Bausch Health ehemals VALEANT PHARMA Allergan-Übernahme beflügelt (Seite 152)
eröffnet am 30.04.14 17:10:46 von
neuester Beitrag 10.04.24 21:16:45 von
neuester Beitrag 10.04.24 21:16:45 von
Beiträge: 4.274
ID: 1.193.944
ID: 1.193.944
Aufrufe heute: 17
Gesamt: 390.061
Gesamt: 390.061
Aktive User: 0
ISIN: CA0717341071 · WKN: A2JQ1X · Symbol: BVF
6,5110
EUR
0,00 %
0,0000 EUR
Letzter Kurs 16.05.24 Tradegate
Neuigkeiten
15.05.24 · Accesswire |
14.05.24 · Accesswire |
09.05.24 · Accesswire |
02.05.24 · Accesswire |
25.04.24 · wO Chartvergleich |
Werte aus der Branche Pharmaindustrie
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 0,5100 | +134,48 | |
| 0,7000 | +75,00 | |
| 3,0000 | +42,18 | |
| 15,348 | +19,53 | |
| 0,5800 | +16,00 |
| Wertpapier | Kurs | Perf. % |
|---|---|---|
| 28,60 | -24,06 | |
| 1,3500 | -25,62 | |
| 0,7800 | -29,73 | |
| 2.849,50 | -83,34 | |
| 5,0100 | -90,93 |
Beitrag zu dieser Diskussion schreiben
Antwort auf Beitrag Nr.: 56.600.774 von marty44 am 03.01.18 15:21:57strong cash flow from operations
Oh das kommt mir doch sehr bekannt vor. Vor den Q3 Zahlen....
Oh das kommt mir doch sehr bekannt vor. Vor den Q3 Zahlen....
Valeant To Present At The 36th Annual J.P. Morgan Healthcare Conference
Tue Gutes und sprich darüber:https://ih.advfn.com/p.php?pid=nmona&article=76397934
LAVAL, Quebec, Jan. 3, 2018 /CNW/ -- Valeant Pharmaceuticals International, Inc. (NYSE: VRX and TSX: VRX) ("Valeant") today announced that Joseph C. Papa, chairman and chief executive officer, is scheduled to present at the 36th Annual J.P. Morgan Healthcare Conference in San Francisco on Wednesday, Jan. 10, 2018, at 10:30 a.m. PST (1:30 p.m. EST).
A live webcast and audio archive of the event will be available on the Investor Relations page of the Valeant web site at: http://ir.valeant.com/events-and-presentations/2018.
Valeant Pays Down $300 Million Of Senior Secured Term Loans
https://ih.advfn.com/p.php?pid=nmona&article=76398584LAVAL, Quebec, Jan. 3, 2018 /CNW/ -- Valeant Pharmaceuticals International, Inc. (NYSE: VRX and TSX: VRX) ("Valeant" or the "Company") today announced it has paid down an additional $300 million of its senior secured term loans, using cash on hand.
In aggregate, Valeant has now reduced its debt by more than $6.5 billion since the end of the first quarter of 2016. As of Dec. 31, 2017, the Company's total debt is approximately $25.7 billion.
"We have reduced our debt by an additional $300 million due to ongoing strong cash flow from operations," said Joseph C. Papa, chairman and CEO, Valeant. "In 2018, we will continue to invest in the core businesses that will drive the future growth of the Company, including durable product franchises and our innovative pipeline."
Antwort auf Beitrag Nr.: 56.599.691 von Berliner_Landstreicher am 03.01.18 14:11:41Hier in D gibt es ja hier ein Forum, auch wenn die Kohle von hier nicht Valeant zukommt:
https://www.psoriasis-netz.de/community/topic/24048-kyntheum…

https://www.psoriasis-netz.de/community/topic/24048-kyntheum…


Antwort auf Beitrag Nr.: 56.599.691 von Berliner_Landstreicher am 03.01.18 14:11:41Danke. Hatte ich, glaube ich, schon Anfang Dezember gepostet. Das Feedback zu Silq/ Brodalumab in den Patientenforen ist noch mager, aber der Dezember war da schon deutlich besser.
Bei den Krebsforen scheinen die Patienten offensichtlich deutlich aktiver zu schreiben.
Ich denke, daß bei Brodalumab der nachhaltige Verkaufsanstieg erst nach der Mund zu Mund Propaganda der Ärzte bei Erfolgserlebnissen kommt. Dann sicher aber gewaltig.
Die Shorties segeln auf dünnem Eis, wenn einige große Investoren bei VRX auf Turnaroundinvestment setzen.
Bei den Krebsforen scheinen die Patienten offensichtlich deutlich aktiver zu schreiben.
Ich denke, daß bei Brodalumab der nachhaltige Verkaufsanstieg erst nach der Mund zu Mund Propaganda der Ärzte bei Erfolgserlebnissen kommt. Dann sicher aber gewaltig.
Die Shorties segeln auf dünnem Eis, wenn einige große Investoren bei VRX auf Turnaroundinvestment setzen.
https://www.drugs.com/comments/brodalumab/siliq-for-plaque-p…
"I started Siliq 6 months ago at 90 % body coverage of plaque psoriasis. I cannot thank my derm enough for recommending this medication. For the first time in 20 years I can wear a dress!! After the first 3 months of usage I had less than 30 percent dryness on my skin. Highly recommended, you wont be disappointed."
Mein bescheidener sachlicher Beitrag.
"I started Siliq 6 months ago at 90 % body coverage of plaque psoriasis. I cannot thank my derm enough for recommending this medication. For the first time in 20 years I can wear a dress!! After the first 3 months of usage I had less than 30 percent dryness on my skin. Highly recommended, you wont be disappointed."
Mein bescheidener sachlicher Beitrag.

http://shortvolume.com
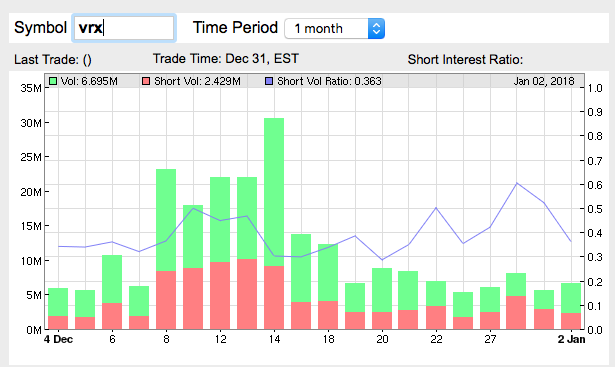
Das ist das schöne an den hohen Shortraten. Das Feuerwerk, wenn die Jungs zurückkaufen müssen.

Das ist das schöne an den hohen Shortraten. Das Feuerwerk, wenn die Jungs zurückkaufen müssen.

Ten most-read glaucoma articles usher in Glaucoma Awareness Month
https://www.healio.com/ophthalmology/glaucoma/news/online/%7…
pSivida to Present at the Annual Biotech Showcase Conference
https://www.econotimes.com/pSivida-to-Present-at-the-Annual-…Tuesday, January 2, 2018 9:01 PM UTC 0 comments
WATERTOWN, Mass., Jan. 02, 2018 -- pSivida Corp. (NASDAQ:PSDV) (ASX:PVA), a leader in the development of sustained release drug delivery products, announced today that Nancy Lurker, President and Chief Executive Officer, will present at the Annual Biotech Showcase conference on Monday, January 8, 2018 at 3:00 p.m. PT in San Francisco, CA.
A live audio and/or webcast and subsequent archived replay of pSivida’s presentations may be accessed via the Investors section of the Company’s website under “Resources - Events & Presentations” at www.psivida.com. The replay will be available for 90 days after the event.
About pSivida Corp.
pSivida Corp. (www.psivida.com), headquartered in Watertown, MA, is a leader in the development of sustained release drug products for treating eye diseases. pSivida has developed three of only four FDA-approved sustained-release treatments for back-of-the-eye diseases. The most recent, ILUVIEN®, a micro-insert for diabetic macular edema, licensed to Alimera Sciences, is currently sold directly in the U.S. and three EU countries. Retisert ®, an implant for posterior uveitis, is licensed to and sold by Bausch & Lomb. pSivida's lead product candidate, Durasert™ micro-insert for posterior segment uveitis, is being independently developed. Two pivotal Phase 3 studies with Durasert achieved their primary efficacy endpoint of prevention of recurrence of uveitis at six months of follow-up with statistical significance, and the Company plans to file an NDA in early January 2018. pSivida's pre-clinical development program is focused on using its core platform technology Durasert™ to deliver drugs to treat wet age-related macular degeneration, glaucoma, osteoarthritis and other diseases. To learn more about pSivida, please visit www.psivida.com and connect on Twitter, LinkedIn, Facebook and Google+.
Bausch + Lomb receives 510(k) clearance for Crystalsert 2.6 injector
https://www.healio.com/ophthalmology/ophthalmic-business/new…
14:00 Uhr · Accesswire · Bausch Health Companies |
25.04.24 · wO Chartvergleich · BAVARIA Industries Group Akt |





















