Der Genanalysespezialist Pacific Biosciences Of California - 500 Beiträge pro Seite
eröffnet am 08.12.11 21:55:55 von
neuester Beitrag 02.02.16 21:37:54 von
neuester Beitrag 02.02.16 21:37:54 von
Beiträge: 35
ID: 1.170.938
ID: 1.170.938
Aufrufe heute: 0
Gesamt: 1.647
Gesamt: 1.647
Aktive User: 0
Top-Diskussionen
| Titel | letzter Beitrag | Aufrufe |
|---|---|---|
| heute 19:46 | 6756 | |
| vor 1 Stunde | 5393 | |
| vor 48 Minuten | 4549 | |
| vor 40 Minuten | 4226 | |
| vor 1 Stunde | 2947 | |
| heute 19:32 | 2179 | |
| heute 14:53 | 1971 | |
| vor 26 Minuten | 1717 |
Meistdiskutierte Wertpapiere
| Platz | vorher | Wertpapier | Kurs | Perf. % | Anzahl | ||
|---|---|---|---|---|---|---|---|
| 1. | 1. | 18.178,00 | +0,57 | 229 | |||
| 2. | 3. | 0,1885 | -0,26 | 96 | |||
| 3. | 2. | 1,1800 | -14,49 | 87 | |||
| 4. | 5. | 9,3600 | +0,29 | 60 | |||
| 5. | 4. | 168,17 | -1,18 | 55 | |||
| 6. | Neu! | 0,4250 | -1,16 | 38 | |||
| 7. | Neu! | 4,8025 | +6,45 | 34 | |||
| 8. | Neu! | 11,828 | +13,73 | 32 |

Pacific Biosciences of California Inc, hinter diesem zugegebenermaßen etwas sperrigen Firmennamen verbirgt sich eines der interessantesten Unternehmen der Medizintechnik. Pacific Biosciences entwickelt sozusagen die Schlüsseltechnologie, um mit erschwinglichen Geräten zur Genanalyse in eine Serienfertigung zu gehen.
Der IPO vor 1 1/2 Jahren lag aus Anlegersicht sehr ungünstig, denn wer zeichnete, hat zum Ende des Hypes einen kräftigen Aufschlag auf die Zukunftsträchtigkeit des Produktes gezahlt. Seither ging es stetig abwärts und inzwischen bemühen sich eine ganze Anzahl von Anwaltsfirmen mit Haifischmentalität um frustrierte Erstinvestoren, um über vermeintlich erfolgsaussichtenreiche Sammelklagen den Anlegern Genugtuung und sich selbst ein Salär zu verschaffen.
Pacific Biosciences hat - von den Börsenturbulenzen unbeeindruckt - in der Zwischenzeit tatsächlich ein Aggregat geschaffen, das die hohen technischen Ansprüche erfüllt und das die Grundlage für eine Serienfertigung bilden kann.
Pacific Biosciences ist auf einem sehr guten Weg - mal sehen, wann sich die bald wieder überzeugten Investoren rückbesinnen, auf den 08. Dez. 2011, dem Tag, als die Aktie für nur 2,60 USD gekauft werden konnte.
Pacific Biosciences Appoints John F. Milligan to Board of Directors
Gilead Sciences Executive Recognized for Financial Leadership in Biotech Industry
GlobeNewswirePress Release: Pacific Biosciences of California, Inc. – Thu, Jul 18, 2013 3:00 PM EDT
RELATED QUOTES
Symbol Price Change
PACB 2.75 +0.09
MENLO PARK, Calif., July 18, 2013 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc. (PACB), provider of the PacBio(R) RS II DNA Sequencing System, today announced that John F. Milligan, Ph.D. has joined the Company's Board of Directors. Dr. Milligan currently serves as President and Chief Operating Officer of Gilead Sciences.
Dr. Milligan joined Gilead Sciences in 1990 as a research scientist and was made Director of Project Management and Project Team Leader for the Gilead Hoffmann-La Roche Tamiflu(R) collaboration in 1996. In 2002, Dr. Milligan was appointed Chief Financial Officer. He was named Chief Operating Officer in 2007 and President in 2008.
Dr. Milligan was named "Bay Area CFO of the Year" in 2006 for companies with revenues greater than $500 million, and he was named the top biotechnology industry CFO in the United States by Institutional Investor magazine in 2006, 2007 and 2008. In 2008, Dr. Milligan joined the board of Biotechnology Industry Organization (BIO), the largest biotechnology industry organization. Dr. Milligan is a Trustee of Ohio Wesleyan University. He received his BA from Ohio Wesleyan University, his Ph.D. in biochemistry from the University of Illinois and was an American Cancer Society postdoctoral fellow at the University of California at San Francisco.
"John is a prominent and well-respected figure in the biotechnology industry, and we are delighted to have him join our Board of Directors," said Michael Hunkapiller, CEO and Chairman of the Board of Directors of Pacific Biosciences. "The combination of scientific and business expertise he brings will be a valuable addition to PacBio."
Dr. Milligan commented: "I'm very pleased to join the Board of Pacific Biosciences, and I look forward to working with my fellow directors and the senior management team to further the company's mission of delivering tools and technology that are helping to drive scientific and medical innovation."
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio(R) RS II DNA Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT(R)) technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems. More information is available at www.pacb.com.
Gilead Sciences Executive Recognized for Financial Leadership in Biotech Industry
GlobeNewswirePress Release: Pacific Biosciences of California, Inc. – Thu, Jul 18, 2013 3:00 PM EDT
RELATED QUOTES
Symbol Price Change
PACB 2.75 +0.09
MENLO PARK, Calif., July 18, 2013 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc. (PACB), provider of the PacBio(R) RS II DNA Sequencing System, today announced that John F. Milligan, Ph.D. has joined the Company's Board of Directors. Dr. Milligan currently serves as President and Chief Operating Officer of Gilead Sciences.
Dr. Milligan joined Gilead Sciences in 1990 as a research scientist and was made Director of Project Management and Project Team Leader for the Gilead Hoffmann-La Roche Tamiflu(R) collaboration in 1996. In 2002, Dr. Milligan was appointed Chief Financial Officer. He was named Chief Operating Officer in 2007 and President in 2008.
Dr. Milligan was named "Bay Area CFO of the Year" in 2006 for companies with revenues greater than $500 million, and he was named the top biotechnology industry CFO in the United States by Institutional Investor magazine in 2006, 2007 and 2008. In 2008, Dr. Milligan joined the board of Biotechnology Industry Organization (BIO), the largest biotechnology industry organization. Dr. Milligan is a Trustee of Ohio Wesleyan University. He received his BA from Ohio Wesleyan University, his Ph.D. in biochemistry from the University of Illinois and was an American Cancer Society postdoctoral fellow at the University of California at San Francisco.
"John is a prominent and well-respected figure in the biotechnology industry, and we are delighted to have him join our Board of Directors," said Michael Hunkapiller, CEO and Chairman of the Board of Directors of Pacific Biosciences. "The combination of scientific and business expertise he brings will be a valuable addition to PacBio."
Dr. Milligan commented: "I'm very pleased to join the Board of Pacific Biosciences, and I look forward to working with my fellow directors and the senior management team to further the company's mission of delivering tools and technology that are helping to drive scientific and medical innovation."
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio(R) RS II DNA Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT(R)) technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems. More information is available at www.pacb.com.
UCDAVIS-100K Genome Project unveils 20 more foodborne pathogen genomes
July 22, 2013 The 100K Genome Project, led by the University of California, Davis, the U.S. Food and Drug Administration’s Center for Food Safety and Applied Nutrition, and Agilent Technologies, today announced that it has added 20 newly completed genome sequences of foodborne disease-causing microorganisms to its public database at the National Center for Biotechnology Information.
The genomes were determined using Single Molecule, Real-Time (SMRT®) Sequencing technology from Pacific Biosciences of California, Inc.
This brings to 30 the number of genomic sequences completed by the 100K Genome Project, which aims to sequence the genomes of 100,000 bacterial and viral genomes. This genome sequencing effort is focused on speeding the diagnosis and treatment of foodborne diseases, as well as shortening the duration and limiting the spread of foodborne illness outbreaks. In the United States alone, foodborne diseases annually sicken around 48 million people and kill approximately 3,000, according to the Centers for Disease Control and Prevention.
The newly deposited sequences include several isolates of Salmonella, Listeria, Campylobacter, and Vibrio, as well as a full characterization of their epigenomes – a diagnostic feature that defines how the DNA is chemically modified and changes how the organism behaves.
“These finished genome sequences represent the highest quality standard, with each strain closed in a single bacterial chromosome and the associated mobile DNA,” said Bart Weimer, director of the 100K Genome Project and professor at the school of veterinary medicine at UC Davis. “They also contain complete associated phage or plasmid elements, which are critical for understanding pathogenicity, drug resistance and other biologically important traits that are linked to survival. (For full story and links,go to IHUB PACB board)
July 22, 2013 The 100K Genome Project, led by the University of California, Davis, the U.S. Food and Drug Administration’s Center for Food Safety and Applied Nutrition, and Agilent Technologies, today announced that it has added 20 newly completed genome sequences of foodborne disease-causing microorganisms to its public database at the National Center for Biotechnology Information.
The genomes were determined using Single Molecule, Real-Time (SMRT®) Sequencing technology from Pacific Biosciences of California, Inc.
This brings to 30 the number of genomic sequences completed by the 100K Genome Project, which aims to sequence the genomes of 100,000 bacterial and viral genomes. This genome sequencing effort is focused on speeding the diagnosis and treatment of foodborne diseases, as well as shortening the duration and limiting the spread of foodborne illness outbreaks. In the United States alone, foodborne diseases annually sicken around 48 million people and kill approximately 3,000, according to the Centers for Disease Control and Prevention.
The newly deposited sequences include several isolates of Salmonella, Listeria, Campylobacter, and Vibrio, as well as a full characterization of their epigenomes – a diagnostic feature that defines how the DNA is chemically modified and changes how the organism behaves.
“These finished genome sequences represent the highest quality standard, with each strain closed in a single bacterial chromosome and the associated mobile DNA,” said Bart Weimer, director of the 100K Genome Project and professor at the school of veterinary medicine at UC Davis. “They also contain complete associated phage or plasmid elements, which are critical for understanding pathogenicity, drug resistance and other biologically important traits that are linked to survival. (For full story and links,go to IHUB PACB board)
Piper Jaffray upgraded Pacific Biosciences to Neutral citing increased stability following the company's Q2 results. The firm raised its price target for shares to $3.10 from $2.
Pacific Biosciences: Higher Expectations Return As Support Grows For This Gene Sequencer
Aug 4 2013, 05:26 | about: PACB (Pacific Biosciences of California)

Recovering From The First Blow
Unfortunately, it has been a rough journey trading on the public markets for Pacific Biosciences. Following its IPO in late 2010 at $16/share, the company soon ran into problems in regards to its novel system appropriately named the PacBio RS. Plagued with system reliability and performance issues, the company was severely set back leading to a tarnished brand.
The effect was only multiplied by the fact that Pacific Biosciences was introducing a very new approach to genetic sequencing. Indeed, orders for new systems fell dramatically over this troubling time period. In Q2 2012, Pacific Biosciences only booked one system order as the company went about addressing the issues.
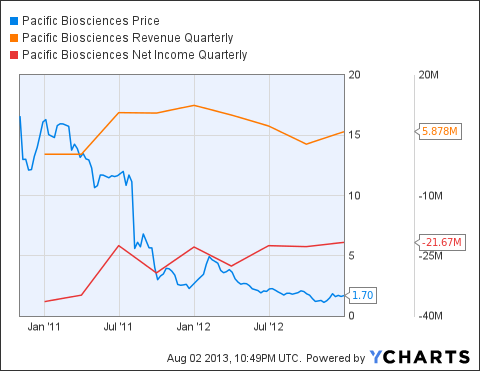
As a consequence, the company's stock price fell dramatically. Seen in the chart above, Pacific Biosciences fell almost 90% from its IPO to the end of 2012 as revenues slowed. This was especially harmful for a company that has yet to make a profit and whose momentum remained heavily dependent on revenue growth.
Tackling the Problem
By Q3 2012, management had made meaningful steps towards addressing the initial woes experienced under the PacBio RS. The flaws themselves could be tracked down to hardware and software bugs which were corrected under a new release. CEO Michael Hunkapiller had this to say in the Q3 2012 earnings call found here:
In terms of system reliability, we've come a long way in just a few quarters. Beginning of the year, our customers were having issues that stem from hardware and software bugs as well as training. In many cases, this resulted in low system availability and low utilization. Since then, we upgraded the installed base with a comprehensive release called C2, which included a host of reliability improvements and we have continued to provide focused training and support through our field and technical support organizations. While we will always continue to work to improve reliability, our system uptime is now very good and comparable to that of some of the more mature life science tools.
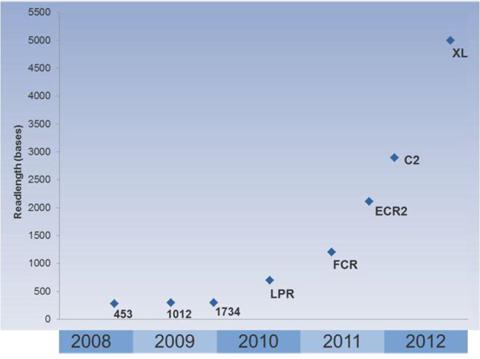
In an industry where accuracy is king, the inability for the PacBio RS to perform at par was crippling. Nevertheless, while the company was releasing its C2 comprehensive release in order to address these reliability issues, at the same time it went about to improve its capabilities as well. The release doubled the read lengths allowed by the system to approximately 3,000 base pairs on average with 5% of those reads exceeding 8,000 bases.
To date, the improvement trend has only continued. According to the latest conference call, the PacBio system was now able to deliver sequencing of whole bacterial genomes with at least 99.999% accuracy. Likewise, according to the brochure found here, the latest PacBio RS II now show typical results averaging 4,606 base pairs. The maximum read length of the system stands as 23,297 base pairs. The graphic below shows the rate at which the company's technology has improved.
The Uniqueness of the PacBio RS
What makes the company's system so unique ultimately comes down to its new approach. SMRT technology allows for the real-time analysis of biomolecules with single molecule resolution. The technology has the potential to evolve scientific research beyond DNA sequencing. It can transform our understanding of biological systems by providing a detailed perspective in ways which were not previously open for scientific study. For instance, in commercial applications it may allow the study of chemical and structural modifications of DNA. It may also allow for the processing of RNA and proteins. Several key advantages to the latest version of the PacBio RS II are as follows:
Long Reads. The system carries the industry's longest reads with over 20,000 base pairs.
High Accuracy. The system can generate finished genome assemblies with 99.999% accuracy.
High Sensitivity. The system can characterize genomic variations that are present at a frequency of less than 0.1%.
Able to Discover DNA Base Modifications. The system is the only commercial system able to detect DNA oxidative damage and other modified bases in the same sequencing run that reads the base sequence.
Shortest Run Time. The system can run a sequence in as few as 30 minutes.
In comparison to these characteristics, the competition fails to impress as seen in the graphic below which was accurate as of 2012 according to the article found here. Pacific Biosciences competes most directly with companies like Life Technologies (LIFE), which bought Ion Torrent, and Illumina (ILMN). At the time, Ion Torrent was only capable of reading up to 200 base pairs just as Illumina was only able to read up to 250 base pairs.
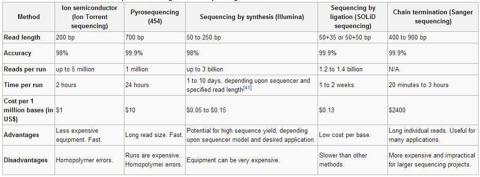
The relative effect of this smaller read length capability is akin to trying to solve a 5,000-piece puzzle compared to a 100-piece puzzle utilizing the PacBio RS. The ability to see larger chunks of the code allows for more effective processing. Another key advantage is seen in the processing time. This stood at 2 hours for Ion Torrent and 1-to-10 days for Illumina where as the PacBio RS II now runs sequences in 30 minutes.
A Look Going Forward
As the latest jump in the company's stock price indicates, the Q2 2013 earnings report was well received by investors. This was reiterated by the fact that Piper Jaffray upgraded the company from an 'Underweight' rating to that of 'Neutral'. Several key points taken from the earnings call transcript are found below:
New order bookings rose to 7 systems, up from 4 bookings in Q1 2013.
The company also booked 23 new orders for RSII upgrades.
Consumable revenue for the quarter was $1.9 million, up 60% from Q2 2012.
Several major publications vouch for the high quality performance of the PacBio RS. The company has seen a clear turnaround in terms of its reputation.
Some researchers demonstrated that the PacBio was 100% effective in detecting cancer mutations that were present in 1.5% of a DNA sample. In contrast, Illumina's MiSeq technology yielded 10 false-positives due to systematic bias and even failed to detect one of the mutations in the sample.
System utilization remains stable in Q2 after jumping significantly in Q1. Consumables are expected to increase in the future.
Management affirms that the company expects to double its 2012 bookings in 2013.
The company continues on its path to recovery, and more so in redefining the industry standard. Though previously believed to have an inaccurate sequencing system, Pacific Biosciences is beginning to prove to academia that it now has the most accurate system available. This is being simultaneously shown in the increased bookings of the company which are expected to translate into increased revenue growth.
Financial Overview
Pacific Biosciences now trades with a $173.35 million market capitalization at its current price of $2.85. The company's total revenue for Q2 2013 was $6.05 million resulting in a gross profit of $1.06 million. The company's profitability continues to improve when compared against Q2 2012 which yielded $7.29 million in revenues and $275,000 in gross profit. This was despite the lack of high-margin grant revenue still present in 2012.
The company now carries $106.97 million in cash and investments as of June 2013 compared to $100.58 million as of December 2012. Nevertheless, shareholder equity has fallen over the same time period from $109.38 million to $100.40 million. The company continues to accrue losses with a net loss of $0.70 in Q2 2013. Though this loss marks an improvement from the prior year, investors should continue to expect a loss until revenues significantly improve.
Conclusion
Pacific Biosciences now offers one of the more compelling recovery stories on the market. The company has turned itself around from the a failing brand into an industry-leading player. Ironically, this stands in a very hot industry that has gained much attention. In April 2013, Thermo Fisher recently purchased Life Technologies for $13.6 billion in one of the year's largest corporate takeovers.
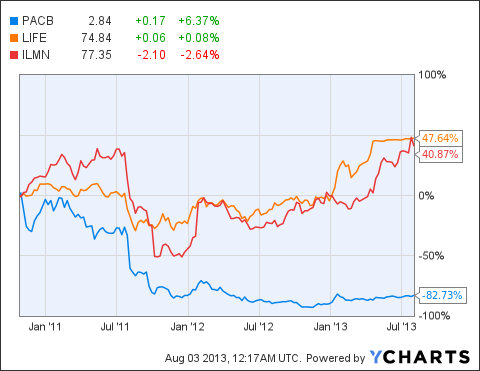
As more investors begin to take note of the PacBio RS II, Pacific Biosciences can arguably be attributed with a greater trading momentum in light of its expected revenue growth. This is especially so in light of the numerous advantages the company's next-generation system carries over the existing technology showcased by names such as Life Technologies and Illumina.
Nevertheless, Pacific Biosciences remains far from being a healthy enterprise. The company's balance sheet continues to deteriorate in light of its accrued losses. While the company has enough cash on hand to endure for several more quarters, there is very little room for failure going forward. As long as the company continues to progress, there is a very realistic expectation that its profitability is sure to follow as well. As it stands, Pacific Biosciences carries the leading technology platform when it comes to its industry.
Disclosure: I am long PACB. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Quelle: http://seekingalpha.com/article/1602542-pacific-biosciences-…
Aug 4 2013, 05:26 | about: PACB (Pacific Biosciences of California)

Recovering From The First Blow
Unfortunately, it has been a rough journey trading on the public markets for Pacific Biosciences. Following its IPO in late 2010 at $16/share, the company soon ran into problems in regards to its novel system appropriately named the PacBio RS. Plagued with system reliability and performance issues, the company was severely set back leading to a tarnished brand.
The effect was only multiplied by the fact that Pacific Biosciences was introducing a very new approach to genetic sequencing. Indeed, orders for new systems fell dramatically over this troubling time period. In Q2 2012, Pacific Biosciences only booked one system order as the company went about addressing the issues.

As a consequence, the company's stock price fell dramatically. Seen in the chart above, Pacific Biosciences fell almost 90% from its IPO to the end of 2012 as revenues slowed. This was especially harmful for a company that has yet to make a profit and whose momentum remained heavily dependent on revenue growth.
Tackling the Problem
By Q3 2012, management had made meaningful steps towards addressing the initial woes experienced under the PacBio RS. The flaws themselves could be tracked down to hardware and software bugs which were corrected under a new release. CEO Michael Hunkapiller had this to say in the Q3 2012 earnings call found here:
In terms of system reliability, we've come a long way in just a few quarters. Beginning of the year, our customers were having issues that stem from hardware and software bugs as well as training. In many cases, this resulted in low system availability and low utilization. Since then, we upgraded the installed base with a comprehensive release called C2, which included a host of reliability improvements and we have continued to provide focused training and support through our field and technical support organizations. While we will always continue to work to improve reliability, our system uptime is now very good and comparable to that of some of the more mature life science tools.
In an industry where accuracy is king, the inability for the PacBio RS to perform at par was crippling. Nevertheless, while the company was releasing its C2 comprehensive release in order to address these reliability issues, at the same time it went about to improve its capabilities as well. The release doubled the read lengths allowed by the system to approximately 3,000 base pairs on average with 5% of those reads exceeding 8,000 bases.
To date, the improvement trend has only continued. According to the latest conference call, the PacBio system was now able to deliver sequencing of whole bacterial genomes with at least 99.999% accuracy. Likewise, according to the brochure found here, the latest PacBio RS II now show typical results averaging 4,606 base pairs. The maximum read length of the system stands as 23,297 base pairs. The graphic below shows the rate at which the company's technology has improved.

The Uniqueness of the PacBio RS
What makes the company's system so unique ultimately comes down to its new approach. SMRT technology allows for the real-time analysis of biomolecules with single molecule resolution. The technology has the potential to evolve scientific research beyond DNA sequencing. It can transform our understanding of biological systems by providing a detailed perspective in ways which were not previously open for scientific study. For instance, in commercial applications it may allow the study of chemical and structural modifications of DNA. It may also allow for the processing of RNA and proteins. Several key advantages to the latest version of the PacBio RS II are as follows:
Long Reads. The system carries the industry's longest reads with over 20,000 base pairs.
High Accuracy. The system can generate finished genome assemblies with 99.999% accuracy.
High Sensitivity. The system can characterize genomic variations that are present at a frequency of less than 0.1%.
Able to Discover DNA Base Modifications. The system is the only commercial system able to detect DNA oxidative damage and other modified bases in the same sequencing run that reads the base sequence.
Shortest Run Time. The system can run a sequence in as few as 30 minutes.
In comparison to these characteristics, the competition fails to impress as seen in the graphic below which was accurate as of 2012 according to the article found here. Pacific Biosciences competes most directly with companies like Life Technologies (LIFE), which bought Ion Torrent, and Illumina (ILMN). At the time, Ion Torrent was only capable of reading up to 200 base pairs just as Illumina was only able to read up to 250 base pairs.

The relative effect of this smaller read length capability is akin to trying to solve a 5,000-piece puzzle compared to a 100-piece puzzle utilizing the PacBio RS. The ability to see larger chunks of the code allows for more effective processing. Another key advantage is seen in the processing time. This stood at 2 hours for Ion Torrent and 1-to-10 days for Illumina where as the PacBio RS II now runs sequences in 30 minutes.
A Look Going Forward
As the latest jump in the company's stock price indicates, the Q2 2013 earnings report was well received by investors. This was reiterated by the fact that Piper Jaffray upgraded the company from an 'Underweight' rating to that of 'Neutral'. Several key points taken from the earnings call transcript are found below:
New order bookings rose to 7 systems, up from 4 bookings in Q1 2013.
The company also booked 23 new orders for RSII upgrades.
Consumable revenue for the quarter was $1.9 million, up 60% from Q2 2012.
Several major publications vouch for the high quality performance of the PacBio RS. The company has seen a clear turnaround in terms of its reputation.
Some researchers demonstrated that the PacBio was 100% effective in detecting cancer mutations that were present in 1.5% of a DNA sample. In contrast, Illumina's MiSeq technology yielded 10 false-positives due to systematic bias and even failed to detect one of the mutations in the sample.
System utilization remains stable in Q2 after jumping significantly in Q1. Consumables are expected to increase in the future.
Management affirms that the company expects to double its 2012 bookings in 2013.
The company continues on its path to recovery, and more so in redefining the industry standard. Though previously believed to have an inaccurate sequencing system, Pacific Biosciences is beginning to prove to academia that it now has the most accurate system available. This is being simultaneously shown in the increased bookings of the company which are expected to translate into increased revenue growth.
Financial Overview
Pacific Biosciences now trades with a $173.35 million market capitalization at its current price of $2.85. The company's total revenue for Q2 2013 was $6.05 million resulting in a gross profit of $1.06 million. The company's profitability continues to improve when compared against Q2 2012 which yielded $7.29 million in revenues and $275,000 in gross profit. This was despite the lack of high-margin grant revenue still present in 2012.
The company now carries $106.97 million in cash and investments as of June 2013 compared to $100.58 million as of December 2012. Nevertheless, shareholder equity has fallen over the same time period from $109.38 million to $100.40 million. The company continues to accrue losses with a net loss of $0.70 in Q2 2013. Though this loss marks an improvement from the prior year, investors should continue to expect a loss until revenues significantly improve.
Conclusion
Pacific Biosciences now offers one of the more compelling recovery stories on the market. The company has turned itself around from the a failing brand into an industry-leading player. Ironically, this stands in a very hot industry that has gained much attention. In April 2013, Thermo Fisher recently purchased Life Technologies for $13.6 billion in one of the year's largest corporate takeovers.

As more investors begin to take note of the PacBio RS II, Pacific Biosciences can arguably be attributed with a greater trading momentum in light of its expected revenue growth. This is especially so in light of the numerous advantages the company's next-generation system carries over the existing technology showcased by names such as Life Technologies and Illumina.
Nevertheless, Pacific Biosciences remains far from being a healthy enterprise. The company's balance sheet continues to deteriorate in light of its accrued losses. While the company has enough cash on hand to endure for several more quarters, there is very little room for failure going forward. As long as the company continues to progress, there is a very realistic expectation that its profitability is sure to follow as well. As it stands, Pacific Biosciences carries the leading technology platform when it comes to its industry.
Disclosure: I am long PACB. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Quelle: http://seekingalpha.com/article/1602542-pacific-biosciences-…
Monday, August 19, 2013
Scientists Assess Error Modes in Sequencing Platforms and Find SMRT Sequencing ‘Least Biased’
A paper from scientists at the Broad Institute reports a rigorous study of bias across all major sequencing platforms. In “Characterizing and measuring bias in sequence data,” published in Genome Biology, lead author Michael Ross and his colleagues report that SMRT® Sequencing on the PacBio® sequencer is the “least biased” in coverage of all the technologies studied.
The authors assessed sequences for coverage bias, or uniformity of read distribution, and error bias, or incorrect call at a given position. For coverage bias, they report that PacBio performed best in extreme GC content (both GC-rich and GC-poor) and suggest this may be related to the lack of an amplification step in the sequencing process. Regarding error bias, the scientists describe shifting error rates based on genome sequence; GC-rich or homopolymer regions, for example, tended to change the rate of errors for each platform. “In general, the sequence context dependence of error rates varied considerably from technology to technology,” they write.
Ross et al. note that each platform’s bias rate changes with technology development, but note that at the time of their work, “single-molecule data from Pacific Biosciences” had “the clear edge.”
Quelle: PacBio Blog
*********************
Ergänzung:
We note that the single statistic of relative coverage for the GC ≥ 85% motif provided a suitable assay for bias on R. sphaeroides, with Pacific Biosciences scoring 0.87 (best), Illumina 0.60 and Ion Torrent 0.10 (worst), while GC ≥ 75% did not clearly distinguish between Illumina and Pacific Biosciences data. The GC ≤ 10% motif was similarly useful for P. falciparum, with Pacific Biosciences scoring 0.89 (best), Illumina 0.58, and Ion Torrent 0.39 (worst). For these data, the (AT)15 motif also stood out, with Pacific Biosciences at 0.85, Illumina at 0.43, and Ion Torrent at 0.11. Importantly, just these few statistics provided a meaningful readout on the performance of the different technologies.
Quelle: yhoo PACB message board
Scientists Assess Error Modes in Sequencing Platforms and Find SMRT Sequencing ‘Least Biased’
A paper from scientists at the Broad Institute reports a rigorous study of bias across all major sequencing platforms. In “Characterizing and measuring bias in sequence data,” published in Genome Biology, lead author Michael Ross and his colleagues report that SMRT® Sequencing on the PacBio® sequencer is the “least biased” in coverage of all the technologies studied.
The authors assessed sequences for coverage bias, or uniformity of read distribution, and error bias, or incorrect call at a given position. For coverage bias, they report that PacBio performed best in extreme GC content (both GC-rich and GC-poor) and suggest this may be related to the lack of an amplification step in the sequencing process. Regarding error bias, the scientists describe shifting error rates based on genome sequence; GC-rich or homopolymer regions, for example, tended to change the rate of errors for each platform. “In general, the sequence context dependence of error rates varied considerably from technology to technology,” they write.
Ross et al. note that each platform’s bias rate changes with technology development, but note that at the time of their work, “single-molecule data from Pacific Biosciences” had “the clear edge.”
Quelle: PacBio Blog
*********************
Ergänzung:
We note that the single statistic of relative coverage for the GC ≥ 85% motif provided a suitable assay for bias on R. sphaeroides, with Pacific Biosciences scoring 0.87 (best), Illumina 0.60 and Ion Torrent 0.10 (worst), while GC ≥ 75% did not clearly distinguish between Illumina and Pacific Biosciences data. The GC ≤ 10% motif was similarly useful for P. falciparum, with Pacific Biosciences scoring 0.89 (best), Illumina 0.58, and Ion Torrent 0.39 (worst). For these data, the (AT)15 motif also stood out, with Pacific Biosciences at 0.85, Illumina at 0.43, and Ion Torrent at 0.11. Importantly, just these few statistics provided a meaningful readout on the performance of the different technologies.
Quelle: yhoo PACB message board
Roche und Pacific Bioscience steuern
gemeinsam Diagnostikmarkt an
Fast schien die Schweizer Roche AG nach
dem gescheiterten feindlichen 6,8 Mrd.
US-$-Übernahmeangebot für Sequen-
cing-Weltmarktführer Illumina geschlagen.
Doch nun melden sich die ins US-amerikani-
sche Pleasanton umgesiedelten Roche-Ex-
perten für das Next-Generation-Sequenci-
ng (NGS) mit einem unerwarteten Schwenk
zurück. Statt länger auf passende Gele-
genheiten für Großakquisitionen zu war-
ten, schloss die nach der Zerschlagung von
Roche Applied Science (transkript 6/2013)
auf ein Zehntel der Belegschaft geschrumpf-
te Sequencing-Kern einheit Ende September
einen exklusiven Lizenzvertrag mit der kali-
fornischen Pacific Biosciences Inc., um den
vielversprechenden Diagnostikmarkt zu er-
schließen. Wenige Tage später wurde über
eine Pflichtmeldung bekannt, dass die Ba-
seler sämtliche 130 Mitarbeiter der 2007 ge-
kauften Sequencing-Firma 454 Life Scien-
ces in Branford bis Ende 2015 entlassen und
den Vertrieb der bisher vermarketen 454-Se-
quencer einstellen wollen. „Die derzeitigen
454-Systeme werden Mitte 2016 schrittwei-
se aus dem Programm genommen“, bestä-
tigte 454-Sprecherin Carla Moita.
Mit einer Anfangsinvestition von nur 35 Mio.
US-$ hat sich Roche den Exklusivzugriff auf Pa-
cific Biosciences (PacBio) Sequenziertechnolo-
gie SMRT (single molecule real time) für Diag-
nostikanwendungen gesichert. Die Kalifornier
vermarkten derzeit als einzige Firma eine 3rd
Generation Sequencing-Technologie, die ein-
zelne DNA-Moleküle ohne den vorherigen feh-
leranfälligen PCR-Schritt schnell, genau und
mit bisher unerreichter Leselänge (5.000 Ba-
sen durchschnittlich) sowie der besten Consen-
sus-Genauigkeit am Markt sequenzieren kann
(NATURE METHODS, doi: 10.1038/nmeth.2474).
Ziel der Kooperation ist es, einen neuen
SMRT-Sequencer sowie Verbrauchsmateria-
lien zu entwickeln, die einen diagnostischen
Einsatz des Sequencings ermöglichen. Um
den auch von Illumina, Thermo und Qiagen
angepeilten lukrativen Diagnostikmarkt ex-
klusiv mit PacBios Technologie bedienen zu
können, stellt Roche seinem neuen Partner
einiges in Aussicht: 40 Mio. US-$ zusätzliche
Meilensteinzahlungen für die Entwicklung des
neuen, robusten Systems; die Zusicherung,
selbst kein Konkurrenzsystem zu entwickeln,
sowie Verkaufsanteile an den von Roche über
sein weltweites Distributionsnetzwerk ver-
markteten diagnostischen Produkten in un-
genannter Höhe. Zudem darf PacBio alle
anderen, nicht-diagnostischen Sequencing-
Anwendungen eigenständig vertreiben.
Mit dem exklusiven Bündnis von David
und Goliath entsteht ein neuer Konkurrent
zu den großen NGS-Anbietern Illumina und
Thermo, die derzeit den Forschungsmarkt
dominieren und auch nach dem Diagnos-
tikmarkt greifen. „Das Sequencing ist eine
Schlüsseltechnologie, um die wachsende
Nachfrage nach genetischen und genomi-
schen Lösungen für den klinischen Einsatz
zu befriedigen”, stellte Dan Zabrowski, der
neue Kopf des Roche-Sequenzierungsteams,
klar. PacBios CEO Mike Hunkapillars Erwar-
tungen an die Partnerschaft mit den Diag-
nostikspezialisten sind hoch: „Als Welt-
marktführer in der In-vitro-Diagnostik bringt
Roche die Fähigkeit mit, klinische Produkte
zu entwickeln, zuzulassen und weltweit zu
vermarkten.”
Quelle: Iranskript Nr. 11, 19. Jahrgang 2013
gemeinsam Diagnostikmarkt an
Fast schien die Schweizer Roche AG nach
dem gescheiterten feindlichen 6,8 Mrd.
US-$-Übernahmeangebot für Sequen-
cing-Weltmarktführer Illumina geschlagen.
Doch nun melden sich die ins US-amerikani-
sche Pleasanton umgesiedelten Roche-Ex-
perten für das Next-Generation-Sequenci-
ng (NGS) mit einem unerwarteten Schwenk
zurück. Statt länger auf passende Gele-
genheiten für Großakquisitionen zu war-
ten, schloss die nach der Zerschlagung von
Roche Applied Science (transkript 6/2013)
auf ein Zehntel der Belegschaft geschrumpf-
te Sequencing-Kern einheit Ende September
einen exklusiven Lizenzvertrag mit der kali-
fornischen Pacific Biosciences Inc., um den
vielversprechenden Diagnostikmarkt zu er-
schließen. Wenige Tage später wurde über
eine Pflichtmeldung bekannt, dass die Ba-
seler sämtliche 130 Mitarbeiter der 2007 ge-
kauften Sequencing-Firma 454 Life Scien-
ces in Branford bis Ende 2015 entlassen und
den Vertrieb der bisher vermarketen 454-Se-
quencer einstellen wollen. „Die derzeitigen
454-Systeme werden Mitte 2016 schrittwei-
se aus dem Programm genommen“, bestä-
tigte 454-Sprecherin Carla Moita.
Mit einer Anfangsinvestition von nur 35 Mio.
US-$ hat sich Roche den Exklusivzugriff auf Pa-
cific Biosciences (PacBio) Sequenziertechnolo-
gie SMRT (single molecule real time) für Diag-
nostikanwendungen gesichert. Die Kalifornier
vermarkten derzeit als einzige Firma eine 3rd
Generation Sequencing-Technologie, die ein-
zelne DNA-Moleküle ohne den vorherigen feh-
leranfälligen PCR-Schritt schnell, genau und
mit bisher unerreichter Leselänge (5.000 Ba-
sen durchschnittlich) sowie der besten Consen-
sus-Genauigkeit am Markt sequenzieren kann
(NATURE METHODS, doi: 10.1038/nmeth.2474).
Ziel der Kooperation ist es, einen neuen
SMRT-Sequencer sowie Verbrauchsmateria-
lien zu entwickeln, die einen diagnostischen
Einsatz des Sequencings ermöglichen. Um
den auch von Illumina, Thermo und Qiagen
angepeilten lukrativen Diagnostikmarkt ex-
klusiv mit PacBios Technologie bedienen zu
können, stellt Roche seinem neuen Partner
einiges in Aussicht: 40 Mio. US-$ zusätzliche
Meilensteinzahlungen für die Entwicklung des
neuen, robusten Systems; die Zusicherung,
selbst kein Konkurrenzsystem zu entwickeln,
sowie Verkaufsanteile an den von Roche über
sein weltweites Distributionsnetzwerk ver-
markteten diagnostischen Produkten in un-
genannter Höhe. Zudem darf PacBio alle
anderen, nicht-diagnostischen Sequencing-
Anwendungen eigenständig vertreiben.
Mit dem exklusiven Bündnis von David
und Goliath entsteht ein neuer Konkurrent
zu den großen NGS-Anbietern Illumina und
Thermo, die derzeit den Forschungsmarkt
dominieren und auch nach dem Diagnos-
tikmarkt greifen. „Das Sequencing ist eine
Schlüsseltechnologie, um die wachsende
Nachfrage nach genetischen und genomi-
schen Lösungen für den klinischen Einsatz
zu befriedigen”, stellte Dan Zabrowski, der
neue Kopf des Roche-Sequenzierungsteams,
klar. PacBios CEO Mike Hunkapillars Erwar-
tungen an die Partnerschaft mit den Diag-
nostikspezialisten sind hoch: „Als Welt-
marktführer in der In-vitro-Diagnostik bringt
Roche die Fähigkeit mit, klinische Produkte
zu entwickeln, zuzulassen und weltweit zu
vermarkten.”
Quelle: Iranskript Nr. 11, 19. Jahrgang 2013
Wednesday, April 16, 2014
Innovation Centre in Quebec Uses SMRT Sequencing for
Cost-Effective, Complete Microbial Genomes
At the McGill University and Génome Québec Innovation Centre, many projects conducted in the sequencing core facility fall under the umbrella of life sciences rather than biomedical research. To the scientists responsible for making the core facility operate as smoothly as possible, that makes a world of difference.

“When you’re in the life sciences in addition to human biomedical [research], you’re out there in the world of things that haven’t been sequenced before, or haven’t been sequenced particularly well,” says Ken Dewar, a principal investigator at the Innovation Centre.
To navigate this type of uncharted territory, scientists at the center rely on long-read sequencing from their PacBio® RS II platform to cost-effectively close microbial genomes, traverse repeat-heavy genomic regions, and perform full-length transcript sequencing. By leveraging the dramatically increased read lengths PacBio sequencing provides, they have driven down costs and improved completeness of their assemblies.
At the core facility, Alexandre Montpetit is dedicated to running the next-generation sequencing platforms. His primary affiliation is with Génome Québec, and he has an adjunct appointment at McGill. He and his colleagues have been champions of long-read sequencing for years, so when PacBio unveiled its platform with industry-leading read length, it was an obvious choice for the center to adopt the technology.
“We’ve always had a focus on sequencing things for the first time or assembling genomes for the first time, not for the thousand-and-first time,” Dewar says. “PacBio was a natural fit.”
At the center, SMRT Sequencing has been used in diverse research areas. Some examples include generation of high-quality assemblies in microbial sequencing, analysis of long, repetitive genomic regions, and sequencing of full-length human gene isoforms. Microbial sequencing encompasses a number of applications, including biotech industry efforts to improve microbial biofermentation and microbiome studies, from environmental remediation projects on Alberta tar sands to veterinary research on microbes present in cattle rumen.
In the two years they’ve been running the SMRT Sequencing platform, the Innovation Centre scientists have seen remarkable progress in what they have been able to achieve. Continued improvements in read lengths — partly due to new reagent kits from PacBio and partly due to more streamlined sample prep protocols developed at the center — have already made a major difference.
One major step was achieving complete bacterial sequencing and assembly in less than a day, a feat that may enable the core facility to serve as a rapid response center for organizations that study pathogen outbreaks and other urgent problems. In 2013, tests conducted with researchers at the Canadian Food Inspection Agency and other government agencies demonstrated that the Innovation Centre scientists could sequence a sample and fully assemble the genome and plasmid elements — all in 20 hours or less.
Indeed, the Innovation Centre team is routinely able to deliver affordable, high-quality, finished genomes. “A single bacterial genome, a library prep, and two SMRT Cells of sequencing — which is generally a little bit overkill — is less than $1,000,” Dewar says. “More and more often, we are getting a completely closed, finished-quality genome for that.”
High-quality assemblies aren’t just for bacteria. “We’ve shown recently that we can assemble a fungal genome of 20 or 30 megabases with four or eight SMRT Cells and get only 10 or 20 contigs — which often represents the number of chromosomes in the genome,” Montpetit says.
Read the full case study to learn more about how the Innovation Centre has deployed SMRT Sequencing, their shift from hybrid to PacBio-only assemblies, and how they differentiate their bioinformatics analysis service.
Quelle: PacBio Blog
Innovation Centre in Quebec Uses SMRT Sequencing for
Cost-Effective, Complete Microbial Genomes
At the McGill University and Génome Québec Innovation Centre, many projects conducted in the sequencing core facility fall under the umbrella of life sciences rather than biomedical research. To the scientists responsible for making the core facility operate as smoothly as possible, that makes a world of difference.
“When you’re in the life sciences in addition to human biomedical [research], you’re out there in the world of things that haven’t been sequenced before, or haven’t been sequenced particularly well,” says Ken Dewar, a principal investigator at the Innovation Centre.
To navigate this type of uncharted territory, scientists at the center rely on long-read sequencing from their PacBio® RS II platform to cost-effectively close microbial genomes, traverse repeat-heavy genomic regions, and perform full-length transcript sequencing. By leveraging the dramatically increased read lengths PacBio sequencing provides, they have driven down costs and improved completeness of their assemblies.
At the core facility, Alexandre Montpetit is dedicated to running the next-generation sequencing platforms. His primary affiliation is with Génome Québec, and he has an adjunct appointment at McGill. He and his colleagues have been champions of long-read sequencing for years, so when PacBio unveiled its platform with industry-leading read length, it was an obvious choice for the center to adopt the technology.
“We’ve always had a focus on sequencing things for the first time or assembling genomes for the first time, not for the thousand-and-first time,” Dewar says. “PacBio was a natural fit.”
At the center, SMRT Sequencing has been used in diverse research areas. Some examples include generation of high-quality assemblies in microbial sequencing, analysis of long, repetitive genomic regions, and sequencing of full-length human gene isoforms. Microbial sequencing encompasses a number of applications, including biotech industry efforts to improve microbial biofermentation and microbiome studies, from environmental remediation projects on Alberta tar sands to veterinary research on microbes present in cattle rumen.
In the two years they’ve been running the SMRT Sequencing platform, the Innovation Centre scientists have seen remarkable progress in what they have been able to achieve. Continued improvements in read lengths — partly due to new reagent kits from PacBio and partly due to more streamlined sample prep protocols developed at the center — have already made a major difference.
One major step was achieving complete bacterial sequencing and assembly in less than a day, a feat that may enable the core facility to serve as a rapid response center for organizations that study pathogen outbreaks and other urgent problems. In 2013, tests conducted with researchers at the Canadian Food Inspection Agency and other government agencies demonstrated that the Innovation Centre scientists could sequence a sample and fully assemble the genome and plasmid elements — all in 20 hours or less.
Indeed, the Innovation Centre team is routinely able to deliver affordable, high-quality, finished genomes. “A single bacterial genome, a library prep, and two SMRT Cells of sequencing — which is generally a little bit overkill — is less than $1,000,” Dewar says. “More and more often, we are getting a completely closed, finished-quality genome for that.”
High-quality assemblies aren’t just for bacteria. “We’ve shown recently that we can assemble a fungal genome of 20 or 30 megabases with four or eight SMRT Cells and get only 10 or 20 contigs — which often represents the number of chromosomes in the genome,” Montpetit says.
Read the full case study to learn more about how the Innovation Centre has deployed SMRT Sequencing, their shift from hybrid to PacBio-only assemblies, and how they differentiate their bioinformatics analysis service.
Quelle: PacBio Blog
Tue, May 13, 2014, 11:50AM EDT -
Anthony Nolan Revolutionizes HLA Typing With Breakthrough DNA Sequencing Technology From Pacific Biosciences
Pacific Biosciences of California
HAMPSTEAD, LONDON and MENLO PARK, Calif., May 13, 2014 (GLOBE NEWSWIRE) -- Anthony Nolan, the UK blood cancer charity, and Pacific Biosciences of California, Inc., (PACB) announced today that Anthony Nolan is the world's first stem cell registry to invest in an innovative new technology for advanced tissue typing.1 The charity, which led the way 40 years ago when it created the world's first bone marrow register, is continuing its record for innovation by purchasing two PacBio(R) RS II systems, which enable Single Molecule, Real-Time (SMRT(R)) DNA Sequencing of full-length HLA genes.
Anthony Nolan will offer unparalleled detail and accuracy across its entire tissue typing service. The PacBio RS II system was selected because it is the only system available that can sequence full-length HLA genes due to its industry-leading read lengths and consensus accuracy.
Professor Steven Marsh, Anthony Nolan's Director of Bioinformatics, said: "Anthony Nolan has, from its inception, always been a scientifically pioneering organization. Investment in Pacific Biosciences SMRT technology will enable us to conduct allele-level typing, as standard. By providing the highest resolution typing available, we will be able to unambiguously phase HLA alleles for research in tissue transplantation and other applications, with the goal of making bone marrow and blood stem cell transplants more successful."
Professor Marsh continued: "Anthony Nolan intends to use this new technology to comprehensively HLA type new and existing donors as well as improve and extend services to our current customer base. Allied with this, Anthony Nolan's strategy seeks to offer services to new customers requiring full HLA typing for first-time donors, re-typing existing donors, confirmatory typing when donor/patient matches have been found, and typing for HLA-related disease association and drug hypersensitivity. This ground-breaking technology means Anthony Nolan staff and our customers will gain extra confidence that they have the most comprehensive data available as we strive toward ultimately improving transplant outcomes for patients in the future."
"We are proud to have innovative leaders like Anthony Nolan adopt our platform as we bring our installed base to more than 100 systems worldwide," said Michael Hunkapiller, President and Chief Executive Officer of Pacific Biosciences. "Together with Professor Steven Marsh, the designer and curator of the worldwide IMGT/HLA Database, we are excited that Anthony Nolan will now begin enhancing this critically important resource with full-length HLA genes. We anticipate that the unique advantages of SMRT Sequencing will also provide significant contributions to the IPD-KIR Sequence Database."
About Anthony Nolan
Anthony Nolan, now in its 40th anniversary year, was the world's first bone marrow register. The blood cancer charity has been saving lives for four decades by matching remarkable people willing to donate their bone marrow to patients in desperate need of a transplant.
About blood cancer
Every 20 minutes someone in the UK is diagnosed with a blood cancer. Around 1,800 people in the UK need a bone marrow (or stem cell) transplant each year. This is usually their last chance of survival. 63% of UK patients will not find a matching donor from within their families; instead they turn to Anthony Nolan to find them an unrelated donor.
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio(R) RS II DNA Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT(R)) technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems.
_____________________
1 Tissue typing is a process carried out at the time potential donors join the register, before a blood stem cell transplant. Transplants are used to treat blood cancers (e.g. leukemia) and other serious blood disorders. The human leukocyte antigen (HLA) of the donor must exactly, or very closely, match the HLA (tissue type) of the patient requiring the transplant.
View photo
.
Contact:
For Anthony Nolan:
Media:
Peter Zarko-Flynn
+44 (0) 7525 053795
info@zarko-flynn.com
For Pacific Biosciences:
Media:
Nicole Litchfield
For Pacific Biosciences
415.793.6468
nicole@bioscribe.com
Investors:
Trevin Rard
Pacific Biosciences
650.521.8450
ir@pacificbiosciences.com
Anthony Nolan Revolutionizes HLA Typing With Breakthrough DNA Sequencing Technology From Pacific Biosciences
Pacific Biosciences of California
HAMPSTEAD, LONDON and MENLO PARK, Calif., May 13, 2014 (GLOBE NEWSWIRE) -- Anthony Nolan, the UK blood cancer charity, and Pacific Biosciences of California, Inc., (PACB) announced today that Anthony Nolan is the world's first stem cell registry to invest in an innovative new technology for advanced tissue typing.1 The charity, which led the way 40 years ago when it created the world's first bone marrow register, is continuing its record for innovation by purchasing two PacBio(R) RS II systems, which enable Single Molecule, Real-Time (SMRT(R)) DNA Sequencing of full-length HLA genes.
Anthony Nolan will offer unparalleled detail and accuracy across its entire tissue typing service. The PacBio RS II system was selected because it is the only system available that can sequence full-length HLA genes due to its industry-leading read lengths and consensus accuracy.
Professor Steven Marsh, Anthony Nolan's Director of Bioinformatics, said: "Anthony Nolan has, from its inception, always been a scientifically pioneering organization. Investment in Pacific Biosciences SMRT technology will enable us to conduct allele-level typing, as standard. By providing the highest resolution typing available, we will be able to unambiguously phase HLA alleles for research in tissue transplantation and other applications, with the goal of making bone marrow and blood stem cell transplants more successful."
Professor Marsh continued: "Anthony Nolan intends to use this new technology to comprehensively HLA type new and existing donors as well as improve and extend services to our current customer base. Allied with this, Anthony Nolan's strategy seeks to offer services to new customers requiring full HLA typing for first-time donors, re-typing existing donors, confirmatory typing when donor/patient matches have been found, and typing for HLA-related disease association and drug hypersensitivity. This ground-breaking technology means Anthony Nolan staff and our customers will gain extra confidence that they have the most comprehensive data available as we strive toward ultimately improving transplant outcomes for patients in the future."
"We are proud to have innovative leaders like Anthony Nolan adopt our platform as we bring our installed base to more than 100 systems worldwide," said Michael Hunkapiller, President and Chief Executive Officer of Pacific Biosciences. "Together with Professor Steven Marsh, the designer and curator of the worldwide IMGT/HLA Database, we are excited that Anthony Nolan will now begin enhancing this critically important resource with full-length HLA genes. We anticipate that the unique advantages of SMRT Sequencing will also provide significant contributions to the IPD-KIR Sequence Database."
About Anthony Nolan
Anthony Nolan, now in its 40th anniversary year, was the world's first bone marrow register. The blood cancer charity has been saving lives for four decades by matching remarkable people willing to donate their bone marrow to patients in desperate need of a transplant.
About blood cancer
Every 20 minutes someone in the UK is diagnosed with a blood cancer. Around 1,800 people in the UK need a bone marrow (or stem cell) transplant each year. This is usually their last chance of survival. 63% of UK patients will not find a matching donor from within their families; instead they turn to Anthony Nolan to find them an unrelated donor.
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio(R) RS II DNA Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT(R)) technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems.
_____________________
1 Tissue typing is a process carried out at the time potential donors join the register, before a blood stem cell transplant. Transplants are used to treat blood cancers (e.g. leukemia) and other serious blood disorders. The human leukocyte antigen (HLA) of the donor must exactly, or very closely, match the HLA (tissue type) of the patient requiring the transplant.
View photo
.
Contact:
For Anthony Nolan:
Media:
Peter Zarko-Flynn
+44 (0) 7525 053795
info@zarko-flynn.com
For Pacific Biosciences:
Media:
Nicole Litchfield
For Pacific Biosciences
415.793.6468
nicole@bioscribe.com
Investors:
Trevin Rard
Pacific Biosciences
650.521.8450
ir@pacificbiosciences.com
PacBio Blog - Tuesday, May 6, 2014--Retroviral Study Reveals Potential for Influencing HIV Replication
Scientists from the Icahn School of Medicine at Mount Sinai in New York City and the MRC National Institute for Medical Research in London published a paper using Single Molecule, Real-Time (SMRT®) Sequencing to gain a better understanding of how human endogenous retroviruses may be interacting with HIV infection. They pursued a new avenue of research that could shed light on how to interfere with HIV replication.
The scientists conducted a study uniquely suited to the extremely long reads provided by the PacBio® platform, noting that this technology was needed to accurately parse the complexity in expression among a specific group of human endogenous retroviruses (HERVs). In this project, the scientists dug deeper into evidence that expression of the endogenous retroviruses that make up almost 5% of the human genome is upregulated when a person is infected with HIV-1. “HIV-1 infection in human cells is equivalent to a co-infection by several retroviruses,” -------------The team found nearly 4,000 HERV-K sequences in these lymphocytes, compared to a previous study from other scientists that found fewer than 1,000 of these sequences in 11 samples. They posit that the higher number seen here reflects the greater sensitivity of PacBio sequencing as well as the difference in cell types analyzed.
In all, the authors identified more than 30 different transcripts for HERV-K envelopes, including two that produce full-length proteins — one of which was found to incorporate into HIV-1 particles. “These findings imply that some HERV-Ks interact specifically with HIV possibly shaping the properties of the lentivirus,” they write. “Future studies are needed to determine the extent of their influence on the HIV-1 life cycle and whether their expression can be harnessed to hinder HIV-1 replication.”
(for full story,go to PACB web site, click on blog)
Scientists from the Icahn School of Medicine at Mount Sinai in New York City and the MRC National Institute for Medical Research in London published a paper using Single Molecule, Real-Time (SMRT®) Sequencing to gain a better understanding of how human endogenous retroviruses may be interacting with HIV infection. They pursued a new avenue of research that could shed light on how to interfere with HIV replication.
The scientists conducted a study uniquely suited to the extremely long reads provided by the PacBio® platform, noting that this technology was needed to accurately parse the complexity in expression among a specific group of human endogenous retroviruses (HERVs). In this project, the scientists dug deeper into evidence that expression of the endogenous retroviruses that make up almost 5% of the human genome is upregulated when a person is infected with HIV-1. “HIV-1 infection in human cells is equivalent to a co-infection by several retroviruses,” -------------The team found nearly 4,000 HERV-K sequences in these lymphocytes, compared to a previous study from other scientists that found fewer than 1,000 of these sequences in 11 samples. They posit that the higher number seen here reflects the greater sensitivity of PacBio sequencing as well as the difference in cell types analyzed.
In all, the authors identified more than 30 different transcripts for HERV-K envelopes, including two that produce full-length proteins — one of which was found to incorporate into HIV-1 particles. “These findings imply that some HERV-Ks interact specifically with HIV possibly shaping the properties of the lentivirus,” they write. “Future studies are needed to determine the extent of their influence on the HIV-1 life cycle and whether their expression can be harnessed to hinder HIV-1 replication.”
(for full story,go to PACB web site, click on blog)
Single Molecule, Real-Time (SMRT) DNA Sequencing Technology From Pacific Biosciences to be Featured in 20 Presentations at the American Society for Microbiology 2014 Annual Meeting
Winner of Last Year's SMRT Grant Program Announced; "SMRTest Microbe" Grant Program Begins Worldwide
Pacific Biosciences of California, Inc.
MENLO PARK, Calif., May 14, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc., (PACB) provider of the PacBio(R) RS II system, announced today that its Single Molecule, Real-Time (SMRT(R)) DNA Sequencing technology will be featured in seven podium and 13 poster presentations at the 2014 American Society for Microbiology (ASM) annual meeting in Boston.
Of note, Nobel Laureate Sir Richard Roberts will host a session entitled "Bacterial Methylomes" (#227) on Tuesday, May 20 from 2-4:30 p.m. The session will include talks from scientists representing the Joint Genome Institute, Mount Sinai School of Medicine, the US Food and Drug Administration, Harvard School of Public Health and New England Biolabs. Also on May 20, at 4:00 p.m., Julie Segre of the National Institutes of Health will discuss her work using SMRT Sequencing in a talk entitled "Tracking Hospital Patients and Environment with Complete Genome Sequencing of Carbapenem-Resistant Klebsiella pneumoniae and other Enterobacteriaceae."
Jonas Korlach, Chief Scientific Officer for Pacific Biosciences commented: "SMRT Sequencing simplifies the genetic characterization of microbes by making high-accuracy finished de novo genome assembly rapid and affordable. Factors affecting virulence such as structural variation, horizontal gene transfer and methylation become readily apparent through comparison of these assemblies. These unique advantages have made SMRT Sequencing the gold standard for microbial sequencing, which is also evidenced by the rapidly increasing number of papers and genome announcements being published."
At last year's ASM annual meeting, Pacific Biosciences initiated a SMRT Grant Program to reward interesting proposals from attendees with free SMRT Cells and project support. The company announced today that the winner from last year was Michael Nelson of the University of Connecticut for his entry to sequence a putative novel Bacteroidete genus with importance to the digestive tract. Nelson experienced difficulty sequencing the genome of the type strain using short-read technologies, but using PacBio technology he was able to sequence the genome completely in one contig.
This year, Pacific Biosciences has opened up the SMRTest Microbe Grant Program to any eligible scientist. The scientist with the winning application will receive a free sequencing run on the PacBio RS II system using up to one SMRT Cell 8Pac and up to four library constructions for their project. More details, including the official rules of the Grant Program, are available at: www.pacb.com/smrtgrant.
A full listing of the podium and poster presentations featuring SMRT technology is listed here, and more information about the ASM annual meeting is available at: http://gm.asm.org/. Pacific Biosciences will be exhibiting its technology at Booth #1034.
About the PacBio RS II and SMRT(R) Sequencing
Pacific Biosciences' Single Molecule, Real-Time (SMRT) Sequencing technology achieves the industry's longest read lengths, highest consensus accuracyi,ii and the least degree of bias.iii These characteristics, combined with the ability to detect many types of DNA base modifications (e.g., methylation) as part of the sequencing process, make the PacBio RS II an essential tool for many scientists studying genetic and genomic variation. The PacBio platform provides a sequencing solution that can address a growing number of complex medical, agricultural and industrial problems.
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio RS II DNA Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT) technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems.
i Koren et al., "Reducing assembly complexity of microbial genomes with single-molecule sequencing." Genome Biology, 14:R10.1 (2013).
ii Chin et al., "Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data." Nature Methods, 10; 563-569 (2013).
iii Ross et al. Characterizing and measuring bias in sequence data. Genome Biol 14: R51 (2013).
View photo
.
Contact:
For Pacific Biosciences:
Media:
Nicole Litchfield
For Pacific Biosciences
415.793.6468
nicole@bioscribe.com
Investors:
Trevin Rard
Pacific Biosciences
650.521.8450
ir@pacificbiosciences.com
Winner of Last Year's SMRT Grant Program Announced; "SMRTest Microbe" Grant Program Begins Worldwide
Pacific Biosciences of California, Inc.
MENLO PARK, Calif., May 14, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc., (PACB) provider of the PacBio(R) RS II system, announced today that its Single Molecule, Real-Time (SMRT(R)) DNA Sequencing technology will be featured in seven podium and 13 poster presentations at the 2014 American Society for Microbiology (ASM) annual meeting in Boston.
Of note, Nobel Laureate Sir Richard Roberts will host a session entitled "Bacterial Methylomes" (#227) on Tuesday, May 20 from 2-4:30 p.m. The session will include talks from scientists representing the Joint Genome Institute, Mount Sinai School of Medicine, the US Food and Drug Administration, Harvard School of Public Health and New England Biolabs. Also on May 20, at 4:00 p.m., Julie Segre of the National Institutes of Health will discuss her work using SMRT Sequencing in a talk entitled "Tracking Hospital Patients and Environment with Complete Genome Sequencing of Carbapenem-Resistant Klebsiella pneumoniae and other Enterobacteriaceae."
Jonas Korlach, Chief Scientific Officer for Pacific Biosciences commented: "SMRT Sequencing simplifies the genetic characterization of microbes by making high-accuracy finished de novo genome assembly rapid and affordable. Factors affecting virulence such as structural variation, horizontal gene transfer and methylation become readily apparent through comparison of these assemblies. These unique advantages have made SMRT Sequencing the gold standard for microbial sequencing, which is also evidenced by the rapidly increasing number of papers and genome announcements being published."
At last year's ASM annual meeting, Pacific Biosciences initiated a SMRT Grant Program to reward interesting proposals from attendees with free SMRT Cells and project support. The company announced today that the winner from last year was Michael Nelson of the University of Connecticut for his entry to sequence a putative novel Bacteroidete genus with importance to the digestive tract. Nelson experienced difficulty sequencing the genome of the type strain using short-read technologies, but using PacBio technology he was able to sequence the genome completely in one contig.
This year, Pacific Biosciences has opened up the SMRTest Microbe Grant Program to any eligible scientist. The scientist with the winning application will receive a free sequencing run on the PacBio RS II system using up to one SMRT Cell 8Pac and up to four library constructions for their project. More details, including the official rules of the Grant Program, are available at: www.pacb.com/smrtgrant.
A full listing of the podium and poster presentations featuring SMRT technology is listed here, and more information about the ASM annual meeting is available at: http://gm.asm.org/. Pacific Biosciences will be exhibiting its technology at Booth #1034.
About the PacBio RS II and SMRT(R) Sequencing
Pacific Biosciences' Single Molecule, Real-Time (SMRT) Sequencing technology achieves the industry's longest read lengths, highest consensus accuracyi,ii and the least degree of bias.iii These characteristics, combined with the ability to detect many types of DNA base modifications (e.g., methylation) as part of the sequencing process, make the PacBio RS II an essential tool for many scientists studying genetic and genomic variation. The PacBio platform provides a sequencing solution that can address a growing number of complex medical, agricultural and industrial problems.
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio RS II DNA Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT) technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems.
i Koren et al., "Reducing assembly complexity of microbial genomes with single-molecule sequencing." Genome Biology, 14:R10.1 (2013).
ii Chin et al., "Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data." Nature Methods, 10; 563-569 (2013).
iii Ross et al. Characterizing and measuring bias in sequence data. Genome Biol 14: R51 (2013).
View photo
.
Contact:
For Pacific Biosciences:
Media:
Nicole Litchfield
For Pacific Biosciences
415.793.6468
nicole@bioscribe.com
Investors:
Trevin Rard
Pacific Biosciences
650.521.8450
ir@pacificbiosciences.com
May 16, 2014
Pacific Biosciences is being awarded a sole source contract for the PacBio RS II Sequencer System from the National Heart, Lung, and Blood Institute (NHLBI) Office of Acquisition (OA) on behalf of the National Human Genome Research Institute, (NHGRI).
The National Human Genome Research Institute NHGRI), NIH Intramural Sequencing Center (NISC) is a high-throughput sequencing center supporting all institutes within the NIH. NISC has served the NIH community for over 15 years as a source for high-quality large-scale sequencing and analysis.
“As next generation sequencing applications expand, so does the demand for NISC services. NISC currently has one (1) HiSeq 25000, three (3) MiSeq Systems, and four (4) HiSeq 2000 Systems which are proven platforms for genetic analysis and functional genomics. However these instruments provide a maximum read length of 300 bases,” states the announcement. “Complex genomes cannot be de novo assembled into one piece with reads of this length. Typically even small microbial genomes are in 50-300 pieces even with 100x coverage.” Source: FBO.gov
Pacific Biosciences is being awarded a sole source contract for the PacBio RS II Sequencer System from the National Heart, Lung, and Blood Institute (NHLBI) Office of Acquisition (OA) on behalf of the National Human Genome Research Institute, (NHGRI).
The National Human Genome Research Institute NHGRI), NIH Intramural Sequencing Center (NISC) is a high-throughput sequencing center supporting all institutes within the NIH. NISC has served the NIH community for over 15 years as a source for high-quality large-scale sequencing and analysis.
“As next generation sequencing applications expand, so does the demand for NISC services. NISC currently has one (1) HiSeq 25000, three (3) MiSeq Systems, and four (4) HiSeq 2000 Systems which are proven platforms for genetic analysis and functional genomics. However these instruments provide a maximum read length of 300 bases,” states the announcement. “Complex genomes cannot be de novo assembled into one piece with reads of this length. Typically even small microbial genomes are in 50-300 pieces even with 100x coverage.” Source: FBO.gov
schon älterer Artikel aber nicht weniger interessant:
Blitzschnell in die Tiefe
Produktübersicht: Next Generation Sequencing
Bei kaum einer anderen Technik tut sich derzeit soviel wie bei der DNA-Sequenzierung.
Lange Zeit entwickelte sich die DNA-Sequenzierung in einem sehr gemächlichen Tempo. Noch in den späten achtziger Jahren musste man bei der von Sanger 1977 vorgestellten Kettenabbruchmethode mit riesigen, schlabberigen Sequenziergelen hantieren und mühsam Banden auswerten. Erleichterung brachten schließlich die 1990 eingeführten Kapillarelektrophorese-Sequenzierer, die die Sanger-Sequenzierung automatisierten (CE-Sequenzierung). Moderne CE-Sequenzierer erreichen Leseweiten von etwa 1000 Basenpaaren und lesen die DNA-Sequenz sehr präzise. Bis heute gilt ihre Genauigkeit deshalb als Goldstandard. Sie können jedoch nur einen Lesevorgang gleichzeitig ausführen und arbeiten daher sehr langsam.
Zu langsam für den amerikanischen Sequenzier-Spezialisten Jonathan Rothberg, der während seines Vaterschaftsurlaubs 1999 darüber nachdachte, wie er die Sequenzierung beschleunigen könnte. Dabei kam er auf die Idee, Millionen kurzer DNA-Fragmente in den Vertiefungen einer Picotiterplatte mit dem Pyrosequenzierungs-Verfahren parallel zu sequenzieren. Die Pyrosequenzierung fußt auf einer simplen biochemischen Reaktion: jedes mal wenn die Polymerase ein Nukleotid in den Matrizenstrang einbaut, wird ein Pyrophosphat (PPi)-Molekül abgespalten. Das freigewordene PPi setzt nachfolgend eine Luciferase-katalysierte Sekundär-Reaktion in Gang, in deren Verlauf ein Lumineszenz-Signal entsteht.
Massives paralleles Sequenzieren
Das Problem, Millionen von Reaktionsansätzen gleichzeitig ausführen zu müssen, löste Rothberg mit einer auf Kügelchen basierenden Emulsions-PCR (em-PCR). Bei dieser wird jeweils ein einzelnes DNA-Fragment je Kügelchen amplifiziert. Die Kügelchen mit den anhaftenden DNA-Amplikons verteilt man nachfolgend auf einer Picotiterplatte, die so konstruiert ist, dass jeweils nur ein Kügelchen in einem Näpfchen Platz findet. Um die Pyrosequenzierung zu starten, setzt man schließlich die nötigen Reagenzien und eines der vier Nukleotide zu und detektiert die Lichtsignale in den Näpfchen der Picotiterplatte.
Rothberg vermarktete seine Idee des massiven parallelen Sequenzierens (Next Generation Sequencing, NGS) zunächst über die Firma 454 Life Sciences, die er 2004 gründete. Aber schon drei Jahre später schnappte sich Roche, Rothenbergs Firma und gliederte sie in ihr Firmenportfolio ein.
454-Sequenzierer glänzen mit Leseweiten von bis zu 1000 Basen. Die langen Reads erkauft sich der Anwender jedoch sehr teuer. Eine chinesische Gruppe vom Beijing Genome Institut verglich die Kosten verschiedener NGS-Systeme, die pro eine Million Basenpaare (1 MB) entstehen. Bei dem 454 GS FLX-Sequenzierer kam sie auf etwa 10$ (Liu et al., Journal of Biomedicine and Biotechnology, Volume 2012, doi:10.1155/2012/251364). Ähnliche Zahlen findet man auch im „NGS Field Guide“ des amerikanischen Biologen Travis Glenn, (www.molecularecologist.com).
Unterschiedliche Stärken und Schwächen
Wesentlich günstiger sind die Sequenzierkosten pro Mb bei den 454-Konkurrenzsystemen von Illumina (HiSeq, MiSeq-Sequenzierer) und Life Technologies (SOLID-Sequenzierer). Was jedoch nicht automatisch bedeutet, dass diese immer die bessere Wahl sind. So produziert zum Beispiel die Sequenzierung-durch-Ligation-Methode der SOLID-Sequenzierer große Datenmengen (120 Gb pro lauf) und sehr kurze Leseweiten von 50 bis 75 Basen. Mit viel Rechenaufwand müssen diese zu einer sinnvollen DNA-Sequenz zusammengesetzt werden.
Keines der NGS-Flaggschiffe von 454, Illumina, und Life Technologies, das die Chinesen testeten, erreicht im übrigen die Genauigkeit eines „popeligen“ CE-Sequenzierers (ABI 3730xl), die bei etwa 99,999% liegt.
Die Preise für die Spitzengeräte von Roche, Illumina und Life Technologies liegen bei etwa 300.000 bis 500.000 €. Die Geräte in dieser Liga sind im Grunde nur etwas für Sequenzier-Zentren und Sequenzier-Dienstleister. Da dieser Markt recht überschaubar ist, treiben die Hersteller die Entwicklung kostengünstiger, kompakter NGS-Sequenzierer voran.
Hier war es einmal mehr Jonathan Rothberg, der 2010 mit dem Ion Torrent Sequenzierer die Nase vorn hatte. Wie das 454-System basiert auch dieser auf der Pyrosequenzierung mit einer Kügelchen-basierten emPCR als erstem Schritt. Revolutionär neu ist jedoch der weitere Verlauf. Die Myriaden Kügelchen mit klonal amplifizierten DNA-Fragmenten werden in den winzigen, Abermillionen Vertiefungen eines Halbleiterchips immobilisiert. Der Chip ist mit Protonen-sensitivem Tantaloxid beschichtet, das einzelne Protonen registriert, die während der Pyrosequenzierung beim Einbau der Nukleotide in den Matrizenstrang freigesetzt werden.
Im August 2010 nahm Life Technologies Ion Torrent unter seine Fittiche, mit Rothberg als neuem alten Chef. Kurze Zeit später kam die von Rothbergs Team weiterentwickelte Ion Torrent-Variante Ion PGM auf den Markt, die mittlerweile zum Verkaufsschlager avancierte. Am 10. Januar diesen Jahres stellte Life Technologies schließlich mit großem Tam-Tam auf der Konsum-Elektronik-Messe in Las Vegas das Nachfolgemodell Ion Proton vor, das laut Pressemeldung von Life Technologies das gesamte humane Genom an einem Tag zu einem Preis von 1000$ sequenzieren kann. Tatsache ist jedoch, dass auch Ion Torrent Sequenzierer Schwächen haben. Ihr größtes Manko, das sie mit ihrem 454-Verwandten teilen, sind hohe Fehlerraten bei homopolymeren DNA-Regionen, die zu Insertionen und Deletionen (Indels) führen. Wie sich der Ion PGM-Sequenzierer unter realen Laborbedingungen gegenüber seinen Rivalen von Illumina und Pacific Bioscience schlägt, kann man unter anderem bei Quail et al. nachlesen (BMC Genomics 2012, 13:341).
Nanoporen Sequenzierer
Kaum war der Hype um Life Technologies Ion Proton abgeebbt, da trat im Februar die von dem englischen Membranprotein-Spezialisten Hagan Bayley 2005 mitgegründete Firma Oxford Nanopore Technologies ins Rampenlicht und präsentierte einen Nanoporen-Sequenzierer.
Das Konzept der Nanoporen-Sequenzierung ist nicht neu. Bereits 1996 demonstrierten John Kasianowicz, Eric Brandin, Daniel Branton und David Deamer von der Harvard Universität, dass man einen DNA-Einzelstrang mit der Kraft eines elektrischen Feldes durch einen alpha-Hämolysin-Kanal bugsieren kann.
Auch Oxford Nanopore Technologies nutzt alpha-Hämolysin-Poren in synthetischen Membranen für die Sequenzierung. Schließt man eine Spannung an die Membran an, können Ionen die Hämolysin-Kanäle passieren, woraus ein messbarer Stromfluss resultiert. Auch größere Moleküle, etwa Nukleotide, passen durch den Kanal, blockieren jedoch einen Teil des Ionenflusses und reduzieren die gemessene Stromstärke. Dies nutzt Oxford Nanopore Technologies für zwei unterschiedliche Sequenzierungsstrategien: die Exonuklase Sequenzierung und die Strangsequenzierung. Bei ersterer koppelt man eine Exonuklease an die Hämolysin-Pore, die ein Nukleotid nach dem anderen von dem DNA-Strang abspaltet und in den Kanal schleust. Die einzelnen Nukleotide passieren den Kanal innerhalb weniger Millisekunden und lösen eine charakteristische Änderung des Stromsignals aus. Bei der Strangsequenzierung füttert eine Polymerase die Pore mit einem DNA-Einzelstrang. Dabei interagieren die durch den Kanal hindurch tretenden Basen des DNA-Strangs mit einer spezifischen Domäne des rekombinanten Kanalproteins. Das hierbei entstehende Signal ordnet ein angeschlossener Computer der entsprechenden Base zu.
Es braucht nicht viel Phantasie, um sich vorzustellen, dass bereits wenige tausend Poren genügen, um Millionen Basen in kürzester Zeit zu sequenzieren. Der zweite Trumpf, den die Nanoporen-Sequenzierung ausspielen kann, sind lange Leseweiten. Sequenzdaten von Oxford Nanopore Bioscience zufolge, erreichte ein Prototyp Leseweiten von 5 kB bei einem Durchsatz von 150 Mb in der Stunde. Das hört sich prima an, wäre nicht die hohe Fehlerrate von vier Prozent. Bis zur Markteinführung will die Firma die Fehlerquote jedoch deutlich senken.
Weniger Fehler mit Tags
Ein Grund für die vielen Fehler sind die geringen Unterschiede zwischen den Nukleotiden. Ein interessantes Konzept, wie man dieses Problem lösen könnte, verfolgt das kalifornische Nanoporen-Startup Genia Technologies, das der deutsche Stefan Röver 2009 mitgründete (ja, das ist der gleiche Stefan Röver, der sich auf dem Höhepunkt der Dotcom-Blase an der Pleite seiner Firma Brokat eine goldene Nase verdiente und die Aktionäre gründlich abzockte).
In Zusammenarbeit mit dem amerikanischen NGS-Guru George Church, dem Porenexperten John Kasianowicz und dem Spezialisten für Fluoreszenztags Jingyue Ju will die Firma einen Sequenzierer konstruieren, der auf dem Prinzip der Nanoporen-basierten Sequenzierung-durch-Synthese (NanoSBS) aufbaut.
Die Grundidee ist folgende: Eine am Eingang des Kanals fixierte Polymerase baut ein Nukleotid nach dem anderen in den Einzelstrang ein. Die Nukleotide sind mit jeweils unterschiedlichen Tags versehen, die beim Einbau abgespalten und in die Pore geschleust werden. Da die Tags größere Unterschiede untereinander aufweisen als einzelne Nukleotide, ist auch die Änderung der Stromstärke beim Passieren des Kanals wesentlich größer. Das Gerät sollte sie also genauer auseinander halten können und deutlich weniger Fehler machen. Soweit zumindest der Plan von Genia Technologies, das mit seinem Sequenzierer alle bisherigen Geräte in den Schatten stellen will.
(Erstveröffentlichung: H. Zähringer, Laborjournal 11/2012, Stand: Oktober 2012, alle Angaben ohne Gewähr)
Blitzschnell in die Tiefe
Produktübersicht: Next Generation Sequencing
Bei kaum einer anderen Technik tut sich derzeit soviel wie bei der DNA-Sequenzierung.
Lange Zeit entwickelte sich die DNA-Sequenzierung in einem sehr gemächlichen Tempo. Noch in den späten achtziger Jahren musste man bei der von Sanger 1977 vorgestellten Kettenabbruchmethode mit riesigen, schlabberigen Sequenziergelen hantieren und mühsam Banden auswerten. Erleichterung brachten schließlich die 1990 eingeführten Kapillarelektrophorese-Sequenzierer, die die Sanger-Sequenzierung automatisierten (CE-Sequenzierung). Moderne CE-Sequenzierer erreichen Leseweiten von etwa 1000 Basenpaaren und lesen die DNA-Sequenz sehr präzise. Bis heute gilt ihre Genauigkeit deshalb als Goldstandard. Sie können jedoch nur einen Lesevorgang gleichzeitig ausführen und arbeiten daher sehr langsam.
Zu langsam für den amerikanischen Sequenzier-Spezialisten Jonathan Rothberg, der während seines Vaterschaftsurlaubs 1999 darüber nachdachte, wie er die Sequenzierung beschleunigen könnte. Dabei kam er auf die Idee, Millionen kurzer DNA-Fragmente in den Vertiefungen einer Picotiterplatte mit dem Pyrosequenzierungs-Verfahren parallel zu sequenzieren. Die Pyrosequenzierung fußt auf einer simplen biochemischen Reaktion: jedes mal wenn die Polymerase ein Nukleotid in den Matrizenstrang einbaut, wird ein Pyrophosphat (PPi)-Molekül abgespalten. Das freigewordene PPi setzt nachfolgend eine Luciferase-katalysierte Sekundär-Reaktion in Gang, in deren Verlauf ein Lumineszenz-Signal entsteht.
Massives paralleles Sequenzieren
Das Problem, Millionen von Reaktionsansätzen gleichzeitig ausführen zu müssen, löste Rothberg mit einer auf Kügelchen basierenden Emulsions-PCR (em-PCR). Bei dieser wird jeweils ein einzelnes DNA-Fragment je Kügelchen amplifiziert. Die Kügelchen mit den anhaftenden DNA-Amplikons verteilt man nachfolgend auf einer Picotiterplatte, die so konstruiert ist, dass jeweils nur ein Kügelchen in einem Näpfchen Platz findet. Um die Pyrosequenzierung zu starten, setzt man schließlich die nötigen Reagenzien und eines der vier Nukleotide zu und detektiert die Lichtsignale in den Näpfchen der Picotiterplatte.
Rothberg vermarktete seine Idee des massiven parallelen Sequenzierens (Next Generation Sequencing, NGS) zunächst über die Firma 454 Life Sciences, die er 2004 gründete. Aber schon drei Jahre später schnappte sich Roche, Rothenbergs Firma und gliederte sie in ihr Firmenportfolio ein.
454-Sequenzierer glänzen mit Leseweiten von bis zu 1000 Basen. Die langen Reads erkauft sich der Anwender jedoch sehr teuer. Eine chinesische Gruppe vom Beijing Genome Institut verglich die Kosten verschiedener NGS-Systeme, die pro eine Million Basenpaare (1 MB) entstehen. Bei dem 454 GS FLX-Sequenzierer kam sie auf etwa 10$ (Liu et al., Journal of Biomedicine and Biotechnology, Volume 2012, doi:10.1155/2012/251364). Ähnliche Zahlen findet man auch im „NGS Field Guide“ des amerikanischen Biologen Travis Glenn, (www.molecularecologist.com).
Unterschiedliche Stärken und Schwächen
Wesentlich günstiger sind die Sequenzierkosten pro Mb bei den 454-Konkurrenzsystemen von Illumina (HiSeq, MiSeq-Sequenzierer) und Life Technologies (SOLID-Sequenzierer). Was jedoch nicht automatisch bedeutet, dass diese immer die bessere Wahl sind. So produziert zum Beispiel die Sequenzierung-durch-Ligation-Methode der SOLID-Sequenzierer große Datenmengen (120 Gb pro lauf) und sehr kurze Leseweiten von 50 bis 75 Basen. Mit viel Rechenaufwand müssen diese zu einer sinnvollen DNA-Sequenz zusammengesetzt werden.
Keines der NGS-Flaggschiffe von 454, Illumina, und Life Technologies, das die Chinesen testeten, erreicht im übrigen die Genauigkeit eines „popeligen“ CE-Sequenzierers (ABI 3730xl), die bei etwa 99,999% liegt.
Die Preise für die Spitzengeräte von Roche, Illumina und Life Technologies liegen bei etwa 300.000 bis 500.000 €. Die Geräte in dieser Liga sind im Grunde nur etwas für Sequenzier-Zentren und Sequenzier-Dienstleister. Da dieser Markt recht überschaubar ist, treiben die Hersteller die Entwicklung kostengünstiger, kompakter NGS-Sequenzierer voran.
Hier war es einmal mehr Jonathan Rothberg, der 2010 mit dem Ion Torrent Sequenzierer die Nase vorn hatte. Wie das 454-System basiert auch dieser auf der Pyrosequenzierung mit einer Kügelchen-basierten emPCR als erstem Schritt. Revolutionär neu ist jedoch der weitere Verlauf. Die Myriaden Kügelchen mit klonal amplifizierten DNA-Fragmenten werden in den winzigen, Abermillionen Vertiefungen eines Halbleiterchips immobilisiert. Der Chip ist mit Protonen-sensitivem Tantaloxid beschichtet, das einzelne Protonen registriert, die während der Pyrosequenzierung beim Einbau der Nukleotide in den Matrizenstrang freigesetzt werden.
Im August 2010 nahm Life Technologies Ion Torrent unter seine Fittiche, mit Rothberg als neuem alten Chef. Kurze Zeit später kam die von Rothbergs Team weiterentwickelte Ion Torrent-Variante Ion PGM auf den Markt, die mittlerweile zum Verkaufsschlager avancierte. Am 10. Januar diesen Jahres stellte Life Technologies schließlich mit großem Tam-Tam auf der Konsum-Elektronik-Messe in Las Vegas das Nachfolgemodell Ion Proton vor, das laut Pressemeldung von Life Technologies das gesamte humane Genom an einem Tag zu einem Preis von 1000$ sequenzieren kann. Tatsache ist jedoch, dass auch Ion Torrent Sequenzierer Schwächen haben. Ihr größtes Manko, das sie mit ihrem 454-Verwandten teilen, sind hohe Fehlerraten bei homopolymeren DNA-Regionen, die zu Insertionen und Deletionen (Indels) führen. Wie sich der Ion PGM-Sequenzierer unter realen Laborbedingungen gegenüber seinen Rivalen von Illumina und Pacific Bioscience schlägt, kann man unter anderem bei Quail et al. nachlesen (BMC Genomics 2012, 13:341).
Nanoporen Sequenzierer
Kaum war der Hype um Life Technologies Ion Proton abgeebbt, da trat im Februar die von dem englischen Membranprotein-Spezialisten Hagan Bayley 2005 mitgegründete Firma Oxford Nanopore Technologies ins Rampenlicht und präsentierte einen Nanoporen-Sequenzierer.
Das Konzept der Nanoporen-Sequenzierung ist nicht neu. Bereits 1996 demonstrierten John Kasianowicz, Eric Brandin, Daniel Branton und David Deamer von der Harvard Universität, dass man einen DNA-Einzelstrang mit der Kraft eines elektrischen Feldes durch einen alpha-Hämolysin-Kanal bugsieren kann.
Auch Oxford Nanopore Technologies nutzt alpha-Hämolysin-Poren in synthetischen Membranen für die Sequenzierung. Schließt man eine Spannung an die Membran an, können Ionen die Hämolysin-Kanäle passieren, woraus ein messbarer Stromfluss resultiert. Auch größere Moleküle, etwa Nukleotide, passen durch den Kanal, blockieren jedoch einen Teil des Ionenflusses und reduzieren die gemessene Stromstärke. Dies nutzt Oxford Nanopore Technologies für zwei unterschiedliche Sequenzierungsstrategien: die Exonuklase Sequenzierung und die Strangsequenzierung. Bei ersterer koppelt man eine Exonuklease an die Hämolysin-Pore, die ein Nukleotid nach dem anderen von dem DNA-Strang abspaltet und in den Kanal schleust. Die einzelnen Nukleotide passieren den Kanal innerhalb weniger Millisekunden und lösen eine charakteristische Änderung des Stromsignals aus. Bei der Strangsequenzierung füttert eine Polymerase die Pore mit einem DNA-Einzelstrang. Dabei interagieren die durch den Kanal hindurch tretenden Basen des DNA-Strangs mit einer spezifischen Domäne des rekombinanten Kanalproteins. Das hierbei entstehende Signal ordnet ein angeschlossener Computer der entsprechenden Base zu.
Es braucht nicht viel Phantasie, um sich vorzustellen, dass bereits wenige tausend Poren genügen, um Millionen Basen in kürzester Zeit zu sequenzieren. Der zweite Trumpf, den die Nanoporen-Sequenzierung ausspielen kann, sind lange Leseweiten. Sequenzdaten von Oxford Nanopore Bioscience zufolge, erreichte ein Prototyp Leseweiten von 5 kB bei einem Durchsatz von 150 Mb in der Stunde. Das hört sich prima an, wäre nicht die hohe Fehlerrate von vier Prozent. Bis zur Markteinführung will die Firma die Fehlerquote jedoch deutlich senken.
Weniger Fehler mit Tags
Ein Grund für die vielen Fehler sind die geringen Unterschiede zwischen den Nukleotiden. Ein interessantes Konzept, wie man dieses Problem lösen könnte, verfolgt das kalifornische Nanoporen-Startup Genia Technologies, das der deutsche Stefan Röver 2009 mitgründete (ja, das ist der gleiche Stefan Röver, der sich auf dem Höhepunkt der Dotcom-Blase an der Pleite seiner Firma Brokat eine goldene Nase verdiente und die Aktionäre gründlich abzockte).
In Zusammenarbeit mit dem amerikanischen NGS-Guru George Church, dem Porenexperten John Kasianowicz und dem Spezialisten für Fluoreszenztags Jingyue Ju will die Firma einen Sequenzierer konstruieren, der auf dem Prinzip der Nanoporen-basierten Sequenzierung-durch-Synthese (NanoSBS) aufbaut.
Die Grundidee ist folgende: Eine am Eingang des Kanals fixierte Polymerase baut ein Nukleotid nach dem anderen in den Einzelstrang ein. Die Nukleotide sind mit jeweils unterschiedlichen Tags versehen, die beim Einbau abgespalten und in die Pore geschleust werden. Da die Tags größere Unterschiede untereinander aufweisen als einzelne Nukleotide, ist auch die Änderung der Stromstärke beim Passieren des Kanals wesentlich größer. Das Gerät sollte sie also genauer auseinander halten können und deutlich weniger Fehler machen. Soweit zumindest der Plan von Genia Technologies, das mit seinem Sequenzierer alle bisherigen Geräte in den Schatten stellen will.
(Erstveröffentlichung: H. Zähringer, Laborjournal 11/2012, Stand: Oktober 2012, alle Angaben ohne Gewähr)
Pacific Biosciences Reports Second Quarter 2014 Financial Results
GlobeNewswire
Pacific Biosciences of California, Inc. 4 minutes ago
MENLO PARK, Calif., July 24, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc. (PACB) today reported an 89% increase in revenue to $11.4 million for the second quarter, compared to $6.0 million for the second quarter of 2013.
Second quarter 2014 revenue reflects the delivery of eight PacBio(R) RS II systems, compared to three systems during the second quarter of 2013. Total revenue for the second quarter of 2014 also included $1.7 million of contractual revenue. The Company booked five PacBio RS II instrument orders during the period and ended the quarter with 10 instruments in backlog.
Gross profit for the quarter increased 195% to $3.1 million, resulting in a gross margin of 27.4%, compared to gross profit of $1.1 million and a gross margin of 17.5% for the second quarter of 2013.
Operating expenses totaled $21.4 million for the quarter, compared to $21.1 million for the second quarter of 2013. Operating expenses for the second quarters of 2014 and 2013 included non-cash stock-based compensation of $2.1 million and $2.3 million, respectively.
The net loss for the quarter was $19.1 million, compared to $20.5 million for the second quarter of 2013.
Cash and investments at June 30, 2014 totaled $105.0 million, compared to $112.5 million at December 31, 2013.
Quarterly Conference Call Information
Management will host a quarterly conference call to discuss its second quarter 2014 results today at 4:30 p.m. Eastern Time. Investors may listen to the call by dialing 1.888.366.7247, or if outside the U.S., by dialing +1.707.287.9330. The call will be webcast live and will be available for replay at Pacific Biosciences' website at http://investor.pacificbiosciences.com/.
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio(R) RS II Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT(R)) Sequencing technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems.
GlobeNewswire
Pacific Biosciences of California, Inc. 4 minutes ago
MENLO PARK, Calif., July 24, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc. (PACB) today reported an 89% increase in revenue to $11.4 million for the second quarter, compared to $6.0 million for the second quarter of 2013.
Second quarter 2014 revenue reflects the delivery of eight PacBio(R) RS II systems, compared to three systems during the second quarter of 2013. Total revenue for the second quarter of 2014 also included $1.7 million of contractual revenue. The Company booked five PacBio RS II instrument orders during the period and ended the quarter with 10 instruments in backlog.
Gross profit for the quarter increased 195% to $3.1 million, resulting in a gross margin of 27.4%, compared to gross profit of $1.1 million and a gross margin of 17.5% for the second quarter of 2013.
Operating expenses totaled $21.4 million for the quarter, compared to $21.1 million for the second quarter of 2013. Operating expenses for the second quarters of 2014 and 2013 included non-cash stock-based compensation of $2.1 million and $2.3 million, respectively.
The net loss for the quarter was $19.1 million, compared to $20.5 million for the second quarter of 2013.
Cash and investments at June 30, 2014 totaled $105.0 million, compared to $112.5 million at December 31, 2013.
Quarterly Conference Call Information
Management will host a quarterly conference call to discuss its second quarter 2014 results today at 4:30 p.m. Eastern Time. Investors may listen to the call by dialing 1.888.366.7247, or if outside the U.S., by dialing +1.707.287.9330. The call will be webcast live and will be available for replay at Pacific Biosciences' website at http://investor.pacificbiosciences.com/.
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio(R) RS II Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT(R)) Sequencing technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems.
Es wird auch charttechnisch spannend...

William Blair Maintains Market Perfom On Pacific Biosciences Following 2Q14 Results
July 25, 2014 5:02 PM EDT by SA Staff in Exclusive Area, Healthcare
After the markets closed on Thursday, July 24, Pacific Biosciences (PACB) reported second-quarter results that were in line with expectations, though the company’s bookings fell short of expectations (five bookings versus our estimate of nine).
In reaction to the results, William Blair analyst Amanda Murphy today maintained a Market Perform rating on PACB and no price target was given.
Murphy noted, “Revenue of $11.4 million was slightly below our estimate of $11.9 million and essentially in line with Street consensus of $11.3 million. Net loss per share was in line with our and Street consensus at $0.27. Despite the light bookings,which management attributed to customer logistical and tender delays, management reiterated full-year revenue guidance of 70% ($47 million to $48 million)”. She continued, “PacBio has established a leading position in long-read technology, which has proved useful in certain applications (e.g., HLA typing, de novo bacterial sequencing, transcript isoform sequencing). Management also stated that the Roche (RHHBY) partnership continues to track as planned and expects a milestone payment later this year. Though, at current valuations, estimates imply meaningful revenue growth beyond 2014, we continue to believe PacBio ultimately operates in a niche market and might be limited by the size and cost of its platform”.
Quelle: http://www.smarteranalyst.com/2014/07/25/william-blair-maint…
July 25, 2014 5:02 PM EDT by SA Staff in Exclusive Area, Healthcare
After the markets closed on Thursday, July 24, Pacific Biosciences (PACB) reported second-quarter results that were in line with expectations, though the company’s bookings fell short of expectations (five bookings versus our estimate of nine).
In reaction to the results, William Blair analyst Amanda Murphy today maintained a Market Perform rating on PACB and no price target was given.
Murphy noted, “Revenue of $11.4 million was slightly below our estimate of $11.9 million and essentially in line with Street consensus of $11.3 million. Net loss per share was in line with our and Street consensus at $0.27. Despite the light bookings,which management attributed to customer logistical and tender delays, management reiterated full-year revenue guidance of 70% ($47 million to $48 million)”. She continued, “PacBio has established a leading position in long-read technology, which has proved useful in certain applications (e.g., HLA typing, de novo bacterial sequencing, transcript isoform sequencing). Management also stated that the Roche (RHHBY) partnership continues to track as planned and expects a milestone payment later this year. Though, at current valuations, estimates imply meaningful revenue growth beyond 2014, we continue to believe PacBio ultimately operates in a niche market and might be limited by the size and cost of its platform”.
Quelle: http://www.smarteranalyst.com/2014/07/25/william-blair-maint…
Time to Focus on Pacific Biosciences of California (PACB) for Strong Earnings Growth Potential
July 31, 2014
by Zacks Equity Research Published on July 31, 2014
PACB
Growth stocks can be some of the most exciting picks in the market, as these high-flyers can captivate investors’ attention, and produce big gains as well. However, these can also lead on the downside when the growth story is over, so it is important to find companies which are still seeing strong growth prospects in their businesses.
One such company that might be well-positioned for future earnings growth is Pacific Biosciences of California, Inc. (PACB - Snapshot Report). This firm, which is in healthcare sector, saw EPS growth of 25.4% last year, and is looking great for this year too.
In fact, the current growth estimate for this year calls for earnings-per-share growth of 15.1%. Furthermore, the long-term growth rate is currently an impressive 30%, suggesting pretty good prospects for the long haul.
And if this wasn’t enough, the stock has actually seen estimates rise over the past month for the current fiscal year by a significant margin. Thanks to this rise in earnings estimates, PACB has a Zacks Rank #2 (Buy) which further underscores the potential for outperformance in this company.
So if you are looking for a fast growing stock that is still seeing plenty of opportunities on the horizon, make sure to consider PACB. Not only does it have double digit earnings growth prospect, but its impressive Zacks Rank suggests that analysts believe better days are ahead for PACB as well.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report >>
July 31, 2014
by Zacks Equity Research Published on July 31, 2014
PACB
Growth stocks can be some of the most exciting picks in the market, as these high-flyers can captivate investors’ attention, and produce big gains as well. However, these can also lead on the downside when the growth story is over, so it is important to find companies which are still seeing strong growth prospects in their businesses.
One such company that might be well-positioned for future earnings growth is Pacific Biosciences of California, Inc. (PACB - Snapshot Report). This firm, which is in healthcare sector, saw EPS growth of 25.4% last year, and is looking great for this year too.
In fact, the current growth estimate for this year calls for earnings-per-share growth of 15.1%. Furthermore, the long-term growth rate is currently an impressive 30%, suggesting pretty good prospects for the long haul.
And if this wasn’t enough, the stock has actually seen estimates rise over the past month for the current fiscal year by a significant margin. Thanks to this rise in earnings estimates, PACB has a Zacks Rank #2 (Buy) which further underscores the potential for outperformance in this company.
So if you are looking for a fast growing stock that is still seeing plenty of opportunities on the horizon, make sure to consider PACB. Not only does it have double digit earnings growth prospect, but its impressive Zacks Rank suggests that analysts believe better days are ahead for PACB as well.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report >>
ist hier ein beitrag entfernt worden?
und zwar der hier??
http://www.xconomy.com/san-francisco/2014/06/11/genia-sold-r…
Auszug:
..."The Genia acquisition gives Roche “a differentiated sequencing pipeline,” Zabrowski says. In its drive to offer its diagnostic tests on a sequencing system of its own, Roche doesn’t need to choose between the Genia instrument and PacBio’s, he says.
“It’s very possible the two platforms will complement each other,” Zabrowski says. PacBio is developing a new sequencer for Roche’s exclusive use in human genetic analysis, he says. “What we’ll see going forward is what are the best applications to be used with these technologies.”...
...Roever, a software entrepreneur before he founded Genia, envisions some day creating a handheld instrument that could sequence a human genome for $100—a sort of health care equivalent of Apple’s iPhone. He foresees that doctors will use such units to get quick answers during patient visits, and that public health workers in the field will quickly identify microbes causing infectious outbreaks.
Roever says the winners in the sequencing market will, like Apple, control all aspects of the user experience from hardware to diagnostic applications.
“That’s an opportunity that we have and Roche has,” Roever says...."
und zwar der hier??
http://www.xconomy.com/san-francisco/2014/06/11/genia-sold-r…
Auszug:
..."The Genia acquisition gives Roche “a differentiated sequencing pipeline,” Zabrowski says. In its drive to offer its diagnostic tests on a sequencing system of its own, Roche doesn’t need to choose between the Genia instrument and PacBio’s, he says.
“It’s very possible the two platforms will complement each other,” Zabrowski says. PacBio is developing a new sequencer for Roche’s exclusive use in human genetic analysis, he says. “What we’ll see going forward is what are the best applications to be used with these technologies.”...
...Roever, a software entrepreneur before he founded Genia, envisions some day creating a handheld instrument that could sequence a human genome for $100—a sort of health care equivalent of Apple’s iPhone. He foresees that doctors will use such units to get quick answers during patient visits, and that public health workers in the field will quickly identify microbes causing infectious outbreaks.
Roever says the winners in the sequencing market will, like Apple, control all aspects of the user experience from hardware to diagnostic applications.
“That’s an opportunity that we have and Roche has,” Roever says...."
28-Aug-2014
Other Events
ITEM 8.01. OTHER EVENTS.
As previously disclosed, on September 23, 2013, Pacific Biosciences of California, Inc. (the "Company") entered into a Development, Commercialization and License Agreement (the "Agreement") with F. Hoffman-La Roche Ltd ("Roche"), pursuant to which the Company: (i) agreed to develop diagnostic products for clinical use including sequencing systems and consumables based on the Company's proprietary Single Molecule, Real-Time (SMRT�) technology; (ii) granted Roche an exclusive right to commercialize, and an exclusive license to sell, the developed diagnostic products for clinical use, the exclusivity of which is contingent on achieving sales minimums to be established in the future and contingent on Roche not selling for clinical use any new sequencing instrument that competes with any diagnostic instrument system developed under the Agreement; and (iii) agreed to manufacture and supply certain products intended for clinical use as the exclusive supplier to Roche. Pursuant to the Agreement, the Company received from Roche a non-refundable up-front payment of $35 million and is eligible to receive up to an additional $40 million based upon the achievement of development milestones. The Agreement has an initial term of 13 years and provisions allowing Roche 5-year renewals.
On August 27, 2014, the Company achieved a development milestone under the Agreement, entitling the Company to receive from Roche a development milestone payment of $10 million. The proceeds from the development milestone will be recognized as contractual revenue in the Company's financial statements for the quarter ending September 30, 2014. The Company may receive up to an additional $30 million based on the achievement of additional development milestones in future periods.
Other Events
ITEM 8.01. OTHER EVENTS.
As previously disclosed, on September 23, 2013, Pacific Biosciences of California, Inc. (the "Company") entered into a Development, Commercialization and License Agreement (the "Agreement") with F. Hoffman-La Roche Ltd ("Roche"), pursuant to which the Company: (i) agreed to develop diagnostic products for clinical use including sequencing systems and consumables based on the Company's proprietary Single Molecule, Real-Time (SMRT�) technology; (ii) granted Roche an exclusive right to commercialize, and an exclusive license to sell, the developed diagnostic products for clinical use, the exclusivity of which is contingent on achieving sales minimums to be established in the future and contingent on Roche not selling for clinical use any new sequencing instrument that competes with any diagnostic instrument system developed under the Agreement; and (iii) agreed to manufacture and supply certain products intended for clinical use as the exclusive supplier to Roche. Pursuant to the Agreement, the Company received from Roche a non-refundable up-front payment of $35 million and is eligible to receive up to an additional $40 million based upon the achievement of development milestones. The Agreement has an initial term of 13 years and provisions allowing Roche 5-year renewals.
On August 27, 2014, the Company achieved a development milestone under the Agreement, entitling the Company to receive from Roche a development milestone payment of $10 million. The proceeds from the development milestone will be recognized as contractual revenue in the Company's financial statements for the quarter ending September 30, 2014. The Company may receive up to an additional $30 million based on the achievement of additional development milestones in future periods.
Krasses Insider Trading in PACB. Insider kaufen was das Zeug hält... da sollte doch mindestens der IPO-Preis von 16 US$ und darüber möglich sein.
http://finance.yahoo.com/q/it?s=PACB+Insider+Transactions
http://finance.yahoo.com/q/it?s=PACB+Insider+Transactions
New Alignment Method to Speed Up De Novo Assembly of Whole Genomes
By Bio-IT World Staff
August 15, 2014 | This Thursday, a team of bioinformaticians from the National Biodefense Analysis and Countermeasures Center, the University of Maryland College Park, and sequencing company Pacific Biosciences posted information on their tool MHAP to the life sciences preprint server bioRxiv. MHAP, or MinHash Alignment Process, is a dramatically faster method for ordering DNA fragments sequenced on long-read technologies like the PacBio RS II Sequencer or the Oxford Nanopore MinION, making it easier to assemble whole genomes from scratch without the use of a reference genome. Bio-IT World previously covered MHAP following a presentation by senior author Adam Phillippy at the PacBio User Group Meeting this June; however, the newly released paper features much greater detail, including assemblies of the human genome and four important model organisms.
As the price of DNA sequencing continues to fall, the barrier to analyzing genomes is increasingly the compute power needed to make sense of the raw sequencing data. This is especially true for de novo whole genome assembly, where software tools have typically fallen back on the brute force, computationally demanding method of comparing every DNA fragment's entire sequence against every other fragment in search of overlaps.
MHAP, created by Konstantin Berlin and Sergey Koren, instead renders each fragment as a short series of numbers generated through hash functions. Briefly, every DNA fragment fed into MHAP is split into a string of k-mers (the authors recommend 16-mers for a human genome). Each k-mer is then subjected to the same series of hash functions, which output short numbers. For each hash function, only the smallest of these numbers is stored to identify the DNA fragment. Thus, the fragment is reduced to one number for each hash function, and each of those numbers corresponds to a single k-mer. MHAP can then search for fragments with many of the same identifying numbers, indicating that they have k-mers in common; close matches are overlapped to assemble the whole genome.
According to the authors, this process is so fast that an E. coli genome can be assembled de novo using MHAP in essentially the same amount of time it would take to create a reference-guided assembly of the same genome from short Illumina reads. This would put de novo assembly within reach for small labs that previously only had access to the computing resources for reference-guided assembly. MHAP sees even greater gains in efficiency with larger, eukaryotic genomes.
The authors used MHAP for assembly of a human genome, plus genomes of the bacterium E. coli, the yeast S. cerevisiae, the fruit fly D. melanogaster, and the plant A. thaliana. All these assemblies will be made freely available in GenBank. While none of the assemblies has yet been thoroughly vetted, the authors suggest that their assemblies may have closed gaps in the D. melanogaster, A. thaliana and even human reference genomes, thanks to their ability to use long-read sequencers. The most significant benefit of MHAP, however, is the time and cost savings to genomics labs. Both the D. melanogaster and A. thaliana genomes were assembled on a desktop computer in a matter of days; compared to an assembly of the fruit fly genome using the previous alignment tool BLASR, MHAP used 600 times less computing power.
Quelle: http://www.bio-itworld.com/2014/8/15/new-alignment-method-sp…
By Bio-IT World Staff
August 15, 2014 | This Thursday, a team of bioinformaticians from the National Biodefense Analysis and Countermeasures Center, the University of Maryland College Park, and sequencing company Pacific Biosciences posted information on their tool MHAP to the life sciences preprint server bioRxiv. MHAP, or MinHash Alignment Process, is a dramatically faster method for ordering DNA fragments sequenced on long-read technologies like the PacBio RS II Sequencer or the Oxford Nanopore MinION, making it easier to assemble whole genomes from scratch without the use of a reference genome. Bio-IT World previously covered MHAP following a presentation by senior author Adam Phillippy at the PacBio User Group Meeting this June; however, the newly released paper features much greater detail, including assemblies of the human genome and four important model organisms.
As the price of DNA sequencing continues to fall, the barrier to analyzing genomes is increasingly the compute power needed to make sense of the raw sequencing data. This is especially true for de novo whole genome assembly, where software tools have typically fallen back on the brute force, computationally demanding method of comparing every DNA fragment's entire sequence against every other fragment in search of overlaps.
MHAP, created by Konstantin Berlin and Sergey Koren, instead renders each fragment as a short series of numbers generated through hash functions. Briefly, every DNA fragment fed into MHAP is split into a string of k-mers (the authors recommend 16-mers for a human genome). Each k-mer is then subjected to the same series of hash functions, which output short numbers. For each hash function, only the smallest of these numbers is stored to identify the DNA fragment. Thus, the fragment is reduced to one number for each hash function, and each of those numbers corresponds to a single k-mer. MHAP can then search for fragments with many of the same identifying numbers, indicating that they have k-mers in common; close matches are overlapped to assemble the whole genome.
According to the authors, this process is so fast that an E. coli genome can be assembled de novo using MHAP in essentially the same amount of time it would take to create a reference-guided assembly of the same genome from short Illumina reads. This would put de novo assembly within reach for small labs that previously only had access to the computing resources for reference-guided assembly. MHAP sees even greater gains in efficiency with larger, eukaryotic genomes.
The authors used MHAP for assembly of a human genome, plus genomes of the bacterium E. coli, the yeast S. cerevisiae, the fruit fly D. melanogaster, and the plant A. thaliana. All these assemblies will be made freely available in GenBank. While none of the assemblies has yet been thoroughly vetted, the authors suggest that their assemblies may have closed gaps in the D. melanogaster, A. thaliana and even human reference genomes, thanks to their ability to use long-read sequencers. The most significant benefit of MHAP, however, is the time and cost savings to genomics labs. Both the D. melanogaster and A. thaliana genomes were assembled on a desktop computer in a matter of days; compared to an assembly of the fruit fly genome using the previous alignment tool BLASR, MHAP used 600 times less computing power.
Quelle: http://www.bio-itworld.com/2014/8/15/new-alignment-method-sp…
Human Longevity Inc. Adds PacBio(R) Sequencing to Enable Additional Insight Into Human Genetic Variation
Purchase of Two PacBio RS II Sequencing Systems Will Be Used to Help Build HLI's Genotype/Phenotype Database
Pacific Biosciences of California, Inc.
MENLO PARK, Calif., Oct. 6, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc., (PACB) provider of the PacBio(R) RS II DNA Sequencing System, today announced that Human Longevity Inc. (HLI) has added Single Molecule, Real-Time (SMRT(R)) Sequencing technology to the repertoire of DNA sequencing technologies they are using to compile the most comprehensive and complete human genotype, microbiome, and phenotype database.
HLI selected the new instruments in recognition of the unique characteristics of SMRT Sequencing, which cannot be found in other available DNA sequencing technologies. J. Craig Venter, PhD, HLI's Co-founder, Chairman, and Chief Executive Officer, said: "Each new sequencing technology has its own unique strengths, and we recognize the value of very long PacBio reads for covering regions of the human genome that we saw with Sanger sequencing. Long-read sequencing also provides higher confidence for calling structural variants and phasing haplotypes, both of which we think will be critically important for understanding the human genome. To get to a medical grade genome, or a genome that can be used for clinical diagnostic purposes, we need to have the most accurate and complete genome for each individual. We believe that the PacBio SMRT machines will help us reach this goal."
A number of recent publications and presentations have highlighted the advantages of using long-read SMRT Sequencing data to extend the view of human genetic variation. The long sequence reads provided by the PacBio RS II are complementary to short reads from high-throughput machines because they enable human genome researchers to, for example, sequence complex genomic regions, resolve structural variation, phase haplotypes, improve the utility and mapping ability of reference genomes, fill gaps, and validate variant calls. This provides the most comprehensive view possible of the human genome.
Michael Hunkapiller, President and Chief Executive Officer of Pacific Biosciences, said: "Even organizations like Craig's with access to high-throughput sequencing instruments are realizing that in order to capitalize on their investment they need PacBio sequencing to see what all other DNA sequencing technologies are missing. As we recently demonstrated with our Macrogen instrument sales, this is creating a great market opportunity for us. We are excited to be part of these large-scale initiatives and to be providing unique value to our customers."
About the PacBio RS II and SMRT Sequencing
Pacific Biosciences' SMRT Sequencing technology achieves the industry's longest read lengths, highest consensus accuracy, and the least degree of bias. These characteristics, combined with the ability to detect many types of DNA base modifications (e.g., methylation) as part of the sequencing process, make the PacBio RS II an essential tool for many scientists studying genetic and genomic variation. The PacBio platform provides a sequencing solution that can address a growing number of complex medical, agricultural, and industrial problems.
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio RS II DNA Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT) technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate, and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems. More information is available at www.pacb.com
Purchase of Two PacBio RS II Sequencing Systems Will Be Used to Help Build HLI's Genotype/Phenotype Database
Pacific Biosciences of California, Inc.
MENLO PARK, Calif., Oct. 6, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc., (PACB) provider of the PacBio(R) RS II DNA Sequencing System, today announced that Human Longevity Inc. (HLI) has added Single Molecule, Real-Time (SMRT(R)) Sequencing technology to the repertoire of DNA sequencing technologies they are using to compile the most comprehensive and complete human genotype, microbiome, and phenotype database.
HLI selected the new instruments in recognition of the unique characteristics of SMRT Sequencing, which cannot be found in other available DNA sequencing technologies. J. Craig Venter, PhD, HLI's Co-founder, Chairman, and Chief Executive Officer, said: "Each new sequencing technology has its own unique strengths, and we recognize the value of very long PacBio reads for covering regions of the human genome that we saw with Sanger sequencing. Long-read sequencing also provides higher confidence for calling structural variants and phasing haplotypes, both of which we think will be critically important for understanding the human genome. To get to a medical grade genome, or a genome that can be used for clinical diagnostic purposes, we need to have the most accurate and complete genome for each individual. We believe that the PacBio SMRT machines will help us reach this goal."
A number of recent publications and presentations have highlighted the advantages of using long-read SMRT Sequencing data to extend the view of human genetic variation. The long sequence reads provided by the PacBio RS II are complementary to short reads from high-throughput machines because they enable human genome researchers to, for example, sequence complex genomic regions, resolve structural variation, phase haplotypes, improve the utility and mapping ability of reference genomes, fill gaps, and validate variant calls. This provides the most comprehensive view possible of the human genome.
Michael Hunkapiller, President and Chief Executive Officer of Pacific Biosciences, said: "Even organizations like Craig's with access to high-throughput sequencing instruments are realizing that in order to capitalize on their investment they need PacBio sequencing to see what all other DNA sequencing technologies are missing. As we recently demonstrated with our Macrogen instrument sales, this is creating a great market opportunity for us. We are excited to be part of these large-scale initiatives and to be providing unique value to our customers."
About the PacBio RS II and SMRT Sequencing
Pacific Biosciences' SMRT Sequencing technology achieves the industry's longest read lengths, highest consensus accuracy, and the least degree of bias. These characteristics, combined with the ability to detect many types of DNA base modifications (e.g., methylation) as part of the sequencing process, make the PacBio RS II an essential tool for many scientists studying genetic and genomic variation. The PacBio platform provides a sequencing solution that can address a growing number of complex medical, agricultural, and industrial problems.
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio RS II DNA Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT) technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate, and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems. More information is available at www.pacb.com
Pacific Biosciences Releases New DNA Sequencing Chemistry to Enhance Read Length and Accuracy for the Study of Human and Other Complex Genomes
New Chemistry Boosts Accuracy and Increases Average Read Length to 10,000 to 15,000 Bases Accelerating Innovative Genomic Research
Pacific Biosciences of California, Inc. 10 hours ago GlobeNewswire
MENLO PARK, Calif., Oct. 15, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc., (PACB) provider of the PacBio(R) RS II DNA Sequencing System, today announced the release of new chemistry and software designed to enhance the performance and output of the platform by 45%. The latest release increases read length and improves accuracy to further accelerate innovative genomic research in complex organisms such as humans, plants, and animals.
The new release, P6-C4, which represents the company's 6th generation of polymerase and 4th generation chemistry, further extends the industry's leading average read length to 10,000 - 15,000 bases, with the longest reads exceeding 40,000 bases. The throughput with the new chemistry is expected to be between 500 million to 1 billion bases per SMRT(R) Cell, depending on the sample being sequenced. By providing an increasing number of longer reads per instrument run, the new chemistry enables users to assemble genomes to a higher quality.
A complete genome is an extremely valuable tool in many types of scientific research, yet most organisms do not have their complete genome assembled — including humans. Instead, complex genomes are only partially completed with difficult repetitive regions and longer structural variants excluded from the assembly, resulting in highly fragmented, incomplete genomes. To sequence the complete genome of an organism, very long sequencing reads are required to span these regions, which can be critical in understanding gene function. This missing information is becoming increasingly recognized as important to a full understanding of any organism. The ability of Single Molecule, Real-Time (SMRT) Sequencing to generate ultra-long reads with unbiased coverage allows researchers to characterize previously undetected structural variants, highly repetitive regions, and distant genetic elements.
Among users with early access to the chemistry are researchers sequencing large genomes including Macrogen, who recently purchased multiple PacBio instruments to de novo assemble a Korean human genome to establish a better reference and expand their service offering.
The P6-C4 chemistry will replace the P5-C3 chemistry and is recommended for all SMRT Sequencing applications, including de novo assembly, targeted sequencing, isoform sequencing, minor variant detection, scaffolding, long-repeat spanning, SNP phasing, and structural variant analysis.
This new release also includes improvements to the SMRT Analysis software suite for long amplicon analysis and the Iso-Seq(TM) method. Together with chemistry enhancements, these advances boost accuracy, speed up analysis, and support sequencing of multiplexed amplicons of different sizes.
"The performance of this new chemistry reflects our commitment to consistently deliver significant improvements in throughput and accuracy to our expanding user base," said Kevin Corcoran, Senior Vice President of Market Development at Pacific Biosciences. "With the longest reads now exceeding 40,000 bases, SMRT Sequencing is rapidly becoming the go-to platform for phasing complex genomic regions like full-length HLA genes, and generating gold-standard genome assemblies."
Researchers attending the 2014 American Society of Human Genetics annual meeting in San Diego can attend a workshop on October 21 from 12:30-2:00 p.m. titled "A New Look at the Human Genome -- Novel Insights with Long-read PacBio(R) Sequencing" to learn more about the PacBio RS II and hear from customers who are using the system.
More information about the PacBio RS II and the latest product enhancements is available at: www.pacb.com.
New Chemistry Boosts Accuracy and Increases Average Read Length to 10,000 to 15,000 Bases Accelerating Innovative Genomic Research
Pacific Biosciences of California, Inc. 10 hours ago GlobeNewswire
MENLO PARK, Calif., Oct. 15, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc., (PACB) provider of the PacBio(R) RS II DNA Sequencing System, today announced the release of new chemistry and software designed to enhance the performance and output of the platform by 45%. The latest release increases read length and improves accuracy to further accelerate innovative genomic research in complex organisms such as humans, plants, and animals.
The new release, P6-C4, which represents the company's 6th generation of polymerase and 4th generation chemistry, further extends the industry's leading average read length to 10,000 - 15,000 bases, with the longest reads exceeding 40,000 bases. The throughput with the new chemistry is expected to be between 500 million to 1 billion bases per SMRT(R) Cell, depending on the sample being sequenced. By providing an increasing number of longer reads per instrument run, the new chemistry enables users to assemble genomes to a higher quality.
A complete genome is an extremely valuable tool in many types of scientific research, yet most organisms do not have their complete genome assembled — including humans. Instead, complex genomes are only partially completed with difficult repetitive regions and longer structural variants excluded from the assembly, resulting in highly fragmented, incomplete genomes. To sequence the complete genome of an organism, very long sequencing reads are required to span these regions, which can be critical in understanding gene function. This missing information is becoming increasingly recognized as important to a full understanding of any organism. The ability of Single Molecule, Real-Time (SMRT) Sequencing to generate ultra-long reads with unbiased coverage allows researchers to characterize previously undetected structural variants, highly repetitive regions, and distant genetic elements.
Among users with early access to the chemistry are researchers sequencing large genomes including Macrogen, who recently purchased multiple PacBio instruments to de novo assemble a Korean human genome to establish a better reference and expand their service offering.
The P6-C4 chemistry will replace the P5-C3 chemistry and is recommended for all SMRT Sequencing applications, including de novo assembly, targeted sequencing, isoform sequencing, minor variant detection, scaffolding, long-repeat spanning, SNP phasing, and structural variant analysis.
This new release also includes improvements to the SMRT Analysis software suite for long amplicon analysis and the Iso-Seq(TM) method. Together with chemistry enhancements, these advances boost accuracy, speed up analysis, and support sequencing of multiplexed amplicons of different sizes.
"The performance of this new chemistry reflects our commitment to consistently deliver significant improvements in throughput and accuracy to our expanding user base," said Kevin Corcoran, Senior Vice President of Market Development at Pacific Biosciences. "With the longest reads now exceeding 40,000 bases, SMRT Sequencing is rapidly becoming the go-to platform for phasing complex genomic regions like full-length HLA genes, and generating gold-standard genome assemblies."
Researchers attending the 2014 American Society of Human Genetics annual meeting in San Diego can attend a workshop on October 21 from 12:30-2:00 p.m. titled "A New Look at the Human Genome -- Novel Insights with Long-read PacBio(R) Sequencing" to learn more about the PacBio RS II and hear from customers who are using the system.
More information about the PacBio RS II and the latest product enhancements is available at: www.pacb.com.
Pacific Biosciences of California, Inc.
October 23, 2014 4:00 PM
GlobeNewswire
. ˠ
➕
✕
.
..
.
.
MENLO PARK, Calif., Oct. 23, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc. (PACB) today reported a 178% increase in revenue to $20.6 million for the third quarter ended September 30, 2014, compared to $7.4 million for the third quarter of 2013. Third quarter 2014 revenue included the achievement of the $10.0 million milestone related to Roche along with the $1.7 million quarterly amortization from the upfront Roche payment in addition to the delivery of six PacBio(R) RS II systems. Third quarter 2013 revenue reflected the delivery of six systems and did not include any Roche related revenue. The Company also reported booking orders for 16 PacBio RS II instruments during the period, ending the quarter with 20 instruments in backlog.
Gross profit increased $12.0 million to $13.2 million for the quarter, resulting in a gross margin of 63.8%, compared to gross profit of $1.2 million and a gross margin of 16.7% for the third quarter of 2013. Gross margin increased due to an increase in Roche contractual revenue.
Operating expenses totaled $21.6 million for the quarter, compared to $21.2 million for the third quarter of 2013. Operating expenses for the third quarters of 2014 and 2013 included non-cash stock-based compensation of $2.2 million and $2.1 million, respectively.
The net loss for the quarter was $9.2 million, compared to $20.5 million for the third quarter of 2013.
Cash and investments at September 30, 2014 totaled $99.3 million, compared to $112.5 million at December 31, 2013.
October 23, 2014 4:00 PM
GlobeNewswire
. ˠ
➕
✕
.
..
.
.
MENLO PARK, Calif., Oct. 23, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc. (PACB) today reported a 178% increase in revenue to $20.6 million for the third quarter ended September 30, 2014, compared to $7.4 million for the third quarter of 2013. Third quarter 2014 revenue included the achievement of the $10.0 million milestone related to Roche along with the $1.7 million quarterly amortization from the upfront Roche payment in addition to the delivery of six PacBio(R) RS II systems. Third quarter 2013 revenue reflected the delivery of six systems and did not include any Roche related revenue. The Company also reported booking orders for 16 PacBio RS II instruments during the period, ending the quarter with 20 instruments in backlog.
Gross profit increased $12.0 million to $13.2 million for the quarter, resulting in a gross margin of 63.8%, compared to gross profit of $1.2 million and a gross margin of 16.7% for the third quarter of 2013. Gross margin increased due to an increase in Roche contractual revenue.
Operating expenses totaled $21.6 million for the quarter, compared to $21.2 million for the third quarter of 2013. Operating expenses for the third quarters of 2014 and 2013 included non-cash stock-based compensation of $2.2 million and $2.1 million, respectively.
The net loss for the quarter was $9.2 million, compared to $20.5 million for the third quarter of 2013.
Cash and investments at September 30, 2014 totaled $99.3 million, compared to $112.5 million at December 31, 2013.
Pacific Biosciences of California, Inc.
October 23, 2014 4:00 PM
GlobeNewswire
MENLO PARK, Calif., Oct. 23, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc. (PACB) today reported a 178% increase in revenue to $20.6 million for the third quarter ended September 30, 2014, compared to $7.4 million for the third quarter of 2013. Third quarter 2014 revenue included the achievement of the $10.0 million milestone related to Roche along with the $1.7 million quarterly amortization from the upfront Roche payment in addition to the delivery of six PacBio(R) RS II systems. Third quarter 2013 revenue reflected the delivery of six systems and did not include any Roche related revenue. The Company also reported booking orders for 16 PacBio RS II instruments during the period, ending the quarter with 20 instruments in backlog.
Gross profit increased $12.0 million to $13.2 million for the quarter, resulting in a gross margin of 63.8%, compared to gross profit of $1.2 million and a gross margin of 16.7% for the third quarter of 2013. Gross margin increased due to an increase in Roche contractual revenue.
Operating expenses totaled $21.6 million for the quarter, compared to $21.2 million for the third quarter of 2013. Operating expenses for the third quarters of 2014 and 2013 included non-cash stock-based compensation of $2.2 million and $2.1 million, respectively.
The net loss for the quarter was $9.2 million, compared to $20.5 million for the third quarter of 2013.
Cash and investments at September 30, 2014 totaled $99.3 million, compared to $112.5 million at December 31, 2013.
October 23, 2014 4:00 PM
GlobeNewswire
MENLO PARK, Calif., Oct. 23, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc. (PACB) today reported a 178% increase in revenue to $20.6 million for the third quarter ended September 30, 2014, compared to $7.4 million for the third quarter of 2013. Third quarter 2014 revenue included the achievement of the $10.0 million milestone related to Roche along with the $1.7 million quarterly amortization from the upfront Roche payment in addition to the delivery of six PacBio(R) RS II systems. Third quarter 2013 revenue reflected the delivery of six systems and did not include any Roche related revenue. The Company also reported booking orders for 16 PacBio RS II instruments during the period, ending the quarter with 20 instruments in backlog.
Gross profit increased $12.0 million to $13.2 million for the quarter, resulting in a gross margin of 63.8%, compared to gross profit of $1.2 million and a gross margin of 16.7% for the third quarter of 2013. Gross margin increased due to an increase in Roche contractual revenue.
Operating expenses totaled $21.6 million for the quarter, compared to $21.2 million for the third quarter of 2013. Operating expenses for the third quarters of 2014 and 2013 included non-cash stock-based compensation of $2.2 million and $2.1 million, respectively.
The net loss for the quarter was $9.2 million, compared to $20.5 million for the third quarter of 2013.
Cash and investments at September 30, 2014 totaled $99.3 million, compared to $112.5 million at December 31, 2013.
Mon, Nov 10, 2014, 1:31pm EST - US Markets close in 2 hrs and 29 mins
Recent
PACB +0.77% 6.57
Researchers Use PacBio Sequencing to Create More Complete Human Genome Reference and Discover New Forms of Structural Variation; Paper Published in Nature
Several New Studies Highlight Growing Trend of Scientists Using SMRT(R) Sequencing Technology to Reveal Complexity and Medically Relevant Information in the Human Genome
Pacific Biosciences of California, Inc. 2 hours ago GlobeNewswire
MENLO PARK, Calif., Nov. 10, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc., (PACB) provider of the PacBio(R) RS II Sequencing System, announced that a paper published online today in Nature1 entitled "Resolving the complexity of the human genome using single-molecule sequencing" demonstrates how researchers used Single Molecule, Real-Time (SMRT(R)) Sequencing technology to identify and resolve missing or misassembled regions of the human genome and to access more complex forms of variation not covered by other sequencing technologies.
Despite extensive efforts in human genome sequencing, more than 160 gaps remain in the human genome reference, and aspects of the structural architecture of human genomes are poorly understood due to limitations of the Sanger and short-read technologies. Researchers at the University of Washington led by Dr. Evan Eichler, Professor of Genome Sciences, collaborating with scientists from the University of Bari Aldo Moro, the University of Pittsburgh, and Pacific Biosciences used SMRT Sequencing data from a human genome cell line that is designated as a target for a 'platinum genome' reference assembly to close or extend 55% of the remaining gaps in the human GRCh37 reference genome. The team also resolved at the base-pair level the complete sequence of more than 26,000 structural variants—75% of which have not been previously reported—and discovered and validated other categories of complex variation that have been difficult to assess.
"The characteristics of the data produced by SMRT Sequencing technology differ significantly from other sequencing platforms," said Dr. Eichler. "With read lengths up to two orders of magnitude longer than second-generation sequencing technologies, we achieved high-confidence mapping across a greater percentage of the genome as well as accurate reconstruction of more complex genetic variation. More comprehensive access to human genetic variation is likely key to understanding disease and disease susceptibility."
Dr. Eichler's laboratory has also been using SMRT Sequencing to interrogate specific regions of human and chimpanzee genomes associated with rapid evolution and certain diseases. Xander Nuttle, a graduate student of Dr. Eichler, recently reported at the American Society of Human Genetics annual meeting on their work to resolve regions that are deleted, duplicated, or repeated in some humans and are associated with predisposition to one of the most common causes of autism. Critical to their discoveries was the use of the PacBio RS II Sequencing System, which allowed the team to accurately sequence and assemble these complex stretches of the human genome.
This paper joins other recent publications that highlight the power of SMRT Sequencing to elucidate the complex structure of human genomes and gene products, and their importance for understanding disease. A team of scientists from Brown University and the Icahn School of Medicine at Mt. Sinai reported in the journal Bioinformatics2 the development of new algorithms to detect structural genetic variation with high sensitivity and specificity. In another recent study, Dr. Flora Tassone and colleagues from the UC Davis MIND Institute reported in the Journal of Medical Genetics3 that different RNA isoforms of the FMR1 gene, which is responsible for Fragile X syndrome (the most common heritable form of cognitive impairment), premature menopause, ataxia syndrome and other disorders, can be directly sequenced and quantified using PacBio's Iso-Seq(TM) approach. This study provides, for the first time, a comprehensive map of the complex landscape of full-length FMR1 gene products, providing new insights that could play relevant roles in the pathology of these important disorders.
"A growing body of scientific evidence supports the use of SMRT Sequencing to obtain the most comprehensive view of the human genome, facilitating the most detailed understanding of the genetics of complex human diseases at both the DNA and RNA level," said Jonas Korlach, Chief Scientific Officer for Pacific Biosciences. "We are very excited by the success of our users who are uncovering important missing information in genomes and better characterizing genetic variation, which will benefit researchers, and ultimately patients, worldwide."
About the PacBio RS II and SMRT Sequencing
Pacific Biosciences' SMRT Sequencing technology achieves the industry's longest read lengths, highest consensus accuracy, and the least degree of bias. These characteristics, combined with the ability to detect many types of DNA base modifications (e.g., methylation) as part of the sequencing process, make the PacBio RS II an essential tool for many scientists studying genetic and genomic variation. The PacBio platform provides a sequencing solution that can address a growing number of complex medical, agricultural, and industrial problems.
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio RS II DNA Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT) technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate, and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems. More information is available at www.pacb.com
Recent
PACB +0.77% 6.57
Researchers Use PacBio Sequencing to Create More Complete Human Genome Reference and Discover New Forms of Structural Variation; Paper Published in Nature
Several New Studies Highlight Growing Trend of Scientists Using SMRT(R) Sequencing Technology to Reveal Complexity and Medically Relevant Information in the Human Genome
Pacific Biosciences of California, Inc. 2 hours ago GlobeNewswire
MENLO PARK, Calif., Nov. 10, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc., (PACB) provider of the PacBio(R) RS II Sequencing System, announced that a paper published online today in Nature1 entitled "Resolving the complexity of the human genome using single-molecule sequencing" demonstrates how researchers used Single Molecule, Real-Time (SMRT(R)) Sequencing technology to identify and resolve missing or misassembled regions of the human genome and to access more complex forms of variation not covered by other sequencing technologies.
Despite extensive efforts in human genome sequencing, more than 160 gaps remain in the human genome reference, and aspects of the structural architecture of human genomes are poorly understood due to limitations of the Sanger and short-read technologies. Researchers at the University of Washington led by Dr. Evan Eichler, Professor of Genome Sciences, collaborating with scientists from the University of Bari Aldo Moro, the University of Pittsburgh, and Pacific Biosciences used SMRT Sequencing data from a human genome cell line that is designated as a target for a 'platinum genome' reference assembly to close or extend 55% of the remaining gaps in the human GRCh37 reference genome. The team also resolved at the base-pair level the complete sequence of more than 26,000 structural variants—75% of which have not been previously reported—and discovered and validated other categories of complex variation that have been difficult to assess.
"The characteristics of the data produced by SMRT Sequencing technology differ significantly from other sequencing platforms," said Dr. Eichler. "With read lengths up to two orders of magnitude longer than second-generation sequencing technologies, we achieved high-confidence mapping across a greater percentage of the genome as well as accurate reconstruction of more complex genetic variation. More comprehensive access to human genetic variation is likely key to understanding disease and disease susceptibility."
Dr. Eichler's laboratory has also been using SMRT Sequencing to interrogate specific regions of human and chimpanzee genomes associated with rapid evolution and certain diseases. Xander Nuttle, a graduate student of Dr. Eichler, recently reported at the American Society of Human Genetics annual meeting on their work to resolve regions that are deleted, duplicated, or repeated in some humans and are associated with predisposition to one of the most common causes of autism. Critical to their discoveries was the use of the PacBio RS II Sequencing System, which allowed the team to accurately sequence and assemble these complex stretches of the human genome.
This paper joins other recent publications that highlight the power of SMRT Sequencing to elucidate the complex structure of human genomes and gene products, and their importance for understanding disease. A team of scientists from Brown University and the Icahn School of Medicine at Mt. Sinai reported in the journal Bioinformatics2 the development of new algorithms to detect structural genetic variation with high sensitivity and specificity. In another recent study, Dr. Flora Tassone and colleagues from the UC Davis MIND Institute reported in the Journal of Medical Genetics3 that different RNA isoforms of the FMR1 gene, which is responsible for Fragile X syndrome (the most common heritable form of cognitive impairment), premature menopause, ataxia syndrome and other disorders, can be directly sequenced and quantified using PacBio's Iso-Seq(TM) approach. This study provides, for the first time, a comprehensive map of the complex landscape of full-length FMR1 gene products, providing new insights that could play relevant roles in the pathology of these important disorders.
"A growing body of scientific evidence supports the use of SMRT Sequencing to obtain the most comprehensive view of the human genome, facilitating the most detailed understanding of the genetics of complex human diseases at both the DNA and RNA level," said Jonas Korlach, Chief Scientific Officer for Pacific Biosciences. "We are very excited by the success of our users who are uncovering important missing information in genomes and better characterizing genetic variation, which will benefit researchers, and ultimately patients, worldwide."
About the PacBio RS II and SMRT Sequencing
Pacific Biosciences' SMRT Sequencing technology achieves the industry's longest read lengths, highest consensus accuracy, and the least degree of bias. These characteristics, combined with the ability to detect many types of DNA base modifications (e.g., methylation) as part of the sequencing process, make the PacBio RS II an essential tool for many scientists studying genetic and genomic variation. The PacBio platform provides a sequencing solution that can address a growing number of complex medical, agricultural, and industrial problems.
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio RS II DNA Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT) technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate, and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems. More information is available at www.pacb.com
die 8US$ sollten doch in diesem Jahr noch mindestens möglich sein

Sehr interessante Vergangenheit...
Tue, Dec 23, 2014, 4:46 AM EST - U.S. Markets open in 4 hrs 44 mins
Recent
PACB +2.67% 7.70
Pacific Biosciences Appoints Kathy Ordonez to Board of Directors
Industry Veteran Previously Served as SVP at Quest Diagnostics and CEO of Celera and of Roche Molecular Systems
Pacific Biosciences of California, Inc. December 18, 2014 9:00 AM GlobeNewswire
MENLO PARK, Calif., Dec. 18, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc., (PACB) provider of the PacBio(R) RS II Sequencing System, today announced that it has appointed industry veteran Kathy Ordonez to its Board of Directors. Ordonez has a 30 year history of leadership in the life sciences and diagnostics industries, including positions as Senior Vice President of Quest Diagnostics, Chief Executive Officer of Celera, founder of Celera Diagnostics, and President and Chief Executive Officer for Roche Molecular Systems.
In 2011 Ordonez joined Quest as part of its acquisition of Celera and was responsible for the company's Diagnostic Solutions businesses, including diagnostic products, life insurer services, clinical trials and healthcare information technology products until she retired in 2013. Previously, she managed Quest's innovation pipeline and diagnostic products businesses, led the Celera business (including Berkeley HeartLab) and was responsible for driving the company's focus on personalizing disease management through diagnostic products and services. Prior to the acquisition by Quest, Ordonez served as Chief Executive Officer of Celera and was a founder of Celera Diagnostics. Before joining Celera's parent company, Applera, in December 2000, Ordonez held a number of senior positions over a 15-year period with Hoffmann-La Roche. She oversaw the formation of Roche Molecular Systems, serving as President and Chief Executive Officer, and led the application of polymerase chain reaction technology to the diagnostic, research and forensic fields.
"With its exquisite sensitivity and accuracy, long-read sequencing offers tremendous promise for future success in clinical diagnostics," said Kathy Ordonez. "The remarkable advances Pacific Biosciences has demonstrated, which are already transforming biological studies in the research market, will be critical for driving the molecular diagnostics field forward. Demand for sequencing in that market is taking off, and I look forward to being involved with the company that stands to deliver unprecedented accuracy and completeness for such a critical application."
"Kathy is a highly respected life science and diagnostics executive and we are delighted that she's joining our Board," said Michael Hunkapiller, Chairman and Chief Executive Officer of Pacific Biosciences. "Her vision and guidance will be instrumental in shaping the next phase for PacBio as our products become more widely established both in the research market and, in the longer term, through our partnership with Roche Diagnostics."
About the PacBio RS II and SMRT Sequencing
Pacific Biosciences' SMRT Sequencing technology achieves the industry's longest read lengths, highest consensus accuracy, and the least degree of bias. These characteristics, combined with the ability to detect many types of DNA base modifications (e.g., methylation) as part of the sequencing process, make the PacBio RS II an essential tool for many scientists studying genetic and genomic variation. The PacBio platform provides a sequencing solution that can address a growing number of complex medical, agricultural, and industrial problems.
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio RS II DNA Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT) technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate, and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems. More information is available at www.pacb.com
View photo
.
Contact:
For Pacific Biosciences:
Media:
Nicole Litchfield
415.793.6468
nicole@bioscribe.com
Investors:
Trevin Rard
650.521.8450
ir@pacificbiosciences.com
Tue, Dec 23, 2014, 4:46 AM EST - U.S. Markets open in 4 hrs 44 mins
Recent
PACB +2.67% 7.70
Pacific Biosciences Appoints Kathy Ordonez to Board of Directors
Industry Veteran Previously Served as SVP at Quest Diagnostics and CEO of Celera and of Roche Molecular Systems
Pacific Biosciences of California, Inc. December 18, 2014 9:00 AM GlobeNewswire
MENLO PARK, Calif., Dec. 18, 2014 (GLOBE NEWSWIRE) -- Pacific Biosciences of California, Inc., (PACB) provider of the PacBio(R) RS II Sequencing System, today announced that it has appointed industry veteran Kathy Ordonez to its Board of Directors. Ordonez has a 30 year history of leadership in the life sciences and diagnostics industries, including positions as Senior Vice President of Quest Diagnostics, Chief Executive Officer of Celera, founder of Celera Diagnostics, and President and Chief Executive Officer for Roche Molecular Systems.
In 2011 Ordonez joined Quest as part of its acquisition of Celera and was responsible for the company's Diagnostic Solutions businesses, including diagnostic products, life insurer services, clinical trials and healthcare information technology products until she retired in 2013. Previously, she managed Quest's innovation pipeline and diagnostic products businesses, led the Celera business (including Berkeley HeartLab) and was responsible for driving the company's focus on personalizing disease management through diagnostic products and services. Prior to the acquisition by Quest, Ordonez served as Chief Executive Officer of Celera and was a founder of Celera Diagnostics. Before joining Celera's parent company, Applera, in December 2000, Ordonez held a number of senior positions over a 15-year period with Hoffmann-La Roche. She oversaw the formation of Roche Molecular Systems, serving as President and Chief Executive Officer, and led the application of polymerase chain reaction technology to the diagnostic, research and forensic fields.
"With its exquisite sensitivity and accuracy, long-read sequencing offers tremendous promise for future success in clinical diagnostics," said Kathy Ordonez. "The remarkable advances Pacific Biosciences has demonstrated, which are already transforming biological studies in the research market, will be critical for driving the molecular diagnostics field forward. Demand for sequencing in that market is taking off, and I look forward to being involved with the company that stands to deliver unprecedented accuracy and completeness for such a critical application."
"Kathy is a highly respected life science and diagnostics executive and we are delighted that she's joining our Board," said Michael Hunkapiller, Chairman and Chief Executive Officer of Pacific Biosciences. "Her vision and guidance will be instrumental in shaping the next phase for PacBio as our products become more widely established both in the research market and, in the longer term, through our partnership with Roche Diagnostics."
About the PacBio RS II and SMRT Sequencing
Pacific Biosciences' SMRT Sequencing technology achieves the industry's longest read lengths, highest consensus accuracy, and the least degree of bias. These characteristics, combined with the ability to detect many types of DNA base modifications (e.g., methylation) as part of the sequencing process, make the PacBio RS II an essential tool for many scientists studying genetic and genomic variation. The PacBio platform provides a sequencing solution that can address a growing number of complex medical, agricultural, and industrial problems.
About Pacific Biosciences
Pacific Biosciences of California, Inc. (PACB) offers the PacBio RS II DNA Sequencing System to help scientists solve genetically complex problems. Based on its novel Single Molecule, Real-Time (SMRT) technology, the company's products enable: targeted sequencing to more comprehensively characterize genetic variations; de novo genome assembly to more fully identify, annotate, and decipher genomic structures; and DNA base modification identification to help characterize epigenetic regulation and DNA damage. By providing access to information that was previously inaccessible, Pacific Biosciences enables scientists to increase their understanding of biological systems. More information is available at www.pacb.com
View photo
.
Contact:
For Pacific Biosciences:
Media:
Nicole Litchfield
415.793.6468
nicole@bioscribe.com
Investors:
Trevin Rard
650.521.8450
ir@pacificbiosciences.com
Antwort auf Beitrag Nr.: 48.288.031 von Hiobsbote am 11.11.14 16:52:40
Bingo!
11.11.2014 = 6,62$
30.12.2014 = 8,00$
Pacific Biosciences of California, Inc. (PACB)
-NasdaqGS Watchlist
8.00 Up 0.23(2.96%) 10:05AM EST -
Wahnsinn!
Zitat von Hiobsbote: die 8US$ sollten doch in diesem Jahr noch mindestens möglich sein
Bingo!
11.11.2014 = 6,62$
30.12.2014 = 8,00$
Pacific Biosciences of California, Inc. (PACB)
-NasdaqGS Watchlist
8.00 Up 0.23(2.96%) 10:05AM EST -
Wahnsinn!
Antwort auf Beitrag Nr.: 42.457.774 von Biobrandschutz am 08.12.11 21:55:55
Macht bis heute einen satten Kursgewinn von 5,08 USD/Aktie. An dieser Stelle blicke ich gerne wieder auf mein Eröffnungszitat zurück.
Zitat von Biobrandschutz:
"Pacific Biosciences of California Inc, hinter diesem zugegebenermaßen etwas sperrigen Firmennamen verbirgt sich eines der interessantesten Unternehmen der Medizintechnik. Pacific Biosciences entwickelt sozusagen die Schlüsseltechnologie, um mit erschwinglichen Geräten zur Genanalyse in eine Serienfertigung zu gehen.
Der IPO vor 1 1/2 Jahren lag aus Anlegersicht sehr ungünstig, denn wer zeichnete, hat zum Ende des Hypes einen kräftigen Aufschlag auf die Zukunftsträchtigkeit des Produktes gezahlt. Seither ging es stetig abwärts und inzwischen bemühen sich eine ganze Anzahl von Anwaltsfirmen mit Haifischmentalität um frustrierte Erstinvestoren, um über vermeintlich erfolgsaussichtenreiche Sammelklagen den Anlegern Genugtuung und sich selbst ein Salär zu verschaffen.
Pacific Biosciences hat - von den Börsenturbulenzen unbeeindruckt - in der Zwischenzeit tatsächlich ein Aggregat geschaffen, das die hohen technischen Ansprüche erfüllt und das die Grundlage für eine Serienfertigung bilden kann.
Pacific Biosciences ist auf einem sehr guten Weg - mal sehen, wann sich die bald wieder überzeugten Investoren rückbesinnen, auf den 08. Dez. 2011, dem Tag, als die Aktie für nur 2,60 USD gekauft werden konnte."
Macht bis heute einen satten Kursgewinn von 5,08 USD/Aktie. An dieser Stelle blicke ich gerne wieder auf mein Eröffnungszitat zurück.
und so schnell kann es gehen... PACB läuft und läuft und läuft...
Why Pacific Biosciences Pushed Higher Today
The sequencer maker highlights its new machine at the American Society of Human Genetics.
Brian Orelli
(TMFBiologyFool)
Oct 7, 2015 at 3:59PM
Images
What: Pacific Biosciences of California (NASDAQ:PACB), which is unveiling its new DNA sequencer at the American Society of Human Genetics (ASHG) this week, rose as much as 25% today before giving a little of it back in afternoon trading.
So what: ASHG is the perfect place for Pacific Biosciences to unveil its new Sequel System since most of its potential customers will be at the scientific meeting.
Pacbiosequel
Sequel System. Souce: PacBio
Today the company held a "workshop" -- a sales pitch by any other name -- highlighting how the longer sequence reads that its sequencer can produce help fill in parts of the genome that are difficult to sequence. It's also possible to make de novo assemblies of the sequences from a whole genome. The shorter sequence reads from rival Illumina (NASDAQ:ILMN) require a reference genome that can introduce bias and cause variations to be missed.
The longer-reads-are-better concept isn't really new. It's the main -- perhaps only -- reason that researchers would buy the company's old PacBio RS II. Despite the issues with shorter sequences, Illumina's system can sequence a genome at a substantially lower cost.
The new Sequel System is cheaper than the PacBio RS II. The company has disclosed the list price for the system of $350,000, but that doesn't tell you how much the actual sequencing will cost since there are consumables used by the machine that factor into the cost.
Now what: The lower cost should result in more sales of the Sequel System compared to the 150 PacBio RS systems Pacific Biosciences has in place. The ultimate cost per genome, which should continue to come down as the company makes more tweaks to the new system, will ultimately determine how much of Illumina's business Pacific Biosciences can take.
While researchers are important, the larger market for the system will likely come from Pacific Biosciences' partnership with Roche, which is developing diagnostics to be used on the machines. The initial launch for clinical research will happen in the second half of 2016 followed by actual diagnostics run on the Sequel System.
Why Pacific Biosciences Pushed Higher Today
The sequencer maker highlights its new machine at the American Society of Human Genetics.
Brian Orelli
(TMFBiologyFool)
Oct 7, 2015 at 3:59PM
Images
What: Pacific Biosciences of California (NASDAQ:PACB), which is unveiling its new DNA sequencer at the American Society of Human Genetics (ASHG) this week, rose as much as 25% today before giving a little of it back in afternoon trading.
So what: ASHG is the perfect place for Pacific Biosciences to unveil its new Sequel System since most of its potential customers will be at the scientific meeting.
Pacbiosequel
Sequel System. Souce: PacBio
Today the company held a "workshop" -- a sales pitch by any other name -- highlighting how the longer sequence reads that its sequencer can produce help fill in parts of the genome that are difficult to sequence. It's also possible to make de novo assemblies of the sequences from a whole genome. The shorter sequence reads from rival Illumina (NASDAQ:ILMN) require a reference genome that can introduce bias and cause variations to be missed.
The longer-reads-are-better concept isn't really new. It's the main -- perhaps only -- reason that researchers would buy the company's old PacBio RS II. Despite the issues with shorter sequences, Illumina's system can sequence a genome at a substantially lower cost.
The new Sequel System is cheaper than the PacBio RS II. The company has disclosed the list price for the system of $350,000, but that doesn't tell you how much the actual sequencing will cost since there are consumables used by the machine that factor into the cost.
Now what: The lower cost should result in more sales of the Sequel System compared to the 150 PacBio RS systems Pacific Biosciences has in place. The ultimate cost per genome, which should continue to come down as the company makes more tweaks to the new system, will ultimately determine how much of Illumina's business Pacific Biosciences can take.
While researchers are important, the larger market for the system will likely come from Pacific Biosciences' partnership with Roche, which is developing diagnostics to be used on the machines. The initial launch for clinical research will happen in the second half of 2016 followed by actual diagnostics run on the Sequel System.
Pacific Biosciences (PACB) is Reiterated by Cantor Fitzgerald to Buy, Raises Price Target to $18
January 4, 2016 ·
Pacific Biosciences (PACB) is Reiterated by Cantor Fitzgerald to Buy according to the research report released to the investors. The brokerage firm has raised the Price Target to $18 from a previous price target of $11 . The shares recommendation by the Brokerage Firm was released on Jan-4-2016.
January 4, 2016 ·
Pacific Biosciences (PACB) is Reiterated by Cantor Fitzgerald to Buy according to the research report released to the investors. The brokerage firm has raised the Price Target to $18 from a previous price target of $11 . The shares recommendation by the Brokerage Firm was released on Jan-4-2016.
Illumina falls on downgrade, new competition
October 2, 2015
Shares of gene-sequencing giant Illumina (NASDAQ:ILMN) tumbled to a nearly one-year low Thursday after the stock was downgraded and a competing product arrived earlier than expected.
Leerink analyst Dan Leonard downgraded Illumina to market perform from outperform and lowered his price target to 185 from 225, as he did not see any near-term catalysts for the stock.
Illumina stock was down 9.5% in afternoon trading, near 159.
“We think the outlook for nextgen sequencing (NGS) in biomedical research remains strong; that said, we think it unclear that the HiSeq 3000/4000 product cycle will compel upgrades/replacements at the same rate we saw with previous product cycles,” Leonard wrote in his research note.
Leonard also pointed out in a separate note that Pacific Biosciences (NASDAQ:PACB) had launched its new Sequel high-throughput gene-sequencing system late Wednesday, a week ahead of schedule. The Sequel competes with Illumina’s NextSeq system, but Leonard didn’t see it as that big a threat.
“Until we learn otherwise, we continue to believe it difficult to make money in this market for those not named Illumina, at least when it comes to direct instrument competition,” Leonard wrote.
Nonetheless, Pacific Biosciences stock was up 50% by Thursday afternoon, though it was still trading below 6
October 2, 2015
Shares of gene-sequencing giant Illumina (NASDAQ:ILMN) tumbled to a nearly one-year low Thursday after the stock was downgraded and a competing product arrived earlier than expected.
Leerink analyst Dan Leonard downgraded Illumina to market perform from outperform and lowered his price target to 185 from 225, as he did not see any near-term catalysts for the stock.
Illumina stock was down 9.5% in afternoon trading, near 159.
“We think the outlook for nextgen sequencing (NGS) in biomedical research remains strong; that said, we think it unclear that the HiSeq 3000/4000 product cycle will compel upgrades/replacements at the same rate we saw with previous product cycles,” Leonard wrote in his research note.
Leonard also pointed out in a separate note that Pacific Biosciences (NASDAQ:PACB) had launched its new Sequel high-throughput gene-sequencing system late Wednesday, a week ahead of schedule. The Sequel competes with Illumina’s NextSeq system, but Leonard didn’t see it as that big a threat.
“Until we learn otherwise, we continue to believe it difficult to make money in this market for those not named Illumina, at least when it comes to direct instrument competition,” Leonard wrote.
Nonetheless, Pacific Biosciences stock was up 50% by Thursday afternoon, though it was still trading below 6
Roche eyes acquisition of Pacific Biosciences-sources
Reuters
31 minutes ago
Related Quotes
PACB12.48+22.47%
ROG.VX260.70-1.36%
Pacific Biosciences of Califor …
Watchlist
12.48+2.29(22.47%)
NASDAQ3:31 PM EST
Roche eyes acquisition of Pacific Biosciences-sources Reuters 32 mins ago
Q4 2015 Pacific Biosciences of California Inc Earnings Release - After Market Close CCBN 8 hrs ago
More
Feb 2 (Reuters) - Roche Holding AG has in recent weeks approached Pacific Biosciences of California Inc to discuss acquiring the U.S. company, spurred by interest in its advanced gene-sequencing technology, people familiar with the matter said.
Talks between the two companies have not yet advanced because of disagreements over the price of a potential deal, the people said this week. There is no certainty that Roche will continue to pursue Pacific Biosciences, the people added.
The sources asked not to be identified because the discussions have not been public. Roche declined to comment, while Pacific Biosciences did not respond to requests for comment.
Reuters
31 minutes ago
Related Quotes
PACB12.48+22.47%
ROG.VX260.70-1.36%
Pacific Biosciences of Califor …
Watchlist
12.48+2.29(22.47%)
NASDAQ3:31 PM EST
Roche eyes acquisition of Pacific Biosciences-sources Reuters 32 mins ago
Q4 2015 Pacific Biosciences of California Inc Earnings Release - After Market Close CCBN 8 hrs ago
More
Feb 2 (Reuters) - Roche Holding AG has in recent weeks approached Pacific Biosciences of California Inc to discuss acquiring the U.S. company, spurred by interest in its advanced gene-sequencing technology, people familiar with the matter said.
Talks between the two companies have not yet advanced because of disagreements over the price of a potential deal, the people said this week. There is no certainty that Roche will continue to pursue Pacific Biosciences, the people added.
The sources asked not to be identified because the discussions have not been public. Roche declined to comment, while Pacific Biosciences did not respond to requests for comment.
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 236 | ||
| 97 | ||
| 96 | ||
| 63 | ||
| 57 | ||
| 40 | ||
| 34 | ||
| 32 | ||
| 30 | ||
| 24 |
| Wertpapier | Beiträge | |
|---|---|---|
| 23 | ||
| 19 | ||
| 19 | ||
| 18 | ||
| 18 | ||
| 18 | ||
| 17 | ||
| 17 | ||
| 16 | ||
| 16 |





















