OSE.PA (MKap €89 M ) Covid 19 Vac / Positive P3 bei Lungenkebs (Seite 5)
eröffnet am 05.07.20 21:55:44 von
neuester Beitrag 28.02.24 09:43:02 von
neuester Beitrag 28.02.24 09:43:02 von
Beiträge: 44
ID: 1.327.334
ID: 1.327.334
Aufrufe heute: 0
Gesamt: 5.032
Gesamt: 5.032
Aktive User: 0
ISIN: FR0012127173 · WKN: A14QXP · Symbol: 6OP
5,6200
EUR
-0,35 %
-0,0200 EUR
Letzter Kurs 25.04.24 Tradegate
Neuigkeiten
16.04.24 · globenewswire |
10.04.24 · globenewswire |
02.04.24 · globenewswire |
27.03.24 · globenewswire |
18.03.24 · Business Wire (engl.) |
Werte aus der Branche Biotechnologie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 1,9000 | +59,66 | |
| 0,7200 | +40,60 | |
| 11,620 | +40,00 | |
| 4,7450 | +35,57 | |
| 6,9200 | +35,42 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 3,4000 | -11,69 | |
| 8,0100 | -12,85 | |
| 2,3150 | -14,58 | |
| 9,8300 | -15,84 | |
| 1,6052 | -30,21 |
Beitrag zu dieser Diskussion schreiben
In USA hätte so eine Nachricht für einen ordentlichen Kurssprung gesorgt aber OSE scheint immer noch nicht auf dem Schirm zu sein .
OSE Immunotherapeutics Receives €200,000 from Nantes Metropole to Develop CoVepiT, its COVID-19 Prophylactic Vaccine Program
https://finance.yahoo.com/news/ose-immunotherapeutics-receiv…
“We thank Nantes Metropole for supporting us with a grant that will allow us to accelerate the development of a vaccine candidate against COVID-19. Our teams are diligently working to finalize the preclinical phase of this program based on a T lymphocyte response that can last over time to eliminate infected cells and avoid developing serious forms. We expect the first preclinical results confirming vaccine protection during this summer. Based on these results, we could launch the clinical phase at the end of the year, provided we have the funding required for this clinical study,” commented Alexis Peyroles, Chief Executive Officer of OSE Immunotherapeutics.
OSE Immunotherapeutics Receives €200,000 from Nantes Metropole to Develop CoVepiT, its COVID-19 Prophylactic Vaccine Program
https://finance.yahoo.com/news/ose-immunotherapeutics-receiv…
“We thank Nantes Metropole for supporting us with a grant that will allow us to accelerate the development of a vaccine candidate against COVID-19. Our teams are diligently working to finalize the preclinical phase of this program based on a T lymphocyte response that can last over time to eliminate infected cells and avoid developing serious forms. We expect the first preclinical results confirming vaccine protection during this summer. Based on these results, we could launch the clinical phase at the end of the year, provided we have the funding required for this clinical study,” commented Alexis Peyroles, Chief Executive Officer of OSE Immunotherapeutics.
Antwort auf Beitrag Nr.: 64.331.015 von Biohero am 08.07.20 10:56:19Neues Update fon Edison
Encouraging data from novel preclinical projects....8 July 2020
https://www.edisongroup.com/publication/encouraging-data-fro…
OSE has a well-balanced R&D pipeline in terms of technology and asset stage (from discovery to Phase III). At the American Association of Cancer Research (AACR) Virtual Annual Meeting II in late June, OSE announced new data from its two more interesting preclinical programmes. C-type lectin receptor (CLEC-1) is a newly disclosed myeloid checkpoint target that tumour cells use to inhibit myeloid cells phagocytosis, a ‘don’t eat me’ signal. Anti-CLEC-1 antibodies restored the phagocytosis function of macrophages and dendritic cells (a similar effect to SIRPα/CD47 axis inhibition). New data from OSE’s bispecifics platform BiCKI were also presented, including with its first drug candidate BiCKI IL-7, an anti-PD-1 antibody fused with IL-7 interleukin. Our valuation is €230m or €15.3/share.
Encouraging data from novel preclinical projects....8 July 2020
https://www.edisongroup.com/publication/encouraging-data-fro…
OSE has a well-balanced R&D pipeline in terms of technology and asset stage (from discovery to Phase III). At the American Association of Cancer Research (AACR) Virtual Annual Meeting II in late June, OSE announced new data from its two more interesting preclinical programmes. C-type lectin receptor (CLEC-1) is a newly disclosed myeloid checkpoint target that tumour cells use to inhibit myeloid cells phagocytosis, a ‘don’t eat me’ signal. Anti-CLEC-1 antibodies restored the phagocytosis function of macrophages and dendritic cells (a similar effect to SIRPα/CD47 axis inhibition). New data from OSE’s bispecifics platform BiCKI were also presented, including with its first drug candidate BiCKI IL-7, an anti-PD-1 antibody fused with IL-7 interleukin. Our valuation is €230m or €15.3/share.
Antwort auf Beitrag Nr.: 64.300.199 von Biohero am 05.07.20 21:55:44Ich habe die Firma angeschrieben und folgendes gefragt 1) Ob man für Tedopi auf basis der ersten Phase 3 daten den Zulassungsantrag stellen kann 2) Wann die Daten von ihrem Covid Vaccine genau kommen und 3) Wie es mit Nasdaq listing aussieht / (unten sind die antworten ):
Dear xxxxxxxx,
Thank you for your email, we thank you for your confidence and support to OSE Immunotherapeutics.
You will find below the answers to your questions and we remain at your disposal for any further information.
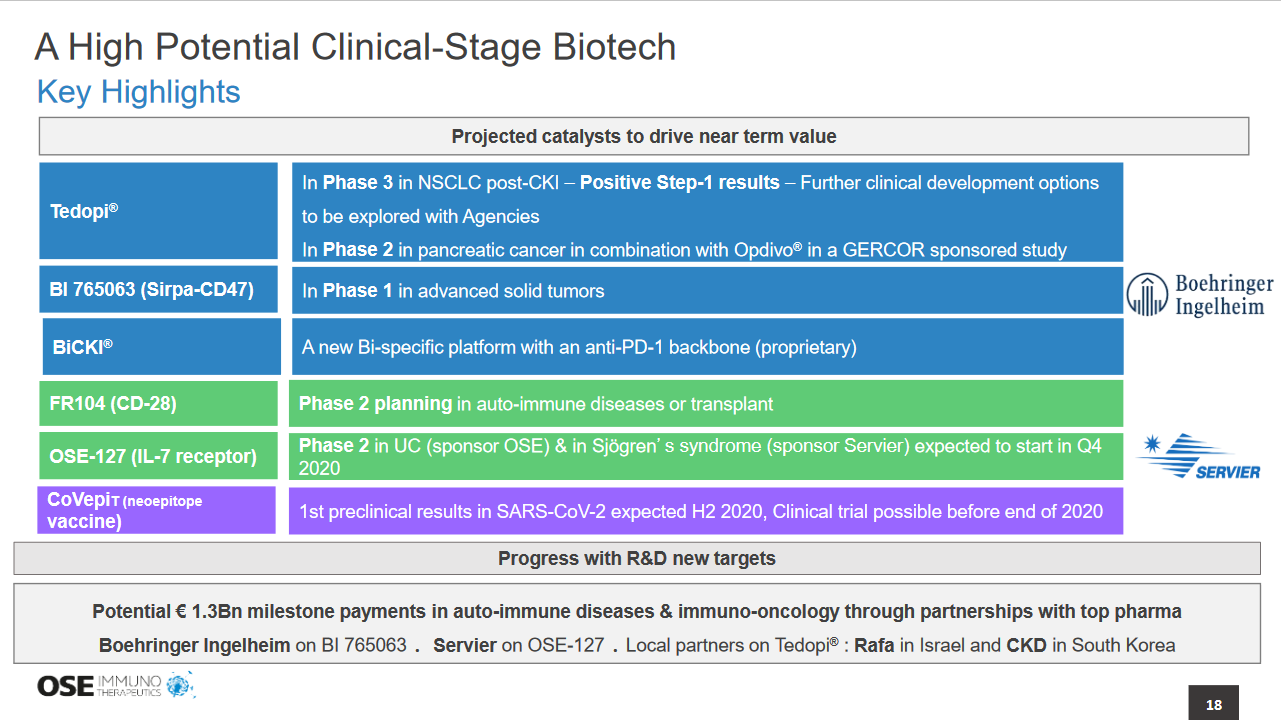
1) There are no product approved in NSCLC post checkpoint failure (in 2nd and 3rd line) so there is clearly a need for new treatment in this setting. Tedopi has reached the primary endpoint of the first step in the Atalante trial with 46% of the 63 patients treated with Tedopi reaching at least 12 months of survival with a good safety. The benefit/risk ratio is therefore interesting and we plan to provide agencies with these full results in this second semester to get their feedback.
2) We expect the results of the pre-clinical program on Covid 19 at the end of Q3 / early Q4.
3) We believe that a listing on Nasdaq is always a possibility for European biotechs to enlarge their investor base and raise additional capital. It must be done when the market capitalization and the track record in terms of positive clinical read-outs are sufficient to attract the Top tiers US investors. With the positive results of the Step-1 of Tedopi, OSE immunotherapeutics had its first positive clinical results and the rest of the portfolio is developing well for additional read-out in the next two years.
Best regards,
Sylvie Détry
Corporate communication – Secretary general
OSE Immunotherapeutics
Paris office: 100, avenue de Suffren – 75015 Paris - France
Tel + 33 (0) 153 198 757
Dear xxxxxxxx,
Thank you for your email, we thank you for your confidence and support to OSE Immunotherapeutics.
You will find below the answers to your questions and we remain at your disposal for any further information.
1) There are no product approved in NSCLC post checkpoint failure (in 2nd and 3rd line) so there is clearly a need for new treatment in this setting. Tedopi has reached the primary endpoint of the first step in the Atalante trial with 46% of the 63 patients treated with Tedopi reaching at least 12 months of survival with a good safety. The benefit/risk ratio is therefore interesting and we plan to provide agencies with these full results in this second semester to get their feedback.
2) We expect the results of the pre-clinical program on Covid 19 at the end of Q3 / early Q4.
3) We believe that a listing on Nasdaq is always a possibility for European biotechs to enlarge their investor base and raise additional capital. It must be done when the market capitalization and the track record in terms of positive clinical read-outs are sufficient to attract the Top tiers US investors. With the positive results of the Step-1 of Tedopi, OSE immunotherapeutics had its first positive clinical results and the rest of the portfolio is developing well for additional read-out in the next two years.
Best regards,
Sylvie Détry
Corporate communication – Secretary general
OSE Immunotherapeutics
Paris office: 100, avenue de Suffren – 75015 Paris - France
Tel + 33 (0) 153 198 757
Eröffne einen neuen Thread nachdem mein vorheriger historisch geworden ist.
kleines Update :
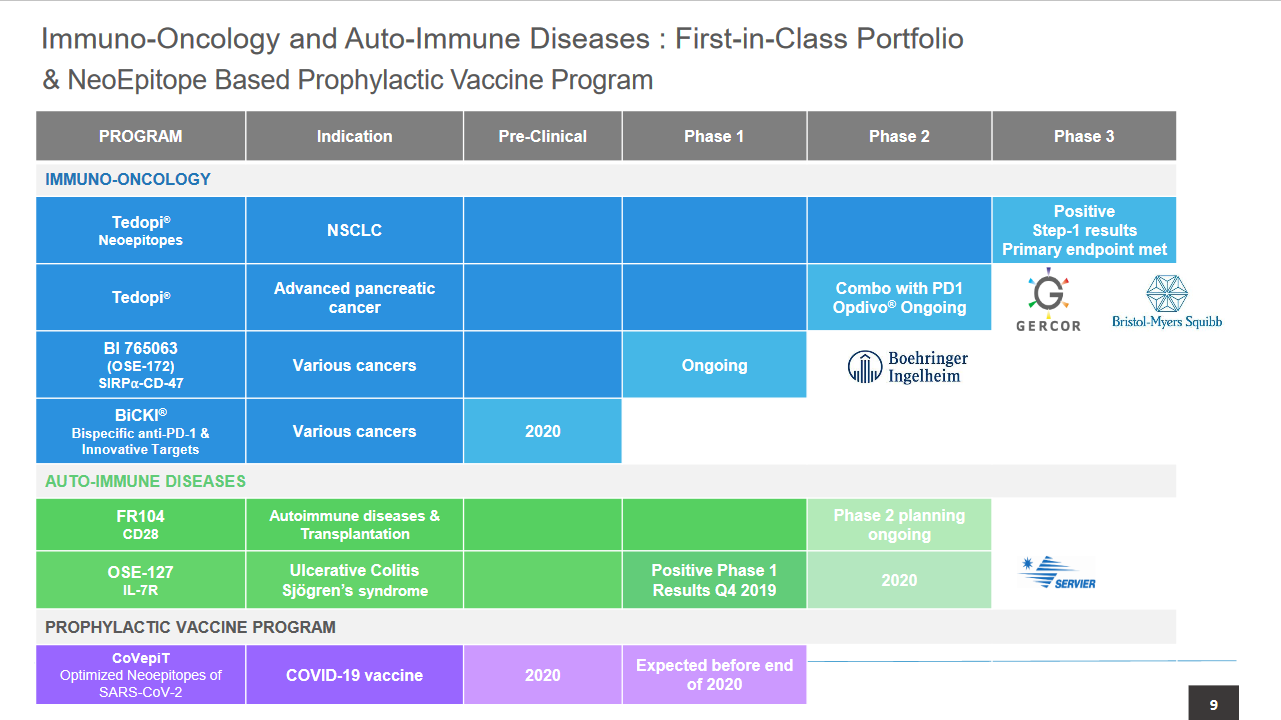
In April wurden Positive Phase 3 Daten für Tedopi bei fortgeschrittenem Lungenkrebs (NSCLC after CKI failure) gemeldet . Im laufe des monats sollen präklinische Daten von ihrem Covid 19 Vaccine veröffentlicht werden .
Ich habe mir letzte Woche ein paar ins Depot gelegt in erster linie wegen den Covid 19 Daten die bevorstehen das könnte die Aktie ordentlich pushen .Aber auch ohne Covid ist die Aktie interessant und Unterbewertet .
Ose Immunotherapeutics (OSE.PA)
Marktkap: €89 M
Cash: €26 M ( ausreichend bis Q3 2021)
Kurs:€5,80
Aktienanzahl: 15,4 M
Präsentation
https://ose-immuno.com/wp-content/uploads/2020/06/OSE_Corpor…
OSE Immunotherapeutics Announces COVID-19 Prophylactic Vaccine Program
https://www.finanznachrichten.de/nachrichten-2020-05/4956693…
Vaccine leverages expertise in peptide selection and optimization and proprietary Memopi technology to explore a T lymphocyte immune response for COVID-19
Uses artificial intelligence algorithm from MAbSilico to accelerate optimization of epitopes able to induce a robust cell memory immunity
First preclinical results expected start of H2 2020, possible clinical trial by year end
Phase 3 Clinical Trial of Tedopi®: OSE Immunotherapeutics Announces Positive Top-Line Results for Step-1 of its trial ‘Atalante 1’ in Non-Small Cell Lung Cancer
https://www.globenewswire.com/news-release/2020/04/01/201020…
Tedopi Vaccine Shows Positive Survival in HLA-A2-Positive Advanced NSCLC
https://www.targetedonc.com/view/tedopi-vaccine-shows-positi…
Positive Tedopi step 1, rethinking its future
https://www.edisongroup.com/publication/positive-tedopi-step…
On 1 April 2020, OSE announced that the primary endpoint was met in the predefined step 1 analysis of the Phase III Atalante 1 trial with OSE’s cancer vaccine Tedopi in HLA-A2 positive, non-small cell lung cancer (NSCLC) patients after they failed checkpoint inhibitors (CPIs, anti-PD-1 or anti-PD-L1). The patients (n=99) were randomised and received treatment at least 12 months before step1 analysis. The 12-month survival rate in the Tedopi arm was 46% (29 out of 63; CI 33–59%), well above the predefined futility threshold of 25%, so a statistically strong result. In the chemotherapy control arm, the 12-month survival rate was 36% (13 out of 36). Due to the COVID-19 pandemic, OSE has decided to terminate enrolment into the step 2 part of the trial, as NSCLC patients are vulnerable to coronavirus infections, and there was therefore a substantial risk of data loss. OSE will focus on regulatory interactions and partnering discussions given the availability of new data. Our valuation is virtually unchanged at €230m or €15.3/share.
kleines Update :
In April wurden Positive Phase 3 Daten für Tedopi bei fortgeschrittenem Lungenkrebs (NSCLC after CKI failure) gemeldet . Im laufe des monats sollen präklinische Daten von ihrem Covid 19 Vaccine veröffentlicht werden .
Ich habe mir letzte Woche ein paar ins Depot gelegt in erster linie wegen den Covid 19 Daten die bevorstehen das könnte die Aktie ordentlich pushen .Aber auch ohne Covid ist die Aktie interessant und Unterbewertet .
Ose Immunotherapeutics (OSE.PA)
Marktkap: €89 M
Cash: €26 M ( ausreichend bis Q3 2021)
Kurs:€5,80
Aktienanzahl: 15,4 M
Präsentation
https://ose-immuno.com/wp-content/uploads/2020/06/OSE_Corpor…
OSE Immunotherapeutics Announces COVID-19 Prophylactic Vaccine Program
https://www.finanznachrichten.de/nachrichten-2020-05/4956693…
Vaccine leverages expertise in peptide selection and optimization and proprietary Memopi technology to explore a T lymphocyte immune response for COVID-19
Uses artificial intelligence algorithm from MAbSilico to accelerate optimization of epitopes able to induce a robust cell memory immunity
First preclinical results expected start of H2 2020, possible clinical trial by year end
Phase 3 Clinical Trial of Tedopi®: OSE Immunotherapeutics Announces Positive Top-Line Results for Step-1 of its trial ‘Atalante 1’ in Non-Small Cell Lung Cancer
https://www.globenewswire.com/news-release/2020/04/01/201020…
Tedopi Vaccine Shows Positive Survival in HLA-A2-Positive Advanced NSCLC
https://www.targetedonc.com/view/tedopi-vaccine-shows-positi…
Positive Tedopi step 1, rethinking its future
https://www.edisongroup.com/publication/positive-tedopi-step…
On 1 April 2020, OSE announced that the primary endpoint was met in the predefined step 1 analysis of the Phase III Atalante 1 trial with OSE’s cancer vaccine Tedopi in HLA-A2 positive, non-small cell lung cancer (NSCLC) patients after they failed checkpoint inhibitors (CPIs, anti-PD-1 or anti-PD-L1). The patients (n=99) were randomised and received treatment at least 12 months before step1 analysis. The 12-month survival rate in the Tedopi arm was 46% (29 out of 63; CI 33–59%), well above the predefined futility threshold of 25%, so a statistically strong result. In the chemotherapy control arm, the 12-month survival rate was 36% (13 out of 36). Due to the COVID-19 pandemic, OSE has decided to terminate enrolment into the step 2 part of the trial, as NSCLC patients are vulnerable to coronavirus infections, and there was therefore a substantial risk of data loss. OSE will focus on regulatory interactions and partnering discussions given the availability of new data. Our valuation is virtually unchanged at €230m or €15.3/share.


OSE.PA (MKap €89 M ) Covid 19 Vac / Positive P3 bei Lungenkebs





















