticoagulant with superior outcomes versus warfarin."
Wall Street analysts have said that, based on clinical efficacy and safety data, they believe Eliquis will become the dominant player in an estimated $10 billion market for the new blood thinners once it receives U.S. approval.
Pfizer Chief Executive Ian Read, in a statement, said he believes Eliquis "has the potential to transform the standard of care in stroke prevention in nonvalvular atrial fibrillation".
Pfizer shares were up 5 cents at $24.19 and Bristol-Myers shares were up 2 cents at $32.05, in afternoon trading on the New York Stock Exchange.
Nächstes Jahr gehts dann natürlich noch um die wichtigere US-Zulassung.
Pfizer, Bristol get EU nod for blood clot preventer
Tue, Nov 20 2012
By Bill Berkrot
(Reuters) - European health regulators on Tuesday approved an eagerly anticipated blood thinner developed by Bristol-Myers Squibb Co and Pfizer Inc for preventing strokes and blood clots in patients with an irregular heartbeat known as atrial fibrillation, the companies said.
The drug Eliquis, also known as apixaban, is widely considered one of the most important new products for the two U.S. drugmakers, with multibillion-dollar annual sales potential.
The European approval was expected after an advisory panel this year recommended it for atrial fibrillation.
"It's not unexpected, but it's positive to finally get an afib approval under the belt for Eliquis," said MKM Partners analyst Jon Lecroy, who sees annual sales reaching $2 billion by about 2017. "We're looking for a March approval or earlier in the U.S. for the same indication," he added.
The U.S. Food and Drug Administration is expected to decide on the proposed use of the drug in the world's biggest market by March 17, after delaying a decision in June to review more information from clinical trials.
The European Commission approval marks the first regulatory approval for Eliquis for stroke prevention in atrial fibrillation patients in any market, the companies said.
Eliquis belongs to a new class of medicines designed to replace decades old warfarin for preventing blood clots in heart patients and after hip or knee replacement surgery.
It already was approved in 27 European Union countries for prevention of certain blood clots called venous thromboembelisms following elective hip or knee replacement surgery.
But atrial fibrillation, which greatly raises the risk of strokes, is considered by far the largest and most important use for the new drugs, that include Xarelto from Bayer and Johnson & Johnson, and Pradaxa from privately held Boehringer Ingelheim.
Eliquis, like Xarelto, works by inhibiting a protein called Factor Xa that plays a critical role in blood clotting. Pradaxa has a slightly different mechanism of action.
About 6 million people in Europe and another 5.8 million in the United States suffer from atrial fibrillation, the most common form of heart arrhythmia, or irregular heartbeat.
In clinical trials, Eliquis demonstrated superiority over warfarin in reducing the risk of strokes, major bleeding and death.
Warfarin, widely used for more than half a century, is inexpensive and works well, but requires close and regular patient monitoring as well as lifestyle and dietary changes that are not necessary with the newer medicines.
"Patients with atrial fibrillation have a five times greater risk of stroke and there remains a critical public health need for improved treatment options to reduce this risk," Lars Wallentin, director of cardiology at Uppsala Clinical Research Centre and University Hospital in Sweden, said in a statement.
He called Eliquis "an important new treatment option for health care professionals, who now have an oral anticoagulant with superior outcomes versus warfarin."
Wall Street analysts have said that, based on clinical efficacy and safety data, they believe Eliquis will become the dominant player in an estimated $10 billion market for the new blood thinners once it receives U.S. approval.
Pfizer Chief Executive Ian Read, in a statement, said he believes Eliquis "has the potential to transform the standard of care in stroke prevention in nonvalvular atrial fibrillation".
Pfizer shares were up 5 cents at $24.19 and Bristol-Myers shares were up 2 cents at $32.05, in afternoon trading on the New York Stock Exchange.
Bei Seeking Alpha ist ein detailreicher Artikel erschienen, der Chancen und Potentiale von Eliquis auslotet.
Ich finde ihn sehr gut geschrieben und kopiere ihn hier deshalb vollständig rein.
Der Artikel geht mit der allgemeinen Auffassung konform, dass Eliquis seinen Konkurrenten Xarelto und Pradaxa überlegen ist.
Eliquis ist nach allem was man wissen kann "best in class".
Es wird auch klar, wie die Analysten zu ihren recht verschiedenen Umsatzschätzungen für Eliquis kommen (die Bandbreite schwankt ja zwischen 2 und 7 Milliarden Dollar Peak Sales).
Der Markt an sich ist riesig (bis zu 20 Milliarden). Die Frage ist aber, welchen Anteil die Faktor Xa-Hemmer erobern werden und welchen Anteil Eliquis in dieser Wirkstoffklasse erreichen wird.
Hier wird man einfach abwarten müssen. Sicher darf man nicht erwarten, dass die Ärzte jetzt sämtliche Patienten, die auf Warfarin eingestellt sind auf die neuen Präparate umstellen. Vor allem dort nicht, wo die Leute mit Warfarin recht gut zurecht kommen.
Ich gehe daher davon aus, dass die Eliquis-Umsätze verhältnismäßig langsam, dafür aber stetig wohl über die gesamte Patentlaufzeit hinweg immer weiter ansteigen werden.
(Eigentlich unnötig zu wiederholen: Für den großen Durchbruch ist die Zulassung durch die FDA unabdingbar - und daran fehlt es bisher. Im Q1/2013 wird sich insofern das Schicksal von Eliquis entscheiden.)
Eliquis (Apixaban) Approved In Europe - A Blockbuster Opportunity For Bristol-Myers, Pfizer
Yesterday, the European Medicine agency granted approval to Bristol-Myers (BMY) and Pfizer's (PFE) blood thinning drug - Eliquis for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Eliquis is still awaiting a US approval, the PDUFA data is set as March 17, 2013. Stroke prevention in atrial fibrillation (SPAF) is a large market and Eliquis is expected to take majority share by virtue of a superior profile compared to competing drugs.
Eliquis which belongs to a class of drugs called Factor Xa inhibitors is a new generation oral anticoagulant / blood thinning drug and is expected to replace warfarin the current gold standard treatment for stroke prevention in atrial fibrillation. Eliquis is to be taken twice daily. Besides Eliquis, two other new generation oral anticoagulants - Bayer's (BAYRY.PK) / Johnson and Johnson's (JNJ) Xarelto (approved in 2011) and Boehringer Ingelheim's Pradaxa (direct thrombin inhibitor, approved in 2010) are also on the market and are vying to replace warfarin.
The clinical data on Eliquis in atrial fibrillation is most impressive, when compared to Xarelto or Pradaxa and looks all set to grab majority market share. You can see a cross trial comparison of Pradaxa, Xarelto and Eliquis here
The Unmet Need in Stroke Prevention in Atrial Fibrillation
Warfarin - The current standard of care has several limitations and would be cannibalized by the new generation oral anti-coagulants which have demonstrated better efficacy and safety when compared to warfarin. A major limitation with warfarin is that patients taking warfarin need to be monitored frequently for their INR control. This is not the case with new generation oral anticoagulants - Pradaxa, Eliquis or Xarelto. The full form of INR is international normalized ratio. The higher the INR, the thinner the blood is. For warfarin to be effective and at the same time avoid bleeding risk, the INR should be between 2.0 and 3.0.
About 35% of the eligible AF patients are currently not treated with warfarin because the typical constraints - drug-drug /dietary interactions and difficulty in INR control. Thus warfarin is a safe and effective oral anticoagulant if a therapeutic international normalized ratio is properly maintained. In practice, however, it is difficult to manage because its therapeutic level is affected by many factors including diet, medications, illnesses, and genetics. Warfarin's narrow therapeutic range predisposes to many adverse effects from both under anticoagulation (resulting in thrombus formation) and over anticoagulation (leading to hemorrhage).
To ensure that a therapeutic INR is achieved, frequent monitoring is required that is inconvenient for patients and physicians and costly for the healthcare system. Specialized anticoagulation management clinics are needed to address the complexity in managing warfarin. Additional warfarin drawbacks include its slow onset and offset of action. It takes 72-96 hours to become effective and requires overlap with a rapidly acting parenteral anticoagulant or "bridging" until a therapeutic INR is achieved. Its slower offset, with an effective half-life of approximately 40 hours, makes it difficult to manage before any surgical procedures. Safety concerns and therapy complexity lead many physicians to
under use warfarin, prescribing it to only two thirds of appropriate candidate.
Why Eliquis is best poised among the New Generation Anticoagulants to grab a larger pie
Eliquis is the only anticoagulant to have demonstrated statistically significant superiority over warfarin in the reduction of the composite of stroke / Systemic Embolism / all-cause death and major bleeding. Xarelto which was approved last year for SPAF has shown non-inferiority compared to warfarin on both efficacy (stroke reduction) and safety(major bleeding). Pradaxa which was approved in 2010 is superior to warfarin as far as reduction in the risk of stroke is concerned, but does not reduce the risk of major bleeding. Unfortunately the larger concern of cardiologists with the use of warfarin is the higher risk of bleeding / Intra cranial hemorrhage, while they are more or less satisfied with the efficacy of warfarin (reduction in stroke). Hence as far as current unmet need is concerned, cardiologists prefer an oral anticoagulant that is atleast as efficacious as warfarin, but significantly reduces the risk of major bleeding. Eliquis seems to have exactly hit this sweet spot and is poised for a breakout once launched. In the pivotal Phase 3 study Eliquis was superior to warfarin in preventing stroke or systemic embolism (the primary end point) and was also associated with less bleeding and lower mortality than warfarin.
In nutshell although both Pradaxa and Xarelto have also shown benefits over warfarin in the pivotal Phase 3 trials, but Eliquis is the only one to have shown definite reductions in each of the major outcomes of stroke, bleeding, and mortality.
Market Potential and Eliquis Peak Sales
Stroke Prevention in Atrial Fibrillation represents a very large market, as besides a very large prevalence, there is a very clear unmet need. SPAF affects about 4.5m people in the EU, 2.2m in the US and about 1 million Japanese. Atrial fibrillation patients are at 3 to 5 fold increased risk of stroke and 75,000 strokes per year are attributable to atrial fibrillation.
If we are to expect a 50% market share shift from existing treatments warfarin and aspirin to new generation oral anticoagulants (Pradaxa, Xarelto and Eliquis), the annual market size works out to be $10b. Pradaxa and Xarelto which are already on the market have been priced at an annual cost of $2500. Assuming a similar price for Eliquis and a 25 percent market share, the peak sales of Eliquis will reach about $5 billion.
Impact of Eliquis on Pfizer and Bristol-Myers Earnings
With regard to Eliquis, Pfizer and Bristol-Myers have entered into an agreement under which the companies will jointly develop the clinical and marketing strategy of Eliquis, and will share commercialization expenses and profits/losses equally on a global basis.
At $5 billion peak sales, we expect Eliquis to generate peak post tax profitability of 75 percent, which would translate into $3.8b in annual profits. As per the agreement Pfizer and BMY will share the profits equally. For BMY, $1.9b in annual profits translates into an incremental EPS of $1.1 per share, which is about 56 percent of 2012 estimated EPS. For a company of the size of Pfizer as well, Eliquis is pretty significant as it would add about $0.25 per share in incremental EPS, which is about 12 percent of its 2012 estimated EPS. Both PFE and BMY have already shed significant sales and profits owing to loss of patent on their key drugs (Plavix and Lipitor) and there are no major patent expiries on the anvil except Abilify for BMY in 2014. Pfizer currently trades at 12x 2012 earnings, while BMY trades at 17x 2012 earnings. Looking at the significant impact of Eliquis on the bottom line of both these companies, one should consider a long position with a horizon of about 3 years.
On an NPV basis, it is worth about $20b assuming a cost of capital of 7 percent. Since Pfizer and Eliquis would be sharing the profits from the sales of Eliquis, we attribute $9billion of NPV each to Bristol Myers and Pfizer. From a per share perspective, Eliquis is worth $5.3 per share for BMY, and it is worth $1.2 per share for Pfizer.
http://seekingalpha.com/article/1023711-eliquis-apixaban-app…
Ich finde ihn sehr gut geschrieben und kopiere ihn hier deshalb vollständig rein.
Der Artikel geht mit der allgemeinen Auffassung konform, dass Eliquis seinen Konkurrenten Xarelto und Pradaxa überlegen ist.
Eliquis ist nach allem was man wissen kann "best in class".
Es wird auch klar, wie die Analysten zu ihren recht verschiedenen Umsatzschätzungen für Eliquis kommen (die Bandbreite schwankt ja zwischen 2 und 7 Milliarden Dollar Peak Sales).
Der Markt an sich ist riesig (bis zu 20 Milliarden). Die Frage ist aber, welchen Anteil die Faktor Xa-Hemmer erobern werden und welchen Anteil Eliquis in dieser Wirkstoffklasse erreichen wird.
Hier wird man einfach abwarten müssen. Sicher darf man nicht erwarten, dass die Ärzte jetzt sämtliche Patienten, die auf Warfarin eingestellt sind auf die neuen Präparate umstellen. Vor allem dort nicht, wo die Leute mit Warfarin recht gut zurecht kommen.
Ich gehe daher davon aus, dass die Eliquis-Umsätze verhältnismäßig langsam, dafür aber stetig wohl über die gesamte Patentlaufzeit hinweg immer weiter ansteigen werden.
(Eigentlich unnötig zu wiederholen: Für den großen Durchbruch ist die Zulassung durch die FDA unabdingbar - und daran fehlt es bisher. Im Q1/2013 wird sich insofern das Schicksal von Eliquis entscheiden.)
Eliquis (Apixaban) Approved In Europe - A Blockbuster Opportunity For Bristol-Myers, Pfizer
Yesterday, the European Medicine agency granted approval to Bristol-Myers (BMY) and Pfizer's (PFE) blood thinning drug - Eliquis for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Eliquis is still awaiting a US approval, the PDUFA data is set as March 17, 2013. Stroke prevention in atrial fibrillation (SPAF) is a large market and Eliquis is expected to take majority share by virtue of a superior profile compared to competing drugs.
Eliquis which belongs to a class of drugs called Factor Xa inhibitors is a new generation oral anticoagulant / blood thinning drug and is expected to replace warfarin the current gold standard treatment for stroke prevention in atrial fibrillation. Eliquis is to be taken twice daily. Besides Eliquis, two other new generation oral anticoagulants - Bayer's (BAYRY.PK) / Johnson and Johnson's (JNJ) Xarelto (approved in 2011) and Boehringer Ingelheim's Pradaxa (direct thrombin inhibitor, approved in 2010) are also on the market and are vying to replace warfarin.
The clinical data on Eliquis in atrial fibrillation is most impressive, when compared to Xarelto or Pradaxa and looks all set to grab majority market share. You can see a cross trial comparison of Pradaxa, Xarelto and Eliquis here
The Unmet Need in Stroke Prevention in Atrial Fibrillation
Warfarin - The current standard of care has several limitations and would be cannibalized by the new generation oral anti-coagulants which have demonstrated better efficacy and safety when compared to warfarin. A major limitation with warfarin is that patients taking warfarin need to be monitored frequently for their INR control. This is not the case with new generation oral anticoagulants - Pradaxa, Eliquis or Xarelto. The full form of INR is international normalized ratio. The higher the INR, the thinner the blood is. For warfarin to be effective and at the same time avoid bleeding risk, the INR should be between 2.0 and 3.0.
About 35% of the eligible AF patients are currently not treated with warfarin because the typical constraints - drug-drug /dietary interactions and difficulty in INR control. Thus warfarin is a safe and effective oral anticoagulant if a therapeutic international normalized ratio is properly maintained. In practice, however, it is difficult to manage because its therapeutic level is affected by many factors including diet, medications, illnesses, and genetics. Warfarin's narrow therapeutic range predisposes to many adverse effects from both under anticoagulation (resulting in thrombus formation) and over anticoagulation (leading to hemorrhage).
To ensure that a therapeutic INR is achieved, frequent monitoring is required that is inconvenient for patients and physicians and costly for the healthcare system. Specialized anticoagulation management clinics are needed to address the complexity in managing warfarin. Additional warfarin drawbacks include its slow onset and offset of action. It takes 72-96 hours to become effective and requires overlap with a rapidly acting parenteral anticoagulant or "bridging" until a therapeutic INR is achieved. Its slower offset, with an effective half-life of approximately 40 hours, makes it difficult to manage before any surgical procedures. Safety concerns and therapy complexity lead many physicians to
under use warfarin, prescribing it to only two thirds of appropriate candidate.
Why Eliquis is best poised among the New Generation Anticoagulants to grab a larger pie
Eliquis is the only anticoagulant to have demonstrated statistically significant superiority over warfarin in the reduction of the composite of stroke / Systemic Embolism / all-cause death and major bleeding. Xarelto which was approved last year for SPAF has shown non-inferiority compared to warfarin on both efficacy (stroke reduction) and safety(major bleeding). Pradaxa which was approved in 2010 is superior to warfarin as far as reduction in the risk of stroke is concerned, but does not reduce the risk of major bleeding. Unfortunately the larger concern of cardiologists with the use of warfarin is the higher risk of bleeding / Intra cranial hemorrhage, while they are more or less satisfied with the efficacy of warfarin (reduction in stroke). Hence as far as current unmet need is concerned, cardiologists prefer an oral anticoagulant that is atleast as efficacious as warfarin, but significantly reduces the risk of major bleeding. Eliquis seems to have exactly hit this sweet spot and is poised for a breakout once launched. In the pivotal Phase 3 study Eliquis was superior to warfarin in preventing stroke or systemic embolism (the primary end point) and was also associated with less bleeding and lower mortality than warfarin.
In nutshell although both Pradaxa and Xarelto have also shown benefits over warfarin in the pivotal Phase 3 trials, but Eliquis is the only one to have shown definite reductions in each of the major outcomes of stroke, bleeding, and mortality.
Market Potential and Eliquis Peak Sales
Stroke Prevention in Atrial Fibrillation represents a very large market, as besides a very large prevalence, there is a very clear unmet need. SPAF affects about 4.5m people in the EU, 2.2m in the US and about 1 million Japanese. Atrial fibrillation patients are at 3 to 5 fold increased risk of stroke and 75,000 strokes per year are attributable to atrial fibrillation.
If we are to expect a 50% market share shift from existing treatments warfarin and aspirin to new generation oral anticoagulants (Pradaxa, Xarelto and Eliquis), the annual market size works out to be $10b. Pradaxa and Xarelto which are already on the market have been priced at an annual cost of $2500. Assuming a similar price for Eliquis and a 25 percent market share, the peak sales of Eliquis will reach about $5 billion.
Impact of Eliquis on Pfizer and Bristol-Myers Earnings
With regard to Eliquis, Pfizer and Bristol-Myers have entered into an agreement under which the companies will jointly develop the clinical and marketing strategy of Eliquis, and will share commercialization expenses and profits/losses equally on a global basis.
At $5 billion peak sales, we expect Eliquis to generate peak post tax profitability of 75 percent, which would translate into $3.8b in annual profits. As per the agreement Pfizer and BMY will share the profits equally. For BMY, $1.9b in annual profits translates into an incremental EPS of $1.1 per share, which is about 56 percent of 2012 estimated EPS. For a company of the size of Pfizer as well, Eliquis is pretty significant as it would add about $0.25 per share in incremental EPS, which is about 12 percent of its 2012 estimated EPS. Both PFE and BMY have already shed significant sales and profits owing to loss of patent on their key drugs (Plavix and Lipitor) and there are no major patent expiries on the anvil except Abilify for BMY in 2014. Pfizer currently trades at 12x 2012 earnings, while BMY trades at 17x 2012 earnings. Looking at the significant impact of Eliquis on the bottom line of both these companies, one should consider a long position with a horizon of about 3 years.
On an NPV basis, it is worth about $20b assuming a cost of capital of 7 percent. Since Pfizer and Eliquis would be sharing the profits from the sales of Eliquis, we attribute $9billion of NPV each to Bristol Myers and Pfizer. From a per share perspective, Eliquis is worth $5.3 per share for BMY, and it is worth $1.2 per share for Pfizer.
http://seekingalpha.com/article/1023711-eliquis-apixaban-app…
Heute bei fierce erschienen.
Aber Vorsicht: Das Lesen des Artikels ist gefährlich für liebgewonnene Vorurteile:
Bristol-Myers' Sigal outshines pharma rivals in R&D chief ranking
December 19, 2012 | By Ryan McBride
Pharma faces a colossal conundrum of how to score more approvals without breaking the bank. The quants have exposed massive inefficiencies in the R&D groups of Big Pharma, but some companies have faired better than others. And a recent analysis ranks R&D chiefs based on the performance of their companies.
Bristol-Myers Squibb's ($BMY) Elliot Sigal topped his counterparts at other large pharma companies--which included Novartis ($NVS), GlaxoSmithKline ($GSK), Amgen ($AMGN), and Merck ($MRK), respectively--in an analysis of R&D productivity from 2004 to 2011, The Wall Street Journal reports, citing the numbers from Informa-Scrip Group. During the period analyzed, Bristol spent $28.41 billion on R&D and racked up 7 FDA approvals of new drugs or biologics, costing the company $4.06 billion per approval.

Elliot Sigal, CSO and president, R&D
Just over $4 billion might seem like too much money for an approval, yet consider that Sigal's counterpart at Merck, Peter Kim, presided over an R&D organization that spent $12.72 billion for each new FDA approval of a new drug or biologic (or $7.27 billion per approval if you count approvals of Merck's vaccines). Bristol-Myers, despite struggling to recover from a patent cliff, shows how more can be done with less relative to its industry peers. And in Informa-Scrip Group's Christopher Bowe's view, Sigal deserves lots of credit.
"Individuals matter," Bowe noted, as quoted by the WSJ. "One person can make a difference in a huge company, in a big world, within the infinite realm of science. There are better R&D chiefs than others; it's a fact. We must set up new ways to develop these unusual individual talents, so that companies don't settle and find them."
It's also a fact that Bristol has benefitted from savvy dealmaking and extramural activities with partners. Bristol gained rights to at least a couple drugs that were approved by the FDA during the analysis period--the skin cancer immunotherapy Yervoy via Medarex and the anti-tumor antibody Erbitux from ImClone Systems--through partnerships or acquisitions.
In fact, pharma chiefs have copied Bristol's "String of Pearls" strategy that emphasized bolt-on acquisitions rather than megadeals. Teva Pharmaceutical ($TEVA) this year poached Jeremy Levin, Bristol's former strategy chief, to steer the growth of that company as CEO. Does Levin deserve some credit for Sigal's success?
Aber Vorsicht: Das Lesen des Artikels ist gefährlich für liebgewonnene Vorurteile:
Bristol-Myers' Sigal outshines pharma rivals in R&D chief ranking
December 19, 2012 | By Ryan McBride
Pharma faces a colossal conundrum of how to score more approvals without breaking the bank. The quants have exposed massive inefficiencies in the R&D groups of Big Pharma, but some companies have faired better than others. And a recent analysis ranks R&D chiefs based on the performance of their companies.
Bristol-Myers Squibb's ($BMY) Elliot Sigal topped his counterparts at other large pharma companies--which included Novartis ($NVS), GlaxoSmithKline ($GSK), Amgen ($AMGN), and Merck ($MRK), respectively--in an analysis of R&D productivity from 2004 to 2011, The Wall Street Journal reports, citing the numbers from Informa-Scrip Group. During the period analyzed, Bristol spent $28.41 billion on R&D and racked up 7 FDA approvals of new drugs or biologics, costing the company $4.06 billion per approval.

Elliot Sigal, CSO and president, R&D
Just over $4 billion might seem like too much money for an approval, yet consider that Sigal's counterpart at Merck, Peter Kim, presided over an R&D organization that spent $12.72 billion for each new FDA approval of a new drug or biologic (or $7.27 billion per approval if you count approvals of Merck's vaccines). Bristol-Myers, despite struggling to recover from a patent cliff, shows how more can be done with less relative to its industry peers. And in Informa-Scrip Group's Christopher Bowe's view, Sigal deserves lots of credit.
"Individuals matter," Bowe noted, as quoted by the WSJ. "One person can make a difference in a huge company, in a big world, within the infinite realm of science. There are better R&D chiefs than others; it's a fact. We must set up new ways to develop these unusual individual talents, so that companies don't settle and find them."
It's also a fact that Bristol has benefitted from savvy dealmaking and extramural activities with partners. Bristol gained rights to at least a couple drugs that were approved by the FDA during the analysis period--the skin cancer immunotherapy Yervoy via Medarex and the anti-tumor antibody Erbitux from ImClone Systems--through partnerships or acquisitions.
In fact, pharma chiefs have copied Bristol's "String of Pearls" strategy that emphasized bolt-on acquisitions rather than megadeals. Teva Pharmaceutical ($TEVA) this year poached Jeremy Levin, Bristol's former strategy chief, to steer the growth of that company as CEO. Does Levin deserve some credit for Sigal's success?
Eine Einschätzung von Goldman Sachs zu den wichtigsten Punkten in 2013:
Analysts at Goldman Sachs lowered the price target and earnings estimates on pharmaceutical company Bristol Myers Squibb Co. (BMY) on Wednesday, but kept its “Buy” rating.
Goldman Sachs now see shares of BMY reaching $39, down from its previous target of $40. The new valuation is a +18% upside to Tuesday’s closing price of $33.02.
A Goldman Sachs analyst noted, “For 2013, a year of new launches for BMY, we revise our assumptions for spending and modestly lower our 2013 EPS estimate from $1.90 to $1.80, on higher SG&A, versus $1.84 for consensus.
Importantly, we leave our longer term estimates unchanged and continue to see BMY as the only company in our sector with the potential to more than double its EPS by the end of the decade. BMY has been a laggard this year owing to the Eliquis delays and the Inhibitex drug failure. We believe execution will be essential to outperformance, the success of which should increase confidence in BMY’s long-term growth story.
Specifically, we look to the following drivers:
(1) Eliquis approval and launch by 1Q13 (already approved in EU with a superior label),
(2) the EU launch of Forxiga/Dapa (expect US resubmission by mid-2013), and
(3) data on key pipeline drugs anti-PD1 (ASCO in June) and HepC (filing in Japan).
We view global Eliquis consensus sales of $350 mn as a low hurdle. For Forxiga, our estimate remains unchanged with only OUS sales in our forecast, however this leaves room for upside on a potential 2014 US launch (could be $1 bn in peak sales).
Regarding anti-PD1, BMY will exit 2012 with five P3 trials underway in lung, renal and melanoma, with PD1 in combination with Yervoy to start next year.
Additionally, potential for positive P3 results in the Japanese trial for HepC may result in consensus numbers moving up.”
Analysts at Goldman Sachs lowered the price target and earnings estimates on pharmaceutical company Bristol Myers Squibb Co. (BMY) on Wednesday, but kept its “Buy” rating.
Goldman Sachs now see shares of BMY reaching $39, down from its previous target of $40. The new valuation is a +18% upside to Tuesday’s closing price of $33.02.
A Goldman Sachs analyst noted, “For 2013, a year of new launches for BMY, we revise our assumptions for spending and modestly lower our 2013 EPS estimate from $1.90 to $1.80, on higher SG&A, versus $1.84 for consensus.
Importantly, we leave our longer term estimates unchanged and continue to see BMY as the only company in our sector with the potential to more than double its EPS by the end of the decade. BMY has been a laggard this year owing to the Eliquis delays and the Inhibitex drug failure. We believe execution will be essential to outperformance, the success of which should increase confidence in BMY’s long-term growth story.
Specifically, we look to the following drivers:
(1) Eliquis approval and launch by 1Q13 (already approved in EU with a superior label),
(2) the EU launch of Forxiga/Dapa (expect US resubmission by mid-2013), and
(3) data on key pipeline drugs anti-PD1 (ASCO in June) and HepC (filing in Japan).
We view global Eliquis consensus sales of $350 mn as a low hurdle. For Forxiga, our estimate remains unchanged with only OUS sales in our forecast, however this leaves room for upside on a potential 2014 US launch (could be $1 bn in peak sales).
Regarding anti-PD1, BMY will exit 2012 with five P3 trials underway in lung, renal and melanoma, with PD1 in combination with Yervoy to start next year.
Additionally, potential for positive P3 results in the Japanese trial for HepC may result in consensus numbers moving up.”
Zulassung von Eliquis in Japan:
Wednesday, December 26, 2012 7:30 am EST
PRINCETON, N.J. & NEW YORK--(BUSINESS WIRE)--Bristol-Myers Squibb (NYSE: BMY) and Pfizer Inc. (NYSE: PFE) announced today that the Japanese Ministry of Health, Labor and Welfare (MHLW) has approved ELIQUIS® (apixaban) for the prevention of ischemic stroke and systemic embolism in patients with nonvalvular atrial fibrillation (NVAF). ELIQUIS is a novel anticoagulant that has demonstrated risk reductions versus warfarin in three important outcomes of stroke, major bleeding and all-cause death. ELIQUIS is an oral direct Factor Xa inhibitor, part of a novel therapeutic class. This is the third approval for ELIQUIS for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, following approvals in the European Union and Canada.
http://news.bms.com/press-release/partnering-news/eliquis-ap…
Wednesday, December 26, 2012 7:30 am EST
PRINCETON, N.J. & NEW YORK--(BUSINESS WIRE)--Bristol-Myers Squibb (NYSE: BMY) and Pfizer Inc. (NYSE: PFE) announced today that the Japanese Ministry of Health, Labor and Welfare (MHLW) has approved ELIQUIS® (apixaban) for the prevention of ischemic stroke and systemic embolism in patients with nonvalvular atrial fibrillation (NVAF). ELIQUIS is a novel anticoagulant that has demonstrated risk reductions versus warfarin in three important outcomes of stroke, major bleeding and all-cause death. ELIQUIS is an oral direct Factor Xa inhibitor, part of a novel therapeutic class. This is the third approval for ELIQUIS for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, following approvals in the European Union and Canada.
http://news.bms.com/press-release/partnering-news/eliquis-ap…
Durchaus überraschend ist die Zulassung von Eliquis nun auch in den USA erfolgt - drei Monate eher als erwartet:
von Bloomberg:
Pfizer, Bristol-Myers Blood Thinner Approved by U.S. FDA
By Drew Armstrong - Dec 28, 2012
Pfizer Inc. (PFE) and Bristol-Myers Squibb Co. (BMY)’s blood thinner Eliquis was approved by U.S. regulators after more than a year of review, giving the companies a new potential blockbuster heart drug.
Eliquis was cleared by the Food and Drug Administration for the prevention of blood clots that develop in patients with the heart rhythm disorder atrial fibrillation, the agency said today in a statement. The approval comes three months before the FDA’s most-recent March deadline for a ruling on the drug, allowing the two New York-based companies to start sales sooner.
“The new anticoagulants are really exciting developments,” William Zoghbi, president of the American College of Cardiology, said in a telephone interview. “They address some of the concerns that traditional warfarin has offered.” Warfarin is the half-century-old drug that has been the standard of care before new medicines like Eliquis.
The government’s sign-off ends several setbacks in the efforts by Pfizer and Bristol-Myers to win sales clearance for the blood thinner. The FDA delayed approval in March 2012 and rejected the therapy in June, saying it needed more data from the companies’ clinical trials. Pfizer, the world’s largest drugmaker, and Bristol-Myers will split sales, which may total $5.2 billion by 2020, Catherine Arnold, an analyst at Credit Suisse in New York, said in a note to clients.
Pfizer shares rose less than 1 percent in extended trading to $24.99 at 4:57 p.m. New York time, after closing at $24.89. Bristol-Myers rose 1.9 percent after closing at $31.90.
Common Condition
About 2.66 million people in the U.S. have atrial fibrillation, the most common type of irregular heart beat, according to the Centers for Disease Control and Prevention in Atlanta. The disease becomes more common with age. Patients have a higher risk of blood clots, which can cause strokes, and of heart failure.
Eliquis will provide another option for the prevention of blood clots and strokes, and the drug showed in clinical trials that it was more effective than warfarin, the FDA said in its statement. It is among a group of new blood thinners such as Boehringer Ingelheim GmbH’s Pradaxa and Johnson & Johnson (JNJ) and Bayer AG (BAYN)’s Xarelto, that are potential replacements for warfarin, which requires regular blood monitoring by doctors.
The new drugs carry some risk of bleeding, as does warfarin. Bleeding can happen in the stomach, under the surface of the skin, or most dangerously, in the brain.
The new blood thinners offer a better trade-off between bleeding and stroke-prevention, said Andrew Epstein, a professor of medicine at the University of Pennsylvania in Philadelphia. “One intracranial bleed is a small price for the great decrease in morbidity and mortality,” Epstein said in a telephone interview. “There ain’t any free lunch.”
Eliquis has been approved in Europe and Japan. Pfizer and Bristol-Myers also want to gain approval for use in clots elsewhere in the body, including the legs and lungs.
-----------------
Das Jahr 2013 wird nun zeigen, wie gut es BMS/Pfizer gelingen wird, das Mittel im Markt gegen Warfarin und vor allem gegen die Konkurrenz von JNJ und Boehringer zu plazieren.
von Bloomberg:
Pfizer, Bristol-Myers Blood Thinner Approved by U.S. FDA
By Drew Armstrong - Dec 28, 2012
Pfizer Inc. (PFE) and Bristol-Myers Squibb Co. (BMY)’s blood thinner Eliquis was approved by U.S. regulators after more than a year of review, giving the companies a new potential blockbuster heart drug.
Eliquis was cleared by the Food and Drug Administration for the prevention of blood clots that develop in patients with the heart rhythm disorder atrial fibrillation, the agency said today in a statement. The approval comes three months before the FDA’s most-recent March deadline for a ruling on the drug, allowing the two New York-based companies to start sales sooner.
“The new anticoagulants are really exciting developments,” William Zoghbi, president of the American College of Cardiology, said in a telephone interview. “They address some of the concerns that traditional warfarin has offered.” Warfarin is the half-century-old drug that has been the standard of care before new medicines like Eliquis.
The government’s sign-off ends several setbacks in the efforts by Pfizer and Bristol-Myers to win sales clearance for the blood thinner. The FDA delayed approval in March 2012 and rejected the therapy in June, saying it needed more data from the companies’ clinical trials. Pfizer, the world’s largest drugmaker, and Bristol-Myers will split sales, which may total $5.2 billion by 2020, Catherine Arnold, an analyst at Credit Suisse in New York, said in a note to clients.
Pfizer shares rose less than 1 percent in extended trading to $24.99 at 4:57 p.m. New York time, after closing at $24.89. Bristol-Myers rose 1.9 percent after closing at $31.90.
Common Condition
About 2.66 million people in the U.S. have atrial fibrillation, the most common type of irregular heart beat, according to the Centers for Disease Control and Prevention in Atlanta. The disease becomes more common with age. Patients have a higher risk of blood clots, which can cause strokes, and of heart failure.
Eliquis will provide another option for the prevention of blood clots and strokes, and the drug showed in clinical trials that it was more effective than warfarin, the FDA said in its statement. It is among a group of new blood thinners such as Boehringer Ingelheim GmbH’s Pradaxa and Johnson & Johnson (JNJ) and Bayer AG (BAYN)’s Xarelto, that are potential replacements for warfarin, which requires regular blood monitoring by doctors.
The new drugs carry some risk of bleeding, as does warfarin. Bleeding can happen in the stomach, under the surface of the skin, or most dangerously, in the brain.
The new blood thinners offer a better trade-off between bleeding and stroke-prevention, said Andrew Epstein, a professor of medicine at the University of Pennsylvania in Philadelphia. “One intracranial bleed is a small price for the great decrease in morbidity and mortality,” Epstein said in a telephone interview. “There ain’t any free lunch.”
Eliquis has been approved in Europe and Japan. Pfizer and Bristol-Myers also want to gain approval for use in clots elsewhere in the body, including the legs and lungs.
-----------------
Das Jahr 2013 wird nun zeigen, wie gut es BMS/Pfizer gelingen wird, das Mittel im Markt gegen Warfarin und vor allem gegen die Konkurrenz von JNJ und Boehringer zu plazieren.
Antwort auf Beitrag Nr.: 43.966.988 von SLGramann am 29.12.12 08:08:15Hier noch ein sehr ausgewogener Artikel der NYT:
F.D.A. Clears Anticlotting Drug by Bristol and Pfizer
By KATIE THOMAS
The Food and Drug Administration on Friday approved Eliquis, an anticlotting drug that has been highly anticipated by cardiologists and is expected to be a blockbuster for Bristol-Myers Squibb, which will make the drug, and Pfizer, which will help market it.
The agency approved Eliquis for reducing the risk of stroke and dangerous blood clots in people with atrial fibrillation, a common heart arrhythmia that afflicts millions of people in the United States.
The drug, also known as apixaban, is the third anticlotting medicine to be approved in recent years and the companies are expected to aggressively compete to pitch their products as a replacement for warfarin, an older treatment that requires more careful monitoring. Warfarin is also known by the brand name Coumadin.
“The marketing games will now begin,” said Dr. Sanjay Kaul, a cardiologist at the Cedars-Sinai Medical Center in Los Angeles, who was not involved in the development of any of the drugs.
He said that cardiologists would now have to sort out the differences among Eliquis and its competitors already on the market: Pradaxa, sold by Boehringer Ingelheim, and Xarelto, sold by Johnson & Johnson and Bayer.
While some experts have argued that Eliquis offers the best balance between the drug’s benefits and risks, Dr. Kaul said since there have been no clinical trials comparing the three new drugs, “it is impossible to adjudicate which of these new agents is the preferred one.”
Bristol-Myers and Pfizer issued a brief statement Friday saying they were pleased with the approval. In a news release in November announcing the drug’s approval in Europe, Bristol-Myers noted that Eliquis was the only drug in the group that has shown an advantage over warfarin in reducing the risk of stroke and dangerous blood clots, major bleeding and death.
The agency also warned that patients with prosthetic heart valves should not take Eliquis, nor should patients with atrial fibrillation that is caused by a heart valve problem.
Despite the promise of Eliquis and the other new drugs, some cautioned against prescribing them too enthusiastically.
Dr. Garret FitzGerald, a cardiologist and chairman of pharmacology at the University of Pennsylvania, said the trial results for Eliquis were impressive. But he added in an e-mail on Friday: “What matters to a patient is the individual effect in them.”
He noted that patients taking Eliquis also suffered major bleeding episodes and said all drugs that prevent clotting carried a risk of bleeding. “Thus the F.D.A.’s warning to be on the lookout for bleeding seems just as appropriate as approval of Eliquis,” he said.
Dr. Kaul says he tends to wait to prescribe new drugs until he learns more from the experience of colleagues, and Eliquis will be no exception. “I’m going to sit back by the sidelines and see how it pans out,” he said, adding that he had begun to prescribe Pradaxa and Xarelto to patients.
Eliquis’s entry into the United States market has been eagerly anticipated by Bristol-Myers and Pfizer after a succession of delays this year. Bristol-Myers, in particular, has been struggling since its best-selling blood-thinner drug, Plavix, lost its patent protection in May. Sales of Plavix, which Bristol-Myers sells in partnership with Sanofi, fell 96 percent in the third quarter of this year after cheaper generic alternatives flooded the market and the company has struggled to replace the lost sales.
Eliquis is “very important for Bristol because it’s one of the legs of their investment case, that they can leapfrog over the Plavix expiration,” said Les Funtleyder, the fund manager of Poliwogg, a private equity and hedge fund. He said he expected Eliquis to eventually earn more than $1 billion for its sellers.
In such a crowded field of competitors, Mr. Funtleyder said, consumers and doctors should brace themselves for a marketing onslaught. “I wonder if we’ll see our first Eliquis commercial before the new year,” he said.
F.D.A. Clears Anticlotting Drug by Bristol and Pfizer
By KATIE THOMAS
The Food and Drug Administration on Friday approved Eliquis, an anticlotting drug that has been highly anticipated by cardiologists and is expected to be a blockbuster for Bristol-Myers Squibb, which will make the drug, and Pfizer, which will help market it.
The agency approved Eliquis for reducing the risk of stroke and dangerous blood clots in people with atrial fibrillation, a common heart arrhythmia that afflicts millions of people in the United States.
The drug, also known as apixaban, is the third anticlotting medicine to be approved in recent years and the companies are expected to aggressively compete to pitch their products as a replacement for warfarin, an older treatment that requires more careful monitoring. Warfarin is also known by the brand name Coumadin.
“The marketing games will now begin,” said Dr. Sanjay Kaul, a cardiologist at the Cedars-Sinai Medical Center in Los Angeles, who was not involved in the development of any of the drugs.
He said that cardiologists would now have to sort out the differences among Eliquis and its competitors already on the market: Pradaxa, sold by Boehringer Ingelheim, and Xarelto, sold by Johnson & Johnson and Bayer.
While some experts have argued that Eliquis offers the best balance between the drug’s benefits and risks, Dr. Kaul said since there have been no clinical trials comparing the three new drugs, “it is impossible to adjudicate which of these new agents is the preferred one.”
Bristol-Myers and Pfizer issued a brief statement Friday saying they were pleased with the approval. In a news release in November announcing the drug’s approval in Europe, Bristol-Myers noted that Eliquis was the only drug in the group that has shown an advantage over warfarin in reducing the risk of stroke and dangerous blood clots, major bleeding and death.
The agency also warned that patients with prosthetic heart valves should not take Eliquis, nor should patients with atrial fibrillation that is caused by a heart valve problem.
Despite the promise of Eliquis and the other new drugs, some cautioned against prescribing them too enthusiastically.
Dr. Garret FitzGerald, a cardiologist and chairman of pharmacology at the University of Pennsylvania, said the trial results for Eliquis were impressive. But he added in an e-mail on Friday: “What matters to a patient is the individual effect in them.”
He noted that patients taking Eliquis also suffered major bleeding episodes and said all drugs that prevent clotting carried a risk of bleeding. “Thus the F.D.A.’s warning to be on the lookout for bleeding seems just as appropriate as approval of Eliquis,” he said.
Dr. Kaul says he tends to wait to prescribe new drugs until he learns more from the experience of colleagues, and Eliquis will be no exception. “I’m going to sit back by the sidelines and see how it pans out,” he said, adding that he had begun to prescribe Pradaxa and Xarelto to patients.
Eliquis’s entry into the United States market has been eagerly anticipated by Bristol-Myers and Pfizer after a succession of delays this year. Bristol-Myers, in particular, has been struggling since its best-selling blood-thinner drug, Plavix, lost its patent protection in May. Sales of Plavix, which Bristol-Myers sells in partnership with Sanofi, fell 96 percent in the third quarter of this year after cheaper generic alternatives flooded the market and the company has struggled to replace the lost sales.
Eliquis is “very important for Bristol because it’s one of the legs of their investment case, that they can leapfrog over the Plavix expiration,” said Les Funtleyder, the fund manager of Poliwogg, a private equity and hedge fund. He said he expected Eliquis to eventually earn more than $1 billion for its sellers.
In such a crowded field of competitors, Mr. Funtleyder said, consumers and doctors should brace themselves for a marketing onslaught. “I wonder if we’ll see our first Eliquis commercial before the new year,” he said.
BMS die Zahlen zum vierten Quartal gemeldet. Mit 47 Cent pro Aktien (non-GAAP) liegen sie über den Erwartungen des Marktes.
Die „Hoffnungsträger“ haben sich im vierten Quartal besser als erwartet entwickelt (abgesehen von Erbitux, das ich falsch eingeschätzt habe).
Ich bin mit den Zahlen sehr zufrieden!
Die Reaktion des Marktes ist recht positiv.
Durch den Plavix-Verlust hat sich eine Umsatzeinbuße in Höhe von 23% im Vergleich zum Q4/2011 ergeben. Unter Ausklammerung von Plavix und Avapro ist BMS aber mit 13% gewachsen.
Im Einzelnen:
Im Saldo sind damit nach dieser Rechnung von Q1 bis Q4/2012 auf Quartalsbasis immer noch 1,467 Milliarden Dollar Umsatzausfall zu beklagen.
Wie bereits beim letzten Mal geschrieben, ist der Tiefpunkt aber bereits im 3. Quartal 2012 durchschritten worden.
Der Umsatzverlust hat sich bereits reduziert und diese Entwicklung wird in 2013 verstärkt weitergehen, insbesondere auch getrieben durch die Zulassung von Eliquis.
Die „Hoffnungsträger“ haben sich im vierten Quartal besser als erwartet entwickelt (abgesehen von Erbitux, das ich falsch eingeschätzt habe).
Ich bin mit den Zahlen sehr zufrieden!
Die Reaktion des Marktes ist recht positiv.
Durch den Plavix-Verlust hat sich eine Umsatzeinbuße in Höhe von 23% im Vergleich zum Q4/2011 ergeben. Unter Ausklammerung von Plavix und Avapro ist BMS aber mit 13% gewachsen.
Im Einzelnen:
Q1/2012 Q2/2012 Q3/2012 Q4/2012
Avapro 207 117 95 84
Plavix 1.693 741 64 49
Gesamt 1.900 858 159 133
Umsatzverlust durch Patentabläufe bis Q4/12: -1.767
Was konnte davon bisher durch „Hoffnungsträger“ kompensiert werden?
Q1/2012 Q2/2012 Q3/2012 Q4/2012
Baraclude 325 357 346 360
Erbitux 179 179 173 171
Sprycel 231 244 263 281
Yveroy 154 162 179 211
Orencia 254 290 307 325
Onglyza 161 172 178 198
Eliquis 0 1 0 1
Bydureon 0 0 20 58
Gesamt 1.305 1.405 1.466 1.605
Umsatzgewinne durch “Hoffnungsträger” bis Q4/12: +300
Im Saldo sind damit nach dieser Rechnung von Q1 bis Q4/2012 auf Quartalsbasis immer noch 1,467 Milliarden Dollar Umsatzausfall zu beklagen.
Wie bereits beim letzten Mal geschrieben, ist der Tiefpunkt aber bereits im 3. Quartal 2012 durchschritten worden.
Der Umsatzverlust hat sich bereits reduziert und diese Entwicklung wird in 2013 verstärkt weitergehen, insbesondere auch getrieben durch die Zulassung von Eliquis.
Nachdem Eliquis jetzt in allen wichtigen Märkten zugelassen ist, richten sich die Blicke nunmehr auf den zweiten großen Hoffnungsträger BMS-936558, der nunmehr als Nivolumab firmiert.
Inzwischen sind fünf P III-Studien am rekrutieren. Ergebnisse aus diesen Studien werden zwischen 2014 und 2016 erwartet:
http://clinicaltrials.gov/ct2/results/displayOpt?flds=a&flds…
Zur ASCO 2013 wird es weitere P I / II - Daten geben.
Inzwischen sind fünf P III-Studien am rekrutieren. Ergebnisse aus diesen Studien werden zwischen 2014 und 2016 erwartet:
http://clinicaltrials.gov/ct2/results/displayOpt?flds=a&flds…
Zur ASCO 2013 wird es weitere P I / II - Daten geben.
Ja, das kommt jetzt in die interessante Phase. Der Fokus auf Specialty Care wird immer klarer, dazu kommt ja auch der Ausstieg aus einigen PC und OTC Aktivitaeten. Wenn es auf dem ASCO interessantes gibt werde ich mir die entsprechenden Veranstaltungen sicher buchen. Allerdings wuerde ich nicht vor Mitte 2014 ernsthaft an einen Einstieg denken, zu viel Unsicherheit ueber einen zu langen Zeitraum...
Antwort auf Beitrag Nr.: 44.123.055 von Norbi2 am 09.02.13 19:37:18Allerdings wuerde ich nicht vor Mitte 2014 ernsthaft an einen Einstieg denken, zu viel Unsicherheit ueber einen zu langen Zeitraum...
Das kann ich nachvollziehen.
Ich gehe aber davon aus, dass inzwischen schon ein wenig BMS-936558 eingepreist ist und bis Mitte 2014 noch einiges mehr eingepreist sein wird (gute Daten natürlich vorausgesetzt).
Das ist sicher eine Frage der persönlichen Risikoneigung, wie viel Zukunft man vorweg nehmen will. Für mich wars im Sommer 2012 soweit - für Dich eventuell im Sommer 2014.
Für eine ordentliche Rendite kann aber wahrscheinlich auch ein Kauf im Sommer 2014 noch ausreichen, wenn schon ziemlich klar sein wird, ob und - wenn ja - wann BMS-936558 zugelassen werden könnte.
Das kann ich nachvollziehen.
Ich gehe aber davon aus, dass inzwischen schon ein wenig BMS-936558 eingepreist ist und bis Mitte 2014 noch einiges mehr eingepreist sein wird (gute Daten natürlich vorausgesetzt).
Das ist sicher eine Frage der persönlichen Risikoneigung, wie viel Zukunft man vorweg nehmen will. Für mich wars im Sommer 2012 soweit - für Dich eventuell im Sommer 2014.
Für eine ordentliche Rendite kann aber wahrscheinlich auch ein Kauf im Sommer 2014 noch ausreichen, wenn schon ziemlich klar sein wird, ob und - wenn ja - wann BMS-936558 zugelassen werden könnte.
Hammer heute mit einem recht grundsätzlichen Text zu Entwicklungen im "war on cancer":
http://www.orf-blog.com/major-trends-in-cancer-drug-developm…
Auszüge, die Nivolumab betreffen:
(Ich wiederhole mich, aber ich habe die begründete Hoffnung, dass sich hier ein wirklich ganz großer Schritt im Kampf gegen den Krebs ankündigt. Wenn die Entwicklungen im Bereich PD-1 halten können, was sie bisher versprechen, wäre das für viele Patienten in vielen verschiedenen Krebsindikationen ein Durchbruch, der dann - zu recht - auch den Investoren entsprechender Unternehmen zugute kommen würde.) Da mit PD-1-mabs solide Tumore verschiedenster Art adressiert werden können, könnte das Umsatzpotential ganz außerordentlich groß sein. Vielleicht sollte Roche darüber nachdenken, BMS zu übernehmen...
The PD-1 race
The first plenary session focused on drugs that inhibit PD-1 signaling. This class of drugs is probably the most exciting thing in oncology, based on unprecedented results across several tumor types. At the session, it was interesting to see the various approaches pursued by different companies in terms of choice of therapeutic agent and clinical strategy.
...
4 PD1 programs were presented: BMS’s (BMY) nivolumab (anti-PD1), Merck’s (MRK) MK-3475 (anti-PD1), Genentech’s MPDL3280A (anti PD-L1) and GSK (GSK)/Amplimmune’s AMP-224 (PD-L2-Fc fusion). The first 2 are in late stage development based on positive phase I data. The other two are still in phase I and initial data are expected this year.
David Feltquate (BMS) reviewed published results with nivolumab (BMS-936558), a fully human PD1 antibody. Nivolumab demonstrated remarkable activity in melanoma, lung and renal cancer, which I previously discussed in my ASCO 2012 summary. Dr. Feltquate emphasized the depth of responses (many responders are progression free after 2 years) and the unusual kinetics of response (some patients respond after initial alleged progression, some maintain long term responses after very short exposure to the drug).
Alexander Eggermont (Institut Gustave Roussy) presented phase I data for MK-3475, a humanized anti-PD1 in melanoma. Response rate was very high (51%) and appeared numerically better than that observed with nivolumab. As this trial started in 2011, many of the patients had been treated with Yervoy, another immune checkpoint antibody. Response rate in this subpopulation was still quite high (41%), which proves that failure on one form of immunotherapy does preclude benefit with another immunotherapy. Durability of responses also appeared very high, similarly to that seen with nivolumab.
...
Everybody agrees that results with both nivolumab and MK-3475 are one of the most impressive data sets ever seen with a single drug in solid tumors. The safety profile was also remarkably good, substantially better than that associated with other immunotherapies (Yervoy, high dose IL-2).
When asked whether PD-1 antibodies are going to cure metastatic melanoma, Michael Atkins replied: “Yes, in 25% of patients”. To put this in context, patients with metastatic melanoma have a life expectancy of 11-13 months with recently approved drugs (Zelboraf, Yervoy). To date, curing these patients has been either impossible or required complicated, expensive and toxic treatments (e.g. IL-2, TILs). For the first time, patients have a 25% chance of long term remissions with a safe drug that can be simply injected to them at every clinic.
My take home message from this session is that BMS is still well positioned with nivolumab, which has the most comprehensive data set and development program (5 phase III trials). Merck’s MK-3475 looks very active and numerically better (in melanoma) and is not too far behind with a very large (500 patients) randomized phase II trial. The trial is evaluating 2 dosing regimens of MK-3475 vs. chemotherapy and might enable accelerated approval.
Genentech’s and GSK’s approaches, although scientifically interesting, are both 1-2 years behind with products that might be safer but could also be inferior in terms of clinical benefit. Given the indications are fatal cancers and the benign safety profile seen with nivolumab and MK-3475, it will be hard to beat nivolumab or MK-3475 based on a better safety profile.
------------------
http://www.orf-blog.com/major-trends-in-cancer-drug-developm…
Auszüge, die Nivolumab betreffen:
(Ich wiederhole mich, aber ich habe die begründete Hoffnung, dass sich hier ein wirklich ganz großer Schritt im Kampf gegen den Krebs ankündigt. Wenn die Entwicklungen im Bereich PD-1 halten können, was sie bisher versprechen, wäre das für viele Patienten in vielen verschiedenen Krebsindikationen ein Durchbruch, der dann - zu recht - auch den Investoren entsprechender Unternehmen zugute kommen würde.) Da mit PD-1-mabs solide Tumore verschiedenster Art adressiert werden können, könnte das Umsatzpotential ganz außerordentlich groß sein. Vielleicht sollte Roche darüber nachdenken, BMS zu übernehmen...
The PD-1 race
The first plenary session focused on drugs that inhibit PD-1 signaling. This class of drugs is probably the most exciting thing in oncology, based on unprecedented results across several tumor types. At the session, it was interesting to see the various approaches pursued by different companies in terms of choice of therapeutic agent and clinical strategy.
...
4 PD1 programs were presented: BMS’s (BMY) nivolumab (anti-PD1), Merck’s (MRK) MK-3475 (anti-PD1), Genentech’s MPDL3280A (anti PD-L1) and GSK (GSK)/Amplimmune’s AMP-224 (PD-L2-Fc fusion). The first 2 are in late stage development based on positive phase I data. The other two are still in phase I and initial data are expected this year.
David Feltquate (BMS) reviewed published results with nivolumab (BMS-936558), a fully human PD1 antibody. Nivolumab demonstrated remarkable activity in melanoma, lung and renal cancer, which I previously discussed in my ASCO 2012 summary. Dr. Feltquate emphasized the depth of responses (many responders are progression free after 2 years) and the unusual kinetics of response (some patients respond after initial alleged progression, some maintain long term responses after very short exposure to the drug).
Alexander Eggermont (Institut Gustave Roussy) presented phase I data for MK-3475, a humanized anti-PD1 in melanoma. Response rate was very high (51%) and appeared numerically better than that observed with nivolumab. As this trial started in 2011, many of the patients had been treated with Yervoy, another immune checkpoint antibody. Response rate in this subpopulation was still quite high (41%), which proves that failure on one form of immunotherapy does preclude benefit with another immunotherapy. Durability of responses also appeared very high, similarly to that seen with nivolumab.
...
Everybody agrees that results with both nivolumab and MK-3475 are one of the most impressive data sets ever seen with a single drug in solid tumors. The safety profile was also remarkably good, substantially better than that associated with other immunotherapies (Yervoy, high dose IL-2).
When asked whether PD-1 antibodies are going to cure metastatic melanoma, Michael Atkins replied: “Yes, in 25% of patients”. To put this in context, patients with metastatic melanoma have a life expectancy of 11-13 months with recently approved drugs (Zelboraf, Yervoy). To date, curing these patients has been either impossible or required complicated, expensive and toxic treatments (e.g. IL-2, TILs). For the first time, patients have a 25% chance of long term remissions with a safe drug that can be simply injected to them at every clinic.
My take home message from this session is that BMS is still well positioned with nivolumab, which has the most comprehensive data set and development program (5 phase III trials). Merck’s MK-3475 looks very active and numerically better (in melanoma) and is not too far behind with a very large (500 patients) randomized phase II trial. The trial is evaluating 2 dosing regimens of MK-3475 vs. chemotherapy and might enable accelerated approval.
Genentech’s and GSK’s approaches, although scientifically interesting, are both 1-2 years behind with products that might be safer but could also be inferior in terms of clinical benefit. Given the indications are fatal cancers and the benign safety profile seen with nivolumab and MK-3475, it will be hard to beat nivolumab or MK-3475 based on a better safety profile.
------------------
Die Aktie ist gestern bei hohen Umsätzen auf über 40 Dollar gestiegen.
Bei aller Wertschätzung finde ich das für Anfang 2013 etwas zu viel. Ich weiß auch nicht, woher die extrem gute Stimmung kommt.
Läuft die Markteinführung von Eliquis besser als gedacht? Wird jetzt doch schon Nivolumab massiv eingepreist?
Was auch immer - Eliquis und Nivolumab brauchen ihre Zeit. Der Markt nimmt jetzt schon sehr viel vorweg.
Die Analysten beginnen nun jedenfalls auch langsam, das Potential von Nivolumab zu erkennen:
Goldman believes Bristol-Myers earnings could double ty the end of the decade given by PD-1 contribution. The firm believes investors are underestimating PD-1 and that peak sales could reach $7B by 2025. The firm reiterates its Conviction Buy rating and raised its price target to $44 from $40.
(Persönlich glaube ich, dass Nivolumab nicht bis 2025 braucht, um 7 Mrd. Umsatz zu machen und die Peak Sales könnten auch einiges über 7 Mrd. liegen - dennoch, Zeit braucht man natürlich schon und man muss jetzt nicht schon zu viel im Kurs vorwegnehmen.)
Bei aller Wertschätzung finde ich das für Anfang 2013 etwas zu viel. Ich weiß auch nicht, woher die extrem gute Stimmung kommt.
Läuft die Markteinführung von Eliquis besser als gedacht? Wird jetzt doch schon Nivolumab massiv eingepreist?
Was auch immer - Eliquis und Nivolumab brauchen ihre Zeit. Der Markt nimmt jetzt schon sehr viel vorweg.
Die Analysten beginnen nun jedenfalls auch langsam, das Potential von Nivolumab zu erkennen:
Goldman believes Bristol-Myers earnings could double ty the end of the decade given by PD-1 contribution. The firm believes investors are underestimating PD-1 and that peak sales could reach $7B by 2025. The firm reiterates its Conviction Buy rating and raised its price target to $44 from $40.
(Persönlich glaube ich, dass Nivolumab nicht bis 2025 braucht, um 7 Mrd. Umsatz zu machen und die Peak Sales könnten auch einiges über 7 Mrd. liegen - dennoch, Zeit braucht man natürlich schon und man muss jetzt nicht schon zu viel im Kurs vorwegnehmen.)
Analystenerwartungen fürs Q1:
(morgen können wir dann mit der Realität vergleichen)
Analysts have become increasingly bearish on Bristol Myers Squibb (BMY) in the month leading up to the company’s first quarter earnings announcement scheduled for Thursday, April 25, 2013. The consensus analyst estimate has moved from 43 cents a share to the current prediction of earnings of 41 cents a share.
The current estimate reflects a 35.9% decrease from the year-ago quarter, when Bristol Myers reported earnings of 64 cents per share.
The consensus estimate has fallen over the past three months, from 43 cents. Analysts are expecting earnings of $1.83 per share for the fiscal year. A year after being $5.25 billion, analysts expect revenue to fall 26.1% year-over-year to $3.88 billion for the quarter. For the year, revenue is expected to come in at $16.54 billion.
(morgen können wir dann mit der Realität vergleichen)
Analysts have become increasingly bearish on Bristol Myers Squibb (BMY) in the month leading up to the company’s first quarter earnings announcement scheduled for Thursday, April 25, 2013. The consensus analyst estimate has moved from 43 cents a share to the current prediction of earnings of 41 cents a share.
The current estimate reflects a 35.9% decrease from the year-ago quarter, when Bristol Myers reported earnings of 64 cents per share.
The consensus estimate has fallen over the past three months, from 43 cents. Analysts are expecting earnings of $1.83 per share for the fiscal year. A year after being $5.25 billion, analysts expect revenue to fall 26.1% year-over-year to $3.88 billion for the quarter. For the year, revenue is expected to come in at $16.54 billion.
BMS die Zahlen zum ersten Quartal 2013 gemeldet. Mit 41 Cent pro Aktien (non-GAAP) liegen sie ungefähr bei den Erwartungen des Marktes. Umsatzentwicklung war aber etwas mau.
Durch den Plavix-Verlust hat sich eine Umsatzeinbuße in Höhe von 27% im Vergleich zum Q1/2012 ergeben. Unter Ausklammerung von Plavix und Avapro ist BMS mit 10% gewachsen.
Die Umsatzentwicklung der „Hoffnungsträger“ war im ersten Quartal verhalten. Bydureon finde ich besonders enttäuschend. Yveroy sieht stark aus, ist aber durch einen Buchungseffet deutlich überzeichnet.
Insgesamt ein recht schwaches Quartal.
Bin gespannt, welche Dynamik Eliquis in den nächsten Quartalen zeigen wird. Ist jetzt eben ein Marketingkrieg.
Im Einzelnen:
Umsatzverlust durch Patentabläufe bis Q1/13: -1.763
Was konnte davon bisher durch „Hoffnungsträger“ kompensiert werden?
Umsatzgewinne durch “Hoffnungsträger” bis Q1/13: +338
Im Saldo sind damit nach dieser Rechnung von Q1/2012 bis Q1/2013 auf Quartalsbasis immer noch 1,425 Milliarden Dollar Umsatzausfall zu beklagen.
Was sonst wichtig war:
Nivolumab hat die fast track designation der FDA erhalten, so dass es nach Abschluss der PIII-Studien eine zügige Entscheidung geben wird, ob das Produkt zugelassen wird.
Im HCV-Bereich gibt man nicht auf. Ein eigenes „triple direct-acting antiviral treatment regime hat gute PII-Ergebnisse gezeigt und zwar ohne Interferon und(!) ohne Ribavirin-Beigabe.
Zwar ist Gilead der unumstrittene Platzhirsch im HCV-Segment, aber eine „ribavirin-free therapie“ wäre ein nicht zu unterschätzendes Plus für BMS. PIII soll noch in diesem Jahr beginnen.
Durch den Plavix-Verlust hat sich eine Umsatzeinbuße in Höhe von 27% im Vergleich zum Q1/2012 ergeben. Unter Ausklammerung von Plavix und Avapro ist BMS mit 10% gewachsen.
Die Umsatzentwicklung der „Hoffnungsträger“ war im ersten Quartal verhalten. Bydureon finde ich besonders enttäuschend. Yveroy sieht stark aus, ist aber durch einen Buchungseffet deutlich überzeichnet.
Insgesamt ein recht schwaches Quartal.
Bin gespannt, welche Dynamik Eliquis in den nächsten Quartalen zeigen wird. Ist jetzt eben ein Marketingkrieg.
Im Einzelnen:
Medikament Q1/2012 Q2/2012 Q3/2012 Q4/2012 Q1/2013
Avapro 207 117 95 84 46
Plavix 1.693 741 64 49 91
Gesamt 1.900 858 159 133 137
Umsatzverlust durch Patentabläufe bis Q1/13: -1.763
Was konnte davon bisher durch „Hoffnungsträger“ kompensiert werden?
Medikament Q1/2012 Q2/2012 Q3/2012 Q4/2012 Q1/2013
Baraclude 325 357 346 360 366
Erbitux 179 179 173 171 162
Sprycel 231 244 263 281 287
Yveroy 154 162 179 211 229
Orencia 254 290 307 325 320
Onglyza 161 172 178 198 202
Eliquis 0 1 0 1 22
Bydureon 0 0 20 58 52
Forxiga 0 0 0 0 3
Gesamt 1.305 1.405 1.466 1.605 1.643
Umsatzgewinne durch “Hoffnungsträger” bis Q1/13: +338
Im Saldo sind damit nach dieser Rechnung von Q1/2012 bis Q1/2013 auf Quartalsbasis immer noch 1,425 Milliarden Dollar Umsatzausfall zu beklagen.
Was sonst wichtig war:
Nivolumab hat die fast track designation der FDA erhalten, so dass es nach Abschluss der PIII-Studien eine zügige Entscheidung geben wird, ob das Produkt zugelassen wird.
Im HCV-Bereich gibt man nicht auf. Ein eigenes „triple direct-acting antiviral treatment regime hat gute PII-Ergebnisse gezeigt und zwar ohne Interferon und(!) ohne Ribavirin-Beigabe.
Zwar ist Gilead der unumstrittene Platzhirsch im HCV-Segment, aber eine „ribavirin-free therapie“ wäre ein nicht zu unterschätzendes Plus für BMS. PIII soll noch in diesem Jahr beginnen.
BMS testet jetzt Nivolumab in Kombination mit Yervoy beim Melanom in einer neuen P III:
http://www.clinicaltrials.gov/ct2/show/NCT01844505?term=NCT0…
Damit laufen jetzt 6 P III-Studien parallel.
Zur ASCO gibt es P I-Daten der Nivolumab/Yervoy-Kombi. Da gerade eine P III gestartet wurde, kann man da wohl mit positiven Daten rechnen.
http://www.clinicaltrials.gov/ct2/show/NCT01844505?term=NCT0…
Damit laufen jetzt 6 P III-Studien parallel.
Zur ASCO gibt es P I-Daten der Nivolumab/Yervoy-Kombi. Da gerade eine P III gestartet wurde, kann man da wohl mit positiven Daten rechnen.
Bloomberg zur neuen Wirkstoffklasse PD(L)-1:
(meiner Meinung nach wird die gesamte Wirkstoffklasse auf lange Sicht einen Umsatz von weit jenseits der 10 Milliarden Dollar Marke generieren. BMS wird sich einen fetten Anteil sichern, wahrscheinlich den Löwenanteil.
Nebenbei bemerkt: Nie sind Aktionäre schlimmer betrogen worden, als bei dem billigen Aufkauf von Medarex durch BMS für ein paar Pfennige. Denn wie der baldige Blockbuster Yervoy ist auch Nivolumab ein Medarex-Produkt.)
Human Immune-Boosting Cancer Drugs Seen Extending Lives
By Robert Langreth - May 13
Merck & Co. (MRK), Bristol-Myers Squibb Co. (BMY) and Roche Holding AG (ROG) have opened a new front against cancer with the next generation of experimental drugs that use the human immune system to seek and destroy tumor cells.
The new therapies have the potential to reap billions of dollars in sales while lengthening patient remissions, said doctors and analysts awaiting study results to be released this week as part of the American Society of Clinical Oncology meeting that starts May 31.
Building on the success of Bristol-Myers’ Yervoy drug for melanoma that reached the market in 2011, drugmakers are devising more potent immune therapies or combining treatments for maximum effectiveness. They are also testing the new medicines in more types of cancers, including lung and breast.
If the new generation of immune therapies lives up to its promise, “this is going to be a paradigm shift for treating cancer,” said Merck senior vice president Gary Gilliland in an interview. “We are pretty good at shrinking tumors, but not good at getting rid of them. Immune therapy is a way to begin to approach that.”
It will take at least a year before scientists will be able to determine whether the new drugs can extend lives. Still, the strategy offers scientists the first major new avenue for attacking cancer in a decade. In recent years, researchers have focused on treatments that targeted specific genetic processes that create uncontrolled cell growth. While this approach produced some successes, advanced tumors are often able to evade attack within a few months by producing mutations.
‘Fair Fight’
Now many believe that by strengthening the immune system’s ability to identify and kill cancer cells, they can broaden the attack so it will fight any dangerous malignancy.
“You’re setting up a fair fight” with the disease, said Nils Lonberg, a senior vice president at Bristol-Myers, in a telephone interview. “The immune system is just as adaptable as the cancer.”
The financial stakes are high. The targeted drug Avastin, for instance, made by Roche, had $5.8 billion in sales last year for use against colon and other tumors. Nivolumab, a new immune-boosting cancer drug candidate that Bristol-Myers is developing could generate an “Avastin-like sales number, maybe even better,” if it’s found to work in lung cancer, said Tony Butler, a New York-based analyst at Barclays Plc (BARC), by phone.
Off Switch
The new drugs are designed to prevent flicking what is essentially an off switch, called PD-1, for immune system T-cells, the body’s key defenders against attacks from dangerous germs, infections or other biological bad guys.
“Tumors have this Harry Potter cloak of invisibility,” Merck’s Gilliland said. The new class of drugs “lifts the cloaking device and allows the immune system to attack.”
Yervoy from Bristol-Myers was the first drug proven to extend survival in advanced melanoma, the most deadly form of skin cancer.
Data presented in September on the medicine showed it doubled the number of patients that survived four years to 19 percent. Now, the New York-based drugmaker is seeing positive early results from nivolumab, a new experimental immune therapy for skin, lung and kidney tumors.
In June of last year, Bristol-Myers reported that nivolumab shrank tumors in people with advanced lung, kidney and skin cancer in 18 percent to 28 percent of patients who had failed on other treatments.
So far, nivolumab is leading the next wave of immune-boosting cancer medicines with six final-stage trials in 3,300 patients with lung cancer, kidney tumors and melanoma.
Close Competition
At least six other companies are testing immune therapy drugs in patients with advanced cancer, said Jedd Wolchok director of immunotherapy at the Ludwig Center at Memorial Sloan-Kettering Cancer Center in New York. Wolchok’s team is testing seven treatments from six companies in 10 trials, up from just two drugs five years ago, he said in an interview.
“A couple of years ago the big story was that immunotherapy can work,” he said, referring to Yervoy’s initial success. ‘Now immunotherapy has entered the mainstream.”
Merck, of Whitehouse Station, New Jersey, is in second-stage testing on its immune therapy, lambrolizumab, in melanoma. If successful, the 500-patient trial may be large enough to gain approval from U.S. regulators without completing the usual required third-stage of testing, putting it in a virtual dead heat with Bristol’s nivolumab in melanoma.
Merck Success
In November, doctors at a melanoma research conference presented early data showing lambrolizumab shrank tumors in 51 percent of patients with advanced skin tumors. Merck has also begun studies of its drug in cancers of the lung, head and neck, and breast.
The best way to keep the PD-1 system from turning off immune cells is not yet clear, which is why the upcoming trial results are so important. In addition, both Bristol-Myers and Merck compounds have shown side effects including the potential for lung inflammation in some cases.
Meanwhile, a medicine under development at the Basel, Switzerland drugmaker Roche targets a related protein on tumor cells called PD-L1, which the company believes may be a safer approach. Results from an initial trial of the drug in patients with various types of advanced cancer will be presented at the meeting. Based on the results to date, Roche plans to begin a final-stage study of its drug in lung cancer.
Patient Demand
Merck’s Gilliland said patient demand for the PD-1 drugs is so intense that the company had to step up the pace of opening trial sites. Some patients with advanced melanoma have skipped over approved treatments to jump right to Merck’s experimental drug, he said.
The enthusiasm is understandable because immune drugs hold the promise of producing long-lasting remissions, said Michael Gordon, director of research at Pinnacle Oncology Hematology, in Scottsdale, Arizona, who has been involved in testing the Roche drug.
Cancer doctors “are accustomed to moving from one therapy to another” as tumors rapidly develop resistance,” he said in a telephone interview. “It is generating tremendous excitement to have a drug class that might well be able to provide long term control of metastatic cancer.”
(meiner Meinung nach wird die gesamte Wirkstoffklasse auf lange Sicht einen Umsatz von weit jenseits der 10 Milliarden Dollar Marke generieren. BMS wird sich einen fetten Anteil sichern, wahrscheinlich den Löwenanteil.
Nebenbei bemerkt: Nie sind Aktionäre schlimmer betrogen worden, als bei dem billigen Aufkauf von Medarex durch BMS für ein paar Pfennige. Denn wie der baldige Blockbuster Yervoy ist auch Nivolumab ein Medarex-Produkt.)
Human Immune-Boosting Cancer Drugs Seen Extending Lives
By Robert Langreth - May 13
Merck & Co. (MRK), Bristol-Myers Squibb Co. (BMY) and Roche Holding AG (ROG) have opened a new front against cancer with the next generation of experimental drugs that use the human immune system to seek and destroy tumor cells.
The new therapies have the potential to reap billions of dollars in sales while lengthening patient remissions, said doctors and analysts awaiting study results to be released this week as part of the American Society of Clinical Oncology meeting that starts May 31.
Building on the success of Bristol-Myers’ Yervoy drug for melanoma that reached the market in 2011, drugmakers are devising more potent immune therapies or combining treatments for maximum effectiveness. They are also testing the new medicines in more types of cancers, including lung and breast.
If the new generation of immune therapies lives up to its promise, “this is going to be a paradigm shift for treating cancer,” said Merck senior vice president Gary Gilliland in an interview. “We are pretty good at shrinking tumors, but not good at getting rid of them. Immune therapy is a way to begin to approach that.”
It will take at least a year before scientists will be able to determine whether the new drugs can extend lives. Still, the strategy offers scientists the first major new avenue for attacking cancer in a decade. In recent years, researchers have focused on treatments that targeted specific genetic processes that create uncontrolled cell growth. While this approach produced some successes, advanced tumors are often able to evade attack within a few months by producing mutations.
‘Fair Fight’
Now many believe that by strengthening the immune system’s ability to identify and kill cancer cells, they can broaden the attack so it will fight any dangerous malignancy.
“You’re setting up a fair fight” with the disease, said Nils Lonberg, a senior vice president at Bristol-Myers, in a telephone interview. “The immune system is just as adaptable as the cancer.”
The financial stakes are high. The targeted drug Avastin, for instance, made by Roche, had $5.8 billion in sales last year for use against colon and other tumors. Nivolumab, a new immune-boosting cancer drug candidate that Bristol-Myers is developing could generate an “Avastin-like sales number, maybe even better,” if it’s found to work in lung cancer, said Tony Butler, a New York-based analyst at Barclays Plc (BARC), by phone.
Off Switch
The new drugs are designed to prevent flicking what is essentially an off switch, called PD-1, for immune system T-cells, the body’s key defenders against attacks from dangerous germs, infections or other biological bad guys.
“Tumors have this Harry Potter cloak of invisibility,” Merck’s Gilliland said. The new class of drugs “lifts the cloaking device and allows the immune system to attack.”
Yervoy from Bristol-Myers was the first drug proven to extend survival in advanced melanoma, the most deadly form of skin cancer.
Data presented in September on the medicine showed it doubled the number of patients that survived four years to 19 percent. Now, the New York-based drugmaker is seeing positive early results from nivolumab, a new experimental immune therapy for skin, lung and kidney tumors.
In June of last year, Bristol-Myers reported that nivolumab shrank tumors in people with advanced lung, kidney and skin cancer in 18 percent to 28 percent of patients who had failed on other treatments.
So far, nivolumab is leading the next wave of immune-boosting cancer medicines with six final-stage trials in 3,300 patients with lung cancer, kidney tumors and melanoma.
Close Competition
At least six other companies are testing immune therapy drugs in patients with advanced cancer, said Jedd Wolchok director of immunotherapy at the Ludwig Center at Memorial Sloan-Kettering Cancer Center in New York. Wolchok’s team is testing seven treatments from six companies in 10 trials, up from just two drugs five years ago, he said in an interview.
“A couple of years ago the big story was that immunotherapy can work,” he said, referring to Yervoy’s initial success. ‘Now immunotherapy has entered the mainstream.”
Merck, of Whitehouse Station, New Jersey, is in second-stage testing on its immune therapy, lambrolizumab, in melanoma. If successful, the 500-patient trial may be large enough to gain approval from U.S. regulators without completing the usual required third-stage of testing, putting it in a virtual dead heat with Bristol’s nivolumab in melanoma.
Merck Success
In November, doctors at a melanoma research conference presented early data showing lambrolizumab shrank tumors in 51 percent of patients with advanced skin tumors. Merck has also begun studies of its drug in cancers of the lung, head and neck, and breast.
The best way to keep the PD-1 system from turning off immune cells is not yet clear, which is why the upcoming trial results are so important. In addition, both Bristol-Myers and Merck compounds have shown side effects including the potential for lung inflammation in some cases.
Meanwhile, a medicine under development at the Basel, Switzerland drugmaker Roche targets a related protein on tumor cells called PD-L1, which the company believes may be a safer approach. Results from an initial trial of the drug in patients with various types of advanced cancer will be presented at the meeting. Based on the results to date, Roche plans to begin a final-stage study of its drug in lung cancer.
Patient Demand
Merck’s Gilliland said patient demand for the PD-1 drugs is so intense that the company had to step up the pace of opening trial sites. Some patients with advanced melanoma have skipped over approved treatments to jump right to Merck’s experimental drug, he said.
The enthusiasm is understandable because immune drugs hold the promise of producing long-lasting remissions, said Michael Gordon, director of research at Pinnacle Oncology Hematology, in Scottsdale, Arizona, who has been involved in testing the Roche drug.
Cancer doctors “are accustomed to moving from one therapy to another” as tumors rapidly develop resistance,” he said in a telephone interview. “It is generating tremendous excitement to have a drug class that might well be able to provide long term control of metastatic cancer.”
Die Yervoy/Nivolumab-Kombi hat zur ASCO hervorragende (vorläufige) Daten beim Melanom geliefert.
Nivolumab ist jetzt bereits merklich im Aktienkurs eingepreist. Um einiges früher, als ich mal gedacht habe.
Melanoma Treatment Harnesses Immune System to Combat Cancer Cells
By ANDREW POLLACK
Cancer researchers are growing increasingly enthusiastic about harnessing the body’s own immune system to fight tumors. And new research shows that two drugs that use this approach may be even better than one.
Researchers reported on Wednesday that a combination of two drugs from Bristol-Myers Squibb shrank tumors significantly in about 41 percent of patients with advanced melanoma in a small study. In few of the 52 patients in the study, tumors disappeared completely, at least as could be determined by imaging.
“I think it was really the rapidity and the magnitude of the responses that was impressive to us,” Dr. Jedd D. Wolchok of the Memorial Sloan-Kettering Cancer Center, said in a telephone news conference organized by the American Society of Clinical Oncology.
Dr. Wolchok’s study, and others on the immune system drugs, will be perhaps the most closely watched items at the society’s annual meeting, which begins on May 31 in Chicago.
The drugs are also generating huge interest on Wall Street, which projects billions of dollars in annual sales. While Bristol is generally considered to have a lead, Merck and Roche are not far behind with similar drugs.
Data released Wednesday from an early-stage study of Roche’s drug, which is known as MPDL3280A, showed significant tumor shrinkage in 21 percent of 140 patients who had a variety of cancers including lung, melanoma and kidney cancer.
The studies are small and they did not compare the drugs with a placebo or with another treatment, and it is unclear if they will lengthen lives. Moreover, it is unclear how long the effects will last, though there are signs that for many patients, it could be a year or more.
Cancer cells often successfully hide from the body’s immune system by preventing T-cells from attacking them. The new drugs basically work by disabling brakes on the immune system, allowing the T-cells to attack the tumors.
One of the drugs in Bristol-Myers’ combination is Yervoy, which was approved as a treatment for melanoma in 2011. Yervoy disables an immune system brake called CTLA-4. It shrinks tumors in only about 10 percent of patients, but the effects can last for a long time.
The other drug in its combination is nivolumab, which is not yet on the market. It disables a brake known as PD-1, which sits on the surface of T-cells. Tumors can produce a protein called PD-L1, which binds to PD-1 and makes the T-cells inactive.
Nivolumab, and the drug being developed by Merck, called MK-3475, are antibodies that bind to PD-1, while Roche’s drug binds to PD-L1. It is not clear yet which approach is better.
It may be possible to test tumors for the presence of PD-L1, and use the drugs mainly for those patients, where it is expected to work more effectively.
It is also not clear yet how many types of tumors the drugs will work for. All the companies are targeting melanoma, a deadly skin cancer, because there is evidence that it is sometimes controlled spontaneously by the immune system. The companies also have data for lung and kidney cancer. Roche’s study showed some effect in colorectal and head and neck cancer as well.
Bristol-Myers’s stock rose 5 percent on Wednesday, even though the results of the study were not released until 6 p.m., after the close of regular trading.
Mark Schoenebaum, the pharmaceutical analyst at ISI Group, said investors were hoping the combination of the two Bristol drugs would significantly shrink tumors in at least 50 percent of patients.
He said in a note on Wednesday that the overall shrinkage rate was perhaps a bit below expectations but added that for many patients, the shrinkage was more than 80 percent.
“The point is that the depth of those responses is pretty incredible,” he wrote.
Some experts say that tumor shrinkage, a measure that evaluates conventional chemotherapy drugs that poison cancer cells, may understate the effect of these new drugs.
“Sometimes it takes awhile for the immune system to be revved up,” said Dr. Gary Gilliland, who leads cancer drug development at Merck.
Nivolumab ist jetzt bereits merklich im Aktienkurs eingepreist. Um einiges früher, als ich mal gedacht habe.
Melanoma Treatment Harnesses Immune System to Combat Cancer Cells
By ANDREW POLLACK
Cancer researchers are growing increasingly enthusiastic about harnessing the body’s own immune system to fight tumors. And new research shows that two drugs that use this approach may be even better than one.
Researchers reported on Wednesday that a combination of two drugs from Bristol-Myers Squibb shrank tumors significantly in about 41 percent of patients with advanced melanoma in a small study. In few of the 52 patients in the study, tumors disappeared completely, at least as could be determined by imaging.
“I think it was really the rapidity and the magnitude of the responses that was impressive to us,” Dr. Jedd D. Wolchok of the Memorial Sloan-Kettering Cancer Center, said in a telephone news conference organized by the American Society of Clinical Oncology.
Dr. Wolchok’s study, and others on the immune system drugs, will be perhaps the most closely watched items at the society’s annual meeting, which begins on May 31 in Chicago.
The drugs are also generating huge interest on Wall Street, which projects billions of dollars in annual sales. While Bristol is generally considered to have a lead, Merck and Roche are not far behind with similar drugs.
Data released Wednesday from an early-stage study of Roche’s drug, which is known as MPDL3280A, showed significant tumor shrinkage in 21 percent of 140 patients who had a variety of cancers including lung, melanoma and kidney cancer.
The studies are small and they did not compare the drugs with a placebo or with another treatment, and it is unclear if they will lengthen lives. Moreover, it is unclear how long the effects will last, though there are signs that for many patients, it could be a year or more.
Cancer cells often successfully hide from the body’s immune system by preventing T-cells from attacking them. The new drugs basically work by disabling brakes on the immune system, allowing the T-cells to attack the tumors.
One of the drugs in Bristol-Myers’ combination is Yervoy, which was approved as a treatment for melanoma in 2011. Yervoy disables an immune system brake called CTLA-4. It shrinks tumors in only about 10 percent of patients, but the effects can last for a long time.
The other drug in its combination is nivolumab, which is not yet on the market. It disables a brake known as PD-1, which sits on the surface of T-cells. Tumors can produce a protein called PD-L1, which binds to PD-1 and makes the T-cells inactive.
Nivolumab, and the drug being developed by Merck, called MK-3475, are antibodies that bind to PD-1, while Roche’s drug binds to PD-L1. It is not clear yet which approach is better.
It may be possible to test tumors for the presence of PD-L1, and use the drugs mainly for those patients, where it is expected to work more effectively.
It is also not clear yet how many types of tumors the drugs will work for. All the companies are targeting melanoma, a deadly skin cancer, because there is evidence that it is sometimes controlled spontaneously by the immune system. The companies also have data for lung and kidney cancer. Roche’s study showed some effect in colorectal and head and neck cancer as well.
Bristol-Myers’s stock rose 5 percent on Wednesday, even though the results of the study were not released until 6 p.m., after the close of regular trading.
Mark Schoenebaum, the pharmaceutical analyst at ISI Group, said investors were hoping the combination of the two Bristol drugs would significantly shrink tumors in at least 50 percent of patients.
He said in a note on Wednesday that the overall shrinkage rate was perhaps a bit below expectations but added that for many patients, the shrinkage was more than 80 percent.
“The point is that the depth of those responses is pretty incredible,” he wrote.
Some experts say that tumor shrinkage, a measure that evaluates conventional chemotherapy drugs that poison cancer cells, may understate the effect of these new drugs.
“Sometimes it takes awhile for the immune system to be revved up,” said Dr. Gary Gilliland, who leads cancer drug development at Merck.
Die Wirkstoffklasse PD(L)-1 - und damit Nivolumab, als der am fortgeschrittenste Vertreter - wird wohl der Star der diesjährigen ASCO werden.
Analysten trauen sich jetzt langsam mit Prognosen zu künftigen Spitzenumsätzen heraus, die nun wirklich das gesamte Potential dieses Ansatzes abbilden. Das übertrifft sogar meine bisherigen Hoffnungen. Vor einem Jahr wäre man mit solchen Erwartungen wohl einfach für deppert erklärt worden:
Immune system cancer drugs tipped to be a $35 billion market
Ben HirschlerReuters
5:52 a.m. EDT, May 22, 2013
LONDON (Reuters) - A new wave of medicines that tap the power of the immune system to fight cancer could become the biggest drug class in history, with potential sales of $35 billion a year.
That bullish sales forecast by analysts at U.S. bank Citigroup highlights the growing excitement surrounding so-called immunotherapy after positive results from clinical trials conducted by companies such as Bristol-Myers Squibb and Roche Holding.
"We believe this market will generate sales of up to $35 billion (a year) over the next 10 years and be used in some way in the management of up to 60 percent of all cancers," Citi analyst Andrew Baum said on Wednesday.
Citi's forecast is considerably higher than current market consensus, but if it proves correct, then cancer immunotherapy would exceed the peak market value of top blockbuster drug classes such as statins for high cholesterol.
After years of puzzling over how to get the body's immune system to respond more effectively against tumor cells, scientists are now finding a number of promising avenues.
The new drugs are designed to target areas that act as brakes on the immune system. By interfering with these brakes, the drugs free the immune system to attack and kill cancer cells.
Bristol-Myers Squibb's nivolumab and Roche's MPDL3280A are two leading contenders in the field. Both had an impressive effect against a variety of cancers, according to preliminary trial results released last week.
Further details of the studies will be presented at a meeting of the American Society of Clinical Oncology in Chicago early next month.
MANAGEABLE CANCER
Conventional chemotherapy and other cancer drugs often have a powerful effect in shrinking tumors, but the effect is typically short-lived. The effect of immunotherapy can last much longer because the immune system has effectively been reset to remember how to keep fighting cancer cells.
Citigroup said that immunotherapy has the potential to transform a significant percentage of cancers into something akin to a chronic disease, in a similar way to how HIV drugs have made the viral disease a manageable condition.
On the back of its upbeat prediction for the immunotherapy market, Citigroup has upgraded shares in Bristol-Myers Squibb and Roche to "buy" from "neutral".
Roche stock was trading 1.7 percent higher by 0914 GMT (5.14 a.m. EDT), outperforming a flat European drugs sector.
Other leading players with a range of drugs, vaccines and cell therapy treatments in the cancer immunotherapy field include GlaxoSmithKline, AstraZeneca, Novartis, Merck & Co and Amgen.
In addition to the progress being made in research, analysts believe that the immunotherapy field could also benefit from a new U.S. Food and Drug Administration initiative to speed approval of important and innovative drugs.
The U.S. watchdog recently started a scheme to allow quicker studies of life-saving therapies designated as a "breakthrough", provided that clinical data is compelling.
(Editing by David Goodman)
Analysten trauen sich jetzt langsam mit Prognosen zu künftigen Spitzenumsätzen heraus, die nun wirklich das gesamte Potential dieses Ansatzes abbilden. Das übertrifft sogar meine bisherigen Hoffnungen. Vor einem Jahr wäre man mit solchen Erwartungen wohl einfach für deppert erklärt worden:
Immune system cancer drugs tipped to be a $35 billion market
Ben HirschlerReuters
5:52 a.m. EDT, May 22, 2013
LONDON (Reuters) - A new wave of medicines that tap the power of the immune system to fight cancer could become the biggest drug class in history, with potential sales of $35 billion a year.
That bullish sales forecast by analysts at U.S. bank Citigroup highlights the growing excitement surrounding so-called immunotherapy after positive results from clinical trials conducted by companies such as Bristol-Myers Squibb and Roche Holding.
"We believe this market will generate sales of up to $35 billion (a year) over the next 10 years and be used in some way in the management of up to 60 percent of all cancers," Citi analyst Andrew Baum said on Wednesday.
Citi's forecast is considerably higher than current market consensus, but if it proves correct, then cancer immunotherapy would exceed the peak market value of top blockbuster drug classes such as statins for high cholesterol.
After years of puzzling over how to get the body's immune system to respond more effectively against tumor cells, scientists are now finding a number of promising avenues.
The new drugs are designed to target areas that act as brakes on the immune system. By interfering with these brakes, the drugs free the immune system to attack and kill cancer cells.
Bristol-Myers Squibb's nivolumab and Roche's MPDL3280A are two leading contenders in the field. Both had an impressive effect against a variety of cancers, according to preliminary trial results released last week.
Further details of the studies will be presented at a meeting of the American Society of Clinical Oncology in Chicago early next month.
MANAGEABLE CANCER
Conventional chemotherapy and other cancer drugs often have a powerful effect in shrinking tumors, but the effect is typically short-lived. The effect of immunotherapy can last much longer because the immune system has effectively been reset to remember how to keep fighting cancer cells.
Citigroup said that immunotherapy has the potential to transform a significant percentage of cancers into something akin to a chronic disease, in a similar way to how HIV drugs have made the viral disease a manageable condition.
On the back of its upbeat prediction for the immunotherapy market, Citigroup has upgraded shares in Bristol-Myers Squibb and Roche to "buy" from "neutral".
Roche stock was trading 1.7 percent higher by 0914 GMT (5.14 a.m. EDT), outperforming a flat European drugs sector.
Other leading players with a range of drugs, vaccines and cell therapy treatments in the cancer immunotherapy field include GlaxoSmithKline, AstraZeneca, Novartis, Merck & Co and Amgen.
In addition to the progress being made in research, analysts believe that the immunotherapy field could also benefit from a new U.S. Food and Drug Administration initiative to speed approval of important and innovative drugs.
The U.S. watchdog recently started a scheme to allow quicker studies of life-saving therapies designated as a "breakthrough", provided that clinical data is compelling.
(Editing by David Goodman)
Hallo SLGRamann,
ich habe eine Frage, die hier ganz gut hinpasst. Hast du dir Ono Pharmaceuticals mal angesehen? Die könnten langfristig vielleicht sehr von Nivolumab profitieren. So wie ich das verstehe, hatten sie mal die weltweiten Rechte (außer USA/Medarex) und haben dann in einem Deal EU etc. an BMS abgegeben.
Halten aber noch die Rechte in Jp und anderen asiatischen Ländern. Zusätzlich bekommen sie Royalties für die Verkäufe in den abgegeben Territorien (?) und evtl. auch comarketing in gewissen Indikationen (?). So ganz schlau werde ich da aber nicht draus. Weißt du hier mehr?
Zusätzlich haben sie auch die Rechte an Kyprolis in Jp.
Natürlich ist die Pipeline auch insgesamt recht breit.
Ohad hammer hatte sich z.B. bzgl. des BTK Inhibitors recht positiv geaüßert:
Japan-based Ono will have results for its Btk inhibitor, which appears promising based on preliminary efficacy mentioned in the abstracts. In CLL, 7/10 patients had a nodal response including 2 PRs whereas 3/6 MCL patients experienced a PR. Even if Ono’s Btk inhibitor proves to be comparable to ibrutinib, it still trails in development by at least 2 years. As ibrutinib will become approved in all the major indications within 1-2 years, Ono will have to run comparative trials to demonstrate its drug’s non-inferiority. In some indications, these trials will take years to read out.
http://www.ono.co.jp/eng/investor/pdf/dp/20131112.pdf
http://www.ono.co.jp/eng/investor/pdf/ar/2013/07.pdf
SG kmastra
ich habe eine Frage, die hier ganz gut hinpasst. Hast du dir Ono Pharmaceuticals mal angesehen? Die könnten langfristig vielleicht sehr von Nivolumab profitieren. So wie ich das verstehe, hatten sie mal die weltweiten Rechte (außer USA/Medarex) und haben dann in einem Deal EU etc. an BMS abgegeben.
Halten aber noch die Rechte in Jp und anderen asiatischen Ländern. Zusätzlich bekommen sie Royalties für die Verkäufe in den abgegeben Territorien (?) und evtl. auch comarketing in gewissen Indikationen (?). So ganz schlau werde ich da aber nicht draus. Weißt du hier mehr?
Zusätzlich haben sie auch die Rechte an Kyprolis in Jp.
Natürlich ist die Pipeline auch insgesamt recht breit.
Ohad hammer hatte sich z.B. bzgl. des BTK Inhibitors recht positiv geaüßert:
Japan-based Ono will have results for its Btk inhibitor, which appears promising based on preliminary efficacy mentioned in the abstracts. In CLL, 7/10 patients had a nodal response including 2 PRs whereas 3/6 MCL patients experienced a PR. Even if Ono’s Btk inhibitor proves to be comparable to ibrutinib, it still trails in development by at least 2 years. As ibrutinib will become approved in all the major indications within 1-2 years, Ono will have to run comparative trials to demonstrate its drug’s non-inferiority. In some indications, these trials will take years to read out.
http://www.ono.co.jp/eng/investor/pdf/dp/20131112.pdf
http://www.ono.co.jp/eng/investor/pdf/ar/2013/07.pdf
SG kmastra
Antwort auf Beitrag Nr.: 46.023.748 von kmastra am 10.12.13 22:08:41Hallo kmastra,
ich denke, diese PM hilft schon weiter:
http://news.bms.com/press-release/partnering-news/bristol-my…
Für mich war damals entscheidend, dass BMS die wesentlichen Rechte inne haben. Mit ONO habe ich mich nicht weiter beschäftigt.
Mich würde aber Deine Einschätzung zu ONO interessieren. Vor allem Bewertung und Pipeline. Auf den ersten Blick scheinen sie sehr(!) teuer zu sein. KGV über 40? Inwiefern lässt sich das rechtfertigen?
Wie hoch ist die Marktkapitalisierung usw.?
Vielleicht machst Du da einen Thread auf? Oder gibt es schon einen?
Gruß
SLG
ich denke, diese PM hilft schon weiter:
http://news.bms.com/press-release/partnering-news/bristol-my…
Für mich war damals entscheidend, dass BMS die wesentlichen Rechte inne haben. Mit ONO habe ich mich nicht weiter beschäftigt.
Mich würde aber Deine Einschätzung zu ONO interessieren. Vor allem Bewertung und Pipeline. Auf den ersten Blick scheinen sie sehr(!) teuer zu sein. KGV über 40? Inwiefern lässt sich das rechtfertigen?
Wie hoch ist die Marktkapitalisierung usw.?
Vielleicht machst Du da einen Thread auf? Oder gibt es schon einen?
Gruß
SLG
bin jetzt auch mit ein paar Ansichtsstücken dabei
Sehr schöner Artikel zu den Entwicklungen im Bereich immunologischer Wirkstoffe bei Krebs:
http://www.orf-blog.com/immuno-oncology-key-themes-for-2014/
BMS ist ganz vorne mit dabei, vor allem auch bei den Kombitherapien, die die Szenerie prägen werden.
http://www.orf-blog.com/immuno-oncology-key-themes-for-2014/
BMS ist ganz vorne mit dabei, vor allem auch bei den Kombitherapien, die die Szenerie prägen werden.
wenn man auf fortschritte durch immunologische wirkstoffe bei krebs setzen will, bietet sich ein korb aus folgenden vier pharmakonzernen an:
bristol-myers sqibb
merck & co
roche
astra zeneca
bristol-myers sqibb
merck & co
roche
astra zeneca
Bristol-Myers Squibb Receives FDA Approval for Opdivo(nivolumab), the Only Treatment to Deliver Significant Overall Survival in Advanced Renal Cell Carcinoma vs. a Standard of Care, in Patients Who Have Received Prior Anti-Angiogenic Therapy1
www.wallstreet-online.de/nachricht/8148075-bristol-myers-squ…
www.wallstreet-online.de/nachricht/8148075-bristol-myers-squ…
Antwort auf Beitrag Nr.: 51.159.150 von Popeye82 am 24.11.15 07:13:02Mit dem Nivolumab haben sie einen absoluten Kracher gelandet...
Gegenüber Ipilimumab besser verträglich und wohl auch besser wirksam und bei mehr soliden Tumoren einsetzbar und bei immer mehr Tumoren mit Wirksamkeitsnachweis. Ich denke die Zulassung beim HCC/Leberkrebs und beim fortgeschritten metastasierten Kolonkarzinom mit Mikrosatelliteninstabilität wird auch kommen, letztlich also ein großer Markt.
Und mit den kommenden Zulassungen werden "hoffentlich" auch die Kosten für die Therapie sinken.
In D ca. 13000€ pro Infusion und das alle 3 Wochen, so ca. 150000€ pro Jahr derzeit. Und bei Wirksamkeit muss das ja nunmal fortgesetzt werden. Und plötzlich gibt es zB beim fortgeschrittenen Melanom Langzeitüberlebende von 40% nach 5 Jahren bei überschauberem NW-Profil!!!!!!!!
Ich komme mir beim Nivolumab und wahrscheinlich ja auch beim Pembrolizumab / Merck vor wie vor 4-5 Jahren, als die ersten Ergebnisse vom Sofosbuvir (Inhaltsstoff von Harvoni-Gilead) kamen, als klar wurde, dass die Hepatitis C plötzlich für praktisch alle heilbar wurde.
Ich warte mit dem Einstieg auf kleineren Rücksetzer. Na ja wenn er denn kommt.
Nepokong
Gegenüber Ipilimumab besser verträglich und wohl auch besser wirksam und bei mehr soliden Tumoren einsetzbar und bei immer mehr Tumoren mit Wirksamkeitsnachweis. Ich denke die Zulassung beim HCC/Leberkrebs und beim fortgeschritten metastasierten Kolonkarzinom mit Mikrosatelliteninstabilität wird auch kommen, letztlich also ein großer Markt.
Und mit den kommenden Zulassungen werden "hoffentlich" auch die Kosten für die Therapie sinken.
In D ca. 13000€ pro Infusion und das alle 3 Wochen, so ca. 150000€ pro Jahr derzeit. Und bei Wirksamkeit muss das ja nunmal fortgesetzt werden. Und plötzlich gibt es zB beim fortgeschrittenen Melanom Langzeitüberlebende von 40% nach 5 Jahren bei überschauberem NW-Profil!!!!!!!!
Ich komme mir beim Nivolumab und wahrscheinlich ja auch beim Pembrolizumab / Merck vor wie vor 4-5 Jahren, als die ersten Ergebnisse vom Sofosbuvir (Inhaltsstoff von Harvoni-Gilead) kamen, als klar wurde, dass die Hepatitis C plötzlich für praktisch alle heilbar wurde.
Ich warte mit dem Einstieg auf kleineren Rücksetzer. Na ja wenn er denn kommt.
Nepokong
Antwort auf Beitrag Nr.: 51.199.452 von Nepokong am 29.11.15 13:13:33
Hallo,
bist Du/Sie Arzt,Mediziner, oder Betroffener?
Hallo,
bist Du/Sie Arzt,Mediziner, oder Betroffener?
Antwort auf Beitrag Nr.: 51.202.194 von Popeye82 am 30.11.15 09:12:35hallo Popeye, zum Glück nicht Betroffener. Ne ich bin ersteres.
Nepokong
Nepokong
den Rücksetzter hab ich mal genutzt, längerfristig habe ich diese Aktie ganz gern im Depot.
Der Thread wurde im August 2012 eröffnet - bei einem Aktienkurs von 32 Dollar.
Auch nach dem jetzigen Knall steht noch eine Verdoppelung zu Buche.
Die erste Reaktion auf die Threaderöffnung von User "Norbi2" "lohnt" es nochmals zitiert zu werden:
"BMS ist hoch spekulativ. In den USA und Europa verlieren sie die Blockbuster die aus dem Patent laufen, in Emerging Markets sind sie nicht stark aufgestellt. Die Pipeline zeigt wenig hoffnungsvolle Kandidaten. Wie man hier von Koenig der Koenige sprechen kann ist schwer nachzuvollziehen. Eliquis wird hoechstens im Premiumbereich Fuss fassen, Plavix und seine Nachahmer haben sich zum Golden Standard entwickelt. Bisher sehen die Daten auch noch nicht so gut aus. Sollte sich BMS 936558 entwickeln wie geplant ergibt sich ein mittelfristiges Potential mit attraktiven Dimensionen. Allerdings ist das wohl auch schon eingepreist (KGV). Bei einem KGV ueber 17 wuerde ich jederzeit Roche, Sanofi und Novartis vorziehen. Alle drei stehen besser da, haben bessere Pipelines und eine gute Emerging Market Praesenz. Roche hat ebenso ein praesentes und potentielles Onkologieportfolio mit grossem Potential. Ebenso sind alle drei dividendenstark."
All diese Aussagen haben sich als Unsinn erwiesen.
Eliquis ist ein Blockbuster geworden.
Opdivo (wie es heute heißt) war natürlich keineswegs voll eingepreist.
Und wie ist der Vergleich zu den drei genannten "besseren Alternativen" ausgegangen?
So:
Mehr als sachlich-fachlichen Unsinn und später folgende Beschimpfungen hatte Norbi2 hier nicht zu bieten.
Auch nach dem jetzigen Knall steht noch eine Verdoppelung zu Buche.
Die erste Reaktion auf die Threaderöffnung von User "Norbi2" "lohnt" es nochmals zitiert zu werden:
"BMS ist hoch spekulativ. In den USA und Europa verlieren sie die Blockbuster die aus dem Patent laufen, in Emerging Markets sind sie nicht stark aufgestellt. Die Pipeline zeigt wenig hoffnungsvolle Kandidaten. Wie man hier von Koenig der Koenige sprechen kann ist schwer nachzuvollziehen. Eliquis wird hoechstens im Premiumbereich Fuss fassen, Plavix und seine Nachahmer haben sich zum Golden Standard entwickelt. Bisher sehen die Daten auch noch nicht so gut aus. Sollte sich BMS 936558 entwickeln wie geplant ergibt sich ein mittelfristiges Potential mit attraktiven Dimensionen. Allerdings ist das wohl auch schon eingepreist (KGV). Bei einem KGV ueber 17 wuerde ich jederzeit Roche, Sanofi und Novartis vorziehen. Alle drei stehen besser da, haben bessere Pipelines und eine gute Emerging Market Praesenz. Roche hat ebenso ein praesentes und potentielles Onkologieportfolio mit grossem Potential. Ebenso sind alle drei dividendenstark."
All diese Aussagen haben sich als Unsinn erwiesen.
Eliquis ist ein Blockbuster geworden.
Opdivo (wie es heute heißt) war natürlich keineswegs voll eingepreist.
Und wie ist der Vergleich zu den drei genannten "besseren Alternativen" ausgegangen?
So:
Einfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
Mehr als sachlich-fachlichen Unsinn und später folgende Beschimpfungen hatte Norbi2 hier nicht zu bieten.
Antwort auf Beitrag Nr.: 53.005.981 von HA_LUX am 06.08.16 21:31:39
Wieso Verdoppelung? Bauchgefühl oder womit begründet?
Zitat von HA_LUX: Der Thread wurde im August 2012 eröffnet - bei einem Aktienkurs von 32 Dollar.
Auch nach dem jetzigen Knall steht noch eine Verdoppelung zu Buche.
Wieso Verdoppelung? Bauchgefühl oder womit begründet?

ist doch wohl nicht so erstaunlich, dass bei bms nun bewertung abgebaut wird. vergleicht mal mit merck, roche, novartis und sanofi, auch wenn diese nicht alle voellig vergleichbar sind. im vergleich zu merck hat bms mit dem von der neuentwicklung zu addressierenden markt zu hoch gepokert und dabei verloren.
Antwort auf Beitrag Nr.: 53.014.813 von El_Matador am 08.08.16 18:24:57Das rechtfertigt aber wohl keinen Verlust von 20 mrd
Antwort auf Beitrag Nr.: 53.015.029 von darkwingduck am 08.08.16 18:55:40du redest von wert, ich von bewertung. eine bewertungsanpassung kann sich hier u.u. auf 20 mrd belaufen. schau dir doch mal das mond kgv von bms an. das ist quasi so als wenn merck & co und roche in onkologie gegenueber bms den anschluss verpasst haetten, haben sie aber nicht.
diesen Thread nicht historisch werden lassen...
heute TA Bastian Galuschka:https://www.godmode-trader.de/analyse/pharmawert-mit-guter-a…
So wie ich das sehe, gibt es (in den USA) zwei grosse onkologische Arzneimittel/Therapiekomplexe ("immunotherapies"):
(a) Merck mt Keytruda und
(b) BMS mit Opdivo
Die Idee hier wäre nun, dass eben nichts alles bei Opdivo für BMS verloren ist (was eben auch faktisch so ist), und sich weitere marktfähige Medikamente/Therapien für Opdivo finden lassen.
Und dass BMS beim klinischen Prozess bei Opdivo (Stichwort "Precision medicine") dazugelernt hat:
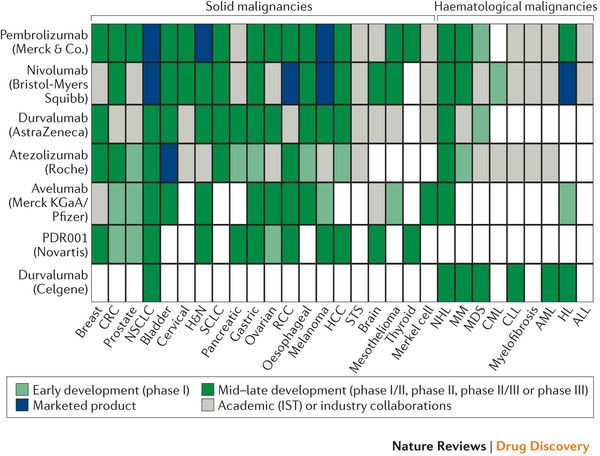
Hintergrund:
http://blogs.sciencemag.org/pipeline/archives/2016/10/10/key…
p.s.: bei Merck klappt auch nicht alles: https://www.reuters.com/article/us-merck-co-keytruda-idUSKBN…
Antwort auf Beitrag Nr.: 55.539.102 von faultcode am 16.08.17 20:40:05(die Ruhe hier zeigt mal wieder: das Publikum ist zu oft in den falschen Aktien  )
)
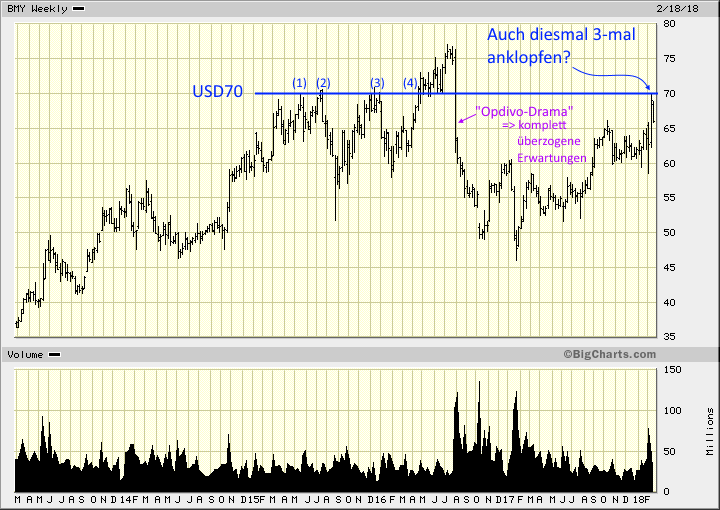
 )
)
Antwort auf Beitrag Nr.: 57.105.030 von faultcode am 23.02.18 18:46:213 Monate vergangen - und kein neuer Kommentar --> das ist sehr typisch für die deutsche "Biotech-Anlegerschaft"
=> dabei sieht es - nach 2016 und 2017 - zum ersten Mal wieder besser aus mMn -- so ganz allgemein bei derzeitiger Bewertung:
https://www.bloomberg.com/quote/BMY:US
Bloomberg PE = 15.85 <---> Ende 2017 nach FactSet ~100! => daher auch einige Alt-Angaben wie auf SA mit PE > 90....
=> das PEG ist ein bischen hoch für ein Schnäppchen:
Bloomberg (BEst) PEG Ratio = 1.7090
--> die Erwartungshaltung - in Summe - ist allseits gedämpft.
--> Cramer mag sie z.B. auch nicht z.Z.: 15.5.
https://www.cnbc.com/2018/05/15/cramers-lightning-round-take…
"Bristol-Myers right now does not have what we're looking for in terms of that cancer franchise that's going to Merck. I've got to tell you, down here, with a 3 percent yield, it gets attractive. But because of interest rates going higher, I'm going to have to take a pass for the moment."
--> ein Grund für den Crash im April: Pfizer mag (als Übernehmer) nicht mehr offensichtlich - oder das was der Markt davon denkt:
Why Bristol-Myers Squibb Stock Crashed in April
https://www.fool.com/investing/2018/05/09/why-bristol-myers-…
--> hier wird ernsthaft eine mögliche Übernahme durch Gilead Sciences erwogen:
Just because Pfizer isn't interested, though, doesn't necessarily mean that Bristol won't get taken out this year. Gilead Sciences, for instance, could very well emerge as a suitor due to the relatively slow start for its cellular immunotherapy endeavor and accelerating declines in its hep C franchise. Gilead also has the financial capacity necessary to take on such a large deal.
Apart from the possibility of a buyout, Bristol looks like a great pick up at these rock bottom prices simply for its strong organic growth. Opdivo is continuing to rack up new indications at record pace and Eliquis' sales are also showing no signs of slowing down. As such, bargain hunters may want to take advantage of this dip to grab some shares soon.
GILD:
• MC = USD88b
• Enterprise Value to EBITDA = 6.14
• Debt-to-EBITDA = 2.0
• Piotroski F-Score = 6/9
• Price-to-Free-Cash-Flow = 8.46
BMY:
• MC = USD85b
• Enterprise Value to EBITDA = 13.46
• Debt-to-EBITDA = 1.31
• Piotroski F-Score = 6/9
• Price-to-Free-Cash-Flow = 18.78
(FactSet, GuruFocus)
=> ich finde, beide schenken sich nichts. Aber GILD als Übernehmer von BMY? Ich weiss nicht. bmy BMY kommt mir arg gross für GILD vor: BMY hat z.B. mehr als doppelt so viele MA's wie GILD...
=> dabei sieht es - nach 2016 und 2017 - zum ersten Mal wieder besser aus mMn -- so ganz allgemein bei derzeitiger Bewertung:
Einfaches Einfügen von wallstreetONLINE Charts: So funktionierts.
https://www.bloomberg.com/quote/BMY:US
Bloomberg PE = 15.85 <---> Ende 2017 nach FactSet ~100! => daher auch einige Alt-Angaben wie auf SA mit PE > 90....
=> das PEG ist ein bischen hoch für ein Schnäppchen:
Bloomberg (BEst) PEG Ratio = 1.7090
--> die Erwartungshaltung - in Summe - ist allseits gedämpft.
--> Cramer mag sie z.B. auch nicht z.Z.: 15.5.
https://www.cnbc.com/2018/05/15/cramers-lightning-round-take…
"Bristol-Myers right now does not have what we're looking for in terms of that cancer franchise that's going to Merck. I've got to tell you, down here, with a 3 percent yield, it gets attractive. But because of interest rates going higher, I'm going to have to take a pass for the moment."
--> ein Grund für den Crash im April: Pfizer mag (als Übernehmer) nicht mehr offensichtlich - oder das was der Markt davon denkt:
Why Bristol-Myers Squibb Stock Crashed in April
https://www.fool.com/investing/2018/05/09/why-bristol-myers-…
--> hier wird ernsthaft eine mögliche Übernahme durch Gilead Sciences erwogen:
Just because Pfizer isn't interested, though, doesn't necessarily mean that Bristol won't get taken out this year. Gilead Sciences, for instance, could very well emerge as a suitor due to the relatively slow start for its cellular immunotherapy endeavor and accelerating declines in its hep C franchise. Gilead also has the financial capacity necessary to take on such a large deal.
Apart from the possibility of a buyout, Bristol looks like a great pick up at these rock bottom prices simply for its strong organic growth. Opdivo is continuing to rack up new indications at record pace and Eliquis' sales are also showing no signs of slowing down. As such, bargain hunters may want to take advantage of this dip to grab some shares soon.
GILD:
• MC = USD88b
• Enterprise Value to EBITDA = 6.14
• Debt-to-EBITDA = 2.0
• Piotroski F-Score = 6/9
• Price-to-Free-Cash-Flow = 8.46
BMY:
• MC = USD85b
• Enterprise Value to EBITDA = 13.46
• Debt-to-EBITDA = 1.31
• Piotroski F-Score = 6/9
• Price-to-Free-Cash-Flow = 18.78
(FactSet, GuruFocus)
=> ich finde, beide schenken sich nichts. Aber GILD als Übernehmer von BMY? Ich weiss nicht. bmy BMY kommt mir arg gross für GILD vor: BMY hat z.B. mehr als doppelt so viele MA's wie GILD...
Bristol-Myers says FDA has approved opdivo as treatment for small cell lung cancer
more to come...
Antwort auf Beitrag Nr.: 58.472.632 von faultcode am 17.08.18 13:09:11https://news.bms.com/press-release/corporatefinancial-news/u…
=>
U.S. Food and Drug Administration Approves Opdivo® (nivolumab) as the First New Medication in Nearly 20 Years for Certain Patients with Previously Treated Small Cell Lung Cancer
-- Opdivo is now the first Immuno-Oncology treatment approved for small cell lung cancer (SCLC) patients who received platinum-based chemotherapy and at least one other line of therapy
-- Approval based on overall response rate and duration of response from the SCLC cohort of the Phase 1/2 CheckMate -032 trial
...
=>
U.S. Food and Drug Administration Approves Opdivo® (nivolumab) as the First New Medication in Nearly 20 Years for Certain Patients with Previously Treated Small Cell Lung Cancer
-- Opdivo is now the first Immuno-Oncology treatment approved for small cell lung cancer (SCLC) patients who received platinum-based chemotherapy and at least one other line of therapy
-- Approval based on overall response rate and duration of response from the SCLC cohort of the Phase 1/2 CheckMate -032 trial
...
..und wieder gecrasht
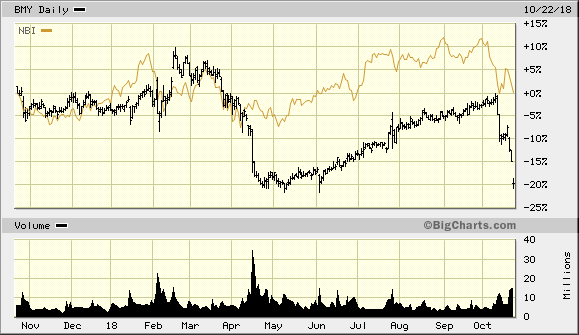
=>
man kann's sehr kurz so zusammenfassen:
• starke Konkurrenz v.a. durch Merck & Co. -- aber nicht nur
• ein paar nicht so starke Studiendaten
• Druck durch Medicare (andere Quelle: https://seekingalpha.com/news/3399212-bristol-myers-squibb-3…
15.10.
Cancer Research Highlight: Yet Again, It's Bristol Drawing The Short Stick In Lung Cancer
https://seekingalpha.com/article/4211700-cancer-research-hig…
Antwort auf Beitrag Nr.: 59.023.633 von faultcode am 22.10.18 20:11:50ich werde den Verdacht nicht los, daß da einige Marktteilnehmer schon früher wussten, was auf BMY zukommen wird, nämlich die anvisierte Celgene-Übernahme
!
Dieser Beitrag wurde von CommunitySupport moderiert. Grund: Auf Wunsch des Autors entfernt.
Antwort auf Beitrag Nr.: 59.553.396 von faultcode am 03.01.19 21:43:02..und jetzt doch nicht?!?
=>
27.2.
Platzt jetzt der Mega-Deal? Größter Bristol-Aktionär gegen Celgene-Kauf
https://www.wallstreet-online.de/nachricht/11275180-platzt-m…
=>
...Es sollte die größte Übernahme in der US-Pharmaindustrie werden: Zu Jahresbeginn hatte der Branchenriese Bristol-Myers Squibb (BMS) angekündigt, den Biopharma-Spezialisten Celgene für rund 74 Milliarden US-Dollar kaufen zu wollen. Am Mittwoch nach Handelsschluss an den US-Börsen dann der Paukenschlag: Der größte Aktionär von BMS sagt nein.
Die US-Investmentfirma Wellington Management, die fast neun Prozent an dem Pharmakonzern hält, stört sich dabei an mehreren Dingen. So sollen die BMS-Aktionäre nach ihrer Ansicht zu große Risiken schultern, wie es in einer Stellungnahme hieß.
Außerdem würden unter den Bedingungen der Übernahme den Celgene-Anteilseignern BMS-Papiere zu billig überlassen. Celgene-Aktionäre sollen pro eigener Aktie ein BMS-Papier und 50 Dollar erhalten.
Zudem befürchten die Investmentexperten, dass das Management des Pharmariesen mit Blick auf die erfolgreiche Umsetzung der Übernahme zu optimistisch sein könnte.
Und schließlich gebe es alternative Wege zur Schaffung von Wert für die BMS-Aktionäre, die attraktiver sein könnten.
Der Kapitalmarkt schätzt die Erfolgsaussichten von Wellington, den Deal noch zu verhindern, offensichtlich als durchaus nicht klein ein. So sprang der BMS-Kurs in einer ersten Reaktion um fast zwei Prozent nach oben.
Celgene hingegen brachen um mehr als 9 Prozent ein. BMS will sich mit Celgene vor allem auf lukrative Krebsmedikamente, Entzündungshemmer sowie auf Erkrankungen des Immunsystems und der Blutgefäße konzentrieren. Die Unternehmen erwarten den Vollzug des Geschäfts eigentlich im dritten Quartal 2019, wenn die Aktionäre und Behörden grünes Licht geben. Die Konzerne wollen bis zum Jahr 2022 jährliche Einsparungen von 2,5 Milliarden Dollar erzielen. Bristol-Myers will nach dem Abschluss zudem einen beschleunigten Aktienrückkauf über bis zu 5 Milliarden Dollar durchziehen. Bereits im ersten vollen Jahr nach Abschluss werde sich der Deal zudem in einem um 40 Prozent höheren Gewinn je Aktie niederschlagen. Für 2019 peilt BMS - noch ohne Celgene - einen Gewinn zwischen 3,75 und 3,85 Dollar an. Das ist etwas mehr als von Analysten mit 3,50 Dollar im Schnitt erwartet.
=>
27.2.
Platzt jetzt der Mega-Deal? Größter Bristol-Aktionär gegen Celgene-Kauf
https://www.wallstreet-online.de/nachricht/11275180-platzt-m…
=>
...Es sollte die größte Übernahme in der US-Pharmaindustrie werden: Zu Jahresbeginn hatte der Branchenriese Bristol-Myers Squibb (BMS) angekündigt, den Biopharma-Spezialisten Celgene für rund 74 Milliarden US-Dollar kaufen zu wollen. Am Mittwoch nach Handelsschluss an den US-Börsen dann der Paukenschlag: Der größte Aktionär von BMS sagt nein.
Die US-Investmentfirma Wellington Management, die fast neun Prozent an dem Pharmakonzern hält, stört sich dabei an mehreren Dingen. So sollen die BMS-Aktionäre nach ihrer Ansicht zu große Risiken schultern, wie es in einer Stellungnahme hieß.
Außerdem würden unter den Bedingungen der Übernahme den Celgene-Anteilseignern BMS-Papiere zu billig überlassen. Celgene-Aktionäre sollen pro eigener Aktie ein BMS-Papier und 50 Dollar erhalten.
Zudem befürchten die Investmentexperten, dass das Management des Pharmariesen mit Blick auf die erfolgreiche Umsetzung der Übernahme zu optimistisch sein könnte.
Und schließlich gebe es alternative Wege zur Schaffung von Wert für die BMS-Aktionäre, die attraktiver sein könnten.
Der Kapitalmarkt schätzt die Erfolgsaussichten von Wellington, den Deal noch zu verhindern, offensichtlich als durchaus nicht klein ein. So sprang der BMS-Kurs in einer ersten Reaktion um fast zwei Prozent nach oben.
Celgene hingegen brachen um mehr als 9 Prozent ein. BMS will sich mit Celgene vor allem auf lukrative Krebsmedikamente, Entzündungshemmer sowie auf Erkrankungen des Immunsystems und der Blutgefäße konzentrieren. Die Unternehmen erwarten den Vollzug des Geschäfts eigentlich im dritten Quartal 2019, wenn die Aktionäre und Behörden grünes Licht geben. Die Konzerne wollen bis zum Jahr 2022 jährliche Einsparungen von 2,5 Milliarden Dollar erzielen. Bristol-Myers will nach dem Abschluss zudem einen beschleunigten Aktienrückkauf über bis zu 5 Milliarden Dollar durchziehen. Bereits im ersten vollen Jahr nach Abschluss werde sich der Deal zudem in einem um 40 Prozent höheren Gewinn je Aktie niederschlagen. Für 2019 peilt BMS - noch ohne Celgene - einen Gewinn zwischen 3,75 und 3,85 Dollar an. Das ist etwas mehr als von Analysten mit 3,50 Dollar im Schnitt erwartet.
BMY könnte man mal im Auge behalten - ob die ~USD44 auch halten bei Vollzug der CELG-Übernahme:
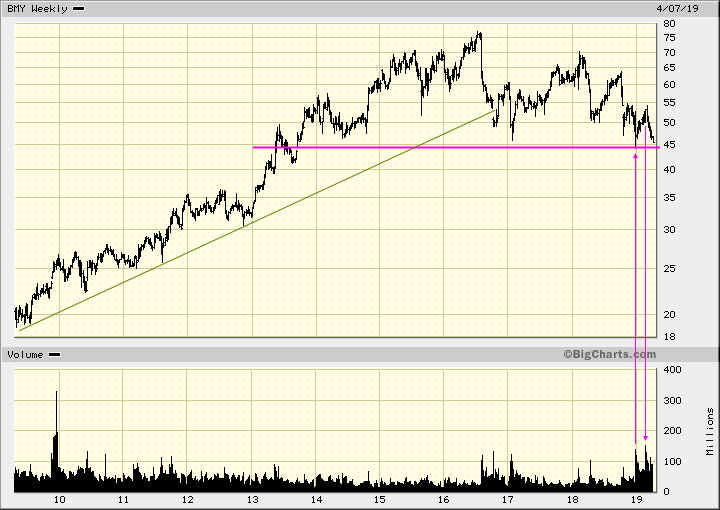

Antwort auf Beitrag Nr.: 60.361.012 von faultcode am 15.04.19 19:07:42
https://www.marketwatch.com/story/bristol-myers-offers-updat…
=>
Bristol-Myers Squibb Co. shares fell 4% in premarket trade Monday, after the company said a late-stage study of its Opdivo as a treatment for a type of liver cancer failed to meet its main goals.
The company said the Phase 3 study called CheckMate -459 evaluating Opdivo versus sorafenib as a first-line treatment in patients with unresectable hepatocellular carcinoma, or HCC, failed to meet its primary endpoint of overall survival, or OS.
"While CheckMate -459 did not reach its pre-specified primary endpoint, the results showed a clear trend towards improvement in OS for patients treated with Opdivo compared to sorafenib, a current standard of care," the company said in a statement.
The full study results will be presented at a coming medical meeting, said the statement. HCC is the fastest rising cause of cancer-related death in the U.S. Separately, Bristol-Myers said it is planning to sell Otezla, a treatment for certain types of psoriasis and psoriatic arthritis, in an effort to address Federal Trade Commission concerns about its pending merger with Celgene Corp.
"The proceeds of the OTEZLA sale will allow Bristol-Myers Squibb to accelerate its post-closing deleveraging plans," the company said. The companies are expecting to close the deal at year-end or in early 2020...
UPDATE: Bristol-Myers offers update on liver cancer trial, to sell Otezla to hasten close of Celgene deal
24.6.https://www.marketwatch.com/story/bristol-myers-offers-updat…
=>
Bristol-Myers Squibb Co. shares fell 4% in premarket trade Monday, after the company said a late-stage study of its Opdivo as a treatment for a type of liver cancer failed to meet its main goals.
The company said the Phase 3 study called CheckMate -459 evaluating Opdivo versus sorafenib as a first-line treatment in patients with unresectable hepatocellular carcinoma, or HCC, failed to meet its primary endpoint of overall survival, or OS.
"While CheckMate -459 did not reach its pre-specified primary endpoint, the results showed a clear trend towards improvement in OS for patients treated with Opdivo compared to sorafenib, a current standard of care," the company said in a statement.
The full study results will be presented at a coming medical meeting, said the statement. HCC is the fastest rising cause of cancer-related death in the U.S. Separately, Bristol-Myers said it is planning to sell Otezla, a treatment for certain types of psoriasis and psoriatic arthritis, in an effort to address Federal Trade Commission concerns about its pending merger with Celgene Corp.
"The proceeds of the OTEZLA sale will allow Bristol-Myers Squibb to accelerate its post-closing deleveraging plans," the company said. The companies are expecting to close the deal at year-end or in early 2020...
Antwort auf Beitrag Nr.: 61.015.538 von faultcode am 12.07.19 19:39:39es fängt an, bedrohlich zu werden:
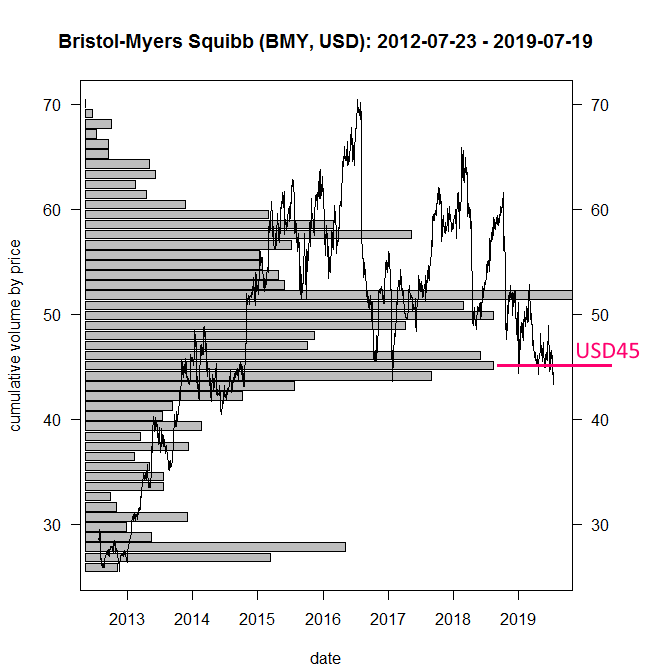


Denke BMY wird bei 40/42 USD seinen Boden finden. Heute erste Position aufgebaut. Schön das du hier auch aktiv dabei bist @faulcode.
ich danke auch. Wenn ich mir aber die Bedingungen der Übernahme ansehe und die Kursdifferenz, würde ich im Augenblick eher Celgene kaufen.
Wirklich gute Pharmacompanies sorgen auch für gute Nachrichten , Kursanstieg nicht ganz so irre wie bei Biogen , aber immerhin ,erstes Kursziel erreicht !!
Antwort auf Beitrag Nr.: 61.099.231 von FinanceWatch am 24.07.19 21:59:18
Läuft ja sehr gut. Werde bald noch mal etwas mehr zukaufen. Preise nach wie vor sehr billig. Bei 85 Dollar dann wieder fair bepreist und Teilverkauf geplant, wenn die Angst vor den Wahlen nicht ein Strich durch die Rechnung macht.
Zitat von FinanceWatch: Denke BMY wird bei 40/42 USD seinen Boden finden. Heute erste Position aufgebaut. Schön das du hier auch aktiv dabei bist @faulcode.
Läuft ja sehr gut. Werde bald noch mal etwas mehr zukaufen. Preise nach wie vor sehr billig. Bei 85 Dollar dann wieder fair bepreist und Teilverkauf geplant, wenn die Angst vor den Wahlen nicht ein Strich durch die Rechnung macht.
Entwickelt sich wirklich schön seit dem Sommer. Langsam aber stetig geht's nach Norden!
ja, dazu kommen noch die Dividenden in jedem Quartal.
Meine einzige Sorge ist, wie es mit dem Zusammenschluss mit Celgene weiter geht, ob das alles gut verdaut wird.
Meine einzige Sorge ist, wie es mit dem Zusammenschluss mit Celgene weiter geht, ob das alles gut verdaut wird.
Antwort auf Beitrag Nr.: 61.770.233 von xylophon am 25.10.19 23:37:01Das es jedes Quartal eine Dividende gibt, war mir noch gar nicht bewusst!
Eine kurze Frage dazu - ich habe am 1.8. meine Aktien gekauft, bei der Oktoberdividende war ich leider noch nicht dabei.
Liegt dies daran, dass ich bereits am 29.05.19 die Aktien hätte haben müssen (das war der Tag der Hauptversammlung) um die Dividenden zu erhalten?
Im Netz finde ich dazu widersprüchliches, z.B. auch die Information, dass der Ex-Dividende Tag der 3.10. war.
Eine kurze Frage dazu - ich habe am 1.8. meine Aktien gekauft, bei der Oktoberdividende war ich leider noch nicht dabei.
Liegt dies daran, dass ich bereits am 29.05.19 die Aktien hätte haben müssen (das war der Tag der Hauptversammlung) um die Dividenden zu erhalten?
Im Netz finde ich dazu widersprüchliches, z.B. auch die Information, dass der Ex-Dividende Tag der 3.10. war.
Antwort auf Beitrag Nr.: 61.771.457 von JohnnyJetset am 26.10.19 11:16:01Bitte schau doch mal auf die Investor relations Seite von Bristol. Dort steht ein "Record" Datum für die jeweilige Quartalsdividende.
Record Day ist der Tag an dem du die Aktie im Depot haben musst damit du die Dividende bekommst.
Record Day ist der Tag an dem du die Aktie im Depot haben musst damit du die Dividende bekommst.
Danke für den Tipp. Ich habe es nun rausgefunden:
Ex-Div Date: 03 October 2019
Pay Date: 01 November 2019
Ex-Div Date: 03 October 2019
Pay Date: 01 November 2019
15.11.
FTC makes Celgene's Otezla divestiture official condition for Bristol-Myers acquisition
https://www.marketwatch.com/story/ftc-makes-celgenes-otezla-…
=>
Celgene Corp. is required to divest its most popular psoriasis treatment in order to be acquired by Bristol-Myers Squibb Co., the Federal Trade Commission announced Friday.
For Bristol-Myers to close on its $74 billion acquisition, Celgene will have to sell its drug Otezla for $13.4 billion to Amgen Inc., the FTC said. "The Commission has ordered BMS to divest Otezla to preserve BMS's incentive to continue developing its own oral product for treating moderate-to-severe psoriasis," said FTC Chairman Joseph Simons in a statement.
"The antitrust laws protect not only competition today, but competition in the future, especially when it comes to the development of new treatments for chronic conditions."
The companies already anticipated the FTC's concerns and agreed to the deal back in August.
psoriasis = Schuppenflechte
FTC makes Celgene's Otezla divestiture official condition for Bristol-Myers acquisition
https://www.marketwatch.com/story/ftc-makes-celgenes-otezla-…
=>
Celgene Corp. is required to divest its most popular psoriasis treatment in order to be acquired by Bristol-Myers Squibb Co., the Federal Trade Commission announced Friday.
For Bristol-Myers to close on its $74 billion acquisition, Celgene will have to sell its drug Otezla for $13.4 billion to Amgen Inc., the FTC said. "The Commission has ordered BMS to divest Otezla to preserve BMS's incentive to continue developing its own oral product for treating moderate-to-severe psoriasis," said FTC Chairman Joseph Simons in a statement.
"The antitrust laws protect not only competition today, but competition in the future, especially when it comes to the development of new treatments for chronic conditions."
The companies already anticipated the FTC's concerns and agreed to the deal back in August.
psoriasis = Schuppenflechte
Antwort auf Beitrag Nr.: 43.533.178 von ipollit am 25.08.12 15:38:58
Schöne Analyse zu Bristol-Myers Squibb
in der auf Aussichten, Kennzahlen, Bewertung und den fairen Wert eingegangen wird. https://modernvalueinvesting.de/bristol-myers-squibb-dank-ce… Sehr interessant zu lesen.
Bristol-Myers Squibb CEO on the drug pipeline, 2020 outlook and more
https://www.cnbc.com/video/2020/01/13/bristol-myers-squibb-c…
https://www.cnbc.com/video/2020/01/13/bristol-myers-squibb-c…
Bristol Myers CEO on company’s cancer treatment pipeline
https://www.cnbc.com/video/2020/05/29/bristol-myers-ceo-on-t…
https://www.cnbc.com/video/2020/05/29/bristol-myers-ceo-on-t…
Frage mich echt, wann hier endlich Mal der Ausbruch kommt. Seit Jahren geht hier nichts. Langsam echt nervig.
Großes Potenzial bei Bristol Myers Squibb
Warren Buffet investierte über eine Milliarde in Bristol Myers Squibbs. Das Unternehmen stellt Produkte her für die Behandlung von Herz- und Kreislaufproblemen, Krebs & Gelenkschmerzen. Mit einem Umsatzwachstum von über 10% p.a. in den letzten 3 Jahren gehört Bristol Myers Squibbs zu den schnellst wachsenden Pharmaunternehmen. Problem dabei: Das Wachstum wurde fast ausschließlich anorganisch durch M&A (z.B. von IFM Therapeutics, Celgene) erzielt. Organisch ist Bristol Myers nur 1% gewachsen. Diese M&A wurden damals stark kritisiert, weil ein deutlicher Aufschlag auf die Unternehmen gezahlt wurde. Doch durch die Zukäufe konnte man nicht nur den Umsatz stark steigern, sondern auch die EBIT-Marge von 17% (2017) auf 25% (2020) steigern. Das KUV ist mit 3,45 das niedrigste seit langer Zeit in der Unternehmenshistorie und auch besonders günstig im Pharma-Peer-Group-Vergleich. Wer mehr zu den Risiken und fairen Bewertung erfahren möchte empfehle ich dieses Video: Warum Buffet BMS gekauft hat (1,8 Mrd Dollar)
Warren Buffet investierte über eine Milliarde in Bristol Myers Squibbs. Das Unternehmen stellt Produkte her für die Behandlung von Herz- und Kreislaufproblemen, Krebs & Gelenkschmerzen. Mit einem Umsatzwachstum von über 10% p.a. in den letzten 3 Jahren gehört Bristol Myers Squibbs zu den schnellst wachsenden Pharmaunternehmen. Problem dabei: Das Wachstum wurde fast ausschließlich anorganisch durch M&A (z.B. von IFM Therapeutics, Celgene) erzielt. Organisch ist Bristol Myers nur 1% gewachsen. Diese M&A wurden damals stark kritisiert, weil ein deutlicher Aufschlag auf die Unternehmen gezahlt wurde. Doch durch die Zukäufe konnte man nicht nur den Umsatz stark steigern, sondern auch die EBIT-Marge von 17% (2017) auf 25% (2020) steigern. Das KUV ist mit 3,45 das niedrigste seit langer Zeit in der Unternehmenshistorie und auch besonders günstig im Pharma-Peer-Group-Vergleich. Wer mehr zu den Risiken und fairen Bewertung erfahren möchte empfehle ich dieses Video:
Absoluter Rohrkrepierer diese Aktie. So ein herumgeeier hab ich selten gesehen. Seit Jahren kommt die nicht aus der Soße. Es nervt einfach nur noch. Stelle sich mal einer vor, sie würden keine Dividende mehr zahlen, dann wäre hier im Kurs das Licht komplett aus. Echt traurig für diese eigentlich recht gut aufgestellte Firma.
Warum Buffet in Bristol Myers Ssquibb investiert ist
Warren Buffet investierte über eine Milliarde in Bristol Myers Squibbs. Das Unternehmen stellt Produkte her für die Behandlung von Herz- und Kreislaufproblemen, Krebs & Gelenkschmerzen. Mit einem Umsatzwachstum von über 10% p.a. in den letzten 3 Jahren gehört Bristol Myers Squibbs zu den schnellst wachsenden Pharmaunternehmen. Problem dabei: Das Wachstum wurde fast ausschließlich anorganisch durch M&A (z.B. von IFM Therapeutics, Celgene) erzielt. Organisch ist Bristol Myers nur 1% gewachsen. Diese M&A wurden damals stark kritisiert, weil ein deutlicher Aufschlag auf die Unternehmen gezahlt wurde. Doch durch die Zukäufe konnte man nicht nur den Umsatz stark steigern, sondern auch die EBIT-Marge von 17% (2017) auf 25% (2020) steigern. Das KUV ist mit 3,45 das niedrigste seit langer Zeit in der Unternehmenshistorie und auch besonders günstig im Pharma-Peer-Group-Vergleich. Wer mehr zu den Risiken und fairen Bewertung erfahren möchte empfehle ich dieses Video:
Paul MacDonald discusses Bristol-Myers
https://www.bnnbloomberg.ca/video/paul-macdonald-discusses-b…
https://www.bnnbloomberg.ca/video/paul-macdonald-discusses-b…
Bristol-Myers Squibb Co. übertrifft im vierten Quartal mit einem Gewinn je Aktie von $1,46 die Analystenschätzungen von $1,41. Umsatz mit $11,07 Mrd. über den Erwartungen von $10,73 Mrd. / Quelle: Guidants News https://news.guidants.com
DZ Bank erhöht fairen Wert für Bristol-Myers Squibb von $74 auf $76. Buy / Quelle: Guidants News https://news.guidants.com
DZ Bank erhöht fairen Wert für Bristol-Myers Squibb von $74 auf $76. Buy / Quelle: Guidants News https://news.guidants.com
Enttäuschender Kursverlauf bei Bristol Meyers Squibb.
Seit 1 Monat fällt BMY von 67$ auf unter 60$.
Hier sollte ein Boden gefunden sein. Hoffe ich mal.
Aktuell wird wohl in BioNtech,Moderna, Curevac usw. investiert.
Aber irgendwann laufen auch wieder BMY, Amgen, Vertex und andere ....
Seit 1 Monat fällt BMY von 67$ auf unter 60$.
Hier sollte ein Boden gefunden sein. Hoffe ich mal.
Aktuell wird wohl in BioNtech,Moderna, Curevac usw. investiert.
Aber irgendwann laufen auch wieder BMY, Amgen, Vertex und andere ....
Warren Buffet investierte über eine Milliarde in Bristol Myers Squibbs. Das Unternehmen stellt Produkte her für die Behandlung von Herz- und Kreislaufproblemen, Krebs & Gelenkschmerzen. Mit einem Umsatzwachstum von über 10% p.a. in den letzten 3 Jahren gehört Bristol Myers Squibbs zu den schnellst wachsenden Pharmaunternehmen. Problem dabei: Das Wachstum wurde fast ausschließlich anorganisch durch M&A (z.B. von IFM Therapeutics, Celgene) erzielt. Organisch ist Bristol Myers nur 1% gewachsen. Diese M&A wurden damals stark kritisiert, weil ein deutlicher Aufschlag auf die Unternehmen gezahlt wurde. Doch durch die Zukäufe konnte man nicht nur den Umsatz stark steigern, sondern auch die EBIT-Marge von 17% (2017) auf 25% (2020) steigern. Das KUV ist mit 3,45 das niedrigste seit langer Zeit in der Unternehmenshistorie und auch besonders günstig im Pharma-Peer-Group-Vergleich. Wer mehr zu den Risiken und fairen Bewertung erfahren möchte empfehle ich dieses Video: Warum Warren Buffet die Bristol Myers Squibb Aktie gekauft hat (1,8 Mrd. $) - YouTube
Dieser Kurs ist und bleibt einfach ein Witz
Antwort auf Beitrag Nr.: 69.323.191 von Crazystefg am 14.09.21 18:10:11hast du recht, als wir an den 60€ waren vor 6 wochen, da dachte ich , jetzt steigen wir auf den fairen wert bei 90-110$
Antwort auf Beitrag Nr.: 69.486.613 von bigdulli am 02.10.21 19:06:02Gestern Kurszielanpassung von Barclays von 71 auf 68 USD, Beibehaltung gleichgewichten.
Das Chartbild inzwischen desolat mit bearish auf allen drei Zeitzonen.
Sehr hoher Umsatz gestern mit über 27 Millionen Stück, insgesamt für mich sehr enttäuschend, weil ich keinen speziellen Grund dafür erkennen kann.
Das Chartbild inzwischen desolat mit bearish auf allen drei Zeitzonen.
Sehr hoher Umsatz gestern mit über 27 Millionen Stück, insgesamt für mich sehr enttäuschend, weil ich keinen speziellen Grund dafür erkennen kann.
Oh man, das einzige Fehlinvestment in meinem Depot 🤦🏼♂️
Die heutigen Quartalszahlen werden schlecht aufgenommen.
Umsatz Q3 plus 10 % auf 11,624 Milliarden
EPS GAAP minus 16 % auf 0,69
EPS Non GAAP plus 23 % auf 2,00
Marge erhöht von 76,3 auf 80,3 %
EPS guidants 2021 nach unten angepasst von 2,77 - 2,97 auf 2,68 - 2,83 USD
EPS Non GAAP 2021 von 7,35 - 7,55 auf 7,40 - 7,55 leicht angehoben.
Hier ist weiterhin Geduld gefragt, ich werde bei anhaltender Schwäche weiter zukaufen...
Umsatz Q3 plus 10 % auf 11,624 Milliarden
EPS GAAP minus 16 % auf 0,69
EPS Non GAAP plus 23 % auf 2,00
Marge erhöht von 76,3 auf 80,3 %
EPS guidants 2021 nach unten angepasst von 2,77 - 2,97 auf 2,68 - 2,83 USD
EPS Non GAAP 2021 von 7,35 - 7,55 auf 7,40 - 7,55 leicht angehoben.
Hier ist weiterhin Geduld gefragt, ich werde bei anhaltender Schwäche weiter zukaufen...
Ob die noch jemals steigen wird 🙄
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Investoren beobachten auch:
| Wertpapier | Perf. % |
|---|---|
| +0,65 | |
| -0,98 | |
| -1,72 | |
| +1,15 | |
| -0,72 | |
| +0,22 | |
| +2,28 | |
| +0,77 | |
| -0,23 | |
| +0,17 |
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 49 | ||
| 43 | ||
| 39 | ||
| 17 | ||
| 16 | ||
| 13 | ||
| 12 | ||
| 12 | ||
| 10 | ||
| 10 |
05.05.24 · wallstreetONLINE Redaktion · Allianz |
02.05.24 · Der Finanzinvestor · Bristol-Myers Squibb |
25.04.24 · dpa-AFX · Bristol-Myers Squibb |
25.04.24 · dpa-AFX · Bristol-Myers Squibb |
25.04.24 · wO Newsflash · ATOSS Software |
25.04.24 · dpa-AFX · Bristol-Myers Squibb |
| Zeit | Titel |
|---|---|
| 19:32 Uhr |