Sellas Life Sciences Group (ehemals Galena Biopharma) (Seite 204)
eröffnet am 30.10.12 22:43:19 von
neuester Beitrag 17.05.24 07:21:04 von
neuester Beitrag 17.05.24 07:21:04 von
Beiträge: 2.942
ID: 1.177.530
ID: 1.177.530
Aufrufe heute: 1
Gesamt: 345.967
Gesamt: 345.967
Aktive User: 0
ISIN: US81642T2096 · WKN: A2PU3T · Symbol: SLS
1,4600
USD
-0,68 %
-0,0100 USD
Letzter Kurs 02:00:00 Nasdaq
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 2,25 | +63,04 | |
| 0,8000 | +45,45 | |
| 1,0000 | +42,86 | |
| 6,2500 | +24,75 | |
| 0,8200 | +15,49 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 114,60 | -13,61 | |
| 23,700 | -19,52 | |
| 28,60 | -24,06 | |
| 1,3500 | -25,62 | |
| 0,7800 | -29,73 |
Beitrag zu dieser Diskussion schreiben
Antwort auf Beitrag Nr.: 49.570.496 von Growth2012 am 15.04.15 16:24:00Alles Gut growth, hab mein Säckel bei GALE zu 3/4 voll. Nur bei nem nochmaligen Absacker wird noch mal nachgekauft.
Aber von mir aus kann das Teil jetzt wieder abzwitschern
Aber von mir aus kann das Teil jetzt wieder abzwitschern

Sorry alfarr27, habe deine Handel "geschändet" beim letzen posting ... 
Ihr kennt dieses evtl. schon, aber so leise Schwartz ist zurzeit, seine letzte Aussagen tragen immer noch Gewicht (13.4.15)
Galena CEO: 'A lot of noise,' but the biotech company is 'on target'

http://www.bizjournals.com/portland/blog/health-care-inc/201…
Watch this space....
[/If Galena were to license its products, it would have to give up “a great percentage of value,” Schwartz said. “By having our own sales force and commercial channel, we can retain a significant amount of value downstream.”
It could also partner with a pharmaceutical company, he said.
“We’re building deep relationships across the spectrum of oncology,” he said
b]

Ihr kennt dieses evtl. schon, aber so leise Schwartz ist zurzeit, seine letzte Aussagen tragen immer noch Gewicht (13.4.15)
Galena CEO: 'A lot of noise,' but the biotech company is 'on target'

http://www.bizjournals.com/portland/blog/health-care-inc/201…
Watch this space....

[/If Galena were to license its products, it would have to give up “a great percentage of value,” Schwartz said. “By having our own sales force and commercial channel, we can retain a significant amount of value downstream.”
It could also partner with a pharmaceutical company, he said.
“We’re building deep relationships across the spectrum of oncology,” he said
b]
Antwort auf Beitrag Nr.: 49.563.158 von alfarr27 am 14.04.15 20:14:05So siehts aus Alfaar
Das Chartbild (Zehntägig) & ansteigende Handelsvolumina lässt vermuten...
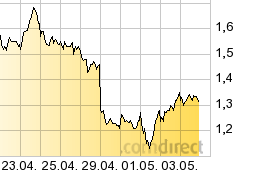
Nasdaq 1,45 14.04.15 22:00 7,88 Mio bei 6.250 Handlungen
...dass die shorties bald kapitulieren werden (habe gestern die Finger erneut-aber nun auch letztmalig- im Hönigtopf drin gehabt (will ja nichts bereuen müssen 'hinterher' )
)
...und zudem, wir sind nun in Gales Berichtungs-Saison angekommen!

Das Chartbild (Zehntägig) & ansteigende Handelsvolumina lässt vermuten...
Nasdaq 1,45 14.04.15 22:00 7,88 Mio bei 6.250 Handlungen
...dass die shorties bald kapitulieren werden (habe gestern die Finger erneut-aber nun auch letztmalig- im Hönigtopf drin gehabt (will ja nichts bereuen müssen 'hinterher'
 )
) ...und zudem, wir sind nun in Gales Berichtungs-Saison angekommen!
volumen ist doch in ordnung - jetzt schon das mittel der letzten 3 monate erreicht...und der überflieger war die meldung heute auch nicht - phase II, zeitrahmen 2018! insofern sind die 5-6 prozent wenn sie denn bis handelsschluss halten schon i.O.
bischen wenig Volumen....
Antwort auf Beitrag Nr.: 49.563.059 von patg am 14.04.15 19:59:43Naja nichts tun würde ich dazu jetzt nicht sagen 



 Warum tut sich nix,,,...das kostet echt nerven....
Warum tut sich nix,,,...das kostet echt nerven....
Galena Biopharma Completes Over-Enrollment of NeuVax(TM) (nelipepimut-S) Phase 3 PRESENT Clinical Trial
PORTLAND, Ore., April 14, 2015 (GLOBE NEWSWIRE) -- Galena Biopharma, Inc. (GALE), a biopharmaceutical company developing and commercializing innovative, targeted oncology therapeutics that address major medical needs across the full spectrum of cancer care, today announced the completion of enrollment in the NeuVax(TM) (nelipepimut-S) Phase 3 PRESENT (Prevention of Recurrence in Early-Stage, Node-Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment) clinical trial. NeuVax(TM) is a first-in-class, HER2-directed cancer immunotherapy under evaluation to prevent breast cancer recurrence after standard of care treatment in the adjuvant setting.
As anticipated, Galena over-enrolled the trial by 7.7% with a total of 758 patients now in the intent-to-treat (ITT) population. The protocol for the PRESENT trial, being conducted under an FDA approved Special Protocol Assessment (SPA), called for 700 patients; and, the Company expects this higher number of ITT patients will increase the confidence in both the timing and quality of the statistics and the final outcome of the trial. The primary endpoint is currently expected to be reached in 2018, after the last patient dosed reaches her 36th month of treatment, or a total of 141 events (recurrence or death) occur, whichever comes later.
"Completion of enrollment in our Phase 3 PRESENT trial is a landmark event for Galena and for breast cancer patients worldwide," said Mark W. Schwartz, Ph.D., President and Chief Executive Officer. "As we look forward to reaching our interim analysis by the end of this year or in Q1, 2016, it is important to note the significant medical need that Galena aims to address with NeuVax. Despite advances in the diagnosis and treatment of breast cancer, approximately 25% of node positive patients have a recurrence within three years after achieving no evidence of disease. NeuVax is designed to prevent these often fatal recurrences, and we anticipate that data from the PRESENT study, as well as our ongoing combination studies of NeuVax in breast cancer, will demonstrate this capability."
"The women in the PRESENT trial are part of the approximately fifty percent of breast cancer patients who have tumors that are HER2 1+ or 2+, and currently have no available treatment options to maintain their disease-free status after their standard of care therapy. Based on our early work with NeuVax, we believe this agent can have a meaningful impact for these women. I am grateful to the hundreds of women who participated in this study and for all of the sites and investigators who devoted their time to help us accomplish this milestone," added Elizabeth A. Mittendorf, M.D., Ph.D., Associate Professor, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center and the Principal Investigator of the PRESENT trial.
PRESENT is a randomized, double blind, placebo controlled, international, Phase 3 trial and is the most advanced study in Galena's pipeline. The trial is being run in 13 countries at more than 140 sites. The PRESENT trial targets the approximately 50%-60% of women with breast cancer who have low to intermediate (immunohistochemistry [IHC] 1+/2+ or fluorescence in situ hybridization [FISH] < 2.0) HER2 expression and achieved no evidence of disease following current standard of care treatment (surgery, chemotherapy, and radiation therapy). Patients enrolled must be lymph node positive, haplotype (HLA) A2 or A3 positive, and have Stage IIa-IIIa breast cancer. Once patients completed their current standard of care treatment, they are administered an injection once a month for six months (Primary Vaccine Series), then receive five booster injections once every six months for a total of eleven injections over a three year period. Currently there are no other treatment options for these patients to maintain their disease-free status.
About NeuVax(TM) (nelipepimut-S)
NeuVax(TM) (nelipepimut-S) is a first-in-class, HER2-directed cancer immunotherapy under evaluation to prevent breast cancer recurrence after standard of care treatment in the adjuvant setting. It is the immunodominant peptide derived from the extracellular domain of the HER2 protein, a well-established target for therapeutic intervention in breast carcinoma. NeuVax has been shown to bind to HLA-A2 and A3, as well as HLA-A24 and A26 molecules. The nelipepimut-S sequence stimulates specific CD8+ cytotoxic T lymphocytes (CTLs) following binding to specific HLA molecules on antigen presenting cells (APC). These activated specific CTLs recognize, neutralize and destroy, through cell lysis, HER2 expressing cancer cells, including occult cancer cells and micrometastatic foci. The nelipepimut-S immune response can also generate CTLs to other immunogenic peptides through inter- and intra-antigenic epitope spreading leading to a broader, more robust anti-tumor immune response.
NeuVax is currently in an international, Phase 3 study called PRESENT (Prevention of Recurrence in Early-Stage, Node-Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment) being conducted under a Special Protocol Assessment (SPA) granted by the U.S. Food and Drug Administration (FDA). Additional information on the PRESENT trial can be found at www.neuvax.com (clinicaltrials.gov identifier: NCT01479244). Galena has two additional breast cancer studies ongoing with NeuVax in combination with trastuzumab (Herceptin(R); Genentech/Roche): a Phase 2b trial in node positive and high risk node negative HER2 IHC 1+/2+ (clinicaltrials.gov identifier: NCT01570036); and, a Phase 2 trial in neoadjuvantly treated node positive and negative HER2 IHC 3+ patients not achieving a pathological complete response (pCR) or adjuvantly treated node positive HER2 IHC 3+ patients (clinicaltrials.gov identifier: NCT02297698).
About HER2 1+/2+ Breast Cancer
According to the National Cancer Institute, over 230,000 women in the U.S. are diagnosed with breast cancer annually. Of these women, only about 25% are HER2 positive (IHC 3+). NeuVax targets approximately 50%-60% of these women who are HER2 low to intermediate (IHC 1+/2+ or FISH < 2.0) and achieve remission with current standard of care, but have no available HER2-targeted adjuvant treatment options to maintain their disease-free status.
quelle: GlobeNewswire
PORTLAND, Ore., April 14, 2015 (GLOBE NEWSWIRE) -- Galena Biopharma, Inc. (GALE), a biopharmaceutical company developing and commercializing innovative, targeted oncology therapeutics that address major medical needs across the full spectrum of cancer care, today announced the completion of enrollment in the NeuVax(TM) (nelipepimut-S) Phase 3 PRESENT (Prevention of Recurrence in Early-Stage, Node-Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment) clinical trial. NeuVax(TM) is a first-in-class, HER2-directed cancer immunotherapy under evaluation to prevent breast cancer recurrence after standard of care treatment in the adjuvant setting.
As anticipated, Galena over-enrolled the trial by 7.7% with a total of 758 patients now in the intent-to-treat (ITT) population. The protocol for the PRESENT trial, being conducted under an FDA approved Special Protocol Assessment (SPA), called for 700 patients; and, the Company expects this higher number of ITT patients will increase the confidence in both the timing and quality of the statistics and the final outcome of the trial. The primary endpoint is currently expected to be reached in 2018, after the last patient dosed reaches her 36th month of treatment, or a total of 141 events (recurrence or death) occur, whichever comes later.
"Completion of enrollment in our Phase 3 PRESENT trial is a landmark event for Galena and for breast cancer patients worldwide," said Mark W. Schwartz, Ph.D., President and Chief Executive Officer. "As we look forward to reaching our interim analysis by the end of this year or in Q1, 2016, it is important to note the significant medical need that Galena aims to address with NeuVax. Despite advances in the diagnosis and treatment of breast cancer, approximately 25% of node positive patients have a recurrence within three years after achieving no evidence of disease. NeuVax is designed to prevent these often fatal recurrences, and we anticipate that data from the PRESENT study, as well as our ongoing combination studies of NeuVax in breast cancer, will demonstrate this capability."
"The women in the PRESENT trial are part of the approximately fifty percent of breast cancer patients who have tumors that are HER2 1+ or 2+, and currently have no available treatment options to maintain their disease-free status after their standard of care therapy. Based on our early work with NeuVax, we believe this agent can have a meaningful impact for these women. I am grateful to the hundreds of women who participated in this study and for all of the sites and investigators who devoted their time to help us accomplish this milestone," added Elizabeth A. Mittendorf, M.D., Ph.D., Associate Professor, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center and the Principal Investigator of the PRESENT trial.
PRESENT is a randomized, double blind, placebo controlled, international, Phase 3 trial and is the most advanced study in Galena's pipeline. The trial is being run in 13 countries at more than 140 sites. The PRESENT trial targets the approximately 50%-60% of women with breast cancer who have low to intermediate (immunohistochemistry [IHC] 1+/2+ or fluorescence in situ hybridization [FISH] < 2.0) HER2 expression and achieved no evidence of disease following current standard of care treatment (surgery, chemotherapy, and radiation therapy). Patients enrolled must be lymph node positive, haplotype (HLA) A2 or A3 positive, and have Stage IIa-IIIa breast cancer. Once patients completed their current standard of care treatment, they are administered an injection once a month for six months (Primary Vaccine Series), then receive five booster injections once every six months for a total of eleven injections over a three year period. Currently there are no other treatment options for these patients to maintain their disease-free status.
About NeuVax(TM) (nelipepimut-S)
NeuVax(TM) (nelipepimut-S) is a first-in-class, HER2-directed cancer immunotherapy under evaluation to prevent breast cancer recurrence after standard of care treatment in the adjuvant setting. It is the immunodominant peptide derived from the extracellular domain of the HER2 protein, a well-established target for therapeutic intervention in breast carcinoma. NeuVax has been shown to bind to HLA-A2 and A3, as well as HLA-A24 and A26 molecules. The nelipepimut-S sequence stimulates specific CD8+ cytotoxic T lymphocytes (CTLs) following binding to specific HLA molecules on antigen presenting cells (APC). These activated specific CTLs recognize, neutralize and destroy, through cell lysis, HER2 expressing cancer cells, including occult cancer cells and micrometastatic foci. The nelipepimut-S immune response can also generate CTLs to other immunogenic peptides through inter- and intra-antigenic epitope spreading leading to a broader, more robust anti-tumor immune response.
NeuVax is currently in an international, Phase 3 study called PRESENT (Prevention of Recurrence in Early-Stage, Node-Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment) being conducted under a Special Protocol Assessment (SPA) granted by the U.S. Food and Drug Administration (FDA). Additional information on the PRESENT trial can be found at www.neuvax.com (clinicaltrials.gov identifier: NCT01479244). Galena has two additional breast cancer studies ongoing with NeuVax in combination with trastuzumab (Herceptin(R); Genentech/Roche): a Phase 2b trial in node positive and high risk node negative HER2 IHC 1+/2+ (clinicaltrials.gov identifier: NCT01570036); and, a Phase 2 trial in neoadjuvantly treated node positive and negative HER2 IHC 3+ patients not achieving a pathological complete response (pCR) or adjuvantly treated node positive HER2 IHC 3+ patients (clinicaltrials.gov identifier: NCT02297698).
About HER2 1+/2+ Breast Cancer
According to the National Cancer Institute, over 230,000 women in the U.S. are diagnosed with breast cancer annually. Of these women, only about 25% are HER2 positive (IHC 3+). NeuVax targets approximately 50%-60% of these women who are HER2 low to intermediate (IHC 1+/2+ or FISH < 2.0) and achieve remission with current standard of care, but have no available HER2-targeted adjuvant treatment options to maintain their disease-free status.
quelle: GlobeNewswire
Antwort auf Beitrag Nr.: 49.541.666 von alfarr27 am 11.04.15 08:49:59Moin alfarr, ich hatte gestern insgesamt drei lebendige orders drin, wurde leider nur eins davon bedient...aber wer weiss, vielleicht kommt ein "weiteren dip" im Laufe der nächsten Woche wegen die "Sammelklage Überschrift"
http://finance.yahoo.com/news/notice-summons-complaint-again…
Bin jedenfalls weiterhin im Sammel & Lauerstellung befindlich....
http://finance.yahoo.com/news/notice-summons-complaint-again…
Bin jedenfalls weiterhin im Sammel & Lauerstellung befindlich....























